WO2009052132A1 - Human amniotic fluid derived mesenchymal stem cells - Google Patents
Human amniotic fluid derived mesenchymal stem cells Download PDFInfo
- Publication number
- WO2009052132A1 WO2009052132A1 PCT/US2008/079916 US2008079916W WO2009052132A1 WO 2009052132 A1 WO2009052132 A1 WO 2009052132A1 US 2008079916 W US2008079916 W US 2008079916W WO 2009052132 A1 WO2009052132 A1 WO 2009052132A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- amniotic fluid
- human
- mscs
- cells
- stem cells
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0603—Embryonic cells ; Embryoid bodies
- C12N5/0605—Cells from extra-embryonic tissues, e.g. placenta, amnion, yolk sac, Wharton's jelly
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N1/00—Preservation of bodies of humans or animals, or parts thereof
- A01N1/02—Preservation of living parts
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N1/00—Preservation of bodies of humans or animals, or parts thereof
- A01N1/02—Preservation of living parts
- A01N1/0205—Chemical aspects
- A01N1/021—Preservation or perfusion media, liquids, solids or gases used in the preservation of cells, tissue, organs or bodily fluids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/48—Reproductive organs
- A61K35/50—Placenta; Placental stem cells; Amniotic fluid; Amnion; Amniotic stem cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0662—Stem cells
- C12N5/0668—Mesenchymal stem cells from other natural sources
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
Definitions
- the invention relates to methods for the collection and culture-expansion of human mesenchymal stem cells (huMSCs) from human amniotic fluid in the absence of non- human animal products, and the cryopreservation of huMSCs to yield a composition of expanded amniotic fluid derived mesenchymal stem cells (MSCs) for tissue engineering, tissue repair, and wound healing in humans.
- HuMSCs can be stored frozen, subsequently thawed and used for differentiation into a variety of mesenchymal lineage tissues for tissue engineering and tissue repairs in humans.
- Human stem cells are totipotential or pluripotential precursor cells capable of generating a variety of mature human cell lineages. This ability serves as the basis for the cellular differentiation and specialization necessary for organ and tissue development.
- stem cells can be employed to repopulate many, if not all, tissues and restore physiologic and anatomic functionality.
- tissue engineering, gene therapy delivery and cell therapeutics is also advancing rapidly.
- stem cells Many different types of mammalian stem cells have been characterized. For example, embryonic stem cells, embryonic germ cells, adult stem cells or other committed stem cells are known. Certain stem cells have not only been isolated and characterized but have also been cultured under conditions to allow differentiation to a limited extent.
- Stem cells are in critically short supply. These are important for the treatment of a wide variety of disorders, including malignancies, inborn errors of metabolism, congenital tissue defects, hemoglobinopathies, and immunodeficiencies.
- FBS fetal calf serum
- FBS fetal bovine serum
- FCS or FBS fetal stem cells
- the amniotic fluid can be a good source of human stem cells.
- the human amniotic fluid represents a rich source of a variety of stem cells, for example, hematopoietic stem cells and MSCs (Fauza DO., Best Pract. Res. Clin. Obstet. Gynaecol., 2004,18: 877-91).
- stem cells derived from human amniotic fluid can free clinicians, researchers, and scientists from the ethical concerns associated with human embryonic cells (Holden, C. Science 2007, 315: 170).
- MSCs are generally recognized as pluripotential cells which are capable of dividing many times to produce progeny cells that can eventually give rise to mesoderm derived tissues, including cartilage, bone, tendon, ligament, marrow stroma and connective tissue.
- these MSCs are generally considered to not be governed by, or are not limited to, a fixed number of mitotic divisions (Caplan, 1991, J. Orthopaed. Res. 9:641-650).
- U.S. Pat. Nos. 5,197,985 and 5,226,914 both described processes for isolating and replicating human bone marrow-derived MSCs in culture, and activating them so that they differentiate either into bone or, purportedly, into cartilage.
- U.S. Pat. No. 5,486,359 described human MSC (huMSCs) and monoclonal antibodies to these cells.
- MSCs have been shown to possess remarkable plasticity [1, 2].
- MSCs normally found in the amniotic fluid could be used in tissue engineering strategies for the surgical repair of congenital anomalies in the perinatal period [12, 13].
- tissue engineering strategies for the surgical repair of congenital anomalies in the perinatal period [12, 13].
- tissue grafts could be engineered in parallel to the remainder of the fetus gestation period, so that a child could benefit from having autologous, expanded tissue promptly available for surgical reconstruction, either in the neonatal period or before birth (Fig.
- Embodiments of the invention provide methods for isolating, expanding, and enriching human fetal mesenchymal stem cells (MSCs) from human amniotic fluid in the absence of non-human derived animal products, cryopreserving the human fetal MSC in the absence of non-human derived animal products for future uses, thawing the cryopreserved MSCs for therapeutic use and/or further cell expansion, expanding the thawed previously cryopreserved stem cells in the absence of non-human derived animal products, and differentiating the MSC into several cell lineages including osteogenic, myogenic, adipogenic, chondrogenic, neurogenic, hepatogenic, nephrogenic, urogenic, isletogenic, pancreatogenic, gastroenterogenic, epitheliogenic, thyroidogenic, myocardiogenic, pneumogenic, retinogenic, gametogenic, endotheliogenic, or hematopoietic lineages.
- MSCs human fetal mesenchymal stem cells
- Undifferentiated and differentiated MSC can be used for tissue engineering, tissue repair and/or wound healing.
- the tissue engineering, tissue repair, and wound healing can be performed for/in an autologous individual from which the MSCs were derived, or in HLA type matched individual that is HLA typed matched with the donor of the MSCs.
- embodied in the invention is a method of obtaining a composition enriched in human amniotic fluid derived MSCs comprising isolating MSCs from a sample of human amniotic fluid in the absence of non-human animal derived products and expanding the isolated human amniotic fluid derived MSCs in the absence of non-human derived products.
- the method further comprises cryopreserving the composition enriched in human amniotic fluid derived MSCs.
- the human amniotic fluid can be collected between 5 weeks of gestation to human term or even at birth.
- the fluid can be collected by a skilled physician specialized during routine diagnostic amniocentesis and is performed under sterile conditions.
- a volume of 5-1OmI of amniotic fluid is preferred for the method described herein for the isolation of the human amniotic fluid derived MSCs.
- the human amniotic fluid can be cryopreserved directly, or the cells from the amniotic fluid can be harvested by centrifugation and the harvested cells can then be cryopreserved.
- the isolation and expansion of human amniotic fluid derived MSCs is carried out prior to cryopreservation. The isolation and expansion is performed in absence of non-human animal derived product. In a preferred embodiment, the isolation and expansion is performed in the presence of human serum, either autologous or allogeneic AB serum, or in the presence of human platelet rich plasma supplemented with heparin. In another embodiment, the isolation and expansion is performed under serum-free conditions.
- the composition enriched in human amniotic fluid derived MSCs is cryopreserved.
- the cryopreserved composition of enriched in human amniotic fluid derived MSCs is thawed such that the MSCs are viable.
- MSCs are at least 90% CD29, CD73, and CD44 positive, at least 50% CD90 and CD105 positive, and at most 5% CD34 and CD45 positive.
- the method for obtaining a composition enriched in human amniotic fluid derived MSCs further comprises thawing the cryopreserved composition of enriched in human amniotic fluid derived MSCs such that the stem cells are viable.
- a method of proliferating a composition enriched in human amniotic fluid derived MSCs comprising selecting at least one single MSC from a sample of human amniotic fluid in the absence of non-human animal derived products, introducing at least one single MSC to a culture medium containing no non-human animal derived product, and proliferating at least one single MSC to a culture medium containing no non-human animal derived product.
- Also envisioned in the invention is a method of storing a composition enriched in human amniotic fluid derived MSCs comprising obtaining a composition enriched in human amniotic fluid derived MSCs according to the methods described herein and cryopreserving the composition enriched in human amniotic fluid derived MSCs.
- the invention provides an isolated human amniotic fluid derived MSC prepared according to the methods described herein.
- the invention provides for a kit for obtaining a composition enriched in human amniotic derived MSCs from human amniotic fluid comprising a container for the collection of human amniotic fluid, a coated container for the isolation and primary expansion of human amniotic fluid derived MSCs, human serum for the culture- expansion of human amniotic fluid derived MSC, and instructions for the isolation, identification, and expansion of human amniotic fluid derived MSCs.
- the kit further comprises culture medium reagents for reconstituting a culture medium containing human serum for use in the isolation and expansion of the MSCs.
- the invention provides for a method for producing differentiated human amniotic fluid derived MSC preparations comprising obtaining a composition enriched in human amniotic fluid derived MSCs according to the methods described herein and culturing the composition enriched in human amniotic fluid derived MSCs in a culture medium containing differentiation factors for a period sufficient for the MSCs to differentiate and express specific tissue markers.
- a variety of differentiating factors can be used and they are selected from a group consisting of osteogenic, myogenic, adipogenic or chondrogenic, neurogenic, hepatogenic, nephrogenic, urogenic, isletogenic, pancreatogenic, gastroenterogenic, epitheliogenic, thyroidogenic, myocardiogenic, pneumogenic, retinogenic, gametogenic endotheliogenic, or hematopoietic factors.
- the MSCs are seeded on a scaffold during the differentiation process.
- the invention provides a method of promoting wound healing and/or tissue repair in a human in need thereof comprising administering a preparation comprising a composition enriched in human amniotic fluid derived MSCs wherein the stem cell preparation is applied directly to the wound and/or tissue needing repair.
- a preparation comprising a composition enriched in human amniotic fluid derived MSCs wherein the stem cell preparation is applied directly to the wound and/or tissue needing repair.
- the MSCs preparation is embedded in a wound dressing material such as a gauze and the seeded wound dressing material is applied on to the wound.
- the invention provides a method of storing human amniotic fluid derived cells comprising harvesting the cells from a sample of human amniotic fluid and cryopreserving the cells such that the cells remain viable upon thawing.
- cryopreservation agents such as DMSO and glycerol are added to the harvested cells that are at a density of at least 3 X 10 cells/ml and the temperature of the mixture of cells is lowered slowly, for example, at a rate of one degree per minute to -196 0 C.
- 5 X 10 6 cells/ml are cryopreserved in PlasmalyteA with 2.5% human serum albumin and 10% DMSO.
- 20 xlO 6 cells are cryopreserved per cryovials.
- cryopreserved pharmaceutical composition comprising a viable composition enriched in human amniotic fluid derived MSCs obtained according to the method described herein, wherein the MSCs are present in an amount sufficient to effect tissue engineering or wound healing; an amount of cryopreservative sufficient for cryopreservation of said cells; and a pharmaceutically acceptable carrier.
- a pharmaceutical composition comprising a viable composition enriched in human amniotic fluid derived MSCs obtained according to the methods described herein, wherein the MSCs are present in an amount sufficient to effect tissue engineering or wound healing and a pharmaceutically acceptable carrier.
- Figure 1 A nanofiber bone construct engineered with electrospun poly(L-lactic) acid nanofibrous scaffolds and expanded amniotic MSCs.
- Bone mineral density in the nanofiber engineered bone construct is directly related to the period spent in culture and differentiation but is not directly related to the initial number of expanded amniotic MSCs seeded on the nanofiber scaffold during culture and differentiation.
- Figure 4 Osteogenic differentiation of the expanded MSCs seeded on the nanofiber bone construct as determined by quantitative alkaline phosphastase activity.
- Figure 5 The clinical concept of amniotic fluid-based fetal tissue engineering for the surgical treatment of congenital anomalies: fetal MSCs isolated from the amniotic fluid are expanded ex vivo and used in an implantable engineered construct either later in gestation, or in postnatal life, for the treatment of a prenatally diagnosed defect.
- Figure 6 Graph of the logarithmic cell expansion rates based on the number of days since the first cell passage (relative days). There were no statistical differences in the growth kinetics of amniotic fluid-derived MSCs cultured in fetal bovine serum when compared to those grown in human AB serum (P>0.05).
- FBS fetal bovine serum
- HAB human AB serum.
- Figure 7 Representative ungated flow cytometry analyses of expanded huMSCs isolated from amniotic fluid. There were no differences in the immunophenotypic profiles of cells grown in fetal bovine serum (thinner line) compared to those cultured in human AB serum (thicker line).
- AF amniotic fluid
- BM neonatal bone marrow
- CB prenatal umbilical cord blood
- AF amniotic fluid
- BM neonatal bone marrow
- CB prenatal umbilical cord blood
- Figure 9 Total DNA levels, expressed as means + SEM, of native fetal hyaline cartilage (hyaline), native fetal elastic cartilage (elastic), engineered cartilage from neonatal bone marrow (BM)-derived MSCs (MSCs), engineered cartilage from preterm umbilical cord blood (CB)-derived MSCs, and engineered cartilage from amniotic fluid (AF)-derived MSCs.
- BM bone marrow
- CB preterm umbilical cord blood
- AF amniotic fluid
- FIG. 10 Sulfated glycosaminoglycan levels, expressed as means + SEM, in native fetal hyaline cartilage (hyaline),native fetal elastic cartilage (elastic), engineered cartilage from neonatal bone marrow (BM)-derived MSCs (MSCs), engineered cartilage from preterm umbilical cord blood (CB)-derived MSCs, and engineered cartilage from amniotic fluid (AF)- derived MSCs. (*p ⁇ 0.05 compared to native hyaline cartilage; # p ⁇ 0.05 compared to native elastic cartilage) (magnification, x 400).
- FIG. 11 Pepsin-soluble collagen levels, expressed as means + SEM, in native fetal hyaline cartilage (hyaline), native fetal elastic cartilage (elastic), engineered cartilage from neonatal bone marrow (BM)-derived MSCs (MSCs), engineered cartilage from preterm umbilical cord blood (CB)-derived MSCs, and engineered cartilage from amniotic fluid (AF)- derived MSCs. (*p ⁇ 0.05 compared to native hyaline cartilage; # p ⁇ 0.05 compared to native elastic cartilage) (magnification, x 400). [0040] Figure 12.
- Elastin levels expressed as means + SEM, in native fetal hyaline cartilage (hyaline), native fetal elastic cartilage (elastic), engineered cartilage from neonatal bone marrow (BM)-derived MSCs (MSCs), engineered cartilage from preterm umbilical cord blood (CB)-derived MSCs, and engineered cartilage from amniotic fluid (AF)-derived MSCs.
- hyaline native fetal hyaline cartilage
- BM bone marrow
- CB preterm umbilical cord blood
- AF amniotic fluid
- Embodiments of the invention provide methods for isolating, expanding, and enriching human fetal MSCs from human amniotic fluid in the absence of non-human animal derived products, cryopreserving the human fetal MSCs in the absence of non-human animal derived products for future uses, thawing the cryopreserved stem cells for therapeutic use and/or further cell expansion, expanding the thawed previously cryopreserved stem cells in the absence of non-human animal derived products, and differentiating the MSCs into several cell lineages including osteogenic, myogenic, adipogenic, chondrogenic, neurogenic, hepatogenic, nephrogenic, urogenic, isletogenic, pancreatogenic, gastroenterogenic, epitheliogenic, thyroidogenic, myocardiogenic, pneumogenic, retinogenic, gametogenic, endotheliogenic, or hematopoietic lineages.
- Undifferentiated and differentiated MSCs can be used for tissue engineering, tissue repair and wound healing.
- the tissue engineering, tissue repair, and wound healing can be performed in an autologous individual from which the MSCs were derived, or in HLA type matched individual, that is HLA typed matched with the donor of the MSCs.
- the invention provides a multi stage process for obtaining a composition enriched in human amniotic fluid derived MSCs, and the composition of MSCs can be further differentiated for tissue engineering, tissue repair, and/or wound repair of a future recipient.
- the method of obtaining a composition enriched in human amniotic fluid derived MSCs comprise the steps of: (a) isolating MSCs from a sample of human amniotic fluid in the absence of non-human animal derived products; and (b) expanding the isolated human amniotic fluid derived MSCs in the absence of non-human animal derived products.
- MSCs can be autologous to the future recipient of the composition, that is derived from the same individual.
- the composition of MSCs can be non-autologous to the future recipient of the composition, that is the donor of the human amniotic fluid derived MSCs is different from the recipient of the MSCs. It is envisioned that proper human leukocyte antigen (HLA) matching between the donor and the recipient be conducted prior to the use of the composition of MSCs.
- HLA human leukocyte antigen
- the multi stage process comprises: (stage-1) isolating MSCs from a sample of human amniotic fluid in the absence of non-human animal derived products; (stage 2) expanding the isolated human amniotic fluid derived MSCs in the absence of non-human animal derived products; and (stage 3) cryopreserving the composition enriched in human amniotic fluid derived MSCs.
- the multi stage process can be implemented before the birth of a baby. This is especially useful when there is in-utero indication that there can be some congenital anomalies with the fetus through routine sonogram of the fetus during prenatal visits.
- congenital anomalies are neural tube defects, congenital heart defects, oral facial clefts, congenital diaphragmatic hernia and limb reduction defects.
- the typical procedure to correct some of these congenital anomalies is surgical repair of the defective tissue by using inert substitute tissue materials such as Teflon, in absence of any human donor tissue or human autologous tissue.
- an additional amount of 5 -10 ml of amniotic fluid can be collected for implementing the multi stage process.
- the goal of the multi stage process is to produce a permanent source of pluripotent cells for correcting congenital defects and for possible future needs due to disease and/or injury.
- the multi stage process can produce a permanent source of pluripotent cells for that are autologous to the baby with congenital defects.
- the cryopreserved human amniotic fluid derived MSCs at stage 3 becomes the permanent source of pluripotent cells.
- a sample of the cryopreserved MSCs can be thawed and further expanded to provide MSCs in sufficiently large quantities for the tissue engineering of the tissues that is needed for repairing the congenital defects, or loss through disease and/or injury.
- MSCs can be expanded and differentiated into the right tissue type for correcting and repairing the congenital defects. For example, if a baby is diagnosed with congenital diaphragmatic hernia, the baby's MSCs is first isolated from the amniotic fluid during a routine amniocentesis, expanded and "banked" by cryopreservation. Several weeks before the birth of the baby, the banked MSCs is retrieved, thawed, expanded to increase the number of cells needed for seeding a scaffold and differentiating into tendon and/or muscle cells, and then tissue engineered into a diaphragmatic tendon and/or muscle tissue.
- This engineered diaphragmatic tendon and/or muscle tissue can then be used to surgically repair the missing part of the diaphragm that is the cause of the congenital diaphragmatic hernia. Since the engineered tissue is autologous to the baby, the baby is less likely to develop any immune rejection of the engineered tissue. Moreover since the repair material consists of living tissue, the repair material has a greater amount of flexibility and elasticity liken to the baby's natural tissue compared to inert substitute tissue materials such as Teflon. In addition, the repair material will grow with the baby. Accordingly, the long term prognosis of such repair graft is better than inert substitute tissue materials such as Teflon, as the autologous repair grafts need less secondary repair.
- the MSCs used are autologous. Their use in tissue engineering helps overcome the potential problems of immune rejection or transfer of infectious agents.
- the harvested cells can be expanding in vitro, providing large amount of cells necessary for tissue engineering.
- the engineered tissue can be prepared in advance and timed with the approximate delivery date of the baby. This facilitates the repair of congenital defects shortly after birth.
- a sample of the permanent source of human amniotic derived MSCs can be thawed and expanded to provide the much needed cells.
- the permanent source of human amniotic derived MSCs can be used for tissue engineering, tissue repair, and/or wound healing in HLA-matched individuals.
- Amniotic fluid is the watery liquid surrounding and cushioning a growing fetus within the amnion. It allows the fetus to move freely without the walls of the uterus being too tight against the fetus's body.
- the amnion grows and begins to fill, mainly with water, around two weeks after fertilization. After a further 10 weeks the liquid contains proteins, carbohydrates, lipids and phospholipids, urea and electrolytes, all which aid in the growth of the fetus.
- the fetus also sheds cells into the amniotic fluid. In the late stages of gestation much of the amniotic fluid consists of fetal urine.
- the amniotic fluid can be a plentiful source of non-embryonic stem cells.
- Hematopoietic stem cells and MSCs are two examples of the fetal cell types found in amniotic fluid.
- MSCs are the formative pluripotential blast cells found inter alia in bone marrow, blood, dermis and periosteum that are capable of differentiating into any of the specific types of mesenchymal or connective tissues (i.e. the tissues of the body that support the specialized elements; particularly adipose, osseous, cartilaginous, muscular, elastic, and fibrous connective tissues) depending upon various influences from bioactive factors, such as cytokines.
- amniotic fluid from the first trimester through to human term at 38 weeks of gestation (Pieternella S. in 't Anker, et. al., 2004, Stem Cells, 22:1338-1345). Accordingly, it is possible to harvest amniotic fluid from 5 weeks through to human term at 38 weeks of gestation for the isolation of MSCs.
- the human amniotic fluid is collected between 5 weeks of gestation to human term. In another embodiment, the human amniotic fluid is collected at birth. In yet another embodiment, the collected human amniotic fluid is cryopreserved. In a further embodiment, the human amniotic fluid is used for the isolation and expansion of human amniotic fluid derived MSCs.
- the method of obtaining a composition enriched in human amniotic fluid derived MSCs further comprise cryopreserving the composition enriched in human amniotic fluid derived MSCs.
- the invention discloses an isolated human amniotic fluid derived MSC prepared according to a method comprising the steps of: (a) isolating MSCs from a sample of human amniotic fluid in the absence of non-human animal derived products; and (b) expanding the isolated human amniotic fluid derived MSCs in the absence of non-human animal derived products.
- the isolated human amniotic fluid derived MSC can be cryopreserved by methods known to one of ordinary skill in the art, or is further expanded to obtain an increase number of the MSCs and then cryopreserved thereafter.
- the cryopreserved MSCs can be thawed and used for tissue engineering, tissue repair and/or wound healing, or expanded further prior to use in tissue engineering, tissue repair and/or wound healing.
- the invention provides a method of proliferating a composition enriched in human amniotic fluid derived MSCs comprising the steps of: (a) selecting at least one single MSC from a sample of human amniotic fluid in the absence of non- human animal derived products; (b) introducing at least one single MSC to a culture medium containing no non-human animal derived product; and (c) proliferating at least one single MSC to a culture medium containing no non-human animal derived product.
- the proliferation of human amniotic fluid derived MSC served to increase the number of such pluoripotent cells for tissue engineering, tissue repair, wound healing, as well as for cryopreserving the MSCs to provide a permanent source of such cells.
- the invention provides a method of storing a composition enriched in human amniotic fluid derived MSCs comprising the steps of: (a) obtaining a composition enriched in human amniotic fluid derived MSCs according to the method described herein; and (b) cryopreserving the composition enriched in human amniotic fluid derived MSCs.
- the cryopreservation of a composition enriched in human amniotic fluid derived MSCs functions to provide a permanent source of such cells. When such cells are needed, an aliquot of the frozen cell in storage can be thawed for use in tissue engineering, tissue repair, and wound healing.
- the invention provides a method of storing human amniotic fluid derived MSCs comprising harvesting MSCs from a sample of human amniotic fluid and cryopreserving the MSCs such that the cells remain viable upon thawing.
- cyropreservatives such as DMSO at 10% final concentration and a 1-3 °C/min, slow and gradual reduction in the temperature of the MSCs to -196 0 C help ensure that the MSCs are not damaged during the cryopreservation process and such cells can remain viable upon thawing.
- kits for obtaining a composition enriched in human amniotic derived MSCs from human amniotic fluid comprising some of the components, but not limited to: (a) a container for the collection of human amniotic fluid; (b) a coated container for the isolation and primary expansion of human amniotic fluid derived MSCs; (c) human serum for the culture-expansion of human amniotic fluid derived MSCs; and (d) instructions for the isolation, identification, and expansion of human amniotic fluid derived MSCs.
- the container for the collection of human amniotic fluid should be sterile , preferably sealed, and have injection ports.
- the amniotic fluid that is collected within the amniocentesis syringe can be injected directly into the container via the injection port.
- the container is also a centrifuge tube. Once the amniotic fluid is injected inside the container, the container can be centrifuged to pellet the cells in the amniotic fluid. The supernatant fluid can be aspirated and fresh media can be added to the tube to resuspend the cells in the pellet. The suspension of cells and media is poured under sterile conditions into a coated container provided in the kit for the isolation and primary expansion of human amniotic fluid derived MSCs.
- the kit provides the reagents for the reconstitution of a culture media for the culture expansion of the MSCs, reagents for the cryopreservation of the MSCs, and instructions for the isolation, identification, and expansion of human amniotic fluid derived MSCs.
- the kit comprises instructions for: (a) reconstituting a culture media; (b) harvesting the MSCs from a sample of amniotic fluid; (c) plating and counting of the harvested MSCs adhered on a coated container; (d) the expected morphology of the adherent MSCs; (e) the removal of non-adherent cells; (f) the confluency at which to passage the MSCs; (f) detaching, harvesting, and dividing the MSCs to several coated containers - this is known as passaging of cells; and (g) detaching, harvesting, and cryopreserving the MSCs.
- the reagents for the reconstitution of the culture media include but are not limited to stock solutions of antibiotics, antimycotics, glucose, buffered media such as Dulbecco v s Modified Eagle Medium, human serum, and growth factors such as basic fibroblast growth factor.
- the coated containers for the culture expansion of the MSCs can be coated with human collagen, fibronectin, laminin, poly-lysine and the likes used in coating culture plates.
- non-human animal products refer to serum, plasma, growth factors and other cell culturing reagents that are used for the culturing and growth of the MSCs, that are derived from non-humans.
- fetal calf serum, fetal bovine serum, mouse basic fibroblast growth factor and recombinant mouse basic fibroblast growth factor are considered non-human animal products.
- autologous refers to a situation in which the donor and recipient are the same person. Autologous engineered tissue used in a tissue repair of congenital anomalies are made with cells derived from the person with congenital anomalies.
- MSC mesenchymal stem cell
- pluripotency a generalized cell that has pluripotency (descendants can specialize into different cell types), for example, an undifferentiated MSC that is capable of differentiating into more than one specific type of mesoderm-derived cells and regenerating into various tissues in vivo.
- Such cell also has unlimited proliferating and self-renewal capability and can differentiate into osteogenic, myogenic, adipogenic or chondrogenic, neurogenic, hepatogenic, nephrogenic, urogenic, isletogenic, pancreatogenic, gastroenterogenic, epitheliogenic, thyroidogenic, myocardiogenic, pneumogenic, retino genie, gametogenic, endotheliogenic, or hematopoietic lineages.
- the enriched population of human amniotic fluid derived MSCs provided herein can positively express the cell surface markers CD73 (SH3), CD105 (SH2), CD44, CD29, CD 90, CD13, CDlO, CD71, CD49d, CD49e, and/or HLA Class I (A, B, and C). Additionally, the enriched population of MSCs provided herein is negative for the cell surface markers CD8, CD14, CD19, CD31, CD34, CD45, CD56, CD133, and/or HLA-DR (Pitting et. al., 1999, Science 284:143- 147; Kaviani et. al., 2001, J. Pediatr. Surg. 36: 1662-5 ; Kunisaki et. al., 2007, J. Pediatr. Surg. 42(6):974-9).
- culture refers to the in vitro maintenance of cells.
- the cells are cultured in culture medium, which is a nutrient-rich buffered aqueous solution capable of sustaining cell growth.
- Culture media suitable for isolating and expanding human fetal MSCs from amniotic fluid according to the practice described herein include but are not limited to high glucose Dulbecco's Modified Eagles Medium with L-Glutamine.
- the media can be supplemented with recombinant human basic fibroblast growth factor (rhbFGF) and contain sera, such as human serum, and antibiotics (Table 1).
- rhbFGF recombinant human basic fibroblast growth factor
- Table 1 provides manufacturers information of the key ingredients in a typical culture media used for isolating and expanding MSCs.
- Cell cultures are maintained in a CO 2 atmosphere, e.g., 5% to 12%, to maintain pH of the culture fluid, and incubated at 37 0 C in a humid atmosphere.
- a CO 2 atmosphere e.g., 5% to 12%
- Suitable chemically defined serum free media are described in U.S. Ser. No. 08/464,599 and WO96/39487, and "complete media" are described in U.S. Pat. No. 5,486,359 and these are hereby incorporated by reference.
- Chemically defined medium comprises a minimum essential medium such as Iscove's Modified Dulbecco's Medium (IMDM) (Gibco), supplemented with human serum albumin, human Ex Cyte lipoprotein, transferrin, insulin, vitamins, essential and non essential amino acids, sodium pyruvate, glutamine and a mitogen. These media stimulate MSCs growth without differentiation.
- IMDM Iscove's Modified Dulbecco's Medium
- a mitogen refers to an agent that stimulate cell division of a cell.
- An agent can be a chemical, usually some form of a protein, that encourages a cell to commence cell division, triggering mitosis.
- non-human animal derived products refers to products that are not derived directly from human sources or expressed from a human gene.
- fetal bovine serum is derived directly from bovine (e. g. domestic cattle, Bison, Water Buffalo, the Yak, and the four-horned and spiral-horned antelopes) and is therefore a non-human animal derived product.
- Human autologous serum and pooled allogenic human AB serum are human animal derived products.
- Recombinant human basic fibroblast growth factor (rhbFGF) is encoded by a human gene and is also considered a human animal derived product.
- isolated signifies that the cells are placed into conditions other than their natural environment.
- isolated does not preclude the later use of these cells thereafter in combinations or mixtures with other cells.
- expanding refers to increasing the number of like cells through cell division (mitosis).
- proliferating and “expanding” are used interchangeably.
- the term "storing” refers to the cryopreservation of MSCs such that the MSCs are viable and can undergo mitosis and cell differentiation after thaw.
- the time frame of storing the MSCs are in the range of one months to years.
- Amniocentesis or an amniotic fluid test (AFT) is a medical procedure used for prenatal diagnosis of a fetus, in which a small amount of amniotic fluid is extracted from the amniotic cavity around a developing fetus. Amniocentesis can be done as soon as there is enough amniotic fluid surrounding the fetus that a sample can be removed safely. Early amniocentesis can be performed as early as 5 weeks of gestation. Standard amniocentesis is usually performed between 15 and 20 weeks gestation. Amniotic fluid is available for collection from 5 weeks right up to term and birth of the baby, that is from 5,...10,...
- amniotic fluid can also be collected during birth.
- a device described in US Patent No. 4031897 can be used to collect the amniotic fluid when a pregnant mother's water breaks. The collection device is worn unobtrusively and with comfort by a woman in the last stages of pregnancy, which is positioned to receive and retain the amniotic fluids when the fluid is released.
- a diagnostic amniocentesis is routinely performed whenever a fetal abnormality, such as congenital diaphragmatic hernia (CDH), is detected by prenatal ultrasound imaging.
- Amniocentesis is performed by a skilled physician specialized in that area, and amniotic fluid is obtained using a long syringe, guided by ultrasound.
- the syringe is usually inserted into the mother's abdominal wall or at the end of the vagina, and through the uterus wall.
- the physician would aim for an area of the amniotic sac that is away from the fetus so to avoid stabbing it.
- a small amount of amniotic fluid then gets sucked out and the syringe is withdrawn.
- the puncture wound should close up by itself, and the amniotic sac should then automatically replenish the liquid over a day or so.
- An amniotic fluid sample can be obtained during such diagnostic amniocentesis for the practice of the invention described herein without any no additional morbidity to the mother. No modification of the standard procedure, which is performed with sterile technique under ultrasound guidance, is necessary. Collections should be made under sterile conditions. Generally, the first 2 ml of the collected amniotic fluid is discarded and 15-30 ml of amniotic fluid is collected with a sterile syringe. The amniotic fluid is then placed in a sterile plastic 15 ml or 50 ml tube. At this point, the amniotic fluid can be supplemented with antibiotics penicillin and streptomycin. An aliquot, 1/100 volume of the collected amniotic fluid, of a stock penicillin (5000 U/ml) /streptomycin (500mg/ml) can be added to the collected amniotic fluid.
- a collection kit comprising a wide-mouth, graduated seal sterile collection container with antibiotics, with ports for the injection of the collected amniotic fluid and an identification label which identifies the mother/fetus source of the amniotic fluid and the time of collection is provided.
- amniotic fluid collected When fetuses share an amniotic sac, the amniotic fluid collected will be duly noted on the identification label. Since fetuses that shared an amniotic fluid are genetically identical, having arose from a single developing embryo, the MSCs are autologous to both individuals that eventually develop from the two fetuses. [0072] As little as 2 niL of amniotic fluid is sufficient for isolating MSCs that can be expanded to sufficient number of cells for cryopreservation and/or tissue engineering of tissues. However, the larger volume of amniotic fluid, the easier it is to isolate and expand the MSCs.
- amniotic fluid Given the amount of amniotic fluid routinely obtained during amniocentesis in most cases, it is expected that 10-20 mL of amniotic fluid can be available for practice of the invention described herein. In a preferred embodiment, a sample of 5-10 ml of human amniotic fluid is used.
- the sample of amniotic fluid collected is appropriately labeled and can be stored at 4 0 C for up to 48 h.
- the samples are immediately transported to a Good Manufacturing Practice (GMP) facility for isolation, expansion, and/or cryopreservation.
- GMP Good Manufacturing Practice
- the sample should be processed within 48 h and preferably within 24 h of harvest.
- the cells in the collected amniotic fluid are isolated by centrifugation and cryopreserved immediately.
- the sample of amniotic fluid is immediately centrifuged at room temperature at low centrifugal force of 400-1000 x g for 10-15 min, and the supernatant amniotic fluid is discarded.
- DMEM Dulbecco's Modified Eagle Medium
- human AB serum 10% human AB serum
- DMSO 10% DMSO
- the cell culture media is not limited to DMEM.
- Other examples include RPMI 1640 and others described herein.
- Other examples of serum that can substitute for human AB serum include human autologous serum and platelet rich plasma supplemented with heparin (2U/ml).
- the pellet of amniotic fluid cells can be cryopreserved in 90% human serum or plasma or the likes and 10% DMSO.
- the collected amniotic fluid is cryopreserved immediately.
- the sample of amniotic fluid is mixed with DMSO and human serum or plasma or the likes to achieve at least 10% serum and at least 10% DMSO.
- the amniotic fluid can be cryopreserved with serum-free media such as ATHENAESTM cell culture media with at least 10% DMSO as a cryopreservative according to the methods known to one skilled in the art.
- the amniotic fluid derived MSCs are allocated to at least four cryogenic vials such as CRYULES ® (Wheaton, Inc.), two of which are assigned for storage to one freezer and another two cryules to another independently-serviced freezer.
- a fifth cryules contains cells set aside for testing of identify, viability, and function, when the withdrawal of cells from cryopreservation is required for tissue repair, wound healing, and/or cell expansion for tissue engineering.
- recordation of data of the amniotic fluid collected can be performed to ensure accurate identification and evaluation of the collected amniotic fluid.
- the preferred recorded data should include: collection number, name of mother, gender of fetus, date of collection, time of gestation (weeks of pregnancy), processing prior to freezing, freezing date, number of cryules, freezer positions, obstetrical data: reason for amniocentesis, congenital birth defects, and health of mother; test results of amniotic fluid sample and cells.
- MSCs can be used for inspection and testing. For example, routine testing for bacterial contamination, diagnostic screening for pathogenic microorganisms such as the human immunodeficiency virus (HIV), and confirmation of the fetal origin of the cells can be performed.
- routine testing for bacterial contamination diagnostic screening for pathogenic microorganisms such as the human immunodeficiency virus (HIV)
- pathogenic microorganisms such as the human immunodeficiency virus (HIV)
- confirmation of the fetal origin of the cells can be performed.
- the tissue culture plates can be coated with, but not limited to human recombinant collagen (BD Biosciences), human fibronectin, laminin, proteoglycans or poly-D- lysine; the FBS or FCS can be replaced with human autologous serum, pooled allogenic human AB serum, or platelet rich plasma supplemented with heparin (2U/ml); the basic fibroblast growth factor (bFGF) can be replaced with recombinant human basic fibroblast growth factor (rhubFGF).
- human recombinant collagen BD Biosciences
- human fibronectin human fibronectin
- laminin laminin
- the FBS or FCS can be replaced with human autologous serum, pooled allogenic human AB serum, or platelet rich plasma supplemented with heparin (2U/ml)
- the basic fibroblast growth factor (bFGF) can be replaced with recombinant human basic
- MSCs and are referred to herein as "complete media" when supplemented with serum as described below.
- One such medium is an augmented version of Dulbecco's Modified Eagle's Medium (DMEM), which is well known and readily commercially available.
- DMEM Dulbecco's Modified Eagle's Medium
- the type of culture medium is not specifically limited in the present invention to DMEM.
- Other examples include RPMI 1640, Iscove's modified Dubelcco's media (IMDM), and Opti-MEM SFM (Invitrogen Inc.).
- Chemically Defined Medium comprises a minimum essential medium such as Iscove's Modified Dulbecco's Medium (IMDM) (Gibco), supplemented with human serum albumin, human Ex Cyte lipoprotein, transferrin, insulin, vitamins, essential and non essential amino acids, sodium pyruvate, glutamine and a mitogen is also suitable.
- IMDM Iscove's Modified Dulbecco's Medium
- serum free media such as those described in U.S. Ser. No. 08/464,599 and WO96/39487, and the "complete media" as described in U.S. Pat. No. 5,486,359 are contemplated for use with the methods described herein.
- the commercial formulation is supplemented with 3700 mg/1 of sodium bicarbonate and 10 ml/1 of a IOOX (100 times concentrated) antibiotic-antimycotic cocktail containing 10,000 units of penicillin, 10,000 ⁇ g of streptomycin, and 25 ⁇ g of amphotericin B/ml utilizing penicillin G (sodium salt), streptomycin sulfate, and amphotericin B (FUNGIZONE TM) in 0.85% saline.
- IOOX 100 times concentrated antibiotic-antimycotic cocktail containing 10,000 units of penicillin, 10,000 ⁇ g of streptomycin, and 25 ⁇ g of amphotericin B/ml utilizing penicillin G (sodium salt), streptomycin sulfate, and amphotericin B (FUNGIZONE TM) in 0.85% saline.
- the culture-expansion media does not contain any human autologous serum, human AB serum or platelet rich plasma supplemented with heparin (2U/ml).
- cells under such culture condition will grow and multiply but at a slower rate than in the presence of human serum or plasma.
- an adherence/non- adherence separation protocol is followed when isolating amniotic fluid derived MSCs.
- the adhesion/non-adhesion is determined visually under a microscope after a period of culture of the MSCs
- the amniotic fluid of 5-10 ml, obtained by amniocentesis, is spun down at 400-500 x g for 10-15 min at room temperature.
- the cell pellet is collected and suspended in growth culture media consisting of supplemented DMEM, 20% pooled allogenic human AB serum, gentamicin and 5 ng/ml of rhbFGF. Cells are plated into a single 6-well plate coated with human collagen (Fibrogen, Inc.
- Resuspended cells derived from 5-10 ml of amniotic fluid are evenly divided among the 6 wells.
- the wells in the plate are monitored under a light microscope for cell adherence to the plate at least once a day.
- Adherent cells exhibit a spread out cytoplasm and take on the classic elongated spindle shape of a MSC. Upon swirling of the media in the wells, these cells are not detached from their position of attachment in the culture well. Non-adherent cells do not exhibit a spread out cytoplasm and the cells remain spherical in shape. Such non-adherent cells move with the flow of the culture media in the dish.
- the MSCs are characterized by their adherent properties on coated tissue culture plates.
- adherent MSCs can be observed after 24 -48 hrs.
- adherent MSCs are noted only after 1 week in culture.
- the culture plate can be maintained for up to two weeks to allow MSCs to adhere, after which non-adherent cells are removed and fresh growth media can be added. Subsequently, the media is replaced every 3-5 days until the adherent MSCs have divided and 70-80% confluence is reached. It takes about 3 weeks or more to reach 70-80% confluence depending on the amount of MSCs used in the initial plating of the wells.
- the cells on each well are observed morphologically for the classic spindle-like shaped cells of MSCs and for the absence of contamination. Signs of contamination include but are limited to cloudy media, presence of filamentous-like fungi, and bacteria growth.
- Culture wells with the majority of cells having the spindle-like shaped cells and no signs of contamination are selected. At 70-80% confluent, there should be at least 95% of spindle-like shaped cells.
- Cells from the selected plate are then detached with a trypsin-like solution (TrypLE, Invitrogen, Inc.) for 3-5 minutes at 37 0 C, washed and plated in fresh growth media in a flask.
- the MSCs can be cryopreserved or they can be further culture-expanded prior to cryopreservation.
- the media are changed every 3 days till the cells reach 80-90% confluence before the cells are passaged at a ratio of 1:2-10, including all the ratios in between 1:2 and 1:10.
- the expanded MSCs 250-600 x 10 6 cells
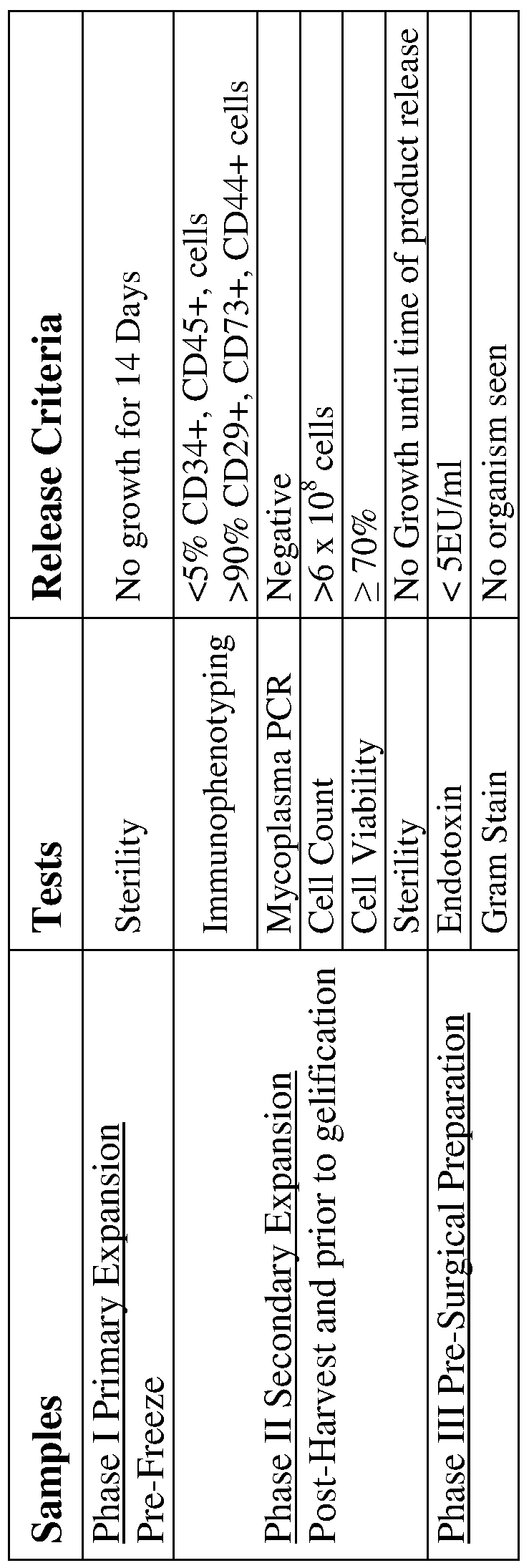
- the expanded MSCs are characterized by flow cytometric analysis of specific surface antigens (cell surface markers) followed by a 14-day sterility and cell viability tests.
- the expanded MSCs can then be cryopreserved in Plasmalyte-A, Human Serum Albumin and 10% DMSO or by other cryopreservation method as described herein.
- the phenotypical identity of these MSCs are determined through multicolor flow cytometry.
- the composition of enriched human amniotic fluid derived MSCs positively expresses CD73, CD105, CD44, CD29, CD90, CD13, CDlO, CD71, CD49d, CD49e, and HLA Class I (A, B, and C) and are negative for CD45, CD34, CD14, CD19, CD8, CD56, CD31, CD133, and HLA-DR.
- the composition of enriched human amniotic fluid derived MSCs is at least 90%, at least 80%, at least 70%, at least 60%, at least 50% and all the percentages in between 50%-90%, positive for the following cell surface antigens: CD29, CD73 and CD44.
- the composition of enriched MSCs is at least 60%, at least 50%, at least 40%, at least 30% at least 20% and all the percentages in between 20%-60%, positive for CD90 and CD105.
- the composition of enriched human amniotic fluid derived MSCs is no more than 5% positive for CD45 and CD34.
- cryopreserved MSCs After the birth of the child or when needed, some of the cryopreserved MSCs can be thawed and plated in growth media, in a flask or a dish containing a culture media as described above.
- the MSCs can be expanded to obtain at least 6 x 10 8 cells in 1-2 passages.
- the MSCs are then ready for pre-surgical implantation preparation such as tissue differentiation and/or tissue engineering.
- a 14-day sterility testing of an isolated human amniotic fluid derived MSCs culture can be performed in accordance with criteria standard to GMP for sterility testing of pharmaceutical products and should be in compliance with the federal guidelines for the final product testing.
- the MSC cultures can be prepared using Millipore's Steritest Filtration System and are incubated in appropriate media for 14 days. The validation of the system, procedural controls, test organisms, and products demonstrated that the Millipore Steritest system is a valid system for the isolation of microorganism contamination of cellular products and/or supplies as low as 10 CFU/ml for test organisms used.
- Millipore TTHVAB210 canisters were used for the test samples.
- the canisters contain a low absorption Durapore membrane filter (0.45 ⁇ m) that is efficient in rinsing away any residual antimicrobial agents from test sample.
- One canister of each set is filled with fluid thioglycolate medium (FTM); the other is filled with soy casein media (SCM).
- FTM media and test samples were incubated at 30- 35° C for 14 days.
- SCM canisters are incubated at room temperature for the same period.
- the canisters are examined for turbidity and evidence of growth on the third, fourth, or fifth day, and on the seventh and fourteenth day of testing. Turbidity is equivalent to identification of positive cultures. All positive cultures are to be sterilized and discarded.
- Bacterial culture To ensure the absence of microbial contamination, established assays known in the art can be performed, such as routine hospital cultures for bacteria under aerobic and anaerobic conditions.
- Diagnostic screening for pathogenic microorganisms To ensure the absence of specific pathogenic microorganisms, various diagnostic tests can be employed. Diagnostic screening for any of the numerous pathogens transmissible through bodily fluids can be done by standard procedures that are known in the art. As one example, the collected amniotic fluid sample can be subjected to diagnostic screening for the presence of the Human Immunodeficiency Virus (HIV), the causative agent of Acquired Immune Deficiency Syndrome (AIDS) (Gallo et al., 1984, Science 224:500-503; Barre-Sinoussi, F., et al., 1983, Science 220:868; Levy, J.
- HAV Human Immunodeficiency Virus
- AIDS Acquired Immune Deficiency Syndrome
- Endotoxin levels can be determined by the gel-clot limulus amebocyte lysate
- the Mycoplasma PCR testing will be performed at a GMP approved facility using MycoSensorTM QPCR Assay Kit (Manufactured by Stratagene).
- the Mesenchymal Stem Cell Characterization Kit (Millipore cat. no. SCROl 8) provides researchers with a convenient means to phenotype MSCs using a panel of antibodies.
- This kit contains reagents to the MSC markers: integrin Bl, CD54, collagen type I and fibronectin, and to the negative markers CD45 and CD14. Also included are mouse and rabbit immunoglobulins for the assessment of background staining.
- MSCs from various species were originally isolated from bone marrow by their ability to adhere to the surface of the culture vessel (Reyes, 2001, Blood, 98:2615-25; Pittenger, 1999, Science, 284:143-7; Marin, 2002, Exp. Hematol., 30:879-86; Kadiyala, 1997, Cell Transplant, 6:125-34; Johnstone, 1998, Exp. Cell. Res., 238:265-72; Wakitani, 1995, Muscle Nerve, 18:1417-26; Berry, 1992, J. Cell. ScL, 101:333-42; Mosca, 2000, Clin. Orthop. Relat. Res. Oct;(379 Suppl):S71-90).
- MSCs of various phenotypes include Sca-1+, CD29+, CD44+, c-Kit+, CD105+, CD45-, CD31+, CD34+ (Sun, S. et. al., 2003, Stem Cells, 21:527-35.) and Sca-1+, CD29+, CD44+, CD81+, CD106+, Nucleostemin+ and CDl 16-, CD34-, CD45-, CD48-, CDl 17- and CD135- (Baddoo, M., et. al., 2003, J. Cell. Biochem. 15;89:1235-49).
- MSCs While the phenotype of murine MSCs within bone marrow remains unknown, cultured MSCs were phenotypically characterized as being negative for CD34, CD44, CD45, c-Kit, MHC-I and MHC-II. Conversely, these MSCs express low levels of FIk-I, Sca-1 and Thy-1, and higher levels of CD 13 and stage specific embryonic antigen-1 (SSEA-I).
- SSEA-I stage specific embryonic antigen-1
- cryopreservation refers to the preservation of cells by cooling to low sub-zero temperatures, such as (typically) 77 K or -196 0 C (the boiling point of liquid nitrogen). Cryopreservation also refers to storing the cells at a temperature between 0-10 0 C in the absence of any cryopreservative agents. At these low temperatures, any biological activity, including the biochemical reactions that would lead to cell death, is effectively stopped. Cryoprotective agents are often used at sub-zero temperatures to preserved the cells from damaged due to freezing at low temperatures or warming to room temperature.
- the invention provides a cryopreserved pharmaceutical composition
- a cryopreserved pharmaceutical composition comprising: (a) a viable composition enriched in human amniotic fluid derived MSCs obtained according to the method described herein in the absence of non-human animal derived products, in which the MSCs are present in an amount sufficient to effect tissue engineering, tissue repair, or wound healing; (b) an amount of cryopreservative sufficient for the cryopreservation of MSCs; and (c) a pharmaceutically acceptable carrier.
- Freezing is destructive to most living cells. Upon cooling, as the external medium freezes, cells equilibrate by losing water, thus increasing intracellular solute concentration. Below about 10°-15° C, intracellular freezing will occur. Both intracellular freezing and solution effects are responsible for cell injury (Mazur, P., 1970, Science 168:939-949). It has been proposed that freezing destruction from extracellular ice is essentially a plasma membrane injury resulting from osmotic dehydration of the cell (Meryman, H. T., et al., 1977, Cryobiology 14:287-302).
- Cryoprotective agents and optimal cooling rates can protect against cell injury.
- Cryoprotection by solute addition is thought to occur by two potential mechanisms: colligatively, by penetration into the cell, reducing the amount of ice formed; or kinetically, by decreasing the rate of water flow out of the cell in response to a decreased vapor pressure of external ice (Meryman, H. T., et al., 1977, Cryobiology 14:287-302).
- Different optimal cooling rates have been described for different cells.
- Various groups have looked at the effect of cooling velocity or cryopreservatives upon the survival or transplantation efficiency of frozen bone marrow cells or red blood cells (Lovelock, J. E. and Bishop, M. W. H., 1959, Nature 183:1394- 1395; Ashwood-Smith, M.
- the injurious effects associated with freezing can be circumvented by (a) use of a cryoprotective agent, (b) control of the freezing rate, and (c) storage at a temperature sufficiently low to minimize degradative reactions.
- Cryoprotective agents which can be used include but are not limited to dimethyl sulfoxide (DMSO) (Lovelock, J. E. and Bishop, M.W.H., 1959, Nature 183:1394-1395; Ashwood-Smith, M. J., 1961, Nature 190:1204-1205), glycerol, polyvinylpyrrolidine (Rinfret, A. P., 1960, Ann. N.Y. Acad. Sci. 85:576), polyethylene glycol (Sloviter, H. A. and Ravdin, R.
- DMSO dimethyl sulfoxide
- glycerol glycerol
- polyvinylpyrrolidine Rost, A. P., 1960, Ann. N.Y. Acad. Sci. 85:576
- polyethylene glycol Rositer, H. A. and Ravdin, R.
- DMSO is used, a liquid which is non-toxic to cells in low concentration. Being a small molecule, DMSO freely permeates the cell and protects intracellular organelles by combining with water to modify its freezability and prevent damage from ice formation.
- Programmable freezing apparatuses allow determination of optimal cooling rates and facilitate standard reproducible cooling.
- Programmable controlled-rate freezers such as Cryomed or Planar permit tuning of the freezing regimen to the desired cooling rate curve.
- the optimal rate is 1 to 3°C/minute from 0° C to -8O 0 C.
- this cooling rate can be used for the amniotic fluid derived MSCs of the invention described herein.
- the container holding the cells must be stable at cryogenic temperatures and allow for rapid heat transfer for effective control of both freezing and thawing.
- Sealed plastic vials e.g., Nunc, Wheaton CRYULES ®
- glass ampules can be used for multiple small amounts (1-2 ml), while larger volumes (100-200 ml) can be frozen in polyolefin bags (e.g., Delmed) held between metal plates for better heat transfer during cooling.
- polyolefin bags e.g., Delmed
- the methanol bath method of cooling can be used.
- the methanol bath method is well-suited to routine cryopreservation of multiple small items on a large scale. The method does not require manual control of the freezing rate nor a recorder to monitor the rate.
- DMSO-treated cells are pre-cooled on ice and transferred to a tray containing chilled methanol which is placed, in turn, in a mechanical refrigerator (e.g., Harris or Revco) at -80° C.
- a mechanical refrigerator e.g., Harris or Revco
- Thermocouple measurements of the methanol bath and the samples indicate the desired cooling rate of 1° to 3°C/minute. After at least two hours, the specimens have reached a temperature of -8O 0 C and can be placed directly into liquid nitrogen (-196° C) for permanent storage.
- MSC samples can be cryogenically stored in liquid nitrogen (-196 0 C) or its vapor (-165° C).
- liquid nitrogen -196 0 C
- vapor -165° C
- the supernatant is aspirated off and the pellet of MSCs is resuspended in 1.5 ml of media.
- a aliquot of 1 ml of 100% DMSO is added to the suspension of MSCs and gently mixed. Then 1 ml aliquots of this suspension of MSCs in DMSO is dispensed into cyrules in preparation for cryopreservation.
- the sterilized storage cryules preferably have their caps threaded inside, allowing easy handling without contamination. Suitable racking systems are commercially available and can be used for cataloguing, storage, and retrieval of individual specimens.
- cryopreservation of viable cells or modifications thereof, are available and envisioned for use (e.g., cold metal-mirror techniques; Livesey, S. A. and Linner, J. G., 1987, Nature 327:255; Linner, J. G., et al., 1986, J. Histochem. Cytochem. 34(9): 1123- 1135; U.S. Pat. No. 4,199,022, 3,753,357, 4,559,298 and are incorporated hereby reference.
- cold metal-mirror techniques e.g., cold metal-mirror techniques; Livesey, S. A. and Linner, J. G., 1987, Nature 327:255; Linner, J. G., et al., 1986, J. Histochem. Cytochem. 34(9): 1123- 1135; U.S. Pat. No. 4,199,022, 3,753,357, 4,559,298 and are incorporated hereby reference.
- Frozen MSCs are preferably thawed quickly (e.g., in a water bath maintained at
- the cryogenic vial containing the frozen MSCs can be immersed up to its neck in a warm water bath; gentle rotation will ensure mixing of the cell suspension as it thaws and increase heat transfer from the warm water to the internal ice mass. As soon as the ice has completely melted, the vial can be immediately placed in ice.
- the thawing procedure after cryopreservation is described in Current Protocols in Stem Cell Biology 2007 (Mick Bhatia, et. al., ed., John Wiley and Sons, Inc.) and is hereby incorporated by reference.
- the vial is rolled between the hands for 10 to 30 sec until the outside of the vial is frost free.
- the vial is then held upright in a 37 0 C water-bath until the contents are visibly thawed.
- the vial is immersed in 95% ethanol or sprayed with 70% ethanol to kill microorganisms from the water-bath and air dry in a sterile hood.
- the contents of the vial is then transferred to a 10-cm sterile culture containing 9 ml of media using sterile techniques.
- the MSCs can then be cultured and further expanded in a incubator at 37 0 C with 5% humidified CO 2 .
- the MSCs are treat in order to prevent cellular clumping upon thawing.
- various procedures can be used, including but not limited to, the addition before and/or after freezing of DNase (Spitzer, G., et al., 1980, Cancer 45:3075- 3085), low molecular weight dextran and citrate, hydroxyethyl starch (Stiff, PJ. , et al., 1983, Cryobiology 20:17-24).
- the cryoprotective agent if toxic in humans, should be removed prior to therapeutic use of the thawed MSCs.
- DMSO dimethyl methacrylate
- the removal is preferably accomplished upon thawing.
- cryoprotective agent is by dilution to an insignificant concentration. This can be accomplished by addition of medium, followed by, if necessary, one or more cycles of centrifugation to pellet the cells, removal of the supernatant, and resuspension of the cells.
- the intracellular DMSO in the thawed cells can be reduced to a level (less than 1%) that will not adversely affect the recovered cells. This is preferably done slowly to minimize potentially damaging osmotic gradients that occur during DMSO removal.
- thawed cells are tested by standard assays of viability (e.g., trypan blue exclusion) and of microbial sterility as described herein, and tested to confirm and/or determine their identity relative to the recipient.
- viability e.g., trypan blue exclusion
- microbial sterility as described herein
- Methods for identity testing which can be used include but are not limited to
- DNA fingerprinting exploits the extensive restriction fragment length polymorphism associated with hypervariable minisatellite regions of human DNA, to enable identification of the origin of a DNA sample, specific to each individual (Jeffreys, A.
- the MSCs recovered for tissue engineering, tissue repair and/or wound healing are to be used in an autologous system
- the MSCs should match exactly the recipient patient from whom the MSCs are originally derived.
- the MSCs are not used in an autologous system but are HLA typed match to the recipient. For example, the HLA type matched for HLA-A, B, C, and D.
- a method for producing differentiated human amniotic fluid derived MSC preparations comprising: (a) obtaining a composition enriched in human amniotic fluid derived MSCs according to the methods described herein in the absences of non-human animal derived products; and (b) culturing the composition enriched in human amniotic fluid derived MSCs in a culture medium containing differentiation factors for a time period sufficient for MSCs to differentiate and express specific tissue markers.
- the differentiation factors are selected from a group consisting of osteogenic, myogenic, adipogenic, chondrogenic, neurogenic, hepatogenic, nephrogenic, urogenic, isletogenic, pancreatogenic, gastroenterogenic, epitheliogenic, thyroidogenic, myocardiogenic, pneumogenic, retinogenic, gametogenic, endotheliogenic, or hematopoietic factors.
- the MSCs can be seeded on a scaffold in the culture conditions described herein.
- a typical seeding density for tissue engineering is at least 1 x 10 cells/cm .
- seeding densities can be a range from 5 x 10 3 cells/cm 2 to 3 x 10 6 cells/cm 2 .
- the seeding density for a solid scaffold is 2 x 10 6 cells/cm 2 .
- the seeding density for a gel-like scaffold e. g. a hydrogel
- the seeding density for a gel-like scaffold is 2.5 x 10 6 cells/ml.
- Many factors including but are not limited to the types of scaffold and availability of MSCs determine the seeding density.
- One of ordinary skill in the art would be able to determine the seeding densities for the various types of scaffold used.
- the composition enriched in MSCs can be differentiated into several cell lineages including osteogenic, myogenic, adipogenic, chondrogenic, neurogenic, hepatogenic, nephrogenic, urogenic, isletogenic, pancreatogenic, gastroenterogenic, epitheliogenic, thyroidogenic, myocardiogenic, pneumogenic, retinogenic, gametogenic, endotheliogenic, or hematopoietic lineages.
- the composition of MSCs can be used therapeutically in their undifferentiated state, such as in tissue engineering, wound healing and tissue repair.
- the Mesenchymal Stem Cell Osteogenesis Kit (Millipore cat. no. SCR028) provides a method for differentiating mesenchymal stem cells to an osteoblast phenotype.
- the kit contains two ECM coating molecules (collagen type I and vitronectin), which have been shown to promote osteogenic differentiation of mesenchymal stem cells (Salasznyk, 2004, J. Biomed. Biotechnol., 2004(l):24-34), and the inducing reagents, dexamethasone, ascorbic acid 2-phosphate and ⁇ -glycerophosphate.
- Alizarin Red Solution a staining solution that is used to detect the presence of calcium in bone.
- the Mesenchymal Stem Cell Adipogenesis Kit (Millipore cat. no. SCR020) contains reagents that readily differentiate mesenchymal stem cells to an adipogenic lineage as assessed with Oil Red O staining of lipid vacuoles in mature adipocytes. These factors include dexamethasone, IBMX, insulin and indomethacin. Along with Oil Red O staining solution, a hematoxylin solution is provided to counterstain the cell nucleus. Using this kit, typically it is possible to obtain > 30% mature adipocytes from the rat bone marrow derived mesenchymal stem cells. [0130] Pancreatic Islet Cell Characterization Kit (Millipore cat. no.
- the kit includes PDX-I (pancreatic duodenal homeobox gene-1), a master regulator of islet cell development and GLUT-2, a glucose transporter present in beta-islet cells.
- PDX-I pancreatic duodenal homeobox gene-1
- GLUT-2 a glucose transporter present in beta-islet cells.
- Pancreatic Cell Development Pathway Kit (Millipore cat. no. SCR046) provides a collection of antibodies that are unique to key transition points along the developmental pathway of pancreatic cells. Included in the kit are antibodies to critical transcription factors expressed during the program of development along with two antibodies to hormones secreted by mature islets cells (FoxA2, Hes-1, Pax 6, IDX-I, Glucagon and Pancreatic Polypeptide).
- Pancreatic Cell DTZ Detection Assay Kit (Millipore cat. no. SCR047) provides a simple and quick method to identify insulin-producing beta cells from a mixed cell culture preparation or from pancreatic tissues, by detecting high levels of zinc (typically contained in pancreatic beta cells), with the use of a zinc-chelating agent, DTZ.
- This kit contains DTZ staining and rinse solutions along with filters and syringes required for live staining reactions.
- Tissue engineering is the use of a combination of cells, engineering and material methods, and suitable biochemical and physiochemical factors to improve or replace biological functions.
- Tissue engineering aims at developing functional cell, tissue, and organ substitutes to repair, replace or enhance biological function that has been lost due to congenital abnormalities, injury, disease, or aging, or repair fascia in hernias.
- the tissue that is engineered is used to repair or replace portions of or whole tissues (i.e., bone, cartilage, blood vessels, bladder, etc.). Often, the tissues involved require certain mechanical and structural properties for proper function.
- Tissue engineering also encompass the efforts to perform specific biochemical functions using cells within an artificially-created support system (e.g. an artificial pancreas, or a bioartificial liver).
- the term regenerative medicine is often used synonymously with tissue engineering, although those involved in regenerative medicine place more emphasis on the use of stem cells to produce tissues.
- Tissue regeneration aims to restore and repair tissue function via the interplay of living cells, an extra-cellular matrix and cell communicators.
- tissue regeneration is an approach in modern medicine that delivers living tissue or cells and stimulates the body's own natural healing process by activating the body's inherent ability to repair and regenerate.
- innovative tissue regeneration therapies are now available that aim to heal or reconstruct diseased tissue and support the regeneration of diseased or injured organs. Doctors use tissue regeneration to speed up healing and to help injuries that will not heal or repair on their own. Tissue regeneration can help heal broken bones, severe burns, chronic wounds, heart damage, nerve damage, and many other diseases.
- the word “repair” means the natural replacement of worn, torn or broken components with newly synthesized components.
- the word “healing”, as used herein, means the returning of torn and broken organs and tissues (wounds) to wholeness. For example, an open wound on the skin can be repaired with the composition of enriched MSCs. It is envisioned healing would be the eventual closing of the open wound with new growth of skin and underlying connective tissues.
- the composition enriched in human amniotic fluid derived MSCs forms the engineering biomaterial needed for tissue repair and tissue engineering.
- the MSCs or the replacement or repair tissue engineered with MSCs is either grown in a patient or outside the patient and then later transplanted into the patient.
- the expanded pluripotent MSCs can be directly implanted to the site needing repair, for example, the heart after suffering a myocardial infarction (Dinender K. Singla, et. al., Am J Physiol Heart Circ Physiol 293: H1308-H1314, 2007).
- the MSCs can be injected into the tissue repair site together with growth factors and differentiation factors that are known in the art to stimulated cell growth and differentiation of the MSC into the appropriate cell type of the recipient tissue.
- suitable growth factors include but are not limited to TGF ⁇ , platelet derived growth factor (PDGF), epidermal growth factor (EGF), bone morpho genie protein (BMP) and fibroblast growth factor (FGF).
- PDGF platelet derived growth factor
- EGF epidermal growth factor
- BMP bone morpho genie protein
- FGF fibroblast growth factor
- compositions of MSCs of the invention can be implantation for the repair of cardiac muscles, blood vessels, kidney, liver, cartilage, bones, brain the pancreas and the connective and support tissues such as ligaments, muscles, tendons and those tissues, such as the collagen-containing tissues which encapsulate organs, to name a few.
- Methods of direct implantation of stem cells for tissue repair are described in Shake JG et, al. 2002 (Ann Thorac Surg. 73:1919-25), Yoshinori Miyaharal, et. al., 2006 (Nature Medicine 12, 459-465), Atta Behfar, et. al., 2005 (Ann. N.Y. Acad. Sci.
- the MSC can be 'seeded' into an artificial structure capable of supporting three-dimensional tissue formation.
- These structures typically called scaffolds, are often critical, both ex vivo as well as in vivo, to recapitulating the in vivo milieu and allowing cells to influence their own microenvironments.
- Scaffold- guided tissue engineering involves seeding highly porous biodegradable scaffolds with MSCs and/or growth factors, then culturing and implanting the scaffolds to induce and direct the growth of new tissue. The goal is for the MSCs to attach to the scaffold, then replicate, differentiate, and organize into normal healthy tissue as the scaffold degrades. This method has been used to create various tissue analogs including skin, cartilage, bone, liver, nerve, vessels, to name a few examples.
- Scaffolds usually serve at least one of the following purposes: (1) allow stem cell attachment and migration; (2) deliver and retain cells and biochemical factors; (3) enable diffusion of vital cell nutrients and expressed products; and (4) exert certain mechanical and biological influences to modify the behavior of the cell phase.
- scaffolds must meet some specific requirements. A high porosity and an adequate pore size are necessary to facilitate cell seeding and diffusion throughout the whole structure of both cells and nutrients. Although not absolutely essential, biodegradability is often a desirable factor since scaffolds should preferably be absorbed by the surrounding tissues without the necessity of a surgical removal. The rate at which degradation occurs has to coincide as much as possible with the rate of tissue formation: this means that while cells are fabricating their own natural matrix structure around themselves, the scaffold is able to provide structural integrity within the body and eventually it will break down leaving the neotissue, newly formed tissue which will take over the mechanical load. Injectability is also important for certain clinical uses.
- Newer biomaterials have been engineered to have ideal properties and functional customization: injectability, synthetic manufacture, biocompatibility, non-immunogenicity, transparency, nano-scale fibers, low concentration, resorption rates, etc. PuraMatrix.
- PLA - polylactic acid This is a polyester which degrades within the human body to form lactic acid, a naturally occurring chemical which is easily removed from the body.
- Similar materials are polyglycolic acid (PGA) and polycaprolactone (PCL): their degradation mechanism is similar to that of PLA, but they exhibit respectively a faster and a slower rate of degradation compared to PLA.
- Scaffolds can also be constructed from natural materials: in particular different derivatives of the extracellular matrix have been studied to evaluate their ability to support cell growth.
- Protein based materials such as collagen or fibrin, and polysaccharidic materials, like chitosan or glycosaminoglycans (GAGs)
- GAGs glycosaminoglycans
- hyaluronic acid possibly in combination with cross linking agents (e.g. glutaraldehyde, water soluble carbodiimide, etc.), is one of the possible choices as scaffold material.
- Functionalized groups of scaffolds can be useful in the delivery of small molecules (drugs) to specific tissues.
- tissue that can be engineered, reconstructed and/or repaired include but are not limited to craniofacial structures such as bone, adipose tissue and facial muscles, cardiac muscle, cardiac valve, skin, bones, skeletal muscles, diaphragmatic muscles and tendons, breast tissue, blood vessels, cartilage, tendons, ligaments, bladder, urether, uterus, ureter, virgina, cervix, trachea, hair, cornea, esophagus and intestines.
- craniofacial structures such as bone, adipose tissue and facial muscles, cardiac muscle, cardiac valve, skin, bones, skeletal muscles, diaphragmatic muscles and tendons, breast tissue, blood vessels, cartilage, tendons, ligaments, bladder, urether, uterus, ureter, virgina, cervix, trachea, hair, cornea, esophagus and intestines.
- Craniofacial structures reconstruction is the regeneration or de novo formation of dental, oral, and craniofacial structures lost to congenital anomalies, trauma, and diseases.
- Virtually all craniofacial structures are derivatives of mesenchymal cells.
- Biological therapies utilize MSCs, delivered or internally recruited, to generate craniofacial structures in temporary scaffolding biomaterials.
- MSCs delivered or internally recruited, to generate craniofacial structures in temporary scaffolding biomaterials.
- Several craniofacial structures such as the mandibular condyle, calvarial bone, cranial suture, and subcutaneous adipose tissue — have been engineered from MSCs, (JJ. Mao, et. al., J Dent Res 85(ll):966-979, 2006) and is hereby incorporated by reference.
- MSCs can also help cardiac tissue to repair itself weeks after a heart attack.
- Embryonic stem cells have been shown to regenerate damaged heart muscle, when transplanted within a 3-dimensional scaffold into the infracted heart.
- the embryonic stem cells were more successful in restoring heart muscle when transplanted within a 3-dimensional matrix into damaged hearts in an animal model of severe infarction.
- Methods of constructing cardiac related structures are described in US Pat. Nos. 5,880,090, 5,899,937, 6,695,879, 6,666,886 , 7,214,371, and US Pat. Publication No. 20040044403 and they are hereby incorporated by reference.
- Tissue regeneration is a multidisciplinary field involving biology, medicine and engineering.
- the products are also used for diagnostic applications where the tissue is made in vitro and used for testing drug metabolism, toxicity, and pathogenicity.
- Various procedures are contemplated for transferring, immobilizing, and activating the culture-expanded, purified MSCs at the site for repair, implantation, etc., including injecting the cells at the site of a skeletal defect, incubating the cells with a prosthesis and implanting the prosthesis, etc.
- the culture-expanded, undifferentiated MSC can be utilized for various therapeutic purposes such as to elucidate cellular, molecular, and genetic disorders in a wide number of metabolic bone diseases, skeletal dysplasias, cartilage defects, ligament and tendon injuries and other musculoskeletal and connective tissue disorders.
- the invention provides a method of promoting wound healing in a human in need thereof comprising administering a preparation comprising a composition enriched in human amniotic fluid derived MSCs according to the methods described herein in the absence of non-human animal products.
- the invention provides a method of promoting tissue repair in a human in need thereof comprising administering a preparation comprising a composition enriched in human amniotic fluid derived MSCs according to the methods described herein in the absence of non-human animal derived products.
- the invention provides a pharmaceutical composition
- a pharmaceutical composition comprising: (a) a viable composition enriched in human amniotic fluid derived mesenchymal stem or cells obtained according to the methods described herein in the absence of non-human animal products, in which the MSCs are present in an amount sufficient to effect tissue engineering, tissue repair, or wound healing; and (b) a pharmaceutically acceptable carrier.
- the pharmaceutical composition of undifferentiated MSCs can be applied directly to wounds to stimulate wound healing.
- wounds For example, pressure ulcers, leg ulcers, abrasions, lacerations, incisions, donor sites and second degree burns on infected wounds, surgical incisions and traumatic wounds.
- the undifferentiated MSCs can be mixed with growth factors for promoting growth at the site of the wound, and the mixture can be applied to the wound.
- the mixture can also be incorporated into a variety of wound dressing products such as wound dressing gauzes.
- the application of undifferentiated MSCs with or without growth factors help promote healing in areas that can have a reduced capability of self-repair and renewal due to variety of medical conditions such as congestive heart failure, poor circulation, obesity, lymphatic obstructions and diabetes.
- the pharmaceutical composition is HLA-typed matched with the recipient prior to the implantation of the composition enriched in MSCs.
- the pharmaceutical composition of undifferentiated MSCs can be applied directly to a tissue needing repair to stimulate tissue repair. For example, after a heart attack or bone fracture.
- the undifferentiated MSCs can be mixed with growth factors for promoting growth at the site of the tissue needing repair, and the mixture can be applied to the tissue.
- tissue that can require repair include but are not limited to the skin, bone, ligaments, tendons, muscles, the heart, and cartilage.
- small grafts of tissues such as bone pieces are incubated with the pharmaceutical composition of undifferentiated MSCs for increasing the population of MSCs in the graft.
- the graft can then be implanted to the respective tissue for tissue repair.
- the small fragments of facial bones can retrieved from the patient, cleaned and culture in vitro with the pharmaceutical composition of undifferentiated MSCs and then used in the facial reconstruction of the patient.
- the use of the pharmaceutical composition of undifferentiated MSCs can be used in combination with tissue engineered structures composed of differentiated MSCs as well as inert implants such as Teflon.
- the pharmaceutical composition of undifferentiated MSCs can be generally administered with a pharmaceutically acceptable carrier or vehicle therefore.
- a pharmaceutically acceptable carrier is one that does not cause an adverse physical reaction upon administration and one in which maintains the viability of the MSC for delivery into the patient or use in tissue engineering.
- the pharmaceutically acceptable carriers that are inherently nontoxic and non-therapeutic.
- the pharmaceutical composition is sterile, is at a physiological pH of between 6-8, and is isotonic to human bodily fluid.
- antioxidants e.g., ascorbic acid
- low molecular weight (less than about ten residues) polypeptides e.g., polyarginine or tripeptides
- proteins such as serum albumin, gelatin, or immunoglobulins
- hydrophilic polymers such as polyvinylpyrrolidone
- amino acids such as glycine, glutamic acid, aspartic acid, or arginine
- monosaccharides, disaccharides, and other carbohydrates including cellulose or its derivatives, glucose, mannose, or dextrins
- chelating agents such as EDTA
- sugar alcohols such as mannitol or sorbitol.
- the therapeutically effective amount and method of adminstering the pharmaceutical composition of undifferentiated MSCs can vary based on the individual patient, the indication being treated and other criteria evident to one of ordinary skill in the art.
- a therapeutically effective amount of pharmaceutical composition of undifferentiated MSCs is one sufficient to induce repair and/or heal a target organ or tissue.
- the route(s) of administration useful in a particular application are apparent to one of ordinary skill in the art.
- Routes of administration include, but are not limited to, topical, transdermal, and parenteral. Topical and transdermal administration is accomplished via a wound dressing impregnated with the MSCs allowing the MSCs to enter the wound and also enter the blood stream.
- Parenteral routes of administration include but are not limited to direct injection such as intravenous, intramuscular, intraperitoneal intracoronary and subcutaneous injection. Parenteral administration can be accomplished using a needle and syringe, using a high pressure, needle free technique, like POWDERJECTTM, or constant infusion pump.
- a method of obtaining a composition enriched in human amniotic fluid derived mesenchymal stem cells comprising the steps of:
- composition enriched in human amniotic fluid derived mesenchymal stem cells are at least 90% CD29, CD73, and CD44 positive.
- a method of proliferating human amniotic fluid derived mesenchymal stem cells comprising the steps of:
- a method of storing a composition enriched in human amniotic fluid derived mesenchymal stem cells comprising the steps of:
- kits for obtaining a composition enriched in human amniotic derived mesenchymal stem cells from human amniotic fluid comprising:
- a method for producing differentiated human amniotic fluid derived mesenchymal stem cell preparations comprising:
- composition enriched in human amniotic fluid derived mesenchymal stem cells in a culture medium containing differentiation factors for a period sufficient for said stem cells to differentiate and express specific tissue markers.
- the differentiation factors are selected from a group consisting of osteogenic, myogenic, adipogenic, chondrogenic, neurogenic, hepatogenic, nephrogenic, urogenic, isletogenic, pancreatogenic, gastroenterogenic, epitheliogenic, thyroidogenic, myocardiogenic, pneumogenic, retinogenic, gametogenic, endotheliogenic, or hematopoietic factors.
- [Q] A method of promoting wound healing or tissue repair in a human in need thereof comprising administering a preparation comprising a composition enriched in human amniotic fluid derived mesenchymal stem cells according to the method of any one of the paragraphs [A]-[J].
- a cryopreserved pharmaceutical composition comprising:
- a viable composition enriched in human amniotic fluid derived mesenchymal stem cells obtained according to the method of any one of the paragraphs [A]-[I], in which said cells are present in an amount sufficient to effect tissue engineering, tissue repair, or wound healing;
- a pharmaceutical composition comprising:
- a viable composition enriched in human amniotic fluid derived mesenchymal stem cells obtained according to the method of any one of the paragraphs [A]-[I], in which said cells are present in an amount sufficient to effect tissue engineering, tissue repair, or wound healing;
- a multi stage amniotic-derived mesenchymal stem cell manufacturing protocol A multi stage amniotic-derived mesenchymal stem cell manufacturing protocol.
- Implementation of a multi stage cell manufacturing process can facilitate the production of sufficient quantities of amniotic derived mesenchymal stem cells for autologous tissue engineering.
- the multi stage cell manufacturing process will include: isolation/primary expansion; cryopreservation; and thawing/secondary expansion. The feasibility of this multi stage cell manufacture protocol and cell yield of such a staged cell manufacturing process, within regulatory guidelines was determined.
- the mesenchymal cell population was isolated from the amniotic samples based on methods as previously described [12-16].
- Culture media included fetal bovine serum (FBS) and bFGF. Briefly, the sample was centrifuged at 400 x g for 15 minutes.
- FBS fetal bovine serum
- bFGF bFGF
- the pellet was resuspended in growth medium (2mL medium per 1OmL amniotic fluid) consisting of high- glucose Dulbeco Modified Eagle Medium with L-glutamine (DMEM; Lonza, Walkersville, MD), 20 % fetal bovine serum (FBS; Hyclone, Logan, UT), Gentamicin (Lonza) and 5 ng/ml of basic Fibroblast Growth Factor (Promega, Madison, WI) and plated into 1 well of a BD BioacoatTM Collagen I-coated 24 well plate (BD Biosciences, San Jose, CA) in a 5% carbon dioxide incubator at 37 0 C.
- growth medium 2mL medium per 1OmL amniotic fluid
- DMEM high- glucose Dulbeco Modified Eagle Medium with L-glutamine
- FBS Hyclone, Logan, UT
- Gentamicin Lonza
- 5 ng/ml of basic Fibroblast Growth Factor Promega, Madison, WI
- Non-adherent cells were removed 48 hours later and cultures were fed as needed until they reached 70-80% confluence. At harvest, cells were washed once with PBS (Invitrogen, Carlsbad, CA) and detached with a trypsin-like solution (TrypLE Express; Invitrogen) for 3-5 minutes at 37 0 C. Cells were passaged in T75 flasks without counting using growth medium. Before being frozen down, cell viability was assessed by Trypan blue exclusion. Viable cells were characterized by flow cytometric analysis of specific surface antigens, 14-day sterility tests, mycoplasma QPCR, and endotoxin assays (details below).
- Cryopreservation - Cells were then seeded at 3xlO 3 cells per cm 2 in 10 x T175 flasks and frozen in Plasmalyte-A (Baxter Healthcare, Charlotte, NC) containing 2.5% human serum albumin (Baxter) and 10% DMSO (Cryoserv; Edwards Lifesciences, Irvine, CA) using a control rate freezer (Cryo, Rockville, MD) and stored in the vapor phase of a liquid nitrogen tank. Cryopreservation was for 3-5 months. Ideally, there is >70% viability and > 6xlO 8 cells after the secondary expansion.
- the data was acquired using the 6-color BD FACSCanto system (BD Biosciences) and analyzed with FlowJo (Treestar Inc., Ashland, OR). Cell release criteria at the different phases included, as appropriate: >90% CD29+, CD73+, and CD44+; ⁇ 5% CD34+ and CD45+.
- Millipore TTHVAB210 canisters (Millipore) were used for the test samples. These canisters contain a low absorption Durapore membrane filter (0.45 ⁇ m) that is efficient in rinsing away any residual antimicrobial agents from a test sample.
- FTM Fluid Thioglycolate Medium
- SCM Soy Casein Media
- Endotoxin Assay - Endotoxin levels were determined by the gel-clot limulus amebocyte lysate (LAL) test method in compliance with the FDA's GMP regulations, 21 CFR ⁇ 211. Acceptable release criteria are endotoxin level of is 5.0 EU/mL or less.
- Bone engineering from human amniotic mesenchymal cells Possible application in the surgical treatment of congenital anomalies.
- porous scaffold materials have been used for tissue engineering, for example, collagen and fibrin, some linear aliphatic polyesters such as PLA - polylactic acid, polyglycolide acid (PGA) and and polycaprolactone (PCL), polysaccharidic materials, like chitosan or glycosaminoglycans (GAGs).
- PLA polylactic acid
- PGA polyglycolide acid
- PCL polycaprolactone
- GAGs glycosaminoglycans
- Cell seeding densities were 5x10 3 , 2x10 4 , 6x10 4 , or 12x10 4 cells/cm 2 , equally distributed among the constructs.
- constructs were left in culture for 4, 8, 12, or 16 weeks, at which point they underwent multiple analyses.
- Osteogenic differentiation was assessed by Alizarin Red staining and quantitative alkaline phosphatase activity.
- Construct mineralization was assessed by spectrophotometric measurements of extracellular calcium levels.
- Statistical analysis was by ANOVA and Student's t test (p ⁇ 0.05).
- Example of a nanofiber-engineered bone is shown in Figure 1.
- Fig. 2 There was a statistically significant increase in construct mineralization over time at all cell seeding densities (Fig. 2), with no difference among the seeding densities at each time point (Fig. 3).
- Alkaline phosphatase activity was significantly higher at 12 weeks in the 2xlO 4 and 6xlO 4 cells/cm 2 constructs, but no different across most other cell densities and time points (Fig. 4).
- Fig. 4 Interestingly, at 16 weeks, all constructs reached equally low levels of alkaline phosphatase activity, regardless of cell seeding density, suggesting minimal ongoing remodeling.
- Electrospun nanofibrous scaffolds are suitable for bone engineering with human amniotic mesenchymal stem cells. Construct mineralization is directly proportional to time in culture, but not to cell seeding density. Engineered nanobone remodeling stabilizes after 16 weeks in culture. Fetal bone engineered with amniotic mesenchymal stem cells and nanofibers can be useful for the treatment of congenital skeletal anomalies.
- Human amniotic derived mesenchymal stems cells isolated and expanded with human serum.
- the pellets were then resuspended in growth medium (2 mL medium per 10 mL initial amniotic fluid) containing either 20% defined FBS (Hyclone, Logan, UT), or 20% allogeneic pooled human AB serum (ABS; Cambrex BioScience, Walkersville, MD).
- the growth medium consisted of high-glucose Dulbecco's Modified Eagle Medium with L-glutamine, gentamycin (50 ⁇ g/ml) (all from Cambrex BioScience), and 5 ng/mL human recombinant basic fibroblast growth factor (Promega, Madison, WI, catalog # G5071).
- the growth kinetics of the MSCs was studied for up to 50 days in vitro. Briefly, the adherent cells were detached using 0.05% trypsin/0.53 mM EDTA (Invitrogen Corp., Carlsbad, CA) when 80-90% confluent. Cells were counted and re-seeded in growth media containing 20% FBS or 20% ABS, typically 0.5xl0 6 cells in 25 ml in a T162 dish (Corning Inc. Life Sciences, Acton, MA). All subsequent passages were performed similarly. Cell expansion was assessed over time based on the relative days in culture, with day 0 defined as the first passage.
- Fluorescence-activated cell sorting analysis was performed on 7 samples of human mesenchymal amniocytes grown in parallel with either FBS or ABS. Approximately 0.5- IxIO 6 cells per staining were incubated with fluorescent-labeled mouse monoclonal antibodies for 30 minutes and then washed twice with PBS. The following antibodies were used: CD9, CDlO, CD13, CD29, CD31/PECAM-1, CD44, CD45, CD49a, CD71, CD73 (SH3), CD90/Thy- 1, CD106, CDl 17, HLA-A,B,C (all from BD Biosciences), CD105 (SH2) (eBiosciences, San Diego, CA), and CD166/ ALCAM (Ancell Corp.
- Colonies composed of several spindle-shaped, fibroblastoid cells of equivalent size could be successfully isolated and cultured from all the 7 amniotic fluid samples that were processed within 48 hours of procurement.
- Amniotic fluid specimens processed after 48 hours did not reliably grow mesenchymal cell colonies in either medium.
- Viable populations of rapidly expanding cells were obtained from samples harvested at any gestational age (mean, 31.6 weeks; range, 20-37 weeks gestation) and from as little as 5.5 mL of amniotic fluid. There were no differences in cell morphology based on the type of culture medium used, at any passage (data not shown). Based on a single well containing cells harvested from approximately 10 mL of amniotic fluid, the time required until the first passage was on average 12.1 + 3.1 days.
- the isolated cells stained consistently positive for several cell surface markers, including CD73 (SH3), CD105 (SH2), CD 166, CD44, CD29, CD90, CD13, CDlO, and CD71, in a profile compatible with a mesenchymal identity. As expected, these cells also stained positive for HLA-A, B, and C but were negative for CD45, CD34, CD14, CD19, CD8, CD56, and CD31. There were no differences in antigen expression based on gestational age or the type of culture medium used (Fig. 7).
- amniotic fluid-derived MSCs can be isolated relatively easily. These cells proliferate quickly under standard culture conditions.
- the commercially available serum derived from allogeneic, pooled human donors can be used to reliably isolate and expand amniotic fluid-derived MSCs ex vivo, regardless of gestational age, at rates comparable to that of cells cultured in FBS. Regardless of the serum used, the mesenchymal amniocytes expressed the exact same markers, which were consistent with a mesenchymal stem/ phenotype, including CD29, CD44, CD90, and CD105.
- the data demonstrate that a small sample of amniotic fluid, as little as 5 mL, can easily produce enough cells (i.e. greater than 100 million) required to engineer a surgically implantable construct in a relatively short period of time. Since a diagnostic amniocentesis is routinely offered to any mother with a fetus in whom a congenital anomaly has been diagnosed by prenatal imaging, a small additional aliquot of amniotic fluid could be effortlessly obtained at that time for the engineering of different tissue grafts, without any further maternal morbidity.
- the data shows that human mesenchymal amniocytes retain their phenotype and can be dependably expanded ex vivo in the absence of animal serum.
- Cartilage engineered from different perinatal mesenchymal stem cells Cartilage engineered from different perinatal mesenchymal stem cells.
- cartilage engineering utilizing chondrocytes, or select mesenchymal stem cells (MSCs)
- MSCs mesenchymal stem cells
- CB umbilical cord blood
- AF amniotic fluid
- the purpose of this study was to compare the in vitro properties of cartilaginous constructs engineered with MSCs isolated from diverse perinatal sources, namely AF, preterm CB, and neonatal bone marrow (BM), as well as to compare them to native fetal hyaline and elastic cartilage.
- the expansion kinetics of MSCs obtained from these different perinatal sources were also compared.
- BM and CB samples were stored in high-glucose Dulbecco's Modified Eagle Medium (DMEM) containing 5% preservative-free heparin (Elkins Sinn, Cherry Hill, NJ). All samples were transported on ice and processed within 4 hours of harvesting.
- DMEM Dulbecco's Modified Eagle Medium
- MSCs adherent, morphologically distinct MSCs were expanded in tissue culture dishes in a humidified 5% CO 2 incubator at 37 0 C.
- the growth medium consisted of DMEM with L-glutamine, 10% fetal bovine serum (FBS), 10,000 U/ml penicillin G sodium, 10 mg/mL streptomycin sulfate (all from Sigma- Aldrich, St. Louis, MO), and 5 ng/mL human recombinant basic fibroblast growth factor (Promega, Madison, WI, catalog #G5071).
- FACS Fluorescence-activated cell sorting
- CD29 (dilution 1:10; VMRD, Pullman, WA), CD31/PECAM-1 (dilution 1:2; Serotec, Oxford, UK), CD44 (Serotec), CD90/Thy-l (dilution 1:10; BD Biosciences, Bedford, MA), and CD105/SH2/endoglin (dilution 1:10; BD Biosciences).
- the samples were washed in DMEM containing 5% FBS, frozen in distilled water for 1 hour at - 80° C, and then quickly thawed at 37° C prior to measurement of total DNA using Hoechst 33258 dye and a VersaFluor Cuvette Fluorometer (Bio-Rad Laboratories, Hercules, CA) with calf thymus DNA (Sigma-Aldrich) used as the standard. DNA concentrations were converted to normalized cell counts based on a conversion factor of 8 pg of DNA per cell. Population doubling times were calculated using a formula as described elsewhere [60] .
- osteogenic differentiation was assessed according to a modified von Kossa staining protocol [61]. Briefly, the cells were fixed in 4% paraformaldehyde for 10 min. and stained with 2% silver nitrate (Fisher Scientific, Fairlawn, NJ) for 30 min under ultraviolet light. After 3 washes in distilled water, the cells were placed in 2.5% sodium thiosulfate (Sigma- Aldrich) for 5 min., washed once more in distilled water, and assessed qualitatively for silver stained mineralization under an inverted microscope.
- MSCs at passage 8 from all 3 groups were plated at a density of 3-5xlO 4 cells per cm 2 in 6-well plates containing DMEM, 10% FBS, 10,000 U/ml penicillin G sodium, 10 mg/mL streptomycin sulfate, 60 ⁇ M indomethacin, l ⁇ M dexamethasone, 10 ⁇ g/mL insulin, and 0.5 mM 3-isobutyl-l-methylxanthine (all from Sigma- Aldrich). As negative controls, MSCs were cultured in parallel in standard medium.
- the chondrogenic medium consisted of DMEM containing 10 ng/mL of transforming growth factor- ⁇ (TGF- ⁇ l - R&D Systems, Minneapolis, MN; or TGF- ⁇ 2 - Peprotech, Rocky Hill, NJ), 10-7 M dexamethasone, 50 ⁇ g/mL ascorbic acid 2-phosphate, 10,000 U/mL penicillin G sodium, 10 mg/mL streptomycin sulfate, 100 ⁇ g/mL sodium pyruvate, 40 ⁇ g/mL L-proline (all from Sigma- Aldrich), and insulin transferrin selenium-plus (ITS-plus - BD Biosciences).
- the media also contained 100 ng/ml of insulin growth factor- 1 (IGF-I, Peprotech).
- GAG levels were determined at 656 nm using the Blyscan dye reagent (Biocolor, Harbor, UK) containing dimethylmethylene blue.
- Collagen type II concentrations were measured at 540 nm using the Sircol dye reagent (Biocolor) containing sirius red.
- Elastin measurements were obtained at 513 nm using the Fastin dye kit (Biocolor) containing tetraphenyl porphine sulfonate.
- MSCs ovine perinatal mesenchymal cells
- FACS analyses of isolated cells from AF, BM, and CB showed similar forward and side scatter profiles (data not shown).
- Immunophenotypic profiles of ungated MSCs from amniotic fluid, neonatal bone marrow, and preterm umbilical cord blood were similar. Greater than 90 percent of cells in all 3 groups were strongly positive for CD29 and CD44 (data not shown).
- CD90/Thy-l and CD105/SH2/endoglin were positive or weak expression of CD90/Thy-l and CD105/SH2/endoglin.
- fetal dermal fibroblasts did not express any detectable levels of CD90/Thy-l and CD105/SH2/endoglin (data not shown).
- CD31/PECAM-1 was not found in any sample.
- MSCs from all 3 groups cultured under osteogenic conditions displayed osteogenic differentiation, as confirmed by von Kossa staining. All 4 samples in each group showed evidence of dark calcium aggregates. The osteogenic phenotype appeared to be less robust in 2 of the samples containing either BM- or CB-derived MSCs although this was not formally quantified. Osteogenic differentiation was not observed in MSCs from any group grown for 21 days in standard medium (control).
- Adipogenic differentiation was confirmed in all 3 MSC groups cultured in adipogenic media, as demonstrated by the presence of numerous cells containing lipid-rich vacuoles on Oil-O-Red staining. Three of 4 samples from AF and 4 of 4 samples from BM contained greater than 20 percent of cells with an obvious adipogenic phenotype. However, only 1 of 4 samples tested from CB could be induced into the adipogenic differentiation pathway. Adipogenic differentiation was not observed in MSCs from any group grown for 21 days in standard medium (control).
- the ECM was more basophilic in both BM- and CB-derived specimens than in AF-based grafts ((data not shown). There were no obvious differences in the AF-based constructs related to whether they were cultured with or without IGF-I in the chondrogenic medium. None of the engineered constructs had an identical appearance to either hyaline or elastic native cartilage. The chondrocytes present in native fetal elastic cartilage were more densely packed when compared to specimens from all other groups (data not shown).
- EVG staining showed varying degrees of elastin fiber formation within the engineered constructs. Histologically, elastin fibers appeared to be more abundant within the interstitial matrix of native fetal elastic cartilage and AF-based engineered cartilage, when compared to all the other groups (data not shown). Quantitative assays confirmed that AF- derived constructs had elevated levels of ⁇ -elastin (156.0+120.4 ⁇ g/mg), which were statistically comparable to that of native elastic cartilage (235.8+54.2 ⁇ g/mg) and were significantly higher than that of native hyaline cartilage as well as that of the other two types of engineered grafts (Fig. 12).
- MSCs from 3 perinatal cell sources namely AF, neonatal BM, and preterm umbilical CB were analysed.
- the data indicate that MSCs from all these sources are morphologically and phenotypically similar. Regardless of the source, these cells expressed markers consistent with a mesenchymal stem/ phenotype, including CD29, CD44, CD90, and CD105. None of the isolated cells expressed CD31, pointing to the absence of endothelial cells in our cultures.
- the nature of these MSCs was further confirmed by their differentiation into at least 2 different mesenchymal cell lineages, regardless of the source. AF-based MSCs appeared to have osteogenic and adipogenic potentials equivalent to that of BM-derived MSCs, even after 8 passages.
- the AF- based MSCs proliferated more than twice as quickly in culture than BM- and CB-derived MSCs grown under identical conditions. For example, a 5 mL aliquot of AF obtained during a routine diagnostic amniocentesis is all that would be needed for one to obtain several hundred million cells in 3-4 weeks' time. The ability to produce a very large number of cells, in a short period of time, from a diminutive sample is generally highly desirable for the clinical translation of cell- based therapies.
- AF-based constructs contained comparable levels of pepsin soluble collagen to the other constructs, they also had the highest concentrations of both sulfated GAG and elastin among all engineered specimens. Their elastin levels were actually equivalent to that of native elastic cartilage, while being significantly richer in GAG than that type of native tissue.
- proteoglycans are primarily responsible for providing mechanical compressive strength, elastin confers pliability, and collagen type II imparts tensile strength and acts as a natural scaffold for tissue remodeling.
- the singular ECM profile of AF-based construct should be particularly suited to surgical reconstructive procedures in which elastic cartilage would be desirable.
- Prockop DJ Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997; 276: 71-4.
- Kaviani A Guleserian K, Perry TE, et al.: Fetal tissue engineering from amniotic fluid. J Am Coll Surg 2003; 196: 592-7. 14. Fuchs JR, Kaviani A, Oh JT, et al.: Diaphragmatic reconstruction with autologous tendon engineered from mesenchymal amniocytes. J Pediatr Surg 2004; 39: 834-8; discussion 834-8.
- Prusa AR and Hengstschlager M Amniotic fluid cells and human stem cell research: a new connection. Med Sci Monit 2002; 8: RA253-7.
- Prusa AR, Marton E, Rosner M, et al. Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Hum Reprod 2003; 18: 1489-93.
- Prusa AR, Marton E, Rosner M, et al. Neurogenic cells in human amniotic fluid. Am J Obstet Gynecol 2004; 191: 309-14.
- Milunsky A Amniotic fluid cell culture, in Genetic disorder of the fetus, Milunsky A, Editor. New York: Plenum Press, 1979. p. 75-84.
- Prusa AR, Marton E, Rosner M, et al. Stem cell marker expression in human trisomy 21 amniotic fluid cells and trophoblasts. J Neural Transm Suppl 2003: 235-42.
- Torricelli F Brizzi L
- Bernabei PA et al.: Identification of hematopoietic cells in human amniotic fluid before the 12th week of gestation. Ital J Anat Embryol 1993; 98: 119-26.
- Fauza DO Amniotic fluid and placental stem cells. Best Pract Res Clin Obstet Gynaecol 2004; 18: 877-91.
- Kern S, Eichler H, Stoeve J, Kluter H, and Bieback K Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24, 1294-301, 2006.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Developmental Biology & Embryology (AREA)
- General Health & Medical Sciences (AREA)
- Biotechnology (AREA)
- Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Genetics & Genomics (AREA)
- Cell Biology (AREA)
- Organic Chemistry (AREA)
- Reproductive Health (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Dentistry (AREA)
- Environmental Sciences (AREA)
- Gynecology & Obstetrics (AREA)
- Pregnancy & Childbirth (AREA)
- Rheumatology (AREA)
- Immunology (AREA)
- Virology (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Abstract
The invention provide methods for isolating, expanding, and enriching human fetal mesenchymal stem cells (MSCs) from human amniotic fluid in the absence of non-human derived animal products, cryopreserving the human fetal MSC in the absence of non-human derived animal products for future uses, thawing the cryopreserved MSCs for therapeutic use and/or further cell expansion, expanding the thawed previously cryopreserved stem cells in the absence of non-human derived animal products, and differentiating the MSCs.
Description
HUMAN AMNIOTIC FLUID DERIVED MESENCHYMAL STEM CELLS
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit under 35 U. S. C. § 119(e) of U.S. provisional application No. 60/979,938 filed October 15, 2007, the contents of which are incorporated herein by reference in its entirety.
GOVERNMENT SUPPORT
[0002] This invention was made with Government Support under U24 HL074355-01A1 awarded by the National Institute of Health. The Government has certain rights in the invention.
FIELD OF INVENTION
[0003] The invention relates to methods for the collection and culture-expansion of human mesenchymal stem cells (huMSCs) from human amniotic fluid in the absence of non- human animal products, and the cryopreservation of huMSCs to yield a composition of expanded amniotic fluid derived mesenchymal stem cells (MSCs) for tissue engineering, tissue repair, and wound healing in humans. HuMSCs can be stored frozen, subsequently thawed and used for differentiation into a variety of mesenchymal lineage tissues for tissue engineering and tissue repairs in humans.
BACKGROUND OF INVENTION
[0004] There is considerable interest in the identification, isolation and generation of human stem cells. Human stem cells are totipotential or pluripotential precursor cells capable of generating a variety of mature human cell lineages. This ability serves as the basis for the cellular differentiation and specialization necessary for organ and tissue development.
[0005] Recent success at transplanting such stem cells have provided new clinical tools to reconstitute and/or supplement bone marrow after myeloablation due to disease, exposure to toxic chemical and/or radiation. Further evidence exists that demonstrates that stem cells can be employed to repopulate many, if not all, tissues and restore physiologic and anatomic functionality. The application of stem cells in tissue engineering, gene therapy delivery and cell therapeutics is also advancing rapidly.
[0006] Many different types of mammalian stem cells have been characterized. For example, embryonic stem cells, embryonic germ cells, adult stem cells or other committed stem cells are known. Certain stem cells have not only been isolated and characterized but have also been cultured under conditions to allow differentiation to a limited extent. A basic problem remains, however, in that obtaining sufficient quantities and populations of human stem cells which are capable of differentiating into many cell types is near impossible. Stem cells are in critically short supply. These are important for the treatment of a wide variety of disorders, including malignancies, inborn errors of metabolism, congenital tissue defects, hemoglobinopathies, and immunodeficiencies.
[0007] Another problem associated with the clinical applications of human stem cell- based tissue engineering and human stem cell-based cell therapy is the fact that almost all stems cells isolated and expanded in vitro or ex vivo cultures are done using non-human animal products in the culture medium, such as fetal calf serum (FCS) and fetal bovine serum (FBS). Previous experimental and clinical data have shown that exposure of human cells to FBS, for example, results in fixation of animal proteins on the cell surface, rendering the human recipient more prone to adverse immune-mediated and/or inflammatory events, including anaphylactic reactions [17-19]. Moreover, the threat of disease transmission by bacterial, viral, and prion pathogens from the contact with animal-based products remains a valid concern [20] . In fact, the United States Food and Drug Administration Agency (FDA) normally defer the approval of novel cell-based therapies that include cell exposure to xenogeneic materials.
[0008] While the obvious solution to the use of non-human animal products is to find a substitute for FCS or FBS, there has been much controversy about what constitutes a suitable FCS or FBS substitute, and whether sufficient cells can be produced for the various therapies in the absence of the likes of FCS. Some studies have experimented using serum- free media supplemented with growth factors and cytokines. These studies have concluded that serum- free media cannot promote the growth of mesenchymal stem cells (MSCs) (Gronthos, S. et. al., 1995, Blood, 85: 929-40; Kuznetsov, S. et. al., 1997, Br. J. Haematol., 97:561-570). There have been some studies using autologous serum and they all have variable success (Spees, JL., et. al., 2004, MoI. Ther., 9:747-56; Stute, N., et. al., 2004, Exp. Hematol., 32: 1212-25; Anselme, K., et. al., 2002, Tiss. Eng., 8: 941-53; Kuznetsov, S. et. al., 1997, Br. J. Haematol., 97:561-570). Yet, this approach is limited by the amount of autologous serum necessary to expand the stem cells for clinical use (Sotiropoulou PA., et. al., 2006, Stem Cells, 24:1409-10) and the variability of serum used, especially for patients receiving prior chemotherapy. There are some studies that
have been successful in isolating and expanding stem cells using human AB serum (Anselme, K., et. al., 2002, Tiss. Eng., 8: 941-53; Yamaguchi, A., et. al., 2002, Transfusion, 42:921-7). However, others have reported growth arrest of stem cells after the first passage (Spees, JL., et. al., 2004, MoI. Ther., 9:747-56; Shahdadfar A., et. al., 2005, Stem Cells, 23: 1357-66). It appears that the direct substitution of FCS or FBS with human serum produces unpredictable results. This can also be due to the source of the stem cells and the age of the MSCs, for example, adult stem cells versus fetal stem cells. Hence it remains unclear if large scale production of expanded human stem cells for human therapy is feasible.
[0009] Beside the bone marrow, adipose tissue, human placenta, and umbilical cord, the amniotic fluid can be a good source of human stem cells. The human amniotic fluid represents a rich source of a variety of stem cells, for example, hematopoietic stem cells and MSCs (Fauza DO., Best Pract. Res. Clin. Obstet. Gynaecol., 2004,18: 877-91). Using stem cells derived from human amniotic fluid can free clinicians, researchers, and scientists from the ethical concerns associated with human embryonic cells (Holden, C. Science 2007, 315: 170).
[0010] MSCs are generally recognized as pluripotential cells which are capable of dividing many times to produce progeny cells that can eventually give rise to mesoderm derived tissues, including cartilage, bone, tendon, ligament, marrow stroma and connective tissue. By definition, these MSCs are generally considered to not be governed by, or are not limited to, a fixed number of mitotic divisions (Caplan, 1991, J. Orthopaed. Res. 9:641-650). U.S. Pat. Nos. 5,197,985 and 5,226,914 both described processes for isolating and replicating human bone marrow-derived MSCs in culture, and activating them so that they differentiate either into bone or, purportedly, into cartilage. Likewise, U.S. Pat. No. 5,486,359 described human MSC (huMSCs) and monoclonal antibodies to these cells.
[0011] In addition to their capacity for multi lineage differentiation, MSCs have been shown to possess remarkable plasticity [1, 2]. As a result, over the last decade there has been increasing interest by multiple groups in the use of MSCs in regenerative therapies for a variety of disorders [3-11]. Moreover, the MSCs normally found in the amniotic fluid could be used in tissue engineering strategies for the surgical repair of congenital anomalies in the perinatal period [12, 13]. Translated clinically, from a simple amniocentesis, different tissue grafts could be engineered in parallel to the remainder of the fetus gestation period, so that a child could benefit from having autologous, expanded tissue promptly available for surgical reconstruction, either in the neonatal period or before birth (Fig. 5).
[0012] Cell-based therapies have been traditionally under explored in the perinatal period. Yet, major congenital anomalies are present in approximately 3% of all newborns [65]. Those diseases are responsible for nearly 20% of deaths occurring in the neonatal period and even higher morbidity rates later in childhood [66]. Tissue engineering and other cell-based therapies have great potential in the treatment of different congenital anomalies [44-47, 49, 51, 52, 54]. To this end, the amniotic fluid derived MSCs make a good cell source for autologous stem cells for human tissue engineering and transplantation. Further applications of MSCs would include wound healing and tissue repair enhancement.
