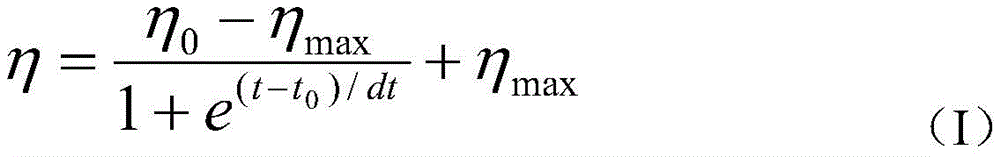CN109799170B - Method for judging relative speed of curing reaction of poly-nitrogen glycidyl ether adhesive - Google Patents
Method for judging relative speed of curing reaction of poly-nitrogen glycidyl ether adhesive Download PDFInfo
- Publication number
- CN109799170B CN109799170B CN201811625177.5A CN201811625177A CN109799170B CN 109799170 B CN109799170 B CN 109799170B CN 201811625177 A CN201811625177 A CN 201811625177A CN 109799170 B CN109799170 B CN 109799170B
- Authority
- CN
- China
- Prior art keywords
- curing reaction
- glycidyl ether
- viscosity
- determining
- ether adhesive
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Abstract
The invention provides a method for judging the relative speed of the curing reaction of a poly azide glycidyl ether (GAP) adhesive, which comprises the steps of firstly testing the viscosity values of the GAP adhesive and a curing agent at different time points of the curing reaction by adopting a rheological method, and then fitting according to a Boltzmann equation to obtain a curve of the change of the viscosity along with the timeAnd each parameter in the equation, and the time constant t representing that the curing reaction viscosity value reaches 50 percent of the maximum viscosity value in the equation0To determine qualitatively the magnitude of the relative rate of cure reaction of the GAP adhesive. The method has the advantages of simple experimental process, easy operation and quick and simple data processing, and can be used for judging the relative speed of the curing reaction of the polyazide glycidyl ether adhesive.
Description
Technical Field
The invention relates to the technical field of adhesive curing, in particular to a method for judging the relative speed of a curing reaction of a poly-nitrogen glycidyl ether adhesive.
Background
Journal literature "interfacial reaction mechanism of HTPB/TDI lining and NEPE propellant" ("solid rocket technology", 2010, volume 33, phase 1), proposes to measure the concentration of isocyanate groups (-NCO) on the surface of the lining at different reaction times by infrared spectroscopy, calculate the chemical reaction degree of the isocyanate groups and hydroxyl groups at different reaction times, and obtain the reaction speed of different curing reaction systems.
Journal literature "curing reaction kinetics research of active hydrogen component in NEPE propellant" ("solid rocket technology", 2010, volume 33, phase 5) proposes that a chemical titration method is adopted to respectively measure reaction kinetics curves of-NCO concentration in an end-to-end hydroxyl Polyether (PEG)/Phenylisocyanate (PI), a bonding agent (NPBA)/PI and a stabilizer (MNA))/PI system at different temperatures along with the change of reaction time, so as to calculate and obtain reaction rate constants and activation energy of the corresponding systems at different temperatures, compare the reaction rate constants, and obtain the reaction speeds of different systems.
Journal literature, "kinetics of curing reaction of HTPB and TDI with different molecular weights" ("energetic materials", vol 21, No. 6, 2013) proposes to use non-isothermal Differential Scanning Calorimetry (DSC) to study the kinetics of curing reaction of HTPB and TDI with different molecular weights, and the method assumes that the curing reaction rate of thermosetting resin is directly proportional to the heat release rate thereof, so as to obtain a rate equation of the curing reaction kinetics of thermosetting resin, and compare the curing reaction rates of HTPB systems with different molecular weights.
The above publication reflects the state of the art of the method for determining the curing reaction rate of a curing system of an HTPB adhesive, which has disadvantages: the absorption characteristic peak of the isocyanate group in the infrared spectroscopy is independent and is not interfered by other peaks, only a sample before a gel point can be tested, and the whole curing process of the HTPB curing system cannot be monitored; chemical reagents are needed in the detection process of the chemical analysis method, the measurement process is complicated, time-consuming and polluted, and the solidification reaction system researched by the thermal analysis method can detect obvious heat release in the reaction process.
However, the infrared test of the polyazide glycidyl ether (GAP) adhesive has the problems that the characteristic absorption peak of isocyanate groups is interfered by the characteristic absorption peak of azido groups, the heat emitted by a thermal analysis method cannot be measured, and the like.
Disclosure of Invention
The invention aims to overcome the defects of the prior art and provide a method for judging the relative curing reaction rate of a poly-azido glycidyl ether (GAP) adhesive, which can overcome the defects, avoid the problems that the characteristic absorption peak of an isocyanate group in infrared spectrum is interfered by the characteristic absorption peak of azido, the heat emitted in a thermal analysis method cannot be detected and the like, and realize the simple and rapid judgment of the relative curing reaction rate of the GAP adhesive.
The above purpose of the invention is realized by the following technical scheme:
the invention provides a method for judging the relative speed of a curing reaction of a polyazide glycidyl ether adhesive, which comprises the following steps:
(1) weighing polyaziridin glycidyl ethers with different hydroxyl values, correspondingly adding a catalyst and a curing agent to form curing reaction systems with different batch numbers, and then carrying out curing reaction at constant temperature;
(2) sampling one batch number and testing the viscosity value of the batch number at different time points in the curing reaction process until the viscosity test of all the batch numbers is completed, and obtaining a viscosity-time scatter diagram of each batch number;
(3) fitting the viscosity-time scatter points of each batch number according to a Boltzmann equation to obtain a viscosity-time fitting curve and fitting parameters of each batch number;
(4) the time constant at which the viscosity reached 50% of the maximum viscosity value during the curing reaction was used to determine the relative rate of curing reaction of the polyazide glycidyl ether adhesive.
According to the method for determining the relative speed of the curing reaction of the polyazide glycidyl ether adhesive, in the step (1), the catalyst is triphenyl bismuth, and the mass fraction of the catalyst in the curing reaction system is 0.1-0.6%.
According to the method for determining the relative rate of the curing reaction of the polyazide glycidyl ether adhesive, in the step (1), the curing agent is toluene diisocyanate.
According to the method for judging the relative speed of the curing reaction of the polyazide glycidyl ether adhesive, the toluene diisocyanate comprises toluene-2, 4-diisocyanate and toluene-2, 6-diisocyanate, and the mass ratio of the toluene-2, 4-diisocyanate to the toluene-2, 6-diisocyanate is 4: 1.
According to the method for determining the relative rate of the curing reaction of the polyazide glycidyl ether adhesive, the adding amount of the toluene diisocyanate is determined according to the amount that the mass ratio of the hydroxyl groups in the polyazide glycidyl ether to the-NCO groups in the toluene diisocyanate is 1: 1.
According to the method for determining the relative rate of the curing reaction of the polyazide glycidyl ether adhesive, in the step (1), the curing reaction is carried out in an oil bath oven, and the temperature of the oil bath oven is 45-60 ℃.
According to the method for determining the relative speed of the curing reaction of the polyazide glycidyl ether adhesive, in the step (2), a rheometer is adopted to test the viscosity value, the frequency of the rheometer is set to be 1Hz, and the testing temperature is the same as the curing reaction temperature.
In some embodiments of the invention, the length of the sampling interval is determined by how fast the viscosity changes; the viscosity change is fast, the sampling interval time is short, and conversely, the sampling interval time is long.
According to the method for determining the relative speed of the curing reaction of the polyazide glycidyl ether adhesive, in the step (3), the viscosity-time fitting curve is shown as the formula (I),
wherein eta is0And ηmaxMinimum and maximum viscosity limit values, t, for the model curve interval, respectively0Is viscosity equal to (η)0+ηmax) Time constant at/2, i.e. when the viscosity value reaches 50% of the maximum viscosity value;
the fitting parameters include η0、ηmaxAnd t0。
According to the method for judging the relative speed of the curing reaction of the polyazide glycidyl ether adhesive, the smaller the time constant is, the larger the relative speed of the curing reaction of the polyazide glycidyl ether adhesive corresponding to the batch number is.
The viscosity-time fit curve is an equation and a curve obtained by Boltzmann model fitting according to origin software.
The invention has the beneficial effects that: the invention has simple experimental process, easy operation, quick and simple data processing and greatly saves experimental time and chemical reagents. And in the experiment, the use of a solvent is avoided, and the pollution is reduced. The method solves the problem that the relative speed of the curing reaction of the GAP adhesive can not be judged, provides a new method for testing the relative speed of the curing reaction of the poly-nitrogen glycidyl ether adhesive, and is beneficial to the formula design research of the GAP elastomer.
Drawings
FIG. 1 is a graph of viscosity versus time for three different GAP-TDI curing systems of the invention,
wherein a is GAP-TDI-1; b is GAP-TDI-2; c is GAP-TDI-3.
Detailed Description
The present invention will be further described with reference to the following examples.
(1) Weighing 20g of each of 3 GAP adhesives with different hydroxyl values, respectively placing the GAP adhesives in three beakers, adding 0.06g of catalyst triphenylbismuth, uniformly stirring, accurately adding 1.16g of toluene diisocyanate, 0.73g of toluene diisocyanate and 0.42g of toluene diisocyanate according to the mass ratio of the isocyanate groups to the hydroxyl groups of 1:1 to form three curing reaction systems of GAP-TDI-1, GAP-TDI-2 and GAP-TDI-3, continuously stirring to uniformly mix the curing reaction systems, and immediately placing the curing reaction systems in an oil bath oven at 50 ℃ for curing reaction.
(2) At different time points during the curing reaction, a small sample of the curing system GAP-TDI-1 was taken out of the oven, the viscosity number of this sample was immediately tested using a rheometer, and the other two systems were cured in the oven. The above tests were also carried out on the curing system GAP-TDI-2 and the curing system GAP-TDI-3. The frequency of the rheometer was set to 1 Hz; the test temperature was set at 50 ℃ and the jig was a flat plate.
The sampling interval time is determined by the speed of viscosity change. The viscosity change is fast, and the sampling interval time is short; on the contrary, the sampling interval is long.
Obtain viscosity-time scatter diagrams of three curing reaction systems GAP-TDI-1, GAP-TDI-2 and GAP-TDI-3.
(3) After the curing reaction is finished, adopting oringin8.6 software to fit the viscosity-time dispersion point of each batch number according to a Boltzmann equation to obtain a viscosity-time fitting curve and fitting parameters of each batch number; the fitted curve is shown in formula (I).
(4) The time constant value t of the viscosity value of the curing system reaching 50 percent of the maximum viscosity value in the curing reaction process0To determine the relative rate of the GAP adhesive curing reaction for different hydroxyl numbers, a smaller time constant indicates a greater relative rate of the adhesive curing reaction.
Fitting curves of the three curing systems are shown in FIG. 1, and the related parameter eta of the curve equation0、ηmax、t0And the correlation coefficient R are shown in table 1. According to t in Table 10The relative rates of the curing reactions of the three batches of curing reaction systems are judged as follows: GAP-TDI-1 > GAP-TDI-2 > GAP-TDI-3.
TABLE 1
| Curing system | η0/pa·s | ηmax/pa·s | t0/h | Coefficient of correlation R |
| GAP-TDI-1 | 0.87 | 12253 | 65 | 0.9786 |
| GAP-TDI-2 | 1.43 | 22011 | 111 | 0.9740 |
| GAP-TDI-3 | 1.45 | 12039 | 199 | 0.9904 |
Claims (8)
1. A method of determining the relative rate of cure reaction of a polyaziridinyl glycidyl ether adhesive comprising the steps of:
(1) weighing polyaziridin glycidyl ethers with different hydroxyl values, correspondingly adding a catalyst and a curing agent to form curing reaction systems with different batch numbers, and then carrying out curing reaction at constant temperature;
(2) sampling one batch number and testing the viscosity value of the batch number at different time points in the curing reaction process until the viscosity test of all the batch numbers is completed, and obtaining a viscosity-time scatter diagram of each batch number;
(3) fitting the viscosity-time scatter points of each batch number according to a Boltzmann equation to obtain a viscosity-time fitting curve and fitting parameters of each batch number;
(4) the viscosity reaches (eta) in the process of curing reaction0+ηmax) Determining the relative rate of curing reaction of the polynitrogen glycidyl ether adhesive according to the time constant at/2;
in the step (3), the viscosity-time fitting curve is shown as a formula (I),
wherein eta is0And ηmaxThe minimum and maximum viscosity values, t, of the model curve interval, respectively0Is viscosity equal to (η)0+ηmax) Time constant at/2;
the fitting parameters include η0、ηmaxAnd t0。
2. The method of determining the relative rate of curing reaction of a polyazide glycidyl ether adhesive according to claim 1, wherein: in the step (1), the catalyst is triphenyl bismuth, and the mass fraction of the catalyst in the curing reaction system is 0.1-0.6%.
3. The method of determining the relative rate of curing reaction of a polyazide glycidyl ether adhesive according to claim 1, wherein: in the step (1), the curing agent is toluene diisocyanate.
4. The method of determining the relative rate of curing reaction of a polyazide glycidyl ether adhesive according to claim 3, wherein: the toluene diisocyanate comprises toluene-2, 4-diisocyanate and toluene-2, 6-diisocyanate, and the mass ratio of the toluene-2, 4-diisocyanate to the toluene-2, 6-diisocyanate is 4: 1.
5. The method of determining the relative rate of curing reaction of a polyazide glycidyl ether adhesive according to claim 3, wherein: the amount of the toluene diisocyanate added was determined in accordance with the amount of the substance in which the ratio of the hydroxyl group in the polyaziridinyl glycidyl ether to the-NCO group in the toluene diisocyanate was 1: 1.
6. The method of determining the relative rate of curing reaction of a polyazide glycidyl ether adhesive according to claim 1, wherein: in the step (1), the curing reaction is carried out in an oil bath oven, and the temperature of the oil bath oven is 45-60 ℃.
7. The method of determining the relative rate of curing reaction of a polyazide glycidyl ether adhesive according to claim 1, wherein: in the step (2), the viscosity value is tested by a rheometer, the frequency of the rheometer is set to be 1Hz, and the testing temperature is the same as the curing reaction temperature.
8. The method for determining the relative rate of curing reaction of a polyazide glycidyl ether adhesive according to any one of claims 1 to 7, wherein: the smaller the time constant, the greater the relative rate of cure reaction for its corresponding lot of polyglycidyl ether adhesive.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201811625177.5A CN109799170B (en) | 2018-12-28 | 2018-12-28 | Method for judging relative speed of curing reaction of poly-nitrogen glycidyl ether adhesive |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201811625177.5A CN109799170B (en) | 2018-12-28 | 2018-12-28 | Method for judging relative speed of curing reaction of poly-nitrogen glycidyl ether adhesive |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN109799170A CN109799170A (en) | 2019-05-24 |
| CN109799170B true CN109799170B (en) | 2021-09-03 |
Family
ID=66558103
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201811625177.5A Active CN109799170B (en) | 2018-12-28 | 2018-12-28 | Method for judging relative speed of curing reaction of poly-nitrogen glycidyl ether adhesive |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN109799170B (en) |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6220083B1 (en) * | 1997-10-17 | 2001-04-24 | Board Of Supervisors Of Louisiana State University And Agricultural And Mechanical College | Elongational rheometer and on-line process controller |
| CN102490370A (en) * | 2011-11-15 | 2012-06-13 | 中国人民解放军国防科学技术大学 | Liquid model molding technology for preparing polymer matrix composite material |
| CN104280359A (en) * | 2013-07-29 | 2015-01-14 | 湖北航天化学技术研究所 | Method for determining reaction kinetic parameters of lining and interface of solid propellant |
| CN106950156A (en) * | 2017-03-31 | 2017-07-14 | 广东省长大公路工程有限公司 | Deck paving epoxy resin composition viscosity Fast Evaluation device and method |
-
2018
- 2018-12-28 CN CN201811625177.5A patent/CN109799170B/en active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6220083B1 (en) * | 1997-10-17 | 2001-04-24 | Board Of Supervisors Of Louisiana State University And Agricultural And Mechanical College | Elongational rheometer and on-line process controller |
| CN102490370A (en) * | 2011-11-15 | 2012-06-13 | 中国人民解放军国防科学技术大学 | Liquid model molding technology for preparing polymer matrix composite material |
| CN104280359A (en) * | 2013-07-29 | 2015-01-14 | 湖北航天化学技术研究所 | Method for determining reaction kinetic parameters of lining and interface of solid propellant |
| CN106950156A (en) * | 2017-03-31 | 2017-07-14 | 广东省长大公路工程有限公司 | Deck paving epoxy resin composition viscosity Fast Evaluation device and method |
Non-Patent Citations (2)
| Title |
|---|
| A Numerical Model of the Viscosity of an Epoxy Prepreg Resin System;R.P.THERIAULT AND T.A.OSSWALD;《POLYMER COMPOSITES》;19991031;第20卷(第5期);第628-633页 * |
| HTPB/N100体系的聚合反应动力学和粘度变化;郑申声 等;《含能材料》;20110625;第19卷(第3期);摘要、第291页、第293-294页 * |
Also Published As
| Publication number | Publication date |
|---|---|
| CN109799170A (en) | 2019-05-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Bach et al. | Extensional viscosity for polymer melts measured in the filament stretching rheometer | |
| CN101435772B (en) | Epoxide-resin glue curing degree and hardness detecting and analyzing method | |
| Santhosh et al. | Rheokinetic characterization of polyurethane formation in a highly filled composite solid propellant | |
| Lucio et al. | Kinetic and chemorheological modelling of the polymerization of 2, 4-Toluenediisocyanate and ferrocene-functionalized hydroxyl-terminated polybutadiene | |
| CN109799170B (en) | Method for judging relative speed of curing reaction of poly-nitrogen glycidyl ether adhesive | |
| Erdmann et al. | Cure conversion of structural epoxies by cure state analysis and in situ cure kinetics using nondestructive NIR spectroscopy | |
| Chen et al. | Improvement of mechanical properties of in situ-prepared HTPE binder in propellants | |
| Zhongliang et al. | A novel approach on the study of cure kinetics for rheological isothermal and non-isothermal methods | |
| Abd elall et al. | Synthesis of long chain bonding agent of CSRP and its effect on propellant mechanical properties | |
| CN104280359A (en) | Method for determining reaction kinetic parameters of lining and interface of solid propellant | |
| CN101552307B (en) | Preparation method of solar energy solar panel | |
| CN110921629B (en) | AlH3Surface coating method of (1) and coated AlH3And uses thereof | |
| Gallant et al. | Graded polymer composites using twin-screw extrusion: a combinatorial approach to developing new energetic materials | |
| Laviolette et al. | Monitoring the aging dynamics of glycidyl azide polyurethane by NMR relaxation times | |
| CN104422623A (en) | Quantitative analysis method for effect of bonding agent in solid propellant | |
| Wei et al. | Using rheometry to study the curing kinetics of glycidyl azide polymer spherical propellant by non-isothermal method | |
| CN108507968B (en) | Method for testing content of HTPB-based crosslinking system curing agent | |
| Urbaniak | Glass transition temperature-cure temperature-transformation (TgTT) diagram for EPY® epoxy system | |
| Lucio et al. | Rheological kinetics of ferrocenylsilane functionalized polyurethanes based on 4, 4'‐methylenediphenyl diisocyanate for advanced energetic materials | |
| CN112198147B (en) | PBT-TDI mixed system curing reaction in-situ test method | |
| CN106338486A (en) | Non-destructive detection method of storage ageing performance of propellant/liner bonding interface | |
| Fountain | Characterization and control of a structural epoxy adhesive | |
| CN117567719A (en) | Polyurethane elastomer and preparation method and application thereof | |
| CN102297820B (en) | Method for measuring content of short-chain branches in polyethylene copolymer | |
| Lucio et al. | Catalytic effects over formation of functional thermoplastic elastomers for rocket propellants |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |