EP0131394B1 - Hard surface acid cleaner - Google Patents
Hard surface acid cleaner Download PDFInfo
- Publication number
- EP0131394B1 EP0131394B1 EP84303999A EP84303999A EP0131394B1 EP 0131394 B1 EP0131394 B1 EP 0131394B1 EP 84303999 A EP84303999 A EP 84303999A EP 84303999 A EP84303999 A EP 84303999A EP 0131394 B1 EP0131394 B1 EP 0131394B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- hard surface
- cleaner
- alkyl
- surface acid
- acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- 0 *CCCC1*C(*)(CCC(CCCS(O)(=O)=O)OC(CC*2*C3CCC2)CCC3(CC*)S(O)(=O)=O)C*1 Chemical compound *CCCC1*C(*)(CCC(CCCS(O)(=O)=O)OC(CC*2*C3CCC2)CCC3(CC*)S(O)(=O)=O)C*1 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/43—Solvents
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/02—Anionic compounds
- C11D1/12—Sulfonic acids or sulfuric acid esters; Salts thereof
- C11D1/22—Sulfonic acids or sulfuric acid esters; Salts thereof derived from aromatic compounds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/02—Anionic compounds
- C11D1/12—Sulfonic acids or sulfuric acid esters; Salts thereof
- C11D1/22—Sulfonic acids or sulfuric acid esters; Salts thereof derived from aromatic compounds
- C11D1/24—Sulfonic acids or sulfuric acid esters; Salts thereof derived from aromatic compounds containing ester or ether groups directly attached to the nucleus
Definitions
- hard surface cleaners in the art, containing some sort of anionic surfactant, solvent, perhaps a dye and a fragrance to impart a pleasing color and odor, and mostly water.
- solvent some sort of anionic surfactant
- solvent perhaps a dye and a fragrance to impart a pleasing color and odor
- Most of these prior cart cleaners suffer from one or more disadvantages.
- some cleaners are effective only on hard water stains.
- An example of this class of cleaner is a highly acidic toilet bowl cleaner containing hydrochloric acid.
- Hard water stains are mineral stains caused by the deposition of calcium or magnesium salts present in hard water.
- Certain other cleaners may be effective only against soap scum stains, caused when a fatty acid soap, such as a sodium lauryl fatty acid soap precipitates in hard water containing alkaline earth metal salts, such as calcium, magnesium, or barium, causing the familiar soap scum stain. Still other cleaners may be effective only on greasy/oily stains. Generally speaking, these are cleaners which have either at least some water miscible solvent, and/or some higher amount of nonionic and/or anionic surfactants.
- Linear alkyl benzene sulfonic acid has been well known in the detergent and cleaner field as storage compound which, upon neutralization with generally, an alkali metal salt, is available for use as an anionic surfactant.
- Kappler, et al, U.S.-A-3,969,282 which utilized alkyl benzene sulfonic acid in combination with a nonionic surfactant as a storage compound to be neutralized with alkali metal salts prior to using them to launder fabrics.
- Reid, U.S.-A-2,463,936 showed that linear alkyl benzene sulfonic acids could be neutralized with triethanolamine to act as a cleaner.
- linear alkyl benzene sulfonic acids can be utilized as hard surface cleaners themselves.
- This invention relates to an improved, hard surface acid cleaner comprising
- the hard surface acid cleaner further comprises an antimicrobial compound selected from:
- the hard surface cleaner further comprises a thickener selected from gums, polysaccharides and resins.
- the hard surface acid cleaner further comprises a defoamer selected from the dialkyl polysiloxane polymers.
- the hard surface acid cleaner of this invention can also include at least one other cleaning adjuvant selected from dyes, pigments, fragrances, and builders.
- the hard surface acid cleaner of this invention has proven to be both effective and fast- acting against all three major problem stains, namely, (1) soap scums; (2) hard water stains; and (3) greasy/ oily stains.
- the present invention is a hard surface acid cleaner which has surprisingly effective and fast cleaning results on all three problem areas: (1) soap scums; (2) hard water stains; and (3) greasy/oily stains.
- alkyl aryl sulfonic acid compounds most particularly, linear alkyl benzene sulfonic acids, (“HLAS") were responsible for the improved cleaning in all three areas.
- HLAS linear alkyl benzene sulfonic acids
- linear alkyl benzene sulfonic acids used in this invention are, as previously mentioned, commonly neutralized with sodium or other alkali metal salts to make common cleaners.
- Linear alkyl benzene sulfonic acid itself is produced by a two step synthesis, in which benzene is first alkylated with some alkyl chloride in the presence of catalyst. Next, the alkylated benzene is reacted with sulfonating reactant.
- a third step which does not concern the present invention, can occur when the thus produced linear alkyl benzene sulfonic acid is neutralized with an alkali metal hyroxide, such as NaOH, to produce the sodium salt, which is commonly called "LAS".
- Linear alkyl benzene sulfonic acid - which was introduced for heavy industrial use, after it was discovered that branched alkyl benzene sulfonic ("ABS") was significantly less bio-degradable - is produced by many companies, including Continental Oil Company under the brand name of Conoco SA-597, and Pilot Chemical Company under the brand name Calsoft LAS-99, Witco Chemical Corporation under the brand name of Witco 1298 Soft Acid and Stepan Chemical Company under the brand name of Bio Soft S-100.
- ABS branched alkyl benzene sulfonic
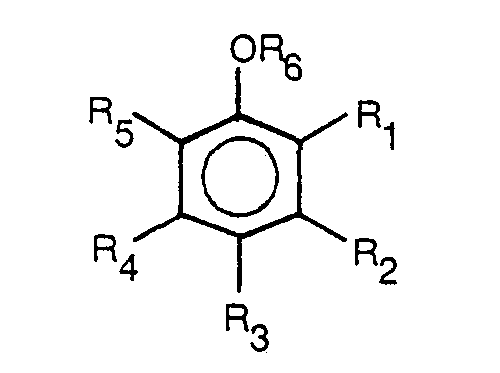
- Linear alkyl benzene sulfonic acid has the general structure (I) above.
- dodecylbenzene sulfonic acids wherein R averages about 11.4 carbon atoms in length.
- alkyl aryl sulfonic acids suitable for use in this invention include alkylated diphenyl oxide disulfonates of the general structure (II) above.
- compositions of this invention Preferably, a range of 0.001 % to 50.0% particularly, 1.0% to 30.0% and most preferably 5.0% to 15% by weight of acid surfactant is present in compositions of this invention. Further, the preferred surfactant of this invention will cause the formulas of this invention to have.pH's of no more than 6.5, and preferably, below 3.
- Solvents appropriate for use in this invention include C l - lo alkanols such as straight chain, primary, secondary and tertiary C 1-10 alkanols, C S - 1o alicyclic alcohols, C 2 - 8 dialkyl ethers, C 3 - 20 glycol ethers such as C 3 - 20 acyl and aryl glycol ethers and mixtures thereof.
- solvents examples include propylene glycol methyl ether, dipropylene glycol methyl ether, tripropylene glycol methyl ether, propylene glycol isobutyl ether, ethylene glycol methyl ether, ethylane glycol ethyl ether, ethylene glycol butyl ether, diethylene glycol methyl ether, diethylene glycol ethyl ether, diethylene glycol butyl ether, ethylene glycol phenyl ether, and propylene glycol phenol ether.
- glycol ethers are especially preferred because they are colorless liquids with mild, pleasant odors. They are very miscible with water, and have been found to be especially stable with the acid surfactants noted above.
- antimicrobial compounds are added to the novel hard surface cleaners of this invention which are selected from substituted phenols and quaternary ammonium compounds.
- quaternary ammonium compounds some of which are cationic surfactants, having essentially positively charged species in aqueous solution, would react with the anionic surfactants in equilibrium with the acid surfactants disclosed above and cause a precipitate to form.
- the quaternary ammonium compounds such as antimicrobial compounds in this invention are miscible with the mixture of acid surfactant and solvent in the formula and do not precipitate. Instead, a thickening is seen to occur when a preferred percentage of up to 5.0% of quaternary ammonium compound. Surpassing 5.0% has a thixotropic effect on the cleaner, and his latter application may be suitable for use as very substantive cleaners, i.e., bathroom cleaners.
- Suitable quaternary ammonium compounds include the ammonium salts of the general structure wherein R and R, are alkyls of 5 to 20 carbon atoms, aryl of 6 to 20 carbon atoms or alkylaryl of 7 to 20 carbon atoms provided that only one of R and R, is aryl or alkylaryl; R, may also be hydrogen and A- is an acid stable anion.
- Preferred anions include CI-, Br-, I-, SO 4 - , CI0 4 -, CI0 3 -, and N0 3 -.
- R can also be an alkyl benzyl group (in which case R is preferably a methyl group).
- R and R are both alkyl groups.
- C 7 to C 12 dialkyl dimethyl ammonium salts are particularly preferred.
- Other cationic surfactants notably other quaternary ammonium salts and tertiary amine surfactants may be suitable for use as disinfectant compounds.
- cationic surfactants used as antimicrobial compounds in this invention are compatible with the acid surfactants.
- a proposed theory, which is not meant herein to be binding is that these acid surfactants, not being neutralized by any alkali metal salts as the more common anionic surfactants are, may exhibit nonionic moieties in solution which act to solublize the cationic surfactants (quaternary ammonium compounds) and keep them from precipitating with the anion form of the acidic surfactants herein.
