CN101774977A - Synthesis method of timolol maleate intermediates - Google Patents
Synthesis method of timolol maleate intermediates Download PDFInfo
- Publication number
- CN101774977A CN101774977A CN201010101684A CN201010101684A CN101774977A CN 101774977 A CN101774977 A CN 101774977A CN 201010101684 A CN201010101684 A CN 201010101684A CN 201010101684 A CN201010101684 A CN 201010101684A CN 101774977 A CN101774977 A CN 101774977A
- Authority
- CN
- China
- Prior art keywords
- dissolvent
- reaction
- timolol maleate
- timolol
- ring compound
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Landscapes
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
The invention discloses a synthesis method of timolol maleate intermediates, which takes a cyclic compound as a dissolvent. The synthesis method is characterized in that the cyclic compound is taken as the dissolvent, and amine diol and benzaldehyde generates oxazole product by cyclization; and the timolol maleate intermediate is obtained after distillation, wherein the molar ratio of the cyclic compound as the dissolvent to the amine diol is 1:2-6.5. The invention adopts the cyclic compound as the dissolvent to replace a highly toxic benzene dissolvent so as to obtain high-quality and high-yield timolol maleate intermediates. The synthesis method of the invention has simple operation in industrial production, repeatedly recycled dissolvent, and environment protection, and is more favorable for large-scale industrial production.
Description
Technical field:
The invention belongs to the organic chemistry synthesis technical field, relate to the preparation method of medicine intermediate, a kind of synthetic method of making the synthetic timolol maleate intermediate of solvent of ring compound of saying so more specifically.
Background technology:
Secondary glaucoma is some eye diseases and the complication of some hologathy in the eye appearance, can be caused by late period cataract or lens-dislocation.Increase (hypermature cataract) with age growth lens expanding volume, push the iris reach, block the angle, room, form crystal shape glaucoma.The pharmacological agent of secondary glaucoma can be divided into local application and systemic administration according to the administering mode difference.Local application's timolol maleate wherein is a kind of non-selective β-adrenergic receptor retarding agent, does not have tangible endogenous to intend sympathetic activity and local anesthetic action, and cardiac muscle is not had direct repression.Particularly the timolol maleate eye drops has good reduction intraocular pressure curative effect to primary open angle glaucoma.For some secondary glaucoma, ocular hypertension, part primary angle-closure glaucoma and other drug and the invalid glaucoma of performing the operation, its reduction intraocular pressure cutter reason really it be unclear that, and the reducing iop of tonography and aqueous humor fluorophotometric research prompting timolol maleate is relevant with the generation of minimizing aqueous humor.
Timolol Chinese another name: thiophene Xi'an, timolol, thiophene must be pacified, thiophene Ma Luoer etc.Be white crystalline powder; Odorless; Bitter.199~203 ℃ of fusing points (decomposing during fusion).In water or methyl alcohol, dissolve, molten in the ethanol part omitted, slightly soluble in chloroform, almost insoluble in ether.The aqueous solution is more stable when ph=12.Timolol is the β adrenergic receptor antagonist, effect of unrestraint cardiac muscle and endogenous plan sympathetic activity.Clinical pharmacology studies confirm that the beta receptor antagonist can change resting heart rate and the reaction of heart rate during to Body Position Change, suppresses the tachycardia that Racemic isoproterenol causes, changes the reaction to the test of Wa Er Savall, the variation of heart rate and blood pressure when taking in sail.And reduce positivity variable force due to the beta receptor agonist, when positivity becomes, segmental bronchus and vasorelaxation action.The degree of this reduction effect is directly proportional with sympathetic tone and the concentration in the receptor site thereof.Also can reduce healthy people and heart disease patient's cardiac output.For the patient that serious cardiac damage is arranged, beta-blocker can reduce sympathetic nervous system and keep the excitation that necessary heart function produces.It acts on segmental bronchus and bronchiole, can cause that Raw air way resistance increases.This effect has the patient of bronchospasm situation to have potentially dangerous for asthma and other.Timolol has hypotensive effect as novel non-selective beta-blockers, and can reduce angina pectoris attacks and pannonit consumption effectively, can reduce mortality ratio and recurrence rate for Acute Myocardial Infarction. and early stage application can reduce infarction size.This paper result confirms, timolol is more remarkable to controlling the anginal symptom of fatigue, improve ischemic type electrocardiogram(ECG, increase effect such as mobility endurance, and be better than Proprasylyte, or not be not worth clinical further application earlier for the anginal a kind of medicine preferably of treatment fatigue.
Treatment, prevention of migraine that the timolol early development is mainly used in after essential hypertension, stenocardia or the myocardial infarction are the beta-2 adrenoceptor retarding agent, found afterwards that this product still had the effect of tangible reduction intraocular pressure, action principle it is reported it mainly is because aqueous humor generates minimizing.Oral back 2 hours blood concentration peakings, about 5 hours of plasma half-life.Clinical essential hypertension, stenocardia, tachycardia and the glaucoma of being used for the treatment of.To light, the moderate hypertension curative effect is better, non-evident effect can share with diuretic(s).Myocardial infarction patient can reduce re-infarction incidence and mortality ratio after taking this product for a long time.To glaucoma, particularly primary, open angle glaucoma have good result, are better than traditional hypotensive agents, its characteristics be rapid-action, side effect is little, better tolerance.20 minutes intraocular pressures promptly begin to descend behind the eye drip, reach maximum effect through 1~2 hour, act on sustainable 24 hours.Pupil size light reflex and eyesight there is not influence.Some patient, this product and some anti-glaucoma medicines have summation action.In addition, aphakic glaucoma, some secondary glaucoma, ocular hypertension and other also there is certain curative effect to the medicine and the invalid glaucoma of performing the operation.
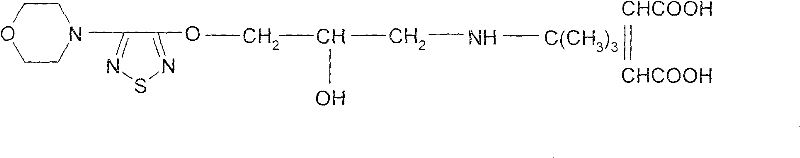
Timolol maleate Chinese name: S-(-)-1-(uncle's fourth amino)-3-[(4-morpholinyl-1,2,5-thiadiazoles-3-yl) oxygen]-2-propyl alcohol maleic acid salt.
Chemical name: S-(-)-1-(t-butyl)-3-[(4-morpholino-1,2,5-thiadiazole-3-) oxy]-2-propanol.
Structural formula:
About the synthetic method of chiral drug timolol maleate, according to bibliographical information, (S)-(-)-the chirality synthetic method of Timolol is summarized as follows
Route one: with D-Glycerose is that raw material and TERTIARY BUTYL AMINE catalytic hydrogenation in the presence of Pd/C get S-(-)-propylene glycol sulfonamide derivatives (1), yield 54%.Resulting S-(-)-propylene glycol sulfonamide derivatives and 3-chloro-4-(N-morpholine)-1,2, the reaction of 5-thiadiazoles obtains S-(-)-1-TERTIARY BUTYL AMINE base-3-[(4-morpholino-1,2,5-thiadiazoles-3-yl) oxygen]-2-propyl alcohol (2), obtain (S)-(-)-Timolol with the maleic acid salify again, but it is very low to go on foot yield.
Route two: route one is simple, convenient; but exist the low shortcoming of yield; for improving yield; can be by the alcoholic extract hydroxyl group on compound S-(-)-propylene glycol sulfonamide derivatives 1 (S) side chain be protected; form optically active intermediate 5 again; 5 and 3-chloro-4-(N-morpholine)-1; 2; the sodium salt reaction of 5-thiadiazoles; obtain S-(-)-1-TERTIARY BUTYL AMINE base-3-[(4-morpholino-1,2,5-thiadiazoles-3-yl) oxygen]-2-propyl alcohol (2); (2) obtain (S)-(-)-Timolol with the maleic acid salify again, yield 36%.
Route three: replace isomer for avoiding producing N-, improve yield, S-(-)-propylene glycol sulfonamide derivatives (1) and phenyl aldehyde condensation can be formed azoles alkane (8), azoles alkane (8) again with 3-chloro-4-(N-morpholine)-1,2, the 5-thiadiazoles reacts under the effect of potassium tert.-butoxide and generates S-(-)-1-TERTIARY BUTYL AMINE base-3-[(4-morpholino-1,2,5-thiadiazoles-3-yl) oxygen]-2-propyl alcohol (2), (2) obtain (S)-(-)-Timolol with the maleic acid salify again, yield 50%.
Route four: for avoiding the shortcoming of D-Glycerose source difficulty, available D-mannitol-1,2,5,6-two acetonylidenes (6) replace.D-mannitol-1,2,5,6-two acetonylidenes (6) generate (R)-Glycerose acetonylidene (7) of 2 molecules under the effect of lead tetra-acetate, (7) obtaining yield by reduction, amination and hydrolysis is 70% (1), (1) form azoles alkane with the phenyl aldehyde condensation, azoles alkane again with 3-chloro-4-(N-morpholine)-1,2, the 5-thiadiazoles reacts generation (2) under the effect of potassium tert.-butoxide, (2) obtain (S)-(-)-Timolol with the maleic acid salify again.
It is benzene that prior art is carried out this when reaction step selected solvent, and its toxicity is big, and consumption is big.Benzene reclaims difficulty.And product is not easily separated.We do solvent with ring compound, and glycol amine and phenyl aldehyde cyclization generate the azoles alkane derivative, again with the thiadiazoles condensation, through hydrolysis, finally draw S-type timolol.On the basis of reference and inspection information, through exploring the synthetic method of having determined this step, and synthetic method is optimized, eliminate toxicity, reduced cost, simplified operating process, ring compound of the present invention refers to: toluene, ethylbenzene, dioxane, 1, pyrimidine, pyrazine, pyrans, pyridine or hexanaphthene etc. are easy to reclaim, and can use repeatedly.Organic synthesis send out should in, ring compound is compared with other organic solvents, has solvent toxicity than low two ranks of benzene, and equipment is not had any infringement.Recyclable use repeatedly.Product is easily separated, after reaction is finished, as long as distillation can be used repeatedly.The characteristics that do not have exhaust gas emission.
Summary of the invention:
The objective of the invention is in order to reduce toxicity, guarantee the healthy of employee, reduced the environment that uses many solvents, the method for the synthetic timolol maleate intermediate of a kind of economy, easy, environmental protection is provided, and guarantees the quality of intermediate and higher yield.For achieving the above object, the invention provides following technical scheme:
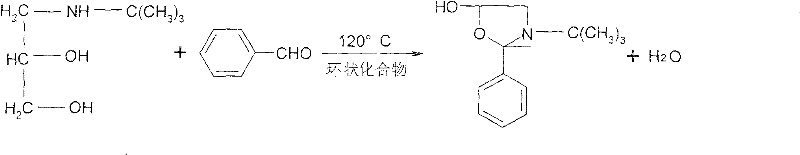
A kind of synthetic method of timolol maleate intermediate, it is characterized in that: in the ring compound solvent, glycol amine and phenyl aldehyde cyclization are generated the oxazole thing, obtain the timolol maleate intermediate after the distillation, wherein the mol ratio of ring compound solvent and glycol amine is 1: 2-6.5, glycol amine and phenyl aldehyde mol ratio 0.2-0.4: 0.91-1.82.
Synthetic method of the present invention, wherein glycol amine and ring compound mol ratio are 1: 2-4.5, reaction times 8-15 hour, preferred return time was 10-12 hour.Temperature of reaction is 113-150 ℃, and preferable reaction temperature is at 120-140 ℃.
Synthetic method of the present invention, wherein the ring compound solvent refers to: toluene, ethylbenzene, dioxane, 1, pyrimidine, pyrazine, pyrans, pyridine or hexanaphthene.
Reaction process of the present invention is following:
Ring compound of the present invention refers to that atom is often referred to organic compound with the compound of circular permutation in the molecule, as benzene.Several atoms are arranged in the ring, and this ring just claims " several units ring ".The number of " many rings " finger ring surpasses 1.For example, toluene, ethylbenzene, dioxane, 1, pyrimidine, pyrazine, pyrans, pyridine or hexanaphthene.
The consumption of ring compound and the mol ratio of glycol amine are 1: 2-6.5 is preferably 1: 2-3, and reaction times 8-15 hour, be preferably 10 hours, temperature of reaction is 113-150 ℃, is preferably 120-130 ℃.
The characteristics that synthetic method of the present invention is had compared with prior art:
(1) synthetic method of the present invention is easy and simple to handle on industrial production, and solvent can reclaim use repeatedly, and environmental protection helps large-scale industrial production.
(2) the present invention has reduced the toxic action of solvent, reduces the pollution to environment.
(3) because this intermediate does not have inspecting standard, this intermediate can only be continued to accomplish elaboration, the quality through checking this intermediate reaches does not have influence to final product quality, and gained elaboration steady quality, the yield height.
Embodiment
For simple and purpose clearly, hereinafter appropriate omission the description of known technology, in order to avoid those unnecessary details influences are to the description of the technical program.The present invention is described further below in conjunction with example.Wherein the glycol amine that is adopted, phenyl aldehyde, thiadiazoles all have commercially available.
Preparation embodiment 1.
