CN111139003A - High-temperature-resistant adhesive and high-temperature-resistant protective adhesive film formed by coating high-temperature-resistant adhesive - Google Patents
High-temperature-resistant adhesive and high-temperature-resistant protective adhesive film formed by coating high-temperature-resistant adhesive Download PDFInfo
- Publication number
- CN111139003A CN111139003A CN202010154270.3A CN202010154270A CN111139003A CN 111139003 A CN111139003 A CN 111139003A CN 202010154270 A CN202010154270 A CN 202010154270A CN 111139003 A CN111139003 A CN 111139003A
- Authority
- CN
- China
- Prior art keywords
- tert
- temperature
- acrylic
- adhesive
- film
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J133/00—Adhesives based on homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides, or nitriles thereof; Adhesives based on derivatives of such polymers
- C09J133/04—Homopolymers or copolymers of esters
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J7/00—Adhesives in the form of films or foils
- C09J7/30—Adhesives in the form of films or foils characterised by the adhesive composition
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J7/00—Adhesives in the form of films or foils
- C09J7/40—Adhesives in the form of films or foils characterised by release liners
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2201/00—Properties
- C08L2201/08—Stabilised against heat, light or radiation or oxydation
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2205/00—Polymer mixtures characterised by other features
- C08L2205/02—Polymer mixtures characterised by other features containing two or more polymers of the same C08L -group
- C08L2205/025—Polymer mixtures characterised by other features containing two or more polymers of the same C08L -group containing two or more polymers of the same hierarchy C08L, and differing only in parameters such as density, comonomer content, molecular weight, structure
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2205/00—Polymer mixtures characterised by other features
- C08L2205/03—Polymer mixtures characterised by other features containing three or more polymers in a blend
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2205/00—Polymer mixtures characterised by other features
- C08L2205/03—Polymer mixtures characterised by other features containing three or more polymers in a blend
- C08L2205/035—Polymer mixtures characterised by other features containing three or more polymers in a blend containing four or more polymers in a blend
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J2203/00—Applications of adhesives in processes or use of adhesives in the form of films or foils
- C09J2203/326—Applications of adhesives in processes or use of adhesives in the form of films or foils for bonding electronic components such as wafers, chips or semiconductors
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Laminated Bodies (AREA)
Abstract
A high-temperature resistant adhesive and a high-temperature resistant protective adhesive film formed by coating the same structurally comprise a high-temperature resistant plastic film layer (or called a substrate film layer), a high-temperature resistant adhesive layer (or called a glue layer) and a release film layer from top to bottom. The high temperature resistant protective adhesive film of the invention can be used for protecting the contact surface of different materials of a circuit board, such as raw copper, or the surface after rough and super treatment, such as copper oxide, and the surfaces of other insulating materials, such as polyimide film, insulating preimpregnation material (Prepreg), and the like, and has high adhesiveness. And the subsequent process requirements of the circuit board, such as high-temperature conditions or acid-base processes, still have good peeling strength and peeling retention, and can be easily peeled from the surfaces of different materials after the processes are finished without adhesive residues.
Description
Technical Field
A high-temperature resistant adhesive and a high-temperature resistant protective adhesive film formed by coating the same structurally comprise a high-temperature resistant plastic film layer, a high-temperature resistant adhesive layer and a release film layer from top to bottom. The high temperature resistant protective adhesive film of the invention can be used for protecting the contact surface of different materials of a circuit board, such as raw copper, or the surface after rough and super treatment, such as copper oxide, and the surfaces of other insulating materials, such as polyimide film, insulating preimpregnation material (Prepreg), and the like, and has high adhesiveness. And the subsequent process requirements of the circuit board, such as high-temperature conditions or acid-base processes, still have good peeling strength and peeling retention, and can be easily peeled from the surfaces of different materials after the processes are finished without adhesive residues.
Background
In the circuit board manufacturing process, special protection film is often required to be fixed in the manufacturing process and at high temperatureAnd masking by acid-base treatment. This requirement is widely seen in the protection of circuit board golden fingers, electroplating insulation or the opening process of rigid-flex boards. The application requires good initial adhesion and long-term high-temperature process (150-260)oC, 1-3 hr) and acid and alkali processes, and the cohesive energy of the adhesive is also high enough, so that the protective film is peeled off after the processes are completed and no adhesive residue is left on the substrate.
The protective adhesive film on the market usually adopts rubber type glue or acrylic acid series solvent type adhesive as the adhesive. However, the rubber-type adhesive has disadvantages that it cannot resist 260 ℃ or higher temperature and cannot maintain fixed viscosity at high temperature, and the rubber-type adhesive needs to be coated to a certain thickness to have proper adhesive strength, so that it is not suitable for the requirement of the process of thinning the circuit board. The rubber type heat-resistant adhesive on the market at present is provided with CM8R and CM8G high-temperature shielding paper-plastic composite adhesive tapes of four-dimensional adhesive tape company, and the highest temperature of the application is 250oC and can only last 1 minute.
The common acrylic acid series solvent-type adhesives have the advantages of excellent adhesive property, no toxicity, low cost and the like, but have common heat resistance, and the peel strength of the adhesive tape is gradually reduced along with the rise of temperature. In addition, some single-component acrylic adhesives increase the initial adhesion of the system by virtue of the reduction of molecular weight, but residual adhesive remains on the substrate after stripping, which not only affects the appearance and electrical properties of the product, but also cannot be subjected to subsequent processing operation. In addition, some two-component adhesives improve the cohesive strength of the system by adding a curing agent so as to reduce the residual adhesive amount, but the initial adhesion of the system is extremely low, the rapid adhesion cannot be realized, and the production efficiency is influenced.
In addition, the base material and the adhesive selected for the high-temperature-resistant adhesive tape have many problems, such as: the substrate is mostly a single layer of conventional materials, such as fabrics, foams, plastic films and non-woven fabrics with general heat and humidity resistance, and the mechanical properties thereof cannot meet the requirements of client-side processes such as heat resistance and cleaness of clean rooms.
Under the present circumstances, it is an urgent need to develop an adhesive with high initial adhesion, high permanent adhesion at high temperature and different acid and alkali processes, and high cohesive strength to meet the needs of the circuit board market.
Disclosure of Invention
The invention discloses a high-temperature-resistant polyacrylic resin adhesive protective film, which comprises a high-temperature-resistant base material layer, a high-temperature-resistant adhesive layer and a release layer; the high-temperature-resistant pressure glue layer comprises the following materials in parts by weight: 50-80 parts of acrylic resin, 1-10 parts of epoxy resin and 2-10 parts of organic peroxide. The present case carries out the free radical polymerization modification through the formula component to acrylic resin, uses epoxy to improve the high temperature resistance and mechanical toughness and the cohesion of glue film simultaneously to guarantee that the protection film possesses excellent high peel strength, high just adhesion, hold the adhesion and peel off the back simultaneously and do not have the cull.
The formula of the raw materials of the polyacrylic resin adhesive comprises the following materials in parts by weight: 50-80 parts of acrylic resin, 1-10 parts of epoxy resin and 2-10 parts of organic peroxide.
The formula of the acrylic resin is further prepared by free radical polymerization of acrylic monomers, wherein the acrylic monomers comprise the following raw materials in percentage by weight: 1) 10-20% of a hard acrylic monomer, wherein the hard acrylic monomer comprises any one or more of methyl acrylate, vinyl acetate, styrene, and methyl methacrylate; 2) 10-30% of an acrylic acid soft monomer, wherein the acrylic acid soft monomer comprises any one or more of ethyl acrylate, butyl methacrylate, 2-ethylhexyl acrylate and isooctyl acrylate; 3) 0.5-2.5% of acrylic acid functional monomer, wherein the acrylic acid functional monomer comprises any one or two of acrylic acid and methacrylic acid; 4) 0.5-5% of acrylic acid crosslinking monomer, wherein the acrylic acid crosslinking monomer comprises: any one or more of hydroxyethyl acrylate, hydroxypropyl acrylate, hydroxyethyl methacrylate, hydroxypropyl methacrylate, glycidyl acrylate, ethylene glycol diacrylate, isooctyl methacrylate acrylamide; 5) 0.2-2.0% of initiator, wherein the initiator comprises any one or two of azo initiator and peroxide initiator; 6) 0.01-0.2% of chain transfer agent, wherein the chain transfer agent is a mercaptan chain transfer agent; and 7) 40-60% of the solvent comprises: any one or more of ethyl acetate, toluene, xylene, and the like.
The acrylic resin is further polymerized by mixing an acrylic hard monomer, an acrylic soft monomer, an acrylic functional monomer and an acrylic crosslinking monomer to form a monomer mixture, adding the monomer mixture accounting for 30-40% of the total weight of the monomer mixture, an initiator accounting for 30-40% of the total weight of the initiator and a solvent accounting for 60-70% of the total weight of the solvent into a reaction kettle, heating to 80-85 ℃, reacting for 1-1.5 hours, then dropwise adding a mixed solution formed by mixing all the rest monomer mixture, the solvent and the initiator into a reaction system in the reaction kettle gradually within 3-4 hours, preserving heat at 85-90 ℃, curing for 3-4 hours, adding a chain transfer agent, and then cooling to room temperature to obtain the acrylic resin.
Further, the curable epoxy resins required in the present invention are liquid epoxy resins which are liquid at room temperature, including bisphenol A type liquid epoxy resins, bisphenol fluorine type epoxy resins, and novolac type epoxy resins and cycloaliphatic epoxy resins, such as 1, 2-epoxyethyl-3, 4-epoxycyclohexane, 3, 4-epoxycyclohexylcarboxylic acid-3, 4-epoxyhexylmethyl and bis (3, 4-epoxy-6-methylcyclohexylmethyl) adipic acid; glycidyl ether type epoxy resins such as diglycidyl ether hexahydrophthalate; 3-methyl-diglycidyl ether hexahydrophthalate and diglycidyl ether hexahydroterephthalate; glycidyl amine type epoxy resins such as diglycidyl aniline, diglycidyl toluidine, triglycidyl-p-aminobenzene, tetraglycidyl-m-xylenediamine and tetraglycidyl bis (aminomethyl) cyclohexane; and hydantoin type epoxy resins such as 1, 3-diglycidyl-5-ethylhydantoin.
Further, the organic peroxides required by the present invention are benzoyl peroxide, t-butyl peroxybenzoate, t-amyl peroxyacetate, t-butyl peroxy3, 5, 5-trimethylhexanoate, t-butyl peroxyisopropylcarbonate, t-butyl peroxy2-ethylhexyl carbonate, t-amyl peroxy2-ethylhexylcarbonate, ethyl 3, 3-peroxy (t-butylperoxy) butyrate, ethyl 3, 3-peroxy (t-pentylperoxy) butyrate, n-butyl 4, 4-peroxy (t-butylperoxy) valerate, 1-peroxy (t-butylperoxy) cyclohexane, 1-peroxy (t-pentylperoxy) cyclohexane, 1-di-t-pentylperoxy-3, 3, 5-trimethylcyclohexane, 1, 1-di-tert-butylperoxy-3, 3, 5-trimethylcyclohexane, 1-di-tert-butylperoxy-hexane, 2, 5-dimethyl-2, 5-o- (tert-butylperoxy) -3-hexyne, di-tert-butyl peroxide, di-tert-amyl peroxide, tert-butylperoxy-cumyl benzene, di- (tert-butylperoxy-propyl) benzene, 2, 5-dimethyl-2, 5-o- (tert-butylperoxy) hexane, and di-l-cumyl peroxide.
The second technical scheme adopted by the invention is to mix 50-80 parts of acrylic resin, 1-10 parts of epoxy resin and 2-10 parts of organic peroxide with the same weight of solvent to form glue solution, then to coat the glue solution on a high temperature resistant film layer by using a coating method, and finally to press a release layer film.
The preferable technical scheme is as follows: the thickness of the high-temperature resistant polyacrylic resin adhesive layer is 10-100 micrometers.
The preferable technical scheme is as follows: the thickness of the high-temperature-resistant base material layer is 10-100 micrometers.
Wherein the high temperature resistant film layer of the present invention is composed of a polymer having a heat resistant temperature of at least 200 deg.CoTypical membranes that are not thermally deformable for C-3 hours include Polyetherimide (PBI) membranes, Polyetherimide (PI) membranes, Polyamideimide (PAI) membranes, polyphenylene sulfide (PPS) membranes, and Polytetrafluoroethylene (PTFE) membranes.
The release film layer of the present invention is made of a polymer, and the material of the release film layer is not particularly limited, and is preferably selected from a thermosetting resin material or a photo-curing resin material, and more preferably, a thermosetting resin material such as polyimide, Polyethylene terephthalate (PET), Polyaniline (PAN), Polyethylene Naphthalate (PEN), triacetin (TAc), or Polycarbonate resin (PC), and is preferably selected from polyimide, Polyethylene terephthalate (PET).
The present invention relates to a composite material with high temperature resistance, acid and alkali resistance, stripping property and fixed adhesion, which is prepared by synthesizing a novel composite acrylic resin, adding a main component of epoxy resin and peroxide, coating the mixture on a high temperature resistant substrate, and laminating a separation membrane with a release layer.
1. The high temperature resistance and the chemical resistance are suitable for the shielding requirement.
2. After use, the adhesive tape was removed without leaving any adhesive residue on the surface of the substrate.
Wherein the technical index
(1) The adhesion on the surface of different substrates is more than 20g/25 mm and less than 100g/25 mm
(2) Repeated thermal cycle lamination process capable of withstanding high temperature up to 200 DEG C
(3) No adhesive residue after stripping on the surface of the base material
Additional features and advantages of the invention will be set forth in the description which follows, and in part will be obvious from the description, or may be learned by practice of the invention. The objectives and other advantages of the invention will be realized and attained by the structure particularly pointed out in the written description and claims hereof as well as the appended drawings.
In order to make the aforementioned and other objects, features and advantages of the present invention comprehensible, preferred embodiments accompanied with figures are described in detail below.
Drawings
In order to more clearly illustrate the embodiments of the present invention or the technical solutions in the prior art, the drawings used in the description of the embodiments or the prior art will be briefly described below, and it is obvious that the drawings in the following description are some embodiments of the present invention, and other drawings can be obtained by those skilled in the art without creative efforts.
FIG. 1 is a structural diagram of a high temperature resistant protective film according to an embodiment of the present invention;
FIG. 2 is a schematic view illustrating a high temperature resistant protective film pre-attached to a protected layer according to an embodiment of the present invention;
FIG. 3 is a schematic diagram illustrating a high temperature resistant protective film and a Prepeg hot-pressed to a protected layer according to an embodiment of the present invention;
FIG. 4 is a diagram illustrating the completion of hot pressing the high temperature resistant protective film and the Prepeg to the protected layer according to the embodiment of the present invention.
Detailed Description
In order to make the objects, technical solutions and advantages of the embodiments of the present invention clearer, the technical solutions in the embodiments of the present invention will be clearly and completely described below with reference to the drawings in the embodiments of the present invention, and it is obvious that the described embodiments are some, but not all, embodiments of the present invention. The components of embodiments of the present invention generally described and illustrated in the figures herein may be arranged and designed in a wide variety of different configurations. Thus, the following detailed description of the embodiments of the present invention, presented in the figures, is not intended to limit the scope of the invention, as claimed, but is merely representative of selected embodiments of the invention. All other embodiments, which can be derived by a person skilled in the art from the embodiments given herein without making any creative effort, shall fall within the protection scope of the present invention.
It should be noted that: like reference numbers and letters refer to like items in the following figures, and thus, once an item is defined in one figure, it need not be further defined and explained in subsequent figures.
In the description of the present invention, it should be noted that the terms "center", "upper", "lower", "left", "right", "vertical", "horizontal", "inner", "outer", etc. indicate orientations or positional relationships based on the orientations or positional relationships shown in the drawings or the orientations or positional relationships that the products of the present invention are conventionally placed in use, but are only used for convenience in describing the present invention and simplifying the description, and do not indicate or imply that the devices or components that are referred to must have specific orientations, be constructed and operated in specific orientations, and thus, should not be construed as limiting the present invention. Furthermore, the terms "first," "second," "third," and the like are used solely to distinguish one from another and are not to be construed as indicating or implying relative importance.
In the description of the present invention, it should also be noted that, unless otherwise explicitly specified or limited, the terms "disposed," "mounted," "connected," and "connected" are to be construed broadly and may, for example, be fixedly connected, detachably connected, or integrally connected; can be mechanically or electrically connected; the two components can be directly connected or indirectly connected through an intermediate medium, and the two components can be communicated with each other. The specific meanings of the above terms in the present invention can be understood in specific cases to those skilled in the art.
In the present invention, unless otherwise expressly stated or limited, "above" or "below" a first feature means that the first and second features are in direct contact, or that the first and second features are not in direct contact but are in contact with each other via another feature therebetween. Also, the first feature being "on," "above" and "over" the second feature includes the first feature being directly on and obliquely above the second feature, or merely indicating that the first feature is at a higher level than the second feature. A first feature being "under," "below," and "beneath" a second feature includes the first feature being directly under and obliquely below the second feature, or simply meaning that the first feature is at a lesser elevation than the second feature.
All embodiments, implementations and features of the invention can be combined with each other in the invention without contradiction or conflict. In the present invention, conventional devices, apparatuses, components, etc. are either commercially available or self-made according to the present disclosure. In the present invention, some conventional operations and apparatuses, devices, components are omitted or only briefly described in order to highlight the importance of the present invention.
The first embodiment is as follows:
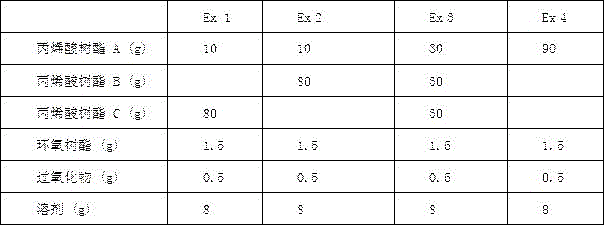
in the case of one kg of gum, 0.3g of the organic peroxide according to claim 5 is first mixed in an equal weight of the solvent according to claim 2. Then 1.5 g of the epoxy resin according to claim 4 is mixed in an equal weight of the solvent according to claim 2; then mixing the two solutions and adding 90 g of the polyacrylic resin as defined in claim 1; finally, adding the solvent to prepare the components into glue solution with solid content of about 50 percent; finally coated on the high temperature resistant film of claim 10 and finally pressed onto the release film of claim 11. Based on the formula design, we select two optimized formulas as examples; in addition, comparative examples 3 and 4, each having a different resin ratio, were added for comparison in order to compare the advantages of the present invention. The test conditions are as follows
Tensile value at room temperature
175oC tensile value and
230oc tensile value
Wherein the tensile strength is compared between the surface of Polyimide film, electrolytic copper and rolled copper. And hot-pressing on the black PI surface at a relative temperature for 2hrs, tearing the sample, and observing whether residual adhesive exists.
Example of the watch
Second, third and fourth tables show the combination of the formula of the first embodiment with different surface materials and tensile values at different temperatures, wherein the surface materials include Polyimide film electrolytic copper and calendered copper
Tensile value at room temperature of table II
| Peel As Received(g/25 mm) | Ex 1 | Ex 2 | Ex 3 | Ex 4 |
| Polyimide | 20 | 70 | 109 | 15 |
| ED Cu | 22 | 75 | 113 | 18 |
| RA Cu | 21 | 73 | 111 | 16 |
Watch III 175oC tensile value
| Peel@ 175oC(g/25 mm) | Ex 1 | Ex 2 | Ex 3 | Ex 4 |
| Polyimide | 25 | 82 | 112 | 23 |
| ED Cu | 27 | 90 | 119 | 25 |
| RA Cu | 23 | 87 | 117 | 22 |
TABLE IV 230oC tensile value
| Peel@ 230oC(g/25 mm) | Ex 1 | Ex 2 | Ex 3 | Ex 4 |
| Polyimide | 28 | 90 | 115 | 28 |
| ED Cu | 30 | 98 | 122 | 22 |
| RA Cu | 25 | 96 | 119 | 27 |
And fifthly, after the formula in the embodiment of the table is pressed on the Polyimide film, the surface is torn off to observe whether the residual glue exists on the surface.
Watch five residual gum
| Ex 1 | Ex 2 | Ex 3 | Ex 4 | |
| Residual gum (175)oC-2hrs) | ○ | ○ | X | X |
| Remnant glue (230)oC-2hrs) | ○ | ○ | X | X |
From the results of the five tables, it can be seen that the optimized formula of example two has the most suitable pressing tension, and no adhesive residue is left after the film surface is torn.
Finally, it should be noted that: the above-mentioned embodiments are only specific embodiments of the present invention, which are used for illustrating the technical solutions of the present invention and not for limiting the same, and the protection scope of the present invention is not limited thereto, although the present invention is described in detail with reference to the foregoing embodiments, those skilled in the art should understand that: any person skilled in the art can modify or easily conceive the technical solutions described in the foregoing embodiments or equivalent substitutes for some technical features within the technical scope of the present disclosure; such modifications, changes or substitutions do not depart from the spirit and scope of the embodiments of the present invention, and they should be construed as being included therein. Therefore, the protection scope of the present invention shall be subject to the protection scope of the claims.
Claims (10)
1. The method of claim 1: the invention relates to a high-temperature-resistant adhesive and a high-temperature-resistant protective adhesive film coated and formed by the same, wherein the adhesive comprises the following raw materials in parts by weight: 50-80 parts of acrylic resin, 1-10 parts of epoxy resin and 2-10 parts of organic peroxide.
2. According to claim 1: the formula of the acrylic resin is prepared by free radical polymerization of acrylic monomers, wherein the raw materials comprise the following components in percentage by weight: 1) 10-20% of a hard acrylic monomer, wherein the hard acrylic monomer comprises any one or more of methyl acrylate, vinyl acetate, styrene, and methyl methacrylate; 2) 10-30% of an acrylic acid soft monomer, wherein the acrylic acid soft monomer comprises any one or more of ethyl acrylate, butyl methacrylate, 2-ethylhexyl acrylate and isooctyl acrylate; 3) 0.5-2.5% of acrylic acid functional monomer, wherein the acrylic acid functional monomer comprises any one or two of acrylic acid and methacrylic acid; 4) 0.5-5% of acrylic acid crosslinking monomer, wherein the acrylic acid crosslinking monomer comprises: any one or more of hydroxyethyl acrylate, hydroxypropyl acrylate, hydroxyethyl methacrylate, hydroxypropyl methacrylate, glycidyl acrylate, ethylene glycol diacrylate, isooctyl methacrylate acrylamide; 5) 0.2-2.0% of initiator, wherein the initiator comprises any one or two of azo initiator and peroxide initiator; 6) 0.01-0.2% of chain transfer agent, wherein the chain transfer agent is a mercaptan chain transfer agent; and 7) 40-60% of the solvent comprises: any one or more of ethyl acetate, toluene, xylene, and the like.
3. According to the method of claim 1, the acrylic resin is prepared by mixing the acrylic hard monomer, the acrylic soft monomer, the acrylic functional monomer and the acrylic crosslinking monomer to form a monomer mixture, adding the monomer mixture accounting for 30-40% of the total weight of the monomer mixture, the initiator accounting for 30-40% of the total weight of the initiator and the solvent accounting for 60-70% of the total weight of the solvent into a reaction kettle, heating to 80-85 ℃, reacting for 1-1.5 hours, then dropwise adding a mixed solution formed by mixing all the rest monomer mixture, the solvent and the initiator into a reaction system in the reaction kettle gradually within 3-4 hours, keeping the temperature at 85-90 ℃, curing for 3-4 hours, adding the chain transfer agent, and cooling to room temperature.
4. The curable epoxy resin composition according to claim 1 is a liquid epoxy resin which is liquid at ordinary temperature, including bisphenol A type liquid epoxy resins, bisphenol fluorine type epoxy resins, and novolak type epoxy resins and cycloaliphatic epoxy resins such as 1, 2-epoxyethyl-3, 4-epoxycyclohexane, 3, 4-epoxycyclohexylcarboxylic acid-3, 4-epoxyhexylmethyl and bis (3, 4-epoxy-6-methylcyclohexylmethyl) adipic acid; glycidyl ether type epoxy resins such as diglycidyl ether hexahydrophthalate; 3-methyl-diglycidyl ether hexahydrophthalate and diglycidyl ether hexahydroterephthalate; glycidyl amine type epoxy resins such as diglycidyl aniline, diglycidyl toluidine, triglycidyl-p-aminobenzene, tetraglycidyl-m-xylenediamine and tetraglycidyl bis (aminomethyl) cyclohexane; and hydantoin type epoxy resins such as 1, 3-diglycidyl-5-ethylhydantoin.
5. The organic peroxide as claimed in claim 1, which is benzoyl peroxide, tert-butyl peroxybenzoate, tert-amyl peroxyacetate, tert-butyl peroxy3, 5, 5-trimethylhexanoate, tert-butyl peroxyl-propyl carbonate, tert-butyl peroxy2-ethylhexyl carbonate, tert-amyl peroxy2-ethylhexyl carbonate, ethyl 3, 3-peroxy (tert-butylperoxy) butyrate, ethyl 3, 3-peroxy (tert-amylperoxy) butyrate, n-butyl 4, 4-peroxy (tert-butylperoxy) valerate, 1-peroxy (tert-butylperoxy) cyclohexane, 1-peroxy (tert-amylperoxy) cyclohexane, 1-di-tert-amylperoxy-3, 3, 5-trimethylcyclohexane, 1, 1-di-tert-butylperoxy-3, 3, 5-trimethyl-hexane, 1-di-tert-butylperoxy-hexane, 2, 5-dimethyl-2, 5-o- (tert-butylperoxy) -3-hexyne, di-tert-butyl peroxide, di-tert-amyl peroxide, tert-butyl cumyl peroxide, di- (tert-butylperoxy-propyl) benzene, 2, 5-dimethyl-2, 5-o- (tert-butylperoxy) hexane and di-l cumyl peroxide.
6. In order to achieve the purpose, the second technical scheme adopted by the invention is to mix 50-80 parts of acrylic resin, 1-10 parts of epoxy resin and 2-10 parts of organic peroxide with the same weight of solvent into glue solution, then coat the glue solution on a high temperature resistant film layer by using a coating method, and finally press a release layer film.
7. The preferable technical scheme is as follows: the thickness of the high-temperature resistant polyacrylic resin adhesive layer is 10-100 micrometers.
8. The preferable technical scheme is as follows: the thickness of the high-temperature-resistant substrate layer is 10-100 microns.
9. The high temperature resistant film substrate layer is made of polymer, and the commonly used film with low heat resistant temperature which needs to reach 200 ℃ to 3 hours without heat deformation comprises polyetherimide film (PBI), polyether imide film (PI), polyamide imide film (PAI), polyphenylene sulfide film (PPS) and polytetrafluoroethylene film (PTFE).
10. The release film layer is made of a polymer, and the material thereof is not particularly limited, and is preferably selected from a thermosetting resin material or a photo-curing resin material, and more preferably, a thermosetting resin material such as polyimide, Polyethylene terephthalate (PET), Polyaniline (PAN), Polyethylene Naphthalate (PEN), triacetin (TAc), or Polycarbonate resin (PC), and is preferably selected from polyimide, Polyethylene terephthalate (PET).
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010154270.3A CN111139003A (en) | 2020-03-07 | 2020-03-07 | High-temperature-resistant adhesive and high-temperature-resistant protective adhesive film formed by coating high-temperature-resistant adhesive |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010154270.3A CN111139003A (en) | 2020-03-07 | 2020-03-07 | High-temperature-resistant adhesive and high-temperature-resistant protective adhesive film formed by coating high-temperature-resistant adhesive |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN111139003A true CN111139003A (en) | 2020-05-12 |
Family
ID=70528456
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202010154270.3A Pending CN111139003A (en) | 2020-03-07 | 2020-03-07 | High-temperature-resistant adhesive and high-temperature-resistant protective adhesive film formed by coating high-temperature-resistant adhesive |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN111139003A (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114149763A (en) * | 2021-11-29 | 2022-03-08 | 东莞市龙美电子科技有限公司 | Electrolyte-resistant adhesive tape for capacitor and preparation method thereof |
-
2020
- 2020-03-07 CN CN202010154270.3A patent/CN111139003A/en active Pending
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114149763A (en) * | 2021-11-29 | 2022-03-08 | 东莞市龙美电子科技有限公司 | Electrolyte-resistant adhesive tape for capacitor and preparation method thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10471682B2 (en) | Adhesive composition and laminate with adhesive layer using same | |
| KR102647985B1 (en) | Adhesive composition and laminate with adhesive layer using the same | |
| JP7335559B2 (en) | Adhesive composition and coverlay film, bonding sheet, copper clad laminate and electromagnetic wave shielding material using the same | |
| CN106536658B (en) | Laminate with adhesive layer, and flexible copper-clad laminate and flexible flat cable using same | |
| CN111040709B (en) | Resin composition and flexible copper-clad plate using same | |
| CN109415609B (en) | Adhesive composition and adhesive sheet | |
| CN111139003A (en) | High-temperature-resistant adhesive and high-temperature-resistant protective adhesive film formed by coating high-temperature-resistant adhesive | |
| JP6709103B2 (en) | Adhesive composition and adhesive sheet using the same | |
| TWI764998B (en) | Coverlay film and cell phone and electronic device using the same | |
| JP7287545B2 (en) | Low dielectric adhesive composition | |
| JP2019169484A (en) | Electromagnetic wave shield material | |
| JP7100299B2 (en) | Low Dielectric Adhesive Composition | |
| JP7348673B2 (en) | Resin composition, and coverlay film, adhesive sheet, resin-coated metal foil, metal-clad laminate, or printed wiring board using the same | |
| KR102478142B1 (en) | Adhesive composition and bonding sheet having the same | |
| CN113372835B (en) | High-temperature-resistant ultraviolet-curing adhesive reducing film and preparation method thereof | |
| JP7163259B2 (en) | Adhesive sheet | |
| KR100730984B1 (en) | Adhesive composition for flexible copper clad laminated film | |
| TWI839686B (en) | Adhesive composition and coating film, bonding sheet, copper-clad laminate and electromagnetic wave shielding material using the same | |
| TWI839685B (en) | Adhesive composition and coating film, bonding sheet, copper-clad laminate and electromagnetic wave shielding material using the same | |
| KR960014766B1 (en) | Adhesive composition for flexible p.c.b. | |
| WO2022102506A1 (en) | Low dielectric adhesive composition | |
| JP2022137124A (en) | Low-dielectric adhesive composition | |
| JP2022133351A (en) | Low-dielectric adhesive composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |