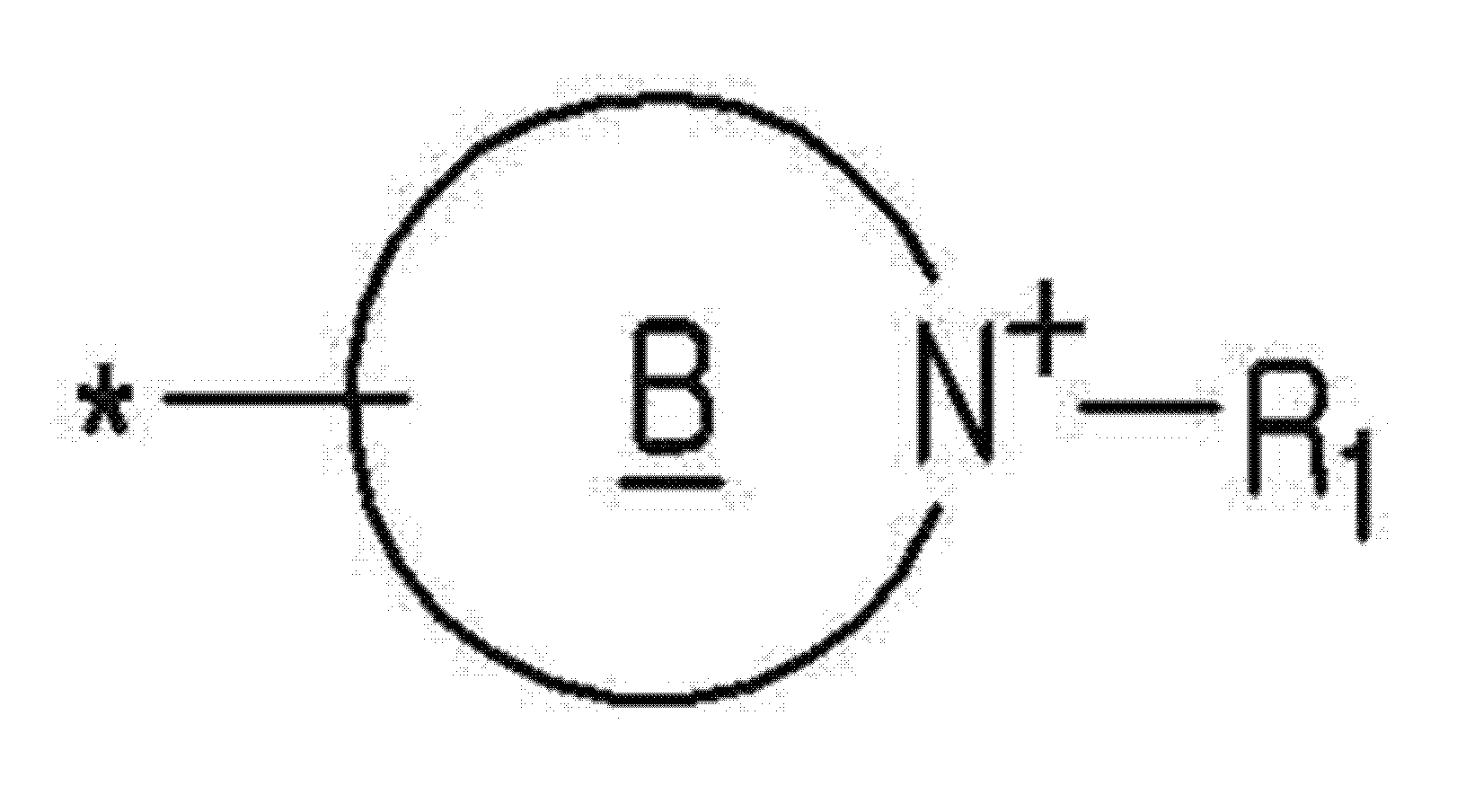WO2017073927A1 - Hydrochromic polydiacetylene polymer patch and manufacturing method therefor - Google Patents
Hydrochromic polydiacetylene polymer patch and manufacturing method therefor Download PDFInfo
- Publication number
- WO2017073927A1 WO2017073927A1 PCT/KR2016/011399 KR2016011399W WO2017073927A1 WO 2017073927 A1 WO2017073927 A1 WO 2017073927A1 KR 2016011399 W KR2016011399 W KR 2016011399W WO 2017073927 A1 WO2017073927 A1 WO 2017073927A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- formula
- polymer
- hydrochromic
- carbon atoms
- patch
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F138/00—Homopolymers of compounds having one or more carbon-to-carbon triple bonds
- C08F138/02—Acetylene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J5/00—Manufacture of articles or shaped materials containing macromolecular substances
- C08J5/18—Manufacture of films or sheets
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L49/00—Compositions of homopolymers or copolymers of compounds having one or more carbon-to-carbon triple bonds; Compositions of derivatives of such polymers
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N21/00—Investigating or analysing materials by the use of optical means, i.e. using sub-millimetre waves, infrared, visible or ultraviolet light
- G01N21/75—Systems in which material is subjected to a chemical reaction, the progress or the result of the reaction being investigated
- G01N21/77—Systems in which material is subjected to a chemical reaction, the progress or the result of the reaction being investigated by observing the effect on a chemical indicator
- G01N21/78—Systems in which material is subjected to a chemical reaction, the progress or the result of the reaction being investigated by observing the effect on a chemical indicator producing a change of colour
Definitions
- the present invention relates to polydiacetylene, and more particularly to a hydrochromic polydiacetylene polymer patch.
- Polydiacetylene is a polymer of diacetylene monomers, and is a conjugated polymer having characteristics that are produced through photopolymerization such as ultraviolet or gamma irradiation when the diacetylene monomers are arranged through self-assembly. .
- Such polydiacetylenes have alternating double and triple bonds in the polymer main chain, and generally have a maximum absorption wavelength at about 640 nm, which is blue, and exhibits external conditions (heat, solvent, pH, force, molecular recognition, etc.). As a result, the maximum absorption wavelength shifts to about 540 nm and turns red.
- Various kinds of sensors have been researched and developed by using the discoloration feature of the polydiacetylene.
- the prior art has the disadvantage that the base material used in the production of hydrochromic polydiacetylene thin film is glass, PET film and OHP film, and the film is easily peeled off or peeled off after the production of the thin film.
- the amount of the die acetylene complex required a large amount and may be generated unevenly, there was a disadvantage that the sensor function may be lost by sensitively reacting to moisture in the atmosphere when the thin film is manufactured and stored.
- an object of the present invention is to provide a polydiacetylene-containing polymer patch which suppresses detachment of polydiacetylene from a base material and decreases sensitivity to humidity.
- the hydrochromic polymer patch may include a hydrophilic polymer matrix; And a polydiacetylene having a repeating unit represented by the following Chemical Formula 5 located in the hydrophilic polymer matrix.
- a is an integer of 1 to 20
- b is an integer of 1 to 20
- L 1 is , , , , , , , or E, E 1 , and E 2 are each independently O or S, c is an integer of 0 to 2, L 2 is an alkylene having 1 to 10 carbon atoms or an arylene group having 5 to 12 carbon atoms, d is an integer from 0 to 1, IG is an ionic functional group, R - and, R - - M +, or R + X is an anion carboxylate, M + is an alkali metal cation, R + is a quaternary ammonium group, and , X - is F -, Cl -, Br - , I -, PF 6 -, BF 4 -, Tf 2 N - (bis (trifluoromethane) sulfonimide), TfO - (trifluoromethanesulfonate), SCN -, or CH 3 COO - to be.
- IG is an ionic functional group
- the polymer may be polyethylene oxide, polyvinyl alcohol or polyvinylpyrrolidone.
- the polymer may be contained in an amount of about 1.5 to 3.5 parts by weight based on 1 part by weight of the polydiacetylene.
- the hydrochromic polymer patch may further include a hydrophilic oligomer or a plasticizer.
- the polydiacetylene having a repeating unit of Formula 5 may be a polydiacetylene having a repeating unit represented by the following formula (6).
- a, b, R -, and M + is the a, b, R Formula 5, and each may be the same as M +.
- M + may be cesium ions or rubinium ions.
- the polymer may be polyethylene oxide, polyvinyl alcohol or polyvinylpyrrolidone.
- polydiacetylene having a repeating unit represented by Formula 5 may be a polydiacetylene having a repeating unit represented by the following Formula 7.
- R + may be N + -R 1 -heterocyclic quaternary ammonium represented by the following Chemical Formula 2a.
- R 1 may be cyanoalkyl having 1 to 16 carbon atoms, haloalkyl having 1 to 16 carbon atoms, hydroxyalkyl having 1 to 16 carbon atoms, or aminoalkyl having 1 to 16 carbon atoms.
- N + -R 1 of formula 2a - heterocyclic quaternary ammonium is N + -R 1 shown in the following formula 2b or 2c to the formula - may be a heterocyclic ring quaternary ammonium.
- Ring C is a 5-membered or 6-membered unsaturated heterocyclic compound having N of 2 to 3 as a hetero member
- Ring D has N of 2 to 3 as heteromember Is a 5-membered or 6-membered unsaturated heterocyclic compound
- R 1 and R 1 ′ are each independently a cyanoalkyl having 1 to 16 carbon atoms, a haloalkyl having 1 to 16 carbon atoms, or a hydroxyl having 1 to 16 carbon atoms.
- the N + -R 1 -heterocyclic quaternary ammonium is N + -R 1 -azolium, N + -R 1 -azinium, or N + -R 1 R 2 -piperazini It may be piperazinium.
- the N + -R 1 - Ah sleepiness is N + -R 1 - can be a tri-O sleepiness (triazolium) - imidazolium diamond (diazolium) or N + -R 1.
- the N + -R 1 - imidazolium diamond is to N + -R 1 represented by formula 2-1 may be a pyrazolyl imidazolium-imidazolidin N + -R 1 represented by the following Formula 2-2 or sleepiness.
- R a may be an alkylene group having 1 to 16 carbon atoms
- Y a may be a cyan group, a halogen, a hydroxy group, or an amine group.
- the hydrochromic polymer patch may exhibit blue color.
- the hydrochromic polymer patch may be a pore mapping patch.
- Another aspect of the present invention to achieve the above technical problem provides a method for producing a hydrochromic polymer patch.
- the diacetylene monomer solution represented by Formula 1 below is mixed with the diacetylene monomer solution contained in the first solvent and the polymer solution containing the polymer in the second solvent.
- the mixture is molded into a film.
- the film is dried to form a polymer patch including the matrix of the polymer and the diacetylene monomers self-assembled therein.
- Ultraviolet or gamma radiation is applied to the polymer patch to photopolymerize the diacetylene monomers to form polydiacetylene.
- a, b, c, d, L 1, L 2, and IG is the same and each of Formula 5 a, b, c, d , L 1, L 2, and IG.
- the first solvent and the second solvent may be a volatile solvent mixed with each other. Further, the first solvent and the second solvent may be a nonpolar solvent having an amphiphilic solvent or a dipole moment irrespective of each other.
- the diacetylene monomer represented by Formula 1 may be a diacetylene monomer represented by Formula 3 below.
- the diacetylene monomer represented by Formula 1 may be a diacetylene monomer represented by Formula 4 below.
- the polymer patch including the hydrochromic polydiacetylene in the hydrophilic polymer may be suppressed from detachment of the polydiacetylene from the base material and the sensitivity to humidity may be reduced.
- FIG. 1 is a schematic diagram showing a polydiacetylene-containing polymer patch production method and a water discoloration reaction of the polymer patch according to an embodiment of the present invention.
- Fig. 2 is a photograph of a PEO film (a) containing DA-Im obtained according to Hydrochromic patch Preparation Example 1-1b and a PEO film (b) containing PDA-Im obtained by light irradiation on the film.
- FIG. 3 is a graph showing Raman spectra of a PEO film containing DA-Im obtained according to Preparation Example 1-1b and a PEO film containing PDA-Im obtained by light irradiation of the film.
- FIG. 5 is a photograph showing an optical image after a finger is touched on a PEO film containing PDA-Im obtained according to hydrochromic patch Preparation Examples 1-1a to 1-1f.
- FIG. 6 is a photograph showing an optical image after contacting the nose, the entire palm, and the sole of the foot on a PEO film containing PDA-Im obtained in Preparation Example 1-1b.
- Figure 7 is a photograph showing the degree of water discoloration according to the relative humidity of the PEO film containing PDA-Im obtained according to the hydrochromic patch Preparation Example 1-1b.
- FIG. 8 is a photograph (a) of a polymer film containing PDA-Im obtained according to hydrochromic patch preparation examples 1-1b, 1-2, 1-3, and 1-4, and an optical image after contact with a finger (b ), And a fluorescence microscope image (c).
- FIG. 9 is a photograph of a polymer film containing PDA-Cs obtained according to the hydrochromic patches Preparation Examples 2-1 to 2-4.
- alkyl refers to an aliphatic hydrocarbon group and may be “saturated alkyl” that does not include a double bond or a triple bond. Saturated alkyl groups can be linear.
- alkylene refers to a divalent group which is a radical of an alkane which is saturated hydrocarbon, and may be linear alkylene.
- halogen or "halo” is an element belonging to Group 17, specifically, it may be a fluorine, chlorine, bromine, or iodine group.
- FIG. 1 is a schematic diagram showing a polydiacetylene-containing polymer patch production method and a water discoloration reaction of the polymer patch according to an embodiment of the present invention.
- a polymer patch (A) having a polymer matrix and diacetylene monomers having ionic functional groups at ends thereof self-assembled may be formed.
- the polymer patch (A) may mix the diacetylene monomer solution containing the diacetylene monomers in the first solvent and the polymer solution containing the polymer in the second solvent, and then shape the mixed solution into a film form.
- the molding in the form of a film may be selected from the group consisting of drop-casting, injection, spin coating on a substrate, ink jet printing, doctor blade and immersion-impression method.
- the polymer patch (A) comprising the diacetylene monomers may be dried to remove at least some or substantially the solvent (s). Such drying may proceed at about 1-5 ° C. and may proceed for about 10-14 hours. During this drying process, the self-assembly of the die acetylene monomers is maintained may be in a semi-crystalline or crystalline state.
- the polymer patch (A) may be a colorless opaque film.
- the die acetylene monomer may be a compound represented by the following formula (1).
- a may be an integer of 1 to 20.
- a may be an integer of 6 to 18, specifically 10 to 12.
- b may be an integer from 1 to 20.
- b may be an integer of 2 to 12, specifically 2 to 8.
- L 1 is , , , , , , , , or
- E, E 1 , and E 2 may be O or S irrespective of each other.
- c may be an integer from 0 to 2. As an example, c may be 1.
- L 2 is an alkylene having 1 to 10 carbon atoms or an arylene group having 5 to 12 carbon atoms, (Wherein e may be an integer of 1 to 10, as an example 1 to 5, more specifically 2 to 4) or benzenediyl and specifically 1,3 benzenediyl. d may be an integer from 0 to 1.
- R - may be - M +, or R + X.
- R - M + may be a carboxylate anion as an anionic functional group.
- M + may be an alkali metal cation as a counter cation, specifically a cation of one or more metals selected from the group consisting of cesium, rubidium, potassium, sodium and lithium.
- M + may be cesium ion or rubinium ion.
- R + X - be a heterocyclic ring quaternary ammonium (heterocyclic quarternary ammonium) -
- R + is a cationic functional group and a quaternary ammonium group, it said quaternary ammonium is one example, to N + -R 1 represented by Formula 2a as have.
- Ring B may be a 5-membered or 6-membered heterocyclic compound, a saturated or unsaturated heterocyclic compound, at least one N, specifically 1 to N of 3 and 0 of 0 may be provided as a hetero member.
- R 1 may be cyanoalkyl having 1 to 16 carbon atoms, haloalkyl having 1 to 16 carbon atoms, hydroxyalkyl having 1 to 16 carbon atoms, or aminoalkyl having 1 to 16 carbon atoms.
- R 1 may be represented by * -R a -Y a , wherein * is a bond, and R a is an alkylene group having 1 to 16 carbon atoms as an example, 1 to 6 carbon atoms specifically, 1 to 3 carbon atoms May be an alkylene group, and Y a may be a cyan group, a halogen, a hydroxy group, or an amine group.
- N + -R 1 of formula 2a - heterocyclic quaternary ammonium is N + -R 1 shown in the following formula 2b or 2c to the formula - may be a heterocyclic ring quaternary ammonium.
- Formula 2b is * -N + -R 1 -heterocyclic quaternary ammonium, ring C is a 5-member or 6-membered unsaturated heterocyclic compound, it may be provided with N of 2 to 3 as a hetero member.
- Ring D is a 5-membered or 6-membered unsaturated heterocyclic compound, and may have N of 2 to 3 as a hetero member.
- R 1 and R 1 ′ may be cyanoalkyl having 1 to 16 carbon atoms, haloalkyl having 1 to 16 carbon atoms, hydroxyalkyl having 1 to 16 carbon atoms, or aminoalkyl having 1 to 16 carbon atoms, irrespective of each other.
- R a and Y a may be as defined above.
- the N + -R 1 -heterocyclic quaternary ammonium is, for example, N + -R 1 -azolium, N + -R 1 -azinium, or N + -R 1 R 2 It may be piperazinium.
- the N + 1 -R - N + O sleepiness is -R 1 - can be a tri-O sleepiness (triazolium) - diamond sleepiness (diazolium) or N + -R 1.
- N + -R 1 - imidazolium diamond is N + -R 1 - imidazolium (imidazolium), or N + -R 1 - pyrazol may be sleepiness (pyrazolium), the N + -R 1 - imidazolium has the formula It may be represented by 2-1, the N + -R 1 -pyrazolium may be represented by the formula 2-2.
- N + -R 1 -triazolium can be represented by the following formula 2-3.
- N + -R 1 - Ah Genie Titanium is N + -R 1 - can be a help pyrazol Genie (pyrazinium) - pyrimidinyl minyum (pyridiminium) or N + -R 1.
- N + -R 1 -pyrimidinium may be represented by the following Chemical Formula 2-7 or 2-8, and N + -R 1 -pyrazinium may be represented by the following Chemical Formula 2-9.
- N + -R 1 R 2 -piperazinium may be * -N + -R 1 R 2 -piperazinium, which may be represented by the following formula (2-14).
- R a and Y a are as described above, and R a1 and R a2 have 1 to 16 carbon atoms regardless of each other.
- Alkylene group as an example may be an alkylene group having 1 to 6 carbon atoms specifically, Y a1 and Y a2 may be a cyan group, a halogen, a hydroxyl group, or an amine group irrespective of each other.
- R b may be an alkylene group having 1 to 16 carbon atoms, for example, an alkylene group having 1 to 6 carbon atoms, and Y b may be hydrogen, a cyan group, a halogen, a hydroxyl group, or an amine group. .
- X - is a counter anion (counter anion) Specifically, F -, Cl -, Br - , I -, PF 6 -, BF 4 -, Tf 2 N - (bis (trifluoromethane) sulfonimide), TfO - (trifluoromethanesulfonate) , SCN -, or CH 3 COO - it can be.
- the diacetylene monomer may exhibit amphiphilic by containing an ionic functional group at one end of an aliphatic hydrocarbon chain.
- These diacetylene monomers may be self-assembled between the polymer chains of the matrix polymer.
- aliphatic hydrocarbon chains including the diacetylene groups of the diacetylene monomers may be disposed adjacent to each other by van der Waals interaction, and ionic functional groups may be disposed adjacent to each other on one side thereof.
- the matrix polymer may be a polar polymer having a dipole moment (ie, having a dipole moment greater than zero) or a polar polymer.
- the matrix polymer has a polar functional group or an electron withdrawing group (or electron donating group) in the main chain or side chain, for example, an ether group, a carboxyl group, an alcohol group, an amine group, an imine group, an amide group, a blood group in the main chain or side chain.
- the matrix polymer may have a hydrophilic functional group in its main chain or side chain, and the hydrophilic functional group may be a polarized or charged functional group.
- hydrophilic polymers may be polyethylene oxide, polyvinyl alcohol or polyvinylpyrrolidone.
- the polymer may be contained in an amount of about 1.5 to 3.5, specifically about 2 to 3 parts by weight, based on 1 part by weight of the diacetylene monomer.
- the first solvent and the second solvent may be mixed with each other, and may be volatile solvents.
- the first solvent and the second solvent may be the same volatile organic solvent.
- the first solvent and the second solvent may be a polar, amphiphilic solvent, or a nonpolar solvent having a dipole moment (non-zero dipole moment) irrespective of each other. These solvents may vary depending on the type of diacetylene monomer.
- the polymer patch (A) having the diacetylene monomers may further include a hydrophilic oligomer or a plasticizer.
- a hydrophilic oligomer may be polyethylene glycol having a molecular weight (Mn) of 100 to 1000.
- Mn molecular weight
- Such polyethylene glycol may be contained in an amount of about 0.125 to 0.5 parts by weight based on 1 part by weight of the diacetylene monomer.
- the diacetylene monomer represented by Formula 1 may be represented by the following formula (3).
- A, b, R ⁇ , and M + of Formula 3 may be the same as a, b, R ⁇ , and M + described in Formula 1, respectively.
- the diacetylene monomer may be an alkali metal salt of PCDA (10,12-pentacosadiynoic acid), an alkali metal salt of TCDA (10,12-tricosadiynoic acid), or an alkali metal salt of HCDA (8,10-heneicosadiynoic acid).
- the first solvent may be a polar or amphiphilic solvent.
- the second solvent may be an amphiphilic solvent or water.
- the first solvent is an amphiphilic solvent and the second solvent is water, and in another example, both the first solvent and the second solvent may be an amphiphilic solvent or the same solvent.
- Amphiphilic solvents include, for example, acetone; Alcohol, such as methanol, ethanol, isopropanol, dimethyl ether, or tetrahydrofuran (THF).
- the matrix polymer may be polyethylene oxide, polyvinyl alcohol, polyvinyl chloride or polyvinylpyrrolidone.
- an alkali metal hydroxide solution and a diacetylene carboxylic acid monomer solution are prepared as an example of an alkali metal salt solution, and the alkali metal hydroxide aqueous solution is used as the diacetylene carboxylic acid monomer solution. It can be prepared by mixing while dropping drops.
- the alkali metal salt may be mixed in the range of 0.1 to 3 moles with respect to the diacetylene carboxylic acid monomer, and preferably in 0.5 to 2 moles.
- the alkali metal hydroxide and the diacetylene carboxylic acid monomer may form the diacetylene monomer represented by Chemical Formula 1 by an acid-base reaction.
- diacetylene monomer represented by Formula 1 may be represented by the following Formula 4.
- the first solvent may be a nonpolar solvent.
- the second solvent may also be a nonpolar solvent.
- the first solvent and the second solvent may be the same solvent.
- the nonpolar solvent may be, for example, chloroform as a nonpolar solvent having a dipole moment.
- the matrix polymer may be polyethylene oxide.
- the polymer patch (A) is irradiated with light to photopolymerize the diacetylene monomers to form polydiacetylene, thereby forming a polymer patch (B) having polydiacetylene in the polymer matrix.
- the light may be ultraviolet rays, specifically, 250-260 nm ultraviolet rays, specifically 254 nm ultraviolet rays, or gamma rays, and may be irradiated for 1 to 300 seconds.
- polyacetylene can be formed by photopolymerizing self-assembled and adjacently disposed diacetylene monomers.
- the polydiacetylene may have a repeating unit represented by the following Formula 5.
- a, b, c, d, L 1 , L 2 , and IG may be the same as each of a, b, c, d, L 1 , L 2 , and IG of Formula 1.
- the polymer patch (B) containing such polydiacetylene exhibits a maximum absorption wavelength at about 600 nm to 680 nm, specifically about 620 nm to 660 nm, for example about 640 nm, which indicates blue color. This is because it is a conjugated polymer with a highly ⁇ -conjugated backbone due to alternating double and triple bonds.
- the polydiacetylene may have a repeating unit represented by the following formula (6). It may be a polydiacetylene polymerized diacetylene monomer represented by the formula (3).
- a, b, R ⁇ , and M + may be the same as each of a, b, R ⁇ , and M + of Formula 1 above.
- the polydiacetylene may have a repeating unit represented by the following Chemical Formula 7. It may be polydiacetylene polymerized diacetylene monomer represented by the formula (4).
- the polydiacetylene-containing polymer patch (B) when moisture is applied to the polydiacetylene-containing polymer patch (B), the polydiacetylene in contact with moisture is geometrically deformed to exhibit a reddish color through ⁇ -conjugated main chain structure breakdown. (C). In other words, it may show a hydrochromic reaction.
- polydiacetylene in case of contact with moisture, polydiacetylene has a maximum absorption wavelength of about 490 to about 590 nm, specifically, 520 to about 570 nm, for example, blue shifted to 540 nm. Can change.
- the hydrochromic polydiacetylene may generate fluorescence.
- the polymer patch (B) containing the polydiacetylene may also be referred to as a hydrochromic patch.
- This water discoloration is supposed to form voids as the diacetylene monomer which remained as a monomer without forming a polymer is dissolved in water, and this causes polydiacetylene to exhibit geometric deformation, but is not limited to this theory.
- the polydiacetylene-containing polymer patch (B) exhibits a color change by contact with moisture (liquid or gas), and thus, may function sufficiently as a moisture sensor, specifically, a hydrochromic patch.
- moisture liquid or gas
- a hydrochromic patch Specifically, humidity may be sensed using the hydrochromic patch, or moisture in an organic solvent may be sensed.
- the hydrochromic patch may be discolored from blue to red even by a very small amount of water (nano litres) coming out of the pores, and thus may be used as a fingerprint recognition application or a pores mapping paper. Specifically, it is possible to effectively map the pores of the entire body area, such as palms, soles, back, face, as well as fingers, thereby expanding the application field of the existing technology.
- the biological information such as the distribution of the pores of the body
- it can be used in the medical field, beauty field, or criminal investigation field.
- it may be used in the medical field, such as active pores distribution analysis or analysis of age-specific pores activity of hyperhidrosis patients, the cosmetic field for the development of deodorant or sweat inhibitors, and the field of criminal investigation through analysis of the pores map of fingerprints.
- the elution position can be accurately identified through color or fluorescence changes in response to moisture eluted from various water pipes, microcracked buildings, and experimental equipment, and thus can be widely used for commercial purposes.
- the polymer patch (B) can produce not only hard hydrochromic patches, but also improved hydrochromic patches with improved ductility, by adjusting the physical properties of the matrix polymer. It can be usefully used for detecting moisture of the skin.
- PCDA-NHS 2,5-dioxopyrrolidin-1-yl pentacosa-10,12-diynoate
- the PCDA-NHS (0.94 g, 2 mmol) and triethylamine (TEA, 0.51 g, 5 mmol) were dissolved in 10 ml of methylene chloride to obtain a first solution, and in 10 ml of methylene chloride in the first solution.
- N- (3- (1H-imidazol-1-yl) propyl) pentacosa-10,12-diyneamide (0.70 g, in 20 ml of acetonitrile containing bromoacetonitrile (0.28 g, 2.32 mmol) 1.45 mmol) was added and refluxed with stirring overnight. After that, it was concentrated in vacuo to give a solid, which was washed three times with hexane to give DA- Im (0.75 g, 86%) as a yellowish powder.
- DA-Im solution a chloroform solution (hereinafter referred to as DA-Im solution) containing 2wt% of DA-Im.
- polymer solution chloroform solution
- DA-Im solution and the polymer solution were mixed at a volume ratio of 1: 1, sonicated for 20 minutes, and then cast on a glass petri dish. Thereafter, the film obtained by drying at 2 ° C. for 12 hours was peeled off to obtain a polymer film containing DA-Im.
- DA-Im was polymerized into a polymer film containing DA-Im by UV irradiation (254 nm, 1 mWcm-2) for 10 seconds to obtain a polymer film containing PDA-Im.
- DA-Cs solution which is a 5 wt% transparent homogeneous solution obtained in the diacetylene monomer synthesis example 2
- the polymer solution were mixed at a volume ratio of 1: 1, sonicated for 20 minutes, and then cast on a glass petri dish. It was.
- the film obtained by drying at 2 ° C. for 12 hours was peeled off to obtain a polymer film containing DA-Cs.
- the polymer film containing DA-Cs was polymerized with DA-Cs by UV irradiation (254 nm, 1 mWcm-2) for 10 seconds to obtain a polymer film containing PDA-Cs.
- Fig. 2 is a photograph of a PEO film (a) containing DA-Im obtained according to Hydrochromic patch Preparation Example 1-1b and a PEO film (b) containing PDA-Im obtained by light irradiation on the film.
- the PEO film (a) containing DA-Im was a film containing DA-Im and PEO in a weight ratio of 1: 2.5 and having a colorless opaque thickness of about 10 ⁇ m.
- the film (b) irradiated with UV showed blue color.
- the blue color may mean that diacetylene (DA-Im) is polymerized to form polydiacetylene (PDA-Im).
- DA-Im diacetylene
- PDA-Im polydiacetylene
- both the PEO film (a) containing DA-Im and the PEO film (b) containing PDA-Im are flexible films and do not produce visible cracks by bending.
- FIG. 3 is a graph showing Raman spectra of a PEO film containing DA-Im obtained according to Preparation Example 1-1b and a PEO film containing PDA-Im obtained by light irradiation of the film.
- the PEO film containing PDA-Im obtained according to Hydrochromic patch Preparation Example 1-1b shows water-promoted blue-to-red color change by water (a) After the moisture had dried, it did not return to blue again.
- the maximum absorption wavelength before exposure to moisture is about 636 nm
- the maximum absorption wavelength is shifted to about 537 nm after exposure to moisture (c).
- the Raman spectrum shows that the 2080 and 1453 cm -1 bands corresponding to the alkyne-alkene bands were shifted to 2120 and 1515 cm -1 bands, respectively, after hydration compared to before hydration. Can be.
- FIG. 5 is a photograph showing an optical image after a finger is touched on a PEO film containing PDA-Im obtained according to hydrochromic patch Preparation Examples 1-1a to 1-1f. Fluorescence microdots showing sweat-secreting active pores were analyzed using a fluorescence spectrometer (510-550 nm excitation).
- a red fluorescence image expressed by sweat at the pore position may be confirmed. This is evident throughout the PEO films containing PDA-Im formed using PEOs with different molecular weights of 100K to 4000K. From this it can be seen that it is possible to implement a flexible yet moisture-sensitive hydrochromic patch irrespective of the molecular weight of the matrix polymer. However, when a low molecular weight (eg 100 K) matrix polymer is used, there may be a slight possibility that the hydrochromic film is torn, and when a high molecular weight (eg 300 K or more) matrix polymer is used, Due to the viscosity, there may be some difficulties in obtaining a film of uniform thickness.
- a low molecular weight eg 100 K
- a high molecular weight eg 300 K or more
- FIG. 6 is a photograph showing an optical image after contacting the nose, the entire palm, and the sole of the foot on a PEO film containing PDA-Im obtained in Preparation Example 1-1b. Fluorescence microdots showing sweat-secreting active pores were analyzed using a fluorescence spectrometer.
- the PEO film containing PDA-Im may be in contact with the nostril (a), the entire palm (b) including the depression (d) of the palm, and the depression (c) of the sole.
- its flexibility allows almost perfect contact with the curved parts of the human body, greatly improving the pores mapping efficiency.
- Figure 7 is a photograph showing the degree of water discoloration according to the relative humidity of the PEO film containing PDA-Im obtained according to the hydrochromic patch Preparation Example 1-1b. Specifically, it shows the degree of water discoloration in a state exposed for 5 minutes at a specific relative humidity.
- the PEO film containing PDA-Im obtained according to the hydrochromic patch Preparation Example 1-1b did not discolor even when exposed for 5 minutes at a relative humidity of 20 to 95%. However, when exposed to 100% relative humidity, it immediately turned red. From this, it can be seen that the hydrochromic patch according to the present embodiment is hydrochromic only when directly exposed to water without being greatly affected by the humidity of the surrounding environment.
- FIG. 8 is a photograph (a) of a polymer film containing PDA-Im obtained according to hydrochromic patch preparation examples 1-1b, 1-2, 1-3, and 1-4, and an optical image after contact with a finger (b ), And a fluorescence microscope image (c).
- FIG. 9 is a photograph of a polymer film containing PDA-Cs obtained according to the hydrochromic patches Preparation Examples 2-1 to 2-4.
- DA-Cs is polymerized by UV to form PDA-Cs. This may mean that DA-Cs can be self-assembled in the polymers. In addition, in all cases, flexibility was excellent, such as no bending or cracking when bending force was applied.
- FIG. 10 is a photograph showing the degree of water discoloration according to the relative humidity of the polymer film containing the PDA-Cs obtained according to the hydrochromic patch Preparation Examples 3-1 to 3-4. Specifically, it shows the degree of water discoloration in a state exposed for 5 minutes at a specific relative humidity.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Physics & Mathematics (AREA)
- Manufacturing & Machinery (AREA)
- Plasma & Fusion (AREA)
- Materials Engineering (AREA)
- Life Sciences & Earth Sciences (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- General Physics & Mathematics (AREA)
- Immunology (AREA)
- Pathology (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Medicinal Preparation (AREA)
- Compositions Of Macromolecular Compounds (AREA)
Abstract
Provided are a hydrochromic polydiacetylene polymer patch and a manufacturing method therefor. The hydrochromic polymer patch comprises: a polymer matrix; and a polydiacetylene located in the polymer matrix. The polydiacetylene includes an ionic functional group in a repeating unit. The ionic functional group is represented by R-M+ or R+X-, wherein R- is a carboxylate anion; M+ is an alkali metal cation; R+ is a quaternary ammonium group; and X- is F-, Cl-, Br-, I-, PF6
-, BF4
-, bis(trifluoromethane)sulfonimide (Tf2N-), trifluoromethanesulfonate (TfO-), SCN-, or CH3COO-.
Description
본 발명은 폴리다이아세틸렌에 관한 것으로, 보다 상세하게는 수변색 폴리다이아세틸렌 고분자 패치에 관한 것이다.The present invention relates to polydiacetylene, and more particularly to a hydrochromic polydiacetylene polymer patch.
폴리다이아세틸렌(Polydiacetylene)은 다이아세틸렌(Diacetylene) 단량체의 중합체로, 다이아세틸렌 단량체들이 자가조립을 통해 배열되어있을 때 자외선 혹은 감마선 조사 등의 광중합을 통하여 만들어지는 특징을 갖는 공액고분자(Conjugated polymer)이다. 이러한 폴리다이아세틸렌은 고분자 주쇄에 이중결합과 삼중결합이 교대로 존재하며, 일반적으로 약 640 nm에서 최대흡수파장을 가지면서 청색을 나타내고 외부환경 (열, 용매, pH, 힘, 분자인식 등)의 변화에 의해 최대흡수파장이 약 540 nm로 이동하며 적색으로 변한다. 이러한 폴리다이아세틸렌의 변색특징을 이용하여 다양한 종류의 센서들이 연구 및 개발되고 있다.Polydiacetylene is a polymer of diacetylene monomers, and is a conjugated polymer having characteristics that are produced through photopolymerization such as ultraviolet or gamma irradiation when the diacetylene monomers are arranged through self-assembly. . Such polydiacetylenes have alternating double and triple bonds in the polymer main chain, and generally have a maximum absorption wavelength at about 640 nm, which is blue, and exhibits external conditions (heat, solvent, pH, force, molecular recognition, etc.). As a result, the maximum absorption wavelength shifts to about 540 nm and turns red. Various kinds of sensors have been researched and developed by using the discoloration feature of the polydiacetylene.
종래기술은 수변색 폴리다이아세틸렌 박막필름 제작 시 사용되는 모재가 유리, PET 필름 및 OHP 필름으로 박막필름 제작 후 필름이 떨어지거나 쉽게 벗겨지는 단점과, 대면적으로 박막 필름제작 시 사용되는 수변색 폴리다이아세틸렌 복합체의 양이 많이 필요하고 불균일하게 생성될 가능성, 박막필름 제조 후 보관 시 분위기 중의 수분에도 민감하게 반응하여 센서기능을 상실할 수 있다는 단점이 있었다.The prior art has the disadvantage that the base material used in the production of hydrochromic polydiacetylene thin film is glass, PET film and OHP film, and the film is easily peeled off or peeled off after the production of the thin film. The amount of the die acetylene complex required a large amount and may be generated unevenly, there was a disadvantage that the sensor function may be lost by sensitively reacting to moisture in the atmosphere when the thin film is manufactured and stored.
따라서, 본 발명이 해결하고자 하는 과제는, 모재로부터 폴리다이아세틸렌의 탈리를 억제하고 습도에 대한 민감도를 저감시킨 폴리다이아세틸렌 함유 고분자 패치를 제공함에 있다.Accordingly, an object of the present invention is to provide a polydiacetylene-containing polymer patch which suppresses detachment of polydiacetylene from a base material and decreases sensitivity to humidity.
