EP0023402B1 - Fluidized catalytic cracking with reduced dilute-phase temperature and/or composition gradients in the regenerator - Google Patents
Fluidized catalytic cracking with reduced dilute-phase temperature and/or composition gradients in the regenerator Download PDFInfo
- Publication number
- EP0023402B1 EP0023402B1 EP19800302404 EP80302404A EP0023402B1 EP 0023402 B1 EP0023402 B1 EP 0023402B1 EP 19800302404 EP19800302404 EP 19800302404 EP 80302404 A EP80302404 A EP 80302404A EP 0023402 B1 EP0023402 B1 EP 0023402B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- catalyst
- regeneration
- bed
- zone
- gas
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- 239000000203 mixture Substances 0.000 title claims description 27
- 238000004523 catalytic cracking Methods 0.000 title claims description 9
- 239000003054 catalyst Substances 0.000 claims description 255
- 230000008929 regeneration Effects 0.000 claims description 196
- 238000011069 regeneration method Methods 0.000 claims description 196
- 239000007789 gas Substances 0.000 claims description 120
- 229930195733 hydrocarbon Natural products 0.000 claims description 91
- 150000002430 hydrocarbons Chemical class 0.000 claims description 91
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 63
- 239000001301 oxygen Substances 0.000 claims description 63
- 229910052760 oxygen Inorganic materials 0.000 claims description 63
- 238000006243 chemical reaction Methods 0.000 claims description 59
- 238000012546 transfer Methods 0.000 claims description 36
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 claims description 33
- 238000000034 method Methods 0.000 claims description 33
- 239000004215 Carbon black (E152) Substances 0.000 claims description 32
- 230000008569 process Effects 0.000 claims description 26
- 238000005336 cracking Methods 0.000 claims description 20
- 238000002485 combustion reaction Methods 0.000 claims description 19
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 claims description 10
- 229910002091 carbon monoxide Inorganic materials 0.000 claims description 9
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 claims description 6
- 229910052757 nitrogen Inorganic materials 0.000 claims description 5
- 229910021529 ammonia Inorganic materials 0.000 claims description 3
- 239000001257 hydrogen Substances 0.000 claims description 3
- 229910052739 hydrogen Inorganic materials 0.000 claims description 3
- 230000001172 regenerating effect Effects 0.000 claims description 3
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims 1
- 239000012071 phase Substances 0.000 description 98
- 239000003546 flue gas Substances 0.000 description 26
- 239000002245 particle Substances 0.000 description 15
- 238000000926 separation method Methods 0.000 description 14
- 239000000571 coke Substances 0.000 description 13
- 229910002092 carbon dioxide Inorganic materials 0.000 description 12
- 230000000694 effects Effects 0.000 description 11
- 239000003921 oil Substances 0.000 description 10
- 229910052799 carbon Inorganic materials 0.000 description 9
- 239000000463 material Substances 0.000 description 9
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 8
- 238000004231 fluid catalytic cracking Methods 0.000 description 7
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 6
- 230000001105 regulatory effect Effects 0.000 description 6
- 239000007787 solid Substances 0.000 description 6
- 239000012530 fluid Substances 0.000 description 5
- 238000002347 injection Methods 0.000 description 5
- 239000007924 injection Substances 0.000 description 5
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 4
- 229910000323 aluminium silicate Inorganic materials 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 239000002808 molecular sieve Substances 0.000 description 4
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 4
- 238000010276 construction Methods 0.000 description 3
- 238000013461 design Methods 0.000 description 3
- 238000010586 diagram Methods 0.000 description 3
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 3
- CPLXHLVBOLITMK-UHFFFAOYSA-N Magnesium oxide Chemical compound [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 2
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 2
- 230000003197 catalytic effect Effects 0.000 description 2
- 230000001276 controlling effect Effects 0.000 description 2
- 230000009849 deactivation Effects 0.000 description 2
- 230000002950 deficient Effects 0.000 description 2
- 238000005243 fluidization Methods 0.000 description 2
- 239000007792 gaseous phase Substances 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 125000004435 hydrogen atom Chemical class [H]* 0.000 description 2
- 239000003701 inert diluent Substances 0.000 description 2
- 239000011261 inert gas Substances 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 239000000377 silicon dioxide Substances 0.000 description 2
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000010426 asphalt Substances 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 230000005587 bubbling Effects 0.000 description 1
- UNYSKUBLZGJSLV-UHFFFAOYSA-L calcium;1,3,5,2,4,6$l^{2}-trioxadisilaluminane 2,4-dioxide;dihydroxide;hexahydrate Chemical compound O.O.O.O.O.O.[OH-].[OH-].[Ca+2].O=[Si]1O[Al]O[Si](=O)O1.O=[Si]1O[Al]O[Si](=O)O1 UNYSKUBLZGJSLV-UHFFFAOYSA-L 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 239000003575 carbonaceous material Substances 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 229910052676 chabazite Inorganic materials 0.000 description 1
- 239000003245 coal Substances 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 239000010779 crude oil Substances 0.000 description 1
- 239000002178 crystalline material Substances 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 229910001882 dioxygen Inorganic materials 0.000 description 1
- 238000007599 discharging Methods 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 230000003628 erosive effect Effects 0.000 description 1
- 239000012013 faujasite Substances 0.000 description 1
- 239000000446 fuel Substances 0.000 description 1
- 239000003502 gasoline Substances 0.000 description 1
- 235000003642 hunger Nutrition 0.000 description 1
- 238000005984 hydrogenation reaction Methods 0.000 description 1
- 238000005470 impregnation Methods 0.000 description 1
- -1 kerogen Substances 0.000 description 1
- 239000000395 magnesium oxide Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229910044991 metal oxide Inorganic materials 0.000 description 1
- 150000004706 metal oxides Chemical class 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 238000003801 milling Methods 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 238000005120 petroleum cracking Methods 0.000 description 1
- JTJMJGYZQZDUJJ-UHFFFAOYSA-N phencyclidine Chemical class C1CCCCN1C1(C=2C=CC=CC=2)CCCCC1 JTJMJGYZQZDUJJ-UHFFFAOYSA-N 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 238000011027 product recovery Methods 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 239000003870 refractory metal Substances 0.000 description 1
- 239000003079 shale oil Substances 0.000 description 1
- 239000011949 solid catalyst Substances 0.000 description 1
- 230000037351 starvation Effects 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G11/00—Catalytic cracking, in the absence of hydrogen, of hydrocarbon oils
- C10G11/14—Catalytic cracking, in the absence of hydrogen, of hydrocarbon oils with preheated moving solid catalysts
- C10G11/18—Catalytic cracking, in the absence of hydrogen, of hydrocarbon oils with preheated moving solid catalysts according to the "fluidised-bed" technique
- C10G11/187—Controlling or regulating
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G11/00—Catalytic cracking, in the absence of hydrogen, of hydrocarbon oils
- C10G11/14—Catalytic cracking, in the absence of hydrogen, of hydrocarbon oils with preheated moving solid catalysts
- C10G11/18—Catalytic cracking, in the absence of hydrogen, of hydrocarbon oils with preheated moving solid catalysts according to the "fluidised-bed" technique
Definitions
- the present invention relates to the regeneration of catalysts employed in a fluid catalytic cracking process. More particularly, this invention relates to the combustion of volatile hydrocarbons in mixture with spent fluid catalytic cracking catalyst prior to said mixture entering the regeneration zone.
- the fluidized catalytic cracking of hydrocarbons is well known in the prior art and may be accomplished in a variety of processes which employ fluidized solid techniques. Normally in such processes, suitably preheated, relatively high molecular weight hydrocarbon liquids and/or vapors are contacted with hot, finely- divided, solid catalyst particles either in a fluidized bed reaction zone or in an elongated riser reaction zone, and maintained at an elevated temperature in a fluidized state for a period of time sufficient to effect the desired degree of cracking to lower molecular weight hydrocarbons typical of those present in motor gasolines and distillate fuels.
- the catalyst is transferred from the reaction zone into a regeneration zone.
- Typical regeneration zones comprise large vertical cylindrical vessels wherein the spent catalyst is maintained as a fluidized bed by the upward passage of an oxygen-containing regeneration gas, such as air.
- the fluidized catalyst forms a dense phase catalyst bed in the lower portion of the vessel and a dilute catalyst phase containing entrained catalyst particles above, with an interface existing between the two phases.
- the catalyst is contacted with the oxygen-containing regeneration gas under conditions sufficient to burn at least a portion, preferably a major portion, of the coke from the catalyst.
- Flue gas which normally comprises gases arising from the combustion of the coke on the spent catalyst, inert gases such as nitrogen from air, any unconverted oxygen and entrained catalyst particles, is then passed from the dilute catalyst phase into solid-gas separation means within the regeneration zone (e.g., cyclone separators) to prevent excessive losses of the entrained catalyst particles.
- the catalyst particles separated from the flue gas are returned to the dense phase catalyst bed.
- a substantially catalyst-free flue gas may then be passed from the separation means to equipment downstream thereof, for example to a plenum chamber, or be discharged directly from the top of the regeneration zone.
- the regenerated catalyst is subsequently withdrawn from the regeneration zone and reintroduced into the reaction zone for reaction with additional hydrocarbon feed.
- spent catalyst from the reaction zone is passed therefrom to a stripping zone for removal of volatile hydrocarbons from the catalyst particles prior to transferring the catalyst to the regeneration zone.
- the volatile hydrocarbons not recovered as product from the reaction zone will pass with the spent catalyst into the regeneration zone wherein they are combusted in preference to the carbon on the spent catalyst. This results in exhaustion of the oxygen in the regeneration gas in the area where the spent catalyst and volatile hydrocarbons enter the regeneration zone.
- the spent catalyst and volatile hydrocarbons enter the regeneration zone at an off-center location to avoid interference with the regeneration overflow well and/or auxiliary heating air section.
- one area of the dense phase bed is essentially starved of oxygen such that CO rather than C0 2 will be formed.
- an excess of oxygen is present in the remaining portion of the dense phase bed since volatile hydrocarbons are not present therein.
- Such high temperatures in the dilute catalyst phase can cause deactivation of the small amounts of catalyst still present, thereby requiring additional catalyst replacement to the process in order to maintain a desired catalytic activity in the hydrocarbon reaction zone. Additionally, these high temperatures may cause damage to mechanical components of the regeneration zone, particularly in that portion of the regeneration zone in contact with the substantially catalyst-free flue gas wherein the temperature may increase to 982.1 °C (1800°F) or greater.
- Such high temperatures are realized because the reaction shown in equation (1) proceeds rapidly within the substantially catalyst-free flue gas since there is very little entrained catalyst present to absorb the heat released, and thereby reduce the rise in temperature. Thus, in that portion of the regeneration zone wherein the flue gas is substantially catalyst-free, there will occur a rapidly accelerating rise in temperature due to the heat released as complete combustion of carbon monoxide occurs in the absence of any means to moderate the temperature therein.
- US-A-4062762 describes a fluidized catalytic cracking process comprising:
- the reaction zone is contained in a reactor vessel and the regeneration zone is contained in a regenerator vessel.
- Coke-contaminated catalyst circulates from the bottom region of the reactor vessel to the regeneration zone via a U-shaped transfer-line which serves to prevent the transfer of gases and vapours between the vessels.
- a gas or vapour is passed into the bottom region of the vertical riser arm of the 'U' beneath the regenerator vessel to reduce the density of catalyst in the vertical riser arm relative to the density of catalyst in the other vertical arm of the 'U' beneath the reactor vessel whereby catalyst is propelled upwardly into the regeneration zone by an action analogous to that of a water lift pump.
- Air is used for convenience and low cost as the gas for reducing the density of catalyst to promote circulation, and, as indicated, the air is passed into the bottom region of the vertical riser arm.
- the circulation of hot regenerated catalyst from the regenerator vessel to the reaction zone is similarly effected through a second U-shaped transfer-line, and the hydrocarbon feed is passed into one arm of the transfer-line adjacent to the reactor vessel at a level lower than the top level of the reaction zone so that the vapours generated by the contact of the feed with the hot regenerated catalyst passing to the reaction zone reduce the density of the regenerated catalyst thereby enhancing catalyst circulation through the second U-shaped transfer-line to the reaction zone from the regenerator vessel.
- the present invention provides a fluidized catalytic cracking process which comprises:
- the present invention is based on the discovery that the formation of CO in the dense phase catalyst bed of the regeneration zone of a fluid catalytic cracking process due to the volatile hydrocarbons in the spent catalyst mixture entering said regeneration zone may be reduced and/or minimized by using a minor portion of the regeneration gas to combust said hydrocarbons prior to said mixture entering the regeneration zone or to combust said hydrocarbons at or near the point where the mixture is released into the regeneration zone.
- the amount of regeneration gas used to combust the hydrocarbons is not critical, but will be within the range of from 2 to 20% of the regeneration gas normally employed.
- combustion of the hydrocarbons will occur in the line transferring spent catalyst from the reaction zone to the regeneration zone.
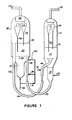
- Figures 1 and 2 disclose a catalyst cracking system in which the catalyst circulation rate is controlled either by adjusting the differential pressure between the zones by suitable control means or by varying the catalyst density in the riser entering the regeneration zone.
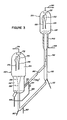
- Figure 3 illustrates a system in which the catalyst flow rate between the reaction and regeneration zones is controlled by a slide valve.
- the subject invention is not limited to these type systems and is equally applicable to other type cracking systems and to other zone configurations and positions such as upflow and downflow regeneration systems with and without slide valves.
- Various items such as valves, pumps, compressors, steam lines, instrumentation and other process equipment and control means have been omitted from the figures for the sake of simplicity. Variations obvious to those having ordinary skill in the art of catalyst regeneration processes are included within the broad scope of the present invention.
- Hydrocarbon feedstocks that can be suitably employed in a fluid catalytic cracking process include naphthas, light gas oils, heavy gas oils, wide-cut gas oils, vacuum gas oils, kerosenes, decanted oils, residual fractions, reduced crude oils, cycle oils derived from any of these, as well as suitable fractions derived from shale oil, kerogen, tar sands bitumen processing, synthetic oils, coal hydrogenation, and the like.
- Such feedstocks may be employed singly, separately in parallel reaction zones, or in any desired combination.
- Hydrocarbon gas and vapors passing through fluid bed 12 maintain the bed in a dense turbulent fluidized condition having the appearance of a boiling liquid.
- the cracking catalyst becomes spent during contact with the hydrocarbon feedstock due to the deposition of coke thereon.
- the terms "spent” or “coke-contaminated” catalyst as used herein generally refer to catalyst which has passed through a reaction zone and which contains a sufficient quantity of coke thereon to cause activity loss, thereby requiring regeneration.
- the coke content of spent catalyst can vary anywhere from 0.5 to 5 wt.% or more.
- spent catalyst coke contents vary from 0.5 to 1.5 wt.%.
- the spent catalyst Prior to actual regeneration, the spent catalyst is usually passed from the reaction zone into a stripping zone 18 and contacted therein with a stripping gas, which is introduced into the lower portion of zone 18 via line 20.
- the stripping gas which is usually introduced at a pressure of from about 10 to about 50 psig, serves to remove most of the volatile hydrocarbons from the spent catalyst.
- a preferred stripping gas is steam, although nitrogen, other inert gases or flue gas may be employed.
- the stripping zone is maintained at essentially the same temperature as the reaction zone, i.e., from 454.4°C (850°F) to 593.3°C (1100°F).
- Stripped spent catalyst from which most of the volatile hydrocarbons have been stripped is then passed from the bottom of stripping zone 18, through a spent catalyst transfer line, such as U-bend 22 and interconnected vertical riser 24, which extends into the lower portion of a regeneration zone 26.
- a spent catalyst transfer line such as U-bend 22 and interconnected vertical riser 24, which extends into the lower portion of a regeneration zone 26.
- Riser 24 is shown entering regeneration zone 26 off-center to avoid interference with the auxiliary heating air from section 31 of the regeneration zone. In the embodiment shown, only one riser 24 is utilized. It is, however, within the contemplation of the subject invention that a plurality of risers may be used.
- Air is added to riser 24 through line 41 and line 28 in an amount sufficient to reduce the density of the catalyst flowing therein, thus causing the catalyst to flow upward into the regeneration zone 26 by simple hydraulic balance.
- the regeneration zone is a separate vessel (arranged at approximately the same level as reaction zone 10) containing a dense phase catalyst bed 30 having a level indicated at 32, which is undergoing regeneration to burn off coke deposits formed in the reaction zone during the cracking reaction, above which is a dilute catalyst phase 34.
- An oxygen-containing regeneration gas enters the lower portion of regeneration zone 26 via line 36 and passes up through a grid 38 and the dense phase catalyst bed 30, maintaining said bed in a turbulent fluidized condition similar to that present in reaction zone 10.
- one embodiment of the present invention resides in passing a minor portion of said regeneration gas via lines 41 and 40 into riser 24 relatively close to where the riser enters regeneration zone 26 to combust the volatile hydrocarbons present therein prior to their entering the dense phase bed.
- Oxygen-containing regeneration gases which may be employed in the process of the present invention are those gases which contain molecular oxygen in admixture with a substantial portion of an inert diluent gas.
- Air is a particularly suitable regeneration gas.
- An additional gas which may be employed is air enriched with oxygen.
- steam may be added to the dense phase bed along with the regeneration gas or separately therefrom to provide additional inert diluents and/or fluidization gas.
- the specific vapor velocity of the regeneration gas will be in the range of from 24.4 to 183 cm/sec (0.8 to 6.0 feet/sec.), preferably from 45.7 to 122 cm/sec (1.5 to about 4 feet/sec.).
- Regenerated catalyst from the dense phase catalyst bed 30 in the regeneration zone 26 flows downward through standpipe 42 and passes through U-bend 44 into the reaction zone 10 by way of the transfer line 46 which joins U-bend 44 at the level of the oil injection line 16 above the U-bend.
- regenerated catalyst is meant catalyst leaving the regeneration zone which has contacted an oxygen-containing gas causing at least a portion, preferably a substantial portion, of the coke present on the catalyst to be removed. More specifically, the carbon content of the regenerated catalyst can vary anywhere from 0.01 to 0.2 wt.%, but preferably is from 0.01 to 0.1.
- the hydrocarbon feedstock for the cracking process is injected into line 46 through line 16 to form an oil and catalyst mixture which is passed into the fluid bed 12 within the reaction zone 10.
- Product vapors containing entrained catalyst particles pass overhead from fluid bed 12 into a gas-solid separation means 48 wherein the entrained catalyst particles are separated therefrom and returned through diplegs 50 leading back into fluid bed 12.
- the product vapors are then conveyed through line 52 into the product recovery system.
- flue gases formed during regeneration of the spent catalyst pass from the dense phase catalyst bed 30 into the dilute catalyst phase 34 along with entrained catalyst particles.
- the catalyst particles are separated from the flue gas by a suitable gas-solid separation means 54 and returned to the dense phase catalyst bed 30 via diplegs 56.
- the substantially catalyst-free flue gas then passes into a plenum chamber 58 prior to discharge from the regeneration zone 26 through line 60.
- the flue gas will contain less than about 0.2, preferably less than 0.1, and more preferably less than 0.05 volume % carbon monoxide.
- the oxygen content will vary from 0.4 to 7 vol.%, preferably from 0.8 to 5 vol.%, more preferably from 1 to 3 vol.%, most preferably from 1.0 to 2 vol.%.
- transverse oxygen gradients i.e., gradients in the direction perpendicular to the flow of the regeneration gas
- the gradient may be especially pronounced where only one asymmetric riser 24 is used.
- undesirable or excessive afterburning is meant to mean obtaining temperatures in the substantially catalyst-free flue gas system due to the combustion of carbon monoxide that exceed permissible catalyst deactivation, or materials of construction limitations and the like.
- undesirable or excessive afterburning corresponds to temperatures above 787.8°C (1450°F).
- the temperature should be maintained below about 771.1°C (1420°F), more preferably below about 760°C (1400°F), to avoid the undesirable effects of excessive afterburning.
- the exact location at which the regeneration gas is injected is rather critical.
- the air should be injected at a point at which it will have little, if any, effect on circulation rate.
- the regeneration gas preferably is injected into riser 24 as close to regeneration zone 26 as mechanical considerations will permit. In a typical system, this point may be 152 to 244 cm (5-8 feet) from the discharge point of riser 24 into bed 30.
- the amount of regeneration gas employed to conduct the volatile hydrocarbons is not critical. However, the amount of regeneration gas injected into the spent catalyst transfer line should be regulated somewhat to assure that only the approximate amount of regeneration gas required for combustion of the volatile hydrocarbons is added. Injection of an insufficient amount of regeneration gas will result in the continued presence of transverse oxygen gradients in regeneration zone 26. Injection of an excess amount of regeneration gas will cause excessive catalyst entrainment which might overload gas-solid separation means 54. Moreover, if a considerable excess of regeneration gas were injected into riser 24, complete combustion of the volatile hydrocarbon and the coke would be effected in the vicinity of the point where the riser enters the vessel, but incomplete combustion would occur in other areas of the regeneration zone.
- a transverse oxygen gradient would be created and once again afterburning would occur in dilute catalyst phase 34.
- the amount of regeneration gas injected into riser 24 preferably is regulated by monitoring the transverse oxygen gradient, the transverse CO gradient, or a transverse temperature gradient.
- the amount of air utilized may be dependent in part on the type of regeneration system utilized and where the minor portion of the regeneration gas is injected to combust the volatile hydrocarbons. Where the minor portion of the regeneration gas is injected into the spent catalyst transfer line, typically this will correspond to from 2 to 20%, preferably from 4 to 15%, of the regeneration gas. In the embodiment of Figure 1, from 0 to 12% of the total regeneration gas enters through line 28 to control catalyst circulation, while 2 to 10%, and preferably 3% to 6%, of the total regeneration gas enters through line 40 to combust the volatile hydrocarbons.
- FIG. 2 shows spent catalyst from a stripping zone (not shown) being introduced into the dense phase catalyst bed 30 of regeneration zone 26 via line 22. Also shown is regeneration gas in line 36 passing up through grid 38 and into bed 30 where it reacts with the carbon on the spent catalyst therein such that a regenerated catalyst is passed from zone 26 via line 42.
- valve 68 controls the catalyst density which, in turn, governs the catalyst circulation rate. Operation of valve 68 may be controlled by a signal Eg from a comparison means 70 which compares control signal E,, corresponding to the desired temperature in reaction zone 10, to signal E 6 , transmitting the actual temperature in the reaction zone. Typically, valve 68 is opened further to increase the catalyst circulation rate when the temperature in reaction zone 10 is too low, and, conversely, the opening in the valve is decreased when the temperature in zone 10 is too high. The catalyst circulation rate also may be varied to control other process variables such as the temperature in regeneration zone 26.
- oxygen concentrations could be sensed at, for example, spaced-apart points (1) and (2).
- concentration of hydrocarbon components or nonhydro- carbon components such as carbon monoxide, ammonia, hydrogen, or oxides of nitrogen, which are oxidizable in regeneration zone 26, alternatively could be sensed.
- a temperature gradient and a C0 2 gradient also will be formed which alternatively may be sensed by spaced-apart points (1) and (2).
- points (1) and (2) are preferably disposed in a horizontal plane generally transverse to the direction of flow of the regeneration gas.
- points (1) and (2) could be located at any spaced-apart locations in dilute phase 34 at which differences resulting from the oxygen gradient could be detected.
- signals E, and E 2 corresponding to the sensed temperatures or oxygen concentrations at points (1) and (2), respectively, could be developed and passed into a computation means 62 suitable for calculating a transverse oxygen or temperature gradient.
- Suitable computation means can be selected from a variety of digital and/or analog computing devices, depending upon the particular application.
- the computation means could be a large computer capable of controlling an entire refinery complex or, if desired, a minicomputer designed for more limited applications. Such computation means are well known articles of commerce and thus are readily available in the marketplace.
- the oxygen, temperature or other gradient thus calculated can then be developed into a control signal E 3 and sent to a comparison means 64 which compares signal E 3 with a signal E 4 corresponding to the desired transverse oxygen, temperature or other gradient at the points being monitored such that a control signal Eg is generated.
- the control signal Eg is then applied to a control means 66 which regulates the amount of regeneration gas introduced into the spent catalyst line via line 40.
- the greater the deviation from the desired oxygen gradient i.e., the greater the amount of volatile hydrocarbon introduced into bed 30
- riser reaction zone 110 comprises a tubular, vertical extending vessel having a relatively large height in relation to its diameter.
- Reaction zone 110 communicates with a disengagement zone 120, shown located a substantial height above regeneration zone 150.
- the catalyst circulation rate is controlled by a valve means, such as slide valve 180, located in spent catalyst transfer line 140 extending between disengagement zone 120 and regeneration zone 1 50.
- hydrocarbon feedstock is injected through line 112 into riser reaction zone 110 filled with catalyst to catalytically crack the feedstock.
- Steam may be injected through lines 160 and 162 into return line 158 extending between regeneration zone 150 and reaction zone 110 to serve as a diluent, to provide a motive force for moving the hydrocarbon feedstock upwardly and for keeping the catalyst in a fluidized condition.
- the vaporized, cracked feedstock products pass upwardly into disengagement zone 120 where a substantial portion of the entrained catalyst is separated.
- the gaseous stream then passes through a gas-solid separation means, such as two stage cyclone 122, which further separates out entrained catalyst and returns it to the disengagement zone through diplegs 124, 126.
- the gaseous stream passed into plenum chamber 132 and exits through line 130 for further processing (not shown).
- the upwardly moving catalyst in reaction zone 110 gradually becomes coated with carbonaceous material which decreases its catalytic activity.
- Flue gas formed during the regeneration of the spent catalyst passes from the dense phase catalyst bed 152 into dilute catalyst phase 154.
- the flue gas then passes through cyclone 170 into plenum chamber 172 prior to discharge through line 174.
- Catalyst entrained in the flue gas is removed by cyclone 170 and is returned to catalyst bed 152 through diplegs 176, 178.
- hydrocarbons not removed from the spent catalyst in stripping zone 140 are combusted in preference to the coke on the spent catalyst in dense phase catalyst bed 152.
- the area where the spent catalyst is discharged into dense phase catalyst bed becomes deficient in oxygen resulting in the formation of CO rather than CO 2 , while excess oxygen will be present in other areas of dense phase catalyst bed 152.
- excess oxygen is present, the coke is completely converted to C0 2 and free oxygen also passes into the dilute catalyst phase 154, thereby resulting in the formation of dilute phase transverse oxygen gradients.
- the CO from the oxygen deficient area contacts the excess oxygen in the dilute catalyst phase, undesired afterburning results from the conversion of the CO to C0 2 .
- the afterburning can be significantly reduced by more completely combusting the volatile hydrocarbons prior to their entry into dense phase bed 152. This may be accomplished by introducing a minor portion of the regeneration gas through line 190 into spent catalyst transfer line 142.
- the point at which the regeneration gas is injected into transfer line 142 may be less critical in this embodiment than that in the previous embodiment since here the catalyst recirculation rate is controlled by slide valve 180, rather than by the pressure in the transfer line.
- the regeneration gas preferably is injected downstream of slide valve 180, most preferably close to regeneration zone 150 to minimize the effect of the regeneration gas on the catalyst flow rate and preclude operational problems.
- One method of discharging the regeneration gas into transfer line 142 relatively close to regeneration zone 150 is to at least partially dispose a conduit means 192 communicating with line 190 in spent catalyst transfer line 142, with the conduit means terminating substantially near the terminus of the transfer line in dense phase catalyst bed 152.
- the amount of regeneration gas added through line 190 preferably should be regulated as in the previous embodiment to minimize catalyst entrainment, and also to minimize transverse oxygen gradients.
- the relative amount of regeneration gas added through lines 164 and 190 can be regulated by control valve 194 in line 190.
- Valve 194 is controlled in a manner similar to valve 66 shown in Figure 2, the schematic control drawing for the embodiment of Figure 1. Typically, it is believed that 4% to 16% of the total regeneration gas should be added to spent catalyst transfer line 142 through line 190, and preferably between 8% and 14% of the total regeneration gas.
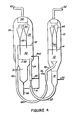
- FIG 4 a cracking system substantially similar to that of Figure 1 is shown.
- a main portion of the regeneration gas is added at or near the point where the spent catalyst mixture is discharged into the regeneration zone, whereas in Figure 1 the main portion of the regeneration gas is injected into the transfer line.
- all elements except for the line through which a minor portion of the regeneration gas is added to combust the hydrocarbons are similar to those of Figure 1 and have similar reference numbers.
- the line through which the minor portion of the regeneration gas is injected into the regeneration zone is denoted as line 240.
- the operation of the cracking system is substantially similar to that of Figure 1 except that in this embodiment a minor portion of the regeneration gas passes through lines 41 and 240 and is released into the localized area where the spent cata- lyst/volatile hydrocarbon mixture from the stripping zone is released into the dense phase catalyst bed of the regeneration zone.
- the term "localized area” is defined to be that area within a locus of about one diameter of the spent catalyst transfer line from the terminus of the spent catalyst transfer line in dense phase catalyst bed 30. Normally, the spent catalyst transfer line has a diameter of 45.6 cm (18 inches) to 106.8 cm (42 inches) depending upon the desired catalyst flow rate and velocity, and the method of circulating the spent catalyst to regeneration zone 26.
- the amount of regeneration gas employed to combust the volatile hydrocarbons is also not critical and need be only an amount sufficent to at least partially combust the volatile hydrocarbons in the mixture with spent catalyst released into the dense phase bed.
- the amount of regeneration gas employed to combust the volatile hydrocarbons preferably is regulated to assure that only the approximate amount of regeneration gas required for combustion of the volatile hydrocarbons is added for the reasons previously indicated in connection with the discussion of the embodiment of Figure 1.
- the amount of regeneration gas injected into dense phase catalyst bed 30 through line 240 will correspond to from 2 to 20%, preferably from about 5 to about 9%, of the total regeneration gas.
- a little more regeneration gas is required through line 240 relative to the embodiments of Figures 1 and 3 where a minor portion of the regeneration gas was injected into the spent catalyst transfer line.
- a portion of the carbon on the spent catalyst will be combusted in addition to the volatile hydrocarbons.
- introducing the regeneration gas directly into the bed rather than into the spent catalyst line has certain safety advantages since there is a large reservoir of catalyst available to absorb excessive amounts of heat released.
- an additional 4-12% of the total regeneration gas in line 36 is directed through lines 41 and 28 into riser 24 to fluidize the spent catalyst and regulate the spent catalyst circulation rate.
- the subject invention is equally applicable to other type cracking systems and to other zone configurations and positions, such as upflow and downflow regeneration systems, including those which incorporate a slide valve to control the catalyst circulation rate.
- a minor portion of the regeneration gas in line 36 is shown being passed via lines 240 and 41 into the dense phase catalyst bed in the localized area where the spent catalyst is released into said bed to combust volatile hydrocarbons not removed in said stripping zone which otherwise would react preferentially with the oxygen passing through grid 38, thereby causing a depletion of the oxygen in a localized area where the spent catalyst enters bed 30 such that CO rather than C0 2 is formed.
- This would create transverse oxygen gradients in not only bed 30, but in dilute catalyst phase 34, when gases are passed from bed 30. Hence, undesirable or excessive afterburning could result.
- the transverse oxygen gradients would be especially pronounced where the spent catalyst enters regeneration zone 26 off-center, as in the embodiment shown.
- oxygen concentrations could be sensed at, for example, spaced apart points (1) and (2).
- concentration of hydrocarbon components or non-hydrocarbon components, such as carbon monoxide, ammonia, hydrogen, or oxides of nitrogen, which are oxidizable in regeneration zone 26 alternatively could be sensed.
- a temperature gradient also will be formed which alternatively may be sensed by spaced-apart points (1) and (2).
- points (1) and (2) preferably are disposed in a horizontal plane generally transverse to the direction of flow of the gas.
- points (1) and (2) could be disposed at any spaced-apart locations in dilute phase 34 at which the oxygen or other concentrations or the temperature differences resulting from the oxygen gradient could be detected.
- Signals E, and E 2 corresponding to the sensed temperature differences or oxygen or other concentration differences between points (1) and (2), respectively, could be developed and passed into a computation means 62 suitable for calculating a transverse temperature or oxygen gradient.
- Suitable computation means can be selected from a variety of digital and/or analog computing devices, depending upon the particular application.
- the computation means could be a large computer capable of controlling an entire refinery complex or, if desired, a minicomputer designed for more limited applications. Such computation means are well known articles of commerce and thus are readily available in the marketplace.
- the temperature, oxygen, or other gradient thus calculated can then be developed into a control signal E 3 and sent to a comparison means 64 which compares signal E 3 with a signal E 4 corresponding to the desired transverse gradient at the points being monitored such that a control signal Eg is generated.
- the control signal Eg is then applied to a control means 66 which regulates the amount of regeneration gas introduced into the dense phase bed via line 240 preferably to minimize the measured gradient.
- the greater the deviation from the desired gradient i.e., the greater the amount of volatile hydrocarbon introduced into bed 30
- the greater will be the amount of regeneration gas employed in line 240.
- the amount of regeneration gas used in line 240 could, alternatively, be regulated according to other variables in dilute catalyst phase 34.
- combustion of the volatile hydrocarbons in mixture with the spent catalyst prior to said mixture entering the dense phase catalyst bed or within a localized area where said mixture enters the dense phase catalyst bed insures that the regeneration gas passing upward through the grid in said bed will burn only the carbon on the catalyst which is mixed throughout the bed.
- the present invention serves to correct an imbalance in the amount of combustible material present in a localized area of the dense phase catalyst bed by removing a portion of said combustible material (the volatile hydrocarbons) immediately after their entering said bed.
