CN1288002A - Method for preparing biocide alkyl-substituted (hetero)-aryl-ketoxime-o-ether and its intermediate ketone or oxime compounds - Google Patents
Method for preparing biocide alkyl-substituted (hetero)-aryl-ketoxime-o-ether and its intermediate ketone or oxime compounds Download PDFInfo
- Publication number
- CN1288002A CN1288002A CN 99115557 CN99115557A CN1288002A CN 1288002 A CN1288002 A CN 1288002A CN 99115557 CN99115557 CN 99115557 CN 99115557 A CN99115557 A CN 99115557A CN 1288002 A CN1288002 A CN 1288002A
- Authority
- CN
- China
- Prior art keywords
- alkyl
- aryl
- heteroaryl
- group
- formula
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Abstract
The present invention relates to preparation method of alkye-substituted (netero)-aryl-ketoxime-o-ether compound series expressed by general formula (I), and series intermediate ketone, oxime compounts expressed by general formulas (II) and (III). The compounds expressed by general formala (I) have excellent biological actirity, especially in preventing and treating insect pest, and no harm to crops. The intermediates expressed by general formulas (II) and (III) also possess better biological activity.
Description
The present invention relates to a series of biocidal alkyl-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds and preparation method thereof.
Relevant alkyl-replacement (mixing)-aryl-ketoxime-O-ether compound all has the lot of documents report both at home and abroad as the research and the application of agricultural chemicals, also has commercially available agricultural chemical to come out simultaneously, relevant document and patent are as Bull, M.J., Davics, J.H., Searle, J.G., Henry, A.G.Pestic.Sci., II, 249 (1980); Paul, JillHelaine et al., Eur.Pat.Appl.4,754, US Appl.891,991; Jpn.Kokai Tokkyo Koho80,115,864; B. storehouse grace, G. Sa Bake etc., CN 1077709A (DE P4213149.9); CN1059515A; DE 4442730A; DE 2806664A; Xi Gangmin heros etc. are openly speciallyyed permit communique, 55-17323, and the 54-13852. commercially available agricultural chemical is as oxime ether chrysanthemum ester (CA registration number 69043-27-2) etc.Alkyl-replacement (mixing)-aryl-ketoxime-O-ether compound has good biocidal activity.It not only has characteristics such as wide spectrum, efficient and low toxicity, and the biological activity characteristics that also have pyrethroid pesticides such as knocking down fast that have.Alkyl-replacement (mixing)-aryl-ketoxime-O-ether compound obtains developing more widely and using as a class novel agrochemical.For finding new alkyl-replacement (mixing)-aryl-ketoxime-O-ethers pesticide species, Hunan Chemical Research Institute has carried out extensive research to this compounds, and with regard to some bioactive new compound application is arranged Chinese invention patent (application number is: 98 112665.0), by further work, have found that a series of fabulous agricultural chemicals character that have, particularly new alkyl-replacement (the mixing)-aryl-ketoxime-O-ether compound of insecticidal properties.
The present invention is shown in the logical formula I-a series of intermediate ketones, the oxime compounds shown in serial alkyl-replacement (mixing)-aryl-ketoxime-O-ether and logical formula II and the logical formula III:
General formula (I) is in (II) and (III): I .Ar
1And Ar
2Be identical or different, and representative
(a) (C
6-C
12The heteroaryl of)-aryl or 10 carbon atoms of band as many as, or
(b) as at I .a) in determined implication, be selected from case of necessity following in 5 identical or different substituting groups of as many as replace:
Halogen, nitro, cyano group, (C
1-C
6)-alkyl, (C
1-C
6)-haloalkyl, cyano group-(C
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkyl, (C
1-C
6)-alkylthio-(C
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkoxyl group, (C
1-C
6)-halogenated alkoxy, (C
1-C
6)-halogenated alkoxy alkyl, (C
1-C
6)-alkylthio, (C
1-C
6)-halogenated alkylthio, (C
1-C
6)-alkyl sulphonyl, (C
1-C
6)-alkyl sulphinyl, (C
1-C
6)-alkoxy carbonyl, (C
1-C
6)-alkylamino, two-(C
1-C
6)-alkylamino, (C
2-C
6)-alkenyl, (C
2-C
6)-alkenyl oxy, (C
2-C
6)-alkenyl oxy alkyl, (C
2-C
6)-halogenated alkenyl, (C
2-C
6)-halogenated alkenyl oxy, (C
2-C
6)-halogenated alkenyl oxy alkyl, (C
2-C
6)-alkynyl, (C
2-C
6)-alkynyloxy base, (C
2-C
6)-halo alkynyl, (C
2-C
6)-halo alkynyloxy base, (C
3-C
8)-cycloalkyl, (C
3-C
8)-cycloalkyl oxy, (C
3-C
8)-cycloalkyl amino, (C
6-C
12)-aryl, (C
6-C
12)-aryloxy, (C
6-C
12)-artyl sulfo, (C
6-C
12)-aryl-(C
1-C
4)-alkyl, (C
6-C
12)-aryloxy carbonyl, (C
6-C
12)-aryl sulfonyl, (C
6-C
12)-aryl sulfonyl kia, (C
6-C
12)-arylamino, heteroaryl, heteroaryl oxygen base, heteroaryl-(C
1-C
4)-alkyl, the heteroaryl sulfenyl, heteroaryl oxygen base carbonyl, heteroarylsulfonyl, the heteroaryl sulfinyl, and
When substituting group is aryl or heteroaryl 1) I .b), sometimes can be by-individual or a plurality of (C that are selected from
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group, (C
1-C
6)-haloalkyl, (C
1-C
6The identical or different group of)-halogenated alkoxy and halogen replaces, and heteroaryl is the heteroaryl of 10 carbon atoms of band as many as,
2) cycloalkyl I .b) can be selected from halogen, (C by 5 of as many as sometimes
1-C
4The identical or different group of)-alkyl replaces,
3) represent methylenedioxy group or ethylenedioxy for substituent 2 I .b), methylenedioxy group or ethylenedioxy sometimes with 1 or 2 identical or different be selected from halogen and (C
1-C
6The substituting group of)-alkyl,
4) I .a) and I .b) in determined the aryl and the partly or entirely hydrogenation of heteroaryl of implication, wherein 1 or 2 CH
2Group can be replaced by CO, II .R
1And R
2Be identical or different, and representative
Hydrogen, halogen, (C
1-C
6)-alkyl, (C
1-C
6)-haloalkyl, cyano group-(C
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkyl, (C
1-C
6)-alkylthio-(C
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkoxyl group, (C
1-C
6)-alkoxyl group, (C
1-C
6)-alkylthio, (C
1-C
6)-alkyl sulphonyl, (C
1-C
6)-alkyl sulphinyl, (C
1-C
6)-alkoxy carbonyl, (C
1-C
6)-alkylamino, two-(C
1-C
6)-alkylamino, (C
1-C
6)-halogenated alkoxy, (C
2-C
6)-alkenyl, (C
2-C
6)-alkenyl oxy, (C
2-C
6)-halogenated alkenyl, (C
2-C
6)-halogenated alkenyl oxy, (C
2-C
6)-alkynyl, (C
2-C
6)-alkynyloxy base, (C
2-C
6)-halo alkynyl, (C
2-C
6)-halo alkynyloxy base, (C
3-C
8)-cycloalkyl, (C
3-C
8)-cycloalkyl oxy, (C
3-C
8)-cycloalkyl amino, (C
6-C
12)-aryl, (C
6-C
12)-aryloxy, (C
6-C
12)-aryl-(C
1-C
4)-alkyl, (C
6-C
12)-artyl sulfo, (C
6-C
12)-aryloxy carbonyl, (C
6-C
12)-aryl sulfonyl, (C
6-C
12)-aryl sulfonyl kia, (C
6-C
12)-arylamino, heteroaryl, heteroaryl oxygen base, heteroaryl-(C
1-C
4)-alkyl, heteroaryl sulfenyl, heteroaryl oxygen base carbonyl, heteroarylsulfonyl, heteroaryl sulfinyl, III .R
3Representative
(a) hydrogen,
(b) (C
1-C
6)-alkyl, (C
6-C
12)-aryl, the heteroaryl of 10 carbon atoms of band as many as, (C
2-C
6)-alkenyl, (C
2-C
6)-alkynyl, (C
1-C
4)-acyl group
III .b) group described in can be selected from halogen, (C sometimes
1-C
4)-alkyl, (C
1-C
4)-alkoxyl group, (C
1-C
4)-haloalkyl, (C
1-C
4)-alkylthio, (C
1-C
4Identical or the different groups of)-halogenated alkoxy replace, and IV .X represents O, S, SO, SO
2, NH, NR
a[R
aBe (C
1-C
4)-alkyl], V .Y represents hydrogen, cyano group, halogen, (C
1-C
4)-alkyl, (C
1-C
4)-alkoxyl group, VI .M are a hydrogen or an alkali metal atom, and VII .m or n represent 0,1,2 or 3.
All abovementioned alkyls, alkenyl and alkynyl can be straight chain also can be side chain, also be applicable to simultaneously, as by alkyl derivative group alkoxyl group, alkoxy carbonyl, alkylthio, haloalkyl and arylalkyl by they derivative groups.
Haloalkyl, halogenated alkenyl and halo alkynyl are meant wherein 1, the alkyl that a plurality of or all hydrogen atoms are replaced by halogen, alkenyl and alkynyl.Also be applicable to by they derivative groups simultaneously halogenated alkoxy is arranged, halogenated alkylthio, halo alkoxy carbonyl and aryl halide substituted alkyl as the group that derives from by haloalkyl.
Halogen is meant fluorine, chlorine, bromine or iodine, preferred fluorine, chlorine or bromine.
(C
6-C
12The preferred phenyl of)-aryl and by its derivative group, as naphthyl, xenyl etc.
The heteroaryl preferred one or the aryl bicyclic of 10 carbon atoms of band as many as have 1 N at least, O and/or S in the formula, as thienyl, benzothienyl, furyl, benzofuryl, pyrryl, indyl, imidazolyl, pyrazolyl, pyridyl, pyrazinyl, pyrimidyl, pyridazinyl, oxazolyl, isoxazolyl, thiazolyl and isothiazolyl.
(C
6-C
12The partly or entirely hydrogenation of heteroaryl of)-aryl and 10 carbon atoms of band as many as, wherein 1 or 2 CH
2-Ji is replaced by CO, as cyclohexenyl, and cyclohexanedione base etc.
At logical formula I, in (II) and (III), Ar
1Preferred following groups:
Z is O, S or NR
5, R
5Be hydrogen, (C
1-C
6)-alkyl, (C
1-C
6)-haloalkyl, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkyl, (C
1-C
6)-alkylthio-(C
1-C
6)-alkyl, cyano group-(C
1-C
6)-alkyl, (C
1-C
6)-alkoxy carbonyl, (C
2-C
6)-alkenyl, (C
2-C
6)-halogenated alkenyl, (C
2-C
6)-alkynyl, (C
2-C
6)-halo alkynyl, (C
3-C
8)-cycloalkyl, and cycloalkyl can be selected from halogen or (C by 5 of as many as sometimes
1-C
4The identical or different group of)-alkyl replaces (C
6-C
12)-aryl, (C
6-C
12)-aryl-(C
1-C
4)-alkyl, (C
6-C
12)-aryloxycarbonyl, the heteroaryl of 10 carbon atoms of band as many as, heteroaryl oxygen base carbonyl, heteroaryl-(C
1-C
4)-alkyl,
P is one 0 to 5 a integer,
R
4Be identical or different, and represent hydrogen, halogen, nitro, cyano group, (C
1-C
6)-alkyl, (C
1-C
6)-haloalkyl, cyano group-(C
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkyl, (C
1-C
6)-alkylthio-(C
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkoxyl group, (C
1-C
6)-halogenated alkoxy, (C
1-C
6)-halogenated alkoxy alkyl, (C
1-C
6)-alkylthio, (C
1-C
6)-halogenated alkylthio, (C
1-C
6)-alkyl sulphonyl, (C
1-C
6)-alkyl sulphinyl, (C
1-C
6)-alkoxy carbonyl, (C
1-C
6)-alkylamino, two-(C
1-C
6)-alkylamino, (C
2-C
6)-alkenyl, (C
2-C
6)-alkenyl oxy, (C
2-C
6)-alkenyl oxy alkyl, (C
2-C
6)-halogenated alkenyl, (C
2-C
6)-halogenated alkenyl oxy, (C
2-C
6)-halogenated alkenyl oxy alkyl, (C
2-C
6)-alkynyl, (C
2-C
6)-alkynyloxy base, (C
2-C
6)-halo alkynyl, (C
2-C
6)-halo alkynyloxy base, (C
3-C
8)-cycloalkyl, (C
3-C
8)-cycloalkyl oxy, (C
3-C
8)-cycloalkyl amino, (C
6-C
12)-aryl, (C
6-C
12)-aryloxy, (C
6-C
12)-artyl sulfo, (C
6-C
12)-aryl-(C
1-C
4)-alkyl, (C
6-C
12)-aryloxy carbonyl, (C
6-C
12)-aryl sulfonyl, (C
6-C
12)-aryl sulfonyl kia, (C
6-C
12)-arylamino, heteroaryl, heteroaryl oxygen base, heteroaryl-(C
1-C
4)-alkyl, the heteroaryl sulfenyl, heteroaryl oxygen base carbonyl, heteroarylsulfonyl, the heteroaryl sulfinyl,
R
4Preferred group is a hydrogen, halogen, (C
1-C
6)-alkyl, (C
1-C
6)-haloalkyl, (C
1-C
6)-alkoxyl group, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkoxyl group, (C
1-C
6)-halogenated alkoxy, (C
1-C
6)-alkyl sulphonyl, (C
2-C
6)-alkenyl, (C
2-C
6)-alkenyl oxy, (C
2-C
6)-halogenated alkenyl, (C
2-C
6)-halogenated alkenyl oxy, (C
2-C
6)-alkynyl, (C
2-C
6)-alkynyloxy base, (C
2-C
6)-halo alkynyl, (C
2-C
6)-halo alkynyloxy base, (C
3-C
8)-cycloalkyl, (C
3-C
8)-cycloalkyl oxy, (C
3-C
8)-halogenated cycloalkyl, (C
6-C
12)-aryl, (C
6-C
12)-aryloxy, (C
6-C
12)-aryl-(C
1-C
4)-alkyl, (C
6-C
12)-artyl sulfo, the heteroaryl of 10 carbon atoms of band as many as, heteroaryl oxygen base, heteroaryl-(C
1-C
4)-alkyl.And
1) R
4Described in substituting group when being aryl or heteroaryl, sometimes can be by one or more (C that are selected from
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group, (C
1-C
6)-haloalkyl, (C
1-C
6The identical or different group of)-halogenated alkoxy and halogen replaces, and heteroaryl is the heteroaryl of 10 carbon atoms of band as many as,
2) R
4Described in cycloalkyl can be selected from halogen, (C by 5 of as many as sometimes
1-C
4Identical or the group not of)-alkyl replaces,
3) R
4In state substituent 2 and represent methylenedioxy group or ethylenedioxy, methylenedioxy group or ethylenedioxy sometimes with 1 or 2 identical or different be selected from halogen and (C
1-C
6The substituting group of)-alkyl, at logical formula I, in (II) and (III), Ar
1Preferred especially following groups:
Z is O in the formula, S or NR
5, R
5Be hydrogen, (C
1-C
6)-alkyl, (C
1-C
6)-haloalkyl, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkyl, (C
1-C
6)-alkoxy carbonyl, (C
2-C
6)-alkenyl, (C
2-C
6)-halogenated alkenyl, (C
2-C
6)-alkynyl, (C
2-C
6)-halo alkynyl.
R
4Be hydrogen, halogen, (C
1-C
6)-alkyl, (C
1-C
6)-haloalkyl, (C
1-C
6)-alkoxyl group, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkoxyl group, (C
1-C
6)-halogenated alkoxy, (C
1-C
6)-alkyl sulphonyl, (C
2-C
6)-alkenyl, (C
2-C
6)-alkenyl oxy, (C
2-C
6)-halogenated alkenyl, (C
2-C
6)-halogenated alkenyl oxy, (C
2-C
6)-alkynyl, (C
2-C
6)-alkynyloxy base, (C
2-C
6)-halo alkynyl, (C
2-C
6)-halo alkynyloxy base, (C
3-C
8)-cycloalkyl, (C
3-C
8)-halogenated cycloalkyl.
R
4Represent methylenedioxy group for substituent 2, methylenedioxy group sometimes with 1 or 2 identical or different be selected from halogen and (C
1-C
6The substituting group of)-alkyl.
At logical formula I, in (II) and (III), R
1And R
2Be identical or different, and preferred hydrogen, halogen, (C
1-C
4)-alkyl, (C
1-C
4)-alkoxyl group, (C
1-C
4)-haloalkyl, (C
1-C
4)-alkylthio, (C
1-C
4)-alkenyl, (C
1-C
4)-alkynyl, (C
1-C
4)-halogenated alkenyl, (C
1-C
4)-halo alkynyl, (C
1-C
4)-alkyl sulphonyl, (C
1-C
4)-alkyl sulphinyl, (C
1-C
4)-alkylamino,
At logical formula I, in (II) and (III), R
3Preferred hydrogen, (C
1-C
4)-alkyl, (C
1-C
4)-haloalkyl, (C
1-C
4)-alkenyl, (C
1-C
4)-alkynyl, (C
1-C
4)-halogenated alkenyl, (C
1-C
4)-halo alkynyl, (C
6-C
12)-aryl,
At logical formula I, in (II) and (III), m is preferred 0, and n preferred 1.
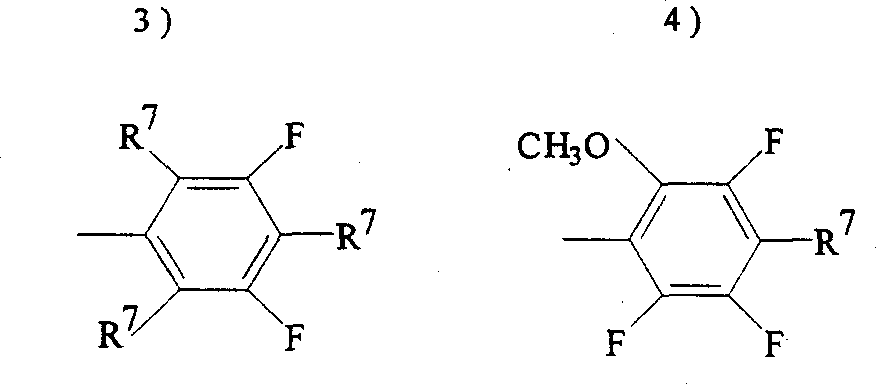
At logical formula I, in (II) and (III), Ar
2Preferably:
R represents N or CH, and q is O, S, NH or CH
2, preferred O or CH
2, R
6Be hydrogen, halogen, allyl group oxygen base, propargyloxy,
F represents fluorine,
T is identical or different with g, represents 0,1,2,3 or 4, and t and g and be not more than 5,
R
7Be identical or different, and represent hydrogen, halogen, (C
1-C
6)-alkyl, (C
1-C
6)-haloalkyl, (C
1-C
6)-alkoxyl group, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkoxyl group, (C
1-C
6)-halogenated alkoxy, (C
1-C
6)-alkyl sulphonyl, (C
2-C
6)-alkenyl, (C
2-C
6)-alkenyl oxy, (C
2-C
6)-halogenated alkenyl, (C
2-C
6)-halogenated alkenyl oxy, (C
2-C
6)-alkynyl, (C
2-C
6)-alkynyloxy base, (C
2-C
6)-halo alkynyl, (C
2-C
6)-halo alkynyloxy base, (C
3-C
8)-cycloalkyl, (C
3-C
8)-cycloalkyl oxy, (C
3-C
8)-halogenated cycloalkyl, (C
6-C
12)-aryl, (C
6-C
12)-aryloxy, (C
6-C
12)-aryl-(C
1-C
4)-alkyl, (C
6-C
12)-artyl sulfo, the heteroaryl of 10 carbon atoms of band as many as, heteroaryl oxygen base, heteroaryl-(C
1-C
4)-alkyl.
W is 1,2 or 3 in the formula,
Z is O, S or NR
5, R
5Be hydrogen, (C
1-C
6)-alkyl, (C
1-C
6)-haloalkyl, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkyl, (C
1-C
6)-alkylthio-(C
1-C
6)-alkyl, cyano group (C
1-C
6)-alkyl, (C
1-C
6)-alkoxy carbonyl, (C
2-C
6)-alkenyl, (C
2-C
6)-halogenated alkenyl, (C
2-C
6)-alkynyl, (C
2-C
6)-halo alkynyl, (C
3-C
8)-cycloalkyl, and cycloalkyl can be selected from halogen or (C by 5 of as many as sometimes
1-C
4The identical or different group of)-alkyl replaces (C
6-C
12)-aryl, (C
6-C
12)-aryl-(C
1-C
4)-alkyl, (C
6-C
12)-aryloxycarbonyl, the heteroaryl of 10 carbon atoms of band as many as, heteroaryl carbonyl, heteroaryl-(C
1-C
4)-alkyl,
R
8Be identical or different, and represent hydrogen, halogen, (C
1-C
6)-alkyl, (C
1-C
6)-haloalkyl, (C
1-C
6)-alkoxyl group, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkoxyl group, (C
1-C
6)-halogenated alkoxy, (C
2-C
6)-alkenyl, (C
2-C
6)-alkenyl oxy, (C
2-C
6)-halogenated alkenyl, (C
2-C
6)-halogenated alkenyl oxy, (C
2-C
6)-alkynyl, (C
2-C
6)-alkynyloxy base, (C
2-C
6)-halo alkynyl, (C
2-C
6)-halo alkynyloxy base, (C
6-C
12)-aryloxy, (C
6-C
12)-aryl-(C
1-C
4)-alkyl, (C
6-C
12)-artyl sulfo, the heteroaryloxy of 10 carbon atoms of band as many as, heteroaryl sulfenyl, heteroaryl-(C
1-C
4)-alkyl, and R
8In aryl or heteroaryl, can be selected from (C sometimes
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group, (C
1-C
6)-haloalkyl, (C
1-C
6The identical or different group of)-halogenated alkoxy and halogen replaces,
R
8Preferred especially benzyl, phenoxy group, propenyl, proyl, methyl or trifluoromethyl,
R is (C
1-C
6)-alkyl, (C
1-C
6)-haloalkyl, preferable methyl,
A is an oxygen, sulphur or NH,
B is nitrogen or CH,
At logical formula I, in (II) and (III), particularly preferred Ar
2Be:
R
6Be hydrogen, the 3-fluorine, the 4-fluorine, 4-chlorine,
R
7Be (C
1-C
6)-alkyl, preferred especially CH
3, (C
1-C
6)-haloalkyl, (C
1-C
6)-alkoxyl group, (C
1-C
6)-halogenated alkoxy.
R is a methyl,
A is an oxygen, sulphur or NH,
B is nitrogen or CH,
Particularly preferred formula I, the compound of formula II and formula III is: Ar
1Be to replace or unsubstituted aryl or heteroaryl Ar
2Be above-mentioned particularly preferred Ar
2, R
1And R
2Be hydrogen, methyl or ethyl, R
3Be methyl, m is 0, and n is 1, and X is S, O, SO
2
Compound shown in formula I and the formula III can two kinds of geometrical isomer forms occur: " cis " (Z formula) and " trans " (E formula) form, " cis " (Z formula) is oxime-oxygen and aryl or heteroaryl Ar
1Be in the two key homonymies of C=N, " trans " (E formula) is oxime-oxygen and aryl or heteroaryl Ar
1Be in the two key heteropleurals of C=N, during the oxime alkylation, do not change its rotamerism, obtain corresponding " cis " (Z formula) (I) formula oxime ether during the alkylation of i.e. " cis " (Z formula) (III) formula oxime, and obtain corresponding " trans " (E formula) (I) formula oxime ether during the alkylation of " trans " (E formula) (III) formula oxime.
In addition, intermediate ketone, oxime compound shown in alkyl-replacement (mixing)-aryl-ketoxime-O-ether shown in the logical formula I and logical formula II and (III), as have one or more unsymmetrical carbons, it can be a racemic modification, diastereomer or pure optical isomer
Alkyl-replacement (mixing)-aryl-ketoxime-O-ether shown in the logical formula I and the intermediate oxime compound shown in the logical formula III not only relate to cis (Z formula), also relate to trans (E formula) and both mixtures,
Intermediate ketone, oxime compound shown in alkyl-replacement (mixing)-aryl-ketoxime-O-ether shown in the logical formula I and logical formula II and (III) not only relates to racemic modification, and diastereomer also relates to pure optical isomer,
Intermediate ketone, oxime compound shown in alkyl-replacement (mixing)-aryl-ketoxime-O-ether shown in the logical formula I and logical formula II and (III) not only relates to pure isomer, also relates to various mixture of isomers.
Intermediate ketone, oxime compound shown in alkyl-replacement (mixing)-aryl-ketoxime-O-ether shown in the part formula I provided by the invention and logical formula II and (III) sees Table 1, in the table 1, and group Ar
2Simplify with GK (K=1-35) abbreviation expression.G
kSee Table 2.But formula (I), the compound of formula II and formula III is not limited only to the compound shown in the table 1.
The present invention also provides the intermediate ketone shown in alkyl-replacement (the mixing)-aryl-ketoxime-O-ether shown in the formula I and logical formula II and (III), the preparation method of oxime compound, and the preparation method of formula I compound is:
A) adopt compound shown in the formula III and the compound shown in the formula IV
In the formula IV, L representative-individual leavings group is if M is a hydrogen in the formula III, at an appropriate base such as alkali metal hydroxide, alkaline carbonate, alkali metal hydrocarbonate, alkali metal alcoholates, basic metal, alkalimetal hydride, pyridine, tertiary amine exists down, adds suitable solvent such as suitable solvent and be to be selected from the following solvents one or both: water, tetrahydrofuran (THF), toluene, benzene, acetonitrile, dimethyl sulfoxide (DMSO) etc., add phase-transfer catalyst PTC such as tetrabutylammonium iodide again, Tetrabutyl amonium bromide, tetraethylammonium bromide, hexaoxacyclooctadecane-6-6, in normal pressure, reaction is 1-20 hour and get under the 0-120 ℃ of condition.
When in the formula III during M=alkali metal atom, the alkylated reaction of its oximate carries out under identical conditions, does not need the existence of appropriate base.Or
B) compound shown in the formula II, in case of necessity in the presence of an appropriate base with the compound shown in the formula (V) or with their reactant salt
The preparation method of formula III compound provided by the invention is:
Compound shown in the formula II and hydroxylamine hydrochloride or hydroxylamine sulfate, in normal pressure, 0-100 ℃ of reaction and getting in a suitable solvent preferably water and/or alcohol, also need add alkali metal hydroxide, alkaline carbonate, alkali metal hydrocarbonate in case of necessity, appropriate base such as pyridine are synthesized
The preparation method of formula II compound provided by the invention is: the logical formula II compound of different structure need select proper raw material and corresponding synthetic method to carry out the synthetic of (II).In logical formula II compound, when X=S, the compound shown in the available formula VI
Ar in the formula
1And R
1, R
2, X, R
3, n, definition in m such as the claim 1, with the sodium salt of corresponding mercaptan in water solvent, in normal pressure, 30-100 ℃ of reaction 2-10 hour and getting.
Alkyl provided by the invention-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds, biologically active and the compound that has have fabulous biological activity.Particularly showing high reactivity aspect the preventing and treating of insects such as agricultural, gardening, flowers and health, and damage to crops not, all is being gratifying aspect desinsection and lasting insecticidal effect fast.Harmful organism described here include but not limited to this:
Harmful insect: Orthoptera such as blattaria, Thysanoptera such as cotton thrips, rice thrips, melon thrips, Homoptera such as leafhopper, plant hopper, aphid, lepidopteran such as oriental armyworm, prodenia litura, small cabbage moth, cabbage caterpillar, Hymenoptera such as sawfly larva, Diptera such as yellow-fever mosquito, culex, fly, acarina such as tangerine gold melon mite, cotton spider mites;
Harmful pathogenic bacteria: the phytophthora kind, white powder belongs to kind, Gibberella kind, Venturia kind, species of Monilinia fructicola, Rhizoctonia kind, Staphlosporonites kind, Pyricularia Sacc. kind, fusarium kind;
Injurious weed: gramineous weeds such as barnyard grass, amur foxtail, brooklet astilbe root or herb, broad leaved weeds such as lamb's-quarters, bitter cress.
With alkyl provided by the invention-replacement (mixing)-aryl-ketoxime-O-ether or intermediate ketone, oxime compounds, Agrotechnical formulation as effective ingredient, can make desirable any formulation such as missible oil, wettable powder, suspension agent, granula, suitable auxiliary agent comprises carrier (thinner) and other auxiliary such as spreader-sticker, emulsifying agent, wetting agent, dispersion agent, tackiness agent and decomposition agent.
Agrotechnical formulation prepares embodiment:
Preparation missible oil: with 20 parts (by weight) alkyl provided by the invention-replacement (mixing)-aryl-ketoxime-O-ether compound or intermediate ketone, oxime compound, 73 parts of thinners such as dimethylbenzene and 7 parts of suitable auxiliary agent uniform mixing can prepare missible oil, and dilute with water can be used.
Preparation wettable powder: with 20 parts (by weight) alkyl provided by the invention-replacement (mixing)-aryl-ketoxime-O-ether compound or intermediate ketone, oxime compound, 53 parts of clays, 20 parts of white carbon blacks, 5 parts of lignin silicate and 2 parts of polyoxy ethyl alkyl oxide mixing are worn into fine powder and can be made wettable powder.
The invention will be further described below in conjunction with embodiment.
Embodiment 1:1-(4-chloro-phenyl-)-2-methylthio group-O-[(3-phenoxy phenyl) methyl] synthetic [No. 30 logical formula I compound in the table 1] (systhesis of 1-propanone, 1-(4-chlorophenyl)-2-methylthio-O-[(3-phenoxyphenyl) methyl] oxime) of acetoxime ether
Take by weighing 1-(4-chloro-phenyl-)-2-methylthio group acetoxime { 1-propanone, 1-(4-chlorophenyl)-2-methylthio) oxime} (mixture of Z and E isomer, content 90%) 1.7 grams (0.007 mole) are in 18 milliliters of anhydrous acetonitriles that are dissolved with 0.20 gram sodium Metal 99.5,30~40 ℃ the reaction 2~3 hours between after, add 0.3 gram tetrabutylammonium iodide, the back drips m-phenoxy benzyl chloride (content 90%) 1.91 grams (0.008 mole), after dropwising, slowly be warming up to 60~65 ℃ of reactions 8~9 hours.After the processing oily crude product 2.85 gram (mixture of Z and E isomer), purify with the petroleum ether-ethyl acetate column chromatography for separation that reduces pressure, can get Z and E pure isomer, product is faint yellow viscous liquid, Z and E pure isomer weigh 1.49 grams altogether.Purity Z body: 92% E body 93% (gas spectrum), yield 47%.
Hydrogen spectrum δ
CDCl3 TMS:
E ' isomer: 1.26 (d, 3H, CH
3), 2.05 (s, 3H, SCH
3), 4.76 (q, 1H ,-CH-S-), 5.15 (s, 2H, C=N-O-CH
2-), 6.96-7.66 (m, 13H, hydrogen on the phenyl ring).
Z isomer: 1.27 (d, 3H, CH
3), 1.93 (s, 3H, SCH
3), 3.60 (q, 1H ,-CH-S-), 5.02 (s, 2H, C=N-O-CH
2-), 6.88-7.38 (m, 13H, hydrogen on the phenyl ring).
Results of elemental analyses: (measured value/theoretical value): C%67.41/67.07 H%5.06/5.35
N%3.32/3.40???S%7.25/7.77
Mass spectrum: molecular ion peak M
+(411).
Embodiment 2.1-(4-chloro-phenyl-)-2-methylthio group-O-[(3-phenoxy phenyl) methyl] synthetic [No. 5 logical formula I compound in the table 1] (synthesis of ethanone, 1-(4-chlorophenyl)-2-methylthio-O-[(3-phenoxyphenyl) methyl] oxime) of second ketoxime ether
Take by weighing 1-(4-chloro-phenyl-)-2-methylmercaptan ethyl ketoxime { ethanone, 1-(4-chlorophenyl)-2-methylthio) oxime} (E isomer, content 90%) 1.7 grams (0.007 mole), m-phenoxy benzyl chloride (content 90%) 1.76 grams (0.007 mole), 0.3 gram tetrabutylammonium iodide, 4 milliliters of toluene add the solution that is made into by 4 ml waters and 0.63 gram sodium hydroxide in batches under stirring in reaction flask, slowly be warming up to 58~60 ℃ of reactions 7~8 hours.After the processing oily crude product 2.67 gram (E isomer), purify with the petroleum ether-ethyl acetate column chromatography for separation that reduces pressure, faint yellow viscous liquid 1.39 grams (E body).Purity 94% (gas spectrum), yield 44%.
Hydrogen spectrum δ
CDCl3 TMS: 1.98 (s, 3H, SCH
3), 3.72 (s, 2H ,-CH
2-S-), 5.19 (s, 2H, C=N-O-CH
2-), 6.89-7.66 (m, 13H, hydrogen on the phenyl ring).
Results of elemental analyses: (measured value/theoretical value): C%66.41/66.08 H%5.06/4.88
N%3.52/3.50???S%8.05/8.54
Mass spectrum: molecular ion peak M
+(397).
Synthetic (synthesis of ethanone, 1-(4-chlorophenyl)-2-methylthio oxime) [No. 5 logical formula III compound in the table 1] of embodiment 3.1-(4-chloro-phenyl-)-2-methylmercaptan ethyl ketoxime
To reflux condensing tube is housed, thermometer, add 1-(4-chloro-phenyl-)-2-methylthio group ethyl ketone 27.0 grams (0.13 mole) in 250 milliliters of there-necked flasks of stirring magneton, 35 milliliters of ethanol, 14 milliliters in water, oxammonium hydrochloride 12.80 grams (0.18 mole), add solid sodium hydroxide 16.0 grams (0.4 mole) under stirring in batches, finish, keep room temperature (about 25 ℃) reaction 15~20 minutes, after slowly be warming up to 45 ℃ the reaction 45 minutes, reheat was to reflux temperature reaction 5~6 hours. and cooling gets canescence needle-like crystal 29 grams (mixture of Z body and E body), purity 90% (gas spectrum) after aftertreatment, yield 90%, fusing point: 93.6~96.6 ℃.
Results of elemental analyses: (measured value/theoretical value) C%:50.42/50.11 H%:4.83/4.64
N%:6.47/6.49?S%:13.82/14.80
Mass spectrum: molecular ion peak M
+(215).
Synthetic (the synthesis of ethanone of embodiment 4.1-(4-chloro-phenyl-)-2-methylthio group ethyl ketone (or claiming 4-chloro-2-methylthio group-methyl phenyl ketone), 1-(4-chlorophenyl)-2-methylthio or title acetophenone, 4 '-chloro-2-methylthio) [No. 5 logical formula II compound in the table 1]
To reflux condensing tube is housed, thermometer, add the sodium methyl mercaptide aqueous solution (content 37%) 41.0 grams (0.217 mole) in 250 milliliters of there-necked flasks of stirring magneton, add 1-(4-chloro-phenyl-)-2-chloroethene ketone (content 95%) 24.75 grams (0.124 mole) under the room temperature, reacted 3~5 hours under 60~80 ℃ of conditions in normal pressure the back in batches, cooling, after aftertreatment, get oily liquids product 27 grams, purity 90% (gas spectrum), yield 97%.
Results of elemental analyses: (measured value/theoretical value) C%:53.80/53.86 H%:4.30/4.52
S%:15.28/15.97
Mass spectrum: molecular ion peak M
+(200).
The synthetic synthesis of ethanone of embodiment 5.1-(4-chloro-phenyl-)-2-chloroethene ketone (or claiming 4 '-chloro-2-chloro-methyl phenyl ketone), and 1-(4-chlorophenyl)-2-chloro (or 4 '-chloro-2-chloro)-acetophenone
To the reflux condensing tube (drying tube links to each other with the alkali absorption liquid) that band Calcium Chloride Powder Anhydrous drying tube is housed, thermometer, dropping funnel, add chlorobenzene 135 grams (1.2 moles) in 500 milliliters of there-necked flasks of stirring magneton, aluminum trichloride (anhydrous) 88.8 grams (0.667 mole), in slowly dripping after chloroacetyl chloride 69.5 gram (0.615 mole) dropwises from the water clock bucket under 5~15 ℃, slowly be warming up to and react about 70 ℃ to emission-free emitting, cooling, get 118 gram white needle-like crystals through aftertreatment, purity 90% (gas spectrum), yield 91%, fusing point: 97.9~99.0 ℃ (literature value: 98~100 ℃).
Results of elemental analyses: (measured value/theoretical value) C%:50.38/50.82 H%:3.02/3.19
Mass spectrum: molecular ion peak M
+(188).
All formula I, the compound of formula II and formula III can be synthetic with similar approach, and institute's synthetic compound is all through the hydrogen spectrum, and is infrared, and mass spectrum is or/and the ultimate analysis conclusive evidence.
(the Z body is different with the biological activity of E body, and the biological activity of general E body is better than the Z body, and its activity data is generally the activity data of E body or Z and E mixture for biological activity determination embodiment.)
The biological activity test of 6 pairs of mythimna separatas of embodiment (Mythimne separata)
Will be by the missible oil or the wettable powder of alkyl provided by the invention-replacement (the mixing)-aryl-ketoxime-O-ether of above-mentioned Agrotechnical formulation embodiment method preparation or intermediate ketone, oxime active compound, dilute with water is made into the pesticidal solutions of predetermined concentration, choosing 10 mythimna separatas and 5 one cun long maize leafs is put in the culture dish and quantitatively sprays, dry the back and move into normal raising the in the greenhouse, statistics survival and death toll after 24 hours.Experiment repeats 3 times, results averaged.Discovery is numbered 5,6, and 7,8,9,10,11,12,21,22,23,30,31,32,33,34,35,36,37,46,47,48,213,216,229,230,231,238,254,629, a series of logical formula I compounds mortality ratio when 500ppm (in effective component content) such as 632,645,646,647,654,657 reach 100%.Be numbered logical formula I compound such as 5,8,30 grades in compound that has such as the table 1 and under lower concentration, still can make mythimna separata 100% death.
The activity experiment of 7 pairs of bean aphids of embodiment (Aphis fabae)
Will be by the missible oil or the wettable powder of alkyl provided by the invention-replacement (the mixing)-aryl-ketoxime-O-ether of above-mentioned Agrotechnical formulation embodiment method preparation or intermediate ketone, oxime active compound, dilute with water is made into the pesticidal solutions of predetermined concentration, bean aphid is connected on the bean seedlings that just have been unearthed, every strain connects more than 15, then bean seedlings are dipped in the soup together with the examination worm, take out after 5 seconds, unnecessary soup is removed in suction, insert in the sponge of suction, cover with glass-tube, check survival and dead borer population after 24 hours.Repeat results averaged 3 times.Found that to be numbered 5,8 that a series of logical formula I compounds lethality rates to bean aphid when 250ppm (in effective component content) such as 21,22,23,30,33,46,47,48 reach 100%.Be numbered logical formula I compound such as 5,8,30 grades etc. in compound that has such as the table 1 and under lower concentration, still can make bean aphid 100% death.
The insecticidal test of 8 pairs of rice green leafhoppers of embodiment (Nephotetlix cimeticeps)
Will be by the missible oil or the wettable powder of alkyl provided by the invention-(the mixing)-aryl-ketoxime-O-ether of above-mentioned Agrotechnical formulation embodiment method preparation or intermediate ketone, oxime active compound, dilute with water is made into the pesticidal solutions of predetermined concentration, choosing two core rice seedlings immerses in the soup, take out after 5 seconds and dry, place Boiling tube, 20 rice green leafhopper nymphs in 5 age are introduced in every pipe 20 strains then, the mouth of pipe is placed under the greenhouse experiment with white gauze wrapping, checks survival and dead borer population after 24 hours.Experiment repeats 3 times.Found that to be numbered 5,6 that a series of logical formula I compounds lethality rates to leafhopper when 250ppm (in effective component content) such as 7,8,9,10,11,12,21,22,23,30,31,32,33,34,36,37,46,47,48,188 reach 100%.In compound that has such as the table 1 No. 5, No. 8,30,33, No. 188 logical formula I compounds such as grade etc. still can make leafhopper 100% death under lower concentration.
Institute's synthetic compound also has advantages of high activity to other insect:
The biological activity of No. 5 logical formula I compound in the table 1: beet armyworm E body LC
50(ppm)<5 cabbage looper E body LC
50(ppm)<5 U.S. state tobacco budworm E body LC
50(ppm)<10 black peach aphid E body LC
50(ppm)>100.
The biological activity of No. 30 logical formula I compound in the table 1: beet armyworm E body LC
50(ppm)<5 Z body LC
50(ppm)>150 cabbage looper E body LC
50(ppm)<1.5 Z body LC
50(ppm)>150 U.S. state tobacco budworm E body LC
50(ppm)<5 Z body LC
50(ppm)>150 black peach aphid E body LC
50(ppm)<15 Z body LC
50(ppm)>150.
Embodiment 9 stripped fungicidal activity determination tests
Alkyl provided by the invention-replacement (mixing)-aryl-ketoxime-O-ether or intermediate ketone, oxime active compound are made into the mother liquor of predetermined concentration with suitable thinner (as acetone), get 1 milliliter of soup in 50 milliliters of PDA substratum that dissolve with transfer pipet, after fully shaking up, pour into respectively in 2 culture dish and repeat as 2 times, cooling back is that 4 millimeters bacterium cake places culture dish central authorities with inoculating needle picking diameter, establishes thinner simultaneously for contrasting.Put into the incubator of optimal temperature after inoculation finishes, measure the mycelial growth diameter after 72 hours, calculate mycelial growth inhibition rate.Found that being numbered a series of logical formula III compounds such as 5,7 reaches more than 90% one or more crop pathogens mycelial growth inhibition rates when 100ppm (in effective component content).
The experiment of embodiment 10 weeding activity
Will be by alkyl provided by the invention-replacement (the mixing)-aryl-ketoxime-O-ether or the intermediate ketone of above-mentioned Agrotechnical formulation embodiment method preparation, the missible oil of oxime active compound or wettable powder, dilute with water is made into the mother liquor of predetermined concentration, choosing that behind presoaking and germinating bud is about is that 0.3 centimetre even seed is (as barnyard grass, amaranth) placing the diameter that is lined with filter paper in advance is in 9 centimetres the culture dish, and then covering one deck filter paper, add in the ware with a certain amount of soup of pipette, extract, loam cake is placed in the illumination box of optimal temperature, it is long to measure root after 3-4 days, long and the fresh weight of bud calculates inhibiting rate.Found that the growth to one or more unifacial leaves or broadleaf weed when 100ppm (in effective component content) of some compound has restraining effect or growth promoting function.
Group Ar in the formula I
2Simplify and use G
K(K=1-35) abbreviation expression, the then logical formula I compound of available general formula (VII) expression in table 1.
Segment bounds (VII) is that the compound of formula I, formula II and formula III shows as table 1, but formula I, the compound of formula II and formula III is not limited only to the compound shown in the table 1.
Claims (14)
1. biocidal alkyl-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds,
It is characterized in that: a series of alkyl-(mixing)-aryl-ketoxime-O-ether compound shown in the logical formula I and a series of intermediate ketones, the oxime compound shown in logical formula II and the logical formula III are provided:
Logical formula I, in (II) and (III):
I .Ar
1And Ar
2Be identical or different, and representative
(a) (C
6-C
12The heteroaryl of)-aryl or 10 carbon atoms of band as many as, or
(b) as at I .a) in determined implication, be selected from case of necessity following in 5 identical or different substituting groups of as many as replace:
Halogen, nitro, cyano group, (C1-C
6)-alkyl, (C1-C
6)-haloalkyl, cyano group-(C1-C
6)-alkyl, (C1-C
6)-alkoxyl-(C1-C
6)-alkyl, (C1-C
6)-alkylthio group-(C1-C
6)-alkyl, (C1-C
6)-alkoxyl, (C1-C
6)-alkoxyl-(C1-C
6)-alkoxyl, (C1-C
6)-halogenated alkoxy, (C1-C
6)-halogenated alkoxy alkyl, (C1-C
6)-alkylthio group, (C1-C
6)-alkyl halide sulfenyl, (C1-C
6)-alkyl sulfonyl base, (C1-C
6)-alkyl sulfinyl, (C1-C
6)-alkoxyl carbonyl, (C1-C
6)-alkyl is amino, two-(C1-C
6)-alkyl is amino, (C2-C
6)-alkenyl, (C2-C
6)-alkenyl oxy, (C2-C
6) the alkenyl oxy alkyl, (C2-C
6)-halogenated alkenyl, (C2-C
6)-halogenated alkenyl oxy, (C2-C
6)-halogenated alkenyl oxy alkyl, (C2-C
6)-alkynyl, (C2-C
6)-alkynyl oxygen base, (C2-C
6)-halo alkynyl, (C2-C
6)-halo alkynyl oxygen base, (C3-C
8)-cycloalkyl, (C3-C
8)-cycloalkyl oxy, (C3-C
8)-cycloalkyl is amino, (C6-C
12)-aryl, (C6-C
12)-aryloxy, (C6-C
12)-aryl sulfenyl, (C6-C
12)-aryl-(C1-C
4)-alkyl, (C6-C
12)-aryloxy carbonyl, (C6-C
12)-arylsulfonyl base, (C6-C
12)-aryl sulfonyl kia, (C6-C
12)-aryl is amino, heteroaryl, heteroaryl oxygen base, heteroaryl-(C1-C
4)-alkyl, heteroaryl sulfenyl, heteroaryl oxygen base carbonyl, heteroaryl sulfonyl, heteroaryl sulfinyl, and 1) I .b) described in substituting group while being aryl or heteroaryl, sometimes can be by one or more (C that are selected from1-
C
6)-alkyl, (C1-C
6)-alkoxyl, (C1-C
6)-haloalkyl, (C1-C
6The identical or different group of)-halogenated alkoxy and halogen replaces, and heteroaryl is the heteroaryl with 10 carbon atoms of as many as, 2) I .b), cycloalkyl can be selected from halogen, (C by 5 of as many as sometimes1-C
4The identical or different group of)-alkyl replaces, 3) I .b), substituent 2 represent methylenedioxy group or ethylenedioxy, and methylenedioxy group or ethylenedioxy are sometimes with 1 or 2 identical or different halogen and (C of being selected from1-C
6The substituting group of)-alkyl, 4) I.a) and I .b) in determined aryl and the partly or entirely hydrogenation of heteroaryl of implication, wherein 1 or 2 CH2Group can be replaced by CO, II .R1And R2Be identical or different, and represent hydrogen, halogen, (C1-C
6)-alkyl, (C1-C
6)-haloalkyl, cyano group-(C1-C
6)-alkyl, (C1-C
6)-alkoxyl-(C1-C
6)-alkyl, (C1-C
6)-alkylthio group-(C1-C
6)-alkyl, (C1-C
6)-alkoxyl-(C1-C
6)-alkoxyl, (C1-C
6)-alkoxyl, (C1-C
6)-alkylthio group, (C1-C
6)-alkyl sulfonyl base, (C1-C
6)-alkyl sulfinyl, (C1-C
6)-alkoxyl carbonyl, (C1-C
6)-alkyl is amino, two-(C1-C
6)-alkyl is amino, (C1-C
6)-halogenated alkoxy, (C2-C
6)-alkenyl, (C2-C
6)-alkenyl oxy, (C2-C
6)-halogenated alkenyl, (C2-C
6)-halogenated alkenyl oxy, (C2-C
6)-alkynyl, (C2-C
6)-alkynyl oxygen base, (C2-C
6)-halo alkynyl, (C2-C
6)-halo alkynyl oxygen base, (C3-C
8)-cycloalkyl, (C3-C
8)-cycloalkyl oxy, (C3-C
8)-cycloalkyl is amino, (C6-C
12)-aryl, (C6-C
12)-aryloxy, (C6-C
12)-aryl-(C1-C
4)-alkyl, (C6-C
12)-aryl sulfenyl, (C6-C
12)-aryloxy carbonyl, (C6-C
12)-arylsulfonyl base, (C6-C
12)-aryl sulfonyl kia, (C6-C
12)-aryl is amino, heteroaryl, heteroaryl oxygen base, heteroaryl-(C1-C
4)-alkyl, heteroaryl sulfenyl, heteroaryl oxygen base carbonyl, heteroaryl sulfonyl, heteroaryl sulfinyl, III .R3Representative
(a) hydrogen,
(b) (C
1-C
6)-alkyl, (C
6-C
12)-aryl, the heteroaryl of 10 carbon atoms of band as many as, (C
2-C
6)-alkenyl, (C
2-C
6)-alkynyl, (C
1-C
4)-acyl group,
And group III .b) can be selected from halogen, (C sometimes
1-C
4)-alkyl, (C
1-C
4)-alkoxyl group, (C
1-C
4)-haloalkyl, (C
1-C
4)-alkylthio, (C
1-C
4Identical or the different groups of)-halogenated alkoxy replace, and IV .X represents O, S, SO, SO
2, NH, NR
a[R
aBe (C
1-C
4)-alkyl], V .Y represents hydrogen, cyano group, halogen, (C
1-C
4)-alkyl, (C
1-C
4)-alkoxyl group, VI .M are a hydrogen or an alkali metal atom, and VII .m or n represent 0,1,2 or 3.
All abovementioned alkyls, alkenyl and alkynyl can be straight chain also can be side chain, also be applicable to simultaneously, as by alkyl derivative group alkoxyl group, alkoxy carbonyl, alkylthio, haloalkyl and arylalkyl by they derivative groups.
Haloalkyl, halogenated alkenyl and halo alkynyl are meant wherein 1, the alkyl that a plurality of or all hydrogen atoms are replaced by halogen, alkenyl and alkynyl.Also be applicable to by they derivative groups simultaneously halogenated alkoxy is arranged, halogenated alkylthio, halo alkoxy carbonyl and aryl halide substituted alkyl as the group that derives from by haloalkyl.
Halogen is meant fluorine, chlorine, bromine or iodine, preferred fluorine, chlorine or bromine.
(C
6-C
12The preferred phenyl of)-aryl and by its derivative group, as naphthyl, xenyl etc.
The heteroaryl preferred one or the aryl bicyclic of 10 carbon atoms of band as many as have 1 N at least, O and/or S in the formula, as thienyl, benzothienyl, furyl, benzofuryl, pyrryl, indyl, imidazolyl, pyrazolyl, pyridyl, pyrazinyl, pyrimidyl, pyridazinyl, oxazolyl, isoxazolyl, thiazolyl and isothiazolyl.
(C
6-C
12The partly or entirely hydrogenation of heteroaryl of)-aryl and 10 carbon atoms of band as many as, wherein 1 or 2 CH
2-Ji is replaced by CO, as cyclohexenyl, and cyclohexanedione base etc.
2. alkyl according to claim 1-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds is characterized in that logical formula I, Ar in (II) and (III)
1A group shown in being preferably as follows:
Shown in above-mentioned in the group:
Z is O, S or NR
5, R
5Be hydrogen, (C
1-C
6)-alkyl, (C
1-C
6)-haloalkyl, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkyl, (C
1-C
6)-alkylthio-(C
1-C
6)-alkyl, cyano group (C
1-C
6)-alkyl, (C
1-C
6)-alkoxy carbonyl, (C
2-C
6)-alkenyl, (C
2-C
6)-halogenated alkenyl, (C
2-C
6)-alkynyl, (C
2-C
6)-halo alkynyl, (C
3-C
8)-cycloalkyl, and cycloalkyl can be selected from halogen or (C by 5 of as many as sometimes
1-C
4The identical or different group of)-alkyl replaces (C
6-C
12)-aryl, (C
6-C
12)-aryl-(C
1-C
4)-alkyl, (C
6-C
12)-aryloxycarbonyl, the heteroaryl of 10 carbon atoms of band as many as, heteroaryl oxygen base carbonyl, heteroaryl-(C
1-C
4)-alkyl,
P is one 0 to 5 a integer,
R
4Be identical or different, and represent hydrogen, halogen, nitro, cyano group, (C
1-C
6)-alkyl, (C
1-C
6)-haloalkyl, cyano group-(C
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkyl, (C
1-C
6)-alkylthio-(C
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkoxyl group, (C
1-C
6)-halogenated alkoxy, (C
1-C
6)-halogenated alkoxy alkyl, (C
1-C
6)-alkylthio, (C
1-C
6)-halogenated alkylthio, (C
1-C
6)-alkyl sulphonyl, (C
1-C
6)-alkyl sulphinyl, (C
1-C
6)-alkoxy carbonyl, (C
1-C
6)-alkylamino, two-(C
1-C
6)-alkylamino, (C
2-C
6)-alkenyl, (C
2-C
6)-alkenyl oxy, (C
2-C
6)-alkenyl oxy alkyl, (C
2-C
6)-halogenated alkenyl, (C
2-C
6)-halogenated alkenyl oxy, (C
2-C
6)-halogenated alkenyl oxy alkyl, (C
2-C
6)-alkynyl, (C
2-C
6)-alkynyloxy base, (C
2-C
6)-halo alkynyl, (C
2-C
6)-halo alkynyloxy base, (C
3-C
8)-cycloalkyl, (C
3-C
8)-cycloalkyl oxy, (C
3-C
8)-cycloalkyl amino, (C
6-C
12)-aryl, (C
6-C
12)-aryloxy, (C
6-C
12)-artyl sulfo, (C
6-C
12)-aryl-(C
1-C
4)-alkyl, (C
6-C
12)-aryloxy carbonyl, (C
6-C
12)-aryl sulfonyl, (C
6-C
12)-aryl sulfonyl kia, (C
6-C
12)-arylamino, heteroaryl, heteroaryl oxygen base, heteroaryl-(C
1-C
4)-alkyl, the heteroaryl sulfenyl, heteroaryl oxygen base carbonyl, heteroarylsulfonyl, the heteroaryl sulfinyl, and
1) R
4Described in substituting group when being aryl or heteroaryl, sometimes can be by one or more (C that are selected from
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group, (C
1-C
6)-haloalkyl, (C
1-C
6The identical or different group of)-halogenated alkoxy and halogen replaces, and heteroaryl is the heteroaryl of 10 carbon atoms of band as many as,
2) R
4Described in cycloalkyl can be selected from halogen, (C by 5 of as many as sometimes
1-C
4The identical or different group of)-alkyl replaces,
3) R
4Described in substituent 2 represent methylenedioxy group or ethylenedioxy, methylenedioxy group or ethylenedioxy sometimes with 1 or 2 identical or different be selected from halogen and (C
1-C
6The substituting group of)-alkyl,
4. alkyl according to claim 1-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds is characterized in that logical formula I, R in (II) and (III)
1And R
2Be identical or different, and preferred hydrogen, halogen, (C
1-C
4)-alkyl, (C
1-C
4)-alkoxyl group, (C
1-C
4)-haloalkyl, (C
1-C
4)-alkylthio, (C
1-C
4)-alkenyl, (C
1-C
4)-alkynyl, (C
1-C
4)-halogenated alkenyl, (C
1-C
4)-halo alkynyl, (C
1-C
4)-alkyl sulphonyl, (C
1-C
4)-alkyl sulphinyl, (C
1-C
4)-alkylamino,
5. according to the described alkyl of claim 1-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds, it is characterized in that logical formula I, (II) and (III) middle R
3Preferred hydrogen, (C
1-C
4)-alkyl, (C
1-C
4)-haloalkyl, (C
1-C
4)-alkenyl, (C
1-C
4)-alkynyl, (C
1-C
4)-halogenated alkenyl, (C
1-C
4)-halo alkynyl, (C
6-C
12)-aryl,
6. according to the described alkyl of claim 1-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds, it is characterized in that Ar in the logical formula I
2Preferably:
R represents N or CH, and q is O, S, NH or CH
2, preferred O or CH
2, R
6Be hydrogen, halogen, allyl group oxygen base, propargyloxy,
F represents fluorine,
T is identical or different with g, represents 0,1,2,3 or 4, and t and g sum be not more than 5,
R
7Be identical or different, and represent hydrogen, halogen, (C
1-C
6)-alkyl, (C
1-C
6)-haloalkyl, (C
1-C
6)-alkoxyl group, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkoxyl group, (C
1-C
6)-halogenated alkoxy, (C
1-C
6)-alkyl sulphonyl, (C
2-C
6)-alkenyl, (C
2-C
6)-alkenyl oxy, (C
2-C
6)-alkenyl oxy alkyl, (C
2-C
6)-halogenated alkenyl, (C
2-C
6)-halogenated alkenyl oxy, (C
2-C
6)-halogenated alkenyl oxy alkyl, (C
2-C
6)-alkynyl, (C
2-C
6)-alkynyloxy base, (C
2-C
6)-halo alkynyl, (C
2-C
6)-halo alkynyloxy base, (C
3-C
8)-cycloalkyl, (C
3-C
8)-cycloalkyl oxy, (C
3-C
8)-halogenated cycloalkyl, (C
6-C
12)-aryl, (C
6-C
12)-aryloxy, (C
6-C
12)-aryl-(C
1-C
4)-alkyl, (C
6-C
12)-artyl sulfo, the heteroaryl of 10 carbon atoms of band as many as, heteroaryl oxygen base, heteroaryl-(C
1-C
4)-alkyl,
Z is O, S or NR
5, R
5As R in the claim 2
5Definition, w is 0,1,2 or 3,
R
8Be identical or different, and represent hydrogen, halogen, (C
1-C
6)-alkyl, (C
1-C
6)-haloalkyl, (C
1-C
6)-alkoxyl group, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group-(C
1-C
6)-alkoxyl group, (C
1-C
6)-halogenated alkoxy, (C
2-C
6)-alkenyl, (C
2-C
6)-alkenyl oxy, (C
2-C
6)-halogenated alkenyl, (C
2-C
6)-halogenated alkenyl oxy, (C
2-C
6)-alkynyl, (C
2-C
6)-alkynyloxy base, (C
2-C
6)-halo alkynyl, (C
2-C
6)-halo alkynyloxy base, (C
6-C
12)-aryloxy, (C
6-C
12)-aryl-(C
1-C
4)-alkyl, (C
6-C
12)-artyl sulfo, the heteroaryl oxygen base of 10 carbon atoms of band as many as, heteroaryl sulfenyl, heteroaryl-(C
1-C
4)-alkyl,
And R
8In aryl or heteroaryl, can be selected from (C sometimes
1-C
6)-alkyl, (C
1-C
6)-alkoxyl group, (C
1-C
6)-haloalkyl, (C
1-C
6The identical or different group of)-halogenated alkoxy and halogen replaces,
R is (C
1-C
6)-alkyl, (C
1-C
6)-haloalkyl, preferable methyl,
A is an oxygen, sulphur or NH,
B is nitrogen or CH,
7. according to the described alkyl of claim 1-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds, the preparation method of logical formula I compound is characterized in that:
A) with a compound shown in the formula III
Ar in the formula IV
2With definition in Y such as the claim 1, L represents a leavings group, if M is a hydrogen in the formula III, then in the presence of an appropriate base, add suitable solvent, add phase-transfer catalyst PTC again, in normal pressure, reaction is 1-20 hour and get under the 0-120 ℃ of condition, if M is alkali metal atom such as sodium in the formula III, then in a suitable solvent, add phase-transfer catalyst PTC, in normal pressure, reaction is 1-20 hour and get under the 0-120 ℃ of condition.Or
B) with the compound shown in the formula II
In case of necessity in the presence of an appropriate base with the compound shown in the formula (V) or with their reactant salt
Y and Ar in the formula
2As definition in the claim 1.
8. according to the described alkyl of claim 1-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds, the preparation method of logical formula III compound is characterized in that:
With compound shown in the formula II and hydroxylamine hydrochloride or hydroxylamine sulfate, in normal pressure, 0-100 ℃, in a suitable solvent preferably water and/or alcohol the reaction and get, also need add an appropriate base such as metal hydroxides in case of necessity, alkaline carbonate, alkali metal hydrocarbonate, appropriate base such as pyridine are synthesized.
9. according to the described alkyl of claim 1-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds, the preparation method of logical formula II compound selects proper raw material and corresponding synthetic method to carry out the synthetic of (II) as the case may be.In logical formula II, the preparation method of X=S compound is characterized in that:
Ar in the formula
1And R
1, R
2, n, definition in m such as the claim 1, with the sodium salt of corresponding mercaptan in the aqueous solution, in normal pressure, 30-100 ℃ of reaction 2-10 hour and getting.
10. according to the described alkyl of claim 1-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds, the preparation method of formula I compound, it is characterized in that preparing the used appropriate base of oxime ether compound is alkali metal hydroxide, alkaline carbonate, alkali metal hydrocarbonate, alkali metal alcoholates, basic metal, alkalimetal hydride, pyridine, tertiary amine, phase-transfer catalyst PTC is a tetrabutylammonium iodide, Tetrabutyl amonium bromide, tetraethylammonium bromide, hexaoxacyclooctadecane-6-6, suitable solvent is to be selected from the following solvents one or both: water, tetrahydrofuran (THF), toluene, benzene, acetonitrile, dimethyl sulfoxide (DMSO) etc., suitable leavings group is a chlorine, bromine, OSO
2Deng.
11. according to the described alkyl of claim 1-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds, it is characterized in that: the compound shown in formula I and the formula III can two kinds of geometrical isomer forms occur: " cis " (Z formula) and " trans " (E formula) form, " cis " (Z formula) is oxime-oxygen and aryl or heteroaryl Ar
1Be in the two key homonymies of C=N, " trans " (E formula) is oxime-oxygen and aryl or heteroaryl Ar
1Be in the two key heteropleurals of C=N,
The described alkyl of claim 1-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds, as have one or more unsymmetrical carbons, it can be a racemic modification, diastereomer or pure optical isomer,
The described alkyl of claim 1-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds not only relate to cis (Z formula), also relate to trans (E formula) and both mixtures,
The described alkyl of claim 1-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds not only relate to racemic modification, and diastereomer also relates to pure optical isomer,
The described alkyl of claim 1-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds not only relate to pure isomer, also relate to various mixture of isomers.
12. according to the described alkyl of claim 1 to 6-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds, it is characterized in that: general formula is (I), the compound of (II) and (III) does harm to mite class, pathogenic fungi and weeds biologically active to insect.And the compound that has has excellent biological activity.
13. contain at least-individually kill harmful organism medicament and this medicament thereof insect, evil mite, the purposes on pathogenic bacteria and the control of weeds according to the compound in the claim 1 and conventional additive and auxiliary material.
14. according to the described alkyl of claim 1 to 6-replacement (mixing)-aryl-ketoxime-O-ether and intermediate ketone, oxime compounds, formula II and formula III compound are except that the intermediate as logical formula I active substance preparation, also as biologically active substance.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CNB991155572A CN1159290C (en) | 1999-09-10 | 1999-09-10 | Method for preparing biocide alkyl-substituted (hetero)-aryl-ketoxime-o-ether and its intermediate ketone or oxime compounds |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CNB991155572A CN1159290C (en) | 1999-09-10 | 1999-09-10 | Method for preparing biocide alkyl-substituted (hetero)-aryl-ketoxime-o-ether and its intermediate ketone or oxime compounds |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN1288002A true CN1288002A (en) | 2001-03-21 |
| CN1159290C CN1159290C (en) | 2004-07-28 |
Family
ID=5278499
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNB991155572A Expired - Lifetime CN1159290C (en) | 1999-09-10 | 1999-09-10 | Method for preparing biocide alkyl-substituted (hetero)-aryl-ketoxime-o-ether and its intermediate ketone or oxime compounds |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN1159290C (en) |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005056518A1 (en) * | 2003-12-12 | 2005-06-23 | Hunan Research Institute Of Chemical Industry | Sulfur- or oxygen-containing oxime ether compounds with biocidal activity |
| CN102030680A (en) * | 2010-10-20 | 2011-04-27 | 湖北省生物农药工程研究中心 | Novel oxime ether or ester derivatives with insecticidal activity, preparation method and application thereof |
| CN102228044A (en) * | 2011-05-11 | 2011-11-02 | 青岛海利尔药业有限公司 | Insecticidal composition containing sufluoxime |
| CN102228028A (en) * | 2011-05-30 | 2011-11-02 | 青岛海利尔药业有限公司 | Pesticide composition containing sulfur-fluorine oxime ether and part of amide pesticides |
| CN102239875A (en) * | 2011-05-17 | 2011-11-16 | 海利尔药业集团股份有限公司 | Insecticidal composition containing 1-(3-fluoro-4-chlorphenyl)-2-methylthioethanone-O-(2-hydroxymethyl-3-methyl)aether and partial nicotine compounds |
| CN102246778A (en) * | 2011-05-18 | 2011-11-23 | 海利尔药业集团股份有限公司 | Efficiency enhanced insecticidal composition, and application thereof |
| CN102911093A (en) * | 2012-10-25 | 2013-02-06 | 湖南化工研究院 | Preparation method for sulfur fluorine oxime ether |
| CN103734180A (en) * | 2013-12-30 | 2014-04-23 | 孙蒙蒙 | Bactericidal and insecticidal composite containing sulfur-fluorine oxime ether |
| CN108137568A (en) * | 2015-10-13 | 2018-06-08 | 日本农药株式会社 | Fused heterocyclic compound or its esters with oximido and contain the salt, garderning pesticide of the compound and its application method |
| CN112661732A (en) * | 2020-12-23 | 2021-04-16 | 湖南加法检测有限公司 | Furanol derivative and preparation method and application thereof |
-
1999
- 1999-09-10 CN CNB991155572A patent/CN1159290C/en not_active Expired - Lifetime
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005056518A1 (en) * | 2003-12-12 | 2005-06-23 | Hunan Research Institute Of Chemical Industry | Sulfur- or oxygen-containing oxime ether compounds with biocidal activity |
| CN102030680A (en) * | 2010-10-20 | 2011-04-27 | 湖北省生物农药工程研究中心 | Novel oxime ether or ester derivatives with insecticidal activity, preparation method and application thereof |
| CN102228044B (en) * | 2011-05-11 | 2013-04-24 | 海利尔药业集团股份有限公司 | Insecticidal composition containing sufluoxime |
| CN102228044A (en) * | 2011-05-11 | 2011-11-02 | 青岛海利尔药业有限公司 | Insecticidal composition containing sufluoxime |
| CN102239875A (en) * | 2011-05-17 | 2011-11-16 | 海利尔药业集团股份有限公司 | Insecticidal composition containing 1-(3-fluoro-4-chlorphenyl)-2-methylthioethanone-O-(2-hydroxymethyl-3-methyl)aether and partial nicotine compounds |
| CN102246778A (en) * | 2011-05-18 | 2011-11-23 | 海利尔药业集团股份有限公司 | Efficiency enhanced insecticidal composition, and application thereof |
| CN102228028A (en) * | 2011-05-30 | 2011-11-02 | 青岛海利尔药业有限公司 | Pesticide composition containing sulfur-fluorine oxime ether and part of amide pesticides |
| CN102911093A (en) * | 2012-10-25 | 2013-02-06 | 湖南化工研究院 | Preparation method for sulfur fluorine oxime ether |
| CN102911093B (en) * | 2012-10-25 | 2014-08-13 | 湖南化工研究院 | Preparation method for sulfur fluorine oxime ether |
| CN103734180A (en) * | 2013-12-30 | 2014-04-23 | 孙蒙蒙 | Bactericidal and insecticidal composite containing sulfur-fluorine oxime ether |
| CN103734180B (en) * | 2013-12-30 | 2015-12-30 | 刘一平 | A kind of Fungicidal insecticidal composition containing sufluoxime |
| CN108137568A (en) * | 2015-10-13 | 2018-06-08 | 日本农药株式会社 | Fused heterocyclic compound or its esters with oximido and contain the salt, garderning pesticide of the compound and its application method |
| CN112661732A (en) * | 2020-12-23 | 2021-04-16 | 湖南加法检测有限公司 | Furanol derivative and preparation method and application thereof |
| CN112661732B (en) * | 2020-12-23 | 2023-08-04 | 湖南化研院检测技术有限公司 | Furanol derivative and preparation method and application thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CN1159290C (en) | 2004-07-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1039079C (en) | Pesticidal 1-arylpyrroles | |
| JP2000516917A (en) | Carboanilide used as a pesticide | |
| WO2013097518A1 (en) | Thiazole methylamino pyridine compounds and preparation method therefor | |
| CN1288002A (en) | Method for preparing biocide alkyl-substituted (hetero)-aryl-ketoxime-o-ether and its intermediate ketone or oxime compounds | |
| CN1117071C (en) | Pesticides | |
| CN1100768C (en) | Substituted diphenyl oxazolines | |
| CN1046503C (en) | 2-aryl-5-trifluoromethyl)-2-pyrroline compounds and process for the manufacture of insecticidal, 2-aryl-1-(alkoxymethyl)-4-halo-5-(trifluoromethyl) pyrroles | |
| JP3833281B2 (en) | 2,6-dichloroisonicotinic acid benzylamide derivative and plant disease control agent | |
| JPH06172348A (en) | Noxious life controlling agent | |
| CN1670016A (en) | Aryl pyrrole compounds with insecticidal, miticidal and fungicidal bioactivities and process for preparing same | |
| JPH07224062A (en) | Insecticidal nitro compound | |
| CN1071322C (en) | Process for producing 5,6-dihydro-1,3-oxazines | |
| CN1546462A (en) | Insecticidal antibacterial sulphur , oxygen and oxime-containing ether compounds | |
| CN1680342A (en) | Diazosulfide derivative and its synthesis and screening method for inducing anti-disease activity | |
| CN1049894C (en) | Substd. (1,4)-dioxino[2,3-F] benzoimidazole derivs. and their use in pest control | |
| CN1288459A (en) | Organic nitrile derivatives and their use as pesticides | |
| EP1125931A1 (en) | Biocidal alkyl-substituted-(hetero)aryl-ketoxime-O-ethers and the production method thereof | |
| KR100311195B1 (en) | Flurovinyl-substituted propenoic ester and amide compound having oxime bridge, process for preparing same and antifungal composition comprising sae | |
| JP2001247539A (en) | Biocidal alkyl-substituted (hetero)aryl-ketoxime-o-ether, and method of producing the same | |
| CN1117728A (en) | Substituted oxazoles | |
| CN1148848A (en) | N-aryl and N-alkylsulphonylaminals as pesticides | |
| KR20090024394A (en) | Synthesis of new fluoroaromatic formamidines and their biological activity | |
| JPS63253084A (en) | Novel heterocyclic compound | |
| KR100311196B1 (en) | Fluropropenyl-substituted propenoic ester and amide compound having oxime bridge, process for preparing same and antifungal composition comprising same | |
| KR100379761B1 (en) | New propenoic ester and amide compound having oxime side chain, process for preparing same and antifungal composition comprising same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C06 | Publication | ||
| PB01 | Publication | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| CX01 | Expiry of patent term |
Granted publication date: 20040728 |
|
| CX01 | Expiry of patent term |