본 발명의 기술적 과제들은 이상에서 언급한 기술적 과제로 제한되지 않으며, 언급되지 않은 또 다른 기술적 과제들은 아래의 기재로부터 당업자에게 명확하게 이해될 수 있을 것이다.Technical problems of the present invention are not limited to the technical problems mentioned above, and other technical problems not mentioned will be clearly understood by those skilled in the art from the following description.
상기 기술적 과제를 이루기 위하여 본 발명의 일 측면은 수변색 고분자 패치를 제공한다. 상기 수변색 고분자 패치는 친수성 고분자 매트릭스; 및 상기 친수성 고분자 매트릭스 내에 위치하는 하기 화학식 5로 나타낸 반복단위를 구비하는 폴리다이아세틸렌을 구비한다.One aspect of the present invention to achieve the above technical problem provides a hydrochromic polymer patch. The hydrochromic polymer patch may include a hydrophilic polymer matrix; And a polydiacetylene having a repeating unit represented by the following Chemical Formula 5 located in the hydrophilic polymer matrix.
[화학식 5][Formula 5]
상기 화학식 5에서, a는 1 내지 20의 정수이고, b는 1 내지 20의 정수이고, In Formula 5, a is an integer of 1 to 20, b is an integer of 1 to 20,
L1은 , , , , , , , , 또는 이고, E, E1, 및 E2는 서로에 관계없이 O 또는 S이고, c는 0 내지 2의 정수이고, L2은 탄소수 1 내지 10의 알킬렌 또는 탄소수 5 내지 12의 아릴렌기이고, d는 0 내지 1의 정수이고, IG는 이온성 작용기로서, R-M+이거나 R+X-이고, R-는 카르복실레이트 음이온이고, M+는 알칼리 금속 양이온이고, R+은 4차 암모늄기이고, X-는 F-, Cl-, Br-, I-, PF6
-, BF4
-, Tf2N-(bis(trifluoromethane)sulfonimide), TfO-(trifluoromethanesulfonate), SCN-, 또는 CH3COO-이다. L 1 is , , , , , , , , or E, E 1 , and E 2 are each independently O or S, c is an integer of 0 to 2, L 2 is an alkylene having 1 to 10 carbon atoms or an arylene group having 5 to 12 carbon atoms, d is an integer from 0 to 1, IG is an ionic functional group, R - and, R - - M +, or R + X is an anion carboxylate, M + is an alkali metal cation, R + is a quaternary ammonium group, and , X - is F -, Cl -, Br - , I -, PF 6 -, BF 4 -, Tf 2 N - (bis (trifluoromethane) sulfonimide), TfO - (trifluoromethanesulfonate), SCN -, or CH 3 COO - to be.
상기 고분자는 폴리에틸렌 옥사이드, 폴리비닐알코올 혹은 폴리비닐피롤리돈일 수 있다. 상기 폴리다이아세틸렌 1 중량부에 대해 상기 고분자는 약 1.5 내지 3.5의 중량부로 함유될 수 있다. 상기 수변색 고분자 패치는 친수성 올리고머 혹은 가소제를 더 포함할 수 있다.The polymer may be polyethylene oxide, polyvinyl alcohol or polyvinylpyrrolidone. The polymer may be contained in an amount of about 1.5 to 3.5 parts by weight based on 1 part by weight of the polydiacetylene. The hydrochromic polymer patch may further include a hydrophilic oligomer or a plasticizer.
일 예에서, 상기 화학식 5의 반복단위를 갖는 폴리다이아세틸렌은 하기 화학식 6으로 표시된 반복단위를 갖는 폴리다이아세틸렌일 수 있다.In one example, the polydiacetylene having a repeating unit of Formula 5 may be a polydiacetylene having a repeating unit represented by the following formula (6).
[화학식 6][Formula 6]
상기 화학식 6에서, a, b, R-, 및 M+는 상기 화학식 5의 a, b, R-, 및 M+와 각각 같을 수 있다. 상기 M+는 세슘 이온 또는 루비늄 이온일 수 있다. 이 때, 상기 고분자는 폴리에틸렌 옥사이드, 폴리비닐알코올 혹은 폴리비닐피롤리돈일 수 있다.In Formula 6, a, b, R -, and M + is the a, b, R Formula 5, and each may be the same as M +. M + may be cesium ions or rubinium ions. In this case, the polymer may be polyethylene oxide, polyvinyl alcohol or polyvinylpyrrolidone.
다른 예에서, 상기 화학식 5로 나타낸 반복단위를 갖는 폴리다이아세틸렌은 하기 화학식 7로 표시된 반복단위를 갖는 폴리다이아세틸렌일 수 있다.In another example, the polydiacetylene having a repeating unit represented by Formula 5 may be a polydiacetylene having a repeating unit represented by the following Formula 7.
[화학식 7][Formula 7]
상기 화학식 7의 a, b, L1, c, e, R+, 및 X-는 상기 화학식 5의 a, b, L1, c, e, R+, 및 X-와 각각 같을 수 있다. 구체적으로, R+은 하기 화학식 2a로 나타낸 N+-R1-헤테로고리 4차 암모늄(heterocyclic quarternary ammonium)일 수 있다.Of Formula 7 a, b, L 1, c, e, R +, and X - is of Formula 5 a, b, L 1, c, e, R +, and X - may be the same with each. Specifically, R + may be N + -R 1 -heterocyclic quaternary ammonium represented by the following Chemical Formula 2a.
[화학식 2a][Formula 2a]
상기 화학식 2a에서, *는 결합이고, 고리 B는 1 내지 3의 N과 0 내지 1의 O를 헤테로멤버로서 구비하는 5-멤버 혹은 6-멤버 헤테로고리화합물(5-membered or 6-membered heterocyclic compound)로서 포화 또는 불포화 헤테로고리화합물이고, R1은 탄소수 1 내지 16의 시아노알킬, 탄소수 1 내지 16의 할로알킬, 탄소수 1 내지 16의 하이드록시알킬, 또는 탄소수 1 내지 16의 아미노알킬일 수 있다.In Formula 2a, * is a bond, and ring B is a 5-membered or 6-membered heterocyclic compound having N of 1 to 3 and O of 0 to 1 as a hetero member. ) Is a saturated or unsaturated heterocyclic compound, and R 1 may be cyanoalkyl having 1 to 16 carbon atoms, haloalkyl having 1 to 16 carbon atoms, hydroxyalkyl having 1 to 16 carbon atoms, or aminoalkyl having 1 to 16 carbon atoms. .
상기 화학식 2a로 나타낸 N+-R1-헤테로고리 4차 암모늄은 하기 화학식 2b 또는 하기 화학식 2c에 나타낸 N+-R1-헤테로고리 4차 암모늄일 수 있다.N + -R 1 of formula 2a - heterocyclic quaternary ammonium is N + -R 1 shown in the following formula 2b or 2c to the formula - may be a heterocyclic ring quaternary ammonium.
[화학식 2b][Formula 2b]
[화학식 2c][Formula 2c]
상기 화학식 2b에서, 고리 C는 2 내지 3의 N을 헤테로멤버로서 구비하는 5-멤버 혹은 6-멤버 불포화 헤테로고리화합물이고, 상기 화학식 2c에서, 고리 D는 2 내지 3의 N을 헤테로멤버로서 구비하는 5-멤버 혹은 6-멤버 불포화 헤테로고리화합물이고, R1과 R1′는 서로에 관계없이, 탄소수 1 내지 16의 시아노알킬, 탄소수 1 내지 16의 할로알킬, 탄소수 1 내지 16의 하이드록시알킬, 또는 탄소수 1 내지 16의 아미노알킬일 수 있다.In Formula 2b, Ring C is a 5-membered or 6-membered unsaturated heterocyclic compound having N of 2 to 3 as a hetero member, and in Formula 2c, Ring D has N of 2 to 3 as heteromember Is a 5-membered or 6-membered unsaturated heterocyclic compound, R 1 and R 1 ′ are each independently a cyanoalkyl having 1 to 16 carbon atoms, a haloalkyl having 1 to 16 carbon atoms, or a hydroxyl having 1 to 16 carbon atoms. Alkyl, or aminoalkyl having 1 to 16 carbon atoms.
상기 N+-R1-헤테로고리 4차 암모늄은 N+-R1-아졸리움(azolium), N+-R1-아지니움(azinium), 또는 N+-R1R2-피페라지니움(piperazinium)일 수 있다. 상기 N+-R1-아졸리움은 N+-R1-다이아졸리움(diazolium) 또는 N+-R1-트라이아졸리움(triazolium)일 수 있다. 상기 N+-R1-다이아졸리움은 하기 화학식 2-1로 나타낸 N+-R1-이미다졸리움 또는 하기 화학식 2-2로 나타낸 N+-R1-피라졸리움일 수 있다.The N + -R 1 -heterocyclic quaternary ammonium is N + -R 1 -azolium, N + -R 1 -azinium, or N + -R 1 R 2 -piperazini It may be piperazinium. The N + -R 1 - Ah sleepiness is N + -R 1 - can be a tri-O sleepiness (triazolium) - imidazolium diamond (diazolium) or N + -R 1. The N + -R 1 - imidazolium diamond is to N + -R 1 represented by formula 2-1 may be a pyrazolyl imidazolium-imidazolidin N + -R 1 represented by the following Formula 2-2 or sleepiness.
상기 화학식 2-1 및 2-2에서, Ra는 탄소수 1 내지 16의 알킬렌기이고, Ya는 시안기, 할로겐, 하이드록시기, 또는 아민기일 수 있다.In Formulas 2-1 and 2-2, R a may be an alkylene group having 1 to 16 carbon atoms, and Y a may be a cyan group, a halogen, a hydroxy group, or an amine group.
상기 수변색 고분자 패치는 청색을 나타낼 수 있다. 상기 수변색 고분자 패치는 땀구멍 맵핑 패치일 수 있다.The hydrochromic polymer patch may exhibit blue color. The hydrochromic polymer patch may be a pore mapping patch.
상기 기술적 과제를 이루기 위하여 본 발명의 다른 측면은 수변색 고분자 패치 제조방법을 제공한다. 먼저, 하기 화학식 1로 표시된 다이아세틸렌 단량체들이 제1 용매 내에 함유된 다이아세틸렌 단량체 용액과 고분자가 제2 용매 내에 함유된 고분자 용액을 서로 혼합한다. 상기 혼합액을 필름으로 성형한다. 상기 필름을 건조하여 상기 고분자의 매트릭스 및 그의 내부에 자기조립되어 배치된 상기 다이아세틸렌 단량체들을 구비하는 고분자 패치를 형성한다. 상기 고분자 패치에 자외선 또는 감마선을 조사하여 상기 다이아세틸렌 단량체들을 광중합하여 폴리다이아세틸렌을 형성한다.Another aspect of the present invention to achieve the above technical problem provides a method for producing a hydrochromic polymer patch. First, the diacetylene monomer solution represented by Formula 1 below is mixed with the diacetylene monomer solution contained in the first solvent and the polymer solution containing the polymer in the second solvent. The mixture is molded into a film. The film is dried to form a polymer patch including the matrix of the polymer and the diacetylene monomers self-assembled therein. Ultraviolet or gamma radiation is applied to the polymer patch to photopolymerize the diacetylene monomers to form polydiacetylene.
[화학식 1][Formula 1]
상기 화학식 1에서, a, b, c, d, L1, L2, 및 IG는 상기 화학식 5의 a, b, c, d, L1, L2, 및 IG와 각각 같다.In Formula 1, a, b, c, d, L 1, L 2, and IG is the same and each of Formula 5 a, b, c, d , L 1, L 2, and IG.
상기 제1 용매와 상기 제2 용매는 서로 섞임성 있는 휘발성 용매일 수 있다. 나아가, 상기 제1 용매와 상기 제2 용매는 서로에 관계없이 양친성 용매 또는 다이폴모멘트를 갖는 비극성 용매일 수 있다.The first solvent and the second solvent may be a volatile solvent mixed with each other. Further, the first solvent and the second solvent may be a nonpolar solvent having an amphiphilic solvent or a dipole moment irrespective of each other.
일 예에서, 상기 화학식 1로 표시된 다이아세틸렌 단량체는 하기 화학식 3으로 표시되는 다이아세틸렌 단량체일 수 있다.In one example, the diacetylene monomer represented by Formula 1 may be a diacetylene monomer represented by Formula 3 below.
[화학식 3][Formula 3]
상기 화학식 3의 a, b, R-, 및 M+는 상기 화학식 1의 a, b, R-, 및 M+와 각각 같을 수 있다.Of Formula 3 a, b, R -, and M + is the a, b, R the general formula (1) -, and each may be the same as M +.
다른 예에서, 상기 화학식 1로 표시된 다이아세틸렌 단량체는 하기 화학식 4로 표시되는 다이아세틸렌 단량체일 수 있다.In another example, the diacetylene monomer represented by Formula 1 may be a diacetylene monomer represented by Formula 4 below.
[화학식 4][Formula 4]
상기 화학식 4의 a, b, L1, c, e, R+, 및 X-는 상기 화학식 1의 a, b, L1, c, e, R+, 및 X-와 각각 같을 수 있다.Of a, b, L 1, c , e, R +, and the general formula X 4 - is of a, b, L 1, c , e, R +, and the general formula X 1 - may be the same with each.
상술한 바와 같이 본 발명에 따르면, 친수성 고분자 내에 수변색 폴리다이아세틸렌을 구비하는 고분자 패치는 모재로부터 폴리다이아세틸렌의 탈리가 억제되고 습도에 대한 민감도가 저감될 수 있다.As described above, according to the present invention, the polymer patch including the hydrochromic polydiacetylene in the hydrophilic polymer may be suppressed from detachment of the polydiacetylene from the base material and the sensitivity to humidity may be reduced.
그러나, 본 발명의 효과들은 이상에서 언급한 효과로 제한되지 않으며, 언급되지 않은 또 다른 효과들은 아래의 기재로부터 당업자에게 명확하게 이해될 수 있을 것이다.However, the effects of the present invention are not limited to the above-mentioned effects, and other effects not mentioned will be clearly understood by those skilled in the art from the following description.
도 1은 본 발명의 일 실시예에 따른 폴리다이아세틸렌 함유 고분자 패치 제조방법 및 상기 고분자 패치의 수변색 반응을 개략적으로 나타낸 모식도이다.1 is a schematic diagram showing a polydiacetylene-containing polymer patch production method and a water discoloration reaction of the polymer patch according to an embodiment of the present invention.
도 2는 수변색 패치 제조예 1-1b에 따라 얻어진 DA-Im을 함유하는 PEO 필름(a)과 이 필름에 광조사하여 얻은 PDA-Im을 함유하는 PEO 필름(b)을 촬영한 사진이다.Fig. 2 is a photograph of a PEO film (a) containing DA-Im obtained according to Hydrochromic patch Preparation Example 1-1b and a PEO film (b) containing PDA-Im obtained by light irradiation on the film.
도 3은 수변색 패치 제조예 1-1b에 따라 얻어진 DA-Im을 함유하는 PEO 필름과 이 필름에 광조사하여 얻은 PDA-Im을 함유하는 PEO 필름에 대한 라만 스펙트럼을 나타낸 그래프이다.FIG. 3 is a graph showing Raman spectra of a PEO film containing DA-Im obtained according to Preparation Example 1-1b and a PEO film containing PDA-Im obtained by light irradiation of the film.
도 4는 수변색 패치 제조예 1-1b에 따라 얻어진 PDA-Im을 함유하는 PEO 필름에 수분을 가하기 전과 가한 후의 a) 사진, b) 폴리다이아세틸렌의 주쇄 변형, c) UV-vis 흡수 스펙트럼, 및 d) 라만 스펙트럼을 나타낸다.4 is a) photograph, b) backbone modification of polydiacetylene, c) UV-vis absorption spectrum, before and after adding water to a PEO film containing PDA-Im obtained according to Preparation Example 1-1b of hydrochromic patch. And d) Raman spectrum.
도 5는 수변색 패치 제조예 1-1a 내지 1-1f에 따라 얻어진 PDA-Im을 함유하는 PEO 필름 상에 손가락을 접촉시킨 후의 광학이미지를 나타낸 사진이다.FIG. 5 is a photograph showing an optical image after a finger is touched on a PEO film containing PDA-Im obtained according to hydrochromic patch Preparation Examples 1-1a to 1-1f.
도 6은 수변색 패치 제조예 1-1b에 따라 얻어진 PDA-Im을 함유하는 PEO 필름 상에 코, 손바닥 전체, 그리고 발바닥에 접촉시킨 후의 광학이미지를 나타낸 사진이다.FIG. 6 is a photograph showing an optical image after contacting the nose, the entire palm, and the sole of the foot on a PEO film containing PDA-Im obtained in Preparation Example 1-1b.
도 7은 수변색 패치 제조예 1-1b에 따라 얻어진 PDA-Im을 함유하는 PEO 필름의 상대습도에 따른 수변색 정도를 보여주는 사진이다.Figure 7 is a photograph showing the degree of water discoloration according to the relative humidity of the PEO film containing PDA-Im obtained according to the hydrochromic patch Preparation Example 1-1b.
도 8은 수변색 패치 제조예 1-1b, 1-2, 1-3, 및 1-4에 따라 얻어진 PDA-Im을 함유하는 고분자 필름의 사진(a), 손가락을 접촉한 후의 광학 이미지(b), 및 형광 현미경 이미지(c)이다.8 is a photograph (a) of a polymer film containing PDA-Im obtained according to hydrochromic patch preparation examples 1-1b, 1-2, 1-3, and 1-4, and an optical image after contact with a finger (b ), And a fluorescence microscope image (c).
도 9는 수변색 패치 제조예 2-1 내지 2-4에 따라 얻어진 PDA-Cs을 함유하는 고분자 필름의 사진이다.9 is a photograph of a polymer film containing PDA-Cs obtained according to the hydrochromic patches Preparation Examples 2-1 to 2-4.
도 10은 수변색 패치 제조예 3-1 내지 3-4에 따라 얻어진 PDA-Cs을 함유하는 고분자 필름의 상대습도에 따른 수변색 정도를 보여주는 사진이다.10 is a photograph showing the degree of water discoloration according to the relative humidity of the polymer film containing the PDA-Cs obtained according to the hydrochromic patch Preparation Examples 3-1 to 3-4.
이하, 본 발명을 보다 구체적으로 설명하기 위하여 본 발명에 따른 바람직한 실시예를 첨부된 도면을 참조하여 보다 상세하게 설명한다. 그러나, 본 발명은 여기서 설명되어지는 실시예에 한정되지 않고 다른 형태로 구체화될 수도 있다. 도면들에 있어서, 층이 다른 층 또는 기판 "상"에 있다고 언급되어지는 경우에 그것은 다른 층 또는 기판 상에 직접 형성될 수 있거나 또는 그들 사이에 제 3의 층이 개재될 수도 있다. 본 실시예들에서 "제1", "제2", 또는 "제3"는 구성요소들에 어떠한 한정을 가하려는 것은 아니며, 다만 구성요소들을 구별하기 위한 용어로서 이해되어야 할 것이다.Hereinafter, exemplary embodiments of the present invention will be described in detail with reference to the accompanying drawings in order to describe the present invention in more detail. However, the present invention is not limited to the embodiments described herein but may be embodied in other forms. In the figures, where a layer is said to be "on" another layer or substrate, it may be formed directly on the other layer or substrate, or a third layer may be interposed therebetween. In the present embodiments, "first", "second", or "third" is not intended to impose any limitation on the components, but should be understood as a term for distinguishing the components.
본 명세서에서 "알킬"은 별도의 정의가 없는 한, 지방족 탄화수소기를 의미하며, 이중결합이나 삼중결합을 포함하고 있지 않은 "포화 알킬(saturated alkyl)"일 수 있다. 포화 알킬기는 선형일 수 있다.As used herein, unless otherwise defined, "alkyl" refers to an aliphatic hydrocarbon group and may be "saturated alkyl" that does not include a double bond or a triple bond. Saturated alkyl groups can be linear.
본 명세서에서 "알킬렌"은 별도의 정의가 없는 한, 포화탄화수소인 알칸의 라디칼인 2가기를 지칭하며, 선형 알킬렌일 수 있다.As used herein, unless otherwise defined, "alkylene" refers to a divalent group which is a radical of an alkane which is saturated hydrocarbon, and may be linear alkylene.
본 명세서에서 "탄소수 X 내지 탄소수 Y"라고 기재한 경우에는, 탄소수 X와 탄소수 Y 사이의 모든 정수에 해당하는 수의 탄소수를 갖는 경우도 함께 기재된 것으로 해석되어야 한다.In the present specification, when " carbon number X to carbon number Y ", the case having the number of carbon atoms corresponding to all integers between carbon number X and carbon number Y should also be interpreted as being described together.
본 명세서에서 "할로겐" 또는 "할로"는 17족에 속하는 원소들로서, 구체적으로는 불소, 염소, 브롬, 또는 요오드기일 수 있다.As used herein, "halogen" or "halo" is an element belonging to Group 17, specifically, it may be a fluorine, chlorine, bromine, or iodine group.
본 명세서에서 "X 내지 Y"라고 기재한 경우에는, X와 Y 사이의 모든 정수에 해당하는 수도 함께 기재된 것으로 해석되어야 한다.In the present specification, when "X to Y" is described, the number corresponding to all integers between X and Y should be interpreted as being described together.
도 1은 본 발명의 일 실시예에 따른 폴리다이아세틸렌 함유 고분자 패치 제조방법 및 상기 고분자 패치의 수변색 반응을 개략적으로 나타낸 모식도이다.1 is a schematic diagram showing a polydiacetylene-containing polymer patch production method and a water discoloration reaction of the polymer patch according to an embodiment of the present invention.
다이아세틸렌Diacetylene
단량체 함유 고분자 패치 Monomer-containing polymer patch
도 1을 참조하면, 고분자 매트릭스와 그의 내부에 자기조립되어 배치된 말단에 이온성 작용기를 가지는 다이아세틸렌 단량체들을 구비하는 고분자 패치(A)를 형성할 수 있다.Referring to FIG. 1, a polymer patch (A) having a polymer matrix and diacetylene monomers having ionic functional groups at ends thereof self-assembled may be formed.
일 예로서, 상기 고분자 패치(A)는 상기 다이아세틸렌 단량체들이 제1 용매 내에 함유된 다이아세틸렌 단량체 용액과 고분자가 제2 용매 내에 함유된 고분자 용액을 서로 혼합한 후, 혼합액을 필름 형태로 성형하여 형성할 수 있다. 상기 필름 형태의 성형은 드롭-캐스팅(drop-casting), 사출, 기재 상에 스핀 코팅, 잉크젯 프린팅, 닥터브레이드 및 침지-인상법으로 이루어진 군에서 선택될 수 있다.As an example, the polymer patch (A) may mix the diacetylene monomer solution containing the diacetylene monomers in the first solvent and the polymer solution containing the polymer in the second solvent, and then shape the mixed solution into a film form. Can be formed. The molding in the form of a film may be selected from the group consisting of drop-casting, injection, spin coating on a substrate, ink jet printing, doctor blade and immersion-impression method.
상기 다이아세틸렌 단량체들을 구비하는 고분자 패치(A)를 건조하여 상기 용매(들)을 적어도 일부 혹은 실질적으로 제거할 수 있다. 이러한 건조는 약 1 내지 5℃에서 진행할 수 있고, 약 10 내지 14시간동안 진행할 수 있다. 이러한 건조과정 동안 상기 다이아세틸렌 단량체들의 자기조립은 유지되어 반결정 혹은 결정상태에 있을 수 있다. 상기 고분자 패치(A)는 무색의 불투명한 필름일 수 있다.The polymer patch (A) comprising the diacetylene monomers may be dried to remove at least some or substantially the solvent (s). Such drying may proceed at about 1-5 ° C. and may proceed for about 10-14 hours. During this drying process, the self-assembly of the die acetylene monomers is maintained may be in a semi-crystalline or crystalline state. The polymer patch (A) may be a colorless opaque film.
상기 다이아세틸렌 단량체는 하기 화학식 1로 나타낸 화합물일 수 있다.The die acetylene monomer may be a compound represented by the following formula (1).
[화학식 1][Formula 1]
상기 화학식 1에서, a는 1 내지 20의 정수일 수 있다. 일 예로서, a는 6 내지 18, 구체적으로는 10 내지 12의 정수일 수 있다. b는 1 내지 20의 정수일 수 있다. 일 예로서, b는 2 내지 12, 구체적으로는 2 내지 8의 정수일 수 있다. In Formula 1, a may be an integer of 1 to 20. As an example, a may be an integer of 6 to 18, specifically 10 to 12. b may be an integer from 1 to 20. As an example, b may be an integer of 2 to 12, specifically 2 to 8.
L1은 , , , , , , , , 또는 이고, E, E1, 및 E2는 서로에 관계없이 O 또는 S일 수 있다. c는 0 내지 2의 정수일 수 있다. 일 예로서, c는 1일 수 있다.L 1 is , , , , , , , , or And E, E 1 , and E 2 may be O or S irrespective of each other. c may be an integer from 0 to 2. As an example, c may be 1.
L2은 탄소수 1 내지 10의 알킬렌 또는 탄소수 5 내지 12의 아릴렌기 구체적으로, (여기서, e는 1 내지 10, 일 예로서 1 내지 5, 더 구체적으로 2내지 4의 정수일 수 있다) 또는 벤젠다이일(benzenediyl) 구체적으로 1,3 벤젠다이일일 수 있다. d는 0 내지 1의 정수일 수 있다.L 2 is an alkylene having 1 to 10 carbon atoms or an arylene group having 5 to 12 carbon atoms, (Wherein e may be an integer of 1 to 10, as an example 1 to 5, more specifically 2 to 4) or benzenediyl and specifically 1,3 benzenediyl. d may be an integer from 0 to 1.
IG는 이온성 작용기로서, R-M+이거나 R+X-일 수 있다. IG is an ionic functional group, R - may be - M +, or R + X.
R-M+에서, R-는 음이온성 작용기로서 카르복실레이트 음이온일 수 있다. M+는 상대 양이온으로 알칼리 금속 양이온일 수 있고, 구체적으로는 세슘, 루비듐, 칼륨, 소듐 및 리튬으로 구성된 군으로부터 선택된 하나 이상의 금속의 양이온일 수 있다. 일 예로서, M+는 세슘이온이거나 루비늄이온일 수 있다.In R - M + , R - may be a carboxylate anion as an anionic functional group. M + may be an alkali metal cation as a counter cation, specifically a cation of one or more metals selected from the group consisting of cesium, rubidium, potassium, sodium and lithium. As an example, M + may be cesium ion or rubinium ion.
R+X-에서, R+은 양이온성 작용기로서 4차 암모늄기이고, 상기 4차 암모늄은 일 예로서, 하기 화학식 2a로 나타낸 N+-R1-헤테로고리 4차 암모늄(heterocyclic quarternary ammonium) 일 수 있다. R + X - be a heterocyclic ring quaternary ammonium (heterocyclic quarternary ammonium) - In, R + is a cationic functional group and a quaternary ammonium group, it said quaternary ammonium is one example, to N + -R 1 represented by Formula 2a as have.
[화학식 2a][Formula 2a]
상기 화학식 2a에서, 고리 B는 5-멤버 혹은 6-멤버 헤테로고리화합물(5-membered or 6-membered heterocyclic compound)로서 포화 또는 불포화 헤테로고리화합물일 수 있고, 적어도 하나의 N, 구체적으로는 1 내지 3의 N과 0 내지 1의 O를 헤테로멤버로서 구비할 수 있다. 또한, 상기 화학식 2a에서 상기 R1은 탄소수 1 내지 16의 시아노알킬, 탄소수 1 내지 16의 할로알킬, 탄소수 1 내지 16의 하이드록시알킬, 또는 탄소수 1 내지 16의 아미노알킬일 수 있다. 구체적으로, R1은 *-Ra-Ya로 나타낼 수 있는데, 이 때, *는 결합이고, Ra는 탄소수 1 내지 16의 알킬렌기 일 예로서 탄소수 1 내지 6 구체적으로는 탄소수 1 내지 3의 알킬렌기일 수 있고, Ya는 시안기, 할로겐, 하이드록시기, 또는 아민기 일 수 있다. In Formula 2a, Ring B may be a 5-membered or 6-membered heterocyclic compound, a saturated or unsaturated heterocyclic compound, at least one N, specifically 1 to N of 3 and 0 of 0 may be provided as a hetero member. In addition, in Formula 2a, R 1 may be cyanoalkyl having 1 to 16 carbon atoms, haloalkyl having 1 to 16 carbon atoms, hydroxyalkyl having 1 to 16 carbon atoms, or aminoalkyl having 1 to 16 carbon atoms. Specifically, R 1 may be represented by * -R a -Y a , wherein * is a bond, and R a is an alkylene group having 1 to 16 carbon atoms as an example, 1 to 6 carbon atoms specifically, 1 to 3 carbon atoms May be an alkylene group, and Y a may be a cyan group, a halogen, a hydroxy group, or an amine group.
상기 화학식 2a로 나타낸 N+-R1-헤테로고리 4차 암모늄은 하기 화학식 2b 또는 하기 화학식 2c에 나타낸 N+-R1-헤테로고리 4차 암모늄일 수 있다.N + -R 1 of formula 2a - heterocyclic quaternary ammonium is N + -R 1 shown in the following formula 2b or 2c to the formula - may be a heterocyclic ring quaternary ammonium.
[화학식 2b][Formula 2b]
[화학식 2c][Formula 2c]
상기 화학식 2b는 *-N+-R1-헤테로고리 4차 암모늄으로, 고리 C는 5-멤버 혹은 6-멤버 불포화 헤테로고리화합물이고, 2 내지 3의 N을 헤테로멤버로서 구비할 수 있다.Formula 2b is * -N + -R 1 -heterocyclic quaternary ammonium, ring C is a 5-member or 6-membered unsaturated heterocyclic compound, it may be provided with N of 2 to 3 as a hetero member.
상기 화학식 2c에서, 고리 D는 5-멤버 혹은 6-멤버 불포화 헤테로고리화합물이고, 2 내지 3의 N을 헤테로멤버로서 구비할 수 있다. 또한, R1과 R1′는 서로에 관계없이, 탄소수 1 내지 16의 시아노알킬, 탄소수 1 내지 16의 할로알킬, 탄소수 1 내지 16의 하이드록시알킬, 또는 탄소수 1 내지 16의 아미노알킬일 수 있고, *-Ra-Ya으로 나타낼 수 있다. Ra와 Ya는 위에서 정의한 바와 같을 수 있다.In Formula 2c, Ring D is a 5-membered or 6-membered unsaturated heterocyclic compound, and may have N of 2 to 3 as a hetero member. In addition, R 1 and R 1 ′ may be cyanoalkyl having 1 to 16 carbon atoms, haloalkyl having 1 to 16 carbon atoms, hydroxyalkyl having 1 to 16 carbon atoms, or aminoalkyl having 1 to 16 carbon atoms, irrespective of each other. And * -R a -Y a . R a and Y a may be as defined above.
상기 N+-R1-헤테로고리 4차 암모늄은 일 예로서, N+-R1-아졸리움(azolium), N+-R1-아지니움(azinium), 또는 N+-R1R2-피페라지니움(piperazinium)일 수 있다. The N + -R 1 -heterocyclic quaternary ammonium is, for example, N + -R 1 -azolium, N + -R 1 -azinium, or N + -R 1 R 2 It may be piperazinium.
상기 N+-R1-아졸리움은 N+-R1-다이아졸리움(diazolium) 또는 N+-R1-트라이아졸리움(triazolium)일 수 있다. N+-R1-다이아졸리움은 N+-R1-이미다졸리움(imidazolium) 또는 N+-R1-피라졸리움(pyrazolium)일 수 있고, 상기 N+-R1-이미다졸리움은 하기 화학식 2-1로 나타낼 수 있고, 상기 N+-R1-피라졸리움은 하기 화학식 2-2로 나타낼 수 있다. 한편, N+-R1-트라이아졸리움은 하기 화학식 2-3으로 나타낼 수 있다.The N + 1 -R - N + O sleepiness is -R 1 - can be a tri-O sleepiness (triazolium) - diamond sleepiness (diazolium) or N + -R 1. N + -R 1 - imidazolium diamond is N + -R 1 - imidazolium (imidazolium), or N + -R 1 - pyrazol may be sleepiness (pyrazolium), the N + -R 1 - imidazolium has the formula It may be represented by 2-1, the N + -R 1 -pyrazolium may be represented by the formula 2-2. On the other hand, N + -R 1 -triazolium can be represented by the following formula 2-3.
상기 N+-R1-아지니움은 N+-R1-피리미디미늄(pyridiminium) 또는 N+-R1-피라지니움(pyrazinium)일 수 있다. N+-R1-피리미디미늄은 하기 화학식 2-7 또는 2-8로 나타낼 수 있고, N+-R1-피라지니움은 하기 화학식 2-9로 나타낼 수 있다.The N + -R 1 - Ah Genie Titanium is N + -R 1 - can be a help pyrazol Genie (pyrazinium) - pyrimidinyl minyum (pyridiminium) or N + -R 1. N + -R 1 -pyrimidinium may be represented by the following Chemical Formula 2-7 or 2-8, and N + -R 1 -pyrazinium may be represented by the following Chemical Formula 2-9.
N+-R1R2-피페라지니움(piperazinium)은 *-N+-R1R2-피페라지니움일 수 있고, 이는 하기 화학식 2-14로 나타낼 수 있다.N + -R 1 R 2 -piperazinium may be * -N + -R 1 R 2 -piperazinium, which may be represented by the following formula (2-14).
상기 화학식들 2-1 내지 2-3, 2-7 내지 2-9, 및 2-14에서, Ra 와 Ya는 앞서 설명한 바와 같고, Ra1과 Ra2는 서로에 관계없이 탄소수 1 내지 16의 알킬렌기 일 예로서 탄소수 1 내지 6 구체적으로는 탄소수 1 내지 3의 알킬렌기일 수 있고, Ya1과 Ya2는 서로에 관계없이 시안기, 할로겐, 하이드록시기, 또는 아민기일 수 있다. Rb는 탄소수 1 내지 16의 알킬렌기 일 예로서 탄소수 1 내지 6 구체적으로는 탄소수 1 내지 3의 알킬렌기일 수 있고, Yb는 수소, 시안기, 할로겐, 하이드록시기, 또는 아민기일 수 있다.In Formulas 2-1 to 2-3, 2-7 to 2-9, and 2-14, R a and Y a are as described above, and R a1 and R a2 have 1 to 16 carbon atoms regardless of each other. Alkylene group as an example may be an alkylene group having 1 to 6 carbon atoms specifically, Y a1 and Y a2 may be a cyan group, a halogen, a hydroxyl group, or an amine group irrespective of each other. R b may be an alkylene group having 1 to 16 carbon atoms, for example, an alkylene group having 1 to 6 carbon atoms, and Y b may be hydrogen, a cyan group, a halogen, a hydroxyl group, or an amine group. .
X-는 상대 음이온(counter anion)으로 구체적으로는 F-, Cl-, Br-, I-, PF6
-, BF4
-, Tf2N-(bis(trifluoromethane)sulfonimide), TfO-(trifluoromethanesulfonate), SCN-, 또는 CH3COO-일 수 있다. X - is a counter anion (counter anion) Specifically, F -, Cl -, Br - , I -, PF 6 -, BF 4 -, Tf 2 N - (bis (trifluoromethane) sulfonimide), TfO - (trifluoromethanesulfonate) , SCN -, or CH 3 COO - it can be.
이와 같이, 상기 다이아세틸렌 단량체는 지방족 탄화수소 사슬 일측 말단에 이온성 작용기를 함유함에 따라 양친매성(amphiphilic)을 나타낼 수 있다. 이러한 다이아세틸렌 단량체는 매트릭스 고분자의 고분자 사슬들 사이에서 자기조립되어 배치될 수 있다. 구체적으로, 상기 다이아세틸렌 단량체들의 다이아세틸렌기를 포함한 지방족 탄화수소 사슬들은 반데르 발스 상호작용에 의해 서로 인접하여 배치될 수 있고, 그의 일측에 이온성 작용기들이 서로 인접하여 배치될 수 있다.As such, the diacetylene monomer may exhibit amphiphilic by containing an ionic functional group at one end of an aliphatic hydrocarbon chain. These diacetylene monomers may be self-assembled between the polymer chains of the matrix polymer. Specifically, aliphatic hydrocarbon chains including the diacetylene groups of the diacetylene monomers may be disposed adjacent to each other by van der Waals interaction, and ionic functional groups may be disposed adjacent to each other on one side thereof.
