CN105636982B - Bi-or multispecific polypeptides that bind immune effector cell surface antigens and HBV antigens for the treatment of HBV infections and related conditions - Google Patents
Bi-or multispecific polypeptides that bind immune effector cell surface antigens and HBV antigens for the treatment of HBV infections and related conditions Download PDFInfo
- Publication number
- CN105636982B CN105636982B CN201480050976.8A CN201480050976A CN105636982B CN 105636982 B CN105636982 B CN 105636982B CN 201480050976 A CN201480050976 A CN 201480050976A CN 105636982 B CN105636982 B CN 105636982B
- Authority
- CN
- China
- Prior art keywords
- cells
- hbv
- polypeptide
- antigen
- complementarity determining
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000000427 antigen Substances 0.000 title claims abstract description 160
- 108091007433 antigens Proteins 0.000 title claims abstract description 157
- 102000036639 antigens Human genes 0.000 title claims abstract description 157
- 108090000765 processed proteins & peptides Proteins 0.000 title claims abstract description 114
- 229920001184 polypeptide Polymers 0.000 title claims abstract description 107
- 102000004196 processed proteins & peptides Human genes 0.000 title claims abstract description 107
- 229940121354 immunomodulator Drugs 0.000 title claims abstract description 46
- 239000012642 immune effector Substances 0.000 title claims abstract description 45
- 210000004027 cell Anatomy 0.000 title abstract description 257
- 208000015181 infectious disease Diseases 0.000 title abstract description 34
- 241000700721 Hepatitis B virus Species 0.000 claims abstract description 155
- 108010047041 Complementarity Determining Regions Proteins 0.000 claims abstract description 69
- 239000000203 mixture Substances 0.000 claims abstract description 30
- 206010073071 hepatocellular carcinoma Diseases 0.000 claims abstract description 23
- 231100000844 hepatocellular carcinoma Toxicity 0.000 claims abstract description 10
- 206010016654 Fibrosis Diseases 0.000 claims abstract description 8
- 230000007882 cirrhosis Effects 0.000 claims abstract description 8
- 208000019425 cirrhosis of liver Diseases 0.000 claims abstract description 8
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 claims description 55
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 claims description 55
- 239000012634 fragment Substances 0.000 claims description 18
- 238000006471 dimerization reaction Methods 0.000 claims description 17
- 101000917839 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-B Proteins 0.000 claims description 14
- 101000917858 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 claims description 13
- 102100029185 Low affinity immunoglobulin gamma Fc region receptor III-B Human genes 0.000 claims description 13
- 101000581981 Homo sapiens Neural cell adhesion molecule 1 Proteins 0.000 claims description 11
- 108020003175 receptors Proteins 0.000 claims description 11
- 102100027347 Neural cell adhesion molecule 1 Human genes 0.000 claims description 9
- 125000006850 spacer group Chemical group 0.000 claims description 9
- 239000008194 pharmaceutical composition Substances 0.000 claims description 7
- 238000000338 in vitro Methods 0.000 claims description 6
- 208000002672 hepatitis B Diseases 0.000 claims description 5
- 201000007270 liver cancer Diseases 0.000 claims description 5
- 208000014018 liver neoplasm Diseases 0.000 claims description 5
- 108020004707 nucleic acids Proteins 0.000 claims description 5
- 102000039446 nucleic acids Human genes 0.000 claims description 5
- 150000007523 nucleic acids Chemical class 0.000 claims description 5
- 108060003951 Immunoglobulin Proteins 0.000 claims description 4
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 claims description 4
- 102000018358 immunoglobulin Human genes 0.000 claims description 4
- 230000002265 prevention Effects 0.000 claims description 4
- 238000002360 preparation method Methods 0.000 claims description 2
- 108700017737 hepatitis B virus L Proteins 0.000 claims 3
- 239000003446 ligand Substances 0.000 abstract description 33
- 230000027455 binding Effects 0.000 abstract description 31
- 238000000034 method Methods 0.000 abstract description 20
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 abstract description 16
- 210000001744 T-lymphocyte Anatomy 0.000 description 70
- 102000017420 CD3 protein, epsilon/gamma/delta subunit Human genes 0.000 description 67
- 108050005493 CD3 protein, epsilon/gamma/delta subunit Proteins 0.000 description 67
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 66
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 description 35
- 210000000822 natural killer cell Anatomy 0.000 description 33
- 102000000588 Interleukin-2 Human genes 0.000 description 29
- 108010002350 Interleukin-2 Proteins 0.000 description 29
- 150000001413 amino acids Chemical class 0.000 description 29
- 238000003501 co-culture Methods 0.000 description 28
- 239000002609 medium Substances 0.000 description 27
- 230000008030 elimination Effects 0.000 description 22
- 238000003379 elimination reaction Methods 0.000 description 22
- 230000035899 viability Effects 0.000 description 22
- 239000012636 effector Substances 0.000 description 20
- 235000001014 amino acid Nutrition 0.000 description 19
- 229940024606 amino acid Drugs 0.000 description 19
- 230000028327 secretion Effects 0.000 description 19
- GOZMBJCYMQQACI-UHFFFAOYSA-N 6,7-dimethyl-3-[[methyl-[2-[methyl-[[1-[3-(trifluoromethyl)phenyl]indol-3-yl]methyl]amino]ethyl]amino]methyl]chromen-4-one;dihydrochloride Chemical compound Cl.Cl.C=1OC2=CC(C)=C(C)C=C2C(=O)C=1CN(C)CCN(C)CC(C1=CC=CC=C11)=CN1C1=CC=CC(C(F)(F)F)=C1 GOZMBJCYMQQACI-UHFFFAOYSA-N 0.000 description 18
- 230000004913 activation Effects 0.000 description 18
- 239000006228 supernatant Substances 0.000 description 18
- 102100032937 CD40 ligand Human genes 0.000 description 16
- 238000002474 experimental method Methods 0.000 description 15
- 102000004127 Cytokines Human genes 0.000 description 14
- 108090000695 Cytokines Proteins 0.000 description 14
- 230000000694 effects Effects 0.000 description 14
- 230000000638 stimulation Effects 0.000 description 14
- 101000851370 Homo sapiens Tumor necrosis factor receptor superfamily member 9 Proteins 0.000 description 13
- 102100036856 Tumor necrosis factor receptor superfamily member 9 Human genes 0.000 description 13
- 102100032852 Natural cytotoxicity triggering receptor 3 Human genes 0.000 description 12
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 12
- 230000009089 cytolysis Effects 0.000 description 12
- 230000002147 killing effect Effects 0.000 description 12
- 102100022153 Tumor necrosis factor receptor superfamily member 4 Human genes 0.000 description 11
- 206010028980 Neoplasm Diseases 0.000 description 10
- 238000007792 addition Methods 0.000 description 10
- 102000005962 receptors Human genes 0.000 description 10
- 101001109501 Homo sapiens NKG2-D type II integral membrane protein Proteins 0.000 description 9
- 102100035133 Lysosome-associated membrane glycoprotein 1 Human genes 0.000 description 9
- 102100022680 NKG2-D type II integral membrane protein Human genes 0.000 description 9
- 101710165473 Tumor necrosis factor receptor superfamily member 4 Proteins 0.000 description 9
- 230000001404 mediated effect Effects 0.000 description 9
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 9
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 8
- 108010004222 Natural Cytotoxicity Triggering Receptor 3 Proteins 0.000 description 8
- 210000004369 blood Anatomy 0.000 description 8
- 239000008280 blood Substances 0.000 description 8
- 230000003833 cell viability Effects 0.000 description 8
- 230000004069 differentiation Effects 0.000 description 8
- 238000004519 manufacturing process Methods 0.000 description 8
- 108090000623 proteins and genes Proteins 0.000 description 8
- 102000003390 tumor necrosis factor Human genes 0.000 description 8
- 238000002965 ELISA Methods 0.000 description 7
- 101710116782 Lysosome-associated membrane glycoprotein 1 Proteins 0.000 description 7
- 102000018697 Membrane Proteins Human genes 0.000 description 7
- 108010052285 Membrane Proteins Proteins 0.000 description 7
- 241000699670 Mus sp. Species 0.000 description 7
- 230000006037 cell lysis Effects 0.000 description 7
- 239000003550 marker Substances 0.000 description 7
- 239000013612 plasmid Substances 0.000 description 7
- 108020004414 DNA Proteins 0.000 description 6
- 231100000433 cytotoxic Toxicity 0.000 description 6
- 230000001472 cytotoxic effect Effects 0.000 description 6
- 230000003013 cytotoxicity Effects 0.000 description 6
- 231100000135 cytotoxicity Toxicity 0.000 description 6
- 230000003247 decreasing effect Effects 0.000 description 6
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 6
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 6
- 239000013642 negative control Substances 0.000 description 6
- 239000002245 particle Substances 0.000 description 6
- 102000004169 proteins and genes Human genes 0.000 description 6
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 6
- 230000002195 synergetic effect Effects 0.000 description 6
- 102100035360 Cerebellar degeneration-related antigen 1 Human genes 0.000 description 5
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 5
- 241001465754 Metazoa Species 0.000 description 5
- 230000006870 function Effects 0.000 description 5
- 230000004927 fusion Effects 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 4
- 108091036055 CccDNA Proteins 0.000 description 4
- 108010087819 Fc receptors Proteins 0.000 description 4
- 102000009109 Fc receptors Human genes 0.000 description 4
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 4
- 101000589307 Homo sapiens Natural cytotoxicity triggering receptor 3 Proteins 0.000 description 4
- 102100037850 Interferon gamma Human genes 0.000 description 4
- 108010074328 Interferon-gamma Proteins 0.000 description 4
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 4
- 241000700605 Viruses Species 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 210000004899 c-terminal region Anatomy 0.000 description 4
- 239000002299 complementary DNA Substances 0.000 description 4
- 230000001419 dependent effect Effects 0.000 description 4
- 239000010432 diamond Substances 0.000 description 4
- 239000012091 fetal bovine serum Substances 0.000 description 4
- 230000001900 immune effect Effects 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- 125000003729 nucleotide group Chemical group 0.000 description 4
- 235000018102 proteins Nutrition 0.000 description 4
- 238000010186 staining Methods 0.000 description 4
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- 101000991061 Homo sapiens MHC class I polypeptide-related sequence B Proteins 0.000 description 3
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 3
- 229930182816 L-glutamine Natural products 0.000 description 3
- 102100030300 MHC class I polypeptide-related sequence B Human genes 0.000 description 3
- 229930182555 Penicillin Natural products 0.000 description 3
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 3
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 3
- 125000000539 amino acid group Chemical group 0.000 description 3
- 239000003443 antiviral agent Substances 0.000 description 3
- 210000000170 cell membrane Anatomy 0.000 description 3
- 238000003570 cell viability assay Methods 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- 108091008034 costimulatory receptors Proteins 0.000 description 3
- 210000002472 endoplasmic reticulum Anatomy 0.000 description 3
- 230000008029 eradication Effects 0.000 description 3
- 230000001747 exhibiting effect Effects 0.000 description 3
- 210000003494 hepatocyte Anatomy 0.000 description 3
- 210000004408 hybridoma Anatomy 0.000 description 3
- 229960000890 hydrocortisone Drugs 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 238000002955 isolation Methods 0.000 description 3
- JTEGQNOMFQHVDC-NKWVEPMBSA-N lamivudine Chemical compound O=C1N=C(N)C=CN1[C@H]1O[C@@H](CO)SC1 JTEGQNOMFQHVDC-NKWVEPMBSA-N 0.000 description 3
- 229910052618 mica group Inorganic materials 0.000 description 3
- 239000002773 nucleotide Substances 0.000 description 3
- 229940049954 penicillin Drugs 0.000 description 3
- 229960005322 streptomycin Drugs 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- IQFYYKKMVGJFEH-CSMHCCOUSA-N telbivudine Chemical compound O=C1NC(=O)C(C)=CN1[C@H]1O[C@@H](CO)[C@H](O)C1 IQFYYKKMVGJFEH-CSMHCCOUSA-N 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 2
- 108010029697 CD40 Ligand Proteins 0.000 description 2
- 108020004635 Complementary DNA Proteins 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- 101100166600 Homo sapiens CD28 gene Proteins 0.000 description 2
- 101001023379 Homo sapiens Lysosome-associated membrane glycoprotein 1 Proteins 0.000 description 2
- 101000638251 Homo sapiens Tumor necrosis factor ligand superfamily member 9 Proteins 0.000 description 2
- 108010073807 IgG Receptors Proteins 0.000 description 2
- 102000009490 IgG Receptors Human genes 0.000 description 2
- 102000018071 Immunoglobulin Fc Fragments Human genes 0.000 description 2
- 108010091135 Immunoglobulin Fc Fragments Proteins 0.000 description 2
- 102000012745 Immunoglobulin Subunits Human genes 0.000 description 2
- 108010079585 Immunoglobulin Subunits Proteins 0.000 description 2
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 2
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 2
- 241000699666 Mus <mouse, genus> Species 0.000 description 2
- 241000699660 Mus musculus Species 0.000 description 2
- 239000012124 Opti-MEM Substances 0.000 description 2
- 239000012980 RPMI-1640 medium Substances 0.000 description 2
- 108700019146 Transgenes Proteins 0.000 description 2
- 102100032101 Tumor necrosis factor ligand superfamily member 9 Human genes 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- WOZSCQDILHKSGG-UHFFFAOYSA-N adefovir depivoxil Chemical compound N1=CN=C2N(CCOCP(=O)(OCOC(=O)C(C)(C)C)OCOC(=O)C(C)(C)C)C=NC2=C1N WOZSCQDILHKSGG-UHFFFAOYSA-N 0.000 description 2
- 229960003205 adefovir dipivoxil Drugs 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 230000000840 anti-viral effect Effects 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 238000002869 basic local alignment search tool Methods 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- KQNZDYYTLMIZCT-KQPMLPITSA-N brefeldin A Chemical compound O[C@@H]1\C=C\C(=O)O[C@@H](C)CCC\C=C\[C@@H]2C[C@H](O)C[C@H]21 KQNZDYYTLMIZCT-KQPMLPITSA-N 0.000 description 2
- JUMGSHROWPPKFX-UHFFFAOYSA-N brefeldin-A Natural products CC1CCCC=CC2(C)CC(O)CC2(C)C(O)C=CC(=O)O1 JUMGSHROWPPKFX-UHFFFAOYSA-N 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 230000020411 cell activation Effects 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 230000006690 co-activation Effects 0.000 description 2
- 238000011260 co-administration Methods 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 230000007402 cytotoxic response Effects 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- QDGZDCVAUDNJFG-FXQIFTODSA-N entecavir (anhydrous) Chemical compound C1=2NC(N)=NC(=O)C=2N=CN1[C@H]1C[C@H](O)[C@@H](CO)C1=C QDGZDCVAUDNJFG-FXQIFTODSA-N 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 230000013595 glycosylation Effects 0.000 description 2
- 238000006206 glycosylation reaction Methods 0.000 description 2
- 102000049018 human NCAM1 Human genes 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 210000004020 intracellular membrane Anatomy 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 229960001627 lamivudine Drugs 0.000 description 2
- 210000005229 liver cell Anatomy 0.000 description 2
- 108020004999 messenger RNA Proteins 0.000 description 2
- 230000002503 metabolic effect Effects 0.000 description 2
- 238000012544 monitoring process Methods 0.000 description 2
- 230000009871 nonspecific binding Effects 0.000 description 2
- 238000011580 nude mouse model Methods 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 239000002953 phosphate buffered saline Substances 0.000 description 2
- 230000004481 post-translational protein modification Effects 0.000 description 2
- 230000000770 proinflammatory effect Effects 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 230000010076 replication Effects 0.000 description 2
- 230000003362 replicative effect Effects 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 230000003248 secreting effect Effects 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 229960005311 telbivudine Drugs 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- 239000012096 transfection reagent Substances 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 210000004881 tumor cell Anatomy 0.000 description 2
- 230000003612 virological effect Effects 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- KQNZDYYTLMIZCT-KFKPYADVSA-N (2e,7s,10e,12r,13r,15s)-12,15-dihydroxy-7-methyl-8-oxabicyclo[11.3.0]hexadeca-2,10-dien-9-one Chemical compound O[C@@H]1\C=C\C(=O)O[C@@H](C)CCC\C=C\C2C[C@H](O)C[C@H]21 KQNZDYYTLMIZCT-KFKPYADVSA-N 0.000 description 1
- FDKWRPBBCBCIGA-REOHCLBHSA-N (2r)-2-azaniumyl-3-$l^{1}-selanylpropanoate Chemical compound [Se]C[C@H](N)C(O)=O FDKWRPBBCBCIGA-REOHCLBHSA-N 0.000 description 1
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 1
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- 108010011170 Ala-Trp-Arg-His-Pro-Gln-Phe-Gly-Gly Proteins 0.000 description 1
- 108010083359 Antigen Receptors Proteins 0.000 description 1
- 102000006306 Antigen Receptors Human genes 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 1
- 101100454808 Caenorhabditis elegans lgg-2 gene Proteins 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 108010067225 Cell Adhesion Molecules Proteins 0.000 description 1
- 102000016289 Cell Adhesion Molecules Human genes 0.000 description 1
- 108010019670 Chimeric Antigen Receptors Proteins 0.000 description 1
- 108020004638 Circular DNA Proteins 0.000 description 1
- 108091026890 Coding region Proteins 0.000 description 1
- 102100031673 Corneodesmosin Human genes 0.000 description 1
- FDKWRPBBCBCIGA-UWTATZPHSA-N D-Selenocysteine Natural products [Se]C[C@@H](N)C(O)=O FDKWRPBBCBCIGA-UWTATZPHSA-N 0.000 description 1
- 150000008574 D-amino acids Chemical class 0.000 description 1
- 238000001712 DNA sequencing Methods 0.000 description 1
- 101100193633 Danio rerio rag2 gene Proteins 0.000 description 1
- -1 Entaliv) Chemical compound 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- XCLCVBYNGXEVDU-WHFBIAKZSA-N Gly-Asn-Ser Chemical compound NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O XCLCVBYNGXEVDU-WHFBIAKZSA-N 0.000 description 1
- BCCRXDTUTZHDEU-VKHMYHEASA-N Gly-Ser Chemical group NCC(=O)N[C@@H](CO)C(O)=O BCCRXDTUTZHDEU-VKHMYHEASA-N 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101000599940 Homo sapiens Interferon gamma Proteins 0.000 description 1
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 1
- 101001109508 Homo sapiens NKG2-A/NKG2-B type II integral membrane protein Proteins 0.000 description 1
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 1
- 229930010555 Inosine Natural products 0.000 description 1
- UGQMRVRMYYASKQ-KQYNXXCUSA-N Inosine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C2=NC=NC(O)=C2N=C1 UGQMRVRMYYASKQ-KQYNXXCUSA-N 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102000006992 Interferon-alpha Human genes 0.000 description 1
- 108010047761 Interferon-alpha Proteins 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 102100026878 Interleukin-2 receptor subunit alpha Human genes 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 1
- ZFOMKMMPBOQKMC-KXUCPTDWSA-N L-pyrrolysine Chemical compound C[C@@H]1CC=N[C@H]1C(=O)NCCCC[C@H]([NH3+])C([O-])=O ZFOMKMMPBOQKMC-KXUCPTDWSA-N 0.000 description 1
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 101100193635 Mus musculus Rag2 gene Proteins 0.000 description 1
- 230000004988 N-glycosylation Effects 0.000 description 1
- 108010057466 NF-kappa B Proteins 0.000 description 1
- 102000003945 NF-kappa B Human genes 0.000 description 1
- 102000000835 NK Cell Lectin-Like Receptor Subfamily B Human genes 0.000 description 1
- 108010001882 NK Cell Lectin-Like Receptor Subfamily B Proteins 0.000 description 1
- 102100022682 NKG2-A/NKG2-B type II integral membrane protein Human genes 0.000 description 1
- 108010069196 Neural Cell Adhesion Molecules Proteins 0.000 description 1
- 102000001068 Neural Cell Adhesion Molecules Human genes 0.000 description 1
- 230000004989 O-glycosylation Effects 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 1
- 238000012408 PCR amplification Methods 0.000 description 1
- 108090000526 Papain Proteins 0.000 description 1
- 108091093037 Peptide nucleic acid Proteins 0.000 description 1
- 102000003992 Peroxidases Human genes 0.000 description 1
- 206010035226 Plasma cell myeloma Diseases 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 229940096437 Protein S Drugs 0.000 description 1
- 239000012979 RPMI medium Substances 0.000 description 1
- PLXBWHJQWKZRKG-UHFFFAOYSA-N Resazurin Chemical compound C1=CC(=O)C=C2OC3=CC(O)=CC=C3[N+]([O-])=C21 PLXBWHJQWKZRKG-UHFFFAOYSA-N 0.000 description 1
- 239000006146 Roswell Park Memorial Institute medium Substances 0.000 description 1
- RJFAYQIBOAGBLC-BYPYZUCNSA-N Selenium-L-methionine Chemical compound C[Se]CC[C@H](N)C(O)=O RJFAYQIBOAGBLC-BYPYZUCNSA-N 0.000 description 1
- RJFAYQIBOAGBLC-UHFFFAOYSA-N Selenomethionine Natural products C[Se]CCC(N)C(O)=O RJFAYQIBOAGBLC-UHFFFAOYSA-N 0.000 description 1
- 101710137302 Surface antigen S Proteins 0.000 description 1
- 230000006044 T cell activation Effects 0.000 description 1
- 230000024932 T cell mediated immunity Effects 0.000 description 1
- 108091008874 T cell receptors Proteins 0.000 description 1
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 239000004473 Threonine Substances 0.000 description 1
- 108010028230 Trp-Ser- His-Pro-Gln-Phe-Glu-Lys Proteins 0.000 description 1
- 108060008683 Tumor Necrosis Factor Receptor Proteins 0.000 description 1
- 206010058874 Viraemia Diseases 0.000 description 1
- 108010003533 Viral Envelope Proteins Proteins 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- 108010031318 Vitronectin Proteins 0.000 description 1
- 230000001594 aberrant effect Effects 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000001464 adherent effect Effects 0.000 description 1
- 150000001371 alpha-amino acids Chemical class 0.000 description 1
- 235000008206 alpha-amino acids Nutrition 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 230000009227 antibody-mediated cytotoxicity Effects 0.000 description 1
- 235000009582 asparagine Nutrition 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- 229940002637 baraclude Drugs 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 1
- 150000001576 beta-amino acids Chemical class 0.000 description 1
- 238000010804 cDNA synthesis Methods 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- 230000005889 cellular cytotoxicity Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 239000012881 co-culture medium Substances 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 230000009260 cross reactivity Effects 0.000 description 1
- 239000012228 culture supernatant Substances 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 230000016396 cytokine production Effects 0.000 description 1
- 238000002784 cytotoxicity assay Methods 0.000 description 1
- 231100000263 cytotoxicity test Toxicity 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 231100000517 death Toxicity 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 238000000432 density-gradient centrifugation Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- 230000000447 dimerizing effect Effects 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 230000001094 effect on targets Effects 0.000 description 1
- 229960000980 entecavir Drugs 0.000 description 1
- 229940074057 epivir hbv Drugs 0.000 description 1
- 239000013604 expression vector Substances 0.000 description 1
- 210000004700 fetal blood Anatomy 0.000 description 1
- 229960002518 gentamicin Drugs 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 210000002288 golgi apparatus Anatomy 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 210000002767 hepatic artery Anatomy 0.000 description 1
- 239000000833 heterodimer Substances 0.000 description 1
- 102000053350 human FCGR3B Human genes 0.000 description 1
- 230000028996 humoral immune response Effects 0.000 description 1
- 229960002591 hydroxyproline Drugs 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 230000008629 immune suppression Effects 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 239000002955 immunomodulating agent Substances 0.000 description 1
- 239000003018 immunosuppressive agent Substances 0.000 description 1
- 229940125721 immunosuppressive agent Drugs 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 210000003000 inclusion body Anatomy 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 239000002054 inoculum Substances 0.000 description 1
- 229960003786 inosine Drugs 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 102000006495 integrins Human genes 0.000 description 1
- 108010044426 integrins Proteins 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 229940047124 interferons Drugs 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000008774 maternal effect Effects 0.000 description 1
- MYWUZJCMWCOHBA-VIFPVBQESA-N methamphetamine Chemical compound CN[C@@H](C)CC1=CC=CC=C1 MYWUZJCMWCOHBA-VIFPVBQESA-N 0.000 description 1
- 239000010445 mica Substances 0.000 description 1
- 239000011325 microbead Substances 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 239000012452 mother liquor Substances 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 201000000050 myeloid neoplasm Diseases 0.000 description 1
- POFWRMVFWIJXHP-UHFFFAOYSA-N n-benzyl-9-(oxan-2-yl)purin-6-amine Chemical compound C=1C=CC=CC=1CNC(C=1N=C2)=NC=NC=1N2C1CCCCO1 POFWRMVFWIJXHP-UHFFFAOYSA-N 0.000 description 1
- 210000003061 neural cell Anatomy 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 230000003472 neutralizing effect Effects 0.000 description 1
- 238000010606 normalization Methods 0.000 description 1
- 239000002777 nucleoside Substances 0.000 description 1
- 150000003833 nucleoside derivatives Chemical class 0.000 description 1
- 229960003104 ornithine Drugs 0.000 description 1
- 229940055729 papain Drugs 0.000 description 1
- 235000019834 papain Nutrition 0.000 description 1
- 230000010412 perfusion Effects 0.000 description 1
- 230000008823 permeabilization Effects 0.000 description 1
- 108040007629 peroxidase activity proteins Proteins 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 230000007420 reactivation Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- HSSLDCABUXLXKM-UHFFFAOYSA-N resorufin Chemical compound C1=CC(=O)C=C2OC3=CC(O)=CC=C3N=C21 HSSLDCABUXLXKM-UHFFFAOYSA-N 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 235000016491 selenocysteine Nutrition 0.000 description 1
- 229940055619 selenocysteine Drugs 0.000 description 1
- ZKZBPNGNEQAJSX-UHFFFAOYSA-N selenocysteine Natural products [SeH]CC(N)C(O)=O ZKZBPNGNEQAJSX-UHFFFAOYSA-N 0.000 description 1
- 229960002718 selenomethionine Drugs 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 210000004989 spleen cell Anatomy 0.000 description 1
- 239000008174 sterile solution Substances 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 230000009897 systematic effect Effects 0.000 description 1
- SGOIRFVFHAKUTI-ZCFIWIBFSA-N tenofovir (anhydrous) Chemical compound N1=CN=C2N(C[C@@H](C)OCP(O)(O)=O)C=NC2=C1N SGOIRFVFHAKUTI-ZCFIWIBFSA-N 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- FGMPLJWBKKVCDB-UHFFFAOYSA-N trans-L-hydroxy-proline Natural products ON1CCCC1C(O)=O FGMPLJWBKKVCDB-UHFFFAOYSA-N 0.000 description 1
- 230000005945 translocation Effects 0.000 description 1
- 102000003298 tumor necrosis factor receptor Human genes 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 229940063032 tyzeka Drugs 0.000 description 1
- 241001430294 unidentified retrovirus Species 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 230000029812 viral genome replication Effects 0.000 description 1
- 210000002845 virion Anatomy 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2809—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against the T-cell receptor (TcR)-CD3 complex
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/46—Cellular immunotherapy
- A61K39/461—Cellular immunotherapy characterised by the cell type used
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/46—Cellular immunotherapy
- A61K39/464—Cellular immunotherapy characterised by the antigen targeted or presented
- A61K39/464838—Viral antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
- C07K16/081—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses from DNA viruses
- C07K16/082—Hepadnaviridae, e.g. hepatitis B virus
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2818—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD28 or CD152
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/283—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against Fc-receptors, e.g. CD16, CD32, CD64
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0636—T lymphocytes
- C12N5/0638—Cytotoxic T lymphocytes [CTL] or lymphokine activated killer cells [LAK]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0646—Natural killers cells [NK], NKT cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
- A61K2039/507—Comprising a combination of two or more separate antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K39/46
- A61K2239/31—Indexing codes associated with cellular immunotherapy of group A61K39/46 characterized by the route of administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K39/46
- A61K2239/38—Indexing codes associated with cellular immunotherapy of group A61K39/46 characterised by the dose, timing or administration schedule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K39/46
- A61K2239/46—Indexing codes associated with cellular immunotherapy of group A61K39/46 characterised by the cancer treated
- A61K2239/53—Liver
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/10—Immunoglobulins specific features characterized by their source of isolation or production
- C07K2317/14—Specific host cells or culture conditions, e.g. components, pH or temperature
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/31—Immunoglobulins specific features characterized by aspects of specificity or valency multispecific
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/35—Valency
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
- C07K2317/524—CH2 domain
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
- C07K2317/526—CH3 domain
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/622—Single chain antibody (scFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
- C07K2317/732—Antibody-dependent cellular cytotoxicity [ADCC]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
Abstract
The present invention relates to polypeptides comprising (a) a first set of 6 Complementarity Determining Regions (CDRs) configured to bind a first antigen; and (b) (ba) a second set of 6 CDRs configured to bind a second antigen; or (bb) a ligand capable of binding a second antigen; wherein (i) the first antigen is selected from the group consisting of Hepatitis B Virus (HBV) small surface antigen; surface antigens in HBV; and HBV large surface antigen; and (ii) the second antigen is selected from surface antigens presented by immune effector cells such as Natural Killer (NK) cells and Cytotoxic T Lymphocytes (CTLs). Also provided are compositions for use in methods of treating or preventing HBV infection and/or a condition caused by said HBV infection, said condition caused by HBV infection being selected from cirrhosis and hepatocellular carcinoma.
Description
Technical Field
The present invention relates to polypeptides comprising (a) a first set of 6 Complementarity Determining Regions (CDRs) configured to bind a first antigen; and (b) (ba) a second set of 6 CDRs configured to bind a second antigen; or (bb) a ligand capable of binding a second antigen; wherein (i) the first antigen is selected from the group consisting of Hepatitis B Virus (HBV) small surface antigen; surface antigens in HBV; and HBV large surface antigen; and (ii) the second antigen is selected from surface antigens presented by immune effector cells such as Natural Killer (NK) cells and Cytotoxic T Lymphocytes (CTLs).
In this specification, a number of documents are cited, including patent applications and manufacturer manuals. The disclosures of these documents, when not considered relevant to the patentability of the present invention, are incorporated herein by reference in their entirety. More specifically, all cited documents are incorporated by reference to the same extent as if each individual document was specifically and individually indicated to be incorporated by reference.
Background
Approximately 3.5 million humans are chronically infected with Hepatitis B Virus (HBV). HBV infection may include cirrhosis and hepatocellular carcinoma (HCC), which results in approximately one million deaths each year (Ganem et al, Hepatitis B viral infection-natural and clinical sequences. N Engl J Med; 350:1118-29 (2004)). Infection with HBV is currently not controlled in about 5% of adult patients and about 90% of neonates. In this case, HBV infection becomes chronic. The possible cause is insufficient cellular immune response. Currently available antiviral drugs for the treatment of HBV infection inhibit viral replication. However, covalently closed circular dna (cccdna) remains in the nucleus of infected hepatocytes and may trigger reactivation of HBV infection once the patient stops taking medication. Therefore, it is essential to eliminate HBV infected cells carrying the cccDNA if the infection is to be cured completely (Protzer et al, Nat Immunol Rev12:2013-213 (2012)).
However, such toxic elimination of HBV infected cells (by cytotoxic T lymphocytes or Natural Killer (NK) cells) does not occur or does not occur to an adequate extent.
Infected cells carrying HBV cccDNA display viral surface proteins on their surface. The same is true even if the virus is released into intracellular vesicles, since many HBV surface proteins remain integrated within the intracellular membrane of the endoplasmic reticulum. During vesicle transfer, the intracellular membrane can fuse with the cell membrane, with the result that HBV surface proteins are displayed on the surface of infected cells.
Bohne et al (T Cell redirected against liver cells with surface Antigen induced hepatology. gateway. 134:239-247(2008)) and Krebs et al (T Cell expression a chiral Antigen Receptor with liver cells with viral envelope Proteins with surface expression on T cells (2013)) describe Chimeric Antigen receptors That, when presented by retroviruses, can cause primary human and murine T cells to recognize hepatocytes exhibiting HBV small surface antigens and lyse cells replicating HBV.
Bispecific antibodies are commonly used in the field of oncology. See, by way of example, Hartmann et al (treatment of recovery Hodgkin's disease with an anti-CD16/CD30bispecific, blood; 89:2042-7 (1997)).
EP 2524699 a1 describes trifunctional antibodies. These antibodies "have a functional Fc portion" and "must be composed of heavy immunoglobulin chains of different subclasses". On the other hand Hornig andthe structure of scFv lacking an Fc moiety is described in chapter 40 of "antibody engineering" (ed. Patrick channels, human Press, 2012).
Liao et al (Oncology Reports 3,637-644(1996)) describe bispecific monoclonal antibodies which are retargeted to effector cells for the lysis of human liver cancer xenografts in nude mice. The bispecific antibodies described are produced by fusion of two hybridomas, which results in the production of a heavy/light chain combination of hybridoma cell lines expressing two different antibodies. This can result in pairing of two different heavy chains, but can also result in pairing of the same heavy chain, resulting in a random mixture of monospecific parent and bispecific antibodies. The bispecific antibody contains a heavy chain and a light chain and dimerizes to form an Ig molecule (which is not a single polypeptide chain).
With reference to the prior art, it is seen as a technical problem to provide alternative or improved measures and methods for treating HBV infection as well as conditions caused by HBV infection such as cirrhosis or hepatocellular carcinoma. Expressed in terms of cell biology, the technical problem seen is to provide means and methods for eradicating HBV cccDNA bearing cells. Such technical problem is solved by the appended claims.
Disclosure of Invention
Accordingly, the present invention relates in a first aspect to a polypeptide comprising (a) a first set of 6 Complementarity Determining Regions (CDRs) configured to bind to a first antigen; and (b) (ba) a second set of 6 CDRs configured to bind a second antigen; or (bb) a ligand capable of binding a second antigen; wherein (i) the first antigen is selected from HBV small surface antigen; surface antigens in HBV; and HBV large surface antigen; and (ii) the second antigen is selected from surface antigens presented by immune effector cells such as Natural Killer (NK) cells and Cytotoxic T Lymphocytes (CTLs).
The term "polypeptide" defines a condensation polymer molecule of amino acids that forms a single chain having one N-terminus and one C-terminus, the constituent amino acids of which include the naturally occurring proteinogenic amino acids of 20. preferably, the polypeptide consists only of the naturally occurring proteinogenic amino acids, in view of this, the term extends to molecules that, in addition to the naturally occurring proteinogenic amino acids, contain up to 20%, 10%, 5%, 2%, or 1% of amino acids selected from the group consisting of non-naturally occurring α -amino acids, β -amino acids, D-amino acids, selenocysteine, selenomethionine, hydroxyproline, pyrrolysine and ornithine. it is also to be understood that amino acids (such as 2, 3, 4, 5, 6, 7, 8, 9 or 10 amino acids) may be phosphorylated the latter are particularly suitable for serine, threonine and tyrosine. there may also be other post-translational modifications known in the art, including glycosylation including N-linked glycosylation (typically at asparagine) and O-linked glycosylation (typically at the amino acid residue or amino acid residue, including the amino acid residue at the C-terminal side chain, 369, 7, 9, or 1% of a polypeptide that includes one or more amino acid that is not a peptide bond that is present at the C-terminal side chain, e.g. 7, 369, a peptide bond, a peptide bond.
In general, there is no upper limit to the number of amino acids in a polypeptide. As shown by the exemplary polypeptide sequences contained in the sequence listing, the polypeptides of the invention typically contain several hundred amino acids, preferably 250 to 1000, 400 to 900, or 700 to 800 amino acids. It is common to distinguish between a peptide on the one hand and a polypeptide on the other hand, where a peptide has 30 or fewer amino acids and a polypeptide has more than 30 amino acids.
The term "complementarity determining region," abbreviated "CDR," has a meaning known in the art, these are short subsequences, typically ranging from about 3 to about 25 amino acids, that confer the ability of an antibody to specifically recognize an epitope of an antigen, in general, the variable domain of an antibody light chain provides 3 CDRs, and the variable domain of an antibody heavy chain provides 3 CDRs, although CDRs are typically part of an immunoglobulin domain, the invention is not required in this regard, as long as the amino acid sequence comprising the CDRs is sufficient, provided that the amino acid sequence is capable of presenting the CDRs in spatial proximity when folded under physiological conditions and retaining its ability to recognize a cognate antigen.
The first set of 6 CDRs and the second set of 6 CDRs each define a binding site.
It is understood that no additional CDRs are present in the polypeptides of the invention outside said first and second sets.
The term "antigen" has the meaning known in the art. It refers to a group of 6 CDRs (usually presented by immunoglobulin domains) specifically recognized and bound molecules. The specific portion of the antigen that the CDRs recognize and bind is known as an epitope.
The term "ligand" has the meaning known in the art. Ligands are the opposite structure of receptors. More specifically, the ligand is capable of binding (preferably specifically binding) to its cognate receptor. According to the invention, the ligand is preferably an immunoligand. An immunoligand is a ligand that is capable of binding to a receptor present on the surface of an immune effector cell. Preferred immune effector cells are (as described above), NK cells and CTL. Preferred are immunological ligands which exert a stimulatory and/or co-stimulatory effect when bound to their cognate receptors on the surface of immune effector cells. The terms "activate" and "stimulate" are used equivalently in this context. Preferred receptors to which the immunoligand binds are described in further detail below.
HBV S/M/L surface proteins are small, medium and large surface antigens in the outer envelope of HBV (Stibbe, W., and W.H.Gerlich.structural relationships between minor and major proteins of hepatitis B surface antigen.J.Virol.198346: 626-.
Three HBV surface antigens are transcribed and translated from one reading frame and differ from each other by the length of the N-terminal part. Thus, the large surface antigen comprises a portion that is not present in the small and medium surface antigens, and the medium surface antigen comprises a portion that is not present in the small antigen (but is contained in the large antigen). The small antigen consists of a sequence that is included in the C-terminal part of the neutralizing large antigen.
The large HBV surface antigens can be inserted into the cytoplasmic membrane in two ways. The N-terminus or C-terminus may be located on the extracellular side. Both formulations were found in HBV infected cells.
The second antigen mentioned is a surface antigen presented by immune effector cells, preferably specifically by NK cells and/or CTLs. Immune effector cells are cells that are to be redirected towards HBV infected cells presenting the mentioned HBV surface antigens on their surface.
It is particularly preferred that the binding of the invention is specific, in particular between the CDRs and the antigen and between the ligand and the antigen. The terms "specifically binds" and "specifically binds" (having the same meaning as "specific interaction") as used herein mean that these binding moieties do not (or substantially do not) cross-react with an epitope or structure similar to the target antigen. The cross-reactivity of a group of molecules under investigation can be tested, for example, by assessing the binding of the group of molecules to epitopes of interest under conventional conditions, as well as their binding to some more or less (structurally and/or functionally) closely related epitopes. Only those molecules that bind an epitope of interest (e.g., a particular motif in a protein structure) in their relevant context, but do not or substantially do not bind any other epitope, will be considered specific for the epitope of interest.
The first aspect encompasses embodiments wherein items (a) and (ba) together are the only binding sites present on said polypeptide, and embodiments wherein items (a) and (bb) together are the only binding sites present on said polypeptide.
Chronic HBV infection is characterized by an immune-tolerant state. More specifically, complete eradication of infected cells or complete control of HBV replication or complete elimination of HBV does not occur under the action of CTL and NK cells of the patient. The polypeptides of the invention are bispecific molecules in the sense that they specifically recognize HBV surface antigens on the one hand and immune effector cell surface antigens on the other hand. Such bispecific molecules can be considered to confer artificial specificity against immune effector cells. In fact, the polypeptides of the invention target CTL and NK cells (also referred to as "bispecific") so that they are used for HBV infected cells and kill them.
The binding of the polypeptides of the invention to HBV infected cells on the one hand and the use of immune effector cells on the other hand may occur in any order or may also occur simultaneously.
In particular, it is desirable to apply the polypeptides of the invention systemically, either by injection or as an oral application, and to enable them to bind to HBV-infected or HBV antigen expressing target T cells and to use the immune effector cells on the target T cells.
In view of this, it is also contemplated to contact a polypeptide of the invention with an immune effector cell (or a population of peripheral blood mononuclear cells comprising the effector cell) such that the effector cell is loaded with the polypeptide. Such effector cells that have been loaded in vitro or ex vivo (or a PBMC population comprising such loaded effector cells) may then be administered to a patient suffering from HBV infection (or a condition associated therewith and defined below). Such administration may be effected intravenously, for example in the hepatic artery. Immune effector cells (having a polypeptide of the invention bound to an antigen indicative of said immune effector cell) are also aspects of the invention. This aspect is further disclosed below.
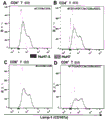
Such killing, particularly in combination with antiviral immune mediators (e.g., cytokines) secreted by immune cells, provides eradication of HBV infection or sustained control of HBV infection or elimination of tumor cells expressing HBV surface antigens. The preferred or exemplary bispecific polypeptides of the invention provide surprisingly high kill rates against HBV-infected cells or hepatoma cells replicating HBV or expressing HBV surface antigens (also referred to as hepatoma cells); see the examples disclosed herein.
Given that the bispecific polypeptides of the invention provide a tailored specificity for immune effector cells, the specificity inherent in the immune effector cells in nature or to which the antigen is presented becomes irrelevant. Thus, a large number of candidate effector cells can be modified to be retargeted. In addition, the polypeptides of the invention have at least comparable bioavailability and half-life to monoclonal antibodies.
In preferred embodiments (a) the first set of 6 CDRs is comprised in a first scFv fragment; and/or (b) (ba) said second set of 6 CDRs is comprised in a second scFv fragment; alternatively (BB) the ligand is an immunological ligand, preferably capable of binding to NKG2D/CD314 (e.g.ligand MICA, MICB, ULBP1-6), NKp30/NCR3/CD337 (e.g.ligand B7-H6), 4-1BB/CD137 (e.g.ligand 4-1BB-L/CD137L) or OX40/CD134 (e.g.ligand OX40-L/CD 252). Slashes ("/") separate different designations in the art. Representative of a given antigen species are provided in parentheses.
The term "scFv" is well known in the art. The abbreviation stands for "single-chain variable fragment" of an antibody and defines a polypeptide capable of specifically recognizing and binding an epitope of an antigen. As described above, the variable domain (V) of the antibody light chainL) Presents 3 CDRs, and the variable domain of an antibody heavy chain (V)H) 3 CDRs are presented. In scFv two variable domains are linked to each other by a peptide linker. The resulting fusion construct is a single polypeptide chain. This provides for easy expression of scFv molecules. The schematic view can be seen in fig. 1.
The term "VHDomains "and" VLDomain "is used according to the definitions given in the art. Thus, they refer to the variable regions of the heavy chain of immunoglobulins (V)H) And light chain variable region (V)L). In general, VHAnd VLThe domains each comprise 3 Complementarity Determining Regions (CDRs), where CDRs are highly variable regions primarily responsible for antigen binding.
Peptide linkers are preferably used to link the variable region of the scFv or to link the scFv to a dimerization and/or spacer region, preferably to the Fc. Typically the peptide linker has a length of 3 to 30 amino acids, preferably 5 to 25 or 10 to 20 amino acids long. This is preferredDoes not affect, or does not substantially affect, the structure and/or function of the linked domains or polypeptides (the linking results in a single, continuous polypeptide chain). Linkers include Gly-rich linkers such as (Gly)4Ser)3(SEQ ID NO:47) linker for use with preferred polypeptides of the invention to connect V of CTL or NK specific scFvH/VLA domain; and Yol linker (SEQ ID NO: 48; AKTTPKLEEGEFSEARV, as described in Sellrie et al, Journal of Biochemistry and molecular Biology, Vol.40, No.6, November 2007, pp.875-880), for use in a preferred polypeptide of the invention to link V of scFv specific for HBV surface antigenH/VLA domain. Also (Gly)4Ser)4Linker (SEQ ID NO:49) useful for linking V of scFv specific for HBV surface antigenH/VLA domain.
The term "antibody" as used herein has its art-known meaning. Preferably, it refers to monoclonal antibodies. By, for example, initially atMonoclonal antibodies are prepared by techniques described in Milstein, Nature 256(1975),495 and Galfr, meth.enzymol.73(1981),3, which involve the fusion of mouse myeloma cells to spleen cells derived from immunized mammals, with modifications developed in the prior art. In addition, the aforementioned Antibody or fragment thereof against HBV surface protein can be obtained by a method described in, for example, Harlow and Lane "Antibody, A Laboratory Manual", CSH Press, Cold Spring Harbor, 1988. The production of chimeric antibodies is described, for example, in WO 89/09622. A further source of antibodies for use in the present invention are so-called xenogeneic antibodies. The general principle of generating xenogenous antibodies, such as human antibodies in mice, is described in, for example, WO 91/10741, WO 94/02602, WO 96/34096 and WO 96/33735. The antibodies to be used in the present invention or their corresponding immunoglobulin chains may be further modified using conventional techniques known in the art, for example, by using amino acid deletions, insertions, substitutions, additions, and/or recombinations and/or any other modification known in the art (alone or in combination). In the formation of immune ballsMethods for introducing such modifications into the DNA or polypeptide sequence of the amino acid sequence of a protein chain are well known to those skilled in the art; see, e.g., Sambrook, Molecular Cloning, Laboratory Manual, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, 1989. Modifications of polypeptides also include post-translational modifications such as glycosylation.
In a further preferred embodiment, the first set of 6 CDRs binds an epitope of the first antigen that is located: (a) the HBV small surface antigen; or (b) a portion of said HBV large surface antigen not comprised by said HBV small surface antigen; or (c) in a portion of said HBV large surface antigen which is structurally altered from said HBV small surface antigen.
Item (a) refers to an epitope present in the HBV small surface antigen. Based on the above-described relationship between the small, medium and large HBV surface antigens, the entire sequence of the small antigen is contained in the medium and large antigen. In general (but not necessarily), the three-dimensional epitopes presented by small surface antigens will also be presented by the medium and/or large surface antigens.
For item (b), it is preferred that said portion of said HBV large surface antigen is also not comprised by said HBV medium surface antigen. For item (c), it is understood that "structural alteration" comprises an epitope of the HBV large surface antigen comprising or consisting of a sequence that is part of the HBV small surface antigen sequence, wherein said epitope is not present on the HBV small surface antigen. For item (c), it is further preferred that the epitope is a portion of the HBV large surface antigen which also differs in structure from the HBV surface antigen.
Said item (a), i.e. said first antigen (being said HBV small surface antigen), is particularly preferably in combination with all aspects and embodiments of the present invention.
According to items (b) and (c), the polypeptide will specifically recognize the large surface antigen of HBV.
In a further preferred embodiment, said surface antigen presented by immune effector cells is selected from the group consisting of CD3, CD28, 4-1BB, OX40, CD16, CD56, NKG2D, and NKp30/NCR 3. Accordingly, the present invention provides a polypeptide comprising (a) a first set of 6 Complementarity Determining Regions (CDRs) configured to bind a first antigen; and (b) (ba) a second set of 6 CDRs configured to bind a second antigen; or (bb) a ligand capable of binding a second antigen; wherein (i) the first antigen is selected from the group consisting of Hepatitis B Virus (HBV) small surface antigen; surface antigens in HBV; and HBV large surface antigen; and (ii) the second antigen is selected from a surface antigen presented by immune effector cells such as Natural Killer (NK) cells and Cytotoxic T Lymphocytes (CTLs), wherein (c) the 6 CDRs of the first set are comprised in a first scFv fragment; and (d) (da) the second set of 6 CDRs is comprised in a second scFv fragment; or (db) the ligand is an immunological ligand capable of binding NKG2D such as the ligands MICA, MICB, ULBP 1-6; NKp30 such as ligand B7-H6, 4-1BB such as ligand 4-1 BB-L; or OX40 such as ligand OX 40-L; and wherein the surface antigen presented by the immune effector cell is selected from the group consisting of CD3, CD28, 4-1BB, OX40, CD16, CD56, NKG2D, and NKp 30.
CD3 represents the CD3 epsilon-chain, which is part of the CD 3-T-cell receptor complex. (Borst, J. et al, the delta-and epsilon-chains of the human T3/T-cell receptor complex polypeptides, Nature.1984.312: 455-458).
CD28 is the major T cell co-stimulatory receptor. (Lesslauer, W. et al, T90/44(9.3 antigen.) Acell surface mobile with a function in human T cell activation Eur.J. Immunol.1986.16: 1289-1296).
4-1BB (CD137) is a co-stimulatory receptor for activated T cells and NK cells. (Kwon, B.S. et al, cDNAsequences of two inductor T-cell genes. Proc. Natl. Acad. Sci. U.S. A.1989.86: 1963-.
OX40(CD134) is a secondary costimulatory receptor. (Arch, R.H. et al, mol.cell.biol.1998.18: 558-565). 4-1BB and OX40 are members of the Tumor Necrosis Factor (TNF) receptor family, which bind TNF receptor-associated ligands and activate the nuclear factor kappa B.
CD16(FcgRIIIa) is a low affinity Fc receptor expressed by NK cells (a subset of activated cytotoxic T cells) and by cells from the myeloid monocyte lineage that binds the Fc domain of IgG molecules. (Lanier, L.L. et al, Functional properties of a unique subset of cytoxic CD3+ Tlympcytes that express Fc receptors for IgG (CD16/Leu-11antigen). J.Exp.Med.1985.162: 2089-.
CD56(NCAM) is a cell adhesion molecule expressed by NK cells. (Lanier, L.L.et al, Identityof Leu-19(CD56) Leu differentiation antigen and neural cell addition molecule. J.Exp.Med.1989.169: 2233-.
NKG2D is an activating receptor expressed by NK cells (Houchins, J.et al, DNA sequencing analysis of NKG2, a family of related cDNA clones encoding type II integrin ligand cell 1991.J.Exp.Med.173:1017 cells 1020).
NKp30(NCR3) is a receptor expressed by NK cells (Pende, D.et al., Identification and molecular characterization of NKp30, a novel triggerering receptor involved in cellular cytotoxicity mediated by human neural killers.2000.J.Exp.Med.192: 337-346).
CD3, CD28, 4-1BB and OX40 were present on the surface of CTL. Binding of the polypeptides of the invention to any of these surface antigens is involved in activation or co-activation of CTLs.
CD16, CD56, NKG2D, NKp30/NCR3 and 4-1BB are present on the surface of NK cells. Binding of the polypeptides of the invention to any of these surface antigens is involved in the activation or co-activation of NK cells.
For human CTLs, CD3 and CD28 are preferred. For human NK cells, CD16 and CD56 are preferred.
The surface antigens mentioned are named according to names known in the art (see Kenneth Murphy, Janeway's immunology, 7)thedition,Garland Science;William E.Paul,FundamentalImmnology,7thedition,Lippincott Williams&Wilkins)。
In a further preferred embodiment, the polypeptide further comprises a dimerization domain. The dimerization domain may provide covalent and/or non-covalent dimerization.
By dimerizing, the bispecific bivalent antibody becomes bispecific tetravalent (or even tetraspecific tetravalent if different bispecific antibodies are co-expressed in the producer cell). The bispecific tetravalent agents described herein are expected to have enhanced avidity similar to conventional monospecific antibodies, since they are able to engage two antigen molecules with their N-terminal side and their C-terminal side, respectively.
In a particularly preferred embodiment, said dimerization region connecting two polypeptides of the invention consists of the hinge region of an IgG heavy chain or comprises cysteine residues responsible for the dimerization of the antibody heavy chains. Preferably, the dimerisation domain consists of a subsequence of 32 amino acids in length, the so-called hinge region of the heavy chain (EPKSSDKTHT)CPPCPAPEFEGAPSVFLFPPKP, see SEQ ID NOs 43-46), and contains two cysteine residues (underlined in the above sequence) responsible for heavy chain dimerization. Preferably, a single cysteine within the IgG heavy chain hinge region, which mediates the intermolecular disulfide bond between the IgG heavy and light chain constant domains in the native antibody, is mutated to serine in order to prevent aberrant disulfide bridges.
Dimerization domains suitable for non-covalent dimerization are known in the art and include leucine zippers.
In a further preferred embodiment, the polypeptide further comprises a spacer region, preferably comprising a CH2 domain and a CH3 domain, located between: (i) the first scFv fragment and (ii) the recombinant ligand in the amino acid sequence of the second scFv fragment or the polypeptide.
It is advantageous to include or consist of a CH2 domain and a CH3 domain (in particular from IgG). Their ability to bind protein a provides for sufficient secretion from the producer cell and/or subsequent purification from the reagent.
The CH2 and CH3 domains on the one hand and the dimerisation domain on the other hand may be provided by corresponding regions of IgG, preferably IgG1 or IgG2 molecules, more preferably human IgG1 or lgG2(hIgG1, hlgG2) molecules. Preferred subsequences of hIgG1 molecules to provide the CH2 domain, CH3 domain and dimerization domain can be found in sequences 43 to 46. Preferably (and this applies to the sequences mentioned) parts of hIgG1 (in particular the CH2 domain) are mutagenized at various positions in order to reduce or eliminate binding to Fc receptors (indicated in bold italics in the sequences given further below). More generally, the Fc region, particularly the CH2 domain and/or the CH3 domain, may be mutated at one or more positions to reduce or eliminate binding to an Fc receptor. This process is known in the art and is described, for example, in Armour et al, Recombinant human IgG molecules binding and hybridizing activity microorganisms Eur.J.Immunol.1999.29: 2613-. This is advantageous because it is preferred in the present invention that antibody-dependent cell-mediated cytotoxicity (ADCC) is not induced.
In other words, antibody Fc fragments can be used to achieve the spacer region and dimerization region. The term "Fc fragment" is known to those skilled in the art and defines an IgG fragment obtained by papain cleavage and comprising the CH2 and CH3 domains.
Between the first scFv fragment and the spacer region and/or between the spacer region and the second scFv fragment there is (a) a linker sequence. Preferred linker sequences are disclosed above. As can be seen from the preferred sequences contained in the sequence listing (in particular the sequences SEQ ID NO:43 to 46), such linker sequences may consist of glycine or of glycine and serine.
FIG. 2 shows the molecular framework of a preferred polypeptide of the invention comprising a dimerization region (hIgG hinge region) and CH2 and CH3 regions separating the two scFv fragments from each other.
The terms "CH 2 domain" and "CH 3 domain" have meanings known in the art. They refer to the second and third constant domains of the antibody heavy chain.
It will be appreciated that a particularly preferred embodiment relates to a polypeptide comprising (a) a first set of 6 Complementarity Determining Regions (CDRs) configured to bind a first antigen; and (b) (ba) a second set of 6 CDRs configured to bind a second antigen; or (bb) a ligand capable of binding a second antigen; wherein (i) the first antigen is selected from the group consisting of Hepatitis B Virus (HBV) small surface antigen; surface antigens in HBV; and HBV large surface antigen; and (ii) the second antigen is selected from a surface antigen presented by immune effector cells such as Natural Killer (NK) cells and Cytotoxic T Lymphocytes (CTLs), wherein (c) the 6 CDRs of the first set are comprised in a first scFv fragment; and (d) (da) the second set of 6 CDRs is comprised in a second scFv fragment; or (db) the ligand is an immunological ligand, preferably capable of binding NKG2D such as the ligands MICA, MICB, ULBP 1-6; NKp30 such as ligand B7-H6; 4-1BB, e.g., ligand 4-1 BB-L; or OX40 such as ligand OX40-L, wherein the surface antigen presented by an immune effector cell is selected from the group consisting of CD3, CD28, 4-1BB, OX40, CD16, CD56, NKG2D, NKp30 and 4-1BB, and wherein the polypeptide further comprises a dimerization region and a spacer region, preferably as further defined above.
In a further preferred embodiment, (a) the first set of 6 CDRs has the sequence of SEQ ID NOs 1-6, 7-12, or 13-18; and/or (b) the second set of 6 CDRs has the sequence of SEQ ID NOs 19-24, 25-30, 31-36, or 37-42.
As is common in the art, and as indicated by the appended sequence listing, the order of the CDRs in the 6 CDRs of each set, as described above, is as follows: the CDR1 of the heavy chain, CDR2 of the heavy chain, CDR3 of the heavy chain, CDR1 of the light chain, CDR2 of the light chain, and CDR3 of the light chain.
C8, 5F9, 5a19, OKT3, 9.3, a9 and NCAM29.2 as used in the sequence listing each indicate from which antibody each CDR originates, and refer to a preferred anti-HBs antibody, a second different anti-HBs antibody, an antibody against HBV large surface antigen, an antibody against human CD3, an antibody against human CD28, an antibody against human CD16 and an antibody against human CD56, respectively. "HBs" means HBV small surface antigen.
It is particularly preferred that the polypeptide comprises or consists of the amino acid sequence: any one of the amino acid sequences of SEQ ID NOS 43 to 46 or an amino acid sequence exhibiting at least 80% identity to any one of the amino acid sequences of SEQ ID NOS 43 to 46, with the proviso that the CDRs of the amino acid sequence exhibiting at least 80% identity to any one of the amino acid sequences of SEQ ID NOS 43 to 46 are identical to the CDRs contained in any one of SEQ ID NOS 43 to 46, respectively. In SEQ ID NO 43, the last three residues "GNS" are optional.
Preferred levels of sequence identity include at least 85%, at least 90%, at least 91%, at least 92%, at least 93%, at least 94%, at least 95%, at least 96%, at least 97%, at least 98%, and at least 99%. Measures and methods for determining sequence identity are well known in the art. A preferred algorithm for determining pairwise sequence identity is the Basic Local Alignment Search Tool (BLAST), as described, for example, in McGinnis and Madden (nucleic acids Research 32, W20-W25 (2004)).
The position of the CDRs in a given sequence (i.e., in the present case in the sequences of SEQ ID NOS: 43 to 46) can be determined by methods known in the art, including systems of Chothia, Kabat, and LeFranc/IMGT, respectively. Without any statement to the contrary, it is understood that the CDRs in the particularly preferred embodiments described above are those defined above, i.e. a first set having the sequence of SEQ ID NOs 1 to 6, 7 to 12 or 13-18 and a second set having the sequence of SEQ ID NOs 19 to 24, 25 to 30, 31 to 36 or 37 to 42. These particular CDR sequences (underlined in the sequences given below) are indeed comprised in the sequences of SEQ ID NO 43 to 46, as indicated by the sequences comprised in the attached sequence Listing.
The sequences of SEQ ID NO 1 to 6 define the CDRs, while SEQ ID NO 37 to 40 define bispecific polypeptides capable of binding to specific epitopes within the small surface antigen of HBV. This epitope is located in the a-epitope, which is exposed on the surface of infected cells and virions, respectively. The term "a-epitope" is used to denote the region within the small surface antigen of HBV in which the major epitopes for inducing a protective humoral immune response are located. These CDRs, as well as the polypeptides of SEQ ID NO 43 to 46, have advantages in that they can be used for all HBV serotypes.
In a second aspect, the invention provides a nucleic acid encoding the above polypeptide. Preferred embodiments of the polypeptides lead to corresponding preferred embodiments of the nucleic acids.
The term "nucleic acid" has a meaning known in the art and is not particularly limited. Preferred are DNA such as genomic DNA or cDNA and RNA such as mRNA. Although not preferred, the use of nucleotide derivatives including 2 'derived nucleotides such as 2' methyl nucleotides is also contemplated; peptide nucleotides present in peptide nucleic acids and the like.
In a third aspect, the present invention provides a covalently linked complex comprising or consisting of a first and a second polypeptide, wherein at least one covalent link between said first and said second polypeptide, preferably at least one disulfide bridge between a Cys residue of said first polypeptide and a Cys residue of said second polypeptide, is provided, said first and second polypeptides being as defined herein.
Preferably there are two covalent linkages, preferably two disulfide bonds as shown in figure 2, between said first and said second polypeptide.
Also provided are complexes comprising or consisting of a first and a second polypeptide, wherein the first and the second polypeptide are non-covalently bound to each other.
An exemplary diagram of such a covalently linked complex is shown in figure 2. Preferably the complex is a dimer.
In a fourth aspect, the present invention provides a composition comprising or consisting of: one or more polypeptides of the invention and/or one or more complexes of the invention, with the proviso that the composition comprises at least two polypeptides which differ from each other in the first antigen and/or the second antigen to which they bind.
In a preferred embodiment of the fourth aspect, the two polypeptides are (a) (i) a polypeptide that binds HBV small or large surface antigen and CD 3; and (ii) a polypeptide that binds to HBV small or large surface antigen and CD 28; or (b) (i) a polypeptide that binds to HBV small or large surface antigen and CD 16; and (ii) a polypeptide that binds to HBV small or large surface antigen and CD 56.
The selections (a) and (b) of this preferred embodiment provide very high elimination rates up to 95% (in particular to the extent that they are related to polypeptides binding to HBV small surface antigens) compared to the negative control. This is expected to provide complete eradication of HBV infected cells or HBV-antigen positive tumor cells, especially after repeated use in an in vivo situation.
It has been found that the combined use of bispecific molecules that bind two different CTL markers or NK markers provides a synergistic effect. Figures 3 and 4B show a comparison of specific target cell lysis following administration of bispecific constructs.
In a particularly preferred embodiment, the two polypeptides comprise or consist of the following sequences: (a) 43 and 44; or (b) SEQ ID NOS: 45 and 46.
Each of the sequences of SEQ ID NO 43 to 46 will allow the formation of two disulfide bridges when forming homodimers. In view of this, it is considered that heterodimers are also formed. An example of a heterodimer would be a complex of two polypeptides of the invention covalently linked, where the first polypeptide would bind to a first marker present on HBV surface antigen and immune effector cells, and the second polypeptide would bind to a second marker present on HBV surface antigen and immune effector cells. Two of the markers of immune effector cells may be, for example, CD3 and CD28, or CD16 and CD 56.
In a further aspect, the present invention provides a pharmaceutical composition comprising or consisting of: one or more polypeptides of the invention, one or more complexes of the invention and/or one or more compositions of the invention.
The pharmaceutical composition may further comprise a pharmaceutically acceptable carrier, excipient and/or diluent. Suitable examples of pharmaceutical carriers, excipients, and/or diluents are well known in the art and include phosphate buffered saline solutions, water, emulsions such as oil/water emulsions, various types of wetting agents, sterile solutions, and the like. Compositions containing such carriers may be formulated by conventional methods well known in the art. These pharmaceutical compositions may be administered to a subject in a suitable dosage. Administration of suitable compositions can be achieved in different ways, for example, by intravenous, subcutaneous or oral administration, preferably these three options, and additionally by intraperitoneal, intramuscular, topical, intradermal, intranasal, or intrabronchial administration. Formulations for oral administration include tablets and syrups. It is particularly preferred to carry out the administration by injection. The composition may also be applied directly to the target site, for example by gene gun delivery to an internal or external target site. The dosage regimen will be determined by the attending physician and clinical factors. It is well known in the medical arts that administration to any one patient depends on many factors. Including the size of the patient, the body surface area, age, the particular compound to be administered, sex, time and route of administration, general health, and other drugs administered concurrently. The protein pharmaceutical active may be present in an amount of 1ng to 10mg/kg body weight per dose; however, dosages below or above the ranges of this example are also contemplated, particularly in view of the above factors. If the regimen is continuous perfusion, it should also be in the range of 1. mu.g to 10mg units per kg body weight per minute.
Intravenous administration is particularly preferred.
In a further aspect, the invention provides the use of one or more polypeptides of the invention, one or more complexes of the invention and/or one or more of any one of the compositions of the invention in a method for the treatment or prevention of an HBV infection and/or a condition caused by said HBV infection, said condition caused by HBV infection being selected from cirrhosis, hepatocellular carcinoma, and liver cancer, said liver cancer being characterized by expression of one or more HBV surface antigens. Preferably the hepatocellular carcinoma is characterized by expression of one or more HBV surface antigens as defined above.
In a further aspect, the present invention provides a method for the treatment or prevention of an HBV infection and/or a condition caused by said HBV infection, said condition caused by said HBV infection being selected from cirrhosis and hepatocellular carcinoma, said method comprising administering to a patient in need thereof a therapeutically or prophylactically effective amount of one or more polypeptides of the invention, one or more complexes of the invention and/or one or more compositions of the invention, respectively.
Preferably the pharmaceutical composition, the polypeptide/complex/composition for use in a method of treatment and the method of treatment, the polypeptide, complex and/or composition referred to is the only pharmaceutically active substance contained therein or used.
Such other pharmaceutically active substances may be selected from interferons or other immunomodulators (such as, for example, interferon- α 2a or 2B, interferon- λ), direct acting antiviral agents such as nucleotide (nucleoside) analogues (such as, for example, lamivudine (Epivir-HBV, Zeffix or hepcodin), adefovir dipivoxil (hepera, Preveon), entecavir (Baraclude, Entaliv), telbivudine (Tyzeka, Sebivo), tenofovir (Viread)), entry inhibitors (such as, for example, myrlux-B), other antiviral agents, or cytokines such as interleukin-2.
In a further aspect, the invention provides an in vitro method of killing HBV infected cells, the method comprising culturing said HBV infected cells with: (i) immune effector cells and (ii) one or more polypeptides of the invention, one or more complexes of the invention and/or one or more compositions of the invention.
In a preferred embodiment of the in vitro method, the immune effector cells are (i) contained in peripheral blood mononuclear cells; or (ii) is or comprises NK cells and/or CTLs.
In a further aspect, the invention provides an in vitro or ex vivo immune effector cell having a polypeptide of the invention or a complex of the invention bound to a surface antigen of said immune effector cell. Preferred immune effector cells and preferred immune effector cells present surface antigens as described above. Such immune effector cells may be used for administration to a patient suffering from HBV infection, cirrhosis or hepatocellular carcinoma. Thus also provided is a pharmaceutical composition comprising or consisting of: an immune effector cell that binds a polypeptide of the invention or a complex of the invention to its surface antigen. Also provided are methods of using immune effector cells that bind a polypeptide of the invention or a complex of the invention to a surface antigen thereof for the treatment or prevention of HBV infection, cirrhosis, or hepatocellular carcinoma.
Sequences disclosed in the present application
Drawings
The figures illustrate the invention.
FIG. 1 scFv fragments obtained by fusion of two variable domains. Fusion involves the use of flexible peptide linkers that do not affect, or do not substantially affect, the structure of each variable domain.
FIG. 2 two polypeptides of the invention dimerize by forming disulfide bonds. Each polypeptide comprises a bispecific bivalent antibody. Dimerization of the natural antibody in the endoplasmic reticulum of the producer cell may result in the formation of a bispecific tetravalent antibody, or a tri-or tetra-specific, tetravalent antibody if two bispecific bivalent antibodies are co-expressed (not shown).
FIG. 3 is a graph comparing the synergistic effect of specific elimination of hepatoma target cells producing HBV surface antigen after using a single bispecific antibody with the simultaneous administration of two CTL-specific or two NK cell-specific bispecific antibodies. The CellTiter-Blue cell viability assay was used.
FIG. 4A) cytokine secretion indicates activation of immune effector cells in the presence of bispecific antibodies of the invention. HBV infected HepaRG cells were co-cultured with PBMCs in the presence or absence of the indicated bispecific antibody. B) Specific elimination of HBV-infected target cells co-cultured with immune effector cells and bispecific antibodies.
FIG. 5 viability of target cells co-cultured with PBMC in presence of a single HBs-active bispecific antibody A, C, E effects of stimulation with α HBs x α CD3(A), α HBs x α CD28 (C) or summarized (E). B, D, F effects of stimulation with α HBs x α CD3[ Fc. DELTA. ADCC ] (B), effects of stimulation with α HBs x α CD28[ Fc. DELTA. ADCC ] (D) or summarized (F). arrow indicates addition of PBMC and bispecific antibody.dotted line represents HBs-transfected HuH7-S cells, curve with diamonds represents HuH7 parental hepatoma cells.xCELLIGEN real-time cytotoxicity assay was used.normalized time: 0 h.
FIG. 6 viability of target cells co-cultured with PBMC in presence of HBs-active bispecific antibody combination of bispecific antibodies mediates large scale killing of target cells A: effect of stimulation with α HBs x α CD3 and α HBs x α CD 28B: effect of stimulation with α HBs x α CD3[ Fc. DELTA. ADCC ] and α HBs x α CD28[ Fc. DELTA. ADCC ] C, D: effect of single bispecific antibody compared to combination arrow indicates addition of PBMC and bispecific antibody dotted line represents HuH7-S cells, curve with diamonds is HuH7 cells normalized time of cell index: 0 h.
FIG. 7 viability of target cells co-cultured with PBMC in the presence of different concentrations of bispecific antibody mixture α HBs x α CD3/α HBs x α CD28(A), or α HBs x α CD3[ Fc. DELTA. ADCC ]/α HBsx α CD28[ Fc. DELTA. ADCC ] (B) of antibody-containing supernatant induced lysis of target cells earlier than 25. mu.l/25. mu.l mixture, indicating dose dependent effects.
FIG. 8 viability of target cells co-cultured with varying amounts of PBMC in the presence of a mixture of α HBs x α CD3 and α HBs x α CD28 2x105PBMC mediation ratio 1x105Significantly earlier HuH7-S with PBMCAnd (4) eliminating the cells. Arrows indicate addition of PBMC and bispecific antibody. The dashed line represents the HuH7-S cell, the curve with diamonds is the HuH7 cell. Normalization time of cell index was 0 h.
FIG. 9: viability of target cells co-cultured with PBMCs in the presence of α HBs x α CD3/α HBs x α CD28 mixtures for different time periods after stimulation for a given time period, removal of supernatant containing bispecific antibody.4 h stimulation resulted in only a small decrease in target cell viability (end point viability of 78.5%), PBMCs induced elimination of target cells with bispecific antibody stimulation for 8h or longer after 8h and 12h stimulation, killing of target cells delayed compared to 24h or 48h stimulation indicating continuous activation of effector cells also retargeted, however, HuH7-S end point viability at 48h was comparable 8h stim.: 14.7%; 12h stim 11.7%, 24h stim.: 5.1%, 48h stim.: 3.2%; arrows indicate PBMCs and time of cell viability normalized by HuH 7-S.
FIG. 10 IL-2, IFN- γ and TNF- α secretion from PBMCs after coculture with HuH7-S/HuH7 cells in the presence of α HBs x α CD3/α HBs x α CD28 at various time points, IL-2 concentration increased over time and reached a plateau at a concentration of about 1550pg/ml at about 24h IFN- γ secretion started between 8h and 12h and increased to 12000pg/ml (48 h). production of C: TNF- α was detectable after 4h, continued to increase, peaked at 24h (1700/ml) and decayed to 1400pg/ml after 48h, high background TNF- α secretion was detectable in the absence of HBs (HuH7 cells), and the highest concentration (-70/ml) after 4h decreased to 9pg/ml after 48h coculture.
FIG. 11 LAMP-1 staining after co-culture of PBMC with HuH7-S/HuH7 cells in the presence of bispecific antibody at α HBs x α CD3/α HBs x α CD28(A, C) or α HBs x α CD3[ Fc. DELTA. ADCC]/αHBs xαCD28[FcΔADCC](B, D) in the presence of HuH7-S (black line) and HuH7 (gray line) cells, and then in CD4+(A, B) and CD8+(C, D) surface expression of the inclusion body degranulation marker LAMP-1 was detected on T cells.
FIG. 12 PBMC with PBMC at 8h, 12h and 24h in the presence of α HBs x α CD3/α HBs x α CD28FACS analysis of co-cultures of HuH7-S or HuH7 cells. A, B; IFN gamma+/IL-2+/TNFα+/CD154+CD4+T cell (A) or IFN gamma+/IL-2+/TNFα+/CD154+CD8+(B) Percentage of T cells. C, D IFN gamma+,IL-2+And/or TNF α+CD4+(C) Or IFN gamma+,IL-2+And/or TNF α+CD8+(D) Boolean combinatorial gate of T cells.
FIG. 13-HBs x α CD3[ Fc. DELTA. ADCC at α]/αHBs xαCD28[FcΔADCC]24h and 48h, FACS analysis of PBMC co-cultured with fixed or soluble HBsAg. A, B; IFN gamma+/IL-2+/TNFα+/CD154+CD4+T cell (A) or IFN gamma+/IL-2+/TNFα+/CD154+CD8+(B) Percentage of T cells. C, D IFN gamma+,IL-2+And/or TNF α+CD4+(C) Or IFN gamma+,IL-2+And/or TNF α+CD8+(D) Boolean combinatorial gate of T cells.
FIG. 14 HBsAg in supernatants of HuH7-S cells (110.8S/CO), HepG2.2.15 cells (41.7S/CO) and HBV infected HepaRG cells (16.5S/CO).
FIG. 15 viability of HBV infected/uninfected HepaRG cells co-cultured with PBMC in the presence of bispecific antibody α HBs x α CD3(A) and α HBs x α CD3/α HBs x α CD28(B) mediated significant target cell lysis end point viability of untreated cells 65.9% (HBV +) and 62.9% (HBV-). arrows indicate addition of PBMC and bispecific construct dotted line represents HBV infected HepaRG cells, curves with diamonds are uninfected HepaRG cells normalized time of cell index in xCELLigence assay 0 h.
FIG. 16 reduction of tumor size in animals treated with bispecific antibody mice bearing HBV-positive subcutaneous HepG2.2.15 tumors were treated with human PBMC and a mixture of α HBs x α CD3 and α HBs x α CD28 bispecific antibody for four consecutive days.
The examples illustrate the invention.
Example 1
Materials and methods for example 2
Cloning and production of bispecific antibodies
Complementary DNA encoding the variable heavy and variable light chains of anti-CD 3(OKT3), anti-CD 28(9.3), anti-CD16 (a9) and anti-CD 56(NCAM29.2) were obtained by PCR amplification from reverse transcribed mRNA of each hybridoma, using a set of primers encompassing all VH and Vk/Vl subtypes. The PCR products were ligated into pCR2.1-TOPO (Invitrogen, Life Technologies) and sequenced. anti-HBsAg scFvC8 is provided in codon-optimized form in plasmid pMP 71-C8. The variable heavy and variable light chain cdnas encoding the above antibodies were combined into scFvs with glycine-serine linkers using primers containing appropriate restriction sites on the 5 'and 3' sides. OKT3, 9.3, A9 and NCAM29.2scFvs (N-terminal extended (Gly)3-4) The 3 'end of the cDNA presented in pBluescript KS II + (Stratagene) cloned into the Fc domain (hinge, CH2, CH3) encoding human IgG1 was extended at the 5' end with the glycine-serine linker GlyAsnSer (Gly4Ser)3AlaSier and StrepTag sequences (WSHPQFEK), and in the second series of constructs, it was extended at the 3' end by an additional glycine-serine linker (Gly4Ser)3. The C8scFv coding sequence was cloned 5 'to the 5' glycine-serine linker. The complete scFv-linker-hIgG 1 Fc-linker-scFv sequence was subcloned into the mammalian expression vector pcDNA3.1(-) (Invitrogen). HEK293 cells were transfected with Maxi-prep plasmid DNA using peqFECT transfection reagent (Peqlab). Stable transfectants were selected and expanded using 0.8-1.0mg/ml G418. Supernatants from HEK transfectants were collected and analyzed for the concentration of secreted bispecific antibody by ELISA and the integrity of secreted antibody by western blot (using goat anti-human IgG-Fc specific, peroxidase labeled antibody.
Cell culture conditions and HBV infection
HuH7 hepatoma cells (Nakabayaski, et al 1982.Growth of human hepatoma cells with differentiated functions in chemically defined medium. cancer Res.42:3858-3863) and HEK293 cells were stored in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS), penicillin (100U/mL), streptomycin (100. mu.g/mL), and L-glutamine (2mmol/L) (all from GIBCO, Life Technologies).
Peripheral Blood Mononuclear Cells (PBMCs) were isolated from heparinized whole blood by density gradient centrifugation using LSM1077 lymphocyte isolation medium (PAA). 25ml of blood was layered over 13ml of LSM 1077. After centrifugation at 2000rpm for 20min at room temperature (without pause), PBMC were harvested and cultured in RPMI 1640 medium supplemented with 10% Fetal Bovine Serum (FBS), penicillin (100U/mL), streptomycin (100. mu.g/mL), and L-glutamine (2mmol/L) (all from GIBCO). After the overnight resting step, PBMC or sorted NK cells were used for co-culture experiments.
The HepaRG cells were maintained in Williams E medium (Invitrogen GmbH, Karlsruhe, Germany) supplemented with L-glutamine (5mmol/L), glucose (0.06% [ wt/vol ]), HEPES (23mmol/L, pH7.4), gentamicin (50. mu.g/ml), penicillin (50I U/ml), streptomycin (50. mu.g/ml), inosine (37. mu. mol/L), hydrocortisone (4.8. mu.g/ml), and insulin (1. mu.g/ml). HepaRG cells were differentiated for 4 weeks using differentiation medium (Williams E medium (described above) supplemented with DMSO (1.75%)) prior to infection. HepaRG cells were infected in differentiation medium with final m.o.i.200 HBV mother liquor and PEG (5%). After overnight incubation the infecting inoculum was removed and replaced with differentiation medium and cultured for 6 days. For co-culture with redirected T cells, we exchanged the differentiation medium for a cortisol-free medium two days before starting the co-culture to avoid cortisol-mediated immune suppression.
Transfection with HBV surface antigen-encoding plasmids
HuH-7 cells were transfected with plasmids encoding various surface antigens using FuGene transfection reagent (Promega). For 8 wells of a 96 well plate, 3. mu.l of FuGENE, 1. mu.g of plasmid DNA were added to 100. mu.l of OptiMEM (Gibco). The transfection solution was incubated at room temperature for 15min to allow binding of FuGENE to the plasmid DNA. After adding additional OptiMEM and incubating for at least 24 hours, a final volume of 100 μ Ι per well was applied.
Magnetically Activated Cell Sorting (MACS) of NK cells
Using human CD56+CD16+NK cell isolation kit (Miltenyi) NK cells were isolated from PBMC. In the first negative screening step, all non-NK cells were removed by monoclonal antibodies directed against antigens not expressed on the NK cell surface. In a second positive screening step, NK cells were isolated by monoclonal CD16 antibody conjugated to iron oxide microbeads and retained in a magnetic field. After isolation, the NK cells were cultured in RPMI-1640 medium as described above.
Co-culture of HBV-positive target cells and redirected effector cells
Target cells were cultured in 96-well plates at full confluency. Will be 1x105To 100. mu.l of medium per well volume. Mu.l HEK supernatant containing bispecific antibody was applied to each well. To determine the synergistic effect, 50 μ l of each bispecific antibody supernatant was added to each well. Untreated target cells co-cultured with 200. mu.l of medium or with effector cells only or bispecific antibody only were used as negative controls.
Enzyme-linked immunosorbent assay (ELISA) for effector cell activation
Cytokine secretion caused by effector cell activation was detected by ELISA. Use of human IFN-gamma ELISAMAXTM(BioLegend). The absorption at 450nm was detected using the programs Magellan6 and InfiniteF200 (Tecan).
Target cell viability assay
Cell Titer-Blue cell viability assay (Promega) was used to determine target cell viability after co-culture. This assay is based on the ability of living cells to convert redox dyes (Resazurin) to fluorescent end products (resorufin) due to metabolic activity. Non-viable cells rapidly lose their metabolic capacity and do not produce a fluorescent signal. After removing the supernatant, 100. mu.l of colorless DMEM (containing 20% of cell Titer-Blue reagent) per well was added to the co-culture and incubated at 37 ℃ for 2 hours. Fluorescence signals were recorded at 569nm using InfiniteF200 (Tecan).
Example 2
Results
In the first line of experiments, we evaluated the activity of bispecific antibody constructs against CTL surface antigens CD3 and CD28 and against NK cell surface antigens CD16 and CD 56. We used plasmid transfected hepatoma cell lines producing HBV surface antigen. After HBV protein expression is established, these target cells are co-cultured with immune effector cells (i.e., PBMCs and isolated NK cells) and bispecific antibody constructs. PBMC contain approximately 70% T cells but only 7% NK cells. Thus, we magnetically separated CD16+CD56+NK cells. As a negative control we analyzed co-cultures with HBV-negative target cells, which had been pre-incubated with supernatants containing HBV and subviral particles. We used this control to exclude activation of effector cells due to non-specific binding of HBV particles on the surface of HBV-negative target cells. In addition, we co-cultured HBV-positive target cells with immune effector cells (in the absence of bispecific constructs) in order to assess non-specific background cytotoxicity. To rule out the cytotoxic effect of the bispecific constructs, we prepared HBV-positive target cells without immune effector cells in the presence of the bispecific constructs.
These experiments showed specific activation of CTL upon co-cultivation in the presence of CD 3-or CD 28-specific constructs, as determined by secretion of the pro-inflammatory cytokine interferon-g (IFN-g) up to 7000 pg/ml. This effect was further enhanced by co-administration of CD 3-and CD 28-specific constructs, demonstrating a synergistic effect.
In addition, the bispecific construct mediated specific cytotoxic elimination of the HBsAg-producing HuH7 hepatoma cell line (fig. 3), with up to a 90% reduction in target cell viability compared to controls. This cytotoxic response was observed in the co-culture of PBMC and HBV-positive target cells with bispecific constructs against CD3 and CD28, and in isolated NK-cells with constructs against CD16 and CD 56. Co-administration of CTL-and NK-cell specific constructs further synergistically increased cytotoxic effects to over 95% elimination. We observed non-specific background cytotoxicity of 15% to 40% for CTL and NK cells, respectively.
In a second round of experiments, we utilized HBV infected HepaRG hepatoma cells. This cell line was allowed to be used for HBV infection after 4 weeks of differentiation and reflected the natural status of HBV infected tissues. Generally, the infection rate of HepaRG cells never reaches 100% and this mixture of infected and uninfected cells mimics the situation of HBV infected individuals in antiviral treatment, carrying infected and uninfected cells in the presence of extracellular viral particles.
In the co-culture of immune effector cells with co-administered bispecific constructs, HBV-infected HepaRG cells mediated efficient activation of both CTL and NK cells, with a suppressed amount of IFN-g secretion of up to 60,000pg/ml (fig. 4A). In this experiment, we did not isolate or enrich NK cells prior to co-cultivation.
In addition, bispecific antibody constructs resulted in a cytotoxic response of activated immune effector cells, leading to the specific elimination of HBV-infected target cells (fig. 4B). We observed 50% to 70% elimination of NK-cells and CTL, respectively. There was a lack of nonspecific cytotoxicity in these experiments.
Example 3
Method for example 4
In order to analyze the therapeutic potential of bispecific antibody constructs to successfully retarget T cells to HBV-positive cells, in vitro co-culture experiments were performed and analyzed in detail We utilized bispecific antibody constructs containing a single chain binding domain against human CD3(α CD3) and human CD28(α CD28), and additionally at their FcThe spacer domain contains a construct (Δ ADCC) with directed mutations that abrogate antibody-dependent cellular cytotoxicity (by avoiding Fc γ receptor binding). These are constructed to serve as safety measures to exclude non-specific activation of natural killer cells. On the other side, all bispecific antibody constructs carried HBV S-protein (HBsAg) specificityBinding domain C8. Peripheral Blood Mononuclear Cells (PBMCs) isolated from fresh venous blood of healthy donors were co-cultured with different human hepatoma cell lines for replacement of HBV-infection. We used HuH7-S (HBV S-antigen transgene) and the maternal cell line HuH7 as a negative control and HBV infected or uninfected HepaRG cells as a control. Hepg2.2.15(HBV genomic transgene) cells were used as controls for quantification of HBV markers. To provide bispecific antibody constructs, the supernatant of a bispecific antibody-containing producer cell line is added. To visualize the change in target cell viability over time due to bispecific antibody mediated cytotoxicity, the xcelligene system was used. This technique allows real-time monitoring of cell viability over long periods of culture. Thus, target hepatoma cells were seeded on specially designed microtiter plates containing staggered gold microelectrodes to non-invasively monitor the viability of adherent target cells (using electrical impedance as a readout). The elimination of cytotoxicity results in a change in impedance, which can be converted into a so-called Cell Index (CI) value for monitoring cell viability.
Co-culture with target cells
On day 0, 3 × 10 was seeded per well in 96-well plates4HuH7-S/HuH7 cells (E-Plate 96). On day 1, the supernatant was removed and 100 μ l of 1 × 10 in PBMC medium was added5Primary human PBMC or just 100 μ l of medium as a control were added to each well. In addition, 100. mu.l of the bispecific antibody-containing supernatant (alone or in combination) was added. As a negative control, 100. mu.l of DMEM medium was added to the wells to give a total volume of 200. mu.l. Co-cultivation was monitored for 48h or 72h in the xCELLigence system.
HepaRG cells were cultured to confluency (confluency), differentiated for 21 days and infected with HBV prior to immunotherapy experiments.
To infect HepaRG cells, virus stocks were prepared in PEG-containing differentiation media and 50 μ Ι were added to each well. The final concentration of PEG was 5% and the MOI of the virus stocks was set to 200(7,5X 10)6Viral particles/well). After adding infection mixture (master mix) for 16 hours, the cells were washed with PBSCell 3 times to remove residual virus. Differentiation medium was added and the medium was changed every 3 days for a total of 12 days. Prior to the co-culture experiments, the medium was changed to co-culture medium (lacking the immunosuppressive agent hydrocortisone). Successful infection of HepaRG cells by HBV was tested by measuring hbsag (axsym) and hbeag (bep iiisystem) in the supernatant of infected cells.
PBMC preparation
PBMCs for co-culture experiments were isolated from whole blood. Heparinized fresh blood was diluted 1:1 with RPMI wash medium. A25 ml layer of diluted blood was spread onto 15ml of Percoll and centrifuged at 960g in a rotary centrifuge for 20min without pause. PBMCs were isolated and transferred to 50ml with RPMI medium. After washing, cells were resuspended in 10ml of PBMC medium and cell number was determined. Adjust the concentration to 2x106Cells/ml to ensure optimal conditions. PBMC were left to stand at 37 ℃ overnight.
Fluorescence Activated Cell Sorting (FACS)
FACS analysis was performed to examine the effector function of redirected PBMCs, therefore, the secretion of proinflammatory cytokines IFN-. gamma., IL-2 and TNF- α, and the expression of the activation marker CD154(CD40L) and the degranulation marker LAMP-1(CD107a), respectively, were analyzed, cytokine production was measured using intercellular cytokine staining, 0.2. mu.g/ml of Brefeldin A (BFA) was therefore applied to the cells and incubated at 37 ℃ for 4 hours.
BFA blocks forward transfer between the endoplasmic reticulum and golgi apparatus, resulting in inhibition of extracellular secretion of cytokines. In case LAMP-1 is to be stained simultaneously, the antibody is applied 1h before addition of BFA (so that LAMP-1 can translocate to the cell surface). Subsequently, cells were transferred to 96-well plates (round bottom) and washed twice in 200 μ l FACS buffer. To Stain surviving cells and exclude DEAD cells, the LIVE/DEAD Fixable Near-IR Cell Stain kit was used. For fixation and permeabilization, cells were resuspended in 100. mu.l Cytofix/Cytoperm reagent and iced on ice in the dark for 20 min. After washing, cells were resuspended in prepared antibody cocktail or stained with only individual single color for systematic compensation. Staining was performed in the dark on ice for 30 min. After washing, cells were resuspended in 200 μ l FACS buffer and transferred to FACS tubes for acquisition (acquisition). FACSCanto II or LSRFortessa. The data were recorded using FACS Diva software and analyzed using FlowJo software.
Animal experiments
For the first in vivo testing of bispecific constructs, experiments were performed with immunodeficient Rag2/IL2R gamma nude mice (international nomenclature: B10; B6-Rag2tm1Fwa II2rgtm1 Wjl). We injected 6 weeks HBV-transgenic human hepatoma cell line HepG2.2.15 5x10 to aged mice6A cell. Cells were measured by subcutaneous injection into animals. This resulted in the formation of tumors over a 14 day period. HBV replication in tumors was monitored by determining HBV viremia. Human PBMCs were isolated from fresh human umbilical cord blood and plated at 0.25x10 on plates pre-coated with antibodies to human CD3 and CD286Cell concentrations of PBMC/ml were stimulated for 3 days. The cells were then maintained in cell culture medium containing 300U/ml IL-2 for 7 days.
At day 14 post-tumor induction, mice were injected intraperitoneally with 2x107PBMC/mice, and mice received 100 μ l of α CD3/α CD28 bispecific antibody construct per mouse (in the supernatant of HEK-producing cells) from the tail vein for 4 consecutive days.
Example 4
Bispecific antibodies mediate specific elimination of target cells expressing HBV surface proteins (HuH7-S)
To test whether the bispecific antibody construct successfully retards T cells against HBsAg-expressing target cells and induces target cell lysis, isolated PBMCs were co-cultured with HuH7-S cells in the presence of the bispecific antibody construct. HuH7-S cells were stably transfected to express HBsAg and thus mimic HBV-infected hepatocytes. This results in the production and secretion of subviral particles into the supernatant, and the incorporation of HBsAg into the cell membrane. Untransfected HuH7 cells were used as negative controls.
Single bispecific antibodies provoke killing of target cells
To analyze whether a bispecific antibody is able to stimulate activation of T cells and mediate lysis of target cells, PBMCs were co-cultured with HuH α 0-S/HuH α cells in the presence of α HBs x α CD3, α 0HBs x α CD α, α 2HBs x α CD α [ Fc Δ ADCC ] or α 4HBs x α CD α [ Fc Δ ADCC ] bispecific tetravalent antibodies to co-culture PBMC with HuH α -S/HuH α cells, stimulation of effector cells by a single bispecific antibody results in specific killing of target cells expressing the bsag (fig. 5) the elimination of target cells mediated by bispecific antibodies against CD α is earlier and stronger than the construct against CD α, because the terminal point viability of HuH α -S cells treated with α CD α is only 6% earlier than and stronger than the construct for CD α than for CD α (α Δ α: 15.5%) while the terminal viability of CD α cells treated with α CD α is only 6% as 6% compared to CD α% for which the terminal viability is only 6.4% (α CD α a CD α) and a further decrease in the time observed after the start of activation of hbh CD α and the start of the activation of CD α -soluble target cells after the start of the CD α is a further CD α -soluble with the CD α and the start of the CD α -soluble target cells after the start of the CD α cell lysis is shown by a certain time of the CD α - α CD α is not detectable by a CD α - α CD α and the CD α - α CD.
This data demonstrates that stimulation with each individual bispecific antibody provokes elimination of the target cells (no further co-stimulation).
Bispecific antibodies mediate target cell lysis in a synergistic manner
To further analyze whether the combination of bispecific constructs would result in enhanced activity and thus enhanced cytotoxicity of effector cells, PBMC were co-cultured with HuH7-S/HuH7 cells in the presence of α CD3/α CD28 or α 0CD3 Δ ADCC/α 1CD28 Δ ADCC combinations as shown in fig. 6, the combination of bispecific constructs resulted in large-scale killing of target cells expressing HBsAg with residual viability of 1.2% (α CD3/α CD28) and 4.4% (α CD3 Δ ADCC/α CD28 Δ ADCC), while almost no HuH7 cells were eliminated (end viability of HuH7 cells: α CD3/α CD28: 92.4%; α CD3 Δ ADCC/α CD28 Δ ADCC: 100.4%).
Again, α CD3/α CD28 mediated lysis of target cells was faster than that induced by constructs with mutated Fc regions, even though killing of target cells began at about the same time (after about 11 h) (fig. 6A, B.) the combination of bispecific antibodies resulted in faster elimination of target cells (fig. 6C, D) compared to lysis induced by a single bispecific construct, which is expected because T cells received not only one signal in the presence of a single construct, but both activating and co-stimulating signals if antibodies to CD3 and CD28 were present.
Thus, the combination of bispecific constructs mediates specific lysis of target cells expressing HBV surface proteins in a synergistic manner.
Bispecific antibodies provoke the elimination of target cells in a concentration-dependent manner
To examine whether the amount of bispecific antibody has an effect on target cell lysis, co-culture was performed using two different amounts of bispecific construct. Thus, a typical amount of antibody was used (100. mu.l in total)) And half thereof (50. mu.l in total)) Lower amounts of bispecific antibody were also able to induce lysis of target cells (end point viability of HuH7-S cells: α CD3/α CD28: 12.6%; α 0CD3 Δ ADCC/α 1CD28 Δ ADCC: 15.9%), while higher amounts caused faster elimination of target cells (fig. 7), with only 1.5% (α CD3/α CD28) and 2.1% (α CD3 Δ ADCC/α CD28 Δ ADCC) of remaining viable cells HuH7 cells unaffected in both cases the combination of α CD3/α CD28 or α CD3 Δ ADCC/α CD28 Δ ADCC provokes killing of target cells in a concentration-dependent manner.
Increased effector cell concentration enhances lysis of target cells
It is of further interest to know whether the number of effector cells will have an effect on the elimination of target cells. Thus, conventional amounts of PBMC (1X 10) will be used for co-culture5) And double the quantity (2x 10)5) In comparison, as demonstrated in fig. 8, higher numbers of PBMC induced lysis of HuH7-S cells significantly faster in the presence of α CD3/α CD28, with an end point viability of 4.5% compared to 11.7%, but more HuH7 cells were killed if double folds of PBMC were present (end point viability of HuH7 cells: 2x10 cells)5PBMC:83.8%;1x105PBMC:102.7%)。
This data indicates that the elimination of target cells is dependent on the amount of effector cells.
Bispecific antibodies mediate killing of target cells only after 8 hours of co-culture
To study how long the bispecific antibody needs to be present in the co-culture to activate T cells and thereby induce cytotoxicity, co-culture supernatants containing the bispecific antibody were removed and new DMEM standard medium was added at different time periods PBMC induced only a small decrease in target cell viability (78.5%) but failed to provoke lysis of target cells if the supernatant containing α CD3/α CD28 was removed after 4h (fig. 9) PBMC could cause elimination of target cells if the supernatant containing the bispecific antibody was present 8h or longer, as shown in fig. 10 PBMC required more time to induce target cell lysis, if stimulation with α CD3/α CD28 lasted 8h or 12h (compared to 24h or 48h), but the effect after 48h was almost similar (HuH7-S end point viability: 8h: 14.7%; 12h: 11.7%, 24h: 5.1%, 48h: 3.2%).
Bispecific antibodies mediate effector function of T cells during co-culture with HBsAg or HuH7-S cells
To investigate the activation and functionality of T cells during co-culture experiments, cytokine secretion was examined by ELISA or FACS analysis.
Bispecific constructs mediate secretion of IFN-. gamma.TNF- α and IL-2
In the timeline experiment it was analyzed when PBMC started to secrete cytokines after contact with bispecific antibody and how kinetics developed over time therefore, 4h, 8h, 12h, 24h and 48h after addition of PBMC and α CD3/α CD28 the co-cultured supernatants were removed, the production of cytokines IL-2, IFN- γ and TNF- α was measured by ELISA, the secretion of IL-2 increased over time, but almost no IL-2 was detected after 4h, the concentration had been 316pg/ml after 8h, and the concentration had become almost four times (1119/ml) after the subsequent 4h, there was almost no further increase between 24h and 48h, while the concentration of IL-pg 2 appeared to reach a plateau around 1550pg/ml (FIG. 10A), IFN- γ secretion (FIG. 10B) required more time, only low levels were monitored after 8h, between 8h and 12h, T-pg 2 reached a plateau around 1550 g/ml, the highest IFN- γ concentration had been observed for both cell production and negative IFN- γ (48h) and the highest IFN- γ -secretion was observed after 8h, the concentration had reached around 10000 h.
The secretion of TNF- α (fig. 10C) was elevated to 24h, where it reached its peak concentration (1700pg/ml), then it was reduced and reached only 1400pg/ml after 48h, in contrast TNF- α secretion was earlier than the other starts, about 100pg/ml after 4h, then steadily elevated to 24h, interestingly, the production of TNF- α on HuH7 cells proceeded in a completely opposite manner, with a relatively high background concentration compared to the other cytokines, which showed the highest concentration (-70 pg/ml) after 4h, then decreased with time and decreased to 9 g/ml after 48h pbmc was induced to secrete IL-2, IFN- γ and TNF- α by contact with α CD3/α CD28 during co-culture with cells expressing HBsAg, while the secretion kinetics differed between the different individual cytokines.
Bispecific constructs activate CD8+T cells and CD4+T cells
To analyze whether PBMCs also show degranulation of cytotoxic vesicles, the shift in the degranulation marker LMAP-1(CD107a) was studied at α CD3/α CD28 or α CD3 Δ ADCC/α CD28CD8 after coculture with HuH7-S/HuH7 cells in the presence of Δ ADCC+T cells showed clear migration of LAMP-1 staining, while the signal in α CD3 Δ ADCC/α CD28 Δ ADCC stimulated samples was stronger than α CD3/α CD28 (fig. 11C, D)+The same results were also observed for T cells (FIGS. 11A, B). for α CD3/α CD28, the ratio of LAMP-1 translocation was at CD8+T cells are more prominent, and the opposite is true for α CD3 Δ ADCC/α CD28 Δ ADCC.
This data demonstrates that not only CD8 was present after contact with bispecific antibody and HBsAg+T cell, CD4+Is also induced to secrete cytotoxic particles.
To examine the versatility of T cells after co-culture experiments, PBMCs were directed against IFN-. gamma., IL-2 and TNF- α and against the activation marker CD154(CD40L) (which is predominantly CD 4) 8h, 12h and 24h after addition of PBMCs and α CD3/α CD28+Expressed on T cells) were stained (fig. 12). CD4+T cells showed a stable increase: IFN-gamma+T cells (9.3% after 24h), IL-2+T cells (11.3% after 24h), TNF- α+T cells (14.7% after 24h) and CD154+T cells (28.0% after 24h), while the major boost occurred between 12h and 24h (fig. 12A).
CD8+The same is true for T cells, and IFN-. gamma.+And IL-2+The percentage of cells was 18.4% and 11.3% over CD4+TNF- α+And CD154+CD8+The amount of T cells compared to CD4+T cell reduction was 10.1% and 6.25% (fig. 12B). PBMCs on HuH7 cells showed no activation in any of the samples. Cytokine-secreting T cells were further analyzed using the boolean combination gate (fig. 12C, D). After 24h, 3.1% CD4+T cells and 2.1% CD8+The T cell is IFN gamma+,IL-2+And TNF α+Thus, α CD3/α CD28 mediates PBMC activation during co-culture with HuH7-S/HuH7 cells, resulting in multifunctional CD4+And CD8+T cells.
To exclude FACS analysis periodsThe possibility of detecting false positive signals on non-specific binding of antibody to dead target cells, PBMC were cultured in the presence of immobilized HBsAg, the effect of soluble HBsAg was additionally examined, since HBV infected patients exhibited high levels of HBsAg in their blood, PBMC were again stained for IFN- γ, IL-2 and TNF- α, and CD154, but only 24h and 48h after addition of PBMC and α CD3 Δ ADCC/α CD28 Δ ADCC (fig. 13)+T cells show IFN-. gamma.+Increase in T cells (3.4% after 24h, 6.8% after 48h), and CD154+Increase in T cells (17.2% after 24 hours, 19.9% after 48 hours). IL-2 after 48h+T cells (4.9%) were fewer than after 24h (5.5%), TNF- α+T cells were also decreased (14.9% after 24 hours, 8.1% after 48 hours) (fig. 13A). CD8+T cells only in TNF- α+T cells showed a decrease (12.6% after 24 hours, 7.4% after 48 hours) which was also observed in ELISA (figure 10). IFN-gamma+、IL-2+And CD154+CD8+The percentage of T cells was elevated between 24h and 48h (IFN. gamma.)+4.7% after 24 hours, 8.5% after 48 hours, IL-2+5.1% after 24 hours, 7.2% after 48 hours, CD154+8.3% after 24 hours and 10.4% after 48 hours (FIG. 13B). IFN-gamma+And IL-2+CD8+The percentage of T cells exceeded CD4 again+T cells, and TNF- α+And CD154+CD8+Amount of T cells and CD4+Also after 48h, some T cells appeared to be activated by soluble HBsAg, since TNF α+T cells reached 1.1% (CD 4)+T cells) and 1.0% (CD 8)+T cell), CD154+T cell 2.7% (CD 4)+T cells) and 3.1% (CD 8)+T cell), IFN γ+CD8+T cells 1.2% and IL-2+CD8+T cells 1.4%. Again, boolean gates were used to further analyze cytokine-secreting T cells (fig. 13C, D). 0.35% (after 24h) and 0.63% (after 48h) of CD4+T cells, 0.3% (after 24h) and 1.0% (after 48h) of CD8+The T cell is IFN-gamma+,IL-2+And TNF- α+Indicates thatα CD3 Δ DCC/α CD28 Δ ADCC mediate the activation of PBMC during co-culture with immobilized HBsAg cells, resulting in multifunctional CD4+And CD8+T cells. Activation of soluble HBsAg remains low.
Bispecific antibody mediated IFN gamma secretion and killing of HBV infected HepaRG cells
Finally, it was of interest whether the bispecific antibody could re-target T cells to HBV infected HepaRG cells. Success of infection was tested by measuring HBsAg in the supernatant of infected cells. The HBsAg concentration produced by HBV infected HepaRG cells was very low compared to results from HuH7-S or HepG2.2.15 cells. The other relatively high standard deviation indicates that the values in different wells differ greatly (fig. 14).
Nevertheless, infection was successful and PBMCs were co-cultured with HepaRG cells in the presence of bispecific antibodies as shown in figure 15, the viability of untreated cells decreased over time, the remaining viability after 56h was 65.9% (HBV +) and 62.9% (HBV-), compared to α CD3 and combination α CD3/α CD28 mediated the specific lysis of HBV infected HepaRG cells. α CD28 failed to induce specific elimination of target cells by itself if α CD3 was present during co-culture, the viability of HBV infected cells decreased to 25.3%, while uninfected HepaRG cells remained at 53.5% (figure 15A), the stimulation of effector cells by α CD3/α CD28 also resulted in significant killing of HBV infected HepaRG cells (figure 15B), where 37.5% of target cells remained viable (62.4% of uninfected HepaRG cells).
Thus, α CD3 or α CD3/α CD28 induce specific lysis of HBV infected HepaRG cells.
Bispecific antibodies mediate the reduction of HBV positive tumors in vivo
To immunodeficient mice injected with the human HBV-transgenic hepatoma cell line hepg2.2.15 to develop subcutaneous HBV-positive tumors, human PBMC and bispecific constructs against CD3 and CD28 were injected (fig. 16). This treatment resulted in a significant reduction in tumor size (compared to untreated or mock-treated (animals receiving PBMC and PBS)). Tumor size was reduced by approximately 50% in treated animals.
Claims (13)
1. A polypeptide comprising
(a) A first set of 6 complementarity determining regions configured to bind a first antigen, wherein the first set of 6 complementarity determining regions bind hepatitis B virus small surface antigen and are represented by the sequences of SEQ ID NOS: 1-6, or
The first group of 6 complementarity determining regions bind to the hepatitis B virus small surface antigen and are as shown in SEQ ID NO 7-12, or
The first group of 6 complementarity determining regions bind to hepatitis B virus large surface antigen and are represented by the sequences of SEQ ID NOS 13-18; and
(b) a second set of 6 complementarity determining regions configured to bind a second antigen, wherein the second set of 6 complementarity determining regions bind CD3 and are as set forth in the sequences of SEQ ID NOS 19-24, or
The second set of 6 complementarity determining regions bind CD28 and are as set forth in the sequences of SEQ ID NOS 25-30, or
The second set of 6 complementarity determining regions bind CD16 and are as set forth in the sequences of SEQ ID NOS 31-36, or
The second set of 6 complementarity determining regions bind CD56 and are as set forth in the sequences of SEQ ID NOS 37-42;
wherein the order of each of the 6 complementarity determining regions of the first and second sets is as follows: heavy chain complementarity determining region 1, heavy chain complementarity determining region 2, heavy chain complementarity determining region 3, light chain complementarity determining region 1, light chain complementarity determining region 2, and light chain complementarity determining region 3; and is
Wherein the complementarity determining region is part of an immunoglobulin domain.
2. The polypeptide of claim 1, wherein
(a) The first set of 6 complementarity determining regions is comprised in a first scFv fragment; and/or
(b) The second set of 6 complementarity determining regions is contained within a second scFv fragment.
3. The polypeptide of claim 1, wherein the first set of 6 complementarity determining regions bind to an epitope of the first antigen that is located:
(a) the hepatitis B virus small surface antigen;
(b) (ii) a portion of said hepatitis b virus large surface antigen not comprised by said hepatitis b virus small surface antigen; or
(c) In the portion of the hepatitis B virus large surface antigen, the portion is structurally different from the hepatitis B virus small surface antigen.
4. The polypeptide of any one of claims 1-3, wherein said polypeptide further comprises a dimerization domain, wherein said dimerization domain provides covalent and/or non-covalent dimerization.
5. The polypeptide of claim 2, wherein the polypeptide further comprises a spacer region comprising a CH2 domain and a CH3 domain, the spacer region being located between:
(i) the first scFv fragment; and
(ii) (ii) the second scFv fragment is,
and the CH2 domain and/or the CH3 domain is mutated at one or more positions to reduce or eliminate FcBinding of the receptor.
6. A nucleic acid encoding the polypeptide of any one of claims 1-5.
7. A complex comprising or consisting of a first and a second polypeptide as defined in any one of claims 1 to 5, wherein
(a) At least one covalent linkage between said first and said second polypeptide, said covalent linkage being at least one disulfide bridge between a cysteine residue of said first polypeptide and a cysteine residue of said second polypeptide; or
(b) Said first and said second polypeptides are non-covalently bound to each other.
8. A composition comprising or consisting of: one or more polypeptides according to any one of claims 1-5 and/or one or more complexes according to claim 7, with the proviso that at least two polypeptides are comprised in the composition, which two polypeptides differ from each other with respect to the first antigen and/or the second antigen to which they bind.
9. The composition of claim 8, wherein said polypeptide is
(a) (ii) (i) a polypeptide that binds to the hepatitis b virus small or large surface antigen and CD 3; and
(ii) a polypeptide that binds to the hepatitis b virus small or large surface antigen and CD 28; or
(b) (ii) (i) a polypeptide that binds to the hepatitis b virus small or large surface antigen and CD 16; and
(ii) a polypeptide that binds to the small or large surface antigen of hepatitis b virus and CD 56.
10. The complex of claim 7 or the composition of claim 8 or 9, wherein the complex and the composition comprise or consist of a bispecific, trispecific or tetraspecific tetravalent antibody.
11. A pharmaceutical composition comprising or consisting of: one or more polypeptides according to any one of claims 1 to 5, one or more complexes according to claim 7 and/or one or more compositions according to claim 8 or 9.
12. Use of one or more polypeptides according to any one of claims 1 to 5, one or more complexes according to claim 7 and/or one or more compositions according to claim 8 or 9 for the preparation of a pharmaceutical composition for the treatment or prevention of a hepatitis b virus infection and/or a condition caused by said hepatitis b virus infection, said condition caused by hepatitis b virus infection being selected from the group consisting of cirrhosis, hepatocellular carcinoma, and liver cancer, said liver cancer being characterized by the expression of one or more hepatitis b virus surface antigens.
13. An in vitro or ex vivo immune effector cell having the polypeptide of any one of claims 1-5 or the complex of claim 7 bound to a surface antigen of said immune effector cell.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010449532.9A CN111606998A (en) | 2013-09-16 | 2014-09-16 | Bi-or multispecific polypeptides for treating HBV infections and related conditions |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP13184635.4 | 2013-09-16 | ||
| EP13184635 | 2013-09-16 | ||
| PCT/EP2014/069675 WO2015036606A1 (en) | 2013-09-16 | 2014-09-16 | Bi- or multispecific polypeptides binding immune effector cell surface antigens and hbv antigens for treating hbv infections and associated conditions |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202010449532.9A Division CN111606998A (en) | 2013-09-16 | 2014-09-16 | Bi-or multispecific polypeptides for treating HBV infections and related conditions |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN105636982A CN105636982A (en) | 2016-06-01 |
| CN105636982B true CN105636982B (en) | 2020-06-23 |
Family
ID=49165642
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202010449532.9A Pending CN111606998A (en) | 2013-09-16 | 2014-09-16 | Bi-or multispecific polypeptides for treating HBV infections and related conditions |
| CN201480050976.8A Active CN105636982B (en) | 2013-09-16 | 2014-09-16 | Bi-or multispecific polypeptides that bind immune effector cell surface antigens and HBV antigens for the treatment of HBV infections and related conditions |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202010449532.9A Pending CN111606998A (en) | 2013-09-16 | 2014-09-16 | Bi-or multispecific polypeptides for treating HBV infections and related conditions |
Country Status (8)
| Country | Link |
|---|---|
| US (2) | US10059767B2 (en) |
| EP (1) | EP3046938B1 (en) |
| JP (1) | JP6599338B2 (en) |
| CN (2) | CN111606998A (en) |
| CA (1) | CA2924252C (en) |
| ES (1) | ES2773306T3 (en) |
| RU (1) | RU2671089C2 (en) |
| WO (1) | WO2015036606A1 (en) |
Families Citing this family (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10865233B2 (en) | 2008-12-18 | 2020-12-15 | Dana-Farber Cancer Institute, Inc. | NKG2D-fc for immunotherapy |
| CA2924252C (en) | 2013-09-16 | 2022-01-04 | Helmholtz Zentrum Munchen - Deutsches Forschungszentrum Fur Gesundheit Und Umwelt (Gmbh) | Bi- or multispecific polypeptides binding immune effector cell surface antigens and hbv antigens for treating hbv infections and associated conditions |
| CN107223135B (en) * | 2015-03-16 | 2021-11-02 | 亥姆霍兹慕尼黑中心-德国环境健康研究中心(Gmbh) | Trispecific binding molecules for the treatment of HBV infections and related disorders |
| CN106349391A (en) * | 2015-07-17 | 2017-01-25 | 中国科学院深圳先进技术研究院 | HBV specific double-targeted antibody as well as preparation method and application thereof, minicircle DNA containing double-targeted antibody expression box and application of minicircle DNA |
| AU2016353231B2 (en) | 2015-11-13 | 2020-08-13 | Dana-Farber Cancer Institute, Inc. | An NKG2D-Ig fusion protein for cancer immunotherapy |
| CN105505872B (en) * | 2016-02-16 | 2019-01-15 | 广州赛莱拉干细胞科技股份有限公司 | Method and composition for sensitizing NK cells |
| CN107129537A (en) * | 2016-02-29 | 2017-09-05 | 北京赛诺泰生物科技有限公司 | The immunoreactive cell of anti-hepatitis B virus |
| US11566063B2 (en) * | 2016-09-30 | 2023-01-31 | Baylor College Of Medicine | Antibody based gene therapy with tissue-directed expression |
| EP3519443A4 (en) * | 2016-09-30 | 2020-06-10 | Baylor College of Medicine | Chimeric antigen receptor therapy with reduced cytotoxicity for viral disease |
| CN110944651A (en) | 2017-02-08 | 2020-03-31 | 蜻蜓疗法股份有限公司 | Multispecific binding proteins for natural killer cell activation and therapeutic uses thereof for treating cancer |
| FI3582806T3 (en) | 2017-02-20 | 2023-09-07 | Dragonfly Therapeutics Inc | Proteins binding her2, nkg2d and cd16 |
| SG11202007482WA (en) | 2018-02-08 | 2020-09-29 | Dragonfly Therapeutics Inc | Antibody variable domains targeting the nkg2d receptor |
| CN109265559B (en) * | 2018-09-25 | 2021-12-07 | 山东兴瑞生物科技有限公司 | Chimeric antigen receptor, preparation method thereof, NK cell modified by same and application of chimeric antigen receptor in treating HBV infection |
| SG11202105948TA (en) * | 2018-12-19 | 2021-07-29 | Regeneron Pharma | Bispecific anti-muc16 x anti-cd28 antibodies and uses thereof |
| GB201904328D0 (en) * | 2019-03-28 | 2019-05-15 | Immunocore Ltd | Specific binding molecules |
| KR20230031981A (en) | 2019-05-14 | 2023-03-07 | 프로벤션 바이오, 인코포레이티드 | Methods and compositions for preventing type 1 diabetes |
| CN112592902B (en) * | 2020-12-16 | 2022-03-29 | 熊猫乳品集团股份有限公司 | Bioactive peptide ASEPPVLDVKRPFLC, and preparation method and application thereof |
| CN114573705A (en) * | 2022-03-17 | 2022-06-03 | 杭州师范大学 | Bispecific antibody for specifically starting anti-hepatitis B virus T cell immunity and application thereof |
| WO2024005713A1 (en) * | 2022-06-27 | 2024-01-04 | Scg Cell Therapy Pte. Ltd. | Bispecific antibodies for hbv surface antigen(hbsag)and cd3 and uses thereof |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101687915A (en) * | 2007-04-03 | 2010-03-31 | 米克罗麦特股份公司 | Cross-species-specific cd 3-epsilon binding domain |
| EP2524699A1 (en) * | 2011-05-17 | 2012-11-21 | Trion Research GmbH | Vaccine preparation containing trifunctional antibodies with antigen immunogenicity enhancer properties |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB8928874D0 (en) * | 1989-12-21 | 1990-02-28 | Celltech Ltd | Humanised antibodies |
| EA013564B1 (en) * | 2000-08-03 | 2010-06-30 | Терапеутик Хьюман Поликлоналз Инк. | Humanized immunoglobulin and pharmaceutical composition comprising thereof |
| CN1294148C (en) | 2001-04-11 | 2007-01-10 | 中国科学院遗传与发育生物学研究所 | Single-stranded cyctic trispecific antibody |
| FR2844513B1 (en) * | 2002-09-13 | 2007-08-03 | Lab Francais Du Fractionnement | ANTIBODIES FOR ADCC AND INDUCING PRODUCTION OF CYTOKINS. |
| ATE504599T1 (en) * | 2004-01-16 | 2011-04-15 | Regeneron Pharma | FUSION POLYPEPTIDES CAPABLE OF ACTIVATING RECEPTORS |
| CN100376599C (en) | 2004-04-01 | 2008-03-26 | 北京安波特基因工程技术有限公司 | Recombining single chained three specific antibodies of anti CCA, anti CD 3, anti CD 28 through genetic engineering |
| CA2683370C (en) * | 2007-04-03 | 2022-12-13 | Micromet Ag | Cross-species-specific binding domain |
| US8999398B2 (en) * | 2009-11-06 | 2015-04-07 | Transtarget Inc. | Polyclonal bispecific antibody compositions and method of use |
| WO2012156430A1 (en) * | 2011-05-17 | 2012-11-22 | Trion Research Gmbh | Vaccine preparation containing trifunctional antibodies with antigen immunogenicity enhancer properties |
| WO2013102123A2 (en) * | 2011-12-28 | 2013-07-04 | Novelmed Therapeutics, Inc. | Aglycosylated human antibody and fusion protein and uses thereof |
| CA2924252C (en) | 2013-09-16 | 2022-01-04 | Helmholtz Zentrum Munchen - Deutsches Forschungszentrum Fur Gesundheit Und Umwelt (Gmbh) | Bi- or multispecific polypeptides binding immune effector cell surface antigens and hbv antigens for treating hbv infections and associated conditions |
-
2014
- 2014-09-16 CA CA2924252A patent/CA2924252C/en active Active
- 2014-09-16 US US15/021,916 patent/US10059767B2/en active Active
- 2014-09-16 ES ES14771250T patent/ES2773306T3/en active Active
- 2014-09-16 CN CN202010449532.9A patent/CN111606998A/en active Pending
- 2014-09-16 WO PCT/EP2014/069675 patent/WO2015036606A1/en active Application Filing
- 2014-09-16 EP EP14771250.9A patent/EP3046938B1/en active Active
- 2014-09-16 CN CN201480050976.8A patent/CN105636982B/en active Active
- 2014-09-16 JP JP2016542337A patent/JP6599338B2/en active Active
- 2014-09-16 RU RU2016114504A patent/RU2671089C2/en active
-
2018
- 2018-08-24 US US16/112,431 patent/US10730943B2/en active Active
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101687915A (en) * | 2007-04-03 | 2010-03-31 | 米克罗麦特股份公司 | Cross-species-specific cd 3-epsilon binding domain |
| EP2524699A1 (en) * | 2011-05-17 | 2012-11-21 | Trion Research GmbH | Vaccine preparation containing trifunctional antibodies with antigen immunogenicity enhancer properties |
Non-Patent Citations (1)
| Title |
|---|
| T Cells Redirected Against Hepatitis B Virus Surface Proteins Eliminate;FELIX BOHNE等;《Gastroenterology》;20081231;第134卷;第239-246页 * |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2924252A1 (en) | 2015-03-19 |
| US10059767B2 (en) | 2018-08-28 |
| CA2924252C (en) | 2022-01-04 |
| US20160200798A1 (en) | 2016-07-14 |
| EP3046938A1 (en) | 2016-07-27 |
| CN105636982A (en) | 2016-06-01 |
| ES2773306T3 (en) | 2020-07-10 |
| JP6599338B2 (en) | 2019-10-30 |
| RU2671089C2 (en) | 2018-10-29 |
| WO2015036606A1 (en) | 2015-03-19 |
| EP3046938B1 (en) | 2019-12-04 |
| RU2016114504A (en) | 2017-10-23 |
| RU2016114504A3 (en) | 2018-04-25 |
| US10730943B2 (en) | 2020-08-04 |
| JP2016530889A (en) | 2016-10-06 |
| US20180362649A1 (en) | 2018-12-20 |
| CN111606998A (en) | 2020-09-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN105636982B (en) | Bi-or multispecific polypeptides that bind immune effector cell surface antigens and HBV antigens for the treatment of HBV infections and related conditions | |
| RU2733496C2 (en) | Trispecific binding molecules for treating viral hepatitis b infection and bound states | |
| US11840577B2 (en) | Antigen binding proteins specifically binding MAGE-A | |
| TW202018083A (en) | Diverse antigen binding domains, novel platforms and other enhancements for cellular therapy | |
| KR20230113578A (en) | CLDN18.2 antibody and uses thereof | |
| US20230272110A1 (en) | Antibodies that bind psma and gamma-delta t cell receptors | |
| CA3135306A1 (en) | Magea1 specific t cell receptors and their use | |
| CN113166251A (en) | Anti-human TIM-3 monoclonal antibody and application thereof | |
| CN115361968A (en) | Increasing antigen negative cell death in antigen-targeted immunotherapy | |
| CA3217738A1 (en) | Antigen binding proteins specifically binding prame | |
| US20230192903A1 (en) | Bispecific antibody against cldn18.2 and cd3 | |
| WO2021170146A1 (en) | Preparation of new-type anti-cd19 antibody and cd19-car-t cell, and use thereof | |
| WO2024035341A1 (en) | Cd30 antigen-binding molecules | |
| AU2022233150A1 (en) | MOLECULES THAT BIND TO CD66e POLYPEPTIDES | |
| KR20240006506A (en) | Anti-vaccinia virus antigen antibodies and related compositions and methods | |
| JP2024511642A (en) | Novel TNFR2 binding molecule |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |