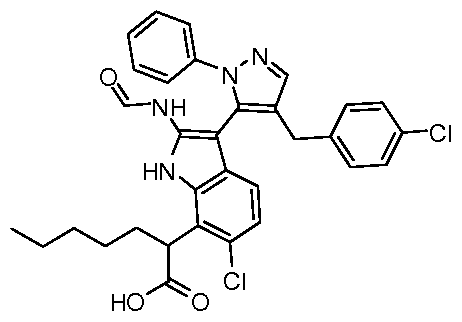
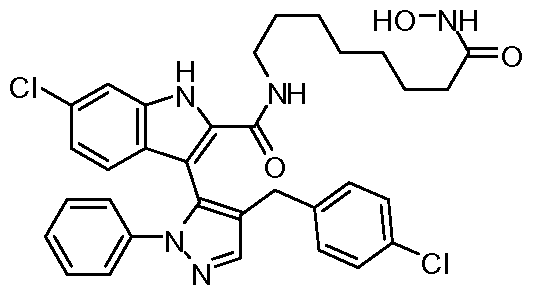
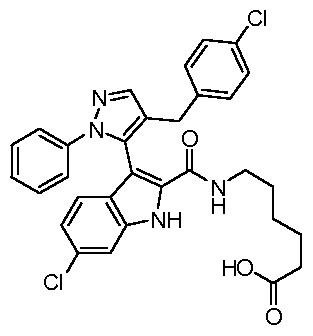
WO2011106650A2 - Novel p53-mdm2/p53-mdm4 antagonists to treat proliferative disease - Google Patents
Novel p53-mdm2/p53-mdm4 antagonists to treat proliferative disease Download PDFInfo
- Publication number
- WO2011106650A2 WO2011106650A2 PCT/US2011/026251 US2011026251W WO2011106650A2 WO 2011106650 A2 WO2011106650 A2 WO 2011106650A2 US 2011026251 W US2011026251 W US 2011026251W WO 2011106650 A2 WO2011106650 A2 WO 2011106650A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- indole
- nmr
- mhz
- wash
- Prior art date
Links
- 0 *C(c([n]c1c2cc(*)c(Cl)c1)c2-c1c(-c2ccccc2)nc[n]1Cc1ccc(*)cc1)=O Chemical compound *C(c([n]c1c2cc(*)c(Cl)c1)c2-c1c(-c2ccccc2)nc[n]1Cc1ccc(*)cc1)=O 0.000 description 3
- STGOYQKURJLDLJ-UHFFFAOYSA-N CCOC(c([nH]c1c2ccc(Cl)c1)c2-c([n](Cc(cc1)ccc1Cl)nc1)c1-c1ccccc1)=O Chemical compound CCOC(c([nH]c1c2ccc(Cl)c1)c2-c([n](Cc(cc1)ccc1Cl)nc1)c1-c1ccccc1)=O STGOYQKURJLDLJ-UHFFFAOYSA-N 0.000 description 1
- PWDMKUQFFWIVGY-UHFFFAOYSA-N CCOC(c([nH]c1cc(Cl)ccc11)c1-c1c(Cc(cc2)ccc2Cl)cn[n]1-c1ccccc1)=O Chemical compound CCOC(c([nH]c1cc(Cl)ccc11)c1-c1c(Cc(cc2)ccc2Cl)cn[n]1-c1ccccc1)=O PWDMKUQFFWIVGY-UHFFFAOYSA-N 0.000 description 1
- VHYFNPMBLIVWCW-UHFFFAOYSA-N CN(C)c1ccncc1 Chemical compound CN(C)c1ccncc1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 1
- YYDNBUBMBZRNQQ-UHFFFAOYSA-N Cc(cc1)ccc1S(C)(=O)=O Chemical compound Cc(cc1)ccc1S(C)(=O)=O YYDNBUBMBZRNQQ-UHFFFAOYSA-N 0.000 description 1
- YLPLVVAXXZWTIR-UHFFFAOYSA-N Cc(cc1)ccc1S(N(CC1)CC1OS(c1ccc(C)cc1)(=O)=O)(=O)=O Chemical compound Cc(cc1)ccc1S(N(CC1)CC1OS(c1ccc(C)cc1)(=O)=O)(=O)=O YLPLVVAXXZWTIR-UHFFFAOYSA-N 0.000 description 1
- XZLSAAMVVHBVKH-UHFFFAOYSA-N OC(c([nH]c1c2ccc(Cl)c1)c2-c([n](Cc1ccccc1)nc1)c1-c1ccccc1)=O Chemical compound OC(c([nH]c1c2ccc(Cl)c1)c2-c([n](Cc1ccccc1)nc1)c1-c1ccccc1)=O XZLSAAMVVHBVKH-UHFFFAOYSA-N 0.000 description 1
- DKIDKTWRGZQBNG-UHFFFAOYSA-N OC(c([nH]c1cc(Cl)ccc11)c1-c([n](Cc(cc1)ccc1Cl)nc1)c1-c(cc1)ccc1F)=O Chemical compound OC(c([nH]c1cc(Cl)ccc11)c1-c([n](Cc(cc1)ccc1Cl)nc1)c1-c(cc1)ccc1F)=O DKIDKTWRGZQBNG-UHFFFAOYSA-N 0.000 description 1
- BLKLUPGQXPXVHK-UHFFFAOYSA-N OCCS(NC(c([nH]c1cc(Cl)ccc11)c1-c([n](Cc(cc1F)ccc1Cl)nc1)c1-c1ccccc1)=O)(=O)=O Chemical compound OCCS(NC(c([nH]c1cc(Cl)ccc11)c1-c([n](Cc(cc1F)ccc1Cl)nc1)c1-c1ccccc1)=O)(=O)=O BLKLUPGQXPXVHK-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/04—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings directly linked by a ring-member-to-ring-member bond
Definitions
- the tumor suppressor protein p53 acts as a checkpoint in the cell cycle, either preventing or initiating programmed cell death.
- the occurrence of many human cancers can be linked to impaired nonfunctional p53 protein, with as many as 50% of all cancers being associated with some disruption of the gene that encodes p53. In these cancers, therefore, p53 is most likely inactivated and is unable to initiate programmed cell death.
- the oncoprotein MDM2 is believed to be the main negative regulator of this protein.
- MDM2/p53 association has been implicated to play a role in drug resistance that has become a major problem in anti-cancer therapy.
- some classes of compounds have been identified as inhibitors of the MDM2-p53 complex. These are the Nutlin -3 class of compounds, the spiro-oxindoles and compounds that have a benzodiazepinone core and others. Biological studies have shown, however, that compounds within each of these classes are of weak cellular activity with GI 50 values in the 0.9-50 ⁇ range.
- MDM4 protein MDM4
- Novartis discloses a small molecule antagonists of MDM4-p53 complex that contains an imidazole -indole core. Still, the K; values for these compounds was in the high micromolar range. Previous work by one of the present inventors has shown, however, that binding of the imidazole-indole class of compounds can be improved by placing appropriate substitutent groups.
- WASH 7720093.1 WO 2008/130614 relates nanomolar antagonists of the MDM4-p53 complex that have the imidazole-indole core.
- the present invention provides a new class of molecules that are individual and MDM2-p53 and MDM4-p53 antagonists, as well as methods for synthesizing these compounds and formulations for administering compounds in accordance with the invention to treat cell proliferative disorders.
- the present invention relates to novel compounds that are potent agonists of p53- MDM2/p53-MDM4 complexes.
- the present invention also relates to a pharmaceutical composition comprising a therapeutically effective amount of at least one compound according to Formula I their pharmaceutically acceptable salts, solvates, steroisomers, or tautomers and a pharmaceutically acceptable carrier, as well as to a method for treating a cell proliferative disorder using a compound in accordance with the invention.
- R ls is an indole
- R 2 and R 3 are different from each other and are phenyl or benzyl.
- the dashed circle -' indicates the presence of one or more optional double bonds and substituent groups R 4 and R 5 are independently selected from the group consisting of H, (Ci-Cg)alkyl, and (C 3 -Ci 4 )aryl.
- A, B, D and E are independently -CR1 , -CR 2, , -CR 4 , -CR 5 , -N, -O, or -S, provided that when A, D and E are each -N then B is -CR 2 , or when A is -N, B is -NR 2 , and D is - CR 5 then E is -CRi. Alternatively, when A is -NR 4 , B is N, and D is -CR 5 then E is -CRi.
- (C3-C 6 )heterocycloalkyl, (C 3 -C 6 )heteroaryl or benzyl groups may be substituted with one or more members selected from the group consisting of halogen, -OH, oxo, -COOR a , -C(0)R a , - (Ci-C 8 )alkyl-COOR a , -(Ci-C 8 )alkyl-NR a R b , S(0) 2 NR a R b ,-C(0)NR a R b , (C 3 -Ci 4 )aryl(Ci- C 6 )alkylene-, -CN, -N0 2 , NR a R b , (Ci-C 6 )alkyl-S-, (C 3 -Ci 4 )cycloalkyl, (C 3 - Ci 4 )heterocycloalkyl, (C 3 -Ci 4 )aryl
- Substituent groups R a and R b are independently selected from the group consisting of hydrogen, (Ci-C 8 )alkyl, hydroxide, (Ci-C 8 )alkylS(0) 2 -, (Ci-C 8 )haloalkylS(0) 2 -,
- Substituent R c is selected from the group consisting H, (Ci-C 8 )alkyl; -NH(OH).
- the present invention also provides compounds that conform to Formula IA, Formula II, Formula IIIA - IIIC, Formula IV, or Formula V, as well as their pharmaceutically acceptable salts, solvates, steroisomers, and tautomers and pharmaceutical compositions thereof.
- Figure 1 shows a stereo view of (A) MDM2-p53 complex and (B) MDM4-p53 complex.
- FIG. 2 shows inhibitors bound to MDM2 and MDM4 proteins.
- the inhibitors are shown in stick models with carbon atoms colored yellow, nitrogen blue, and oxygen red. Key side-chains of the proteins are labeled. Hydrogen bonds are depicted as yellow dashed lines.
- (A) Compound WW298 binds to MDM4 by filling its Trp23 subpocket with the 6- chloroindole group. The 4-phenyl group is located in the Phel9 and l-(4-chlorobenzyl) group in the Leu26 pockets, respectively. Two hydrogen bonds to Met53 and His54 are formed.
- the His96-Tyrl00 region has the most pronounced differences in the shape of the Leu26 pocket, but the position of l-(4-chlorobenzyl ) is not altered in (A) and (B).
- a hydrogen bond is formed between the indole of WK23 and the Leu54 carbonyl oxygen of MDM2.
- C The MI-63 inhibitor binds to MDM2 also by nesting the chlorophenyl substructure of the 6-chlorooxindole into the Trp26 subpocket.
- the Leu26 subpocket is filled by the 2-fluoro-3 -chlorophenyl ring.
- This ring is located as in WW298 and WK23 (a and b) but its plane is rotated to allow the phenyl substituent atoms to fill the bottom of the MDM2 pocket.
- the neopentyl fragment fills the Phel9 pocket and is a cause of a substantial induced- fit reshaping of the binding cleft.
- the Tyr67 side-chain is rotated to form a much steeper wall "closing" the binding region.
- the whole Tyr67-His73 region acquires a different fold to allow the Tyr67 movement.
- the compound forms two hydrogen bonds with Leu54 and His96.
- the ethyl-morpholino part of the compound is not taking part in the binding and is not seen in the electron density.
- FIG. 3 shows binding of the MDM4 inhibitor (WW298),with respect to the native p53 binding.
- the inhibitor is shown with yellow carbon atoms.
- MDM4 bound to the native p53 peptide is presented as a dark blue ribbon plot with most important residues shown in stick model.
- MDM4 bound to WW298 is similarly shown in light grey-blue.
- side-chains of Phel9, Trp23 and Leu26 of p53 are shown (green, labeled in italics).
- the Trp23 side-chain and the 6-chloroindole part of the inhibitor are bound in a nearly identical position both forming hydrogen bonds with the Met53 carbonyl oxygen.
- WASH 7720093.1 Trp23 subpocket undergoes induced-fit changes to accommodate the larger 6-chloroindole group.
- Leu56, Leu98, and Leul02 are retracted from the native peptide complex position to make space for an inhibitor.
- the position of the Tyr99 side-chain is altered but remains in a "closed” orientation.
- An additional hydrogen bond is formed between the inhibitor and His54.
- FIG. 4 shows that MDM4-inhibitor WW298 and MDM2 inhibitor WK23 bind in an identical mode.
- WW298 bound to MDM4 is shown with yellow carbon atoms.
- MDM2 is shown as a red ribbon plot with important residues shown as stick model.
- MDM4 is shown similarly in light blue. Important residues are labelled in italics, if different from MDM4, or their numbers given in parentheses, if of the same type as in MDMX.
- MDM2 and MDM4 especially in the Pro95-Tyr99 region of MDM4 and the corresponding His96-Tyrl00 of MDM2, the ligands are bound in a nearly identical way.
- FIG. 5 A shows the MDM2 and MDM4 inhibitors used for structural study. WK23 and WW298 differ only by an additional amide moiety attached to the 2-position of the indole ring. MI-63 is a spirooxindole compound.
- Figure 5B shows binding curves for MDM2 and MDM4 inhibitors based on data from a fluorescence polarization assay. All compounds show much lower affinity for MDM4.
- Figure 6 shows the binding to MI-63 to MDM2. Binding of MI-63 causes an induced fit rearrangement that causes changes in the conformation of the protein.
- Figure 7 illustrates results of an ex vivo cell growth inhibition study that was carried out against the National Cancer Institute's (NCI60) panel of cancer cell lines for three of the
- Alkyl refers to a straight or branched chain, saturated hydrocarbon having the indicated number of carbon atoms.
- (Ci-Ce)alkyl is meant to include, but is not limited to methyl, ethyl, propyl, isopropyl, butyl, sec-butyl, tert-butyl, pentyl, isopentyl, neopentyl, hexyl, isohexyl, and neohexyl.
- An alkyl group can be unsubstituted or optionally substituted with one or more substituents as described herein throughout.
- alkenyl refers to a straight or branched chain unsaturated hydrocarbon having the indicated number of carbon atoms and at least one double bond.
- Examples of a (C2-C 8 )alkenyl group include, but are not limited to, ethylene, propylene, 1-butylene, 2- butylene, isobutylene, sec-butylene, 1-pentene, 2-pentene, isopentene, 1-hexene, 2-hexene, 3- hexene, isohexene, 1-heptene, 2-heptene, 3-heptene, isoheptene, 1-octene, 2-octene, 3-octene, 4-octene, and isooctene.
- An alkenyl group can be unsubstituted or optionally substituted with one or more substituents as described herein below.
- alkynyl refers to a straight or branched chain unsaturated hydrocarbon having the indicated number of carbon atoms and at least one triple bond.
- Examples of a (C 2 - Cg)alkynyl group include, but are not limited to, acetylene, propyne, 1-butyne, 2-butyne, 1- pentyne, 2-pentyne, 1-hexyne, 2-hexyne, 3-hexyne, 1-heptyne, 2-heptyne, 3-heptyne, 1- octyne, 2-octyne, 3-octyne and 4-octyne.
- An alkynyl group can be unsubstituted or optionally substituted with one or more substituents as described herein below.
- halogen refers to -F, -CI, -Br or -I.
- alkoxy refers to an -O-alkyl group having the indicated number of carbon atoms.
- a (Ci-Ce)alkoxy group includes -O-methyl, -O-ethyl, -O-propyl, -O-isopropyl, -O-butyl, -O-sec -butyl, -O-tert-butyl, -O-pentyl, -O-isopentyl, -O-neopentyl, - O-hexyl, -O-isohexyl, and -O-neohexyl.
- aryl refers to a 6- to 14-membered monocyclic, bicyclic or tricyclic aromatic hydrocarbon ring system. Examples of an aryl group include phenyl and naphthyl. An aryl group can be unsubstituted or optionally substituted with one or more substituents as described herein throughout.
- Cycloalkyl denotes a 3- to 14-membered saturated or unsaturated non-aromatic monocyclic, bicyclic or tricyclic hydrocarbon ring system. Included in this class are cycloalkyl groups which are fused to a benzene ring.
- heteroaryl refers to an aromatic heterocycle ring of 3 to 14 members and having at least one heteroatom selected from nitrogen, oxygen and sulfur, and containing at least 1 carbon atom, including monocyclic, bicyclic, and tricyclic ring systems.
- heteroaryls are triazolyl, oxadiazolyl, pyridyl, furyl, benzofuranyl, thiophenyl, benzothiophenyl, quinolinyl, pyrrolyl, indolyl, oxazolyl, benzoxazolyl, imidazolyl, benzimidazolyl, thiazolyl, benzothiazolyl, isoxazolyl, pyrazolyl, isothiazolyl, pyridazinyl, pyrimidinyl, pyrazinyl, triazinyl, cinnolinyl, phthalazinyl, quinazolinyl, pyrimidyl, azepinyl, oxepinyl, quinoxalinyl and oxazolyl.
- a heteroaryl group can be unsubstituted or optionally substituted with one or more substituents as described throughout.
- heterocycle refers to 3- to 14-membered ring systems that are either saturated, unsaturated, or aromatic, and that contains from 1 to 4 heteroatoms independently selected from nitrogen, oxygen and sulfur, where the nitrogen and sulfur heteroatoms can be optionally oxidized and the nitrogen heteroatom can be optionally quaternized, including monocyclic, bicyclic, and tricyclic ring systems.
- the bicyclic and tricyclic ring systems may encompass a heterocycle or heteroaryl fused to a benzene ring.
- the heterocycle can be attached via any heteroatom or carbon atom.
- Heterocycles include heteroaryls as defined above.
- heterocycloalkyl by itself or combined with other terms, represents cyclic versions of “hetero alkyl.” Additionally, a heteroatom can occupy the position at which the heterocycle is attached to the remainder of the molecule.
- amine or amino refers to an -NR a R b group wherein R a and R b each independently refer to a hydrogen, (Ci-Cg)alkyl, aryl, (C 3 -C 6 )heterocycloalkyl, a
- R a and R b together with the nitrogen atom to which they are bound can form a (C 3 -C 6 )heterocycloalkyl, or a
- 'nitrile or cyano can be used interchangeably and refer to a -CN group which is bound to a carbon atom of a heteroaryl ring, aryl ring and a heterocycloalkyl ring.
- hydroxyalkyl refers to an alkyl group having the indicated number of carbon atoms wherein one or more of the alkyl group's hydrogen atoms is replaced with an - OH group.
- hydroxyalkyl groups include, but are not limited to, -CH 2 OH, - CH 2 CH 2 OH, -CH 2 CH 2 CH 2 OH, -CH 2 CH 2 CH 2 CH 2 OH, -CH 2 CH 2 CH 2 CH 2 CH 2 OH, - CH 2 CH 2 CH 2 CH 2 CH 2 OH, and branched versions thereof.
- haloalkyl refers to an alkyl group having the indicated number of carbon atoms wherein one or more hydrogen atoms in the alkyl group is replaced with a halogen atom, which can be the same or different.
- amide refers to C(0)NR a R b group wherein R a and R b are independently hydrogen, (C 3 -C 6 )heterocycloalkyl, a (C 3 -Ce)heteroaryl (Ci-Ce)alkyl, or R a and R b together with the nitrogen atom to which they are bound can form a (C 3 -C 6 )heterocycloalkyl, or a (C 3 -C 6 )heteroaryl.
- sulfonamide refers to an -NR a S(0) 2 R b group where R a and R b are each independently refer to a hydrogen, (Ci-C 8 )alkyl, aryl, heteroaryl, heterocycloalkyl, (Ci- C 8 )haloalkyl, and (Ci-Ce)hydroxyalkyl group.
- t R and "r.t” are used interchangeably and refer to the run time in a chromatogram. That is, these terms indicate the time interval at which a given compound elutes as identified by a peak in the chromatogram.
- the present invention provides a new class of molecules that are dual antagonists of MDM2/p53 and MDM4/p53 interactions. To design potent dual antagonists of the
- MDM2/MDM4 proteins the inventors juxtaposed deduced X-ray coordinates for the interaction of p53 with MDM4/MDM2 with X-ray coordinates for two compounds belonging to Formula I.
- Figure 1 shows a stereo view of the p53-MDM2 and p53-MDM4 complexes.
- Three amino acids from the hydrophobic surface of the p53 peptide, namely Phel9, Trp23 and Leu26 are appropriately placed to enter the hydrophobic pocket of MDM2/MDM4 proteins and are believed to be important for protein-peptide binding interactions.
- the X-ray data also revealed that the dimensions of the p53 binding pocket ( ⁇ 18 A), was similar in size to a small organic molecule, thereby illuminating the prospect of designing compounds that could interfere with binding of p53 to MDM2 and MDM4 proteins.
- the present inventors tested the ability of two structurally close 6-chloroindole-imidazole analogs and a structurally dissimilar spiro-oxindole compound to bind these proteins.
- WW298 an indole-imidazole
- MDM4 the deduced X-ray coordinates for this co-crystal revealed that WW298 mimics to some extent the binding interaction of p53 with MDM4 (see Figures 1 and 2).
- the 6-chloroindole group interacts with residues in the pocket normally occupied by Trp 23 residue of p53 (Trp23 sub-pocket). Based on the crystallographic coordinates, the -NH group in the indole ring is within hydrogen bonding distance with the carbonyl of Met53.
- the crystal structure also provides insights about the position and interactions of the 4-chlorobenzyl and phenyl rings of WW298.
- the 4-chlorobenzyl group is positioned to penetrate the cleft occupied by Leu26 upon binding of p53 (Leu26 sub-pocket), while the phenyl group of WW298 occupies the same pocket as Phel9 of p53 (Phel9 sub-pocket).
- the crystal structure further shows that there are changes in the three dimensional structure of the binding pocket of MDM4 when occupied by WW298, rather than the native ligand p53.
- the MDM4 binding pocket undergoes and induced fit change to accommodate the bulky hydrophobic chloro group on the indole ring.
- the presence of chlorine also causes changes in the position of residues more distant from the Trp23 sub- pocket, as well. For example, retraction of the side chain of Leu26 by 0.81 A as well as topographical changes in the main chains of helices that form the binding pocket are attributed to the occupancy of chlorine in the Trp23 sub-pocket.
- the 6-chloroindole group of WW298 also causes a shift in the position of the al helix by 0.56 A near the Met53 Ca atom, while the ⁇ 2' helix is displaced by 1.03 A at Leu98.
- the X-ray crystallographic data provided information that can be used, however, to tweak the potency of compounds that are suitable as candidate therapeutics for treating diseases related to improper p53 function, such as cell proliferative diseases.
- the 4-chlorobenzyl group occupies the Leu26 pocket and is in greater proximity (-2.16 A), to TyrlOO than the corresponding Cy atom of the native Leu26.
- the position of the 4-cholorbenzyl group is very similar to the bromophenyl ring of Nutlin-2, with the distance between the CI and Br atoms of 1.06 A and the angle between aromatic ring planes of 13°.
- the phenyl group of WK23 is oriented perpendicular to the plane of Phel9 in p53, and fills the aperture to the Phel9 sub-pocket. This is in stark contrast to the orientation of the Phel9 residue of p53, where the phenyl ring penetrates deeper into the binding pocket by approximately 2°A.
- the complex of MDM2 with the MI-63 inhibitor gave crystals with the asymmetric unit that contained three separate MI-63 -MDM2 complexes. Despite different crystal contacts and environments, the three complexes show no differences in structures with the exception of changes at the N-terminus, the TyrlOO side-chain (in proximity of it), and the Glu69-Gln71 loop.
- crystallographic data indicate that for the spiro-oxindole analog MI-63, the 6- chloroindole group is located in the Trp23 sub-pocket and is within hydrogen bonding distance to the carbonyl of Leu54.
- the plane of the indole ring is rotated, however, by 10° when compared to Trp23 of the native ligand p53 or WK23.
- the 2-fluoro-3-chlorophenyl ring is situated in the Leu26 sub-pocket with the bulky chlorine atom 0.52 A closer to the ⁇ 2' helix that the phenyl ring in WK23 or Nutlin-2. Similar to WK23, TyrlOO adapts an open conformation, but crystallographic data indicate that the side chain of this residue adopts
- the pyrrolidine ring of MI-63 extends the hydrophobic interaction with Val93 in a way similar to the imidazole ring of the WK23 inhibitor.
- the amide group of MI-63 forms a short (2.23 A) hydrogen bond between its carbonyl oxygen and His96 ⁇ . This hydrogen bond, although located in a solvent accessible region, is likely to be beneficial to the binding energy.
- the 6-chlorooxindole substituent is rigidly tied-up to the pyrrolidine core by a spiro connection, while in compounds WK23 and WW298 there is rotational freedom between the chloroindole and imidazole rings.
- MI-63 causes significant ligand-binding induced changes in the shape of the Phel9 pocket.
- This and the rigid connection between the chloroindole and pyrrolidine rings may well explain its high specificity in binding to MDM2 and a dramatic loss of its potency towards MDMX, as neither the protein nor the ligand seems to able to undergo necessary structural rearrangements for effective binding to MDMX.
- the plane of the chloroindole ring is rotated by 14.8° in MDM4 relative to MDM2, the energy cost for such a rotation is effectively contributed from the gain in binding energy due to the presence of the bulky chlorine on the indole ring.
- Another difference between the MDM4 and MDM2 proteins appears to be in the Phel9 sub-pocket, particularly at the outer rim of this pocket.
- for effective binding of the inventive compounds there appears to be no need for the phenyl group of the heterocycle to penetrate the Phel9 sub-pocket. Rather, hydrophobic interactions between the phenyl ring and the proteins hydrophobic surface are all that is required for optimal interactions.
- WASH 7720093.1 ray crystallo graphic data shows that while Tyr99 forces the MDMX protein to adopt a closed conformation, the position of the equivalent residue, TyrlOO in MDM2 protein can vary depending on the structure of the inhibitor complexed to MDM2. That is, for MDM4- inhibitor complex, Tyr99 forces the protein to adopt a closed conformation, while for MDM2, binding of WK23 does not cause the protein to adopt a closed conformation. Accordingly, candidate dual action antagonists to MDM2-p53 and MDM4-p53 complexes can be developed by optimizing the structure and chemical nature of substituent groups surrounding the heterocyclic core.
- the present invention provides compounds that comport with Formula 1A as well as a method for synthesizing these compounds (Scheme 1).
- R'" is either a H or a (Ci-Ce)alkyl and NRR' is .
- Formula 1A compounds can be prepared as shown in Scheme 1.
- the first step involves the synthesis of ethyl 6-chloro-lH-indole-2-carboxylate as follows. To a mixture of potassium (7.2 g, 185 mmol) in diethyl ether (60 mL) was added ethanol (40 mL) in diethyl ether (100 mL), followed by a solution of diethyl oxalate (27.8 g, 190 mmol) in diethyl ether (100 mL), and finally, a solution of 4-chloro-2-nitrotoluene (27.4 g, 160 mmol) in diethyl ether (40 mL).
- reaction mixture was stirred for 15 h and then sonicated for 7 h.
- the reaction mixture was poured into 1 N HCl (200 mL) at 0°C. Then the mixture was extracted with ethyl acetate. The combined organic layers were washed with brine, dried (anhydrous sodium sulfate) and concentrated to afford the intermediate ethyl 3- (4-chloro-2-nitrophenyl)-2-oxopropanoate which was used directly in the next step.
- 1H NMR of the crude product indicates that the conversion is about 80 %.
- the present invention also provides a methodology for generating (S)-N ,N ,N - trimethyl-N -(pyrrolidin-3-yl)propane-l,3-diamine (compound (A)).
- the first step involves the synthesis of (i?)-l-tosylpyrrolidin-3-yl 4- methylbenzenesulfonate according to the methodology illustrated in Scheme 1 A.
- 1 g of i?-Prolinol (11 mmol) is dissolved in 50 mL of dichloromethane, 4.4 g of pyridine (56 mmol) and 122 mg of dimethylaminopyridine (1 mmol) are added at room temperature.
- the reaction is cooled in an ice bath followed by addition of 5.4 g of toluenesulfonyl chloride (28 mmol).
- 13 C-NMR (CD 3 OD, 150.92 MHz): ⁇ 63.4, 54.0, 44.5, 42.3, 19.9, 19.5.
- the present invention provides compounds in which the imidazole core is replaced by other 5- membered heterocycles, such as a triazole, a pyrazole, an isoxazole or a thiazole. Inventive compounds belonging to the above classes should mimic the binding geometry of the imidazole analogs. The present invention also provides methods for synthesizing these compounds as well as formulations of the same.
- the present invention provides synthetic strategies for generating compounds that contain a pyrazole core and comport with Formulae 3A-3C.
- Scheme 4 show a method for synthesizing compounds in accordance with Formula 3 A.
- reacting the appropriately protected 6-chloroindole with the acetal of N,N- dimethylformamide results in pyrazole analogs.
- inventive compounds are generated by reacting 6-chloroindole with a benzylhydrazide as shown in Scheme 4.
- the present invention further provides a methodology for synthesizing
- the present invention also provides methodology for synthesizing compounds in accordance with Formulae 3B and 3C, as shown below in Schemes 5 and 6, respectively.
- Inventive compounds are potent antagonists of the p53/MDM2 and p53/MDM4 complex based on fluorescent polarization analysis, AIDA NMR and HSQCNMR analysis.
- Figure 5 compares the binding curve for an inventive compound that belongs to the imidazole-indole class (WK23), to the binding curves of known antagonists, namely, WW298, Nutlin-3 and a spiro-oxindole compound (MI-63).
- WK23 imidazole-indole class
- MI-63 spiro-oxindole compound
- the inventive compound WK23 had weaker binding affinity to MDM2 when compared to the affinity of other known antagonists, and yet WK23 showed greater affinity for MDM4 than Nutlin-3 and had a similar affinity for MDM4 as WW298.
- Table 1 provides protein inhibition data for additional compounds that belong to the imidazole -indole class, while Table 2 presents data from protein inhibition studies for a representative group of the pyrazolo-indole-class compounds.
- n.a. could not be measured because of the high, fluorescence of the compound.
- compounds in accordance with the present invention have affinity for both MDM2 and MDM4 proteins. Accordingly, the inventive compounds are dual action antagonists and are candidate therapeutics for treating cell proliferative disorders such as cancer.
- Exemplary inventive compounds also were tested for their ability to disrupt p53- hMDM2 protein-protein interaction, using a fluorescent based biosensor assay described, for example, by Dudgeon et al., J. Biomol. Screen. 15 : 766-82 (2010), and by Dudgeon et al., Assay and Drug Development Technologies 8: 437-58 (2010). Briefly, U20S cells are CO-
- WASH 7720093.1 infected with adenovirus constructs expressing a p53-monomeric TagGFP protein having a nuclear localization sequence (NLS) and a hDM2-monomeric Tag RFP cytoplasm-nucleus shuttling protein interaction partner having a NLS and a nuclear export sequence (NES).
- NLS nuclear localization sequence
- NES nuclear export sequence
- the hDM2 protein component binds to the p53 component and both proteins are localized in the nucleolus.
- Treatment of cells using an inventive compound disrupts the p53- hDM2 protein-protein interaction, which results in an export and redistribution of the unbound hDM2-RFP component in the cellular cytoplasm.
- the disruption of the hDM2-p53 biosensor interaction is determined via a molecular translocation image analysis algorithm that measures the difference in the average fluorescent intensity associated with hDM2-RFP component in the cytoplasm and nuclear compartments. See Dudgeon et al., J. Biomol. Screen. 15: 766-82 (2010), and Dudgeon et al., Assay and Drug Development Technologies 8: 437-58 (2010).
- inventive compounds were potent disrupters of cellular protein-protein interactions with potencies in the low micromolar range.
- the NMR-based technique "Antagonist Induced Dissociation Assay (AIDA-NMR)," can be used to study an antagonist's ability to disrupt a protein-protein interaction. See Bista et al, JACS, Vol. 131, pp7500-7501, (2009).
- An advantage to using AIDA to study disruption of protein-protein interactions is that this NMR based method provides unambiguous information on whether the antagonist activity of a compound is strong enough to dissociate a protein-protein complex and whether dissociation is through precipitation, denaturation or release of a protein in its functional folded state.
- AIDA has revealed that compounds in accordance with the present invention are potent disrupters of MDM2- or MDMX-p53 protein complexes and are thus suitable as therapeutics directed to the treatment of cell proliferative disorders.
- the present invention provides a method for treating cell proliferative disorders using an agent that is selected from a compound comporting with Formula 1 , its salt, prodrug, solvate, stereoisomer, or tautomer or by using a pharmaceutically acceptable formulation of such agent with a pharmaceutically acceptable carrier.
- FIG. 7A-7C representative compound of this invention for each cancer cell line included in the screen is graphically represented in Figures 7A-7C.
- the data illustrate that the inventive compounds do arrest the growth of various cancer cells in culture, with compound YH265 exhibiting the most potency against colon, melanoma, and CNS cancer cells.
- compounds of the invention and their pharmaceutically acceptable formulations provide a novel method for treating cancer.
- Pharmaceutically acceptable formulations in accordancewith the present invention are manufactured using the inventive compounds, its prodrug or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer and a pharmaceutically acceptable carrier.
- the polar amino and sulfonamide groups can promote hydrophilic interactions, such as hydrogen bonding with conventional aqueous carrier solvents, such as saline.
- aqueous carrier solvents such as saline.
- Inventive compounds that do not readily dissolve in an aqueous medium can be formulated by adding to the aqueous medium, pharmaceutically acceptable hydrophobic solvents, such as poly-alkylene glycol gelatin, gum arabic, lactose, starch, petroleum jelly and vegetable oil.
- aqueous carriers can be used to formulate the hydrophilic Formulae III compounds. Accordingly, several Formula I compounds showed good aqueous solubility in pH 7.0, phosphate buffer. See Table 3.
- excipients such as flavoring agents, preservatives, stabilizers, emulsifying agents, buffers and the like may be added in accordance with accepted practices of pharmaceutical compounding.
- a formulation of a compound of this invention can be administered intravenously, intraperitoneally, orally, bucally, or by parenteral administration.
- the compounds can be formulated, alone or together with other known antineoplastic agents, in suitable unit dosage formulations that may contain conventional non-toxic pharmaceutically acceptable carriers, adjuvants and vehicles, as described above, which are appropriate for each route of administration.
- the invention also contemplates administration of the compounds of the invention in a depot formulation, in which the active ingredient is released over a defined time period.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
High-resolution structural information delineates key interactions between MDM2 or MDM4 and heterocyclic compounds that bind to these proteins. These compounds are potent agonists of the p53-MDM2/p53-MDM4 complex.
Description
NOVEL P53-MDM2/P53-MDM4 ANTAGONISTS
TO TREAT PROLIFERATIVE DISEASE
CROSS-REFERENCE TO RELATED PATENT APPLICATIONS
[0001] This application claims priority to United States provisional application number 61/308,925, filed February 27, 2010, the entire contents of which are incorporated herein by reference.
BACKGROUND OF THE INVENTION
[0002] The tumor suppressor protein p53 acts as a checkpoint in the cell cycle, either preventing or initiating programmed cell death. The occurrence of many human cancers can be linked to impaired nonfunctional p53 protein, with as many as 50% of all cancers being associated with some disruption of the gene that encodes p53. In these cancers, therefore, p53 is most likely inactivated and is unable to initiate programmed cell death.
[0003] Of a variety of biological molecules that are capable of inactivating p53, the oncoprotein MDM2 is believed to be the main negative regulator of this protein.
Additionally, MDM2/p53 association has been implicated to play a role in drug resistance that has become a major problem in anti-cancer therapy. To date some classes of compounds have been identified as inhibitors of the MDM2-p53 complex. These are the Nutlin -3 class of compounds, the spiro-oxindoles and compounds that have a benzodiazepinone core and others. Biological studies have shown, however, that compounds within each of these classes are of weak cellular activity with GI50 values in the 0.9-50 μΜ range.
[0004] Recently, another protein MDM4 (MDMX), has been identified as a negative regulator of p53. Published PCT WO 2008/119741 (Novartis), discloses a small molecule antagonists of MDM4-p53 complex that contains an imidazole -indole core. Still, the K; values for these compounds was in the high micromolar range. Previous work by one of the present inventors has shown, however, that binding of the imidazole-indole class of compounds can be improved by placing appropriate substitutent groups. Thus,
WASH 7720093.1
WO 2008/130614 relates nanomolar antagonists of the MDM4-p53 complex that have the imidazole-indole core.
[0005] The present invention provides a new class of molecules that are individual and MDM2-p53 and MDM4-p53 antagonists, as well as methods for synthesizing these compounds and formulations for administering compounds in accordance with the invention to treat cell proliferative disorders.
SUMMARY OF THE INVENTION
[0006] The present invention relates to novel compounds that are potent agonists of p53- MDM2/p53-MDM4 complexes. The present invention also relates to a pharmaceutical composition comprising a therapeutically effective amount of at least one compound according to Formula I their pharmaceutically acceptable salts, solvates, steroisomers, or tautomers and a pharmaceutically acceptable carrier, as well as to a method for treating a cell proliferative disorder using a compound in accordance with the invention.
[0007] Accordingly, compounds are provided that conform to Formula I.
[0008] For compounds of Formula I, Rls is an indole, R2 and R3 are different from each other and are phenyl or benzyl. The dashed circle -' indicates the presence of one or more optional double bonds and substituent groups R4 and R5 are independently selected from the group consisting of H, (Ci-Cg)alkyl, and (C3-Ci4)aryl.
[0009] A, B, D and E are independently -CR1 , -CR2,, -CR4, -CR5, -N, -O, or -S, provided that when A, D and E are each -N then B is -CR2, or when A is -N, B is -NR2, and D is - CR5 then E is -CRi. Alternatively, when A is -NR4, B is N, and D is -CR5 then E is -CRi.
[0010] In another embodiment when A is -N, B is -O, and D is -CR5 then E is -CRi, or alternatively, when A is -N, B is -S, and D is -CR5 then E is -CRi.
WASH 7720093.1
[0011] For Formula 1 compounds any indole, phenyl, (C3-C8)cycloalkyl,
(C3-C6)heterocycloalkyl, (C3-C6)heteroaryl or benzyl groups may be substituted with one or more members selected from the group consisting of halogen, -OH, oxo, -COORa, -C(0)Ra, - (Ci-C8)alkyl-COORa, -(Ci-C8)alkyl-NRaRb, S(0)2 NRaRb,-C(0)NRaRb, (C3-Ci4)aryl(Ci- C6)alkylene-, -CN, -N02, NRaRb, (Ci-C6)alkyl-S-, (C3-Ci4)cycloalkyl, (C3- Ci4)heterocycloalkyl, (C3-Ci4)aryl, (C3-Ci4)heteroaryl, -C(0)NH-(Ci-C6)alkyl, - NHC(O)- (Ci-C6)alkyl, (Ci-C6)alkyl, (C2-C8)alkenyl, (C2-C8)alkynyl, (Ci-C6)alkoxy, (Ci-C6)haloalkyl, -C(0)-(C3-C8)cycloalkyl-NRaRb and (Ci-C6)hydroxyalkyl.
[0012] Substituent groups Ra and Rb are independently selected from the group consisting of hydrogen, (Ci-C8)alkyl, hydroxide, (Ci-C8)alkylS(0)2-, (Ci-C8)haloalkylS(0)2-,
(Ci-C8)hydroxyalkylS(0)2-, -(Ci-C8)alkyl-COORc, -(Ci-C8)alkyl-CORc and optionally substituted (C3-C8)cycloalkyl or Ra and Rb together with the nitrogen atom to which they are bound can form a (C3-C6)heterocycloalkyl, or a (C3-C6)heteroaryl.
[0013] Substituent Rc is selected from the group consisting H, (Ci-C8)alkyl; -NH(OH).
[0014] The present invention also provides compounds that conform to Formula IA, Formula II, Formula IIIA - IIIC, Formula IV, or Formula V, as well as their pharmaceutically acceptable salts, solvates, steroisomers, and tautomers and pharmaceutical compositions thereof.
Illb,
BRIEF DESCRIPTION OF THE DRAWINGS
[0015] Figure 1 shows a stereo view of (A) MDM2-p53 complex and (B) MDM4-p53 complex.
-3-
WASH 7720093.1
[0016] Figure 2 shows inhibitors bound to MDM2 and MDM4 proteins. The inhibitors are shown in stick models with carbon atoms colored yellow, nitrogen blue, and oxygen red. Key side-chains of the proteins are labeled. Hydrogen bonds are depicted as yellow dashed lines. (A) Compound WW298 binds to MDM4 by filling its Trp23 subpocket with the 6- chloroindole group. The 4-phenyl group is located in the Phel9 and l-(4-chlorobenzyl) group in the Leu26 pockets, respectively. Two hydrogen bonds to Met53 and His54 are formed. The Ν,Ν-dimethylpropylamine part of the WW298 molecule folds over Gly57 and Met61, forming additional hydrophobic protection of the binding cleft. Tyr99 closes the Leu26 subpocket. (B) shows an inhibitor in accordance with the invention (WK23), bound to the MDM2 protein. The conformation of WK23 is similar to the conformation of WW298 as shown in Figure 2(A), despite different shapes of the p53 binding sites in MDM2 and
MDM4. The His96-Tyrl00 region has the most pronounced differences in the shape of the Leu26 pocket, but the position of l-(4-chlorobenzyl ) is not altered in (A) and (B). A hydrogen bond is formed between the indole of WK23 and the Leu54 carbonyl oxygen of MDM2. (C) The MI-63 inhibitor binds to MDM2 also by nesting the chlorophenyl substructure of the 6-chlorooxindole into the Trp26 subpocket. The Leu26 subpocket is filled by the 2-fluoro-3 -chlorophenyl ring. This ring is located as in WW298 and WK23 (a and b) but its plane is rotated to allow the phenyl substituent atoms to fill the bottom of the MDM2 pocket. The neopentyl fragment fills the Phel9 pocket and is a cause of a substantial induced- fit reshaping of the binding cleft. The Tyr67 side-chain is rotated to form a much steeper wall "closing" the binding region. The whole Tyr67-His73 region acquires a different fold to allow the Tyr67 movement. The compound forms two hydrogen bonds with Leu54 and His96. The ethyl-morpholino part of the compound is not taking part in the binding and is not seen in the electron density.
[0017] Figure 3 shows binding of the MDM4 inhibitor (WW298),with respect to the native p53 binding. The inhibitor is shown with yellow carbon atoms. MDM4 bound to the native p53 peptide is presented as a dark blue ribbon plot with most important residues shown in stick model. MDM4 bound to WW298 is similarly shown in light grey-blue. For readability purposes only side-chains of Phel9, Trp23 and Leu26 of p53 are shown (green, labeled in italics). The Trp23 side-chain and the 6-chloroindole part of the inhibitor are bound in a nearly identical position both forming hydrogen bonds with the Met53 carbonyl oxygen. The
-4-
WASH 7720093.1
Trp23 subpocket undergoes induced-fit changes to accommodate the larger 6-chloroindole group. Leu56, Leu98, and Leul02 are retracted from the native peptide complex position to make space for an inhibitor. The position of the Tyr99 side-chain is altered but remains in a "closed" orientation. An additional hydrogen bond is formed between the inhibitor and His54.
[0018] Figure 4 shows that MDM4-inhibitor WW298 and MDM2 inhibitor WK23 bind in an identical mode. WW298 bound to MDM4 is shown with yellow carbon atoms. For WK23 the carbon atoms are in salmon. MDM2 is shown as a red ribbon plot with important residues shown as stick model. MDM4 is shown similarly in light blue. Important residues are labelled in italics, if different from MDM4, or their numbers given in parentheses, if of the same type as in MDMX. Despite significant structural differences between MDM2 and MDM4, especially in the Pro95-Tyr99 region of MDM4 and the corresponding His96-Tyrl00 of MDM2, the ligands are bound in a nearly identical way. Only the position of the chlorophenyl substituent in the Leu26 pocket is shifted between the two structures to adjust to different proteins environment. Clearly, weaker binding of the compounds towards MDM4 compared to MDM2 is caused by the sub-optimal interaction with the Leu26 subpocket.
[0019] Figure 5 A shows the MDM2 and MDM4 inhibitors used for structural study. WK23 and WW298 differ only by an additional amide moiety attached to the 2-position of the indole ring. MI-63 is a spirooxindole compound. Figure 5B shows binding curves for MDM2 and MDM4 inhibitors based on data from a fluorescence polarization assay. All compounds show much lower affinity for MDM4.
[0020] Figure 6 shows the binding to MI-63 to MDM2. Binding of MI-63 causes an induced fit rearrangement that causes changes in the conformation of the protein.
[0021] Figure 7 illustrates results of an ex vivo cell growth inhibition study that was carried out against the National Cancer Institute's (NCI60) panel of cancer cell lines for three of the
-5-
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Definitions
[0022] Unless indicated otherwise, the terms and phrases used in this description have the following meanings:
[0023] "Alkyl" refers to a straight or branched chain, saturated hydrocarbon having the indicated number of carbon atoms. For example, (Ci-Ce)alkyl is meant to include, but is not limited to methyl, ethyl, propyl, isopropyl, butyl, sec-butyl, tert-butyl, pentyl, isopentyl, neopentyl, hexyl, isohexyl, and neohexyl. An alkyl group can be unsubstituted or optionally substituted with one or more substituents as described herein throughout.
[0024] The term "alkenyl" refers to a straight or branched chain unsaturated hydrocarbon having the indicated number of carbon atoms and at least one double bond. Examples of a (C2-C8)alkenyl group include, but are not limited to, ethylene, propylene, 1-butylene, 2- butylene, isobutylene, sec-butylene, 1-pentene, 2-pentene, isopentene, 1-hexene, 2-hexene, 3- hexene, isohexene, 1-heptene, 2-heptene, 3-heptene, isoheptene, 1-octene, 2-octene, 3-octene, 4-octene, and isooctene. An alkenyl group can be unsubstituted or optionally substituted with one or more substituents as described herein below.
[0025] The term "alkynyl" refers to a straight or branched chain unsaturated hydrocarbon having the indicated number of carbon atoms and at least one triple bond. Examples of a (C2- Cg)alkynyl group include, but are not limited to, acetylene, propyne, 1-butyne, 2-butyne, 1- pentyne, 2-pentyne, 1-hexyne, 2-hexyne, 3-hexyne, 1-heptyne, 2-heptyne, 3-heptyne, 1- octyne, 2-octyne, 3-octyne and 4-octyne. An alkynyl group can be unsubstituted or optionally substituted with one or more substituents as described herein below.
-6-
WASH 7720093.1
[0026] The term "halogen" and "halo" refers to -F, -CI, -Br or -I.
[0027] The term "alkoxy" refers to an -O-alkyl group having the indicated number of carbon atoms. For example, a (Ci-Ce)alkoxy group includes -O-methyl, -O-ethyl, -O-propyl, -O-isopropyl, -O-butyl, -O-sec -butyl, -O-tert-butyl, -O-pentyl, -O-isopentyl, -O-neopentyl, - O-hexyl, -O-isohexyl, and -O-neohexyl.
[0028] The term "aryl" refers to a 6- to 14-membered monocyclic, bicyclic or tricyclic aromatic hydrocarbon ring system. Examples of an aryl group include phenyl and naphthyl. An aryl group can be unsubstituted or optionally substituted with one or more substituents as described herein throughout.
[0029] "Cycloalkyl" denotes a 3- to 14-membered saturated or unsaturated non-aromatic monocyclic, bicyclic or tricyclic hydrocarbon ring system. Included in this class are cycloalkyl groups which are fused to a benzene ring.
[0030] The term "heteroaryl" refers to an aromatic heterocycle ring of 3 to 14 members and having at least one heteroatom selected from nitrogen, oxygen and sulfur, and containing at least 1 carbon atom, including monocyclic, bicyclic, and tricyclic ring systems.
Representative heteroaryls are triazolyl, oxadiazolyl, pyridyl, furyl, benzofuranyl, thiophenyl, benzothiophenyl, quinolinyl, pyrrolyl, indolyl, oxazolyl, benzoxazolyl, imidazolyl, benzimidazolyl, thiazolyl, benzothiazolyl, isoxazolyl, pyrazolyl, isothiazolyl, pyridazinyl, pyrimidinyl, pyrazinyl, triazinyl, cinnolinyl, phthalazinyl, quinazolinyl, pyrimidyl, azepinyl, oxepinyl, quinoxalinyl and oxazolyl. A heteroaryl group can be unsubstituted or optionally substituted with one or more substituents as described throughout.
[0031] The term "heterocycle" refers to 3- to 14-membered ring systems that are either saturated, unsaturated, or aromatic, and that contains from 1 to 4 heteroatoms independently selected from nitrogen, oxygen and sulfur, where the nitrogen and sulfur heteroatoms can be optionally oxidized and the nitrogen heteroatom can be optionally quaternized, including monocyclic, bicyclic, and tricyclic ring systems. The bicyclic and tricyclic ring systems may encompass a heterocycle or heteroaryl fused to a benzene ring. The heterocycle can be attached via any heteroatom or carbon atom. Heterocycles include heteroaryls as defined above.
-7-
WASH 7720093.1
[0032] Unless otherwise stated, the term "heterocycloalkyl," by itself or combined with other terms, represents cyclic versions of "hetero alkyl." Additionally, a heteroatom can occupy the position at which the heterocycle is attached to the remainder of the molecule.
[0033] The term "amine or amino" refers to an -NRaRb group wherein Ra and Rb each independently refer to a hydrogen, (Ci-Cg)alkyl, aryl, (C3-C6)heterocycloalkyl, a
(C3-Ce)heteroaryl and (Ci-Ce)hydroxyalkyl group. In addition, Ra and Rb together with the nitrogen atom to which they are bound can form a (C3-C6)heterocycloalkyl, or a
(C3-C6)heteroaryl.
[0034] The term 'nitrile or cyano" can be used interchangeably and refer to a -CN group which is bound to a carbon atom of a heteroaryl ring, aryl ring and a heterocycloalkyl ring.
[0035] The term "oxo" refers to a =0 atom attached to a saturated or unsaturated (C3-C8) cyclic or a (Ci-C8) acyclic moiety. The =0 atom can be attached to a carbon, sulfur, and nitrogen atom that is part of the cyclic or acyclic moiety.
[0036] The term "hydroxyalkyl," refers to an alkyl group having the indicated number of carbon atoms wherein one or more of the alkyl group's hydrogen atoms is replaced with an - OH group. Examples of hydroxyalkyl groups include, but are not limited to, -CH2OH, - CH2CH2OH, -CH2CH2CH2OH, -CH2CH2CH2CH2OH, -CH2CH2CH2CH2CH2OH, - CH2CH2CH2CH2CH2CH2OH, and branched versions thereof.
[0037] The term "haloalkyl," refers to an alkyl group having the indicated number of carbon atoms wherein one or more hydrogen atoms in the alkyl group is replaced with a halogen atom, which can be the same or different.
[0038] The term "amide" refers to C(0)NRaRb group wherein Ra and Rb are independently hydrogen, (C3-C6)heterocycloalkyl, a (C3-Ce)heteroaryl (Ci-Ce)alkyl, or Ra and Rb together with the nitrogen atom to which they are bound can form a (C3-C6)heterocycloalkyl, or a (C3-C6)heteroaryl.
[0039] The term "sulfonamide" refers to an -NRaS(0)2Rb group where Ra and Rb are each independently refer to a hydrogen, (Ci-C8)alkyl, aryl, heteroaryl, heterocycloalkyl, (Ci- C8)haloalkyl, and (Ci-Ce)hydroxyalkyl group.
[0040]
-8-
WASH 7720093.1
[0041] The terms "tR" and "r.t" are used interchangeably and refer to the run time in a chromatogram. That is, these terms indicate the time interval at which a given compound elutes as identified by a peak in the chromatogram.
[0042] The present invention provides a new class of molecules that are dual antagonists of MDM2/p53 and MDM4/p53 interactions. To design potent dual antagonists of the
MDM2/MDM4 proteins the inventors juxtaposed deduced X-ray coordinates for the interaction of p53 with MDM4/MDM2 with X-ray coordinates for two compounds belonging to Formula I.
[0043] Figure 1 shows a stereo view of the p53-MDM2 and p53-MDM4 complexes. Three amino acids from the hydrophobic surface of the p53 peptide, namely Phel9, Trp23 and Leu26 are appropriately placed to enter the hydrophobic pocket of MDM2/MDM4 proteins and are believed to be important for protein-peptide binding interactions. The X-ray data also revealed that the dimensions of the p53 binding pocket (~18 A), was similar in size to a small organic molecule, thereby illuminating the prospect of designing compounds that could interfere with binding of p53 to MDM2 and MDM4 proteins. Accordingly, the present inventors tested the ability of two structurally close 6-chloroindole-imidazole analogs and a structurally dissimilar spiro-oxindole compound to bind these proteins. Thus, when WW298, an indole-imidazole, was co-crystallized with MDM4 to determine its binding interactions, the deduced X-ray coordinates for this co-crystal revealed that WW298 mimics to some extent the binding interaction of p53 with MDM4 (see Figures 1 and 2).
-9-
WASH 7720093.1
[0044] For example, the 6-chloroindole group interacts with residues in the pocket normally occupied by Trp 23 residue of p53 (Trp23 sub-pocket). Based on the crystallographic coordinates, the -NH group in the indole ring is within hydrogen bonding distance with the carbonyl of Met53. The crystal structure also provides insights about the position and interactions of the 4-chlorobenzyl and phenyl rings of WW298. As seen in Figures 2A and 3, the 4-chlorobenzyl group is positioned to penetrate the cleft occupied by Leu26 upon binding of p53 (Leu26 sub-pocket), while the phenyl group of WW298 occupies the same pocket as Phel9 of p53 (Phel9 sub-pocket).
[0045] The crystal structure further shows that there are changes in the three dimensional structure of the binding pocket of MDM4 when occupied by WW298, rather than the native ligand p53. For example, the MDM4 binding pocket undergoes and induced fit change to accommodate the bulky hydrophobic chloro group on the indole ring. The presence of chlorine also causes changes in the position of residues more distant from the Trp23 sub- pocket, as well. For example, retraction of the side chain of Leu26 by 0.81 A as well as topographical changes in the main chains of helices that form the binding pocket are attributed to the occupancy of chlorine in the Trp23 sub-pocket. The 6-chloroindole group of WW298 also causes a shift in the position of the al helix by 0.56 A near the Met53 Ca atom, while the α2' helix is displaced by 1.03 A at Leu98.
[0046] Similar changes are seen for other residues in the vicinity of the 4-chlorobenzyl group. To accommodate this bulky hydrophobic group, the Ca of Tyr99 is shifted by 1.27 A while the phenyl side chain of this residue is pushed outside the binding pocket resulting in a partially open conformation for the binding pocket. See Figure 3.
-10-
WASH 7720093.1
[0047] These results generally comport with binding of 2FEA a 6-chlorotryptophan peptide as disclosed by Kallen et al, J. Biol. Chem. 284: 8812 (2009). One major difference between the orientation of 2FEA and WW298 is that when 2FEA is bound, the side chain of Tyr99 in the MDM4 pocket is flipped outside the pocket which is now forced to adopt an open conformation rather than the partially closed conformation adopted upon binding of WW298. Taken together, these results suggest that the weaker binding potency of WW298 to MDMX when compared to p53, may likely be from energetically unfavorable changes in the position of certain residues in MDM4's pocket.
[0048] The X-ray crystallographic data provided information that can be used, however, to tweak the potency of compounds that are suitable as candidate therapeutics for treating diseases related to improper p53 function, such as cell proliferative diseases.
[0049] To verify that compounds of the imidazole-indole class also are capable of disrupting the p53-MDM2 complex, the present inventors deduced the X-ray crystallographic coordinates of a crystalline complex of MDM2 with WK23.
[0050] As illustrated in Figures 2B and 4, the binding of WK23 to MDM2 is predominantly through hydrophobic interactions and is not significantly different from the binding of p53. The crystallographic data does indicate, however, that Leu54 in the binding pocket is within hydrogen bonding distance from nitrogen of the 6-chloroindole group. Based on the deduced structure it is evident that the bulky chlorine atom penetrates into the pocket, and occupies a position similar to the position of a bromine group in the crystal structure of MDM2 complexed to the known antagonist Nutlin-3.
[0051] In addition to these binding interactions, there are other subtle changes in the position of various residues within the binding pocket when WK23 is present. For example,
-11-
WASH 7720093.1
for the MDM2-WK23 complex, the 4-chlorobenzyl group occupies the Leu26 pocket and is in greater proximity (-2.16 A), to TyrlOO than the corresponding Cy atom of the native Leu26. The position of the 4-cholorbenzyl group is very similar to the bromophenyl ring of Nutlin-2, with the distance between the CI and Br atoms of 1.06 A and the angle between aromatic ring planes of 13°.
[0052] Crystallographic data further indicated that binding of WK23 forces the plane of the phenyl ring of TyrlOO to rotate by approximately 75°. The orientation of TyrlOO side chain in MDM2, however, is in a "closed" conformation, similar to the position of this group in the Nutlin-2-MDM2 complex. In general, the Leu26 pocket is filled more completely in the WK23-MDM2 and to a greater depth by the 4-chlorobenzyl substituent than by the aliphatic Leu26 side-chain of p53, with only minor induced- fit changes in the MDM2 structure.
[0053] Other changes in the proteins 3-D conformation upon binding WK23 include a flip in the side chain of Met62 which now rests outside the binding pocket as well as changes in the orientation of residues Tyr67 to Gln72 that line the Phel9 sub-pocket.
[0054] As seen in Figures 2B and 4, the phenyl group of WK23 is oriented perpendicular to the plane of Phel9 in p53, and fills the aperture to the Phel9 sub-pocket. This is in stark contrast to the orientation of the Phel9 residue of p53, where the phenyl ring penetrates deeper into the binding pocket by approximately 2°A.
[0055] The complex of MDM2 with the MI-63 inhibitor gave crystals with the asymmetric unit that contained three separate MI-63 -MDM2 complexes. Despite different crystal contacts and environments, the three complexes show no differences in structures with the exception of changes at the N-terminus, the TyrlOO side-chain (in proximity of it), and the Glu69-Gln71 loop.
[0056] Thus, crystallographic data indicate that for the spiro-oxindole analog MI-63, the 6- chloroindole group is located in the Trp23 sub-pocket and is within hydrogen bonding distance to the carbonyl of Leu54. The plane of the indole ring is rotated, however, by 10° when compared to Trp23 of the native ligand p53 or WK23. The 2-fluoro-3-chlorophenyl ring is situated in the Leu26 sub-pocket with the bulky chlorine atom 0.52 A closer to the α2' helix that the phenyl ring in WK23 or Nutlin-2. Similar to WK23, TyrlOO adapts an open conformation, but crystallographic data indicate that the side chain of this residue adopts
-12-
WASH 7720093.1
different conformations for each molecule present in the asymmetric unit. These results implicate increased flexibility for TyrlOO. In contrast to WK23, the neopentyl group of MI- 63 occupies the Phel9 sub-pocket. Yet, because the neopentyl group is more flexible and is less bulky, it does not occupy the entire space of this sub-pocket, allowing Tyr67 from the protein to fill up the unoccupied space. This causes the main chain of Tyr67 to rotate by 0.83 A into the binding pocket. To allow the Tyr67 ring to enter the binding site, the main chain of His73 is retracted by 2.02 A (at Ca position) outward from the binding pocket. Altogether, these changes extensively reshape the Phel9 pocket, forming a "massive" induced fit rearrangement (Figures 2C and 6).
[0057] The pyrrolidine ring of MI-63 extends the hydrophobic interaction with Val93 in a way similar to the imidazole ring of the WK23 inhibitor. The amide group of MI-63 forms a short (2.23 A) hydrogen bond between its carbonyl oxygen and His96 Νε. This hydrogen bond, although located in a solvent accessible region, is likely to be beneficial to the binding energy. It should be noted that the 6-chlorooxindole substituent is rigidly tied-up to the pyrrolidine core by a spiro connection, while in compounds WK23 and WW298 there is rotational freedom between the chloroindole and imidazole rings. Because of differences in the shape of the p53 binding cleft of MDM4, the lack of freedom of the 6-chloroindole group may weaken the interactions of MI-63 to MDM4. On the other hand, these reduced degrees of rotational freedom also could contribute the very high affinity of MI-63 to MDM2 by entropy reduction.
[0058] The remaining part of the MI-63 molecule, including the morpholine ring and the aliphatic fragment attached to the amide group, is not visible in the electron density map. This indicates high flexibility of this segment of the MI-63 molecule and the lack of any direct interaction with MDM2. Clearly this substituent can be used to improve other drug- required properties such as water solubility, pKa or lipophilicity.
-13-
WASH 7720093.1
[0059] In summary, MI-63 causes significant ligand-binding induced changes in the shape of the Phel9 pocket. This and the rigid connection between the chloroindole and pyrrolidine rings may well explain its high specificity in binding to MDM2 and a dramatic loss of its potency towards MDMX, as neither the protein nor the ligand seems to able to undergo necessary structural rearrangements for effective binding to MDMX.
[0060] An unexpected insight from the crystallographic data for the WK23-MDM2 complex is that the imidazole ring of WK23 plays a role in binding. Based on the
crystallographic data, strong hydrophobic interactions should exist between the aromatic imidazole ring and the side chain of Val93. The crystallographic data also show that the plane of the imidazole ring is tilted when compared to the plane of the imidazoline in Nutlin- 3, which results in the imidazole group to shift by 1.03 A towards the a2 helix. Thus, for WK23 these changes in the orientation of imidazole force the 5-membered ring to pucker so that three atoms of the ring are forced to remain in the same plane. As a result, the 4- chlorobenzyl group attached to the ring is pushed into the Leu26 sub-pocket of MDM2.
[0061] Taken together, the data suggest that binding interactions between MDM2 and small heterocyclic scaffolds can be further improved by promoting hydrophobic, hydrogen bonding, and salt bridge interactions between the heterocyclic scaffold and side chains of amino acids surrounding the heterocycle in the binding pocket. Moreover, crystallographic studies implicate an overlap in the binding orientation of indole-imidazole heterocycles to MDM4 and MDM2 proteins. It is evident, therefore, that the 6-chloroindole group is well- suited to bind to the Trp23 sub-pocket present in both proteins. Although, the plane of the chloroindole ring is rotated by 14.8° in MDM4 relative to MDM2, the energy cost for such a rotation is effectively contributed from the gain in binding energy due to the presence of the bulky chlorine on the indole ring. Another difference between the MDM4 and MDM2 proteins appears to be in the Phel9 sub-pocket, particularly at the outer rim of this pocket. As stated above, for effective binding of the inventive compounds there appears to be no need for the phenyl group of the heterocycle to penetrate the Phel9 sub-pocket. Rather, hydrophobic interactions between the phenyl ring and the proteins hydrophobic surface are all that is required for optimal interactions.
[0062] The significant challenge in developing dual action antagonists is understood to arise from differences in the Leu26 sub-pocket, particularly with respect to the α2' helix. X-
-14-
WASH 7720093.1
ray crystallo graphic data shows that while Tyr99 forces the MDMX protein to adopt a closed conformation, the position of the equivalent residue, TyrlOO in MDM2 protein can vary depending on the structure of the inhibitor complexed to MDM2. That is, for MDM4- inhibitor complex, Tyr99 forces the protein to adopt a closed conformation, while for MDM2, binding of WK23 does not cause the protein to adopt a closed conformation. Accordingly, candidate dual action antagonists to MDM2-p53 and MDM4-p53 complexes can be developed by optimizing the structure and chemical nature of substituent groups surrounding the heterocyclic core.
SYNTHESIS
1. The Imidazole Scaffold
[0063] In one aspect, therefore, the present invention provides compounds that comport with Formula 1A as well as a method for synthesizing these compounds (Scheme 1).
X = H, D, F, Me, CI, Br
Y = OR'" or NRR' 1A
compounds, R'" is either a H or a (Ci-Ce)alkyl and NRR' is
. Formula 1A compounds can be prepared as shown in Scheme 1.
-15-
WASH 7720093.1
Scheme 1
Ethyl 6-chloro-lH-indole-2-carboxylate
[0065] The first step involves the synthesis of ethyl 6-chloro-lH-indole-2-carboxylate as follows. To a mixture of potassium (7.2 g, 185 mmol) in diethyl ether (60 mL) was added ethanol (40 mL) in diethyl ether (100 mL), followed by a solution of diethyl oxalate (27.8 g, 190 mmol) in diethyl ether (100 mL), and finally, a solution of 4-chloro-2-nitrotoluene (27.4 g, 160 mmol) in diethyl ether (40 mL). The reaction mixture was stirred for 15 h and then sonicated for 7 h. The reaction mixture was poured into 1 N HCl (200 mL) at 0°C. Then the mixture was extracted with ethyl acetate. The combined organic layers were washed with brine, dried (anhydrous sodium sulfate) and concentrated to afford the intermediate ethyl 3- (4-chloro-2-nitrophenyl)-2-oxopropanoate which was used directly in the next step. 1H NMR of the crude product indicates that the conversion is about 80 %.
[0066] To crude ethyl 3-(4-chloro-2-nitrophenyl)-2-oxopropanoate (ca 130 mmol) in ethanol (260 mL) and glacial acetic acid (260 mL) was added iron powder (74.4 g, 1.33 mol) and the reaction mixture was heated to reflux for 4 h. The mixture was filtered and evaporated. The residue was partitioned between dichloromethane and 1 N HCl. The organic layer was washed with 1 N HCl and brine and dried (anhydrous sodium sulfate). Evaporation of solvent gave the title product as a pale yellow solid 23 g, (65 %) over 2 steps. 1H NMR (d6-DMSO, 600 MHz): δ 12.02 (s, 1H), 7.68 (d, J = 8.4 Hz, 1H), 7.46 (s, 1H), 7.17 (s, 1H),
-16-
WASH 7720093.1
7.09 (d, J = 8.4 Hz, 1H), 4.34 (t, J = 7.2 Hz, 2H), 1.33 (q, J = 7.2 Hz, 3H) ppm; 13C NMR (d6- DMSO, 150.92 MHz): δ 161.0, 137.5, 129.2, 128.3, 125.4, 123.7, 120.7, 111.9, 107.8, 60.6, 14.2 ppm.
Ethyl 6-chloro-3-formyl-lH-indole-2-carboxylate
[0067] To a reaction of ethyl 6-chloro-lH-indole-2-carboxylate (4.46 g, 20 mmol) and phosphorus oxychloride (3.68 g, 24 mmol) in N,N-dimethyl formamide (15 mL), was added in a 100 mL round bottom flask equipped with stir bar. The reaction was heated to 50°C for 30 h. After completion, the reaction mixture is quenched with saturated sodium bicarbonate solution (50 mL) and extracted with diethyl ether (3 x 50 mL). The combined organic phase was washed with brine and dried (anhydrous sodium sulfate). The solvent was evaporated and the crude product was purified by recrystallization (ethyl acetate/hexane) to produce 3.11 g (62 %) title compound as light yellow solid. 1H NMR (d6-DMSO, 600 MHz): δ 12.99 (brs, 1H), 10.63 (s, 1H), 8.26 (d, J = 9.0 Hz, 1H), 7.62 (d, J = 1.8 Hz, 1H), 7.38 (dd, J = 9.0, 1.8 Hz, 1H), 4.51 (q, J = 7.2 Hz, 2H), 1.46 (t, J = 7.2 Hz, 3H) ppm; 13C NMR (d6-DMSO, 150.92 MHz): δ 187.4, 159.9, 136.1, 133.5, 130.4, 124.0, 123.9, 123.4, 118.2, 112.6 ppm.
Ethyl 6-chloro-3-(l-(4-chlorobenzyl)-4-phenyl-lH-imidazol-5-yl)-lH-indole-2- carboxylate
[0068] A 20 mL vial with stir bar was charged with ethyl 6-chloro-3-formyl-lH-indole-2- carboxylate (1.00 g, 4.0 mmol), l-(isocyano(phenyl)methylsulfonyl)-4-methylbenzene (1.10 g, 4.0 mmol), 4-chlorobenzylamine (0.57 g, 4.0 mmol) and triethylamine (0.41 g, 4.0 mmol) in ethanol (10 mL). The reaction was heated to 60 °C for 3 h. Then the solvent was removed in vacuum and the crude product was purified by silica gel chromatography (0-5 % methanol in ethyl acetate) to produce 1.80 g (92 %) of the title compound as the light white solid. 1H NMR (de-DMSO, 600 MHz): δ 12.41 (s, 1H), 8.13 (s, 1H), 7.55 (s, 1H), 7.40 (d, J = 7.2 Hz, 2H), 7.12-7.19 (m, 4H), 7.09 (t, J = 6.6 Hz, 1H), 7.02 (s, 2H), 6.82 (d, J = 8.4 Hz, 2H), 5.00 (s, 2H), 4.08-4.13 (m, 2H), 1.10 (t, J = 7.2 Hz, 3H) ppm; 13C NMR (d6-DMSO, 150.92 MHz): δ 160.2, 138.7, 138.3, 136.5, 136.1, 135.0, 131.7, 129.7, 128.7, 128.0, 127.9, 126.8, 125.8, 125.0, 121.9, 121.4, 119.5, 60.4, 47.5, 13.7 ppm; HRMS ESL-TOF for C27H22CI2N3O2 (M+H+) found: m/z: 490.1090; Calc. Mass: 490.1089.
-17-
WASH 7720093.1
6-Chloro-3-(l-(4-chlorobenzyl)-4-phenyl-lH-imidazol-5-yl)-lH-indole-2-carboxylic acid K23)
[0069] Hydrolysis of ethyl 6-chloro-3-(l-(4-chlorobenzyl)-4-phenyl-lH-imidazol-5-yl)-lH- indole-2-carboxylate is carried out as follows. To 900 mg (2 mmol), of the ester is added NaOH solution (2M, 35 mL) in ethanol (35 mL) and the reaction mixture is stirred followed by reflux for 2.5 h. The resulting mixture is poured into a mixture of ice and water. Then 25 mL 4M HC1 was added and 3 x extracted with ethyl acetate (a 50 mL). The combined organic phase was washed with brine and dried over sodium sulfate. The solvent was removed in vacuum to produce the title compound, 880 mg (95 %) as light yellow crystals. 1H NMR (de- DMSO, 600 MHz): δ 12.65 (s, 1H), 9.71 (s, 1H), 7.53 (d, J = 1.8 Hz, 1H), 7.40-7.44 (m, 2H), 7.25-7.30 (m, 3H), 7.11 (d, J = 8.4 Hz, 2H), 7.02 (d, J = 8.4 Hz, 1H), 6.95 (dd, J = 8.4, 1.2 Hz, 1H), 6.89 (d, J = 8.4 Hz, 2H), 5.27 (d, J = 15.0 Hz, 1H), 5.17 (d, J = 15.0 Hz, 1H) ppm; 13C NMR (d6-DMSO, 150.92 MHz): δ 161.2, 136.4, 136.2, 133.2, 132.7, 131.2, 129.8, 129.5, 129.3, 129.0, 128.9, 128.1, 127.2, 126.2, 125.5, 122.1, 121.7, 121.3, 49.5 ppm; HRMS ESL- TOF for C25Hi8Ci2N302 (M+) found: m/z: 462.0746 ; Calc. Mass: 462.0776.
2. The Pyrrolidine Scaffold (^-^^^-trimethyl-TN^-i yrrolidin-S-ylJ ro ane-l^-diamine hydrobromide
1 1 3
[0070] The present invention also provides a methodology for generating (S)-N ,N ,N - trimethyl-N -(pyrrolidin-3-yl)propane-l,3-diamine (compound (A)).
-18-
WASH 7720093.1
[0071] The first step involves the synthesis of (i?)-l-tosylpyrrolidin-3-yl 4- methylbenzenesulfonate according to the methodology illustrated in Scheme 1 A. Thus, 1 g of i?-Prolinol (11 mmol) is dissolved in 50 mL of dichloromethane, 4.4 g of pyridine (56 mmol) and 122 mg of dimethylaminopyridine (1 mmol) are added at room temperature. The reaction is cooled in an ice bath followed by addition of 5.4 g of toluenesulfonyl chloride (28 mmol).
Scheme 1A
[0072] Then reaction is allowed to come to room temperature and stirred overnight. It is then filtered and diluted with water (50ml), washed with brine, dried and purified by column chromatography (100% PE to PE:EtOAc=3: l) to give 4 g of product (90% yield).
Ci8H2iN05S2, 1H-NMR (CDC13, 600 MHz): δ = 1.98 (IH, m, J=3.90 Hz), 2.02 (IH, t, J=1.89 Hz), 2.45(3H, s), 2.47(3H, s), 3.23 (IH, dt, J=6.92, 4.88 Hz), 3.37 (IH, td, J=1.41, 12,4 Hz), 3.44 (IH, ddd, J=3.00 8.35, 97.2Hz), 3.48 (IH, dd, J=4.95, 12.27 Hz), 4.95 (IH, d, J=4.74 Hz), 4.95 (IH, t, J=4.98 Hz), 7.33 (2H, d, J=8.16 Hz), 7.34 (2H, d, J=8.52 Hz), 7.67 (2H, d, J=5.29 Hz), 7.68 (2H, d, J=5.10 Hz). 13C-NMR (CDCI3, 150.92 MHz): δ = 14.2(EtOAc), 21.1(EtOAc), 21.6, 21.7, 32.2, 45.8, 53.5, 60.4(EtOAc), 79.4, 127.6, 127.7, 129.8, 130.0, 133.2, 133.4, 143.9, 145.2, 171.2(EtOAc).
Conversion of bis-sulphonate to (SJ-N^N^^-trimethyl-^-il-tosylpyrrolidin-S-yl) propane-l,3-diamine
[0073] To 1 g of Bisulfonate intermediate (2.5 mmol) is dissolved in 3 ml of Ν,Ν,Ν - trimethylpropane- 1,3 -diamine in a sealed tube and heated to 90 °C in an oil bath for 12 h. The
-19-
WASH 7720093.1
mixture was separated by silica column chromatography (100% DCM to
DCM/methanol=100:5), to give 700 mg of the target compound (80% yield). C17H29N3O2S, 1H-NMR (CDCI3, 600 MHz): δ =1.58 (2H, m), 1.66 (IH, m), 2.00 (IH, m), 2.15 (3H, s), 2.20 (6H, s), 2.22 (2H, t, J=7.44 Hz), 2.32 (IH, m), 2.44 (3H, s), 2.86 (IH, dt, J=7.15, 7.67 Hz), 2.95 (IH, t, J=8.85 Hz), 3.19 (IH, dt, J=7.23, 4.83 Hz), 3.36 (IH, dt, J=2.95, 4.70 Hz), 3.49 (IH, dd, J=7.11, 9.33 Hz), 7.33 (2H, d, J=8.25 Hz), 7.71 (2H, d, J=8.31 Hz). 13C-NMR (CDCI3, 150.92 MHz): δ = 21.5, 25.1, 29.3, 39.5, 45.5, 46.8, 51.2, 53.4, 53.6, 57.7, 63.6, 127.6, 129.7, 133.3, 143.5.
(^-^^^-trimethyl-TN^-i yrrolidin-S-ylJ ro ane-l^-diamine hydrobromide salt
[0074] Monosulfonate intermediate (500 mg, 1.47 mmol) is dissolved in a 33% HBr/AcOH (2 ml) solution and heated to reflux for 2.5 h. The reaction is cooled to room temperature. The precipitate is isolated by filtration, washed with ethylacetate and dried to give 450 mg of the target compound (71 > yield) as a yellow power which is used in the next step without further purification.
[0075] CioH23N3, 1H-NMR (CD3OD, 600 MHz): δ =2.33 (2H, t, J=7.26 Hz), 2.44 (IH, m), 2.64 (IH, w), 2.98(3H, s), 3.02 (2H, s), 3.43 (IH, m), 3.67 (IH, m), 3.75 (IH, dd, J=7.92, 12.66 Hz), 3.89 (IH, dd, J=8.69, 12.46 Hz), 4.35 (IH, t, J=7.18 Hz). 13C-NMR (CD3OD, 150.92 MHz): δ =63.4, 54.0, 44.5, 42.3, 19.9, 19.5.
[0076] In another aspect, the present invention provides compounds in which the imidazole core is replaced by other 5- membered heterocycles, such as a triazole, a pyrazole, an isoxazole or a thiazole. Inventive compounds belonging to the above classes should mimic the binding geometry of the imidazole analogs. The present invention also provides methods for synthesizing these compounds as well as formulations of the same.
-20-
WASH 7720093.1
3. The Triazole Scaffold
[0077] In one embodiment, therefore, compounds that have a 1,2,4-triazole core and comport with Formula 2 are synthesized as shown in Schemes 2 and 3.
Bioorganic & Medicinal Chemistry Letters 18 (2008) 2799-2804
-21-
WASH 7720093.1
Scheme 3
[0078] According to Scheme 2, therefore, compounds having the triazole core can be synthesized by reacting the appropriately protected indole with 2-(4-chlorophenylethylidene) benzohydrazonoyl chloride. Pursuant to Scheme 3, however, the triazole scaffold is synthesized by reacting an appropriately protected 6-chloroindole with benzohydrazide.
4. The Pyrazole Scaffold
[0079] In another embodiment, the present invention provides synthetic strategies for generating compounds that contain a pyrazole core and comport with Formulae 3A-3C.
[0080] Scheme 4 show a method for synthesizing compounds in accordance with Formula 3 A. Thus, reacting the appropriately protected 6-chloroindole with the acetal of N,N- dimethylformamide results in pyrazole analogs. Alternatively, the inventive compounds are generated by reacting 6-chloroindole with a benzylhydrazide as shown in Scheme 4.
-22-
WASH 7720093.1
[0081] The present invention further provides a methodology for synthesizing
carboxamides of the inventive compounds as shown below in Scheme 4A. Accordingly, amidation of compound (A) with the desired amino alkanoic acid followed by reaction of the product with hydroxylamine gave the desired carboxamide.
Sche
[0082] The present invention also provides methodology for synthesizing compounds in accordance with Formulae 3B and 3C, as shown below in Schemes 5 and 6, respectively.
-23-
WASH 7720093.1
Scheme 5
[0083] Carboxamides of the inventive pyrazole analogs shown above can be readily obtained using a protocol analogous to the one illustrated above in Scheme 4A.
Scheme 6
[0084] The following inventive compounds having a pyrazolo core were synthesized using the methodologies described above. Analytical data characterizing the synthesized compounds is shown below.
[0085] Ethyl 6-chloro-3-(l-(4-chlorobenzyl)-4-phenyl-lH-pyrazol-5-yl)-lH-indole-2- carboxylate (YH215)
-24-
[0086] HPLC/MS: tR = 19.47 min; m/z = 490.1 [M+H] ; HRMS: C27H22CI2N3O2, 490.1089 (calcd.), 490.1062 (found).
[0087] 1H NMR (600 MHz, CDC13): 9.59 (s, 1H), 7.94 (s, 1H), 7.49 (1H, s), 7.10-7.19 (m, 8H), 6.85 (d, 2H, J = 7.8 Hz), 5.16 (1H, ABd, J = 15.6 Hz), 5.00 (1H, ABd, J = 15.0 Hz), 3.97-4.11 (m, 2H), 1.05 (t, 3H, J = 7.2 Hz).
[0088] 13C NMR (150 MHz, CDC13): 160.9, 138.0, 136.0, 135.2, 133.5, 132.9, 132.3, 131.4, 128.9, 128.5, 128.4, 126.6, 126.4, 126.2, 123.4, 123.0, 122.2, 112.1, 110.6, 61.4, 53.2,
13.8.
[0089] 6-chloro-3-(l-(4-chlorobenzyl)-4-phenyl-lH-pyrazol-5-yl)-lH-indole-2- carboxylic acid (YH245)
[0090] HPLC/MS: tR = 18.60 min; m/z = 462.1 [M+H] ; HRMS: C25Hi7Cl2N302,
461.069782 (calcd.), 461.069688 (found).
[0091] 1H NMR (600 MHz, Acetone): 12.09 (s, 1H), 7.92 (s, 1H), 7.10-7.23 (m, 5H), 6.62- 6.88 (m 8H), 5.17-5.28 (m, 2H).
[0092] 13C NMR (150 MHz, Acetone): 171.2, 137.4, 137.0, 134.0, 131.9, 129.4, 129.1, 128.0, 127.7, 126.2, 125.9, 125.2, 121.1, 121.0, 120.1, 112.2, 52.3.
-25-
WASH 7720093.1
[0093] (6-chloro-3-(l-(4-chlorobenzyl)-4-phenyl-lH-pyrazol-5-yl)-lH-indol-2-yl)((S)-3- ((3-(dimethylamino)propyl)(methyl)amino)pyrrolidin-l-yl)methanone (YH249)
[0094] HPLC/MS: tR = 10.73 min; m/z = 629.0 [M+H]+.
[0095] 1H NMR (600 MHz, MeOD): 1.28 (m, 1H), 1.76-1.86 (m, 2H), 2.55 (m, 2H), 2.76- 78 (m, 6H), 2.84-2.97 (m, 2H), 3.10-3.22 (m, 2H), 3.29 (m, 4H), 3.51 (m, 1H), 3.84 (m, 1H), 5.16 (m, 2H), 6.94 (m, 3H), 7.08-7.21 (m, 6H), 7.51 (m, 1H), 7.95 (m, 1H), 8.14 (s, 1H), 8.74
(br.s, 1H).
[0096] C NMR (150 MHz, MeOD): 20.1, 33.8, 40.7, 41.6, 42.1, 51.2, 52.1, 53.4, 62.8, 110.3, 119.1, 120.0, 124.1, 124.8, 126.6, 126.9, 127.0, 127.4, 128.3, 129.6, 131.0, 131.6, 133.9, 134.7, 136.1.
[0097] Methyl 7-(6-chloro-3-(l-(4-chlorobenzyl)-4-phenyl-lH-pyrazol-5-yl)-lH-indole- 2-carboxamido)heptanoate (YH250)
[0098] HRMS : C33H32N403Cl2Na, 625.1749 (calcd.), 625.1729 (found).
[0099] 1H NMR (600 MHz, CDC13): 0.98 (m, 2H), 1.11-1.21 (m, 4H), 1.52-1.55 (m, 2H), 2.27-2.29 (m, 2H), 2.93-3.15 (2H, m), 3.69 (s, 3H), 4.94 (1H, ABd, J = 15.0 Hz), 5.14 (1H, ABd, J = 15.0 Hz), 5.72 (1H, m), 6.87 (m, 2H), 7.13-7.20 (8H, m), 7.59 (s, 1H), 8.03 (s, 1H), 10.88 (br.s, 1H).
-26-
WASH 7720093.1
[0100] 1JC NMR (150 MHz, CDC13): 8.6, 24.7, 26.3, 28.7, 28.8, 33.9, 39.4, 45.8, 51.5, 53.4, 104.1, 112.7, 121.3, 122.8, 123.6, 126.1, 126.5, 127.1, 128.7, 128.9, 129.3, 129.9, 130.5, 131.3, 131.4, 134.0, 134.5, 135.9, 138.6, 160.3, 174.1.
[0101] 7-(6-chloro-3-(l-(4-chlorobenzyl)-4-phenyl-lH-pyrazol-5-yl)-lH-indole-2- carboxamido)heptanoic acid (YH254)
[0102] HPLC/MS: tR = 12.35 min; m/z = 589.2 [M+H] ; HRMS: C32H3oN403Cl2Na, 611.1593 (calcd.), 611.1566 (found).
[0103] 1H NMR (600 MHz, CDC13): 0.85-1.10 (m, 8H), 1.27-1.41 (m, 4H), 2.14 (m, 2H), 2.78-3.03 (m, 2H), 4.88 (1H, ABd, J = 14.4 Hz), 5.02 (1H, ABd, J = 13.8 Hz), 5.68 (s, 1H), 6.78-6.79 (m, 2H), 7.05-7.13 (m, 8H), 7.54 (s, 1H), 7.99 (s, 1H), 11.71 (br.s, 1H).
[0104] 13C NMR (150 MHz, CDCl3): 25.1, 27.8, 28.5, 29.7, 38.1, 53.1, 104.0, 112.7, 121.2, 122.6, 123.4, 126.1, 126.4, 127.0, 128.6, 128.9, 129.2, 129.8, 130.7, 131.1, 131.4, 133.9, 134.5, 136.1, 138.6, 160.6.
[0105] 6-chloro-3-(l-(4-chlorobenzyl)-4-phenyl-lH-pyrazol-5-yl)-N-(7- (hydroxyamino)-7-oxoheptyl)-lH-indole-2-carboxamide (YH256)
[0106] HRMS: C32H3iN503Cl2Na, 626.1702 (calcd.), 626.1694 (found).
[0107] 1H NMR (600 MHz, CDC13): 0.88-1.51 (m, 8H), 2.04-2.27 (m, 2H), 3.01-3.05 (m, 2H), 4.94 (1H, ABd, J = 16.2 Hz), 5.08 (1H, ABd, J = 15.0 Hz), 5.73 (s, 1H), 6.81-6.84 (m, 1H), 7.14 (m, 8H), 7.72 (m, 3H), 8.01 (s, 1H).
-27-
WASH 7720093.1
[0108] C NMR (150 MHz, CDC13): 14.1, 22.7, 28.5, 29.4, 29.7, 31.9, 53.3, 103.9, 112.6,
121.3, 122.8, 123.7, 126.2, 126.4, 127.2, 128.7, 129.0, 129.3, 130.0, 130.8, 131.4, 134.0,
134.4, 138.6.
[0109] Ethyl 3-(l-benzyl-4-phenyl-lH-pyrazol-5-yl)-6-chloro-lH-indole-2-carboxylate
(YH214)
[0110] HPLC/MS: tR = 12.65 min; m/z = 456.3 [M+H] ; HRMS: C27H23C1N302, 456.1479 (calcd.), 456.1486 (found).
[0111] 1H NMR (600 MHz, CDC13): 9.35 (s, 1H), 7.93 (s, 1H), 7.49 (1H, s), 7.08-7.21 (m, 9H), 6.91 (m, 2H), 5.21 (1H, ABd, J = 15.0 Hz), 5.02 (1H, ABd, J = 15.6 Hz), 3.90-4.07 (m, 2H), 1.02 (t, 3H, J = 7.2 Hz).
[0112] 13C NMR (150 MHz, CDC13): 13.7, 54.0, 61.3, 110.8, 112.0, 122.3, 123.0, 123.2, 126.1, 126.3, 126.5, 126.7, 127.5, 127.6, 128.3, 128.4, 131.4, 132.2, 133.1, 135.9, 136.7, 137.8, 161.0.
[0113] 3-(l-benzyl-4-phenyl-lH-pyrazol-5-yl)-6-chloro-lH-indole-2-carboxylic acid
(YH262)
[0114] HPLC/MS: tR = 11.78 min; m/z = 428.1 [M+H]+
[0115] 1H NMR (600 MHz, MeOD): 7.89 (s, 1H), 7.42 (1H, s), 7.26-7.27 (m, 2H), 7.01-7.09 (m, 6H), 6.88 (m, 2H), 6.71 (m, 2H), 5.39 (1H, ABd, J = 15.6 Hz), 5.21 (1H, ABd, J = 15.6 Hz).
[0116] 13C NMR (150 MHz, MeOD): 53.2, 105.7, 111.2, 120.0, 120.9, 121.9, 125.3, 126.2, 126.3, 126.7, 127.0, 127.8, 128.7, 133.4, 134.6, 135.4, 136.6, 137.3, 166.8.
-28-
WASH 7720093.1
[0117] Ethyl 6-chloro-3-(l-(4-chlorobenzyl)-4-(4-fluorophenyl)-lH-pyrazol-5-yl)-lH- indole-2-carboxylate (YH263)
[0118] HPLC/MS: tR = 12.59 min; m/z = 508.2 [M+H] ; HRMS: C27H20N3O2FCI2, 507.091661 (calcd.), 507.092044 (found).
[0119] 1H NMR (600 MHz, CDC13): 9.35 (s, 1H), 7.89 (s, 1H), 7.52 (s, 1H), 7.09-7.18 (m, 6H), 6.83-6.86 (m, 4H), 5.16 (1H, ABd, J = 15.0 Hz), 5.00 (1H, ABd, J = 15.0 Hz), 4.01-4.16 (m, 2H), 1.08 (t, 3H, J = 7.2 Hz).
[0120] 13C NMR (150 MHz, CDCI3): 13.9, 53.3, 61.4, 110.4, 112.1, 115.3, 122.1, 122.5, 123.2, 126.3, 126.6, 127.88, 127.93, 128.4, 128.9, 131.3, 132.5, 133.5, 135.2, 135.9, 137.8,
160.6.
[0121] 6-chloro-3-(l-(4-chlorobenzyl)-4-(4-fluorophenyl)-lH-pyrazol-5-yl)-lH-indole- 2-carboxylic acid (YH264)
[0122] HPLC/MS: tR = 12.21 min; m/z = 480.1 [M+H]+.
[0123] 1H NMR (600 MHz, MeOD): 8.57 (s, 1H), 7.88 (s, 1H), 7.43 (s, 1H), 7.27 (m, 2H), 7.04 (m, 2H), 6.69-6.87 (m, 6H), 5.32 (1H, ABd, J = 15.6 Hz), 5.19 (1H, ABd, J = 15.6 Hz).
[0124] 13C NMR (150 MHz, MeOD): 52.6, 105.2, 111.4, 114.3, 114.5, 120.2, 120.6, 121.2, 126.2, 127.8, 127.9, 128.0, 128.7, 128.9, 132.6, 134.6, 135.4, 136.0, 136.7, 160.4, 166.6,
169.0.
-29-
WASH 7720093.1
[0125] (6-chloro-3-(l-(4-chlorobenzyl)-4-(4-fluorophenyl)-lH-pyrazol-5-yl)-lH-indol-
2-yl)((S)-3-((3-(dimethylamino)propyl)(methyl)amino)pyrrolidin-l-yl)methanone
(YH265)
[0126] HPLC/MS: tR = 10.23 min; m/z = 647.4 [M+H] ; HRMS: CssHsgNeOCbF, 647.2468 (calcd.), 647.2477 (found).
[0127] 1H NMR (600 MHz, CDC13): 1.28-1.62 (m, 12H), 1.85-2.07 (m, 6H), 2.14-2.23 (m, 24H), 2.26-2.74 (m, 12H), 2.93-3.75 (m, 12H), 5.07-5.15 (m, 4H), 5.24-5.33 (m, 2H), 6.88- 6.98 (m, 16H), 7.00-7.21 (m, 12H), 7.34 (m, 2H), 7.47-7.50 (m, 3H), 7.85-8.04 (m, 1H), 10.64 (br.s, 1H), 10.79 (br.s, 1H).
[0128] 13C NMR (150 MHz, CDC13): 24.6, 25.0, 28.2, 28.5, 29.7, 30.8, 39.2, 39.3, 40.0, 45.4, 45.5, 45.8, 51.3, 52.7, 53.5, 57.3, 57.7, 62.6, 63.8, 105.8, 112.0, 115.6, 115.8, 115.9, 119.7, 121.5, 121.8, 122.3, 125.1, 127.2, 127.7, 128.5, 128.6, 128.7, 129.5, 130.7, 131.1, 132.2, 132.4, 133.5, 134.7, 136.2, 138.2, 138.4, 162.0.
[0129] Methyl 7-(6-chloro-3-(l-(4-chlorobenzyl)-4-(4-fluorophenyl)-lH-pyrazol-5-yl)- lH-indole-2-carboxamido)heptanoate (YH266)
643.1655 (calcd.), 643.1614 (found).
[0131] 1H NMR (600 MHz, CDCI3): 0.99-1.02 (m, 2H), 1.13-1.24 (m, 4H), 1.54-1.57 (m, 2H), 2.29-2.31 (m, 2H), 2.95-3.17 (2H, m), 3.71 (s, 3H), 4.95 (1H, ABd, J = 15.0 Hz), 5.16
-30-
WASH 7720093.1
(1H, ABd, J = 15.0 Hz), 5.63 (1H, m), 6.87-6.90 (m, 4H), 7.14-7.19 (m, 6H), 7.61 (s, 1H), 8.00 (s, 1H), 10.65 (br.s, 1H).
[0132] 13C NMR (150 MHz, CDC13): 24.7, 26.3, 28.7, 28.8, 33.9, 38.6, 39.4, 51.6, 53.5, 103.8, 112.6, 115.9, 116.0, 121.2, 122.7, 122.9, 126.5, 127.7, 127.8, 128.7, 129.3, 129.9, 130.3, 131.5, 134.1, 134.4, 135.8, 138.4, 160.1, 174.1.
[0133] 7-(6-chloro-3-(l-(4-chlorobenzyl)-4-(4-fluorophenyl)-lH-pyrazol-5-yl)-lH- indole-2-carboxamido)heptanoic acid (YH267)
[0134] HPLC/MS: iR = 12.29 min; m/z = 607.1 [M+H] ; HRMS: C32H29N403Cl2FNa, 629.1498 (calcd.), 629.1552 (found).
[0135] 1H NMR (600 MHz, CDC13): 0.92-1.28 (m, 8H), 1.57-1.60 (m, 2H), 2.35 (m, 2H), 2.92-3.14 (m, 2H), 4.94 (1H, ABd, J = 15.0 Hz), 5.15 (1H, ABd, J = 15.0 Hz), 5.66 (s, 1H), 6.86-6.89 (m, 4H), 7.14-7.33 (m, 6H), 7.62 (s, 1H), 8.01 (s, 1H), 10.95 (br.s, 1H).
[0136] 13C NMR (150 MHz, CDC13): 24.6, 26.1, 28.5, 28.7, 33.8, 38.6, 39.3, 53.5, 103.9, 112.8, 115.9, 116.0, 121.1, 122.8, 122.9, 126.4, 127.7, 127.9, 128.7, 129.3, 129.7, 131.5, 134.1, 134.4, 135.9, 138.4, 160.3, 177.9.
[0137] 6-chloro-3-(l-(4-chlorobenzyl)-4-(4-fluorophenyl)-lH-pyrazol-5-yl)-N-(7- (hydroxyamino)-7-oxoheptyl)-lH-indole-2-carboxamide (YH268)
[0138] HRMS: C32H30N5O3Cl2FNa, 644.1607 (calcd.), 644.1568 (found).
-31-
WASH 7720093.1
[0139] 1H NMR (600 MHz, CDC13): 0.89-1.46 (m, 8H), 2.00 (m, 2H), 2.83-2.86 (m, 2H), 3.05-3.10 (m, 2H), 4.92-5.03 (m, 2H), 5.69 (s, 1H), 6.80-6.88 (m, 4H), 7.08-7.16 (m, 6H), 7.58 (s, 1H), 7.96 (s, 1H).
[0140] 13C NMR (150 MHz, CDC13): 14.1, 22.7, 28.5, 29.4, 29.7, 31.9, 33.9, 36.5, 53.2, 56.0, 103.9, 115.8, 115.9, 121.1, 122.6, 123.0, 126.3, 127.8, 128.6, 128.7, 129.3, 130.7, 133.9, 134.5, 138.5, 160.6, 160.9.
[0141] Methyl 8-(6-chloro-3-(l-(4-chlorobenzyl)-4-(4-fluorophenyl)-lH-pyrazol-5-yl)- lH-indole-2-carboxamido)octanoate (YH271)
[0142] HPLC/MS: iR = 13.18 min; m/z = 635.3 [M+H] ; HRMS: C34H33Cl2FN403Na, 657.1811 (calcd.), 657.1833 (found).
[0143] 1H NMR (600 MHz, CDC13): 0.99-1.01 (m, 2H), 1.13-1.27 (m, 6H), 1.59-1.63 (m, 2H), 2.31-2.33 (m, 2H), 2.98-3.17 (m, 2H), 3.70 (s, 3H), 4.94 (1H, ABd, J = 15.0 Hz), 5.15 (1H, ABd, J = 14.4 Hz), 5.67 (m, 1H), 6.86-6.88 (m, 4H), 7.14-7.19 (m, 6H), 7.61 (s, 1H), 8.00 (s, 1H).
[0144] 13C NMR (150 MHz, CDC13): 24.8, 26.4, 28.8, 28.9, 34.0, 38.6, 39.5, 51.5, 53.4,
103.8, 112.8, 115.8, 116.0, 121.1, 122.7, 122.8, 126.4, 127.6, 127.7, 127.8, 128.7, 129.3,
129.9, 130.4, 131.4, 134.1, 134.5, 136.0, 138.4, 160.3, 161.0, 162.6, 174.2.
[0145] 8-(6-chloro-3-(l-(4-chlorobenzyl)-4-(4-fluorophenyl)-lH-pyrazol-5-yl)-lH- indole-2-carboxamido)octanoic acid (YH272)
-32-
WASH 7720093.1
[0146] HPLC/MS: tR = 12.35 min; m/z = 621.2 [M+H] ; HRMS: C33H3iCl2FN403Na, 643.1655 (calcd.), 643.1678 (found).
[0147] 1H NMR (600 MHz, CDC13): 0.92-0.98 (m, 2H), 1.08-1.28 (m, 6H), 1.60-1.64 (m, 2H), 1.87-1.89 (m, 2H), 2.36-2.39 (m, 2H), 2.88-3.12 (m, 2H), 3.78 (m, 1H), 4.92 (1H, ABd, J = 15.0 Hz), 5.14 (1H, ABd, J = 15.0 Hz), 5.67 (m, 1H), 6.84-6.88 (m, 4H), 7.12-7.17 (m, 6H), 7.61 (s, 1H), 8.00 (s, 1H), 11.19 (br.s, 1H).
[0148] 13C NMR (150 MHz, CDC13): 24.8, 25.6, 26.0, 28.4, 28.6, 38.6, 39.3, 53.4, 68.0, 103.9, 112.8, 115.9, 116.0, 121.1, 122.8, 122.9, 126.3, 127.4, 127.7, 127.8, 128.7, 129.3, 129.7, 130.5, 131.5, 134.1, 134.4, 136.0, 138.4, 160.4, 161.0, 162.7.
[0149] 6-chloro-3-(l-(4-chlorobenzyl)-4-(4-fluorophenyl)-lH-pyrazol-5-yl)-N-(8- (hydroxyamino)-8-oxooctyl)-lH-indole-2-carboxamide (YH273)
[0150] HPLC/MS: tR = 12.31 min; m/z = 621.3 [M+H] ; HRMS: C33H32Cl2FN503Na, 658.1764 (calcd.), 658.1772 (found).
[0151] 1H NMR (600 MHz, CDC13): 0.81-1.44 (m, 8H), 2.02-2.14 (m, 2H), 2.83-2.86 (m, 2H), 2.80-3.03 (m, 2H), 4.89-5.07 (m, 2H), 5.94 (s, 1H), 6.74-6.79 (m, 3H), 7.03-7.18 (m, 8H), 7.53 (s, 1H), 7.94 (s, 1H).
[0152] 13C NMR (150 MHz, CDC13): 25.9, 26.0, 28.5, 28.8, 29.1, 29.7, 39.1, 39.2, 53.2, 104.2, 112.7, 115.8, 121.1, 122.4, 126.3, 127.8, 128.59, 128.61, 129.18, 129.23, 130.1, 131.1, 133.8, 134.6, 136.3, 138.3, 160.5, 160.8.
[0153] Methyl 6-(6-chloro-3-(l-(4-chlorobenzyl)-4-(4-fluorophenyl)-lH-pyrazol-5-yl)- lH-indole-2-carboxamido)hexanoate (YH275)
-33-
[0154] HPLC/MS: tR = 12.73 min; m/z = 607.2 [M+H] ; HRMS: C32H29Cl2FN403Na, 629.1498 (calcd.), 629.1500 (found).
[0155] 1H NMR (600 MHz, CDC13): 1.01-1.05 (m, 2H), 1.14-1.20 (m, 2H), 1.51-1.55 (m, 2H), 2.24-2.26 (m, 2H), 2.96-3.18 (m, 2H), 3.71 (s, 3H), 4.94 (1H, ABd, J = 15.0 Hz), 5.16 (1H, ABd, J = 15.0 Hz), 5.67 (m, 1H), 6.87-6.90 (m, 4H), 7.14-7.19 (m, 6H), 7.61 (s, 1H), 8.01 (s, 1H), 10.87 (br.s, 1H).
[0156] 13C NMR (150 MHz, CDC13): 24.5, 26.1, 28.7, 33.8, 39.3, 51.7, 53.5, 103.8, 112.7, 115.9, 116.1, 121.2, 122.7, 122.9, 126.4, 127.49, 127.51, 127.7, 127.8, 128.7, 129.3, 129.8, 130.3, 131.5, 134.1, 134.4, 135.9, 138.4, 160.2, 161.0, 162.6, 173.9.
[0157] 6-(6-chloro-3-(l-(4-chlorobenzyl)-4-(4-fluorophenyl)-lH-pyrazol-5-yl)-lH- indole-2-carboxamido)hexanoic acid (YH276)
[0158] HPLC/MS: tR (run time) = 11.98 min; m/z = 593.3 [M+H]+.
[0159] 1H NMR (600 MHz, CDC13): 1.01-1.05 (m, 2H), 1.13-1.19 (m, 2H), 1.55-1.59 (m, 2H), 2.31 (m, 2H), 2.96-3.14 (m, 2H), 4.95 (1H, ABd, J = 15.0 Hz), 5.16 (1H, ABd, J = 15.0 Hz), 5.67 (m, 1H), 6.84-6.89 (m, 4H), 7.13-7.18 (m, 6H), 7.62 (s, 1H), 8.02 (s, 1H), 11.09 (s, 1H).
[0160] 13C NMR (150 MHz, CDC13): 24.4, 25.9, 28.7, 29.7, 39.1, 53.5, 68.0, 103.9, 112.8, 115.9, 116.1, 121.1, 123.0, 126.3, 127.8, 128.7, 129.3, 129.6, 131.5, 134.1, 134.3, 136.0, 138.5, 160.4, 161.1, 162.7.
-34-
WASH 7720093.1
[0161] 6-chloro-3-(l-(4-chlorobenzyl)-4-(4-fluorophenyl)-lH-pyrazol-5-yl)-N-(6- (hydroxyamino)-6-oxohexyl)-lH-indole-2-carboxamide (YH277)
[0162] HPLC/MS: tR = 11.98 min; m/z = 608.2 [M+H]+.
[0163] 1H NMR (600 MHz, CDC13): 0.99-1.02 (m, 4H), 1.20-1.25 (m, 2H), 2.00-2.07 (m, 2H), 2.70-3.02 (m, 2H), 4.88 (1H, ABd, J = 13.8 Hz), 4.97 (1H, ABd, J = 13.8 Hz), 5.70 (m, 1H), 6.68-6.76 (m, 4H), 6.99-7.06 (m, 6H), 7.50 (s, 1H), 7.93 (s, 1H).
[0164] 13C NMR (150 MHz, CDC13): 19.2, 22.7, 25.5, 28.8, 29.7, 53.1, 103.8, 112.7, 115.6, 115.8, 121.1, 122.4, 122.6, 126.3, 127.8, 128.6, 129.2, 129.8, 131.2, 133.8, 134.5, 136.3, 138.5, 160.8.
[0165] 6-chloro-3-(l-(4-chlorobenzyl)-4-(4-fluorophenyl)-lH-pyrazol-5-yl)-N- (methylsulfonyl)-lH-indole-2-carboxamide (YH392)
[0166] The mixture of 6-chloro-3-(l-(4-chlorobenzyl)-4-(4-fiuorophenyl)-lH-pyrazol-5- yl)-lH-indole-2-carboxylic acid (YH264, 0.05 mmol, 24 mg) and CDI (0.1 mmol, 16 mg) in 1 mL of dry THF was stirring under 50 °C for 2 hours. The mixture was cooled to ambient temperature and the sulphonamide (0.1 mmol, 9.5 mg) was added. The reaction mixture was stirred for 10 min and diazabicyclo[5.4.0]undec-7-en (DBU) (0.1 mmol, 15 μί) was added. After stirring at room temperature overnight, the residue was purified by silica gel chromatography (EtOAc/methanol, 5/1) to give the product as yellow solids (12 mg, 43% yield).
-35-
WASH 7720093.1
[0167] Schematically the reaction is depicted as follows:
[0168] HPLC/MS: iR = 14.03 min; m/z = 556.6 [M+H]+.
[0169] Carboxamides of the following pyrazole derivatives were synthesized
analogous synthetic route
[0170] 6-chloro-3-(l-(4-chlorobenzyl)-4-phenyl-lH-pyrazol-5-yl)-N- ((trifluoromethyl)sulfonyl)-lH-indole-2-carboxamide:
[0171] 6-chloro-3-(l-(4-chlorobenzyl)-4-phenyl-lH-pyrazol-5-yl)-N-((2- hydroxyethyl)sulfonyl)-lH-indole-2-carboxamide:
[0172] 6-chloro-3-(l-(3,4-difluorobenzyl)-4-phenyl-lH-pyrazol-5-yl)-N-((2- hydroxyethyl)sulfonyl)-lH-indole-2-carboxamide:
[0173] 6-chloro-N-((2-hydroxyethyl)sulfonyl)-3-(4-phenyl-l-(3,4,5-trifluorobenzyl)-lH- pyrazol-5-yl)-lH-indole-2-carboxamide:
-36-
WASH 7720093.1
[0174] 6-chloro-N-((3-hydroxypropyl)sulfonyl)-3-(4-phenyl-l-(3,4,5-trifluorobenzyl)- lH-pyrazol-5-yl)-lH-indole-2-carboxamide:
[0175] 6-chloro-3-(l-(4-chloro-3,5-difluorobenzyl)-4-phenyl-lH-pyrazol-5-yl)-N-((3- hydroxypropyl)sulfonyl)-lH-indole-2-carboxamide:
[0176] 6-chloro-3-(l-(4-chloro-3,5-difluorobenzyl)-4-phenyl-lH-pyrazol-5-yl)-N-((2- hydroxyethyl)sulfonyl)-lH-indole-2-carboxamide:
[0177] 6-chloro-3-(l-(4-chloro-3-fluorobenzyl)-4-phenyl-lH-pyrazol-5-yl)-N-((2- hydroxyethyl)sulfonyl)-lH-indole-2-carboxamide:
[0178] 6-chloro-3-(l-(4-chlorobenzyl)-4-phenyl-lH-pyrazol-5-yl)-N-((2- hydroxypropyl)sulfonyl)-lH-indole-2-carboxamide:
-37-
WASH 7720093.1
[0179] 6-chloro-3-(l-(4-chlorobenzyl)-4-phenyl-lH-pyrazol-5-yl)-N-sulfamoyl-lH- indole-2-carboxamide:
[0180] 6-chloro-3-(l-(4-chlorobenzyl)-4-phenyl-lH-pyrazol-5-yl)-N-(N- methylsulfamoyl)-lH-indole-2-carboxamide:
[0181] 6-chloro-3-(l-(4-chlorobenzyl)-4-phenyl-lH-pyrazol-5-yl)-N-(N,N- dimethylsulfamoyl)-lH-indole-2-carboxamide:
[0182] 6-chloro-N-(N,N-dimethylsulfamoyl)-3-(4-(4-fluorophenyl)-l-(3,4,5- trifluorobenzyl)-lH-pyrazol-5-yl)-lH-indole-2-carboxamide:
[0183] 6-chloro-3-(4-(4-fluorophenyl)-l-(3,4,5-trifluorobenzyl)-lH-pyrazol-5-yl)-N-(N- methylsulfamoyl)-lH-indole-2-carboxamide:
-38-
WASH 7720093.1
[0184] 6-chloro-3-(4-(4-fluorophenyl)-l-(3,4,5-trifluorobenzyl)-lH-pyrazol-5-yl)-N- sulfamoyl-lH-indole-2-carboxamide:
[0185] Ethyl 6-chloro-3-(3-(4-chlorophenyl)propanoyl)-lH-indole-2- carboxylate(WW482)
[0186] 1H-NMR (CDCls, 600 MHz): δ =1.41 (3H, t, J=7.14 Hz), 3.07 (2H, t, J=7.62 Hz), 3.43 (2H, t, J=7.62 Hz), 4.44 (2H, q, J=7.05 Hz), 7.18 (2H, d, J=8.31 Hz), 7.22 (IH, d, J=1.68 Hz), 7.24 (2H, d, J=8.34 Hz), 7.43 (IH, d, J=1.62 Hz), 7.88 (IH, d, J=8.69 Hz), 9.05 (IH, w).
[0187] 13C-NMR (CDC13, 150.92 MHz): δ =14.2, 29.9, 45.2, 62.2, 111.5, 121.6, 123.8, 123.8, 125.3, 126.4, 128.5, 129.8, 131.7, 132.4, 135.1, 139.7, 160.2.
[0188] Ethyl 6-chloro-3-(4-(4-chlorobenzyl)-l-(phenyl)-lH-pyrazol-5-yl)-lH-indole-2- carboxylate (WW509)
[0189] HRMS (ESI-TOF) m/z (calc): 489.1011, (found) [M+H] : 490.1134.
[0190] 1H-NMR (CDCI3, 600 MHz): 5 =1.11 (3H, t, J=7.14 Hz), 3.61 (IH, d, J=15.86 Hz), 3.71 (IH, d, J=15.90 Hz), 4.01 (IH, m), 4.10 (IH, m), 6.89 (2H, d, J=8.31 Hz), 7.10 (2H, d, J=8.34 Hz), 7.14 (2H, m), 7.18 (3H, m), 7.35 (IH, d, J=8.69 Hz), 7.45 (IH, m), 7.68 (IH, d, J=2.27 Hz), 9.10 (lH, w).
[0191] 13C-NMR (CDC13, 150.92 MHz): δ =14.0, 29.8, 61.3, 110.9, 112.0, 121.9, 122.1, 122.9, 123.2, 126.3, 126.5, 126.8, 128.1, 128.6, 129.6, 131.6, 132.2, 139.1, 140.2, 140.3.
-39-
WASH 7720093.1
[0192] 6-chloro-3-(4-(4-chlorobenzyl)-l-phenyl-lH-pyrazol-5-yl)-lH-indole-2- carboxylic acid (WW512)
[0193] HRMS (ESI-TOF) m/z (calc): 461.0698, (found) [M+H] : 462.0795; HPLC-MS r.t: 12.28, m/z [M+H]+: 462.3.
[0194] 1H-NMR (MeOD, 600 MHz): δ =3.70 (2H, dd, J=15.81, 31.36 Hz), 6.96 (2H, d, J=8.31 Hz), 7.07 (3H, m), 7.20 (5H, m), 7.47 (1H, t, J=1.8Hz), 7.66 (1H, d, J=2.44 Hz).
[0195] 13C-NMR (CDC13, 150.92 MHz): δ =29.3, 109.5, 111.9, 121.2, 121.5, 121.9, 124.0, 126.2, 127.1, 127.5, 127.7, 128.2, 129.6, 130.9, 131.2, 134.0, 136.3, 139.3, 139.7, 140.0, 161.7.
[0196] (6-chloro-3-(4-(4-chlorobenzyl)-l-phenyl-lH-pyrazol-5-yl)-lH-indol-2-yl)((S)-3- ((3-(dimethylamino)propyl)(methyl)amino)pyrrolidin-l-yl)methanone (WW536)
[0197] HRMS (ESI-TOF) m/z (calc): 628.2484, (found) [M+H] : 629.2556; HPLC-MS r_t: 15.63, m/z [M+H]+: 629.2.
[0198] 1H-NMR (CD3OD, 600 MHz): δ =0.80 (1H, m), 1.21 (1H, m), 1.60-1.70 (1H, m), 1.75-1.90 (2H, m), 2.00-2.10 (2H, m), 2.24-2.30 (3H, m), 2.31-2.5 (2H, m), 2.61-2.99 (10H, m), 3.09-3.20 (3H, m), 3.36-3.7 (3H, m), 3.87-3.90 (2H, m), 6.89-7.35 (10H, m), 7.35-7.52 (2H, m), 7.60-7.72 (1H, m), 7.94-8.06 (1H, m).
[0199] 13C-NMR (CD3OD, 150.92 MHz): δ =13.1, 19.5, 19.7, 20.1, 21.0, 21.1, 21.2, 27.2, 28.2, 28.9, 29.0, 29.1, 29.4, 30.3, 35.6, 37.5, 38.0, 38.3, 38.4, 42.2, 42.2, 50.3, 52.1, 52.3, 56.7, 56.8, 60.1, 62.3, 63.4, 63.6, 105.9, 106.0, 111.7, 111.8, 120.9, 121.0, 121.2, 121.5, 123.0, 124.1, 124.1, 125.1, 126.3, 127.5, 127.7, 128.0, 128.1, 128.4, 128.7, 128.8, 129.5,
-40-
WASH 7720093.1
129.6, 129.8, 129.8, 129.9, 130.0, 131.0, 131.4, 132.8, 133.6, 133.7, 136.1, 137.6, 139.2, 139.5, 140.5, 140.9, 144.0, 161.4, 163.3, 163.5, 171.6.
[0200] Methyl 7-(6-chloro-3-(4-(4-chlorobenzyl)-l-phenyl-lH-pyrazol-5-yl)-lH-indole- 2-carboxamido)heptanoate (WW535)
[0201] HRMS (ESI-TOF) m/z (calc): 602.1851, (found) [M+Na] : 625.1753.
[0202] 1H-NMR (CDC13, 600 MHz): δ =1.14 (0.6H, s), 1.32 (5.1H, d, J=39.67 Hz), 1.61 (3.3H, d, J=27.13 Hz), 2.30 (2.2H, d, J=7.32 Hz), 2.81 (1.2H, d, J=6.80 Hz), 2.89 (l .OH, d, J=6.72 Hz), 2.99 (3.0H, s), 3.12 (0.4H, s), 3.19 (0.7H, s), 3.67 (3.1H, s), 5.53 (0.7H, d, J=5.29 Hz), 5.76 (0.3H, s), 6.89 (1.8H, t, J=7.18 Hz), 7.13 (7.3H, d, J=45.70 Hz), 7.48 (0.6H, s), 7.81 (0.5H, s), 8.03 (0.2H, s), 10.63 (0.5H, s).
[0203] 7-(6-chloro-3-(4-(4-chlorobenzyl)-l-phenyl-lH-pyrazol-5-yl)-lH-indole-2- carboxamido)heptanoic acid (WW540)
[0204] HRMS (ESI-TOF) m/z (calc): 588.1695, (found) [M+Na] : 611.1650;
[0205] HPLC-MS run time (r.t): 18.14, m/z [M+H]+: 589.2.
[0206] 1H-NMR (CD3OD, 600 MHz): δ =0.90 (2H, m), 1.14 (6H, m), 1.67(2H, m), 2.04 (2H, m), 3.08 (1H, m), 3.21 (1H, m), 3.62 (1H, d, J=15Hz), 3.72 (1H, d, J=15Hz), 5.54 (1H, s), 6.90(2H, d, J=8.4 Hz), 7.11(2H, d, J=8.4 Hz), 7.14-7.19(4H, m), 7.51(1H, s), 7.84(1H, s), 10.53(1H, s).
[0207] 13C-NMR (CD3OD, 150.92 MHz): δ =24.3, 24.8, 29.1, 29.3, 29.4, 29.5, 29.6, 29.7, 29.8, 31.9, 33.7, 33.9, 39.4, 103.1, 104.6, 112.5, 114.5, 117.8, 121.2, 122.7, 123.0, 123.6,
-41-
WASH 7720093.1
126.6, 127.6, 128.6, 129.0, 129.6, 129.7, 131.2, 131.6, 132.1, 135.6, 138.5, 139.3, 141.0, 160.6, 178.0.
[0208] 6-chloro-3-(4-(4-chlorobenzyl)-l-phenyl-lH-pyrazol-5-yl)-N-(7- (hydroxyamino)-7-oxoheptyl)-lH-indole-2-carboxamide (WW538)
[0209] HRMS (ESI-TOF) m/z (calc): 603.1804, (found) [M+H]+:
[0210] 1H-NMR (CDCls, 600 MHz): δ =0.89 (IH, s), 0.99 (2H, s), 1.24 (5H, m), 1.55 (3H, s), 2.01 (2H, s), 2.98 (IH, s), 3.28 (IH, s), 3.60 (IH, d, J=15.30 Hz), 3.70 (IH, d, J=15.24 Hz), 5.55 (IH, s), 6.88 (2H, d, J=7.86 Hz), 7.08 (2H, d, J=8.40 Hz), 7.13 (2H, d, J=7.20 Hz), 7.18 (2H, s), 7.47 (IH, s), 7.83 (IH, s).
[0211] Ethyl 6-chloro-3-(4-(4-chlorobenzyl)-l-(4-fluorophenyl)-lH-pyrazol-5-yl)-lH- indole-2-carboxylate (WW515)
[0212] HRMS (ESI-TOF) m/z (calc): 507.0917, (found) [M+H] : 508.1045.
[0213] 1H-NMR (CDCI3, 600 MHz): 5=1.13 (3H, t, J=7.14 Hz), 3.60 (IH, d, J=15.86 Hz), 3.70 (IH, d, J=15.84 Hz), 4.05 (IH, m), 4.12 (IH, m), 6.87 (IH, t, J=8.49 Hz), 6.88 (2H, d, J=8.22 Hz), 7.10 (2H, d, J=8.69 Hz), 7.16 (3H, m), 7.32 (IH, d, J=8.69 Hz), 7.45 (IH, d, J=1.51 Hz), 7.67 (IH, s), 9.33 (IH, s).
[0214] 13C-NMR (CDC13, 150.92 MHz): δ =14.0, 29.8, 61.3, 110.7, 112.1, 115.4, 115.6, 121.9, 122.0, 123.0, 125.0, 125.0, 126.3, 126.4, 128.2, 129.6, 131.7, 132.3, 132.4, 135.8, 136.3, 139.0, 140.4, 160.4, 160.5, 162.0.
-42-
WASH 7720093.1
[0215] 6-chloro-3-(4-(4-chlorobenzyl)-l-(4-fluorophenyl)-lH-pyrazol-5-yl)-lH-indole- 2-carboxylic acid (WW519)
[0216] HRMS (ESI-TOF) m/z (calc): 479.0604, (found) [M+H] : 629.2556; HPLC-MS r.t: 12.23, m/z [M+H]+: 480.1.
[0217] 1H-NMR (MeOD, 600 MHz): δ =3.70 (2H, dd, J=15.78, 33.85 Hz), 6.95 (2H, d, J=8.22 Hz), 6.98 (1H, d, J=8.52 Hz), 7.06-7.08 (3H, m), 7.19-7.22 (3H, m), 7.48 (1H, d, J=1.13 Hz), 7.66 (1H, s).
[0218] 13C-NMR (MeOH, 150.92 MHz): δ =29.2, 111.9, 114.9, 115.0, 121.2, 121.6, 121.9, 126.1, 126.1, 126.2, 127.7, 129.6, 130.9, 131.2, 134.2, 136.3, 139.3, 139.8, 152.4, 152.5, 160.8, 161.7, 162.4.
[0219] (6-chloro-3-(4-(4-chlorobenzyl)-l-(4-fluorophenyl)-lH-pyrazol-5-yl)-lH-indol-
2-yl)((S)-3-((3-(dimethylamino)propyl)(methyl)amino)pyrrolidin-l-yl)methanone
(WW529)
[0220] HRMS (ESI-TOF) m/z (calc): 646.239, (found) [M+H] : 629.2556; HPLC-MS r_t: 9.95, m/z [M+H]+: 647.3.
[0221] Methyl 7-(6-chloro-3-(4-(4-chlorobenzyl)-l-(4-fluorophenyl)-lH-pyrazol-5-yl)- lH-indole-2-carboxamido)heptanoate (WW 543)
-43-
WASH 7720093.1
[0222] HRMS (ESI-TOF) m/z (calc): 620.1757, (found) [M+H] : 643.1620;
[0223] 1H-NMR (CDC13, 600 MHz): δ =1.17 (2H, m), 1.29 (4.0H, m), 1.61 (2H, m, J=7.65 Hz), 2.31 (2H, t, J=7.37 Hz), 3.15 (IH, td, J=6.48, 19.89 Hz), 3.25 (IH, td, J=6.77, 20.45 Hz), 3.62 (IH, d, J=15.49 Hz), 3.69 (3H, s), 3.71 (IH, d, J=15.42 Hz), 5.54 (IH, t, J=5.67 Hz), 6.86 (2H, t, J=8.69 Hz), 6.89 (2H, d, J=8.28 Hz), 7.11 (IH, d, J=8.31 Hz), 7.13(1H, s), 7.17 (2H, dd, J=4.41, 8.67 Hz), 7.51(1H, s), 7.81(1H, s).
[0224] 13C-NMR (CDCI3, 150.92 MHz): δ =14.2, 21.1, 24.7, 26.4, 28.7, 29.1, 29.8, 33.9, 39.6, 51.5, 60.4, 104.2, 112.5, 115.9, 116.0, 121.1, 122.7, 123.6, 124.7, 124.8, 126.6, 128.6, 129.6, 129.8, 131.2, 131.7, 132.2, 135.6, 135.6, 135.7, 138.4, 141.0, 160.4, 160.6, 162.3, 171.2, 174.0.
[0225] 7-(6-chloro-3-(4-(4-chlorobenzyl)-l-(4-fluorophenyl)-lH-pyrazol-5-yl)-lH- indole-2-carboxamido)heptanoic acid (WW544)
[0226] HRMS (ESI-TOF) m/z (calc): 606.1601, (found) [M+H] : 605.1530; HPLC-MS r.t: 12.35, m/z [M+H]+: 607.3.
[0227] 1H-NMR (CDC13, 600 MHz): δ =1.11 (2H, m), 1.27 (3H, m), 1.59 (2H, w), 2.37 (2H, w), 2.82 (IH, s), 3.10 (IH, w), 3.20 (IH, w), 3.60 (IH, d, J=15.18 Hz), 3.69 (IH, d, J=15.18 Hz), 5.55 (IH, s), 6.86 (4H, m), 7.12 (5H, m), 7.51 (IH, w), 7.81 (IH, s).
[0228] 13C-NMR (CDC13, 150.92 MHz): δ =26.0, 28.3, 28.9, 29.8, 38.6, 39.2, 104.3, 112.6, 115.9, 116.0, 121.0, 122.7, 123.6, 124.8, 124.8 126.4, 128.6, 129.6, 129.7, 131.3, 131.8, 132.1, 135.5, 135.9, 138.4, 141.0, 160.6, 162.3.
-44-
WASH 7720093.1
[0229] 6-chloro-3-(4-(4-chlorobenzyl)-l-(4-fluorophenyl)-lH-pyrazol-5-yl)-N-(7- (hydroxyamino)-7-oxoheptyl)-lH-indole-2-carboxamide (WW558)
[0230] HRMS (ESI-TOF) m/z (calc): 621.1710, (found) [M+H] : 622.1808; HPLC-MS r.t: 11.85, m/z [M+H]+: 622.3.
[0231] 1H-NMR (CDC13, 600 MHz): δ =1.02 (2H, m), 1.27 (5H, m), 1.58 (2H, m), 2.07 (2H, m), 3.01 (IH, m), 3.26 (IH, m), 3.60 (IH, d, J=15.18 Hz), 3.71 (IH, d, J=15.24 Hz), 5.53 (IH, s), 6.87 (3H, m), 7.13 (5H, s), 7.51 (IH, m), 7.82 (IH, s).
[0232] 13C-NMR (CDC13, 150.92 MHz): δ = 28.7, 29.8, 104.2, 111.0, 112.6, 116.0, 116.1, 118.3, 121.0, 122.2, 122.8, 123.6, 125.0, 126.4, 128.6, 129.6, 131.3, 132.1, 132.2, 135.3, 136.6, 138.3, 140.9, 152.1, 160.5.
[0233] Methyl 8-(6-chloro-3-(4-(4-chlorobenzyl)-l-phenyl-lH-pyrazol-5-yl)-lH-indole- 2-carboxamido)octanoate (WW582)
[0234] HRMS (ESI-TOF) m/z (calc): 616.2008.
[0235] 1H-NMR (CDC13, 600 MHz): δ = 1.14 (12H, m), 1.30 (6H, s), 1.47 (IH, m), 1.63 (IH, m), 1.73 (IH, m), 2.33 (2H, t, J=7.50 Hz), 3.14 (IH, m), 3.24 (IH, m), 3.63 (IH, d, J=15.11 Hz), 3.70 (3H, s), 3.71 (IH, m), 5.56 (IH, t, J=5.48 Hz), 6.91 (IH, d, J=8.31 Hz), 7.11 (IH, d, J=8.58 Hz), 7.14 (2H, d, J=7.98 Hz), 7.18 (4H, d, J=15.90 Hz), 7.50 (IH, s), 7.83 (IH, s).
[0236] 13C-NMR (CDC13, 150.92 MHz): δ =24.8, 24.9, 26.5, 27.0, 28.9, 29.0, 29.1, 29.2, 29.9, 34.0, 34.1, 39.6, 40.3, 51.5, 104.5, 112.5, 121.2, 122.6, 123.0, 123.6, 126.6, 127.6,
-45-
WASH 7720093.1
128.6, 128.7, 129.0, 129.6, 129.8, 129.9, 131.1, 131.6, 132.1, 135.7, 138.5, 139.4, 141.0, 160.5, 174.2.
[0237] 8-(6-chloro-3-(4-(4-chlorobenzyl)-l-phenyl-lH-pyrazol-5-yl)-lH-indole-2- carboxamido)octanoic acid (WW587)
[0238] HRMS (ESI-TOF) m/z (calc): 602.1851, (found) [M+K] : 641.1529.
[0239] 1H-NMR (CDCls, 600 MHz): 5 = 1.11 (2H, m), 1.28 (9H, m), 1.65 (IH, m), 2.37 (2H, t, J=7.37 Hz), 3.09 (IH, td, J=6.55, 20.13 Hz), 3.20 (IH, td, J=6.58, 20.07 Hz), 3.62 (IH, d, J=15.49 Hz), 3.72 (IH, d, J=15.36 Hz), 5.54 (IH, t, J=5.67 Hz), 6.89 (2H, d, J=8.31 Hz), 7.12 (IH, d, J=8.31 Hz), 7.14 (2H, d, J=8.28 Hz), 7.18 (5H, m), 7.51 (IH, s), 7.84 (IH, s).
[0240] ^C-NMR (CDCI3, 150.92 MHz): δ =24.6, 26.3, 28.7, 28.8, 29.0, 29.8, 33.8, 39.5, 104.6, 112.5, 121.2, 122.7, 123.0, 123.6, 126.6, 127.6, 128.6, 129.0, 129.6, 129.7, 131.2, 131.6, 132.1, 135.6, 138.5, 139.3, 141.0, 160.5, 177.9.
[0241] 6-chloro-3-(4-(4-chlorobenzyl)-l-phenyl-lH-pyrazol-5-yl)-N-(8- (hydroxyamino)-8-oxooctyl)-lH-indole-2-carboxamide (WW588)
[0242] HRMS (ESI-TOF) m/z (calc): 617.1960.
[0243] 1H-NMR (CDCI3, 600 MHz): δ = 0.96 (IH, m), 1.00(2H, m), 1.23 (7H, m), 1.53 (7H, m), 2.08 (2H, m), 2.21 (2H, m), 3.04 (IH, s), 3.18 (IH, s), 3.36 (IH, m), 3.59 (IH, d, J=18.60 Hz), 3.68 (IH, d, J=16.86 Hz), 5.59 (IH, s), 6.87 (IH, m), 7.15 (6H, m), 7.46 (IH, s), 7.77- 7.82 (IH, m).
-46-
WASH 7720093.1
[0244] Methyl 6-(6-chloro-3-(4-(4-chlorobenzyl)-l-phenyl-lH-pyrazol-5-yl)-lH-indole- 2-carboxamido)hexanoate (WW582)
[0245] HPLC-MS r_t: 12.73, m/z [M+H]+: 589.2.
[0246] 1H-NMR (CDCls, 600 MHz): 5 = 1.17 (2H, m), 1.32 (2H, m), 1.61 (2H, m), 1.68 (2H, s), 2.30 (2H, t, J=7.49 Hz), 3.12 (IH, td, J=6.57, 20.10 Hz), 3.24 (IH, td, J=6.66, 20.29 Hz), 3.63 (IH, d, J=15.42 Hz), 3.70 (3H, s), 3.72 (IH, d, J=15.42 Hz), 5.53 (IH, t, J=5.67 Hz), 6.90 (2H, d, J=8.31 Hz), 7.11-7.13 (IH, m), 7.13 (2H, d, J=1.74 Hz), 7.15 (5H, m), 7.49 (IH, d, J=1.51 Hz), 7.82 (IH, s), 10.44 (IH, s).
[0247] 13C-NMR (CDCI3, 150.92 MHz): δ =24.5, 26.2, 29.0, 29.9, 33.8, 39.4, 51.6, 104.6, 112.4, 121.3, 122.6, 122.9, 123.6, 126.7, 127.6, 128.6, 129.0, 129.6, 129.8, 131.2, 131.5, 132.1, 135.6, 138.5, 139.4, 141.0, 160.4, 173.9.
[0248] 6-(6-chloro-3-(4-(4-chlorobenzyl)-l-phenyl-lH-pyrazol-5-yl)-lH-indole-2- carboxamido)hexanoic acid (WW585)
[0249] HPLC-MS r.t: 12.39, m/z [M+K] : 603.2.
[0250] 6-chloro-3-(4-(4-chlorobenzyl)-l-phenyl-lH-pyrazol-5-yl)-N-(6- (hydroxyamino)-6-oxohexyl)-lH-indole-2-carboxamide (WW590)
[0251] HPLC-MS r.t: 11.86, m z [M+H] : 590.3.
-47-
WASH 7720093.1
[0252] 1H-NMR (CDCI3, 600 MHz): δ =0.89-0.97 (2H, d, m), 1.28 (3H, s), 1.73 (7H, m), 3.13 (1H, m), 3.36(1H, m), 3.65 (1H, m), 5.57 (1H, s), 6.87 (1H, s), 7.15 (3H, m), 7.46 (1H, s), 7.88 (1H, s).
5. The Isoxazole and Isothiazole Scaffolds
[0253] In another aspect of this invention, are provided methods for generating inventive compounds that have an isoxazole core (Formula 4), or a isothiazole core (Formula 5).
[0254] Synthesis of compounds that comport to Formula 4 will be achieved as illustrated in Scheme 7.
Scheme 7
[0255] Thus, reaction of the indole with aldehyde followed by Dieckmann condensation of (a) with a benzyl ester results in the α,β-diketone (b), which is allowed to react with a hydroxylamine to give the corresponding isoxazole. The isothiazoles are obtained in a similar manner by reacting (b) with a thiohydroxyl amine as shown in Scheme 8.
-48-
WASH 7720093.1
Scheme 8
BIOLOGICAL ACTIVITY OF INVENTIVE COMPOUNDS
a. Protein Inhibition Studies
[0256] Inventive compounds are potent antagonists of the p53/MDM2 and p53/MDM4 complex based on fluorescent polarization analysis, AIDA NMR and HSQCNMR analysis. Figure 5 compares the binding curve for an inventive compound that belongs to the imidazole-indole class (WK23), to the binding curves of known antagonists, namely, WW298, Nutlin-3 and a spiro-oxindole compound (MI-63). The structures for compounds used in this study are as follows:
-49-
WASH 7720093.1
[0257] As illustrated in this figure, the inventive compound WK23 had weaker binding affinity to MDM2 when compared to the affinity of other known antagonists, and yet WK23 showed greater affinity for MDM4 than Nutlin-3 and had a similar affinity for MDM4 as WW298. Table 1 provides protein inhibition data for additional compounds that belong to the imidazole -indole class, while Table 2 presents data from protein inhibition studies for a representative group of the pyrazolo-indole-class compounds.
-50-
WASH 7720093.1
Table 1: Fluorescence polarization binding assay. Measured binding constants of MDM2 and MDMX li ands.
n.i. no interaction.
n.a. could not be measured because of the high, fluorescence of the compound.
-51-
WASH 7720093.1
-53-
WASH 7720093.1
-55-
-56-
[0258] As illustrated in Table 1 above, compounds in accordance with the present invention have affinity for both MDM2 and MDM4 proteins. Accordingly, the inventive compounds are dual action antagonists and are candidate therapeutics for treating cell proliferative disorders such as cancer.
[0259] Exemplary inventive compounds also were tested for their ability to disrupt p53- hMDM2 protein-protein interaction, using a fluorescent based biosensor assay described, for example, by Dudgeon et al., J. Biomol. Screen. 15 : 766-82 (2010), and by Dudgeon et al., Assay and Drug Development Technologies 8: 437-58 (2010). Briefly, U20S cells are CO-
-57-
WASH 7720093.1
infected with adenovirus constructs expressing a p53-monomeric TagGFP protein having a nuclear localization sequence (NLS) and a hDM2-monomeric Tag RFP cytoplasm-nucleus shuttling protein interaction partner having a NLS and a nuclear export sequence (NES). Initially, the hDM2 protein component binds to the p53 component and both proteins are localized in the nucleolus. Treatment of cells using an inventive compound disrupts the p53- hDM2 protein-protein interaction, which results in an export and redistribution of the unbound hDM2-RFP component in the cellular cytoplasm. The disruption of the hDM2-p53 biosensor interaction is determined via a molecular translocation image analysis algorithm that measures the difference in the average fluorescent intensity associated with hDM2-RFP component in the cytoplasm and nuclear compartments. See Dudgeon et al., J. Biomol. Screen. 15: 766-82 (2010), and Dudgeon et al., Assay and Drug Development Technologies 8: 437-58 (2010).
[0260] As shown in Table 3 below, the inventive compounds were potent disrupters of cellular protein-protein interactions with potencies in the low micromolar range.
Table 3
-58-
WASH 7720093.1
[0261] Alternatively, the NMR-based technique "Antagonist Induced Dissociation Assay (AIDA-NMR)," can be used to study an antagonist's ability to disrupt a protein-protein interaction. See Bista et al, JACS, Vol. 131, pp7500-7501, (2009). An advantage to using AIDA to study disruption of protein-protein interactions is that this NMR based method provides unambiguous information on whether the antagonist activity of a compound is strong enough to dissociate a protein-protein complex and whether dissociation is through precipitation, denaturation or release of a protein in its functional folded state. AIDA has revealed that compounds in accordance with the present invention are potent disrupters of MDM2- or MDMX-p53 protein complexes and are thus suitable as therapeutics directed to the treatment of cell proliferative disorders.
[0262] Accordingly, the present invention provides a method for treating cell proliferative disorders using an agent that is selected from a compound comporting with Formula 1 , its salt, prodrug, solvate, stereoisomer, or tautomer or by using a pharmaceutically acceptable formulation of such agent with a pharmaceutically acceptable carrier. b. NCI60 Cancer Cell Screening
[0263] Compounds in accordance with this invention were tested for their ability to arrest the growth of cancer cells in culture. Specifically, exemplary compounds were submitted to the National Institute of Cancer for inclusion into their screening assay encompassing a variety of different cancer cell lines. See Figures 7A-7C. Briefly, a single 10 μΜ dose of the test compound dissolved in a suitable solvent is added to each well of a tissue culture plate containing the desired cancer cells plated at a known cell density in a tissue culture plate.
[0264] After incubation for a fixed period of time the cell density and viability is determined for each well of the tissue culture plate. The percent inhibition by a
representative compound of this invention for each cancer cell line included in the screen is graphically represented in Figures 7A-7C. The data illustrate that the inventive compounds do arrest the growth of various cancer cells in culture, with compound YH265 exhibiting the most potency against colon, melanoma, and CNS cancer cells.
-59-
WASH 7720093.1
PHARMACEUTICALLY ACCEPTABLE FORMULATIONS
[0265] Despite evidence suggesting that the regulatory role of the p53 tumor suppressor protein is compromised in many cancer cells by sequestration to its negative regulators, MDM2 and MDMX, there are no known anticancer therapies that target the disruption of MDM2-p53 or MDMX-p53 protein-protein interactions. In some embodiments, compounds of the invention and their pharmaceutically acceptable formulations provide a novel method for treating cancer. Pharmaceutically acceptable formulations in accordancewith the present invention are manufactured using the inventive compounds, its prodrug or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer and a pharmaceutically acceptable carrier. For Formula I compounds, the polar amino and sulfonamide groups can promote hydrophilic interactions, such as hydrogen bonding with conventional aqueous carrier solvents, such as saline. Inventive compounds that do not readily dissolve in an aqueous medium, however, can be formulated by adding to the aqueous medium, pharmaceutically acceptable hydrophobic solvents, such as poly-alkylene glycol gelatin, gum arabic, lactose, starch, petroleum jelly and vegetable oil. By the same token, aqueous carriers can be used to formulate the hydrophilic Formulae III compounds. Accordingly, several Formula I compounds showed good aqueous solubility in pH 7.0, phosphate buffer. See Table 3.
Additional excipients, such as flavoring agents, preservatives, stabilizers, emulsifying agents, buffers and the like may be added in accordance with accepted practices of pharmaceutical compounding.
[0266] A formulation of a compound of this invention can be administered intravenously, intraperitoneally, orally, bucally, or by parenteral administration. The compounds can be formulated, alone or together with other known antineoplastic agents, in suitable unit dosage formulations that may contain conventional non-toxic pharmaceutically acceptable carriers, adjuvants and vehicles, as described above, which are appropriate for each route of administration. The invention also contemplates administration of the compounds of the invention in a depot formulation, in which the active ingredient is released over a defined time period.
-60-
WASH 7720093.1
Claims
1. A compound according to formula I
Ri, is an indole;
R2 and R3 are different from each other and are phenyl or benzyl; indicates the presence of one or more optional double bonds;
R4 and R5 are independently selected from the group consisting of H, (Ci-C8)alkyl, and (C3-Ci4)aryl;
Ra and Rb are independently selected from the group consisting of hydrogen, (Ci-Cg)alkyl, hydroxide, (Ci-C8)alkylS(0)2-, (Ci-C8)haloalkylS(0)2-,
(Ci-C8)hydroxyalkylS(0)2-, -(Ci-C8)alkyl-COORc, -(Ci-C8)alkyl-CORc and optionally substituted (C3-C8)cycloalkyl;
wherein
Ra and Rb together with the nitrogen atom to which they are bound can form a
(C3-C6)heterocycloalkyl, or a (C3-Ce)heteroaryl;
Rc is selected from the group consisting H, (Ci-C8)alkyl; -NH(OH); and
A, B, D and E are independently -CR1, -CR2,, -CR4, -CR5, -N, -O, or -S, provided that
when A, D and E are each -N then B is -CR2,
when A is -N, B is -NR2, and D is -CR5 then E is -CRi, and
when A is -NR4, B is N, and D is -CR5 then E is -CRi,
when in A is -N, B is -O, and D is -CR5 then E is -CRi, and
when A is -N, B is -S, and D is -CR5 then E is -CRi,
-61-
WASH 7720093.1 and wherein
the aforementioned indole, phenyl, (C3-C8)cycloalkyl, (C3-C6)heterocycloalkyl, (C3-Ce)heteroaryl or benzyl groups may be substituted with one or more members selected from the group consisting of halogen, -OH, oxo, -COORa, -C(0)Ra, -(Ci-C8)alkyl-COORa, - (Ci-C8)alkyl-NRaRb, S(0)2 NRaRb,-C(0)NRaRb, (C3-Ci4)aryl(Ci-C6)alkylene-, -CN, -N02, NRaRb, (Ci-C6)alkyl-S-, (C3-Ci4)cycloalkyl, (C3-Ci4)heterocycloalkyl, (C3-Ci4)aryl, (C3- Ci4)heteroaryl, -C(0)NH-(Ci-C6)alkyl, - NHC(0)-(Ci-C6)alkyl, (Ci-C6)alkyl, (C2-C8)alkenyl, (C2-C8)alkynyl, (Ci-C6)alkoxy, (Ci-C6)haloalkyl, -C(0)-(C3-C8)cycloalkyl-NRaRb and (C i -C6)hydroxyalkyl .
2. The compound according to claim 1, wherein said compound is a substituted imidazole represented by Formula IA
3. The compound according to claim 2, wherein Ri is 6-chloroindole, R2 is 4- chlorobenzyl and R3 is phenyl.
4. The compound according to claim 1, wherein said compound is a substituted triazole represented by Formula II
5. The compound according to claim 1, wherein said compound is a substituted pyrazole re resented by Formulae IIIA, IIIB or IIIC
-62-
WASH 7720093.1
6. The compound according to claim 1, wherein said compound is a substituted isoxazole represented by Formulae IV
7. The compound according to claim 1, wherein said compound is a substituted isothiazole represented by Formulae V
8. A pharmaceutical composition that comprises (A) a therapeutically effective amount of at least one compound according to any of claims 1 to 7 and (B) a
pharmaceutically acceptable carrier therefor.
9. A method for treating a cell proliferative disorder, comprising contacting a cell with at least one compound according to any of claims 1 to 7.
-63-
WASH 7720093.1
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US30892510P | 2010-02-27 | 2010-02-27 | |
| US61/308,925 | 2010-02-27 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2011106650A2 true WO2011106650A2 (en) | 2011-09-01 |
| WO2011106650A3 WO2011106650A3 (en) | 2012-01-19 |
Family
ID=44507586
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2011/026251 WO2011106650A2 (en) | 2010-02-27 | 2011-02-25 | Novel p53-mdm2/p53-mdm4 antagonists to treat proliferative disease |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2011106650A2 (en) |
Cited By (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8518984B2 (en) | 2009-11-12 | 2013-08-27 | The Regents Of The University Of Michigan | Spiro-oxindole MDM2 antagonists |
| CN103497164A (en) * | 2013-09-23 | 2014-01-08 | 西安近代化学研究所 | Anthracene derivative and preparation method thereof |
| US8629141B2 (en) | 2011-05-11 | 2014-01-14 | The Regents Of The University Of Michigan | Spiro-oxindole MDM2 antagonists |
| US8680132B2 (en) | 2010-11-12 | 2014-03-25 | The Regents Of The University Of Michigan | Spiro-oxindole MDM2 antagonists |
| US8859723B2 (en) | 2010-08-13 | 2014-10-14 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles |
| US8889632B2 (en) | 2007-01-31 | 2014-11-18 | Dana-Farber Cancer Institute, Inc. | Stabilized p53 peptides and uses thereof |
| US8927500B2 (en) | 2012-02-15 | 2015-01-06 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles |
| US8987414B2 (en) | 2012-02-15 | 2015-03-24 | Aileron Therapeutics, Inc. | Triazole-crosslinked and thioether-crosslinked peptidomimetic macrocycles |
| US9096684B2 (en) | 2011-10-18 | 2015-08-04 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles |
| US9604919B2 (en) | 2012-11-01 | 2017-03-28 | Aileron Therapeutics, Inc. | Disubstituted amino acids and methods of preparation and use thereof |
| US9745253B2 (en) | 2015-03-13 | 2017-08-29 | Forma Therapeutics, Inc. | Alpha-cinnamide compounds and compositions as HDAC8 inhibitors |
| WO2017201449A1 (en) | 2016-05-20 | 2017-11-23 | Genentech, Inc. | Protac antibody conjugates and methods of use |
| US10023613B2 (en) | 2015-09-10 | 2018-07-17 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles as modulators of MCL-1 |
| EP3265073A4 (en) * | 2015-03-03 | 2018-09-26 | Shuttle Pharmaceuticals, LLC | Dual function molecules for histone deacetylase inhibition and ataxia telangiectasia mutated activation and methods of use thereof |
| WO2018189340A1 (en) | 2017-04-14 | 2018-10-18 | Italfarmaco S.P.A. | Selective hdac6 inhibitors |
| US10253067B2 (en) | 2015-03-20 | 2019-04-09 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles and uses thereof |
| US10301351B2 (en) | 2007-03-28 | 2019-05-28 | President And Fellows Of Harvard College | Stitched polypeptides |
| EP3371173A4 (en) * | 2015-11-02 | 2019-06-19 | Carmel-Haifa University Economic Corporation Ltd. | Apoptosis related protein in the tgf-beta signaling pathway (arts) mimetic compounds, compositions, methods and uses thereof in induction of differentiation and/or apoptosis of premalignant and malignant cells, thereby restoring their normal-like phenotype |
| US10471120B2 (en) | 2014-09-24 | 2019-11-12 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles and uses thereof |
| US10905739B2 (en) | 2014-09-24 | 2021-02-02 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles and formulations thereof |
| US11034667B2 (en) | 2017-01-09 | 2021-06-15 | Shuttle Pharmaceuticals, Inc. | Selective histone deacetylase inhibitors for the treatment of human disease |
| US11034669B2 (en) | 2018-11-30 | 2021-06-15 | Nuvation Bio Inc. | Pyrrole and pyrazole compounds and methods of use thereof |
| US11407723B2 (en) | 2018-01-09 | 2022-08-09 | Shuttle Pharmaceuticals, Inc. | Selective histone deacetylase inhibitors for the treatment of human disease |
| US11584733B2 (en) | 2017-01-09 | 2023-02-21 | Shuttle Pharmaceuticals, Inc. | Selective histone deacetylase inhibitors for the treatment of human disease |
| WO2023056069A1 (en) | 2021-09-30 | 2023-04-06 | Angiex, Inc. | Degrader-antibody conjugates and methods of using same |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060293319A1 (en) * | 2005-06-24 | 2006-12-28 | Kim-Chun Luk | Oxindole derivatives |
| WO2008119741A2 (en) * | 2007-03-29 | 2008-10-09 | Novartis Ag | 3-imidazolyl-indoles for the treatment of proliferative diseases |
| WO2008130614A2 (en) * | 2007-04-20 | 2008-10-30 | University Of Pittsburg-Of The Commonwealth System Of Higher Education | Selective and dual-action p53/mdm2/mdm4 antagonists |
| US20090143364A1 (en) * | 2004-05-18 | 2009-06-04 | Nader Fotouhi | Chiral cis-imidazolines |
-
2011
- 2011-02-25 WO PCT/US2011/026251 patent/WO2011106650A2/en active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20090143364A1 (en) * | 2004-05-18 | 2009-06-04 | Nader Fotouhi | Chiral cis-imidazolines |
| US20060293319A1 (en) * | 2005-06-24 | 2006-12-28 | Kim-Chun Luk | Oxindole derivatives |
| WO2008119741A2 (en) * | 2007-03-29 | 2008-10-09 | Novartis Ag | 3-imidazolyl-indoles for the treatment of proliferative diseases |
| WO2008130614A2 (en) * | 2007-04-20 | 2008-10-30 | University Of Pittsburg-Of The Commonwealth System Of Higher Education | Selective and dual-action p53/mdm2/mdm4 antagonists |
Cited By (45)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8889632B2 (en) | 2007-01-31 | 2014-11-18 | Dana-Farber Cancer Institute, Inc. | Stabilized p53 peptides and uses thereof |
| US9527896B2 (en) | 2007-01-31 | 2016-12-27 | Dana-Farber Cancer Institute, Inc. | Stabilized p53 peptides and uses thereof |
| US10301351B2 (en) | 2007-03-28 | 2019-05-28 | President And Fellows Of Harvard College | Stitched polypeptides |
| US8518984B2 (en) | 2009-11-12 | 2013-08-27 | The Regents Of The University Of Michigan | Spiro-oxindole MDM2 antagonists |
| US8877796B2 (en) | 2009-11-12 | 2014-11-04 | The Regents Of The University Of Michigan | Spiro-oxindole MDM2 antagonists |
| US9079913B2 (en) | 2009-11-12 | 2015-07-14 | The Regents Of The University Of Michigan | Spiro-oxindole MDM2 antagonists |
| US11008366B2 (en) | 2010-08-13 | 2021-05-18 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles |
| US10703780B2 (en) | 2010-08-13 | 2020-07-07 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles |
| US8859723B2 (en) | 2010-08-13 | 2014-10-14 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles |
| US9957299B2 (en) | 2010-08-13 | 2018-05-01 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles |
| US9302120B2 (en) | 2010-11-12 | 2016-04-05 | The Regents Of The University Of Michigan | Spiro-oxindole MDM2 antagonists |
| US8680132B2 (en) | 2010-11-12 | 2014-03-25 | The Regents Of The University Of Michigan | Spiro-oxindole MDM2 antagonists |
| US8629141B2 (en) | 2011-05-11 | 2014-01-14 | The Regents Of The University Of Michigan | Spiro-oxindole MDM2 antagonists |
| US9096684B2 (en) | 2011-10-18 | 2015-08-04 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles |
| US9522947B2 (en) | 2011-10-18 | 2016-12-20 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles |
| US10308699B2 (en) | 2011-10-18 | 2019-06-04 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles |
| US8927500B2 (en) | 2012-02-15 | 2015-01-06 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles |
| US8987414B2 (en) | 2012-02-15 | 2015-03-24 | Aileron Therapeutics, Inc. | Triazole-crosslinked and thioether-crosslinked peptidomimetic macrocycles |
| US9505804B2 (en) | 2012-02-15 | 2016-11-29 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles |
| US10213477B2 (en) | 2012-02-15 | 2019-02-26 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles |
| US10227380B2 (en) | 2012-02-15 | 2019-03-12 | Aileron Therapeutics, Inc. | Triazole-crosslinked and thioether-crosslinked peptidomimetic macrocycles |
| US9845287B2 (en) | 2012-11-01 | 2017-12-19 | Aileron Therapeutics, Inc. | Disubstituted amino acids and methods of preparation and use thereof |
| US10669230B2 (en) | 2012-11-01 | 2020-06-02 | Aileron Therapeutics, Inc. | Disubstituted amino acids and methods of preparation and use thereof |
| US9604919B2 (en) | 2012-11-01 | 2017-03-28 | Aileron Therapeutics, Inc. | Disubstituted amino acids and methods of preparation and use thereof |
| CN103497164A (en) * | 2013-09-23 | 2014-01-08 | 西安近代化学研究所 | Anthracene derivative and preparation method thereof |
| US10905739B2 (en) | 2014-09-24 | 2021-02-02 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles and formulations thereof |
| US10471120B2 (en) | 2014-09-24 | 2019-11-12 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles and uses thereof |
| EP3265073A4 (en) * | 2015-03-03 | 2018-09-26 | Shuttle Pharmaceuticals, LLC | Dual function molecules for histone deacetylase inhibition and ataxia telangiectasia mutated activation and methods of use thereof |
| US10508077B2 (en) | 2015-03-13 | 2019-12-17 | Forma Therapeutics, Inc. | Alpha-cinnamide compounds and compositions as HDAC8 inhibitors |
| US10266487B2 (en) | 2015-03-13 | 2019-04-23 | Forma Therapeutics, Inc. | Alpha-cinnamide compounds and compositions as HDAC8 inhibitors |
| US11919839B2 (en) | 2015-03-13 | 2024-03-05 | Valo Health, Inc. | Alpha-cinnamide compounds and compositions as HDAC8 inhibitors |
| US10988441B2 (en) | 2015-03-13 | 2021-04-27 | Valo Early Discovery, Inc. | Alpha-cinnamide compounds and compositions as HDAC8 inhibitors |
| US9745253B2 (en) | 2015-03-13 | 2017-08-29 | Forma Therapeutics, Inc. | Alpha-cinnamide compounds and compositions as HDAC8 inhibitors |
| US10253067B2 (en) | 2015-03-20 | 2019-04-09 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles and uses thereof |
| US10023613B2 (en) | 2015-09-10 | 2018-07-17 | Aileron Therapeutics, Inc. | Peptidomimetic macrocycles as modulators of MCL-1 |
| EP3371173A4 (en) * | 2015-11-02 | 2019-06-19 | Carmel-Haifa University Economic Corporation Ltd. | Apoptosis related protein in the tgf-beta signaling pathway (arts) mimetic compounds, compositions, methods and uses thereof in induction of differentiation and/or apoptosis of premalignant and malignant cells, thereby restoring their normal-like phenotype |
| US11866409B2 (en) | 2015-11-02 | 2024-01-09 | Carmel-Haifa University Economic Corporation Ltd. | Apoptosis related protein in the tgf-beta signaling pathway (ARTS) mimetic compounds, compositions, methods and uses thereof in induction of differentiation and/or apoptosis of premalignant and malignant cells, thereby restoring their normal-like phenotype |
| WO2017201449A1 (en) | 2016-05-20 | 2017-11-23 | Genentech, Inc. | Protac antibody conjugates and methods of use |
| US11584733B2 (en) | 2017-01-09 | 2023-02-21 | Shuttle Pharmaceuticals, Inc. | Selective histone deacetylase inhibitors for the treatment of human disease |
| US11034667B2 (en) | 2017-01-09 | 2021-06-15 | Shuttle Pharmaceuticals, Inc. | Selective histone deacetylase inhibitors for the treatment of human disease |
| US11351178B2 (en) | 2017-04-14 | 2022-06-07 | Italfarmaco Spa | Selective HDAC6 inhibitors |
| WO2018189340A1 (en) | 2017-04-14 | 2018-10-18 | Italfarmaco S.P.A. | Selective hdac6 inhibitors |
| US11407723B2 (en) | 2018-01-09 | 2022-08-09 | Shuttle Pharmaceuticals, Inc. | Selective histone deacetylase inhibitors for the treatment of human disease |
| US11034669B2 (en) | 2018-11-30 | 2021-06-15 | Nuvation Bio Inc. | Pyrrole and pyrazole compounds and methods of use thereof |
| WO2023056069A1 (en) | 2021-09-30 | 2023-04-06 | Angiex, Inc. | Degrader-antibody conjugates and methods of using same |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2011106650A3 (en) | 2012-01-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2011106650A2 (en) | Novel p53-mdm2/p53-mdm4 antagonists to treat proliferative disease | |
| JP7337883B2 (en) | Small molecule inhibitors of lactate dehydrogenase and methods of use thereof | |
| WO2020190774A1 (en) | Novel small molecule inhibitors of tead transcription factors | |
| JP2016525075A (en) | Heteroaryl substituted pyrazoles | |
| Liu et al. | Synthesis and structure-activity relationship study of water-soluble carbazole sulfonamide derivatives as new anticancer agents | |
| CN107176933B (en) | Indoleamine-2,3-dioxygenase inhibitor containing nitrogen alkylated and arylated sulfoximine | |
| JP2016536311A (en) | Heteroaryl substituted pyrazoles | |
| AU2017226005A1 (en) | Inhibitors of WDR5 protein-protein binding | |
| JP2008516905A (en) | 1,5-naphthyridine azolidinone having CDK1 antiproliferative activity | |
| WO2011135303A2 (en) | Ubiquitination modulators | |
| EP3154982B1 (en) | Compounds comprising 1,1',2,5'-tetrahydrospiro[indole-3,2'-pyrrole]-2,5'-dione system as inhibitors p53-mdm2 protein-protein interaction | |
| BR112019021794A2 (en) | compound, pharmaceutical composition, and use of the compound | |
| WO2020020288A1 (en) | Sulfoximine compound as bromodomain protein inhibitor and pharmaceutical composition and medical use thereof | |
| KR20210130753A (en) | Amide derivatives useful for the treatment of HBV infection or HBV-induced disease | |
| CA3197470A1 (en) | Bicyclic compounds | |
| WO2021055936A1 (en) | Small-molecule inhibitors for the β-catenin/b-cell lymphoma 9 protein−protein interaction | |
| ES2927529T3 (en) | condensed heterocyclic compound | |
| JP5583845B2 (en) | Imidazopyridazinyl compounds and their use against cancer | |
| ES2788660T3 (en) | 2,3-dihydro-1h-inden-1-one derivatives as orphan receptor antagonists related to gamma retinoic acid (ROR gamma) for the treatment of multiple sclerosis | |
| JP2019523237A (en) | 1H-pyrazol-1-yl-thiazole as inhibitors of lactate dehydrogenase and methods for their use | |
| WO2015189433A1 (en) | Pyridazinones for the treatment of cancer | |
| US20220348595A1 (en) | Compounds and methods for the targeted degradation of estrogen receptors | |
| CN114269720A (en) | acetyl-CoA synthetase short chains2(ACSS2) Small molecule inhibitors of | |
| KR102636651B1 (en) | Thiazolopyridine or pharmaceutically acceptable salts thereof, and uses thereof | |
| JP7384669B2 (en) | Pharmaceutical compositions in which a substituted dihydropyrrolopyrazole compound and an immunotherapeutic agent are administered in combination |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11748150 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase in: |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11748150 Country of ref document: EP Kind code of ref document: A2 |