EP0198775B1 - Process for the continuous monitoring of the dissolved metal concentration in a molten salts bath and its use in the continuous feeding of these metal salts to an electrolysis cell - Google Patents
Process for the continuous monitoring of the dissolved metal concentration in a molten salts bath and its use in the continuous feeding of these metal salts to an electrolysis cell Download PDFInfo
- Publication number
- EP0198775B1 EP0198775B1 EP86420087A EP86420087A EP0198775B1 EP 0198775 B1 EP0198775 B1 EP 0198775B1 EP 86420087 A EP86420087 A EP 86420087A EP 86420087 A EP86420087 A EP 86420087A EP 0198775 B1 EP0198775 B1 EP 0198775B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- bath
- metal
- cell
- dissolved
- potential
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- 229910052751 metal Inorganic materials 0.000 title claims abstract description 32
- 239000002184 metal Substances 0.000 title claims abstract description 32
- 238000000034 method Methods 0.000 title claims abstract description 15
- 238000005868 electrolysis reaction Methods 0.000 title claims description 16
- 150000003839 salts Chemical class 0.000 title description 8
- 238000012544 monitoring process Methods 0.000 title 1
- 150000001805 chlorine compounds Chemical class 0.000 claims abstract description 15
- 229910052723 transition metal Inorganic materials 0.000 claims abstract description 13
- 150000003624 transition metals Chemical class 0.000 claims abstract description 13
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 claims abstract description 7
- 229910052731 fluorine Inorganic materials 0.000 claims abstract description 7
- 239000011737 fluorine Substances 0.000 claims abstract description 7
- 150000002222 fluorine compounds Chemical class 0.000 claims abstract description 5
- 229910052783 alkali metal Inorganic materials 0.000 claims abstract 2
- 150000001340 alkali metals Chemical class 0.000 claims abstract 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 claims description 8
- 238000004519 manufacturing process Methods 0.000 claims description 2
- 229910021381 transition metal chloride Inorganic materials 0.000 claims 1
- 230000007704 transition Effects 0.000 abstract 1
- 229910052735 hafnium Inorganic materials 0.000 description 12
- VBJZVLUMGGDVMO-UHFFFAOYSA-N hafnium atom Chemical compound [Hf] VBJZVLUMGGDVMO-UHFFFAOYSA-N 0.000 description 11
- 235000002639 sodium chloride Nutrition 0.000 description 7
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 4
- 239000004020 conductor Substances 0.000 description 4
- 239000000203 mixture Substances 0.000 description 4
- 239000003513 alkali Substances 0.000 description 3
- 239000000460 chlorine Substances 0.000 description 3
- 229910052801 chlorine Inorganic materials 0.000 description 3
- PDPJQWYGJJBYLF-UHFFFAOYSA-J hafnium tetrachloride Chemical class Cl[Hf](Cl)(Cl)Cl PDPJQWYGJJBYLF-UHFFFAOYSA-J 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical class [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 description 1
- 229910001514 alkali metal chloride Inorganic materials 0.000 description 1
- 229910001515 alkali metal fluoride Inorganic materials 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 238000009434 installation Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 229910052758 niobium Inorganic materials 0.000 description 1
- 239000010955 niobium Substances 0.000 description 1
- GUCVJGMIXFAOAE-UHFFFAOYSA-N niobium atom Chemical compound [Nb] GUCVJGMIXFAOAE-UHFFFAOYSA-N 0.000 description 1
- 230000010287 polarization Effects 0.000 description 1
- 235000011164 potassium chloride Nutrition 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052715 tantalum Inorganic materials 0.000 description 1
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 229910052720 vanadium Inorganic materials 0.000 description 1
- LEONUFNNVUYDNQ-UHFFFAOYSA-N vanadium atom Chemical compound [V] LEONUFNNVUYDNQ-UHFFFAOYSA-N 0.000 description 1
- 239000006200 vaporizer Substances 0.000 description 1
- 229910052726 zirconium Inorganic materials 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25C—PROCESSES FOR THE ELECTROLYTIC PRODUCTION, RECOVERY OR REFINING OF METALS; APPARATUS THEREFOR
- C25C7/00—Constructional parts, or assemblies thereof, of cells; Servicing or operating of cells
- C25C7/06—Operating or servicing
Definitions
- the present invention which results from work carried out in the laboratories of the lich Nationale Su Southerneure d'Electrochimie et d'Electrométallurgie de Grenoble, relates to a process for continuous control of the content of transition metal dissolved in a bath of molten salts and its application to the continuous supply of an electrolysis cell with salts of said metal.
- transition metals can be obtained industrially by continuous electrolysis in a cell of at least one of their chlorides previously dissolved in a bath of molten salts constituted by alkali chlorides and / or alkaline earth. He also knows from FR-A-1154129 that a mixture of alkali metal chloride and alkali metal fluoride can be used as molten salts.
