CN1314909A - Aryl phosphate derivatives of d4T having anti-HIV activity - Google Patents
Aryl phosphate derivatives of d4T having anti-HIV activity Download PDFInfo
- Publication number
- CN1314909A CN1314909A CN99810072A CN99810072A CN1314909A CN 1314909 A CN1314909 A CN 1314909A CN 99810072 A CN99810072 A CN 99810072A CN 99810072 A CN99810072 A CN 99810072A CN 1314909 A CN1314909 A CN 1314909A
- Authority
- CN
- China
- Prior art keywords
- hiv
- compound
- composition
- cell
- aryl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7068—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid
- A61K31/7072—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid having two oxo groups directly attached to the pyrimidine ring, e.g. uridine, uridylic acid, thymidine, zidovudine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H19/00—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof
- C07H19/02—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof sharing nitrogen
- C07H19/04—Heterocyclic radicals containing only nitrogen atoms as ring hetero atom
- C07H19/06—Pyrimidine radicals
- C07H19/10—Pyrimidine radicals with the saccharide radical esterified by phosphoric or polyphosphoric acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H19/00—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof
- C07H19/02—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof sharing nitrogen
- C07H19/04—Heterocyclic radicals containing only nitrogen atoms as ring hetero atom
- C07H19/16—Purine radicals
- C07H19/20—Purine radicals with the saccharide radical esterified by phosphoric or polyphosphoric acids
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Molecular Biology (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Virology (AREA)
- Communicable Diseases (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oncology (AREA)
- Tropical Medicine & Parasitology (AREA)
- AIDS & HIV (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Saccharide Compounds (AREA)
Abstract
Aryl phosphate nucleosise derivatives of formula (I) show potent activity against HIV without undesirable levels of cytotoxic activity. Examples of aryl phosphate nucleoside derivatives include aryl phosphate derivatives of d4T with para-bromo substitution on the aryl group. In particular, these derivatives are potent inhibitors of HIV reverse transcriptase. In a preferred aspect of the present invention, the phosphorus of the aryl phosphate group is further substituted with an amino acid residue that may be esterified or substituted, such as a methoxy alaninyl group. In said formula, Y is oxygen or sulfur; R1 is unsubstituted aryl or aryl substituted with an electron-withdrawing group; R2 is a nucleoside of formula (II or III).
Description
Invention field
The present invention relates to the aryl phosphate nucleoside derivates, 2 ' particularly, 3 '-dihydro-2 ', the aryl phosphate derivatives of 3 '-dideoxy thymidine (after this claiming " d4T "), this material has effective activity to human immune deficiency virus (HIV), for example as the inhibitor of hiv reverse transcriptase.
Background of invention
The propagation of AIDS and control this virus constantly to make great efforts be best proof.The method of a kind of HIV of control is exactly to suppress the activity (RT) of its reversed transcriptive enzyme.Therefore, need new, effectively and optionally HIV RT inhibitor is as useful medicine.Known valid HIVRT inhibitor comprises 2 ', the analogue of 5 ' of 3 '-di-deoxynucleoside-triguaiacyl phosphate (" ddN ").These active RT inhibitor are to produce by acting in the cell of nucleoside kinase and nucleoside monophosphate kinase.Therefore, think that in the research of anti-HIV reagent ddN compound such as AZT and d4T are very likely.
3 '-azido--3 '-deoxythymidine (azidothymidine, AZT) rate-limiting step that is converted into its bioactive metabolites AZT triguaiacyl phosphate is that its phosplate derivative is converted into the bisphosphate derivative, and it was reported, in cell, produce bioactive 2 ', 3 '-two dehydrogenation-2 ', the rate-limiting step of the metabolite d4T-triguaiacyl phosphate of 3 '-two dehydrogenation thymidine (d4T) is that this nucleosides is converted into its phosplate derivative (Balzarini etc., 1989, J.Biol.Chem.264:6127; McGuigan etc., 1996, J.Med.Chem.39:1748).This mechanism that proposes in prior art is seen Fig. 1.
In order to overcome the dependence of nucleosides kinase activator in the ddN analogue pair cell, McGuigan etc. have prepared AZT (McGuigan etc., 1993, J.Med. Chem.36:1048; McGuigan etc., 1992, Antiviral Res., 17:311) and d4T (McGuigan etc., 1996, J.Med.Chem.39:1748; McGuigan etc., 1996, aryl phosphate methoxyl group alaninyl ester derivative Bioorg.Med.Chem.Lett.6:1183).The result shows that this compounds produces the phosplate derivative through hydrolysis in the cell, and described derivative obtains bioactive triguaiacyl phosphate derivative by the further phosphorylation of the effect of thymidylate kinase under the pattern that does not rely on thymidine kinase (TK)., the aryl phosphate derivatives that all replacements by various aryl moieties up to the present further improve d4T render a service and do not follow the Cytotoxic trial that increases them all fail (McGuigan etc., 1996, J.Med.Chem.39:1748).
The present invention finds to have the substituting group of electrophilic part on the aryl moiety of the aryl phosphate derivatives of nucleosides, during as the contraposition bromine substituent since this substituent electrophilic character can improve d4T nucleoside derivates bear hydrolysis ability.The phosphoric acid phenylester nucleoside derivates that replaces presents effective and special antiviral activity.
Summary of the invention
The present invention relates to the aryl phosphate nucleoside derivates, 2 ' particularly, 3 '-two dehydrogenation-2 ', the aryl phosphate derivatives of 3 '-dideoxy thymidine (after this claiming " d4T "), this derivative has effective activity to HIV, for example as the inhibitor of hiv reverse transcriptase.Go out to exhale and expect the aryl phosphate derivatives that d4T is found on ground, for example have the derivative that an electrophilic replaces as has a right-bromo to replace on aryl, it is renderd a service obviously and strengthens as a kind of inverase, and does not have the cytotoxicity of undesirable level.In detail, these derivatives are effective inhibitor of hiv reverse transcriptase.Of the present invention one preferred aspect, can further replace phosphorus in the aryl phosphate ester group with amino-acid residue, described amino-acid residue can esterification or replacement, as the methoxyl group alaninyl.
For example, the phosphoric acid phenyl methoxyl group alaninyl ester derivative of the d4T that replaces of right-bromo as a kind of effective anti-hiv agent suppress effectively HIV in peripheral blood lymphocytes (PBMNC) and TK-defective type CEM T-cell duplicate and do not have can detected cytotoxicity.In addition, this new d4T derivative d4T-5 '-(phosphoric acid right-bromophenyl methoxyl group alaninyl ester) is a kind of HIV-1 bacterial strain to AZT and NNI-tolerance to RTMDR-1, has effective antiviral activity, and HIV-2 is had medium activity.Equally, accordingly the phosphoric acid phenyl methoxyl group alaninyl ester AZT derivative that bromine is replaced shows effective anti-HIV activity in PBMNC and TK defective type CEM T-cell, and is still invalid to AZT-and NNI-resistance RTMDR-1 or HIV-2.With these d4T and the contrast of AZT derivative, corresponding 3dT derivative-3dT-5 '-(phosphoric acid right-bromophenyl methoxyl group alaninyl ester) presents the modified activity above 3dT, but ineffective as d4T and AZT derivative in PBMNC or TK-defective type CEM T-cell.As far as we know, this is a unheeded structure-activity relationship before the report for the first time, and this relation has determined the drug effect of the phosphoric acid phenyl ester derivatives of d4T and AZT.
Lead compound d4T-5 '-(phosphoric acid right-bromophenyl methoxyl group alaninyl ester) and AZT-5 '-(phosphoric acid right-bromophenyl methoxyl group alaninyl ester) provides the basis for effective HIV therapeutic strategy design, this strategy can suppress duplicating of HIV, particularly suppresses its duplicating in the TK-deficient cell.
The accompanying drawing summary
Fig. 1 is the d4T aryl phosphate derivatives metabolic pathway synoptic diagram that prior art proposes.
Fig. 2 A and 2B diagram can improve the electrophilic hypothesis of substituted benzene ring hydrolysis.
Fig. 2 C is an elution profile, illustrates each test-compound hydrolysis and produces A-d4T: compound 2, wherein X=H (square hollow); Compound 3, wherein X=OCH
3(solid squares); With compound 4, X=Br (solid circles) wherein.
Fig. 2 D is an elution profile, the susceptibility of the enzymically hydrolyse that the diagram test-compound causes the liver esterase of pig.
Fig. 3 is an elution profile, and hydrolysis in the cell of compound 2-4 in TK-defective type cem cell is described.In the lysate sample aliquot of the cem cell of hatching with compound 4, detect metabolite peak only corresponding to the 680pmol of A-d4T-MP.
The chemical structure of Fig. 4 A-4F graphic compound 6c (Fig. 4 A) and compound 7c (Fig. 4 B); Compound 6c (Fig. 4 C) and compound 7c (Fig. 4 D) are to the anti-HIV activity of HTL VIII B in PBMNC or TK-defective type CEM T-cell; Reach compound 6c (Fig. 4 E) and compound 7c (Fig. 4 F) antiviral activity to HIV-1 (HTLV VIII B), HIV-2 and RTMDR-1.The % that duplicates with inhibition HIV represents antiviral activity, measures according to the activity of RT in the cells infected.
Fig. 5 A and 5B are synoptic diagram, are shown in the resonance effect (electron delocalization) on the phenyl ring, therefore expect that the para-orienting group on the phenyl ring has identical electronic effect with ortho-substituent.
Detailed Description Of The Invention
We find that beyond expectationly the aryl phosphate derivatives of some replacement of nucleosides has the HIV-resistant activity of increase and keeps simultaneously low-level cytotoxicity. Therefore, these derivatives are specially adapted to the activating agent of the method that infects as the activating agent of antiviral composition and as treatment virus infections such as HIV. Compound of the present invention
As discussing more fully in following examples, the compounds of this invention is the aryl phosphate nucleosise derivative with effective antiviral activity, is the derivative of d4T and AZT in detail. The nucleoside derivates that is suitable for the present composition and the inventive method is following formula: compound or its pharmaceutically acceptable salt:Wherein Y is oxygen or sulphur, preferred oxygen; R1Aryl for unsubstituted aryl or electron withdraw group replacement; R2Nucleosides for formula II or III:R wherein6Be purine or pyrimidine, preferred pyrimidine; And R7、R
8、R
9、R
10、R
11And R12Independent be hydrogen, hydroxyl, halogen, azido ,-NO2、-NR
13R
14Or-N (OR15)R
16, R wherein13、R
14、R
15And R16Independent is hydrogen, acyl group, alkyl or cycloalkyl; R3Be hydrogen, acyl group, alkyl or cycloalkyl; R4Be amino acid whose side chain; Or R3And R4Can be in conjunction with the side chain that forms proline or hydroxy-proline; And R5Be hydrogen, alkyl, cycloalkyl or aryl.
Term " aryl " comprises aryl radical such as phenyl, comprises the aromatic rings such as the naphthyl that condense. This class group can not be substituted on aromatic rings or be replaced by an electron withdraw group, such as halogen (bromine, chlorine, fluorine, iodo), NO2Or acyl group. The aryl of an electron withdraw group replacement is preferably bromophenyl, more preferably the 4-bromophenyl.
Term " acyl group " comprises formula R
17C (O)-substituting group, R wherein
17Be hydrogen, alkyl or cycloalkyl.
Term " alkyl " comprises the straight or branched representative examples of saturated aliphatic hydrocarbon chain that contains 1 to 6 carbon atom, as methyl, ethyl, propyl group, sec.-propyl (1-methylethyl), butyl, tertiary butyl (1, the 1-dimethyl ethyl) or the like.This class group can not be substituted or by hydroxyl, halogen, azido-,-NO
2,-NR
13R
14Or-N (OR
15) R
16Replace, wherein R
13, R
14, R
15And R
16As preceding definition.
Term " cycloalkyl " comprises the saturated aliphatic hydrocarbon ring that contains 3 to 7 carbon atoms, as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl etc.This class group can not be substituted or by hydroxyl, halogen, azido-,-NO
2,-NR
13R
14Or-N (OR
15) R
16Replace, wherein R
13, R
14, R
15And R
16As preceding definition.
Term " purine " comprises VITAMIN B4 and guanine.
Term " pyrimidine " comprises uridylic, thymus pyrimidine and cytosine(Cyt).Described pyrimidine is preferably thymus pyrimidine.
Term " amino acid whose side chain " refers to the amino acid group that changes comprise for example side chain of glycine, L-Ala, arginine, l-asparagine, aspartic acid, halfcystine, Gelucystine, L-glutamic acid, glutamine, hydroxylysine, Isoleucine, leucine, Methionin, methionine(Met), phenylalanine, Serine, Threonine, tryptophane, tyrosine, Xie Ansuan etc.Described amino acid side chain is preferably L-Ala or tryptophane side chain.
Usually, contain the compound of electron-withdrawing substituent, for example at the ortho position shown in Fig. 5 A and the 5B or contraposition by halogen or NO
2Replace, more effectively hydrolysis provides the activity inhibitor compound.Preferred halogen replaces, and most preferably right-bromo replaces.
In order to set forth the compositions and methods of the invention comprehensively, the d4T derivative is described.The d4T derivative contains aryl phosphate and replaces, and aryl wherein contains an electrophilic and replaces, as ortho position or contraposition halogen (Br, Cl, F, I) substituting group or NO
2Substituting group.Example, wherein a R have been shown below
18Be one can esterification or the amino-acid residue of replacement as-NHCH (CH
3) COOCH
3Or its pharmacy acceptable salt or ester.
Synthesizing of d4T derivative:
In order to set forth the synthetic of The compounds of this invention, the synthetic of d4T derivative described.Can be prepared as follows the d4T derivative.Be used in Mansuri etc., 1989, J.Med.Chem.32, the method for discussing in 461 prepares d4T by thymidine, and document disclosure is attached to herein by reference.By at McGuigan etc., 1992, the method for discussing among the Antiviral Res.17:311 can prepare chloro phosphoric acid (phosphorochloridate) the substituted aryl ester of suitable replacement, and document disclosure is attached to herein by reference.The chloro phosphoric acid ester is joined in the anhydrous tetrahydrofuran solution that contains the N-Methylimidazole of d4T so that form the product of needs.
Adopt the suitable compositions form to give the patient with the d4T derivative, said composition contains as the derivative of the d4T of promoting agent or AZT and pharmaceutically acceptable carrier, adjuvant or thinner.Can use slow release formulation if desired.Said composition is needed antiviral patient according to suitable antiviral dosage, and for example, described dosage suppresses hiv reverse transcriptase effectively and/or suppresses HIV duplicating at host cell.According to the administration of appropriate dosage scheme.
Embodiment
Further explain the present invention with the following example, should not be considered as these embodiment and limit the present invention.
Synthetic and the evaluation of d4T derivative
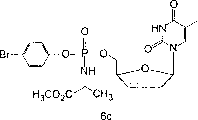
According to Mansuri, etc., 1989, J.Med.Chem.32,461 method prepares d4T 1 with thymidine.According to McGuigan etc., 1992, Antiviral Res., the phenylmethoxyalaninyl phosphorochloridate that the preparation of 17:311 reported method suitably replaces.According to summarizing synthetic compound 2-4 in the scheme 1 below.
Scheme 1. joins phenylmethoxyalaninyl phosphorochloridate in anhydrous tetrahydro furan (THF) solution of d4T and 1-Methylimidazole and at room temperature stirred this mixture 5-6 hour.Reaction mixture provides needed derivative with good yield.Adopt column chromatography to obtain purified compound.
By the physical data of HPLC measurement synthetic compound, the condition of carrying out of HPLC is C184 * 250mm LiChrospher post, and as eluent, flow velocity was 1ml/ minute with 70: 30 water/acetonitriles.Purity by the HPLC following compounds surpasses 96%.Because diastereo-isomerism body and function asterisk mark
13C NMR peak is a splitted.
Compound 2: productive rate 81%; IR (Neat): 3222,2985,2954,1743,1693,1593,1491,1456,1213,1153,1039,931,769 cm
-1 1H NMR (CDCl
3) δ 9.30 (brs, 1H), 7.30-7.10 (m, 6H), 6.85-6.82 (m, 1H), 6.36-6.26 (m, 1H), 5.91-5.85 (m, 1H), 5.00 (br m, 1H), 4.19-3.68 (m, 4H), 3.72,3.71 (s, 3H), 1.83,1.80 (d, 3H), 1.38-1.25 (m, 3H);
13C NMR (CDCl
3) δ 173.9,163.7,150.7,149.7,135.7
*, 133.2
*, 129.6
*, 127.3
*, 125.0
*, 120.0,111.1,89.6
*, 84.5
*, 66.9
*, 52.5
*, 50.0
*, 20.9 and 12.3;
31P NMR (CDCl
3) δ 2.66,3.20; MALDI-TOF mass spectrum m/e 487.9 (M+Na); HPLC retention time: 5.54 and 5.85 minutes.
Compound 3: productive rate 92%; IR (Neat): 3223,3072,2999,2953,2837,1743,1693,1506,1443,1207,1153,1111,1034,937,837 and 756 cm
-1 1H NMR (CDCl
3) δ 9.40 (br s, 1H), 7.30-7.00 (m, 5H), 6.83-6.81 (m, 1H), 6.37-6.27 (m, 1H), 5.91-5.86 (m, 1H), 5.00 (br m, 1H), 4.40-4.30 (m, 2H), 4.20-4.10 (m, 2H), 3.95-3.93 (s, 3H), 3.82-3.80 (s, 3H), 1.85-1.81 (s, 3H), and 1.39-1.29 (m, 3H);
13C NMR (CDCl
3) δ 174.0,163.9,156.6,150.8,143.5,135.8
*, 133.3
*, 127.4
*, 121.2
*, 114.5,111.2,89.7
*, 84.5,66.9
*, 55.5,52.5,50.6
*, 20.9 and 12.3;
31P NMR (CDCl
3) δ 3.82,3.20; MALDI-TOF mass spectrum m/e 518.2 (M+Na); HPLC retention time: 5.83 and 6.26 minutes.
Compound 4: productive rate 83%; IR (Neat): 3203,3070,2954,2887,2248,1743,1693,1485,1221,1153,1038,912,835,733 cm
-1 1H NMR (CDCl
3) δ 9.60-9.58 (br s, 1H), 7.45-7.42 (m, 2H), 7.30-7.09 (m, 4H), 6.37-6.27 (m, 1H), 5.93-5.88 (m, 1H), 5.04-5.01 (br m, 1H), 4.35-4.33 (m, 2H), 4.27-3.98 (m, 2H), 3.71-3.70 (s, 3H), 1.85-1.81 (s, 3H), and 1.37-1.31 (m, 3H);
13C NMR (CDCl
3) δ 173.7,163.8,150.8,149.7
*, 135.6
*, 133.1
*, 127.4
*, 121.9
*, 11 8.0,111.2
*, 89.7
*, 84.4
*, 67.8
*, 52.5,50.0
*, 20.7 and 12.3;
31P NMR (CDCl
3) δ 3.41,2.78; MALDI-TOF mass spectrum m/e 567.1 (M+Na); HPLC retention time: 12.04 and 12.72 minutes.
Embodiment 2
Compound 2-4 is to the susceptibility of hydrolysis
Fig. 2 A and 2B are shown in the electronic effect of the para-orienting group on the phenyl ring of meta-bolites precursor B (see figure 1).In order to assess the susceptibility of compound to hydrolysis, compound 2-4 is dissolved in the methyl alcohol, handle with 0.002N NaOH then.Keep constant concentration and with the generation of HPLC monitoring hydrolysate A-d4T-MP.Carry out the HPLC chromatography with the Lichrospher post.Under isocratic condition, carry out wash-out and elution profile is seen Fig. 2 C with the mixed solvent coupled columns of 70: 30 water/acetonitriles.
The hydrolysis of test compound under the liver esterase system of pig.Data show in Fig. 2 C.Under 37 ℃, the compound 2 and 4 (1mM in Tris-HCl) and the Tris-HCl damping fluid (pH7.4) of the pig liver esterase (Sigma) of 100U were hatched 2 hours.By adding acetone and cooling off this reaction mixture reaction is stopped.15, under the 000xg centrifugal after, detect the active metabolite that exists in the 0.1ml equal portions reaction mixture with a kind of quantitative analysis HPLC method that can detect the 50pmol meta-bolites.0.1ml the reaction product of the compound 4 of equal portions contains the A-d4T-MP of 1.4nmol, and does not detect this meta-bolites in the reaction product of compound 2.
Shown in Fig. 2 A and 2B, the existence of the electron-withdrawing substituent in the contraposition of phenyl moiety has increased the hydrolysis rate (Fig. 2 A and 2B) of phenoxy group group among the meta-bolites precursor B probably, precursor B is that the first step (Fig. 1, A to B) in the metabolic pathway of the phosphoric acid phenyl ester derivatives of d4T depends on Procaine esterase and produces.Single bromine substituent in the phenyl ring contraposition can not influence identification and the hydrolysis (A to B step in Fig. 1) of Procaine esterase to this compound.The electronic effect that is caused by electrophilic contraposition bromine substituent can increase the hydrolysis generation D of the C of phenoxy group group and produce the precursor that E is crucial meta-bolites A-d4T-MP subsequently.In order to test described hydrolysis, by measuring the generation of alaninyl-d4T-phosplate (A-d4T-MP), we have compared unsubstituted compound 2, right-methoxyl group (OCH
3) compound 4 that replaces of the compound 3 that replaces and right-bromo (=d4T-5 '-[phosphoric acid right-bromophenyl methoxyl group alaninyl ester] or d4T-pBPMAP) in the speed of handling the back chemical hydrolysis with 0.002N NaOH.
Shown in Fig. 2 C, the compound 4 with right-bromine substituent presents hydrolysis rate faster than unsubstituted compound 2, and has electron donating group-OCH in contraposition
3Compound 3 have slower hydrolysing rate than any in these two kinds of compounds.Similarly, 4 pairs of enzymically hydrolyses that caused by the liver esterase of pig of lead compound are than compound 2 more responsive (Fig. 2 D).
The endocellular metabolism of compound 2-4 in T-defective type cem cell
For the endocellular metabolism of analysis of compounds 2-4 in T-defective type cem cell, with 1 * 10
6Cem cell and compound 2-4 (100uM) were hatched 3 hours, checked phosphodiester meta-bolites, the alaninyl d4T phosplate of partial hydrolysis subsequently with HPLC.Amount that it should be noted that described meta-bolites in the cem cell of handling with compound 4 is apparently higher than the amount (680pmol/10 with the described meta-bolites in compound 2 or 3 cem cells of handling
6Cell and<50pmol/10
6Cell; Fig. 3).
In the substratum that RPMI, 10% foetal calf serum and 1% penicillin/streptomycin are formed, cultivate cem cell.Under 37 ℃, according to 10
6The density of cell/ml is with 1 * 10
6These compounds of cell and 100uM were hatched 3 hours.After hatching, with twice ice-cooled of PBS washed cell and add 0.5ml 60% methanol extraction.The lysate of cell preserved down at-20 ℃ spend the night, then 15, centrifugal lysate 10 minutes is to remove cell debris under the 000xg.These lysates of every part 100 μ L are injected directly into HPLC.This HPLC system has Hewlett Packard (HP) 1100 series of four-stage pump, an automatic sampler, an electronic degasser, a diode-array detector and the computer with the chemstation software program that is used for data analysis by one and forms.With described sample wash-out on 250 * 4.6mm Sulpelco LC-DB C18 post.Utilize the solvent gradient to resolve meta-bolites and parent compound, it contains the mixture (pH3.7) of methyl alcohol and 10mM ammonium phosphate.Carry out gradient elution with 1ml/ minute flow velocity, preceding 10 minutes with 5 to 35% methanol-eluted fractions, continues with 35% methanol-eluted fractions 5 minutes, uses 35 to 100% methyl alcohol linear gradient wash-out 20 minutes more at last.The detection wavelength is 270nm.Only detect a retention time and be 8.7 minutes metabolite peak in the equal portions of the cem cell lysate of hatching with compound 4, this peak is corresponding to the A-d4T-MP of 680pmol.
Because the hydrolysis susceptibility of its raising supposes that it is a more effective anti-HIV reagent that compound 4 is compared with other compound.In peripheral blood lymphocytes and TK-defective type CEM T-cell, with aforesaid method (Zarling etc., 1990 Nature 347:92; Erice etc., 1993Antimicrob.Agents Chemother.37: 835; Uckun etc., 1998 Antimicrob.Agents Chemother.42:383) test compounds 2-4 and parent compound d4T (1) suppress the ability that HIV duplicates.The p24 of the p24 of the cells infected by relatively being tried mass treatment and RT activity value and the cells infected that is untreated and RT activity value calculate the percentage that suppresses virus replication.Simultaneously, the same with the microculture tetrazolium assay (MTA) of cell proliferation as what in the article of above Zarling, Enrice and Uckum, describe, detect the cytotoxicity of described compound.
Table 1 shows the similar IC that inhibition HIV-1 duplicates
50Value provides when testing in hiv-1-infected peripheral blood lymphocytes, and the d4T aryl phosphate derivatives is unlike this parent compound d4T effective evidences more.Consistent with former report, in fact d4T suppresses the ability reduction that HIV-1 duplicates in TK-defective type cem cell.Otherwise d4T suppresses to produce the IC of p24
50Value is 18nM in peripheral blood lymphocytes, is 556nM in TK-defective type cem cell.Similarly, suppress the active IC of RT
50Value is increased to 2355nM (table 1) from 40nM.Although 3 kinds of all aryl phosphate derivatives are all effective than d4T in TK-defective type cem cell, there is the compound 4 (d4T-5 '-[phosphoric acid right-bromophenyl methoxyl group alaninyl ester]) of a right-bromine substituent suppressing in the p24 generation than the strong 12.6 times of (IC of d4T at aryl moiety
50Value: 44nM is to 556nM) and suppressing on the RT activity than d4T strong 41.3 times (the IC50 value: 57nM is to 2355nM) (table 1).
Table 1
| ????PBMNC | ????CEM | ||||||
| Compound | X | ?IC 50[p24] | IC 50[RT] | IC 50[MTA] | IC 50[p24] | IC 50[RT] | IC 50[MTA] |
| ?1(=d4T) | ?0.018 | ??0.040 | >10 | ?0.556 | ?2.355 | >10 | |
| ?2 | ?H | ?ND | ??ND | >10 | ?0.145 | ?0.133 | >10 |
| ?3 | ?-OCH 3 | ?0.033 | ??0.033 | >10 | ?0.106 | ?0.320 | >10 |
| ?4 | ?Br | ?0.022 | ??0.042 | >10 | ?0.044 | ?0.057 | >10 |
Measure by MTA, up to 10, during 000nM peripheral blood lymphocytes or cem cell being presented without any one can detected cytotoxicity in concentration for all test-compounds.What is interesting is to have the substituent compound 3 of right-methoxyl group at aryl moiety and hanging down 5.6 times of (IC than compound 4 aspect the RT of the TK-defective type cem cell that suppresses the HIV infection activity
50Value: 320nM is to 57nM), though these two compounds present similar activity (IC in peripheral blood lymphocytes
50Value: 33nM is to 42nM).Therefore, the consistent HIV (human immunodeficiency virus)-resistant activity of aryl phosphate derivatives in the TK-deficient cell that influences d4T that show of contraposition-substituting group.As far as we know, this be for the first time demonstration by introduce at aryl moiety single right-in fact bromine substituent can improve the drug effect and the selectivity index of the aryl phosphate derivatives of d4T.The structure-activity relation of the aryl moiety decision of in the past unknown phosphate derivative by d4T be possible more effective d4T analogue design provide the foundation.
Embodiment 4
Compound 4 and the AZT activity in the MDR cell
With compound 4 (d4T-5 '-[phosphoric acid right-bromophenyl methoxyl group alaninyl ester]) anti-HIV-MDR cell activity and AZT-5 '-[phosphoric acid right-bromophenyl methoxyl group alaninyl ester] (P-AZT) and AZT compare.Use embodiment 4 described hatching and analytical procedure.
Just as shown in table 2, P-AZT has similar activity, IC with AZT
50Value is respectively 1.5 and 2.0nM.More effective 100 times of the activity of compound 4 (0.02nM) than AZT (2.0nM).
Table 2
| ????HIV-2 | ????HIV-MDR | |
| Compound | ????IC 50[RT] | ???IC 50??[RT] |
| ????4 | ????0.4 | ????0.02 |
| ????P-AZT | ????3.9 | ????1.5 |
| ????AZT | ????2.4 | ????2.0 |
Embodiment 5
Synthesizing of 3dT aryl phosphate derivatives
By further comparison, research is in the active influence of the various substituting group antagonism HIV of the aryl moiety of the aryl phosphate derivatives of 3 '-deoxythymidine (3dT).Shown in scheme 2,, prepare d4T 1 from thymidine with literature method (Mansuri etc., 1989 J.Med.Chem.32:461-466) by d4T 1 preparation 3dT 5.In ethanol, at H
2And carry out 1 hydrogenization under the existence of the 5%Pd/C of catalytic amount and obtain 3dT 5, yield is 85%.Also according to McGuigan etc., the phenylmethoxyalaninyl phosphorochloridate that the preparation of 1992 Antiviral Res 17:311-321 reported method suitably replaces, and according to the method synthetic compound 6-11 that in scheme 2, describes.
The phenylmethoxyalaninyl phosphorochloridate that scheme 2. will suitably replace joins in the mixture of anhydrous tetrahydro furan (THF) of 3dT and 1-Methylimidazole.At room temperature stirred this reaction mixture 12 hours and removed and desolvate.The gum that produces is dissolved in the chloroform again and with 1M HCl, saturated sodium hydrogen carbonate solution (NO
2The derivative situation makes an exception) and water washing.Organic phase MgSO
4Dry and under vacuum, remove and desolvate.This crude product of silica gel rapid column chromatography purifying in order to the chloroformic solution wash-out of 5% methyl alcohol obtains purified compound 6-11 with good yield.
Measured the physical data of described synthetic compound.The condition of carrying out of HPLC is C184 * 250mm LiChrospher post, and as eluent, flow velocity was 1ml/ minute with 70: 30 water/acetonitriles.Because HPLC, the purity of following compounds surpasses 96%.Because diastereo-isomerism body and function asterisk mark
13C NMR peak is a splitted.
Compound 5: productive rate 85%;
1H NMR (CDCl
3) δ 11.1 (br s, 1H), 7.82 (s, 1H), 5.97-5.94 (m, 1H), 5.10 (br s, 1H), 4.05-3.95 (m, 1H), 3.72-3.52 (m, 2H), 2.30-1.86 (m, 4H), 1.77 (s, 3H);
13C NMR (CDCl
3) δ 163.9,150.4,136.4,108.7,84.8,81.4,62.2,31.8,25.1 and 12.5.
Compound 6: productive rate 96%; IR (neat): 3211,2955,2821,1689,1491,1265,1211,1153,1043 and 933 cm
-1 1H NMR (CDCl
3) δ 10.1 (br s, 1H), 7.47 (s, 1H), 7.32-7.12 (m, 5H), 6.14-6.08 (m, 1H), and 4.41-4.21 (m, 4H), 4.05-4.00 (m, 1H), 3.70,3.69 (s, 3H), and 2.37-2.32 (m, 1H), 2.05-1.89 (m, 7H), 1.38-1.35 (dd, 3H);
13C NMR (CDCl
3) δ 173.6
*, 163.8,150.3,150.1
*, 135.2,129.4
*, 124.7,119.8
*, 110.5
*, 85.7
*, 78.3
*, 67.2
*, 52.3,50.1
*, 31.6
*, 25.4
*, 20.7
*With 12.4
* 31P NMR (CDCl
3) δ 2.82﹠amp; 3.11; MS (MALDI-TOF): 490.4 (M+Na); HPLC retention time=6.86,7.35 minute.
Compound 7: productive rate 96%; IR (neat): 3217,2954,2821,1743,1689,1489,1265,1217,1153,1092,1012,926 and 837cm
-1 1H NMR (CDCl
3) δ 9.40 (br s, 1H), 7.43-7.41 (m, 1H), 7.30-7.14 (m, 4H), 6.13-6.07 (m, 1H), 4.39-4.00 (m, 5H), 3.71,3.70 (s, 3H), 2.38-2.36 (m, 2H), 2.09-1.89 (m, 5H), 1.39-1.36 (dd, 3H);
13C NMR (CDCl
3) δ 173.6
*, 163.7,150.2,148.8
*, 135.3,129.5-129.0,121.5-121.3,116.3,110.6,86.0
*, 78.4
*, 67.7
*, 52.6
*, 50.2
*, 31.8
*, 25.4
*, 20.9
*With 12.5;
31P NMR (CDCl
3) δ 2.87﹠amp; 3.09; MS (MALDI-TOF): 524.9 (M+Na); HPLC retention time=14.05,14.89 minute.
Compound 8: heavy-gravity oily matter, productive rate 96%; λ
Max: 223 (ε 3338) and 269 (ε 4695) nm; IR (neat): 3211,2955,1743,1693,1500,1569,1265,1197,1153,1045,923 and 843cm
-1 1H NMR (CDCl
3) δ 9.40 (br s, 1H), 7.45-7.43 (d, 1H), 7.19-7.01 (m, 4H), 6.14-6.06 (m, 1H), 4.39-3.97 (nm, 5H), 3.71,3.70 (s, 3H), 2.38-1.89 (m, 7H), 1.39-1.35 (t, 3H);
13C NMR (CDCl
3) δ 173.6
*, 163.7,150.2,150.1
*, 135.3,121.5
*, 116.3
*, 110.6
*, 85.9
*, 78.4
*, 67.7
*, 52.6,50.2
*, 31.8
*, 25.6
*, 20.9
*With 12.5;
31P NMR (CDCl
3) δ 3.13 and 3.37; MS (MALDI-TOF): 508.2 (M+Na); HPLC retention time=8.38,8.80 minute.
Compound 9: productive rate 83%; IR (neat): 3211,2954,1743,1689,1485,1265,1217,1153,1010,923 and 833cm
-1 1H NMR (CDCl
3) δ 9.82 (br s, 1H), 7.45-7.41 (m, 3H), 7.15-7.11 (m, 2H), 6.14-6.06 (m, 1H), 4.39-4.00 (m, 5H), 3.71,3.70 (s, 3H), 2.38-1.89 (m, 7H), 1.39-1.35 (dd, 3H);
13C NMR (CDCl
3) δ 173.6
*, 163.8,150.3,148.5
*, 135.2,132.6
*, 121.8
*, 117.7,110.6
*, 85.9
*, 78.3
*, 67.2
*, 52.5,50.2
*, 31.6
*, 25.6
*, 20.8
*With 12.5;
31PNMR (CDCl
3) δ 2.83 and 3.05; MS (MALDI-TOF): 570.0 (M+2+Na); HPLC retention time=15.50,16.57 minute.
Compound 10: productive rate 87%; IR (neat): 3203,2955,1743,1684,1593,1522,1348,1265,1153,1101,920 and 860cm
-1 1H NMR (CDCl
3) δ 9.51 (br s, 1H), 8.24-8.21 (m, 2H), 7.42-7.37 (m, 3H), 6.13-6.08 (m, 1H), 4.39-4.03 (nm, 5H), 3.72,3.71 (s, 3H), 2.38-1.89 (m, 7H), 1.41-1.38 (dd, 3H);
13CNMR (CDCl
3) δ 173.4
*, 163.7,155.2
*, 150.2
*, 144.4,135.3,125.9-125.4,120.6
*, 115.4,110.6
*, 86.1
*, 78.4
*, 68.1
*, 52.7,50.2
*, 31.7
*, 25.8
*, 20.9
*With 12.5;
31P NMR (CDCl
3) δ 2.60 and 2.81; MS (MALDI-TOF): 535.0 (M+Na); HPLC retention time=8.12,10.14 minute.
Compound 11: productive rate 100%; IR (neat): 3209,2954,1743,1506,1468,1265,1207,1153,1036,937 and 835 cm
-1 1H NMR (CDCl
3) δ 9.89 (br s, 1H), 7.49-7.47 (m, 1H), 7.16-7.11 (m, 2H), 6.84-6.80 (m, 2H), 6.15-6.09 (m, 1H), 4.39-4.02 (m, 5H), 3.77,3.76 (s, 3H), 3.74,3.73 (s, 3H), 2.38-1.89 (m, 7H), 1.38-1.33 (t, 3H);
13C NMR (CDCl
3) δ 173.7
*, 163.9,156.3,150.3,143.7
*, 135.2,120.7
*, 114.3
*, 110.5,85.7
*, 78.4
*, 67.3
*, 55.4,52.4,50.1
*, 31.8
*, 25.4
*, 20.8
*With 12.4
* 31P NMR (CDCl
3) δ 3.27 and 3.52; MS (MALDI-TOF): 521.3 (M+1+Na); HPLC retention time=7.15,7.66 minute.
Embodiment 6
The antiviral activity of 3dT compound 6-11
Method (Zarling etc., 1990 with former description; Erice etc., 1993; Uckun etc., 1998, the same) in peripheral blood lymphocytes and TK-defective type CEM T-cell, to suppress the ability that HIV-1 duplicates with parallel mode test compounds 6-11 and the parent compound 3dT relatively of d4T.
The activity of 3dT and its derivative is than d4T low (table 3) in peripheral blood lymphocytes and TK-defective type CEM T-cell.It should be noted that the IC of compound 6-11 in peripheral blood lymphocytes
50[RT] value is higher than the IC of 3dT
50[RT] value (1.2-3.1 to 0.7, table 3) hints that these prodrugs are sufficiently stable, does not rely on the step of TK in their metabolism, and perhaps their enzymically hydrolyse may be the rate-limiting step that produces actives.By contrast, the aryl phosphate derivatives of report d4T is more effective than d4T, hint rely on TK produce the rate-limiting step that the d4T phosplate is its metabolic activation (McGuigan etc., 1996a).According to bioactive bibliographical information result (McGuigan etc. about the aryl phosphate derivatives of d4T and AZT, 1993,1996a), the aryl phosphate derivatives of 3dT in the TK-deficient cell, suppress HIV-1 duplicate more effective than its parent compound 3dT, though still have high micromole IC
50[RT] is worth (table 3).
Because the activity of compound 6-11 is low in peripheral blood lymphocytes (PBMNC) in TK-defective type CEM T-cell, be to carry out with lower speed so the supposition 3dT phosplate that these prodrugs produce when lacking TK is converted into its active triguaiacyl phosphate.By comparison, the aryl phosphate derivatives of d4T presents similar activity (McGuigan etc., 1996 Bioorg.Med.Chem.Lett.6:1183-1186) in normal cell and TK-deficient cell.The HIV (human immunodeficiency virus)-resistant activity of the aryl phosphate derivatives of 3 '-deoxythymidine (6-11) in normal peripheral blood mononuclear cells (PBMNC) and TK-defective type CEM T-cell.All data are represented with uM and are represented the concentration that suppresses virus replication, measure by the RT activation analysis, with 50% (IC
50[RT])
9Or 50% cytotoxicity concentration represent, by MTA (IC
50[MTA]) measurement (Mansuri etc., 1989 J.Med.Chem.32:461).
Table 3
| ????PBMNC | ????CEM | ||||
| Compound | ????X | ????IC 50????[RT] | ?IC 50?[MTA] | ????IC 50????[RT] | ????IC 50????[MTA] |
| ????6 | ????H | ????2.1 | ????>100 | ????7.5 | ????>100 |
| ????7 | ????Cl | ????2.1 | ????>100 | ????21.9 | ????>100 |
| ????8 | ????F | ????3.1 | ????>100 | ????32.7 | ????>100 |
| ????9 | ????Br | ????1.2 | ????>100 | ????22.8 | ????>100 |
| ????10 | ????NO | ????2.0 | ????>100 | ????22.6 | ????>100 |
| ????11 | ????OMe | ????1.3 | ????>100 | ????19.7 | ????>100 |
| ????3dT | ????- | ????0.7 | ????>100 | ????91.2 | ????>100 |
| ?d4T | ????- | ????0.004 | ????>100 | ????2.335 | ????>100 |
Shown in Fig. 5 A and 5B, the electronic effect of para-orienting group may influence that the B hydrolysis is converted into D in the metabolic pathway of the aryl phosphate derivatives of the 3dT that Fig. 1 describes on the phenyl ring.The existence that is expected at the contraposition electron-withdrawing substituent of phenyl moiety among the compound 7-10 (Fig. 2 A and 2B) will increase the speed of the phenoxy group group hydrolysis that replaces.; these compounds are unlike the compound 6 that does not have para-orientation or to have a compound 11 of the sub-para-orienting group of power supply more effective, and the effect that reaches speed limit of a key is played in therefore prompting hypothesis depends on Procaine esterase in their new old generation (A is to B in Fig. 1) the first step hydrolysis for the generation of active 3dT meta-bolites.Therefore, according to the metabolic pathway that proposes for the aryl phosphate methoxyl group alaninyl ester derivative of nucleoside analog, compound 7-10 can be used as the less relatively substrate (McIntee etc., 1997 J.Med.Chem.40:3323-3331) of Procaine esterase that supposition causes its hydrolysis.
Embodiment 7
The HIV (human immunodeficiency virus)-resistant activity of d4T, AZT and 3dT derivative
Shown in scheme 1, the usefulness literature method (Mansuri etc., 1989, the same) prepare d4T 1 from thymidine.At H
2And in ethanol, carrying out 1 hydrogenization under the existence of the 5%Pd/C of catalytic amount, the yield with 85% obtains 3dT 3 (scheme 1).
Prepare AZT 2 with literature method (Chu etc., United States Patent (USP) the 4th, 841, No. 039) from thymidine.(McGuigan etc. 1992 partly to have ddN phosphoric acid agent 5a, the 5b of different substituents and 5c by two step programs (scheme 2) preparations at its phenoxy group with the obtainable phenol in commercial channel, the same), reported compound 4a, 4b, 5a, 5b, 7a and 7b in the past.Compound 4c and 5c are new compound and synthetic method and the characteristic of reporting them below.
Shown in scheme 3, carry out synthetic (McGuigan etc., 1992) of the phosphoric acid phenyl methoxyl group alaninyl ester derivative of d4T 1, AZT 2 and 3dT 3 according to following document condition.Conventional synthetic method is as follows: the phenylmethoxyalaninyl phosphorochloridate 5 that will suitably replace joins in anhydrous tetrahydro furan (THF) mixture of the ddN (1,2 or 3) of needs and 1-Methylimidazole.At room temperature stirred this reaction mixture 12 hours, and removed then and desolvate.The gum that obtains is dissolved in the chloroform again and with 1M HCl, saturated sodium hydrogen carbonate solution and water washing.Organic phase MgSO
4Dry and under vacuum, remove and desolvate.With the mixed solvent of methyl alcohol and chloroform this crude product of silica gel rapid column chromatography purifying, obtain the neat compounds that needs with good yield as eluent.
Synthesizing of scheme 2. phenylmethoxyalaninyl phosphorochloridates.
Synthesizing of the phosphoric acid phenyl methoxyl group alaninyl ester derivative of scheme 3.ddN.
The phosphoric acid dichloro is right-bromophenyl ester 4c. is according to McGuigan etc., 1993, the same, the method for description, under 0 ℃, nitrogen atmosphere, in 3 hours with right-bromophenol (13.20g; 76.30mmol) and the anhydrous Et of distillatory triethylamine (10.65ml)
2O (165ml) drips of solution be added to vigorous stirring phosphoryl chloride (8.5ml; 91.2mmol) anhydrous Et
2In O (83ml) solution.Subsequently the mixture that produces is warmed to room temperature gradually, at room temperature stirs and spend the night, be heated to then and refluxed two hours.Reaction mixture is cooled to room temperature and under aspirator pressure, filters.Use anhydrous Et
2O (2 * 50ml) washing precipitations.With the Et that merges
2The O layer is evaporated to the dried thick product 4c that obtains in rotatory evaporator, a kind of lurid oily matter carries out this oily matter vacuum distilling then and obtains purified 4c (14.05g; 63.5% productive rate), a kind of colourless thickness oily matter (bp.110-115 ℃/2mmHg).IR(Neat):3095,1481,1303,1187,948,829cm
-1;
1H?NMR(300MHz,CDCl
3)δ?7.50(2H,d,J=9.0Hz),7.15(2H,d,J=9.0Hz)。GC/MS(m/e)290(M
+),254(M
+-Cl),173(M
+-POCl
2,
81Br),171(M
+-POCl
2,
79Br),156(M
+-PO
2Cl
2,
81Br),154(M
+-PO
2Cl
2,
79Br)。Chloro phosphoric acid is right-bromophenyl methoxyl group alaninyl ester 5c.According to McGuigan etc., the same, the method for description under-70 ℃, nitrogen environment, will be distilled triethylamine (8.80ml in 3 hours; 63.14mmol) anhydrous CH
2Cl
2(180ml) solution through the phosphoric acid dichloro that an addition funnel is added drop-wise to vigorous stirring right-bromophenyl ester 4c (8.69g; 29.97mmol) and L-alanine methyl ester hydrochloride (4.19g; 30.02mmol) anhydrous CH
2Cl
2(250ml) in the solution.Subsequently the mixture that produces is warmed to gradually room temperature and at room temperature stirs and spend the night.In rotatory evaporator, remove and desolvate.Add anhydrous Et
2O (300ml) dissolves this resistates and filters so that remove white solid under aspirator pressure.Use anhydrous Et
2O (2 * 60ml) flushing white solids.Merge Et
2The O layer also is evaporated to driedly, obtains the 5c (10.7g) of quantitative yield, a kind of light pink xanchromatic thickness oily matter.Need not be further purified and this product can be directly used in next step reaction.IR (Neat): 3212,2989,2952,1747,1483,1270,1209,1147,927,831,757cm
-1 1H NMR (300MHz, CDCl
3) δ 8.70 (1H, br, Ala-NH), 7.48 (2H, d, J=9.0Hz, aryl H), 7.16 (2H, d, J=9.0Hz, aryl H), 3.79 and 3.77 (3H, s﹠amp; S ,-OCH
3), 1.51 and 1.40 (3H, d﹠amp; D, Ala-CH
3).MS(CI,m/e)357.9(M
+,
81Br),355.9(M
+,
79Br),322.0(M
+-Cl,
81Br),320.0(M
+-Cl,
79Br),297.9(M
+-COOCH
3,
81Br),295.9(M
+-COOCH
3,
79Br),184.0(M
+-BrC
6H
4O)。The characteristic of the phosphoric acid phenyl methoxyl group alaninyl ester derivative of AZT 1, d4T 2 and 3dT 3: the condition of carrying out of HPLC is C18 4 * 250mm LiChrospher post, and as eluent, flow velocity was 1ml/ minute with 70: 30 water/acetonitriles.Because HPLC, the purity of following compounds surpasses 96%.Because the diastereomer that the stereocenter of phosphorus causes, with star-like sign flag
13C NMR peak is a splitted.Compound 6a: productive rate 81%; IR (Neat): 3222,2985,2954,1743,1693,1593,1491,1456,1213,1153,1039,931,769cm
-1 1H NMR (CDCl
3) δ 9.30 (brs, 1H), 7.30-7.10 (m, 6H), 6.85-6.82 (m, 1H), 6.36-6.26 (m, 1H), 5.91-5.85 (m, 1H), 5.00 (br m, 1H), 4.19-3.68 (m, 4H), 3.72,3.71 (s, 3H), 1.83,1.80 (d, 3H), 1.38-1.25 (m, 3H);
13C NMR (CDCl
3) δ 173.9,163.7,150.7,149.7,135.7
*, 133.2
*, 129.6
*, 127.3
*, 125.0
*, 120.0,111.1,89.6
*, 84.5
*, 66.9
*, 52.5
*, 50.0
*, 20.9 and 12.3;
31P NMR (CDCl
3) δ 2.66,3.20; MALDI-TOF mass spectrum m/e:487.9 (M+Na); HPLC retention time: 5.54 and 5.85 minutes.Compound 6b: productive rate 92%; IR (Neat): 3223,3072,2999,2953,2837,1743,1693,1506,1443,1207,1153,1111,1034,937,837 and 756cm
-1 1H NMR (CDCl
3) δ 9.40 (br s, 1H), 7.30-7.00 (m, 5H), 6.83-6.81 (m, 1H), 6.37-6.27 (m, 1H), 5.91-5.86 (m, 1H), 5.00 (br m, 1H), 4.40-4.30 (m, 2H), 4.20-4.10 (m, 2H), 3.95-3.93 (s, 3H), 3.82-3.80 (s, 3H), 1.85-1.81 (s, 3H) and 1.39-1.29 (ml, 3H);
13C NMR (CDCl
3) δ 174.0,163.9,156.6,150.8,143.5,135.8
*, 133.3
*, 127.4
*, 121.2
*, 114.5,111.2,89.7
*, 84.5,66.9
*, 55.5,52.5,50.6
*, 20.9 and 12.3;
31P NMR (CDCl
3) δ 3.82,3.20; MALDI-TOF mass spectrum m/e:518.2 (M+Na); HPLC retention time: 5.83 and 6.26 minutes.Compound 6c: productive rate 83%; IR (Neat): 3203,3070,2954,2887,2248,1743,1693,1485,1221,1153,1038,912,835,733cm
-1 1H NMR (CDCl
3) δ 9.60-9.58 (br s, 1H), 7.45-7.42 (m, 2H), 7.30-7.09 (m, 4H), 6.37-6.27 (m, 1H), 5.93-5.88 (m, 1H), 5.04-5.01 (br m, 1H), 4.35-4.33 (m, 2H), 4.27-3.98 (m, 2H), 3.71-3.70 (s, 3H), 1.85-1.81 (s, 3H), and 1.37-1.31 (m, 3H);
13C NMR (CDCl
3) δ 173.7,163.8,150.8,149.7
*, 135.6
*, 133.1
*, 127.4
*, 121.9
*, 118.0,111.2
*, 89.7
*, 84.4
*, 67.8
*, 52.5,50.0
*, 20.7 and 12.3;
31P NMR (CDCl
3) δ 3.41,2.78; MALDI-TOF mass spectrum m/e:567.1 (M+Na); HPLC retention time: 12.04 and 12.72 minutes.Compound 7c: productive rate 95%; IR (Neat): 3205.7,3066.3,2954.5,2109.8,1745.3,1691.3,1484.9,1270.9,1153.2,1010.5 and 926.1cm
-1 1HNMR (300MHz, CDCl
3) δ 8.69 (1H, br, 3-NH), 7.45 (2H, d, J=9.0Hz, aryl H), 7.34 and 7.32 (1H, s﹠amp; S, vinyl H), 7.11 (2H, d, J=9.0Hz, aryl H), 6.18﹠amp; 6.13 (1H, t﹠amp; T, J=6.6﹠amp; 6.6Hz, C-1 ' position H), 4.44-3.77 (6H, m, C-3 ', 4 ' and 5 ' H, Ala-NH and Ala-CH), 3.73﹠amp; 3.72 (3H, s﹠amp; S ,-COOCH
3), 2.51-2.20 (2H, m, C-2 ' position H), 2.18 (3H, s, C-5 position-CH
3), 1.39﹠amp; 1.36 (3H, d﹠amp; D, Ala-CH
3);
13C NMR (75MHZ, CDCl
3) δ 173.6,163.6,150.1,149.2,149.1,135.2,132.4,121.6,117.8,111.1,85.0,84.7,81.9,81.8,65.5,60.1,59.9,52.4,50.0,49.9,36.9,20.6,20.5,12.2.MS (CI, m/e): 589.1 (M
+,
81Br) and 587.1 (M
+,
79Br).Compound 8a: productive rate 96%; IR (Neat): 3211,2955,2821,1689,1491,1265,1211,1153,1043 and 933cm
-1 1H NMR (CDCl
3) δ 10.1 (br s, 1H), 7.47 (s, 1H), 7.32-7.12 (m, 5H), 6.14-6.08 (m, 1H), and 4.41-4.21 (m, 4H), 4.05-4.00 (m, 1H), 3.70,3.69 (s, 3H), and 2.37-2.32 (m, 1H), 2.05-1.89 (m, 7H), 1.38-1.35 (dd, 3H);
13C NMR (CDCl
3) δ 173.6
*, 163.8,150.3,150.1
*, 135.2,129.4
*, 124.7,119.8
*, 110.5
*, 85.7
*, 78.3
*, 67.2
*, 52.3,50.1
*, 31.6
*, 25.4
*, 20.7
*With 12.4
* 31P NMR (CDCl
3) δ 2.82﹠amp; 3.11; MS (MALDI-TOF): 490.4 (M+Na); HPLC retention time=6.86,7.35 minute.Compound 8b: productive rate 100%; IR (Neat): 3209,2954,1743,1506,1468,1265,1207,1153,1036,937 and 835cm
-1 1H NMR (CDCl
3) δ 9.89 (br s, 1H), 7.49-7.47 (m, 1H), 7.16-7.11 (m, 2H), 6.84-6.80 (m, 2H), 6.15-6.09 (m, 1H), 4.39-4.02 (m, 5H), 3.77,3.76 (s, 3H), 3.74,3.73 (s, 3H), 2.38-1.89 (m, 7H), 1.38-1.33 (t, 3H);
13C NMR (CDCl
3) δ 173.7
*, 163.9,156.3,150.3,143.7
*, 135.2,120.7
*, 114.3
*, 110.5,85.7
*, 78.4
*, 67.3
*, 55.4,52.4,50.1
*, 31.8
*, 25.4
*, 20.8
*With 12.4
* 31P NMR (CDCl
3) δ 3.27,3.52; MS (MALDI-TOF): 521.3 (M+1+Na); HPLC retention time=7.15,7.66 minute.Compound 8c: productive rate 83%; IR (Neat): 3211,2954,1743,1689,1485,1265,1217,1153,1010,923 and 833cm
-1 1H NMR (CDCl
3) δ 9.82 (br s, 1H), 7.45-7.41 (m, 3H), 7.15-7.11 (m, 2H), 6.14-6.06 (m, 1H), 4.39-4.00 (m, 5H), 3.71,3.70 (s, 3H), 2.38-1.89 (m, 7H), 1.39-1.35 (dd, 3H);
13C NMR (CDCl
3) δ 173.6
*, 163.8,150.3,148.5
*, 135.2,132.6
*, 121.8
*, 117.7,110.6
*, 85.9
*, 78.3
*, 67.2
*, 52.5,50.2
*, 31.6
*, 25.6
*, 20.8
*With 12.5;
31P NMR (CDCl
3) δ 2.83,3.05; MS (MALDI-TOF): 570.0 (M+2+Na); HPLC retention time=15.50,16.57 minute.HTL VIII B), AZT-and NNI-resistance HIV-1 (virus strain: RTMDR-1) (be so kind as to give by Dr.Brendan Larder HIV (human immunodeficiency virus)-resistant activity and Cytotoxic cell analysis are with the HIV-1 of AZT-sensitivity (virus strain:, NIH AIDS Research and Reference Reagent Program, DIV.AIDS, NIAID, NIH; Cat.#2529) or HIV-2 (virus strain: CBL-20) the TK-defective type CEM T-cell that infects of peripheral blood lymphocytes of Gan Raning (PBMNC) and HTL VIII B, reach 50% needed compound concentration assessment HIV (human immunodeficiency virus)-resistant activity by measuring the inhibition virus replication, this mensuration is the activation analysis (IC based on reversed transcriptive enzyme
50[RT]).The RT activity value of the cells infected by relatively being tried mass treatment and the RT activity value of untreated cells infected (being virus control) calculate the percentage that suppresses virus replication.By microculture tetrazolium assay (MTA), adopt 2, two (2-methoxyl group-4-nitro-5-sulfur phenenyl)-5-[phenylamino-carbonyls of 3-]-2H-tetrazolium oxyhydroxide (XTT), detect 50% cytotoxicity (CC of described compound
50[MTA]) (Zarling etc., 1990; Erice etc., 1993, Uckun etc., 1998, the same).D4T-5 '-(phosphoric acid right-bromophenyl methoxyl group alaninyl ester) and AZT-5 '-(phosphoric acid right-bromophenyl methoxyl group alaninyl ester) is as the effectively evaluation of anti-hiv agent
When testing among the PBMNC that infects at HIV-1, d4T phosphoric acid phenyl ester derivatives is more effective unlike its parent compound d4T.The ability that D4T inhibition HIV-1 duplicates in TK-defective type cem cell obviously descends.D4T suppresses the active IC of RT
50Value is 40nM in PBNMC, and is 2400nM (table 4 and Fig. 4 A-4F) in TK-defective type cem cell.Although all three kinds of phosphoric acid phenyl ester derivatives are more effective than d4T in TK-defective type cem cell, the compound 6c (d4T-5 '-[phosphoric acid right-bromophenyl methoxyl group alaninyl ester]) that has right-bromine substituent at phenyl moiety suppress RT active aspect than the strong 60 times of (IC of d4T
50Value: 60nM is to 2400nM) (table 4).
Measure by MTA, up to 10, during 000nM PBMNC or cem cell being presented without any one can detected cytotoxicity in concentration for all compounds.What is interesting is to have the substituent compound 6b of right-methoxyl group at phenyl moiety and hanging down 5 times of (IC than compound 6c aspect the RT of the TK-defective type cem cell that suppresses the HIV infection activity
50Value: 300nM is to 60nM), so these two compounds present similar activity (IC in peripheral blood lymphocytes
50Value: 30nM is to 40nM) (table 4).
Test compounds 7a, 7b, 7c and their parent compound AZT 2 suppress the ability (table 4) that HIV duplicates in PBMNC and TK-defective type CEM T-cell.The RT activity value of the cells infected by relatively being tried mass treatment and the RT activity value of untreated cells infected calculate the percentage that suppresses virus replication.Simultaneously, cultivate the cytotoxicity that tetrazolium analytical method (MTA) detects described compound with the trace of hyperplasia.The ability that AZT2 inhibition HIV-1 duplicates in TK-defective type cem cell significantly descends.AZT suppresses the active IC of RT
50Value is 3nM in PBNMC, is 200nM in TK-defective type cem cell.Be different from corresponding d4T derivative, when testing in the TK-defective type CEM T-cell that infects at HIV-1, the phosphoric acid phenyl ester derivatives of the unsubstituted and para-orientation of AZT is more effective unlike parent compound AZT., the phosphoric acid of AZT is right-and phenyl ester derivatives that bromine replaces is AZT-5 '-(phosphoric acid right-bromophenyl methoxyl group alaninyl ester) 7c, and the validity of duplicating at the HIV that suppresses TK-defective type cem cell is 5 times of (IC of AZT
50[RT] value: 0.04uM is to 0.2uM).Measure by MTA, up to 10, during 000nM PBMNC or cem cell being presented without any a compound can detected cytotoxicity in concentration.
Relatively parallel with 4TT 1, test compounds 8a-c and parent compound 3dT 3 thereof suppress the ability that HIV-1 duplicates in PBMNC and TK-defective type CEMT-cell.The activity of 3dT and derivative thereof is than d4T low (table 4) in peripheral blood lymphocytes and TK-defective type CEM T-cell.It should be noted that in peripheral blood lymphocytes the IC of compound 8a-c
50[RT] value is higher than the IC of 3dT
50[RT] value (1.2-3.1 to 0.7, table 4), pointing out these prodrugs is that step sufficiently stable and that in their metabolism, perhaps do not rely on TK in its enzymically hydrolyse may be the rate-limiting step that produces actives.According to bioactive bibliographical information result about the phosphoric acid phenyl ester derivatives of d4T and AZT, the phosphoric acid phenyl ester derivatives of 3dT in the TK-deficient cell, suppress HIV-1 duplicate more effective than its parent compound 3dT, though still have high micromole IC
50[RT] is worth (table 4 and Fig. 4 A-4F).Because the activity of compound 8a-c is low in PBMNC in TK-defective type CEM T-cell, so we infer that these prodrugs produce when lacking TK 3dT phosplate is converted into it and has active triguaiacyl phosphate and carry out with low-down speed.
The HIV (human immunodeficiency virus)-resistant activity of the phosphoric acid phenyl methoxyl group alaninyl ester derivative of table 4d4T, AZT and 3dT in normal peripheral blood lymphocytes (PBMNC) and TK-defective type CEM T-cell
| ????PBMNC | ????CEM | ||||
| Compound | ????X | ????IC 50????[RT] | ??IC 50??[MTA] | ????IC 50????[RT] | IC 50[MTA] |
| ????6a | ????H | ????N.D | ????N.D | ????0.1 | ????>10 |
| ????6b | ????OCH 3 | ????0.03 | ????>10 | ????0.3 | ????>10 |
| ????6c | ????Br | ????0.04 | ????>10 | ????0.06 | ????>10 |
| ????7a | ????H | ????N.D | ????N.D | ????1.7 | ????>10 |
| ????7b | ????OMe | ????0.1 | ????>10 | ????4.1 | ????>10 |
| ????7c | ????Br | ????0.004 | ????>10 | ????0.04 | ????>10 |
| ????8a | ????H | ????2.1 | ????>10 | ????7.5 | ????>10 |
| ????8b | ????OMe | ????1.3 | ????>10 | ????19.7 | ????>10 |
| ????8c | ????Br | ????1.2 | ????>10 | ????22.8 | ????>10 |
| ?1(d4T) | ????- | ????0.04 | ????>10 | ????2.4 | ????>10 |
| ?2(AZT) | ????- | ????0.003 | ????>10 | ????0.2 | ????>10 |
| ?3(3dT) | ????- | ????0.7 | ????>10 | ????91.2 | ????>10 |
The activity of lead compound d4T-5 '-(phosphoric acid right-bromophenyl methoxyl group alaninyl ester) and AZT-5 '-(phosphoric acid right-bromophenyl methoxyl group alaninyl ester) anti-HIV-2 and RTMDR-1
With AZT 2 parallel comparisons, the inhibition ability (table 5) that test compounds 6c and 7c duplicate the HIV of RTMDR-1 in PBMNC (a kind of AZT and NNI resistance HIV-1 strain) and HIV-2.New d4T derivative 6c is d4T-5 '-(phosphoric acid right-bromophenyl methoxyl group alaninyl ester), and RTMDR-1 is had effective antiviral activity and HIV-2 is had moderate activity., the phosphoric acid of Dui Ying AZT right-bromine substituted-phenyl methoxyl group alaninyl ester derivative 7c and its parent compound AZT 2 can not anti-effectively AZT resistance RTMDR-1 or anti-HIV-2.
Table 5 lead compound 6c and the 7c HIV (human immunodeficiency virus)-resistant activity in HIV-2 and RTMDR-1 cell
| ????HIV-2 | ????RTMDR-1 | |
| Compound | ?IC 50???[RT] | ????IC 50????[RT] |
| ????6c | ????0.4 | ????0.02 |
| ????7c | ????3.9 | ????1.5 |
| ????2(AZT) | ????2.4 | ????2.0 |
Concentration (the IC of inhibition virus replication 50% needs is represented and represented to all data with uM
50[RT]), measure by the RT activation analysis.
Compound 6a, 6b and 6c are more effective than their parent compound d4T 1 in TK-defective type cem cell, and in the PBMNC that HIV-1 infects these d4T phosphoric acid phenyl ester derivatives (6a, 6b and 6c) unlike their parent compound d4T 1 more effective (table 4).Relatively more all d4T phosphoric acid phenyl methoxyl group alaninyl ester derivatives, d4T-5 ' in TK-defective type cem cell-(phosphoric acid right-bromophenyl methoxyl group alaninyl ester) 6c is the most effective anti-hiv agent.Described observations may be owing to the right-bromine substituent on the phenyl moiety of 6c, because the ability that the phosphorus that the electrophilic character (Fig. 2) of bromine substituent makes this substituting group increase 6c is hydrolyzed and cause in TK-defective type CEM T-cell, producing obviously the more crucial meta-bolites d4T phosplate (McIntee etc. of a large amount, 1997, J.Med.Chem.40:3233-3331).
The drug effect of the phosphoric acid phenylester of AZT, phosphate methoxy phenylester and phosphoric acid bromophenyl ester derivative also has the same drug effect trend with the d4T derivative, just 7c (bromophenyl ester)>7a (phenylester)>7b (p-methoxy-phenyl ester) in TK-defective type cem cell., in these three kinds of AZT phosphoric acid phenyl methoxyl group alaninyl ester derivatives (7a, 7b and 7c), in TK-defective type cem cell, have only 7c to have the drug effect (IC higher than AZT
50Value: 40nM is to 200nM).For 3dT phosphoric acid phenyl methoxyl group alaninyl ester derivative, expect that phenoxy group that existence at the contraposition electron-withdrawing substituent of phenyl moiety will increase the replacement of compound 8c rolls into a ball the speed (for example the B among Fig. 2 is to C) of hydrolysis.; therefore 8c is unlike the 8a compound that does not have para-orientation or to have a 8b compound of the sub-para-orienting group of power supply more effective, and propose such hypothesis: first hydrolysing step (for example the A among Fig. 2 is to B) that depends on Procaine esterase in their metabolism plays the effect that reaches speed limit of a key for the generation of active 3dT meta-bolites.For the phosphoric acid phenyl methoxyl group alaninyl ester derivative (Fig. 2) of nucleoside analog, we infer that according to the metabolic pathway that is proposed, compound 8a, 8b and 8c may work the relatively poor substrate of inferring the carboxylicesters that causes hydrolysis.The effect of 3dT aryl phosphate derivatives is not desired as metabolism and active open works about the closely similar nucleoside analog d4T of prodrug type.Make that we are very surprised to be, the 3dT aryl phosphate derivatives does not cause HIV (human immunodeficiency virus)-resistant activity likely in normal peripheral blood mononuclear cells that HIV-1 infects or TK-defective type CEM T-clone.
In a word, think that d4T-5 '-(phosphoric acid right-bromophenyl methoxyl group alaninyl ester) 6c and AZT-5 '-(phosphoric acid right-bromophenyl methoxyl group alaninyl ester) 7c is the promoting agent of anti-HIV, can suppress effectively in TK-defective type CEM T-cell that HIV duplicates and without any can detected cytotoxicity.In addition, described new d4T derivative 6c is that a kind of AZT and NNI resistance HIV-1 strain have effective antiviral activity and HIV-2 is had moderate activity to RTMDR-1.Compare with the AZT derivative with these d4T, corresponding 3dT derivative is that 3dT-5 '-(phosphoric acid right-bromophenyl methoxyl group alaninyl ester) does not have significant HIV (human immunodeficiency virus)-resistant activity in PBMNC or TK-defective type CEM T-cell.As far as we know, this is the structure-activity relationship in the past do not recognized of roundup first, the effectiveness of the phosphoric acid phenyl ester derivatives of this relation decision d4T and AZT.The further exploitation of lead compound 6c and 7c can provide basis for designing effective HIV therapeutic strategy, and this strategy can suppress HIV and duplicate in the TK-deficient cell.
Although detailed description of the present invention more than is provided, the present invention is not limited thereto.Can revise the present invention described here, comprise flexible embodiment, this is conspicuous for a person skilled in the art.Connect following claim, should think that all these class accommodations all within the spirit and scope of the present invention.
Claims (30)
1. method that is suppressed at the hiv reverse transcriptase in the HIV cells infected, this method comprise and give the following formula: compound that cells infected effectively suppresses dosage:
Wherein Y is oxygen or sulphur; R
1Be unsubstituted aryl or the aryl that electron withdrawing group replaces; R
2Nucleosides for formula II or III:
Wherein
R
6Be purine or pyrimidine;
R
7, R
8, R
9, R
10, R
11And R
12Independent be hydrogen, hydroxyl, halogen, azido-,-NO
2,-NR
13R
14Or-N (OR
15) R
16, R wherein
13, R
14, R
15And R
16Independent is hydrogen, acyl group, alkyl or cycloalkyl; R
3Be hydrogen, acyl group, alkyl-cycloalkyl; R
4Be amino acid whose side chain; Or R
3And R
4Can be in conjunction with forming proline(Pro) or oxyproline side chain; And R
5Be hydrogen, alkyl, cycloalkyl or aryl.
2. the process of claim 1 wherein that Y is an oxygen.
3. the process of claim 1 wherein R
1Be bromophenyl.
4. the process of claim 1 wherein R
6Be thymus pyrimidine, cytosine(Cyt) or uridylic.
5. the process of claim 1 wherein R
4Side chain for L-Ala or tryptophane.
7. the method for claim 6, wherein X is halogen or NO
2
8. the method for claim 7, wherein X is a bromo.
9. the method for claim 8, wherein bromo is a para-orientation.
11. the method for claim 10, wherein R
18For-NHCH (CH
3) COOCH
3
13. the method for claim 12, wherein X is halogen or NO
2
14. the method for claim 13, wherein X is a bromo.
15. the method for claim 14, wherein bromo is a para-orientation.
17. the method for claim 16, wherein R
18For-NHCH (CH
3) COOCH
3
18. one kind is suppressed the composition that HIV duplicates in host cell, said composition comprises the following formula: compound and the pharmaceutically acceptable carrier of significant quantity, and described dosage can effectively suppress HIV and duplicate in host cell:
Wherein Y is oxygen or sulphur; R
1Be unsubstituted aryl or the aryl that electron withdrawing group replaces; R
2Nucleosides for formula II or III:
Wherein
R
6Be purine or pyrimidine;
R
7, R
8, R
9, R
10, R
11And R
12Independent be hydrogen, hydroxyl, halogen, azido-,-NO
2,-NR
13R
14Or-N (OR
15) R
16, R wherein
13, R
14, R
15And R
16Independent is hydrogen, acyl group, alkyl or cycloalkyl; R
3Be hydrogen, acyl group, alkyl or cycloalkyl; R
4Be amino acid whose side chain; Or R
3And R
4Can be in conjunction with forming proline(Pro) or oxyproline side chain; And R
5Be hydrogen, alkyl, cycloalkyl or aryl.
19. the composition of claim 18, wherein Y is an oxygen.
20. the composition of claim 18, wherein R
1Be bromophenyl.
21. the composition of claim 18, wherein R
6Be thymus pyrimidine, cytosine(Cyt) or uridylic.
22. the composition of claim 18, wherein R
4Be L-Ala or tryptophane side chain.
23. one kind is suppressed the composition that HIV duplicates in host cell, said composition comprises formula IV or the V compound and the pharmaceutically acceptable carrier of effective dose, and described dosage can effectively suppress HIV and duplicate in host cell:
Wherein X is an electron-withdrawing group.
24. the composition of claim 23, wherein X is halogen or NO
2
25. the composition of claim 24, wherein X is a bromo.
26. the composition of claim 25, wherein bromo is a para-orientation.
27. one kind is suppressed the composition that HIV duplicates in host cell, said composition comprises the following formula: compound and the pharmaceutically acceptable carrier of effective dose, and described dosage can effectively suppress HIV and duplicate in host cell:
R wherein
18It is an amino-acid residue.
28. the composition of claim 27, wherein R
18For-NHCH (CH
3) COOCH
3
29. one kind is used for suppressing the composition that HIV duplicates at host cell, said composition comprises the following formula: compound and the pharmaceutically acceptable carrier of effective dose, and described dosage can effectively suppress HIV and duplicate in host cell:
Wherein X is substituted in the para or ortho position electron-withdrawing group.
30. the composition of claim 29, wherein X is halogen or NO
2
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/107,716 US6030957A (en) | 1998-06-29 | 1998-06-29 | Aryl phosphate derivatives of d4T having anti-HIV activity |
| US09/107716 | 1998-06-30 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN1314909A true CN1314909A (en) | 2001-09-26 |
Family
ID=22318071
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN99810072A Pending CN1314909A (en) | 1998-06-29 | 1999-06-29 | Aryl phosphate derivatives of d4T having anti-HIV activity |
Country Status (14)
| Country | Link |
|---|---|
| US (7) | US6030957A (en) |
| EP (1) | EP1090018B1 (en) |
| JP (1) | JP2002519355A (en) |
| KR (1) | KR20010071673A (en) |
| CN (1) | CN1314909A (en) |
| AT (1) | ATE277940T1 (en) |
| AU (1) | AU4846999A (en) |
| BR (1) | BR9911685A (en) |
| CA (1) | CA2336285A1 (en) |
| DE (1) | DE69920696T2 (en) |
| HU (1) | HUP0102527A3 (en) |
| IL (1) | IL140388A0 (en) |
| NO (1) | NO20006685L (en) |
| WO (1) | WO2000000501A1 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103403014A (en) * | 2011-01-03 | 2013-11-20 | 南京迈勒克生物技术研究中心 | O-(replaced benzyl)phosphoramidate and use thereof |
Families Citing this family (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20070015733A1 (en) * | 1998-06-29 | 2007-01-18 | Parker Hughes Institute | Aryl Phosphate Derivatives of d4T having Activity Against Resistant HIV Strains |
| US7144874B2 (en) * | 2002-10-25 | 2006-12-05 | Parker Hughes Institute | Aryl phosphate derivatives of d4T having activity against resistant HIV strains |
| US6030957A (en) * | 1998-06-29 | 2000-02-29 | Wayne Hughes Institute | Aryl phosphate derivatives of d4T having anti-HIV activity |
| WO2000056750A1 (en) * | 1999-03-19 | 2000-09-28 | Parker Hughes Institute | One pot synthesis of 5'-hydroxy phosphorylated nucleoside derivatives, and compounds formed thereby |
| US6825177B2 (en) * | 2000-10-30 | 2004-11-30 | Parker Hughes Institute | Aryl phosphate derivatives of d4T with potent anti-viral activity |
| DK1334108T3 (en) * | 2000-11-13 | 2005-07-11 | Parker Hughes Inst | Aryl phosphate derivatives of d4T |
| CA2487419A1 (en) * | 2002-05-28 | 2003-12-04 | Regents Of The University Of Minnesota | Crisp polypeptides as contraceptives and inhibitors of sperm capacitation |
| US20030236218A1 (en) * | 2002-06-12 | 2003-12-25 | Uckun Fatih M. | Aryl phosphate derivatives of d4T with selective activity against adenovirus and HIV |
| US20040076931A1 (en) * | 2002-10-18 | 2004-04-22 | Cashflow Technologies, Inc. | Educational interactive games |
| US20040077607A1 (en) * | 2002-10-21 | 2004-04-22 | Uckun Fatih M. | Aryl phosphate derivatives of d4T with potent anti-viral activity against hemorrhagic fever viruses |
| GB0401088D0 (en) | 2004-01-19 | 2004-02-18 | Univ Cardiff | Phosphoramidate derivatives |
| CA2571079A1 (en) * | 2004-06-24 | 2006-02-02 | Merck & Co., Inc. | Nucleoside aryl phosphoramidates for the treatment of rna-dependent rna viral infection |
| WO2006026656A1 (en) * | 2004-08-31 | 2006-03-09 | Parker Hughes Institute | Cytotoxic nucleoside analog compound 003 for treating cancer |
| JP2008523082A (en) * | 2004-12-09 | 2008-07-03 | リージェンツ オブ ザ ユニバーシティ オブ ミネソタ | Nucleotides having antibacterial and anticancer activities |
| BRPI0608363A2 (en) * | 2005-03-09 | 2011-05-31 | Univ California | nanocomposite membranes and methods of fabrication and use thereof |
| US7892618B2 (en) | 2005-03-21 | 2011-02-22 | Sony Corporation | Deterring theft of optical media |
| GB0623493D0 (en) * | 2006-11-24 | 2007-01-03 | Univ Cardiff | Chemical compounds |
| JP2012521359A (en) * | 2009-03-20 | 2012-09-13 | アリオス バイオファーマ インク. | Substituted nucleoside and nucleotide analogs |
| CN103209987B (en) | 2010-09-22 | 2017-06-06 | 艾丽奥斯生物制药有限公司 | Substituted nucleotide analog |
| AU2012358804B2 (en) | 2011-12-22 | 2018-04-19 | Alios Biopharma, Inc. | Substituted phosphorothioate nucleotide analogs |
| WO2013142124A1 (en) | 2012-03-21 | 2013-09-26 | Vertex Pharmaceuticals Incorporated | Solid forms of a thiophosphoramidate nucleotide prodrug |
| EP2827876A4 (en) | 2012-03-22 | 2015-10-28 | Alios Biopharma Inc | Pharmaceutical combinations comprising a thionucleotide analog |
| WO2014169280A2 (en) | 2013-04-12 | 2014-10-16 | Achillion Pharmaceuticals, Inc. | Deuterated nucleoside prodrugs useful for treating hcv |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4707362A (en) | 1985-02-15 | 1987-11-17 | Biotek, Inc. | Sustained release composition |
| US4841039A (en) | 1986-05-01 | 1989-06-20 | Emory University | 2',3'-dideoxy-5-substituted uridines and related compounds as antiviral agents |
| US4983393A (en) | 1987-07-21 | 1991-01-08 | Maximed Corporation | Intra-vaginal device and method for sustained drug release |
| JPH06189998A (en) | 1992-11-06 | 1994-07-12 | C & Co:Yugen | Condom |
| AU5691094A (en) | 1992-12-18 | 1994-07-19 | University Of Alberta, The | Dihydropyrimidine nucleosides with antiviral properties |
| ZA947572B (en) | 1993-09-29 | 1995-09-28 | Hampton Roads Medical College | Contraceptive compositions |
| US5672698A (en) * | 1993-11-15 | 1997-09-30 | Bristol-Myers Squibb Co. | Preparation of 2',3'-didehydro-3'-deoxythymidine from 5-methyluridine |
| US5659023A (en) * | 1995-02-01 | 1997-08-19 | Gilead Sciences, Inc. | Nucleotide analogues |
| GB9505025D0 (en) * | 1995-03-13 | 1995-05-03 | Medical Res Council | Chemical compounds |
| AU723081B2 (en) | 1996-05-09 | 2000-08-17 | Infectio Recherche Inc. | Formulation for use in the prevention of pathogen induced diseases including HIV and HSV |
| US6030957A (en) | 1998-06-29 | 2000-02-29 | Wayne Hughes Institute | Aryl phosphate derivatives of d4T having anti-HIV activity |
-
1998
- 1998-06-29 US US09/107,716 patent/US6030957A/en not_active Expired - Fee Related
-
1999
- 1999-06-29 HU HU0102527A patent/HUP0102527A3/en unknown
- 1999-06-29 EP EP99932080A patent/EP1090018B1/en not_active Expired - Lifetime
- 1999-06-29 DE DE69920696T patent/DE69920696T2/en not_active Expired - Fee Related
- 1999-06-29 WO PCT/US1999/014774 patent/WO2000000501A1/en active IP Right Grant
- 1999-06-29 AU AU48469/99A patent/AU4846999A/en not_active Abandoned
- 1999-06-29 IL IL14038899A patent/IL140388A0/en unknown
- 1999-06-29 BR BR9911685-5A patent/BR9911685A/en not_active Application Discontinuation
- 1999-06-29 KR KR1020007014949A patent/KR20010071673A/en not_active Application Discontinuation
- 1999-06-29 JP JP2000557262A patent/JP2002519355A/en active Pending
- 1999-06-29 CN CN99810072A patent/CN1314909A/en active Pending
- 1999-06-29 AT AT99932080T patent/ATE277940T1/en not_active IP Right Cessation
- 1999-06-29 CA CA002336285A patent/CA2336285A1/en not_active Abandoned
- 1999-11-29 US US09/450,082 patent/US6503890B1/en not_active Expired - Fee Related
- 1999-12-15 US US09/464,516 patent/US6350736B1/en not_active Expired - Fee Related
-
2000
- 2000-04-13 US US09/548,492 patent/US6528495B1/en not_active Expired - Fee Related
- 2000-04-13 US US09/548,496 patent/US6537975B1/en not_active Expired - Fee Related
- 2000-04-13 US US09/548,494 patent/US6670336B1/en not_active Expired - Fee Related
- 2000-12-28 NO NO20006685A patent/NO20006685L/en not_active Application Discontinuation
-
2003
- 2003-12-01 US US10/726,073 patent/US7071176B2/en not_active Expired - Fee Related
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103403014A (en) * | 2011-01-03 | 2013-11-20 | 南京迈勒克生物技术研究中心 | O-(replaced benzyl)phosphoramidate and use thereof |
| CN103403014B (en) * | 2011-01-03 | 2016-07-06 | 河南美泰宝生物制药有限公司 | O-(benzyl being substituted) phosphoramidate compounds and therapeutic use thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20010071673A (en) | 2001-07-31 |
| IL140388A0 (en) | 2002-02-10 |
| NO20006685L (en) | 2001-02-28 |
| EP1090018A1 (en) | 2001-04-11 |
| US6350736B1 (en) | 2002-02-26 |
| BR9911685A (en) | 2001-12-18 |
| US6670336B1 (en) | 2003-12-30 |
| WO2000000501A1 (en) | 2000-01-06 |
| US6537975B1 (en) | 2003-03-25 |
| HUP0102527A3 (en) | 2002-04-29 |
| EP1090018B1 (en) | 2004-09-29 |
| AU4846999A (en) | 2000-01-17 |
| DE69920696T2 (en) | 2006-03-02 |
| JP2002519355A (en) | 2002-07-02 |
| ATE277940T1 (en) | 2004-10-15 |
| NO20006685D0 (en) | 2000-12-28 |
| US6030957A (en) | 2000-02-29 |
| DE69920696D1 (en) | 2004-11-04 |
| US7071176B2 (en) | 2006-07-04 |
| US6503890B1 (en) | 2003-01-07 |
| HUP0102527A2 (en) | 2001-12-28 |
| CA2336285A1 (en) | 2000-01-06 |
| US20040171577A1 (en) | 2004-09-02 |
| US6528495B1 (en) | 2003-03-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1314909A (en) | Aryl phosphate derivatives of d4T having anti-HIV activity | |
| Balakrishna Pai et al. | Inhibition of hepatitis B virus by a novel L-nucleoside, 2'-fluoro-5-methyl-beta-L-arabinofuranosyl uracil | |
| US6949522B2 (en) | β-2′- or 3′-halonucleosides | |
| CN103403014B (en) | O-(benzyl being substituted) phosphoramidate compounds and therapeutic use thereof | |
| CN1076021C (en) | L-2',3'-dideoxy nucleoside analogs as anti-hepatitis B (HBV) and anti-HIV agents | |
| FI85978C (en) | FOERFARANDE FOER FRAMSTAELLNING AV ETT TERAPEUTISKT ANVAENDBART SALT ELLER ESTER AV 3'-AZIDO-3'DEOXITYMIDIN. | |
| McIntee et al. | Probing the mechanism of action and decomposition of amino acid phosphomonoester amidates of antiviral nucleoside prodrugs | |
| JP5042996B2 (en) | A1 adenosine receptor agonist | |
| EP3455205A1 (en) | Peptidomimetics for the treatment of norovirus infection | |
| MX2013013570A (en) | Purine monophosphate prodrugs for treatment of viral infections. | |
| JP2005503358A5 (en) | ||
| CN1292795A (en) | Certain dinucleotides and their use as membrana mucosa cilium clearing and cilium vibration frequency regulating agent | |
| CN1679612A (en) | Enantiomerically pure beta-d-dioxolane nucleosides with selective anti-hepatitus b virus activity | |
| Krecmerova et al. | Synthesis of ester prodrugs of 9-(S)-[3-hydroxy-2-(phosphonomethoxy) propyl]-2, 6-diaminopurine (HPMPDAP) as anti-poxvirus agents | |
| US20060009417A1 (en) | A1 adenosine receptor agonists | |
| Choi et al. | Structure− activity relationships of (e)-5-(2-bromovinyl) uracil and related pyrimidine nucleosides as antiviral agents for herpes viruses | |
| CN1216893C (en) | Nucleosides with anti-hepatitis B virus activity | |
| Boyle et al. | Synthesis of 2 ‘, 3 ‘-Dideoxynucleoside 5 ‘-α-P-Borano-β, γ-(difluoromethylene) triphosphates and Their Inhibition of HIV-1 Reverse Transcriptase | |
| Zacharie et al. | Synthesis and activity of 6-substituted purine linker amino acid immunostimulants | |
| Nguyen-Ba et al. | Design and SAR study of a novel class of nucleotide analogues as potent anti-HCMV agents | |
| US11618765B2 (en) | Broad-spectrum antiviral nucleoside derivatives | |
| Ikejiri et al. | 5′-O-Masked 2′-deoxyadenosine analogues as lead compounds for hepatitis C virus (HCV) therapeutic agents | |
| Sommadossi | ANTIVIRAL 3-L-NUCLEOSIDES SPECIFIC FOR HEPATITIS B VIRUS | |
| CN114209706A (en) | Use of vitamin D analogues for treating or preventing viral hepatitis | |
| Bryant et al. | Antiviral β-L-nucleosides specific for hepatitis B virus infection |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| C10 | Entry into substantive examination | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| C12 | Rejection of a patent application after its publication | ||
| RJ01 | Rejection of invention patent application after publication |