CN103120650A - Rupatadine fumarate tablet - Google Patents
Rupatadine fumarate tablet Download PDFInfo
- Publication number
- CN103120650A CN103120650A CN2011103707788A CN201110370778A CN103120650A CN 103120650 A CN103120650 A CN 103120650A CN 2011103707788 A CN2011103707788 A CN 2011103707788A CN 201110370778 A CN201110370778 A CN 201110370778A CN 103120650 A CN103120650 A CN 103120650A
- Authority
- CN
- China
- Prior art keywords
- rupatadine fumarate
- rupatadine
- tablet
- add
- ferric oxide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Landscapes
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
The invention provides a rupatadine fumarate tablet. Each 1000 tablets comprise 12.8g of rupatadine fumarate, 30-120g of lactose, 3-15g of pregelatinized starch, 3-15g pf hydroxypropylcellulose, 0.03-0.30g of ferric oxide and 0.3-12g of wetting agent. According to the embodiment of the invention, looseness and color change and the like can be improved, and indissolvable main medicine is dissolved quickly, so that the in vivo bioavailability is ensured, and furthermore, good effect in clinical use is obtained.
Description
Technical field
The invention belongs to technical field of medicine, relate to particularly a kind of Rupatadine fumarate tablet and preparation method thereof.
Background technology
Anaphylactic disease is a kind of multi-factor disease of complexity, along with the control of infectious disease and the raising of industrialization degree, is the trend that increases year by year in worldwide.30 national anaphylactic disease Epidemiological study results that world's allergy tissue is announced show: in 1,200,000,000 total populations of these countries, 22,%(2 100,011,000 people) suffer from various anaphylactic diseases, as allergic rhinitis, asthma, urticaria, eczema etc.
With regard to present China at present clinically with regard to treatment of allergic rhinitis and anaphylaxis urticaria medicine, mainly contain antihistamine drug chlorphenamine (flutter and quick), astemizole (hismanal), terfenadine (terfenadine), cetirizine (cetirizine HCl), loratadine (loratadine) etc., chlorphenamine has stronger sedation, can cause drowsiness and weak, hismanal, terfenadine can cause serious cardiac toxicity, however, these three kinds are still used at present clinically in a large number.The pharmaceutical market low along with cardiac toxicity such as loratadine, cetirizines, that side effect is little is promoted, and the listing of other new varieties, and these medicines will step down from the stage of history gradually.
As Claritin, Rupatadine has antihistamine and antagonism platelet activating factor (PAF) dual function.Studies show that, irritated and diseases associated with inflammation is by the generation of multiple different medium and discharges the multifactor complex process that produces, histamine is exactly that irritated early symptom contains maximum inflammatory mediators when occurring, disease symptoms such as sneeze, rhinocnesmus, with tears in one's eyes, the great majority such as watery nasal discharge, skin pruritus and welt are all by histamine H
1Receptor causes.And PAF can cause that also bronchoconstriction and vascular permeability increase, thereby causes watery nasal discharge, nasal congestion, welt and pruritus, is also simultaneously the main cause that causes asthma.The Claritin that uses clinically only has the anti-histamine activity effect, and there is no the PAF antagonism.Obviously simultaneously the medicine of blocking histamine and PAF will have a better clinical effectiveness than blocking-up only is wherein a kind of.Rupatadine is the Claritin that not only has antihistamine effect but also antagonism PAF activity of present unique listing, without cardiovascular and the calm side effect of maincenter, has certain advantage than cetirizine and loratadine, be expected to become the first-line drug for the treatment of of allergic rhinitis and anaphylaxis urticaria, have potential applicability in clinical practice preferably.
The chemistry of Rupatadine fumarate is by name: 8-chloro-11-[1-[(5-methyl-3-pyridine radicals) methyl] piperidines-4-thiazolinyl]-6,11-dihydro-5H-benzo [5,6] ring [1,2-b] pyridine fumarate salt in heptan, its structural formula is as follows:
The Rupatadine fumarate preparation of listing is tablet abroad at present, and specification is 10mg.Tablet have take, easy to carry, cost is low, the patient is easy to accept, be easy to the characteristics such as suitability for industrialized production.Rupatadine fumarate is white, off-white color or show slightly pink crystalline powder, odorless, and mildly bitter flavor, soluble,very slightly in water should be noted the problems such as dissolution of outward appearance, mouldability, slightly solubility principal agent when making tablet.
Summary of the invention
The object of the present invention is to provide a kind of Rupatadine fumarate tablet, every 1000 of described tablet contains following composition:
Above-mentioned Rupatadine fumarate tablet, every 1000 contain following composition:
Above-mentioned Rupatadine fumarate tablet, every 1000 contain following composition:
Above-mentioned Rupatadine fumarate tablet, described lubricant are selected from one or more in stearic acid, magnesium stearate, calcium stearate, Pulvis Talci, boric acid, sodium benzoate, sodium acetate, sodium chloride, polyoxyethylene monostearate, Brij30, DL-leucine, sodium laurylsulfate, magnesium laurylsulfate, Macrogol 4000 or polyethylene glycol 6000.
Above-mentioned Rupatadine fumarate tablet, described ferrum oxide is selected from one or both in yellow ferric oxide and red ferric oxide.
Rupatadine fumarate tablet provided by the invention, preferred every 1000 contain following composition:
Rupatadine fumarate tablet provided by the invention, more preferably every 1000 contain following composition:
Above-mentioned Rupatadine fumarate tablet adopts 30 POVIDONE K 30 BP/USP
3085% alcoholic solution as binding agent.
The preparation method of the Rupatadine fumarate tablet that another object of the present invention is to provide above-mentioned said method comprising the steps of:
(1) Rupatadine fumarate is crossed 100 mesh sieves, lactose, pregelatinized Starch, hyprolose were pulverized 80 mesh sieves, and be standby;
(2) get 30 POVIDONE K 30 BP/USP
30, add 85% appropriate amount of ethanol dissolving, make 10% solution, standby;
(3) red ferric oxide and yellow ferric oxide are mixed, adopt the equivalent mix homogeneously that progressively increases with appropriate lactose, then add Rupatadine fumarate, pregelatinized Starch, hyprolose and residue lactose mix homogeneously, add 10% 30 POVIDONE K 30 BP/USP
3085% alcoholic solution is made soft material in right amount, and 20 mesh sieves are granulated, and 50 ℃~70 ℃ dryings are controlled moisture 1.0%~3.0%, with 20 mesh sieve granulate;
(4) add mix lubricant even, measure granule content, tabletting, control strip focus on labelled amount ± 5% in, packing, and get final product.
By enforcement of the present invention, can improve the situations such as loose sheet, variable color, and make the stripping of slightly solubility principal agent rapid, thereby guarantee bioavailability in body, clinical use has also obtained good effect.
The specific embodiment
In order to understand better the present invention, the below will be described in detail and illustrate the present invention and advantage thereof and beneficial effect by embodiments of the invention and experimental data, but these embodiment are not limited to the present invention.
The preparation of embodiment 1-tablet of the present invention
Prescription:
Method for making:
1, Rupatadine fumarate is crossed 100 mesh sieves, lactose, pregelatinized Starch, hyprolose were pulverized 80 mesh sieves, and be standby;
2, get 30 POVIDONE K 30 BP/USP
30, add 85% appropriate amount of ethanol dissolving, make 10% solution, standby;
3, red ferric oxide and yellow ferric oxide are mixed, adopt the equivalent mix homogeneously that progressively increases with appropriate lactose, then add Rupatadine fumarate, pregelatinized Starch, hyprolose and residue lactose mix homogeneously, add 10% 30 POVIDONE K 30 BP/USP
3085% alcoholic solution is made soft material in right amount, and 20 mesh sieves are granulated, and 50 ℃~70 ℃ dryings are controlled moisture 1.0%~3.0%, with 20 mesh sieve granulate;
4, add magnesium stearate and Pulvis Talci mix homogeneously, measure granule content, calculate sheet heavy;
5, tabletting, control strip focus in labelled amount ± 5%;
6, detect (character, content, related substance, uniformity of dosage units, dissolution etc.);
7, packing, get finished product.
