WO2016198613A1 - N-(thio)acylimino compounds - Google Patents
N-(thio)acylimino compounds Download PDFInfo
- Publication number
- WO2016198613A1 WO2016198613A1 PCT/EP2016/063318 EP2016063318W WO2016198613A1 WO 2016198613 A1 WO2016198613 A1 WO 2016198613A1 EP 2016063318 W EP2016063318 W EP 2016063318W WO 2016198613 A1 WO2016198613 A1 WO 2016198613A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- spp
- het
- alkoxy
- group
- Prior art date
Links
- 0 C*C=CC(*)=CC=N/C=S/C Chemical compound C*C=CC(*)=CC=N/C=S/C 0.000 description 16
- LUFOBNXHXHLURC-SJDXMKNESA-N NC(/C=C\C=C/NCc(cn1)ccc1Cl)I Chemical compound NC(/C=C\C=C/NCc(cn1)ccc1Cl)I LUFOBNXHXHLURC-SJDXMKNESA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/14—Ectoparasiticides, e.g. scabicides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing three or more hetero rings
Definitions
- Pesticidal compounds which are similar to those of US 2013/0150414 are known from WO 2013/129688.
- R 4 is a group -L 1 -C ⁇ C-R A or a group -L 2 -C ⁇ N; wherein L 1 is a bond or is a bridging group selected from -O-, -S-, -N(R 9a )-,
- phenyl, benzyl, pyridyl, phenoxy, benzyloxy and pyridyloxy wherein the six last- mentioned radicals may carry 1, 2, 3, 4 or 5 substituents independently selected from halogen, C 1 -C 6 -alkyl, C 1 -C 6 -haloalkyl, C 1 -C 6 -alkoxy, C 1 -C 6 -haloalkoxy and (C1-C6-alkoxy)carbonyl,
- a process for the preparation of a veterinary composition for treating, controlling, preventing or protecting animals against infestation or infection by parasites which comprises formulating a compound of formula (I) or a stereoisomer, tautomer and/or veterinary acceptable salt thereof with a carrier composition suitable for veterinary use;
- R 1 and R 2 independently of each other, are selected from the group consisting of hydrogen, halogen, CN, C1-C6-alkyl, C3-C6-cycloalkyl, C1-C6-haloalkyl and C3-C6- halocycloalkyl; or
- R 6a is as defined above (i.e. in the summary of invention) or in any of embodiments 12 or 13.
- R 4 is as defined above (i.e. in the summary of invention) or in any of embodiments 4 to 7;
- alkyl as used herein and in the alkyl moieties of alkoxy, alkylthio, alkylsulfinyl, alkylsulfonyl, alkylcarbonyl, alkoxycarbonyl, alkylamino, dialkylamino and the like refers to saturated straight-chain or branched hydrocarbon radicals having 1 to 2 ("C 1 -C 2 -alkyl"), 1 to 3 ("C 1 -C 3 -alkyl”),1 to 4 (“C 1 -C 4 -alkyl”), 1 to 6 (“C 1 -C 6 -alkyl”), 1 to 8 (“C 1 -C 8 -alkyl”) or 1 to 10 (“C 1 -C 10 -alkyl”) carbon atoms.
- LG 2 examples include, but are not limited to: halogen, alkyl sulfonate, haloalkyl sulfonate, aryl sulfonate, alkyl phosphonate and various activated esters derived from the reaction of a free carboxylic acid with a peptide coupling reagent in the presence of an amine base; representative reaction conditions can be found in the following review, and the references cited therein: Chem. Rev., 2011, 111 (11), 6557- 6602. A reversal of the order of these two steps would also result in an acceptable synthesis of the desired compounds.
- invertebrate pests are preferably selected from arthropods, gastropods and nematodes, more preferably from harmful insects, arachnids and nematodes, and even more preferably from insects, acarids and nematodes. In the sense of the present invention, “invertebrate pests” are most preferably insects.
- latiferreana Dalaca noctuides, Datana integerrima, Dasychira pinicola, Dendrolimus spp. such as D. pini, D. spectabilis, D. sibiricus; Desmia funeralis, Diaphania spp. such as D. nitidalis, D. hyalinata; Diatraea grandiosella, Diatraea saccharalis, Diphthera festiva, Earias spp. such as E. insulana, E.
- Diaspis spp. such as D. bromeliae; Dichelops furcatus, Diconocoris hewetti, Doralis spp., Dreyfusia nordmannianae, Dreyfusia piceae, Drosicha spp., Dysaphis spp. such as D.
- Tibraca spp. Tomaspis spp., Toxoptera spp. such as T. aurantii
- Trialeurodes spp. such as T. abutilonea, T. ricini, T. vaporariorum
- Triatoma spp. Trioza spp., Typhlocyba spp., Unaspis spp. such as U. citri, U. yanonensis
- Viteus vitifolii
- Dustable powders (DP, DS)
- either individual components of the composition according to the invention or partially premixed components e. g. components comprising compounds of the present invention and/or mixing partners as defined above or below, can be applied jointly (e.g. after tank mix) or consecutively.
- the compounds of the present invention can be applied as such or in form of compositions comprising them as defined above. Furthermore, the compounds of the present invention can be applied together with a mixing partner as defined above or in form of compositions comprising said mixtures as defined above.
- the components of said mixture can be applied simultaneously, jointly or separately, or in succession, that is immediately one after another and thereby creating the mixture“ in situ” on the desired location, e.g. the plant, the sequence, in the case of separate application, generally not having any effect on the result of the control measures.
- crop refers to both, growing and harvested crops.
- pesticidally effective amount means the amount of active ingredient needed to achieve an observable effect on growth, including the effects of necrosis, death, retardation, prevention, and removal, destruction, or otherwise diminishing the occurrence and activity of the target organism.
- the pesticidally effective amount can vary for the various compounds/compositions used in the invention.
- a pesticidally effective amount of the compositions will also vary according to the prevailing conditions such as desired pesticidal effect and duration, weather, target species, locus, mode of application, and the like.
- an anti-freeze agent from 5 to 20 % of an anti-freeze agent, from 0 to 15 % by weight, e.g.1 to 15 % by weight of a pigment and/or a dye, from 0 to 40 % by weight, e.g.1 to 40 % by weight of a binder (sticker /adhesion agent), optionally up to 5 % by weight, e.g. from 0.1 to 5 % by weight of a thickener, optionally from 0.1 to 2 % of an anti-foam agent, and optionally a preservative such as a biocide, antioxidant or the like, e.g. in an amount from 0.01 to 1 % by weight and a filler/vehicle up to 100 % by weight.
- a binder sticker /adhesion agent
- a preservative such as a biocide, antioxidant or the like, e.g. in an amount from 0.01 to 1 % by weight and a filler/
- the compounds of the present invention are suitable for use in treating or protecting animals against infestation or infection by parasites. Therefore, the present invention also relates to the use of a compound of the present invention for the manufacture of a medicament for the treatment or protection of animals against infestation or infection by parasites. Furthermore, the present invention relates to a method of treating or protecting animals against infestation and infection by parasites, which comprises orally, topically or parenterally administering or applying to the animals a parasiticidally effective amount of a compound of the present invention. The present invention also relates to the non-therapeutic use of compounds of the present invention for treating or protecting animals against infestation and infection by parasites. Moreover, the present invention relates to a non-therapeutic method of treating or protecting animals against infestation and infection by parasites, which comprises applying to a locus a parasiticidally effective amount of a compound of the present invention.
Landscapes
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Tropical Medicine & Parasitology (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
Abstract
The present invention relates to N-(thio)acylimino compoundsof formula (I) wherein the variables are as defined in the claims and the description, and the stereoisomers, tautomers and salts thereof. The invention further relates to agricultural or veterinary compositions comprising such compounds, to the use of the compounds for combating invertebrate pests, and to methods of combating invertebrate pests, which comprises applying such compounds.
Description
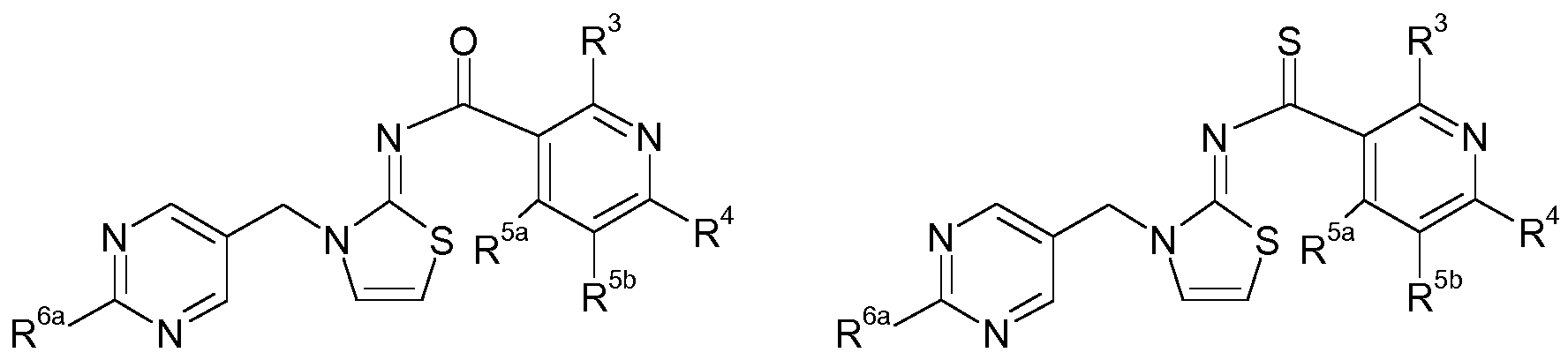
N-(Thio)Acylimino compounds The present invention relates to N-(thio)acylimino compounds carrying an alkynyl- or cyano-substituted 6-membered heteroaromatic ring, including their stereoisomers, tautomers and salts, and to agricultural or veterinary compositions comprising such compounds. The invention also relates to the use of the N-acylimino heterocyclic compounds, their stereoisomers, their tautomers and their salts for combating invertebrate pests. Furthermore the invention relates to methods of combating invertebrate pests, which comprises applying such compounds. Background of the invention Invertebrate pests, such as insects, acaridae and nematode pests, destroy growing and harvested crops and attack wooden dwelling and commercial structures, causing large economic loss to the food supply and to property. While a large number of pesticidal agents are known, due to the ability of target pests to develop resistance to said agents, there is an ongoing need for new agents for combating animal pests. In particular, animal pests such as insects and acaridae are difficult to be effectively controlled.
EP 259738 discloses compounds of the formula A, which have insecticidal activity:
(A)
where W is a substituted pyridyl radical or a 5-or 6-membered heterocyclic radical, R is hydrogen or alkyl, T together with the atoms to which it is bound forms a 5- or 6- membered heterocyclic ring, Y is inter alia a nitrogen atom and Z is an electron withdrawing group selected from nitro and cyano.
Pesticidal compounds, which are similar to those of EP 259738, are known from EP 639569, where the moiety Z is an electron withdrawing group such as
alkoxcarbonyl, arylcarbonyl, heterocyclic carbonyl, C1-C4-alkylsulfonyl, sulfamoyl or C1-C4-acyl.
wherein Ar is an aryl or 5- or 6-membered heterocyclic group, Ra is hydrogen or alkyl, Y' is hydrogen, halogen, a hydroxyl group, an alkyl group or an alkoxy group and Rb is an alkyl group substituted with halogen or an alkoxy group, optionally substituted with halogen.
Pesticidal compounds which are similar to those of US 2013/0150414 are known from WO 2013/129688.
WO 2015/028630) describes compounds similar to the compounds of formula (B), where Ar is an optionally substituted 3-tetrahydrofuryl radical and Rb is a heterocyclic radical.
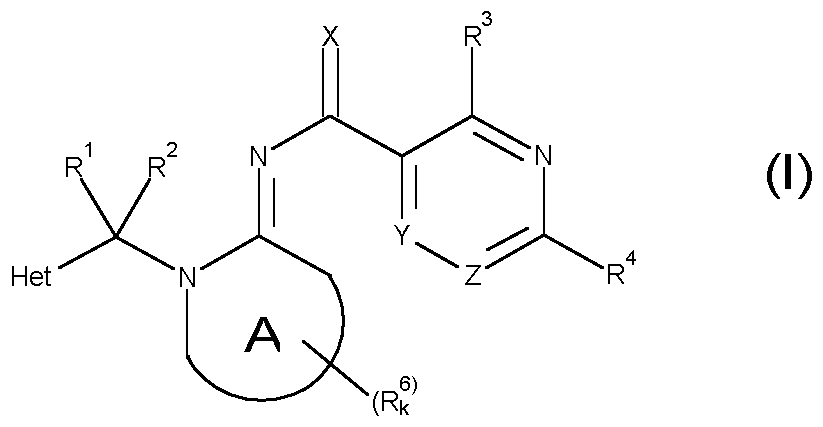
The pesticidal activity of the compounds is not yet satisfactory. It is therefore an object of the present invention to provide compounds having a good pesticidal activity, especially against difficult to control insects and acarid pests. Summary of Invention It has been found that these objects are solved by N-(thio)acyl-imino compounds of the general formula (I) described below, by their stereoisomers, their tautomers and their salts. Therefore, the present invention relates to N-(thio)acylimino compounds of formula (I):
wherein Het is a 5- or 6-membered heteroaromatic ring containing 1, 2, 3 or 4 heteroatoms selected from N, O and S as ring members, where the heteroaromatic ring is optionally substituted by 1, 2, 3 or 4 radicals R6a;
A is a 5- or 6-membered saturated, partly unsaturated or maximally unsaturated N-heterocyclic ring which may contain 1 or 2 further heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members; X is O or S; Y is N or CR5a; Z is N or CR5b; with the proviso that at most one of Y and Z is N; R1 and R2, independently of each other, are selected from the group consisting of hydrogen, halogen, cyano, nitro, SCN, C1-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl, C3-C6-cycloalkyl, wherein the 4 last-mentioned aliphatic and cycloaliphatic radicals may be partly or completely halogenated and/or may carry one or more substituents R7;
OR8, OSO2R8, S(O)nR8, S(O)nNR9aR9b, NR9aR9b, C(=O)R7a, C(=S)R7a, C(=O)OR8, C(=O)NR9aR9b, C(=S)NR9aR9b, Si(R11)2R12,
phenyl, optionally substituted with 1, 2, 3, 4 or 5 substituents R10, and
a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents R10;
or
R1 and R2 form, together with the carbon atom they are attached to, a 3-, 4-, 5- or 6-membered saturated or partly unsaturated carbocyclic or heterocyclic ring, where the heterocyclic ring contains 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the carbocyclic or heterocyclic ring is unsubstituted or carries 1 or 2 radicals R10; or
R1 and R2 form together a group =O, =CR13R14; =S; =NR9a, =NOR8 or =NNR9aR9b; R3 is selected from the group consisting of hydrogen, halogen, cyano, azido, nitro, SCN, SF5, C1-C10-alkyl, C2-C10-alkenyl, C2-C10-alkynyl, C3-C8-cycloalkyl, wherein the 4 last-mentioned aliphatic and cycloaliphatic radicals may be partly or completely halogenated and/or may carry one or more radicals R7;
C(=O)R7a, C(=S)R7a, C(=NR17)R7a, C(=O)OR8, C(=S)OR8, C(=S)SR8,
C(=O)NR9aR9b, C(=S)NR9aR9b, C(=NR17)NR9aR9b, Si(R11)2R12;
phenyl, optionally substituted with 1, 2, 3, 4 or 5 radicals R10; and
a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, wherein the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 radicals R10; R4 is a group -L1-C ^C-RA or a group -L2-C ^N; wherein L1 is a bond or is a bridging group selected from -O-, -S-, -N(R9a)-,
-C1-C10-alkylene-, -O-C1-C10-alkylene-, -S-C1-C10-alkylene- and -N(R9a)-C1-C10-alkylene-; L2 is a bond or is a bridging group selected from -C1-C10-alkylene-,
-O-C1-C10-alkylene-, -S-C1-C10-alkylene- and -N(R9a)-C1-C10-alkylene-; and RA is selected from the group consisting of hydrogen, C1-C10-alkyl, C2-C10- alkenyl, C2-C10-alkynyl, C3-C8-cycloalkyl, wherein the 4 last-mentioned aliphatic and cycloaliphatic radicals may be partly or completely halogenated and/or may carry one or more radicals R7,
OR8, S(O)nR8, S(O)nNR9aR9b, NR9aR9b, C(=O)R7a, C(=S)R7a, C(=NR17)R7a, C(=O)OR8, C(=S)OR8, C(=S)SR8, C(=O)NR9aR9b, C(=S)NR9aR9b, C(=NR17)NR9aR9b, Si(R11)2R12;
phenyl, optionally substituted with 1, 2, 3, 4 or 5 radicals R10; and a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, wherein the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 radicals R10; R5a and R5b, independently of each other, are selected from hydrogen, halogen, cyano, azido, nitro, SCN, SF5, C1-C10-alkyl, C2-C10-alkenyl, C2-C10-alkynyl, C3-C8- cycloalkyl, wherein the 4 last-mentioned aliphatic and cycloaliphatic radicals may be partly or completely halogenated and/or may carry one or more radicals R7, OR8, OSO2R8, S(O)nR8, S(O)nNR9aR9b, NR9aR9b, C(=O)R7a, C(=S)R7a,
C(=NR17)R7a, C(=O)OR8, C(=S)OR8, C(=S)SR8, C(=O)NR9aR9b, C(=S)NR9aR9b, C(=NR17)NR9aR9b, Si(R11)2R12;
phenyl, optionally substituted with 1, 2, 3, 4 or 5 radicals R10; and
a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom
groups selected from N, O, S, NO, SO and SO2 as ring members, wherein the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 radicals R10; each R6 is independently selected from the group consisting of halogen, cyano, azido, nitro, SCN, SF5, C1-C10-alkyl, C2-C10-alkenyl, C2-C10-alkynyl, C3-C8-cycloalkyl, wherein the 4 last-mentioned aliphatic and cycloaliphatic radicals may be partly or completely halogenated and/or may carry one or more radicals R7,
OR8, NR9aR9b, S(O)nR8, S(O)nNR9aR9b, C(=O)R7a, C(=S)R7a, C(=NR17)R7a, C(=O)OR8, C(=S)OR8, C(=S)SR8, C(=O)NR9aR9b, C(=S)NR9aR9b,
C(=NR17)NR9aR9b, and Si(R11)2R12;
or two radicals R6 present on the same ring carbon may form together a group =O, =CR13R14, =S, =NR17, =NOR16 or =NNR9aR9b;
or two radicals R6 bound on adjacent ring atoms, together with the atoms they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partly unsaturated or maximally unsaturated carbocyclic or heterocyclic ring, where the heterocyclic ring contains 1 or 2 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members; and where said carbocyclic or heterocyclic ring is unsubstituted or carries 1, 2, 3 or 4 radicals selected from halogen, C1-C6- alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-haloalkenyl, C2-C6-alkynyl, C2-C6- haloalkynyl, C3-C8-cycloalkyl, C3-C8-halocycloalkyl, C1-C6-alkoxy, C1-C6- haloalkoxy, C1-C6-alkylthio, C1-C6-haloalkylthio, phenyl, optionally substituted with 1, 2, 3, 4 or 5 substituents R10, and a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents R10; each R6a has independently one of the meanings given for R6; each R7 is independently selected from the group consisting of cyano, azido, nitro, - SCN, SF5, Si(R11)2R12, OR16, OSO2R16, S(O)nR16, S(O)nNR17aR17b, NR17aR17b, C(=O)R15, C(=S)R15, C(=NR17)R15, C(=NOR16)R15, C(=O)OR16, C(=S)OR16, C(=O)NR17aR17b, C(=S)NR17aR17b,
C3-C8-cycloalkyl, C3-C8-halocycloalkyl,
phenyl, optionally substituted with 1, 2, 3, 4 or 5 substituents R10, and
a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, wherein the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents R10;
or
two R7 present on the same carbon atom may form together a group =O, =CR13R14; =S; =S(O)nR16, =S(O)nNR17aR17b, =NR17, =NOR16 or =NNR17aR17b; or
two R7 bound on the same or adjacent carbon atom(s), together with the carbon atom(s) they are bound to, may form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, or partly unsaturated carbocyclic or heterocyclic ring, where the heterocyclic ring contains 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the carbocyclic or heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents R10; and
R7 as a substituent on a cycloaliphatic radical is additionally selected from the group consisting of C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-haloalkenyl, C2-C6-alkynyl and C2-C6-haloalkynyl; each R7a is independently selected from the group consisting of hydrogen, halogen, C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-haloalkenyl, C2-C6-alkynyl, C2-C6-haloalkynyl, C3-C8-cycloalkyl, C3-C8-halocycloalkyl, C3-C8-cycloalkyl-C1-C4- alkyl, C3-C8-halocycloalkyl-C1-C4-alkyl, C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6- alkylthio, C1-C6-haloalkylthio, C1-C6-alkylsulfinyl, C1-C6-haloalkylsulfinyl, C1-C6- alkylsulfonyl, C1-C6-haloalkylsulfonyl,
phenyl, phenyl-C1-C4-alkyl, where the phenyl ring in the two last-mentioned radicals is optionally substituted with 1, 2, 3, 4 or 5 radicals R10, and
a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents R10; each R8 is independently selected from the group consisting of hydrogen, CN, C1-C6- alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-haloalkenyl, C2-C6-alkynyl, C2-C6- haloalkynyl, C3-C8-cycloalkyl, C3-C8-halocycloalkyl, C3-C8-cycloalkyl-C1-C4-alkyl, C3-C8-halocycloalkyl-C1-C4-alkyl, C(=O)R15, C(=S)R15, C(=O)OR16, C(=S)OR16, C(=O)NR17aR17b, C(=S)NR17aR17b,
phenyl, phenyl-C1-C4-alkyl, where the phenyl ring in the two last-mentioned radicals is unsubstituted or substituted with 1, 2, 3, 4 or 5 substituents R10; and a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents R10; and
R8 as a substituent on a sulfur atom is additionally selected from the group consisting of C1-C6-alkoxy and C1-C6-haloalkoxy; R9a and R9b, independently of each other and independently of each occurrence, are selected from the group consisting of hydrogen, C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-haloalkenyl, C2-C6-alkynyl, C2-C6-haloalkynyl, C3-C8- cycloalkyl, C3-C8-halocycloalkyl, C3-C8-cycloalkyl-C1-C4-alkyl, C3-C8- halocycloalkyl-C1-C4-alkyl,
OR16, S(O)nR16, NR17aR17b, -S(O)nNR17aR17b, C(=O)R15, C(=S)R15, C(=NR17)R15, C(=O)OR16, C(=S)SR16, C(=O)NR17aR17b, C(=S)NR17aR17b, P(=O)(R18)2, phenyl, benzyl, 1-phenylethyl, 2-phenylethyl, where the phenyl ring in the four last-mentioned radicals is unsubstituted or substituted with 1, 2, 3, 4 or 5 substituents R10, and
a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated C-bound heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents R10;
or
R9a and R9b, together with the nitrogen atom they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring, where the heterocyclic ring may contain one or two further heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, and where the heterocyclic ring may be substituted with 1, 2, 3 or 4 radicals selected from halogen, C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-haloalkenyl, C2-C6-alkynyl, C2-C6-haloalkynyl, C3-C8-cycloalkyl, C3-C8- halocycloalkyl, C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6-alkylthio, C1-C6- haloalkylthio, phenyl, optionally substituted with 1, 2, 3, 4 or 5 substituents R10, and a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents R10;
or
R9a and R9b together may form a group =CR13R14, =S(O)nR16, =S(O)nNR17aR17b, =NR17 or =NOR16; each R10 is independently selected from the group consisting of halogen, cyano, azido, nitro, SCN, SF5, C1-C10-alkyl, C2-C10-alkenyl, C2-C10-alkynyl, C3-C8-cycloalkyl,
wherein the 4 last-mentioned aliphatic and cycloaliphatic radicals may be partly or completely halogenated and/or may carry 1, 2, 3, 4 or 5 radicals R19,
Si(R11)2R12, OR16, OS(O)nR16, -S(O)nR16, S(O)nNR17aR17b, NR17aR17b, C(=O)R15, C(=S)R15, C(=NR17)R15, C(=O)OR16, C(=O)NR17aR17b, C(=S)NR17aR17b, phenyl, optionally substituted with 1, 2, 3, 4 or 5 radicals independently selected from OH, halogen, cyano, nitro, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy, and
a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the heterocyclic ring is unsubstituted or may be substituted with 1, 2, 3, 4 or 5 substituents independently selected from OH, halogen, cyano, nitro, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy;
or
two R10 present on the same carbon ring atom of a saturated or partly
unsaturated carbocyclic or heterocyclic radical may form together a group =O, =CR13R14, =S, =NR17, =NOR16 or =NNR17aR17b;
or
two R10 bound on adjacent ring atoms may form together a bivalent radical selected from CH2CH2CH2CH2, CH=CH-CH=CH, N=CH-CH=CH, CH=N-CH=CH, N=CH-N=CH, OCH2CH2CH2, OCH=CHCH2, CH2OCH2CH2, OCH2CH2O,
OCH2OCH2, CH2CH2CH2, CH=CHCH2, CH2CH2O, CH=CHO, CH2OCH2,
CH2C(=O)O, C(=O)OCH2, O(CH2)O, SCH2CH2CH2, SCH=CHCH2, CH2SCH2CH2, SCH2CH2S, SCH2SCH2, CH2CH2S, CH=CHS, CH2SCH2, CH2C(=S)S,
C(=S)SCH2, S(CH2)S, CH2CH2NR17a, CH2CH=N, CH=CH-NR17a, OCH=N and SCH=N, thus forming together with the carbon atoms to which the two R10 are bound a 5-membered or 6-membered saturated, partly unsaturated or maximally unsaturated carbocyclic or heterocyclic ring, wherein the ring may optionally be substituted with one or two substituents independently selected from =O, OH, CH3, OCH3, halogen, cyano, halomethyl and halomethoxy; R11 and R12, independently of each other and independently of each occurrence, are selected from the group consisting of C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-haloalkenyl, C2-C6-alkynyl, C2-C6-haloalkynyl, C3-C8-cycloalkyl, C3-C8- halocycloalkyl, C3-C8-cycloalkyl-C1-C4-alkyl, C3-C8-halocycloalkyl-C1-C4-alkyl, C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6-alkoxy-C1-C4-alkyl, C1-C6-haloalkoxy- C1-C4-alkyl, phenyl and benzyl, where phenyl ring in last two radicals is unsubstituted or substituted with 1, 2, 3, 4 or 5 radicals independently selected
from OH, halogen, cyano, nitro, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy; R13 and R14, independently of each other and independently of each occurrence, are selected from the group consisting of hydrogen, halogen, CN, C1-C6-alkyl, C1-C6- haloalkyl, C3-C6-cycloalkyl, C1-C4-alkoxy-C1-C4-alkyl, phenyl and benzyl; each R15 is independently selected from the group consisting of hydrogen, halogen, C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-haloalkenyl, C2-C6-alkynyl, C2-C6-haloalkynyl, C3-C8-cycloalkyl, C3-C8-halocycloalkyl, C1-C6-alkoxy, C1-C6- haloalkoxy, C1-C6-alkylthio, C1-C6-haloalkylthio, C1-C6-alkylsulfinyl, C1-C6- haloalkylsulfinyl, C1-C6-alkylsulfonyl, C1-C6-haloalkylsulfonyl,
phenyl, optionally substituted with 1, 2, 3, 4 or 5 radicals independently selected from OH, halogen, cyano, nitro, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy, and
a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents
independently selected from OH, halogen, cyano, nitro, C1-C6-alkyl, C1-C6- haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy; each R16 is independently selected from the group consisting of hydrogen, C1-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl, C3-C8-cycloalkyl, C3-C8-cycloalkyl-C1-C4-alkyl, wherein the five last-mentioned aliphatic and cycloaliphatic radicals may be partly or fully halogenated and/or may carry 1 or 2 radicals independently selected from =O and C1-C4-alkoxy;
phenyl, benzyl and pyridyl, wherein the last three radicals may be unsubstituted or may carry 1, 2, 3, 4 or 5 substituents independently selected from halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy, C1-C6-haloalkoxy, (C1-C6- alkoxy)carbonyl, (C1-C6-alkyl)amino and di-(C1-C6-alkyl)amino; each R17 is independently selected from the group consisting of hydrogen, C1-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl, C3-C8-cycloalkyl, C3-C8-cycloalkyl-C1-C4-alkyl, C1-C6-alkoxy, C2-C6-alkenyloxy, C2-C6-alkynyloxy, C3-C8-cycloalkoxy, C3-C8- cycloalkyl-C1-C4-alkoxy, C1-C6-alkylthio, C1-C6-alkylsulfinyl, C1-C6-alkylsulfonyl, wherein the aliphatic and cycloaliphatic moieties in the 13 last-mentioned radicals may be partly or fully halogenated and/or may carry 1 or 2 radicals independently selected from =O and C1-C4-alkoxy;
phenyl, benzyl, pyridyl, phenoxy, benzyloxy and pyridyloxy, wherein the six last- mentioned radicals may carry 1, 2, 3, 4 or 5 substituents independently selected from halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy, C1-C6-haloalkoxy and (C1-C6-alkoxy)carbonyl; R17a and R17b, independently of each other and independently of each occurrence, are selected from the group consisting of hydrogen, C1-C6-alkyl, C2-C6-alkenyl, C2-C6- alkynyl, C3-C8-cycloalkyl, C3-C8-cycloalkyl-C1-C4-alkyl, C1-C6-alkoxy, C2-C6- alkenyloxy, C2-C6-alkynyloxy, C3-C8-cycloalkoxy, C3-C8-cycloalkyl-C1-C4-alkoxy, C1-C6-alkylthio, C1-C6-alkylsulfinyl, C1-C6-alkylsulfonyl, wherein the aliphatic and cycloaliphatic moieties in the 13 last-mentioned radicals may be partly or fully halogenated and/or may carry 1 or 2 radicals independently selected from =O and C1-C4-alkoxy;
phenyl, benzyl, pyridyl, phenoxy, benzyloxy and pyridyloxy, wherein the six last- mentioned radicals may carry 1, 2, 3, 4 or 5 substituents independently selected from halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy, C1-C6-haloalkoxy and (C1-C6-alkoxy)carbonyl,
or
R17a and R17b, together with the nitrogen atom they are bound to, form a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring, wherein the heterocyclic ring may contain 1 or 2 further heteroatoms selected from or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, and wherein the heterocyclic ring may be substituted with one or more substituents independently selected from halogen, C1-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy and C1-C4-haloalkoxy;
or
R17a and R17b together may form a group =CR13R14, =NR17 or =NOR16; each R18 is independently selected from the group consisting of hydrogen, C1-C6-alkyl, C1-C6-alkoxy, C3-C6-cycloalkyl, C3-C6-cycloalkoxy, phenyl and phenoxy; each R19 is independently selected from the group consisting of cyano, azido, nitro, -SCN, SF5, Si(R11)2R12, OR16, OSO2R16, S(O)nR16, S(O)nNR17aR17b, NR17aR17b, C(=O)NR17aR17b, C(=S)NR17aR17b, C(=O)OR16, C(=O)R15, C(=S)R15, C(=NR17)R15, C(=NOR16)R15,
C3-C8-cycloalkyl, C3-C8-halocycloalkyl, C3-C8-cycloalkyl-C1-C4-alkyl, C3-C8- halocycloalkyl-C1-C4-alkyl,
phenyl, phenyl-C1-C4-alkyl, where the phenyl ring in the two last-mentioned radicals is optionally substituted with 1, 2, 3, 4 or 5 substituents selected from
CN, halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy; and
a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, wherein the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents selected from CN, halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6- haloalkoxy;
or
two R19 present on the same carbon atom may form together a group =O, =CR13R14; =S; =S(O)nR16, =S(O)nNR17aR17b, =NR17, =NOR16 or =NNR17aR17b; or
two R19 bound on the same or adjacent carbon atom(s), together with the carbon atom(s) they are bound to, may form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated or partly unsaturated carbocyclic or heterocyclic ring, where the heterocyclic ring contains 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, and where the carbocyclic or heterocyclic ring may be substituted with 1, 2, 3, 4 or 5 substituents selected from CN, halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy; and
R19 as a substituent on a cycloaliphatic radical is additionally selected from the group consisting of C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-haloalkenyl, C2-C6-alkynyl and C2-C6-haloalkynyl; k is 0, 1, 2, 3 or 4; and n is 0, 1 or 2; and the stereoisomers, tautomers and the agriculturally or veterinarily acceptable salts thereof. Moreover, the present invention relates to and includes the following
embodiments:
- agricultural and veterinary compositions comprising at least one compound of the formula (I) or a stereoisomer, tautomer or salt thereof;
- the use of the compounds of formula (I), the stereoisomers, the tautomers or the salts thereof for combating invertebrate pests;
- the use of the compounds of formula (I), the stereoisomers, the tautomers or the salts thereof for protecting growing plants from attack or infestation by
invertebrate pests;
- the use of the compounds of formula (I), the stereoisomers, the tautomers or the salts, thereof for protecting plant proparagation material, especially seeds, from soil insects;
- the use of the compounds of formula (I), the stereoisomers, the tautomers or the salts thereof for protecting the seedlings roots and shoots of plants from soil and foliar insects;
- a method for combating or controlling invertebrate pests, which method
comprises contacting said pest or its food supply, habitat or breeding grounds with a pesticidally effective amount of at least one compound of the formula (I) or a stereoisomer, a tautomer or salt thereof (where the method does however not include the (medical) treatment of the human or animal body);
- a method for protecting growing plants from attack or infestation by invertebrate pests, which method comprises contacting a plant, or soil or water in which the plant is growing, with a pesticidally effective amount of at least one compound of the formula (I) or a stereoisomer, a tautomer or salt thereof, in particular a method protecting crop plants from attack or infestation by animal pests, which comprises contacting the crop plants with a pesticidally effective amount of at least one compound of the formula (I) or stereoisomer, a tautomer or salt thereof; - a method for the protection of plant propagation material, especially seeds, from soil insects and of the seedlings' roots and shoots from soil and foliar insects comprising contacting the seeds before sowing and/or after pregermination with at least one compound of the formula (I) or stereoisomer, a tautomer or salt thereof;
- seeds comprising a compound of the formula (I) or an enantiomer, diastereomer or salt thereof;
- the use of compounds of formula (I), the stereoisomers, the tautomers or the salts, in particular the veterinary acceptable salts, thereof for combating parasites in and on animals, in particular for the use in the treatment of animals infested or infected by parasites, for preventing animals of getting infected or infested by parasites or for protecting animals against infestation or infection by parasites; - a method for treating animals infested or infected by parasites or preventing
animals of getting infected or infested by parasites or protecting animals against infestation or infection by parasites which comprises administering or applying to the animals a parasiticidally effective amount of a compound of formula (I) or the stereoisomers and/or salts, in particular veterinary acceptable salts, thereof; - a process for the preparation of a veterinary composition for treating, controlling, preventing or protecting animals against infestation or infection by parasites which comprises formulating a compound of formula (I) or a stereoisomer,
tautomer and/or veterinary acceptable salt thereof with a carrier composition suitable for veterinary use;
- the use of a compound of formula (I) or the stereoisomers, tautomers and/or veterinary acceptable salt thereof for the preparation of a medicament for treating, controlling, preventing or protecting animals against infestation or infection by parasites.
The present invention also relates to plant propagation materials, in particular as mentioned above to seeds, containing at least one compound of formula (I), a stereoisomer, a tautomer and/or an agriculturally acceptable salt thereof. Embodiments of Invention
The invention comprises the following particular embodiments: Embodiment 1:
The compound of formula I as defined above (i.e. in the summary of invention), where R1 and R2, independently of each other, are selected from the group consisting of hydrogen, halogen, CN, C1-C6-alkyl, C3-C6-cycloalkyl, C1-C6-haloalkyl and C3-C6- halocycloalkyl; or
R1 and R2 may together be =CR13R14; or
R1 and R2 form, together with the carbon atom which they attached to, a 3-, 4-or 5-membered saturated carbocyclic ring;
where R13 and R14 are as defined above (i.e. in the summary of invention). Embodiment 2:
The compound of formula I as defined in embodiment 1, where both R1 and R2 are hydrogen. Embodiment 3:
The compound of formula I as defined above (i.e. in the summary of invention) or in any of embodiments 1 or 2, where R3 is selected from the group consisting of hydrogen, halogen, C1-C6-alkyl and C1-C6-haloalkyl. Embodiment 4:
The compound of formula I as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 3, where R4 is a group
-L1-C ^C-RA, where
L1 is a bond or -O-CH2-; in particular a bond; and
RA is selected from the group consisting of hydrogen, C1-C6-alkyl, C1-C6- haloalkyl, C1-C6-alkyl which carries one radical R7,
OR8, S(O)nR8, C(=O)R7a, C(=O)OR8 and C(=O)NR9aR9b;
where R7, R7a, R8, R9a, R9b and n are as defined above (i.e. in the summary of invention). Embodiment 5:
The compound of formula I as defined in embodiment 4, where
RA is selected from the group consisting of hydrogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkyl which carries one radical R7,
C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6-alkylthio, C1-C6-haloalkylthio, C1-C6- alkylsulfinyl, C1-C6-haloalkylsulfinyl, C1-C6-alkylsulfonyl, C1-C6-haloalkylsulfonyl, C1-C6-alkylcarbonyl, C1-C6-haloalkylcarbonyl, C1-C6-alkoxycarbonyl and C1-C6- haloalkoxycarbonyl;
where
R7 is selected from cyano, C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6-alkylthio, C1- C6-haloalkylthio, C1-C6-alkylsulfinyl, C1-C6-haloalkylsulfinyl, C1-C6- alkylsulfonyl, C1-C6-haloalkylsulfonyl, C1-C6-alkylcarbonyl, C1-C6- haloalkylcarbonyl, C1-C6-alkoxycarbonyl and C1-C6-haloalkoxycarbonyl. Embodiment 6:
The compound of formula I as defined in embodiment 5, where
RA is selected from the group consisting of hydrogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkyl which carries one radical R7,
C1-C6-alkylsulfonyl and C1-C6-haloalkylsulfonyl;
where
R7 is selected from cyano, C1-C6-alkoxy and C1-C6-haloalkoxy. Embodiment 7:
The compound of formula I as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 3, where R4 is cyano. Embodiment 8:
The compound of formula I as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 7, where R5a and R5b, independently of each other, are selected from hydrogen, halogen, cyano, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy, in particular from hydrogen, C1-C6-alkyl and C1-C6-alkoxy and specifically from hydrogen and C1-C6-alkyl.
Embodiment 9:
The compound of formula I as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 8, where each R6 is independently selected from halogen, C1- C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy and C1-C4-haloalkoxy. Embodiment 10:
The compound of formula I as defined in embodiment 9, where each R6 is
independently selected from C1-C4-alkyl. Embodiment 11:
The compound of formula I as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 10, where k is 0, 1 or 2, in particular 0 or 1; specifically 0. Embodiment 12:
The compound of formula I as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 11, where each R6a is independently selected from halogen, C1-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy and C1-C4-haloalkoxy; in particular from halogen and C1-C2-haloalkyl. Embodiment 13:
The compound of formula I as defined in embodiment 12, where each R6a is independently selected from Cl, Br or CF3, and is specifically Cl. Embodiment 14:
The compound of formula I as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 13, where X is O. Embodiment 15:
The compound of formula I as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 13, where X is S. Embodiment 16:
The compound of formula I as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 15, where Y is N and Z is CR5b, where R5b is as defined above (i.e. in the summary of invention) or in embodiment 8. Embodiment 17:
The compound of formula I as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 16, where Het is selected from Het-1 to Het-7:
Het-1 Het-2 Het-3
Het-4 Het-5 Het-6 Het-7 wherein
# denotes the attachment point to the remainder of the molecule;
j is 0, 1 or 2; and
R6a is as defined above (i.e. in the summary of invention) or in any of embodiments 12 or 13. Embodiment 18:
The compound as defined in embodiment 17, where Het is selected from Het-1, Het-2, - -
Het-1 Het-2 Het-4 Het-7a wherein
# denotes the attachment point to the remainder of the molecule;
j is 0, 1 or 2; and
R6a is as defined above (i.e. in the summary of invention) or in any of embodiments 12 or 13. Embodiment 19:
Het-1a Het-4a Het-7aa wherein
# denotes the attachment point to the remainder of the molecule; and
R6a is halogen or C1-C2-haloalkyl, in particular Cl, Br or CF3, specifically Cl. Embodiment 20:
The compound of formula I as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 19 wherein the moiet of the formula W.Het
W.Het-18 W.Het-19 W.Het-20 W.Het-21 wherein
# denotes the attachment point to the remainder of the molecule;
R1 and R2 are as defined above (i.e. in the summary of invention) or in any of embodiments 1 or 2;
R6 is as defined in above (i.e. in the summary of invention) or in any of embodiments 9 or 10;
Het is as defined above (i.e. in the summary of invention) or in any of embodiments 12, 13 or 17 to 19;
X is as defined above (i.e. in the summary of invention) or in any of embodiments 14 or 15; and
each R6b is independently hydrogen or has one of the meanings given above (i.e. in the summary of invention) or in any of embodiments 9 or 10 for R6, with the proviso that R6b is not halogen, azido, nitro, SCN or SF5;
each R6c has independently one of the meanings given above (i.e. in the summary of invention) or in any of embodiments 9 or 10 for R6, and
k is 0, 1 or 2. Embodiment 21:
The compound as defined in embodiment 20, where the moiety W-Het is selected from moieties of formulae W.Het-1, W.Het-2, W.Het-3, W.Het 4, W.Het 13 and W-Het-19, and in particular from W.Het-13 and W.Het-19, where in particular in W-Het-19 R6c is methyl and k is 0, 1 or 2. Embodiment 22:
The compound of formula I as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 19 wherein the moiet of the formula W.Het
is selected from moieties of formulae W.Het-1 to W.Het-15 and W.Het-18 to W.Het-21 as defined in embodiment 20, where in W.Het-19 k is 0 or 1. Embodiment 23:
The compound as defined in embodiment 22, where the moiety W-Het is selected from moieties of formulae W.Het-1, W.Het-2, W.Het-3, W.Het 4, W.Het 13 and W-Het-19, and in particular from W.Het-13 and W.Het-19, where in particular in W-Het-19 k is 0. Embodiment 24:
The compound of formula I as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 23, of formula I.1
(I.1)
4
Y1 is CH or N;
X is as defined above (i.e. in the summary of invention) or in any of embodiments 14 or 15;
R3 is as defined above (i.e. in the summary of invention) or in embodiment 3;
R4 is as defined above (i.e. in the summary of invention) or in any of embodiments 4 to 7;
R5b is as defined above (i.e. in the summary of invention) or in embodiment 8; and R6a is as defined above (i.e. in the summary of invention) or in any of embodiments 12, 13 or 19;
Y1 is CH or N;
X is as defined above (i.e. in the summary of invention) or in any of embodiments 14 or 15;
R3 is as defined above (i.e. in the summary of invention) or in embodiment 3;
R4 is as defined above (i.e. in the summary of invention) or in any of embodiments 4 to 7;
R5b is as defined above (i.e. in the summary of invention) or in embodiment 8;
R6a is as defined above (i.e. in the summary of invention) or in any of embodiments 12, 13 or 19; and
R6c1 and R6c2, independently of each other, are hydrogen or have one of the meanings given above (i.e. in the summary of invention) or in any of embodiments 9 or 10 for R6; or of formula I.3
X is as defined above (i.e. in the summary of invention) or in any of embodiments 14 or 15;
R3 is as defined above (i.e. in the summary of invention) or in embodiment 3;
R4 is as defined above (i.e. in the summary of invention) or in any of embodiments 4 to 7;
R5b is as defined above (i.e. in the summary of invention) or in embodiment 8; and R6a is as defined above (i.e. in the summary of invention) or in any of embodiments 12, 13 or 19; or of formula I.4
X is as defined above (i.e. in the summary of invention) or in any of embodiments 14 or 15;
R3 is as defined above (i.e. in the summary of invention) or in embodiment 3;
R4 is as defined above (i.e. in the summary of invention) or in any of embodiments 4 to 7;
R5b is as defined above (i.e. in the summary of invention) or in embodiment 8;
R6a is as defined above (i.e. in the summary of invention) or in any of embodiments 12, 13 or 19; and
R6c1 and R6c2, independently of each other, are hydrogen or have one of the meanings given above (i.e. in the summary of invention) or in any of embodiments 9 or 10 for R6. Embodiment 25:
An agricultural or veterinary composition comprising at least one compound of formula I as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 24, a stereoisomer, a tautomer and/or at least one agriculturally or veterinarily acceptable salt thereof, and at least one inert liquid and/or solid agriculturally or veterinarily acceptable carrier. Embodiment 26:
The use of a compound as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 24, of a stereoisomer, a tautomer and/or of an agriculturally or veterinarily acceptable salt thereof for combating or controlling invertebrate pests, for protecting growing plants from attack or infestation by invertebrate pests, for protecting plant propagation material, especially seeds, from soil insects, or for protecting the seedlings, roots and shoots of plants from soil and foliar insects. Embodiment 27:
A method for combating or controlling invertebrate pests, which method comprises contacting said pest or its food supply, habitat or breeding grounds with a pesticidally effective amount of at least one compound as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 24, of a stereoisomer, a tautomer and/or of an agriculturally or veterinarily acceptable salt thereof. Embodiment 28:
A method for protecting growing plants from attack or infestation by invertebrate pests, which method comprises contacting a plant, or soil or water in which the plant is growing with a pesticidally effective amount of at least one compound as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 24, of a stereoisomer, a tautomer and/or of an agriculturally or veterinarily acceptable salt thereof. Embodiment 29:
A method for the protection of plant propagation material, especially seeds, from soil insects and of the seedlings roots and shoots from soil and foliar insects comprising contacting the plant propagation material before sowing and/or after pregermination
with at least one compound as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 24, a stereoisomer, tautomer and/or an agriculturally or veterinarily acceptable salt thereof. Embodiment 30:
The compound as defined above (i.e. in the summary of invention) or in any of embodiments 1 to 24, a stereoisomer, tautomer and/or an agriculturally or veterinarily acceptable salt thereof, for the use in the treatment of animals infested or infected by parasites, for preventing animals of getting infected or infested by parasites or for protecting animals against infestation or infection by parasites. Detailed Description of Invention
The present invention relates to every possible stereoisomer of the compounds of formula (I), i.e. to single enantiomers, diastereomers and E/Z-isomers as well as to mixtures thereof and also to the salts thereof. The present invention relates to each isomer alone, or mixtures or combinations of the isomers in any proportion to each other. Suitable compounds of the formula (I) also include all possible geometrical stereoisomers (cis/trans isomers) and mixtures thereof. In particular, the C=N double bond may lead to Z/E isomers (the moiety -C(X)-ring may be Z or E to the ring nitrogen atom of ring A). Depending on the substitution pattern, the compounds of the formula (I) may have one or more centers of chirality, in which case they are present as mixtures of enantiomers or diastereomers. The invention provides both the pure enantiomers or diastereomers and their mixtures and the use according to the invention of the pure enantiomers or diastereomers of the compound I or its mixtures.
The present invention also relates to potential tautomers of the compounds of formula (I) and also to the salts of such tautomers. The present invention relates to the tautomer as such as well as to mixtures or combinations of the tautomers in any proportion to each other. The term "tautomers" encompasses isomers which are derived from the compounds of formula (I) by the shift of a H-atom involving at least one H-atom located at a nitrogen, oxygen or sulphur atom. Examples of tautomeric forms are keto-enol forms, imine-enamine forms, urea-isourea forms, thiourea- isothiourea forms, (thio)amide-(thio)imidate forms etc.
The compounds of the present invention, i.e. the compounds of formula (I), their stereoisomers, their tautomers as well as their salts, in particular their agriculturally acceptable salts and their veterinarily acceptable salts, may be amorphous or may exist in one ore more different crystalline states (polymorphs) or modifications which may have a different macroscopic properties such as stability or show different biological properties such as activities. The present invention includes both amorphous and crystalline compounds of the formula (I), mixtures of different crystalline states or
modifications of the respective stereoisomers or tautomers, as well as amorphous or crystalline salts thereof.
Salts of the compounds of the formula (I) are preferably agriculturally as well as veterinarily acceptable salts. They can be formed in a customary method, e.g. by reacting the compound with an acid of the anion in question if the compound of formula (I) has a basic functionality or by reacting an acidic compound of formula (I) with a suitable base.
Suitable agriculturally or veterinary useful salts are especially the salts of those cations or anions, in particular the acid addition salts of those acids, whose cations and anions, respectively, do not have any adverse effect on the action of the compounds according to the present invention. Suitable cations are in particular the ions of the alkali metals, preferably lithium, sodium and potassium, of the alkaline earth metals, preferably calcium, magnesium and barium, and of the transition metals, preferably manganese, copper, zinc and iron, and also ammonium (NH4+) and substituted ammonium in which one to four of the hydrogen atoms are replaced by C1-C4-alkyl, C1-C4-hydroxyalkyl, C1-C4-alkoxy, C1-C4-alkoxy-C1-C4-alkyl, hydroxy-C1-C4-alkoxy- C1-C4-alkyl, phenyl or benzyl. Examples of substituted ammonium ions comprise methylammonium, isopropylammonium, dimethylammonium, diisopropylammonium, trimethylammonium, tetramethylammonium, tetraethylammonium,
tetrabutylammonium, 2-hydroxyethylammonium, 2-(2-hydroxyethoxy)ethyl-ammonium, bis(2-hydroxyethyl)ammonium, benzyltrimethylammonium and
benzyltriethylammonium, furthermore phosphonium ions, sulfonium ions, preferably tri(C1-C4-alkyl)sulfonium, and sulfoxonium ions, preferably tri(C1-C4-alkyl)sulfoxonium.
Anions of useful acid addition salts are primarily chloride, bromide, fluoride, hydrogen sulfate, sulfate, dihydrogen phosphate, hydrogen phosphate, phosphate, nitrate, hydrogen carbonate, carbonate, hexafluorosilicate, hexafluorophosphate, benzoate, and the anions of C1-C4-alkanoic acids, preferably formate, acetate, propionate and butyrate. They can be formed by reacting the compounds of the formulae I with an acid of the corresponding anion, preferably of hydrochloric acid, hydrobromic acid, sulfuric acid, phosphoric acid or nitric acid.
The term "invertebrate pest" (also termed "animal pest") as used herein encompasses animal populations, such as insects, arachnids and nematodes, which may attack plants, thereby causing substantial damage to the plants attacked, as well as ectoparasites which may infest animals, in particular warm blooded animals such as e.g. mammals or birds, or other higher animals such as reptiles, amphibians or fish, thereby causing substantial damage to the animals infested. For more details see below.
The term "plant propagation material" is to be understood to denote all the generative parts of the plant such as seeds and vegetative plant material such as
cuttings and tubers (e. g. potatoes), which can be used for the multiplication of the plant. This includes seeds, roots, fruits, tubers, bulbs, rhizomes, shoots, sprouts and other parts of plants, including seedlings and young plants, which are to be transplan- ted after germination or after emergence from soil. The plant propagation materials may be treated prophylactically with a plant protection compound either at or before planting or transplanting. Said young plants may also be protected before
transplantation by a total or partial treatment by immersion or pouring.
The term "plants" comprises any types of plants including "non-cultivated plants" and in particular "cultivated plants".
The term "non-cultivated plants" refers to any wild type species or related species or related genera of a cultivated plant.
The term "cultivated plants" is to be understood as including plants which have been modified by breeding, mutagenesis or genetic engineering including but not limiting to agricultural biotech products on the market or in development (cf.
http://www.bio.org/speeches/pubs/er/agri_products.asp). For more details see below.
The organic moieties mentioned in the above definitions of the variables are - like the term halogen - collective terms for individual listings of the individual group members. The prefix Cn-Cm indicates in each case the possible number of carbon atoms in the group.
"Halogen" will be taken to mean fluoro, chloro, bromo and iodo.
The term "partially or fully halogenated" will be taken to mean that 1 or more, e.g.1, 2, 3, 4 or 5 or all of the hydrogen atoms of a given radical have been replaced by a halogen atom, in particular by fluorine or chlorine. For example, partially or fully halogenated alkyl is also termed haloalkyl, partially or fully halogenated cycloalkyl is also termed halocycloalkyl, partially or fully halogenated alkylenyl is also termed haloalkenyl, partially or fully halogenated alkylynyl is also termed haloalkynyl, partially or fully halogenated alkoxy is also termed haloalkoxy, partially or fully halogenated alkylthio is also termed haloalkthio, partially or fully halogenated alkylsulfinyl is also termed haloalkylsulfinyl, partially or fully halogenated alkylsulfonyl is also termed haloalsulfonyl, partially or fully halogenated cycloalkylalkyl is also termed
halocycloalkylalkyl.
The term "alkyl" as used herein and in the alkyl moieties of alkoxy, alkylthio, alkylsulfinyl, alkylsulfonyl, alkylcarbonyl, alkoxycarbonyl, alkylamino, dialkylamino and the like refers to saturated straight-chain or branched hydrocarbon radicals having 1 to 2 ("C1-C2-alkyl"), 1 to 3 ("C1-C3-alkyl"),1 to 4 ("C1-C4-alkyl"), 1 to 6 ("C1-C6-alkyl"), 1 to 8 ("C1-C8-alkyl") or 1 to 10 ("C1-C10-alkyl") carbon atoms. C1-C2-Alkyl is methyl or ethyl. C1-C3-Alkyl is additionally propyl and isopropyl. C1-C4-Alkyl is additionally n-butyl, 1-methylpropyl (sec-butyl), 2-methylpropyl (isobutyl) or 1,1-dimethylethyl (tert-butyl). C1-C6-Alkyl is additionally also, for example, pentyl, 1-methylbutyl, 2-methylbutyl,
3-methylbutyl, 2,2-dimethylpropyl, 1-ethylpropyl, 1,1-dimethylpropyl, 1,2-dimethylpropyl, hexyl, 1-methylpentyl, 2-methylpentyl, 3-methylpentyl, 4- methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 1,3-dimethylbutyl, 2,2-dimethylbutyl, 2,3-dimethylbutyl, 3,3-dimethylbutyl, 1-ethylbutyl, 2-ethylbutyl, 1,1,2-trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-1-methylpropyl, or 1-ethyl-2-methylpropyl. C1-C8-Alkyl is additionally also, for example, heptyl, octyl, 2-ethylhexyl and positional isomers thereof. C1-C10-Alkyl is additionally also, for example, nonyl, decyl and positional isomers thereof.
The term "haloalkyl" as used herein, which is also expressed as "alkyl which is partially or fully halogenated", refers to straight-chain or branched alkyl groups having 1 to 2 ("C1-C2-haloalkyl"), 1 to 3 ("C1-C3-haloalkyl"), 1 to 4 ("C1-C4-haloalkyl"), 1 to 6 ("C1-C6-haloalkyl"), 1 to 8 ("C1-C8-haloalkyl") or 1 to 10 ("C1-C10-haloalkyl") carbon atoms (as mentioned above), where some or all of the hydrogen atoms in these groups are replaced by halogen atoms as mentioned above: in particular C1-C2-haloalkyl, such as chloromethyl, bromomethyl, dichloromethyl, trichloromethyl, fluoromethyl, difluoromethyl, trifluoromethyl, chlorofluoromethyl, dichlorofluoromethyl,
chlorodifluoromethyl, 1-chloroethyl, 1-bromoethyl, 1-fluoroethyl, 2-fluoroethyl,
2,2-difluoroethyl, 2,2,2-trifluoroethyl, 2-chloro-2-fluoroethyl, 2-chloro-2,2-difluoroethyl, 2,2-dichloro-2-fluoroethyl, 2,2,2-trichloroethyl or pentafluoroethyl. C1-C3-haloalkyl is additionally, for example, 1-fluoropropyl, 2-fluoropropyl, 3-fluoropropyl,
1,1-difluoropropyl, 2,2-difluoropropyl, 1,2-difluoropropyl, 3,3-difluoropropyl,
3,3,3-trifluoropropyl, heptafluoropropyl, 1,1,1-trifluoroprop-2-yl, 3-chloropropyl and the like. Examples for C1-C4-haloalkyl are, apart those mentioned for C1-C3-haloalkyl, 4-chlorobutyl and the like.
"Halomethyl" is methyl in which 1, 2 or 3 of the hydrogen atoms are replaced by halogen atoms. Examples are bromomethyl, chloromethyl, fluoromethyl,
dichloromethyl, trichloromethyl, difluoromethyl, trifluoromethyl, chlorofluoromethyl, dichlorofluoromethyl, chlorodifluoromethyl and the like.
The term "alkenyl" as used herein refers to monounsaturated straight-chain or branched hydrocarbon radicals having 2 to 3 ("C2-C3-alkenyl"), 2 to 4 ("C2-C4-alkenyl"), 2 to 6 ("C2-C6-alkenyl"), 2 to 8 ("C2-C8-alkenyl") or 2 to 10 ("C2-C10-alkenyl") carbon atoms and a double bond in any position, for example C2-C3-alkenyl, such as ethenyl, 1-propenyl, 2-propenyl or 1-methylethenyl; C2-C4-alkenyl, such as ethenyl, 1-propenyl, 2-propenyl, 1-methylethenyl, 1-butenyl, 2-butenyl, 3-butenyl, 1-methyl-1-propenyl, 2-methyl-1-propenyl, 1-methyl-2-propenyl or 2-methyl-2-propenyl; C2-C6-alkenyl, such as ethenyl, 1-propenyl, 2-propenyl, 1-methylethenyl, 1-butenyl, 2-butenyl, 3-butenyl, 1-methyl-1-propenyl, 2-methyl-1-propenyl, 1-methyl-2-propenyl, 2-methyl-2-propenyl, 1-pentenyl, 2-pentenyl, 3-pentenyl, 4-pentenyl, 1-methyl-1-butenyl, 2-methyl-1-butenyl, 3-methyl-1-butenyl, 1-methyl-2-butenyl, 2-methyl-2-butenyl, 3-methyl-2-butenyl,
1-methyl-3-butenyl, 2-methyl-3-butenyl, 3-methyl-3-butenyl, 1,1-dimethyl-2-propenyl, 1,2-dimethyl-1-propenyl, 1,2-dimethyl-2-propenyl, 1-ethyl-1-propenyl, 1-ethyl-2- propenyl, 1-hexenyl, 2-hexenyl, 3-hexenyl, 4-hexenyl, 5-hexenyl, 1-methyl-1-pentenyl, 2-methyl-1-pentenyl, 3-methyl-1-pentenyl, 4-methyl-1-pentenyl, 1-methyl-2-pentenyl, 2-methyl-2-pentenyl, 3-methyl-2-pentenyl, 4-methyl-2-pentenyl, 1-methyl-3-pentenyl, 2-methyl-3-pentenyl, 3-methyl-3-pentenyl, 4-methyl-3-pentenyl, 1-methyl-4-pentenyl, 2-methyl-4-pentenyl, 3-methyl-4-pentenyl, 4-methyl-4-pentenyl, 1,1-dimethyl-2-butenyl, 1,1-dimethyl-3-butenyl, 1,2-dimethyl-1-butenyl, 1,2-dimethyl-2-butenyl, 1,2-dimethyl-3- butenyl, 1,3-dimethyl-1-butenyl, 1,3-dimethyl-2-butenyl, 1,3-dimethyl-3-butenyl, 2,2-dimethyl-3-butenyl, 2,3-dimethyl-1-butenyl, 2,3-dimethyl-2-butenyl, 2,3-dimethyl-3- butenyl, 3,3-dimethyl-1-butenyl, 3,3-dimethyl-2-butenyl, 1-ethyl-1-butenyl, 1-ethyl-2- butenyl, 1-ethyl-3-butenyl, 2-ethyl-1-butenyl, 2-ethyl-2-butenyl, 2-ethyl-3-butenyl, 1,1,2-trimethyl-2-propenyl, 1-ethyl-1-methyl-2-propenyl, 1-ethyl-2-methyl-1-propenyl, 1-ethyl-2-methyl-2-propenyl and the like, or C2-C10-alkenyl, such as the radicals mentioned for C2-C6-alkenyl and additionally 1-heptenyl, 2-heptenyl, 3-heptenyl, 1-octenyl, 2-octenyl, 3-octenyl, 4-octenyl, 1-nonenyl, 2-nonenyl, 3-nonenyl, 4-nonenyl, 1-decenyl, 2-decenyl, 3-decenyl, 4-decenyl, 5-decenyl and the positional isomers thereof.
The term "haloalkenyl" as used herein, which is also expressed as "alkenyl which is partially or fully halogenated", refers to unsaturated straight-chain or branched hydrocarbon radicals having 2 to 3 ("C2-C3-haloalkenyl"), 2 to 4 ("C2-C4-haloalkenyl"), 2 to 6 ("C2-C6-haloalkenyl"), 2 to 8 ("C2-C6-haloalkenyl") or 2 to 10 ("C2-C10-haloalkenyl") carbon atoms and a double bond in any position (as mentioned above), where some or all of the hydrogen atoms in these groups are replaced by halogen atoms as mentioned above, in particular fluorine, chlorine and bromine, for example chlorovinyl, chloroallyl and the like.
The term "alkynyl" as used herein refers to straight-chain or branched
hydrocarbon groups having 2 to 3 ("C2-C3-alkynyl"), 2 to 4 ("C2-C4-alkynyl"), 2 to 6 ("C2_C6-alkynyl"), 2 to 8 ("C2-C8-alkynyl"), or 2 to 10 ("C2-C10-alkynyl") carbon atoms and one or two triple bonds in any position, for example C2-C3-alkynyl, such as ethynyl, 1-propynyl or 2-propynyl; C2-C4-alkynyl, such as ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl, 3-butynyl, 1-methyl-2-propynyl and the like, C2-C6-alkynyl, such as ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl, 3-butynyl, 1-methyl-2-propynyl, 1-pentynyl, 2-pentynyl, 3-pentynyl, 4-pentynyl, 1-methyl-2-butynyl, 1-methyl-3-butynyl, 2-methyl-3-butynyl, 3-methyl-1-butynyl, 1,1-dimethyl-2-propynyl, 1-ethyl-2-propynyl, 1-hexynyl, 2-hexynyl, 3-hexynyl, 4-hexynyl, 5-hexynyl, 1-methyl-2-pentynyl, 1-methyl-3- pentynyl, 1-methyl-4-pentynyl, 2-methyl-3-pentynyl, 2-methyl-4-pentynyl, 3-methyl-1- pentynyl, 3-methyl-4-pentynyl, 4-methyl-1-pentynyl, 4-methyl-2-pentynyl, 1,1-dimethyl- 2-butynyl, 1,1-dimethyl-3-butynyl, 1,2-dimethyl-3-butynyl, 2,2-dimethyl-3-butynyl,
3,3-dimethyl-1-butynyl, 1-ethyl-2-butynyl, 1-ethyl-3-butynyl, 2-ethyl-3-butynyl, 1-ethyl-1- methyl-2-propynyl and the like;
The term "haloalkynyl" as used herein, which is also expressed as "alkynyl which is partially or fully halogenated", refers to unsaturated straight-chain or branched hydrocarbon radicals having 2 to 3 ("C2-C3-haloalkynyl"), 2 to 4 ("C2-C4-haloalkynyl"), 3 to 4 ("C3-C4-haloalkynyl"), 2 to 6 ("C2-C6-haloalkynyl"), 2 to 8 ("C2-C8-haloalkynyl") or 2 to 10 ("C2-C10-haloalkynyl") carbon atoms and one or two triple bonds in any position (as mentioned above), where some or all of the hydrogen atoms in these groups are replaced by halogen atoms as mentioned above, in particular fluorine, chlorine and bromine;
The term "cycloalkyl" as used herein refers to mono- or bicyclic saturated hydrocarbon radicals having 3 to 8 ("C3-C8-cycloalkyl"), in particular 3 to 6 ("C3-C6- cycloalkyl") or 3 to 5 ("C3-C5-cycloalkyl") or 3 to 4 ("C3-C4-cycloalkyl") carbon atoms. Examples of monocyclic radicals having 3 to 4 carbon atoms comprise cyclopropyl and cyclobutyl. Examples of monocyclic radicals having 3 to 5 carbon atoms comprise cyclopropyl, cyclobutyl and cyclopentyl. Examples of monocyclic radicals having 3 to 6 carbon atoms comprise cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl. Examples of monocyclic radicals having 3 to 8 carbon atoms comprise cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl and cyclooctyl. Examples of bicyclic radicals having 7 or 8 carbon atoms comprise bicyclo[2.2.1]heptyl, bicyclo[3.1.1]heptyl, bicyclo[2.2.2]octyl and bicyclo[3.2.1]octyl. Preferably, the term cycloalkyl denotes a monocyclic saturated hydrocarbon radical.
The term "halocycloalkyl" as used herein, which is also expressed as "cycloalkyl which is partially or fully halogenated", refers to mono- or bicyclic saturated
hydrocarbon groups having 3 to 8 ("C3-C8-halocycloalkyl" ) or preferably 3 to 6 ("C3-C6- halocycloalkyl") or 3 to 5 ("C3-C5-halocycloalkyl") or 3 to 4 ("C3-C4-halocycloalkyl") carbon ring members (as mentioned above) in which some or all of the hydrogen atoms are replaced by halogen atoms as mentioned above, in particular fluorine, chlorine and bromine.
The term "cycloalkyl-C1-C4-alkyl" refers to a C3-C8-cycloalkyl group ("C3-C8- cycloalkyl-C1-C4-alkyl"), preferably a C3-C6-cycloalkyl group ("C3-C6-cycloalkyl-C1-C4- alkyl"), more preferably a C3-C4-cycloalkyl group ("C3-C4-cycloalkyl-C1-C4-alkyl") as defined above (preferably a monocyclic cycloalkyl group) which is bound to the remainder of the molecule via a C1-C4-alkyl group, as defined above. Examples for C3-C4-cycloalkyl-C1-C4-alkyl are cyclopropylmethyl, cyclopropylethyl, cyclopropylpropyl, cyclobutylmethyl, cyclobutylethyl and cyclobutylpropyl, Examples for C3-C6-cycloalkyl- C1-C4-alkyl, apart those mentioned for C3-C4-cycloalkyl-C1-C4-alkyl, are
cyclopentylmethyl, cyclopentylethyl, cyclopentylpropyl, cyclohexylmethyl,
cyclohexylethyl and cyclohexylpropyl. Examples for C3-C8-cycloalkyl-C1-C4-alkyl, apart
those mentioned for C3-C6-cycloalkyl-C1-C4-alkyl, are cycloheptylmethyl,
cycloheptylethyl, cyclooctylmethyl and the like.
The term "C3-C6-cycloalkyl-methyl" refers to a C3-C6-cycloalkyl group which is bound to the remainder of the molecule via a methylene group (CH2). Examples are cyclopropylmethyl, cyclobutylmethyl, cyclopentylmethyl and cyclohexylmethyl.
The term "C3-C8-halocycloalkyl-C1-C4-alkyl" refers to a C3-C8-halocycloalkyl group as defined above which is bound to the remainder of the molecule via a C1-C4-alkyl group, as defined above. "C3-C6-halocycloalkyl-C1-C4-alkyl" refers to a C3-C6- halocycloalkyl group as defined above which is bound to the remainder of the molecule via a C1-C4-alkyl group, as defined above.
The term "C1-C2-alkoxy" is a C1-C2-alkyl group, as defined above, attached via an oxygen atom. The term "C1-C3-alkoxy" is a C1-C3-alkyl group, as defined above, attached via an oxygen atom. The term "C1-C4-alkoxy" is a C1-C4-alkyl group, as defined above, attached via an oxygen atom. The term "C1-C6-alkoxy" is a C1-C6-alkyl group, as defined above, attached via an oxygen atom. The term "C1-C10-alkoxy" is a C1-C10-alkyl group, as defined above, attached via an oxygen atom. C1-C2-Alkoxy is methoxy or ethoxy. C1-C3-Alkoxy is additionally, for example, n-propoxy and
1-methylethoxy (isopropoxy). C1-C4-Alkoxy is additionally, for example, butoxy, 1-methylpropoxy (sec-butoxy), 2-methylpropoxy (isobutoxy) or 1,1-dimethylethoxy (tert- butoxy). C1-C6-Alkoxy is additionally, for example, pentoxy, 1-methylbutoxy,
2-methylbutoxy, 3-methylbutoxy, 1,1-dimethylpropoxy, 1,2-dimethylpropoxy,
2,2-dimethylpropoxy, 1-ethylpropoxy, hexoxy, 1-methylpentoxy, 2-methylpentoxy, 3-methylpentoxy, 4-methylpentoxy, 1,1-dimethylbutoxy, 1,2-dimethylbutoxy,
1,3-dimethylbutoxy, 2,2-dimethylbutoxy, 2,3-dimethylbutoxy, 3,3-dimethylbutoxy, 1-ethylbutoxy, 2-ethylbutoxy, 1,1,2-trimethylpropoxy, 1,2,2-trimethylpropoxy, 1-ethyl-1- methylpropoxy or 1-ethyl-2-methylpropoxy. C1-C8-Alkoxy is additionally, for example, heptyloxy, octyloxy, 2-ethylhexyloxy and positional isomers thereof. C1-C10-Alkoxy is additionally, for example, nonyloxy, decyloxy and positional isomers thereof.
The term "C1-C2-haloalkoxy" is a C1-C2-haloalkyl group, as defined above, attached via an oxygen atom. The term "C1-C3-haloalkoxy" is a C1-C3-haloalkyl group, as defined above, attached via an oxygen atom. The term "C1-C4-haloalkoxy" is a C1-C4-haloalkyl group, as defined above, attached via an oxygen atom. The term "C1-C6-haloalkoxy" is a C1-C6-haloalkyl group, as defined above, attached via an oxygen atom. The term "C1-C10-haloalkoxy" is a C1-C10-haloalkyl group, as defined above, attached via an oxygen atom. C1-C2-Haloalkoxy is, for example, OCH2F, OCHF2, OCF3, OCH2Cl, OCHCl2, OCCl3, chlorofluoromethoxy, dichlorofluoromethoxy, chlorodifluoromethoxy, 2-fluoroethoxy, 2-chloroethoxy, 2-bromoethoxy, 2-iodoethoxy, 2,2-difluoroethoxy, 2,2,2-trifluoroethoxy, 2-chloro-2-fluoroethoxy, 2-chloro-2,2- difluoroethoxy, 2,2-dichloro-2-fluoroethoxy, 2,2,2-trichloroethoxy or OC2F5. C1-C3-
Haloalkoxy is additionally, for example, 2-fluoropropoxy, 3-fluoropropoxy,
2,2-difluoropropoxy, 2,3-difluoropropoxy, 2-chloropropoxy, 3-chloropropoxy,
2,3-dichloropropoxy, 2-bromopropoxy, 3-bromopropoxy, 3,3,3-trifluoropropoxy, 3,3,3-trichloropropoxy, OCH2-C2F5, OCF2-C2F5, 1-(CH2F)-2-fluoroethoxy, 1-(CH2Cl)-2- chloroethoxy or 1-(CH2Br)-2-bromoethoxy. C1-C4-Haloalkoxy is additionally, for example, 4-fluorobutoxy, 4-chlorobutoxy, 4-bromobutoxy or nonafluorobutoxy. C1-C6- Haloalkoxy is additionally, for example, 5-fluoropentoxy, 5-chloropentoxy,
5_brompentoxy, 5-iodopentoxy, undecafluoropentoxy, 6-fluorohexoxy, 6-chlorohexoxy, 6-bromohexoxy, 6-iodohexoxy or dodecafluorohexoxy.
The term "C1-C4-alkoxy-C1-C4-alkyl" as used herein, refers to a straight-chain or branched alkyl group having 1 to 4 carbon atoms, as defined above, where one hydrogen atom is replaced by a C1-C4-alkoxy group, as defined above. The term "C1-C6-alkoxy-C1-C4-alkyl" as used herein, refers to a straight-chain or branched alkyl group having 1 to 4 carbon atoms, as defined above, where one hydrogen atom is replaced by a C1-C6-alkoxy group, as defined above. Examples are methoxymethyl, ethoxymethyl, propoxymethyl, isopropoxymethyl, n-butoxymethyl, sec-butoxymethyl, isobutoxymethyl, tert-butoxymethyl, 1-methoxyethyl, 1-ethoxyethyl, 1-propoxyethyl, 1-isopropoxyethyl, 1-n-butoxyethyl, 1-sec-butoxyethyl, 1-isobutoxyethyl, 1-tert- butoxyethyl, 2-methoxyethyl, 2-ethoxyethyl, 2-propoxyethyl, 2-isopropoxyethyl, 2-n- butoxyethyl, 2-sec-butoxyethyl, 2-isobutoxyethyl, 2-tert-butoxyethyl, 1-methoxypropyl, 1-ethoxypropyl, 1-propoxypropyl, 1-isopropoxypropyl, 1-n-butoxypropyl, 1-sec- butoxypropyl, 1-isobutoxypropyl, 1-tert-butoxypropyl, 2-methoxypropyl, 2-ethoxypropyl, 2-propoxypropyl, 2-isopropoxypropyl, 2-n-butoxypropyl, 2-sec-butoxypropyl,
2-isobutoxypropyl, 2-tert-butoxypropyl, 3-methoxypropyl, 3-ethoxypropyl,
3-propoxypropyl, 3-isopropoxypropyl, 3-n-butoxypropyl, 3-sec-butoxypropyl,
3-isobutoxypropyl, 3-tert-butoxypropyl and the like.
The term "C1-C4-alkoxy-methyl" as used herein, refers to methyl in which one hydrogen atom is replaced by a C1-C4-alkoxy group, as defined above. The term "C1-C6-alkoxy-methyl" as used herein, refers to methyl in which one hydrogen atom is replaced by a C1-C6-alkoxy group, as defined above. Examples are methoxymethyl, ethoxymethyl, propoxymethyl, isopropoxymethyl, n-butoxymethyl, sec-butoxymethyl, isobutoxymethyl, tert-butoxymethyl, pentyloxymethyl, hexyloxymethyl and the like.
C1-C6-Haloalkoxy-C1-C4-alkyl is a straight-chain or branched alkyl group having from 1 to 4 carbon atoms, wherein one of the hydrogen atoms is replaced by a C1-C6- alkoxy group and wherein at least one, e.g.1, 2, 3, 4 or all of the remaining hydrogen atoms (either in the alkoxy moiety or in the alkyl moiety or in both) are replaced by halogen atoms. C1-C4-Haloalkoxy-C1-C4-alkyl is a straight-chain or branched alkyl group having from 1 to 4 carbon atoms, wherein one of the hydrogen atoms is replaced by a C1-C4-alkoxy group and wherein at least one, e.g.1, 2, 3, 4 or all of the remaining
hydrogen atoms (either in the alkoxy moiety or in the alkyl moiety or in both) are replaced by halogen atoms. Examples are difluoromethoxymethyl (CHF2OCH2), trifluoromethoxymethyl, 1-difluoromethoxyethyl, 1-trifluoromethoxyethyl,
2-difluoromethoxyethyl, 2-trifluoromethoxyethyl, difluoro-methoxy-methyl (CH3OCF2), 1,1-difluoro-2-methoxyethyl, 2,2-difluoro-2-methoxyethyl and the like.
The term "C2-C6-alkenyloxy" is a C2-C6-alkenyl group, as defined above, attached via an oxygen atom. Examples are ethenyloxy, propen-1-yloxy, allyloxy (propen-2-yl- oxy), buten-1-oxy, buten-2-oxy, buten-3-oxy, and the like.
The term "C2-C6-haloalkenyloxy" is a C2-C6-haloalkenyl group, as defined above, attached via an oxygen atom.
The term "C2-C6-alkynyloxy" is a C2-C6-alkynyl group, as defined above, attached via an oxygen atom. Examples are ethynyloxy, propyn-1-yloxy, propargyloxy (propyn-2- yl-oxy), butyn-1-oxy, butyn-2-oxy, butyn-3-oxy, and the like.
The term "C2-C6-haloalkynyloxy" is a C2-C6-haloalkynyl group, as defined above, attached via an oxygen atom.
The term "C3-C6-cycloalkoxy" denotes a C3-C6-cycloalkyl group, as defined above, which is attached via an oxygen atom. The term "C3-C8-cycloalkoxy" denotes a C3-C8-cycloalkyl group, as defined above, which is attached via an oxygen atom.
Examples of C3-C6-cycloalkoxy comprise cyclopropoxy, cyclobutoxy, cyclopentoxy and cyclohexoxy. Examples of C3-C8-cycloalkoxy comprise, in addition to those mentioned for C3-C6-cycloalkoxy, cycloheptoxy and cyclooctyloxy.
The term "C3-C6-halocycloalkoxy" denotes a C3-C6-halocycloalkyl group, as defined above, which is attached via an oxygen atom. The term "C3-C8- halocycloalkoxy" denotes a C3-C8-halocycloalkyl group, as defined above, which is attached via an oxygen atom.
C3-C8-Cycloalkyl-C1-C4-alkoxy is C1-C4-alkoxy, as defined above wherein one of the hydrogen atoms is replaced by a C3-C8-cycloalkyl group.
C3-C8-Halocycloalkyl-C1-C4-alkoxy is C1-C4-alkoxy, as defined above wherein one of the hydrogen atoms is replaced by a C3-C8-cycloalkyl group and wherein at least one, e.g.1, 2, 3, 4 or all of the remaining hydrogen atoms (either in the cycloalkyl moiety or in the alkoxy moiety or in both) are replaced by halogen atoms.
The term "C1-C2-alkylthio" is a C1-C2-alkyl group, as defined above, attached via a sulfur atom. The term "C1-C3-alkylthio" is a C1-C3-alkyl group, as defined above, attached via a sulfur atom. The term "C1-C4-alkylthio" is a C1-C4-alkyl group, as defined above, attached via a sulfur atom. The term "C1-C6-alkylthio" is a C1-C6-alkyl group, as defined above, attached via a sulfur atom. The term "C1-C10-alkylthio" is a C1-C10-alkyl group, as defined above, attached via a sulfur atom. C1-C2-Alkylthio is methylthio or ethylthio. C1-C3-Alkylthio is additionally, for example, n-propylthio or 1-methylethylthio (isopropylthio). C1-C4-Alkylthio is additionally, for example, butylthio, 1-methylpropylthio
(sec-butylthio), 2-methylpropylthio (isobutylthio) or 1,1-dimethylethylthio (tert-butylthio). C1-C6-Alkylthio is additionally, for example, pentylthio, 1-methylbutylthio,
2-methylbutylthio, 3-methylbutylthio, 1,1-dimethylpropylthio, 1,2-dimethylpropylthio, 2,2-dimethylpropylthio, 1-ethylpropylthio, hexylthio, 1-methylpentylthio,
2-methylpentylthio, 3-methylpentylthio, 4-methylpentylthio, 1,1-dimethylbutylthio, 1,2-dimethylbutylthio, 1,3-dimethylbutylthio, 2,2-dimethylbutylthio, 2,3-dimethylbutylthio, 3,3-dimethylbutylthio, 1-ethylbutylthio, 2-ethylbutylthio, 1,1,2-trimethylpropylthio, 1,2,2-trimethylpropylthio, 1-ethyl-1-methylpropylthio or 1-ethyl-2-methylpropylthio.
C1-C8-Alkylthio is additionally, for example, heptylthio, octylthio, 2-ethylhexylthio and positional isomers thereof. C1-C10-Alkylthio is additionally, for example, nonylthio, decylthio and positional isomers thereof.
The term "C1-C2-haloalkylthio" is a C1-C2-haloalkyl group, as defined above, attached via a sulfur atom. The term "C1-C3-haloalkylthio" is a C1-C3-haloalkyl group, as defined above, attached via a sulfur atom. The term "C1-C4-haloalkylthio" is a C1-C4- haloalkyl group, as defined above, attached via a sulfur atom. The term "C1-C6- haloalkylthio" is a C1-C6-haloalkyl group, as defined above, attached via a sulfur atom. The term "C1-C10-haloalkylthio" is a C1-C10-haloalkyl group, as defined above, attached via a sulfur atom. C1-C2-Haloalkylthio is, for example, SCH2F, SCHF2, SCF3, SCH2Cl, SCHCl2, SCCl3, chlorofluoromethylthio, dichlorofluoromethylthio,
chlorodifluoromethylthio, 2-fluoroethylthio, 2-chloroethylthio, 2-bromoethylthio,
2-iodoethylthio, 2,2-difluoroethylthio, 2,2,2-trifluoroethylthio, 2-chloro-2-fluoroethylthio, 2-chloro-2,2-difluoroethylthio, 2,2-dichloro-2-fluoroethylthio, 2,2,2-trichloroethylthio or SC2F5. C1-C3-Haloalkylthio is additionally, for example, 2-fluoropropylthio,
3-fluoropropylthio, 2,2-difluoropropylthio, 2,3-difluoropropylthio, 2-chloropropylthio, 3-chloropropylthio, 2,3-dichloropropylthio, 2-bromopropylthio, 3-bromopropylthio, 3,3,3-trifluoropropylthio, 3,3,3-trichloropropylthio, SCH2-C2F5, SCF2-C2F5, 1-(CH2F)-2- fluoroethylthio, 1-(CH2Cl)-2-chloroethylthio or 1-(CH2Br)-2-bromoethylthio. C1-C4- Haloalkylthio is additionally, for example, 4-fluorobutylthio, 4-chlorobutylthio,
4-bromobutylthio or nonafluorobutylthio. C1-C6-Haloalkylthio is additionally, for example, 5-fluoropentylthio, 5-chloropentylthio, 5-brompentylthio, 5-iodopentylthio, undecafluoropentylthio, 6-fluorohexylthio, 6-chlorohexylthio, 6-bromohexylthio,
6-iodohexylthio or dodecafluorohexylthio.
The term "C1-C2-alkylsulfinyl" is a C1-C2-alkyl group, as defined above, attached via a sulfinyl [S(O)] group. The term "C1-C4-alkylsulfinyl" is a C1-C4-alkyl group, as defined above, attached via a sulfinyl [S(O)] group. The term "C1-C6-alkylsulfinyl" is a C1-C6-alkyl group, as defined above, attached via a sulfinyl [S(O)] group. The term "C1-C10-alkylsulfinyl" is a C1-C10-alkyl group, as defined above, attached via a sulfinyl [S(O)] group. C1-C2-Alkylsulfinyl is methylsulfinyl or ethylsulfinyl. C1-C4-Alkylsulfinyl is additionally, for example, n-propylsulfinyl, 1-methylethylsulfinyl (isopropylsulfinyl),
butylsulfinyl, 1-methylpropylsulfinyl (sec-butylsulfinyl), 2-methylpropylsulfinyl
(isobutylsulfinyl) or 1,1-dimethylethylsulfinyl (tert-butylsulfinyl). C1-C6-Alkylsulfinyl is additionally, for example, pentylsulfinyl, 1-methylbutylsulfinyl, 2-methylbutylsulfinyl, 3-methylbutylsulfinyl, 1,1-dimethylpropylsulfinyl, 1,2-dimethylpropylsulfinyl,
2,2-dimethylpropylsulfinyl, 1-ethylpropylsulfinyl, hexylsulfinyl, 1-methylpentylsulfinyl, 2-methylpentylsulfinyl, 3-methylpentylsulfinyl, 4-methylpentylsulfinyl,
1,1-dimethylbutylsulfinyl, 1,2-dimethylbutylsulfinyl, 1,3-dimethylbutylsulfinyl,
2,2-dimethylbutylsulfinyl, 2,3-dimethylbutylsulfinyl, 3,3-dimethylbutylsulfinyl,
1-ethylbutylsulfinyl, 2-ethylbutylsulfinyl, 1,1,2-trimethylpropylsulfinyl,
1,2,2-trimethylpropylsulfinyl, 1-ethyl-1-methylpropylsulfinyl or 1-ethyl-2- methylpropylsulfinyl. C1-C8-Alkylsulfinyl is additionally, for example, heptylsulfinyl, octylsulfinyl, 2-ethylhexylsulfinyl and positional isomers thereof. C1-C10-Alkylsulfinyl is additionally, for example, nonylsulfinyl, decylsulfinyl and positional isomers thereof.
The term "C1-C2-haloalkylsulfinyl" is a C1-C2-haloalkyl group, as defined above, attached via a sulfinyl [S(O)] group. The term "C1-C4-haloalkylsulfinyl" is a C1-C4- haloalkyl group, as defined above, attached via a sulfinyl [S(O)] group. The term "C1-C6-haloalkylsulfinyl" is a C1-C6-haloalkyl group, as defined above, attached via a sulfinyl [S(O)] group. The term "C1-C10-haloalkylsulfinyl" is a C1-C10-haloalkyl group, as defined above, attached via a sulfinyl [S(O)] group. C1-C2-Haloalkylsulfinyl is, for example, S(O)CH2F, S(O)CHF2, S(O)CF3, S(O)CH2Cl, S(O)CHCl2, S(O)CCl3, chlorofluoromethylsulfinyl, dichlorofluoromethylsulfinyl, chlorodifluoromethylsulfinyl, 2-fluoroethylsulfinyl, 2-chloroethylsulfinyl, 2-bromoethylsulfinyl, 2-iodoethylsulfinyl, 2,2-difluoroethylsulfinyl, 2,2,2-trifluoroethylsulfinyl, 2-chloro-2-fluoroethylsulfinyl, 2-chloro-2,2-difluoroethylsulfinyl, 2,2-dichloro-2-fluoroethylsulfinyl,
2,2,2-trichloroethylsulfinyl or S(O)C2F5. C1-C4-Haloalkylsulfinyl is additionally, for example, 2-fluoropropylsulfinyl, 3-fluoropropylsulfinyl, 2,2-difluoropropylsulfinyl, 2,3-difluoropropylsulfinyl, 2-chloropropylsulfinyl, 3-chloropropylsulfinyl,
2,3-dichloropropylsulfinyl, 2-bromopropylsulfinyl, 3-bromopropylsulfinyl,
3,3,3-trifluoropropylsulfinyl, 3,3,3-trichloropropylsulfinyl, S(O)CH2-C2F5, S(O)CF2-C2F5, 1-(CH2F)-2-fluoroethylsulfinyl, 1-(CH2Cl)-2-chloroethylsulfinyl, 1-(CH2Br)-2- bromoethylsulfinyl, 4-fluorobutylsulfinyl, 4-chlorobutylsulfinyl, 4-bromobutylsulfinyl or nonafluorobutylsulfinyl. C1-C6-Haloalkylsulfinyl is additionally, for example,
5-fluoropentylsulfinyl, 5-chloropentylsulfinyl, 5-brompentylsulfinyl, 5-iodopentylsulfinyl, undecafluoropentylsulfinyl, 6-fluorohexylsulfinyl, 6-chlorohexylsulfinyl,
6-bromohexylsulfinyl, 6-iodohexylsulfinyl or dodecafluorohexylsulfinyl.
The term "C1-C2-alkylsulfonyl" is a C1-C2-alkyl group, as defined above, attached via a sulfonyl [S(O)2] group. The term "C1-C3-alkylsulfonyl" is a C1-C3-alkyl group, as defined above, attached via a sulfonyl [S(O)2] group. The term "C1-C4-alkylsulfonyl" is a C1-C4-alkyl group, as defined above, attached via a sulfonyl [S(O)2] group. The term
"C1-C6-alkylsulfonyl" is a C1-C6-alkyl group, as defined above, attached via a sulfonyl [S(O)2] group. The term "C1-C10-alkylsulfonyl" is a C1-C10-alkyl group, as defined above, attached via a sulfonyl [S(O)2] group. C1-C2-Alkylsulfonyl is methylsulfonyl or ethylsulfonyl. C1-C3-Alkylsulfonyl is additionally, for example, n-propylsulfonyl or 1-methylethylsulfonyl (isopropylsulfonyl). C1-C4-Alkylsulfonyl is additionally, for example, butylsulfonyl, 1-methylpropylsulfonyl (sec-butylsulfonyl),
2-methylpropylsulfonyl (isobutylsulfonyl) or 1,1-dimethylethylsulfonyl (tert-butylsulfonyl). C1-C6-Alkylsulfonyl is additionally, for example, pentylsulfonyl, 1-methylbutylsulfonyl, 2-methylbutylsulfonyl, 3-methylbutylsulfonyl, 1,1-dimethylpropylsulfonyl,
1,2-dimethylpropylsulfonyl, 2,2-dimethylpropylsulfonyl, 1-ethylpropylsulfonyl, hexylsulfonyl, 1-methylpentylsulfonyl, 2-methylpentylsulfonyl, 3-methylpentylsulfonyl, 4-methylpentylsulfonyl, 1,1-dimethylbutylsulfonyl, 1,2-dimethylbutylsulfonyl,
1,3-dimethylbutylsulfonyl, 2,2-dimethylbutylsulfonyl, 2,3-dimethylbutylsulfonyl,
3,3-dimethylbutylsulfonyl, 1-ethylbutylsulfonyl, 2-ethylbutylsulfonyl,
1,1,2-trimethylpropylsulfonyl, 1,2,2-trimethylpropylsulfonyl, 1-ethyl-1- methylpropylsulfonyl or 1-ethyl-2-methylpropylsulfonyl. C1-C8-Alkylsulfonyl is additionally, for example, heptylsulfonyl, octylsulfonyl, 2-ethylhexylsulfonyl and positional isomers thereof. C1-C10-Alkylsulfonyl is additionally, for example,
nonylsulfonyl, decylsulfonyl and positional isomers thereof.
The term "C1-C2-haloalkylsulfonyl" is a C1-C2-haloalkyl group, as defined above, attached via a sulfonyl [S(O)2] group. The term "C1-C3-haloalkylsulfonyl" is a C1-C3- haloalkyl group, as defined above, attached via a sulfonyl [S(O)2] group. The term "C1-C4-haloalkylsulfonyl" is a C1-C4-haloalkyl group, as defined above, attached via a sulfonyl [S(O)2] group. The term "C1-C6-haloalkylsulfonyl" is a C1-C6-haloalkyl group, as defined above, attached via a sulfonyl [S(O)2] group. The term "C1-C10- haloalkylsulfonyl" is a C1-C10-haloalkyl group, as defined above, attached via a sulfonyl [S(O)2] group. C1-C2-Haloalkylsulfonyl is, for example, S(O)2CH2F, S(O)2CHF2,
S(O)2CF3, S(O)2CH2Cl, S(O)2CHCl2, S(O)2CCl3, chlorofluoromethylsulfonyl,
dichlorofluoromethylsulfonyl, chlorodifluoromethylsulfonyl, 2-fluoroethylsulfonyl, 2-chloroethylsulfonyl, 2-bromoethylsulfonyl, 2-iodoethylsulfonyl,
2,2-difluoroethylsulfonyl, 2,2,2-trifluoroethylsulfonyl, 2-chloro-2-fluoroethylsulfonyl, 2-chloro-2,2-difluoroethylsulfonyl, 2,2-dichloro-2-fluoroethylsulfonyl,
2,2,2-trichloroethylsulfonyl or S(O)2C2F5. C1-C3-Haloalkylsulfonyl is additionally, for example, 2-fluoropropylsulfonyl, 3-fluoropropylsulfonyl, 2,2-difluoropropylsulfonyl, 2,3-difluoropropylsulfonyl, 2-chloropropylsulfonyl, 3-chloropropylsulfonyl,
2,3-dichloropropylsulfonyl, 2-bromopropylsulfonyl, 3-bromopropylsulfonyl,
3,3,3-trifluoropropylsulfonyl, 3,3,3-trichloropropylsulfonyl, S(O)2CH2-C2F5, S(O)2CF2- C2F5, 1-(CH2F)-2-fluoroethylsulfonyl, 1-(CH2Cl)-2-chloroethylsulfonylor 1-(CH2Br)-2- bromoethylsulfonyl. C1-C4-Haloalkylsulfonyl is additionally, for example,
4-fluorobutylsulfonyl, 4-chlorobutylsulfonyl, 4-bromobutylsulfonyl or nonafluorobutylsulfonyl. C1-C6-Haloalkylsulfonyl is additionally, for example,
5-fluoropentylsulfonyl, 5-chloropentylsulfonyl, 5-brompentylsulfonyl,
5-iodopentylsulfonyl, undecafluoropentylsulfonyl, 6-fluorohexylsulfonyl,
6-chlorohexylsulfonyl, 6-bromohexylsulfonyl, 6-iodohexylsulfonyl or
dodecafluorohexylsulfonyl.
The substituent "oxo" replaces a CH2 group by a C(=O) group.
The term "alkylcarbonyl" is a C1-C6-alkyl ("C1-C6-alkylcarbonyl"), preferably a C1-C4-alkyl ("C1-C4-alkylcarbonyl") group, as defined above, attached via a carbonyl [C(=O)] group. Examples are acetyl (methylcarbonyl), propionyl (ethylcarbonyl), propylcarbonyl, isopropylcarbonyl, n-butylcarbonyl and the like.
The term "haloalkylcarbonyl" is a C1-C6-haloalkyl ("C1-C6-haloalkylcarbonyl"), preferably a C1-C4-haloalkyl ("C1-C4-haloalkylcarbonyl") group, as defined above, attached via a carbonyl [C(=O)] group. Examples are trifluoromethylcarbonyl,
2,2,2-trifluoroethylcarbonyl and the like.
The term "alkoxycarbonyl" is a C1-C6-alkoxy ("C1-C6-alkoxycarbonyl"), preferably a C1-C4-alkoxy ("C1-C4-alkoxycarbonyl") group, as defined above, attached via a carbonyl [C(=O)] group. Examples are methoxycarbonyl, ethoxycarbonyl,
propoxycarbonyl, isopropoxycarbonyl, n-butoxycarbonyl and the like.
The term "haloalkoxycarbonyl" is a C1-C6-haloalkoxy ("C1-C6- haloalkoxycarbonyl"), preferably a C1-C4-haloalkoxy ("C1-C4-haloalkoxycarbonyl") group, as defined above, attached via a carbonyl [C(=O)] group. Examples are trifluoromethoxycarbonyl, 2,2,2-trifluoroethoxycarbonyl and the like.
The term "C1-C6-alkylamino" is a group -N(H)C1-C6-alkyl. Examples are methylamino, ethylamino, propylamino, isopropylamino, butylamino and the like.
The term "di-(C1-C6-alkyl)amino" is a group -N(C1-C6-alkyl)2. Examples are dimethylamino, diethylamino, ethylmethylamino, dipropylamino, diisopropylamino, methylpropylamino, methylisopropylamino, ethylpropylamino, ethylisopropylamino, dibutylamino and the like.
Phenyl-C1-C4-alkyl is a C1-C4-alkyl group, as defined above, wherein one of the hydrogen atoms is replaced by a phenyl ring. Examples are benzyl, phenyl-1-ethyl and phenethyl (phenyl-2-ethyl).
C1-C10-Alkylene is a linear or branched divalent alkyl radical having 1 to 10 carbon atoms. Examples are -CH2-, -CH2CH2-, -CH(CH3)-, -CH2CH2CH2-,
-CH(CH3)CH2-, -CH2CH(CH3)-, -C(CH3)2-, -CH2CH2CH2CH2-, -CH(CH3)CH2CH2-, -CH2CH2CH(CH3)-, -C(CH3)2CH2-, -CH2C(CH3)2-, -(CH2)5-, -(CH2)6-, -(CH2)7-, -(CH2)8, -(CH2)9-, -(CH2)10- and the branched isomers.
The term "3-, 4-, 5-, 6- or 7-membered saturated, partially unsaturated or maximally unsaturated heterocyclic rings containing 1, 2 or 3 heteroatoms or
heteroatom groups selected from N, O, S, NO, SO and SO2, as ring members" denotes a 3-, 4-, 5-, 6- or 7-membered saturated, partially unsaturated or maximum unsaturated heteromonocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2, as ring members.
Unsaturated rings contain at least one C-C and/or C-N and/or N-N double bond(s). Maximally unsaturated rings contain as many conjugated C-C and/or C-N and/or N-N double bonds as allowed by the ring size. Partly unsaturated rings contain less conjugated C-C and/or C-N and/or N-N double bonds than maximally allowed by the ring size. Maximally unsaturated 5- or 6-membered heterocyclic rings are aromatic. 7-membered rings cannot be aromatic; they are homoaromatic (3 double bonds). The heterocyclic ring may be attached to the remainder of the molecule via a carbon ring member or via a nitrogen ring member. As a matter of course, the heterocyclic ring contains at least one carbon ring atom. If the ring contains more than one O ring atom, these are not adjacent.
Examples of a 3-, 4-, 5-, 6- or 7-membered saturated heterocyclic ring include: Oxiranyl, thiiranyl, aziridinyl, oxetanyl, thietanyl, azetidinyl, tetrahydrofuran-2-yl, tetrahydrofuran-3-yl, tetrahydrothien-2-yl, tetrahydrothien-3-yl, pyrrolidin-1-yl, pyrrolidin- 2-yl, pyrrolidin-3-yl, pyrazolidin-1-yl, pyrazolidin-3-yl, pyrazolidin-4-yl, pyrazolidin-5-yl, imidazolidin-1-yl, imidazolidin-2-yl, imidazolidin-4-yl, oxazolidin-2-yl, oxazolidin-3-yl, oxazolidin-4-yl, oxazolidin-5-yl, isoxazolidin-2-yl, isoxazolidin-3-yl, isoxazolidin-4-yl, isoxazolidin-5-yl, thiazolidin-2-yl, thiazolidin-3-yl, thiazolidin-4-yl, thiazolidin-5-yl, isothiazolidin-2-yl, isothiazolidin-3-yl, isothiazolidin-4-yl, isothiazolidin-5-yl,
1,2,4-oxadiazolidin-3-yl, 1,2,4-oxadiazolidin-5-yl, 1,2,4-thiadiazolidin-3-yl,
1,2,4-thiadiazolidin-5-yl, 1,2,4-triazolidin-3-yl, 1,3,4-oxadiazolidin-2-yl,
1,3,4-thiadiazolidin-2-yl, 1,3,4-triazolidin-1-yl, 1,3,4-triazolidin-2-yl, 1,2,3,4-tetrazolidin- 1-yl, 1,2,3,4-tetrazolidin-2-yl, 1,2,3,4-tetrazolidin-5-yl, 2-tetrahydropyranyl,
4-tetrahydropyranyl, 1,3-dioxan-5-yl, 1,4-dioxan-2-yl, piperidin-1-yl, piperidin-2-yl, piperidin-3-yl, piperidin-4-yl, hexahydropyridazin-3-yl, hexahydropyridazin-4-yl, hexahydropyrimidin-2-yl, hexahydropyrimidin-4-yl, hexahydropyrimidin-5-yl, piperazin- 1-yl, piperazin-2-yl, 1,3,5-hexahydrotriazin-1-yl, 1,3,5-hexahydrotriazin-2-yl and 1,2,4-hexahydrotriazin-3-yl, morpholin-2-yl, morpholin-3-yl, morpholin-4-yl,
thiomorpholin-2-yl, thiomorpholin-3-yl, thiomorpholin-4-yl, 1-oxothiomorpholin-2-yl, 1-oxothiomorpholin-3-yl, 1-oxothiomorpholin-4-yl, 1,1-dioxothiomorpholin-2-yl,
1,1-dioxothiomorpholin-3-yl, 1,1-dioxothiomorpholin-4-yl, azepan-1-, -2-, -3- or -4-yl, oxepan-2-, -3-, -4- or -5-yl, hexahydro-1,3-diazepinyl, hexahydro-1,4-diazepinyl, hexahydro-1,3-oxazepinyl, hexahydro-1,4-oxazepinyl, hexahydro-1,3-dioxepinyl, hexahydro-1,4-dioxepinyl and the like.
Examples of a 3-, 4-, 5-, 6- or 7-membered partially unsaturated heterocyclic ring include: 2,3-dihydrofur-2-yl, 2,3-dihydrofur-3-yl, 2,4-dihydrofur-2-yl, 2,4-dihydrofur-3-yl,
2,3-dihydrothien-2-yl, 2,3-dihydrothien-3-yl, 2,4-dihydrothien-2-yl, 2,4-dihydrothien-3-yl, 2-pyrrolin-2-yl, 2-pyrrolin-3-yl, 3-pyrrolin-2-yl, 3-pyrrolin-3-yl, 2-isoxazolin-3-yl,
3-isoxazolin-3-yl, 4-isoxazolin-3-yl, 2-isoxazolin-4-yl, 3-isoxazolin-4-yl, 4-isoxazolin-4-yl, 2-isoxazolin-5-yl, 3-isoxazolin-5-yl, 4-isoxazolin-5-yl, 2-isothiazolin-3-yl, 3-isothiazolin- 3-yl, 4-isothiazolin-3-yl, 2-isothiazolin-4-yl, 3-isothiazolin-4-yl, 4-isothiazolin-4-yl, 2-isothiazolin-5-yl, 3-isothiazolin-5-yl, 4-isothiazolin-5-yl, 2,3-dihydropyrazol-1-yl, 2,3-dihydropyrazol-2-yl, 2,3-dihydropyrazol-3-yl, 2,3-dihydropyrazol-4-yl,
2,3-dihydropyrazol-5-yl, 3,4-dihydropyrazol-1-yl, 3,4-dihydropyrazol-3-yl,
3,4-dihydropyrazol-4-yl, 3,4-dihydropyrazol-5-yl, 4,5-dihydropyrazol-1-yl,
4,5-dihydropyrazol-3-yl, 4,5-dihydropyrazol-4-yl, 4,5-dihydropyrazol-5-yl,
2,3-dihydrooxazol-2-yl, 2,3-dihydrooxazol-3-yl, 2,3-dihydrooxazol-4-yl,
2,3-dihydrooxazol-5-yl, 3,4-dihydrooxazol-2-yl, 3,4-dihydrooxazol-3-yl,
3,4-dihydrooxazol-4-yl, 3,4-dihydrooxazol-5-yl, 3,4-dihydrooxazol-2-yl,
3,4-dihydrooxazol-3-yl, 3,4-dihydrooxazol-4-yl, 2,3-dihydro-1,2,4-triazol-1-, -2-, -3- or - 5-yl, 4,5-dihydro-1,3,4-triazol-1-, -2-, -4- or -5-yl, 2,5-dihydro-1,3,4-triazol-1-, -2- or -5- yl, 4,5-dihydro-1,2,3-triazol-1-, -4- or -5-yl, 2,5-dihydro-1,2,3-triazol-1-, -2- or -5-yl, 2,3-dihydro-1,2,3-triazol-1-, -2-, -3-, -4- or -5-yl, 2,3-dihydro-1,2,3,4-tetrazol-1-, -2-, -3- or -5-yl, 2,5-dihydro-1,2,3,4-tetrazol-1-, -2- or -5-yl, 4,5-dihydro-1,2,3,4-tetrazol-1-, -4- or -5-yl, 2-, 3-, 4-, 5- or 6-di- or tetrahydropyridinyl, 3-di- or tetrahydropyridazinyl, 4-di- or tetrahydropyridazinyl, 2-di- or tetrahydropyrimidinyl, 4-di- or tetrahydropyrimidinyl, 5-di- or tetrahydropyrimidinyl, di- or tetrahydropyrazinyl, 1,3,5-di- or tetrahydrotriazin-2- yl, 1,2,4-di- or tetrahydrotriazin-3-yl, 2,3,4,5-tetrahydro[1H]azepin-1-, -2-, -3-, -4-, -5-, -6- or -7-yl, 3,4,5,6-tetrahydro[2H]azepin-2-, -3-, -4-, -5-, -6- or -7-yl,
2,3,4,7-tetrahydro[1H]azepin-1-, -2-, -3-, -4-, -5-, -6- or -7-yl,
2,3,6,7-tetrahydro[1H]azepin-1-, -2-, -3-, -4-, -5-, -6- or -7-yl, tetrahydrooxepinyl, such as 2,3,4,5-tetrahydro[1H]oxepin-2-, -3-, -4-, -5-, -6- or -7-yl, 2,3,4,7- tetrahydro[1H]oxepin-2-, -3-, -4-, -5-, -6- or -7-yl, 2,3,6,7-tetrahydro[1H]oxepin-2-, -3-, -4-, -5-, -6- or -7-yl, tetrahydro-1,3-diazepinyl, tetrahydro-1,4-diazepinyl, tetrahydro-1,3- oxazepinyl, tetrahydro-1,4-oxazepinyl, tetrahydro-1,3-dioxepinyl and tetrahydro-1,4- dioxepinyl.
Examples for a 3-, 4-, 5-, 6- or 7-membered maximally unsaturated (including aromatic) heterocyclic ring are 5- or 6-membered heteroaromatic rings, such as 2-furyl, 3-furyl, 2-thienyl, 3-thienyl, 1-pyrrolyl, 2-pyrrolyl, 3-pyrrolyl, 1-pyrazolyl, 3-pyrazolyl, 4-pyrazolyl, 5-pyrazolyl, 2-oxazolyl, 4-oxazolyl, 5-oxazolyl, 2-thiazolyl, 4-thiazolyl, 5-thiazolyl, 1-imidazolyl, 2-imidazolyl, 4-imidazolyl, 1,3,4-triazol-1-yl, 1,3,4-triazol-2-yl, 1,2,3,4-1H-tetrazol-1-yl, 1,2,3,4-1H-tetrazol-5-yl, 1,2,3,4-2H-tetrazol-2-yl, 1,2,3,4-2H- tetrazol-5-yl, 2-pyridinyl, 3-pyridinyl, 4-pyridinyl, 1-oxopyridin-2-yl, 1-oxopyridin-3-yl, 1-oxopyridin-4-yl,3-pyridazinyl, 4-pyridazinyl, 2-pyrimidinyl, 4-pyrimidinyl, 5-pyrimidinyl, 2-pyrazinyl, 1,3,5-triazin-2-yl, 1,2,4-triazin-3-yl and 1,2,4-triazin-5-yl, and also
homoaromatic radicals, such as 1H-azepine, 1H-[1,3]-diazepine and 1H-[1,4]- diazepine.
In addition to the above-listed 5- or 6-membered heteroaromatic rings, examples for 5- or 6-membered heteroaromatic rings containing 1, 2 ,3 or 4 heteroatoms selected from O, S and N include 1,2,3,4-1H-tetrazol-1-yl, 1,2,3,4-1H-tetrazol-5-yl, 1,2,3,4-2H- tetrazol-2-yl, 1,2,3,4-2H-tetrazol-5-yl, 1,2,3,4-tetrazin-5-yl, 1,2,4,5-tetrazin-3-yl and 1,2,3,5-tetrazin-4-yl.
When # appears in a formula showing a substructure of a compound of the present invention, it denotes the attachment bond in the remainder molecule.
The remarks made below concerning preferred embodiments of the variables of the compounds of formula I, especially with respect to their substituents A, Het, X, Y, Z, R1, R2, R3, R4, R5a, R5b, R6, R6a, R7, R7a, R8, R9a, R9b, R10, R11, R12, R13, R14, R5, R16, R17, R17a, R17b, R18, R19, RA, L1, L2, k and n, the features of the use and method according to the invention and of the composition of the invention are valid both on their own and, in particular, in every possible combination with each other. In a preferred embodiment,
R1 and R2, independently of each other, are selected from the group consisting of
hydrogen, halogen, CN, C1-C6-alkyl, C3-C6-cycloalkyl, C1-C6-haloalkyl and C3-C6- halocycloalkyl; or
R1 and R2 may together be =CR13R14; or
R1 and R2 form, together with the carbon atom which they attached to, a 3-, 4-or 5-membered saturated carbocyclic ring;
where R13 and R14 have one of the above meanings.
In particular, R1 and R2, independently of each other, are selected from hydrogen, halogen and methyl. Specifically, both R1 and R2 are hydrogen. In a preferred embodiment, R3 is selected from the group consisting of hydrogen, halogen, C1-C6-alkyl and C1-C6-haloalkyl. In particular, R3 is selected from hydrogen, halogen and C1-C4-alkyl. Specifically, R3 is selected from hydrogen, Cl, Br and methyl. In one preferred embodiment, R4 is a group -L1-C ^C-RA. In another preferred embodiment, R4 is CN. However, more preferably, R4 is a group -L1-C ^C-RA. In this group -L1-C ^C-RA, L1 is preferably a bond or -O-CH2-; and is in particular a bond.
RA is preferably selected from the group consisting of hydrogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkyl which carries one radical R7, OR8, S(O)nR8, C(=O)R7a, C(=O)OR8 and C(=O)NR9aR9b;
where R7, R7a, R8, R9a, R9b and n have one of the above general or, in particular, one of the below preferred meanings.
More preferably, RA is selected from the group consisting of hydrogen, C1-C6- alkyl, C1-C6-haloalkyl, C1-C6-alkyl which carries one radical R7, C1-C6-alkoxy, C1-C6- haloalkoxy, C1-C6-alkylthio, C1-C6-haloalkylthio, C1-C6-alkylsulfinyl, C1-C6- haloalkylsulfinyl, C1-C6-alkylsulfonyl, C1-C6-haloalkylsulfonyl, C1-C6-alkylcarbonyl, C1-C6-haloalkylcarbonyl, C1-C6-alkoxycarbonyl and C1-C6-haloalkoxycarbonyl, where R7 has one of the above general or, in particular, one of the below preferred meanings.
Specifically, RA is selected from the group consisting of hydrogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkyl which carries one radical R7, C1-C6-alkylsulfonyl and C1-C6- haloalkylsulfonyl,
where R7 has one of the above general or, in particular, one of the below preferred meanings.
In the above preferred, more preferred and specific embodiments of RA, R7 is preferably selected from cyano, C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6-alkylthio, C1-C6- haloalkylthio, C1-C6-alkylsulfinyl, C1-C6-haloalkylsulfinyl, C1-C6-alkylsulfonyl, C1-C6- haloalkylsulfonyl, C1-C6-alkylcarbonyl, C1-C6-haloalkylcarbonyl, C1-C6-alkoxycarbonyl and C1-C6-haloalkoxycarbonyl; more preferably from C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6-alkylthio, C1-C6-haloalkylthio, C1-C6-alkylsulfinyl, C1-C6-haloalkylsulfinyl, C1-C6- alkylsulfonyl and C1-C6-haloalkylsulfonyl; and in particular from cyano, C1-C6-alkoxy and C1-C6-haloalkoxy.
In the above preferred, more preferred and specific embodiments of RA, L1 is preferably a bond or -O-CH2-; and is in particular a bond. R5a and R5b, independently of each other, are preferably selected from hydrogen, halogen, cyano, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy, in particular from hydrogen, C1-C6-alkyl and C1-C6-alkoxy (especially from hydrogen, methyl and methoxy), specifically from hydrogen and C1-C6-alkyl, and are very specifically hydrogen or methyl. Each R6 is independently preferably selected from halogen, C1-C4-alkyl, C1-C4- haloalkyl, C1-C4-alkoxy and C1-C4-haloalkoxy. In particular, each R6 is independently selected from C1-C4-alkyl, and is specifically methyl.
k is preferably 0, 1 or 2, in particular 0 or 1; specifically 0. Each R6a is independently preferably selected from halogen, C1-C4-alkyl, C1-C4- haloalkyl, C1-C4-alkoxy and C1-C4-haloalkoxy; in particular from halogen and C1-C2- haloalkyl; specifically from Cl, Br and CF3, and is very specifically Cl.
In one preferred embodiment, X is O. In another preferred embodiment, X is S. In one preferred embodiment Y is N and Z is CR5b, where R5b has one of the above general or, in particular, one of the above preferred meanings. Preferably, Y is N and Z is CR5b, where R5b is selected from hydrogen, halogen, cyano, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy, in particular from hydrogen, C1-C6- alkyl and C1-C6-alkoxy (especially from hydrogen, methyl and methoxy), specifically from hydrogen and C1-C6-alkyl, and very specifically from hydrogen and methyl. In a preferred embodiment, Het is selected from rings of formulae Het-1 to Het-7:
Het-4 Het-5 Het-6 Het-7 wherein
# denotes the attachment point to the remainder of the molecule;
j is 0, 1 or 2; and
R6a has one of the above general or, in particular, one of the above preferred
Het-1 Het-2 Het-4 Het-7a wherein
# denotes the attachment point to the remainder of the molecule;
j is 0, 1 or 2; and
R6a has one of the above general or, in particular, one of the above preferred meanings. In particular, Het is selected from Het-1a, Het-4a and Het 7-aa:
Het-1a Het-4a Het-7aa wherein
# denotes the attachment point to the remainder of the molecule; and
R6a is halogen or C1-C2-haloalkyl, in particular Cl, Br or CF3, and is specifically Cl. Specifically, Het is selected from Het-1a and Het-4a, wherein R6a is halogen or C1-C2-haloalkyl, in particular Cl, Br or CF3, and is specifically Cl. In a preferred embodiment, the moiety of the formula W.Het
# denotes the attachment point to the remainder of the molecule;
R1, R2, R6, Het and X have one of the above general or, in particular, one of the above preferred meanings; and
each R6b is independently hydrogen or has one of the general or, in particular, one of the preferred meanings given above for R6, with the proviso that R6b is not halogen, azido, nitro, SCN or SF5;
each R6c has independently one of the general or, in particular, one of the preferred meanings given above for R6; and
k is 0, 1 or 2. In particular, R6b is selected from hydrogen, C1-C4-alkyl and C1-C4-haloalkyl, more particularly from hydrogen and C1-C4-alkyl, and specifically from hydrogen and methyl. In particular, R6c is selected from halogen, C1-C4-alkyl, C1-C4-haloalkyl, C1-C4- alkoxy and C1-C4-haloalkoxy, where k is 0, 1 or 2, in particular 0 or 1, specifically 0. More preferably, the moiety W-Het is selected from moieties of formulae W.Het-1, W.Het-2, W.Het-3, W.Het 4, W.Het 13 and W-Het-19, and in particular from W.Het-13 and W.Het-19. In W.Het-19, R6c is in particular methyl and k is 0, 1 or 2, in particular 0 or 1, specifically 0. Specifically, W-Het is W.Het 13. In a particular emb pound of formula I.1
(I.1)
Y1 is CH or N; and
X, R3, R4, R5b and R6a have one of the above general or, in particular, one of the above preferred meanings. In another particular embodiment, the compound of formula I is a compound of formula I.2
Y1 is CH or N;
X, R3, R4, R5b and R6a have one of the above general or, in particular, one of the above preferred meanings; and
R6c1 and R6c2, independently of each other, are hydrogen or have one of the general or, in particular, one of the preferred meanings given above for R6. In another particular embodiment, the compound of formula I is a compound of formula I.3
where X, R3, R4, R5b and R6a have one of the above general or, in particular, one of the above preferred meanings. In another particular embodiment, the compound of formula I is a compound of formula I.4
X, R3, R4, R5b and R6a have one of the above general or, in particular, one of the above preferred meanings; and
R6c1 and R6c2, independently of each other, are hydrogen or have one of the general or, in particular, one of the preferred meanings given above for R6. If not specified otherwise above, the variables R7, R7a, R8, R9a, R9b, R10, R11, R12, R13, R14, R16, R16, R17, R17a, R17b, R18 and R19 have following preferred meanings:
Prefeably, R7 and R19, independently of each other and independently of their occurrence, are selected from the group consisting of CN, C1-C6-alkoxy, C1-C6- haloalkoxy, C3-C6-cycloalkyl, C3-C6-halocycloalkyl, C1-C6-alkylthio, C1-C6-haloalkylthio, C1-C6-alkylsulfinyl, C1-C6-haloalkylsulfinyl, C1-C6-alkylsulfonyl, haloalkylsulfonyl, C1-C6- alkylcarbonyl, C1-C6-haloalkylcarbonyl, C1-C6-alkoxycarbonyl and C1-C6- haloalkoxycarbonyl.
Preferably, R7a and R15, independently of each other and independently of their occurrence, are selected from the group consisting of hydrogen, C1-C4-alkyl, C1-C4- haloalkyl, C3-C6-cycloalkyl, C3-C6-halocycloalkyl, C3-C6-cycloalkyl-C1-C4-alkyl, C3-C6- halocycloalkyl-C1-C4-alkyl, phenyl and benzyl, where the phenyl ring in the last two radicals is unsubstitued or substituted by 1, 2 or 3 radicals independently selected from the group consisting of halogen, CN, C1-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy and C1-C4-haloalkoxy.
Preferably R8 and R16, independently of each other and independently of their occurrence, are selected from the group consisting of hydrogen, C1-C4-alkyl, C1-C4- haloalkyl, C3-C6-cycloalkyl, C3-C6-halocycloalkyl, C3-C6-cycloalkyl-C1-C4-alkyl, C3-C6- halocycloalkyl-C1-C4-alkyl, phenyl and benzyl, where the phenyl ring in the last two radicals is unsubstitued or substituted by 1, 2 or 3 radicals independently selected from the group consisting of halogen, CN, C1-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy and C1-C4-haloalkoxy.
R9a, R9b, R17, R17a and R17b, independently of each other and independently of their occurrence, are preferably selected from the group consisting of hydrogen, C1-C4- alkyl, C1-C4-haloalkyl, C3-C6-cycloalkyl, C3-C6-halocycloalkyl, C3-C6-cycloalkyl-C1-C4- alkyl, C3-C6-halocycloalkyl-C1-C4-alkyl, phenyl and benzyl, where the phenyl ring in the last two radicals is unsubstitued or substituted by 1, 2 or 3 radicals independently selected from the group consisting of halogen, CN, C1-C4-alkyl, C1-C4-haloalkyl, C1-C4- alkoxy and C1-C4-haloalkoxy; or R9a and R9b, or R17a and R17b, in each case together with nitrogen atom they are bound to, form a saturated N-bound 3-, 4-, 5- or 6- membered heterocyclic ring, which in addition to the nitrogen atom may contain 1 further heteroatom selected from O and N as ring member, and where the N-bound 3-, 4-, 5- or 6-membered heterocyclic may be unsubstituted or carry 1, 2 or 3 radicals independently selected from halogen, CN, C1-C4-alkyl and C1-C4-haloalkyl.
Preferably each R10 is independently selected from the group consisting of halogen, CN, C1-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy and C1-C4-haloalkoxy.
Preferably, R11 and R12, independently of each other and independently of their occurrence, are selected from the group consisting of C1-C6-alkyl, C1-C6-alkoxy, C3-C8- cycloalkyl, C3-C8-cycloalkyl-C1-C4-alkyl, phenyl and benzyl, where the phenyl ring in last two radicals are unsubstituted or substituted with 1, 2, or 3 radicals selected from fluorine, chlorine, C1-C3-alkyl, C1-C2-haloalkyl, C1-C2-alkoxy and C1-C2-haloalkoxy.
Preferably, R13 and R14, independently of each other and independently of their occurrence, are selected from the group consisting of hydrogen, fluorine, chlorine, CN, C1-C4-alkyl, C3-C6-cycloalkyl and phenyl.
Preferably, each R18 is selected from the group consisting of C1-C4-alkyl and C1-C4-alkoxy. Examples of preferred compounds are compounds of the following formulae Ia.1 to Ia.70, where R3, R4, R5a, R5b and R6a, have one of the general or preferred meanings given above. Examples of preferred compounds are the individual compounds compiled in the tables 1 to 31500 below. Moreover, the meanings mentioned below for the individual variables in the tables are per se, independently of the combination in which they are mentioned, a particularly preferred embodiment of the substituents in question.
(Ia.23) (Ia.24)
(Ia.67) (Ia.68)
(Ia.69) (Ia.70) Table 1
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-H, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 2
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 3
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 4
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-CH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 5
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-CH(CH3)2, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 6
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-CH2CH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 7
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-C(CH3)3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 8
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-CH2Cl, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 9
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-(CH2)2Cl, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 10
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-(CH2)3Cl, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 11
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-(CH2)4Cl, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 12
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-CH2-CN, and the
combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 13
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-(CH2)2-CN, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 14
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-(CH2)3-CN, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 15
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-(CH2)4-CN, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 16
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-OCH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 17
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-OCH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 18
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-OCH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 19
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-OCH(CH3)2, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 20
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-SCH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 21
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-SCH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 22
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-SCH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 23
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-SCH(CH3)2, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 24
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-S(O)CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 25
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-S(O)CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 26
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-S(O)CH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 27
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-S(O)CH(CH3)2, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 28
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-S(O)2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 29
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-S(O)CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 30
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-S(O)2CH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 31
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-S(O)2CH(CH3)2,
and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 32
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-C(O)CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 33
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-C(O)CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 34
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-C(O)CH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 35
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-C(O)CH(CH3)2, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 36
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-C(O)OCH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 37
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-C(O)OCH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 38
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-C ^C-C(O)OCH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 39
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-C(O)OCH(CH3)2, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 40
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-CH2OCH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 41
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-CH2OCH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 42
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-CH2OCH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 43
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-CH2OCH(CH3)2, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 44
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -C ^C-CH2CH2OCH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 45
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-H, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 46
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 47
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 48
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-CH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 49
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-CH(CH3)2, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 50
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-CH2CH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 51
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-C(CH3)3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 52
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-CH2Cl, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 53
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-(CH2)2Cl, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 54
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-(CH2)3Cl, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 55
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-(CH2)4Cl, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 56
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-CH2-CN, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 57
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-(CH2)2-CN, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 58
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-(CH2)3-CN, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 59
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-(CH2)4-CN, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 60
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-OCH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 61
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-OCH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 62
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
OCH2-C ^C-OCH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 63
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-OCH(CH3)2, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 64
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-SCH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 65
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-SCH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 66
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-SCH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 67
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-SCH(CH3)2, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 68
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-S(O)CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 69
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-S(O)CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 70
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-S(O)CH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 71
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-S(O)CH(CH3)2, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 72
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-S(O)2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 73
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-S(O)CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 74
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-S(O)2CH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 75
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-S(O)2CH(CH3)2, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 76
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-C(O)CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 77
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-C(O)CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 78
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-C(O)CH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 79
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-C(O)CH(CH3)2, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 80
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -OCH2-C ^C-C(O)OCH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 81
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-C(O)OCH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 82
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-C(O)OCH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 83
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-C(O)OCH(CH3)2, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 84
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-CH2OCH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 85
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-CH2OCH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 86
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-CH2OCH2CH2CH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 87
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-CH2OCH(CH3)2, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 88
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is
-OCH2-C ^C-CH2CH2OCH3, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 89
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -CN, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Table 90
Compounds of the formula Ia.1 in which R6a is hydrogen, R4 is -CH2-CN, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 91 to 180
Compounds of the formula Ia.1 in which R6a is F, R4 has one of the meanings given in tables 1 to 90, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 181 to 270
Compounds of the formula Ia.1 in which R6a is Cl, R4 has one of the meanings given in tables 1 to 90, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 271 to 360
Compounds of the formula Ia.1 in which R6a is Br, R4 has one of the meanings given in tables 1 to 90, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 361 to 450
Compounds of the formula Ia.1 in which R6a is CF3, R4 has one of the meanings given in tables 1 to 90, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 451 to 900
Compounds of the formula Ia.2 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 901 to 1350
Compounds of the formula Ia.3 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 1351 to 1800
Compounds of the formula Ia.4 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 1801 to 2250
Compounds of the formula Ia.5 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 2251 to 2700
Compounds of the formula Ia.6 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 2701 to 3150
Compounds of the formula Ia.7 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 3151 to 3600
Compounds of the formula Ia.8 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 3601 to 4050
Compounds of the formula Ia.9 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 4051 to 4500
Compounds of the formula Ia.10 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 4501 to 4950
Compounds of the formula Ia.11 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 4951 to 5400
Compounds of the formula Ia.12 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 5401 to 5850
Compounds of the formula Ia.13 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 5851 to 6300
Compounds of the formula Ia.14 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 6301 to 6750
Compounds of the formula Ia.15 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 6751 to 7200
Compounds of the formula Ia.16 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 7201 to 7650
Compounds of the formula Ia.17 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 7651 to 8100
Compounds of the formula Ia.18 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 8101 to 8550
Compounds of the formula Ia.19 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 8551 to 9000
Compounds of the formula Ia.20 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 9001 to 9450
Compounds of the formula Ia.21 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 9451 to 9900
Compounds of the formula Ia.22 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 9901 to 10350
Compounds of the formula Ia.23 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 10351 to 10800
Compounds of the formula Ia.24 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 10801 to 11250
Compounds of the formula Ia.25 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 11251 to 11700
Compounds of the formula Ia.26 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 11701 to 12150
Compounds of the formula Ia.27 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 12151 to 12600
Compounds of the formula Ia.28 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 12601 to 13050
Compounds of the formula Ia.29 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 13051 to 13500
Compounds of the formula Ia.30 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 13501 to 13950
Compounds of the formula Ia.31 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 13951 to 14400
Compounds of the formula Ia.32 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 14401 to 14850
Compounds of the formula Ia.33 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 14851 to 15300
Compounds of the formula Ia.34 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 15301 to 15750
Compounds of the formula Ia.35 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 15751 to 16200
Compounds of the formula Ia.36 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 16201 to 16650
Compounds of the formula Ia.37 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 16651 to 17100
Compounds of the formula Ia.38 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 17101 to 17550
Compounds of the formula Ia.39 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 17551 to 18000
Compounds of the formula Ia.40 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 18001 to 18450
Compounds of the formula Ia.41 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 18451 to 18900
Compounds of the formula Ia.42 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 18901 to 19350
Compounds of the formula Ia.43 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 19351 to 19800
Compounds of the formula Ia.44 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 19801 to 20250
Compounds of the formula Ia.45 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 20251 to 20700
Compounds of the formula Ia.46 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 20701 to 21150
Compounds of the formula Ia.47 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 21151 to 21600
Compounds of the formula Ia.48 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 21601 to 22050
Compounds of the formula Ia.49 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 22051 to 22500
Compounds of the formula Ia.50 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 22501 to 22950
Compounds of the formula Ia.51 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 22951 to 23400
Compounds of the formula Ia.52 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5b for a compound corresponds in each case to one row of Table A
Tables 23401 to 23850
Compounds of the formula Ia.53 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 23851 to 24300
Compounds of the formula Ia.54 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 24301 to 24750
Compounds of the formula Ia.55 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 24751 to 25200
Compounds of the formula Ia.56 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3, R5a and R5b for a compound corresponds in each case to one row of Table B
Tables 25201 to 25650
Compounds of the formula Ia.57 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 25651 to 26100
Compounds of the formula Ia.58 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 26101 to 26550
Compounds of the formula Ia.59 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 26551 to 27000
Compounds of the formula Ia.60 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 27001 to 27450
Compounds of the formula Ia.61 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 27451 to 27900
Compounds of the formula Ia.62 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 27901 to 28350
Compounds of the formula Ia.63 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 28351 to 28800
Compounds of the formula Ia.64 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 28801 to 29250
Compounds of the formula Ia.65 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 29251 to 29700
Compounds of the formula Ia.66 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 29701 to 30150
Compounds of the formula Ia.67 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 30151 to 30600
Compounds of the formula Ia.68 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 30601 to 31050
Compounds of the formula Ia.69 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Tables 31051 to 31500
Compounds of the formula Ia.70 in which R6a and R4 have one of the meanings given in tables 1 to 450, and the combination of R3 and R5a for a compound corresponds in each case to one row of Table A
Among the above compounds, preference is given to compounds Ia.1, Ia.2, Ia.3 and Ia.4, and especially to compounds Ia.1. In a specific embodiment, the compounds I are selected from the compounds specified in the examples, either as a free base or in form of an agriculturally or veterinarily acceptable salt, an N-oxide or a stereoisomer thereof. The compounds of the formula (I) can be prepared by the methods as described in the below schemes or and in the synthesis descriptions of the working examples, or by standard methods of organic chemistry. The substituents, variables and indices are as defined above for formula (I), if not otherwise specified.
The definitions of A, Het, X, Y, Z, R1, R2, R3, R4, R6 and k of the molecular structures given in the schemes are as defined above. Room temperature means a temperature range of from ca.20 to 25 °C.
An examples of a general method for the preparation of compounds of formula (I) is shown below in Scheme A. Thus, construction of the heterocyclic element 3 present in compounds of formula (I) can be achieved, for example, by alkylation of the appropriate 2-amino heterocycle precursor 1 with the appropriate reagent of formula 2. The transformation is preferably carried out in polar solvents such as acetonitrile, acetone, dichloromethane, 1,4-dioxane, tetrahydrofuran, N,N-dimethylformamide, N,N-dimethylacetamide, N-methylpyrrolidinone or a C1-C6-alcohol ranging between room temperature and the reflux temperature of the solvent. Representative reaction conditions for the alkylation of compounds analogous to formula 1 are given in
Tetrahedron Lett.2011, 52(23), 3033-3037. The synthesis of compounds of formula I can be achieved by acylation of the amine functionality in compounds of formula 3 using carboxylic acid derivatives 4 which are activated in situ. The transformation is preferably carried out in polar solvents such as acetonitrile, acetone, 1,4-dioxane, tetrahydrofuran, N,N-dimethylformamide, N,N-dimethylacetamide, N- methylpyrrolidinone or in an inert solvent such as dichloromethane, 1,2-dichloroethane, or 1,2-dimethoxyethane at temperatures ranging between room temperature and the reflux temperature of the solvent. A representative procedure condition for the acylation is given in Journal of Medicinal Chemistry, 2012, 55, 7378-7391. Examples of suitable leaving groups (LG) in formula 2 include, but are not limited to: halogen, alkyl sulfonate, haloalkyl sulfonate, aryl sulfonate, alkyl phosphonate. Examples of suitable leaving groups (LG2) in formula 4 include, but are not limited to: halogen, alkyl sulfonate, haloalkyl sulfonate, aryl sulfonate, alkyl phosphonate and various activated esters derived from the reaction of a free carboxylic acid with a peptide coupling reagent in the presence of an amine base; representative reaction conditions can be found in the following review, and the references cited therein: Chem. Rev., 2011, 111 (11), 6557- 6602. A reversal of the order of these two steps would also result in an acceptable synthesis of the desired compounds. Scheme A:
In cases where X is a sulfur atom, the sulfur atom is best installed in a subsequent step from the compound where X is an oxygen atom as detailed in scheme B.
Scheme B.
Here the carbonyl compound I' (compound I wherein X = O) is transformed into the corresponding thiocarbonyl compound I'' (compound I wherein X = S) The transformation is preferably using a reagent of substructure 7 in polar solvents such as acetonitrile, acetone, tetrahydrofuran, N,N-dimethylformamide, or in an inert solvent such as dichloromethane, 1,2-dichloroethane, or 1,2-dimethoxyethane at temperatures ranging between room temperature and the reflux temperature of the solvent. Suitable Ra groups for 7 are: thio, alkyl, aryl or substituted aryl. For example, Lawesson's reagent can be used (Ra = 4-methoxyphenyl). Representative reaction conditions for the thionation of analogous substrates are given in European Journal of Organic Chemistry, 2000, 3273-3278, and Chemical Reviews, 2007, 107, 5210-5278.
As a rule, the compounds of formula I including their stereoisomers, salts, and N- oxides, and their precursors in the synthesis process, can be prepared by the methods described above. If individual compounds can not be prepared via the above-described routes, they can be prepared by derivatization of other compounds I or the respective precursor or by customary modifications of the synthesis routes described. For example, in individual cases, certain compounds of formula (I) can advantageously be prepared from other compounds of formula (I) by derivatization, e.g. by ester hydrolysis, amidation, esterification, ether cleavage, olefination, reduction, oxidation and the like, or by customary modifications of the synthesis routes described.
The reaction mixtures are worked up in the customary manner, for example by mixing with water, separating the phases, and, if appropriate, purifying the crude products by chromatography, for example on alumina or on silica gel. Some of the intermediates and end products may be obtained in the form of colorless or pale brown viscous oils which are freed or purified from volatile components under reduced pressure and at moderately elevated temperature. If the intermediates and end products are obtained as solids, they may be purified by recrystallization or trituration. Due to their excellent activity, the compounds of the present invention may be used for controlling invertebrate pests.
Accordingly, the present invention also provides a method for combatting or controlling invertebrate pests which method comprises treating the pests, their food supply, their habitat or their breeding ground or a cultivated plant, plant propagation materials (such as seed), soil, area, material or environment in which the pests are growing or may grow, or the materials, cultivated plants, plant propagation materials (such as seed), soils, surfaces or spaces to be protected from pest attack or infestation with a pesticidally effective amount of a compound of the present invention or a composition as defined above. The invention also relates to the use of a compound of the invention, of a stereoisomer and/or of an agriculturally or veterinarily acceptable salt thereof for combating invertebrate pests
Preferably, the method of the invention serves for protecting plant propagation material (such as seed) and the plant which grows therefrom from invertebrate pest attack or infestation and comprises treating the plant propagation material (such as seed) with a pesticidally effective amount of a compound of the present invention as defined above or with a pesticidally effective amount of an agricultural composition as defined above and below. The method of the invention is not limited to the protection of the "substrate" (plant, plant propagation materials, soil material etc.) which has been treated according to the invention, but also has a preventive effect, thus, for example, according protection to a plant which grows from a treated plant propagation materials (such as seed), the plant itself not having been treated.
Alternatively preferably, the method of the invention serves for protecting plants from attack or infestation by invertebrate pests, which method comprises treating the plants with a pesticidally effective amount of at least one compound of the invention, a stereoisomer thereof and/or at least one agriculturally acceptable salt thereof.
In the sense of the present invention, "invertebrate pests" are preferably selected from arthropods, gastropods and nematodes, more preferably from harmful insects, arachnids and nematodes, and even more preferably from insects, acarids and nematodes. In the sense of the present invention, "invertebrate pests" are most preferably insects.
The invention further provides an agricultural composition for combating invertebrate pests, which comprises such an amount of at least one compound according to the invention and at least one inert liquid and/or solid agronomically acceptable carrier that has a pesticidal action and, if desired, at least one surfactant. Such a composition may comprise a single active compound of the present invention or a mixture of several active compounds of the present invention. The composition according to the present invention may comprise an individual isomer or mixtures of isomers or a salt as well as individual tautomers or mixtures of tautomers. Pests:
The compounds of the present invention, including their salts, stereoisomers and tautomers, are in particular suitable for efficiently controlling animal pests such as arthropods, gastropods and nematodes including but not limited to:
insects from the order of Lepidoptera, for example Achroia grisella, Acleris spp. such as A. fimbriana, A. gloverana, A. variana; Acrolepiopsis assectella, Acronicta major, Adoxophyes spp. such as A. cyrtosema, A. orana; Aedia leucomelas, Agrotis spp. such as A. exclamationis, A. fucosa, A. ipsilon, A. orthogoma, A. segetum, A. subterranea; Alabama argillacea, Aleurodicus dispersus, Alsophila pometaria, Ampelophaga rubiginosa, Amyelois transitella, Anacampsis sarcitella, Anagasta kuehniella, Anarsia lineatella, Anisota senatoria, Antheraea pernyi, Anticarsia (=Thermesia) spp. such as A. gemmatalis; Apamea spp., Aproaerema modicella, Archips spp. such as A.
argyrospila, A. fuscocupreanus, A. rosana, A. xyloseanus; Argyresthia conjugella, Argyroploce spp., Argyrotaenia spp. such as A. velutinana; Athetis mindara,
Austroasca viridigrisea, Autographa gamma, Autographa nigrisigna, Barathra brassicae, Bedellia spp., Bonagota salubricola, Borbo cinnara, Bucculatrix thurberiella, Bupalus piniarius, Busseola spp., Cacoecia spp. such as C. murinana, C. podana; Cactoblastis cactorum, Cadra cautella, Calingo braziliensis, Caloptilis theivora, Capua reticulana, Carposina spp. such as C. niponensis, C. sasakii; Cephus spp.,
Chaetocnema aridula, Cheimatobia brumata, Chilo spp. such as C. Indicus, C.
suppressalis, C. partellus; Choreutis pariana, Choristoneura spp. such as C.
conflictana, C. fumiferana, C. longicellana, C. murinana, C. occidentalis, C. rosaceana; Chrysodeixis (=Pseudoplusia) spp. such as C. eriosoma, C. includens; Cirphis unipuncta, Clysia ambiguella, Cnaphalocerus spp., Cnaphalocrocis medinalis,
Cnephasia spp., Cochylis hospes, Coleophora spp., Colias eurytheme, Conopomorpha spp., Conotrachelus spp., Copitarsia spp., Corcyra cephalonica, Crambus
caliginosellus, Crambus teterrellus, Crocidosema (=Epinotia) aporema, Cydalima (=Diaphania) perspectalis, Cydia (=Carpocapsa) spp. such as C. pomonella, C.
latiferreana; Dalaca noctuides, Datana integerrima, Dasychira pinicola, Dendrolimus spp. such as D. pini, D. spectabilis, D. sibiricus; Desmia funeralis, Diaphania spp. such as D. nitidalis, D. hyalinata; Diatraea grandiosella, Diatraea saccharalis, Diphthera festiva, Earias spp. such as E. insulana, E. vittella; Ecdytolopha aurantianu, Egira (=Xylomyges) curialis, Elasmopalpus lignosellus, Eldana saccharina, Endopiza viteana, Ennomos subsignaria, Eoreuma loftini, Ephestia spp. such as E. cautella, E. elutella, E. kuehniella; Epinotia aporema, Epiphyas postvittana, Erannis tiliaria, Erionota thrax, Etiella spp., Eulia spp., Eupoecilia ambiguella, Euproctis chrysorrhoea, Euxoa spp., Evetria bouliana, Faronta albilinea, Feltia spp. such as F. subterranean; Galleria mellonella, Gracillaria spp., Grapholita spp. such as G. funebrana, G. molesta, G.
inopinata; Halysidota spp., Harrisina americana, Hedylepta spp., Helicoverpa spp. such as H. armigera (=Heliothis armigera), H. zea (=Heliothis zea); Heliothis spp. such as H.
assulta, H. subflexa, H. virescens; Hellula spp. such as H. undalis, H. rogatalis;
Helocoverpa gelotopoeon, Hemileuca oliviae, Herpetogramma licarsisalis, Hibernia defoliaria, Hofmannophila pseudospretella, Homoeosoma electellum, Homona magnanima, Hypena scabra, Hyphantria cunea, Hyponomeuta padella, Hyponomeuta malinellus, Kakivoria flavofasciata, Keiferia lycopersicella, Lambdina fiscellaria fiscellaria, Lambdina fiscellaria lugubrosa, Lamprosema indicata, Laspeyresia molesta, Leguminivora glycinivorella, Lerodea eufala, Leucinodes orbonalis, Leucoma salicis, Leucoptera spp. such as L. coffeella, L. scitella; Leuminivora lycinivorella, Lithocolletis blancardella, Lithophane antennata, Llattia octo (=Amyna axis), Lobesia botrana, Lophocampa spp., Loxagrotis albicosta, Loxostege spp. such as L. sticticalis, L.
cereralis; Lymantria spp. such as L. dispar, L. monacha; Lyonetia clerkella, Lyonetia prunifoliella, Malacosoma spp. such as M. americanum, M. californicum, M.
constrictum, M. neustria; Mamestra spp. such as M. brassicae, M. configurata;
Mamstra brassicae, Manduca spp. such as M. quinquemaculata, M. sexta; Marasmia spp, Marmara spp., Maruca testulalis, Megalopyge lanata, Melanchra picta, Melanitis leda, Mocis spp. such as M. lapites, M. repanda; Mocis latipes, Monochroa fragariae, Mythimna separata, Nemapogon cloacella, Neoleucinodes elegantalis, Nepytia spp., Nymphula spp., Oiketicus spp., Omiodes indicata, Omphisa anastomosalis,
Operophtera brumata, Orgyia pseudotsugata, Oria spp., Orthaga thyrisalis, Ostrinia spp. such as O. nubilalis; Oulema oryzae, Paleacrita vernata, Panolis flammea, Parnara spp., Papaipema nebris, Papilio cresphontes, Paramyelois transitella, Paranthrene regalis, Paysandisia archon, Pectinophora spp. such as P. gossypiella; Peridroma saucia, Perileucoptera spp., such as P. coffeella; Phalera bucephala, Phryganidia californica, Phthorimaea spp. such as P. operculella; Phyllocnistis citrella, Phyllonorycter spp. such as P. blancardella, P. crataegella, P. issikii, P. ringoniella; Pieris spp. such as P. brassicae, P. rapae, P. napi; Pilocrocis tripunctata, Plathypena scabra, Platynota spp. such as P. flavedana, P. idaeusalis, P. stultana; Platyptilia carduidactyla, Plebejus argus, Plodia interpunctella, Plusia spp, Plutella maculipennis, Plutella xylostella, Pontia protodica, Prays spp., Prodenia spp., Proxenus lepigone, Pseudaletia spp. such as P. sequax, P. unipuncta; Pyrausta nubilalis, Rachiplusia nu, Richia albicosta, Rhizobius ventralis, Rhyacionia frustrana, Sabulodes aegrotata, Schizura concinna, Schoenobius spp., Schreckensteinia festaliella, Scirpophaga spp. such as S. incertulas, S. innotata; Scotia segetum, Sesamia spp. such as S. inferens, Seudyra subflava, Sitotroga cerealella, Sparganothis pilleriana, Spilonota lechriaspis, S. ocellana, Spodoptera (=Lamphygma) spp. such as S. cosmoides, S. eridania, S. exigua, S. frugiperda, S. latisfascia, S. littoralis, S. litura, S. omithogalli; Stigmella spp., Stomopteryx subsecivella, Strymon bazochii, Sylepta derogata, Synanthedon spp. such as S. exitiosa, Tecia solanivora, Telehin licus, Thaumatopoea pityocampa, Thaumatotibia (=Cryptophlebia) leucotreta, Thaumetopoea pityocampa, Thecla spp.,
Theresimima ampelophaga, Thyrinteina spp, Tildenia inconspicuella, Tinea spp. such as T. cloacella, T. pellionella; Tineola bisselliella, Tortrix spp. such as T. viridana;
Trichophaga tapetzella, Trichoplusia spp. such as T. ni; Tuta (=Scrobipalpula) absoluta, Udea spp. such as U. rubigalis, U. rubigalis; Virachola spp., Yponomeuta padella, and Zeiraphera canadensis;
insects from the order of Coleoptera, for example Acalymma vittatum,
Acanthoscehdes obtectus, Adoretus spp., Agelastica alni, Agrilus spp. such as A. anxius, A. planipennis, A. sinuatus; Agriotes spp. such as A. fuscicollis, A. lineatus, A. obscurus; Alphitobius diaperinus, Amphimallus solstitialis, Anisandrus dispar, Anisoplia austriaca, Anobium punctatum, Anomala corpulenta, Anomala rufocuprea,
Anoplophora spp. such as A. glabripennis; Anthonomus spp. such as A. eugenii, A. grandis, A. pomorum; Anthrenus spp., Aphthona euphoridae, Apion spp., Apogonia spp., Athous haemorrhoidalis, Atomaria spp. such as A. linearis; Attagenus spp., Aulacophora femoralis, Blastophagus piniperda, Blitophaga undata, Bruchidius obtectus, Bruchus spp. such as B. lentis, B. pisorum, B. rufimanus; Byctiscus betulae, Callidiellum rufipenne, Callopistria floridensis, Callosobruchus chinensis, Cameraria ohridella, Cassida nebulosa, Cerotoma trifurcata, Cetonia aurata, Ceuthorhynchus spp. such as C. assimilis, C. napi; Chaetocnema tibialis, Cleonus mendicus, Conoderus spp. such as C. vespertinus; Conotrachelus nenuphar, Cosmopolites spp., Costelytra zealandica, Crioceris asparagi, Cryptolestes ferrugineus, Cryptorhynchus lapathi, Ctenicera spp. such as C. destructor; Curculio spp., Cylindrocopturus spp.,
Cyclocephala spp., Dactylispa balyi, Dectes texanus, Dermestes spp., Diabrotica spp. such as D. undecimpunctata, D. speciosa, D. longicornis, D. semipunctata, D. virgifera; Diaprepes abbreviates, Dichocrocis spp., Dicladispa armigera, Diloboderus abderus, Diocalandra frumenti (Diocalandra stigmaticollis), Enaphalodes rufulus, Epilachna spp. such as E. varivestis, E. vigintioctomaculata; Epitrix spp. such as E. hirtipennis, E. similaris; Eutheola humilis, Eutinobothrus brasiliensis, Faustinus cubae, Gibbium psylloides, Gnathocerus cornutus, Hellula undalis, Heteronychus arator, Hylamorpha elegans, Hylobius abietis, Hylotrupes bajulus, Hypera spp. such as H. brunneipennis, H. postica; Hypomeces squamosus, Hypothenemus spp., Ips typographus,
Lachnosterna consanguinea, Lasioderma serricorne, Latheticus oryzae, Lathridius spp., Lema spp. such as L. bilineata, L. melanopus; Leptinotarsa spp. such as L.
decemlineata; Leptispa pygmaea, Limonius californicus, Lissorhoptrus oryzophilus, Lixus spp., Luperodes spp., Lyctus spp. such as L. bruneus; Liogenys fuscus,
Macrodactylus spp. such as M. subspinosus; Maladera matrida, Megaplatypus mutates, Megascelis spp., Melanotus communis, Meligethes spp. such as M. aeneus; Melolontha spp. such as M. hippocastani, M. melolontha; Metamasius hemipterus, Microtheca spp., Migdolus spp. such as M. fryanus, Monochamus spp. such as M. alternatus; Naupactus xanthographus, Niptus hololeucus, Oberia brevis, Oemona hirta,
Oryctes rhinoceros, Oryzaephilus surinamensis, Oryzaphagus oryzae, Otiorrhynchus sulcatus, Otiorrhynchus ovatus, Otiorrhynchus sulcatus, Oulema melanopus, Oulema oryzae, Oxycetonia jucunda, Phaedon spp. such as P. brassicae, P. cochleariae;
Phoracantha recurva, Phyllobius pyri, Phyllopertha horticola, Phyllophaga spp. such as P. helleri; Phyllotreta spp. such as P. chrysocephala, P. nemorum, P. striolata, P.
vittula; Phyllopertha horticola, Popillia japonica, Premnotrypes spp., Psacothea hilaris, Psylliodes chrysocephala, Prostephanus truncates, Psylliodes spp., Ptinus spp., Pulga saltona, Rhizopertha dominica, Rhynchophorus spp. such as R. billineatus, R.
ferrugineus, R. palmarum, R. phoenicis, R. vulneratus; Saperda candida, Scolytus schevyrewi, Scyphophorus acupunctatus, Sitona lineatus, Sitophilus spp. such as S. granaria, S. oryzae, S. zeamais; Sphenophorus spp. such as S. levis; Stegobium paniceum, Sternechus spp. such as S. subsignatus; Strophomorphus ctenotus, Symphyletes spp., Tanymecus spp., Tenebrio molitor, Tenebrioides mauretanicus, Tribolium spp. such as T. castaneum; Trogoderma spp., Tychius spp., Xylotrechus spp. such as X. pyrrhoderus; and, Zabrus spp. such as Z. tenebrioides;
insects from the order of Diptera for example Aedes spp. such as A. aegypti, A.
albopictus, A. vexans; Anastrepha ludens, Anopheles spp. such as A. albimanus, A. crucians, A. freeborni, A. gambiae, A. leucosphyrus, A. maculipennis, A. minimus, A. quadrimaculatus, A. sinensis; Bactrocera invadens, Bibio hortulanus, Calliphora erythrocephala, Calliphora vicina, Ceratitis capitata, Chrysomyia spp. such as C.
bezziana, C. hominivorax, C. macellaria; Chrysops atlanticus, Chrysops discalis, Chrysops silacea, Cochliomyia spp. such as C. hominivorax; Contarinia spp. such as C. sorghicola; Cordylobia anthropophaga, Culex spp. such as C. nigripalpus, C.
pipiens, C. quinquefasciatus, C. tarsalis, C. tritaeniorhynchus; Culicoides furens, Culiseta inornata, Culiseta melanura, Cuterebra spp., Dacus cucurbitae, Dacus oleae, Dasineura brassicae, Dasineura oxycoccana, Delia spp. such as D. antique, D.
coarctata, D. platura, D. radicum; Dermatobia hominis, Drosophila spp. such as D. suzukii, Fannia spp. such as F. canicularis; Gastraphilus spp. such as G. intestinalis; Geomyza tipunctata, Glossina spp. such as G. fuscipes, G. morsitans, G. palpalis, G. tachinoides; Haematobia irritans, Haplodiplosis equestris, Hippelates spp., Hylemyia spp. such as H. platura; Hypoderma spp. such as H. lineata; Hyppobosca spp., Hydrellia philippina, Leptoconops torrens, Liriomyza spp. such as L. sativae, L. trifolii; Lucilia spp. such as L. caprina, L. cuprina, L. sericata; Lycoria pectoralis, Mansonia titillanus, Mayetiola spp. such as M. destructor; Musca spp. such as M. autumnalis, M. domestica; Muscina stabulans, Oestrus spp. such as O. ovis; Opomyza florum, Oscinella spp. such as O. frit; Orseolia oryzae, Pegomya hysocyami, Phlebotomus argentipes, Phorbia spp. such as P. antiqua, P. brassicae, P. coarctata; Phytomyza gymnostoma, Prosimulium mixtum, Psila rosae, Psorophora columbiae, Psorophora discolor, Rhagoletis spp. such as R. cerasi, R. cingulate, R. indifferens, R. mendax, R.
pomonella; Rivellia quadrifasciata, Sarcophaga spp. such as S. haemorrhoidalis;
Simulium vittatum, Sitodiplosis mosellana, Stomoxys spp. such as S. calcitrans;
Tabanus spp. such as T. atratus, T. bovinus, T. lineola, T. similis; Tannia spp., Thecodiplosis japonensis, Tipula oleracea, Tipula paludosa, and Wohlfahrtia spp; insects from the order of Thysanoptera for example, Baliothrips biformis,
Dichromothrips corbetti, Dichromothrips ssp., Echinothrips americanus, Enneothrips flavens, Frankliniella spp. such as F. fusca, F. occidentalis, F. tritici; Heliothrips spp., Hercinothrips femoralis, Kakothrips spp., Microcephalothrips abdominalis,
Neohydatothrips samayunkur, Pezothrips kellyanus, Rhipiphorothrips cruentatus, Scirtothrips spp. such as S. citri, S. dorsalis, S. perseae; Stenchaetothrips spp, Taeniothrips cardamoni, Taeniothrips inconsequens, Thrips spp. such as T. imagines, T. hawaiiensis, T. oryzae, T. palmi, T. parvispinus, T. tabaci;
insects from the order of Hemiptera for example, Acizzia jamatonica, Acrosternum spp. such as A. hilare; Acyrthosipon spp. such as A. onobrychis, A. pisum; Adelges laricis, Adelges tsugae, Adelphocoris spp., such as A. rapidus, A. superbus;
Aeneolamia spp., Agonoscena spp., Aulacorthum solani, Aleurocanthus woglumi, Aleurodes spp., Aleurodicus disperses, Aleurolobus barodensis, Aleurothrixus spp., Amrasca spp., Anasa tristis, Antestiopsis spp., Anuraphis cardui, Aonidiella spp., Aphanostigma piri, Aphidula nasturtii, Aphis spp. such as A. craccivora, A. fabae, A. forbesi, A. gossypii, A. grossulariae, A. maidiradicis, A. pomi, A. sambuci, A.
schneideri, A. spiraecola; Arboridia apicalis, Arilus critatus, Aspidiella spp., Aspidiotus spp., Atanus spp., Aulacaspis yasumatsui, Aulacorthum solani, Bactericera cockerelli (Paratrioza cockerelli), Bemisia spp. such as B. argentifolii, B. tabaci (Aleurodes tabaci); Blissus spp. such as B. leucopterus; Brachycaudus spp. such as B. cardui, B. helichrysi, B. persicae, B. prunicola; Brachycolus spp., Brachycorynella asparagi, Brevicoryne brassicae, Cacopsylla spp. such as C. fulguralis, C. pyricola (Psylla piri); Calligypona marginata, Calocoris spp., Campylomma livida, Capitophorus horni, Carneocephala fulgida, Cavelerius spp., Ceraplastes spp., Ceratovacuna lanigera, Ceroplastes ceriferus, Cerosipha gossypii, Chaetosiphon fragaefolii, Chionaspis tegalensis, Chlorita onukii, Chromaphis juglandicola, Chrysomphalus ficus, Cicadulina mbila, Cimex spp. such as C. hemipterus, C. lectularius; Coccomytilus halli, Coccus spp. such as C. hesperidum, C. pseudomagnoliarum; Corythucha arcuata, Creontiades dilutus, Cryptomyzus ribis, Chrysomphalus aonidum, Cryptomyzus ribis, Ctenarytaina spatulata, Cyrtopeltis notatus, Dalbulus spp., Dasynus piperis, Dialeurodes spp. such as D. citrifolii; Dalbulus maidis, Diaphorina spp. such as D. citri; Diaspis spp. such as D. bromeliae; Dichelops furcatus, Diconocoris hewetti, Doralis spp., Dreyfusia nordmannianae, Dreyfusia piceae, Drosicha spp., Dysaphis spp. such as D.
plantaginea, D. pyri, D. radicola; Dysaulacorthum pseudosolani, Dysdercus spp. such as D. cingulatus, D. intermedius; Dysmicoccus spp., Edessa spp., Geocoris spp.,
Empoasca spp. such as E. fabae, E. solana; Epidiaspis leperii, Eriosoma spp. such as E. lanigerum, E. pyricola; Erythroneura spp., Eurygaster spp. such as E. integriceps; Euscelis bilobatus, Euschistus spp. such as E. heros, E. impictiventris, E. servus; Fiorinia theae, Geococcus coffeae, Glycaspis brimblecombei, Halyomorpha spp. such as H. halys; Heliopeltis spp., Homalodisca vitripennis (=H. coagulata), Horcias nobilellus, Hyalopterus pruni, Hyperomyzus lactucae, Icerya spp. such as I. purchase; Idiocerus spp., Idioscopus spp., Laodelphax striatellus, Lecanium spp., Lecanoideus floccissimus, Lepidosaphes spp. such as L. ulmi; Leptocorisa spp., Leptoglossus phyllopus, Lipaphis erysimi, Lygus spp. such as L. hesperus, L. lineolaris, L. pratensis; Maconellicoccus hirsutus, Marchalina hellenica, Macropes excavatus, Macrosiphum spp. such as M. rosae, M. avenae, M. euphorbiae; Macrosteles quadrilineatus, Mahanarva fimbriolata, Megacopta cribraria, Megoura viciae, Melanaphis pyrarius, Melanaphis sacchari, Melanocallis (=Tinocallis) caryaefoliae, Metcafiella spp.,
Metopolophium dirhodum, Monellia costalis, Monelliopsis pecanis, Myzocallis coryli, Murgantia spp., Myzus spp. such as M. ascalonicus, M. cerasi, M. nicotianae, M.
persicae, M. varians; Nasonovia ribis-nigri, Neotoxoptera formosana, Neomegalotomus spp, Nephotettix spp. such as N. malayanus, N. nigropictus, N. parvus, N. virescens; Nezara spp. such as N. viridula; Nilaparvata lugens, Nysius huttoni, Oebalus spp. such as O. pugnax; Oncometopia spp., Orthezia praelonga, Oxycaraenus hyalinipennis, Parabemisia myricae, Parlatoria spp., Parthenolecanium spp. such as P. corni, P. persicae; Pemphigus spp. such as P. bursarius, P. populivenae; Peregrinus maidis, Perkinsiella saccharicida, Phenacoccus spp. such as P. aceris, P. gossypii;
Phloeomyzus passerinii, Phorodon humuli, Phylloxera spp. such as P. devastatrix, Piesma quadrata, Piezodorus spp. such as P. guildinii; Pinnaspis aspidistrae,
Planococcus spp. such as P. citri, P. ficus; Prosapia bicincta, Protopulvinaria pyriformis, Psallus seriatus, Pseudacysta persea, Pseudaulacaspis pentagona, Pseudococcus spp. such as P. comstocki; Psylla spp. such as P. mali; Pteromalus spp., Pulvinaria amygdali, Pyrilla spp., Quadraspidiotus spp., such as Q. perniciosus; Quesada gigas, Rastrococcus spp., Reduvius senilis, Rhizoecus americanus,
Rhodnius spp., Rhopalomyzus ascalonicus, Rhopalosiphum spp. such as R.
pseudobrassicas, R. insertum, R. maidis, R. padi; Sagatodes spp., Sahlbergella singularis, Saissetia spp., Sappaphis mala, Sappaphis mali, Scaptocoris spp.,
Scaphoides titanus, Schizaphis graminum, Schizoneura lanuginosa, Scotinophora spp., Selenaspidus articulatus, Sitobion avenae, Sogata spp., Sogatella furcifera, Solubea insularis, Spissistilus festinus (=Stictocephala festina), Stephanitis nashi, Stephanitis pyrioides, Stephanitis takeyai, Tenalaphara malayensis, Tetraleurodes perseae, Therioaphis maculate, Thyanta spp. such as T. accerra, T. perditor; Tibraca spp., Tomaspis spp., Toxoptera spp. such as T. aurantii; Trialeurodes spp. such as T.
abutilonea, T. ricini, T. vaporariorum; Triatoma spp., Trioza spp., Typhlocyba spp., Unaspis spp. such as U. citri, U. yanonensis; and Viteus vitifolii,
Insects from the order Hymenoptera for example Acanthomyops interjectus, Athalia rosae, Atta spp. such as A. capiguara, A. cephalotes, A. cephalotes, A. laevigata, A. robusta, A. sexdens, A. texana, Bombus spp., Brachymyrmex spp., Camponotus spp. such as C. floridanus, C. pennsylvanicus, C. modoc; Cardiocondyla nuda, Chalibion sp, Crematogaster spp., Dasymutilla occidentalis, Diprion spp., Dolichovespula maculata, Dorymyrmex spp., Dryocosmus kuriphilus, Formica spp., Hoplocampa spp. such as H. minuta, H. testudinea; Iridomyrmex humilis, Lasius spp. such as L. niger, Linepithema humile, Liometopum spp., Leptocybe invasa, Monomorium spp. such as M. pharaonis, Monomorium, Nylandria fulva, Pachycondyla chinensis, Paratrechina longicornis, Paravespula spp., such as P. germanica, P. pennsylvanica, P. vulgaris; Pheidole spp. such as P. megacephala; Pogonomyrmex spp. such as P. barbatus, P. californicus, Polistes rubiginosa, Prenolepis impairs, Pseudomyrmex gracilis, Schelipron spp., Sirex cyaneus, Solenopsis spp. such as S. geminata, S.invicta, S. molesta, S. richteri, S. xyloni, Sphecius speciosus, Sphex spp., Tapinoma spp. such as T. melanocephalum, T. sessile; Tetramorium spp. such as T. caespitum, T. bicarinatum, Vespa spp. such as V. crabro; Vespula spp. such as V. squamosal; Wasmannia auropunctata, Xylocopa sp;
Insects from the order Orthoptera for example Acheta domesticus, Calliptamus italicus, Chortoicetes terminifera, Ceuthophilus spp., Diastrammena asynamora, Dociostaurus maroccanus, Gryllotalpa spp. such as G. africana, G. gryllotalpa; Gryllus spp., Hieroglyphus daganensis, Kraussaria angulifera, Locusta spp. such as L.
migratoria, L. pardalina; Melanoplus spp. such as M. bivittatus, M. femurrubrum, M. mexicanus, M. sanguinipes, M. spretus; Nomadacris septemfasciata, Oedaleus senegalensis, Scapteriscus spp., Schistocerca spp. such as S. americana, S. gregaria, Stemopelmatus spp., Tachycines asynamorus, and Zonozerus variegatus;
Pests from the Class Arachnida for example Acari,e.g. of the families Argasidae, Ixodidae and Sarcoptidae, such as Amblyomma spp. (e.g. A. americanum, A.
variegatum, A. maculatum), Argas spp. such as A. persicu), Boophilus spp. such as B. annulatus, B. decoloratus, B. microplus, Dermacentor spp. such as D.silvarum, D. andersoni, D. variabilis, Hyalomma spp. such as H. truncatum, Ixodes spp. such as I. ricinus, I. rubicundus, I. scapularis, I. holocyclus, I. pacificus, Rhipicephalus
sanguineus, Ornithodorus spp. such as O. moubata, O. hermsi, O. turicata,
Ornithonyssus bacoti, Otobius megnini, Dermanyssus gallinae, Psoroptes spp. such as P. ovis, Rhipicephalus spp. such as R. sanguineus, R. appendiculatus, Rhipicephalus evertsi, Rhizoglyphus spp., Sarcoptes spp. such asS. Scabiei; and Family Eriophyidae including Aceria spp. such as A. sheldoni, A. anthocoptes, Acallitus spp., Aculops spp. such as A. lycopersici, A. pelekassi; Aculus spp. such as A. schlechtendali; Colomerus
vitis, Epitrimerus pyri, Phyllocoptruta oleivora; Eriophytes ribis and Eriophyes spp. such as Eriophyes sheldoni; Family Tarsonemidae including Hemitarsonemus spp., Phytonemus pallidus and Polyphagotarsonemus latus, Stenotarsonemus spp.
Steneotarsonemus spinki; Family Tenuipalpidae including Brevipalpus spp. such as B. phoenicis; Family Tetranychidae including Eotetranychus spp., Eutetranychus spp., Oligonychus spp., Petrobia latens, Tetranychus spp. such as T. cinnabarinus, T.
evansi, T. kanzawai, T, pacificus, T. phaseulus, T. telarius and T. urticae; Bryobia praetiosa; Panonychus spp. such as P. ulmi, P. citri; Metatetranychus spp. and Oligonychus spp. such as O. pratensis, O. perseae, Vasates lycopersici; Raoiella indica, Family Carpoglyphidae including Carpoglyphus spp.; Penthaleidae spp. such as Halotydeus destructor; Family Demodicidae with species such as Demodex spp.; Family Trombicidea including Trombicula spp.; Family Macronyssidae including Ornothonyssus spp.; Family Pyemotidae including Pyemotes tritici; Tyrophagus putrescentiae; Family Acaridae including Acarus siro; Family Araneida including Latrodectus mactans, Tegenaria agrestis, Chiracanthium sp, Lycosa sp Achaearanea tepidariorum and Loxosceles reclusa;
Pests from the Phylum Nematoda, for example, plant parasitic nematodes such as root-knot nematodes, Meloidogyne spp. such as M. hapla, M. incognita, M. javanica; cyst-forming nematodes, Globodera spp. such as G. rostochiensis; Heterodera spp. such as H. avenae, H. glycines, H. schachtii, H. trifolii; Seed gall nematodes, Anguina spp.; Stem and foliar nematodes, Aphelenchoides spp. such as A. besseyi; Sting nematodes, Belonolaimus spp. such as B. longicaudatus; Pine nematodes,
Bursaphelenchus spp. such as B. lignicolus, B. xylophilus; Ring nematodes, Criconema spp., Criconemella spp. such as C. xenoplax and C. ornata; and, Criconemoides spp. such as Criconemoides informis; Mesocriconema spp.; Stem and bulb nematodes, Ditylenchus spp. such as D. destructor, D. dipsaci; Awl nematodes, Dolichodorus spp.; Spiral nematodes, Heliocotylenchus multicinctus; Sheath and sheathoid nematodes, Hemicycliophora spp. and Hemicriconemoides spp.; Hirshmanniella spp.; Lance nematodes, Hoploaimus spp.; False rootknot nematodes, Nacobbus spp.; Needle nematodes, Longidorus spp. such as L. elongatus; Lesion nematodes, Pratylenchus spp. such as P. brachyurus, P. neglectus, P. penetrans, P. curvitatus, P. goodeyi; Burrowing nematodes, Radopholus spp. such as R. similis; Rhadopholus spp.;
Rhodopholus spp.; Reniform nematodes, Rotylenchus spp. such as R. robustus, R. reniformis; Scutellonema spp.; Stubby-root nematode, Trichodorus spp. such as T. obtusus, T. primitivus; Paratrichodorus spp. such as P. minor; Stunt nematodes, Tylenchorhynchus spp. such as T. claytoni, T. dubius; Citrus nematodes, Tylenchulus spp. such as T. semipenetrans; Dagger nematodes, Xiphinema spp.; and other plant parasitic nematode species;
Insects from the order Isoptera for example Calotermes flavicollis, Coptotermes spp. such as C. formosanus, C. gestroi, C. acinaciformis; Cornitermes cumulans,
Cryptotermes spp. such as C. brevis, C. cavifrons; Globitermes sulfureus,
Heterotermes spp. such as H. aureus, H. longiceps, H. tenuis; Leucotermes flavipes, Odontotermes spp., Incisitermes spp. such as I. minor, I. Snyder; Marginitermes hubbardi, Mastotermes spp. such as M. darwiniensis Neocapritermes spp. such as N. opacus, N. parvus; Neotermes spp., Procornitermes spp., Zootermopsis spp. such as Z. angusticollis, Z. nevadensis, Reticulitermes spp. such as R. hesperus, R. tibialis, R. speratus, R. flavipes, R. grassei, R. lucifugus, R. santonensis, R. virginicus; Termes natalensis,
Insects from the order Blattaria for example Blatta spp. such as B. orientalis, B.
lateralis; Blattella spp. such as B. asahinae, B. germanica; Leucophaea maderae, Panchlora nivea, Periplaneta spp. such as P. americana, P. australasiae, P. brunnea, P. fuligginosa, P. japonica; Supella longipalpa, Parcoblatta pennsylvanica, Eurycotis floridana, Pycnoscelus surinamensis,
Insects from the order Siphonoptera for example Cediopsylla simples, Ceratophyllus spp., Ctenocephalides spp. such as C. felis, C. canis, Xenopsylla cheopis, Pulex irritans, Trichodectes canis, Tunga penetrans, and Nosopsyllus fasciatus,
Insects from the order Thysanura for example Lepisma saccharina , Ctenolepisma urbana, and Thermobia domestica,
Pests from the class Chilopoda for example Geophilus spp., Scutigera spp. such as Scutigera coleoptrata;
Pests from the class Diplopoda for example Blaniulus guttulatus, Julus spp., Narceus spp.,
Pests from the class Symphyla for example Scutigerella immaculata,
Insects from the order Dermaptera, for example Forficula auricularia,
Insects from the order Collembola, for example Onychiurus spp., such as Onychiurus armatus,
Pests from the order Isopoda for example, Armadillidium vulgare, Oniscus asellus, Porcellio scaber,
Insects from the order Phthiraptera, for example Damalinia spp., Pediculus spp. such as Pediculus humanus capitis, Pediculus humanus corporis, Pediculus humanus humanus; Pthirus pubis, Haematopinus spp. such as Haematopinus eurysternus, Haematopinus suis; Linognathus spp. such as Linognathus vituli; Bovicola bovis, Menopon gallinae, Menacanthus stramineus and Solenopotes capillatus, Trichodectes spp.,
Examples of further pest species which may be controlled by compounds of fomula (I) include: from the Phylum Mollusca, class Bivalvia, for example, Dreissena spp.; class Gastropoda, for example, Arion spp., Biomphalaria spp., Bulinus spp., Deroceras spp.,
Galba spp., Lymnaea spp., Oncomelania spp., Pomacea canaliclata, Succinea spp.; from the class of the helminths, for example, Ancylostoma duodenale, Ancylostoma ceylanicum, Acylostoma braziliensis, Ancylostoma spp., Ascaris lubricoides, Ascaris spp., Brugia malayi, Brugia timori, Bunostomum spp., Chabertia spp., Clonorchis spp., Cooperia spp., Dicrocoelium spp., Dictyocaulus filaria, Diphyllobothrium latum, Dracunculus medinensis, Echinococcus granulosus, Echinococcus multilocularis, Enterobius vermicularis, Faciola spp., Haemonchus spp. such as Haemonchus contortus; Heterakis spp., Hymenolepis nana, Hyostrongulus spp., Loa Loa,
Nematodirus spp., Oesophagostomum spp., Opisthorchis spp., Onchocerca volvulus, Ostertagia spp., Paragonimus spp., Schistosomen spp., Strongyloides fuelleborni, Strongyloides stercora lis, Stronyloides spp., Taenia saginata, Taenia solium,
Trichinella spiralis, Trichinella nativa, Trichinella britovi, Trichinella nelsoni, Trichinella pseudopsiralis, Trichostrongulus spp., Trichuris trichuria, Wuchereria bancrofti. Formulations
The invention also relates to agrochemical compositions comprising an auxiliary and at least one compound of the present invention or a mixture thereof.
An agrochemical composition comprises a pesticidally effective amount of a compound of the present invention or a mixture thereof. The term "pesticidally effective amount" is defined below.
The compounds of the present invention or the mixtures thereof can be converted into customary types of agrochemical compositions, e. g. solutions, emulsions, suspensions, dusts, powders, pastes, granules, pressings, capsules, and mixtures thereof. Examples for composition types are suspensions (e.g. SC, OD, FS), emulsifiable concentrates (e.g. EC), emulsions (e.g. EW, EO, ES, ME), capsules (e.g. CS, ZC), pastes, pastilles, wettable powders or dusts (e.g. WP, SP, WS, DP, DS), pressings (e.g. BR, TB, DT), granules (e.g. WG, SG, GR, FG, GG, MG), insecticidal articles (e.g. LN), as well as gel formulations for the treatment of plant propagation materials such as seeds (e.g. GF). These and further compositions types are defined in the "Catalogue of pesticide formulation types and international coding system", Technical Mono-graph No.2, 6th Ed. May 2008, CropLife International.
The compositions are prepared in a known manner, such as described by Mollet and Grubemann, Formulation technology, Wiley VCH, Weinheim, 2001; or Knowles, New developments in crop protection product formulation, Agrow Reports DS243, T&F Informa, London, 2005.
Examples for suitable auxiliaries are solvents, liquid carriers, solid carriers or fillers, surfatants, dispersants, emulsifiers, wetters, adjuvants, solubilizers, penetration enhancers, protective colloids, adhesion agents, thickeners, humectants, repellents,
attractants, feeding stimulants, compatibilizers, bactericides, anti-freezing agents, anti- foaming agents, colorants, tackifiers and binders.
Suitable solvents and liquid carriers are water and organic solvents, such as mineral oil fractions of medium to high boiling point, e.g. kerosene, diesel oil; oils of vegetable or animal origin; aliphatic, cyclic and aromatic hydrocarbons, e. g. toluene, paraffin, tetrahydronaphthalene, alkylated naphthalenes; alcohols, e.g. ethanol, propanol, butanol, benzylalcohol, cyclo¬hexanol; glycols; DMSO; ketones, e.g.
cyclohexanone; esters, e.g. lactates, carbonates, fatty acid esters, gamma- butyrolactone; fatty acids; phosphonates; amines; amides, e.g. N-methylpyrrolidone, fatty acid dimethylamides; and mixtures thereof.
Suitable solid carriers or fillers are mineral earths, e.g. silicates, silica gels, talc, kaolins, limestone, lime, chalk, clays, dolomite, diatomaceous earth, bentonite, calcium sulfate, magnesium sulfate, magnesium oxide; polysaccharide powders, e.g. cellulose, starch; fertilizers, e.g. ammonium sulfate, ammonium phosphate, ammonium nitrate, ureas; products of vegetable origin, e.g. cereal meal, tree bark meal, wood meal, nutshell meal, and mixtures thereof.
Suitable surfactants are surface-active compounds, such as anionic, cationic, nonionic and amphoteric surfactants, block polymers, polyelectrolytes, and mixtures thereof. Such surfactants can be used as emusifier, dispersant, solubilizer, wetter, penetration enhancer, protective colloid, or adjuvant. Examples of surfactants are listed in McCutcheon's, Vol.1: Emulsifiers & Detergents, McCutcheon's Directories, Glen Rock, USA, 2008 (International Ed. or North American Ed.).
Suitable anionic surfactants are alkali, alkaline earth or ammonium salts of sulfonates, sulfates, phosphates, carboxylates, and mixtures thereof. Examples of sulfonates are alkylarylsulfonates, diphenylsulfonates, alpha-olefin sulfonates, lignine sulfonates, sulfonates of fatty acids and oils, sulfonates of ethoxylated alkylphenols, sulfonates of alkoxylated arylphenols, sulfonates of condensed naphthalenes, sulfonates of dodecyl- and tridecylbenzenes, sulfonates of naphthalenes and alkyl¬naphthalenes, sulfosuccinates or sulfosuccinamates. Examples of sulfates are sulfates of fatty acids and oils, of ethoxylated alkylphenols, of alcohols, of ethoxylated alcohols, or of fatty acid esters. Examples of phosphates are phosphate esters.
Examples of carboxylates are alkyl carboxylates, and carboxylated alcohol or alkylphenol ethoxylates.
Suitable nonionic surfactants are alkoxylates, N-substituted fatty acid amides, amine oxides, esters, sugar-based surfactants, polymeric surfactants, and mixtures thereof. Examples of alkoxylates are compounds such as alcohols, alkylphenols, amines, amides, arylphenols, fatty acids or fatty acid esters which have been alkoxylated with 1 to 50 equivalents. Ethylene oxide and/or propylene oxide may be employed for the alkoxylation, preferably ethylene oxide. Examples of N-subsititued
fatty acid amides are fatty acid glucamides or fatty acid alkanolamides. Examples of esters are fatty acid esters, glycerol esters or monoglycerides. Examples of sugar- based surfactants are sorbitans, ethoxylated sorbitans, sucrose and glucose esters or alkylpolyglucosides. Examples of polymeric surfactants are homo- or copolymers of vinylpyrrolidone, vinylalcohols, or vinylacetate.
Suitable cationic surfactants are quaternary surfactants, for example quaternary ammonium compounds with one or two hydrophobic groups, or salts of long-chain primary amines. Suitable amphoteric surfactants are alkylbetains and imidazolines. Suitable block polymers are block polymers of the A-B or A-B-A type comprising blocks of polyethylene oxide and polypropylene oxide, or of the A-B-C type comprising alkanol, polyethylene oxide and polypropylene oxide. Suitable polyelectrolytes are polyacids or polybases. Examples of polyacids are alkali salts of polyacrylic acid or polyacid comb polymers. Examples of polybases are polyvinylamines or
polyethyleneamines.
Suitable adjuvants are compounds, which have a neglectable or even no pesticidal activity themselves, and which improve the biological performance of the compounds of the present invention on the target. Examples are surfactants, mineral or vegetable oils, and other auxilaries. Further examples are listed by Knowles, Adjuvants and additives, Agrow Reports DS256, T&F Informa UK, 2006, chapter 5.
Suitable thickeners are polysaccharides (e.g. xanthan gum,
carboxymethylcellulose), anorganic clays (organically modified or unmodified), polycarboxylates, and silicates.
Suitable bactericides are bronopol and isothiazolinone derivatives such as alkylisothiazolinones and benzisothiazolinones.
Suitable anti-freezing agents are ethylene glycol, propylene glycol, urea and glycerin.
Suitable anti-foaming agents are silicones, long chain alcohols, and salts of fatty acids.
Suitable colorants (e.g. in red, blue, or green) are pigments of low water solubility and water-soluble dyes. Examples are inorganic colorants (e.g. iron oxide, titan oxide, iron hexacyanoferrate) and organic colorants (e.g. alizarin-, azo- and phthalocyanine colorants).
Suitable tackifiers or binders are polyvinylpyrrolidons, polyvinylacetates, polyvinyl alcohols, polyacrylates, biological or synthetic waxes, and cellulose ethers.
Examples for composition types and their preparation are:
i) Water-soluble concentrates (SL, LS)
10-60 wt% of a compound I according to the invention and 5-15 wt% wetting agent (e.g. alcohol alkoxylates) are dissolved in water and/or in a water-soluble solvent (e.g. alcohols) up to 100 wt%. The active substance dissolves upon dilution with water.
ii) Dispersible concentrates (DC)
5-25 wt% of a compound I according to the invention and 1-10 wt% dispersant (e. g. polyvinylpyrrolidone) are dissolved in up to 100 wt% organic solvent (e.g.
cyclohexanone). Dilution with water gives a dispersion.
iii) Emulsifiable concentrates (EC)
15-70 wt% of a compound I according to the invention and 5-10 wt% emulsifiers (e.g. calcium dodecylbenzenesulfonate and castor oil ethoxylate) are dissolved in up to 100 wt% water-insoluble organic solvent (e.g. aromatic hydrocarbon). Dilution with water gives an emulsion.
iv) Emulsions (EW, EO, ES)
5-40 wt% of a compound I according to the invention and 1-10 wt% emulsifiers (e.g. calcium dodecylbenzenesulfonate and castor oil ethoxylate) are dissolved in 20-40 wt% water-insoluble organic solvent (e.g. aromatic hydrocarbon). This mixture is introduced into up to 100 wt% water by means of an emulsifying machine and made into a homogeneous emulsion. Dilution with water gives an emulsion.
v) Suspensions (SC, OD, FS)
In an agitated ball mill, 20-60 wt% of a compound I according to the invention are comminuted with addition of 2-10 wt% dispersants and wetting agents (e.g. sodium lignosulfonate and alcohol ethoxylate), 0,1-2 wt% thickener (e.g. xanthan gum) and up to 100 wt% water to give a fine active substance suspension. Dilution with water gives a stable suspension of the active substance. For FS type composition up to 40 wt% binder (e.g. polyvinylalcohol) is added.
vi) Water-dispersible granules and water-soluble granules (WG, SG)
50-80 wt% of a compound I according to the invention are ground finely with addition of up to 100 wt% dispersants and wetting agents (e.g. sodium lignosulfonate and alcohol ethoxylate) and prepared as water-dispersible or water-soluble granules by means of technical appliances (e. g. extrusion, spray tower, fluidized bed). Dilution with water gives a stable dispersion or solution of the active substance.
vii) Water-dispersible powders and water-soluble powders (WP, SP, WS) 50-80 wt% of a compound I according to the invention are ground in a rotor-stator mill with addition of 1-5 wt% dispersants (e.g. sodium lignosulfonate), 1-3 wt% wetting agents (e.g. alcohol ethoxylate) and up to 100 wt% solid carrier, e.g. silica gel. Dilution with water gives a stable dispersion or solution of the active substance.
viii) Gel (GW, GF)
In an agitated ball mill, 5-25 wt% of a compound I according to the invention are comminuted with addition of 3-10 wt% dispersants (e.g. sodium lignosulfonate), 1-5 wt% thickener (e.g. carboxymethylcellulose) and up to 100 wt% water to give a fine suspension of the active substance. Dilution with water gives a stable suspension of the active substance.
ix) Microemulsion (ME)
5-20 wt% of a compound I according to the invention are added to 5-30 wt% organic solvent blend (e.g. fatty acid dimethylamide and cyclohexanone), 10-25 wt% surfactant blend (e.g. alkohol ethoxylate and arylphenol ethoxylate), and water up to 100 %. This mixture is stirred for 1 h to produce spontaneously a thermodynamically stable microemulsion.
x) Microcapsules (CS)
An oil phase comprising 5-50 wt% of a compound I according to the invention, 0-40 wt% water insoluble organic solvent (e.g. aromatic hydrocarbon), 2-15 wt% acrylic monomers (e.g. methylmethacrylate, methacrylic acid and a di- or triacrylate) are dispersed into an aqueous solution of a protective colloid (e.g. polyvinyl alcohol).
Radical polymerization initiated by a radical initiator results in the formation of poly(meth)acrylate microcapsules. Alternatively, an oil phase comprising 5-50 wt% of a compound I according to the invention, 0-40 wt% water insoluble organic solvent (e.g. aromatic hydrocarbon), and an isocyanate monomer (e.g. diphenylmethene-4,4'- diisocyanatae) are dispersed into an aqueous solution of a protective colloid (e.g.
polyvinyl alcohol). The addition of a polyamine (e.g. hexamethylenediamine) results in the formation of a polyurea microcapsule. The monomers amount to 1-10 wt%. The wt% relate to the total CS composition.
xi) Dustable powders (DP, DS)
1-10 wt% of a compound I according to the invention are ground finely and mixed intimately with up to 100 wt% solid carrier, e.g. finely divided kaolin.
xii) Granules (GR, FG)
0.5-30 wt% of a compound I according to the invention is ground finely and associated with up to 100 wt% solid carrier (e.g. silicate). Granulation is achieved by extrusion, spray-drying or the fluidized bed.
xiii) Ultra-low volume liquids (UL)
1-50 wt% of a compound I according to the invention are dissolved in up to 100 wt% organic solvent, e.g. aromatic hydrocarbon.
The compositions types i) to xi) may optionally comprise further auxiliaries, such as 0.1-1 wt% bactericides, 5-15 wt% anti-freezing agents, 0.1-1 wt% anti-foaming agents, and 0.1-1 wt% colorants.
The agrochemical compositions generally comprise between 0.01 and 95%, preferably between 0.1 and 90%, and most preferably between 0.5 and 75%, by weight of active substance. The active substances are employed in a purity of from 90% to 100%, preferably from 95% to 100% (according to NMR spectrum).
Various types of oils, wetters, adjuvants, fertilizer, or micronutrients, and other pesticides (e.g. herbicides, insecticides, fungicides, growth regulators, safeners) may be added to the active substances or the compositions com¬prising them as premix or,
if appropriate not until immediately prior to use (tank mix). These agents can be admixed with the compositions according to the invention in a weight ratio of 1:100 to 100:1, preferably 1:10 to 10:1.
The user applies the composition according to the invention usually from a predosage device, a knapsack sprayer, a spray tank, a spray plane, or an irrigation system. Usually, the agrochemical composition is made up with water, buffer, and/or further auxiliaries to the desired application concentration and the ready-to-use spray liquor or the agrochemical composition according to the invention is thus obtained. Usually, 20 to 2000 liters, preferably 50 to 400 liters, of the ready-to-use spray liquor are applied per hectare of agricultural useful area.
According to one embodiment, individual components of the composition according to the invention such as parts of a kit or parts of a binary or ternary mixture may be mixed by the user himself in a spray tank and further auxiliaries may be added, if appropriate.
In a further embodiment, either individual components of the composition according to the invention or partially premixed components, e. g. components comprising compounds of the present invention and/or mixing partners as defined above or below, may be mixed by the user in a spray tank and further auxiliaries and additives may be added, if appropriate.
In a further embodiment, either individual components of the composition according to the invention or partially premixed components, e. g. components comprising compounds of the present invention and/or mixing partners as defined above or below, can be applied jointly (e.g. after tank mix) or consecutively. Application methods
The compounds of the present invention are suitable for use in protecting crops, plants, plant propagation materials, such as seeds, or soil or water, in which the plants are growing, from attack or infestation by animal pests. Therefore, the present invention also relates to a plant protection method, which comprises contacting crops, plants, plant propagation materials, such as seeds, or soil or water, in which the plants are growing, to be protected from attack or infestation by animal pests, with a pesticidally effective amount of a compound of the present invention.
The compounds of the present invention are also suitable for use in combating or controlling animal pests. Therefore, the present invention also relates to a method of combating or controlling animal pests, which comprises contacting the animal pests, their habitat, breeding ground, or food supply, or the crops, plants, plant propagation materials, such as seeds, or soil, or the area, material or environment in which the animal pests are growing or may grow, with a pesticidally effective amount of a compound of the present invention.
The compounds of the present invention are effective through both contact and ingestion. Furthermore, the compounds of the present invention can be applied to any and all developmental stages, such as egg, larva, pupa, and adult.
The compounds of the present invention can be applied as such or in form of compositions comprising them as defined above. Furthermore, the compounds of the present invention can be applied together with a mixing partner as defined above or in form of compositions comprising said mixtures as defined above. The components of said mixture can be applied simultaneously, jointly or separately, or in succession, that is immediately one after another and thereby creating the mixture“ in situ” on the desired location, e.g. the plant, the sequence, in the case of separate application, generally not having any effect on the result of the control measures.
The application can be carried out both before and after the infestation of the crops, plants, plant propagation materials, such as seeds, soil, or the area, material or environment by the pests.
Suitable application methods include inter alia soil treatment, seed treatment, in furrow application, and foliar application. Soil treatment methods include drenching the soil, drip irrigation (drip application onto the soil), dipping roots, tubers or bulbs, or soil injection. Seed treatment techniques include seed dressing, seed coating, seed dusting, seed soaking, and seed pelleting. In furrow applications typically include the steps of making a furrow in cultivated land, seeding the furrow with seeds, applying the pesticidally active compound to the furrow, and closing the furrow. Foliar application refers to the application of the pesticidally active compound to plant foliage, e.g.
through spray equipment. For foliar applications, it can be advantageous to modify the behavior of the pests by use of pheromones in combination with the compounds of the present invention. Suitable pheromones for specific crops and pests are known to a skilled person and publicly available from databases of pheromones and
semiochemicals, such as http://www.pherobase.com.
As used herein, the term "contacting" includes both direct contact (applying the compounds/compositions directly on the animal pest or plant - typically to the foliage, stem or roots of the plant) and indirect contact (applying the compounds/compositions to the locus, i.e. habitat, breeding ground, plant, seed, soil, area, material or environment in which a pest is growing or may grow, of the animal pest or plant).
The term "animal pest" includes arthropods, gastropods, and nematodes.
Preferred animal pests according to the invention are arthropods, preferably insects and arachnids, in particular insects. Insects, which are of particular relevance for crops, are typically referred to as crop insect pests.
The term "crop" refers to both, growing and harvested crops.
The term "plant" includes cereals, e.g. durum and other wheat, rye, barley, triticale, oats, rice, or maize (fodder maize and sugar maize / sweet and field corn);
beet, e.g. sugar beet or fodder beet; fruits, such as pomes, stone fruits or soft fruits, e.g. apples, pears, plums, peaches, nectarines, almonds, cherries, papayas, strawberries, raspberries, blackberries or gooseberries; leguminous plants, such as beans, lentils, peas, alfalfa or soybeans; oil plants, such as rapeseed (oilseed rape), turnip rape, mustard, olives, sunflowers, coconut, cocoa beans, castor oil plants, oil palms, ground nuts or soybeans; cucurbits, such as squashes, pumpkins, cucumber or melons; fiber plants, such as cotton, flax, hemp or jute; citrus fruit, such as oranges, lemons, grapefruits or mandarins; vegetables, such as eggplant, spinach, lettuce (e.g. iceberg lettuce), chicory, cabbage, asparagus, cabbages, carrots, onions, garlic, leeks, tomatoes, potatoes, cucurbits or sweet peppers; lauraceous plants, such as avocados, cinnamon or camphor; energy and raw material plants, such as corn, soybean, rapeseed, sugar cane or oil palm; tobacco; nuts, e.g. walnuts; pistachios; coffee; tea; bananas; vines (table grapes and grape juice grape vines); hop; sweet leaf (also called Stevia); natural rubber plants or ornamental and forestry plants, such as flowers (e.g. carnation, petunias, geranium/pelargoniums, pansies and impatiens), shrubs, broad- leaved trees (e.g. poplar) or evergreens, e.g. conifers; eucalyptus; turf; lawn; grass such as grass for animal feed or ornamental uses. Preferred plants include potatoes sugar beets, tobacco, wheat, rye, barley, oats, rice, corn, cotton, soybeans, rapeseed, legumes, sunflowers, coffee or sugar cane; fruits; vines; ornamentals; or vegetables, such as cucumbers, tomatoes, beans or squashes.
The term "plant" is to be understood as including wild type plants and plants which have been modified by either conventional breeding or mutagenesis or genetic engineering, or by a combination thereof.
Plants, which have been modified by mutagenesis or genetic engineering, and are of particular commercial importance, include alfalfa, rapeseed (e.g. oilseed rape), bean, carnation, chicory, cotton, eggplant, eucalyptus, flax, lentil, maize, melon, papaya, petunia, plum, poplar, potato, rice, soybean, squash, sugar beet, sugarcane, sunflower, sweet pepper, tobacco, tomato, and cereals (e.g. wheat), in particular maize, soybean, cotton, wheat, and rice. In plants, which have been modified by mutagenesis or genetic engineering, one or more genes have been mutagenized or integrated into the genetic material of the plant. The one or more mutagenized or integrated genes are preferably selected from pat, epsps, cry1Ab, bar, cry1Fa2, cry1Ac, cry34Ab1, cry35AB1, cry3A, cryF, cry1F, mcry3a, cry2Ab2, cry3Bb1, cry1A.105, dfr, barnase, vip3Aa20, barstar, als, bxn, bp40, asn1, and ppo5. The mutagenesis or integration of the one or more genes is performed in order to improve certain properties of the plant. Such properties, also known as traits, include abiotic stress tolerance, altered growth/yield, disease resistance, herbicide tolerance, insect resistance, modified product quality, and pollination control. Of these properties, herbicide tolerance, e.g. imidazolinone tolerance, glyphosate tolerance, or glufosinate
tolerance, is of particular importance. Several plants have been rendered tolerant to herbicides by mutagenesis, for example Clearfield® oilseed rape being tolerant to imidazolinones, e.g. imazamox. Alternatively, genetic engineering methods have been used to render plants, such as soybean, cotton, corn, beets and oil seed rape, tolerant to herbicides, such as glyphosate and glufosinate, some of which are commercially available under the trade names RoundupReady® (glyphosate) and LibertyLink® (glufosinate). Furthermore, insect resistance is of importance, in particular lepidopteran insect resistance and coleopteran insect resistance. Insect resistance is typically achieved by modifying plants by integrating cry and/or vip genes, which were isolated from Bacillus thuringiensis (Bt), and code for the respective Bt toxins. Genetically modified plants with insect resistance are commercially available under trade names including WideStrike®, Bollgard®, Agrisure®, Herculex®, YieldGard®, Genuity®, and Intacta®. Plants may be modified by mutagenesis or genetic engineering either in terms of one property (singular traits) or in terms of a combination of properties (stacked traits). Stacked traits, e.g. the combination of herbicide tolerance and insect resistance, are of increasing importance. In general, all relevant modified plants in connection with singular or stacked traits as well as detailed information as to the mutagenized or integrated genes and the respective events are available from websites of the organizations "International Service for the Acquisition of Agribiotech Applications (ISAAA)" (http://www.isaaa.org/gmapprovaldatabase) and "Center for Environmental Risk Assessment (CERA)" (http://cera-gmc.org/GMCropDatabase). It has surprisingly been found that the pesticidal activity of the compounds of the present invention may be enhanced by the insecticidal trait of a modified plant.
Furthermore, it has been found that the compounds of the present invention are suitable for preventing insects to become resistant to the insecticidal trait or for combating pests, which already have become resistant to the insecticidal trait of a modified plant. Moreover, the compounds of the present invention are suitable for combating pests, against which the insecticidal trait is not effective, so that a complementary insecticidal activity can advantageously be used.
The term "plant propagation material" refers to all the generative parts of the plant such as seeds and vegetative plant material such as cuttings and tubers (e.g. potatoes), which can be used for the multiplication of the plant. This includes seeds, roots, fruits, tubers, bulbs, rhizomes, shoots, sprouts and other parts of plants.
Seedlings and young plants, which are to be transplanted after germination or after emergence from soil, may also be included. These plant propagation materials may be treated prophylactically with a plant protection compound either at or before planting or transplanting.
The term "seed" embraces seeds and plant propagules of all kinds including but not limited to true seeds, seed pieces, suckers, corms, bulbs, fruit, tubers, grains, cuttings, cut shoots and the like, and means in a preferred embodiment true seeds.
In general, "pesticidally effective amount" means the amount of active ingredient needed to achieve an observable effect on growth, including the effects of necrosis, death, retardation, prevention, and removal, destruction, or otherwise diminishing the occurrence and activity of the target organism. The pesticidally effective amount can vary for the various compounds/compositions used in the invention. A pesticidally effective amount of the compositions will also vary according to the prevailing conditions such as desired pesticidal effect and duration, weather, target species, locus, mode of application, and the like.
In the case of soil treatment, in furrow application or of application to the pests dwelling place or nest, the quantity of active ingredient ranges from 0.0001 to 500 g per 100 m2, preferably from 0.001 to 20 g per 100 m2.
For use in treating crop plants, e.g. by foliar application, the rate of application of the active ingredients of this invention may be in the range of 0.0001 g to 4000 g per hectare, e.g. from 1 g to 2 kg per hectare or from 1 g to 750 g per hectare, desirably from 1 g to 100 g per hectare, more desirably from 10 g to 50 g per hectare, e.g., 10 to 20 g per hectare, 20 to 30 g per hectare, 30 to 40 g per hectare, or 40 to 50 g per hectare. The compounds of the present invention are particularly suitable for use in the treatment of seeds in order to protect the seeds from insect pests, in particular from soil-living insect pests, and the resulting seedling's roots and shoots against soil pests and foliar insects. The present invention therefore also relates to a method for the protection of seeds from insects, in particular from soil insects, and of the seedling's roots and shoots from insects, in particular from soil and foliar insects, said method comprising treating the seeds before sowing and/or after pregermination with a compound of the present invention. The protection of the seedling's roots and shoots is preferred. More preferred is the protection of seedling’ s shoots from piercing and sucking insects, chewing insects and nematodes.
The term "seed treatment" comprises all suitable seed treatment techniques known in the art, such as seed dressing, seed coating, seed dusting, seed soaking, seed pelleting, and in-furrow application methods. Preferably, the seed treatment application of the active compound is carried out by spraying or by dusting the seeds before sowing of the plants and before emergence of the plants.
The present invention also comprises seeds coated with or containing the active compound. The term "coated with and/or containing" generally signifies that the active ingredient is for the most part on the surface of the propagation product at the time of
application, although a greater or lesser part of the ingredient may penetrate into the propagation product, depending on the method of application. When the said propagation product is (re)planted, it may absorb the active ingredient.
Suitable seed is for example seed of cereals, root crops, oil crops, vegetables, spices, ornamentals, for example seed of durum and other wheat, barley, oats, rye, maize (fodder maize and sugar maize / sweet and field corn), soybeans, oil crops, crucifers, cotton, sunflowers, bananas, rice, oilseed rape, turnip rape, sugarbeet, fodder beet, eggplants, potatoes, grass, lawn, turf, fodder grass, tomatoes, leeks, pumpkin/squash, cabbage, iceberg lettuce, pepper, cucumbers, melons, Brassica species, melons, beans, peas, garlic, onions, carrots, tuberous plants such as potatoes, sugar cane, tobacco, grapes, petunias, geranium/pelargoniums, pansies and impatiens.
In addition, the active compound may also be used for the treatment of seeds from plants, which have been modified by mutagenisis or genetic engineering, and which e.g. tolerate the action of herbicides or fungicides or insecticides. Such modified plants have been described in detail above.
Conventional seed treatment formulations include for example flowable concentrates FS, solutions LS, suspoemulsions (SE), powders for dry treatment DS, water dispersible powders for slurry treatment WS, water-soluble powders SS and emulsion ES and EC and gel formulation GF. These formulations can be applied to the seed diluted or undiluted. Application to the seeds is carried out before sowing, either directly on the seeds or after having pregerminated the latter. Preferably, the formulations are applied such that germination is not included.
The active substance concentrations in ready-to-use formulations, which may be obtained after two-to-tenfold dilution, are preferably from 0.01 to 60% by weight, more preferably from 0.1 to 40 % by weight.
In a preferred embodiment a FS formulation is used for seed treatment. Typically, a FS formulation may comprise 1-800 g/l of active ingredient, 1-200 g/l Surfactant, 0 to 200 g/l antifreezing agent, 0 to 400 g/l of binder, 0 to 200 g/l of a pigment and up to 1 liter of a solvent, preferably water.
Especially preferred FS formulations of the compounds of the present invention for seed treatment usually comprise from 0.1 to 80% by weight (1 to 800 g/l) of the active ingredient, from 0.1 to 20 % by weight (1 to 200 g/l) of at least one surfactant, e.g.0.05 to 5 % by weight of a wetter and from 0.5 to 15 % by weight of a dispersing agent, up to 20 % by weight, e.g. from 5 to 20 % of an anti-freeze agent, from 0 to 15 % by weight, e.g.1 to 15 % by weight of a pigment and/or a dye, from 0 to 40 % by weight, e.g.1 to 40 % by weight of a binder (sticker /adhesion agent), optionally up to 5 % by weight, e.g. from 0.1 to 5 % by weight of a thickener, optionally from 0.1 to 2 % of an anti-foam agent, and optionally a preservative such as a biocide, antioxidant or the
like, e.g. in an amount from 0.01 to 1 % by weight and a filler/vehicle up to 100 % by weight.
In the treatment of seed, the application rates of the compounds of the invention are generally from 0.1 g to 10 kg per 100 kg of seed, preferably from 1 g to 5 kg per 100 kg of seed, more preferably from 1 g to 1000 g per 100 kg of seed and in particular from 1 g to 200 g per 100 kg of seed, e.g. from 1 g to 100 g or from 5 g to 100 g per 100 kg of seed.
The invention therefore also relates to seed comprising a compound of the present invention, or an agriculturally useful salt thereof, as defined herein. The amount of the compound of the present invention or the agriculturally useful salt thereof will in general vary from 0.1 g to 10 kg per 100 kg of seed, preferably from 1 g to 5 kg per 100 kg of seed, in particular from 1 g to 1000 g per 100 kg of seed. For specific crops such as lettuce the rate can be higher. The compounds of the present invention may also be used for improving the health of a plant. Therefore, the present invention also relates to a method for improving plant health by treating a plant, plant propagation material and/or the locus where the plant is growing or is to grow with an effective and non-phytotoxic amount of a compound of the present invention.
As used herein "an effective and non-phytotoxic amount" means that the compound is used in a quantity which allows to obtain the desired effect but which does not give rise to any phytotoxic symptom on the treated plant or on the plant grown from the treated propagule or treated soil.
The terms "plant" and "plant propagation material" are defined above.
"Plant health" is defined as a condition of the plant and/or its products which is determined by several aspects alone or in combination with each other such as yield (for example increased biomass and/or increased content of valuable ingredients), quality (for example improved content or composition of certain ingredients or shelf life), plant vigour (for example improved plant growth and/or greener leaves ("greening effect"), tolerance to abiotic (for example drought) and/or biotic stress (for example disease) and production efficiency (for example, harvesting efficiency, processability).
The above identified indicators for the health condition of a plant may be interdependent and may result from each other. Each indicator is defined in the art and can be determined by methods known to a skilled person. The compounds of the invention are also suitable for use against non-crop insect pests. For use against said non-crop pests, compounds of the present invention can be used as bait composition, gel, general insect spray, aerosol, as ultra-low volume
application and bed net (impregnated or surface applied). Furthermore, drenching and rodding methods can be used.
As used herein, the term "non-crop insect pest" refers to pests, which are particularly relevant for non-crop targets, such as ants, termites, wasps, flies, ticks, mosquitos, crickets, or cockroaches.
The bait can be a liquid, a solid or a semisolid preparation (e.g. a gel). The bait employed in the composition is a product, which is sufficiently attractive to incite insects such as ants, termites, wasps, flies, mosquitos, crickets etc. or cockroaches to eat it. The attractiveness can be manipulated by using feeding stimulants or sex pheromones. Food stimulants are chosen, for example, but not exclusively, from animal and/or plant proteins (meat-, fish- or blood meal, insect parts, egg yolk), from fats and oils of animal and/or plant origin, or mono-, oligo- or polyorganosaccharides, especially from sucrose, lactose, fructose, dextrose, glucose, starch, pectin or even molasses or honey. Fresh or decaying parts of fruits, crops, plants, animals, insects or specific parts thereof can also serve as a feeding stimulant. Sex pheromones are known to be more insect specific. Specific pheromones are described in the literature (e.g.
http://www.pherobase.com), and are known to those skilled in the art.
For use in bait compositions, the typical content of active ingredient is from 0.001 weight % to 15 weight %, desirably from 0.001 weight % to 5% weight % of active compound.
Formulations of the compounds of the present invention as aerosols (e.g in spray cans), oil sprays or pump sprays are highly suitable for the non-professional user for controlling pests such as flies, fleas, ticks, mosquitos or cockroaches. Aerosol recipes are preferably composed of the active compound, solvents, furthermore auxiliaries such as emulsifiers, perfume oils, if appropriate stabilizers, and, if required, propellants.
The oil spray formulations differ from the aerosol recipes in that no propellants are used.
For use in spray compositions, the content of active ingredient is from 0.001 to 80 weights %, preferably from 0.01 to 50 weight % and most preferably from 0.01 to 15 weight %.
The compounds of the present invention and its respective compositions can also be used in mosquito and fumigating coils, smoke cartridges, vaporizer plates or long- term vaporizers and also in moth papers, moth pads or other heat-independent vaporizer systems.
Methods to control infectious diseases transmitted by insects (e.g. malaria, dengue and yellow fever, lymphatic filariasis, and leishmaniasis) with compounds of the present invention and its respective compositions also comprise treating surfaces of huts and houses, air spraying and impregnation of curtains, tents, clothing items, bed nets, tsetse-fly trap or the like. Insecticidal compositions for application to fibers, fabric,
knitgoods, nonwovens, netting material or foils and tarpaulins preferably comprise a mixture including the insecticide, optionally a repellent and at least one binder.
The compounds of the present invention and its compositions can be used for protecting wooden materials such as trees, board fences, sleepers, frames, artistic artifacts, etc. and buildings, but also construction materials, furniture, leathers, fibers, vinyl articles, electric wires and cables etc. from ants and/or termites, and for controlling ants and termites from doing harm to crops or human being (e.g. when the pests invade into houses and public facilities).
Customary application rates in the protection of materials are, for example, from 0.001 g to 2000 g or from 0.01 g to 1000 g of active compound per m2 treated material, desirably from 0.1 g to 50 g per m2.
Insecticidal compositions for use in the impregnation of materials typically contain from 0.001 to 95 weight %, preferably from 0.1 to 45 weight %, and more preferably from 1 to 25 weight % of at least one repellent and/or insecticide. Animal health
The compounds of the present invention are suitable for use in treating or protecting animals against infestation or infection by parasites. Therefore, the present invention also relates to the use of a compound of the present invention for the manufacture of a medicament for the treatment or protection of animals against infestation or infection by parasites. Furthermore, the present invention relates to a method of treating or protecting animals against infestation and infection by parasites, which comprises orally, topically or parenterally administering or applying to the animals a parasiticidally effective amount of a compound of the present invention. The present invention also relates to the non-therapeutic use of compounds of the present invention for treating or protecting animals against infestation and infection by parasites. Moreover, the present invention relates to a non-therapeutic method of treating or protecting animals against infestation and infection by parasites, which comprises applying to a locus a parasiticidally effective amount of a compound of the present invention.
The compounds of the present invention are further suitable for use in combating or controlling parasites in and on animals. Furthermore, the present invention relates to a method of combating or controlling parasites in and on animals, which comprises contacting the parasites with a parasitically effective amount of a compound of the present invention.
The present invention also relates to the non-therapeutic use of compounds of the present invention for controlling or combating parasites. Moreover, the present invention relates to a non-therapeutic method of combating or controlling parasites,
which comprises applying to a locus a parasiticidally effective amount of a compound of the present invention.
The compounds of the present invention can be effective through both contact (via soil, glass, wall, bed net, carpet, blankets or animal parts) and ingestion (e.g. baits). Furthermore, the compounds of the present invention can be applied to any and all developmental stages.
The compounds of the present invention can be applied as such or in form of compositions comprising the compounds of the present invention.
The compounds of the present invention can also be applied together with a mixing partner, which acts against pathogenic parasites, e.g. with synthetic coccidiosis compounds, polyetherantibiotics such as Amprolium, Robenidin, Toltrazuril, Monensin, Salinomycin, Maduramicin, Lasalocid, Narasin or Semduramicin, or with other mixing partners as defined above, or in form of compositions comprising said mixtures.
The compounds of the present invention and compositions comprising them can be applied orally, parenterally or topically, e.g. dermally. The compounds of the present invention can be systemically or non-systemically effective.
The application can be carried out prophylactically, therapeutically or non- therapeutically. Furthermore, the application can be carried out preventively to places at which occurrence of the parasites is expected.
As used herein, the term "contacting" includes both direct contact (applying the compounds/compositions directly on the parasite, including the application directly on the animal or excluding the application directly on the animal, e.g. at it's locus for the latter) and indirect contact (applying the compounds/compositions to the locus of the parasite). The contact of the parasite through application to its locus is an example of a non-therapeutic use of the compounds of the present invention.
The term "locus" means the habitat, food supply, breeding ground, area, material or environment in which a parasite is growing or may grow outside of the animal.
As used herein, the term "parasites" includes endo- and ectoparasites. In some embodiments of the present invention, endoparasites can be preferred. In other embodiments, ectoparasites can be preferred. Infestations in warm-blooded animals and fish include, but are not limited to, lice, biting lice, ticks, nasal bots, keds, biting flies, muscoid flies, flies, myiasitic fly larvae, chiggers, gnats, mosquitoes and fleas. The compounds of the present invention are especially useful for combating parasites of the following orders and species, respectively:
fleas (Siphonaptera), e.g. Ctenocephalides felis, Ctenocephalides canis, Xenopsylla cheopis, Pulex irritans, Tunga penetrans, and Nosopsyllus fasciatus; cockroaches (Blattaria - Blattodea), e.g. Blattella germanica, Blattella asahinae, Periplaneta americana, Periplaneta japonica, Periplaneta brunnea, Periplaneta fuligginosa, Periplaneta australasiae, and Blatta orientalis; flies, mosquitoes (Diptera), e.g. Aedes
aegypti, Aedes albopictus, Aedes vexans, Anastrepha ludens, Anopheles
maculipennis, Anopheles crucians, Anopheles albimanus, Anopheles gambiae, Anopheles freeborni, Anopheles leucosphyrus, Anopheles minimus, Anopheles quadrimaculatus, Calliphora vicina, Chrysomya bezziana, Chrysomya hominivorax, Chrysomya macellaria, Chrysops discalis, Chrysops silacea, Chrysops atlanticus, Cochliomyia hominivorax, Cordylobia anthropophaga, Culicoides furens, Culex pipiens, Culex nigripalpus, Culex quinquefasciatus, Culex tarsalis, Culiseta inornata, Culiseta melanura, Dermatobia hominis, Fannia canicularis, Gasterophilus intestinalis, Glossina morsitans, Glossina palpalis, Glossina fuscipes, Glossina tachinoides, Haematobia irritans, Haplodiplosis equestris, Hippelates spp., Hypoderma lineata, Leptoconops torrens, Lucilia caprina, Lucilia cuprina, Lucilia sericata, Lycoria pectoralis, Mansonia spp., Musca domestica, Muscina stabulans, Oestrus ovis, Phlebotomus argentipes, Psorophora columbiae, Psorophora discolor, Prosimulium mixtum, Sarcophaga haemorrhoidalis, Sarcophaga sp., Simulium vittatum, Stomoxys calcitrans, Tabanus bovinus, Tabanus atratus, Tabanus lineola, and Tabanus similis; lice (Phthiraptera), e.g. Pediculus humanus capitis, Pediculus humanus corporis, Pthirus pubis,
Haematopinus eurysternus, Haematopinus suis, Linognathus vituli, Bovicola bovis, Menopon gallinae, Menacanthus stramineus and Solenopotes capillatus; ticks and parasitic mites (Parasitiformes): ticks (Ixodida), e.g. Ixodes scapularis, Ixodes holocyclus, Ixodes pacificus, Rhiphicephalus sanguineus, Dermacentor andersoni, Dermacentor variabilis, Amblyomma americanum, Ambryomma maculatum,
Ornithodorus hermsi, Ornithodorus turicata and parasitic mites (Mesostigmata), e.g. Ornithonyssus bacoti and Dermanyssus gallinae; Actinedida (Prostigmata) und Acaridida (Astigmata), e.g. Acarapis spp., Cheyletiella spp., Ornithocheyletia spp., Myobia spp., Psorergates spp., Demodex spp., Trombicula spp., Listrophorus spp., Acarus spp., Tyrophagus spp., Caloglyphus spp., Hypodectes spp., Pterolichus spp., Psoroptes spp., Chorioptes spp., Otodectes spp., Sarcoptes spp., Notoedres spp.,Knemidocoptes spp., Cytodites spp., and Laminosioptes spp; Bugs
(Heteropterida): Cimex lectularius, Cimex hemipterus, Reduvius senilis, Triatoma spp., Rhodnius ssp., Panstrongylus ssp., and Arilus critatus; Anoplurida, e.g. Haematopinus spp., Linognathus spp., Pediculus spp., Phtirus spp., and Solenopotes spp.;
Mallophagida (suborders Arnblycerina and Ischnocerina), e.g. Trimenopon spp., Menopon spp., Trinoton spp., Bovicola spp., Werneckiella spp., Lepikentron spp., Trichodectes spp., and Felicola spp.; Roundworms Nematoda: Wipeworms and Trichinosis (Trichosyringida), e.g. Trichinellidae (Trichinella spp.), (Trichuridae) Trichuris spp., Capillaria spp.; Rhabditida, e.g. Rhabditis spp., Strongyloides spp., Helicephalobus spp.; Strongylida, e.g. Strongylus spp., Ancylostoma spp., Necator americanus, Bunostomum spp. (Hookworm), Trichostrongylus spp., Haemonchus contortus, Ostertagia spp., Cooperia spp., Nematodirus spp., Dictyocaulus spp.,
Cyathostoma spp., Oesophagostomum spp., Stephanurus dentatus, Ollulanus spp., Chabertia spp., Stephanurus dentatus, Syngamus trachea, Ancylostoma spp., Uncinaria spp., Globocephalus spp., Necator spp., Metastrongylus spp., Muellerius capillaris, Protostrongylus spp., Angiostrongylus spp., Parelaphostrongylus spp., Aleurostrongylus abstrusus, and Dioctophyma renale; Intestinal roundworms
(Ascaridida), e.g. Ascaris lumbricoides, Ascaris suum, Ascaridia galli, Parascaris equorum, Enterobius vermicularis (Threadworm), Toxocara canis, Toxascaris leonine, Skrjabinema spp., and Oxyuris equi; Camallanida, e.g. Dracunculus medinensis (guinea worm); Spirurida, e.g. Thelazia spp., Wuchereria spp., Brugia spp.,
Onchocerca spp., Dirofilari spp.a, Dipetalonema spp., Setaria spp., Elaeophora spp., Spirocerca lupi, and Habronema spp.; Thorny headed worms (Acanthocephala), e.g. Acanthocephalus spp., Macracanthorhynchus hirudinaceus and Oncicola spp.;
Planarians (Plathelminthes): Flukes (Trematoda), e.g. Faciola spp., Fascioloides magna, Paragonimus spp., Dicrocoelium spp., Fasciolopsis buski, Clonorchis sinensis, Schistosoma spp., Trichobilharzia spp., Alaria alata, Paragonimus spp., and
Nanocyetes spp.; Cercomeromorpha, in particular Cestoda (Tapeworms), e.g.
Diphyllobothrium spp., Tenia spp., Echinococcus spp., Dipylidium caninum, Multiceps spp., Hymenolepis spp., Mesocestoides spp., Vampirolepis spp., Moniezia spp., Anoplocephala spp., Sirometra spp., Anoplocephala spp., and Hymenolepis spp.. As used herein, the term“ animal” includes warm-blooded animals (including humans) and fish. Preferred are mammals, such as cattle, sheep, swine, camels, deer, horses, pigs, poultry, rabbits, goats, dogs and cats, water buffalo, donkeys, fallow deer and reindeer, and also in fur-bearing animals such as mink, chinchilla and raccoon, birds such as hens, geese, turkeys and ducks and fish such as fresh- and salt-water fish such as trout, carp and eels. Particularly preferred are domestic animals, such as dogs or cats.
In general, "parasiticidally effective amount" means the amount of active ingredient needed to achieve an observable effect on growth, including the effects of necrosis, death, retardation, prevention, and removal, destruction, or otherwise diminishing the occurrence and activity of the target organism. The parasiticidally effective amount can vary for the various compounds/compositions used in the invention. A parasiticidally effective amount of the compositions will also vary according to the prevailing conditions such as desired parasiticidal effect and duration, target species, mode of application, and the like.
Generally, it is favorable to apply the compounds of the present invention in total amounts of 0.5 mg/kg to 100 mg/kg per day, preferably 1 mg/kg to 50 mg/kg per day. For oral administration to warm-blooded animals, the formula I compounds may be formulated as animal feeds, animal feed premixes, animal feed concentrates, pills, solutions, pastes, suspensions, drenches, gels, tablets, boluses and capsules. In
addition, the formula I compounds may be administered to the animals in their drinking water. For oral administration, the dosage form chosen should provide the animal with 0.01 mg/kg to 100 mg/kg of animal body weight per day of the formula I compound, preferably with 0.5 mg/kg to 100 mg/kg of animal body weight per day.
Alternatively, the formula I compounds may be administered to animals parenterally, for example, by intraruminal, intramuscular, intravenous or subcutaneous injection. The formula I compounds may be dispersed or dissolved in a physiologically acceptable carrier for subcutaneous injection. Alternatively, the formula I compounds may be formulated into an implant for subcutaneous administration. In addition the formula I compound may be transdermally administered to animals. For parenteral
administration, the dosage form chosen should provide the animal with 0.01 mg/kg to 100 mg/kg of animal body weight per day of the formula I compound.
The formula I compounds may also be applied topically to the animals in the form of dips, dusts, powders, collars, medallions, sprays, shampoos, spot-on and pour-on formulations and in ointments or oil-in-water or water-in-oil emulsions. For topical application, dips and sprays usually contain 0.5 ppm to 5,000 ppm and preferably 1 ppm to 3,000 ppm of the formula I compound. In addition, the formula I compounds may be formulated as ear tags for animals, particularly quadrupeds such as cattle and sheep.
Suitable preparations are:
- Solutions such as oral solutions, concentrates for oral administration after dilution, solutions for use on the skin or in body cavities, pouring-on formulations, gels;
- Emulsions and suspensions for oral or dermal administration; semi-solid
preparations;
- Formulations in which the active compound is processed in an ointment base or in an oil-in-water or water-in-oil emulsion base;
- Solid preparations such as powders, premixes or concentrates, granules, pellets, tablets, boluses, capsules; aerosols and inhalants, and active compound-containing shaped articles.
Compositions suitable for injection are prepared by dissolving the active ingredient in a suitable solvent and optionally adding further auxiliaries such as acids, bases, buffer salts, preservatives, and solubilizers. Suitable auxiliaries for injection solutions are known in the art. The solutions are filtered and filled sterile.
Oral solutions are administered directly. Concentrates are administered orally after prior dilution to the use concentration. Oral solutions and concentrates are prepared according to the state of the art and as described above for injection solutions, sterile procedures not being necessary.
Solutions for use on the skin are trickled on, spread on, rubbed in, sprinkled on or sprayed on. Solutions for use on the skin are prepared according to the state of the art
and according to what is described above for injection solutions, sterile procedures not being necessary.
Gels are applied to or spread on the skin or introduced into body cavities. Gels are prepared by treating solutions which have been prepared as described in the case of the injection solutions with sufficient thickener that a clear material having an ointment- like consistency results. Suitable thickeners are known in the art.
Pour-on formulations are poured or sprayed onto limited areas of the skin, the active compound penetrating the skin and acting systemically. Pour-on formulations are prepared by dissolving, suspending or emulsifying the active compound in suitable skin-compatible solvents or solvent mixtures. If appropriate, other auxiliaries such as colorants, bioabsorption-promoting substances, antioxidants, light stabilizers, adhesives are added. Suitable such auxiliaries are known in the art.
Emulsions can be administered orally, dermally or as injections. Emulsions are either of the water-in-oil type or of the oil-in-water type. They are prepared by dissolving the active compound either in the hydrophobic or in the hydrophilic phase and
homogenizing this with the solvent of the other phase with the aid of suitable emulsifiers and, if appropriate, other auxiliaries such as colorants, absorption- promoting substances, preservatives, antioxidants, light stabilizers, viscosity-enhancing substances. Suitable hydrophobic phases (oils), suitable hydrophilic phases, suitable emulsifiers, and suitable further auxiliaries for emulsions are known in the art.
Suspensions can be administered orally or topically/dermally. They are prepared by suspending the active compound in a suspending agent, if appropriate with addition of other auxiliaries such as wetting agents, colorants, bioabsorption-promoting substances, preservatives, antioxidants, light stabilizers. Suitable suspending agents, and suitable other auxiliaries for suspensions including wetting agents are known in the art.
Semi-solid preparations can be administered orally or topically/dermally. They differ from the suspensions and emulsions described above only by their higher viscosity. For the production of solid preparations, the active compound is mixed with suitable excipients, if appropriate with addition of auxiliaries, and brought into the desired form. Suitable auxiliaries for this purpose are known in the art.
The compositions which can be used in the invention can comprise generally from about 0.001 to 95% of the compound of the present invention.
Ready-to-use preparations contain the compounds acting against parasites, preferably ectoparasites, in concentrations of 10 ppm to 80 per cent by weight, preferably from 0.1 to 65 per cent by weight, more preferably from 1 to 50 per cent by weight, most preferably from 5 to 40 per cent by weight.
Preparations which are diluted before use contain the compounds acting against ectoparasites in concentrations of 0.5 to 90 per cent by weight, preferably of 1 to 50 per cent by weight.
Furthermore, the preparations comprise the compounds of formula I against endoparasites in concentrations of 10 ppm to 2 per cent by weight, preferably of 0.05 to 0.9 per cent by weight, very particularly preferably of 0.005 to 0.25 per cent by weight. Topical application may be conducted with compound-containing shaped articles such as collars, medallions, ear tags, bands for fixing at body parts, and adhesive strips and foils.
Generally it is favorable to apply solid formulations which release compounds of the present invention in total amounts of 10 mg/kg to 300 mg/kg, preferably 20 mg/kg to 200 mg/kg, most preferably 25 mg/kg to 160 mg/kg body weight of the treated animal in the course of three weeks. Mixtures
The present invention also relates to a mixture of at least one compound of the present invention with at least one mixing partner as defined herein after. Preferred are binary mixtures of one compound of the present invention as component I with one mixing partner as defined herein after as component II. Preferred weight ratios for such binary mixtures are from 5000:1 to 1:5000, preferably from 1000:1 to 1:1000, more preferably from 100:1 to 1:100, particularly preferably from 10:1 to 1:10. In such binary mixtures, components I and II may be used in equal amounts, or an excess of component I, or an excess of component II may be used.
Also, in the method of the present invention compounds of formula (I) may be applied with other active ingredients (mixing partners). These additional ingredients may be used sequentially or in combination with the above-described compositions, if appropriate also added only immediately prior to use (tank mix). For example, the plant(s) may be sprayed with a composition of this invention either before or after being treated with other active ingredients.
Mixing partners can be selected from pesticides, in particular insecticides,
nematicides, and acaricides, fungicides, herbicides, plant growth regulators, fertilizers, and the like. Preferred mixing partners are insecticides, nematicides and fungicides. The following list M of pesticides, grouped and numbered according the Mode of Action Classification of the Insecticide Resistance Action Committee (IRAC), together with which the compounds of the present invention can be used and with which potential synergistic effects might be produced, is intended to illustrate the possible combinations, but not to impose any limitation:
M.1 Acetylcholine esterase (AChE) inhibitors from the class of: M.1A carbamates, for example aldicarb, alanycarb, bendiocarb, benfuracarb, butocarboxim, butoxycarboxim, carbaryl, carbofuran, carbosulfan, ethiofencarb, fenobucarb, formetanate, furathiocarb, isoprocarb, methiocarb, methomyl, metolcarb, oxamyl, pirimicarb, propoxur, thiodicarb, thiofanox, trimethacarb, XMC, xylylcarb and triazamate; or from the class of M.1B organophosphates, for example acephate, azamethiphos, azinphos-ethyl,
azinphosmethyl, cadusafos, chlorethoxyfos, chlorfenvinphos, chlormephos,
chlorpyrifos, chlorpyrifos-methyl, coumaphos, cyanophos, demeton-S-methyl, diazinon, dichlorvos/ DDVP, dicrotophos, dimethoate, dimethylvinphos, disulfoton, EPN, ethion, ethoprophos, famphur, fenamiphos, fenitrothion, fenthion, fosthiazate, heptenophos, imicyafos, isofenphos, isopropyl O- (methoxyaminothio-phosphoryl) salicylate, isoxathion, malathion, mecarbam, methamidophos, methidathion, mevinphos, monocrotophos, naled, omethoate, oxydemeton-methyl, parathion, parathion-methyl, phenthoate, phorate, phosalone, phosmet, phosphamidon, phoxim, pirimiphos- methyl, profenofos, propetamphos, prothiofos, pyraclofos, pyridaphenthion, quinalphos, sulfotep, tebupirimfos, temephos, terbufos, tetrachlorvinphos, thiometon, triazophos, trichlorfon and vamidothion;
M.2. GABA-gated chloride channel antagonists such as: M.2A cyclodiene
organochlorine compounds, as for example endosulfan or chlordane; or M.2B fiproles (phenylpyrazoles), as for example ethiprole, fipronil, flufiprole, pyrafluprole and pyriprole;
M.3 Sodium channel modulators from the class of M.3A pyrethroids, for example acrinathrin, allethrin, d-cis-trans allethrin, d-trans allethrin, bifenthrin, bioallethrin, bioallethrin S-cylclopentenyl, bioresmethrin, cycloprothrin, cyfluthrin, beta-cyfluthrin, cyhalothrin, lambda-cyhalothrin, gamma-cyhalothrin, cypermethrin, alpha-cypermethrin, beta-cypermethrin, theta-cypermethrin, zeta-cypermethrin, cyphenothrin, deltamethrin, empenthrin, esfenvalerate, etofenprox, fenpropathrin, fenvalerate, flucythrinate, flumethrin, tau-fluvalinate, halfenprox, heptafluthrin, imiprothrin,
meperfluthrin,metofluthrin, momfluorothrin, permethrin, phenothrin, prallethrin, profluthrin, pyrethrin (pyrethrum), resmethrin, silafluofen, tefluthrin, tetramethylfluthrin, tetramethrin, tralomethrin and transfluthrin; or M.3B sodium channel modulators such as DDT or methoxychlor;
M.4 Nicotinic acetylcholine receptor agonists (nAChR) from the class of M.4A neonicotinoids, for example acetamiprid, clothianidin, cycloxaprid, dinotefuran, imidacloprid, nitenpyram, thiacloprid and thiamethoxam; or the compounds M.4A.2: (2E-)-1-[(6-Chloropyridin-3-yl)methyl]-N'-nitro-2-pentylidenehydrazinecarboximidamide; or M4.A.3: 1-[(6-Chloropyridin-3-yl)methyl]-7-methyl-8-nitro-5-propoxy-1,2,3,5,6,7- hexahydroimidazo[1,2-a]pyridine; or from the class M.4B nicotine;
M.5 Nicotinic acetylcholine receptor allosteric activators from the class of spinosyns,
for example spinosad or spinetoram;
M.6 Chloride channel activators from the class of avermectins and milbemycins, for example abamectin, emamectin benzoate, ivermectin, lepimectin or milbemectin;
M.7 Juvenile hormone mimics, such as M.7A juvenile hormone analogues as hydroprene, kinoprene and methoprene; or others as M.7B fenoxycarb or M.7C pyriproxyfen;
M.8 miscellaneous non-specific (multi-site) inhibitors, for example M.8A alkyl halides as methyl bromide and other alkyl halides, or M.8B chloropicrin, or M.8C sulfuryl fluoride, or M.8D borax, or M.8E tartar emetic;
M.9 Selective homopteran feeding blockers, for example M.9B pymetrozine, or M.9C flonicamid;
M.10 Mite growth inhibitors, for example M.10A clofentezine, hexythiazox and diflovidazin, or M.10B etoxazole;
M.11 Microbial disruptors of insect midgut membranes, for example bacillus thuringiensis or bacillus sphaericus and the insecticdal proteins they produce such as bacillus thuringiensis subsp. israelensis, bacillus sphaericus, bacillus thuringiensis subsp. aizawai, bacillus thuringiensis subsp. kurstaki and bacillus thuringiensis subsp. tenebrionis, or the Bt crop proteins: Cry1Ab, Cry1Ac, Cry1Fa, Cry2Ab, mCry3A, Cry3Ab, Cry3Bb and Cry34/35Ab1;
M.12 Inhibitors of mitochondrial ATP synthase, for example M.12A diafenthiuron, or M.12B organotin miticides such as azocyclotin, cyhexatin or fenbutatin oxide, or M.12C propargite, or M.12D tetradifon;
M.13 Uncouplers of oxidative phosphorylation via disruption of the proton gradient, for example chlorfenapyr, DNOC or sulfluramid;
M.14 Nicotinic acetylcholine receptor (nAChR) channel blockers, for example nereistoxin analogues as bensultap, cartap hydrochloride, thiocyclam or thiosultap sodium;
M.15 Inhibitors of the chitin biosynthesis type 0, such as benzoylureas as for example bistrifluron, chlorfluazuron, diflubenzuron, flucycloxuron, flufenoxuron, hexaflumuron, lufenuron, novaluron, noviflumuron, teflubenzuron or triflumuron;
M.16 Inhibitors of the chitin biosynthesis type 1, as for example buprofezin;
M.17 Moulting disruptors, Dipteran, as for example cyromazine;
M.18 Ecdyson receptor agonists such as diacylhydrazines, for example
methoxyfenozide, tebufenozide, halofenozide, fufenozide or chromafenozide;
M.19 Octopamin receptor agonists, as for example amitraz;
M.20 Mitochondrial complex III electron transport inhibitors, for example M.20A hydramethylnon, or M.20B acequinocyl, or M.20C fluacrypyrim;
M.21 Mitochondrial complex I electron transport inhibitors, for example M.21A METI acaricides and insecticides such as fenazaquin, fenpyroximate, pyrimidifen, pyridaben, tebufenpyrad or tolfenpyrad, or M.21B rotenone;
M.22 Voltage-dependent sodium channel blockers, for example M.22A indoxacarb, or M.22B metaflumizone, or M.22B.1: 2-[2-(4-Cyanophenyl)-1-[3-(trifluoromethyl)phenyl]- ethylidene]-N-[4-(difluoromethoxy)phenyl]-hydrazinecarboxamide or M.22B.2: N-(3- Chloro-2-methylphenyl)-2-[(4-chlorophenyl)[4-[methyl(methylsulfonyl)amino]phenyl]- methylene]-hydrazinecarboxamide;
M.23 Inhibitors of the of acetyl CoA carboxylase, such as Tetronic and Tetramic acid derivatives, for example spirodiclofen, spiromesifen or spirotetramat;
M.24 Mitochondrial complex IV electron transport inhibitors, for example M.24A phosphine such as aluminium phosphide, calcium phosphide, phosphine or zinc phosphide, or M.24B cyanide;
M.25 Mitochondrial complex II electron transport inhibitors, such as beta-ketonitrile derivatives, for example cyenopyrafen or cyflumetofen;
M.28 Ryanodine receptor-modulators from the class of diamides, as for example flubendiamide, chlorantraniliprole (rynaxypyr®), cyantraniliprole (cyazypyr®), tetraniliprole, or the phthalamide compounds M.28.1: (R)-3-Chlor-N1-{2-methyl-4- [1,2,2,2-tetrafluor-1-(trifluormethyl)ethyl]phenyl}-N2-(1-methyl-2- methylsulfonylethyl)phthalamid and M.28.2: (S)-3-Chlor-N1-{2-methyl-4-[1,2,2,2– tetrafluor-1-(trifluormethyl)ethyl]phenyl}-N2-(1-methyl-2-methylsulfonylethyl)phthalamid, or the compound M.28.3: 3-bromo-N-{2-bromo-4-chloro-6-[(1- cyclopropylethyl)carbamoyl]phenyl}-1-(3-chlorpyridin-2-yl)-1H-pyrazole-5-carboxamide (proposed ISO name: cyclaniliprole), or the compound M.28.4: methyl-2-[3,5-dibromo- 2-({[3-bromo-1-(3-chlorpyridin-2-yl)-1H-pyrazol-5-yl]carbonyl}amino)benzoyl]-1,2- dimethylhydrazinecarboxylate; or a compound selected from M.28.5a) to M.28.5d) and M.28.5h) to M.28.5l): M.28.5a) N-[4,6-dichloro-2-[(diethyl-lambda-4-sulfanylidene)- carbamoyl]-phenyl]-2-(3-chloro-2-pyridyl)-5-(trifluoromethyl)pyrazole-3-carboxamide; M.28.5b) N-[4-chloro-2-[(diethyl-lambda-4-sulfanylidene)carbamoyl]-6-methyl-phenyl]- 2-(3-chloro-2-pyridyl)-5-(trifluoromethyl)pyrazole-3-carboxamide; M.28.5c) N-[4-chloro- 2-[(di-2-propyl-lambda-4-sulfanylidene)carbamoyl]-6-methyl-phenyl]-2-(3-chloro-2- pyridyl)-5-(trifluoromethyl)pyrazole-3-carboxamide; M.28.5d) N-[4,6-dichloro-2-[(di-2- propyl-lambda-4-sulfanylidene)carbamoyl]-phenyl]-2-(3-chloro-2-pyridyl)-5-(trifluoro- methyl)pyrazole-3-carboxamide; M.28.5h) N-[4,6-dibromo-2-[(diethyl-lambda-4- sulfanylidene)carbamoyl]-phenyl]-2-(3-chloro-2-pyridyl)-5-(trifluoromethyl)pyrazole-3- carboxamide; M.28.5i) N-[2-(5-Amino-1,3,4-thiadiazol-2-yl)-4-chloro-6-methylphenyl]-3- bromo-1-(3-chloro-2-pyridinyl)-1H-pyrazole-5-carboxamide; M.28.5j) 3-Chloro-1-(3- chloro-2-pyridinyl)-N-[2,4-dichloro-6-[[(1-cyano-1-methylethyl)amino]carbonyl]phenyl]- 1H-pyrazole-5-carboxamide; M.28.5k) 3-Bromo-N-[2,4-dichloro-6-
(methylcarbamoyl)phenyl]-1-(3,5-dichloro-2-pyridyl)-1H-pyrazole-5-carboxamide;
M.28.5l) N-[4-Chloro-2-[[(1,1-dimethylethyl)amino]carbonyl]-6-methylphenyl]-1-(3- chloro-2-pyridinyl)-3-(fluoromethoxy)-1H-pyrazole-5-carboxamide; or
M.28.6: cyhalodiamide;
M.29. insecticidal active compounds of unknown or uncertain mode of action, as for example afidopyropen, afoxolaner, azadirachtin, amidoflumet, benzoximate, bifenazate, broflanilide, bromopropylate, chinomethionat, cryolite, dicloromezotiaz, dicofol, flufenerim, flometoquin, fluensulfone, fluhexafon, fluopyram, flupyradifurone, fluralaner, metoxadiazone, piperonyl butoxide, pyflubumide, pyridalyl, pyrifluquinazon, sulfoxaflor, tioxazafen, triflumezopyrim, or the compounds
M.29.3: 11-(4-chloro-2,6-dimethylphenyl)-12-hydroxy-1,4-dioxa-9-azadispiro[4.2.4.2]- tetradec-11-en-10-one, or the compound
M.29.4: 3-(4'-fluoro-2,4-dimethylbiphenyl-3-yl)-4-hydroxy-8-oxa-1-azaspiro[4.5]dec-3- en-2-one, or the compound
M.29.5: 1-[2-fluoro-4-methyl-5-[(2,2,2-trifluoroethyl)sulfinyl]phenyl]-3-(trifluoromethyl)- 1H-1,2,4-triazole-5-amine, or actives on basis of bacillus firmus (Votivo, I-1582); or a compound selected from the group of M.29.6, wherein the compound is selected from M.29.6a) to M.29.6k): M.29.6a) (E/Z)-N-[1-[(6-chloro-3-pyridyl)methyl]-2- pyridylidene]-2,2,2-trifluoro-acetamide; M.29.6b) (E/Z)-N-[1-[(6-chloro-5-fluoro-3- pyridyl)methyl]-2-pyridylidene]-2,2,2-trifluoro-acetamide; M.29.6c) (E/Z)-2,2,2-trifluoro- N-[1-[(6-fluoro-3-pyridyl)methyl]-2-pyridylidene]acetamide; M.29.6d) (E/Z)-N-[1-[(6- bromo-3-pyridyl)methyl]-2-pyridylidene]-2,2,2-trifluoro-acetamide; M.29.6e) (E/Z)-N-[1- [1-(6-chloro-3-pyridyl)ethyl]-2-pyridylidene]-2,2,2-trifluoro-acetamide; M.29.6f) (E/Z)-N- [1-[(6-chloro-3-pyridyl)methyl]-2-pyridylidene]-2,2-difluoro-acetamide; M.29.6g) (E/Z)-2- chloro-N-[1-[(6-chloro-3-pyridyl)methyl]-2-pyridylidene]-2,2-difluoro-acetamide;
M.29.6h) (E/Z)-N-[1-[(2-chloropyrimidin-5-yl)methyl]-2-pyridylidene]-2,2,2-trifluoro- acetamide; M.29.6i) (E/Z)-N-[1-[(6-chloro-3-pyridyl)methyl]-2-pyridylidene]-2,2,3,3,3- pentafluoro-propanamide.); M.29.6j) N-[1-[(6-chloro-3-pyridyl)methyl]-2-pyridylidene]- 2,2,2-trifluoro-thioacetamide; or M.29.6k) N-[1-[(6-chloro-3-pyridyl)methyl]-2- pyridylidene]-2,2,2-trifluoro-N'-isopropyl-acetamidine; or the compounds
M.29.8: fluazaindolizine; or the compounds
M.29.9.a): 4-[5-(3,5-dichlorophenyl)-5-(trifluoromethyl)-4H-isoxazol-3-yl]-2-methyl-N- (1-oxothietan-3-yl)benzamide; or M.29.9.b): fluxametamide; or
M.29.10: 5-[3-[2,6-dichloro-4-(3,3-dichloroallyloxy)phenoxy]propoxy]-1H-pyrazole; or a compound selected from the group of M.29.11, wherein the compound is selected from M.29.11b) to M.29.11p): M.29.11.b) 3-(benzoylmethylamino)-N-[2-bromo-4- [1,2,2,3,3,3-hexafluoro-1-(trifluoromethyl)propyl]-6-(trifluoromethyl)phenyl]-2-fluoro- benzamide; M.29.11.c) 3-(benzoylmethylamino)-2-fluoro-N-[2-iodo-4-[1,2,2,2- tetrafluoro-1-(trifluoromethyl)ethyl]-6-(trifluoromethyl)phenyl]-benzamide; M.29.11.d) N-
[3-[[[2-iodo-4-[1,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl]-6- (trifluoromethyl)phenyl]amino]carbonyl]phenyl]-N-methyl-benzamide; M.29.11.e) N-[3- [[[2-bromo-4-[1,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl]-6- (trifluoromethyl)phenyl]amino]carbonyl]-2-fluorophenyl]-4-fluoro-N-methyl-benzamide; M.29.11.f) 4-fluoro-N-[2-fluoro-3-[[[2-iodo-4-[1,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl]- 6-(trifluoromethyl)phenyl]amino]carbonyl]phenyl]-N-methyl-benzamide; M.29.11.g) 3- fluoro-N-[2-fluoro-3-[[[2-iodo-4-[1,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl]-6- (trifluoromethyl)phenyl]amino]carbonyl]phenyl]-N-methyl-benzamide; M.29.11.h) 2- chloro-N-[3-[[[2-iodo-4-[1,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl]-6- (trifluoromethyl)phenyl]amino]carbonyl]phenyl]- 3-pyridinecarboxamide; M.29.11.i) 4- cyano-N-[2-cyano-5-[[2,6-dibromo-4-[1,2,2,3,3,3-hexafluoro-1-(trifluoromethyl)- propyl]phenyl]carbamoyl]phenyl]-2-methyl-benzamide; M.29.11.j) 4-cyano-3-[(4-cyano- 2-methyl-benzoyl)amino]-N-[2,6-dichloro-4-[1,2,2,3,3,3-hexafluoro-1-(trifluoromethyl)- propyl]phenyl]-2-fluoro-benzamide; M.29.11.k) N-[5-[[2-chloro-6-cyano-4-[1,2,2,3,3,3- hexafluoro-1-(trifluoromethyl)propyl]phenyl]carbamoyl]-2-cyano-phenyl]-4-cyano-2- methyl-benzamide; M.29.11.l) N-[5-[[2-bromo-6-chloro-4-[2,2,2-trifluoro-1-hydroxy-1- (trifluoromethyl)ethyl]phenyl]carbamoyl]-2-cyano-phenyl]-4-cyano-2-methyl-benzamide; M.29.11.m) N-[5-[[2-bromo-6-chloro-4-[1,2,2,3,3,3-hexafluoro-1-(trifluoromethyl)- propyl]phenyl]carbamoyl]-2-cyano-phenyl]-4-cyano-2-methyl-benzamide; M.29.11.n) 4- cyano-N-[2-cyano-5-[[2,6-dichloro-4-[1,2,2,3,3,3-hexafluoro-1-(trifluoromethyl)- propyl]phenyl]carbamoyl]phenyl]-2-methyl-benzamide; M.29.11.o) 4-cyano-N-[2-cyano- 5-[[2,6-dichloro-4-[1,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl]phenyl]carbamoyl]phenyl]- 2-methyl-benzamide; M.29.11.p) N-[5-[[2-bromo-6-chloro-4-[1,2,2,2-tetrafluoro-1- (trifluoromethyl)ethyl]phenyl]carbamoyl]-2-cyano-phenyl]-4-cyano-2-methyl-benzamide; or
a compound selected from the group of M.29.12, wherein the compound is selected from M.29.12a) to M.29.12m): M.29.12.a) 2-(1,3-Dioxan-2-yl)-6-[2-(3-pyridinyl)-5- thiazolyl]-pyridine; M.29.12.b) 2-[6-[2-(5-Fluoro-3-pyridinyl)-5-thiazolyl]-2-pyridinyl]- pyrimidine; M.29.12.c) 2-[6-[2-(3-Pyridinyl)-5-thiazolyl]-2-pyridinyl]-pyrimidine;
M.29.12.d) N-Methylsulfonyl-6-[2-(3-pyridyl)thiazol-5-yl]pyridine-2-carboxamide;
M.29.12.e) N-Methylsulfonyl-6-[2-(3-pyridyl)thiazol-5-yl]pyridine-2-carboxamide;
M.29.12.f) N-Ethyl-N-[4-methyl-2-(3-pyridyl)thiazol-5-yl]-3-methylthio-propanamide; M.29.12.g) N-Methyl-N-[4-methyl-2-(3-pyridyl)thiazol-5-yl]-3-methylthio-propanamide; M.29.12.h) N,2-Dimethyl-N-[4-methyl-2-(3-pyridyl)thiazol-5-yl]-3-methylthio- propanamide; M.29.12.i) N-Ethyl-2-methyl-N-[4-methyl-2-(3-pyridyl)thiazol-5-yl]-3- methylthio-propanamide; M.29.12.j) N-[4-Chloro-2-(3-pyridyl)thiazol-5-yl]-N-ethyl-2- methyl-3-methylthio-propanamide; M.29.12.k) N-[4-Chloro-2-(3-pyridyl)thiazol-5-yl]-N,2- dimethyl-3-methylthio-propanamide; M.29.12.l) N-[4-Chloro-2-(3-pyridyl)thiazol-5-yl]-N-
methyl-3-methylthio-propanamide; M.29.12.m) N-[4-Chloro-2-(3-pyridyl)thiazol-5-yl]-N- ethyl-3-methylthio-propanamide; or the compounds
M.29.14a) 1-[(6-Chloro-3-pyridinyl)methyl]-1,2,3,5,6,7-hexahydro-5-methoxy-7- methyl-8-nitro-imidazo[1,2-a]pyridine; or M.29.14b) 1-[(6-Chloropyridin-3-yl)methyl]-7- methyl-8-nitro-1,2,3,5,6,7-hexahydroimidazo[1,2-a]pyridin-5-ol; or the compounds M.29.16a) 1-isopropyl-N,5-dimethyl-N-pyridazin-4-yl-pyrazole-4-carboxamide; or M.29.16b) 1-(1,2-dimethylpropyl)-N-ethyl-5-methyl-N-pyridazin-4-yl-pyrazole-4- carboxamide; M.29.16c) N,5-dimethyl-N-pyridazin-4-yl-1-(2,2,2-trifluoro-1-methyl- ethyl)pyrazole-4-carboxamide; M.29.16d) 1-[1-(1-cyanocyclopropyl)ethyl]-N-ethyl-5- methyl-N-pyridazin-4-yl-pyrazole-4-carboxamide; M.29.16e) N-ethyl-1-(2-fluoro-1- methyl-propyl)-5-methyl-N-pyridazin-4-yl-pyrazole-4-carboxamide; M.29.16f) 1-(1,2- dimethylpropyl)-N,5-dimethyl-N-pyridazin-4-yl-pyrazole-4-carboxamide; M.29.16g) 1-[1- (1-cyanocyclopropyl)ethyl]-N,5-dimethyl-N-pyridazin-4-yl-pyrazole-4-carboxamide; M.29.16h) N-methyl-1-(2-fluoro-1-methyl-propyl]-5-methyl-N-pyridazin-4-yl-pyrazole-4- carboxamide; M.29.16i) 1-(4,4-difluorocyclohexyl)-N-ethyl-5-methyl-N-pyridazin-4-yl- pyrazole-4-carboxamide; or M.29.16j) 1-(4,4-difluorocyclohexyl)-N,5-dimethyl-N- pyridazin-4-yl-pyrazole-4-carboxamide; or
M.29.17 a compound selected from the compounds M.29.17a) to M.29.17j):
M.29.17a) N-(1-methylethyl)-2-(3-pyridinyl)-2H-indazole-4-carboxamide; M.29.17b) N- cyclopropyl-2-(3-pyridinyl)-2H-indazole-4-carboxamide; M.29.17c) N-cyclohexyl-2-(3- pyridinyl)-2H-indazole-4-carboxamide; M.29.17d) 2-(3-pyridinyl)-N-(2,2,2-trifluoroethyl)- 2H-indazole-4-carboxamide; M.29.17e) 2-(3-pyridinyl)-N-[(tetrahydro-2-furanyl)methyl]- 2H-indazole-5-carboxamide; M.29.17f) methyl 2-[[2-(3-pyridinyl)-2H-indazol-5- yl]carbonyl]hydrazinecarboxylate; M.29.17g) N-[(2,2-difluorocyclopropyl)methyl]-2-(3- pyridinyl)-2H-indazole-5-carboxamide; M.29.17h) N-(2,2-difluoropropyl)-2-(3-pyridinyl)- 2H-indazole-5-carboxamide; M.29.17i) 2-(3-pyridinyl )-N-(2-pyrimidinylmethyl )-2H- indazole-5-carboxamide; M.29.17j) N-[(5-methyl-2-pyrazinyl)methyl]-2-(3-pyridinyl)-2H- indazole-5-carboxamide, or
M.29.18 a compound selected from the compounds M.29.18a) to M.29.18d):
M.29.18a) N-[3-chloro-1-(3-pyridyl)pyrazol-4-yl]-N-ethyl-3-(3,3,3- trifluoropropylsulfanyl)propanamide; M.29.18b) N-[3-chloro-1-(3-pyridyl)pyrazol-4-yl]-N- ethyl-3-(3,3,3-trifluoropropylsulfinyl)propanamide; M.29.18c) N-[3-chloro-1-(3- pyridyl)pyrazol-4-yl]-3-[(2,2-difluorocyclopropyl)methylsulfanyl]-N-ethyl-propanamide; M.29.18d) N-[3-chloro-1-(3-pyridyl)pyrazol-4-yl]-3-[(2,2- difluorocyclopropyl)methylsulfinyl]-N-ethyl-propanamide; or the compound
M.29.19 sarolaner , or the compound
M.29.20 lotilaner.
The commercially available compounds of the group M listed above may be found in The Pesticide Manual, 16th Edition, C. MacBean, British Crop Protection Council
(2013) among other publications. The online Pesticide Manual is updated regularly and is accessible through http://bcpcdata.com/pesticide-manual.html.
Another online data base for pesticides providing the ISO common names is http://www.alanwood.net/pesticides.
The M.4 neonicotinoid cycloxaprid is known from WO2010/069266 and
WO2011/069456, the neonicotinoid M.4A.2, sometimes also to be named as guadipyr, is known from WO2013/003977, and the neonicotinoid M.4A.3 (approved as paichongding in China) is known from WO2007/101369. The metaflumizone analogue M.22B.1 is described in CN10171577 and the analogue M.22B.2 in CN102126994. The phthalamides M.28.1 and M.28.2 are both known from WO2007/101540. The anthranilamide M.28.3 is described in WO2005/077934. The hydrazide compound M.28.4 is described in WO2007/043677. The anthranilamides M.28.5a) to M.28.5d) and M.28.5h) are described in WO 2007/006670, WO2013/024009 and
WO2013/024010, the anthranilamide M.28.5i) is described in WO2011/085575, M.28.5j) in WO2008/134969, M.28.5k) in US2011/046186 and M.28.5l) in
WO2012/034403. The diamide compound M.28.6 can be found in WO2012/034472. The spiroketal-substituted cyclic ketoenol derivative M.29.3 is known from
WO2006/089633 and the biphenyl-substituted spirocyclic ketoenol derivative M.29.4 from WO2008/067911. The triazoylphenylsulfide M.29.5 is described in
WO2006/043635, and biological control agents on the basis of bacillus firmus are described in WO2009/124707. The compounds M.29.6a) to M.29.6i) listed under M.29.6 are described in WO2012/029672, and M.29.6j) and M.29.6k) in
WO2013/129688. The nematicide M.29.8 is known from WO2013/055584. The isoxazoline M.29.9.a) is described in WO2013/050317. The isoxazoline M.29.9.b) is described in WO2014/126208. The pyridalyl-type analogue M.29.10 is known from WO2010/060379. The carboxamides broflanilide and M.29.11.b) to M.29.11.h) are described in WO2010/018714, and the carboxamides M.29.11i) to M.29.11.p) in WO2010/127926. The pyridylthiazoles M.29.12.a) to M.29.12.c) are known from WO2010/006713, M.29.12.d) and M.29.12.e) are known from WO2012/000896, and M.29.12.f) to M.29.12.m) from WO2010/129497. The compounds M.29.14a) and M.29.14b) are known from WO2007/101369. The pyrazoles M.29.16.a) to M.29.16h) are described in WO2010/034737, WO2012/084670, and WO2012/143317, respectively, and the pyrazoles M.29.16i) and M.29.16j) are described in US
61/891437. The pyridinylindazoles M.29.17a) to M.29.17.j) are described in
WO2015/038503. The pyridylpyrazoles M.29.18a) to M.29.18d) are described in US2014/0213448. The isoxazoline M.29.19 is described in WO2014/036056. The isoxazoline M.29.20 is known from WO2014/090918.
The following list of fungicides, in conjunction with which the compounds of the present invention can be used, is intended to illustrate the possible combinations but
does not limit them:
A) Respiration inhibitors
- Inhibitors of complex III at Qo site (e. g. strobilurins): azoxystrobin (A.1.1), coumethoxystrobin (A.1.2), coumoxystrobin (A.1.3), dimoxystrobin (A.1.4), enestroburin (A.1.5), fenaminstrobin (A.1.6), fenoxystrobin/flufenoxystrobin (A.1.7), fluoxastrobin (A.1.8), kresoxim-methyl (A.1.9), mandestrobin (A.1.10), metominostrobin (A.1.11), orysastrobin (A.1.12), picoxy.strobin (A.1.13), pyraclostrobin (A.1.14), pyrametostrobin (A.1.15), pyraoxystrobin (A.1.16), trifloxystrobin (A.1.17), 2-(2-(3-(2,6-dichlorophenyl)- 1-methyl-allylideneaminooxymethyl)-phenyl)-2-methoxyimino-N-methyl-acetamide (A.1.18), pyribencarb (A.1.19), triclopyricarb/chlorodincarb (A.1.20), famoxadone (A.1.21), fenamidone (A.1.21), methyl-N-[2-[(1,4-dimethyl-5-phenyl-pyrazol-3- yl)oxylmethyl]phenyl]-N-methoxy-carbamate (A.1.22), 1-[3-chloro-2-[[1-(4- chlorophenyl)-1H-pyrazol-3-yl]oxymethyl]phenyl]-4-methyl-tetrazol-5-one (A.1.23), 1-[3- bromo-2-[[1-(4-chlorophenyl)pyrazol-3-yl]oxymethyl]phenyl]-4-methyl-tetrazol-5-one (A.1.24), 1-[2-[[1-(4-chlorophenyl)pyrazol-3-yl]oxymethyl]-3-methyl-phenyl]-4-methyl- tetrazol-5-one (A.1.25), 1-[2-[[1-(4-chlorophenyl)pyrazol-3-yl]oxymethyl]-3-fluoro- phenyl]-4-methyl-tetrazol-5-one (A.1.26), 1-[2-[[1-(2,4-dichlorophenyl)pyrazol-3- yl]oxymethyl]-3-fluoro-phenyl]-4-methyl-tetrazol-5-one (A.1.27), 1-[2-[[4-(4- chlorophenyl)thiazol-2-yl]oxymethyl]-3-methyl-phenyl]-4-methyl-tetrazol-5-one (A.1.28), 1-[3-chloro-2-[[4-(p-tolyl)thiazol-2-yl]oxymethyl]phenyl]-4-methyl-tetrazol-5-one
(A.1.29), 1-[3-cyclopropyl-2-[[2-methyl-4-(1-methylpyrazol-3-yl)phenoxy]methyl]phenyl]- 4-methyl-tetrazol-5-one (A.1.30), 1-[3-(difluoromethoxy)-2-[[2-methyl-4- (1-methylpyrazol-3-yl)phenoxy]methyl]phenyl]-4-methyl-tetrazol-5-one (A.1.31), 1- methyl-4-[3-methyl-2-[[2-methyl-4-(1-methylpyrazol-3- yl)phenoxy]methyl]phenyl]tetrazol-5-one (A.1.32), 1-methyl-4-[3-methyl-2-[[1-[3- (trifluoromethyl)phenyl]-ethylideneamino]oxymethyl]phenyl]tetrazol-5-one (A.1.33), (Z,2E)-5-[1-(2,4-dichlorophenyl)pyrazol-3-yl]oxy-2-methoxyimino-N,3-dimethyl-pent-3- enamide (A.1.34), (Z,2E)-5-[1-(4-chlorophenyl)pyrazol-3-yl]oxy-2-methoxyimino-N,3- dimethyl-pent-3-enamide (A.1.35), (Z,2E)-5-[1-(4-chloro-2-fluoro-phenyl)pyrazol-3- yl]oxy-2-methoxyimino-N,3-dimethyl-pent-3-enamide (A.1.36),
- inhibitors of complex III at Qi site: cyazofamid (A.2.1), amisulbrom (A.2.2),
[(3S,6S,7R,8R)-8-benzyl-3-[(3-acetoxy-4-methoxy-pyridine-2-carbonyl)amino]- 6-methyl-4,9-dioxo-1,5-dioxonan-7-yl] 2-methylpropanoate (A.2.3), [(3S,6S,7R,8R)-8- benzyl-3-[[3-(acetoxymethoxy)-4-methoxy-pyridine-2-carbonyl]amino]-6-methyl-4,9- dioxo-1,5-dioxonan-7-yl] 2-methylpropanoate (A.2.4), [(3S,6S,7R,8R)-8-benzyl-3-[(3- isobutoxycarbonyloxy-4-methoxy-pyridine-2-carbonyl)amino]-6-methyl-4,9-dioxo-1,5- dioxonan-7-yl] 2-methylpropanoate (A.2.5), [(3S,6S,7R,8R)-8-benzyl-3-[[3-(1,3-ben- zodioxol-5-ylmethoxy)-4-methoxy-pyridine-2-carbonyl]amino]-6-methyl-4,9-dioxo-1,5- dioxonan-7-yl] 2-methylpropanoate (A.2.6); (3S,6S,7R,8R)-3-[[(3-hydroxy-4-methoxy-2-
pyridinyl)carbonyl]amino]-6-methyl-4,9-dioxo-8-(phenylmethyl)-1,5-dioxonan-7-yl 2- methylpropanoate (A.2.7), (3S,6S,7R,8R)-8-benzyl-3-[3-[(isobutyryloxy)methoxy]-4- methoxypicolinamido]-6-methyl-4,9-dioxo-1,5-dioxonan-7-yl isobutyrate (A.2.8);
- inhibitors of complex II (e. g. carboxamides): benodanil (A.3.1), benzovindiflupyr (A.3.2), bixafen (A.3.3), boscalid (A.3.4), carboxin (A.3.5), fenfuram (A.3.6), fluopyram (A.3.7), flutolanil (A.3.8), fluxapyroxad (A.3.9), furametpyr (A.3.10), isofetamid (A.3.11), isopyrazam (A.3.12), mepronil (A.3.13), oxycarboxin (A.3.14), penflufen (A.3.14), penthiopyrad (A.3.15), sedaxane (A.3.16), tecloftalam (A.3.17), thifluzamide (A.3.18), N-(4'-trifluoromethylthiobiphenyl-2-yl)-3-difluoromethyl-1-methyl-1H-pyrazole-4- carboxamide (A.3.19), N-(2-(1,3,3-trimethyl-butyl)-phenyl)-1,3-dimethyl-5-fluoro-1H-pyr- azole-4-carboxamide (A.3.20), 3-(difluoromethyl)-1-methyl-N-(1,1,3-trimethylindan-4- yl)pyrazole-4-carboxamide (A.3.21), 3-(trifluoromethyl)-1-methyl-N-(1,1,3-trimethyl- indan-4-yl)pyrazole-4-carboxamide (A.3.22), 1,3-dimethyl-N-(1,1,3-trimethylindan-4- yl)pyrazole-4-carboxamide (A.3.23), 3-(trifluoromethyl)-1,5-dimethyl-N-(1,1,3- trimethylindan-4-yl)pyrazole-4-carboxamide (A.3.24), 1,3,5-trimethyl-N-(1,1,3- trimethylindan-4-yl)pyrazole-4-carboxamide (A.3.25), N-(7-fluoro-1,1,3-trimethyl-indan- 4-yl)-1,3-dimethyl-pyrazole-4-carboxamide (A.3.26), N-[2-(2,4-dichlorophenyl)-2- methoxy-1-methyl-ethyl]-3-(difluoromethyl)-1-methyl-pyrazole-4-carboxamide (A.3.27); - other respiration inhibitors (e. g. complex I, uncouplers): diflumetorim (A.4.1), (5,8-difluoroquinazolin-4-yl)-{2-[2-fluoro-4-(4-trifluoromethylpyridin-2-yloxy)-phenyl]- ethyl}-amine (A.4.2); nitrophenyl derivates: binapacryl (A.4.3), dinobuton (A.4.4), dinocap (A.4.5), fluazinam (A.4.6); ferimzone (A.4.7); organometal compounds: fentin salts, such as fentin-acetate (A.4.8), fentin chloride (A.4.9) or fentin hydroxide (A.4.10); ametoctradin (A.4.11); and silthiofam (A.4.12);
B) Sterol biosynthesis inhibitors (SBI fungicides)
- C14 demethylase inhibitors (DMI fungicides): triazoles: azaconazole (B.1.1), bitertanol (B.1.2), bromuconazole (B.1.3), cyproconazole (B.1.4), difenoconazole (B.1.5), diniconazole (B.1.6), diniconazole-M (B.1.7), epoxiconazole (B.1.8), fenbuconazole (B.1.9), fluquinconazole (B.1.10), flusilazole (B.1.11), flutriafol (B.1.12), hexaconazole (B.1.13), imibenconazole (B.1.14), ipconazole (B.1.15), metconazole (B.1.17), myclobutanil (B.1.18), oxpoconazole (B.1.19), paclobutrazole (B.1.20), penconazole (B.1.21), propiconazole (B.1.22), prothioconazole (B.1.23), simeconazole (B.1.24), tebuconazole (B.1.25), tetraconazole (B.1.26), triadimefon (B.1.27), triadimenol (B.1.28), triticonazole (B.1.29), uniconazole (B.1.30), 1-[rel-(2S;3R)-3-(2- chlorophenyl)-2-(2,4-difluorophenyl)-oxiranylmethyl]-5-thiocyanato-1H-[1,2,4]triazolo (B.1.31), 2-[rel-(2S;3R)-3-(2-chlorophenyl)-2-(2,4-difluorophenyl)-oxiranylmethyl]- 2H-[1,2,4]triazole-3-thiol (B.1.32), 2-[2-chloro-4-(4-chlorophenoxy)phenyl]-1-(1,2,4- triazol-1-yl)pentan-2-ol (B.1.33), 1-[4-(4-chlorophenoxy)-2-(trifluoromethyl)phenyl]- 1-cyclopropyl-2-(1,2,4-triazol-1-yl)ethanol (B.1.34), 2-[4-(4-chlorophenoxy)-2-
(trifluoromethyl)phenyl]-1-(1,2,4-triazol-1-yl)butan-2-ol (B.1.35), 2-[2-chloro-4-(4- chlorophenoxy)phenyl]-1-(1,2,4-triazol-1-yl)butan-2-ol (B.1.36), 2-[4-(4-chlorophenoxy)- 2-(trifluoromethyl)phenyl]-3-methyl-1-(1,2,4-triazol-1-yl)butan-2-ol (B.1.37), 2-[4-(4- chlorophenoxy)-2-(trifluoromethyl)phenyl]-1-(1,2,4-triazol-1-yl)propan-2-ol (B.1.38), 2- [2-chloro-4-(4-chlorophenoxy)phenyl]-3-methyl-1-(1,2,4-triazol-1-yl)butan-2-ol (B.1.39), 2-[4-(4-chlorophenoxy)-2-(trifluoromethyl)phenyl]-1-(1,2,4-triazol-1-yl)pentan-2-ol (B.1.40), 2-[4-(4-fluorophenoxy)-2-(trifluoromethyl)phenyl]-1-(1,2,4-triazol-1-yl)propan- 2-ol (B.1.41), 2-[2-chloro-4-(4-chlorophenoxy)phenyl]-1-(1,2,4-triazol-1-yl)pent-3-yn-2-ol (B.1.51); imidazoles: imazalil (B.1.42), pefurazoate (B.1.43), prochloraz (B.1.44), triflumizol (B.1.45); pyrimidines, pyridines and piperazines: fenarimol (B.1.46), nuarimol (B.1.47), pyrifenox (B.1.48), triforine (B.1.49), [3-(4-chloro-2-fluoro-phenyl)-5-(2,4- difluorophenyl)isoxazol-4-yl]-(3-pyridyl)methanol (B.1.50);
- Delta14-reductase inhibitors: aldimorph (B.2.1), dodemorph (B.2.2), dodemorph- acetate (B.2.3), fenpropimorph (B.2.4), tridemorph (B.2.5), fenpropidin (B.2.6), piperalin (B.2.7), spiroxamine (B.2.8);
- Inhibitors of 3-keto reductase: fenhexamid (B.3.1);
C) Nucleic acid synthesis inhibitors
- phenylamides or acyl amino acid fungicides: benalaxyl (C.1.1), benalaxyl-M (C.1.2), kiralaxyl (C.1.3), metalaxyl (C.1.4), metalaxyl-M (mefenoxam, C.1.5), ofurace (C.1.6), oxadixyl (C.1.7);
- others: hymexazole (C.2.1), octhilinone (C.2.2), oxolinic acid (C.2.3), bupirimate (C.2.4), 5-fluorocytosine (C.2.5), 5-fluoro-2-(p-tolylmethoxy)pyrimidin-4-amine (C.2.6), 5-fluoro-2-(4-fluorophenylmethoxy)pyrimidin-4-amine (C.2.7);
D) Inhibitors of cell division and cytoskeleton
- tubulin inhibitors, such as benzimidazoles, thiophanates: benomyl (D1.1), carbendazim (D1.2), fuberidazole (D1.3), thiabendazole (D1.4), thiophanate-methyl (D1.5); triazolopyrimidines: 5-chloro-7-(4-methylpiperidin-1-yl)-6-(2,4,6-trifluorophenyl)- [1,2,4]triazolo[1,5-a]pyrimidine (D1.6);
- other cell division inhibitors: diethofencarb (D2.1), ethaboxam (D2.2), pencycuron (D2.3), fluopicolide (D2.4), zoxamide (D2.5), metrafenone (D2.6), pyriofenone (D2.7); E) Inhibitors of amino acid and protein synthesis
- methionine synthesis inhibitors (anilino-pyrimidines): cyprodinil (E.1.1), mepani- pyrim (E.1.2), pyrimethanil (E.1.3);
- protein synthesis inhibitors: blasticidin-S (E.2.1), kasugamycin (E.2.2), kasugamycin hydrochloride-hydrate (E.2.3), mildiomycin (E.2.4), streptomycin (E.2.5), oxytetracyclin (E.2.6), polyoxine (E.2.7), validamycin A (E.2.8);
F) Signal transduction inhibitors
- MAP / histidine kinase inhibitors: fluoroimid (F.1.1), iprodione (F.1.2),
procymidone (F.1.3), vinclozolin (F.1.4), fenpiclonil (F.1.5), fludioxonil (F.1.6);
- G protein inhibitors: quinoxyfen (F.2.1);
G) Lipid and membrane synthesis inhibitors
- Phospholipid biosynthesis inhibitors: edifenphos (G.1.1), iprobenfos (G.1.2), pyrazophos (G.1.3), isoprothiolane (G.1.4);
- lipid peroxidation: dicloran (G.2.1), quintozene (G.2.2), tecnazene (G.2.3), tolclofos-methyl (G.2.4), biphenyl (G.2.5), chloroneb (G.2.6), etridiazole (G.2.7);
- phospholipid biosynthesis and cell wall deposition: dimethomorph (G.3.1), flumorph (G.3.2), mandipropamid (G.3.3), pyrimorph (G.3.4), benthiavalicarb (G.3.5), iprovalicarb (G.3.6), valifenalate (G.3.7) and N-(1-(1-(4-cyano-phenyl)ethanesulfonyl)- but-2-yl) carbamic acid-(4-fluorophenyl) ester (G.3.8);
- compounds affecting cell membrane permeability and fatty acides: propamocarb (G.4.1);
- fatty acid amide hydrolase inhibitors: oxathiapiprolin (G.5.1), 2-{3-[2-(1-{[3,5- bis(difluoromethyl-1H-pyrazol-1-yl]acetyl}piperidin-4-yl)-1,3-thiazol-4-yl]-4,5-dihydro- 1,2-oxazol-5-yl}phenyl methanesulfonate (G.5.2), 2-{3-[2-(1-{[3,5-bis(difluoromethyl)- 1H-pyrazol-1-yl]acetyl}piperidin-4-yl) 1,3-thiazol-4-yl]-4,5-dihydro-1,2-oxazol-5-yl}-3- chlorophenyl methanesulfonate (G.5.3);
H) Inhibitors with Multi Site Action
- inorganic active substances: Bordeaux mixture (H.1.1), copper acetate (H.1.2), copper hydroxide (H.1.3), copper oxychloride (H.1.4), basic copper sulfate (H.1.5), sulfur (H.1.6);
- thio- and dithiocarbamates: ferbam (H.2.1), mancozeb (H.2.2), maneb (H.2.3), metam (H.2.4), metiram (H.2.5), propineb (H.2.6), thiram (H.2.7), zineb (H.2.8), ziram (H.2.9);
- organochlorine compounds (e. g. phthalimides, sulfamides, chloronitriles):
anilazine (H.3.1), chlorothalonil (H.3.2), captafol (H.3.3), captan (H.3.4), folpet (H.3.5), dichlofluanid (H.3.6), dichlorophen (H.3.7), hexachlorobenzene (H.3.8),
pentachlorphenole (H.3.9) and its salts, phthalide (H.3.10), tolylfluanid (H.3.11), N-(4- chloro-2-nitro-phenyl)-N-ethyl-4-methyl-benzenesulfonamide (H.3.12);
- guanidines and others: guanidine (H.4.1), dodine (H.4.2), dodine free base (H.4.3), guazatine (H.4.4), guazatine-acetate (H.4.5), iminoctadine (H.4.6),
iminoctadine-triacetate (H.4.7), iminoctadine-tris(albesilate) (H.4.8), dithianon (H.4.9), 2,6-dimethyl-1H,5H-[1,4]dithiino[2,3-c:5,6-c']dipyrrole-1,3,5,7(2H,6H)-tetraone (H.4.10); I) Cell wall synthesis inhibitors
- inhibitors of glucan synthesis: validamycin (I.1.1), polyoxin B (I.1.2);
- melanin synthesis inhibitors: pyroquilon (I.2.1), tricyclazole (I.2.2), carpropamid (I.2.3), dicyclomet (I.2.4), fenoxanil (I.2.5);
J) Plant defence inducers
- acibenzolar-S-methyl (J.1.1), probenazole (J.1.2), isotianil (J.1.3), tiadinil (J.1.4),
prohexadione-calcium (J.1.5); phosphonates: fosetyl (J.1.6), fosetyl-aluminum (J.1.7), phosphorous acid and its salts (J.1.8), potassium or sodium bicarbonate (J.1.9);
K) Unknown mode of action
- bronopol (K.1.1), chinomethionat (K.1.2), cyflufenamid (K.1.3), cymoxanil (K.1.4), dazomet (K.1.5), debacarb (K.1.6), diclomezine (K.1.7), difenzoquat (K.1.8), difenzoquat-methylsulfate (K.1.9), diphenylamin (K.1.10), fenpyrazamine (K.1.11), flumetover (K.1.12), flusulfamide (K.1.13), flutianil (K.1.14), methasulfocarb (K.1.15), nitrapyrin (K.1.16), nitrothal-isopropyl (K.1.18), oxathiapiprolin (K.1.19), tolprocarb (K.1.20), oxin-copper (K.1.21), proquinazid (K.1.22), tebufloquin (K.1.23), tecloftalam (K.1.24), triazoxide (K.1.25), 2-butoxy-6-iodo-3-propylchromen-4-one (K.1.26), 2-[3,5- bis(difluoromethyl)-1H-pyrazol-1-yl]-1-[4-(4-{5-[2-(prop-2-yn-1-yloxy)phenyl]-4,5- dihydro-1,2-oxazol-3-yl}-1,3-thiazol-2-yl)piperidin-1-yl]ethanone (K.1.27), 2-[3,5- bis(difluoromethyl)-1H-pyrazol-1-yl]-1-[4-(4-{5-[2-fluoro-6-(prop-2-yn-1-yloxy)phenyl]- 4,5-dihydro-1,2-oxazol-3-yl}-1,3-thiazol-2-yl)piperidin-1-yl]ethanone (K.1.28), 2-[3,5- bis(difluoromethyl)-1H-pyrazol-1-yl]-1-[4-(4-{5-[2-chloro-6-(prop-2-yn-1-yloxy)phenyl]- 4,5-dihydro-1,2-oxazol-3-yl}-1,3-thiazol-2-yl)piperidin-1-yl]ethanone (K.1.29), N-(cyclo- propylmethoxyimino-(6-difluoro-methoxy-2,3-difluoro-phenyl)-methyl)-2-phenyl acetamide (K.1.30), N'-(4-(4-chloro-3-trifluoromethyl-phenoxy)-2,5-dimethyl-phenyl)-N- ethyl-N-methyl formamidine (K.1.31), N'-(4-(4-fluoro-3-trifluoromethyl-phenoxy)-2,5- dimethyl-phenyl)-N-ethyl-N-methyl formamidine (K.1.32), N'-(2-methyl-5-trifluoromethyl- 4-(3-trimethylsilanyl-propoxy)-phenyl)-N-ethyl-N-methyl formamidine (K.1.33), N'-(5- difluoromethyl-2-methyl-4-(3-trimethylsilanyl-propoxy)-phenyl)-N-ethyl-N-methyl formamidine (K.1.34), methoxy-acetic acid 6-tert-butyl-8-fluoro-2,3-dimethyl-quinolin-4- yl ester (K.1.35), 3-[5-(4-methylphenyl)-2,3-dimethyl-isoxazolidin-3-yl]-pyridine (K.1.36), 3-[5-(4-chloro-phenyl)-2,3-dimethyl-isoxazolidin-3-yl]-pyridine (pyrisoxazole) (K.1.37), N-(6-methoxy-pyridin-3-yl) cyclopropanecarboxylic acid amide (K.1.38), 5-chloro- 1-(4,6-dimethoxy-pyrimidin-2-yl)-2-methyl-1H-benzoimidazole (K.1.39), 2-(4-chloro- phenyl)-N-[4-(3,4-dimethoxy-phenyl)-isoxazol-5-yl]-2-prop-2-ynyloxy-acetamide, ethyl (Z)-3-amino-2-cyano-3-phenyl-prop-2-enoate (K.1.40), picarbutrazox (K.1.41), pentyl N-[6-[[(Z)-[(1-methyltetrazol-5-yl)-phenyl-methylene]amino]oxymethyl]-2- pyridyl]carbamate (K.1.42), 2-[2-[(7,8-difluoro-2-methyl-3-quinolyl)oxy]-6-fluoro- phenyl]propan-2-ol (K.1.43), 2-[2-fluoro-6-[(8-fluoro-2-methyl-3-quinolyl)oxy]phen- yl]propan-2-ol (K.1.44), 3-(5-fluoro-3,3,4,4-tetramethyl-3,4-dihydroisoquinolin-1-yl)- quinoline (K.1.45), 3-(4,4-difluoro-3,3-dimethyl-3,4-dihydroisoquinolin-1-yl)quinoline (K.1.46), 3-(4,4,5-trifluoro-3,3-dimethyl-3,4-dihydroisoquinolin-1-yl)quinoline (K.1.47), 9- fluoro-2,2-dimethyl-5-(3-quinolyl)-3H-1,4-benzoxazepine (K.1.48).
The fungicides described by common names, their preparation and their activity e.g. against harmful fungi is known (cf.: http://www.alanwood.net/pesticides/); these substances are commercially available.
The fungicides described by IUPAC nomenclature, their preparation and their pesticidal activity is also known (cf. Can. J. Plant Sci.48(6), 587-94, 1968;
EP-A 141317; EP-A 152031; EP-A 226917; EP-A 243970; EP-A 256503;
EP-A 428941; EP-A 532022; EP-A 1028125; EP-A 1035122; EP-A 1201648; EP-A 1122244, JP 2002316902; DE 19650197; DE 10021412; DE 102005009458; US 3,296,272; US 3,325,503; WO 98/46608; WO 99/14187; WO 99/24413;
WO 99/27783; WO 00/29404; WO 00/46148; WO 00/65913; WO 01/54501;
WO 01/56358; WO 02/22583; WO 02/40431; WO 03/10149; WO 03/11853;
WO 03/14103; WO 03/16286; WO 03/53145; WO 03/61388; WO 03/66609;
WO 03/74491; WO 04/49804; WO 04/83193; WO 05/120234; WO 05/123689;
WO 05/123690; WO 05/63721; WO 05/87772; WO 05/87773; WO 06/15866;
WO 06/87325; WO 06/87343; WO 07/82098; WO 07/90624, WO 11/028657,
WO2012/168188, WO 2007/006670, WO 2011/77514; WO13/047749, WO 10/069882, WO 13/047441, WO 03/16303, WO 09/90181, WO 13/007767, WO 13/010862, WO 13/127704, WO 13/024009, WO 13/024010 and WO 13/047441, WO 13/162072, WO 13/092224, WO 11/135833). The present invention is now illustrated in further detail by the following examples, without imposing any limitation thereto. Examples The following abbreviations are used:
TFA: trifluoroacetic acid
EtOAc: ethyl acetate
HPLC: high performance liquid chromatography
MS: mass spectrometry
MeOH: methanol
tR: retention time The compound examples were characterized by coupled High Performance Liquid Chromatography with mass spectrometry (HPLC/MS) or by their melting point.
Method A: Analytical HPLC column 1: RP-18 column Chromolith Speed ROD (from Merck KgaA, Germany). Elution: acetonitrile + 0.1 % TFA acid/ water + 0.1 % TFA in a ratio of from 5:95 to 95:5 in 5 minutes at 40 °C.
Method B:
Analytical UPLC column: Phenomenex Kinetex 1,7 µm XB-C18100A; 50 x 2.1 mm; mobile phase: A: water+ 0.1 % TFA; B: acetonitrile + 0.1 % TFA; gradient: 5-100% B in 1.50 minutes; 100% B 0.20 min; flow: 0,8-1,0mL/min in 1,50 minutes at 60°C.
MS-method: ESI positive. A. Preparation examples
a) Preparation of intermediate compounds
a.1) Synthesis of amine salt a.1
A solution of 2-chloro-5-(chloromethyl)-pyridine (16.20 g, 100 mmol) and 2- amino-pyridine (9.60 g, 102 mmol) in ethanol (100 mL) was refluxed for 24 hours. The reaction was then cooled to room temperature, and concentrated in vacuo. Then 100 mL of toluene were added to the residue, and the mixture was concentrated in vacuo. 75 mL of CH2Cl2 were added to the residue and the mixture was stirred rapidly for 15 minutes, during which time a precipitate formed. The precipitate was then filtered, washed with CH2Cl2 (50 mL) and diethyl ether (50 mL), and dried under vacuum to afford the title product as a pale yellow solid (14.0g, 55% yield).
LC-MS: mass calculated. for C11H11ClN3 [M]+ 220.1, found 220.1; tR= 0.529 min. a.2) Synthesis of methyl 5-pent-1-ynylpyrazine-2-carboxylate
To a solution of methyl 5-chloropyrazine-2-carboxylate (0.500g, 2.90 mmol) and 1-pentyne (0.470g, 6.92 mmol) in triethlyamine (6.70 mL) at room temperature was sequentially added copper(I) iodide (0.005g, 0.020 mmol) and Pd(PPh3)4 (0.020g, 0.030 mmol). The reaction was then heated to 50°C and stirred for 18 hours. The reaction was concentrated in vacuo to afford a residue. To the residue was then added 10% aqueous HCl (100 mL) and CH2Cl2 (100 mL); the layers were separated, and the aqueous phase was extracted twice with CH2Cl2 (100 mL). The combined organic phases were dried over Na2SO4 and concentrated in vacuo to afford the title product as an off white solid (0.320g, 53%), which was sufficiently pure to carry on without further purification
LC-MS: mass calculated for C11H13N2O2 [M+H]+ 204.1, found 204.8; tR= 1.004 min. a.3) Synthesis of 5-pent-1-ynylpyrazine-2-carboxylic acid
To a solution of methyl 5-pent-1-ynylpyrazine-2-carboxylate (0.120g, 0.590 mmol) from example a.2) in dioxane (1 mL) and water (1 mL) at room temperature was added lithium hydroxide (0.060g, 2.50 mmoL) as a solid. The reaction was then stirred at room temperature for 18 hours. The reaction was then diluted with water (50 mL) and the pH was adjusted to 4 by the addition of 10% aqueous HCl. The aqeous phase
was then extracted three times with EtOAC (100 mL each). The combined organic phases were dried over Na2SO4 and concentrated in vacuo to afford the title product as an off white solid (0.80g, 72%), which was sufficiently pure to carry on without further purification
LC-MS: mass calculated for C10H11N2O2 [M+H]+ 190.1, found 190.8; tR= 0.838 min. b) Preparation of final compounds b.1) (E)-N-[1-[(6-Chloro-3-pyridyl)methyl]-2-pyridylidene]-5-pent-1-ynyl-pyrazine-2- carboxamide (compound of formula 1-1)
To a suspension of amine salt a.1 (0.430g, 1.68 mmol) from example a.1), 5-pent-1- ynylpyrazine-2-carboxylic acid from example a.3) (0.380g, 1.19 mmol), and
triethylamine (0.420g, 4.20 mmol) in CH2Cl2 (13 mL) at room temperature was added a solution of propyl phosphoric anhydride (1.28g, 2.01 mmol, a 50% wt solution in DMF was used). The reaction was then heated in the mircrowave at 90°C for 2 hrs. The reaction was then dilluted with EtOAc (100 mL), washed with saturated aqueous NaHCO3 (100 mL) and water (100mL), the layers were separated, and the organic layer was dried over Na2SO4 and concentrated in vacuo to afford a residue, which was purified using column chromatography over silica gel (0 ^30% MeOH/ CH2Cl2) to afford the desired product as a beige solid (0.080g, 12% yield).
LC-MS: mass calculated for C21H19N5OCl [M+H]+ 392.1, found 392.6; tR= 0.909 min. The following compounds were synthesized in analogy to the above synthetic path:
0
00 00 87 34 1
1 12 RT [min] m/z [M+H]+ Method
0.909 392.6 B
0.714 350.3 B
0,906 393,1 B
0,775 364,1 B
0,768 394,9 B
0,878 399 B
0,904 443,8 B
00 0 0,837 395 B 0
87 4 1,026 - B 31 0,811 378,9 B
0.751 350.7 B
0,806 379 B
0,85 393 B
0.799 417.1 B
12 0.902 426.1 B 2 0.978 406.1 B
0.950 440.0 B
1.037 442.0 B
1.120 422.0 B
1.045 408.0 B
1.053 456.0 B
0.948 406.1 B
00 0 5-chloropent-1-ynyl CH3 0.906 440.0 B 0
87 4 5-cyanopent-1-ynyl CH3 0.813 430.8 B 31 5-cyanopent-1-ynyl CH3 0.795 431.8 B
pent-1-ynyl CH3 1.077 421.8 B
5-cyanopent-1-ynyl CH3 0.897 447.0 B
5-chloropent-1-ynyl CH3 1.029 456.0 B
5-cyanopent-1-ynyl CH3 0.831 437.0 B
1 32
00 70 48 13
(C.2) 1 42 R6c2 RT [min] m/z [M+H]+ Method
H 0.912 355.9 B
CH3 1.126 412.0 B
H 1.185 412.0 B
CH3 1.202 426.2 B
The activity of the compounds of formula I of the present invention could be demonstrated and evaluated in biological tests described in the following.
If not otherwise specified the test solutions were prepared as follows:
The active compound was dissolved at the desired concentration in a mixture of 1:1 (vol:vol) distilled water : aceton. The test solution was prepared at the day of use and in general at concentrations of ppm (wt/vol). B1 Boll weevil (Anthonomus grandis)
For evaluating control of boll weevil (Anthonomus grandis) the test unit consisted of 96-well-microtiter plates containing an insect diet and 5-10 A. grandis eggs.
The compounds were formulated using a solution containing 75% v/v water and 25% v/v DMSO. Different concentrations of formulated compounds were sprayed onto the insect diet at 5 µl, using a custom built micro atomizer, at two replications.
After application, microtiter plates were incubated at about 25 + 1°C and about 75 + 5 % relative humidity for 5 days. Egg and larval mortality was then visually assessed. In this test, compounds 1-2, 1-4, 1-6, 1-16, 1-23, 1-28, 2-2, 2-3 at 1415 ppm showed over 75 % mortality in comparison with untreated controls. B2 Colorado Potato Beetle (Leptinotarsa decemlineata)
The active compounds were formulated by a Tecan liquid handler in 100% cyclohexanone as a 10,000 ppm solution supplied in tubes. The 10,000 ppm solution was serially diluted in 100% cyclohexanone to make interim solutions. These served as stock solutions for which final dilutions were made by the Tecan in 50% acetone : 50% water (v/v) into 10 or 20ml glass vials. A nonionic surfactant (Kinetic®) was included in the solution at a volume of 0.01% (v/v). The vials were then inserted into an automated electrostatic sprayer equipped with an atomizing nozzle for application to
plants/insects.
Eggplants were grown 2 plants to a pot and were selected for treatment at the 1st true leaf stage. Test solutions were sprayed onto the foliage by an automated electrostatic plant sprayer equipped with an atomizing spray nozzle. The plants were dried in the sprayer fume hood and then removed from the sprayer. The treated foliage was then cut and removed from the pot and placed in a Petri dish lined with moistened filter paper. Five beetle larvae were introduced into each Petri dish and the dish was covered by a Petri dish lid. Petri dishes were maintained in a growth room at about 25°C and about 20-40% relative humidity for 4 days, avoiding direct exposure to fluorescent light (24 hour photoperiod) to prevent trapping of heat inside the dishes.
Mortality and reduced feeding were assessed 4 days after treatment, compared to untreated control plants. B3 Cotton aphid (Aphis gossypii) I
The active compounds were formulated by a Tecan liquid handler in 100% cyclohexanone as a 10,000 ppm solution supplied in tubes. The 10,000 ppm solution was serially diluted in 100% cyclohexanone to make interim solutions. These served as stock solutions for which final dilutions were made by the Tecan in 50% acetone:50% water (v/v) into 10 or 20ml glass vials. A nonionic surfactant (Kinetic®) was included in the solution at a volume of 0.01% (v/v). The vials were then inserted into an automated electrostatic sprayer equipped with an atomizing nozzle for application to
plants/insects.
Cotton plants at the cotyledon stage were infested with aphids prior to treatment by placing a heavily infested leaf from the main aphid colony on top of each cotyledon. Aphids were allowed to transfer overnight to accomplish an infestation of 80-100 aphids per plant and the host leaf was removed. The infested plants were then sprayed by an automated electrostatic plant sprayer equipped with an atomizing spray nozzle. The plants were dried in the sprayer fume hood, removed from the sprayer, and then maintained in a growth room under fluorescent lighting in a 24 h photoperiod at 25°C and 20-40% relative humidity. Aphid mortality on the treated plants, relative to mortality on untreated control plants, was determined after 5 days.
In this test, compounds 1-21, 1-28, 2-2 at 300 ppm showed over 75 % mortality in comparison with untreated controls. B4 Cotton aphid (Aphis gossypii) II for seed treatment
The active compounds were prepared and formulated in 50% acetone : 50% water (vol:vol) in glass vials.
Cotton seeds were placed in the glass vials and mixed with the formulated compounds. Solvent blank control seeds were treated with 50% acetone : 50% water (vol:vol). Treated seeds were then air-dried. The cotton seeds were planted in Metro Mix® potting mix in pots, 2 seeds per pot, and maintained in the greenhouse.
Seedling plants were thinned to one plant per pot. At the cotyledon stage, six plants were infested with Aphis gossypii by manually transferring circa 25 aphids to each plant on a piece of leaf tissue cut from a donor plant infested with aphids. Infested plants were maintained on light carts. Four days after infestation, live aphids on each plant were counted. B5 Cowpea aphid (Aphis craccivora)
The active compound was dissolved at the desired concentration in a mixture of 1:1 (vol:vol) distilled water : acetone. Surfactant (Kinetic® HV) was added at a rate of 0.01% (vol/vol). The test solution is prepared at the day of use.
Potted cowpea plants were colonized with approximately 30 - 50 aphids of various stages by manually transferring a leaf tissue cut from infested plant 24 hours before application. Plants were sprayed with the test solutions using a DeVilbiss® hand atomizer at 20- 30 psi (≈ 1.38 to 2.07 bar) after the pest population has been checked. Treated plants are maintained on light carts at about 25- 26°C. Percent mortality was assessed after 72 hours.
In this test, compounds 1-1, 1-2, 1-3, 1-6, 1-7, 1-10, 1-11, 1-13, 1-15, 1-16, 1-17, 1-20, 1-21, 1-26, 1-27, 1-28, 1-29, 2-2 at 300 ppm showed over 75 % mortality in comparison with untreated controls. B6 Diamond back moth (Plutella xylostella)
The active compound was dissolved at the desired concentration in a mixture of 1:1 (vol:vol) distilled water : acetone. Surfactant (Kinetic® HV) was added at a rate of 0.01% (vol/vol).The test solution was prepared at the day of use.
Leaves of cabbage were dipped in test solution and air-dried. Treated leaves were placed in petri dishes lined with moist filter paper and inoculated with ten 3rd instar larvae. Mortality was recorded 72 hours after treatment. Feeding damages were also recorded using a scale of 0-100%.
In this test, compounds 1-1, 1-3, 1-10 at 300 ppm showed over 75 % mortality in comparison with untreated controls. B7A Green Peach Aphid (Myzus persicae)
The active compounds were formulated by a Tecan liquid handler in 100%
cyclohexanone as a 10,000 ppm solution supplied in tubes. The 10,000 ppm solution was serially diluted in 100% cyclohexanone to make interim solutions. These served as stock solutions for which final dilutions were made by the Tecan in 50% acetone:50% water (v/v) into 10or 20ml glass vials. A nonionic surfactant (Kinetic®) was included in the solution at a volume of 0.01% (v/v). The vials were then inserted into an automated electrostatic sprayer equipped with an atomizing nozzle for application to
plants/insects. Bell pepper plants at the first true-leaf stage were infested prior to treatment by placing heavily infested leaves from the main colony on top of the treatment plants. Aphids were allowed to transfer overnight to accomplish an infestation of 30-50 aphids per plant and the host leaves were removed. The infested plants were then sprayed by an automated electrostatic plant sprayer equipped with an atomizing spray nozzle. The plants were dried in the sprayer fume hood, removed, and then maintained in a growth
room under fluorescent lighting in a 24-hr photoperiod at about 25ºC and about 20-40% relative humidity. Aphid mortality on the treated plants, relative to mortality on untreated control plants, was determined after 5 days. B7B Green Peach Aphid (Myzus persicae)
For evaluating control of green peach aphid (Myzus persicae) through systemic means the test unit consisted of 96-well-microtiter plates containing liquid artificial diet under an artificial membrane.
The compounds were formulated using a solution containing 75% v/v water and 25% v/v DMSO. Different concentrations of formulated compounds were pipetted into the aphid diet, using a custom built pipetter, at two replications.
After application, 5– 8 adult aphids were placed on the artificial membrane inside the microtiter plate wells. The aphids were then allowed to suck on the treated aphid diet and incubated at about 23 + 1°C and about 50 + 5 % relative humidity for 3 days. Aphid mortality and fecundity was then visually assessed.
In this test, compounds 1-1, 1-2, 1-3, 1-4, 1-5, 1-6, 1-7, 1-8, 1-9, 1-10, 1-11, 1-12, 1-13, 1-14, 1-15, 1-16, 1-17, 1-18, 1-19, 1-20, 1-21, 1-22, 1-23, 1-24, 1-25, 1-26, 1-27, 1-28, 1-29, 1-30, 2-1, 2-2, 2-4 at 2500 ppm showed over 75% mortality in comparison with untreated controls. B8 Green Soldier Stink Bug (Nezara viridula)
The active compound was dissolved at the desired concentration in a mixture of 1:1 (vol:vol) distilled water : aceteone. Surfactant (Kinetic® HV) was added at a rate of 0.01% (vol/vol). The test solution was prepared at the day of use.
Soybean pods were placed in 90 x 50 mm glass Petri dishes lined with moist filter paper and inoculated with ten late 3rd instar N. viridula. Using a hand atomizer, an approximately 2 ml solution is sprayed into each Petri dish. Treated cups were kept at about 25-26°C and relative humidity of about 65-70%. Percent mortality was recorded after 5 days.
In this test, compounds 1-3, 1-20, 1-21 at 300 ppm showed over 75 % mortality in comparison with untreated controls.
In this test, compound 1-1 at 500 ppm showed over 75 % mortality in comparison with untreated controls. B10 Mediterranean fruitfly (Ceratitis capitata)
For evaluating control of Mediterranean fruitfly (Ceratitis capitata) the test unit consisted of microtiter plates containing an insect diet and 50-80 C. capitata eggs. The compounds were formulated using a solution containing 75% v/v water and 25% v/v DMSO. Different concentrations of formulated compounds were sprayed onto the insect diet at 5 µl, using a custom built micro atomizer, at two replications.
After application, microtiter plates were incubated at about 28 + 1°C and about 80 + 5 % relative humidity for 5 days. Egg and larval mortality was then visually assessed.
In this test, compounds 1-24, 1-28, 2-2 at 1415 ppm showed over 75 % mortality in comparison with untreated controls. B11 Orchid thrips (dichromothrips corbetti)
Dichromothrips corbetti adults used for bioassay were obtained from a colony maintained continuously under laboratory conditions. For testing purposes, the test compound was diluted in a 1:1 mixture of acetone:water (vol:vol), plus Kinetic® HV at a rate of 0.01% v/v.
Thrips potency of each compound was evaluated by using a floral-immersion technique. All petals of individual, intact orchid flowers were dipped into treatment solution and allowed to dry in Petri dishes. Treated petals were placed into individual re-sealable plastic along with about 20 adult thrips. All test arenas were held under continuous light and a temperature of about 28°C for duration of the assay. After 3 days, the numbers of live thrips were counted on each petal. The percent mortality was recorded 72 hours after treatment.
In this test, compounds 1-1, 1-2, 1-3, 1-5, 1-10, 1-11, 1-12, 1-13, 1-15, 1-16, 1-17, 1-18, 1-19, 1-20, 1-21, 1-22, 1-24, 1-25, 1-26, 1-27, 2-2 at 300 ppm showed over 75 % mortality in comparison with untreated controls. B12 Red spider Mite (Tetranychus kanzawai)
The active compound was dissolved at the desired concentration in a mixture of 1:1 (vol:vol) distilled water : acetone. Surfactant (Kinetic® HV) was added at a rate of 0.01% (vol/vol). The test solution was prepared at the day of use.
Potted cowpea beans of 4-5 days of age were cleaned with tap water and sprayed with 1-2 ml of the test solution using air driven DeVilbiss® hand atomizer at 20- 30 psi (≈ 1,38 to 2,07 bar). The treated plants were allowed to air dry and afterwards inoculated with 30 or more mites by clipping a cassava leaf section from rearing population. Treated plants were placed inside a holding room at about 25-26°C and about 65-70% relative humidity. Percent mortality was assessed 72 hours after treatment.. B13 Rice green leafhopper (Nephotettix virescens)
Four to five-week old rice seedlings with cut upper leaf portion were cleaned and washed 24 hours before spraying. The active compounds were formulated in 1:1 acetone:water (vol:vol), and 0.01% vol/vol surfactant (Kinetic® HV) was added. Potted rice seedlings were sprayed with 5-6 ml test solution, air dried, covered with Mylar
cages and inoculated with 10 adults. Treated rice plants were kept at about 28-29°C and relative humidity of about 50-60%. Percent mortality was recorded after 72 hours.
In this test, compounds 1-1, 1-3, 1-11, 1-12, 1-13, 1-17, 1-20, 1-21 at 300 ppm showed over 75 % mortality in comparison with untreated controls. B14 Rice brown plant hopper (Nilaparvata lugens)
Four to five-week old rice seedlings were cleaned and washed 24 hours before spraying. The active compounds were formulated in 1:1 acetone:water (vol:vol) and 0.01% vol/vol surfactant (Kinetic® HV) was added. Potted rice seedlings were sprayed with 5- 6 ml test solution, air dried, covered with Mylar cages and inoculated with 10 adults. Treated rice plants were kept at about 28-29°C and relative humidity of about 50-60%. Percent mortality was recorded after 72 hours.
In this test, compounds 1-1, 1-12, 1-13, 1-20, 1-21 at 300 ppm showed over 75 % mortality in comparison with untreated controls. B15 Silverleaf whitefly (Bemisia argentifolii)
The active compounds were formulated by a Tecan liquid handler in 100% cyclohexanone as a 10,000 ppm solution supplied in tubes. The 10,000 ppm solution was serially diluted in 100% cyclohexanone to make interim solutions. These served as stock solutions for which final dilutions were made by the Tecan in 50% acetone:50% water (v/v) into 5 or 10ml glass vials. A nonionic surfactant (Kinetic®) was included in the solution at a volume of 0.01% (v/v). The vials were then inserted into an automated electrostatic sprayer equipped with an atomizing nozzle for application to
plants/insects.
Cotton plants at the cotyledon stage (one plant per pot) were sprayed by an automated electrostatic plant sprayer equipped with an atomizing spray nozzle. The plants were dried in the sprayer fume hood and then removed from the sprayer. Each pot was pla-ced into a plastic cup and about 10 to 12 whitefly adults (approximately 3-5 days old) were introduced. The insects were collected using an aspirator and a nontoxic Tygon® tubing connected to a barrier pipette tip. The tip, containing the collected insects, was then gently inserted into the soil containing the treated plant, allowing insects to crawl out of the tip to reach the foliage for feeding. Cups were covered with a reusable screened lid. Test plants were maintained in a growth room at about 25°C and about 20-40% relative humidity for 3 days, avoiding direct exposure to fluorescent light (24 hour photoperiod) to prevent trapping of heat inside the cup.
Mortality was assessed 3 days after treatment, compared to untreated control plants.
In this test, compounds 1-15, 1-21, 1-25, 1-29, 2-1 at 300 ppm showed over 75 % mortality in comparison with untreated controls.
B16 Southern armyworm (Spodoptera eridania), 2nd instar larvae
The active compounds were formulated by a Tecan liquid handler in 100% cyclohexanone as a 10,000 ppm solution supplied in tubes. The 10,000 ppm solution was serially diluted in 100% cyclohexanone to make interim solutions. These served as stock solutions for which final dilutions were made by the Tecan in 50% acetone:50% water (v/v) into 10 or 20ml glass vials. A nonionic surfactant (Kinetic®) was included in the solution at a volume of 0.01% (v/v). The vials were then inserted into an automated electrostatic sprayer equipped with an atomizing nozzle for application to
plants/insects.
Lima bean plants (variety Sieva) were grown 2 plants to a pot and selected for treatment at the 1st true leaf stage. Test solutions were sprayed onto the foliage by an automated electrostatic plant sprayer equipped with an atomizing spray nozzle. The plants were dried in the sprayer fume hood and then removed from the sprayer. Each pot was placed into perforated plastic bags with a zip closure. About 10 to 11 armyworm larvae were placed into the bag and the bags zipped closed. Test plants were maintained in a growth room at about 25°C and about 20-40% relative humidity for 4 days, avoiding direct exposure to fluorescent light (24 hour photoperiod) to prevent trapping of heat inside the bags. Mortality and reduced feeding were assessed 4 days after treatment, compared to untreated control plants.
In this test, compounds 1-1, 1-15, 1-16, 1-17, 1-18, 1-19, 1-20, 1-21, 1-22, 1-26, 1-27, 1-29, 1-30, 2-2 at 300 ppm showed over 75 % mortality in comparison with untreated controls. B17 Striped Stem Borer (Chilo suppressalis)
The active compound was dissolved at the desired concentration in a mixture of 1:1 (vol:vol) distilled water : acetone. Surfactant (Kinetic® HV) was added at a rate of 0.01% (vol/vol). The test solution was prepared at the day of use. Ovicidal Effect:
Day old egg masses on 0.5- 1 inch (≈ 1.27 to 2.54 cm) rice leaf section were soaked in the test solution. The treated egg masses were transferred in petri dishes lined with moist filter paper. Percent hatch and percent mortality of larvae were both recorded after 120 hours. Contact Activity:
Ten first-instar larvae were allowed to crawl on sprayed petriplates for 1 minute and then provided with one freshly cut rice straw per plate. After 10 minutes when all of the larvae were inside the straw were then covered with Petri lid. Percent mortality was recorded after 72 hours after treatment.
B18 Tobacco budworm (Heliothis virescens) I
For evaluating control of tobacco budworm (Heliothis virescens) the test unit consisted of 96-well-microtiter plates containing an insect diet and 15-25 H. virescens eggs.
The compounds were formulated using a solution containing 75% v/v water and 25% v/v DMSO. Different concentrations of formulated compounds were sprayed onto the insect diet at 10 µl, using a custom built micro atomizer, at two replications.
After application, microtiter plates were incubated at about 28 + 1°C and about 80 + 5 % relative humidity for 5 days. Egg and larval mortality was then visually assessed.
In this test, compounds 1-1, 1-3, 1-12, 1-13, 1-15, 1-18, 1-19, 1-20, 1-24, 1-25, 1-27, 1-28, 2-2, 2-3, 2-4 at 2500 ppm showed over 75 % mortality in comparison with untreated controls. B19 Tobacco Budworm (Heliothis virescens) II
The active compounds were formulated by a Tecan liquid handler in 100% cyclohexanone as a 10,000 ppm solution supplied in tubes. The 10,000 ppm solution was serially diluted in 100% cyclohexanone to make interim solutions. These served as stock solutions for which final dilutions were made by the Tecan in 50% acetone:50% water (v/v) into 10or 20ml glass vials. A nonionic surfactant (Kinetic®) was included in the solution at a volume of 0.01% (v/v). The vials were then inserted into an automated electrostatic sprayer equipped with an atomizing nozzle for application to
plants/insects.
Cotton plants were grown 2 plants to a pot and selected for treatment at the cotyledon stage. Test solutions were sprayed onto the foliage by an automated electrostatic plant sprayer equipped with an atomizing spray nozzle. The plants were dried in the sprayer fume hood and then removed from the sprayer. Each pot was placed into perforated plastic bags with a zip closure. About 10 to 11 budworm larvae were placed into the bag and the bags zipped closed. Test plants were maintained in a growth room at about 25°C and about 20-40% relative humidity for 4 days, avoiding direct exposure to fluorescent light (24 hour photoperiod) to prevent trapping of heat inside the bags. Mortality and reduced feeding were assessed 4 days after treatment, compared to untreated control plants. B20 Two-spotted spider mite (tetranychus urticae); (special test: OP-resistant strain) The active compounds were formulated by a Tecan liquid handler in 100% cyclohexanone as a 10,000 ppm solution supplied in tubes. The 10,000 ppm solution was serially diluted in 100% cyclohexanone to make interim solutions. These served as stock solutions for which final dilutions were made by the Tecan in 50% acetone:50%
water (v/v) into 10 or 20 ml glass vials. A nonionic surfactant (Kinetic®) was included in the solution at a volume of 0.01% (v/v). The vials were then inserted into an automated electrostatic sprayer equipped with an atomizing nozzle for application to
plants/insects.
Sieva lima bean plants with primary leaves expanded to 7-12 cm were infested by placing on each a small piece from an infested leaf (with about 100 mites) taken from the main colony. This was done at about 2 hours before treatment to allow the mites to move over to the test plant to lay eggs. The piece of leaf used to transfer the mites was removed. The newly-infested plants were dipped in the test solution and allowed to dry. The test plants were kept under fluorescent light (24 hour photoperiod) at about 25°C and about 20-40% relative humidity. After 5 days, one leaf was removed and mortality counts were made. B21 Vetch aphid (Megoura viciae)
For evaluating control of vetch aphid (Megoura viciae) through contact or systemic means the test unit consisted of 24-well-microtiter plates containing broad bean leaf disks.
The compounds were formulated using a solution containing 75% v/v water and 25% v/v DMSO. Different concentrations of formulated compounds were sprayed onto the leaf disks at 2.5 µl, using a custom built micro atomizer, at two replications.
After application, the leaf disks were air-dried and 5– 8 adult aphids placed on the leaf disks inside the microtiter plate wells. The aphids were then allowed to suck on the treated leaf disks and incubated at about 23 + 1°C and about 50 + 5 % relative humidity for 5 days. Aphid mortality and fecundity was then visually assessed.
In this test, compounds 1-1, 1-2, 1-3, 1-4, 1-5, 1-6, 1-7, 1-8, 1-9, 1-10, 1-11, 1-12, 1-13, 1-14, 1-15, 1-16, 1-17, 1-18, 1-19, 1-20, 1-21, 1-22, 1-23, 1-28, 1-29, 1-30, 2-1, 2-2 at 2500 ppm showed over 75 % mortality in comparison with untreated controls. B22 Western corn rootworm assay (Diabrotica virgifera virgifera) Soil incorporation against western corn rootworm
The active compound was applied in acetone at rates of 5 and 50 ppm a.i./soil (w/w). Treatments were applied in solution to sifted, North Carolina loamy sand (Sandhill soil) in a plastic bag. Treatments were thoroughly incorporated by sealing and shaking each bag by hand and allowing the solution to soak through the soil mass for at least 10 minutes before unsealing. The bags were then kept open in a fume hood overnight to evaporate the solvent from the soil.
One day after treatment (DAT) distilled water for moisture and water-soaked millet seed (Panicum miliaceum 'white millet') as a food source were added to each
bag and mixed in thoroughly.11 cm3 of millet and soil mixture were dispensed into a 1 oz. plastic cup. Each cup was infested with 10 western corn rootworm second-instar larvae. Each cup or group of four cells was a replicate, and replication was 3x. The test was maintained in incubators at 26°C in the dark. Mortality was evaluated 3 days after infestation (DAI) and mean percent mortality was calculated. B23 Western flower thrips (Frankliniella occidentalis)
Serial dilutions of each technical grade AI were made in pure acetone.0.5 ml of the treatment solution was deposited into the bottom of a glass vial (scintillation vial). The cap was screwed back onto the vial and inverted for about five seconds. The cap was subsequently removed and the vial laid on its side and rolled constantly, on a hot dog roller, until all the acetone had flashed off and the inner surface of the vial was dry. Cotton leave discs were also dipped simultaneously into the treatment solutions and allowed to dry. After the vials were dried, the leave discs were placed into the vials to serve as a food/water source for the thrips. Each treatment was replicated 5-fold.
Western flower thrips were aspirated into the vials, approximately 5 larvae or adults / vial. Following treatment application the vials were held in a holding room under fluorescent light and constant 26°C.
Thrips mortality was assessed at 2 DAT (days after treatment), counting all thrips both dead and alive. B24 Yellow fever mosquito (Aedes aegypti)
For evaluating control of yellow fever mosquito (Aedes aegypti) the test unit consisted of 96-well-microtiter plates containing 200µl of tap water per well and 5-15 freshly hatched A. aegypti larvae.
The active compounds were formulated using a solution containing 75% (v/v) water and 25% (v/v) DMSO. Different concentrations of formulated compounds or mixtures were sprayed onto the insect diet at 2.5µl, using a custom built micro atomizer, at two replications.
After application, microtiter plates were incubated at 28 + 1°C, 80 + 5 % relative humidity for 2 days. Larval mortality was then visually assessed.
In this test, compounds 1-3, 1-8, 1-9, 1-15, 1-28, 1-29, 1-30, 2-2 at 2500 ppm showed over 75 % mortality in comparison with untreated controls.
Claims
We claim: 1. An N-(thio)acylimino compound of formula (I):
heteroatoms selected from N, O and S as ring members, where the heteroaromatic ring is optionally substituted by 1, 2, 3 or 4 radicals R6a; A is a 5- or 6-membered saturated, partly unsaturated or maximally
unsaturated N-heterocyclic ring which may contain 1 or 2 further heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members; X is O or S; Y is N or CR5a; Z is N or CR5b; with the proviso that at most one of Y and Z is N; R1 and R2, independently of each other, are selected from the group consisting of hydrogen, halogen, cyano, nitro, SCN, C1-C6-alkyl, C2-C6-alkenyl, C2-C6- alkynyl, C3-C6-cycloalkyl, wherein the 4 last-mentioned aliphatic and cycloaliphatic radicals may be partly or completely halogenated and/or may carry one or more substituents R7;
OR8, OSO2R8, S(O)nR8, S(O)nNR9aR9b, NR9aR9b, C(=O)R7a, C(=S)R7a, C(=O)OR8, C(=O)NR9aR9b, C(=S)NR9aR9b, Si(R11)2R12,
phenyl, optionally substituted with 1, 2, 3, 4 or 5 substituents R10, and
a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents R10;
or
R1 and R2 form, together with the carbon atom they are attached to, a 3-, 4-, 5- or 6-membered saturated or partly unsaturated carbocyclic or heterocyclic ring, where the heterocyclic ring contains 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the carbocyclic or heterocyclic ring is unsubstituted or carries 1 or 2 radicals R10;
or
R1 and R2 form together a group =O, =CR13R14; =S; =NR9a, =NOR8 or =NNR9aR9b; R3 is selected from the group consisting of hydrogen, halogen, cyano, azido, nitro, SCN, SF5, C1-C10-alkyl, C2-C10-alkenyl, C2-C10-alkynyl, C3-C8- cycloalkyl, wherein the 4 last-mentioned aliphatic and cycloaliphatic radicals may be partly or completely halogenated and/or may carry one or more radicals R7;
C(=O)R7a, C(=S)R7a, C(=NR17)R7a, C(=O)OR8, C(=S)OR8, C(=S)SR8, C(=O)NR9aR9b, C(=S)NR9aR9b, C(=NR17)NR9aR9b, Si(R11)2R12;
phenyl, optionally substituted with 1, 2, 3, 4 or 5 radicals R10; and a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, wherein the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 radicals R10; R4 is a group -L1-C ^C-RA or a group -L2-C ^N; wherein L1 is a bond or is a bridging group selected from -O-, -S-, -N(R9a)-,
-C1-C10-alkylene-, -O-C1-C10-alkylene-, -S-C1-C10-alkylene- and -N(R9a)-C1-C10-alkylene-; L2 is a bond or is a bridging group selected from -C1-C10-alkylene-,
-O-C1-C10-alkylene-, -S-C1-C10-alkylene- and -N(R9a)-C1-C10-alkylene-; and
RA is selected from the group consisting of hydrogen, C1-C10-alkyl, C2-C10-alkenyl, C2-C10-alkynyl, C3-C8-cycloalkyl, wherein the 4 last- mentioned aliphatic and cycloaliphatic radicals may be partly or completely halogenated and/or may carry one or more radicals R7, OR8, S(O)nR8, S(O)nNR9aR9b, NR9aR9b, C(=O)R7a, C(=S)R7a,
C(=NR17)R7a, C(=O)OR8, C(=S)OR8, C(=S)SR8, C(=O)NR9aR9b, C(=S)NR9aR9b, C(=NR17)NR9aR9b, Si(R11)2R12;
phenyl, optionally substituted with 1, 2, 3, 4 or 5 radicals R10; and a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, wherein the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 radicals R10; R5a and R5b, independently of each other, are selected from hydrogen, halogen, cyano, azido, nitro, SCN, SF5, C1-C10-alkyl, C2-C10-alkenyl, C2-C10-alkynyl, C3-C8-cycloalkyl, wherein the 4 last-mentioned aliphatic and cycloaliphatic radicals may be partly or completely halogenated and/or may carry one or more radicals R7,
OR8, OSO2R8, S(O)nR8, S(O)nNR9aR9b, NR9aR9b, C(=O)R7a, C(=S)R7a, C(=NR17)R7a, C(=O)OR8, C(=S)OR8, C(=S)SR8, C(=O)NR9aR9b,
C(=S)NR9aR9b, C(=NR17)NR9aR9b, Si(R11)2R12;
phenyl, optionally substituted with 1, 2, 3, 4 or 5 radicals R10; and a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, wherein the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 radicals R10; each R6 is independently selected from the group consisting of halogen, cyano, azido, nitro, SCN, SF5, C1-C10-alkyl, C2-C10-alkenyl, C2-C10-alkynyl, C3-C8- cycloalkyl, wherein the 4 last-mentioned aliphatic and cycloaliphatic radicals may be partly or completely halogenated and/or may carry one or more radicals R7,
OR8, NR9aR9b, S(O)nR8, S(O)nNR9aR9b, C(=O)R7a, C(=S)R7a, C(=NR17)R7a, C(=O)OR8, C(=S)OR8, C(=S)SR8, C(=O)NR9aR9b, C(=S)NR9aR9b,
C(=NR17)NR9aR9b, and Si(R11)2R12;
or two radicals R6 present on the same ring carbon may form together a group =O, =CR13R14, =S, =NR17, =NOR16 or =NNR9aR9b;
or two radicals R6 bound on adjacent ring atoms, together with the atoms they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partly unsaturated or maximally unsaturated carbocyclic or heterocyclic ring, where the heterocyclic ring contains 1 or 2 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members; and where said carbocyclic or heterocyclic ring is unsubstituted or carries 1, 2, 3 or 4 radicals selected from halogen, C1-C6-alkyl, C1-C6-haloalkyl, C2-C6- alkenyl, C2-C6-haloalkenyl, C2-C6-alkynyl, C2-C6-haloalkynyl, C3-C8- cycloalkyl, C3-C8-halocycloalkyl, C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6- alkylthio, C1-C6-haloalkylthio, phenyl, optionally substituted with 1, 2, 3, 4 or 5 substituents R10, and a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents R10; each R6a has independently one of the meanings given for R6; each R7 is independently selected from the group consisting of cyano, azido, nitro, -SCN, SF5, Si(R11)2R12, OR16, OSO2R16, S(O)nR16, S(O)nNR17aR17b, NR17aR17b, C(=O)R15, C(=S)R15, C(=NR17)R15, C(=NOR16)R15, C(=O)OR16, C(=S)OR16, C(=O)NR17aR17b, C(=S)NR17aR17b,
C3-C8-cycloalkyl, C3-C8-halocycloalkyl,
phenyl, optionally substituted with 1, 2, 3, 4 or 5 substituents R10, and a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, wherein the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents R10;
or
two R7 present on the same carbon atom may form together a group =O, =CR13R14; =S; =S(O)nR16, =S(O)nNR17aR17b, =NR17, =NOR16 or =NNR17a; or
two R7 bound on the same or adjacent carbon atom(s), together with the carbon atom(s) they are bound to, may form a 3-, 4-, 5-, 6-, 7- or 8- membered saturated, or partly unsaturated carbocyclic or heterocyclic ring, where the heterocyclic ring contains 1, 2 or 3 heteroatoms or heteroatom
groups selected from N, O, S, NO, SO and SO2 as ring members, where the carbocyclic or heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents R10; and
R7 as a substituent on a cycloaliphatic radical is additionally selected from the group consisting of C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6- haloalkenyl, C2-C6-alkynyl and C2-C6-haloalkynyl; each R7a is independently selected from the group consisting of hydrogen,
halogen, C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-haloalkenyl, C2-C6-alkynyl, C2-C6-haloalkynyl, C3-C8-cycloalkyl, C3-C8-halocycloalkyl, C3-C8-cycloalkyl-C1-C4-alkyl, C3-C8-halocycloalkyl-C1-C4-alkyl, C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6-alkylthio, C1-C6-haloalkylthio, C1-C6-alkylsulfinyl, C1-C6-haloalkylsulfinyl, C1-C6-alkylsulfonyl, C1-C6-haloalkylsulfonyl, phenyl, phenyl-C1-C4-alkyl, where the phenyl ring in the two last-mentioned radicals is optionally substituted with 1, 2, 3, 4 or 5 radicals R10, and a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents R10; each R8 is independently selected from the group consisting of hydrogen, CN, C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-haloalkenyl, C2-C6- alkynyl, C2-C6-haloalkynyl, C3-C8-cycloalkyl, C3-C8-halocycloalkyl, C3-C8- cycloalkyl-C1-C4-alkyl, C3-C8-halocycloalkyl-C1-C4-alkyl, C(=O)R15,
C(=S)R15, C(=O)OR16, C(=S)OR16, C(=O)NR17aR17b, C(=S)NR17aR17b, phenyl, phenyl-C1-C4-alkyl, where the phenyl ring in the two last-mentioned radicals is unsubstituted or substituted with 1, 2, 3, 4 or 5 substituents R10; and
a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents R10; and
R8 as a substituent on a sulfur atom is additionally selected from the group consisting of C1-C6-alkoxy and C1-C6-haloalkoxy; R9a and R9b, independently of each other and independently of each occurrence, are selected from the group consisting of hydrogen, C1-C6-alkyl, C1-C6-
haloalkyl, C2-C6-alkenyl, C2-C6-haloalkenyl, C2-C6-alkynyl, C2-C6- haloalkynyl, C3-C8-cycloalkyl, C3-C8-halocycloalkyl, C3-C8-cycloalkyl-C1-C4- alkyl, C3-C8-halocycloalkyl-C1-C4-alkyl,
OR16, S(O)nR16, NR17aR17b, -S(O)nNR17aR17b, C(=O)R15, C(=S)R15,
C(=NR17)R15, C(=O)OR16, C(=S)SR16, C(=O)NR17aR17b, C(=S)NR17aR17b, P(=O)(R18)2,
phenyl, benzyl, 1-phenylethyl, 2-phenylethyl, where the phenyl ring in the four last-mentioned radicals is unsubstituted or substituted with 1, 2, 3, 4 or 5 substituents R10, and
a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated C-bound heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents R10;
or
R9a and R9b, together with the nitrogen atom they are bound to, form a 3-, 4-, 5-, 6-, 7- or 8-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring, where the heterocyclic ring may contain one or two further heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, and where the heterocyclic ring may be substituted with 1, 2, 3 or 4 radicals selected from halogen, C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-haloalkenyl, C2-C6-alkynyl, C2-C6- haloalkynyl, C3-C8-cycloalkyl, C3-C8-halocycloalkyl, C1-C6-alkoxy, C1-C6- haloalkoxy, C1-C6-alkylthio, C1-C6-haloalkylthio, phenyl, optionally substituted with 1, 2, 3, 4 or 5 substituents R10, and a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents R10; or
R9a and R9b together may form a group =CR13R14, =S(O)nR16,
=S(O)nNR17aR17b, =NR17 or =NOR16; each R10 is independently selected from the group consisting of halogen, cyano, azido, nitro, SCN, SF5, C1-C10-alkyl, C2-C10-alkenyl, C2-C10-alkynyl, C3-C8- cycloalkyl, wherein the 4 last-mentioned aliphatic and cycloaliphatic radicals may be partly or completely halogenated and/or may carry 1, 2, 3, 4 or 5 radicals R19,
Si(R11)2R12, OR16, OS(O)nR16, -S(O)nR16, S(O)nNR17aR17b, NR17aR17b, C(=O)R15, C(=S)R15, C(=NR17)R15, C(=O)OR16, C(=O)NR17aR17b,
C(=S)NR17aR17b,
phenyl, optionally substituted with 1, 2, 3, 4 or 5 radicals independently selected from OH, halogen, cyano, nitro, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy, and
a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the heterocyclic ring is unsubstituted or may be substituted with 1, 2, 3, 4 or 5 substituents independently selected from OH, halogen, cyano, nitro, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy;
or
two R10 present on the same carbon ring atom of a saturated or partly unsaturated carbocyclic or heterocyclic radical may form together a group =O, =CR13R14, =S, =NR17, =NOR16 or =NNR17aR17b;
or
two R10 bound on adjacent ring atoms may form together a bivalent radical selected from CH2CH2CH2CH2, CH=CH-CH=CH, N=CH-CH=CH, CH=N- CH=CH, N=CH-N=CH, OCH2CH2CH2, OCH=CHCH2, CH2OCH2CH2, OCH2CH2O, OCH2OCH2, CH2CH2CH2, CH=CHCH2, CH2CH2O, CH=CHO, CH2OCH2, CH2C(=O)O, C(=O)OCH2, O(CH2)O, SCH2CH2CH2,
SCH=CHCH2, CH2SCH2CH2, SCH2CH2S, SCH2SCH2, CH2CH2S, CH=CHS, CH2SCH2, CH2C(=S)S, C(=S)SCH2, S(CH2)S, CH2CH2NR17a, CH2CH=N, CH=CH-NR17a, OCH=N and SCH=N, thus forming together with the carbon atoms to which the two R10 are bound a 5-membered or 6-membered saturated, partly unsaturated or maximally unsaturated carbocyclic or heterocyclic ring, wherein the ring may optionally be substituted with one or two substituents independently selected from =O, OH, CH3, OCH3, halogen, cyano, halomethyl and halomethoxy; R11 and R12, independently of each other and independently of each occurrence, are selected from the group consisting of C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-haloalkenyl, C2-C6-alkynyl, C2-C6-haloalkynyl, C3-C8- cycloalkyl, C3-C8-halocycloalkyl, C3-C8-cycloalkyl-C1-C4-alkyl, C3-C8- halocycloalkyl-C1-C4-alkyl, C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6-alkoxy- C1-C4-alkyl, C1-C6-haloalkoxy-C1-C4-alkyl, phenyl and benzyl, where phenyl ring in last two radicals is unsubstituted or substituted with 1, 2, 3, 4 or 5
radicals independently selected from OH, halogen, cyano, nitro, C1-C6- alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy; R13 and R14, independently of each other and independently of each occurrence, are selected from the group consisting of hydrogen, halogen, CN, C1-C6- alkyl, C1-C6-haloalkyl, C3-C6-cycloalkyl, C1-C4-alkoxy-C1-C4-alkyl, phenyl and benzyl; each R15 is independently selected from the group consisting of hydrogen,
halogen, C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6-haloalkenyl, C2-C6-alkynyl, C2-C6-haloalkynyl, C3-C8-cycloalkyl, C3-C8-halocycloalkyl, C3-C8-cycloalkyl-C1-C4-alkyl, C3-C8-halocycloalkyl-C1-C4-alkyl, C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6-alkylthio, C1-C6-haloalkylthio, C1-C6-alkylsulfinyl, C1-C6-haloalkylsulfinyl, C1-C6-alkylsulfonyl, C1-C6-haloalkylsulfonyl, phenyl, phenyl-C1-C4-alkyl, where the phenyl ring in the two last-mentioned radicals is optionally substituted with 1, 2, 3, 4 or 5 radicals independently selected from OH, halogen, cyano, nitro, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy, and
a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, where the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents independently selected from OH, halogen, cyano, nitro, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy; each R16 is independently selected from the group consisting of hydrogen, C1-C6- alkyl, C2-C6-alkenyl, C2-C6-alkynyl, C3-C8-cycloalkyl, C3-C8-cycloalkyl-C1-C4- alkyl, wherein the five last-mentioned aliphatic and cycloaliphatic radicals may be partly or fully halogenated and/or may carry 1 or 2 radicals independently selected from =O and C1-C4-alkoxy;
phenyl, benzyl and pyridyl, wherein the last three radicals may be unsubstituted or may carry 1, 2, 3, 4 or 5 substituents independently selected from halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy, C1-C6- haloalkoxy, (C1-C6-alkoxy)carbonyl, (C1-C6-alkyl)amino and di-(C1-C6- alkyl)amino; each R17 is independently selected from the group consisting of hydrogen, C1-C6- alkyl, C2-C6-alkenyl, C2-C6-alkynyl, C3-C8-cycloalkyl, C3-C8-cycloalkyl-C1-C4- alkyl, C1-C6-alkoxy, C2-C6-alkenyloxy, C2-C6-alkynyloxy, C3-C8-cycloalkoxy,
C3-C8-cycloalkyl-C1-C4-alkoxy, C1-C6-alkylthio, C1-C6-alkylsulfinyl, C1-C6- alkylsulfonyl, wherein the aliphatic and cycloaliphatic moieties in the 13 last-mentioned radicals may be partly or fully halogenated and/or may carry 1 or 2 radicals independently selected from =O and C1-C4-alkoxy;
phenyl, benzyl, pyridyl, phenoxy, benzyloxy and pyridyloxy, wherein the six last-mentioned radicals may carry 1, 2, 3, 4 or 5 substituents independently selected from halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy, C1-C6- haloalkoxy and (C1-C6-alkoxy)carbonyl; R17a and R17b, independently of each other and independently of each
occurrence, are selected from the group consisting of hydrogen, C1-C6- alkyl, C2-C6-alkenyl, C2-C6-alkynyl, C3-C8-cycloalkyl, C3-C8-cycloalkyl-C1-C4- alkyl, C1-C6-alkoxy, C2-C6-alkenyloxy, C2-C6-alkynyloxy, C3-C8-cycloalkoxy, C3-C8-cycloalkyl-C1-C4-alkoxy, C1-C6-alkylthio, C1-C6-alkylsulfinyl, C1-C6- alkylsulfonyl, wherein the aliphatic and cycloaliphatic moieties in the 13 last-mentioned radicals may be partly or fully halogenated and/or may carry 1 or 2 radicals independently selected from =O and C1-C4-alkoxy;
phenyl, benzyl, pyridyl, phenoxy, benzyloxy and pyridyloxy, wherein the six last-mentioned radicals may carry 1, 2, 3, 4 or 5 substituents independently selected from halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy, C1-C6- haloalkoxy and (C1-C6-alkoxy)carbonyl,
or,
R17a and R17b, together with the nitrogen atom they are bound to, form a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring, wherein the heterocyclic ring may contain 1 or 2 further heteroatoms selected from or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, and wherein the heterocyclic ring may be substituted with one or more substituents independently selected from halogen, C1-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy and C1-C4-haloalkoxy;
or
R17a and R17b together may form a group =CR13R14, =NR17 or =NOR16; each R18 is independently selected from the group consisting of hydrogen, C1-C6- alkyl, C1-C6-alkoxy, C3-C6-cycloalkyl, C3-C6-cycloalkoxy, phenyl and phenoxy; each R19 is independently selected from the group consisting of cyano, azido, nitro, -SCN, SF5, Si(R11)2R12, OR16, OSO2R16, S(O)nR16, S(O)nNR17aR17b,
NR17aR17b, C(=O)NR17aR17b, C(=S)NR17aR17b, C(=O)OR16, C(=O)R15, C(=S)R15, C(=NR17)R15, C(=NOR16)R15,
C3-C8-cycloalkyl, C3-C8-halocycloalkyl,
phenyl, optionally substituted with 1, 2, 3, 4 or 5 substituents selected from CN, halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6- haloalkoxy; and
a 3-, 4-, 5-, 6- or 7-membered saturated, partly unsaturated or maximally unsaturated heterocyclic ring containing 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, wherein the heterocyclic ring is optionally substituted with 1, 2, 3 or 4 substituents selected from CN, halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy;
or
two R19 present on the same carbon atom may form together a group =O, =CR13R14; =S; =S(O)nR16, =S(O)nNR17aR17b, =NR17, =NOR16 or =NNR17a; or
two R19 bound on the same or adjacent carbon atom(s), together with the carbon atom(s) they are bound to, may form a 3-, 4-, 5-, 6-, 7- or 8- membered saturated or partly unsaturated carbocyclic or heterocyclic ring, where the heterocyclic ring contains 1, 2 or 3 heteroatoms or heteroatom groups selected from N, O, S, NO, SO and SO2 as ring members, and where the carbocyclic or heterocyclic ring may be substituted with 1, 2, 3, 4 or 5 substituents selected from CN, halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy; and
R19 as a substituent on a cycloaliphatic radical is additionally selected from the group consisting of C1-C6-alkyl, C1-C6-haloalkyl, C2-C6-alkenyl, C2-C6- haloalkenyl, C2-C6-alkynyl and C2-C6-haloalkynyl; k is 0, 1, 2, 3 or 4; and n is 0, 1 or 2; and the stereoisomers, tautomers and the agriculturally or veterinarily acceptable salts thereof.
2. The compound as claimed in claim 1, where
R1 and R2, independently of each other, are selected from the group consisting of hydrogen, halogen, CN, C1-C6-alkyl, C3-C6-cycloalkyl, C1-C6-haloalkyl and C3-C6-halocycloalkyl; or
R1 and R2 may together be =CR13R14; or
R1 and R2 form, together with the carbon atom which they attached to, a 3-, 4-or 5-membered saturated carbocyclic ring;
where R13 and R14 are as defined in claim 1; and where in particular both R1 and R2 are hydrogen.
3. The compound as claimed in any of the preceding claims, where R3 is selected from the group consisting of hydrogen, halogen, C1-C6-alkyl and C1-C6-haloalkyl.
4. The compound as claimed in any of the preceding claims, where R4 is a group -L1-C ^C-RA, where
L1 is a bond or -O-CH2-; in particular a bond; and
RA is selected from the group consisting of hydrogen, C1-C6-alkyl, C1-C6- haloalkyl, C1-C6-alkyl which carries one radical R7,
C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6-alkylthio, C1-C6-haloalkylthio, C1-C6- alkylsulfinyl, C1-C6-haloalkylsulfinyl, C1-C6-alkylsulfonyl, C1-C6- haloalkylsulfonyl, C1-C6-alkylcarbonyl, C1-C6-haloalkylcarbonyl, C1-C6- alkoxycarbonyl and C1-C6-haloalkoxycarbonyl;
where
R7 is selected from cyano, C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6- alkylthio, C1-C6-haloalkylthio, C1-C6-alkylsulfinyl, C1-C6- haloalkylsulfinyl, C1-C6-alkylsulfonyl, C1-C6-haloalkylsulfonyl, C1-C6- alkylcarbonyl, C1-C6-haloalkylcarbonyl, C1-C6-alkoxycarbonyl and C1-C6-haloalkoxycarbonyl.
5. The compound as claimed in any of claims 1 to 3, where R4 is cyano.
6. The compound as claimed in any of the preceding claims, where R5a and R5b, independently of each other, are selected from hydrogen, halogen, cyano, C1-C6- alkyl, C1-C6-haloalkyl, C1-C6-alkoxy and C1-C6-haloalkoxy, in particular from hydrogen, C1-C6-alkyl and C1-C6-alkoxy and specifically from hydrogen and C1-C6-alkyl.
7. The compound as claimed in any of the preceding claims, where each R6 is
independently selected from halogen, C1-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy and C1-C4-haloalkoxy; and k is 0, 1 or 2; in particular 0 or 1, specifically 0.
8. The compound as claimed in any of the preceding claims, where each R6a is independently selected from halogen, C1-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy and C1-C4-haloalkoxy; in particular from halogen and C1-C2-haloalkyl; specifically from Cl, Br and CF3, and is very specifically Cl.
9. The compound as claimed in any of the preceding claims, where Y is N and Z is CR5b, where R5b is as defined in claim 1 or 6.
10. The compound as claimed in any of the preceding claims, where Het is selected from Het-1 to Het-7:
Het-1 Het-2 Het-3
Het-4 Het-5 Het-6 Het-7 wherein
# denotes the attachment point to the remainder of the molecule;
j is 0, 1 or 2; and
R6a is as defined in claim 1 or 8.
Het-1a Het-4a Het-7aa wherein
# denotes the attachment point to the remainder of the molecule; and R6a is halogen or C1-C2-haloalkyl, in particular Cl, Br or CF3.
wherein
# denotes the attachment point to the remainder of the molecule;
R1 and R2 are as defined in claims 1 or 2;
R6 is as defined in claim 1 or 7;
Het is as defined is claim 1, 8, 10 or 11;
X is as defined in claim 1; and
each R6b is independently hydrogen or has one of the meanings given in claim 1 or 7 for R6, with the proviso that R6b is not halogen, azido, nitro, SCN or SF5;
each R6c has independently one of the meanings given in claim 1 or 7 for R6, and k is 0, 1 or 2.
13. The compound as claimed in claim 12, where the moiety W-Het is selected from moieties of formulae W.Het-1, W.Het-2, W.Het-3, W.Het 4, W.Het 13 and W-Het- 19, and in particular from W.Het-13 and W.Het-19, where in particular in W-Het- 19 each R6c is independently methyl, and k is 0, 1 or 2.
14. The compound as claimed in an of the recedin claims of formula I.1
Y1 is CH or N;
X is as defined in claim 1;
R3 is as defined in claim 1 or 3;
R4 is as defined in claim 1, 4 or 5;
R5b is as defined in claim 1 or 6; and
R6a is as defined in claim 1, 8 or 11; or of formula I.2
Y1 is CH or N;
X is as defined in claim 1;
R3 is as defined in claim 1 or 3;
R4 is as defined in claim 1, 4 or 5;
R5b is as defined in claim 1 or 6;
R6a is as defined in claim 1, 8 or 11; and
R6c1 and R6c2, independently of each other, are hydrogen or have one of the meanings given in claim 1 or 7 for R6; or of formula I.3
X is as defined in claim 1;
R3 is as defined in claim 1 or 3;
R4 is as defined in claim 1, 4 or 5;
R5b is as defined in claim 1 or 6; and
X is as defined in claim 1;
R3 is as defined in claim 1 or 3;
R4 is as defined in claim 1, 4 or 5;
R5b is as defined in claim 1 or 6;
R6a is as defined in claim 1, 8 or 11; and
R6c1 and R6c2, independently of each other, are hydrogen or have one of the
meanings given in claim 1 or 7 for R6.
15. An agricultural or veterinary composition comprising at least one compound as defined in any of claims 1 to 14, a stereoisomer, a tautomer and/or at least one agriculturally or veterinarily acceptable salt thereof, and at least one inert liquid and/or solid agriculturally or veterinarily acceptable carrier.
16. The use of a compound as defined in any of claims 1 to 14, of a stereoisomer, a tautomer and/or of an agriculturally or veterinarily acceptable salt thereof for combating or controlling invertebrate pests, for protecting growing plants from attack or infestation by invertebrate pests, for protecting plant propagation material, especially seeds, from soil insects, or for protecting the seedlings, roots and shoots of plants from soil and foliar insects.
17. A method for combating or controlling invertebrate pests, which method
comprises contacting said pest or its food supply, habitat or breeding grounds with a pesticidally effective amount of at least one compound as defined in any of claims 1 to 14, of a stereoisomer, a tautomer and/or of an agriculturally or veterinarily acceptable salt thereof; or
for protecting growing plants from attack or infestation by invertebrate pests, which method comprises contacting a plant, or soil or water in which the plant is growing with a pesticidally effective amount of at least one compound as defined in any of claims 1 to 14, of a stereoisomer, a tautomer and/or of an agriculturally or veterinarily acceptable salt thereof; or
for the protection of plant propagation material, especially seeds, from soil insects
and of the seedlings roots and shoots from soil and foliar insects comprising contacting the plant propagation material before sowing and/or after pregermination with at least one compound as defined in any one of claims 1 to 14, a stereoisomer, tautomer and/or an agriculturally or veterinarily acceptable salt thereof.
18. The compound as defined in any of claims 1 to 14, a stereoisomer, tautomer and/or an agriculturally or veterinarily acceptable salt thereof, for the use in the treatment of animals infested or infected by parasites, for preventing animals of getting infected or infested by parasites or for protecting animals against infestation or infection by parasites.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562173978P | 2015-06-11 | 2015-06-11 | |
| US62/173,978 | 2015-06-11 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016198613A1 true WO2016198613A1 (en) | 2016-12-15 |
Family
ID=56116458
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2016/063318 WO2016198613A1 (en) | 2015-06-11 | 2016-06-10 | N-(thio)acylimino compounds |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2016198613A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10440953B2 (en) | 2015-08-07 | 2019-10-15 | Basf Se | Control of pests in maize by ginkgolides and bilobalide |
| US11297837B2 (en) | 2016-02-19 | 2022-04-12 | Basf Se | Pesticidally activi mixtures comprising anthranilamide compounds |
Citations (108)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3296272A (en) | 1965-04-01 | 1967-01-03 | Dow Chemical Co | Sulfinyl- and sulfonylpyridines |
| US3325503A (en) | 1965-02-18 | 1967-06-13 | Diamond Alkali Co | Polychloro derivatives of mono- and dicyano pyridines and a method for their preparation |
| EP0141317A2 (en) | 1983-10-21 | 1985-05-15 | BASF Aktiengesellschaft | 7-Amino-azolo[1,5-a]pyrimidines and fungicides containing them |
| EP0152031A2 (en) | 1984-02-03 | 1985-08-21 | Shionogi & Co., Ltd. | Azolyl cycloalkanol derivatives and agricultural fungicides |
| EP0226917A1 (en) | 1985-12-20 | 1987-07-01 | BASF Aktiengesellschaft | Acrylic acid esters and fungicides containing these compounds |
| EP0243970A1 (en) | 1986-05-02 | 1987-11-04 | Stauffer Chemical Company | Fungicidal pyridyl imidates |
| EP0256503A2 (en) | 1986-08-12 | 1988-02-24 | Mitsubishi Kasei Corporation | Pyridinecarboxamide derivatives and their use as fungicide |
| EP0259738A2 (en) | 1986-09-10 | 1988-03-16 | Nihon Tokushu Noyaku Seizo K.K. | Heterocyclic compounds |
| EP0428941A1 (en) | 1989-11-10 | 1991-05-29 | Agro-Kanesho Co., Ltd. | Hexahydrotriazine compounds and insecticides |
| EP0532022A1 (en) | 1991-09-13 | 1993-03-17 | Ube Industries, Ltd. | Acrylate compound, preparation process thereof and fungicide using the same |
| EP0639569A1 (en) | 1991-03-11 | 1995-02-22 | Nippon Soda Co., Ltd. | Novel heterocyclic compound |
| DE19650197A1 (en) | 1996-12-04 | 1998-06-10 | Bayer Ag | 3-thiocarbamoylpyrazole derivatives |
| WO1998046608A1 (en) | 1997-04-14 | 1998-10-22 | American Cyanamid Company | Fungicidal trifluoromethylalkylamino-triazolopyrimidines |
| WO1999014187A1 (en) | 1997-09-18 | 1999-03-25 | Basf Aktiengesellschaft | Benzamidoxim derivatives, intermediate products and methods for preparing and using them as fungicides |
| WO1999024413A2 (en) | 1997-11-12 | 1999-05-20 | Bayer Aktiengesellschaft | Isothiazole carboxylic acid amides and the application thereof in order to protect plants |
| WO1999027783A1 (en) | 1997-12-04 | 1999-06-10 | Dow Agrosciences Llc | Fungicidal compositions and methods, and compounds and methods for the preparation thereof |
| WO2000029404A1 (en) | 1998-11-17 | 2000-05-25 | Kumiai Chemical Industry Co., Ltd. | Pyrimidinylbenzimidazole and triazinylbenzimidazole derivatives and agricultura/horticultural bactericides |
| WO2000046148A1 (en) | 1999-02-02 | 2000-08-10 | Sintokogio, Ltd. | Silica gel carrying titanium oxide photocatalyst in high concentration and method for preparation thereof |
| EP1028125A1 (en) | 1998-11-30 | 2000-08-16 | Isagro Ricerca S.r.l. | Dipeptide compounds having fungicidal activity and their agronomic use |
| EP1035122A1 (en) | 1999-03-11 | 2000-09-13 | Rohm And Haas Company | Heterocyclic subsituted isoxazolidines and their use as fungicides |
| WO2000065913A1 (en) | 1999-04-28 | 2000-11-09 | Takeda Chemical Industries, Ltd. | Sulfonamide derivatives |
| DE10021412A1 (en) | 1999-12-13 | 2001-06-21 | Bayer Ag | Fungicidal active ingredient combinations |
| WO2001054501A2 (en) | 2000-01-25 | 2001-08-02 | Syngenta Participations Ag | Herbicidal composition |
| EP1122244A1 (en) | 2000-02-04 | 2001-08-08 | Sumitomo Chemical Company, Limited | Uracil compounds and their use |
| WO2001056358A2 (en) | 2000-01-28 | 2001-08-09 | Rohm And Haas Company | Enhanced propertied pesticides |
| WO2002022583A2 (en) | 2000-09-18 | 2002-03-21 | E. I. Du Pont De Nemours And Company | Pyridinyl amides and imides for use as fungicides |
| EP1201648A1 (en) | 1999-08-05 | 2002-05-02 | Kumiai Chemical Industry Co., Ltd. | Carbamate derivatives and agricultural/horticultural bactericides |
| WO2002040431A2 (en) | 2000-11-17 | 2002-05-23 | Dow Agrosciences Llc | Compounds having fungicidal activity and processes to make and use same |
| JP2002316902A (en) | 2001-04-20 | 2002-10-31 | Sumitomo Chem Co Ltd | Plant blight-preventing agent composition |
| WO2003010149A1 (en) | 2001-07-25 | 2003-02-06 | Bayer Cropscience Ag | Pyrazolylcarboxanilides as fungicides |
| WO2003011853A1 (en) | 2001-07-30 | 2003-02-13 | Dow Agrosciences Llc | 6-aryl-4-aminopicolinates and their use as herbicides |
| WO2003014103A1 (en) | 2001-08-03 | 2003-02-20 | Bayer Cropscience S.A. | Iodobenzopyran-4-one derivatives having fungicidal activity |
| WO2003016286A1 (en) | 2001-08-17 | 2003-02-27 | Sankyo Agro Company, Limited | 3-phenoxy-4-pyridazinol derivative and herbicide composition containing the same |
| WO2003016303A1 (en) | 2001-08-20 | 2003-02-27 | Dainippon Ink And Chemicals, Inc. | Tetrazoyl oxime derivative and agricultural chemical containing the same as active ingredient |
| WO2003053145A1 (en) | 2001-12-21 | 2003-07-03 | Nissan Chemical Industries, Ltd. | Bactericidal composition |
| WO2003061388A1 (en) | 2002-01-18 | 2003-07-31 | Sumitomo Chemical Takeda Agro Company, Limited | Fused heterocyclic sulfonylurea compound, herbicide containing the same, and method of controlling weed with the same |
| WO2003066609A1 (en) | 2002-02-04 | 2003-08-14 | Bayer Cropscience Aktiengesellschaft | Disubstituted thiazolyl carboxanilides and their use as microbicides |
| WO2003074491A1 (en) | 2002-03-05 | 2003-09-12 | Syngenta Participations Ag | O-cyclopropyl-carboxanilides and their use as fungicides |
| WO2004049804A2 (en) | 2002-11-29 | 2004-06-17 | Syngenta Participations Ag | Fungicidal combinations for crop potection |
| WO2004083193A1 (en) | 2003-03-17 | 2004-09-30 | Sumitomo Chemical Company, Limited | Amide compound and bactericide composition containing the same |
| WO2005063721A1 (en) | 2003-12-19 | 2005-07-14 | E.I. Dupont De Nemours And Company | Herbicidal pyrimidines |
| WO2005077934A1 (en) | 2004-02-18 | 2005-08-25 | Ishihara Sangyo Kaisha, Ltd. | Anthranilamides, process for the production thereof, and pest controllers containing the same |
| WO2005087772A1 (en) | 2004-03-10 | 2005-09-22 | Basf Aktiengesellschaft | 5,6-dialkyl-7-amino-triazolopyrimidines, method for their production, their use for controlling pathogenic fungi and agents containing said compounds |
| WO2005087773A1 (en) | 2004-03-10 | 2005-09-22 | Basf Aktiengesellschaft | 5,6-dialkyl-7-amino-triazolopyrimidines, method for their production, their use for controlling pathogenic fungi and agents containing said compounds |
| WO2005120234A2 (en) | 2004-06-03 | 2005-12-22 | E.I. Dupont De Nemours And Company | Fungicidal mixtures of amidinylphenyl compounds |
| WO2005123690A1 (en) | 2004-06-18 | 2005-12-29 | Basf Aktiengesellschaft | 1-methyl-3-difluoromethyl-pyrazol-4-carbonic acid-(ortho-phenyl)-anilides, and use thereof as a fungicide |
| WO2005123689A1 (en) | 2004-06-18 | 2005-12-29 | Basf Aktiengesellschaft | 1-methyl-3-trifluoromethyl-pyrazole-4-carboxylic acid (ortho-phenyl)-anilides and to use thereof as fungicide |
| WO2006015866A1 (en) | 2004-08-12 | 2006-02-16 | Syngenta Participations Ag | Method for protecting useful plants or plant propagation material |
| WO2006043635A1 (en) | 2004-10-20 | 2006-04-27 | Kumiai Chemical Industry Co., Ltd. | 3-triazolylphenyl sulfide derivative and insecticide/acaricide/nematicide containing the same as active ingredient |
| WO2006087325A1 (en) | 2005-02-16 | 2006-08-24 | Basf Aktiengesellschaft | 5-alkoxyalkyl-6-alkyl-7-amino-azolopyrimidines, method for their production, their use for controlling pathogenic fungi and agents containing said substances |
| WO2006087343A1 (en) | 2005-02-16 | 2006-08-24 | Basf Aktiengesellschaft | Pyrazole carboxylic acid anilides, method for the production thereof and agents containing them for controlling pathogenic fungi |
| WO2006089633A2 (en) | 2005-02-22 | 2006-08-31 | Bayer Cropscience Ag | Spiroketal-substituted cyclic ketoenols |
| DE102005009458A1 (en) | 2005-03-02 | 2006-09-07 | Bayer Cropscience Ag | pyrazolylcarboxanilides |
| WO2007006670A1 (en) | 2005-07-07 | 2007-01-18 | Basf Aktiengesellschaft | N-thio-anthranilamid compounds and their use as pesticides |
| WO2007043677A1 (en) | 2005-10-14 | 2007-04-19 | Sumitomo Chemical Company, Limited | Hydrazide compound and pesticidal use of the same |
| WO2007082098A2 (en) | 2006-01-13 | 2007-07-19 | Dow Agrosciences Llc | 6-(poly-substituted aryl)-4-aminopicolinates and their use as herbicides |
| WO2007090624A2 (en) | 2006-02-09 | 2007-08-16 | Syngenta Participations Ag | A method of protecting a plant propagation material, a plant, and/or plant organs |
| WO2007101540A1 (en) | 2006-03-06 | 2007-09-13 | Bayer Cropscience Ag | Combinations of active ingredients with insecticidal properties |
| WO2007101369A1 (en) | 2006-03-09 | 2007-09-13 | East China University Of Science And Technology | Preparation method and use of compounds having high biocidal activities |
| WO2008067911A1 (en) | 2006-12-04 | 2008-06-12 | Bayer Cropscience Ag | Biphenyl-substituted spirocyclic ketoenols |
| WO2008134969A1 (en) | 2007-04-30 | 2008-11-13 | Sinochem Corporation | Benzamide compounds and applications thereof |
| WO2009090181A2 (en) | 2008-01-15 | 2009-07-23 | Bayer Cropscience Sa | Pesticide composition comprising a tetrazolyloxime derivative and a fungicide or an insecticide active substance |
| WO2009124707A2 (en) | 2008-04-07 | 2009-10-15 | Bayer Cropscience Ag | Combinations of biological control agents and insecticides or fungicides |
| WO2010006713A2 (en) | 2008-07-17 | 2010-01-21 | Bayer Cropscience Ag | Heterocyclic compounds used as pesticides |
| WO2010018714A1 (en) | 2008-08-13 | 2010-02-18 | 三井化学アグロ株式会社 | Amide derivative, pest control agent containing the amide derivative and use of the pest control agent |
| WO2010034737A1 (en) | 2008-09-24 | 2010-04-01 | Basf Se | Pyrazole compounds for controlling invertebrate pests |
| CN101715777A (en) | 2010-01-12 | 2010-06-02 | 惠州市中迅化工有限公司 | Pesticide composition containing ethirimol and thiophanate-methyl, and application thereof |
| WO2010060379A1 (en) | 2008-11-28 | 2010-06-03 | 中国中化集团公司 | Ether compounds with nitrogen-containing 5-member heterocycle and the uses thereof |
| WO2010069882A1 (en) | 2008-12-17 | 2010-06-24 | Syngenta Participations Ag | Isoxazole derivatives for use as fungicides |
| WO2010069266A1 (en) | 2008-12-19 | 2010-06-24 | 华东理工大学 | Heterocyclic nitrogenous or oxygenous compounds with insecticidal activity formed from dialdehydes and their preparation and uses thereof |
| WO2010127926A1 (en) | 2009-05-06 | 2010-11-11 | Syngenta Participations Ag | 4 -cyano- 3 -benzoylamino-n- phenyl-benzamides for use in pest control |
| WO2010129497A1 (en) | 2009-05-05 | 2010-11-11 | Dow Agrosciences Llc | Pesticidal compositions |
| US20110046186A1 (en) | 2008-07-07 | 2011-02-24 | Bin Li | 1-Substituted Pyridyl-Pyrazolyl Amide Compounds and Uses Thereof |
| WO2011028657A1 (en) | 2009-09-01 | 2011-03-10 | Dow Agrosciences Llc | Synergistic fungicidal compositions containing a 5-fluoropyrimidine derivative for fungal control in cereals |
| WO2011069456A1 (en) | 2009-12-09 | 2011-06-16 | 华东理工大学 | Divalent and oxabridged heterocyclic neonicotinoid compounds and preparation methods thereof |
| WO2011077514A1 (en) | 2009-12-22 | 2011-06-30 | 三井化学アグロ株式会社 | Plant disease control composition and method for controlling plant diseases by applying the composition |
| US20110172433A1 (en) * | 2008-07-01 | 2011-07-14 | Meiji Seika Kaisha, Ltd. | Novel imino derivatives, methods for producing the same and insecticides containing the same |
| CN102126994A (en) | 2010-01-19 | 2011-07-20 | 中化蓝天集团有限公司 | Benzophenone hydrazone derivative and preparation method and application thereof |
| WO2011085575A1 (en) | 2010-01-15 | 2011-07-21 | 江苏省农药研究所股份有限公司 | Ortho-heterocyclyl formanilide compounds, their synthesis methods and use |
| WO2011135833A1 (en) | 2010-04-28 | 2011-11-03 | Sumitomo Chemical Company, Limited | Plant disease control composition and its use |
| WO2012000896A2 (en) | 2010-06-28 | 2012-01-05 | Bayer Cropscience Ag | Heterocyclic compounds as agents for pest control |
| WO2012029672A1 (en) | 2010-08-31 | 2012-03-08 | Meiji Seikaファルマ株式会社 | Noxious organism control agent |
| WO2012034472A1 (en) | 2010-09-13 | 2012-03-22 | 中化蓝天集团有限公司 | Cyano benzenedicarboxamide compounds, preparing methods and as agricultural insecticides uses thereof |
| WO2012034403A1 (en) | 2010-09-14 | 2012-03-22 | 中化蓝天集团有限公司 | Fluoromethoxypyrazole anthranilamide compounds, synthesization methods and uses thereof |
| WO2012084670A1 (en) | 2010-12-20 | 2012-06-28 | Basf Se | Pesticidal active mixtures comprising pyrazole compounds |
| WO2012143317A1 (en) | 2011-04-21 | 2012-10-26 | Basf Se | Novel pesticidal pyrazole compounds |
| WO2012168188A1 (en) | 2011-06-07 | 2012-12-13 | Bayer Intellectual Property Gmbh | Active compound combinations |
| WO2013003977A1 (en) | 2011-07-01 | 2013-01-10 | 合肥星宇化学有限责任公司 | Compound of 2,5-disubstituted-3-nitroimino-1,2,4-triazoline and preparation method and use as pesticide thereof |
| WO2013007767A1 (en) | 2011-07-13 | 2013-01-17 | Basf Se | Fungicidal substituted 2-[2-halogenalkyl-4-(phenoxy)-phenyl]-1-[1,2,4]triazol-1-yl-ethanol compounds |
| WO2013010862A1 (en) | 2011-07-15 | 2013-01-24 | Basf Se | Fungicidal alkyl-substituted 2-[2-chloro-4-(4-chloro-phenoxy)-phenyl]-1-[1,2,4]triazol-1-yl-ethanol compounds |
| WO2013024009A1 (en) | 2011-08-12 | 2013-02-21 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| WO2013024010A1 (en) | 2011-08-12 | 2013-02-21 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| WO2013047441A1 (en) | 2011-09-26 | 2013-04-04 | 日本曹達株式会社 | Agricultural and horticultural bactericide composition |
| WO2013047749A1 (en) | 2011-09-29 | 2013-04-04 | 三井化学アグロ株式会社 | Production method for 4, 4-difluoro-3,4-dihydroisoquinoline derivative |
| WO2013050317A1 (en) | 2011-10-03 | 2013-04-11 | Syngenta Limited | Polymorphs of an isoxazoline derivative |
| WO2013055584A1 (en) | 2011-10-13 | 2013-04-18 | E. I. Du Pont De Nemours And Company | Solid forms of nematocidal sulfonamides |
| US20130102637A1 (en) * | 2010-07-07 | 2013-04-25 | Gifu University | Pest control agent |
| WO2013092224A1 (en) | 2011-12-21 | 2013-06-27 | Basf Se | Use of strobilurin type compounds for combating phytopathogenic fungi resistant to qo inhibitors |
| WO2013129688A1 (en) | 2012-02-29 | 2013-09-06 | Meiji Seika Pharma Co., Ltd. | Pest control composition including novel iminopyridine derivative |
| WO2013127704A1 (en) | 2012-02-27 | 2013-09-06 | Bayer Intellectual Property Gmbh | Active compound combinations containing a thiazoylisoxazoline and a fungicide |
| WO2013144223A1 (en) * | 2012-03-30 | 2013-10-03 | Basf Se | N-substituted pyrimidinylidene compounds and derivatives for combating animal pests |
| WO2013162072A1 (en) | 2012-04-27 | 2013-10-31 | Sumitomo Chemical Company, Limited | Tetrazolinone compounds and its use as pesticides |
| WO2014036056A1 (en) | 2012-08-31 | 2014-03-06 | Zoetis Llc | Crystalline forms of 1-(5'-(5-(3,5-dichloro-4-fluorophenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-yl)-3'h-spiro[azetidine-3,1'-isobenzofuran]-1-yl)-2-(methylsulfonyl)ethanone |
| WO2014090918A1 (en) | 2012-12-13 | 2014-06-19 | Novartis Ag | Process for the enantiomeric enrichment of diaryloxazoline derivatives |
| US20140213448A1 (en) | 2012-04-27 | 2014-07-31 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| WO2014126208A1 (en) | 2013-02-14 | 2014-08-21 | 日産化学工業株式会社 | Crystalline polymorph of isoxazoline-substituted benzamide compound, and method for producing same |
| WO2015028630A1 (en) | 2013-08-30 | 2015-03-05 | Basf Se | N-substituted pyridylidene compounds and derivatives for combating animal pests |
| WO2015038503A1 (en) | 2013-09-13 | 2015-03-19 | E. I. Du Pont De Nemours And Company | Heterocycle-substituted bicyclic azole pesticides |
-
2016
- 2016-06-10 WO PCT/EP2016/063318 patent/WO2016198613A1/en active Application Filing
Patent Citations (109)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3325503A (en) | 1965-02-18 | 1967-06-13 | Diamond Alkali Co | Polychloro derivatives of mono- and dicyano pyridines and a method for their preparation |
| US3296272A (en) | 1965-04-01 | 1967-01-03 | Dow Chemical Co | Sulfinyl- and sulfonylpyridines |
| EP0141317A2 (en) | 1983-10-21 | 1985-05-15 | BASF Aktiengesellschaft | 7-Amino-azolo[1,5-a]pyrimidines and fungicides containing them |
| EP0152031A2 (en) | 1984-02-03 | 1985-08-21 | Shionogi & Co., Ltd. | Azolyl cycloalkanol derivatives and agricultural fungicides |
| EP0226917A1 (en) | 1985-12-20 | 1987-07-01 | BASF Aktiengesellschaft | Acrylic acid esters and fungicides containing these compounds |
| EP0243970A1 (en) | 1986-05-02 | 1987-11-04 | Stauffer Chemical Company | Fungicidal pyridyl imidates |
| EP0256503A2 (en) | 1986-08-12 | 1988-02-24 | Mitsubishi Kasei Corporation | Pyridinecarboxamide derivatives and their use as fungicide |
| EP0259738A2 (en) | 1986-09-10 | 1988-03-16 | Nihon Tokushu Noyaku Seizo K.K. | Heterocyclic compounds |
| EP0428941A1 (en) | 1989-11-10 | 1991-05-29 | Agro-Kanesho Co., Ltd. | Hexahydrotriazine compounds and insecticides |
| EP0639569A1 (en) | 1991-03-11 | 1995-02-22 | Nippon Soda Co., Ltd. | Novel heterocyclic compound |
| EP0532022A1 (en) | 1991-09-13 | 1993-03-17 | Ube Industries, Ltd. | Acrylate compound, preparation process thereof and fungicide using the same |
| DE19650197A1 (en) | 1996-12-04 | 1998-06-10 | Bayer Ag | 3-thiocarbamoylpyrazole derivatives |
| WO1998046608A1 (en) | 1997-04-14 | 1998-10-22 | American Cyanamid Company | Fungicidal trifluoromethylalkylamino-triazolopyrimidines |
| WO1999014187A1 (en) | 1997-09-18 | 1999-03-25 | Basf Aktiengesellschaft | Benzamidoxim derivatives, intermediate products and methods for preparing and using them as fungicides |
| WO1999024413A2 (en) | 1997-11-12 | 1999-05-20 | Bayer Aktiengesellschaft | Isothiazole carboxylic acid amides and the application thereof in order to protect plants |
| WO1999027783A1 (en) | 1997-12-04 | 1999-06-10 | Dow Agrosciences Llc | Fungicidal compositions and methods, and compounds and methods for the preparation thereof |
| WO2000029404A1 (en) | 1998-11-17 | 2000-05-25 | Kumiai Chemical Industry Co., Ltd. | Pyrimidinylbenzimidazole and triazinylbenzimidazole derivatives and agricultura/horticultural bactericides |
| EP1028125A1 (en) | 1998-11-30 | 2000-08-16 | Isagro Ricerca S.r.l. | Dipeptide compounds having fungicidal activity and their agronomic use |
| WO2000046148A1 (en) | 1999-02-02 | 2000-08-10 | Sintokogio, Ltd. | Silica gel carrying titanium oxide photocatalyst in high concentration and method for preparation thereof |
| EP1035122A1 (en) | 1999-03-11 | 2000-09-13 | Rohm And Haas Company | Heterocyclic subsituted isoxazolidines and their use as fungicides |
| WO2000065913A1 (en) | 1999-04-28 | 2000-11-09 | Takeda Chemical Industries, Ltd. | Sulfonamide derivatives |
| EP1201648A1 (en) | 1999-08-05 | 2002-05-02 | Kumiai Chemical Industry Co., Ltd. | Carbamate derivatives and agricultural/horticultural bactericides |
| DE10021412A1 (en) | 1999-12-13 | 2001-06-21 | Bayer Ag | Fungicidal active ingredient combinations |
| WO2001054501A2 (en) | 2000-01-25 | 2001-08-02 | Syngenta Participations Ag | Herbicidal composition |
| WO2001056358A2 (en) | 2000-01-28 | 2001-08-09 | Rohm And Haas Company | Enhanced propertied pesticides |
| EP1122244A1 (en) | 2000-02-04 | 2001-08-08 | Sumitomo Chemical Company, Limited | Uracil compounds and their use |
| WO2002022583A2 (en) | 2000-09-18 | 2002-03-21 | E. I. Du Pont De Nemours And Company | Pyridinyl amides and imides for use as fungicides |
| WO2002040431A2 (en) | 2000-11-17 | 2002-05-23 | Dow Agrosciences Llc | Compounds having fungicidal activity and processes to make and use same |
| JP2002316902A (en) | 2001-04-20 | 2002-10-31 | Sumitomo Chem Co Ltd | Plant blight-preventing agent composition |
| WO2003010149A1 (en) | 2001-07-25 | 2003-02-06 | Bayer Cropscience Ag | Pyrazolylcarboxanilides as fungicides |
| WO2003011853A1 (en) | 2001-07-30 | 2003-02-13 | Dow Agrosciences Llc | 6-aryl-4-aminopicolinates and their use as herbicides |
| WO2003014103A1 (en) | 2001-08-03 | 2003-02-20 | Bayer Cropscience S.A. | Iodobenzopyran-4-one derivatives having fungicidal activity |
| WO2003016286A1 (en) | 2001-08-17 | 2003-02-27 | Sankyo Agro Company, Limited | 3-phenoxy-4-pyridazinol derivative and herbicide composition containing the same |
| WO2003016303A1 (en) | 2001-08-20 | 2003-02-27 | Dainippon Ink And Chemicals, Inc. | Tetrazoyl oxime derivative and agricultural chemical containing the same as active ingredient |
| WO2003053145A1 (en) | 2001-12-21 | 2003-07-03 | Nissan Chemical Industries, Ltd. | Bactericidal composition |
| WO2003061388A1 (en) | 2002-01-18 | 2003-07-31 | Sumitomo Chemical Takeda Agro Company, Limited | Fused heterocyclic sulfonylurea compound, herbicide containing the same, and method of controlling weed with the same |
| WO2003066609A1 (en) | 2002-02-04 | 2003-08-14 | Bayer Cropscience Aktiengesellschaft | Disubstituted thiazolyl carboxanilides and their use as microbicides |
| WO2003074491A1 (en) | 2002-03-05 | 2003-09-12 | Syngenta Participations Ag | O-cyclopropyl-carboxanilides and their use as fungicides |
| WO2004049804A2 (en) | 2002-11-29 | 2004-06-17 | Syngenta Participations Ag | Fungicidal combinations for crop potection |
| WO2004083193A1 (en) | 2003-03-17 | 2004-09-30 | Sumitomo Chemical Company, Limited | Amide compound and bactericide composition containing the same |
| WO2005063721A1 (en) | 2003-12-19 | 2005-07-14 | E.I. Dupont De Nemours And Company | Herbicidal pyrimidines |
| WO2005077934A1 (en) | 2004-02-18 | 2005-08-25 | Ishihara Sangyo Kaisha, Ltd. | Anthranilamides, process for the production thereof, and pest controllers containing the same |
| WO2005087772A1 (en) | 2004-03-10 | 2005-09-22 | Basf Aktiengesellschaft | 5,6-dialkyl-7-amino-triazolopyrimidines, method for their production, their use for controlling pathogenic fungi and agents containing said compounds |
| WO2005087773A1 (en) | 2004-03-10 | 2005-09-22 | Basf Aktiengesellschaft | 5,6-dialkyl-7-amino-triazolopyrimidines, method for their production, their use for controlling pathogenic fungi and agents containing said compounds |
| WO2005120234A2 (en) | 2004-06-03 | 2005-12-22 | E.I. Dupont De Nemours And Company | Fungicidal mixtures of amidinylphenyl compounds |
| WO2005123690A1 (en) | 2004-06-18 | 2005-12-29 | Basf Aktiengesellschaft | 1-methyl-3-difluoromethyl-pyrazol-4-carbonic acid-(ortho-phenyl)-anilides, and use thereof as a fungicide |
| WO2005123689A1 (en) | 2004-06-18 | 2005-12-29 | Basf Aktiengesellschaft | 1-methyl-3-trifluoromethyl-pyrazole-4-carboxylic acid (ortho-phenyl)-anilides and to use thereof as fungicide |
| WO2006015866A1 (en) | 2004-08-12 | 2006-02-16 | Syngenta Participations Ag | Method for protecting useful plants or plant propagation material |
| WO2006043635A1 (en) | 2004-10-20 | 2006-04-27 | Kumiai Chemical Industry Co., Ltd. | 3-triazolylphenyl sulfide derivative and insecticide/acaricide/nematicide containing the same as active ingredient |
| WO2006087325A1 (en) | 2005-02-16 | 2006-08-24 | Basf Aktiengesellschaft | 5-alkoxyalkyl-6-alkyl-7-amino-azolopyrimidines, method for their production, their use for controlling pathogenic fungi and agents containing said substances |
| WO2006087343A1 (en) | 2005-02-16 | 2006-08-24 | Basf Aktiengesellschaft | Pyrazole carboxylic acid anilides, method for the production thereof and agents containing them for controlling pathogenic fungi |
| WO2006089633A2 (en) | 2005-02-22 | 2006-08-31 | Bayer Cropscience Ag | Spiroketal-substituted cyclic ketoenols |
| DE102005009458A1 (en) | 2005-03-02 | 2006-09-07 | Bayer Cropscience Ag | pyrazolylcarboxanilides |
| WO2007006670A1 (en) | 2005-07-07 | 2007-01-18 | Basf Aktiengesellschaft | N-thio-anthranilamid compounds and their use as pesticides |
| WO2007043677A1 (en) | 2005-10-14 | 2007-04-19 | Sumitomo Chemical Company, Limited | Hydrazide compound and pesticidal use of the same |
| WO2007082098A2 (en) | 2006-01-13 | 2007-07-19 | Dow Agrosciences Llc | 6-(poly-substituted aryl)-4-aminopicolinates and their use as herbicides |
| WO2007090624A2 (en) | 2006-02-09 | 2007-08-16 | Syngenta Participations Ag | A method of protecting a plant propagation material, a plant, and/or plant organs |
| WO2007101540A1 (en) | 2006-03-06 | 2007-09-13 | Bayer Cropscience Ag | Combinations of active ingredients with insecticidal properties |
| WO2007101369A1 (en) | 2006-03-09 | 2007-09-13 | East China University Of Science And Technology | Preparation method and use of compounds having high biocidal activities |
| WO2008067911A1 (en) | 2006-12-04 | 2008-06-12 | Bayer Cropscience Ag | Biphenyl-substituted spirocyclic ketoenols |
| WO2008134969A1 (en) | 2007-04-30 | 2008-11-13 | Sinochem Corporation | Benzamide compounds and applications thereof |
| WO2009090181A2 (en) | 2008-01-15 | 2009-07-23 | Bayer Cropscience Sa | Pesticide composition comprising a tetrazolyloxime derivative and a fungicide or an insecticide active substance |
| WO2009124707A2 (en) | 2008-04-07 | 2009-10-15 | Bayer Cropscience Ag | Combinations of biological control agents and insecticides or fungicides |
| US20110172433A1 (en) * | 2008-07-01 | 2011-07-14 | Meiji Seika Kaisha, Ltd. | Novel imino derivatives, methods for producing the same and insecticides containing the same |
| US20110046186A1 (en) | 2008-07-07 | 2011-02-24 | Bin Li | 1-Substituted Pyridyl-Pyrazolyl Amide Compounds and Uses Thereof |
| WO2010006713A2 (en) | 2008-07-17 | 2010-01-21 | Bayer Cropscience Ag | Heterocyclic compounds used as pesticides |
| WO2010018714A1 (en) | 2008-08-13 | 2010-02-18 | 三井化学アグロ株式会社 | Amide derivative, pest control agent containing the amide derivative and use of the pest control agent |
| WO2010034737A1 (en) | 2008-09-24 | 2010-04-01 | Basf Se | Pyrazole compounds for controlling invertebrate pests |
| WO2010060379A1 (en) | 2008-11-28 | 2010-06-03 | 中国中化集团公司 | Ether compounds with nitrogen-containing 5-member heterocycle and the uses thereof |
| WO2010069882A1 (en) | 2008-12-17 | 2010-06-24 | Syngenta Participations Ag | Isoxazole derivatives for use as fungicides |
| WO2010069266A1 (en) | 2008-12-19 | 2010-06-24 | 华东理工大学 | Heterocyclic nitrogenous or oxygenous compounds with insecticidal activity formed from dialdehydes and their preparation and uses thereof |
| WO2010129497A1 (en) | 2009-05-05 | 2010-11-11 | Dow Agrosciences Llc | Pesticidal compositions |
| WO2010127926A1 (en) | 2009-05-06 | 2010-11-11 | Syngenta Participations Ag | 4 -cyano- 3 -benzoylamino-n- phenyl-benzamides for use in pest control |
| WO2011028657A1 (en) | 2009-09-01 | 2011-03-10 | Dow Agrosciences Llc | Synergistic fungicidal compositions containing a 5-fluoropyrimidine derivative for fungal control in cereals |
| WO2011069456A1 (en) | 2009-12-09 | 2011-06-16 | 华东理工大学 | Divalent and oxabridged heterocyclic neonicotinoid compounds and preparation methods thereof |
| WO2011077514A1 (en) | 2009-12-22 | 2011-06-30 | 三井化学アグロ株式会社 | Plant disease control composition and method for controlling plant diseases by applying the composition |
| CN101715777A (en) | 2010-01-12 | 2010-06-02 | 惠州市中迅化工有限公司 | Pesticide composition containing ethirimol and thiophanate-methyl, and application thereof |
| WO2011085575A1 (en) | 2010-01-15 | 2011-07-21 | 江苏省农药研究所股份有限公司 | Ortho-heterocyclyl formanilide compounds, their synthesis methods and use |
| CN102126994A (en) | 2010-01-19 | 2011-07-20 | 中化蓝天集团有限公司 | Benzophenone hydrazone derivative and preparation method and application thereof |
| WO2011135833A1 (en) | 2010-04-28 | 2011-11-03 | Sumitomo Chemical Company, Limited | Plant disease control composition and its use |
| WO2012000896A2 (en) | 2010-06-28 | 2012-01-05 | Bayer Cropscience Ag | Heterocyclic compounds as agents for pest control |
| US20130102637A1 (en) * | 2010-07-07 | 2013-04-25 | Gifu University | Pest control agent |
| WO2012029672A1 (en) | 2010-08-31 | 2012-03-08 | Meiji Seikaファルマ株式会社 | Noxious organism control agent |
| US20130150414A1 (en) | 2010-08-31 | 2013-06-13 | Meiji Seika Pharma Co., Ltd. | Pest control agent |
| WO2012034472A1 (en) | 2010-09-13 | 2012-03-22 | 中化蓝天集团有限公司 | Cyano benzenedicarboxamide compounds, preparing methods and as agricultural insecticides uses thereof |
| WO2012034403A1 (en) | 2010-09-14 | 2012-03-22 | 中化蓝天集团有限公司 | Fluoromethoxypyrazole anthranilamide compounds, synthesization methods and uses thereof |
| WO2012084670A1 (en) | 2010-12-20 | 2012-06-28 | Basf Se | Pesticidal active mixtures comprising pyrazole compounds |
| WO2012143317A1 (en) | 2011-04-21 | 2012-10-26 | Basf Se | Novel pesticidal pyrazole compounds |
| WO2012168188A1 (en) | 2011-06-07 | 2012-12-13 | Bayer Intellectual Property Gmbh | Active compound combinations |
| WO2013003977A1 (en) | 2011-07-01 | 2013-01-10 | 合肥星宇化学有限责任公司 | Compound of 2,5-disubstituted-3-nitroimino-1,2,4-triazoline and preparation method and use as pesticide thereof |
| WO2013007767A1 (en) | 2011-07-13 | 2013-01-17 | Basf Se | Fungicidal substituted 2-[2-halogenalkyl-4-(phenoxy)-phenyl]-1-[1,2,4]triazol-1-yl-ethanol compounds |
| WO2013010862A1 (en) | 2011-07-15 | 2013-01-24 | Basf Se | Fungicidal alkyl-substituted 2-[2-chloro-4-(4-chloro-phenoxy)-phenyl]-1-[1,2,4]triazol-1-yl-ethanol compounds |
| WO2013024010A1 (en) | 2011-08-12 | 2013-02-21 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| WO2013024009A1 (en) | 2011-08-12 | 2013-02-21 | Basf Se | N-thio-anthranilamide compounds and their use as pesticides |
| WO2013047441A1 (en) | 2011-09-26 | 2013-04-04 | 日本曹達株式会社 | Agricultural and horticultural bactericide composition |
| WO2013047749A1 (en) | 2011-09-29 | 2013-04-04 | 三井化学アグロ株式会社 | Production method for 4, 4-difluoro-3,4-dihydroisoquinoline derivative |
| WO2013050317A1 (en) | 2011-10-03 | 2013-04-11 | Syngenta Limited | Polymorphs of an isoxazoline derivative |
| WO2013055584A1 (en) | 2011-10-13 | 2013-04-18 | E. I. Du Pont De Nemours And Company | Solid forms of nematocidal sulfonamides |
| WO2013092224A1 (en) | 2011-12-21 | 2013-06-27 | Basf Se | Use of strobilurin type compounds for combating phytopathogenic fungi resistant to qo inhibitors |
| WO2013127704A1 (en) | 2012-02-27 | 2013-09-06 | Bayer Intellectual Property Gmbh | Active compound combinations containing a thiazoylisoxazoline and a fungicide |
| WO2013129688A1 (en) | 2012-02-29 | 2013-09-06 | Meiji Seika Pharma Co., Ltd. | Pest control composition including novel iminopyridine derivative |
| WO2013144223A1 (en) * | 2012-03-30 | 2013-10-03 | Basf Se | N-substituted pyrimidinylidene compounds and derivatives for combating animal pests |
| WO2013162072A1 (en) | 2012-04-27 | 2013-10-31 | Sumitomo Chemical Company, Limited | Tetrazolinone compounds and its use as pesticides |
| US20140213448A1 (en) | 2012-04-27 | 2014-07-31 | Dow Agrosciences Llc | Pesticidal compositions and processes related thereto |
| WO2014036056A1 (en) | 2012-08-31 | 2014-03-06 | Zoetis Llc | Crystalline forms of 1-(5'-(5-(3,5-dichloro-4-fluorophenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-yl)-3'h-spiro[azetidine-3,1'-isobenzofuran]-1-yl)-2-(methylsulfonyl)ethanone |
| WO2014090918A1 (en) | 2012-12-13 | 2014-06-19 | Novartis Ag | Process for the enantiomeric enrichment of diaryloxazoline derivatives |
| WO2014126208A1 (en) | 2013-02-14 | 2014-08-21 | 日産化学工業株式会社 | Crystalline polymorph of isoxazoline-substituted benzamide compound, and method for producing same |
| WO2015028630A1 (en) | 2013-08-30 | 2015-03-05 | Basf Se | N-substituted pyridylidene compounds and derivatives for combating animal pests |
| WO2015038503A1 (en) | 2013-09-13 | 2015-03-19 | E. I. Du Pont De Nemours And Company | Heterocycle-substituted bicyclic azole pesticides |
Non-Patent Citations (12)
| Title |
|---|
| ""Catalogue of pesticide formulation types and international coding system", Technical Mono-graph No. 2, 6th Ed.", May 2008, CROPLIFE INTERNATIONAL |
| C. MACBEAN: "The Pesticide Manual, 16th edition", 2013, BRITISH CROP PROTECTION COUNCIL |
| CAN. J. PLANT SCI, vol. 48, no. 6, 1968, pages 587 - 94 |
| CHEM. REV., vol. 111, no. 11, 2011, pages 6557 - 6602 |
| CHEMICAL REVIEWS, vol. 107, 2007, pages 5210 - 5278 |
| EUROPEAN JOURNAL OF ORGANIC CHEMISTRY, 2000, pages 3273 - 3278 |
| JOURNAL OF MEDICINAL CHEMISTRY, vol. 55, 2012, pages 7378 - 7391 |
| KNOWLES: "Agrow Reports DS243", 2005, T&F INFORMA, article "New developments in crop protection product formulation" |
| KNOWLES: "Agrow Reports DS256", 2006, T&F INFORMA UK, article "Adjuvants and additives" |
| MCCUTCHEON'S: "Emulsifiers & Detergents, McCutcheon's Directories", vol. 1, 2008 |
| MOLLET; GRUBEMANN: "Formulation technology", 2001, WILEY VCH |
| TETRAHEDRON LETT., vol. 52, no. 23, 2011, pages 3033 - 3037 |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10440953B2 (en) | 2015-08-07 | 2019-10-15 | Basf Se | Control of pests in maize by ginkgolides and bilobalide |
| US11297837B2 (en) | 2016-02-19 | 2022-04-12 | Basf Se | Pesticidally activi mixtures comprising anthranilamide compounds |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10273231B2 (en) | Azoline compounds substituted by a condensed ring system | |
| US10023563B2 (en) | Cyclic compounds substituted by a condensed ring system | |
| EP3204390A1 (en) | Substituted pyrimidinium compounds for combating animal pests | |
| US10085448B2 (en) | Azoline compounds substituted by a carbocyclic condensed ring system | |
| US11142514B2 (en) | Imino compounds with a 2-chloropyrimidin-5-yl substituent as pest-control agents | |
| US10327446B2 (en) | Naphthyl- or isoquinolinyl-substituted isothiazoline compounds | |
| WO2016062678A1 (en) | N-acylimino heterocyclic compounds | |
| WO2016198613A1 (en) | N-(thio)acylimino compounds | |
| WO2016198611A1 (en) | N-(thio)acylimino heterocyclic compounds | |
| EP3013808B1 (en) | Isothiazoline compounds substituted with a carbobicyclic group | |
| WO2016062680A1 (en) | N-acylimino heterocyclic compounds |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16728056 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 16728056 Country of ref document: EP Kind code of ref document: A1 |