EP0167682A1 - High performance papers comprised of fibrils of thermotropic liquid crystal polymers - Google Patents
High performance papers comprised of fibrils of thermotropic liquid crystal polymers Download PDFInfo
- Publication number
- EP0167682A1 EP0167682A1 EP84302959A EP84302959A EP0167682A1 EP 0167682 A1 EP0167682 A1 EP 0167682A1 EP 84302959 A EP84302959 A EP 84302959A EP 84302959 A EP84302959 A EP 84302959A EP 0167682 A1 EP0167682 A1 EP 0167682A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- paper
- moiety
- mole percent
- approximately
- fibrils
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
Classifications
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H5/00—Special paper or cardboard not otherwise provided for
- D21H5/12—Special paper or cardboard not otherwise provided for characterised by the use of special fibrous materials
- D21H5/20—Special paper or cardboard not otherwise provided for characterised by the use of special fibrous materials of organic non-cellulosic fibres too short for spinning, with or without cellulose fibres
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H13/00—Pulp or paper, comprising synthetic cellulose or non-cellulose fibres or web-forming material
- D21H13/10—Organic non-cellulose fibres
- D21H13/20—Organic non-cellulose fibres from macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
- D21H13/24—Polyesters
Definitions
- the present invention is directed to high performance papers comprised of fibrils of thermotropic liquid crystal polymers.
- Papers comprised of polymeric materials have been employed for many purposes including filters and electrical insulation, etc. See, for example, U.S. Patent Nos. 2,988,782; 3,080,272; and 3,101,294 as well as Glen, W. "Papers from Synthetic Fibers" Paper Technology, Vol. 5, No. 2, pages 137-142, 1964.
- Such papers are frequently not appropriate for use in a high temperature environment (e.g., temperatures in excess of about 200°C.) or in an environment where the paper will come into contact with corrosive chemicals or solvents. It is therefore desirable to provide a paper comprised of a polymeric material which is resistant to solvents or corrosive chemicals and suitable for use at high temperatures.
- papers which exhibit desirable thermal stability and chemical and solvent resistance comprised of fibrils of a polymer which is capable of forming an anisotropic melt phase.
- Thermotropic liquid crystal polymers are polymers which are liquid crystalline (i.e., anisotropic) in the melt phase. These polymers have been described by various terms, including “liquid crystalline”, “liquid crystal” and anisotropic". Briefly, the polymers of this class are thought to involve a parallel ordering of the molecular chains. The state wherein the molecules are so ordered is often referred to either as the liquid crystal state or the nematic phase of the liquid crystalline material. These polymers are prepared from monomers which are generally long, flat and fairly rigid along the long axis of the molecule and commonly have chain-extending linkages that are either coaxial or parallel.
- Such polymers readily form liquid crystals (i.e., exhibit anisotropic properties) in the melt phase. Such properties may be confirmed by conventional polarized light techniques whereby crossed polarizers are utilized. More specifically, the anisotropic melt phase may be confirmed by the use of a Leitz polarizing microscope at a magnification of 40X with the sample on a Leitz hot stage and under nitrogen atmosphere.
- the polymer is optically anisotropic; i.e., it transmits light when examined between crossed.polarizers. Polarized light is transmitted when the sample is optically anisotropic even in the static state.
- thermotropic liquid crystal polymers suitable for use in the present invention include but are not limited to wholly aromatic polyesters, aromatic-aliphatic polyesters, aromatic polyazomethines, wholly and non-wholly aromatic poly(ester- amide)s and aromatic polyester-carbonates.
- the wholly aromatic thermotropic liquid crystal polymers are comprised of moieties which contribute at least one aromatic ring to the polymer backbone and which enable the polymer to exhibit anisotropic properties in the melt phase.
- moieties include but are not limited to aromatic diols, aromatic amines, aromatic diacids and aromatic hydroxy acids.
- Moieties which may be present in the thermotropic liquid crystal polymers employed in the present invention include but are not limited to the following:
- thermotropic liquid crystal polymers which are employed comprise not less than about 10 mole percent of recurring units which include a naphthalene moiety.
- Preferred naphthalene moieties include 6-oxy-2-naphthoyl; 2,6-dioxynaphthalene, and 2,6-dicarboxynaphthalene.
- suitable aromatic-aliphatic polyesters are copolymers of polyethylene terephthalate and hydroxybenzoic acid as disclosed in Polyester X7G-A Self Reinforced Thermoplastic, by W.J. Jackson, Jr., H.F. Kuhfuss, and T.F. Gray, Jr., 30th Anniversary Technical Conference, 1975 Reinforced Plas- tics/Composites Institute, The Society of the Plastics Industry, Inc., Section 17- D , Pages 1-4.
- a further disclosure of such copolymers can be found in "Liquid Crystal Polymers: I. Preparation and Properties of p-Hydroxybenzoic Acid Copolymers," Journal of Polymer Science, Polymer Chemistry Edition, Vol. 14, pp. 2043-58 (1976), by W.J. Jackson, Jr. and H.F. Kuhfuss.
- the above-cited references are herein incorporated by reference in their entirety.
- Aromatic polyazomethines and processes of preparing the same are disclosed in the U.S. Patent Nos. 3,493,522; 3,493,524; 3,503,739; 3,516,970; 3,516,971; 3,526,611; 4,048,148; and 4,122,070. Each of these patents is herein incorporated by reference in its'entirety.
- polymers include poly(nitrilo-2-methyl-1,4-phenyl-enenitriloethylidyne-1,4-phenyleneethylidyne); poly(nitrolo-2-methyl-1,4-phenylene- nitrilomethylidyne-1,4-phenylene-methylidyne); and poly(nitrilo-2-chloro-1,4-phenylenenitrilomethylidyne-1,4-phenylene- methylidyne).
- Aromatic polyester-carbonates are disclosed in U.S. Patent No. 4,107,143, which is herein incorporated by reference in its entirety.
- Examples of such polymers include those consisting essentially of hydroxybenzoic acid units, hydroquinone units, carbonate units, and aromatic carboxylic acid units.
- the liquid crystal polymers which are preferred for use in the present invent'ion include thermotropic wholly aromatic polyesters.
- Recent publications disclosing such polyesters include (a) Belgian Pat. Nos. 828,935 and 828,936, (b) Dutch Pat. No. 7505551, (c) West German Pat. Nos. 2,520,819, 2,520,820, and 2,722,120, (d) Japanese Pat. Nos. 43-223, 2132-116, 3017-692, and 3021-293, (e) U.S. Pat. Nos.
- Wholly aromatic polymers which are preferred for use in the present invention include wholly aromatic polyesters and poly(ester-amide)s which are disclosed in commonly-assigned U.S. Patent Nos. 4,067,852; 4,083,829; 4,130;545; 4,161,470; 4,184,996 4,219,461; 4,238,599; 4,224,433; 4,256,624 and 4,279,803; and in commonly-assigned U.S. Application Serial Nos. 91,003, filed November 5, 1979; 128,759, filed March 10, 1980; and 214,557, filed December 9, 1980.
- the disclosures of all of the above- identified commonly-assigned U.S. patents and applications are herein incorporated by reference in their entirety.
- the wholly aromatic polymers disclosed therein typically are capable of forming an anisotropic melt phase at a temperature below approximately 400°C., and preferably below approximately 350°C.
- the wholly aromatic polymers including wholly aromatic polyesters and poly(ester-amide)s which are suitable for use in the present invention may be formed by a variety of ester-forming techniques whereby organic monomer compounds possessing functional groups which, upon condensation, form the requisite recurring moieties are reacted.
- the functional groups of the organic monomer compounds may be carboxylic acid groups, hydroxyl groups, ester groups, acyloxy groups, acid halides, amine groups, etc.
- the organic monomer compounds may be reacted in the absence of a heat exchange fluid via a melt acidolysis procedure. They, accordingly, may be heated initially to form a melt solution of the reactants with the reaction continuing as said polymer particles are suspended therein.
- a vacuum may be applied to facilitate removal of volatiles formed during the final stage of the condensation (e.g., acetic acid or water).
- the organic monomer reactants from which the wholly aromatic polyesters are derived may be initially provided in a modified form whereby the usual hydroxy groups of such monomers are esterified (i.e., they are provided as lower acyl esters).
- the lower acyl groups preferably have from about two to about four carbon atoms.
- the acetate esters of organic monomer reactants are provided.
- Representative catalysts which optionally may be employed in either the melt acidolysis procedure or in the slurry _procedure of U.S. Patent No. 4,083,829 include dialkyl tin oxide (e.g., dibutyl tin oxide), diaryl tin oxide, titanium dioxide, antimony trioxide, alkoxy titanium silicates, titanium alkoxides, alkali and alkaline earth metal salts of carboxylic acids (e.g., zinc acetate), the gaseous acid catalysts such as Lewis acids (e. g. , BF 3 ), hydrogen halides (e.g., HC1), etc.
- the quantity of catalyst utilized typically is about 0.001 to 1 percent by weight based upon the total monomer weight, and most commonly about 0.01 to 0.2 percent by weight.
- the wholly aromatic polymers suitable for use in the present invention tend to be substantially insoluble in common solvents and accordingly are not susceptible to solution processing. As discussed previously, they can be readily processed by common melt processing techniques. Most suitable wholly aromatic polymers are soluble in pentafluorophenol.
- the wholly aromatic polyesters which are preferred for . use in the present invention commonly exhibit a weight average molecular weight of about 2,000 to 200,000, and preferably about 10,000' to 50,000, and most preferably about 20,000 to 25,000.
- the wholly aromatic poly(ester-amide)s which are preferred commonly exhibit a molecular weight of about 5000 to 50,000 and preferably about 10,000 to 30,000; e.g., 15,000 to 17,000.
- Such molecular weight may be determined by gel permeation chromatography as well as by other standard techniques not involving the solutioning of the polymer, e.g., by end group determination via infrared spectroscopy on compression molded films. Alternatively, light scattering techniques in a pentafluorophenol solution may be employed to determine the molecular weight.