- transition metal is understood here to mean any metal belonging to columns IVa, Va, Via of the specific classification of Mendeleev and in particular titanium, zirconium, hafnium, tantalum, niobium and vanadium.
- Continuous electrolysis is also understood to mean a process in which the deposition and extraction of the metal at the cathode and the release of chlorine at the anode are constantly compensated by an addition of fresh chloride; this contribution being intended to maintain the content of metal to be produced dissolved in the bath at a relatively constant value and preferably optimal, that is to say the most favorable for a good functioning of the cell.
- the cell In such a process, if it is actually wanted to keep the dissolved metal content at a constant value, the cell must be supplied with a quantity of fresh chlorides corresponding exactly to the quantity consumed by the cell.
- the generally adopted solution consists in periodically taking bath samples, analyzing them and adjusting accordingly the amounts of chloride in the metal to be fed. But this operation is not simple and above all its response is not immediate, so that if the drifts are more or less reduced periodically, the chloride content of the metal dissolved in the bath is rarely equal to the optimal content.
- the method therefore consists, knowing the optimal metal content to ensure proper functioning of the cell, to calculate from this content and the molar ratio claimed the amount of fluorides to be added.
- This fluoride is introduced into the bath at the time of its constitution.
- the cell being equipped with an indicator electrode and a reference electrode, the optimal amount of chloride of the transition metal to be deposited is charged and after a time sufficient to allow the dissolution, the potential is measured before starting the electrolysis properly said.
- the dissolved metal content can be confirmed by analysis of the bath.
- the cell is then put into service and regular operation can be achieved by supplying it with chlorides of the metal so that the measured potential remains constant. It is easy to see the application that can be made of this measurement to the continuous supply of the cell. It suffices to compare the potential measured at all times at the setpoint potential corresponding to the optimal content of the bath and to control the supply of chlorides accordingly. In this way, one can very finely regulate the flow of chlorides of the metal and have an extremely precise dissolved metal content throughout the electrolysis.
- the internal wall of the cell is metallic, it has been found that it could be used as an indicator electrode. Indeed, knowing that this must be constituted by the metal to be deposited, we tried during a preelectrolysis in the presence of the chloride of the metal to be deposited, to pass a direct current between the anode of the cell and the wall of the tank. Under these conditions, it has been found that the deposit of metal obtained on the tank can play perfectly thereafter the role of indicator electrode. If necessary, this deposit on the wall can be periodically reconstituted during electrolysis or even created permanently by cathodic polarization of the cell.
- the applicant has also sought to eliminate the installation of a reference electrode; it achieved this by replacing it with the cell anode.
- a reference electrode it achieved this by replacing it with the cell anode.
- an ohmic drop is established in the potential control circuit due to the electrolysis current I which passes through the anode. This disturbs the measurement and gives an incorrect indication of the actual content of dissolved chlorides in the bath.
- the applicant incorporates between the indicator electrode and the anode an ohmic drop corrector, the constitution of which is described below.
- a direct current source (13) whose positive pole is connected to the anode and the negative pole to the cathode of the cell respectively by the conductors (14) and (15);
- the terminals A and D of the device (16) are connected to a comparator (19) of the measured potential to the reference potential which sends a signal via the conductor (20) to the motor (11) in the event that the measured potential is in absolute value greater than the absolute value of the reference potential.
- the ohmic drop corrector comprising an operational amplifier denoted AOP, two resistors of the same value R1, a variable and adjustable resistance Rv.
- a calibration is carried out which consists in adjusting the value of Rv so as to obtain:
- This signal S is sent to the comparator (19).
- the invention finds its application in all cases where it is desired to manufacture transition metals by continuous electrolysis of their chlorides in the molten baths of alkali or alkaline earth chlorides.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Electrolytic Production Of Metals (AREA)
Abstract
Description
La présente invention qui résulte de travaux effectués dans les laboratoires de l'Ecole Nationale Supérieure d'Electrochimie et d'Electromé- tallurgie de Grenoble, est relative à un procédé de contrôle en continu de la teneur en métal de transition dissous dans un bain de sels fondus et à son application à l'alimentation continue d'une cellule d'électrolyse en sels dudit métal.The present invention which results from work carried out in the laboratories of the Ecole Nationale Supérieure d'Electrochimie et d'Electrométallurgie de Grenoble, relates to a process for continuous control of the content of transition metal dissolved in a bath of molten salts and its application to the continuous supply of an electrolysis cell with salts of said metal.