- Such combustion of the volatile hydrocarbons also serves to prevent or minimize localized starvation of oxygen in the dense phase catalyst bed such that formation of CO rather than CO 2 is minimized.
- any commercial catalytic cracking catalyst designed for high thermal stability could be suitably employed in the present invention.
- Such catalysts include those containing silica and/or alumina. Catalysts containing combustion promoters such as platinum also can be used. Other refractory metal oxides such as magnesia or zirconia may be employed and are limited only by their ability to be effectively regenerated under the selected conditions.
- preferred catalysts include the combinations of silica and alumina, containing 10 to 50 wt.% alumina, and particularly their admixtures with molecular sieves or crystalline aluminosilicates.
- Suitable molecular sieves include both naturally occurring and synthetic aluminosilicate materials, such as faujasite, chabazite, X-type and Y-type aluminosilicate materials and ultra stable, large pore crystalline aluminosilicate materials.
- the molecular sieve content of the finished fresh catalyst particles is suitably within the range from 5--15 wt.%, preferably 8-10 wt.%.
- An equilibrium molecular sieve cracking catalyst may contain as little as about 1 wt.% crystalline material. Admixtures of clay- extended aluminas may also be employed.
- Such catalysts may be prepared in any suitable method such as by impregnation, milling, co- gelling and the like, subject only to provision of the finished catalyst in a physical form capable of fluidization.
- the regeneration zone employed in the present invention normally comprises vertical cylindrical vessels wherein the catalyst to be regenerated is maintained as a fluidized bed by the upward passage of an oxygen-containing regeneration gas thereby forming a dense phase catalyst bed and a dilute catalyst phase with an interface in between.
- the dense phase bed which is usually located in the lower portion of the regeneration zone, is maintained at a temperature in the range of from 621.1°C to 732.2°C (1150 1- 1350 0 F), preferably from 676.7 to 715.6°C (1250-1320°F).
- the density of the dense phase bed may range from 0.1281 to 0.481 g/cm 3 (8 to 30 Ib/cu. ft).
- the dilute catalyst phase is the primarily gaseous phase volume located above the dense phase bed within the regeneration zone. Specifically, the dilute phase contains relatively small quantities of catalyst compared to the dense phase bed.
- the density of the dilute phase zone ranges from 16.02 ⁇ 10 -4 to 16.02x 10- 3 g/cm 3 (0.1 to 1.0 Ib/cu. ft.) at the inlet to the separation means and from 16.02x10- 3 to 80.10x10-3 g/cm 3 (1 to 5 Ib/cu. ft.) near the interface between the dense bed phase and the dilute catalyst phase.
- the overall flow in the dilute phase is a concurrent flow of catalyst entrained with flue gases.
- the dilute catalyst phase can include substantial quantities of the dense bed material which passes into that phase from excessive agitation or bubbling of gaseous materials through the dense bed.
- the temperature in the dilute catalyst phase is at least that in the dense bed phase and is advantageously maintained within the range from 648.9 to 787.7°C (1200° to 1450°F.), preferably from 710 to 760°C (1310° to 1400°F).
- substantially catalyst-free flue gas is the gaseous phase volume located within or downstream of the catalyst separation means within the regeneration zone. Specifically, the “substantially catalyst-free flue gas” comprises flue gas from the dilute catalyst phase from which entrained catalyst particles have been substantially removed. This corresponds to the gaseous effluent from the separation means within the regeneration zone wherein the concentration of entrained catalyst particles will be less than about 1 grain (0.06479 g) preferably less than about 0.2 grains (0.12959 g) per actual cubic foot (28.32 I).
- actual cubic foot (28.32 I) refers to the volume measured at actual operating conditions without correction to a standard temperature and pressure.
- the substantially catalyst-free flue gas from the separation means may be discharged to a variety of downstream equipment such as a dispersion means to redistribute the flue gas, stack valves, a plenum chamber and the like, prior to leaving the regeneration zone.
- downstream equipment such as a dispersion means to redistribute the flue gas, stack valves, a plenum chamber and the like, prior to leaving the regeneration zone.
- substantial afterburning, and hence excessive temperatures in that portion of the regeneration zone wherein the flue gas is substantially catalyst-free may be avoided.
- the temperature in that portion of said regeneration zone is maintained at least equal to that of the dilute catalyst phase at the inlet to the separation devices but no more than 27.8°C (50°F), preferably no more than 16.7°C (30°F), and most preferably no more than 11.1°C (20°F) above that at said inlet.
- extraneous cooling means such as steam may be employed to further reduce the temperature and thereby inhibit the afterburning reaction in that portion of the
- One or more gas-solids separation means may be utilized in the dilute catalyst phase to separate entrained regenerated catalyst particles from the regeneration gas.
- Preferred separation means will be cyclone separators, multiclones or the like whose design and construction are well known in the art. In the case of cyclone separators, a single cyclone may be used, but preferably, more than one cyclone will be used in parallel or in series flow to effect the desired degree of separation.
- the construction of the regeneration zone can be made with any material sufficiently able to withstand the relatively high temperatures involved when afterburning is encountered within the vessel and the high attrition conditions which are inherent in systems wherein fluidized catalyst is regenerated and transported.
- metals are contemplated which may or may not be lined.
- ceramic liners are contemplated within any and all portions of the regeneration zone together with alloy use and structural designs in order to withstand the erosive conditions and temperatures of about 760°C (1400°F.) and, for reasonably short periods of time, temperatures which may be as high as 982.2°C (1800°F).
- the gauge pressure in the regeneration zone is usually maintained in a range from atmospheric pressure to 3.5155 kg/cm 2 (50 psig.), preferably from 0.7031 to 3.5155 kg/cm 2 (10 to 50 psig). It is preferred, however, to design the regeneration zone to withstand gauge pressures of up to about 7.031 kg/cm 2 (100 psig). Operation of the regeneration zone at increased pressure has the effect of promoting the conversion of carbon monoxide to carbon dioxide and reducing the temperature level within the dense bed phase at which the substantially complete combustion of carbon monoxide can be accomplished. The higher pressure also lowers the equilibrium level of carbon on regenerated catalyst at a given regeneration temperature.
- the residence time of the spent catalyst in the regeneration zone is not critical. In general, it can vary from about 1 to about 6 minutes; typically, from about 2 to about 4 minutes.
- the contact time or residence time of the flue gas in the dilute catalyst phase established the extent to which the combustion reaction can reach equilibrium.
- the residence time of the flue gas may vary from about 10 to about 60 seconds in the regeneration zone and from about 2 to about 15 seconds in the dense bed phase.
- the residence time of the flue gas varies from about 15 to about 20 seconds in the regeneration zone and from about 6 to about 10 seconds in the dense bed.
- the present invention may be applied beneficially to any type of fluid cat cracking unit with little or no modifications and without limitations as to the spatial arrangement of the reaction, stripping, and regeneration zones thereof.
- the regeneration zone of a catalytic cracking unit can be designed independently from the reaction zone since the regeneration zone merely receives spent catalyst, oxidizes the coke thereon to regenerate the catalyst, and returns the regenerated catalyst to the reaction zone. Therefore, the reaction zone can be a pure transfer line, i.e. one in which the reaction occurs in a single pipe type vessel directly terminating in a rough cut cyclone or cyclones as in Figure 3, a conventional dilute riser/dense bed combination as in Figures 1 and 4 or a dense bed alone.
- temperatures in °F are converted to °C by subtracting 32 and then dividing by 1.8. Temperature differences in °F are converted to °C by dividing by 1.8.
- Lengths expressed in inches are converted to cms by multiplying by 2.54, and lengths in feet are converted to cms by multiplying by 30.48.
- Weights in grains are converted to grams by multiplying by 0.06479891.
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Production Of Liquid Hydrocarbon Mixture For Refining Petroleum (AREA)
- Catalysts (AREA)
Description
- The present invention relates to the regeneration of catalysts employed in a fluid catalytic cracking process. More particularly, this invention relates to the combustion of volatile hydrocarbons in mixture with spent fluid catalytic cracking catalyst prior to said mixture entering the regeneration zone.
- The fluidized catalytic cracking of hydrocarbons is well known in the prior art and may be accomplished in a variety of processes which employ fluidized solid techniques. Normally in such processes, suitably preheated, relatively high molecular weight hydrocarbon liquids and/or vapors are contacted with hot, finely- divided, solid catalyst particles either in a fluidized bed reaction zone or in an elongated riser reaction zone, and maintained at an elevated temperature in a fluidized state for a period of time sufficient to effect the desired degree of cracking to lower molecular weight hydrocarbons typical of those present in motor gasolines and distillate fuels.
- During the cracking reaction, coke is deposited on the catalyst particles in the reaction zone thereby reducing the activity of the catalyst for cracking and the selectivity of the catalyst for producing gasoline blending stock. In order to restore a portion, preferably a major portion, of the activity to the coke-contaminated or spent catalyst, the catalyst is transferred from the reaction zone into a regeneration zone. Typical regeneration zones comprise large vertical cylindrical vessels wherein the spent catalyst is maintained as a fluidized bed by the upward passage of an oxygen-containing regeneration gas, such as air. The fluidized catalyst forms a dense phase catalyst bed in the lower portion of the vessel and a dilute catalyst phase containing entrained catalyst particles above, with an interface existing between the two phases. The catalyst is contacted with the oxygen-containing regeneration gas under conditions sufficient to burn at least a portion, preferably a major portion, of the coke from the catalyst. Flue gas, which normally comprises gases arising from the combustion of the coke on the spent catalyst, inert gases such as nitrogen from air, any unconverted oxygen and entrained catalyst particles, is then passed from the dilute catalyst phase into solid-gas separation means within the regeneration zone (e.g., cyclone separators) to prevent excessive losses of the entrained catalyst particles. The catalyst particles separated from the flue gas are returned to the dense phase catalyst bed. A substantially catalyst-free flue gas may then be passed from the separation means to equipment downstream thereof, for example to a plenum chamber, or be discharged directly from the top of the regeneration zone. The regenerated catalyst is subsequently withdrawn from the regeneration zone and reintroduced into the reaction zone for reaction with additional hydrocarbon feed.
- Commonly, spent catalyst from the reaction zone is passed therefrom to a stripping zone for removal of volatile hydrocarbons from the catalyst particles prior to transferring the catalyst to the regeneration zone. However, the volatile hydrocarbons not recovered as product from the reaction zone will pass with the spent catalyst into the regeneration zone wherein they are combusted in preference to the carbon on the spent catalyst. This results in exhaustion of the oxygen in the regeneration gas in the area where the spent catalyst and volatile hydrocarbons enter the regeneration zone. Normally, the spent catalyst and volatile hydrocarbons enter the regeneration zone at an off-center location to avoid interference with the regeneration overflow well and/or auxiliary heating air section. Thus, one area of the dense phase bed is essentially starved of oxygen such that CO rather than C02 will be formed. In contrast, an excess of oxygen is present in the remaining portion of the dense phase bed since volatile hydrocarbons are not present therein.
- The CO thus formed in this localized area passes from the dense phase bed into the dilute catalyst phase where it is reacted with oxygen leaving the oxygen-rich portions from other parts of the dense phase bed according to the following equation, an exothermic reaction:
- Thus, in view of the undesirable consequences resulting from the combustion of volatile hydrocarbons in the regeneration zone, it would be desirable to have a simple and convenient method for removing said hydrocarbons prior to their entering said regeneration zone.