- the wholly aromatic polyesters and poly(ester-amide)s additionally commonly exhibit an inherent viscosity (i.e.,'I.V.) of at least approximately 2.0 dl./g., e.g., approximately 2.0 to 10.0 dl./g., when dissolved in a concentration of 0.1 percent by weight in pentafluorophenol at 60°C.
- an inherent viscosity i.e.,'I.V.
- Especially preferred wholly aromatic polymers are those which are disclosed in above-noted U.S. Patent Nos. 4,161,470, 4,184,996, 4,219,461, 4,238,599 and 4,256,624 and Application Serial No. 214,55'
- the aromatic rings which are included in the polymer backbones of the polymer components employed in the present invention may include substitution of at least some of the hydrogen atoms present upon an aromatic ring.
- substituents include alkyl groups of up to four carbon atoms; alkoxy groups having up to four carbon atoms; halogens; and additional aromatic rings, such as phenyl and substituted phenyl.
- Preferred halogens include fluorine, chlorine, and bromine. Although bromine atoms tend to be released from organic compounds at high temperatures, bromine is more stable on aromatic rings than on aliphatic chains, and therefore is suitable for inclusion'as a possible substituent on the aromatic rings.
- the wholly aromatic polyester which is disclosed in U.S. Patent No. 4,161,470 is a melt processable wholly aromatic polyester capable of forming an anisotropic melt phase at a temperature below approximately 350°C.
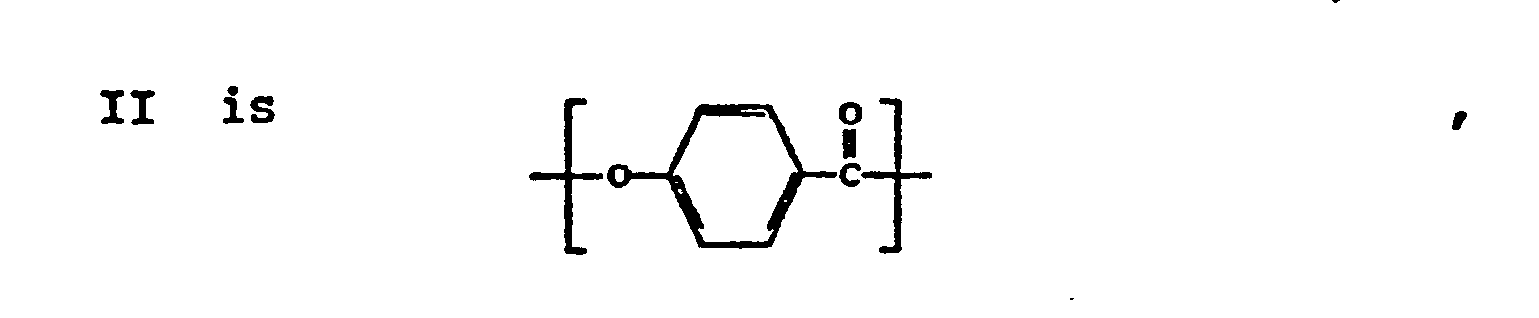
- the polyester consists essentially'of the recurring moieties I and II wherein:
- the polyester comprises approximately 10 to 90 mole percent of moiety I, and approximately 10 to 90 mole percent of moiety II.
- moiety II is present in a concentration of approximately 65 to 85 mole percent, and preferably in a concentration of approximately 70 to 80 mole percent, e.g., approximately 75 mole percent.
- moiety II is present in a lesser proportion of approximately 15 to 35 mole percent, and preferably in a concentration of approximately 20 to 30 mole percent.
- the hydrogen atoms present upon the rings optionally may be replaced by substitution selected from the group consisting of an alkyl group of 1 to 4 carbon atoms, an alkoxy group of 1 to 4 carbon atoms, halogen, phenyl, substituted phenyl, and mixtures thereof.
- the wholly aromatic polyester which is disclosed in U.S. Patent No. 4,184,996 is a melt processable wholly aromatic polyester capable of forming an anisotropic melt phase at a temperature below approximately 325°C.
- the polyester consists essentially of the recurring moieties I, II, and III wherein: The polyester comprises approximately 30 to 70 mole percent of moiety I.
- the polyester preferably comprises approximately 40 to 60 mole percent of moiety I, approximately 20 to 30 mole percent of moiety II, and approximately 20 to 30 mole percent of moiety III.
- the hydrogen atoms present upon the rings optionally may be replaced by substitution selected from the group consisting of an alkyl group of 1 to 4 carbon atoms, an alkoxy group of 1 to 4 carbon atoms, halogen, phenyl, substituted phenyl, and mixtures thereof.
- the wholly aromatic polyester which is disclosed in U.S. Patent No. 4,238,599 is a melt processable polyester capable of forming an anisotropic melt phase at a temperature no higher than approximately 320°C. consisting essentially of the recurring moieties I, II, III and IV wherein: R is methyl, chloro, bromo, or mixtures thereof, and is substituted for a hydrogen atom present upon the aromatic ring, and wherein said polyester comprises approximately 20 to 60 mole percent of moiety I, approximately 5 to 18 mole percent of moiety II, approximately 5 to 35 mole percent of moiety III, and approximately 20 to 40 mole percent of moiety IV.
- the polyester preferably comprises approximately 35 to 45 mole percent of moiety I, approximately 10 to 15 mole percent of moiety II, approximately 15 to 25 mole percent of moiety III, and approximately 25 to 35 mole percent of moiety IV, with the proviso that the total molar concentration of moieties II and III is substantially identical to that of moiety IV.
- at least some of the hydrogen atoms present upon the rings optionally may be replaced by substitution selected from the group consisting of an alkyl group of 1 to 4 carbon atoms, an alkoxy group of 1 to 4 carbon atoms, halogen, phenyl, substituted phenyl, and mixtures thereof.
- This wholly aromatic polyester commonly exhibits an inherent viscosity of at least 2.0 dl./g., e.g., 2.0 to 10.0 dl./g., when dissolved in a concentration of 0.1 weight/volume .percent in pentafluorphenol at 60°C.
- the polyester disclosed in U.S. Patent No. 4,219,461 is a melt processable wholly aromatic polyester which is capable of forming an anisotropic melt phase at a temperature below approximately 320°C.
- the polyester consists essentially of the recurring moieties I, II, III, and IV wherein:
- Moieties III and IV are preferably symmetrical in the sense that the divalent bonds which join these moieties to other moieties in the main polymer chain are symmetrically disposed on one or more aromatic rings (e.g., are para to each other or diagonally disposed when present on a naphthalene ring).
- non-symmetrical moieties such as those derived from resorcinol and isophthalic acid, may also be used.
- Preferred moieties III and IV are set forth in above noted U.S. Patent No. 4,219,461.
- the preferred dioxy aryl moiety III is: and the preferred dicarboxy aryl moiety IV is:
- the polyester disclosed in U.S. Patent No. 4,256,624 is a melt processable wholly aromatic polyester which is capable of forming an anisotropic melt phase at a temperature below approximately 400°C.
- the polyester consists essentially of the recurring moieties I, II, and III wherein:
- moieties II and III of the polyester described immediately above may be symmetrical or nonsymmetrical, but are preferably symmetrical.
- Preferred moieties II and III are set forth in above-noted U.S. Patent 4,256,624.
- the preferred dioxy aryl moiety II is: and the preferred dicarboxy aryl moiety III is:
- Preferred moieties II, III and IV are set forth in above-noted U.S. Application Serial No. 214,557.
- the preferred dicarboxy aryl moiety II is: the preferred moiety III is: and the preferred dioxy aryl moiety IV is:
- the papers of the present invention are comprised of fibrils of thermotropic liquid crystal polymers (i.e., polymers which are capable of forming an anisotropic melt phase).
- the fibrils may be produced by several methods including mechanically masticating shaped articles including as-spun thermotropic liquid crystal polymer fibers. Since the as-spun fibers are highly oriented along their longitudinal axis, the fibers are able to withstand much less stress along the transverse axis as opposed to along the longitudinal axis. Accordingly, the fibers readily break up length-wise into much narrower fibrils as they are masticated to form a pulp.
- the fibers are preferably masticated in the form of an aqueous slurry.
- the pulp which is formed is a mass of fibrils which are generally frayed, fibrillated and/or branched. Such characteristics permit the fibrils to become intertangled such that a thin, yet coherent mat may be formed upon simple screening of the slurry.
- the term "pulp" is intended to refer to a mass of fibers or other shaped articles which have been mechanically masticated or ground, causing the fiber or article to separate, split, fray, fibrillate and/or shred into generally finer diameter units. The intertangling between the units, or "interfelting", is thereby enhanced, thus allowing the formation of thin, yet coherent sheets.
- the fibers and fragments may, in addition, become crimped, branched, or multiply bifurcated to improve the interfelting.
- a typical solids content of the pulp ranges from about 0.5 to 50 grams of polymer per liter of liquid (e.g., water),. i.e., from about 0.05 to 5 percent by weight of solids.
- a preferred solids content ranges from about 0.5 to 2 percent by weight of solids.
- the term "masticating" as used herein is intended to include various mechanical processes whereby the shaped article is subjected to grinding or shearing forces of sufficient magnitude to break up the shaped article into fibrils.
- the mastication step may involve a series of techniques rather than a single operation. Such processes include but are not limited to the use of fluid or air turbulence (i.e., air fibrillation), use of high shear (i.e., as in a pulp refiner and open disk emulsifier), repeated chopping in conjunction with fluid shear (e.g., as in a Waring blender), and crushing in conjunction with mechanical shear (e.g., as in a Jordan "beater”).
- the use of surface wetting agents or 50.percent isopropanol or ethanol may facilitate the fibrillation of the polymer.
- the fibrils which are produced commonly exhibit a ratio of length to diameter which is generally greater than that exhibited by the fibers.
- the length to diameter ratio exhibited by the fibers generally ranges from about 30:1 to about 300:1, while the corresponding ratio for the fibrils ranges from about 50:1 to about 600:1.
- the fibrils preferably exhibit a diameter of about 0.5 to about 5 microns and a length of about 50 microns to about 3 millimeters.