L'homme de l'art sait qu'on peut obtenir de façon industrielle, les métaux de transition par électrolyse en continu dans une cellule d'un au moins de leurs chlorures préalablement dissous dans un bain de sels fondus constitués par des chlorures alcalins et/ou alcalino-terreux. Il sait aussi de FR-A-1154129 qu'on peut utiliser comme sels fondus un mélange de chlorure de métal alcalin et de fluorure de métal alcalin.A person skilled in the art knows that the transition metals can be obtained industrially by continuous electrolysis in a cell of at least one of their chlorides previously dissolved in a bath of molten salts constituted by alkali chlorides and / or alkaline earth. He also knows from FR-A-1154129 that a mixture of alkali metal chloride and alkali metal fluoride can be used as molten salts.
On entend ici par métal de transition, tout métal appartenant aux colonnes IVa, Va, Via de la classification spécifique de Mendeleev et notamment le titane, le zirconium, le hafnium, le tantale, le niobium et le vanadium.The term “transition metal” is understood here to mean any metal belonging to columns IVa, Va, Via of the specific classification of Mendeleev and in particular titanium, zirconium, hafnium, tantalum, niobium and vanadium.
On entend également par électrolyse en continu, un procédé dans lequel le dépôt et l'extraction du métal à la cathode et le dégagement de chlore à l'anode sont en permanence compensés par un apport de chlorure frais ; cet apport étant destiné à maintenir la teneur en métal à produire dissous dans le bain à une valeur relativement constante et de préférence optimale, c'est-à-dire la plus favorable à un bon fonctionnement de la cellule.Continuous electrolysis is also understood to mean a process in which the deposition and extraction of the metal at the cathode and the release of chlorine at the anode are constantly compensated by an addition of fresh chloride; this contribution being intended to maintain the content of metal to be produced dissolved in the bath at a relatively constant value and preferably optimal, that is to say the most favorable for a good functioning of the cell.
Dans un tel procédé, si l'on veut effectivement maintenir la teneur en métal dissous à une valeur constante, il faut alimenter la cellule avec une quantité de chlorures frais correspondant exactement à la quantité consommée par la cellule.In such a process, if it is actually wanted to keep the dissolved metal content at a constant value, the cell must be supplied with a quantity of fresh chlorides corresponding exactly to the quantity consumed by the cell.
Théoriquement, cette quantité étant proportionnelle à la quantité de métal déposé à la cathode et donc à la quantité de courant électrique passant dans la cellule, il semble à première vue logique de se servir des mesures de l'intensité du courant d'électrolyse et du temps qui s'est écoulé pour déterminer la quantité de chlorures à alimenter. En réalité, du fait de fluctuations inévitables du courant, de l'alimentation en chlorure du métal et du rendement, on constate qu'une telle méthode conduit à des dérives de la teneur en métal dissous par rapport à la teneur optimale, d'autant plus importantes que le temps de fonctionnement de la cellule est long. C'est pourquoi il s'avère nécessaire de recourir à d'autres moyens pour contrôler de manière efficace la teneur du bain en métal dissous.Theoretically, this quantity being proportional to the quantity of metal deposited at the cathode and therefore to the quantity of electric current passing through the cell, it seems at first sight logical to use measurements of the intensity of the electrolysis current and of the time that has elapsed to determine the quantity of chlorides to be supplied. In reality, due to inevitable fluctuations in the current, the chloride supply of the metal and the yield, it is found that such a method leads to deviations in the dissolved metal content compared to the optimal content, all the more more important that the operating time of the cell is long. This is why it is necessary to resort to other means to effectively control the content of the dissolved metal bath.
La solution généralement adoptée consiste à effectuer périodiquement des prélèvements de bain, à les analyser et à ajuster en conséquence les quantités de chlorure du métal à alimenter. Mais cette opération n'est pas simple et surtout sa réponse n'est pas immédiate, de sorte que si les dérives sont plus au moins réduites périodiquement, la teneur en chlorure du métal dissous dans le bain est rarement égale à la teneur optimale.The generally adopted solution consists in periodically taking bath samples, analyzing them and adjusting accordingly the amounts of chloride in the metal to be fed. But this operation is not simple and above all its response is not immediate, so that if the drifts are more or less reduced periodically, the chloride content of the metal dissolved in the bath is rarely equal to the optimal content.
Une solution plus intéressante dans le sens qu'elle permet un contrôle continu de la composition du bain et donc une forte atténuation des dérivés a été également proposée. Elle consiste à utiliser des électrodes indicatrices du métal dissous constituées par une tige du métal à déposer et plongées dans le bain de sels. Ces électrodes en effet prennent normalement un potentiel qui est fonction de la teneur du bain en métal dissous. Il suffit alors de mesurer ce potentiel par rapport à une électrode de référence pour connaître la teneur que l'on cherche à contrôler. Malheureusement, on constate que pour une variation d'un facteur 10 de la teneur en métal dissous, la variation du potentiel ne dépasse pas quelques dizaines de mV, ce qui est très insuffisant pour permettre un contrôle précis.A more interesting solution in the sense that it allows continuous control of the composition of the bath and therefore a strong attenuation of the derivatives has also been proposed. It consists in using electrodes indicating the dissolved metal constituted by a rod of the metal to be deposited and immersed in the salt bath. These electrodes in fact normally take a potential which is a function of the dissolved metal content of the bath. It then suffices to measure this potential with respect to a reference electrode to know the content that one seeks to control. Unfortunately, it is found that for a variation of a factor of 10 of the dissolved metal content, the variation of the potential does not exceed a few tens of mV, which is very insufficient to allow precise control.
La demanderesse ayant cherché à améliorer la sensibilité d'un tel procédé a trouvé que dans la gamme des teneurs en métal de transition dissous généralement mis en oeuvre dans les bains d'électrolyse, c'est-à-dire entre 1 et 10 % en poids, la variation de ce potentiel pouvait être fortement amplifiée par l'ajout dans le bain de quantités relativement faibles de certains ions.Applicants who have sought to improve the sensitivity of such a process have found that in the range of contents of dissolved transition metal generally used in electrolysis baths, that is to say between 1 and 10% by By weight, the variation of this potential could be greatly amplified by adding relatively small amounts of certain ions to the bath.
C'est ainsi qu'elle a mis au point un procédé caractérisé en ce que l'on introduit dans le bain une quantité de fluorures alcalins et/ou alcalino- terreux telle que le rapport molaire du fluor contenu à la quantité de métal de transition dissous dans le bain soit compris entre 2,5 et 15. Toutefois, les valeurs comprises entre 4 et 8 conduisent à des sensibilités beaucoup plus grandes. Une telle addition permet d'obtenir une variation de potentiel de plusieurs centaines de millivolts pour une variation de + ou - 2,5 % de la teneur en métal dissous, ce qui permet un contrôle précis de cette teneur.This is how it has developed a process characterized in that an amount of alkali and / or alkaline earth fluorides is introduced into the bath, such as the molar ratio of fluorine contained to the amount of transition metal. dissolved in the bath is between 2.5 and 15. However, the values between 4 and 8 lead to much greater sensitivities. Such an addition makes it possible to obtain a variation in potential of several hundred millivolts for a variation of + or - 2.5% of the content of dissolved metal, which allows precise control of this content.
De façon pratique, le procédé consiste donc, connaissant la teneur optimale en métal pour assurer un bon fonctionnement de la cellule, à calculer à partir de cette teneur et du rapport molaire revendiqué la quantité de fluorures à ajouter. Ce fluorure est introduit dans le bain au moment de sa constitution.Practically, the method therefore consists, knowing the optimal metal content to ensure proper functioning of the cell, to calculate from this content and the molar ratio claimed the amount of fluorides to be added. This fluoride is introduced into the bath at the time of its constitution.
La cellule étant équipée d'une électrode indicatrice et d'une électrode de référence, on charge la quantité optimale de chlorure du métal de transition à déposer et après un temps suffisant pour permettre la dissolution on mesure le potentiel avant de démarrer l'électrolyse proprement dite. La teneur en métal dissous peut être confirmée par une analyse du bain.The cell being equipped with an indicator electrode and a reference electrode, the optimal amount of chloride of the transition metal to be deposited is charged and after a time sufficient to allow the dissolution, the potential is measured before starting the electrolysis properly said. The dissolved metal content can be confirmed by analysis of the bath.
La cellule est alors mise en service et un fonctionnement régulier peut être réalisé en l'alimentant en chlorures du métal de manière telle que le potentiel mesuré reste constant. On conçoit aisément l'application qui peut être faite de cette mesure à l'alimentation continue de la cellule. Il suffit en effet de comparer le potentiel mesuré à chaque instant au potentiel de consigne correspondant à la teneur optimale du bain et de commander en conséquence l'alimentation en chlorures. De cette manière, on peut régler très finement le débit de chlorures du métal et avoir une teneur en métal dissous extrêmement précise tout au long de l'électrolyse.The cell is then put into service and regular operation can be achieved by supplying it with chlorides of the metal so that the measured potential remains constant. It is easy to see the application that can be made of this measurement to the continuous supply of the cell. It suffices to compare the potential measured at all times at the setpoint potential corresponding to the optimal content of the bath and to control the supply of chlorides accordingly. In this way, one can very finely regulate the flow of chlorides of the metal and have an extremely precise dissolved metal content throughout the electrolysis.
Il se peut qu'il ne soit pas aisé d'incorporer dans une cellule une électrode indicatrice et une électrode de référence, c'est pourquoi la demanderesse a cherché à utiliser les moyens existant dans les cellules classiques.It may not be easy to incorporate an indicator electrode and a reference electrode into a cell, this is why the applicant has sought to use the means existing in conventional cells.
Dans le cas où la paroi interne de la cellule est métallique, on a trouvé qu'on pouvait l'utiliser comme électrode indicatrice. En effet, sachant que celle-ci doit être constituée par le métal à déposer, on a essayé au cours d'une préélectro- lyse en présence du chlorure du métal à déposer, de faire passer un courant continu ente l'anode de la cellule et la paroi de la cuve. Dans ces conditions, on a constaté que le dépôt de métal obtenu sur la cuve peut jouer parfaitement par la suite le rôle d'électrode indicatrice. Si nécessaire, ce dépôt sur la paroi peut être périodiquement reconstitué au cours de l'électrolyse ou même créé en permanence par polarisation cathodique de la cuve.In the case where the internal wall of the cell is metallic, it has been found that it could be used as an indicator electrode. Indeed, knowing that this must be constituted by the metal to be deposited, we tried during a preelectrolysis in the presence of the chloride of the metal to be deposited, to pass a direct current between the anode of the cell and the wall of the tank. Under these conditions, it has been found that the deposit of metal obtained on the tank can play perfectly thereafter the role of indicator electrode. If necessary, this deposit on the wall can be periodically reconstituted during electrolysis or even created permanently by cathodic polarization of the cell.
De plus, la demanderesse a également cherché à supprimer l'installation d'une électrode de référence ; elle y est parvenue en lui substituant l'anode de la cellule. Toutefois, dans ce cas, il s'établit alors dans le circuit de contrôle du potentiel une chute ohmique due au courant d'électrolyse I qui traverse l'anode. Ceci perturbe la mesure et donne une indication erronée sur la teneur réelle du bain en chlorures dissous. C'est pourquoi la demanderesse incorpore entre l'électrode indicatrice et l'anode un correcteur de chute ohmique dont la constitution est décrite ci-après.In addition, the applicant has also sought to eliminate the installation of a reference electrode; it achieved this by replacing it with the cell anode. However, in this case, an ohmic drop is established in the potential control circuit due to the electrolysis current I which passes through the anode. This disturbs the measurement and gives an incorrect indication of the actual content of dissolved chlorides in the bath. This is why the applicant incorporates between the indicator electrode and the anode an ohmic drop corrector, the constitution of which is described below.
L'invention sera mieux comprise à l'aide des dessins joints qui représentent :
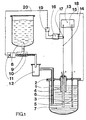
- figure 1 : un schéma montrant une cellule d'électrolyse utilisée pour l'élaboration du hafnium avec son système d'alimentation en chlorures dans laquelle le procédé selon l'invention est mis en oeuvre au moyen d'une électrode indicatrice constituée par la cuve d'électrolyse et d'une électrode de référence constituée par l'anode et d'un correcteur de chute ohmique.
- figure 2 : un schéma du correcteur.
- figure 3 : une courbe de variation du potentiel E de la cuve (électrode indicatrice) par rapport à la référence (anode + correcteur) en fonction de la teneur en hafnium dissous dans le bain.
- Figure 1: a diagram showing an electrolysis cell used for the development of hafnium with its chlorides supply system in which the method according to the invention is implemented by means of an indicator electrode constituted by the cell d electrolysis and a reference electrode constituted by the anode and an ohmic drop corrector.
- Figure 2: a diagram of the corrector.
- Figure 3: a variation curve of the potential E of the tank (indicator electrode) relative to the reference (anode + corrector) as a function of the content of hafnium dissolved in the bath.
Sur la figure 1, on distingue :
- 1. une cellule d'électrolyse formée d'une cuve métallique (1) fermée par un couvercle étanche (2) sur lequel sonf fixées une anode (3) munie d'une cloche (4) de récupération du chlore dégagé, une cathode (5) et une tuyauterie (6) d'alimentation en chlorures de hafnium gazeux ; chacun de ces éléments plongent dans le bain de sels constitués par un mélange de chlorures de potassium et de sodium.
- 2. un système d'alimentation comprenant un récipient (8) fermé renfermant le chlorure de hafnium en poudre (9) et muni à sa base d'une tubulure communiquant avec une vis sans fin (10) mue par un moteur (11) qui transporte le chlorure du récipient (8) vers un vaporisateur (12) communiquant avec la tuyauterie (6).
- 3. le système d'alimentation électrique de la cellule, le contrôle du potentiel et de commande d'alimentation de la cellule en chlorures comprenant :
- 1. an electrolysis cell formed by a metal cell (1) closed by a tight cover (2) on which is fixed an anode (3) provided with a bell (4) for recovering the chlorine released, a cathode ( 5) and a pipe (6) for supplying gaseous hafnium chlorides; each of these elements is immersed in the salt bath consisting of a mixture of potassium and sodium chlorides.
- 2. a supply system comprising a closed container (8) containing the powdered hafnium chloride (9) and provided at its base with a tube communicating with a worm (10) driven by a motor (11) which transports the chloride from the container (8) to a vaporizer (12) communicating with the piping (6).