상기 매트릭스 고분자는 다이폴 모멘트를 갖는 (다시 말해서, 0 초과의 다이폴 모멘트를 갖는) 혹은 극성 고분자일 수 있다. 구체적으로, 상기 매트릭스 고분자는 주쇄 또는 측쇄에 극성 작용기, 혹은 전자 구인성기(또는 전자 공여성기), 일 예로서, 주쇄 또는 측쇄에 에테르기, 카르복시기, 알코올기, 아민기, 이민기, 아마이드기, 피롤리돈기, 클로라이드기, 또는 벤젠기를 갖는 폴리에틸렌옥사이드, 폴리비닐피롤리돈, 폴리비닐알코올, 폴리비닐클로다이드. 폴리 아크릴레이트, 폴리스티렌, 또는 이의 공중합체일 수 있다. 나아가, 상기 매트릭스 고분자는 그의 주쇄 또는 측쇄에 친수성 작용기를 가질 수 있는데, 친수성 작용기는 극성 또는 차지를 갖는 작용기(charged functional group)일 수 있다. 이러한 친수성 고분자는 폴리에틸렌옥사이드, 폴리비닐알코올 혹은 폴리비닐피롤리돈일 수 있다. The matrix polymer may be a polar polymer having a dipole moment (ie, having a dipole moment greater than zero) or a polar polymer. Specifically, the matrix polymer has a polar functional group or an electron withdrawing group (or electron donating group) in the main chain or side chain, for example, an ether group, a carboxyl group, an alcohol group, an amine group, an imine group, an amide group, a blood group in the main chain or side chain. Polyethylene oxide, polyvinylpyrrolidone, polyvinyl alcohol, polyvinyl chloride which has a rollidone group, a chloride group, or a benzene group. Polyacrylates, polystyrenes, or copolymers thereof. Furthermore, the matrix polymer may have a hydrophilic functional group in its main chain or side chain, and the hydrophilic functional group may be a polarized or charged functional group. Such hydrophilic polymers may be polyethylene oxide, polyvinyl alcohol or polyvinylpyrrolidone.
상기 다이아세틸렌 단량체 1 중량부에 대해 상기 고분자는 약 1.5 내지 3.5, 구체적으로는 약 2 내지 3 중량부로 함유될 수 있다. 상기 제1 용매와 상기 제2 용매는 서로 섞임성 있는 용매로서, 휘발성 용매일 수 있다. 일 예로서, 상기 제1 용매와 상기 제2 용매는 동일한 휘발성 유기 용매일 수 있다. 구체적으로, 상기 제1 용매와 상기 제2 용매는 서로에 관계없이 극성, 양친성 용매 또는 다이폴모멘트를 갖는(다이폴모멘트가 0이 아닌) 비극성 용매일 수 있다. 이러한 용매들은 상기 다이아세틸렌 단량체의 종류에 따라 달라질 수 있다. The polymer may be contained in an amount of about 1.5 to 3.5, specifically about 2 to 3 parts by weight, based on 1 part by weight of the diacetylene monomer. The first solvent and the second solvent may be mixed with each other, and may be volatile solvents. As an example, the first solvent and the second solvent may be the same volatile organic solvent. Specifically, the first solvent and the second solvent may be a polar, amphiphilic solvent, or a nonpolar solvent having a dipole moment (non-zero dipole moment) irrespective of each other. These solvents may vary depending on the type of diacetylene monomer.
상기 다이아세틸렌 단량체들을 구비하는 고분자 패치(A)는 친수성 올리고머 또는 가소제를 더 포함할 수 있다. 이 경우, 고분자 패치(A)의 친수성 또는 유연성이 더 향상될 수 있다. 상기 친수성 올리고머는 분자량(Mn)이 100 내지 1000인 폴리에틸렌글리콜일 수 있다. 이러한 폴리에틸렌글리콜은 상기 다이아세틸렌 단량체 1 중량부에 대해 약 0.125 내지 0.5 중량부로 함유될 수 있다.The polymer patch (A) having the diacetylene monomers may further include a hydrophilic oligomer or a plasticizer. In this case, the hydrophilicity or flexibility of the polymer patch (A) can be further improved. The hydrophilic oligomer may be polyethylene glycol having a molecular weight (Mn) of 100 to 1000. Such polyethylene glycol may be contained in an amount of about 0.125 to 0.5 parts by weight based on 1 part by weight of the diacetylene monomer.
일 예에서, 상기 화학식 1로 표시된 다이아세틸렌 단량체는 하기 화학식 3으로 나타낼 수 있다.In one example, the diacetylene monomer represented by Formula 1 may be represented by the following formula (3).
[화학식 3][Formula 3]
상기 화학식 3의 a, b, R-, 및 M+는 상기 화학식 1에서 설명한 a, b, R-, 및 M+와 각각 같을 수 있다. 상기 다이아세틸렌 단량체는 PCDA(10,12-pentacosadiynoic acid)의 알칼리 금속 염, TCDA (10,12-tricosadiynoic acid)의 알칼리 금속 염, 또는 HCDA (8,10-heneicosadiynoic acid)의 알칼리 금속 염일 수 있다. A, b, R − , and M + of Formula 3 may be the same as a, b, R − , and M + described in Formula 1, respectively. The diacetylene monomer may be an alkali metal salt of PCDA (10,12-pentacosadiynoic acid), an alkali metal salt of TCDA (10,12-tricosadiynoic acid), or an alkali metal salt of HCDA (8,10-heneicosadiynoic acid).
상기 화학식 1로 표시된 다이아세틸렌 단량체를 사용할 때, 상기 제1 용매는 극성 또는 양친성 용매일 수 있다. 상기 제2 용매는 양친성 용매 또는 물일 수 있다. 일 예에서, 상기 제1 용매는 양친성 용매이고 상기 제2 용매는 물이고, 다른 예에서 상기 제1 용매와 상기 제2 용매는 모두 양친성 용매 나아가 동일한 용매일 수 있다. 양친성 용매는 일 예로서, 아세톤(acetone); 메탄올, 에탄올, 이소프로판올 등의 알코올, 다이메틸 에테르(dimethyl ether) 또는 테트라하이드로퓨란(tetrahydrofuran; THF)일 수 있다. 한편, 상기 매트릭스 고분자는 폴리에틸렌옥사이드, 폴리비닐알코올, 폴리비닐클로라이드 혹은 폴리비닐피롤리돈일 수 있다.When using the diacetylene monomer represented by Formula 1, the first solvent may be a polar or amphiphilic solvent. The second solvent may be an amphiphilic solvent or water. In one example, the first solvent is an amphiphilic solvent and the second solvent is water, and in another example, both the first solvent and the second solvent may be an amphiphilic solvent or the same solvent. Amphiphilic solvents include, for example, acetone; Alcohol, such as methanol, ethanol, isopropanol, dimethyl ether, or tetrahydrofuran (THF). On the other hand, the matrix polymer may be polyethylene oxide, polyvinyl alcohol, polyvinyl chloride or polyvinylpyrrolidone.
상기 화학식 1로 표시된 다이아세틸렌 단량체는 알칼리 금속염 용액 일 예로서 알칼리 금속 히드록사이드(alkali metal hydroxide) 수용액과 다이아세틸렌 카르복시산 단량체 용액을 준비하고, 상기 알칼리 금속 히드록사이드 수용액을 상기 다이아세틸렌 카르복시산 단량체 용액에 방울방울 떨어뜨리면서 혼합하여 제조할 수 있다. 상기 알칼리 금속염은 다이아세틸렌 카르복시산 단량체에 대하여 0.1 내지 3몰의 범위로 혼합할 수 있고 바람직하게는 0.5 내지 2몰로 혼합할 수 있다. 또한, 상기 알칼리 금속 히드록사이드와 다이아세틸렌 카르복시산 단량체는 산-염기 반응에 의해 상기 화학식 1로 표시된 다이아세틸렌 단량체를 형성할 수 있다.As the diacetylene monomer represented by Formula 1, an alkali metal hydroxide solution and a diacetylene carboxylic acid monomer solution are prepared as an example of an alkali metal salt solution, and the alkali metal hydroxide aqueous solution is used as the diacetylene carboxylic acid monomer solution. It can be prepared by mixing while dropping drops. The alkali metal salt may be mixed in the range of 0.1 to 3 moles with respect to the diacetylene carboxylic acid monomer, and preferably in 0.5 to 2 moles. In addition, the alkali metal hydroxide and the diacetylene carboxylic acid monomer may form the diacetylene monomer represented by Chemical Formula 1 by an acid-base reaction.
또 다른 예에서, 상기 화학식 1로 표시된 다이아세틸렌이 단량체는 하기 화학식 4로 나타낼 수 있다.In another example, the diacetylene monomer represented by Formula 1 may be represented by the following Formula 4.
[화학식 4][Formula 4]
상기 화학식 4의 a, b, L1, c, e, R+, 및 X-는 상기 화학식 1에서 설명한 a, b, L1, c, e, R+, 및 X-와 각각 같을 수 있다.Of Formula 4 a, b, L 1, c, e, R +, and X - is described a, b, L 1, c , e, R +, and X in the formula (1) may be the same, respectively and.
상기 화학식 4로 표시된 다이아세틸렌이 단량체를 사용할 때, 상기 제1 용매는 비극성 용매일 수 있다. 상기 제2 용매 또한 비극성 용매일 수 있다. 일 예에서, 상기 제1 용매와 상기 제2 용매는 동일한 용매일 수 있다. 상기 비극성 용매는 일 예로서, 다이폴 모멘트를 갖는 비극성 용매로서 클로로포름일 수 있다. 한편, 상기 매트릭스 고분자는 폴리에틸렌옥사이드일 수 있다.When the diacetylene represented by Formula 4 uses a monomer, the first solvent may be a nonpolar solvent. The second solvent may also be a nonpolar solvent. In one example, the first solvent and the second solvent may be the same solvent. The nonpolar solvent may be, for example, chloroform as a nonpolar solvent having a dipole moment. On the other hand, the matrix polymer may be polyethylene oxide.
폴리다이아세틸렌Polydiacetylene
함유 고분자 패치 Containing polymer patch
다시 도 1을 참조하면, 건조된 고분자 패치(A)에 광을 조사하여 다이아세틸렌 단량체들을 광중합시켜 폴리다이아세틸렌을 형성하여, 고분자 매트릭스 내에 폴리다이아세틸렌을 구비하는 고분자 패치(B)가 형성될 수 있다. Referring back to FIG. 1, the polymer patch (A) is irradiated with light to photopolymerize the diacetylene monomers to form polydiacetylene, thereby forming a polymer patch (B) having polydiacetylene in the polymer matrix. have.
상기 광은 자외선 구체적으로 250 내지 260 nm의 자외선 구체적으로, 254 nm의 자외선, 또는 감마선일 수 있고, 1 내지 300초 조사할 수 있다. 그 결과, 자기조립되어 인접하여 배치된 다이아세틸렌 단량체들을 광중합시켜 폴리다이아세틸렌을 형성할 수 있다. The light may be ultraviolet rays, specifically, 250-260 nm ultraviolet rays, specifically 254 nm ultraviolet rays, or gamma rays, and may be irradiated for 1 to 300 seconds. As a result, polyacetylene can be formed by photopolymerizing self-assembled and adjacently disposed diacetylene monomers.
상기 폴리다이아세틸렌은 하기 화학식 5로 표시된 반복단위를 가질 수 있다.The polydiacetylene may have a repeating unit represented by the following Formula 5.
[화학식 5][Formula 5]
상기 화학식 5에서, a, b, c, d, L1, L2, 및 IG는 상기 화학식 1의 a, b, c, d, L1, L2, 및 IG와 각각 같을 수 있다.In Formula 5, a, b, c, d, L 1 , L 2 , and IG may be the same as each of a, b, c, d, L 1 , L 2 , and IG of Formula 1.
이러한 폴리다이아세틸렌을 함유하는 고분자 패치(B)는 약 600 nm 내지 680 nm, 구체적으로 약 620 nm 내지 660 nm, 일 예로서 약 640 nm에서 최대 흡수 파장을 나타내어 청색을 나타내는데, 이는 폴리다이아세틸렌이 교호로 배치된 이중 및 삼중 결합으로 인한 고도로 π-컨쥬게이션된 주쇄를 갖는 공액 고분자이기 때문이다. The polymer patch (B) containing such polydiacetylene exhibits a maximum absorption wavelength at about 600 nm to 680 nm, specifically about 620 nm to 660 nm, for example about 640 nm, which indicates blue color. This is because it is a conjugated polymer with a highly π-conjugated backbone due to alternating double and triple bonds.
일 예에서, 상기 폴리다이아세틸렌은 하기 화학식 6으로 표시된 반복단위를 가질 수 있다. 이는 화학식 3으로 표시된 다이아세틸렌 단량체가 고분자화된 폴리다이아세틸렌일 수 있다.In one example, the polydiacetylene may have a repeating unit represented by the following formula (6). It may be a polydiacetylene polymerized diacetylene monomer represented by the formula (3).
[화학식 6][Formula 6]
상기 화학식 6에서, a, b, R-, 및 M+는 상기 화학식 1의 a, b, R-, 및 M+와 각각 같을 수 있다.In Formula 6, a, b, R − , and M + may be the same as each of a, b, R − , and M + of Formula 1 above.
예에서, 상기 폴리다이아세틸렌은 하기 화학식 7로 표시된 반복단위를 가질 수 있다. 이는 화학식 4로 표시된 다이아세틸렌 단량체가 고분자화된 폴리다이아세틸렌일 수 있다. In an example, the polydiacetylene may have a repeating unit represented by the following Chemical Formula 7. It may be polydiacetylene polymerized diacetylene monomer represented by the formula (4).
[화학식 7][Formula 7]
상기 화학식 7의 a, b, L1, c, e, R+, 및 X-는 상기 화학식 1의 a, b, L1, c, e, R+, 및 X-와 각각 같을 수 있다.The formula (7) of a, b, L 1, c , e, R +, and X-is the a, b, L 1, c , e, R +, and the general formula X 1 - may be the same with each.
폴리다이아세틸렌Polydiacetylene
함유 고분자 패치의 Containing polymer patches
수변색Water discoloration
반응 reaction
다시 도 1을 참조하면, 상기 폴리다이아세틸렌 함유 고분자 패치(B)에 수분을 가하면, 수분에 접촉된 폴리다이아세틸렌이 기하학적으로 변형되어 π-컨쥬게이션된 주쇄 구조 와해를 통해 적색 계열의 색을 나타낼 수 있다(C). 다시 말해서 수변색 반응을 나타낼 수 있다. 구체적으로, 수분에 접촉한 경우 폴리다이아세틸렌은 최대흡수파장이 약 490 내지 약 590 nm, 구체적으로 520 내지 약 570 nm, 일 예로서 540 nm로 청색 이동되어 육안으로 보이는 색은 적색 계열의 색으로 변할 수 있다. 이와 동시에, 수변색된 폴리다이아세틸렌은 형광을 발생시킬 수도 있다. 이에 따라, 상기 폴리다이아세틸렌을 함유하는 고분자 패치(B)는 수변색 패치로도 명명할 수도 있다.Referring back to FIG. 1, when moisture is applied to the polydiacetylene-containing polymer patch (B), the polydiacetylene in contact with moisture is geometrically deformed to exhibit a reddish color through π-conjugated main chain structure breakdown. (C). In other words, it may show a hydrochromic reaction. Specifically, in case of contact with moisture, polydiacetylene has a maximum absorption wavelength of about 490 to about 590 nm, specifically, 520 to about 570 nm, for example, blue shifted to 540 nm. Can change. At the same time, the hydrochromic polydiacetylene may generate fluorescence. Accordingly, the polymer patch (B) containing the polydiacetylene may also be referred to as a hydrochromic patch.
이러한 수변색은 폴리머를 형성하지 않고 단량체로 남아있던 다이아세틸렌 단량체가 물에 녹으면서 보이드를 형성하고 이로 인해 폴리다이아세틸렌이 기하학적 변형이 나타나는 것으로 추측되나, 이러한 이론에 구속되는 것은 아니다.This water discoloration is supposed to form voids as the diacetylene monomer which remained as a monomer without forming a polymer is dissolved in water, and this causes polydiacetylene to exhibit geometric deformation, but is not limited to this theory.
이와 같이, 폴리다이아세틸렌 함유 고분자 패치(B)는 수분(액체 또는 기체)의 접촉에 의해 색변화를 나타냄에 수분 센서 구체적으로는 수변색 패치로서의 역할을 충분히 수행할 수 있다. 구체적으로, 상기 수변색 패치를 사용하여 습도를 감지하거나, 유기 용매 내의 수분을 감지할 수도 있다. 나아가, 상기 수변색 패치는 땀구멍으로부터 나오는 극소량의 수분(나노리터의 수분)에 의해서도 청색에서 적색으로 변색될 수 있어, 지문 인식 용도 혹은 땀구멍 맵핑지로서도 사용될 수 있다. 구체적으로, 손가락뿐만 아니라 손바닥, 발바닥, 등, 얼굴 등 신체 전영역의 땀구멍을 효과적으로 맵핑할 수 있어 기존 기술의 응용분야를 확대할 수 있다. 이와 같이, 신체의 땀구멍 분포 등 의 생체 정보 분석이 가능하므로, 의료 분야, 미용분야, 또는 범죄 수사 분야에서 이용될 수 있다. 구체적으로, 다한증 환자의 활성 땀구멍 분포 분석 혹은 연령별 땀구멍 활성도 분석 등의 의료 분야, 데오드란트나 땀 억제제 등 개발을 위한 미용분야, 지문 등 의 땀구멍 지도 분석을 통한 범죄 수사 분야에 이용될 수 있다. 또한, 각종 송수관, 미세균열 건축물, 실험장비 등에서 용출되는 수분에 감응하여 나타나는 색 또는 형광 변화를 통하여 용출 위치를 정확히 파악할 수 있어 상업적 용도로 광범위하게 사용할 수 있다. 나아가, 상기 고분자 패치(B)는 매트릭스 고분자의 물성을 조절하면, 단단한 수변색 패치 뿐 아니라, 연성이 향상된 수변색 패치를 제조할 수 있는데, 연성이 향상된 수변색 패치의 경우 얼굴과 같은 곡면 형태의 피부의 수분 검출을 위해 유용하게 사용될 수 있다.As described above, the polydiacetylene-containing polymer patch (B) exhibits a color change by contact with moisture (liquid or gas), and thus, may function sufficiently as a moisture sensor, specifically, a hydrochromic patch. Specifically, humidity may be sensed using the hydrochromic patch, or moisture in an organic solvent may be sensed. Further, the hydrochromic patch may be discolored from blue to red even by a very small amount of water (nano litres) coming out of the pores, and thus may be used as a fingerprint recognition application or a pores mapping paper. Specifically, it is possible to effectively map the pores of the entire body area, such as palms, soles, back, face, as well as fingers, thereby expanding the application field of the existing technology. In this way, it is possible to analyze the biological information such as the distribution of the pores of the body, it can be used in the medical field, beauty field, or criminal investigation field. Specifically, it may be used in the medical field, such as active pores distribution analysis or analysis of age-specific pores activity of hyperhidrosis patients, the cosmetic field for the development of deodorant or sweat inhibitors, and the field of criminal investigation through analysis of the pores map of fingerprints. In addition, the elution position can be accurately identified through color or fluorescence changes in response to moisture eluted from various water pipes, microcracked buildings, and experimental equipment, and thus can be widely used for commercial purposes. In addition, the polymer patch (B) can produce not only hard hydrochromic patches, but also improved hydrochromic patches with improved ductility, by adjusting the physical properties of the matrix polymer. It can be usefully used for detecting moisture of the skin.
이하, 본 발명의 이해를 돕기 위하여 바람직한 실험예(example)를 제시한다. 다만, 하기의 실험예는 본 발명의 이해를 돕기 위한 것일 뿐, 본 발명이 하기의 실험예에 의해 한정되는 것은 아니다.Hereinafter, preferred examples are provided to aid the understanding of the present invention. However, the following experimental examples are only for helping understanding of the present invention, and the present invention is not limited to the following experimental examples.
<<
다이아세틸렌Diacetylene
단량체 Monomer
합성예들Synthesis Examples
>>
합성예Synthesis Example
1 : DA- 1: DA-
ImIm
[3-( [3- (
CyanomethylCyanomethyl
)-1-(3-() -1- (3- (
pentacosapentacosa
-10,12--10,12-
diynamidodiynamido
) propyl)-1H-imidazol-3-ium bromide]의 합성) propyl) -1H-imidazol-3-ium bromide]
메틸렌클로라이드(methylene chloride) 20 ㎖ 내에 PCDA (10,12-pentacosadiynoic acid, 0.75 g, 2 mmol)과 N-hydrosuccinimide (NHS, 0.35 g, 3 mmol), EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, 0.77 g, 4 mmol)을 녹인 용액을 상온에서 하룻밤 동안 교반하였다. 그 후, 진공상태에서 농축하여 잔류물을 에틸 아세테이트에 다시 용해시키고, 용액을 물과 함께 분별깔때기에 넣어 분리한 뒤 유기용액 층을 따로 얻어내었다. 상기 유기용액을 탈수 시킨 후 진공상태에서 농축하여 흰색 가루상태의 PCDA-NHS(2,5-dioxopyrrolidin-1-yl pentacosa-10,12-diynoate)를 얻었다. 메틸렌클로라이드(methylene chloride) 10 ㎖ 내에 상기 PCDA-NHS(0.94 g, 2 mmol)과 트라이에틸아민(TEA, 0.51 g, 5 mmol)을 녹여 제1 용액을 얻고, 제1 용액 내에 메틸렌클로라이드 10 ㎖ 내에 1-(3-아미노프로필)이미다졸(1-(3-aminopropyl)imidazole, 0.38 g, 3.00 mmol)을 녹여 얻은 제2 용액을 더한 후, 상온에서 하룻밤 동안 교반하였다. 그 후, 진공에서 농축하여 잔류물(residue)을 얻고, 상기 잔류물을 실리카겔 컬럼 크로마토그래피(메틸렌클로라이드/메탄올, 96/4)에 넣어 흰색의 고형물질로서 N-(3-(1H-이미다졸-1-일)프로필)펜타코사-10,12-다이인아마이드(N-(3-(1H-imidazol-1-yl)propyl)pentacosa-10,12- diynamide, 0.77 g, 80%) 를 얻었다.In 20 mL of methylene chloride, PCDA (10,12-pentacosadiynoic acid, 0.75 g, 2 mmol), N-hydrosuccinimide (NHS, 0.35 g, 3 mmol), EDC (1-ethyl-3- (3-dimethylaminopropyl) ) carbodiimide, 0.77 g, 4 mmol) was dissolved at room temperature overnight. Thereafter, the mixture was concentrated in vacuo, and the residue was dissolved in ethyl acetate again, and the solution was separated with a separatory funnel with water to obtain an organic solution layer. The organic solution was dehydrated and concentrated in vacuo to give white powdery PCDA-NHS (2,5-dioxopyrrolidin-1-yl pentacosa-10,12-diynoate). The PCDA-NHS (0.94 g, 2 mmol) and triethylamine (TEA, 0.51 g, 5 mmol) were dissolved in 10 ml of methylene chloride to obtain a first solution, and in 10 ml of methylene chloride in the first solution. A second solution obtained by dissolving 1- (3-aminopropyl) imidazole (1- (3-aminopropyl) imidazole, 0.38 g, 3.00 mmol) was added thereto, followed by stirring at room temperature overnight. After that, it was concentrated in vacuo to give a residue, which was put into silica gel column chromatography (methylene chloride / methanol, 96/4) to give N- (3- (1H-imidazole) as a white solid. -1-yl) propyl) pentacosa-10,12-diyneamide (N- (3- (1H-imidazol-1-yl) propyl) pentacosa-10,12-diynamide, 0.77 g, 80%) was obtained. .
브로모아세토니트릴(0.28 g, 2.32 mmol)을 함유한 아세토니트릴 20㎖ 내에 N-(3-(1H-이미다졸-1-일)프로필)펜타코사-10,12-다이인아마이드 (0.70 g, 1.45 mmol)을 넣고 하룻밤동안 교반하면서 환류하였다. 그 후, 진공에서 농축하여 고형물을 얻고, 상기 고형물을 헥산으로 세 번 세척하여 노르스름한 파우더(yellowish powder)로서 DA-
Im(0.75 g, 86%)을 얻었다. m.p.: 89 ℃, IR (KBr, cm -1 ): ν max 611, 624, 652, 719, 757, 860, 927, 1022, 1168, 1382, 1423, 1453, 1467, 1538, 1642, 1651, 2267, 2849, 2919, 3070, 3094, 3255, 3358. 1 H NMR (600 MHz, dimethyl sulfoxide- d 6 , δ ): 9.39 (s, 1H), 7.96 (t, J = 6 Hz, 1H), 7.95 (t, J = 1.8 Hz, 1H), 7.93 (t, J = 1.8 Hz, 1H), 5.63 (s, 2H), 4.22 (t, J = 6.6 Hz, 2H), 3.05 (q, J = 6 Hz, 2H), 2.26 (t, J = 7.2 Hz, 4H), 2.06 (t, J = 7.8 Hz, 2H), 1.92 (quint, J = 6.6 Hz, 2H), 1.50-.40 (m, 6H), 1.30-.23 (m, 26H), 0.85 (t, J = 7.2 Hz, 3H); 13 C NMR (75 MHz, CDCl 3, δ ): 174.82, 137.88, 123.53, 123.22, 114.08, 65.50, 65.44, 48.29, 38.71, 36.70, 35.77, 32.14, 29.88, 29.86, 29.72, 29.58, 29.34, 29.24, 29.11, 28.60, 26.04, 22.92, 19.44, 14.37.N- (3- (1H-imidazol-1-yl) propyl) pentacosa-10,12-diyneamide (0.70 g, in 20 ml of acetonitrile containing bromoacetonitrile (0.28 g, 2.32 mmol) 1.45 mmol) was added and refluxed with stirring overnight. After that, it was concentrated in vacuo to give a solid, which was washed three times with hexane to give DA- Im (0.75 g, 86%) as a yellowish powder. mp: 89 ° C., IR (KBr, cm −1): ν max 611, 624, 652, 719, 757, 860, 927, 1022, 1168, 1382, 1423, 1453, 1467, 1538, 1642, 1651, 2267, 2849, 2919, 3070, 3094, 3255, 3358. 1 H NMR (600 MHz, dimethyl sulfoxide- d 6, δ): 9.39 (s, 1H), 7.96 (t, J = 6 Hz, 1H), 7.95 (t , J = 1.8 Hz, 1H), 7.93 (t, J = 1.8 Hz, 1H), 5.63 (s, 2H), 4.22 (t, J = 6.6 Hz, 2H), 3.05 (q, J = 6 Hz, 2H ), 2.26 (t, J = 7.2 Hz, 4H), 2.06 (t, J = 7.8 Hz, 2H), 1.92 (quint, J = 6.6 Hz, 2H), 1.50-.40 (m, 6H), 1.30- .23 (m, 26H), 0.85 (t, J = 7.2 Hz, 3H); 13 C NMR (75 MHz, CDCl 3, δ): 174.82, 137.88, 123.53, 123.22, 114.08, 65.50, 65.44, 48.29, 38.71, 36.70, 35.77, 32.14, 29.88, 29.86, 29.72, 29.58, 29.34, 29.24, 29.11 , 28.60, 26.04, 22.92, 19.44, 14.37.
합성예Synthesis Example
2 : DA-Cs [Cesium salt of 10,12- 2: DA-Cs [Cesium salt of 10,12-
pentacosadiynoicpentacosadiynoic
acid]의 합성 acid] synthesis
CsOH 0.187g을 Di-워터(deionized water) 0.4mL에 녹인 다음, PCDA (10,12-pentacosadiynoic acid) 0.4658g을 10mL의 THF(tetrahydrofuran, 테트라히드로푸란) 용매에 녹인 후 위의 CsOH 수용액을 PCDA 용액에 한 방울씩 떨어뜨려 섞었다 (5 wt% 용액). 골고루 섞이도록 상온에서 1 시간동안 교반하여 투명한 균질용액인 DA-Cs용액을 제조하였다.0.187 g of CsOH was dissolved in 0.4 mL of Di-deionized water, 0.4658 g of PCDA (10,12-pentacosadiynoic acid) was dissolved in 10 mL of THF (tetrahydrofuran, tetrahydrofuran), and the aqueous CsOH solution was dissolved in PCDA solution. Drop by drop into and mix (5 wt% solution). It was stirred for 1 hour at room temperature to mix evenly to prepare a transparent homogeneous solution DA-Cs solution.
<수변색 패치 제조예>Hydrochromic patch manufacturing example
수변색 패치 제조예 1Hydrochromic patch preparation example 1
DA-Im을 클로로포름에 녹여 2wt%의 DA-Im을 함유하는 클로로포름 용액(이하, DA-Im 용액이라 함)을 준비하였다. 한편, PEO (poly(ethylene oxide), MW = 100K, 200K, 300K, 600K, 1000K, 4000K), PVP (poly(vinyl pyrrolidone), MW = 360K), PMMA (poly(methyl methacrylate), MW = ca. 120K), PVC (poly(vinyl chloride), MW = ca. 43,000, average MW = ca. 22,000), 및 PS (polystyrene, MW = 280,000)중 어느 하나의 고분자를 클로로포름에 녹여 5wt%의 고분자를 함유하는 클로로포름 용액(이하, 고분자 용액이라 함)을 준비하였다. 상기 DA-Im 용액과 고분자 용액을 1:1의 부피비로 섞고, 20분 동안 소니케이션(sonication)한 후, 유리 페트리 디쉬 상에 캐스팅하였다. 이 후, 2℃에서 12시간 동안 건조하여 얻은 필름을 필-오프하여 DA-Im을 함유하는 고분자 필름을 얻었다. 이 후, DA-Im을 함유하는 고분자 필름에 10초간의 UV 조사(254 nm, 1 mWcm-2)에 의해 DA-Im을 고분자화하여, PDA-Im을 함유하는 고분자 필름을 얻었다.DA-Im was dissolved in chloroform to prepare a chloroform solution (hereinafter referred to as DA-Im solution) containing 2wt% of DA-Im. On the other hand, PEO (poly (ethylene oxide), MW = 100K, 200K, 300K, 600K, 1000K, 4000K), PVP (poly (vinyl pyrrolidone), MW = 360K), PMMA (poly (methyl methacrylate), MW = ca. 120K), PVC (poly (vinyl chloride), MW = ca. 43,000, average MW = ca. 22,000), and PS (polystyrene, MW = 280,000) dissolved in chloroform containing 5wt% of polymer A chloroform solution (hereinafter referred to as polymer solution) was prepared. The DA-Im solution and the polymer solution were mixed at a volume ratio of 1: 1, sonicated for 20 minutes, and then cast on a glass petri dish. Thereafter, the film obtained by drying at 2 ° C. for 12 hours was peeled off to obtain a polymer film containing DA-Im. Thereafter, DA-Im was polymerized into a polymer film containing DA-Im by UV irradiation (254 nm, 1 mWcm-2) for 10 seconds to obtain a polymer film containing PDA-Im.
수변색 패치 제조예 2Hydrochromic patch preparation example 2
PEO (poly(ethylene oxide), MW = 200K), PVP (poly(vinyl pyrrolidone), MW = 360K), PVA (poly(vinyl alcohol), MW = 89K), 및 PVC (poly(vinyl chloride), MW = ca. 43K, average MW = ca. 22K) 중 하나의 고분자를 기재된 용매 내에 녹여 5wt%의 고분자 용액을 준비하였다. 한편, 다이아세틸렌 단량체 합성예 2에서 얻어진 5wt%의 투명한 균질용액인 DA-Cs 용액과 고분자 용액을 1:1의 부피비로 섞고, 20분 동안 소니케이션(sonication)한 후, 유리 페트리 디쉬 상에 캐스팅하였다. 이 후, 2℃에서 12시간 동안 건조하여 얻은 필름을 필-오프하여 DA-Cs을 함유하는 고분자 필름을 얻었다. 이 후, DA-Cs를 함유하는 고분자 필름에 10초간의 UV 조사(254 nm, 1 mWcm-2)에 의해 DA-Cs을 고분자화하여, PDA-Cs을 함유하는 고분자 필름을 얻었다.PEO (poly (ethylene oxide), MW = 200K), PVP (poly (vinyl pyrrolidone), MW = 360K), PVA (poly (vinyl alcohol), MW = 89K), and PVC (poly (vinyl chloride), MW = 43 K, average MW = ca. 22 K) was dissolved in the solvent described to prepare a 5 wt% polymer solution. Meanwhile, the DA-Cs solution, which is a 5 wt% transparent homogeneous solution obtained in the diacetylene monomer synthesis example 2, and the polymer solution were mixed at a volume ratio of 1: 1, sonicated for 20 minutes, and then cast on a glass petri dish. It was. Thereafter, the film obtained by drying at 2 ° C. for 12 hours was peeled off to obtain a polymer film containing DA-Cs. Thereafter, the polymer film containing DA-Cs was polymerized with DA-Cs by UV irradiation (254 nm, 1 mWcm-2) for 10 seconds to obtain a polymer film containing PDA-Cs.
수변색 패치 제조예 3Hydrochromic patch preparation example 3
PVP (MW = 360K)를 증류수 내에 녹인 5wt% PVP 수용액 10 ㎖ 내에 PEG (MW = 0.6K)를 0.20㎖ (제조예 3-1), 0.15㎖ (제조예 3-2), 0.10㎖ (제조예 3-3), 또는 0.05㎖ (제조예 3-4)를 혼합하여 고분자 용액을 제조한 것을 제외하고는 수변색 패치 제조예 2와 동일한 방법을 사용하여 PDA-Cs을 함유하는 고분자 필름을 얻었다.0.20 ml of PEG (MW = 0.6K), 0.15 ml (Preparation 3-2), 0.10 ml (Preparation Example) 3-3) or a polymer film containing PDA-Cs was obtained in the same manner as in Preparation Example 2 except that the polymer solution was prepared by mixing 0.05 ml (Preparation Example 3-4).
| 수변색 패치 제조예Hydrochromic patch manufacturing example | 다이아세틸렌 단량체 용액Diacetylene Monomer Solution | 고분자 용액Polymer solution | |||
| 다이아세틸렌 단량체Diacetylene Monomer | 용매menstruum | 고분자Polymer | 용매menstruum | ||
| 1One | 1-1a1-1a | DA-ImDA-Im | 클로로포름chloroform | PEO (MW = 100K)PEO (MW = 100K) | 클로로포름chloroform |
| 1-1b1-1b | PEO (MW = 200K)PEO (MW = 200K) | ||||
| 1-1c1-1c | PEO (MW = 300K)PEO (MW = 300K) | ||||
| 1-1d1-1d | PEO (MW = 600K)PEO (MW = 600K) | ||||
| 1-1e1-1e | PEO (MW = 1000K)PEO (MW = 1000K) | ||||
| 1-1f1-1f | PEO (MW = 4000K)PEO (MW = 4000K) | ||||
| 1-21-2 | PMMA (MW = 120K)PMMA (MW = 120K) | ||||
| 1-31-3 | PS (MW= 280K)PS (MW = 280K) | ||||
| 1-41-4 | PVC (MW = 430K)PVC (MW = 430K) | ||||
| 22 | 2-12-1 | DA-CsDA-Cs | THF, H2OTHF, H2O | PEO (MW = 200K)PEO (MW = 200K) | H2OH2O |
| 2-22-2 | PVP (MW = 360K)PVP (MW = 360K) | H2OH2O | |||
| 2-32-3 | PVA (MW = 89K)PVA (MW = 89K) | H2OH2O | |||
| 2-42-4 | PVC (MW = 430K)PVC (MW = 430K) | THFTHF | |||
| 33 | 3-13-1 | PVP (MW = 360K) +PEG (MW = 0.6K)PVP (MW = 360K) + PEG (MW = 0.6K) | H20H20 | ||
| 3-23-2 | |||||
| 3-33-3 | |||||
| 3-43-4 | |||||
도 2는 수변색 패치 제조예 1-1b에 따라 얻어진 DA-Im을 함유하는 PEO 필름(a)과 이 필름에 광조사하여 얻은 PDA-Im을 함유하는 PEO 필름(b)을 촬영한 사진이다.Fig. 2 is a photograph of a PEO film (a) containing DA-Im obtained according to Hydrochromic patch Preparation Example 1-1b and a PEO film (b) containing PDA-Im obtained by light irradiation on the film.
도 2를 참조하면, DA-Im을 함유하는 PEO 필름(a)은 DA-Im과 PEO를 1:2.5의 무게비로 함유하며, 무색의 불투명한 두께가 약 10 ㎛인 필름이었다. 한편, UV가 조사된 필름(b)은 청색을 나타내었다. 이러한 청색은 다이아세틸렌(DA-Im)이 고분자화되어 폴리다이아세틸렌(PDA-Im)이 형성하였음을 의미할 수 있다. 이와 더불어서, DA-Im을 함유하는 PEO 필름(a)과 PDA-Im을 함유하는 PEO 필름(b) 모두 유연한 필름이며, 굽힘에 의해 가시적인 크랙을 생성하지 않음을 알 수 있다.Referring to FIG. 2, the PEO film (a) containing DA-Im was a film containing DA-Im and PEO in a weight ratio of 1: 2.5 and having a colorless opaque thickness of about 10 μm. On the other hand, the film (b) irradiated with UV showed blue color. The blue color may mean that diacetylene (DA-Im) is polymerized to form polydiacetylene (PDA-Im). In addition, it can be seen that both the PEO film (a) containing DA-Im and the PEO film (b) containing PDA-Im are flexible films and do not produce visible cracks by bending.
도 3은 수변색 패치 제조예 1-1b에 따라 얻어진 DA-Im을 함유하는 PEO 필름과 이 필름에 광조사하여 얻은 PDA-Im을 함유하는 PEO 필름에 대한 라만 스펙트럼을 나타낸 그래프이다.FIG. 3 is a graph showing Raman spectra of a PEO film containing DA-Im obtained according to Preparation Example 1-1b and a PEO film containing PDA-Im obtained by light irradiation of the film.
도 3을 참조하면, PEO 필름 자체 (PEO)와 DA-Im을 함유하는 PEO 필름(PEO+DA) 대비 PDA-Im을 함유하는 PEO 필름(PEO+PDA)은 컨쥬게이트된 알켄을 나타내는 1453 cm-1 피크와 컨쥬게이트된 알킨을 나타내는 2080cm-1 피크를 특징적으로 나타냄을 알 수 있다. 이로부터 PDA-Im을 함유하는 PEO 필름 내의 폴리다이아세틸렌은 이중결합과 삼중결합이 교호배치되어 고도로 π-컨쥬게이트된 주쇄를 가짐을 알 수 있다.Referring to FIG. 3, the PEO film (PEO + PDA) containing PDA-Im compared with the PEO film itself (PEO) and the PEO film (PEO + DA) containing DA-Im conjugated with a 1453 cm −1 peak representing the conjugated alkenes. It can be seen that the 2080 cm -1 peak representing the gated alkyne is characteristically represented. From this, it can be seen that the polydiacetylene in the PEO film containing PDA-Im has a highly π-conjugated backbone by alternating double bonds and triple bonds.
도 4는 수변색 패치 제조예 1-1b에 따라 얻어진 PDA-Im을 함유하는 PEO 필름에 수분을 가하기 전과 가한 후의 a) 사진, b) 폴리다이아세틸렌의 주쇄 변형, c) UV-vis 흡수 스펙트럼, 및 d) 라만 스펙트럼을 나타낸다.4 is a) photograph, b) backbone modification of polydiacetylene, c) UV-vis absorption spectrum, before and after adding water to a PEO film containing PDA-Im obtained according to Preparation Example 1-1b of hydrochromic patch. And d) Raman spectrum.
도 4를 참조하면, 수변색 패치 제조예 1-1b에 따라 얻어진 PDA-Im을 함유하는 PEO 필름은 수분에 의한 청색에서 적색 변환(water-promoted blue-to-red color change)을 나타내고(a), 수분이 건조된 후에도 청색으로 다시 되돌가가지 않았다. 한편, UV-vis 흡수 스펙트럼에서 수분 노출 전 최대 흡수파장이 약 636 nm 에서 수분 노출 후 최대 흡수파장이 약 537 nm으로 이동하게 됨을 알 수 있다(c). 또한, 라만 스펙트럼에서 수분 노출 전 대비 수분 노출 후 즉 수화된 경우에는 알카인-알켄(alkyne-alkene) 밴드에 해당하는 2080 및 1453 cm-1 밴드가 2120 및 1515 cm-1 밴드로 각각 쉬프트되었음을 알 수 있다. 이러한 결과들은 수분에 의해 폴리다이아세틸렌의 주쇄에 뒤틀어짐이 발생하여 π-오비탈 어레이의 중첩에 일부 왜곡이 생성되었음을 의미할 수 있다(b). Referring to FIG. 4, the PEO film containing PDA-Im obtained according to Hydrochromic patch Preparation Example 1-1b shows water-promoted blue-to-red color change by water (a) After the moisture had dried, it did not return to blue again. On the other hand, in the UV-vis absorption spectrum, the maximum absorption wavelength before exposure to moisture is about 636 nm, the maximum absorption wavelength is shifted to about 537 nm after exposure to moisture (c). In addition, the Raman spectrum shows that the 2080 and 1453 cm -1 bands corresponding to the alkyne-alkene bands were shifted to 2120 and 1515 cm -1 bands, respectively, after hydration compared to before hydration. Can be. These results may mean that the distortion occurs in the main chain of the polydiacetylene due to moisture, so that some distortion is generated in the superposition of the π-orbital array (b).
<땀구멍 매핑(sweat pore mapping)예><Sweat pore mapping example>
상기 수변색 패치 제조예 1-1b에 따라 얻어진 PDA-Im을 함유하는 PEO 필름 상에 손가락 끝을 부드럽게 접촉시켜 땀구멍을 매핑 정도를 관찰하였다.The degree of mapping of the pores was observed by gently contacting the fingertips on the PEO film containing PDA-Im obtained in Preparation Example 1-1b.
도 5는 수변색 패치 제조예 1-1a 내지 1-1f에 따라 얻어진 PDA-Im을 함유하는 PEO 필름 상에 손가락을 접촉시킨 후의 광학이미지를 나타낸 사진이다. 땀-분비 활성구멍을 나타내는 형광 마이크로 닷들을 형광 분광기(510 ~ 550 nm 여기)를 사용하여 분석하였다.FIG. 5 is a photograph showing an optical image after a finger is touched on a PEO film containing PDA-Im obtained according to hydrochromic patch Preparation Examples 1-1a to 1-1f. Fluorescence microdots showing sweat-secreting active pores were analyzed using a fluorescence spectrometer (510-550 nm excitation).
도 5를 참조하면, 땀구멍 위치에서 땀에 의해 발현된 붉은색 형광이미지를 확인할 수 있다. 이는 100K 내지 4000K의 서로 다른 분자량을 갖는 PEO들을 사용하여 형성한 PDA-Im을 함유하는 PEO 필름들 전체에서 확인가능하다. 이로부터 매트릭스 고분자의 분자량에 관계없이 플렉서블하면서도 수분에 민감한 수변색 패치를 구현할 수 있음을 알 수 있다. 다만, 저분자량(예를 들어, 100K인)의 매트릭스 고분자를 사용한 경우, 수변색 필름이 찢어질 가능성이 다소 있을 수 있고 고분자량(예를 들어, 300K 이상인)의 매트릭스 고분자를 사용한 경우 용액의 높은 점도로 인해 균일한 두께의 필름을 얻는데 어려움이 다소 있을 수 있다.Referring to FIG. 5, a red fluorescence image expressed by sweat at the pore position may be confirmed. This is evident throughout the PEO films containing PDA-Im formed using PEOs with different molecular weights of 100K to 4000K. From this it can be seen that it is possible to implement a flexible yet moisture-sensitive hydrochromic patch irrespective of the molecular weight of the matrix polymer. However, when a low molecular weight (eg 100 K) matrix polymer is used, there may be a slight possibility that the hydrochromic film is torn, and when a high molecular weight (eg 300 K or more) matrix polymer is used, Due to the viscosity, there may be some difficulties in obtaining a film of uniform thickness.
도 6은 수변색 패치 제조예 1-1b에 따라 얻어진 PDA-Im을 함유하는 PEO 필름 상에 코, 손바닥 전체, 그리고 발바닥에 접촉시킨 후의 광학이미지를 나타낸 사진이다. 땀-분비 활성구멍을 나타내는 형광 마이크로 닷들을 형광 분광기를 사용하여 분석하였다.FIG. 6 is a photograph showing an optical image after contacting the nose, the entire palm, and the sole of the foot on a PEO film containing PDA-Im obtained in Preparation Example 1-1b. Fluorescence microdots showing sweat-secreting active pores were analyzed using a fluorescence spectrometer.
도 6을 참조하면, PDA-Im을 함유하는 PEO 필름은 콧등(a), 손바닥의 움푹 패인 부분(d)을 포함한 손바닥 전체(b), 그리고 발바닥의 움푹 패인 부분(c)에 접촉시킨 경우에도, 그 유연성으로 인해 인체의 굴곡진 부분과 거의 완벽하게 접촉할 수 있어 땀구멍 매핑 효율이 크게 향상될 수 있다.Referring to FIG. 6, the PEO film containing PDA-Im may be in contact with the nostril (a), the entire palm (b) including the depression (d) of the palm, and the depression (c) of the sole. In addition, its flexibility allows almost perfect contact with the curved parts of the human body, greatly improving the pores mapping efficiency.
도 7은 수변색 패치 제조예 1-1b에 따라 얻어진 PDA-Im을 함유하는 PEO 필름의 상대습도에 따른 수변색 정도를 보여주는 사진이다. 구체적으로, 특정 상대습도에서 5분간 노출시킨 상태의 수변색 정도를 보여준다.Figure 7 is a photograph showing the degree of water discoloration according to the relative humidity of the PEO film containing PDA-Im obtained according to the hydrochromic patch Preparation Example 1-1b. Specifically, it shows the degree of water discoloration in a state exposed for 5 minutes at a specific relative humidity.
도 7을 참조하면, 수변색 패치 제조예 1-1b에 따라 얻어진 PDA-Im을 함유하는 PEO 필름는 20 내지 95%에 이르는 상대습도에서 5분간 노출하여도 변색되지 않았다. 그러나, 상대습도 100%에 노출하였을 때에는 바로 붉은색으로 변색되었다. 이로부터, 본 실시예에 따른 수변색 패치는 주변환경의 습도에 크게 영향받지 않고 물에 직접적으로 노출되었을 때에만 수변색되는 것을 알 수 있다.Referring to FIG. 7, the PEO film containing PDA-Im obtained according to the hydrochromic patch Preparation Example 1-1b did not discolor even when exposed for 5 minutes at a relative humidity of 20 to 95%. However, when exposed to 100% relative humidity, it immediately turned red. From this, it can be seen that the hydrochromic patch according to the present embodiment is hydrochromic only when directly exposed to water without being greatly affected by the humidity of the surrounding environment.
도 8은 수변색 패치 제조예 1-1b, 1-2, 1-3, 및 1-4에 따라 얻어진 PDA-Im을 함유하는 고분자 필름의 사진(a), 손가락을 접촉한 후의 광학 이미지(b), 및 형광 현미경 이미지(c)이다.8 is a photograph (a) of a polymer film containing PDA-Im obtained according to hydrochromic patch preparation examples 1-1b, 1-2, 1-3, and 1-4, and an optical image after contact with a finger (b ), And a fluorescence microscope image (c).
도 8 참조하면, 매트릭스 고분자로서 PEO, PMMA, PS, 및 PVC를 사용한 경우 모두 푸른색 필름을 나타내는 것으로보아, UV에 의해 DA-Im이 고분자화되어 PDA-Im을 형성한 것을 알 수 있다. 이는 DA-Im이 상기 고분자들 내에서 자기조립될 수 있음을 의미할 수 있다. 또한, 매트릭스 고분자로서 PS와 PVC를 사용한 경우에는 굽힘력(bending force)이 가해졌을 때 부서지거나 혹은 크랙이 발생되는 것이 관찰되었으나 수변색을 나타내는 것에는 문제가 없었다.Referring to FIG. 8, when PEO, PMMA, PS, and PVC are used as the matrix polymer, all of them show a blue film, indicating that DA-Im is polymerized by UV to form PDA-Im. This may mean that DA-Im may self-assemble in the polymers. In addition, when PS and PVC were used as the matrix polymer, it was observed that cracking or cracking occurred when a bending force was applied, but there was no problem in indicating water discoloration.
한편, PDA-Im을 함유하는 고분자 필름에 손가락을 접촉하였을 때, 매트릭스 고분자로서 PEO를 사용한 경우에는 광학 이미지와 형광 현미경 이미지 모두에서 땀구멍을 포함하는 손가락 지문이 선명하게 나타나는 반면, 매트릭스 고분자로서 PMMA 및 PVC를 사용한 경우에는 약간의 땀구멍이, 그리고 PS를 사용한 경우에는 전혀 땀구멍이 관찰되지 않았다. 이는 매트릭스 고분자의 친수성 정도에 따른 것으로 추정되었다. 즉, PS는 소수성이 매우 큰 고분자이고, PMMA와 PVC 또한 소수성 고분자로 알려져 있다.On the other hand, when PEO is used as the matrix polymer when the finger is in contact with the polymer film containing PDA-Im, finger fingerprints including pores appear clearly in both optical and fluorescence microscope images, whereas PMMA and Slight pores were observed with PVC and no pores with PS. This was estimated according to the degree of hydrophilicity of the matrix polymer. In other words, PS is a very hydrophobic polymer, and PMMA and PVC are also known as hydrophobic polymers.
도 9는 수변색 패치 제조예 2-1 내지 2-4에 따라 얻어진 PDA-Cs을 함유하는 고분자 필름의 사진이다.9 is a photograph of a polymer film containing PDA-Cs obtained according to the hydrochromic patches Preparation Examples 2-1 to 2-4.
도 9를 참조하면, 매트릭스 고분자로서 PEO, PVP, PVA, 및 PVC를 사용한 경우 모두 푸른색 필름을 나타내는 것으로보아, UV에 의해 DA-Cs가 고분자화되어 PDA-Cs를 형성한 것을 알 수 있다. 이는 DA-Cs가 상기 고분자들 내에서 자기조립될 수 있음을 의미할 수 있다. 또한, 모든 경우에 굽힘력(bending force)이 가해졌을 때 부서지거나 혹은 크랙이 발생하지 않는 등 유연성이 뛰어났다.Referring to FIG. 9, when PEO, PVP, PVA, and PVC are used as the matrix polymer, all of them show a blue film, and it can be seen that DA-Cs is polymerized by UV to form PDA-Cs. This may mean that DA-Cs can be self-assembled in the polymers. In addition, in all cases, flexibility was excellent, such as no bending or cracking when bending force was applied.
도 10은 수변색 패치 제조예 3-1 내지 3-4에 따라 얻어진 PDA-Cs을 함유하는 고분자 필름의 상대습도에 따른 수변색 정도를 보여주는 사진이다. 구체적으로, 특정 상대습도에서 5분간 노출시킨 상태의 수변색 정도를 보여준다.10 is a photograph showing the degree of water discoloration according to the relative humidity of the polymer film containing the PDA-Cs obtained according to the hydrochromic patch Preparation Examples 3-1 to 3-4. Specifically, it shows the degree of water discoloration in a state exposed for 5 minutes at a specific relative humidity.
도 10을 참조하면, 매트릭스 고분자로서 PVP를 사용한 상태에서 친수성이 크고 흡습성을 갖는 PEG의 함량을 증가시킴에 따라, 수변색이 나타나는 상대 습도값이 작아짐을 알 수 있다. 구체적으로, PEG를 0.05ml 첨가한 경우와 0.10ml 첨가한 경우 70%RH에서 수변색이 나타나기 시작하는 것으로 보이고, PEG를 0.15ml 첨가한 경우 60%RH에서 수변색이 나타나기 시작하는 것으로 보이고, PEG를 0.20ml 첨가한 경우 50%RH에서 수변색이 나타나기 시작하는 것으로 보인다.Referring to FIG. 10, it can be seen that as the content of PEG having hydrophilicity and hygroscopicity is increased in the state of using PVP as the matrix polymer, the relative humidity value in which water discoloration occurs is reduced. Specifically, when 0.05 ml of PEG and 0.10 ml were added, water discoloration began to appear at 70% RH, and when 0.15 ml of PEG appeared to start at water discoloration at 60% RH. When 0.20 ml of Hg added, water discoloration began to appear at 50% RH.
이상, 본 발명을 바람직한 실시예를 들어 상세하게 설명하였으나, 본 발명은 상기 실시예에 한정되지 않고, 본 발명의 기술적 사상 및 범위 내에서 당 분야에서 통상의 지식을 가진 자에 의하여 여러가지 변형 및 변경이 가능하다.In the above, the present invention has been described in detail with reference to preferred embodiments, but the present invention is not limited to the above embodiments, and various modifications and changes by those skilled in the art within the spirit and scope of the present invention. This is possible.
Claims (20)
- 고분자 매트릭스; 및Polymer matrix; And상기 고분자 매트릭스 내에 위치하는 하기 화학식 5로 나타낸 반복단위를 구비하는 폴리다이아세틸렌을 구비하는 수변색 고분자 패치:Hydrochromic polymer patch having a polydiacetylene having a repeating unit represented by the formula (5) located in the polymer matrix:[화학식 5][Formula 5]상기 화학식 5에서, In Chemical Formula 5,a는 1 내지 20의 정수이고, b는 1 내지 20의 정수이고, a is an integer from 1 to 20, b is an integer from 1 to 20,L1은 , , , , , , , , 또는 이고, E, E1, 및 E2는 서로에 관계없이 O 또는 S이고, c는 0 내지 2의 정수이고,L 1 is , , , , , , , , or E, E 1 , and E 2 are O or S irrespective of each other, c is an integer from 0 to 2,L2은 탄소수 1 내지 10의 알킬렌 또는 탄소수 5 내지 12의 아릴렌기이고, d는 0 내지 1의 정수이고,L 2 is an alkylene having 1 to 10 carbon atoms or an arylene group having 5 to 12 carbon atoms, d is an integer of 0 to 1,IG는 이온성 작용기로서, R-M+이거나 R+X-이고, And, - IG is an ionic functional group, R - M +, or R + XR-는 카르복실레이트 음이온이고, M+는 알칼리 금속 양이온이고,R − is a carboxylate anion, M + is an alkali metal cation,R+은 4차 암모늄기이고, X-는 F-, Cl-, Br-, I-, PF6 -, BF4 -, Tf2N-(bis(trifluoromethane)sulfonimide), TfO-(trifluoromethanesulfonate), SCN-, 또는 CH3COO-이다. R + is a quaternary ammonium group, X - is F -, Cl -, Br - , I -, PF 6 -, BF 4 -, Tf 2 N - (bis (trifluoromethane) sulfonimide), TfO - (trifluoromethanesulfonate), SCN - or CH 3 COO - it is.
- 제1항에 있어서,The method of claim 1,상기 고분자는 폴리에틸렌 옥사이드, 폴리비닐알코올 혹은 폴리비닐피롤리돈인 수변색 고분자 패치.The polymer is a hydrochromic polymer patch of polyethylene oxide, polyvinyl alcohol or polyvinylpyrrolidone.
- 제1항에 있어서,The method of claim 1,상기 폴리다이아세틸렌 1 중량부에 대해 상기 고분자는 약 1.5 내지 3.5의 중량부로 함유되는 수변색 고분자 패치.The hydrochromic polymer patch containing about 1.5 to 3.5 parts by weight of the polymer relative to 1 part by weight of the polydiacetylene.
- 제1항에 있어서,The method of claim 1,친수성 올리고머 혹은 가소제를 더 포함하는 수변색 고분자 패치.Hydrochromic polymer patch further comprising a hydrophilic oligomer or plasticizer.
- 제1항에 있어서,The method of claim 1,상기 화학식 5의 반복단위를 갖는 폴리다이아세틸렌은 하기 화학식 6으로 표시된 반복단위를 갖는 폴리다이아세틸렌인 수변색 고분자 패치:The polydiacetylene having a repeating unit of Formula 5 is a polydiacetylene having a repeating unit represented by the following Formula 6 hydrochromic polymer patch:[화학식 6][Formula 6]상기 화학식 6에서, a, b, R-, 및 M+는 상기 화학식 5의 a, b, R-, 및 M+와 각각 같다.In Formula 6, a, b, R -, and M + is of Formula 5 a, b, R - and the like, respectively, and M +.
- 제5항에 있어서,The method of claim 5,상기 M+는 세슘 이온 또는 루비늄 이온인 수변색 고분자 패치.Wherein M + is cesium ion or rubinium ion.
- 제5항에 있어서,The method of claim 5,상기 고분자는 폴리에틸렌 옥사이드, 폴리비닐알코올 혹은 폴리비닐피롤리돈인 수변색 고분자 패치.The polymer is a hydrochromic polymer patch of polyethylene oxide, polyvinyl alcohol or polyvinylpyrrolidone.
- 제1항에 있어서,The method of claim 1,상기 화학식 5로 나타낸 반복단위를 갖는 폴리다이아세틸렌은 하기 화학식 7로 표시된 반복단위를 갖는 폴리다이아세틸렌인 수변색 고분자 패치:The polydiacetylene having a repeating unit represented by Formula 5 is a polydiacetylene having a repeating unit represented by Formula 7 below:[화학식 7][Formula 7]상기 화학식 7의 a, b, L1, c, e, R+, 및 X-는 상기 화학식 5의 a, b, L1, c, e, R+, 및 X-와 각각 같다.Formula 7, a, b, L 1, c , e, R +, and X - is of Formula 5 a, b, L 1, c, e, R +, and X - and the like, respectively.
- 제8항에 있어서,The method of claim 8,R+은 하기 화학식 2a로 나타낸 N+-R1-헤테로고리 4차 암모늄(heterocyclic quarternary ammonium)인 수변색 고분자 패치: R + is a hydrochromic polymer patch of N + -R 1 -heterocyclic quaternary ammonium represented by Formula 2a:[화학식 2a][Formula 2a]상기 화학식 2a에서, In Chemical Formula 2a,*는 결합이고,* Is a combination,고리 B는 1 내지 3의 N과 0 내지 1의 O를 헤테로멤버로서 구비하는 5-멤버 혹은 6-멤버 헤테로고리화합물(5-membered or 6-membered heterocyclic compound)로서 포화 또는 불포화 헤테로고리화합물이고,Ring B is a 5-membered or 6-membered heterocyclic compound having 1 to 3 N and 0 to 1 as O-membered hetero member, and a saturated or unsaturated heterocyclic compound,R1은 탄소수 1 내지 16의 시아노알킬, 탄소수 1 내지 16의 할로알킬, 탄소수 1 내지 16의 하이드록시알킬, 또는 탄소수 1 내지 16의 아미노알킬이다.R 1 is cyanoalkyl having 1 to 16 carbon atoms, haloalkyl having 1 to 16 carbon atoms, hydroxyalkyl having 1 to 16 carbon atoms, or aminoalkyl having 1 to 16 carbon atoms.
- 제9항에 있어서,The method of claim 9,상기 화학식 2a로 나타낸 N+-R1-헤테로고리 4차 암모늄은 하기 화학식 2b 또는 하기 화학식 2c에 나타낸 N+-R1-헤테로고리 4차 암모늄인 수변색 고분자 패치: Of formula 2a N + -R 1 - heterocyclic quaternary ammonium is the formula 2b or to N + -R 1 shown in Chemical Formula 2c - number of heterocyclic quaternary ammonium polymer discolored patches:[화학식 2b][Formula 2b][화학식 2c][Formula 2c]상기 화학식 2b에서, 고리 C는 2 내지 3의 N을 헤테로멤버로서 구비하는 5-멤버 혹은 6-멤버 불포화 헤테로고리화합물이고, In Formula 2b, Ring C is a 5-membered or 6-membered unsaturated heterocyclic compound having N of 2 to 3 as a hetero member,상기 화학식 2c에서, 고리 D는 2 내지 3의 N을 헤테로멤버로서 구비하는 5-멤버 혹은 6-멤버 불포화 헤테로고리화합물이고, In Formula 2c, Ring D is a 5-membered or 6-membered unsaturated heterocyclic compound having N of 2 to 3 as a hetero member,R1과 R1′는 서로에 관계없이, 탄소수 1 내지 16의 시아노알킬, 탄소수 1 내지 16의 할로알킬, 탄소수 1 내지 16의 하이드록시알킬, 또는 탄소수 1 내지 16의 아미노알킬이다.R 1 and R 1 ′ are each independently cyanoalkyl having 1 to 16 carbon atoms, haloalkyl having 1 to 16 carbon atoms, hydroxyalkyl having 1 to 16 carbon atoms, or aminoalkyl having 1 to 16 carbon atoms.
- 제10항에 있어서,The method of claim 10,상기 N+-R1-헤테로고리 4차 암모늄은 N+-R1-아졸리움(azolium), N+-R1-아지니움(azinium), 또는 N+-R1R2-피페라지니움(piperazinium)인 수변색 고분자 패치. The N + -R 1 -heterocyclic quaternary ammonium is N + -R 1 -azolium, N + -R 1 -azinium, or N + -R 1 R 2 -piperazini Hydrochromic polymer patch that is piperazinium.
- 제11항에 있어서,The method of claim 11,상기 N+-R1-아졸리움은 N+-R1-다이아졸리움(diazolium) 또는 N+-R1-트라이아졸리움(triazolium)인 수변색 고분자 패치.The N + -R 1 -azolium is N + -R 1 -diazolium or N + -R 1 -triazolium (hydroachromic polymer patch).
- 제12항에 있어서,The method of claim 12,상기 N+-R1-다이아졸리움은 하기 화학식 2-1로 나타낸 N+-R1-이미다졸리움 또는 하기 화학식 2-2로 나타낸 N+-R1-피라졸리움인 수변색 고분자 패치.The N + -R 1 - imidazolium diamond is to N + -R 1 represented by Formula 2-1-imidazolidin N + -R 1 represented by the following Formula 2-2 or imidazolium-pyrazolidine may imidazolium discoloration polymer patches.상기 화학식 2-1 및 2-2에서, Ra는 탄소수 1 내지 16의 알킬렌기이고,In Formulas 2-1 and 2-2, R a is an alkylene group having 1 to 16 carbon atoms,Ya는 시안기, 할로겐, 하이드록시기, 또는 아민기이다.Y a is a cyan group, a halogen, a hydroxy group, or an amine group.
- 제1항에 있어서,The method of claim 1,상기 수변색 고분자 패치는 청색을 나타내는 수변색 고분자 패치.The hydrochromic polymer patch may be blue.
- 제1항에 있어서,The method of claim 1,상기 수변색 고분자 패치는 땀구멍 맵핑 패치인 수변색 고분자 패치.The hydrochromic polymer patch is a hydrochromic polymer patch.
- 하기 화학식 1로 표시된 다이아세틸렌 단량체들이 제1 용매 내에 함유된 다이아세틸렌 단량체 용액과 고분자가 제2 용매 내에 함유된 고분자 용액을 서로 혼합하는 단계;Mixing the diacetylene monomer solution containing the diacetylene monomers represented by Formula 1 in the first solvent and the polymer solution containing the polymer in the second solvent;상기 혼합액을 필름으로 성형하는 단계;Molding the mixed solution into a film;상기 필름을 건조하여 상기 고분자의 매트릭스 및 그의 내부에 자기조립되어 배치된 상기 다이아세틸렌 단량체들을 구비하는 고분자 패치를 형성하는 단계; 및Drying the film to form a polymer patch comprising the matrix of the polymer and the diacetylene monomers self-assembled therein; And상기 고분자 패치에 자외선 또는 감마선을 조사하여 상기 다이아세틸렌 단량체들을 광중합하여 폴리다이아세틸렌을 형성하는 단계를 포함하는 수변색 고분자 패치 제조방법:Method for producing a hydrochromic polymer patch comprising irradiating the polymer patch with ultraviolet or gamma rays to photopolymerize the diacetylene monomers to form a polydiacetylene.[화학식 1][Formula 1]상기 화학식 1에서, In Chemical Formula 1,a는 1 내지 20의 정수이고, b는 1 내지 20의 정수이고, a is an integer from 1 to 20, b is an integer from 1 to 20,L1은 , , , , , , , , 또는 이고, E, E1, 및 E2는 서로에 관계없이 O 또는 S이고, c는 0 내지 2의 정수이고,L 1 is , , , , , , , , or E, E 1 , and E 2 are O or S irrespective of each other, c is an integer from 0 to 2,L2은 탄소수 1 내지 10의 알킬렌 또는 탄소수 5 내지 12의 아릴렌기이고, d는 0 내지 1의 정수이고,L 2 is an alkylene having 1 to 10 carbon atoms or an arylene group having 5 to 12 carbon atoms, d is an integer of 0 to 1,IG는 이온성 작용기로서, R-M+이거나 R+X-이고, And, - IG is an ionic functional group, R - M +, or R + XR-는 카르복실레이트 음이온이고, M+는 알칼리 금속 양이온이고,R − is a carboxylate anion, M + is an alkali metal cation,R+은 4차 암모늄기이고, X-는 F-, Cl-, Br-, I-, PF6 -, BF4 -, Tf2N-(bis(trifluoromethane)sulfonimide), TfO-(trifluoromethanesulfonate), SCN-, 또는 CH3COO-이다. R + is a quaternary ammonium group, X - is F -, Cl -, Br - , I -, PF 6 -, BF 4 -, Tf 2 N - (bis (trifluoromethane) sulfonimide), TfO - (trifluoromethanesulfonate), SCN - or CH 3 COO - it is.
- 제16항에 있어서,The method of claim 16,상기 제1 용매와 상기 제2 용매는 서로 섞임성 있는 휘발성 용매인 수변색 고분자 패치 제조방법.The first solvent and the second solvent is a hydrochromic polymer patch manufacturing method that is a volatile solvent mixed with each other.
- 제17항에 있어서,The method of claim 17,상기 제1 용매와 상기 제2 용매는 서로에 관계없이 극성 용매, 양친성 용매 또는 다이폴모멘트를 갖는 비극성 용매인 수변색 고분자 패치 제조방법.The first solvent and the second solvent is a non-polar solvent having a polar solvent, an amphiphilic solvent or a dipole moment irrespective of each other.
- 제16항에 있어서,The method of claim 16,상기 화학식 1로 표시된 다이아세틸렌 단량체는 하기 화학식 3으로 표시되는 다이아세틸렌 단량체인 수변색 고분자 패치 제조방법:The diacetylene monomer represented by Chemical Formula 1 is a diacetylene monomer represented by Chemical Formula 3:[화학식 3][Formula 3]상기 화학식 3의 a, b, R-, 및 M+는 상기 화학식 1의 a, b, R-, 및 M+와 각각 같다.Of Formula 3 a, b, R -, and M + is of the formula 1 a, b, R - and the like, respectively, and M +.
- 제16항에 있어서,The method of claim 16,상기 화학식 1로 표시된 다이아세틸렌 단량체는 하기 화학식 4로 표시되는 다이아세틸렌 단량체인 수변색 고분자 패치 제조방법:The diacetylene monomer represented by Chemical Formula 1 is a diacetylene monomer represented by Chemical Formula 4:[화학식 4][Formula 4]상기 화학식 4의 a, b, L1, c, e, R+, 및 X-는 상기 화학식 1의 a, b, L1, c, e, R+, 및 X-와 각각 같다.Of Formula 4 a, b, L 1, c, e, R +, and X - is of the formula 1 a, b, L 1, c, e, R +, and X - and the like, respectively.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/772,189 US10954380B2 (en) | 2015-10-30 | 2016-10-12 | Hydrochromic polydiacetylene polymer patch and method of manufacturing the same |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR20150151930 | 2015-10-30 | ||
| KR10-2015-0151930 | 2015-10-30 | ||
| KR10-2016-0126159 | 2016-09-30 | ||
| KR1020160126159A KR101802091B1 (en) | 2015-10-30 | 2016-09-30 | Hydrochromic polydiacetylene polymer patch and fabrication method thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017073927A1 true WO2017073927A1 (en) | 2017-05-04 |
Family
ID=58631913
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/KR2016/011399 WO2017073927A1 (en) | 2015-10-30 | 2016-10-12 | Hydrochromic polydiacetylene polymer patch and manufacturing method therefor |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2017073927A1 (en) |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030103905A1 (en) * | 2000-06-23 | 2003-06-05 | Ribi Hans O. | Methods and compositions for preparing consumables with optical shifting properties |
| KR100781599B1 (en) * | 2006-10-24 | 2007-12-05 | 안동준 | Sensor using polydiacetylene-embedded polymer film and process for preparing the same |
| KR20150083814A (en) * | 2013-01-18 | 2015-07-20 | 한양대학교 산학협력단 | Hydrochromic polydiacetylene composite, hydrochromic thin film using the same and use thereof |
-
2016
- 2016-10-12 WO PCT/KR2016/011399 patent/WO2017073927A1/en active Application Filing
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030103905A1 (en) * | 2000-06-23 | 2003-06-05 | Ribi Hans O. | Methods and compositions for preparing consumables with optical shifting properties |
| KR100781599B1 (en) * | 2006-10-24 | 2007-12-05 | 안동준 | Sensor using polydiacetylene-embedded polymer film and process for preparing the same |
| KR20150083814A (en) * | 2013-01-18 | 2015-07-20 | 한양대학교 산학협력단 | Hydrochromic polydiacetylene composite, hydrochromic thin film using the same and use thereof |
Non-Patent Citations (4)
| Title |
|---|
| PARK, D. -H. ET AL.: "Inkjet-Printable Amphiphilic Polydiacetylene Precursor for Hydrochromic Imaging on Paper", ADVANCED FUNCTIONAL MATERIALS, vol. 26, no. 4, 2016, pages 498 - 506, [retrieved on 20151215] * |
| PYO, M. ET AL.: "Sweat Pore Mapping Using a Fluorescein-polymer Composite Film for Fingerprint Analysis", CHEMICAL COMMUNICATIONS, vol. 51, no. 15, 6 January 2015 (2015-01-06), pages 3177 - 3180, XP055379639 * |
| XU, Q. ET AL.: "Polydiacetylene-based Colorimetric and Fluorescent Chemosensor for the Detection of Carbon Dioxide", JOURNAL OF THE AMERICAN CHEMICAL SOCIETY, vol. 135, no. 47, 6 November 2013 (2013-11-06), pages 17751 - 17754, XP055379629 * |
| XU, Q. ET AL.: "Visual Detection of Copper Ions Based on Azide-and Alkyne-functionalized Polydiacetylene Vesicles", JOURNAL OF MATERIALS CHEMISTRY, vol. 21, no. 39, 2011, pages 15214 - 15217, XP055379637 * |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU774664B2 (en) | Amine derivatives as protease inhibitors | |
| KR101802091B1 (en) | Hydrochromic polydiacetylene polymer patch and fabrication method thereof | |
| WO2016159734A1 (en) | Bleed-free injection needle coated with crosslinked chitosan having introduced catechol group and oxidized catechol group | |
| WO2011096701A2 (en) | Novel fluorinated compound, a composition comprising the same, and a production method for a film using the same | |
| WO2016163741A1 (en) | Polymer containing aziridine and method for producing same | |
| WO2014104480A1 (en) | Monomer, hard mask composition comprising said monomer, and method for forming pattern using said hard mask composition | |
| WO2012111964A2 (en) | Solventless composition and method for preparing same | |
| WO2017073927A1 (en) | Hydrochromic polydiacetylene polymer patch and manufacturing method therefor | |
| WO2018190493A1 (en) | Method for synthesizing sucralfate and sucralfate thereby | |
| WO2018128336A1 (en) | Emulsion particle, emulsion comprising same, method for manufacturing emulsion | |
| WO2021090997A1 (en) | Photo-crosslinked hyaluronic acid sponge and manufacturing method thereof | |
| WO2021075800A1 (en) | Method for preparing water-soluble acrylic-modified phenolic-modified epoxy resin, water-soluble acrylic-modified phenolic-modified epoxy resin prepared thereby, and aqueous coating composition comprising same | |
| WO2016159600A1 (en) | Method for preparing superabsorbent resin | |
| WO2017073926A1 (en) | Diacetylene aqueous ink, hydrochromic polydiacetylene test paper manufactured using same, and use thereof | |
| WO2018186683A1 (en) | Biosensor substrate, method for producing same, and biosensor comprising same | |
| JP4974412B2 (en) | Diol preparation process | |
| WO2009107987A2 (en) | Ph-sensitive polyethylene oxide co-polymer and synthetic method thereof | |
| WO2020046056A1 (en) | Adhesive composition for label, method for preparing same, adhesive sheet comprising same, and article product | |
| WO2020060194A1 (en) | Poly(allyl glycidyl ether)-based redox polymer and electrochemical biosensor using same | |
| WO2015108271A1 (en) | Novel sulfonyl imide salt compound, production method for same, and photoacid generator and light-sensitive resin composition comprising same | |
| WO2018004292A1 (en) | Crosslinked polysilsesquioxane random copolymer absorbing both uv-a and uv-b, and method for preparing same | |
| CN109422881A (en) | Containing the isocyanurate-modified organic siliconresin of epoxy group, photosensitive resin composition, photo-conductive film, laminated body and pattern forming method | |
| WO2022010288A1 (en) | Functionalized thermoplastic polyurethane, method for producing same, method for producing highly functional medical composite material using same, and medical device including same | |
| KR102101773B1 (en) | Gel nail polish composition comprising acrylate containing acetal group, photoradical initiator and photoacid generator as effective component and uses thereof | |
| JP7270486B2 (en) | Stretchable membrane and method for forming the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16860111 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15772189 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 16860111 Country of ref document: EP Kind code of ref document: A1 |