EP0074983B1 - High efficiency organosolv saccharification process - Google Patents
High efficiency organosolv saccharification process Download PDFInfo
- Publication number
- EP0074983B1 EP0074983B1 EP19820900956 EP82900956A EP0074983B1 EP 0074983 B1 EP0074983 B1 EP 0074983B1 EP 19820900956 EP19820900956 EP 19820900956 EP 82900956 A EP82900956 A EP 82900956A EP 0074983 B1 EP0074983 B1 EP 0074983B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- acetone
- sugars
- hydrolysis
- sugar
- liquor
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- 238000000034 method Methods 0.000 title claims abstract description 26
- 230000008569 process Effects 0.000 title claims abstract description 20
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 claims abstract description 136
- 235000000346 sugar Nutrition 0.000 claims abstract description 85
- 230000007062 hydrolysis Effects 0.000 claims abstract description 63
- 238000006460 hydrolysis reaction Methods 0.000 claims abstract description 63
- 150000008163 sugars Chemical class 0.000 claims abstract description 41
- 229920005610 lignin Polymers 0.000 claims abstract description 22
- 239000000463 material Substances 0.000 claims abstract description 18
- 230000015556 catabolic process Effects 0.000 claims abstract description 12
- 238000006731 degradation reaction Methods 0.000 claims abstract description 11
- 150000007513 acids Chemical class 0.000 claims abstract description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 32
- 239000007787 solid Substances 0.000 claims description 10
- 238000001704 evaporation Methods 0.000 claims description 9
- 229920002678 cellulose Polymers 0.000 claims description 8
- 239000001913 cellulose Substances 0.000 claims description 8
- 238000010411 cooking Methods 0.000 claims description 8
- 239000000203 mixture Substances 0.000 claims description 7
- 238000004519 manufacturing process Methods 0.000 claims description 2
- 239000012978 lignocellulosic material Substances 0.000 claims 2
- 238000006243 chemical reaction Methods 0.000 abstract description 23
- 239000011877 solvent mixture Substances 0.000 abstract description 7
- 238000000926 separation method Methods 0.000 abstract description 6
- 238000001816 cooling Methods 0.000 abstract description 5
- 150000001875 compounds Chemical class 0.000 abstract 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 29
- 239000002253 acid Substances 0.000 description 29
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 22
- 239000002023 wood Substances 0.000 description 17
- 239000000243 solution Substances 0.000 description 15
- 238000011084 recovery Methods 0.000 description 14
- 230000004083 survival effect Effects 0.000 description 14
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 11
- 229920000742 Cotton Polymers 0.000 description 10
- 230000000694 effects Effects 0.000 description 10
- 239000002904 solvent Substances 0.000 description 9
- SRBFZHDQGSBBOR-IOVATXLUSA-N D-xylopyranose Chemical compound O[C@@H]1COC(O)[C@H](O)[C@H]1O SRBFZHDQGSBBOR-IOVATXLUSA-N 0.000 description 8
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 8
- 239000000706 filtrate Substances 0.000 description 8
- 239000008103 glucose Substances 0.000 description 8
- 239000000047 product Substances 0.000 description 8
- 239000000758 substrate Substances 0.000 description 8
- 239000003039 volatile agent Substances 0.000 description 8
- -1 alkyl glucosides Chemical class 0.000 description 7
- WQZGKKKJIJFFOK-QTVWNMPRSA-N D-mannopyranose Chemical compound OC[C@H]1OC(O)[C@@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-QTVWNMPRSA-N 0.000 description 6
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 6
- 238000004090 dissolution Methods 0.000 description 6
- 230000008020 evaporation Effects 0.000 description 6
- 239000011521 glass Substances 0.000 description 6
- 239000000413 hydrolysate Substances 0.000 description 6
- PYMYPHUHKUWMLA-UHFFFAOYSA-N arabinose Natural products OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 description 5
- SRBFZHDQGSBBOR-UHFFFAOYSA-N beta-D-Pyranose-Lyxose Natural products OC1COC(O)C(O)C1O SRBFZHDQGSBBOR-UHFFFAOYSA-N 0.000 description 5
- 238000009835 boiling Methods 0.000 description 5
- 238000010438 heat treatment Methods 0.000 description 5
- 150000002972 pentoses Chemical class 0.000 description 5
- 239000006188 syrup Substances 0.000 description 5
- 235000020357 syrup Nutrition 0.000 description 5
- 241000183024 Populus tremula Species 0.000 description 4
- 238000005903 acid hydrolysis reaction Methods 0.000 description 4
- 238000004817 gas chromatography Methods 0.000 description 4
- 235000011167 hydrochloric acid Nutrition 0.000 description 4
- 229920001223 polyethylene glycol Polymers 0.000 description 4
- 230000035484 reaction time Effects 0.000 description 4
- 238000001256 steam distillation Methods 0.000 description 4
- 230000004580 weight loss Effects 0.000 description 4
- 238000010626 work up procedure Methods 0.000 description 4
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- 235000014466 Douglas bleu Nutrition 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 229920002488 Hemicellulose Polymers 0.000 description 3
- 241000218683 Pseudotsuga Species 0.000 description 3
- 235000005386 Pseudotsuga menziesii var menziesii Nutrition 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 238000001914 filtration Methods 0.000 description 3
- 229930182830 galactose Natural products 0.000 description 3
- 230000003301 hydrolyzing effect Effects 0.000 description 3
- 239000011541 reaction mixture Substances 0.000 description 3
- 241000894007 species Species 0.000 description 3
- 229910001220 stainless steel Inorganic materials 0.000 description 3
- 239000010935 stainless steel Substances 0.000 description 3
- ZXSQEZNORDWBGZ-UHFFFAOYSA-N 1,3-dihydropyrrolo[2,3-b]pyridin-2-one Chemical compound C1=CN=C2NC(=O)CC2=C1 ZXSQEZNORDWBGZ-UHFFFAOYSA-N 0.000 description 2
- KIWBPDUYBMNFTB-UHFFFAOYSA-N Ethyl hydrogen sulfate Chemical compound CCOS(O)(=O)=O KIWBPDUYBMNFTB-UHFFFAOYSA-N 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 2
- 239000003377 acid catalyst Substances 0.000 description 2
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical compound OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 description 2
- VSCWAEJMTAWNJL-UHFFFAOYSA-K aluminium trichloride Chemical compound Cl[Al](Cl)Cl VSCWAEJMTAWNJL-UHFFFAOYSA-K 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 150000001720 carbohydrates Chemical class 0.000 description 2
- 235000014633 carbohydrates Nutrition 0.000 description 2
- 239000007795 chemical reaction product Substances 0.000 description 2
- 238000010924 continuous production Methods 0.000 description 2
- 238000004821 distillation Methods 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- IDGUHHHQCWSQLU-UHFFFAOYSA-N ethanol;hydrate Chemical compound O.CCO IDGUHHHQCWSQLU-UHFFFAOYSA-N 0.000 description 2
- 238000000855 fermentation Methods 0.000 description 2
- 230000004151 fermentation Effects 0.000 description 2
- 229930182478 glucoside Natural products 0.000 description 2
- 238000006386 neutralization reaction Methods 0.000 description 2
- 238000005325 percolation Methods 0.000 description 2
- 239000003208 petroleum Substances 0.000 description 2
- 238000006116 polymerization reaction Methods 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- OVARTBFNCCXQKS-UHFFFAOYSA-N propan-2-one;hydrate Chemical compound O.CC(C)=O OVARTBFNCCXQKS-UHFFFAOYSA-N 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 229910001958 silver carbonate Inorganic materials 0.000 description 2
- LKZMBDSASOBTPN-UHFFFAOYSA-L silver carbonate Substances [Ag].[O-]C([O-])=O LKZMBDSASOBTPN-UHFFFAOYSA-L 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 238000011282 treatment Methods 0.000 description 2
- NWUYHJFMYQTDRP-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;1-ethenyl-2-ethylbenzene;styrene Chemical compound C=CC1=CC=CC=C1.CCC1=CC=CC=C1C=C.C=CC1=CC=CC=C1C=C NWUYHJFMYQTDRP-UHFFFAOYSA-N 0.000 description 1
- 239000002028 Biomass Substances 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 229920001503 Glucan Polymers 0.000 description 1
- 229910021578 Iron(III) chloride Inorganic materials 0.000 description 1
- 240000000111 Saccharum officinarum Species 0.000 description 1
- 235000007201 Saccharum officinarum Nutrition 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 150000008043 acidic salts Chemical class 0.000 description 1
- 238000007792 addition Methods 0.000 description 1
- 230000001476 alcoholic effect Effects 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 238000006136 alcoholysis reaction Methods 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- PYMYPHUHKUWMLA-WDCZJNDASA-N arabinose Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)C=O PYMYPHUHKUWMLA-WDCZJNDASA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 239000003729 cation exchange resin Substances 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 230000009918 complex formation Effects 0.000 description 1
- 235000008504 concentrate Nutrition 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 239000002178 crystalline material Substances 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000007599 discharging Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000002360 explosive Substances 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 239000011121 hardwood Substances 0.000 description 1
- 150000002402 hexoses Chemical class 0.000 description 1
- 230000036571 hydration Effects 0.000 description 1
- 238000006703 hydration reaction Methods 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- RBTARNINKXHZNM-UHFFFAOYSA-K iron trichloride Chemical compound Cl[Fe](Cl)Cl RBTARNINKXHZNM-UHFFFAOYSA-K 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000000622 liquid--liquid extraction Methods 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 239000012452 mother liquor Substances 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 230000020477 pH reduction Effects 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 235000011007 phosphoric acid Nutrition 0.000 description 1
- 150000003016 phosphoric acids Chemical class 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 238000005204 segregation Methods 0.000 description 1
- 238000007086 side reaction Methods 0.000 description 1
- 239000011122 softwood Substances 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 238000010025 steaming Methods 0.000 description 1
- 239000011550 stock solution Substances 0.000 description 1
- 230000002277 temperature effect Effects 0.000 description 1
- 229910001428 transition metal ion Inorganic materials 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
Classifications
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21C—PRODUCTION OF CELLULOSE BY REMOVING NON-CELLULOSE SUBSTANCES FROM CELLULOSE-CONTAINING MATERIALS; REGENERATION OF PULPING LIQUORS; APPARATUS THEREFOR
- D21C3/00—Pulping cellulose-containing materials
- D21C3/20—Pulping cellulose-containing materials with organic solvents or in solvent environment
-
- C—CHEMISTRY; METALLURGY
- C13—SUGAR INDUSTRY
- C13K—SACCHARIDES OBTAINED FROM NATURAL SOURCES OR BY HYDROLYSIS OF NATURALLY OCCURRING DISACCHARIDES, OLIGOSACCHARIDES OR POLYSACCHARIDES
- C13K1/00—Glucose; Glucose-containing syrups
- C13K1/02—Glucose; Glucose-containing syrups obtained by saccharification of cellulosic materials
Definitions
- the invention relates to a process for the production of sugars from particulate cellulose material by treating the material in a pressure vessel with a mixture of acetone and water containing a small amount of an acidic compound at elevated temperatures.
- GB-A-416416 describes the recovery of cellulose by the extraction of lignin from a lignocellulose material with acetone or a mixture of acetone and water.
- acetone in volume concentrations in water of greater than 70% with a catalytic amount of an acid greatly accelerates the hydrolysis rates of cellulosic materials at elevated temperatures by sugar-acetone-complex formation.
- acetone complexes are found to hydrolyse roughly 500 times faster than the alkyl glucosides and polyglucan described in the prior art.
- Further benefit of the acetone-sugar-complexes is their facile separation into individual sugar species based on such simple processes as volatilization, selective hydrolysis and liquid-liquid extraction. Complex formation of monomeric sugars in anhydrous acetone in the presence of mineral acids at room temperature is described in Methods in Carbohydrate Chemistry, Vol. II, pp. 318.
- cellulosic material includes materials of vegetable and woody origin, i.e. cellulose and lignocellulose materials.
- reaction vessels with inert linings are used to eliminate the sugar degradation catalyzing effects of transition metal ions such as Ni, Co, Cr, Fe and Cu which may be components of metallic vessel walls, tubing and other control elements with which the hot liquor comes into contact.
- transition metal ions such as Ni, Co, Cr, Fe and Cu which may be components of metallic vessel walls, tubing and other control elements with which the hot liquor comes into contact.
- the temperature of the reaction mixture be rapidly lowered to under 100°C to avoid unwanted degradation of the sugars. This is best accomplished by controlled flashing-off of the volatiles since sugar degradation was found to be insignificant below the boiling point of water even in the presence of dilute acids.
- the cooling of the liquor can be continued to ambient temperatures or less (25°C) before fermentation or further processing.
- the liquor-to-wood ratio can be kept constant at 10:1 as by necessity successive additions both wood and liquor will carry hydrolyzates of the residuals already within the reactor.
- This also establishes sugar concentrations to' be in the order of 37 to 40% following flash evaporation of the volatiles.
- Such high sugar solids concentrations were hitherto possible only with strong acid hydrolysis systems but not with dilute acid hydrolysis.
- liquor to wood ratio is extremely important in organosolv and acid hydrolysis processes since it directly relates to energy inputs during the hydrolysis and solvent recovery as well as during alcohol recovery from the resulting aqueous solution following fermentation of the sugars to ethanol or other organic solvents.
- liquor to wood ratio will have a profound effect on the economics of biomass conversion to liquid chemicals as well as the energy efficiency (energy gained over energy expanded in conversion) of the process.
- Saccharification power and sugar survival were compared for three competitive systems namely: acidified water (aqueous weak acid), acidified aqueous ethanol and acidified aqueous acetone in the following example.
- the combined filtrates were diluted to 100 ml with water and a half milliliter aliquot was placed in a test tube with 3 ml of 2.0 Normal sulfuric acid added and subjected to a secondary hydrolysis at 100°C by heating in a boiling water bath for 40 minutes.
- the solution was neutralized on cooling and the sugars present in the solution were determined by their reducing power.
- the results were thus uniform based essentially on the resultant monosaccharides liberated during the hydrolysis process.
- Theoretical percentage of reducing sugars available after the hydrolysis of the substrate was determined by difference between the known chemical composition of the starting material and the weight loss incurred due to the hydrolysis.
- Solid residues less than 50% in yield show high degree of crystallinity (87%) and are pure white, have a DP (degree of polymerization) of 130 to 350.
- the invention allows facile segregation and nearly quantitative isolation of the five major wood sugars, if so desired.
- the mixed nature of the sugar derivatives in aqueous hydrolyzates if such thorough and detailed separation is desired, it is always necessary to neutralize the recovered aqueous sugar wort after removal of the volatiles and concentrate the wort to a syrup.
- the syrup is then redissolved in anhydrous acetone containing 3 percent acid, allowed to stand at least 6 hr until all sugars formed their respective di-acetone complexes before attempting the detailed separation as described below.
- the separated sugar complexes are readily hydrolyzed in dilute acid on boiling at least 20 to 40 minutes.
- the combined filtrate (127 ml) was neutralized and subjected to steam distillation in an all glass apparatus and approximately 35 ml distillate was collected. Both the distillate and residual solution were made up to 100 ml and 0.5 ml portions of each were acidified with sulfuric acid to 3 percent acid and boiled for 40 min on a water bath. The solutions were neutralized and the sugar reducing power determined by the Somogyi method. The yield of sugars was 1.89 g in the distillate and 1.96 g from the residual liquor.
- Hydrolysate No. 3 contained only traces of lignin after evaporation of the acetone solvent too small to collect and determine gravimetrically. It was removed by centrifuging. The aqueous residue (97 ml) was acidified to 3 percent acid with sulfuric acid, boiled for 40 min and after neutralization filtered and made up to 100 ml. The reducing sugar content of the filtrate was determined by the Somogyi method to be 1.83 g. GC analysis of the alditol acetates determined on an aliquot sample indicated mainly glucose with traces of mannose and galactose.
- Hydrolysate No. 4 and 5 were processed and analyzed in the same manner as No. 3. H-4 yielded 1.73 g reducing sugars and H-5 yielded 1.40 g sugars both being composed only of glucose as evidenced by GC analysis of an aliquot sample.
- Example VI In a similar hydrolysis arrangement to Example VI 10 g OD Douglas-fir sawdust (to pass a 10 mesh screen), pre-extracted with dichloromethane and air dried to 8 percent moisture content in a controlled humidity room, was hydrolyzed with 80:20 acetone:water solvent containing 0.05 Normal Hydrochloric acid in five consecutive steps. Each reaction step consisted of three minutes at a reaction temperature of 200°C. The heating up time was 7 minutes. Again Hydrolysate No. 1 and 2 were combined whereas the subsequent fractions were analyzed separately.
- the combined liquor of H-1 and H-2 yielded 2.39 g lignin on low temperature evaporation of the volatiles and 135 ml of aqueous liquor was collected on filtration of the powdered lignin.
- the dried lignin had a weight average molecular weight of 3200.
- the filtrate was neutralized to pH 8 and subjected to steam distillation in all glass apparatus.
- the 28 ml distillate which was collected contained 0.62 g pentoses which after passing the filtrate through a cation exchange resin in the acid form and repeated steam distillation of the filtrate yielded 0.58 g xylose as determined by GC analysis.
- the ethanol-petroleum ether solution was extracted with 5 ml portions of water and the collected aqueous layer combined with the syrup removed from the crystalline product above.
- the solution was briefly heated to expel the alcohol, made up to 3 percent acid with hydrochloric acid, boiled for 40 min, neutralized with silver carbonate and alditol acetates were prepared for GC analysis.
- the combined syrup and filtrate contained a total of 58 g sugars of which 0.29 g was galactose, 0.25 g was glucose and 0.04 g was mannose.
- Hydrolysate No. 4 gave 1.66 g of pure glucose with only very small traces of lignin, whereas H-5 gave 1.85 g of glucose and no lignin. The undissolved residue was 0.18 g and was composed of 99 percent glucose.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Emergency Medicine (AREA)
- General Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biochemistry (AREA)
- Organic Chemistry (AREA)
- Saccharide Compounds (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
Description
- The invention relates to a process for the production of sugars from particulate cellulose material by treating the material in a pressure vessel with a mixture of acetone and water containing a small amount of an acidic compound at elevated temperatures.
- Such a process wherein an acidified 30 to 70% by volume acetone containing mixture of acetone and water is used as the cooking liquor is described in GB-A-2003478.
- Further GB-A-416416 describes the recovery of cellulose by the extraction of lignin from a lignocellulose material with acetone or a mixture of acetone and water.
- The object of the invention is to rapidly and quantitatively solubilize and recover sugars from cellulosic materials.
- This object is attained by the process of the claims.
- Unexpectedly it has been found that acetone in volume concentrations in water of greater than 70% with a catalytic amount of an acid greatly accelerates the hydrolysis rates of cellulosic materials at elevated temperatures by sugar-acetone-complex formation. In addition there is substantially no degradation of sugars during the saccharification process at the selected conditions, although the acetone complexes are found to hydrolyse roughly 500 times faster than the alkyl glucosides and polyglucan described in the prior art. Further benefit of the acetone-sugar-complexes is their facile separation into individual sugar species based on such simple processes as volatilization, selective hydrolysis and liquid-liquid extraction. Complex formation of monomeric sugars in anhydrous acetone in the presence of mineral acids at room temperature is described in Methods in Carbohydrate Chemistry, Vol. II, pp. 318.
- The term "cellulosic material" includes materials of vegetable and woody origin, i.e. cellulose and lignocellulose materials.
- The acidic compounds can be of inorganic or organic origin and should be inert with respect to the solvent. Strong inorganic acids as sulfuric, hydrochloric and phosphoric acids are preferred; acidic salts such as aluminum chloride and sulfate, ferric chloride and organic acids such as trifluoroacetic acid can also be used. For very rapid hydrolysis acid concentrations of 0.04 to 0.06 normal, acetone concentrations of about 80% and temperatures over 200°C can be used. However, for near theoretical sugar yields, low acid concentration of 0.02 normal and less, high acetone concentration of above 80% and high temperature of above 200°C are most suitable.
- Preferably reaction vessels with inert linings are used to eliminate the sugar degradation catalyzing effects of transition metal ions such as Ni, Co, Cr, Fe and Cu which may be components of metallic vessel walls, tubing and other control elements with which the hot liquor comes into contact.
- Notwithstanding these process options the recovery of pentoses from the reaction mixture is generally by flash-evaporation of the major fraction of the acetone first with continued distillation under reduced pressure or by steam stripping to yield the pentose sugar complexes in the distillate. Separation of pentoses and hexoses by such simple means is made possible by the largely differing boiling points of their acetone-sugar complexes which form even in the presence of water during the high temperature hydrolysis step in the process of the invention provided the acetone concentration exceeds 70% by volume.
- After hydrolyzing the cellulosic material at elevated temperature for a limited period of time, it is very important, that the temperature of the reaction mixture be rapidly lowered to under 100°C to avoid unwanted degradation of the sugars. This is best accomplished by controlled flashing-off of the volatiles since sugar degradation was found to be insignificant below the boiling point of water even in the presence of dilute acids. Usually, the cooling of the liquor can be continued to ambient temperatures or less (25°C) before fermentation or further processing.
- The above described process can be operated in continuous or semi-continuous manner using batch cooking principles for the latter. Semi-continuous saccharification would employ a battery of pressure vessels each at various stage of hydrolysis to simulate a continuous process. In continuous operation, all stages of hydrolysis are accomplished in a single pressure vessel and the product mix is always determined by the particular saccharification program set. Comminuted wood solids and the cooking liquor are fed continuously to the pressure vessel at such a rate that the time elapsed between feeding and exit of the products would not exceed that determined earlier to obtain 50% hydrolysis of solid residue at any one stage considered for the process. Thus the residence time would be always fitted to the most sensitive stage in order to provide sugar recoveries exceeding 90% for that particular stage. The three major stages of saccharification to be considered are:
- (a) bulk delignification and pre-hydrolysis; during this stage up to 75% of the lignin and 95% of the governing hemicelluloses (xylose in hard-woods and mannose in soft-woods) may be removed. The solid residue yield is invariably above 50% of the starting material;
- (b) continued delignification and cellulose purification stage; during this stage delignification is largely completed and the rest of the hemicellulose sugars and some of the amorphous glucan are removed. The solid residue at this stage is generally less than 35% and is predominantly crystalline in nature;
- (c) proceeding to total saccharification; the residual cellulose of stage (b) is decomposed to monomeric sugars. This step may take more than one liquor change to accomplish a better than 90% sugar recovery.
- In continuous operation liquors collected from the various stages of hydrolysis may contain sugars from all stages (a) to (c) which is the situation with an apparatus having no means of separating the top pre-hydrolysis liquor from the rest of the liquor pumped in with the chips. With the present invention such separation for purification of the sugars is unnecessary because the sugars occur as complexes, pentoses having a different volatility than the hexose sugars with which they may be mixed. The lignin is separated on basis of its insolubility in water and is recovered outside the reactor on flash-evaporation of the organic vqlatiles.
- In practical hydrolysis, based on the semi-continuous process, five liquor changes would be required to cause total saccharification and dissolution and provide mass recoveries better than 95%. The preferred liquor-to-wood ratio is 7:1 to 10:1. Due to the shrinking mass bed the total amount of liquor required for hydrolysis of 100 kg of aspen wood at a constant liquor-to-wood ratio of 7:1 is 1356 kg for an overall liquor-to-wood ratio of 13,56:1. Under these conditions the average sugar concentration in the combined residual aqueous phase (271 kg) is 30% (82,3 kg of recovered sugars).
- In continuous percolation, the liquor-to-wood ratio can be kept constant at 10:1 as by necessity successive additions both wood and liquor will carry hydrolyzates of the residuals already within the reactor. This also establishes sugar concentrations to' be in the order of 37 to 40% following flash evaporation of the volatiles. Such high sugar solids concentrations were hitherto possible only with strong acid hydrolysis systems but not with dilute acid hydrolysis.
- Discussion of the liquor to wood ratio is extremely important in organosolv and acid hydrolysis processes since it directly relates to energy inputs during the hydrolysis and solvent recovery as well as during alcohol recovery from the resulting aqueous solution following fermentation of the sugars to ethanol or other organic solvents. Thus the liquor to wood ratio will have a profound effect on the economics of biomass conversion to liquid chemicals as well as the energy efficiency (energy gained over energy expanded in conversion) of the process.
- Steaming of the comminuted cellulosic material before mixing with the hydrolysis liquor can be used to advantage to expel trapped air. Such treatment will aid rapid liquor penetration. Such practice is well known from the prior art.
- Saccharification power and sugar survival were compared for three competitive systems namely: acidified water (aqueous weak acid), acidified aqueous ethanol and acidified aqueous acetone in the following example.
- In every case purified cotton linters having TAPPI 0.5 percent viscosity of 35 cP and 73 percent crystallinity index at 7 percent moisture content were used. Acidification was affected with sulfuric acid by making up stock solutions of the various solvent systems each being 0.04 Normal with respect to the acid. Hydrolysis conditions were as follows:
- In a series of experiments one gram samples of cotton linters (oven dry weight) were placed in glass lined stainless steel vessels of 20 ml capacity along with 10 ml of the solvent mixture and heated at 180°C for various lengths of time and residual solids and detected sugars in solution were plotted on graph paper. The times to obtain dissolution of about 99, 75, 50 and 25 percent of the substrate were read from the graphs and shown in Table 1. At the end of the reaction periods heating was interrupted, the vessel chilled and its cold contents filtered through medium porosity glass crucible, the undissolved residue first washed with warm water followed by rinsing with several 5 ml portions of acetone and finally by warm water. The residue weight was determined gravimetrically after drying at 105°C.
- For comparative analytical purposes the combined filtrates were diluted to 100 ml with water and a half milliliter aliquot was placed in a test tube with 3 ml of 2.0 Normal sulfuric acid added and subjected to a secondary hydrolysis at 100°C by heating in a boiling water bath for 40 minutes. The solution was neutralized on cooling and the sugars present in the solution were determined by their reducing power. The results were thus uniform based essentially on the resultant monosaccharides liberated during the hydrolysis process. Theoretical percentage of reducing sugars available after the hydrolysis of the substrate was determined by difference between the known chemical composition of the starting material and the weight loss incurred due to the hydrolysis. To account for the weight increase of the carbohydrate fraction due to hydration of the polymer on breakdown into monomeric sugars, the weight loss is normally multiplied by 1.1111, the weight percentage (11.11%) of the added water to the cellulose in hydrolysis to monomeric sugars.
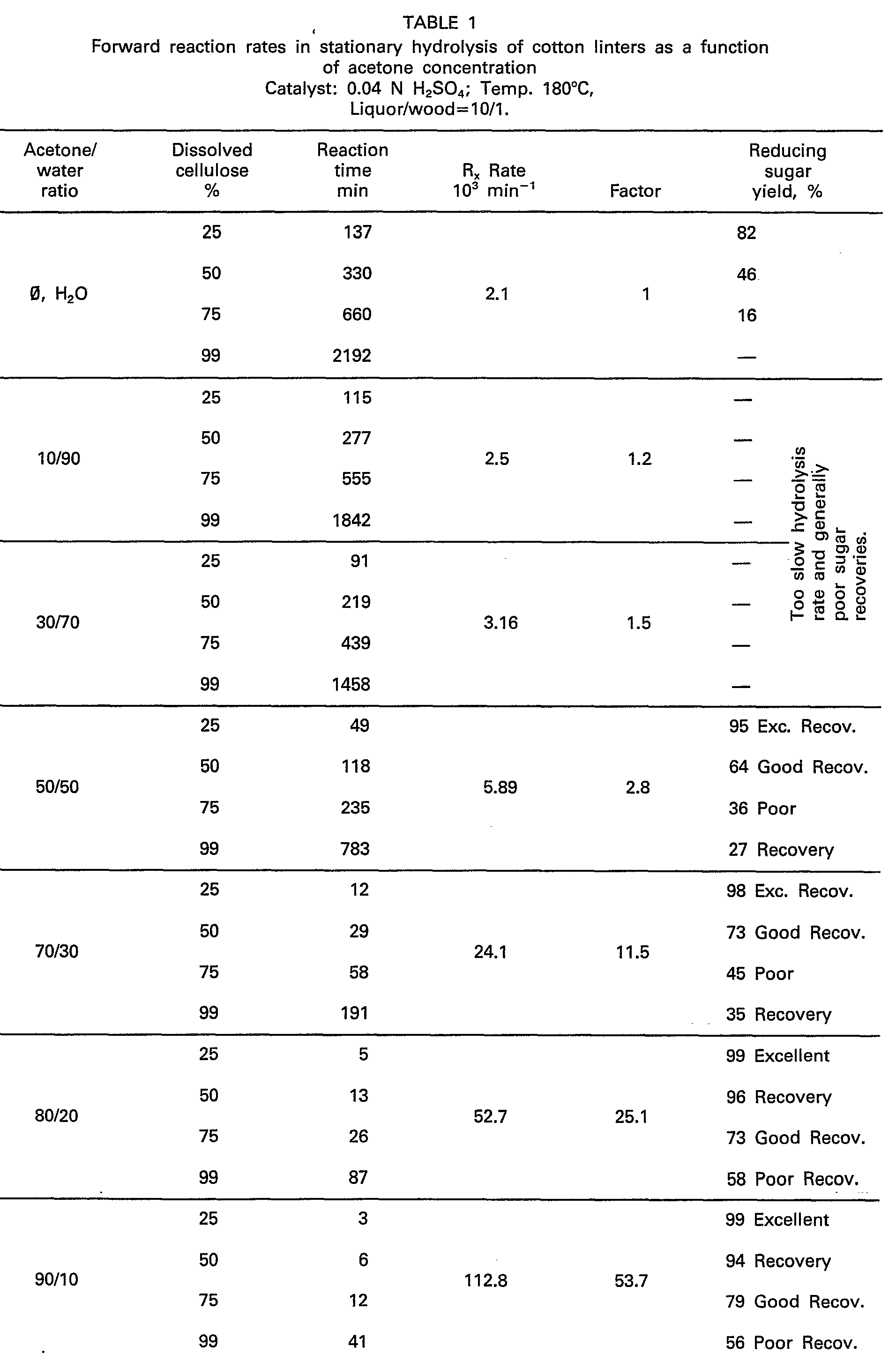
- As evidenced from Table I, hydrolysis rates improved constantly as the acetone concentration increased to 50 percent. However, significant improvements were observed only as the acetone concentration was raised above 70 percent by volume of the acidified solvent mixture. Very rapid hydrolysis rates were obtained with nearly anhydrous acetone solutions. The dissolved sugars were found to be most stable when using a solvent mixture of between 80 to 90 percent acetone even though the relative half lives were relatively short. Sugar survivals over 90 percent are obtained as long as the reaction time at temperature is kept below that required for hydrolyzing 50 percent of the substrate to dissolved products. The time required to hydrolyze 50 percent of the substrate to dissolved products is called half life of sugar survival. This criteria holds regardless of what stage of hydrolysis is considered. The solvent effect both on the hydrolysis rate and sugar survival for limited hydrolysis times was the most surprising discovery of the present invention whereby maxima were found around 80 to 90 percent acetone concentration in the reaction mixture. At higher acetone concentrations, the response of the hydrolysis rate to increase in temperature and acid concentration was observed to follow well known kinetic principles in contrast to both the aqueous dilute acid and acidified aqueous ethanol systems in which the balance of increase in higher hydrolysis rates and sugar degradation did not improve with an increase in these parameters especially that of the temperature. The improved sugar survival with increase in acetone concentration is attributed to formation of acetone sugar complexes which have improved stability at high temperature. The complexes are very readily and safely hydrolyzable to free sugars on heating with dilute acid at 100°C for a limited amount of time.
- In identical stationary acidified ethanol-water cooks, in which the ethanol concentration was higher than 80 percent neither delignification nor hydrolysis was obtained due to the fact that the acid catalyst was quickly consumed by reaction with the alcohol by formation of ethyl hydrogen sulfate (C2H5-O-S02-OH) and formation of diethyl ether via condensation of two ethanol molecules. Ether formation was quite substantial under these conditions. Also alkyl glucosides formed in high concentration alcohol solutions are substantially more difficult to hydrolyse to free sugars than the corresponding acetone complexes, and alcoholysis results in oligomeric sugars rather than monomers as is the case in acetone-water solutions. Thus alcohols prove to be largely unsuited for hydrolysis media due to the unwanted solvent loss and general danger from the explosive ether. With lignified materials the low delignification power of acidified alcohol solutions is clearly a drawback. With 80:20 ethanol:water cooks in the presence of 0.190 percent (0.04 Normal) sulfuric acid at 180°C the hydrolysis rate was 5.47x 103 min-1 and the half life of cotton linters decomposition was 126.8 minutes. A maximum of 76 percent could be dissolved in 254 minutes, the crystalline residue showing substantial resistance to hydrolysis in the alcoholic solvent. Residual acid concentration was found to be one fourth of that originally applied, i.e., 0.01 Normal, the balance possibly consumed in the various side reactions.
- It is evident from the data that under identical hydrolysis conditions excessively long hydrolysis times are required for complete dissolution of cotton linters both by acidified water and acidified aqueous ethanol media. An increase of the ethanol concentration from 50 percent to 80 percent did not improve the hydrolysis rate or improve particularly the sugar survival. The hydrolysis rate in ethanol water was only marginally better than in dilute acid in water.
- These examples clearly show that a high acetone concentration over 70 percent is mandatory for high speed hydrolysis and high sugar survival. Under the conditions indicated for sugar recoveries better than 90 percent, reaction times (or high temperature exposure times of less than indicated for half lives are preferred). Thus according to these data, total saccharification and quantitative sugar recovery would dictate a percolation or pass through process wherein the liquor residence time would not exceed 10 minutes when 80:20 acetone:water with 0.04 Normal sulfuric acid is used as solvent mixture at 180°C temperature. The residence time would have to be substantially shortened when higher temperatures and larger acid concentrations are used as shown in the following examples.
-
- The effect of acid concentration on the rate of hydrolysis and sugar survival in 80:20 acetone:water solvent mixtures was studied at 180°C temperature using cotton linters as substrate.
- In stationary cooks one gram samples (oven dry) of cotton linters were hydrolyzed in glass lined stainless steel pressure vessels along with 10 ml of the appropriate hydrolysis liquor and heated until the original substrate mass was hydrolyzed and dissolved. The levels of 25, 50, 75 and 99 percent of hydrolysis were determined by graphing as in Example I.
- Work-up of the reaction products followed the same procedure as outlined in Example I. The results are indicated in Table 2.
- Increased acid concentration resulted in higher hydrolysis rates within the range studied and a somewhat faster degradation of the sugars as the single stage hydrolysis times exceeded those indicated as half lives for the solid residue. Equal concentrations of sulfuric and hydrochloric acid were found to give largely comparable results. The increased acid concentrations showed a substantial hydrolysis accelerating effect as evidenced by the rapidly decreasing half lives. Thus the hydrolysis rate can be readily controlled by limited acid concentrations, all other conditions being held constant.
- Temperature effects on hydrolysis of cotton linters were studied with acidified aqueous acetone solutions containing 0.04 Normal sulfuric acid in 80:20 acetone:water at different hydrolysis times so that weight losses of 25, 50, 75 and 99 percent could be determined as in Example I. All cooks were preconditioned to 35°C before being placed in the oil bath to minimize the effect of heating-up time at the various temperature levels studied.
- Work-up of the products and analysis followed the same procedure as described in Example I and the results are summarized in Table 3.
- The data indicate that increased temperature had the most profound accelerating effect of the hydrolysis rate and generally in such single stage batch cooks reaction times exceeding sugar dissolution half lives at any stage of the hydrolysis increased somewhat the rate of sugar degradation at the higher temperature regimes used. However, it was learned that such high temperature hydrolyses afford practically instantaneous high-yield hydrolysis to be carried out on even such difficult to hydrolyze substrate as cotton linters. The rate of sugar degradation can be offset somewhat by lowering the acid concentration and by increasing the liquor to wood ratio whereby the forward reaction rate (k,) in hydrolysis remains unaffected but the sugar degradation rate (k2) is lowered. Thereby sugar survival, which depends on the ratio of k,/k2 is largely improved especially if high acetone concentrations are used.
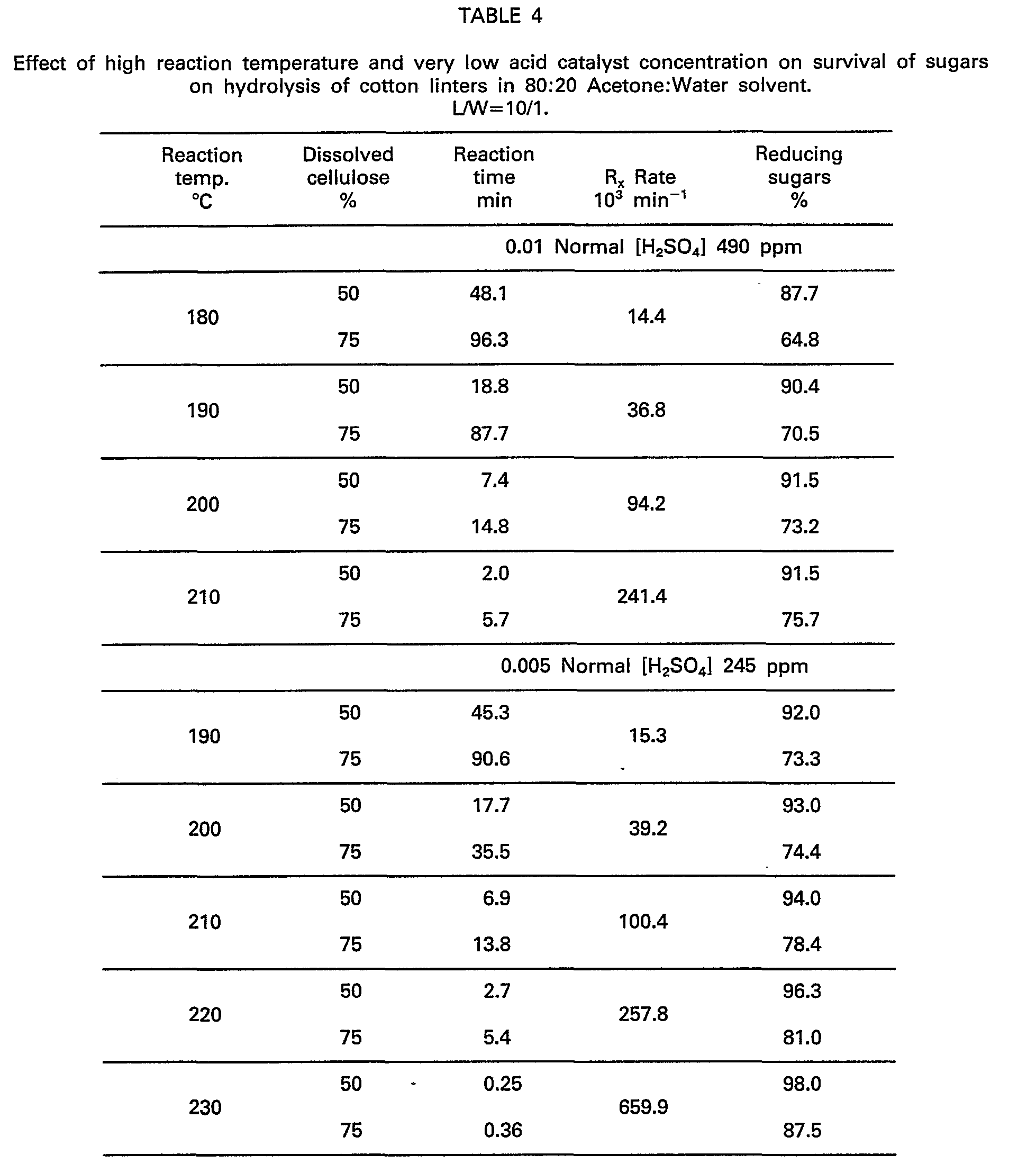
- Cooks reported in this example explore the hitherto unobserved relationship of increasing the sugar survival at reduced acid concentration and increased reaction temperatures without any reduction in the high hydrolysis rates disclosed herein. This unusual discovery is demonstrated in the data of Table 4.
- The effect of reduced acid concentration but high reaction temperature is demonstrated by cooking one gram samples of cotton linters (oven-dry weight) in glass lined stainless steel pressure vessels along with 10 ml of 80:20 acetone:water cooking liquor containing 0.01 and 0.005 Normal H2S04 with respect to the solvent mixture, and heated until 50 percent and 75 percent dissolution of the substrate was obtained at 190 to 220°C reaction temperature.
- Cooling and work-up of the reaction products to determine sugar survival and reaction rates were performed as outlined in Example I..
- The data indicate that acid concentration can be successfully reduced and traded by increasing the reaction temperature without loss in reaction rate with a concomittant increase in sugar yield (survival) when hydrolysis liquors of at least 80 percent acetone content are used. Such a trend is clearly against all previously published scientific results (Seamen, J.F., ACS, Honolulu 1979; Bio-Energy, Atlanta 1980) where the increase in hydrolysis rates and sugar survival was a function of both increased acid concentration and higher temperature. The surprising solvent effect of the acetone water system has never been observed or reported in scientific literature or the prior art before.
- One gram samples of several wood species were hydrolyzed in 80:20 acetone:water containing 0.04 Normal sulfuric acid at 180°C. Hydrolysis rates were calculated only for the crystalline cellulose fractions to avoid the confounding effect of easily hydrolyzable lignin and hemicelluloses. Times to mass losses of 25, 50, 75 and 99 percent of the original oven dry mass along with the calculated reaction rates are recorded in Table 4.
- Work-up of the products followed the same procedure as indicated in Example I except that after removal of the volatiles by distillation it was necessary to remove the precipitated lignins by filtration or centrifuging.
- It is quite evident that under identical conditions the hydrolysis rates for wood are roughly twice that of cotton linters. Due to the increased forward reaction rates sugar recoveries became quite impressive indeed.
- The rate of Douglas-fir hydrolysis was somewhat slower than that of aspen and sugarcane rind. However, when hydrolysis in a purely aqueous system was attempted under otherwise exactly matching conditions (same temperature and acid catalyst content) a hydrolysis rate of 0.5x103 min-1 was obtained and only 6 percent weight loss was recorded for a 280 min long cook at 180°C the usual dilute acid hydrolysis temperature. Thus the high acetone content hydrolysis liquor allowed at least 100 times faster hydrolysis of Douglas fir by simultaneous dissolution of the lignin than possible in purely aqueous systems.
- Among the products of partial saccharification of wood, solid residues of about 30 to 35% yield are pure white, devoid of residual lignin. This cellulosic fraction has a crystallinity index of 80% from aspen wood and a degree of polymerization (DP) of between 80 to 280. Similar results are obtained with the other wood species.
- It is found to be a further advantage of the present invention that the high acetone concentration clearly favors formation of relatively stable acetone-sugar complexes in spite of the presence of water. The better stability of the sugar complexes at high temperature profoundly affects survival of the dissolved sugars. The improvements are quite evident from the data in Table 1.
- Further due to the differences in volatility and solubility of the various sugar complexes the invention allows facile segregation and nearly quantitative isolation of the five major wood sugars, if so desired. However, due to the mixed nature of the sugar derivatives in aqueous hydrolyzates, if such thorough and detailed separation is desired, it is always necessary to neutralize the recovered aqueous sugar wort after removal of the volatiles and concentrate the wort to a syrup. The syrup is then redissolved in anhydrous acetone containing 3 percent acid, allowed to stand at least 6 hr until all sugars formed their respective di-acetone complexes before attempting the detailed separation as described below. The separated sugar complexes are readily hydrolyzed in dilute acid on boiling at least 20 to 40 minutes.
- Thus 10 g (OD) coarse aspen wood sawdust (passing a 5 mesh screen) was charged with 100 ml of hydrolyzing liquor made up to 80:20 acetone:water and 0.04 Normal sulfuric acid as catalyst. The bomb was brought to 180°C temperature by immersing it into a hot glycerol bath within 9 min and heating was continued until the required reaction times were reached.
- In another larger bomb 450 ml of hydrolysis liquor containing 80:20 acetone:water and 0.04 Normal sulfuric acid was also preheated and connected through a syphon tube and shut-off valve to the reaction vessel. Following three minutes at reaction temperature (9+3=12 min total) the reaction liquor was drained into a small beaker containing 75 g crushed ice. The reaction vessel was immediately recharged with hot liquor from the stand-by vessel and the reaction was allowed to proceed for an additional 3 minutes before again discharging the reactor contents as above. In all, five liquor changes were effected and the liquors collected for analysis. The chilled reactor contents were analyzed as follows:
- Hydrolysate No. 1 and 2 were combined before evaporation of the low boiling volatiles. Flash evaporation of the acetone at low temperature (50°C) and reduced pressure resulted in precipitation of a flocculant lignin which aggregated to small clusters of granules on standing. The lignin was carefully filtered off the mother liquor, washed with two portions of water and dried in vacuo to constant weight as a powder. The lignin powder collected weighed 1.67 g and had a weight average molecular weight of 2800.
- The combined filtrate (127 ml) was neutralized and subjected to steam distillation in an all glass apparatus and approximately 35 ml distillate was collected. Both the distillate and residual solution were made up to 100 ml and 0.5 ml portions of each were acidified with sulfuric acid to 3 percent acid and boiled for 40 min on a water bath. The solutions were neutralized and the sugar reducing power determined by the Somogyi method. The yield of sugars was 1.89 g in the distillate and 1.96 g from the residual liquor.
- Gas chromatographic determination of alditol acetates of the sugars from the steam distillate indicated mainly xylose and arabinose whereas from the residual solution glucose, mannose and galactose with only minor traces of xylose were indicated.
- Hydrolysate No. 3 contained only traces of lignin after evaporation of the acetone solvent too small to collect and determine gravimetrically. It was removed by centrifuging. The aqueous residue (97 ml) was acidified to 3 percent acid with sulfuric acid, boiled for 40 min and after neutralization filtered and made up to 100 ml. The reducing sugar content of the filtrate was determined by the Somogyi method to be 1.83 g. GC analysis of the alditol acetates determined on an aliquot sample indicated mainly glucose with traces of mannose and galactose.
- Hydrolysate No. 4 and 5 were processed and analyzed in the same manner as No. 3. H-4 yielded 1.73 g reducing sugars and H-5 yielded 1.40 g sugars both being composed only of glucose as evidenced by GC analysis of an aliquot sample.
- The undissolved residue was 0.12 g following 2 h drying in an oven at 105°C.
-
- In a similar hydrolysis arrangement to Example VI 10 g OD Douglas-fir sawdust (to pass a 10 mesh screen), pre-extracted with dichloromethane and air dried to 8 percent moisture content in a controlled humidity room, was hydrolyzed with 80:20 acetone:water solvent containing 0.05 Normal Hydrochloric acid in five consecutive steps. Each reaction step consisted of three minutes at a reaction temperature of 200°C. The heating up time was 7 minutes. Again Hydrolysate No. 1 and 2 were combined whereas the subsequent fractions were analyzed separately.
- The combined liquor of H-1 and H-2 yielded 2.39 g lignin on low temperature evaporation of the volatiles and 135 ml of aqueous liquor was collected on filtration of the powdered lignin. The dried lignin had a weight average molecular weight of 3200. The filtrate was neutralized to pH 8 and subjected to steam distillation in all glass apparatus. The 28 ml distillate which was collected contained 0.62 g pentoses which after passing the filtrate through a cation exchange resin in the acid form and repeated steam distillation of the filtrate yielded 0.58 g xylose as determined by GC analysis.
- The residue remaining behind after the above steam distillation (128 ml) was neutralized on an ion exchange column, the filtrate concentrated to a syrup, seeded with some crystalline mannose and left standing overnight. The crystalline material was collected by filtration and recrystallized from ethanol-petroleum ether. The crystals were re-dissolved in water, acidified to 3 percent acid and boiled for 40 min to liberate the free sugars. After neutralization with silver carbonate the solution was analyzed by GC alditol acetates to determine the sugar concentration. The only sugar detected by GC was mannose and the yield was calculated as 1.00 g.
- The ethanol-petroleum ether solution was extracted with 5 ml portions of water and the collected aqueous layer combined with the syrup removed from the crystalline product above. The solution was briefly heated to expel the alcohol, made up to 3 percent acid with hydrochloric acid, boiled for 40 min, neutralized with silver carbonate and alditol acetates were prepared for GC analysis. The combined syrup and filtrate contained a total of 58 g sugars of which 0.29 g was galactose, 0.25 g was glucose and 0.04 g was mannose.
- Hydrolysate No. 3 gave 1.89 g pure glucose with 0.4 g of lignin precipitate on removal of the volatiles.
- Hydrolysate No. 4 gave 1.66 g of pure glucose with only very small traces of lignin, whereas H-5 gave 1.85 g of glucose and no lignin. The undissolved residue was 0.18 g and was composed of 99 percent glucose.
-
- Total Sugar Recovery: 7.60 g=95.95% (of theoretical)
- Lignin Recovery: 98%
- Under large scale industrial conditions chilling of the recovered sugar solutions is best accomplished by controlled flash evaporation of the volatiles. Cooling of the liquor samples outside of the pressure vessel in Examples VI and VII with crushed ice was adapted as matter of convenience for small scale treatments.
Claims (4)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT82900956T ATE23364T1 (en) | 1981-03-26 | 1982-03-26 | VERY EFFECTIVE SUGARING PROCESS USING ORGANIC SOLVENTS. |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US06/248,023 US4409032A (en) | 1977-08-31 | 1981-03-26 | Organosolv delignification and saccharification process for lignocellulosic plant materials |
| CA395820 | 1982-02-09 | ||
| CA000395820A CA1201115A (en) | 1981-03-26 | 1982-02-09 | High efficiency organosolv saccharification process |
| US248023 | 1994-05-24 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0074983A1 EP0074983A1 (en) | 1983-03-30 |
| EP0074983B1 true EP0074983B1 (en) | 1986-11-05 |
Family
ID=25669563
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP19820900956 Expired EP0074983B1 (en) | 1981-03-26 | 1982-03-26 | High efficiency organosolv saccharification process |
Country Status (6)
| Country | Link |
|---|---|
| EP (1) | EP0074983B1 (en) |
| JP (1) | JPH0785720B2 (en) |
| AR (1) | AR227462A1 (en) |
| BR (1) | BR8207243A (en) |
| DE (1) | DE3274120D1 (en) |
| WO (1) | WO1982003409A1 (en) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| HU197774B (en) * | 1983-02-16 | 1989-05-29 | Laszlo Paszner | Organic solvent process for the hydrolytic saccharification of vegetable materials of starch type |
| BR9600672A (en) * | 1996-03-08 | 1997-12-30 | Dedini S A Administracao E Par | Acid hydrolysis process of lignocellulosic material and hydrolysis reactor |
| BRPI0505212A (en) * | 2005-11-01 | 2007-08-07 | Dedini Sa Ind De Base | improvements in fast acid hydrolysis process of lignocellulosic material and hydrolysis reactor |
| ES2545754T3 (en) | 2006-10-26 | 2015-09-15 | Kawasaki Jukogyo Kabushiki Kaisha | Method and system for hydrolytic saccharification of a cellulosic biomass |
| NZ580751A (en) * | 2007-05-31 | 2012-08-31 | Lignol Innovations Ltd | Continuous counter-current organosolv processing of lignocellulosic feedstocks |
| JP2010081917A (en) * | 2008-10-02 | 2010-04-15 | Toyota Motor Corp | Biomass treatment method and method for producing organic acid or alcohol |
| CA2818041C (en) * | 2010-11-25 | 2015-10-13 | Studiengesellschaft Kohle Mbh | Method for the acid-catalyzed depolymerization of cellulose |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB416549A (en) * | 1933-03-07 | 1934-09-07 | Henry Dreyfus | Improvements in the manufacture of cellulose and lignocellulosic materials |
| CH202534A (en) * | 1937-05-27 | 1939-01-31 | F Reemtsma Philipp | Process for the production of a sugar-containing product not intended for feeding people or animals by digesting cellulose-containing masses by means of hydrogen halide in the presence of a liquid organic solvent for the latter. |
| DE2737118A1 (en) * | 1977-08-17 | 1979-03-01 | Projektierung Chem Verfahrenst | METHOD FOR OBTAINING SUGAR, CELLULOSE AND LIGNIN, WHEREAS, FROM LIGNOCELLULOSIC VEGETABLE RAW MATERIALS |
| JPS5526279A (en) * | 1978-08-11 | 1980-02-25 | Paszner Laszlo | Treatment of lignocellulose containing substance |
| CA1150012A (en) * | 1980-07-25 | 1983-07-19 | Pei-Ching Chang | Aqueous catalysed solvent pulping of lignocellulose |
-
1982
- 1982-03-23 AR AR28884882A patent/AR227462A1/en active
- 1982-03-26 JP JP57501134A patent/JPH0785720B2/en not_active Expired - Lifetime
- 1982-03-26 DE DE8282900956T patent/DE3274120D1/en not_active Expired
- 1982-03-26 BR BR8207243A patent/BR8207243A/en not_active IP Right Cessation
- 1982-03-26 WO PCT/EP1982/000068 patent/WO1982003409A1/en not_active Ceased
- 1982-03-26 EP EP19820900956 patent/EP0074983B1/en not_active Expired
Also Published As
| Publication number | Publication date |
|---|---|
| EP0074983A1 (en) | 1983-03-30 |
| WO1982003409A1 (en) | 1982-10-14 |
| BR8207243A (en) | 1983-03-01 |
| DE3274120D1 (en) | 1986-12-11 |
| AR227462A1 (en) | 1982-10-29 |
| JPS58500431A (en) | 1983-03-24 |
| JPH0785720B2 (en) | 1995-09-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4470851A (en) | High efficiency organosolv saccharification process | |
| AU579094B2 (en) | Improved organosolv process for hydrolytic decomposition of lignocellulosic and starch materials | |
| US5395455A (en) | Process for the production of anhydrosugars from lignin and cellulose containing biomass by pyrolysis | |
| US5730837A (en) | Method of separating lignocellulosic material into lignin, cellulose and dissolved sugars | |
| US3212933A (en) | Hydrolysis of lignocellulose materials with solvent extraction of the hydrolysate | |
| Dominguez et al. | Dilute acid hemicellulose hydrolysates from corn cobs for xylitol production by yeast | |
| EP0824616B1 (en) | A process for rapid acid hydrolysis of lignocellulosic material and hydrolysis reactor | |
| US4160695A (en) | Process for the production of glucose from cellulose-containing vegetable raw materials | |
| CA1100266A (en) | Organosolv delignification and saccharification process for lignocellulosic plant materials | |
| US5125977A (en) | Two-stage dilute acid prehydrolysis of biomass | |
| SE440086B (en) | PROCEDURE FOR THE EXTRACTION OF SUGAR, LIGNIN AND EVEN CELLULOSA FROM LIGNOCELLULOSOUS VEGETABLE | |
| US2959500A (en) | Process for the saccharification of cellulose and cellulosic materials | |
| US4181796A (en) | Process for obtaining xylan and fibrin from vegetable raw material containing xylan | |
| JP2010531215A (en) | Biomass component separation process | |
| CA1105457A (en) | Process for the winning of xylose by hydrolysis of residues of annuals | |
| NO312070B1 (en) | Process of a process for the production of processable sugar from cellulosic raw materials | |
| EP0074983B1 (en) | High efficiency organosolv saccharification process | |
| SU1701115A3 (en) | Method of producing sugar from cellulose containing stock | |
| BR102022005698A2 (en) | PROCESS FOR TREATMENT OF LIGNOCELLULOSIC BIOMASS | |
| CA1201115A (en) | High efficiency organosolv saccharification process | |
| US4916242A (en) | Combined process for thermally and chemically treating lignocellulose-containing biomass and for producing furfural | |
| EP0096497B1 (en) | Solubilisation and hydrolysis of cellulose-containing materials | |
| EP3822407B1 (en) | Process for the production of bioproducts from lignocellulosic material | |
| RU2740098C1 (en) | Method for hydrolysis of hemicelluloses of plant materials for producing xylose solutions | |
| Aravamuthan et al. | Ethanol from southern hardwoods: the role of presulfonation in the acid hydrolysis process |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB LI NL SE |
|
| 17P | Request for examination filed |
Effective date: 19830317 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE FR GB LI NL SE |
|
| REF | Corresponds to: |
Ref document number: 23364 Country of ref document: AT Date of ref document: 19861115 Kind code of ref document: T |
|
| REF | Corresponds to: |
Ref document number: 3274120 Country of ref document: DE Date of ref document: 19861211 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| EAL | Se: european patent in force in sweden |
Ref document number: 82900956.2 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19950925 Year of fee payment: 14 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19951130 Year of fee payment: 14 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Effective date: 19960326 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 19960326 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19961203 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19980925 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19980928 Year of fee payment: 17 Ref country code: CH Payment date: 19980928 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19980930 Year of fee payment: 17 Ref country code: AT Payment date: 19980930 Year of fee payment: 17 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990326 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990327 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990331 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990331 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990331 |
|
| BERE | Be: lapsed |
Owner name: BAU- UND FORSCHUNGSG- THERMOFORM A.G. Effective date: 19990331 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19991011 Year of fee payment: 19 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 82900956.2 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19991130 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 82900956.2 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20011001 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 20011001 |