- 3. the electrical supply system of the cell, the potential control and control of the supply of chlorides to the cell comprising:
une source de courant continu (13) dont le pôle positif est relié à l'anode et le pôle négatif à la cathode de la cellule respectivement par les conducteurs (14) et (15) ;a direct current source (13) whose positive pole is connected to the anode and the negative pole to the cathode of the cell respectively by the conductors (14) and (15);
un correcteur (16) de chute ohmique relié d'une part au conducteur (14) par l'intermédiaire des bornes A et B d'un shunt (17), d'autre part à un conducteur (18) en liaison avec la cuve (1) par l'intermédiaire de la borne C ;an ohmic drop corrector (16) connected on the one hand to the conductor (14) via the terminals A and B of a shunt (17), on the other hand to a conductor (18) in connection with the tank (1) via terminal C;
les bornes A et D du dispositif (16) sont reliées à un comparateur (19) du potentiel mesuré au potentiel de référence qui envoie un signal par l'intermédiaire du conducteur (20) au moteur (11) dans le cas où lé potentiel mesuré est en valeur absolue supérieur à la valeur absolue du potentiel de référence.the terminals A and D of the device (16) are connected to a comparator (19) of the measured potential to the reference potential which sends a signal via the conductor (20) to the motor (11) in the event that the measured potential is in absolute value greater than the absolute value of the reference potential.
Sur la figure 2 est représenté le correcteur de chute ohmique comprenant un amplificateur opérationnel noté AOP, deux résistances de même valeur R1, une résistance variable et réglable Rv.In FIG. 2 is shown the ohmic drop corrector comprising an operational amplifier denoted AOP, two resistors of the same value R1, a variable and adjustable resistance Rv.
Entre l'anode et l'électrode indicatrice (cuve de la cellule), bornes A et C est mesurée une tension - U qui comprend le potentiel E que l'on désire connaître, plus un terme de chute ohmique R.I. Cette tension s'écrit : U = E + RI. R est une résistance constante qui ne dépend que de la géométrie, de la nature et de la température du bain fondu.Between the anode and the indicator electrode (cell of the cell), terminals A and C is measured a voltage - U which includes the potential E which one wishes to know, plus an ohmic fall term RI This voltage is written : U = E + RI. R is a constant resistance which only depends on the geometry, the nature and the temperature of the molten bath.
Pour soustraire le terme RI quelle que soit d'ailleurs la valeur de I, nous mesurons par l'intermédiaire d'un shunt r, figure_ repère (17) une -tension -proportionnelle à I de valeur rl.To subtract the term RI whatever the value of I, we measure by means of a shunt r, figure_ mark (17) a -tension -proportional to I of value rl.
Les lois des mailles et des noeuds appliquées au circuit figure 2 donnent pour résultat de la tension de sortie S : soit
Au départ de l'opération, un étalonnage est effectué qui consiste à régler la valeur de Rv de manière à obtenir :
A ce moment, on a alors S = E qui est indépendant de la valeur de et donc de ses fluctuations et qui ne dépend que de la teneur en métal dissous comme il est représenté figure 3.At this time, we then have S = E which is independent of the value of and therefore of its fluctuations and which only depends on the content of dissolved metal as shown in FIG. 3.
Ce signal S est envoyé au comparateur (19).This signal S is sent to the comparator (19).
Sur la figure 3, on voit une courbe qui représente les variations de potentiel E ou S en volts de la cuve (électrode indicatrice en hafnium) par rapport à l'anode corrigée de la chute ohmique (référence normale à chlore) en fonction de la teneur en % d'Hf dissous dans le bain et du rapport molaire R de la quantité de fluor à la quantité de hafnium. Cette courbe a été établie avec un bain équimoléculaire (KCI, NaCI (56 % KCI, 44 % NaCI en poids) fondu à 750 °C auquel on avait ajouté 1,1 % de fluor sous forme de NaF (2, 5 % en poids). On constate une variation de potentiel de 750 mV quand la teneur en hafnium dissous dans le bain passe de 0,5 à 5 % en poids.In FIG. 3, we see a curve which represents the variations of potential E or S in volts of the tank (indicator electrode in hafnium) by ratio to the anode corrected for the ohmic drop (normal reference to chlorine) as a function of the% Hf content dissolved in the bath and of the molar ratio R of the quantity of fluorine to the quantity of hafnium. This curve was established with an equimolecular bath (KCI, NaCI (56% KCI, 44% NaCI by weight) melted at 750 ° C. to which 1.1% of fluorine was added in the form of NaF (2.5% by weight A variation in potential of 750 mV is noted when the content of hafnium dissolved in the bath goes from 0.5 to 5% by weight.
D'autres courbes non représentées ici montrent qu'avec une addition de 4,1 % de fluor, on obtient la même variation de potentiel pour une variation de poids de hafnium de 1 à 8 %.Other curves not shown here show that with an addition of 4.1% of fluorine, the same variation in potential is obtained for a variation in weight of hafnium from 1 to 8%.
Plus généralement, les maxima de variation et donc de prévision sont obtenus pour un rapport molaire fluor/métal dissous compris entre 4 et 8 mais des résultats encore fort acceptables peuvent être obtenus avec des rapports compris entre 2,5 et 15.More generally, the maxima of variation and therefore of prediction are obtained for a fluorine / dissolved metal molar ratio of between 4 and 8 but still very acceptable results can be obtained with ratios of between 2.5 and 15.
L'invention trouve son application dans tous les cas où on désire fabriquer des métaux de transition par électrolyse en continu de leurs chlorures dans les bains fondus de chlorures alcalins ou alcalino-terreux.The invention finds its application in all cases where it is desired to manufacture transition metals by continuous electrolysis of their chlorides in the molten baths of alkali or alkaline earth chlorides.
Claims (5)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT86420087T ATE34586T1 (en) | 1985-03-28 | 1986-03-26 | METHOD OF CONTINUOUSLY MONITORING THE CONTENT OF A METAL DISSOLVED IN A MOLTEN SALT BATH AND ITS APPLICATION TO CONTINUOUSLY FEED THESE METAL SALTS INTO AN ELECTROLYTIC CELL. |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR8505196A FR2579629B1 (en) | 1985-03-28 | 1985-03-28 | METHOD FOR THE CONTINUOUS CONTROL OF THE METAL CONTENT DISSOLVED IN A MOLTEN SALT BATH AND ITS APPLICATION TO THE CONTINUOUS SUPPLY OF A SALT ELECTROLYSIS CELL |
| FR8505196 | 1985-03-28 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0198775A1 EP0198775A1 (en) | 1986-10-22 |
| EP0198775B1 true EP0198775B1 (en) | 1988-05-25 |
Family
ID=9317977
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP86420087A Expired EP0198775B1 (en) | 1985-03-28 | 1986-03-26 | Process for the continuous monitoring of the dissolved metal concentration in a molten salts bath and its use in the continuous feeding of these metal salts to an electrolysis cell |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US4657643A (en) |
| EP (1) | EP0198775B1 (en) |
| JP (1) | JPS61227191A (en) |
| AT (1) | ATE34586T1 (en) |
| CA (1) | CA1251161A (en) |
| DE (1) | DE3660222D1 (en) |
| FR (1) | FR2579629B1 (en) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2691169B1 (en) * | 1992-05-12 | 1994-07-01 | Cezus Co Europ Zirconium | REFRACTORY METAL ALLOYS SUITABLE FOR TRANSFORMATION INTO HOMOGENEOUS AND PURE INGOTS AND METHODS FOR OBTAINING SAID ALLOYS. |
| JP2015098626A (en) * | 2013-11-19 | 2015-05-28 | 住友電気工業株式会社 | Method of producing refined metal |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2828251A (en) * | 1953-09-30 | 1958-03-25 | Horizons Titanium Corp | Electrolytic cladding process |
| FR1154129A (en) * | 1955-05-31 | 1958-04-02 | Union Carbide & Carbon Corp | Semi-continuous electrolytic process |
| US2975111A (en) * | 1958-03-19 | 1961-03-14 | New Jersey Zinc Co | Production of titanium |
| FR2494725A1 (en) * | 1980-11-27 | 1982-05-28 | Armand Marcel | NEW DEVICE AND METHOD FOR THE TICL4 POWERING OF ELECTROLYTIC CELLS FOR THE PREPARATION OF TITANIUM |
-
1985
- 1985-03-28 FR FR8505196A patent/FR2579629B1/en not_active Expired
-
1986
- 1986-03-12 US US06/839,131 patent/US4657643A/en not_active Expired - Lifetime
- 1986-03-25 CA CA000504974A patent/CA1251161A/en not_active Expired
- 1986-03-26 JP JP61068271A patent/JPS61227191A/en active Granted
- 1986-03-26 EP EP86420087A patent/EP0198775B1/en not_active Expired
- 1986-03-26 DE DE8686420087T patent/DE3660222D1/en not_active Expired
- 1986-03-26 AT AT86420087T patent/ATE34586T1/en not_active IP Right Cessation
Also Published As
| Publication number | Publication date |
|---|---|
| JPS61227191A (en) | 1986-10-09 |
| FR2579629B1 (en) | 1987-05-07 |
| CA1251161A (en) | 1989-03-14 |
| EP0198775A1 (en) | 1986-10-22 |
| DE3660222D1 (en) | 1988-06-30 |
| US4657643A (en) | 1987-04-14 |
| JPH033753B2 (en) | 1991-01-21 |
| ATE34586T1 (en) | 1988-06-15 |
| FR2579629A1 (en) | 1986-10-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4240879A (en) | Methods of measuring the concentrations of ions | |
| EP0198775B1 (en) | Process for the continuous monitoring of the dissolved metal concentration in a molten salts bath and its use in the continuous feeding of these metal salts to an electrolysis cell | |
| EP0814181B1 (en) | Process for regulating the alumina content of bath in cells for producing aluminium | |
| CN110579524B (en) | Method for cleaning, adjusting, calibrating and/or regulating a current sensor | |
| EP0201438A1 (en) | Process for accurately regulating the low alumina content of an igneous electrolysis cell for aluminium production | |
| GB1522410A (en) | Determination of heavy metal ion concentrations | |
| EP0201837B1 (en) | Process and apparatus for the readjustment of the operational setting in an electrolysis cell | |
| JPH0525957B2 (en) | ||
| EP0227517B1 (en) | Process and apparatus for controlling the amount of metal electrolytically deposited on a continuously moving strip | |
| FR2629205A1 (en) | DEVICE AND METHOD FOR MEASURING THE CONCENTRATION OF HYDROGEN IN WATER | |
| EP0972860B1 (en) | Electrolytic recovery of metal from solution | |
| US4693790A (en) | Method for monitoring the quality of ZnSO4 electrolyte containing Sb (V) | |
| JP4217077B2 (en) | Stabilization method of diaphragm type electrode | |
| FR2494725A1 (en) | NEW DEVICE AND METHOD FOR THE TICL4 POWERING OF ELECTROLYTIC CELLS FOR THE PREPARATION OF TITANIUM | |
| Porter et al. | The Electrolytic Decomposition of Dilute Amalgams | |
| CN106290503A (en) | A kind of silver/silver chloride reference electrode and preparation method thereof | |
| Zaitsev et al. | Determination of selenium (IV) by cathodic stripping voltammetry using a copper-modified mercury-film electrode modified with copper | |
| FR3065969B1 (en) | METHOD FOR CONTROLLING AN ALUMINUM ELECTROLYSIS TANK | |
| FR2605410A1 (en) | METHOD AND DEVICE FOR THE ELECTROCHEMICAL MEASUREMENT OF OXIDE ION CONCENTRATION IN A FAT-BASED HALIDE BATH | |
| FR2633048A1 (en) | METHOD FOR THE AUTOMATIC ONLINE CALIBRATION OF A SENSOR FOR MEASURING THE CONCENTRATION OF AN ELECTROCHEMICAL SPECIES DISSOLVED IN LIQUID PHASE | |
| FR2830875A1 (en) | Regulation of an electrolytic cell for the production of aluminum involves controlled addition of alumina as a function of the amount of undissolved alumina in the molten bath | |
| Chabala et al. | In situ and real time X-ray diffraction study of an electrocrystallisation process: Ag electrodeposition on Au (111) | |
| US4416736A (en) | Procedure for the enrichment of the element of interest from a solution for nonflame atomic absorption spectroscopy | |
| Guo | Kinetics of Oxidation Processes on Lead Electrode in H 2 SO 4: II. The Growth of the and Layer | |
| Czajkowski et al. | Automatic apparatus for precise measuring and recording of pzc value of liquid electrodes and its application |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE GB IT LI LU NL SE |
|
| 17P | Request for examination filed |
Effective date: 19861115 |
|
| 17Q | First examination report despatched |
Effective date: 19870820 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE GB IT LI LU NL SE |
|
| REF | Corresponds to: |
Ref document number: 34586 Country of ref document: AT Date of ref document: 19880615 Kind code of ref document: T |
|
| ITF | It: translation for a ep patent filed | ||
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) | ||
| REF | Corresponds to: |
Ref document number: 3660222 Country of ref document: DE Date of ref document: 19880630 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19910213 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19910214 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: LU Payment date: 19910220 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19910328 Year of fee payment: 6 |
|
| EPTA | Lu: last paid annual fee | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19920326 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Effective date: 19920327 |
|
| ITTA | It: last paid annual fee | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Effective date: 19920331 Ref country code: CH Effective date: 19920331 Ref country code: BE Effective date: 19920331 |
|
| BERE | Be: lapsed |
Owner name: PECHINEY Effective date: 19920331 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 19940325 Year of fee payment: 9 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 86420087.8 Effective date: 19921005 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Effective date: 19950326 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732E |
|
| NLS | Nl: assignments of ep-patents |
Owner name: COMPAGNIE GENERALE DES MATIERES NUCLEAIRES;COMPAGN |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20010306 Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20010307 Year of fee payment: 16 Ref country code: DE Payment date: 20010307 Year of fee payment: 16 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020326 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20021001 Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20021001 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20020326 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 20021001 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 20050326 |