WO2017157332A1 - Aromatic amide derivative, preparation method therefor, and pharmaceutical applications thereof - Google Patents
Aromatic amide derivative, preparation method therefor, and pharmaceutical applications thereof Download PDFInfo
- Publication number
- WO2017157332A1 WO2017157332A1 PCT/CN2017/077114 CN2017077114W WO2017157332A1 WO 2017157332 A1 WO2017157332 A1 WO 2017157332A1 CN 2017077114 W CN2017077114 W CN 2017077114W WO 2017157332 A1 WO2017157332 A1 WO 2017157332A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- alkyl
- aryl
- cycloalkyl
- heteroaryl
- Prior art date
Links
- 0 C*(C1CCC2(CCC2)CC1)=C Chemical compound C*(C1CCC2(CCC2)CC1)=C 0.000 description 23
- CVISMXYMUGVLJB-UHFFFAOYSA-N CCS(c1cnc(CNC(c(cc2)cc3c2[n](C2CC2)c(C[n]2nc(C)cc2C)c3)=O)cc1)(=O)=O Chemical compound CCS(c1cnc(CNC(c(cc2)cc3c2[n](C2CC2)c(C[n]2nc(C)cc2C)c3)=O)cc1)(=O)=O CVISMXYMUGVLJB-UHFFFAOYSA-N 0.000 description 2
- GWVMLCQWXVFZCN-UHFFFAOYSA-N C1NCc2ccccc12 Chemical compound C1NCc2ccccc12 GWVMLCQWXVFZCN-UHFFFAOYSA-N 0.000 description 1
- WBUJZHRLDMGOTJ-UHFFFAOYSA-N CC(C)[n](c(Cc1c(C(F)(F)F)cccc1)cc1c2)c1ccc2C(O)=O Chemical compound CC(C)[n](c(Cc1c(C(F)(F)F)cccc1)cc1c2)c1ccc2C(O)=O WBUJZHRLDMGOTJ-UHFFFAOYSA-N 0.000 description 1
- LKOBCRBZIPNCNC-UHFFFAOYSA-N CC(C)[n](c(Cc1c(C(F)(F)F)cccc1)cc1c2)c1ccc2C(OC)=O Chemical compound CC(C)[n](c(Cc1c(C(F)(F)F)cccc1)cc1c2)c1ccc2C(OC)=O LKOBCRBZIPNCNC-UHFFFAOYSA-N 0.000 description 1
- UGUBYDRVJOZWSK-UHFFFAOYSA-N CC(C1)CC1NC Chemical compound CC(C1)CC1NC UGUBYDRVJOZWSK-UHFFFAOYSA-N 0.000 description 1
- YIXSKNWSNPIUBN-UHFFFAOYSA-N CC1(C)Nc(cccc2)c2OC1 Chemical compound CC1(C)Nc(cccc2)c2OC1 YIXSKNWSNPIUBN-UHFFFAOYSA-N 0.000 description 1
- KSGXWBUPARAZPX-UHFFFAOYSA-N CC1(C)Nc(ccnc2)c2OC1 Chemical compound CC1(C)Nc(ccnc2)c2OC1 KSGXWBUPARAZPX-UHFFFAOYSA-N 0.000 description 1
- RAPAJSMLVOFBHP-UHFFFAOYSA-N CCC1C(CC2)C2CC1 Chemical compound CCC1C(CC2)C2CC1 RAPAJSMLVOFBHP-UHFFFAOYSA-N 0.000 description 1
- VPWMFJRXSIYYGF-UHFFFAOYSA-N CCS(c1cc(Cl)c(CNC(c(cc2)cc3c2[n](C(C)C)c(Cc2ccc(C(F)(F)F)cc2)c3)=O)cc1)(=O)=O Chemical compound CCS(c1cc(Cl)c(CNC(c(cc2)cc3c2[n](C(C)C)c(Cc2ccc(C(F)(F)F)cc2)c3)=O)cc1)(=O)=O VPWMFJRXSIYYGF-UHFFFAOYSA-N 0.000 description 1
- HZGCZYVVTXVNAA-UHFFFAOYSA-N CCS(c1ccc(CNC(c(c(Cl)c2)cc3c2[n](C2CC2)c(Cc(cc2)c(C)cc2F)c3)=O)cc1)(=O)=O Chemical compound CCS(c1ccc(CNC(c(c(Cl)c2)cc3c2[n](C2CC2)c(Cc(cc2)c(C)cc2F)c3)=O)cc1)(=O)=O HZGCZYVVTXVNAA-UHFFFAOYSA-N 0.000 description 1
- RJTXLIBXUQVLKA-UHFFFAOYSA-N CCS(c1ccc(CNC(c(cc2)cc3c2[n](C(C)C)c(Cc2c(C)cccc2)c3)=O)cc1)(=O)=O Chemical compound CCS(c1ccc(CNC(c(cc2)cc3c2[n](C(C)C)c(Cc2c(C)cccc2)c3)=O)cc1)(=O)=O RJTXLIBXUQVLKA-UHFFFAOYSA-N 0.000 description 1
- VWODIXOWLZQABY-UHFFFAOYSA-N CCS(c1ccc(CNC(c(cc2)cc3c2[n](C(C)C)c(Cc2cccc(C(F)(F)F)c2)n3)=O)cc1)(=O)=O Chemical compound CCS(c1ccc(CNC(c(cc2)cc3c2[n](C(C)C)c(Cc2cccc(C(F)(F)F)c2)n3)=O)cc1)(=O)=O VWODIXOWLZQABY-UHFFFAOYSA-N 0.000 description 1
- YPDMWRSPHLRGOM-UHFFFAOYSA-N CCS(c1ccc(CNC(c(cc2)cc3c2[n](C)c(Cc(ccc(C)c2)c2F)c3)=O)cc1)(=O)=O Chemical compound CCS(c1ccc(CNC(c(cc2)cc3c2[n](C)c(Cc(ccc(C)c2)c2F)c3)=O)cc1)(=O)=O YPDMWRSPHLRGOM-UHFFFAOYSA-N 0.000 description 1
- UCKMMPMKBSUTEW-UHFFFAOYSA-N COC(c1ccc2[nH]c(Cc3ccccc3C(F)(F)F)cc2c1)=O Chemical compound COC(c1ccc2[nH]c(Cc3ccccc3C(F)(F)F)cc2c1)=O UCKMMPMKBSUTEW-UHFFFAOYSA-N 0.000 description 1
- DRYBMFJLYYEOBZ-UHFFFAOYSA-N COC(c1ccc2[nH]ccc2c1)=O Chemical compound COC(c1ccc2[nH]ccc2c1)=O DRYBMFJLYYEOBZ-UHFFFAOYSA-N 0.000 description 1
- JICVFOZWBQICDN-AFPNSQJFSA-N C[C@H](C1)CC2C1CCNC2 Chemical compound C[C@H](C1)CC2C1CCNC2 JICVFOZWBQICDN-AFPNSQJFSA-N 0.000 description 1
- WRLIXOXLMFOGDX-UHFFFAOYSA-N C[N](C)(C)C1CCC1 Chemical compound C[N](C)(C)C1CCC1 WRLIXOXLMFOGDX-UHFFFAOYSA-N 0.000 description 1
- TXVVVEUSVBLDED-UHFFFAOYSA-N FC(c1c(CBr)cccc1)(F)F Chemical compound FC(c1c(CBr)cccc1)(F)F TXVVVEUSVBLDED-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
- A61K31/4184—1,3-Diazoles condensed with carbocyclic rings, e.g. benzimidazoles
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/02—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom condensed with one carbocyclic ring
- C07D209/04—Indoles; Hydrogenated indoles
- C07D209/10—Indoles; Hydrogenated indoles with substituted hydrocarbon radicals attached to carbon atoms of the hetero ring
- C07D209/12—Radicals substituted by oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D235/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings
- C07D235/02—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings condensed with carbocyclic rings or ring systems
- C07D235/04—Benzimidazoles; Hydrogenated benzimidazoles
- C07D235/06—Benzimidazoles; Hydrogenated benzimidazoles with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached in position 2
- C07D235/10—Radicals substituted by halogen atoms or nitro radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D235/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings
- C07D235/02—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings condensed with carbocyclic rings or ring systems
- C07D235/04—Benzimidazoles; Hydrogenated benzimidazoles
- C07D235/06—Benzimidazoles; Hydrogenated benzimidazoles with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached in position 2
- C07D235/12—Radicals substituted by oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/06—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a carbon chain containing only aliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
Definitions
- the invention belongs to the field of medicine, and relates to an aromatic amide derivative, a preparation method thereof and application thereof in medicine.
- the present invention relates to an aromatic amide derivative represented by the formula (I), a process for producing the same, and a pharmaceutical composition containing the same, which are useful as ROR modulators and for preventing and/or treating autoimmune diseases and the like the use of.
- RORs Retinoic acid-related orphan nuclear receptors
- the ROR family contains three types of ROR ⁇ , ROR ⁇ , and ROR ⁇ .
- Three different RORs can be expressed in different tissues and control different physiological processes.
- ROR ⁇ is mainly distributed in liver, skeletal muscle, skin, lung, adipose tissue, kidney, thymus and brain.
- ROR ⁇ has a small range of action, mainly acting on In the central nervous system, ROR ⁇ can be expressed in many tissues, including liver, animal fat, and skeletal muscle. The lack of ROR ⁇ in mammals shows a decrease in blood glucose.
- ROR ⁇ 1 is expressed in many tissues such as thymus, muscle, kidney and liver, while ROR ⁇ 2 is expressed only in immune cells, and ROR ⁇ 2 is thought to control T cell-assisted T cell 17 (Th17) differentiation.
- Th17 is a class of helper T cells that produce interleukin-17 (IL-17) and other cytokines. Th-17 has been found to be associated with human inflammatory diseases and immune disorders, such as multiple sclerosis, Rheumatoid arthritis, psoriasis, clonal diseases and asthma are related. Now, it is reported in the literature that ROR ⁇ may be related to the occurrence and development of prostate cancer.
- ROR ⁇ t is a subtype of ROR ⁇ specifically expressed on immune cells. It is a major transcription factor of human and murine Th17 cells, which not only promotes the differentiation of Th17 cells, but also regulates the expression and secretion of the specific effector factor IL-17 of Th17 cells. ROR ⁇ t is closely related to the occurrence and development of various immune diseases, infectious diseases and tumors.
- ROR ⁇ particularly the ROR ⁇ t type
- Vanov et al. found that ROR ⁇ t is an important transcription factor for Th17 cell differentiation in mouse experiments. Their study showed that mice were difficult to induce the formation of an EAE model in the absence of ROR ⁇ t.
- ROR ⁇ t was also confirmed to have a similar important role. The technological discovery caused people to attach great importance to ROR ⁇ t.
- the inventors found that in the compound of the formula (I) of the present invention, the change of the ortho group of the ring A changes its regulation effect, when the ring A is ortho
- the group is a group having a small steric hindrance (for example, H and F)
- the compound represented by the formula (I) is an inverse agonist
- the ring A ortho group is halogenated
- a compound represented by the general formula (I) is a compound having a sterically hindered group such as an alkyl group (for example, a trifluoromethyl group), an alkyl group (for example, an ethyl group), and a halogenated alkoxy group (for example, a trifluoromethoxy group).
- the present invention has developed a new generation of ROR modulators, and further studies have found that structural changes in compounds can modulate different mechanisms.
- X, Y and Z are the same or different and are each independently CR 9 or N;
- Ring A and Ring B are the same or different and are each independently selected from the group consisting of a cycloalkyl group, a heterocyclic group, an aryl group, and a heteroaryl group;
- R 1 and R 2 are the same or different and are each independently selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen, an amino group, a nitro group, a hydroxyl group, a cyano group, and a heterocyclic ring.
- Alkyl, cycloalkyl, alkoxy, haloalkyl, heterocyclyl, aryl and heteroaryl are each independently optionally selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, Substituted with one or more substituents of alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl;
- R 3 and R 4 are the same or different and are each independently selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen group, an amino group, a nitro group, a hydroxyl group, a cyano group, and a heterocyclic ring.
- a base an aryl group, a heteroaryl group, -OR 8 , -C(O)R 8 , -C(O)OR 8 and -S(O) m R 8 , wherein said alkyl group, cycloalkyl group, halogenated alkane
- the radical, heterocyclyl, aryl and heteroaryl are each independently selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, Substituted by one or more substituents in the cycloalkyl, heterocyclyl, aryl and heteroaryl groups;
- R 3 and R 4 form an oxo group
- R 5 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, an amino group, an alkenyl group, an alkynyl group, an aryl group, a heteroaryl group, -OR 8 , -C(O)R 8 , —C(O)OR 8 and —S(O) m R 8 , wherein said alkyl, cycloalkyl, haloalkyl, aryl and heteroaryl are each independently optionally selected from alkyl, One or more of haloalkyl, halogen, amino, nitro, cyano, hydroxy, alkenyl, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl Substituted by a substituent;
- R 6 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen, an amino group, a nitro group, a hydroxyl group, a cyano group, a heterocyclic group, an aryl group, a heteroaryl group, -OR 8 , —C(O)R 8 , —C(O)OR 8 and —S(O) m R 8 , wherein said alkyl, cycloalkyl, haloalkyl, heterocyclyl, aryl and heteroaryl
- the groups are each independently optionally selected from the group consisting of alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, ary
- R 7 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a heterocyclic group, an aryl group, and a heteroaryl group;
- R 8 is selected from the group consisting of a hydrogen atom, an alkyl group, a halogenated alkyl group, an alkoxy group, a hydroxyalkyl group, a hydroxyl group, an amino group, a cycloalkyl group, a heterocyclic group, an aryl group, and a heteroaryl group, wherein the alkyl group, the amino group, and the ring are The alkyl group, heterocyclic group, aryl group and heteroaryl group are each independently selected from the group consisting of alkyl, halogen, hydroxy, amino, carboxylate, nitro, cyano, alkoxy, hydroxyalkyl, cyclo Substituted by one or more substituents of an alkyl group, a heterocyclic group, an aryl group, and a heteroaryl group;
- R 9 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, an amino group, an alkenyl group, an alkynyl group, an aryl group and a heteroaryl group, wherein the alkyl group, the cycloalkyl group And haloalkyl, aryl and heteroaryl are each independently selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkenyl, alkoxy, haloalkoxy, hydroxyalkyl Substituting one or more substituents of a cycloalkyl, a heterocyclic group, an aryl group, and a heteroaryl group;
- n 0, 1 or 2;
- x 0, 1, 2, 3 or 4;
- y 0, 1, 2 or 3;
- z 0, 1, 2, 3 or 4.
- the compound of the formula (I) is a compound of the formula (II):
- X is CR 9 or N
- Y is CH or N
- R 9 is a hydrogen atom or an alkyl group
- Ring A, Ring B, R 1 to R 7 , x, y and z are as defined in the general formula (I).
- the compound of the formula (I) wherein A ring and The B rings are the same or different and are each independently selected from the group consisting of a heterocyclic group, an aryl group, and a heteroaryl group.
- the compound of the formula (I) is a compound of the formula (II-A):
- X is CR 9 or N; R 9 is a hydrogen atom or an alkyl group;
- G is CH or N
- R a is selected from the group consisting of alkyl, cycloalkyl, haloalkyl, alkoxy, haloalkoxy, bromo, amino, nitro, hydroxy, cyano, heterocyclyl, aryl, heteroaryl, -OR 8 , - C(O)R 8 , —C(O)NHR 8 , —C(O)OR 8 and —S(O) m R 8 , wherein said alkyl group, cycloalkyl group, alkoxy group, haloalkyl group,
- the heterocyclic group, the aryl group and the heteroaryl group are each independently selected from the group consisting of alkyl, haloalkyl, halogen, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkane. Substituted by one or more substituents in the group, heterocyclic group, aryl group and heteroary
- p 0, 1, 2 or 3;
- Ring A, R 1 to R 8 , m, y and z are as defined in the general formula (I).
- the compound of the formula (I) is a compound of the formula (III):
- X is CR 9 or N
- Y is CH or N
- R 9 is a hydrogen atom or an alkyl group
- Ring A, R 1 to R 7 , x, y and z are as defined in the general formula (I).
- the compound of the formula (I) is a compound of the formula (IV):
- X is CR 9 or N
- Y is CH or N
- R 9 is a hydrogen atom or an alkyl group
- R 1 to R 7 , x, y and z are as defined in the formula (I).
- the compound represented by the formula (I) is a compound represented by the formula (IV-A), the formula (IV-B) or the formula (IV-C). :
- X is CR 9 or N
- Y is CH or N
- R 9 is a hydrogen atom or an alkyl group
- R a is selected from the group consisting of alkyl, cycloalkyl, haloalkyl, alkoxy, haloalkoxy, amino, nitro, hydroxy, cyano, heterocyclyl, aryl, heteroaryl, -OR 8 , -C ( O) R 8 , -C(O)NHR 8 , -C(O)OR 8 and -S(O) m R 8 , wherein said alkyl group, cycloalkyl group, alkoxy group, haloalkyl group, heterocyclic ring
- the aryl group, the aryl group and the heteroaryl group are each independently selected from the group consisting of alkyl, haloalkyl, halogen, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, Substituting one or more substituents in a heterocyclic group, an aryl group, and
- R b is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen, an amino group, a nitro group, a hydroxyl group, a cyano group, a heterocyclic group, an aryl group, a heteroaryl group, -OR 8 , —C(O)R 8 , —C(O)NHR 8 , —C(O)OR 8 and —S(O) m R 8 , wherein said alkyl group, cycloalkyl group, alkoxy group, Haloalkyl, heterocyclyl, aryl and heteroaryl are each independently optionally selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl Substituting one
- p 0, 1, 2 or 3;
- q 0, 1, or 2;
- R 1 to R 8 , m, y and z are as defined in the formula (I).
- the compound of the formula (IV-A) is a compound of the formula (IV-A-1):
- R 1 to R 7 , p, y and z are as defined in the formula (IV-A).
- R 1 is selected from the group consisting of an alkyl group, a halogen, a halogenated alkyl group, an alkoxy group, and a halogenated alkoxy group
- R 8 is selected from a hydrogen atom or an alkyl group.
- R 5 is selected from the group consisting of alkyl, cycloalkyl, haloalkyl, alkenyl and aryl, wherein the alkyl group
- the cycloalkyl and aryl groups are each independently optionally substituted with one or more substituents selected from the group consisting of alkyl, halogen, alkenyl and hydroxy; preferably R 5 is isopropyl or cyclopropyl.
- Typical compounds of formula (I) include, but are not limited to:
- a tautomer a meso form, a racemate, an enantiomer, a diastereomer or a mixture thereof, or a pharmaceutically acceptable salt thereof.
- the invention further provides a preparation according to the compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer, or a mixture thereof, Or an intermediate thereof, or a compound of the formula (V):
- X, Y and Z are the same or different and are each independently CR 9 or N;
- Ring A is selected from the group consisting of a cycloalkyl group, a heterocyclic group, an aryl group, and a heteroaryl group;
- R 1 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen, an amino group, a nitro group, a hydroxyl group, a cyano group, a heterocyclic group, an aryl group, a heteroaryl group, -OR 8 , —C(O)R 8 , —C(O)NHR 8 , —C(O)OR 8 and —S(O) m R 8 , wherein said alkyl group, cycloalkyl group, alkoxy group, Haloalkyl, heterocyclyl, aryl and heteroaryl are each independently optionally selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl Substituting one or
- R 3 and R 4 are the same or different and are each independently selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen group, an amino group, a nitro group, a hydroxyl group, a cyano group, and a heterocyclic ring.
- a base an aryl group, a heteroaryl group, -OR 8 , -C(O)R 8 , -C(O)OR 8 and -S(O) m R 8 , wherein said alkyl group, cycloalkyl group, halogenated alkane
- the radical, heterocyclyl, aryl and heteroaryl are each independently selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, Substituted by one or more substituents in the cycloalkyl, heterocyclyl, aryl and heteroaryl groups;
- R 3 and R 4 form an oxo group
- R 5 is selected from the group consisting of alkyl, cycloalkyl, haloalkyl, alkoxy, haloalkoxy, amino, alkenyl, alkynyl, aryl, heteroaryl, -OR 8 , -C(O)R 8 ,- C(O)OR 8 and -S(O) m R 8 , wherein said alkyl, cycloalkyl, haloalkyl, aryl and heteroaryl are each independently optionally selected from alkyl, haloalkyl, One or more of halogen, amino, nitro, cyano, hydroxy, alkenyl, alkynyl, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl Substituted by a substituent;
- R 6 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen, an amino group, a nitro group, a hydroxyl group, a cyano group, a heterocyclic group, an aryl group, a heteroaryl group, -OR 8 , —C(O)R 8 , —C(O)OR 8 and —S(O) m R 8 , wherein said alkyl, cycloalkyl, haloalkyl, heterocyclyl, aryl and heteroaryl
- the groups are each independently optionally selected from the group consisting of alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, ary
- R 8 is selected from the group consisting of a hydrogen atom, an alkyl group, a halogenated alkyl group, an alkoxy group, a hydroxyalkyl group, a hydroxyl group, an amino group, a cycloalkyl group, a heterocyclic group, an aryl group, and a heteroaryl group, wherein the alkyl group, the amino group, and the ring are The alkyl group, heterocyclic group, aryl group and heteroaryl group are each independently selected from the group consisting of alkyl, halogen, hydroxy, amino, carboxylate, nitro, cyano, alkoxy, hydroxyalkyl, cyclo Substituted by one or more substituents of an alkyl group, a heterocyclic group, an aryl group, and a heteroaryl group;
- R 9 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, an amino group, an alkenyl group, an alkynyl group, an aryl group and a heteroaryl group, wherein the alkyl group, the cycloalkyl group And haloalkyl, aryl and heteroaryl are each independently selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkenyl, alkoxy, haloalkoxy, hydroxyalkyl Substituting one or more substituents of a cycloalkyl, a heterocyclic group, an aryl group, and a heteroaryl group;
- n 0, 1 or 2;
- x 0, 1, 2, 3 or 4;
- y 0, 1, 2 or 3.
- the invention further provides a preparation of a compound according to formula (II-A) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer, or a mixture thereof
- An intermediate of the form, or a pharmaceutically acceptable salt thereof, is a compound of the formula (II-A-1):
- Ring A, X, R a , R 1 , R 3 to R 6 , p and y are as defined in the formula (II-A).
- the invention further provides a process for the preparation of said compound of formula (I), which process comprises:
- Ring A, Ring B, X, Y, Z, R 1 to R 7 , x, y and z are as defined in the general formula (I).
- the invention further provides a process for the preparation of the compound of the formula (II-A), which process comprises:
- a compound of the formula (II-A-1) is condensed with a compound of the formula (II-A-2) to give a compound of the formula (II-A);
- Rings A, G, X, R a , R 1 to R 7 , p, y and z are as defined in the formula (II-A).
- Another aspect of the invention relates to a pharmaceutical composition
- a pharmaceutical composition comprising a therapeutically effective amount of the formula (I), (II), (III), (IV), (IV-A), (IV-B), a compound represented by IV-C) or (V) or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer, or a mixture thereof, or A pharmaceutically acceptable salt, and one or more pharmaceutically acceptable carriers, diluents or excipients.
- the invention further relates to a process for the preparation of the above composition
- a process for the preparation of the above composition comprising the general formulae (I), (II), (III), (IV), (IV-A), (IV-B), (IV-C) Or a compound represented by (V) or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer, or a mixture thereof, or a pharmaceutically acceptable form thereof
- the salt is mixed with a pharmaceutically acceptable carrier, diluent or excipient.
- the invention further relates to a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, for use as a ROR modulator in the manufacture of a medicament for the prevention and/or treatment of inflammation, autoimmune diseases, tumors or cancer.
- the present invention further relates to a compound represented by the formula (I) or a tautomer thereof, a mesogen, and an external a racemate, an enantiomer, a diastereomer or a mixture thereof, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition comprising the same, for use as a ROR inverse agonist in the preparation for prophylaxis and/or treatment Use in medicines for inflammation and autoimmune diseases.
- the invention further relates to a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, for use as a ROR agonist in the manufacture of a medicament for the prevention and/or treatment of a tumor or cancer.
- the invention further relates to the use of a compound of the formula (IV-A) as an ROR agonist for the preparation of a medicament for the prevention and/or treatment of a tumor or cancer.
- the invention further relates to a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, for use in the manufacture of a medicament for the treatment of a tumor or cancer with a combination of an ROR agonist and an anti-PD-1 antibody.
- the invention further relates to the use of a compound of the formula (IV-A) as an ROR agonist for the preparation of a medicament for the treatment of a tumor or cancer in combination with an anti-PD-1 antibody.
- the invention further relates to a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer or a mixture thereof, or Use of a pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, in the manufacture of a medicament for the modulation of ROR.
- the invention further relates to a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer or a mixture thereof, or Use of a pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, in the manufacture of a medicament for an ROR agonist.
- the invention further relates to the use of a compound of the formula (IV-A) for the manufacture of a medicament for an ROR agonist.
- the invention further relates to a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer or a mixture thereof, or Use of a pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, for the manufacture of a medicament for the prevention and/or treatment of inflammatory and autoimmune diseases.
- the inflammatory and autoimmune diseases are selected from the group consisting of psoriasis, rheumatoid arthritis, psoriatic arthritis, multiple sclerosis, inflammatory bowel disease, ankylosing spondylitis, chronic obstructive pulmonary disease , glomerulonephritis, myocarditis, thyroiditis, dry eye, uveitis, Behcet's disease, asthma, allergic skin Inflammation, acne, Crohn's disease, ulcerative colitis, systemic lupus erythematosus, scleroderma, bronchitis and dermatomyositis allergic rhinitis.
- the invention further relates to a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer or a mixture thereof, or Use of a pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, in the manufacture of a medicament for the prevention and/or treatment of a tumor or cancer.
- the cancer and tumor are selected from the group consisting of non-Hodgkin's lymphoma, diffuse large B-cell lymphoma, follicular lymphoma, synovial sarcoma, breast cancer, cervical cancer, colon cancer, lung cancer, stomach cancer, rectum Cancer, pancreatic cancer, brain cancer, skin cancer, oral cancer, prostate cancer, bone cancer, kidney cancer, ovarian cancer, bladder cancer, liver cancer, fallopian tube tumor, ovarian tumor, peritoneal tumor, stage IV melanoma, solid tumor, nerve glue Tumor, glioblastoma, hepatocellular carcinoma, papillary renal tumor, head and neck cancer, leukemia, lymphoma, myeloma and non-small cell lung cancer, especially non-Hodgkin's lymphoma, diffuse large B-cell lymph Tumor, follicular lymphoma, synovial sarcoma.
- the invention further relates to a compound of the formula (I) or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer or In the form of a mixture, or a pharmaceutically acceptable salt thereof.
- the present invention also relates to a compound of the formula (I) or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, for use in the prevention and/or treatment of an inflammatory or autoimmune disease, wherein the inflammatory and autoimmune diseases are as defined above.
- the present invention also relates to a compound of the formula (I) or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, for use in the prevention and/or treatment of a tumor or cancer, wherein said tumor and cancer are as defined above.
- the present invention also relates to a compound represented by the formula (IV-A) for use in the prevention and/or treatment of a tumor or a cancer, wherein the tumor and the cancer are as defined above.
- the invention further relates to a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, is for use in a ROR agonist and an anti-PD-1 antibody composition for preventing and/or treating a tumor or cancer, wherein the tumor and cancer are as defined above.
- the present invention also relates to a compound represented by the formula (IV-A) and an anti-PD-1 antibody composition for use in the prevention and/or treatment of a tumor or a cancer, wherein the tumor and the cancer are as defined above.
- the present invention also relates to a method for the prophylaxis and/or treatment of a prophylactic or autoimmune disease comprising administering to a patient in need thereof a therapeutically effective amount of a compound of the formula (I) or a tautomer thereof. , a meso form, a racemate, an enantiomer, a diastereomer or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same.
- a compound of the formula (I) or a tautomer thereof a meso form, a racemate, an enantiomer, a diastereomer or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same.
- the inflammatory and autoimmune diseases described therein are as defined above.
- the present invention also relates to a method for the prophylactic and/or therapeutic treatment of preventing tumors or cancer comprising administering to a patient in need thereof a therapeutically effective amount of a compound of the formula (I) or a tautomer thereof, internal elimination A form of a polar body, a racemate, an enantiomer, a diastereomer or a mixture thereof, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition comprising the same.
- the tumors and cancers described therein are as defined above.
- the present invention also relates to a method of treating prevention and/or treatment for preventing tumor or cancer comprising administering to a patient in need thereof a therapeutically effective amount of a compound of the formula (IV-A).
- a method of treating prevention and/or treatment for preventing tumor or cancer comprising administering to a patient in need thereof a therapeutically effective amount of a compound of the formula (IV-A).
- the present invention also relates to a method for the prophylactic and/or therapeutic treatment of preventing tumors or cancer comprising administering to a patient in need thereof a therapeutically effective amount of a compound of the formula (IV-A) and an anti-PD-1 antibody composition.
- a compound of the formula (IV-A) and an anti-PD-1 antibody composition comprising administering to a patient in need thereof a therapeutically effective amount of a compound of the formula (IV-A) and an anti-PD-1 antibody composition.
- the tumors and cancers described therein are as defined above.
- the present invention also relates to a compound of the formula (I) or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, for use in modulating ROR.
- the invention further relates to compounds of the general formula (IV-A) for use in ROR agonists.
- the present invention also relates to a compound of the formula (IV-A) and an anti-PD-1 antibody composition for use in an ROR agonist.
- the invention also relates to a method of modulating ROR comprising administering to a patient in need thereof a therapeutically effective amount of a compound of formula (I) or a tautomer, a mesogen, a racemate thereof, An enantiomer, a diastereomer or a mixture thereof, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition comprising the same.
- the active ingredient-containing pharmaceutical composition may be in a form suitable for oral administration, such as tablets, dragees, lozenges, aqueous or oily suspensions, dispersible powders or granules, emulsions, hard or soft capsules, or syrups or Tincture.
- Oral compositions can be prepared according to any method known in the art for preparing pharmaceutical compositions, such compositions may contain one or more ingredients selected from the group consisting of sweeteners, flavoring agents, coloring agents, and preservatives, To provide a pleasing and tasty pharmaceutical preparation. Tablets contain the active ingredient and non-toxic pharmaceutically acceptable excipients suitable for the preparation of a tablet for admixture.
- excipients may be inert excipients such as calcium carbonate, sodium carbonate, lactose, calcium phosphate or sodium phosphate; granulating agents and disintegrating agents such as microcrystalline cellulose, croscarmellose sodium, corn Starch or alginic acid; a binder such as starch, gelatin, polyvinylpyrrolidone or gum arabic; and a lubricant such as magnesium stearate, stearic acid or talc.
- These tablets may be uncoated or may be packaged by a known technique that provides a sustained release effect over a longer period of time by masking the taste of the drug or delaying disintegration and absorption in the gastrointestinal tract. clothes.
- water-soluble taste masking materials such as hydroxypropylmethylcellulose or hydroxypropylcellulose, or extended-time materials such as ethylcellulose, cellulose acetate butyrate may be used.
- hard gelatin capsules in which the active ingredient is mixed with an inert solid diluent such as calcium carbonate, calcium phosphate or kaolin, or in which the active ingredient is mixed with a water-soluble carrier such as polyethylene glycol or an oil vehicle such as peanut oil, liquid paraffin or olive oil.
- Soft gelatin capsules provide oral preparations.
- the aqueous suspension contains the active substance and excipients suitable for the preparation of the aqueous suspension for mixing.
- excipients are suspending agents such as sodium carboxymethylcellulose, methylcellulose, hydroxypropylmethylcellulose, sodium alginate, polyvinylpyrrolidone and acacia; dispersing or wetting agents may be naturally occurring a phospholipid such as lecithin, or a condensation product of an alkylene oxide with a fatty acid such as polyoxyethylene stearate, or a condensation product of ethylene oxide with a long chain fatty alcohol, such as heptadecylethyleneoxy cetyl alcohol (heptadecaethyleneoxy cetanol), or a condensation product of ethylene oxide with a partial ester derived from a fatty acid and a hexitol, such as polyethylene oxide sorbitol monooleate, or ethylene oxide with derivatives derived from fatty acids and hexitols
- the aqueous suspensions may also contain one or more preservatives such as ethylparaben or n-propylparaben, one or more coloring agents, one or more flavoring agents, and one or more sweetening agents.
- preservatives such as ethylparaben or n-propylparaben
- coloring agents such as ethylparaben or n-propylparaben
- flavoring agents such as sucrose, saccharin or aspartame.
- the oil suspension can be formulated by suspending the active ingredient in a vegetable oil such as peanut oil, olive oil, sesame oil or coconut oil, or a mineral oil such as liquid paraffin.
- the oily suspensions may contain a thickening agent, such as beeswax, hard paraffin or cetyl alcohol.
- the above sweeteners and flavoring agents may be added to provide a palatable preparation.
- These compositions can be preserved by the addition of an anti-oxidant such as butylated hydroxyanisole or alpha-tocopherol.
- the dispersible powders and granules suitable for the preparation of aqueous suspensions can be provided by the addition of water to provide the active ingredient and dispersing or wetting agents, suspending agents or one or more preservatives. Suitable dispersing or wetting agents and suspending agents can be used to illustrate the above examples. Other excipients such as sweetening, flavoring, and coloring agents may also be added. These compositions are preserved by the addition of an anti-oxidant such as ascorbic acid.
- the pharmaceutical compositions of the invention may also be in the form of an oil-in-water emulsion.
- the oil phase may be a vegetable oil such as olive oil or peanut oil, or a mineral oil such as liquid paraffin or a mixture thereof.
- Suitable emulsifiers may be naturally occurring phospholipids, such as soy lecithin and esters or partial esters derived from fatty acids and hexitol anhydrides such as sorbitan monooleate, and condensation products of the partial esters and ethylene oxide, For example, polyethylene oxide sorbitol monooleate.
- the emulsions may also contain sweeteners, flavoring agents, preservatives, and antioxidants.
- Syrups and elixirs may be formulated with sweetening agents such as glycerol, propylene glycol, sorbitol or sucrose. Such formulations may also contain a demulcent, a preservative, a colorant, and an antioxidant.
- sweetening agents such as glycerol, propylene glycol, sorbitol or sucrose.
- Such formulations may also contain a demulcent, a preservative, a colorant, and an antioxidant.
- the pharmaceutical composition may be in the form of a sterile injectable aqueous solution.
- acceptable vehicles or solvents that may be employed are water, Ringer's solution, and isotonic sodium chloride solution.
- the sterile injectable preparation may be a sterile injectable oil-in-water microemulsion in which the active ingredient is dissolved in the oily phase.
- the active ingredient is dissolved in a mixture of soybean oil and lecithin.
- the oil solution is then added to a mixture of water and glycerin to form a microemulsion.
- the injection or microemulsion can be injected into the bloodstream of the patient by a local injection.
- the solution and microemulsion are preferably administered in a manner that maintains a constant circulating concentration of the compound of the invention.
- a continuous intravenous delivery device can be used. This type of equipment An example of this is the Deltec CADD-PLUS.TM.5400 intravenous pump.
- the pharmaceutical composition may be in the form of a sterile injectable aqueous or oily suspension for intramuscular and subcutaneous administration.
- the suspension may be formulated according to known techniques using those suitable dispersing or wetting agents and suspending agents.
- the sterile injectable preparation may also be a sterile injectable solution or suspension, such as a solution prepared in 1,3-butanediol, in a parenterally acceptable non-toxic diluent or solvent.
- sterile fixed oils may conveniently be employed as a solvent or suspension medium. For this purpose, any blended fixed oil including synthetic mono- or diglycerides can be used.
- fatty acids such as oleic acid can also be prepared as an injection.
- the compounds of the invention may be administered in the form of a suppository for rectal administration.
- These pharmaceutical compositions can be prepared by mixing the drug with a suitable non-irritating excipient which is solid at ordinary temperatures but liquid in the rectum and thus dissolves in the rectum to release the drug.
- suitable non-irritating excipient include a mixture of cocoa butter, glycerin gelatin, hydrogenated vegetable oil, polyethylene glycols of various molecular weights, and fatty acid esters of polyethylene glycol.
- the dosage of the drug to be administered depends on a variety of factors including, but not limited to, the following factors: the activity of the particular compound used, the age of the patient, the weight of the patient, the health of the patient, and the patient's behavior.
- the dosage, the patient's diet, the time of administration, the mode of administration, the rate of excretion, the combination of drugs, etc.; in addition, the optimal treatment mode such as the mode of treatment, the daily dosage of the compound of formula (I) or a pharmaceutically acceptable salt The type can be verified according to traditional treatment options.
- alkyl refers to a saturated aliphatic hydrocarbon group which is a straight or branched chain group containing from 1 to 20 carbon atoms, preferably an alkyl group having from 1 to 12 carbon atoms, more preferably from 1 to 6 carbons.
- the alkyl group of the atom is a saturated aliphatic hydrocarbon group which is a straight or branched chain group containing from 1 to 20 carbon atoms, preferably an alkyl group having from 1 to 12 carbon atoms, more preferably from 1 to 6 carbons.
- Non-limiting examples include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, sec-butyl, n-pentyl, 1,1-dimethylpropyl, 1 ,2-dimethylpropyl, 2,2-dimethylpropyl, 1-ethylpropyl, 2-methylbutyl, 3-methylbutyl, n-hexyl, 1-ethyl-2- Methylpropyl, 1,1,2-trimethylpropyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1,3 - dimethylbutyl, 2-ethylbutyl, 2-methylpentyl, 3-methylpentyl, 4-methylpentyl, 2,3-dimethylbutyl, n-heptyl, 2 -methylhexyl, 3-methylhexyl, 4-methylhexyl,
- lower alkyl groups having from 1 to 6 carbon atoms, non-limiting examples including methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, sec-butyl Base, n-pentyl, 1,1-dimethylpropyl, 1,2-dimethylpropyl, 2,2-dimethylpropyl, 1-ethylpropyl, 2-methylbutyl, 3-methylbutyl, n-hexyl, 1-ethyl-2-methylpropyl, 1,1,2-trimethylpropyl, 1,1-dimethylbutyl, 1,2-dimethyl Butyl, 2,2-dimethylbutyl, 1,3-dimethylbutyl, 2-ethylbutyl, 2-methylpentyl, 3-methylpentyl, 4-methylpentyl, 2,3-dimethylbutyl, and the like.
- the alkyl group may be substituted or unsubstituted, and when substituted, the substituent may be substituted at any available point of attachment, preferably one or more of the following groups independently selected from the group consisting of an alkane Base, alkenyl, alkynyl, alkoxy, alkylthio, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, naphthenic An oxy group, a heterocycloalkoxy group, a cycloalkylthio group, a heterocycloalkylthio group, an oxo group, a carboxyl group or a carboxylate group.
- an alkane Base alkenyl, alkynyl, alkoxy, alkylthio, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, hetero
- alkylene means that one hydrogen atom of the alkyl group is further substituted, for example, "methylene” refers to -CH 2 -, "ethylene” refers to -(CH 2 ) 2 -, "propylene” Refers to -(CH 2 ) 3 -, "butylene” means -(CH 2 ) 4 - and the like.
- alkenyl refers to an alkyl group as defined above consisting of at least two carbon atoms and at least one carbon-carbon double bond, such as ethenyl, 1-propenyl, 2-propenyl, 1-, 2- or -butenyl and the like.
- the alkenyl group may be substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups independently selected from the group consisting of alkyl, alkenyl, alkynyl, alkoxy, alkylthio, Alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio, heterocycle Alkylthio group.
- cycloalkyl refers to a saturated or partially unsaturated monocyclic or polycyclic cyclic hydrocarbon substituent containing from 3 to 20 carbon atoms, preferably from 3 to 12 carbon atoms, more preferably from 3 to 6 carbon atoms. One carbon atom.
- Non-limiting examples of monocyclic cycloalkyl groups include cyclopropyl, cyclobutyl, cyclopentyl, cyclopentenyl, cyclohexyl, cyclohexenyl, cyclohexadienyl, cycloheptyl, cycloheptatriene
- a polycycloalkyl group includes a spiro ring, a fused ring, and a cycloalkyl group.
- spirocycloalkyl refers to a polycyclic group that shares a carbon atom (referred to as a spiro atom) between 5 to 20 members of a single ring, which may contain one or more double bonds, but none of the rings have a fully conjugated ⁇ electronic system. It is preferably 6 to 14 members, more preferably 7 to 10 members.
- the spirocycloalkyl group is classified into a monospirocycloalkyl group, a bispirocycloalkyl group or a polyspirocycloalkyl group, preferably a monospirocycloalkyl group and a bispirocycloalkyl group, depending on the number of common spiro atoms between the rings.
- spirocycloalkyl groups include:
- fused cycloalkyl refers to 5 to 20 members, and each ring in the system shares an all-carbon polycyclic group of an adjacent pair of carbon atoms with other rings in the system, wherein one or more of the rings may contain one or Multiple double bonds, but none of the rings have a fully conjugated ⁇ -electron system. It is preferably 6 to 14 members, more preferably 7 to 10 members.
- fused cycloalkyl groups include:
- bridged cycloalkyl refers to an all-carbon polycyclic group of 5 to 20 members, any two rings sharing two carbon atoms which are not directly bonded, which may contain one or more double bonds, but none of the rings have complete Conjugate ⁇ -electron system. It is preferably 6 to 14 members, more preferably 7 to 10 members. Depending on the number of constituent rings, it may be classified into a bicyclic, tricyclic, tetracyclic or polycyclic bridged cycloalkyl group, preferably a bicyclic ring, a tricyclic ring or a tetracyclic ring, and more preferably a bicyclic ring or a tricyclic ring.
- bridged cycloalkyl groups include:
- the cycloalkyl ring may be fused to an aryl, heteroaryl or heterocycloalkyl ring, wherein the ring to which the parent structure is attached is a cycloalkyl group, non-limiting examples include indanyl, tetrahydronaphthalene Base, benzocycloheptyl and the like.
- the cycloalkyl group may be optionally substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups independently selected from the group consisting of alkyl, alkenyl, alkynyl, alkoxy, alkane Thio, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio a heterocycloalkylthio group, an oxo group, a carboxyl group or a carboxylate group.
- heterocyclyl refers to a saturated or partially unsaturated monocyclic or polycyclic cyclic hydrocarbon substituent containing from 3 to 20 ring atoms wherein one or more ring atoms are selected from nitrogen, oxygen or S(O).
- a hetero atom of m (where m is an integer of 0 to 2), but excluding the ring moiety of -OO-, -OS- or -SS-, the remaining ring atoms being carbon.
- ring atoms Preferably comprising from 3 to 12 ring atoms, wherein from 1 to 4 are heteroatoms; most preferably from 3 to 8 ring atoms, wherein from 1 to 3 are heteroatoms; most preferably from 3 to 6 ring atoms, wherein from 1 to 2 It is a hetero atom.
- monocyclic heterocyclic groups include pyrrolidinyl, imidazolidinyl, tetrahydrofuranyl, tetrahydrothiophenyl, dihydroimidazolyl, dihydrofuranyl, dihydropyrazolyl, dihydropyrrolyl, piperidine.
- the group, piperazinyl, morpholinyl, thiomorpholinyl, homopiperazinyl, pyranyl and the like are preferably piperidinyl, piperazinyl or morpholinyl.
- Polycyclic heterocyclic groups include spiro, fused, and bridged heterocyclic groups.
- spiroheterocyclyl refers to a polycyclic heterocyclic group in which one atom (called a spiro atom) is shared between 5 to 20 members of a single ring, wherein one or more ring atoms are selected from nitrogen, oxygen or S (O). ) m (where m is an integer 0 to 2) heteroatoms, and the remaining ring atoms are carbon. It may contain one or more double bonds, but none of the rings have a fully conjugated pi-electron system. It is preferably 6 to 14 members, more preferably 7 to 10 members.
- the spiroheterocyclyl group is classified into a monospiroheterocyclic group, a dispiroheterocyclic group or a polyspirocyclic group according to the number of shared spiro atoms between the ring and the ring, and is preferably a monospiroheterocyclic group and a dispiroheterocyclic group. More preferably, it is 4 yuan / 4 yuan, 4 yuan / 5 yuan, 4 yuan / 6 yuan, 5 yuan / 5 yuan or 5 yuan / 6-membered monospiroheterocyclic group.
- Non-limiting examples of spiroheterocyclyl groups include:
- fused heterocyclyl refers to 5 to 20 members, and each ring in the system shares an adjacent pair of atomic polycyclic heterocyclic groups with other rings in the system, and one or more rings may contain one or more Double bond, but none of the rings have a fully conjugated ⁇ -electron system in which one or more ring atoms are heteroatoms selected from nitrogen, oxygen or S(O) m (where m is an integer from 0 to 2), and the remaining rings
- the atom is carbon. It is preferably 6 to 14 members, more preferably 7 to 10 members.
- fused heterocyclic groups include:
- bridge heterocyclyl refers to a polycyclic heterocyclic group of 5 to 14 members, any two rings sharing two atoms which are not directly bonded, which may contain one or more double bonds, but none of the rings have a total A ⁇ -electron system of a yoke in which one or more ring atoms are heteroatoms selected from nitrogen, oxygen or S(O) m (where m is an integer from 0 to 2), the remaining ring atoms being carbon. It is preferably 6 to 14 members, more preferably 7 to 10 members.
- bridge heterocyclic groups include:
- the heterocyclyl ring may be fused to an aryl, heteroaryl or cycloalkyl ring, wherein the ring to which the parent structure is attached is a heterocyclic group, non-limiting examples of which include:
- the heterocyclic group may be optionally substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups independently selected from alkyl, alkenyl, alkynyl, alkoxy, alkane Thio, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio a heterocycloalkylthio group, an oxo group, a carboxyl group or a carboxylate group.
- aryl refers to a 6 to 14 membered all-carbon monocyclic or fused polycyclic ring (ie, a ring that shares a pair of adjacent carbon atoms) having a conjugated ⁇ -electron system, preferably 6 to 10 members, such as benzene. Base and naphthyl. More preferred is phenyl.
- the aryl ring may be fused to a heteroaryl, heterocyclyl or cycloalkyl ring, wherein the ring to which the parent structure is attached is an aryl ring, non-limiting examples of which include:
- the aryl group may be substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups independently selected from the group consisting of alkyl, alkenyl, alkynyl, alkoxy, alkylthio, Alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio, heterocycle An alkylthio group, a carboxyl group or a carboxylate group.
- heteroaryl refers to a heteroaromatic system containing from 1 to 4 heteroatoms, from 5 to 14 ring atoms, wherein the heteroatoms are selected from the group consisting of oxygen, sulfur and nitrogen.
- the heteroaryl group is preferably 5 to 10 members, and has 1 to 3 hetero atoms; more preferably 5 or 6 members, and 1 to 2 hetero atoms; preferably, for example, imidazolyl, furyl, thienyl, thiazolyl, pyridyl Azolyl, oxazolyl, pyrrolyl, tetrazolyl, pyridyl, pyrimidinyl, thiadiazole, pyrazinyl, etc., preferably imidazolyl, tetrazolyl, pyridyl, thienyl, pyrazolyl or pyrimidinyl , thiazolyl; more selective pyridyl.
- the heteroaryl ring may be fuse
- the heteroaryl group may be optionally substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups independently selected from the group consisting of alkyl, alkenyl, alkynyl, alkoxy, alkane Thio, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio a heterocycloalkylthio group, a carboxyl group or a carboxylate group.
- alkoxy refers to -O-(alkyl) and -O-(unsubstituted cycloalkyl), wherein alkyl is as defined above.
- alkoxy groups include: methoxy, ethoxy, propoxy, butoxy, cyclopropoxy, cyclobutoxy, cyclopentyloxy, cyclohexyloxy.
- the alkoxy group may be optionally substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups independently selected from the group consisting of alkyl, alkenyl, alkynyl, alkoxy, alkane Thio, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio a heterocycloalkylthio group, a carboxyl group or a carboxylate group.
- haloalkyl refers to an alkyl group substituted by one or more halogens, wherein alkyl is as defined above.
- haloalkoxy refers to an alkoxy group substituted by one or more halogens, wherein alkoxy is as defined above.
- hydroxyalkyl refers to an alkyl group substituted with a hydroxy group, wherein alkyl is as defined above.
- hydroxy refers to an -OH group.
- halogen means fluoro, chloro, bromo or iodo.
- amino means -NH 2.
- cyano refers to -CN.
- nitro refers to -NO 2 .
- isocyanato refers to -NCO.
- carboxylate group refers to -C(O)O(alkyl) or -C(O)O(cycloalkyl), wherein alkyl, cycloalkyl are as defined above.
- acyl halide refers to a compound containing a -C(O)-halogen group.
- X is selected from A, B, or C
- X is selected from A, B, and C
- X is A, B, or C
- X is A, B, and C
- heterocyclic group optionally substituted by an alkyl group It is meant that an alkyl group may, but need not, be present, and the description includes the case where the heterocyclic group is substituted with an alkyl group and the case where the heterocyclic group is not substituted with an alkyl group.
- Substituted refers to one or more hydrogen atoms in the group, preferably up to 5, more preferably 1 to 3, hydrogen atoms, independently of each other, substituted by a corresponding number of substituents. It goes without saying that the substituents are only in their possible chemical positions, and those skilled in the art will be able to determine (by experiment or theory) substitutions that may or may not be possible without undue effort. For example, an amino group or a hydroxyl group having a free hydrogen may be unstable when combined with a carbon atom having an unsaturated (e.g., olefinic) bond.
- “Pharmaceutical composition” means a mixture comprising one or more of the compounds described herein, or a physiologically/pharmaceutically acceptable salt or prodrug thereof, and other chemical components, as well as other components such as physiological/pharmaceutically acceptable carriers. And excipients.
- the purpose of the pharmaceutical composition is to promote the administration of the organism, which facilitates the absorption of the active ingredient and thereby exerts biological activity.
- “Pharmaceutically acceptable salt” refers to a salt of a compound of the invention which is safe and effective for use in a mammal and which possesses the desired biological activity.
- the preparation method of the salt used includes the following steps:
- a compound of the formula (IA), a compound of the formula (IB), bis(cyanium dichloride) and norbornene in a polar solvent, and heating the reaction under basic conditions to obtain a compound of the formula (IC),
- the alkaline reagent of this condition is preferably sodium hydrogencarbonate, and the polar solvent is preferably N,N-dimethylacetamide; the obtained compound of the general formula (IC) is reacted with methyl iodide and chlorinated compound under heating and basic conditions to obtain a pass.
- a compound of the formula (ID) wherein the alkaline reagent of the condition is preferably sodium hydride; the obtained compound of the formula (ID) is hydrolyzed under basic conditions to give a compound of the formula (V).
- the alkaline reagent of this condition is preferably sodium hydroxide; the obtained compound of the formula (V) is reacted with a compound of the formula (VI), 1-hydroxybenzotriazole and N,N-diisopropylethylamine to give a general formula. (I) a compound.
- the reagents providing basic conditions include organic bases including, but not limited to, triethylamine, N,N-diisopropylethylamine, n-butyllithium, lithium diisopropylamide, and inorganic bases. And potassium acetate, sodium t-butoxide or potassium t-butoxide.
- the inorganic bases include, but are not limited to, sodium hydride, sodium hydroxide, potassium phosphate, sodium carbonate, sodium hydrogencarbonate, potassium carbonate or cesium carbonate.
- R x is selected from the group consisting of alkyl, haloalkyl, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl, wherein said alkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl Each is optionally independently selected from the group consisting of alkyl, halogen, hydroxy, amino, carboxylate, nitro, cyano, alkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl Substituted by one or more substituents;
- Ring A, Ring B, X, Y, Z, R 1 to R 7 , x, y and z are as defined in the general formula (I).
- the alkaline reagent of the condition is preferably sodium hydride; the obtained compound of the formula (II-4) is hydrolyzed under basic conditions to give the formula (II-A).
- -1) a compound, the alkaline reagent of this condition is preferably sodium hydroxide; the obtained compound of the formula (II-A-1) and the compound of the formula (II-A-2), 1-hydroxybenzotriazole and N, Reaction with N-diisopropylethylamine gives the compound of the formula (II-A).
- the reagents providing basic conditions include organic bases and inorganic bases, including but not limited to Triethylamine, N,N-diisopropylethylamine, n-butyllithium, lithium diisopropylamide, potassium acetate, sodium t-butoxide or potassium t-butoxide, the inorganic bases include but are not limited to Sodium hydride, sodium hydroxide, potassium phosphate, sodium carbonate, sodium hydrogencarbonate, potassium carbonate or cesium carbonate.
- R x is selected from the group consisting of alkyl, haloalkyl, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl, wherein said alkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl Each is optionally independently selected from the group consisting of alkyl, halogen, hydroxy, amino, carboxylate, nitro, cyano, alkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl Substituted by one or more substituents;
- Rings A, G, X, R a , R 1 to R 7 , p, y and z are as defined in the formula (II-A).
- the structure of the compound is determined by nuclear magnetic resonance (NMR) or/and mass spectrometry (MS).
- NMR shift ( ⁇ ) is given in units of 10 -6 (ppm).
- NMR was measured using a Bruker AVANCE-400 nuclear magnetic apparatus, and the solvent was deuterated dimethyl sulfoxide (DMSO-d 6 ), deuterated chloroform (CDCl 3 ), deuterated methanol (CD 3 OD), internal standard was four.
- DMSO-d 6 dimethyl sulfoxide
- CDCl 3 deuterated chloroform
- CD 3 OD deuterated methanol
- TMS Methyl silane
- the measurement of the MS was carried out using a FINNIGAN LCQAd (ESI) mass spectrometer (manufacturer: Thermo, model: Finnigan LCQ advantage MAX).
- ESI FINNIGAN LCQAd
- the HPLC was measured using an Agilent 1200 DAD high pressure liquid chromatograph (Sunfire C18 150 x 4.6 mm column) and a Waters 2695-2996 high pressure liquid chromatograph (Gimini C18 150 x 4.6 mm column).
- Chiral HPLC analysis assays were performed using LC-10A vp (Shimadzu) or SFC-analytical (Berger Instruments Inc.).
- Thin layer chromatography silica gel plate uses Yantai Yellow Sea HSGF254 or Qingdao GF254 silica gel plate.
- the specification of silica gel plate used for thin layer chromatography (TLC) is 0.15mm ⁇ 0.2mm.
- the specification for thin layer chromatography separation and purification is 0.4mm. ⁇ 0.5mm.
- the known starting materials of the present invention may be synthesized by or according to methods known in the art, or may be purchased from ABCR GmbH & Co. KG, Acros Organics, Aldrich Chemical Company, ⁇ Companies such as Accela ChemBio Inc., Dary Chemicals.
- the reactions can all be carried out under an argon atmosphere or a nitrogen atmosphere.
- An argon atmosphere or a nitrogen atmosphere means that the reaction flask is connected to an argon or nitrogen balloon having a volume of about 1 L.
- the hydrogen atmosphere means that the reaction flask is connected to a hydrogen balloon of about 1 L volume.
- the pressurized hydrogenation reaction was carried out using a Parr Model 3916EKX hydrogenation apparatus and a clear blue QL-500 type hydrogen generator or a HC2-SS type hydrogenation apparatus.
- the hydrogenation reaction is usually evacuated, charged with hydrogen, and operated three times.
- the microwave reaction used a CEM Discover-S Model 908860 microwave reactor.
- the solution means an aqueous solution.
- reaction temperature is room temperature and is 20 ° C to 30 ° C.
- the progress of the reaction in the examples was monitored by thin layer chromatography (TLC).
- TLC thin layer chromatography
- the system used for the reaction was: A: dichloromethane and methanol system, B: n-hexane and ethyl acetate system, C: petroleum ether And the ethyl acetate system, D: acetone, the volume ratio of the solvent is adjusted depending on the polarity of the compound.
- Purification compounds using column chromatography eluent systems and thin layer chromatography developer systems include: A: dichloromethane and methanol systems, B: n-hexane and ethyl acetate systems, C: dichloromethane and acetone
- A dichloromethane and methanol systems
- B n-hexane and ethyl acetate systems
- C dichloromethane and acetone
- the volume ratio of the solvent is adjusted depending on the polarity of the compound, and a small amount of an alkaline or acidic reagent such as triethylamine or acetic acid may be added for adjustment.
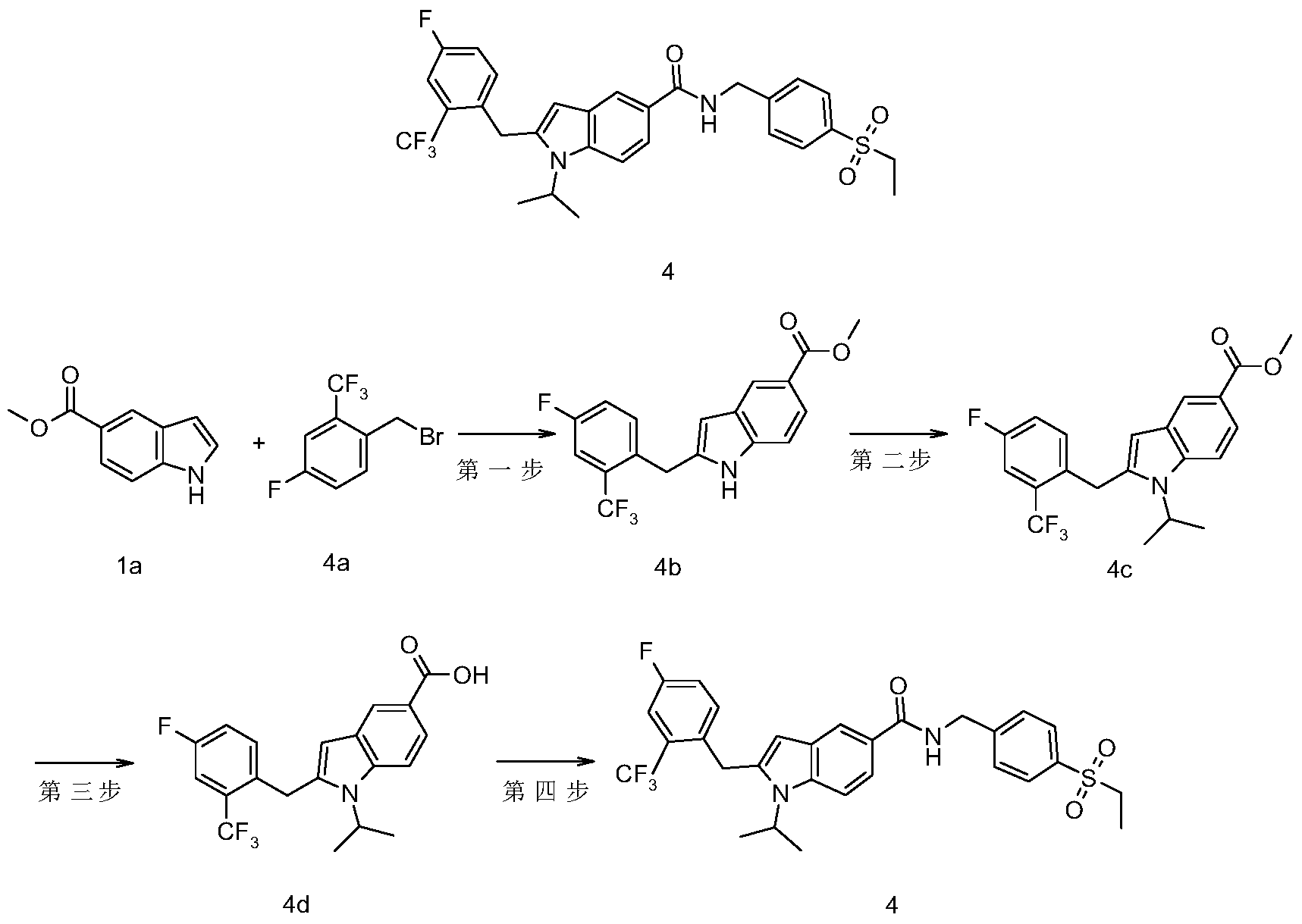
- the crude product 14c (67 mg, 0.18 mmol) was dissolved in 3 mL of methanol, and 1 mL of a 2M potassium hydroxide solution was added thereto, and the mixture was heated to reflux and stirred for 3 hours.
- the reaction solution was cooled to room temperature, concentrated under reduced pressure, and water was added to the residue, and concentrated hydrochloric acid was added dropwise to pH 5-6, extracted with ethyl acetate (20 mL ⁇ 3), and the organic phase was combined with water and saturated sodium chloride solution The mixture was washed with EtOAc EtOAc EtOAc.
- the crude product 19a (0.2 g, 0.6 mmol) was dissolved in 5 mL of N,N-dimethylformamide, sodium hydride (48 mg, 1.2 mmol, 60% in oil) was added, and the reaction was stirred at 0 ° C for 1 hour.
- the alkane (0.19 g, 1.2 mmol) was heated to 50 ° C and stirred for 15 hours.
- the reaction mixture was cooled to room temperature, then added with 50 mL of EtOAc (EtOAc (EtOAc)
- EtOAc EtOAc
- the crude title product 19b (63 mg, yellow solid).
- the crude product 22a (28 mg, 0.07 mmol) was dissolved in 3 mL of methanol, and 1 mL of a 2M potassium hydroxide solution was added thereto, and the mixture was heated to reflux and stirred for 2 hours.
- the reaction solution was cooled to room temperature, concentrated under reduced pressure, and water was added to the residue, and concentrated hydrochloric acid was added dropwise to pH 5-6, extracted with ethyl acetate (20 mL ⁇ 3), and the organic phase was combined with water and saturated sodium chloride solution The mixture was washed with EtOAc EtOAc EtOAc.
- the crude product 23a (26 mg, 0.07 mmol) was dissolved in 3 mL of methanol, and 1 mL of a 2M potassium hydroxide solution was added thereto, and the mixture was heated to reflux and stirred for 2 hours.
- the reaction mixture was cooled to room temperature, and the mixture was evaporated, evaporated, evaporated, evaporated.
- the filtrate was concentrated under reduced pressure to give crystals crystals crystals crystals crystals crystals
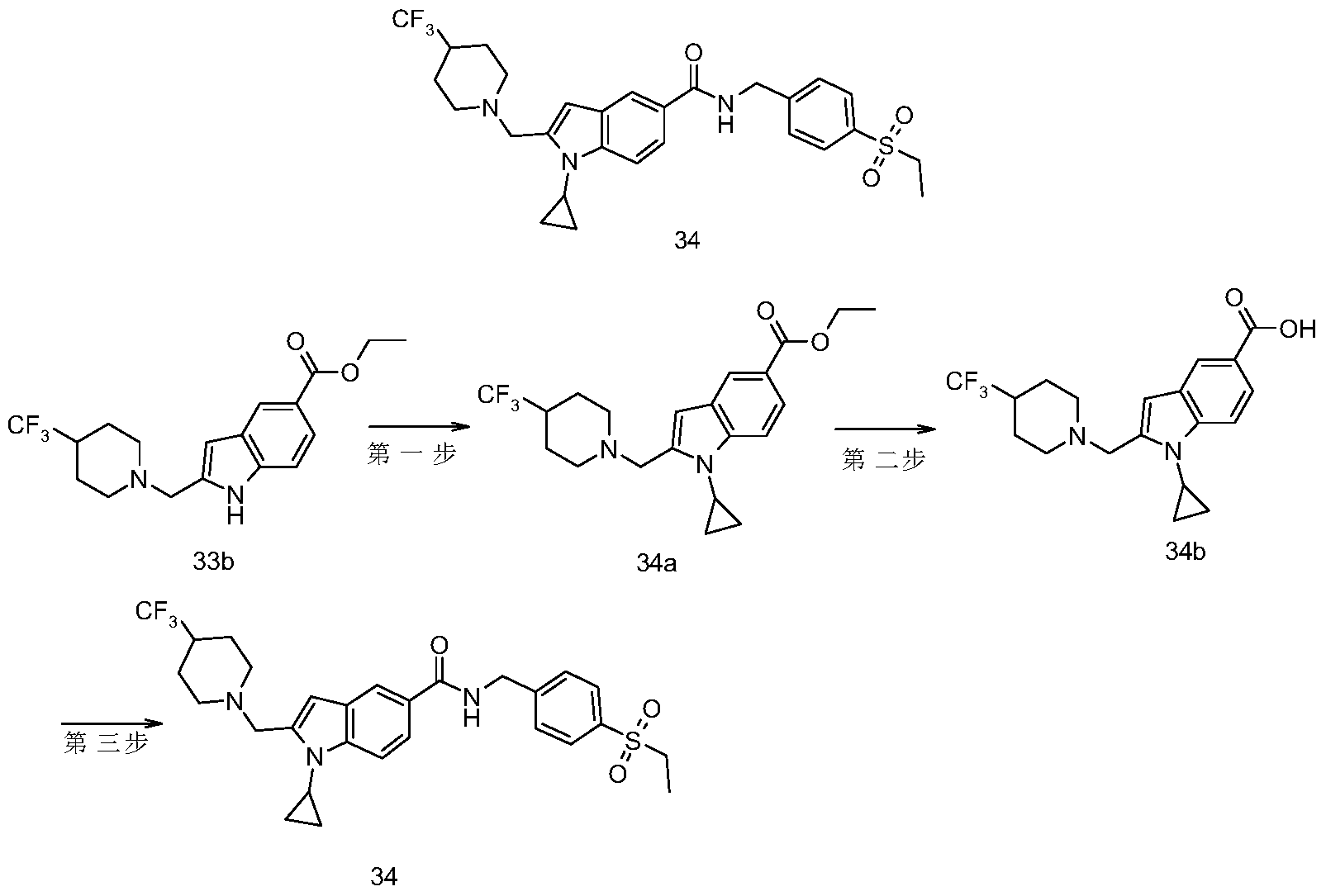
- the crude product 33b (220 mg, 0.62 mmol) was dissolved in 1,2-dichloroethane, followed by copper acetate monohydrate (178 mg, 0.93 mmol), 2,2'-bipyridine (145 mg, 0.93 mmol), sodium carbonate (99 mg, 0.93 mmol) and cyclopropylboronic acid (39 mg, 0.45 mmol), warmed to 70 ° C, and stirred for 12 hours. Further, cyclopropylboric acid (39 mg, 0.45 mmol), copper acetate monohydrate (178 mg, 0.93 mmol) and 2,2'-bipyridine (145 mg, 0.93 mmol) were added and reacted for 12 hours.
- 5-bromo-1H-indole-2-carboxylic acid 38b (600 mg, 2.5 mmol, prepared by the well-known method "Journal of Medicinal Chemistry, 2009, 52 (23), 7512-7527") was dissolved in 15 mL of tetrahydrofuran.
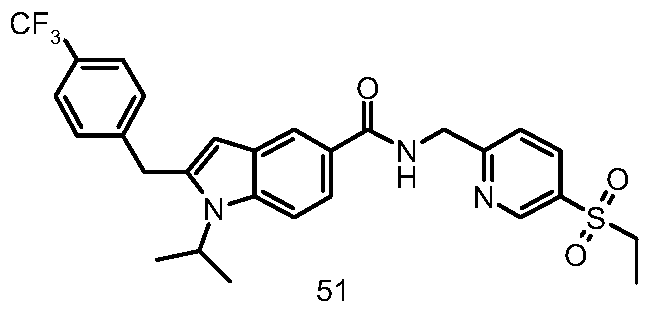
- the crude product 38 g (30 mg, 0.08 mmol) was dissolved in 1.5 mL of N,N-dimethylformamide, and 11a (32.8 mg, 0.16 mmol), 2-(7-benzotriazole)-N, N N', N'-tetramethyluronium hexafluorophosphate (62.23 mg, 0.16 mmol) and N,N-diisopropylethylamine (31.69 mg, 0.25 mmol) were added, and the reaction was stirred for 16 hours. The reaction mixture was concentrated under reduced vacuolulululululululululululululululululululululululululululululu
- the title material 51 (11 mg) was obtained from the title compound (4-(ethylsulfonyl)phenyl)methylamine.
- Example 42 Using the synthetic route of Example 42, the first step starting material 42a was replaced with 2-(3-(trifluoromethyl)phenyl)acetic acid (using a known method "Angewandte Chemie, International Edition, 2010, 49 (27), 4665-4668, S4665/1-S4665/60" prepared to give the title product 56 (4.6 mg)
- the first step starting material 42a was replaced with 2-(2-fluoro-4-(trifluoromethyl)phenyl)acetic acid (Admas) to give the title product 57 (4.6 mg).
- Example 42 Using the synthesis route of Example 42, the first step starting material 42a was replaced with 4-fluorophenylacetic acid (prepared by the known method "RSC Advances, 2016, 6(8), 6719-6723”) to obtain the title product 58 ( 4.6mg).
- Example 8 Using the procedure of Example 8, the first step starting material 8a was replaced with 4-bromo-2-chlorobenzylcarbamic acid tert-butyl ester, and the second step starting material 6e was replaced with 14d to give the title product 59 (16 mg).
- the first step starting material 42a was replaced with 4-trifluoromethoxyphenylacetic acid using the procedure of Example 42 to give the title product 60 (15 mg).
- Example 42 Using the synthetic route of Example 42, the first step starting material 42a was replaced with 4-trifluoromethylphenylacetic acid, and 42b was replaced with methyl 3-amino-4-(ethylamino)benzoate (using a known method "Bioorganic & Medicinal Chemistry , 2005, 13(5), 1587-1597 "prepared to give the title product 62 (15 mg).
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
An aromatic amide derivative represented by formula (I), a preparation method therefor, a pharmaceutical composition comprising the derivative, uses thereof as an ROR regulator, and users thereof in preventing or treating inflammatory disease, autoimmune diseases, cancers and other diseases. The aromatic amide derivative has an agitation or anti-agitation activity on ROR. The combined medication of the aromatic amide derivative and a PD-1 antibody can be used in treatment of tumors or cancers.
Description
本发明属于医药领域,涉及芳香酰胺类衍生物、其制备方法及其在医药上的应用。特别地,本发明涉及通式(I)所示的芳香酰胺类衍生物、其制备方法、含有该衍生物的药物组合物,其作为ROR调节剂以及其预防和/或治疗自身免疫性等疾病的用途。The invention belongs to the field of medicine, and relates to an aromatic amide derivative, a preparation method thereof and application thereof in medicine. In particular, the present invention relates to an aromatic amide derivative represented by the formula (I), a process for producing the same, and a pharmaceutical composition containing the same, which are useful as ROR modulators and for preventing and/or treating autoimmune diseases and the like the use of.
维甲酸相关孤儿核受体(ROR)是核受体家族的成员之一,它能够调控多种生理和生活过程。ROR家族包含三种类型RORα、RORβ以及RORγ。三种不同的ROR可以在不同的组织中表达并且控制不同的生理过程,RORα主要分布在肝脏、骨骼肌、皮肤、肺、脂肪组织、肾脏、胸腺和大脑,RORβ作用范围很小,主要作用于中枢神经系统,RORγ可以在许多组织中表达,包括肝脏、动物脂肪和骨骼肌。哺乳动物缺乏RORγ表现出血糖降低的现象。Retinoic acid-related orphan nuclear receptors (RORs) are members of the nuclear receptor family that regulate a variety of physiological and life processes. The ROR family contains three types of RORα, RORβ, and RORγ. Three different RORs can be expressed in different tissues and control different physiological processes. RORα is mainly distributed in liver, skeletal muscle, skin, lung, adipose tissue, kidney, thymus and brain. RORβ has a small range of action, mainly acting on In the central nervous system, RORγ can be expressed in many tissues, including liver, animal fat, and skeletal muscle. The lack of RORγ in mammals shows a decrease in blood glucose.
RORγ有两种亚型:RORγ1和RORγ2。RORγ1在许多组织,如:胸腺、肌肉、肾脏和肝脏中表达,而RORγ2则只有在免疫细胞内表达,RORγ2被认为能够控制T细胞辅助T细胞17(Th17)的分化。Th17是一类辅助T细胞的细胞,这种细胞可以产生白介素17(IL-17)和其他细胞因子,已经发现Th-17已经被发现与人类炎症疾病和免疫紊乱,如,多发性硬化症、风湿性关节炎、银屑病、克隆疾病和哮喘等疾病有关系,现在又文献报道RORγ可能与前列腺癌的发生与发展有关系。There are two subtypes of RORγ: RORγ1 and RORγ2. RORγ1 is expressed in many tissues such as thymus, muscle, kidney and liver, while RORγ2 is expressed only in immune cells, and RORγ2 is thought to control T cell-assisted T cell 17 (Th17) differentiation. Th17 is a class of helper T cells that produce interleukin-17 (IL-17) and other cytokines. Th-17 has been found to be associated with human inflammatory diseases and immune disorders, such as multiple sclerosis, Rheumatoid arthritis, psoriasis, clonal diseases and asthma are related. Now, it is reported in the literature that RORγ may be related to the occurrence and development of prostate cancer.
RORγt是RORγ特异性表达在免疫细胞上的亚型,是人和鼠Th17细胞的主要转录因子,不仅能促进Th17细胞分化,还能调节Th17细胞的特异性效应因子IL-17的表达和分泌,RORγt与多种免疫性疾病、感染性疾病和肿瘤等的发生、发展密切相关。RORγt is a subtype of RORγ specifically expressed on immune cells. It is a major transcription factor of human and murine Th17 cells, which not only promotes the differentiation of Th17 cells, but also regulates the expression and secretion of the specific effector factor IL-17 of Th17 cells. RORγt is closely related to the occurrence and development of various immune diseases, infectious diseases and tumors.
RORγ,特别是RORγt型,已确定是Th17细胞的分化的一个重要的转录调节因子。2006年Vanov等人的研究发现,在小鼠实验中,RORγt是Th17细胞分化的一个重要的转录因子。他们的研究显示小鼠在缺乏RORγt时很难诱导形成EAE模型。而在人类Th17细胞分化过程中,RORγt也很快被证实有类似的重要作用,开创性的发现引起了人们对RORγt高度重视。RORγ, particularly the RORγt type, has been identified as an important transcriptional regulator of the differentiation of Th17 cells. In 2006, Vanov et al. found that RORγt is an important transcription factor for Th17 cell differentiation in mouse experiments. Their study showed that mice were difficult to induce the formation of an EAE model in the absence of RORγt. In the process of human Th17 cell differentiation, RORγt was also confirmed to have a similar important role. The groundbreaking discovery caused people to attach great importance to RORγt.
目前,ROR作为抑制剂在医药界已得到高度的重视,成为研究的热点问题,现已公开的的专利申请包括WO2015171610、WO22015171558、WO2015131035、WO2013169864、WO2014179564、WO2015116904等。At present, ROR has been highly regarded as an inhibitor in the medical field and has become a hot issue of research. The patent applications that have been published include WO2015171610, WO22015171558, WO2015131035, WO2013169864, WO2014179564, WO2015116904 and the like.
发明人在研究ROR调节剂的过程中,发现了在本发明所述的通式(I)所示的化合物中,环A邻位基团的变化会改变其调节效果,当环A邻位基团为位阻较小的基团(例如H和F)时通式(I)所示的化合物为反激动剂,当环A邻位基团为卤代
烷基(例如三氟甲基)、烷基(例如乙基)和卤代烷氧基(例如三氟甲氧基)类位阻较大的基团时通式(I)所示的化合物为ROR激动剂,由此本发明开发出了新一代的ROR调节剂,并且进一步的研究发现化合物结构上的变化可以调节不同的机制。In the process of studying the ROR regulator, the inventors found that in the compound of the formula (I) of the present invention, the change of the ortho group of the ring A changes its regulation effect, when the ring A is ortho When the group is a group having a small steric hindrance (for example, H and F), the compound represented by the formula (I) is an inverse agonist, and when the ring A ortho group is halogenated
A compound represented by the general formula (I) is a compound having a sterically hindered group such as an alkyl group (for example, a trifluoromethyl group), an alkyl group (for example, an ethyl group), and a halogenated alkoxy group (for example, a trifluoromethoxy group). Thus, the present invention has developed a new generation of ROR modulators, and further studies have found that structural changes in compounds can modulate different mechanisms.
发明内容Summary of the invention
本发明的目的在于提供一种通式(I)所示的化合物:It is an object of the present invention to provide a compound of the formula (I):
或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用的盐,Or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer or a mixture thereof, or a pharmaceutically acceptable salt thereof,
其中:among them:
X、Y和Z相同或不同,且各自独立地为CR9或N;X, Y and Z are the same or different and are each independently CR 9 or N;
环A和环B相同或不同,且各自独立地选自环烷基、杂环基、芳基和杂芳基;Ring A and Ring B are the same or different and are each independently selected from the group consisting of a cycloalkyl group, a heterocyclic group, an aryl group, and a heteroaryl group;
R1和R2相同或不同,且各自独立地选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、卤素、氨基、硝基、羟基、氰基、杂环基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)NHR8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、烷氧基、卤代烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 1 and R 2 are the same or different and are each independently selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen, an amino group, a nitro group, a hydroxyl group, a cyano group, and a heterocyclic ring. a base, an aryl group, a heteroaryl group, -OR 8 , -C(O)R 8 , -C(O)NHR 8 , -C(O)OR 8 and -S(O) m R 8 , wherein said Alkyl, cycloalkyl, alkoxy, haloalkyl, heterocyclyl, aryl and heteroaryl are each independently optionally selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, Substituted with one or more substituents of alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl;
R3和R4相同或不同,且各自独立地选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、卤素、氨基、硝基、羟基、氰基、杂环基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、卤代烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 3 and R 4 are the same or different and are each independently selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen group, an amino group, a nitro group, a hydroxyl group, a cyano group, and a heterocyclic ring. a base, an aryl group, a heteroaryl group, -OR 8 , -C(O)R 8 , -C(O)OR 8 and -S(O) m R 8 , wherein said alkyl group, cycloalkyl group, halogenated alkane The radical, heterocyclyl, aryl and heteroaryl are each independently selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, Substituted by one or more substituents in the cycloalkyl, heterocyclyl, aryl and heteroaryl groups;
或者R3和R4形成氧代基;Or R 3 and R 4 form an oxo group;
R5选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、氨基、烯基、炔基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、卤代烷基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烯基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;
R 5 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, an amino group, an alkenyl group, an alkynyl group, an aryl group, a heteroaryl group, -OR 8 , -C(O)R 8 , —C(O)OR 8 and —S(O) m R 8 , wherein said alkyl, cycloalkyl, haloalkyl, aryl and heteroaryl are each independently optionally selected from alkyl, One or more of haloalkyl, halogen, amino, nitro, cyano, hydroxy, alkenyl, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl Substituted by a substituent;
R6选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、卤素、氨基、硝基、羟基、氰基、杂环基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、卤代烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 6 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen, an amino group, a nitro group, a hydroxyl group, a cyano group, a heterocyclic group, an aryl group, a heteroaryl group, -OR 8 , —C(O)R 8 , —C(O)OR 8 and —S(O) m R 8 , wherein said alkyl, cycloalkyl, haloalkyl, heterocyclyl, aryl and heteroaryl The groups are each independently optionally selected from the group consisting of alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and Substituting one or more substituents in the heteroaryl;
R7选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、杂环基、芳基和杂芳基;R 7 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a heterocyclic group, an aryl group, and a heteroaryl group;
R8选自氢原子、烷基、卤代烷基、烷氧基、羟烷基、羟基、氨基、环烷基、杂环基、芳基和杂芳基,其中所述的烷基、氨基、环烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤素、羟基、氨基、羧酸酯基、硝基、氰基、烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 8 is selected from the group consisting of a hydrogen atom, an alkyl group, a halogenated alkyl group, an alkoxy group, a hydroxyalkyl group, a hydroxyl group, an amino group, a cycloalkyl group, a heterocyclic group, an aryl group, and a heteroaryl group, wherein the alkyl group, the amino group, and the ring are The alkyl group, heterocyclic group, aryl group and heteroaryl group are each independently selected from the group consisting of alkyl, halogen, hydroxy, amino, carboxylate, nitro, cyano, alkoxy, hydroxyalkyl, cyclo Substituted by one or more substituents of an alkyl group, a heterocyclic group, an aryl group, and a heteroaryl group;
R9选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、氨基、烯基、炔基、芳基和杂芳基,其中所述的烷基、环烷基、卤代烷基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烯基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 9 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, an amino group, an alkenyl group, an alkynyl group, an aryl group and a heteroaryl group, wherein the alkyl group, the cycloalkyl group And haloalkyl, aryl and heteroaryl are each independently selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkenyl, alkoxy, haloalkoxy, hydroxyalkyl Substituting one or more substituents of a cycloalkyl, a heterocyclic group, an aryl group, and a heteroaryl group;
m为0、1或2;m is 0, 1 or 2;
x为0、1、2、3或4;x is 0, 1, 2, 3 or 4;
y为0、1、2或3;且y is 0, 1, 2 or 3;
z为0、1、2、3或4。z is 0, 1, 2, 3 or 4.
在本发明一个优选的实施方案中,所述的通式(I)所示的化合物为通式(II)所示的化合物:In a preferred embodiment of the invention, the compound of the formula (I) is a compound of the formula (II):
其中:among them:
X为CR9或N;X is CR 9 or N;
Y为CH或N;Y is CH or N;
R9为氢原子或烷基;且R 9 is a hydrogen atom or an alkyl group;
环A、环B、R1~R7、x、y和z如通式(I)中所定义。Ring A, Ring B, R 1 to R 7 , x, y and z are as defined in the general formula (I).
在本发明一个优选的实施方案中,所述的通式(I)所示的化合物,其中A环和
B环相同或不同,且各自独立地选自杂环基、芳基和杂芳基。In a preferred embodiment of the invention, the compound of the formula (I) wherein A ring and
The B rings are the same or different and are each independently selected from the group consisting of a heterocyclic group, an aryl group, and a heteroaryl group.
在本发明一个优选的实施方案中,所述的通式(I)所示的化合物,其为通式(II-A)所示的化合物:In a preferred embodiment of the invention, the compound of the formula (I) is a compound of the formula (II-A):
其中:among them:
X为CR9或N;R9为氢原子或烷基;X is CR 9 or N; R 9 is a hydrogen atom or an alkyl group;
G为CH或N;G is CH or N;
Ra选自烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、溴、氨基、硝基、羟基、氰基、杂环基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)NHR8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、烷氧基、卤代烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R a is selected from the group consisting of alkyl, cycloalkyl, haloalkyl, alkoxy, haloalkoxy, bromo, amino, nitro, hydroxy, cyano, heterocyclyl, aryl, heteroaryl, -OR 8 , - C(O)R 8 , —C(O)NHR 8 , —C(O)OR 8 and —S(O) m R 8 , wherein said alkyl group, cycloalkyl group, alkoxy group, haloalkyl group, The heterocyclic group, the aryl group and the heteroaryl group are each independently selected from the group consisting of alkyl, haloalkyl, halogen, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkane. Substituted by one or more substituents in the group, heterocyclic group, aryl group and heteroaryl group;
p为0、1、2或3;p is 0, 1, 2 or 3;
环A、R1~R8、m、y和z如通式(I)中所定义。Ring A, R 1 to R 8 , m, y and z are as defined in the general formula (I).
在本发明一个优选的实施方案中,所述的通式(II-A)所示的化合物,其中选自
In a preferred embodiment of the invention, the compound of the formula (II-A), wherein Selected from
在本发明一个优选的实施方案中,所述的通式(I)所示的化合物为通式(III)所示的化合物:
In a preferred embodiment of the invention, the compound of the formula (I) is a compound of the formula (III):
其中:among them:
X为CR9或N;X is CR 9 or N;
Y为CH或N;Y is CH or N;
R9为氢原子或烷基;且R 9 is a hydrogen atom or an alkyl group;
环A、R1~R7、x、y和z如通式(I)中所定义。Ring A, R 1 to R 7 , x, y and z are as defined in the general formula (I).
在本发明一个优选的实施方案中,所述的通式(I)所示的化合物为通式(IV)所示的化合物:In a preferred embodiment of the invention, the compound of the formula (I) is a compound of the formula (IV):
其中:among them:
X为CR9或N;X is CR 9 or N;
Y为CH或N;Y is CH or N;
R9为氢原子或烷基;R 9 is a hydrogen atom or an alkyl group;
R1~R7、x、y和z如通式(I)中所定义。R 1 to R 7 , x, y and z are as defined in the formula (I).
在本发明一个优选的实施方案中,所述的通式(I)所示的化合物为通式(IV-A)、通式(IV-B)或通式(IV-C)所示的化合物:In a preferred embodiment of the present invention, the compound represented by the formula (I) is a compound represented by the formula (IV-A), the formula (IV-B) or the formula (IV-C). :
其中:among them:
X为CR9或N;X is CR 9 or N;
Y为CH或N;Y is CH or N;
R9为氢原子或烷基;R 9 is a hydrogen atom or an alkyl group;
Ra选自烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、氨基、硝基、羟基、氰基、杂环基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)NHR8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、烷氧基、卤代烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R a is selected from the group consisting of alkyl, cycloalkyl, haloalkyl, alkoxy, haloalkoxy, amino, nitro, hydroxy, cyano, heterocyclyl, aryl, heteroaryl, -OR 8 , -C ( O) R 8 , -C(O)NHR 8 , -C(O)OR 8 and -S(O) m R 8 , wherein said alkyl group, cycloalkyl group, alkoxy group, haloalkyl group, heterocyclic ring The aryl group, the aryl group and the heteroaryl group are each independently selected from the group consisting of alkyl, haloalkyl, halogen, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, Substituting one or more substituents in a heterocyclic group, an aryl group, and a heteroaryl group;
Rb选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、卤素、氨基、硝基、羟基、氰基、杂环基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)NHR8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、烷氧基、卤代烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R b is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen, an amino group, a nitro group, a hydroxyl group, a cyano group, a heterocyclic group, an aryl group, a heteroaryl group, -OR 8 , —C(O)R 8 , —C(O)NHR 8 , —C(O)OR 8 and —S(O) m R 8 , wherein said alkyl group, cycloalkyl group, alkoxy group, Haloalkyl, heterocyclyl, aryl and heteroaryl are each independently optionally selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl Substituting one or more substituents of a cycloalkyl, a heterocyclic group, an aryl group, and a heteroaryl group;
p为0、1、2或3;p is 0, 1, 2 or 3;
q为0、1或2;且q is 0, 1, or 2;
R1~R8、m、y和z如通式(I)中所定义。R 1 to R 8 , m, y and z are as defined in the formula (I).
在本发明一个优选的实施方案中,所述的通式(IV-A)所示的化合物,其为通式(IV-A-1)所示的化合物:In a preferred embodiment of the invention, the compound of the formula (IV-A) is a compound of the formula (IV-A-1):
其中:among them:
R1~R7、p、y和z如通式(IV-A)中所定义。R 1 to R 7 , p, y and z are as defined in the formula (IV-A).
在本发明一个优选的实施方案中,所述的通式(I)所示的化合物,其中R1选自
氢原子、烷基、环烷基、烷氧基、卤代烷基、卤代烷氧基、卤素、氰基、杂环基和-C(O)NHR8,其中所述的烷基、环烷基、烷氧基和杂环基各自独立地任选被选自烷基、卤素、羟基、氨基和氰基中的一个或多个取代基所取代;优选R1选自烷基、卤素、卤代烷基、烷氧基和卤代烷氧基;R8选自氢原子或烷基。In a preferred embodiment of the invention, the compound of the formula (I), wherein R 1 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, an alkoxy group, a halogenated alkyl group, a halogenated alkoxy group, a halogen And a cyano group, a heterocyclic group, and -C(O)NHR 8 , wherein each of the alkyl group, the cycloalkyl group, the alkoxy group and the heterocyclic group is independently selected from the group consisting of an alkyl group, a halogen group, a hydroxyl group, and an amino group. And one or more substituents in the cyano group are substituted; preferably, R 1 is selected from the group consisting of an alkyl group, a halogen, a halogenated alkyl group, an alkoxy group, and a halogenated alkoxy group; and R 8 is selected from a hydrogen atom or an alkyl group.
在本发明一个优选的实施方案中,所述的通式(I)所示的化合物,其中R2为卤素;优选氟或氯。In a preferred embodiment of the invention, the compound of the formula (I), wherein R 2 is halogen; preferably fluorine or chlorine.
在本发明一个优选的实施方案中,所述的通式(I)所示的化合物,其中R3或R4相同或不同,且各自独立地选自氢原子、烷基、卤代烷基和羟基,或者R3和R4一起形成氧代基。In a preferred embodiment of the invention, the compound of the formula (I), wherein R 3 or R 4 are the same or different, and each independently selected from a hydrogen atom, an alkyl group, a halogenated alkyl group and a hydroxyl group, Or R 3 and R 4 together form an oxo group.
在本发明一个优选的实施方案中,所述的通式(I)所示的化合物,其中R5选自烷基、环烷基、卤代烷基、烯基和芳基,其中所述的烷基、环烷基和芳基各自独立地任选被选自烷基、卤素、烯基和羟基中的一个或多个取代基所取代;优选R5为异丙基或环丙基。In a preferred embodiment of the invention, the compound of the formula (I), wherein R 5 is selected from the group consisting of alkyl, cycloalkyl, haloalkyl, alkenyl and aryl, wherein the alkyl group The cycloalkyl and aryl groups are each independently optionally substituted with one or more substituents selected from the group consisting of alkyl, halogen, alkenyl and hydroxy; preferably R 5 is isopropyl or cyclopropyl.
在本发明一个优选的实施方案中,所述的通式(I)所示的化合物,其中R6为卤素;优选氟。In a preferred embodiment of the invention, the compound of the formula (I), wherein R 6 is halogen; preferably fluorine.
在本发明一个优选的实施方案中,所述的通式(I)所示的化合物,其中R7为任选被选自卤素、环烷基和羟基中的一个或多个取代基所取代的烷基;优选R7为乙基。In a preferred embodiment of the invention, the compound of the formula (I), wherein R 7 is optionally substituted by one or more substituents selected from the group consisting of halogen, cycloalkyl and hydroxy Alkyl; preferably R 7 is ethyl.
典型的通式(I)的化合物,包括但不限于:Typical compounds of formula (I) include, but are not limited to:
或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐。Or a tautomer, a meso form, a racemate, an enantiomer, a diastereomer or a mixture thereof, or a pharmaceutically acceptable salt thereof.
本发明另外提供一种制备根据通式(I)化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体、或其混合物形式、或其可药用盐的中间体,即通式(V)所示化合物:The invention further provides a preparation according to the compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer, or a mixture thereof, Or an intermediate thereof, or a compound of the formula (V):
或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐,Or a tautomer, a meso form, a racemate, an enantiomer, a diastereomer or a mixture thereof, or a pharmaceutically acceptable salt thereof,
其中:among them:
X、Y和Z相同或不同,且各自独立地为CR9或N;X, Y and Z are the same or different and are each independently CR 9 or N;
环A选自环烷基、杂环基、芳基和杂芳基;Ring A is selected from the group consisting of a cycloalkyl group, a heterocyclic group, an aryl group, and a heteroaryl group;
R1选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、卤素、氨基、硝基、羟基、氰基、杂环基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)NHR8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、烷氧基、卤代烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 1 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen, an amino group, a nitro group, a hydroxyl group, a cyano group, a heterocyclic group, an aryl group, a heteroaryl group, -OR 8 , —C(O)R 8 , —C(O)NHR 8 , —C(O)OR 8 and —S(O) m R 8 , wherein said alkyl group, cycloalkyl group, alkoxy group, Haloalkyl, heterocyclyl, aryl and heteroaryl are each independently optionally selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl Substituting one or more substituents of a cycloalkyl, a heterocyclic group, an aryl group, and a heteroaryl group;
R3和R4相同或不同,且各自独立地选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、卤素、氨基、硝基、羟基、氰基、杂环基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、卤代烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 3 and R 4 are the same or different and are each independently selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen group, an amino group, a nitro group, a hydroxyl group, a cyano group, and a heterocyclic ring. a base, an aryl group, a heteroaryl group, -OR 8 , -C(O)R 8 , -C(O)OR 8 and -S(O) m R 8 , wherein said alkyl group, cycloalkyl group, halogenated alkane The radical, heterocyclyl, aryl and heteroaryl are each independently selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, Substituted by one or more substituents in the cycloalkyl, heterocyclyl, aryl and heteroaryl groups;
或者R3和R4形成氧代基;
Or R 3 and R 4 form an oxo group;
R5选自烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、氨基、烯基、炔基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、卤代烷基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烯基、炔基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 5 is selected from the group consisting of alkyl, cycloalkyl, haloalkyl, alkoxy, haloalkoxy, amino, alkenyl, alkynyl, aryl, heteroaryl, -OR 8 , -C(O)R 8 ,- C(O)OR 8 and -S(O) m R 8 , wherein said alkyl, cycloalkyl, haloalkyl, aryl and heteroaryl are each independently optionally selected from alkyl, haloalkyl, One or more of halogen, amino, nitro, cyano, hydroxy, alkenyl, alkynyl, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl Substituted by a substituent;
R6选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、卤素、氨基、硝基、羟基、氰基、杂环基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、卤代烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 6 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen, an amino group, a nitro group, a hydroxyl group, a cyano group, a heterocyclic group, an aryl group, a heteroaryl group, -OR 8 , —C(O)R 8 , —C(O)OR 8 and —S(O) m R 8 , wherein said alkyl, cycloalkyl, haloalkyl, heterocyclyl, aryl and heteroaryl The groups are each independently optionally selected from the group consisting of alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and Substituting one or more substituents in the heteroaryl;
R8选自氢原子、烷基、卤代烷基、烷氧基、羟烷基、羟基、氨基、环烷基、杂环基、芳基和杂芳基,其中所述的烷基、氨基、环烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤素、羟基、氨基、羧酸酯基、硝基、氰基、烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 8 is selected from the group consisting of a hydrogen atom, an alkyl group, a halogenated alkyl group, an alkoxy group, a hydroxyalkyl group, a hydroxyl group, an amino group, a cycloalkyl group, a heterocyclic group, an aryl group, and a heteroaryl group, wherein the alkyl group, the amino group, and the ring are The alkyl group, heterocyclic group, aryl group and heteroaryl group are each independently selected from the group consisting of alkyl, halogen, hydroxy, amino, carboxylate, nitro, cyano, alkoxy, hydroxyalkyl, cyclo Substituted by one or more substituents of an alkyl group, a heterocyclic group, an aryl group, and a heteroaryl group;
R9选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、氨基、烯基、炔基、芳基和杂芳基,其中所述的烷基、环烷基、卤代烷基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烯基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 9 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, an amino group, an alkenyl group, an alkynyl group, an aryl group and a heteroaryl group, wherein the alkyl group, the cycloalkyl group And haloalkyl, aryl and heteroaryl are each independently selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkenyl, alkoxy, haloalkoxy, hydroxyalkyl Substituting one or more substituents of a cycloalkyl, a heterocyclic group, an aryl group, and a heteroaryl group;
m为0、1或2;m is 0, 1 or 2;
x为0、1、2、3或4;且x is 0, 1, 2, 3 or 4;
y为0、1、2或3。y is 0, 1, 2 or 3.
本发明另外提供一种制备根据通式(II-A)化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体、或其混合物形式、或其可药用盐的中间体,即通式(II-A-1)所示化合物:The invention further provides a preparation of a compound according to formula (II-A) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer, or a mixture thereof An intermediate of the form, or a pharmaceutically acceptable salt thereof, is a compound of the formula (II-A-1):
或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐,Or a tautomer, a meso form, a racemate, an enantiomer, a diastereomer or a mixture thereof, or a pharmaceutically acceptable salt thereof,
其中:环A、X、Ra、R1、R3~R6、p和y如通式(II-A)中所定义。
Wherein: Ring A, X, R a , R 1 , R 3 to R 6 , p and y are as defined in the formula (II-A).
本发明另外提供一种制备所述的通式(I)化合物的方法,该方法包括:The invention further provides a process for the preparation of said compound of formula (I), which process comprises:
通式(V)化合物与通式(VI)化合物发生缩合反应,得到通式(I)化合物;a compound of the formula (V) is condensed with a compound of the formula (VI) to give a compound of the formula (I);
其中:among them:
环A、环B、X、Y、Z、R1~R7、x、y和z如通式(I)中所定义。Ring A, Ring B, X, Y, Z, R 1 to R 7 , x, y and z are as defined in the general formula (I).
本发明另外提供一种制备所述的通式(II-A)化合物的方法,该方法包括:The invention further provides a process for the preparation of the compound of the formula (II-A), which process comprises:
通式(II-A-1)化合物与通式(II-A-2)化合物发生缩合反应,得到通式(II-A)化合物;a compound of the formula (II-A-1) is condensed with a compound of the formula (II-A-2) to give a compound of the formula (II-A);
其中:among them:
环A、G、X、Ra、R1~R7、p、y和z如通式(II-A)中所定义。Rings A, G, X, R a , R 1 to R 7 , p, y and z are as defined in the formula (II-A).
本发明的另一方面涉及一种药物组合物,其含有治疗有效剂量的通式(I)、(II)、(III)、(IV)、(IV-A)、(IV-B)、(IV-C)或(V)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体、或其混合物形式、或可药用的盐,以及一种或多种药学上可接受的载体、稀释剂或赋形剂。本发明还涉及一种制备上述组合物的方法,其包括将通式(I)、(II)、(III)、(IV)、(IV-A)、(IV-B)、(IV-C)或(V)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体、或其混合物形式、或其可药用的盐与药学上可接受的载体、稀释剂或赋形剂相混合。Another aspect of the invention relates to a pharmaceutical composition comprising a therapeutically effective amount of the formula (I), (II), (III), (IV), (IV-A), (IV-B), a compound represented by IV-C) or (V) or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer, or a mixture thereof, or A pharmaceutically acceptable salt, and one or more pharmaceutically acceptable carriers, diluents or excipients. The invention further relates to a process for the preparation of the above composition comprising the general formulae (I), (II), (III), (IV), (IV-A), (IV-B), (IV-C) Or a compound represented by (V) or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer, or a mixture thereof, or a pharmaceutically acceptable form thereof The salt is mixed with a pharmaceutically acceptable carrier, diluent or excipient.
本发明进一步涉及通式(I)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐,或包含其的药物组合物在作为ROR调节剂在制备用于预防和/或治疗炎症、自身免疫性疾病、肿瘤或癌症的药物中的用途。The invention further relates to a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, for use as a ROR modulator in the manufacture of a medicament for the prevention and/or treatment of inflammation, autoimmune diseases, tumors or cancer.
本发明进一步涉及通式(I)所示的化合物或其互变异构体、内消旋体、外
消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐,或包含其的药物组合物在作为ROR反激动剂在制备用于预防和/或治疗炎症和自身免疫性疾病的药物中的用途。The present invention further relates to a compound represented by the formula (I) or a tautomer thereof, a mesogen, and an external
a racemate, an enantiomer, a diastereomer or a mixture thereof, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition comprising the same, for use as a ROR inverse agonist in the preparation for prophylaxis and/or treatment Use in medicines for inflammation and autoimmune diseases.
本发明进一步涉及通式(I)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐,或包含其的药物组合物在作为ROR激动剂在制备用于预防和/或治疗肿瘤或癌症的药物中的用途。The invention further relates to a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, for use as a ROR agonist in the manufacture of a medicament for the prevention and/or treatment of a tumor or cancer.
本发明进一步涉及通式(IV-A)所示的化合物作为ROR激动剂在制备用于预防和/或治疗肿瘤或癌症的药物中的用途。The invention further relates to the use of a compound of the formula (IV-A) as an ROR agonist for the preparation of a medicament for the prevention and/or treatment of a tumor or cancer.
本发明进一步涉及通式(I)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐,或包含其的药物组合物在制备用于ROR激动剂与抗PD-1抗体组合治疗肿瘤或癌症的药物中的用途。The invention further relates to a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, for use in the manufacture of a medicament for the treatment of a tumor or cancer with a combination of an ROR agonist and an anti-PD-1 antibody.
本发明进一步涉及通式(IV-A)所示的化合物作为ROR激动剂在制备用于与抗PD-1抗体组合治疗肿瘤或癌症的药物中的用途。The invention further relates to the use of a compound of the formula (IV-A) as an ROR agonist for the preparation of a medicament for the treatment of a tumor or cancer in combination with an anti-PD-1 antibody.
本发明进一步涉及通式(I)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐,或包含其的药物组合物在制备调节ROR的药物中的用途。The invention further relates to a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer or a mixture thereof, or Use of a pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, in the manufacture of a medicament for the modulation of ROR.
本发明进一步涉及通式(I)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐,或包含其的药物组合物在制备ROR激动剂的药物中的用途。The invention further relates to a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer or a mixture thereof, or Use of a pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, in the manufacture of a medicament for an ROR agonist.
本发明进一步涉及通式(IV-A)所示的化合物在制备ROR激动剂的药物中的用途。The invention further relates to the use of a compound of the formula (IV-A) for the manufacture of a medicament for an ROR agonist.
本发明进一步涉及通式(I)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐,或包含其的药物组合物在制备用于预防和/或治疗炎症和自身免疫性疾病的药物中的用途。The invention further relates to a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer or a mixture thereof, or Use of a pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, for the manufacture of a medicament for the prevention and/or treatment of inflammatory and autoimmune diseases.
在本文中,所述的炎症和自身免疫性疾病选自银屑病、类风湿性关节炎、银屑病性关节炎、多发性硬化、炎性肠病、强直性脊柱炎、慢性阻塞性肺病、血管球性肾炎、心肌炎、甲状腺炎、干眼症、葡萄膜炎、白塞病、哮喘、过敏性皮肤
炎、粉刺、克隆氏病、溃疡性结肠炎、系统性红斑狼疮、硬皮病、支气管炎和皮肌炎过敏性鼻炎。In the present context, the inflammatory and autoimmune diseases are selected from the group consisting of psoriasis, rheumatoid arthritis, psoriatic arthritis, multiple sclerosis, inflammatory bowel disease, ankylosing spondylitis, chronic obstructive pulmonary disease , glomerulonephritis, myocarditis, thyroiditis, dry eye, uveitis, Behcet's disease, asthma, allergic skin
Inflammation, acne, Crohn's disease, ulcerative colitis, systemic lupus erythematosus, scleroderma, bronchitis and dermatomyositis allergic rhinitis.
本发明进一步涉及通式(I)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐,或包含其的药物组合物在制备用于预防和/或治疗肿瘤或癌症的药物中的用途。The invention further relates to a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer or a mixture thereof, or Use of a pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, in the manufacture of a medicament for the prevention and/or treatment of a tumor or cancer.
在本文中,所述的癌症和肿瘤选自非霍奇金淋巴瘤、弥漫大B细胞淋巴瘤、滤泡性淋巴瘤、滑膜肉瘤、乳腺癌、宫颈癌、结肠癌、肺癌、胃癌、直肠癌、胰腺癌、脑癌、皮肤癌、口腔癌、前列腺癌、骨癌、肾癌、卵巢癌、膀胱癌、肝癌、输卵管肿瘤、卵巢瘤、腹膜肿瘤、IV期黑色素瘤、实体瘤、神经胶质瘤、神经胶母细胞瘤、肝细胞癌、乳突肾性瘤、头颈部肿瘤、白血病、淋巴瘤、骨髓瘤和非小细胞肺癌,特别是非霍奇金淋巴瘤、弥漫大B细胞淋巴瘤、滤泡性淋巴瘤、滑膜肉瘤。In the present context, the cancer and tumor are selected from the group consisting of non-Hodgkin's lymphoma, diffuse large B-cell lymphoma, follicular lymphoma, synovial sarcoma, breast cancer, cervical cancer, colon cancer, lung cancer, stomach cancer, rectum Cancer, pancreatic cancer, brain cancer, skin cancer, oral cancer, prostate cancer, bone cancer, kidney cancer, ovarian cancer, bladder cancer, liver cancer, fallopian tube tumor, ovarian tumor, peritoneal tumor, stage IV melanoma, solid tumor, nerve glue Tumor, glioblastoma, hepatocellular carcinoma, papillary renal tumor, head and neck cancer, leukemia, lymphoma, myeloma and non-small cell lung cancer, especially non-Hodgkin's lymphoma, diffuse large B-cell lymph Tumor, follicular lymphoma, synovial sarcoma.
本发明进一步涉及一种用作药物的通式(I)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式、或其可药用盐。The invention further relates to a compound of the formula (I) or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer or In the form of a mixture, or a pharmaceutically acceptable salt thereof.
本发明还涉及通式(I)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐,或包含其的药物组合物,其用于预防和/或治疗炎症或自身免疫性疾病,其中所述的炎症和自身免疫性疾病如上所定义。The present invention also relates to a compound of the formula (I) or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, for use in the prevention and/or treatment of an inflammatory or autoimmune disease, wherein the inflammatory and autoimmune diseases are as defined above.
本发明还涉及通式(I)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐,或包含其的药物组合物,其用于预防和/或治疗肿瘤或癌症,其中所述的肿瘤和癌症如上所定义。The present invention also relates to a compound of the formula (I) or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, for use in the prevention and/or treatment of a tumor or cancer, wherein said tumor and cancer are as defined above.
本发明还涉及通式(IV-A)所示的化合物,其用于预防和/或治疗肿瘤或癌症,其中所述的肿瘤和癌症如上所定义。The present invention also relates to a compound represented by the formula (IV-A) for use in the prevention and/or treatment of a tumor or a cancer, wherein the tumor and the cancer are as defined above.
本发明进一步涉及通式(I)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐,或包含其的药物组合物用于ROR激动剂与抗PD-1抗体组合物,其用于预防和/或治疗肿瘤或癌症,其中所述的肿瘤和癌症如上所定义。The invention further relates to a compound of the formula (I) or a tautomer, a mesomer, a racemate, an enantiomer, a diastereomer or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, is for use in a ROR agonist and an anti-PD-1 antibody composition for preventing and/or treating a tumor or cancer, wherein the tumor and cancer are as defined above.
本发明还涉及通式(IV-A)所示的化合物与抗PD-1抗体组合物,其用于预防和/或治疗肿瘤或癌症,其中所述的肿瘤和癌症如上所定义。The present invention also relates to a compound represented by the formula (IV-A) and an anti-PD-1 antibody composition for use in the prevention and/or treatment of a tumor or a cancer, wherein the tumor and the cancer are as defined above.
本发明还涉及一种治疗预防和/或治疗预防炎症或自身免疫性疾病的方法,其包括向需要其的患者施用治疗有效剂量的通式(I)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可
药用盐,或包含其的药物组合物。其中所述的炎症和自身免疫性疾病如上所定义。The present invention also relates to a method for the prophylaxis and/or treatment of a prophylactic or autoimmune disease comprising administering to a patient in need thereof a therapeutically effective amount of a compound of the formula (I) or a tautomer thereof. , a meso form, a racemate, an enantiomer, a diastereomer or a mixture thereof, or
A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same. The inflammatory and autoimmune diseases described therein are as defined above.
本发明还涉及一种治疗预防和/或治疗预防肿瘤或癌症的方法,其包括向需要其的患者施用治疗有效剂量的通式(I)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐,或包含其的药物组合物。其中所述的肿瘤和癌症如上所定义。The present invention also relates to a method for the prophylactic and/or therapeutic treatment of preventing tumors or cancer comprising administering to a patient in need thereof a therapeutically effective amount of a compound of the formula (I) or a tautomer thereof, internal elimination A form of a polar body, a racemate, an enantiomer, a diastereomer or a mixture thereof, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition comprising the same. The tumors and cancers described therein are as defined above.
本发明还涉及一种治疗预防和/或治疗预防肿瘤或癌症的方法,其包括向需要其的患者施用治疗有效剂量的通式(IV-A)所示的化合物。其中所述的肿瘤和癌症如上所定义。The present invention also relates to a method of treating prevention and/or treatment for preventing tumor or cancer comprising administering to a patient in need thereof a therapeutically effective amount of a compound of the formula (IV-A). The tumors and cancers described therein are as defined above.
本发明还涉及一种治疗预防和/或治疗预防肿瘤或癌症的方法,其包括向需要其的患者施用治疗有效剂量的通式(IV-A)所示的化合物与抗PD-1抗体组合物。其中所述的肿瘤和癌症如上所定义。The present invention also relates to a method for the prophylactic and/or therapeutic treatment of preventing tumors or cancer comprising administering to a patient in need thereof a therapeutically effective amount of a compound of the formula (IV-A) and an anti-PD-1 antibody composition. . The tumors and cancers described therein are as defined above.
本发明还涉及通式(I)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐,或包含其的药物组合物,其用于调节ROR。The present invention also relates to a compound of the formula (I) or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer or a mixture thereof, or A pharmaceutically acceptable salt, or a pharmaceutical composition comprising the same, for use in modulating ROR.
本发明还涉及通式(IV-A)所示的化合物,其用于ROR激动剂。The invention further relates to compounds of the general formula (IV-A) for use in ROR agonists.
本发明还涉及通式(IV-A)所示的化合物与抗PD-1抗体组合物,其用于ROR激动剂。The present invention also relates to a compound of the formula (IV-A) and an anti-PD-1 antibody composition for use in an ROR agonist.
本发明还涉及一种调节ROR的方法,其包括向需要其的患者施用治疗有效剂量的通式(I)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐,或包含其的药物组合物。The invention also relates to a method of modulating ROR comprising administering to a patient in need thereof a therapeutically effective amount of a compound of formula (I) or a tautomer, a mesogen, a racemate thereof, An enantiomer, a diastereomer or a mixture thereof, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition comprising the same.
含活性成分的药物组合物可以是适用于口服的形式,例如片剂、糖锭剂、锭剂、水或油混悬液、可分散粉末或颗粒、乳液、硬或软胶囊,或糖浆剂或酏剂。可按照本领域任何已知制备药用组合物的方法制备口服组合物,此类组合物可含有一种或多种选自以下的成分:甜味剂、矫味剂、着色剂和防腐剂,以提供悦目和可口的药用制剂。片剂含有活性成分和用于混合的适宜制备片剂的无毒的可药用的赋形剂。这些赋形剂可以是惰性赋形剂,如碳酸钙、碳酸钠、乳糖、磷酸钙或磷酸钠;造粒剂和崩解剂,例如微晶纤维素、交联羧甲基纤维素钠、玉米淀粉或藻酸;粘合剂,例如淀粉、明胶、聚乙烯吡咯烷酮或阿拉伯胶;和润滑剂,例如硬脂酸镁、硬脂酸或滑石粉。这些片剂可以不包衣或可通过掩盖药物的味道或在胃肠道中延迟崩解和吸收,因而在较长时间内提供缓释作用的已知技术将其包
衣。例如,可使用水溶性味道掩蔽物质,例如羟丙基甲基纤维素或羟丙基纤维素,或延长时间物质例如乙基纤维素、醋酸丁酸纤维素。The active ingredient-containing pharmaceutical composition may be in a form suitable for oral administration, such as tablets, dragees, lozenges, aqueous or oily suspensions, dispersible powders or granules, emulsions, hard or soft capsules, or syrups or Tincture. Oral compositions can be prepared according to any method known in the art for preparing pharmaceutical compositions, such compositions may contain one or more ingredients selected from the group consisting of sweeteners, flavoring agents, coloring agents, and preservatives, To provide a pleasing and tasty pharmaceutical preparation. Tablets contain the active ingredient and non-toxic pharmaceutically acceptable excipients suitable for the preparation of a tablet for admixture. These excipients may be inert excipients such as calcium carbonate, sodium carbonate, lactose, calcium phosphate or sodium phosphate; granulating agents and disintegrating agents such as microcrystalline cellulose, croscarmellose sodium, corn Starch or alginic acid; a binder such as starch, gelatin, polyvinylpyrrolidone or gum arabic; and a lubricant such as magnesium stearate, stearic acid or talc. These tablets may be uncoated or may be packaged by a known technique that provides a sustained release effect over a longer period of time by masking the taste of the drug or delaying disintegration and absorption in the gastrointestinal tract.
clothes. For example, water-soluble taste masking materials such as hydroxypropylmethylcellulose or hydroxypropylcellulose, or extended-time materials such as ethylcellulose, cellulose acetate butyrate may be used.
也可用其中活性成分与惰性固体稀释剂例如碳酸钙、磷酸钙或高岭土混合的硬明胶胶囊,或其中活性成分与水溶性载体例如聚乙二醇或油溶媒例如花生油、液体石蜡或橄榄油混合的软明胶胶囊提供口服制剂。It is also possible to use hard gelatin capsules in which the active ingredient is mixed with an inert solid diluent such as calcium carbonate, calcium phosphate or kaolin, or in which the active ingredient is mixed with a water-soluble carrier such as polyethylene glycol or an oil vehicle such as peanut oil, liquid paraffin or olive oil. Soft gelatin capsules provide oral preparations.
水悬浮液含有活性物质和用于混合的适宜制备水悬浮液的赋形剂。此类赋形剂是悬浮剂,例如羧基甲基纤维素钠、甲基纤维素、羟丙基甲基纤维素、藻酸钠、聚乙烯吡咯烷酮和阿拉伯胶;分散剂或湿润剂可以是天然产生的磷脂例如卵磷脂,或烯化氧与脂肪酸的缩合产物例如聚氧乙烯硬脂酸酯,或环氧乙烷与长链脂肪醇的缩合产物,例如十七碳亚乙基氧基鲸蜡醇(heptadecaethyleneoxy cetanol),或环氧乙烷与由脂肪酸和己糖醇衍生的部分酯的缩合产物,例如聚环氧乙烷山梨醇单油酸酯,或环氧乙烷与由脂肪酸和己糖醇酐衍生的偏酯的缩合产物,例如聚环氧乙烷脱水山梨醇单油酸酯。水混悬液也可以含有一种或多种防腐剂例如尼泊金乙酯或尼泊金正丙酯、一种或多种着色剂、一种或多种矫味剂和一种或多种甜味剂,例如蔗糖、糖精或阿司帕坦。The aqueous suspension contains the active substance and excipients suitable for the preparation of the aqueous suspension for mixing. Such excipients are suspending agents such as sodium carboxymethylcellulose, methylcellulose, hydroxypropylmethylcellulose, sodium alginate, polyvinylpyrrolidone and acacia; dispersing or wetting agents may be naturally occurring a phospholipid such as lecithin, or a condensation product of an alkylene oxide with a fatty acid such as polyoxyethylene stearate, or a condensation product of ethylene oxide with a long chain fatty alcohol, such as heptadecylethyleneoxy cetyl alcohol (heptadecaethyleneoxy cetanol), or a condensation product of ethylene oxide with a partial ester derived from a fatty acid and a hexitol, such as polyethylene oxide sorbitol monooleate, or ethylene oxide with derivatives derived from fatty acids and hexitols A condensation product of a partial ester such as polyethylene oxide sorbitan monooleate. The aqueous suspensions may also contain one or more preservatives such as ethylparaben or n-propylparaben, one or more coloring agents, one or more flavoring agents, and one or more sweetening agents. Flavoring agents such as sucrose, saccharin or aspartame.
油混悬液可通过使活性成分悬浮于植物油如花生油、橄榄油、芝麻油或椰子油,或矿物油例如液体石蜡中配制而成。油悬浮液可含有增稠剂,例如蜂蜡、硬石蜡或鲸蜡醇。可加入上述的甜味剂和矫味剂,以提供可口的制剂。可通过加入抗氧化剂例如丁羟茴醚或α-生育酚保存这些组合物。The oil suspension can be formulated by suspending the active ingredient in a vegetable oil such as peanut oil, olive oil, sesame oil or coconut oil, or a mineral oil such as liquid paraffin. The oily suspensions may contain a thickening agent, such as beeswax, hard paraffin or cetyl alcohol. The above sweeteners and flavoring agents may be added to provide a palatable preparation. These compositions can be preserved by the addition of an anti-oxidant such as butylated hydroxyanisole or alpha-tocopherol.
通过加入水可使适用于制备水混悬也的可分散粉末和颗粒提供活性成分和用于混合的分散剂或湿润剂、悬浮剂或一种或多种防腐剂。适宜的分散剂或湿润剂和悬浮剂可说明上述的例子。也可加入其他赋形剂例如甜味剂、矫味剂和着色剂。通过加入抗氧化剂例如抗坏血酸保存这些组合物。The dispersible powders and granules suitable for the preparation of aqueous suspensions can be provided by the addition of water to provide the active ingredient and dispersing or wetting agents, suspending agents or one or more preservatives. Suitable dispersing or wetting agents and suspending agents can be used to illustrate the above examples. Other excipients such as sweetening, flavoring, and coloring agents may also be added. These compositions are preserved by the addition of an anti-oxidant such as ascorbic acid.
本发明的药物组合物也可以是水包油乳剂的形式。油相可以是植物油例如橄榄油或花生油,或矿物油例如液体石蜡或其混合物。适宜的乳化剂可以是天然产生的磷脂,例如大豆卵磷脂和由脂肪酸和己糖醇酐衍生的酯或偏酯例如山梨坦单油酸酯,和所述偏酯和环氧乙烷的缩合产物,例如聚环氧乙烷山梨醇单油酸酯。乳剂也可以含有甜味剂、矫味剂、防腐剂和抗氧剂。可用甜味剂例如甘油、丙二醇、山梨醇或蔗糖配制糖浆和酏剂。此类制剂也可含有缓和剂、防腐剂、着色剂和抗氧剂。The pharmaceutical compositions of the invention may also be in the form of an oil-in-water emulsion. The oil phase may be a vegetable oil such as olive oil or peanut oil, or a mineral oil such as liquid paraffin or a mixture thereof. Suitable emulsifiers may be naturally occurring phospholipids, such as soy lecithin and esters or partial esters derived from fatty acids and hexitol anhydrides such as sorbitan monooleate, and condensation products of the partial esters and ethylene oxide, For example, polyethylene oxide sorbitol monooleate. The emulsions may also contain sweeteners, flavoring agents, preservatives, and antioxidants. Syrups and elixirs may be formulated with sweetening agents such as glycerol, propylene glycol, sorbitol or sucrose. Such formulations may also contain a demulcent, a preservative, a colorant, and an antioxidant.
药物组合物可以是无菌注射水溶液形式。可以使用的可接受的溶媒或溶剂有水、林格氏液和等渗氯化钠溶液。无菌注射制剂可以是其中活性成分溶于油相的无菌注射水包油微乳。例如将活性成分溶于大豆油和卵磷脂的混合物中。然后将油溶液加入水和甘油的混合物中处理形成微乳。可通过局部大量注射,将注射液或微乳注入患者的血流中。或者,最好按可保持本发明化合物恒定循环浓度的方式给予溶液和微乳。为保持这种恒定浓度,可使用连续静脉内递药装置。这种装
置的实例是Deltec CADD-PLUS.TM.5400型静脉注射泵。The pharmaceutical composition may be in the form of a sterile injectable aqueous solution. Among the acceptable vehicles or solvents that may be employed are water, Ringer's solution, and isotonic sodium chloride solution. The sterile injectable preparation may be a sterile injectable oil-in-water microemulsion in which the active ingredient is dissolved in the oily phase. For example, the active ingredient is dissolved in a mixture of soybean oil and lecithin. The oil solution is then added to a mixture of water and glycerin to form a microemulsion. The injection or microemulsion can be injected into the bloodstream of the patient by a local injection. Alternatively, the solution and microemulsion are preferably administered in a manner that maintains a constant circulating concentration of the compound of the invention. To maintain this constant concentration, a continuous intravenous delivery device can be used. This type of equipment
An example of this is the Deltec CADD-PLUS.TM.5400 intravenous pump.
药物组合物可以是用于肌内和皮下给药的无菌注射水或油混悬液的形式。可按已知技术,用上述那些适宜的分散剂或湿润剂和悬浮剂配制该混悬液。无菌注射制剂也可以是在肠胃外可接受的无毒稀释剂或溶剂中制备的无菌注射溶液或混悬液,例如1,3-丁二醇中制备的溶液。此外,可方便地用无菌固定油作为溶剂或悬浮介质。为此目的,可使用包括合成甘油单或二酯在内的任何调和固定油。此外,脂肪酸例如油酸也可以制备注射剂。The pharmaceutical composition may be in the form of a sterile injectable aqueous or oily suspension for intramuscular and subcutaneous administration. The suspension may be formulated according to known techniques using those suitable dispersing or wetting agents and suspending agents. The sterile injectable preparation may also be a sterile injectable solution or suspension, such as a solution prepared in 1,3-butanediol, in a parenterally acceptable non-toxic diluent or solvent. In addition, sterile fixed oils may conveniently be employed as a solvent or suspension medium. For this purpose, any blended fixed oil including synthetic mono- or diglycerides can be used. In addition, fatty acids such as oleic acid can also be prepared as an injection.
可按用于直肠给药的栓剂形式给予本发明化合物。可通过将药物与在普通温度下为固体但在直肠中为液体,因而在直肠中会溶化而释放药物的适宜的无刺激性赋形剂混合来制备这些药物组合物。此类物质包括可可脂、甘油明胶、氢化植物油、各种分子量的聚乙二醇和聚乙二醇的脂肪酸酯的混合物。The compounds of the invention may be administered in the form of a suppository for rectal administration. These pharmaceutical compositions can be prepared by mixing the drug with a suitable non-irritating excipient which is solid at ordinary temperatures but liquid in the rectum and thus dissolves in the rectum to release the drug. Such materials include a mixture of cocoa butter, glycerin gelatin, hydrogenated vegetable oil, polyethylene glycols of various molecular weights, and fatty acid esters of polyethylene glycol.
如本领域技术人员所熟知的,药物的给药剂量依赖于多种因素,包括但并非限定于以下因素:所用具体化合物的活性、患者的年龄、患者的体重、患者的健康状况、患者的行被、患者的饮食、给药时间、给药方式、排泄的速率、药物的组合等;另外,最佳的治疗方式如治疗的模式、通式化合物(I)的日用量或可药用的盐的种类可以根据传统的治疗方案来验证。As is well known to those skilled in the art, the dosage of the drug to be administered depends on a variety of factors including, but not limited to, the following factors: the activity of the particular compound used, the age of the patient, the weight of the patient, the health of the patient, and the patient's behavior. The dosage, the patient's diet, the time of administration, the mode of administration, the rate of excretion, the combination of drugs, etc.; in addition, the optimal treatment mode such as the mode of treatment, the daily dosage of the compound of formula (I) or a pharmaceutically acceptable salt The type can be verified according to traditional treatment options.
发明的详细说明Detailed description of the invention
除非有相反陈述,在说明书和权利要求书中使用的术语具有下述含义。Terms used in the specification and claims have the following meanings unless stated to the contrary.
术语“烷基”指饱和脂肪族烃基团,其为包含1至20个碳原子的直链或支链基团,优选含有1至12个碳原子的烷基,更优选含有1至6个碳原子的烷基。非限制性实例包括甲基、乙基、正丙基、异丙基、正丁基、异丁基、叔丁基、仲丁基、正戊基、1,1-二甲基丙基、1,2-二甲基丙基、2,2-二甲基丙基、1-乙基丙基、2-甲基丁基、3-甲基丁基、正己基、1-乙基-2-甲基丙基、1,1,2-三甲基丙基、1,1-二甲基丁基、1,2-二甲基丁基、2,2-二甲基丁基、1,3-二甲基丁基、2-乙基丁基、2-甲基戊基、3-甲基戊基、4-甲基戊基、2,3-二甲基丁基、正庚基、2-甲基己基、3-甲基己基、4-甲基己基、5-甲基己基、2,3-二甲基戊基、2,4-二甲基戊基、2,2-二甲基戊基、3,3-二甲基戊基、2-乙基戊基、3-乙基戊基、正辛基、2,3-二甲基己基、2,4-二甲基己基、2,5-二甲基己基、2,2-二甲基己基、3,3-二甲基己基、4,4-二甲基己基、2-乙基己基、3-乙基己基、4-乙基己基、2-甲基-2-乙基戊基、2-甲基-3-乙基戊基、正壬基、2-甲基-2-乙基己基、2-甲基-3-乙基己基、2,2-二乙基戊基、正癸基、3,3-二乙基己基、2,2-二乙基己基,及其各种支链异构体等。更优选的是含有1至6个碳原子的低级烷基,非限制性实施例包括甲基、乙基、正丙基、异丙基、正丁基、异丁基、叔丁基、仲丁基、正戊基、1,1-二甲基丙基、1,2-二甲基丙基、2,2-二甲基丙基、1-乙基丙基、2-甲基丁基、3-甲基丁基、正己基、1-乙基-2-甲基丙基、1,1,2-三甲基丙基、1,1-二甲基丁基、1,2-二甲基丁基、2,2-二甲基丁基、1,3-二甲基丁基、
2-乙基丁基、2-甲基戊基、3-甲基戊基、4-甲基戊基、2,3-二甲基丁基等。烷基可以是取代的或非取代的,当被取代时,取代基可以在任何可使用的连接点上被取代,所述取代基优选为一个或多个以下基团,其独立地选自烷基、烯基、炔基、烷氧基、烷硫基、烷基氨基、卤素、巯基、羟基、硝基、氰基、环烷基、杂环烷基、芳基、杂芳基、环烷氧基、杂环烷氧基、环烷硫基、杂环烷硫基、氧代基、羧基或羧酸酯基。The term "alkyl" refers to a saturated aliphatic hydrocarbon group which is a straight or branched chain group containing from 1 to 20 carbon atoms, preferably an alkyl group having from 1 to 12 carbon atoms, more preferably from 1 to 6 carbons. The alkyl group of the atom. Non-limiting examples include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, sec-butyl, n-pentyl, 1,1-dimethylpropyl, 1 ,2-dimethylpropyl, 2,2-dimethylpropyl, 1-ethylpropyl, 2-methylbutyl, 3-methylbutyl, n-hexyl, 1-ethyl-2- Methylpropyl, 1,1,2-trimethylpropyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1,3 - dimethylbutyl, 2-ethylbutyl, 2-methylpentyl, 3-methylpentyl, 4-methylpentyl, 2,3-dimethylbutyl, n-heptyl, 2 -methylhexyl, 3-methylhexyl, 4-methylhexyl, 5-methylhexyl, 2,3-dimethylpentyl, 2,4-dimethylpentyl, 2,2-dimethyl Pentyl, 3,3-dimethylpentyl, 2-ethylpentyl, 3-ethylpentyl, n-octyl, 2,3-dimethylhexyl, 2,4-dimethylhexyl, 2 , 5-dimethylhexyl, 2,2-dimethylhexyl, 3,3-dimethylhexyl, 4,4-dimethylhexyl, 2-ethylhexyl, 3-ethylhexyl, 4-B Hexyl, 2-methyl-2-ethylpentyl, 2-methyl-3-ethylpentyl, n-decyl, 2-methyl-2-ethylhexyl, 2-methyl-3-B Hexyl group, 2,2- Diethylpentyl, n-decyl, 3,3-diethylhexyl, 2,2-diethylhexyl, and various branched isomers thereof. More preferred are lower alkyl groups having from 1 to 6 carbon atoms, non-limiting examples including methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, sec-butyl Base, n-pentyl, 1,1-dimethylpropyl, 1,2-dimethylpropyl, 2,2-dimethylpropyl, 1-ethylpropyl, 2-methylbutyl, 3-methylbutyl, n-hexyl, 1-ethyl-2-methylpropyl, 1,1,2-trimethylpropyl, 1,1-dimethylbutyl, 1,2-dimethyl Butyl, 2,2-dimethylbutyl, 1,3-dimethylbutyl,
2-ethylbutyl, 2-methylpentyl, 3-methylpentyl, 4-methylpentyl, 2,3-dimethylbutyl, and the like. The alkyl group may be substituted or unsubstituted, and when substituted, the substituent may be substituted at any available point of attachment, preferably one or more of the following groups independently selected from the group consisting of an alkane Base, alkenyl, alkynyl, alkoxy, alkylthio, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, naphthenic An oxy group, a heterocycloalkoxy group, a cycloalkylthio group, a heterocycloalkylthio group, an oxo group, a carboxyl group or a carboxylate group.
术语“亚烷基”是指烷基的一个氢原子进一步被取代,例如:“亚甲基”指-CH2-、“亚乙基”指-(CH2)2-、“亚丙基”指-(CH2)3-、“亚丁基”指-(CH2)4-等。The term "alkylene" means that one hydrogen atom of the alkyl group is further substituted, for example, "methylene" refers to -CH 2 -, "ethylene" refers to -(CH 2 ) 2 -, "propylene" Refers to -(CH 2 ) 3 -, "butylene" means -(CH 2 ) 4 - and the like.
术语“烯基”指由至少由两个碳原子和至少一个碳-碳双键组成的如上定义的烷基,例如乙烯基、1-丙烯基、2-丙烯基、1-、2-或3-丁烯基等。烯基可以是取代的或非取代的,当被取代时,取代基优选为一个或多个以下基团,其独立地选自烷基、烯基、炔基、烷氧基、烷硫基、烷基氨基、卤素、巯基、羟基、硝基、氰基、环烷基、杂环烷基、芳基、杂芳基、环烷氧基、杂环烷氧基、环烷硫基、杂环烷硫基。The term "alkenyl" refers to an alkyl group as defined above consisting of at least two carbon atoms and at least one carbon-carbon double bond, such as ethenyl, 1-propenyl, 2-propenyl, 1-, 2- or -butenyl and the like. The alkenyl group may be substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups independently selected from the group consisting of alkyl, alkenyl, alkynyl, alkoxy, alkylthio, Alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio, heterocycle Alkylthio group.
术语“环烷基”指饱和或部分不饱和单环或多环环状烃取代基,环烷基环包含3至20个碳原子,优选包含3至12个碳原子,更优选包含3至6个碳原子。单环环烷基的非限制性实例包括环丙基、环丁基、环戊基、环戊烯基、环己基、环己烯基、环己二烯基、环庚基、环庚三烯基、环辛基等;多环环烷基包括螺环、稠环和桥环的环烷基。The term "cycloalkyl" refers to a saturated or partially unsaturated monocyclic or polycyclic cyclic hydrocarbon substituent containing from 3 to 20 carbon atoms, preferably from 3 to 12 carbon atoms, more preferably from 3 to 6 carbon atoms. One carbon atom. Non-limiting examples of monocyclic cycloalkyl groups include cyclopropyl, cyclobutyl, cyclopentyl, cyclopentenyl, cyclohexyl, cyclohexenyl, cyclohexadienyl, cycloheptyl, cycloheptatriene A polycycloalkyl group includes a spiro ring, a fused ring, and a cycloalkyl group.
术语“螺环烷基”指5至20元的单环之间共用一个碳原子(称螺原子)的多环基团,其可以含有一个或多个双键,但没有一个环具有完全共轭的π电子系统。优选为6至14元,更优选为7至10元。根据环与环之间共用螺原子的数目将螺环烷基分为单螺环烷基、双螺环烷基或多螺环烷基,优选为单螺环烷基和双螺环烷基。更优选为4元/4元、4元/5元、4元/6元、5元/5元或5元/6元单螺环烷基。螺环烷基的非限制性实例包括:The term "spirocycloalkyl" refers to a polycyclic group that shares a carbon atom (referred to as a spiro atom) between 5 to 20 members of a single ring, which may contain one or more double bonds, but none of the rings have a fully conjugated π electronic system. It is preferably 6 to 14 members, more preferably 7 to 10 members. The spirocycloalkyl group is classified into a monospirocycloalkyl group, a bispirocycloalkyl group or a polyspirocycloalkyl group, preferably a monospirocycloalkyl group and a bispirocycloalkyl group, depending on the number of common spiro atoms between the rings. More preferably, it is 4 yuan / 4 yuan, 4 yuan / 5 yuan, 4 yuan / 6 yuan, 5 yuan / 5 yuan or 5 yuan / 6-membered monospirocycloalkyl group. Non-limiting examples of spirocycloalkyl groups include:
术语“稠环烷基”指5至20元,系统中的每个环与体系中的其他环共享毗邻的一对碳原子的全碳多环基团,其中一个或多个环可以含有一个或多个双键,但没有一个环具有完全共轭的π电子系统。优选为6至14元,更优选为7至10元。根据组成环的数目可以分为双环、三环、四环或多环稠环烷基,优选为双环或三环,更优选为5元/5元或5元/6元双环烷基。稠环烷基的非限制性实例包括:
The term "fused cycloalkyl" refers to 5 to 20 members, and each ring in the system shares an all-carbon polycyclic group of an adjacent pair of carbon atoms with other rings in the system, wherein one or more of the rings may contain one or Multiple double bonds, but none of the rings have a fully conjugated π-electron system. It is preferably 6 to 14 members, more preferably 7 to 10 members. Depending on the number of constituent rings, it may be classified into a bicyclic, tricyclic, tetracyclic or polycyclic fused ring alkyl group, preferably a bicyclic or tricyclic ring, more preferably a 5-membered/5-membered or 5-membered/6-membered bicycloalkyl group. Non-limiting examples of fused cycloalkyl groups include:
术语“桥环烷基”指5至20元,任意两个环共用两个不直接连接的碳原子的全碳多环基团,其可以含有一个或多个双键,但没有一个环具有完全共轭的π电子系统。优选为6至14元,更优选为7至10元。根据组成环的数目可以分为双环、三环、四环或多环桥环烷基,优选为双环、三环或四环,更有选为双环或三环。桥环烷基的非限制性实例包括:The term "bridged cycloalkyl" refers to an all-carbon polycyclic group of 5 to 20 members, any two rings sharing two carbon atoms which are not directly bonded, which may contain one or more double bonds, but none of the rings have complete Conjugate π-electron system. It is preferably 6 to 14 members, more preferably 7 to 10 members. Depending on the number of constituent rings, it may be classified into a bicyclic, tricyclic, tetracyclic or polycyclic bridged cycloalkyl group, preferably a bicyclic ring, a tricyclic ring or a tetracyclic ring, and more preferably a bicyclic ring or a tricyclic ring. Non-limiting examples of bridged cycloalkyl groups include:
所述环烷基环可以稠合于芳基、杂芳基或杂环烷基环上,其中与母体结构连接在一起的环为环烷基,非限制性实例包括茚满基、四氢萘基、苯并环庚烷基等。环烷基可以是任选取代的或非取代的,当被取代时,取代基优选为一个或多个以下基团,其独立地选自烷基、烯基、炔基、烷氧基、烷硫基、烷基氨基、卤素、巯基、羟基、硝基、氰基、环烷基、杂环烷基、芳基、杂芳基、环烷氧基、杂环烷氧基、环烷硫基、杂环烷硫基、氧代基、羧基或羧酸酯基。The cycloalkyl ring may be fused to an aryl, heteroaryl or heterocycloalkyl ring, wherein the ring to which the parent structure is attached is a cycloalkyl group, non-limiting examples include indanyl, tetrahydronaphthalene Base, benzocycloheptyl and the like. The cycloalkyl group may be optionally substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups independently selected from the group consisting of alkyl, alkenyl, alkynyl, alkoxy, alkane Thio, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio a heterocycloalkylthio group, an oxo group, a carboxyl group or a carboxylate group.
术语“杂环基”指饱和或部分不饱和单环或多环环状烃取代基,其包含3至20个环原子,其中一个或多个环原子为选自氮、氧或S(O)m(其中m是整数0至2)的杂原子,但不包括-O-O-、-O-S-或-S-S-的环部分,其余环原子为碳。优选包含3至12个环原子,其中1~4个是杂原子;最优选包含3至8个环原子,其中1~3是杂原子;最优选包含3至6个环原子,其中1~2是杂原子。单环杂环基的非限制性实例包括吡咯烷基、咪唑烷基、四氢呋喃基、四氢噻吩基、二氢咪唑基、二氢呋喃基、二氢吡唑基、二氢吡咯基、哌啶基、哌嗪基、吗啉基、硫代吗啉基、高哌嗪基、吡喃基等,优选哌啶基、哌嗪基或吗啉基。多环杂环基包括螺环、稠环和桥环的杂环基。The term "heterocyclyl" refers to a saturated or partially unsaturated monocyclic or polycyclic cyclic hydrocarbon substituent containing from 3 to 20 ring atoms wherein one or more ring atoms are selected from nitrogen, oxygen or S(O). A hetero atom of m (where m is an integer of 0 to 2), but excluding the ring moiety of -OO-, -OS- or -SS-, the remaining ring atoms being carbon. Preferably comprising from 3 to 12 ring atoms, wherein from 1 to 4 are heteroatoms; most preferably from 3 to 8 ring atoms, wherein from 1 to 3 are heteroatoms; most preferably from 3 to 6 ring atoms, wherein from 1 to 2 It is a hetero atom. Non-limiting examples of monocyclic heterocyclic groups include pyrrolidinyl, imidazolidinyl, tetrahydrofuranyl, tetrahydrothiophenyl, dihydroimidazolyl, dihydrofuranyl, dihydropyrazolyl, dihydropyrrolyl, piperidine. The group, piperazinyl, morpholinyl, thiomorpholinyl, homopiperazinyl, pyranyl and the like are preferably piperidinyl, piperazinyl or morpholinyl. Polycyclic heterocyclic groups include spiro, fused, and bridged heterocyclic groups.
术语“螺杂环基”指5至20元的单环之间共用一个原子(称螺原子)的多环杂环基团,其中一个或多个环原子为选自氮、氧或S(O)m(其中m是整数0至2)的杂原子,其余环原子为碳。其可以含有一个或多个双键,但没有一个环具有完全共轭的π电子系统。优选为6至14元,更优选为7至10元。根据环与环之间共用螺原子的数目将螺杂环基分为单螺杂环基、双螺杂环基或多螺杂环基,优选为单螺杂环基和双螺杂环基。更优选为4元/4元、4元/5元、4元/6元、5元/5元或5
元/6元单螺杂环基。螺杂环基的非限制性实例包括:The term "spiroheterocyclyl" refers to a polycyclic heterocyclic group in which one atom (called a spiro atom) is shared between 5 to 20 members of a single ring, wherein one or more ring atoms are selected from nitrogen, oxygen or S (O). ) m (where m is an integer 0 to 2) heteroatoms, and the remaining ring atoms are carbon. It may contain one or more double bonds, but none of the rings have a fully conjugated pi-electron system. It is preferably 6 to 14 members, more preferably 7 to 10 members. The spiroheterocyclyl group is classified into a monospiroheterocyclic group, a dispiroheterocyclic group or a polyspirocyclic group according to the number of shared spiro atoms between the ring and the ring, and is preferably a monospiroheterocyclic group and a dispiroheterocyclic group. More preferably, it is 4 yuan / 4 yuan, 4 yuan / 5 yuan, 4 yuan / 6 yuan, 5 yuan / 5 yuan or 5 yuan / 6-membered monospiroheterocyclic group. Non-limiting examples of spiroheterocyclyl groups include:
术语“稠杂环基”指5至20元,系统中的每个环与体系中的其他环共享毗邻的一对原子的多环杂环基团,一个或多个环可以含有一个或多个双键,但没有一个环具有完全共轭的π电子系统,其中一个或多个环原子为选自氮、氧或S(O)m(其中m是整数0至2)的杂原子,其余环原子为碳。优选为6至14元,更优选为7至10元。根据组成环的数目可以分为双环、三环、四环或多环稠杂环基,优选为双环或三环,更优选为5元/5元或5元/6元双环稠杂环基。稠杂环基的非限制性实例包括:The term "fused heterocyclyl" refers to 5 to 20 members, and each ring in the system shares an adjacent pair of atomic polycyclic heterocyclic groups with other rings in the system, and one or more rings may contain one or more Double bond, but none of the rings have a fully conjugated π-electron system in which one or more ring atoms are heteroatoms selected from nitrogen, oxygen or S(O) m (where m is an integer from 0 to 2), and the remaining rings The atom is carbon. It is preferably 6 to 14 members, more preferably 7 to 10 members. Depending on the number of constituent rings, it may be classified into a bicyclic, tricyclic, tetracyclic or polycyclic fused heterocyclic group, preferably a bicyclic or tricyclic ring, more preferably a 5-membered/5-membered or 5-membered/6-membered bicyclic fused heterocyclic group. Non-limiting examples of fused heterocyclic groups include:
术语“桥杂环基”指5至14元,任意两个环共用两个不直接连接的原子的多环杂环基团,其可以含有一个或多个双键,但没有一个环具有完全共轭的π电子系统,其中一个或多个环原子为选自氮、氧或S(O)m(其中m是整数0至2)的杂原子,其余环原子为碳。优选为6至14元,更优选为7至10元。根据组成环的数目可以分为双环、三环、四环或多环桥杂环基,优选为双环、三环或四环,更有选为双环或三环。桥杂环基的非限制性实例包括:The term "bridge heterocyclyl" refers to a polycyclic heterocyclic group of 5 to 14 members, any two rings sharing two atoms which are not directly bonded, which may contain one or more double bonds, but none of the rings have a total A π-electron system of a yoke in which one or more ring atoms are heteroatoms selected from nitrogen, oxygen or S(O) m (where m is an integer from 0 to 2), the remaining ring atoms being carbon. It is preferably 6 to 14 members, more preferably 7 to 10 members. Depending on the number of constituent rings, it may be classified into a bicyclic, tricyclic, tetracyclic or polycyclic bridged heterocyclic group, preferably a bicyclic ring, a tricyclic ring or a tetracyclic ring, and more preferably a bicyclic ring or a tricyclic ring. Non-limiting examples of bridge heterocyclic groups include:
所述杂环基环可以稠合于芳基、杂芳基或环烷基环上,其中与母体结构连接在一起的环为杂环基,其非限制性实例包括:
The heterocyclyl ring may be fused to an aryl, heteroaryl or cycloalkyl ring, wherein the ring to which the parent structure is attached is a heterocyclic group, non-limiting examples of which include:
杂环基可以是任选取代的或非取代的,当被取代时,取代基优选为一个或多个以下基团,其独立地选自烷基、烯基、炔基、烷氧基、烷硫基、烷基氨基、卤素、巯基、羟基、硝基、氰基、环烷基、杂环烷基、芳基、杂芳基、环烷氧基、杂环烷氧基、环烷硫基、杂环烷硫基、氧代基、羧基或羧酸酯基。The heterocyclic group may be optionally substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups independently selected from alkyl, alkenyl, alkynyl, alkoxy, alkane Thio, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio a heterocycloalkylthio group, an oxo group, a carboxyl group or a carboxylate group.
术语“芳基”指具有共轭的π电子体系的6至14元全碳单环或稠合多环(也就是共享毗邻碳原子对的环)基团,优选为6至10元,例如苯基和萘基。更优选苯基。所述芳基环可以稠合于杂芳基、杂环基或环烷基环上,其中与母体结构连接在一起的环为芳基环,其非限制性实例包括:The term "aryl" refers to a 6 to 14 membered all-carbon monocyclic or fused polycyclic ring (ie, a ring that shares a pair of adjacent carbon atoms) having a conjugated π-electron system, preferably 6 to 10 members, such as benzene. Base and naphthyl. More preferred is phenyl. The aryl ring may be fused to a heteroaryl, heterocyclyl or cycloalkyl ring, wherein the ring to which the parent structure is attached is an aryl ring, non-limiting examples of which include:
芳基可以是取代的或非取代的,当被取代时,取代基优选为一个或多个以下基团,其独立地选自烷基、烯基、炔基、烷氧基、烷硫基、烷基氨基、卤素、巯基、羟基、硝基、氰基、环烷基、杂环烷基、芳基、杂芳基、环烷氧基、杂环烷氧基、环烷硫基、杂环烷硫基、羧基或羧酸酯基。The aryl group may be substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups independently selected from the group consisting of alkyl, alkenyl, alkynyl, alkoxy, alkylthio, Alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio, heterocycle An alkylthio group, a carboxyl group or a carboxylate group.
术语“杂芳基”指包含1至4个杂原子、5至14个环原子的杂芳族体系,其中杂原子选自氧、硫和氮。杂芳基优选为5至10元,含1至3个杂原子;更优选为5元或6元,含1至2个杂原子;优选例如咪唑基、呋喃基、噻吩基、噻唑基、吡唑基、噁唑基、吡咯基、四唑基、吡啶基、嘧啶基、噻二唑、吡嗪基等,优选为咪唑基、四唑基、吡啶基、噻吩基、吡唑基或嘧啶基、噻唑基;更有选吡啶基。所述杂芳基环可以稠合于芳基、杂环基或环烷基环上,其中与母体结构连接在一起的环为杂芳基环,其非限制性实例包括:The term "heteroaryl" refers to a heteroaromatic system containing from 1 to 4 heteroatoms, from 5 to 14 ring atoms, wherein the heteroatoms are selected from the group consisting of oxygen, sulfur and nitrogen. The heteroaryl group is preferably 5 to 10 members, and has 1 to 3 hetero atoms; more preferably 5 or 6 members, and 1 to 2 hetero atoms; preferably, for example, imidazolyl, furyl, thienyl, thiazolyl, pyridyl Azolyl, oxazolyl, pyrrolyl, tetrazolyl, pyridyl, pyrimidinyl, thiadiazole, pyrazinyl, etc., preferably imidazolyl, tetrazolyl, pyridyl, thienyl, pyrazolyl or pyrimidinyl , thiazolyl; more selective pyridyl. The heteroaryl ring may be fused to an aryl, heterocyclic or cycloalkyl ring, wherein the ring to which the parent structure is attached is a heteroaryl ring, non-limiting examples of which include:
杂芳基可以是任选取代的或非取代的,当被取代时,取代基优选为一个或多个以下基团,其独立地选自烷基、烯基、炔基、烷氧基、烷硫基、烷基氨基、卤素、巯基、羟基、硝基、氰基、环烷基、杂环烷基、芳基、杂芳基、环烷氧基、杂环烷氧基、环烷硫基、杂环烷硫基、羧基或羧酸酯基。The heteroaryl group may be optionally substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups independently selected from the group consisting of alkyl, alkenyl, alkynyl, alkoxy, alkane Thio, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio a heterocycloalkylthio group, a carboxyl group or a carboxylate group.
术语“烷氧基”指-O-(烷基)和-O-(非取代的环烷基),其中烷基的定义如上所述。烷氧基的非限制性实例包括:甲氧基、乙氧基、丙氧基、丁氧基、环丙氧基、环丁氧基、环戊氧基、环己氧基。烷氧基可以是任选取代的或非取代的,当被取代时,取代基优选为一个或多个以下基团,其独立地选自烷基、烯基、炔基、烷氧基、烷硫基、烷基氨基、卤素、巯基、羟基、硝基、氰基、环烷基、杂环烷基、芳基、杂芳基、环烷氧基、杂环烷氧基、环烷硫基、杂环烷硫基、羧基或羧酸酯基。The term "alkoxy" refers to -O-(alkyl) and -O-(unsubstituted cycloalkyl), wherein alkyl is as defined above. Non-limiting examples of alkoxy groups include: methoxy, ethoxy, propoxy, butoxy, cyclopropoxy, cyclobutoxy, cyclopentyloxy, cyclohexyloxy. The alkoxy group may be optionally substituted or unsubstituted, and when substituted, the substituent is preferably one or more of the following groups independently selected from the group consisting of alkyl, alkenyl, alkynyl, alkoxy, alkane Thio, alkylamino, halogen, fluorenyl, hydroxy, nitro, cyano, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cycloalkoxy, heterocycloalkoxy, cycloalkylthio a heterocycloalkylthio group, a carboxyl group or a carboxylate group.
术语“卤代烷基”指被一个或多个卤素取代的烷基,其中烷基如上所定义。The term "haloalkyl" refers to an alkyl group substituted by one or more halogens, wherein alkyl is as defined above.
术语“卤代烷氧基”指被一个或多个卤素取代的烷氧基,其中烷氧基如上所定义。The term "haloalkoxy" refers to an alkoxy group substituted by one or more halogens, wherein alkoxy is as defined above.
术语“羟烷基”指被羟基取代的烷基,其中烷基如上所定义。The term "hydroxyalkyl" refers to an alkyl group substituted with a hydroxy group, wherein alkyl is as defined above.
术语“羟基”指-OH基团。The term "hydroxy" refers to an -OH group.
术语“卤素”指氟、氯、溴或碘。The term "halogen" means fluoro, chloro, bromo or iodo.
术语“氨基”指-NH2。The term "amino" means -NH 2.
术语“氰基”指-CN。The term "cyano" refers to -CN.
术语“硝基”指-NO2。The term "nitro" refers to -NO 2 .
术语“氧代基”指=O。The term "oxo" refers to =0.
术语“羰基”指C=O。The term "carbonyl" refers to C=O.
术语“羧基”指-C(O)OH。The term "carboxy" refers to -C(O)OH.
术语“异氰酸基”指-NCO。The term "isocyanato" refers to -NCO.
术语“肟基”指=N-OH。The term "mercapto" refers to =N-OH.
术语“羧酸酯基”指-C(O)O(烷基)或-C(O)O(环烷基),其中烷基、环烷基如上所定义。The term "carboxylate group" refers to -C(O)O(alkyl) or -C(O)O(cycloalkyl), wherein alkyl, cycloalkyl are as defined above.
术语“酰卤”指含有-C(O)-卤素的基团的化合物。The term "acyl halide" refers to a compound containing a -C(O)-halogen group.
“X选自A、B、或C”、“X选自A、B和C”、“X为A、B或C”、“X为A、B和C”等不同用语均表达了相同的意义,即表示X可以是A、B、C中任意一种或几种。"X is selected from A, B, or C", "X is selected from A, B, and C", "X is A, B, or C", and "X is A, B, and C" and the like are expressed in the same language. Meaning, that is, X can be any one or several of A, B, and C.
“任选”或“任选地”意味着随后所描述的事件或环境可以但不必发生,该说明包括该事件或环境发生或不发生的场合。例如,“任选被烷基取代的杂环基团”
意味着烷基可以但不必须存在,该说明包括杂环基团被烷基取代的情形和杂环基团不被烷基取代的情形。"Optional" or "optionally" means that the subsequently described event or environment may, but need not, occur, including where the event or environment occurs or does not occur. For example, "heterocyclic group optionally substituted by an alkyl group"
It is meant that an alkyl group may, but need not, be present, and the description includes the case where the heterocyclic group is substituted with an alkyl group and the case where the heterocyclic group is not substituted with an alkyl group.
“取代的”指基团中的一个或多个氢原子,优选为最多5个,更优选为1~3个氢原子彼此独立地被相应数目的取代基取代。不言而喻,取代基仅处在它们的可能的化学位置,本领域技术人员能够在不付出过多努力的情况下确定(通过实验或理论)可能或不可能的取代。例如,具有游离氢的氨基或羟基与具有不饱和(如烯属)键的碳原子结合时可能是不稳定的。"Substituted" refers to one or more hydrogen atoms in the group, preferably up to 5, more preferably 1 to 3, hydrogen atoms, independently of each other, substituted by a corresponding number of substituents. It goes without saying that the substituents are only in their possible chemical positions, and those skilled in the art will be able to determine (by experiment or theory) substitutions that may or may not be possible without undue effort. For example, an amino group or a hydroxyl group having a free hydrogen may be unstable when combined with a carbon atom having an unsaturated (e.g., olefinic) bond.
“药物组合物”表示含有一种或多种本文所述化合物或其生理学上/可药用的盐或前体药物与其他化学组分的混合物,以及其他组分例如生理学/可药用的载体和赋形剂。药物组合物的目的是促进对生物体的给药,利于活性成分的吸收进而发挥生物活性。"Pharmaceutical composition" means a mixture comprising one or more of the compounds described herein, or a physiologically/pharmaceutically acceptable salt or prodrug thereof, and other chemical components, as well as other components such as physiological/pharmaceutically acceptable carriers. And excipients. The purpose of the pharmaceutical composition is to promote the administration of the organism, which facilitates the absorption of the active ingredient and thereby exerts biological activity.
“可药用盐”是指本发明化合物的盐,这类盐用于哺乳动物体内时具有安全性和有效性,且具有应有的生物活性。"Pharmaceutically acceptable salt" refers to a salt of a compound of the invention which is safe and effective for use in a mammal and which possesses the desired biological activity.
本发明化合物的合成方法Method for synthesizing the compound of the present invention
为了完成本发明的目的,本发明采用如下技术方案:In order to accomplish the object of the present invention, the present invention adopts the following technical solutions:
本发明通式(I)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用的盐的制备方法,包括以下步骤:a compound of the formula (I) of the present invention or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer or a mixture thereof, or a drug thereof The preparation method of the salt used includes the following steps:
将通式(I-A)化合物、和通式(I-B)化合物、双乙氰二氯化钯和降冰片烯溶解到极性溶剂中,在碱性条件下加热反应,得到通式(I-C)化合物,该条件的碱性试剂优选碳酸氢钠,极性溶剂优选N,N-二甲基乙酰胺;得到的通式(I-C)化合物在加热、碱性条件下与碘甲烷和氯代物反应,得到通式(I-D)化合物,该条件的碱性试剂优选氢化钠;得到的通式(I-D)化合物在碱性条件下,水解得到通式(V)化合物,
该条件的碱性试剂优选氢氧化钠;得到的通式(V)化合物与通式(VI)化合物、1-羟基苯并三唑和N,N-二异丙基乙胺反应,得到通式(I)化合物。Dissolving a compound of the formula (IA), a compound of the formula (IB), bis(cyanium dichloride) and norbornene in a polar solvent, and heating the reaction under basic conditions to obtain a compound of the formula (IC), The alkaline reagent of this condition is preferably sodium hydrogencarbonate, and the polar solvent is preferably N,N-dimethylacetamide; the obtained compound of the general formula (IC) is reacted with methyl iodide and chlorinated compound under heating and basic conditions to obtain a pass. a compound of the formula (ID), wherein the alkaline reagent of the condition is preferably sodium hydride; the obtained compound of the formula (ID) is hydrolyzed under basic conditions to give a compound of the formula (V).
The alkaline reagent of this condition is preferably sodium hydroxide; the obtained compound of the formula (V) is reacted with a compound of the formula (VI), 1-hydroxybenzotriazole and N,N-diisopropylethylamine to give a general formula. (I) a compound.
提供碱性条件的试剂包括有机碱和无机碱类,所述的有机碱类包括但不限于三乙胺、N,N-二异丙基乙胺、正丁基锂、二异丙基氨基锂、醋酸钾、叔丁醇钠或叔丁醇钾,所述的无机碱类包括但不限于氢化钠、氢氧化钠、磷酸钾、碳酸钠、碳酸氢钠、碳酸钾或碳酸铯。The reagents providing basic conditions include organic bases including, but not limited to, triethylamine, N,N-diisopropylethylamine, n-butyllithium, lithium diisopropylamide, and inorganic bases. And potassium acetate, sodium t-butoxide or potassium t-butoxide. The inorganic bases include, but are not limited to, sodium hydride, sodium hydroxide, potassium phosphate, sodium carbonate, sodium hydrogencarbonate, potassium carbonate or cesium carbonate.
其中:among them:
Rx选自烷基、卤代烷基、羟烷基、环烷基、杂环基、芳基和杂芳基,其中所述的烷基、环烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤素、羟基、氨基、羧酸酯基、硝基、氰基、烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R x is selected from the group consisting of alkyl, haloalkyl, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl, wherein said alkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl Each is optionally independently selected from the group consisting of alkyl, halogen, hydroxy, amino, carboxylate, nitro, cyano, alkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl Substituted by one or more substituents;
环A、环B、X、Y、Z、R1~R7、x、y和z如通式(I)中所定义。Ring A, Ring B, X, Y, Z, R 1 to R 7 , x, y and z are as defined in the general formula (I).
本发明通式(II-A)所示的化合物或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用的盐的制备方法,包括以下步骤:a compound of the formula (II-A) of the present invention or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer or a mixture thereof, or A method for preparing a pharmaceutically acceptable salt, comprising the steps of:
将通式(II-1)化合物、和通式(II-2)化合物、双乙氰二氯化钯和降冰片烯溶解到极性溶剂中,在碱性条件下加热反应,得到通式(II-3)化合物,该条件的碱性试剂优选碳酸氢钠,极性溶剂优选N,N-二甲基乙酰胺;得到的通式(II-3)化合物在加热、碱性条件下与碘甲烷和氯代物反应,得到通式(II-4)化合物,该条件的碱性试剂优选氢化钠;得到的通式(II-4)化合物在碱性条件下,水解得到通式(II-A-1)化合物,该条件的碱性试剂优选氢氧化钠;得到的通式(II-A-1)化合物与通式(II-A-2)化合物、1-羟基苯并三唑和N,N-二异丙基乙胺反应,得到通式(II-A)化合物。Dissolving the compound of the formula (II-1), and the compound of the formula (II-2), bis-cyanide palladium dichloride and norbornene in a polar solvent, and heating the reaction under basic conditions to obtain a formula ( II-3) a compound, the alkaline reagent of the condition is preferably sodium hydrogencarbonate, the polar solvent is preferably N,N-dimethylacetamide; and the obtained compound of the formula (II-3) is heated under alkaline conditions with iodine. Methane and a chlorinated product are reacted to obtain a compound of the formula (II-4). The alkaline reagent of the condition is preferably sodium hydride; the obtained compound of the formula (II-4) is hydrolyzed under basic conditions to give the formula (II-A). -1) a compound, the alkaline reagent of this condition is preferably sodium hydroxide; the obtained compound of the formula (II-A-1) and the compound of the formula (II-A-2), 1-hydroxybenzotriazole and N, Reaction with N-diisopropylethylamine gives the compound of the formula (II-A).
提供碱性条件的试剂包括有机碱和无机碱类,所述的有机碱类包括但不限于
三乙胺、N,N-二异丙基乙胺、正丁基锂、二异丙基氨基锂、醋酸钾、叔丁醇钠或叔丁醇钾,所述的无机碱类包括但不限于氢化钠、氢氧化钠、磷酸钾、碳酸钠、碳酸氢钠、碳酸钾或碳酸铯。The reagents providing basic conditions include organic bases and inorganic bases, including but not limited to
Triethylamine, N,N-diisopropylethylamine, n-butyllithium, lithium diisopropylamide, potassium acetate, sodium t-butoxide or potassium t-butoxide, the inorganic bases include but are not limited to Sodium hydride, sodium hydroxide, potassium phosphate, sodium carbonate, sodium hydrogencarbonate, potassium carbonate or cesium carbonate.
其中:among them:
Rx选自烷基、卤代烷基、羟烷基、环烷基、杂环基、芳基和杂芳基,其中所述的烷基、环烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤素、羟基、氨基、羧酸酯基、硝基、氰基、烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R x is selected from the group consisting of alkyl, haloalkyl, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl, wherein said alkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl Each is optionally independently selected from the group consisting of alkyl, halogen, hydroxy, amino, carboxylate, nitro, cyano, alkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl Substituted by one or more substituents;
环A、G、X、Ra、R1~R7、p、y和z如通式(II-A)中所定义。Rings A, G, X, R a , R 1 to R 7 , p, y and z are as defined in the formula (II-A).
图1化合物实施例30在C57BL/6小鼠中对MC38结肠直肠肿瘤生长的影响。Effect of Compound Example 30 of Figure 1 on MC38 colorectal tumor growth in C57BL/6 mice.
以下结合实施例进一步描述本发明,但这些实施例并非限制着本发明的范围。The invention is further described in the following examples, which are not intended to limit the scope of the invention.
实施例Example
化合物的结构是通过核磁共振(NMR)或/和质谱(MS)来确定的。NMR位移(δ)以10-6(ppm)的单位给出。NMR的测定是用Bruker AVANCE-400核磁仪,测定溶剂为氘代二甲基亚砜(DMSO-d6),氘代氯仿(CDCl3),氘代甲醇(CD3OD),内标为四甲基硅烷(TMS)。The structure of the compound is determined by nuclear magnetic resonance (NMR) or/and mass spectrometry (MS). The NMR shift (δ) is given in units of 10 -6 (ppm). NMR was measured using a Bruker AVANCE-400 nuclear magnetic apparatus, and the solvent was deuterated dimethyl sulfoxide (DMSO-d 6 ), deuterated chloroform (CDCl 3 ), deuterated methanol (CD 3 OD), internal standard was four. Methyl silane (TMS).
MS的测定用FINNIGAN LCQAd(ESI)质谱仪(生产商:Thermo,型号:Finnigan LCQ advantage MAX)。The measurement of the MS was carried out using a FINNIGAN LCQAd (ESI) mass spectrometer (manufacturer: Thermo, model: Finnigan LCQ advantage MAX).
HPLC的测定使用安捷伦1200DAD高压液相色谱仪(Sunfire C18 150×4.6mm色谱柱)和Waters 2695-2996高压液相色谱仪(Gimini C18 150×4.6mm色谱柱)。The HPLC was measured using an Agilent 1200 DAD high pressure liquid chromatograph (Sunfire C18 150 x 4.6 mm column) and a Waters 2695-2996 high pressure liquid chromatograph (Gimini C18 150 x 4.6 mm column).
手性HPLC分析测定使用LC-10A vp(Shimadzu)或者SFC-analytical(Berger Instruments Inc.)。Chiral HPLC analysis assays were performed using LC-10A vp (Shimadzu) or SFC-analytical (Berger Instruments Inc.).
薄层层析硅胶板使用烟台黄海HSGF254或青岛GF254硅胶板,薄层色谱法(TLC)使用的硅胶板采用的规格是0.15mm~0.2mm,薄层层析分离纯化产品采用的规格是0.4mm~0.5mm。Thin layer chromatography silica gel plate uses Yantai Yellow Sea HSGF254 or Qingdao GF254 silica gel plate. The specification of silica gel plate used for thin layer chromatography (TLC) is 0.15mm~0.2mm. The specification for thin layer chromatography separation and purification is 0.4mm. ~0.5mm.
柱层析一般使用烟台黄海硅胶200~300目硅胶为载体。Column chromatography generally uses Yantai Huanghai silica gel 200-300 mesh silica gel as a carrier.
手性制备柱层析使用Prep Star SD-1(Varian Instruments Inc.)或SFC-multigram(Berger Instruments Inc.)。Chiral preparative column chromatography was performed using Prep Star SD-1 (Varian Instruments Inc.) or SFC-multigram (Berger Instruments Inc.).
激酶平均抑制率及IC50值的测定用NovoStar酶标仪(德国BMG公司)。The average value of 50 measured kinase inhibition rate and IC NovoStar using a microplate reader (BMG, Germany).
本发明的已知的起始原料可以采用或按照本领域已知的方法来合成,或可购买自ABCR GmbH&Co.KG,Acros Organics,Aldrich Chemical Company,韶远
化学科技(Accela ChemBio Inc)、达瑞化学品等公司。The known starting materials of the present invention may be synthesized by or according to methods known in the art, or may be purchased from ABCR GmbH & Co. KG, Acros Organics, Aldrich Chemical Company, 韶远
Companies such as Accela ChemBio Inc., Dary Chemicals.
实施例中无特殊说明,反应能够均在氩气氛或氮气氛下进行。Unless otherwise specified in the examples, the reactions can all be carried out under an argon atmosphere or a nitrogen atmosphere.
氩气氛或氮气氛是指反应瓶连接一个约1L容积的氩气或氮气气球。An argon atmosphere or a nitrogen atmosphere means that the reaction flask is connected to an argon or nitrogen balloon having a volume of about 1 L.
氢气氛是指反应瓶连接一个约1L容积的氢气气球。The hydrogen atmosphere means that the reaction flask is connected to a hydrogen balloon of about 1 L volume.
加压氢化反应使用Parr 3916EKX型氢化仪和清蓝QL-500型氢气发生器或HC2-SS型氢化仪。The pressurized hydrogenation reaction was carried out using a Parr Model 3916EKX hydrogenation apparatus and a clear blue QL-500 type hydrogen generator or a HC2-SS type hydrogenation apparatus.
氢化反应通常抽真空,充入氢气,反复操作3次。The hydrogenation reaction is usually evacuated, charged with hydrogen, and operated three times.
微波反应使用CEM Discover-S 908860型微波反应器。The microwave reaction used a CEM Discover-S Model 908860 microwave reactor.
实施例中无特殊说明,溶液是指水溶液。Unless otherwise stated in the examples, the solution means an aqueous solution.
实施例中无特殊说明,反应的温度为室温,为20℃~30℃。There is no particular description in the examples, and the reaction temperature is room temperature and is 20 ° C to 30 ° C.
实施例中的反应进程的监测采用薄层色谱法(TLC),反应所使用的展开剂的体系有:A:二氯甲烷和甲醇体系,B:正己烷和乙酸乙酯体系,C:石油醚和乙酸乙酯体系,D:丙酮,溶剂的体积比根据化合物的极性不同而进行调节。纯化化合物采用的柱层析的洗脱剂的体系和薄层色谱法的展开剂体系包括:A:二氯甲烷和甲醇体系,B:正己烷和乙酸乙酯体系,C:二氯甲烷和丙酮体系,溶剂的体积比根据化合物的极性不同而进行调节,也可以加入少量的三乙胺和醋酸等碱性或酸性试剂进行调节。The progress of the reaction in the examples was monitored by thin layer chromatography (TLC). The system used for the reaction was: A: dichloromethane and methanol system, B: n-hexane and ethyl acetate system, C: petroleum ether And the ethyl acetate system, D: acetone, the volume ratio of the solvent is adjusted depending on the polarity of the compound. Purification compounds using column chromatography eluent systems and thin layer chromatography developer systems include: A: dichloromethane and methanol systems, B: n-hexane and ethyl acetate systems, C: dichloromethane and acetone In the system, the volume ratio of the solvent is adjusted depending on the polarity of the compound, and a small amount of an alkaline or acidic reagent such as triethylamine or acetic acid may be added for adjustment.
实施例1Example 1
N-(4-(乙磺酰基)苄基)-1-异丙基-2-(2-(三氟甲氧基)苄基)-1H-吲哚-5-甲酰胺1N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-(2-(trifluoromethoxy)benzyl)-1H-indole-5-carboxamide 1
第一步first step
2-(2-(三氟甲氧基)苄基)-1H-吲哚-5-甲酸甲酯1cMethyl 2-(2-(trifluoromethoxy)benzyl)-1H-indole-5-carboxylate 1c
将1H-吲哚-5-甲酸甲酯1a(50mg,0.28mmol),1-(溴甲基)-2-三氟甲氧基苯1b
(71mg,0.28mmol),双乙腈二氯化钯(7mg,0.03mmol),降冰片烯(54mg,0.57mmol),碳酸氢钠(72mg,0.86mmol)溶于N,N-二甲基乙酰胺中,升至70℃搅拌反应12小时。反应液冷却至室温,加入过量水,用乙酸乙酯萃取(20mL×3),合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物1c(70mg,黄色固体),产率:70%。1H-indole-5-carboxylic acid methyl ester 1a (50 mg, 0.28 mmol), 1-(bromomethyl)-2-trifluoromethoxybenzene 1b
(71 mg, 0.28 mmol), diacetonitrile palladium dichloride (7 mg, 0.03 mmol), norbornene (54 mg, 0.57 mmol), sodium bicarbonate (72 mg, 0.86 mmol) dissolved in N,N-dimethylacetamide The reaction was stirred at 70 ° C for 12 hours. The reaction mixture was cooled to room temperature, and water was added, and the mixture was applied to ethyl acetate (20 mL × 3), and the organic phase was combined, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered, The residue obtained was purified to give titled product 1c (yield: 70 mg, yellow solid).
第二步Second step
1-异丙基-2-(2-(三氟甲氧基)苄基)-1H-吲哚-5-甲酸甲酯1dMethyl 1-isopropyl-2-(2-(trifluoromethoxy)benzyl)-1H-indole-5-carboxylate 1d
将1c(60mg,0.17mmol)溶于N,N-二甲基甲酰胺中,加入催化量碘化钾,再加入氢化钠(10mg,0.26mmol,60%in oil),搅拌反应30分钟后加入2-溴丙烷(103mg,0.86mmol),升至60℃封管反应12小时。反应液冷却至室温,加入过量水,用乙酸乙酯萃取(20mL×3),合并有机相,依次用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物1d(24mg,黄色油状物),产率:36%。1c (60mg, 0.17mmol) was dissolved in N,N-dimethylformamide, a catalytic amount of potassium iodide was added, sodium hydride (10mg, 0.26mmol, 60% in oil) was added, and the reaction was stirred for 30 minutes, then added 2- Bromopropane (103 mg, 0.86 mmol) was stirred at 60 ° C for 12 hours. The reaction mixture was cooled to room temperature, and water was added, and the mixture was combined with ethyl acetate (20 mL×3). The resulting residue was purified with EtOAc EtOAcjjjjj
第三步third step
1-异丙基-2-(2-(三氟甲氧基)苄基)-1H-吲哚-5-甲酸1e1-isopropyl-2-(2-(trifluoromethoxy)benzyl)-1H-indole-5-carboxylic acid 1e
将1d(25mg,0.064mmol)溶于7mL甲醇和四氢呋喃(V:V=5:2)混合溶剂中,加入4M氢氧化钠溶液2mL,60℃搅拌反应2小时。反应液冷却至室温,滴加浓盐酸至pH为4,用乙酸乙酯萃取(20mL×2),合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物1e(20mg,黄色固体),产率:83%。1 d (25 mg, 0.064 mmol) was dissolved in 7 mL of a mixed solvent of methanol and tetrahydrofuran (V: V = 5:2), 2 mL of a 4 M sodium hydroxide solution was added, and the reaction was stirred at 60 ° C for 2 hours. The reaction mixture was cooled to room temperature, and then concentrated EtOAc EtOAc (EtOAc m. The resulting residue was purified by EtOAc EtOAcjjjjj
第四步the fourth step
N-(4-(乙磺酰基)苄基)-1-异丙基-2-(2-(三氟甲氧基)苄基)-1H-吲哚-5-甲酰胺1N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-(2-(trifluoromethoxy)benzyl)-1H-indole-5-carboxamide 1
将1e(20mg,0.053mmol),(4-(乙磺酰基)苯基)甲胺(16mg,0.08mmol,采用专利申请“WO2015/17335”公开的方法制备而得),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(15mg,0.08mmol),1-羟基苯并三唑(11mg,0.08mmol),N,N-二异丙基乙胺(21mg,0.16mmol)溶于二氯甲烷中,搅拌反应12小时。加入水,用二氯甲烷和甲醇(V:V=8:1)混合溶剂萃取(20mL×3),合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物1(20mg,白色固体),产率:65%。1e (20 mg, 0.053 mmol), (4-(ethylsulfonyl)phenyl)methanamine (16 mg, 0.08 mmol, prepared by the method disclosed in the patent application "WO2015/17335"), 1-(3-) Methylaminopropyl)-3-ethylcarbodiimide hydrochloride (15 mg, 0.08 mmol), 1-hydroxybenzotriazole (11 mg, 0.08 mmol), N,N-diisopropylethylamine (21 mg , 0.16 mmol) was dissolved in dichloromethane, and the reaction was stirred for 12 hours. Add water, extract with a mixed solvent of dichloromethane and methanol (V: V = 8:1) (20 mL × 3), combine the organic phase, wash with saturated sodium chloride solution, dry over anhydrous sodium sulfate, The residue was purified by EtOAc EtOAc EtOAc:
MS m/z(ESI):559.4[M+1];MS m/z (ESI): 559.4 [M+1];
1H NMR(400MHz,CDCl3)δ8.06(s,1H),7.87(d,2H),7.64(d,1H),7.53-7.57(m,3H),7.31(d,2H),7.17-7.21(m,1H),7.03(d,1H),6.64(t,1H),6.34(s,1H),4.79(d,2H),4.41-4.48(m,1H),4.20(s,2H),3.11(q,2H),1.49(d,6H),1.28(t,3H)。 1 H NMR (400MHz, CDCl 3 ) δ8.06 (s, 1H), 7.87 (d, 2H), 7.64 (d, 1H), 7.53-7.57 (m, 3H), 7.31 (d, 2H), 7.17- 7.21 (m, 1H), 7.03 (d, 1H), 6.64 (t, 1H), 6.34 (s, 1H), 4.79 (d, 2H), 4.41-4.48 (m, 1H), 4.20 (s, 2H) , 3.11 (q, 2H), 1.49 (d, 6H), 1.28 (t, 3H).
实施例2
Example 2
N-(4-(乙磺酰基)苄基)-1-异丙基-2-(2-甲氧基苄基)-1H-吲哚-5-甲酰胺2N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-(2-methoxybenzyl)-1H-indole-5-carboxamide 2
第一步first step
2-(2-甲氧基苄基)-1H-吲哚-5-甲酸甲酯2b2-(2-methoxybenzyl)-1H-indole-5-carboxylic acid methyl ester 2b
将1a(250mg,1.43mmol),1-(溴甲基)-2-甲氧基苯2a(301mg,1.5mmol,采用公知的方法“Journal of the American Chemical Society 2013,135(30),10934-10937”制备而得),双乙腈二氯化钯(37mg,0.14mmol),降冰片烯(268mg,2.86mmol)溶于N,N-二甲基乙酰胺中,加入碳酸氢钠(240mg,2.86mmol),升至70℃搅拌反应12小时。反应液冷却至室温,加入过量水,用乙酸乙酯萃取(20mL×3),合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得标题产物2b(120mg,白色固体),产率:29%。1a (250 mg, 1.43 mmol), 1-(bromomethyl)-2-methoxybenzene 2a (301 mg, 1.5 mmol, using a known method "Journal of the American Chemical Society 2013, 135 (30), 10934- Prepared by 10937", diacetonitrile palladium dichloride (37 mg, 0.14 mmol), norbornene (268 mg, 2.86 mmol) dissolved in N,N-dimethylacetamide, sodium bicarbonate (240 mg, 2.86) Methyl), the reaction was stirred at 70 ° C for 12 hours. The reaction mixture was cooled to room temperature, and the mixture was combined with EtOAc EtOAc. The residue was purified with EtOAc EtOAc (EtOAc)
第二步Second step
1-异丙基-2-(2-甲氧基苄基)-1H-吲哚-5-甲酸甲酯2c1-isopropyl-2-(2-methoxybenzyl)-1H-indole-5-carboxylic acid methyl ester 2c
将2b(110mg,0.37mmol)溶于N,N-二甲基甲酰胺中,加入催化量碘化钾,再加入氢化钠(30mg,0.74mmol,60%in oil),搅拌反应30分钟后加入2-溴丙烷(183mg,1.49mmol),升至55℃搅拌反应12小时。反应液冷却至室温,加入过量水,用乙酸乙酯萃取(20mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物2c(25mg,黄色油状物),产率:20%。2b (110mg, 0.37mmol) was dissolved in N,N-dimethylformamide, a catalytic amount of potassium iodide was added, then sodium hydride (30 mg, 0.74 mmol, 60% in oil) was added, and the reaction was stirred for 30 minutes, then 2- Bromopropane (183 mg, 1.49 mmol) was stirred at 55 ° C for 12 hours. The reaction mixture was cooled to room temperature, and the mixture was evaporated, evaporated, evaporated, evaporated, evaporated Chromatography of the residue obtained from EtOAc (EtOAc)
第三步third step
1-异丙基-2-(2-甲氧基苄基)-1H-吲哚-5-甲酸2d1-isopropyl-2-(2-methoxybenzyl)-1H-indole-5-carboxylic acid 2d
将2c(25mg,0.085mmol)溶于6mL甲醇和四氢呋喃(V:V=4:2)混合溶剂中,
加入4M氢氧化钠溶液2mL,60℃搅拌反应1小时。反应液冷却至室温,滴加浓盐酸至pH为4,用乙酸乙酯萃取(20mL×3),合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物2d(23mg,黄色固体),产率:95%。2c (25 mg, 0.085 mmol) was dissolved in 6 mL of a mixed solvent of methanol and tetrahydrofuran (V:V=4:2).
2 mL of a 4 M sodium hydroxide solution was added, and the reaction was stirred at 60 ° C for 1 hour. The reaction mixture was cooled to room temperature. EtOAc was evaporated. The residue obtained was purified by EtOAc (EtOAc) eluting
第四步the fourth step
N-(4-(乙磺酰基)苄基)-1-异丙基-2-(2-甲氧基苄基)-1H-吲哚-5-甲酰胺2N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-(2-methoxybenzyl)-1H-indole-5-carboxamide 2
将2d(23mg,0.08mmol),(4-(乙磺酰基)苯基)甲胺(24mg,0.12mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(21mg,0.12mmol),1-羟基苯并三唑(17mg,0.12mmol),N,N-二异丙基乙胺(53mg,0.41mmol)溶于二氯甲烷中,搅拌反应12小时。加入水,用二氯甲烷和甲醇(V:V=8:1)混合溶剂萃取(20mL×3),合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物2(21mg,白色固体),产率:50%。2d (23 mg, 0.08 mmol), (4-(ethylsulfonyl)phenyl)methanamine (24 mg, 0.12 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide salt Acid salt (21 mg, 0.12 mmol), 1-hydroxybenzotriazole (17 mg, 0.12 mmol), N,N-diisopropylethylamine (53 mg, 0.41 mmol) dissolved in dichloromethane. . Add water, extract with a mixed solvent of dichloromethane and methanol (V: V = 8:1) (20 mL × 3), combine the organic phase, wash with saturated sodium chloride solution, dry over anhydrous sodium sulfate, The residue was purified by EtOAc EtOAc EtOAc.
MS m/z(ESI):505.4[M+1];MS m/z (ESI): 505.4 [M + 1];
1H NMR(400MHz,CDCl3)δ8.03(s,1H),7.87(d,2H),7.62(d,1H),7.53-7.57(m,3H),7.23(t,1H),6.91(t,2H),6.85(t,1H),6.64(t,1H),6.30(s,1H),4.79(d,2H),4.50-4.56(m,1H),4.12(s,2H),3.88(s,3H),3.11(q,2H),1.51(d,6H),1.27(t,3H)。 1 H NMR (400 MHz, CDCl 3 ) δ 8.03 (s, 1H), 7.78 (d, 2H), 7.62 (d, 1H), 7.53-7.57 (m, 3H), 7.23 (t, 1H), 6.91 ( t,2H), 6.85(t,1H), 6.64(t,1H), 6.30(s,1H), 4.79(d,2H),4.50-4.56(m,1H),4.12(s,2H),3.88 (s, 3H), 3.11 (q, 2H), 1.51 (d, 6H), 1.27 (t, 3H).
实施例3Example 3
1-乙基-N-(4-(乙磺酰基)苄基)-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺31-ethyl-N-(4-(ethylsulfonyl)benzyl)-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 3
第一步first step
2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酸甲酯3bMethyl 2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxylate 3b
将1a(2.7g,15.41mmol),1-(溴甲基)-2-(三氟甲基)苯3a(3.87g,16.18mmol),双乙腈二氯化钯(399mg,1.54mmol),降冰片烯(2.9g,30.82mmol),碳酸氢钠(2.59g,30.82mmol)溶于N,N-二甲基乙酰胺中,升至70℃搅拌反应12小时。反应液冷却至室温,反应液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得标题产物3b(4.4g,浅黄色固体),产率:86%。1a (2.7 g, 15.41 mmol), 1-(bromomethyl)-2-(trifluoromethyl)benzene 3a (3.87 g, 16.18 mmol), diacetonitrile palladium dichloride (399 mg, 1.54 mmol) The borneol (2.9 g, 30.82 mmol), sodium hydrogencarbonate (2.59 g, 30.82 mmol) was dissolved in N,N-dimethylacetamide, and the mixture was stirred at 70 ° C for 12 hours. The reaction mixture was cooled to room temperature, and then evaporated, evaporated, evaporated.
第二步Second step
1-乙基-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酸甲酯3cMethyl 1-ethyl-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxylate 3c
将3b(4.4g,13.2mmol)溶于N,N-二甲基甲酰胺中,加入氢化钠(1.06g,26.4mmol,60%in oil)和碘乙烷(10.29g,66.01mmol),搅拌反应12小时。将反应液倒入水中,用乙酸乙酯萃取(50mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得到标题产物3c(4.2g,浅黄色固体),产率:88%。3b (4.4g, 13.2mmol) was dissolved in N,N-dimethylformamide, sodium hydride (1.06g, 26.4mmol, 60% in oil) and ethyl iodide (10.29g, 66.01mmol) were added and stirred. Reaction for 12 hours. The reaction mixture was poured into water and extracted with ethyl acetate (50 mL×3). The residue obtained was purified to afford titled product 3c (4.2 g, pale yellow solid).
第三步third step
1-乙基-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酸3d1-ethyl-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxylic acid 3d
将3c(1.4g,3.87mmol)溶于15mL甲醇中,加入2M氢氧化钾溶液5mL,升温至回流反应12小时。反应液冷却至室温,滴加浓盐酸至pH为4,用乙酸乙酯萃取(50mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得到粗品标题产物3d(1.1g,浅黄色固体),产品不经纯化直接进行下一步反应。3c (1.4 g, 3.87 mmol) was dissolved in 15 mL of methanol, 5 mL of 2M potassium hydroxide solution was added, and the mixture was warmed to reflux for 12 hours. The reaction solution was cooled to room temperature, and concentrated hydrochloric acid was added dropwise to pH 4, and ethyl acetate (50 mL×3), and the organic phase was combined, washed with water, saturated sodium chloride solution, dried over anhydrous sodium sulfate Concentration gave the crude title product 3d (1.1 g, pale yellow solid).
第四步the fourth step
1-乙基-N-(4-(乙磺酰基)苄基)-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺31-ethyl-N-(4-(ethylsulfonyl)benzyl)-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 3
将粗品3d(1.1g,3.17mmol),4-(乙磺酰基)苯基)甲胺(757.31mg,3.8mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(1.21g,6.33mmol),1-羟基苯并三唑(856g,6.33mmol),三乙胺(641mg,6.33mmol)溶于二氯甲烷中,搅拌反应12小时。反应液减压浓缩,用硅胶柱色谱法以洗脱剂体系A纯化所得残余物,用高效液相色谱法纯化所得粗品,得到标题产物3(1g,浅灰白色固体),产率:60%。3d (1.1 g, 3.17 mmol), 4-(ethylsulfonyl)phenyl)methanamine (757.31 mg, 3.8 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbazide Amine hydrochloride (1.21 g, 6.33 mmol), 1-hydroxybenzotriazole (856 g, 6.33 mmol), triethylamine (641 mg, 6.33 mmol). The reaction mixture was concentrated under reduced pressure. EtOAcjjjjjjjjj
MS m/z(ESI):529.9[M+1];MS m/z (ESI): 529.9 [M + 1];
1H NMR(400MHz,CDCl3)δ8.11(s,1H),7.91(d,2H),7.72-7.77(m,2H),7.61(d,2H),7.37-7.49(m,3H),7.16(d,1H),6.67(t,1H),6.34(s,1H),4.83(d,2H),4.37(d,2H),4.05-4.10(m,2H),3.14(q,2H),1.25-1.33(m,6H)。 1 H NMR (400MHz, CDCl 3 ) δ8.11 (s, 1H), 7.91 (d, 2H), 7.72-7.77 (m, 2H), 7.61 (d, 2H), 7.37-7.49 (m, 3H), 7.16(d,1H), 6.67(t,1H), 6.34(s,1H),4.83(d,2H), 4.37(d,2H),4.05-4.10(m,2H),3.14(q,2H) , 1.25-1.33 (m, 6H).
实施例4Example 4
N-(4-(乙磺酰基)苄基)-2-(4-氟-2-(三氟甲基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺4
N-(4-(ethylsulfonyl)benzyl)-2-(4-fluoro-2-(trifluoromethyl)benzyl)-1-isopropyl-1H-indole-5-carboxamide 4
第一步first step
2-(4-氟-2-(三氟甲基)苄基)-1H-吲哚-5-甲酸甲酯4bMethyl 2-(4-fluoro-2-(trifluoromethyl)benzyl)-1H-indole-5-carboxylate 4b
将1a(200mg,1.14mmol),1-(溴甲基)-4-氟-2-(三氟甲基)苯4a(308.1mg,1.2mmol),双乙腈二氯化钯(29.6mg,0.14mmol),降冰片烯(215mg,2.28mmol),碳酸氢钠(191.8mg,2.28mmol)溶于N,N-二甲基乙酰胺中,升至70℃搅拌反应12小时。反应液冷却至室温,减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得标题产物4b(141mg,浅黄色固体),产率:35%。1a (200 mg, 1.14 mmol), 1-(bromomethyl)-4-fluoro-2-(trifluoromethyl)benzene 4a (308.1 mg, 1.2 mmol), diacetonitrile palladium dichloride (29.6 mg, 0.14 Methyl), norbornene (215 mg, 2.28 mmol), sodium hydrogencarbonate (191.8 mg, 2.28 mmol) was dissolved in N,N-dimethylacetamide, and the mixture was stirred at 70 ° C for 12 hours. The reaction mixture was cooled to EtOAc EtOAc m.
第二步Second step
2-(4-氟-2-(三氟甲基)苄基)-1-异丙基-1H-吲哚-5-甲酸甲酯4c2-(4-Fluoro-2-(trifluoromethyl)benzyl)-1-isopropyl-1H-indole-5-carboxylic acid methyl ester 4c
将4b(50mg,0.142mmol)溶于N,N-二甲基甲酰胺中,加入氢化钠(12mg,0.285mmol,60%in oil),2-溴丙烷(87.53mg,0.711mmol),50℃搅拌反应12小时。反应液冷却至室温,倒入水中,用乙酸乙酯萃取(50mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得到标题产物4c(34mg,浅黄色固体),产率:61%。4b (50mg, 0.142mmol) was dissolved in N,N-dimethylformamide, sodium hydride (12mg, 0.285mmol, 60% in oil), 2-bromopropane (87.53mg, 0.711mmol), 50°C The reaction was stirred for 12 hours. The reaction mixture was cooled to room temperature, poured into water, EtOAc (EtOAc m. Chromatography of the residue obtained from EtOAc (EtOAc)
第三步third step
2-(4-氟-2-(三氟甲基)苄基)-1-异丙基-1H-吲哚-5-甲酸4d2-(4-Fluoro-2-(trifluoromethyl)benzyl)-1-isopropyl-1H-indole-5-carboxylic acid 4d
将4c(34mg,0.09mmol)溶于3mL甲醇中,加入2M氢氧化钾溶液1mL,升温至回流反应12小时。反应液冷却至室温,滴加浓盐酸至pH为4,用乙酸乙酯萃取(50mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得到粗品标题产物4d(26mg,浅黄色固体),产品不经纯化直接进行下一步反应。
4c (34 mg, 0.09 mmol) was dissolved in 3 mL of methanol, 1 mL of 2M potassium hydroxide solution was added, and the mixture was warmed to reflux for 12 hours. The reaction solution was cooled to room temperature, and concentrated hydrochloric acid was added dropwise to pH 4, and ethyl acetate (50 mL×3), and the organic phase was combined, washed with water, saturated sodium chloride solution, dried over anhydrous sodium sulfate Concentration gave the crude title product 4d (26 mg, pale yellow solid).
第四步the fourth step
N-(4-(乙磺酰基)苄基)-2-(4-氟-2-(三氟甲基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺4N-(4-(ethylsulfonyl)benzyl)-2-(4-fluoro-2-(trifluoromethyl)benzyl)-1-isopropyl-1H-indole-5-carboxamide 4
将粗品4d(26mg,0.068mmol),4-(乙磺酰基)苯基)甲胺(16.4mg,0.082mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(26.28mg,0.137mmol),1-羟基苯并三唑(18.52mg,0.137mmol),三乙胺(13.87mg,0.137mmol)溶于二氯甲烷中,搅拌反应12小时。反应液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物4(13mg,黄色固体),产率:34%。Crude 4d (26 mg, 0.068 mmol), 4-(ethylsulfonyl)phenyl)methanamine (16.4 mg, 0.082 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (26.28 mg, 0.137 mmol), 1-hydroxybenzotriazole (18.52 mg, 0.137 mmol), triethylamine (13.87 mg, 0.137 mmol) was dissolved in dichloromethane. The reaction mixture was concentrated under reduced vacuo. EtOAcjjjjjjj
MS m/z(ESI):561.3[M+1];MS m/z (ESI): 561.3 [M + 1];
1H NMR(400MHz,CDCl3)δ8.10(s,1H),7.91(d,2H),7.69(dd,1H),7.58-7.61(m,3H),7.48(dd,1H),7.16(dt,1H),7.08(d,1H),6.67(t,1H),6.34(s,1H),4.83(d,2H),4.40-4.47(m,1H),4.33(s,2H),3.14(q,2H),1.53(d,6H),1.31(t,3H)。 1 H NMR (400MHz, CDCl 3 ) δ8.10 (s, 1H), 7.91 (d, 2H), 7.69 (dd, 1H), 7.58-7.61 (m, 3H), 7.48 (dd, 1H), 7.16 ( Dt,1H),7.08(d,1H),6.67(t,1H),6.34(s,1H),4.83(d,2H),4.40-4.47(m,1H),4.33(s,2H),3.14 (q, 2H), 1.53 (d, 6H), 1.31 (t, 3H).
实施例5Example 5
2-(4-氯-2-(三氟甲基)苄基)-N-(4-(乙磺酰基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺52-(4-Chloro-2-(trifluoromethyl)benzyl)-N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-1H-indole-5-carboxamide 5
第一步first step
2-(4-氯-2-(三氟甲基)苄基)-1H-吲哚-5-甲酸甲酯5bMethyl 2-(4-chloro-2-(trifluoromethyl)benzyl)-1H-indole-5-carboxylate 5b
将1a(200mg,1.14mmol),1-(溴甲基)-4-氯-2-(三氟甲基)苯5a(327.83mg,1.2mmol),双乙腈二氯化钯(29.6mg,0.14mmol),降冰片烯(215mg,2.28mmol),碳酸氢钠(191.8mg,2.28mmol)溶于N,N-二甲基乙酰胺中,升至70℃搅拌反应12小时。反应液冷却至室温,减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得标题产物5b(136mg,浅黄色固体),产率:33%。
1a (200 mg, 1.14 mmol), 1-(bromomethyl)-4-chloro-2-(trifluoromethyl)benzene 5a (327.83 mg, 1.2 mmol), diacetonitrile palladium dichloride (29.6 mg, 0.14 Methyl), norbornene (215 mg, 2.28 mmol), sodium hydrogencarbonate (191.8 mg, 2.28 mmol) was dissolved in N,N-dimethylacetamide, and the mixture was stirred at 70 ° C for 12 hours. The reaction mixture was cooled to EtOAc EtOAc m.
第二步Second step
2-(4-氯-2-(三氟甲基)苄基)-1-异丙基-1H-吲哚-5-甲酸甲酯5cMethyl 2-(4-chloro-2-(trifluoromethyl)benzyl)-1-isopropyl-1H-indole-5-carboxylate 5c
将5b(50mg,0.136mmol)溶于N,N-二甲基甲酰胺中,加入氢化钠(11mg,0.272mmol,60%in oil),2-溴丙烷(84mg,0.68mmol),50℃搅拌反应12小时。反应液冷却至室温,倒入水中,用乙酸乙酯萃取(50mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得到标题产物5c(38mg,浅黄色固体),产率:68%。5b (50mg, 0.136mmol) was dissolved in N,N-dimethylformamide, sodium hydride (11mg, 0.272mmol, 60% in oil), 2-bromopropane (84mg, 0.68mmol), stirred at 50 ° C Reaction for 12 hours. The reaction mixture was cooled to room temperature, poured into water, EtOAc (EtOAc m. Chromatography of the residue obtained from EtOAc (EtOAc)
第三步third step
2-(4-氯-2-(三氟甲基)苄基)-1-异丙基-1H-吲哚-5-甲酸5d2-(4-Chloro-2-(trifluoromethyl)benzyl)-1-isopropyl-1H-indole-5-carboxylic acid 5d
将5c(28mg,0.071mmol)溶于3mL甲醇中,加入2M氢氧化钾溶液1mL,升温至回流反应12小时。反应液冷却至室温,滴加浓盐酸至pH为4,用乙酸乙酯萃取(50mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得到粗品标题产物5d(14mg,浅黄色固体),产品不经纯化直接进行下一步反应。5c (28 mg, 0.071 mmol) was dissolved in 3 mL of methanol, 1 mL of 2M potassium hydroxide solution was added, and the mixture was warmed to reflux for 12 hours. The reaction solution was cooled to room temperature, and concentrated hydrochloric acid was added dropwise to pH 4, and ethyl acetate (50 mL×3), and the organic phase was combined, washed with water, saturated sodium chloride solution, dried over anhydrous sodium sulfate Concentration gave the crude title product 5d (14 mg, pale yellow solid).
第四步the fourth step
2-(4-氯-2-(三氟甲基)苄基)-N-(4-(乙磺酰基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺52-(4-Chloro-2-(trifluoromethyl)benzyl)-N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-1H-indole-5-carboxamide 5
将粗品5d(28mg,0.07mmol),4-(乙磺酰基)苯基)甲胺(16.92mg,0.085mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(27.12mg,0.141mmol),1-羟基苯并三唑(19.12mg,0.101mmol),三乙胺(14.32mg,0.141mmol)溶于二氯甲烷中,搅拌反应12小时。反应液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物5(14mg,黄色固体),产率:34%。Crude 5d (28 mg, 0.07 mmol), 4-(ethylsulfonyl)phenyl)methanamine (16.92 mg, 0.085 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (27.12 mg, 0.141 mmol), 1-hydroxybenzotriazole (19.12 mg, 0.101 mmol), triethylamine (14.32 mg, 0.141 mmol) was dissolved in dichloromethane. The reaction mixture was concentrated under reduced vacuo. EtOAcjjjjjjj
MS m/z(ESI):577.4[M+1];MS m/z (ESI): 577.4 [M+1];
1H NMR(400MHz,CDCl3)δ8.08(s,1H),7.81(d,2H),7.65-7.71(m,2H),7.53-7.56(m,3H),7.39(dd,1H),7.00(d,1H),,6.78(t,1H),6.30(s,1H),4.78(d,2H),4.35-4.42(m,1H),4.29(s,2H),3.10(q,2H),1.50(d,6H),1.28(t,3H)。 1 H NMR (400MHz, CDCl 3 ) δ8.08 (s, 1H), 7.81 (d, 2H), 7.65-7.71 (m, 2H), 7.53-7.56 (m, 3H), 7.39 (dd, 1H), 7.00 (d, 1H), 6.78 (t, 1H), 6.30 (s, 1H), 4.78 (d, 2H), 4.35-4.42 (m, 1H), 4.29 (s, 2H), 3.10 (q, 2H) ), 1.50 (d, 6H), 1.28 (t, 3H).
实施例6Example 6
N-(4-(乙磺酰基)苄基)-1-异丙基-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺6N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 6
第一步first step
2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酸甲酯6aMethyl 2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxylate 6a
将1a(3g,17.12mmol),1-(溴甲基)-2-(三氟甲基)苯3b(4.3g,17.98mmol),双乙腈二氯化钯(444mg,1.71mmol),降冰片烯(3.22g,34.25mmol),碳酸氢钠(2.88g,34.25mmol)溶于N,N-二甲基乙酰胺中,升至70℃搅拌反应12小时。反应液冷却至室温,减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得标题产物6a(4.6g,浅黄色固体),产率:81%。1a (3g, 17.12mmol), 1-(bromomethyl)-2-(trifluoromethyl)benzene 3b (4.3g, 17.98mmol), diacetonitrile palladium dichloride (444mg, 1.71mmol), borneol The olefin (3.22 g, 34.25 mmol), sodium hydrogencarbonate (2.88 g, 34.25 mmol) was dissolved in N,N-dimethylacetamide, and the mixture was stirred at 70 ° C for 12 hours. The reaction mixture was cooled to EtOAc EtOAc m.
第二步Second step
1-异丙基-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酸甲酯6bMethyl 1-isopropyl-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxylate 6b
将6a(4.6g,13.8mmol)溶于20mL N,N-二甲基甲酰胺中,加入氢化钠(1.1g,27.6mmol,60%in oil),2-溴丙烷(84mg,0.68mmol),50℃搅拌反应12小时。反应液冷却至室温,倒入水中,用乙酸乙酯萃取(50mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得到标题产物6b(2.9g,浅黄色固体),产率:75%。6a (4.6 g, 13.8 mmol) was dissolved in 20 mL of N,N-dimethylformamide, sodium hydride (1.1 g, 27.6 mmol, 60% in oil), 2-bromopropane (84 mg, 0.68 mmol). The reaction was stirred at 50 ° C for 12 hours. The reaction mixture was cooled to room temperature, poured into water, EtOAc (EtOAc m. Chromatography The residue obtained was purified with EtOAc EtOAc (EtOAc)
第三步third step
1-异丙基-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酸6c1-isopropyl-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxylic acid 6c
将6b(1.5g,4mmol)溶于15mL甲醇中,加入2M氢氧化钾溶液5mL,升温至回流搅拌反应2小时。反应液冷却至室温,滴加浓盐酸至pH为4,用乙酸乙酯萃取(50mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得到粗品标题产物6c(1.33g,浅黄色固体),产品不经纯化直接进行下一步反应。6b (1.5 g, 4 mmol) was dissolved in 15 mL of methanol, 5 mL of 2M potassium hydroxide solution was added, and the mixture was heated to reflux and stirred for 2 hours. The reaction solution was cooled to room temperature, and concentrated hydrochloric acid was added dropwise to pH 4, and ethyl acetate (50 mL×3), and the organic phase was combined, washed with water, saturated sodium chloride solution, dried over anhydrous sodium sulfate Concentration gave the crude title product 6c (1.33 g, pale yellow solid).
第四步the fourth step
N-(4-(乙磺酰基)苄基)-1-异丙基-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺6N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 6
将粗品6c(1.33g,3.68mmol),4-(乙磺酰基)苯基)甲胺(880.11mg,0.42mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(1.41g,7.36mmol),1-羟基苯并三唑(994.66mg,7.36mmol),三乙胺(744.87mg,7.36mmol)溶于二氯甲烷中,搅拌反
应12小时。反应液减压浓缩,用高效液相色谱法纯化所得残余物,得到标题产物6(1.02g,白色固体),产率:51%。Crude 6c (1.33 g, 3.68 mmol), 4-(ethylsulfonyl)phenyl)methanamine (880.11 mg, 0.42 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbazide Amine hydrochloride (1.41 g, 7.36 mmol), 1-hydroxybenzotriazole (994.66 mg, 7.36 mmol), triethylamine (744.87 mg, 7.36 mmol) dissolved in dichloromethane
Should be 12 hours. The reaction mixture was concentrated under reduced vacuo.
MS m/z(ESI):544.4[M+1];MS m/z (ESI): 544.4 [M + 1];
1H NMR(400MHz,CDCl3)δ8.11(s,1H),7.89(d,2H),7.76(d,1H),7.69(dd,1H),7.59(d,2H),7.38-7.46(m,2H),7.10(d,1H),6.74(t,1H),6.35(s,1H),4.83(d,2H),4.42-4.49(m,1H),4.38(s,2H),3.14(q,2H),1.52(d,6H),1.31(t,3H)。 1 H NMR (400MHz, CDCl 3 ) δ8.11 (s, 1H), 7.89 (d, 2H), 7.76 (d, 1H), 7.69 (dd, 1H), 7.59 (d, 2H), 7.38-7.46 ( m, 2H), 7.10 (d, 1H), 6.74 (t, 1H), 6.35 (s, 1H), 4.83 (d, 2H), 4.42-4.49 (m, 1H), 4.38 (s, 2H), 3.14 (q, 2H), 1.52 (d, 6H), 1.31 (t, 3H).
实施例7Example 7
N-(4-((环丙基甲基)磺酰基)苄基)-1-异丙基-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺7N-(4-((cyclopropylmethyl)sulfonyl)benzyl)-1-isopropyl-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 7
第一步first step
4-氰基苯亚磺酸钠7bSodium 4-cyanobenzenesulfinate 7b
将4-氰基苯磺酰氯7a(1.01g,5mmol),亚硫酸钠(1.26g,10mmol),碳酸氢钠(0.84g,10mmol)加入到20mL水中,75℃搅拌反应5小时。反应液冷却至室温,减压浓缩,得到粗品标题产物7b(946mg,白色固体),产品不经纯化直接进行下一步反应。4-Cyanobenzenesulfonyl chloride 7a (1.01 g, 5 mmol), sodium sulfite (1.26 g, 10 mmol), sodium hydrogencarbonate (0.84 g, 10 mmol) was added to 20 mL of water, and the mixture was stirred at 75 ° C for 5 hours. The reaction mixture was cooled to room temperature and then evaporated, evaporated, evaporated
第二步Second step
4-((环丙基甲基)磺酰基)苯甲腈7c4-((cyclopropylmethyl)sulfonyl)benzonitrile 7c
将粗品7b(946mg,5mmol),(溴甲基)环丙烷(2.03g,15mmol)和催化量的碘甲烷加入到30mL N,N-二甲基甲酰胺中,75℃搅拌反应12小时。反应液冷却至室温,加入100mL水,用乙酸乙酯萃取(50mL×3),合并有机相用无水硫酸钠干燥,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得到标题产物7c(670mg,白色固体),产率:61%。
The crude 7b (946 mg, 5 mmol), (bromomethyl)cyclopropane (2.03 g, 15 mmol) and a catalytic amount of methyl iodide were added to 30 mL of N,N-dimethylformamide, and the reaction was stirred at 75 ° C for 12 hours. The reaction solution was cooled to room temperature, and then added with 100 mL of water, EtOAc (EtOAc (EtOAc) The residue was obtained to give the title compound (jjjjjjj
第三步third step
(4-((环丙基甲基)磺酰基)苯基)甲胺7d(4-((cyclopropylmethyl)sulfonyl)phenyl)methanamine 7d
将7c(670mg,3mmol)溶于30mL甲醇中,加入5mL氨水,再加入催化量的雷尼镍,氢气置换三次,搅拌反应12小时。将反应液过滤,滤液减压浓缩,得到粗品标题产物7d(0.6g,淡黄色油状物),产品不经纯化直接进行下一步反应。7c (670 mg, 3 mmol) was dissolved in 30 mL of methanol, 5 mL of aqueous ammonia was added, and a catalytic amount of Raney nickel was added thereto, and the hydrogen was replaced three times, and the reaction was stirred for 12 hours. The reaction solution was filtered, and the filtrate was evaporated. mjjjjjjjjj
第四步the fourth step
N-(4-((环丙基甲基)磺酰基)苄基)-1-异丙基-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺7N-(4-((cyclopropylmethyl)sulfonyl)benzyl)-1-isopropyl-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 7
将粗品7d(28.06mg,0.12mmol),粗品6e(30mg,0.083mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(32mg,0.166mmol),1-羟基苯并三唑(23mg,0.166mmol),N,N-二异丙基乙胺(22mg,0.166mmol)溶于二氯甲烷中,搅拌反应12小时。反应液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物7(16mg,浅黄色固体),产率:34%。Crude 7d (28.06 mg, 0.12 mmol), crude 6e (30 mg, 0.083 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (32 mg, 0.166 mmol) 1-Hydroxybenzotriazole (23 mg, 0.166 mmol), N,N-diisopropylethylamine (22 mg, 0.166 mmol) was dissolved in dichloromethane. The reaction mixture was concentrated under reduced vacuo. EtOAcjjjjjjj
MS m/z(ESI):569.2[M+1];MS m/z (ESI): 569.2 [M+1];
1H NMR(400MHz,CDCl3)δ8.13(s,1H),7.89(d,2H),7.76(d,1H),7.71(d,1H),7.57(t,3H),7.38-7.47(m,2H),7.10(d,1H),6.86(t,1H),6.34(s,1H),4.82(d,2H),4.42-4.49(m,1H),4.37(s,2H),3.03(d,2H),1.52(d,6H),0.99-1.02(m,1H),0.57-0.62(m,2H),0.17-0.21(m,2H)。 1 H NMR (400MHz, CDCl 3 ) δ8.13 (s, 1H), 7.89 (d, 2H), 7.76 (d, 1H), 7.71 (d, 1H), 7.57 (t, 3H), 7.38-7.47 ( m, 2H), 7.10 (d, 1H), 6.86 (t, 1H), 6.34 (s, 1H), 4.82 (d, 2H), 4.42-4.49 (m, 1H), 4.37 (s, 2H), 3.03 (d, 2H), 1.52 (d, 6H), 0.99-1.02 (m, 1H), 0.57-0.62 (m, 2H), 0.17-0.21 (m, 2H).
实施例8Example 8
N-(4-(乙磺酰基)-2-氟苄基)-1-异丙基-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺8N-(4-(ethylsulfonyl)-2-fluorobenzyl)-1-isopropyl-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 8
第一步first step
4-(乙磺酰基)-2-氟苄基氨基甲酸叔丁酯8b4-(ethylsulfonyl)-2-fluorobenzylcarbamic acid tert-butyl ester 8b
将4-溴-2-氟苄基氨基甲酸叔丁酯8a(400mg,1.32mmol,采用专利申请“EP991638”公开的方法制备而得),乙基亚磺酸钠(229mg,1.97mmol),碳酸铯(642.7mg,1.97mmol)溶于二甲基亚砜中,加入催化量的碘化亚铜和L-脯氨酸,升温至120℃搅拌反应1小时。反应液冷却至室温,加入适量乙酸乙酯,过滤,滤液依次用水、饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得到标题产物8b(340mg,浅黄色油状物),产率:82%。4-Bromo-2-fluorobenzylcarbamic acid tert-butyl ester 8a (400 mg, 1.32 mmol, prepared by the method disclosed in the patent application "EP991638"), sodium ethyl sulfinate (229 mg, 1.97 mmol), carbonic acid The hydrazine (642.7 mg, 1.97 mmol) was dissolved in dimethyl sulfoxide, and a catalytic amount of cuprous iodide and L-valine was added thereto, and the mixture was heated to 120 ° C and stirred for 1 hour. The reaction solution was cooled to room temperature, and the mixture was filtered and evaporated. The residue was obtained to give the title compound (jjjjjjj
第二步Second step
(4-(乙磺酰基)-2-氟苯基)甲胺三氟乙酸盐8c(4-(ethylsulfonyl)-2-fluorophenyl)methanamine trifluoroacetate 8c
将8b(340mg,1.12mmol)溶于10mL二氯甲烷中,加入2mL三氟乙酸,搅拌反应3小时。反应液减压浓缩,得粗品标题产物8c(340mg,浅棕色固体),产品不经纯化直接进行下一步反应。8b (340 mg, 1.12 mmol) was dissolved in 10 mL of dichloromethane, 2 mL of trifluoroacetic acid was added, and the reaction was stirred for 3 hours. The reaction mixture was concentrated to dryness crystals crystals crystals crystals
第三步third step
N-(4-(乙磺酰基)-2-氟苄基)-1-异丙基-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺8N-(4-(ethylsulfonyl)-2-fluorobenzyl)-1-isopropyl-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 8
将粗品8c(21.64mg,0.1mmol),6e(30mg,0.083mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(31.83mg,0.166mmol),1-羟基苯并三唑(22.44mg,0.166mmol),N,N-二异丙基乙胺(21.46mg,0.166mmol)溶于二氯甲烷中,搅拌反应12小时。将反应液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物8(10mg,黄色固体),产率:21%。The crude product 8c (21.64 mg, 0.1 mmol), 6e (30 mg, 0.083 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (31.83 mg, 0.166 mmol), 1-Hydroxybenzotriazole (22.44 mg, 0.166 mmol), N,N-diisopropylethylamine (21.46 mg, 0.166 mmol) was dissolved in dichloromethane. The reaction mixture was concentrated under reduced vacuo. EtOAcjjjjjjj
MS m/z(ESI):561.1[M+1];MS m/z (ESI): 561.1 [M + 1];
1H NMR(400MHz,CDCl3)δ8.10(s,1H),7.95(s,1H),7.75-7.78(m,2H),7.67-7.71(m,2H),7.59(d,1H),7.38-7.48(m,2H),7.10(d,1H),6.89(t,1H),6.35(s,1H),4.85(d,2H),4.42-4.49(m,1H),4.38(s,2H),3.15(q,2H),1.52(d,6H),1.31(t,
3H)。 1 H NMR (400MHz, CDCl 3 ) δ8.10 (s, 1H), 7.95 (s, 1H), 7.75-7.78 (m, 2H), 7.67-7.71 (m, 2H), 7.59 (d, 1H), 7.38-7.48 (m, 2H), 7.10 (d, 1H), 6.89 (t, 1H), 6.35 (s, 1H), 4.85 (d, 2H), 4.42-4.49 (m, 1H), 4.38 (s, 2H), 3.15 (q, 2H), 1.52 (d, 6H), 1.31 (t, 3H).
实施例9Example 9
1-环丙基-N-(4-(乙磺酰基)苄基)-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺91-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 9
第一步first step
1-环丙基-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酸甲酯9aMethyl 1-cyclopropyl-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxylate 9a
将6c(100mg,0.3mmol)溶于1,2-二氯乙烷中,分别加入环丙基硼酸(39mg,0.45mmol),一水合醋酸铜(90mg,0.45mmol),2,2'-联吡啶(70mg,0.45mmol)和碳酸钠(64mg,0.6mmol),升温至70℃搅拌反应24小时。反应液冷却至室温,减压浓缩,向所得残余物中加入水,用乙酸乙酯萃取(20mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得到标题产物9a(65mg,黄色固体),产率:58%。6c (100 mg, 0.3 mmol) was dissolved in 1,2-dichloroethane, respectively, cyclopropylboronic acid (39 mg, 0.45 mmol), copper acetate monohydrate (90 mg, 0.45 mmol), 2,2'-linked Pyridine (70 mg, 0.45 mmol) and sodium carbonate (64 mg, 0.6 mmol) were warmed to 70 ° C and stirred for 24 hours. The reaction mixture was cooled to room temperature, and the mixture was evaporated, evaporated, evaporated, evaporated. The filtrate was concentrated under reduced pressure. EtOAcjjjjjjjj
第二步Second step
1-环丙基-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酸9b1-cyclopropyl-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxylic acid 9b
将9a(65mg,0.17mmol)溶于7mL甲醇和四氢呋喃(V:V=5:2)混合溶剂中,加入4M氢氧化钠溶液2mL,升温至60℃搅拌反应1小时。反应液冷却至室温,滴加浓盐酸至pH为4,用乙酸乙酯萃取(20mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得标题产物9b(55mg,白色固体),产品不经纯化直接进行下一步反应。9a (65 mg, 0.17 mmol) was dissolved in 7 mL of a mixed solvent of methanol and tetrahydrofuran (V: V = 5:2), 2 mL of 4 M sodium hydroxide solution was added, and the mixture was heated to 60 ° C and stirred for 1 hour. The reaction solution was cooled to room temperature, and concentrated hydrochloric acid was added dropwise to pH 4, and ethyl acetate was evaporated to ethyl acetate (20 mL, 3), and the organic phase was combined, washed with water, saturated sodium chloride solution, dried over anhydrous sodium sulfate Concentration gave the title product 9b (55 mg, white solid).
第三步third step
1-环丙基-N-(4-(乙磺酰基)苄基)-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺9
1-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 9
将粗品9b(55mg,0.15mmol),4-(乙磺酰基)苯基)甲胺(40mg,0.2mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(44mg,0.23mmol),1-羟基苯并三唑(31mg,0.23mmol),N,N-二异丙基乙胺(59mg,0.46mmol)溶于二氯甲烷中,搅拌反应12小时。加入水和少量甲醇,用二氯甲烷和甲醇(V:V=8:1)混合溶剂萃取(20mL×2),合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物9(40mg,白色固体),产率:48%。Crude 9b (55 mg, 0.15 mmol), 4-(ethylsulfonyl)phenyl)methanamine (40 mg, 0.2 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide salt Acid salt (44 mg, 0.23 mmol), 1-hydroxybenzotriazole (31 mg, 0.23 mmol), N,N-diisopropylethylamine (59 mg, 0.46 mmol) dissolved in dichloromethane. . Add water and a small amount of methanol, and extract with a mixed solvent of dichloromethane and methanol (V: V = 8:1) (20 mL × 2), the organic phase is combined, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated under reduced pressure. EtOAcjjjjjjj
MS m/z(ESI):541.4[M+1];MS m/z (ESI): 541.4 [M+1];
1H NMR(400MHz,DMSO-d6)δ9.03(t,1H),8.06(s,1H),7.83(d,2H),7.79(d,1H),7.72(d,1H),7.63(t,1H),7.57(d,3H),7.51(t,1H),7.26(d,1H),6.01(s,1H),4.58(d,2H),4.44(s,2H),3.25(q,2H),3.08-3.13(m,1H),1.10-1.15(m,2H),1.08(t,3H),0.94-0.98(m,2H)。 1 H NMR (400 MHz, DMSO-d 6 ) δ 9.03 (t, 1H), 8.06 (s, 1H), 7.83 (d, 2H), 7.79 (d, 1H), 7.72 (d, 1H), 7.63 ( t,1H),7.57(d,3H),7.51(t,1H),7.26(d,1H),6.01(s,1H),4.58(d,2H),4.44(s,2H),3.25(q , 2H), 3.08-3.13 (m, 1H), 1.10-1.15 (m, 2H), 1.08 (t, 3H), 0.94-0.98 (m, 2H).
实施例10Example 10
2-(2-(乙基苄基)-N-(4-(乙磺酰基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺102-(2-(ethylbenzyl)-N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-1H-indole-5-carboxamide 10
第一步first step
2-(2-(乙基苄基)-1H-吲哚-5-甲酸甲酯10b2-(2-(ethylbenzyl)-1H-indole-5-carboxylic acid methyl ester 10b
将1a(150mg,0.856mmol),1-(溴甲基)-2-乙基苯10a(179mg,0.899mmol),双乙腈二氯化钯(22.2mg,0.086mmol),降冰片烯(161.24mg,1.71mmol),碳酸氢钠(143.86mg,1.71mmol)溶于N,N-二甲基乙酰胺中,升至70℃搅拌反应12小时。反应液冷却至室温,减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得标题产物10b(68mg,浅黄色固体),产率:27%。
1a (150 mg, 0.856 mmol), 1-(bromomethyl)-2-ethylbenzene 10a (179 mg, 0.899 mmol), diacetonitrile palladium dichloride (22.2 mg, 0.086 mmol), norbornene (161.24 mg) , 1.71 mmol), sodium hydrogencarbonate (143.86 mg, 1.71 mmol) was dissolved in N,N-dimethylacetamide, and the mixture was stirred at 70 ° C for 12 hours. The reaction mixture was cooled to EtOAc EtOAc m.
第二步Second step
2-(2-(乙基苄基)-1-异丙基-1H-吲哚-5-甲酸甲酯10c2-(2-(ethylbenzyl)-1-isopropyl-1H-indole-5-carboxylic acid methyl ester 10c
将10b(67mg,0.23mmol)溶于5mL N,N-二甲基甲酰胺中,加入氢化钠(18.27mg,0.46mmol,60%in oil),2-溴丙烷(140.45mg,1.14mmol),50℃搅拌反应12小时。反应液冷却至室温,倒入水中,用乙酸乙酯萃取(20mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得到标题产物10c(46mg,浅黄色固体),产率:60%。10b (67 mg, 0.23 mmol) was dissolved in 5 mL of N,N-dimethylformamide, sodium hydride (18.27mg, 0.46mmol, 60% in oil), 2-bromopropane (140.45mg, 1.14mmol), The reaction was stirred at 50 ° C for 12 hours. The reaction mixture was cooled to room temperature, poured into water, EtOAc (EtOAc (EtOAc) Chromatography The residue was purified with EtOAc EtOAc (EtOAc)
第三步third step
2-(2-(乙基苄基)-1-异丙基-1H-吲哚-5-甲酸10d2-(2-(ethylbenzyl)-1-isopropyl-1H-indole-5-carboxylic acid 10d
将10c(46mg,0.137mmol)溶于5mL甲醇中,加入2M氢氧化钾溶液2mL,升温至回流搅拌反应3小时。反应液冷却至室温,减压浓缩,向所得残余物中加入水,滴加浓盐酸至pH为4-5,用乙酸乙酯萃取(20mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩得到粗品标题产物10d(42mg,浅黄色固体),产品不经纯化直接进行下一步反应。10c (46 mg, 0.137 mmol) was dissolved in 5 mL of methanol, 2 mL of 2M potassium hydroxide solution was added, and the mixture was heated to reflux and stirred for 3 hours. The reaction solution was cooled to room temperature, and concentrated under reduced pressure. Water was evaporated, evaporated, evaporated, evaporated. The solution was washed with EtOAc EtOAc (EtOAc m.
第四步the fourth step
2-(2-(乙基苄基)-N-(4-(乙磺酰基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺102-(2-(ethylbenzyl)-N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-1H-indole-5-carboxamide 10
将粗品10d(42mg,0.13mmol),(4-(乙磺酰基)苯基)甲胺(31.25mg,0.16mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(50.01mg,0.26mmol),1-羟基苯并三唑(35.31mg,0.26mmol),三乙胺(26.45mg,0.26mmol)溶于二氯甲烷中,搅拌反应12小时。将反应液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物10(16mg,浅黄色固体),产率:24%。Crude 10d (42 mg, 0.13 mmol), (4-(ethylsulfonyl)phenyl)methanamine (31.25 mg, 0.16 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbazide The amine hydrochloride (50.01 mg, 0.26 mmol), 1-hydroxybenzotriazole (35.31 mg, 0.26 mmol), triethylamine (26.45 mg, 0.26 mmol) was dissolved in dichloromethane. The reaction mixture was concentrated under reduced vacuolululululululululululululululu
MS m/z(ESI):503.5[M+1];MS m/z (ESI): 503.5 [M + 1];
1H NMR(400MHz,CDCl3)δ8.04(s,1H),7.91(d,2H),7.67(d,1H),7.58-7.61(m,3H),7.27-7.31(m,2H),7.16(t,1H),6.99(d,1H),6.62(t,1H),6.18(s,1H),4.83(d,2H),4.54-4.61(m,1H),4.16(s,2H),3.14(q,2H),2.74(q,2H),1.60(d,6H),1.25-1.31(m,6H)。 1 H NMR (400MHz, CDCl 3 ) δ8.04 (s, 1H), 7.91 (d, 2H), 7.67 (d, 1H), 7.58-7.61 (m, 3H), 7.27-7.31 (m, 2H), 7.16(t,1H), 6.99(d,1H), 6.62(t,1H), 6.18(s,1H),4.83(d,2H),4.54-4.61(m,1H),4.16(s,2H) , 3.14 (q, 2H), 2.74 (q, 2H), 1.60 (d, 6H), 1.25-1.31 (m, 6H).
实施例11Example 11
N-((5-(乙磺酰基)吡啶-2-基)甲基)-1-异丙基-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺11N-((5-(ethylsulfonyl)pyridin-2-yl)methyl)-1-isopropyl-2-(2-(trifluoromethyl)benzyl)-1H-indole-5- Amide 11
将粗品6e(42mg,0.11mmol),5-(乙磺酰基)吡啶-2-基)甲胺11a(26.47mg,0.13mmol,采用专利申请“WO201517335”公开的方法制备而得),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(42.45mg,0.22mmol),1-羟基苯并三唑(29.92mg,0.22mmol),三乙胺(22.41mg,0.22mmol)溶于二氯甲烷中,搅拌反应12小时。将反应液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物11(16mg,浅黄色固体),产率:25.8%。Crude 6e (42 mg, 0.11 mmol), 5-(ethylsulfonyl)pyridin-2-yl)methylamine 11a (26.47 mg, 0.13 mmol, obtained by the method disclosed in the patent application "WO201517335"), 1-( 3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (42.45 mg, 0.22 mmol), 1-hydroxybenzotriazole (29.92 mg, 0.22 mmol), triethylamine (22.41 mg, 0.22 mmol) was dissolved in dichloromethane, and the reaction was stirred for 12 hours. The reaction mixture was concentrated under reduced vacuo. EtOAcjjjjjjj
MS m/z(ESI):544.4[M+1]。MS m/z (ESI): 544.4 [M+1].
实施例12Example 12
2-(4-氯-2-(三氟甲基)苄基)-1-环丙基-N-(4-(乙磺酰基)苄基)-1H-吲哚-5-甲酰胺122-(4-Chloro-2-(trifluoromethyl)benzyl)-1-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-1H-indole-5-carboxamide 12
第一步first step
2-(4-氯-2-(三氟甲基)苄基)-1-环丙基-1H-吲哚-5-甲酸甲酯12a2-(4-Chloro-2-(trifluoromethyl)benzyl)-1-cyclopropyl-1H-indole-5-carboxylic acid methyl ester 12a
将5c(150mg,0.408mmol)溶于1,2-二氯乙烷中,分别加入环丙基硼酸(180mg,2.04mmol),醋酸铜(14.9mg,0.08mmol),2,2'-联吡啶(318.5mg,2.04mmol)和碳酸钠(86.5mg,0.816mmol),升温至70℃搅拌反应12小时。反应液冷却至室温,减压浓缩,向所得残余物中加入乙酸乙酯,过滤,滤液减压浓缩,用硅胶柱色谱
法以洗脱剂体系B纯化所得残余物,得到标题产物12a(21mg,浅黄色油状物),产率:13%。5c (150 mg, 0.408 mmol) was dissolved in 1,2-dichloroethane, respectively, cyclopropylboronic acid (180 mg, 2.04 mmol), copper acetate (14.9 mg, 0.08 mmol), 2,2'-bipyridine (318.5 mg, 2.04 mmol) and sodium carbonate (86.5 mg, 0.816 mmol) were heated to 70 ° C and stirred for 12 hours. The reaction mixture was cooled to room temperature and then evaporated.
The residue was purified with EtOAc EtOAc (EtOAc)
第二步Second step
2-(4-氯-2-(三氟甲基)苄基)-1-环丙基-1H-吲哚-5-甲酸12b2-(4-Chloro-2-(trifluoromethyl)benzyl)-1-cyclopropyl-1H-indole-5-carboxylic acid 12b
将12a(21mg,0.05mmol)溶于2mL甲醇中,加入2M氢氧化钾溶液2mL,升温至回流搅拌反应3小时。反应液冷却至室温,减压浓缩,向所得残余物中加入水,滴加浓盐酸至pH为5-6,用乙酸乙酯萃取(20mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得到粗品标题产物12b(16mg,浅黄色油状物),产品不经纯化直接进行下一步反应。12a (21 mg, 0.05 mmol) was dissolved in 2 mL of methanol, 2 mL of 2M potassium hydroxide solution was added, and the mixture was heated to reflux and stirred for 3 hours. The reaction solution was cooled to room temperature, and concentrated under reduced pressure. Water was evaporated, evaporated, evaporated, evaporated, The solution was washed with EtOAc EtOAc (EtOAc m.
第三步third step
2-(4-氯-2-(三氟甲基)苄基)-1-环丙基-N-(4-(乙磺酰基)苄基)-1H-吲哚-5-甲酰胺122-(4-Chloro-2-(trifluoromethyl)benzyl)-1-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-1H-indole-5-carboxamide 12
将粗品12b(16mg,0.04mmol),(4-(乙磺酰基)苯基)甲胺(12.2mg,0.06mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(15.6mg,0.08mmol),1-羟基苯并三唑(11mg,0.08mmol),三乙胺(9mg,0.08mmol)溶于二氯甲烷中,搅拌反应12小时。将反应液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物12(13mg,黄色固体),产率:56%。Crude 12b (16 mg, 0.04 mmol), (4-(ethylsulfonyl)phenyl)methanamine (12.2 mg, 0.06 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbazide Amine hydrochloride (15.6 mg, 0.08 mmol), 1-hydroxybenzotriazole (11 mg, 0.08 mmol), triethylamine (9 mg, 0.08 mmol) was dissolved in dichloromethane. The reaction mixture was concentrated under reduced EtOAc.
MS m/z(ESI):575.4[M+1];MS m/z (ESI): 575.4 [M + 1];
1H NMR(400MHz,CDCl3)δ8.02(s,1H),7.87(d,2H),7.68-7.71(m,2H),7.56-7.61(m,3H),7.41(dd,1H),7.02(d,1H),6.62(t,1H),6.20(s,1H),4.79(d,2H),4.42(s,2H),3.10(q,2H),2.96-3.00(m,1H),1.28(t,3H),1.10-1.14(m,2H),1.97-1.01(m,2H)。 1 H NMR (400MHz, CDCl 3 ) δ8.02 (s, 1H), 7.87 (d, 2H), 7.68-7.71 (m, 2H), 7.56-7.61 (m, 3H), 7.41 (dd, 1H), 7.02(d,1H), 6.62(t,1H), 6.20(s,1H),4.79(d,2H),4.42(s,2H),3.10(q,2H),2.96-3.00(m,1H) , 1.28 (t, 3H), 1.10 - 1.14 (m, 2H), 1.97-1.01 (m, 2H).
实施例13Example 13
2-(4-氟-2-(三氟甲基)苄基)-1-环丙基-N-(4-(乙磺酰基)苄基)-1H-吲哚-5-甲酰胺132-(4-Fluoro-2-(trifluoromethyl)benzyl)-1-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-1H-indole-5-carboxamide 13
第一步first step
2-(4-氟-2-(三氟甲基)苄基)-1-环丙基-1H-吲哚-5-甲酸甲酯13aMethyl 2-(4-fluoro-2-(trifluoromethyl)benzyl)-1-cyclopropyl-1H-indole-5-carboxylate 13a
将4c(100mg,0.285mmol)溶于1,2-二氯乙烷中,分别加入环丙基硼酸(125.4mg,1.42mmol),醋酸铜(103.4mg,0.569mmol),2,2'-联吡啶(222.3mg,1.42mmol)和碳酸钠(60.4mg,0.569mmol),升温至80℃搅拌反应12小时。反应液冷却至室温,减压浓缩,向所得残余物中加入乙酸乙酯,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得到标题产物13a(71mg,浅黄色油状物),产率:64%。4c (100 mg, 0.285 mmol) was dissolved in 1,2-dichloroethane, respectively, cyclopropylboronic acid (125.4 mg, 1.42 mmol), copper acetate (103.4 mg, 0.569 mmol), 2,2'-linked Pyridine (222.3 mg, 1.42 mmol) and sodium carbonate (60.4 mg, 0.569 mmol) were heated to 80 ° C and stirred for 12 hours. The reaction mixture was cooled to room temperature, EtOAc was evaporated, evaporated, evaporated, evaporated. Light yellow oil), Yield: 64%.
第二步Second step
2-(4-氟-2-(三氟甲基)苄基)-1-环丙基-1H-吲哚-5-甲酸13b2-(4-Fluoro-2-(trifluoromethyl)benzyl)-1-cyclopropyl-1H-indole-5-carboxylic acid 13b
将13a(71mg,0.174mmol)溶于3mL甲醇中,加入2M氢氧化钾溶液3mL,升温至回流搅拌反应3小时。反应液冷却至室温,减压浓缩,向所得残余物中加入水,滴加浓盐酸至pH为5-6,用乙酸乙酯萃取(20mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得到粗品标题产物13b(56mg,浅黄色油状物),产品不经纯化直接进行下一步反应。13a (71 mg, 0.174 mmol) was dissolved in 3 mL of methanol, and 3 mL of 2M potassium hydroxide solution was added thereto, and the mixture was heated to reflux and stirred for 3 hours. The reaction solution was cooled to room temperature, and concentrated under reduced pressure. Water was evaporated, evaporated, evaporated, evaporated, The solution was washed with EtOAc EtOAc (EtOAc m.
第三步third step
2-(4-氟-2-(三氟甲基)苄基)-1-环丙基-N-(4-(乙磺酰基)苄基)-1H-吲哚-5-甲酰胺132-(4-Fluoro-2-(trifluoromethyl)benzyl)-1-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-1H-indole-5-carboxamide 13
将粗品13b(56mg,0.148mmol),(4-(乙磺酰基)苯基)甲胺(44.36mg,0.222mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(56.9mg,0.297mmol),1-羟基苯并三唑(40.11mg,0.297mmol),三乙胺(30mg,0.297mmol)溶于二氯甲烷中,搅拌反应12小时。将反应液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得到标题产物13(36mg,白色固体),产率:43%。Crude 13b (56 mg, 0.148 mmol), (4-(ethylsulfonyl)phenyl)methanamine (44.36 mg, 0.222 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbazide The amine hydrochloride (56.9 mg, 0.297 mmol), 1-hydroxybenzotriazole (40.11 mg, 0.297 mmol), triethylamine (30 mg, 0.297 mmol) was dissolved in dichloromethane. The reaction mixture was concentrated under reduced EtOAc.
MS m/z(ESI):557.1[M-1];MS m/z (ESI): 557.1 [M-1];
1H NMR(400MHz,CDCl3)δ8.05(s,1H),7.91(d,2H),7.72(dd,1H),7.59-7.65(m,3H),7.48(dd,1H),7.18(dt,1H),6.64(t,1H),6.21(s,1H),4.82(d,2H),4.45(s,2H),3.14(q,2H),3.00-3.06(m,1H),1.32(t,3H),1.14-1.19(m,2H),1.02-1.05(m,
2H)。 1 H NMR (400MHz, CDCl 3 ) δ8.05 (s, 1H), 7.91 (d, 2H), 7.72 (dd, 1H), 7.59-7.65 (m, 3H), 7.48 (dd, 1H), 7.18 ( Dt, 1H), 6.64 (t, 1H), 6.21 (s, 1H), 4.82 (d, 2H), 4.45 (s, 2H), 3.14 (q, 2H), 3.00-3.06 (m, 1H), 1.32 (t, 3H), 1.14-1.19 (m, 2H), 1.02-1.05 (m, 2H).
实施例14Example 14
N-(4-(乙磺酰基)苄基)-1-异丙基-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺14N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 14
第一步first step
2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酸甲酯14b2-(4-(Trifluoromethyl)benzyl)-1H-indole-5-carboxylic acid methyl ester 14b
将1a(200mg,1.14mmol),1-(溴甲基)-4-三氟甲基)苯14a(286mg,1.97mmol),双乙腈二氯化钯(16mg,0.057mmol),降冰片烯(214mg,2.28mmol),碳酸氢钠(144mg,1.71mmol)溶于N,N-二甲基乙酰胺中,升至70℃搅拌反应12小时。反应液冷却至室温,倒入水中,用乙酸乙酯萃取(20mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得标题产物14b(94mg,浅黄色油状物),产率:25%。1a (200 mg, 1.14 mmol), 1-(bromomethyl)-4-trifluoromethyl)benzene 14a (286 mg, 1.97 mmol), diacetonitrile palladium dichloride (16 mg, 0.057 mmol), norbornene ( 214 mg, 2.28 mmol), sodium hydrogencarbonate (144 mg, 1.71 mmol) was dissolved in N,N-dimethylacetamide, and the mixture was stirred at 70 ° C for 12 hours. The reaction mixture was cooled to room temperature, poured into water, EtOAc (EtOAc (EtOAc) The resulting residue was purified with EtOAc EtOAcjjjjj
第二步Second step
2-(4-(三氟甲基)苄基)-1-异丙基-1H-吲哚-5-甲酸甲酯14cMethyl 2-(4-(trifluoromethyl)benzyl)-1-isopropyl-1H-indole-5-carboxylate 14c
将14b(94mg,0.28mmol)溶于3mL N,N-二甲基甲酰胺中,加入氢化钠(27mg,0.56mmol,60%in oil),2-溴丙烷(70mg,0.56mmol),80℃搅拌反应12小时。反应液冷却至室温,倒入水中,用乙酸乙酯萃取(20mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得到粗品标题产物14c(67mg,浅黄色固体),产品不经纯化直接进行下一步反应。14b (94mg, 0.28mmol) was dissolved in 3mL of N,N-dimethylformamide, sodium hydride (27mg, 0.56mmol, 60% in oil), 2-bromopropane (70mg, 0.56mmol), 80°C The reaction was stirred for 12 hours. The reaction mixture was cooled to room temperature, poured into water, EtOAcjjjjjjjjjjjjjjjjjj Product 14c (67 mg, pale yellow solid).
第三步third step
2-(4-(三氟甲基)苄基)-1-异丙基-1H-吲哚-5-甲酸14d
2-(4-(Trifluoromethyl)benzyl)-1-isopropyl-1H-indole-5-carboxylic acid 14d
将粗品14c(67mg,0.18mmol)溶于3mL甲醇中,加入2M氢氧化钾溶液1mL,升温至回流搅拌反应3小时。反应液冷却至室温,减压浓缩,向残余物中加入水,滴加浓盐酸至pH为5-6,用乙酸乙酯萃取(20mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得到粗品标题产物14d(54mg,无色油状物),产品不经纯化直接进行下一步反应。The crude product 14c (67 mg, 0.18 mmol) was dissolved in 3 mL of methanol, and 1 mL of a 2M potassium hydroxide solution was added thereto, and the mixture was heated to reflux and stirred for 3 hours. The reaction solution was cooled to room temperature, concentrated under reduced pressure, and water was added to the residue, and concentrated hydrochloric acid was added dropwise to pH 5-6, extracted with ethyl acetate (20 mL×3), and the organic phase was combined with water and saturated sodium chloride solution The mixture was washed with EtOAc EtOAc EtOAc.
第四步the fourth step
N-(4-(乙磺酰基)苄基)-1-异丙基-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺14N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 14
将粗品14d(54mg,0.15mmol),(4-(乙磺酰基)苯基)甲胺(36mg,0.18mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(57mg,0.3mmol),1-羟基苯并三唑(39mg,0.3mmol),三乙胺(33mg,0.3mmol)溶于二氯甲烷中,搅拌反应12小时。将反应液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物14(23.7mg,白色固体),产率:29%。Crude 14d (54 mg, 0.15 mmol), (4-(ethylsulfonyl)phenyl)methanamine (36 mg, 0.18 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (57 mg, 0.3 mmol), 1-hydroxybenzotriazole (39 mg, 0.3 mmol), triethylamine (33 mg, 0.3 mmol) was dissolved in dichloromethane. The reaction mixture was concentrated under reduced vacuo. mjjjjjjjj
MS m/z(ESI):543.9[M+1];MS m/z (ESI): 543.9 [M + 1];
1H NMR(400MHz,CDCl3)δ8.08(s,1H),7.84(d,2H),7.65(d,1H),7.52-7.58(m,5H),7.30(d,2H),6.74(t,1H),6.34(s,1H),4.78(d,2H),4.47-4.50(m,1H),4.22(s,2H),3.10(q,2H),1.47(d,6H),1.27(t,3H)。 1 H NMR (400MHz, CDCl 3 ) δ8.08 (s, 1H), 7.84 (d, 2H), 7.65 (d, 1H), 7.52-7.58 (m, 5H), 7.30 (d, 2H), 6.74 ( t,1H), 6.34 (s, 1H), 4.78 (d, 2H), 4.47-4.50 (m, 1H), 4.22 (s, 2H), 3.10 (q, 2H), 1.47 (d, 6H), 1.27 (t, 3H).
实施例15Example 15
N-(4-(乙磺酰基)苄基)-2-(2-氟-4-(三氟甲基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺15N-(4-(ethylsulfonyl)benzyl)-2-(2-fluoro-4-(trifluoromethyl)benzyl)-1-isopropyl-1H-indole-5-carboxamide 15
第一步first step
2-(2-氟-4-(三氟甲基)苄基)-1H-吲哚-5-甲酸甲酯15b2-(2-Fluoro-4-(trifluoromethyl)benzyl)-1H-indole-5-carboxylic acid methyl ester 15b
将1a(200mg,1.14mmol),1-(溴甲基)-2-氟-4-三氟甲基)苯15a(322.8mg,1.26mmol),双乙腈二氯化钯(13mg,0.057mmol),降冰片烯(12mg,0.114mmol),碳酸氢钠(144mg,1.71mmol)溶于N,N-二甲基乙酰胺中,升至70℃搅拌反应12小时。反应液冷却至室温,减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得标题产物15b(104mg,浅黄色油状物),产率:25%。1a (200 mg, 1.14 mmol), 1-(bromomethyl)-2-fluoro-4-trifluoromethyl)benzene 15a (322.8 mg, 1.26 mmol), bis acetonitrile palladium chloride (13 mg, 0.057 mmol) The norbornene (12 mg, 0.114 mmol), sodium hydrogencarbonate (144 mg, 1.71 mmol) was dissolved in N,N-dimethylacetamide, and the mixture was stirred at 70 ° C for 12 hours. The reaction mixture was cooled to EtOAc.
第二步Second step
2-(2-氟-4-(三氟甲基)苄基)-1-异丙基-1H-吲哚-5-甲酸甲酯15c2-(2-Fluoro-4-(trifluoromethyl)benzyl)-1-isopropyl-1H-indole-5-carboxylic acid methyl ester 15c
将15b(131mg,0.37mmol)溶于N,N-二甲基甲酰胺中,加入氢化钠(30mg,0.75mmol,60%in oil),2-溴丙烷(92mg,0.75mmol),50℃搅拌反应12小时。反应液冷却至室温,倒入水中,用乙酸乙酯萃取(20mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得到标题产物15c(72mg,无色油状物),产率:49%。15b (131mg, 0.37mmol) was dissolved in N,N-dimethylformamide, sodium hydride (30mg, 0.75mmol, 60% in oil), 2-bromopropane (92mg, 0.75mmol), stirred at 50 °C Reaction for 12 hours. The reaction mixture was cooled to room temperature, poured into water, EtOAc (EtOAc (EtOAc) The residue was purified by EtOAc EtOAc (EtOAc)
第三步third step
2-(2-氟-4-(三氟甲基)苄基)-1-异丙基-1H-吲哚-5-甲酸15d2-(2-Fluoro-4-(trifluoromethyl)benzyl)-1-isopropyl-1H-indole-5-carboxylic acid 15d
将15c(72mg,0.183mmol)溶于3mL甲醇中,加入2M氢氧化钾溶液1mL,升温至70℃搅拌反应3小时。反应液冷却至室温,减压浓缩,向残余物中加入水,滴加浓盐酸至pH为5-6,用乙酸乙酯萃取(20mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得到粗品标题产物15d(47mg,无色油状物),产品不经纯化直接进行下一步反应。15c (72 mg, 0.183 mmol) was dissolved in 3 mL of methanol, 1 mL of 2M potassium hydroxide solution was added, and the mixture was heated to 70 ° C and stirred for 3 hours. The reaction solution was cooled to room temperature, concentrated under reduced pressure, and water was added to the residue, and concentrated hydrochloric acid was added dropwise to pH 5-6, extracted with ethyl acetate (20 mL×3), and the organic phase was combined with water and saturated sodium chloride solution The mixture was washed with EtOAc EtOAc EtOAc.
第四步the fourth step
N-(4-(乙磺酰基)苄基)-2-(2-氟-4-(三氟甲基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺15N-(4-(ethylsulfonyl)benzyl)-2-(2-fluoro-4-(trifluoromethyl)benzyl)-1-isopropyl-1H-indole-5-carboxamide 15
将粗品15d(47mg,0.12mmol),(4-(乙磺酰基)苯基)甲胺(30mg,0.15mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(48mg,0.25mmol),1-羟基苯并三唑(34mg,0.25mmol),三乙胺(26mg,0.25mmol)溶于二氯甲烷中,搅拌反应12小时。将反应液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物15(3.1mg,白色固体),产率:5%。Crude 15d (47 mg, 0.12 mmol), (4-(ethylsulfonyl)phenyl)methanamine (30 mg, 0.15 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (48 mg, 0.25 mmol), 1-hydroxybenzotriazole (34 mg, 0.25 mmol), triethylamine (26 mg, 0.25 mmol) was dissolved in dichloromethane. The reaction mixture was concentrated under reduced vacuo.
MS m/z(ESI):561.5[M+1];MS m/z (ESI): 561.5 [M + 1];
1HNMR(400MHz,CDCl3)δ8.10(s,1H),7.90(d,2H),7.69(d,1H),7.57-7.61(m,3H),7.42(d,1H),7.37(d,1H),7.20(t,1H),6.69(t,1H),6.36(s,1H),4.82(d,2H),4.50-4.57(m,1H),4.25(s,2H),3.14(q,2H),1.57(d,6H),1.31(t,3H)。 1 H NMR (400 MHz, CDCl 3 ) δ 8.10 (s, 1H), 7.90 (d, 2H), 7.69 (d, 1H), 7.57-7.61 (m, 3H), 7.42 (d, 1H), 7.37 (d) , 1H), 7.20 (t, 1H), 6.69 (t, 1H), 6.36 (s, 1H), 4.82 (d, 2H), 4.50-4.57 (m, 1H), 4.25 (s, 2H), 3.14 ( q, 2H), 1.57 (d, 6H), 1.31 (t, 3H).
实施例16Example 16
2-(2-氯-4-(三氟甲基)苄基)-N-(4-(乙磺酰基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺
162-(2-Chloro-4-(trifluoromethyl)benzyl)-N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-1H-indole-5-carboxamide
16
第一步first step
2-(2-氯-4-(三氟甲基)苄基)-1H-吲哚-5-甲酸甲酯16b2-(2-chloro-4-(trifluoromethyl)benzyl)-1H-indole-5-carboxylic acid methyl ester 16b
将1a(200mg,1.14mmol),1-(溴甲基)-2-氯-4-三氟甲基)苯16a(328mg,1.2mmol),双乙腈二氯化钯(28mg,0.11mmol),降冰片烯(215mg,2.28mmol),碳酸氢钠(144mg,1.71mmol)溶于N,N-二甲基乙酰胺中,升至70℃搅拌反应12小时。反应液冷却至室温,减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得标题产物16b(89mg,浅黄色油状物),产率:22%。1a (200 mg, 1.14 mmol), 1-(bromomethyl)-2-chloro-4-trifluoromethyl)benzene 16a (328 mg, 1.2 mmol), bis acetonitrile dichloride (28 mg, 0.11 mmol) Norbornene (215 mg, 2.28 mmol), sodium hydrogencarbonate (144 mg, 1.71 mmol) was dissolved in N,N-dimethylacetamide, and the mixture was stirred at 70 ° C for 12 hours. The reaction mixture was cooled to EtOAc EtOAc m.
第二步Second step
2-(2-氯-4-(三氟甲基)苄基)-1-异丙基-1H-吲哚-5-甲酸甲酯16c2-(2-chloro-4-(trifluoromethyl)benzyl)-1-isopropyl-1H-indole-5-carboxylic acid methyl ester 16c
将16b(89mg,0.24mmol)溶于N,N-二甲基甲酰胺中,加入氢化钠(20mg,0.48mmol,60%in oil),2-溴丙烷(60mg,0.48mmol),50℃搅拌反应12小时。反应液冷却至室温,倒入水中,用乙酸乙酯萃取(20mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩得到粗品标题产物16c(41mg,无色油状物),产品不经纯化直接进行下一步反应。16b (89mg, 0.24mmol) was dissolved in N,N-dimethylformamide, sodium hydride (20mg, 0.48mmol, 60% in oil), 2-bromopropane (60mg, 0.48mmol), stirred at 50 ° C Reaction for 12 hours. The reaction mixture was cooled to room temperature, poured into water, EtOAcjjjjjjjjjjjjjjjjjjjjj 16c (41 mg, colorless oil), product was taken to the next step without purification.
第三步third step
2-(2-氯-4-(三氟甲基)苄基)-1-异丙基-1H-吲哚-5-甲酸16d2-(2-chloro-4-(trifluoromethyl)benzyl)-1-isopropyl-1H-indole-5-carboxylic acid 16d
将粗品16c(41mg,0.1mmol)溶于3mL甲醇中,加入2M氢氧化钾溶液1mL,70℃搅拌反应12小时。反应液冷却至室温,减压浓缩,向残余物中加入水,滴加浓盐酸至pH为5-6,用乙酸乙酯萃取(20mL×3),合并有机相,用水,饱和氯化钠
溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩得到粗品标题产物16d(34mg,无色油状物),产品不经纯化直接进行下一步反应。The crude 16c (41 mg, 0.1 mmol) was dissolved in 3 mL of methanol, 1 mL of 2M potassium hydroxide solution was added, and the reaction was stirred at 70 ° C for 12 hours. The reaction solution was cooled to room temperature, concentrated under reduced pressure. Water was evaporated, evaporated, evaporated, evaporated,
The solution was washed with EtOAc EtOAc (EtOAc m.
第四步the fourth step
2-(2-氯-4-(三氟甲基)苄基)-N-(4-(乙磺酰基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺162-(2-Chloro-4-(trifluoromethyl)benzyl)-N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-1H-indole-5-carboxamide 16
将粗品16d(34mg,0.086mmol),(4-(乙磺酰基)苯基)甲胺(26mg,0.13mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(33mg,0.17mmol),1-羟基苯并三唑(23mg,0.17mmol),三乙胺(17.4mg,0.17mmol)溶于二氯甲烷中,搅拌反应12小时。减压浓缩用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物16(6.5mg,白色固体),产率:13%。Crude 16d (34 mg, 0.086 mmol), (4-(ethylsulfonyl)phenyl)methanamine (26 mg, 0.13 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (33 mg, 0.17 mmol), 1-hydroxybenzotriazole (23 mg, 0.17 mmol), triethylamine (17.4 mg, 0.17 mmol) was dissolved in dichloromethane. The residue was purified by EtOAc EtOAc (EtOAc)
MS m/z(ESI):576.9[M-1]。MS m/z (ESI): 576.9 [M-1].
实施例17Example 17
N-(4-(乙磺酰基)苄基)-1-异丙基-2-(3-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺17N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-(3-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 17
第一步first step
2-(3-(三氟甲基)苄基)-1H-吲哚-5-甲酸甲酯17bMethyl 2-(3-(trifluoromethyl)benzyl)-1H-indole-5-carboxylate 17b
将1a(200mg,1.13mmol),1-(溴甲基)-3-三氟甲基)苯17a(286mg,1.2mmol),双乙腈二氯化钯(16mg,0.06mmol),降冰片烯(218mg,2.3mmol),碳酸氢钠(134mg,1.71mmol)溶于N,N-二甲基乙酰胺中,升至70℃搅拌反应12小时。反应液冷却至室温,减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得标题产物17b(134mg,浅黄色固体),产率:32%。1a (200 mg, 1.13 mmol), 1-(bromomethyl)-3-trifluoromethyl)benzene 17a (286 mg, 1.2 mmol), diacetonitrile palladium dichloride (16 mg, 0.06 mmol), norbornene ( 218 mg, 2.3 mmol), sodium hydrogencarbonate (134 mg, 1.71 mmol) was dissolved in N,N-dimethylacetamide, and the mixture was stirred at 70 ° C for 12 hours. The reaction mixture was cooled to EtOAc EtOAc m.
第二步
Second step
2-(3-(三氟甲基)苄基)-1-异丙基-1H-吲哚-5-甲酸17c2-(3-(Trifluoromethyl)benzyl)-1-isopropyl-1H-indole-5-carboxylic acid 17c
将17b(134mg,0.4mmol)溶于N,N-二甲基甲酰胺中,加入氢化钠(32mg,0.8mmol,60%in oil),2-溴丙烷(99mg,0.8mmol),70℃搅拌反应12小时。反应液冷却至室温,减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得到标题产物17c(108mg,浅黄色固体),产率:72%。17b (134mg, 0.4mmol) was dissolved in N,N-dimethylformamide, sodium hydride (32mg, 0.8mmol, 60% in oil), 2-bromopropane (99mg, 0.8mmol), and stirred at 70 ° C Reaction for 12 hours. The reaction mixture was cooled to EtOAc.
第三步third step
N-(4-(乙磺酰基)苄基)-1-异丙基-2-(3-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺17N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-(3-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 17
将17c(30mg,0.08mmol),(4-(乙磺酰基)苯基)甲胺(20mg,0.1mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(33mg,0.17mmol),1-羟基苯并三唑(23mg,0.17mmol),三乙胺(17mg,0.17mmol)溶于二氯甲烷中,搅拌反应12小时。减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物17(11mg,白色固体),产率:24%。17c (30 mg, 0.08 mmol), (4-(ethylsulfonyl)phenyl)methanamine (20 mg, 0.1 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide salt The acid salt (33 mg, 0.17 mmol), 1-hydroxybenzotriazole (23 mg, 0.17 mmol), triethylamine (17 mg, 0.17 mmol) was dissolved in dichloromethane. The residue was purified by EtOAc EtOAcjjjjjjj
MS m/z(ESI):543.5[M+1];MS m/z (ESI): 543.5 [M+1];
1HNMR(400MHz,CDCl3)δ8.08(s,1H),7.85(d,2H),7.65(d,1H),7.50-7.57(m,3H),7.41-7.47(m,2H),7.36(d,1H),6.75(t,1H),6.33(s,1H),4.78(d,2H),4.45-4.53(m,1H),4.22(s,2H),4.10-4.16(m,1H),3.10(q,2H),1.47(d,6H),1.27(t,3H)。 1 HNMR (400MHz, CDCl 3) δ8.08 (s, 1H), 7.85 (d, 2H), 7.65 (d, 1H), 7.50-7.57 (m, 3H), 7.41-7.47 (m, 2H), 7.36 (d, 1H), 6.75 (t, 1H), 6.33 (s, 1H), 4.78 (d, 2H), 4.45-4.53 (m, 1H), 4.22 (s, 2H), 4.10-4.16 (m, 1H) ), 3.10 (q, 2H), 1.47 (d, 6H), 1.27 (t, 3H).
实施例18Example 18
N-(4-(乙磺酰基)苄基)-1-异丙基-2-(4-(三氟甲氧基)苄基)-1H-吲哚-5-甲酰胺18N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-(4-(trifluoromethoxy)benzyl)-1H-indole-5-carboxamide 18
第一步first step
2-(4-(三氟甲氧基)苄基)-1H-吲哚-5-甲酸甲酯18bMethyl 2-(4-(trifluoromethoxy)benzyl)-1H-indole-5-carboxylate 18b
将1a(500mg,2.85mmol),1-(溴甲基)-4-三氟甲氧基苯18a(716mg,3mmol),双乙腈二氯化钯(39mg,0.14mmol),降冰片烯(534mg,5.7mmol),碳酸氢钠(360
mg,4.28mmol)溶于N,N-二甲基乙酰胺中,升至70℃搅拌反应12小时。反应液冷却至室温,减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得到标题产物18b(520mg,浅黄色固体),产率:55%。1a (500 mg, 2.85 mmol), 1-(bromomethyl)-4-trifluoromethoxybenzene 18a (716 mg, 3 mmol), diacetonitrile palladium dichloride (39 mg, 0.14 mmol), norbornene (534 mg) , 5.7mmol), sodium bicarbonate (360
The mg, 4.28 mmol) was dissolved in N,N-dimethylacetamide, and the reaction was stirred at 70 ° C for 12 hours. The reaction mixture was cooled to EtOAc EtOAc m.
第二步Second step
1-异丙基-2-(4-(三氟甲氧基)苄基)-1H-吲哚-5-甲酸18c1-isopropyl-2-(4-(trifluoromethoxy)benzyl)-1H-indole-5-carboxylic acid 18c
将18b(200mg,0.573mmol)溶于N,N-二甲基甲酰胺中,加入氢化钠(46mg,0.15mmol,60%in oil),2-溴丙烷(140.84mg,1.15mmol),于70℃搅拌反应12小时。反应液冷却至室温,减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物18c(92mg,浅黄色油状物),产率:41%。18b (200mg, 0.573mmol) was dissolved in N,N-dimethylformamide, sodium hydride (46mg, 0.15mmol, 60% in oil), 2-bromopropane (140.84mg, 1.15mmol), The reaction was stirred at ° C for 12 hours. The reaction mixture was cooled to EtOAc EtOAc m.
第三步third step
N-(4-(乙磺酰基)苄基)-1-异丙基-2-(4-(三氟甲氧基)苄基)-1H-吲哚-5-甲酰胺18N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-(4-(trifluoromethoxy)benzyl)-1H-indole-5-carboxamide 18
将18c(30mg,0.08mmol),(4-(乙磺酰基)苯基)甲胺(20mg,0.1mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(31mg,0.16mmol),1-羟基苯并三唑(21mg,0.16mmol),三乙胺(17mg,0.16mmol)溶于二氯甲烷中,搅拌反应12小时。减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物18(13mg,白色固体),产率:29%。18c (30 mg, 0.08 mmol), (4-(ethylsulfonyl)phenyl)methanamine (20 mg, 0.1 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide salt The acid salt (31 mg, 0.16 mmol), 1-hydroxybenzotriazole (21 mg, 0.16 mmol), triethylamine (17 mg, 0.16 mmol) was dissolved in dichloromethane. The residue was purified by EtOAc EtOAcjjjjjjj
MS m/z(ESI):559.5[M+1];MS m/z (ESI): 559.5 [M + 1];
1HNMR(400MHz,CDCl3)δ8.08(s,1H),7.85(d,2H),7.65(d,1H),7.50-7.57(m,3H),7.41-7.47(m,2H),7.36(d,1H),6.75(t,1H),6.33(s,1H),4.78(d,2H),4.45-4.53(m,1H),4.22(s,2H),4.10-4.16(m,1H),3.10(q,2H),1.47(d,6H),1.27(t,3H)。 1 HNMR (400MHz, CDCl 3) δ8.08 (s, 1H), 7.85 (d, 2H), 7.65 (d, 1H), 7.50-7.57 (m, 3H), 7.41-7.47 (m, 2H), 7.36 (d, 1H), 6.75 (t, 1H), 6.33 (s, 1H), 4.78 (d, 2H), 4.45-4.53 (m, 1H), 4.22 (s, 2H), 4.10-4.16 (m, 1H) ), 3.10 (q, 2H), 1.47 (d, 6H), 1.27 (t, 3H).
实施例19Example 19
1-乙基-N-(4-(乙磺酰基)苄基)-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺191-ethyl-N-(4-(ethylsulfonyl)benzyl)-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 19
第一步first step
2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酸甲酯19aMethyl 2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxylate 19a
将1a(1g,5.71mmol),14b(1.43g,5.99mmol),双乙腈二氯化钯(150mg,0.571mmol),降冰片烯(1.1g,11.42mmol),碳酸氢钠(0.63g,7.42mmol)溶于N,N-二甲基乙酰胺中,升至70℃搅拌反应16小时。反应液冷却至室温,加入100mL水,用乙酸乙酯萃取(100mL×3),合并有机相,用饱和氯化钠溶液洗涤(100mL),无水硫酸钠干燥,过滤,滤液减压浓缩,,得粗品标题产物19a(1.5g,黄色固体),产品不经纯化直接进行下一步反应。1a (1g, 5.71mmol), 14b (1.43g, 5.99mmol), diacetonitrile palladium dichloride (150mg, 0.571mmol), norbornene (1.1g, 11.42mmol), sodium bicarbonate (0.63g, 7.42) Methyl) was dissolved in N,N-dimethylacetamide and the reaction was stirred at 70 ° C for 16 hours. The reaction mixture was cooled to room temperature, EtOAc (EtOAc) (EtOAc) The crude title product 19a (1.5 g, yellow solid) was obtained.
第二步Second step
1-乙基-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酸甲酯19bMethyl 1-ethyl-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxylate 19b
将粗品19a(0.2g,0.6mmol)溶于5mL N,N-二甲基甲酰胺中,加入氢化钠(48mg,1.2mmol,60%in oil),于0℃搅拌反应1小时,加入碘乙烷(0.19g,1.2mmol),升温至50℃搅拌反应15小时。反应液冷却至室温,加入50mL水,用乙酸乙酯萃取(30mL×3),合并有机相,用饱和氯化钠溶液洗涤(50mL),无水硫酸钠干燥,过滤,滤液减压浓缩,得到粗品标题产物19b(63mg,黄色固体),产品不经纯化直接进行下一步反应。The crude product 19a (0.2 g, 0.6 mmol) was dissolved in 5 mL of N,N-dimethylformamide, sodium hydride (48 mg, 1.2 mmol, 60% in oil) was added, and the reaction was stirred at 0 ° C for 1 hour. The alkane (0.19 g, 1.2 mmol) was heated to 50 ° C and stirred for 15 hours. The reaction mixture was cooled to room temperature, then added with 50 mL of EtOAc (EtOAc (EtOAc) The crude title product 19b (63 mg, yellow solid).
第三步third step
1-乙基-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酸19c1-ethyl-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxylic acid 19c
将粗品19b(63mg,0.74mmol)溶于11mL甲醇和水(V:V=10:1)的混合溶剂中,加入氢氧化钠(35mg,0.872mmol),60℃搅拌反应16小时。反应液冷却至室温,滴加2M盐酸至pH为3-4,减压浓缩,用薄层色谱法以展开剂体系A纯化所得残余物,得到标题产物19c(40g,白色固体),产率:67%。The crude product 19b (63 mg, 0.74 mmol) was dissolved in a mixed solvent of 11 mL of methanol and water (V: V = 10:1), sodium hydroxide (35 mg, 0.872 mmol) was added, and the reaction was stirred at 60 ° C for 16 hours. The reaction mixture was cooled to room temperature, EtOAc EtOAc (EtOAc m. 67%.
第四步the fourth step
1-乙基-N-(4-(乙磺酰基)苄基)-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺191-ethyl-N-(4-(ethylsulfonyl)benzyl)-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 19
将19c(10mg,0.029mmol),(4-(乙磺酰基)苯基)甲胺(11.6mg,0.058mmol),
2-(7-偶氮苯并三氮唑)-N,N,N',N'-四甲基脲六氟磷酸酯(16mg,0.044mmol),N,N-二异丙基乙胺(18.7mg,0.044mmol)溶于5mL N,N-二甲基甲酰胺中,搅拌反应16小时。向反应液加入50mL水,用二氯甲烷萃取(30mL×3),合并有机相,用饱和氯化钠溶液洗涤(60mL),无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物19(8mg,白色固体),产率:40%。19c (10 mg, 0.029 mmol), (4-(ethylsulfonyl)phenyl)methanamine (11.6 mg, 0.058 mmol),
2-(7-Azobenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate (16 mg, 0.044 mmol), N,N-diisopropylethylamine ( 18.7 mg, 0.044 mmol) was dissolved in 5 mL of N,N-dimethylformamide and the reaction was stirred for 16 hours. 50 ml of water was added to the reaction solution, and the mixture was extracted with dichloromethane (30 mL × 3). The organic phase was combined, washed with saturated sodium chloride solution (60 mL), dried over anhydrous sodium sulfate The residue was purified with EtOAc EtOAc (EtOAc)
MS m/z(ESI):529.5[M+1];MS m/z (ESI): 529.5 [M+1];
1HNMR(400MHz,CDCl3)δ8.07(s,1H),7.88(d,2H),7.69(dd,1H),7.56-7.60(m,4H),7.32-7.35(m,3H),6.64(t,1H),6.34(s,1H),4.80(d,2H),4.21(s,2H),4.08(q,2H),3.11(q,2H),1.29(t,3H),1.21(t,3H)。 1 H NMR (400 MHz, CDCl 3 ) δ 8.07 (s, 1H), 7.88 (d, 2H), 7.69 (dd, 1H), 7.56-7.60 (m, 4H), 7.32-7.35 (m, 3H), 6.64 (t, 1H), 6.34 (s, 1H), 4.80 (d, 2H), 4.21 (s, 2H), 4.08 (q, 2H), 3.11 (q, 2H), 1.29 (t, 3H), 1.21. t, 3H).
实施例20Example 20
1-环丙基-N-(4-(乙磺酰基)苄基)-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺201-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 20
第一步first step
1-环丙基-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酸甲酯20aMethyl 1-cyclopropyl-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxylate 20a
将19c(75mg,0.22mmol)溶于1,2-二氯乙烷中,分别加入环丙基硼酸(39mg,0.45mmol),一水合醋酸铜(45mg,0.22mmol),2,2'-联吡啶(35mg,0.22mmol)和碳酸钠(36mg,0.34mmol),升温至70℃搅拌反应24小时。反应液冷却至室温,减压浓缩,向所得残余物中加入水,用乙酸乙酯萃取(30mL×3),合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物20a(60mg,黄色固体),产率:71%。19c (75 mg, 0.22 mmol) was dissolved in 1,2-dichloroethane, respectively, cyclopropylboronic acid (39 mg, 0.45 mmol), copper acetate monohydrate (45 mg, 0.22 mmol), 2,2'-linked Pyridine (35 mg, 0.22 mmol) and sodium carbonate (36 mg, 0.34 mmol) were warmed to 70 ° C and stirred for 24 hours. The reaction mixture was cooled to room temperature, EtOAc was evaporated, evaporated, evaporated, evaporated, evaporated, evaporated The residue was purified by EtOAc EtOAcjjjjjjj
第二步Second step
1-环丙基-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酸20b
1-cyclopropyl-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxylic acid 20b
将20a(80mg,0.21mmol)溶于8mL甲醇和四氢呋喃(V:V=1:1)混合溶剂中,加入4M氢氧化钠溶液2mL,60℃搅拌反应2小时。反应液冷却至室温,滴加浓盐酸至pH为5-6,用乙酸乙酯萃取(20mL×3),合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得到粗品标题产物20b(60mg,淡黄色油固体),产品不经纯化直接进行下一步反应。20a (80 mg, 0.21 mmol) was dissolved in 8 mL of a mixed solvent of methanol and tetrahydrofuran (V: V = 1:1), 2 mL of 4M sodium hydroxide solution was added, and the reaction was stirred at 60 ° C for 2 hours. The reaction solution was cooled to room temperature, and concentrated hydrochloric acid was added dropwise to pH 5-6, and ethyl acetate (20 mL×3), and the organic phase was combined, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered, filtrate reduced Concentration under pressure gave the crude title product 20b (60 mg, pale yellow oil solid).
第三步third step
1-环丙基-N-(4-(乙磺酰基)苄基)-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺201-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 20
将粗品20b(60mg,0.17mmol),(4-(乙磺酰基)苯基)甲胺(50mg,0.25mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(48mg,0.25mmol),1-羟基苯并三唑(34mg,0.25mmol),N,N-二异丙基乙胺(65mg,0.50mmol)溶于二氯甲烷中,搅拌反应12小时。加入水,用二氯甲烷和甲醇(V:V=8:1)混合溶剂萃取(20mL×3),合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系A纯化所得残余物,得到标题产物20(20mg,白色固体),产率:21%。Crude 20b (60 mg, 0.17 mmol), (4-(ethylsulfonyl)phenyl)methanamine (50 mg, 0.25 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (48 mg, 0.25 mmol), 1-hydroxybenzotriazole (34 mg, 0.25 mmol), N,N-diisopropylethylamine (65 mg, 0.50 mmol) dissolved in dichloromethane. hour. Add water, extract with a mixed solvent of dichloromethane and methanol (V: V = 8:1) (20 mL × 3), combine the organic phase, wash with saturated sodium chloride solution, dry over anhydrous sodium sulfate, The residue was purified by EtOAc EtOAc EtOAc:
MS m/z(ESI):541.4[M+1];MS m/z (ESI): 541.4 [M+1];
1H NMR(400MHz,DMSO-d6)δ9.03(t,1H),8.09(s,1H),7.84(d,2H),7.70(d,3H),7.58(t,2.5H),7.52(t,2.5H),6.24(s,1H),4.58(d,2H),4.37(s,2H),3.25(q,2H),2.94-2.99(m,1H),1.12-1.17(m,2H),1.08(t,3H),0.97-1.01(m,2H)。 1 H NMR (400 MHz, DMSO-d 6 ) δ 9.03 (t, 1H), 8.09 (s, 1H), 7.84 (d, 2H), 7.70 (d, 3H), 7.58 (t, 2.5H), 7.52 (t, 2.5H), 6.24 (s, 1H), 4.58 (d, 2H), 4.37 (s, 2H), 3.25 (q, 2H), 2.94-2.99 (m, 1H), 1.12-1.17 (m, 2H), 1.08 (t, 3H), 0.97-1.01 (m, 2H).
实施例21Example 21
N-(4-(乙磺酰基)苄基)-1-甲基-2-(4-(三氟甲氧基)苄基)-1H-吲哚-5-甲酰胺21N-(4-(ethylsulfonyl)benzyl)-1-methyl-2-(4-(trifluoromethoxy)benzyl)-1H-indole-5-carboxamide 21
第一步first step
2-(4-(三氟甲氧基)苄基)-1-甲基-1H-吲哚-5-甲酸甲酯21aMethyl 2-(4-(trifluoromethoxy)benzyl)-1-methyl-1H-indole-5-carboxylate 21a
将18c(50mg,0.14mmol)溶于5mL四氢呋喃中,加入氢化钠(12mg,0.28
mmol,60%in oil),搅拌反应5分钟,加入碘甲烷(30.5mg,0.21mmol),搅拌反应30分钟。反应液加入水中,用乙酸乙酯萃取(30mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得到粗品标题产物21a(28mg,浅黄色油状物),产品不经纯化直接进行下一步反应。18c (50mg, 0.14mmol) was dissolved in 5mL of tetrahydrofuran, sodium hydride (12mg, 0.28)
Methanol, 60% in oil), the reaction was stirred for 5 minutes, methyl iodide (30.5 mg, 0.21 mmol) was added, and the reaction was stirred for 30 minutes. The reaction mixture was poured into EtOAc (3 mL, EtOAc (EtOAc) Light yellow oil), the product was directly subjected to the next reaction without purification.
第二步Second step
1-甲基-2-(4-(三氟甲氧基)苄基)-1H-吲哚-5-甲酸21b1-methyl-2-(4-(trifluoromethoxy)benzyl)-1H-indole-5-carboxylic acid 21b
将粗品21a(28mg,0.07mmol)溶于3mL甲醇中,加入2M氢氧化钾溶液1mL,70℃搅拌反应2小时。反应液冷却至室温,减压浓缩,向所得残余物中加入水,用乙酸乙酯萃取(30mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得到粗品标题产物21b(22mg,浅黄色油状物),产品不经纯化直接进行下一步反应。The crude product 21a (28 mg, 0.07 mmol) was dissolved in 3 mL of methanol, 1 mL of 2M potassium hydroxide solution was added, and the reaction was stirred at 70 ° C for 2 hours. The reaction mixture was cooled to room temperature, and the mixture was evaporated, evaporated, evaporated, evaporated. The filtrate was concentrated under reduced pressure to give the title compound (jjjjjjjjj
第三步third step
N-(4-(乙磺酰基)苄基)-1-甲基-2-(4-(三氟甲氧基)苄基)-1H-吲哚-5-甲酰胺21N-(4-(ethylsulfonyl)benzyl)-1-methyl-2-(4-(trifluoromethoxy)benzyl)-1H-indole-5-carboxamide 21
将粗品21b(22mg,0.063mmol),(4-(乙磺酰基)苯基)甲胺(25.1mg,0.126mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(24mg,0.126mmol),1-羟基苯并三唑(17mg,0.126mmol),三乙胺(13mg,0.126mmol)溶于二氯甲烷中,搅拌反应12小时。将反应液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物21(12mg,浅黄色固体),产率:36%。Crude 21b (22 mg, 0.063 mmol), (4-(ethylsulfonyl)phenyl)methanamine (25.1 mg, 0.126 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbazide The amine hydrochloride (24 mg, 0.126 mmol), 1-hydroxybenzotriazole (17 mg, 0.126 mmol), triethylamine (13 mg, 0.126 mmol) was dissolved in dichloromethane. The reaction mixture was concentrated under reduced vacuo. EtOAcjjjjjjj
MS m/z(ESI):530.9[M+1];MS m/z (ESI): 530.9 [M+1];
1HNMR(400MHz,CDCl3)δ8.07(s,1H),7.85(d,2H),7.69(d,1H),7.55(d,2H),7.30(d,1H),7.16-7.23(m,4H),6.72(t,1H),6.35(s,1H),4.78(d,2H),4.16(s,2H),3.60(s,3H),3.10(q,2H),1.27(t,3H)。 1 HNMR (400MHz, CDCl 3) δ8.07 (s, 1H), 7.85 (d, 2H), 7.69 (d, 1H), 7.55 (d, 2H), 7.30 (d, 1H), 7.16-7.23 (m , 4H), 6.72 (t, 1H), 6.35 (s, 1H), 4.78 (d, 2H), 4.16 (s, 2H), 3.60 (s, 3H), 3.10 (q, 2H), 1.27 (t, 3H).
实施例22Example 22
1-乙基-N-(4-(乙磺酰基)苄基)-2-(2-氟-4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺221-ethyl-N-(4-(ethylsulfonyl)benzyl)-2-(2-fluoro-4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 22
第一步first step
1-乙基-2-(2-氟-4-(三氟甲基)苄基)-1H-吲哚-5-甲酸甲酯22aMethyl 1-ethyl-2-(2-fluoro-4-(trifluoromethyl)benzyl)-1H-indole-5-carboxylate 22a
将15c(50mg,0.14mmol)溶于5mL四氢呋喃中,加入氢化钠(12mg,0.28mmol,60%in oil),碘乙烷(33.3mg,0.21mmol),50℃搅拌反应2小时。反应液冷却至室温,减压浓缩,得到粗品标题产物22a(28mg,浅黄色油状物),产品不经纯化直接进行下一步反应。15c (50 mg, 0.14 mmol) was dissolved in 5 mL of tetrahydrofuran, sodium hydride (12 mg, 0.28 mmol, 60% in oil), ethyl iodide (33.3 mg, 0.21 mmol) was added, and the reaction was stirred at 50 ° C for 2 hours. The reaction mixture was cooled to room temperature and then evaporated.
第二步Second step
1-乙基-2-(2-氟-4-(三氟甲基)苄基)-1H-吲哚-5-甲酸22b1-ethyl-2-(2-fluoro-4-(trifluoromethyl)benzyl)-1H-indole-5-carboxylic acid 22b
将粗品22a(28mg,0.07mmol)溶于3mL甲醇中,加入2M氢氧化钾溶液1mL,升温至回流搅拌反应2小时。反应液冷却至室温,减压浓缩,向残余物中加入水,滴加浓盐酸至pH为5-6,用乙酸乙酯萃取(20mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得到粗品标题产物22b(24mg,浅黄色油状物),产品不经纯化直接进行下一步反应。The crude product 22a (28 mg, 0.07 mmol) was dissolved in 3 mL of methanol, and 1 mL of a 2M potassium hydroxide solution was added thereto, and the mixture was heated to reflux and stirred for 2 hours. The reaction solution was cooled to room temperature, concentrated under reduced pressure, and water was added to the residue, and concentrated hydrochloric acid was added dropwise to pH 5-6, extracted with ethyl acetate (20 mL×3), and the organic phase was combined with water and saturated sodium chloride solution The mixture was washed with EtOAc EtOAc EtOAc.
第三步third step
1-乙基-N-(4-(乙磺酰基)苄基)-2-(2-氟-4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺221-ethyl-N-(4-(ethylsulfonyl)benzyl)-2-(2-fluoro-4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 22
将粗品22b(24mg,0.066mmol),(4-(乙磺酰基)苯基)甲胺(26.2mg,0.131mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(26mg,0.131mmol),1-羟基苯并三唑(18mg,0.131mmol),三乙胺(13.3mg,0.131mmol)溶于二氯甲烷中,搅拌反应12小时。反应液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物22(7mg,浅黄色固体),产率:21%。Crude 22b (24 mg, 0.066 mmol), (4-(ethylsulfonyl)phenyl)methanamine (26.2 mg, 0.131 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbazide The amine hydrochloride (26 mg, 0.131 mmol), 1-hydroxybenzotriazole (18 mg, 0.131 mmol), triethylamine (13.3 mg, 0.131 mmol) was dissolved in dichloromethane. The reaction mixture was concentrated under reduced vacuo.
MS m/z(ESI):547.9[M+1]。MS m/z (ESI): 547.9 [M+].
实施例23Example 23
1-乙基-N-(4-(乙磺酰基)苄基)-2-(4-(三氟甲氧基)苄基)-1H-吲哚-5-甲酰胺23
1-ethyl-N-(4-(ethylsulfonyl)benzyl)-2-(4-(trifluoromethoxy)benzyl)-1H-indole-5-carboxamide 23
第一步first step
1-乙基-2-(4-(三氟甲氧基)苄基)-1H-吲哚-5-甲酸甲酯23aMethyl 1-ethyl-2-(4-(trifluoromethoxy)benzyl)-1H-indole-5-carboxylate 23a
将18c(50mg,0.14mmol)溶于5mL四氢呋喃中,加入氢化钠(12mg,0.28mmol,60%in oil),碘乙烷(33.5mg,0.21mmol),搅拌反应2小时。将反应液减压浓缩,得到粗品标题产物23a(29mg,浅黄色油状物),产品不经纯化直接进行下一步反应。18c (50 mg, 0.14 mmol) was dissolved in 5 mL of tetrahydrofuran, sodium hydride (12 mg, 0.28 mmol, 60% in oil), ethyl iodide (33.5 mg, 0.21 mmol), and the reaction was stirred for 2 hours. The reaction mixture was concentrated under reduced pressure to give crystal crystal crystal crystal crystal crystal crystal crystal
第二步Second step
1-乙基-2-(4-(三氟甲氧基)苄基)-1H-吲哚-5-甲酸23b1-ethyl-2-(4-(trifluoromethoxy)benzyl)-1H-indole-5-carboxylic acid 23b
将粗品23a(26mg,0.07mmol)溶于3mL甲醇中,加入2M氢氧化钾溶液1mL,升温至回流搅拌反应2小时。反应液冷却至室温,减压浓缩,向所得残余物中加入水,用乙酸乙酯萃取(30mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得到粗品标题产物23b(21mg,浅黄色油状物),产品不经纯化直接进行下一步反应。The crude product 23a (26 mg, 0.07 mmol) was dissolved in 3 mL of methanol, and 1 mL of a 2M potassium hydroxide solution was added thereto, and the mixture was heated to reflux and stirred for 2 hours. The reaction mixture was cooled to room temperature, and the mixture was evaporated, evaporated, evaporated, evaporated. The filtrate was concentrated under reduced pressure to give crystals crystals crystals crystals crystals
第三步third step
1-乙基-N-(4-(乙磺酰基)苄基)-2-(4-(三氟甲氧基)苄基)-1H-吲哚-5-甲酰胺231-ethyl-N-(4-(ethylsulfonyl)benzyl)-2-(4-(trifluoromethoxy)benzyl)-1H-indole-5-carboxamide 23
将粗品23b(21mg,0.057mmol),(4-(乙磺酰基)苯基)甲胺(23mg,0.115mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(22mg,0.115mmol),1-羟基苯并三唑(15mg,0.115mmol),三乙胺(12mg,0.115mmol)溶于二氯甲烷中,搅拌反应12小时。将反应液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物23(8mg,浅黄色固体),产率:25%。Crude 23b (21 mg, 0.057 mmol), (4-(ethylsulfonyl)phenyl)methanamine (23 mg, 0.115 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (22 mg, 0.115 mmol), 1-hydroxybenzotriazole (15 mg, 0.115 mmol), triethylamine (12 mg, 0.115 mmol) was dissolved in dichloromethane. The reaction mixture was concentrated under reduced vacuo. EtOAcjjjjjjjj
MS m/z(ESI):545.9[M+1]。
MS m/z (ESI): 545.9 [M+1].
实施例24Example 24
N-(4-(乙磺酰基)苄基)-1-(2-羟乙基)-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺24N-(4-(ethylsulfonyl)benzyl)-1-(2-hydroxyethyl)-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 24
第一步first step
1-(2-((叔丁基二甲基硅基)氧基)乙基)-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酸甲酯24aMethyl 1-(2-((tert-butyldimethylsilyl)oxy)ethyl)-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxylate 24a
将14c(60mg,0.18mmol),碳酸铯(117mg,0.36mmol),(2-溴乙氧基)-叔丁基二甲基硅烷(86mg,0.36mmol)和催化量的碘化钾溶于3mL N,N-二甲基甲酰胺中,80℃微波反应1小时。反应液冷却至室温,加入60mL乙酸乙酯,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物24a(38mg,粘稠状物),产率:43%。14c (60 mg, 0.18 mmol), cesium carbonate (117 mg, 0.36 mmol), (2-bromoethoxy)-tert-butyldimethylsilane (86 mg, 0.36 mmol) and a catalytic amount of potassium iodide dissolved in 3 mL of N, In N-dimethylformamide, microwave reaction was carried out at 80 ° C for 1 hour. The reaction solution was cooled to room temperature, and then added with ethyl acetate (60 mL). Title product 24a (38 mg, viscous), yield: 43%.
第二步Second step
1-(2-羟乙基)-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酸24b1-(2-hydroxyethyl)-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxylic acid 24b
将24a(38mg,0.077mmol)溶于6mL甲醇中,加入2M氢氧化钠溶液1.1mL,65℃搅拌反应12小时。反应液冷却至室温,减压浓缩去除大部分溶剂,加入5mL四氢呋喃,0℃下滴加1M盐酸至pH为4,减压浓缩得到粗品标题产物24b(14mg,粉色固体),产品不经纯化直接进行下一步反应。24a (38 mg, 0.077 mmol) was dissolved in 6 mL of methanol, and 1 mL of a 2M sodium hydroxide solution was added thereto, and the reaction was stirred at 65 ° C for 12 hours. The reaction mixture was cooled to room temperature, and then concentrated, evaporated, evaporated, evaporated, evaporated, evaporated, evaporated, evaporated, evaporated. Carry out the next reaction.
第三步third step
N-(4-(乙磺酰基)苄基)-1-(2-羟乙基)-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺24N-(4-(ethylsulfonyl)benzyl)-1-(2-hydroxyethyl)-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 24
将粗品24b(14mg,0.038mmol),(4-(乙磺酰基)苯基)甲胺(10mg,0.046mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(11mg,0.057mmol),1-羟基苯并三唑(8mg,0.057mmol),三乙胺(19mg,0.19mmol)溶于二氯甲烷中,搅拌反应12小
时。加入40mL乙酸乙酯,用水(20mL),饱和氯化钠溶液(20mL)洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物24(9mg,白色固体),产率:43%。Crude 24b (14 mg, 0.038 mmol), (4-(ethylsulfonyl)phenyl)methanamine (10 mg, 0.046 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (11 mg, 0.057 mmol), 1-hydroxybenzotriazole (8 mg, 0.057 mmol), triethylamine (19 mg, 0.19 mmol) dissolved in dichloromethane.
Time. After adding 40 mL of ethyl acetate, it was washed with water (20 mL), EtOAc (EtOAc) Title product 24 (9 mg, white solid), yield: 43%.
MS m/z(ESI):545.5[M+1];MS m/z (ESI): 545.5 [M+1];
1HNMR(400MHz,CDCl3)δ8.05(s,1H),7.88-7.83(m,2H),7.65(d,1H),7.50-7.53(m,4H),7.32-7.34(m,3H),6.74(brs,1H),6.30(s,1H),4.77(d,2H),4.29(s,2H),4.19(t,2H),3.92(s,2H),3.85(t,2H),3.10(q,2H),1.27(t,3H)。 1 H NMR (400 MHz, CDCl 3 ) δ 8.05 (s, 1H), 7.88-7.83 (m, 2H), 7.65 (d, 1H), 7.50-7.53 (m, 4H), 7.32-7.34 (m, 3H) , 6.74 (brs, 1H), 6.30 (s, 1H), 4.77 (d, 2H), 4.29 (s, 2H), 4.19 (t, 2H), 3.92 (s, 2H), 3.85 (t, 2H), 3.10 (q, 2H), 1.27 (t, 3H).
实施例25Example 25
1-乙基-N-(4-(乙磺酰基)苄基)-2-((6-(三氟甲基)吡啶-3-基)甲基)-1H-吲哚-5-甲酰胺251-ethyl-N-(4-(ethylsulfonyl)benzyl)-2-((6-(trifluoromethyl)pyridin-3-yl)methyl)-1H-indole-5-carboxamide 25
第一步first step
2-((6-(三氟甲基)吡啶-3-基)甲基)-1H-吲哚-5-甲酸甲酯25b2-((6-(Trifluoromethyl)pyridin-3-yl)methyl)-1H-indole-5-carboxylic acid methyl ester 25b
将1a(300mg,1.71mmol),5-(溴甲基)-2-(三氟甲基)吡啶25a(431.58mg,1.8mmol),双乙腈二氯化钯(44.43mg,0.17mmol),降冰片烯(322.48mg,3.42mmol),碳酸氢钠(281.71mg,3.42mmol)溶于N,N-二甲基乙酰胺中,升至70℃搅拌反应12小时。反应液冷却至室温,减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得标题产物25b(480mg,浅黄色固体),产率:84%。1a (300 mg, 1.71 mmol), 5-(bromomethyl)-2-(trifluoromethyl)pyridine 25a (431.58 mg, 1.8 mmol), diacetonitrile palladium dichloride (44.43 mg, 0.17 mmol) The borneol (322.48 mg, 3.42 mmol), sodium hydrogencarbonate (281.71 mg, 3.42 mmol) was dissolved in N,N-dimethylacetamide, and the mixture was stirred at 70 ° C for 12 hours. The reaction mixture was cooled to EtOAc EtOAc m.
第二步Second step
1-乙基2-((6-(三氟甲基)吡啶-3-基)甲基)-1H-吲哚-5-甲酸甲酯25c1-ethyl 2-((6-(trifluoromethyl)pyridin-3-yl)methyl)-1H-indole-5-carboxylic acid methyl ester 25c
将25b(50mg,2.149mmol)溶于5mL乙腈中,加入碘乙烷(116.64mg,0.748mmol)和碳酸钾(41.34mg,0.3mmol),50℃搅拌反应12小时。反应液冷却至室温,
过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得标题产物25c(36mg,浅黄色固体),产率:66%。25b (50 mg, 2.149 mmol) was dissolved in 5 mL of acetonitrile, ethyl iodide (116.64 mg, 0.748 mmol) and potassium carbonate (41.34 mg, 0.3 mmol) were added, and the reaction was stirred at 50 ° C for 12 hours. The reaction solution was cooled to room temperature.
Filtration, the filtrate was concentrated under reduced pressure. EtOAcjjjjjjjjj
第三步third step
1-乙基2-((6-(三氟甲基)吡啶-3-基)甲基)-1H-吲哚-5-甲酸25d1-ethyl 2-((6-(trifluoromethyl)pyridin-3-yl)methyl)-1H-indole-5-carboxylic acid 25d
将25c(36mg,0.1mmol)溶于3mL甲醇中,加入2M氢氧化钠溶液1mL,70℃搅拌反应2小时。反应液冷却至室温,减压浓缩,向所得残余物中加入水,滴加浓盐酸至pH为4-5,用乙酸乙酯萃取(30mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩得到粗品标题产物25d(27mg,浅黄色油状物),产品不经纯化直接进行下一步反应。25c (36 mg, 0.1 mmol) was dissolved in 3 mL of methanol, 1 mL of 2M sodium hydroxide solution was added, and the reaction was stirred at 70 ° C for 2 hours. The reaction solution was cooled to room temperature, and concentrated under reduced pressure. Water was evaporated, evaporated, evaporated, evaporated. The solution was washed with EtOAc EtOAc (EtOAc m.
第四步the fourth step
1-乙基-N-(4-(乙磺酰基)苄基)-2-((6-(三氟甲基)吡啶-3-基)甲基)-1H-吲哚-5-甲酰胺251-ethyl-N-(4-(ethylsulfonyl)benzyl)-2-((6-(trifluoromethyl)pyridin-3-yl)methyl)-1H-indole-5-carboxamide 25
将粗品25d(27mg,0.077mmol),(4-(乙磺酰基)苯基)甲胺(23.17mg,0.116mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(29.7mg,0.155mmol),1-羟基苯并三唑(21mg,0.155mmol),三乙胺(15.69mg,0.155mmol)溶于二氯甲烷中,搅拌反应12小时。将反应液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物25(22mg,浅黄色固体),产率:54%。Crude 25d (27 mg, 0.077 mmol), (4-(ethylsulfonyl)phenyl)methanamine (23.17 mg, 0.116 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbazide The amine hydrochloride (29.7 mg, 0.155 mmol), 1-hydroxybenzotriazole (21 mg, 0.155 mmol), triethylamine (15.69 mg, 0.155 mmol) was dissolved in dichloromethane. The reaction mixture was concentrated under reduced vacuo.
MS m/z(ESI):530.3[M+1];MS m/z (ESI): 530.3 [M + 1];
1HNMR(400MHz,CDCl3)δ8.68(s,1H),8.07(s,1H),7.87(d,2H),7.64-7.72(m,3H),7.57(d,2H),7.35(d,1H),6.64(t,1H),6.29(s,1H),4.79(d,2H),4.23(s,2H),4.11(q,2H),3.11(q,2H),1.26-1.30(m,6H)。 1 H NMR (400 MHz, CDCl 3 ) δ 8.68 (s, 1H), 8.07 (s, 1H), 7.87 (d, 2H), 7.64 - 7.72 (m, 3H), 7.57 (d, 2H), 7.35 (d) , 1H), 6.64 (t, 1H), 6.29 (s, 1H), 4.79 (d, 2H), 4.23 (s, 2H), 4.11 (q, 2H), 3.11 (q, 2H), 1.26-1.30 ( m, 6H).
实施例26Example 26
1-乙基-N-(4-(乙磺酰基)苄基)-2-(4-(三氟甲基)苯甲酰基)-1H-吲哚-5-甲酰胺261-ethyl-N-(4-(ethylsulfonyl)benzyl)-2-(4-(trifluoromethyl)benzoyl)-1H-indole-5-carboxamide 26
第一步first step
1-乙基-2-(4-(三氟甲基)苯甲酰基)-1H-吲哚-5-甲酸甲酯26aMethyl 1-ethyl-2-(4-(trifluoromethyl)benzoyl)-1H-indole-5-carboxylate 26a
将19d(180mg,0.5mmol)溶于20mL1,4-二氧六环中,加入二氧化锰(2.18g,25mmol),100℃搅拌反应12小时,补加二氧化锰(2.18g,25mmol),100℃搅拌反应36小时。反应液冷却至室温,硅藻土过滤,滤饼用乙酸乙酯洗涤,滤液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物26a(50mg,白色固体),产率:27%。19d (180 mg, 0.5 mmol) was dissolved in 20 mL of 1,4-dioxane, manganese dioxide (2.18 g, 25 mmol) was added, and the reaction was stirred at 100 ° C for 12 hours, and manganese dioxide (2.18 g, 25 mmol) was added. The reaction was stirred at 100 ° C for 36 hours. The reaction mixture was cooled to room temperature, EtOAc (EtOAc m.) Yield: 27%.
第二步Second step
1-乙基-2-(4-(三氟甲基)苯甲酰基)-1H-吲哚-5-甲酸26b1-ethyl-2-(4-(trifluoromethyl)benzoyl)-1H-indole-5-carboxylic acid 26b
将26a(15mg,0.04mmol)溶于5mL甲醇中,加入1M氢氧化钠溶液0.6mL,60℃搅拌反应12小时。反应液冷却至室温,减压浓缩除去甲醇,向所得残余物中加入2mL四氢呋喃,滴加1M盐酸至pH为4-5,减压浓缩,得到粗品标题产物26b(14mg,白色固体),产品不经纯化直接进行下一步反应。26a (15 mg, 0.04 mmol) was dissolved in 5 mL of methanol, 0.6 mL of 1 M sodium hydroxide solution was added, and the reaction was stirred at 60 ° C for 12 hours. The reaction mixture was cooled to room temperature, and the mixture was evaporated to dryness.mjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjj The next reaction was carried out directly by purification.
第三步third step
1-乙基-N-(4-(乙磺酰基)苄基)-2-(4-(三氟甲基)苯甲酰基)-1H-吲哚-5-甲酰胺261-ethyl-N-(4-(ethylsulfonyl)benzyl)-2-(4-(trifluoromethyl)benzoyl)-1H-indole-5-carboxamide 26
将粗品26b(14mg,0.04mmol),(4-(乙磺酰基)苯基)甲胺(10mg,0.048mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(14mg,0.072mmol),1-羟基苯并三唑(10mg,0.072mmol),三乙胺(16mg,0.16mmol)溶于二氯甲烷中,搅拌反应12小时。加入20mL乙酸乙酯,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物26(16mg,浅黄色固体),产率:73%。Crude 26b (14 mg, 0.04 mmol), (4-(ethylsulfonyl)phenyl)methanamine (10 mg, 0.048 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (14 mg, 0.072 mmol), 1-hydroxybenzotriazole (10 mg, 0.072 mmol), triethylamine (16 mg, 0.16 mmol) was dissolved in dichloromethane. After adding 20 mL of ethyl acetate, the mixture was washed with EtOAc EtOAc EtOAc (HHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHH Light yellow solid), Yield: 73%.
MS m/z(ESI):543.3[M+1];MS m/z (ESI): 543.3 [M+1];
1HNMR(400MHz,CDCl3)δ8.22(s,1H),8.00(d,2H),7.86-7.91(m,3H),7.79-7.81(d,2H),7.58-7.53(m,3H),7.07(s,1H),6.67-6.69(m,1H),4.80(d,2H),4.67(q,2H),3.11(q,2H),1.51(t,3H),1.29(t,3H)。 1 H NMR (400 MHz, CDCl 3 ) δ 8.22 (s, 1H), 8.00 (d, 2H), 7.86-7.91 (m, 3H), 7.79-7.81 (d, 2H), 7.58-7.53 (m, 3H) , 7.07 (s, 1H), 6.67-6.69 (m, 1H), 4.80 (d, 2H), 4.67 (q, 2H), 3.11 (q, 2H), 1.51 (t, 3H), 1.29 (t, 3H) ).
实施例27
Example 27
1-环丙基-N-(4-(乙磺酰基)苄基)-2-(2-氟-4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺271-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-(2-fluoro-4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 27
第一步first step
1-环丙基-2-(2-氟-4-(三氟甲基)苄基)-1H-吲哚-5-甲酸甲酯27aMethyl 1-cyclopropyl-2-(2-fluoro-4-(trifluoromethyl)benzyl)-1H-indole-5-carboxylate 27a
将15c(150mg,0.427mmol)溶于1,2-二氯乙烷中,分别加入环丙基硼酸(183.39mg,2.13mmol),一水合醋酸铜(15.51mg,0.085mmol),2,2'-联吡啶(133.38mg,0.854mmol)和碳酸钠(90.51mg,0.853mmol),升温至70℃搅拌反应12小时。反应液冷却至室温,加入二氯甲烷,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得到标题产物27a(96mg,无色油状物),产率:57%。15c (150 mg, 0.427 mmol) was dissolved in 1,2-dichloroethane, respectively, cyclopropylboronic acid (183.39 mg, 2.13 mmol), copper acetate monohydrate (15.51 mg, 0.085 mmol), 2, 2' Bipyridine (133.38 mg, 0.854 mmol) and sodium carbonate (90.51 mg, 0.853 mmol) were heated to 70 ° C and stirred for 12 hours. The reaction mixture was cooled to room temperature, dichloromethane was added, EtOAcjjjjjjjjjjjjjjjjjj 57%.
第二步Second step
1-环丙基-2-(2-氟-4-(三氟甲基)苄基)-1H-吲哚-5-甲酸27b1-cyclopropyl-2-(2-fluoro-4-(trifluoromethyl)benzyl)-1H-indole-5-carboxylic acid 27b
将27a(96mg,0.254mmol)溶于4mL甲醇和水(V:V=1:1)的混合溶剂中,加入氢氧化锂(13mg,0.508mmol),升温至70℃搅拌反应2小时。反应液冷却至室温,减压浓缩,向所得残余物中加入水,滴加浓盐酸至pH为4-5,用乙酸乙酯萃取(30mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得到粗品标题产物27b(31mg,浅黄色油状物),产品不经纯化直接进行下一步反应。27a (96 mg, 0.254 mmol) was dissolved in 4 mL of a mixed solvent of methanol and water (V: V = 1:1), and lithium hydroxide (13 mg, 0.508 mmol) was added thereto, and the mixture was heated to 70 ° C and stirred for 2 hours. The reaction solution was cooled to room temperature, and concentrated under reduced pressure. Water was evaporated, evaporated, evaporated, evaporated. The solution was washed with EtOAc EtOAc EtOAc.
第三步third step
1-环丙基-N-(4-(乙磺酰基)苄基)-2-(2-氟-4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺271-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-(2-fluoro-4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 27
将粗品27b(31mg,0.082mmol),(4-(乙磺酰基)苯基)甲胺(19.6mg,0.099mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(31.5mg,0.164mmol),1-羟基
苯并三唑(22.2mg,0.164mmol),三乙胺(16.6mg,0.164mmol)溶于二氯甲烷中,搅拌反应12小时。将反应液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物27(13mg,白色固体),产率:28%。Crude 27b (31 mg, 0.082 mmol), (4-(ethylsulfonyl)phenyl)methanamine (19.6 mg, 0.099 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbazide Amine hydrochloride (31.5 mg, 0.164 mmol), 1-hydroxyl
Benzotriazole (22.2 mg, 0.164 mmol), triethylamine (16.6 mg, 0.164 mmol) was dissolved in dichloromethane and stirred for 12 hr. The reaction mixture was concentrated under reduced vacuo. EtOAcjjjjjjj
MS m/z(ESI):559.4[M+1];MS m/z (ESI): 559.4 [M+1];
1HNMR(400MHz,CDCl3)δ8.06(s,1H),7.87(d,2H),7.72(d,1H),7.56-7.62(m,3H),7.40(t,2H),7.28(t,1H),6.79(t,1H),6.28(s,1H),4.81(d,2H),4.36(s,2H),3.13(q,2H),3.02-3.07(m,1H),1.30(t,3H),1.20-1.25(m,2H),1.07-1.11(m,2H)。 1 H NMR (400 MHz, CDCl 3 ) δ 8.06 (s, 1H), 7.78 (d, 2H), 7.72 (d, 1H), 7.56-7.62 (m, 3H), 7.40 (t, 2H), 7.28 (t) , 1H), 6.79 (t, 1H), 6.28 (s, 1H), 4.81 (d, 2H), 4.36 (s, 2H), 3.13 (q, 2H), 3.02-3.07 (m, 1H), 1.30 ( t, 3H), 1.20-1.25 (m, 2H), 1.07-1.11 (m, 2H).
实施例28Example 28
1-环丙基-N-(4-(乙磺酰基)苄基)-2-(4-(三氟甲氧基)苄基)-1H-吲哚-5-甲酰胺281-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-(4-(trifluoromethoxy)benzyl)-1H-indole-5-carboxamide 28
第一步first step
1-环丙基-2-(4-(三氟甲氧基)苄基)-1H-吲哚-5-甲酸甲酯28aMethyl 1-cyclopropyl-2-(4-(trifluoromethoxy)benzyl)-1H-indole-5-carboxylate 28a
将18c(100mg,0.286mmol)溶于二氯甲烷中,分别加入环丙基硼酸(126.12mg,1.43mmol),醋酸铜(10.4mg,0.057mmol),2,2'-联吡啶(223.5mg,1.43mmol)和碳酸钠(60.7mg,1.572mmol),升温至70℃搅拌反应12小时。将反应液冷却至室温,减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得到标题产物28a(71mg,浅黄色油状物),产率:64%。18c (100 mg, 0.286 mmol) was dissolved in dichloromethane and cyclopropylboronic acid (126.12 mg, 1.43 mmol), copper acetate (10.4 mg, 0.057 mmol), 2,2'-bipyridine (223.5 mg, 1.43 mmol) and sodium carbonate (60.7 mg, 1.572 mmol) were heated to 70 ° C and stirred for 12 hours. The reaction mixture was cooled to EtOAc EtOAc m.
第二步Second step
1-环丙基-2-(4-(三氟甲氧基)苄基)-1H-吲哚-5-甲酸28b1-cyclopropyl-2-(4-(trifluoromethoxy)benzyl)-1H-indole-5-carboxylic acid 28b
将28a(71mg,0.182mmol)溶于3mL甲醇中,加入3mL水,加入氢氧化钾(35.5mg,0.91mmol),室温搅拌反应12小时。将反应液减压浓缩,向所得残余物
中加入水,滴加浓盐酸至pH为5-6,用乙酸乙酯萃取(20mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得到粗品标题产物28b(56mg,白色固体),产品不经纯化直接进行下一步反应。28a (71 mg, 0.182 mmol) was dissolved in 3 mL of methanol, 3 mL of water was added, and potassium hydroxide (35.5 mg, 0.91 mmol) was added, and the reaction was stirred at room temperature for 12 hours. The reaction solution was concentrated under reduced pressure to give a residue.
Add water, add concentrated hydrochloric acid to pH 5-6, extract with ethyl acetate (20 mL×3), combine the organic phase, wash with water, saturated sodium chloride solution, dry over anhydrous sodium sulfate, Concentration gave the crude title product 28b (56 mg, white solid).
第三步third step
1-环丙基-N-(4-(乙磺酰基)苄基)-2-(4-(三氟甲氧基)苄基)-1H-吲哚-5-甲酰胺281-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-(4-(trifluoromethoxy)benzyl)-1H-indole-5-carboxamide 28
将粗品28b(56mg,0.149mmol),(4-(乙磺酰基)苯基)甲胺(44.6mg,0.223mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(57.2mg,0.298mmol),1-羟基苯并三唑(40.3mg,0.298mmol),三乙胺(30.2mg,0.298mmol)溶于二氯甲烷中,搅拌反应12小时。将反应液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得到标题产物28(36mg,白色固体),产率:43%。Crude 28b (56 mg, 0.149 mmol), (4-(ethylsulfonyl)phenyl)methanamine (44.6 mg, 0.223 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbazide Amine hydrochloride (57.2 mg, 0.298 mmol), 1-hydroxybenzotriazole (40.3 mg, 0.298 mmol), triethylamine (30.2 mg, 0.298 mmol) was dissolved in dichloromethane. The reaction mixture was concentrated under reduced EtOAc.
MS m/z(ESI):557.3[M+1];MS m/z (ESI): 557.3 [M+1];
1HNMR(400MHz,CDCl3)δ8.02(s,1H),7.87(d,2H),7.67(d,1H),7.56(d,3H),7.25(d,2H),7.16(d,2H),6.64(t,1H),6.26(s,1H),4.79(d,2H),4.25(s,2H),3.10(q,2H),2.92-2.96(m,1H),1.28(t,3H),1.13-1.17(m,2H),1.02-1.06(m,2H)。 1 H NMR (400 MHz, CDCl 3 ) δ 8.02 (s, 1H), 7.87 (d, 2H), 7.67 (d, 1H), 7.56 (d, 3H), 7.25 (d, 2H), 7.16 (d, 2H) ), 6.64(t,1H), 6.26(s,1H), 4.79(d,2H), 4.25(s,2H),3.10(q,2H),2.92-2.96(m,1H),1.28(t, 3H), 1.13-1.17 (m, 2H), 1.02-1.06 (m, 2H).
实施例29Example 29
1-环丙基-N-((1-(乙磺酰基)哌啶-4-基)甲基)-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺291-cyclopropyl-N-((1-(ethylsulfonyl)piperidin-4-yl)methyl)-2-(4-(trifluoromethyl)benzyl)-1H-indole-5- Formamide 29
第一步first step
4-((1-环丙基-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰氨基)甲基)哌啶-1-甲酸叔丁酯29b4-((1-Cyclopropyl-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamido)methyl)piperidine-1-carboxylic acid tert-butyl ester 29b
将20b(23mg,0.064mmol),4-(氨基甲基)哌啶-1-甲酸叔丁酯29a(21mg,0.096
mmol,采用公知的方法“Bioorganic&Medicinal Chemistry,2002,10(5),1347-1359”制备而得),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(18mg,0.096mmol),1-羟基苯并三唑(13mg,0.096mmol)和N,N-二异丙基乙胺(42mg,0.32mmol)溶于N,N-二甲基甲酰胺中,搅拌反应12小时。向反应液中加入水,再用二氯甲烷和甲醇混合溶剂(V:V=8:1)萃取(20mL×3),饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系A纯化所得残余物,得到标题产物29b(35.0mg,产率:98%)。20b (23 mg, 0.064 mmol), tert-butyl 4-(aminomethyl)piperidine-1-carboxylate 29a (21 mg, 0.096
Ment, prepared by the well-known method "Bioorganic & Medicinal Chemistry, 2002, 10 (5), 1347-1359"), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride ( 18 mg, 0.096 mmol), 1-hydroxybenzotriazole (13 mg, 0.096 mmol) and N,N-diisopropylethylamine (42 mg, 0.32 mmol) dissolved in N,N-dimethylformamide, stirred Reaction for 12 hours. Water was added to the reaction mixture, and the mixture was extracted with a mixed solvent of dichloromethane and methanol (V:V=8:1) (20 mL×3), washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate The residue was purified by EtOAc EtOAc (EtOAc)
MS m/z(ESI):554.2[M-1];MS m/z (ESI): 554.2 [M-1];
第二步Second step
1-环丙基-N-(哌啶-4-基甲基)-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺29c1-cyclopropyl-N-(piperidin-4-ylmethyl)-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 29c
将29b(350mg,0.063mmol)溶于5mL二氯甲烷中,再向反应液中加入1mL三氟乙酸,搅拌反应1小时,向反应液中加入饱和碳酸氢钠至中性,再用二氯甲烷和甲醇混合溶剂(V:V=8:1)萃取(20mL×3),饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得粗品标题产物29c(25mg),产品不经纯化直接用于下一步反应。29b (350mg, 0.063mmol) was dissolved in 5mL of dichloromethane, 1mL of trifluoroacetic acid was added to the reaction solution, and the reaction was stirred for 1 hour. Saturated sodium hydrogencarbonate was added to the reaction solution to neutral, and then dichloromethane was used. It is extracted with a methanol mixed solvent (V: V = 8:1) (20 mL × 3), washed with a saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered, and the filtrate is concentrated under reduced pressure to give crude title product 29c (25 mg) It was used in the next reaction without purification.
MS m/z(ESI):456.3[M+1];MS m/z (ESI): 456.3 [M + 1];
第三步third step
1-环丙基-N-((1-(乙磺酰基)哌啶-4-基)甲基)-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺291-cyclopropyl-N-((1-(ethylsulfonyl)piperidin-4-yl)methyl)-2-(4-(trifluoromethyl)benzyl)-1H-indole-5- Formamide 29
依次将粗品29c(25mg,0.055mmol),三乙胺(8.5mg,0.082mmol)和乙磺酰氯29d(8mg,0.066mmol,采用公知的方法“Journal of Organic Chemistry,2007,72(15),5847-5850”制备而得)溶于5mL二氯甲烷中,搅拌反应3小时。加入饱和碳酸氢钠溶液,用二氯甲烷和甲醇(V:V=8:1)混合溶剂萃取(20mL×3),合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系A纯化所得残余物,得到标题产物29(27mg,产率:90%)。The crude product 29c (25 mg, 0.055 mmol), triethylamine (8.5 mg, 0.082 mmol) and ethanesulfonyl chloride 29d (8 mg, 0.066 mmol, by the known method "Journal of Organic Chemistry, 2007, 72 (15), 5847). -5850" was prepared) dissolved in 5 mL of dichloromethane and stirred for 3 hours. Adding a saturated sodium hydrogencarbonate solution, and extracting with a mixed solvent of dichloromethane and methanol (V: V = 8:1) (20 mL × 3), and the organic phase is combined, washed with a saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered The filtrate was concentrated under reduced vacuo.
MS m/z(ESI):548.5[M+1]MS m/z (ESI): 548.5 [M+1]
1H NMR(400MHz,DMSO-d6)δ8.39(s,1H),8.02(s,1H),7.66-7.71(m,2H),7.63-7.64(d,1H),7.48-7.51(m,3H),6.22(s,1H),4.34(s,2H),3.58(d,2H),3.17(t,2H),3.01(q,2H),2.90-2.98(m,1H),2.75(t,2H),1.73(m,3H),1.12-1.33(m,7H),0.98(t,2H)。 1 H NMR (400 MHz, DMSO-d 6 ) δ 8.39 (s, 1H), 8.02 (s, 1H), 7.66-7.71 (m, 2H), 7.63-7.64 (d, 1H), 7.48-7.51 (m) , 3H), 6.22 (s, 1H), 4.34 (s, 2H), 3.58 (d, 2H), 3.17 (t, 2H), 3.01 (q, 2H), 2.90-2.98 (m, 1H), 2.75 ( t, 2H), 1.73 (m, 3H), 1.12-1.33 (m, 7H), 0.98 (t, 2H).
实施例30Example 30
2-(4-氯-2-(三氟甲基)苄基)-N-(4-(乙磺酰基)苄基)-1-(2-氟乙基)-1H-吲哚-5-甲酰胺30
2-(4-Chloro-2-(trifluoromethyl)benzyl)-N-(4-(ethylsulfonyl)benzyl)-1-(2-fluoroethyl)-1H-indole-5- Formamide 30
第一步first step
2-(4-氯-2-(三氟甲基)苄基)-1-(2-氟乙基)-1H-吲哚-5-甲酸甲酯30aMethyl 2-(4-chloro-2-(trifluoromethyl)benzyl)-1-(2-fluoroethyl)-1H-indole-5-carboxylate 30a
将5b(1g,2.72mmol)溶于5mL N,N-二甲基甲酰胺中,再加入1-溴-2-氟乙烷(345.23mg,2.72mmol)和碳酸铯(1782.95mg,5.44mmol),100℃条件下,微波反应1小时。将反应液冷却至室温,减压浓缩,用硅胶柱色谱法以洗脱剂体系C纯化所得残余物,得到标题产物30a(300mg,产率:23.97%)。5b (1g, 2.72mmol) was dissolved in 5mL of N,N-dimethylformamide, then added 1-bromo-2-fluoroethane (345.23mg, 2.72mmol) and cesium carbonate (1782.95mg, 5.44mmol) The microwave was reacted at 100 ° C for 1 hour. The reaction mixture was cooled to room temperature, then evaporated, evaporated, evaporated
MS m/z(ESI):412.1[M-1]MS m/z (ESI): 412.1 [M-1]
第二步Second step
2-(4-氯-2-(三氟甲基)苄基)-1-(2-氟乙基)-1H-吲哚-5-甲酸30b2-(4-Chloro-2-(trifluoromethyl)benzyl)-1-(2-fluoroethyl)-1H-indole-5-carboxylic acid 30b
将30a(2g,4.83mmol)溶于110mL乙醇和水混合溶液中(V:V=3:8),加入氢氧化钠(580mg,14.5mmol),80℃条件下,反应1小时。反应液减压浓缩,加入30mL水,滴加1M盐酸至pH为2,用乙酸乙酯萃取(30mL×2),合并有机相,有机相用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得粗品标题产物30b(1500mg),产物不经纯化直接用于下一步反应。30a (2 g, 4.83 mmol) was dissolved in 110 mL of a mixed solution of ethanol and water (V: V = 3:8), and sodium hydroxide (580 mg, 14.5 mmol) was added thereto, and the mixture was reacted at 80 ° C for 1 hour. The reaction mixture was concentrated under reduced pressure. EtOAc (EtOAc m. Filtration and concentration of the filtrate under reduced pressure afforded crude title product 30b (1500 mg).
MS m/z(ESI):400.4[M-1]MS m/z (ESI): 400.4 [M-1]
第三步third step
2-(4-氯-2-(三氟甲基)苄基)-N-(4-(乙磺酰基)苄基)-1-(2-氟乙基)-1H-吲哚-5-甲酰胺302-(4-Chloro-2-(trifluoromethyl)benzyl)-N-(4-(ethylsulfonyl)benzyl)-1-(2-fluoroethyl)-1H-indole-5- Formamide 30
将粗品30b(1.5g,3.75mmol),(4-(乙磺酰基)苯基)甲胺(44.6mg,0.223mmol),2-(7-氧化苯并三氮唑)-N,N,N',N'-四甲基脲六氟磷酸酯(2138.73mg,5.63mmol),三乙胺(1136.9mg,11.26mmol)溶于30mL N,N-二甲基甲酰胺中,搅拌反应12小时。反应液中加入50mL水,乙酸乙酯萃取(50mL×2),合并有机相,有机相用饱和氯
化钠水溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用高效液相色谱法纯化所得残余物,得到标题产物30(1100mg,产率:49.45%)。Crude 30b (1.5 g, 3.75 mmol), (4-(ethylsulfonyl)phenyl)methanamine (44.6 mg, 0.223 mmol), 2-(7-benzobenzotriazole)-N,N,N ', N'-Tetramethylurea hexafluorophosphate (2138.73 mg, 5.63 mmol), triethylamine (1136.9 mg, 11.26 mmol) was dissolved in 30 mL of N,N-dimethylformamide, and the reaction was stirred for 12 hours. 50 mL of water was added to the reaction solution, and the mixture was extracted with ethyl acetate (50 mL×2), and the organic phase was combined.
The title compound (30 mg, yield: 49.45%) was obtained.
MS m/z(ESI):579.1[M-1]MS m/z (ESI): 579.1 [M-1]
1H NMR(400MHz,DMSO-d6)δ8.98-9.01(m,1H),8.05(s,1H),7.82-7.86(m,3H),7.69-7.73(m,2H),7.53-7.57(m,3H),7.31-7.33(m,1H),5.99(s,1H),4.71-4.72(m,1H),4.57-4.61(m,3H),4.44-4.52(m,2H),4.33(s,2H),3.22-3.27(m,2H),1.05-1.09(m,3H)。 1 H NMR (400MHz, DMSO- d 6) δ8.98-9.01 (m, 1H), 8.05 (s, 1H), 7.82-7.86 (m, 3H), 7.69-7.73 (m, 2H), 7.53-7.57 (m, 3H), 7.31-7.33 (m, 1H), 5.99 (s, 1H), 4.71-4.72 (m, 1H), 4.57-4.61 (m, 3H), 4.44-4.52 (m, 2H), 4.33 (s, 2H), 3.22-3.27 (m, 2H), 1.05-1.09 (m, 3H).
实施例31Example 31
1-环丙基-N-(4-(乙磺酰基)苄基)-2-((3-甲基-5-(三氟甲基)-1H-吡唑-1-基)甲基)-1H-吲哚-5-甲酰胺311-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-((3-methyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methyl) -1H-吲哚-5-carboxamide 31
第一步first step
2-(((叔丁基二甲基硅基)氧基)甲基)-1H-吲哚-5-甲酸乙酯31c2-(((tert-Butyldimethylsilyl)oxy)methyl)-1H-indole-5-carboxylic acid ethyl ester 31c
氩气氛下,依次将3-碘-4-(2,2,2-三氟乙酰氨基)苯甲酸乙酯31b(1.0g,2.58mmol,采用公知的方法“Journal of the Chemical Society,Perkin Transactions 1:Organic and Bio-Organic Chemistry,1997(14),2059-2063”制备而得)、叔丁基二甲基(丙-2-炔-1-基氧基)硅烷31a(660mg,3.88mmol,采用公知的方法“Journal of the American Chemical Society,2016,138(24),7532-7535”制备而得)、碘化亚铜(99mg,0.52mmol)、双三苯基磷二氯化钯(362mg,0.52mmol)和三乙胺(1.3g,12.9mmol)溶于N,N-二甲基甲酰胺中,加毕,升温至60℃搅拌反应3小时。反应液冷却至室
温,硅藻土过滤,滤液中加入水,用乙酸乙酯萃取(50mL×3),合并有机相,用水、饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得标题产物31c(460mg,产率:54%)。Under an argon atmosphere, ethyl 3-iodo-4-(2,2,2-trifluoroacetamido)benzoate 31b (1.0 g, 2.58 mmol, by a known method "Journal of the Chemical Society, Perkin Transactions 1" :Organic and Bio-Organic Chemistry, 1997 (14), 2059-2063 "prepared", tert-butyl dimethyl (prop-2-yn-1-yloxy) silane 31a (660 mg, 3.88 mmol, using A well-known method "Prepared by Journal of the American Chemical Society, 2016, 138(24), 7532-7535"), cuprous iodide (99 mg, 0.52 mmol), bistriphenylphosphine palladium dichloride (362 mg, 0.52 mmol) and triethylamine (1.3 g, 12.9 mmol) were dissolved in N,N-dimethylformamide, and the mixture was warmed to 60 ° C and stirred for 3 hours. The reaction solution is cooled to the chamber
The mixture was filtered with Celite (EtOAc) (EtOAc)EtOAc. The resulting residue was purified to silica gel elut elut elut elut elut elut elut
MS m/z(ESI):334.2[M+1];MS m/z (ESI): 334.2 [M + 1];
第二步Second step
2-(((叔丁基二甲基硅基)氧基)甲基)-1-环丙基-1H-吲哚-5-甲酸乙酯31dEthyl 2-(((tert-butyldimethylsilyl)oxy)methyl)-1-cyclopropyl-1H-indole-5-carboxylate 31d
将31c(460mg,1.4mmol)溶于1,2-二氯乙烷中,分别加入环丙基硼酸(178mg,2.1mmol),一水合醋酸铜(393mg,2.1mmol),2,2'-联吡啶(328mg,2.1mmol)和碳酸钠(223mg,2.1mmol),升温至70℃搅拌反应12小时。再补加环丙基硼酸(178mg,2.1mmol),一水合醋酸铜(393mg,2.1mmol),2,2'-联吡啶(328mg,2.1mmol)和碳酸钠(223mg,2.1mmol),搅拌反应6小时,将反应液冷却至室温,硅藻土过滤,滤液减压浓缩,残余物中加入水,用乙酸乙酯萃取(50mL×3),合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得标题产物31d(329mg,产率:64%)。31c (460 mg, 1.4 mmol) was dissolved in 1,2-dichloroethane, respectively, cyclopropylboronic acid (178 mg, 2.1 mmol), copper acetate monohydrate (393 mg, 2.1 mmol), 2,2'-linked Pyridine (328 mg, 2.1 mmol) and sodium carbonate (223 mg, 2.1 mmol) were heated to 70 ° C and stirred for 12 hours. Further, cyclopropylboronic acid (178 mg, 2.1 mmol), copper acetate monohydrate (393 mg, 2.1 mmol), 2,2'-bipyridine (328 mg, 2.1 mmol) and sodium carbonate (223 mg, 2.1 mmol) were added and stirred. After 6 hours, the reaction solution was cooled to room temperature, celite was filtered, filtered, evaporated, evaporated, evaporated, evaporated, evaporated The residue was dried over EtOAc (EtOAc m.)
第三步third step
1-环丙基-2-(羟甲基)-1H-吲哚-5-甲酸乙酯31f1-cyclopropyl-2-(hydroxymethyl)-1H-indole-5-carboxylic acid ethyl ester 31f
将31d(329mg,0.88mmol)溶于四氢呋喃中,滴加1mL 1M四丁基氟化铵,搅拌反应1小时。乙酸乙酯萃取(10mL×3),合并有机相,有机相减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得标题产物31f(120mg,产率:52%)。31 d (329 mg, 0.88 mmol) was dissolved in tetrahydrofuran, 1 mL of 1 M tetrabutylammonium fluoride was added dropwise, and the reaction was stirred for 1 hour. The ethyl acetate was extracted (10 mL × 3), EtOAc (EtOAc m.
第四步the fourth step
1-环丙基-2-((3-甲基-5-(三氟甲基)-1H-吡唑-1-基)甲基)-1H-吲哚-5-甲酸乙酯31g1-cyclopropyl-2-((3-methyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methyl)-1H-indole-5-carboxylic acid ethyl ester 31 g
氩气氛下,将31f(120mg,0.46mmol)、3-甲基-5-(三氟甲基)-1H-吡唑31e(104mg,0.69mmol,采用公知的方法“Tetrahedron Letters,2016,57(14),1555-1559”制备而得)和三苯基膦(181mg,0.69mmol)溶于10mL四氢呋喃,再加入偶氮二甲酸二乙酯(120mg,0.69mmol),加毕,搅拌反应12小时。反应液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,再用薄层色谱法纯化以展开剂体系B纯化所得粗品,得标题产物31g(60mg,产率:33.1%)。Under an argon atmosphere, 31f (120 mg, 0.46 mmol), 3-methyl-5-(trifluoromethyl)-1H-pyrazole 31e (104 mg, 0.69 mmol, using a known method "Tetrahedron Letters, 2016, 57 ( 14), 1555-1559 "prepared" and triphenylphosphine (181 mg, 0.69 mmol) dissolved in 10 mL of tetrahydrofuran, then added diethyl azodicarboxylate (120 mg, 0.69 mmol), added, stirred for 12 hours . The reaction mixture was concentrated under reduced pressure. EtOAcjjjjjjjjjjj ).
MS m/z(ESI):390.1[M-1];MS m/z (ESI): 390.1 [M-1];
第五步the fifth step
1-环丙基-2-((3-甲基-5-(三氟甲基)-1H-吡唑-1-基)甲基)-1H-吲哚-5-甲酸31h1-cyclopropyl-2-((3-methyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methyl)-1H-indole-5-carboxylic acid 31h
将31g(60mg,0.15mmol)溶于6mL甲醇和四氢呋喃混合溶液中(V:V=5:1),加入2mL的4M氢氧化钠溶液,60℃条件下,反应3小时。滴加浓盐酸至pH为3,用乙酸乙酯萃取(30mL×3),合并有机相,有机相用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得粗品标题产物31h(55mg),产物不经纯化直接用于下一步反应。
31 g (60 mg, 0.15 mmol) was dissolved in 6 mL of a mixed solution of methanol and tetrahydrofuran (V: V = 5:1), and 2 mL of a 4 M sodium hydroxide solution was added thereto, and the mixture was reacted at 60 ° C for 3 hours. Concentrated hydrochloric acid was added dropwise to pH 3, which was extracted with ethyl acetate (30 mL×3). The organic phase was combined and evaporated. Product 31 h (55 mg).
MS m/z(ESI):362.1[M-1];MS m/z (ESI): 362.1 [M-1];
第六步Step 6
1-环丙基-N-(4-(乙磺酰基)苄基)-2-((3-甲基-5-(三氟甲基)-1H-吡唑-1-基)甲基)-1H-吲哚-5-甲酰胺311-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-((3-methyl-5-(trifluoromethyl)-1H-pyrazol-1-yl)methyl) -1H-吲哚-5-carboxamide 31
将粗品31h(30mg,0.083mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(24mg,0.12mmol),1-羟基苯并三唑(17mg,0.12mmol),N,N-二异丙基乙胺(53mg,0.41mmol)溶于二氯甲烷中,滴加31i(4-(乙磺酰基)苯基)甲胺盐酸盐(44.6mg,0.223mmol,采用专利申请“US20150291607A1”公开的方法制备而得),加毕,搅拌反应12小时。反应液中加入水,用二氯甲烷和甲醇(V:V=8:1)混合溶剂萃取(20mL×3),合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系A纯化所得残余物,得到标题产物31(30mg,产率:66%)。31h (30mg, 0.083mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (24mg, 0.12mmol), 1-hydroxybenzotriazole (17mg, 0.12 mmol), N,N-diisopropylethylamine (53 mg, 0.41 mmol) was dissolved in dichloromethane, and <RTI ID=0.0>> 0.223 mmol was prepared by the method disclosed in the patent application "US20150291607A1", and the reaction was stirred for 12 hours. Water was added to the reaction mixture, and the mixture was extracted with a mixture of dichloromethane and methanol (V:V=8:1) (20 mL×3). The organic phase was combined, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated under reduced pressure. EtOAcjjjjjjjj
MS m/z(ESI):545.5[M+1]MS m/z (ESI): 545.5 [M+1]
1H NMR(400MHz,DMSO-d6)δ9.05(t,1H),8.11(s,1H),7.84(d,2H),7.75(d,1H),7.57-7.61(t,3H),6.79(s,1H),6.16(s,1H),5.72(s,2H),4.59(d,2H),3.27(q,2H),3.20-3.23(m,1H),2.21(s,3H),1.15-1.21(m,2H),1.09(t,3H),0.98-1.05(m,2H)。 1 H NMR (400MHz, DMSO- d 6) δ9.05 (t, 1H), 8.11 (s, 1H), 7.84 (d, 2H), 7.75 (d, 1H), 7.57-7.61 (t, 3H), 6.79 (s, 1H), 6.16 (s, 1H), 5.72 (s, 2H), 4.59 (d, 2H), 3.27 (q, 2H), 3.20-3.23 (m, 1H), 2.21 (s, 3H) , 1.15 - 1.21. (m, 2H), 1.09 (t, 3H), 0.98 - 1.05 (m, 2H).
实施例32Example 32
1-环丙基-N-(4-(乙磺酰基)苄基)-2-((5-(三氟甲基)-1H-吡唑-1-基)甲基)-1H-吲哚-5-甲酰胺321-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-((5-(trifluoromethyl)-1H-pyrazol-1-yl)methyl)-1H-indole -5-carboxamide 32
第一步first step
2-(((叔丁基二甲基硅基)氧基)甲基)-1H-吲哚-5-甲酸甲酯32b2-(((tert-Butyldimethylsilyl)oxy)methyl)-1H-indole-5-carboxylic acid methyl ester 32b
氩气氛下,依次将3-碘-4-(2,2,2-三氟乙酰氨基)苯甲酸甲酯32a(13.0g,34.85mmol,采用公知的方法“Journal of Medicinal Chemistry,2005,48(5),1314-1317”制备而得)、31a(8.9g,52.27mmol)、碘化亚铜(1.33g,6.97mmol)、双三苯基磷二氯化钯(4.89g,6.97mmol)和三乙胺(17.63g,174.23mmol)溶于150mL N,N-二甲基甲酰胺中,加毕,升温至60℃搅拌反应3小时。反应液中加入水,用乙酸乙酯萃取(50mL×3),合并有机相,用水、饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得标题产物32b(7.0g,产率:63%)。Under an argon atmosphere, methyl 3-iodo-4-(2,2,2-trifluoroacetamido)benzoate 32a (13.0 g, 34.85 mmol, using a known method "Journal of Medicinal Chemistry, 2005, 48" 5), 1314-1317 "prepared", 31a (8.9 g, 52.27 mmol), cuprous iodide (1.33 g, 6.97 mmol), bistriphenylphosphine palladium dichloride (4.89 g, 6.97 mmol) and Triethylamine (17.63 g, 174.23 mmol) was dissolved in 150 mL of N,N-dimethylformamide, and the mixture was warmed to 60 ° C and stirred for 3 hours. Water was added to the reaction mixture, and the mixture was evaporated. EtOAcjjjjjjjjjjjjjjjj The obtained residue was purified to give the titled product 32b (yield: (yield: 63%).
第二步Second step
2-(((叔丁基二甲基硅基)氧基)甲基)-1-环丙基-1H-吲哚-5-甲酸甲酯32c2-(((tert-Butyldimethylsilyl)oxy)methyl)-1-cyclopropyl-1H-indole-5-carboxylic acid methyl ester 32c
将32b(956mg,2.99mmol)、环丙基硼酸(1.3g,14.96mmol)、醋酸铜(1.14g,6.28mmol)、2,2’-联吡啶(1.03g,6.58mmol)和碳酸钠(698mg,6.58mmol)溶于1,2-二氯乙烷中,升温至70℃搅拌反应12小时。反应液冷却至室温,减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得标题产物32c(500mg,产率:46%)。32b (956 mg, 2.99 mmol), cyclopropylboronic acid (1.3 g, 14.96 mmol), copper acetate (1.14 g, 6.28 mmol), 2,2'-bipyridine (1.03 g, 6.58 mmol) and sodium carbonate (698 mg) , 6.58 mmol) was dissolved in 1,2-dichloroethane, and the mixture was heated to 70 ° C and stirred for 12 hours. The reaction mixture was cooled to room temperature, and then evaporated.
第三步third step
1-环丙基-2-(羟甲基)-1H-吲哚-5-甲酸甲酯32d1-cyclopropyl-2-(hydroxymethyl)-1H-indole-5-carboxylic acid methyl ester 32d
将32c(500mg,1.39mmol)溶于5mL四氢呋喃中,降温至0℃,滴加2.8mL1M四丁基氟化铵四氢呋喃溶液,滴加完毕,搅拌0.5小时。向反应液中加入水,水相用乙酸乙酯萃取(50mL×3),合并有机相,有机相用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得粗品标题产物32d(300mg)。产品不经纯化,直接投下一步反应。
32c (500 mg, 1.39 mmol) was dissolved in 5 mL of tetrahydrofuran, and the mixture was cooled to 0 ° C. 2.8 mL of 1M tetrabutylammonium fluoride tetrahydrofuran solution was added dropwise, and the mixture was stirred for 0.5 hour. Water was added to the reaction mixture, and the aqueous layer was extracted with ethyl acetate (50 mL×3). Title product 32d (300 mg). The product was directly subjected to the next reaction without purification.
第四步the fourth step
1-环丙基-2-((5-(三氟甲基)-1H-吡唑-1-基)甲基)-1H-吲哚-5-甲酸甲酯32f1-cyclopropyl-2-((5-(trifluoromethyl)-1H-pyrazol-1-yl)methyl)-1H-indole-5-carboxylic acid methyl ester 32f
氩气氛下,将粗品32d(120mg,0.53mmol)、3-甲基-1H-吡唑32e(104mg,0.69mmol)和三苯基膦(208mg,0.795mmol)溶于3mL四氢呋喃,再加入偶氮二甲酸二乙酯(138mg,0.795mmol),加毕,搅拌反应12小时。反应液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得标题产物32f(51mg,产率:26%)。The crude product 32d (120mg, 0.53mmol), 3-methyl-1H-pyrazole 32e (104mg, 0.69mmol) and triphenylphosphine (208mg, 0.795mmol) were dissolved in 3mL of tetrahydrofuran under argon, then azo was added. Diethyl dicarboxylate (138 mg, 0.795 mmol) was added and the reaction was stirred for 12 hours. The reaction mixture was concentrated under reduced pressure. EtOAcjjjjjjj
第五步the fifth step
1-环丙基-2-((5-(三氟甲基)-1H-吡唑-1-基)甲基)-1H-吲哚-5-甲酸32g1-cyclopropyl-2-((5-(trifluoromethyl)-1H-pyrazol-1-yl)methyl)-1H-indole-5-carboxylic acid 32g
将32f(51mg,0.14mmol)溶于2mL甲醇溶液中,加入1.4mL的2M氢氧化钾溶液,70℃条件下,反应3小时。反应液冷却至室温,滴加1M盐酸至pH为1-2,用二氯甲烷萃取(30mL×3),合并有机相,有机相用水和饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得粗品标题产物32g(45mg),产物不经纯化直接用于下一步反应。32f (51 mg, 0.14 mmol) was dissolved in 2 mL of a methanol solution, and 1.4 mL of a 2 M potassium hydroxide solution was added thereto, and the mixture was reacted at 70 ° C for 3 hours. The reaction solution was cooled to room temperature, and 1 M hydrochloric acid was added dropwise to pH 1-2, and extracted with dichloromethane (30 mL × 3). The organic phase was combined, and the organic phase was washed with water and saturated sodium chloride The filtrate was concentrated under reduced pressure to give the crude title compound (32 g)
第六步Step 6
1-环丙基-N-(4-(乙磺酰基)苄基)-2-((5-(三氟甲基)-1H-吡唑-1-基)甲基)-1H-吲哚-5-甲酰胺321-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-((5-(trifluoromethyl)-1H-pyrazol-1-yl)methyl)-1H-indole -5-carboxamide 32
将粗品32g(20mg,0.057mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(22mg,0.115mmol),1-羟基苯并三唑(16mg,0.118mmol),三乙胺(23mg,0.227mmol)溶于二氯甲烷中,加入(4-(乙磺酰基)苯基)甲胺(23mg,0.115mmol),加毕,搅拌反应12小时。反应液减压浓缩,用高效液相色谱法纯化所得残余物,得到标题产物32(15mg,产率:50%)。32 g (20 mg, 0.057 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (22 mg, 0.115 mmol), 1-hydroxybenzotriazole (16 mg, 0.118 mmol), triethylamine (23 mg, 0.227 mmol) was dissolved in dichloromethane, (4-(ethylsulfonyl)phenyl)methanamine (23 mg, 0.115 mmol) was added, and the reaction was stirred for 12 hours. The reaction mixture was concentrated under reduced vacuo.
MS m/z(ESI):531.2[M+1];MS m/z (ESI): 531.2 [M + 1];
1H NMR(400MHz,CDCl3)δ8.04(s,1H),7.88-7.86(d,2H),7.75-7.72(d,1H),7.65-7.61(m,2H),7.57-7.55(d,2H),6.81-6.78(m,1H),6.75(s,1H),6.31(s,1H),5.76(s,2H),4.81-4.79(d,2H),3.29-3.24(m,1H),3.16-3.10(q,2H),1.32-1.28(t,3H)1.27-1.24(m,2H),1.12.40-1.08(m,2H). 1 H NMR (400 MHz, CDCl 3 ) δ 8.04 (s, 1H), 7.88-7.86 (d, 2H), 7.75-7.72 (d, 1H), 7.65-7.61 (m, 2H), 7.57-7.55 (d , 2H), 6.81-6.78 (m, 1H), 6.75 (s, 1H), 6.31 (s, 1H), 5.76 (s, 2H), 4.81-4.79 (d, 2H), 3.29-3.24 (m, 1H) ), 3.16-3.10 (q, 2H), 1.32-1.28 (t, 3H) 1.27-1.24 (m, 2H), 1.12.40-1.08 (m, 2H).
实施例33Example 33
N-(4-(乙磺酰基)苄基)-1-异丙基-2-((4-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酰胺33N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-((4-(trifluoromethyl)piperidin-1-yl)methyl)-1H-indole-5- Formamide 33
第一步first step
2-((4-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酸乙酯33b2-((4-(Trifluoromethyl)piperidin-1-yl)methyl)-1H-indole-5-carboxylic acid ethyl ester 33b
氩气氛下,将1-(丙-2-炔-1-基)-4-(三氟甲基)哌啶33a(950mg,6.2mmol,采用专利公开的方法“WO2003093253”制备而得)、31b(1.2g,3.1mmol)、碘化亚铜(118mg,0.62mmol)、双三苯基磷二氯化钯(217mg,0.31mmol)和三乙胺(1.57g,15.5mmol)溶于N,N-二甲基甲酰胺中,加毕,升温至60℃搅拌反应5小时。反应液冷却至室温,反应液中加入水,用乙酸乙酯萃取(50mL×3),合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得粗品标题产物33b(920mg),产品不经纯化直接用于下一步反应。1-(Propan-2-yn-1-yl)-4-(trifluoromethyl)piperidine 33a (950 mg, 6.2 mmol, prepared by the method disclosed in WO2003093253), under the argon atmosphere, 31b (1.2 g, 3.1 mmol), cuprous iodide (118 mg, 0.62 mmol), bistriphenylphosphine palladium dichloride (217 mg, 0.31 mmol) and triethylamine (1.57 g, 15.5 mmol) dissolved in N, N After the addition of dimethylformamide, the mixture was heated to 60 ° C and stirred for 5 hours. The reaction mixture was cooled to room temperature, and the mixture was evaporated. EtOAcjjjjjjjjjjjjj Column chromatography The residue obtained was purified with EtOAc EtOAc.
第二步Second step
1-异丙基-2-((4-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酸乙酯33c1-isopropyl-2-((4-(trifluoromethyl)piperidin-1-yl)methyl)-1H-indole-5-carboxylic acid ethyl ester 33c
将粗品33b(140mg,0.40mmol)溶于5mL N,N-二甲基甲酰胺中,加入氢化钠(24mg,0.60mmol,60%in oil),搅拌反应5分钟,加入2-碘丙烷(67mg,0.40mmol),60℃封管条件下,搅拌反应12小时。反应液冷却至室温,加入水,用乙酸乙酯萃取(30mL×3),合并有机相,用水,饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系B纯化所得残余物,得标题产物33c(105mg,产率:67%)。The crude product 33b (140 mg, 0.40 mmol) was dissolved in 5 mL of N,N-dimethylformamide, sodium hydride (24 mg, 0.60 mmol, 60% in oil) was added, and the reaction was stirred for 5 minutes, and 2-iodopropane (67 mg) was added. , 0.40 mmol), and the reaction was stirred for 12 hours under 60 ° C sealing conditions. The reaction mixture was cooled to room temperature, water was added, and ethyl acetate (30 mL × 3) was evaporated. The obtained residue was purified to afford titled product (yield: 67%).
第三步third step
1-异丙基-2-((4-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酸33d1-isopropyl-2-((4-(trifluoromethyl)piperidin-1-yl)methyl)-1H-indole-5-carboxylic acid 33d
将33c(105mg,0.26mmol)溶于10mL乙醇中,加入3mL 4M氢氧化钠溶液,60℃条件下,搅拌反应12小时。反应液冷却至室温,滴加浓盐酸至pH为4,用二氯甲烷和甲醇混合溶液(V:V=1:1)萃取(20mL×3),合并有机相,减压浓缩,得到粗品标题产物33d(95mg),产品不经纯化直接进行下一步反应。33c (105 mg, 0.26 mmol) was dissolved in 10 mL of ethanol, and 3 mL of a 4 M sodium hydroxide solution was added thereto, and the reaction was stirred at 60 ° C for 12 hours. The reaction solution was cooled to room temperature, and concentrated hydrochloric acid was added dropwise to pH 4, and the mixture was extracted with methylene chloride and methanol (V:V = 1:1) (20 mL × 3). Product 33d (95 mg) was taken directly to the next product without purification.
MS m/z(ESI):367.2[M-1];MS m/z (ESI): 367.2 [M-1];
第四步the fourth step
N-(4-(乙磺酰基)苄基)-1-异丙基-2-((4-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酰胺33
N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-((4-(trifluoromethyl)piperidin-1-yl)methyl)-1H-indole-5- Formamide 33
将粗品33d(55mg,0.15mmol),(4-(乙磺酰基)苯基)甲胺(45mg,0.22mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(43mg,0.22mmol),N,N-二异丙基乙胺(97mg,0.75mmol)溶于N,N-二甲基甲酰胺中,搅拌反应12小时。加入水,用二氯甲烷和甲醇(V:V=8:1)混合溶剂萃取(20mL×3),合并有机相,用水、饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用高效液相色谱法纯化所得残余物,得到标题产物33(40mg,产率:50%)。Crude 33d (55 mg, 0.15 mmol), (4-(ethylsulfonyl)phenyl)methanamine (45 mg, 0.22 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (43 mg, 0.22 mmol), N,N-diisopropylethylamine (97 mg, 0.75 mmol) was dissolved in N,N-dimethylformamide, and the reaction was stirred for 12 hours. Add water, extract with a mixed solvent of dichloromethane and methanol (V: V = 8:1) (20 mL × 3), combine the organic phase, wash with water, saturated sodium chloride solution, dry over anhydrous sodium sulfate, filter, filtrate The mixture was concentrated under reduced pressure.
MS m/z(ESI):550.4[M+1]MS m/z (ESI): 550.4 [M+1]
1H NMR(400MHz,DMSO-d6)δ9.02(t,1H),8.12(s,1H),7.85-7.95(m,2H),7.66(s,2H),7.56-7.59(m,2H),6.42(s,1H),4.91-4.95(m,1H),4.59(d,2H),6.65(s,2H),3.25(q,2H),2.88(d,2H),2.26(brs,1H),1.97(t,2H),1.79(d,2H),1.56(d,6H),1.36-1.39(m,2H),1.08(t,3H)。 1 H NMR (400 MHz, DMSO-d 6 ) δ 9.02 (t, 1 H), 8.12 (s, 1H), 7.85-7.95 (m, 2H), 7.66 (s, 2H), 7.56-7.59 (m, 2H) ), 6.42 (s, 1H), 4.91-4.95 (m, 1H), 4.59 (d, 2H), 6.65 (s, 2H), 3.25 (q, 2H), 2.88 (d, 2H), 2.26 (brs, 1H), 1.97 (t, 2H), 1.79 (d, 2H), 1.56 (d, 6H), 1.36-1.39 (m, 2H), 1.08 (t, 3H).
实施例34Example 34
1-环丙基-N-(4-(乙磺酰基)苄基)-2-((4-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酰胺341-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-((4-(trifluoromethyl)piperidin-1-yl)methyl)-1H-indole-5- Formamide 34
第一步first step
1-环丙基-2-((4-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酸乙酯34a1-cyclopropyl-2-((4-(trifluoromethyl)piperidin-1-yl)methyl)-1H-indole-5-carboxylic acid ethyl ester 34a
将粗品33b(220mg,0.62mmol)溶于1,2-二氯乙烷中,依次加入一水合醋酸铜(178mg,0.93mmol),2,2'-联吡啶(145mg,0.93mmol),碳酸钠(99mg,0.93mmol)和环丙基硼酸(39mg,0.45mmol),升温至70℃,搅拌反应12小时。再加入环丙基硼酸(39mg,0.45mmol),一水合醋酸铜(178mg,0.93mmol)和2,2'-联吡啶(145mg,0.93mmol),反应12小时。反应液冷却至室温,硅藻土过滤,乙酸乙酯洗涤,
滤液减压浓缩,向所得残余物中加入水,用乙酸乙酯萃取(30mL×3),合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用硅胶柱色谱法以洗脱剂体系B纯化所得残余物,得到标题产物34a(175mg,产率:71%)。The crude product 33b (220 mg, 0.62 mmol) was dissolved in 1,2-dichloroethane, followed by copper acetate monohydrate (178 mg, 0.93 mmol), 2,2'-bipyridine (145 mg, 0.93 mmol), sodium carbonate (99 mg, 0.93 mmol) and cyclopropylboronic acid (39 mg, 0.45 mmol), warmed to 70 ° C, and stirred for 12 hours. Further, cyclopropylboric acid (39 mg, 0.45 mmol), copper acetate monohydrate (178 mg, 0.93 mmol) and 2,2'-bipyridine (145 mg, 0.93 mmol) were added and reacted for 12 hours. The reaction solution was cooled to room temperature, filtered over Celite, ethyl acetate.
The filtrate was concentrated under reduced pressure. EtOAc was evaporated, evaporated, evaporated, evaporated. The obtained residue was purified to silicagel elut elut elut elut elut elut elut
MS m/z(ESI):395.2[M+1];MS m/z (ESI): 395.2 [M + 1];
第二步Second step
1-环丙基-2-((4-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酸34b1-cyclopropyl-2-((4-(trifluoromethyl)piperidin-1-yl)methyl)-1H-indole-5-carboxylic acid 34b
将34a(175mg,0.44mmol)溶于15mL甲醇和四氢呋喃(V:V=2:1)混合溶剂中,加入4mL 4M氢氧化钠溶液,60℃搅拌反应12小时。反应液冷却至室温,滴加浓盐酸至pH为4,用二氯甲烷和甲醇混合溶液(V:V=1:1)洗涤,合并有机相,减压浓缩,得到粗品标题产物34b(170mg),产品不经纯化直接进行下一步反应。34a (175 mg, 0.44 mmol) was dissolved in 15 mL of a mixed solvent of methanol and tetrahydrofuran (V: V = 2:1), 4 mL of 4M sodium hydroxide solution was added, and the reaction was stirred at 60 ° C for 12 hours. The reaction mixture was cooled to room temperature, and concentrated hydrochloric acid was added dropwise to pH 4, and washed with dichloromethane and methanol (V: V = 1:1). The product was directly subjected to the next reaction without purification.
MS m/z(ESI):367.0[M+1];MS m/z (ESI): 367.0 [M+1];
第三步third step
1-环丙基-N-(4-(乙磺酰基)苄基)-2-((4-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酰胺341-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-((4-(trifluoromethyl)piperidin-1-yl)methyl)-1H-indole-5- Formamide 34
将粗品34b(80mg,0.22mmol),(4-(乙磺酰基)苯基)甲胺(65mg,0.32mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(61mg,0.32mmol),N,N-二异丙基乙胺(142mg,1.1mmol)溶于N,N-二甲基甲酰胺中,搅拌反应12小时。加入水,用二氯甲烷和甲醇(V:V=8:1)混合溶剂萃取(20mL×3),合并有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,用薄层色谱法以展开剂体系A纯化所得残余物,得到标题产物34(55mg,产率:46%)。Crude 34b (80 mg, 0.22 mmol), (4-(ethylsulfonyl)phenyl)methanamine (65 mg, 0.32 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (61 mg, 0.32 mmol), N,N-diisopropylethylamine (142 mg, 1.1 mmol) was dissolved in N,N-dimethylformamide, and the reaction was stirred for 12 hours. Add water, extract with a mixed solvent of dichloromethane and methanol (V: V = 8:1) (20 mL × 3), combine the organic phase, wash with saturated sodium chloride solution, dry over anhydrous sodium sulfate, The residue was purified by EtOAc EtOAc (EtOAc)
MS m/z(ESI):548.5[M+1]MS m/z (ESI): 548.5 [M+1]
1H NMR(400MHz,DMSO-d6)δ9.03(t,1H),8.11(s,1H),7.83-7.85(m,2H),7.54-7.59(m,4H),6.45(s,1H),4.59(d,2H),3.71(d,2H),3.25(q,2H),3.01(m,1H),2.28(brs,1H),2.06(t,2H),1.78(d,2H),1.45(q,2H),1.15(t,2H),1.05-1.10(m,7H)。 1 H NMR (400 MHz, DMSO-d 6 ) δ 9.03 (t, 1H), 8.11 (s, 1H), 7.83-7.85 (m, 2H), 7.54-7.59 (m, 4H), 6.45 (s, 1H) ), 4.59 (d, 2H), 3.71 (d, 2H), 3.25 (q, 2H), 3.01 (m, 1H), 2.28 (brs, 1H), 2.06 (t, 2H), 1.78 (d, 2H) , 1.45 (q, 2H), 1.15 (t, 2H), 1.05-1.10 (m, 7H).
实施例35Example 35
2-((2-乙基-4,4-二氟哌啶-1-基)甲基)-N-(4-(乙磺酰基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺352-((2-Ethyl-4,4-difluoropiperidin-1-yl)methyl)-N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-1H-indole -5-formamide 35
从2-乙基-4-氧代哌啶-1-甲酸叔丁酯35a出发,采用实施例33类似合成路线,制得标题产物35(4mg)。Starting from 2-ethyl-4-oxopiperidine-l-carboxylic acid tert-butyl ester 35a, the title compound 35 (4 mg)
MS m/z(ESI):545.5[M+1];MS m/z (ESI): 545.5 [M+1];
1H NMR(400MHz,CDCl3)δ9.07(s,1H),8.16-8.18(m,1H),8.09(s,1H),
7.71-7.73(m,1H),7.58-7.62(m,2H),7.34-7.37(m,1H),6.49(s,1H),4.91-4.92(m,2H),4.22-4.25(m,1H),3.58-3.62(m,1H),3.25-3.28(m,1H),3.14-3.20(m,2H),2.87-2.93(m,1H),2.61-2.63(m,1H),2.40-2.43(m,1H),2.08-2.12(m,1H),1.84-1.93(m,3H),0.89-1.35(m,12H)。 1 H NMR (400MHz, CDCl 3 ) δ9.07 (s, 1H), 8.16-8.18 (m, 1H), 8.09 (s, 1H), 7.71-7.73 (m, 1H), 7.58-7.62 (m, 2H ), 7.34-7.37 (m, 1H), 6.49 (s, 1H), 4.91-4.92 (m, 2H), 4.22-4.25 (m, 1H), 3.58-3.62 (m, 1H), 3.25-3.28 (m , 1H), 3.14-3.20 (m, 2H), 2.87-2.93 (m, 1H), 2.61-2.63 (m, 1H), 2.40-2.43 (m, 1H), 2.08-2.12 (m, 1H), 1.84 -1.93 (m, 3H), 0.89-1.35 (m, 12H).
实施例36Example 36
1-环丙基-N-(4-(乙磺酰基)苄基)-2-(4-氟-2-(三氟甲氧基)苄基)-1H-吲哚-5-甲酰胺361-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-(4-fluoro-2-(trifluoromethoxy)benzyl)-1H-indole-5-carboxamide 36
从1-(溴甲基)-4-氟-2-(三氟甲氧基)苯出发,采用实施例1类似合成路线,制得标题产物36(35mg)。Starting from 1-(bromomethyl)-4-fluoro-2-(trifluoromethoxy)benzene, the title compound 36 (35 mg) was obtained using the procedure
MS m/z(ESI):575.3[M+1]。1H NMR(400MHz,DMSO-d6)δ9.00(t,1H),7.99(s,1H),7.82(d,2H),7.70(d,1H),7.52-7.5 8(m,4H),7.33-7.39(m,2H),5.78(s,1H),4.56(d,2H),4.31(s,2H),3.22-3.30(m,3H),1.26(q,2H),1.05-1.09(m,5H)。MS m/z (ESI): 575.3 [M + 1]. 1 H NMR (400MHz, DMSO- d 6) δ9.00 (t, 1H), 7.99 (s, 1H), 7.82 (d, 2H), 7.70 (d, 1H), 7.52-7.5 8 (m, 4H) ,7.33-7.39(m,2H), 5.78(s,1H),4.56(d,2H),4.31(s,2H),3.22-3.30(m,3H),1.26(q,2H),1.05-1.09 (m, 5H).
实施例37Example 37
1-环丙基-N-(4-(乙磺酰基)苄基)-2-(1-(4-(三氟甲基)苯基)乙基)-1H-吲哚-5-甲酰胺371-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-(1-(4-(trifluoromethyl)phenyl)ethyl)-1H-indole-5-carboxamide 37
从1-(1-溴乙基)-4-(三氟甲基)苯出发,采用实施例1类似合成路线,制得标题产物37(12mg)。MS m/z(ESI):555.3[M+1]Starting from 1-(1-bromoethyl)-4-(trifluoromethyl)benzene, the title compound (37 mg, m. MS m/z (ESI): 555.3 [M+1]
1H NMR(400MHz,CDCl3)δ7.85-7.81(m,3H),7.57-7.55(m,1H),7.52-7.48(m,5H),7.37(d,2H),7.02(s,1H),6.62(t,1H),4.72(d,2H),4.42(q,1H),3.37-3.35(m,1H),3.09(q,2H),1.68(d,3H),1.26(t,3H),1.11-1.09(m,2H),1.04-1.03(m,2H)。 1 H NMR (400MHz, CDCl 3 ) δ7.85-7.81 (m, 3H), 7.57-7.55 (m, 1H), 7.52-7.48 (m, 5H), 7.37 (d, 2H), 7.02 (s, 1H ), 6.62 (t, 1H), 4.72 (d, 2H), 4.42 (q, 1H), 3.37-3.35 (m, 1H), 3.09 (q, 2H), 1.68 (d, 3H), 1.26 (t, 3H), 1.11-1.09 (m, 2H), 1.04-1.03 (m, 2H).
实施例38,39Example 38, 39
(R)-1-环丙基-N-((5-(乙磺酰基)吡啶-2-基)甲基)-2-((2-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酰胺38(R)-1-cyclopropyl-N-((5-(ethylsulfonyl)pyridin-2-yl)methyl)-2-((2-(trifluoromethyl)piperidin-1-yl) Methyl)-1H-indole-5-carboxamide 38
(S)-1-环丙基-N-((5-(乙磺酰基)吡啶-2-基)甲基)-2-((2-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酰胺39
(S)-1-cyclopropyl-N-((5-(ethylsulfonyl)pyridin-2-yl)methyl)-2-((2-(trifluoromethyl)piperidin-1-yl) Methyl)-1H-indole-5-carboxamide 39
第一步first step
(5-溴-1H-吲哚-2-基)(2-(三氟甲基)哌啶-1-基)甲酮38c(5-bromo-1H-indol-2-yl)(2-(trifluoromethyl)piperidin-1-yl)methanone 38c
将5-溴-1H-吲哚-2-甲酸38b(600mg,2.5mmol,采用公知的方法“Journal of Medicinal Chemistry,2009,52(23),7512-7527”制备而得)溶于15mL四氢呋喃中,加入2-(三氟甲基)哌啶38a(382.8mg,2.5mmol,采用公知的方法“Tetrahedron,2011,67(1),69-74”制备而得)、2-(7-氧化苯并三氮唑)-N,N,N',N'-四甲基脲六氟磷酸酯(1424.7mg,3.75mmol)和N,N-二异丙基乙胺(967.3mg,7.5mmol),加毕,搅拌反应18小时。反应液减压浓缩,用硅胶柱色谱法以洗脱剂体系A纯化所得残余物,得到标题产物38c(400mg,产率:42.66%)。5-bromo-1H-indole-2-carboxylic acid 38b (600 mg, 2.5 mmol, prepared by the well-known method "Journal of Medicinal Chemistry, 2009, 52 (23), 7512-7527") was dissolved in 15 mL of tetrahydrofuran. 2-(trifluoromethyl)piperidine 38a (382.8 mg, 2.5 mmol, prepared by a known method "Tetrahedron, 2011, 67 (1), 69-74"), 2-(7-phenylene oxide) And triazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate (1424.7 mg, 3.75 mmol) and N,N-diisopropylethylamine (967.3 mg, 7.5 mmol), After the addition was completed, the reaction was stirred for 18 hours. The reaction mixture was concentrated under reduced pressure. EtOAcjjjjjj
MS m/z(ESI):375.3[M+1];MS m/z (ESI): 375.3 [M + 1];
第二步Second step
5-溴-2-((2-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚38d5-bromo-2-((2-(trifluoromethyl)piperidin-1-yl)methyl)-1H-indole 38d
将38c(400mg,1.07mmol)溶于N,N-二甲基甲酰胺中,加入氢化铝锂(121.38mg,3.2mmol),加毕,搅拌反应18小时。反应液减压浓缩,得到粗品标题产物38d(120mg),产品不经纯化直接进行下一步反应。38c (400 mg, 1.07 mmol) was dissolved in N,N-dimethylformamide, lithium aluminum hydride (121.38 mg, 3.2 mmol) was added, and the reaction was stirred for 18 hours. The reaction mixture was concentrated under reduced pressure to give titled md.
MS m/z(ESI):361.4[M+1];MS m/z (ESI): 361.4 [M+1];
第三步third step
5-溴-1-环丙基-2-((2-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚38e5-bromo-1-cyclopropyl-2-((2-(trifluoromethyl)piperidin-1-yl)methyl)-1H-indole 38e
将粗品38d(120mg,0.33mmol)溶于5mL 1,2-二氯乙烷中,加入环丙基硼酸
(42.81mg,0.50mmol)、2,2'-联吡啶(77.83mg,0.50mmol)、醋酸铜(99.17mg,0.50mmol)和碳酸钠(52.82mg,0.50mmol),加毕,70℃条件下,搅拌反应16小时。反应液减压浓缩,用硅胶柱色谱法以洗脱剂体系C纯化所得残余物,得到标题产物38e(300mg,产率:22.5%)。The crude product 38d (120 mg, 0.33 mmol) was dissolved in 5 mL of 1,2-dichloroethane, and cyclopropylboronic acid was added.
(42.81 mg, 0.50 mmol), 2,2'-bipyridine (77.83 mg, 0.50 mmol), copper acetate (99.17 mg, 0.50 mmol) and sodium carbonate (52.82 mg, 0.50 mmol), added, 70 ° C The reaction was stirred for 16 hours. The reaction mixture was concentrated under reduced pressure. EtOAcjjjjjjj
第四步the fourth step
1-环丙基-2-((2-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酸乙酯38f1-cyclopropyl-2-((2-(trifluoromethyl)piperidin-1-yl)methyl)-1H-indole-5-carboxylic acid ethyl ester 38f
将38e(100mg,0.25mmol)溶于2mL乙醇和二甲基亚砜(V:V=1:1)混合溶剂中,加入醋酸钯(11.23mg,0.05mmol)、1,3-双(二苯基膦)丙烷(25.7mg,0.06mmol)和三乙胺(25.17mg,0.25mmol),加毕,一氧化碳气氛下,80℃条件下,搅拌反应16小时。反应液冷却至室温,减压浓缩,用硅胶柱色谱法以洗脱剂体系C纯化所得残余物,得到标题产物38f(80mg,产率:81.39%)。38e (100mg, 0.25mmol) was dissolved in 2mL ethanol and dimethyl sulfoxide (V: V = 1:1) mixed solvent, palladium acetate (11.23mg, 0.05mmol), 1,3-bis(diphenyl) The phosphine)propane (25.7 mg, 0.06 mmol) and triethylamine (25.17 mg, 0.25 mmol) were added, and the reaction was stirred at 80 ° C for 16 hours under a carbon monoxide atmosphere. The reaction mixture was cooled to room temperature, and then evaporated.
第五步the fifth step
1-环丙基-2-((2-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酸38g1-cyclopropyl-2-((2-(trifluoromethyl)piperidin-1-yl)methyl)-1H-indole-5-carboxylic acid 38g
将38f(30mg,0.08mmol)溶于2.1mL甲醇和四氢呋喃(V:V=20:1)混合溶剂中,加入氢氧化钠(30.42mg,0.76mmol),加毕,45℃条件下,搅拌反应3小时。反应液减压浓缩,滴加1M盐酸至反应液的pH为3,减压浓缩,得粗品标题产物38g(27mg),产品不经纯化直接进行下一步反应。38f (30mg, 0.08mmol) was dissolved in a mixed solvent of 2.1mL methanol and tetrahydrofuran (V:V=20:1), sodium hydroxide (30.42mg, 0.76mmol) was added, and the reaction was stirred at 45 °C. 3 hours. The reaction mixture was concentrated under reduced pressure. EtOAc was evaporated.
MS m/z(ESI):365.4[M-1];MS m/z (ESI): 365.4 [M-1];
第六步Step 6
(R)-1-环丙基-N-((5-(乙磺酰基)吡啶-2-基)甲基)-2-((2-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酰胺38(R)-1-cyclopropyl-N-((5-(ethylsulfonyl)pyridin-2-yl)methyl)-2-((2-(trifluoromethyl)piperidin-1-yl) Methyl)-1H-indole-5-carboxamide 38
(S)-1-环丙基-N-((5-(乙磺酰基)吡啶-2-基)甲基)-2-((2-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酰胺39(S)-1-cyclopropyl-N-((5-(ethylsulfonyl)pyridin-2-yl)methyl)-2-((2-(trifluoromethyl)piperidin-1-yl) Methyl)-1H-indole-5-carboxamide 39
将粗品38g(30mg,0.08mmol)溶于1.5mL N,N-二甲基甲酰胺中,加入11a(32.8mg,0.16mmol)、2-(7-氧化苯并三氮唑)-N,N,N',N'-四甲基脲六氟磷酸酯(62.23mg,0.16mmol)和N,N-二异丙基乙胺(31.69mg,0.25mmol),加毕,搅拌反应16小时。反应液减压浓缩,用硅胶柱色谱法以洗脱剂体系C纯化所得残余物,得到标题产物38(12mg,产率:36.7%)和39(12mg,产率:36.7%)。The crude product 38 g (30 mg, 0.08 mmol) was dissolved in 1.5 mL of N,N-dimethylformamide, and 11a (32.8 mg, 0.16 mmol), 2-(7-benzotriazole)-N, N N', N'-tetramethyluronium hexafluorophosphate (62.23 mg, 0.16 mmol) and N,N-diisopropylethylamine (31.69 mg, 0.25 mmol) were added, and the reaction was stirred for 16 hours. The reaction mixture was concentrated under reduced vacuolululululululululululululululululululululululululululululululululululu
化合物38:Compound 38:
MS m/z(ESI):549.1[M+1];MS m/z (ESI): 549.1 [M+1];
手性HPLC分析:保留时间16.910分钟,手性纯度:98%(色谱柱:Lux Amylose-3(AD),4.6*150cm Length,5um;流动相:乙醇/正己烷=60/40(v/v))。Chiral HPLC analysis: retention time 16.910 min, chiral purity: 98% (column: Lux Amylose-3 (AD), 4.6*150 cm Length, 5 um; mobile phase: ethanol/n-hexane = 60/40 (v/v) )).
1H NMR(400MHz,CDCl3)δ9.06(s,1H),8.15-8.17(m,1H),8.09(s,1H),7.71-7.73(m,1H),7.57-7.62(m,2H),7.35-7.39(m,1H),6.47(s,1H),4.91-4.92(m,2H),4.07-4.15(m,2H),3.28-3.30(m,2H),3.14-3.19(m,2H),2.86-2.92(m,1H),2.63-2.66(m,1H),1.90-1.93(m,1H),1.75-1.79(m,1H),1.56-1.64(m,3H),1.31-1.35
(m,4H),1.15-1.17(m,2H),1.08-1.10(m,2H)。 1 H NMR (400MHz, CDCl 3 ) δ9.06 (s, 1H), 8.15-8.17 (m, 1H), 8.09 (s, 1H), 7.71-7.73 (m, 1H), 7.57-7.62 (m, 2H ), 7.35-7.39 (m, 1H), 6.47 (s, 1H), 4.91-4.92 (m, 2H), 4.07-4.15 (m, 2H), 3.28-3.30 (m, 2H), 3.14 - 3.19 (m , 2H), 2.86-2.92 (m, 1H), 2.63-2.66 (m, 1H), 1.90- 1.93 (m, 1H), 1.75-1.79 (m, 1H), 1.56-1.64 (m, 3H), 1.31 -1.35 (m, 4H), 1.15 - 1.17 (m, 2H), 1.08-1.10 (m, 2H).
化合物39:Compound 39:
MS m/z(ESI):549.1[M+1];MS m/z (ESI): 549.1 [M+1];
手性HPLC分析:保留时间11.940分钟,手性纯度:98%(色谱柱:Lux Amylose-3(AD),4.6*150cm Length,5um;流动相:乙醇/正己烷=60/40(v/v))。Chiral HPLC analysis: retention time 11.940 minutes, chiral purity: 98% (column: Lux Amylose-3 (AD), 4.6*150 cm Length, 5 um; mobile phase: ethanol / n-hexane = 60/40 (v/v) )).
1H NMR(400MHz,CDCl3)δ9.06(s,1H),8.15-8.18(m,1H),8.09(s,1H),7.71-7.74(m,1H),7.58-7.62(m,2H),7.34-7.37(m,1H),6.47(s,1H),4.91-4.92(m,2H),4.08-4.15(m,2H),3.27-3.33(m,2H),3.14-3.19(m,2H),2.86-2.92(m,1H),2.63-2.66(m,1H),1.90-1.94(m,1H),1.75-1.79(m,1H),1.56-1.61(m,3H),1.31-1.35(m,4H),1.15-1.18(m,2H),1.08-1.10(m,2H)。 1 H NMR (400MHz, CDCl 3 ) δ9.06 (s, 1H), 8.15-8.18 (m, 1H), 8.09 (s, 1H), 7.71-7.74 (m, 1H), 7.58-7.62 (m, 2H ), 7.34-7.37 (m, 1H), 6.47 (s, 1H), 4.91-4.92 (m, 2H), 4.08-4.15 (m, 2H), 3.27-3.33 (m, 2H), 3.14 - 3.19 (m , 2H), 2.86-2.92 (m, 1H), 2.63-2.66 (m, 1H), 1.90- 1.94 (m, 1H), 1.75-1.79 (m, 1H), 1.56-1.61 (m, 3H), 1.31 - 1.35 (m, 4H), 1.15-1.18 (m, 2H), 1.08-1.10 (m, 2H).
实施例40Example 40
2-((2-二乙基哌啶-1-基)甲基)-N-(4-(乙磺酰基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺402-((2-Diethylpiperidin-1-yl)methyl)-N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-1H-indole-5-carboxamide 40
从2-乙基哌啶40a出发,采用实施例33类似合成路线,制得标题产物40(70mg)。Starting from 2-ethylpiperidine 40a, the title compound 40 (70 mg) was obtained using the procedure of Example 33.
MS m/z(ESI):510.5[M+1];MS m/z (ESI): 510.5 [M + 1];
1H NMR(400MHz,CDCl3)δ8.08(s,1H),7.90(d,2H),7.65-7.66(m,1H),7.59(s,2H),6.67(s,1H),6.40(s,1H),5.14-5.16(m,1H),4.82(s,2H),4.09(d,1H),3.42(d,1H),3.14(q,2H),2.61-2.63(m,1H),2.27-2.29(m,1H),2.09-2.11(m,1H),1.62-1.71(m,10H),1.39-1.49(m,4H),1.31(t,3H),0.94-0.96(m,3H)。 1 H NMR (400MHz, CDCl 3 ) δ8.08 (s, 1H), 7.90 (d, 2H), 7.65-7.66 (m, 1H), 7.59 (s, 2H), 6.67 (s, 1H), 6.40 ( s,1H),5.14-5.16(m,1H),4.82(s,2H),4.09(d,1H), 3.42(d,1H),3.14(q,2H),2.61-2.63(m,1H) , 2.27-2.29 (m, 1H), 2.09-2.11 (m, 1H), 1.62-1.71 (m, 10H), 1.39-1.49 (m, 4H), 1.31 (t, 3H), 0.94-0.96 (m, 3H).
实施例41Example 41
N-(4-(乙磺酰基)苄基)-1-异丙基-2-((2-(三氟甲基)吡啶-3-基)甲基)-1H-吲哚-5-甲酰胺41N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-((2-(trifluoromethyl)pyridin-3-yl)methyl)-1H-indole-5-A Amide 41
采用实施例2合成路线,将第一步原料2a替换为3-(溴甲基)-2-(三氟甲基)吡啶,得到标题产物41(10mg)。Using the procedure of Example 2, the first step starting material 2a was replaced with 3-(bromomethyl)-2-(trifluoromethyl)pyridine to give the title product 41 (10 mg).
MS m/z(ESI):544.3[M+1];
MS m/z (ESI): 544.3 [M + 1];
1H NMR(400MHz,CDCl3)δ8.62-8.61(m,1H),8.07(s,1H),7.87(d,2H),7.68-7.66(m,1H),7.57-7.55(m,3H),7.46-7.42(m,2H),6.65(t,1H),6.30(s,1H),4.79(d,2H),4.43-4.40(m,1H),4.37(s,2H),3.10(q,2H),1.51(d,6H),1.28(t,3H)。 1 H NMR (400MHz, CDCl 3 ) δ8.62-8.61 (m, 1H), 8.07 (s, 1H), 7.87 (d, 2H), 7.68-7.66 (m, 1H), 7.57-7.55 (m, 3H ), 7.46-7.42 (m, 2H), 6.65 (t, 1H), 6.30 (s, 1H), 4.79 (d, 2H), 4.43-4.40 (m, 1H), 4.37 (s, 2H), 3.10 ( q, 2H), 1.51 (d, 6H), 1.28 (t, 3H).
实施例42Example 42
N-(4-(乙磺酰基)苄基)-1-异丙基-2-(2-(三氟甲基)苄基)-1H-苯并[d]咪唑-5-甲酰胺42N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-(2-(trifluoromethyl)benzyl)-1H-benzo[d]imidazole-5-carboxamide 42
从2-(2-(三氟甲基)苯基)乙酸42a(采用公开的方法“Tetrahedron,2002,58(50),9925-9932”制备而得)和3-氨基-4-(异丙基氨基)出发,采用实施例1类似合成路线,制得标题产物42(15mg)。From 2-(2-(trifluoromethyl)phenyl)acetic acid 42a (prepared by the published method "Tetrahedron, 2002, 58(50), 9925-9932") and 3-amino-4-(isopropyl) Starting from the base amino group, the title product 42 (15 mg) was obtained using a similar synthetic route from Example 1.
MS m/z(ESI):544.4[M+1];1H NMR(400MHz,DMSO-d6)δ9.13(t,1H),8.17(s,1H),7.77-7.85(m,5H),7.59-7.62(m,3H),7.51-7.57(m,1H),7.22(d,1H),4.68-4.72(m,1H),4.59(d,2H),4.49(s,2H),3.25(q,2H),1.51(d,6H),1.09(t,3H)。MS m/z (ESI): 544.4 [M + 1]; 1 H NMR (400 MHz, DMSO-d 6 ) δ 9.13 (t, 1H), 8.17 (s, 1H), 7.77-7.85 (m, 5H) , 7.59-7.62 (m, 3H), 7.51-7.57 (m, 1H), 7.22 (d, 1H), 4.68-4.72 (m, 1H), 4.59 (d, 2H), 4.49 (s, 2H), 3.25 (q, 2H), 1.51 (d, 6H), 1.09 (t, 3H).
实施例43Example 43
6-氯-1-环丙基-N-(4-(乙磺酰基)苄基)-2-(4-氟-2-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺436-Chloro-1-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-(4-fluoro-2-(trifluoromethyl)benzyl)-1H-indole-5- Formamide 43
从6-氯-1H-吲哚-5-甲酸甲酯(采用专利申请公开的方法“WO2004022712A2”制备而得)出发,采用实施例4类似合成路线,制得产物43(2.8mg)。Starting from 6-chloro-1H-indole-5-carboxylic acid methyl ester (prepared by the method disclosed in the patent application "WO2004022712A2"), a similar synthetic route of Example 4 was used to obtain the product 43 (2.8 mg).
MS m/z(ESI):593.2[M+1]MS m/z (ESI): 593.2 [M+1]
实施例44Example 44
N-(2-氯-4-(乙磺酰基)苯基)-1-环丙基-2-((4-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酰胺44N-(2-chloro-4-(ethylsulfonyl)phenyl)-1-cyclopropyl-2-((4-(trifluoromethyl)piperidin-1-yl)methyl)-1H-indole哚-5-formamide 44
从4-溴-2-氯苄基氨基甲酸叔丁酯(采用专利申请“WO2005082859A1”公开的方法制备而得)出发,采用实施例8类似合成路线,制得产物44(10mg)。Starting from tert-butyl 4-bromo-2-chlorobenzylcarbamate (prepared by the method disclosed in the patent application "WO2005082859A1"), a product of the same procedure as in Example 8 was used to obtain the product 44 (10 mg).
MS m/z(ESI):582.2[M+1];MS m/z (ESI): 582.2 [M + 1];
1H NMR(400MHz,CD3OD)δ8.11(s,1H),7.95(s,1H),7.83-7.81(m,1H),7.74-7.72(m,1H),7.67-7.63(m,2H),6.48(s,1H),4.75(s,2H),3.80(s,2H),3.29-3.21(m,3H),3.12-3.09(m,2H),2.19-2.09(m,3H),1.88-1.85(m,2H),1.64-1.54(m,2H),1.24-1.20(m,5H),1.12-1.08(m,2H)。 1 H NMR (400 MHz, CD 3 OD) δ 8.11 (s, 1H), 7.95 (s, 1H), 7.83-7.81 (m, 1H), 7.74-7.72 (m, 1H), 7.67-7.63 (m, 2H), 6.48 (s, 1H), 4.75 (s, 2H), 3.80 (s, 2H), 3.29-3.21 (m, 3H), 3.12-3.09 (m, 2H), 2.19-2.09 (m, 3H) , 1.88-1.85 (m, 2H), 1.64-1.54 (m, 2H), 1.24-1.20 (m, 5H), 1.12-1.08 (m, 2H).
实施例45Example 45
1-异丙基-N-(4-(甲磺酰基)苄基)-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺451-isopropyl-N-(4-(methylsulfonyl)benzyl)-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 45
采用实施例14的合成路线,将第四步原料(4-(乙磺酰基)苯基)甲胺替换为(4-(甲磺酰基)苯基)甲胺(采用专利申请“US20160122318A1”公开的方法制备而得),得到标题产物45(17.1mg)。Using the synthetic route of Example 14, the fourth step starting material (4-(ethylsulfonyl)phenyl)methanamine was replaced by (4-(methylsulfonyl)phenyl)methanamine (disclosed by the patent application "US20160122318A1") The title compound was obtained by the method to give the title product 45 (17.1 mg).
MS m/z(ESI):529.5[M+1]MS m/z (ESI): 529.5 [M+1]
实施例46Example 46
N-(4-(乙磺酰基)苄基)-2-(4-氟苄基)-1-异丙基-1H-吲哚-5-甲酰胺46N-(4-(ethylsulfonyl)benzyl)-2-(4-fluorobenzyl)-1-isopropyl-1H-indole-5-carboxamide 46
采用实施例14的合成路线,将第一步原料14a替换为1-溴甲基-4-氟苯,得到产物46(26mg)。Using the synthetic route of Example 14, the first step starting material 14a was replaced with 1-bromomethyl-4-fluorobenzene to give the product 46 (26 mg).
MS m/z(ESI):493.5[M+1];MS m/z (ESI): 493.5 [M+1];
1H NMR(400MHz,CDCl3)δ8.11(s,1H),7.85(d,2H),7.67(d,1H),7.53-7.57(m,3H),7.15-7.19(m,2H),7.01-7.05(m,2H),6.87(t,1H),6.3 6(s,1H),4.80(d,2H),4.51-4.58(m,1H),4.16(s,2H),3.11(q,2H),1.49(d,6H),1.30(t,3H)。 1 H NMR (400MHz, CDCl 3 ) δ8.11 (s, 1H), 7.85 (d, 2H), 7.67 (d, 1H), 7.53-7.57 (m, 3H), 7.15-7.19 (m, 2H), 7.01-7.05(m,2H),6.87(t,1H),6.3 6(s,1H), 4.80(d,2H),4.51-4.58(m,1H),4.16(s,2H),3.11(q , 2H), 1.49 (d, 6H), 1.30 (t, 3H).
实施例47Example 47
N-(4-(乙磺酰基)苄基)-1-甲基-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺47
N-(4-(ethylsulfonyl)benzyl)-1-methyl-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 47
采用实施例14的合成路线,将第二步原料2-溴丙烷替换为碘甲烷,得到标题产物47(26mg)。Using the synthetic route of Example 14, the second step starting material, 2-bromopropane, was replaced with methyl iodide to give the title product 47 (26 mg).
MS m/z(ESI):515.5[M+1];MS m/z (ESI): 515.5 [M+1];
1H NMR(400MHz,CD3OD)δ8.10(s,1H),7.88(d,2H),7.71-7.61(m,5H),7.44-7.39(m,3H),6.35(s,1H),4.70(s,2H),4.31(s,2H),3.63(s,3H),3.19(q,2H),0.90(t,3H)。 1 H NMR (400 MHz, CD 3 OD) δ 8.10 (s, 1H), 7.88 (d, 2H), 7.71-7.61 (m, 5H), 7.44-7.39 (m, 3H), 6.35 (s, 1H) , 4.70 (s, 2H), 4.31 (s, 2H), 3.63 (s, 3H), 3.19 (q, 2H), 0.90 (t, 3H).
实施例48Example 48
2-(4-溴苄基)-N-(4-(乙磺酰基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺482-(4-Bromobenzyl)-N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-1H-indole-5-carboxamide 48
采用实施例2的合成路线,将第一步原料2a替换为1-溴-4-(溴甲基)苯,得到标题产物48(58mg)。Using the synthetic route of Example 2, the first step starting material 2a was replaced with 1-bromo-4-(bromomethyl)benzene to give the title product 48 (58 mg).
MS m/z(ESI):552.9[M+1]MS m/z (ESI): 552.9 [M+1]
实施例49Example 49
2-(4-氰苄基)-N-(4-(乙磺酰基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺492-(4-cyanobenzyl)-N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-1H-indole-5-carboxamide 49
将48(50mg,0.09mmol)、氰化亚铜(17mg,0.18mmol)和碘化亚铜(17.1mg,0.09mmol)溶于1mL N,N-二甲基乙酰胺,200℃条件下,微波反应30分钟。反应液冷却至室温,减压浓缩,用薄层色谱法以展开剂体系C纯化所得残余物,得标题产物49(39mg,产率:86.7%)。
48 (50 mg, 0.09 mmol), cuprous cyanide (17 mg, 0.18 mmol) and cuprous iodide (17.1 mg, 0.09 mmol) were dissolved in 1 mL of N,N-dimethylacetamide at 200 ° C under microwave Reaction for 30 minutes. The reaction mixture was cooled to EtOAc.
MS m/z(ESI):500.5[M+1]MS m/z (ESI): 500.5 [M+1]
实施例50Example 50
2-(4-氨基甲酰基苄基)-N-(4-(乙磺酰基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺502-(4-carbamoylbenzyl)-N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-1H-indole-5-carboxamide 50
将49(20mg,0.04mmol)溶于甲醇中,加入0.1mL 2M氢氧化钾溶液和0.1mL双氧水,加毕,反应15分钟。将反应液倒入水中,乙酸乙酯萃取(20mL×3),合并有机相,有机相用水、饱和氯化钠溶液洗涤,无水硫酸钠干燥,过滤,滤液减压浓缩,得粗品标题产物50(16mg)。49 (20 mg, 0.04 mmol) was dissolved in methanol, 0.1 mL of 2M potassium hydroxide solution and 0.1 mL of hydrogen peroxide were added, and the reaction was carried out for 15 minutes. The reaction mixture was poured into water, ethyl acetate (20 mL×3), and the organic phase was combined, and the organic phase was washed with water and saturated sodium chloride (16mg).
MS m/z(ESI):518.2[M+1]MS m/z (ESI): 518.2 [M+1]
实施例51Example 51
N-((5-(乙磺酰基)吡啶-2-基)甲基)-1-异丙基-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺51N-((5-(ethylsulfonyl)pyridin-2-yl)methyl)-1-isopropyl-2-(4-(trifluoromethyl)benzyl)-1H-indole-5- Amide 51
采用实施例14合成路线,将第四步原料(4-(乙磺酰基)苯基)甲胺替换为11a,得标题产物51(11mg)。The title material 51 (11 mg) was obtained from the title compound (4-(ethylsulfonyl)phenyl)methylamine.
MS m/z(ESI):544.5[M+1]MS m/z (ESI): 544.5 [M+1]
实施例52Example 52
N-(4-(乙磺酰基)苄基)-1-异丙基-3-甲基-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺52
N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-3-methyl-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 52
采用实施例14合成路线,将第一步原料1a替换为3-甲基-1H-吲哚-5-甲酸甲酯(采用公知的方法“RSC Advances,2015,5(86),70329-70332”制备而得),得标题产物52(9mg)。Using the synthetic route of Example 14, the first step starting material 1a was replaced with methyl 3-methyl-1H-indole-5-carboxylate (using the well-known method "RSC Advances, 2015, 5(86), 70329-70332" The title product 52 (9 mg) was obtained.
MS m/z(ESI):557.5[M+1]MS m/z (ESI): 557.5 [M+1]
实施例53Example 53
1-环戊基-N-(4-(乙磺酰基)苄基)-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺531-cyclopentyl-N-(4-(ethylsulfonyl)benzyl)-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 53
采用实施例14合成路线,将第二步原料2-溴丙烷替换为溴代环戊烷,得标题产物53(23mg)。Using the procedure of Example 14, the second step of the starting material, 2-bromopropane, was replaced with the bromocyclopentane to give the title product 53 (23 mg).
MS m/z(ESI):569.5[M+1]MS m/z (ESI): 569.5 [M+1]
实施例54Example 54
N-(4-(乙磺酰基)-2-氟苄基)-1-异丙基-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺54N-(4-(ethylsulfonyl)-2-fluorobenzyl)-1-isopropyl-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 54
采用实施例14合成路线,将第四步原料(4-(乙磺酰基)苯基)甲胺替换为8c,得标题产物54(11mg)。Using the procedure of Example 14, the fourth step starting material (4-(ethylsulfonyl)phenyl)methylamine was replaced by 8c to give the title product 54 (11 mg).
MS m/z(ESI):561.5[M+1];MS m/z (ESI): 561.5 [M + 1];
1H NMR(400MHz,CDCl3)δ8.10(d,1H),7.59-7.70(m,7H),7.31-7.35(m,2H),6.82(t,1H),6.38(s,1H),4.83(d,2H),4.50-4.53(m,1H),4.25(s,1H),3.15(q,2H),1.49(d,6H),1.32(t,3H)。 1 H NMR (400MHz, CDCl 3 ) δ8.10 (d, 1H), 7.59-7.70 (m, 7H), 7.31-7.35 (m, 2H), 6.82 (t, 1H), 6.38 (s, 1H), 4.83 (d, 2H), 4.50-4.53 (m, 1H), 4.25 (s, 1H), 3.15 (q, 2H), 1.49 (d, 6H), 1.32 (t, 3H).
实施例55Example 55
N-(4-(乙磺酰基)苄基)-1-苯基-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺55
N-(4-(ethylsulfonyl)benzyl)-1-phenyl-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 55
采用实施例20合成路线,将实施例20第一步原料环丙基硼酸替换为苯基硼酸,制得标题产物55(2.7mg)Using the synthesis route of Example 20, the first step starting material of the example 20, cyclopropylboronic acid, was replaced with phenylboronic acid to give the title product 55 (2.7 mg).
MS m/z(ESI):577.1[M+1]MS m/z (ESI): 577.1 [M+1]
1H NMR(400MHz,CDCl3)δ8.76-8.74(dd,1H),8.44-8.41(dd,1H),8.12(s,1H),7.89-7.87(d,2H),7.61-7.53(m,3H),7.49-7.43(m,4H),7.19-7.17(m,2H),7.11-7.06(m,2H),6.64-6.61(t,1H),6.62(s,1H),6.48(s,1H),4.80-4.78(d,2H),2.25-2.18(m,2H),0.91-0.87(t,3H)。 1 H NMR (400 MHz, CDCl 3 ) δ 8.76-8.74 (dd, 1H), 8.44-8.41 (dd, 1H), 8.12 (s, 1H), 7.89-7.87 (d, 2H), 7.61-7.53 (m) , 3H), 7.49-7.43 (m, 4H), 7.19-7.17 (m, 2H), 7.11-7.06 (m, 2H), 6.64-6.61 (t, 1H), 6.62 (s, 1H), 6.48 (s , 1H), 4.80-4.78 (d, 2H), 2.25-2.18 (m, 2H), 0.91 - 0.87 (t, 3H).
实施例56Example 56
N-(4-(乙磺酰基)苄基)-1-异丙基-2-(3-(三氟甲基)苄基)-1H-苯并[d]咪唑-5-甲酰胺56N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-(3-(trifluoromethyl)benzyl)-1H-benzo[d]imidazole-5-carboxamide 56
采用实施例42的合成路线,将第一步原料42a替换为2-(3-(三氟甲基)苯基)乙酸(采用公知的方法“Angewandte Chemie,International Edition,2010,49(27),4665-4668,S4665/1-S4665/60”制备而得),得标题产物56(4.6mg)Using the synthetic route of Example 42, the first step starting material 42a was replaced with 2-(3-(trifluoromethyl)phenyl)acetic acid (using a known method "Angewandte Chemie, International Edition, 2010, 49 (27), 4665-4668, S4665/1-S4665/60" prepared to give the title product 56 (4.6 mg)
MS m/z(ESI):544.5[M+1];MS m/z (ESI): 544.5 [M + 1];
1H NMR(400MHz,CDCl3)δ8.18(s,1H),7.89-7.87(dd,1H),7.83-7.81(dd,1H),7.58-7.39(m,7H),6.72(s,1H),4.81-4.79(d,2H),4.62-4.55(m,1H),4.42(s,2H),3.14-3.08(m,2H),1.47-1.45(d,6H),1.30-1.262(t,3H)。 1 H NMR (400MHz, CDCl 3 ) δ8.18 (s, 1H), 7.89-7.87 (dd, 1H), 7.83-7.81 (dd, 1H), 7.58-7.39 (m, 7H), 6.72 (s, 1H ), 4.81-4.79 (d, 2H), 4.62-4.55 (m, 1H), 4.42 (s, 2H), 3.14 - 3.08 (m, 2H), 1.47-1.45 (d, 6H), 1.30-1.262 (t , 3H).
实施例57Example 57
N-(4-(乙磺酰基)苄基)-2-(2-氟-4-(三氟甲基)苄基)-1-异丙基-1H-苯并[d]咪唑-5-甲酰胺57N-(4-(ethylsulfonyl)benzyl)-2-(2-fluoro-4-(trifluoromethyl)benzyl)-1-isopropyl-1H-benzo[d]imidazole-5- Formamide 57
采用实施例42合成路线,将第一步原料42a替换为2-(2-氟-4-(三氟甲基)苯基)乙酸(Admas),得标题产物57(4.6mg)。
The first step starting material 42a was replaced with 2-(2-fluoro-4-(trifluoromethyl)phenyl)acetic acid (Admas) to give the title product 57 (4.6 mg).
MS m/z(ESI):562.5[M+1];MS m/z (ESI): 562.5 [M + 1];
1H NMR(400MHz,CDCl3)δ8.18(s,1H),7.84-7.81(m,3H),7.59-7.52(m,3H),7.39-7.27(m,3H),6.87-6.85(t,1H),4.78-4.76(d,2H),4.66-4.59(m,1H),4.39(s,2H),3.12-3.06(m,2H),1.55-1.53(d,6H),2.28-1.24(t,3H)。 1 H NMR (400 MHz, CDCl 3 ) δ 8.18 (s, 1H), 7.84-7.81 (m, 3H), 7.59-7.52 (m, 3H), 7.39-7.27 (m, 3H), 6.87-6.85 (t , 1H), 4.78-4.76 (d, 2H), 4.66-4.59 (m, 1H), 4.39 (s, 2H), 3.12-3.06 (m, 2H), 1.55-1.53 (d, 6H), 2.28-1.24 (t, 3H).
实施例58Example 58
N-(4-(乙磺酰基)苄基)-2-(4-氟苄基)-1-异丙基-1H-苯并[d]咪唑-5-甲酰胺58N-(4-(ethylsulfonyl)benzyl)-2-(4-fluorobenzyl)-1-isopropyl-1H-benzo[d]imidazole-5-carboxamide 58
采用实施例42合成路线,将第一步原料42a替换为4-氟苯乙酸(采用公知的方法“RSC Advances,2016,6(8),6719-6723”制备而得),得标题产物58(4.6mg)。Using the synthesis route of Example 42, the first step starting material 42a was replaced with 4-fluorophenylacetic acid (prepared by the known method "RSC Advances, 2016, 6(8), 6719-6723") to obtain the title product 58 ( 4.6mg).
MS m/z(ESI):494.5[M+1];MS m/z (ESI): 494.5 [M + 1];
1H NMR(400MHz,CDCl3)δ8.18-8.09(d,2H),7.80-7.75(dd,3H),7.54(s,2H),7.24(s,2H),7.07-7.06(d,2H),4.80-4.73(d,3H),4.61(s,2H),3.11-3.06(m,2H),1.55-1.53(d,6H),1.31-1.23(t,3H)。 1 H NMR (400MHz, CDCl 3 ) δ 8.18-8.09 (d, 2H), 7.80-7.75 (dd, 3H), 7.54 (s, 2H), 7.24 (s, 2H), 7.07-7.06 (d, 2H) ), 4.80-4.73 (d, 3H), 4.61 (s, 2H), 3.11-3.06 (m, 2H), 1.55-1.53 (d, 6H), 1.31-1.23 (t, 3H).
实施例59Example 59
N-(2-氯4-(乙磺酰基)苄基)-1-异丙基-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺59N-(2-Chloro-4-(ethylsulfonyl)benzyl)-1-isopropyl-2-(4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 59
采用实施例8合成路线,将第一步原料8a替换为4-溴-2-氯苄基氨基甲酸叔丁酯,第二步原料6e替换为14d,制得标题产物59(16mg)。Using the procedure of Example 8, the first step starting material 8a was replaced with 4-bromo-2-chlorobenzylcarbamic acid tert-butyl ester, and the second step starting material 6e was replaced with 14d to give the title product 59 (16 mg).
MS m/z(ESI):577.5[M+1];MS m/z (ESI): 577.5 [M + 1];
1H NMR(400MHz,CDCl3)δ8.08(s,1H),7.52-7.74(m,7H),7.29-7.31(m,2H),6.93(t,1H),6.34(s,1H),4.81(d,2H),4.22(s,2H),3.48(m,1H),3.11(q,2H),1.47(d,6H),1.26(t,3H)。 1 H NMR (400MHz, CDCl 3 ) δ8.08 (s, 1H), 7.52-7.74 (m, 7H), 7.29-7.31 (m, 2H), 6.93 (t, 1H), 6.34 (s, 1H), 4.81 (d, 2H), 4.22 (s, 2H), 3.48 (m, 1H), 3.11 (q, 2H), 1.47 (d, 6H), 1.26 (t, 3H).
实施例60Example 60
N-(4-(乙磺酰基)苄基)-1-异丙基-2-(4-(三氟甲氧基)苄基)-1H-苯并[d]咪唑-5-甲酰胺60
N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-2-(4-(trifluoromethoxy)benzyl)-1H-benzo[d]imidazole-5-carboxamide 60
采用实施例42合成路线,将第一步原料42a替换为4-三氟甲氧基苯乙酸,得标题产物60(15mg)。The first step starting material 42a was replaced with 4-trifluoromethoxyphenylacetic acid using the procedure of Example 42 to give the title product 60 (15 mg).
MS m/z(ESI):559.9[M+1];MS m/z (ESI): 559.9 [M+1];
1H NMR(400MHz,CDCl3)δ8.52(s,2H),8.20-8.18(d,1H),8.00(s,1H),7.83-7.76(m,3H),7.57-7.55(d,2H),7.35-7.33(d,2H),7.26-7.24(d,2H),4.84-4.81(m,2H),4.68(s,2H),3.16-3.09(m,2H),1.58-1.57(d,6H),1.30-1.26(t,3H)。 1 H NMR (400MHz, CDCl 3 ) δ 8.52 (s, 2H), 8.20-8.18 (d, 1H), 8.00 (s, 1H), 7.83-7.76 (m, 3H), 7.57-7.55 (d, 2H) ), 7.35-7.33 (d, 2H), 7.26-7.24 (d, 2H), 4.84-4.81 (m, 2H), 4.68 (s, 2H), 3.16-3.09 (m, 2H), 1.58-1.57 (d , 6H), 1.30-1.26 (t, 3H).
实施例61Example 61
N-(4-(乙磺酰基)苄基)-2-(2-氟-4-(三氟甲基)苄基)-1-甲基-1H-吲哚-5-甲酰胺61N-(4-(ethylsulfonyl)benzyl)-2-(2-fluoro-4-(trifluoromethyl)benzyl)-1-methyl-1H-indole-5-carboxamide 61
采用实施例15合成路线,将第二步原料2-溴丙烷替换为碘甲烷,得标题产物61(15mg)。Using the synthetic route of Example 15, the second step of the starting material, 2-bromopropane, was replaced with methyl iodide to give the title product 61 (15 mg).
MS m/z(ESI):532.9[M+1];MS m/z (ESI): 532.9 [M + 1];
1H NMR(400MHz,CDCl3)δ8.10(s,1H),7.87(m,2H),7.74(d,1H),7.57(m,2H),7.30-7.37(m,3H),7.21(t,1H),6.83(t,1H),6.38(s,1H),4.80(d,2H),4.25(s,2H),3.68(s,3H),3.12(q,2H),1.32(t,3H)。 1 H NMR (400MHz, CDCl 3 ) δ8.10 (s, 1H), 7.87 (m, 2H), 7.74 (d, 1H), 7.57 (m, 2H), 7.30-7.37 (m, 3H), 7.21 ( t,1H), 6.83(t,1H), 6.38(s,1H), 4.80(d,2H), 4.25(s,2H), 3.68(s,3H),3.12(q,2H), 1.32(t , 3H).
实施例62Example 62
1-乙基-N-(4-(乙磺酰基)苄基)-2-(4-(三氟甲基)苄基)-1H-苯并[d]咪唑-5-甲酰胺621-ethyl-N-(4-(ethylsulfonyl)benzyl)-2-(4-(trifluoromethyl)benzyl)-1H-benzo[d]imidazole-5-carboxamide 62
采用实施例42合成路线,将第一步原料42a替换为4-三氟甲基苯乙酸,42b替换为3-氨基-4-(乙基氨基)苯甲酸甲酯(采用公知的方法“Bioorganic&Medicinal Chemistry,2005,13(5),1587-1597”制备而得),得标题产物62(15mg)。Using the synthetic route of Example 42, the first step starting material 42a was replaced with 4-trifluoromethylphenylacetic acid, and 42b was replaced with methyl 3-amino-4-(ethylamino)benzoate (using a known method "Bioorganic & Medicinal Chemistry , 2005, 13(5), 1587-1597 "prepared to give the title product 62 (15 mg).
MS m/z(ESI):530.4[M+1]MS m/z (ESI): 530.4 [M+1]
1H NMR(400MHz,DMSO-d6)δ9.16(t,1H),9.21(s,1H),7.83-7.85(m,3H),7.71(d,2H),7.55-7.62(m,5H),4.60(d,2H),4.47(s,2H),4.27(t,2H),3.26(q,2H),1.23(t,
3H),1.09(t,3H)。 1 H NMR (400 MHz, DMSO-d 6 ) δ 9.16 (t, 1H), 9.21 (s, 1H), 7.83-7.85 (m, 3H), 7.71 (d, 2H), 7.55-7.62 (m, 5H) ), 4.60 (d, 2H), 4.47 (s, 2H), 4.27 (t, 2H), 3.26 (q, 2H), 1.23 (t, 3H), 1.09 (t, 3H).
实施例63Example 63
1-环丙基-N-((1-(环丙基磺酰基)哌啶-4-基)甲基)-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺631-cyclopropyl-N-((1-(cyclopropylsulfonyl)piperidin-4-yl)methyl)-2-(4-(trifluoromethyl)benzyl)-1H-indole- 5-formamide 63
从(哌啶-4-基甲基)氨基甲酸叔丁酯(采用专利申请“WO2001096303A1”公开的方法制备而得)出发,采用实施例19类似合成路线,制得标题产物63(15mg)。Starting from tert-butyl (piperidin-4-ylmethyl)carbamate (prepared by the method disclosed in the patent application "WO2001096303A1"), the title product 63 (15 mg) was obtained using a similar synthetic route of Example 19.
MS m/z(ESI):560.5[M+1];MS m/z (ESI): 560.5 [M + 1];
1H NMR(400MHz,CDCl3)δ7.95(m,1H),7..63-7.53(m,4H),7.35-7.33(m,2H),6.32-6.27(m,2H),4.31(s,2H),3.85-3.82(m,3H),3.42-3.39(t,2H),3.10-3.07(t,1H),2.93-2.90(m,1H),2.85-2.78(m,3H),2.29-2.23(m,1H),1.28-1.25(m,2H),1.18-1.16(m,4H),1.06-1.00(m,2H),0.98-0.96(m,2H)。 1 H NMR (400 MHz, CDCl 3 ) δ 7.95 (m, 1H), 7.. 63-7.53 (m, 4H), 7.35-7.33 (m, 2H), 6.32-6.27 (m, 2H), 4.31 ( s, 2H), 3.85-3.82 (m, 3H), 3.42-3.39 (t, 2H), 3.10-3.07 (t, 1H), 2.93-2.90 (m, 1H), 2.85-2.78 (m, 3H), 2.29-2.23 (m, 1H), 1.28-1.25 (m, 2H), 1.18-1.16 (m, 4H), 1.06-1.00 (m, 2H), 0.98-0.96 (m, 2H).
实施例64Example 64
1-烯丙基-N-(4-(乙磺酰基)苄基)-2-(2-氟-4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺641-allyl-N-(4-(ethylsulfonyl)benzyl)-2-(2-fluoro-4-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 64
采用实施例15合成路线,将第二步原料2-溴丙烷替换为烯丙基溴,得第四步产物64(13mg)。Using the synthetic route of Example 15, the second step starting material, 2-bromopropane, was replaced with allyl bromide to give the fourth step product 64 (13 mg).
MS m/z(ESI):559.4[M+1];MS m/z (ESI): 559.4 [M+1];
1H NMR(400MHz,CDCl3)δ8.11(s,1H),7.89-7.91(m,2H),7.58-7.63(m,3H),7.37-7.41(m,2H),7.30-7.34(m,2H),6.70(t,1H),5.99(m,1H),5.01(dd,2H),4.81(d,2H),4.21(s,3H),3.61(d,2H),3.14(q,2H),1.31(t,3H)。 1 H NMR (400 MHz, CDCl 3 ) δ 8.11 (s, 1H), 7.89-7.91 (m, 2H), 7.58-7.63 (m, 3H), 7.37-7.41 (m, 2H), 7.30-7.34 (m) , 2H), 6.70 (t, 1H), 5.99 (m, 1H), 5.01 (dd, 2H), 4.81 (d, 2H), 4.21 (s, 3H), 3.61 (d, 2H), 3.14 (q, 2H), 1.31 (t, 3H).
实施例65Example 65
1-环丙基-N-((5-(乙磺酰基)吡啶-2-基)甲基)-2-((4-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酰胺65
1-cyclopropyl-N-((5-(ethylsulfonyl)pyridin-2-yl)methyl)-2-((4-(trifluoromethyl)piperidin-1-yl)methyl)- 1H-吲哚-5-carboxamide 65
采用实施例20合成路线,将实施例20的第一步原料19c替换为33b,将第三步原料(4-(乙磺酰基)苯基)甲胺替换为11a,制得标题产物65(38mg)。Using the synthesis scheme of Example 20, the first step starting material 19c of Example 20 was replaced with 33b, and the third step starting material (4-(ethylsulfonyl)phenyl)methanamine was replaced with 11a to give the title product 65 (38 mg). ).
MS m/z(ESI):549.5[M+1];MS m/z (ESI): 549.5 [M + 1];
1H NMR(400MHz,DMSO-d6)δ9.13(brs,1H),8.97(s,1H),8.27(d,1H),8.15(s,1H),7.75(d,1H),7.59(d,2H),6.46(s,1H),4.69(d,2H),3.73(s,2H),3.38(q,2H),3.27(brs,1H),3.01(d,2H),2.28(brs,1H),2.07(t,2H),1.78(d,2H),1.47(q,2H),1.06-1.17(m,7H)。 1 H NMR (400MHz, DMSO- d 6) δ9.13 (brs, 1H), 8.97 (s, 1H), 8.27 (d, 1H), 8.15 (s, 1H), 7.75 (d, 1H), 7.59 ( d, 2H), 6.46 (s, 1H), 4.69 (d, 2H), 3.73 (s, 2H), 3.38 (q, 2H), 3.27 (brs, 1H), 3.01 (d, 2H), 2.28 (brs) , 1H), 2.07 (t, 2H), 1.78 (d, 2H), 1.47 (q, 2H), 1.06-1.17 (m, 7H).
实施例66Example 66
1-乙基-N-(4-(乙磺酰基)苄基)-2-(哌啶-1-甲基)-1H-吲哚-5-甲酰胺661-ethyl-N-(4-(ethylsulfonyl)benzyl)-2-(piperidin-1-methyl)-1H-indole-5-carboxamide 66
从(丙-2-炔-1-基氧基)四氢-2H-吡喃(采用公知的方法“ChemCatChem,2016,8(18),2912-2915”制备而得)出发,采用实施例33类似合成路线,制得标题产物66(21mg)。 Starting from (prop-2-yn-1-yloxy)tetrahydro-2H-pyran (prepared by the known method "ChemCatChem, 2016, 8 (18), 2912-2915"), Example 33 was employed. A similar synthetic route gave the title product 66 (21 mg).
MS m/z(ESI):468.4[M+1];MS m/z (ESI): 468.4 [M+1];
1H NMR(400MHz,DMSO-d6)δ9.06(brs,1H),8.13(s,1H),7.83(d,2H),7.73(brs,1H),7.58(d,2H),7.49(brs,1H),6.41(s,1H),4.58(d,2H),4.27(brs,2H),3.58(brs,2H),3.25(q,2H),2.36(brs,3H),1.79(brs,2H),1.48(brs,2H),1.39(brs,2H),1.31(brs,2H),1.23(brs,2H),1.08(t,3H)。 1 H NMR (400 MHz, DMSO-d 6 ) δ 9.06 (brs, 1H), 8.13 (s, 1H), 7.83 (d, 2H), 7.73 (brs, 1H), 7.58 (d, 2H), 7.49 ( Brs, 1H), 6.41 (s, 1H), 4.58 (d, 2H), 4.27 (brs, 2H), 3.58 (brs, 2H), 3.25 (q, 2H), 2.36 (brs, 3H), 1.79 (brs) , 2H), 1.48 (brs, 2H), 1.39 (brs, 2H), 1.31 (brs, 2H), 1.23 (brs, 2H), 1.08 (t, 3H).
实施例67Example 67
1-乙基-N-(4-(乙磺酰基)苄基)-2-(吗啉甲基)-1H-吲哚-5-甲酰胺671-ethyl-N-(4-(ethylsulfonyl)benzyl)-2-(morpholinylmethyl)-1H-indole-5-carboxamide 67
采用实施例66合成路线,将第三步原料哌啶替换为吗啉,制得标题产物67(21mg)。The title product 67 (21 mg) was obtained by the procedure of the procedure of Example 66, which was obtained from the title compound.
MS m/z(ESI):470.4[M+1];MS m/z (ESI): 470.4 [M+1];
1H NMR(400MHz,DMSO-d6)δ9.06(brs,1H),8.16(brs,1H),7.83(d,2H),7.73(brs,
1H),7.58(d,2H),7.50(brs,1H),6.47(brs,1H),4.59(d,2H),4.32(brs,2H),3.57-3.64(m,4H),3.36(brs,4H),3.27(q,2H),2.41(brs,2H),1.30(brs,3H),1.08(t,3H)。 1 H NMR (400MHz, DMSO- d 6) δ9.06 (brs, 1H), 8.16 (brs, 1H), 7.83 (d, 2H), 7.73 (brs, 1H), 7.58 (d, 2H), 7.50 ( Brs, 1H), 6.47 (brs, 1H), 4.59 (d, 2H), 4.32 (brs, 2H), 3.57-3.64 (m, 4H), 3.36 (brs, 4H), 3.27 (q, 2H), 2.41 (brs, 2H), 1.30 (brs, 3H), 1.08 (t, 3H).
实施例68Example 68
1-乙基-N-(4-(乙磺酰基)苄基)-2-((4-甲基哌嗪-1-基)甲基)-1H-吲哚-5-甲酰胺681-ethyl-N-(4-(ethylsulfonyl)benzyl)-2-((4-methylpiperazin-1-yl)methyl)-1H-indole-5-carboxamide 68
从哌嗪-1-羧酸叔丁酯68a(采用公知的方法“Chemical Communications(Cambridge,United Kingdom),2013,49(61),6867-6869”制备而得)出发,采用实施例33类似合成路线,制得标题产物68(11mg)。Starting from piperazine-1-carboxylic acid tert-butyl ester 68a (prepared by the known method "Chemical Communications (Cambridge, United Kingdom), 2013, 49 (61), 6867-6869"), similar synthesis was carried out using Example 33 Route, the title product 68 (11 mg) was obtained.
MS m/z(ESI):483.4[M+1];MS m/z (ESI): 483.4 [M+1];
1H NMR(400MHz,DMSO-d6)δ9.06(brs,1H),8.15(s,1H),7.83-7.85(m,2H),7.72(d,1H),7.58(d,2H),7.49(d,1H),6.49(s,1H),4.589(d,2H),4.28(q,2H),3.7(s,2H),3.42(brs,4H),3.23(q,2H),2.85(brs,4H),2.60(s,3H),1.31(t,3H),1.09(t,3H) 1 H NMR (400 MHz, DMSO-d 6 ) δ 9.06 (brs, 1H), 8.15 (s, 1H), 7.83-7.85 (m, 2H), 7.72 (d, 1H), 7.58 (d, 2H), 7.49 (d, 1H), 6.49 (s, 1H), 4.589 (d, 2H), 4.28 (q, 2H), 3.7 (s, 2H), 3.42 (brs, 4H), 3.23 (q, 2H), 2.85 (brs, 4H), 2.60 (s, 3H), 1.31 (t, 3H), 1.09 (t, 3H)
实施例69Example 69
N-(4-(乙磺酰基)苄基)-1-甲基-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺69N-(4-(ethylsulfonyl)benzyl)-1-methyl-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 69
采用实施例3的合成路线,将第二步原料碘乙烷替换为碘甲烷,制得标题产物69(8mg)。Using the synthetic route of Example 3, the second step of the starting material iodoethane was replaced with methyl iodide to give the title product 69 (8 mg).
MS m/z(ESI):515.4[M+1];MS m/z (ESI): 515.4 [M+1];
实施例70Example 70
2-(2-溴苄基)-N-(4-(乙磺酰基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺702-(2-Bromobenzyl)-N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-1H-indole-5-carboxamide 70
采用实施例1的合成路线,将第一步原料1b替换为1-溴-2-(溴甲基)苯,制得标题产物70(21mg)。Using the synthetic route of Example 1, the first starting material 1b was replaced with 1-bromo-2-(bromomethyl)benzene to give the title product 70 (21 mg).
MS m/z(ESI):553.3[M+1];
MS m/z (ESI): 553.3 [M+1];
1H NMR(400MHz,CDCl3)δ8.03(s,1H),7.85(d,2H),7.60-7.64(m,2H),7.53-7.56(m,3H),7.18-7.20(m,1H),7.12-7.14(m,1H),6.97-6.99(m,1H),6.65(t,1H),6.25(s,1H),4.77(d,2H),4.44-4.49(m,1H),4.23(s,2H),3.09(q,2H),1.53(d,6H),1.28(t,3H)。 1 H NMR (400MHz, CDCl 3 ) δ8.03 (s, 1H), 7.85 (d, 2H), 7.60-7.64 (m, 2H), 7.53-7.56 (m, 3H), 7.18-7.20 (m, 1H ), 7.12-7.14 (m, 1H), 6.97-6.99 (m, 1H), 6.65 (t, 1H), 6.25 (s, 1H), 4.77 (d, 2H), 4.44-4.49 (m, 1H), 4.23 (s, 2H), 3.09 (q, 2H), 1.53 (d, 6H), 1.28 (t, 3H).
实施例71Example 71
2-(2-氰苄基)-N-(4-(乙磺酰基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺712-(2-cyanobenzyl)-N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-1H-indole-5-carboxamide 71
采用实施例1的合成路线,将第一步原料1b替换为2-(溴甲基)苯甲腈,制得标题产物71(15mg)。Using the synthetic route of Example 1, the first step starting material 1b was replaced with 2-(bromomethyl)benzonitrile to give the title product 71 (15 mg).
MS m/z(ESI):500.2[M+1]MS m/z (ESI): 500.2 [M+1]
实施例72Example 72
2-(2-环丙基苄基)-N-(4-(乙磺酰基)苄基)-1-异丙基-1H-吲哚-5-甲酰胺722-(2-Cyclopropylbenzyl)-N-(4-(ethylsulfonyl)benzyl)-1-isopropyl-1H-indole-5-carboxamide 72
从1-溴-2-(溴甲基)苯出发,采用实施例1的类似合成路线,制得标题产物72(9mg)。Starting from 1-bromo-2-(bromomethyl)benzene, a similar synthetic route from Example 1 was used to afford the title product 72 (9 mg).
MS m/z(ESI):515.3[M+1];MS m/z (ESI): 515.3 [M + 1];
实施例73Example 73
N-(4-(乙磺酰基)-2-氯苄基)-1-异丙基-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺73N-(4-(ethylsulfonyl)-2-chlorobenzyl)-1-isopropyl-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 73
采用实施例8的合成路线,将第一步原料8a替换为4-溴-2-氯苄基氨基甲酸叔丁酯,制得标题产物73(11mg)。Using the synthetic route of Example 8, the first starting material 8a was replaced with 4-bromo-2-chlorobenzylcarbamic acid tert-butyl ester to give the title product 73 (11 mg).
MS m/z(ESI):577.1[M+1];MS m/z (ESI): 577.1 [M + 1];
1H NMR(400MHz,CDCl3)δ8.07(d,1H),7.88(d,1H),7.53-7.73(m,6H),7.35-7.44(m,2H),7.06(d,1H),6.31(s,1H),4.79(d,2H),4.38-4.45(m,1H),4.34(s,2H),3.11
(q,2H),1.48(d,6H),1.27(t,3H) 1 H NMR (400MHz, CDCl 3 ) δ8.07 (d, 1H), 7.88 (d, 1H), 7.53-7.73 (m, 6H), 7.35-7.44 (m, 2H), 7.06 (d, 1H), 6.31(s,1H), 4.79(d,2H), 4.38-4.45(m,1H), 4.34(s,2H),3.11 (q,2H), 1.48(d,6H),1.27(t,3H)
实施例74Example 74
1-环丙基-2-((4,4-二氟哌啶-1-基)甲基)-N-((5-(乙磺酰基)吡啶-2-基)甲基)-1H-吲哚-5-甲酰胺741-cyclopropyl-2-((4,4-difluoropiperidin-1-yl)methyl)-N-((5-(ethylsulfonyl)pyridin-2-yl)methyl)-1H-吲哚-5-formamide 74
第一步first step
1-环丙基-2-((4,4-二氟哌啶-1-基)甲基)-1H-吲哚-5-甲腈74b1-cyclopropyl-2-((4,4-difluoropiperidin-1-yl)methyl)-1H-indole-5-carbonitrile 74b
从4,4-二氟哌啶出发,采用实施例38类似合成路线,制得标题产物74(10mg)。MS m/z(ESI):517.5[M+1];Starting from 4,4-difluoropiperidine, the title compound 74 (10 mg) was obtained using the procedure of the procedure of Example 38. MS m/z (ESI): 517.5 [M + 1];
实施例75Example 75
6-氯-1-环丙基-N-((1-(乙磺酰基)哌啶-4-基)甲基)-2-(4-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺756-Chloro-1-cyclopropyl-N-((1-(ethylsulfonyl)piperidin-4-yl)methyl)-2-(4-(trifluoromethyl)benzyl)-1H-indole哚-5-formamide 75
从6-氯-1H-吲哚-5-甲酸甲酯出发,采用实施例19类似合成路线,制得标题产物75(5mg)。Starting from 6-chloro-1H-indole-5-carboxylic acid methyl ester, the title compound (yield: 75 mg)
MS m/z(ESI):582.2[M+1]MS m/z (ESI): 582.2 [M+1]
实施例76Example 76
1-环丙基-N-(4-(乙磺酰基)苄基)-2-(4-(三氟甲基)苯甲酰基)-1H-吲哚-5-甲酰胺761-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-(4-(trifluoromethyl)benzoyl)-1H-indole-5-carboxamide 76
从20a出发,采用实施例1类似合成路线,得到标题产物76(35mg)。Starting from 20a, a similar synthetic route to Example 1 was used to give the title product 76 (35 mg).
MS m/z(ESI):555.3[M+1];
MS m/z (ESI): 555.3 [M + 1];
1H NMR(400MHz,DMSO-d6)δ9.21(t,1H),8.33(s,1H),8.11(d,2H),7.96-7.98(m,3H),7.84(d,2H),7.74(d,1H),7.59(d,2H),7.13(s,1H),4.60(d,2H),3.62-3.67(m,1H),3.25(q,2H),1.15(brs,2H),1.08(t,3H),0.80-0.84(m,2H) 1 H NMR (400 MHz, DMSO-d 6 ) δ 9.21 (t, 1H), 8.33 (s, 1H), 8.11 (d, 2H), 7.96-7.98 (m, 3H), 7.84 (d, 2H), 7.74 (d, 1H), 7.59 (d, 2H), 7.13 (s, 1H), 4.60 (d, 2H), 3.62-3.67 (m, 1H), 3.25 (q, 2H), 1.15 (brs, 2H) , 1.08 (t, 3H), 0.80-0.84 (m, 2H)
实施例77Example 77
1-环丙基-N-(4-(乙磺酰基)苄基)-2-(羟基(4-(三氟甲基)苯基)甲基)-1H-吲哚-5-甲酰胺771-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-(hydroxy(4-(trifluoromethyl)phenyl)methyl)-1H-indole-5-carboxamide 77
将76(10mg,0.018mmol)溶于4mL甲醇和四氢呋喃的混合溶剂(V:V=3:1),加入硼氢化钠(1.5mg,0.036mmol),搅拌反应2小时。反应减压浓缩,用薄层色谱法以展开剂体系A纯化所得残余物,得标题产物77(9.7mg,产率:96%)。76 (10 mg, 0.018 mmol) was dissolved in 4 mL of a mixed solvent of methanol and tetrahydrofuran (V: V = 3:1), sodium borohydride (1.5 mg, 0.036 mmol) was added, and the reaction was stirred for 2 hours. The reaction mixture was concentrated under reduced pressure. EtOAcjjjjjjj
MS m/z(ESI):557.4[M+1];MS m/z (ESI): 557.4 [M + 1];
1H NMR(400MHz,DMSO-d6)δ9.06(brs,1H),8.11(s,1H),7.83(d,2H),7.73-7.75(m,3H),7.67(d,2H),7.56-7.58(m,3H),6.27(d,1H),6.24(d,1H),6.18(s,1H),4.57(d,2H),3.25(q,2H),3.02-3.07(m,1H),1.15(brs,2H),1.07(t,3H),0.84(m,2H). 1 H NMR (400 MHz, DMSO-d 6 ) δ 9.06 (brs, 1H), 8.11 (s, 1H), 7.83 (d, 2H), 7.73-7.75 (m, 3H), 7.67 (d, 2H), 7.56-7.58 (m, 3H), 6.27 (d, 1H), 6.24 (d, 1H), 6.18 (s, 1H), 4.57 (d, 2H), 3.25 (q, 2H), 3.02-3.07 (m, 1H), 1.15 (brs, 2H), 1.07 (t, 3H), 0.84 (m, 2H).
实施例78Example 78
(S)-1-环丙基-N-(4-(乙磺酰基)苄基)-2-((2-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酰胺78(S)-1-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-((2-(trifluoromethyl)piperidin-1-yl)methyl)-1H-indole哚-5-formamide 78
采用实施例39的合成路线,将第六步原料11a替换为4-(乙磺酰基)苯基)甲胺,得到标题产物78(20mg)。The sixth step starting material 11a was replaced with 4-(ethylsulfonyl)phenyl)methylamine using the procedure of Example 39 to give the title product 78 (20 mg).
MS m/z(ESI):548.6[M+1];MS m/z (ESI): 548.6 [M+1];
1H NMR(400MHz,CDCl3)δ8.07(s,1H),7.85-7.87(m,2H),7.70-7.73(m,1H),
7.62-7.64(m,1H),7.4-7.56(m,2H),6.82-6.85(m,1H),6.63(s,1H),4.79-4.80(m,2H),4.40-4.54(m,2H),3.83-3.86(m,1H),3.33-3.40(m,3H),3.08-3.14(m,2H),2.85-2.89(m,1H),2.01-2.04(m,2H),1.63-1.80(m,5H),1.42-1.43(m,1H),1.03-1.08(m,2H),0.87-0.90(m,2H)。 1 H NMR (400MHz, CDCl 3 ) δ8.07 (s, 1H), 7.85-7.87 (m, 2H), 7.70-7.73 (m, 1H), 7.62-7.64 (m, 1H), 7.4-7.56 (m , 2H), 6.82-6.85 (m, 1H), 6.63 (s, 1H), 4.79-4.80 (m, 2H), 4.40-4.54 (m, 2H), 3.83-3.86 (m, 1H), 3.33-3.40 (m, 3H), 3.08-3.14 (m, 2H), 2.85-2.89 (m, 1H), 2.01-2.04 (m, 2H), 1.63-1.80 (m, 5H), 1.42-1.43 (m, 1H) , 1.03-1.08 (m, 2H), 0.87-0.90 (m, 2H).
实施例79Example 79
N-(4-(乙磺酰基)苄基)-6-氟-1-异丙基-2-(2-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺79N-(4-(ethylsulfonyl)benzyl)-6-fluoro-1-isopropyl-2-(2-(trifluoromethyl)benzyl)-1H-indole-5-carboxamide 79
从6-氟-1-(三异丙基硅基)-1H-吲哚-5-甲酸甲酯79a(采用公知的方法“European Journal of Organic Chemistry,2006,(13),2956-2969”制备而得)出发,采用实施例6类似合成路线,制得标题产物79(6mg)。Methyl 6-fluoro-1-(triisopropylsilyl)-1H-indole-5-carboxylate 79a (prepared by a known method "European Journal of Organic Chemistry, 2006, (13), 2956-2969" Starting from the same procedure as in Example 6, the title product 79 (6 mg) was obtained.
MS m/z(ESI):561.4[M+1];MS m/z (ESI): 561.4 [M+1];
1H NMR(400MHz,CDCl3)δ8.38(d,1H),7.91(d,2H),7.76(d,1H),7.61(d,2H),7.47(t,1H),7.42(s,1H),7.30-7.35(m,1H),7.24(d,1H),7.10(d,1H),6.35(s,1H),4.85(d,2H),4.39-4.42(m,1H),4.34(s,2H),1.34(q,2H),1.49(d,6H),1.32(t,3H)。 1 H NMR (400MHz, CDCl 3 ) δ8.38 (d, 1H), 7.91 (d, 2H), 7.76 (d, 1H), 7.61 (d, 2H), 7.47 (t, 1H), 7.42 (s, 1H), 7.30-7.35 (m, 1H), 7.24 (d, 1H), 7.10 (d, 1H), 6.35 (s, 1H), 4.85 (d, 2H), 4.39-4.42 (m, 1H), 4.34 (s, 2H), 1.34 (q, 2H), 1.49 (d, 6H), 1.32 (t, 3H).
实施例80Example 80
1-环丙基-N-((5-(乙磺酰基)吡啶-2-基)甲基)-2-(4-(-(三氟甲基)苄基)-1H-吲哚-5-甲酰胺801-cyclopropyl-N-((5-(ethylsulfonyl)pyridin-2-yl)methyl)-2-(4-(-(trifluoromethyl)benzyl)-1H-indole-5 -formamide 80
采用实施例20合成路线,将第三步原料(4-(乙磺酰基)苯基)甲胺替换为11a,得标题产物80(60mg)。Using the procedure of Example 20, the third step starting material (4-(ethylsulfonyl)phenyl)methanamine was replaced by 11a to give the title product 80 (60 mg).
MS m/z(ESI):542.4[M+1];MS m/z (ESI): 542.4 [M + 1];
1H NMR(400MHz,DMSO-d6)δ9.09(t,1H),8.95(d,1H),8.25(dd,1H),8.11(d,1H),7.70-7.73(m,3H),7.57(t,2H),7.51(d,2H),6.25(s,1H),4.68(d,2H),4.38(s,2H),3.39(q,2H),2.97-2.99(m,1H),1.11-1.16(m,5H),1.00-1.02(m,2H)。 1 H NMR (400MHz, DMSO- d 6) δ9.09 (t, 1H), 8.95 (d, 1H), 8.25 (dd, 1H), 8.11 (d, 1H), 7.70-7.73 (m, 3H), 7.57(t,2H), 7.51(d,2H), 6.25(s,1H), 4.68(d,2H), 4.38(s,2H), 3.39(q,2H),2.97-2.99(m,1H) , 1.11-1.16 (m, 5H), 1.00-1.02 (m, 2H).
实施例81Example 81
2-(4-氯-2-(三氟甲基)苄基)-N-(4-(乙磺酰基)苄基)-6-氟-1-(2-氟乙基)-1H-吲哚-5-甲
酰胺812-(4-Chloro-2-(trifluoromethyl)benzyl)-N-(4-(ethylsulfonyl)benzyl)-6-fluoro-1-(2-fluoroethyl)-1H-indole哚-5-A
Amide 81
采用实施例5合成路线,将第一步原料5a替换为79b,将第二步原料2-溴丙烷替换为1-溴-2-氟乙烷,制得标题产物81(55mg)。Using the procedure of Example 5, the first step starting material 5a was replaced with 79b, and the second step starting material 2-bromopropane was replaced with 1-bromo-2-fluoroethane to give the title product 81 (55 mg).
MS m/z(ESI):599.4[M+1];MS m/z (ESI): 599.4 [M+1];
1H NMR(400MHz,DMSO-d6)δ8.76(brs,1H),7.84-7.86(m,3H),7.79(d,1H),7.76(d,1H),7.58-7.60(m,2H),7.48(d,1H),7.34(d,1H),5.94(s,1H),4.73(brs,1H),4.57-4.61(m,3H),4.51(brs,1H),4.44(brs,1H),4.31(s,2H),3.26(q,2H),1.09(t,3H)。 1 H NMR (400MHz, DMSO- d 6) δ8.76 (brs, 1H), 7.84-7.86 (m, 3H), 7.79 (d, 1H), 7.76 (d, 1H), 7.58-7.60 (m, 2H ), 7.48 (d, 1H), 7.34 (d, 1H), 5.94 (s, 1H), 4.73 (brs, 1H), 4.57-4.61 (m, 3H), 4.51 (brs, 1H), 4.44 (brs, 1H), 4.31 (s, 2H), 3.26 (q, 2H), 1.09 (t, 3H).
实施例82Example 82
1-环丙基-N-(4-(乙磺酰基)苄基)-2-((6-(三氟甲基)吡啶-3-基)甲基)-1H-吲哚-5-甲酰胺821-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-((6-(trifluoromethyl)pyridin-3-yl)methyl)-1H-indole-5-A Amide 82
从5-(溴甲基)-2-(三氟甲基)吡啶出发,采用实施例1类似合成路线,制得标题产物82(55mg)。Starting from 5-(bromomethyl)-2-(trifluoromethyl)pyridine, the title compound 82 (55 mg) was obtained.
MS m/z(ESI):542.2[M+1];MS m/z (ESI): 542.2 [M + 1];
1H NMR(400MHz,DMSO-d6)δ9.03(t,1H),8.77(s,1H),8.08(s,1H),7.94(d,1H),7.83-7.89(m,3H),7.71(d,1H),7.54-7.59(m,3H),6.18(s,1H),4.58(d,2H),4.42(s,2H),3.25(q,2H),3.02-3.06(m,1H),1.10-1.12(m,2H),1.08(t,3H),1.02-1.03(m,2H)。 1 H NMR (400 MHz, DMSO-d 6 ) δ 9.03 (t, 1H), 8.77 (s, 1H), 8.08 (s, 1H), 7.94 (d, 1H), 7.83-7.89 (m, 3H), 7.71 (d, 1H), 7.54 - 7.59 (m, 3H), 6.18 (s, 1H), 4.58 (d, 2H), 4.42 (s, 2H), 3.25 (q, 2H), 3.02-3.06 (m, 1H), 1.10 - 1.12 (m, 2H), 1.08 (t, 3H), 1.02-1.03 (m, 2H).
实施例83Example 83
1-环丙基-N-(4-(乙磺酰基)苄基)-2-((5-(三氟甲基)吡啶-2-基)甲基)-1H-吲哚-5-甲酰胺83
1-cyclopropyl-N-(4-(ethylsulfonyl)benzyl)-2-((5-(trifluoromethyl)pyridin-2-yl)methyl)-1H-indole-5-A Amide 83
从2-(溴甲基)-5-(三氟甲基)吡啶(采用专利申请“WO2009103478A1”公开的方法制备而得)出发,采用实施例1类似合成路线,制得标题产物83(12mg)。Starting from 2-(bromomethyl)-5-(trifluoromethyl)pyridine (prepared by the method disclosed in the patent application "WO2009103478A1"), using the similar synthetic route of Example 1, the title product 83 (12 mg) was obtained. .
MS m/z(ESI):542.2[M+1]MS m/z (ESI): 542.2 [M+1]
实施例84Example 84
1-环丙基-N-((5-(乙磺酰基)吡啶-2-基)甲基)-6-氟-2-((4-(三氟甲基)哌啶-1-基)甲基)-1H-吲哚-5-甲酰胺841-cyclopropyl-N-((5-(ethylsulfonyl)pyridin-2-yl)methyl)-6-fluoro-2-((4-(trifluoromethyl)piperidin-1-yl) Methyl)-1H-indole-5-carboxamide 84
从4-氨基-2-氟苯甲酸甲酯出发,采用实施例33类似合成路线,得到标题产物84(27mg)。Starting from methyl 4-amino-2-fluorobenzoate, a similar synthetic route of Example 33 was used to give the title product 84 (27 mg).
MS m/z(ESI):566.5[M+1]MS m/z (ESI): 566.5 [M+1]
实施例85Example 85
1-环丙基-N-((5-(乙磺酰基)吡啶-2-基)甲基)-2-((3-甲基-5-(三氟甲基)-1H-吡唑-1-基)甲基)-1H-吲哚-5-甲酰胺851-cyclopropyl-N-((5-(ethylsulfonyl)pyridin-2-yl)methyl)-2-((3-methyl-5-(trifluoromethyl)-1H-pyrazole- 1-yl)methyl)-1H-indole-5-carboxamide 85
采用实施例31合成路线,将第六步原料31i替换为11a,制得标题产物85(30mg)。Using the synthesis route of Example 31, the sixth step starting material 31i was replaced with 11a to give the title product 85 (30 mg).
MS m/z(ESI):546.5[M+1];MS m/z (ESI): 546.5 [M + 1];
1H NMR(400MHz,DMSO-d6)δ9.12(brs,1H),8.95(s,1H),8.23-8.25(m,1H),8.13(s,1H),7.78(d,1H),7.57-7.62(m,2H),6.79(s,1H),6.17(s,1H),5.72(s,2H),4.67(s,2H),3.39(d,2H),3.21(brs,1H),2.20(s,3H),1.11-1.16(m,5H),1.01(brs,2H)。
1 H NMR (400MHz, DMSO- d 6) δ9.12 (brs, 1H), 8.95 (s, 1H), 8.23-8.25 (m, 1H), 8.13 (s, 1H), 7.78 (d, 1H), 7.57-7.62 (m, 2H), 6.79 (s, 1H), 6.17 (s, 1H), 5.72 (s, 2H), 4.67 (s, 2H), 3.39 (d, 2H), 3.21 (brs, 1H) , 2.20 (s, 3H), 1.11-1.16 (m, 5H), 1.01 (brs, 2H).
生物学评价Biological evaluation
以下结合测试例进一步描述解释本发明,但这些实施例并非意味着限制本发明的范围。The invention is further described below in conjunction with the test examples, but these examples are not intended to limit the scope of the invention.
测试例1、本发明实施例化合物对RORγ体外活性的测定Test Example 1. Determination of in vitro activity of RORγ by the compounds of the examples of the present invention
一、实验材料及仪器First, experimental materials and instruments
2.RORγLBD(AB Vector)2.RORγLBD(AB Vector)
3.DMSO(SigmaAldrich)3. DMSO (Sigma Aldrich)
4.384孔细胞培养板(Perkin Elmer)4.384-well cell culture plate (Perkin Elmer)
5.酶标仪(Tecan)5. Microplate reader (Tecan)
二、实验步骤Second, the experimental steps
采用LanthaScreen TR-FRET(时间分辨荧光能量共振转移)RORγ共激活体系筛选本发明的化合物对RORγ活性的调节。Modulation of RORy activity by the compounds of the invention was screened using a LanthaScreen TR-FRET (Time Resolved Fluorescence Energy Resonance Transfer) RORγ co-activation system.
首先配制完整缓冲液D(complete TR-FRET Coregulator)(Life Technologies)包含终浓度5mM DTT。DMSO终浓度为2%。将待测化合物在含有2%DMSO的完整缓冲液D中连续稀释为2x终浓度,最高剂量为的60μm。10μl/孔加入384孔板的试验孔(PerkinElmer)。每个检测化合物在相同浓度下设置2个平行对照孔。准备4X RORγLBD(AB Vector)。使用完整缓冲液D稀释RORγLBD浓度为1ng/μL。5μl/孔加入384孔测定板的试验孔。阴性对照孔为5μL完整缓冲液D,无RORγLBD。使用完全缓冲液D配制含有0.6μM荧光素-D22(4X)和8nM铽(Tb)标记的抗GST抗体(4X)(Life Technologies)混合液,将5μL混合液加入到384孔板中。总反应体系为20μL。在振荡器上轻轻混匀该384孔板并在室温下避光孵育2-4小时。The Complete TR-FRET Coregulator (Life Technologies) was first formulated to contain a final concentration of 5 mM DTT. The final concentration of DMSO was 2%. The test compound was serially diluted to 2 x final concentration in intact buffer D containing 2% DMSO at a maximum dose of 60 μm. 10 μl/well was added to the test well of a 384-well plate (PerkinElmer). Two parallel control wells were placed at the same concentration for each test compound. Prepare 4X RORγLBD (AB Vector). The ROR γLBD concentration was diluted to 1 ng/μL using intact buffer D. 5 μl/well was added to the test well of a 384-well assay plate. The negative control wells were 5 [mu]L of intact buffer D without ROR[gamma]LBD. A mixture of 0.6 μM fluorescein-D22 (4X) and 8 nM 铽 (Tb)-labeled anti-GST antibody (4X) (Life Technologies) was prepared using Complete Buffer D, and 5 μL of the mixture was added to a 384-well plate. The total reaction system was 20 μL. The 384-well plate was gently mixed on a shaker and incubated for 2-4 hours at room temperature in the dark.
使用Tecan Infinite M1000检测荧光读数,通过GraphPad Prism 6.0软件绘制发射波长520nm/495nm的比值与化合物浓度的对数曲线,计算待测化合物的IC50/EC50值。Fluorescence readings were detected using a Tecan Infinite M1000, and a logarithmic curve of the ratio of the emission wavelength of 520 nm / 495 nm to the concentration of the compound was plotted by GraphPad Prism 6.0 software to calculate the IC 50 /EC 50 value of the test compound.
本发明化合物对RORγ体外活性通过以上的试验进行测定,测得的IC50/EC50值见表1。The in vitro activity of the compounds of the present invention against RORγ was determined by the above test, and the measured IC 50 /EC 50 values are shown in Table 1.
表1 本发明化合物对RORγ体外活性的IC50/EC50值Table 1 IC 50 /EC 50 values of the compounds of the invention for RORγ in vitro activity
a:如果是反激动剂,数值标示为IC50;如果是激动剂,数值标示为EC50。 a: If it is an inverse agonist, the value is indicated as IC 50 ; if it is an agonist, the value is indicated as EC 50 .
结论:本发明化合物对RORγ体外活性具有明显的调节作用,在实验结果中显示在通式(I)所示的化合物中A环取代基的改变对RORγ体外活性的调节显示不同的机制,在A环的邻位有较大的取代基(例如:三氟甲基、甲氧基、乙基、三氟甲氧基)时,表现出激动的效果(见实施例1、2、4、6和10等),在A环的邻位有较小的取代基(例如:氢原子、氟)时,表现出抑制的效果(见实施例14、15、17和18等)。Conclusion: The compound of the present invention has a significant regulatory effect on the in vitro activity of RORγ. It is shown in the experimental results that the modification of the A ring substituent in the compound represented by the general formula (I) shows different mechanisms for the regulation of the activity of RORγ in vitro, in A. When the ortho position of the ring has a larger substituent (for example: trifluoromethyl, methoxy, ethyl, trifluoromethoxy), it exhibits an agonistic effect (see Examples 1, 2, 4, 6 and 10, etc., exhibits an inhibitory effect when there are smaller substituents (for example, hydrogen atom or fluorine) in the ortho position of the ring A (see Examples 14, 15, 17, and 18, etc.).
测试例2、本发明实施例化合物对IL-17A酶联免疫定量分析活性测定Test Example 2: Quantitative Analysis of Activity of IL-17A Enzyme-Linked Immunosorbent by Compounds of the Present Invention
一、实验材料及仪器
First, experimental materials and instruments
1.人外周血单核细胞(PBMC)(Zenbio)1. Human peripheral blood mononuclear cells (PBMC) (Zenbio)
2.淋巴细胞培养基(Zenbio)2. Lymphocyte medium (Zenbio)
3.TexMACS(Miltenyi Biotec)3.TexMACS (Miltenyi Biotec)
4.人Cytostim(Miltenyi Biotec)4. People Cytostim (Miltenyi Biotec)
5.人IL-17酶联免疫试剂盒(R&D系统)5. Human IL-17 enzyme-linked immunoassay kit (R&D system)
6.CO2培养箱(Fisher Scientific)6.CO 2 incubator (Fisher Scientific)
7.离心机(Fisher Scientific)7. Centrifuge (Fisher Scientific)
8.96孔细胞培养板(Fisher Scientific)8.96-well cell culture plate (Fisher Scientific)
9.酶标仪(Tecan)9. Microplate reader (Tecan)
二、实验步骤Second, the experimental steps
将冻存的人外周血单核细胞(PBMC)在预热的淋巴细胞培养基中快速复苏,离心1000rpm,10min,除去细胞培养上清,将细胞轻轻悬浮于TexMACS培养基中,计数细胞。在细胞悬液中按比例加入T细胞激活试剂cytostim(10μl/ml),然后以1×105外周血单核细胞/孔的密度将细胞种植于96孔细胞培养板中。使用TexMACS培养基梯度稀释待测化合物,分别加入各实验孔中,每组2-3个平行孔。准备只含细胞不含cytostim的阴性对照孔,以得到背景读数。将细胞培养板放置于5%二氧化碳37℃培养箱孵育3天。药物处理3天后收取细胞培养上清液,离心去除悬浮物。然后使用IL-17A酶联免疫试剂盒定量上清液中IL-17A。使用GraphPad Prism 6.0计算待测化合物的IC50/EC50值。Frozen human peripheral blood mononuclear cells (PBMC) were rapidly resuscitated in pre-warmed lymphocyte medium, centrifuged at 1000 rpm for 10 min, and the cell culture supernatant was removed, and the cells were gently suspended in TexMACS medium, and the cells were counted. The T cell activation reagent cytostim (10 μl/ml) was added in proportion to the cell suspension, and then the cells were seeded in 96-well cell culture plates at a density of 1 × 105 peripheral blood mononuclear cells/well. The test compounds were diluted with TexMACS medium and added to each test well, with 2-3 parallel wells per group. Prepare a negative control well containing only cells without cytostim to obtain a background reading. The cell culture plates were placed in a 5% carbon dioxide incubator at 37 ° C for 3 days. The cell culture supernatant was collected 3 days after the drug treatment, and the suspension was removed by centrifugation. The supernatant was then used to quantify IL-17A in the supernatant using the IL-17A enzyme-linked immunosorbent assay kit. Calculated using GraphPad Prism 6.0 50 / EC 50 value of the test compound IC.
本发明化合物对IL-17A酶联免疫定量分析通过以上的试验进行测定,测得的IC50/EC50值见表2。Quantitative analysis of the IL-17A enzyme-linked immunoassay of the compound of the present invention was carried out by the above test, and the measured IC 50 /EC 50 values are shown in Table 2.
表2 本发明化合物对IL-17A酶联免疫定量分析的IC50/EC50值Table 2 IC 50 /EC 50 values for quantitative analysis of IL-17A by immunoassay of the compounds of the invention
a:如果是反激动剂,数值标示为IC50;如果是激动剂,数值标示为EC50。a: If it is an inverse agonist, the value is indicated as IC 50 ; if it is an agonist, the value is indicated as EC 50 .
结论:本发明化合物对IL-17A酶联免疫定量分析活性具有明显的调节作用。Conclusion: The compounds of the present invention have a significant regulatory effect on the activity of IL-17A enzyme-linked immunoassay.
测试例3、本发明实施例化合物体内药效研究Test Example 3: In vivo pharmacodynamic study of the compound of the present invention
一、实验材料及仪器First, experimental materials and instruments
1.抗鼠-PD-1抗体(BioXcell)1. Anti-mouse-PD-1 antibody (BioXcell)
2.鼠IgG2a(BioXcell)2. Mouse IgG2a (BioXcell)
二、实验步骤Second, the experimental steps
通过检测MC38小鼠结肠肿瘤在同系C57BL/6小鼠上的生长情况,评估单独使用实施例30或实施例30与抗鼠-PD-1抗体联合用药的体内抗肿瘤活性。The in vivo antitumor activity of the combination of Example 30 or Example 30 alone with the anti-mouse-PD-1 antibody was evaluated by measuring the growth of MC38 mouse colon tumors on syngeneic C57BL/6 mice.
将MC38细胞(5×105)植入每只小鼠的右侧腹部皮下,待5天后,当肿瘤生长至40-80mm3后,将小鼠随机分成4组,分别给药。分组如下:MC38 cells (5 × 10 5 ) were implanted subcutaneously into the right abdomen of each mouse. After 5 days, when the tumors grew to 40-80 mm 3 , the mice were randomly divided into 4 groups and administered separately. Grouped as follows:
第①组:空白组,为CMC-Na溶剂配方与IgG2a同型对照抗体联合给药。其中CMC-Na溶剂配方的给药方案同第②组药物单用组,IgG2a同型对照抗体的给药方案同第③组抗体单用组。Group 1: The blank group was administered in combination with the IgG2a isotype control antibody for the CMC-Na solvent formulation. The dosage regimen of the CMC-Na solvent formulation is the same as that of the second group drug monotherapy group, and the administration scheme of the IgG2a isotype control antibody is the same as the third group antibody monotherapy group.
第②组:药物单用组,每天给药(实施例30,12.5mg/kg)2次,连续给药21天。Group 2: The drug alone group was administered twice a day (Example 30, 12.5 mg/kg) for 21 consecutive days.
第③组:抗体单用组,即图1中“抗PD-1抗体”组,在小鼠种瘤后第5,8,11,14天,对携带MC38肿瘤的小鼠腹腔(i.p.)注射抗鼠PD-1(CD279)抗体(BioXcell)(5mg/只)。
Group 3: The antibody alone group, the "anti-PD-1 antibody" group in Figure 1, was injected intraperitoneally (ip) to mice bearing MC38 tumors on days 5, 8, 11, and 14 after tumor implantation in mice. Anti-mouse PD-1 (CD279) antibody (BioXcell) (5 mg/head).
第④组:抗体与实施例30化合物联合用药组,即图1中“抗PD-1抗体+实施例30”。其中抗体用药同第③组抗体单用给药方案,实施例30化合物用药同第②组药物单用给药方案。Group 4: The antibody was administered in combination with the compound of Example 30, i.e., "anti-PD-1 antibody + Example 30" in Figure 1. The antibody administration is the same as the third group antibody single administration protocol, the compound of the third embodiment is the same as the second group drug single administration protocol.
用卡尺在三个维度中测量肿瘤体积,然后根据下式计算:The tumor volume is measured in three dimensions with a caliper and then calculated according to the following formula:
肿瘤体积(mm3)=l×w×h×0.5236,其中,1表示肿瘤长度,w表示肿瘤的宽度,h表示肿瘤的高度,单位为毫米。Tumor volume (mm 3 ) = l × w × h × 0.5236, where 1 represents the length of the tumor, w represents the width of the tumor, and h represents the height of the tumor in millimeters.
如图1所示,单独给药实施例30 12.5mg/kg时,TGI为34%。单独注射抗鼠PD-1(CD279)抗体(5mg/只)时,TGI为25%。当实施例30(12.5mg/kg)与抗鼠PD-1单克隆抗体(5mg/只)联合用药时,表现出较强的协同效应(TGI为68%)。As shown in Fig. 1, when 12.5 mg/kg of Example 30 was administered alone, the TGI was 34%. When the anti-mouse PD-1 (CD279) antibody (5 mg/mouse) was injected alone, the TGI was 25%. When Example 30 (12.5 mg/kg) was administered in combination with an anti-mouse PD-1 monoclonal antibody (5 mg/mouse), a strong synergistic effect (TGI of 68%) was exhibited.
TGI%=100x(TV对照-TV肿瘤-TV初始)/(TV对照-TV初始),其中TGI=肿瘤生长抑制率;TV对照=对照组的肿瘤体积;TV肿瘤=治疗组的肿瘤体积;TV初始=5天时的肿瘤体积。TGI%=100x (TV control- TV tumor- TV initial )/(TV control- TV initial ), where TGI=tumor growth inhibition rate; TV control =control group tumor volume; TV tumor =treatment group tumor volume; TV Initial = tumor volume at 5 days.
这些数据表明了在MC38结肠肿瘤模型中,单独施用实施例30表现出抑瘤活性,同时实施例30与PD-1抗体联合用药表现出较强的协同作用,这也表明实施例30具有与RORγ激活(而非抑制)一致的生物活性,为提高免疫治疗的疗效开辟了新途径。
These data indicate that in the MC38 colon tumor model, Example 30 alone showed antitumor activity, while Example 30 showed a strong synergistic effect with the PD-1 antibody combination, which also indicates that Example 30 has RORγ. Activating (rather than inhibiting) consistent biological activity opens up new avenues for improving the efficacy of immunotherapy.
Claims (29)
- 一种通式(I)所示的化合物:A compound of the formula (I):或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用的盐,Or a tautomer, a mesophil, a racemate, an enantiomer, a diastereomer or a mixture thereof, or a pharmaceutically acceptable salt thereof,其中:among them:X、Y和Z相同或不同,且各自独立地为CR9或N;X, Y and Z are the same or different and are each independently CR 9 or N;环A和环B相同或不同,且各自独立地选自环烷基、杂环基、芳基和杂芳基;Ring A and Ring B are the same or different and are each independently selected from the group consisting of a cycloalkyl group, a heterocyclic group, an aryl group, and a heteroaryl group;R1和R2相同或不同,且各自独立地选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、卤素、氨基、硝基、羟基、氰基、杂环基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)NHR8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、烷氧基、卤代烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 1 and R 2 are the same or different and are each independently selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen, an amino group, a nitro group, a hydroxyl group, a cyano group, and a heterocyclic ring. a base, an aryl group, a heteroaryl group, -OR 8 , -C(O)R 8 , -C(O)NHR 8 , -C(O)OR 8 and -S(O) m R 8 , wherein said Alkyl, cycloalkyl, alkoxy, haloalkyl, heterocyclyl, aryl and heteroaryl are each independently optionally selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, Substituted with one or more substituents of alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl;R3和R4相同或不同,且各自独立地选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、卤素、氨基、硝基、羟基、氰基、杂环基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、卤代烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 3 and R 4 are the same or different and are each independently selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen group, an amino group, a nitro group, a hydroxyl group, a cyano group, and a heterocyclic ring. a base, an aryl group, a heteroaryl group, -OR 8 , -C(O)R 8 , -C(O)OR 8 and -S(O) m R 8 , wherein said alkyl group, cycloalkyl group, halogenated alkane The radical, heterocyclyl, aryl and heteroaryl are each independently selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, Substituted by one or more substituents in the cycloalkyl, heterocyclyl, aryl and heteroaryl groups;或者R3和R4形成氧代基;Or R 3 and R 4 form an oxo group;R5选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、氨基、烯基、炔基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、卤代烷基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烯基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 5 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, an amino group, an alkenyl group, an alkynyl group, an aryl group, a heteroaryl group, -OR 8 , -C(O)R 8 , —C(O)OR 8 and —S(O) m R 8 , wherein said alkyl, cycloalkyl, haloalkyl, aryl and heteroaryl are each independently optionally selected from alkyl, One or more of haloalkyl, halogen, amino, nitro, cyano, hydroxy, alkenyl, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl Substituted by a substituent;R6选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、卤素、氨基、硝基、羟基、氰基、杂环基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、卤代烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代; R 6 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen, an amino group, a nitro group, a hydroxyl group, a cyano group, a heterocyclic group, an aryl group, a heteroaryl group, -OR 8 , —C(O)R 8 , —C(O)OR 8 and —S(O) m R 8 , wherein said alkyl, cycloalkyl, haloalkyl, heterocyclyl, aryl and heteroaryl The groups are each independently optionally selected from the group consisting of alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and Substituting one or more substituents in the heteroaryl;R7选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、杂环基、芳基和杂芳基;R 7 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a heterocyclic group, an aryl group, and a heteroaryl group;R8选自氢原子、烷基、卤代烷基、烷氧基、羟烷基、羟基、氨基、环烷基、杂环基、芳基和杂芳基,其中所述的烷基、氨基、环烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤素、羟基、氨基、羧酸酯基、硝基、氰基、烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 8 is selected from the group consisting of a hydrogen atom, an alkyl group, a halogenated alkyl group, an alkoxy group, a hydroxyalkyl group, a hydroxyl group, an amino group, a cycloalkyl group, a heterocyclic group, an aryl group, and a heteroaryl group, wherein the alkyl group, the amino group, and the ring are The alkyl group, heterocyclic group, aryl group and heteroaryl group are each independently selected from the group consisting of alkyl, halogen, hydroxy, amino, carboxylate, nitro, cyano, alkoxy, hydroxyalkyl, cyclo Substituted by one or more substituents of an alkyl group, a heterocyclic group, an aryl group, and a heteroaryl group;R9选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、氨基、烯基、炔基、芳基和杂芳基,其中所述的烷基、环烷基、卤代烷基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烯基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 9 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, an amino group, an alkenyl group, an alkynyl group, an aryl group and a heteroaryl group, wherein the alkyl group, the cycloalkyl group And haloalkyl, aryl and heteroaryl are each independently selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkenyl, alkoxy, haloalkoxy, hydroxyalkyl Substituting one or more substituents of a cycloalkyl, a heterocyclic group, an aryl group, and a heteroaryl group;m为0、1或2;m is 0, 1 or 2;x为0、1、2、3或4;x is 0, 1, 2, 3 or 4;y为0、1、2或3;且y is 0, 1, 2 or 3;z为0、1、2、3或4。z is 0, 1, 2, 3 or 4.
- 根据权利要求1所述的通式(I)所示的化合物,其为通式(II)所示的化合物:The compound of the formula (I) according to claim 1, which is a compound of the formula (II):其中:among them:X为CR9或N;X is CR 9 or N;Y为CH或N;Y is CH or N;R9为氢原子或烷基;且R 9 is a hydrogen atom or an alkyl group;环A、环B、R1~R7、x、y和z如权利要求1中所定义。Ring A, Ring B, R 1 to R 7 , x, y and z are as defined in claim 1.
- 根据权利要求1或2所述的通式(I)所示的化合物,其中A环和B环相同或不同,且各自独立地选自杂环基、芳基和杂芳基。The compound of the formula (I) according to claim 1 or 2, wherein the A ring and the B ring are the same or different and each is independently selected from a heterocyclic group, an aryl group and a heteroaryl group.
- 根据权利要求1~3中任一项所述的通式(I)所示的化合物,其为通式(II-A)所示的化合物: The compound of the formula (I) according to any one of claims 1 to 3, which is a compound represented by the formula (II-A):其中:among them:X为CR9或N;R9为氢原子或烷基;X is CR 9 or N; R 9 is a hydrogen atom or an alkyl group;G为CH或N;G is CH or N;Ra选自烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、溴、氨基、硝基、羟基、氰基、杂环基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)NHR8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、烷氧基、卤代烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R a is selected from the group consisting of alkyl, cycloalkyl, haloalkyl, alkoxy, haloalkoxy, bromo, amino, nitro, hydroxy, cyano, heterocyclyl, aryl, heteroaryl, -OR 8 , - C(O)R 8 , —C(O)NHR 8 , —C(O)OR 8 and —S(O) m R 8 , wherein said alkyl group, cycloalkyl group, alkoxy group, haloalkyl group, The heterocyclic group, the aryl group and the heteroaryl group are each independently selected from the group consisting of alkyl, haloalkyl, halogen, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkane. Substituted by one or more substituents in the group, heterocyclic group, aryl group and heteroaryl group;p为0、1、2或3;p is 0, 1, 2 or 3;环A、R1~R8、m、y和z如权利要求1中所定义。Rings A, R 1 to R 8 , m, y and z are as defined in claim 1.
- 根据权利要求1~3中任一项所述的通式(I)所示的化合物,其为通式(III)所示的化合物: The compound of the formula (I) according to any one of claims 1 to 3, which is a compound represented by the formula (III):其中:among them:X为CR9或N;X is CR 9 or N;Y为CH或N;Y is CH or N;R9为氢原子或烷基;且R 9 is a hydrogen atom or an alkyl group;环A、R1~R7、x、y和z如权利要求1中所定义。Ring A, R 1 to R 7 , x, y and z are as defined in claim 1.
- 根据权利要求1~3和6中任一项所述的通式(I)所示的化合物,其为通式(IV)所示的化合物:The compound of the formula (I) according to any one of claims 1 to 3 and 6, which is a compound represented by the formula (IV):其中among themX为CR9或N;X is CR 9 or N;Y为CH或N;Y is CH or N;R9为氢原子或烷基;且R 9 is a hydrogen atom or an alkyl group;R1~R7、x、y和z如权利要求1中所定义。R 1 to R 7 , x, y and z are as defined in claim 1.
- 根据权利要求1~3和6~7中任一项所述的通式(I)所示的化合物,其为通式(IV-A)所示的化合物:The compound of the formula (I) according to any one of claims 1 to 3 and 6 to 7, which is a compound represented by the formula (IV-A):其中:among them:X为CR9或N;X is CR 9 or N;Y为CH或N;Y is CH or N;R9为氢原子或烷基; R 9 is a hydrogen atom or an alkyl group;Ra选自烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、氨基、硝基、羟基、氰基、杂环基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)NHR8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、烷氧基、卤代烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R a is selected from the group consisting of alkyl, cycloalkyl, haloalkyl, alkoxy, haloalkoxy, amino, nitro, hydroxy, cyano, heterocyclyl, aryl, heteroaryl, -OR 8 , -C ( O) R 8 , -C(O)NHR 8 , -C(O)OR 8 and -S(O) m R 8 , wherein said alkyl group, cycloalkyl group, alkoxy group, haloalkyl group, heterocyclic ring The aryl group, the aryl group and the heteroaryl group are each independently selected from the group consisting of alkyl, haloalkyl, halogen, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, Substituting one or more substituents in a heterocyclic group, an aryl group, and a heteroaryl group;p为0、1、2或3;且p is 0, 1, 2 or 3;R1~R8、m、y和z如权利要求1中所定义。R 1 to R 8 , m, y and z are as defined in claim 1.
- 根据权利要求1~9中任一项所述的通式(I)所示的化合物,其中R1选自氢原子、烷基、环烷基、烷氧基、卤代烷基、卤代烷氧基、卤素、氰基、杂环基和-C(O)NHR8,其中所述的烷基、环烷基、烷氧基和杂环基各自独立地任选被选自烷基、卤素、羟基、氨基和氰基中的一个或多个取代基所取代;优选R1选自烷基、卤素、卤代烷基、烷氧基和卤代烷氧基;R8选自氢原子或烷基。The compound of the formula (I) according to any one of claims 1 to 9, wherein R 1 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, an alkoxy group, a halogenated alkyl group, a halogenated alkoxy group, and a halogen. And a cyano group, a heterocyclic group, and -C(O)NHR 8 , wherein each of the alkyl group, the cycloalkyl group, the alkoxy group and the heterocyclic group is independently selected from the group consisting of an alkyl group, a halogen group, a hydroxyl group, and an amino group. And one or more substituents in the cyano group are substituted; preferably, R 1 is selected from the group consisting of an alkyl group, a halogen, a halogenated alkyl group, an alkoxy group, and a halogenated alkoxy group; and R 8 is selected from a hydrogen atom or an alkyl group.
- 根据权利要求1~10中任一项所述的通式(I)所示的化合物,其中R2为卤素;优选氟或氯。The compound of the formula (I) according to any one of claims 1 to 10, wherein R 2 is a halogen; preferably fluorine or chlorine.
- 根据权利要求1~11中任一项所述的通式(I)所示的化合物,其中R3和R4相同或不同,且各自独立地选自氢原子、烷基、卤代烷基和羟基,或者R3和R4一起形成氧代基。The compound of the formula (I) according to any one of claims 1 to 11, wherein R 3 and R 4 are the same or different and are each independently selected from a hydrogen atom, an alkyl group, a halogenated alkyl group and a hydroxyl group. Or R 3 and R 4 together form an oxo group.
- 根据权利要求1~12中任一项所述的通式(I)所示的化合物,其中R5选自烷基、环烷基、卤代烷基、烯基和芳基,其中所述的烷基、环烷基和芳基各自独立地任选被选自烷基、卤素、烯基和羟基中的一个或多个取代基所取代;优选R5为异丙基或环丙基。 The compound of the formula (I) according to any one of claims 1 to 12, wherein R 5 is selected from the group consisting of alkyl, cycloalkyl, haloalkyl, alkenyl and aryl, wherein said alkyl group The cycloalkyl and aryl groups are each independently optionally substituted with one or more substituents selected from the group consisting of alkyl, halogen, alkenyl and hydroxy; preferably R 5 is isopropyl or cyclopropyl.
- 根据权利要求1~13中任一项所述的通式(I)所示的化合物,其中R7为任选被选自卤素、环烷基和羟基中的一个或多个取代基所取代的烷基;优选R7为乙基。The compound of the formula (I) according to any one of claims 1 to 13, wherein R 7 is optionally substituted by one or more substituents selected from the group consisting of halogen, cycloalkyl and hydroxy. Alkyl; preferably R 7 is ethyl.
- 一种通式(V)所示化合物:A compound of the formula (V):或其互变异构体、内消旋体、外消旋体、对映异构体、非对映异构体或其混合物形式,或其可药用盐,Or a tautomer, a meso form, a racemate, an enantiomer, a diastereomer or a mixture thereof, or a pharmaceutically acceptable salt thereof,其中:among them:X、Y和Z相同或不同,且各自独立地为CR9或N;X, Y and Z are the same or different and are each independently CR 9 or N;环A选自环烷基、杂环基、芳基和杂芳基;Ring A is selected from the group consisting of a cycloalkyl group, a heterocyclic group, an aryl group, and a heteroaryl group;R1选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、卤素、氨基、硝基、羟基、氰基、杂环基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)NHR8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、烷氧基、卤代烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 1 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen, an amino group, a nitro group, a hydroxyl group, a cyano group, a heterocyclic group, an aryl group, a heteroaryl group, -OR 8 , —C(O)R 8 , —C(O)NHR 8 , —C(O)OR 8 and —S(O) m R 8 , wherein said alkyl group, cycloalkyl group, alkoxy group, Haloalkyl, heterocyclyl, aryl and heteroaryl are each independently optionally selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl Substituting one or more substituents of a cycloalkyl, a heterocyclic group, an aryl group, and a heteroaryl group;R3和R4相同或不同,且各自独立地选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、卤素、氨基、硝基、羟基、氰基、杂环基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、卤代烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 3 and R 4 are the same or different and are each independently selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen group, an amino group, a nitro group, a hydroxyl group, a cyano group, and a heterocyclic ring. a base, an aryl group, a heteroaryl group, -OR 8 , -C(O)R 8 , -C(O)OR 8 and -S(O) m R 8 , wherein said alkyl group, cycloalkyl group, halogenated alkane The radical, heterocyclyl, aryl and heteroaryl are each independently selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, Substituted by one or more substituents in the cycloalkyl, heterocyclyl, aryl and heteroaryl groups;或者R3和R4形成氧代基;Or R 3 and R 4 form an oxo group;R5选自烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、氨基、烯基、炔基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、卤代烷基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烯基、炔基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 5 is selected from the group consisting of alkyl, cycloalkyl, haloalkyl, alkoxy, haloalkoxy, amino, alkenyl, alkynyl, aryl, heteroaryl, -OR 8 , -C(O)R 8 ,- C(O)OR 8 and -S(O) m R 8 , wherein said alkyl, cycloalkyl, haloalkyl, aryl and heteroaryl are each independently optionally selected from alkyl, haloalkyl, One or more of halogen, amino, nitro, cyano, hydroxy, alkenyl, alkynyl, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl Substituted by a substituent;R6选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、卤素、氨基、硝基、羟基、氰基、杂环基、芳基、杂芳基、-OR8、-C(O)R8、-C(O)OR8和-S(O)mR8,其中所述的烷基、环烷基、卤代烷基、杂环基、芳基和杂芳基各自独立地任选被 选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 6 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, a halogen, an amino group, a nitro group, a hydroxyl group, a cyano group, a heterocyclic group, an aryl group, a heteroaryl group, -OR 8 , —C(O)R 8 , —C(O)OR 8 and —S(O) m R 8 , wherein said alkyl, cycloalkyl, haloalkyl, heterocyclyl, aryl and heteroaryl The groups are each independently optionally selected from the group consisting of alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkoxy, haloalkoxy, hydroxyalkyl, cycloalkyl, heterocyclyl, aryl and Substituting one or more substituents in the heteroaryl;R8选自氢原子、烷基、卤代烷基、烷氧基、羟烷基、羟基、氨基、环烷基、杂环基、芳基和杂芳基,其中所述的烷基、氨基、环烷基、杂环基、芳基和杂芳基各自独立地任选被选自烷基、卤素、羟基、氨基、羧酸酯基、硝基、氰基、烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 8 is selected from the group consisting of a hydrogen atom, an alkyl group, a halogenated alkyl group, an alkoxy group, a hydroxyalkyl group, a hydroxyl group, an amino group, a cycloalkyl group, a heterocyclic group, an aryl group, and a heteroaryl group, wherein the alkyl group, the amino group, and the ring are The alkyl group, heterocyclic group, aryl group and heteroaryl group are each independently selected from the group consisting of alkyl, halogen, hydroxy, amino, carboxylate, nitro, cyano, alkoxy, hydroxyalkyl, cyclo Substituted by one or more substituents of an alkyl group, a heterocyclic group, an aryl group, and a heteroaryl group;R9选自氢原子、烷基、环烷基、卤代烷基、烷氧基、卤代烷氧基、氨基、烯基、炔基、芳基和杂芳基,其中所述的烷基、环烷基、卤代烷基、芳基和杂芳基各自独立地任选被选自烷基、卤代烷基、卤素、氨基、硝基、氰基、羟基、烯基、烷氧基、卤代烷氧基、羟烷基、环烷基、杂环基、芳基和杂芳基中的一个或多个取代基所取代;R 9 is selected from the group consisting of a hydrogen atom, an alkyl group, a cycloalkyl group, a halogenated alkyl group, an alkoxy group, a halogenated alkoxy group, an amino group, an alkenyl group, an alkynyl group, an aryl group and a heteroaryl group, wherein the alkyl group, the cycloalkyl group And haloalkyl, aryl and heteroaryl are each independently selected from alkyl, haloalkyl, halo, amino, nitro, cyano, hydroxy, alkenyl, alkoxy, haloalkoxy, hydroxyalkyl Substituting one or more substituents of a cycloalkyl, a heterocyclic group, an aryl group, and a heteroaryl group;m为0、1或2;m is 0, 1 or 2;x为0、1、2、3或4;且x is 0, 1, 2, 3 or 4;y为0、1、2或3。y is 0, 1, 2 or 3.
- 一种制备根据权利要求1所述的通式(I)化合物的方法,该方法包括:A method of preparing a compound of formula (I) according to claim 1 comprising:通式(V)化合物与通式(VI)化合物发生缩合反应,得到通式(I)化合物;a compound of the formula (V) is condensed with a compound of the formula (VI) to give a compound of the formula (I);其中:among them:环A、环B、X、Y、Z、R1~R7、x、y和z如权利要求1中所定义。Ring A, Ring B, X, Y, Z, R 1 to R 7 , x, y and z are as defined in claim 1.
- 一种药物组合物,其含有治疗有效量的根据权利要求1~15中任一项所述的通式(I)所示的化合物,以及一种或多种药学上可接受的载体、稀释剂或赋形剂。A pharmaceutical composition comprising a therapeutically effective amount of a compound of the formula (I) according to any one of claims 1 to 15, together with one or more pharmaceutically acceptable carriers, diluents Or an excipient.
- 根据权利要求1~15中任一项所述的通式(I)所示的化合物或根据权利要求18所述的药物组合物作为ROR调节剂在制备用于预防和/或治疗炎症、自身免疫性疾病、肿瘤或癌症的药物中的用途。The compound of the formula (I) according to any one of claims 1 to 15 or the pharmaceutical composition according to claim 18 as a ROR regulator for the preparation of a prophylactic and/or therapeutic inflammatory, autoimmune Use in drugs for sexual diseases, tumors or cancer.
- 根据权利要求1~15中任一项所述的通式(I)所示的化合物或根据权利要求18所述的药物组合物作为ROR反激动剂在制备用于预防和/或治疗炎症或自身免疫性疾病的药物中的用途。 The compound of the formula (I) according to any one of claims 1 to 15 or the pharmaceutical composition according to claim 18 as an ROR inverse agonist for preparation for preventing and/or treating inflammation or itself Use in medicines for immune diseases.
- 根据权利要求1~15中任一项所述的通式(I)所示的化合物或根据权利要求18所述的药物组合物作为ROR激动剂在制备用于预防和/或治疗肿瘤或癌症的药物中的用途。The compound of the formula (I) according to any one of claims 1 to 15 or the pharmaceutical composition according to claim 18 as an ROR agonist for the preparation of a tumor or a cancer for preventing and/or treating Use in medicine.
- 根据权利要求8~9中任一项所述的通式(IV-A)所示的化合物作为ROR激动剂在制备用于预防和/或治疗肿瘤或癌症的药物中的用途。Use of the compound of the formula (IV-A) according to any one of claims 8 to 9 as an ROR agonist for the preparation of a medicament for preventing and/or treating a tumor or cancer.
- 根据权利要求1~15中任一项所述的通式(I)所示的化合物或根据权利要求18所述的药物组合物在制备用于ROR激动剂与抗PD-1抗体组合治疗肿瘤或癌症的药物中的用途。The compound of the formula (I) according to any one of claims 1 to 15 or the pharmaceutical composition according to claim 18 for the preparation of a tumor for the treatment of a tumor with a combination of an ROR agonist and an anti-PD-1 antibody Use in cancer drugs.
- 根据权利要求8~9所述的通式(IV-A)所示的化合物作为ROR激动剂在制备用于与抗PD-1抗体组合治疗肿瘤或癌症的药物中的用途。Use of the compound of the formula (IV-A) according to claims 8 to 9 as an ROR agonist for the preparation of a medicament for the treatment of a tumor or cancer in combination with an anti-PD-1 antibody.
- 根据权利要求1~15中任一项所述的通式(I)所示的化合物或根据权利要求18所述的药物组合物在制备调节ROR的药物中的用途。Use of the compound of the formula (I) according to any one of claims 1 to 15 or the pharmaceutical composition according to claim 18 for the preparation of a medicament for regulating ROR.
- 根据权利要求1~15中任一项所述的通式(I)所示的化合物或根据权利要求18所述的药物组合物在制备ROR激动剂的药物中的用途;Use of the compound of the formula (I) according to any one of claims 1 to 15 or the pharmaceutical composition according to claim 18 for the preparation of a medicament for an ROR agonist;
- 根据权利要求8~9所述的通式(IV-A)所示的化合物在制备ROR激动剂的药物中的用途。Use of the compound of the formula (IV-A) according to claims 8 to 9 for the preparation of a medicament for an ROR agonist.
- 根据权利要求20所述的用途,其中所述的炎症或自身免疫性疾病选自银屑病、类风湿性关节炎、银屑病性关节炎、多发性硬化、炎性肠病、强直性脊柱炎、慢性阻塞性肺病、血管球性肾炎、心肌炎、甲状腺炎、干眼症、葡萄膜炎、白塞病、哮喘、过敏性皮肤炎、粉刺、克隆氏病、溃疡性结肠炎、系统性红斑狼疮、硬皮病、支气管炎和皮肌炎过敏性鼻炎。The use according to claim 20, wherein the inflammatory or autoimmune disease is selected from the group consisting of psoriasis, rheumatoid arthritis, psoriatic arthritis, multiple sclerosis, inflammatory bowel disease, ankylosing spine Inflammation, chronic obstructive pulmonary disease, glomerulonephritis, myocarditis, thyroiditis, dry eye, uveitis, Behcet's disease, asthma, allergic dermatitis, acne, Crohn's disease, ulcerative colitis, systemic erythema Allergic rhinitis of lupus, scleroderma, bronchitis and dermatomyositis.
- 根据权利要求21~24中任一项所述的用途,其中所述的肿瘤或癌症选自非霍奇金淋巴瘤、弥漫大B细胞淋巴瘤、滤泡性淋巴瘤、滑膜肉瘤、乳腺癌、宫颈癌、结肠癌、肺癌、胃癌、直肠癌、胰腺癌、脑癌、皮肤癌、口腔癌、前列腺癌、骨癌、肾癌、卵巢癌、膀胱癌、肝癌、输卵管肿瘤、卵巢瘤、腹膜肿瘤、IV期黑色素瘤、实体瘤、神经胶质瘤、神经胶母细胞瘤、肝细胞癌、乳突肾性瘤、头颈部肿瘤、白血病、淋巴瘤、骨髓瘤和非小细胞肺癌。 The use according to any one of claims 21 to 24, wherein the tumor or cancer is selected from the group consisting of non-Hodgkin's lymphoma, diffuse large B-cell lymphoma, follicular lymphoma, synovial sarcoma, breast cancer , cervical cancer, colon cancer, lung cancer, stomach cancer, rectal cancer, pancreatic cancer, brain cancer, skin cancer, oral cancer, prostate cancer, bone cancer, kidney cancer, ovarian cancer, bladder cancer, liver cancer, fallopian tube tumor, ovarian tumor, peritoneum Tumor, stage IV melanoma, solid tumor, glioma, glioblastoma, hepatocellular carcinoma, papillary renal tumor, head and neck tumor, leukemia, lymphoma, myeloma and non-small cell lung cancer.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201780001328.7A CN107531679B (en) | 2016-03-18 | 2017-03-17 | Aromatic amide derivative, preparation method and medical application thereof |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201610158675.8 | 2016-03-18 | ||
| CN201610158675 | 2016-03-18 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017157332A1 true WO2017157332A1 (en) | 2017-09-21 |
Family
ID=59850085
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CN2017/077114 WO2017157332A1 (en) | 2016-03-18 | 2017-03-17 | Aromatic amide derivative, preparation method therefor, and pharmaceutical applications thereof |
Country Status (3)
| Country | Link |
|---|---|
| CN (1) | CN107531679B (en) |
| TW (1) | TW201733982A (en) |
| WO (1) | WO2017157332A1 (en) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019007382A1 (en) * | 2017-07-06 | 2019-01-10 | 江苏恒瑞医药股份有限公司 | Indole-formamide derivative, preparation method therefor and use thereof in medicine |
| WO2019052440A1 (en) * | 2017-09-12 | 2019-03-21 | 江苏恒瑞医药股份有限公司 | Deuterium atom-substituted indole formamide derivative, preparation method therefor, and medical applications thereof |
| CN110151760A (en) * | 2019-06-06 | 2019-08-23 | 上海海洋大学 | Bis-benzazolyl compounds FGFC1 is preparing the application in anti-non-small cell lung cancer drug |
| WO2020011147A1 (en) * | 2018-07-10 | 2020-01-16 | Sunshine Lake Pharma Co., Ltd. | RORγ ANTAGONIST AND APPLICATION THEREOF IN MEDICINE |
| WO2020140960A1 (en) * | 2019-01-04 | 2020-07-09 | 江苏恒瑞医药股份有限公司 | Crystal form for indole-formamide derivative and preparation method for crystal form |
| WO2020182109A1 (en) * | 2019-03-11 | 2020-09-17 | 江苏恒瑞医药股份有限公司 | Crystal form of deuterium atom-substituted indole formamide derivative and preparation method therefor |
| WO2021063362A1 (en) * | 2019-09-30 | 2021-04-08 | 上海辉启生物医药科技有限公司 | Sulfo-substituted biaryl compound or salt thereof, preparation method therefor, and use thereof |
| CN112745268A (en) * | 2019-10-31 | 2021-05-04 | 江苏恒瑞医药股份有限公司 | Crystal form of benzimidazole derivative and preparation method thereof |
| WO2021228215A1 (en) * | 2020-05-15 | 2021-11-18 | 上海辉启生物医药科技有限公司 | BIARYL COMPOUND CAPABLE OF SERVING AS RORγ REGULATOR |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN110872243B (en) * | 2018-08-31 | 2023-03-31 | 四川科伦博泰生物医药股份有限公司 | Sulfonamide compound, preparation method and application thereof |
Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4496572A (en) * | 1981-08-26 | 1985-01-29 | Pfizer Inc. | Benzo-fused thromboxane synthetase inhibitors |
| CN1503787A (en) * | 2001-04-20 | 2004-06-09 | Novel compounds | |
| CN1553909A (en) * | 2000-12-21 | 2004-12-08 | Benzimidazole and pyridylimidazole derivatives as ligands for GABA receptors | |
| US20060148830A1 (en) * | 2002-10-03 | 2006-07-06 | Ono Pharmaceutical Co., Ltd. | Lpa receptor antagonist |
| CN101421235A (en) * | 2006-04-12 | 2009-04-29 | 霍夫曼-拉罗奇有限公司 | 5-amido-2-carboxamide indoles |
| WO2010051245A1 (en) * | 2008-11-03 | 2010-05-06 | Merck Sharp & Dohme Corp. | Benzimidazole and aza-benzimidazole carboxamides |
| CN102498114A (en) * | 2009-09-03 | 2012-06-13 | 阿勒根公司 | Compounds as tyrosine kinase modulators |
| CN102574836A (en) * | 2009-08-07 | 2012-07-11 | 中外制药株式会社 | Aminopyrazole derivative |
| WO2013087805A1 (en) * | 2011-12-14 | 2013-06-20 | Boehringer Ingelheim International Gmbh | Piperazine derivatives and their use as positive allosteric modulators of mglu5 receptors |
| CN104640846A (en) * | 2012-09-21 | 2015-05-20 | 赛诺菲 | Benzoimidazole-carboxylic acid amide derivatives as APJ receptor modulators |
| WO2015175845A1 (en) * | 2014-05-15 | 2015-11-19 | Peloton Therapeutics, Inc. | Benzimidazole derivatives and uses thereof |
| WO2017024018A1 (en) * | 2015-08-05 | 2017-02-09 | Vitae Pharmaceuticals, Inc. | Modulators of ror-gamma |
-
2017
- 2017-03-17 WO PCT/CN2017/077114 patent/WO2017157332A1/en active Application Filing
- 2017-03-17 CN CN201780001328.7A patent/CN107531679B/en active Active
- 2017-03-20 TW TW106109163A patent/TW201733982A/en unknown
Patent Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4496572A (en) * | 1981-08-26 | 1985-01-29 | Pfizer Inc. | Benzo-fused thromboxane synthetase inhibitors |
| CN1553909A (en) * | 2000-12-21 | 2004-12-08 | Benzimidazole and pyridylimidazole derivatives as ligands for GABA receptors | |
| CN1503787A (en) * | 2001-04-20 | 2004-06-09 | Novel compounds | |
| US20060148830A1 (en) * | 2002-10-03 | 2006-07-06 | Ono Pharmaceutical Co., Ltd. | Lpa receptor antagonist |
| CN101421235A (en) * | 2006-04-12 | 2009-04-29 | 霍夫曼-拉罗奇有限公司 | 5-amido-2-carboxamide indoles |
| WO2010051245A1 (en) * | 2008-11-03 | 2010-05-06 | Merck Sharp & Dohme Corp. | Benzimidazole and aza-benzimidazole carboxamides |
| CN102574836A (en) * | 2009-08-07 | 2012-07-11 | 中外制药株式会社 | Aminopyrazole derivative |
| CN102498114A (en) * | 2009-09-03 | 2012-06-13 | 阿勒根公司 | Compounds as tyrosine kinase modulators |
| WO2013087805A1 (en) * | 2011-12-14 | 2013-06-20 | Boehringer Ingelheim International Gmbh | Piperazine derivatives and their use as positive allosteric modulators of mglu5 receptors |
| CN104640846A (en) * | 2012-09-21 | 2015-05-20 | 赛诺菲 | Benzoimidazole-carboxylic acid amide derivatives as APJ receptor modulators |
| WO2015175845A1 (en) * | 2014-05-15 | 2015-11-19 | Peloton Therapeutics, Inc. | Benzimidazole derivatives and uses thereof |
| WO2017024018A1 (en) * | 2015-08-05 | 2017-02-09 | Vitae Pharmaceuticals, Inc. | Modulators of ror-gamma |
Non-Patent Citations (4)
| Title |
|---|
| AKGUN, H. ET AL.: "Synthesis of Some New Benzimidazole-5(6)-Carboxylic Acids.", J. HETEROCYCLIC CHEM., vol. 32, 31 December 1995 (1995-12-31), pages 1767 - 1773, XP002695652 * |
| DRAGOVICH, S.P. ET AL.: "Fragment-Based Design of 3-Aminopyridine-Derived Amides as Potent Inhibitors of Human Nicotinamide Phosphoribosyltransferase (NAMPT", BIOORGANIC & MEDICINAL CHEMISTRY LETTERS, vol. 24, 21 December 2013 (2013-12-21), pages 954 - 962, XP028819878, ISSN: 0960-894X * |
| LUO, YU ET AL.: "Synthesis and Anti-Hepatitis B Virus Activity of a Novel Class of Thiazolylbenzimidazole Derivatives", ARCH. PHARM. CHEM. LIFE SCI., vol. 2, 31 December 2001 (2001-12-31), pages 78 - 83, XP055424584 * |
| PAGE, D. ET AL.: "Novel Benzimidazole Derivatives as Selective CB2 Agonists", BIOORGANIC & MEDICINAL CHEMISTRY LETTERS, vol. 18, no. 13, 22 May 2008 (2008-05-22), pages 3695 - 3700, XP022716278, ISSN: 0960-894X * |
Cited By (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019007382A1 (en) * | 2017-07-06 | 2019-01-10 | 江苏恒瑞医药股份有限公司 | Indole-formamide derivative, preparation method therefor and use thereof in medicine |
| WO2019052440A1 (en) * | 2017-09-12 | 2019-03-21 | 江苏恒瑞医药股份有限公司 | Deuterium atom-substituted indole formamide derivative, preparation method therefor, and medical applications thereof |
| CN109963836A (en) * | 2017-09-12 | 2019-07-02 | 江苏恒瑞医药股份有限公司 | Indole carboxamides analog derivative, preparation method and its application in medicine that D-atom replaces |
| WO2020011147A1 (en) * | 2018-07-10 | 2020-01-16 | Sunshine Lake Pharma Co., Ltd. | RORγ ANTAGONIST AND APPLICATION THEREOF IN MEDICINE |
| WO2020140960A1 (en) * | 2019-01-04 | 2020-07-09 | 江苏恒瑞医药股份有限公司 | Crystal form for indole-formamide derivative and preparation method for crystal form |
| CN113227048B (en) * | 2019-01-04 | 2022-04-12 | 江苏恒瑞医药股份有限公司 | Crystal form of indole carboxamide derivative and preparation method thereof |
| CN113227048A (en) * | 2019-01-04 | 2021-08-06 | 江苏恒瑞医药股份有限公司 | Crystal form of indole carboxamide derivative and preparation method thereof |
| CN113365980A (en) * | 2019-03-11 | 2021-09-07 | 江苏恒瑞医药股份有限公司 | Crystal form of deuterium atom substituted indole formamide derivative and preparation method thereof |
| CN113365980B (en) * | 2019-03-11 | 2022-04-12 | 江苏恒瑞医药股份有限公司 | Crystal form of deuterium atom substituted indole formamide derivative and preparation method thereof |
| WO2020182109A1 (en) * | 2019-03-11 | 2020-09-17 | 江苏恒瑞医药股份有限公司 | Crystal form of deuterium atom-substituted indole formamide derivative and preparation method therefor |
| CN110151760B (en) * | 2019-06-06 | 2022-02-11 | 上海海洋大学 | Application of bisindole compound FGFC1 in preparation of non-small cell lung cancer resistant medicine |
| CN110151760A (en) * | 2019-06-06 | 2019-08-23 | 上海海洋大学 | Bis-benzazolyl compounds FGFC1 is preparing the application in anti-non-small cell lung cancer drug |
| WO2021063362A1 (en) * | 2019-09-30 | 2021-04-08 | 上海辉启生物医药科技有限公司 | Sulfo-substituted biaryl compound or salt thereof, preparation method therefor, and use thereof |
| CN112745268A (en) * | 2019-10-31 | 2021-05-04 | 江苏恒瑞医药股份有限公司 | Crystal form of benzimidazole derivative and preparation method thereof |
| CN112745268B (en) * | 2019-10-31 | 2022-09-16 | 江苏恒瑞医药股份有限公司 | Crystal form of benzimidazole derivative and preparation method thereof |
| WO2021228215A1 (en) * | 2020-05-15 | 2021-11-18 | 上海辉启生物医药科技有限公司 | BIARYL COMPOUND CAPABLE OF SERVING AS RORγ REGULATOR |
Also Published As
| Publication number | Publication date |
|---|---|
| CN107531679B (en) | 2021-07-02 |
| CN107531679A (en) | 2018-01-02 |
| TW201733982A (en) | 2017-10-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2017157332A1 (en) | Aromatic amide derivative, preparation method therefor, and pharmaceutical applications thereof | |
| WO2020238791A1 (en) | Hydropyridopyrimidine derivative, preparation method therefor and medical use thereof | |
| CN110049982A (en) | Heterocyclic FXR regulator | |
| WO2017084494A1 (en) | Benzofuran derivative, preparation method thereof and use thereof in medicine | |
| KR101944686B1 (en) | Phenyl-3-aza-bicyclo[3.1.0]hex-3-yl-methanones and the use thereof as medicament | |
| CN110730661A (en) | ASK1 inhibitor compounds and uses thereof | |
| WO2016169421A1 (en) | Imidazo isoindole derivative, preparation method therefor and medical use thereof | |
| WO2019020070A1 (en) | Piperazine heteroaryl derivative, preparation method therefor and use of same in medicine | |
| CN109952298B (en) | Indole carboxamide derivative, preparation method and medical application thereof | |
| CN114423753A (en) | Heterobicyclic amides as CD38 inhibitors | |
| KR20110091550A (en) | Novel pyrazole-3-carboxamide derivative having 5-ht2b receptor antagonist activity | |
| WO2019154247A1 (en) | Steroid derivative regulators, method for preparing the same, and uses thereof | |
| WO2023098832A1 (en) | Pyridopyrimidine derivatives serving as small gtp enzyme kras mutation inhibitors | |
| CN108779115B (en) | Five-membered heteroaromatic ring bridged ring derivative, preparation method thereof and application thereof in medicine | |
| WO2020011220A1 (en) | Heteroaryl derivative, preparation method therefor, and medical use thereof | |
| WO2017025493A1 (en) | Quinoline ezh2 inhibitors | |
| WO2022089389A1 (en) | Heterocyclic compound, preparation method therefor, pharmaceutical composition thereof and application thereof | |
| TWI601726B (en) | Pyrazolopyridine derivatives as ttx-s blockers | |
| TW201900617A (en) | Spirocyclic methotrexate derivatives, preparation method thereof and application thereof in medicine | |
| WO2021027647A1 (en) | Bridged heterocyclyl-substituted pyrimidine compound, preparation method therefor, and pharmaceutical use thereof | |
| CN109485595B (en) | Hydrophilic group substituted indole formamide derivative, preparation method and medical application thereof | |
| WO2016026372A1 (en) | Thienocycloalkyl or thienoheterocyclic derivatives, preparation method thereof and use thereof in medicine | |
| WO2022170947A1 (en) | Tetrahydronaphthyridine derivatives as kras mutant g12c inhibitors, preparation method therefor, and application thereof | |
| WO2023274396A1 (en) | Benzazepine heterocyclic compound and application thereof in medicine | |
| TW201835079A (en) | 6-pyrazol-[1,2,4]triazolo[4,3-a]pyridine-3-carboxamide derivatives, a preparation method thereof and pharmaceutical use thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17765874 Country of ref document: EP Kind code of ref document: A1 |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 17765874 Country of ref document: EP Kind code of ref document: A1 |