WO2013091595A1 - Pharmaceutical formulation of prasugrel hydrobromide - Google Patents
Pharmaceutical formulation of prasugrel hydrobromide Download PDFInfo
- Publication number
- WO2013091595A1 WO2013091595A1 PCT/CZ2012/000139 CZ2012000139W WO2013091595A1 WO 2013091595 A1 WO2013091595 A1 WO 2013091595A1 CZ 2012000139 W CZ2012000139 W CZ 2012000139W WO 2013091595 A1 WO2013091595 A1 WO 2013091595A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- weight
- amount
- pharmaceutical composition
- composition according
- prototype
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2013—Organic compounds, e.g. phospholipids, fats
- A61K9/2018—Sugars, or sugar alcohols, e.g. lactose, mannitol; Derivatives thereof, e.g. polysorbates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/4353—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems
- A61K31/4365—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems the heterocyclic ring system having sulfur as a ring hetero atom, e.g. ticlopidine
Definitions
- the invention relates to an oral pharmaceutical composition containing prasugrel h drobromide of form C of formula I as the active substance.
- EP 2 100 609 describes a formulation that contains a specific size of particles of these auxiliary substances. According to the authors this selection provides a dosage form with enhanced weight uniformity.
- the issue of mannitol quality in the formulation is also mentioned in WO 2010137613 by the same company, which says that using alpha-mannitol in the formulation is more convenient.
- the document EP 2 100 610 describes the use of low substituted hydroxypropyl cellulose, which improves solubility of the formulation.
- EP 2 100 606 describes a film coating consisting of polyvinyl alcohol, sodium salt of carboxymethyl cellulose and pullulan, which considerably enhance stability of the formulation according to the authors; according to EP 2 100 607 the stability enhancing effect is also provided by addition of water soluble polymers such as polyvinyl pyrrolidone or hydroxypropyl cellulose.
- Another document describing preparation of a dosage form using the dry granulation method is EP 2 100 608.
- EP 1 896 019 exposing either free prasugrel or prasugrel in the form of a salt to air humidity and air oxygen leads to degradation and this problem is solved
- the application WO 2010094471 describes a formulation that does not contain lactose and mannitol as according to this application a stable formulation is difficult to achieve in the case of mannitol and/or lactose due to possible interactions with the excipients.
- This invention provides an oral pharmaceutical composition of prasugrel hydrobromide of form C in the form of a coated tablet, which contains mannitol and is highly stable and guarantees a suitable dissolution profile.
- the pharmaceutical composition consists of prasugrel hydrobromide of form C, exhibiting in an X-ray pattern the characteristic reflections 8.0; 14.5; 22.0; 27.1 and 29.9° 2 ⁇ , measured using a CuKa radiation, mannitol, a disintegrant, a lubricant, a filler and a coating.
- This invention provides a formulation of highly stable prasugrel hydrobromide of form C, which, as table 1 shows, exhibits higher chemical stability during stability studies, especially in comparison to the hydrochloride of form B, which is contained in the original product Effient.
- the polymorphous form C of the hydrobromide shows the following characteristic peaks in the X-ray powder diffraction pattern: 7.8; 8.0; 13.4; 14.5; 16.8; 22.0; 25.1 ; 27.1 and 29.9° 20. The main reflections were observed at 8.0; 14.5; 22.0; 27.1 and 29.9° 20.
- the hydrobromide form C can be formulated with using standardly supplied mannitol without any specific requirements for this excipient.
- the pharmaceutical composition in accordance with this invention is a coated tablet, which consists of prasugrel hydrochloride in an amount of 3 - 20% by weight, mannitol in an amount of 85 - 5% by weight, a disintegrant in an amount of 1 - 20% by weight, a lubricant in an amount of 5 - 0% by weight, a filler in an amount of 5 - 50% by weight and a coating in an amount of 1 - 5% by weight of the total weight of the tablet.
- auxiliary substances are employed as the disintegrants, e.g. starch, carboxymethyl starch sodium salt, carboxymethyl cellulose sodium salt, carboxymethyl cellulose calcium salt, preferably crosscarmellose sodium salt.
- the lubricants used include, e.g., talc, calcium stearate, stearic acid, sodium stearyl fumarate; magnesium stearate is especially preferable.
- fillers such as lactose, corn starch, sucrose, calcium phosphate, especially microcrystalline cellulose.
- Common coating mixtures are used as the coating, which consist of, e.g., polyvinyl alcohol, polyethylene glycol 3350, talc, titanium dioxide, and lecithin such as Opadry II® 85G.
- the formulation in accordance with this invention is produced using the following procedure: prasugrel hydrobromide is dry granulated with mannitol and the lubricant, the other ingredients are added to the resulting granulate, and the mixture is homogenized. The mixture treated this way is then compressed into cores that are subsequently coated. The compression of cores is done e.g. using a rotatory tabletting press. The resulting cores are then covered with the coating mixture. in the case of the described composition (details - see prototype 5) a suitable dissolution profile was achieved. Significant acceleration of releasing of the active substance was caused by a change of the compacting mixture in this case.
- the active substance is only compacted with a water-soluble excipient - mannitol.
- a water-soluble excipient - mannitol Such a composition enables faster penetration of water through the structure of individual granules, their quicker disintegration and subsequent release of the active substance.
- another way of releasing of the active substance was selected. In one case the concentration of the disintegrant was increased. However, this method did not have any significant impact on the speed of releasing, which means that it is not suitable for further use in the formulation of a therapeutic product. Then, the active substance, which was subject to micronization before the use in the formulation, was tested. But even a smaller size related to a larger specific surface of the particles did not bring the desired acceleration of the dissolution profile. The micronized substance is less suitable (worse bulking capability, higher dustiness) also from the technological point of view.
- the innovator uses, for the manufacture of its product Effient, the active substance in the form of a salt - prasugrel hydrochloride form B.
- the manufacturing procedure is described in US 2010/01791 184A1.
- the manufacture comprises mixing of the active substance, mannitol, hydroxypropyl methyl cellulose, crosscarmellose sodium, microcrystalline cellulose and magnesium stearate. This mixture is compacted by means of a cylinder compactor. Additional amount of crosscarmellose sodium, microcrystalline cellulose and magnesium stearate is admixed to the resulting granulate. The resulting tabletting matter is compressed into tablets. The tablets are coated with the commercially available mixture OPADRY AMB ®. The final product is adjusted in Alu/Alu under an inert atmosphere of N 2 .
- the original product achieves the following dissolution profile (measured in 500ml of SGF, 50/150 rpm, in baskets):
- prototype 1 (batch 01051 1) having the following composition was prepared: Core:
- Hypromellose (methocel E5)
- the manufacturing method of the batch (01051 1) consists in compaction of a mixture of the active substance and all the above mentioned excipients. Magnesium stearate was divided into two parts. 40% of the total amount of MgSt was added to the mixture before and 60% after the compaction. The final tabletting matter was compressed into biconvex cores with a diameter of 8 mm and a weight of ca. 183.3mg. The last production step comprised coating with the coating mixture Opadry II® 85G white. The resulting weight of the coated tablet containing the active substance prasugrel hydrobromide form B is 188.8 mg.
- the active substance with the following particle size characteristic was used: d(90) ⁇ 35 ⁇ (Malvern).
- the specific surface of the substance used is 15.4 m 2 /g.
- Prototype I. (batch 01051 1) achieves the following dissolution profile (measured in 500ml of SGF, 50/150 rpm, in baskets): Prasugrel 10 mg
- the above mentioned dissolution profile can be described as slower in comparison to the reference product Efient. In comparison of the amount of the active substance released at minute 15 there is an average difference between the individual preparations of 24.3 %.
- Lecithin The production method of the batch (020511) consists in compaction of a mixture of the active substance and all the above mentioned excipients. Magnesium stearate was divided into two parts. 40% of the total amount of MgSt was added to the mixture before and 60% after the compaction. The final tabletting matter was compressed into biconvex cores with a diameter of 8 mm and a weight of ca. 210 mg. The last manufacturing step was coating with the coating mixture Opadry II® 85G white. The resulting weight of the coated tablet containing the active substance prasugrel hydrobromide form B is 216.3 mg.
- prototype 2 (batch 02051 1) does not contain hypromellose, which acts as a binder in the composition. For the dry granulation method this excipient is not necessary and therefore it can be omitted without any replacement. Further, prototype 2 (batch 02051 1) contains ca. 24% more microcrystalline cellulose and 6% more mannitol than prototype 1. Both the auxiliary substances fulfil the binder function in the formulation.
- the active substance with the following particle size characteristic was used: d(90) ⁇ 35 ⁇ (Malvern).
- the specific surface of the substance used is 15.4 m 2 /g.
- Prototype 2 (batch 02051 1) achieves the following dissolution profile (measured in 500 ml of SGF, 50/150 rpm, in baskets):
- the production method of the batch (040511) consists in compaction of a mixture of the active substance and all the above mentioned excipients. Magnesium stearate was divided into two parts. 40% of the total amount of MgSt was added to the mixture before and 60% after the compaction. The final tabletting matter was compressed into biconvex cores with a diameter of 8 mm and a weight of ca. 210 mg. The last production step was coating with the coating mixture Opadry II® 85G white. The resulting weight of the coated tablet containing the active substance prasugrel hydrobromide of form B is 216.3 mg.
- the active substance with the following particle size characteristic was used: d(90) ⁇ 35 ⁇ (Malvern).
- the specific surface of the substance used is 15.4 m /g.
- the produced tablets can be further characterized with the average content of the active substance of 10.2 mg.
- Prototype 3 (batch 04061 1) achieves the following dissolution profile (measured in 500ml of SGF, 50/150 rpm, in baskets):
- Prototype 4 issues from the same composition as prototype 3:
- prototype 3 only consists in the production procedure.
- the qualitative and quantitative composition remains the same as in prototype 3.
- prototype 4 it is only the mixture of the active substance with mannitol that is compacted.
- the other excipients crosscarmellose sodium, microcrystalline cellulose and magnesium stearate
- the produced tabletting matter is compressed into biconvex cores with a diameter of 8 mm and a weight of ca. 210 mg.
- the last step is coating with the coating mixture Opadry II® 85G white.
- the weight of the coated tablet containing the active substance prasugrel hydrobromide of form B is 216.3 mg.
- the active substance with the following particle size characteristics was used: d(90) ⁇ 35 ⁇ (Malvern).
- the specific surface of the substance used is 15.4 m 2 /g.
- the produced tablets can be further characterized with the average content of the active substance of 10.0 mg and the AV value of 6.84.
- Prototype 4 (batch 05061 1) achieves the following dissolution profile (measured in 500 ml of SGF, 50/150 rpm, in baskets):
- prototype 2 the fastest of all the development prototypes
- the difference at minute 15 amounts to 10%.
- the profile of prototype 4 is 27.3% faster at minute 15 than the profile of prototype 3.
- the production method of the batch of prototype 5 (04051 1) is the same as that of prototype 2. It consists in compaction of a mixture of the active substance and all the above mentioned excipients. Magnesium stearate was divided into two parts. 40% of the total amount of MgSt was added to the mixture before and 60% after the compaction. The final tabletting matter was compressed into biconvex cores with a diameter of 8 mm and a weight of ca. 210 mg. The last production step was coating with the coating mixture Opadry II® 85 G white. The resulting weight of a coated tablet containing the active substance prasugrel hydrobromide of form B is 216.3 mg.
- the active substance with the following particle size characteristic was used: d(90) ⁇ 2,4 ⁇ (Malvern).
- the size or distribution of particles, respectively, is the main change as compared to prototype 2.
- the specific surface of the used substance is 23.1 m /g.
- Prototype 5 (batch 06061 1) achieves the following dissolution profile (measured in 500 ml of SGF, 50/150 rpm, in baskets):
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Medicinal Preparation (AREA)
Abstract
A pharmaceutical composition in the form of coated tablets containing as the active substance prasugrel hydrobromide form C, which exhibits the following characteristic reflections in an X-ray pattern: 8.0; 14.5; 22.0; 27.1 and 29.9° 2θ, measured using CuKα radiation. The composition consists of prasugrel hydrobromide form C in an amount of 3 to 20% by weight, mannitol in an amount of 85 to 5% by weight, a disintegrant in an amount of 1 to 20% by weight, a lubricant in an amount of 5 to 0% by weight, a filler in an amount of 5 to 50% by weight and a coating in an amount of 1 to 5% of the total weight of a tablet.
Description
Pharmaceutical formulation of prasugrel hydrobromide Technical Field:
The invention relates to an oral pharmaceutical composition containing prasugrel h drobromide of form C of formula I as the active substance.
(I)
Background Art:
Prasugrel I, developed by Eli-Lilly in cooperation with Daiichi, was first mentioned in the patent EP 0 542 41 1. This document also describes its application as an anti-aggregation agent for patients with a risk of vessel clogging by a blood clot. EP 1 298 132 and EP 1 728 794 define basic types of formulation of pharmaceutically acceptable salts of prasugrel, where, among the applicable excipients, inter alia, lactose and mannitol are mentioned. These documents describe that prasugrel hydrochloride and maleate provide unexpected advantages from the point of view of stability profiles. Daiichi has published several documents that deal with the composition and preparation procedures of an oral formulation. EP 2 100 609 describes a formulation that contains a specific size of particles of these auxiliary substances. According to the authors this selection provides a dosage form with enhanced weight uniformity. The issue of mannitol quality in the formulation is also mentioned in WO 2010137613 by the same company, which says that using alpha-mannitol in the formulation is more convenient. The document EP 2 100 610 describes the use of low substituted hydroxypropyl cellulose, which improves solubility of the formulation. The application EP 2 100 606 describes a film coating consisting of polyvinyl alcohol, sodium salt of carboxymethyl cellulose and pullulan, which considerably enhance stability of the formulation according to the authors; according to EP 2 100 607 the stability enhancing effect is also provided by addition of water soluble polymers such as polyvinyl pyrrolidone or hydroxypropyl cellulose. Another document describing preparation of a dosage form using the
dry granulation method is EP 2 100 608. According to EP 1 896 019 exposing either free prasugrel or prasugrel in the form of a salt to air humidity and air oxygen leads to degradation and this problem is solved
The application WO 2010094471 describes a formulation that does not contain lactose and mannitol as according to this application a stable formulation is difficult to achieve in the case of mannitol and/or lactose due to possible interactions with the excipients.
Disclosure of Invention: This invention provides an oral pharmaceutical composition of prasugrel hydrobromide of form C in the form of a coated tablet, which contains mannitol and is highly stable and guarantees a suitable dissolution profile. The pharmaceutical composition consists of prasugrel hydrobromide of form C, exhibiting in an X-ray pattern the characteristic reflections 8.0; 14.5; 22.0; 27.1 and 29.9° 2Θ, measured using a CuKa radiation, mannitol, a disintegrant, a lubricant, a filler and a coating.
The teachings of prior documents suggest that formulation of a salt of prasugrel is not a trivial matter, as the prasugrel base or its salts are compounds sensitive to humidity, air oxygen, and can react to various excipients. This problem may be solved by careful combination of excipients and/or selection of a new form of prasugrel that by itself guarantees better chemical stability and thus higher stability of the dosage form.
This invention provides a formulation of highly stable prasugrel hydrobromide of form C, which, as table 1 shows, exhibits higher chemical stability during stability studies, especially in comparison to the hydrochloride of form B, which is contained in the original product Effient.
Stability after 4 months' storage (double PE foil, dark top layer, desiccant, 25°C):
HBr form C HC1 form B (orig.)
Purity 0 months 99.73 99.4
Purity after 4 months 99.69 98.8
Water content 0 months 0.14% 0.21%
Water content after 4
0.14% 0.29%
months
During standard tests the increase of emerging impurities only amounted to 0.04% in the case of the hydrobromide as compared to 0.6% of impurities in hydrochloride B.
It can be inferred from a comparison of the HBr C and HC1 B dissolution profiles that this form will provide comparable bioavailability to the already marketed form. A comparison of the two forms only shows a small difference in the amount of the substance released into the dissolution media. The difference between prasugrel hydrobromide, form C, and the hydrochloride, form B, is 0.005 mg/cm2/min. (See the following table - comparison of true dissolution of various forms of prasugrel, incl. free base).
Comparison of true dissolution of various forms of prasugrel
The polymorphous form C of the hydrobromide shows the following characteristic peaks in the X-ray powder diffraction pattern: 7.8; 8.0; 13.4; 14.5; 16.8; 22.0; 25.1 ; 27.1 and 29.9° 20. The main reflections were observed at 8.0; 14.5; 22.0; 27.1 and 29.9° 20. The diffraction pattern was obtained in an X'PERT PRO MPD PANalytical diffractometer with a graphite monochromator, CuKa (λ= 1.542 χ 10"10 m (1.542 A)) radiation used, excitation voltage: 45 kV, anode current: 40 niA, measured range: 2 - 40° 2Θ, increment: 0.01 ° 2Θ with the reflection dwell of 50 s; the measurement was carried out in a flat sample with the area/thickness of 10/0.5 mm.
The hydrobromide form C can be formulated with using standardly supplied mannitol without any specific requirements for this excipient.
The pharmaceutical composition in accordance with this invention is a coated tablet, which consists of prasugrel hydrochloride in an amount of 3 - 20% by weight, mannitol in an amount of 85 - 5% by weight, a disintegrant in an amount of 1 - 20% by weight, a lubricant in an
amount of 5 - 0% by weight, a filler in an amount of 5 - 50% by weight and a coating in an amount of 1 - 5% by weight of the total weight of the tablet.
Commonly used auxiliary substances are employed as the disintegrants, e.g. starch, carboxymethyl starch sodium salt, carboxymethyl cellulose sodium salt, carboxymethyl cellulose calcium salt, preferably crosscarmellose sodium salt.
The lubricants used include, e.g., talc, calcium stearate, stearic acid, sodium stearyl fumarate; magnesium stearate is especially preferable.
Commonly used fillers are employed as the fillers, such as lactose, corn starch, sucrose, calcium phosphate, especially microcrystalline cellulose. Common coating mixtures are used as the coating, which consist of, e.g., polyvinyl alcohol, polyethylene glycol 3350, talc, titanium dioxide, and lecithin such as Opadry II® 85G.
Experimental tests have confirmed that for prasugrel hydrobromide form C the following composition is preferable:
Our previous work has shown that for prasugrel hydrobromide form C the following composition is preferable:
The formulation in accordance with this invention is produced using the following procedure: prasugrel hydrobromide is dry granulated with mannitol and the lubricant, the other ingredients are added to the resulting granulate, and the mixture is homogenized. The mixture treated this way is then compressed into cores that are subsequently coated. The compression of cores is done e.g. using a rotatory tabletting press. The resulting cores are then covered with the coating mixture.
in the case of the described composition (details - see prototype 5) a suitable dissolution profile was achieved. Significant acceleration of releasing of the active substance was caused by a change of the compacting mixture in this case. While in the other cases a mixture of all the excipients is compacted and the corresponding releasing profiles are too slow, in the above mentioned composition the active substance is only compacted with a water-soluble excipient - mannitol. Such a composition enables faster penetration of water through the structure of individual granules, their quicker disintegration and subsequent release of the active substance. In the case of the other development prototypes another way of releasing of the active substance was selected. In one case the concentration of the disintegrant was increased. However, this method did not have any significant impact on the speed of releasing, which means that it is not suitable for further use in the formulation of a therapeutic product. Then, the active substance, which was subject to micronization before the use in the formulation, was tested. But even a smaller size related to a larger specific surface of the particles did not bring the desired acceleration of the dissolution profile. The micronized substance is less suitable (worse bulking capability, higher dustiness) also from the technological point of view.
Reference product
Composition of the Effient product according to SPC:
Core:
Prasugrel HC1
Mannitol
Hypromellose
Crosscarmellose sodium
Microcrystalline cellulose
Magnesium stearate
Coating:
Hypromellose
Lactose monohydrate
Triacetin (El 518)
Titanium dioxide (El 71)
Red ferric oxide (El 72)
Yellow iron oxide
Talc
The innovator (Eli Lilly) uses, for the manufacture of its product Effient, the active substance in the form of a salt - prasugrel hydrochloride form B.
The manufacturing procedure is described in US 2010/01791 184A1. The manufacture comprises mixing of the active substance, mannitol, hydroxypropyl methyl cellulose, crosscarmellose sodium, microcrystalline cellulose and magnesium stearate. This mixture is compacted by means of a cylinder compactor. Additional amount of crosscarmellose sodium, microcrystalline cellulose and magnesium stearate is admixed to the resulting granulate. The resulting tabletting matter is compressed into tablets. The tablets are coated with the commercially available mixture OPADRY AMB ®. The final product is adjusted in Alu/Alu under an inert atmosphere of N2.
The original product achieves the following dissolution profile (measured in 500ml of SGF, 50/150 rpm, in baskets):
Prototype 1
Based on the knowledge of composition and manufacturing procedure of the original product, prototype 1 (batch 01051 1) having the following composition was prepared:
Core:
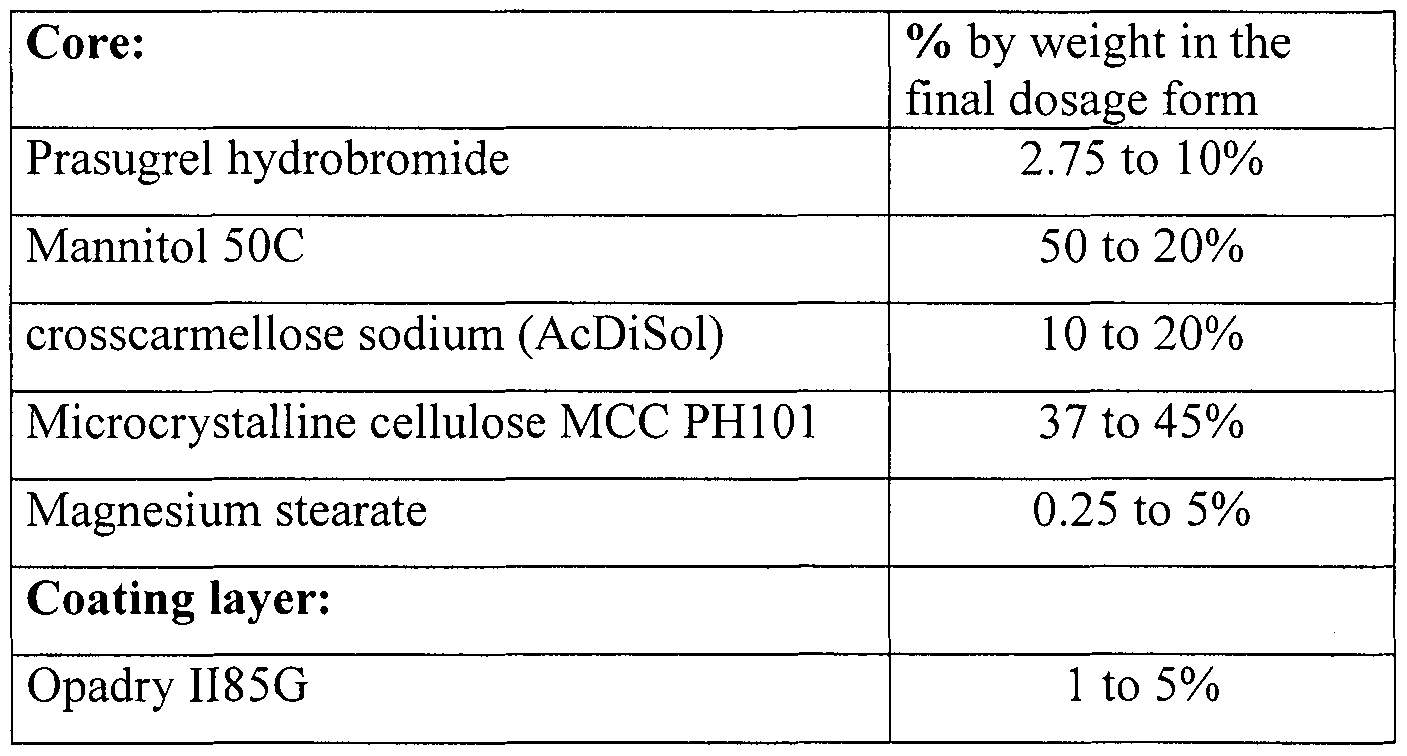
Prasugrel hydrobromide
Mannitol 50C
Hypromellose (methocel E5)
Crosscarmellose sodium (AcDiSol)
Microcrystalline cellulose MCC PHI 01
Magnesium stearate
Coating:
Polyvinyl alcohol
Polyethylene glycol 3350
Talc
Titanium dioxide
Lecithin
The manufacturing method of the batch (01051 1) consists in compaction of a mixture of the active substance and all the above mentioned excipients. Magnesium stearate was divided into two parts. 40% of the total amount of MgSt was added to the mixture before and 60% after the compaction. The final tabletting matter was compressed into biconvex cores with a diameter of 8 mm and a weight of ca. 183.3mg. The last production step comprised coating with the coating mixture Opadry II® 85G white. The resulting weight of the coated tablet containing the active substance prasugrel hydrobromide form B is 188.8 mg.
For the manufacture of the above mentioned prototype (batch 01051 1) the active substance with the following particle size characteristic was used: d(90) < 35 μηι (Malvern). The specific surface of the substance used is 15.4 m2/g. The produced tablets can be further characterized by the average content of the active substance of 9.84mg; AV value = 14.5; sum of impurities of 0.68 % and the moisture content of 2.5 % (TGA).
Prototype I. (batch 01051 1) achieves the following dissolution profile (measured in 500ml of SGF, 50/150 rpm, in baskets):
Prasugrel 10 mg
b.01051 1
% of released active
(min) substance.
0 0
5 39.6
10 53.0
15 61.1
20 66.6
30 76.0
45 83.6
60 92.8
The above mentioned dissolution profile can be described as slower in comparison to the reference product Efient. In comparison of the amount of the active substance released at minute 15 there is an average difference between the individual preparations of 24.3 %.
Prototype 2
A significant improvement of the dissolution profile was achieved in prototype 2 (batch 02051 1) the composition of which is described in the table below:
Core:
Prasugrel hydrobromide
Mannitol 50C
Crosscarmellose sodium (AcDiSol)
Microcrystalline cellulose MCC PHI 01
Magnesium stearate
Coating:
Polyvinyl alcohol
Polyethylene glycol 3350
Titanium dioxide
Lecithin
The production method of the batch (020511) consists in compaction of a mixture of the active substance and all the above mentioned excipients. Magnesium stearate was divided into two parts. 40% of the total amount of MgSt was added to the mixture before and 60% after the compaction. The final tabletting matter was compressed into biconvex cores with a diameter of 8 mm and a weight of ca. 210 mg. The last manufacturing step was coating with the coating mixture Opadry II® 85G white. The resulting weight of the coated tablet containing the active substance prasugrel hydrobromide form B is 216.3 mg.
Unlike prototype 1 (batch 01051 1), prototype 2 (batch 02051 1) does not contain hypromellose, which acts as a binder in the composition. For the dry granulation method this excipient is not necessary and therefore it can be omitted without any replacement. Further, prototype 2 (batch 02051 1) contains ca. 24% more microcrystalline cellulose and 6% more mannitol than prototype 1. Both the auxiliary substances fulfil the binder function in the formulation.
For the production of prototype 2 (batch 02051 1) the active substance with the following particle size characteristic was used: d(90) < 35 μπι (Malvern). The specific surface of the substance used is 15.4 m2/g. The produced tablets can be further characterized by means of: an average content of the active substance at the level of 9.99mg; AV value = 4.22; sum of impurities of 0.74% and the moisture content of 2.7%.
Prototype 2 (batch 02051 1) achieves the following dissolution profile (measured in 500 ml of SGF, 50/150 rpm, in baskets):
Prasugrel 10 mg
b.02051 1
% of released active
(min)
substance.
0 0
5 42.7
10 58.3
15 67.7
20 74.3
30 84.9
45 93.4
60 92.9
Although the above mentioned dissolution profile at minute 15 is 14.4% faster than that of prototype 1 , it is still more than 10% slower than the reference product. Prototype 3
Another experiment the objective of which was to improve the dissolution profile consisted in increasing the disintegrant content in the formulation. This change was tested in prototype 3. Compared to prototype 2, the concentration of the disintegrant (sodium salt of crosscarmellose) was increased in its composition from ca. 10% (m/m) to ca. 15% (m/m). The qualitative composition of prototype 3 is the same as that of prototype 2.
Core:
Prasugrel hydrobromide
Mannitol 50C
Crosscarmellose sodium (AcDiSol)
Microcrystalline cellulose MCC PHI 01
Magnesium stearate
Coating:
Polyvinyl alcohol
Polyethylene glycol 3350
Tide
Titanium dioxide
Lecithin
The production method of the batch (040511) consists in compaction of a mixture of the active substance and all the above mentioned excipients. Magnesium stearate was divided into two parts. 40% of the total amount of MgSt was added to the mixture before and 60% after the compaction. The final tabletting matter was compressed into biconvex cores with a diameter of 8 mm and a weight of ca. 210 mg. The last production step was coating with the coating mixture Opadry II® 85G white. The resulting weight of the coated tablet containing the active substance prasugrel hydrobromide of form B is 216.3 mg.
For the production of prototype 3 (batch 04061 1) the active substance with the following particle size characteristic was used: d(90) < 35μηι (Malvern). The specific surface of the substance used is 15.4 m /g. The produced tablets can be further characterized with the average content of the active substance of 10.2 mg.
Prototype 3 (batch 04061 1) achieves the following dissolution profile (measured in 500ml of SGF, 50/150 rpm, in baskets):
The above mentioned change of the disintegrant content did not bring the desired impact on dissolution. The profile of prototype 4 at minute 15 is nearly 18% slower than that of prototype 2. Compared to the profile of prototype 1 it is more than 1 1% slower. Compared to the reference preparation this means up to 34% difference at minute 15.
Prototype 4
Prototype 4 issues from the same composition as prototype 3:
Core:
Prasugrel hydrobromide
Mannitol 50C
Crosscarmellose sodium (AcDiSol)
Microcrystalline cellulose MCC PHI 01
Magnesium stearate
Coating:
Polyvinyl alcohol
Polyethylene glycol 3350
Talc
Titanium dioxide
Lecithin
The difference from prototype 3 only consists in the production procedure. The qualitative and quantitative composition remains the same as in prototype 3. In prototype 4 it is only the mixture of the active substance with mannitol that is compacted. The other excipients (crosscarmellose sodium, microcrystalline cellulose and magnesium stearate) are later admixed to the resulting compaction product. The produced tabletting matter is compressed into biconvex cores with a diameter of 8 mm and a weight of ca. 210 mg. Like in the other prototypes the last step is coating with the coating mixture Opadry II® 85G white. The weight of the coated tablet containing the active substance prasugrel hydrobromide of form B is 216.3 mg.
For the production of prototype 4 (batch 04061 1) the active substance with the following particle size characteristics was used: d(90) < 35 μπι (Malvern). The specific surface of the substance used is 15.4 m2/g. The produced tablets can be further characterized with the average content of the active substance of 10.0 mg and the AV value of 6.84.
Prototype 4 (batch 05061 1) achieves the following dissolution profile (measured in 500 ml of SGF, 50/150 rpm, in baskets):
Prasugrel 10 mg
b.05061 1
% of released active
(min)
substance
0 0
5 47.8
10 66.5
15 77.1
20 84.9
30 94.7
45 96.6
60 95.5
The above mentioned change of the production method of the concerned pharmaceutical composition brought about a considerable improvement of the dissolution profile. The acceleration of the dissolution profile was caused by granulation of the insoluble active substance together with an insoluble excipient (mannitol). The function of the soluble excipient consists in quicker penetration of water through individual granules and their faster disintegration. The difference between the profiles of prototype 4 and the batch of the reference product no. A743732 is only as low as 0.08%.
If prototype 2 (the fastest of all the development prototypes) is used for the comparison, the difference at minute 15 amounts to 10%. The profile of prototype 4 is 27.3% faster at minute 15 than the profile of prototype 3.
Prototype 5
The composition of prototype 5 is described in the table below.
The production method of the batch of prototype 5 (04051 1) is the same as that of prototype 2. It consists in compaction of a mixture of the active substance and all the above mentioned excipients. Magnesium stearate was divided into two parts. 40% of the total amount of MgSt was added to the mixture before and 60% after the compaction. The final tabletting matter was compressed into biconvex cores with a diameter of 8 mm and a weight of ca. 210 mg. The last production step was coating with the coating mixture Opadry II® 85 G white. The resulting weight of a coated tablet containing the active substance prasugrel hydrobromide of form B is 216.3 mg.
For the production of prototype 5 (batch 06061 1) the active substance with the following particle size characteristic was used: d(90) < 2,4 μηι (Malvern). The size or distribution of particles, respectively, is the main change as compared to prototype 2. The specific surface of the used substance is 23.1 m /g. The produced tablets can be further characterized: with the average content of the active substance of 9.9 mg; AV value = 6.56; sum of impurities of 0.79 % and the moisture content of 2.6 %.
Prototype 5 (batch 06061 1) achieves the following dissolution profile (measured in 500 ml of SGF, 50/150 rpm, in baskets):
The dissolution profile of prototype 5 does not virtually differ from the profile of prototype 2 (difference at minute 15 = 0.5%). Thus, in comparison with the reference product the difference amounts to more than 10%.
It can be concluded that micronization of the active substance does not have any significant effect on dissolution behaviour of this product.
Claims
1 . A pharmaceutical composition in the form of coated tablets containing as the active substance prasugrel hydrobromide of form C, characterized in that the prasugrel hydrobromide form C used exhibits the following characteristic reflections in an X-ray pattern: 8.0; 14.5; 22.0; 27.1 and 29.9° 20, measured using CuKa radiation.
2. The pharmaceutical composition according to claim 1, characterized in that it consists of
prasugrel hydrobromide form C in an amount of 3 to 20% by weight,
mannitol in an amount of 85 to 5% by weight,
a disintegrant in an amount of 1 to 20% by weight,
a lubricant in an amount of 5 to 0% by weight,
a filler in an amount of 5 to 50% by weight,
and a coating in an amount of 1 to 5% by weight of the total weight of the tablet.
3. The pharmaceutical composition according to claim 2, characterized in that the disintegrant is crosscarmellose sodium.
4. The pharmaceutical composition according to claim 2, characterized in that the lubricant is magnesium stearate.
5. The pharmaceutical composition according to claim 2, characterized in that the filler is microcrystalline cellulose.
6. The pharmaceutical composition according to claim 2, characterized in that a mixture is used as the coating which consists of polyvinyl alcohol, polyethylene glycol 3350, talc, titanium dioxide and lecithin.
7. The pharmaceutical composition according to claims 1 to 6, characterized in that it consists of
prasugrel hydrobromide in an amount of 2.75 to 10% by weight,
mannitol in an amount of 50 to 20% by weight, crosscarmellose sodium in an amount of 10 to 20% by weight,
magnesium stearate in an amount of 0.25 to 5% by weight
and a coating in an amount of 1 to 5% by weight of the total tablet weight.
8. A method of manufacturing the pharmaceutical composition according to claims 1 to 7, characterized in that prasugrel hydrobromide is dry granulated with mannitol and the lubricant, the other constituents are added to the resulting granulate and the mixture is homogenized, followed by compressing thus treated mixture into cores and coating said cores.
9. The method according to claim 8, characterized in that the dry granulation is compaction.
10. The pharmaceutical composition according to claims 1 to 6, characterized by the following rate of releasing of the active substance:
1 1 . A pharmaceutical composition prepared according to claims 8 and 9.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CZ20110872A CZ2011872A3 (en) | 2011-12-22 | 2011-12-22 | Pharmaceutical formulation of prasugrel hydrobromide |
| CZPV2011-872 | 2011-12-22 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013091595A1 true WO2013091595A1 (en) | 2013-06-27 |
Family
ID=47665757
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CZ2012/000139 WO2013091595A1 (en) | 2011-12-22 | 2012-12-19 | Pharmaceutical formulation of prasugrel hydrobromide |
Country Status (2)
| Country | Link |
|---|---|
| CZ (1) | CZ2011872A3 (en) |
| WO (1) | WO2013091595A1 (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104370934A (en) * | 2013-08-13 | 2015-02-25 | 上海科胜药物研发有限公司 | Prasugrel salt and preparation method thereof |
| EP3106151A1 (en) | 2015-06-19 | 2016-12-21 | Sanovel Ilac Sanayi ve Ticaret A.S. | Pharmaceutical compositions of prasugrel hydrobromide |
| CN107961221A (en) * | 2016-10-20 | 2018-04-27 | 长春海悦药业股份有限公司 | A kind of pharmaceutical composition containing prasugrel hydrochloride and preparation method thereof |
Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0542411A2 (en) | 1991-09-09 | 1993-05-19 | Sankyo Company Limited | Tetrahydrothienopyridine derivatives, furo and pyrrolo analogs thereof and their preparation and uses for inhibiting blood platelet aggregation |
| EP1298132A1 (en) | 2000-07-06 | 2003-04-02 | Sankyo Company, Limited | Hydropyridine derivative acid addition salts |
| EP1896019A2 (en) | 2005-06-10 | 2008-03-12 | Eli Lilly And Company | Formulation of a thienopyridine platelet aggregation inhibitor |
| EP2100607A1 (en) | 2006-12-07 | 2009-09-16 | Daiichi Sankyo Company, Limited | Pharmaceutical composition having improved storage stability |
| EP2100609A1 (en) | 2006-12-07 | 2009-09-16 | Daiichi Sankyo Company, Limited | Solid medicinal preparation containing mannitol or lactose |
| EP2100606A1 (en) | 2006-12-07 | 2009-09-16 | Daiichi Sankyo Company, Limited | Film-coated preparation having improved stability |
| EP2100608A1 (en) | 2006-12-07 | 2009-09-16 | Daiichi Sankyo Company, Limited | Method for producing solid preparation |
| EP2100610A1 (en) | 2006-12-07 | 2009-09-16 | Daiichi Sankyo Company, Limited | Pharmaceutical composition containing low-substituted hydroxypropylcellulose |
| US20100179184A1 (en) | 2006-12-07 | 2010-07-15 | Matthew John Moon | Article of manufacture for prasugrel |
| WO2010094471A1 (en) | 2009-02-17 | 2010-08-26 | Krka, D. D., Novo Mesto | Pharmaceutical compositions comprising prasugrel base or its pharmaceutically acceptable acid addition salts and processes for their preparation |
| WO2010137613A1 (en) | 2009-05-28 | 2010-12-02 | 第一三共株式会社 | Solid preparation having improved storage stability |
| WO2011004392A1 (en) * | 2009-07-06 | 2011-01-13 | Glenmark Generics Limited | Crystalline form of prasugrel hydrobromide, preparation and application thereof |
-
2011
- 2011-12-22 CZ CZ20110872A patent/CZ2011872A3/en unknown
-
2012
- 2012-12-19 WO PCT/CZ2012/000139 patent/WO2013091595A1/en active Application Filing
Patent Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0542411A2 (en) | 1991-09-09 | 1993-05-19 | Sankyo Company Limited | Tetrahydrothienopyridine derivatives, furo and pyrrolo analogs thereof and their preparation and uses for inhibiting blood platelet aggregation |
| EP1298132A1 (en) | 2000-07-06 | 2003-04-02 | Sankyo Company, Limited | Hydropyridine derivative acid addition salts |
| EP1728794A1 (en) | 2000-07-06 | 2006-12-06 | Sankyo Company Limited | Maleate addition salt of hydropyridine derivatives |
| EP1896019A2 (en) | 2005-06-10 | 2008-03-12 | Eli Lilly And Company | Formulation of a thienopyridine platelet aggregation inhibitor |
| EP2100607A1 (en) | 2006-12-07 | 2009-09-16 | Daiichi Sankyo Company, Limited | Pharmaceutical composition having improved storage stability |
| EP2100609A1 (en) | 2006-12-07 | 2009-09-16 | Daiichi Sankyo Company, Limited | Solid medicinal preparation containing mannitol or lactose |
| EP2100606A1 (en) | 2006-12-07 | 2009-09-16 | Daiichi Sankyo Company, Limited | Film-coated preparation having improved stability |
| EP2100608A1 (en) | 2006-12-07 | 2009-09-16 | Daiichi Sankyo Company, Limited | Method for producing solid preparation |
| EP2100610A1 (en) | 2006-12-07 | 2009-09-16 | Daiichi Sankyo Company, Limited | Pharmaceutical composition containing low-substituted hydroxypropylcellulose |
| US20100179184A1 (en) | 2006-12-07 | 2010-07-15 | Matthew John Moon | Article of manufacture for prasugrel |
| WO2010094471A1 (en) | 2009-02-17 | 2010-08-26 | Krka, D. D., Novo Mesto | Pharmaceutical compositions comprising prasugrel base or its pharmaceutically acceptable acid addition salts and processes for their preparation |
| WO2010137613A1 (en) | 2009-05-28 | 2010-12-02 | 第一三共株式会社 | Solid preparation having improved storage stability |
| WO2011004392A1 (en) * | 2009-07-06 | 2011-01-13 | Glenmark Generics Limited | Crystalline form of prasugrel hydrobromide, preparation and application thereof |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104370934A (en) * | 2013-08-13 | 2015-02-25 | 上海科胜药物研发有限公司 | Prasugrel salt and preparation method thereof |
| CN104370934B (en) * | 2013-08-13 | 2018-04-06 | 上海科胜药物研发有限公司 | A kind of prasugrel salt and preparation method thereof |
| EP3106151A1 (en) | 2015-06-19 | 2016-12-21 | Sanovel Ilac Sanayi ve Ticaret A.S. | Pharmaceutical compositions of prasugrel hydrobromide |
| WO2016203018A1 (en) | 2015-06-19 | 2016-12-22 | Sanovel Ilac Sanayi Ve Ticaret A.S. | Pharmaceutical compositions of prasugrel hydrobromide |
| CN107961221A (en) * | 2016-10-20 | 2018-04-27 | 长春海悦药业股份有限公司 | A kind of pharmaceutical composition containing prasugrel hydrochloride and preparation method thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CZ2011872A3 (en) | 2013-07-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4773456B2 (en) | Oral preparation with improved bioavailability | |
| EP3606511B1 (en) | Pharmaceutical composition comprising lenvatinib mesylate | |
| WO2008072534A1 (en) | Solid medicinal preparation containing mannitol or lactose | |
| EP2732810A1 (en) | Spherical particles of clopidogrel bisulfate, pharmaceutical composition including same, and method for manufacturing same | |
| WO2008027600A2 (en) | Imatinib compositions | |
| WO2013091595A1 (en) | Pharmaceutical formulation of prasugrel hydrobromide | |
| EP2603288A1 (en) | Pharmaceutical granulate comprising imatinib mesylate | |
| EP3860606B1 (en) | Pharmaceutical composition comprising lenvatinib esylate or tosylate | |
| US7959948B2 (en) | Pharmaceutical composition of quetiapine fumarate | |
| EP2744484A1 (en) | Pharmaceutical composition comprising 4-[4[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]phenoxy]-n-methylpyridine-2-carboxamide | |
| WO2003055467A1 (en) | Simvastatin dosage forms | |
| EP3419605A1 (en) | Dasatinib formulation | |
| EP1803457A1 (en) | Pharmaceutical composition containing montelukast | |
| WO2014040577A1 (en) | A stable pharmaceutical formulation containing vardenafil hydrochloride | |
| JP4643899B2 (en) | Ibuprofen-containing tablet and method for producing the same | |
| US20120121700A1 (en) | Pharmaceutical formulations comprising valganciclovir | |
| TW201431553A (en) | Pharmaceutical formulation of N-[5-[2-(3,5-dimethoxyphenyl)ethyl]-2H-pyrazol-3-yl]-4-[(3R,5S)-3,5-dimethylpiperazin-1-yl]benzamide | |
| JP2018104425A (en) | Tablet containing dabigatran etexilate or a pharmaceutically acceptable salt thereof | |
| KR101944085B1 (en) | Solid oral dosage form containing valsartan, and preparation method therefor | |
| JP6298435B2 (en) | Orally disintegrating tablet containing an angiotensin II receptor antagonist | |
| AU2022342749A1 (en) | Pharmaceutical composition of bempedoic acid | |
| EP4321154A1 (en) | A tablet of tolvaptan and at least one binder processed with spray granulation | |
| JP2022090391A (en) | Release-controlled tablet containing mirabegron and method for producing the same | |
| JP2024007837A (en) | Pharmaceutical composition including lacosamide and use thereof | |
| KR20240052937A (en) | Soluble pharmaceutical composition comprising salts of disubstituted 1, 2, 4-triazine compounds |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12821234 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 12821234 Country of ref document: EP Kind code of ref document: A1 |