- the fibers which are masticated are generally from 1/32 to 1/4 inch in length and 14 to 35 microns in diameter.
- the denier of the fiber is preferably from about 2 to 10. Fibers of a much broader size can also be employed, such as, for example, 0.5 to 100 denier (7 to 100 microns in diameter).
- a typical starting length ranges from 1/32 to 1/4 inch. If a starting length of greater than 1/8 inch is employed, the pulping method employed should be capable of length reduction as well as fibrillation to avoid clumping of the pulp particles.
- the length of the fibrils which are produced will generally be proportional to the length of the fibers employed.
- thermotropic liquid crystal polymer Pulp and Method of Production Thereof comprising Recurring Units Which Contain A 2,6-Dioxyanthraquinone Moiety
- Said Polymer Comprises Recurring Units Which Contain A 2,6-Dioxyanthraquinone Moiety
- the presence of at least about 5 mole percent of recurring units which contain a . 2,6-dioxyanthraquinone moiety in the thermotropic liquid crystal polymer enables the fibers to be readily broken up into fibrils of submicron size while minimizing the use of time and energy with respect to the fibrillation process.
- the polymer may also be in the form of pellets or sheets, etc.
- shaped article as used herein is intended to include particles, pellets, filaments, staple fibers, films, chips, sheets and other extruded, molded, cast or otherwise formed shaped articles.
- the fibrils which are produced can subsequently be slurried with a liquid which is a non-solvent for the polymer of which the fibrils are comprised such as water and collected (e.g., filtered) onto a web or screen to provide a random (i.e., multi-dimensional) array or sheet of fibrils.
- a liquid which is a non-solvent for the polymer of which the fibrils are comprised such as water and collected (e.g., filtered) onto a web or screen to provide a random (i.e., multi-dimensional) array or sheet of fibrils.
- webs may be formed by air lay processes wherein the fibrous material is entrained in and deposited from a moving air stream. Since the fibrils which are produced are generally bifurcated or branched along the longitudinal axis, they tend to become attached to one another as they are deposited.
- Appropriate methods are employed to bond the fibrils together to form a paper having the desired degree of structural. integrity. That is, the paper at a minimum supports its own weight and preferably is pulled apart only with difficulty. Such a paper will accordingly be in the form of a sheet or web.
- the fibrils may be thermally bonded to one another at a suitable temperature by conventional means such as heat pressing or calendering to at least bond the fibrils together at their crossover points. Such fusion bonding does not result in any significant loss of orientation (and, accordingly, loss of strength) since the polymer of which the fibrils are comprised forms an anisotropic melt phase. Such a characteristic is in direct contrast to conventional thermoplastic polymers which do not form an anisotropic melt phase and which readily lose their orientation upon being heated to temperatures in excess of their melting temperature.
- Heat pressing is essentially a batch process wherein the web of fibrils is pressed between two heated plates. Calendering involves the passage of the fibrils in the form of a web between heated rolls. The use of a padded backup roll against a heated metal roll is preferred. The thermal bonding temperature will generally range from about 100 to about 250°C.
- the fibrils may also be bonded together by means of adhesives including but not limited to the following: epoxies, thermosetting or thermoplastic resins including thermosetting polyesters, water soluble adhesives such as casine, guar gum or polyacrylic acid, solvent-based adhesives and emulsion or latex- based adhesives such as styrene/butyl/acrylic copolymer systems.
- adhesives including but not limited to the following: epoxies, thermosetting or thermoplastic resins including thermosetting polyesters, water soluble adhesives such as casine, guar gum or polyacrylic acid, solvent-based adhesives and emulsion or latex- based adhesives such as styrene/butyl/acrylic copolymer systems.
- the adhesive may be coated onto the web or array of fibrils by use of kiss rolls. Alternatively, the adhesive may be sprayed upon or deposited upon the web by known emulsion techniques (for use with wet laid paper). The use of adhesives in such methods
- the temperature as well as the method of thermal bonding employed affects the physical characteristics exhibited by the paper which is produced. For example, when temperatures below about 140°C. are used, an opaque paper is provided. Such. papers are essentially a mat of intertangled fibrous particulates (the fibrils) which exhibits substantial porosity and low density. On the other hand, when temperatures in excess of about 140°C. are employed in conjunction with a pressing or calendering step (and especially in the range- of 170°C. or so), a transparent paper in the form of a film or membrane resembling a glassine film is produced which exhibits reduced porosity.
- temperatures and pressures should generally be avoided during calendering if a paper of substantial porosity is desired since such temperatures and pressures increase the degree of fusion and compaction the fibrils to each other while correspondingly decreasing the porosity.
- the amount of pressure applied as well as the duration of the application of pressure can also influence the degree of fusion.
- the papers of the present invention possess many advantageous properties due to the presence of thermotropic liquid crystal polymers therein. Since the liquid crystal'polymers are highly oriented as spun, the fibrils which comprise the papers of the present invention possess relatively high tensile strength and high modulus. Accordingly, papers comprised of such fibrils similarly exhibit relatively high modulus and high tensile strength. In addition, the papers exhibit such tensile strength and modulus in a multi-dimensional manner due to the multi-dimensional (i.e., random) orientation of the fibrils within the paper.
- the papers can vary in weight, porosity, thickness, etc. depending upon the amount of fibrils employed, the pressure used to thermally bond the fibrils together, etc.
- the papers typically, however, can exhibit a weight ranging from 3 to 20 ounces/square yard.
- the mechanical properties of the papers produced in accordance with the present invention can be improved still further by subjecting the papers to a heat treatment following . formation thereof.
- the heat treatment improves the properties of the paper by increasing the molecular weight of the liquid crystalline polymer which comprises the fibrils and increasing the degree of crystallinity thereof while also increasing the melting temperature of the polymer. Such heat treatment can also serve to bond the fibrils together.
- the papers may be thermally treated in an inert atmosphere (e.g., nitrogen, carbon dioxide, argon, helium) or alternatively, in a flowing oxygen-containing atmosphere (e.g., air).
- an inert atmosphere e.g., nitrogen, carbon dioxide, argon, helium
- a flowing oxygen-containing atmosphere e.g., air
- the use of a non-oxidizing substantially moisture-free atmosphere is preferred to avoid the possibility of thermal degradation.
- the paper may be brought to a temperature approximately 10 to 30 centigrade degrees below the melting temperature of the liquid crystal polymer, at which temperature the fibrils remain solid. It is preferable for the temperature of the heat treatment to be as high as possible without equaling or exceeding the melting temperature of the polymer. It is most preferable to gradually increase the temperature, of heat treatment in accord-- ance with the increase of the melting temperature of the polymer during heat treatment.
- the duration of the heat treatment will commonly range from a few minutes to a number of days, e.g., from 0.5 to 200 hours, or more.
- the heat treatment is conducted for a time of 1 to 48 hours and typically from about 5 to 30 hours.
- the duration of heat treatment varies depending upon the heat treatment temperature; that is, a shorter treatment time is required as a higher treatment is used.
- the duration of the heat treatment can be shortened for higher melting polymers, since higher heat treatment temperatures can be applied without melting the polymer.
- the heat treatment is conducted under conditions sufficient to increase the melting temperature of the polymer at least 10 centigrade degrees.
- the melting temperature of the liquid crystal polymer is increased from between about 20 to about 50 centigrade degrees as a result of the heat treatment. The amount of increase which is obtained is dependent upon the temperature used in the heat treatment, with higher heat treatment temperatures giving greater increases.
- the chemical resistance of the liquid crystal polymer also increases with the heat treatment and the solubility in pentafluorophenol, one of the rare solvents for thermotropic liquid crystal polymers, continuously decreases with increasing heat treatment time such that eventually the polymer does not dissolve even minimally (such as in amounts of 0.1 percent by weight).
- the physical characteristics of the paper may be varied by the addition of various additives to the pulp in the web formation process.
- additives can also include reinforcing fibers of various materials including thermotropic liquid crystal polymeric fibers, glass fibers, microglass fibers, wood pulp, cotton and other cellulosic fibers, asbestos, mineral fibers, ceramic fibers, metallic fibers such as steel fibers, carbon fibers and synthetic non-liquid crystalline fibers such as viscous rayon, polyester, polyolefin, nylon and polytetrafluoroethylene.
- Such fibers can be incorporated over a wide range of proportions to provide products having the desired characteristics.
- Exemplary proportions generally are less than 50 percent by weight such that at least about 50 percent by weight of the paper is comprised of fibrils of thermotropic liquid crystal polymers.
- the paper consists essentially of fibrils of a thermotropic liquid crystal polymer in order to maximize the advantageous effect of such polymers.
- Dimensions of common reinforcing fibers range from 1 micron to 50 microns in diameter and from 1/32 to several inches in length, depending on the type of fiber and the physical characteristics desired in the paper product.
- a fiber comprised of a thermotropic liquid crystal polymer consisting of 40 mole percent of a p-oxybenzoyl moiety and 60 mole percent of a 6-oxy-2-naphthoyl moiety are cut into lengths ranging from about 1/8 to 1/4 inch.
- the cut fibers are admixed with three quarts of water and subjected to grinding in a Waring blender for about one hour to provide a coarse pulp comprised of fibrils.
- the coarse pulp in slurry form is further ground in a Ross and Sons mixer/emulsifier at the highest speed possible to provide a finer pulp.
- the fibrils in the finer pulp generally exhibit a length 1/2 to 1/4 of the length of the as cut fibers and a diameter 1/2 to 1/5 that of the as cut fibers. Grinding occurs in batches containing about 2 grams each of the coarse pulp.
- Each batch is further diluted with water and formed into a single sheet using a standard hand sheet mold equipped with a plastic screen.
- Each of the sheets are dried and calendered at a temperature between about 100 and 170°C. to produce paper sheets of increasing density and strength as the calendering temperature increases.
- thermotropic liquid crystal polymer consisting of 40 mole percent-of p-oxybenzoyl moieties and 60 mole percent of 6-oxy-2-naphthoyl moieties are added to a batch of fine ground pulp in an amount of 25 percent by weight prior to the sheet forming step.
- the fibers are heat treated by heating the fibers to a temperature of about 275°C. over a period of about 16 hours and maintaining the fibers at about 275°C. for several hours.
- the sheet formed therefrom is calendered at temperatures similar to those employed before to produce sheets of improved strength in comparison to the non-reinforced sheets.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Paper (AREA)
Abstract
Papers are provided comprised of fibrils of thermotropic liquid crystal polymers. The papers exhibit high temperature stability and resistance to solvents and chemical degradation.
Description
- The present invention is directed to high performance papers comprised of fibrils of thermotropic liquid crystal polymers.
- Papers comprised of polymeric materials have been employed for many purposes including filters and electrical insulation, etc. See, for example, U.S. Patent Nos. 2,988,782; 3,080,272; and 3,101,294 as well as Glen, W. "Papers from Synthetic Fibers" Paper Technology, Vol. 5, No. 2, pages 137-142, 1964. However, such papers are frequently not appropriate for use in a high temperature environment (e.g., temperatures in excess of about 200°C.) or in an environment where the paper will come into contact with corrosive chemicals or solvents. It is therefore desirable to provide a paper comprised of a polymeric material which is resistant to solvents or corrosive chemicals and suitable for use at high temperatures.
- It is also known to those skilled in the art that the heat treatment of shaped articles of liquid crystal polymers increases the melting temperature, molecular weight and. mechanical properties of the polymer. See, for example, U.S. Patent Nos. 3,975,487; 4,183,895; and 4,247,514.
- It is therefore an object of the present invention to provide a paper which exhibits desirable temperature stability.
- It is also an object of the present invention to provide a paper which exhibits desirable chemical and solvent resistance.
- It is also an object.of the present invention to obviate the disadvantages of the prior art discussed above..
- In accordance with the present invention, there are thus provided papers which exhibit desirable thermal stability and chemical and solvent resistance comprised of fibrils of a polymer which is capable of forming an anisotropic melt phase.
- Thermotropic liquid crystal polymers are polymers which are liquid crystalline (i.e., anisotropic) in the melt phase. These polymers have been described by various terms, including "liquid crystalline", "liquid crystal" and anisotropic". Briefly, the polymers of this class are thought to involve a parallel ordering of the molecular chains. The state wherein the molecules are so ordered is often referred to either as the liquid crystal state or the nematic phase of the liquid crystalline material. These polymers are prepared from monomers which are generally long, flat and fairly rigid along the long axis of the molecule and commonly have chain-extending linkages that are either coaxial or parallel.
- Such polymers readily form liquid crystals (i.e., exhibit anisotropic properties) in the melt phase. Such properties may be confirmed by conventional polarized light techniques whereby crossed polarizers are utilized. More specifically, the anisotropic melt phase may be confirmed by the use of a Leitz polarizing microscope at a magnification of 40X with the sample on a Leitz hot stage and under nitrogen atmosphere. The polymer is optically anisotropic; i.e., it transmits light when examined between crossed.polarizers. Polarized light is transmitted when the sample is optically anisotropic even in the static state.
- Those thermotropic liquid crystal polymers suitable for use in the present invention include but are not limited to wholly aromatic polyesters, aromatic-aliphatic polyesters, aromatic polyazomethines, wholly and non-wholly aromatic poly(ester- amide)s and aromatic polyester-carbonates.
- The wholly aromatic thermotropic liquid crystal polymers are comprised of moieties which contribute at least one aromatic ring to the polymer backbone and which enable the polymer to exhibit anisotropic properties in the melt phase. Such moieties include but are not limited to aromatic diols, aromatic amines, aromatic diacids and aromatic hydroxy acids. Moieties which may be present in the thermotropic liquid crystal polymers employed in the present invention include but are not limited to the following:
-
- Specific examples of suitable aromatic-aliphatic polyesters are copolymers of polyethylene terephthalate and hydroxybenzoic acid as disclosed in Polyester X7G-A Self Reinforced Thermoplastic, by W.J. Jackson, Jr., H.F. Kuhfuss, and T.F. Gray, Jr., 30th Anniversary Technical Conference, 1975 Reinforced Plas- tics/Composites Institute, The Society of the Plastics Industry, Inc., Section 17-D, Pages 1-4. A further disclosure of such copolymers can be found in "Liquid Crystal Polymers: I. Preparation and Properties of p-Hydroxybenzoic Acid Copolymers," Journal of Polymer Science, Polymer Chemistry Edition, Vol. 14, pp. 2043-58 (1976), by W.J. Jackson, Jr. and H.F. Kuhfuss. The above-cited references are herein incorporated by reference in their entirety.
- Aromatic polyazomethines and processes of preparing the same are disclosed in the U.S. Patent Nos. 3,493,522; 3,493,524; 3,503,739; 3,516,970; 3,516,971; 3,526,611; 4,048,148; and 4,122,070. Each of these patents is herein incorporated by reference in its'entirety. Specific examples of such polymers include poly(nitrilo-2-methyl-1,4-phenyl-enenitriloethylidyne-1,4-phenyleneethylidyne); poly(nitrolo-2-methyl-1,4-phenylene- nitrilomethylidyne-1,4-phenylene-methylidyne); and poly(nitrilo-2-chloro-1,4-phenylenenitrilomethylidyne-1,4-phenylene- methylidyne).
- Aromatic polyester-carbonates are disclosed in U.S. Patent No. 4,107,143, which is herein incorporated by reference in its entirety. Examples of such polymers include those consisting essentially of hydroxybenzoic acid units, hydroquinone units, carbonate units, and aromatic carboxylic acid units.
- The liquid crystal polymers which are preferred for use in the present invent'ion include thermotropic wholly aromatic polyesters. Recent publications disclosing such polyesters include (a) Belgian Pat. Nos. 828,935 and 828,936, (b) Dutch Pat. No. 7505551, (c) West German Pat. Nos. 2,520,819, 2,520,820, and 2,722,120, (d) Japanese Pat. Nos. 43-223, 2132-116, 3017-692, and 3021-293, (e) U.S. Pat. Nos. 3,991,013; 3,991,014; 4,057,597; 4,066,620; 4,075,262; 4,118,372; 4,146,702; 4,153,779; 4,156,070; 4,159,365; 4,169,933; 4,181,792; 4,188,476; 4,201,856 ; 4,226,970; 4,232,143; 4,232,144; 4,245,082; 4,238,600; 4,242,496; 4,267,304; and 4,269,965; and (f) U.K. Application No. 2,002,404.
- Wholly aromatic polymers which are preferred for use in the present invention include wholly aromatic polyesters and poly(ester-amide)s which are disclosed in commonly-assigned U.S. Patent Nos. 4,067,852; 4,083,829; 4,130;545; 4,161,470; 4,184,996 4,219,461; 4,238,599; 4,224,433; 4,256,624 and 4,279,803; and in commonly-assigned U.S. Application Serial Nos. 91,003, filed November 5, 1979; 128,759, filed March 10, 1980; and 214,557, filed December 9, 1980. The disclosures of all of the above- identified commonly-assigned U.S. patents and applications are herein incorporated by reference in their entirety. The wholly aromatic polymers disclosed therein typically are capable of forming an anisotropic melt phase at a temperature below approximately 400°C., and preferably below approximately 350°C.
- The wholly aromatic polymers including wholly aromatic polyesters and poly(ester-amide)s which are suitable for use in the present invention may be formed by a variety of ester-forming techniques whereby organic monomer compounds possessing functional groups which, upon condensation, form the requisite recurring moieties are reacted. For instance, the functional groups of the organic monomer compounds may be carboxylic acid groups, hydroxyl groups, ester groups, acyloxy groups, acid halides, amine groups, etc. The organic monomer compounds may be reacted in the absence of a heat exchange fluid via a melt acidolysis procedure. They, accordingly, may be heated initially to form a melt solution of the reactants with the reaction continuing as said polymer particles are suspended therein. A vacuum may be applied to facilitate removal of volatiles formed during the final stage of the condensation (e.g., acetic acid or water).
- Commonly-assigned U.S. Patent No. 4,083,829, entitled "Melt Processable Thermotropic Wholly Aromatic Polyester," describes a slurry polymerization process which may be employed to form the wholly aromatic polyesters which are preferred for use in the present invention. According to such a process, the solid product is suspended in a heat exchange medium. The disclosure of this patent has previously been incorporated herein by reference in its entirety.
- When employing either the melt acidolysis procedure or the slurry procedure of U.S. Patent No. 4,083,829, the organic monomer reactants from which the wholly aromatic polyesters are derived may be initially provided in a modified form whereby the usual hydroxy groups of such monomers are esterified (i.e., they are provided as lower acyl esters). The lower acyl groups preferably have from about two to about four carbon atoms. Preferably, the acetate esters of organic monomer reactants are provided.
- Representative catalysts which optionally may be employed in either the melt acidolysis procedure or in the slurry _procedure of U.S. Patent No. 4,083,829 include dialkyl tin oxide (e.g., dibutyl tin oxide), diaryl tin oxide, titanium dioxide, antimony trioxide, alkoxy titanium silicates, titanium alkoxides, alkali and alkaline earth metal salts of carboxylic acids (e.g., zinc acetate), the gaseous acid catalysts such as Lewis acids (e.g., BF3), hydrogen halides (e.g., HC1), etc. The quantity of catalyst utilized typically is about 0.001 to 1 percent by weight based upon the total monomer weight, and most commonly about 0.01 to 0.2 percent by weight.
- The wholly aromatic polymers suitable for use in the present invention tend to be substantially insoluble in common solvents and accordingly are not susceptible to solution processing. As discussed previously, they can be readily processed by common melt processing techniques. Most suitable wholly aromatic polymers are soluble in pentafluorophenol.
- The wholly aromatic polyesters which are preferred for . use in the present invention commonly exhibit a weight average molecular weight of about 2,000 to 200,000, and preferably about 10,000' to 50,000, and most preferably about 20,000 to 25,000. The wholly aromatic poly(ester-amide)s which are preferred commonly exhibit a molecular weight of about 5000 to 50,000 and preferably about 10,000 to 30,000; e.g., 15,000 to 17,000. Such molecular weight may be determined by gel permeation chromatography as well as by other standard techniques not involving the solutioning of the polymer, e.g., by end group determination via infrared spectroscopy on compression molded films. Alternatively, light scattering techniques in a pentafluorophenol solution may be employed to determine the molecular weight.
