EP0157612B1 - Rubber toughened polyvinyl alcohol films - Google Patents
Rubber toughened polyvinyl alcohol films Download PDFInfo
- Publication number
- EP0157612B1 EP0157612B1 EP85302217A EP85302217A EP0157612B1 EP 0157612 B1 EP0157612 B1 EP 0157612B1 EP 85302217 A EP85302217 A EP 85302217A EP 85302217 A EP85302217 A EP 85302217A EP 0157612 B1 EP0157612 B1 EP 0157612B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- polyvinyl alcohol
- microdomains
- film
- polymeric matrix
- envelope
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D17/00—Detergent materials or soaps characterised by their shape or physical properties
- C11D17/04—Detergent materials or soaps characterised by their shape or physical properties combined with or containing other objects
- C11D17/041—Compositions releasably affixed on a substrate or incorporated into a dispensing means
- C11D17/042—Water soluble or water disintegrable containers or substrates containing cleaning compositions or additives for cleaning compositions
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L29/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an alcohol, ether, aldehydo, ketonic, acetal or ketal radical; Compositions of hydrolysed polymers of esters of unsaturated alcohols with saturated carboxylic acids; Compositions of derivatives of such polymers
- C08L29/02—Homopolymers or copolymers of unsaturated alcohols
- C08L29/04—Polyvinyl alcohol; Partially hydrolysed homopolymers or copolymers of esters of unsaturated alcohols with saturated carboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L21/00—Compositions of unspecified rubbers
Definitions
- the present invention relates generally to polyvinyl alcohol compositions useful for making packaging films and the like, and more particularly to polyvinyl alcohol compositions with an additional component therein having improved tensile strength and impact resistance when formed into films.
- Polyvinyl alcohol compositions are known and useful for a variety of applications.
- polyvinyl alcohol films are used in packaging materials, such as granulated laundry detergent and pulverulent pesticides and insecticides.
- Packages prepared from these films separate the contents from exposure to the immediate surroundings and provide premeasured amounts of the packaged materials.
- Polyvinyl alcohol compositions are water soluble, and rapidly dissolved in hot water.
- packages with materials designed for being slurried, dispensed or dissolved in water may be conveniently added to hot water, such as to the wash water of a washing machine when the package contains a laundry aid. As the package dissolves, the contents are dispensed.
- polyvinyl alcohol film are brittle at low temperatures and low relative humidities. They have thus not found wide commercial use as soluble films in packaging consumer products which may be stored under conditions conducive to package breakage prior to use.
- U.S. Patent 4,115,292 inventors Richardson et al., issued September 19, 1978, discloses packets of detergent composition in polyvinyl alcohol films which are useful in automatic dishwashers.
- U.S. Patent No. 3,892,905 inventor Albert, issued July 1, 1975 and U.S. Patent No. 4,155,971, inventor Wysong, issued May 22,1979, both disclose polyvinyl alcohol film-forming compositions which dissolve in cold water.
- the former compositions are made from a polymer mixture of polyvinyl alcohol or polyvinyl pyrrolidone and a water-soluble plasticizer such as glycerol or polyethylene glycol.
- the latter discloses polyvinyl alcohol compositions including polyethylene glycol plasticizers which are said to impart enhanced resistance to package breakage.
- polyvinyl alcohol compositions which may be formed into films having greatly enhanced impact resistance at low temperatures and low relative humidities.
- inventive polyvinyl alcohol compositions be suitable for forming water soluble or dispersible films for non-aqueous liquids, such as detergents having substantially no free water, as well as for packaging granulated or pulverulent materials.
- a sealed envelope comprises:
- an aqueous polymer composition castable as a thin, self-supporting film comprises:
- an article useful for treating fabrics comprises:
- Articles made from compositions in accordance with the present invention are resistant to breakage at low temperature and low relative humidity, and are particularly suitable as packaging or delivery pouches for products such as granulated or non-aqueous, liquid laundry detergents.
- Articles made from these films are preferably water-soluble, or dispersible, and when containing a laundry aid may be conveniently added to the wash water with the film dissolving and dispensing the article's contents.
- the present invention provides a method of increasing the tensile strength of polyvinyl alcohol films characterised in that it comprises:
- Polyvinyl alcohol is an excellent film forming material, and has good strength and pliability under most conditions. Polyvinyl alcohol film formation occurs readily by simply evaporating water from the solution.
- polyvinyl alcohol compositions for casting as films vary in molecular weight and degree of hydrolysis. For most film applications, molecular weights in the range of 10,000 to 100,000 are used. Hydrolysis is the percent by which acetate groups of the polyvinyl alcohol have been substituted with hydroxyl. For film applications, the range of hydrolysis typically is 70% up to 100%. Thus, the term "polyvinyl alcohol” usually includes polyvinyl acetate compounds.
- polyvinyl alcohol can be characterised by glass transition temperature (Tg).
- Tg glass transition temperature
- the glass transition temperature is the temperature at which amorphous domains of a polymer take on characteristic properties of the glassy state - brittleness, stiffness and rigidity. At temperature above Tg, localised, or segment, movement of the polymer macromolecules occurs and the polymer becomes ductile.
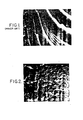
- FIG 1 illustrates the photomicrograph taken by a scanning electron microscope of a prior art polyvinyl alcohol film. As may be seen by FIG 1, the polyvinyl alcohol film has a homogeneous texture and has fractured along a plurality curves.
- plasticizers for polyvinyl alcohol.
- ethylene glycol, polyethylene glycol, glycerin and other ether polyols have been known as useful to impart various properties to the films.
- plasticizers have not satisfactorily overcome the brittleness problem at low temperatures and relative humidities. And especially after contact with liquids, plasticizer depletion from a polyvinyl alcohol film has been found to present a serious problem.
- the present invention provides polyvinyl alcohol films which have been toughened by a plurality of discrete, rubbery microdomains.
- Films in accordance with the invention are resistant to breakage at low temperature and low relative humidity, and may be formed into shapes such as envelopes or pouches (typically having wall thicknesses of from 0.05 mm to 0.5 mm) and sealed by means such as heat or pressure after moistening.
- FIG 2 illustrates a film in accordance with the present invention.
- the scale of both FIGS 1 and 2 is whereby about 1.5 centimeters represents 10 microns (1 x 10- 1 m).
- the microdomains in accordance with compositions of the invention have a glass transition temperature of less than 0°C, and more preferably below -18°C. Thus, even under very cold conditions, the microdomain material is rubbery.
- the discrete microdomains are generally of a size less than 10 microns (1 x 10- S m), more preferably between 0.5 to 2 microns (x 10- s m), and most preferably 0.5 to 1 micron (x 10-sm
- Preferred rubbery materials are acrylate and styrene-butadiene copolymer.
- the rubbery material, or rubber component will be at least 3 wt % of films in accordance with the present invention, more preferably from 5 wt % to 15 wt %, and most preferably 5 wt % to 10 wt %.
- the microdomains are dispersed in a polymeric matrix comprising at least 50 wt % polyvinyl alcohol, more preferably polyvinyl alcohol in an amount of from 70 wt % to 97 wt %.
- the polyvinyl alcohol component of the polymeric matrix is preferably in a weight ratio with respect to the plurality of microdomains, or rubber component, of between 32:1 to 2.3:1, more preferably between 19:1 to 6:1.
- Films in accordance with the present invention may be prepared from aqueous polymer compositions comprising a polyvinyl alcohol component and a rubber component.
- the polyvinyl alcohol component preferably has a molecular weight from 10,000 to 50,000, and is 70% to 100% hydrolyzed. Where the films are desirably water soluble, or dispersible, then hydrolysis is preferably 80% to 90%.
- the rubber component (which forms the discrete microdomains in the film when cast) is at least partially incompatible with the polyvinyl alcohol component.
- microdomains of the inventive films improve tensile strength and impact resistance, particularly at low temperatures and relative humidities, by permitting microscopic "crazing" of the polymer matrix adjacent the microdomains when the films are subject to deformation.
- Aqueous polymer compositions in accordance with the invention may include dispersing agents, such as sorbitol, mannitol, dextran, glycerin, and one or more wetting agents, such as non-ionic surfactants or the like.
- dispersing agents such as sorbitol, mannitol, dextran, glycerin
- wetting agents such as non-ionic surfactants or the like.
- Example I illustrates an aqueous polymer composition embodiment of the invention and the formation of a thin, self-supporting film cast therefrom.
- the polyvinyl alcohol component was provided by an 88% hydrolyzed, 10,000 molecular weight polymer from Aldrich Chemical Co.
- One part of non-ionic surfactant Triton X-114 was dissolved in 79 parts of demineralized water and the solution heated to 80°C with agitation. Twenty parts of the polyvinyl alcohol were then added slowly in several portions to avoid particle agglomeration.
- a dispersing solution was prepared by dissolving 50 parts of sorbitol in 50 parts of demineralized water.
- Example I The photomicrograph of FIG 2 was taken of a film prepared as just-described in Example I.
- This film embodiment will sometimes hereinafter be referred to as the "Example I embodiment", and is a preferred embodiment.
- Films of the invention are typically hazy (due to the phase separation between microdomains and polymeric matrix) and show two sets of glass transition temperatures.
- One glass transition is attributable to the rubber component, or rubbery phase, and the other to the polyvinyl alcohol component, or polymer phase.
- the latter typically varies depending upon relative humidity, but is at a significantly higher temperature than the former.
- Example I embodiment differential scanning calorimetry of the Example I embodiment at a heating rate of 5°C per minute (conducted over a temperature range between -40°C to 60°C) and at 40% relative humidity, showed a Tg of between -31°C to -21°C (attributable to the rubber component, or microdomains), and then another Tg of about 28°C attributable to the polyvinyl alcohol matrix.
- inventive film embodiments were prepared utilizing various rubber components to form the dispersed microdomains.
- inventive, toughened polyvinyl alcohol films were prepared with another acrylate polymer (Hycar 2600 X 171, having about 4.5% acid groups before neutralization, Tg of -43°C, available from B.F. Goodrich), with a styrene-butadiene copolymer (Goodright 2505, Tg of -48°C, available from B.F. Goodrich), with isoprene polymer (Hartex 102, Tg of -70°C, available from Firestone Rubber Co.) and with a chloroprene polymer (i.e. neoprene, Tg of -50°C, available from Firestone Rubber Co.).
- Tables I and II Resistance to breakage at different temperatures and relative humidities for films in accordance with the present invention are illustrated by Tables I and II, below.
- the data of both Tables was gathered by testing at least ten samples of the Example I embodiment by dropping a metal dart of a fixed weight from increasing heights until the film being tested ruptured. Films were conditioned at specified R.H. and temperature before being tested, and the impact tests were performed in a constant temperature room. The tested films had a film thickness of 2.1 mil (-0.0053 cm).
- polyvinyl alcohol films with plasticizer (6 wt.% trimethylpropane) but without the rubber component (that is, without microdomains) were also tested under the same sets of temperature and relative humidity conditions as in Table At -17.8°C (R.H.
- the inventive films demonstrated significantly improved impact resistance at low temperatures and low relative humidities with respect to the comparison, polyvinyl alcohol films.
- the impact resistances of both the inventive films and the comparison films were similar at temperatures at and above about 23.9°C (75°F) and R.H. of 50% or higher.
- Table II illustrates data from tests similar to those illustrated by Table I, but with the difference that the inventive films were exposed for 12 days to a liquid laundry detergent before the impact testing.
- the inventive films were more resistant to impact after having been exposed to detergent than without such exposure at low temperatures and R.H. and generally retain impact resistance after detergent exposure over a very wide range of temperature and relative humidity conditions.
- comparison polyvinyl alcohol films exposed to detergent for 12 days were ruptured at 4.4°C (and R.H. of 20%) by the 55 gram dart's falling from a height of only about 2.54 cm. It is believed that this may be due to plasticizer migration from the comparison films into the detergent.
- Example I embodiment The tensile strength and percent elongation of the Example I embodiment were also determined based on American Standards Testing Materials method at a two inch (5.08 cm) per minute cross head speed, with 35% R.H. and 21.1°C, and were found to have substantially improved tensile strength and elongation properties with respect to comparison polyvinyl alcohol films.
- Table III below, illustrates the data of two different embodiments of the invention and of a comparison film (including plasticizer but no rubber component), all of which were tested for tensile strength and elongation. As can be seen from the Table III data, above, a comparison polyvinyl alcohol film tested at the same temperature and relative humidity conditions had significantly less tensile strength and about the same percent elongation properties as the inventive film embodiments.
- Samples of the Example embodiment were also studied for time to disperse and to dissolve by testing in a standard laundry solution (including Borate) at 21.1°C.
- the films broke up and completely dissolved in less than 30 seconds.
Description
- The present invention relates generally to polyvinyl alcohol compositions useful for making packaging films and the like, and more particularly to polyvinyl alcohol compositions with an additional component therein having improved tensile strength and impact resistance when formed into films.
- Polyvinyl alcohol compositions are known and useful for a variety of applications. For example, polyvinyl alcohol films are used in packaging materials, such as granulated laundry detergent and pulverulent pesticides and insecticides. Packages prepared from these films separate the contents from exposure to the immediate surroundings and provide premeasured amounts of the packaged materials.
- Polyvinyl alcohol compositions are water soluble, and rapidly dissolved in hot water. Thus, packages with materials designed for being slurried, dispensed or dissolved in water may be conveniently added to hot water, such as to the wash water of a washing machine when the package contains a laundry aid. As the package dissolves, the contents are dispensed.
- However, polyvinyl alcohol film are brittle at low temperatures and low relative humidities. They have thus not found wide commercial use as soluble films in packaging consumer products which may be stored under conditions conducive to package breakage prior to use.
- U.S. Patent 4,115,292, inventors Richardson et al., issued September 19, 1978, discloses packets of detergent composition in polyvinyl alcohol films which are useful in automatic dishwashers. U.S. Patent No. 3,892,905, inventor Albert, issued July 1, 1975 and U.S. Patent No. 4,155,971, inventor Wysong, issued May 22,1979, both disclose polyvinyl alcohol film-forming compositions which dissolve in cold water. The former compositions are made from a polymer mixture of polyvinyl alcohol or polyvinyl pyrrolidone and a water-soluble plasticizer such as glycerol or polyethylene glycol. The latter discloses polyvinyl alcohol compositions including polyethylene glycol plasticizers which are said to impart enhanced resistance to package breakage.
- U.K. Patent application 2,090,603A, published July 14, 1982, inventor Sonenstein, discloses water soluble films comprising a uniform or homogeneous blend of water soluble polyvinyl alcohol and polyacrylic acid. The compositions are said to have high rates of solubility in both cold and hot water and to be of reduced sensitivity to humidity.
- Nevertheless, the known water-soluble polyvinyl alcohol films useful as packaging or delivery pouches have continued to present problems or brittleness at low temperatures and low relative humidities.
- Accordingly, it is an object of the present invention to provide polyvinyl alcohol compositions which may be formed into films having greatly enhanced impact resistance at low temperatures and low relative humidities.
- It is another object that the inventive polyvinyl alcohol compositions be suitable for forming water soluble or dispersible films for non-aqueous liquids, such as detergents having substantially no free water, as well as for packaging granulated or pulverulent materials.
- The present invention relates to a self-supporting film characterised in that it comprises:
- a polymeric matrix, the polymeric matrix including polyvinyl alcohol and having a molecular weight from 10,000 to 100,000; and
- a plurality of microdomains dispersed throughout the polymeric matrix and being at least partially incompatible with the polyvinyl alcohol, the microdomains being from 0.5 to 10 microns (x 10-sm) in size, the microdomains consisting essentially of a rubbery material having a glass transition temperature of less than 0°C and being at least 3 wt % of the film, the polyvinyl alcohol of the polymeric matrix being in a weight ratio with respect to the plurality of microdomains of between 19:1 to 6:1.
- In one aspect of this invention, a sealed envelope comprises:
- a self-supporting film, the film formed by a polymeric matrix comprising at least 50 wt % polyvinyl alcohol having a plurality of microdomains dispersed there throughout, the polyvinyl alcohol having a molecular weight of from 10,000 to 100,000, the plurality of microdomains consisting essentially of a rubbery material which is at least partially incompatible with the polyvinyl alcohol and has a glass transition temperature of less than 0°C, the microdomains being at least 3 wt. % of the film and the polyvinyl alcohol of the polymeric matrix being in a weight ratio with respect to the plurality of microdomains of between 19:1 and 6:1. Such an envelope may contain a liquid detergent which has substantially no free water.
- In another aspect of the present invention, an aqueous polymer composition castable as a thin, self-supporting film comprises:
- a polyvinyl alcohol component and a rubber component, the polyvinyl alcohol component having a molecular weight from 10,000 to 100,000 and being 70% to 100% hydrolyzed, the rubber component having a glass transition temperature of less than 0°C and being at least partially incompatible when cast in film form with the polyvinyl alcohol component; and
- an aqueous solution in which the polyvinyl alcohol component and the rubber component are dispersed.
- In a further aspect, an article useful for treating fabrics comprises:
- a flexible pouch, the pouch being resistant to breakage at low temperature and low relative humidity, the pouch formed by a plastic phase and a rubber phase, the plastic phase including polyvinyl alcohol and being in an amount of from 70 wt % to 97 wt % of the pouch, the rubber phase being at least partially incompatible with the polyvinyl alcohol and having a glass transition temperature at or below -18°C and being in an amount of from at least 3 wt % to 30 wt % of the pouch, and the rubber phase being in a weight ratio with respect to the plastic phase of from 19:1 to 6:1; and
- a laundering aid disposed within the pouch.
- Articles made from compositions in accordance with the present invention are resistant to breakage at low temperature and low relative humidity, and are particularly suitable as packaging or delivery pouches for products such as granulated or non-aqueous, liquid laundry detergents. Articles made from these films are preferably water-soluble, or dispersible, and when containing a laundry aid may be conveniently added to the wash water with the film dissolving and dispensing the article's contents.
- Another aspect of the present invention relates to a self-supporting film characterised in that it comprises:
- a polymeric matrix, the polymeric matrix including polyvinyl alcohol of a molecular weight from 10,000 to 100,000; and
- a plurality of microdomains dispersed throughout the polymeric matrix, the microdomains being from 0.5 to 10 microns (x 10-sm) in size, the microdomains having a glass transition temperature of less than 0°C, the microdomains being at least 3 wt % of the film, the polyvinyl alcohol of the polymeric matrix being in a weight ratio with respect to the plurality of microdomains of between 19:1 to 6: 1.
- Also, the present invention provides a method of increasing the tensile strength of polyvinyl alcohol films characterised in that it comprises:
- providing a polyvinyl alcohol solution, the polyvinyl alcohol having a molecular weight of from 10,000 to 100,000;
- admixing a rubber solution with the polyvinyl alcohol solution, the rubber solution being at least partially incompatible with the polyvinyl alcohol solution; and
- casting the admixture such that a self-supporting film forms in which the polyvinyl alcohol has formed a polymeric matrix and the rubber solution has formed a plurality of microdomains throughout the polymeric matrix, the polyvinyl alcohol of the polymeric matrix being in a weight ratio with respect to the plurality of microdomains of between 19:1 to 6:1.
- The invention will be further explained with reference to the following detailed description, which includes mention of photomicrographs in which:
- FIG 1 illustrates a prior art film at a magnification of 1600 times; and
- FIG 2 illustrates a film in accordance with the present invention at a magnification of 1600 times.
- Polyvinyl alcohol is an excellent film forming material, and has good strength and pliability under most conditions. Polyvinyl alcohol film formation occurs readily by simply evaporating water from the solution.
- Commercially available polyvinyl alcohol compositions for casting as films vary in molecular weight and degree of hydrolysis. For most film applications, molecular weights in the range of 10,000 to 100,000 are used. Hydrolysis is the percent by which acetate groups of the polyvinyl alcohol have been substituted with hydroxyl. For film applications, the range of hydrolysis typically is 70% up to 100%. Thus, the term "polyvinyl alcohol" usually includes polyvinyl acetate compounds.
- Like other polymeric materials, polyvinyl alcohol can be characterised by glass transition temperature (Tg). The glass transition temperature is the temperature at which amorphous domains of a polymer take on characteristic properties of the glassy state - brittleness, stiffness and rigidity. At temperature above Tg, localised, or segment, movement of the polymer macromolecules occurs and the polymer becomes ductile.
- Temperature alone does not determine the point at which polyvinyl alcohol becomes brittle, since polyvinyl alcohol readily absorbs water from the atmosphere. The absorbed water acts as a plasticizer, and assists in preventing brittleness. However, at low temperatures and low relative humidity polyvinyl alochol becomes brittle and has very little impact resistance. Even at 20°C, polyvinyl alcohol becomes brittle when the relative humidity is less than 15%, and at lower temperatures the situation is exacerbated. FIG 1 illustrates the photomicrograph taken by a scanning electron microscope of a prior art polyvinyl alcohol film. As may be seen by FIG 1, the polyvinyl alcohol film has a homogeneous texture and has fractured along a plurality curves.
- A wide variety of materials are known and used as plasticizers for polyvinyl alcohol. For example, ethylene glycol, polyethylene glycol, glycerin and other ether polyols have been known as useful to impart various properties to the films. But plasticizers have not satisfactorily overcome the brittleness problem at low temperatures and relative humidities. And especially after contact with liquids, plasticizer depletion from a polyvinyl alcohol film has been found to present a serious problem.
- Broadly, the present invention provides polyvinyl alcohol films which have been toughened by a plurality of discrete, rubbery microdomains. Films in accordance with the invention are resistant to breakage at low temperature and low relative humidity, and may be formed into shapes such as envelopes or pouches (typically having wall thicknesses of from 0.05 mm to 0.5 mm) and sealed by means such as heat or pressure after moistening.
- FIG 2 illustrates a film in accordance with the present invention. As may be seen, there are a plurality of microdomains, or phase separated, heterogeneous areas, dispersed throughout the polyvinyl matrix, by contrast to the homogeneous, or single phase, of the prior polyvinyl alcohol film illustrated by FIG 1. The scale of both FIGS 1 and 2 is whereby about 1.5 centimeters represents 10 microns (1 x 10-1m).
- The microdomains in accordance with compositions of the invention have a glass transition temperature of less than 0°C, and more preferably below -18°C. Thus, even under very cold conditions, the microdomain material is rubbery. The discrete microdomains are generally of a size less than 10 microns (1 x 10-Sm), more preferably between 0.5 to 2 microns (x 10-sm), and most preferably 0.5 to 1 micron (x 10-sm
- Suitable rubbery materials for microdomains in accordance with the present invention include polydimethylsiloxane (Tg=-123°C), polyethylene (Tg=-115°C), polyoxymethylene (Tg=-85°C), natural rubber (Tg=-73°C), polyisobutylene (Tg=-73°C), poly(ethylene oxide) (Tg=-67°C), neoprene (Tg=-50°C), styrene-butadiene copolymer (Tg=-48°C), polypropylene (Tg=-20°C), poly(vinyl fluoride) (Tg=-20°C), poly(vinylidene chloride) (Tg=-19°C) and acrylate (Tg= from -18°C to -43°C, depending upon percent acid groups). Preferred rubbery materials are acrylate and styrene-butadiene copolymer.
- The rubbery material, or rubber component, will be at least 3 wt % of films in accordance with the present invention, more preferably from 5 wt % to 15 wt %, and most preferably 5 wt % to 10 wt %.
- The microdomains are dispersed in a polymeric matrix comprising at least 50 wt % polyvinyl alcohol, more preferably polyvinyl alcohol in an amount of from 70 wt % to 97 wt %. The polyvinyl alcohol component of the polymeric matrix is preferably in a weight ratio with respect to the plurality of microdomains, or rubber component, of between 32:1 to 2.3:1, more preferably between 19:1 to 6:1.
- Films in accordance with the present invention may be prepared from aqueous polymer compositions comprising a polyvinyl alcohol component and a rubber component. The polyvinyl alcohol component preferably has a molecular weight from 10,000 to 50,000, and is 70% to 100% hydrolyzed. Where the films are desirably water soluble, or dispersible, then hydrolysis is preferably 80% to 90%. The rubber component (which forms the discrete microdomains in the film when cast) is at least partially incompatible with the polyvinyl alcohol component.
- It is believed that microdomains of the inventive films improve tensile strength and impact resistance, particularly at low temperatures and relative humidities, by permitting microscopic "crazing" of the polymer matrix adjacent the microdomains when the films are subject to deformation.
- Aqueous polymer compositions in accordance with the invention may include dispersing agents, such as sorbitol, mannitol, dextran, glycerin, and one or more wetting agents, such as non-ionic surfactants or the like.
- Example I, below, illustrates an aqueous polymer composition embodiment of the invention and the formation of a thin, self-supporting film cast therefrom.
- The rubber component was provided by Carboset 515 (acrylate polymer having about 8% acid groups before neutralization and a Tg of -18°C, available from B.F. Goodrich). 40 parts of the Carboset 515 were added with vigorous agitation to a solution containing 2.0 parts of concentrated ammonia (for neutralization of acid groups), 0.5 parts of non-ionic surfactant (Triton X-114 available from Rohm and Haas Co.) and 57.5 parts of demineralized water. The Carboset 515 dissolved in about one hour with pH at 7.7. The resultant rubber component solution was a clear, viscous syrup (" = 600 cps/mPa.s at 20 rpm, No. 3 spindle of a Brookfield viscometer).
- The polyvinyl alcohol component was provided by an 88% hydrolyzed, 10,000 molecular weight polymer from Aldrich Chemical Co. One part of non-ionic surfactant (Triton X-114) was dissolved in 79 parts of demineralized water and the solution heated to 80°C with agitation. Twenty parts of the polyvinyl alcohol were then added slowly in several portions to avoid particle agglomeration. The resultant polyvinyl alcohol solution was a clear, viscous syrup. (" = 600 cps/mPa.s at 20 rpm, No. 3 spindle of a Brookfield viscometer).
- A dispersing solution was prepared by dissolving 50 parts of sorbitol in 50 parts of demineralized water.
- Two parts of the dispersing solution, 2.5 parts of the rubber component solution and 40 parts of the polyvinyl alcohol component solution were admixed with gentle stirring to avoid generating bubbles. The admixture (a cloudy emulsion) was then cast as a 2.4 mil (-0.0061 cm) thick film on a glass plate and the film dried at about 50°C or above.
- The photomicrograph of FIG 2 was taken of a film prepared as just-described in Example I. This film embodiment will sometimes hereinafter be referred to as the "Example I embodiment", and is a preferred embodiment.
- Films of the invention are typically hazy (due to the phase separation between microdomains and polymeric matrix) and show two sets of glass transition temperatures. One glass transition is attributable to the rubber component, or rubbery phase, and the other to the polyvinyl alcohol component, or polymer phase. The latter typically varies depending upon relative humidity, but is at a significantly higher temperature than the former. For example, differential scanning calorimetry of the Example I embodiment at a heating rate of 5°C per minute (conducted over a temperature range between -40°C to 60°C) and at 40% relative humidity, showed a Tg of between -31°C to -21°C (attributable to the rubber component, or microdomains), and then another Tg of about 28°C attributable to the polyvinyl alcohol matrix.
- In an analogous manner, other inventive film embodiments were prepared utilizing various rubber components to form the dispersed microdomains. For example, inventive, toughened polyvinyl alcohol films were prepared with another acrylate polymer (Hycar 2600 X 171, having about 4.5% acid groups before neutralization, Tg of -43°C, available from B.F. Goodrich), with a styrene-butadiene copolymer (Goodright 2505, Tg of -48°C, available from B.F. Goodrich), with isoprene polymer (Hartex 102, Tg of -70°C, available from Firestone Rubber Co.) and with a chloroprene polymer (i.e. neoprene, Tg of -50°C, available from Firestone Rubber Co.).
- Resistance to breakage at different temperatures and relative humidities for films in accordance with the present invention are illustrated by Tables I and II, below. The data of both Tables was gathered by testing at least ten samples of the Example I embodiment by dropping a metal dart of a fixed weight from increasing heights until the film being tested ruptured. Films were conditioned at specified R.H. and temperature before being tested, and the impact tests were performed in a constant temperature room. The tested films had a film thickness of 2.1 mil (-0.0053 cm).
- Table II, below, illustrates data from tests similar to those illustrated by Table I, but with the difference that the inventive films were exposed for 12 days to a liquid laundry detergent before the impact testing.
- The tensile strength and percent elongation of the Example I embodiment were also determined based on American Standards Testing Materials method at a two inch (5.08 cm) per minute cross head speed, with 35% R.H. and 21.1°C, and were found to have substantially improved tensile strength and elongation properties with respect to comparison polyvinyl alcohol films. Table III, below, illustrates the data of two different embodiments of the invention and of a comparison film (including plasticizer but no rubber component), all of which were tested for tensile strength and elongation.
- Samples of the Example embodiment were also studied for time to disperse and to dissolve by testing in a standard laundry solution (including Borate) at 21.1°C. The films broke up and completely dissolved in less than 30 seconds.
Claims (17)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US06/595,980 US4608187A (en) | 1984-04-02 | 1984-04-02 | Rubber toughened polyvinyl alcohol film compositions |
| US595980 | 1984-04-02 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0157612A2 EP0157612A2 (en) | 1985-10-09 |
| EP0157612A3 EP0157612A3 (en) | 1986-10-01 |
| EP0157612B1 true EP0157612B1 (en) | 1989-05-31 |
Family
ID=24385509
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP85302217A Expired EP0157612B1 (en) | 1984-04-02 | 1985-03-29 | Rubber toughened polyvinyl alcohol films |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US4608187A (en) |
| EP (1) | EP0157612B1 (en) |
| JP (1) | JPH0676532B2 (en) |
| AR (1) | AR242364A1 (en) |
| AU (1) | AU570102B2 (en) |
| BR (1) | BR8501365A (en) |
| CA (1) | CA1249688A (en) |
| DE (1) | DE3570685D1 (en) |
| ES (4) | ES8700299A1 (en) |
| TR (1) | TR22291A (en) |
Families Citing this family (39)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4797221A (en) * | 1985-09-12 | 1989-01-10 | S. C. Johnson & Son, Inc. | Polymer sheet for delivering laundry care additive and laundry care product formed from same |
| EP0221651A1 (en) * | 1985-09-27 | 1987-05-13 | Kao Corporation | Water soluble polyvinyl alcohol derivative |
| GB8612706D0 (en) * | 1986-05-23 | 1986-07-02 | Unilever Plc | Sealable container |
| US5078301A (en) * | 1987-10-02 | 1992-01-07 | Ecolab Inc. | Article comprising a water soluble bag containing a multiple use amount of a pelletized functional material and methods of its use |
| US5234615A (en) * | 1987-10-02 | 1993-08-10 | Ecolab Inc. | Article comprising a water soluble bag containing a multiple use amount of a pelletized functional material and methods of its use |
| US4849256A (en) * | 1988-03-11 | 1989-07-18 | The Clorox Company | Process for plasticizing polyvinyl alcohol resin |
| US4806261A (en) * | 1988-04-11 | 1989-02-21 | Colgate-Palmolive Co. | Detersive article |
| US5196132A (en) * | 1989-03-03 | 1993-03-23 | Fabritec International Corporation | Unit-dose drycleaning product |
| US5055215A (en) * | 1989-03-03 | 1991-10-08 | Fabritec International Corporation | Unit-dose drycleaning product and method |
| EP0389695A1 (en) * | 1989-03-28 | 1990-10-03 | Neste Oy | Gastight material |
| JP2860129B2 (en) * | 1990-02-01 | 1999-02-24 | 日本合成化学工業株式会社 | Manufacturing method of molded products with excellent impact resistance |
| US5126400A (en) * | 1990-07-30 | 1992-06-30 | Dow Corning Corporation | Reinforced polyorganosiloxane elastomers |
| US5126403A (en) * | 1990-07-30 | 1992-06-30 | Dow Corning Corporation | Reinforced polyorganosiloxane elastomers |
| AU661491B2 (en) | 1991-05-14 | 1995-07-27 | Ecolab Inc. | Two part chemical concentrate |
| JPH05329276A (en) * | 1992-05-20 | 1993-12-14 | Mitsui & Co Ltd | Water-soluble chromatic balloon |
| US5349000A (en) * | 1993-02-25 | 1994-09-20 | Air Products And Chemicals, Inc. | Extrudable polyvinyl alcohol compositions containing polyester-polyether block copolymers |
| US5649326A (en) * | 1994-11-18 | 1997-07-22 | Johnson & Johnson Professional, Inc. | Flexible hydrophilic coating for orthopaedic casting gloves and method for making such gloves |
| US6673765B1 (en) | 1995-05-15 | 2004-01-06 | Ecolab Inc. | Method of making non-caustic solid cleaning compositions |
| DE69700854T2 (en) * | 1996-04-10 | 2000-05-18 | Kuraray Co | Molded objects made of polyvinyl alcohol |
| US6150324A (en) | 1997-01-13 | 2000-11-21 | Ecolab, Inc. | Alkaline detergent containing mixed organic and inorganic sequestrants resulting in improved soil removal |
| US6258765B1 (en) * | 1997-01-13 | 2001-07-10 | Ecolab Inc. | Binding agent for solid block functional material |
| GB9906176D0 (en) * | 1999-03-17 | 1999-05-12 | Unilever Plc | Process for producing a water soluble package |
| GB9906169D0 (en) | 1999-03-17 | 1999-05-12 | Unilever Plc | A process for producing a water soluble package |
| GB9906171D0 (en) | 1999-03-17 | 1999-05-12 | Unilever Plc | A process for producing a water soluble package |
| US6387870B1 (en) | 1999-03-29 | 2002-05-14 | Ecolab Inc. | Solid pot and pan detergent |
| JP2003504491A (en) * | 1999-07-09 | 2003-02-04 | ヘンケル・コマンディットゲゼルシャフト・アウフ・アクチエン | Packaging of laundry detergent / dishwasher detergent |
| WO2001079417A1 (en) * | 2000-04-14 | 2001-10-25 | Unilever N.V. | Water soluble package and liquid contents thereof |
| GB2368584A (en) * | 2000-10-18 | 2002-05-08 | Reckitt Benckiser Nv | An ethoxylated PVA for packaging a composition |
| US6638902B2 (en) * | 2001-02-01 | 2003-10-28 | Ecolab Inc. | Stable solid enzyme compositions and methods employing them |
| US6492312B1 (en) | 2001-03-16 | 2002-12-10 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Water soluble sachet with a dishwashing enhancing particle |
| US6632291B2 (en) | 2001-03-23 | 2003-10-14 | Ecolab Inc. | Methods and compositions for cleaning, rinsing, and antimicrobial treatment of medical equipment |
| GB2414958A (en) * | 2004-06-11 | 2005-12-14 | Reckitt Benckiser Nv | A process for preparing a water soluble article. |
| US7964544B2 (en) * | 2005-10-31 | 2011-06-21 | Ecolab Usa Inc. | Cleaning composition and method for preparing a cleaning composition |
| US20070179073A1 (en) * | 2005-11-09 | 2007-08-02 | Smith Kim R | Detergent composition for removing polymerized food soils and method for cleaning polymerized food soils |
| US20070253926A1 (en) * | 2006-04-28 | 2007-11-01 | Tadrowski Tami J | Packaged cleaning composition concentrate and method and system for forming a cleaning composition |
| MX2010010247A (en) | 2008-04-07 | 2010-10-20 | Ecolab Inc | Ultra-concentrated solid degreaser composition. |
| US9701931B2 (en) | 2013-09-30 | 2017-07-11 | Chemlink Laboratories, Llc | Environmentally preferred antimicrobial compositions |
| DE102016201498B4 (en) | 2016-02-01 | 2017-08-17 | Norbert Kuhl | OXYGEN-CONTAINED FOOD CONTAINER |
| CN112334535B (en) * | 2018-06-20 | 2023-03-28 | 株式会社可乐丽 | Polyvinyl alcohol film, stretched film, and method for producing polyvinyl alcohol film |
Family Cites Families (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2804395A (en) * | 1953-09-04 | 1957-08-27 | Setrak K Boyajian | Envelopes and the like with remoistenable adhesive comprising polyvinyl alcohol |
| NL266245A (en) * | 1960-06-22 | |||
| GB927963A (en) * | 1961-01-06 | 1963-06-06 | Kurashiki Rayon Kk | Method of preventing water-soluble films of polyvinyl alcohol derivatives from adhering and non-sticky films made thereby |
| GB1003178A (en) * | 1961-05-27 | 1965-09-02 | Kurashiki Rayon Kk | Method of manufacturing water soluble film |
| US3113674A (en) * | 1961-08-28 | 1963-12-10 | Eastman Kodak Co | Composition comprising sodium cellulose acetate sulfate and a polymer and unit package preparted therefrom |
| US3220991A (en) * | 1962-03-28 | 1965-11-30 | Monsanto Co | Heat stabilization of polyvinyl alcohol with aliphatic polycarboxylic acid |
| US3374195A (en) * | 1964-03-03 | 1968-03-19 | Mono Sol Division | Polyvinyl alcohol compositions containing a plasticizer mixture |
| US3413229A (en) * | 1964-03-03 | 1968-11-26 | Mono Sol Division Baldwin Mont | Polyvinyl alcohol compositions |
| US3409598A (en) * | 1964-06-18 | 1968-11-05 | Denki Kagaku Kogyo Kk | Process for the manufacture of water soluble polyvinyl alcohol film |
| US3366592A (en) * | 1964-06-24 | 1968-01-30 | Du Pont | Polyvinyl alcohol plasticized with 2, 2-diethyl-1, 3-propanediol |
| US3300546A (en) * | 1965-10-05 | 1967-01-24 | American Cyanamid Co | Water soluble envelope prepared from a graft polymer of alkyl acrylate on a polyvinyl alcohol/polyvinyl acetate co-polymer |
| CA813298A (en) * | 1966-07-08 | 1969-05-20 | W. Gray Frederick | Bleaching packets |
| US3505303A (en) * | 1966-12-30 | 1970-04-07 | Air Reduction | Water soluble modified polyvinyl alcohol films |
| US3563244A (en) * | 1968-03-15 | 1971-02-16 | Hajime Moribe | Condoms |
| US3632786A (en) * | 1968-11-12 | 1972-01-04 | Monsanto Co | Polyvinyl alcohol adhesive composition with high wet tack containing a boron compound and a cis 1 2 polyol compound |
| US3607812A (en) * | 1968-12-17 | 1971-09-21 | Denki Kagaku Kogyo Kk | Method of manufacturing polyvinyl alcohol films and product |
| US3892905A (en) * | 1970-08-12 | 1975-07-01 | Du Pont | Cold water soluble plastic films |
| JPS5117936A (en) * | 1974-08-05 | 1976-02-13 | Kuraray Co | Shisu 1*44 horiisopurengomusuiseibunsantaino seiho |
| JPS5117933A (en) * | 1974-08-05 | 1976-02-13 | Kuraray Co | Gomujobutsushitsuno suiseibunsantaino seizoho |
| JPS6011736B2 (en) * | 1974-08-05 | 1985-03-27 | 株式会社クラレ | Manufacturing method of aqueous dispersion |
| JPS5810438B2 (en) * | 1975-01-31 | 1983-02-25 | ユニチカ株式会社 | Polyvinyl alcohol silicone hot melt |
| US4155971A (en) * | 1976-08-18 | 1979-05-22 | E. I. Du Pont De Nemours And Company | Method of making water-soluble films from polyvinyl alcohol compositions |
| US4082678A (en) * | 1976-11-10 | 1978-04-04 | The Procter & Gamble Company | Fabric conditioning articles and process |
| US4115292A (en) * | 1977-04-20 | 1978-09-19 | The Procter & Gamble Company | Enzyme-containing detergent articles |
| GB1583082A (en) * | 1977-05-18 | 1981-01-21 | Unilever Ltd | Detergent products |
| US4289815A (en) * | 1978-06-26 | 1981-09-15 | Airwick Industries, Inc. | Cold water-insoluble polyvinyl alcohol pouch for the controlled release of active ingredients |
| MX151028A (en) * | 1978-11-17 | 1984-09-11 | Unilever Nv | IMPROVEMENTS IN INSOLUBLE BAG BUT PERMEABLE TO WATER THAT HAS A DISPERSIBLE PROTECTIVE LAYER OR SOLUBLE IN WATER, WHICH CONTAINS A PARTICULATE DETERGENT COMPOSITION |
| JPS5831106B2 (en) * | 1979-04-24 | 1983-07-04 | 出光興産株式会社 | thermoplastic resin composition |
| NZ199130A (en) * | 1980-12-15 | 1985-07-31 | Colgate Palmolive Co | Water-soluble film;mixture of polyvinyl alcohol and polyacrylic acid |
| US4374035A (en) * | 1981-07-13 | 1983-02-15 | The Procter & Gamble Company | Accelerated release laundry bleach product |
| US4416791A (en) * | 1981-11-11 | 1983-11-22 | Lever Brothers Company | Packaging film and packaging of detergent compositions therewith |
| US4410441A (en) * | 1982-04-26 | 1983-10-18 | Lever Brothers Company | Product for treating fabrics in a washing machine |
-
1984
- 1984-04-02 US US06/595,980 patent/US4608187A/en not_active Expired - Lifetime
-
1985
- 1985-03-22 TR TR13977A patent/TR22291A/en unknown
- 1985-03-26 CA CA000477486A patent/CA1249688A/en not_active Expired
- 1985-03-26 BR BR8501365A patent/BR8501365A/en not_active IP Right Cessation
- 1985-03-27 AR AR85299900A patent/AR242364A1/en active
- 1985-03-27 JP JP60063173A patent/JPH0676532B2/en not_active Expired - Lifetime
- 1985-03-29 EP EP85302217A patent/EP0157612B1/en not_active Expired
- 1985-03-29 DE DE8585302217T patent/DE3570685D1/en not_active Expired
- 1985-04-01 AU AU40556/85A patent/AU570102B2/en not_active Ceased
- 1985-04-02 ES ES542542A patent/ES8700299A1/en not_active Expired
- 1985-04-02 ES ES551883A patent/ES8704192A1/en not_active Expired
- 1985-04-02 ES ES551884A patent/ES8800967A1/en not_active Expired
- 1985-04-02 ES ES542541A patent/ES8707266A1/en not_active Expired
Also Published As
| Publication number | Publication date |
|---|---|
| AU4055685A (en) | 1985-10-10 |
| JPH0676532B2 (en) | 1994-09-28 |
| ES8800967A1 (en) | 1987-12-01 |
| ES8700299A1 (en) | 1986-10-01 |
| ES8707266A1 (en) | 1987-07-16 |
| CA1249688A (en) | 1989-01-31 |
| AR242364A1 (en) | 1993-03-31 |
| ES551883A0 (en) | 1987-04-01 |
| AU570102B2 (en) | 1988-03-03 |
| BR8501365A (en) | 1985-11-19 |
| TR22291A (en) | 1987-01-02 |
| ES542541A0 (en) | 1987-07-16 |
| EP0157612A3 (en) | 1986-10-01 |
| US4608187A (en) | 1986-08-26 |
| JPS60248763A (en) | 1985-12-09 |
| ES542542A0 (en) | 1986-10-01 |
| US4608187B1 (en) | 1988-02-09 |
| ES551884A0 (en) | 1987-12-01 |
| EP0157612A2 (en) | 1985-10-09 |
| ES8704192A1 (en) | 1987-04-01 |
| DE3570685D1 (en) | 1989-07-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0157612B1 (en) | Rubber toughened polyvinyl alcohol films | |
| JP4017982B2 (en) | Rapidly dissolving polymer films and articles made therefrom | |
| EP0079712B2 (en) | Borate solution soluble polyvinyl alcohol films | |
| EP1319706B1 (en) | Unit dose products | |
| US3413229A (en) | Polyvinyl alcohol compositions | |
| CA2115244A1 (en) | Cold water soluble films and film forming compositions | |
| US3374195A (en) | Polyvinyl alcohol compositions containing a plasticizer mixture | |
| JPH0214376B2 (en) | ||
| EP0284334A2 (en) | Rinse soluble polymer film composition for wash additives | |
| US5322880A (en) | Thixotropic adhesive gel | |
| US20020198125A1 (en) | Water soluble package and liquid contents thereof | |
| JPS63260435A (en) | Polymer for rinse-release of washing additive | |
| CN101484569A (en) | Laundry articles | |
| EP1397478B1 (en) | Water soluble package and liquid contents thereof | |
| BRPI0509682B1 (en) | Dispensing system, and methods for releasing a liquid active laundry washer on a target and for releasing a liquid washing agent on a textile material | |
| CN111433135A (en) | Film for packaging drug and package | |
| JPH0665463A (en) | Polyvinyl alcohol composition and film obtained by using the same | |
| DE60201398T2 (en) | Water-soluble packaging and associated liquid contents | |
| JP7162010B2 (en) | Aqueous Membrane Sealing Compositions and Methods of Using the Compositions or Membranes | |
| JP7050804B2 (en) | Free-standing film | |
| JP3010506B2 (en) | Antifogging composition for polyethylene resin film | |
| JP3069586B2 (en) | Detergent packaging film | |
| EP3630643B1 (en) | Free standing dispersant film | |
| JPH0517653A (en) | Polyvinyl alcohol film |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): BE CH DE FR GB IT LI LU NL SE |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): BE CH DE FR GB IT LI LU NL SE |
|
| 17P | Request for examination filed |
Effective date: 19861204 |
|
| 17Q | First examination report despatched |
Effective date: 19880208 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): BE CH DE FR GB IT LI LU NL SE |
|
| ITF | It: translation for a ep patent filed |
Owner name: ING. C. GREGORJ S.P.A. |
|
| REF | Corresponds to: |
Ref document number: 3570685 Country of ref document: DE Date of ref document: 19890706 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19900331 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: LU Payment date: 19900420 Year of fee payment: 6 |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19930215 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19930217 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19930225 Year of fee payment: 9 |
|
| ITTA | It: last paid annual fee | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19930331 Year of fee payment: 9 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19940330 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Effective date: 19940331 Ref country code: CH Effective date: 19940331 Ref country code: BE Effective date: 19940331 |
|
| BERE | Be: lapsed |
Owner name: THE CLOROX CY Effective date: 19940331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Effective date: 19941001 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee | ||
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| EUG | Se: european patent has lapsed |
Ref document number: 85302217.6 Effective date: 19941010 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20040318 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20040324 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20040430 Year of fee payment: 20 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20050328 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 |