- the antimicrobial compounds can apparently be used in combination. No loss in stability is seen by combining these two antimicrobial compounds. Further, either or both of these antimicrobial compounds may be present in the invention from 0.001 to 15.0% by weight. Particularly preferred percentages of these antimicrobial compounds are from 0.1 to 10% by weight.
- cleaning adjuvants include thickeners selected from gums, resins and other polysaccharides, such as xanthan gums, starch, and mixtures.
- Further cleaning adjuvants include defoamers, such as dialkyl polysiloxane polymers. Particularly preferred as the defoamers are those sold by Dow-Corning under the trade name DB 100 for 100% dimethylpolysiloxane. Further defoamers may be applicable for use in this invention, including various cationic and nonionic surfactants.
- Still further cleaning adjuvants include dyes, pigments, fragrances and builders.
- the dyes and pigments in this invention are merely limited to those which will not substantially deposit and stain the surface to be cleaned. Fragrances selected must generally be those which will not be degraded by the low pH of the hard surface acid cleaner.
- Builders can include many inorganic and organic builders, such as sodium ethylenediaminetetraacetate or HEDTA (hydroxyethyl ethylenediaminetriacetic acid). Further builders include many organic acids and their alkali metal salts, eg., citric acid, sodium citrate, sodium lactate, sodium maleate, etc.
- a standard soap scum suspension was prepared using the following ingredients:
- the tiles were cleaned by applying approximately 4 grams of various formulations of the hard surface acid cleaner, scrubbed and rinsed ("Scrubbing Test"). Impartial panelists then graded the cleaning results on a 0 to 5 scale, as previously discussed. Competitive products were also compared. Further, in a second test, "Soak Test", formulations were allowed to soak the target stain for time intervals of 30, 20, 10, 5, 3, and 1 minutes, or 10, 5, 3, 2, minutes and 45 seconds to demonstrate rapid cleaning efficency. The results are reported in TABLES I, II and III below. Participants noted also how quickly effective cleaning occurred at the various time intervals.
- This mixture was applied in a thin layer to pre-cleaned white enamalled metal sheets (which material is the same as used for manufacturing porcelain kitchen sinks) and allowed to dry (age) for approximatley 24 hours.
- the preferred germicidal or antimicrobial compounds are chosen from substituted phenols, quaternary ammonium compounds, or mixtures thereof. Surprisingly, not only were the phenols stably miscible with the hard surface acid cleaners, but the quaternary ammonium compounds as well. Since the quaternary ammonium compounds are cationic species, i.e., positively charged species in aqueous solutions, it was presumed that precipitation would occur upon combination with the acid surfactants of the present invention.
- TABLES IV-V disclosed the even greater cleaning efficiency when using a variety of different solvents of varying structures. Further, using more than one type of solvent as in Example 48 is possible, but in the interest of cost effectiveness may not be as desirable, although such increases still constitute a part of this invention.
- TABLE VI shows that effective antimicrobial action is obtained by adding either a substituted phenol, a quaternary ammonium compound, or both, with surprisingly no loss in stability from addition of the quaternary ammonium compound.
- the present invention therefore provides a multipurpose, fast acting and effective hard surface acid cleaner which is effective over the three major problem cleaning areas.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Detergent Compositions (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Compositions Of Macromolecular Compounds (AREA)
Abstract
Description
- There are many examples of hard surface cleaners in the art, containing some sort of anionic surfactant, solvent, perhaps a dye and a fragrance to impart a pleasing color and odor, and mostly water. Most of these prior cart cleaners suffer from one or more disadvantages. For example, some cleaners are effective only on hard water stains. An example of this class of cleaner is a highly acidic toilet bowl cleaner containing hydrochloric acid. Hard water stains are mineral stains caused by the deposition of calcium or magnesium salts present in hard water. Certain other cleaners may be effective only against soap scum stains, caused when a fatty acid soap, such as a sodium lauryl fatty acid soap precipitates in hard water containing alkaline earth metal salts, such as calcium, magnesium, or barium, causing the familiar soap scum stain. Still other cleaners may be effective only on greasy/oily stains. Generally speaking, these are cleaners which have either at least some water miscible solvent, and/or some higher amount of nonionic and/or anionic surfactants.
- None of the prior art cleaners have addressed all three of these problematic areas together. As mentioned, a particular cleaner may be effective against a particular stain or cleaning problem, but not against the others. Thus, ineffective cleaning results may occur against certain stains using some of the cleaners, requiring purchase of other cleaners which will effectively remove the target stain. This will result in added expense as a particular cleaner must be purchased for a particular stain.
- Furthermore, may of the prior art cleaners are very slow-acting. That is, after being applied to a target stain for long periods of time, they may show some cleaning effect, but this slow action is considered a great disadvantage and inconvenience.