The preparation of embodiment 2-tablet of the present invention
Prescription:
Method for making:
1, Rupatadine fumarate is crossed 100 mesh sieves, lactose, pregelatinized Starch, hyprolose were pulverized 80 mesh sieves, and be standby;
2, get 30 POVIDONE K 30 BP/USP
30, add 85% appropriate amount of ethanol dissolving, make 10% solution, standby;
3, red ferric oxide and yellow ferric oxide are mixed, adopt the equivalent mix homogeneously that progressively increases with appropriate lactose, then add Rupatadine fumarate, pregelatinized Starch, hyprolose and residue lactose mix homogeneously, add 10% 30 POVIDONE K 30 BP/USP
3085% alcoholic solution is made soft material in right amount, and 20 mesh sieves are granulated, and 50 ℃~70 ℃ dryings are controlled moisture 1.0%~3.0%, with 20 mesh sieve granulate;
4, add magnesium stearate and Pulvis Talci mix homogeneously, measure granule content, calculate sheet heavy;
5, tabletting, control strip focus in labelled amount ± 5%;
6, detect (character, content, related substance, uniformity of dosage units, dissolution etc.);
7, packing, get finished product.
The preparation of embodiment 3-tablet of the present invention
Prescription:
Method for making:
1, Rupatadine fumarate is crossed 100 mesh sieves, lactose, pregelatinized Starch, hyprolose were pulverized 80 mesh sieves, and be standby;
2, get 30 POVIDONE K 30 BP/USP
30, add 85% appropriate amount of ethanol dissolving, make 10% solution, standby;
3, red ferric oxide and yellow ferric oxide are mixed, adopt the equivalent mix homogeneously that progressively increases with appropriate lactose, then add Rupatadine fumarate, pregelatinized Starch, hyprolose and residue lactose mix homogeneously, add 10% 30 POVIDONE K 30 BP/USP
3085% alcoholic solution is made soft material in right amount, and 20 mesh sieves are granulated, and 50 ℃~70 ℃ dryings are controlled moisture 1.0%~3.0%, with 20 mesh sieve granulate;
4, add magnesium stearate and stearic acid mix homogeneously, measure granule content, calculate sheet heavy;
5, tabletting, control strip focus in labelled amount ± 5%;
6, detect (character, content, related substance, uniformity of dosage units, dissolution etc.);
7, packing, get finished product.
The preparation of embodiment 4-tablet of the present invention
Prescription:
Method for making:
1, Rupatadine fumarate is crossed 100 mesh sieves, lactose, pregelatinized Starch, hyprolose were pulverized 80 mesh sieves, and be standby;
2, get 30 POVIDONE K 30 BP/USP
30, add 85% appropriate amount of ethanol dissolving, make 10% solution, standby;
3, red ferric oxide and yellow ferric oxide are mixed, adopt the equivalent mix homogeneously that progressively increases with appropriate lactose, then add Rupatadine fumarate, pregelatinized Starch, hyprolose and residue lactose mix homogeneously, add 10% 30 POVIDONE K 30 BP/USP
3085% alcoholic solution is made soft material in right amount, and 20 mesh sieves are granulated, and 50 ℃~70 ℃ dryings are controlled moisture 1.0%~3.0%, with 20 mesh sieve granulate;
4, add magnesium stearate and Pulvis Talci mix homogeneously, measure granule content, calculate sheet heavy;
5, tabletting, control strip focus in labelled amount ± 5%;
6, detect (character, content, related substance, uniformity of dosage units, dissolution etc.);
7, packing, get finished product.
Prescription and the technical study of experimental example-Rupatadine tablet of the present invention
The present invention finds in R﹠D process, the sample hardness of formula preparation does not reach requirement improperly, loose sheet phenomenon is arranged, have in synthetic or put procedure because of crude drug simultaneously and become peach phenomenon, cause preparation that the phenomenon of variable color is arranged, in order to solve sample pine sheet phenomenon and sample metachromatism, we re-start research to the formulation and technology of Rupatadine tablet.
