EP0150235B1 - Derivatives of 3-pyrrolidinopropiophenone and a process for preparation thereof - Google Patents
Derivatives of 3-pyrrolidinopropiophenone and a process for preparation thereof Download PDFInfo
- Publication number
- EP0150235B1 EP0150235B1 EP84100827A EP84100827A EP0150235B1 EP 0150235 B1 EP0150235 B1 EP 0150235B1 EP 84100827 A EP84100827 A EP 84100827A EP 84100827 A EP84100827 A EP 84100827A EP 0150235 B1 EP0150235 B1 EP 0150235B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- pyrrolidinopropiophenone
- drug
- formula
- represented
- addition salts
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- 238000000034 method Methods 0.000 title claims description 9
- WRSWNYCNECGVNL-UHFFFAOYSA-N 1-phenyl-3-pyrrolidin-1-ylpropan-1-one Chemical class C=1C=CC=CC=1C(=O)CCN1CCCC1 WRSWNYCNECGVNL-UHFFFAOYSA-N 0.000 title claims description 8
- 238000002360 preparation method Methods 0.000 title claims description 5
- RWRDLPDLKQPQOW-UHFFFAOYSA-N Pyrrolidine Chemical compound C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 claims description 24
- 239000002253 acid Substances 0.000 claims description 15
- 239000003814 drug Substances 0.000 claims description 13
- 150000003839 salts Chemical class 0.000 claims description 13
- 229940079593 drug Drugs 0.000 claims description 10
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 10
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 claims description 9
- 239000003937 drug carrier Substances 0.000 claims description 8
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 claims description 8
- -1 ethyl- Chemical group 0.000 claims description 6
- 239000004480 active ingredient Substances 0.000 claims description 5
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 claims description 4
- 229920002472 Starch Polymers 0.000 claims description 4
- 239000000969 carrier Substances 0.000 claims description 4
- 239000008101 lactose Substances 0.000 claims description 4
- 235000019359 magnesium stearate Nutrition 0.000 claims description 4
- KRIOVPPHQSLHCZ-UHFFFAOYSA-N propiophenone Chemical class CCC(=O)C1=CC=CC=C1 KRIOVPPHQSLHCZ-UHFFFAOYSA-N 0.000 claims description 4
- 239000008107 starch Substances 0.000 claims description 4
- 235000019698 starch Nutrition 0.000 claims description 4
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 3
- 239000006187 pill Substances 0.000 claims description 3
- 239000003826 tablet Substances 0.000 claims description 3
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 claims description 2
- 108010010803 Gelatin Proteins 0.000 claims description 2
- 240000007472 Leucaena leucocephala Species 0.000 claims description 2
- 235000010643 Leucaena leucocephala Nutrition 0.000 claims description 2
- 235000019483 Peanut oil Nutrition 0.000 claims description 2
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 claims description 2
- 239000008116 calcium stearate Substances 0.000 claims description 2
- 235000013539 calcium stearate Nutrition 0.000 claims description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 claims description 2
- 125000004432 carbon atom Chemical group C* 0.000 claims description 2
- 239000000839 emulsion Substances 0.000 claims description 2
- 239000008273 gelatin Substances 0.000 claims description 2
- 229920000159 gelatin Polymers 0.000 claims description 2
- 235000019322 gelatine Nutrition 0.000 claims description 2
- 235000011852 gelatine desserts Nutrition 0.000 claims description 2
- 239000008187 granular material Substances 0.000 claims description 2
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 claims description 2
- 239000007788 liquid Substances 0.000 claims description 2
- 239000006194 liquid suspension Substances 0.000 claims description 2
- 239000007937 lozenge Substances 0.000 claims description 2
- 239000004006 olive oil Substances 0.000 claims description 2
- 235000008390 olive oil Nutrition 0.000 claims description 2
- 239000000312 peanut oil Substances 0.000 claims description 2
- 239000008159 sesame oil Substances 0.000 claims description 2
- 235000011803 sesame oil Nutrition 0.000 claims description 2
- 239000007787 solid Substances 0.000 claims description 2
- 239000008174 sterile solution Substances 0.000 claims description 2
- 239000000829 suppository Substances 0.000 claims description 2
- 239000006188 syrup Substances 0.000 claims description 2
- 235000020357 syrup Nutrition 0.000 claims description 2
- 239000000454 talc Substances 0.000 claims description 2
- 229910052623 talc Inorganic materials 0.000 claims description 2
- 235000012222 talc Nutrition 0.000 claims description 2
- 239000000546 pharmaceutical excipient Substances 0.000 claims 2
- 150000001875 compounds Chemical class 0.000 description 30
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 27
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 23
- 230000000694 effects Effects 0.000 description 21
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 18
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 15
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 14
- 239000000203 mixture Substances 0.000 description 11
- 206010021118 Hypotonia Diseases 0.000 description 10
- 230000036640 muscle relaxation Effects 0.000 description 10
- 239000000243 solution Substances 0.000 description 9
- SQUNAWUMZGQQJD-UHFFFAOYSA-N 1-(4-ethylphenyl)-2-methyl-3-(piperidin-1-yl)propan-1-one Chemical compound C1=CC(CC)=CC=C1C(=O)C(C)CN1CCCCC1 SQUNAWUMZGQQJD-UHFFFAOYSA-N 0.000 description 8
- 241001465754 Metazoa Species 0.000 description 8
- 241000699670 Mus sp. Species 0.000 description 8
- 229960002565 eperisone Drugs 0.000 description 8
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 8
- IXCSERBJSXMMFS-UHFFFAOYSA-N hydrogen chloride Substances Cl.Cl IXCSERBJSXMMFS-UHFFFAOYSA-N 0.000 description 7
- 229910000041 hydrogen chloride Inorganic materials 0.000 description 7
- FSKFPVLPFLJRQB-UHFFFAOYSA-N 2-methyl-1-(4-methylphenyl)-3-(1-piperidinyl)-1-propanone Chemical compound C=1C=C(C)C=CC=1C(=O)C(C)CN1CCCCC1 FSKFPVLPFLJRQB-UHFFFAOYSA-N 0.000 description 6
- 229930040373 Paraformaldehyde Natural products 0.000 description 6
- 238000004458 analytical method Methods 0.000 description 6
- 229920002866 paraformaldehyde Polymers 0.000 description 6
- 239000002904 solvent Substances 0.000 description 6
- SNICXCGAKADSCV-JTQLQIEISA-N (-)-Nicotine Chemical compound CN1CCC[C@H]1C1=CC=CN=C1 SNICXCGAKADSCV-JTQLQIEISA-N 0.000 description 5
- 206010010904 Convulsion Diseases 0.000 description 5
- 230000036461 convulsion Effects 0.000 description 5
- 229960002715 nicotine Drugs 0.000 description 5
- SNICXCGAKADSCV-UHFFFAOYSA-N nicotine Natural products CN1CCCC1C1=CC=CN=C1 SNICXCGAKADSCV-UHFFFAOYSA-N 0.000 description 5
- 239000011541 reaction mixture Substances 0.000 description 5
- 229960005334 tolperisone Drugs 0.000 description 5
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 4
- RSDOPYMFZBJHRL-UHFFFAOYSA-N Oxotremorine Chemical compound O=C1CCCN1CC#CCN1CCCC1 RSDOPYMFZBJHRL-UHFFFAOYSA-N 0.000 description 4
- FCLZCOCSZQNREK-UHFFFAOYSA-N Pyrrolidine, hydrochloride Chemical compound Cl.C1CCNC1 FCLZCOCSZQNREK-UHFFFAOYSA-N 0.000 description 4
- 206010044565 Tremor Diseases 0.000 description 4
- 229910000027 potassium carbonate Inorganic materials 0.000 description 4
- 230000003389 potentiating effect Effects 0.000 description 4
- 231100000419 toxicity Toxicity 0.000 description 4
- 230000001988 toxicity Effects 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 238000001816 cooling Methods 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- MVEAAGBEUOMFRX-UHFFFAOYSA-N ethyl acetate;hydrochloride Chemical compound Cl.CCOC(C)=O MVEAAGBEUOMFRX-UHFFFAOYSA-N 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 229910052500 inorganic mineral Inorganic materials 0.000 description 3
- WSFSSNUMVMOOMR-NJFSPNSNSA-N methanone Chemical compound O=[14CH2] WSFSSNUMVMOOMR-NJFSPNSNSA-N 0.000 description 3
- 239000011707 mineral Substances 0.000 description 3
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 2
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 206010043994 Tonic convulsion Diseases 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 231100000215 acute (single dose) toxicity testing Toxicity 0.000 description 2
- 238000011047 acute toxicity test Methods 0.000 description 2
- 125000001931 aliphatic group Chemical group 0.000 description 2
- 125000005907 alkyl ester group Chemical group 0.000 description 2
- 238000009835 boiling Methods 0.000 description 2
- 208000035475 disorder Diseases 0.000 description 2
- 238000001647 drug administration Methods 0.000 description 2
- FKRCODPIKNYEAC-UHFFFAOYSA-N ethyl propionate Chemical compound CCOC(=O)CC FKRCODPIKNYEAC-UHFFFAOYSA-N 0.000 description 2
- 230000002440 hepatic effect Effects 0.000 description 2
- 230000003387 muscular Effects 0.000 description 2
- 229910017604 nitric acid Inorganic materials 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 0 ***1CCCCC1 Chemical compound ***1CCCCC1 0.000 description 1
- BGJSXRVXTHVRSN-UHFFFAOYSA-N 1,3,5-trioxane Chemical compound C1OCOCO1 BGJSXRVXTHVRSN-UHFFFAOYSA-N 0.000 description 1
- XWLMJQYEEYESHD-UHFFFAOYSA-N 1-(4-butylphenyl)-2-methyl-3-pyrrolidin-1-ylpropan-1-one Chemical compound C1=CC(CCCC)=CC=C1C(=O)C(C)CN1CCCC1 XWLMJQYEEYESHD-UHFFFAOYSA-N 0.000 description 1
- HLYLZPUXULIVQP-UHFFFAOYSA-N 1-(4-butylphenyl)propan-1-one Chemical compound CCCCC1=CC=C(C(=O)CC)C=C1 HLYLZPUXULIVQP-UHFFFAOYSA-N 0.000 description 1
- FDXQBKHOYXKTQZ-UHFFFAOYSA-N 1-(4-cyclohexylphenyl)propan-1-one Chemical compound C1=CC(C(=O)CC)=CC=C1C1CCCCC1 FDXQBKHOYXKTQZ-UHFFFAOYSA-N 0.000 description 1
- VGQRIILEZYZAOE-UHFFFAOYSA-N 1-(4-ethylphenyl)propan-1-one Chemical compound CCC(=O)C1=CC=C(CC)C=C1 VGQRIILEZYZAOE-UHFFFAOYSA-N 0.000 description 1
- HUQTUHKXMAKTEH-UHFFFAOYSA-N 1-(4-propan-2-ylphenyl)propan-1-one Chemical compound CCC(=O)C1=CC=C(C(C)C)C=C1 HUQTUHKXMAKTEH-UHFFFAOYSA-N 0.000 description 1
- FDSYYILHBJMPTN-UHFFFAOYSA-N 1-(4-propylphenyl)propan-1-one Chemical compound CCCC1=CC=C(C(=O)CC)C=C1 FDSYYILHBJMPTN-UHFFFAOYSA-N 0.000 description 1
- GPZIKPBHFMNTOH-UHFFFAOYSA-N 1-[4-(2-methylpropyl)phenyl]propan-1-one Chemical compound CCC(=O)C1=CC=C(CC(C)C)C=C1 GPZIKPBHFMNTOH-UHFFFAOYSA-N 0.000 description 1
- WQVADQGLWGUDKH-UHFFFAOYSA-N 2-methyl-1-(4-propan-2-ylphenyl)-3-pyrrolidin-1-ylpropan-1-one Chemical compound C1=CC(C(C)C)=CC=C1C(=O)C(C)CN1CCCC1 WQVADQGLWGUDKH-UHFFFAOYSA-N 0.000 description 1
- SSTIFRPGHVHVGP-UHFFFAOYSA-N 2-methyl-1-[4-(2-methylpropyl)phenyl]-3-pyrrolidin-1-ylpropan-1-one Chemical compound C1=CC(CC(C)C)=CC=C1C(=O)C(C)CN1CCCC1 SSTIFRPGHVHVGP-UHFFFAOYSA-N 0.000 description 1
- WTFUTSCZYYCBAY-SXBRIOAWSA-N 6-[(E)-C-[[4-[2-(2,3-dihydro-1H-inden-2-ylamino)pyrimidin-5-yl]piperazin-1-yl]methyl]-N-hydroxycarbonimidoyl]-3H-1,3-benzoxazol-2-one Chemical compound C1C(CC2=CC=CC=C12)NC1=NC=C(C=N1)N1CCN(CC1)C/C(=N/O)/C1=CC2=C(NC(O2)=O)C=C1 WTFUTSCZYYCBAY-SXBRIOAWSA-N 0.000 description 1
- 208000008035 Back Pain Diseases 0.000 description 1
- ZAFNJMIOTHYJRJ-UHFFFAOYSA-N Diisopropyl ether Chemical compound CC(C)OC(C)C ZAFNJMIOTHYJRJ-UHFFFAOYSA-N 0.000 description 1
- 206010019909 Hernia Diseases 0.000 description 1
- 238000005684 Liebig rearrangement reaction Methods 0.000 description 1
- 208000008930 Low Back Pain Diseases 0.000 description 1
- 206010062575 Muscle contracture Diseases 0.000 description 1
- MKYBYDHXWVHEJW-UHFFFAOYSA-N N-[1-oxo-1-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)propan-2-yl]-2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidine-5-carboxamide Chemical compound O=C(C(C)NC(=O)C=1C=NC(=NC=1)NCC1=CC(=CC=C1)OC(F)(F)F)N1CC2=C(CC1)NN=N2 MKYBYDHXWVHEJW-UHFFFAOYSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 244000184734 Pyrus japonica Species 0.000 description 1
- 206010041415 Spastic paralysis Diseases 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- KXKVLQRXCPHEJC-UHFFFAOYSA-N acetic acid trimethyl ester Natural products COC(C)=O KXKVLQRXCPHEJC-UHFFFAOYSA-N 0.000 description 1
- 231100000403 acute toxicity Toxicity 0.000 description 1
- 230000007059 acute toxicity Effects 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 230000001476 alcoholic effect Effects 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 238000013329 compounding Methods 0.000 description 1
- 208000006111 contracture Diseases 0.000 description 1
- 229920005565 cyclic polymer Polymers 0.000 description 1
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 235000011087 fumaric acid Nutrition 0.000 description 1
- KVUKDCFEXVWYBN-JDXPBYPHSA-N gamma-sanshool Chemical compound C\C=C\C=C\C=C/CC\C=C\C=C\C(=O)NCC(C)C KVUKDCFEXVWYBN-JDXPBYPHSA-N 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 229940071870 hydroiodic acid Drugs 0.000 description 1
- VNFAARJCGSAROU-UHFFFAOYSA-N inaperisone Chemical compound C1=CC(CC)=CC=C1C(=O)C(C)CN1CCCC1 VNFAARJCGSAROU-UHFFFAOYSA-N 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 239000002050 international nonproprietary name Substances 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 230000003155 kinesthetic effect Effects 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 239000003158 myorelaxant agent Substances 0.000 description 1
- 125000004971 nitroalkyl group Chemical group 0.000 description 1
- MCSAJNNLRCFZED-UHFFFAOYSA-N nitroethane Chemical compound CC[N+]([O-])=O MCSAJNNLRCFZED-UHFFFAOYSA-N 0.000 description 1
- LYGJENNIWJXYER-UHFFFAOYSA-N nitromethane Chemical compound C[N+]([O-])=O LYGJENNIWJXYER-UHFFFAOYSA-N 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 239000012429 reaction media Substances 0.000 description 1
- 230000011514 reflex Effects 0.000 description 1
- 230000001373 regressive effect Effects 0.000 description 1
- 208000001413 spine osteoarthritis Diseases 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D295/00—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms
- C07D295/04—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms
- C07D295/10—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by doubly bound oxygen or sulphur atoms
- C07D295/104—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by doubly bound oxygen or sulphur atoms with the ring nitrogen atoms and the doubly bound oxygen or sulfur atoms attached to the same carbon chain, which is not interrupted by carbocyclic rings
- C07D295/108—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by doubly bound oxygen or sulphur atoms with the ring nitrogen atoms and the doubly bound oxygen or sulfur atoms attached to the same carbon chain, which is not interrupted by carbocyclic rings to an acyclic saturated chain
Definitions
- This invention relates to novel derivatives of 3-pyrrolidinopropiophenone and the pharmaceutically acceptable acid addition salts thereof, which exhibit a potent activity on muscle relaxation and to a process for preparation thereof.
- this invention relates to the derivatives of 3-pyrrolidinopropiophenone represented by formula (I): wherein R represents an ethyl-, propyl-, isopropyl-, butyl- and isobutylgroup or a cycloalkylgroup having 5-7 carbon atoms and the pharmaceutically acceptable acid addition salts thereof, as well as to a process for preparation thereof.
- formula (I) wherein R represents an ethyl-, propyl-, isopropyl-, butyl- and isobutylgroup or a cycloalkylgroup having 5-7 carbon atoms and the pharmaceutically acceptable acid addition salts thereof, as well as to a process for preparation thereof.
- Tolperisone (generic name, Merck Index, 9th Edition 9219) represented by formula (II): was on the market and been widely provided for clinical usage of the treatment of muscular contractive and spastic paralysis.
- Eperisone of formula (fll) shows an improvement in muscle relaxation activity. However, Eperisone does not show an improvement in toxicity and has just the same strong toxicity as Tolperison. Further concerning to the side effect, Eperisan causes hepatic and kidney functional disorder, while Tolperisone possesses only the hepatic functional disorder. Therefore, Eperisone is rather regressive than Tolperisone in respect to the unwanted side effects.
- examples of cycloalkylgroup shown by R are a cyclopentyl-, cyclohexyl-and cycloheptylgroup.
- the compounds represented by formula (I) can be converted into the corresponding pharmaceutically acceptable acid addition salts in a conventional manner and the base can be liberated from the acid addition salts so prepared, if necessary.
- Examples of the pharmaceutically acceptable acid addition salts of the compounds represented by formula (I), are salts with a mineral acid, such as hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid and phosphoric acid, and with an organic acid, such as acetic acid, maleic acid, fumaric acid, citric acid, oxalic acid and tartaric acid.
- a mineral acid such as hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid and phosphoric acid
- an organic acid such as acetic acid, maleic acid, fumaric acid, citric acid, oxalic acid and tartaric acid.
- the novel derivative of 3-pyrrolidinopropiophenone represented by formula (I) can be prepared by reacting a derivative of propiophenone represented by formula (IV): wherein R is as defined above, with a formaldehyde and pyrrolidine represented by formula (V): or the salt thereof in a solvent.
- formaldehyde As a formaldehyde to be used for the preparation, formaldehyde, linear or cyclic polymer of formaldehyde, such as paraformaldehyde and trioxane, can be considered.
- Pyrrolidine is used usually as a salt of a mineral acid such as hydrochloric acid, hydrobromic acid and nitric acid, whereas the pyrrolidine in the form of base is reacted in acid reaction medium by adding a sufficient amount of a mineral acid to the reaction mixture.
- a mineral acid such as hydrochloric acid, hydrobromic acid and nitric acid
- the mole ratio of the reactants can be chosen freely. However, 1 mol of pyrrolidine represented by formula (V) is reacted with at least 1 mol, preferably 1.1 moles of a derivative of propiophenone represented by formula (IV) and at least 1 mol, preferably 1.5 moles of formaldehyde, so that the pyrrolidine used can be eliminated from the reaction mixture, which should be submitted to an aftertreatment.
- Solvent used in the process of this invention is an alcoholic solvent such as methanol, ethanol, propanol and isopropanol, a nitroalkane solvent, such as nitromethane and nitroethane, a lower alkyl ester of a lower aliphatic acid, such as methyl acetate, ethyl acetate and ethyl propionate.
- a alcoholic solvent such as methanol, ethanol, propanol and isopropanol
- a nitroalkane solvent such as nitromethane and nitroethane
- a lower alkyl ester of a lower aliphatic acid such as methyl acetate, ethyl acetate and ethyl propionate.
- the lower alkyl ester of a lower aliphatic acid is used.
- the reaction can be carried out at a temperature between room temperature and the boiling point of the solvent used, preferably at the boiling point of the solvent.
- the thus prepared derivatives of 3-pyrrolidinopropiophenone represented by formula (I) and pharmaceutically-acceptable acid addition salts thereof exhibit an effective activity on muscle relaxation, inhibition of spinal reflex, nicotine-induced convulsion and oxotremorine-induced tremor and can be used extremely favorably as a medicine for treating of spasmodic muscular contracture by disease of kinesthetic organ such as low back pain, hernia of intervertebral dice and osteoarthritis of the spine.
- the compounds of this invention exhibit strong muscle relaxant activity with minimized side effects.
- the high order of these activities of the active agent of this invention is evidenced by tests in lower animals, representative of which are reported herein.
- the compound of this invention can be administered per os, e.g., in the form of pills or tablets, in which it may be present together with the usual pharmaceutical carriers, conventionally by compounding the compounds of this invention together with a customary carrier or adjuvant, such as talc, magnesium stearate, starch, lactose, gelatin and any of numerous gums.
- a non-toxic pharmaceutical carrier in addition to the active ingredient of this invention.
- Exemplary solid carriers are lactose, magnesium stearate, calcium stearate, starch, terra alba or dicalcium acacia.
- liquid carriers are peanut oil, sesame oil, olive oil or water.
- the active agents of this invention can be conveniently administered in such compositions containing active ingredient so as to eventually be within the dosage range illustrated hereafter.
- a wide variety of pharmaceutical forms suitable for many modes of administration and dosages may be employed.
- the active ingredient and pharmaceutical forms suitable for many modes of administration and dosages may be employed.
- the active ingredient and pharmaceutical carrier may, for example, take the form of a granule, pill, tablet, lozenge, elixir, syrup, or other liquid suspension or emulsion, whereas, for parenteral administration, the composition may be in the form of a sterile solution or suppository.
- a method of using the compounds of this invention comprises internally or externally administering the compounds of this invention, preferably orally or parenterally and preferably admixed with the pharmaceutical carrier, for example, in the form of any of the above compositions, or filled into a.capsule, to alleviate conditions to be treated and symptoms thereof in a living animal body.
- the pharmaceutical carrier for example, in the form of any of the above compositions, or filled into a.capsule.
- it may be used in an amount of about 1 to about 100 mg per unit dose, preferably 30 to 80 mg for an oral dose, while parenteral dosages are usually less and ordinarily about one-half of the oral dose.
- the unit dose is preferably given a suitable number of times daily, typically three times. The daily dose may vary depending upon the number of times given.
- Fig. 1, Table 1 and 2 The effective activity on muscle relaxation (rotating rod test), nicotine-induced convulsion and oxotremorine-induced tremor is shown in Fig. 1, Table 1 and 2 respectively as one example representing the potentiating pharmacological effect of the inventive compounds.
- the acute toxicity has been determined as shown in Table 3, whereby as a reference compound, Eperisone, market product, represented by formula (III) is used.
- the mice were tested previously by using rotating rod of 3 cm in diameter at the rate of 10 r.p.m. and the mice which can stay on the rotating rod during more than 60 seconds were selected for the test.
- the compounds were administered per os at a dose of 200 mg/kg and after 10, 20, 30 and 60 minutes of drug administration the mice were moved on the rotating rod. The times (seconds) of staying on the rod have been measured. It has been judged that the compound is effective, if the mice have fallen from the rod before 60 sec. has elapsed. The results are shown in the Figure 1.
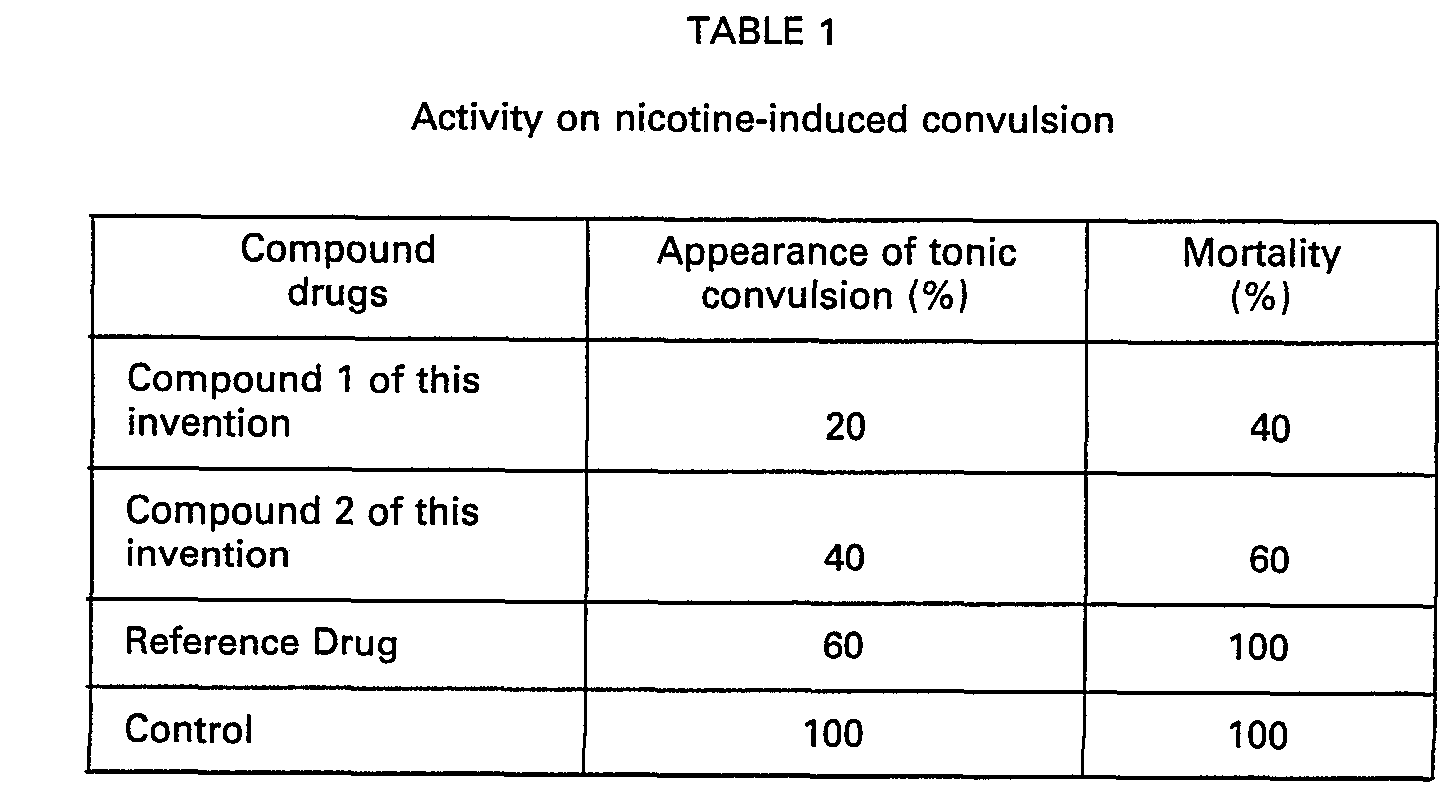
- mice Male ddY mice 5 weeks old were used as 5 animals in a group. The compounds were administered per os at a dose of 25 mg/kg. After 15 minutes of drug administration, Nicotine tartarate was administered intraveneously at an amount of 3 mg/kg and the tonic convulsion and animals dying because of the convulsion were measured. The effect of the compounds was indicated by the percentage of appearance of tonic convulsion and the mortality.
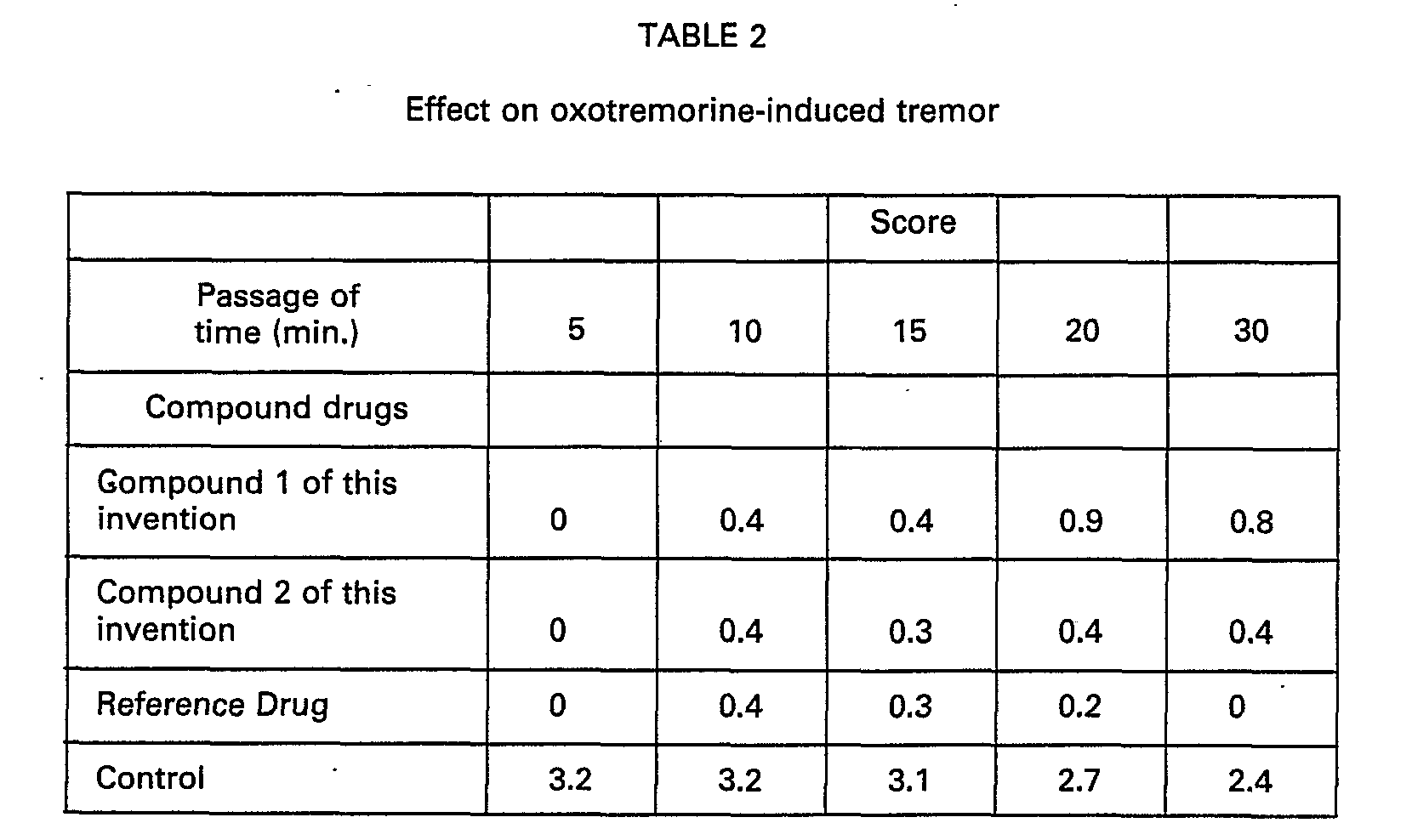
- mice Male ddY mice 5 weeks old were used as 5 animals in a group. The compounds were administered per os at a dose of 100 mg/kg and thereafter 15 minutes oxotremorine has been intraperitoneal administered at a dose of 1 mg/kg. The strength of the induced tremor has been observed by the method of Suzuki et al (Folia Pharmacologica Japonica, 83, 127 (1983) and scored with the passage of time as follows:
- mice Male ddY mice 5 weeks old were used as 5 animals in a group. The compounds were administered orally with each dosage. LD 50 was determined by the Probit method from animals dying within 7 days.
- the acute toxicity test shows that the compounds of the invention have a higher LD 50 value as compared with Eperisone. Therefore, it is clear that the compounds of the invention are very useful as a medicine for clinical usage because of the superior pharmacological effects and of the lower toxicity.
- reaction mixture was extracted with water.
- the water layer was made alkaline with potassium carbonate and extracted with ethyl acetate.
- the mixture was acidified by the addition of gaseous hydrogen chloride and refluxed for 2 hours. After cooling, the reaction mixture was extracted with aqueous hydrochloric acid. The water layer was made alkaline with potassium carbonate and extracted with ethyl acetate. The extract was washed with water, dried and evaporated. The residue was dissolved in ethanol and acidified with ethanolic hydrogen chloride. The solution was evaporated and the residue was washed with ethyl acetate and then recrystallized from methyl ethyl ketone to give 3.00 og of hydrochloride of the desired compound as colorless needles, mp 152-153°C.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Description
- This invention relates to novel derivatives of 3-pyrrolidinopropiophenone and the pharmaceutically acceptable acid addition salts thereof, which exhibit a potent activity on muscle relaxation and to a process for preparation thereof.
- More particularly, this invention relates to the derivatives of 3-pyrrolidinopropiophenone represented by formula (I):
-
-
- Eperisone of formula (fll) shows an improvement in muscle relaxation activity. However, Eperisone does not show an improvement in toxicity and has just the same strong toxicity as Tolperison. Further concerning to the side effect, Eperisan causes hepatic and kidney functional disorder, while Tolperisone possesses only the hepatic functional disorder. Therefore, Eperisone is rather regressive than Tolperisone in respect to the unwanted side effects.
- Tolperisone and Eperisone, as medicines which are commercially available, are not yet satisfactory.
- As a result of extensive investigation on new compounds having a potent activity on muscle relaxation, it has been found that the compounds of formula (I) have extremely weak toxicity and possess an effective activity on muscle relaxation as compared to those of formula (II) and (III), and thus this invention has been accomplished.
- In the foregoing formula (I), examples of cycloalkylgroup shown by R are a cyclopentyl-, cyclohexyl-and cycloheptylgroup.
- The compounds represented by formula (I) can be converted into the corresponding pharmaceutically acceptable acid addition salts in a conventional manner and the base can be liberated from the acid addition salts so prepared, if necessary.
- Examples of the pharmaceutically acceptable acid addition salts of the compounds represented by formula (I), are salts with a mineral acid, such as hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid and phosphoric acid, and with an organic acid, such as acetic acid, maleic acid, fumaric acid, citric acid, oxalic acid and tartaric acid.
- According to this invention, the novel derivative of 3-pyrrolidinopropiophenone represented by formula (I) can be prepared by reacting a derivative of propiophenone represented by formula (IV):
- As a formaldehyde to be used for the preparation, formaldehyde, linear or cyclic polymer of formaldehyde, such as paraformaldehyde and trioxane, can be considered.
- Pyrrolidine is used usually as a salt of a mineral acid such as hydrochloric acid, hydrobromic acid and nitric acid, whereas the pyrrolidine in the form of base is reacted in acid reaction medium by adding a sufficient amount of a mineral acid to the reaction mixture.
- The mole ratio of the reactants can be chosen freely. However, 1 mol of pyrrolidine represented by formula (V) is reacted with at least 1 mol, preferably 1.1 moles of a derivative of propiophenone represented by formula (IV) and at least 1 mol, preferably 1.5 moles of formaldehyde, so that the pyrrolidine used can be eliminated from the reaction mixture, which should be submitted to an aftertreatment.
- Solvent used in the process of this invention is an alcoholic solvent such as methanol, ethanol, propanol and isopropanol, a nitroalkane solvent, such as nitromethane and nitroethane, a lower alkyl ester of a lower aliphatic acid, such as methyl acetate, ethyl acetate and ethyl propionate. Preferably the lower alkyl ester of a lower aliphatic acid is used.
- The reaction can be carried out at a temperature between room temperature and the boiling point of the solvent used, preferably at the boiling point of the solvent..
- The derivatives of propiophenone represented by formula (IV), which can. be used as the starting materials for the process of thisinvention, are all known, and can be prepared in a manner as described, for example, in the literature, Pharmazie, 24, 735 (1969), Journal of the American Chemical Society, 78, 5899 (1953), Annalen der Chemie, Justus Liebigs, 546, 273 (1941), DBP 2059618.
- The thus prepared derivatives of 3-pyrrolidinopropiophenone represented by formula (I) and pharmaceutically-acceptable acid addition salts thereof exhibit an effective activity on muscle relaxation, inhibition of spinal reflex, nicotine-induced convulsion and oxotremorine-induced tremor and can be used extremely favorably as a medicine for treating of spasmodic muscular contracture by disease of kinesthetic organ such as low back pain, hernia of intervertebral dice and osteoarthritis of the spine.
- The compounds of this invention exhibit strong muscle relaxant activity with minimized side effects.
- The high order of these activities of the active agent of this invention is evidenced by tests in lower animals, representative of which are reported herein. The compound of this invention can be administered per os, e.g., in the form of pills or tablets, in which it may be present together with the usual pharmaceutical carriers, conventionally by compounding the compounds of this invention together with a customary carrier or adjuvant, such as talc, magnesium stearate, starch, lactose, gelatin and any of numerous gums. Thus, in their most advantageous form, the compositions of this invention will contain a non-toxic pharmaceutical carrier in addition to the active ingredient of this invention. Exemplary solid carriers are lactose, magnesium stearate, calcium stearate, starch, terra alba or dicalcium acacia.
- Representative liquid carriers are peanut oil, sesame oil, olive oil or water. The active agents of this invention can be conveniently administered in such compositions containing active ingredient so as to eventually be within the dosage range illustrated hereafter. Thus, a wide variety of pharmaceutical forms suitable for many modes of administration and dosages may be employed.
- For oral administration, the active ingredient and pharmaceutical forms suitable for many modes of administration and dosages may be employed. For oral administration, the active ingredient and pharmaceutical carrier may, for example, take the form of a granule, pill, tablet, lozenge, elixir, syrup, or other liquid suspension or emulsion, whereas, for parenteral administration, the composition may be in the form of a sterile solution or suppository.
- .A method of using the compounds of this invention comprises internally or externally administering the compounds of this invention, preferably orally or parenterally and preferably admixed with the pharmaceutical carrier, for example, in the form of any of the above compositions, or filled into a.capsule, to alleviate conditions to be treated and symptoms thereof in a living animal body. Illustratively, it may be used in an amount of about 1 to about 100 mg per unit dose, preferably 30 to 80 mg for an oral dose, while parenteral dosages are usually less and ordinarily about one-half of the oral dose. The unit dose is preferably given a suitable number of times daily, typically three times. The daily dose may vary depending upon the number of times given. Naturally, a suitable clinical dose must be adjusted in accordance with the condition, age, and weight of the patient, and it goes without saying that the enhanced activities of the compounds of this invention, together with their reduced side effects, also make it suitable for wide variations, and the invention therefore should not be limited by the exact ranges stated. The exact dosage, both unit dosage and daily dosage, will of course have to be determined according to established medical principles.
- The effective activity on muscle relaxation (rotating rod test), nicotine-induced convulsion and oxotremorine-induced tremor is shown in Fig. 1, Table 1 and 2 respectively as one example representing the potentiating pharmacological effect of the inventive compounds.
- The acute toxicity has been determined as shown in Table 3, whereby as a reference compound, Eperisone, market product, represented by formula (III) is used.
- Test Compounds:
- Compound of Invention 1 (Example 3)
- Compound of Invention 2 (Example 6)
- Reference Drug (Eperisone hydrochloride)
- Male ddY mice 5 weeks old were used as 10 animals at a group. The mice were tested previously by using rotating rod of 3 cm in diameter at the rate of 10 r.p.m. and the mice which can stay on the rotating rod during more than 60 seconds were selected for the test. The compounds were administered per os at a dose of 200 mg/kg and after 10, 20, 30 and 60 minutes of drug administration the mice were moved on the rotating rod. The times (seconds) of staying on the rod have been measured. It has been judged that the compound is effective, if the mice have fallen from the rod before 60 sec. has elapsed. The results are shown in the Figure 1.
- Male ddY mice 5 weeks old were used as 5 animals in a group. The compounds were administered per os at a dose of 25 mg/kg. After 15 minutes of drug administration, Nicotine tartarate was administered intraveneously at an amount of 3 mg/kg and the tonic convulsion and animals dying because of the convulsion were measured. The effect of the compounds was indicated by the percentage of appearance of tonic convulsion and the mortality.
-
- Male ddY mice 5 weeks old were used as 5 animals in a group. The compounds were administered per os at a dose of 100 mg/kg and thereafter 15 minutes oxotremorine has been intraperitoneal administered at a dose of 1 mg/kg. The strength of the induced tremor has been observed by the method of Suzuki et al (Folia Pharmacologica Japonica, 83, 127 (1983) and scored with the passage of time as follows:
-
- Male ddY mice 5 weeks old were used as 5 animals in a group. The compounds were administered orally with each dosage. LD50 was determined by the Probit method from animals dying within 7 days.
-
- It is clearly seen from the results above that the compounds of this invention exhibit potent activity on muscle relaxation and excellent activity on nicotine-induced convulsion as compared to reference drug.
- Further, the acute toxicity test shows that the compounds of the invention have a higher LD50 value as compared with Eperisone. Therefore, it is clear that the compounds of the invention are very useful as a medicine for clinical usage because of the superior pharmacological effects and of the lower toxicity.
- This invention will be described in detail with reference to the examples below:
- To a solution of 5.00 g of 4'-Propylpropiophenone in 10 ml of isopropanol were added 3.00 g of pyrrolidine hydrochloride, 1.30 g of paraformaldehyde and 0.5 ml of 40% ethanolic hydrogen chloride, and the mixture was refluxed for 4.5 hours and evaporated.
- The residue was dissolved in aqueous hydrochloric acid and washed with ether. The aqueous layer was made alkaline with potassium carbonate and extracted with ether. The extract was washed with water, dried and evaporated. The residue was dissolved in ether and acidified with 40% ethanolic hydrogen chloride. The precipitate was filtered, washed with a mixture of ethanol and ether, and recrystallized from methyl ethyl ketone to give 1.50 g of hydrochloride of the desired compound as colorless needles, m.p. 151-152°C.
-
- To a solution of 10.00 g of 4'-isopropylpropiophenone in 80 ml of ethyl acetate were added 2.00 g of pyrrolidine and 2.50 g of paraformaldehyde. The mixture was acidified by the addition of gaseous hydrogen chloride and refluxed for 4 hours.
- After cooling, the reaction mixture was extracted with water. The water layer was made alkaline with potassium carbonate and extracted with ethyl acetate.
- The extract was washed with water, dried and evaporated. The residue was dissolved in ethanol and acidified with 15% ethanolic hydrogen chloride. The solution was evaporated and the residue was washed with a mixture of acetone and ether and then recrystallized from methyl ethyl ketone to give 3.58 g of hydrochloride of the desired compound as colorless needles, mp 131-133°C.
-
- To a solution of 5.40 g of 4'-butylpropiophenone in 15 ml of ethyl acetate were added 3.00 g of pyrrolidine hydrochloride, 1.30 g of parformaldehyde and 0.5 ml of 22% hydrogen chloride-ethyl acetate and the mixture was refluxed for 4.5 hours. After cooling, the reaction mixture was extracted with aqueous hydrochloric acid. The water layer was made alkaline with potassium carbonate and extracted with ether. The extract was washed with water, dried and evaporated. The residue was dissolved in ethanol and acidified with 40% ethanolic hydrogen chloride.
- The solution was evaporated and the residue was washed with isopropyl ether and then recrystallized from methyl ethyl ketone to give 2.93 g of hydrochloride of the desired compound as colorless scales, mp 116-117°C.
-
- A solution of 5.40 g of 4'-isobutylpropiophenone in 15 ml of ethyl acetate, 3.00 g of pyrrolidine hydrochloride, 1.30 g of paraformaldehyde and 0.5 ml of 22% hydrogen chloride-ethyl acetate were treated by the same manner as that described for Example 3 to give 3.53 g of hydrochloride of the desired compound which were recrystallized from methyl ethyl ketone as colorless scales, mp 127-128°C.
-
- A solution of 5.10 g of 4'-cyclohexylpropiophenone in 15 ml of ethyl acetate, 2.50 g of pyrrolidine hydrochloride, 1.30 g of paraformaldehyde and 0.5 ml of 22% hydrogen chloride-ethyl acetate were treated by the same manner as that described for example 3 to give 3.65 g of hydrochloride of the desired compound, which were recrystallized from a mixture of ethanol and ether as colorless needles, mp 186-187°C.
-
- To a solution of 6.80 g of 4'-ethylpropiophenone in 60 ml of ethyl acetate were added 1.50 g of pyrrolidine and 1.90 g of paraformaldehyde.
- The mixture was acidified by the addition of gaseous hydrogen chloride and refluxed for 2 hours. After cooling, the reaction mixture was extracted with aqueous hydrochloric acid. The water layer was made alkaline with potassium carbonate and extracted with ethyl acetate. The extract was washed with water, dried and evaporated. The residue was dissolved in ethanol and acidified with ethanolic hydrogen chloride. The solution was evaporated and the residue was washed with ethyl acetate and then recrystallized from methyl ethyl ketone to give 3.00 og of hydrochloride of the desired compound as colorless needles, mp 152-153°C.
-
Claims (7)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| HU33131D HU33131A (en) | 1984-01-26 | ||
| US06/573,862 US4638009A (en) | 1984-01-26 | 1984-01-25 | Derivatives of 3-pyrrolidinopropiophenone and a process for preparation thereof |
| EP84100827A EP0150235B1 (en) | 1984-01-26 | 1984-01-26 | Derivatives of 3-pyrrolidinopropiophenone and a process for preparation thereof |
| DE8484100827T DE3466689D1 (en) | 1984-01-26 | 1984-01-26 | Derivatives of 3-pyrrolidinopropiophenone and a process for preparation thereof |
| FR8402469A FR2559771B1 (en) | 1984-01-26 | 1984-02-17 | 3-PYRROLIDINOPROPIOPHENONE DERIVATIVES AND PROCESS FOR THEIR PREPARATION |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP84100827A EP0150235B1 (en) | 1984-01-26 | 1984-01-26 | Derivatives of 3-pyrrolidinopropiophenone and a process for preparation thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0150235A1 EP0150235A1 (en) | 1985-08-07 |
| EP0150235B1 true EP0150235B1 (en) | 1987-10-07 |
Family
ID=8191722
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP84100827A Expired EP0150235B1 (en) | 1984-01-26 | 1984-01-26 | Derivatives of 3-pyrrolidinopropiophenone and a process for preparation thereof |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US4638009A (en) |
| EP (1) | EP0150235B1 (en) |
| DE (1) | DE3466689D1 (en) |
| FR (1) | FR2559771B1 (en) |
| HU (1) | HU33131A (en) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0637389B2 (en) * | 1986-12-26 | 1994-05-18 | 北陸製薬株式会社 | Treatment for frequent urination |
| JPH0720866B2 (en) * | 1987-05-15 | 1995-03-08 | 三生製薬株式会社 | Transdermal preparation containing eperisone or tolperisone or their salts |
| JPH03115267A (en) * | 1989-09-28 | 1991-05-16 | Maruho Kk | Propiophenone derivative, its production and central muscle relaxant and antispasmodic agent containing the same |
| DE10123129A1 (en) * | 2001-05-02 | 2002-11-14 | Berolina Drug Dev Ab Svedala | Deuterated 3-piperidinopropiophenones and medicinal products containing these compounds |
| EP2865381A1 (en) * | 2006-05-18 | 2015-04-29 | Pharmacyclics, Inc. | ITK inhibitors for treating blood cell malignancies |
| US7858666B2 (en) | 2007-06-08 | 2010-12-28 | Mannkind Corporation | IRE-1α inhibitors |
| KR101462468B1 (en) | 2012-11-26 | 2014-11-18 | 주식회사 엔지켐생명과학 | Process for preparing Eperisone hydrochloride |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3252996A (en) * | 1962-10-02 | 1966-05-24 | Ciba Geigy Corp | Alpha-pyrrolidinomethyl valero and caprophenones |
| US3151124A (en) * | 1961-11-20 | 1964-09-29 | Ciba Geigy Corp | Alpha oxy-beta alkyl-gamma tertiary amino-alpha phenyl propanes |
| US3995047A (en) * | 1973-12-14 | 1976-11-30 | Eisai Co., Ltd. | Propiophenone derivatives in the treatment of pathological muscular conditions |
| JPS5527914B2 (en) * | 1973-12-14 | 1980-07-24 | ||
| JPS54125630A (en) * | 1978-02-22 | 1979-09-29 | Nippon Zoki Pharmaceutical Co | Novel propanone derivative*its manufacture and medical composition containing it as active component |
| DE3019497A1 (en) * | 1980-05-22 | 1981-11-26 | Bayer Ag, 5090 Leverkusen | AMINOPROPIOPHENONE DERIVATIVES, METHOD FOR THE PRODUCTION THEREOF AND THEIR USE AS FUNGICIDES |
-
0
- HU HU33131D patent/HU33131A/hu unknown
-
1984
- 1984-01-25 US US06/573,862 patent/US4638009A/en not_active Expired - Lifetime
- 1984-01-26 DE DE8484100827T patent/DE3466689D1/en not_active Expired
- 1984-01-26 EP EP84100827A patent/EP0150235B1/en not_active Expired
- 1984-02-17 FR FR8402469A patent/FR2559771B1/en not_active Expired
Also Published As
| Publication number | Publication date |
|---|---|
| DE3466689D1 (en) | 1987-11-12 |
| US4638009A (en) | 1987-01-20 |
| HU33131A (en) | 1984-10-29 |
| EP0150235A1 (en) | 1985-08-07 |
| FR2559771B1 (en) | 1986-12-26 |
| FR2559771A1 (en) | 1985-08-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4518712A (en) | Piperazine derivative and analgesic composition containing the same | |
| GB2243833A (en) | 2-substituted 4-amino-5-chloro-n-[2-(diethylamino)ethyl]-benzamides | |
| IL30782A (en) | Amino guanidines | |
| EP0150235B1 (en) | Derivatives of 3-pyrrolidinopropiophenone and a process for preparation thereof | |
| US4983633A (en) | Amide compounds, process for preparing the same, and composition for activating gastric motor function containing the same | |
| US4034113A (en) | Treatment of senile geriatric patients to restore performance | |
| US2278123A (en) | Antispasmodics | |
| HU181147B (en) | Process for preparing substituted heterocyclin phenoxamines | |
| US4636513A (en) | Isoxazole derivatives and medicaments containing these compounds | |
| US4105769A (en) | Inhibition of indoleamine-N-methyl transferase by 2-iminopyridines | |
| US2918407A (en) | Anti-spasmodics specific for upper gastrointestinal pain and spasm | |
| EP0042366A1 (en) | Diphenylbutyl-1-acylpiperazines | |
| US4478838A (en) | 1-(3,4,5-Trimethoxycinnamoyl)-4-alkylaminocarbonylethyl piperazines | |
| CA1218374A (en) | Derivatives of 3-pyrrolidinopropiophenone and a process for preparation thereof | |
| US3925469A (en) | Tetiary-alkylamino-lower-acyl-xylidide local anaesthetics | |
| US4055664A (en) | Pharmaceutical preparations containing 4-(4-biphenylyl) butylamines and treatment of the animal organism therewith | |
| KR900006096B1 (en) | Process for preparing 3-pyrolidino propiophenone derivative | |
| US3274248A (en) | N-1, 1-bis-[aminophenyl]-propyl-amines and salts thereof | |
| US3334017A (en) | Phenylalkylhydrazine compositions | |
| US3553267A (en) | 3-dimethylamino-1,2,3,4-tetrahydrofluorene | |
| US3973017A (en) | Imidazothiazines, derivatives and analogues thereof | |
| JPH03115267A (en) | Propiophenone derivative, its production and central muscle relaxant and antispasmodic agent containing the same | |
| US3485924A (en) | Pharmaceutical compositions and methods for reducing appetite in animals | |
| US3037982A (en) | Phenylpiperazinylalkyl propionanilides | |
| US3642793A (en) | 3-hydrocarbon-4-tertiary amino methyl sydnones |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): BE CH DE GB IT LI NL SE |
|
| 17P | Request for examination filed |
Effective date: 19860124 |
|
| 17Q | First examination report despatched |
Effective date: 19870225 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): BE CH DE GB IT LI NL SE |
|
| ITF | It: translation for a ep patent filed | ||
| REF | Corresponds to: |
Ref document number: 3466689 Country of ref document: DE Date of ref document: 19871112 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| ITTA | It: last paid annual fee | ||
| EAL | Se: european patent in force in sweden |
Ref document number: 84100827.9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19990107 Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19990128 Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19990131 Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19990201 Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19990210 Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19990324 Year of fee payment: 16 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20000126 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20000127 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20000131 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20000131 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20000131 |
|
| BERE | Be: lapsed |
Owner name: HOKURIKU PHARMACEUTICAL CO. LTD Effective date: 20000131 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20000801 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 84100827.9 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20000126 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 20000801 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20001101 |