WO2010050634A1 - Manufacturing method for peracetic acid solution using column-type reactor and coil-tube-type aging reactor - Google Patents
Manufacturing method for peracetic acid solution using column-type reactor and coil-tube-type aging reactor Download PDFInfo
- Publication number
- WO2010050634A1 WO2010050634A1 PCT/KR2008/006423 KR2008006423W WO2010050634A1 WO 2010050634 A1 WO2010050634 A1 WO 2010050634A1 KR 2008006423 W KR2008006423 W KR 2008006423W WO 2010050634 A1 WO2010050634 A1 WO 2010050634A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- peracetic acid
- hydrogen peroxide
- stage
- acid solution
- aging
- Prior art date
Links
- KFSLWBXXFJQRDL-UHFFFAOYSA-N Peracetic acid Chemical compound CC(=O)OO KFSLWBXXFJQRDL-UHFFFAOYSA-N 0.000 title claims abstract description 310
- 230000032683 aging Effects 0.000 title claims abstract description 59
- 238000004519 manufacturing process Methods 0.000 title claims abstract description 24
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 claims abstract description 183
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 claims abstract description 155
- 239000000243 solution Substances 0.000 claims abstract description 107
- 238000000034 method Methods 0.000 claims abstract description 39
- 238000002156 mixing Methods 0.000 claims abstract description 26
- 238000006243 chemical reaction Methods 0.000 claims abstract description 21
- NWUYHJFMYQTDRP-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;1-ethenyl-2-ethylbenzene;styrene Chemical compound C=CC1=CC=CC=C1.CCC1=CC=CC=C1C=C.C=CC1=CC=CC=C1C=C NWUYHJFMYQTDRP-UHFFFAOYSA-N 0.000 claims abstract description 20
- 239000007795 chemical reaction product Substances 0.000 claims abstract description 17
- 239000003729 cation exchange resin Substances 0.000 claims abstract description 12
- 238000010790 dilution Methods 0.000 claims abstract description 9
- 239000012895 dilution Substances 0.000 claims abstract description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 26
- 239000000376 reactant Substances 0.000 claims description 18
- 239000008213 purified water Substances 0.000 claims description 10
- 238000004401 flow injection analysis Methods 0.000 claims description 4
- 239000012535 impurity Substances 0.000 claims description 2
- 238000002347 injection Methods 0.000 claims description 2
- 239000007924 injection Substances 0.000 claims description 2
- 125000001273 sulfonato group Chemical group [O-]S(*)(=O)=O 0.000 claims description 2
- 229940023913 cation exchange resins Drugs 0.000 claims 2
- 230000000087 stabilizing effect Effects 0.000 claims 1
- 238000002360 preparation method Methods 0.000 abstract description 21
- 238000004659 sterilization and disinfection Methods 0.000 abstract description 15
- 150000007522 mineralic acids Chemical class 0.000 abstract description 12
- 230000001954 sterilising effect Effects 0.000 abstract description 12
- 239000003054 catalyst Substances 0.000 abstract description 8
- 238000005260 corrosion Methods 0.000 abstract description 8
- 239000011541 reaction mixture Substances 0.000 abstract description 3
- 239000002085 irritant Substances 0.000 abstract description 2
- 231100000021 irritant Toxicity 0.000 abstract description 2
- 238000003912 environmental pollution Methods 0.000 abstract 1
- 239000000645 desinfectant Substances 0.000 description 22
- 239000000203 mixture Substances 0.000 description 14
- 230000007423 decrease Effects 0.000 description 12
- 239000007864 aqueous solution Substances 0.000 description 11
- 239000003456 ion exchange resin Substances 0.000 description 11
- 229920003303 ion-exchange polymer Polymers 0.000 description 11
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 10
- 239000003112 inhibitor Substances 0.000 description 10
- 230000008961 swelling Effects 0.000 description 10
- 238000003756 stirring Methods 0.000 description 9
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical group [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 8
- 230000007797 corrosion Effects 0.000 description 7
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 6
- 238000000354 decomposition reaction Methods 0.000 description 6
- 239000003381 stabilizer Substances 0.000 description 6
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 5
- 230000000249 desinfective effect Effects 0.000 description 5
- 238000001704 evaporation Methods 0.000 description 5
- 230000008020 evaporation Effects 0.000 description 5
- 239000003377 acid catalyst Substances 0.000 description 4
- 229910000019 calcium carbonate Inorganic materials 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 238000002474 experimental method Methods 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- 238000003860 storage Methods 0.000 description 3
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 208000003322 Coinfection Diseases 0.000 description 2
- SXRSQZLOMIGNAQ-UHFFFAOYSA-N Glutaraldehyde Chemical compound O=CCCCC=O SXRSQZLOMIGNAQ-UHFFFAOYSA-N 0.000 description 2
- 239000005708 Sodium hypochlorite Substances 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 230000007613 environmental effect Effects 0.000 description 2
- 239000006260 foam Substances 0.000 description 2
- 210000003734 kidney Anatomy 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- SUKJFIGYRHOWBL-UHFFFAOYSA-N sodium hypochlorite Chemical compound [Na+].Cl[O-] SUKJFIGYRHOWBL-UHFFFAOYSA-N 0.000 description 2
- 239000011550 stock solution Substances 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- FDCJDKXCCYFOCV-UHFFFAOYSA-N 1-hexadecoxyhexadecane Chemical compound CCCCCCCCCCCCCCCCOCCCCCCCCCCCCCCCC FDCJDKXCCYFOCV-UHFFFAOYSA-N 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- DBVJJBKOTRCVKF-UHFFFAOYSA-N Etidronic acid Chemical compound OP(=O)(O)C(O)(C)P(O)(O)=O DBVJJBKOTRCVKF-UHFFFAOYSA-N 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- 239000001888 Peptone Substances 0.000 description 1
- 108010080698 Peptones Proteins 0.000 description 1
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical compound OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- IYFATESGLOUGBX-YVNJGZBMSA-N Sorbitan monopalmitate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O IYFATESGLOUGBX-YVNJGZBMSA-N 0.000 description 1
- 238000002441 X-ray diffraction Methods 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- JLPULHDHAOZNQI-JLOPVYAASA-N [(2r)-3-hexadecanoyloxy-2-[(9e,12e)-octadeca-9,12-dienoyl]oxypropyl] 2-(trimethylazaniumyl)ethyl phosphate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCC\C=C\C\C=C\CCCCC JLPULHDHAOZNQI-JLOPVYAASA-N 0.000 description 1
- 230000001464 adherent effect Effects 0.000 description 1
- 239000003463 adsorbent Substances 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 230000003444 anaesthetic effect Effects 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 239000007844 bleaching agent Substances 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 239000007809 chemical reaction catalyst Substances 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- BNIILDVGGAEEIG-UHFFFAOYSA-L disodium hydrogen phosphate Chemical compound [Na+].[Na+].OP([O-])([O-])=O BNIILDVGGAEEIG-UHFFFAOYSA-L 0.000 description 1
- 229910000397 disodium phosphate Inorganic materials 0.000 description 1
- 235000019800 disodium phosphate Nutrition 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 231100000053 low toxicity Toxicity 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 239000007769 metal material Substances 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- WSFSSNUMVMOOMR-NJFSPNSNSA-N methanone Chemical compound O=[14CH2] WSFSSNUMVMOOMR-NJFSPNSNSA-N 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 210000003097 mucus Anatomy 0.000 description 1
- 239000002736 nonionic surfactant Substances 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 235000019319 peptone Nutrition 0.000 description 1
- -1 poly oxy ethylene Polymers 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 238000005086 pumping Methods 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- PODWXQQNRWNDGD-UHFFFAOYSA-L sodium thiosulfate pentahydrate Chemical compound O.O.O.O.O.[Na+].[Na+].[O-]S([S-])(=O)=O PODWXQQNRWNDGD-UHFFFAOYSA-L 0.000 description 1
- 239000001570 sorbitan monopalmitate Substances 0.000 description 1
- 229940031953 sorbitan monopalmitate Drugs 0.000 description 1
- 235000011071 sorbitan monopalmitate Nutrition 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 239000001974 tryptic soy broth Substances 0.000 description 1
- 108010050327 trypticase-soy broth Proteins 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N37/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids
- A01N37/02—Saturated carboxylic acids or thio analogues thereof; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N59/00—Biocides, pest repellants or attractants, or plant growth regulators containing elements or inorganic compounds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C407/00—Preparation of peroxy compounds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C409/00—Peroxy compounds
- C07C409/24—Peroxy compounds the —O—O— group being bound between a >C=O group and hydrogen, i.e. peroxy acids
- C07C409/26—Peracetic acid
Definitions
- the present invention relates to a method for preparing a disinfectant cleansing solution containing peracetic acid, which is superior in sterilizing, cleansing or disinfecting medical devices and is highly stable.
- the disinfectant cleansing solution may be used to cleanse, disinfect or sterilize dialysis equipments, specialized endoscope equipments, surgical instruments, anesthetic equipments, obstetric and gynecologic instruments, dental instruments, and other general medical devices.
- Examples of disinfectants for medical devices include sodium hypochlorite, formaldehyde, glutaraldehyde and acetic acid.
- glutaraldehyde and formaldehyde have a pungent odor and are irritant to skin and eyes.
- sodium hypochlorite and acetic acid may result in corrosion of the medical devices. For these reasons, a neglected maintenance of medical devices often results in a fatal consequence during medical care, as well as secondary infections.
- peracetic acid solution has few corrosion problems and provides good disinfecting ability, it is used in cleansing and disinfecting of various medical equipments as an alternative to the aforesaid disinfectants. Since it exhibits broad activities on microorganisms, it is also widely used for disinfecting water for cooling towers, water for food processing, water for pulp- and papermaking, etc., in addition to sterilization, dis- infection and cleansing of medical equipments.
- Peracetic acid-based disinfectant cleansing solutions for medical devices containing peracetic acid, acetic acid and hydrogen peroxide are disclosed in WO 88/08667, WO 93/07909, WO 94/14321, EP 193,426, EP 596,493, USP 6,168,808, KR 2000-49667, or the like.
- Those peracetic acid-based disinfectant cleansing solutions further include a stabilizer such as phosphoric acid, phosphonic acid, etc., a non-ionic surfactant such as sorbitan monopalmitate, poly oxy ethylene cetyl ether, etc., an amine oxide, or the like, in addition to the main components - peracetic acid, acetic acid, hydrogen peroxide and purified water.
- a stabilizer such as phosphoric acid, phosphonic acid, etc.
- a non-ionic surfactant such as sorbitan monopalmitate, poly oxy ethylene cetyl ether, etc., an amine oxide, or the like
- the peracetic acid-based disinfectant cleansing solutions are suitable for general cleansing or automatic cleansing, but they have some problems in practical applications.
- a disinfectant cleansing solution with a low peracetic acid content in general, less than 1 wt%) may not effectively cope with the contamination by the patient's mucus.
- disinfectant cleansing solutions containing peracetic acid are usually used for endoscope equipments, forceps, surgical instruments, or the like.
- endoscope equipments for endoscope equipments, forceps, surgical instruments, or the like.
- peracetic acid-based disinfectant cleansing solutions may result in a corrosion problem.
- high-concentration peracetic acid solutions are prepared by distilling peracetic acid at relatively low concentration obtained from the reaction of hydrogen peroxide and acetic acid in the presence of an acid catalyst.
- UK 776,758 discloses a method for preparing a peracetic acid solution using a cation-exchange resin as catalyst instead of an inorganic acid such as sulfuric acid or phosphoric acid.
- US 4,647,678 discloses a method for preparing a peracetic acid solution using a strongly acidic cation-exchange resin as catalyst for use as disinfectant and bleach.
- inorganic acid such as sulfuric acid or phosphoric acid is typically used as catalyst. Accordingly, the peracetic acid solution used for the cleansing of medical devices contains a small amount of inorganic acid, which has the problems of corrosion and irritation to the human body.
- the inventors of the present invention developed an apparatus for the preparation of a peracetic acid solution capable of preparing a peracetic acid solution continuously by means of flow injection in an integrated space cut off from the air, and a method for preparing a peracetic acid solution using the same.
- the reactants acetic acid and hydrogen peroxide are continuously purified at a constant flow rate and mixed with each other at an accurate concentration ratio. Then, they react while passing through a tubular reactor filled with a strongly acidic ion-exchange resin at 20-50 0 C. After passing through a first coil tube type aging reactor, the product is mixed with purified water. Then, after passing through a second coil tube type aging reactor, it may be used in real time or stored for later use.
- the method for preparing a peracetic acid solution includes: a preliminary stage of purifying the reactants acetic acid and hydrogen peroxide using a first cation-exchange resin as an adsorbent; a mixing stage of mixing the reactants by means of flow injection while controlling the relative and absolute injection amount to continuously prepare a reactant solution having a acetic acid/hydrogen peroxide mixing proportion adequate for an intended use; a main reaction stage of injecting the reactant solution to a column type reactor in which a second cation-exchange resin is filled as a reaction catalyst to produce peracetic acid; a first aging stage of aging the reaction product that has passed through the main reaction stage in a first coil tube type aging reactor; a dilution stage of mixing the reaction product that has passed through the first aging stage with purified water to prepare diluted peracetic acid solution; and a second aging stage of aging the diluted peracetic acid solution again in a second coil tube type aging reactor.
- the respective stages may be performed consistently and interconnectedly in an integrated space cut off from the air.
- the method for preparing a peracetic acid solution according to the present invention is characterized by an integrated preparation process of a peracetic acid solution. Differently from the existing production method wherein disinfectant cleansing solutions are produced in batches according to uses, peracetic acid solutions with a stabilized equilibrium concentration of peracetic acid can be prepared easily and conveniently, while changing the mixing proportion of acetic acid and hydrogen peroxide in real time depending on various required uses.
- the peracetic acid disinfectant prepared according to the method of the present invention is advantageous in that the control of peracetic acid concentration and the change of the mixing proportion of acetic acid and hydrogen peroxide may be carried out continuously. Specifically, for example, the relative amount of acetic acid in the reaction mixture of acetic acid and hydrogen peroxide is increased first to remove hardly soluble carbonates (scales). Immediately thereafter, the relative amount of hydrogen peroxide and peracetic acid is increased to make it easier to remove, sterilize or disinfect biofilms. As a result, descaling and sterilization of medical devices can be performed effectively through cleansing and disinfection using a relatively small amount of disinfectant cleansing solution.
- the cleansing disinfectant prepared in accordance with the present invention may comprise 1.0-35 wt% of acetic acid, 1.0-35 wt% of hydrogen peroxide, 0.1-20 wt% of peracetic acid and 10-97.9 wt% of reverse-osmosed water.
- the method for preparing a peracetic acid solution according to the present invention is particularly advantageous in that a stable cleansing disinfectant with an adequate composition for various uses can be prepared continuously and that a peracetic acid solution capable of maintaining a sufficient stability can be prepared without adding a swelling inhibitor or a stabilizer to the reactants since the reactants are purified in advance.
- FIG. 1 schematically illustrates an integrated manufacturing system for realizing the continuous preparation method of a peracetic acid solution according to the present invention. Best Mode for Carrying out the Invention
- FIG. 1 schematically illustrates an integrated manufacturing system for realizing the continuous preparation method of a peracetic acid solution according to the present invention.
- all the reaction apparatuses excluding storage tanks 1, 2 for acetic acid and hydrogen peroxide may be disposed inside an isolated space cut off from the air (enclosed by a square box in the figure).
- the temperature of the isolated space and all the apparatuses disposed therein may be controlled within 20 and 5O 0 C.
- the method for preparing a peracetic acid solution according to the present invention comprises a preliminary stage, a mixing stage, a main reaction stage, a first aging stage, a dilution stage and a second aging stage.
- acetic acid and hydrogen peroxide supplied from first and second storage tanks 1, 2 are passed through column type purifiers 3, 6 to remove metallic impurities including divalent and trivalent cations and to preheat the reactants for reaction.
- the flow rate of acetic acid and hydrogen peroxide may be effectively controlled using flow meters 4, 5 in the range from 0.1 to 50 mL/min.
- the acetic acid and hydrogen peroxide that have passed through the flow meters 4, 5 are mixed at a molar ratio determined by the controlled flow rate to prepare a reactant solution.
- the reactant solution is passed through a column type reactor 7 filled with a cation-exchange resin to prepare a reaction product containing peracetic acid.
- the cation-exchange resin may be an ion-exchange resin having sulfonate groups.
- the reaction product is aged as it passes through a first coil tube type aging reactor 8 maintained at a constant temperature until the peracetic acid reaction reaches an equilibrium state. As a result, the peracetic acid concentration of the reaction product may be maintained constant.
- the reaction product is mixed with purified water 10 the supply of which is controlled by a purified water flow meter 9, thereby preparing a diluted peracetic acid stock solution.
- the purified water 10 may be nearly pure water.
- reverse-osmosed water or deionized distilled water may be used.
- the diluted peracetic acid stock solution is subjected again to an equilibrium reaction as it passed through a second coil tube type aging reactor 11.
- a final peracetic acid solution the peracetic acid concentration of which is maintained stably at a desired level is obtained.
- the final peracetic acid solution may be used as a cleansing disinfectant immediately after the preparation. Alternatively, it may be stored in a storage container equipped outside the integrated reaction apparatus for later use.
- composition of the components of the peracetic acid solution for cleansing and disinfection prepared according to the preparation method of the present invention comprising the aforesaid stages may be adjusted for different uses to be provided for cleansing of various medical devices. For example, when removing scales whose main component is calcium carbonate, it is preferable to treat with a peracetic acid solution containing less than 0.1 mol of hydrogen peroxide per 10 mol of acetic acid at first, and then gradually use a peracetic acid solution with a higher hydrogen peroxide content. For another example, when disinfection or sterilization is the main purpose, it will be effective to use a peracetic acid solution having a molar ratio of acetic acid to hydrogen peroxide of 2:1.
- peracetic acid solution having a molar ratio of acetic acid to hydrogen peroxide of about 1:2 to 1:11.
- peracetic acid solution it may be the most effective not to fix the composition of the components but to perform cleansing or disinfection while gradually changing the composition.
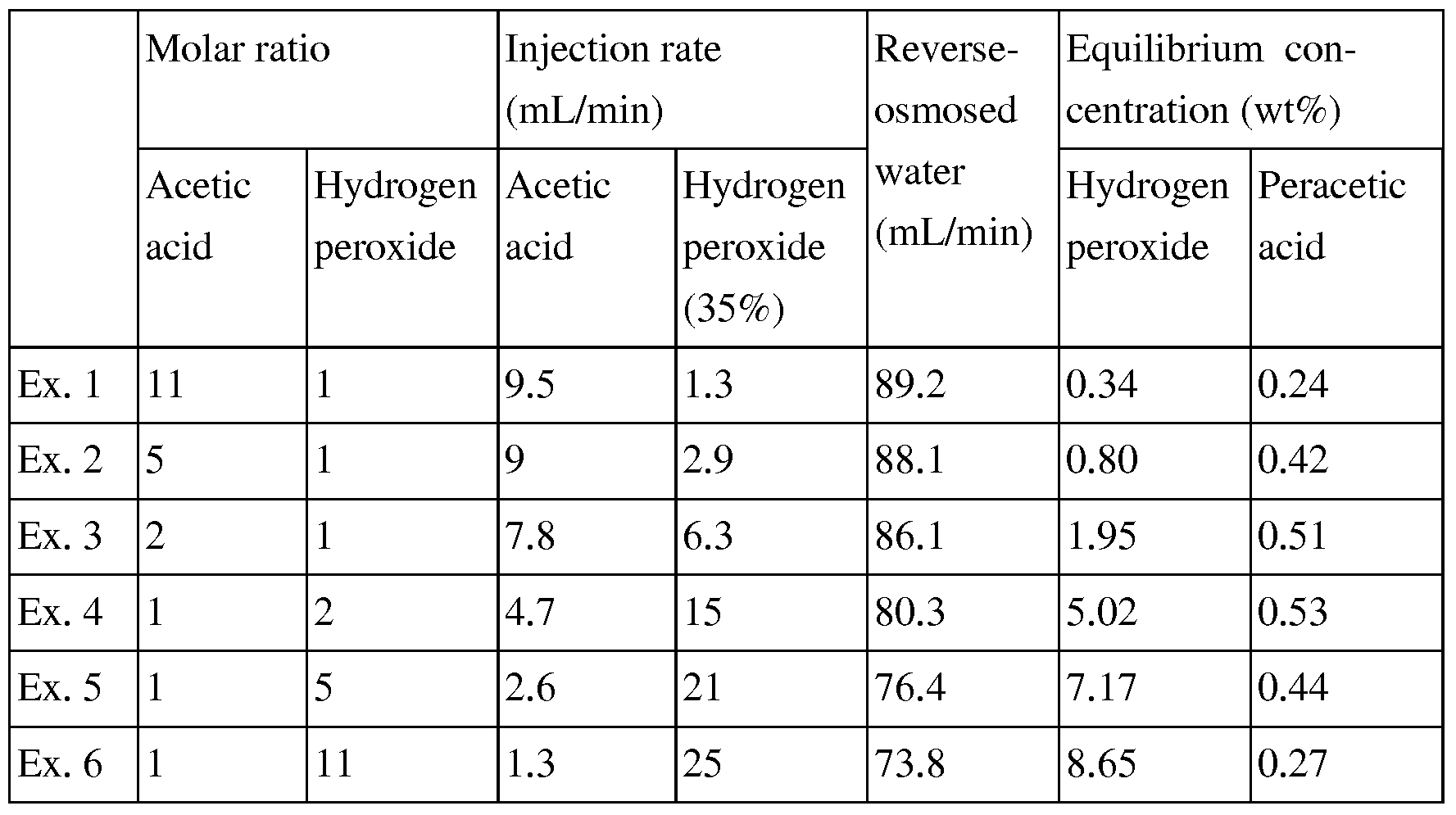
- Example 1 Preparation of peracetic acid solution with molar ratio of acetic acid to hydrogen peroxide of 11 : 1
- the temperature of an isolated space in which an integrated reaction system for preparation of peracetic acid is located is maintained at 35 0 C.
- Acetic acid and hydrogen peroxide are respectively passed through a tube type purifier filled with a sulfonate ion-exchange resin.
- acetic acid is passed at a flow rate of 9.5 mL/min and hydrogen peroxide (35% aqueous solution) is passed at a flow rate of 1.3 mL/min, so that the mixing molar ratio of acetic acid to hydrogen peroxide is 11:1.
- the resultant reactant solution is injected into a column type reactor filled with a sulfonate ion-exchange resin at a rate of 10 mL/min to produce peracetic acid by means of a continuous reaction.
- a reaction product flowing out of the column type reactor is passed through a first coil tube type aging reactor, so that the production of peracetic acid reaches nearly an equilibrium state.
- the reaction product flowing out of the first coil tube type aging reactor is mixed with reverse-osmosed water supplied from a purified water flow meter at a rate of 89.2 mL/ min to prepare a diluted peracetic acid solution.
- the diluted peracetic acid solution is passed through a second coil tube type aging reactor, so that the production of peracetic acid reaches nearly an equilibrium state again.
- Example 2 Preparation of peracetic acid solution with molar ratio of acetic acid to hydrogen peroxide of 5: 1
- Example 1 The procedure of Example 1 is repeated, except that acetic acid is passed at a flow rate of 9 mL/min and hydrogen peroxide (35% aqueous solution) is passed at a flow rate of 2.9 mL/min, so that the mixing molar ratio of acetic acid to hydrogen peroxide is 5 : 1 , and that reverse-osmosed water is mixed at a rate of 88.1 mL/min in the dilution stage.
- hydrogen peroxide 35% aqueous solution
- Example 3 The procedure of Example 1 is repeated, except that acetic acid is passed at a flow rate of 7.8 mL/min and hydrogen peroxide (35% aqueous solution) is passed at a flow rate of 6.3 mL/min, so that the mixing molar ratio of acetic acid to hydrogen peroxide is 2: 1 , and that reverse-osmosed water is mixed at a rate of 86.1 mL/min in the dilution stage.
- Example 4 Preparation of peracetic acid solution with molar ratio of acetic acid to hydrogen peroxide of 1:2
- Example 1 The procedure of Example 1 is repeated, except that acetic acid is passed at a flow rate of 4.7 mL/min and hydrogen peroxide (35% aqueous solution) is passed at a flow rate of 15 mL/min, so that the mixing molar ratio of acetic acid to hydrogen peroxide is 1:2, and that reverse-osmosed water is mixed at a rate of 80.3 mL/min in the dilution stage.
- Example 5 Preparation of peracetic acid solution with molar ratio of acetic acid to hydrogen peroxide of 1:5 [43] The procedure of Example 1 is repeated, except that acetic acid is passed at a flow rate of 2.6 mL/min and hydrogen peroxide (35% aqueous solution) is passed at a flow rate of 21 mL/min, so that the mixing molar ratio of acetic acid to hydrogen peroxide is 1:5, and that reverse-osmosed water is mixed at a rate of 76.4 mL/min in the dilution stage.
- Example 6 Preparation of peracetic acid solution with molar ratio of acetic acid to hydrogen peroxide of 1 : 11 [45] The procedure of Example 1 is repeated, except that acetic acid is passed at a flow rate of 1.3 mL/min and hydrogen peroxide (35% aqueous solution) is passed at a flow rate of 24.9 mL/min, so that the mixing molar ratio of acetic acid to hydrogen peroxide is 1:11, and that reverse-osmosed water is mixed at a rate of 73.8 mL/min in the dilution stage.
- Table 1 summarizes the preparation of peracetic acid solutions with different molar proportions of acetic acid and hydrogen peroxide according to Examples 1 to 6. [47] Table 1 [Table 1] [Table ]
- Example 7 Change of equilibrium concentration depending on addition of swelling inhibitor
- etidronic acid is added as a swelling inhibitor at 0, 0.5 and 1 wt%, and the result is monitored.
- the experiment is carried out with the molar ratio of acetic acid to hydrogen peroxide fixed at 2: 1 (as in Example 3).
- the initial total volume of acetic acid, hydrogen peroxide, the swelling inhibitor and water is set as 50 mL.
- Table 2 summarizes the measurement of the equilibrium concentration depending on the addition of the swelling inhibitor. [52] Table 2 [Table 2] [Table ]
- Example 8 Peracetic acid production depending on aging of reaction product following main reaction stage
- Reaction products are obtained as in Examples 1 to 6 through the preliminary stage, mixing stage and main reaction stage, but without the aging stage, with the weight proportion of the reactants acetic acid and hydrogen peroxide (35% aqueous solution) at 20: 17.
- the reaction products are aged using either a stirring type aging reactor or a coil tube type aging reactor.
- the concentration of hydrogen peroxide and peracetic acid in the resultant final peracetic acid solutions are determined.
- Aging using the stirring type aging reactor is carried out while stirring in a sealed reactor at 45 0 C using a magnetic bar.
- Aging using the coil tube type aging reactor is carried out while circulating the reaction product in a sealed coil tube type aging reactor (coil with diameter 1 mm or less) at 45 0 C.
- Table 3 summarizes stability of the peracetic acid solutions obtained by aging the stirring type aging reactor and the coil tube type aging reactor. [57] Table 3 [Table 3] [Table ]
- H 2 O 2 hydrogen peroxide
- PAA peracetic acid
- the peracetic acid concentration increases significantly faster when the coil tube type aging reactor is used as compared to when the stirring type aging reactor. Specifically, for example, after 1 hour of aging, the coil tube type aging reactor produces 13.7 wt% of peracetic acid, whereas the stirring type aging reactor produces 10.5 wt% of peracetic acid.
- peracetic acid concentration in the equilibrium state may be maximized while minimizing loss of hydrogen peroxide.
- Example 10 Biofilm removal ability of peracetic acid solution depending on composition
- Example 11 Sterilizing power of peracetic acid solution depending on composition
- the sterilizing power of the peracetic acid solutions prepared according to Examples 1 to 6 against bacteria and fungi is tested. Specifically, 0.1 mL of bacterial solution incubated for 24 hours in nutrient broth and tryptic soy broth is added to 10 mL of the peracetic acid solutions prepared according to Examples 1 to 6, each diluted 10 times with purified water.
- Table 5 summarizes the sterilizing power of the peracetic acid solutions with different compositions.
- a peracetic acid solution appropriate for the purpose of cleansing or sterilization may be prepared safely and quickly.
- the preparation method enables a continuous preparation of peracetic acid solutions with desired mixing proportion of hydrogen peroxide and acetic acid and peracetic acid concentration, appropriate for various uses, while changing them in real time. Accordingly, an effective cleansing and disinfection is possible by using a disinfectant cleansing solution the composition of which is adjusted in real time depending on the desired environmental factors of medical devices or equipments or depending on the required cleansing or sterilization requirements.
- the peracetic acid aqueous solution prepared in accordance with the present invention is an environment-friendly disinfectant in the true sense of the word, since it exhibits a desired cleansing and disinfection effect without using a commonly used catalyst such as phosphoric acid, a stabilizer or a swelling inhibitor, which are added to improve stability of the peracetic acid concentration, or other chemical substances.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Zoology (AREA)
- Pest Control & Pesticides (AREA)
- Plant Pathology (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Dentistry (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Agronomy & Crop Science (AREA)
- Environmental Sciences (AREA)
- Inorganic Chemistry (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
Abstract
A method for continuous preparation of a peracetic acid solution is provided. The preparation method includes a preliminary stage, a mixing stage, a main reaction stage, a first aging stage, a dilution stage, and a second aging stage. In the main reaction stage, a reaction mixture solution of hydrogen peroxide and acetic acid is continuously injected to a column type reactor filled with a cation-exchange resin to produce peracetic acid. In the first and second aging stages, the reaction product is passed through a coil tube type aging reactor until the peracetic acid production reaches an equilibrium state to stabilize the peracetic acid solution. The preparation method is capable of continuously preparing peracetic acid solutions appropriate for various uses such as descaling, sterilization, disinfection, etc. by changing the mixing proportion of acetic acid and hydrogen peroxide, without interrupting the production of peracetic acid. The method is capable of reducing loss of peracetic acid solution and preventing environmental pollution. Further, since the prepared peracetic acid solution does not contain an inorganic acid, which is commonly used as catalyst for the production of peracetic acid, it is less irritant and has superior anti-corrosion property.
Description
Description
MANUFACTURING METHOD FOR PERACETIC ACID SOLUTION USING COLUMN-TYPE REACTOR AND COIL- TUBE-TYPE AGING REACTOR
Technical Field
[1] The present invention relates to a method for preparing a disinfectant cleansing solution containing peracetic acid, which is superior in sterilizing, cleansing or disinfecting medical devices and is highly stable. The disinfectant cleansing solution may be used to cleanse, disinfect or sterilize dialysis equipments, specialized endoscope equipments, surgical instruments, anesthetic equipments, obstetric and gynecologic instruments, dental instruments, and other general medical devices. Background Art
[2] The development of electronic devices has led to the development in the medical field, providing more convenient and effective medical care for patients. The conventional medical equipments having simple structure can be easily sterilized and disinfected without having to consider the environment, heat or toxicity issue of the related disinfectant. However, recently, as the medical equipments adopt more complicated and various materials, the right use and thorough post-management of the medical equipments are critical in improving the quality of medical care. Accordingly, hospitals are hiring experts and having them managing the medical equipments. The importance of cleansing, sterilization and disinfection of machines and devices is appreciated a lot recently. In addition to the fact that they determine the quality of medical care, use of stable and superior cleansing disinfectants is essential in that the use of improperly disinfected or cleansed medical instruments may result in a fatal secondary infection.
[3] Examples of disinfectants for medical devices include sodium hypochlorite, formaldehyde, glutaraldehyde and acetic acid. However, glutaraldehyde and formaldehyde have a pungent odor and are irritant to skin and eyes. And, sodium hypochlorite and acetic acid may result in corrosion of the medical devices. For these reasons, a neglected maintenance of medical devices often results in a fatal consequence during medical care, as well as secondary infections.
[4] Because peracetic acid solution has few corrosion problems and provides good disinfecting ability, it is used in cleansing and disinfecting of various medical equipments as an alternative to the aforesaid disinfectants. Since it exhibits broad activities on microorganisms, it is also widely used for disinfecting water for cooling towers, water for food processing, water for pulp- and papermaking, etc., in addition to sterilization, dis-
infection and cleansing of medical equipments.
[5] Peracetic acid-based disinfectant cleansing solutions for medical devices containing peracetic acid, acetic acid and hydrogen peroxide are disclosed in WO 88/08667, WO 93/07909, WO 94/14321, EP 193,426, EP 596,493, USP 6,168,808, KR 2000-49667, or the like. Those peracetic acid-based disinfectant cleansing solutions further include a stabilizer such as phosphoric acid, phosphonic acid, etc., a non-ionic surfactant such as sorbitan monopalmitate, poly oxy ethylene cetyl ether, etc., an amine oxide, or the like, in addition to the main components - peracetic acid, acetic acid, hydrogen peroxide and purified water. With quick sterilizing ability, the peracetic acid-based disinfectant cleansing solutions are suitable for general cleansing or automatic cleansing, but they have some problems in practical applications. For example, a disinfectant cleansing solution with a low peracetic acid content (in general, less than 1 wt%) may not effectively cope with the contamination by the patient's mucus. Further, general- use disinfectant cleansing solutions containing peracetic acid are usually used for endoscope equipments, forceps, surgical instruments, or the like. In case of medical devices made, for example, with copper- or zinc-containing compounds the peracetic acid-based disinfectant cleansing solutions may result in a corrosion problem.
[6] Recently, a method for preparing a disinfectant composition comprising a first aqueous solution comprising peracetic acid and a second aqueous solution comprising a corrosion inhibitor and a peracetic acid stabilizer has been developed. According to USP 5,624,634, since it has remarkably improved corrosion inhibiting ability, the two- component peracetic acid composition may be used to cleanse and disinfect medical equipments wherein corrosion- susceptible metals are used.
[7] Also, methods for preparing high-concentration peracetic acid solutions are reported.
According to USP 3,264,345, UK 789,016, UK 1,014,361, EP 296,328, EP 789,016 and EP 1,004,576, high-concentration peracetic acid solutions are prepared by distilling peracetic acid at relatively low concentration obtained from the reaction of hydrogen peroxide and acetic acid in the presence of an acid catalyst.
[8] An example of the most frequently employed method for the preparation of peracetic acid aqueous solutions is to mix acetic acid, hydrogen peroxide and a stabilizer at various proportions in the presence of an inorganic acid catalyst. This method results in various products adequate for various purposes, with significantly improved stability and functionality. However, the method is problematic in that the use of the inorganic acid such as phosphoric acid or sulfuric acid may cause severe corrosion of metallic materials. In consequence, the characteristic advantages of peracetic acid such as environment-friendliness and low toxicity are deteriorated and the production cost increases remarkably. In particular, the method of preparing high-concentration peracetic acid solutions by distilling peracetic acid produced in the presence of the
inorganic acid catalyst is disadvantageous in the aspects of environment and production cost due to excessive use of the inorganic acid catalyst.
[9] For these reasons, a method for preparing a peracetic acid solution without using an inorganic acid was developed. UK 776,758 discloses a method for preparing a peracetic acid solution using a cation-exchange resin as catalyst instead of an inorganic acid such as sulfuric acid or phosphoric acid. And, US 4,647,678 discloses a method for preparing a peracetic acid solution using a strongly acidic cation-exchange resin as catalyst for use as disinfectant and bleach.
[10] According to the aforesaid methods for preparing peracetic acid solutions using ion- exchange resins, (1) an ion-exchange resin is added to a mixture solution of acetic acid and hydrogen peroxide, and then stirred for several hours, or (2) a reaction mixture is continuously passed through a tubular reactor filled with an ion-exchange resin by means of pumping and pressure control. However, these methods are problematic in that the mixing proportion of acetic acid and hydrogen peroxide is not precisely controlled in real time so as to be adequate for various purposes, and in that a long time of aging reaction is required. Therefore, it was difficult to obtain peracetic acid solutions with stabilized peracetic acid concentration in real time for various purposes.
[11] In addition, the existing methods for preparing peracetic acid solutions are problematic in that a swelling inhibitor or a stabilizer is used to maintain the catalytic property of the ion-exchange resin and thereby to attain stabilized peracetic acid concentration. The use of such compounds results in deterioration of the properties of the peracetic acid solutions, as well as environmental problems. Disclosure of Invention
Technical Problem
[12] When producing peracetic acid by reacting acetic acid and hydrogen peroxide, inorganic acid such as sulfuric acid or phosphoric acid is typically used as catalyst. Accordingly, the peracetic acid solution used for the cleansing of medical devices contains a small amount of inorganic acid, which has the problems of corrosion and irritation to the human body. Technical Solution
[13] One of the methods to solve the problems caused by the use of inorganic acid as catalyst is to use a cation-exchange resin instead of the inorganic acid. The methods for producing peracetic acid using an ion-exchange resin as catalyst are disclosed in several literatures, but they are not applied widely. The reason is that the concentration of acetic acid, hydrogen peroxide and peracetic acid in the produced peracetic acid solution is unstable and that the productivity is not high as compared to the method using the inorganic acid. Specifically, according to the method for preparing peracetic
acid using an ion-exchange resin as catalyst disclosed in the literature, a very long aging time is necessary to stabilize the peracetic acid concentration and it is difficult to maintain the mixing proportion of acetic acid and hydrogen peroxide constant in real time. Besides, exposure of the reactants and product before and after the production of peracetic acid to outside, as well as purification of the reactants, also acts as a factor making the final peracetic acid product unstable.
[14] To overcome the problems of the existing techniques, the inventors of the present invention developed an apparatus for the preparation of a peracetic acid solution capable of preparing a peracetic acid solution continuously by means of flow injection in an integrated space cut off from the air, and a method for preparing a peracetic acid solution using the same. According to the preparation method, the reactants acetic acid and hydrogen peroxide are continuously purified at a constant flow rate and mixed with each other at an accurate concentration ratio. Then, they react while passing through a tubular reactor filled with a strongly acidic ion-exchange resin at 20-500C. After passing through a first coil tube type aging reactor, the product is mixed with purified water. Then, after passing through a second coil tube type aging reactor, it may be used in real time or stored for later use.
[15] More specifically, the method for preparing a peracetic acid solution according to the present invention includes: a preliminary stage of purifying the reactants acetic acid and hydrogen peroxide using a first cation-exchange resin as an adsorbent; a mixing stage of mixing the reactants by means of flow injection while controlling the relative and absolute injection amount to continuously prepare a reactant solution having a acetic acid/hydrogen peroxide mixing proportion adequate for an intended use; a main reaction stage of injecting the reactant solution to a column type reactor in which a second cation-exchange resin is filled as a reaction catalyst to produce peracetic acid; a first aging stage of aging the reaction product that has passed through the main reaction stage in a first coil tube type aging reactor; a dilution stage of mixing the reaction product that has passed through the first aging stage with purified water to prepare diluted peracetic acid solution; and a second aging stage of aging the diluted peracetic acid solution again in a second coil tube type aging reactor. The respective stages may be performed consistently and interconnectedly in an integrated space cut off from the air. The method for preparing a peracetic acid solution according to the present invention is characterized by an integrated preparation process of a peracetic acid solution. Differently from the existing production method wherein disinfectant cleansing solutions are produced in batches according to uses, peracetic acid solutions with a stabilized equilibrium concentration of peracetic acid can be prepared easily and conveniently, while changing the mixing proportion of acetic acid and hydrogen peroxide in real time depending on various required uses.
Advantageous Effects
[16] Differently from the existing batch type method, the peracetic acid disinfectant prepared according to the method of the present invention is advantageous in that the control of peracetic acid concentration and the change of the mixing proportion of acetic acid and hydrogen peroxide may be carried out continuously. Specifically, for example, the relative amount of acetic acid in the reaction mixture of acetic acid and hydrogen peroxide is increased first to remove hardly soluble carbonates (scales). Immediately thereafter, the relative amount of hydrogen peroxide and peracetic acid is increased to make it easier to remove, sterilize or disinfect biofilms. As a result, descaling and sterilization of medical devices can be performed effectively through cleansing and disinfection using a relatively small amount of disinfectant cleansing solution.
[17] The cleansing disinfectant prepared in accordance with the present invention may comprise 1.0-35 wt% of acetic acid, 1.0-35 wt% of hydrogen peroxide, 0.1-20 wt% of peracetic acid and 10-97.9 wt% of reverse-osmosed water. The method for preparing a peracetic acid solution according to the present invention is particularly advantageous in that a stable cleansing disinfectant with an adequate composition for various uses can be prepared continuously and that a peracetic acid solution capable of maintaining a sufficient stability can be prepared without adding a swelling inhibitor or a stabilizer to the reactants since the reactants are purified in advance. Brief Description of Drawings
[18] The above and other aspects, features and advantages of the disclosed exemplary embodiments will be more apparent from the following detailed description taken in conjunction with the accompanying drawing in which:
[19] Fig. 1 schematically illustrates an integrated manufacturing system for realizing the continuous preparation method of a peracetic acid solution according to the present invention. Best Mode for Carrying out the Invention
[20] Exemplary embodiments now will be described more fully hereinafter with reference to the accompanying drawings, in which exemplary embodiments are shown. This disclosure may, however, be embodied in many different forms and should not be construed as limited to the exemplary embodiments set forth therein. Rather, these exemplary embodiments are provided so that this disclosure will be thorough and complete, and will fully convey the scope of this disclosure to those skilled in the art. In the description, details of well-known features and techniques may be omitted to avoid unnecessarily obscuring the presented embodiments.
[21] The terminology used herein is for the purpose of describing particular embodiments
only and is not intended to be limiting of this disclosure. As used herein, the singular forms "a", "an" and "the" are intended to include the plural forms as well, unless the context clearly indicates otherwise. Furthermore, the use of the terms a, an, etc. does not denote a limitation of quantity, but rather denotes the presence of at least one of the referenced item. The use of the terms "first", "second", and the like does not imply any particular order, but they are included to identify individual elements. Moreover, the use of the terms first, second, etc. does not denote any order or importance, but rather the terms first, second, etc. are used to distinguish one element from another. It will be further understood that the terms "comprises" and/or "comprising", or "includes" and/ or "including" when used in this specification, specify the presence of stated features, regions, integers, steps, operations, elements, and/or components, but do not preclude the presence or addition of one or more other features, regions, integers, steps, operations, elements, components, and/or groups thereof.
[22] Unless otherwise defined, all terms (including technical and scientific terms) used herein have the same meaning as commonly understood by one of ordinary skill in the art. It will be further understood that terms, such as those defined in commonly used dictionaries, should be interpreted as having a meaning that is consistent with their meaning in the context of the relevant art and the present disclosure, and will not be interpreted in an idealized or overly formal sense unless expressly so defined herein.
[23] Fig. 1 schematically illustrates an integrated manufacturing system for realizing the continuous preparation method of a peracetic acid solution according to the present invention.
[24] As seen in Fig. 1, all the reaction apparatuses excluding storage tanks 1, 2 for acetic acid and hydrogen peroxide may be disposed inside an isolated space cut off from the air (enclosed by a square box in the figure). The temperature of the isolated space and all the apparatuses disposed therein may be controlled within 20 and 5O0C.
[25] The method for preparing a peracetic acid solution according to the present invention comprises a preliminary stage, a mixing stage, a main reaction stage, a first aging stage, a dilution stage and a second aging stage.
[26] In the preliminary stage, acetic acid and hydrogen peroxide supplied from first and second storage tanks 1, 2 are passed through column type purifiers 3, 6 to remove metallic impurities including divalent and trivalent cations and to preheat the reactants for reaction. The flow rate of acetic acid and hydrogen peroxide may be effectively controlled using flow meters 4, 5 in the range from 0.1 to 50 mL/min.
[27] In the mixing stage, the acetic acid and hydrogen peroxide that have passed through the flow meters 4, 5 are mixed at a molar ratio determined by the controlled flow rate to prepare a reactant solution.
[28] In the main reaction stage, the reactant solution is passed through a column type
reactor 7 filled with a cation-exchange resin to prepare a reaction product containing peracetic acid. The cation-exchange resin may be an ion-exchange resin having sulfonate groups.
[29] In the first aging stage, the reaction product is aged as it passes through a first coil tube type aging reactor 8 maintained at a constant temperature until the peracetic acid reaction reaches an equilibrium state. As a result, the peracetic acid concentration of the reaction product may be maintained constant.
[30] In the mixing stage, the reaction product is mixed with purified water 10 the supply of which is controlled by a purified water flow meter 9, thereby preparing a diluted peracetic acid stock solution. The purified water 10 may be nearly pure water. For example, reverse-osmosed water or deionized distilled water may be used.
[31] In the second aging stage, the diluted peracetic acid stock solution is subjected again to an equilibrium reaction as it passed through a second coil tube type aging reactor 11. As a result, a final peracetic acid solution the peracetic acid concentration of which is maintained stably at a desired level is obtained. The final peracetic acid solution may be used as a cleansing disinfectant immediately after the preparation. Alternatively, it may be stored in a storage container equipped outside the integrated reaction apparatus for later use.
[32] The composition of the components of the peracetic acid solution for cleansing and disinfection prepared according to the preparation method of the present invention comprising the aforesaid stages may be adjusted for different uses to be provided for cleansing of various medical devices. For example, when removing scales whose main component is calcium carbonate, it is preferable to treat with a peracetic acid solution containing less than 0.1 mol of hydrogen peroxide per 10 mol of acetic acid at first, and then gradually use a peracetic acid solution with a higher hydrogen peroxide content. For another example, when disinfection or sterilization is the main purpose, it will be effective to use a peracetic acid solution having a molar ratio of acetic acid to hydrogen peroxide of 2:1. For another example, when cleansing medical devices to which biofilms or excess organic substances are adhered, it will be effective to use a peracetic acid solution having a molar ratio of acetic acid to hydrogen peroxide of about 1:2 to 1:11. For practical use, peracetic acid solution it may be the most effective not to fix the composition of the components but to perform cleansing or disinfection while gradually changing the composition. Mode for the Invention
[33] The examples and experiments will now be described. The following examples and experiments are for illustrative purposes only and not intended to limit the scope of this disclosure.
[34] Example 1 : Preparation of peracetic acid solution with molar ratio of acetic acid to hydrogen peroxide of 11 : 1
[35] The temperature of an isolated space in which an integrated reaction system for preparation of peracetic acid is located is maintained at 350C. Acetic acid and hydrogen peroxide are respectively passed through a tube type purifier filled with a sulfonate ion-exchange resin. Using flow meters for acetic acid and hydrogen peroxide, acetic acid is passed at a flow rate of 9.5 mL/min and hydrogen peroxide (35% aqueous solution) is passed at a flow rate of 1.3 mL/min, so that the mixing molar ratio of acetic acid to hydrogen peroxide is 11:1. The resultant reactant solution is injected into a column type reactor filled with a sulfonate ion-exchange resin at a rate of 10 mL/min to produce peracetic acid by means of a continuous reaction. A reaction product flowing out of the column type reactor is passed through a first coil tube type aging reactor, so that the production of peracetic acid reaches nearly an equilibrium state. The reaction product flowing out of the first coil tube type aging reactor is mixed with reverse-osmosed water supplied from a purified water flow meter at a rate of 89.2 mL/ min to prepare a diluted peracetic acid solution. The diluted peracetic acid solution is passed through a second coil tube type aging reactor, so that the production of peracetic acid reaches nearly an equilibrium state again.
[36] Example 2: Preparation of peracetic acid solution with molar ratio of acetic acid to hydrogen peroxide of 5: 1
[37] The procedure of Example 1 is repeated, except that acetic acid is passed at a flow rate of 9 mL/min and hydrogen peroxide (35% aqueous solution) is passed at a flow rate of 2.9 mL/min, so that the mixing molar ratio of acetic acid to hydrogen peroxide is 5 : 1 , and that reverse-osmosed water is mixed at a rate of 88.1 mL/min in the dilution stage.
[38] Example 3: Preparation of peracetic acid solution with molar ratio of acetic acid to hydrogen peroxide of 2: 1
[39] The procedure of Example 1 is repeated, except that acetic acid is passed at a flow rate of 7.8 mL/min and hydrogen peroxide (35% aqueous solution) is passed at a flow rate of 6.3 mL/min, so that the mixing molar ratio of acetic acid to hydrogen peroxide is 2: 1 , and that reverse-osmosed water is mixed at a rate of 86.1 mL/min in the dilution stage.
[40] Example 4: Preparation of peracetic acid solution with molar ratio of acetic acid to hydrogen peroxide of 1:2
[41] The procedure of Example 1 is repeated, except that acetic acid is passed at a flow rate of 4.7 mL/min and hydrogen peroxide (35% aqueous solution) is passed at a flow rate of 15 mL/min, so that the mixing molar ratio of acetic acid to hydrogen peroxide is 1:2, and that reverse-osmosed water is mixed at a rate of 80.3 mL/min in the dilution
stage.
[42] Example 5: Preparation of peracetic acid solution with molar ratio of acetic acid to hydrogen peroxide of 1:5 [43] The procedure of Example 1 is repeated, except that acetic acid is passed at a flow rate of 2.6 mL/min and hydrogen peroxide (35% aqueous solution) is passed at a flow rate of 21 mL/min, so that the mixing molar ratio of acetic acid to hydrogen peroxide is 1:5, and that reverse-osmosed water is mixed at a rate of 76.4 mL/min in the dilution stage.
[44] Example 6: Preparation of peracetic acid solution with molar ratio of acetic acid to hydrogen peroxide of 1 : 11 [45] The procedure of Example 1 is repeated, except that acetic acid is passed at a flow rate of 1.3 mL/min and hydrogen peroxide (35% aqueous solution) is passed at a flow rate of 24.9 mL/min, so that the mixing molar ratio of acetic acid to hydrogen peroxide is 1:11, and that reverse-osmosed water is mixed at a rate of 73.8 mL/min in the dilution stage.
[46] Table 1 summarizes the preparation of peracetic acid solutions with different molar proportions of acetic acid and hydrogen peroxide according to Examples 1 to 6. [47] Table 1 [Table 1] [Table ]
[48] As seen in Table 1, by controlling the flow rate of acetic acid and hydrogen peroxide injected to the integrated reaction system, the equilibrium concentration of hydrogen peroxide and peracetic acid in the final peracetic acid solution may be changed variously.
[49] Example 7: Change of equilibrium concentration depending on addition of swelling inhibitor [50] In order to measure the effect of the swelling of the sulfonate ion-exchange resin on the equilibrium of the peracetic acid production reaction, etidronic acid is added as a swelling inhibitor at 0, 0.5 and 1 wt%, and the result is monitored. The experiment is carried out with the molar ratio of acetic acid to hydrogen peroxide fixed at 2: 1 (as in Example 3). The initial total volume of acetic acid, hydrogen peroxide, the swelling inhibitor and water is set as 50 mL.
[51] Table 2 summarizes the measurement of the equilibrium concentration depending on the addition of the swelling inhibitor. [52] Table 2 [Table 2] [Table ]
[53] As seen in Table 2, the peracetic acid concentration of the final peracetic acid solution in the equilibrium state does not show significant change depending on the addition amount of the swelling inhibitor. In addition, no significant instability of peracetic acid production is observed when the swelling inhibitor is not added.
[54] Example 8: Peracetic acid production depending on aging of reaction product following main reaction stage [55] Reaction products are obtained as in Examples 1 to 6 through the preliminary stage, mixing stage and main reaction stage, but without the aging stage, with the weight proportion of the reactants acetic acid and hydrogen peroxide (35% aqueous solution) at 20: 17. The reaction products are aged using either a stirring type aging reactor or a coil tube type aging reactor. The concentration of hydrogen peroxide and peracetic acid in the resultant final peracetic acid solutions are determined. Aging using the stirring type aging reactor is carried out while stirring in a sealed reactor at 450C using
a magnetic bar. Aging using the coil tube type aging reactor is carried out while circulating the reaction product in a sealed coil tube type aging reactor (coil with diameter 1 mm or less) at 450C.
[56] Table 3 summarizes stability of the peracetic acid solutions obtained by aging the stirring type aging reactor and the coil tube type aging reactor. [57] Table 3 [Table 3] [Table ]
[58] H2O2: hydrogen peroxide; PAA: peracetic acid [59] As seen in Table 3, the peracetic acid concentration increases significantly faster when the coil tube type aging reactor is used as compared to when the stirring type aging reactor. Specifically, for example, after 1 hour of aging, the coil tube type aging reactor produces 13.7 wt% of peracetic acid, whereas the stirring type aging reactor produces 10.5 wt% of peracetic acid.
[60] This suggests that the rate of decrease of the hydrogen peroxide concentration in the aged peracetic acid solution due to the production of peracetic acid or simple decomposition and/or evaporation may be different depending on the aging method. This may be quantitatively analyzed by the following equations considering the molecular weights of hydrogen peroxide and peracetic acid.
[61] (1) Decrease of hydrogen peroxide due to production of peracetic acid (wt%) = increase of peracetic acid (wt%) x 34 ÷ 76 [62] (2) Decrease of hydrogen peroxide due to decomposition and/or evaporation (wt%) = experimentally determined decrease of hydrogen peroxide (wt%) - decrease of hydrogen peroxide due to production of peracetic acid (wt%)
[63] When the stirring type aging reactor is used, the experimentally determined decrease of hydrogen peroxide is 7.58 wt% and the decrease of hydrogen peroxide due to production of peracetic acid is 6.03 wt%. Therefore, it can be seen that the decrease of hydrogen peroxide due to decomposition and/or evaporation is 1.55 wt%. In contrast, when the coil tube type aging reactor is used, the experimentally determined decrease is 7.48 wt% and the decrease due to production of peracetic acid is 6.89 wt%. Thus, the decrease of hydrogen peroxide due to decomposition and/or evaporation is only 0.59 wt%.
[64] When the stirring type aging reactor is used, the decomposition rate of hydrogen peroxide relatively increases even when the stirring is performed in a sealed state. As a result, generation of oxygen foams increases and, consequently, the hydrogen peroxide in the solution decreases. This results in a decreased peracetic acid concentration in the peracetic acid solution in the equilibrium state. In contrast, when the coil tube type aging reactor is used, generation of the foams may be suppressed as much as possible since the reaction product flows through a path provided by the thin coil tube having a uniform temperature distribution. The experimental results reveal the advantage of the coil tube type aging reactor as an apparatus capable of minimizing hydrogen peroxide loss during aging due to decomposition and/or evaporation. Likewise, by diluting the reaction product that has passed through the first aging stage by means of the first coil tube type aging reactor with purified water to considering the intended use, and then performing the second aging stage using the second coil tube type aging reactor, peracetic acid concentration in the equilibrium state may be maximized while minimizing loss of hydrogen peroxide.
[65] Example 9: Descaling ability of peracetic acid solution depending on composition
[66] In order to evaluate the ability of removing scales from an artificial kidney that has been used for a long time, samples taken from the artificial kidney machine and the tube are analyzed by X-ray diffractometry. The main components of the scales are calcium carbonate and calcium sulfate. In order to measure the descaling ability of the peracetic acid solutions prepared according to Examples 1 to 6, 0.1 g of artificially prepared calcium carbonate powder is added to 10 mL of each peracetic acid solution of Examples 1 to 6, and the time required for the calcium carbonate powder to be completely dissolved is measured.
[67] Table 4 summarizes the descaling ability of the peracetic acid solutions.
[68] Table 4
[Table 4] [Table ]
[69] As seen in Table 4, the descaling ability of the peracetic acid solutions prepared by increasing the molar ratio of the reactants acetic acid to hydrogen peroxide from 11:1 to 1:11 increases in proportion to the molar ratio.
[70] Example 10: Biofilm removal ability of peracetic acid solution depending on composition
[71] In order to evaluate the biofilm removal ability of peracetic acid solutions with different compositions, a stainless steel plate is cut to a size of 1 cm x 1 cm and sterilized, and an adherent microcolony is prepared thereon. The microcolony is observed after cleansing and disinfecting with the peracetic acid solutions prepared according to Examples 1 to 6. As a result, it is revealed that the peracetic acid solution prepared according to Example 6 exhibits the most superior biofilm removal ability.
[72] Example 11 : Sterilizing power of peracetic acid solution depending on composition
[73] In order to measure the sterilizing power of the peracetic acid solutions with different compositions, the sterilizing power of the peracetic acid solutions prepared according to Examples 1 to 6 against bacteria and fungi is tested. Specifically, 0.1 mL of bacterial solution incubated for 24 hours in nutrient broth and tryptic soy broth is added to 10 mL of the peracetic acid solutions prepared according to Examples 1 to 6, each diluted 10 times with purified water. After mixing by agitation for 30 seconds, followed by allowing to stand for 1 minute, 1 mL of the reaction solution is taken and immediately added a test tube containing 9 mL of a neutralizer (4.26% sodium phosphate dibasic, 3% Tween, 0.5% sodium thiosulfate pentahydrate, 0.3% L-α-lecithin and 0.1% L- histidine). Then, 1 mL is taken from the test tube and diluted 10 times with peptone water. For comparative experiment depending on concentration, each 0.1 mL of the solution from the test tube and the 10x diluted solution thereof are applied on a previously prepared plate medium. The samples are incubated in a 30-350C incubator for 3 days. After counting the number of colonies in all samples, the number is divided by the number of plates to calculate the average number of colonies per plate.
[74] Table 5 summarizes the sterilizing power of the peracetic acid solutions with different compositions.
[75] Table 5
[Table 5] [Table ]
Industrial Applicability
[76] According to the present invention, a peracetic acid solution appropriate for the purpose of cleansing or sterilization may be prepared safely and quickly. The preparation method enables a continuous preparation of peracetic acid solutions with desired mixing proportion of hydrogen peroxide and acetic acid and peracetic acid concentration, appropriate for various uses, while changing them in real time. Accordingly, an effective cleansing and disinfection is possible by using a disinfectant cleansing solution the composition of which is adjusted in real time depending on the desired environmental factors of medical devices or equipments or depending on the required cleansing or sterilization requirements. In addition, the peracetic acid aqueous solution prepared in accordance with the present invention is an environment-friendly disinfectant in the true sense of the word, since it exhibits a desired cleansing and disinfection effect without using a commonly used catalyst such as phosphoric acid, a stabilizer or a swelling inhibitor, which are added to improve stability of the peracetic acid concentration, or other chemical substances.
[77] While the exemplary embodiments have been shown and described, it will be understood by those skilled in the art that various changes in form and details may be made thereto without departing from the spirit and scope of this disclosure as defined by the appended claims.
[78] In addition, many modifications can be made to adapt a particular situation or material to the teachings of this disclosure without departing from the essential scope thereof. Therefore, it is intended that this disclosure not be limited to the particular
exemplary embodiments disclosed as the best mode contemplated for carrying out this disclosure, but that this disclosure will include all embodiments falling within the scope of the appended claims.
Claims
[1] A method for preparing a peracetic acid solution comprising: a preliminary stage of passing hydrogen peroxide and acetic acid respectively through a tube type purifier filled with a first cation-exchange resin, thereby removing metallic impurities included in the hydrogen peroxide and acetic acid and stabilizing temperature; a mixing stage of mixing the hydrogen peroxide and acetic acid by means of continuous flow injection at a molar ratio of 1:20 to 20:1, thereby preparing a reactant solution; a main reaction stage of continuously injecting the reactant solution to a column type reactor filled with a second cation-exchange resin, thereby producing peracetic acid; a first aging stage of continuously passing the resulting reaction product through a first coil tube type aging reactor until the peracetic acid production reaches an equilibrium state; a dilution stage of mixing the reaction product with purified water, thereby preparing a diluted peracetic acid solution; and a second aging stage of continuously passing the diluted peracetic acid solution through a second coil tube type aging reactor until the peracetic acid production reaches an equilibrium state again, thereby preparing a final peracetic acid solution having a stabilized peracetic acid concentration.
[2] The method for preparing a peracetic acid solution according to claim 1, wherein the tube type purifier, the column type reactor, and the first and second coil tube type aging reactors are disposed in an integrated space cut off from the air the temperature of which is maintained at 20-500C.
[3] The method for preparing a peracetic acid solution according to claim 1, wherein the change of the molar ratio of acetic acid to hydrogen peroxide of the reactant solution in the mixing stage is performed continuously by changing the relative injection volume of acetic acid and hydrogen peroxide supplied by means of flow injection, without interrupting the production of peracetic acid.
[4] The method for preparing a peracetic acid solution according to claim 1, wherein the first and second cation-exchange resins are cation-exchange resins having sulfonate groups.
[5] A peracetic acid solution prepared by the method according to claim 1, which comprises 1.0-35 wt% of acetic acid, 1.0-35 wt% of hydrogen peroxide, 0.1-20 wt% of peracetic acid, and 10-97.9 wt% of reverse-osmosed water.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/KR2008/006423 WO2010050634A1 (en) | 2008-10-30 | 2008-10-30 | Manufacturing method for peracetic acid solution using column-type reactor and coil-tube-type aging reactor |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/KR2008/006423 WO2010050634A1 (en) | 2008-10-30 | 2008-10-30 | Manufacturing method for peracetic acid solution using column-type reactor and coil-tube-type aging reactor |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2010050634A1 true WO2010050634A1 (en) | 2010-05-06 |
Family
ID=42128985
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/KR2008/006423 WO2010050634A1 (en) | 2008-10-30 | 2008-10-30 | Manufacturing method for peracetic acid solution using column-type reactor and coil-tube-type aging reactor |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2010050634A1 (en) |
Cited By (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8729296B2 (en) | 2010-12-29 | 2014-05-20 | Ecolab Usa Inc. | Generation of peroxycarboxylic acids at alkaline pH, and their use as textile bleaching and antimicrobial agents |
| US8846107B2 (en) | 2010-12-29 | 2014-09-30 | Ecolab Usa Inc. | In situ generation of peroxycarboxylic acids at alkaline pH, and methods of use thereof |
| CN104098494A (en) * | 2014-07-28 | 2014-10-15 | 青岛科技大学 | Device and method for continuous production of propionic acid peroxide |
| US8889900B2 (en) | 2010-12-29 | 2014-11-18 | Ecolab Usa Inc. | Sugar ester peracid on site generator and formulator |
| CN104193662A (en) * | 2014-05-22 | 2014-12-10 | 上海应用技术学院 | Preparing method of peracetic acid |
| US9321664B2 (en) | 2011-12-20 | 2016-04-26 | Ecolab Usa Inc. | Stable percarboxylic acid compositions and uses thereof |
| US9518013B2 (en) | 2014-12-18 | 2016-12-13 | Ecolab Usa Inc. | Generation of peroxyformic acid through polyhydric alcohol formate |
| US9845290B2 (en) | 2014-12-18 | 2017-12-19 | Ecolab Usa Inc. | Methods for forming peroxyformic acid and uses thereof |
| US9926214B2 (en) | 2012-03-30 | 2018-03-27 | Ecolab Usa Inc. | Use of peracetic acid/hydrogen peroxide and peroxide-reducing agents for treatment of drilling fluids, frac fluids, flowback water and disposal water |
| US10031081B2 (en) | 2013-03-05 | 2018-07-24 | Ecolab Usa Inc. | Peroxycarboxylic acid compositions suitable for inline optical or conductivity monitoring |
| US10165774B2 (en) | 2013-03-05 | 2019-01-01 | Ecolab Usa Inc. | Defoamer useful in a peracid composition with anionic surfactants |
| CN109430249A (en) * | 2018-12-11 | 2019-03-08 | 张全 | A kind of low-temperature storage device for reproductive medicine |
| US10893674B2 (en) | 2013-03-05 | 2021-01-19 | Ecolab Usa Inc. | Efficient stabilizer in controlling self accelerated decomposition temperature of peroxycarboxylic acid compositions with mineral acids |
| US11040902B2 (en) | 2014-12-18 | 2021-06-22 | Ecolab Usa Inc. | Use of percarboxylic acids for scale prevention in treatment systems |
| WO2021183516A1 (en) * | 2020-03-09 | 2021-09-16 | Kemira Oyj | Performic acid production systems and methods |
| US11260040B2 (en) | 2018-06-15 | 2022-03-01 | Ecolab Usa Inc. | On site generated performic acid compositions for teat treatment |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994006294A1 (en) * | 1992-09-15 | 1994-03-31 | Solvay Interox Limited | Microbicidal compositions and methods |
| US5368867A (en) * | 1990-02-23 | 1994-11-29 | Peroxidos Do Brasil | Process for the accelerated production of stable solutions, in equilibrium, of peracetic acid in low concentrations |
| US5886216A (en) * | 1996-01-31 | 1999-03-23 | Eka Chemicals Ab | Method for producing a chemical product |
| JP2001199811A (en) * | 2000-01-20 | 2001-07-24 | Mitsubishi Gas Chem Co Inc | Aqueous solution containing peracetic acid |
-
2008
- 2008-10-30 WO PCT/KR2008/006423 patent/WO2010050634A1/en active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5368867A (en) * | 1990-02-23 | 1994-11-29 | Peroxidos Do Brasil | Process for the accelerated production of stable solutions, in equilibrium, of peracetic acid in low concentrations |
| WO1994006294A1 (en) * | 1992-09-15 | 1994-03-31 | Solvay Interox Limited | Microbicidal compositions and methods |
| US5886216A (en) * | 1996-01-31 | 1999-03-23 | Eka Chemicals Ab | Method for producing a chemical product |
| JP2001199811A (en) * | 2000-01-20 | 2001-07-24 | Mitsubishi Gas Chem Co Inc | Aqueous solution containing peracetic acid |
Cited By (50)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9763442B2 (en) | 2010-12-29 | 2017-09-19 | Ecolab Usa Inc. | In situ generation of peroxycarboxylic acids at alkaline pH, and methods of use thereof |
| US11678664B2 (en) | 2010-12-29 | 2023-06-20 | Ecolab Usa Inc. | Water temperature as a means of controlling kinetics of onsite generated peracids |
| US11330818B2 (en) | 2010-12-29 | 2022-05-17 | Ecolab Usa Inc. | Water temperature as a means of controlling kinetics of onsite generated peracids |
| US10201156B2 (en) | 2010-12-29 | 2019-02-12 | Ecolab Usa Inc. | Continuous on-line adjustable disinfectant/sanitizer/bleach generator |
| US8877254B2 (en) | 2010-12-29 | 2014-11-04 | Ecolab Usa Inc. | In situ generation of peroxycarboxylic acids at alkaline pH, and methods of use thereof |
| US8889900B2 (en) | 2010-12-29 | 2014-11-18 | Ecolab Usa Inc. | Sugar ester peracid on site generator and formulator |
| US10477862B2 (en) | 2010-12-29 | 2019-11-19 | Ecolab Usa Inc. | In situ generation of peroxycarboxylic acids at alkaline pH, and methods of use thereof |
| US8933263B2 (en) | 2010-12-29 | 2015-01-13 | Ecolab Usa Inc. | Water temperature as a means of controlling kinetics of onsite generated peracids |
| US9192909B2 (en) | 2010-12-29 | 2015-11-24 | Ecolab USA, Inc. | Sugar ester peracid on site generator and formulator |
| US10244751B2 (en) | 2010-12-29 | 2019-04-02 | Ecolab Usa Inc. | Water temperature as a means of controlling kinetics of onsite generated peracids |
| US9365509B2 (en) | 2010-12-29 | 2016-06-14 | Ecolab Usa Inc. | Continuous on-line adjustable disinfectant/sanitizer/bleach generator |
| US8729296B2 (en) | 2010-12-29 | 2014-05-20 | Ecolab Usa Inc. | Generation of peroxycarboxylic acids at alkaline pH, and their use as textile bleaching and antimicrobial agents |
| US9505715B2 (en) | 2010-12-29 | 2016-11-29 | Ecolab Usa Inc. | Sugar ester peracid on site generator and formulator |
| US11311011B2 (en) | 2010-12-29 | 2022-04-26 | Ecolab Usa Inc. | Continuous on-line adjustable disinfectant/sanitizer/bleach generator |
| US8858895B2 (en) | 2010-12-29 | 2014-10-14 | Ecolab Usa Inc. | Continuous on-line adjustable disinfectant/sanitizer/bleach generator |
| US9861101B2 (en) | 2010-12-29 | 2018-01-09 | Ecolab Usa Inc. | Continuous on-line adjustable disinfectant/sanitizer/bleach generator |
| US8846107B2 (en) | 2010-12-29 | 2014-09-30 | Ecolab Usa Inc. | In situ generation of peroxycarboxylic acids at alkaline pH, and methods of use thereof |
| US9883672B2 (en) | 2010-12-29 | 2018-02-06 | Ecolab Usa Inc. | Sugar ester peracid on site generator and formulator |
| US10010075B2 (en) | 2010-12-29 | 2018-07-03 | Ecolab Usa Inc. | Water temperature as a means of controlling kinetics of onsite generated peracids |
| US9321664B2 (en) | 2011-12-20 | 2016-04-26 | Ecolab Usa Inc. | Stable percarboxylic acid compositions and uses thereof |
| US9926214B2 (en) | 2012-03-30 | 2018-03-27 | Ecolab Usa Inc. | Use of peracetic acid/hydrogen peroxide and peroxide-reducing agents for treatment of drilling fluids, frac fluids, flowback water and disposal water |
| US10017403B2 (en) | 2012-03-30 | 2018-07-10 | Ecolab Usa Inc. | Use of peracetic acid/hydrogen peroxide and peroxide-reducing enzymes for treatment of drilling fluids, frac fluids, flowback water and disposal water |
| US10023484B2 (en) | 2012-03-30 | 2018-07-17 | Ecolab Usa Inc. | Use of peracetic acid/hydrogen peroxide and peroxide-reducing agents for treatment of drilling fluids, frac fluids, flowback water and disposal water |
| US11180385B2 (en) | 2012-10-05 | 2021-11-23 | Ecolab USA, Inc. | Stable percarboxylic acid compositions and uses thereof |
| US11939241B2 (en) | 2012-10-05 | 2024-03-26 | Ecolab Usa Inc. | Stable percarboxylic acid compositions and uses thereof |
| US11026421B2 (en) | 2013-03-05 | 2021-06-08 | Ecolab Usa Inc. | Efficient stabilizer in controlling self accelerated decomposition temperature of peroxycarboxylic acid compositions with mineral acids |
| US11206826B2 (en) | 2013-03-05 | 2021-12-28 | Ecolab Usa Inc. | Defoamer useful in a peracid composition with anionic surfactants |
| US10165774B2 (en) | 2013-03-05 | 2019-01-01 | Ecolab Usa Inc. | Defoamer useful in a peracid composition with anionic surfactants |
| US10031081B2 (en) | 2013-03-05 | 2018-07-24 | Ecolab Usa Inc. | Peroxycarboxylic acid compositions suitable for inline optical or conductivity monitoring |
| US10893674B2 (en) | 2013-03-05 | 2021-01-19 | Ecolab Usa Inc. | Efficient stabilizer in controlling self accelerated decomposition temperature of peroxycarboxylic acid compositions with mineral acids |
| CN104193662A (en) * | 2014-05-22 | 2014-12-10 | 上海应用技术学院 | Preparing method of peracetic acid |
| CN104098494B (en) * | 2014-07-28 | 2016-06-22 | 青岛科技大学 | The continuous production device of a kind of Perpropionic Acid and production method |
| CN104098494A (en) * | 2014-07-28 | 2014-10-15 | 青岛科技大学 | Device and method for continuous production of propionic acid peroxide |
| US10433547B2 (en) | 2014-12-18 | 2019-10-08 | Ecolab Usa Inc. | Generation of peroxyformic acid through polyhydric alcohol formate |
| US10899707B2 (en) | 2014-12-18 | 2021-01-26 | Ecolab Usa Inc. | Methods for forming peroxyformic acid and uses thereof |
| US10542751B2 (en) | 2014-12-18 | 2020-01-28 | Ecolab Usa Inc. | Generation of peroxyformic acid through polyhydric alcohol formate |
| US11040902B2 (en) | 2014-12-18 | 2021-06-22 | Ecolab Usa Inc. | Use of percarboxylic acids for scale prevention in treatment systems |
| US10709131B2 (en) | 2014-12-18 | 2020-07-14 | Ecolab Usa Inc. | Generation of peroxyformic acid through polyhydric alcohol formate |
| US9518013B2 (en) | 2014-12-18 | 2016-12-13 | Ecolab Usa Inc. | Generation of peroxyformic acid through polyhydric alcohol formate |
| US10233149B2 (en) | 2014-12-18 | 2019-03-19 | Ecolab Usa Inc. | Methods for forming peroxyformic acid and uses thereof |
| US10834924B2 (en) | 2014-12-18 | 2020-11-17 | Ecolab Usa Inc. | Generation of peroxyformic acid through polyhydric alcohol formate |
| US11772998B2 (en) | 2014-12-18 | 2023-10-03 | Ecolab Usa Inc. | Use of percarboxylic acids for scale prevention in treatment systems |
| US11325887B2 (en) | 2014-12-18 | 2022-05-10 | Ecolab Usa Inc. | Methods for forming peroxyformic acid and uses thereof |
| US9845290B2 (en) | 2014-12-18 | 2017-12-19 | Ecolab Usa Inc. | Methods for forming peroxyformic acid and uses thereof |
| US11684067B2 (en) | 2014-12-18 | 2023-06-27 | Ecolab Usa Inc. | Generation of peroxyformic acid through polyhydric alcohol formate |
| US11260040B2 (en) | 2018-06-15 | 2022-03-01 | Ecolab Usa Inc. | On site generated performic acid compositions for teat treatment |
| US11771673B2 (en) | 2018-06-15 | 2023-10-03 | Ecolab Usa Inc. | On site generated performic acid compositions for teat treatment |
| CN109430249A (en) * | 2018-12-11 | 2019-03-08 | 张全 | A kind of low-temperature storage device for reproductive medicine |
| CN115334883A (en) * | 2020-03-09 | 2022-11-11 | 凯米拉公司 | Performic acid production system and method |
| WO2021183516A1 (en) * | 2020-03-09 | 2021-09-16 | Kemira Oyj | Performic acid production systems and methods |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2010050634A1 (en) | Manufacturing method for peracetic acid solution using column-type reactor and coil-tube-type aging reactor | |
| AU686127B2 (en) | Compositions and uses thereof | |
| JP4533618B2 (en) | Disinfectant cleaning composition | |
| EP0609266B1 (en) | Compositions and uses thereof | |
| US5696046A (en) | Cold sterilant solution | |
| JP2009507903A (en) | Method for preparing peracid | |
| CN104206413B (en) | A kind of thimerosal for haemodialysis control unit cleaning and sterilizing and preparation method thereof | |
| CN110367275A (en) | A kind of thimerosal and preparation method thereof | |
| KR100876684B1 (en) | Preparation method of acidic antibiotic composition containing peracid and acetyl salicylic acid | |
| JP2023171397A (en) | Method for producing chlorous acid water using material obtained by salt electrolysis as raw material | |
| US6007772A (en) | Cold sterilant solution | |
| JP2010184868A (en) | Peracetic acid aqueous solution excellent in stability | |
| KR101032872B1 (en) | Manufacturing method for peracetic acid solution using column-type reactor and coil-tube-type aging reactor | |
| EP1117294B1 (en) | Multi-part anti-microbial sterilization compositions and methods | |
| CN104041511B (en) | A kind of solution for haemodialysis control unit sterilization and cleaning and preparation method thereof | |
| JP2011502143A (en) | Bactericidal composition comprising peracid and acetylsalicylic acid | |
| KR102307392B1 (en) | Peracetic acid disinfectant composition for medical devices with improved stability | |
| KR102353182B1 (en) | Percitric acid aqueous solution and method for producing the same | |
| KR102382461B1 (en) | Composition of generating pure chlorine dioxide and preparing method of the same | |
| WO2004093859A1 (en) | Dilution-type sterilizer composition | |
| KR20000049667A (en) | A medical instrument cleaning solution using peracetic acid and the manufacturing method of the above solution | |
| CN113749094B (en) | Peracetic acid disinfectant for endoscope and preparation method thereof | |
| JP2004217565A (en) | Fungicide and method for solving bulking and scumming of activated sludge | |
| SU1172514A1 (en) | Method of obtaining desinfection agent | |
| KR102353181B1 (en) | Peracetic acid aqueous solution and method for producing the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 08877793 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 08877793 Country of ref document: EP Kind code of ref document: A1 |