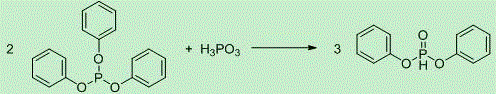Background
Diphenyl phosphite is a commonly used adjuvant in PVC. The literature reports methods for preparing diphenyl phosphite mainly include the following methods:
1, prepared by the acid hydrolysis of diphenylalkyl (ethyl or propyl) phosphite, the reaction equation is as follows:
acid hydrolysis reaction is carried out on diphenyl alkyl phosphite and anhydrous hydrogen chloride, and the main product diphenyl phosphite is obtained after the by-product alkyl chloride is removed. The starting material diphenyl alkyl phosphite of this method is relatively expensive and is not economical in production.
Prepared by reacting triphenyl phosphite with phosphorous acid, the reaction equation is as follows:
walsh 1 mol triphenyl phosphite and 0.5 mol phosphorous acid were mixed at 25 ℃ and the reaction was protected with nitrogen. The reactants naturally release heat and are divided into a solid phase and a liquid phase, and when the temperature is raised to 55 ℃, the solid phase is dissolved, and the reactants become a uniform mixture. And continuously heating to 130 ℃ and 150 ℃, and keeping the temperature for 5 hours for reaction to obtain the product diphenyl phosphite. The method is simple to operate on production, but a large amount of the starting material phosphorous acid is easy to decompose at a higher reaction temperature, yellow phosphorus is generated in equipment, and when the yellow phosphorus is accumulated to a certain degree in long-term production, the risk of fire caused by air is generated, so that a great potential safety hazard exists on production.
And (3) hydrolyzing triphenyl phosphite. The reaction equation is as follows:
the method is mainly used for preparing diphenyl phosphite in the current production. Heating triphenyl phosphite to a certain temperature, dripping water to hydrolyze the triphenyl phosphite, then heating and distilling under reduced pressure to remove phenol which is a byproduct generated by hydrolysis, and finally obtaining diphenyl phosphite. In the method, the generated phenol is usually recovered, triphenyl phosphite is regenerated with phosphorus trichloride, repeated recovery not only continuously wastes energy consumption and reduces production efficiency, but also the phenol recovered for multiple times gradually influences the quality of products, therefore, after the phenol is recovered for two times, the phenol can be continuously used after being refined, and a residual waste liquid which cannot be continuously used is inevitably generated in the process, so that the loss of raw materials is caused, and the environmental cost is increased.
Disclosure of Invention
The technical problem to be solved by the present invention is to provide a method for preparing diphenyl phosphite, aiming at the deficiencies of the prior art. Phosphorus trichloride, phenol and water are used as raw materials, and after the reaction is finished, pressure is reduced, nitrogen is introduced to remove a byproduct, namely hydrogen chloride, so that the diphenyl phosphite is obtained.
In order to solve the technical problems, the technical scheme of the invention is as follows:
a preparation method of diphenyl phosphite comprises the following steps:
A. firstly, filling nitrogen into a reaction device to ensure that all gas in the reaction device is replaced by the nitrogen;
B. adding phenol and water into a reaction device, heating until the materials are homogeneous, wherein the heating temperature is 35-80 ℃, and then uniformly stirring for 0.1-1 hour;
C. dropwise adding phosphorus trichloride into the mixture of the phenol and the water stirred in the step B to form a material, wherein the dropwise adding duration of the phosphorus trichloride is 3-12 hours;
D. c, heating the materials in the step C to be above 100 ℃, and stirring at the temperature of 100-140 ℃ for 0.5-8 hours;
E. and discharging hydrogen chloride gas generated in the material by a method of reduced pressure distillation and nitrogen gas introduction, wherein the vacuum degree is 1-100 mmHg, and the content of chloride ions in the material is below 10 ppm.
In the above diphenyl phosphite preparation method, the nitrogen concentration in step a is greater than 99% by mass.
The preparation method of diphenyl phosphite, wherein the molar ratio of phenol to water in the step B is 1.5-2.3: 0.7 to 1.
The diphenyl phosphite preparation method comprises the following steps of: the molar ratio of phenol to water is 1: 1.5-2.3: 0.7 to 1.5.
Compared with the prior art, the invention has the following beneficial effects:
(1) the production link is simplified, the target product diphenyl phosphite is directly prepared by the phosphorus trichloride one-step method, and the intermediate diphenyl alkyl (ethyl or propyl) phosphite and triphenyl phosphite are not needed to be prepared, so that the production efficiency is improved;
(2) a large amount of phosphorous acid is not needed in the production process, so that the production risk is greatly reduced;
(3) the method does not produce phenol as a byproduct, solves the problems of cost increase and waste liquid treatment caused by repeated recovery of phenol, and reduces the production and environmental costs.
Detailed Description
The present invention will be described in further detail with reference to examples, but the present invention is not limited thereto.
Example 1
A preparation method of diphenyl phosphite comprises the following steps:
A. firstly, fully replacing air in a reaction device by using nitrogen to ensure that all gas in the device is replaced by nitrogen, wherein the concentration of the nitrogen is more than 99 percent;
B. adding phenol and water into a reaction device, heating to 80 ℃, and stirring for 0.1 hour to ensure that the materials are uniform;
C. and C, dropwise adding phosphorus trichloride into the mixture of the phenol and the water stirred in the step B to form a material, wherein the dropwise adding duration of the phosphorus trichloride is 3 hours. In this example, the molar ratio of phosphorus trichloride to phenol to water was 1: 1.5: 1.5;
D. c, heating the materials in the step C to 105 +/-5 ℃, and keeping the temperature and stirring in the temperature range for 8 hours;
E. and discharging hydrogen chloride gas generated in the material by a method of reduced pressure distillation and nitrogen gas introduction, wherein the vacuum degree is 1-10 mmHg, and the content of chloride ions in the material is below 10 ppm.
Performance indexes are as follows: the product yield in this example was 99.9%.
Example 2
A preparation method of diphenyl phosphite comprises the following steps:
A. firstly, fully replacing air in a reaction device by using nitrogen to ensure that all gas in the device is replaced by nitrogen with the nitrogen concentration of more than 99 percent;
B. Adding phenol and water into a reaction device, heating to 35 ℃, and stirring for 1 hour to ensure that the materials are uniform;
C. and C, dropwise adding phosphorus trichloride into the mixture of the phenol and the water which is stirred in the step B, wherein the dropwise adding duration of the phosphorus trichloride is 12 hours. In this example, the molar ratio of phosphorus trichloride to phenol to water was 1: 2.3: 0.7 of the total weight of the mixture;
D. c, heating the materials in the step C to 135 +/-5 ℃, and keeping the temperature and stirring in the temperature range for 0.5 hour;
E. and discharging hydrogen chloride gas generated in the material by a reduced pressure distillation and nitrogen gas introduction method, and controlling the vacuum degree to be 90-100 mmHg so that the content of chloride ions in the material is below 10 ppm.
Performance indexes are as follows: the product yield in this example was 100.2%.
Example 3
A preparation method of diphenyl phosphite comprises the following steps:
A. firstly, nitrogen is used for fully replacing the air in the reaction device, so that the gas in the device is completely replaced by the nitrogen, the concentration of the nitrogen is more than 99 percent,
B. then adding phenol and water into the reaction device, heating to 65 ℃, and stirring for 0.5 hour to ensure that the materials are uniform;
C. and D, dropwise adding phosphorus trichloride into the mixture of the phenol and the water which is stirred in the step B, wherein the dropwise adding duration of the phosphorus trichloride is 8 hours. In this example, the molar ratio of phosphorus trichloride to phenol to water was 1: 1.9: 1.1;
D. And C, heating the materials in the step C to 120 +/-5 ℃, and keeping the temperature and stirring in the temperature range for 4 hours.
And discharging hydrogen chloride gas generated in the material by a reduced pressure distillation and nitrogen gas introduction method, and controlling the vacuum degree to be 50-60 mmHg so that the content of chloride ions in the material is below 10 ppm.
Performance indexes are as follows: the product yield in this example was 100.3%.
The nitrogen in step A and step E is only used for illustrating the present invention, and should not be construed as limiting the present invention, and all gases that do not react with all materials involved in the present invention can be substituted for the nitrogen described in the present invention, such as carbon dioxide, helium, argon.
Example 4
A preparation method of diphenyl phosphite comprises the following steps:
C. firstly, carbon dioxide is used for fully replacing the air in the reaction device, so that the gas in the device is completely replaced by the carbon dioxide, the concentration of the carbon dioxide is more than 99 percent,
D. then adding phenol and water into the reaction device, heating to 65 ℃, and stirring for 0.5 hour to ensure that the materials are uniform;
C. and D, dropwise adding phosphorus trichloride into the mixture of the phenol and the water which is stirred in the step B, wherein the dropwise adding duration of the phosphorus trichloride is 8 hours. In this example, the molar ratio of phosphorus trichloride to phenol to water was 1: 1.9: 1.1;
D. And C, heating the materials in the step C to 120 +/-5 ℃, and keeping the temperature and stirring in the temperature range for 4 hours.
And discharging hydrogen chloride gas generated in the material by a method of reduced pressure distillation and carbon dioxide introduction, and controlling the vacuum degree to be 50-60 mmHg so that the content of chloride ions in the material is below 10 ppm.
Performance indexes are as follows: the product yield in this example was 100.3%.
Example 5
A preparation method of diphenyl phosphite comprises the following steps:
E. firstly, helium is used for fully replacing air in the reaction device, so that the gas in the device is completely replaced by the helium, the concentration of the helium is more than 99 percent,
F. then adding phenol and water into the reaction device, heating to 65 ℃, and stirring for 0.5 hour to ensure that the materials are uniform;
C. and D, dropwise adding phosphorus trichloride into the mixture of the phenol and the water which is stirred in the step B, wherein the dropwise adding duration of the phosphorus trichloride is 8 hours. In this example, the molar ratio of phosphorus trichloride to phenol to water was 1: 1.9: 1.1;
D. and C, heating the materials in the step C to 120 +/-5 ℃, and keeping the temperature and stirring in the temperature range for 4 hours.
And discharging hydrogen chloride gas generated in the material by adopting a method of reduced pressure distillation and helium gas introduction, and controlling the vacuum degree to be 50-60 mmHg so that the content of chloride ions in the material is below 10 ppm.
Performance indexes are as follows: the product yield in this example was 100.3%.
Example 6
A preparation method of diphenyl phosphite comprises the following steps:
G. firstly, argon is used for fully replacing the air in the reaction device, so that the gas in the device is completely replaced by the argon, the concentration of the argon is more than 99 percent,
H. then adding phenol and water into the reaction device, heating to 65 ℃, and stirring for 0.5 hour to ensure that the materials are uniform;
C. and D, dropwise adding phosphorus trichloride into the mixture of the phenol and the water which is stirred in the step B, wherein the dropwise adding duration of the phosphorus trichloride is 8 hours. In this example, the molar ratio of phosphorus trichloride to phenol to water was 1: 1.9: 1.1;
D. and C, heating the materials in the step C to 120 +/-5 ℃, and keeping the temperature and stirring in the temperature range for 4 hours.
And discharging hydrogen chloride gas generated in the material by a reduced pressure distillation and argon introduction method, and controlling the vacuum degree to be 50-60 mmHg so that the content of chloride ions in the material is below 10 ppm.
Performance indexes are as follows: the product yield in this example was 100.3%.
The above description is only for the preferred embodiment of the present invention, but the scope of the present invention is not limited thereto, and any changes or substitutions that can be easily conceived by those skilled in the art within the technical scope of the present invention are also included in the scope of the present invention. Therefore, the protection scope of the present invention shall be subject to the protection scope of the claims.