CN111789846A - Application of L-securinine and its medicinal salt in preparing antidepressant drug - Google Patents
Application of L-securinine and its medicinal salt in preparing antidepressant drug Download PDFInfo
- Publication number
- CN111789846A CN111789846A CN202010799865.4A CN202010799865A CN111789846A CN 111789846 A CN111789846 A CN 111789846A CN 202010799865 A CN202010799865 A CN 202010799865A CN 111789846 A CN111789846 A CN 111789846A
- Authority
- CN
- China
- Prior art keywords
- securinine
- depression
- pharmaceutically acceptable
- mice
- antidepressant
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- SWZMSZQQJRKFBP-WZRBSPASSA-N Securinine Chemical compound N12CCCC[C@@H]2[C@@]23OC(=O)C=C2C=C[C@@H]1C3 SWZMSZQQJRKFBP-WZRBSPASSA-N 0.000 title claims abstract description 94
- 150000003839 salts Chemical class 0.000 title claims abstract description 29
- 239000000935 antidepressant agent Substances 0.000 title claims abstract description 17
- 208000020401 Depressive disease Diseases 0.000 claims abstract description 20
- 239000000243 solution Substances 0.000 claims abstract description 15
- 239000002552 dosage form Substances 0.000 claims abstract description 7
- 239000007924 injection Substances 0.000 claims abstract description 5
- 238000002347 injection Methods 0.000 claims abstract description 5
- 239000006187 pill Substances 0.000 claims abstract description 4
- 239000007921 spray Substances 0.000 claims abstract description 4
- 239000000725 suspension Substances 0.000 claims abstract description 4
- 239000003826 tablet Substances 0.000 claims abstract description 4
- 239000002775 capsule Substances 0.000 claims abstract description 3
- 239000008187 granular material Substances 0.000 claims abstract description 3
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract 5
- 239000000843 powder Substances 0.000 claims abstract 2
- 230000001430 anti-depressive effect Effects 0.000 claims description 12
- 229930013930 alkaloid Natural products 0.000 claims description 11
- 229940005513 antidepressants Drugs 0.000 claims description 10
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 claims description 9
- 238000002360 preparation method Methods 0.000 claims description 9
- 229910002651 NO3 Inorganic materials 0.000 claims description 7
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 claims description 7
- 150000003797 alkaloid derivatives Chemical class 0.000 claims description 7
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 claims description 6
- 230000000694 effects Effects 0.000 abstract description 23
- 239000003814 drug Substances 0.000 abstract description 18
- 239000000203 mixture Substances 0.000 abstract description 7
- 230000004044 response Effects 0.000 abstract description 7
- 231100000331 toxic Toxicity 0.000 abstract description 6
- 230000002588 toxic effect Effects 0.000 abstract description 6
- 241000699670 Mus sp. Species 0.000 description 39
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 24
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 18
- 210000004556 brain Anatomy 0.000 description 18
- 238000002474 experimental method Methods 0.000 description 18
- 230000033001 locomotion Effects 0.000 description 17
- 239000013078 crystal Substances 0.000 description 16
- 229950005774 securinine Drugs 0.000 description 14
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 12
- 238000000034 method Methods 0.000 description 12
- 238000007619 statistical method Methods 0.000 description 12
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 12
- 102000013530 TOR Serine-Threonine Kinases Human genes 0.000 description 11
- 108010065917 TOR Serine-Threonine Kinases Proteins 0.000 description 11
- 238000001543 one-way ANOVA Methods 0.000 description 11
- 230000037361 pathway Effects 0.000 description 11
- 102000047174 Disks Large Homolog 4 Human genes 0.000 description 10
- 108700019745 Disks Large Homolog 4 Proteins 0.000 description 10
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 10
- QZAYGJVTTNCVMB-UHFFFAOYSA-N serotonin Chemical compound C1=C(O)C=C2C(CCN)=CNC2=C1 QZAYGJVTTNCVMB-UHFFFAOYSA-N 0.000 description 10
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 9
- SWZMSZQQJRKFBP-UHFFFAOYSA-N Vivosecurinine Natural products N12CCCCC2C23OC(=O)C=C2C=CC1C3 SWZMSZQQJRKFBP-UHFFFAOYSA-N 0.000 description 9
- 210000004027 cell Anatomy 0.000 description 9
- 229940079593 drug Drugs 0.000 description 9
- 238000001035 drying Methods 0.000 description 9
- 230000002829 reductive effect Effects 0.000 description 9
- 230000009182 swimming Effects 0.000 description 9
- 210000004369 blood Anatomy 0.000 description 8
- 239000008280 blood Substances 0.000 description 8
- 210000002569 neuron Anatomy 0.000 description 8
- 241000699666 Mus <mouse, genus> Species 0.000 description 7
- 230000002093 peripheral effect Effects 0.000 description 7
- 102000004169 proteins and genes Human genes 0.000 description 7
- 108090000623 proteins and genes Proteins 0.000 description 7
- 230000003247 decreasing effect Effects 0.000 description 6
- 239000007928 intraperitoneal injection Substances 0.000 description 6
- 230000001737 promoting effect Effects 0.000 description 6
- 241000132346 Securinega suffruticosa Species 0.000 description 5
- 230000006399 behavior Effects 0.000 description 5
- 210000005013 brain tissue Anatomy 0.000 description 5
- 150000001875 compounds Chemical class 0.000 description 5
- 238000007912 intraperitoneal administration Methods 0.000 description 5
- 238000002156 mixing Methods 0.000 description 5
- 238000000465 moulding Methods 0.000 description 5
- 210000002241 neurite Anatomy 0.000 description 5
- 230000001225 therapeutic effect Effects 0.000 description 5
- 230000004913 activation Effects 0.000 description 4
- 230000004069 differentiation Effects 0.000 description 4
- 238000011156 evaluation Methods 0.000 description 4
- 238000001914 filtration Methods 0.000 description 4
- 239000005457 ice water Substances 0.000 description 4
- 150000002500 ions Chemical class 0.000 description 4
- 238000010172 mouse model Methods 0.000 description 4
- 210000005036 nerve Anatomy 0.000 description 4
- 238000001953 recrystallisation Methods 0.000 description 4
- 230000019491 signal transduction Effects 0.000 description 4
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 4
- 239000006228 supernatant Substances 0.000 description 4
- 210000000225 synapse Anatomy 0.000 description 4
- 238000003260 vortexing Methods 0.000 description 4
- 238000005406 washing Methods 0.000 description 4
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- YQEZLKZALYSWHR-UHFFFAOYSA-N Ketamine Chemical compound C=1C=CC=C(Cl)C=1C1(NC)CCCCC1=O YQEZLKZALYSWHR-UHFFFAOYSA-N 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 3
- 230000009471 action Effects 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 150000001720 carbohydrates Chemical class 0.000 description 3
- 235000014633 carbohydrates Nutrition 0.000 description 3
- 230000003001 depressive effect Effects 0.000 description 3
- 101150069842 dlg4 gene Proteins 0.000 description 3
- 239000011521 glass Substances 0.000 description 3
- 239000001963 growth medium Substances 0.000 description 3
- 229960003299 ketamine Drugs 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 230000000946 synaptic effect Effects 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 2
- 206010003694 Atrophy Diseases 0.000 description 2
- OMFXVFTZEKFJBZ-UHFFFAOYSA-N Corticosterone Natural products O=C1CCC2(C)C3C(O)CC(C)(C(CC4)C(=O)CO)C4C3CCC2=C1 OMFXVFTZEKFJBZ-UHFFFAOYSA-N 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- 229930182555 Penicillin Natural products 0.000 description 2
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 2
- -1 Polypropylene Polymers 0.000 description 2
- 239000004743 Polypropylene Substances 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 235000011114 ammonium hydroxide Nutrition 0.000 description 2
- 230000037444 atrophy Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000024245 cell differentiation Effects 0.000 description 2
- OMFXVFTZEKFJBZ-HJTSIMOOSA-N corticosterone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@H](CC4)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OMFXVFTZEKFJBZ-HJTSIMOOSA-N 0.000 description 2
- 210000003520 dendritic spine Anatomy 0.000 description 2
- 230000000994 depressogenic effect Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 239000011363 dried mixture Substances 0.000 description 2
- 235000019441 ethanol Nutrition 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 230000000971 hippocampal effect Effects 0.000 description 2
- 210000001320 hippocampus Anatomy 0.000 description 2
- 238000003125 immunofluorescent labeling Methods 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- 238000001819 mass spectrum Methods 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 102000002574 p38 Mitogen-Activated Protein Kinases Human genes 0.000 description 2
- 230000001575 pathological effect Effects 0.000 description 2
- 229940049954 penicillin Drugs 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 239000008055 phosphate buffer solution Substances 0.000 description 2
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 2
- 229920001155 polypropylene Polymers 0.000 description 2
- 229920000053 polysorbate 80 Polymers 0.000 description 2
- 210000004129 prosencephalon Anatomy 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 238000010898 silica gel chromatography Methods 0.000 description 2
- 229960005322 streptomycin Drugs 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 239000003643 water by type Substances 0.000 description 2
- 238000001262 western blot Methods 0.000 description 2
- AHOUBRCZNHFOSL-YOEHRIQHSA-N (+)-Casbol Chemical compound C1=CC(F)=CC=C1[C@H]1[C@H](COC=2C=C3OCOC3=CC=2)CNCC1 AHOUBRCZNHFOSL-YOEHRIQHSA-N 0.000 description 1
- RTHCYVBBDHJXIQ-MRXNPFEDSA-N (R)-fluoxetine Chemical compound O([C@H](CCNC)C=1C=CC=CC=1)C1=CC=C(C(F)(F)F)C=C1 RTHCYVBBDHJXIQ-MRXNPFEDSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- 108091006146 Channels Proteins 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 206010010144 Completed suicide Diseases 0.000 description 1
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 description 1
- KDXKERNSBIXSRK-RXMQYKEDSA-N D-lysine Chemical compound NCCCC[C@@H](N)C(O)=O KDXKERNSBIXSRK-RXMQYKEDSA-N 0.000 description 1
- 206010012374 Depressed mood Diseases 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 208000004929 Facial Paralysis Diseases 0.000 description 1
- 241001343888 Ferraria Species 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- 206010024264 Lethargy Diseases 0.000 description 1
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 description 1
- 102000004868 N-Methyl-D-Aspartate Receptors Human genes 0.000 description 1
- 108090001041 N-Methyl-D-Aspartate Receptors Proteins 0.000 description 1
- 208000007443 Neurasthenia Diseases 0.000 description 1
- 102000004108 Neurotransmitter Receptors Human genes 0.000 description 1
- 108090000590 Neurotransmitter Receptors Proteins 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- IOVCWXUNBOPUCH-UHFFFAOYSA-M Nitrite anion Chemical compound [O-]N=O IOVCWXUNBOPUCH-UHFFFAOYSA-M 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 108010019160 Pancreatin Proteins 0.000 description 1
- 229930040373 Paraformaldehyde Natural products 0.000 description 1
- AHOUBRCZNHFOSL-UHFFFAOYSA-N Paroxetine hydrochloride Natural products C1=CC(F)=CC=C1C1C(COC=2C=C3OCOC3=CC=2)CNCC1 AHOUBRCZNHFOSL-UHFFFAOYSA-N 0.000 description 1
- 206010057249 Phagocytosis Diseases 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-L Phosphate ion(2-) Chemical compound OP([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-L 0.000 description 1
- 229920005372 Plexiglas® Polymers 0.000 description 1
- 208000000474 Poliomyelitis Diseases 0.000 description 1
- 108010039918 Polylysine Proteins 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 241000867909 Securinega Species 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 206010042458 Suicidal ideation Diseases 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 102000004243 Tubulin Human genes 0.000 description 1
- 108090000704 Tubulin Proteins 0.000 description 1
- 208000036826 VIIth nerve paralysis Diseases 0.000 description 1
- 210000000683 abdominal cavity Anatomy 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 230000001133 acceleration Effects 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 230000001464 adherent effect Effects 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 150000001449 anionic compounds Chemical class 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 238000013528 artificial neural network Methods 0.000 description 1
- 206010003549 asthenia Diseases 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 230000003542 behavioural effect Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 208000036815 beta tubulin Diseases 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 210000005056 cell body Anatomy 0.000 description 1
- 210000003710 cerebral cortex Anatomy 0.000 description 1
- WORJEOGGNQDSOE-UHFFFAOYSA-N chloroform;methanol Chemical compound OC.ClC(Cl)Cl WORJEOGGNQDSOE-UHFFFAOYSA-N 0.000 description 1
- 229940114081 cinnamate Drugs 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 210000001787 dendrite Anatomy 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 230000000857 drug effect Effects 0.000 description 1
- 239000013583 drug formulation Substances 0.000 description 1
- 238000000132 electrospray ionisation Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 230000002964 excitative effect Effects 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 229960002464 fluoxetine Drugs 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 229940050410 gluconate Drugs 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 230000003400 hallucinatory effect Effects 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 210000004295 hippocampal neuron Anatomy 0.000 description 1
- DKAGJZJALZXOOV-UHFFFAOYSA-N hydrate;hydrochloride Chemical compound O.Cl DKAGJZJALZXOOV-UHFFFAOYSA-N 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 239000012729 immediate-release (IR) formulation Substances 0.000 description 1
- HAJKHJOABGFIGP-UHFFFAOYSA-N indolizidine Chemical class C1CCCN2CCCC21 HAJKHJOABGFIGP-UHFFFAOYSA-N 0.000 description 1
- 229930005307 indolizidine alkaloid Natural products 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 229910001412 inorganic anion Inorganic materials 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 231100000053 low toxicity Toxicity 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 238000013227 male C57BL/6J mice Methods 0.000 description 1
- 239000012046 mixed solvent Substances 0.000 description 1
- 230000036651 mood Effects 0.000 description 1
- 210000003061 neural cell Anatomy 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 230000004770 neurodegeneration Effects 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 150000002891 organic anions Chemical class 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 229940055695 pancreatin Drugs 0.000 description 1
- 229920002866 paraformaldehyde Polymers 0.000 description 1
- 229960002296 paroxetine Drugs 0.000 description 1
- 230000008782 phagocytosis Effects 0.000 description 1
- NIXKBAZVOQAHGC-UHFFFAOYSA-M phenylmethanesulfonate Chemical compound [O-]S(=O)(=O)CC1=CC=CC=C1 NIXKBAZVOQAHGC-UHFFFAOYSA-M 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 229920000656 polylysine Polymers 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 230000001242 postsynaptic effect Effects 0.000 description 1
- 210000002442 prefrontal cortex Anatomy 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 208000020016 psychiatric disease Diseases 0.000 description 1
- 238000010298 pulverizing process Methods 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 229930002330 retinoic acid Natural products 0.000 description 1
- 238000004366 reverse phase liquid chromatography Methods 0.000 description 1
- 230000033764 rhythmic process Effects 0.000 description 1
- WRDRGEDNNRSUNY-UHFFFAOYSA-N securinegine Chemical compound C1C2C34OC(=O)C=C2C=CC1N3CCCC4 WRDRGEDNNRSUNY-UHFFFAOYSA-N 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 229960002073 sertraline Drugs 0.000 description 1
- VGKDLMBJGBXTGI-SJCJKPOMSA-N sertraline Chemical compound C1([C@@H]2CC[C@@H](C3=CC=CC=C32)NC)=CC=C(Cl)C(Cl)=C1 VGKDLMBJGBXTGI-SJCJKPOMSA-N 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 238000012453 sprague-dawley rat model Methods 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 230000003977 synaptic function Effects 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- WBYWAXJHAXSJNI-VOTSOKGWSA-M trans-cinnamate Chemical compound [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 230000014616 translation Effects 0.000 description 1
- 229960001727 tretinoin Drugs 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 238000004704 ultra performance liquid chromatography Methods 0.000 description 1
- 238000002604 ultrasonography Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
Landscapes
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical & Material Sciences (AREA)
- Biomedical Technology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Neurosurgery (AREA)
- Neurology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Psychiatry (AREA)
- Pain & Pain Management (AREA)
- Epidemiology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
The invention discloses application of L-securinine and a medicinal salt thereof in preparing an anti-depression medicament. The antidepressant drug disclosed by the invention is single-component L-securinine and a pharmaceutically acceptable salt thereof or a pharmaceutical composition containing the L-securinine and the pharmaceutically acceptable salt thereof; the medicament or the pharmaceutical composition adopts the dosage forms of tablets, capsules, solutions, granules, powder, pills, sprays, suspensions, injections or instillations. The medicine or the medicine composition and the medicinal salt thereof can be applied to the treatment of acute depression and chronic depression and have the characteristics of obvious curative effect, quick response and small toxic and side effect.
Description
Technical Field
The invention belongs to the field of medicines, and particularly relates to application of L-securinega suffruticosa alkaloid and pharmaceutically acceptable salts thereof in preparation of anti-depression drugs.
Background
Depression is a common mental disorder characterized primarily by a depressed mood, often accompanied by a suicidal tendency (FerrariA J, et al.PLoS Medicine2013, 10(11): e 1001547). According to the data published by the world health organization, about 3.5 million people worldwide have depression, about 100 million depression patients suicide each year, and about 6100 tens of thousands of depression patients in China. With the development of society and the acceleration of life rhythm, the number of patients is expected to increase continuously, which brings heavy economic burden to society (Phillips MR, et al,Lancet, 2009, 373(9680): 2041-2053). The first-line antidepressant drugs used in clinic at present are mainly selective 5-hydroxytryptamine reuptake inhibitors, such as fluoxetine, paroxetine, sertraline and other drugs, and the basic principle is that the 5-hydroxytryptamine level in the brain of depression patients is considered to be lower based on early researches. However, these drugs have two distinct limitations: 1) the drug effect is slow, and the effect is generally generated after the patient takes the drug for 5 to 7 weeks; 2) the response was low, with only about one third of patients responding to the drug. More notably, in some cases, the drug exerts antidepressant therapeutic effects but no increase in 5-hydroxytryptamine is detected. These evidence suggest that levels of 5-hydroxytryptamine may not be directly linked to levels of depression, nor are they the most effective targets for antidepressant drugs.
Studies have shown that multiple regions of the brain in depression patients develop lesions, the most stable of which are neuronal loss, atrophy, decreased dendritic protein expression, decreased dendritic spines and synaptic numbers in the hippocampal and prefrontal cortex sites (Krishnan Vand nester EJ,Nature, 2008, 455(7215): 894; Kang HJ, et al,Nature Medicine, 2012, 18(9): 1413-1417). In animal models of depression, pathological features of the brain consistent with the patient also appear, such as decreased branching of hippocampal neurons, shorter length, decreased dendritic spines, synaptic atrophy and the like (Duman RS, oral,Nature Medicine, 2016, 22(3): 238). The mTOR molecular signaling pathway is used as an important regulation pathway in neurons and can be adjusted by itselfThe processes of phagocytosis, transcription, translation and the like improve the expression of the neurosynaptic protein so as to promote the synaptic function, and various traditional antidepressants also have certain effect of activating mTOR; therefore, mTOR may be an important target for antidepressants (Ign cio ZM, et al, Br J Clin Pharmacol, 2016, 82(5): 1280-1290). The first antidepressant discovered in recent years, ketamine, which is different from a reuptake inhibitor of 5-hydroxytryptamine, has the advantages of quick response and high response. Ketamine can regulate the function of neural networks and neuronal synapses by inhibiting NMDA receptor function and activating mTOR pathway, but the overdose of ketamine can cause hallucinogenic and addictive properties to patients, and the clinical use is limited. Therefore, the development of a novel antidepressant with high efficiency and low toxicity is urgently needed.
Securinine (securinine) belongs to indolizidine alkaloids, and natural securinine includes levo-securinine and dextro-securinine. L-securinega suffruticosa has been found to antagonize neurotransmitter receptor GABAAThe action of the receptors to excite central nerves (Beutler JA, et al,Brain Res1985, 3310(1):135-140), and can be used for treating facial paralysis, neurasthenia, lethargy, and poliomyelitis sequela. However, the application of the L-securinega suffruticosa alkaloid in depression has not been reported. The inventor finds that the L-securinine can improve the mTOR pathway level and the synaptic protein level of the brains of depressed mice, has obvious curative effect on the mice with acute depression and chronic depression, and is expected to be developed into a novel medicament for treating depression.
Disclosure of Invention
The invention aims to provide the application of the L-securinega suffruticosa alkaloid or the medicinal salt thereof in preparing the anti-depression medicament.
In order to achieve the purpose, the invention adopts the following technical scheme:
the preparation method of the L-securinine and the pharmaceutically acceptable salt thereof comprises the following steps:
drying and pulverizing the plant raw material of the securinega suffruticosa, percolating and extracting by using 95% ethanol, and concentrating under reduced pressure to obtain a total extract of the securinega suffruticosa extract; suspending the total extract with water, adding 10% hydrochloric acid water solution to adjust pH to 2-3, extracting with chloroform, adding ammonia water to adjust pH to 9-10, extracting with chloroform, and concentrating chloroform layer under reduced pressure to obtain total alkaloids; purifying the total alkaloids by silica gel column chromatography to obtain an enriched part of L-securinine, and recrystallizing the enriched part to obtain L-securinine crystal.
Slowly adding dilute acid solution into the L-securinine crystal to completely dissolve the L-securinine crystal, cooling in ice water bath, drying under reduced pressure to obtain salt of L-securinine, dissolving with anhydrous ethanol, standing for recrystallization, filtering, and drying to obtain pure product of pharmaceutically acceptable salt of L-securinine.
The pharmaceutically acceptable salts described above include, but are not limited to: inorganic anions such as chloride, bromide, iodide, sulfate, sulfite, nitrate, nitrite, phosphate, and hydrogenphosphate, and the like; organic anions such as acetate, propionate, cinnamate, phenylmethanesulfonate, citrate, lactate, gluconate, and the like.
The L-securinine and its pharmaceutically acceptable salt can be made into various dosage forms, including tablet, pill, lozenge, granule, gel, unguent, solution, suppository, injection, inhalant and spray. These dosage forms can be used for both local or systemic administration and for immediate or sustained release administration. When administered by injection, the compounds can be formulated into solutions, suspensions, and emulsions with water-soluble or lipid-soluble solvents. When administered orally, they can be formulated into a complex with pharmaceutically acceptable excipients using conventional techniques. These excipients can be used to formulate the compounds into a variety of dosage forms acceptable to the patient, such as tablets, pills, capsules, suspensions, gels, and the like.
Compared with the prior art, the invention has the following advantages and beneficial effects:
the invention provides a new application of L-securinine and a medicinal salt thereof in treating depression. The experimental data show that the L-securinega suffruticosa alkaloid has obvious curative effect on mice with acute depression and chronic depression, has effect after single administration, takes effect quickly and has no influence on the autonomous movement of animals. Therefore, the anti-depression drug prepared from the L-securinine and the pharmaceutically acceptable salt thereof has the characteristics of obvious curative effect, quick response and good safety.
Drawings
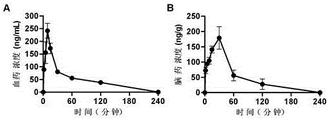
FIG. 1: the pharmacokinetics curve of single intraperitoneal administration of the L-securinine for blood inflow and brain inflow is shown in the specification, wherein A is the blood concentration of the L-securinine after different time of intraperitoneal administration, and B is the brain concentration of the L-securinine after different time of intraperitoneal administration.
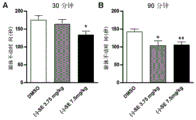
FIG. 2: securinine levorotatory reduced the level of acute depression in mice in a forced swim model: after intraperitoneal administration of L-securinine for 30min (A) or 90min (B), the result of immobility time in the experiment is forced. The result of 15 mice per group experiment is the mean value + -SEM, and the statistical method is one-way ANOVA; p <0.05, p < 0.01.
FIG. 3: l-securinenine activates AKT-mTOR-S6K, ERK and p38 pathways in mouse brain: (A) performing intraperitoneal injection of L-securinine for 90min, homogenizing forebrain parts (mainly including cerebral cortex and hippocampus), performing Western blot experiment, and detecting response of each signal channel. (B-G) statistics of different signaling pathway protein changes; the result is the mean value + -SEM of 6 groups of samples, and the statistical method is one-way ANOVA; p <0.01, p < 0.001.
FIG. 4: the oral administration of the L-securinine has the following effects of resisting acute depression: statistics of forced swimming immobility time after 3 hours of oral administration of securinega suffruticosa alkali. Results are mean ± SEM for 15 mice per group, one-way ANOVA, p <0.05 as statistical method.
FIG. 5: therapeutic effect of securinine levorotatory on chronic depression model mice: after administration of securinine, mice, a chronic depression model, were tested for carbohydrate preference (a), forced swimming (B), mTOR pathway activation (C), and PSD95 expression (D). It can be seen that a single administration improves carbohydrate bias and increases immobility time in forced swimming; the effect is more obvious after multiple times of administration. For the aspects of mTOR pathway activation and PSD95 expression, the effect of single administration is not obvious, and the effect of multiple administrations is obvious. Results were mean ± SEM for 15 mice per group, statistical methods one-way ANOVA, p <0.01, p <0.001, depression model + DMSO vs normal mice group; # p <0.05, # p <0.01, # p <0.001, depressive model + Securinegine levogyration group vs depressive model + DMSO group; a depression model, a single levorotatory securinine group vs depression model and a multiple levorotatory securinine group.
FIG. 6: the L-securinine does not generate toxic and side effects on the autonomous behavior of the mice: after the administration of the L-securinine for 60min, various indexes of the movement of the mice in an open field are tested: (A) a central zone residence time; (B) peripheral zone dwell time; (C) a center region movement distance; (D) peripheral zone movement distance; (E) a total movement distance; (F) a central region movement speed; (G) peripheral region
The speed of movement; (H) the total speed of movement. All indexes have no statistical difference. Results are mean ± SEM of 15 mice per group, one-way ANOVA is the statistical method.
Detailed Description
The present invention will be described in further detail with reference to examples and drawings, but the embodiments of the present invention are not limited thereto.
Example 1: separation and purification of L-securinine
Taking 1 kg of dried securinega suffruticosa branches and leaves, grinding, percolating and extracting with 5L of 95% ethanol for 3 times, mixing the extracting solutions, and concentrating under reduced pressure to obtain 80 g of total extract; suspending the total extract with 800 mL of water, adding 50 mL of 10% hydrochloric acid aqueous solution to adjust the pH to 2-3, extracting with chloroform, adding 30 mL of ammonia water into the acid aqueous layer to adjust the pH to 9-10, extracting with chloroform, and concentrating under reduced pressure in the chloroform layer to obtain 0.9 g of total alkaloids of securinega suffruticosa; purifying the total alkaloids by silica gel column chromatography, eluting with chloroform-methanol mixed solvent, mixing the fractions rich in L-securinine, and recrystallizing to obtain L-securinine crystal of about 200 mg.
Example 2: preparation of L-securinega suffruticosa salt
And slowly adding 100 mg of L-securinine crystals (purity is more than 95%), completely dissolving the crystals in 6 mL of 1% hydrochloric acid solution, standing the mixture for 2 hours in an ice water bath, drying the mixture under reduced pressure, dissolving the dried mixture in 3 mL of absolute ethanol, standing the mixture for recrystallization, washing the crystals with methanol, and filtering and drying the washed crystals to obtain the L-securinine hydrochloride (103 mg).
And slowly adding 100 mg of L-securinine crystals (purity is more than 95%), completely dissolving the crystals in 2 mL of 1% sulfuric acid solution, standing the mixture for 2 hours in an ice-water bath, drying the mixture under reduced pressure, dissolving the dried mixture in 3 mL of absolute ethanol, standing the mixture for recrystallization, washing the crystals with methanol, and filtering and drying the crystals to obtain the L-securinine sulfate (108 mg).
And slowly adding 100 mg of L-securinine crystals (with the purity of more than 95 percent), slowly adding 3 mL of 1 percent nitric acid solution to completely dissolve the crystals, standing for 2 hours in ice-water bath, drying under reduced pressure, dissolving the crystals by using 3 mL of absolute ethyl alcohol, standing for recrystallization, washing the crystals by using methanol, filtering and drying to obtain the L-securinine nitrate (110 mg).
Example 3: nerve cell differentiation promoting activity of L-securinine and its salts
The purpose is as follows: investigating the activity of L-securinine and the nitrate, hydrochloride and sulfate thereof for promoting the differentiation of nerve cells
The method comprises the following steps: neuro-2a cells (purchased from American type culture collection cell bank) were recovered, cultured in growth medium (MEM +10% FBS + 100U/mL penicillin and 100. mu.g/mL streptomycin), plated in a 100 mm dish, and cultured in a constant temperature incubator containing 5% CO2 at 37 ℃. And (3) carrying out passage when the cells grow to 60-70%, sucking out the culture solution in a culture dish, adding a proper amount of PBS (phosphate buffer solution), washing, digesting for 45 seconds by using 0.25% trypsin, adding a growth culture medium to stop digestion after the adherent cells are spherical, uniformly mixing, carrying out passage according to a ratio of 1:10, and carrying out passage for one day in three days. When the differentiation of the nerve cell strain is induced, the planting density of the cells is 2 multiplied by 104Each cell/35 mm culture dish or 1X 104One well (12-well plate), cultured in growth medium for 24 hours, then changed to differentiation medium (MEM +0.5% FBS + 100U/mL penicillin and 100. mu.g/mL streptomycin), and treated with addition of L-securinine or several pharmaceutically acceptable salts thereof for 48 hours. With 4% multimerizationFixing cells with formaldehyde/4% sucrose for 20-30 min, and observing differentiation morphology and neurite of nerve cell strain by using beta-tubulin III (marker protein specifically expressed by neurite) antibody immunofluorescence staining method. And (3) automatically scanning and photographing by adopting a high content instrument, and performing statistical analysis by utilizing Cellomics view software. Cells with neurite length greater than twice the soma were defined as nerve cells, and the average length of total neurite per differentiated cell was statistically analyzed.
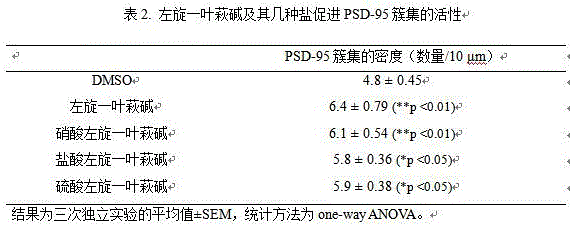
As a result: l-securinine and its nitrate, hydrochloride and sulfate all had similar activities of promoting nerve cell differentiation (Table 1).
TABLE 1 neural cell differentiation-promoting activity of L-securinine and its salts
aThe values in the table are neurite length (unit: μm), the results are mean. + -. SEM of three independent experiments, and the statistical method is one-way ANOVA.bAs a positive control, trans-Retinoic Acid (RA).
Example 4: l-securinine and its medicinal salts for promoting neurosynaptic formation
The purpose is as follows: investigating the neurosynaptic formation promoting activity of L-securinine and nitrate, hydrochloride and sulfate thereof
The method comprises the following steps: collecting hippocampal tissue of fetal rat of Sprague Dawley at 18 days of embryonic stage, digesting with pancreatin to obtain single isolated neuron, and planting on 18 mm glass slide coated with polylysine (Poly-D-Lysine) at density of 0.2 × 105Slide glass, cultured in Neurobasal medium supplemented with 2% B27. The solution was changed every three days (2% B27 in Neurobasal medium) half at a time. DMSO or L-securinine or its several pharmaceutically acceptable salts (10 μ M) was added at the 14 th day of culture, and treated for 48 hours. Fixation was performed with 4% paraformaldehyde for 5 minutes, followed by pre-cooled methanol on ice for 15 minutes. PSD-95 (excitatory postsynaptic nerve)Specifically expressed marker protein) antibody immunofluorescent staining method to evaluate the synapse formation. The number of PSD-95 clusters on the average dendrite length in each group was counted by photographing with a Zeiss Imager A2 fluorescence microscope.
As a result: l-securinine and its nitrate, hydrochloride and sulfate all had similar activity in promoting PSD-95 clustering (Table 2).
Example 5: the pharmacokinetic results of the L-securinine after intraperitoneal injection into blood and brain
The purpose is as follows: study on the pharmacokinetic parameters of L-securinine entering blood and brain
The method comprises the following steps:
male C57BL/6J mice 7-8 months old are fasted for 12 hours before the experiment, freely drink water, and are intraperitoneally injected with L-securinine at a dose of 7.5 mg/kg, and the preparation method is shown in example 3. The eyes were bled and the brain tissue was harvested at 10 time points (3 mice per time point) before (0 min) and 1.5, 5, 9, 15, 30, 60, 120, 240, 480 min after administration, respectively. The blood samples were centrifuged (4 ℃, 3000 rpm, 8 min) and the plasma was separated.
Taking 100 mu L of mouse plasma, adding 600 mu L of acetonitrile (IS) solution containing an internal standard compound, vortexing and oscillating for 5 min, centrifuging for 15 min at 4 ℃ and 15000 rpm, taking supernatant, volatilizing by a vacuum concentrator (40 ℃, 4 h), adding 100 mu L of 50% methanol for redissolving, vortexing for 3 min, performing ultrasound for 5 min, vortexing for 1 min, then centrifuging for 15 min at 15000 rpm, and taking supernatant for LC/MS analysis.
After the surface bloodstain of the brain tissue is washed by normal saline, water is sucked out, the brain tissue is precisely weighed, the normal saline is added according to the mass (g) to volume (mL) ratio of 1:2, and the brain tissue is homogenized. Taking 200 mu L of brain homogenate sample, respectively adding 1200 mu L of acetonitrile solution containing an internal standard compound, vortexing for 5 min, centrifuging for 15 min at the temperature of 4 ℃ and the speed of 15000 rpm, taking supernatant, volatilizing by using a vacuum concentrator, re-dissolving by 100 mu L of 50% methanol, processing the supernatant by using the vacuum concentrator, and then carrying out LC/MS analysis.
Chromatographic conditions are as follows: the method comprises the steps of adopting Waters ACQUITY QTOF four-stage rod series time flight mass spectrum to effectively separate the L-securinine and the internal standard substance, setting the column temperature to be 40 ℃, setting the sample chamber temperature to be 5 ℃, and adopting pure water and acetonitrile as a mobile phase, wherein the water contains 0.1% of formic acid. A chromatographic column: waters ACQUITY UPLC BEH C18 column (1.7 μm, 2.1 mm. times.100 mm) reverse phase chromatography column; time: 6 min; sample introduction amount: 5 μ L (specific conditions see Table below).
Mass spectrum conditions: positive ion scanning mode, electrospray ionization source, ion source temperature 60 ℃; ion spray voltage 3000V; scanning range: 100-500 Da, and the ion pair [ M + H + ] of (+) -SE is 218.12. According to the peak areas of plasma and brain collected at each time point after administration of each tested mouse, the plasma or brain concentration at each time point is calculated through a corresponding standard curve of blood or brain of each compound, each main pharmacokinetic parameter is calculated by using a non-atrioventricular model method, and parameters such as half-life and the like are analyzed through WinNonlin version 6.3 (Pharsight, Mountain View, Calif., USA) software.
As a result: securinine levorotatory can rapidly enter blood and brain in prototype, peak time is 9 minutes and 30 minutes respectively, and elimination time is 4 hours (table 3 and figure 1).
TABLE 3 pharmacokinetic results of L-securinine in blood and brain after intraperitoneal injection
Example 6: therapeutic effect of L-securinine on acute depressive episode induced by forced swimming model
The purpose is as follows: evaluation of anti-acute Depression action of L-securinine
The method comprises the following steps:
1) male mice, C57BL/6J (6-7 weeks) were housed in animal rooms with 12 hours bright (8: 00 to 20: 00), 12 hours dark cycle (20: 00 to 8:00 the next day), and relative humidity 60-70% and were freely fed with water. One week after acclimatization (7-8 weeks), the experiment was started.
2) Mice were placed in a behavioural laboratory in advance to acclimatize for more than 1 hour.
3) Single administration by intraperitoneal injection or oral administration: the injection dosage of the L-securinega suffruticosa alkaloid [ (-) -SE ] is 3.75mg/kg or 7.5 mg/kg, and the oral administration dosage is 10 mg/kg and 30 mg/kg. The preparation method of the medicine comprises the following steps: completely dissolving L-securinine in DMSO at concentrations of 37.5 mg/mL, 75 mg/mL, 100 mg/kg and 300 mg/kg respectively, adding 1 volume of DMSO, adding 1 volume of Tween-80, mixing, adding 98 volumes of physiological saline, mixing to obtain solutions at concentrations of 0.375 mg/mL, 0.75 mg/mL, 1 mg/kg and 3 mg/kg respectively, and injecting or orally administering at a volume of 0.1 mL per 10 g body weight. Blanks were given 1% DMSO, 1% Tween-80 and 98% saline as solvent controls. Prepared fresh before each administration. Each group was set with 15 mice. Forced swimming experiments were performed 30min or 90min after dosing.
4) The video recorder was turned on (angle for side shooter) and the mice were placed in a plexiglass jar of 15 cm diameter, 25 cm height, and 13 cm depth of water (now the mice hind paws were not enough to bottom) at 25 + -1 deg.C. The whole course was videotaped for 6 min, and the immobility time of the mice 4 min later indicated the depressed (despair) mood level of the mice. The mice that completed the experiment were wiped dry with paper, anesthetized with ether, and the brains were immediately removed and stored at-80 ℃ for subsequent pathological experiments. The glass jar was cleaned and water changed before the next mouse experiment.
5) The last 4 minutes of immobility time of the mice in the video was analyzed by forcedslim Version 2.0 (Clever Sys Inc.).
As a result: securinine L-securinine showed antidepressant effect after a single intraperitoneal administration for 30min or 90min at a dose of 7.5 mg/kg, and also showed antidepressant effect after a dose of 3.75mg/kg for 90min (FIG. 1). Brain tissues of a depression group and a depression administration group are simultaneously taken for analysis, and the result shows that an mTOR pathway is obviously activated after administration, and the expression of an important functional protein PSD-95 in the nerve synapse is also up-regulated, which indicates that the nerve synapse function is improved (figure 2). In addition, the oral administration showed antidepressant effect at a dose of 30 mg/kg (administration time of 3 hours) (FIG. 3).
Figure 2 shows that levo-securinine reduces the level of acute depression produced by mice in a forced swim model: retention time in forced swimming test after administering L-securinine to abdominal cavity for 30min (A) or 90min (B). The result of 15 mice per group experiment was the mean ± SEM, and the statistical method was one-way ANOVA; p <0.05, p < 0.01.
FIG. 3 shows that L-securinine activates AKT-mTOR-S6K, ERK and p38 pathway in mouse brain, wherein (A) after administration of L-securinine by intraperitoneal injection for 90min, forebrain parts (mainly cortex and hippocampus) are homogenized and then subjected to Western blot experiment to detect response of each signal pathway. (B-G) statistics of different signaling pathway protein changes; the result is the mean value + -SEM of 6 groups of samples, and the statistical method is one-way ANOVA; p <0.01, p < 0.001.
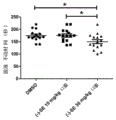
Figure 4 shows the anti-acute depressive effect of oral securinega laevigata base: statistics of forced swimming immobility time after 3 hours of oral administration of L-securinine. Results were mean ± SEM for 15 mice per group, one-way ANOVA, p <0.05 as statistical method.
Example 7: therapeutic action of L-securinine on chronic depression model mice
The purpose is as follows: evaluation of anti-chronic Depression action of L-securinine
The method comprises the following steps:
1) male mice, C57BL/6J (6-7 weeks) were housed in animal rooms with 12 hours bright (8: 00 to 20: 00), 12 hours dark cycle (20: 00 to 8:00 the next day), and relative humidity 60-70% and were freely fed with water. One week after acclimatization (7-8 weeks), the experiment was started.
2) The chronic depression molding method comprises the following steps: mice were sequentially given the stress patterns shown in the following table, with two stress categories per day for 30 days. The mice were weighed every 5 days during molding and tested for depression levels by a sugar water preference test on days 15 and 30.
Stress mode adopted by chronic depression modeling
3) The mice are divided into a normal control group, a depression model and DMSO solvent single administration group, a depression model and L-securinine single administration group, a depression model and DMSO multiple administration group, a depression model and L-securinine multiple administration group, and 15 mice in each group. The single administration group is administered the next day after the molding is finished, and depression indexes are detected after administration for 90 min; the multi-administration group starts to administer the medicament on the 16 th day of the molding, once a day, lasts for 15 days until the molding is finished; the next day depression indicators were measured. The administration modes are intraperitoneal injection, the administration dosage of the L-securinine is 7.5 mg/kg, and the preparation method is shown in example 6.
4) Evaluation tests of the depression index include: forced swimming experiment, sugar water preference experiment, mTOR pathway and PSD-95 expression level detection.
As a result: both single and multiple dose groups showed antidepressant effects including decreased immobility time in forced swim experiments, increased sugar intake in sugar preference experiments, decreased corticosterone levels in serum, and increased mTOR pathway activation and PSD-95 expression levels (fig. 5).
Figure 5 shows the therapeutic effect of levo-securinine on chronic depression model mice: after administration of securinerine laevigata (l-securinine) to chronic depression model mice, carbohydrate preference (a), forced swimming (B), corticosterone level in serum (C), mTOR pathway activation (D), and PSD95 expression (E) were tested. Results are mean ± SEM, statistical methods one-way ANOVA, <0.01, <0.001, < depression model + DMSO group vs normal mice group; # p <0.05, # p <0.01, # p <0.001, depression model + securinine levogyration group vs depression model + DMSO group; and the group of the L-securinine are respectively selected from a group of a depression model and a single L-securinine and a group of a multiple L-securinine and a multiple L-securinine.
Example 8: the L-securinine does not generate toxic and side effects on the autonomous motor behavior of the mice
The purpose is as follows: evaluation of toxic and side effects of L-securinine
The method comprises the following steps:
c57BL/6J (7-8 weeks) male mice were acclimated in the laboratory at 23-25 deg.C for 1 hour, and then administered with 3.75mg/kg or 7.5 mg/kg L-securinine intraperitoneally, and the drug formulation was as described in example 6. The independent behavior of the mice was evaluated by an Open field experiment (Open field) 30min after a single administration, as follows:
1) open-top experimental box of opaque Polypropylene (Polypropylene) of 40 × 40 × 40 cm in open field, take out mouse from cage, put into box center, the top shoots and begins timing, record 30min with video system;
2) data statistics were performed by analyzing the mouse motion trajectory, velocity, residence time in the central region (area 50%) and peripheral region using TopScan Version 3.0 (Clever Sys Inc.) software.
As a result: after the administration of L-securinine (3.75 mg/kg or 7.5 mg/kg), the movement distance, speed and residence time in the center and the periphery of the mice are not abnormal, which indicates that the two doses have no toxic and side effects on the autonomous motor behaviors of the mice (figure 6).
Fig. 6 shows that the L-securinine does not generate toxic and side effects on the autonomous motor behavior of the mice: after the administration of the L-securinine for 30min, the various indexes of the movement of the mice in an open field are tested: (A) a central zone residence time; (B) peripheral zone dwell time; (C) a center region movement distance; (D) peripheral zone movement distance; (E) a total movement distance; (F) a central region movement speed; (G) peripheral zone movement speed; (H) the total speed of movement. All indexes have no statistical difference. Results are mean ± SEM of 15 mice per group, one-way ANOVA is the statistical method.
The above embodiments are preferred embodiments of the present invention, but the present invention is not limited to the above embodiments, and any other changes, modifications, substitutions, combinations, and simplifications which do not depart from the spirit and principle of the present invention should be construed as equivalents thereof, and all such changes, modifications, substitutions, combinations, and simplifications are intended to be included in the scope of the present invention.
Claims (5)
1. Application of L-securinine and its pharmaceutically acceptable salt in preparing antidepressant is provided.
2. L-securineine and its pharmaceutically acceptable salts according to claim 1, wherein pharmaceutically acceptable salts include hydrochloride, sulfate and nitrate.
3. The use of L-securinega suffruticosa alkaloid and its pharmaceutically acceptable salt in the preparation of antidepressant according to claims 1-2, wherein the antidepressant is L-securinega suffruticosa alkaloid and its pharmaceutically acceptable salt or pharmaceutical composition containing the above components.
4. The use of L-securinenine and its pharmaceutically acceptable salts for the preparation of antidepressant for the treatment of depression of claims 1-3, wherein said antidepressant or pharmaceutical composition can be administered in a pharmaceutically acceptable manner and dosage form.
5. The administration mode and dosage form of the L-securinine and the pharmaceutically acceptable salt thereof or the pharmaceutical composition containing the above components in the preparation of antidepressant drug according to claim 4 are characterized in that the administration mode and dosage form can be realized by tablets, capsules, solutions, granules, powders, pills, sprays, suspensions, injections or instillations and the like.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010799865.4A CN111789846A (en) | 2020-08-11 | 2020-08-11 | Application of L-securinine and its medicinal salt in preparing antidepressant drug |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010799865.4A CN111789846A (en) | 2020-08-11 | 2020-08-11 | Application of L-securinine and its medicinal salt in preparing antidepressant drug |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN111789846A true CN111789846A (en) | 2020-10-20 |
Family
ID=72833695
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202010799865.4A Pending CN111789846A (en) | 2020-08-11 | 2020-08-11 | Application of L-securinine and its medicinal salt in preparing antidepressant drug |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN111789846A (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114230582A (en) * | 2021-12-24 | 2022-03-25 | 暨南大学 | Novel securinine dimer and preparation method and application thereof |
-
2020
- 2020-08-11 CN CN202010799865.4A patent/CN111789846A/en active Pending
Non-Patent Citations (2)
| Title |
|---|
| 刘毅等: "一叶萩碱的研究进展", 《中国药事》, vol. 23, no. 8, 31 August 2009 (2009-08-31), pages 817 - 818 * |
| 杨秀伟: "《中药成分的吸收、分布、代谢、排泄、毒性与药效(下册)》", 31 August 2006, pages: 1363 - 1365 * |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114230582A (en) * | 2021-12-24 | 2022-03-25 | 暨南大学 | Novel securinine dimer and preparation method and application thereof |
| CN114230582B (en) * | 2021-12-24 | 2023-01-10 | 暨南大学 | Novel securinine dimer and preparation method and application thereof |
| WO2023116724A1 (en) * | 2021-12-24 | 2023-06-29 | 暨南大学 | New-type securinine dimer, and preparation method therefor and use thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN107684559B (en) | Gold cluster-containing substance and preparation method and application thereof | |
| CN111888435B (en) | Anti-coronavirus traditional Chinese medicine composition and application thereof in treating inflammation | |
| US7078063B2 (en) | Water soluble extract from plant of Solanum genus and the preparation process thereof, and pharmaceutical composition containing the water soluble extract | |
| WO2011140676A1 (en) | Rhizoma gastrodiae plant extract used to prevent and treat alzheimer disease and vascular dementia and mixed type diseases thereof and preparative method thereof | |
| CN111789846A (en) | Application of L-securinine and its medicinal salt in preparing antidepressant drug | |
| JP2010528063A (en) | Method and use for obtaining an extract containing sequoyitol from a plant belonging to the genus Rhododendron, soybean, genus Ginkgo | |
| WO2021093087A1 (en) | Traditional chinese medicine composition having effect of improving cognition, preparation method therefor, and traditional chinese medicine preparation | |
| CN106491680B (en) | A Chinese medicinal composition for preventing or treating senile dementia, and its preparation method | |
| CN107827940B (en) | Uncaria amide A and pharmaceutical composition and application thereof | |
| CN107349244B (en) | Extraction method of malonyl ginsenoside | |
| CN111437323A (en) | Application of vine tea extract in medicine for preventing and treating Alzheimer disease | |
| JPWO2015076286A1 (en) | Ephedrine alkaloid-removed Mao extract, its production and use | |
| CN114832006B (en) | Application of ginsenoside Rh4 in preparation of medicine for inhibiting sleep | |
| CN116019819A (en) | Active ginsenoside composition and preparation method and application thereof | |
| WO2009062374A1 (en) | The pharmaceutical use of liquiritigenin for preparing medicine for treating neurodegenerative diseases | |
| CN111558007B (en) | Corn root extract and preparation method and application thereof | |
| RU2408384C2 (en) | Chinese medicinal composition, method for preparing and administration | |
| CN112940001A (en) | Phthalide isoquinoline alkaloid and preparation method and application thereof | |
| CN109662986B (en) | Persimmon leaf extract and new medical application of preparation thereof | |
| CN113143985A (en) | Application of liposome of radix Tripterygii Wilfordii extract in preparing medicine for preventing and treating behavior cognitive disorder induced by lipopolysaccharide by nasal administration | |
| CN105641363B (en) | Use of Qing' e prescription extract in anti-depression and anti-anxiety | |
| CN111773285B (en) | Application of medicine in preparing medicine for reducing muscle toxicity of statins | |
| CN111939185B (en) | Chinese herbal medicine extract, extraction method and application | |
| CN114767783B (en) | Application of ophiopogon root extract in preparing medicine for preventing or treating parkinsonism | |
| CN113444015B (en) | Cinnamoyl amino acid compound and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| RJ01 | Rejection of invention patent application after publication | ||
| RJ01 | Rejection of invention patent application after publication |
Application publication date: 20201020 |