- Thus, heretofore no single cleaner has been formulated which will satisfactorily clean all types of stains. There is also a need for an effective all purpose'hard surface cleaner which is faster than the prior art cleaners. There is thus a long felt need for a multipurpose household cleaner capable of quickly and effectively cleaning all three types of stain.
- Linear alkyl benzene sulfonic acid has been well known in the detergent and cleaner field as storage compound which, upon neutralization with generally, an alkali metal salt, is available for use as an anionic surfactant. This was recognized in Kappler, et al, U.S.-A-3,969,282 which utilized alkyl benzene sulfonic acid in combination with a nonionic surfactant as a storage compound to be neutralized with alkali metal salts prior to using them to launder fabrics. Further, Reid, U.S.-A-2,463,936 showed that linear alkyl benzene sulfonic acids could be neutralized with triethanolamine to act as a cleaner. However, it was not realized in the art that linear alkyl benzene sulfonic acids can be utilized as hard surface cleaners themselves.
- This invention relates to an improved, hard surface acid cleaner comprising
- (a) alkyl aryl sulfonic acid of formula (I)
- (b) a solvent selected from C1-10 alkanols, C5-10 alicylic alkanols, C2-, dialkyl ethers and C3-20 glycol ethers and mixtures thereof; and
- (c) at least 25.0% by weight water.
- In another embodiment, the hard surface acid cleaner further comprises an antimicrobial compound selected from:
- (i) a substituted phenol of the general structure:
- (ii) a quarternary ammonium compound; and
- (iii) mixtures thereof.
- In yet another embodiment, the hard surface cleaner further comprises a thickener selected from gums, polysaccharides and resins.
- In still another embodiment, the hard surface acid cleaner further comprises a defoamer selected from the dialkyl polysiloxane polymers.
- The hard surface acid cleaner of this invention can also include at least one other cleaning adjuvant selected from dyes, pigments, fragrances, and builders.
- Surprisingly, the hard surface acid cleaner of this invention has proven to be both effective and fast- acting against all three major problem stains, namely, (1) soap scums; (2) hard water stains; and (3) greasy/ oily stains.
- The present invention is a hard surface acid cleaner which has surprisingly effective and fast cleaning results on all three problem areas: (1) soap scums; (2) hard water stains; and (3) greasy/oily stains. The surprising revelation was that alkyl aryl sulfonic acid compounds, most particularly, linear alkyl benzene sulfonic acids, ("HLAS") were responsible for the improved cleaning in all three areas. These alkyl benzene sulfonic acid surfactants are not neutralized with alkali metal, alkaline earth metal, or ammonium salts as are the typical detergents using substituted alkyl benzene sulfonates.
- The linear alkyl benzene sulfonic acids used in this invention are, as previously mentioned, commonly neutralized with sodium or other alkali metal salts to make common cleaners. Linear alkyl benzene sulfonic acid itself is produced by a two step synthesis, in which benzene is first alkylated with some alkyl chloride in the presence of catalyst. Next, the alkylated benzene is reacted with sulfonating reactant. A third step, which does not concern the present invention, can occur when the thus produced linear alkyl benzene sulfonic acid is neutralized with an alkali metal hyroxide, such as NaOH, to produce the sodium salt, which is commonly called "LAS".
- Linear alkyl benzene sulfonic acid - which was introduced for heavy industrial use, after it was discovered that branched alkyl benzene sulfonic ("ABS") was significantly less bio-degradable - is produced by many companies, including Continental Oil Company under the brand name of Conoco SA-597, and Pilot Chemical Company under the brand name Calsoft LAS-99, Witco Chemical Corporation under the brand name of Witco 1298 Soft Acid and Stepan Chemical Company under the brand name of Bio Soft S-100.
- Linear alkyl benzene sulfonic acid has the general structure (I) above.,
- Most preferable for use in the formula of this invention are dodecylbenzene sulfonic acids, wherein R averages about 11.4 carbon atoms in length.
- Other alkyl aryl sulfonic acids suitable for use in this invention include alkylated diphenyl oxide disulfonates of the general structure (II) above.
- Preferably, a range of 0.001 % to 50.0% particularly, 1.0% to 30.0% and most preferably 5.0% to 15% by weight of acid surfactant is present in compositions of this invention. Further, the preferred surfactant of this invention will cause the formulas of this invention to have.pH's of no more than 6.5, and preferably, below 3.
- An example of a suitable alkyl sulfonic acid in Dowfax 2AO manufactured by Dow Chemical Company corresponding to the structure immediately above when R, is H and R2 averages 12 carbons.
- Solvents appropriate for use in this invention include Cl-lo alkanols such as straight chain, primary, secondary and tertiary C1-10 alkanols, CS-1o alicyclic alcohols, C2-8 dialkyl ethers, C3-20 glycol ethers such as C3-20 acyl and aryl glycol ethers and mixtures thereof.
- 1. Alkanols: appropriate alkanol solvents in this invention include those having the general formula R-OH where R can be a straight, or substituted carbon chain of 1-10 carbon atoms. Solvents of this type include methanol, ethanol, n-propanol, isopropanol, n-butanol, sec butanol, tert butanol, hexanol, heptanol, etc.
- 2. Aliphatic Cyclic Alcohols (Alicyclics): Further appropriate solvents herein are ring structures, such as cyclohexanol, cyclooctanol and cyclodecanol and average 5-10 carbon atoms in their ring structures.
- 3. Dialkyl ethers: the dialkyl ethers suitable for use as solvents in this invention have a general structure R-O-R,, wherein R and R1 are equal, and each comprise a carbon chain of at least 1. The dialkyl ethers herein comprise two to eight carbon atoms in average chain length. Examples of this particular group of solvents include dimethyl ether, diethyl ether, and dipropyl ether.
- 4. Glycol ethers: particularly preferred as solvents in this invention are the glycol ethers having the general structure R-0-R,-OH, wherein R is an alkoxy of 1 to 20 carbons atoms, or aryloxy of at least 6 carbon atoms, and R1 is an ether condensate of propylene glycol and/or ethylene glycol having from one to ten glycol monomer units. Preferred are glycol ethers having one to five glycol monomer units. These are C3-20 glycol ethers.
- Examples of particularly preferred solvents include propylene glycol methyl ether, dipropylene glycol methyl ether, tripropylene glycol methyl ether, propylene glycol isobutyl ether, ethylene glycol methyl ether, ethylane glycol ethyl ether, ethylene glycol butyl ether, diethylene glycol methyl ether, diethylene glycol ethyl ether, diethylene glycol butyl ether, ethylene glycol phenyl ether, and propylene glycol phenol ether.
- These glycol ethers are especially preferred because they are colorless liquids with mild, pleasant odors. They are very miscible with water, and have been found to be especially stable with the acid surfactants noted above.
- Addition of 0.001-25.0% by weight of any of the solvents disclosed to this hard surface acid cleaner appears desirable, and especially preferred is 0.1 to 15% by weight of added solvent. As will be further discussed in greater detail, addition of solvents to the hard surface acid cleaners herein provide surprisingly even greater cleaning benefits.
- In yet another embodiment of this invention, antimicrobial compounds are added to the novel hard surface cleaners of this invention which are selected from substituted phenols and quaternary ammonium compounds.
- 1. Substituted phenols: suitable antimicrobial compounds can be selected from the substituted phenols having the general structure
- 2. Quaternary Ammonium Compounds: particular, surprisingly effective antimicrobial compounds suitable for use in this invention are quaternary ammonium compounds.
- One would normally expect that quaternary ammonium compounds, some of which are cationic surfactants, having essentially positively charged species in aqueous solution, would react with the anionic surfactants in equilibrium with the acid surfactants disclosed above and cause a precipitate to form. However, the quaternary ammonium compounds such as antimicrobial compounds in this invention are miscible with the mixture of acid surfactant and solvent in the formula and do not precipitate. Instead, a thickening is seen to occur when a preferred percentage of up to 5.0% of quaternary ammonium compound. Surpassing 5.0% has a thixotropic effect on the cleaner, and his latter application may be suitable for use as very substantive cleaners, i.e., bathroom cleaners.
- Suitable quaternary ammonium compounds include the ammonium salts of the general structure
- Preferably R and R, are both alkyl groups.
- Further particularly preferred are C7 to C12 dialkyl dimethyl ammonium salts. Other cationic surfactants, notably other quaternary ammonium salts and tertiary amine surfactants may be suitable for use as disinfectant compounds.
- As previously discussed, it is generally unknown why the cationic surfactants used as antimicrobial compounds in this invention are compatible with the acid surfactants. One would normally suspect that the quaternary ammonium compounds would co-precipitate with the anionic form of the acid surfactants. However, a proposed theory, which is not meant herein to be binding, is that these acid surfactants, not being neutralized by any alkali metal salts as the more common anionic surfactants are, may exhibit nonionic moieties in solution which act to solublize the cationic surfactants (quaternary ammonium compounds) and keep them from precipitating with the anion form of the acidic surfactants herein.
- Additionally, the antimicrobial compounds can apparently be used in combination. No loss in stability is seen by combining these two antimicrobial compounds. Further, either or both of these antimicrobial compounds may be present in the invention from 0.001 to 15.0% by weight. Particularly preferred percentages of these antimicrobial compounds are from 0.1 to 10% by weight.
- Further, approximately 0.0001-25% by weight of further cleaning adjuvants may be added to the present invention. These cleaning adjuvants include thickeners selected from gums, resins and other polysaccharides, such as xanthan gums, starch, and mixtures.
- Further cleaning adjuvants include defoamers, such as dialkyl polysiloxane polymers. Particularly preferred as the defoamers are those sold by Dow-Corning under the trade name DB 100 for 100% dimethylpolysiloxane. Further defoamers may be applicable for use in this invention, including various cationic and nonionic surfactants.
- Still further cleaning adjuvants include dyes, pigments, fragrances and builders. The dyes and pigments in this invention are merely limited to those which will not substantially deposit and stain the surface to be cleaned. Fragrances selected must generally be those which will not be degraded by the low pH of the hard surface acid cleaner. Builders can include many inorganic and organic builders, such as sodium ethylenediaminetetraacetate or HEDTA (hydroxyethyl ethylenediaminetriacetic acid). Further builders include many organic acids and their alkali metal salts, eg., citric acid, sodium citrate, sodium lactate, sodium maleate, etc.
- Various formulations of the hard surface acid cleaners of this invention were assayed in the three soiling problem areas (1) soap scums; (2) hard water stains; and (3) oily/greasy stains, as described in the following section, under "EXPERIMENTAL". Test methodologies and results of assays are set forth in greater detail below.
-
- This suspension was applied and baked onto tiles which were then cleaned with various formulations of the hard surface acid cleaner (1) using a Gardner Wear Tester (i.e., "Scrubbing Test") and (2) by simple application according to the "Soak Test". Impartial panelists were asked to grade the cleaning of the synthetic soap scum stains by the formulations of this invention as well as the performance of competitive cleaners on a 0 to 5 scale, wherein 0 = no cleaning, 5 = total cleaning. The grading was averaged for a number of trials. These results are reported below in TABLES I, II and III. Competitive products were also tested. Grading was conducted over various time periods to show that the hard surface acid cleaners of this invention clean effectively much more rapidly than competitive products.
-
- Each premix was sprayed on preheated brown tiles, then baked and allowed to cool.
- The tiles were cleaned by applying approximately 4 grams of various formulations of the hard surface acid cleaner, scrubbed and rinsed ("Scrubbing Test"). Impartial panelists then graded the cleaning results on a 0 to 5 scale, as previously discussed. Competitive products were also compared. Further, in a second test, "Soak Test", formulations were allowed to soak the target stain for time intervals of 30, 20, 10, 5, 3, and 1 minutes, or 10, 5, 3, 2, minutes and 45 seconds to demonstrate rapid cleaning efficency. The results are reported in TABLES I, II and III below. Participants noted also how quickly effective cleaning occurred at the various time intervals.
-
- This mixture was applied in a thin layer to pre-cleaned white enamalled metal sheets (which material is the same as used for manufacturing porcelain kitchen sinks) and allowed to dry (age) for approximatley 24 hours.
- Thereafter, each panel was cleaned via the Oil/Grease Stain "Soak Method", wherein approximately 5 grams of various formulations of the hard surface acid cleaner were applied and allowed to soak for time intervals of 30, 20, 10, 5, 3 and 1 minutes. Competitive products were similarly compared. Speed in cleaning, as previously noted, was scrutinized carefully. The results are reported below in TABLES I, II and III below.
-
- TABLE VI; below, illustrates the effect of adding a germicidal compound to the hard surface acid cleaners of this invention. As previously discussed, the preferred germicidal or antimicrobial compounds are chosen from substituted phenols, quaternary ammonium compounds, or mixtures thereof. Surprisingly, not only were the phenols stably miscible with the hard surface acid cleaners, but the quaternary ammonium compounds as well. Since the quaternary ammonium compounds are cationic species, i.e., positively charged species in aqueous solutions, it was presumed that precipitation would occur upon combination with the acid surfactants of the present invention. Surprisingly, no precipitation occurred, and the quaternary ammonium compounds also caused a thickening of the formulas when used in percentages of 5.0% or more. Adding more than 5.0% may cause a thixotrope to form, which helps the cleaning formula stay in place when applied to a vertical surface. This is a substantial benefit over other cleaners which are not as substantive and which tend to drain off. Furthermore, as disclosed in TABLE VI below, the antimicrobial activity of the antimicrobial compounds was very efficacious.
- Review of TABLES I-VI shows that the hard surface acid cleaners of the present invention show surprising efficacy and fast action.
- In direct comparison tests with other commercially available cleaners in TABLES I, II and III, the hard surface acid cleaners showed that total cleaning was consistently achieved, whether a "Scrubbing Test" or "Soak Test" was considered, and cleaning results were achieved faster than when using any of the competitive products. TABLES IV-V disclosed the even greater cleaning efficiency when using a variety of different solvents of varying structures. Further, using more than one type of solvent as in Example 48 is possible, but in the interest of cost effectiveness may not be as desirable, although such increases still constitute a part of this invention. Lastly, TABLE VI shows that effective antimicrobial action is obtained by adding either a substituted phenol, a quaternary ammonium compound, or both, with surprisingly no loss in stability from addition of the quaternary ammonium compound.
- The present invention therefore provides a multipurpose, fast acting and effective hard surface acid cleaner which is effective over the three major problem cleaning areas.
- The trademarks mentioned herein may be registered in some of the Designated States.
Claims (10)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT84303999T ATE32521T1 (en) | 1983-07-07 | 1984-06-13 | ACIDIC CLEANER FOR HARD SURFACES. |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US51210083A | 1983-07-07 | 1983-07-07 | |
| US512100 | 1983-07-07 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0131394A2 EP0131394A2 (en) | 1985-01-16 |
| EP0131394A3 EP0131394A3 (en) | 1985-11-21 |
| EP0131394B1 true EP0131394B1 (en) | 1988-02-17 |
Family
ID=24037670
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP84303999A Expired EP0131394B1 (en) | 1983-07-07 | 1984-06-13 | Hard surface acid cleaner |
Country Status (7)
| Country | Link |
|---|---|
| EP (1) | EP0131394B1 (en) |
| JP (1) | JPH0699704B2 (en) |
| AT (1) | ATE32521T1 (en) |
| CA (1) | CA1217690A (en) |
| DE (1) | DE3469363D1 (en) |
| ES (2) | ES8704887A1 (en) |
| MX (1) | MX159252A (en) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB8508010D0 (en) * | 1985-03-27 | 1985-05-01 | Unilever Plc | Liquid bleaching compositions |
| GB8625103D0 (en) * | 1986-10-20 | 1986-11-26 | Unilever Plc | Disinfectant compositions |
| GB8625974D0 (en) * | 1986-10-30 | 1986-12-03 | Unilever Plc | Non-aqueous liquid detergent |
| US4895669A (en) * | 1986-11-03 | 1990-01-23 | The Clorox Company | Aqueous based acidic hard surface cleaner |
| DE3637711A1 (en) * | 1986-11-05 | 1988-05-11 | Remmers Chemie Gmbh & Co | Composition for cleaning porous mineral building elements |
| US5362422A (en) * | 1993-05-03 | 1994-11-08 | The Procter & Gamble Company | Liquid hard surface detergent compositions containing amphoteric detergent surfactant and specific anionic surfactant |
| EP0988064A1 (en) * | 1997-06-09 | 2000-03-29 | THE PROCTER & GAMBLE CELLULOSE COMPANY (an Ohio corp.) | Improved uncomplexed cyclodextrin compositions for odor control |
| BR9815836A (en) * | 1998-04-27 | 2000-12-26 | Procter & Gamble | Improved decomplexed cyclodextrin compositions for odor control |
| AU740240B2 (en) * | 1998-04-27 | 2001-11-01 | Procter & Gamble Company, The | Improved uncomplexed cyclodextrin compositions for odor control |
| AR017716A1 (en) | 1998-04-27 | 2001-09-12 | Procter & Gamble | ARTICLE OF MANUFACTURE IN THE FORM OF A NON-MANUALLY OPERATED ATOMIZING EXPENDER |
| AU740341B2 (en) * | 1998-04-27 | 2001-11-01 | Procter & Gamble Company, The | Improved uncomplexed cyclodextrin compositions for odor and wrinkle control |
| WO2000030691A1 (en) * | 1998-11-25 | 2000-06-02 | The Procter & Gamble Company | Improved uncomplexed cyclodextrin compositions for odor control |
| US20140271762A1 (en) * | 2013-03-15 | 2014-09-18 | Ecolab Usa Inc. | Non-Sorptive or Minimally Sorptive Disinfectant Wipes |
| CN115772449B (en) * | 2022-12-07 | 2024-09-20 | 上海达潮工贸有限公司 | Outer wall stone retreading treatment cleaning agent and manufacturing method thereof |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3463736A (en) * | 1966-03-04 | 1969-08-26 | Atlantic Richfield Co | Aqueous slurries of triethanolamine salts of linear alkylbenzene sulfonic acids |
| DK132898A (en) * | 1969-10-24 | |||
| JPS4841133A (en) * | 1971-09-29 | 1973-06-16 | ||
| JPS5158405A (en) * | 1974-11-18 | 1976-05-21 | Dai Ichi Kogyo Seiyaku Co Ltd | MUKISAN SENEKYOZONENZAI |
| US3969282A (en) * | 1974-12-23 | 1976-07-13 | Basf Wyandotte Corporation | Acidic surfactant composition, stock surfactant solution prepared therefrom, and method of washing soiled substrates employing the same |
| CA1116059A (en) * | 1978-05-22 | 1982-01-12 | Allied Corporation | Phenol-free and chlorinated hydrocarbon-free photoresist stripper |
| US4414035A (en) * | 1979-05-21 | 1983-11-08 | Petrolite Corporation | Method for the removal of asphaltenic deposits |
-
1983
- 1983-10-21 CA CA000439460A patent/CA1217690A/en not_active Expired
- 1983-12-14 JP JP58235994A patent/JPH0699704B2/en not_active Expired - Lifetime
- 1983-12-15 MX MX199762A patent/MX159252A/en unknown
-
1984
- 1984-06-13 AT AT84303999T patent/ATE32521T1/en not_active IP Right Cessation
- 1984-06-13 DE DE8484303999T patent/DE3469363D1/en not_active Expired
- 1984-06-13 EP EP84303999A patent/EP0131394B1/en not_active Expired
- 1984-07-04 ES ES534403A patent/ES8704887A1/en not_active Expired
-
1987
- 1987-01-15 ES ES557353A patent/ES8802535A1/en not_active Expired
Also Published As
| Publication number | Publication date |
|---|---|
| EP0131394A3 (en) | 1985-11-21 |
| JPS6015499A (en) | 1985-01-26 |
| MX159252A (en) | 1989-05-09 |
| ES8704887A1 (en) | 1987-05-01 |
| ATE32521T1 (en) | 1988-03-15 |
| ES534403A0 (en) | 1987-05-01 |
| JPH0699704B2 (en) | 1994-12-07 |
| DE3469363D1 (en) | 1988-03-24 |
| EP0131394A2 (en) | 1985-01-16 |
| CA1217690A (en) | 1987-02-10 |
| ES8802535A1 (en) | 1988-07-16 |
| ES557353A0 (en) | 1988-07-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4759867A (en) | Hard surface acid cleaner | |
| EP0131394B1 (en) | Hard surface acid cleaner | |
| KR950008565B1 (en) | Builder-incorporated detergent compositions containing polyalkylene glycoliminodiacetic acid | |
| US6281178B1 (en) | Reduced residue hard surface cleaner comprising hydrotrope | |
| EP0273472B1 (en) | Aqueous detergent compositions containing diethyleneglycol monohexyl ether solvent | |
| CA2173435C (en) | Alkaline liquid hard-surface cleaning composition containing a quaternary ammonium disinfectant and selected dicarboxylate sequestrants | |
| RU2515224C2 (en) | Liquid acidic composition for cleaning solid surfaces | |
| US4492646A (en) | Liquid dishwashing detergent containing anionic surfactant, suds stabilizer and highly ethoxylated nonionic drainage promotor | |
| US4316824A (en) | Liquid detergent composition containing alkyl sulfate and alkyl ethoxylated sulfate | |
| US5254290A (en) | Hard surface cleaner | |
| US6255270B1 (en) | Cleaning and disinfecting compositions with electrolytic disinfecting booster | |
| EP0049546B1 (en) | Liquid detergent composition | |
| EP0214868A2 (en) | Surfactants for use in cleaning compositions | |
| IE59208B1 (en) | Liquid detergent composition | |
| US4486329A (en) | Liquid all-purpose cleaner | |
| GB2075043A (en) | Surfactant System | |
| CA1040504A (en) | Germicidal cleaning composition and method | |
| EP0034039B1 (en) | Liquid detergent composition | |
| JP2797571B2 (en) | Detergent composition | |
| US6100231A (en) | Biphenyl based solvents in blooming type hard surface cleaners | |
| US5604192A (en) | Hard surface detergent compositions | |
| JPH01198697A (en) | detergent composition | |
| JPS62252499A (en) | Liquid detergent composition | |
| WO1997044428A1 (en) | Acidic cleaning compositions | |
| CA1170949A (en) | Liquid detergent composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): AT DE FR GB IT |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Designated state(s): AT DE FR GB IT |
|
| 17P | Request for examination filed |
Effective date: 19860329 |
|
| 17Q | First examination report despatched |
Effective date: 19861106 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT DE FR GB IT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 19880217 Ref country code: FR Free format text: THE PATENT HAS BEEN ANNULLED BY A DECISION OF A NATIONAL AUTHORITY Effective date: 19880217 Ref country code: AT Effective date: 19880217 |
|
| REF | Corresponds to: |
Ref document number: 32521 Country of ref document: AT Date of ref document: 19880315 Kind code of ref document: T |
|
| REF | Corresponds to: |
Ref document number: 3469363 Country of ref document: DE Date of ref document: 19880324 |
|
| EN | Fr: translation not filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20000518 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20000519 Year of fee payment: 17 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010613 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20010613 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020403 |