- The wholly aromatic polyesters and poly(ester-amide)s additionally commonly exhibit an inherent viscosity (i.e.,'I.V.) of at least approximately 2.0 dl./g., e.g., approximately 2.0 to 10.0 dl./g., when dissolved in a concentration of 0.1 percent by weight in pentafluorophenol at 60°C.
- Especially preferred wholly aromatic polymers are those which are disclosed in above-noted U.S. Patent Nos. 4,161,470, 4,184,996, 4,219,461, 4,238,599 and 4,256,624 and Application Serial No. 214,55'
- For the purpose of the present invention, the aromatic rings which are included in the polymer backbones of the polymer components employed in the present invention may include substitution of at least some of the hydrogen atoms present upon an aromatic ring. Such substituents include alkyl groups of up to four carbon atoms; alkoxy groups having up to four carbon atoms; halogens; and additional aromatic rings, such as phenyl and substituted phenyl. Preferred halogens include fluorine, chlorine, and bromine. Although bromine atoms tend to be released from organic compounds at high temperatures, bromine is more stable on aromatic rings than on aliphatic chains, and therefore is suitable for inclusion'as a possible substituent on the aromatic rings.
- The wholly aromatic polyester which is disclosed in U.S. Patent No. 4,161,470 is a melt processable wholly aromatic polyester capable of forming an anisotropic melt phase at a temperature below approximately 350°C. The polyester consists essentially'of the recurring moieties I and II wherein:
- The wholly aromatic polyester which is disclosed in U.S. Patent No. 4,184,996 is a melt processable wholly aromatic polyester capable of forming an anisotropic melt phase at a temperature below approximately 325°C. The polyester consists essentially of the recurring moieties I, II, and III wherein:
- The wholly aromatic polyester which is disclosed in U.S. Patent No. 4,238,599 is a melt processable polyester capable of forming an anisotropic melt phase at a temperature no higher than approximately 320°C. consisting essentially of the recurring moieties I, II, III and IV wherein:
R is methyl, chloro, bromo, or mixtures thereof, and is substituted for a hydrogen atom present upon the aromatic ring,
and wherein said polyester comprises approximately 20 to 60 mole percent of moiety I, approximately 5 to 18 mole percent of moiety II, approximately 5 to 35 mole percent of moiety III, and approximately 20 to 40 mole percent of moiety IV. The polyester preferably comprises approximately 35 to 45 mole percent of moiety I, approximately 10 to 15 mole percent of moiety II, approximately 15 to 25 mole percent of moiety III, and approximately 25 to 35 mole percent of moiety IV, with the proviso that the total molar concentration of moieties II and III is substantially identical to that of moiety IV. In addition, at least some of the hydrogen atoms present upon the rings optionally may be replaced by substitution selected from the group consisting of an alkyl group of 1 to 4 carbon atoms, an alkoxy group of 1 to 4 carbon atoms, halogen, phenyl, substituted phenyl, and mixtures thereof. This wholly aromatic polyester commonly exhibits an inherent viscosity of at least 2.0 dl./g., e.g., 2.0 to 10.0 dl./g., when dissolved in a concentration of 0.1 weight/volume .percent in pentafluorphenol at 60°C. -
- III is a dioxy aryl moiety of the formula {-O-Ar-O-} wherein Ar is a divalent radical comprising at least one aromatic ring, and
- IV is a dicarboxy aryl moiety of the formula
- Moieties III and IV are preferably symmetrical in the sense that the divalent bonds which join these moieties to other moieties in the main polymer chain are symmetrically disposed on one or more aromatic rings (e.g., are para to each other or diagonally disposed when present on a naphthalene ring). However, non-symmetrical moieties, such as those derived from resorcinol and isophthalic acid, may also be used.
-
-
- II is a dioxy aryl moiety of the formula O-Ar-O where Ar is a divalent radical comprising at least one aromatic ring, and
- III is a dicarboxy aryl moiety of the formula -OC-Ar'-OC- where Ar' is a divalent radical comprising at least one aromatic ring, and
- As with moieties III and IV of the polyester disclosed in U.S. Patent No..4,219,461, moieties II and III of the polyester described immediately above may be symmetrical or nonsymmetrical, but are preferably symmetrical.
-
- U.S. Application Serial No. 214,557, filed December 9, 1980, discloses a melt processable poly(ester-amide) which is capable of forming an anisotropic melt phase at a temperature below approximately 400°C. The poly(ester-amide) consists essentially of the recurring moieties I, II, III and optionally IV wherein:
- II is -OC-A-OC, where A is a divalent radical comprising at least one aromatic ring or a divalent trans-cyclohexane radical;
- III is Y-Ar-Z, where Ar is a divalent radical comprising at least one aromatic ring, Y is O, NH, or NR, and Z is NH or NR, where R is an alkyl group of 1 to 6 carbon atoms or an aryl group; and
- IV is O-Ar'-O, where Ar' is a divalent radical comprising at least one aromatic ring;
-
- The papers of the present invention are comprised of fibrils of thermotropic liquid crystal polymers (i.e., polymers which are capable of forming an anisotropic melt phase). The fibrils may be produced by several methods including mechanically masticating shaped articles including as-spun thermotropic liquid crystal polymer fibers. Since the as-spun fibers are highly oriented along their longitudinal axis, the fibers are able to withstand much less stress along the transverse axis as opposed to along the longitudinal axis. Accordingly, the fibers readily break up length-wise into much narrower fibrils as they are masticated to form a pulp. The fibers are preferably masticated in the form of an aqueous slurry.
- .. - ' The pulp which is formed is a mass of fibrils which are generally frayed, fibrillated and/or branched. Such characteristics permit the fibrils to become intertangled such that a thin, yet coherent mat may be formed upon simple screening of the slurry. The term "pulp" is intended to refer to a mass of fibers or other shaped articles which have been mechanically masticated or ground, causing the fiber or article to separate, split, fray, fibrillate and/or shred into generally finer diameter units. The intertangling between the units, or "interfelting", is thereby enhanced, thus allowing the formation of thin, yet coherent sheets. The fibers and fragments may, in addition, become crimped, branched, or multiply bifurcated to improve the interfelting.
- A typical solids content of the pulp ranges from about 0.5 to 50 grams of polymer per liter of liquid (e.g., water),. i.e., from about 0.05 to 5 percent by weight of solids. A preferred solids content ranges from about 0.5 to 2 percent by weight of solids. When a wet laid paper mat is produced it is advantageous to cast the paper from a slightly more dilute slurry (e.g., from about 0.01 to 1 percent by weight of solids).
- The term "masticating" as used herein is intended to include various mechanical processes whereby the shaped article is subjected to grinding or shearing forces of sufficient magnitude to break up the shaped article into fibrils. The mastication step may involve a series of techniques rather than a single operation. Such processes include but are not limited to the use of fluid or air turbulence (i.e., air fibrillation), use of high shear (i.e., as in a pulp refiner and open disk emulsifier), repeated chopping in conjunction with fluid shear (e.g., as in a Waring blender), and crushing in conjunction with mechanical shear (e.g., as in a Jordan "beater"). The use of surface wetting agents or 50.percent isopropanol or ethanol may facilitate the fibrillation of the polymer.
- The fibrils which are produced commonly exhibit a ratio of length to diameter which is generally greater than that exhibited by the fibers. For example, the length to diameter ratio exhibited by the fibers generally ranges from about 30:1 to about 300:1, while the corresponding ratio for the fibrils ranges from about 50:1 to about 600:1. The fibrils preferably exhibit a diameter of about 0.5 to about 5 microns and a length of about 50 microns to about 3 millimeters.
- The fibers which are masticated are generally from 1/32 to 1/4 inch in length and 14 to 35 microns in diameter. The denier of the fiber is preferably from about 2 to 10. Fibers of a much broader size can also be employed, such as, for example, 0.5 to 100 denier (7 to 100 microns in diameter). A typical starting length ranges from 1/32 to 1/4 inch. If a starting length of greater than 1/8 inch is employed, the pulping method employed should be capable of length reduction as well as fibrillation to avoid clumping of the pulp particles. The length of the fibrils which are produced will generally be proportional to the length of the fibers employed.
- As noted in copending application Serial No. of John R. Kastelic, Larry F. Charbonneau and Thomas P. Carter, Jr. entitled "Thermotropic Liquid Crystal Polymer Pulp and Method of Production Thereof Wherein Said Polymer Comprises Recurring Units Which Contain A 2,6-Dioxyanthraquinone Moiety" filed , it has been found that the presence of at least about 5 mole percent of recurring units which contain a . 2,6-dioxyanthraquinone moiety in the thermotropic liquid crystal polymer enables the fibers to be readily broken up into fibrils of submicron size while minimizing the use of time and energy with respect to the fibrillation process.
- While the use of the fibers is preferred, it is also possible to use articles of other shapes and configurations. For example, the polymer may also be in the form of pellets or sheets, etc. The term shaped article as used herein is intended to include particles, pellets, filaments, staple fibers, films, chips, sheets and other extruded, molded, cast or otherwise formed shaped articles.
- It should be noted, however, that the more highly oriented is the polymer in the article, the higher the aspect ratio of the fibrils which are formed will be. It is therefore preferable to employ articles in the method of the present invention which are highly oriented as a result of being 'formed (e.g., melt spun fibers) in order to produce fibrils having a high aspect ratio.
- The fibrils which are produced can subsequently be slurried with a liquid which is a non-solvent for the polymer of which the fibrils are comprised such as water and collected (e.g., filtered) onto a web or screen to provide a random (i.e., multi-dimensional) array or sheet of fibrils. In addition to wet laying, webs may be formed by air lay processes wherein the fibrous material is entrained in and deposited from a moving air stream. Since the fibrils which are produced are generally bifurcated or branched along the longitudinal axis, they tend to become attached to one another as they are deposited.
- Appropriate methods are employed to bond the fibrils together to form a paper having the desired degree of structural. integrity. That is, the paper at a minimum supports its own weight and preferably is pulled apart only with difficulty. Such a paper will accordingly be in the form of a sheet or web. The fibrils may be thermally bonded to one another at a suitable temperature by conventional means such as heat pressing or calendering to at least bond the fibrils together at their crossover points. Such fusion bonding does not result in any significant loss of orientation (and, accordingly, loss of strength) since the polymer of which the fibrils are comprised forms an anisotropic melt phase. Such a characteristic is in direct contrast to conventional thermoplastic polymers which do not form an anisotropic melt phase and which readily lose their orientation upon being heated to temperatures in excess of their melting temperature.
- Heat pressing is essentially a batch process wherein the web of fibrils is pressed between two heated plates. Calendering involves the passage of the fibrils in the form of a web between heated rolls. The use of a padded backup roll against a heated metal roll is preferred. The thermal bonding temperature will generally range from about 100 to about 250°C.
- The fibrils may also be bonded together by means of adhesives including but not limited to the following: epoxies, thermosetting or thermoplastic resins including thermosetting polyesters, water soluble adhesives such as casine, guar gum or polyacrylic acid, solvent-based adhesives and emulsion or latex- based adhesives such as styrene/butyl/acrylic copolymer systems. The adhesive may be coated onto the web or array of fibrils by use of kiss rolls. Alternatively, the adhesive may be sprayed upon or deposited upon the web by known emulsion techniques (for use with wet laid paper). The use of adhesives in such methods is known and will not be discussed in greater detail herein.
- The temperature as well as the method of thermal bonding employed affects the physical characteristics exhibited by the paper which is produced. For example, when temperatures below about 140°C. are used, an opaque paper is provided. Such. papers are essentially a mat of intertangled fibrous particulates (the fibrils) which exhibits substantial porosity and low density. On the other hand, when temperatures in excess of about 140°C. are employed in conjunction with a pressing or calendering step (and especially in the range- of 170°C. or so), a transparent paper in the form of a film or membrane resembling a glassine film is produced which exhibits reduced porosity. Accordingly, the use of excessive temperatures and pressures should generally be avoided during calendering if a paper of substantial porosity is desired since such temperatures and pressures increase the degree of fusion and compaction the fibrils to each other while correspondingly decreasing the porosity. The amount of pressure applied as well as the duration of the application of pressure can also influence the degree of fusion.
- The papers of the present invention possess many advantageous properties due to the presence of thermotropic liquid crystal polymers therein. Since the liquid crystal'polymers are highly oriented as spun, the fibrils which comprise the papers of the present invention possess relatively high tensile strength and high modulus. Accordingly, papers comprised of such fibrils similarly exhibit relatively high modulus and high tensile strength. In addition, the papers exhibit such tensile strength and modulus in a multi-dimensional manner due to the multi-dimensional (i.e., random) orientation of the fibrils within the paper. The papers can vary in weight, porosity, thickness, etc. depending upon the amount of fibrils employed, the pressure used to thermally bond the fibrils together, etc. The papers typically, however, can exhibit a weight ranging from 3 to 20 ounces/square yard.
- The mechanical properties of the papers produced in accordance with the present invention can be improved still further by subjecting the papers to a heat treatment following . formation thereof. The heat treatment improves the properties of the paper by increasing the molecular weight of the liquid crystalline polymer which comprises the fibrils and increasing the degree of crystallinity thereof while also increasing the melting temperature of the polymer. Such heat treatment can also serve to bond the fibrils together.
- The papers may be thermally treated in an inert atmosphere (e.g., nitrogen, carbon dioxide, argon, helium) or alternatively, in a flowing oxygen-containing atmosphere (e.g., air). The use of a non-oxidizing substantially moisture-free atmosphere is preferred to avoid the possibility of thermal degradation.. For instance, the paper may be brought to a temperature approximately 10 to 30 centigrade degrees below the melting temperature of the liquid crystal polymer, at which temperature the fibrils remain solid. It is preferable for the temperature of the heat treatment to be as high as possible without equaling or exceeding the melting temperature of the polymer. It is most preferable to gradually increase the temperature, of heat treatment in accord-- ance with the increase of the melting temperature of the polymer during heat treatment.
- The duration of the heat treatment will commonly range from a few minutes to a number of days, e.g., from 0.5 to 200 hours, or more. Preferably, the heat treatment is conducted for a time of 1 to 48 hours and typically from about 5 to 30 hours.
- Generally, the duration of heat treatment varies depending upon the heat treatment temperature; that is, a shorter treatment time is required as a higher treatment is used. Thus, the duration of the heat treatment can be shortened for higher melting polymers, since higher heat treatment temperatures can be applied without melting the polymer.
- Preferably, the heat treatment is conducted under conditions sufficient to increase the melting temperature of the polymer at least 10 centigrade degrees. Most preferably, the melting temperature of the liquid crystal polymer is increased from between about 20 to about 50 centigrade degrees as a result of the heat treatment. The amount of increase which is obtained is dependent upon the temperature used in the heat treatment, with higher heat treatment temperatures giving greater increases.
- Similar advantages can also be obtained by heat treating the fibrils prior to their incorporation into a paper. It is, however, preferable to heat treat the paper subsequent to its formation since the thermal bonding and heat treatment steps can then be combined.
- It should be noted at this point that reference herein to a temperature below which a specific polymer may exhibit anisotropic properties in the melt phase is intended to refer to the temperature below which the polymer exhibits such properties prior to heat treatment thereof.
- The chemical resistance of the liquid crystal polymer also increases with the heat treatment and the solubility in pentafluorophenol, one of the rare solvents for thermotropic liquid crystal polymers, continuously decreases with increasing heat treatment time such that eventually the polymer does not dissolve even minimally (such as in amounts of 0.1 percent by weight).
- The physical characteristics of the paper may be varied by the addition of various additives to the pulp in the web formation process. For example, wetting agents, surface treatment agents, coloring agents and fillers can be added. Such additives can also include reinforcing fibers of various materials including thermotropic liquid crystal polymeric fibers, glass fibers, microglass fibers, wood pulp, cotton and other cellulosic fibers, asbestos, mineral fibers, ceramic fibers, metallic fibers such as steel fibers, carbon fibers and synthetic non-liquid crystalline fibers such as viscous rayon, polyester, polyolefin, nylon and polytetrafluoroethylene. Such fibers can be incorporated over a wide range of proportions to provide products having the desired characteristics. Exemplary proportions generally are less than 50 percent by weight such that at least about 50 percent by weight of the paper is comprised of fibrils of thermotropic liquid crystal polymers. Preferably, the paper consists essentially of fibrils of a thermotropic liquid crystal polymer in order to maximize the advantageous effect of such polymers.
- Dimensions of common reinforcing fibers range from 1 micron to 50 microns in diameter and from 1/32 to several inches in length, depending on the type of fiber and the physical characteristics desired in the paper product.
- The invention is additionally illustrated in connection with the following Example which is to be considered as illustrative of the present invention. It should understood, however, that the invention is not limited to the specific details of.the Example.
- Twenty grams of a fiber comprised of a thermotropic liquid crystal polymer consisting of 40 mole percent of a p-oxybenzoyl moiety and 60 mole percent of a 6-oxy-2-naphthoyl moiety are cut into lengths ranging from about 1/8 to 1/4 inch. The cut fibers are admixed with three quarts of water and subjected to grinding in a Waring blender for about one hour to provide a coarse pulp comprised of fibrils.
- The coarse pulp in slurry form is further ground in a Ross and Sons mixer/emulsifier at the highest speed possible to provide a finer pulp. The fibrils in the finer pulp generally exhibit a length 1/2 to 1/4 of the length of the as cut fibers and a diameter 1/2 to 1/5 that of the as cut fibers. Grinding occurs in batches containing about 2 grams each of the coarse pulp.
- Each batch is further diluted with water and formed into a single sheet using a standard hand sheet mold equipped with a plastic screen. Each of the sheets are dried and calendered at a temperature between about 100 and 170°C. to produce paper sheets of increasing density and strength as the calendering temperature increases.
- The above procedure is repeated except that heat treated chopped fibers of a thermotropic liquid crystal polymer consisting of 40 mole percent-of p-oxybenzoyl moieties and 60 mole percent of 6-oxy-2-naphthoyl moieties are added to a batch of fine ground pulp in an amount of 25 percent by weight prior to the sheet forming step. The fibers are heat treated by heating the fibers to a temperature of about 275°C. over a period of about 16 hours and maintaining the fibers at about 275°C. for several hours. The sheet formed therefrom is calendered at temperatures similar to those employed before to produce sheets of improved strength in comparison to the non-reinforced sheets.
-
- The principles, preferred embodiments and modes of operation of the present invention have been described in the foregoing specification. The invention which is intended to be protected herein, however, is not to be construed as limited to the particular forms disclosed, since these are to be regarded as illustrative rather than restrictive. Variations and changes may be made by those skilled in the art without departing from the spirit of the invention.
wherein the polyester comprises approximately 20 to 40 mole percent of moiety I, in excess of 10 up to about 50 mole percent of moiety II, in excess of 5 up to about 30 mole percent of moiety III, and in excess of 5 up to about 30 mole percent of moiety IV. The polyester preferably comprises approximately 20 to 30 (e.g., approximately 25) mole percent of moiety I, approximately 25 to 40 (e.g., approximately 35) mole percent of moiety II, approximately 15 to 25 (e.g., approximately 20) mole percent of moiety III, and approximately 15 to 25 (e.g. , approximately 20) mole percent of moiety IV. In addition, at least some of the hydrogen atoms present upon the rings optionally may be replaced by substitution selected from the group consisting of an alkyl group of 1 to 4 carbon atoms, an alkoxy group of 1 to 4 carbon atoms, halogen, phenyl, substituted phenyl, and mixtures thereof.
wherein the polyester comprises approximately 10 to 90 mole percent of moiety I, approximately 5 to 45 mole percent of moiety II, and approximately 5 to 45 mole percent of moiety III. The polyester preferably comprises approximately 20 to 80 mole percent of moiety I, approximately 10 to 40 mole percent of moiety II, and approximately 10 to 40 mole percent of moiety III. The polyester more preferably comprises approximately 60 to 80 mole percent of moiety I, approximately 10 to 20 mole percent of moiety II, and approximately 10 to 20 mole percent of moiety III. In addition, at least some of the hydrogen atoms present upon the rings optionally may be replaced by substitution selected from the group consisting of an alkyl group of 1 to 4 carbon atoms, an alkoxy group of 1 to 4 carbon atoms, halogen, phenyl, substituted phenyl, and mixtures thereof.
and wherein said poly(ester-amide) comprises approximately 10 to 90 mole percent of moiety I, approximately 5 to 45 mole percent of moiety II, approximately 5 to 45 mole percent of moiety III, and approximately 0 to 40 mole.percent of moiety IV. In addition, at least some of the hydrogen atoms present upon the rings optionally may be replaced by substitution selected from the group consisting of an alkyl group of 1 to 4 carbon atoms, an alkoxy group of 1 to 4 carbon atoms, halogen, phenyl, substituted phenyl, and mixtures thereof.
Claims (38)
1. A paper which exhibits desirable thermal stability and chemical and solvent resistance comprised of fibrils of a polymer which is capable of forming an anisotropic melt phase, said fibrils being bonded together to an extent sufficient to impart structural integrity to said paper.
2. The paper of claim 1 wherein said polymer is a wholly aromatic polymer.
3. The paper of claim 2 wherein said polymer is a - wholly aromatic polyester.
4. The paper of claim 1 wherein said polymer exhibits an inherent viscosity of at least 2.0 dl./g. when dissolved in a concentration of 0.1 percent by weight in pentafluorophenol at 60.C.
5. The paper of claim 1 wherein said polymer comprises not less than about 10 mole percent of recurring units which include a naphthalene moiety.
6. The paper of claim 5 wherein said naphthalene moiety of said wholly aromatic polymer is selected from the group consisting of a 6-oxy-2-naphthoyl moiety, a 2,6-dioxynaphthalene moiety, and a 2,6-dicarboxynaphthalene moiety.
7. The paper of claim 1 wherein said polymer is capable of forming an anisotropic melt phase at a temperature below approximately 400°C.
8. The paper of claim 1 wherein said polymer comprises a melt processable wholly aromatic polyester which is capable of forming an anisotropic melt phase and consists essentially of the recurring moieties I, II, and III wherein:
wherein said polyester comprises approximately 30 to 70 mole percent of moiety I and wherein at least some of the hydrogen atoms present upon the rings optionally may be replaced by substitution selected from the group consisting of an alkyl group of 1 to 4 carbon atoms, an alkoxy group of 1 to 4 carbon atoms, halogen, phenyl, substituted phenyl, and mixtures thereof.
9.. The paper of claim 8 wherein said polyester comprises approximately 40 to 60 mole percent of moiety I, approximately 20 to 30 mole percent of moiety II, and approximately 20 to 30 mole percent of moiety III.
10. The paper of claim 1 wherein said polyme comprises a melt processable wholly aromatic polyester which is capable of forming an anisotropic melt phase and consists essentially of the recurring moieties I and II wherein:
wherein said polyester comprises approximately 10 to 90 mole percent of moiety I, and approximately 10 to 90 mole percent of moiety II and wherein at least some of the hydrogen atoms present upon the rings optionally may be replaced by substitution selected from the group consisting of an alkyl group of 1 to 4 carbon atoms, an alkoxy group of I to 4 carbon atoms, halogen, phenyl, substituted phenyl, and mixtures thereof.
11. The paper of claim 10 wherein said polyester comprises approximately 65 to 85 mole percent of moiety II.
12. The paper of claim 10 wherein said polyester comprises approximately 15 to 35 mole percent of moiety II.
13. The paper of claim 1 wherein said polymer comprises a melt processable wholly aromatic polyester which is capable of forming an anisotropic melt phase and consists essentially of the recurring moieties I, II, and III wherein:
wherein said polyester comprises approximately 10 to 90 mole percent of moiety I, approximately 5 to 45 mole percent of moiety II, and approximately 5 to 45 mole percent of moiety III and wherein at least some of the hydrogen atoms present upon the rings optionally may be replaced by substitution selected from the group consisting of an alkyl group of 1 to 4 carbon atoms, an alkoxy group of 1 to 4 carbon atoms, halogen, phenyl, substituted phenyl, and mixtures thereof.
II is a dioxy aryl moiety of the formula 0-Ar-0 where Ar is a divalent radical comprising at least one aromatic ring, and
III is a dicarboxy aryl moiety of the formula. (̵C-Ar'-C)̵ where Ar' is a divalent radical comprising at least one aromatic ring, and
wherein said polyester comprises approximately 10 to 90 mole percent of moiety I, approximately 5 to 45 mole percent of moiety II, and approximately 5 to 45 mole percent of moiety III and wherein at least some of the hydrogen atoms present upon the rings optionally may be replaced by substitution selected from the group consisting of an alkyl group of 1 to 4 carbon atoms, an alkoxy group of 1 to 4 carbon atoms, halogen, phenyl, substituted phenyl, and mixtures thereof.
14. The paper of claim 13 wherein said polyester comprises approximately 20 to 80 mole percent of moiety I, approximately 10 to 40 mole percent of moiety II, and approximately 10 to 40 mole percent of moiety III.
15. The paper of claim 1 wherein said polymer comprises a melt processable wholly aromatic polyester which is capable of forming an anisotropic melt phase and consists essen- tially of the recurring moieties I, II, III and IV wherein:
wherein the polyester comprises approximately 20 to 40 mole percent of moiety I, in excess of 10 up to about 50 mole percent of moiety II, in excess of 5 up to about 30 mole percent of moiety III, and in excess of 5 up to about 30 mole percent of moiety IV and wherein at least some of the hydrogen atoms present upon the rings optionally may be replaced by substitution selected from the group consisting of an alkyl group of l'to 4 carbon atoms, an .alkoxy group of 1 to 4 carbon atoms, halogen, phenyl, substituted phenyl, and mixtures thereof.
III is a dioxy aryl moiety of the formula (̵µ-Ar-µ)̵· wherein Ar is a divalent radical comprising at least one aromatic ring, and
IV is a dicarboxy aryl moiety of the formula C0-Ar'-C where Art is a divalent radical comprising at least one aromatic ring, and
wherein the polyester comprises approximately 20 to 40 mole percent of moiety I, in excess of 10 up to about 50 mole percent of moiety II, in excess of 5 up to about 30 mole percent of moiety III, and in excess of 5 up to about 30 mole percent of moiety IV and wherein at least some of the hydrogen atoms present upon the rings optionally may be replaced by substitution selected from the group consisting of an alkyl group of l'to 4 carbon atoms, an .alkoxy group of 1 to 4 carbon atoms, halogen, phenyl, substituted phenyl, and mixtures thereof.
16. The paper of claim 15 wherein said polyester comprises approximately 20 to 30 mole percent of moiety I, approximately 25 to 40 mole percent of moiety II, approximately 15 to 25 mole percent of moiety III and approximately 15 to 25 mole percent of moiety IV.
17. The paper of claim 1 wherein said polymer comprises a melt processable poly(ester-amide) which is capable of forming an anisotropic melt phase and consists essentially of the recurring moieties I, II, III and optionally IV wherein:
where A is a divalent radical comprising at least one aromatic ring or a divalent trans-cyclohexane radical;
and wherein said poly(ester-amide) comprises approximately 10 to 90 mole percent of moiety I, approximately 5 to 45 mole percent of moiety II, approximately 5 to 45 mole percent of moiety III and approximately 0 to 40 mole percent of moiety IV and wherein at least some of the hydrogen atoms present upon the rings optionally may be replaced by substitution selected from the group consisting of an alkyl group of 1 to 4 carbon atoms, an alkoxy group of 1 to 4 carbon atoms, halogen, phenyl, substituted phenyl, and mixtures thereof.
III is (̵Y-Ar-Z)̵, where Ar is a divalent radical comprising at least one aromatic ring, Y is 0, NH, or NR, and Z is NH or NR, where R is an alkyl group of 1 to 6 carbon atoms or an aryl group; and
IV is O-Ar'-O, where Ar' is a divalent radical comprising at least one aromatic ring;
and wherein said poly(ester-amide) comprises approximately 10 to 90 mole percent of moiety I, approximately 5 to 45 mole percent of moiety II, approximately 5 to 45 mole percent of moiety III and approximately 0 to 40 mole percent of moiety IV and wherein at least some of the hydrogen atoms present upon the rings optionally may be replaced by substitution selected from the group consisting of an alkyl group of 1 to 4 carbon atoms, an alkoxy group of 1 to 4 carbon atoms, halogen, phenyl, substituted phenyl, and mixtures thereof.
18. The paper of claim 1 wherein said polymer has been subjected to a heat treatment for a period of time and at a temperature sufficient to increase the melting temperature of the polymer between about 20 to about 50 centigrade degrees.
19. The paper of claim 18 wherein said heat treatment temperature ranges from about 10 to about 30 centigrade degrees below the melting temperature of the polymer.
20. The paper of claim 19 wherein said period of time ranges from about 0.5 to about 200 hours.
21. The paper of claim 20 wherein said period of time ranges from about 1 to about 48 hours.
22. The paper of claim 21 wherein said period of time ranges from about 5 to about 30 hours.
23. The paper of claim 18 wherein said heat treatment occurs in a non-oxidizing atmosphere.
24. The paper of claim 23 wherein said atmosphere is substantially moisture-free.
25. The paper of claim 23 wherein said heat treatment occurs in a nitrogen atmosphere.
26. The paper of claim 1 wherein said fibrils exhibit a ratio of length to diameter ranging from about 50:1 to about 600:1.
27. The paper of claim 1 wherein said fibrils exhibit a diameter ranging from about 0.5 to about 5 microns.
28. The paper of claim 1 wherein said fibrils are thermally bonded together. '
29. The paper of claim 28 wherein said fibrils are thermally bonded together at a temperature in the range of about 100 to 250°C.
30. The paper of claim 28 wherein said thermal bonding occurs under pressure.
31. The paper of claim 30 wherein said fibrils are thermally bonded together by heat pressing.
32. The paper of claim 28 wherein said fibrils are thermally bonded together by calendering.
33. The paper of claim 1 wherein said fibrils are bonded together by use of adhesives.
34. The paper of claim 33 wherein said fibrils are bonded together by use of thermosetting or thermoplastic resins.
35. The paper of claim 1 having a weight of about 3 to 20 ounces/square yard.
36. The paper of claim 1 further comprising reinforcing fibers.
37. The paper of claim 1 consisting essentially of said fibrils.
38. The paper of claim 28 wherein said thermal bonding occurs under pressure.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP84302959A EP0167682A1 (en) | 1984-05-02 | 1984-05-02 | High performance papers comprised of fibrils of thermotropic liquid crystal polymers |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP84302959A EP0167682A1 (en) | 1984-05-02 | 1984-05-02 | High performance papers comprised of fibrils of thermotropic liquid crystal polymers |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| EP0167682A1 true EP0167682A1 (en) | 1986-01-15 |
Family
ID=8192628
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP84302959A Withdrawn EP0167682A1 (en) | 1984-05-02 | 1984-05-02 | High performance papers comprised of fibrils of thermotropic liquid crystal polymers |
Country Status (1)
| Country | Link |
|---|---|
| EP (1) | EP0167682A1 (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5954920A (en) * | 1994-06-07 | 1999-09-21 | Kuraray Co., Ltd. | Paper comprising aromatic polyester and process for producing the same |
| EP1243696A2 (en) * | 2001-03-23 | 2002-09-25 | Sumitomo Chemical Company, Limited | Low hygroscopic paper and method of producing the same |
| WO2003020511A1 (en) * | 2001-08-30 | 2003-03-13 | E.I. Du Pont De Nemours And Company | Solid sheet material especially useful for circuit boards |
| US20150294754A1 (en) * | 2013-05-22 | 2015-10-15 | Murata Manufacturing Co., Ltd. | Fibrillated liquid crystal polymer powder, method of producing fibrillated liquid crystal polymer powder, paste, resin multilayer substrate, and method of producing resin multilayer substrate |
| CN112501954A (en) * | 2020-11-23 | 2021-03-16 | 江苏展宝新材料有限公司 | Preparation method of LCP film |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5335021A (en) * | 1976-09-08 | 1978-04-01 | Teijin Ltd | Polyester particle pulp and its production |
| FR2376501A1 (en) * | 1976-12-23 | 1978-07-28 | Inst Sint Smol | Synthetic fibre electrical insulator sheets - of wet laid sheets of synthetic fibres, e.g. of poly-(meta)-phenylene isophthalamide and fibrids of aromatic bisphenol polyester as binder |
| EP0022182A1 (en) * | 1979-06-11 | 1981-01-14 | Teijin Limited | Synthetic polyester pulp and process for preparing same |
| EP0027516A1 (en) * | 1979-08-24 | 1981-04-29 | Teijin Limited | Artificial pulp particles, process for producing same and paper-like sheet prepared therefrom |
| US4395307A (en) * | 1981-11-09 | 1983-07-26 | Celanese Corporation | Thermotropic liquid crystal polymer pulp and method of preparation thereof wherein said polymer comprises recurring units which contain a 2,6-dioxyanthraquinone moiety |
-
1984
- 1984-05-02 EP EP84302959A patent/EP0167682A1/en not_active Withdrawn
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5335021A (en) * | 1976-09-08 | 1978-04-01 | Teijin Ltd | Polyester particle pulp and its production |
| FR2376501A1 (en) * | 1976-12-23 | 1978-07-28 | Inst Sint Smol | Synthetic fibre electrical insulator sheets - of wet laid sheets of synthetic fibres, e.g. of poly-(meta)-phenylene isophthalamide and fibrids of aromatic bisphenol polyester as binder |
| EP0022182A1 (en) * | 1979-06-11 | 1981-01-14 | Teijin Limited | Synthetic polyester pulp and process for preparing same |
| EP0027516A1 (en) * | 1979-08-24 | 1981-04-29 | Teijin Limited | Artificial pulp particles, process for producing same and paper-like sheet prepared therefrom |
| US4395307A (en) * | 1981-11-09 | 1983-07-26 | Celanese Corporation | Thermotropic liquid crystal polymer pulp and method of preparation thereof wherein said polymer comprises recurring units which contain a 2,6-dioxyanthraquinone moiety |
Non-Patent Citations (1)
| Title |
|---|
| ABSTRACT BULLETIN OF THE INSTITUTE OF PAPER CHEMISTRY, vol. 53, no. 7, January 1983, page 853, abstract no. 7881, Appleton, Wisc., US * |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5954920A (en) * | 1994-06-07 | 1999-09-21 | Kuraray Co., Ltd. | Paper comprising aromatic polyester and process for producing the same |
| EP1243696A2 (en) * | 2001-03-23 | 2002-09-25 | Sumitomo Chemical Company, Limited | Low hygroscopic paper and method of producing the same |
| EP1243696A3 (en) * | 2001-03-23 | 2002-11-20 | Sumitomo Chemical Company, Limited | Low hygroscopic paper and method of producing the same |
| US6843887B2 (en) | 2001-03-23 | 2005-01-18 | Sumitomo Chemical Company, Limited | Low hygroscopic paper and method of producing the same |
| WO2003020511A1 (en) * | 2001-08-30 | 2003-03-13 | E.I. Du Pont De Nemours And Company | Solid sheet material especially useful for circuit boards |
| US20150294754A1 (en) * | 2013-05-22 | 2015-10-15 | Murata Manufacturing Co., Ltd. | Fibrillated liquid crystal polymer powder, method of producing fibrillated liquid crystal polymer powder, paste, resin multilayer substrate, and method of producing resin multilayer substrate |
| US11646127B2 (en) | 2013-05-22 | 2023-05-09 | Murata Manufacturing Co., Ltd. | Fibrillated liquid crystal polymer powder, method of producing fibrillated liquid crystal polymer powder, paste, resin multilayer substrate, and method of producing resin multilayer substrate |
| CN112501954A (en) * | 2020-11-23 | 2021-03-16 | 江苏展宝新材料有限公司 | Preparation method of LCP film |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4540737A (en) | Method for the formation of composite articles comprised of thermotropic liquid crystalline polymers and articles produced thereby | |
| CA1169748A (en) | Multiaxially oriented high performance laminates comprised of uniaxially oriented sheets of thermotropic liquid crystal polymers | |
| CA1197979A (en) | Method for the melt processing of thermotropic liquid crystal polymers | |
| US4479999A (en) | Fabric comprised of fusible and infusible fibers, the former comprising a polymer which is capable of forming an anisotropic melt phase | |
| US4439578A (en) | Use of liquid crystal polymer particulates having a high aspect ratio in polymeric molding resins to suppress melt dripping | |
| US4394498A (en) | Method for providing particulates of liquid crystal polymers and particulates produced therefrom | |
| US4256624A (en) | Polyester of 6-hydroxy-2-naphthoic acid, aromatic diol, and aromatic diacid capable of undergoing melt processing | |
| EP0063880B1 (en) | Poly(esteramide) derived from 6-hydroxy-2-naphthoic acid | |
| JPH0320337A (en) | Total aromatic polyester film | |
| EP0044675A1 (en) | Processing of melt processable liquid crystal polymer by control of thermal history | |
| US4238599A (en) | Polyester of para-hydroxy benzoic acid, 1,2-bis(para-carboxy phenoxy)ethane, terephthalic acid and substituted hydroquinone capable of forming an anisotropic melt which readily undergoes melt processing | |
| EP0169947B1 (en) | Melt blend of non-thermotropic and thermotropic wholly aromatic polyesters | |
| US4395307A (en) | Thermotropic liquid crystal polymer pulp and method of preparation thereof wherein said polymer comprises recurring units which contain a 2,6-dioxyanthraquinone moiety | |
| JP4736548B2 (en) | Nonwoven fabric made of liquid crystalline resin fiber | |
| JPH0633595B2 (en) | High-performance paper made of fibrils of thermotropic liquid crystal polymer | |
| EP0167682A1 (en) | High performance papers comprised of fibrils of thermotropic liquid crystal polymers | |
| US4265802A (en) | Polyester of para-hydroxy benzoic acid, 1,4-bis(para-carboxyphenoxy) benzene, aromatic diol and aromatic diacid capable of undergoing melt processing | |
| JPH0725996B2 (en) | Polyester resin composition | |
| JPH06341014A (en) | Pulp-like staple fiber of liquid crystal polyester | |
| EP0018709A1 (en) | Thermotropic polyesters of 2,6-dihydroxy-anthraquinone, the production thereof, moulding powders, fibres and melts produced from these polyesters | |
| EP0166830B1 (en) | Non-woven articles comprised of thermotropic liquid crystal polymer fibers and method of production thereof | |
| JPS6128059A (en) | Nonwoven product comprising thermotropic liquid crystal polymer fiber and its production | |
| CA1226107A (en) | High performance papers comprised of fibrils of thermotropic liquid crystal polymers | |
| US4457962A (en) | Molded article comprised of a thermotropic liquid crystal line polymer with an inherently weak weld line incorporated therein | |
| US4626584A (en) | Crosslinkable thermotropic polyesters derived from allylically substituted hydroxybenzoic acid and a process for preparing a shaped article of the polyesters |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): DE FR GB |
|
| 17P | Request for examination filed |
Effective date: 19860619 |
|
| 17Q | First examination report despatched |
Effective date: 19870701 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION IS DEEMED TO BE WITHDRAWN |
|
| 18D | Application deemed to be withdrawn |
Effective date: 19871112 |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: CALUNDANN, GORDON W. Inventor name: BUCKLEY, ALAN Inventor name: KASTELIC, JOHN R. |