Rupatadine tablet original prescription is:
According to the original prescription analysis, the reason of the easy loose sheet of this product is that in prescription, the crospolyvinylpyrrolidone proportion is too large, and we reduce the crospolyvinylpyrrolidone consumption on the basis of original prescription, also investigates simultaneously and selects other binding agents, and result of the test sees Table 1.
Table 1 prescription screening is table as a result
Get from result of the test, reduce the crospolyvinylpyrrolidone consumption, slice, thin piece hardness does not still reach requirement, the sheet sub-surface becomes not bright and clean simultaneously, explanation reduces the crospolyvinylpyrrolidone consumption on the original prescription basis can't improve loose sheet phenomenon, need to select other adjuvants as filler, this product crude drug color in synthetic and put procedure easily becomes and shows slightly pink, cause the variation of the color of preparation, hide its variable color so we select coating and add ferrum oxide when investigating prescription, result of the test sees Table 2.
Table 2 prescription screening is table as a result
Got by result of the test, above-mentioned prescription all can improve hardness and surface smoothness, but experimental example 8 is yellow, can not effectively cover pink, 10 days influence factors test of other prescription (temperature 60 C ± 2 ℃, high humidity 75% ± 5%, high humidity 92.5% ± 5%) is investigated result and is shown, the color of experimental example 5-7 by off-white color become show slightly the pink, the color of embodiment 1 does not change, so we select the prescription of embodiment 1 as prescription of the present invention.
Influence factor's test of experimental example-tablet of the present invention
In order to investigate adjuvant to the impact of principal agent stability, the reasonability that the checking prescription forms and feasibility and the stability of production technology, with reference to two appendix XI X C medicine stability test guidelines of Chinese Pharmacopoeia version in 2005, we select high temperature (60 ℃ ± 2 ℃), high humidity (92.5% ± 5%), high humidity (75% ± 5%), high light, and (4500lx ± 500lx) condition is carried out influence factor's test to embodiment 1.The result demonstration, high humidity (92.5% ± 5%) moisture absorption of 5 days weightening finish surpasses 5.0%, and other result of the tests see Table 3.
Table 3 influence factor result of the test table
By influence factor's result of the test as can be known, embodiment 1 after placing 10 days under high temperature, illumination and super-humid conditions, indices with relatively had no significant change in 0 day, show adjuvant on principal agent stability without impact, this prescription composition is reasonable, stable processing technique is feasible.
Formulation and technology checking and the quality inspection of experimental example-tablet of the present invention
For further proof prescription of the present invention is reasonable, process stabilizing is feasible, we have carried out trial production and three batches of production technology checkings in three batches according to prescription and technique (embodiment 1) that the present invention determines, and gained 6 batch samples have been carried out full inspection, the results are shown in Table 4~7.
The production of test agent table as a result in three batches, table 4
The examination table of test agent in three batches, table 5
The production of three batches of production technology verification samples of table 6 is table as a result
The examination table of three batches of production technology verification samples of table 7
In three batches of the prescription of determining according to the present invention, technique preparation, test agent and three batches of process certification sample result show, prescription of the present invention forms rationally, process stabilizing, can produce stay-in-grade sample.
The Dissolution Rate Testing of experimental example-tablet of the present invention
The dissolution of oral solid formulation is a key factor that affects human bioavailability.For insoluble drug, usually because dissolubility in water is minimum, cause stripping slow, in body, bioavailability is low, therefore thereby limited performance and the clinical practice of drug effect, improved dissolution rate and dissolution and be the problem that needs emphasis to consider when insoluble drug is made oral formulations.In order to investigate adjuvant in the present invention prescription and production technology to the impact of the dissolution of slightly solubility principal agent; get tablet of the present invention (embodiment 1); according to dissolution method (two appendix X C the second methods of Chinese Pharmacopoeia version in 2005); take water 500ml as dissolution medium, rotating speed is per minute 50 to turn, operation in accordance with the law; respectively at sampling in 5,10,20,30,45 minutes; filter, adopt the amount of Rupatadine fumarate in the high effective liquid chromatography for measuring subsequent filtrate, calculate dissolution.Result shows, tablet of the present invention (test agent and three batches of production technology verification samples in three batches) is when 10min, dissolution has all reached 90%, show that tablet stripping of the present invention is rapid, prescription composition of the present invention (adjuvant) and production technology have solved the dissolution problem of slightly solubility principal agent effectively, have guaranteed the normal performance of the interior bioavailability of body and drug effect.
The clinical research of experimental example-tablet of the present invention
One, human pharmacokinetics research
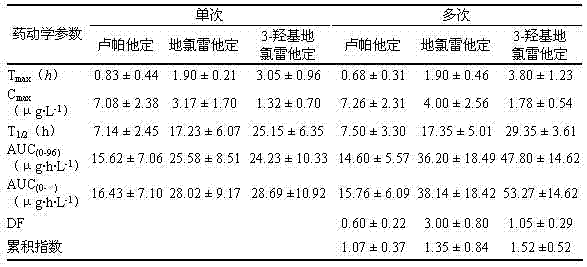
1 of 10 healthy volunteer's single oral Rupatadine fumarate sheet (embodiment 1), experimenter 15min, 30min, 45min, 1h, 1.5h, 2h, 3h, 4h, 6h, 8h, 10h, 12h, 15h, 24h, 36h, 48h, 72h and 96h blood sample collection after (0h) and administration before administration; Entered the multiple dosing test through 7 days cleaning phases after single test is completed, 10 experimenters took medicine 8 days continuously, each 1 of every day 1 time.In took medicine in the 6th, 7,8 day front 0h and took medicine in the 8th day after 15min, 30min, 45min, 1h, 1.5h, 2h, 3h, 4h, 6h, 8h, 10h, 12h, 15h, 24h, 36h, 48h, 72h and 96h blood sample collection, concentration with Rupatadine and main metabolites Desloratadine thereof, 3-hydroxyl Desloratadine in HPLC-MS/MS method mensuration blood plasma, and adopt the DAS program that test data is processed, ask and calculate relevant pharmacokinetic parameters.The results are shown in following table 8.
The main pharmacokinetic parameters of table 8 Rupatadine and major metabolite
Two, treatment seasonality or catarrhus perennialis's efficacy and saferry clinical research
Tablet of the present invention (embodiment 1) has carried out the clinical research of multicenter, random, Double-blind double-dummy, masculine parallel comparison design in 6 hospitals such as Subsidiary Hospital of Eye-Ear-Throat Department, Fudan Univ. in JIUYUE, 2008 to 2009 year June, to estimate tablet in treatment seasonality of the present invention or catarrhus perennialis's efficacy and saferry.Seasonality or catarrhus perennialis experimenter totally 167 examples are included in this test in, press the 1:1 random assortment to 10mg/d Rupatadine group and 10mg/d loratadine group, oral 2 weeks.Result shows:
1. curative effect index (PPS analysis)
Rear 7 days of Rupatadine group treatment and 14 days sings and symptoms total mark effective percentage are respectively 81.82%, 89.61%; The loratadine group is respectively 76.06%, 87.32%.Two groups are relatively, the equal not statistically significant of difference (P〉0.05).
2. secondary efficacy index (PPS analysis)
Rupatadine group treatment after rear 7 days, 14 days symptom and sign always score and descend respectively 5.09 ± 2.49 minutes, 6.49 ± 2.80 minutes; The loratadine group descended respectively 4.77 ± 2.63 minutes, 6.10 ± 2.75 minutes.Two groups are relatively, the equal not statistically significant of difference (P〉0.05).
Two groups of medications sniffle sign disappearance rate after 14 days: Rupatadine group sneeze disappearance rate is 51.35%, and the watery nasal discharge disappearance rate is 32.47%, and the nasal obstruction disappearance rate is 40%, and the rhinocnesmus disappearance rate is 53.33%, and the sign disappearance rate is 11.69%; Loratadine group sneeze disappearance rate is 51.43%, and the watery nasal discharge disappearance rate is 33.80%, and the nasal obstruction disappearance rate is 36.62%, and the rhinocnesmus disappearance rate is 40.58%, and the sign disappearance rate is 11.27%.Two groups relatively, difference not statistically significant (P>0.05).
Two groups of medications curative effect general comment effective percentage after 14 days: the Rupatadine group is 80.52%, the loratadine group be 85.92%, two group relatively, difference not statistically significant (P>0.05).
3. safety indexes
Have 24 examples that untoward reaction occur in process of the test, the Rupatadine group has 12 examples that untoward reaction occur, and incidence rate is 14.63%, shows as drowsiness, xerostomia etc.; The loratadine group has 12 examples that untoward reaction occur, and incidence rate is 14.81%, shows as drowsiness, xerostomia, dizziness etc.; Two groups relatively, no difference of science of statistics.
The medication of Rupatadine group the lab index such as routine blood test, blood biochemistry substantially without the impact.
Three, the efficacy and saferry clinical research for the treatment of chronic idiopathic urticaria
Tablet of the present invention (embodiment 1) has carried out the clinical research of multicenter, random, Double-blind double-dummy, masculine parallel comparison design in 5 hospitals such as Hospital No.1 Attached to Military Medical Univ. No. 3 in JIUYUE, 2008 to 2009 year June, to estimate the efficacy and saferry of tablet in treatment chronic idiopathic urticaria of the present invention.182 routine chronic idiopathic urticaria experimenters press the 1:1 random assortment to 10mg/ day Rupatadine group and 10mg/ day loratadine group, oral taking for 4 weeks.Result shows:
1. curative effect index
Two groups after medication the sings and symptoms total mark all be improved, significant difference (P<0.05) is relatively arranged before and after medication.
After two groups of medications, 28 days sings and symptoms total marks are improved effective percentage relatively (PPS analysis): the Rupatadine group is 76.83%, and the loratadine group is 73.56%, two group and compares, difference not statistically significant (P=0.6385).After two groups of medications, 28 days efficient non-bad effects of sings and symptoms total mark improvement are up to the standards.
2. secondary efficacy index
Two groups of patients treat rear 28 days self evaluation effective percentage (PPS analysis): the Rupatadine group is 81.71%, and the loratadine group is 76.14%, two group and compares, difference not statistically significant (P=0.3387).
Compare before and after the pruritus scoring before and after two groups of tests, two groups of prurituss all have decline after treatment, and significant difference (P<0.05) is arranged.
Pruritus scoring mean difference (PPS analysis) before and after two groups of patients test: the average drop-out value of Rupatadine group pruritus scoring is 4.83 ± 2.59, the average drop-out value of loratadine group pruritus scoring is 4.89 ± 2.60, two groups relatively, difference not statistically significant (P=0.9937).
3. safety indexes
Have 41 examples in test untoward reaction occur, the Rupatadine group has 19 examples time untoward reaction to occur, and incidence rate is 21.11%, is mainly manifested in drowsiness, xerostomia, headache, weak etc., and wherein 14 examples are slightly, 5 routine moderates, 0 routine severe; The loratadine group has 22 examples time untoward reaction to occur, and incidence rate is 24.18%, shows drowsiness, xerostomia, headache, weak etc., and wherein 17 examples are slight, 4 routine moderates, 1 routine severe; Two groups relatively, no difference of science of statistics.
Rupatadine group and loratadine group be lab testing (routine blood test, blood biochemical ALT, AST, BUN, Cr, QT interval) before and after test, more equal not statistically significant relatively and in group between group, illustrate Rupatadine group and matched group medication on lab index (routine blood test, blood biochemistry etc.) substantially without affecting.
Three, clinical trial overall assessment
Human pharmacokinetics studies show that, this product absorbs in healthy human body rapidly, and metabolite Desloratadine and 3-hydroxyl Desloratadine are all active metabolites, and pharmacological action time reaches 24h, and the successive administration Rupatadine was not accumulated in 8 days.
We at home many hospitals in strict accordance with the GCP requirement, two random, Double-blind double-dummies, loratadine tablet parallel control, multi-center clinical trial have been completed, result confirms Rupatadine fumarate sheet treatment seasonality or catarrhus perennialis and chronic idiopathic urticaria, its effectiveness and safety are not less than loratadine, therefore are suitable for treating the treatment of seasonality or catarrhus perennialis and chronic idiopathic urticaria.
Four, discuss
Rupatadine is H
1Receptor antagonist has antihistamine and antagonism platelet activating factor (PAF) dual function.The Claritin of great majority use mainly take antihistamine effect as main, does not have the medicine of clear and definite antagonism PAF so far clinically.Theoretically, simultaneously the medicine of blocking histamine and PAF biological effect will have better clinical effectiveness than blocking histamine effect only.Rupatadine is the Claritin that not only has antihistamine effect but also antagonism PAF activity of present unique listing, than cetirizine and loratadine, certain advantage is arranged.
The clinical test results confirmation, tablet clinical efficacy of the present invention is good, safe, for treating the excellent selection medicine of seasonality or catarrhus perennialis and chronic idiopathic urticaria, will bring huge benefit for the patient, is worthy of popularization.
Claims (9)
4. according to claim 1-3 described Rupatadine fumarate tablets of any one, is characterized in that described lubricant is selected from one or more in stearic acid, magnesium stearate, calcium stearate, Pulvis Talci, boric acid, sodium benzoate, sodium acetate, sodium chloride, polyoxyethylene monostearate, Brij30, DL-leucine, sodium laurylsulfate, magnesium laurylsulfate, Macrogol 4000 or polyethylene glycol 6000.
5. according to claim 1-3 described Rupatadine fumarate tablets of any one, is characterized in that described ferrum oxide is selected from one or both in yellow ferric oxide and red ferric oxide.
8. according to claim 1-7 described Rupatadine fumarate tablets of any one, is characterized in that, adopts 30 POVIDONE K 30 BP/USP
3085% alcoholic solution as binding agent.
9. the preparation method of the described Rupatadine fumarate tablet of claim 1-7 any one, is characterized in that, comprises the following steps:
(1) Rupatadine fumarate is crossed 100 mesh sieves, lactose, pregelatinized Starch, hyprolose were pulverized 80 mesh sieves, and be standby;
(2) get 30 POVIDONE K 30 BP/USP
30, add 85% appropriate amount of ethanol dissolving, make 10% solution, standby;
(3) red ferric oxide and yellow ferric oxide are mixed, adopt the equivalent mix homogeneously that progressively increases with appropriate lactose, then add Rupatadine fumarate, pregelatinized Starch, hyprolose and residue lactose mix homogeneously, add 10% 30 POVIDONE K 30 BP/USP
3085% alcoholic solution is made soft material in right amount, and 20 mesh sieves are granulated, and 50 ℃~70 ℃ dryings are controlled moisture 1.0%~3.0%, with 20 mesh sieve granulate;
(4) add mix lubricant even, measure granule content, tabletting, control strip focus on labelled amount ± 5% in, packing, and get final product.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201110370778.8A CN103120650B (en) | 2011-11-21 | 2011-11-21 | A kind of Rupatadine fumarate tablet |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201110370778.8A CN103120650B (en) | 2011-11-21 | 2011-11-21 | A kind of Rupatadine fumarate tablet |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN103120650A true CN103120650A (en) | 2013-05-29 |
| CN103120650B CN103120650B (en) | 2015-09-23 |
Family
ID=48451922
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201110370778.8A Active CN103120650B (en) | 2011-11-21 | 2011-11-21 | A kind of Rupatadine fumarate tablet |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN103120650B (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN106963738A (en) * | 2017-03-31 | 2017-07-21 | 华益药业科技(安徽)有限公司 | A kind of enalapril maleate piece of stabilization and preparation method thereof |
| CN107550874A (en) * | 2016-07-01 | 2018-01-09 | 扬子江药业集团江苏紫龙药业有限公司 | A kind of Rupatadine fumarate tablet and preparation method thereof |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1883480A (en) * | 2005-06-22 | 2006-12-27 | 北京德众万全医药科技有限公司 | A pharmaceutical composition containing rupatatine |
| CN1985816A (en) * | 2005-12-22 | 2007-06-27 | 汕头大学医学院 | Oral disintegrated rupatadine tablet and its preparing method |
| CN101590050A (en) * | 2008-05-29 | 2009-12-02 | 焦作平光制药有限责任公司 | Medicine of a kind of treatment of allergic rhinitis and preparation method thereof |
-
2011
- 2011-11-21 CN CN201110370778.8A patent/CN103120650B/en active Active
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1883480A (en) * | 2005-06-22 | 2006-12-27 | 北京德众万全医药科技有限公司 | A pharmaceutical composition containing rupatatine |
| CN1985816A (en) * | 2005-12-22 | 2007-06-27 | 汕头大学医学院 | Oral disintegrated rupatadine tablet and its preparing method |
| CN101590050A (en) * | 2008-05-29 | 2009-12-02 | 焦作平光制药有限责任公司 | Medicine of a kind of treatment of allergic rhinitis and preparation method thereof |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107550874A (en) * | 2016-07-01 | 2018-01-09 | 扬子江药业集团江苏紫龙药业有限公司 | A kind of Rupatadine fumarate tablet and preparation method thereof |
| CN106963738A (en) * | 2017-03-31 | 2017-07-21 | 华益药业科技(安徽)有限公司 | A kind of enalapril maleate piece of stabilization and preparation method thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CN103120650B (en) | 2015-09-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN102114044A (en) | Artificially processed bear bile powder and preparation method thereof | |
| CN105998014A (en) | Application of harmine derivative to preparation of drugs for treating cystic echinococcosis | |
| CN103120650B (en) | A kind of Rupatadine fumarate tablet | |
| CN1546027A (en) | Dripping pills for treating allergic disease and its preparation process | |
| CN103751141A (en) | Rupatadine fumarate tablets and preparation method thereof | |
| CN103304542B (en) | Rupatadine fumarate compound | |
| CN103142683B (en) | Be used for the treatment of Chinese medicinal perfusion liquid containing Herba Sophorae alopecuroidis total alkali of bovine mastitis and endometritis and preparation method thereof | |
| CN102988370A (en) | Application of tanshinone I in preparation of medicine for treating psoriasis | |
| CN102813907B (en) | Medicine composition for treating cerebrovascular accident sequela and preparation method and application thereof | |
| CN102342942A (en) | Novel oral solid medicinal composition and preparation method thereof | |
| Ferreira et al. | Clinical, pharmacokinetic and technological aspects of the hydroxychloroquine sulfate | |
| CN107129453B (en) | Compound, muscarinic M receptor antagonist, composition and application | |
| CN108272949B (en) | Application of traditional Chinese medicine composition in preparation of medicine for treating dementia | |
| CN102397281A (en) | A ginsenoside composition for treating cardiovascular and cerebrovascular diseases | |
| CN106727371B (en) | Donepezil hydrochloride pharmaceutical composition and preparation method thereof | |
| CN101002773A (en) | Use of hydrochloride coculine for preparing medicine to treat glioma | |
| CN101194906B (en) | Dropping pills for treating hypersensitivity disease and method for preparing the same | |
| CN102631467B (en) | Honglong antalgic medicinal composition, and preparation method and application thereof | |
| CN103690555B (en) | Pharmaceutical composition for treating acetyl cholinergic urticaria | |
| CN111450144B (en) | Application of ficus microcarpa leaf extract in preparation of medicines for preventing and/or treating diabetes and complications thereof | |
| CN102614326A (en) | Preparation method of Tibetan drug for treating keratoconjunctivitis | |
| CN112741879B (en) | Traditional Chinese medicine composition for treating schizophrenia | |
| CN102319378B (en) | Chinese medicinal composition for antagonizing side effect caused by chlorpromazine and preparation method thereof | |
| Yong et al. | Synergism in pharmacokinetics of retagliptin and metformin observed during clinical trials of their combination therapy | |
| CN112438952A (en) | Rupatadine fumarate tablet and preparation method thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| TR01 | Transfer of patent right |
Effective date of registration: 20210107 Address after: 620010 No.53, south section of Shunjiang Avenue, East District, Meishan Economic Development Zone, Sichuan Province Patentee after: Haisike Pharmaceutical (Meishan) Co.,Ltd. Address before: 611130 No.136 Baili Road, Chengdu cross strait science and Technology Industrial Development Park, Wenjiang District, Chengdu City, Sichuan Province Patentee before: SICHUAN HAISCO PHARMACEUTICAL Co.,Ltd. |
|
| TR01 | Transfer of patent right |