With 30g glycol amine (0.2mol), and the 94ml phenyl aldehyde (3.28 times, 0.91mol), 70ml ring compound (dioxane 0.65mol, 1.82 doubly), drop in the reaction flask, temperature of reaction 113-115 ℃, refluxed 13 hours, decompression is steamed to 130 ℃, gets oxazole thing 47.9g, yield 99.89%.
Preparation embodiment 2.
With 30g glycol amine (0.2mol), and the 94ml phenyl aldehyde (3.28 times, 0.91mol), 62ml ring compound (toluene, 0.57mol, 1.6 times), drop in the reaction flask 120 ℃ of temperature of reaction, refluxed 10 hours, decompression is steamed to 130 ℃, gets oxazole thing 48g, yield 100.3%.
Preparation embodiment 3.
With 30g glycol amine (0.2mol), and the 94ml phenyl aldehyde (3.28 times, 0.91mol), 57ml ring compound (1,1.5 doubly, 0.52mol), drop in the reaction flask temperature of reaction 125-130 ℃, refluxed 8 hours, decompression is steamed to 130 ℃, gets oxazole thing 48.3g, yield 100.3%.
Preparation embodiment 4.
With 60g glycol amine (0.4mol), and the 188ml phenyl aldehyde (3.28 times, 1.82mol), 84ml ring compound (1.1 times of pyridines, 0.77mol), dropping in the reaction flask, 120 ℃ of temperature of reaction refluxed 8 hours, decompression is steamed to 130 ℃, gets oxazole thing 98.8g, yield 103%.
Preparation embodiment 5.
With 60g glycol amine (0.4mol), 188ml phenyl aldehyde (3.28 times of 1.82mol), 84ml ring compound (hexanaphthene, 1.1 times, 0.77mol), dropping in the reaction flask, 120 ℃ of temperature of reaction refluxed 10 hours, decompression is steamed to 130 ℃, gets oxazole thing 94.9g, yield 98.95%.
Preparation embodiment 6.
With 60g glycol amine (0.4mol), 188ml phenyl aldehyde (3.28 times of 1.82mol), the 84ml ring compound (1.1 times of dioxane, 0.77mol), drop in the reaction flask, 120 ℃ of temperature of reaction refluxed 10 hours, decompression is steamed to 130 ℃, gets oxazole thing 95.4g, yield 99.47%.
Reference example 1
Oxazole thing 95.4g that embodiment 1-6 is obtained and-thiadiazoles condensation; the method of reaction is as follows: trimethyl carbinol 150g is under protection of inert gas; react with 3gNa; react completely to Na, be cooled to 35 ℃, add S-(-)-2-phenyl-3-tertiary butyl-5-methylol oxazolidine 27g; isothermal reaction 1h; add 3 chloro-4-morpholine-thiadiazoles 27g, reaction 4h, condensation reaction finishes.
Reference example 2
Underpressure distillation is cooled to below 30 ℃ after going out the trimethyl carbinol, slowly drip 1.2N HCl 220g, 80 ℃ of following hydrolysis 2h again, hydrolyzed solution is isolated brown oil, and with 10%HCl extraction 2 times, merge acidizing fluid, acidizing fluid extracts 4 times with the 70CC hexanaphthene, and water is with extracting 4 times among the 26g NaHCO3 and with the 40g ethyl acetate, merge organic phase, with the dry 12h of the anhydrous CaCl2 of 25g, filter, use the ethyl acetate washing leaching cake, the toxilic acid 10g of porphyrize is added in the above-mentioned filtrate, constantly stir, place 2~3h, make its salify, filter, filter cake washs with ethyl acetate, normal temperature vacuum-drying, and crude product is with the finished product of the dehydrated alcohol recrystallization of 8~10 times of amounts.90.57g, mp199.5-202 ℃, specific optical rotation
-5.9Content
99.9%Total recovery 64.28%.Be consistent with the mass-spectrometric data of document Leader.
Reference:
1. all gold, Sun Shanshan etc.The study on the synthesis of chiral drug timolol.Jining Medical College journal 2006,29 (2), 27-30.
2. Zhou Zhishan, Zhu Liliang. optical activity timolol synthetic. the more chemical journal of high, 1988,9 (7): 743.
3.Weistock?LM.3-sulfonate?ester?of?2,3-dihydroxypropylamine.US4031125。
4. card man has, Li Tong. and the TLC a flat iron plate for making cakes of its intermediate of timolol tall building is other. Chinese Journal of Pharmaceuticals, 1991,22 (4): 174.
5. king's ability and political integrity, Lu Wei uses the state woods, etc. the preliminary study of its reducing iop of synthetic tall building of tartrate timolol, Chinese pharmaceutical chemistry magazine, 1994.4 (4): 289.
Claims (4)
1. the synthetic method of a timolol maleate intermediate, it is characterized in that: in the ring compound solvent, glycol amine and phenyl aldehyde cyclization are generated the oxazole thing, obtain the timolol maleate intermediate after the distillation, wherein the mol ratio of ring compound solvent and glycol amine is 1: 2-6.5, glycol amine and phenyl aldehyde mol ratio 0.2-0.4: 0.91-1.82.
2. the described synthetic method of claim 1, wherein glycol amine and ring compound mol ratio are 1: 2-4.5, reaction times 8-15 hour, temperature of reaction was 113-150 ℃.
3. the described synthetic method of claim 2, wherein cyclisation synthetic temperature of reaction is at 120-140 ℃, and return time is 10-12 hour.
4. claim 1 or 2 described synthetic methods, wherein the ring compound solvent refers to: toluene, dioxane, 1, pyridine or hexanaphthene.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2010101016846A CN101774977B (en) | 2010-01-28 | 2010-01-28 | Synthesis method of timolol maleate intermediates |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2010101016846A CN101774977B (en) | 2010-01-28 | 2010-01-28 | Synthesis method of timolol maleate intermediates |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN101774977A true CN101774977A (en) | 2010-07-14 |
| CN101774977B CN101774977B (en) | 2012-05-30 |
Family
ID=42511588
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN2010101016846A Active CN101774977B (en) | 2010-01-28 | 2010-01-28 | Synthesis method of timolol maleate intermediates |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN101774977B (en) |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1003711B (en) * | 1986-12-29 | 1989-03-29 | 国家医药管理局天津药物研究院 | Improvement in the synthesis of (l) 1, 2-dihydroxyl-3-tert-butylamino-propane |
-
2010
- 2010-01-28 CN CN2010101016846A patent/CN101774977B/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CN101774977B (en) | 2012-05-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| NO144455B (en) | PROCEDURE FOR THE PREPARATION OF THERAPEUTIC ACTIVE NEW PIPERIDE INGREDIENTS | |
| CA2903483A1 (en) | Substituted 3-phenylpropylamine derivatives for the treatment of ophthalmic diseases and disorders | |
| CN103435538B (en) | (R) preparation method of-3-amido piperidine hydrochlorate | |
| CN101062897B (en) | Improved process for preparing 2,3-dihydro-1H-indenes-1-amine and derivative thereof | |
| NO330143B1 (en) | Morpholine derivatives, preparations containing such, such compounds for use as a medicament and the use thereof for the manufacture of medicaments for the treatment of disease | |
| CN110194719A (en) | A kind of preparation method in R- (-)-levels Moses spit of fland | |
| MX2010012896A (en) | Imidazolidine derivatives. | |
| NO753246L (en) | ||
| JPS5989665A (en) | Piperazine derivative, manufacture and medicine | |
| CN101774977B (en) | Synthesis method of timolol maleate intermediates | |
| KR20070094647A (en) | Novel salt form of a dopamine agonist | |
| WO2019100785A1 (en) | Synthesis process for crizotinib intermediate | |
| CN101665441B (en) | Method for preparing l-betaxolol hydrochloride | |
| NO781556L (en) | BENZIMIDAZOLE DERIVATIVES. | |
| CN103058985A (en) | Novel process for preparing rotigotine | |
| JP2018503608A (en) | Polymorph of yonkenafil hydrochloride and its preparation method, composition and use | |
| JP2012505166A (en) | 1-Butyl-2-hydroxyaralkylpiperazine derivatives and their use as antidepressants | |
| CN102875499B (en) | The preparation method of 3-aminomethyl trimethylene oxide and organic acid salt thereof | |
| CN112645945A (en) | Preparation method of Wumei ammonium bromide intermediate | |
| JPH11124376A (en) | Synthesis of organic compound | |
| CN111303046A (en) | Biphenyl diaryl pyrimidine derivative containing chiral hydroxymethylene structure and preparation method and application thereof | |
| NO871744L (en) | NEW BENZOTIAZINO DERIVATIVES, PROCEDURES FOR THEIR PREPARATION, PHARMACEUTICALS CONTAINING THESE AND THEIR USE. | |
| CN109988162A (en) | One kind is according to piperazine Zole derivatives and preparation method thereof | |
| CN115785057B (en) | Preparation method of ticagrelor intermediate compound and salt thereof | |
| WO2024098856A1 (en) | Anti-influenza-virus derivatives and use thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant |