WO2011105069A1 - Actinic radiation curable resin composition and cured products - Google Patents
Actinic radiation curable resin composition and cured products Download PDFInfo
- Publication number
- WO2011105069A1 WO2011105069A1 PCT/JP2011/001020 JP2011001020W WO2011105069A1 WO 2011105069 A1 WO2011105069 A1 WO 2011105069A1 JP 2011001020 W JP2011001020 W JP 2011001020W WO 2011105069 A1 WO2011105069 A1 WO 2011105069A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- meth
- resin composition
- curable resin
- acrylate
- active energy
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F290/00—Macromolecular compounds obtained by polymerising monomers on to polymers modified by introduction of aliphatic unsaturated end or side groups
- C08F290/02—Macromolecular compounds obtained by polymerising monomers on to polymers modified by introduction of aliphatic unsaturated end or side groups on to polymers modified by introduction of unsaturated end groups
- C08F290/06—Polymers provided for in subclass C08G
- C08F290/067—Polyurethanes; Polyureas
-
- H—ELECTRICITY
- H04—ELECTRIC COMMUNICATION TECHNIQUE
- H04M—TELEPHONIC COMMUNICATION
- H04M1/00—Substation equipment, e.g. for use by subscribers
- H04M1/02—Constructional features of telephone sets
- H04M1/23—Construction or mounting of dials or of equivalent devices; Means for facilitating the use thereof
Definitions
- the present invention relates to an active energy ray-curable resin composition applied to applications requiring low warpage, adhesion at high temperature and high humidity, and wear resistance.
- a photocurable resin mainly used for a button of a mobile phone or the like is required to have transparency, low curing shrinkage, and the like. Furthermore, recently, along with the enhancement of functions of such mobile phones, high reliability that does not cause problems even under high temperature and high humidity is required. Further, when used in an outermost case such as a case, in addition to these characteristics, wear resistance, impact resistance, and the like are required.
- a photocurable resin having low curing shrinkage there is a photosensitive resin using a bifunctional urethane acrylate resin (see Patent Document 1 and Patent Document 2). There is also a method using a tri- or higher functional photosensitive resin in order to improve scratch resistance and hardness (see Patent Document 3).
- Monofunctional or bifunctional photosensitive resins are used to achieve low cure shrinkage, but these are inferior in wear resistance and impact resistance.
- tri- or higher functional photosensitive resins are good for improving wear resistance and impact resistance, but these have a problem of large shrinkage in curing and cause large warpage in the molded product of the cured product. is there.
- the present invention provides a cured product having high adhesion to a base material even under high temperature and high humidity without causing warping, and further having abrasion resistance when cured by irradiation with active energy rays.
- An object is to provide an active energy ray-curable resin composition and a cured product obtained by curing the active energy ray-curable resin composition.
- the present inventors have found that there is no warpage, high adhesion to the substrate even when exposed to high temperature and high humidity, and activity that can provide a cured product having wear resistance.
- an energy ray curable resin composition the inventors have conceived an invention having the following contents.
- the active energy ray-curable resin composition of the present invention includes a urethane resin having a plurality of (meth) acrylate groups, a photosensitive amide and / or a derivative of a photosensitive amide, and a cyclic skeleton containing no double bond. It contains a functional (meth) acrylate and a photopolymerization initiator.
- the photosensitive amide and / or the derivative of the photosensitive amide is at least one of N- (2-hydroxyethyl) acrylamide, N- (2-hydroxyethyl) methacrylamide, N-acryloylmorpholine, and N-methacryloylmorpholine. It is characterized by comprising.
- cured material or molded article of this invention is obtained by hardening
- the “durometer hardness D type” refers to a value measured according to JIS K7215.
- (meth) acrylate is a term that collectively refers to acrylate and methacrylate, and the same applies to other similar expressions.
- an acrylate and a methacrylate may be used independently or may be used in mixture of 2 or more types.
- an active energy ray-curable resin composition capable of obtaining a hardened product and a cured product thereof are obtained.
- a plate having a concave portion on its surface, which is used for producing the cured product of the present invention A state in which the concave portion of the plate is filled with the active energy ray resin composition of the present invention.
- the urethane resin having a plurality of (meth) acrylate groups contained in the active energy ray-curable resin composition of the present invention preferably has a weight average molecular weight in the range of 1,000 to 20,000.
- the weight average molecular weight is less than 1,000, the crosslink density caused by the urethane resin having a plurality of (meth) acrylate groups is increased, and the curing shrinkage of the resin is increased, so that the warpage of the cured molded product is also increased.
- the weight average molecular weight is 20,000 If it exceeds 0, the viscosity becomes high, which is not preferable in the application of the present invention.
- urethane resins having a plurality of (meth) acrylate groups a bifunctional urethane (meth) acrylate resin and a trifunctional urethane (meth) acrylate resin are preferable.
- urethane resin having a plurality of (meth) acrylate groups, bifunctional U-108A, UA-112P, UA-5201, UA-512, UA-412A, UA-4200, UA manufactured by Shin-Nakamura Chemical Co., Ltd.
- urethane resins having a plurality of (meth) acrylate groups may be used alone or in admixture of two or more.
- Examples of the photosensitive amide and the photosensitive amide derivative contained in the active energy ray-curable resin composition of the present invention include N-vinylformamide, N-vinylacetamide, (meth) acrylamide, N-methyl (meth) acrylamide, N, N-dimethyl (meth) acrylamide, Nn-propyl (meth) acrylamide, N-isopropyl (meth) acrylamide, N, N-diethyl (meth) acrylamide, N-methyl-Nn-propyl (meth) Acrylamide, N-methyl-N-isopropyl (meth) acrylamide, N-tetrahydrofurfuryl (meth) acrylamide, N-ethoxypropyl (meth) acrylamide, N-ethoxyethyl (meth) acrylamide, N-1-methyl-2- Methoxyethyl (meth) acrylamide, N Morpholinopropyl (meth) acrylamide, N
- it is a photosensitive amide compound having a (meth) acryloyl group, among which N- (2-hydroxyethyl) acrylamide, N- (2-hydroxyethyl) methacrylamide, N-acryloylmorpholine, N-methacryloylmorpholine, At least one of these is particularly preferred from the viewpoint of transparency.
- Such a photosensitive amide and / or a derivative of the photosensitive amide is preferably 15 to 200 parts by mass, more preferably 20 to 100 parts by mass with respect to 100 parts by mass of the urethane resin having a plurality of (meth) acrylate groups. Included in the ratio. If the blending amount of the photosensitive amide and / or the derivative of the photosensitive amide is less than 15 parts by mass, the adhesion is insufficient, while if it exceeds 200 parts by mass, the cured product tends to become brittle.
- the bifunctional (meth) acrylate having a cyclic skeleton having no double bond which is contained in the active energy ray-curable resin composition of the present invention, prevents warpage by having no double bond, thereby preventing the cyclic skeleton. By having, it is possible to enhance the wear resistance.
- the weight average molecular weight is preferably in the range of 150 to 2,000. When the weight average molecular weight is less than 150, the crosslinking density caused by the urethane resin having a plurality of (meth) acrylate groups is increased, and the curing shrinkage of the resin is increased, so that the warpage of the cured molded product is also increased. When the weight average molecular weight exceeds 2,000, the viscosity becomes high, which may not be applicable in the application of the present invention.
- bifunctional (meth) acrylate having a cyclic skeleton having no double bond examples include cyclohexanedimethanol di (meth) acrylate, tricyclodencan dimethanol di (meth) acrylate, and hydrogenated bisphenol A di (meth).
- Bifunctional (meth) having an alicyclic skeleton such as acrylate, hydrogenated bisphenol F di (meth) acrylate, hydrogenated hexafluorobisphenol A di (meth) acrylate, bis (2- (meth) acryloyloxy) hexahydrophthalic acid Heterocycles such as acrylate, 5-ethyl-2- (2-hydroxy-1,1dimethylethyl) -5- (hydroxymethyl) -1,3-dioxane diacrylate, 1,4-di (meth) acryloylpiperazine Bifunctional (meth) acrylate with skeleton, and even this Difunctional (meth) acrylates of ethylene oxide, propylene oxide, and a modified product such as caprolactone.
- the bifunctional (meth) acrylate having a cyclic skeleton having no double bond is preferably 10 to 100 parts by mass, more preferably 100 parts by mass with respect to 100 parts by mass of the urethane resin having a plurality of (meth) acrylate groups. Is contained in a proportion of 20 to 50 parts by mass.
- the blending amount of the bifunctional (meth) acrylate having a cyclic skeleton having no double bond is less than 10 parts by mass, the abrasion resistance is insufficient, and when it exceeds 100 parts by mass, the cured product becomes brittle. It tends to be unfavorable.
- Examples of the photopolymerization initiator contained in the active energy ray-curable resin composition of the present invention include benzoin, benzyl, benzoin methyl ether, benzoin ethyl ether, benzoin n-propyl ether, benzoin isopropyl ether, and benzoin n-butyl ether.
- Benzoins such as benzoin; benzoin alkyl ethers; benzophenones such as benzophenone, p-methylbenzophenone, Michler's ketone, methylbenzophenone, 4,4′-dichlorobenzophenone, 4,4′-bisdiethylaminobenzophenone; acetophenone, 2,2-dimethoxy -2-phenylacetophenone, 2,2-diethoxy-2-phenylacetophenone, 1,1-dichloroacetophenone, 1-hydroxycyclohexyl phenylketo Acetophenones such as 2-methyl- [4- (methylthio) phenyl] -2-morpholino-1-propanone and N, N-dimethylaminoacetophenone; 2,4-dimethylthioxanthone, 2,4-diethylthioxanthone, 2- Thioxanthones such as chlorothioxanthone and 2,4-diisopropyl
- photopolymerization initiators can be used alone or in combination of two or more.
- This photopolymerization initiator is contained in a proportion of preferably 0.1 to 10 parts by mass, more preferably 0.3 to 5 parts by mass with respect to 100 parts by mass of the urethane resin having a plurality of (meth) acrylate groups. .
- the active energy ray-curable resin composition of the present invention can be blended with an inorganic filler as necessary for the purpose of further improving properties such as adhesion, mechanical strength, and linear expansion coefficient of the cured product.
- an inorganic filler such as barium sulfate, barium titanate, silicon oxide powder, finely divided silicon oxide, amorphous silica, talc, clay, magnesium carbonate, calcium carbonate, aluminum oxide, aluminum hydroxide, mica powder, etc.
- Inorganic fillers can be used.
- the blending ratio is preferably 10 to 100 parts by mass with respect to 100 parts by mass of the present active energy ray-curable resin composition.
- Colorants known and conventional photopolymerization inhibitors such as hydroquinone, hydroquinone monomethyl ether, t-butylcatechol, pyrogallol and phenothiazine, known and conventional thickeners such as finely divided silica, organic bentonite and montmorillonite, silicone-based, fluorine-based, high Known and commonly used additives such as defoamers and / or leveling agents such as molecular systems, silane coupling agents such as imidazole systems, thiazole systems, and triazole systems can be blended.
- photopolymerization inhibitors such as hydroquinone, hydroquinone monomethyl ether, t-butylcatechol, pyrogallol and phenothiazine
- known and conventional thickeners such as finely divided silica, organic bentonite and montmorillonite, silicone-based, fluorine-based, high Known and commonly used additives such as defoamers and / or leveling
- the active energy ray-curable resin composition of the present invention preferably has a viscosity of 1 to 50 dPa ⁇ s.
- This viscosity is preferably adjusted in the range of 1 to 100 parts by mass, more preferably 10 to 50 parts by mass with respect to 100 parts by mass of the urethane resin having a plurality of (meth) acrylate groups. Can be provided. If the amount of reactive diluent is 1 part by mass or less, the viscosity is high and workability is lowered. On the other hand, if it is 100 parts by mass or more, the crosslink density of the cured product is reduced and sufficient wear resistance cannot be obtained.
- hydroxyalkyl acrylates such as 2-hydroxyethyl acrylate, 2-hydroxypropyl acrylate, and 4-hydroxybutyl acrylate
- cyclic skeletons such as isobornyl acrylate, tetrahydrofurfuryl acrylate, N-acryloylmorpholine, and N-vinylpyrrolidinone.
- Monofunctional photosensitive monomers having; mono- or diacrylates of glycols such as ethylene glycol, methoxytetraethylene glycol, polyethylene glycol, propylene glycol; N, N-dimethylacrylamide, N-methylolacrylamide, N, N-dimethylaminopropyl Acrylamides such as acrylamide; N, N-dimethylaminoethyl acrylate, N, N-dimethylaminopropyl acrylate A polyhydric alcohol such as hexanediol, trimethylolpropane, pentaerythritol, dipentaerythritol, tris-hydroxyethyl isocyanurate, or a polyhydric acrylate such as an ethylene oxide adduct or a propylene oxide adduct; Phenoxyacrylate, bisphenol A diacrylate, and acrylates such as ethylene oxide adducts or propylene oxide adducts of these phenols;
- the active energy ray-curable resin composition according to the present invention is cured by irradiating active energy rays after being applied to a substrate or the like by the following application method.
- the cured product or molded product preferably has a durometer hardness D type of 75 ° or more from the viewpoint of warpage prevention.
- any method such as a dip coating method, a flow coating method, a roll coating method, a bar coater method, a screen printing method, a curtain coating method, a gravure printing method, and an offset printing method can be applied.
- a low pressure mercury lamp As the irradiation source of the active energy ray, a low pressure mercury lamp, a medium pressure mercury lamp, a high pressure mercury lamp, an ultrahigh pressure mercury lamp, a xenon lamp or a metal halide lamp is suitable.
- laser beams, electron beams, and the like can be used as active energy rays.
- a method for obtaining a molded product by filling the active energy ray-curable resin composition of the present invention using an intaglio, curing the filled resin composition with active energy rays, and taking out the cured product from the recess Illustrate.
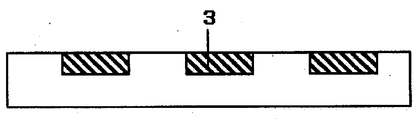
- FIG. 1 shows a plate having a desired shape formed on the surface.
- a metal such as stainless steel is used.
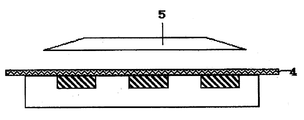
- FIG. 2 shows a state in which this plate is filled with the active energy ray-curable resin composition of the present invention.
- the active energy ray-curable resin composition is filled into the concave portion of the plate using a doctor knife or the like.
- FIG. 3 shows a state in which the active energy ray-curable resin composition of the present invention is cured by covering the base material from above and further irradiating ultraviolet light from the light source on the top.
- a transparent polyethylene terephthalate or polycarbonate film that transmits ultraviolet rays is used as the substrate.
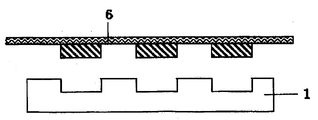
- FIG. 4 shows a state in which the cured product is released from the plate to obtain a molded product.
- a molded product can be continuously produced by using a dedicated apparatus.
- EBECRYL 8807 Aliphatic bifunctional urethane acrylate (manufactured by Daicel-Cytec)
- CN929 aliphatic trifunctional urethane acrylate (manufactured by Sartomer)
- KAYARAD RM-1001 N-acryloylmorpholine (manufactured by Nippon Kayaku Co., Ltd.)
- HEAA N- (2-hydroxyethyl) acrylamide (manufactured by Kojin Co., Ltd.)
- Beam set 770 N-vinylformamide (Arakawa Chemical Industries)
- Aronix M-111 1 mol of ethylene oxide addition nonylphenol acrylate (manufactured by Toagosei Co., Ltd.)
- Light acrylate IB-XA Isoboronyl acrylate (manufactured by Kyoeisha Chemical Co., Ltd.) 4HBA: 4-hydroxybutyl
- the active energy ray-curable resin composition thus prepared was evaluated for warpage, adhesion, and RCA wear by the following evaluation methods. The results are shown in Tables 3 and 4.
- the resin composition is poured into a Teflon (registered trademark) mold having a length of 2 cm, a width of 2 cm, and a depth of 1 mm, covered with an easily adhesive-treated PET film, and a UV conveyor furnace (metal halide lamp, 80 W, 3 lights) is used.
- the film was exposed at an integrated light quantity of 1000 mJ / cm 2 and released from the mold to obtain a cured coating film.
- Warp test Place the cured coating film produced by the above method on a flat base so that the warped side faces upward, press one side with the warp against the base with your finger, and lift it from the base on the opposite side. The height was read and the warpage was measured.
- ⁇ Warpage of cured coating film is less than 5 mm
- Curing of cured coating film is 5 mm or more and less than 10 mm
- Curing of cured coating film is 10 mm or more
- RCA wear test The cured coating film produced by the above method was manufactured by NORMAN TOOL, INC. Using the RCA abrasion tester manufactured by the manufacturer and a dedicated abrasion paper for the RCA abrasion tester, it was worn 50 times under a load of 175 g, and the degree of wear of the cured coating film surface was observed with an optical microscope. ⁇ : Almost no wear ⁇ : Some wear ⁇ : Clear wear
- the resin composition is poured into a stainless steel mold having a diameter of 2.5 cm and a depth of 3 mm, covered with a glass plate, and exposed using a UV conveyor furnace (high pressure mercury lamp, 80 W, 3 lights) at an exposure amount of 1000 mJ / cm 2 .
- the mold was released to obtain a cured coating film.
- the cured coating film produced by the above method was measured according to JIS K7215. The hardness value was read 30 seconds after the load surface was in close contact. At this time, the case of 75 ° or more was regarded as high hardness.
- Examples 1 to 5 are excellent in warpage, adhesion, and wear resistance. Further, since the durometer hardness D type is 75 ° or more, the durometer hardness D type has high hardness and can suppress warpage. Since Comparative Examples 1 to 3 in Table 4 contain a photosensitive amide and / or a derivative of a photosensitive amide, the adhesion is good. However, although the durometer hardness D type is 70 ° or more, the hardness is insufficient and there is a problem with warpage. Moreover, since it does not contain a bifunctional (meth) acrylate having a cyclic skeleton that does not contain a double bond, the wear resistance is poor.
- Comparative Examples 4 and 5 did not contain a photosensitive amide and / or a derivative of the photosensitive amide and a bifunctional (meth) acrylate having a cyclic skeleton containing no double bond, all the characteristics were inferior. Furthermore, since the durometer hardness D type is less than 75 °, the hardness is insufficient and there is a problem of warpage. Comparative Examples 6 and 7 do not contain a photosensitive amide and / or a derivative of the photosensitive amide, but have high adhesion because they contain a photosensitive monomer having a hydroxyl group that is a polar group. However, since the durometer hardness D type is less than 75 °, the hardness is insufficient, and there is a problem with warpage.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Macromonomer-Based Addition Polymer (AREA)
Abstract
Provided is an actinic radiation curable resin composition which, when cured by irradiation with actinic radiation, can yield cured products that exhibit high hardness, low warpage, excellent adhesion under high-temperature and high-humidity conditions, and excellent wear resistance. Also provided are cured products obtained by curing the actinic radiation curable resin composition.
The actinic radiation curable resin composition comprises: a urethane resin that has multiple (meth)acrylate groups; a photosensitive amide and/or a photosensitive amide derivative; a bifunctional (meth)acrylate having a cyclic skeleton that contains no double bond; and a photopolymerization initiator.
Description
本発明は、低反り性、高温高湿下での密着性、及び耐摩耗性とが要求される用途に適用される活性エネルギー線硬化性樹脂組成物に関する。
The present invention relates to an active energy ray-curable resin composition applied to applications requiring low warpage, adhesion at high temperature and high humidity, and wear resistance.
一般的に、主に携帯電話のボタンなどに使用される光硬化性樹脂には、透明性、低硬化収縮性などが求められる。さらに最近では、これら携帯電話などの高機能化に伴い、高温高湿下でも不具合が発生しない高信頼性も必要とされる。
また、ケースなど最外装に使用される場合には、これらの特性に加えて耐磨耗性、耐衝撃性などが要求される。
低硬化収縮性のある光硬化性樹脂としては、二官能ウレタンアクリレート樹脂を用いた感光性樹脂がある(特許文献1・特許文献2参照)。また、耐擦傷性や硬度を向上させるために三官能以上の感光性樹脂を用いる方法もある(特許文献3参照)。 In general, a photocurable resin mainly used for a button of a mobile phone or the like is required to have transparency, low curing shrinkage, and the like. Furthermore, recently, along with the enhancement of functions of such mobile phones, high reliability that does not cause problems even under high temperature and high humidity is required.
Further, when used in an outermost case such as a case, in addition to these characteristics, wear resistance, impact resistance, and the like are required.
As a photocurable resin having low curing shrinkage, there is a photosensitive resin using a bifunctional urethane acrylate resin (seePatent Document 1 and Patent Document 2). There is also a method using a tri- or higher functional photosensitive resin in order to improve scratch resistance and hardness (see Patent Document 3).
また、ケースなど最外装に使用される場合には、これらの特性に加えて耐磨耗性、耐衝撃性などが要求される。
低硬化収縮性のある光硬化性樹脂としては、二官能ウレタンアクリレート樹脂を用いた感光性樹脂がある(特許文献1・特許文献2参照)。また、耐擦傷性や硬度を向上させるために三官能以上の感光性樹脂を用いる方法もある(特許文献3参照)。 In general, a photocurable resin mainly used for a button of a mobile phone or the like is required to have transparency, low curing shrinkage, and the like. Furthermore, recently, along with the enhancement of functions of such mobile phones, high reliability that does not cause problems even under high temperature and high humidity is required.
Further, when used in an outermost case such as a case, in addition to these characteristics, wear resistance, impact resistance, and the like are required.
As a photocurable resin having low curing shrinkage, there is a photosensitive resin using a bifunctional urethane acrylate resin (see
低硬化収縮性を実現するには、単官能もしくは二官能の感光性樹脂などが用いられているが、これらは耐磨耗性・耐衝撃性に劣る。一方、耐磨耗性・耐衝撃性を向上させるには三官能以上の感光性樹脂などが良好であるが、これらは硬化収縮が大きく、硬化物の成型品に大きな反りを生じさせるという問題がある。
本発明は、活性エネルギー線照射により硬化させた際に、反りを生じることなく、高温高湿下においても基材との高い密着性を有し、さらに耐磨耗性も有する硬化物が得られる活性エネルギー線硬化性樹脂組成物と、該活性エネルギー線硬化性樹脂組成物を硬化させることによって得られる硬化物を提供することを目的とする。 Monofunctional or bifunctional photosensitive resins are used to achieve low cure shrinkage, but these are inferior in wear resistance and impact resistance. On the other hand, tri- or higher functional photosensitive resins are good for improving wear resistance and impact resistance, but these have a problem of large shrinkage in curing and cause large warpage in the molded product of the cured product. is there.
The present invention provides a cured product having high adhesion to a base material even under high temperature and high humidity without causing warping, and further having abrasion resistance when cured by irradiation with active energy rays. An object is to provide an active energy ray-curable resin composition and a cured product obtained by curing the active energy ray-curable resin composition.
本発明は、活性エネルギー線照射により硬化させた際に、反りを生じることなく、高温高湿下においても基材との高い密着性を有し、さらに耐磨耗性も有する硬化物が得られる活性エネルギー線硬化性樹脂組成物と、該活性エネルギー線硬化性樹脂組成物を硬化させることによって得られる硬化物を提供することを目的とする。 Monofunctional or bifunctional photosensitive resins are used to achieve low cure shrinkage, but these are inferior in wear resistance and impact resistance. On the other hand, tri- or higher functional photosensitive resins are good for improving wear resistance and impact resistance, but these have a problem of large shrinkage in curing and cause large warpage in the molded product of the cured product. is there.
The present invention provides a cured product having high adhesion to a base material even under high temperature and high humidity without causing warping, and further having abrasion resistance when cured by irradiation with active energy rays. An object is to provide an active energy ray-curable resin composition and a cured product obtained by curing the active energy ray-curable resin composition.
発明者は、上記目的実現のため鋭意研究した結果、反りもなく、高温高湿下にさらされても基材との高い密着性を示し、さらに耐磨耗性を有する硬化物が得られる活性エネルギー線硬化性樹脂組成物として、以下の内容を要旨とする発明に想到した。
As a result of earnest research conducted by the inventor for realizing the above-mentioned object, the present inventors have found that there is no warpage, high adhesion to the substrate even when exposed to high temperature and high humidity, and activity that can provide a cured product having wear resistance. As an energy ray curable resin composition, the inventors have conceived an invention having the following contents.
すなわち、本発明の活性エネルギー線硬化性樹脂組成物は、複数の(メタ)アクリレート基を有するウレタン樹脂、感光性アミド及び/又は感光性アミドの誘導体、二重結合を含まない環状骨格を有する二官能(メタ)アクリレート、及び光重合開始剤を含むことを特徴とする。
また、この感光性アミド及び/又は感光性アミドの誘導体が、N-(2-ヒドロキシエチル)アクリルアミド、N-(2-ヒドロキシエチル)メタアクリルアミド、N-アクリロイルモルホリン、N-メタクリロイルモルホリンの少なくとも1種からなることを特徴とする。
また、本発明の硬化物又は成型品は、上述した活性エネルギー線硬化性樹脂組成物を硬化して得られる。
さらに、本発明の硬化物又は成型品のデュロメータ硬さDタイプが75°以上であることを特徴とする。 That is, the active energy ray-curable resin composition of the present invention includes a urethane resin having a plurality of (meth) acrylate groups, a photosensitive amide and / or a derivative of a photosensitive amide, and a cyclic skeleton containing no double bond. It contains a functional (meth) acrylate and a photopolymerization initiator.
The photosensitive amide and / or the derivative of the photosensitive amide is at least one of N- (2-hydroxyethyl) acrylamide, N- (2-hydroxyethyl) methacrylamide, N-acryloylmorpholine, and N-methacryloylmorpholine. It is characterized by comprising.
Moreover, the hardened | cured material or molded article of this invention is obtained by hardening | curing the active energy ray-curable resin composition mentioned above.
Furthermore, the durometer hardness D type of the cured product or molded product of the present invention is 75 ° or more.
また、この感光性アミド及び/又は感光性アミドの誘導体が、N-(2-ヒドロキシエチル)アクリルアミド、N-(2-ヒドロキシエチル)メタアクリルアミド、N-アクリロイルモルホリン、N-メタクリロイルモルホリンの少なくとも1種からなることを特徴とする。
また、本発明の硬化物又は成型品は、上述した活性エネルギー線硬化性樹脂組成物を硬化して得られる。
さらに、本発明の硬化物又は成型品のデュロメータ硬さDタイプが75°以上であることを特徴とする。 That is, the active energy ray-curable resin composition of the present invention includes a urethane resin having a plurality of (meth) acrylate groups, a photosensitive amide and / or a derivative of a photosensitive amide, and a cyclic skeleton containing no double bond. It contains a functional (meth) acrylate and a photopolymerization initiator.
The photosensitive amide and / or the derivative of the photosensitive amide is at least one of N- (2-hydroxyethyl) acrylamide, N- (2-hydroxyethyl) methacrylamide, N-acryloylmorpholine, and N-methacryloylmorpholine. It is characterized by comprising.
Moreover, the hardened | cured material or molded article of this invention is obtained by hardening | curing the active energy ray-curable resin composition mentioned above.
Furthermore, the durometer hardness D type of the cured product or molded product of the present invention is 75 ° or more.
なお、本明細書及び特許請求の範囲において、「デュロメータ硬さDタイプ」とは、JIS K7215に準拠して測定された値をいう。
また、(メタ)アクリレートとは、アクリレートおよびメタクリレートを総称する用語であり、他の類似の表現についても同様である。ここで、アクリレートおよびメタクリレートは、単独で用いても、2種以上を混合して用いてもよい。 In the present specification and claims, the “durometer hardness D type” refers to a value measured according to JIS K7215.
Moreover, (meth) acrylate is a term that collectively refers to acrylate and methacrylate, and the same applies to other similar expressions. Here, an acrylate and a methacrylate may be used independently or may be used in mixture of 2 or more types.
また、(メタ)アクリレートとは、アクリレートおよびメタクリレートを総称する用語であり、他の類似の表現についても同様である。ここで、アクリレートおよびメタクリレートは、単独で用いても、2種以上を混合して用いてもよい。 In the present specification and claims, the “durometer hardness D type” refers to a value measured according to JIS K7215.
Moreover, (meth) acrylate is a term that collectively refers to acrylate and methacrylate, and the same applies to other similar expressions. Here, an acrylate and a methacrylate may be used independently or may be used in mixture of 2 or more types.
本発明によれば、活性エネルギー線照射により硬化させた際に、反りを生じることなく、高温高湿下にさらされても基材との高い密着性を示し、さらに耐磨耗性をも有する高硬度の硬化物が得られる活性エネルギー線硬化性樹脂組成物と、その硬化物が得られる。
According to the present invention, when cured by active energy ray irradiation, it exhibits high adhesion to a substrate even when exposed to high temperature and high humidity without warping, and also has wear resistance. An active energy ray-curable resin composition capable of obtaining a hardened product and a cured product thereof are obtained.
以下、活性エネルギー線硬化性樹脂組成物を構成する各成分、同組成物を活性エネルギー線照射により硬化させることによって得られる硬化物または成型品について説明する。
Hereinafter, each component constituting the active energy ray-curable resin composition, and a cured product or molded product obtained by curing the composition by active energy ray irradiation will be described.
本発明の活性エネルギー線硬化性樹脂組成物に含まれる、複数の(メタ)アクリレート基を有するウレタン樹脂は、その重量平均分子量が、1,000~20,000の範囲にあることが好ましい。重量平均分子量が1,000未満であると、複数の(メタ)アクリレ
ート基を有するウレタン樹脂によってもたらされる架橋密度が高くなり、樹脂の硬化収縮も大きくなるため、硬化成型物の反りも大きくなる。逆に、重量平均分子量が20,00
0を超えると、粘度が高くなり、本発明の用途では適用できなくなるため、好ましくない。
また、官能基数が大きいと架橋密度が高くなるため、硬化物の反りが大きくなり、強靭性も悪くなる傾向がある。そのため、複数の(メタ)アクリレート基を有するウレタン樹脂の中でも、二官能ウレタン(メタ)アクリレート樹脂や三官能ウレタン(メタ)アクリレート樹脂が好ましい。 The urethane resin having a plurality of (meth) acrylate groups contained in the active energy ray-curable resin composition of the present invention preferably has a weight average molecular weight in the range of 1,000 to 20,000. When the weight average molecular weight is less than 1,000, the crosslink density caused by the urethane resin having a plurality of (meth) acrylate groups is increased, and the curing shrinkage of the resin is increased, so that the warpage of the cured molded product is also increased. Conversely, the weight average molecular weight is 20,000
If it exceeds 0, the viscosity becomes high, which is not preferable in the application of the present invention.
Moreover, since the crosslinking density becomes high when the number of functional groups is large, the warpage of the cured product tends to be large and the toughness tends to be poor. Therefore, among the urethane resins having a plurality of (meth) acrylate groups, a bifunctional urethane (meth) acrylate resin and a trifunctional urethane (meth) acrylate resin are preferable.
ート基を有するウレタン樹脂によってもたらされる架橋密度が高くなり、樹脂の硬化収縮も大きくなるため、硬化成型物の反りも大きくなる。逆に、重量平均分子量が20,00
0を超えると、粘度が高くなり、本発明の用途では適用できなくなるため、好ましくない。
また、官能基数が大きいと架橋密度が高くなるため、硬化物の反りが大きくなり、強靭性も悪くなる傾向がある。そのため、複数の(メタ)アクリレート基を有するウレタン樹脂の中でも、二官能ウレタン(メタ)アクリレート樹脂や三官能ウレタン(メタ)アクリレート樹脂が好ましい。 The urethane resin having a plurality of (meth) acrylate groups contained in the active energy ray-curable resin composition of the present invention preferably has a weight average molecular weight in the range of 1,000 to 20,000. When the weight average molecular weight is less than 1,000, the crosslink density caused by the urethane resin having a plurality of (meth) acrylate groups is increased, and the curing shrinkage of the resin is increased, so that the warpage of the cured molded product is also increased. Conversely, the weight average molecular weight is 20,000
If it exceeds 0, the viscosity becomes high, which is not preferable in the application of the present invention.
Moreover, since the crosslinking density becomes high when the number of functional groups is large, the warpage of the cured product tends to be large and the toughness tends to be poor. Therefore, among the urethane resins having a plurality of (meth) acrylate groups, a bifunctional urethane (meth) acrylate resin and a trifunctional urethane (meth) acrylate resin are preferable.
このような複数の(メタ)アクリレート基を有するウレタン樹脂としては、二官能では新中村化学工業製U-108A、UA-112P、UA-5201、UA-512、UA-412A、UA-4200、UA-4400、UA-340P、UA-2235PE、UA-160TM、UA-122P、UA-512、UA-W2、UA-7000、UA-7100;サートマー製CN962、CN963、CN964、CN965、CN980、CN981、CN982、CN983、CN996、CN9001、CN9002、CN9788、CN9893、CN978、CN9782、CN9783;東亞合成化学工業製M-1100、M-1200、M-1210、M-1310、M-1600;根上工業製UN-9000PEP、UN-9200A、UN-7600、UN-333、UN-1255、UN-6060PTM、UN-6060P、SH-500B;共栄社化学製AH-600、AT-600;ダイセル・サイテック製エベクリル280、エベクリル284、エベクリル402、エベクリル8402、エベクリル8807、エベクリル9270などが挙げられる。
三官能ではサートマー製CN929、CN944B85、CN989、CN9008;ダイセル・サイテック製エベクリル264、エベクリル265、エベクリル1259、エベクリル8201、KRM8296、エベクリル294/25HD、エベクリル4820等が挙げられる。四官能以上では新中村化学工業製U-6HA、U-6H、U-15HA、UA-32P、U-324A、UA-7200;サートマー製CN968、CN9006、CN9010;根上工業製UN-3320HA、UN-3320HB、UN-3320HC、UN-3320HS、UN-904、UN-901T、UN-905、UN-952;ダイセル・サイテック製エベクリル1290、エベクリル1290K、KRM8200、エベクリル5129、エベクリル8210、エベクリル8301、エベクリル8405などが挙げられる。 As such a urethane resin having a plurality of (meth) acrylate groups, bifunctional U-108A, UA-112P, UA-5201, UA-512, UA-412A, UA-4200, UA manufactured by Shin-Nakamura Chemical Co., Ltd. -4400, UA-340P, UA-2235PE, UA-160TM, UA-122P, UA-512, UA-W2, UA-7000, UA-7100; Sartomer CN962, CN963, CN964, CN965, CN980, CN981, CN982 CN983, CN996, CN9001, CN9002, CN9788, CN9873, CN978, CN9782, CN9783; Toagosei Chemical M-1100, M-1200, M-1210, M-1310, M-1600; Negami Kogyo UN-9000PEP , N-9200A, UN-7600, UN-333, UN-1255, UN-6060PTM, UN-6060P, SH-500B; Kyoeisha Chemical Co., Ltd. AH-600, AT-600; 402, Evekril 8402, Evekryl 8807, Evekrill 9270, and the like.
Trifunctional CN929, CN944B85, CN989, CN9008; Daicel-Cytec Evekril 264, Evekril 265, Evekril 1259, Evekril 8201, KRM8296, Evekril 294 / 25HD, Evekril 4820 and the like. For tetrafunctional or higher, Shin-Nakamura Chemical U-6HA, U-6H, U-15HA, UA-32P, U-324A, UA-7200; Sartomer CN968, CN9006, CN9010; Negami Kogyo UN-3320HA, UN- 3320HB, UN-3320HC, UN-3320HS, UN-904, UN-901T, UN-905, UN-952; Daicel Cytec's Evekril 1290, Evekril 1290K, KRM8200, Evekril 5129, Evekril 8210, Evekril 8305, etc. Is mentioned.
三官能ではサートマー製CN929、CN944B85、CN989、CN9008;ダイセル・サイテック製エベクリル264、エベクリル265、エベクリル1259、エベクリル8201、KRM8296、エベクリル294/25HD、エベクリル4820等が挙げられる。四官能以上では新中村化学工業製U-6HA、U-6H、U-15HA、UA-32P、U-324A、UA-7200;サートマー製CN968、CN9006、CN9010;根上工業製UN-3320HA、UN-3320HB、UN-3320HC、UN-3320HS、UN-904、UN-901T、UN-905、UN-952;ダイセル・サイテック製エベクリル1290、エベクリル1290K、KRM8200、エベクリル5129、エベクリル8210、エベクリル8301、エベクリル8405などが挙げられる。 As such a urethane resin having a plurality of (meth) acrylate groups, bifunctional U-108A, UA-112P, UA-5201, UA-512, UA-412A, UA-4200, UA manufactured by Shin-Nakamura Chemical Co., Ltd. -4400, UA-340P, UA-2235PE, UA-160TM, UA-122P, UA-512, UA-W2, UA-7000, UA-7100; Sartomer CN962, CN963, CN964, CN965, CN980, CN981, CN982 CN983, CN996, CN9001, CN9002, CN9788, CN9873, CN978, CN9782, CN9783; Toagosei Chemical M-1100, M-1200, M-1210, M-1310, M-1600; Negami Kogyo UN-9000PEP , N-9200A, UN-7600, UN-333, UN-1255, UN-6060PTM, UN-6060P, SH-500B; Kyoeisha Chemical Co., Ltd. AH-600, AT-600; 402, Evekril 8402, Evekryl 8807, Evekrill 9270, and the like.
Trifunctional CN929, CN944B85, CN989, CN9008; Daicel-Cytec Evekril 264, Evekril 265, Evekril 1259, Evekril 8201, KRM8296, Evekril 294 / 25HD, Evekril 4820 and the like. For tetrafunctional or higher, Shin-Nakamura Chemical U-6HA, U-6H, U-15HA, UA-32P, U-324A, UA-7200; Sartomer CN968, CN9006, CN9010; Negami Kogyo UN-3320HA, UN- 3320HB, UN-3320HC, UN-3320HS, UN-904, UN-901T, UN-905, UN-952; Daicel Cytec's Evekril 1290, Evekril 1290K, KRM8200, Evekril 5129, Evekril 8210, Evekril 8305, etc. Is mentioned.
これらの複数の(メタ)アクリレート基を有するウレタン樹脂は、単独あるいは2種以上を混合して用いても良い。
These urethane resins having a plurality of (meth) acrylate groups may be used alone or in admixture of two or more.
本発明の活性エネルギー線硬化性樹脂組成物に含まれる感光性アミド及び感光性アミドの誘導体には、例えばN-ビニルホルムアミド、N-ビニルアセトアミド、(メタ)アクリルアミド、N-メチル(メタ)アクリルアミド、N,N-ジメチル(メタ)アクリルアミド、N-n-プロピル(メタ)アクリルアミド、N-イソプロピル(メタ)アクリルアミド、N,N-ジエチル(メタ)アクリルアミド、N-メチル-N-n-プロピル(メタ)アクリルアミド、N-メチル-N-イソプロピル(メタ)アクリルアミド、N-テトラヒドロフルフリル(メタ)アクリルアミド、N-エトキシプロピル(メタ)アクリルアミド、N-エトキシエチル(メタ)アクリルアミド、N-1-メチル-2-メトキシエチル(メタ)アクリルアミド、N-モルホリノプロピル(メタ)アクリルアミド、N-メトキシプロピル(メタ)アクリルアミド、N-イソプロポキシプロピル(メタ)アクリルアミド、N-イソプロポキシエチル(メタ)アクリルアミド、N-ヒドロキシメチル(メタ)アクリルアミド、N-(2-ヒドロキシエチル)(メタ)アクリルアミド、N-(3-ヒドロキシプロピル)(メタ)アクリルアミドなどの脂肪族感光性アミド化合物、N-ビニルカプロラクタム、N-ビニルピロリドン、N-(メタ)アクリロイルモルホリン、N-(メタ)アクリロイルピペリジン、などの環状感光性アミド化合物などが挙げられる。好ましくは、(メタ)アクリロイル基を有する感光性アミド化合物であり、なかでもN-(2-ヒドロキシエチル)アクリルアミド、N-(2-ヒドロキシエチル)メタアクリルアミド、N-アクリロイルモルホリン、N-メタクリロイルモルホリン、の少なくとも1種が透明性の点からも特に好ましい。
Examples of the photosensitive amide and the photosensitive amide derivative contained in the active energy ray-curable resin composition of the present invention include N-vinylformamide, N-vinylacetamide, (meth) acrylamide, N-methyl (meth) acrylamide, N, N-dimethyl (meth) acrylamide, Nn-propyl (meth) acrylamide, N-isopropyl (meth) acrylamide, N, N-diethyl (meth) acrylamide, N-methyl-Nn-propyl (meth) Acrylamide, N-methyl-N-isopropyl (meth) acrylamide, N-tetrahydrofurfuryl (meth) acrylamide, N-ethoxypropyl (meth) acrylamide, N-ethoxyethyl (meth) acrylamide, N-1-methyl-2- Methoxyethyl (meth) acrylamide, N Morpholinopropyl (meth) acrylamide, N-methoxypropyl (meth) acrylamide, N-isopropoxypropyl (meth) acrylamide, N-isopropoxyethyl (meth) acrylamide, N-hydroxymethyl (meth) acrylamide, N- (2- Aliphatic photosensitive amide compounds such as hydroxyethyl) (meth) acrylamide, N- (3-hydroxypropyl) (meth) acrylamide, N-vinylcaprolactam, N-vinylpyrrolidone, N- (meth) acryloylmorpholine, N- ( And cyclic photosensitive amide compounds such as (meth) acryloylpiperidine. Preferably, it is a photosensitive amide compound having a (meth) acryloyl group, among which N- (2-hydroxyethyl) acrylamide, N- (2-hydroxyethyl) methacrylamide, N-acryloylmorpholine, N-methacryloylmorpholine, At least one of these is particularly preferred from the viewpoint of transparency.
このような感光性アミド及び/又は感光性アミドの誘導体は、複数の(メタ)アクリレート基を有するウレタン樹脂100質量部に対して、好ましくは15~200質量部、より好ましくは20~100質量部の割合で含まれる。この感光性アミド及び/又は感光性アミドの誘導体の配合量が15質量部未満であると密着性が不足し、一方200質量部を超えると硬化物が脆くなる傾向にあり好ましくない。
Such a photosensitive amide and / or a derivative of the photosensitive amide is preferably 15 to 200 parts by mass, more preferably 20 to 100 parts by mass with respect to 100 parts by mass of the urethane resin having a plurality of (meth) acrylate groups. Included in the ratio. If the blending amount of the photosensitive amide and / or the derivative of the photosensitive amide is less than 15 parts by mass, the adhesion is insufficient, while if it exceeds 200 parts by mass, the cured product tends to become brittle.
本発明の活性エネルギー線硬化性樹脂組成物に含まれる二重結合を有さない環状骨格を有する二官能(メタ)アクリレートは、二重結合を有さないことにより、反りを防止し、環状骨格を有することにより耐磨耗性を強化することができる。その重量平均分子量は、150~2,000の範囲にあることが好ましい。重量平均分子量が150未満であると、複数の(メタ)アクリレート基を有するウレタン樹脂によってもたらされる架橋密度が高くなり、樹脂の硬化収縮が大きくなるため、硬化成型物の反りも大きくなる。重量平均分子量が2,000を超えると、粘度が高くなり、本発明の用途では適用できなくなるおそれがあり好ましくない。
The bifunctional (meth) acrylate having a cyclic skeleton having no double bond, which is contained in the active energy ray-curable resin composition of the present invention, prevents warpage by having no double bond, thereby preventing the cyclic skeleton. By having, it is possible to enhance the wear resistance. The weight average molecular weight is preferably in the range of 150 to 2,000. When the weight average molecular weight is less than 150, the crosslinking density caused by the urethane resin having a plurality of (meth) acrylate groups is increased, and the curing shrinkage of the resin is increased, so that the warpage of the cured molded product is also increased. When the weight average molecular weight exceeds 2,000, the viscosity becomes high, which may not be applicable in the application of the present invention.
この二重結合を有さない環状骨格を有する二官能(メタ)アクリレートには、例えばシクロヘキサンジメタノールジ(メタ)アクリレート、トリシクロデンカンジメタノールジ(メタ)アクリレート、水素化ビスフェノールAジ(メタ)アクリレート、水素化ビスフェノールFジ(メタ)アクリレート、水素化ヘキサフルオロビスフェノールAジ(メタ)アクリレート、ビス(2-(メタ)アクリロイルオキシ)ヘキサヒドロフタル酸などの脂環骨格を有する二官能(メタ)アクリレートや、5-エチル-2-(2-ヒドロキシ-1,1ジメチルエチル)-5-(ヒドロキシメチル)-1,3-ジオキサンジアクリレート、1,4-ジ(メタ)アクリロイルピペラジンなどの複素環骨格を有する二官能(メタ)アクリレート、さらにはこれら二官能(メタ)アクリレートのエチレンオキシド、プロピレンオキシド、カプロラクトンなどの変性物が挙げられる。
Examples of the bifunctional (meth) acrylate having a cyclic skeleton having no double bond include cyclohexanedimethanol di (meth) acrylate, tricyclodencan dimethanol di (meth) acrylate, and hydrogenated bisphenol A di (meth). Bifunctional (meth) having an alicyclic skeleton such as acrylate, hydrogenated bisphenol F di (meth) acrylate, hydrogenated hexafluorobisphenol A di (meth) acrylate, bis (2- (meth) acryloyloxy) hexahydrophthalic acid Heterocycles such as acrylate, 5-ethyl-2- (2-hydroxy-1,1dimethylethyl) -5- (hydroxymethyl) -1,3-dioxane diacrylate, 1,4-di (meth) acryloylpiperazine Bifunctional (meth) acrylate with skeleton, and even this Difunctional (meth) acrylates of ethylene oxide, propylene oxide, and a modified product such as caprolactone.
このような二重結合を有さない環状骨格を有する二官能(メタ)アクリレートは、複数の(メタ)アクリレート基を有するウレタン樹脂100質量部に対して、好ましくは10~100質量部、より好ましくは20~50質量部の割合で含まれる。この二重結合を有さない環状骨格を有する二官能(メタ)アクリレートの配合量が、10質量部未満であると耐磨耗性が不足し、一方100質量部を超えると硬化物が脆くなる傾向にあり好ましくない。
The bifunctional (meth) acrylate having a cyclic skeleton having no double bond is preferably 10 to 100 parts by mass, more preferably 100 parts by mass with respect to 100 parts by mass of the urethane resin having a plurality of (meth) acrylate groups. Is contained in a proportion of 20 to 50 parts by mass. When the blending amount of the bifunctional (meth) acrylate having a cyclic skeleton having no double bond is less than 10 parts by mass, the abrasion resistance is insufficient, and when it exceeds 100 parts by mass, the cured product becomes brittle. It tends to be unfavorable.
本発明の活性エネルギー線硬化性樹脂組成物中に含まれる光重合開始剤としては、例えば、ベンゾイン、ベンジル、ベンゾインメチルエーテル、ベンゾインエチルエーテル、ベンゾインn-プロピルエーテル、ベンゾインイソプロピルエーテル、ベンゾインn-ブチルエーテルなどのベンゾイン類;ベンゾインアルキルエーテル類;ベンゾフェノン、p-メチルベンゾフェノン、ミヒラーズケトン、メチルベンゾフェノン、4,4’-ジクロロベンゾフェノン、4,4’-ビスジエチルアミノベンゾフェノンなどのベンゾフェノン類;アセトフェノン、2,2-ジメトキシ-2-フェニルアセトフェノン、2,2-ジエトキシ-2-フェニルアセトフェノン、1,1-ジクロロアセトフェノン、1-ヒドロキシシクロヘキシルフェニルケトン、2-メチル-[4-(メチルチオ)フェニル]-2-モルフォリノ-1-プロパノン、N,N-ジメチルアミノアセトフェノンなどのアセトフェノン類;2,4-ジメチルチオキサントン、2,4-ジエチルチオキサントン、2-クロロチオキサントン、2,4-ジイソプロピルチオキサントンなどのチオキサントン類;アントラキノン、クロロアントラキノン、2-メチルアントラキノン、2-エチルアントラキノン、2-tert-ブチルアントラキノン、1-クロロアントラキノン、2-アミルアントラキノン、2-アミノアントラキノンなどのアントラキノン類;アセトフェノンジメチルケタール、ベンジルジメチルケタールなどのケタール類;エチル-4-ジメチルアミノベンゾエート、2-(ジメチルアミノ)エチルベンゾエート、p-ジメチル安息香酸エチルエステルなどの安息香酸エステル類;フェニルジスルフィド2-ニトロフルオレン、ブチロイン、アニソインエチルエーテル、アゾビスイソブチロニトリル、テトラメチルチウラムジスルフィド等を挙げることができる。
Examples of the photopolymerization initiator contained in the active energy ray-curable resin composition of the present invention include benzoin, benzyl, benzoin methyl ether, benzoin ethyl ether, benzoin n-propyl ether, benzoin isopropyl ether, and benzoin n-butyl ether. Benzoins such as benzoin; benzoin alkyl ethers; benzophenones such as benzophenone, p-methylbenzophenone, Michler's ketone, methylbenzophenone, 4,4′-dichlorobenzophenone, 4,4′-bisdiethylaminobenzophenone; acetophenone, 2,2-dimethoxy -2-phenylacetophenone, 2,2-diethoxy-2-phenylacetophenone, 1,1-dichloroacetophenone, 1-hydroxycyclohexyl phenylketo Acetophenones such as 2-methyl- [4- (methylthio) phenyl] -2-morpholino-1-propanone and N, N-dimethylaminoacetophenone; 2,4-dimethylthioxanthone, 2,4-diethylthioxanthone, 2- Thioxanthones such as chlorothioxanthone and 2,4-diisopropylthioxanthone; anthraquinone, chloroanthraquinone, 2-methylanthraquinone, 2-ethylanthraquinone, 2-tert-butylanthraquinone, 1-chloroanthraquinone, 2-amylanthraquinone, 2-aminoanthraquinone Anthraquinones such as acetophenone ketals such as dimethyl ketal and benzyl dimethyl ketal; ethyl-4-dimethylaminobenzoate, 2- (dimethylamino) ethylbenzo Over DOO, p- dimethylbenzoic acid ethyl ester benzoic acid esters such as; phenyl disulfide 2-nitrofluorene, butyroin, anisoin ethyl ether, azobisisobutyronitrile, can be mentioned tetramethylthiuram disulfide or the like.
これらの光重合開始材は、1種もしくは2種以上を組み合わせて用いることができる。この光重合開始剤は、複数の(メタ)アクリレート基を有するウレタン樹脂100質量部に対して、好ましくは0.1~10質量部、より好ましくは0.3~5質量部の割合で含まれる。
These photopolymerization initiators can be used alone or in combination of two or more. This photopolymerization initiator is contained in a proportion of preferably 0.1 to 10 parts by mass, more preferably 0.3 to 5 parts by mass with respect to 100 parts by mass of the urethane resin having a plurality of (meth) acrylate groups. .
また、本発明の活性エネルギー線硬化性樹脂組成物には、さらに硬化物の密着性、機械的強度、線膨張係数などの特性を向上させる目的で、必要により無機充填材を配合することができる。無機充填材として、例えば、硫酸バリウム、チタン酸バリウム、酸化ケイ素粉、微粉状酸化ケイ素、無定形シリカ、タルク、クレー、炭酸マグネシウム、炭酸カルシウム、酸化アルミニウム、水酸化アルミニウム、雲母粉などの公知慣用の無機充填剤が使用できる。その配合比率は本活性エネルギー線硬化性樹脂組成物100質量部に対して10~100質量部が好適である。
Further, the active energy ray-curable resin composition of the present invention can be blended with an inorganic filler as necessary for the purpose of further improving properties such as adhesion, mechanical strength, and linear expansion coefficient of the cured product. . Known inorganic fillers such as barium sulfate, barium titanate, silicon oxide powder, finely divided silicon oxide, amorphous silica, talc, clay, magnesium carbonate, calcium carbonate, aluminum oxide, aluminum hydroxide, mica powder, etc. Inorganic fillers can be used. The blending ratio is preferably 10 to 100 parts by mass with respect to 100 parts by mass of the present active energy ray-curable resin composition.
本発明の活性エネルギー線硬化性樹脂組成物には、さらに必要に応じてフタロシアニン・ブルー、フタロシアニン・グリーン、アイオジン・グリーン、ジスアゾイエロー、クリスタルバイオレット、酸化チタン、カーボンブラック、ナフタレンブラックなどの公知慣用の着色剤、ハイドロキノン、ハイドロキノンモノメチルエーテル、t-ブチルカテコール、ピロガロール、フェノチアジンなどの公知慣用の光重合禁止剤、微粉シリカ、有機ベントナイト、モンモリロナイトなどの公知慣用の増粘剤、シリコーン系、フッ素系、高分子系などの消泡剤及び/又はレベリング剤、イミダゾール系、チアゾール系、トリアゾール系等のシランカップリング剤などのような公知慣用の添加剤類を配合することができる。
In the active energy ray-curable resin composition of the present invention, a known and commonly used phthalocyanine blue, phthalocyanine green, iodin green, disazo yellow, crystal violet, titanium oxide, carbon black, naphthalene black, etc. Colorants, known and conventional photopolymerization inhibitors such as hydroquinone, hydroquinone monomethyl ether, t-butylcatechol, pyrogallol and phenothiazine, known and conventional thickeners such as finely divided silica, organic bentonite and montmorillonite, silicone-based, fluorine-based, high Known and commonly used additives such as defoamers and / or leveling agents such as molecular systems, silane coupling agents such as imidazole systems, thiazole systems, and triazole systems can be blended.
本発明の活性エネルギー線硬化性樹脂組成物は、1~50dPa・sの粘度を有することが好ましい。この粘度は、複数の(メタ)アクリレート基を有するウレタン樹脂100質量部に対して、好ましくは1~100質量部、より好ましくは10~50質量部の範囲内で反応性希釈剤の量を調節することにより提供することができる。反応性希釈剤量が1質量部以下では粘度が高く作業性が低下し、一方、100質量部以上では硬化物の架橋密度が低下し、十分な耐磨耗性が得られなくなるので好ましくない。
The active energy ray-curable resin composition of the present invention preferably has a viscosity of 1 to 50 dPa · s. This viscosity is preferably adjusted in the range of 1 to 100 parts by mass, more preferably 10 to 50 parts by mass with respect to 100 parts by mass of the urethane resin having a plurality of (meth) acrylate groups. Can be provided. If the amount of reactive diluent is 1 part by mass or less, the viscosity is high and workability is lowered. On the other hand, if it is 100 parts by mass or more, the crosslink density of the cured product is reduced and sufficient wear resistance cannot be obtained.
反応性希釈剤には公知慣用のものを用いることができる。例えば、2-ヒドロキシエチルアクリレート、2-ヒドロキシプロピルアクリレート、4-ヒドロキシブチルアクリレートなどのヒドロキシアルキルアクリレート類;イソボロニルアクリレート、テトラヒドロフルフリルアクリレート、N-アクリロイルモルホリン、N-ビニルピロリジノンなどの環状骨格を有する単官能感光性モノマー類;エチレングリコール、メトキシテトラエチレングリコール、ポリエチレングリコール、プロピレングリコールなどのグリコールのモノ又はジアクリレート類;N,N-ジメチルアクリルアミド、N-メチロールアクリルアミド、N,N-ジメチルアミノプロピルアクリルアミドなどのアクリルアミド類;N,N-ジメチルアミノエチルアクリレート、N,N-ジメチルアミノプロピルアクリレートなどのアミノアルキルアクリレート類;ヘキサンジオール、トリメチロールプロパン、ペンタエリスリトール、ジペンタエリスリトール、トリス-ヒドロキシエチルイソシアヌレートなどの多価アルコール又はこれらのエチレオキサイド付加物もしくはプロピレンオキサイド付加物などの多価アクリレート類;フェノキシアクリレート、ビスフェノールAジアクリレート、及びこれらのフェノール類のエチレンオキサイド付加物もしくはプロピレンオキサイド付加物などのアクリレート類;グリセリンジグリシジルエーテル、グリセリントリグリシジルエーテル、トリメチロールプロパントリグリシジルエーテル、トリグリシジルイソシアヌレートなどのグリシジルエーテルのアクリレート類;及びメラミンアクリレート、及び/又は上記アクリレートに対応する各メタクリレート類などが挙げられる。これらの中で、特に分子中に1個のエチレン性不飽和基を有する化合物である単官能(メタ)アクリレート化合物が、希釈効果が高く好ましい。
As the reactive diluent, a conventionally known one can be used. For example, hydroxyalkyl acrylates such as 2-hydroxyethyl acrylate, 2-hydroxypropyl acrylate, and 4-hydroxybutyl acrylate; cyclic skeletons such as isobornyl acrylate, tetrahydrofurfuryl acrylate, N-acryloylmorpholine, and N-vinylpyrrolidinone. Monofunctional photosensitive monomers having; mono- or diacrylates of glycols such as ethylene glycol, methoxytetraethylene glycol, polyethylene glycol, propylene glycol; N, N-dimethylacrylamide, N-methylolacrylamide, N, N-dimethylaminopropyl Acrylamides such as acrylamide; N, N-dimethylaminoethyl acrylate, N, N-dimethylaminopropyl acrylate A polyhydric alcohol such as hexanediol, trimethylolpropane, pentaerythritol, dipentaerythritol, tris-hydroxyethyl isocyanurate, or a polyhydric acrylate such as an ethylene oxide adduct or a propylene oxide adduct; Phenoxyacrylate, bisphenol A diacrylate, and acrylates such as ethylene oxide adducts or propylene oxide adducts of these phenols; glycerin diglycidyl ether, glycerin triglycidyl ether, trimethylolpropane triglycidyl ether, triglycidyl isocyanurate, etc. Acrylates of glycidyl ethers; and melamine acrylates and / or Etc. Each methacrylates corresponding to the rate and the like. Among these, a monofunctional (meth) acrylate compound which is a compound having one ethylenically unsaturated group in the molecule is particularly preferable because of its high dilution effect.
本発明にかかる活性エネルギー線硬化性樹脂組成物は、下記の塗布方法で基材などに塗布後、活性エネルギー線を照射し、硬化する。なお、この硬化物又は成型物は、反り防止の点から、デュロメータ硬さDタイプが75°以上であることが好ましい。
The active energy ray-curable resin composition according to the present invention is cured by irradiating active energy rays after being applied to a substrate or the like by the following application method. The cured product or molded product preferably has a durometer hardness D type of 75 ° or more from the viewpoint of warpage prevention.
塗布方法は、ディップコート法、フローコート法、ロールコート法、バーコーター法、スクリーン印刷法、カーテンコート法、グラビア印刷法、オフセット印刷法等の任意の方法を適用することができる。
As the coating method, any method such as a dip coating method, a flow coating method, a roll coating method, a bar coater method, a screen printing method, a curtain coating method, a gravure printing method, and an offset printing method can be applied.
活性エネルギー線の照射光源としては、低圧水銀灯、中圧水銀灯、高圧水銀灯、超高圧水銀灯、キセノンランプ又はメタルハライドランプなどが適当である。その他、レーザー光線、電子線なども活性エネルギー線として利用できる。
As the irradiation source of the active energy ray, a low pressure mercury lamp, a medium pressure mercury lamp, a high pressure mercury lamp, an ultrahigh pressure mercury lamp, a xenon lamp or a metal halide lamp is suitable. In addition, laser beams, electron beams, and the like can be used as active energy rays. *
ここで、凹版を用いて本発明の活性エネルギー線硬化性樹脂組成物を充填し、充填した樹脂組成物を活性エネルギー線により硬化させ、その硬化物を凹部から取り出すことにより成型品を得る方法について例示する。
Here, a method for obtaining a molded product by filling the active energy ray-curable resin composition of the present invention using an intaglio, curing the filled resin composition with active energy rays, and taking out the cured product from the recess. Illustrate.
図1には、表面に所望の形状が形成された版体を示している。一般的には、ステンレスなどの金属製のものが用いられる。
FIG. 1 shows a plate having a desired shape formed on the surface. Generally, a metal such as stainless steel is used.
図2には、この版体に本発明の活性エネルギー線硬化性樹脂組成物を充填する状態を示している。一般的には、ドクターナイフなどを用いて活性エネルギー線硬化性樹脂組成物を版体の凹部へ充填する。
FIG. 2 shows a state in which this plate is filled with the active energy ray-curable resin composition of the present invention. In general, the active energy ray-curable resin composition is filled into the concave portion of the plate using a doctor knife or the like.
図3には、その上部から基材を被せ、さらにその上部の光源から紫外線を照射して本発明の活性エネルギー線硬化性樹脂組成物を硬化させる状態を示している。一般的に、基材には紫外線を透過する透明なポリエチレンテレフタレートやポリカーボネート製のフィルムが用いられる。
FIG. 3 shows a state in which the active energy ray-curable resin composition of the present invention is cured by covering the base material from above and further irradiating ultraviolet light from the light source on the top. Generally, a transparent polyethylene terephthalate or polycarbonate film that transmits ultraviolet rays is used as the substrate.
図4には、硬化物を版体から離型し成型物を得る状態を示している。図1から図4の一連の工程は、専用の装置を用いることで、連続的に成型品を製造することができる。
FIG. 4 shows a state in which the cured product is released from the plate to obtain a molded product. In the series of steps shown in FIGS. 1 to 4, a molded product can be continuously produced by using a dedicated apparatus.
以下に実施例および比較例を示して本発明について具体的に説明するが、本発明が下記実施例に限定されるものではないことはもとよりである。
Hereinafter, the present invention will be described in detail with reference to examples and comparative examples, but the present invention is not limited to the following examples.
(活性エネルギー線硬化性樹脂組成物の調製)
上述の通り作製した樹脂に、表1と表2に示す成分を同表に示す組成で配合し、攪拌して溶解させ、活性エネルギー線硬化性樹脂組成物を調製した。 (Preparation of active energy ray-curable resin composition)
The components shown in Tables 1 and 2 were blended in the resins prepared as described above in the compositions shown in the same table and dissolved by stirring to prepare active energy ray-curable resin compositions.
上述の通り作製した樹脂に、表1と表2に示す成分を同表に示す組成で配合し、攪拌して溶解させ、活性エネルギー線硬化性樹脂組成物を調製した。 (Preparation of active energy ray-curable resin composition)
The components shown in Tables 1 and 2 were blended in the resins prepared as described above in the compositions shown in the same table and dissolved by stirring to prepare active energy ray-curable resin compositions.
なお、表1と表2の成分は次の通りである。
EBECRYL 8807:脂肪族二官能ウレタンアクリレート(ダイセル・サイテック社製)
CN929:脂肪族三官能ウレタンアクリレート(サートマー社製)
KAYARAD RM-1001:N-アクリロイルモルホリン(日本化薬社製)
HEAA:N-(2-ヒドロキシエチル)アクリルアミド(興人社製)
ビームセット770:N-ビニルホルムアミド(荒川化学工業社製)
アロニックスM-111:エチレンオキシド1モル付加ノニルフェノールアクリレート(東亞合成社製)
ライトアクリレートIB-XA:イソボロニルアクリレート(共栄社化学社製)
4HBA:4-ヒドロキシブチルアクリレート(日本化成社製)
アロニックスM-5700:2-ヒドロキシ-3-フェノキシプロピルアクリレート(東亞合成社製)
NKエステルA-DCP:トリシクロデカンジメタノールジアクリレート(新中村化学工業社製)
CD406:シクロヘキサンジメタノールジアクリレート(サートマー社製)
ネオマーBA-641:エチレンオキシド4モル付加ビスフェノールA型ジアクリレート(三洋化成工業社製)
イルガキュア184:1-ヒドロキシ-シクロヘキシルフェニルケトン(チバ・ジャパン社製) The components in Tables 1 and 2 are as follows.
EBECRYL 8807: Aliphatic bifunctional urethane acrylate (manufactured by Daicel-Cytec)
CN929: aliphatic trifunctional urethane acrylate (manufactured by Sartomer)
KAYARAD RM-1001: N-acryloylmorpholine (manufactured by Nippon Kayaku Co., Ltd.)
HEAA: N- (2-hydroxyethyl) acrylamide (manufactured by Kojin Co., Ltd.)
Beam set 770: N-vinylformamide (Arakawa Chemical Industries)
Aronix M-111: 1 mol of ethylene oxide addition nonylphenol acrylate (manufactured by Toagosei Co., Ltd.)
Light acrylate IB-XA: Isoboronyl acrylate (manufactured by Kyoeisha Chemical Co., Ltd.)
4HBA: 4-hydroxybutyl acrylate (manufactured by Nippon Kasei Co., Ltd.)
Aronix M-5700: 2-hydroxy-3-phenoxypropyl acrylate (manufactured by Toagosei Co., Ltd.)
NK ester A-DCP: Tricyclodecane dimethanol diacrylate (manufactured by Shin-Nakamura Chemical Co., Ltd.)
CD406: Cyclohexanedimethanol diacrylate (manufactured by Sartomer)
Neomer BA-641: Ethylene oxide 4 mol addition bisphenol A diacrylate (manufactured by Sanyo Chemical Industries)
Irgacure 184: 1-hydroxy-cyclohexyl phenyl ketone (manufactured by Ciba Japan)
EBECRYL 8807:脂肪族二官能ウレタンアクリレート(ダイセル・サイテック社製)
CN929:脂肪族三官能ウレタンアクリレート(サートマー社製)
KAYARAD RM-1001:N-アクリロイルモルホリン(日本化薬社製)
HEAA:N-(2-ヒドロキシエチル)アクリルアミド(興人社製)
ビームセット770:N-ビニルホルムアミド(荒川化学工業社製)
アロニックスM-111:エチレンオキシド1モル付加ノニルフェノールアクリレート(東亞合成社製)
ライトアクリレートIB-XA:イソボロニルアクリレート(共栄社化学社製)
4HBA:4-ヒドロキシブチルアクリレート(日本化成社製)
アロニックスM-5700:2-ヒドロキシ-3-フェノキシプロピルアクリレート(東亞合成社製)
NKエステルA-DCP:トリシクロデカンジメタノールジアクリレート(新中村化学工業社製)
CD406:シクロヘキサンジメタノールジアクリレート(サートマー社製)
ネオマーBA-641:エチレンオキシド4モル付加ビスフェノールA型ジアクリレート(三洋化成工業社製)
イルガキュア184:1-ヒドロキシ-シクロヘキシルフェニルケトン(チバ・ジャパン社製) The components in Tables 1 and 2 are as follows.
EBECRYL 8807: Aliphatic bifunctional urethane acrylate (manufactured by Daicel-Cytec)
CN929: aliphatic trifunctional urethane acrylate (manufactured by Sartomer)
KAYARAD RM-1001: N-acryloylmorpholine (manufactured by Nippon Kayaku Co., Ltd.)
HEAA: N- (2-hydroxyethyl) acrylamide (manufactured by Kojin Co., Ltd.)
Beam set 770: N-vinylformamide (Arakawa Chemical Industries)
Aronix M-111: 1 mol of ethylene oxide addition nonylphenol acrylate (manufactured by Toagosei Co., Ltd.)
Light acrylate IB-XA: Isoboronyl acrylate (manufactured by Kyoeisha Chemical Co., Ltd.)
4HBA: 4-hydroxybutyl acrylate (manufactured by Nippon Kasei Co., Ltd.)
Aronix M-5700: 2-hydroxy-3-phenoxypropyl acrylate (manufactured by Toagosei Co., Ltd.)
NK ester A-DCP: Tricyclodecane dimethanol diacrylate (manufactured by Shin-Nakamura Chemical Co., Ltd.)
CD406: Cyclohexanedimethanol diacrylate (manufactured by Sartomer)
Neomer BA-641: Ethylene oxide 4 mol addition bisphenol A diacrylate (manufactured by Sanyo Chemical Industries)
Irgacure 184: 1-hydroxy-cyclohexyl phenyl ketone (manufactured by Ciba Japan)
こうして調製した活性エネルギー線硬化性樹脂組成物について、反り、密着性、RCA磨耗性を以下の評価方法で評価した。その結果を表3と4に示す。
The active energy ray-curable resin composition thus prepared was evaluated for warpage, adhesion, and RCA wear by the following evaluation methods. The results are shown in Tables 3 and 4.
(反り試験用硬化塗膜作製方法)
樹脂組成物を縦2cm、横2cm、深さ1mmのテフロン(登録商標)製型に注ぎ、その上を易接着処理済PETフィルムで覆い、UVコンベア炉(メタルハライドランプ、80W、3灯)を用いて積算光量1000mJ/cm2で露光し、離型して硬化塗膜を得た。 (Method for preparing cured coating film for warpage test)
The resin composition is poured into a Teflon (registered trademark) mold having a length of 2 cm, a width of 2 cm, and a depth of 1 mm, covered with an easily adhesive-treated PET film, and a UV conveyor furnace (metal halide lamp, 80 W, 3 lights) is used. The film was exposed at an integrated light quantity of 1000 mJ / cm 2 and released from the mold to obtain a cured coating film.
樹脂組成物を縦2cm、横2cm、深さ1mmのテフロン(登録商標)製型に注ぎ、その上を易接着処理済PETフィルムで覆い、UVコンベア炉(メタルハライドランプ、80W、3灯)を用いて積算光量1000mJ/cm2で露光し、離型して硬化塗膜を得た。 (Method for preparing cured coating film for warpage test)
The resin composition is poured into a Teflon (registered trademark) mold having a length of 2 cm, a width of 2 cm, and a depth of 1 mm, covered with an easily adhesive-treated PET film, and a UV conveyor furnace (metal halide lamp, 80 W, 3 lights) is used. The film was exposed at an integrated light quantity of 1000 mJ / cm 2 and released from the mold to obtain a cured coating film.
(反り試験)
上記方法にて作製した硬化塗膜を、反りを生じた辺が上を向くように平らな台に置き、反りのある一辺を指で台に押さえつけて、反対側の浮き上がった辺の台からの高さを読み取り、反りの大きさを測定した。
○:硬化塗膜の反りが5mm未満
△:硬化塗膜の反りが5mm以上10mm未満
×:硬化塗膜の反りが10mm以上 (Warp test)
Place the cured coating film produced by the above method on a flat base so that the warped side faces upward, press one side with the warp against the base with your finger, and lift it from the base on the opposite side. The height was read and the warpage was measured.
○: Warpage of cured coating film is less than 5 mm Δ: Curing of cured coating film is 5 mm or more and less than 10 mm ×: Curing of cured coating film is 10 mm or more
上記方法にて作製した硬化塗膜を、反りを生じた辺が上を向くように平らな台に置き、反りのある一辺を指で台に押さえつけて、反対側の浮き上がった辺の台からの高さを読み取り、反りの大きさを測定した。
○:硬化塗膜の反りが5mm未満
△:硬化塗膜の反りが5mm以上10mm未満
×:硬化塗膜の反りが10mm以上 (Warp test)
Place the cured coating film produced by the above method on a flat base so that the warped side faces upward, press one side with the warp against the base with your finger, and lift it from the base on the opposite side. The height was read and the warpage was measured.
○: Warpage of cured coating film is less than 5 mm Δ: Curing of cured coating film is 5 mm or more and less than 10 mm ×: Curing of cured coating film is 10 mm or more
[試験方法]
(密着性及びRCA磨耗性試験用硬化物作製方法)
樹脂組成物を、バーコーターを用いて易接着処理済PETに50μmの厚みで塗布し、
上から未処理PETで覆い、UVコンベア炉(高圧水銀灯、80W、3灯)を用いて露光量1000mJ/cm2で露光し、未処理PETを剥がして硬化塗膜を得た。 [Test method]
(Method for preparing cured product for adhesion and RCA abrasion test)
The resin composition was applied to the easy-adhesion-treated PET with a thickness of 50 μm using a bar coater,
It was covered with untreated PET from above, exposed to an exposure amount of 1000 mJ / cm 2 using a UV conveyor furnace (high pressure mercury lamp, 80 W, 3 lamps), and untreated PET was peeled off to obtain a cured coating film.
(密着性及びRCA磨耗性試験用硬化物作製方法)
樹脂組成物を、バーコーターを用いて易接着処理済PETに50μmの厚みで塗布し、
上から未処理PETで覆い、UVコンベア炉(高圧水銀灯、80W、3灯)を用いて露光量1000mJ/cm2で露光し、未処理PETを剥がして硬化塗膜を得た。 [Test method]
(Method for preparing cured product for adhesion and RCA abrasion test)
The resin composition was applied to the easy-adhesion-treated PET with a thickness of 50 μm using a bar coater,
It was covered with untreated PET from above, exposed to an exposure amount of 1000 mJ / cm 2 using a UV conveyor furnace (high pressure mercury lamp, 80 W, 3 lamps), and untreated PET was peeled off to obtain a cured coating film.
(密着性試験)
上記の方法で作製した硬化塗膜を85℃85%RHの恒温槽に10日間保管し、その後取り出して室温に戻す。室温に戻ったら、JIS K 5600-5-6に従って碁盤目状にクロスカットを入れ、次いでセロハン粘着テープによるピーリングテスト後の碁盤目の残り数を以下の基準で評価した。
○:碁盤目の残り数が70以上100以下
△:碁盤目の残り数が30以上70未満
×:碁盤目の残り数が0以上30未満 (Adhesion test)
The cured coating film produced by the above method is stored in a constant temperature bath at 85 ° C. and 85% RH for 10 days, and then taken out and returned to room temperature. When the temperature returned to room temperature, cross cuts were made in a grid pattern according to JIS K 5600-5-6, and the remaining number of grids after the peeling test using cellophane adhesive tape was evaluated according to the following criteria.
○: Remaining number of grids 70 to 100: Δ: Remaining number of grids 30 to less than 70 ×: Remaining number of grids 0 to 30
上記の方法で作製した硬化塗膜を85℃85%RHの恒温槽に10日間保管し、その後取り出して室温に戻す。室温に戻ったら、JIS K 5600-5-6に従って碁盤目状にクロスカットを入れ、次いでセロハン粘着テープによるピーリングテスト後の碁盤目の残り数を以下の基準で評価した。
○:碁盤目の残り数が70以上100以下
△:碁盤目の残り数が30以上70未満
×:碁盤目の残り数が0以上30未満 (Adhesion test)
The cured coating film produced by the above method is stored in a constant temperature bath at 85 ° C. and 85% RH for 10 days, and then taken out and returned to room temperature. When the temperature returned to room temperature, cross cuts were made in a grid pattern according to JIS K 5600-5-6, and the remaining number of grids after the peeling test using cellophane adhesive tape was evaluated according to the following criteria.
○: Remaining number of grids 70 to 100: Δ: Remaining number of grids 30 to less than 70 ×: Remaining number of grids 0 to 30
(RCA磨耗試験)
上記の方法で作製した硬化塗膜をNORMAN TOOL,INC.製RCA磨耗試験機及びRCA磨耗試験機用専用磨耗紙を用いて175gの荷重で50回磨耗し、その後の硬化塗膜表面の磨耗度合いを光学顕微鏡で観察した。
○:ほぼ磨耗していない
△:若干の磨耗あり
×:明らかな磨耗あり (RCA wear test)
The cured coating film produced by the above method was manufactured by NORMAN TOOL, INC. Using the RCA abrasion tester manufactured by the manufacturer and a dedicated abrasion paper for the RCA abrasion tester, it was worn 50 times under a load of 175 g, and the degree of wear of the cured coating film surface was observed with an optical microscope.
○: Almost no wear △: Some wear ×: Clear wear
上記の方法で作製した硬化塗膜をNORMAN TOOL,INC.製RCA磨耗試験機及びRCA磨耗試験機用専用磨耗紙を用いて175gの荷重で50回磨耗し、その後の硬化塗膜表面の磨耗度合いを光学顕微鏡で観察した。
○:ほぼ磨耗していない
△:若干の磨耗あり
×:明らかな磨耗あり (RCA wear test)
The cured coating film produced by the above method was manufactured by NORMAN TOOL, INC. Using the RCA abrasion tester manufactured by the manufacturer and a dedicated abrasion paper for the RCA abrasion tester, it was worn 50 times under a load of 175 g, and the degree of wear of the cured coating film surface was observed with an optical microscope.
○: Almost no wear △: Some wear ×: Clear wear
(デュロメータ硬さDタイプ測定用硬化物作製方法)
樹脂組成物を直径2.5cm、深さ3mmのステンレス製型に注ぎ、その上をガラス板で覆い、UVコンベア炉(高圧水銀灯、80W、3灯)を用いて露光量1000mJ/cm2で露光し、離型して硬化塗膜を得た。
(デュロメータ硬さDタイプ測定方法)
上記方法にて作製した硬化塗膜を、JIS K7215に従って測定した。なお硬度値の読み取りは、荷重面が密着してから30秒後に行った。このとき、75°以上の場合を高硬度とした。 (Durometer Hardness D Type Measurement Cured Material Preparation Method)
The resin composition is poured into a stainless steel mold having a diameter of 2.5 cm and a depth of 3 mm, covered with a glass plate, and exposed using a UV conveyor furnace (high pressure mercury lamp, 80 W, 3 lights) at an exposure amount of 1000 mJ / cm 2 . The mold was released to obtain a cured coating film.
(Durometer hardness D type measurement method)
The cured coating film produced by the above method was measured according to JIS K7215. The hardness value was read 30 seconds after the load surface was in close contact. At this time, the case of 75 ° or more was regarded as high hardness.
樹脂組成物を直径2.5cm、深さ3mmのステンレス製型に注ぎ、その上をガラス板で覆い、UVコンベア炉(高圧水銀灯、80W、3灯)を用いて露光量1000mJ/cm2で露光し、離型して硬化塗膜を得た。
(デュロメータ硬さDタイプ測定方法)
上記方法にて作製した硬化塗膜を、JIS K7215に従って測定した。なお硬度値の読み取りは、荷重面が密着してから30秒後に行った。このとき、75°以上の場合を高硬度とした。 (Durometer Hardness D Type Measurement Cured Material Preparation Method)
The resin composition is poured into a stainless steel mold having a diameter of 2.5 cm and a depth of 3 mm, covered with a glass plate, and exposed using a UV conveyor furnace (high pressure mercury lamp, 80 W, 3 lights) at an exposure amount of 1000 mJ / cm 2 . The mold was released to obtain a cured coating film.
(Durometer hardness D type measurement method)
The cured coating film produced by the above method was measured according to JIS K7215. The hardness value was read 30 seconds after the load surface was in close contact. At this time, the case of 75 ° or more was regarded as high hardness.
表3に示す結果から明らかなように、実施例1~5は、反り、密着性、耐磨耗性が優れている。また、デュロメータ硬さDタイプが75°以上であるため高い硬度を有しており、反りも抑制できていた。
表4の比較例1~3は、感光性アミド及び/又は感光性アミドの誘導体を含有するため密着性は良好である。しかし、デュロメータ硬さDタイプが70°以上はあるが、硬度不足であり、反りにも問題がある。また、二重結合を含まない環状骨格を有する二官能(メタ)アクリレートを含有しないため耐磨耗性が劣る。
比較例4と5は、感光性アミド及び/又は感光性アミドの誘導体と、二重結合を含まない環状骨格を有する二官能(メタ)アクリレートを含有しないため、全ての特性が劣っていた。さらにデュロメータ硬さDタイプが75°未満のため硬度も不足し、反りの問題もある。
比較例6と7は、感光性アミド及び/又は感光性アミドの誘導体を含んでいないが、極性基である水酸基を有する感光性モノマーを含むため密着性は高い。しかし、デュロメータ硬さDタイプが75°未満であるため硬度も不足しており、反りにも問題がある。また、二重結合を含まない環状骨格を有する二官能(メタ)アクリレートを含有しないため、実施例1~3と同様に反りと耐磨耗性が劣っている。
比較例8は、硬化物のデュロメータ硬さDタイプが75°以上と高硬度であり、反りも問題ない。また、感光性アミド及び/又は感光性アミドの誘導体を含有するため、密着性が良好である。しかしながら、環状骨格を有する二官能(メタ)アクリレートが二重結合を含むため、耐磨耗性に劣っていた。 As is apparent from the results shown in Table 3, Examples 1 to 5 are excellent in warpage, adhesion, and wear resistance. Further, since the durometer hardness D type is 75 ° or more, the durometer hardness D type has high hardness and can suppress warpage.
Since Comparative Examples 1 to 3 in Table 4 contain a photosensitive amide and / or a derivative of a photosensitive amide, the adhesion is good. However, although the durometer hardness D type is 70 ° or more, the hardness is insufficient and there is a problem with warpage. Moreover, since it does not contain a bifunctional (meth) acrylate having a cyclic skeleton that does not contain a double bond, the wear resistance is poor.
Since Comparative Examples 4 and 5 did not contain a photosensitive amide and / or a derivative of the photosensitive amide and a bifunctional (meth) acrylate having a cyclic skeleton containing no double bond, all the characteristics were inferior. Furthermore, since the durometer hardness D type is less than 75 °, the hardness is insufficient and there is a problem of warpage.
Comparative Examples 6 and 7 do not contain a photosensitive amide and / or a derivative of the photosensitive amide, but have high adhesion because they contain a photosensitive monomer having a hydroxyl group that is a polar group. However, since the durometer hardness D type is less than 75 °, the hardness is insufficient, and there is a problem with warpage. Further, since it does not contain a bifunctional (meth) acrylate having a cyclic skeleton that does not contain a double bond, warping and wear resistance are inferior as in Examples 1 to 3.
In Comparative Example 8, the durometer hardness D type of the cured product is as high as 75 ° or more, and there is no problem with warping. Moreover, since it contains a photosensitive amide and / or a derivative of a photosensitive amide, the adhesion is good. However, since the bifunctional (meth) acrylate having a cyclic skeleton contains a double bond, the wear resistance is poor.
表4の比較例1~3は、感光性アミド及び/又は感光性アミドの誘導体を含有するため密着性は良好である。しかし、デュロメータ硬さDタイプが70°以上はあるが、硬度不足であり、反りにも問題がある。また、二重結合を含まない環状骨格を有する二官能(メタ)アクリレートを含有しないため耐磨耗性が劣る。
比較例4と5は、感光性アミド及び/又は感光性アミドの誘導体と、二重結合を含まない環状骨格を有する二官能(メタ)アクリレートを含有しないため、全ての特性が劣っていた。さらにデュロメータ硬さDタイプが75°未満のため硬度も不足し、反りの問題もある。
比較例6と7は、感光性アミド及び/又は感光性アミドの誘導体を含んでいないが、極性基である水酸基を有する感光性モノマーを含むため密着性は高い。しかし、デュロメータ硬さDタイプが75°未満であるため硬度も不足しており、反りにも問題がある。また、二重結合を含まない環状骨格を有する二官能(メタ)アクリレートを含有しないため、実施例1~3と同様に反りと耐磨耗性が劣っている。
比較例8は、硬化物のデュロメータ硬さDタイプが75°以上と高硬度であり、反りも問題ない。また、感光性アミド及び/又は感光性アミドの誘導体を含有するため、密着性が良好である。しかしながら、環状骨格を有する二官能(メタ)アクリレートが二重結合を含むため、耐磨耗性に劣っていた。 As is apparent from the results shown in Table 3, Examples 1 to 5 are excellent in warpage, adhesion, and wear resistance. Further, since the durometer hardness D type is 75 ° or more, the durometer hardness D type has high hardness and can suppress warpage.
Since Comparative Examples 1 to 3 in Table 4 contain a photosensitive amide and / or a derivative of a photosensitive amide, the adhesion is good. However, although the durometer hardness D type is 70 ° or more, the hardness is insufficient and there is a problem with warpage. Moreover, since it does not contain a bifunctional (meth) acrylate having a cyclic skeleton that does not contain a double bond, the wear resistance is poor.
Since Comparative Examples 4 and 5 did not contain a photosensitive amide and / or a derivative of the photosensitive amide and a bifunctional (meth) acrylate having a cyclic skeleton containing no double bond, all the characteristics were inferior. Furthermore, since the durometer hardness D type is less than 75 °, the hardness is insufficient and there is a problem of warpage.
Comparative Examples 6 and 7 do not contain a photosensitive amide and / or a derivative of the photosensitive amide, but have high adhesion because they contain a photosensitive monomer having a hydroxyl group that is a polar group. However, since the durometer hardness D type is less than 75 °, the hardness is insufficient, and there is a problem with warpage. Further, since it does not contain a bifunctional (meth) acrylate having a cyclic skeleton that does not contain a double bond, warping and wear resistance are inferior as in Examples 1 to 3.
In Comparative Example 8, the durometer hardness D type of the cured product is as high as 75 ° or more, and there is no problem with warping. Moreover, since it contains a photosensitive amide and / or a derivative of a photosensitive amide, the adhesion is good. However, since the bifunctional (meth) acrylate having a cyclic skeleton contains a double bond, the wear resistance is poor.
1…版体
2…凹部
3…樹脂組成物
4…基材
5…光源
6…硬化成型体 DESCRIPTION OFSYMBOLS 1 ... Plate body 2 ... Concave part 3 ... Resin composition 4 ... Base material 5 ... Light source 6 ... Curing molding
2…凹部
3…樹脂組成物
4…基材
5…光源
6…硬化成型体 DESCRIPTION OF
Claims (3)
- 複数のアクリレート基又はメタクリレート基を有するウレタン樹脂と、
感光性アミド及び/又は感光性アミドの誘導体と、
二重結合を含まない環状骨格を有する二官能アクリレート及び/又は二官能メタクリレートと、
光重合開始剤と、
を含有することを特徴とする活性エネルギー線硬化性樹脂組成物。 A urethane resin having a plurality of acrylate groups or methacrylate groups;
A photosensitive amide and / or a derivative of a photosensitive amide;
A bifunctional acrylate and / or a bifunctional methacrylate having a cyclic skeleton not containing a double bond;
A photopolymerization initiator;
An active energy ray-curable resin composition comprising: - 前記感光性アミド又は前記誘導体が、N-(2-ヒドロキシエチル)アクリルアミド、N-(2-ヒドロキシエチル)メタアクリルアミド、N-アクリロイルモルホリン、N-メタアクリロイルモルホリンの少なくとも1種からなることを特徴とする請求項1に記載の活性エネルギー線硬化性樹脂組成物。 The photosensitive amide or the derivative comprises at least one of N- (2-hydroxyethyl) acrylamide, N- (2-hydroxyethyl) methacrylamide, N-acryloylmorpholine, and N-methacryloylmorpholine. The active energy ray-curable resin composition according to claim 1.
- 請求項1又は2に記載の活性エネルギー線硬化性樹脂組成物を硬化させて得られる硬化物。 A cured product obtained by curing the active energy ray-curable resin composition according to claim 1 or 2.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010038459A JP6068776B2 (en) | 2010-02-24 | 2010-02-24 | Active energy ray-curable resin composition and molded body |
| JP2010-038459 | 2010-02-24 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011105069A1 true WO2011105069A1 (en) | 2011-09-01 |
Family
ID=44506499
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/001020 WO2011105069A1 (en) | 2010-02-24 | 2011-02-23 | Actinic radiation curable resin composition and cured products |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP6068776B2 (en) |
| WO (1) | WO2011105069A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015151495A (en) * | 2014-02-17 | 2015-08-24 | 太陽インキ製造株式会社 | Photocurable composition and molded product |
| JP2020122053A (en) * | 2019-01-29 | 2020-08-13 | 株式会社日本触媒 | Active energy ray-curable resin composition and cured product thereof |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6115100B2 (en) | 2012-11-23 | 2017-04-19 | デクセリアルズ株式会社 | Photocurable composition |
| JP6455115B2 (en) * | 2014-12-10 | 2019-01-23 | 凸版印刷株式会社 | Photo-curable resin molding, polarizing plate, and transmissive liquid crystal display |
| JP6826417B2 (en) * | 2015-11-20 | 2021-02-03 | 三洋化成工業株式会社 | Active energy ray-curable resin composition |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH08183821A (en) * | 1994-12-28 | 1996-07-16 | Takemoto Oil & Fat Co Ltd | Stereolithographic resin and stereolithographic resin composition |
| JPH08183824A (en) * | 1994-12-28 | 1996-07-16 | Takemoto Oil & Fat Co Ltd | Stereolithographic resin and stereolithographic resin composition |
| JPH08183822A (en) * | 1994-12-28 | 1996-07-16 | Takemoto Oil & Fat Co Ltd | Stereolithographic resin |
| JPH08183820A (en) * | 1994-12-28 | 1996-07-16 | Takemoto Oil & Fat Co Ltd | Stereolithographic resin and stereolithographic resin composition |
| JPH08183823A (en) * | 1994-12-28 | 1996-07-16 | Takemoto Oil & Fat Co Ltd | Stereolithographic resin and stereolithographic resin composition |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS62277473A (en) * | 1986-05-26 | 1987-12-02 | Nippon Kayaku Co Ltd | Coating agent for optical glass fiber |
| JP3705508B2 (en) * | 1995-10-31 | 2005-10-12 | ナブテスコ株式会社 | Photo-curable resin composition with excellent heat resistance |
| JP4906336B2 (en) * | 2005-12-16 | 2012-03-28 | シーメット株式会社 | Photocurable resin composition |
-
2010
- 2010-02-24 JP JP2010038459A patent/JP6068776B2/en active Active
-
2011
- 2011-02-23 WO PCT/JP2011/001020 patent/WO2011105069A1/en active Application Filing
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH08183821A (en) * | 1994-12-28 | 1996-07-16 | Takemoto Oil & Fat Co Ltd | Stereolithographic resin and stereolithographic resin composition |
| JPH08183824A (en) * | 1994-12-28 | 1996-07-16 | Takemoto Oil & Fat Co Ltd | Stereolithographic resin and stereolithographic resin composition |
| JPH08183822A (en) * | 1994-12-28 | 1996-07-16 | Takemoto Oil & Fat Co Ltd | Stereolithographic resin |
| JPH08183820A (en) * | 1994-12-28 | 1996-07-16 | Takemoto Oil & Fat Co Ltd | Stereolithographic resin and stereolithographic resin composition |
| JPH08183823A (en) * | 1994-12-28 | 1996-07-16 | Takemoto Oil & Fat Co Ltd | Stereolithographic resin and stereolithographic resin composition |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015151495A (en) * | 2014-02-17 | 2015-08-24 | 太陽インキ製造株式会社 | Photocurable composition and molded product |
| JP2020122053A (en) * | 2019-01-29 | 2020-08-13 | 株式会社日本触媒 | Active energy ray-curable resin composition and cured product thereof |
| JP7240884B2 (en) | 2019-01-29 | 2023-03-16 | 株式会社日本触媒 | Active energy ray-curable resin composition and cured product thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| JP6068776B2 (en) | 2017-01-25 |
| JP2011173982A (en) | 2011-09-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5758472B2 (en) | Curable composition for printed wiring board, cured coating film using the same, and printed wiring board | |
| JP6343439B2 (en) | Curable composition for printed wiring board, cured coating film using the same, and printed wiring board | |
| JP5688129B1 (en) | Curable composition for printed wiring board, cured coating film using the same, and printed wiring board | |
| WO2011105069A1 (en) | Actinic radiation curable resin composition and cured products | |
| JP7300596B2 (en) | Curable composition for inkjet, cured product thereof, and electronic component having cured product thereof | |
| JP6339346B2 (en) | Curable composition for printed wiring board, cured coating film using the same, and printed wiring board | |
| JP5653508B2 (en) | Curable composition for printed wiring board, cured coating film using the same, and printed wiring board | |
| JP6232049B2 (en) | Active energy ray-curable resin composition and cured product thereof | |
| JP2009227778A (en) | Active energy ray curable composition, method for producing cured material, and laminate | |
| CN108141964B (en) | Curable composition for inkjet, cured coating film using same, and printed wiring board | |
| JP2017066302A (en) | Curable composition for inkjet, and cured coating film and printed wiring board using the same | |
| JP6783510B2 (en) | Curable composition for inkjet, cured coating film and printed wiring board using this | |
| JP6440944B2 (en) | Photocurable composition and molded article | |
| JP2009203315A (en) | Active energy ray-curable resin composition for optical article and article | |
| JP6390817B2 (en) | Active energy ray-curable resin composition for optical articles, cured product, and optical sheet | |
| JP5846709B2 (en) | Active energy ray-curable resin composition, and its cured product and transparent molded product | |
| JP2010229366A (en) | Active energy ray-curable resin composition and cured product thereof | |
| JPH11271504A (en) | Composition curable with activation energy beam for sheetlike optical article | |
| JP2009114374A (en) | Composition and hardened product | |
| JP4228768B2 (en) | Active energy ray-curable resin composition for casting polymerization | |
| JP4151477B2 (en) | Curable resin composition for resist | |
| JP2002338637A (en) | Resin composition and cured item thereof | |
| JP2002338638A (en) | Resin composition and cured item thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11747038 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11747038 Country of ref document: EP Kind code of ref document: A1 |