SUMMARY OF THE INVENTION
[0013] Embodiments of the invention provide methods for isolating, expanding, and enriching human fetal mesenchymal stem cells (MSCs) from human amniotic fluid in the absence of non-human derived animal products, cryopreserving the human fetal MSC in the absence of non-human derived animal products for future uses, thawing the cryopreserved MSCs for therapeutic use and/or further cell expansion, expanding the thawed previously cryopreserved stem cells in the absence of non-human derived animal products, and differentiating the MSC into several cell lineages including osteogenic, myogenic, adipogenic, chondrogenic, neurogenic, hepatogenic, nephrogenic, urogenic, isletogenic, pancreatogenic, gastroenterogenic, epitheliogenic, thyroidogenic, myocardiogenic, pneumogenic, retinogenic, gametogenic, endotheliogenic, or hematopoietic lineages. Undifferentiated and differentiated MSC can be used for tissue engineering, tissue repair and/or wound healing. The tissue engineering, tissue repair, and wound healing can be performed for/in an autologous individual from which the MSCs were derived, or in HLA type matched individual that is HLA typed matched with the donor of the MSCs.
[0014] Additionally, embodied in the invention is a method of obtaining a composition enriched in human amniotic fluid derived MSCs comprising isolating MSCs from a sample of human amniotic fluid in the absence of non-human animal derived products and expanding the isolated human amniotic fluid derived MSCs in the absence of non-human derived products. The method further comprises cryopreserving the composition enriched in human amniotic fluid derived MSCs.
[0015] The human amniotic fluid can be collected between 5 weeks of gestation to human term or even at birth. The fluid can be collected by a skilled physician specialized during routine diagnostic amniocentesis and is performed under sterile conditions. A volume of 5-1OmI
of amniotic fluid is preferred for the method described herein for the isolation of the human amniotic fluid derived MSCs.
[0016] Following the collection of the fluid, the human amniotic fluid can be cryopreserved directly, or the cells from the amniotic fluid can be harvested by centrifugation and the harvested cells can then be cryopreserved. In an alternate embodiment, the isolation and expansion of human amniotic fluid derived MSCs is carried out prior to cryopreservation. The isolation and expansion is performed in absence of non-human animal derived product. In a preferred embodiment, the isolation and expansion is performed in the presence of human serum, either autologous or allogeneic AB serum, or in the presence of human platelet rich plasma supplemented with heparin. In another embodiment, the isolation and expansion is performed under serum-free conditions. In a preferred embodiment, the composition enriched in human amniotic fluid derived MSCs is cryopreserved. In another preferred embodiment, the cryopreserved composition of enriched in human amniotic fluid derived MSCs is thawed such that the MSCs are viable.
[0017] In one embodiment, the composition enriched in human amniotic fluid derived
MSCs are at least 90% CD29, CD73, and CD44 positive, at least 50% CD90 and CD105 positive, and at most 5% CD34 and CD45 positive.
[0018] When the cryopreserved MSCs are needed, they are thawed and placed in culture for further expansion to obtain sufficient number of cells. In one embodiment, the method for obtaining a composition enriched in human amniotic fluid derived MSCs further comprises thawing the cryopreserved composition of enriched in human amniotic fluid derived MSCs such that the stem cells are viable.
[0019] Encompassed in the invention is a method of proliferating a composition enriched in human amniotic fluid derived MSCs comprising selecting at least one single MSC from a sample of human amniotic fluid in the absence of non-human animal derived products, introducing at least one single MSC to a culture medium containing no non-human animal derived product, and proliferating at least one single MSC to a culture medium containing no non-human animal derived product.
[0020] Also envisioned in the invention is a method of storing a composition enriched in human amniotic fluid derived MSCs comprising obtaining a composition enriched in human amniotic fluid derived MSCs according to the methods described herein and cryopreserving the composition enriched in human amniotic fluid derived MSCs.
[0021] In one embodiment, the invention provides an isolated human amniotic fluid derived MSC prepared according to the methods described herein.
[0022] In another embodiment, the invention provides for a kit for obtaining a composition enriched in human amniotic derived MSCs from human amniotic fluid comprising a container for the collection of human amniotic fluid, a coated container for the isolation and primary expansion of human amniotic fluid derived MSCs, human serum for the culture- expansion of human amniotic fluid derived MSC, and instructions for the isolation, identification, and expansion of human amniotic fluid derived MSCs. In addition, the kit further comprises culture medium reagents for reconstituting a culture medium containing human serum for use in the isolation and expansion of the MSCs.
[0023] In one embodiment, the invention provides for a method for producing differentiated human amniotic fluid derived MSC preparations comprising obtaining a composition enriched in human amniotic fluid derived MSCs according to the methods described herein and culturing the composition enriched in human amniotic fluid derived MSCs in a culture medium containing differentiation factors for a period sufficient for the MSCs to differentiate and express specific tissue markers. A variety of differentiating factors can be used and they are selected from a group consisting of osteogenic, myogenic, adipogenic or chondrogenic, neurogenic, hepatogenic, nephrogenic, urogenic, isletogenic, pancreatogenic, gastroenterogenic, epitheliogenic, thyroidogenic, myocardiogenic, pneumogenic, retinogenic, gametogenic endotheliogenic, or hematopoietic factors. In another embodiment, the MSCs are seeded on a scaffold during the differentiation process.
[0024] In one embodiment, the invention provides a method of promoting wound healing and/or tissue repair in a human in need thereof comprising administering a preparation comprising a composition enriched in human amniotic fluid derived MSCs wherein the stem cell preparation is applied directly to the wound and/or tissue needing repair. In another embodiment, the MSCs preparation is embedded in a wound dressing material such as a gauze and the seeded wound dressing material is applied on to the wound.
[0025] In one embodiment, the invention provides a method of storing human amniotic fluid derived cells comprising harvesting the cells from a sample of human amniotic fluid and cryopreserving the cells such that the cells remain viable upon thawing. In a preferred embodiment, cryopreservation agents such as DMSO and glycerol are added to the harvested cells that are at a density of at least 3 X 10 cells/ml and the temperature of the mixture of cells
is lowered slowly, for example, at a rate of one degree per minute to -1960C. In one embodiment, 5 X 106 cells/ml are cryopreserved in PlasmalyteA with 2.5% human serum albumin and 10% DMSO. In another embodiment, 20 xlO6 cells are cryopreserved per cryovials.
[0026] Envisioned in the invention is a cryopreserved pharmaceutical composition comprising a viable composition enriched in human amniotic fluid derived MSCs obtained according to the method described herein, wherein the MSCs are present in an amount sufficient to effect tissue engineering or wound healing; an amount of cryopreservative sufficient for cryopreservation of said cells; and a pharmaceutically acceptable carrier.
[0027] Also envisioned in the invention is a pharmaceutical composition comprising a viable composition enriched in human amniotic fluid derived MSCs obtained according to the methods described herein, wherein the MSCs are present in an amount sufficient to effect tissue engineering or wound healing and a pharmaceutically acceptable carrier.
BRIEF DESCRIPTION OF DRAWINGS
[0028] Figure 1. A nanofiber bone construct engineered with electrospun poly(L-lactic) acid nanofibrous scaffolds and expanded amniotic MSCs.
[0029] Figure 2. Mineralization of nanofiber bone constructs as determined by spectrophotometric measurements of extracellular calcium levels.
[0030] Figure 3. Bone mineral density in the nanofiber engineered bone construct is directly related to the period spent in culture and differentiation but is not directly related to the initial number of expanded amniotic MSCs seeded on the nanofiber scaffold during culture and differentiation.
[0031] Figure 4. Osteogenic differentiation of the expanded MSCs seeded on the nanofiber bone construct as determined by quantitative alkaline phosphastase activity.
[0032] Figure 5. The clinical concept of amniotic fluid-based fetal tissue engineering for the surgical treatment of congenital anomalies: fetal MSCs isolated from the amniotic fluid are expanded ex vivo and used in an implantable engineered construct either later in gestation, or in postnatal life, for the treatment of a prenatally diagnosed defect.
[0033] Figure 6. Graph of the logarithmic cell expansion rates based on the number of days since the first cell passage (relative days). There were no statistical differences in the
growth kinetics of amniotic fluid-derived MSCs cultured in fetal bovine serum when compared to those grown in human AB serum (P>0.05). FBS = fetal bovine serum; HAB = human AB serum.
[0034] Figure 7. Representative ungated flow cytometry analyses of expanded huMSCs isolated from amniotic fluid. There were no differences in the immunophenotypic profiles of cells grown in fetal bovine serum (thinner line) compared to those cultured in human AB serum (thicker line).
[0035] Figure 8A. Ex vivo cell proliferation kinetics of ovine perinatal MSCs from amniotic fluid (AF), neonatal bone marrow (BM), and prenatal umbilical cord blood (CB) (n = 4 per group) presented as cell number counts.
[0036] Figure 8B. Ex vivo cell proliferation kinetics of ovine perinatal MSCs from amniotic fluid (AF), neonatal bone marrow (BM), and prenatal umbilical cord blood (CB) (n = 4 per group) presented as population doubling time in hours (*p<0.5).
[0037] Figure 9. Total DNA levels, expressed as means + SEM, of native fetal hyaline cartilage (hyaline), native fetal elastic cartilage (elastic), engineered cartilage from neonatal bone marrow (BM)-derived MSCs (MSCs), engineered cartilage from preterm umbilical cord blood (CB)-derived MSCs, and engineered cartilage from amniotic fluid (AF)-derived MSCs. (*p<0.05 compared to native hyaline cartilage; #p<0.05 compared to native elastic cartilage) (magnification, x 400).
[0038] Figure 10. Sulfated glycosaminoglycan levels, expressed as means + SEM, in native fetal hyaline cartilage (hyaline),native fetal elastic cartilage (elastic), engineered cartilage from neonatal bone marrow (BM)-derived MSCs (MSCs), engineered cartilage from preterm umbilical cord blood (CB)-derived MSCs, and engineered cartilage from amniotic fluid (AF)- derived MSCs. (*p<0.05 compared to native hyaline cartilage; #p<0.05 compared to native elastic cartilage) (magnification, x 400).
[0039] Figure 11. Pepsin-soluble collagen levels, expressed as means + SEM, in native fetal hyaline cartilage (hyaline), native fetal elastic cartilage (elastic), engineered cartilage from neonatal bone marrow (BM)-derived MSCs (MSCs), engineered cartilage from preterm umbilical cord blood (CB)-derived MSCs, and engineered cartilage from amniotic fluid (AF)- derived MSCs. (*p<0.05 compared to native hyaline cartilage; #p<0.05 compared to native elastic cartilage) (magnification, x 400).
[0040] Figure 12. Elastin levels, expressed as means + SEM, in native fetal hyaline cartilage (hyaline), native fetal elastic cartilage (elastic), engineered cartilage from neonatal bone marrow (BM)-derived MSCs (MSCs), engineered cartilage from preterm umbilical cord blood (CB)-derived MSCs, and engineered cartilage from amniotic fluid (AF)-derived MSCs. (*p<0.05 compared to native hyaline cartilage; #p<0.05 compared to native elastic cartilage) (magnification, x 400).
DETAILED DESCRIPTION OF THE INVENTION
[0041] Embodiments of the invention provide methods for isolating, expanding, and enriching human fetal MSCs from human amniotic fluid in the absence of non-human animal derived products, cryopreserving the human fetal MSCs in the absence of non-human animal derived products for future uses, thawing the cryopreserved stem cells for therapeutic use and/or further cell expansion, expanding the thawed previously cryopreserved stem cells in the absence of non-human animal derived products, and differentiating the MSCs into several cell lineages including osteogenic, myogenic, adipogenic, chondrogenic, neurogenic, hepatogenic, nephrogenic, urogenic, isletogenic, pancreatogenic, gastroenterogenic, epitheliogenic, thyroidogenic, myocardiogenic, pneumogenic, retinogenic, gametogenic, endotheliogenic, or hematopoietic lineages. Undifferentiated and differentiated MSCs can be used for tissue engineering, tissue repair and wound healing. The tissue engineering, tissue repair, and wound healing can be performed in an autologous individual from which the MSCs were derived, or in HLA type matched individual, that is HLA typed matched with the donor of the MSCs.
[0042] In one embodiment, the invention provides a multi stage process for obtaining a composition enriched in human amniotic fluid derived MSCs, and the composition of MSCs can be further differentiated for tissue engineering, tissue repair, and/or wound repair of a future recipient. In one embodiment, the method of obtaining a composition enriched in human amniotic fluid derived MSCs comprise the steps of: (a) isolating MSCs from a sample of human amniotic fluid in the absence of non-human animal derived products; and (b) expanding the isolated human amniotic fluid derived MSCs in the absence of non-human animal derived products.
[0043] In one embodiment, the composition enriched in human amniotic fluid derived
MSCs can be autologous to the future recipient of the composition, that is derived from the same individual. In another embodiment, the composition of MSCs can be non-autologous to the future recipient of the composition, that is the donor of the human amniotic fluid derived MSCs
is different from the recipient of the MSCs. It is envisioned that proper human leukocyte antigen (HLA) matching between the donor and the recipient be conducted prior to the use of the composition of MSCs.
[0044] The multi stage process comprises: (stage-1) isolating MSCs from a sample of human amniotic fluid in the absence of non-human animal derived products; (stage 2) expanding the isolated human amniotic fluid derived MSCs in the absence of non-human animal derived products; and (stage 3) cryopreserving the composition enriched in human amniotic fluid derived MSCs.
[0045] The multi stage process can be implemented before the birth of a baby. This is especially useful when there is in-utero indication that there can be some congenital anomalies with the fetus through routine sonogram of the fetus during prenatal visits. Some examples of congenital anomalies are neural tube defects, congenital heart defects, oral facial clefts, congenital diaphragmatic hernia and limb reduction defects. The typical procedure to correct some of these congenital anomalies is surgical repair of the defective tissue by using inert substitute tissue materials such as Teflon, in absence of any human donor tissue or human autologous tissue. During a subsequent diagnostic amniocentesis, an additional amount of 5 -10 ml of amniotic fluid can be collected for implementing the multi stage process. The goal of the multi stage process is to produce a permanent source of pluripotent cells for correcting congenital defects and for possible future needs due to disease and/or injury. When the MSCs is derived from a fetus diagnosed with some congenital defects, the multi stage process can produce a permanent source of pluripotent cells for that are autologous to the baby with congenital defects. The cryopreserved human amniotic fluid derived MSCs at stage 3 becomes the permanent source of pluripotent cells. A sample of the cryopreserved MSCs can be thawed and further expanded to provide MSCs in sufficiently large quantities for the tissue engineering of the tissues that is needed for repairing the congenital defects, or loss through disease and/or injury.
[0046] By harvesting the baby's own MSCs from its surrounding amniotic fluid, the
MSCs can be expanded and differentiated into the right tissue type for correcting and repairing the congenital defects. For example, if a baby is diagnosed with congenital diaphragmatic hernia, the baby's MSCs is first isolated from the amniotic fluid during a routine amniocentesis, expanded and "banked" by cryopreservation. Several weeks before the birth of the baby, the banked MSCs is retrieved, thawed, expanded to increase the number of cells needed for seeding a scaffold and differentiating into tendon and/or muscle cells, and then tissue engineered into a
diaphragmatic tendon and/or muscle tissue. This engineered diaphragmatic tendon and/or muscle tissue can then be used to surgically repair the missing part of the diaphragm that is the cause of the congenital diaphragmatic hernia. Since the engineered tissue is autologous to the baby, the baby is less likely to develop any immune rejection of the engineered tissue. Moreover since the repair material consists of living tissue, the repair material has a greater amount of flexibility and elasticity liken to the baby's natural tissue compared to inert substitute tissue materials such as Teflon. In addition, the repair material will grow with the baby. Accordingly, the long term prognosis of such repair graft is better than inert substitute tissue materials such as Teflon, as the autologous repair grafts need less secondary repair.
[0047] There are several advantages to implementing the multi stage process for any fetus. Firstly, the MSCs used are autologous. Their use in tissue engineering helps overcome the potential problems of immune rejection or transfer of infectious agents. Secondly, by harvesting the MSCs well in advance of the birth of the baby, the harvested cells can be expanding in vitro, providing large amount of cells necessary for tissue engineering. Thirdly, the engineered tissue can be prepared in advance and timed with the approximate delivery date of the baby. This facilitates the repair of congenital defects shortly after birth. Fourthly, there is a permanent source of autologous pluripotent cells available for the baby throughout his or her lifespan. If in the future, there is a need for addition or subsequent tissue repair of the same congenital defects, tissue repair resulting from injury, or a need of autologous stem cells for treating cancer, a disease or disorder, a sample of the permanent source of human amniotic derived MSCs can be thawed and expanded to provide the much needed cells. In another embodiment, the permanent source of human amniotic derived MSCs can be used for tissue engineering, tissue repair, and/or wound healing in HLA-matched individuals.
[0048] Amniotic fluid is the watery liquid surrounding and cushioning a growing fetus within the amnion. It allows the fetus to move freely without the walls of the uterus being too tight against the fetus's body. The amnion grows and begins to fill, mainly with water, around two weeks after fertilization. After a further 10 weeks the liquid contains proteins, carbohydrates, lipids and phospholipids, urea and electrolytes, all which aid in the growth of the fetus. The fetus also sheds cells into the amniotic fluid. In the late stages of gestation much of the amniotic fluid consists of fetal urine. Accordingly, the amniotic fluid can be a plentiful source of non-embryonic stem cells. Hematopoietic stem cells and MSCs are two examples of the fetal cell types found in amniotic fluid.
[0049] MSCs are the formative pluripotential blast cells found inter alia in bone marrow, blood, dermis and periosteum that are capable of differentiating into any of the specific types of mesenchymal or connective tissues (i.e. the tissues of the body that support the specialized elements; particularly adipose, osseous, cartilaginous, muscular, elastic, and fibrous connective tissues) depending upon various influences from bioactive factors, such as cytokines. These cells are present in amniotic fluid from the first trimester through to human term at 38 weeks of gestation (Pieternella S. in 't Anker, et. al., 2004, Stem Cells, 22:1338-1345). Accordingly, it is possible to harvest amniotic fluid from 5 weeks through to human term at 38 weeks of gestation for the isolation of MSCs.
[0050] In one embodiment, the human amniotic fluid is collected between 5 weeks of gestation to human term. In another embodiment, the human amniotic fluid is collected at birth. In yet another embodiment, the collected human amniotic fluid is cryopreserved. In a further embodiment, the human amniotic fluid is used for the isolation and expansion of human amniotic fluid derived MSCs.
[0051] In one embodiment, the method of obtaining a composition enriched in human amniotic fluid derived MSCs further comprise cryopreserving the composition enriched in human amniotic fluid derived MSCs.
[0052] In one embodiment, the invention discloses an isolated human amniotic fluid derived MSC prepared according to a method comprising the steps of: (a) isolating MSCs from a sample of human amniotic fluid in the absence of non-human animal derived products; and (b) expanding the isolated human amniotic fluid derived MSCs in the absence of non-human animal derived products. The isolated human amniotic fluid derived MSC can be cryopreserved by methods known to one of ordinary skill in the art, or is further expanded to obtain an increase number of the MSCs and then cryopreserved thereafter. In another embodiment, the cryopreserved MSCs can be thawed and used for tissue engineering, tissue repair and/or wound healing, or expanded further prior to use in tissue engineering, tissue repair and/or wound healing.
[0053] In one embodiment, the invention provides a method of proliferating a composition enriched in human amniotic fluid derived MSCs comprising the steps of: (a) selecting at least one single MSC from a sample of human amniotic fluid in the absence of non- human animal derived products; (b) introducing at least one single MSC to a culture medium containing no non-human animal derived product; and (c) proliferating at least one single MSC
to a culture medium containing no non-human animal derived product. The proliferation of human amniotic fluid derived MSC served to increase the number of such pluoripotent cells for tissue engineering, tissue repair, wound healing, as well as for cryopreserving the MSCs to provide a permanent source of such cells.
[0054] In another embodiment, the invention provides a method of storing a composition enriched in human amniotic fluid derived MSCs comprising the steps of: (a) obtaining a composition enriched in human amniotic fluid derived MSCs according to the method described herein; and (b) cryopreserving the composition enriched in human amniotic fluid derived MSCs. The cryopreservation of a composition enriched in human amniotic fluid derived MSCs functions to provide a permanent source of such cells. When such cells are needed, an aliquot of the frozen cell in storage can be thawed for use in tissue engineering, tissue repair, and wound healing.
[0055] In yet another embodiment, the invention provides a method of storing human amniotic fluid derived MSCs comprising harvesting MSCs from a sample of human amniotic fluid and cryopreserving the MSCs such that the cells remain viable upon thawing. The use of cyropreservatives such as DMSO at 10% final concentration and a 1-3 °C/min, slow and gradual reduction in the temperature of the MSCs to -1960C help ensure that the MSCs are not damaged during the cryopreservation process and such cells can remain viable upon thawing.
[0056] Encompassed in the invention is a kit for obtaining a composition enriched in human amniotic derived MSCs from human amniotic fluid comprising some of the components, but not limited to: (a) a container for the collection of human amniotic fluid; (b) a coated container for the isolation and primary expansion of human amniotic fluid derived MSCs; (c) human serum for the culture-expansion of human amniotic fluid derived MSCs; and (d) instructions for the isolation, identification, and expansion of human amniotic fluid derived MSCs. The container for the collection of human amniotic fluid should be sterile , preferably sealed, and have injection ports. The amniotic fluid that is collected within the amniocentesis syringe can be injected directly into the container via the injection port. In one embodiment, the container is also a centrifuge tube. Once the amniotic fluid is injected inside the container, the container can be centrifuged to pellet the cells in the amniotic fluid. The supernatant fluid can be aspirated and fresh media can be added to the tube to resuspend the cells in the pellet. The suspension of cells and media is poured under sterile conditions into a coated container provided in the kit for the isolation and primary expansion of human amniotic fluid derived MSCs. In one embodiment, the kit provides the reagents for the reconstitution of a culture media for the culture
expansion of the MSCs, reagents for the cryopreservation of the MSCs, and instructions for the isolation, identification, and expansion of human amniotic fluid derived MSCs. The kit comprises instructions for: (a) reconstituting a culture media; (b) harvesting the MSCs from a sample of amniotic fluid; (c) plating and counting of the harvested MSCs adhered on a coated container; (d) the expected morphology of the adherent MSCs; (e) the removal of non-adherent cells; (f) the confluency at which to passage the MSCs; (f) detaching, harvesting, and dividing the MSCs to several coated containers - this is known as passaging of cells; and (g) detaching, harvesting, and cryopreserving the MSCs. The reagents for the reconstitution of the culture media include but are not limited to stock solutions of antibiotics, antimycotics, glucose, buffered media such as Dulbeccovs Modified Eagle Medium, human serum, and growth factors such as basic fibroblast growth factor. The coated containers for the culture expansion of the MSCs can be coated with human collagen, fibronectin, laminin, poly-lysine and the likes used in coating culture plates.
[0057] Unless otherwise defined herein, scientific and technical terms used in connection with the present application shall have the meanings that are commonly understood by those of ordinary skill in the art. Further, unless otherwise required by context, singular terms shall include pluralities and plural terms shall include the singular.
[0058] It should be understood that embodiments of this invention are not limited to the particular methodology, protocols, and reagents, etc., described herein and as such can vary. The terminology used herein is for the purpose of describing particular embodiments only, and is not intended to limit the scope of the present invention, which is defined solely by the claims.
[0059] As used herein, the term " non-human animal products" refer to serum, plasma, growth factors and other cell culturing reagents that are used for the culturing and growth of the MSCs, that are derived from non-humans. For example, fetal calf serum, fetal bovine serum, mouse basic fibroblast growth factor and recombinant mouse basic fibroblast growth factor are considered non-human animal products.
[0060] As used herein, the term "autologous" refers to a situation in which the donor and recipient are the same person. Autologous engineered tissue used in a tissue repair of congenital anomalies are made with cells derived from the person with congenital anomalies.
[0061] As used herein, the term "mesenchymal stem cell" or abbreviated "MSC" refers to a generalized cell that has pluripotency (descendants can specialize into different cell types), for example, an undifferentiated MSC that is capable of differentiating into more than one
specific type of mesoderm-derived cells and regenerating into various tissues in vivo. Such cell also has unlimited proliferating and self-renewal capability and can differentiate into osteogenic, myogenic, adipogenic or chondrogenic, neurogenic, hepatogenic, nephrogenic, urogenic, isletogenic, pancreatogenic, gastroenterogenic, epitheliogenic, thyroidogenic, myocardiogenic, pneumogenic, retino genie, gametogenic, endotheliogenic, or hematopoietic lineages. The enriched population of human amniotic fluid derived MSCs provided herein can positively express the cell surface markers CD73 (SH3), CD105 (SH2), CD44, CD29, CD 90, CD13, CDlO, CD71, CD49d, CD49e, and/or HLA Class I (A, B, and C). Additionally, the enriched population of MSCs provided herein is negative for the cell surface markers CD8, CD14, CD19, CD31, CD34, CD45, CD56, CD133, and/or HLA-DR (Pitting et. al., 1999, Science 284:143- 147; Kaviani et. al., 2001, J. Pediatr. Surg. 36: 1662-5 ; Kunisaki et. al., 2007, J. Pediatr. Surg. 42(6):974-9).
[0062] As used herein, the term "culture" refers to the in vitro maintenance of cells.
Generally, the cells are cultured in culture medium, which is a nutrient-rich buffered aqueous solution capable of sustaining cell growth. Culture media suitable for isolating and expanding human fetal MSCs from amniotic fluid according to the practice described herein include but are not limited to high glucose Dulbecco's Modified Eagles Medium with L-Glutamine. The media can be supplemented with recombinant human basic fibroblast growth factor (rhbFGF) and contain sera, such as human serum, and antibiotics (Table 1). Table 1 provides manufacturers information of the key ingredients in a typical culture media used for isolating and expanding MSCs. Cell cultures are maintained in a CO2 atmosphere, e.g., 5% to 12%, to maintain pH of the culture fluid, and incubated at 370C in a humid atmosphere. Suitable chemically defined serum free media are described in U.S. Ser. No. 08/464,599 and WO96/39487, and "complete media" are described in U.S. Pat. No. 5,486,359 and these are hereby incorporated by reference. Chemically defined medium comprises a minimum essential medium such as Iscove's Modified Dulbecco's Medium (IMDM) (Gibco), supplemented with human serum albumin, human Ex Cyte lipoprotein, transferrin, insulin, vitamins, essential and non essential amino acids, sodium pyruvate, glutamine and a mitogen. These media stimulate MSCs growth without differentiation. As used herein, a mitogen refers to an agent that stimulate cell division of a cell. An agent can be a chemical, usually some form of a protein, that encourages a cell to commence cell division, triggering mitosis.
[0063] As used herein, the term "non-human animal derived products" refers to products that are not derived directly from human sources or expressed from a human gene. For example,
fetal bovine serum is derived directly from bovine (e. g. domestic cattle, Bison, Water Buffalo, the Yak, and the four-horned and spiral-horned antelopes) and is therefore a non-human animal derived product. Human autologous serum and pooled allogenic human AB serum are human animal derived products. Recombinant human basic fibroblast growth factor (rhbFGF) is encoded by a human gene and is also considered a human animal derived product.
[0064] The term "isolated" as used herein signifies that the cells are placed into conditions other than their natural environment. The term "isolated" does not preclude the later use of these cells thereafter in combinations or mixtures with other cells.
[0065] As used herein, the term "expanding" refers to increasing the number of like cells through cell division (mitosis). The term "proliferating" and "expanding" are used interchangeably.
[0066] As used herein, the term "storing" refers to the cryopreservation of MSCs such that the MSCs are viable and can undergo mitosis and cell differentiation after thaw. The time frame of storing the MSCs are in the range of one months to years.
Amniotic Fluid Collection
[0067] Amniocentesis, or an amniotic fluid test (AFT), is a medical procedure used for prenatal diagnosis of a fetus, in which a small amount of amniotic fluid is extracted from the amniotic cavity around a developing fetus. Amniocentesis can be done as soon as there is enough amniotic fluid surrounding the fetus that a sample can be removed safely. Early amniocentesis can be performed as early as 5 weeks of gestation. Standard amniocentesis is usually performed between 15 and 20 weeks gestation. Amniotic fluid is available for collection from 5 weeks right up to term and birth of the baby, that is from 5,...10,... 14, ...20, ...25...30...31, 32, 33, 34, 35, ...38 weeks of gestation including any time period in between 5 and 38 weeks of gestation. In addition, amniotic fluid can also be collected during birth. A device described in US Patent No. 4031897 can be used to collect the amniotic fluid when a pregnant mother's water breaks. The collection device is worn unobtrusively and with comfort by a woman in the last stages of pregnancy, which is positioned to receive and retain the amniotic fluids when the fluid is released.
[0068] A diagnostic amniocentesis is routinely performed whenever a fetal abnormality, such as congenital diaphragmatic hernia (CDH), is detected by prenatal ultrasound imaging. Amniocentesis is performed by a skilled physician specialized in that area, and amniotic fluid is
obtained using a long syringe, guided by ultrasound. The syringe is usually inserted into the mother's abdominal wall or at the end of the vagina, and through the uterus wall. The physician would aim for an area of the amniotic sac that is away from the fetus so to avoid stabbing it. A small amount of amniotic fluid then gets sucked out and the syringe is withdrawn. The puncture wound should close up by itself, and the amniotic sac should then automatically replenish the liquid over a day or so.
[0069] An amniotic fluid sample can be obtained during such diagnostic amniocentesis for the practice of the invention described herein without any no additional morbidity to the mother. No modification of the standard procedure, which is performed with sterile technique under ultrasound guidance, is necessary. Collections should be made under sterile conditions. Generally, the first 2 ml of the collected amniotic fluid is discarded and 15-30 ml of amniotic fluid is collected with a sterile syringe. The amniotic fluid is then placed in a sterile plastic 15 ml or 50 ml tube. At this point, the amniotic fluid can be supplemented with antibiotics penicillin and streptomycin. An aliquot, 1/100 volume of the collected amniotic fluid, of a stock penicillin (5000 U/ml) /streptomycin (500mg/ml) can be added to the collected amniotic fluid.
[0070] In one embodiment, a collection kit comprising a wide-mouth, graduated seal sterile collection container with antibiotics, with ports for the injection of the collected amniotic fluid and an identification label which identifies the mother/fetus source of the amniotic fluid and the time of collection is provided.
[0071] In the event of multiple pregnancies, additional procedural considerations can be followed. Fetuses that have their own amniotic sac will have their amniotic fluid contained in the sac. Amniocentesis can be performed for each amniotic sac with aid of sonogram guiding the needle. Since fetuses move around during gestation, it may not be possible to accurately identify which amniotic fluid derived MSCs are autologous to which infant in multiple pregnancies. Therefore it is recommended that come time in the future when the expanded MSCs are needed for use in the presumptive autologous infant, some identity testing can be performed prior to implantation of the MSCs or MSCs derived tissue in that infant. When fetuses share an amniotic sac, the amniotic fluid collected will be duly noted on the identification label. Since fetuses that shared an amniotic fluid are genetically identical, having arose from a single developing embryo, the MSCs are autologous to both individuals that eventually develop from the two fetuses.
[0072] As little as 2 niL of amniotic fluid is sufficient for isolating MSCs that can be expanded to sufficient number of cells for cryopreservation and/or tissue engineering of tissues. However, the larger volume of amniotic fluid, the easier it is to isolate and expand the MSCs. Given the amount of amniotic fluid routinely obtained during amniocentesis in most cases, it is expected that 10-20 mL of amniotic fluid can be available for practice of the invention described herein. In a preferred embodiment, a sample of 5-10 ml of human amniotic fluid is used.
[0073] The sample of amniotic fluid collected is appropriately labeled and can be stored at 40C for up to 48 h. In a preferred embodiment, the samples are immediately transported to a Good Manufacturing Practice (GMP) facility for isolation, expansion, and/or cryopreservation. The sample should be processed within 48 h and preferably within 24 h of harvest.
[0074] In one embodiment, the cells in the collected amniotic fluid are isolated by centrifugation and cryopreserved immediately. The sample of amniotic fluid is immediately centrifuged at room temperature at low centrifugal force of 400-1000 x g for 10-15 min, and the supernatant amniotic fluid is discarded. To the pellet of amniotic fluid cells, add Dulbecco's Modified Eagle Medium (DMEM), 10% human AB serum, and 10% DMSO. The cell culture media is not limited to DMEM. Other examples include RPMI 1640 and others described herein. Other examples of serum that can substitute for human AB serum include human autologous serum and platelet rich plasma supplemented with heparin (2U/ml). Other cryopreservatives are described herein. Alternatively, the pellet of amniotic fluid cells can be cryopreserved in 90% human serum or plasma or the likes and 10% DMSO. In another embodiment, the collected amniotic fluid is cryopreserved immediately. The sample of amniotic fluid is mixed with DMSO and human serum or plasma or the likes to achieve at least 10% serum and at least 10% DMSO. In another embodiment, the amniotic fluid can be cryopreserved with serum-free media such as ATHENAES™ cell culture media with at least 10% DMSO as a cryopreservative according to the methods known to one skilled in the art.
[0075] In one embodiment, the amniotic fluid derived MSCs are allocated to at least four cryogenic vials such as CRYULES® (Wheaton, Inc.), two of which are assigned for storage to one freezer and another two cryules to another independently-serviced freezer. A fifth cryules contains cells set aside for testing of identify, viability, and function, when the withdrawal of cells from cryopreservation is required for tissue repair, wound healing, and/or cell expansion for tissue engineering.
[0076] In one embodiment, recordation of data of the amniotic fluid collected can be performed to ensure accurate identification and evaluation of the collected amniotic fluid. The preferred recorded data should include: collection number, name of mother, gender of fetus, date of collection, time of gestation (weeks of pregnancy), processing prior to freezing, freezing date, number of cryules, freezer positions, obstetrical data: reason for amniocentesis, congenital birth defects, and health of mother; test results of amniotic fluid sample and cells.
[0077] In one embodiment, the small portion of the harvested amniotic fluid derived
MSCs can be used for inspection and testing. For example, routine testing for bacterial contamination, diagnostic screening for pathogenic microorganisms such as the human immunodeficiency virus (HIV), and confirmation of the fetal origin of the cells can be performed.
Isolation of mesenchymal stem cells from human amniotic fluid
[0078] The detailed procedure for the isolation of human amniotic fluid derived MSCs is described in Current Protocols in Stem Cell Biology 2007 (Mick Bhatia, et. al., ed., John Wiley and Sons, Inc.) and is hereby incorporated by reference. In accordance with the present invention, all non-human animal derived products are replaced with suitable human product substitute. For example, the tissue culture plates can be coated with, but not limited to human recombinant collagen (BD Biosciences), human fibronectin, laminin, proteoglycans or poly-D- lysine; the FBS or FCS can be replaced with human autologous serum, pooled allogenic human AB serum, or platelet rich plasma supplemented with heparin (2U/ml); the basic fibroblast growth factor (bFGF) can be replaced with recombinant human basic fibroblast growth factor (rhubFGF).
[0079] Several media are particularly well suited to the desired selective attachment of
MSCs and are referred to herein as "complete media" when supplemented with serum as described below. One such medium is an augmented version of Dulbecco's Modified Eagle's Medium (DMEM), which is well known and readily commercially available. The type of culture medium is not specifically limited in the present invention to DMEM. Other examples include RPMI 1640, Iscove's modified Dubelcco's media (IMDM), and Opti-MEM SFM (Invitrogen Inc.). Chemically Defined Medium comprises a minimum essential medium such as Iscove's Modified Dulbecco's Medium (IMDM) (Gibco), supplemented with human serum albumin, human Ex Cyte lipoprotein, transferrin, insulin, vitamins, essential and non essential amino acids, sodium pyruvate, glutamine and a mitogen is also suitable. In one embodiment, serum
free media such as those described in U.S. Ser. No. 08/464,599 and WO96/39487, and the "complete media" as described in U.S. Pat. No. 5,486,359 are contemplated for use with the methods described herein.
[0080] The formulation of standard DMEM/F-12 is as follows: calcium chloride 2H2O
0.154 g/L, cupric sulfate H2O 0.0000013 g/L, ferric nitrate 9H2O 0.00005 g/L, ferrous sulfate 7H2O 0.000417 g/L, magnesium chloride 6H2O 0.0612 g/L, magnesium sulfate 0.04884 g/L, potassium chloride 0.3118 g/L, sodium chloride 6.996 g/L, sodium phosphate dibasic 0.07102 g/L, sodium phosphate monobasic 0.0543 g/L, zinc sulfate 7H2O 0.000432 g/L, amino Acids: L-alanine 0.00445 g/L, L-arginine HCl 0.1475 g/L, L-asparagine H2O 0.0075 g/L, L-aspartic acid 0.00665 g/L L-cystine 2HCl 0.03129 g/L, L-cysteine HCl H2O 0.01756 g/L, L-glutamic acid 0.00735 g/L, L-glutamine 0.365 g/L, glycine 0.01875 g/L, L-histidine HCl H2O 0.03148 g/L, L-isoleucine 0.05447 g/L, L-leucine 0.05905 g/L, L-lysine HCl 0.09125 L-methionine 0.01724 L-phenylalanine 0.03548 L-proline 0.01725 L-serine 0.02625 L-threonine 0.05345 g/L, L-tryptophan 0.00902 g/L, L-tyrosine 2Na 2H2O 0.05579 g/L, L-valine 0.05285 g/L; vitamins: biotin 0.0000035 g/L, choline chloride 0.00898 g/L, folic acid 0.00266 g/L, i-inositol 0.0126 g/L, nicotinamide 0.00202 g/L, D-pantothenic acid Ca 0.00224 g/L, pyridoxine HCl 0.000031 g/L, pyridoxal hydrochloride 0.002 g/L, riboflavin 0.000219 g/L, thiamine HCl 0.00217 g/L, vitamin B12 0.00068 g/L; other: D-glucose 3.15 g/L, hypoxanthine (Na) 0.0021 g/L, linoleic acid 0.000042 g/L, lipoicacid 0.000105 g/L, phenol red 0.0081 g/L, putrescine HCl 0.000081 g/L, pyruvic acid, sodium salt 0.055 g/L, thymidine 0.000365 g/L.
[0081] The commercial formulation is supplemented with 3700 mg/1 of sodium bicarbonate and 10 ml/1 of a IOOX (100 times concentrated) antibiotic-antimycotic cocktail containing 10,000 units of penicillin, 10,000 μg of streptomycin, and 25 μg of amphotericin B/ml utilizing penicillin G (sodium salt), streptomycin sulfate, and amphotericin B (FUNGIZONE ™) in 0.85% saline.
[0082] In one embodiment, the culture-expansion media does not contain any human autologous serum, human AB serum or platelet rich plasma supplemented with heparin (2U/ml). In accordance with the present invention, cells under such culture condition will grow and multiply but at a slower rate than in the presence of human serum or plasma.
[0083] Generally, an adherence/non- adherence separation protocol is followed when isolating amniotic fluid derived MSCs. The adhesion/non-adhesion is determined visually under a microscope after a period of culture of the MSCs The amniotic fluid of 5-10 ml, obtained by
amniocentesis, is spun down at 400-500 x g for 10-15 min at room temperature. The cell pellet is collected and suspended in growth culture media consisting of supplemented DMEM, 20% pooled allogenic human AB serum, gentamicin and 5 ng/ml of rhbFGF. Cells are plated into a single 6-well plate coated with human collagen (Fibrogen, Inc. catalog No.:FG-5016) and incubated at 370C with 5% humidified CO2. Resuspended cells derived from 5-10 ml of amniotic fluid are evenly divided among the 6 wells. The wells in the plate are monitored under a light microscope for cell adherence to the plate at least once a day. Adherent cells exhibit a spread out cytoplasm and take on the classic elongated spindle shape of a MSC. Upon swirling of the media in the wells, these cells are not detached from their position of attachment in the culture well. Non-adherent cells do not exhibit a spread out cytoplasm and the cells remain spherical in shape. Such non-adherent cells move with the flow of the culture media in the dish. The MSCs are characterized by their adherent properties on coated tissue culture plates. In one embodiment, adherent MSCs can be observed after 24 -48 hrs. In another embodiment, adherent MSCs are noted only after 1 week in culture. The culture plate can be maintained for up to two weeks to allow MSCs to adhere, after which non-adherent cells are removed and fresh growth media can be added. Subsequently, the media is replaced every 3-5 days until the adherent MSCs have divided and 70-80% confluence is reached. It takes about 3 weeks or more to reach 70-80% confluence depending on the amount of MSCs used in the initial plating of the wells.
[0084] At this point, the cells on each well are observed morphologically for the classic spindle-like shaped cells of MSCs and for the absence of contamination. Signs of contamination include but are limited to cloudy media, presence of filamentous-like fungi, and bacteria growth. Culture wells with the majority of cells having the spindle-like shaped cells and no signs of contamination are selected. At 70-80% confluent, there should be at least 95% of spindle-like shaped cells. Cells from the selected plate are then detached with a trypsin-like solution (TrypLE, Invitrogen, Inc.) for 3-5 minutes at 370C, washed and plated in fresh growth media in a flask. At this point, the MSCs can be cryopreserved or they can be further culture-expanded prior to cryopreservation.
Expansion of isolated mesenchymal stem cells from human amniotic fluid
[0085] The detailed procedure for the expansion of the isolation of human amniotic fluid derived MSCs is described in Current Protocols in Stem Cell Biology 2007 (Mick Bhatia, et. al., ed., John Wiley and Sons, Inc.) and is hereby incorporated by reference. In accordance with the present invention, the FBS is replaced with human autologous serum, pooled allogenic human
AB serum, or platelet rich plasma supplemented with heparin (2U/ml). Basic fibroblast growth factor (bFGF) can be replaced with recombinant human basic fibroblast growth factor (rhbFGF).
[0086] To expand the cells, successive passages are performed with the same protocol as previously described for the isolation of MSCs except only 10 % serum or supplemented platelet rich plasma can be used for expansion. In one embodiment, the media are changed every 3 days till the cells reach 80-90% confluence before the cells are passaged at a ratio of 1:2-10, including all the ratios in between 1:2 and 1:10. The expanded MSCs (250-600 x 106 cells) are characterized by flow cytometric analysis of specific surface antigens (cell surface markers) followed by a 14-day sterility and cell viability tests. The expanded MSCs can then be cryopreserved in Plasmalyte-A, Human Serum Albumin and 10% DMSO or by other cryopreservation method as described herein.
[0087] Typically, the phenotypical identity of these MSCs are determined through multicolor flow cytometry. The composition of enriched human amniotic fluid derived MSCs positively expresses CD73, CD105, CD44, CD29, CD90, CD13, CDlO, CD71, CD49d, CD49e, and HLA Class I (A, B, and C) and are negative for CD45, CD34, CD14, CD19, CD8, CD56, CD31, CD133, and HLA-DR. In a preferred embodiment, the composition of enriched human amniotic fluid derived MSCs is at least 90%, at least 80%, at least 70%, at least 60%, at least 50% and all the percentages in between 50%-90%, positive for the following cell surface antigens: CD29, CD73 and CD44. In another preferred embodiment, the composition of enriched MSCs is at least 60%, at least 50%, at least 40%, at least 30% at least 20% and all the percentages in between 20%-60%, positive for CD90 and CD105. In yet another preferred embodiment, the composition of enriched human amniotic fluid derived MSCs is no more than 5% positive for CD45 and CD34.
[0088] After the birth of the child or when needed, some of the cryopreserved MSCs can be thawed and plated in growth media, in a flask or a dish containing a culture media as described above. The MSCs can be expanded to obtain at least 6 x 108 cells in 1-2 passages. The MSCs are then ready for pre-surgical implantation preparation such as tissue differentiation and/or tissue engineering.
Sterility Testing
[0089] Prior to cryopreservation, a 14-day sterility testing of an isolated human amniotic fluid derived MSCs culture can be performed in accordance with criteria standard to GMP for
sterility testing of pharmaceutical products and should be in compliance with the federal guidelines for the final product testing. The MSC cultures can be prepared using Millipore's Steritest Filtration System and are incubated in appropriate media for 14 days. The validation of the system, procedural controls, test organisms, and products demonstrated that the Millipore Steritest system is a valid system for the isolation of microorganism contamination of cellular products and/or supplies as low as 10 CFU/ml for test organisms used. For cellular products cultured in the presence of antibiotics such as Gentamicin, Millipore TTHVAB210 canisters were used for the test samples. The canisters contain a low absorption Durapore membrane filter (0.45 μm) that is efficient in rinsing away any residual antimicrobial agents from test sample.
[0090] One canister of each set is filled with fluid thioglycolate medium (FTM); the other is filled with soy casein media (SCM). FTM media and test samples were incubated at 30- 35° C for 14 days. The SCM canisters are incubated at room temperature for the same period. The canisters are examined for turbidity and evidence of growth on the third, fourth, or fifth day, and on the seventh and fourteenth day of testing. Turbidity is equivalent to identification of positive cultures. All positive cultures are to be sterilized and discarded.
[0091] In one embodiment, the following tests on an amniotic fluid derived MSCs can also be performed:
(i) Bacterial culture: To ensure the absence of microbial contamination, established assays known in the art can be performed, such as routine hospital cultures for bacteria under aerobic and anaerobic conditions.
(ii) Diagnostic screening for pathogenic microorganisms: To ensure the absence of specific pathogenic microorganisms, various diagnostic tests can be employed. Diagnostic screening for any of the numerous pathogens transmissible through bodily fluids can be done by standard procedures that are known in the art. As one example, the collected amniotic fluid sample can be subjected to diagnostic screening for the presence of the Human Immunodeficiency Virus (HIV), the causative agent of Acquired Immune Deficiency Syndrome (AIDS) (Gallo et al., 1984, Science 224:500-503; Barre-Sinoussi, F., et al., 1983, Science 220:868; Levy, J. A., et al., 1984, Science 225:840). Any of numerous assay systems that are known in the art can be used, for example, based on the detection of virions, viral-encoded proteins, HIV-specific nucleic acids, or antibodies to HIV proteins.
(iii) Confirmation of neonatal origin of the MSCs: Contamination with maternal blood and maternal cells, not necessarily a contraindication to storage and clinical utility, can be suspected from the obstetrical history. Presence of maternal cells, and of adult blood generally, can be revealed by various tests, including but not limited to I typing (Wiener, A. S., et al., 1965, Am. J. Phys. Anthropol. 23(4): 389-396); analysis on a Coulter Channelyzer, which detects size differences between neonatal and maternal blood cells (Daffos, F., et al., 1985, Am. J. Obstet. Gynecol. 153:655-60); staining procedures for hemoglobin such as the Kleinhauer-Betke technique (Betke, K., 1968, Bibl. Haematologica 29:1085) and others (Clay-ton, E. M., et al., 1970, Obstetrics and Gynecology 35(4):642-645), which detect differences in the types of hemoglobin contained in red blood cells before birth versus in later life. Other methods of distinguishing fetal cells from maternal cells are described in US Pat.No. 5,858,649 and US Pat. Publication No. 20060105353 and these are hereby incorporated by reference.
Endotoxin Assay
[0092] Endotoxin levels can be determined by the gel-clot limulus amebocyte lysate
(LAL) test method in compliance with the US Food and Drug Administration's GMP regulations, 21 CFR § 211. Acceptable endotoxin level is 5.0 EU/ml.
Mycoplasma Assay
[0093] An aliquot of the cells will be taken prior to cryopreservation for mycoplasma
PCR testing. The Mycoplasma PCR testing will be performed at a GMP approved facility using MycoSensor™ QPCR Assay Kit (Manufactured by Stratagene).
Characterization of Mesenchymal Stem Cells
[0094] The Mesenchymal Stem Cell Characterization Kit (Millipore cat. no. SCROl 8) provides researchers with a convenient means to phenotype MSCs using a panel of antibodies. This kit contains reagents to the MSC markers: integrin Bl, CD54, collagen type I and fibronectin, and to the negative markers CD45 and CD14. Also included are mouse and rabbit immunoglobulins for the assessment of background staining.
[0095] MSCs from various species were originally isolated from bone marrow by their ability to adhere to the surface of the culture vessel (Reyes, 2001, Blood, 98:2615-25; Pittenger, 1999, Science, 284:143-7; Marin, 2002, Exp. Hematol., 30:879-86; Kadiyala, 1997, Cell
Transplant, 6:125-34; Johnstone, 1998, Exp. Cell. Res., 238:265-72; Wakitani, 1995, Muscle Nerve, 18:1417-26; Berry, 1992, J. Cell. ScL, 101:333-42; Mosca, 2000, Clin. Orthop. Relat. Res. Oct;(379 Suppl):S71-90). In addition to this methodology, techniques employing panels of monoclonal antibodies have been successfully utilized to define and purify MSC. It is now possible to distinguish and define MSC through the presence of specific cell surface MSC markers and the absence of specific cell surface hematopoietic stem cell markers.
[0096] Using monoclonal antibodies to define the expression pattern of cell surface antigens, a number of phenotypes for cultured human MSCs have been reported. These include CD73+, Stro-1+, CD105+, CD34-, CD45- and CD144- (TuIi, R., et. at., 2003, Stem Cells, 21:681-93); CD34-, CD44(low), CD45-, CD117-, HLA-I- and HLA-DR- (Reyes, M., et. al., 2001, Blood, 98:2615-25; Reyes, M. and Verfaillie CM. Ann N Y Acad Sci. 2001 938:231-3) and SH2+; SH3+, CD29+, CD44+, CD71+, CD90+, CD106+, CD120a+, CD124+, CD14-, CD34- and CD45-1 (Pittenger, MF, et. al., 1999, Science, 284:143-7).
[0097] Jones, EA et. al., (2002, Arthritis and Rhematism, 46:3349-60) described using a two-stage process, involving magnetic separation and multiparameter flow cytometry, where MSCs were isolated directly from human bone marrow. The MSCs were first purified from bone marrow samples using D7-microbeads, then separated by flow cytometry using a monoclonal antibody to CD45. Sorted cells were uniformly positive for CD105, LNGFR, CDlO, CD13, CD90, Stro-1 and Bone Morphogenic Receptor Type IA [BMPRIA], and negative for CD 14, CD34, CDl 17 and CD133. Furthermore, only cells of this phenotype could proliferate and produce adherent monolayers capable of chondrogenic, osteogenic and adipogenic differentiation. This reference is hereby incorporated by reference.
[0098] Similarly, monoclonal antibodies have also been used to characterize murine
MSCs of various phenotypes. These include Sca-1+, CD29+, CD44+, c-Kit+, CD105+, CD45-, CD31+, CD34+ (Sun, S. et. al., 2003, Stem Cells, 21:527-35.) and Sca-1+, CD29+, CD44+, CD81+, CD106+, Nucleostemin+ and CDl 16-, CD34-, CD45-, CD48-, CDl 17- and CD135- (Baddoo, M., et. al., 2003, J. Cell. Biochem. 15;89:1235-49). While the phenotype of murine MSCs within bone marrow remains unknown, cultured MSCs were phenotypically characterized as being negative for CD34, CD44, CD45, c-Kit, MHC-I and MHC-II. Conversely, these MSCs express low levels of FIk-I, Sca-1 and Thy-1, and higher levels of CD 13 and stage specific embryonic antigen-1 (SSEA-I).
Crvopreservation of Cells
[0099] As used herein, "cryopreservation" refers to the preservation of cells by cooling to low sub-zero temperatures, such as (typically) 77 K or -196 0C (the boiling point of liquid nitrogen). Cryopreservation also refers to storing the cells at a temperature between 0-100C in the absence of any cryopreservative agents. At these low temperatures, any biological activity, including the biochemical reactions that would lead to cell death, is effectively stopped. Cryoprotective agents are often used at sub-zero temperatures to preserved the cells from damaged due to freezing at low temperatures or warming to room temperature.
[0100] In one embodiment, the invention provides a cryopreserved pharmaceutical composition comprising: (a) a viable composition enriched in human amniotic fluid derived MSCs obtained according to the method described herein in the absence of non-human animal derived products, in which the MSCs are present in an amount sufficient to effect tissue engineering, tissue repair, or wound healing; (b) an amount of cryopreservative sufficient for the cryopreservation of MSCs; and (c) a pharmaceutically acceptable carrier.
[0101] Freezing is destructive to most living cells. Upon cooling, as the external medium freezes, cells equilibrate by losing water, thus increasing intracellular solute concentration. Below about 10°-15° C, intracellular freezing will occur. Both intracellular freezing and solution effects are responsible for cell injury (Mazur, P., 1970, Science 168:939-949). It has been proposed that freezing destruction from extracellular ice is essentially a plasma membrane injury resulting from osmotic dehydration of the cell (Meryman, H. T., et al., 1977, Cryobiology 14:287-302).
[0102] Cryoprotective agents and optimal cooling rates can protect against cell injury.
Cryoprotection by solute addition is thought to occur by two potential mechanisms: colligatively, by penetration into the cell, reducing the amount of ice formed; or kinetically, by decreasing the rate of water flow out of the cell in response to a decreased vapor pressure of external ice (Meryman, H. T., et al., 1977, Cryobiology 14:287-302). Different optimal cooling rates have been described for different cells. Various groups have looked at the effect of cooling velocity or cryopreservatives upon the survival or transplantation efficiency of frozen bone marrow cells or red blood cells (Lovelock, J. E. and Bishop, M. W. H., 1959, Nature 183:1394- 1395; Ashwood-Smith, M. J., 1961, Nature 190:1204-1205; RoWe, A. W. and Rinfret, A. P., 1962, Blood 20:636; Rowe, A. W. and Fellig, J., 1962, Fed. Proc. 21:157; Rowe, A. W., 1966, Cryobiology 3(1):12-18; Lewis, J. P., et al., 1967, Transfusion 7(l):17-32; Rapatz, G., et al., 1968, Cryobiology 5(l):18-25; Mazur, P., 1970, Science 168:939-949; Mazur, P., 1977,
Cryobiology 14:251-272; Rowe, A. W. and Lenny, L. L., 1983, Cryobiology 20:717; Stiff, P. J., et al., 1983, Cryobiology 20:17-24; Gorin, N. C, 1986, Clinics in Haematology 15(1): 19-48).
[0103] The successful recovery of human bone marrow cells after long-term storage in liquid nitrogen has been described (1983, American Type Culture Collection, Quarterly Newsletter 3(4): 1). In addition, stem cells in bone marrow were shown capable of withstanding cryopreservation and thawing without significant cell death, as demonstrated by the ability to form equal numbers of mixed myeloid-erythroid colonies in vitro both before and after freezing (Fabian, L, et al., 1982, Exp. Hematol 10:119-122). The cryopreservation and thawing of human fetal liver cells (Zuckerman, A. J., et al., 1968, J. Clin. Pathol. (London) 21(1): 109-110), fetal myocardial cells (Robinson, D. M. and Simpson, J. F., 1971, In Vitro 6(5):378), neonatal rat heart cells (Alink, G. M., et al., 1976, Cryobiology 13:295-304), and fetal rat pancreases (Kemp, J. A., et al., 1978, Transplantation 26(4):260-264) have also been reported.
[0104] The injurious effects associated with freezing can be circumvented by (a) use of a cryoprotective agent, (b) control of the freezing rate, and (c) storage at a temperature sufficiently low to minimize degradative reactions.
[0105] Cryoprotective agents which can be used include but are not limited to dimethyl sulfoxide (DMSO) (Lovelock, J. E. and Bishop, M.W.H., 1959, Nature 183:1394-1395; Ashwood-Smith, M. J., 1961, Nature 190:1204-1205), glycerol, polyvinylpyrrolidine (Rinfret, A. P., 1960, Ann. N.Y. Acad. Sci. 85:576), polyethylene glycol (Sloviter, H. A. and Ravdin, R. G., 1962, Nature 196:548), albumin, dextran, sucrose, ethylene glycol, i-erythritol, D-Sorbitol, D-mannitol (Rowe, A. W., et al., 1962, Fed. Proc. 21:157), D-sorbitol, i-inositol, D-lactose, choline chloride (Bender, M. A., et al., 1960, J. Appl. Physiol. 15:520), amino acids (Phan The Tran and Bender, M. A., 1960, Exp. Cell Res. 20:651), methanol, acetamide, glycerol monoacetate (Lovelock, J. E., 1954, Biochem. J. 56:265), and inorganic salts (Phan The Tran and Bender, M. A., 1960, Proc. Soc. Exp. Biol. Med. 104:388; Phan The Tran and Bender, M. A., 1961, in Radiobiology, Proceedings of the Third Australian Conference on Radiobiology, Ilbery, P. L. T., ed., Butterworth, London, p. 59). In a preferred embodiment, DMSO is used, a liquid which is non-toxic to cells in low concentration. Being a small molecule, DMSO freely permeates the cell and protects intracellular organelles by combining with water to modify its freezability and prevent damage from ice formation. Addition of plasma (e.g., to a concentration of 20-25%) can augment the protective effect of DMSO. After addition of DMSO, cells should be kept at 0-40C until freezing, since DMSO concentrations of about 1% are toxic at temperatures above 40C.
[0106] A controlled slow cooling rate is critical. Different cryoprotective agents (Rapatz,
G., et al., 1968, Cryobiology 5(l):18-25) and different cell types have different optimal cooling rates (see e.g., Rowe, A. W. and Rinfret, A. P., 1962, Blood 20:636; Rowe, A. W., 1966, Cryobiology 3(1):12-18; Lewis, J. P., et al., 1967, Transfusion 7(l):17-32; and Mazur, P., 1970, Science 168:939-949 for effects of cooling velocity on survival of marrow-stem cells and on their transplantation potential). The heat of fusion phase where water turns to ice should be minimal. The cooling procedure can be carried out by use of, e.g., a programmable freezing device or a methanol bath procedure.
[0107] Programmable freezing apparatuses allow determination of optimal cooling rates and facilitate standard reproducible cooling. Programmable controlled-rate freezers such as Cryomed or Planar permit tuning of the freezing regimen to the desired cooling rate curve. For example, for marrow cells in 10% DMSO and 20% plasma, the optimal rate is 1 to 3°C/minute from 0° C to -8O0C. In one embodiment, this cooling rate can be used for the amniotic fluid derived MSCs of the invention described herein. The container holding the cells must be stable at cryogenic temperatures and allow for rapid heat transfer for effective control of both freezing and thawing. Sealed plastic vials (e.g., Nunc, Wheaton CRYULES®) or glass ampules can be used for multiple small amounts (1-2 ml), while larger volumes (100-200 ml) can be frozen in polyolefin bags (e.g., Delmed) held between metal plates for better heat transfer during cooling. (Bags of bone marrow cells have been successfully frozen by placing them in -80° C freezers which, fortuitously, gives a cooling rate of approximately 3°C/minute).
[0108] In an alternative embodiment, the methanol bath method of cooling can be used.
The methanol bath method is well-suited to routine cryopreservation of multiple small items on a large scale. The method does not require manual control of the freezing rate nor a recorder to monitor the rate. In a preferred aspect, DMSO-treated cells are pre-cooled on ice and transferred to a tray containing chilled methanol which is placed, in turn, in a mechanical refrigerator (e.g., Harris or Revco) at -80° C Thermocouple measurements of the methanol bath and the samples indicate the desired cooling rate of 1° to 3°C/minute. After at least two hours, the specimens have reached a temperature of -8O0C and can be placed directly into liquid nitrogen (-196° C) for permanent storage.
[0109] After thorough freezing, cells can be rapidly transferred to a long-term cryogenic storage vessel. In a preferred embodiment, MSC samples can be cryogenically stored in liquid nitrogen (-1960C) or its vapor (-165° C). Such storage is greatly facilitated by the availability of highly efficient liquid nitrogen refrigerators, which resemble large Thermos containers with an
extremely low vacuum and internal super insulation, such that heat leakage and nitrogen losses are kept to an absolute minimum
[0110] In a particular embodiment, the cryopreservation procedure described in Current
Protocols in Stem Cell Biology, 2007, (Mick Bhatia, et. al., ed., John Wiley and Sons, Inc.) is used in the methods described herein and is hereby incorporated by reference. Mainly when the MSCs on a 10-cm tissue culture plate have reached approximately 50% confluency, the media within the plate is aspirated and the MSC s are rinsed with phosphate buffered saline. The adherent MSC are then detached by 3 ml of 0.025% trypsin/0.04%EDTA treatment. The trypsin/EDTA is neutralized by 7 ml of media and the detached MSC are collected by centrifugation at 200 x g for 2 min. The supernatant is aspirated off and the pellet of MSCs is resuspended in 1.5 ml of media. A aliquot of 1 ml of 100% DMSO is added to the suspension of MSCs and gently mixed. Then 1 ml aliquots of this suspension of MSCs in DMSO is dispensed into cyrules in preparation for cryopreservation. The sterilized storage cryules preferably have their caps threaded inside, allowing easy handling without contamination. Suitable racking systems are commercially available and can be used for cataloguing, storage, and retrieval of individual specimens.
[0111] Considerations and procedures for the manipulation, cryopreservation, and long- term storage of hematopoietic stem cells, particularly from bone marrow or peripheral blood, are also applicable to the neonatal and fetal MSCs of the invention. Such a discussion can be found, for example, in the following references, incorporated by reference herein: Gorin, N. C, 1986, Clinics In Haematology 15(1): 19-48; Bone-Marrow Conservation, Culture and Transplantation, Proceedings of a Panel, Moscow, July 22-26, 1968, International Atomic Energy Agency, Vienna, pp. 107-186.
[0112] Other methods of cryopreservation of viable cells, or modifications thereof, are available and envisioned for use (e.g., cold metal-mirror techniques; Livesey, S. A. and Linner, J. G., 1987, Nature 327:255; Linner, J. G., et al., 1986, J. Histochem. Cytochem. 34(9): 1123- 1135; U.S. Pat. No. 4,199,022, 3,753,357, 4,559,298 and are incorporated hereby reference.
Recovering mesenchymal stem cells from the frozen state
[0113] Frozen MSCs are preferably thawed quickly (e.g., in a water bath maintained at
37°-41°C) and chilled on ice immediately upon thawing. In particular, the cryogenic vial containing the frozen MSCs can be immersed up to its neck in a warm water bath; gentle rotation will ensure mixing of the cell suspension as it thaws and increase heat transfer from the
warm water to the internal ice mass. As soon as the ice has completely melted, the vial can be immediately placed in ice.
[0114] In a particular embodiment, the thawing procedure after cryopreservation is described in Current Protocols in Stem Cell Biology 2007 (Mick Bhatia, et. al., ed., John Wiley and Sons, Inc.) and is hereby incorporated by reference. Immediately after removing the cryogenic vial from the cryo-freezer, the vial is rolled between the hands for 10 to 30 sec until the outside of the vial is frost free. The vial is then held upright in a 370C water-bath until the contents are visibly thawed. The vial is immersed in 95% ethanol or sprayed with 70% ethanol to kill microorganisms from the water-bath and air dry in a sterile hood. The contents of the vial is then transferred to a 10-cm sterile culture containing 9 ml of media using sterile techniques. The MSCs can then be cultured and further expanded in a incubator at 370C with 5% humidified CO2.
[0115] In some embodiments, the MSCs are treat in order to prevent cellular clumping upon thawing. To prevent clumping, various procedures can be used, including but not limited to, the addition before and/or after freezing of DNase (Spitzer, G., et al., 1980, Cancer 45:3075- 3085), low molecular weight dextran and citrate, hydroxyethyl starch (Stiff, PJ. , et al., 1983, Cryobiology 20:17-24).
[0116] The cryoprotective agent, if toxic in humans, should be removed prior to therapeutic use of the thawed MSCs. In an embodiment employing DMSO as the cryopreservative, it is preferable to omit this step in order to avoid cell loss, since DMSO has no serious toxicity. However, where removal of the cryoprotective agent is desired, the removal is preferably accomplished upon thawing.
[0117] One way in which to remove the cryoprotective agent is by dilution to an insignificant concentration. This can be accomplished by addition of medium, followed by, if necessary, one or more cycles of centrifugation to pellet the cells, removal of the supernatant, and resuspension of the cells. For example, the intracellular DMSO in the thawed cells can be reduced to a level (less than 1%) that will not adversely affect the recovered cells. This is preferably done slowly to minimize potentially damaging osmotic gradients that occur during DMSO removal.
[0118] After removal of the cryoprotective agent, cell count (e.g., by use of a hemocytometer) and viability testing (e.g., by trypan blue exclusion; Kuchler, R. J. 1977,
Biochemical Methods in Cell Culture and Virology, Dowden, Hutchinson & Ross, Stroudsburg,
Pa., pp. 18-19; 1964, Methods in Medical Research, Eisen, H. N., et al., eds., Vol. 10, Year Book Medical Publishers, Inc., Chicago, pp. 39-47) can be done to confirm cell survival.
[0119] Other procedures which can be used, relating to processing of the thawed cells, include enrichment for adherent MSCs and expansion by in vitro culture as described supra.
[0120] In a preferred, but not required, aspect of the invention, thawed cells are tested by standard assays of viability (e.g., trypan blue exclusion) and of microbial sterility as described herein, and tested to confirm and/or determine their identity relative to the recipient.
[0121] Methods for identity testing which can be used include but are not limited to
HLA typing (Bodmer, W., 1973, in Manual of Tissue Typing Techniques, Ray, J. G., et al., eds., DHEW Publication No. (NIH) 74-545, pp. 24-27), and DNA fingerprinting, which can be used to establish the genetic identity of the cells. DNA fingerprinting (Jeffreys, A. J., et al., 1985, Nature 314:67-73) exploits the extensive restriction fragment length polymorphism associated with hypervariable minisatellite regions of human DNA, to enable identification of the origin of a DNA sample, specific to each individual (Jeffreys, A. J., et al., 1985, Nature 316:76; Gill, P., et al., 1985, Nature 318:577; Vassart, G., et al., 1987, Science 235:683), and is thus preferred for use.
[0122] In a specific embodiment of the invention in which the MSCs recovered for tissue engineering, tissue repair and/or wound healing are to be used in an autologous system, the MSCs should match exactly the recipient patient from whom the MSCs are originally derived. In another embodiment, the MSCs are not used in an autologous system but are HLA typed match to the recipient. For example, the HLA type matched for HLA-A, B, C, and D.
Differentiation of human amniotic fluid derived MSCs
[0123] Encompassed in the invention is a method for producing differentiated human amniotic fluid derived MSC preparations comprising: (a) obtaining a composition enriched in human amniotic fluid derived MSCs according to the methods described herein in the absences of non-human animal derived products; and (b) culturing the composition enriched in human amniotic fluid derived MSCs in a culture medium containing differentiation factors for a time period sufficient for MSCs to differentiate and express specific tissue markers. The differentiation factors are selected from a group consisting of osteogenic, myogenic, adipogenic, chondrogenic, neurogenic, hepatogenic, nephrogenic, urogenic, isletogenic, pancreatogenic, gastroenterogenic, epitheliogenic, thyroidogenic, myocardiogenic, pneumogenic, retinogenic,
gametogenic, endotheliogenic, or hematopoietic factors. The MSCs can be seeded on a scaffold in the culture conditions described herein.
[0124] After the composition enriched in human amniotic fluid derived MSCs has been thawed, it can be further expanded to provide sufficient cell number and density necessary for differentiation, tissue engineering, tissue repair and wound healing. A typical seeding density for tissue engineering is at least 1 x 10 cells/cm . In some embodiments, seeding densities can be a range from 5 x 103 cells/cm2 to 3 x 106 cells/cm2. In one preferred embodiment, the seeding density for a solid scaffold is 2 x 106 cells/cm2. In another preferred embodiment, the seeding density for a gel-like scaffold (e. g. a hydrogel) is 2.5 x 106 cells/ml. Many factors including but are not limited to the types of scaffold and availability of MSCs determine the seeding density. One of ordinary skill in the art would be able to determine the seeding densities for the various types of scaffold used.
[0125] The composition enriched in MSCs can be differentiated into several cell lineages including osteogenic, myogenic, adipogenic, chondrogenic, neurogenic, hepatogenic, nephrogenic, urogenic, isletogenic, pancreatogenic, gastroenterogenic, epitheliogenic, thyroidogenic, myocardiogenic, pneumogenic, retinogenic, gametogenic, endotheliogenic, or hematopoietic lineages. In one embodiment, the composition of MSCs can be used therapeutically in their undifferentiated state, such as in tissue engineering, wound healing and tissue repair.
[0126] Differentiation and cell culture conditions necessary to initiate the various differentiation are described in detailed in the osteogenesis differentiation protocol by Millipore which is to be used in conjunction with Millipore's Mesenchymal Stem Cell Osteogenesis Kit; in M. Ahmad, et al., Biomaterials, 1999, 20: 211-20; MJ. Coelho and M.H. Fernandes, Biomaterials, 2000, 21: 1095-102; Paolo De Coppi, et. al., 2007, Nature Biotechnology, 25, 100- 106; B. Johnstone, et al., Exp Cell Res, 1998, 238: 265-72; A.M. Mackay, et al., Tissue Eng, 1998, 4: 415-28; M.E. Nuttall, et al., J Bone Miner Res, (1998), 13: 371-82; P.A. Conget and JJ. Minguell, J Cell Physiol, (1999) 181: 67-73; in Current Protocols in Stem Cell Biology (Mick Bhatia, et. al., ed., John Wiley and Sons, Inc.); Bjδrklund & Lindvall, Nat Neurosci, 3: 537 (2000); Bjδrklund & Lindvall, Nature, 405: 892 (2000); Cameron et al., J Neurobiol, 36: 287 (1998); McKay, Nature, 406: 361 (2000); Wachs et al., Lab Invest. 83: 949 (2003); Heng, BC, et. al., J. Gastro. and Hepatology, (2005) 20: 975-987; Nat R., et. al., 2007, Glia, 55: 385-99; Schulz TC, et. al., Stem Cells (2004) 22:1218-38; Kim D and Dressier GR., J Am Soc Nephrol. (2005) 16:3527-34; Insa S Schroeder, et. al., Nature Protocols 1:495-507 (2006); Alejandro
Soto-Gutierrez, Nature Protocols, (2007) 2: in press; Jiang W, et. al., 2007, Cell Res.(2007) 17: 333-44; Rivas-Carrillo JD, et. al., 2007, Curr Med Chem. (2007) 14: 1573-8; Invernici G., et. al., Exp Cell Res. 2007, Milne HM, et. al., Biochem Biophys Res Commun. (2005) 328: 399-403; Bruce SJ, et. al, (2007) Differentiation, 75: 337-49; Oottamasathien S., et. al., (2007), Dev. Biol. 304: 556-66; Wang D., et. al., Proc Natl Acad Sci U S A. (2007) 104: 4449-54; McCloskey KE, et. al, Methods MoI Biol. (2006), 330: 287-301; Kang SM, et. al, (2007), Stem Cells, 25: 419- 24; Arufe MC, et. al, 1: Endocrinology (2006) 147: 3007-15; Van Vranken BE, et. al., Tissue Eng. (2005)11: 1177-87; Glaser T, et. al, PLoS ONE, (2007) 2:e298; Kolambkar YM, et. al., (2007), J MoI Histol. 2007 Aug 1, in press, in the Current Protocols of Stem Cell Biology, (Mick Bhatia, et. al., ed., John Wiley and Sons, Inc.) and methods described herein. These references are hereby incorporated by reference.
[0127] Differentiation assays and kits that are commercially available include the
Mesenchymal Stem Cell Adipogenesis Kit (Millipore cat. no.SCR020), Pancreatic Islet Cell Characterization Kit (Millipore cat. no. SCR045), Pancreatic Cell Development Pathway Kit (Millipore cat. no. SCR046), Pancreatic Cell DTZ Detection Assay, (Millipore cat. no. SCR047), Mesenchymal Stem Cell Osteogenesis Kit (Millipore cat. no.SCR028) and the Mesenchymal Stem Cell Osteogenesis Kit (Millipore cat. no. SCR028).
[0128] The Mesenchymal Stem Cell Osteogenesis Kit (Millipore cat. no. SCR028) provides a method for differentiating mesenchymal stem cells to an osteoblast phenotype. The kit contains two ECM coating molecules (collagen type I and vitronectin), which have been shown to promote osteogenic differentiation of mesenchymal stem cells (Salasznyk, 2004, J. Biomed. Biotechnol., 2004(l):24-34), and the inducing reagents, dexamethasone, ascorbic acid 2-phosphate and β-glycerophosphate. Also included is Alizarin Red Solution, a staining solution that is used to detect the presence of calcium in bone.
[0129] The Mesenchymal Stem Cell Adipogenesis Kit (Millipore cat. no. SCR020) contains reagents that readily differentiate mesenchymal stem cells to an adipogenic lineage as assessed with Oil Red O staining of lipid vacuoles in mature adipocytes. These factors include dexamethasone, IBMX, insulin and indomethacin. Along with Oil Red O staining solution, a hematoxylin solution is provided to counterstain the cell nucleus. Using this kit, typically it is possible to obtain > 30% mature adipocytes from the rat bone marrow derived mesenchymal stem cells.
[0130] Pancreatic Islet Cell Characterization Kit (Millipore cat. no. SCR045) provides a convenient set of validated antibodies that allows researchers to reliably identify mature pancreatic islets cells. Along with antibodies generated against discrete hormones secreted by alpha, beta, delta and gamma cells of the pancreatic islets, the kit includes PDX-I (pancreatic duodenal homeobox gene-1), a master regulator of islet cell development and GLUT-2, a glucose transporter present in beta-islet cells.
[0131] Pancreatic Cell Development Pathway Kit (Millipore cat. no. SCR046) provides a collection of antibodies that are unique to key transition points along the developmental pathway of pancreatic cells. Included in the kit are antibodies to critical transcription factors expressed during the program of development along with two antibodies to hormones secreted by mature islets cells (FoxA2, Hes-1, Pax 6, IDX-I, Glucagon and Pancreatic Polypeptide).
[0132] Pancreatic Cell DTZ Detection Assay Kit (Millipore cat. no. SCR047) provides a simple and quick method to identify insulin-producing beta cells from a mixed cell culture preparation or from pancreatic tissues, by detecting high levels of zinc (typically contained in pancreatic beta cells), with the use of a zinc-chelating agent, DTZ. This kit contains DTZ staining and rinse solutions along with filters and syringes required for live staining reactions.
Uses of human amniotic fluid derived MSCs
[0133] Encompassed in the invention are the uses of a composition enriched in human amniotic fluid derived MSCs for tissue engineering, tissue repair, regenerative medicine and wound healing in humans. Tissue engineering is the use of a combination of cells, engineering and material methods, and suitable biochemical and physiochemical factors to improve or replace biological functions. Tissue engineering aims at developing functional cell, tissue, and organ substitutes to repair, replace or enhance biological function that has been lost due to congenital abnormalities, injury, disease, or aging, or repair fascia in hernias. The tissue that is engineered is used to repair or replace portions of or whole tissues (i.e., bone, cartilage, blood vessels, bladder, etc.). Often, the tissues involved require certain mechanical and structural properties for proper function. Tissue engineering also encompass the efforts to perform specific biochemical functions using cells within an artificially-created support system (e.g. an artificial pancreas, or a bioartificial liver). The term regenerative medicine is often used synonymously with tissue engineering, although those involved in regenerative medicine place more emphasis on the use of stem cells to produce tissues. Tissue regeneration aims to restore and repair tissue function via the interplay of living cells, an extra-cellular matrix and cell communicators.
[0134] As used herein, the terms "tissue regeneration", "tissue engineering" and
"regenerative medicine" are related terms and used interchangeably.
[0135] Current traditional approaches to treat medical diseases, congenital abnormalities and injury include: drugs, hormones, enzymes, vaccines, prosthetic substitution, surgical reconstruction, and organ transplantation. These methods are all considered essential, but have their limitations. For example, drugs have unwanted side effects, prosthetics are not biologically active and do not integrate or remodel into the body, surgery is invasive, and organ transplantation is limited by donor availability and toxic immunosuppressive cocktails.
[0136] Contrary to traditional approaches, tissue regeneration is an approach in modern medicine that delivers living tissue or cells and stimulates the body's own natural healing process by activating the body's inherent ability to repair and regenerate. Innovative tissue regeneration therapies are now available that aim to heal or reconstruct diseased tissue and support the regeneration of diseased or injured organs. Doctors use tissue regeneration to speed up healing and to help injuries that will not heal or repair on their own. Tissue regeneration can help heal broken bones, severe burns, chronic wounds, heart damage, nerve damage, and many other diseases.
[0137] As used herein, the word "repair", means the natural replacement of worn, torn or broken components with newly synthesized components. The word "healing", as used herein, means the returning of torn and broken organs and tissues (wounds) to wholeness. For example, an open wound on the skin can be repaired with the composition of enriched MSCs. It is envisioned healing would be the eventual closing of the open wound with new growth of skin and underlying connective tissues.
[0138] The composition enriched in human amniotic fluid derived MSCs forms the engineering biomaterial needed for tissue repair and tissue engineering. The MSCs or the replacement or repair tissue engineered with MSCs is either grown in a patient or outside the patient and then later transplanted into the patient. The expanded pluripotent MSCs can be directly implanted to the site needing repair, for example, the heart after suffering a myocardial infarction (Dinender K. Singla, et. al., Am J Physiol Heart Circ Physiol 293: H1308-H1314, 2007). The MSCs can be injected into the tissue repair site together with growth factors and differentiation factors that are known in the art to stimulated cell growth and differentiation of the MSC into the appropriate cell type of the recipient tissue. Suitable growth factors include but are not limited to TGFβ, platelet derived growth factor (PDGF), epidermal growth factor (EGF),
bone morpho genie protein (BMP) and fibroblast growth factor (FGF). Other examples are described in Dijke et al., "Growth Factors for Wound Healing", Bio/Technology, 7:793-798 (1989).
[0139] The compositions of MSCs of the invention can be implantation for the repair of cardiac muscles, blood vessels, kidney, liver, cartilage, bones, brain the pancreas and the connective and support tissues such as ligaments, muscles, tendons and those tissues, such as the collagen-containing tissues which encapsulate organs, to name a few. Methods of direct implantation of stem cells for tissue repair are described in Shake JG et, al. 2002 (Ann Thorac Surg. 73:1919-25), Yoshinori Miyaharal, et. al., 2006 (Nature Medicine 12, 459-465), Atta Behfar, et. al., 2005 (Ann. N.Y. Acad. Sci. 1049: 189-198), Luciano C. Amado, et. al., 2005, (PNAS, 102: 11474-9), Khalil PN, et. al., 2007, (Gastroenterology. 132:944-54), Lee RH, et. al.,
2006 (Proc Natl Acad Sci U S A.;103:17438-43), and Chamberlain J., et. al., 2007, (Hepatology.
2007 Aug 17, in press), S. P. Bruder, et. al., 1998, (J. Bone and Joint Surgery 80:985-96), Pignataro G., et. al., J. Cereb Blood Flow Metab. 2007, 27:919-27 and are hereby incorporated by reference.
[0140] Alternatively, the MSC can be 'seeded' into an artificial structure capable of supporting three-dimensional tissue formation. These structures, typically called scaffolds, are often critical, both ex vivo as well as in vivo, to recapitulating the in vivo milieu and allowing cells to influence their own microenvironments. Scaffold- guided tissue engineering involves seeding highly porous biodegradable scaffolds with MSCs and/or growth factors, then culturing and implanting the scaffolds to induce and direct the growth of new tissue. The goal is for the MSCs to attach to the scaffold, then replicate, differentiate, and organize into normal healthy tissue as the scaffold degrades. This method has been used to create various tissue analogs including skin, cartilage, bone, liver, nerve, vessels, to name a few examples.
[0141] Scaffolds usually serve at least one of the following purposes: (1) allow stem cell attachment and migration; (2) deliver and retain cells and biochemical factors; (3) enable diffusion of vital cell nutrients and expressed products; and (4) exert certain mechanical and biological influences to modify the behavior of the cell phase.
[0142] To achieve the goal of tissue reconstruction, scaffolds must meet some specific requirements. A high porosity and an adequate pore size are necessary to facilitate cell seeding and diffusion throughout the whole structure of both cells and nutrients. Although not absolutely essential, biodegradability is often a desirable factor since scaffolds should preferably be
absorbed by the surrounding tissues without the necessity of a surgical removal. The rate at which degradation occurs has to coincide as much as possible with the rate of tissue formation: this means that while cells are fabricating their own natural matrix structure around themselves, the scaffold is able to provide structural integrity within the body and eventually it will break down leaving the neotissue, newly formed tissue which will take over the mechanical load. Injectability is also important for certain clinical uses.
[0143] Many different materials (natural and synthetic, biodegradable and permanent) have been investigated. Most of these materials have been known in the medical field before the advent of tissue engineering as a research topic, being already employed as bioresorbable sutures. Examples of these materials are collagen or some linear aliphatic polyesters.
[0144] Newer biomaterials have been engineered to have ideal properties and functional customization: injectability, synthetic manufacture, biocompatibility, non-immunogenicity, transparency, nano-scale fibers, low concentration, resorption rates, etc. PuraMatrix.
[0145] A commonly used synthetic material is PLA - polylactic acid. This is a polyester which degrades within the human body to form lactic acid, a naturally occurring chemical which is easily removed from the body. Similar materials are polyglycolic acid (PGA) and polycaprolactone (PCL): their degradation mechanism is similar to that of PLA, but they exhibit respectively a faster and a slower rate of degradation compared to PLA.
[0146] Scaffolds can also be constructed from natural materials: in particular different derivatives of the extracellular matrix have been studied to evaluate their ability to support cell growth. Protein based materials, such as collagen or fibrin, and polysaccharidic materials, like chitosan or glycosaminoglycans (GAGs), have all proved suitable in terms of cell compatibility, but some issues with potential immunogenicity still remains. Among GAGs hyaluronic acid, possibly in combination with cross linking agents (e.g. glutaraldehyde, water soluble carbodiimide, etc.), is one of the possible choices as scaffold material. Functionalized groups of scaffolds can be useful in the delivery of small molecules (drugs) to specific tissues.
[0147] A variety of scaffolds and uses thereof are described in US Pat.Nos. 6,103,255,
6,224,893, 6,228,117, 6,328,990, 6,376,742, 6,432,435, 6,514,515, 6,525,145, 6,541,023, 6,562,374, 6,656,489, 6,689,166, 6,696,575, 6,737,072, and 6,902,932, they are hereby incorporated by reference.
[0148] Examples of tissue that can be engineered, reconstructed and/or repaired include but are not limited to craniofacial structures such as bone, adipose tissue and facial muscles, cardiac muscle, cardiac valve, skin, bones, skeletal muscles, diaphragmatic muscles and tendons, breast tissue, blood vessels, cartilage, tendons, ligaments, bladder, urether, uterus, ureter, virgina, cervix, trachea, hair, cornea, esophagus and intestines. Fetal reconstructions of the tracheal and the diaphragm using tissue engineered autologous cartilage grafts and tendons respectively are fully described by Kunisaki et. al., 2005 and by Fuch et. al., 2004 and these are hereby incorporated by reference.
[0149] Craniofacial structures reconstruction is the regeneration or de novo formation of dental, oral, and craniofacial structures lost to congenital anomalies, trauma, and diseases. Virtually all craniofacial structures are derivatives of mesenchymal cells. Biological therapies utilize MSCs, delivered or internally recruited, to generate craniofacial structures in temporary scaffolding biomaterials. Several craniofacial structures — such as the mandibular condyle, calvarial bone, cranial suture, and subcutaneous adipose tissue — have been engineered from MSCs, (JJ. Mao, et. al., J Dent Res 85(ll):966-979, 2006) and is hereby incorporated by reference.
[0150] MSCs can also help cardiac tissue to repair itself weeks after a heart attack.
Embryonic stem cells have been shown to regenerate damaged heart muscle, when transplanted within a 3-dimensional scaffold into the infracted heart. The embryonic stem cells were more successful in restoring heart muscle when transplanted within a 3-dimensional matrix into damaged hearts in an animal model of severe infarction. Methods of constructing cardiac related structures are described in US Pat. Nos. 5,880,090, 5,899,937, 6,695,879, 6,666,886 , 7,214,371, and US Pat. Publication No. 20040044403 and they are hereby incorporated by reference.
[0151] The procedures for tissue engineering the various tissue types can be found in the methods described in the examples herein, in Koji Kojima, et. al., J. Thorac. Cardiovasc. Surg. 2002, 123:1177-1184, Duxbury MS, et. al., Transplantation, 2004 77:1162-6, US Pat. Nos. 5,700,289, 5,716,404, 6,123,727, 6,171,344, 6,503,273, 6,620,203, 6,666,886, 6,692,761, 6,656,489, 6,840,962, 6,737,053, 7,049,057, 7,049,139, 7,052,514, 7,052,518, 7,112,218, 7,179,287, 7,198,641 and they are hereby incorporated by reference.
[0152] Tissue regeneration is a multidisciplinary field involving biology, medicine and engineering. In addition to having a therapeutic application, where living tissue is provided to
treat disease, the products are also used for diagnostic applications where the tissue is made in vitro and used for testing drug metabolism, toxicity, and pathogenicity.
[0153] Various procedures are contemplated for transferring, immobilizing, and activating the culture-expanded, purified MSCs at the site for repair, implantation, etc., including injecting the cells at the site of a skeletal defect, incubating the cells with a prosthesis and implanting the prosthesis, etc. Thus, by isolating, purifying and greatly expanding the number of MSCs prior to differentiation and then actively controlling the differentiation process by virtue of their positioning at the site of tissue damage or by pretreating in vitro prior to their transplantation, the culture-expanded, undifferentiated MSC can be utilized for various therapeutic purposes such as to elucidate cellular, molecular, and genetic disorders in a wide number of metabolic bone diseases, skeletal dysplasias, cartilage defects, ligament and tendon injuries and other musculoskeletal and connective tissue disorders.
[0154] In one embodiment, the invention provides a method of promoting wound healing in a human in need thereof comprising administering a preparation comprising a composition enriched in human amniotic fluid derived MSCs according to the methods described herein in the absence of non-human animal products.
[0155] In another embodiment, the invention provides a method of promoting tissue repair in a human in need thereof comprising administering a preparation comprising a composition enriched in human amniotic fluid derived MSCs according to the methods described herein in the absence of non-human animal derived products.
[0156] In yet another embodiment, the invention provides a pharmaceutical composition comprising: (a) a viable composition enriched in human amniotic fluid derived mesenchymal stem or cells obtained according to the methods described herein in the absence of non-human animal products, in which the MSCs are present in an amount sufficient to effect tissue engineering, tissue repair, or wound healing; and (b) a pharmaceutically acceptable carrier.
[0157] The pharmaceutical composition of undifferentiated MSCs can be applied directly to wounds to stimulate wound healing. For example, pressure ulcers, leg ulcers, abrasions, lacerations, incisions, donor sites and second degree burns on infected wounds, surgical incisions and traumatic wounds. The undifferentiated MSCs can be mixed with growth factors for promoting growth at the site of the wound, and the mixture can be applied to the wound. The mixture can also be incorporated into a variety of wound dressing products such as wound dressing gauzes. The application of undifferentiated MSCs with or without growth
factors help promote healing in areas that can have a reduced capability of self-repair and renewal due to variety of medical conditions such as congestive heart failure, poor circulation, obesity, lymphatic obstructions and diabetes.
[0158] It is envisioned that the pharmaceutical composition is HLA-typed matched with the recipient prior to the implantation of the composition enriched in MSCs.
[0159] In one embodiment, the pharmaceutical composition of undifferentiated MSCs can be applied directly to a tissue needing repair to stimulate tissue repair. For example, after a heart attack or bone fracture. The undifferentiated MSCs can be mixed with growth factors for promoting growth at the site of the tissue needing repair, and the mixture can be applied to the tissue. Examples of tissue that can require repair include but are not limited to the skin, bone, ligaments, tendons, muscles, the heart, and cartilage.
[0160] In another embodiment, small grafts of tissues such as bone pieces are incubated with the pharmaceutical composition of undifferentiated MSCs for increasing the population of MSCs in the graft. The graft can then be implanted to the respective tissue for tissue repair. For example, in situations of massive facial trauma, the small fragments of facial bones can retrieved from the patient, cleaned and culture in vitro with the pharmaceutical composition of undifferentiated MSCs and then used in the facial reconstruction of the patient. In another embodiment, the use of the pharmaceutical composition of undifferentiated MSCs can be used in combination with tissue engineered structures composed of differentiated MSCs as well as inert implants such as Teflon.
[0161] The pharmaceutical composition of undifferentiated MSCs can be generally administered with a pharmaceutically acceptable carrier or vehicle therefore. A pharmaceutically acceptable carrier is one that does not cause an adverse physical reaction upon administration and one in which maintains the viability of the MSC for delivery into the patient or use in tissue engineering. In one embodiment, the pharmaceutically acceptable carriers that are inherently nontoxic and non-therapeutic. Examples of such carriers include ion exchangers, alumina, aluminum stearate, lecithin, serum proteins, such as human serum albumin, buffer substances such as phosphates, glycine, sorbic acid, potassium sorbate, partial glyceride mixtures of saturated vegetable fatty acids, water, salts, or electrolytes such as protamine sulfate, disodium hydrogen phosphate, potassium hydrogen phosphate, sodium chloride, zinc salts, colloidal silica, magnesium trisilicate, polyvinyl pyrrolidone, cellulose-based substances, and polyethylene glycol.
[0162] In one embodiment, the pharmaceutical composition is sterile, is at a physiological pH of between 6-8, and is isotonic to human bodily fluid.
[0163] In one embodiment, other ingredients can be added to the pharmaceutical composition, including antioxidants, e.g., ascorbic acid; low molecular weight (less than about ten residues) polypeptides, e.g., polyarginine or tripeptides; proteins, such as serum albumin, gelatin, or immunoglobulins; hydrophilic polymers such as polyvinylpyrrolidone; amino acids, such as glycine, glutamic acid, aspartic acid, or arginine; monosaccharides, disaccharides, and other carbohydrates including cellulose or its derivatives, glucose, mannose, or dextrins; chelating agents such as EDTA; and sugar alcohols such as mannitol or sorbitol.
[0164] The therapeutically effective amount and method of adminstering the pharmaceutical composition of undifferentiated MSCs can vary based on the individual patient, the indication being treated and other criteria evident to one of ordinary skill in the art. A therapeutically effective amount of pharmaceutical composition of undifferentiated MSCs is one sufficient to induce repair and/or heal a target organ or tissue. The route(s) of administration useful in a particular application are apparent to one of ordinary skill in the art.
[0165] Routes of administration include, but are not limited to, topical, transdermal, and parenteral. Topical and transdermal administration is accomplished via a wound dressing impregnated with the MSCs allowing the MSCs to enter the wound and also enter the blood stream. Parenteral routes of administration include but are not limited to direct injection such as intravenous, intramuscular, intraperitoneal intracoronary and subcutaneous injection. Parenteral administration can be accomplished using a needle and syringe, using a high pressure, needle free technique, like POWDERJECT™, or constant infusion pump.
[0166] The present invention can also be defined as in any of the following alphabetized paragraphs:
[A] A method of obtaining a composition enriched in human amniotic fluid derived mesenchymal stem cells comprising the steps of:
a. isolating mesenchymal stem cells from a sample of human amniotic fluid in the absence of non-human animal derived products; and
b. expanding said isolated human amniotic fluid derived mesenchymal stem cells in the absence of non-human animal derived products.
[B] The method of paragraph [A], wherein said human amniotic fluid is cryopreserved.
[C] The method of paragraph [A], wherein said human amniotic fluid is collected between 5 weeks of gestation to human term.
[D] The method of paragraph [A], wherein said human amniotic fluid is collected at birth.
[E] The method of paragraph [A], wherein the isolation and expansion of human amniotic fluid derived mesenchymal stem cells is in the presence of human serum.
[F] The method of paragraph [A], further comprising cryopreserving the composition enriched in human amniotic fluid derived mesenchymal stem cells.
[G] The method of paragraph [A], wherein the composition enriched in human amniotic fluid derived mesenchymal stem cells are at least 90% CD29, CD73, and CD44 positive.
[H] The method of paragraph [A], wherein the composition enriched in human amniotic fluid derived mesenchymal stem cells are at most 5% CD34 and CD45 positive.
[I] A method of proliferating human amniotic fluid derived mesenchymal stem cells comprising the steps of:
a. selecting at least one single mesenchymal stem cell from a sample of human amniotic fluid in the absence of non-human animal derived products;
b. introducing said at least one single mesenchymal stem cell to a culture medium containing no non-human animal derived product; and
c. proliferating said at least one single mesenchymal stem cell to a culture medium containing no non-human animal derived product.
[J] A method of storing a composition enriched in human amniotic fluid derived mesenchymal stem cells comprising the steps of:
a. obtaining a composition enriched in human amniotic fluid derived mesenchymal stem cells according to the method of any one of the paragraphs
[A]-[I]; and
b. cryopreserving said composition enriched in human amniotic fluid derived mesenchymal stem cells.
[K] An isolated human amniotic fluid derived mesenchymal stem cell prepared according to the method of any one of the paragraphs [A]-[J].
[L] A kit for obtaining a composition enriched in human amniotic derived mesenchymal stem cells from human amniotic fluid comprising:
a. a container for the collection of human amniotic fluid;
b. a container coated with extracellular matrix protein for the isolation and primary expansion of human amniotic fluid derived mesenchymal stem cells;
c. human serum for the culture-expansion of human amniotic fluid derived mesenchymal stem cells; and
d. instructions for the isolation, identification, and expansion of human amniotic fluid derived mesenchymal stem cells.
[M] The kit of paragraph [L], further comprising reagents for reconstituting a culture medium containing human serum.
[N] A method for producing differentiated human amniotic fluid derived mesenchymal stem cell preparations comprising:
a. obtaining a composition enriched in human amniotic fluid derived mesenchymal stem cells according to the method of any one of the paragraphs [A]-[J]; and
b. culturing said composition enriched in human amniotic fluid derived mesenchymal stem cells in a culture medium containing differentiation factors for a period sufficient for said stem cells to differentiate and express specific tissue markers.
[O] The method of paragraph [N], wherein the differentiation factors are selected from a group consisting of osteogenic, myogenic, adipogenic, chondrogenic, neurogenic, hepatogenic, nephrogenic, urogenic, isletogenic, pancreatogenic, gastroenterogenic,
epitheliogenic, thyroidogenic, myocardiogenic, pneumogenic, retinogenic, gametogenic, endotheliogenic, or hematopoietic factors.
[P] The method of paragraph [N], wherein the stem cells are seeded on a scaffold.
[Q] A method of promoting wound healing or tissue repair in a human in need thereof comprising administering a preparation comprising a composition enriched in human amniotic fluid derived mesenchymal stem cells according to the method of any one of the paragraphs [A]-[J].
[R] The method of paragraph [Q], wherein the composition is applied directly to the wound or tissue.
[S] The method of paragraph [Q], wherein the composition is embedded in a wound dressing material or scaffold, said wound dressing material or scaffold is applied on to the wound.
[T] A cryopreserved pharmaceutical composition comprising:
a. a viable composition enriched in human amniotic fluid derived mesenchymal stem cells obtained according to the method of any one of the paragraphs [A]-[I], in which said cells are present in an amount sufficient to effect tissue engineering, tissue repair, or wound healing;
b. an amount of cryopreservative sufficient for cryopreservation of said cells; and
c. a pharmaceutically acceptable carrier.
[U] A pharmaceutical composition comprising:
a. a viable composition enriched in human amniotic fluid derived mesenchymal stem cells obtained according to the method of any one of the paragraphs [A]-[I], in which said cells are present in an amount sufficient to effect tissue engineering, tissue repair, or wound healing; and
b. a pharmaceutically acceptable carrier.
[0167] This invention is further illustrated by the following examples which should not be construed as limiting. The contents of all references cited throughout this application, as well as the figures and table are incorporated herein by reference.
[0168] It should be understood that this invention is not limited to the particular methodology, protocols, and reagents, etc., described herein and as such can vary. The terminology used herein is for the purpose of describing particular embodiments only, and is not intended to limit the scope of the present invention, which is defined solely by the claims.
[0169] Other than in the operating examples, or where otherwise indicated, all numbers expressing quantities of ingredients or reaction conditions used herein should be understood as modified in all instances by the term "about." The term "about" when used in connection with percentages can mean +1%.
EXAMPLES
EXAMPLE 1
A multi stage amniotic-derived mesenchymal stem cell manufacturing protocol.
[0170] Perinatal amniotic mesenchymal stem cell (MSC)-based tissue engineering has been validated experimentally. It represents an ideal avenue for generating autologous engineered tissues for tissue repair in the new born babies with congenital defects and anomalies. However, one of the biggest hurdle in this avenue is providing sufficient autologous cells for tissue engineering. Often, an ultrasonagram and diagnostic amniocentesis is conducted around 17 weeks of gestation. The period of 4-6-month interval between a diagnostic amniocentesis and birth can be utilized to make sufficient autologous cells and also prepare autologous engineered tissue for use in tissue repair and re- structuring immediately or shortly after birth. Implementation of a multi stage cell manufacturing process can facilitate the production of sufficient quantities of amniotic derived mesenchymal stem cells for autologous tissue engineering. The multi stage cell manufacturing process will include: isolation/primary expansion; cryopreservation; and thawing/secondary expansion. The feasibility of this multi stage cell manufacture protocol and cell yield of such a staged cell manufacturing process, within regulatory guidelines was determined.
Materials and Methods
[0171] The study was approved by Children's Hospital Boston's Institutional Review
Board, under protocol #S04-12-149. Human MSCs were isolated from diagnostic amniocentesis samples obtained at 17-24 weeks gestation (n=ll; volume=1.5-7mL). All cell processing was under FDA-accredited Good Manufacturing Practice (GMP), starting 0-3 days after procurement. An overview of the staged cell manufacturing process and release criteria can be found on Table 2 and Table 3.
[0172] The mesenchymal cell population was isolated from the amniotic samples based on methods as previously described [12-16]. Culture media included fetal bovine serum (FBS) and bFGF. Briefly, the sample was centrifuged at 400 x g for 15 minutes. The pellet was resuspended in growth medium (2mL medium per 1OmL amniotic fluid) consisting of high- glucose Dulbeco Modified Eagle Medium with L-glutamine (DMEM; Lonza, Walkersville, MD), 20 % fetal bovine serum (FBS; Hyclone, Logan, UT), Gentamicin (Lonza) and 5 ng/ml of basic Fibroblast Growth Factor (Promega, Madison, WI) and plated into 1 well of a BD Bioacoat™ Collagen I-coated 24 well plate (BD Biosciences, San Jose, CA) in a 5% carbon dioxide incubator at 370C. Non-adherent cells were removed 48 hours later and cultures were fed as needed until they reached 70-80% confluence. At harvest, cells were washed once with PBS (Invitrogen, Carlsbad, CA) and detached with a trypsin-like solution (TrypLE Express; Invitrogen) for 3-5 minutes at 370C. Cells were passaged in T75 flasks without counting using growth medium. Before being frozen down, cell viability was assessed by Trypan blue exclusion. Viable cells were characterized by flow cytometric analysis of specific surface antigens, 14-day sterility tests, mycoplasma QPCR, and endotoxin assays (details below).
[0173] Cryopreservation - Cells were then seeded at 3xlO3 cells per cm2 in 10 x T175 flasks and frozen in Plasmalyte-A (Baxter Healthcare, Charlotte, NC) containing 2.5% human serum albumin (Baxter) and 10% DMSO (Cryoserv; Edwards Lifesciences, Irvine, CA) using a control rate freezer (Cryo, Rockville, MD) and stored in the vapor phase of a liquid nitrogen tank. Cryopreservation was for 3-5 months. Ideally, there is >70% viability and > 6xlO8 cells after the secondary expansion.
[0174] Secondary Expansion - Frozen cells were thawed and diluted 10 times in the same growth medium as described for isolation and primary expansion and immediately plated at 3xlO3 cells per cm2. The medium was changed the following day to remove dead cells and residual DMSO. Secondary expansion was up to at least 6 x 108 cells, after which cell viability was assessed by Trypan blue exclusion and viable cells were again characterized by flow cytometry, 14-day sterility tests, mycoplasma QPCR, and endotoxin assays (details below).
[0175] Flow Cytometry - At both expansion stages, cells were stained following standard protocol with a panel of 15 antibodies: CD90 FITC, HLA ABC FITC, CD9 FITC (BD Biosciences), CD73 PE, CD106 PE, CD166 PE (BD Biosciences, San Jose, CA), CD45 PerCP- Cy5.5, HLA-DR PerCP, CDl 17 PerCP-Cy5.5 (BD Biosciences), CD34 PE-Cy7, CDlO PE-Cy7 (BD Bioscience), CD44 PE-Cy7 (eBioscience, San Diego, CA), CD29 APC, CD 13 APC (BD Biosciences) and CD105 APC (eBioscience). Nonspecific cell staining was excluded using mouse isotype immunoglobulin controls. The data was acquired using the 6-color BD FACSCanto system (BD Biosciences) and analyzed with FlowJo (Treestar Inc., Ashland, OR). Cell release criteria at the different phases included, as appropriate: >90% CD29+, CD73+, and CD44+; <5% CD34+ and CD45+.
[0176] Sterility Testing - Fourteen-day sterility cultures were performed in accordance with criteria set forth in the FDA's GMP regulations, 21 CFR § 610.12 for sterility testing of pharmaceutical products. This method is in compliance with federal guidelines for final product testing. The cultures were prepared using Millipore's Steritest Filtration System (Millipore, Billerica, MA) and were incubated in appropriate media as per the manufacturer' s instructions for 14 days. The validation of this system, procedural controls, test organisms, and products has demonstrated that the Millipore Steritest system is valid for the isolation of microorganism contamination of cellular products and/or supplies as low as 10 CFU/ml for test organisms used. For cellular products cultured in the presence of antibiotics such as Gentamicin, Millipore TTHVAB210 canisters (Millipore) were used for the test samples. These canisters contain a low absorption Durapore membrane filter (0.45 μm) that is efficient in rinsing away any residual antimicrobial agents from a test sample.
[0177] One canister of each set was filled with Fluid Thioglycolate Medium (FTM); the other was filled with Soy Casein Media (SCM). FTM media and test samples were incubated at 30-35° C for 14 days. The SCM canisters were incubated at room temperature for the same period. The canisters were examined for turbidity and evidence of growth on the third, fourth, or fifth day, and on the seventh and fourteenth day of testing. Turbidity is equivalent to identification of positive cultures. Positive cultures undergo hospital-based gram stain, organism identification and sensitivity testing.
[0178] Mycoplasma Assay - Mycoplasma PCR testing was performed using
MycoSensor™ QPCR Assay Kit (Stratagene, La Jolla, CA), per manufacturer's instructions. The should be negative mycoplasma QPCR.
[0179] Endotoxin Assay - Endotoxin levels were determined by the gel-clot limulus amebocyte lysate (LAL) test method in compliance with the FDA's GMP regulations, 21 CFR § 211. Acceptable release criteria are endotoxin level of is 5.0 EU/mL or less.
Results
[0180] Isolation and expansion of MSCs in sufficient numbers so as to meet the cell release criteria as described in Table 2 was achieved in 54.5% (6/11) of the samples. Both the size of the amniotic fluid sample and the time between procurement and initial processing seemed to have an impact on the feasibility of the cell manufacturing process. At least a 2mL sample was shown to be necessary and cell isolation could only be achieved within 48 hours of procurement.
[0181] As expected, MSCs were found to proliferate quite rapidly. Average cell yield during primary expansion was 223.2 ± 65.4xlO6 cells (44.6-fold expansion), plus a 14.7 x 106- cell backup, after 36.3 ± 7.8 days. Average cell viability post-thaw was 88%. At both stages in which immunophenotyping was performed, all viable cells expressed markers compatible with a multipotent mesenchymal progenitor lineage, including CD73 (SH3), CD105 (SH2), CD44, CD29, CD90, and CD13. As expected, these cells also stained positive for HLA-A,B,C but were negative for CD45 and CD34.
[0182] All samples that met the expansion numbers set forth in the cell release criteria passed the 14-day sterility tests and had negative mycoplasma QPCR and endotoxin assays at the end of both primary and secondary expansions.
[0183] Conclusions: Human amniotic derived MSC can be manufactured in large numbers from diagnostic amniocentesis, by accredited staged processing, under definite procurement guidelines. These data further support the viability of clinical trials of perinatal amniotic mesenchymal stem cell-based therapies.
EXAMPLE 2
Bone engineering from human amniotic mesenchymal cells: Possible application in the surgical treatment of congenital anomalies.
[0184] Purpose: The treatment of many congenital anomalies, including chest wall, craniofacial, vertebral, and limb malformations, can benefit from the ready availability of autologous engineered bone grafts at birth. This study sought to determine whether fetal
mesenchymal stem cells (MSCs) normally found in the amniotic fluid could be used to engineer three-dimensional (3D) bone constructs in vitro.
[0185] Methods: Human fetal MSCs were isolated from amniotic fluid samples procured at 29-36 weeks gestation (n=6). Their mesenchymal phenotype was confirmed by immunocytochemistry and 6-color flow cytometry. Expanded cells were plated in either monolayer or 3D culture in an osteogenic medium containing beta-glycerophosphate, ascorbic acid, and dexamethasone for 3-6 weeks. For 3D culture, cells were seeded at comparable densities in 6 different biodegradable scaffolds, namely demineralized bone matrix (DBM), unwoven polyglycolic acid, collagen sponge, hydroxyapatite-collagen amalgam, and hydroxyapatite-tricalcium phosphate composite (n=4 per scaffold). Differentiation into the osteogenic lineage was assessed by matrix- specific staining, immunohistochemistry, RT-PCR, and/or matrix-specific quantitative assays, as appropriate. Statistical analyses were by the unpaired t-test (P<0.05). Results: Osteogenic differentiation of the amniotic MSCs was seen in both monolayer and 3D culture at all time points, as demonstrated by von Kossa and Alizarin Red staining, as well as by the expression of bone- specific markers, including osteocalcin, collagen type I, and alkaline phosphatase. Bone formation was enhanced in 3D culture when compared to monolayer culture, with DBM showing significantly higher levels of alkaline phosphatase when compared to all other scaffolds. There were no differences in the extracellular matrix profile of the 3D constructs beyond the 4- week time point.
[0186] Conclusions: Human bone grafts can be successfully engineered from amniotic fluid-derived mesenchymal cells. Osteogenicity can be enhanced by the scaffold employed. The amniotic fluid is a practical and minimally invasive cell source for the engineering of bone grafts for use in pediatric surgical reconstruction.
EXAMPLE 3
Human fetal bone engineering with electrospun nanofibers and amniotic derived mesenchymal stem cells.
[0187] Background: A variety of porous scaffold materials have been used for tissue engineering, for example, collagen and fibrin, some linear aliphatic polyesters such as PLA - polylactic acid, polyglycolide acid (PGA) and and polycaprolactone (PCL), polysaccharidic materials, like chitosan or glycosaminoglycans (GAGs). For these scaffold materials, a number of different methods has been described in literature for preparing porous structures to be employed as tissue engineering scaffolds. Here the efficacy of three-dimensional nanofibrous
scaffolds for the engineering of bone constructs with amniotic mesenchymal stem cells (MSCs) was determined and the impact of cell density and time in culture on construct structure was examined.
[0188] Methods: Electrospun poly(L-lactic) acid nanofibrous scaffolds with 200-700 nm in fiber diameter (n=16) were seeded with expanded human MSCs and placed in an osteogenic medium containing beta-glycerophosphate, ascorbic acid, and dexamethasone. Cell seeding densities were 5x103, 2x104, 6x104, or 12x104 cells/cm2, equally distributed among the constructs. At each cell density, constructs were left in culture for 4, 8, 12, or 16 weeks, at which point they underwent multiple analyses. Osteogenic differentiation was assessed by Alizarin Red staining and quantitative alkaline phosphatase activity. Construct mineralization was assessed by spectrophotometric measurements of extracellular calcium levels. Statistical analysis was by ANOVA and Student's t test (p<0.05).
[0189] Results: Osteogenic differentiation of the MSCs was evident in all constructs.
Example of a nanofiber-engineered bone is shown in Figure 1. There was a statistically significant increase in construct mineralization over time at all cell seeding densities (Fig. 2), with no difference among the seeding densities at each time point (Fig. 3). Alkaline phosphatase activity was significantly higher at 12 weeks in the 2xlO4 and 6xlO4 cells/cm2 constructs, but no different across most other cell densities and time points (Fig. 4). Interestingly, at 16 weeks, all constructs reached equally low levels of alkaline phosphatase activity, regardless of cell seeding density, suggesting minimal ongoing remodeling.
[0190] Conclusions: Electrospun nanofibrous scaffolds are suitable for bone engineering with human amniotic mesenchymal stem cells. Construct mineralization is directly proportional to time in culture, but not to cell seeding density. Engineered nanobone remodeling stabilizes after 16 weeks in culture. Fetal bone engineered with amniotic mesenchymal stem cells and nanofibers can be useful for the treatment of congenital skeletal anomalies.
EXAMPLE 4
Human amniotic derived mesenchymal stems cells isolated and expanded with human serum.
[0191] This study was aimed at determining the response of human amniotic MSCs when cultured in the absence of non-human animal products and at comparing it with culture under standard FBS-based medium, as a pre-requisite for fulfilling the basic regulatory requirements needed for initiation of clinical trials of this therapeutic concept.
Materials and Methods
MSC isolation
[0192] This study was approved by the Institutional Review Board of Children' s
Hospital Boston under protocol #S04-12-149. Human amniotic fluid specimens (n=12) were obtained between 20 and 37 weeks gestation by amniocentesis or amnioreduction (5.5-500 mL per sample), in a sterile fashion, from fetuses with a normal karyotype. All samples were stored at 40C prior to further processing at a local, FDA- accredited Good Manufacturing Practice facility. The mesenchymal cell population was then isolated based on methods as previously described [12, 21]. Briefly, the amniotic fluid sample was equally divided into 2 tubes and centrifuged at 40Og for 15 minutes. The pellets were then resuspended in growth medium (2 mL medium per 10 mL initial amniotic fluid) containing either 20% defined FBS (Hyclone, Logan, UT), or 20% allogeneic pooled human AB serum (ABS; Cambrex BioScience, Walkersville, MD). The growth medium consisted of high-glucose Dulbecco's Modified Eagle Medium with L-glutamine, gentamycin (50 μg/ml) (all from Cambrex BioScience), and 5 ng/mL human recombinant basic fibroblast growth factor (Promega, Madison, WI, catalog # G5071). Cells were cultured in collagen type I-coated 6 well plates (Biocoat, BD Biosciences, San Jose, CA) and placed in a 5% CO2 incubator at 37° C for 7-14 days. After 2 days, the non-adherent cells were removed, and 2 ml of fresh media were added to each well.
MSC proliferation
[0193] The growth kinetics of the MSCs was studied for up to 50 days in vitro. Briefly, the adherent cells were detached using 0.05% trypsin/0.53 mM EDTA (Invitrogen Corp., Carlsbad, CA) when 80-90% confluent. Cells were counted and re-seeded in growth media containing 20% FBS or 20% ABS, typically 0.5xl06 cells in 25 ml in a T162 dish (Corning Inc. Life Sciences, Acton, MA). All subsequent passages were performed similarly. Cell expansion was assessed over time based on the relative days in culture, with day 0 defined as the first passage.
Flow cytometry
[0194] Fluorescence-activated cell sorting analysis was performed on 7 samples of human mesenchymal amniocytes grown in parallel with either FBS or ABS. Approximately 0.5- IxIO6 cells per staining were incubated with fluorescent-labeled mouse monoclonal antibodies for 30 minutes and then washed twice with PBS. The following antibodies were used: CD9,
CDlO, CD13, CD29, CD31/PECAM-1, CD44, CD45, CD49a, CD71, CD73 (SH3), CD90/Thy- 1, CD106, CDl 17, HLA-A,B,C (all from BD Biosciences), CD105 (SH2) (eBiosciences, San Diego, CA), and CD166/ ALCAM (Ancell Corp. Bayport, MN). Nonspecific cell staining was excluded using mouse isotype immunoglobulin controls. The data was acquired using the 6- color BD FACSCanto system (BD Biosciences) and analyzed with FlowJo (Treestar Inc., Ashland, OR).
Statistical analysis
[0195] Statistical analyses were performed by the 2-sided Wilcoxon Signed-Rank test and linear regression, as appropriate (P<0.05).
Results
[0196] Colonies composed of several spindle-shaped, fibroblastoid cells of equivalent size could be successfully isolated and cultured from all the 7 amniotic fluid samples that were processed within 48 hours of procurement. Amniotic fluid specimens processed after 48 hours did not reliably grow mesenchymal cell colonies in either medium. Viable populations of rapidly expanding cells were obtained from samples harvested at any gestational age (mean, 31.6 weeks; range, 20-37 weeks gestation) and from as little as 5.5 mL of amniotic fluid. There were no differences in cell morphology based on the type of culture medium used, at any passage (data not shown). Based on a single well containing cells harvested from approximately 10 mL of amniotic fluid, the time required until the first passage was on average 12.1 + 3.1 days.
[0197] Starting from the first passage, amniotic fluid-derived MSCs were found to proliferate rapidly, regardless of the growth medium employed. Overall, there was a greater than 9-fold logarithmic cell expansion in the time period studied (mean, 32.9 days), with no significant differences in the overall cell proliferation rates, based on serum type (P=O.94), or gestational age (P=O.14; Fig. 6). MSCs cultured in FBS appeared to have a smaller variation in growth kinetics compared to those grown in ABS, but this trend was not statistically significant in this initial series. There was no evidence of cell expansion arrest in cultures grown in either FBS or ABS, for up to 38 days after the first passage (a total of up to 50 days in culture).
[0198] At any passage, the isolated cells stained consistently positive for several cell surface markers, including CD73 (SH3), CD105 (SH2), CD 166, CD44, CD29, CD90, CD13, CDlO, and CD71, in a profile compatible with a mesenchymal identity. As expected, these cells also stained positive for HLA-A, B, and C but were negative for CD45, CD34, CD14, CD19,
CD8, CD56, and CD31. There were no differences in antigen expression based on gestational age or the type of culture medium used (Fig. 7).
[0199] The data collected shows that amniotic fluid-derived MSCs can be isolated relatively easily. These cells proliferate quickly under standard culture conditions. The commercially available serum derived from allogeneic, pooled human donors can be used to reliably isolate and expand amniotic fluid-derived MSCs ex vivo, regardless of gestational age, at rates comparable to that of cells cultured in FBS. Regardless of the serum used, the mesenchymal amniocytes expressed the exact same markers, which were consistent with a mesenchymal stem/ phenotype, including CD29, CD44, CD90, and CD105.
[0200] The data also demonstrate that a small sample of amniotic fluid, as little as 5 mL, can easily produce enough cells (i.e. greater than 100 million) required to engineer a surgically implantable construct in a relatively short period of time. Since a diagnostic amniocentesis is routinely offered to any mother with a fetus in whom a congenital anomaly has been diagnosed by prenatal imaging, a small additional aliquot of amniotic fluid could be effortlessly obtained at that time for the engineering of different tissue grafts, without any further maternal morbidity. The data shows that human mesenchymal amniocytes retain their phenotype and can be dependably expanded ex vivo in the absence of animal serum.
EXAMPLE 5
Cartilage engineered from different perinatal mesenchymal stem cells.
[0201] The repair of many congenital anomalies that involve malformation or loss of cartilage, such as tracheal, craniofacial, chest wall, and limb defects, could benefit greatly from the availability of autologous cartilaginous constructs in the perinatal period, through fetal tissue engineering. This therapeutic principle involves the procurement of fetal cells, which are then processed through tissue engineering techniques in parallel to the remainder of gestation, so that a newborn or fetus could benefit from the availability of not only cartilage, but a variety of autologous tissues soon after birth, or even in utero [44-52].
[0202] Despite a plethora of reports on cartilage engineering utilizing chondrocytes, or select mesenchymal stem cells (MSCs), no study to date has directly compared cartilaginous constructs derived from MSCs from different sources. It has been shown that cartilage can be engineered from MSCs present in umbilical cord blood (CB) and amniotic fluid (AF) [53, 54]. The purpose of this study was to compare the in vitro properties of cartilaginous constructs
engineered with MSCs isolated from diverse perinatal sources, namely AF, preterm CB, and neonatal bone marrow (BM), as well as to compare them to native fetal hyaline and elastic cartilage. In addition, the expansion kinetics of MSCs obtained from these different perinatal sources were also compared.
Materials and Methods
[0203] An animal model was chosen for this study primarily because of ethical and logistical barriers to obtaining different samples of human fetal tissue/cells. This study was approved by the Children's Hospital Boston Animal Care and Use Committee and the Harvard Medical School Animal Management Program under protocols #A04-10-130 and #03354, respectively.
Cell isolation and culture
[0204] The different methods for the procurement of the fetal and neonatal cells used here and the documentation of their mesenchymal identity have been previously reported [53, 55, 56]. Briefly, BM (n=6) was obtained by aspirating 15 mL of marrow from the long bones of lambs less than a week after birth, immediately after euthanasia. Samples of AF (50 mL; n=8) and of venous CB (10 mL; n=12) were obtained from fetal lambs at 80-120 days gestation (term=145 days), under general anesthesia. Specimens of ovine tracheal hyaline cartilage (n=10) and auricular elastic cartilage (n=5) no larger than 1 cm2 were collected from fetal lambs immediately after euthanasia, performed at 100-135 days gestation. BM and CB samples were stored in high-glucose Dulbecco's Modified Eagle Medium (DMEM) containing 5% preservative-free heparin (Elkins Sinn, Cherry Hill, NJ). All samples were transported on ice and processed within 4 hours of harvesting.
[0205] After 48-72 hours, adherent, morphologically distinct MSCs were expanded in tissue culture dishes in a humidified 5% CO2 incubator at 370C. The growth medium consisted of DMEM with L-glutamine, 10% fetal bovine serum (FBS), 10,000 U/ml penicillin G sodium, 10 mg/mL streptomycin sulfate (all from Sigma- Aldrich, St. Louis, MO), and 5 ng/mL human recombinant basic fibroblast growth factor (Promega, Madison, WI, catalog #G5071).
Flow Cytometry
[0206] Fluorescence-activated cell sorting (FACS) analysis was performed on isolated cells obtained from AF between passages 3 and 10; from BM between passages 5 and 9; and from CB between passages 7 and 11.
[0207] Approximately 5x105 unfixed cells were detached with cell dissociation reagent
(Sigma-Aldrich) for 15 min., washed in PBS, and incubated for 30 minutes with unconjugated, mouse monoclonal antibodies previously validated for use in sheep [57, 58]. The antibodies used were CD29 (dilution 1:10; VMRD, Pullman, WA), CD31/PECAM-1 (dilution 1:2; Serotec, Oxford, UK), CD44 (Serotec), CD90/Thy-l (dilution 1:10; BD Biosciences, Bedford, MA), and CD105/SH2/endoglin (dilution 1:10; BD Biosciences). After several washes in 0.1% bovine serum albumin (Sigma-Aldrich), the cells were put on ice for an additional 20 minutes with a fluorescein isothiocynate (FITC)-conjugated rabbit anti-mouse immunoglobulin (1:100 dilution, STAR9B, Serotec). Nonspecific cell staining was excluded using a mouse isotype immunoglobulin control. Between 5,000 and 10,000 labeled cells were acquired and analyzed using the Vantage SE cell sorter (BD Biosciences).
MSC Expansion Kinetics
[0208] Cell proliferation rates of the MSCs from the three sources were measured in quadruplicate fashion using Hoechst 33258 dye (Sigma-Aldrich) assay based on methods as previously described [55, 59]. Briefly, MSCs from AF between passages 6 and 10 (n=4); from BM between passages 5 and 8 (n=4); and from CB between passages 7 and 10 (n=4) were cultured in the exact same standard growth medium containing 20% FBS from the same lot, in 24-well plates. The initial plating density was approximately 2.5xlO4 cells per cm2. Cells were then harvested at the 0, 24, 48, and 96 hour time points using 0.05% trypsin/0.53 mM EDTA. The samples were washed in DMEM containing 5% FBS, frozen in distilled water for 1 hour at - 80° C, and then quickly thawed at 37° C prior to measurement of total DNA using Hoechst 33258 dye and a VersaFluor Cuvette Fluorometer (Bio-Rad Laboratories, Hercules, CA) with calf thymus DNA (Sigma-Aldrich) used as the standard. DNA concentrations were converted to normalized cell counts based on a conversion factor of 8 pg of DNA per cell. Population doubling times were calculated using a formula as described elsewhere [60] .
MSC Multipotent Differentiation Potential
[0209] In order to confirm their multipotent differential potential, subsets of MSCs from all 3 groups also underwent culture under osteogenic and adipogenic conditions, as follows.
Osteogenic Differentiation.
[0210] MSCs at passage 8 from all 3 groups (n=4 per group) were plated at a density of
5xlO4 cells per cm2 in 6-well plates containing DMEM, 5% FBS, 10,000 U/ml penicillin G sodium, 10 mg/mL streptomycin sulfate, 5 mM β-glycerophosphate, 50 μM ascorbic acid-2 phosphate, and 100 nM dexamethasone (all from Sigma). As negative controls, MSCs were cultured in parallel in standard culture medium.
[0211] After 21 days, osteogenic differentiation was assessed according to a modified von Kossa staining protocol [61]. Briefly, the cells were fixed in 4% paraformaldehyde for 10 min. and stained with 2% silver nitrate (Fisher Scientific, Fairlawn, NJ) for 30 min under ultraviolet light. After 3 washes in distilled water, the cells were placed in 2.5% sodium thiosulfate (Sigma- Aldrich) for 5 min., washed once more in distilled water, and assessed qualitatively for silver stained mineralization under an inverted microscope.
[0212] Adipogenic Differentation. MSCs at passage 8 from all 3 groups (n=4 per group) were plated at a density of 3-5xlO4 cells per cm2 in 6-well plates containing DMEM, 10% FBS, 10,000 U/ml penicillin G sodium, 10 mg/mL streptomycin sulfate, 60 μM indomethacin, lμM dexamethasone, 10 μg/mL insulin, and 0.5 mM 3-isobutyl-l-methylxanthine (all from Sigma- Aldrich). As negative controls, MSCs were cultured in parallel in standard medium.
[0213] After 21 days, cells were fixed in 10% formalin and stained with 0.5% Oil-O-Red
(Sigma- Aldrich) in 60% isopropanol (Fisher Scientific) for 10 minutes. The cells were washed with distilled water and assessed qualitatively for the presence of orange-red intracellular lipid vacuoles under an inverted microscope.
Scaffold processing
[0214] Scaffolds were made of highly porous, non- woven polyglycolic acid (PGA -
Albany International, Mansfield, MA) at a density of approximately 67 mg/mL, cut to 10x10 mm (thickness 3 mm). Each matrix was sprayed with 3% poly-L-lactic acid (Sigma- Aldrich) in chloroform prior to lyophilization for 48 hrs. They were then placed in 70% ethanol for 2 hours and washed 3 times in cation-free phosphate buffered solution (Invitrogen, Carlsbad, CA). After sterilization, they were treated with 1 N sodium hydroxide for 1 minute, rinsed 3 times in distilled water, and coated with a 0.3% collagen solution (Vitrogen - Cohesion, Palo Alto, CA) for 12 hours at 4 0C. They were stored in a vacuum container until cell seeding.
Scaffold seeding and bioreactor environment
[0215] Scaffolds were dynamically seeded with the AF-, BM-, and CB-derived MSCs at parallel passages, at a density of 40-80 million/cm2 in 250 mL spinner flasks (Dow Corning, Midland, MI) at 37 0C, using a modified protocol as previously described [62]. After 72 hours, the constructs were transferred into 50 mL polypropylene centrifuge tubes (BD Biosciences, Bedford, MA) and maintained in a serum- free chondrogenic medium within a rotating apparatus (ATR, Laurel, MD) at 10 rpm, in a 5% CO2 incubator at 37 ° C for 12 weeks. The chondrogenic medium consisted of DMEM containing 10 ng/mL of transforming growth factor-β (TGF-βl - R&D Systems, Minneapolis, MN; or TGF-β2 - Peprotech, Rocky Hill, NJ), 10-7 M dexamethasone, 50 μg/mL ascorbic acid 2-phosphate, 10,000 U/mL penicillin G sodium, 10 mg/mL streptomycin sulfate, 100 μg/mL sodium pyruvate, 40 μg/mL L-proline (all from Sigma- Aldrich), and insulin transferrin selenium-plus (ITS-plus - BD Biosciences). In 4 (50%) of the scaffolds seeded with AF-derived MSCs, the media also contained 100 ng/ml of insulin growth factor- 1 (IGF-I, Peprotech).
Histological analyses
[0216] All engineered and native cartilage specimens were fixed in 10% neutral buffered formalin for less than 24 hours, paraffin embedded, and sectioned. Slides were stained with hematoxylin and eosin (H&E). In addition, toluidine blue and elastin von Gieson (EVG) stains were used to evaluate the presence of glycosaminoglycans (GAG) and elastin, respectively. Immunohistochemical analysis was performed using antibodies against two cartilage-specific proteoglycans, namely aggrecan (12/21/1-C-6) and cartilage link protein (9/30/8-A-4), both developed by Dr. Bruce Caterson from the Developmental Studies Hybridoma Bank under the auspices of the National Institutes of Health and maintained by the University of Iowa. Sequestered epitopes were exposed by pretreatment with 0.5 U/mL chondroitinase ABC (Sigma- Aldrich) for 30 min at room temperature, 10 mM dithiothreitol (Sigma- Aldrich) for 2 hrs at 37 0C, and 40 mM iodoacetamide (Sigma- Aldrich) for 1 hr at room temperature. Secondary antibody detection was by a multispecies link ultra-streptavidin detection system (Signet Laboratories, Dedham, MA), according to the manufacturer's instructions. Further immunohistochemical staining for collagen types I, II, and X was conducted as previously described [63]. Histological examination was performed under a light microscope (Carl Zeiss) specially equipped with a digital camera (Nikon Coolpix 8700; Nikon, Tokyo, Japan).
DNA content
[0217] The methods for total DNA quantification within tissue specimens are according to that described in [64] . Briefly, engineered and native cartilage specimens were lyophilized overnight, minced, and weighed. Samples were incubated in a 0.5 mg/mL proteinase K solution (Sigma-Aldrich) at 550C for 16 hours. Hoechst 33258 dye (Sigma-Aldrich) was then used to measure the total DNA content using a VersaFluor Cuvette fluorometer (Bio-Rad Laboratories).
Quantitative matrix analyses
[0218] Native and engineered specimens were lyophilized overnight, minced, and weighed. The levels of sulfated GAGs, pepsin soluble collagen (predominantly type II, a cartilage-specific collagen subtype), and α-elastin were quantified using a standard protocol and a spectrophotometer (Thermo Electron, Waltham, MA), as detailed in a previous work [63]. GAG levels were determined at 656 nm using the Blyscan dye reagent (Biocolor, Belfast, UK) containing dimethylmethylene blue. Collagen type II concentrations were measured at 540 nm using the Sircol dye reagent (Biocolor) containing sirius red. Elastin measurements were obtained at 513 nm using the Fastin dye kit (Biocolor) containing tetraphenyl porphine sulfonate.
Statistical analyses
[0219] Statistical analysis of cell proliferation rates was by two-way repeated-measures analysis of covariance using the F-test for comparisons. Statistical comparisons of the DNA and extracellular matrix levels was by the one-way analysis of variance (ANOVA), with Dunnett's multiple comparisons or the Kruskal-Wallis test, as appropriate, using commercially available software (Instat, San Diego, CA). P values of less then 0.05 were considered significant.
Results
MSC Immunophenotypic Profile
[0220] Representative analyses of ovine perinatal mesenchymal cells (MSCs) by flow cytometry. Forward scatter and side scatter profiles of MSCs from amniotic fluid(AF), neonatal bone marrow (BM), and preterm umbilical cord blood (CB). FACS analyses of isolated cells from AF, BM, and CB showed similar forward and side scatter profiles (data not shown). Immunophenotypic profiles of ungated MSCs from amniotic fluid, neonatal bone marrow, and preterm umbilical cord blood were similar. Greater than 90 percent of cells in all 3 groups were strongly positive for CD29 and CD44 (data not shown). Additionally, between 40 and 50
percent of cells from amniotic fluid, bone marrow, and cord blood showed positive or weak expression of CD90/Thy-l and CD105/SH2/endoglin. In contrast, fetal dermal fibroblasts did not express any detectable levels of CD90/Thy-l and CD105/SH2/endoglin (data not shown). CD31/PECAM-1 was not found in any sample.
MSC Expansion Kinetics
[0221] All samples exhibited a logarithmic growth velocity without evidence of a plateau phase during the entire study period. However, there were statistically significant differences in the proliferation rates of MSCs based on perinatal cell source by Hoescht dye assays. By 24 hours, AF-based MSCs proliferated significantly faster in culture when compared to MSCs derived from BM and CB (Fig. 8A). There were no significant differences in the growth rates between BM- and CB-derived MSCs. Overall, the population doubling time for MSCs from AF was 17.8+2.0 hours (Fig. 8B). In contrast, MSCs from BM and CB had significantly lower population doubling times of 43.8+3.8 and 43.9+3.7 hours, respectively.
MSC Multipotent Differentiation Potential
[0222] MSCs from all 3 groups cultured under osteogenic conditions displayed osteogenic differentiation, as confirmed by von Kossa staining. All 4 samples in each group showed evidence of dark calcium aggregates. The osteogenic phenotype appeared to be less robust in 2 of the samples containing either BM- or CB-derived MSCs although this was not formally quantified. Osteogenic differentiation was not observed in MSCs from any group grown for 21 days in standard medium (control).
[0223] Adipogenic differentiation was confirmed in all 3 MSC groups cultured in adipogenic media, as demonstrated by the presence of numerous cells containing lipid-rich vacuoles on Oil-O-Red staining. Three of 4 samples from AF and 4 of 4 samples from BM contained greater than 20 percent of cells with an obvious adipogenic phenotype. However, only 1 of 4 samples tested from CB could be induced into the adipogenic differentiation pathway. Adipogenic differentiation was not observed in MSCs from any group grown for 21 days in standard medium (control).
Gross construct findings
[0224] After 12 weeks in the rotating bioreactor, all engineered constructs maintained the same shape and size of the original PGA-based scaffold, with no differences across the
groups. All constructs were moderately rigid upon routine manipulation. However, constructs engineered from BM and CB MSCs were evidently more brittle than constructs derived from AF MSCs and than both types of native fetal cartilage specimens.
Standard histology
[0225] Representative H&E photomicrographs of the engineered constructs were taken
(data not shown). Morphological evidence of chondrogenic differentiation was present in all 3 engineered groups, with multiple chondrocyte-like lacunae housing a single cell containing clear cytoplasm. Along the periphery of each engineered construct, there was a densely packed, stratified layer of fusiform cells that resembled perichondrium. Small amounts of residual, degrading PGA polymer fibers were found throughout all engineered specimens (data not shown). Unlike constructs seeded with BM or CB MSCs, engineered grafts derived from AF MSCs were surrounded by a fibrous, acidophilic ground substance. The ECM was more basophilic in both BM- and CB-derived specimens than in AF-based grafts ((data not shown). There were no obvious differences in the AF-based constructs related to whether they were cultured with or without IGF-I in the chondrogenic medium. None of the engineered constructs had an identical appearance to either hyaline or elastic native cartilage. The chondrocytes present in native fetal elastic cartilage were more densely packed when compared to specimens from all other groups (data not shown).
Cellularity
[0226] Despite the initial cell seeding at comparable densities across the groups, there was significant variation in the degree of cellularity among them. Total DNA levels were markedly higher in native fetal elastic cartilage when compared to native hyaline and engineered cartilage specimens (Fig. 9). The DNA content of engineered cartilage from AF- and CB- derived MSCs was comparable to that of native hyaline cartilage, while BM-based constructs had the lowest levels of DNA per mass of tissue.
GAG content
[0227] Immunohistochemical staining for aggrecan and link protein were positive in all groups (data not shown). Toluidine blue staining revealed a uniform, metachromatic matrix in all specimens (data not shown). Sulfated GAG levels were significantly higher in native fetal hyaline cartilage when compared to native elastic and engineered cartilage specimens (Fig. 10). Among the engineered samples, levels of sulfated GAGs were highest in constructs seeded with
MSCs derived from AF. There were no significant differences in the sulfated GAG content among AF-constructs cultured with IGF-I when compared to those cultured without IGF-I. The sulfated GAG concentrations in BM- and CB -derived specimens were comparable to that of native elastic cartilage.
Collagen content
[0228] Immunohistochemical staining for collagen type II was strongly positive in all native specimens and in the engineered grafts made from BM- and CB-derived MSCs (data not shown). Histologically, AF-derived constructs displayed a seemingly lower expression of collagen type II and an increased expression of collagen type I. The degree of collagen type X expression was negligible in all 3 groups. Quantitatively, however, the levels of pepsin soluble collagen were comparable in all engineered specimens and not statistically different from that of native samples (Fig. 11). There were no significant differences in the collagen levels between AF-constructs cultured with or without IGF-I.
Elastin content
[0229] EVG staining showed varying degrees of elastin fiber formation within the engineered constructs. Histologically, elastin fibers appeared to be more abundant within the interstitial matrix of native fetal elastic cartilage and AF-based engineered cartilage, when compared to all the other groups (data not shown). Quantitative assays confirmed that AF- derived constructs had elevated levels of α-elastin (156.0+120.4 μg/mg), which were statistically comparable to that of native elastic cartilage (235.8+54.2 μg/mg) and were significantly higher than that of native hyaline cartilage as well as that of the other two types of engineered grafts (Fig. 12). There were no significant differences in the elastin levels between AF-constructs cultured with or without IGF-I. Elastin concentrations were significantly lower in the engineered constructs seeded with BM- and CB-derived MSCs when compared to native fetal elastic cartilage.
Conclusion
[0230] MSCs from 3 perinatal cell sources, namely AF, neonatal BM, and preterm umbilical CB were analysed. The data indicate that MSCs from all these sources are morphologically and phenotypically similar. Regardless of the source, these cells expressed markers consistent with a mesenchymal stem/ phenotype, including CD29, CD44, CD90, and CD105. None of the isolated cells expressed CD31, pointing to the absence of endothelial cells
in our cultures. The nature of these MSCs was further confirmed by their differentiation into at least 2 different mesenchymal cell lineages, regardless of the source. AF-based MSCs appeared to have osteogenic and adipogenic potentials equivalent to that of BM-derived MSCs, even after 8 passages.
[0231] In spite of the many similarities among the different MSCs analyzed, the AF- based MSCs proliferated more than twice as quickly in culture than BM- and CB-derived MSCs grown under identical conditions. For example, a 5 mL aliquot of AF obtained during a routine diagnostic amniocentesis is all that would be needed for one to obtain several hundred million cells in 3-4 weeks' time. The ability to produce a very large number of cells, in a short period of time, from a diminutive sample is generally highly desirable for the clinical translation of cell- based therapies.
[0232] Variations in the differentiation potential of MSCs isolated from assorted anatomical locations have only recently begun to be quantitatively appreciated in monolayer culture systems [67, 70-74]. Here, three-dimensional cartilaginous constructs engineered from different perinatal MSCs were examined, with a translational emphasis on quantitative extracellular matrix (ECM) comparisons. The data show that both the ECM pattern (in particular) and cellularity of these constructs are clearly dependent on cell source.
[0233] AF-based constructs contained comparable levels of pepsin soluble collagen to the other constructs, they also had the highest concentrations of both sulfated GAG and elastin among all engineered specimens. Their elastin levels were actually equivalent to that of native elastic cartilage, while being significantly richer in GAG than that type of native tissue. In general, within cartilage, proteoglycans are primarily responsible for providing mechanical compressive strength, elastin confers pliability, and collagen type II imparts tensile strength and acts as a natural scaffold for tissue remodeling. Of course, the singular ECM profile of AF-based construct should be particularly suited to surgical reconstructive procedures in which elastic cartilage would be desirable. However, the fact that they are rich in both elastin and GAG can in fact be optimal for most, if not all types of reconstruction as well. Indeed, these constructs were not only more pliable, but also noticeably less brittle than the ones derived from BM or CB.
[0234] In addition to diverse ECM patterns, the choice of the best source of MSCs for cartilage engineering applications would also depend on specific clinical scenarios. For the treatment of prenatally diagnosed congenital anomalies, AF-based grafts would add further advantages over other cell sources. An amniocentesis is routinely performed whenever a
structural anomaly is diagnosed by fetal imaging. In skilled hands, the method is associated with a <0.5% spontaneous abortion rate, the lowest of any prenatal invasive procedure [76]. There would be no added risk of obtaining an additional 5-10 mL aliquot of amniotic fluid during a routine diagnostic amniocentesis, which is all that would be required for most fetal tissue engineering applications, including cartilage fabrication [54, 69]. In contrast, harvesting cells from the fetus itself is certainly more invasive and technically difficult. As to fetal CB aspirations, they have been performed safely in many parts of the world but can be associated with as much as 1-5% risk of fetal loss [77]. Of course, less stringent time constraints could allow for the harvesting of MSCs from CB at birth or from BM in the early postnatal setting.
[0235] In humans, all the 3 cell sources evaluated in this study have been shown to contain MSCs expressing mesenchymal -specific markers, including CD73 (SH3), CD90 (Thy- 1), CD105 (SH2), CD44, and CD29, while being negative for hematopoietic- specific markers, including CD31, CD34, and CD45 [69, 78-82].
[0236] All patents and other publications identified are expressly incorporated herein by reference for the purpose of describing and disclosing, for example, the methodologies described in such publications that might be used in connection with the present invention. These publications are provided solely for their disclosure prior to the filing date of the present application. Nothing in this regard should be construed as an admission that the inventors are not entitled to antedate such disclosure by virtue of prior invention or for any other reason. All statements as to the date or representation as to the contents of these documents is based on the information available to the applicants and does not constitute any admission as to the correctness of the dates or contents of these documents.
[0237] References
1. Prockop DJ: Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997; 276: 71-4.
2. Pittenger MF, Mackay AM, Beck SC, et al.: Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143-7.
3. Miyahara Y, Nagaya N, Kataoka M, et al.: Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med 2006; 12: 459-65.
4. Portmann-Lanz CB, Schoeberlein A, Huber A, et al.: Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol 2006; 194: 664-73.
5. Frosch KH, Drengk A, Krause P, et al.: Stem cell-coated titanium implants for the partial joint resurfacing of the knee. Biomaterials 2006; 27: 2542-9.
6. Deng YB, Liu XG, Liu ZG, et al.: Implantation of BM mesenchymal stem cells into injured spinal cord elicits de novo neurogenesis and functional recovery: evidence from a study in rhesus monkeys. Cytotherapy 2006; 8: 210-4.
7. Le Blanc K, Gotherstrom C, Ringden O, et al.: Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation 2005; 79: 1607-14.
8. Koc ON, Day J, Nieder M, et al.: Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant 2002; 30: 215-22.
9. Koc ON, Gerson SL, Cooper BW, et al.: Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000; 18: 307- 16.
10. Awad HA, Butler DL, Boivin GP, et al.: Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng 1999; 5: 267-77 '.
11. Horwitz EM, Prockop DJ, Fitzpatrick LA, et al.: Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 1999; 5: 309-13.
12. Kaviani A, Perry TE, Dzakovic A, et al.: The amniotic fluid as a source of cells for fetal tissue engineering. J Pediatr Surg 2001; 36: 1662-5.
13. Kaviani A, Guleserian K, Perry TE, et al.: Fetal tissue engineering from amniotic fluid. J Am Coll Surg 2003; 196: 592-7.
14. Fuchs JR, Kaviani A, Oh JT, et al.: Diaphragmatic reconstruction with autologous tendon engineered from mesenchymal amniocytes. J Pediatr Surg 2004; 39: 834-8; discussion 834-8.
15. Kunisaki SM, Fuchs JR, Kaviani A, et al.: Diaphragmatic repair through fetal tissue engineering: a comparison between mesenchymal amniocyte- and myoblast-based constructs. J Pediatr Surg 2006; 41: 34-9; discussion 34-9.
16. Kunisaki SM, Freedman DA, and Fauza DO: Fetal tracheal reconstruction with cartilaginous grafts engineered from mesenchymal amniocytes. J Pediatr Surg 2006; 41: 675- 82.
17. Mackensen A, Drager R, Schlesier M, et al.: Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol Immunother 2000; 49: 152-6.
18. Chachques JC, Herreros J, Trainini J, et al.: Autologous human serum for cell culture avoids the implantation of cardioverter-defibrillators in cellular cardiomyoplasty. Int J Cardiol 2004; 95 Suppl l: S29-33.
19. Spees JL, Gregory CA, Singh H, et al.: Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. MoI Ther 2004; 9: 747- 56.
20. Medicinal and other products and human and animal transmissible spongiform encephalopathies: memorandum from a WHO meeting. Bull World Health Organ 1997; 75: 505-13.
21. Kunisaki SM, Jennings RW, and Fauza DO: Fetal cartilage engineering from amniotic mesenchymal cells. Stem Cells Dev 2006; 15: 245-53.
22. Prusa AR and Hengstschlager M: Amniotic fluid cells and human stem cell research: a new connection. Med Sci Monit 2002; 8: RA253-7.
23. In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al.: Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 2003; 102: 1548-9.
24. Prusa AR, Marton E, Rosner M, et al.: Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Hum Reprod 2003; 18: 1489-93.
25. Prusa AR, Marton E, Rosner M, et al.: Neurogenic cells in human amniotic fluid. Am J Obstet Gynecol 2004; 191: 309-14.
26. Tsai MS, Lee JL, Chang YJ, et al.: Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod 2004; 19: 1450-6.
27. Tsai MS, Hwang SM, Tsai YL, et al.: Clonal Amniotic Fluid-Derived Stem Cells Express Characteristics of Both Mesenchymal and Neural Stem Cells. Biol Reprod 2005:
28. Zhao P, Ise H, Hongo M, et al.: Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation 2005; 79: 528-35.
29. Milunsky A: Amniotic fluid cell culture, in Genetic disorder of the fetus, Milunsky A, Editor. New York: Plenum Press, 1979. p. 75-84.
30. Hoehn H and SaIk D: Morphological and biochemical heterogeneity of amniotic fluid cells in culture. Methods Cell Biol 1982; 26: 11-34.
31. Gosden CM: Amniotic fluid cell types and culture. Br Med Bull 1983; 39: 348-54.
32. Prusa AR, Marton E, Rosner M, et al.: Stem cell marker expression in human trisomy 21 amniotic fluid cells and trophoblasts. J Neural Transm Suppl 2003: 235-42.
33. Torricelli F, Brizzi L, Bernabei PA, et al.: Identification of hematopoietic cells in human amniotic fluid before the 12th week of gestation. Ital J Anat Embryol 1993; 98: 119-26.
34. Streubel B, Martucci-Ivessa G, Fleck T, et al.: [In vitro transformation of amniotic cells to muscle cells— background and outlook]. Wien Med Wochenschr 1996; 146: 216-7.
35. Macek M, Hurych J, and Rezacova D: Collagen synthesis in long-term amniotic fluid cell cultures. Nature 1973; 243: 289-90.
36. Hurych J, Macek M, Beniac F, et al.: Biochemical characteristics of collagen produced by long term cultivated amniotic fluid cells. Hum Genet 1976; 31: 335-40.
37. Kaviani A, Jennings RW, and Fauza DO: Amniotic fluid-derived fetal mesenchymal cells differentiate into myogenic precursors in vitro. J Am Coll Surg 2002; 195: S29 [abstract].
38. In't Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al.: Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 2003; 102: 1548-9.
39. Fauza DO: Amniotic fluid and placental stem cells. Best Pract Res Clin Obstet Gynaecol 2004; 18: 877-91.
40. BiIi C, Divane A, Apessos A, et al.: Prenatal diagnosis of common aneuploidies using quantitative fluorescent PCR. Prenat Diagn 2002; 22: 360-5.
41. Kunisaki SM, Fuchs JR, Azpurua H, et al.: A comparison of different perinatal sources of mesenchymal cells: implications for tissue engineering, in Thirty- seventh Annual Meeting of the American Pediatric Surgical Association. Hilton Head, SC 2006.
42. Noort WA, Kruisselbrink AB, in't Anker PS, et al.: Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol 2002; 30: 870-8.
43. Stute N, Holtz K, Bubenheim M, et al.: Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Exp Hematol 2004; 32: 1212-25.
44. Fauza DO, Fishman SJ, Mehegan K, and Atala A. Videofetoscopically assisted fetal tissue engineering: bladder augmentation. J Pediatr Surg 33, 7-12, 1998.
45. Fauza DO, Fishman SJ, Mehegan K, and Atala A. Videofetoscopically assisted fetal tissue engineering: skin replacement. J Pediatr Surg 33, 357-61, 1998.
46. Fauza DO, Marler JJ, Koka R, Forse RA, Mayer JE, and Vacanti JP. Fetal tissue engineering: diaphragmatic replacement. J Pediatr Surg 36, 146-51., 2001.
47. Fuchs JR, Terada S, Ochoa ER, Vacanti JP, and Fauza DO. Fetal tissue engineering: In utero tracheal augmentation in an ovine model. J Pediatr Surg 37, 1000-6, 2002.
48. Fuchs JR, Terada S, Hannouche D, Ochoa ER, Vacanti JP, and Fauza DO. Fetal tissue engineering: chest wall reconstruction. J Pediatr Surg 38, 1188-93, 2003.
49. Fuchs JR, Kaviani A, Oh JT, LaVan D, Udagawa T, Jennings RW, Wilson JM, and Fauza DO. Diaphragmatic reconstruction with autologous tendon engineered from mesenchymal amniocytes. J Pediatr Surg 39, 834-8; discussion 834-8, 2004.
50. Fuchs JR, Nasseri BA, Vacanti JP, and Fauza DO. Postnatal myocardial augmentation with skeletal myoblast-based fetal tissue engineering. Surgery in press, 2006.
51. Kunisaki SM, Fuchs JR, Kaviani A, Oh JT, LaVan DA, Vacanti JP, Wilson JM, and Fauza DO. Diaphragmatic repair through fetal tissue engineering: a comparison between mesenchymal amniocyte- and myoblast-based constructs. J Pediatr Surg 41, 34-9; discussion 34- 9, 2006.
52. Kunisaki SM, Freedman DA, and Fauza DO. Fetal tracheal reconstruction with cartilaginous grafts engineered from mesenchymal amniocytes. J Pediatr Surg 41, 675-82, 2006.
53. Fuchs JR, Hannouche D, Terada S, Zand S, Vacanti JP, and Fauza DO. Cartilage Engineering from Ovine Umbilical Cord Blood Mesenchymal Cells. Stem Cells 23, 958-964, 2005.
54. Kunisaki SM, Jennings RW, and Fauza DO. Fetal cartilage engineering from amniotic mesenchymal cells. Stem Cells Dev 15, 245-53, 2006.
55. Kaviani A, Perry TE, Dzakovic A, Jennings RW, Ziegler MM, and Fauza DO. The amniotic fluid as a source of cells for fetal tissue engineering. J Pediatr Surg 36, 1662-5, 2001.
56. Fuchs JR, Hannouche D, Terada S, Vacanti JP, and Fauza DO. Fetal tracheal augmentation with cartilage engineered from bone marrow-derived mesenchymal cells. J Pediatr Surg 38, 984-7, 2003.
57. Perry TE, Kaushal S, Sutherland FW, Guleserian KJ, Bischoff J, Sacks M, and Mayer JE. Thoracic Surgery Directors Association Award. Bone marrow as a cell source for tissue engineering heart valves. Ann Thorac Surg 75, 761-7; discussion 767, 2003.
58. Krupnick AS, Balsara KR, Kreisel D, Riha M, Gelman AE, Estives MS, Amin KM, Rosengard BR, and Flake AW. Fetal liver as a source of autologous cells for perinatal tissue engineering. Tissue Eng 10, 723-35, 2004.
59. Adams CJStorrie B. A simple DNA-dependent fluorescence enhancement assay for cell number. J Histochem Cytochem 29, 326-8, 1981.
60. Cristofalo VJ, Allen RG, Pignolo RJ, Martin BG, and Beck JC. Relationship between donor age and the replicative lifespan of human cells in culture: a reevaluation. Proc Natl Acad
Sci U S A 95, 10614-9, 1998.
61. Puchtler HMeloan SN. Demonstration of phosphates in calcium deposits: a modification of von Kossa's reaction. Histochemistry 56, 177-85, 1978.
62. Vunjak-Novakovic G, Obradovic B, Martin I, Bursac PM, Langer R, and Freed LE. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog 14, 193-202, 1998.
63. Fuchs JR, Terada S, Hannouche D, Ochoa ER, Vacanti JP, and Fauza DO. Engineered fetal cartilage: structural and functional analysis in vitro. J Pediatr Surg 37, 1720-5, 2002.
64. Kim YJ, Sah RL, Doong JY, and Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem 174, 168-76, 1988.
65. McKusick VA. 1998. Mendelian Inheritance in Man: A Catalog of Human Genes and Genetic Disorders. 11th ed. Baltimore: Johns Hopkins University Press.
66. Contribution of birth defects to infant mortality-United States, 1986. MMWR Morb Mortal WkIy Rep 38, 633-5, 1989.
67. Chang YJ, Shih DT, Tseng CP, Hsieh TB, Lee DC, and Hwang SM. Disparate mesenchyme-lineage tendencies in mesenchymal stem cells from human bone marrow and umbilical cord blood. Stem Cells 24, 679-85, 2006.
68. Kern S, Eichler H, Stoeve J, Kluter H, and Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24, 1294-301, 2006.
69. Kaviani A, Guleserian K, Perry TE, Jennings RW, Ziegler MM, and Fauza DO. Fetal tissue engineering from amniotic fluid. J Am Coll Surg 196, 592-7, 2003.
70. Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, and Fisk NM. Identification of mesenchymal stem/ cells in human first-trimester fetal blood, liver, and bone marrow. Blood 98, 2396-402, 2001.
71. In 't Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, Beekhuizen W, Willemze R, Kanhai HH, and Fibbe WE. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica 88,
845-52, 2003.
72. Koerner J, Nesic D, Romero JD, Brehm W, Mainil-Varlet P, and Grogan SP. Equine peripheral blood-derived s in comparison to bone marrow-derived mesenchymal stem cells. Stem Cells 24, 1613-9, 2006.
73. Miao Z, Jin J, Chen L, Zhu J, Huang W, Zhao J, Qian H, and Zhang X. Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol Int 30, 681-7, 2006.
74. Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, and Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 33, 1402-16, 2005.
75. Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, Tanaka J, and Bunyaratvej A. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun 320, 914-9, 2004.
76. Evans MIWapner RJ. Invasive prenatal diagnostic procedures 2005. Semin Perinatol 29, 215-8, 2005.
77. Tongsong T, Wanapirak C, Kunavikatikul C, Sirirchotiyakul S, Piyamongkol W, and Chanprapaph P. Fetal loss rate associated with cordocentesis at midgestation. Am J Obstet Gynecol 184, 719-23, 2001.
78. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, and Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143-7, 1999.
79. Tsai MS, Lee JL, Chang YJ, and Hwang SM. Isolation of human multipotent mesenchymal stem cells from second- trimester amniotic fluid using a novel two- stage culture protocol. Hum Reprod 19, 1450-6, 2004.
80. In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, and Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 22, 1338-45, 2004.
81. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, and Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 103, 1669-75, 2004.
82. Kunisaki SM, Armant M, Kao GS, Stevenson K, Kim H, and Fauza DO. Tissue engineering from human mesenchymal amniocytes: a prelude to clinical trials. J Pediatr Surg in press, 2006.
83. Fauza D. Amniotic fluid and placental stem cells. Best Pract Res Clin Obstet Gynaecol 18, 877-91, 2004.
Table 1
Reagent Grade Manufacturer
Phosphate Buffered Saline (Ix & 10XPBS) Tissue Culture Invitrogen
25% Human Serum Albumin (Buminate) Clinical Baxter Bioscience
25% Human Serum Albumin (PLASBUMIN®-25) Clinical Talecris Biotherapeutics
DMEM with L-Glutamine Tissue Culture Cambrex Bio Science
TrypLE Tissue Culture Invitrogen
Fetal Bovine Serum Tissue Culture HyClone
Gentamicin Clinical American Pharmaceutical Partners
Basic Fibroblast Growth Factor Tissue Culture Promega
BD Biocoat Collagen I-coated plates Tissue Culture BD Biosciences
Dimethylsulfoxide (DMSO) Research Edwards Lifescience
Plasma-Lyte A Clinical Baxter
-j
K)
Table 3
Claims
1. A method of obtaining a composition enriched in human amniotic fluid derived mesenchymal stem cells comprising the steps of:
a. isolating mesenchymal stem cells from a sample of human amniotic fluid in the absence of non-human animal derived products; and
b. expanding said isolated human amniotic fluid derived mesenchymal stem cells in the absence of non-human animal derived products.
2. The method of claim 1, wherein said human amniotic fluid is cryopreserved.
3. The method of claim 1, wherein said human amniotic fluid is collected between 5 weeks of gestation to human term.
4. The method of claim 1, wherein said human amniotic fluid is collected at birth.
5. The method of claim 1, wherein the isolation and expansion of human amniotic fluid derived mesenchymal stem cells is in the presence of human serum.
6. The method of claim 1, further comprising cryopreserving the composition enriched in human amniotic fluid derived mesenchymal stem cells.
7. The method of claim 1, wherein the composition enriched in human amniotic fluid derived mesenchymal stem cells are at least 90% CD29, CD73, and CD44 positive.
8. The method of claim 1, wherein the composition enriched in human amniotic fluid derived mesenchymal stem cells are at most 5% CD34 and CD45 positive.
9. A method of proliferating human amniotic fluid derived mesenchymal stem cells comprising the steps of:
a. selecting at least one single mesenchymal stem cell from a sample of human amniotic fluid in the absence of non-human animal derived products;
b. introducing said at least one single mesenchymal stem cell to a culture medium containing no non-human animal derived product; and c. proliferating said at least one single mesenchymal stem cell to a culture medium containing no non-human animal derived product.
10. A method of storing a composition enriched in human amniotic fluid derived mesenchymal stem cells comprising the steps of:
a. obtaining a composition enriched in human amniotic fluid derived mesenchymal stem cells according to the method of any one of the claims 1-9; and
b. cryopreserving said composition enriched in human amniotic fluid derived mesenchymal stem cells.
11. An isolated human amniotic fluid derived mesenchymal stem cell prepared according to the method of any one of the claims 1-10.
12. A kit for obtaining a composition enriched in human amniotic derived mesenchymal stem cells from human amniotic fluid comprising:
a. a container for the collection of human amniotic fluid;
b. a container coated with extracellular matrix protein for the isolation and primary expansion of human amniotic fluid derived mesenchymal stem cells;
c. human serum for the culture-expansion of human amniotic fluid derived mesenchymal stem cells; and
d. instructions for the isolation, identification, and expansion of human amniotic fluid derived mesenchymal stem cells.
13. The kit of claim 12, further comprising reagents for reconstituting a culture medium containing human serum.
14. A method for producing differentiated human amniotic fluid derived mesenchymal stem cell preparations comprising:
a. obtaining a composition enriched in human amniotic fluid derived mesenchymal stem cells according to the method of any one of the claim 1-10; and
b. culturing said composition enriched in human amniotic fluid derived mesenchymal stem cells in a culture medium containing differentiation factors for a period sufficient for said stem cells to differentiate and express specific tissue markers.
15. The method of claim 14, wherein the differentiation factors are selected from a group consisting of osteogenic, myogenic, adipogenic, chondrogenic, neurogenic, hepatogenic, nephrogenic, urogenic, isletogenic, pancreatogenic, gastroenterogenic, epitheliogenic, thyroidogenic, myocardiogenic, pneumogenic, retinogenic, gametogenic, endotheliogenic, or hematopoietic factors.
16. The method of claim 14, wherein the stem cells are seeded on a scaffold.
17. A method of promoting wound healing or tissue repair in a human in need thereof comprising administering a preparation comprising a composition enriched in human amniotic fluid derived mesenchymal stem cells according to the method of any one of the claims 1-10.
18. The method of claim 17, wherein the composition is applied directly to the wound or tissue.
19. The method of claim 17, wherein the composition is embedded in a wound dressing material or scaffold, said wound dressing material or scaffold is applied on to the wound.
20. A cryopreserved pharmaceutical composition comprising:
a. a viable composition enriched in human amniotic fluid derived mesenchymal stem cells obtained according to the method of any one of the claims 1-9, in which said cells are present in an amount sufficient to effect tissue engineering, tissue repair, or wound healing;
b. an amount of cryopreservative sufficient for cryopreservation of said cells; and
c. a pharmaceutically acceptable carrier.
21. A pharmaceutical composition comprising:
a. a viable composition enriched in human amniotic fluid derived mesenchymal stem cells obtained according to the method of any one of the claims 1-9, in which said cells are present in an amount sufficient to effect tissue engineering, tissue repair, or wound healing; and b. a pharmaceutically acceptable carrier.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US97993807P | 2007-10-15 | 2007-10-15 | |
| US60/979,938 | 2007-10-15 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2009052132A1 true WO2009052132A1 (en) | 2009-04-23 |
Family
ID=40185017
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2008/079916 WO2009052132A1 (en) | 2007-10-15 | 2008-10-15 | Human amniotic fluid derived mesenchymal stem cells |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2009052132A1 (en) |
Cited By (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8932805B1 (en) | 2011-10-31 | 2015-01-13 | BioDlogics, LLC | Birth tissue material and method of preparation |
| US8969315B2 (en) | 2010-12-31 | 2015-03-03 | Anthrogenesis Corporation | Enhancement of placental stem cell potency using modulatory RNA molecules |
| US9040035B2 (en) | 2011-06-01 | 2015-05-26 | Anthrogenesis Corporation | Treatment of pain using placental stem cells |
| US9198938B2 (en) | 2008-11-19 | 2015-12-01 | Antrhogenesis Corporation | Amnion derived adherent cells |
| WO2017004460A1 (en) * | 2015-06-30 | 2017-01-05 | Surgenex, LLC | Compositions and methods for flowable allograft tissue |
| US9795639B1 (en) | 2013-03-16 | 2017-10-24 | BioDlogics, LLC | Methods for the treatment of erectile dysfunction by human birth tissue material compostion |
| EP3140417A4 (en) * | 2014-05-09 | 2018-05-23 | Reelabs Private Limited | Foetal polymix of mesenchymal stem cells under hypoxic conditions for the treatment of clinical disorders |
| US9993506B1 (en) | 2013-03-16 | 2018-06-12 | BioDlogics, Inc. | Methods for the treatment of degenerative disc diseases by human birth tissue material composition |
| US10016459B1 (en) | 2013-03-13 | 2018-07-10 | BioDlogics, LLC | Platelet-rich plasma derived from human umbilical cord blood |
| US10039792B1 (en) | 2013-03-16 | 2018-08-07 | Brahm Holdings, Llc | Methods for the treatment of inflammation and pain using human birth tissue material composition |
| EP3331989A4 (en) * | 2014-12-31 | 2018-08-08 | American CryoStem Corporation | Human serum for cell culture medium for clinical growth of human adipose stromal cells |
| US10104880B2 (en) | 2008-08-20 | 2018-10-23 | Celularity, Inc. | Cell composition and methods of making the same |
| US10201573B1 (en) | 2014-10-27 | 2019-02-12 | Brahm Holdings, Llc | Human birth tissue material composition and methods for the treatment of damage associated with a cerebral vascular accident |
| US10265438B1 (en) | 2014-11-03 | 2019-04-23 | BioDlogics, LLC | Methods and compositions for the repair and replacement of connective tissue |
| US10517902B2 (en) * | 2011-09-30 | 2019-12-31 | Prime Merger Sub, Llc | Expandable amnion membrane for treating non-healing wounds |
| CN110760572A (en) * | 2019-12-04 | 2020-02-07 | 江苏易诺维生物医学研究院有限公司 | Method and kit for detecting surface molecular marker of human umbilical cord mesenchymal stem cells |
| US10555897B1 (en) | 2013-03-16 | 2020-02-11 | Brahm Holdings Llc | Cosmetic composition and methods of treatment |
| US10596203B1 (en) * | 2014-10-10 | 2020-03-24 | Brahm Holdings, Llc | Customized repair constructs and method of preparation |
| CN113088488A (en) * | 2021-04-14 | 2021-07-09 | 贵州医科大学附属医院 | Preparation and application of human amniotic mesenchymal stem cell suspension |
| US11077229B1 (en) | 2013-03-08 | 2021-08-03 | BioDlogics, LLC | Implant coating composition and method of use |
| EP3909593A1 (en) | 2020-05-15 | 2021-11-17 | Rigshospitalet | Stem cells for treating skin lesions |
| US11180732B2 (en) | 2018-10-03 | 2021-11-23 | Stembiosys, Inc. | Amniotic fluid cell-derived extracellular matrix and uses thereof |
| US11220671B2 (en) | 2019-02-21 | 2022-01-11 | Stembiosys, Inc. | Methods for the maturation of cardiomyocytes on amniotic fluid cell-derived ECM, cellular constructs, and uses for cardiotoxicity and proarrhythmic screening of drug compounds |
| WO2022099472A1 (en) * | 2020-11-10 | 2022-05-19 | 南通市巨久新材料科技有限公司 | Cryopreservation tube containing aloe-polysaccharide-coated layer for amniotic-fluid-derived mesenchymal stem cells |
| US11446334B2 (en) | 2019-10-18 | 2022-09-20 | Amniotics Ab | Use of term amniotic fluid cells for the treatment of acute and chronic respiratory diseases |
| US11542473B2 (en) | 2016-10-21 | 2023-01-03 | Amniotics Ab | Methods and compositions for generating hematopoietic cells |
| WO2023281067A1 (en) * | 2021-07-09 | 2023-01-12 | Boost Pharma Aps | Fetal mesenchymal stem cells |
| WO2023192484A3 (en) * | 2022-04-01 | 2023-11-23 | Bright Cell, Inc. | A mesenchymal stem cells formulation for cosmetic use |
| CN117224577A (en) * | 2023-09-21 | 2023-12-15 | 浙江大学 | Application of stem cell apoptosis corpuscle in preparing medicine for treating recurrent abortion |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005017117A2 (en) * | 2003-08-14 | 2005-02-24 | Martin Haas | Multipotent amniotic fetal stem cells (mafsc) and banking of same |
| US20050118712A1 (en) * | 2003-12-02 | 2005-06-02 | Ming-Song Tsai | Two-stage culture protocol for isolating mesenchymal stem cells from amniotic fluid |
| US20050124003A1 (en) * | 2001-11-15 | 2005-06-09 | Anthony Atala | Methods of isolation, expansion and differentiation of fetal stem cells from chorionic villus, amniotic fluid, and placenta and therapeutic uses thereof |
| WO2007048813A2 (en) * | 2005-10-28 | 2007-05-03 | Universität Zürich | Tissue engineering using pure populations of isolated non-embryoblastic fetal cells |
-
2008
- 2008-10-15 WO PCT/US2008/079916 patent/WO2009052132A1/en active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050124003A1 (en) * | 2001-11-15 | 2005-06-09 | Anthony Atala | Methods of isolation, expansion and differentiation of fetal stem cells from chorionic villus, amniotic fluid, and placenta and therapeutic uses thereof |
| WO2005017117A2 (en) * | 2003-08-14 | 2005-02-24 | Martin Haas | Multipotent amniotic fetal stem cells (mafsc) and banking of same |
| US20050118712A1 (en) * | 2003-12-02 | 2005-06-02 | Ming-Song Tsai | Two-stage culture protocol for isolating mesenchymal stem cells from amniotic fluid |
| WO2007048813A2 (en) * | 2005-10-28 | 2007-05-03 | Universität Zürich | Tissue engineering using pure populations of isolated non-embryoblastic fetal cells |
Non-Patent Citations (5)
| Title |
|---|
| KAVIANI A ET AL: "Fetal tissue engineering from amniotic fluid.", JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS, vol. 196, no. 4, April 2003 (2003-04-01), pages 592 - 597, XP002509643, ISSN: 1072-7515 * |
| KUNISAKI S M ET AL: "Tissue engineering from human mesenchymal amniocytes: a prelude to clinical trials.", JOURNAL OF PEDIATRIC SURGERY, vol. 42, no. 6, June 2007 (2007-06-01), pages 974 - 979, XP002509642, ISSN: 1531-5037 * |
| STEIGMAN S A & FAUZA D O: "Isolation of mesenchymal stem cells from amniotic fluid and placenta.", CURRENT PROTOCOLS IN STEM CELL BIOLOGY, June 2007 (2007-06-01), pages 1E.2.1 - 1E.2.12, XP008100089, ISSN: 1938-8969 * |
| STEIGMAN S A ET AL: "Preclinical regulatory validation of a 3-stage amniotic mesenchymal stem cell manufacturing protocol.", JOURNAL OF PEDIATRIC SURGERY, vol. 43, no. 6, June 2008 (2008-06-01), pages 1164 - 1169, XP002509644, ISSN: 1531-5037 * |
| TSAI MING-SONG ET AL: "Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol.", HUMAN REPRODUCTION, vol. 19, no. 6, June 2004 (2004-06-01), pages 1450 - 1456, XP002404972, ISSN: 0268-1161 * |
Cited By (43)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10104880B2 (en) | 2008-08-20 | 2018-10-23 | Celularity, Inc. | Cell composition and methods of making the same |
| US9198938B2 (en) | 2008-11-19 | 2015-12-01 | Antrhogenesis Corporation | Amnion derived adherent cells |
| US8969315B2 (en) | 2010-12-31 | 2015-03-03 | Anthrogenesis Corporation | Enhancement of placental stem cell potency using modulatory RNA molecules |
| US9040035B2 (en) | 2011-06-01 | 2015-05-26 | Anthrogenesis Corporation | Treatment of pain using placental stem cells |
| US11090339B2 (en) | 2011-06-01 | 2021-08-17 | Celularity Inc. | Treatment of pain using placental stem cells |
| US10517902B2 (en) * | 2011-09-30 | 2019-12-31 | Prime Merger Sub, Llc | Expandable amnion membrane for treating non-healing wounds |
| US10786600B1 (en) | 2011-10-31 | 2020-09-29 | BioDlogics, LLC | Birth tissue material and method of preparation |
| US8932805B1 (en) | 2011-10-31 | 2015-01-13 | BioDlogics, LLC | Birth tissue material and method of preparation |
| US10245349B2 (en) | 2011-10-31 | 2019-04-02 | BioDlogics, LLC | Birth tissue material and method of preparation |
| US11077229B1 (en) | 2013-03-08 | 2021-08-03 | BioDlogics, LLC | Implant coating composition and method of use |
| US10016459B1 (en) | 2013-03-13 | 2018-07-10 | BioDlogics, LLC | Platelet-rich plasma derived from human umbilical cord blood |
| US11224616B1 (en) | 2013-03-13 | 2022-01-18 | BioDlogics, LLC | Platelet-rich plasma derived from human umbilical cord blood |
| US9795639B1 (en) | 2013-03-16 | 2017-10-24 | BioDlogics, LLC | Methods for the treatment of erectile dysfunction by human birth tissue material compostion |
| US11224617B1 (en) | 2013-03-16 | 2022-01-18 | BioDlogics, LLC | Methods for the treatment of degenerative disc diseases by human birth tissue material composition |
| US10039792B1 (en) | 2013-03-16 | 2018-08-07 | Brahm Holdings, Llc | Methods for the treatment of inflammation and pain using human birth tissue material composition |
| US11382859B1 (en) | 2013-03-16 | 2022-07-12 | Brahm Holdings, Llc | Cosmetic composition and methods of treatment |
| US9993506B1 (en) | 2013-03-16 | 2018-06-12 | BioDlogics, Inc. | Methods for the treatment of degenerative disc diseases by human birth tissue material composition |
| US10555897B1 (en) | 2013-03-16 | 2020-02-11 | Brahm Holdings Llc | Cosmetic composition and methods of treatment |
| US10555974B1 (en) | 2013-03-16 | 2020-02-11 | BioDlogics, LLC | Methods for the treatment of erectile dysfunction by human birth tissue material composition |
| US11547731B2 (en) | 2013-03-16 | 2023-01-10 | Brahm Holdings, Llc | Methods for the treatment of inflammation and pain using human birth tissue material composition |
| EP3140417A4 (en) * | 2014-05-09 | 2018-05-23 | Reelabs Private Limited | Foetal polymix of mesenchymal stem cells under hypoxic conditions for the treatment of clinical disorders |
| US11072776B2 (en) | 2014-05-09 | 2021-07-27 | Reelabs Private Limited, a Company Incorporated Under Provisions of The Companies Act 1956 | Foetal polymix of mesenchymal stem cells under hypoxic conditions for the treatment of clinical disorders and diseases |
| US10596203B1 (en) * | 2014-10-10 | 2020-03-24 | Brahm Holdings, Llc | Customized repair constructs and method of preparation |
| US10201573B1 (en) | 2014-10-27 | 2019-02-12 | Brahm Holdings, Llc | Human birth tissue material composition and methods for the treatment of damage associated with a cerebral vascular accident |
| US10265438B1 (en) | 2014-11-03 | 2019-04-23 | BioDlogics, LLC | Methods and compositions for the repair and replacement of connective tissue |
| US10905798B1 (en) | 2014-11-03 | 2021-02-02 | BioDlogics, LLC | Methods and compositions for the repair and replacement of connective tissue |
| EP3331989A4 (en) * | 2014-12-31 | 2018-08-08 | American CryoStem Corporation | Human serum for cell culture medium for clinical growth of human adipose stromal cells |
| WO2017004460A1 (en) * | 2015-06-30 | 2017-01-05 | Surgenex, LLC | Compositions and methods for flowable allograft tissue |
| US10006003B2 (en) | 2015-06-30 | 2018-06-26 | Surgenex, LLC | Compositions and methods for flowable allograft amniotic tissue |
| US11542473B2 (en) | 2016-10-21 | 2023-01-03 | Amniotics Ab | Methods and compositions for generating hematopoietic cells |
| US11180732B2 (en) | 2018-10-03 | 2021-11-23 | Stembiosys, Inc. | Amniotic fluid cell-derived extracellular matrix and uses thereof |
| US11220671B2 (en) | 2019-02-21 | 2022-01-11 | Stembiosys, Inc. | Methods for the maturation of cardiomyocytes on amniotic fluid cell-derived ECM, cellular constructs, and uses for cardiotoxicity and proarrhythmic screening of drug compounds |
| EP4234019A3 (en) * | 2019-10-18 | 2023-09-13 | Amniotics AB | Processes and apparatuses for obtaining amniotic mesenchymal stem cells from amniotic fluid and cells derived thereof |
| US11446334B2 (en) | 2019-10-18 | 2022-09-20 | Amniotics Ab | Use of term amniotic fluid cells for the treatment of acute and chronic respiratory diseases |
| CN110760572A (en) * | 2019-12-04 | 2020-02-07 | 江苏易诺维生物医学研究院有限公司 | Method and kit for detecting surface molecular marker of human umbilical cord mesenchymal stem cells |
| EP3909593A1 (en) | 2020-05-15 | 2021-11-17 | Rigshospitalet | Stem cells for treating skin lesions |
| WO2021229101A1 (en) | 2020-05-15 | 2021-11-18 | Rigshospitalet | Stem cells for treating skin lesions |
| WO2022099472A1 (en) * | 2020-11-10 | 2022-05-19 | 南通市巨久新材料科技有限公司 | Cryopreservation tube containing aloe-polysaccharide-coated layer for amniotic-fluid-derived mesenchymal stem cells |
| CN113088488A (en) * | 2021-04-14 | 2021-07-09 | 贵州医科大学附属医院 | Preparation and application of human amniotic mesenchymal stem cell suspension |
| WO2023281067A1 (en) * | 2021-07-09 | 2023-01-12 | Boost Pharma Aps | Fetal mesenchymal stem cells |
| WO2023192484A3 (en) * | 2022-04-01 | 2023-11-23 | Bright Cell, Inc. | A mesenchymal stem cells formulation for cosmetic use |
| CN117224577A (en) * | 2023-09-21 | 2023-12-15 | 浙江大学 | Application of stem cell apoptosis corpuscle in preparing medicine for treating recurrent abortion |
| CN117224577B (en) * | 2023-09-21 | 2024-02-27 | 浙江大学 | Application of stem cell apoptosis corpuscle in preparing medicine for treating recurrent abortion |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2009052132A1 (en) | Human amniotic fluid derived mesenchymal stem cells | |
| Díaz-Prado et al. | Human amniotic membrane as an alternative source of stem cells for regenerative medicine | |
| Chatzistamatiou et al. | Optimizing isolation culture and freezing methods to preserve W harton's jelly's mesenchymal stem cell (MSC) properties: an MSC banking protocol validation for the H ellenic C ord B lood B ank | |
| US20170157291A1 (en) | Tissue transplant compositions and methods for use | |
| AU2002243980B2 (en) | Renovation and repopulation of decellularized tissues and cadaveric organs by stem cells | |
| Kunisaki et al. | Tissue engineering from human mesenchymal amniocytes: a prelude to clinical trials | |
| Bieback et al. | Clinical protocols for the isolation and expansion of mesenchymal stromal cells | |
| Kunisaki et al. | A comparative analysis of cartilage engineered from different perinatal mesenchymal progenitor cells | |
| IL234879A (en) | Methods of generating tendon in-vitro | |
| WO2011101834A1 (en) | A method for obtaining mesenchymal stem cells, media, methods and composition thereof | |
| AU2002243980A1 (en) | Renovation and repopulation of decellularized tissues and cadaveric organs by stem cells | |
| CA2700613A1 (en) | Angiogenic cells from human placental perfusate | |
| HUE025338T2 (en) | Use of adipose tissue-derived stromal stem cells in treating fistula | |
| V Semenov et al. | Mesenchymal stem cells derived from Wharton's jelly and their potential for cardio-vascular tissue engineering | |
| WO2008077094A2 (en) | Methods for promoting neovascularization | |
| US20050059152A1 (en) | In vitro culture of mesenchymal stem cells (MSC) and a process for the preparation thereof for therapeutic use | |
| CZ200649A3 (en) | Method of culturing human mezenchymal stem cells, particularly for facilitating fracture healing process, and bioreactor for carrying out the method | |
| Xiao et al. | Adipogenic and osteogenic differentiation of Lin− CD271+ Sca-1+ adipose-derived stem cells | |
| Laurent et al. | Holistic approach of Swiss fetal progenitor cell banking: Optimizing safe and sustainable substrates for regenerative medicine and biotechnology | |
| US20110195054A1 (en) | Preparation And Use Of Stromal Cells For Treatment Of Cardiac Diseases | |
| Liu et al. | Construction of tissue-engineered cartilage using human placenta-derived stem cells | |
| Burukçu et al. | The investigation of diverse physiological and therapeutic impact of cellular-based products derived from human cumulus cells | |
| Ramzan et al. | Extracellular Matrix Based 3D Scaffold for Directing the Fate of Adult Stem Cells into Osteogenic Lineage | |
| Özpolat et al. | The effect of basic fibroblast growth factor and adipose tissue-derived mesenchymal stem cells on wound healing, epithelization and angiogenesis in a tracheal resection and end-to-end anastomosis rat model | |
| Bank | TRANSPLANTATION AND CELLULAR ENGINEERING |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 08838852 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 08838852 Country of ref document: EP Kind code of ref document: A1 |