- US-A-4062762 describes a fluidized catalytic cracking process comprising:
- (a) contacting a hydrocarbon feedstock with cracking catalyst in a reaction zone under cracking conditions to produce cracked hydrocarbon vapours and coke-contaminated catalyst;
- (b) contacting the coke-contaminated catalyst with a stripping gas to partially remove volatile hydrocarbons therefrom, thereby forming a mixture of coke-contaminated catalyst and unstripped volatile hydrocarbons;
- (c) passing the mixture from the reaction zone through a transfer line with a terminus in the dense phase catalyst bed of a regeneration zone having a dense phase catalyst bed and a dilute catalyst phase; and
- (d) regenerating the coke-contaminated catalyst by contacting the mixture under regeneration conditions with an oxygen-containing regeneration gas.
- As described in US-A-4062762, the reaction zone is contained in a reactor vessel and the regeneration zone is contained in a regenerator vessel. Coke-contaminated catalyst circulates from the bottom region of the reactor vessel to the regeneration zone via a U-shaped transfer-line which serves to prevent the transfer of gases and vapours between the vessels. A gas or vapour is passed into the bottom region of the vertical riser arm of the 'U' beneath the regenerator vessel to reduce the density of catalyst in the vertical riser arm relative to the density of catalyst in the other vertical arm of the 'U' beneath the reactor vessel whereby catalyst is propelled upwardly into the regeneration zone by an action analogous to that of a water lift pump. Air is used for convenience and low cost as the gas for reducing the density of catalyst to promote circulation, and, as indicated, the air is passed into the bottom region of the vertical riser arm. The circulation of hot regenerated catalyst from the regenerator vessel to the reaction zone is similarly effected through a second U-shaped transfer-line, and the hydrocarbon feed is passed into one arm of the transfer-line adjacent to the reactor vessel at a level lower than the top level of the reaction zone so that the vapours generated by the contact of the feed with the hot regenerated catalyst passing to the reaction zone reduce the density of the regenerated catalyst thereby enhancing catalyst circulation through the second U-shaped transfer-line to the reaction zone from the regenerator vessel.
- The present invention provides a fluidized catalytic cracking process which comprises:
- (a) contacting a hydrocarbon feedstock with cracking catalyst in a reaction zone under cracking conditions to produce cracked hydrocarbon vapours and coke-contaminated catalyst;
- (b) contacting the coke-contaminated catalyst with a stripping gas to partially remove volatile hydrocarbons therefrom, thereby forming a mixture of coke-contaminated catalyst and unstripped volatile hydrocarbons;
- (c) passing the mixture from the reaction zone through a transfer line with a terminus in the dense phase catalyst bed of a regeneration zone having a dense phase catalyst bed and a dilute catalyst phase; and
- (d) regenerating the coke-contaminated catalyst by contacting the mixture under regeneration conditions with an oxygen-containing regeneration gas, characterized by injecting a minor portion of the regeneration gas consisting of from 2 to 20% of the total regeneration gas either into the transfer line at a point immediately adjacent to or close to the regeneration zone or into the localized area of the terminus of the transfer line in the dense phase catalyst bed to cause at least partial combustion of the remaining volatile hydrocarbons from the mixture without substantially influencing the flow rate of catalyst through the transfer line.
- The present invention is based on the discovery that the formation of CO in the dense phase catalyst bed of the regeneration zone of a fluid catalytic cracking process due to the volatile hydrocarbons in the spent catalyst mixture entering said regeneration zone may be reduced and/or minimized by using a minor portion of the regeneration gas to combust said hydrocarbons prior to said mixture entering the regeneration zone or to combust said hydrocarbons at or near the point where the mixture is released into the regeneration zone. The amount of regeneration gas used to combust the hydrocarbons is not critical, but will be within the range of from 2 to 20% of the regeneration gas normally employed. Preferably combustion of the hydrocarbons will occur in the line transferring spent catalyst from the reaction zone to the regeneration zone.
-
- Figure 1 is a flow diagram of an embodiment of the present invention as applied to a pressure controlled type fluid catalytic cracking process where a minor portion of the regeneration gas is used to combust the hydrocarbons prior to the hydrocarbons entering the regeneration zone.
- Figure 2 shows a more detailed view of the embodiment of Figure 1.
- Figure 3 is an alternative embodiment of the present invention as applied to a slide valve type fluid catalytic cracking process where a minor portion of the regeneration gas combusts the hydrocarbons prior to the hydrocarbons entering the regeneration zone.
- Figure 4 is a flow diagram of an embodiment of the present invention where a minor portion of the regeneration gas combusts the hydrocarbons at or near the point where the catalyst mixture enters the regeneration zone.
- Figure 5 is a more detailed view of the embodiment of Figure 4.
- Having thus described the invention in general terms, reference is now made to the Figures which illustrate two embodiments in which the present invention is applied to a fluid catalytic cracking process. Figures 1 and 2 disclose a catalyst cracking system in which the catalyst circulation rate is controlled either by adjusting the differential pressure between the zones by suitable control means or by varying the catalyst density in the riser entering the regeneration zone. Figure 3 illustrates a system in which the catalyst flow rate between the reaction and regeneration zones is controlled by a slide valve.
- In Figures 1-3 a minor portion of the regeneration gas is used to combust the hydrocarbons prior to the hydrocarbons entering the regeneration zone.
- In Figures 4 and 5, another embodiment of the invention is shown in which a minor portion of the regeneration gas is added at or near the point where the catalyst is released into the regeneration zone.
- The subject invention is not limited to these type systems and is equally applicable to other type cracking systems and to other zone configurations and positions such as upflow and downflow regeneration systems with and without slide valves. Various items such as valves, pumps, compressors, steam lines, instrumentation and other process equipment and control means have been omitted from the figures for the sake of simplicity. Variations obvious to those having ordinary skill in the art of catalyst regeneration processes are included within the broad scope of the present invention.
- Referring now to Figure 1, there is shown a vertically arranged
cylindrical reaction zone 10 containing a fluidizedbed 12 of catalyst having a level indicated at 14 in which a hydrocarbon feedstock introduced atline 16 is undergoing catalytic cracking. Hydrocarbon feedstocks that can be suitably employed in a fluid catalytic cracking process include naphthas, light gas oils, heavy gas oils, wide-cut gas oils, vacuum gas oils, kerosenes, decanted oils, residual fractions, reduced crude oils, cycle oils derived from any of these, as well as suitable fractions derived from shale oil, kerogen, tar sands bitumen processing, synthetic oils, coal hydrogenation, and the like. Such feedstocks may be employed singly, separately in parallel reaction zones, or in any desired combination. Hydrocarbon gas and vapors passing throughfluid bed 12 maintain the bed in a dense turbulent fluidized condition having the appearance of a boiling liquid. - In
reaction zone 10, the cracking catalyst becomes spent during contact with the hydrocarbon feedstock due to the deposition of coke thereon. Thus, the terms "spent" or "coke-contaminated" catalyst as used herein generally refer to catalyst which has passed through a reaction zone and which contains a sufficient quantity of coke thereon to cause activity loss, thereby requiring regeneration. Generally, the coke content of spent catalyst can vary anywhere from 0.5 to 5 wt.% or more. Typically, spent catalyst coke contents vary from 0.5 to 1.5 wt.%. - Prior to actual regeneration, the spent catalyst is usually passed from the reaction zone into a stripping
zone 18 and contacted therein with a stripping gas, which is introduced into the lower portion ofzone 18 vialine 20. The stripping gas, which is usually introduced at a pressure of from about 10 to about 50 psig, serves to remove most of the volatile hydrocarbons from the spent catalyst. A preferred stripping gas is steam, although nitrogen, other inert gases or flue gas may be employed. Normally, the stripping zone is maintained at essentially the same temperature as the reaction zone, i.e., from 454.4°C (850°F) to 593.3°C (1100°F). - Stripped spent catalyst from which most of the volatile hydrocarbons have been stripped, is then passed from the bottom of stripping
zone 18, through a spent catalyst transfer line, such as U-bend 22 and interconnectedvertical riser 24, which extends into the lower portion of aregeneration zone 26. -
Riser 24 is shown enteringregeneration zone 26 off-center to avoid interference with the auxiliary heating air fromsection 31 of the regeneration zone. In the embodiment shown, only oneriser 24 is utilized. It is, however, within the contemplation of the subject invention that a plurality of risers may be used. - Air is added to
riser 24 throughline 41 andline 28 in an amount sufficient to reduce the density of the catalyst flowing therein, thus causing the catalyst to flow upward into theregeneration zone 26 by simple hydraulic balance. - In the particular configuration shown in Figure 1, the regeneration zone is a separate vessel (arranged at approximately the same level as reaction zone 10) containing a dense
phase catalyst bed 30 having a level indicated at 32, which is undergoing regeneration to burn off coke deposits formed in the reaction zone during the cracking reaction, above which is adilute catalyst phase 34. An oxygen-containing regeneration gas enters the lower portion ofregeneration zone 26 via line 36 and passes up through a grid 38 and the densephase catalyst bed 30, maintaining said bed in a turbulent fluidized condition similar to that present inreaction zone 10. As will be discussed in more detail hereinbelow, one embodiment of the present invention resides in passing a minor portion of said regeneration gas vialines 41 and 40 intoriser 24 relatively close to where the riser entersregeneration zone 26 to combust the volatile hydrocarbons present therein prior to their entering the dense phase bed. - Oxygen-containing regeneration gases which may be employed in the process of the present invention are those gases which contain molecular oxygen in admixture with a substantial portion of an inert diluent gas. Air is a particularly suitable regeneration gas. An additional gas which may be employed is air enriched with oxygen. Additionally, if desired, steam may be added to the dense phase bed along with the regeneration gas or separately therefrom to provide additional inert diluents and/or fluidization gas. Typically, the specific vapor velocity of the regeneration gas will be in the range of from 24.4 to 183 cm/sec (0.8 to 6.0 feet/sec.), preferably from 45.7 to 122 cm/sec (1.5 to about 4 feet/sec.).
- Regenerated catalyst from the dense
phase catalyst bed 30 in theregeneration zone 26 flows downward throughstandpipe 42 and passes through U-bend 44 into thereaction zone 10 by way of thetransfer line 46 which joins U-bend 44 at the level of theoil injection line 16 above the U-bend. By regenerated catalyst is meant catalyst leaving the regeneration zone which has contacted an oxygen-containing gas causing at least a portion, preferably a substantial portion, of the coke present on the catalyst to be removed. More specifically, the carbon content of the regenerated catalyst can vary anywhere from 0.01 to 0.2 wt.%, but preferably is from 0.01 to 0.1. - The hydrocarbon feedstock for the cracking process is injected into
line 46 throughline 16 to form an oil and catalyst mixture which is passed into thefluid bed 12 within thereaction zone 10. Product vapors containing entrained catalyst particles pass overhead fromfluid bed 12 into a gas-solid separation means 48 wherein the entrained catalyst particles are separated therefrom and returned throughdiplegs 50 leading back intofluid bed 12. The product vapors are then conveyed throughline 52 into the product recovery system. - In
regeneration zone 26, flue gases formed during regeneration of the spent catalyst pass from the densephase catalyst bed 30 into thedilute catalyst phase 34 along with entrained catalyst particles. The catalyst particles are separated from the flue gas by a suitable gas-solid separation means 54 and returned to the densephase catalyst bed 30 viadiplegs 56. The substantially catalyst-free flue gas then passes into aplenum chamber 58 prior to discharge from theregeneration zone 26 throughline 60. Typically, the flue gas will contain less than about 0.2, preferably less than 0.1, and more preferably less than 0.05 volume % carbon monoxide. Typically, the oxygen content will vary from 0.4 to 7 vol.%, preferably from 0.8 to 5 vol.%, more preferably from 1 to 3 vol.%, most preferably from 1.0 to 2 vol.%. - As noted above, most of the volatile hydrocarbons are stripped from the spent catalyst leaving
reaction zone 10. However, the hydrocarbons not removed will be passed in mixture with spent catalyst (and steam) intoregeneration zone 26 wherein said hydrocarbons are combusted in preference to the carbon on the spent catalyst. As such, the localized area where the spent catalyst mixture is released into densephase catalyst bed 30 of the regeneration zone becomes depleted in oxygen. Thus, sufficient oxygen is not present to combust CO to CO2, As such, CO will pass intodilute catalyst phase 34 from said localized area of the dense phase catalyst bed. In contrast, an excess of oxygen will be present in other areas of the dense phase bed (i.e., areas where the volatile hydrocarbons are not present) such that CO will be converted to C02 therein. As such, transverse oxygen gradients (i.e., gradients in the direction perpendicular to the flow of the regeneration gas) will exist in the bed. The gradient may be especially pronounced where only oneasymmetric riser 24 is used. When the CO passed into the dilute catalyst phase contacts the oxygen present therein from the other areas (i.e., oxygen-rich areas) of the bed, undesirable or excessive afterburning could occur according to equation (1). - The expression "undesirable or excessive" afterburning is meant to mean obtaining temperatures in the substantially catalyst-free flue gas system due to the combustion of carbon monoxide that exceed permissible catalyst deactivation, or materials of construction limitations and the like. In general, undesirable or excessive afterburning corresponds to temperatures above 787.8°C (1450°F). Preferably, however, the temperature should be maintained below about 771.1°C (1420°F), more preferably below about 760°C (1400°F), to avoid the undesirable effects of excessive afterburning.
- However, the problems associated with transverse oxygen gradients due to the presence of volatile hydrocarbons in the dense phase bed can be virtually eliminated by combusting said volatile hydrocarbons prior to their entering the dense phase catalyst bed or by combusting the volatile hydrocarbons at and/or near the point where they enter the dense phase bed. This may be accomplished simply and conveniently in the embodiment of Figure 1 by introducing a minor portion of the regeneration gas into the spent catalyst transfer line extending from the stripping
zone 18 to theregeneration zone 26. Other methods for combusting the volatile hydrocarbons will be discussed hereinafter. - For the embodiment of Figure 1 where the catalyst circulation rate is controlled by density variations caused by air injection into
riser 24, the exact location at which the regeneration gas is injected is rather critical. The air should be injected at a point at which it will have little, if any, effect on circulation rate. The regeneration gas preferably is injected intoriser 24 as close toregeneration zone 26 as mechanical considerations will permit. In a typical system, this point may be 152 to 244 cm (5-8 feet) from the discharge point ofriser 24 intobed 30. - The amount of regeneration gas employed to conduct the volatile hydrocarbons is not critical. However, the amount of regeneration gas injected into the spent catalyst transfer line should be regulated somewhat to assure that only the approximate amount of regeneration gas required for combustion of the volatile hydrocarbons is added. Injection of an insufficient amount of regeneration gas will result in the continued presence of transverse oxygen gradients in
regeneration zone 26. Injection of an excess amount of regeneration gas will cause excessive catalyst entrainment which might overload gas-solid separation means 54. Moreover, if a considerable excess of regeneration gas were injected intoriser 24, complete combustion of the volatile hydrocarbon and the coke would be effected in the vicinity of the point where the riser enters the vessel, but incomplete combustion would occur in other areas of the regeneration zone. A transverse oxygen gradient would be created and once again afterburning would occur indilute catalyst phase 34. The amount of regeneration gas injected intoriser 24 preferably is regulated by monitoring the transverse oxygen gradient, the transverse CO gradient, or a transverse temperature gradient. A schematic diagram and a detailed description of such a control system is presented hereinafter. - The amount of air utilized may be dependent in part on the type of regeneration system utilized and where the minor portion of the regeneration gas is injected to combust the volatile hydrocarbons. Where the minor portion of the regeneration gas is injected into the spent catalyst transfer line, typically this will correspond to from 2 to 20%, preferably from 4 to 15%, of the regeneration gas. In the embodiment of Figure 1, from 0 to 12% of the total regeneration gas enters through
line 28 to control catalyst circulation, while 2 to 10%, and preferably 3% to 6%, of the total regeneration gas enters through line 40 to combust the volatile hydrocarbons. - A better understanding of how the present invention may be applied to reducing and/or minimizing excessive or undesirable afterburning may be obtained by reference to Figure 2 which shows spent catalyst from a stripping zone (not shown) being introduced into the dense
phase catalyst bed 30 ofregeneration zone 26 vialine 22. Also shown is regeneration gas in line 36 passing up through grid 38 and intobed 30 where it reacts with the carbon on the spent catalyst therein such that a regenerated catalyst is passed fromzone 26 vialine 42. - A minor portion of the regeneration gas in line 36 is shown passing through
lines control valve 68 therein. Operation ofvalve 68 controls the catalyst density which, in turn, governs the catalyst circulation rate. Operation ofvalve 68 may be controlled by a signal Eg from a comparison means 70 which compares control signal E,, corresponding to the desired temperature inreaction zone 10, to signal E6, transmitting the actual temperature in the reaction zone. Typically,valve 68 is opened further to increase the catalyst circulation rate when the temperature inreaction zone 10 is too low, and, conversely, the opening in the valve is decreased when the temperature inzone 10 is too high. The catalyst circulation rate also may be varied to control other process variables such as the temperature inregeneration zone 26. An additional minor portion of the regeneration gas in line 36 is shown being passed vialines 41 and 40 into the spent catalyst line to combust volatile hydrocarbons not removed in the stripping zone which otherwise would react preferentially with the oxygen passing through grid 38, thereby causing a depletion of the oxygen in a localized area where the spent catalyst entersbed 30 such that CO rather than C02 is formed therein. This, in turn, would create transverse oxygen gradients in not onlybed 30, but indilute catalyst phase 34 when gases are passed frombed 30. Hence, undesirable or excessive afterburning would result. - However, according to one embodiment of the present invention, should such gradients exist in the dilute catalyst phase, oxygen concentrations could be sensed at, for example, spaced-apart points (1) and (2). The concentration of hydrocarbon components or nonhydro- carbon components, such as carbon monoxide, ammonia, hydrogen, or oxides of nitrogen, which are oxidizable in
regeneration zone 26, alternatively could be sensed. Or, since oxygen gradients across the vessel will result in combustion of CO to C02 in the dilute phase, a temperature gradient and a C02 gradient also will be formed which alternatively may be sensed by spaced-apart points (1) and (2). In the embodiment shown, points (1) and (2) are preferably disposed in a horizontal plane generally transverse to the direction of flow of the regeneration gas. However, with suitable biasing, points (1) and (2) could be located at any spaced-apart locations indilute phase 34 at which differences resulting from the oxygen gradient could be detected. In either event, signals E, and E2 corresponding to the sensed temperatures or oxygen concentrations at points (1) and (2), respectively, could be developed and passed into a computation means 62 suitable for calculating a transverse oxygen or temperature gradient. Suitable computation means can be selected from a variety of digital and/or analog computing devices, depending upon the particular application. For example, the computation means could be a large computer capable of controlling an entire refinery complex or, if desired, a minicomputer designed for more limited applications. Such computation means are well known articles of commerce and thus are readily available in the marketplace. - The oxygen, temperature or other gradient thus calculated can then be developed into a control signal E3 and sent to a comparison means 64 which compares signal E3 with a signal E4 corresponding to the desired transverse oxygen, temperature or other gradient at the points being monitored such that a control signal Eg is generated. The control signal Eg is then applied to a control means 66 which regulates the amount of regeneration gas introduced into the spent catalyst line via line 40. Thus, as would be obvious to one skilled in the art, the greater the deviation from the desired oxygen gradient (i.e., the greater the amount of volatile hydrocarbon introduced into bed 30), the greater will be the amount of regeneration gas employed in line 40.
- Referring to Figure 3, an alternative embodiment for practicing the subject invention is disclosed. The operation of this embodiment is generally similar to that previously described in Figures 1 and 2. In this embodiment,
riser reaction zone 110 comprises a tubular, vertical extending vessel having a relatively large height in relation to its diameter.Reaction zone 110 communicates with adisengagement zone 120, shown located a substantial height above regeneration zone 150. The catalyst circulation rate is controlled by a valve means, such asslide valve 180, located in spentcatalyst transfer line 140 extending betweendisengagement zone 120 andregeneration zone 1 50. In this embodiment hydrocarbon feedstock is injected throughline 112 intoriser reaction zone 110 filled with catalyst to catalytically crack the feedstock. Steam may be injected throughlines 160 and 162 intoreturn line 158 extending between regeneration zone 150 andreaction zone 110 to serve as a diluent, to provide a motive force for moving the hydrocarbon feedstock upwardly and for keeping the catalyst in a fluidized condition. - The vaporized, cracked feedstock products pass upwardly into
disengagement zone 120 where a substantial portion of the entrained catalyst is separated. The gaseous stream then passes through a gas-solid separation means, such as twostage cyclone 122, which further separates out entrained catalyst and returns it to the disengagement zone throughdiplegs plenum chamber 132 and exits through line 130 for further processing (not shown). The upwardly moving catalyst inreaction zone 110 gradually becomes coated with carbonaceous material which decreases its catalytic activity. When the catalyst reaches the top ofreaction zone 110 it is redirected by grid 128 into strippingzone 140 in spentcatalyst transfer line 142 where it is contacted by a stripping gas, such as steam, entering throughline 144 to partially remove the remaining volatile hydrocarbons from the spent catalyst. The spent catalyst then passes through spentcatalyst transfer line 142 into densephase catalyst bed 152 of regeneration zone 150. Oxygen containing regeneration gas enters densephase catalyst bed 152 through line 164 to maintain the bed in a turbulent fluidized condition, similar to that inriser reaction zone 110. Regenerated catalyst gradually moves upwardly through densephase catalyst bed 152 eventually flowing into overflow well 156 communicating withreturn line 158.Return line 158 is shown exiting through the center of densephase catalyst bed 152, and communicating withriser reaction zone 110. - Flue gas formed during the regeneration of the spent catalyst passes from the dense
phase catalyst bed 152 intodilute catalyst phase 154. The flue gas then passes throughcyclone 170 intoplenum chamber 172 prior to discharge throughline 174. Catalyst entrained in the flue gas is removed bycyclone 170 and is returned tocatalyst bed 152 throughdiplegs - As indicated for the previous embodiment, hydrocarbons not removed from the spent catalyst in stripping
zone 140 are combusted in preference to the coke on the spent catalyst in densephase catalyst bed 152. Thus, the area where the spent catalyst is discharged into dense phase catalyst bed becomes deficient in oxygen resulting in the formation of CO rather than CO2, while excess oxygen will be present in other areas of densephase catalyst bed 152. Where excess oxygen is present, the coke is completely converted to C02 and free oxygen also passes into thedilute catalyst phase 154, thereby resulting in the formation of dilute phase transverse oxygen gradients. When the CO from the oxygen deficient area contacts the excess oxygen in the dilute catalyst phase, undesired afterburning results from the conversion of the CO to C02. In this embodiment, the afterburning can be significantly reduced by more completely combusting the volatile hydrocarbons prior to their entry intodense phase bed 152. This may be accomplished by introducing a minor portion of the regeneration gas through line 190 into spentcatalyst transfer line 142. The point at which the regeneration gas is injected intotransfer line 142 may be less critical in this embodiment than that in the previous embodiment since here the catalyst recirculation rate is controlled byslide valve 180, rather than by the pressure in the transfer line. The regeneration gas preferably is injected downstream ofslide valve 180, most preferably close to regeneration zone 150 to minimize the effect of the regeneration gas on the catalyst flow rate and preclude operational problems. If the regeneration gas were injected upstream ofslide valve 180, this might cause overfluidization of the catalyst in thetransfer line 142 and enable regeneration gas to enter strippingzone 140 resulting in high heat release. Injection of regeneration gas downstream of, but close to,slide valve 180 also may affect the catalyst flow rate, but here the effect would be much less pronounced. Therefore, to minimize the effect of the regeneration gas on the catalyst flow rate, the gas should be injected relatively close to regeneration zone 150. One method of discharging the regeneration gas intotransfer line 142 relatively close to regeneration zone 150 is to at least partially dispose a conduit means 192 communicating with line 190 in spentcatalyst transfer line 142, with the conduit means terminating substantially near the terminus of the transfer line in densephase catalyst bed 152. Irrespective of whether a conduit means is disposed inline 142, the amount of regeneration gas added through line 190 preferably should be regulated as in the previous embodiment to minimize catalyst entrainment, and also to minimize transverse oxygen gradients. The relative amount of regeneration gas added through lines 164 and 190 can be regulated by control valve 194 in line 190. Valve 194 is controlled in a manner similar tovalve 66 shown in Figure 2, the schematic control drawing for the embodiment of Figure 1. Typically, it is believed that 4% to 16% of the total regeneration gas should be added to spentcatalyst transfer line 142 through line 190, and preferably between 8% and 14% of the total regeneration gas. - Referring now to Figure 4 a cracking system substantially similar to that of Figure 1 is shown. Here a main portion of the regeneration gas is added at or near the point where the spent catalyst mixture is discharged into the regeneration zone, whereas in Figure 1 the main portion of the regeneration gas is injected into the transfer line. In Figure 4 all elements except for the line through which a minor portion of the regeneration gas is added to combust the hydrocarbons are similar to those of Figure 1 and have similar reference numbers. In Figure 4 the line through which the minor portion of the regeneration gas is injected into the regeneration zone is denoted as
line 240. The operation of the cracking system is substantially similar to that of Figure 1 except that in this embodiment a minor portion of the regeneration gas passes throughlines phase catalyst bed 30. Normally, the spent catalyst transfer line has a diameter of 45.6 cm (18 inches) to 106.8 cm (42 inches) depending upon the desired catalyst flow rate and velocity, and the method of circulating the spent catalyst toregeneration zone 26. The amount of regeneration gas employed to combust the volatile hydrocarbons is also not critical and need be only an amount sufficent to at least partially combust the volatile hydrocarbons in the mixture with spent catalyst released into the dense phase bed. However, the amount of regeneration gas employed to combust the volatile hydrocarbons preferably is regulated to assure that only the approximate amount of regeneration gas required for combustion of the volatile hydrocarbons is added for the reasons previously indicated in connection with the discussion of the embodiment of Figure 1. The amount of regeneration gas injected into densephase catalyst bed 30 throughline 240 will correspond to from 2 to 20%, preferably from about 5 to about 9%, of the total regeneration gas. A little more regeneration gas is required throughline 240 relative to the embodiments of Figures 1 and 3 where a minor portion of the regeneration gas was injected into the spent catalyst transfer line. In the embodiment of Figure 4, when the regeneration gas is injected directly into the dense phase bed, a portion of the carbon on the spent catalyst will be combusted in addition to the volatile hydrocarbons. Also, introducing the regeneration gas directly into the bed rather than into the spent catalyst line has certain safety advantages since there is a large reservoir of catalyst available to absorb excessive amounts of heat released. - In the embodiment shown, an additional 4-12% of the total regeneration gas in line 36 is directed through
lines riser 24 to fluidize the spent catalyst and regulate the spent catalyst circulation rate. - While the embodiment shown here discloses a catalyst cracking system in which the catalyst is transferred between the reaction zone and the regeneration zone by pressure balance, the subject invention is equally applicable to other type cracking systems and to other zone configurations and positions, such as upflow and downflow regeneration systems, including those which incorporate a slide valve to control the catalyst circulation rate.
- A better understanding of how the present invention may be applied to reducing and/or minimizing excessive or undesirably afterburning may be obtained by reference to Figure 5 in which spent catalyst from a stripping zone (not shown) is introduced into the dense
phase catalyst bed 30 ofregeneration zone 26 vialine 22. Also shown is a regeneration gas in line 36 passing up through grid 38 and intobed 30 where it reacts with the carbon on the spent catalyst therein such that a regenerated catalyst is passed fromzone 26 vialine 42. A minor portion of the regeneration gas in line 36 is shown being passed vialines bed 30 such that CO rather than C02 is formed. This, in turn, would create transverse oxygen gradients in not onlybed 30, but indilute catalyst phase 34, when gases are passed frombed 30. Hence, undesirable or excessive afterburning could result. The transverse oxygen gradients would be especially pronounced where the spent catalyst entersregeneration zone 26 off-center, as in the embodiment shown. - However, according to one embodiment of the present invention, should such gradients exist in
dilute catalyst phase 34, oxygen concentrations could be sensed at, for example, spaced apart points (1) and (2). The concentration of hydrocarbon components or non-hydrocarbon components, such as carbon monoxide, ammonia, hydrogen, or oxides of nitrogen, which are oxidizable inregeneration zone 26 alternatively could be sensed. Or, since oxygen gradients acrosszone 26 will result in combustion of CO indilute catalyst phase 34, a temperature gradient also will be formed which alternatively may be sensed by spaced-apart points (1) and (2). In the embodiment shown, points (1) and (2) preferably are disposed in a horizontal plane generally transverse to the direction of flow of the gas. However, with suitable biasing, points (1) and (2) could be disposed at any spaced-apart locations indilute phase 34 at which the oxygen or other concentrations or the temperature differences resulting from the oxygen gradient could be detected. Signals E, and E2, corresponding to the sensed temperature differences or oxygen or other concentration differences between points (1) and (2), respectively, could be developed and passed into a computation means 62 suitable for calculating a transverse temperature or oxygen gradient. Suitable computation means can be selected from a variety of digital and/or analog computing devices, depending upon the particular application. For example, the computation means could be a large computer capable of controlling an entire refinery complex or, if desired, a minicomputer designed for more limited applications. Such computation means are well known articles of commerce and thus are readily available in the marketplace. - The temperature, oxygen, or other gradient thus calculated can then be developed into a control signal E3 and sent to a comparison means 64 which compares signal E3 with a signal E4 corresponding to the desired transverse gradient at the points being monitored such that a control signal Eg is generated. The control signal Eg is then applied to a control means 66 which regulates the amount of regeneration gas introduced into the dense phase bed via
line 240 preferably to minimize the measured gradient. Thus, as would be obvious to one skilled in the art, the greater the deviation from the desired gradient (i.e., the greater the amount of volatile hydrocarbon introduced into bed 30), the greater will be the amount of regeneration gas employed inline 240. It should be understood that the amount of regeneration gas used inline 240 could, alternatively, be regulated according to other variables indilute catalyst phase 34. - Therefore, combustion of the volatile hydrocarbons in mixture with the spent catalyst prior to said mixture entering the dense phase catalyst bed or within a localized area where said mixture enters the dense phase catalyst bed insures that the regeneration gas passing upward through the grid in said bed will burn only the carbon on the catalyst which is mixed throughout the bed. As such, the present invention serves to correct an imbalance in the amount of combustible material present in a localized area of the dense phase catalyst bed by removing a portion of said combustible material (the volatile hydrocarbons) immediately after their entering said bed. Such combustion of the volatile hydrocarbons also serves to prevent or minimize localized starvation of oxygen in the dense phase catalyst bed such that formation of CO rather than CO2 is minimized. As such, there will be virtually no transverse oxygen gradients in the gases leaving the dense phase catalyst bed, thereby minimizing or preventing undesirable or excessive afterburning. As illustrated in Figures 2 and 5, this can be done simply and conveniently on a continuous basis.
- In general, any commercial catalytic cracking catalyst designed for high thermal stability could be suitably employed in the present invention. Such catalysts include those containing silica and/or alumina. Catalysts containing combustion promoters such as platinum also can be used. Other refractory metal oxides such as magnesia or zirconia may be employed and are limited only by their ability to be effectively regenerated under the selected conditions. With particular regard to catalytic cracking, preferred catalysts include the combinations of silica and alumina, containing 10 to 50 wt.% alumina, and particularly their admixtures with molecular sieves or crystalline aluminosilicates. Suitable molecular sieves include both naturally occurring and synthetic aluminosilicate materials, such as faujasite, chabazite, X-type and Y-type aluminosilicate materials and ultra stable, large pore crystalline aluminosilicate materials. When admixed with, for example, silica-alumina to provide a petroleum cracking catalyst, the molecular sieve content of the finished fresh catalyst particles is suitably within the range from 5--15 wt.%, preferably 8-10 wt.%. An equilibrium molecular sieve cracking catalyst may contain as little as about 1 wt.% crystalline material. Admixtures of clay- extended aluminas may also be employed. Such catalysts may be prepared in any suitable method such as by impregnation, milling, co- gelling and the like, subject only to provision of the finished catalyst in a physical form capable of fluidization.
- As noted previously, the regeneration zone employed in the present invention normally comprises vertical cylindrical vessels wherein the catalyst to be regenerated is maintained as a fluidized bed by the upward passage of an oxygen-containing regeneration gas thereby forming a dense phase catalyst bed and a dilute catalyst phase with an interface in between. The dense phase bed, which is usually located in the lower portion of the regeneration zone, is maintained at a temperature in the range of from 621.1°C to 732.2°C (11501-13500F), preferably from 676.7 to 715.6°C (1250-1320°F). The density of the dense phase bed may range from 0.1281 to 0.481 g/cm3 (8 to 30 Ib/cu. ft).
- The dilute catalyst phase is the primarily gaseous phase volume located above the dense phase bed within the regeneration zone. Specifically, the dilute phase contains relatively small quantities of catalyst compared to the dense phase bed. For example, the density of the dilute phase zone ranges from 16.02×10-4 to 16.02x 10-3 g/cm3 (0.1 to 1.0 Ib/cu. ft.) at the inlet to the separation means and from 16.02x10-3 to 80.10x10-3 g/cm3 (1 to 5 Ib/cu. ft.) near the interface between the dense bed phase and the dilute catalyst phase. In many instances, the overall flow in the dilute phase is a concurrent flow of catalyst entrained with flue gases. It is contemplated that the dilute catalyst phase can include substantial quantities of the dense bed material which passes into that phase from excessive agitation or bubbling of gaseous materials through the dense bed. In general, the temperature in the dilute catalyst phase is at least that in the dense bed phase and is advantageously maintained within the range from 648.9 to 787.7°C (1200° to 1450°F.), preferably from 710 to 760°C (1310° to 1400°F).
- The term "substantially catalyst-free flue gas" is the gaseous phase volume located within or downstream of the catalyst separation means within the regeneration zone. Specifically, the "substantially catalyst-free flue gas" comprises flue gas from the dilute catalyst phase from which entrained catalyst particles have been substantially removed. This corresponds to the gaseous effluent from the separation means within the regeneration zone wherein the concentration of entrained catalyst particles will be less than about 1 grain (0.06479 g) preferably less than about 0.2 grains (0.12959 g) per actual cubic foot (28.32 I). The term "actual cubic foot" (28.32 I) refers to the volume measured at actual operating conditions without correction to a standard temperature and pressure. The substantially catalyst-free flue gas from the separation means may be discharged to a variety of downstream equipment such as a dispersion means to redistribute the flue gas, stack valves, a plenum chamber and the like, prior to leaving the regeneration zone. By the use of the method of the present invention, substantial afterburning, and hence excessive temperatures in that portion of the regeneration zone wherein the flue gas is substantially catalyst-free, may be avoided. Preferably, the temperature in that portion of said regeneration zone is maintained at least equal to that of the dilute catalyst phase at the inlet to the separation devices but no more than 27.8°C (50°F), preferably no more than 16.7°C (30°F), and most preferably no more than 11.1°C (20°F) above that at said inlet. Although not necessary to the practice of the present invention, extraneous cooling means such as steam may be employed to further reduce the temperature and thereby inhibit the afterburning reaction in that portion of the regeneration zone wherein the flue gas is substantially catalyst-free.
- One or more gas-solids separation means may be utilized in the dilute catalyst phase to separate entrained regenerated catalyst particles from the regeneration gas. Preferred separation means will be cyclone separators, multiclones or the like whose design and construction are well known in the art. In the case of cyclone separators, a single cyclone may be used, but preferably, more than one cyclone will be used in parallel or in series flow to effect the desired degree of separation.
- The construction of the regeneration zone can be made with any material sufficiently able to withstand the relatively high temperatures involved when afterburning is encountered within the vessel and the high attrition conditions which are inherent in systems wherein fluidized catalyst is regenerated and transported. Specifically, metals are contemplated which may or may not be lined. More specifically, ceramic liners are contemplated within any and all portions of the regeneration zone together with alloy use and structural designs in order to withstand the erosive conditions and temperatures of about 760°C (1400°F.) and, for reasonably short periods of time, temperatures which may be as high as 982.2°C (1800°F).
- The gauge pressure in the regeneration zone is usually maintained in a range from atmospheric pressure to 3.5155 kg/cm2 (50 psig.), preferably from 0.7031 to 3.5155 kg/cm2 (10 to 50 psig). It is preferred, however, to design the regeneration zone to withstand gauge pressures of up to about 7.031 kg/cm2 (100 psig). Operation of the regeneration zone at increased pressure has the effect of promoting the conversion of carbon monoxide to carbon dioxide and reducing the temperature level within the dense bed phase at which the substantially complete combustion of carbon monoxide can be accomplished. The higher pressure also lowers the equilibrium level of carbon on regenerated catalyst at a given regeneration temperature.
- The residence time of the spent catalyst in the regeneration zone is not critical. In general, it can vary from about 1 to about 6 minutes; typically, from about 2 to about 4 minutes. The contact time or residence time of the flue gas in the dilute catalyst phase established the extent to which the combustion reaction can reach equilibrium. The residence time of the flue gas may vary from about 10 to about 60 seconds in the regeneration zone and from about 2 to about 15 seconds in the dense bed phase. Preferably, the residence time of the flue gas varies from about 15 to about 20 seconds in the regeneration zone and from about 6 to about 10 seconds in the dense bed.
- The present invention may be applied beneficially to any type of fluid cat cracking unit with little or no modifications and without limitations as to the spatial arrangement of the reaction, stripping, and regeneration zones thereof. The regeneration zone of a catalytic cracking unit can be designed independently from the reaction zone since the regeneration zone merely receives spent catalyst, oxidizes the coke thereon to regenerate the catalyst, and returns the regenerated catalyst to the reaction zone. Therefore, the reaction zone can be a pure transfer line, i.e. one in which the reaction occurs in a single pipe type vessel directly terminating in a rough cut cyclone or cyclones as in Figure 3, a conventional dilute riser/dense bed combination as in Figures 1 and 4 or a dense bed alone.
- In this patent specification temperatures in °F are converted to °C by subtracting 32 and then dividing by 1.8. Temperature differences in °F are converted to °C by dividing by 1.8.
- Lengths expressed in inches are converted to cms by multiplying by 2.54, and lengths in feet are converted to cms by multiplying by 30.48.
- Weights in grains are converted to grams by multiplying by 0.06479891.
- Pressures in pounds per square inch gauge (psig) are converted to kg/cm2 by multiplying by 0.07031, and densities in pounds per cubic foot (lb/cu.ft.) are converted to g/cc by multiplying by 0.0160185.
Claims (10)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US58491 | 1979-07-18 | ||
| US58490 | 1979-07-18 | ||
| US06/058,491 US4243518A (en) | 1979-07-18 | 1979-07-18 | External method for reducing transverse oxygen gradients in FCCU regeneration |
| US06/058,490 US4243517A (en) | 1979-07-18 | 1979-07-18 | Internal method for reducing transverse oxygen gradients in FCCU regeneration |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0023402A1 EP0023402A1 (en) | 1981-02-04 |
| EP0023402B1 true EP0023402B1 (en) | 1984-05-02 |
Family
ID=26737667
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP19800302404 Expired EP0023402B1 (en) | 1979-07-18 | 1980-07-17 | Fluidized catalytic cracking with reduced dilute-phase temperature and/or composition gradients in the regenerator |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP0023402B1 (en) |
| CA (1) | CA1157796A (en) |
| DE (1) | DE3067687D1 (en) |
| ES (1) | ES493507A0 (en) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2515680A1 (en) * | 1981-10-30 | 1983-05-06 | Texaco Development Corp | Controlled catalyst regeneration in FCC regenerators - by adjustment of catalyst bed level in riser reactor |
| IL88582A (en) * | 1987-12-08 | 1993-03-15 | Tosoh Corp | 4,4)-bis (phthalimido) diphenyl sulfone compounds, processes for their production and flame retardant polymer compositions containing them |
| FR2624877B1 (en) * | 1987-12-22 | 1992-01-10 | Inst Francais Du Petrole | METHOD AND DEVICE FOR THE CATALYTIC CRACKING OF HEAVY LOADS COMPRISING A SECOND STRIPPING IN A FLUID BED |
| FR2627187B1 (en) * | 1988-02-15 | 1993-01-22 | Inst Francais Du Petrole | FLUID CRACKING PROCESS OF A HYDROCARBON LOAD |
| GB2250027A (en) * | 1990-07-02 | 1992-05-27 | Exxon Research Engineering Co | Process and apparatus for the simultaneous production of olefins and catalytically cracked hydrocarbon products |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2994659A (en) * | 1959-10-16 | 1961-08-01 | Kellogg M W Co | Method and apparatus for conversion of hydrocarbons |
| US3135700A (en) * | 1959-12-01 | 1964-06-02 | Exxon Research Engineering Co | Method and apparatus for catalyst regeneration |
| US3527694A (en) * | 1968-12-09 | 1970-09-08 | Exxon Research Engineering Co | Fluid solids system |
| US4062761A (en) * | 1975-11-28 | 1977-12-13 | Exxon Research And Engineering Company | Method for varying the catalyst circulation rate in a fluid catalytic cracking process |
| US4062759A (en) * | 1976-05-07 | 1977-12-13 | Texaco Inc. | Fluidized catalytic cracking regeneration process |
| US4035153A (en) * | 1976-05-07 | 1977-07-12 | Texaco Inc. | Fluidized cracking catalyst regeneration apparatus |
-
1980
- 1980-07-17 EP EP19800302404 patent/EP0023402B1/en not_active Expired
- 1980-07-17 DE DE8080302404T patent/DE3067687D1/en not_active Expired
- 1980-07-18 ES ES493507A patent/ES493507A0/en active Granted
- 1980-07-18 CA CA000356570A patent/CA1157796A/en not_active Expired
Also Published As
| Publication number | Publication date |
|---|---|
| ES8105025A1 (en) | 1981-05-16 |
| CA1157796A (en) | 1983-11-29 |
| EP0023402A1 (en) | 1981-02-04 |
| DE3067687D1 (en) | 1984-06-07 |
| ES493507A0 (en) | 1981-05-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4385985A (en) | FCC Reactor with a downflow reactor riser | |
| US4098680A (en) | Method of regenerating a cracking catalyst | |
| CA1305090C (en) | Ultra-short contact time fluidized catalytic cracking process | |
| US4051013A (en) | Fluid catalytic cracking process for upgrading a gasoline-range feed | |
| US6010618A (en) | FCC process with two zone short contact time reaction conduit | |
| US4419221A (en) | Cracking with short contact time and high temperatures | |
| CA1293219C (en) | Downflow fluidized catalytic cracking reactor process and apparatus with quick catalyst separation means in the bottom thereof | |
| US4849091A (en) | Partial CO combustion with staged regeneration of catalyst | |
| US4304659A (en) | Method for controlling regenerator temperature in a fluidized catalytic cracking process | |
| US4260475A (en) | Hydrocarbon cracking process | |
| JPS6359439B2 (en) | ||
| US3948757A (en) | Fluid catalytic cracking process for upgrading a gasoline-range feed | |
| US4180454A (en) | Method for combusting carbon monoxide in a fluid catalytic cracking process | |
| EP0593823A1 (en) | FCC Riser discharge separation and quench | |
| US4430201A (en) | Regeneration of fluidizable catalyst | |
| US3966587A (en) | Method for controlling regenerator temperature in a fluidized cracking process | |
| EP0023402B1 (en) | Fluidized catalytic cracking with reduced dilute-phase temperature and/or composition gradients in the regenerator | |
| US4428822A (en) | Fluid catalytic cracking | |
| US4522704A (en) | Passivation of cracking catalysts | |
| US4243517A (en) | Internal method for reducing transverse oxygen gradients in FCCU regeneration | |
| EP0490453A1 (en) | Process and apparatus for removal of carbonaceous materials from particles containing such materials | |
| US3963603A (en) | Fluid catalytic cracking | |
| US4062761A (en) | Method for varying the catalyst circulation rate in a fluid catalytic cracking process | |
| US4243518A (en) | External method for reducing transverse oxygen gradients in FCCU regeneration | |
| US4895637A (en) | Resid cracking process and apparatus |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): BE DE FR GB IT NL |
|
| 17P | Request for examination filed |
Effective date: 19810106 |
|
| ITF | It: translation for a ep patent filed | ||
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Designated state(s): BE DE FR GB IT NL |
|
| REF | Corresponds to: |
Ref document number: 3067687 Country of ref document: DE Date of ref document: 19840607 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| ITTA | It: last paid annual fee | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19970625 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19970627 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19970630 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19970701 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19970711 Year of fee payment: 18 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980717 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980731 |
|
| BERE | Be: lapsed |
Owner name: EXXON RESEARCH AND ENGINEERING CY Effective date: 19980731 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990201 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 19980717 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990331 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 19990201 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990501 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |