WO2008020435A2 - Compositions and methods for treatment of mood disorders - Google Patents
Compositions and methods for treatment of mood disorders Download PDFInfo
- Publication number
- WO2008020435A2 WO2008020435A2 PCT/IL2007/001013 IL2007001013W WO2008020435A2 WO 2008020435 A2 WO2008020435 A2 WO 2008020435A2 IL 2007001013 W IL2007001013 W IL 2007001013W WO 2008020435 A2 WO2008020435 A2 WO 2008020435A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- mrna
- homo sapiens
- compound
- protein
- gene
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/14—Type of nucleic acid interfering N.A.
Definitions
- the present invention relates to the field of treatment of mood disorders, depression, and conditions which cause depression, as well as neurodegenerative diseases.
- RNA interference RNA interference is a phenomenon involving double-stranded (ds) RNA-dependent gene-specific posttranscriptional gene silencing.
- ds double-stranded
- RNAi RNA interference RNA interference
- RNA interference in mammals is mediated by small interfering RNAs (siRNAs) (Fire et al, Nature 1998, 391 :806) or microRNAs (miRNAs) (Ambros, Nature 2004, 431(7006):350-355; Bartel, Cell 2004, 116(2): 281-97).
- siRNAs small interfering RNAs
- miRNAs microRNAs
- the corresponding process in plants is commonly referred to as specific post-transcriptional gene silencing (PTGS) or RNA silencing and is also referred to as quelling in fungi.
- siRNA is a double-stranded RNA or modified RNA molecule which down-regulates or silences (prevents) the expression of a gene/ mRNA of its endogenous (cellular) counterpart.
- RNA interference The mechanism of RNA interference is detailed infra.
- siRNA therapeutics is effective in vivo in both mammals and in humans. Bitko et al., have shown that specific siRNA molecules directed against the respiratory syncytial virus (RSV) nucleocapsid N gene are effective in treating mice when administered intranasally (Nat. Med. 2005, l l(l):50-55). Recent reviews discussing siRNA therapeutics are available (Barik, et al., J. MoI. Med 2005, 83:764-773; Dallas and Vlassov, Med. Sci. Monitor 2006, 12(4):RA67-74; Chakraborty Current Drug Targets 2007, 8(3):469-82). Mucke (IDrugs 2007 10(l):37-41) presents a review of current therapeutics, including siRNA to various targets, for the treatment of ocular diseases, for example age related macular degeneration (AMD) and glaucoma.
- RSV respiratory syncytial virus
- Clinical depression is a serious brain disorder that affects the way nearly 19 million American adults feel, think, and interact. In contrast to the normal emotional experiences of sadness, loss, or passing mood states, clinical depression is extreme and persistent and can interfere significantly with a person's ability to function.
- disorders There are three main types of clinical depression: major depressive disorder; dysthymic disorder; and bipolar depression, the depressed phase of bipolar disorder. Within these types are variations in the number of associated mental symptoms, and their severity and persistence.
- a person experiencing major depressive disorder suffers from, among other symptoms, a depressed mood or loss of interest in normal activities that lasts most of the day, nearly every day, for at least two weeks. Such episodes may occur only once, but more commonly occur several times in a lifetime.
- dysthymic disorder a chronic but less severe type—doesn't strike in episodes, but is instead characterized by milder, persistent symptoms that may last for years. Although it usually doesn't interfere with everyday tasks, people with this milder form of depression rarely feel like they are functioning at their full capacities.
- a person might act on delusional grand schemes that could range from unwise business decisions to romantic sprees.
- Mania left untreated may deteriorate into a psychotic state. Because the symptoms, course of illness, and response to treatment vary so much among people with depression, doctors believe that depression may have a number of complex and interacting causes.
- Some factors include another medical illness, losing a loved one, stressful life events, and drug or alcohol abuse. Any of these factors also may contribute to recurrent major depressive episodes.
- a person is clinically depressed if he or she has five or more of these symptoms and has not been functioning normally for most days during the same two-week period.
- Dysthymic disorder is diagnosed when depressed mood persists for at least two years (one year in children) and is accompanied by at least two other symptoms of depression.
- the episodes of depression that occur in people with bipolar disorder alternate with mania, which is characterized by abnormally and persistently elevated mood or irritability.
- Symptoms of mania include overly inflated self-esteem, decreased need for sleep, increased talkativeness, racing thoughts, distractibility, physical agitation, and excessive risk-taking. Because bipolar disorder requires different treatment than major depression or dysthymia, obtaining an accurate diagnosis is extremely important.
- the kind of depression that will most likely benefit from treatment with medications is a condition that's prolonged, lasting 2 weeks or more, and interferes with a person's ability to carry on daily tasks and to enjoy activities that previously brought pleasure.
- the depressed person will seem sad, or "down,” or may show a lack of interest in his surroundings. He may have trouble eating and lose weight (although some people eat more and gain weight when depressed). He may sleep too much or too little, have difficulty going to sleep, sleep restlessly, or awaken very early in the morning. He may speak of feeling guilty, worthless, or hopeless. He may complain that his thinking is slowed down. He may lack energy, feeling "everything's too much," or he might be agitated and jumpy. A person who is depressed may cry.
- a depression can range in intensity from mild to severe.
- Antidepressants are used most widely for serious depressions, but they can also be helpful for some milder depressions. Antidepressants, although they are not "uppers” or stimulants, take away or reduce the symptoms of depression and help the depressed person feel the way he did before he became depressed. Antidepressants are also used for disorders characterized principally by anxiety. They can block the symptoms of panic, including rapid heartbeat, terror, dizziness, chest pains, nausea, and breathing problems. They can also be used to treat some phobias.
- the physician chooses the particular antidepressant to prescribe based on the individual patient's symptoms.
- improvement generally will not begin to show immediately. With most of these medications, it will take from 1 to 3 weeks before changes begin to occur. Some symptoms diminish early in treatment; others, later. For instance, a person's energy level or sleeping or eating patterns may improve before his depressed mood lifts. If there is little or no change in symptoms after 5 to 6 weeks, a different medication may be tried. Some people will respond better to one than another. Since there is no certain way of determining beforehand which medication will be effective, the doctor may have to prescribe first one, then another, and an effective medication may not be found. Treatment is continued for a minimum of several months and may last up to a year or more.
- Tricyclic antidepressants are more commonly used for treatment of major depressions than are monoamine oxidase inhibitors (MAOIs); but MAOIs are often helpful in so-called “atypical” depressions in which there are symptoms like oversleeping, anxiety, panic attacks, and phobias.
- MAOIs monoamine oxidase inhibitors
- SSRIs selective serotonin reuptake inhibitors
- fluoxetine Prozac
- fluvoxamine Livox
- paroxetine Paxil
- sertraline Zoloft
- SSRIs' antidepressant effects are due to their action on one specific neurotransmitter, serotonin.
- Two other antidepressants that affect two neurotransmitters serotonin and norepinephrine have also been approved by the FDA. They are venlafaxine (Effexor) and nefazodone (Serzone).
- the tricyclic antidepressant clomipramine (Anafranil) affects serotonin but is not as selective as the SSRIs. It was the first medication specifically approved for use in the treatment of obsessive compulsive disorder (OCD). Prozac and Luvox have now been approved for use with OCD.
- OCD obsessive compulsive disorder
- Another of the newer antidepressants, bupropion (Wellbutrin) is chemically unrelated to the other antidepressants. It has more effect on norepinephrine and dopamine than on serotonin. Wellbutrin has not been associated with weight gain or sexual dysfunction.
- Tricyclic antidepressants There are a number of possible side effects with tricyclic antidepressants that vary, depending on the medication. For example, amitriptyline (Elavil) may make people feel drowsy, while protriptyline (Vivactil) hardly does this at all and, in some people, may have an opposite effect, producing feelings of anxiety and restlessness. Because of this kind of variation in side effects, one antidepressant might be effecive for one person and not recommended for another. Tricyclics may complicate specific heart problems. Other side effects with tricyclics include blurred vision, dry mouth, constipation, weight gain, dizziness when changing position, increased sweating, difficulty urinating, changes in sexual desire, decrease in sexual ability, muscle twitches, fatigue, and weakness.
- Tricyclics may also interact with thyroid hormone, antihypertensive medications, oral contraceptives, blood coagulants, sleeping medications, antipsychotic medications, diuretics, antihistamines, aspirin, bicarbonate of soda, vitamin C, alcohol, and tobacco.
- An overdose of antidepressants is serious and potentially lethal. It requires immediate medical attention. Symptoms of an overdose of tricyclic antidepressant medication develop within an hour and may start with rapid heartbeat, dilated pupils, flushed face, and agitation, and progress to confusion, loss of consciousness, seizures, irregular heart beats, cardiorespiratory collapse, and death.
- MAOIs Monoamine Oxidase Inhibitors
- MAOIs may cause some side effects similar to those of the other antidepressants. Dizziness when changing position and rapid heartbeat are common. MAOIs also react with certain foods and alcoholic beverages (such as aged cheeses, foods containing monosodium glutamate (MSG), Chianti and other red wines), and other medications (such as over-the-counter cold and allergy preparations, local anesthetics, amphetamines, insulin, some narcotics, and antiparkinsonian medications). Signs may include severe high blood pressure, headache, nausea, vomiting, rapid heartbeat, possible confusion, psychotic symptoms, seizures, stroke, and coma. For this reason, people taking MAOIs must stay away from restricted foods, drinks, and medications.
- TCAs tricyclic antidepressants
- MAOIs monoamine oxidase inhibitors
- Newer medications such as the selective serotonin reuptake inhibitors (SSRJs), have fewer side effects, but may also have sundesirable side affects, as indicated above.
- SSRJs selective serotonin reuptake inhibitors
- a non-exhaustive list of such drugs includes: Abilify, Adapin, Adderall, alprazolam, Altruline, amitriptyline, amoxapine, Anafranil, Anatensol, aripiprazole, Aropax, Aroxat, Asenden, Atarax, Atenolol, Ativan, Aurorix, Aventyl, Benadryl, Bupropion, Buspar, Buspirone, Camcolit, Canad, Carbamazepine, Celexa, Chlorpromazine, Chlordiazepoxide, Cipramil,
- Spinal cord injury Spinal cord injury or myelopathy, is a disturbance of the spinal cord that results in loss of sensation and/or mobility.
- the two common types of spinal cord injury are due to trauma and disease. Traumatic injury can be due to automobile accidents, falls, gunshot, diving accidents inter alia, and diseases which can affect the spinal cord include polio, spina bifida, tumors and
- Ischemia reperfusion (IR) injury is one of the leading causes of death in lung allograft recipients.
- An effective therapy to treat the above mentioned diseases and disorders would be of great therapeutic value.
- Brain injury such as trauma and stroke are among the leading causes of mortality and disability in the western world.
- Traumatic brain injury is one of the most serious reasons for hospital admission and disability in modern society. Clinical experience suggests that TBI may be classified into primary damage occurring immediately after injury, and secondary damage, which occurs during several days post injury.
- Current therapy of TBI is either surgical or else mainly symptomatic. Cerebrovascular diseases occur predominately in the middle and late years of life. They cause approximately 200,000 deaths in the United States each year as well as considerable neurologic disability. The incidence of stroke increases with age and affects many elderly people, a rapidly growing segment of the population. These diseases cause either ischemia-infarction or intracranial hemorrhage.
- Stroke is an acute neurologic injury occurring as a result of interrupted blood supply, resulting in an insult to the brain.
- Most cerebrovascular diseases present as the abrupt onset of focal neurologic deficit. The deficit may remain fixed, or it may improve or progressively worsen, leading usually to irreversible neuronal damage at the core of the ischemic focus, whereas neuronal dysfunction in the penumbra may be treatable and/or reversible.
- Prolonged periods of ischemia result in frank tissue necrosis. Cerebral edema follows and progresses over the subsequent 2 to 4 days. If the region of the infarction is large, the edema may produce considerable mass effect with all of its attendant consequences.
- Neuroprotective drugs are being developed in an effort to rescue neurons in the penumbra from dying, though as yet none has been proven efficacious.
- Damage to neuronal tissue can lead to severe disability and death.
- the extent of the damage is primarily affected by the location and extent of the injured tissue. Endogenous cascades activated in response to the acute insult play a role in the functional outcome. Efforts to minimize, limit and/or reverse the damage have the great potential of alleviating the clinical consequences.
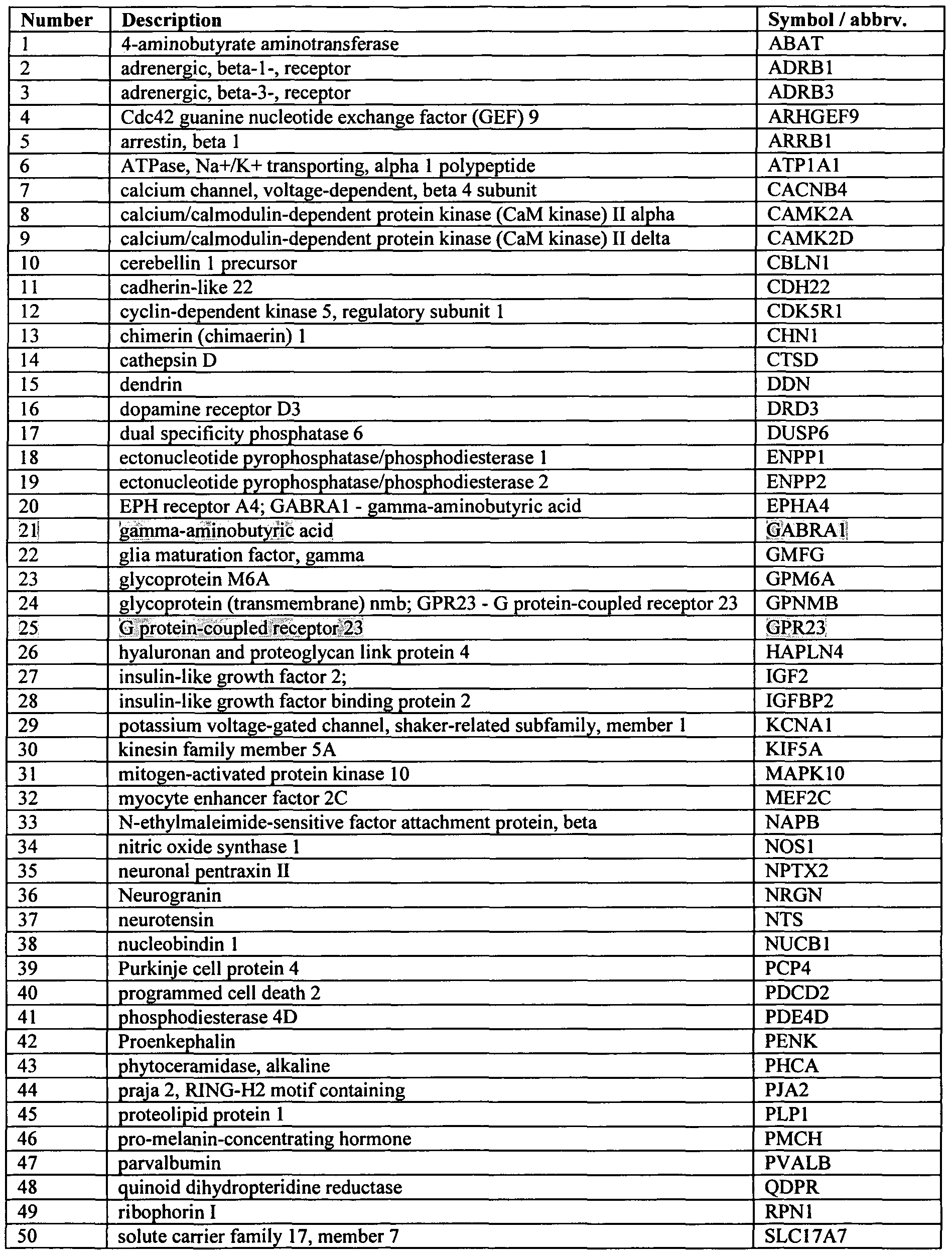
- the present invention provides, in one embodiment, novel double stranded oligonucleotides that inhibit or reduce expression of a gene selected from the group consisting of ABAT; ADRB 1 ; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl
- the present invention further relates to methods for treating or preventing the incidence or severity of various diseases or conditions, particularly mood disorders and neurological conditions, in which gene expression is associated with the etiology or progression of the disease or condition.
- the present invention provides a compound having the structure:
- each of N and N' is a nucleotide which may be modified or unmodified in its sugar residue; wherein each of (N) x and (N') y is an oligonucleotide in which each consecutive N or N' is joined to the next N or N' by a covalent bond; wherein each of x and y is an integer between 18 and 40; wherein each of Z and Z' may be present or absent, but if present is 1-5 nucleotides and is covalently attached at the 3' terminus of the strand in which it is present; and wherein the sequence of (N) x comprises an antisense sequence relative to the mRNA transcribed from a mammalian gene selected from the group consisting of ABAT; ADRBl; ADRB3;
- GPNMB GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB;
- the compound comprises a phosphodiester bond.
- the compound is blunt ended, for example wherein Z and Z' are both absent.
- the compound comprises at least one 3' overhang, wherein at least one of Z or Z' is present.
- the compound comprises one or more ribonucleotides unmodified in their sugar residues.
- the compound comprises at least one ribonucleotide modified in the sugar residue.
- the compound comprises a modification at the 2' position of the sugar residue.
- Modifications in the 2' position of the sugar residue include amino, fluoro, methoxy, alkoxy and alkyl moieties.
- the modification comprises a ribonucleotide comprising a methoxy moiety at the 2' position (2'-O- methyl; 2'-0-Me; 2'-0-CH 3 ) of the sugar residue.
- the compound comprises modified alternating ribonucleotides in one or both of the antisense and the sense strands. In preferred embodiments the compound comprises modified alternating ribonucleotides in the antisense and the sense strands. In some preferred embodiments the middle ribonucleotide of the antisense strand is not modified; e.g. ribonucleotide in position 10 in a 19-mer strand.
- the compound comprises modified ribonucleotides in alternating positions wherein the ribonucleotides at the 5' and 3' termini of the antisense strand are modified in their sugar residues, and the ribonucleotides at the 5' and 3' termini of the sense strand are unmodified in their sugar residues.
- neither the antisense nor the sense strands are phosphorylated at the 3' and 5' termini. In other embodiments either or both the antisense and the sense strands are phosphorylated at the 3' termini.
- the compound comprises an antisense sequence present in Tables A-DN.
- the present invention provides a mammalian expression vector comprising an antisense sequence present in Tables A-DN.
- the present invention provides a compound having the structure
- N and N' 19 and are fully complementary; wherein alternating ribonucleotides in the antisense and the sense strands are modified to result in a 2'-O-methyl modification in the sugar residue of the ribonucleotides; wherein the ribonucleotides at the 5' and 3' termini of the antisense strand are modified; wherein the ribonucleotides at the 5' and 3' termini of the sense strand are unmodified; wherein the antisense and the sense strands are phosphorylated or non-phosphorylated at the 3' and 5' termini; and wherein each of N and N' is selected from the group of oligomers set forth in Table A- Table
- the present invention provides a pharmaceutical composition
- a pharmaceutical composition comprising one or more compounds of the present invention, in an amount effective to inhibit human gene expression wherein the gene is selected from the group consisting of ABAT; ADRBl ; ADRB3;
- ARHGEF9 ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1;
- GPNMB GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB;
- TPTl TPTl
- UGT8 VGT8
- VIP VGT8
- a pharmaceutically acceptable carrier a pharmaceutically acceptable carrier
- the present invention relates to a method for the treatment of a subject in need of treatment for a disease or disorder associated with the expression of a gene wherein the gene is selected from ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2;
- EPHA4 ; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl;
- KIF5A KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2;
- PDE4D PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2;
- SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 and VIP comprising administering to the subject an amount of an siRNA which reduces or inhibits expression of at least one of the genes.
- the present invention provides methods and compositions useful in treating a patient suffering from mood disorders and /or neurological or ischemic conditions such as hypertension, hypertensive cerebral vascular disease, a constriction or obstruction of a blood vessel- as occurs in the case of a thrombus or embolus, angioma, blood dyscrasias, any form of compromised cardiac function including cardiac arrest or failure, systemic hypotension; and diseases such as stroke, Parkinson's disease, epilepsy, depression, ALS, Alzheimer's disease, Huntington's disease and any other disease-induced dementia (such as HIV induced dementia for example).
- mood disorders and /or neurological or ischemic conditions such as hypertension, hypertensive cerebral vascular disease, a constriction or obstruction of a blood vessel- as occurs in the case of a thrombus or embolus, angioma, blood dyscrasias, any form of compromised cardiac function including cardiac arrest or failure, systemic hypotension
- diseases such as stroke, Parkinson's disease, epilepsy, depression, ALS
- the methods of the invention comprise administering to the patient one or more inhibitory compounds which down-regulate expression of a gene selected from the group consisting of ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A
- the present invention provides improved a method for treating or preventing mood disorders and/or depression comprising administering to a subject in need thereof a therapeutically effective amount of at least one siRNA compound that inhibits expression of the mood-disorder associated genes of the present invention.
- the compositions of the invention can also be administered at a suitable interval(s) either prior to, subsequent to, or substantially concurrent with the administration of a second drug required to treat a pre-existing condition the patient is suffering from, said drug being known to cause depression, mood-swings or other neurological diseases which may be treated by inhibiting the genes described herein.
- the siRNA and the additional drug are administered separately.
- the siRNA compound that inhibits a gene of the present invention is administered locally while the depression causing drug is administered systemically.
- the siRNA compounds may be administered prior to, simultaneously with or subsequent to the additional drug.
- the present invention provides a pharmaceutical composition comprising an siRNA that inhibits depression-associated gene expression; and a pharmaceutically acceptable carrier.
- the present invention further relates to the use of compounds which down-regulate the expression of a depression-associated gene, particularly to small interfering RNAs (siRNAs), in the treatment of various diseases, conditions or disorders associated with depression-associated gene expression including depression, mood disorders and neurological diseases.
- a non- exhaustive list of conditions to be treated with the compounds of the present invention includes: ADD (attention deficit disorder), ADHD (attention deficit hyperactivity disorder), Autism, anxiety, panic, bi-polar disorder, depression, GAD (generalized anxiety disorder), OCD (obsessive compulsive disorder), PTSD (post-traumatic stress disorder), Phobias, Schizophrenia, Convulsions, Anxiety, Depression, Mania, Manic-depression, Psychosis and mood disorders. Further, use of the compounds of the present invention may also be aimed at relieveing specific symptoms associated with these diseases and conditions, such as, inet alia, hearing voices and psychosis associated with schizophrenia, or dark moods associated with depression.
- compositions of the present invention can have application in the treatment of any disease in which neuronal degeneration or damage is involved or implicated, such as, inter alia, the following conditions: hypertension, hypertensive cerebral vascular disease, a constriction or obstruction of a blood vessel- as occurs in the case of a thrombus or embolus, angioma, blood dyscrasias, any form of compromised cardiac function including cardiac arrest or failure, systemic hypotension,; and diseases such as stroke, Parkinson's disease, epilepsy, depression, ALS, Alzheimer's disease, Huntington's disease and any other disease-induced dementia (such as HIV induced dementia for example). These conditions are also referred to herein as "neurodegenerative diseases”.
- Trauma to the central nervous system such as rupture of aneurysm, cardiac arrest, cardiogenic shock, septic shock, spinal cord trauma, head trauma, traumatic brain injury (TBI), seizure, bleeding from a tumor, etc., are also referred to herein as "injury to the central nervous system” and may also be treated using the compounds and compositions of the present invention. Additional conditions to be treated by the compounds of the present invention include acute renal failure, hearing loss, acute respiratory distress syndrome, COPD, pressure sores and glaucoma.
- the present invention provides compositions and methods for alleviation or reduction of the symptoms and signs associated with mood disorders or depressive conditions.
- one embodiment of the present invention provides one or more pharmaceutical compositions comprising as an active ingredient an ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1
- An additional embodiment provides a method for treating depression in a patient who suffers from a mood altering condition, comprising administering to the patient a pharmaceutical composition in a dosage sufficient to reduce the damage.
- Yet another embodiment provides for the use of a ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2;
- An additional embodiment provides a method for identifying a chemical compound that modulates depression.
- the present invention in some of its embodiments, provides polynucleotides, polypeptides, small moleculeSjCompositions and methods for alleviation or reduction of the symptoms and signs associated with Depression, mood disorders and any illness in which depression or depressive tendencies are a factor, such as, inter alia, ADD (attention deficit disorder), ADHD (attention deficit hyperactivity disorder), Autism, anxiety, panic, bi-polar disorder, depression, GAD (generalized anxiety disorder), OCD (obsessive compulsive disorder), PTSD (post-traumatic stress disorder), Phobias, Schizophrenia, Convulsions, Anxiety, Depression, Mania, Manic- depression, Psychosis and mood disorders.
- Certain aspects of the present invention provide pharmaceutical compositions which reduce or even completely diminish depression.
- the present invention provides methods leading to functional improvement after mood disorders or depressive events. These effects are achieved by administering an agent that inhibits the biological activity of or the expression of one or more gene targets as disclosed herein.
- Transthyretin is a secreted carrier protein expressed in the choroid plexus and liver.
- Transthyretin is a plasma protein delivering retinol to tissues. Note that CSF concentrations of transthyretin are significantly lowered in the depressed patients;
- Analogues of thyrotropin-releasing hormone produced significant antidepressant effects in rodent model.
- T3 is known to cause cell death in primary neuronal cultures.
- PD2 Synthase Prostaglandin D2 Synthase (PD2 Synthase; prostaglandin-H-2 D-isomerase)
- PD2 Synthase is an enzyme localized to the rough endoplasmic reticulum, nuclear envelope, Golgi apparatus, secretory vesicles and various cytoplasmic domains, and is also secreted.
- PD2 Synthase is expressed in tissues of the blood-brain barrier, and secreted into the cerebrospinal fluid. It is also expressed in the heart, testis, epididymis and prostate, and secreted into the seminal fluid. Additionally, it is expressed in the eye and secreted into the aqueous humor.
- PD2 Synthase catalyzes the conversion of PGH2 to PGD2, a prostaglandin involved in smooth muscle contraction/relaxation and a potent inhibitor of platelet aggregation. It is involved in sedation, NREM sleep and PGE2-induced allodynia, and may have an anti-apoptotic role in oligodendrocytes.
- PD2 Synthase may be involved in development and maintenance of the blood- brain, blood-retina, blood-aqueous humor and blood-testis barrier, and likely plays a role in both maturation and maintenance of the central nervous system and male reproductive system. PD2 Synthase expression is induced by thyroid hormone.
- transgenic mice that overexpress human PGDS gene show changed pattern of NREM sleepand NREM sleep cycle is changed in depressed patients.
- PTGDS catalyses the synthesis of PGD2, which binds to the PTDGD receptor and stimulates cAMP cascade, which can play a role in pathophysiology of depression.
- HMG-CoA 3-hydroxy-3-methylglutaryl CoA reductase
- saturated and unsaturated fatty acids such as Myristic acid, stearic, oleic, linoleic, and linolenic Arachidonic acid
- Adrenergic Receptor beta 3 (Adrb3)
- Adrb3 is an integral membrane receptor localized mainly in adipose tissues. Adrb3 mediates the catecholamine- induced activation of adenylate cyclase through the action of G proteins, and is involved in the regulation of lipolysis and thermogenesis.
- Phosphodiesterase 4D PDE4D
- Phosphodiesterase 4D is a cAMP-specific phosphodiesterase enzyme associated with carotid and cardiogenic stroke. Note that pharmacological inhibition of this enzyme produces antidepressant-like effects in animals.
- PDE4D-/- mice exhibited decreased immobility in tail-suspension and forced-swim tests; Chronic fluoxetine treatment decreased expression of PDE4D in some brain regions.
- GABA Gamma-Aminobutyric acid
- GABRP Gamma-Aminobutyric acid
- GABA A Receptor, pi subunit
- GABA levels are decreased in animal models of depression and in mood disorder patients.
- Citalopram increases pregnanolone sensitivity in patients with premenstrual dysphoric disorder . Diminished GABA(A) Receptor-Binding capacity and a DNA base substitution were found in a patient with treatment-resistant depression.
- BDNF brain-derived neurotrophic factor
- PCMH is a pro-hormone neuromodulator expressed in the Lateral hypothalamus, neocortex, palladium, cerebellum, thymus, brown adipose tissue, duodenum and testis. It is differentially processed in the brain and peripheral organs producing two neuropeptides: NEI and MCH.
- MCH acts as a neurotransmitter or neuromodulator in regulation of goal-directed behavior, such as food intake, and general arousal.
- MCHl-R MCHl receptor
- Dusp ⁇ is an enzyme localized in the cell cytoplasm, ambiguously expressed with highest levels in heart and pancreas. Dusp ⁇ inactivates MAP kinases by dephosphorylating both the phosphoserine/threonine and phosphotyrosine residues. Further, Dusp ⁇ has a certain specificity for the ERK family.
- ERX activation by neurothropic factors such as BDNF has a known positive effect on cell survival and neuroplasticity, therefore down regulation of the DUSP-6 may increase survival pathways and be cytoprotective
- Camk2a/b is an enzyme localized in the cell cytoplasm and expressed in the brain and skeletal muscle.
- Camk2a/b functions in long-term potentiation and neurotransmitter release, possesses Calcium- calmodulin (CaM)-dependent activity, and undergoes autophosphorylation, resulting in CaM- independent activity.
- CaMKII alpha mRNA expression is significantly reduced in the prefrontal cortex of patients with bipolar illness
- VIP Vasoactive Intestinal Polypeptide
- VIP is a secreted hormone expressed in the brain, prostate and lung. VIP stimulates myocardial contractility, causes vasodilation, increases glycogenosis, lowers arterial blood pressure and relaxes the smooth muscles of trachea, stomach and gall bladder. VIP appears to play a role in the temporal organization of sleep.
- VIP levels in non-endogenously depressed patients are significantly lower than those of controls and endogenous depressives.
- VIP injection elicited rapid eye movement sleep.
- Atplal is an integral membrane enzyme transporter, functioning as a catalytic component of the active enzyme (ATPase).
- Atplal catalyzes the hydrolysis of ATP coupled with the exchange of sodium and potassium ions across the plasma membrane and creates the energy for active transport of various nutrients. Mutations in Atplal are related to hypertension.
- MEF2C Myocyte Enhancer Factor 2C
- MEF2C is a transcription factor, localized to the nucleus. MEF2C is involved in cardiac myogenesis, morphogenesis and neurogenesis, regulates the expression of genes that are critical for newly differentiated neurons, and is activated by p38. p38alpha/MEF2 pathway prevents cell death during neuronal differentiation.
- Cdk5r is an enzyme localization either at the cell periphery or cytoplasm dependent on the cellular state, expressed in the brain and neuron specific.
- Cdk5r is a neuron-specific activator of cyclin-dependent kinase 5 (CDK5); the activation of CDK5
- CDK5 is required for proper development of the central nervous system, for neurite outgrowth and cortical lamination.
- the p35 form of Cdk5r is proteolytically cleaved generating a p25 form.
- cleavage of p35 into p25 results in relocalization of the protein from the cell periphery to nuclear and perinuclear regions. P25 deregulates CDK5 activity by prolonging its activation and changing its cellular location. Additionally, the p25 form accumulates in the brain neurons of patients with Alzheimer's disease.
- Nitric Oxide Synthase 1 neuronal
- NOSl is a ubiquitously expressed enzyme which produces nitric oxide (NO) which is a messenger molecule. In the brain and peripheral nervous system, NO displays many properties of a neurotransmitter. NOS 1 is stimulated by calcium/calmodulin. Note that NOS activities were increased in serum of CMS rats.
- the number of NOS-immunoreactive neurons in hypothalamus was significantly reduced in depression.
- Neurogranin is a brain-specific, post-synaptically located protein kinase C (PKC) substrate. It is also a Ca(2+)-sensitive calmodulin (CaM)-binding protein, the CaM-binding affinity being modulated by phosphorylation and oxidation. Neurogranin is expressed in the brain cortex, hippocampus, striatum, and amygdala. Chimerin 1
- Chimerinl is an enzyme, a GTPase activating protein for p21-rac and a phorbol ester receptor, which may play an important role in neuronal signal- transduction mechanisms. Chimerinl is expressed in neurons of brain regions that are involved in learning and memory, and increases in amount during brain development coincident with synaptogenesis.
- Dendrin is induced by sleep deprivation and predicted to serve as a transcription factor. It is expressed exclusively in forebrain structures, with a near exclusive dendritic localization.
- Glia Maturation Factor gamma Glia Maturation Factor gamma
- GMF is a predicted intracellular enzyme, expressed in the lung, heart and placenta.
- Phosphorylated GMF is a potent inhibitor of ERK1/ERK2 subfamily of mitogen-activated protein (MAP) kinase and a strong enhancer of p38 MAP kinase activity in vitro.
- MAP mitogen-activated protein
- ERK Activation of ERK by neurotrophic factors such as BDNF has a known positive effect on cell survival and neuroplasticity, protecting cells from chronic stress damage.
- GMF by inhibiting ERK and promoting p38 MAPK activation may facilitate neuronal cell-apoptosis
- Cacnb4 is an integral membrane channel which contributes to the function of the calcium channel by: increasing peak calcium current, shifting the voltage dependencies of activation and inactivation, modulating G protein inhibition and controlling the alpha- 1 subunit membrane targeting.
- Defects in cacnb4 are a cause of idiopathic generalized epilepsy (IGE), which is characterized by recurrent generalized seizures.
- Defects in cacnb4 are also a cause of juvenile myoclonic epilepsy (JME), which is a common epileptic syndrome characterized by afebrile seizures, onset in adolescence (rather than in childhood) and myoclonic jerks.
- IGE idiopathic generalized epilepsy
- JME juvenile myoclonic epilepsy
- Solute Carrier Family 17 (Sodium-Dependent Inorganic Phosphate Cotransporter).
- Member 7 SLC17A7
- SLC17A7 is a vesicle-bound, sodium-dependent integral membrane phosphate transporter, localized to protein synaptic vesicles, and expressed specifically in neuron-rich regions of the Brain.
- SLCl 7 A7 also functions in glutamate transport.
- Solute Carrier Family 8 (Sodium/Calcium Exchanger), Member 1 (SLC8A1)
- SLC8A1 is an integral membrane transporter which rapidly transports Ca(2+) during excitation- contraction coupling.
- Drd3 Dopamine Receptor D3 (Drd3) Drd3 is an integral membrane receptor expressed in the brain, involved in synaptic transmission.
- Drd3 Inhibits adenylyl cyclase through inhibitory G-proteins, may play a role in cognitive and emotional functions, and serves as a target for drugs which treat schizophrenia and Parkinson disease.
- Polymorphisms in this gene are connected to various normal and pathological behavioral paradigms; additionally, the allele of D2 subtype is related to the response to paroxetine treatment
- MAPKKKK5 Mitogen-Activated Protein Kinase Kinase Kinase Kinase Kinase 5
- MAPKKKK5 is an intracellular enzyme, a member of the MAP kinase family. Further, it is a neuronal-specific form of c-Jun N-terminal kinases (JNKs), and may play a role in the response to environmental stress. It also appears to act upstream of the c-jun N-terminal pathway.
- ARHGAP6 Rho GTPase activating protein 6
- GAP cytoskeletal GTPase-activating protein
- ARHGAP6 undergoes X inactivation and mutations in ARHGAP6 may be a cause of microphthalmia with linear skin defects (MLS) - a dominant male- lethal disorder characterized by eye, skin and central nervous system (CNS) malformations.
- MLS linear skin defects
- CNS central nervous system
- an inhibitor preferably a nucleotide inhibitor, to one or more of the following genes can prevent or ameliorate a depressive condition in a patient: ABAT - 4-aminobutyrate aminotransferase; ADRBl - adrenergic, beta-1-, receptor; ADRB3 - adrenergic, beta-3-, receptor; ARHGEF9 - Cdc42 guanine nucleotide exchange factor (GEF) 9; ARRBl - arrestin, beta 1; ATPlAl - ATPase, Na+/K+ transporting, alpha 1 polypeptide; CACNB4 - calcium channel, voltage-dependent, beta 4 subunit; CAMK2A - calcium/calmodulin-dependent protein kinase (CaM kinase) II alpha; CAMK2D - calcium/calmodulin-dependent protein kinase (CaM kinase) II delta; CBLNl
- inhibitor any molecule, whether a polynucleotide, polypeptide, antibody, or small chemical compound, that prevents or reduces the biological effect of ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR
- the present invention relates generally to compounds which down-regulate expression of genes, particularly to novel small interfering RNAs (siRNAs), and to the use of these siRNAs in the treatment of various diseases and medical conditions.
- diseases and conditions to be treated are depression, mood disorders and neurological diseases.
- Particular genes to be inhibited in order to treat said diseases and conditions are presented in Tables 1-3; the most preferable genes are presented in Table 3.
- Lists of preferred siRNA are provided in Tables A-DN infra. The separate lists of 19-mer and 21- mer siRNAs are prioritized based on their score according to a proprietary algorithm as the best sequences for targeting the human gene expression.
- Lists of 19- and 21-mer sense and corresponding antisense sequences useful in preparation of siRNA compounds are set forth in Tables A-DN.
- a list of preferred siRNA to ABAT is provided in tables A-B, infra.
- a list of preferred siRNA to ADRB 1 is provided in tables C-D, infra.
- a list of preferred siRNA to ADRB3 is provided in tables E-F, infra.
- a list of preferred siRNA to ARHGEF9 is provided in tables G-H, infra.
- a list of preferred siRNA to ARRBl is provided in tables I- J, infra.
- a list of preferred siRNA to ATPlAl is provided in tables K-L, infra.
- a list of preferred siRNA to CACNB4 is provided in tables M-N, infra.
- a list of preferred siRNA to CAMK2A is provided in tables O-P, infra.
- a list of preferred siRNA to CAMK2D is provided in tables Q-R, infra.
- a list of preferred siRNA to CBLNl is provided in tables S-T, infra.
- a list of preferred siRNA to CDH22 is provided in tables U-V, infra.
- a list of preferred siRNA to CDK5R1 is provided in tables W-X, infra.
- a list of preferred siRNA to CHNl is provided in tables Y-Z, infra.
- a list of preferred siRNA to CTSD is provided in tables AA-AB, infra.
- a list of preferred siRNA to DDN is provided in tables AC-AD, infra.
- a list of preferred siRNA to DRD3 is provided in tables AE-AF, infra.
- a list of preferred siRNA to DUSP6 is provided in tables AG-AH, infra.
- a list of preferred siRNA to ENPPl is provided in tables AI-AJ, infra.
- a list of preferred siRNA to ENPP2 is provided in tables AK-AL, infra.
- a list of preferred siRNA to EPHA4 is provided in tables AM-AN, infra.
- a list of preferred siRNA to GABRAl is provided in tables AO-AP, infra.
- a list of preferred siRNA to GMFG is provided in tables AQ-AR, infra.
- a list of preferred siRNA to GPM6A is provided in tables AS-AT, infra.
- a list of preferred siRNA to GPNMB is provided in tables AU-AV, infra.
- a list of preferred siRNA to GPR23 is provided in tables AW-AX, infra.
- a list of preferred siRNA to HAPLN4 is provided in tables AY-AZ, infra.
- a list of preferred siRNA to IGF2 is provided in tables BA-BB, infra.
- a list of preferred siRNA to IGFBP2 is provided in tables BC-BD, infra.
- a list of preferred siRNA to KCNAl is provided in tables BE-BF, infra.
- a list of preferred siRNA to KIF5A is provided in tables BG-BH, infra.
- a list of preferred siRNA to MAPKlO is provided in tables BI-BJ, infra.
- a list of preferred siRNA to MEF2C is provided in tables BK-BL, infra.
- a list of preferred siRNA to NAPB is provided in tables BM-BN, infra.
- a list of preferred siRNA to NOSl is provided in tables BO-BP, infra.
- a list of preferred siRNA to NPTX2 is provided in tables BQ-BR, infra.
- a list of preferred siRNA to NRGN is provided in tables BS-BT, infra.
- a list of preferred siRNA to NTS is provided in tables BU-BV, infra.
- a list of preferred siRNA to NUCB 1 is provided in tables BW-BX, infra.
- a list of preferred siRNA to PCP4 is provided in tables BY-BZ, infra.
- a list of preferred siRNA to PDCD2 is provided in tables CA-CB, infra.
- a list of preferred siRNA to PDE4D is provided in tables CC-CD, infra.
- a list of preferred siRNA to PENK is provided in tables CE-CF, infra.
- a list of preferred siRNA to PHCA is provided in tables CG-CH, infra.
- a list of preferred siRNA to PJA2 is provided in tables CI-CJ, infra.
- a list of preferred siRNA to PLPl is provided in tables CK-CL, infra.
- a list of preferred siRNA to PMCH is provided in tables CM-CN, infra.
- a list of preferred siRNA to PVALB is provided in tables CO-CP, infra.
- a list of preferred siRNA to QDPR is provided in tables CQ-CR, infra.
- a list of preferred siRNA to RPNl is provided in tables CS-CT, infra.
- a list of preferred siRNA to SLC17A7 is provided in tables CU-CV, infra.
- a list of preferred siRNA to SLC28A2 is provided in tables CW-CX, infra.
- a list of preferred siRNA to SLC8A1 is provided in tables CY-CZ, infra.
- a list of preferred siRNA to SNAP91 is provided in tables DA-DB, infra.
- a list of preferred siRNA to SYN2 is provided in tables DC-DD, infra.
- a list of preferred siRNA to SYTl is provided in tables DE-DF, infra.
- a list of preferred siRNA to TKT is provided in tables DG-DH, infra.
- a list of preferred siRNA to TPTl is provided in tables DI-DJ, infra.
- a list of preferred siRNA to UGT8 is provided in tables DK-DL, infra.
- an “inhibitor” is a compound which is capable of inhibiting or reducing the expression or activity of a gene or the product of such gene to an extent sufficient to achieve a desired biological or physiological effect.
- the term “inhibitor” as used herein refers to one or more of an oligonucleotide inhibitor, including siRNA, shRNA, aptamers, antisense molecules, miRNA and ribozymes, as well as antibodies.
- polypeptide refers to, in addition to a polypeptide, an oligopeptide, peptide and a full protein.
- the present invention provides compounds that inhibit one or more isoforms of a gene, in the event that more than one isoforms exits.
- RNA interference and siRNA RNA interference is based on the ability of dsRNA species to enter a cytoplasmic protein complex, where it is then targeted to the complementary cellular RNA and specifically degrade it.
- the RNA interference response features an endonuclease complex containing an siRNA, commonly referred to as an RNA-induced silencing complex (RISC), which mediates cleavage of single-stranded RNA having a sequence complementary to the antisense strand of the siRNA duplex. Cleavage of the target RNA may take place in the middle of the region complementary to the antisense strand of the siRNA duplex (Elbashir et al., Genes Dev., 2001, 15(2): 188-200).
- RISC RNA-induced silencing complex
- dsRNAs are digested into short (17-29 bp) dsRNA fragments (also referred to as short inhibitory RNAs, "siRNAs") by type III RNAses (DICER, DROSHA, etc.; Bernstein et al., Nature, 2001, 409(6818):363-6; Lee et al., Nature, 2003, 425(6956):415-9).
- the RISC protein complex recognizes these fragments and complementary mRNA. The whole process is culminated by endonuclease cleavage of target mRNA (McManus & Sharp, Nature Rev Genet, 2002, 3(10):737-47; Paddison & Hannon, Curr Opin MoI Ther.
- nucleic acid refers to polynucleotides such as deoxyribonucleic acid (DNA), and, where appropriate, ribonucleic acid (RNA).
- DNA deoxyribonucleic acid
- RNA ribonucleic acid
- the terms should also be understood to include, as equivalents, analogs of either RNA or DNA made from nucleotide analogs, such as chemically modified nucleotides and synthetic nucleotides and, as applicable to the embodiment being described, double-stranded polynucleotides and single-stranded polynucleotides such as sense or antisense.
- Oligonucleotide refers to a sequence having from about 2 to about 50 linked nucleotides or linked modified nucleotides, or a combination of modified and unmodified nucleotide. Oligonucleotide includes the terms oligomer, antisense strand and sense strand. "Nucleotide” is meant to encompass deoxyribonucleotides and ribonucleotides, which may be natural or synthetic, and or modified or unmodified. Modifications include changes to the sugar moiety, the base moiety and or the linkages between ribonucleotides in the oligoribonucleotide.
- nucleotides can be selected from naturally occurring or synthetic modified bases.
- Naturally occurring bases include adenine, guanine, cytosine, thymine and uracil.
- Modified bases of nucleotides include inosine, xanthine, hypoxanthine, 2- aminoadenine, 6-methyl, 2-propyl and other alkyl adenines, 5-halo uracil, 5- halo cytosine, 6-aza cytosine and 6-aza thymine, psuedo uracil, 4- thiuracil, 8-halo adenine, 8- aminoadenine, 8-thiol adenine, 8-thiolalkyl adenines, 8-hydroxyl adenine and other 8-substituted adenines, 8-halo guanines, 8-amino guanine, 8-thiol guanine, 8-thioalkyl guanines, 8- hydroxyl guanine and other substituted guanines, other aza and deaza adenines, other aza and deaza guanines, 5-trifluoromethyl uracil and 5- tri
- analogues of polynucleotides can be prepared wherein the structure of one or more nucleotide is fundamentally altered and better suited as therapeutic or experimental reagents.
- An example of a nucleotide analogue is a peptide nucleic acid (PNA) wherein the deoxyribose (or ribose) phosphate backbone in DNA (or RNA is replaced with a polyamide backbone which is similar to that found in peptides.
- PNA analogues have been shown to be resistant to enzymatic degradation and to have extended lives in vivo and in vitro.
- oligonucleotides include polymer backbones, cyclic backbones, acyclic backbones, thiophosphate-D-ribose backbones, triester backbones, thioate backbones, 5'-2' bridged backbone, artificial nucleic acids, morpholino nucleic acids, locked nucleic acid (LNA), glycol nucleic acid (GNA), threose nucleic acid (TNA), arabinoside, and mirror nucleoside (for example, beta-L-deoxynucIeoside instead of beta-D-deoxynucleoside).
- LNA locked nucleic acid
- GNA glycol nucleic acid
- TAA threose nucleic acid
- arabinoside arabinoside
- mirror nucleoside for example, beta-L-deoxynucIeoside instead of beta-D-deoxynucleoside.
- siRNA compounds comprising LNA nucleotides
- a "mirror" nucleotide is a nucleotide with reversed chirality to the naturally occurring or commonly employed nucleotide, i.e., a mirror image (L-nucleotide) of the naturally occurring (D- nucleotide).
- the nucleotide can be a ribonucleotide or a deoxyribonucleotide and my further comprise at least one sugar, base and or backbone modification.
- US patent No. 6,602,858 discloses nucleic acid catalysts comprising at least one L-nucleotides substitution.
- the present invention provides methods and compositions for inhibiting expression of a depression associated gene in vivo.
- the method includes administering oligoribonucleotides, in particular small interfering RNAs (i.e., siRNAs) or a nucleic acid material that can produce siRNA in a cell, that targets an mRNA, in an amount sufficient to down-regulate expression of a target gene by an RNA interference mechanism.
- siRNAs small interfering RNAs
- the subject method can be used to inhibit expression of depression associated gene for treatment of a disease.
- the siRNA compounds or other inhibitors are used as drugs to treat various pathologies.
- the present invention provides double-stranded oligoribonucleotides (eg. siRNAs), which down- regulate the expression of a gene selected from the group consisting of ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR;
- siRNA of the invention is a duplex oligoribonucleotide in which the sense strand is derived from the mRNA sequence of said genes, and the antisense strand is complementary to the sense strand. In general, some deviation from the target mRNA sequence is tolerated without compromising the siRNA activity (see e.g. Czauderna et al., Nuc. Acids Res. 2003, 31(11):2705- 2716).
- An siRNA of the invention inhibits gene expression on a post-transcriptional level with or without destroying the mRNA. Without being bound by theory, siRNA may target the mRNA for specific cleavage and degradation and/ or may inhibit translation from the targeted message.
- the oligoribonucleotide according to the present invention comprises modified siRNA.
- the siRNA comprises an RNA duplex comprising a first strand and a second strand, whereby the first strand comprises a ribonucleotide sequence at least partially complementary to about 18 to about 40 consecutive nucleotides of a target nucleic acid, and the second strand comprises ribonucleotide sequence at least partially complementary to the first strand and wherein said first strand and/or said second strand comprises a plurality of groups of modified ribonucleotides having a modification at the 2'-position of the sugar moiety whereby within each strand each group of modified ribonucleotides is flanked on one or both sides by a group of flanking ribonucleotides whereby each ribonucleotide forming the group of flanking ribonucleotides is selected from an unmodified ribonucleotide or a ribonucle
- the group of modified ribonucleotides and/or the group of flanking ribonucleotides comprise a number of ribonucleotides selected from the group consisting of an integer from 1 to 10. Accordingly, the group thus comprises one nucleotide, two nucleotides, three nucleotides, four nucleotides, five nucleotides, six nucleotides, seven nucleotides, eight nucleotides, nine nucleotides or ten nucleotides.
- the groups of modified nucleotides and flanking nucleotides may be organized in a pattern on at least one of the strands.
- the first and second strands comprise a pattern of modified nucleotides.
- the pattern of modified nucleotides of said first strand is identical relative to the pattern of modified nucleotides of the second strand.
- the pattern of modified nucleotides of said first strand is shifted by one or more nucleotides relative to the pattern of modified nucleotides of the second strand.
- the middle ribonucleotide in the first strand is an unmodified nucleotide.
- ribonucleotide number 10 is unmodified; in a 21 -oligomer antisense strand, ribonucleotide number 11 is unmodified; and in a 23-oligomer antisense strand, ribonucleotide number 12 is unmodified.
- the modifications or pattern of modification, if any, of the siRNA must be planned to allow for this.
- the modifications on the 2' moiety of the sugar residue include amino, fluoro, methoxy alkoxy, alkyl, amino, fluoro, chloro, bromo, CN, CF, imidazole, caboxylate, thioate, Ci to Cio lower alkyl, substituted lower alkyl, alkaryl or aralkyl, OCF 3 , OCN, O-, S-, or N- alkyl; O-, S, or N- alkenyl; SOCH 3 ; SO 2 CH 3 ; ONO 2 ; NO 2 , N 3 ; heterozycloalkyl; heterozycloalkaryl; aminoalkylamino; polyalkylamino or substituted silyl, as described, inter alia, in European patents EP 0 586 520 B 1 and EP 0 618 925 B 1.
- the siRNA is blunt ended, on one or both ends. More specifically, the siRNA may be blunt ended on the end defined by the 5'- terminus of the first strand and the 3'- terminus of the second strand, or the end defined by the 3'-terminus of the first strand and the 5'- terminus of the second strand. In other embodiments at least one of the two strands may have an overhang of at least one nucleotide at the 5'-terminus; the overhang may consist of at least one deoxyribonucleotide. At least one of the strands may also optionally have an overhang of at least one nucleotide at the 3'- terminus. The overhang may consist of from about 1 to about 4 nucleotides.
- RNA duplex is from about 18 to about 40 ribonucleotides, preferably 19 to 23 ribonucleotides. Further, the length of each strand (oligomer) may independently have a length selected from the group consisting of about 15 to about 40 bases, preferably 18 to 23 bases and more preferably 19, 20 or 21 ribonucleotides.
- the complementarity between said first strand and the target nucleic acid can be perfect.
- the strands are substantially complementary, i.e. having one, two or up to three mismatches between said first strand and the target nucleic acid.
- the first strand and the second strand each comprise at least one group of modified ribonucleotides and at least one group of flanking ribonucleotides, whereby each group of modified ribonucleotides comprises at least one ribonucleotide and whereby each group of flanking ribonucleotides comprises at least one ribonucleotide, wherein each group of modified ribonucleotides of the first strand is aligned with a group of flanking ribonucleotides on the second strand, and wherein the 5' most terminal ribonucleotide is selected from a group of modified ribonucleotides, and the 3' most terminal ribonucleotide of the second strand is a selected from the group of flanking ribonucleotide.
- each group of modified ribonucleotides consists of a single ribonucleotide and each group of flanking ribon
- the ribonucleotide forming the group of flanking ribonucleotides on the first strand is an unmodified ribonucleotide arranged in a 3' direction relative to the ribonucleotide forming the group of modified ribonucleotides
- the ribonucleotide forming the group of modified ribonucleotides on the second strand is a modified ribonucleotide which is arranged in 5' direction relative to the ribonucleotide forming the group of flanking ribonucleotides.
- the first strand of the siRNA comprises five to about twenty, eight to twelve, preferably ten or twelve groups of modified ribonucleotides, and the second strand comprises seven to eleven, preferably nine or eleven groups of modified ribonucleotides.
- the first strand and the second strand may be linked by a loop structure, which may be comprised of a non-nucleic acid polymer such as, inter alia, polyethylene glycol.
- the loop structure may be comprised of a nucleic acid, including modified and non- modified ribonucleotides and modified and non-modified deoxyribonucleotides.
- the 5'-terminus of the first strand of the siRNA may be linked to the 3'-terminus of the second strand, or the 3'-terminus of the first strand may be linked to the 5'-terminus of the second strand, said linkage being via a nucleic acid linker typically having a length between 3-100 nucleotides, preferably about 3 to about 10 nucleotides.
- the present invention provides a compound having structure A:
- each N and N' is a ribonucleotide selected from the group consisting of a modified ribonucleotide or an unmodified ribonucleotide and each of (N) x and (N') y is an oligomer in which each consecutive N or N' is joined to the next N or N' by a covalent bond;
- each of x and y is an integer between 18 and 40;
- each of Z and Z' may be present or absent, but if present is comprises 1-5 nucleotides and is covalently attached at the 3' terminus of the strand in which it is present;
- sequence of (N) x comprises an antisense sequence having substantial identity to about 18 to about 40 consecutive ribonucleotides in the mRNA transcribed from a gene selected from the group consisting of ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PM
- the compounds of the present invention consist of a plurality of modified and/or unmodified ribonucleotides, which are linked through covalent linkages.
- Each such covalent linkage may be a phosphodiester linkage, a phosphorothioate linkage, or a combination of both, along the length of the ribonucleotide sequence of the individual strand.
- Other possible backbone modifications are described inter alia in U.S. Patent Nos. 5,587,361; 6,242,589; 6,277,967; 6,326,358; 5,399,676; 5,489,677; and 5,596,086.
- x and y are independently an integer between about 18 to about 40, preferably from about 19 to about 23.
- the compound is blunt ended, for example wherein Z and Z' are both absent.
- the compound comprises at least one 3' overhang, wherein at least one of Z or Z' is present.
- Z and Z' can independently comprise one or more covalently linked modified or non-modified nucleotides, as described herein, for example inverted dT or dA; dT, LNA, mirror nucleotide and the like.
- each of Z and Z' are independently selected from dT and dTdT.
- all of the ribonucleotides of the compound are unmodified in their sugar residues.
- at least one ribonucleotide is modified in its sugar residue, preferably by the addition of a moiety at the 2' position.
- a preferred moiety is selected from the group consisting of amino, fluoro, methoxy, alkoxy and alkyl groups.
- the moiety at the 2' position is methoxy (2'-0-Me).
- alternating ribonucleotides are modified in both the antisense and the sense strands of the compound.
- the exemplified siRNA has been modified such that a 2'-O-methyl (Me) group was present on the first, third, fifth, seventh, ninth, eleventh, thirteenth, fifteenth, seventeenth and nineteenth nucleotide of the antisense strand, whereby the very same modification, i. e. a 2'-0-Me group, was present at the second, fourth, sixth, eighth, tenth, twelfth, fourteenth, sixteenth and eighteenth nucleotide of the sense strand.
- these particular siRNA compounds are also blunt ended.
- the ribonucleotides at the 5' and 3' termini of the antisense strand are modified in their sugar residues, and the ribonucleotides at the 5' and 3' termini of the sense strand are unmodified in their sugar residues.
- the ribonucleotides at the 5' and 3' termini of the sense strand are modified in their sugar residues, and the ribonucleotides at the 5' and 3' termini of the antisense strand are unmodified in their sugar residues.
- it is preferred that the middle nucleotide of the antisense strand is unmodified.
- the antisense and the sense strands of the siRNA are phosphorylated only at the 3 '-terminus and not at the 5 '-terminus.
- the antisense and the sense strands are non- phosphorylated.
- the 5' most ribonucleotide in the sense strand is modified, for example to abolish any possibility of in vivo 5'- phosphorylation.
- the invention further provides a vector capable of expressing any of the aforementioned oligoribonucleotides in unmodified form in a cell after which appropriate modification may be made.
- the cell is a mammalian cell, preferably a human cell.
- Substantially complementary refers to complementarity of greater than about 84%, to another sequence.
- one mismatch results in 94.7% complementarity
- two mismatches results in about 89.5% complementarity
- 3 mismatches results in about 84.2% complementarity, rendering the duplex region substantially complementary.
- substantially identical refers to identity of greater than about 84%, to another sequence.
- the invention provides an antisense oligoribonucleotide wherein one strand comprises consecutive nucleotides having, from 5' to 3', the sequence set forth in Tables A-DN or a homolog thereof wherein in up to two of the ribonucleotides in each terminal region is altered.
- the terminal region of the oligoribonucleotide refers to bases 1-4 and/or 16-19 in the 19-mer sequence and to bases 1-4 and/or 18-21 in the 21-mer sequence.
- the invention provides sense oligoribonucleotides wherein one strand comprises consecutive nucleotides having, from 5' to 3', the sequence set forth in Tables A-DN or a homolog thereof wherein in up to two of the ribonucleotides in each terminal region is altered.
- Tables A-DN provide 19- and 21-mer oligomers useful in the preparation of siRNA compounds targeted against ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; S
- the siRNA is either phosphorylated at 3' termini of both sense and anti-sense strands, or non-phosphorylated at all; or having the 5' most ribonucleotide on the sense strand specifically modified to abolish any possibility of in vivo 5 '-phosphorylation.
- the alternating ribonucleotides are modified at the 2' position of the sugar residue in both the antisense and the sense strands, wherein the moiety at the 2' position is methoxy (2'-O-methyl) and wherein the ribonucleotides at the 5' and 3' termini of the antisense strand are modified in their sugar residues, and the ribonucleotides at the 5' and 3' termini of the sense strand are unmodified in their sugar residues.
- Additional nucleic acids according to the present invention comprise at least 14 contiguous nucleotides of any one of the polynucleotides in Tables A-DN and more preferably 14 contiguous nucleotide base pairs at any end of the double-stranded structure. It will be understood by one skilled in the art that given the potential length of the nucleic acid according to the present invention and particularly of the individual stretches forming such nucleic acid according to the present invention, some shifts relative to the coding sequence of the mammalian genes of the present invention to each side is possible, whereby such shifts can be up to 1, 2, 3, 4, 5 and 6 nucleotides in both directions, and whereby the thus generated double-stranded nucleic acid molecules shall also be within the present invention.
- the compounds of the present invention can be synthesized by any of the methods that are well- known in the art for synthesis of ribonucleic (or deoxyribonucleic) oligonucleotides. Such synthesis is, among others, described in Beaucage and Iyer Tetrahedron 1992; 48: 2223-2311, Beaucage and Iyer, Tetrahedron 1993; 49: 6123-6194 and Caruthers et. al., Methods Enzymol. 1987; 154: 287-313; the synthesis of thioates is, among others, described in Eckstein, Annu. Rev. Biochem.
- oligonucleotides of the present invention can be synthesized separately and joined together post-synthetically, for example, by ligation (Moore et al., 1992, Science 256, 9923; Draper et al., International PCT publication No. WO 93/23569; Shabarova et al., 1991, NAR 19, 4247; Bellon et al., 1997, Nucleosides & Nucleotides, 16, 951; Bellon et al., 1997, Bioconjugate Chem. 8, 204), or by hybridization following synthesis and/or deprotection.
- oligonucleotides are prepared according to the sequences disclosed herein. Overlapping pairs of chemically synthesized fragments can be ligated using methods well known in the art (e.g., see US Patent No. 6,121,426). The strands are synthesized separately and then are annealed to each other in the tube. Then, the double-stranded siRNAs are separated from the single-stranded oligonucleotides that were not annealed (e.g. because of the excess of one of them) by HPLC.
- siRNAs or siRNA fragments of the present invention two or more such sequences can be synthesized and linked together for use in the present invention.
- the compounds of the invention can also be synthesized via a tandem synthesis methodology, as described in US patent application publication No. 2004/0019001 wherein both siRNA strands are synthesized as a single contiguous oligonucleotide fragment or strand separated by a cleavable linker which is subsequently cleaved to provide separate siRNA fragments or strands that hybridize and permit purification of the siRNA duplex.
- the linker can be a polynucleotide linker or a non-nucleotide linker.
- the present invention provides a pharmaceutical composition comprising one or more of the compounds of the invention; and a pharmaceutically acceptable carrier.
- This composition may comprise a mixture of two or more different siRNA compounds.
- the invention further provides a pharmaceutical composition
- a pharmaceutical composition comprising at least one compound of the invention covalently or non-covalently bound to one or more compounds of the invention in an amount effective to inhibit the mammalian depression-associated genes; and a pharmaceutically acceptable carrier.
- the compound may be processed intracellularly by endogenous cellular complexes to produce one or more oligoribonucleotides of the invention.
- the invention further provides a pharmaceutical composition
- a pharmaceutical composition comprising a pharmaceutically acceptable carrier and one or more of the compounds of the invention in an amount effective to down-regulate expression in a cell of a mammalian gene of the present invention, the compound comprising a sequence substantially complementary to the sequence of (N) x
- the subject being treated is a warm-blooded animal and, in particular, mammals including human.
- treatment refers to administration of a therapeutic substance to a subject in need thereof in an amount effective to ameliorate symptoms associated with a disease, to lessen the severity or cure the disease, or to prevent the disease from occurring.
- the invention provides a method of down-regulating the expression of a mammalian gene selected from the group consisting of ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl;
- ATPlAl ATPlAl
- CACNB4 CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23;
- HAPLN4 IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 and VIP by at least 50% as compared to a control comprising contacting a mRNA transcript selected from the group consisting of ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6;
- the compound of the present invention down-regulates one of the mammalian genes selected from the group consisting of ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2
- the compound is down-regulating a mammalian polypeptide, whereby the down-regulation is selected from the group comprising down-regulation of function (which may be examined by an enzymatic assay or a binding assay with a known interactor of the native gene / polypeptide, inter alia), down-regulation of protein (which may be examined by Western blotting, ELISA or immuno-precipitation, inter alia) and down-regulation of mRNA expression (which may be examined by Northern blotting, quantitative RT-PCR, in-situ hybridisation or microarray hybridisation, inter alia
- the down-regulation is selected from the group comprising down-regulation of function (which may be examined by an enzymatic assay or a binding assay with a known interactor of the native gene / polypeptide, inter alia), down-regulation of protein (which may be examined by Western blotting, ELISA or immuno-precipitation, inter alia) and down-regulation of mRNA expression (which may be examined by Northern blotting
- the invention provides a method of treating a patient suffering from a disease accompanied by an elevated level of a mammalian depression associated gene disclosed herein, the method comprising administering to the patient a compound or composition of the invention in a therapeutically effective dose thereby treating the patient.
- the present invention relates to the use of compounds which down-regulate the expression of a mammalian depression associated gene particularly to novel small interfering RNAs (siRNAs), in the treatment of the following diseases or conditions in which inhibition of the expression of the mammalian depression associated gene is beneficial: depression, mood disorders and neurological disorders, such as, inter alia, ADD (attention deficit disorder), ADHD (attention deficit hyperactivity disorder), Autism, anxiety, panic, bi-polar disorder, depression, GAD (generalized anxiety disorder), OCD (obsessive compulsive disorder), PTSD (post-traumatic stress disorder), Phobias, Schizophrenia, Convulsions, Anxiety, Depression, Mania, Manic- depression, Psychosis and mood disorders.
- ADD attention deficit disorder
- ADHD attention deficit hyperactivity disorder
- Autism anxiety, panic, bi-polar disorder
- depression generalized anxiety disorder
- OCD obsessive compulsive disorder
- PTSD post-traumatic stress disorder
- Phobias Schizophrenia
- Treatment refers to both therapeutic treatment and prophylactic or preventative measures, wherein the object is to prevent or slow down (lessen) a mood disorder as listed above.
- Those in need of treatment include those already experiencing the disease or condition, those prone to having the disease or condition, and those in which the disease or condition is to be prevented.
- the compounds of the invention may be administered before, during or subsequent to the onset of the disease or condition.
- the method of the invention includes administering a therapeutically effective amount of one or more compounds which down-regulate expression of a depression associated gene, particularly the novel siRNAs of the present invention, small molecule inhibitors of a depression associated gene or protein or antibodies to depression associated proteins.
- exposure to a toxic agent is meant that the toxic agent is made available to, or comes into contact with, a mammal.
- a toxic agent can be toxic to one or more organs in the body, for example, the ear, kidney, nervous system, liver and the like.
- Exposure to a toxic agent can occur by direct administration, e.g., by ingestion or administration of a food, medicinal, or therapeutic agent, e.g., a chemotherapeutic agent, by accidental contamination, or by environmental exposure, e g., aerial or aqueous exposure.
- the compounds and methods of the invention are useful for treating or preventing the incidence or severity of other diseases and conditions in a patient.
- diseases and conditions include stroke and stroke-like situations (e.g. cerebral, renal, cardiac failure), neuronal cell death, brain injuries with or without reperfusion, chronic degenerative diseases e.g. neurodegenerative disease including Alzheimer's disease, Huntington's disease, Parkinson's disease, multiple sclerosis, amyotrophic lateral sclerosis, spinobulbar atrophy, prion disease, and apoptosis resulting from traumatic brain injury (TBI).
- TBI traumatic brain injury
- the compounds and methods of the invention are directed to providing neuroprotection, cerebroprotection, or to prevent and/or treat cytotoxic T cell and natural killer cell-mediated apoptosis associated with autoimmune disease and transplant rejection, or to prevent cell death of cardiac cells including heart failure, cardiomyopathy, viral infection or bacterial infection of the heart, myocardial ischemia, myocardial infarct, and myocardial ischemia, coronary artery by- pass graft, or to prevent and/or treat mitochondrial drug toxicity e. g. as a result of chemotherapy or HIV therapy, to prevent cell death during viral infection or bacterial infection, or to prevent and/or treat inflammation or inflammatory diseases, inflammatory bowel disease, sepsis and septic shock.
- cytotoxic T cell and natural killer cell-mediated apoptosis associated with autoimmune disease and transplant rejection or to prevent cell death of cardiac cells including heart failure, cardiomyopathy, viral infection or bacterial infection of the heart, myocardial ischemia, myocardial infar
- Additional conditions which may be treated using the compounds of the present invention include hearing loss, acute renal failure, nephritis, glaucoma, Acute Respiratory Distress Syndrome and other acute lung injuries, lung transplantation, spinal cord injury, pressure sores, osteoarthritis and Chronic Obstructive Pulmonary Disease (COPD).
- COPD Chronic Obstructive Pulmonary Disease
- follicle to ovocyte stages for example, methods of freezing and transplanting ovarian tissue, artificial fertilization
- sperm for example, methods of freezing and transplanting ovarian tissue, artificial fertilization
- to preserve fertility in mammals after chemotherapy in particular human mammals, or to prevent and/or treat, macular degeneration, or to prevent and/or treat acute hepatitis, chronic active hepatitis, hepatitis-B, and hepatitis-C, or to prevent hair loss, (e.g.
- hair loss due-to male- pattern baldness, or hair loss due to radiation, chemotherapy or emotional stress or to treat or ameliorate skin damage whereby the skin damage may be due to exposure to high levels of radiation, heat, chemicals, sun, or to burns and autoimmune diseases), or to prevent cell death of bone marrow cells in myelodysplastic syndromes (MDS), or to treat pancreatisis, or to treat, rheumatoid arthritis, psoriasis, glomerulonephritis, atheroscerosis, and graft versus host disease (GVHD), or to treat retinal pericyte apoptosis, retinal damages resulting from ischemia, diabetic retinopathy, or to treat any disease states associated with expression of a depression associated gene, wherein the gene is selected from ABAT; ADRBl ; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22
- the present invention also provides for a process of preparing a pharmaceutical composition, which comprises: providing one or more double stranded compound of the invention ; and admixing said compound with a pharmaceutically acceptable carrier.
- the compound used in the preparation of a pharmaceutical composition is admixed with a carrier in a pharmaceutically effective dose.
- the compound of the present invention is conjugated to a steroid or to a lipid or to another suitable molecule e.g. to cholesterol.
- siRNA molecules of the present invention may be delivered to the target tissue by direct application of the naked molecules prepared with a carrier or a diluent.
- naked siRNA refers to siRNA molecules that are free from any delivery vehicle that acts to assist, promote or facilitate entry into the cell, including viral sequences, viral particles, liposome formulations, lipofectin or precipitating agents and the like.
- PBS is "naked siRNA”.
- siRNA molecules of the invention are delivered in liposome formulations and lipofectin formulations and the like and can be prepared by methods well known to those skilled in the art. Such methods are described, for example, in U.S. Pat. Nos.
- siRNA has recently been successfully used for inhibition of gene expression in primates; (for details see for example,
- Pharmaceutically acceptable carriers, solvents, diluents, excipients, adjuvants and vehicles as well as implant carriers generally refer to inert, non-toxic solid or liquid fillers, diluents or encapsulating material not reacting with the active ingredients of the invention and they include liposomes and microspheres.
- delivery systems useful in the present invention include U.S. Patent Nos. 5,225,182; 5,169,383; 5,167,616; 4,959,217; 4,925,678; 4,487,603; 4,486,194; 4,447,233; 4,447,224; 4,439,196; and 4,475,196. Many other such implants, delivery systems, and modules are well known to those skilled in the art.
- topical and transdermal formulations may be selected.
- the siRNAs or pharmaceutical compositions of the present invention are administered and dosed in accordance with good medical practice, taking into account the clinical condition of the individual patient, the disease to be treated, the site and method of administration, scheduling of administration, patient age, sex, body weight and other factors known to medical practitioners.
- a "therapeutically effective dose” for purposes herein is thus determined by such considerations as are known in the art.
- the dose must be effective to achieve improvement including but not limited to improved survival rate or more rapid recovery, or improvement or elimination of symptoms and other indicators as are selected as appropriate measures by those skilled in the art.
- the active dose of compound for humans is in the range of from lng/kg to about 20- 100 mg/kg body weight per day, preferably about 0.01 mg to about 2-10 mg/kg body weight per day, in a regimen of one dose per day or twice or three or more times per day for a period of 1-4 weeks or longer.
- the compounds of the present invention can be administered by any of the conventional routes of administration. It should be noted that the compound can be administered as the compound or as pharmaceutically acceptable salt and can be administered alone or as an active ingredient in combination with pharmaceutically acceptable carriers, solvents, diluents, excipients, adjuvants and vehicles.
- the compounds can be administered orally, subcutaneously or parenterally including intravenous, intraarterial, intramuscular, intraperitoneally, and intranasal administration as well as intrathecal and infusion techniques. Implants of the compounds are also useful.
- Liquid forms may be prepared for injection, the term including subcutaneous, transdermal, intravenous, intramuscular, intrathecal, and other parental routes of administration.
- the liquid compositions include aqueous solutions, with and without organic co-solvents, aqueous or oil suspensions, emulsions with edible oils, as well as similar pharmaceutical vehicles.
- the administration comprises intravenous administration.
- the administration comprises topical or local administration
- compositions for use in the novel treatments of the present invention may be formed as aerosols, for example for intranasal administration.
- Delivery of inhibitors into the brain can be accomplished by several methods such as, inter alia, neurosurgical implants, blood-brain barrier disruption, lipid mediated transport, carrier mediated influx or efflux, plasma protein-mediated transport, receptor-mediated transcytosis, absorptive-mediated transcytosis, neuropeptide transport at the blood-brain barrier, and genetically engineering "Trojan horses” for drug targeting.
- neurosurgical implants blood-brain barrier disruption, lipid mediated transport, carrier mediated influx or efflux, plasma protein-mediated transport, receptor-mediated transcytosis, absorptive-mediated transcytosis, neuropeptide transport at the blood-brain barrier, and genetically engineering "Trojan horses” for drug targeting.
- the above methods are performed for example as described in "Brain Drug Targeting: the future of brain drug development", W.M. Pardridge, Cambridge University Press, Cambridge, UK (2001).
- the present invention further provides for a pharmaceutical composition
- a pharmaceutical composition comprising two or more siRNA molecules for the treatment of any of the diseases and conditions mentioned herein, whereby said two molecules may be physically mixed together in the pharmaceutical composition in amounts which generate equal or otherwise beneficial activity, or may be covalently or non-covalently bound, or joined together by a nucleic acid linker of a length ranging from 2-100, preferably 2-50 or 2-30 nucleotides.
- the siRNA molecules are comprised of a double-stranded nucleic acid structure as described herein, wherein the two siRNA sequences are selected from Tables A-DN.
- the siRNA molecules are covalently or non-covalently bound or joined by a linker to form a tandem siRNA molecule.
- tandem siRNA molecules comprising two siRNA sequences are typically of 38-150 nucleotides in length, more preferably 38 or 40-60 nucleotides in length, and longer accordingly if more than two siRNA sequences are included in the tandem molecule.
- a longer tandem molecule comprised of two or more longer sequences which encode siRNA produced via internal cellular processing, e.g., long dsRNAs, is also envisaged, as is a tandem molecule encoding two or more shRNAs.
- tandem molecules are also considered to be a part of the present invention.
- siRNA compounds that target any one of the genes disclosed herein may be the main active component in a pharmaceutical composition, or may be one active component of a pharmaceutical composition containing two or more siRNAs (or molecules which encode or endogenously produce two or more siRNAs, be it a mixture of molecules or one or more tandem molecules which encode two or more siRNAs), said pharmaceutical composition further being comprised of one or more additional siRNA molecule which targets one or more additional gene. Simultaneous inhibition of said additional gene(s) will likely have an additive or synergistic effect for treatment of the diseases disclosed herein.
- siRNA compounds disclosed herein or any nucleic acid molecule comprising or encoding such siRNA can be linked or bound (covalently or non-covalently) to antibodies (including aptamer molecules) against cell surface internalizable molecules expressed on the target cells, in order to achieve enhanced targeting for treatment of the diseases disclosed herein.
- anti-Fas antibody preferably a neutralizing antibody
- an aptamer which can act like a ligand/antibody may be combined (covalently or non-covalently) with any siRNA to a gene disclosed herein.
- the compounds of the present invention can be delivered either directly or with viral or non-viral vectors.
- sequences When delivered directly the sequences are generally rendered nuclease resistant. Alternatively the sequences can be incorporated into expression cassettes or constructs such that the sequence is expressed in the cell as discussed herein below. Generally the construct contains the proper regulatory sequence or promoter to allow the sequence to be expressed in the targeted cell.
- Vectors optionally used for delivery of the compounds of the present invention are commercially available, and may be modified for the purpose of delivery of the compounds of the present invention by methods known to one of skill in the art.
- a long oligonucleotide (typically 25-500 nucleotides in length) comprising one or more stem and loop structures, where stem regions comprise the sequences of the oligonucleotides of the invention, may be delivered in a carrier, preferably a pharmaceutically acceptable carrier, and may be processed intracellular ⁇ by endogenous cellular complexes (e.g. by DROSHA and DICER as described above) to produce one or more smaller double stranded oligonucleotides (siRNAs) which are oligonucleotides of the invention.
- This oligonucleotide can be termed a tandem shRNA construct.
- this long oligonucleotide is a single stranded oligonucleotide comprising one or more stem and loop structures, wherein each stem region comprises a sense and corresponding antisense siRNA sequence of the genes of the invention.
- this oligonucleotide comprises sense and antisense siRNA sequences as depicted in Tables A-DN.
- inhibitors contemplated to be used in the methods of the invention to inhibit expression of a gene and to treat the diseases and conditions described herein are inter alia antibodies, preferably neutralizing antibodies or fragments thereof, including single chain antibodies, antisense oligonucleotides, antisense DNA or RNA molecules, ribozymes, proteins, polypeptides and peptides including peptido-mimetics and dominant negatives, and also expression vectors expressing all the above.
- Additional inhibitors may be small chemical molecules, which generally have a molecular weight of less than 2000 daltons, preferably less than 1000 daltons, more preferably less than 500 daltons.
- inhibitors may act as follows: small molecules may affect expression and/or activity; antibodies may affect activity; all kinds of antisense may affect gene expression; and dominant negative polypeptides and peptidomimetics may affect activity; expression vectors may be used inter alia for delivery of antisense or dominant-negative polypeptides or antibodies.
- antibody refers to IgG, IgM, IgD, IgA, and IgE antibody, inter alia.
- the definition includes polyclonal antibodies or monoclonal antibodies. This term refers to whole antibodies or fragments of antibodies comprising an antigen-binding domain, e.g. antibodies without the Fc portion, single chain antibodies, miniantibodies, fragments consisting of essentially only the variable, antigen-binding domain of the antibody, etc.
- antibody may also refer to antibodies against polynucleotide sequences obtained by cDNA vaccination.
- the term also encompasses antibody fragments which retain the ability to selectively bind with their antigen or receptor and are exemplified as follows, inter alia: (1) Fab, the fragment which contains a monovalent antigen-binding fragment of an antibody molecule which can be produced by digestion of whole antibody with the enzyme papain to yield a light chain and a portion of the heavy chain;
- F(ab' 2 ) is a dimer of two Fab fragments held together by two disulfide bonds;
- Fv defined as a genetically engineered fragment containing the variable region of the light chain and the variable region of the heavy chain expressed as two chains
- Single chain antibody defined as a genetically engineered molecule containing the variable region of the light chain and the variable region of the heavy chain linked by a suitable polypeptide linker as a genetically fused single chain molecule.
- AS antisense
- antisense fragment a polynucleotide fragment
- An AS polynucleotide is a polynucleotide which comprises consecutive nucleotides having a sequence of sufficient length and homology to a sequence present within the sequence of the target gene to permit hybridization of the AS to the gene.
- Antisense intervention in the expression of specific genes can be achieved by the use of modified AS oligonucleotide sequences (for recent reports see Lefebvre- d'Hellencourt et al, 1995; Agrawal, 1996; LevLehman et al, 1997).
- AS oligonucleotide sequences may be short sequences of DNA, typically 15-30 mer but may be as small as 7-mer (Wagner et al, Nat. Biotech. 1996, 14(7):840-4), designed to complement a target mRNA of interest and form an RNA:AS duplex. This duplex formation can prevent processing, splicing, transport or translation of the relevant mRNA. Moreover, certain AS nucleotide sequences can elicit cellular RNase H activity when hybridized with their target mRNA, resulting in mRNA degradation (Calabretta et al, Semin Oncol. 1996, 23(l):78-87).
- RNase H will cleave the RNA component of the duplex and can potentially release the AS to further hybridize with additional molecules of the target RNA.
- An additional mode of action results from the interaction of AS with genomic DNA to form a triple helix, which can be transcriptionally inactive.
- the sequence target segment for the antisense oligonucleotide is selected such that the sequence exhibits suitable energy related characteristics important for oligonucleotide duplex formation with their complementary templates, and shows a low potential for self-dimerization or self- complementation (Anazodo et al., 1996, Biochem. Biophys. Res. Comm. 229:305-309).
- the computer program OLIGO (Primer Analysis Software, Version 3.4), can be used to determine antisense sequence melting temperature, free energy properties, and to estimate potential self-dimer formation and self-complimentary properties.
- the program allows the determination of a qualitative estimation of these two parameters (potential self-dimer formation and self- complimentary) and provides an indication of "no potential” or "some potential” or “essentially complete potential”.
- target segments are generally selected that have estimates of no potential in these parameters. However, segments can be used that have "some potential” in one of the categories. A balance of the parameters is used in the selection as is known in the art.
- the oligonucleotides are also selected as needed so that analog substitution does not substantially affect function. Phosphorothioate antisense oligonucleotides do not normally show significant toxicity at concentrations that are effective and exhibit sufficient pharmacodynamic half-lives in animals (Agrawal, et al., PNAS U S A.
- bFGF basic fibroblast growth factor
- a "ribozyme” is an RNA molecule that possesses RNA catalytic ability (see Cech for review) and cleaves a specific site in a target RNA.
- ribozymes which cleave mRNA may be utilized as inhibitors. This may be necessary in cases where antisense therapy is limited by stoichiometric considerations (Sarver et al., 1990, Gene Regulation and Aids, pp. 305-325). Ribozymes can then be used that will target the a gene associated with a bone marrow disease.
- the number of RNA molecules that are cleaved by a ribozyme is greater than the number predicted by stochiochemistry. (Hampel and Tritz, Biochem. 1989, 28(12):4929-33; Uhlenbeck, Nature. 1987 328(6131):596-600).
- Ribozymes catalyze the phosphodiester bond cleavage of RNA.
- ribozyme structural families include Group I introns, RNase P, the hepatitis delta virus ribozyme, hammerhead ribozymes and the hairpin ribozyme originally derived from the negative strand of the tobacco ringspot virus satellite RNA (sTRSV) (US Patent No. 5,225,347).
- the latter two families are derived from viroids and virusoids, in which the ribozyme is believed to separate monomers from oligomers created during rolling circle replication (Symons, 1989 and 1992).
- ribozyme motifs are most commonly adapted for trans-cleavage of mRNAs for gene therapy (Sullivan, 1994).
- the ribozyme has a length of from about 30-100 nucleotides. Delivery of ribozymes is similar to that of AS fragments and/or siRNA molecules.
- Some of the compounds and compositions of the present invention may be used in a screening assay for identifying and isolating compounds that modulate the activity of a gene, in particular compounds that modulate a disorder accompanied by an elevated level of said gene.
- the compounds to be screened comprise inter alia substances such as small chemical molecules and antisense oligonucleotides.
- the inhibitory activity of the compounds of the present invention on a gene or binding of the compounds of the present invention to a gene or mRNA may be used to determine the interaction of a test compound with the sequence e.g., if the test compound competes with the oligonucleotides of the present invention for inhibition of a gene, or if the additional compound rescues said inhibition.
- the inhibition or activation can be tested by various means, such as, inter alia, assaying for the product of the activity of a polypeptide or displacement of binding compound from the a polypeptide in radioactive or fluorescent competition assays.
- the present invention is illustrated in detail below with reference to Examples, but is not to be construed as being limited thereto.
- One aspect of the present invention provides for a pharmaceutical composition
- a pharmaceutical composition comprising as an active ingredient an inhibitor to TTR, PD2 Synthase, Adrb3, PDE4D, GABRP, PMCH, Dusp ⁇ , Camk2a/2b, VIP, Atplal, MEF2C, Cdk5r, NOSl, Neurogranin, Chimerinl, Dendrin, GMF, Cacnb4, SLC17A7, SLC8A1, Drd3, MAPKKKK5, ARHGAP6, CLU, BAMBI, PTGER3, CLSTN2, STARD7, DKK3, CART, SCARA3, NRP2, MGC42105, NRGl, NELL2, CD276, CD81, NTRK2, AKAP5, EFNB3, CNP, ENPP2, SMPD2, CKB, SLC6A15, SLC6A20, SLC22A17, SLC6A17, SLC6A9, TRPC6, CLCN3, EPHA4, MAPKl, PTPRR, TAC
- Another aspect of the present invention concerns a method for treating a patient suffering from a depression or mood disorder, comprising administering to the patient a therapeutically effective amount of an inhibitor to TTR, PD2 Synthase, Adrb3, PDE4D, GABRP, PMCH, Dusp ⁇ , Camk2a/2b, VIP, Atplal, MEF2C, Cdk5r, NOSl, Neurogranin, Chimerinl, Dendrin, GMF, Cacnb4, SLC17A7, SLC8A1, Drd3, MAPKKKK5, ARHGAP6, CLU, BAMBI, PTGER3, CLSTN2, STARD7, DKK3, CART, SCARA3, NRP2, MGC42105, NRGl, NELL2, CD276, CD81, NTRK2, AKAP5, EFNB3, CNP, ENPP2, SMPD2, CKB, SLC6A15, SLC6A20, SLC22A17, SLC6A17, SLC6A9, TRPC6, CLCN
- the inhibitor may be a small chemical compound; a polynucleotide, such as an antisense polynucleotide, or a polynucleotide which is a sense polynucleotide and which encodes a dominant negative peptide, or a polynucleotide that functions as silencing RNA (siRNA), such as an siRNA present in any one of Tables A-DN; a vector comprising any of these polynucleotides; a polypeptide, such as a dominant negative peptide, or an antibody, optionally a polyclonal or a monoclonal antibody.
- siRNA silencing RNA
- the present invention provides a method of regulating a pathology or disease (as recited above) in a patient in need of such treatment by administering to a patient a therapeutically effective dose of at least one inhibitor e.g. at least one antisense (AS) oligonucleotide or at least one siRNA against the nucleic acid sequences or a dominant negative peptide directed against any of the genes described herein, or an antibody directed against a polypeptide encoded by any of the genes described herein, or any of the inhibitors described above.
- AS antisense
- chemical compound small molecule
- chemical molecule small chemical molecule
- small chemical compound refers to chemical moieties of any particular type which may be synthetically produced or obtained from natural sources and typically have a molecular weight of less than 2000 daltons, more preferably less than 1000 daltons or even less than 600 daltons.
- the invention also provides a method of treating a patient suffering from a mood disorder or depression, comprising administering to the patient a composition of the invention in a therapeutically effective dose so as to thereby treat the patient.
- the invention also provides a use of a therapeutically effective dose of one or more compounds of the invention for the preparation of a composition for promoting recovery in a patient suffering from a mood disorder or depression.
- “Mood disorder” includes major depressive disorder; dysthymic disorder; bipolar depression; post-partum depression; and any disorder which causes a subject to be diagnosed as depressed by a clinician, including depression which may be associated with ADD (attention deficit disorder), ADHD (attention deficit hyperactivity disorder), Autism, anxiety, panic, bi-polar disorder, depression, GAD (generalized anxiety disorder), OCD (obsessive compulsive disorder), PTSD (post-traumatic stress disorder), Phobias, Schizophrenia, Convulsions, Anxiety, Mania, Manic- depression and Psychosis, it is also the object of the present invention to treat mental illnesses such as ADD (attention deficit disorder), ADHD (attention deficit hyperactivity disorder), Autism, anxiety, panic, bi-polar disorder, depression, GAD (generalized anxiety disorder), OCD (obsessive compulsive disorder), PTSD (post-traumatic stress disorder), Phobias, Schizophrenia, Convulsions, Anxiety, Depression, Mania, Manic-depression, Psychosis
- siRNA for any of the genes presented in Tables 1-3 can be made using methods known in the art as described herein, based on the known sequence of any of the genes presented in Tables 1-3 and can be made stable by various modifications as described above. For further information, see Example 3.
- additional inhibitory RNA molecules of the present invention which may be used with the methods of the present invention include single stranded oligoribonucleotides preferably comprising stretches of at least 7-14 consecutive nucleotides present in the sequences of any one of the genes detailed in
- oligoribonucleotides being capable of forming and/or said oligoribonucleotides comprising double stranded regions in particular conformations that are recognized by intracellular complexes, leading to the degradation of said oligoribonucleotides into smaller RNA molecules that are capable of exerting inhibition of any of said genes / polypeptides, and DNA molecules encoding such RNA molecules.
- any molecules such as, for example, antisense DNA molecules which comprise the siRNA sequences disclosed herein (with the appropriate nucleic acid modifications) are particularly desirable and may be used in the same capacity as their corresponding siRNAs for all uses and methods disclosed herein.
- any of the siRNA molecules disclosed herein, or any long double-stranded RNA molecules (typically 25-500 nucleotides in length) which are processed by endogenous cellular complexes (such as DICER - see above) to form the siRNA molecules disclosed herein, or molecules which comprise the siRNA molecules disclosed herein, can be employed in the treatment of any disease or disorder.
- the present invention provides a method of treating a patient suffering from a disease or disorder, such as nerodegenerative disorders or Central nervous system disorders, inter alia., comprising administering to the patient a pharmaceutical composition comprising one or more of the siRNAs disclosed herein (or one or more long dsRNA which encodes one or more of said siRNAs, as described above) in a therapeutically effective amount so as to thereby treat the patient.
- a disease or disorder such as nerodegenerative disorders or Central nervous system disorders, inter alia.
- Expression vector refers to vectors that have the ability to incorporate and express heterologous DNA fragments in a foreign cell. Many prokaryotic and eukaryotic expression vectors are known and/or commercially available. Selection of appropriate expression vectors is within the knowledge of those having skill in the art.
- Polypeptide is meant a molecule composed of amino acids and the term includes peptides, polypeptides, proteins and peptidomimetics.
- a peptidomimetic is a compound containing non-peptidic structural elements that is capable of mimicking the biological action(s) of a natural parent peptide. Some of the classical peptide characteristics such as enzymatically scissille peptidic bonds are normally not present in a peptidomimetic.
- amino acid refers to a molecule which consists of any one of the 20 naturally occurring amino acids, amino acids which have been chemically modified (see below), or synthetic amino acids.
- dominant negative peptide refers to a polypeptide encoded by a cDNA fragment that encodes for a part of a protein which can interact with the full protein and inhibit its activity or which can interact with other proteins and inhibit their activity in response to the full protein.
- epitopic determinants an antigenic determinant on an antigen to which the antibody binds.
- Epitopic determinants usually consist of chemically active surface groupings of molecules such as amino acids or sugar side chains and usually have specific three dimensional structural characteristics, as well as specific charge characteristics.
- any one of these pharmaceutical compositions is used for alleviation or reduction of the symptoms and signs associated with any type of depression mood disorder.
- This embodiment concerns a method or process for promoting recovery in a patient who has suffered or suffers from a mood disorder, comprising administering to the patient any one of the pharmaceutical compositions recited above, in a dosage and over a period of time sufficient to reduce the damage or promote recovery.
- This embodiment further provides a method or process for treating a patient who has suffered or suffers from a mood disorder, optionally as a result of any of the conditions described herein, comprising administering to the patient a pharmaceutical composition comprising a therapeutically effective amount of an inhibitor to ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PH
- an additional pharmaceutically effective compound is administered in conjunction with the aforementioned pharmaceutical composition.
- One embodiment of the claimed invention provides for using a therapeutically effective amount of an inhibitor to ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SN
- the inhibitor may be a small chemical compound; a polynucleotide, such as an antisense polynucleotide comprising consecutive nucleotides having a sequence which is an antisense sequence to the sequence of any of the genes disclosed in Tables 1-3, or a polynucleotide which is a sense polynucleotide comprising consecutive nucleotides having a sequence which is a sense sequence to the sequence of any of the genes disclosed in Tables 1-3, and which encodes a dominant negative peptide to said sequence, or a polynucleotide that functions as silencing RNA (siRNA); a vector comprising any of these polynucleotides; a polypeptide, such as a dominant negative peptide, or an antibody, optionally a polyclonal or a monoclonal antibody.
- the pharmaceutical composition may further contain a diluent or carrier.
- the treatment regimen according to the invention is carried out, in terms of administration mode, timing of the administration, and dosage, so that the functional recovery of the patient from the adverse consequences of the mood disorder is improved.
- Administration of a pharmaceutical composition comprising any one of the inhibitors according to the invention can be carried out by any known route of administration, including intravenously, intra-arterially, subcutaneously, intraperitoneally or intracerebrally. Using specialized formulations, it may also be possible to administer these orally or via inhalation. Suitable doses and treatment regimens for administering compositions to an individual in need thereof are discussed in detail below.
- An additional embodiment of the present invention concerns methods and processes for obtaining a species and/or chemical compound that modulates the biological activity of TTR, PD2 Synthase, Adrb3, PDE4D, GABRP, PMCH, Dusp ⁇ , Camk2a/2b, VIP, Atplal, MEF2C, Cdk5r, NOSl, Neurogranin, Chimerinl, Dendrin, GMF, Cacnb4, SLC17A7, SLC8A1, Drd3, MAPKKKK5, ARHGAP6 CLU, BAMBI, PTGER3, CLSTN2, STARD7, DKK3, CART, SCARA3, NRP2, MGC42105, NRGl, NELL2, CD276, CD81, NTRK2, AKAP5, EFNB3, CNP, ENPP2, SMPD2, CKB, SLC6A15, SLC6A20, SLC22A17, SLC6A17, SLC6A9, TRPC6, CL
- One aspect of this embodiment provides a process for obtaining a species and/or chemical compound that modulates the biological activity of any of the genes disclosed in Table 1, optionally present in Tables 2 or 3, which comprises contacting a cell expressing any one of said genes with a species and/or compound and determining the ability of the species and/or compound to modulate the biological activity of said gene, as compared to a control.
- the cell being examined may be modified to express said gene.
- this process may be used in order to prepare a pharmaceutical composition.
- the process then comprises admixing a species or compound obtained by the process recited above or a chemical analog or homolog thereof with a pharmaceutically acceptable carrier.
- cells being "modified to express" as used herein is meant that cells are modified by transfection, transduction, infection or any other known molecular biology method which will cause the cells to express the desired gene. Materials and protocols for carrying out such methods are evident to the skilled artisan.
- An additional aspect of the screening embodiment provides a process of screening a plurality of species or compounds to obtain a species and/or compound that modulates the biological activity of any one of the genes of Table 1, which comprises:
- the cells in the contacting step may be modified to express the polypeptide if said gene.
- this process may be used in order to prepare a pharmaceutical composition.
- the process then comprises admixing a species or compound identified by the process recited above or a chemical analog or homolog thereof with a pharmaceutically acceptable carrier.
- the process may additionally comprise modification of a species or compound found by the above process to produce a compound with improved activity and admixing such compound with a pharmaceutically acceptable carrier.
- This additional act may be performed with a compound discovered by any of the processes which are disclosed in the screening embodiment of the present invention, so as to thereby obtain a pharmaceutical composition comprising a compound with improved activity.
- the screening embodiment of the present invention provides a non cell-based process for obtaining a species or compound which modulates the biological activity of any one of the genes of Table 1, comprising:
- the present invention further provides a method or process for diagnosing depression or a mood disorder in a subject comprising detecting modulation of the expression level of any one of the genes of Table 1, preferably present in Table 2 or 3, for example: by detecting the polypeptide expressed by any one of said genes in an immunoassay, or by detecting an mRNA encoding said gene in the subject, as compared to a control.
- the expression level of the polypeptide can be assessed by assaying for mRNA encoding the any one of the above polypeptides, or by method of an immunoassay using antibodies which detect the polypeptide. Both detection of mRNA and immunoassays can be performed by methods well known in the art. Measurement of level of the desired polypeptide is determined by a method selected from the group consisting of immunohistochemistry (Microscopy, Immunohistochemistry and Antigen Retrieval Methods: For Light and Electron Microscopy, M.A.
- Measurement of level of a desired polynucleotide is determined by a method selected from: RT- PCR analysis, in-situ hybridization ("Introduction to Fluorescence In Situ Hybridization: Principles and Clinical Applications", Andreeff & Pinkel (Editors), John Wiley & Sons Inc., 1999), polynucleotide microarray and Northern blotting (Trayhurn, “Northern blotting”, Proc Nutr Soc 1996; 55(1B): 583-9; Shifman & Stein, "A reliable and sensitive method for nonradioactive Northern blot analysis of nerve growth factor mRNA from brain tissues", Journal of Neuroscience Methods 1995; 59: 205-208).
- This diagnostic method may be useful, inter alia, for diagnosing patients suspected to be suffering from a mood disorder.
- abnormal in the context of protein expression, is meant a difference of at least 10% in the expression levels of the polypeptide as compared to a control.
- the invention provides a method or process of treating depression in a subject which comprises administering to the subject a therapeutically effective amount of a pharmaceutical composition which inhibits the biological activity of any one of the genes detailed in Table 2.
- the invention further provides for the use of a modulator of any one of the genes present in Table 2 in the preparation of a medicament; said medicament may be used for the treatment of a depression.
- nuclease resistance is provided by any method known in the art that does not interfere with biological activity of the AS polynucleotide, siRNA, cDNA and/or ribozymes as needed for the method of use and delivery (Iyer et al., 1990; Eckstein, 1985; Spitzer and Eckstein, 1988; Woolf et al., 1990; Shaw et al., 1991).
- Modifications that can be made to oligonucleotides in order to enhance nuclease resistance include modifying the phophorous or oxygen heteroatom in the phosphate backbone. These include preparing methyl phosphonates, phosphorothioates, phosphorodithioates and morpholino oligomers. In one embodiment it is provided by having phosphorothioate bonds linking between the four to six 3'-terminus nucleotide bases. Alternatively, phosphorothioate bonds link all the nucleotide bases. Other modifications known in the art may be used where the biological activity is retained, but the stability to nucleases is substantially increased.
- nucleotides can be selected from naturally occurring or synthetic modified bases.
- Naturally occurring bases include adenine, guanine, cytosine, thymine and uracil.
- Modified bases of nucleotides include inosine, xanthine, hypoxanthine, 2- aminoadenine, 6- methyl, 2-propyl and other alkyl adenines, 5-halo uracil, 5-halo cytosine, 6-aza cytosine and 6- aza thymine, psuedo uracil, 4- thiuracil, 8-halo adenine, 8-aminoadenine, 8-thiol adenine, 8- thiolalkyl adenines, 8-hydroxyl adenine and other 8-substituted adenines, 8-halo guanines, 8- amino guanine, 8-thiol guanine, 8-thioalkyl guanines, 8- hydroxyl guanine and other substituted guanines, other aza and deaza adenines, other aza and deaza guanines, 5-trifluoromethyl uracil and
- polypeptides employed in the present invention may also be modified, optionally chemically modified, in order to improve their therapeutic activity.
- "Chemically modified" when referring to the polypeptides, means a polypeptide where at least one of its amino acid residues is modified either by natural processes, such as processing or other post-translational modifications, or by chemical modification techniques which are well known in the art.
- modifications typical, but not exclusive examples include: acetylation, acylation, amidation, ADP-ribosylation, glycosylation, GPI anchor formation, covalent attachment of a lipid or lipid derivative, methylation, myristlyation, pegylation, prenylation, phosphorylation, ubiqutination, or any similar process.
- polypeptide modifications include the following:
- Constant substitution refers to the substitution of an amino acid in one class by an amino acid of the same class, where a class is defined by common physicochemical amino acid side chain properties and high substitution frequencies in homologous polypeptides found in nature, as determined, for example, by a standard Dayhoff frequency exchange matrix or BLOSUM matrix.
- Six general classes of amino acid side chains have been categorized and include: Class I (Cys); Class II (Ser, Thr, Pro, Ala, GIy); Class III (Asn, Asp, GIn, GIu); Class IV (His, Arg, Lys); Class V (He, Leu, VaI, Met); and Class VI (Phe, Tyr, Trp).
- substitution of an Asp for another class III residue such as Asn, GIn, or GIu, is a conservative substitution.
- Non-conservative substitution refers to the substitution of an amino acid in one class with an amino acid from another class; for example, substitution of an Ala, a class II residue, with a class III residue such as Asp, Asn, GIu, or GIn.
- “Deletion” - is a change in either nucleotide or amino acid sequence in which one or more nucleotides or amino acid residues, respectively, are absent.
- “Insertion” or “addition” - is that change in a nucleotide or amino acid sequence which has resulted in the addition of one or more nucleotides or amino acid residues, respectively, as compared to the naturally occurring sequence.
- substitution - replacement of one or more nucleotides or amino acids by different nucleotides or amino acids, respectively. As regards amino acid sequences the substitution may be conservative or non- conservative.
- Detection refers to a method of detection of a disease. This term may refer to detection of a predisposition to a disease, or to the detection of the severity of the disease.
- homolog/homology is meant at least about 70%, preferably at least about 75% homology, advantageously at least about 80% homology, more advantageously at least about 90% homology, even more advantageously at least about 95%, e.g., at least about 97%, about 98%, about 99% or even about 100% homology.
- the invention also comprehends that these polynucleotides and polypeptides can be used in the same fashion as the herein or aforementioned polynucleotides and polypeptides.
- homology can refer to the number of positions with identical nucleotides or amino acid residues, divided by the number of nucleotides or amino acid residues in the shorter of the two sequences, wherein alignment of the two sequences can be determined in accordance with the Wilbur and Lipman algorithm ((1983) Proc. Natl. Acad. Sci. USA 80:726); for instance, using a window size of 20 nucleotides, a word length of 4 nucleotides, and a gap penalty of 4, computer-assisted analysis and interpretation of the sequence data, including alignment, can be conveniently performed using commercially available programs (e.g., IntelligeneticsTM Suite, Intelligenetics Inc., CA).
- RNA sequences are said to be similar, or to have a degree of sequence identity or homology with DNA sequences, thymidine (T) in the DNA sequence is considered equal to uracil (U) in the RNA sequence.
- RNA sequences within the scope of the invention can be derived from DNA sequences or their complements, by substituting thymidine (T) in the DNA sequence with uracil (U).
- amino acid sequence similarity or homology can be determined, for instance, using the BlastP program (Altschul et al, Nucl. Acids Res. 25:3389-3402) and available at NCBI.
- the following references provide algorithms for comparing the relative identity or homology of amino acid residues of two polypeptides, and additionally, or alternatively, with respect to the foregoing, the teachings in these references can be used for determining percent homology: Smith et al, (1981) Adv. Appl. Math. 2:482-489; Smith et al, (1983) Nucl. Acids Res. 11:2205-2220; Devereux et al, (1984) Nucl. Acids Res.
- Having at least X% homology refers to the percentage of residues that are identical in the two sequences when the sequences are optimally aligned.
- 90% amino acid sequence identity means that 90% of the amino acids in two or more optimally aligned polypeptide sequences are identical.
- module By the term “modulates” is meant either increases (promotes, enhances) or decreases (prevents, inhibits).
- CMS (chronic mild stress) chip The CMS chip is composed of:
- the probes are prepared from following animals:
- Control animals (4 time groups, 6 different brain structures: Amygdala, Nucleus Accumbens, Hypothalamus, Hippocampus, Pons and Frontal cortex)
- 5- Stressed animals treated with fluoxetine (4 time groups, 2 treatment groups -responders and not responders- and 6 different brain structures: Amygdala, Nucleus Accumbens, Hypothalamus, Hippocampus, Pons and Frontal cortex)
- Imipramine tricyclic class (Positive control in the experiment)
- Chip / hybridizations Hybridization points (days 22, 29, 36, 64)
- PCR Polymerase chain reaction
- Vectors are constructed containing the cDNA of the present invention by those skilled in the art and can contain all expression elements necessary to achieve the desired transcription of the sequences, should transcription be required (see below in specific methods for a more detailed description).
- Other beneficial characteristics can also be contained within the vectors such as mechanisms for recovery of the nucleic acids in a different form.
- Phagemids are a specific example of such beneficial vectors because they can be used either as plasmids or as bacteriophage vectors. Examples of other vectors include viruses such as bacteriophages, baculoviruses and retroviruses, DNA viruses, cosmids, plasmids, liposomes and other recombination vectors.
- the vectors can also contain elements for use in either procaryotic or eucaryotic host systems.
- One of ordinary skill in the art knows which host systems are compatible with a particular vector.
- the vectors are introduced into cells or tissues by any one of a variety of known methods within the art (calcium phosphate transfection; electroporation; lipofection; protoplast fusion; polybrene transfection).
- the host cell can be any eucaryotic and procaryotic cells, which can be transformed with the vector and which supports the production of the polypeptide.
- ELISAs are the preferred immunoassays employed to assess a specimen.
- ELISA assays are well known to those skilled in the art. Both polyclonal and monoclonal antibodies can be used in the assays. Where appropriate other immunoassays, such as radioimmunoassays (RIA) can be used as are known to those in the art.
- RIA radioimmunoassays
- Available immunoassays are extensively described in the patent and scientific literature. See, for example, United States Patent Nos. 3,791,932; 3,839,153; 3,850,752;
- siRNA molecules according to the above specifications may be prepared essentially as described herein.
- siRNAs of the present invention can be synthesized by any of the methods which are well- known in the art for synthesis of ribonucleic (or deoxyribonucleic) oligonucleotides.
- a commercially available machine available, inter alia, from Applied Biosystems
- the oligonucleotides are prepared according to the sequences disclosed herein.
- Overlapping pairs of chemically synthesized fragments can be ligated using methods well known in the art (e.g., see U.S. Patent No. 6,121,426).
- the strands are synthesized separately and then are annealed to each other in the tube.
- the double-stranded siRNAs are separated from the single- stranded oligonucleotides that were not annealed (e.g. because of the excess of one of them) by HPLC.
- siRNAs or siRNA fragments of the present invention two or more such sequences can be synthesized and linked together for use in the present invention.
- siRNA molecules of the invention may be synthesized by procedures known in the art e.g. the procedures as described in Usman et al., 1987, J. Am. Chem. Soc, 109, 7845; Scaringe et al., 1990, Nucleic Acids Res., 18, 5433; Wincott et al., 1995, Nucleic Acids Res. 23, 2677-2684; and Wincott et al., 1997, Methods MoI. Bio., 74, 59, and may make use of common nucleic acid protecting and coupling groups, such as dimethoxytrityl at the 5 '-end, and phosphoramidites at the 3'-end.
- the modified (e.g. 2'-O-methylated) nucleotides and unmodified nucleotides are incorporated as desired.
- nucleic acid molecules of the present invention can be synthesized separately and joined together post-synthetically, for example, by ligation (Moore et al., 1992, Science 256, 9923; Draper et al., International PCT publication No. WO93/23569; Shabarova et al., 1991, Nucleic Acids Research 19, 4247; Bellon et al., 1997, Nucleosides & Nucleotides, 16, 951; Bellon et al., 1997, Bioconjugate Chem. 8, 204), or by hybridization following synthesis and/or deprotection.
- siRNA molecules of the invention can also be synthesized via a tandem synthesis methodology, as described in US patent application publication No. US2004/0019001 (McSwiggen) wherein both siRNA strands are synthesized as a single contiguous oligonucleotide fragment or strand separated by a cleavable linker which is subsequently cleaved to provide separate siRNA fragments or strands that hybridize and permit purification of the siRNA duplex.
- the linker can be a polynucleotide linker or a non-nucleotide linker.
- siRNAs used in the experiments described herein are all 19-mers, having alternating ribonucleotides modified in both the antisense and the sense strands of the compound.
- the modification is such that a 2'-O-methyl (Me) group is present on the first, third, fifth, seventh, ninth, eleventh, thirteenth, fifteenth, seventeenth and nineteenth nucleotide of the antisense strand, whereby the very same modification, i. e. a 2'-0-Me group, is present at the second, fourth, sixth, eighth, tenth, twelfth, fourteenth, sixteenth and eighteenth nucleotide of the sense strand.
- These particular siRNA compounds are also blunt ended and are non-phosphorylated at the termini; however, comparative experiments have shown that siRNA compounds phosphorylated at one or both of the 3'-termini have similar activity.
- Antibodies may be prepared using an intact polypeptide or fragments containing smaller polypeptides as the immunizing antigen. For example, it may be desirable to produce antibodies that specifically bind to the N- or C- terminal or any other suitable domains.
- the polypeptide used to immunize an animal can be derived from translated cDNA or chemical synthesis which can be conjugated to a carrier protein, if desired.
- Such commonly used carriers which are chemically coupled to the polypeptide include keyhole limpet hemocyanin (KLH), thyroglobulin, bovine serum albumin (BSA) and tetanus toxoid. The coupled polypeptide is then used to immunize the animal.
- polyclonal or monoclonal antibodies can be further purified, for example by binding to and elution from a matrix to which the polypeptide or a peptide to which the antibodies were raised is bound.
- a matrix to which the polypeptide or a peptide to which the antibodies were raised is bound.
- Those skilled in the art know various techniques common in immunology for purification and/or concentration of polyclonal as well as monoclonal antibodies (Coligan et al, Unit 9, Current Protocols in Immunology, Wiley Interscience, 1994).
- the antibodies may be humanized antibodies or human antibodies.
- Antibodies can be humanized using a variety of techniques known in the art including CDR- grafting (EP239,400: PCT publication WO.91/09967; U.S. patent Nos.5,225,539;5,530,101; and 5,585,089, veneering or resurfacing (EP 592,106; EP 519,596; Padlan, Molecular Immunology 28(4/5):489-498 (1991); Studnicka et al., Protein Engineering 7(6):805-814 (1994); Roguska et al., PNAS 91 :969-973 (1994)), and chain shuffling (U.S. Patent No. 5,565,332).
- the monoclonal antibodies as defined include antibodies derived from one species (such as murine, rabbit, goat, rat, human, etc.) as well as antibodies derived from two (or more) species, such as chimeric and humanized antibodies.
- Human antibodies are particularly desirable for therapeutic treatment of human patients.
- Human antibodies can be made by a variety of methods known in the art including phage display methods using antibody libraries derived from human immunoglobulin sequences. See also U.S. Patent Nos. 4,444,887 and 4,716,111; and PCT publications WO 98/46645, WO 98/50433, WO 98/24893, WO 98/16654, WO 96/34096, WO 96/33735, and WO 91/10741, each of which is incorporated herein by reference in its entirety.
- the animals are first trained to consume a 1% sucrose solution; training consists of several 1-h baseline tests (twice weekly) in which sucrose solution will is presented, in the home cage, following 14h food and water deprivation. Subsequently, sucrose consumption is monitored, under similar conditions, throughout the duration of the study. On the basis of their sucrose intakes in the final baseline test (Day 0), the animals are divided into two matched groups. One group of animals is subjected to the CMS procedure for a period of 8 consecutive weeks.
- Each week of the stress regime consists of: two periods of food or water deprivation, two periods of 45-degree cage tilt, two periods of intermittent illumination (light on and off every 2h), two periods of soiled cage (250 ml water in sawdust bedding), two periods of paired housing, two periods of low intensity stroboscopic illumination (150 flashes/min), and two periods of no stress. All the stressors are of 10 - 14 h duration and are applied individually and continuously, day and night. Control non-stressed animals are housed in separate rooms and have no contact with the stressed animals. They are deprived of food and water for 14 h before each sucrose test, but otherwise food and water are available at libitum.
- both stressed and control animals are further divided into matched subgroups, and for the subsequent five weeks they receive daily intraperitoneal injections of vehicle (distilled water, 1 ml/kg), imipramine (10 mg/kg), citalopram (10 mg/kg), moclobemide (10 mg/kg) or amphetamine as drug controls.
- vehicle distilled water, 1 ml/kg
- imipramine 10 mg/kg
- citalopram 10 mg/kg
- moclobemide 10 mg/kg
- amphetamine as drug controls.
- the test animals receive one of the active siRNA inhibitors of the invention.
- the drugs are administered at 10.00 and the weekly sucrose tests are carried out 24 h following the last drug injection.
- the control and stressed animals are be decapitated, five brain structures (frontal cortex, hippocampus, Amygdala, nucleus accumbens, hypothalamus, pons) are dissected, frozen and transferred for further molecular analysis.
- the structures are isolated from the following groups:
- the group of rats killed on day 64 will include both animals responding and non-responding to antidepressant treatments.
- the animals receiving the active siRNA display less stress than the control animals (receiving water or receiving known drugs).
- Traumatic injury can be due to automobile accidents, falls, gunshot, diving accidents inter alia, and diseases which can affect the spinal cord include polio, spina bifida, tumors and
- CHI Closed Head Injury
- Transient middle cerebral artery occlusion a 90 to 120 minutes transient focal ischemia is performed in adult, male Sprague Dawley rats, 300-370 gr.
- the method employed is the intraluminal suture MCAO (Longa et al., Stroke, 30, 84, 1989, and
- Permanent middle cerebral artery occlusion (MCAO) - occlusion is permanent, unilateral- induced by electrocoagulation of MCA. Both methods lead to focal brain ischemia of the ipsilateral side of the brain cortex leaving the contralateral side intact (control).
- the left MCA is exposed via a temporal craniectomy, as described for rats by Tamura A.et al., J Cereb Blood Flow Metab. 1981 ;1 :53— 60.
- the MCA and its lenticulostriatal branch are occluded proximally to the medial border of the olfactory tract with microbipolar coagulation.
- the wound is sutured, and animals returned to their home cage in a room warmed at 26°C to 28°C. The temperature of the animals is maintained all the time with an automatic thermostat.
- the efficacy of the inhibitor is determined by mortality rate, weight gain, infarct volume, short and long term clinical and neurophysichological and behavioral (including feeding behavior) outcomes in surviving animals. Infarct volumes are assessed histologically (Knight et al., Stroke, 25, 1252, 1994, and Mintorovitch et al., Magn. Reson. Med. 18, 39, 1991).
- the staircase test Montoya et al., J. Neurosci. Methods 36, 219, 1991
- the motor disability scale according to Bederson's method (Bederson et al., Stroke, 17, 472, 1986) is employed to evaluate the functional outcome following MCAO. The animals are followed for different time points, the longest one being two months.
- mice are sacrificed and cardiac perfusion with 4% formaldehyde in PBS is performed. Brains are removed and serial coronal 200 ⁇ m sections are prepared for processing and paraffin embedding. The sections are stained with suitable dyes such as TCC. The infarct area is measured in these sections using a computerized image analyzer. The results show that the siRNA inhibitor of the invention is efficacious when compared to the controls.
- screening assays are known to those of ordinary skill in the art.
- the specific assay which is chosen depends to a great extent on the activity of the candidate gene or the polypeptide expressed thereby.
- an assay which is based on inhibition (or stimulation) of the enzymatic activity can be used.
- the candidate polypeptide is known to bind to a ligand or other interactor, then the assay can be based on the inhibition of such binding or interaction.
- the candidate gene is a known gene, then many of its properties can also be known, and these can be used to determine the best screening assay.
- the candidate gene is novel, then some analysis and/or experimentation is appropriate in order to determine the best assay to be used to find inhibitors of the activity of that candidate gene.
- the analysis can involve a sequence analysis to find domains in the sequence which shed light on its activity.
- the screening assays can be cell-based or non-cell-based.
- the cell-based assay is performed using eukaryotic cells such as HeLa cells, and such cell-based systems are particularly relevant in order to directly measure the activity of candidate genes which are anti- apoptotic functional genes, i.e., expression of the gene prevents apoptosis or otherwise prevents cell death in target cells.
- One way of running such a cell-based assay uses tetracycline-inducible (Tet- inducible) gene expression. Tet-inducible gene expression is well known in the art; see for example, Hofmann et al, 1996, Proc Natl Acad Sci 93( 11 ):5185-5190.
- Tet-inducible retroviruses have been designed incorporating the Self-inactivating (SIN) feature of a 3' Ltr enhancer/promoter retroviral deletion mutant. Expression of this vector in cells is virtually undetectable in the presence of tetracycline or other active analogs. However, in the absence of Tet, expression is turned on to maximum within 48 hours after induction, with uniform increased expression of the whole population of cells that harbor the inducible retrovirus, thus indicating that expression is regulated uniformly within the infected cell population.
- SI Self-inactivating
- a specific reporter gene construct can be designed such that phosphorylation of this reporter gene product causes its activation, which can be followed by a color reaction.
- the candidate gene can be specifically induced, using the Tet-inducible system discussed above, and a comparison of induced versus non-induced genes provides a measure of reporter gene activation.
- a reporter system can be designed that responds to changes in protein- protein interaction of the candidate protein. If the reporter responds to actual interaction with the candidate protein, a color reaction occurs.
- a specific promoter or regulatory element controlling the activity of a candidate gene is defined by methods well known in the art.
- a reporter gene is constructed which is controlled by the specific candidate gene promoter or regulatory elements. The DNA containing the specific promoter or regulatory agent is actually linked to the gene encoding the reporter. Reporter activity depends on specific activation of the promoter or regulatory element.
- inhibition or stimulation of the reporter is a direct assay of stimulation/inhibition of the reporter gene; see, for example, Komarov et al (1999), Science vol 285,1733-7 and Storz et al (1999) Analytical Biochemistry, 276, 97-104.
- Various non-cell-based screening assays are also well within the skill of those of ordinary skill in the art. For example, if enzymatic activity is to be measured, such as if the candidate protein has a kinase activity, the target protein can be defined and specific phosphorylation of the target can be followed.
- the assay can involve either inhibition of target phosphorylation or stimulation of target phosphorylation, both types of assay being well known in the art; for example see Mohney et al (1998) J.Neuroscience 18, 5285 and Tang et al (1997) J Clin. Invest. 100, 1180 for measurement of kinase activity.
- assays for measuring the enzymatic activity of PD2 Synthase, PDE4D, Dusp ⁇ , Camk2a/2b, Atplal, Cdk5r, NOSl, Chimerinl, MAPKKKK5 and ARHGAP6 are well known in the art.
- the activity of a transporter / channel / receptor can be assessed by measuring the uptake of a relevant ligand / hormone / neurotransmitter, as the case may be, or a downstream signaling molecule which indicates the activation level of said receptor / transporter.
- TTR TTR, PD2 Synthase, Adrb3, PDE4D, GABRP, PMCH, Dusp ⁇ , Camk2a/2b, VIP, Atplal, MEF2C, Cdk5r, NOSl, Neurogranin, Chimerinl, Dendrin, GMF, Cacnb4, SLC17A7, SLC8A1, Drd3, MAPKKKK5 or ARHGAP6 interacts with an enzyme and regulates its enzymatic activity through protein-protein interaction.
- An interactor such as a receptor ligand, is radioactively labeled and added.
- the amount of radioactivity carried on the beads can be measured.
- the assay indicates inhibition of the interaction by measuring the amount of radioactivity on the bead.
- Any of the screening assays, according to the present invention can include a step of identifying the chemical compound (as described above) or other species which tests positive in the assay and can also include the further step of producing as a medicament that which has been so identified. It is considered that medicaments comprising such compounds, or chemical analogs or homologs thereof, are part of the present invention.
- genetic therapy refers to the transfer of genetic material (e.g DNA or RNA) of interest into a host to treat or prevent a genetic or acquired disease or condition phenotype.
- the genetic material of interest encodes a product (e.g. a protein, polypeptide, peptide, functional RNA, antisense) the production of which in vivo is desired.
- the genetic material of interest can encode a hormone, receptor, enzyme, polypeptide or peptide of therapeutic value.
- the genetic material of interest may encode a suicide gene.
- Gene therapy of the present invention can be carried out in vivo or ex vivo.
- Ex vivo gene therapy requires the isolation and purification of cells from a patient, the introduction of a therapeutic gene and the introduction of the genetically altered cells back into the patient.
- a replication-deficient virus such as a modified retrovirus can be used to introduce a therapeutic antisense fragment into such cells.
- mouse Moloney leukemia virus MMLV
- MMLV mouse Moloney leukemia virus
- the therapeutic gene or fragment such as an antisense fragment is typically "packaged" for administration to a patient such as in liposomes or in a replication-deficient virus such as adenovirus as described by Berkner, K. L., in Curr. Top. Microbiol. Immunol., 158, 39-66 (1992) or adeno- associated virus (AAV) vectors as described by Muzyczka, N., in Curr. Top. Microbiol. Immunol., 158, 97-129 (1992) and U.S. Pat. No. 5,252,479.
- adenovirus as described by Berkner, K. L., in Curr. Top. Microbiol. Immunol., 158, 39-66 (1992) or adeno- associated virus (AAV) vectors as described by Muzyczka, N., in Curr. Top. Microbiol. Immunol., 158, 97-129 (1992) and U.S. Pat. No. 5,252,
- naked DNA is administered in which the therapeutic gene or fragment such as an antisense fragment is directly injected into the bloodstream or muscle tissue.
- administration of naked DNA in which the therapeutic gene or fragment such as an antisense fragment is introduced into the target tissue by microparticle bombardment using gold particles coated with the DNA.
- Gene therapy vectors can be delivered to a subject by, for example, intravenous injection, local administration (see U.S. Pat. No. 5,328,470) or by stereotactic injection (see e.g., Chen et al. (1994) PNAS 91:3054-3057).
- the pharmaceutical preparation of the gene therapy vector can include the gene therapy vector in an acceptable diluent, or can comprise a slow release matrix in which the gene delivery vehicle is imbedded.
- the pharmaceutical preparation can include one or more cells which produce the gene delivery system.
- Cell types useful for gene therapy of the present invention include lymphocytes, hepatocytes, myoblasts, fibroblasts, and any cell of the eye such as retinal cells, epithelial and endothelial cells.
- the cells are T lymphocytes drawn from the patient to be treated, hepatocytes, any cell of the eye or respiratory or pulmonary epithelial cells.
- Transfection of pulmonary epithelial cells can occur via inhalation of a neubulized preparation of DNA vectors in liposomes, DNA-protein complexes or replication-deficient adenoviruses. See, e.g., U.S. Patent No. 5,240,846.
- BAMBI Homo sapiens BMP and activin membrane-bound 3 inhibitor homolog (Xenopus laevis) (BAMBI), mRNA NM. _012342 62953114 BAMBI
- Homo sapiens ankyrin repeat domain 17 (ANKRD 17), 4 transcript variant 1 , mRNA NM. _032217 38683806 ANKRD 17
- PITPNA phosphatidylinositol transfer protein, alpha 5
- LAS Homo sapiens lipoic acid synthetase
- nuclear gene encoding mitochondrial protein transcript variant 1
- transcript variant 1 mRNA NM. _006859 37577165 LIAS
- TTR Homo sapiens transthyretin (prealbumin, amyloidosis type I) (TTR), mRNA NM. _000371 4507724 TTR
- ADRB3 Homo sapiens adrenergic, beta-3-, receptor
- GABA gamma-aminobutyric acid
- G6PC3 Homo sapiens glucose 6 phosphatase, catalytic, 3 18 (G6PC3), mRNA NM_ .138387 46852181 G6PC3
- Homo sapiens synaptosomal-associated protein 25kDa 21 (SN AP25), transcript variant 2, mRNA NM_ .130811 18765734 SNAP25
- FLYWCHl Homo sapiens FLYWCH-type zinc finger 1 (FLYWCHl), 22 transcript variant 1 , mRNA NM_ 032296 62953133 FLYWCHl
- GABA gamma-aminobutyric acid
- GABRA3 alpha 3
- CLSTN2 calsyntenin 2
- START domain containing 7 STARD7
- GRM7 Homo sapiens glutamate receptor, metabotropic 7 (GRM7), transcript variant 1 , mRNA NM_000844 32528271 GRM7
- NOSl Homo sapiens nitric oxide synthase 1 (neuronal) (NOSl), mRNA NM_000620 10835172 NOSl
- DKK3 Homo sapiens dickkopf homolog 3 (Xenopus laevis) (DKK3), transcript variant 2, mRNA NM_013253 66346687 DKK3
- HNRPU Hemo sapiens heterogeneous nuclear ribonucleoprotein U (scaffold attachment factor A) (HNRPU), transcript variant 2, mRNA NM_004501 14141160 HNRPU
- Homo sapiens fibroblast growth factor 13 FGF 13
- transcript variant IB mRNA NM_033642 16306542
- FGF 13 PREDICTED Homo sapiens KIAA 1201 protein (KIAA1201), mRNA XM_370660 51468639 K1AA1201
- GABA gamma-aminobutyric acid
- GABA alpha 1
- CBX6 chromobox homolog 6
- CBX6 chromobox homolog 6
- SELS selenoprotein S
- transcript variant 1 mRNA NM_203472 45439348
- SELS Homo sapiens kinectin mRNA, complete cds L25616 409465 Homo sapiens THO complex 2 (THOC2), mRNA NM_020449 52486998 THOC2
- VPS41 Homo sapiens vacuolar protein sorting 41 (yeast) (VPS41 ), transcript variant 1 , mRNA NM_014396 18105059 VPS41
- FLJ14624 Homo sapiens hypothetical protein FLJ 14624 (FLJ14624), mRNA NM_032813 14249503 FLJ14624
- Homo sapiens protein phosphatase 3 (formerly 2B), regulatory subunit B, 19kDa, alpha isoform (calcineurin B, type I) (PPP3R1), mRNA NMJJ00945 45238847 PPP3R1
- ROMl retinal outer segment membrane protein 1
- mRNA NM_000327 19743809 ROMl full-length cDNA clone CS0DI008YL23 of Placenta Cot 25-normalized of Homo sapiens (human) CR607755 50488562
- DCAMKLl Homo sapiens doublecortin and CaM kinase-like 1 (DCAMKLl), mRNA NM_004734 42544237 DCAMKLl
- RNA binding motif protein 10 (RBMlO), transcript variant 2, mRNA NM_152856 23111017 RBMlO
- TNFRSF 16 nerve growth factor receptor (TNFRSF 16) associated protein 1 (NGFRAPl), transcript variant 3, mRNA NM_014380 7657043 TNFRSF 16
- SCARA3 Homo sapiens scavenger receptor class A, member 3 (SCARA3), transcript variant 1, mRNA NM_016240 33598923 SCARA3
- GMFG Homo sapiens glia maturation factor, gamma (GMFG), mRNA NMJJ04877 4758439 GMFG
- CDC42SE1 Homo sapiens CDC42 small effector 1 (CDC42SE1), mRNA NMJ)20239 12965169 CDC42SE1
- Homo sapiens cDNA FLJ46825 fis clone UTERU2000300 AK128665 34536155 Homo sapiens ribosomal protein L5 (RPL5), mRNA NM_000969 71772259 RPL5 Homo sapiens neuropilin 2 (NRP2), transcript variant 2, mRNA NMJ)03872 41872532 NRP2
- MGC42105 Homo sapiens hypothetical protein MGC42105 (MGC42105), mRNA NMJ 53361 37059769 MGC42105
- GAS7 Homo sapiens growth arrest-specific 7 (GAS7), transcript variant b, mRNA NM_201432 41406077 GAS7
- FKBP9 Homo sapiens FK506 binding protein 9, 63 kDa (FKBP9), mRNA NMJ)07270 33469984 FKBP9
- NADH dehydrogenase ubiquinone 1 beta subcomplex, 11, 17.3kDa, mRNA (cDNA clone BCO 10665 14715010 ubiquinone MGC:8855 IMAGE:3870660), complete cds
- RPL 19 Homo sapiens ribosomal protein L 19 (RPL 19), mRNA NM_000981 68216257 RPL19
- NEDD8 90 developmentally down-regulated 8 (NEDD8), mRNA NM_006156 5453759 NEDD8 Homo sapiens SPARC-like 1 (mast9, hevin) (SPARCLl),
- FXR2 98 homolog 2
- Homo sapiens cell division cycle 73 Homo sapiens cell division cycle 73, Pafl/RNA polymerase II complex component, homolog (S.
- SNF8 S. cerevisiae
- FP Protaglandin F receptor
- HNMT histamine N-methyltransferase
- ADRAlD mRNA NM. _000678 15451784 ADRAlD
- CD276 antigen CD276
- transcript variant CD276
- CD34 antigen CD34
- transcript variant 1 CD34 antigen
- TUBB2 Homo sapiens tubulin, beta 2 (TUBB2), mRNA NM. _001069 68299771 TUBB2
- TUBA3 Homo sapiens tubulin, alpha 3
- TUBA3 Homo sapiens adenylate cyclase 2 (brain) (ADCY2)
- C AMK2N 1 120 kinase II inhibitor 1
- mRNA NM_ .018584 31324542 CAMK2N1 120 kinase II inhibitor 1
- CaM kinase II alpha CAMK2A
- CDK5R1 124 subunit 1 (p35) (CDK5R1), mRNA NM_ .003885 34304373 CDK5R1
- CDK5R2 125 subunit 2 (p39) (CDK5R2), mRNA NM_ .003936 42741664 CDK5R2
- KIF20A Homo sapiens kinesin family member 2OA
- KIF5A Homo sapiens kinesin family member 5A
- KIF2 Homo sapiens kinesin heavy chain member 2
- ACTR2 133 (yeast) (ACTR2)
- transcript variant 2 mRNA NM_005722 53692185 ACTR2
- ACTR2 Homo sapiens actin related protein 2/3 complex, subunit
- ARPC5L 134 5-like (ARPC5L), mRNA NM_030978 13569955 ARPC5L Homo sapiens anillin, actin binding protein (scraps)
- ABLIMl Homo sapiens actin binding LIM protein 1
- ACTR3 Homo sapiens mitochondrial ribosomal protein L51
- MRPL51 nuclear gene encoding mitochondrial protein
- CTSD computed tomRNA NMJX
- ANXA2 Homo sapiens annexin A2 (ANXA2), transcript variant 3,
- Homo sapiens wingless-type MMTV integration site Homo sapiens wingless-type MMTV integration site
- WNT2 145 family member 2 (WNT2), mRNA NMJ303391 4507926 WNT2
- HSPDl nuclear gene encoding mitochondrial protein
- transcript variant 1 mRNA NM_002156 41399283 HSPDl
- NTRK2 NTRK2
- AKAP350 A-kinase anchoring protein
- AKAP5 mRNA NMJJ04857 21493042 AKAP5
- EFNB3 Homo sapiens ephrin-B3
- mRNA NM_001406 38201712 EFNB3 Homo sapiens poly(A) polymerase beta (testis specific)
- PAPOLB PAPOLB
- PAIP2 PAIP2
- mRNA NM_016480 19923458 PAIP2 Homo sapiens potassium voltage-gated channel, Shab-
- KCNBl 159 related subfamily, member 1 (KCNBl), mRNA NM_004975 27436972 KCNBl Homo sapiens potassium voltage-gated channel, shaker- related subfamily, beta member 1 (KCNABl), transcript
- KCNA6 165 related subfamily, member 6
- mRNA NM_002235 25952089
- KCNA6 Homo sapiens potassium voltage-gated channel
- KCNC3 166 related subfamily, member 3 (KCNC3), mRNA NM_004977 24497459 KCNC3 Homo sapiens potassium voltage-gated channel, shaker- related subfamily, beta member 2 (KCNAB2), transcript
- KCNAB3 beta member 3
- mRNA NM_004732 27436970 KCNAB3 Homo sapiens potassium inwardly-rectifying channel
- KCNJ5 169 subfamily J, member 5 (KCNJ5), mRNA NM__000890 24797140 KCNJ5 Homo sapiens potassium voltage-gated channel, Shal- related subfamily, member 3 (KCND3), transcript variant
- Homo sapiens potassium voltage-gated channel, shaker- related subfamily, member 1 episodic ataxia with
- KCNJ2 subfamily J, member 2
- mRNA NM_000891 22095339 KCNJ2 Homo sapiens potassium voltage-gated channel, shaker-
- KCNA2 175 related subfamily, member 2
- KCNA2 mRNA NM_004974 25952079
- KCNA2 Homo sapiens cytochrome c oxidase subunit VIIb
- cytochrome c oxidase subunit Via polypeptide 1 (COX6A1), nuclear gene encoding
- mitochondrial protein mRNA NM 004373 17999527 COX6A1 Homo sapiens phosphodiesterase 7A (PDE7A), transcript
- CNP 179 phosphodiesterase
- mRNA NM_033133 38570090
- CNP Homo sapiens ectonucleotid ⁇ pyrophosphatase/phosphodiesterase 2 (autotaxin)
- SMPD2 neutral sphingomyelinase
- glycogen storage disease type II GAA
- Homo sapiens solute carrier family 13 sodium-dependent dicarboxylate transporter
- member 3 SLC 13 A3
- Homo sapiens solute carrier family 8 sodium/calcium
- Homo sapiens solute carrier family 17 sodium-dependent inorganic phosphate cotransporter
- Homo sapiens solute carrier family 25 mitochondrial carrier; adenine nucleotide translocator), member 4 (SLC25A4), nuclear gene encoding mitochondrial protein,
- Homo sapiens solute carrier family 1 high affinity aspartate/glutamate transporter
- member 6 SLCl IA6
- Homo sapiens solute carrier family 22 (organic cation
- Homo sapiens solute carrier family 6 neurotransmitter transporter, betaine/GABA
- member 12 SLC6A12
- Homo sapiens solute carrier family 1 Neuronal/epithelial high affinity glutamate transporter, system Xag
- member 1 Neuronal/epithelial high affinity glutamate transporter, system Xag
- Homo sapiens solute carrier family 1 (glial high affinity
- Homo sapiens solute carrier family 1 (glial high affinity
- nucleoside transporter 216 nucleoside transporter
- member 2 SLC28 A2
- Homo sapiens solute carrier family 23 nucleobase transporters
- member 1 SLC23A1
- transcript variant 1 transcript variant 1
- Homo sapiens solute carrier family 8 sodium-calcium
- RPL27 Homo sapiens ribosomal protein L27 (RPL27), mRNA NM. .000988 17017972 RPL27
- CACNB4 225 subunit
- transcript variant 2 mRNA NM. .000726 54607063
- CACNB4 Homo sapiens calcium channel, voltage-dependent
- CACNG5 gamma subunit 5
- transcript variant 1 mRNA NNMMJ 14455881111 22027550
- CACNG5 Homo sapiens calcium channel, voltage-dependent, alpha
- CACNAlH 227 IH subunit
- transcript variant 2 mRNA NMJ
- CACNAlA 227 IH subunit
- transcript variant 2 mRNA NMJ
- CACNAlA 227 IH subunit
- CACNA2Dl 230 2/delta subunit l
- mRNA NM_000722 54112389 CACNA2D1
- CDH2 mRNA NM_001792 14589888 CDH2
- PCDHGA7 transcript variant 2
- transcript variant 2 mRNA NMJD2087 14196476 PCDHGA7
- PCDH19 Homo sapiens protocadherin 19
- mRNA NM_020766 58037112 PCDH19 full-length cDNA clone CS0DF026YA20 of Fetal brain of
- P2RY1 Homo sapiens purinergic receptor P2Y, G-protein
- P2RY 12 coupled, 12 (P2RY 12), transcript variant 1, mRNA NM_022788 29029603 P2RY12 Homo sapiens purinergic receptor P2X, ligand-gated ion
- calmodulin 2 phosphorylase kinase, delta
- HSPC 196 Homo sapiens hypothetical protein HSPC 196 (HSPC 196),
- HTR2C 247 2C (HTR2C)
- HTR3A transcript variant 248 3A
- transcript variant 248 3A transcript variant 248 3A
- transcript variant 248 3A transcript variant 248 3A
- mRNA NMJJ00869 47519823 HTR3A
- transcript variant b (adenylate cyclase-coupled) (HTR7), transcript variant b,
- HTR2B 250 2B
- mRNA NM_000867 40254430 HTR2B Homo sapiens transient receptor potential cation channel, subfamily C, member 4 associated protein (TRPC4AP),
- TRPC6 252 subfamily C, member 6 (TRPC6), mRNA NMJW4621 19923256 TRPC6 Homo sapiens transient receptor potential cation channel, subfamily V, member 1 (TRPVl), transcript variant 3,
- TAM2 tripartite motif-containing 2
- 262 polypeptide (ATP IAl), transcript variant 1 , mRNA NM 000701 48762680 ATP 1 A 1 Homo sapiens proteasome (prosome, macropain) 26S subunit, non- ATPase, 12 (PSMD 12), transcript variant 1,
- ATPlBl 265 polypeptide (ATPlBl), transcript variant 1, mRNA NM_001677 49574487 ATPlBl
- ATP9A Homo sapiens ATPase, Class II, type 9A (ATP9A),
- RNA NM_006045 65301138 ATP9A Homo sapiens ATPase, Ca++ transporting, cardiac muscle, slow twitch 2 (ATP2A2), transcript variant 1,
- transcript variant 1 mRNA NM_005175 50659067 ATP5G1
- Vl subunit C isoform 2 (ATP6V1C2)
- mRNA NMJ44583 47717097 ATP6V1C2 Homo sapiens ATPase, H+ transporting, lysosomal
- ATPOVlA 271 7OkDa 5 Vl subunit A
- mRNA NM_001690 19913423 ATP6V1A Homo sapiens ATP synthase, H+ transporting, mitochondrial Fl complex, beta polypeptide (ATP5B),
- MT3 neurotrophic 273 (neurotrophic) (MT3), mRNA NM_005954 45580728 MT3 Homo sapiens glutathione S-transferase Ml (GSTMl),
- SCN2B 276 beta
- mRNA NM_004588 56699490 SCN2B Homo sapiens sodium channel, voltage-gated, type II,
- SCN2A2 277 alpha 2
- nucleoporin 155kDa (NUP 155), transcript
- nudix nucleoside diphosphate linked
- NUDT3 282 moiety X)-type motif 3 (NUDT3), mRNA NM_006703 37622350 NUDT3
- RPS21 Homo sapiens ribosomal protein S21 (RPS21), mRNA NMJ)01024 71483115 RPS21
- RPS3A Homo sapiens ribosomal protein S3A (RPS3A), mRNA NMJ)01006 70609888 RPS3A
- RPS25 Homo sapiens ribosomal protein S25 (RPS25), mRNA NM_001028 14591916 RPS25
- EPHA4 Homo sapiens EPH receptor A4
- MAP3K13 289 kinase 13 (MAP3K13), mRNA NMJ)04721 32130538 MAP3K13
- MAP4K5 290 kinase kinase 5 (MAP4K5), transcript variant 1, mRNA NMJ)06575 38570133 MAP4K5
- CLIC4 chloride intracellular channel 4
- PIK3CG 298 gamma polypeptide
- mRNA NMJW2649 21237724 PIK3CG
- ZNF677 300 Homo sapiens zinc finger protein 677 (ZNF677), mRNA NMJ 82609 33438597 ZNF677
- ZNF654 Homo sapiens zinc finger protein 654 (ZNF654), mRNA NMJH8293 8922809 ZNF654
- ZNF655 Homo sapiens zinc finger protein 655
- Homo sapiens zinc fineer nrotein 341 (ZNF341), mRNA NM 032819 40807464 ZNF341
- Homo sapiens zinc finger protein 513 (ZNF513)
- mRNA NM_144631 47419890 ZNF513
- Homo sapiens zinc finger protein 21 (KOX 14) (ZNF21)
- ZNF692 Homo sapiens zinc finger protein 692
- ZNF330 Homo sapiens zinc finger protein 330
- ZNF 189 Homo sapiens zinc finger protein 189
- CHRNA3 311 polypeptide 3 (CHRN A3), mRNA NM_000743 19923121 CHRNA3
- CHRNA2 312 polypeptide 2 (neuronal) (CHRNA2), mRNA NM_000742 4502822 CHRNA2
- 315 protease 30 (MGC 10702) mRNA, partial cds AY893160 60812121 MGC 10702 PREDICTED: Homo sapiens similar to Ubiquitin carboxyl-terminal hydrolase 7 (Ubiquitin thiolesterase 7) (Ubiquitin-specific processing protease 7) (Deubiquitinating enzyme 7) (Herpesvirus associated
- LOC345576 316 ubiquitin-specific protease
- USB 16 Homo sapiens ubiquitin specific protease 16
- ELF2 320 factor 320 factor
- transcript variant 2 mRNA NM_006874 42544175 ELF2
- 322 transcript variant 1 mRNA NM_212535 47157321 PRKCBl Homo sapiens neurogranin (protein kinase C substrate,
- NRGN mRNA NM_006176 5453799 NRGN
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Biomedical Technology (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Molecular Biology (AREA)
- Biotechnology (AREA)
- General Engineering & Computer Science (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Wood Science & Technology (AREA)
- Microbiology (AREA)
- Plant Pathology (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- Physics & Mathematics (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
The invention relates to one or more inhibitors, in particular compounds which down-regulate the expression of a gene selected from the group consisting of ABAT; ADRB1; ADRB3; ARHGEF9; ARRB1; ATP1A1; CACNB4; CAMK2A; CAMK2D; CBLN1; CDH22; CDK5R1; CHN1; CTSD; DDN; DRD3; DUSP6; ENPP1; ENPP2; EPHA4; GABRA1; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNA1; KIF5A; MAPK10; MEF2C; NAPB; NOS1; NPTX2; NRGN; NTS; NUCB1; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLP1; PMCH; PVALB; QDPR; RPN1; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYT1; TKT; TPT1; UGT8 and VIP. The invention also relates to a pharmaceutical composition comprising the compound, or a vector capable of expressing the compound, and a pharmaceutically acceptable carrier. The present invention also contemplates a method of treating a subject comprising administering to the patient the pharmaceutical composition in a therapeutically effective dose so as to thereby treat the patient.
Description
COMPOSITIONS AND METHODS FOR TREATMENT OF MOOD DISORDERS
This application claims priority of United States provisional patent application No. 60/838097, filed 15-Aug-2006; which is hereby incorporated by reference in its entirety.
FIELD OF THE INVENTION
The present invention relates to the field of treatment of mood disorders, depression, and conditions which cause depression, as well as neurodegenerative diseases.
BACKGROUND OF THE INVENTION
RNA interference RNA interference (RNAi) is a phenomenon involving double-stranded (ds) RNA-dependent gene-specific posttranscriptional gene silencing. Originally, attempts to study this phenomenon and to manipulate mammalian cells experimentally were hindered by a non-specific antiviral defense mechanism activated in response to long dsRNA molecules (see Gil et al. Apoptosis 2000, 5:107-114). Later it was discovered that short, synthetic RNA duplexes of 21 nucleotides could mediate gene specific RNAi in mammalian cells, precluding stimulation of the generic antiviral defense mechanisms (see Elbashir et al. Nature 2001, 411:494-498; Caplen et al. PNAS USA 2001, 98:9742-9747). As a result, small interfering RNAs (siRNAs) have become powerful tools in attempting to understand gene function. RNA interference (RNAi) in mammals is mediated by small interfering RNAs (siRNAs) (Fire et al, Nature 1998, 391 :806) or microRNAs (miRNAs) (Ambros, Nature 2004, 431(7006):350-355; Bartel, Cell 2004, 116(2): 281-97). The corresponding process in plants is commonly referred to as specific post-transcriptional gene silencing (PTGS) or RNA silencing and is also referred to as quelling in fungi. An siRNA is a double-stranded RNA or modified RNA molecule which down-regulates or silences (prevents) the expression of a gene/ mRNA of its endogenous (cellular) counterpart. The mechanism of RNA interference is detailed infra.
Several studies have revealed that siRNA therapeutics is effective in vivo in both mammals and in humans. Bitko et al., have shown that specific siRNA molecules directed against the respiratory syncytial virus (RSV) nucleocapsid N gene are effective in treating mice when administered
intranasally (Nat. Med. 2005, l l(l):50-55). Recent reviews discussing siRNA therapeutics are available (Barik, et al., J. MoI. Med 2005, 83:764-773; Dallas and Vlassov, Med. Sci. Monitor 2006, 12(4):RA67-74; Chakraborty Current Drug Targets 2007, 8(3):469-82). Mucke (IDrugs 2007 10(l):37-41) presents a review of current therapeutics, including siRNA to various targets, for the treatment of ocular diseases, for example age related macular degeneration (AMD) and glaucoma.
Depression and mood disorders
Clinical depression is a serious brain disorder that affects the way nearly 19 million American adults feel, think, and interact. In contrast to the normal emotional experiences of sadness, loss, or passing mood states, clinical depression is extreme and persistent and can interfere significantly with a person's ability to function.
There are three main types of clinical depression: major depressive disorder; dysthymic disorder; and bipolar depression, the depressed phase of bipolar disorder. Within these types are variations in the number of associated mental symptoms, and their severity and persistence.
A person experiencing major depressive disorder suffers from, among other symptoms, a depressed mood or loss of interest in normal activities that lasts most of the day, nearly every day, for at least two weeks. Such episodes may occur only once, but more commonly occur several times in a lifetime.
Unlike major depressive disorder, dysthymic disorder—a chronic but less severe type—doesn't strike in episodes, but is instead characterized by milder, persistent symptoms that may last for years. Although it usually doesn't interfere with everyday tasks, people with this milder form of depression rarely feel like they are functioning at their full capacities.
Bipolar disorder cycles between episodes of major depression, similar to those seen in major depressive disorder, and highs known as mania. In a manic phase, a person might act on delusional grand schemes that could range from unwise business decisions to romantic sprees. Mania left untreated may deteriorate into a psychotic state.
Because the symptoms, course of illness, and response to treatment vary so much among people with depression, doctors believe that depression may have a number of complex and interacting causes.
Some factors include another medical illness, losing a loved one, stressful life events, and drug or alcohol abuse. Any of these factors also may contribute to recurrent major depressive episodes.
Modern brain imaging technologies are revealing that neural circuits responsible for the regulation of moods, thinking, sleep, appetite, and behavior fail to function properly in people with depression. Imaging studies also indicate that critical neurotransmitters—chemicals used by nerve cells to communicate— are out of balance.
Moreover, genetics research suggests that vulnerability to depression results from the influence of multiple genes acting together with environmental factors. The hormonal system that regulates the body's response to stress also is overactive in many depressed people.
Research conducted in the fields of psychiatry, behavioral science, neuroscience, biology, and genetics, including studies of twins, lead scientists to believe that the risk of developing mental illness increases if another family member is similarly affected, suggesting a hereditary component.
Diagnosing Depression
Medical professionals generally base a diagnosis of mental illness on the presence of certain symptoms listed in the 4th edition of the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). The symptoms listed for a major depressive episode include:
• sadness
• loss of interest or pleasure in activities once enjoyed • change in appetite or weight
• difficulty sleeping or oversleeping
• physical slowing or agitation
• energy loss
• feelings of worthlessness or inappropriate guilt
• difficulty thinking or concentrating
• recurrent thoughts of death or suicide.
A person is clinically depressed if he or she has five or more of these symptoms and has not been functioning normally for most days during the same two-week period.
Dysthymic disorder is diagnosed when depressed mood persists for at least two years (one year in children) and is accompanied by at least two other symptoms of depression. The episodes of depression that occur in people with bipolar disorder alternate with mania, which is characterized by abnormally and persistently elevated mood or irritability. Symptoms of mania include overly inflated self-esteem, decreased need for sleep, increased talkativeness, racing thoughts, distractibility, physical agitation, and excessive risk-taking. Because bipolar disorder requires different treatment than major depression or dysthymia, obtaining an accurate diagnosis is extremely important.
Drug Treatment
Antidepressant Medications
The kind of depression that will most likely benefit from treatment with medications is a condition that's prolonged, lasting 2 weeks or more, and interferes with a person's ability to carry on daily tasks and to enjoy activities that previously brought pleasure. The depressed person will seem sad, or "down," or may show a lack of interest in his surroundings. He may have trouble eating and lose weight (although some people eat more and gain weight when depressed). He may sleep too much or too little, have difficulty going to sleep, sleep restlessly, or awaken very early in the morning. He may speak of feeling guilty, worthless, or hopeless. He may complain that his thinking is slowed down. He may lack energy, feeling "everything's too much," or he might be agitated and jumpy. A person who is depressed may cry. He may think and talk about killing himself and may even make a suicide attempt. Some people who are depressed have psychotic symptoms, such as delusions (false ideas) that are related to their depression. For instance, a psychotically depressed person might imagine that he is already dead, or "in hell," being punished. Not everyone who is depressed has all these symptoms, but everyone who is depressed has at least some of them.
A depression can range in intensity from mild to severe. Antidepressants are used most widely for serious depressions, but they can also be helpful for some milder depressions. Antidepressants, although they are not "uppers" or stimulants, take away or reduce the symptoms of depression and help the depressed person feel the way he did before he became depressed. Antidepressants are also used for disorders characterized principally by anxiety. They can block the symptoms of panic, including rapid heartbeat, terror, dizziness, chest pains, nausea, and breathing problems. They can also be used to treat some phobias.
The physician chooses the particular antidepressant to prescribe based on the individual patient's symptoms. When someone begins taking an antidepressant, improvement generally will not begin to show immediately. With most of these medications, it will take from 1 to 3 weeks before changes begin to occur. Some symptoms diminish early in treatment; others, later. For instance, a person's energy level or sleeping or eating patterns may improve before his depressed mood lifts. If there is little or no change in symptoms after 5 to 6 weeks, a different medication may be tried. Some people will respond better to one than another. Since there is no certain way of determining beforehand which medication will be effective, the doctor may have to prescribe first one, then another, and an effective medication may not be found. Treatment is continued for a minimum of several months and may last up to a year or more. While some people have one episode of depression and then never have another, or remain symptom-free for years, others have more frequent episodes or very long-lasting depressions that may go on for years. Some people find that their depressions become more frequent and severe as they get older. For these people, continuing (maintenance) treatment with antidepressants can be an effective way of reducing the frequency and severity of depressions.
There are a number of antidepressant medications available. They differ in their side effects and, to some extent, in their level of effectiveness. Tricyclic antidepressants (named for their chemical structure) are more commonly used for treatment of major depressions than are monoamine oxidase inhibitors (MAOIs); but MAOIs are often helpful in so-called "atypical" depressions in which there are symptoms like oversleeping, anxiety, panic attacks, and phobias. The last few years have seen the introduction of a number of new antidepressants. Several of them are called "selective serotonin reuptake inhibitors" (SSRIs). Those available at the present time in the United States are fluoxetine (Prozac), fluvoxamine (Luvox), paroxetine (Paxil), and sertraline (Zoloft). (Luvox has been approved for obsessive-compulsive disorder, and Paxil has
been approved for panic disorder.) Though structurally different from each other, all the SSRIs' antidepressant effects are due to their action on one specific neurotransmitter, serotonin. Two other antidepressants that affect two neurotransmitters serotonin and norepinephrine have also been approved by the FDA. They are venlafaxine (Effexor) and nefazodone (Serzone). AU of these newer antidepressants seem to have less bothersome side effects than the older tricyclic antidepressants. The tricyclic antidepressant clomipramine (Anafranil) affects serotonin but is not as selective as the SSRIs. It was the first medication specifically approved for use in the treatment of obsessive compulsive disorder (OCD). Prozac and Luvox have now been approved for use with OCD. Another of the newer antidepressants, bupropion (Wellbutrin), is chemically unrelated to the other antidepressants. It has more effect on norepinephrine and dopamine than on serotonin. Wellbutrin has not been associated with weight gain or sexual dysfunction. It is contraindicated for individuals with, or at risk for, a seizure disorder or who have been diagnosed with bulimia or anorexia nervosa. However, the current anti-depressive drugs are unsatisfactory as they have man side effects, and have varying efficacy depending on the patient history and exact condition to be treated.
Side Effects of Antidepressant Medications
1. Tricyclic Antidepressants
There are a number of possible side effects with tricyclic antidepressants that vary, depending on the medication. For example, amitriptyline (Elavil) may make people feel drowsy, while protriptyline (Vivactil) hardly does this at all and, in some people, may have an opposite effect, producing feelings of anxiety and restlessness. Because of this kind of variation in side effects, one antidepressant might be effecive for one person and not recommended for another. Tricyclics may complicate specific heart problems. Other side effects with tricyclics include blurred vision, dry mouth, constipation, weight gain, dizziness when changing position, increased sweating, difficulty urinating, changes in sexual desire, decrease in sexual ability, muscle twitches, fatigue, and weakness. Some side effects are similar to symptoms of depression (for instance, fatigue and constipation). Tricyclics may also interact with thyroid hormone, antihypertensive medications, oral contraceptives, blood coagulants, sleeping medications, antipsychotic medications, diuretics, antihistamines, aspirin, bicarbonate of soda, vitamin C, alcohol, and tobacco. An overdose of antidepressants is serious and potentially lethal. It requires immediate medical attention.
Symptoms of an overdose of tricyclic antidepressant medication develop within an hour and may start with rapid heartbeat, dilated pupils, flushed face, and agitation, and progress to confusion, loss of consciousness, seizures, irregular heart beats, cardiorespiratory collapse, and death.
2. The Newer Antidepressants
The most common side effects of these antidepressants are gastrointestinal problems and headache. Others are insomnia, anxiety, and agitation. Because of potentially serious interaction between these medications and monoamine oxidase inhibitors, it is required to stop taking one medication from 2 to 4 or 5 weeks before starting the other, depending on the specific medications involved. In addition, SSRIs have been found to affect metabolism of certain other medications in the liver, creating possible drug interactions.
3. Monoamine Oxidase Inhibitors (MAOIs)
MAOIs may cause some side effects similar to those of the other antidepressants. Dizziness when changing position and rapid heartbeat are common. MAOIs also react with certain foods and alcoholic beverages (such as aged cheeses, foods containing monosodium glutamate (MSG), Chianti and other red wines), and other medications (such as over-the-counter cold and allergy preparations, local anesthetics, amphetamines, insulin, some narcotics, and antiparkinsonian medications). Signs may include severe high blood pressure, headache, nausea, vomiting, rapid heartbeat, possible confusion, psychotic symptoms, seizures, stroke, and coma. For this reason, people taking MAOIs must stay away from restricted foods, drinks, and medications.
existing antidepressant drugs are known to influence the functioning primarily of either or both of two neurotransmitters in the brain—serotonin and norepinephrine. Older medications tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs affect the activity of both of these neurotransmitters simultaneously. As indicated above, their disadvantage is that they can be difficult to tolerate due to significant side effects, or, in the case of MAOIs, dietary and medication restrictions.
Newer medications, such as the selective serotonin reuptake inhibitors (SSRJs), have fewer side effects, but may also have sundesirable side affects, as indicated above. See description of drugs and treatment in The American Psychiatric Publishing Textbook of Clinical Psychiatry, Fourth
Edition (2003), edited by Robert E. Hales, M.D., M.B.A., and Stuart C. Yudofsky, M.D.; and in Kaplan and Sadock's Comprehensive Textbook of Psychiatry (2005), authors Benjamin J. Sadock, MD and Virginia A. Sadock, MD , both of which are hereby incorporated by reference in their entirety.
However, not all patients respond to the existing drug treatment, and as indicated above, there is a problem of side effects with many existing drugs. There is therefore a need to find new drugs and new drug targets; there exists a problem in need of solving as no current modes of therapy provide adequate alleviation from mood disorders or depressive conditions for all patients. It is further within the scope of the present invention to treat mood-disorder patients with combinations therapies consisting of one or more of the novel inhibitors of the present invention in combination with one or more known anti-depressant or mod disorder drug. A non-exhaustive list of such drugs includes: Abilify, Adapin, Adderall, alprazolam, Altruline, amitriptyline, amoxapine, Anafranil, Anatensol, aripiprazole, Aropax, Aroxat, Asenden, Atarax, Atenolol, Ativan, Aurorix, Aventyl, Benadryl, Bupropion, Buspar, Buspirone, Camcolit, Canad, Carbamazepine, Celexa, Chlorpromazine, Chlordiazepoxide, Cipramil,
Citalopram,Clomipramine, Clonazepam, Clorazepate, Clozapine, Clozaril, Cylert, Cyproheptadine, Dalmane, Depakene, Depakote, Deprax,Desipramine, Desyrel, Dexedrine, Dextroamphetamine, Diazepam, Dilantin, Diphenhydramine, Divalproex, Dobupal, Doxepin, Dutonin, Edronax, Elavil, Effexor, Eskalith,Ethosuximide, Eufor,Favarin, Felbamate, Felbatol, Fluanxol, Fluoxetine,Flurpenthixol, Fluphenazine, Flurazepam, Fluvoxamine, Gabapentin, Geodon,Halcion, Haldol, Haloperidol, Helix, Hydroxyzine, Imipramine, Imovane, Klonopin, Lamictal, Lamotrigine, Leponex, Librium, Lithane, lithium carbonate, lithium,Lithobid, Lithonate,Lithotabs, Lorazepam, Loxapine, Loxitane, Ludiomil, Lustral,Luvox, Manerix, Maprotoline, Mellaril, Meprobamate, Mesoridazine, Methotrimeprazine, Methylphenide, Miltown, Mirtazepine, Moclobemide, Modafinil, Mysoline, Nardil, Nefadar, Nefazodone, Neurontin, Norpramin, Nortriptyline,Nortrilen, Nozinan, Olanzapine, Orap, Oxazepam, Pamelor, Parnate, Paroxetine, Paxil, Pemoline, Periactin, Perphenazine, Pertofrane, Phenelzine, Phenytoin, Pimozide, pipotiazine palmate, Piportil, Priadel,Primidone, Prisdal, Prolixin, Protriptyline, Provigil,Prozac, Psiquial,Quetiapine, Reboxetine, Remeron, Restoril, Risperdal, Risperidone, Ritalin, Rivotril, Sabril,Saroten,Serax, Sercerin,Serentil, Seroquel, Seropram,Serotex, Seroxat,
Sertraline,Serzone, Sinequan, Stelazine, Stesolid, Sulpiride, Surmontil, Tegretol, Temazepam, Temesta, Tenormin, Thioridazine, Thorazine, Tofranil, Tolrest,Topamax, Topiramate, Tranxene, Tranylcypromine, Trazodone, Triazolam, Trifluoperazine, Trilafon, Trimipramine, Typtanol,Typtizol, Valium, valproic acid, venlafaxine, Veritina,Vestral, Vigabatrin, Vistaril, Vivactil, Wellbutrin, Xanax, Zarontin, Ziprasidone, Zoloft, Zopiclone, and Zyprexa. The combination pharmaceutical compositions of an inhibitor according to the present invention and a known anti-depressant are also within the scope of the present invention.
Spinal cord injury Spinal cord injury or myelopathy, is a disturbance of the spinal cord that results in loss of sensation and/or mobility. The two common types of spinal cord injury are due to trauma and disease. Traumatic injury can be due to automobile accidents, falls, gunshot, diving accidents inter alia, and diseases which can affect the spinal cord include polio, spina bifida, tumors and
Friedreich's ataxia.
Ischemia reperfusion injury following organ transplantation
Lung transplantation, the only definitive therapy for many patients with end stage lung disease, has poor survival rates in all solid allograft recipients. Ischemia reperfusion (IR) injury is one of the leading causes of death in lung allograft recipients. An effective therapy to treat the above mentioned diseases and disorders would be of great therapeutic value.
Ischemia of the brain
Brain injury such as trauma and stroke are among the leading causes of mortality and disability in the western world.
Traumatic brain injury (TBI) is one of the most serious reasons for hospital admission and disability in modern society. Clinical experience suggests that TBI may be classified into primary damage occurring immediately after injury, and secondary damage, which occurs during several days post injury. Current therapy of TBI is either surgical or else mainly symptomatic.
Cerebrovascular diseases occur predominately in the middle and late years of life. They cause approximately 200,000 deaths in the United States each year as well as considerable neurologic disability. The incidence of stroke increases with age and affects many elderly people, a rapidly growing segment of the population. These diseases cause either ischemia-infarction or intracranial hemorrhage.
Stroke is an acute neurologic injury occurring as a result of interrupted blood supply, resulting in an insult to the brain. Most cerebrovascular diseases present as the abrupt onset of focal neurologic deficit. The deficit may remain fixed, or it may improve or progressively worsen, leading usually to irreversible neuronal damage at the core of the ischemic focus, whereas neuronal dysfunction in the penumbra may be treatable and/or reversible. Prolonged periods of ischemia result in frank tissue necrosis. Cerebral edema follows and progresses over the subsequent 2 to 4 days. If the region of the infarction is large, the edema may produce considerable mass effect with all of its attendant consequences.
Neuroprotective drugs are being developed in an effort to rescue neurons in the penumbra from dying, though as yet none has been proven efficacious.
Damage to neuronal tissue can lead to severe disability and death. The extent of the damage is primarily affected by the location and extent of the injured tissue. Endogenous cascades activated in response to the acute insult play a role in the functional outcome. Efforts to minimize, limit and/or reverse the damage have the great potential of alleviating the clinical consequences.
SUMMARY OF THE INVENTION
The present invention provides, in one embodiment, novel double stranded oligonucleotides that inhibit or reduce expression of a gene selected from the group consisting of ABAT; ADRB 1 ; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 and VIP and pharmaceutical compositions comprising one or more such
oligonucleotides or a vector capable of expressing the oligoribonucleotide. The above genes are also referred to herein as "depression-associated" genes, as are all the genes detailed in Tables 1-
3. The present invention further relates to methods for treating or preventing the incidence or severity of various diseases or conditions, particularly mood disorders and neurological conditions, in which gene expression is associated with the etiology or progression of the disease or condition.
In one aspect the present invention provides a compound having the structure:
5' (N)x - Z 3' (antisense strand)
3' Z'-(N')y 5' (sense strand) wherein each of N and N' is a nucleotide which may be modified or unmodified in its sugar residue; wherein each of (N)x and (N')y is an oligonucleotide in which each consecutive N or N' is joined to the next N or N' by a covalent bond; wherein each of x and y is an integer between 18 and 40; wherein each of Z and Z' may be present or absent, but if present is 1-5 nucleotides and is covalently attached at the 3' terminus of the strand in which it is present; and wherein the sequence of (N)x comprises an antisense sequence relative to the mRNA transcribed from a mammalian gene selected from the group consisting of ABAT; ADRBl; ADRB3;
ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRA 1;GMFG; GPM6A;
GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB;
NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl;
PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT;
TPTl; UGT8 and VIP.
In some embodiments the compound comprises a phosphodiester bond. In various embodiments the compound comprises ribonucleotides wherein x = y and wherein x is an integer selected from the group consisting of 19, 20 and 21. In preferred embodiments x = y =19. In some embodiments the compound is blunt ended, for example wherein Z and Z' are both absent. In an alternative embodiment, the compound comprises at least one 3' overhang, wherein at least one of Z or Z' is present.
In some embodiments the compound comprises one or more ribonucleotides unmodified in their sugar residues. In other embodiments the compound comprises at least one ribonucleotide modified in the sugar residue. In some embodiments the compound comprises a modification at the 2' position of the sugar residue. Modifications in the 2' position of the sugar residue include amino, fluoro, methoxy, alkoxy and alkyl moieties. In certain preferred embodiments the modification comprises a ribonucleotide comprising a methoxy moiety at the 2' position (2'-O- methyl; 2'-0-Me; 2'-0-CH3) of the sugar residue.
In some embodiments the compound comprises modified alternating ribonucleotides in one or both of the antisense and the sense strands. In preferred embodiments the compound comprises modified alternating ribonucleotides in the antisense and the sense strands. In some preferred embodiments the middle ribonucleotide of the antisense strand is not modified; e.g. ribonucleotide in position 10 in a 19-mer strand.
In additional embodiments the compound comprises modified ribonucleotides in alternating positions wherein the ribonucleotides at the 5' and 3' termini of the antisense strand are modified in their sugar residues, and the ribonucleotides at the 5' and 3' termini of the sense strand are unmodified in their sugar residues. In some embodiments, neither the antisense nor the sense strands are phosphorylated at the 3' and 5' termini. In other embodiments either or both the antisense and the sense strands are phosphorylated at the 3' termini.
In various embodiments the compound comprises an antisense sequence present in Tables A-DN. In other embodiments the present invention provides a mammalian expression vector comprising an antisense sequence present in Tables A-DN. In certain preferred embodiments the present invention provides a compound having the structure
5' (N) 3' antisense strand
3' (N') 5' sense strand wherein each of N and N' =19 and are fully complementary; wherein alternating ribonucleotides in the antisense and the sense strands are modified to result in a 2'-O-methyl modification in the sugar residue of the ribonucleotides; wherein the ribonucleotides at the 5' and 3' termini of the antisense strand are modified; wherein the ribonucleotides at the 5' and 3' termini of the sense strand are unmodified; wherein the antisense and the sense strands are phosphorylated or non-phosphorylated at the 3' and 5' termini; and
wherein each of N and N' is selected from the group of oligomers set forth in Table A- Table
DN.
In a second aspect the present invention provides a pharmaceutical composition comprising one or more compounds of the present invention, in an amount effective to inhibit human gene expression wherein the gene is selected from the group consisting of ABAT; ADRBl ; ADRB3;
ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1;
CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A;
GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB;
NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT;
TPTl; UGT8 and VIP; and a pharmaceutically acceptable carrier.
In another aspect, the present invention relates to a method for the treatment of a subject in need of treatment for a disease or disorder associated with the expression of a gene wherein the gene is selected from ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2;
EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl;
KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2;
PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2;
SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 and VIP, comprising administering to the subject an amount of an siRNA which reduces or inhibits expression of at least one of the genes.
More specifically, the present invention provides methods and compositions useful in treating a patient suffering from mood disorders and /or neurological or ischemic conditions such as hypertension, hypertensive cerebral vascular disease, a constriction or obstruction of a blood vessel- as occurs in the case of a thrombus or embolus, angioma, blood dyscrasias, any form of compromised cardiac function including cardiac arrest or failure, systemic hypotension; and diseases such as stroke, Parkinson's disease, epilepsy, depression, ALS, Alzheimer's disease, Huntington's disease and any other disease-induced dementia (such as HIV induced dementia for example).
The methods of the invention comprise administering to the patient one or more inhibitory compounds which down-regulate expression of a gene selected from the group consisting of ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 and VIP; and in particular siRNA in a therapeutically effective dose so as to thereby treat the patient. In various embodiments the inhibitor is selected from the group consisting of an siRNA, shRNA, an aptamer, an antisense molecule, miRNA, a ribozyme, and an antibody. In preferred embodiments the inhibitor is siRNA.
According to one embodiment, the present invention provides improved a method for treating or preventing mood disorders and/or depression comprising administering to a subject in need thereof a therapeutically effective amount of at least one siRNA compound that inhibits expression of the mood-disorder associated genes of the present invention. The compositions of the invention can also be administered at a suitable interval(s) either prior to, subsequent to, or substantially concurrent with the administration of a second drug required to treat a pre-existing condition the patient is suffering from, said drug being known to cause depression, mood-swings or other neurological diseases which may be treated by inhibiting the genes described herein. Preferably, the siRNA and the additional drug are administered separately. In some embodiments the siRNA compound that inhibits a gene of the present invention is administered locally while the depression causing drug is administered systemically. The siRNA compounds may be administered prior to, simultaneously with or subsequent to the additional drug. In some embodiments the present invention provides a pharmaceutical composition comprising an siRNA that inhibits depression-associated gene expression; and a pharmaceutically acceptable carrier.
The present invention further relates to the use of compounds which down-regulate the expression of a depression-associated gene, particularly to small interfering RNAs (siRNAs), in the treatment of various diseases, conditions or disorders associated with depression-associated
gene expression including depression, mood disorders and neurological diseases. A non- exhaustive list of conditions to be treated with the compounds of the present invention includes: ADD (attention deficit disorder), ADHD (attention deficit hyperactivity disorder), Autism, anxiety, panic, bi-polar disorder, depression, GAD (generalized anxiety disorder), OCD (obsessive compulsive disorder), PTSD (post-traumatic stress disorder), Phobias, Schizophrenia, Convulsions, Anxiety, Depression, Mania, Manic-depression, Psychosis and mood disorders. Further, use of the compounds of the present invention may also be aimed at relieveing specific symptoms associated with these diseases and conditions, such as, inet alia, hearing voices and psychosis associated with schizophrenia, or dark moods associated with depression.
The pharmaceutical compositions of the present invention can have application in the treatment of any disease in which neuronal degeneration or damage is involved or implicated, such as, inter alia, the following conditions: hypertension, hypertensive cerebral vascular disease, a constriction or obstruction of a blood vessel- as occurs in the case of a thrombus or embolus, angioma, blood dyscrasias, any form of compromised cardiac function including cardiac arrest or failure, systemic hypotension,; and diseases such as stroke, Parkinson's disease, epilepsy, depression, ALS, Alzheimer's disease, Huntington's disease and any other disease-induced dementia (such as HIV induced dementia for example). These conditions are also referred to herein as "neurodegenerative diseases". Trauma to the central nervous system, such as rupture of aneurysm, cardiac arrest, cardiogenic shock, septic shock, spinal cord trauma, head trauma, traumatic brain injury (TBI), seizure, bleeding from a tumor, etc., are also referred to herein as "injury to the central nervous system" and may also be treated using the compounds and compositions of the present invention. Additional conditions to be treated by the compounds of the present invention include acute renal failure, hearing loss, acute respiratory distress syndrome, COPD, pressure sores and glaucoma.
The present invention provides compositions and methods for alleviation or reduction of the symptoms and signs associated with mood disorders or depressive conditions.
In particular, one embodiment of the present invention provides one or more pharmaceutical compositions comprising as an active ingredient an ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl;
CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 or VIP inhibitor further comprising a pharmaceutically acceptable diluent or carrier.
An additional embodiment provides a method for treating depression in a patient who suffers from a mood altering condition, comprising administering to the patient a pharmaceutical composition in a dosage sufficient to reduce the damage. Yet another embodiment provides for the use of a ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 or VIP inhibitor for the preparation of a medicament for promoting or enhancing recovery in a patient who suffers from a mood altering condition and/or depression.
An additional embodiment provides a method for identifying a chemical compound that modulates depression.
The preferred methods, materials, and examples that will now be described are illustrative only and are not intended to be limiting; materials and methods similar or equivalent to those described herein can be used in practice or testing of the invention. Other features and advantages of the invention will be apparent from the following detailed description, and from the claims.
DETAILED DESCRIPTION OF THE INVENTION
The present invention, in some of its embodiments, provides polynucleotides, polypeptides, small moleculeSjCompositions and methods for alleviation or reduction of the symptoms and signs associated with Depression, mood disorders and any illness in which depression or depressive tendencies are a factor, such as, inter alia, ADD (attention deficit disorder), ADHD (attention
deficit hyperactivity disorder), Autism, anxiety, panic, bi-polar disorder, depression, GAD (generalized anxiety disorder), OCD (obsessive compulsive disorder), PTSD (post-traumatic stress disorder), Phobias, Schizophrenia, Convulsions, Anxiety, Depression, Mania, Manic- depression, Psychosis and mood disorders.. Certain aspects of the present invention provide pharmaceutical compositions which reduce or even completely diminish depression. In additional aspects, the present invention provides methods leading to functional improvement after mood disorders or depressive events. These effects are achieved by administering an agent that inhibits the biological activity of or the expression of one or more gene targets as disclosed herein.
Through use of animal models mimicking depression and mood disorders, and using applicant's proprietary gene discovery technology, the inventors of the present invention discovered that a group of genes are involved in the depressive state, also referred to herein as "depression- associated" genes. These genes are presented in Table 1 , and the preferred genes are presented in Tables 2 and 3. Some of these preferred genes are discussed in brief, below.
A few exemplary genes to be inhibited in accordance with the present invention are discussed below.
Transthyretin
Transthyretin (TTR) is a secreted carrier protein expressed in the choroid plexus and liver.
It is a thyroid hormone-binding protein, likely involved in transporting thyroxine from the bloodstream to the brain. Transthyretin is a plasma protein delivering retinol to tissues. Note that CSF concentrations of transthyretin are significantly lowered in the depressed patients;
Change in thyroid system activity occur during lithium augmentation: increase of TSH levels and a decrease of peripheral T3 and T4 levels.
Mood disturbances are frequent in the elderly with elevated serum TSH levels.
Analogues of thyrotropin-releasing hormone produced significant antidepressant effects in rodent model.
T3 is known to cause cell death in primary neuronal cultures.
Treatment of PC 12 cells with T3 leads to cell death and/or growth arrest.
Prostaglandin D2 Synthase (PD2 Synthase; prostaglandin-H-2 D-isomerase)
PD2 Synthase is an enzyme localized to the rough endoplasmic reticulum, nuclear envelope, Golgi apparatus, secretory vesicles and various cytoplasmic domains, and is also secreted. PD2 Synthase is expressed in tissues of the blood-brain barrier, and secreted into the cerebrospinal fluid. It is also expressed in the heart, testis, epididymis and prostate, and secreted into the seminal fluid. Additionally, it is expressed in the eye and secreted into the aqueous humor.
PD2 Synthase catalyzes the conversion of PGH2 to PGD2, a prostaglandin involved in smooth muscle contraction/relaxation and a potent inhibitor of platelet aggregation. It is involved in sedation, NREM sleep and PGE2-induced allodynia, and may have an anti-apoptotic role in oligodendrocytes. PD2 Synthase may be involved in development and maintenance of the blood- brain, blood-retina, blood-aqueous humor and blood-testis barrier, and likely plays a role in both maturation and maintenance of the central nervous system and male reproductive system. PD2 Synthase expression is induced by thyroid hormone.
Note that transgenic (TG) mice that overexpress human PGDS gene show changed pattern of NREM sleepand NREM sleep cycle is changed in depressed patients. PTGDS catalyses the synthesis of PGD2, which binds to the PTDGD receptor and stimulates cAMP cascade, which can play a role in pathophysiology of depression.
Inhibitors of Prostaglandin D2 Synthase fPD2 Synthase*), (also termed "ptgs" or alternatively "pgd2")
* competitive inhibitors of 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase such as lovastatin, simvastatin and pravastatin;
* compounds disclosed in JP 2004002248 (anthraquinones);
* A cyclooxygenase-2 inhibitor; * anti-PGD2S antibody;
* insulin, insulin-like growth factor- 1, and platelet-derived growth factor
* Biliverdin, Bilirubin, (and other bile pigments), thyroid hormones, steroid hormones
* inorganic selenium compounds (SeC14 and Na2SeO3)
* saturated and unsaturated fatty acids such as Myristic acid, stearic, oleic, linoleic, and linolenic Arachidonic acid
* compounds disclosed in WO2004016223
* compounds disclosed in JP2004051600
* Tranilast [N-(3,4-dimethoxycinnamoyl)anthranilic acid]
* Inhibition of prostaglandinD receptor by 4-benzhydryloxy-l-[3-(lH-tetrazol-5- yl)propyl]piperidine
References for PD2-synthase inhibitors:
J. Neurosci. 2002, JuI 1; 22(13): 5679-86; Brain Research (1993), 623(1), 65-71.; Archives of Biochemistry and Biophysics (1991), 289(1), 161-6.; JP2004051600; Japanese Journal of Pharmacology (1998), 78(1), 11-22; JP2004002248; Biochemistry (1999), 38(25), 8006- 8013.; Biochemistry International (1990), 22(4), 601-5.; Biochemical Pharmacology (1989), 38(16), 2673-6.; Biochimica et Biophysica Acta (1983), 752(2), 251-8.
Adrenergic Receptor, beta 3 (Adrb3)
Adrb3 is an integral membrane receptor localized mainly in adipose tissues. Adrb3 mediates the catecholamine- induced activation of adenylate cyclase through the action of G proteins, and is involved in the regulation of lipolysis and thermogenesis.
Note that decrease in Adrb binding was observed in the polymorphonuclear leukocytes (PMNs) of the depressed patients.
Central administration of isoproterenol, a non-selective beta adrenergic agonist, produces behavioral changes in animal models sensitive to antidepressants.
Phosphodiesterase 4D (PDE4D)
Phosphodiesterase 4D is a cAMP-specific phosphodiesterase enzyme associated with carotid and cardiogenic stroke. Note that pharmacological inhibition of this enzyme produces antidepressant-like effects in animals.
PDE4D-/- mice exhibited decreased immobility in tail-suspension and forced-swim tests; Chronic fluoxetine treatment decreased expression of PDE4D in some brain regions.
Gamma-Aminobutyric acid (GABA) A Receptor, pi subunit (GABRP)
GABRP is an integral membrane receptor channel, expressed in the CNS, uterus, lung, thymus and prostate, which functions as a major inhibitory neurotransmitter in brain, mediating neuronal inhibition by binding to the GABA/Benzodiazepine receptor and opening an integral chloride channel. Note that GABA levels are decreased in animal models of depression and in mood disorder patients.
Fluoxetine significantly decreased responsiveness of PFC neurons to the iontophoretic administration of bicuculline (GABA(A) antagonist) .
.Polymorphisms on GABRAl and GABRA6 genes are associated with mood disorders in female patients.
Citalopram increases pregnanolone sensitivity in patients with premenstrual dysphoric disorder . Diminished GABA(A) Receptor-Binding capacity and a DNA base substitution were found in a patient with treatment-resistant depression. BDNF (brain-derived neurotrophic factor) modulates the activity of GABA(A) receptors.
Pro-Melanin-Concentrating Hormone Precursor fPMCH)
PCMH is a pro-hormone neuromodulator expressed in the Lateral hypothalamus, neocortex, palladium, cerebellum, thymus, brown adipose tissue, duodenum and testis. It is differentially processed in the brain and peripheral organs producing two neuropeptides: NEI and MCH.
MCH acts as a neurotransmitter or neuromodulator in regulation of goal-directed behavior, such as food intake, and general arousal.
SNAP-7941, a selective, high-affinity MCHl receptor (MCHl-R) antagonist has been shown to possess antidepressant, anxiolytic and anorectic properties
Dual Specificity Phosphatase 6 (Duspό) aka MAP Kinase Phosphatase 3
Duspβ is an enzyme localized in the cell cytoplasm, ambiguously expressed with highest levels in heart and pancreas. Duspό inactivates MAP kinases by dephosphorylating both the phosphoserine/threonine and phosphotyrosine residues. Further, Duspό has a certain specificity for the ERK family.
Duspόmay act together with CDK5 in regulating MAP kinase pathway.
ERX activation by neurothropic factors such as BDNF has a known positive effect on cell survival and neuroplasticity, therefore down regulation of the DUSP-6 may increase survival pathways and be cytoprotective
Calcium/Calmodulin-Dependent Protein Kinase II alpha subunit (Camk2a / 2b)
Camk2a/b is an enzyme localized in the cell cytoplasm and expressed in the brain and skeletal muscle.
Camk2a/b functions in long-term potentiation and neurotransmitter release, possesses Calcium- calmodulin (CaM)-dependent activity, and undergoes autophosphorylation, resulting in CaM- independent activity. CaMKII alpha mRNA expression is significantly reduced in the prefrontal cortex of patients with bipolar illness
Vasoactive Intestinal Polypeptide (VIP)
VIP is a secreted hormone expressed in the brain, prostate and lung. VIP stimulates myocardial contractility, causes vasodilation, increases glycogenosis, lowers arterial blood pressure and relaxes the smooth muscles of trachea, stomach and gall bladder. VIP appears to play a role in the temporal organization of sleep.
Note that VIP levels in non-endogenously depressed patients are significantly lower than those of controls and endogenous depressives. In rats VIP injection elicited rapid eye movement sleep.
ATPase. Na+K+ Transporting, alpha 1 (Atplal)
Atplal is an integral membrane enzyme transporter, functioning as a catalytic component of the active enzyme (ATPase).
Atplal catalyzes the hydrolysis of ATP coupled with the exchange of sodium and potassium ions across the plasma membrane and creates the energy for active transport of various nutrients. Mutations in Atplal are related to hypertension.
Myocyte Enhancer Factor 2C (MEF2C)
MEF2C is a transcription factor, localized to the nucleus.
MEF2C is involved in cardiac myogenesis, morphogenesis and neurogenesis, regulates the expression of genes that are critical for newly differentiated neurons, and is activated by p38. p38alpha/MEF2 pathway prevents cell death during neuronal differentiation.
Cyclin-Dependent Kinase 5, Regulatory Subunit 1 (p35) (Cdk5r)
Cdk5r is an enzyme localization either at the cell periphery or cytoplasm dependent on the cellular state, expressed in the brain and neuron specific.
Cdk5r is a neuron-specific activator of cyclin-dependent kinase 5 (CDK5); the activation of
CDK5 is required for proper development of the central nervous system, for neurite outgrowth and cortical lamination. The p35 form of Cdk5r is proteolytically cleaved generating a p25 form.
The cleavage of p35 into p25 results in relocalization of the protein from the cell periphery to nuclear and perinuclear regions. P25 deregulates CDK5 activity by prolonging its activation and changing its cellular location. Additionally, the p25 form accumulates in the brain neurons of patients with Alzheimer's disease.
Nitric Oxide Synthase 1 (NOSl). neuronal
NOSl is a ubiquitously expressed enzyme which produces nitric oxide (NO) which is a messenger molecule. In the brain and peripheral nervous system, NO displays many properties of a neurotransmitter. NOS 1 is stimulated by calcium/calmodulin. Note that NOS activities were increased in serum of CMS rats.
Various inhibitors of NOS have been shown to possess antidepressant- and anxiolytic-like properties in animal models.
The number of NOS-immunoreactive neurons in hypothalamus was significantly reduced in depression.
Neurogranin
Neurogranin is a brain-specific, post-synaptically located protein kinase C (PKC) substrate. It is also a Ca(2+)-sensitive calmodulin (CaM)-binding protein, the CaM-binding affinity being modulated by phosphorylation and oxidation. Neurogranin is expressed in the brain cortex, hippocampus, striatum, and amygdala.
Chimerin 1
Chimerinl is an enzyme, a GTPase activating protein for p21-rac and a phorbol ester receptor, which may play an important role in neuronal signal- transduction mechanisms. Chimerinl is expressed in neurons of brain regions that are involved in learning and memory, and increases in amount during brain development coincident with synaptogenesis.
Dendrin
Dendrin is induced by sleep deprivation and predicted to serve as a transcription factor. It is expressed exclusively in forebrain structures, with a near exclusive dendritic localization.
Glia Maturation Factor gamma (GMF)
GMF is a predicted intracellular enzyme, expressed in the lung, heart and placenta. Phosphorylated GMF is a potent inhibitor of ERK1/ERK2 subfamily of mitogen-activated protein (MAP) kinase and a strong enhancer of p38 MAP kinase activity in vitro. Thus, GMF is an intracellular regulator of signal transduction pathways..
Activation of ERK by neurotrophic factors such as BDNF has a known positive effect on cell survival and neuroplasticity, protecting cells from chronic stress damage. Thus, GMF by inhibiting ERK and promoting p38 MAPK activation may facilitate neuronal cell-apoptosis
Voltage-Dependent Calcium Channel (beta 4 Subunit) (Cacnb4)
Cacnb4 is an integral membrane channel which contributes to the function of the calcium channel by: increasing peak calcium current, shifting the voltage dependencies of activation and inactivation, modulating G protein inhibition and controlling the alpha- 1 subunit membrane targeting. Defects in cacnb4 are a cause of idiopathic generalized epilepsy (IGE), which is characterized by recurrent generalized seizures. Defects in cacnb4 are also a cause of juvenile myoclonic epilepsy (JME), which is a common epileptic syndrome characterized by afebrile seizures, onset in adolescence (rather than in childhood) and myoclonic jerks.
Solute Carrier Family 17 (Sodium-Dependent Inorganic Phosphate Cotransporter). Member 7 (SLC17A7)
SLC17A7 is a vesicle-bound, sodium-dependent integral membrane phosphate transporter, localized to protein synaptic vesicles, and expressed specifically in neuron-rich regions of the Brain. SLCl 7 A7 also functions in glutamate transport.
Solute Carrier Family 8 (Sodium/Calcium Exchanger), Member 1 (SLC8A1)
SLC8A1 is an integral membrane transporter which rapidly transports Ca(2+) during excitation- contraction coupling.
Dopamine Receptor D3 (Drd3) Drd3 is an integral membrane receptor expressed in the brain, involved in synaptic transmission.
Drd3 Inhibits adenylyl cyclase through inhibitory G-proteins, may play a role in cognitive and emotional functions, and serves as a target for drugs which treat schizophrenia and Parkinson disease.
Polymorphisms in this gene are connected to various normal and pathological behavioral paradigms; additionally, the allele of D2 subtype is related to the response to paroxetine treatment
Mitogen-Activated Protein Kinase Kinase Kinase Kinase 5 (MAPKKKK5).
MAPKKKK5 is an intracellular enzyme, a member of the MAP kinase family. Further, it is a neuronal-specific form of c-Jun N-terminal kinases (JNKs), and may play a role in the response to environmental stress. It also appears to act upstream of the c-jun N-terminal pathway.
Rho GTPase activating protein 6 (ARHGAP6) ARHGAP6 is a cytoplasmic, ubiquitously expressed cytoskeletal GTPase-activating protein (GAP) with specificity for RhoA, and also promotes actin remodeling. ARHGAP6 undergoes X inactivation and mutations in ARHGAP6 may be a cause of microphthalmia with linear skin defects (MLS) - a dominant male- lethal disorder characterized by eye, skin and central nervous system (CNS) malformations.
Without being bound by theory, applicants suggest that an inhibitor preferably a nucleotide inhibitor, to one or more of the following genes can prevent or ameliorate a depressive condition in a patient: ABAT - 4-aminobutyrate aminotransferase; ADRBl - adrenergic, beta-1-, receptor; ADRB3 - adrenergic, beta-3-, receptor; ARHGEF9 - Cdc42 guanine nucleotide exchange factor (GEF) 9; ARRBl - arrestin, beta 1; ATPlAl - ATPase, Na+/K+ transporting, alpha 1 polypeptide; CACNB4 - calcium channel, voltage-dependent, beta 4 subunit; CAMK2A - calcium/calmodulin-dependent protein kinase (CaM kinase) II alpha; CAMK2D - calcium/calmodulin-dependent protein kinase (CaM kinase) II delta; CBLNl - cerebellin 1 precursor; CDH22 - cadherin-like 22; CDK5R1 - cycl in-dependent kinase 5, regulatory subunit 1; CHNl - chimerin (chimaerin) 1; CTSD - cathepsin D; DDN - dendrin; DRD3 - dopamine receptor D3; DUSP6 - dual specificity phosphatase 6; ENPPl - ectonucleotide pyrophosphatase/phosphodiesterase 1; ENPP2 - ectonucleotide pyrophosphatase/phosphodiesterase 2; EPHA4 - EPH receptor A4; GABRAl - gamma- aminobutyric acid; GMFG - glia maturation factor, gamma; GPM6A - glycoprotein M6A; GPNMB - glycoprotein (transmembrane) nmb; GPR23 - G protein-coupled receptor 23; HAPLN4 - hyaluronan and proteoglycan link protein 4; IGF2 - insulin-like growth factor 2; IGFBP2 - insulin-like growth factor binding protein 2; KCNAl - potassium voltage-gated channel, shaker-related subfamily, member 1; KIF5A - kinesin family member 5 A; MAPKlO - mitogen-activated protein kinase 10; MEF2C - myocyte enhancer factor 2C; NAPB - N- ethylmaleimide-sensitive factor attachment protein, beta; NOS 1 - nitric oxide synthase 1 ; NPTX2 - neuronal pentraxin II; NRGN - neurogranin; NTS - neurotensin; NUCBl - nucleobindin 1; PCP4 - Purkinje cell protein 4; PDCD2 - programmed cell death 2; PDE4D - phosphodiesterase 4D; PENK - proenkephalin; PHCA - phytoceramidase, alkaline; PJA2 - praja 2, RING-H2 motif containing; PLPl - proteolipid protein 1; PMCH - pro-melanin-concentrating hormone; PVALB - parvalbumin; QDPR - quinoid dihydropteridine reductase; RPNl - ribophorin I; SLC17A7 - solute carrier family 17, member 7 ; SLC28A2 - solute carrier family 28 (sodium-coupled nucleoside transporter), member 2; SLC8A1 - solute carrier family 8 (sodium/calcium exchanger), member 1; SNAP91 - synaptosomal-associated protein, 9IkDa homolog; SYN2 - synapsin II; SYTl - synaptotagmin I; TKT - transketolase; TPTl - tumor protein, translationally- controlled 1; UGT8 - UDP glycosyltransferase 8 and VIP - vasoactive intestinal peptideOf additional interest are inhibitors of any one of the genes presented in Table 1, 2 or 3, inhibitors of
which can also prevent or ameliorate mood disorders or depression.
By "inhibitor" is meant any molecule, whether a polynucleotide, polypeptide, antibody, or small chemical compound, that prevents or reduces the biological effect of ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 or VIP, or any other desired gene such as, inter alia, any gene presented in Table 1. An inhibitor may also be an inhibitor of the promoter or of transcription/translation such as an antisense RNA molecule, siRNA, dominant negative peptide, ribozyme, inter alia.
In one embodiment, the present invention relates generally to compounds which down-regulate expression of genes, particularly to novel small interfering RNAs (siRNAs), and to the use of these siRNAs in the treatment of various diseases and medical conditions. Particular diseases and conditions to be treated are depression, mood disorders and neurological diseases. Particular genes to be inhibited in order to treat said diseases and conditions are presented in Tables 1-3; the most preferable genes are presented in Table 3. Lists of preferred siRNA are provided in Tables A-DN infra. The separate lists of 19-mer and 21- mer siRNAs are prioritized based on their score according to a proprietary algorithm as the best sequences for targeting the human gene expression. Methods, molecules and compositions, which inhibit genes are discussed herein at length, and any of said molecules and/or compositions may be beneficially employed in the treatment of a patient suffering from any of said conditions. Tables A, C, E, G, I, K, M, O, Q, S, U, W, Y, AA, AC, AE, AG, AI, AK, AM, AO, AQ, AS, AU, AW, AY, BA, BC, BE, BG, BI, BK, BM, BO, BQ, BS, BU, BW, BY, CA, CC, CE, CG, CI, CK, CM, CO, CQ, CS, CU, CW, CY, DA, DC, DE, DG, DI, DK and DM set forth 19-mer oligomers. Tables B, D, F, H, J, L, N, P, R, T, V, X, Z, AB, AD, AF, AH, AJ, AL, AN, AP, AR, AT, AV, AX, AZ, BB, BD, BF, BH, BJ, BL, BN, BP, BR, BT, BV, BX, BZ, CB, CD, CF, CH, CJ, CL, CN, CP, CR, CT, CV, CX, CZ, DB, DD, DF, DH, DJ, DL and DN set forth 21-mer oligomers.
Lists of 19- and 21-mer sense and corresponding antisense sequences useful in preparation of siRNA compounds are set forth in Tables A-DN.
A list of preferred siRNA to ABAT is provided in tables A-B, infra. A list of preferred siRNA to ADRB 1 is provided in tables C-D, infra.
A list of preferred siRNA to ADRB3 is provided in tables E-F, infra.
A list of preferred siRNA to ARHGEF9 is provided in tables G-H, infra.
A list of preferred siRNA to ARRBl is provided in tables I- J, infra.
A list of preferred siRNA to ATPlAl is provided in tables K-L, infra. A list of preferred siRNA to CACNB4 is provided in tables M-N, infra.
A list of preferred siRNA to CAMK2A is provided in tables O-P, infra.
A list of preferred siRNA to CAMK2D is provided in tables Q-R, infra.
A list of preferred siRNA to CBLNl is provided in tables S-T, infra.
A list of preferred siRNA to CDH22 is provided in tables U-V, infra. A list of preferred siRNA to CDK5R1 is provided in tables W-X, infra.
A list of preferred siRNA to CHNl is provided in tables Y-Z, infra.
A list of preferred siRNA to CTSD is provided in tables AA-AB, infra.
A list of preferred siRNA to DDN is provided in tables AC-AD, infra.
A list of preferred siRNA to DRD3 is provided in tables AE-AF, infra. A list of preferred siRNA to DUSP6 is provided in tables AG-AH, infra.
A list of preferred siRNA to ENPPl is provided in tables AI-AJ, infra.
A list of preferred siRNA to ENPP2 is provided in tables AK-AL, infra.
A list of preferred siRNA to EPHA4 is provided in tables AM-AN, infra.
A list of preferred siRNA to GABRAl is provided in tables AO-AP, infra. A list of preferred siRNA to GMFG is provided in tables AQ-AR, infra.
A list of preferred siRNA to GPM6A is provided in tables AS-AT, infra.
A list of preferred siRNA to GPNMB is provided in tables AU-AV, infra.
A list of preferred siRNA to GPR23 is provided in tables AW-AX, infra.
A list of preferred siRNA to HAPLN4 is provided in tables AY-AZ, infra. A list of preferred siRNA to IGF2 is provided in tables BA-BB, infra.
A list of preferred siRNA to IGFBP2 is provided in tables BC-BD, infra.
A list of preferred siRNA to KCNAl is provided in tables BE-BF, infra.
A list of preferred siRNA to KIF5A is provided in tables BG-BH, infra.
A list of preferred siRNA to MAPKlO is provided in tables BI-BJ, infra.
A list of preferred siRNA to MEF2C is provided in tables BK-BL, infra. A list of preferred siRNA to NAPB is provided in tables BM-BN, infra.
A list of preferred siRNA to NOSl is provided in tables BO-BP, infra.
A list of preferred siRNA to NPTX2 is provided in tables BQ-BR, infra.
A list of preferred siRNA to NRGN is provided in tables BS-BT, infra.
A list of preferred siRNA to NTS is provided in tables BU-BV, infra. A list of preferred siRNA to NUCB 1 is provided in tables BW-BX, infra.
A list of preferred siRNA to PCP4 is provided in tables BY-BZ, infra.
A list of preferred siRNA to PDCD2 is provided in tables CA-CB, infra.
A list of preferred siRNA to PDE4D is provided in tables CC-CD, infra.
A list of preferred siRNA to PENK is provided in tables CE-CF, infra. A list of preferred siRNA to PHCA is provided in tables CG-CH, infra.
A list of preferred siRNA to PJA2 is provided in tables CI-CJ, infra.
A list of preferred siRNA to PLPl is provided in tables CK-CL, infra.
A list of preferred siRNA to PMCH is provided in tables CM-CN, infra.
A list of preferred siRNA to PVALB is provided in tables CO-CP, infra. A list of preferred siRNA to QDPR is provided in tables CQ-CR, infra.
A list of preferred siRNA to RPNl is provided in tables CS-CT, infra.
A list of preferred siRNA to SLC17A7 is provided in tables CU-CV, infra.
A list of preferred siRNA to SLC28A2 is provided in tables CW-CX, infra.
A list of preferred siRNA to SLC8A1 is provided in tables CY-CZ, infra. A list of preferred siRNA to SNAP91 is provided in tables DA-DB, infra.
A list of preferred siRNA to SYN2 is provided in tables DC-DD, infra.
A list of preferred siRNA to SYTl is provided in tables DE-DF, infra.
A list of preferred siRNA to TKT is provided in tables DG-DH, infra.
A list of preferred siRNA to TPTl is provided in tables DI-DJ, infra. A list of preferred siRNA to UGT8 is provided in tables DK-DL, infra.
A list of preferred siRNA to VIP is provided in tables DM-DN, infra.
Definitions
For convenience certain terms employed in the specification, examples and claims are described herein. An "inhibitor" is a compound which is capable of inhibiting or reducing the expression or activity of a gene or the product of such gene to an extent sufficient to achieve a desired biological or physiological effect. The term "inhibitor" as used herein refers to one or more of an oligonucleotide inhibitor, including siRNA, shRNA, aptamers, antisense molecules, miRNA and ribozymes, as well as antibodies.
As used herein, the term "polypeptide" refers to, in addition to a polypeptide, an oligopeptide, peptide and a full protein. The present invention provides compounds that inhibit one or more isoforms of a gene, in the event that more than one isoforms exits.
RNA interference and siRNA RNA interference (RNAi) is based on the ability of dsRNA species to enter a cytoplasmic protein complex, where it is then targeted to the complementary cellular RNA and specifically degrade it. The RNA interference response features an endonuclease complex containing an siRNA, commonly referred to as an RNA-induced silencing complex (RISC), which mediates cleavage of single-stranded RNA having a sequence complementary to the antisense strand of the siRNA duplex. Cleavage of the target RNA may take place in the middle of the region complementary to the antisense strand of the siRNA duplex (Elbashir et al., Genes Dev., 2001, 15(2): 188-200). In more detail, longer dsRNAs are digested into short (17-29 bp) dsRNA fragments (also referred to as short inhibitory RNAs, "siRNAs") by type III RNAses (DICER, DROSHA, etc.; Bernstein et al., Nature, 2001, 409(6818):363-6; Lee et al., Nature, 2003, 425(6956):415-9). The RISC protein complex recognizes these fragments and complementary mRNA. The whole process is culminated by endonuclease cleavage of target mRNA (McManus & Sharp, Nature Rev Genet, 2002, 3(10):737-47; Paddison & Hannon, Curr Opin MoI Ther. 2003, 5(3):217-24). (For additional information on these terms and proposed mechanisms, see for example Bernstein et al., RNA 2001, 7(l l):1509-21; Nishikura, Cell 2001, 107(4):415-8 and PCT publication WO 01/36646).
The selection and synthesis of siRNA corresponding to known genes has been widely reported; see for example Ui-Tei et al., J Biomed Biotechnol. 2006; 2006: 65052; Chalk et al., Biochem. Biophys. Res. Comm. 2004, 319(1): 264-74; Sioud & Leirdal, Met. MoI Biol.; 2004, 252:457-69; Levenkova et al., Bioinform. 2004, 20(3):430-2; Ui-Tei et al., Nuc. Acid Res. 2004, 32(3):936- 48. For examples of the use of, and production of, modified siRNA see Braasch et al., Biochem., 2003, 42(26):7967-75; Chiu et al., RNA, 2003, 9(9): 1034-48; PCT publications WO 2004/015107 (assigned to Atugen); WO 02/44321 (Tuschl et al), and US Patent Nos. 5,898,031 and 6,107,094. Several groups have described the development of DNA-based vectors capable of generating siRNA within cells. The method generally involves transcription of short hairpin RNAs that are efficiently processed to form siRNAs within cells (Paddison et al. PNAS USA 2002, 99:1443- 1448; Paddison et al. Genes & Dev 2002, 16:948-958; Sui et al. PNAS USA 2002, 8:5515-5520; and Brummelkamp et al. Science 2002, 296:550-553). These reports describe methods to generate siRNAs capable of specifically targeting numerous endogenously and exogenously expressed genes.
As used herein, the term "nucleic acid" refers to polynucleotides such as deoxyribonucleic acid (DNA), and, where appropriate, ribonucleic acid (RNA). The terms should also be understood to include, as equivalents, analogs of either RNA or DNA made from nucleotide analogs, such as chemically modified nucleotides and synthetic nucleotides and, as applicable to the embodiment being described, double-stranded polynucleotides and single-stranded polynucleotides such as sense or antisense.
"Oligonucleotide" refers to a sequence having from about 2 to about 50 linked nucleotides or linked modified nucleotides, or a combination of modified and unmodified nucleotide. Oligonucleotide includes the terms oligomer, antisense strand and sense strand. "Nucleotide" is meant to encompass deoxyribonucleotides and ribonucleotides, which may be natural or synthetic, and or modified or unmodified. Modifications include changes to the sugar moiety, the base moiety and or the linkages between ribonucleotides in the oligoribonucleotide. All analogues of, or modifications to, a nucleotide / oligonucleotide may be employed with the present invention, provided that said analogue or modification does not substantially affect the function of the nucleotide / oligonucleotide. The nucleotides can be selected from naturally occurring or synthetic modified bases. Naturally occurring bases include adenine, guanine,
cytosine, thymine and uracil. Modified bases of nucleotides include inosine, xanthine, hypoxanthine, 2- aminoadenine, 6-methyl, 2-propyl and other alkyl adenines, 5-halo uracil, 5- halo cytosine, 6-aza cytosine and 6-aza thymine, psuedo uracil, 4- thiuracil, 8-halo adenine, 8- aminoadenine, 8-thiol adenine, 8-thiolalkyl adenines, 8-hydroxyl adenine and other 8-substituted adenines, 8-halo guanines, 8-amino guanine, 8-thiol guanine, 8-thioalkyl guanines, 8- hydroxyl guanine and other substituted guanines, other aza and deaza adenines, other aza and deaza guanines, 5-trifluoromethyl uracil and 5- trifluoro cytosine.
In addition, analogues of polynucleotides can be prepared wherein the structure of one or more nucleotide is fundamentally altered and better suited as therapeutic or experimental reagents. An example of a nucleotide analogue is a peptide nucleic acid (PNA) wherein the deoxyribose (or ribose) phosphate backbone in DNA (or RNA is replaced with a polyamide backbone which is similar to that found in peptides. PNA analogues have been shown to be resistant to enzymatic degradation and to have extended lives in vivo and in vitro. Other modifications that can be made to oligonucleotides include polymer backbones, cyclic backbones, acyclic backbones, thiophosphate-D-ribose backbones, triester backbones, thioate backbones, 5'-2' bridged backbone, artificial nucleic acids, morpholino nucleic acids, locked nucleic acid (LNA), glycol nucleic acid (GNA), threose nucleic acid (TNA), arabinoside, and mirror nucleoside (for example, beta-L-deoxynucIeoside instead of beta-D-deoxynucleoside). Examples of siRNA compounds comprising LNA nucleotides are disclosed in Elmen et al., (NAR 2005. 33(1):439- 447).
A "mirror" nucleotide is a nucleotide with reversed chirality to the naturally occurring or commonly employed nucleotide, i.e., a mirror image (L-nucleotide) of the naturally occurring (D- nucleotide). The nucleotide can be a ribonucleotide or a deoxyribonucleotide and my further comprise at least one sugar, base and or backbone modification. US patent No. 6,602,858 discloses nucleic acid catalysts comprising at least one L-nucleotides substitution.
The present invention provides methods and compositions for inhibiting expression of a depression associated gene in vivo. In general, the method includes administering oligoribonucleotides, in particular small interfering RNAs (i.e., siRNAs) or a nucleic acid material that can produce siRNA in a cell, that targets an mRNA, in an amount sufficient to down-regulate expression of a target gene by an RNA interference mechanism. In particular, the
subject method can be used to inhibit expression of depression associated gene for treatment of a disease.
In accordance with the present invention, the siRNA compounds or other inhibitors are used as drugs to treat various pathologies.
The present invention provides double-stranded oligoribonucleotides (eg. siRNAs), which down- regulate the expression of a gene selected from the group consisting of ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 and VIP.
An siRNA of the invention is a duplex oligoribonucleotide in which the sense strand is derived from the mRNA sequence of said genes, and the antisense strand is complementary to the sense strand. In general, some deviation from the target mRNA sequence is tolerated without compromising the siRNA activity (see e.g. Czauderna et al., Nuc. Acids Res. 2003, 31(11):2705- 2716). An siRNA of the invention inhibits gene expression on a post-transcriptional level with or without destroying the mRNA. Without being bound by theory, siRNA may target the mRNA for specific cleavage and degradation and/ or may inhibit translation from the targeted message. In some embodiments the oligoribonucleotide according to the present invention comprises modified siRNA. In various embodiments the siRNA comprises an RNA duplex comprising a first strand and a second strand, whereby the first strand comprises a ribonucleotide sequence at least partially complementary to about 18 to about 40 consecutive nucleotides of a target nucleic acid, and the second strand comprises ribonucleotide sequence at least partially complementary to the first strand and wherein said first strand and/or said second strand comprises a plurality of groups of modified ribonucleotides having a modification at the 2'-position of the sugar moiety whereby within each strand each group of modified ribonucleotides is flanked on one or both sides by a group of flanking ribonucleotides whereby each ribonucleotide forming the group of
flanking ribonucleotides is selected from an unmodified ribonucleotide or a ribonucleotide having a modification different from the modification of the groups of modified ribonucleotides. The group of modified ribonucleotides and/or the group of flanking ribonucleotides comprise a number of ribonucleotides selected from the group consisting of an integer from 1 to 10. Accordingly, the group thus comprises one nucleotide, two nucleotides, three nucleotides, four nucleotides, five nucleotides, six nucleotides, seven nucleotides, eight nucleotides, nine nucleotides or ten nucleotides.
The groups of modified nucleotides and flanking nucleotides may be organized in a pattern on at least one of the strands. In some embodiments the first and second strands comprise a pattern of modified nucleotides. In various embodiments the pattern of modified nucleotides of said first strand is identical relative to the pattern of modified nucleotides of the second strand.
In other embodiments the pattern of modified nucleotides of said first strand is shifted by one or more nucleotides relative to the pattern of modified nucleotides of the second strand. In some preferred embodiments the middle ribonucleotide in the first strand (antisense) is an unmodified nucleotide. For example, in a 19-oligomer antisense strand, ribonucleotide number 10 is unmodified; in a 21 -oligomer antisense strand, ribonucleotide number 11 is unmodified; and in a 23-oligomer antisense strand, ribonucleotide number 12 is unmodified. The modifications or pattern of modification, if any, of the siRNA must be planned to allow for this. The modifications on the 2' moiety of the sugar residue include amino, fluoro, methoxy alkoxy, alkyl, amino, fluoro, chloro, bromo, CN, CF, imidazole, caboxylate, thioate, Ci to Cio lower alkyl, substituted lower alkyl, alkaryl or aralkyl, OCF3, OCN, O-, S-, or N- alkyl; O-, S, or N- alkenyl; SOCH3; SO2CH3; ONO2; NO2, N3; heterozycloalkyl; heterozycloalkaryl; aminoalkylamino; polyalkylamino or substituted silyl, as described, inter alia, in European patents EP 0 586 520 B 1 and EP 0 618 925 B 1.
In some embodiments the siRNA is blunt ended, on one or both ends. More specifically, the siRNA may be blunt ended on the end defined by the 5'- terminus of the first strand and the 3'- terminus of the second strand, or the end defined by the 3'-terminus of the first strand and the 5'- terminus of the second strand. In other embodiments at least one of the two strands may have an overhang of at least one nucleotide at the 5'-terminus; the overhang may consist of at least one deoxyribonucleotide. At
least one of the strands may also optionally have an overhang of at least one nucleotide at the 3'- terminus. The overhang may consist of from about 1 to about 4 nucleotides. The length of RNA duplex is from about 18 to about 40 ribonucleotides, preferably 19 to 23 ribonucleotides. Further, the length of each strand (oligomer) may independently have a length selected from the group consisting of about 15 to about 40 bases, preferably 18 to 23 bases and more preferably 19, 20 or 21 ribonucleotides.
Additionally, in certain preferred embodiments the complementarity between said first strand and the target nucleic acid can be perfect. In some embodiments, the strands are substantially complementary, i.e. having one, two or up to three mismatches between said first strand and the target nucleic acid.
In some embodiments the first strand and the second strand each comprise at least one group of modified ribonucleotides and at least one group of flanking ribonucleotides, whereby each group of modified ribonucleotides comprises at least one ribonucleotide and whereby each group of flanking ribonucleotides comprises at least one ribonucleotide, wherein each group of modified ribonucleotides of the first strand is aligned with a group of flanking ribonucleotides on the second strand, and wherein the 5' most terminal ribonucleotide is selected from a group of modified ribonucleotides, and the 3' most terminal ribonucleotide of the second strand is a selected from the group of flanking ribonucleotide. In some embodiments each group of modified ribonucleotides consists of a single ribonucleotide and each group of flanking ribonucleotides consists of a single nucleotide.
In yet other embodiments the ribonucleotide forming the group of flanking ribonucleotides on the first strand is an unmodified ribonucleotide arranged in a 3' direction relative to the ribonucleotide forming the group of modified ribonucleotides, and the ribonucleotide forming the group of modified ribonucleotides on the second strand is a modified ribonucleotide which is arranged in 5' direction relative to the ribonucleotide forming the group of flanking ribonucleotides. In some embodiments the first strand of the siRNA comprises five to about twenty, eight to twelve, preferably ten or twelve groups of modified ribonucleotides, and the second strand comprises seven to eleven, preferably nine or eleven groups of modified ribonucleotides. The first strand and the second strand may be linked by a loop structure, which may be comprised of a non-nucleic acid polymer such as, inter alia, polyethylene glycol.
Alternatively, the loop structure may be comprised of a nucleic acid, including modified and non- modified ribonucleotides and modified and non-modified deoxyribonucleotides. Further, the 5'-terminus of the first strand of the siRNA may be linked to the 3'-terminus of the second strand, or the 3'-terminus of the first strand may be linked to the 5'-terminus of the second strand, said linkage being via a nucleic acid linker typically having a length between 3-100 nucleotides, preferably about 3 to about 10 nucleotides.
In various embodiments, the present invention provides a compound having structure A:
5' (N)x - Z 3' (antisense strand) 3' Z'-(N')y 5' (sense strand)
wherein each N and N' is a ribonucleotide selected from the group consisting of a modified ribonucleotide or an unmodified ribonucleotide and each of (N)x and (N')y is an oligomer in which each consecutive N or N' is joined to the next N or N' by a covalent bond;
wherein each of x and y is an integer between 18 and 40;
wherein each of Z and Z' may be present or absent, but if present is comprises 1-5 nucleotides and is covalently attached at the 3' terminus of the strand in which it is present;
and wherein the sequence of (N)x comprises an antisense sequence having substantial identity to about 18 to about 40 consecutive ribonucleotides in the mRNA transcribed from a gene selected from the group consisting of ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 and VIP.
In certain embodiments the antisense and sense sequences are selected from the sequences presented in Tables A-DN. All sequences are provided in 5 '-3' orientation. Where cross-species specificity is known, the species is (are) listed, as well.
It will be readily understood by those skilled in the art that the compounds of the present invention consist of a plurality of modified and/or unmodified ribonucleotides, which are linked through covalent linkages. Each such covalent linkage may be a phosphodiester linkage, a phosphorothioate linkage, or a combination of both, along the length of the ribonucleotide sequence of the individual strand. Other possible backbone modifications are described inter alia in U.S. Patent Nos. 5,587,361; 6,242,589; 6,277,967; 6,326,358; 5,399,676; 5,489,677; and 5,596,086.
In particular embodiments, x and y are independently an integer between about 18 to about 40, preferably from about 19 to about 23. In a particular embodiment, x is equal to y (i.e. x = y) and in preferred embodiments x = y = 19, x = y = 20 or x = y = 21. In a particularly preferred embodiment x = y = 19.
. In some embodiments the compound is blunt ended, for example wherein Z and Z' are both absent. In an alternative embodiment, the compound comprises at least one 3' overhang, wherein at least one of Z or Z' is present. Z and Z' can independently comprise one or more covalently linked modified or non-modified nucleotides, as described herein, for example inverted dT or dA; dT, LNA, mirror nucleotide and the like. In some embodiments each of Z and Z' are independently selected from dT and dTdT.
In one embodiment all of the ribonucleotides of the compound are unmodified in their sugar residues. In certain preferred embodiments at least one ribonucleotide is modified in its sugar residue, preferably by the addition of a moiety at the 2' position. A preferred moiety is selected from the group consisting of amino, fluoro, methoxy, alkoxy and alkyl groups. In a presently preferred embodiment the moiety at the 2' position is methoxy (2'-0-Me).
In preferred embodiments of the invention, alternating ribonucleotides are modified in both the antisense and the sense strands of the compound. In particular the exemplified siRNA has been modified such that a 2'-O-methyl (Me) group was present on the first, third, fifth, seventh, ninth,
eleventh, thirteenth, fifteenth, seventeenth and nineteenth nucleotide of the antisense strand, whereby the very same modification, i. e. a 2'-0-Me group, was present at the second, fourth, sixth, eighth, tenth, twelfth, fourteenth, sixteenth and eighteenth nucleotide of the sense strand. Additionally, it is to be noted that these particular siRNA compounds are also blunt ended. In various preferred embodiments of the compounds of the invention having alternating ribonucleotides modified in both the antisense and the sense strands of the compound, for 19- mers and 23-mers the ribonucleotides at the 5' and 3' termini of the antisense strand are modified in their sugar residues, and the ribonucleotides at the 5' and 3' termini of the sense strand are unmodified in their sugar residues. For 21 mers the ribonucleotides at the 5' and 3' termini of the sense strand are modified in their sugar residues, and the ribonucleotides at the 5' and 3' termini of the antisense strand are unmodified in their sugar residues. As mentioned above, it is preferred that the middle nucleotide of the antisense strand is unmodified.
According to one preferred embodiment of the invention, the antisense and the sense strands of the siRNA are phosphorylated only at the 3 '-terminus and not at the 5 '-terminus. According to another preferred embodiment of the invention, the antisense and the sense strands are non- phosphorylated. According to yet another preferred embodiment of the invention, the 5' most ribonucleotide in the sense strand is modified, for example to abolish any possibility of in vivo 5'- phosphorylation. The invention further provides a vector capable of expressing any of the aforementioned oligoribonucleotides in unmodified form in a cell after which appropriate modification may be made. In preferred embodiment the cell is a mammalian cell, preferably a human cell. Substantially complementary refers to complementarity of greater than about 84%, to another sequence. For example in a duplex region consisting of 19 base pairs one mismatch results in 94.7% complementarity, two mismatches results in about 89.5% complementarity and 3 mismatches results in about 84.2% complementarity, rendering the duplex region substantially complementary. Accordingly substantially identical refers to identity of greater than about 84%, to another sequence.
More particularly, the invention provides an antisense oligoribonucleotide wherein one strand comprises consecutive nucleotides having, from 5' to 3', the sequence set forth in Tables A-DN or a homolog thereof wherein in up to two of the ribonucleotides in each terminal region is altered.
The terminal region of the oligoribonucleotide refers to bases 1-4 and/or 16-19 in the 19-mer sequence and to bases 1-4 and/or 18-21 in the 21-mer sequence.
Additionally, the invention provides sense oligoribonucleotides wherein one strand comprises consecutive nucleotides having, from 5' to 3', the sequence set forth in Tables A-DN or a homolog thereof wherein in up to two of the ribonucleotides in each terminal region is altered. Tables A-DN provide 19- and 21-mer oligomers useful in the preparation of siRNA compounds targeted against ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 and VIP. The presently most preferred compound of the invention is a blunt-ended 19-mer siRNA, i.e. x=y=19 and Z and Z' are both absent. The siRNA is either phosphorylated at 3' termini of both sense and anti-sense strands, or non-phosphorylated at all; or having the 5' most ribonucleotide on the sense strand specifically modified to abolish any possibility of in vivo 5 '-phosphorylation. The alternating ribonucleotides are modified at the 2' position of the sugar residue in both the antisense and the sense strands, wherein the moiety at the 2' position is methoxy (2'-O-methyl) and wherein the ribonucleotides at the 5' and 3' termini of the antisense strand are modified in their sugar residues, and the ribonucleotides at the 5' and 3' termini of the sense strand are unmodified in their sugar residues.
Additional nucleic acids according to the present invention comprise at least 14 contiguous nucleotides of any one of the polynucleotides in Tables A-DN and more preferably 14 contiguous nucleotide base pairs at any end of the double-stranded structure. It will be understood by one skilled in the art that given the potential length of the nucleic acid according to the present invention and particularly of the individual stretches forming such nucleic acid according to the present invention, some shifts relative to the coding sequence of the mammalian genes of the present invention to each side is possible, whereby such shifts can be up to 1, 2, 3, 4, 5 and 6 nucleotides in both directions, and whereby the thus generated double-stranded nucleic acid molecules shall also be within the present invention.
The compounds of the present invention can be synthesized by any of the methods that are well- known in the art for synthesis of ribonucleic (or deoxyribonucleic) oligonucleotides. Such synthesis is, among others, described in Beaucage and Iyer Tetrahedron 1992; 48: 2223-2311, Beaucage and Iyer, Tetrahedron 1993; 49: 6123-6194 and Caruthers et. al., Methods Enzymol. 1987; 154: 287-313; the synthesis of thioates is, among others, described in Eckstein, Annu. Rev. Biochem. 1985; 54: 367-402, the synthesis of RNA molecules is described in Sproat ,in Humana Press 2005 edited by Herdewijn; Kap. 2: 17-31 and respective downstream processes are, among others, described in Pingoud et. al., in IRL Press 1989 edited by Oliver; Kap. 7: 183-208 and Sproat, in Humana Press 2005 edited by Herdewijn; Kap. 2: 17-31 (supra). Other synthetic procedures are known in the art e.g. the procedures as described in Usman et al., 1987, J. Am. Chem. Soc, 109, 7845; Scaringe et al., 1990, NAR, 18, 5433; Wincott et al., 1995, NAR, 23, 2677-2684; and Wincott et al., 1997, Methods MoI. Bio., 74, 59, and these procedures may make use of common nucleic acid protecting and coupling groups, such as dimethoxytrityl at the 5'-end, and phosphoramidites at the 3'-end. The modified (e.g. 2'-O-methylated) nucleotides and unmodified nucleotides are incorporated as desired.
The oligonucleotides of the present invention can be synthesized separately and joined together post-synthetically, for example, by ligation (Moore et al., 1992, Science 256, 9923; Draper et al., International PCT publication No. WO 93/23569; Shabarova et al., 1991, NAR 19, 4247; Bellon et al., 1997, Nucleosides & Nucleotides, 16, 951; Bellon et al., 1997, Bioconjugate Chem. 8, 204), or by hybridization following synthesis and/or deprotection.
It is noted that a commercially available machine (available, inter alia, from Applied Biosystems) can be used; the oligonucleotides are prepared according to the sequences disclosed herein. Overlapping pairs of chemically synthesized fragments can be ligated using methods well known in the art (e.g., see US Patent No. 6,121,426). The strands are synthesized separately and then are annealed to each other in the tube. Then, the double-stranded siRNAs are separated from the single-stranded oligonucleotides that were not annealed (e.g. because of the excess of one of them) by HPLC. In relation to the siRNAs or siRNA fragments of the present invention, two or more such sequences can be synthesized and linked together for use in the present invention. The compounds of the invention can also be synthesized via a tandem synthesis methodology, as described in US patent application publication No. 2004/0019001 wherein both siRNA strands are synthesized as a single contiguous oligonucleotide fragment or strand separated by a
cleavable linker which is subsequently cleaved to provide separate siRNA fragments or strands that hybridize and permit purification of the siRNA duplex. The linker can be a polynucleotide linker or a non-nucleotide linker.
Pharmaceutical Compositions
While it may be possible for the compounds of the present invention to be administered as the raw chemical, it is preferable to present them as a pharmaceutical composition. Accordingly the present invention provides a pharmaceutical composition comprising one or more of the compounds of the invention; and a pharmaceutically acceptable carrier. This composition may comprise a mixture of two or more different siRNA compounds.
The invention further provides a pharmaceutical composition comprising at least one compound of the invention covalently or non-covalently bound to one or more compounds of the invention in an amount effective to inhibit the mammalian depression-associated genes; and a pharmaceutically acceptable carrier. The compound may be processed intracellularly by endogenous cellular complexes to produce one or more oligoribonucleotides of the invention.
The invention further provides a pharmaceutical composition comprising a pharmaceutically acceptable carrier and one or more of the compounds of the invention in an amount effective to down-regulate expression in a cell of a mammalian gene of the present invention, the compound comprising a sequence substantially complementary to the sequence of (N)x
Methods of Treatment
In preferred embodiments the subject being treated is a warm-blooded animal and, in particular, mammals including human.
The term "treatment" as used herein refers to administration of a therapeutic substance to a subject in need thereof in an amount effective to ameliorate symptoms associated with a disease, to lessen the severity or cure the disease, or to prevent the disease from occurring.
Additionally, the invention provides a method of down-regulating the expression of a mammalian gene selected from the group consisting of ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl;
ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23;
HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2;
NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 and VIP by at least 50% as compared to a control comprising contacting a mRNA transcript selected from the group consisting of ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 and VIP with one or more of the compounds of the present invention.
In one embodiment the compound of the present invention down-regulates one of the mammalian genes selected from the group consisting of ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 and VIP whereby the down-regulation is selected from the group comprising down-regulation of gene function, down-regulation of polypeptide and down-regulation of mRNA expression.
In one embodiment the compound is down-regulating a mammalian polypeptide, whereby the down-regulation is selected from the group comprising down-regulation of function (which may be examined by an enzymatic assay or a binding assay with a known interactor of the native gene / polypeptide, inter alia), down-regulation of protein (which may be examined by Western blotting, ELISA or immuno-precipitation, inter alia) and down-regulation of mRNA expression (which may be examined by Northern blotting, quantitative RT-PCR, in-situ hybridisation or microarray hybridisation, inter alia
In additional embodiments the invention provides a method of treating a patient suffering from a disease accompanied by an elevated level of a mammalian depression associated gene disclosed herein, the method comprising administering to the patient a compound or composition of the invention in a therapeutically effective dose thereby treating the patient.
The present invention relates to the use of compounds which down-regulate the expression of a mammalian depression associated gene particularly to novel small interfering RNAs (siRNAs), in the treatment of the following diseases or conditions in which inhibition of the expression of the mammalian depression associated gene is beneficial: depression, mood disorders and neurological disorders, such as, inter alia, ADD (attention deficit disorder), ADHD (attention deficit hyperactivity disorder), Autism, anxiety, panic, bi-polar disorder, depression, GAD (generalized anxiety disorder), OCD (obsessive compulsive disorder), PTSD (post-traumatic stress disorder), Phobias, Schizophrenia, Convulsions, Anxiety, Depression, Mania, Manic- depression, Psychosis and mood disorders.
Methods, molecules and compositions which inhibit a mammalian gene or polypeptide of the present invention are discussed herein at length, and any of said molecules and/or compositions may be beneficially employed in the treatment of a patient suffering from any of said conditions. It is to be explicitly understood that known compounds are excluded from the present invention. Novel methods of treatment using known compounds and compositions fall within the scope of the present invention Combination therapies with known treatments for mood and CNS disorders as described above (under DRUG TREATMENT) in conjunction with the novel compounds and therapies described herein are considered part of the current invention .
"Treatment" refers to both therapeutic treatment and prophylactic or preventative measures, wherein the object is to prevent or slow down (lessen) a mood disorder as listed above. Those in need of treatment include those already experiencing the disease or condition, those prone to having the disease or condition, and those in which the disease or condition is to be prevented. The compounds of the invention may be administered before, during or subsequent to the onset of the disease or condition.
The method of the invention includes administering a therapeutically effective amount of one or more compounds which down-regulate expression of a depression associated gene, particularly the novel siRNAs of the present invention, small molecule inhibitors of a depression associated gene or protein or antibodies to depression associated proteins.
By "exposure to a toxic agent" is meant that the toxic agent is made available to, or comes into contact with, a mammal. A toxic agent can be toxic to one or more organs in the body, for example, the ear, kidney, nervous system, liver and the like. Exposure to a toxic agent can occur by direct administration, e.g., by ingestion or administration of a food, medicinal, or therapeutic agent, e.g., a chemotherapeutic agent, by accidental contamination, or by environmental exposure, e g., aerial or aqueous exposure.
In other embodiments the compounds and methods of the invention are useful for treating or preventing the incidence or severity of other diseases and conditions in a patient. These diseases and conditions include stroke and stroke-like situations (e.g. cerebral, renal, cardiac failure), neuronal cell death, brain injuries with or without reperfusion, chronic degenerative diseases e.g. neurodegenerative disease including Alzheimer's disease, Huntington's disease, Parkinson's disease, multiple sclerosis, amyotrophic lateral sclerosis, spinobulbar atrophy, prion disease, and apoptosis resulting from traumatic brain injury (TBI).
In an additional embodiment, the compounds and methods of the invention are directed to providing neuroprotection, cerebroprotection, or to prevent and/or treat cytotoxic T cell and natural killer cell-mediated apoptosis associated with autoimmune disease and transplant rejection, or to prevent cell death of cardiac cells including heart failure, cardiomyopathy, viral infection or bacterial infection of the heart, myocardial ischemia, myocardial infarct, and myocardial ischemia, coronary artery by- pass graft, or to prevent and/or treat mitochondrial drug toxicity e. g. as a result of chemotherapy or HIV therapy, to prevent cell death during viral infection or bacterial infection, or to prevent and/or treat inflammation or inflammatory diseases, inflammatory bowel disease, sepsis and septic shock. Additional conditions which may be treated using the compounds of the present invention include hearing loss, acute renal failure, nephritis, glaucoma, Acute Respiratory Distress Syndrome and other acute lung injuries, lung transplantation, spinal cord injury, pressure sores, osteoarthritis and Chronic Obstructive Pulmonary Disease (COPD). Other uses include prevention of cell death from follicle to ovocyte stages, from ovocyte to mature egg stages and sperm (for example, methods of freezing and transplanting ovarian tissue, artificial fertilization), or to preserve fertility in mammals after chemotherapy, in particular human mammals, or to prevent and/or treat, macular degeneration, or
to prevent and/or treat acute hepatitis, chronic active hepatitis, hepatitis-B, and hepatitis-C, or to prevent hair loss, (e.g. hair loss due-to male- pattern baldness, or hair loss due to radiation, chemotherapy or emotional stress), or to treat or ameliorate skin damage whereby the skin damage may be due to exposure to high levels of radiation, heat, chemicals, sun, or to burns and autoimmune diseases), or to prevent cell death of bone marrow cells in myelodysplastic syndromes (MDS), or to treat pancreatisis, or to treat, rheumatoid arthritis, psoriasis, glomerulonephritis, atheroscerosis, and graft versus host disease (GVHD), or to treat retinal pericyte apoptosis, retinal damages resulting from ischemia, diabetic retinopathy, or to treat any disease states associated with expression of a depression associated gene, wherein the gene is selected from ABAT; ADRBl ; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl ; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl ; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl ; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91 ; SYN2; SYTl; TKT; TPTl; UGT8 and VIP.
The present invention also provides for a process of preparing a pharmaceutical composition, which comprises: providing one or more double stranded compound of the invention ; and admixing said compound with a pharmaceutically acceptable carrier.
In a preferred embodiment, the compound used in the preparation of a pharmaceutical composition is admixed with a carrier in a pharmaceutically effective dose. In a particular embodiment the compound of the present invention is conjugated to a steroid or to a lipid or to another suitable molecule e.g. to cholesterol.
Delivery
The siRNA molecules of the present invention may be delivered to the target tissue by direct application of the naked molecules prepared with a carrier or a diluent. The term "naked siRNA" refers to siRNA molecules that are free from any delivery vehicle that acts to assist, promote or facilitate entry into the cell, including viral sequences, viral particles,
liposome formulations, lipofectin or precipitating agents and the like. For example, siRNA in
PBS is "naked siRNA".
However, in some embodiments the siRNA molecules of the invention are delivered in liposome formulations and lipofectin formulations and the like and can be prepared by methods well known to those skilled in the art. Such methods are described, for example, in U.S. Pat. Nos.
5,593,972, 5,589,466, and 5,580,859, which are herein incorporated by reference.
Delivery systems aimed specifically at the enhanced and improved delivery of siRNA into mammalian cells have been developed (see, for example, Shen et al FEBS Let. 539: 111-114
(2003), Xia et al., Nat. Biotech. 20: 1006-1010 (2002), Reich et al., MoI. Vision 9: 210-216 (2003), Sorensen et al., J. MoI. Biol. 327: 761-766 (2003), Lewis et al., Nat. Gen. 32: 107-108
(2002) and Simeoni et al., NAR 31, 11: 2717-2724 (2003)). siRNA has recently been successfully used for inhibition of gene expression in primates; (for details see for example,
Tolentino et al., Retina 24(1): 132-138).
Respiratory formulations for siRNA are described in US patent application publication No. 2004/0063654. Cholesterol-conjugated siRNAs (and other steroid and lipid conjugated siRNAs) can been used for delivery (see for example Soutschek et al Nature 2004. 432: 173-177.
Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs; and
Lorenz et al. Bioorg. Med. Chem.. Lett. 14:4975-4977 (2004) Steroid and lipid conjugates of siRNAs to enhance cellular uptake and gene silencing in liver cells)
Pharmaceutically acceptable carriers, solvents, diluents, excipients, adjuvants and vehicles as well as implant carriers generally refer to inert, non-toxic solid or liquid fillers, diluents or encapsulating material not reacting with the active ingredients of the invention and they include liposomes and microspheres. Examples of delivery systems useful in the present invention include U.S. Patent Nos. 5,225,182; 5,169,383; 5,167,616; 4,959,217; 4,925,678; 4,487,603; 4,486,194; 4,447,233; 4,447,224; 4,439,196; and 4,475,196. Many other such implants, delivery systems, and modules are well known to those skilled in the art. In one specific embodiment of this invention topical and transdermal formulations may be selected. The siRNAs or pharmaceutical compositions of the present invention are administered and dosed in accordance with good medical practice, taking into account the clinical condition of the individual patient, the disease to be treated, the site and method of administration, scheduling of administration, patient age, sex, body weight and other factors known to medical practitioners.
A "therapeutically effective dose" for purposes herein is thus determined by such considerations as are known in the art. The dose must be effective to achieve improvement including but not limited to improved survival rate or more rapid recovery, or improvement or elimination of symptoms and other indicators as are selected as appropriate measures by those skilled in the art. In general, the active dose of compound for humans is in the range of from lng/kg to about 20- 100 mg/kg body weight per day, preferably about 0.01 mg to about 2-10 mg/kg body weight per day, in a regimen of one dose per day or twice or three or more times per day for a period of 1-4 weeks or longer. The compounds of the present invention can be administered by any of the conventional routes of administration. It should be noted that the compound can be administered as the compound or as pharmaceutically acceptable salt and can be administered alone or as an active ingredient in combination with pharmaceutically acceptable carriers, solvents, diluents, excipients, adjuvants and vehicles. The compounds can be administered orally, subcutaneously or parenterally including intravenous, intraarterial, intramuscular, intraperitoneally, and intranasal administration as well as intrathecal and infusion techniques. Implants of the compounds are also useful. Liquid forms may be prepared for injection, the term including subcutaneous, transdermal, intravenous, intramuscular, intrathecal, and other parental routes of administration. The liquid compositions include aqueous solutions, with and without organic co-solvents, aqueous or oil suspensions, emulsions with edible oils, as well as similar pharmaceutical vehicles. In a particular embodiment, the administration comprises intravenous administration. In another embodiment the administration comprises topical or local administration
In addition, in certain embodiments the compositions for use in the novel treatments of the present invention may be formed as aerosols, for example for intranasal administration.
Delivery of inhibitors into the brain Delivery of compounds into the brain can be accomplished by several methods such as, inter alia, neurosurgical implants, blood-brain barrier disruption, lipid mediated transport, carrier mediated influx or efflux, plasma protein-mediated transport, receptor-mediated transcytosis, absorptive-mediated transcytosis, neuropeptide transport at the blood-brain barrier, and genetically engineering "Trojan horses" for drug targeting. The above methods are performed for
example as described in "Brain Drug Targeting: the future of brain drug development", W.M. Pardridge, Cambridge University Press, Cambridge, UK (2001).
The present invention further provides for a pharmaceutical composition comprising two or more siRNA molecules for the treatment of any of the diseases and conditions mentioned herein, whereby said two molecules may be physically mixed together in the pharmaceutical composition in amounts which generate equal or otherwise beneficial activity, or may be covalently or non-covalently bound, or joined together by a nucleic acid linker of a length ranging from 2-100, preferably 2-50 or 2-30 nucleotides. In one embodiment, the siRNA molecules are comprised of a double-stranded nucleic acid structure as described herein, wherein the two siRNA sequences are selected from Tables A-DN.
The siRNA molecules are covalently or non-covalently bound or joined by a linker to form a tandem siRNA molecule. Such tandem siRNA molecules comprising two siRNA sequences are typically of 38-150 nucleotides in length, more preferably 38 or 40-60 nucleotides in length, and longer accordingly if more than two siRNA sequences are included in the tandem molecule. A longer tandem molecule comprised of two or more longer sequences which encode siRNA produced via internal cellular processing, e.g., long dsRNAs, is also envisaged, as is a tandem molecule encoding two or more shRNAs. Such tandem molecules are also considered to be a part of the present invention. siRNA compounds that target any one of the genes disclosed herein may be the main active component in a pharmaceutical composition, or may be one active component of a pharmaceutical composition containing two or more siRNAs (or molecules which encode or endogenously produce two or more siRNAs, be it a mixture of molecules or one or more tandem molecules which encode two or more siRNAs), said pharmaceutical composition further being comprised of one or more additional siRNA molecule which targets one or more additional gene. Simultaneous inhibition of said additional gene(s) will likely have an additive or synergistic effect for treatment of the diseases disclosed herein.
Additionally, the siRNA compounds disclosed herein or any nucleic acid molecule comprising or encoding such siRNA can be linked or bound (covalently or non-covalently) to antibodies (including aptamer molecules) against cell surface internalizable molecules expressed on the target cells, in order to achieve enhanced targeting for treatment of the diseases disclosed herein.
For example, anti-Fas antibody (preferably a neutralizing antibody) may be combined (covalently or non-covalently) with siRNA. In another example, an aptamer which can act like a ligand/antibody may be combined (covalently or non-covalently) with any siRNA to a gene disclosed herein. The compounds of the present invention can be delivered either directly or with viral or non-viral vectors. When delivered directly the sequences are generally rendered nuclease resistant. Alternatively the sequences can be incorporated into expression cassettes or constructs such that the sequence is expressed in the cell as discussed herein below. Generally the construct contains the proper regulatory sequence or promoter to allow the sequence to be expressed in the targeted cell. Vectors optionally used for delivery of the compounds of the present invention are commercially available, and may be modified for the purpose of delivery of the compounds of the present invention by methods known to one of skill in the art.
It is also envisaged that a long oligonucleotide (typically 25-500 nucleotides in length) comprising one or more stem and loop structures, where stem regions comprise the sequences of the oligonucleotides of the invention, may be delivered in a carrier, preferably a pharmaceutically acceptable carrier, and may be processed intracellular^ by endogenous cellular complexes (e.g. by DROSHA and DICER as described above) to produce one or more smaller double stranded oligonucleotides (siRNAs) which are oligonucleotides of the invention. This oligonucleotide can be termed a tandem shRNA construct. It is envisaged that this long oligonucleotide is a single stranded oligonucleotide comprising one or more stem and loop structures, wherein each stem region comprises a sense and corresponding antisense siRNA sequence of the genes of the invention. In particular, it is envisaged that this oligonucleotide comprises sense and antisense siRNA sequences as depicted in Tables A-DN.
Although the inhibitor may be an siRNA molecule, other inhibitors contemplated to be used in the methods of the invention to inhibit expression of a gene and to treat the diseases and conditions described herein are inter alia antibodies, preferably neutralizing antibodies or fragments thereof, including single chain antibodies, antisense oligonucleotides, antisense DNA or RNA molecules, ribozymes, proteins, polypeptides and peptides including peptido-mimetics and dominant negatives, and also expression vectors expressing all the above. Additional inhibitors may be small chemical molecules, which generally have a molecular weight of less than 2000 daltons, preferably less than 1000 daltons, more preferably less than 500 daltons.
These inhibitors may act as follows: small molecules may affect expression and/or activity; antibodies may affect activity; all kinds of antisense may affect gene expression; and dominant negative polypeptides and peptidomimetics may affect activity; expression vectors may be used inter alia for delivery of antisense or dominant-negative polypeptides or antibodies.
Antibodies
The term "antibody" refers to IgG, IgM, IgD, IgA, and IgE antibody, inter alia. The definition includes polyclonal antibodies or monoclonal antibodies. This term refers to whole antibodies or fragments of antibodies comprising an antigen-binding domain, e.g. antibodies without the Fc portion, single chain antibodies, miniantibodies, fragments consisting of essentially only the variable, antigen-binding domain of the antibody, etc. The term "antibody" may also refer to antibodies against polynucleotide sequences obtained by cDNA vaccination. The term also encompasses antibody fragments which retain the ability to selectively bind with their antigen or receptor and are exemplified as follows, inter alia: (1) Fab, the fragment which contains a monovalent antigen-binding fragment of an antibody molecule which can be produced by digestion of whole antibody with the enzyme papain to yield a light chain and a portion of the heavy chain;
(2) (Fab')2> the fragment of the antibody that can be obtained by treating whole antibody with the enzyme pepsin without subsequent reduction; F(ab'2) is a dimer of two Fab fragments held together by two disulfide bonds;
(3) Fv, defined as a genetically engineered fragment containing the variable region of the light chain and the variable region of the heavy chain expressed as two chains; and
(4) Single chain antibody (SCA), defined as a genetically engineered molecule containing the variable region of the light chain and the variable region of the heavy chain linked by a suitable polypeptide linker as a genetically fused single chain molecule.
Antisense molecules
By the term "antisense" (AS) or "antisense fragment" is meant a polynucleotide fragment
(comprising either deoxyribonucleotides, ribonucleotides or a mixture of both) having inhibitory antisense activity, said activity causing a decrease in the expression of the endogenous genomic copy of the corresponding gene. An AS polynucleotide is a polynucleotide which comprises
consecutive nucleotides having a sequence of sufficient length and homology to a sequence present within the sequence of the target gene to permit hybridization of the AS to the gene. Many reviews have covered the main aspects of antisense (AS) technology and its therapeutic potential (Aboul-Fadl T., Curr Med Chem. 2005, 12(19):2193-214; Crooke ST, Curr MoI Med. 2004, 4(5):465-87; Crooke ST, Ann Rev Med. 2004, 55:61-95; Vacek M et al., Cell MoI Life Sci. 2003, 60(5):825-33; Cho-Chung YS, Arch Pharm Res. 2003, 26(3):183-91. There are further reviews on the chemical (Crooke et al., Hematol Pathol. 1995, 9(2):59-72), cellular (Wagner, Nature. 1994, 372(6504):333-5) and therapeutic (Scanlon, et al, FASEB J. 1995, 9(13): 1288-96) aspects of AS technology. Antisense intervention in the expression of specific genes can be achieved by the use of modified AS oligonucleotide sequences (for recent reports see Lefebvre- d'Hellencourt et al, 1995; Agrawal, 1996; LevLehman et al, 1997).
AS oligonucleotide sequences may be short sequences of DNA, typically 15-30 mer but may be as small as 7-mer (Wagner et al, Nat. Biotech. 1996, 14(7):840-4), designed to complement a target mRNA of interest and form an RNA:AS duplex. This duplex formation can prevent processing, splicing, transport or translation of the relevant mRNA. Moreover, certain AS nucleotide sequences can elicit cellular RNase H activity when hybridized with their target mRNA, resulting in mRNA degradation (Calabretta et al, Semin Oncol. 1996, 23(l):78-87). In that case, RNase H will cleave the RNA component of the duplex and can potentially release the AS to further hybridize with additional molecules of the target RNA. An additional mode of action results from the interaction of AS with genomic DNA to form a triple helix, which can be transcriptionally inactive.
The sequence target segment for the antisense oligonucleotide is selected such that the sequence exhibits suitable energy related characteristics important for oligonucleotide duplex formation with their complementary templates, and shows a low potential for self-dimerization or self- complementation (Anazodo et al., 1996, Biochem. Biophys. Res. Comm. 229:305-309). For example, the computer program OLIGO (Primer Analysis Software, Version 3.4), can be used to determine antisense sequence melting temperature, free energy properties, and to estimate potential self-dimer formation and self-complimentary properties. The program allows the determination of a qualitative estimation of these two parameters (potential self-dimer formation and self- complimentary) and provides an indication of "no potential" or "some potential" or "essentially complete potential". Using this program target segments are generally selected that
have estimates of no potential in these parameters. However, segments can be used that have "some potential" in one of the categories. A balance of the parameters is used in the selection as is known in the art. Further, the oligonucleotides are also selected as needed so that analog substitution does not substantially affect function. Phosphorothioate antisense oligonucleotides do not normally show significant toxicity at concentrations that are effective and exhibit sufficient pharmacodynamic half-lives in animals (Agrawal, et al., PNAS U S A. 1997, 94(6):2620-5) and are nuclease resistant. Antisense oligonucleotide inhibition of basic fibroblast growth factor (bFGF), having mitogenic and angiogenic properties, suppressed 80% of growth in glioma cells (Morrison, J Biol Chem. 1991 266(2):728-34) in a saturable and specific manner. Being hydrophobic, antisense oligonucleotides interact well with phospholipid membranes (Akhter et al., NAR.. 1991, 19:5551-5559). Following their interaction with the cellular plasma membrane, they are actively (or passively) transported into living cells (Loke et al., PNAS 1989, 86(10):3474-8), in a saturable mechanism predicted to involve specific receptors (Yakubov et al., PNAS, 1989 86(17):6454-58).
Ribozymes
A "ribozyme" is an RNA molecule that possesses RNA catalytic ability (see Cech for review) and cleaves a specific site in a target RNA. In accordance with the present invention, ribozymes which cleave mRNA may be utilized as inhibitors. This may be necessary in cases where antisense therapy is limited by stoichiometric considerations (Sarver et al., 1990, Gene Regulation and Aids, pp. 305-325). Ribozymes can then be used that will target the a gene associated with a bone marrow disease. The number of RNA molecules that are cleaved by a ribozyme is greater than the number predicted by stochiochemistry. (Hampel and Tritz, Biochem. 1989, 28(12):4929-33; Uhlenbeck, Nature. 1987 328(6131):596-600).
Ribozymes catalyze the phosphodiester bond cleavage of RNA. Several ribozyme structural families have been identified including Group I introns, RNase P, the hepatitis delta virus ribozyme, hammerhead ribozymes and the hairpin ribozyme originally derived from the negative strand of the tobacco ringspot virus satellite RNA (sTRSV) (US Patent No. 5,225,347). The latter two families are derived from viroids and virusoids, in which the ribozyme is believed to separate monomers from oligomers created during rolling circle replication (Symons, 1989 and 1992).
Hammerhead and hairpin ribozyme motifs are most commonly adapted for trans-cleavage of mRNAs for gene therapy (Sullivan, 1994). In general the ribozyme has a length of from about 30-100 nucleotides. Delivery of ribozymes is similar to that of AS fragments and/or siRNA molecules.
Screening for inhibitors of depression associated gene expression
Some of the compounds and compositions of the present invention may be used in a screening assay for identifying and isolating compounds that modulate the activity of a gene, in particular compounds that modulate a disorder accompanied by an elevated level of said gene. The compounds to be screened comprise inter alia substances such as small chemical molecules and antisense oligonucleotides.
The inhibitory activity of the compounds of the present invention on a gene or binding of the compounds of the present invention to a gene or mRNA may be used to determine the interaction of a test compound with the sequence e.g., if the test compound competes with the oligonucleotides of the present invention for inhibition of a gene, or if the additional compound rescues said inhibition. The inhibition or activation can be tested by various means, such as, inter alia, assaying for the product of the activity of a polypeptide or displacement of binding compound from the a polypeptide in radioactive or fluorescent competition assays. The present invention is illustrated in detail below with reference to Examples, but is not to be construed as being limited thereto.
Citation of any document herein is not intended as an admission that such document is pertinent prior art, or considered material to the patentability of any claim of the present application. Any statement as to content or a date of any document is based on the information available to applicant at the time of filing and does not constitute an admission as to the correctness of such a statement.
One aspect of the present invention provides for a pharmaceutical composition comprising as an active ingredient an inhibitor to TTR, PD2 Synthase, Adrb3, PDE4D, GABRP, PMCH, Duspό, Camk2a/2b, VIP, Atplal, MEF2C, Cdk5r, NOSl, Neurogranin, Chimerinl, Dendrin, GMF, Cacnb4, SLC17A7, SLC8A1, Drd3, MAPKKKK5, ARHGAP6, CLU, BAMBI, PTGER3, CLSTN2, STARD7, DKK3, CART, SCARA3, NRP2, MGC42105, NRGl, NELL2, CD276,
CD81, NTRK2, AKAP5, EFNB3, CNP, ENPP2, SMPD2, CKB, SLC6A15, SLC6A20, SLC22A17, SLC6A17, SLC6A9, TRPC6, CLCN3, EPHA4, MAPKl, PTPRR, TACl, TM, TBXA2R, TDE2, TDEl, GABA(A), BCAN, PTGDR, ZDHHC12, PLXNA2, SNPH, DLGAP3, SCAMP4, SEZ6L, NTS, CPE, TMEM30A, FLJl 0700, LPHN3, GPM6B, PTPRD, UNC5A, PAM, GHITM, SPOCK, MGC20235, FUNDCl, PKIG, CLSTN3, AQP4, EDG2, PPP3CB, PPMlE, GPM6A, ARMCl, AVP, SYNGR3, PENK, C10orf93, SMBP, IGFBP2, GPR23, CBLNl, PHCA, HIGl, GPR22, DLGAPl, FSTLl, RNF103, ABAT; ADRBl; ARHGEF9; ARRBl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 and VIP, or an inhibitor of any of the genes presented in Table 1, preferable present in Table 3, in a therapeutically effective amount, which may be a small chemical compound; a polynucleotide, such as an antisense polynucleotide, or a polynucleotide which is a sense polynucleotide and which encodes a dominant negative peptide or a polynucleotide that functions as silencing RNA (siRNA); a vector comprising any of these polynucleotides; a polypeptide, such as a dominant negative peptide, or an antibody, optionally a polyclonal or a monoclonal antibody. The pharmaceutical composition may further contain a diluent or carrier.
Another aspect of the present invention concerns a method for treating a patient suffering from a depression or mood disorder, comprising administering to the patient a therapeutically effective amount of an inhibitor to TTR, PD2 Synthase, Adrb3, PDE4D, GABRP, PMCH, Duspό, Camk2a/2b, VIP, Atplal, MEF2C, Cdk5r, NOSl, Neurogranin, Chimerinl, Dendrin, GMF, Cacnb4, SLC17A7, SLC8A1, Drd3, MAPKKKK5, ARHGAP6, CLU, BAMBI, PTGER3, CLSTN2, STARD7, DKK3, CART, SCARA3, NRP2, MGC42105, NRGl, NELL2, CD276, CD81, NTRK2, AKAP5, EFNB3, CNP, ENPP2, SMPD2, CKB, SLC6A15, SLC6A20, SLC22A17, SLC6A17, SLC6A9, TRPC6, CLCN3, EPHA4, MAPKl, PTPRR, TACl, TM, TBXA2R, TDE2, TDEl, GABA(A), BCAN, PTGDR, ZDHHC12, PLXNA2, SNPH, DLGAP3, SCAMP4, SEZ6L, NTS, CPE, TMEM30A, FLJl 0700, LPHN3, GPM6B, PTPRD, UNC5A, PAM, GHITM, SPOCK, MGC20235, FUNDCl, PKIG, CLSTN3, AQP4, EDG2, PPP3CB,
PPMlE, GPM6A, ARMCl, AVP, SYNGR3, PENK, C10orf93, SMBP, IGFBP2, GPR23, CBLNl, PHCA, HIGl, GPR22, DLGAPl, FSTLl, RNF103, ABAT; ADRBl; ARHGEF9; ARRBl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 or VIP, so as to thereby treat the patient. Administration may be periodical. The inhibitor may be a small chemical compound; a polynucleotide, such as an antisense polynucleotide, or a polynucleotide which is a sense polynucleotide and which encodes a dominant negative peptide, or a polynucleotide that functions as silencing RNA (siRNA), such as an siRNA present in any one of Tables A-DN; a vector comprising any of these polynucleotides; a polypeptide, such as a dominant negative peptide, or an antibody, optionally a polyclonal or a monoclonal antibody.
Further provided in this aspect is the use of a therapeutically effective amount of an inhibitor to TTR, PD2 Synthase, Adrb3, PDE4D, GABRP, PMCH, Duspό, Camk2a/2b, VIP, Atplal, MEF2C, Cdk5r, NOSl, Neurogranin, Chimerinl, Dendrin, GMF, Cacnb4, SLC17A7, SLC8A1, Drd3, MAPKKKK5, ARHGAP6, CLU, BAMBI, PTGER3, CLSTN2, STARD7, DKK3, CART, SCARA3, NRP2, MGC42105, NRGl, NELL2, CD276, CD81, NTRK2, AKAP5, EFNB3, CNP, ENPP2, SMPD2, CKB, SLC6A15, SLC6A20, SLC22A17, SLC6A17, SLC6A9, TRPC6, CLCN3, EPHA4, MAPKl, PTPRR, TACl, TM, TBXA2R, TDE2, TDEl, GABA(A), BCAN, PTGDR, ZDHHC 12, PLXNA2, SNPH, DLGAP3, SCAMP4, SEZ6L, NTS, CPE, TMEM30A, FLJ 10700, LPHN3, GPM6B, PTPRD, UNC5A, PAM, GHITM, SPOCK, MGC20235, FUNDCl, PKIG, CLSTN3, AQP4, EDG2, PPP3CB, PPMlE, GPM6A, ARMCl, AVP, SYNGR3, PENK, C10orf93, SMBP, IGFBP2, GPR23, CBLNl, PHCA, HIGl, GPR22, DLGAPl, FSTLl, RNF 103, ABAT; ADRBl; ARHGEF9; ARRBl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 or VIP, such as any of the inhibitors detailed above, for the preparation of a
medicament for promoting or enhancing recovery in a patient suffering from a mood disorder.
Additionally, the present invention provides a method of regulating a pathology or disease (as recited above) in a patient in need of such treatment by administering to a patient a therapeutically effective dose of at least one inhibitor e.g. at least one antisense (AS) oligonucleotide or at least one siRNA against the nucleic acid sequences or a dominant negative peptide directed against any of the genes described herein, or an antibody directed against a polypeptide encoded by any of the genes described herein, or any of the inhibitors described above. The terms "chemical compound", "small molecule", "chemical molecule" "small chemical molecule" and "small chemical compound" are used interchangeably herein and are understood to refer to chemical moieties of any particular type which may be synthetically produced or obtained from natural sources and typically have a molecular weight of less than 2000 daltons, more preferably less than 1000 daltons or even less than 600 daltons.
The invention also provides a method of treating a patient suffering from a mood disorder or depression, comprising administering to the patient a composition of the invention in a therapeutically effective dose so as to thereby treat the patient.
The invention also provides a use of a therapeutically effective dose of one or more compounds of the invention for the preparation of a composition for promoting recovery in a patient suffering from a mood disorder or depression.
"Mood disorder" includes major depressive disorder; dysthymic disorder; bipolar depression; post-partum depression; and any disorder which causes a subject to be diagnosed as depressed by a clinician, including depression which may be associated with ADD (attention deficit disorder), ADHD (attention deficit hyperactivity disorder), Autism, anxiety, panic, bi-polar disorder, depression, GAD (generalized anxiety disorder), OCD (obsessive compulsive disorder), PTSD (post-traumatic stress disorder), Phobias, Schizophrenia, Convulsions, Anxiety, Mania, Manic- depression and Psychosis, it is also the object of the present invention to treat mental illnesses such as ADD (attention deficit disorder), ADHD (attention deficit hyperactivity disorder), Autism, anxiety, panic, bi-polar disorder, depression, GAD (generalized anxiety disorder), OCD
(obsessive compulsive disorder), PTSD (post-traumatic stress disorder), Phobias, Schizophrenia, Convulsions, Anxiety, Depression, Mania, Manic-depression, Psychosis and mood disorders in general, and not just in relation to the mood disorder aspect.
siRNA for any of the genes presented in Tables 1-3 can be made using methods known in the art as described herein, based on the known sequence of any of the genes presented in Tables 1-3 and can be made stable by various modifications as described above. For further information, see Example 3.
Further, in relation to the methods of the present invention as described herein, additional inhibitory RNA molecules of the present invention, which may be used with the methods of the present invention include single stranded oligoribonucleotides preferably comprising stretches of at least 7-14 consecutive nucleotides present in the sequences of any one of the genes detailed in
Tables 1-3, said oligoribonucleotides being capable of forming and/or said oligoribonucleotides comprising double stranded regions in particular conformations that are recognized by intracellular complexes, leading to the degradation of said oligoribonucleotides into smaller RNA molecules that are capable of exerting inhibition of any of said genes / polypeptides, and DNA molecules encoding such RNA molecules.
Any molecules, such as, for example, antisense DNA molecules which comprise the siRNA sequences disclosed herein (with the appropriate nucleic acid modifications) are particularly desirable and may be used in the same capacity as their corresponding siRNAs for all uses and methods disclosed herein.
It is to be understood that, in the context of the present invention, any of the siRNA molecules disclosed herein, or any long double-stranded RNA molecules (typically 25-500 nucleotides in length) which are processed by endogenous cellular complexes (such as DICER - see above) to form the siRNA molecules disclosed herein, or molecules which comprise the siRNA molecules disclosed herein, can be employed in the treatment of any disease or disorder. More specifically, the present invention provides a method of treating a patient suffering from a disease or disorder, such as nerodegenerative disorders or Central nervous system disorders, inter alia., comprising
administering to the patient a pharmaceutical composition comprising one or more of the siRNAs disclosed herein (or one or more long dsRNA which encodes one or more of said siRNAs, as described above) in a therapeutically effective amount so as to thereby treat the patient.
The term "Expression vector" refers to vectors that have the ability to incorporate and express heterologous DNA fragments in a foreign cell. Many prokaryotic and eukaryotic expression vectors are known and/or commercially available. Selection of appropriate expression vectors is within the knowledge of those having skill in the art.
By "Polypeptide" is meant a molecule composed of amino acids and the term includes peptides, polypeptides, proteins and peptidomimetics.
A peptidomimetic is a compound containing non-peptidic structural elements that is capable of mimicking the biological action(s) of a natural parent peptide. Some of the classical peptide characteristics such as enzymatically scissille peptidic bonds are normally not present in a peptidomimetic.
The term "amino acid" refers to a molecule which consists of any one of the 20 naturally occurring amino acids, amino acids which have been chemically modified (see below), or synthetic amino acids.
The term "dominant negative peptide" refers to a polypeptide encoded by a cDNA fragment that encodes for a part of a protein which can interact with the full protein and inhibit its activity or which can interact with other proteins and inhibit their activity in response to the full protein.
By the term "epitope" as used in this invention is meant an antigenic determinant on an antigen to which the antibody binds. Epitopic determinants usually consist of chemically active surface groupings of molecules such as amino acids or sugar side chains and usually have specific three dimensional structural characteristics, as well as specific charge characteristics.
In one embodiment of the invention, any one of these pharmaceutical compositions is used for alleviation or reduction of the symptoms and signs associated with any type of depression mood
disorder. This embodiment concerns a method or process for promoting recovery in a patient who has suffered or suffers from a mood disorder, comprising administering to the patient any one of the pharmaceutical compositions recited above, in a dosage and over a period of time sufficient to reduce the damage or promote recovery. This embodiment further provides a method or process for treating a patient who has suffered or suffers from a mood disorder, optionally as a result of any of the conditions described herein, comprising administering to the patient a pharmaceutical composition comprising a therapeutically effective amount of an inhibitor to ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 or VIP, as exemplified herein, in a dosage and over a period of time sufficient to inhibit the gene, so as to thereby treat the patient.
In another aspect of this invention, an additional pharmaceutically effective compound is administered in conjunction with the aforementioned pharmaceutical composition.
By "in conjunction with" is meant that the additional pharmaceutically effective composition is administered prior to, at the same time as, or subsequent to administration of the pharmaceutical composition comprising any of the above inhibitors.
One embodiment of the claimed invention provides for using a therapeutically effective amount of an inhibitor to ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 or VIP in a process for the preparation of a medicament for the treatment of a patient who has suffered or suffers from depression or a mood disorder. The inhibitor may be a small chemical compound; a polynucleotide, such as an antisense polynucleotide comprising consecutive nucleotides having a sequence which is an antisense sequence to the sequence of any of the genes disclosed in Tables
1-3, or a polynucleotide which is a sense polynucleotide comprising consecutive nucleotides having a sequence which is a sense sequence to the sequence of any of the genes disclosed in Tables 1-3, and which encodes a dominant negative peptide to said sequence, or a polynucleotide that functions as silencing RNA (siRNA); a vector comprising any of these polynucleotides; a polypeptide, such as a dominant negative peptide, or an antibody, optionally a polyclonal or a monoclonal antibody. The pharmaceutical composition may further contain a diluent or carrier.
The treatment regimen according to the invention is carried out, in terms of administration mode, timing of the administration, and dosage, so that the functional recovery of the patient from the adverse consequences of the mood disorder is improved.
Administration of a pharmaceutical composition comprising any one of the inhibitors according to the invention can be carried out by any known route of administration, including intravenously, intra-arterially, subcutaneously, intraperitoneally or intracerebrally. Using specialized formulations, it may also be possible to administer these orally or via inhalation. Suitable doses and treatment regimens for administering compositions to an individual in need thereof are discussed in detail below.
An additional embodiment of the present invention, referred to herein as the "screening" embodiment, concerns methods and processes for obtaining a species and/or chemical compound that modulates the biological activity of TTR, PD2 Synthase, Adrb3, PDE4D, GABRP, PMCH, Duspό, Camk2a/2b, VIP, Atplal, MEF2C, Cdk5r, NOSl, Neurogranin, Chimerinl, Dendrin, GMF, Cacnb4, SLC17A7, SLC8A1, Drd3, MAPKKKK5, ARHGAP6 CLU, BAMBI, PTGER3, CLSTN2, STARD7, DKK3, CART, SCARA3, NRP2, MGC42105, NRGl, NELL2, CD276, CD81, NTRK2, AKAP5, EFNB3, CNP, ENPP2, SMPD2, CKB, SLC6A15, SLC6A20, SLC22A17, SLC6A17, SLC6A9, TRPC6, CLCN3, EPHA4, MAPKl, PTPRR, TACl, TM, TBXA2R, TDE2, TDEl, GABA(A), BCAN, PTGDR, ZDHHC 12, PLXNA2, SNPH, DLGAP3, SCAMP4, SEZ6L, NTS, CPE, TMEM30A, FLJ 10700, LPHN3, GPM6B, PTPRD, UNC5A, PAM, GHITM, SPOCK, MGC20235, FUNDCl, PKIG, CLSTN3, AQP4, EDG2, PPP3CB, PPMlE, GPM6A, ARMCl, AVP, SYNGR3, PENK, C10orf93, SMBP, IGFBP2, GPR23, CBLNl, PHCA, HIGl, GPR22, DLGAPl, FSTLl, RNF103, ABAT; ADRBl; ADRB3;
ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 or VIP a mood disorder and/or a depression. One aspect of this embodiment provides a process for obtaining a species and/or chemical compound that modulates the biological activity of any of the genes disclosed in Table 1, optionally present in Tables 2 or 3, which comprises contacting a cell expressing any one of said genes with a species and/or compound and determining the ability of the species and/or compound to modulate the biological activity of said gene, as compared to a control. The cell being examined may be modified to express said gene. In addition, this process may be used in order to prepare a pharmaceutical composition. The process then comprises admixing a species or compound obtained by the process recited above or a chemical analog or homolog thereof with a pharmaceutically acceptable carrier.
By cells being "modified to express" as used herein is meant that cells are modified by transfection, transduction, infection or any other known molecular biology method which will cause the cells to express the desired gene. Materials and protocols for carrying out such methods are evident to the skilled artisan.
An additional aspect of the screening embodiment provides a process of screening a plurality of species or compounds to obtain a species and/or compound that modulates the biological activity of any one of the genes of Table 1, which comprises:
(a) contacting cells expressing any one of the genes of Table 1 with a plurality of species and/or chemical compounds;
(b) determining whether the biological activity of any one of said genes is modulated in the presence of the species and/or compounds, as compared to a control; and if so
(c) separately determining whether the modulation of the biological activity of said gene is effected by each species and/or compound included in the plurality of species and/or
compounds, so as to thereby identify the species and/or compound which modulates the biological activity of said gene.
The cells in the contacting step may be modified to express the polypeptide if said gene. In addition, this process may be used in order to prepare a pharmaceutical composition. The process then comprises admixing a species or compound identified by the process recited above or a chemical analog or homolog thereof with a pharmaceutically acceptable carrier.
The process may additionally comprise modification of a species or compound found by the above process to produce a compound with improved activity and admixing such compound with a pharmaceutically acceptable carrier. This additional act may be performed with a compound discovered by any of the processes which are disclosed in the screening embodiment of the present invention, so as to thereby obtain a pharmaceutical composition comprising a compound with improved activity.
Additionally, the screening embodiment of the present invention provides a non cell-based process for obtaining a species or compound which modulates the biological activity of any one of the genes of Table 1, comprising:
(a) measuring the binding of any one of the genes of Table 1 or polypeptides encoded thereby to an interactor ;
(b) contacting said gene or polypeptide with said species or compound; and
(c) determining whether the binding of said gene or polypeptide to said interactor is affected by said species or compound.
Means of measuring interactions between molecules and determining the strength, affinity, avidity and other parameters of the interaction are well known in the art (see, for example, Lubert Stryer, Biochemistry, W H Freeman & Co.; 5th edition (April 2002); and "Comprehensive Medicinal Chemistry", by various authors and editors, published by Pergamon Press).
The present invention further provides a method or process for diagnosing depression or a mood disorder in a subject comprising detecting modulation of the expression level of any one of the genes of Table 1, preferably present in Table 2 or 3, for example: by detecting the polypeptide expressed by any one of said genes in an immunoassay, or by detecting an mRNA encoding said gene in the subject, as compared to a control.
The expression level of the polypeptide can be assessed by assaying for mRNA encoding the any one of the above polypeptides, or by method of an immunoassay using antibodies which detect the polypeptide. Both detection of mRNA and immunoassays can be performed by methods well known in the art. Measurement of level of the desired polypeptide is determined by a method selected from the group consisting of immunohistochemistry (Microscopy, Immunohistochemistry and Antigen Retrieval Methods: For Light and Electron Microscopy, M.A. Hayat (Author), Kluwer Academic Publishers, 2002; Brown C: "Antigen retrieval methods for immunohistochemistry", Toxicol Pathol 1998; 26(6): 830-1), western blotting (Laemmeli UK: "Cleavage of structural proteins during the assembley of the head of a bacteriophage T4", Nature 1970;227: 680-685; Egger & Bienz, "Protein (western) blotting", MoI Biotechnol 1994; 1(3): 289-305), ELISA (Onorato et al., "Immunohistochemical and ELISA assays for biomarkers of oxidative stress in aging and disease", Ann NY Acad Sci 1998 20; 854: 277-90), antibody microarray hybridization (Huang, "detection of multiple proteins in an antibody-based protein microarray system, Immunol Methods 2001 1; 255 (1-2): 1-13) and targeted molecular imaging (Thomas, Targeted Molecular Imaging in Oncology, Kim et al (Eds)., Springer Verlag, 2001).
Measurement of level of a desired polynucleotide is determined by a method selected from: RT- PCR analysis, in-situ hybridization ("Introduction to Fluorescence In Situ Hybridization: Principles and Clinical Applications", Andreeff & Pinkel (Editors), John Wiley & Sons Inc., 1999), polynucleotide microarray and Northern blotting (Trayhurn, "Northern blotting", Proc Nutr Soc 1996; 55(1B): 583-9; Shifman & Stein, "A reliable and sensitive method for nonradioactive Northern blot analysis of nerve growth factor mRNA from brain tissues", Journal of Neuroscience Methods 1995; 59: 205-208). This diagnostic method may be useful, inter alia, for diagnosing patients suspected to be suffering from a mood disorder.
By "abnormal" in the context of protein expression, is meant a difference of at least 10% in the expression levels of the polypeptide as compared to a control.
Further, the invention provides a method or process of treating depression in a subject which comprises administering to the subject a therapeutically effective amount of a pharmaceutical composition which inhibits the biological activity of any one of the genes detailed in Table 2.
The invention further provides for the use of a modulator of any one of the genes present in Table 2 in the preparation of a medicament; said medicament may be used for the treatment of a depression.
It will be noted that all the polynucleotides to be used in the present invention may undergo modifications so as to possess improved therapeutic properties. Modifications or analogs of nucleotides can be introduced to improve the therapeutic properties of polynucleotides. Improved properties include increased nuclease resistance and/or increased ability to permeate cell membranes. Nuclease resistance, where needed, is provided by any method known in the art that does not interfere with biological activity of the AS polynucleotide, siRNA, cDNA and/or ribozymes as needed for the method of use and delivery (Iyer et al., 1990; Eckstein, 1985; Spitzer and Eckstein, 1988; Woolf et al., 1990; Shaw et al., 1991). Modifications that can be made to oligonucleotides in order to enhance nuclease resistance include modifying the phophorous or oxygen heteroatom in the phosphate backbone. These include preparing methyl phosphonates, phosphorothioates, phosphorodithioates and morpholino oligomers. In one embodiment it is provided by having phosphorothioate bonds linking between the four to six 3'-terminus nucleotide bases. Alternatively, phosphorothioate bonds link all the nucleotide bases. Other modifications known in the art may be used where the biological activity is retained, but the stability to nucleases is substantially increased.
All analogues of, or modifications to, a polynucleotide may be employed with the present invention, provided that said analogue or modification does not substantially affect the function of the polynucleotide. The nucleotides can be selected from naturally occurring or synthetic modified bases. Naturally occurring bases include adenine, guanine, cytosine, thymine and uracil. Modified bases of nucleotides include inosine, xanthine, hypoxanthine, 2- aminoadenine, 6- methyl, 2-propyl and other alkyl adenines, 5-halo uracil, 5-halo cytosine, 6-aza cytosine and 6- aza thymine, psuedo uracil, 4- thiuracil, 8-halo adenine, 8-aminoadenine, 8-thiol adenine, 8-
thiolalkyl adenines, 8-hydroxyl adenine and other 8-substituted adenines, 8-halo guanines, 8- amino guanine, 8-thiol guanine, 8-thioalkyl guanines, 8- hydroxyl guanine and other substituted guanines, other aza and deaza adenines, other aza and deaza guanines, 5-trifluoromethyl uracil and 5- trifluoro cytosine.
The polypeptides employed in the present invention may also be modified, optionally chemically modified, in order to improve their therapeutic activity. "Chemically modified" - when referring to the polypeptides, means a polypeptide where at least one of its amino acid residues is modified either by natural processes, such as processing or other post-translational modifications, or by chemical modification techniques which are well known in the art. Among the numerous known modifications typical, but not exclusive examples include: acetylation, acylation, amidation, ADP-ribosylation, glycosylation, GPI anchor formation, covalent attachment of a lipid or lipid derivative, methylation, myristlyation, pegylation, prenylation, phosphorylation, ubiqutination, or any similar process.
Additional possible polypeptide modifications (such as those resulting from nucleic acid sequence alteration) include the following:
"Conservative substitution" - refers to the substitution of an amino acid in one class by an amino acid of the same class, where a class is defined by common physicochemical amino acid side chain properties and high substitution frequencies in homologous polypeptides found in nature, as determined, for example, by a standard Dayhoff frequency exchange matrix or BLOSUM matrix. Six general classes of amino acid side chains have been categorized and include: Class I (Cys); Class II (Ser, Thr, Pro, Ala, GIy); Class III (Asn, Asp, GIn, GIu); Class IV (His, Arg, Lys); Class V (He, Leu, VaI, Met); and Class VI (Phe, Tyr, Trp). For example, substitution of an Asp for another class III residue such as Asn, GIn, or GIu, is a conservative substitution.
"Non-conservative substitution" - refers to the substitution of an amino acid in one class with an amino acid from another class; for example, substitution of an Ala, a class II residue, with a class III residue such as Asp, Asn, GIu, or GIn.
"Deletion" - is a change in either nucleotide or amino acid sequence in which one or more nucleotides or amino acid residues, respectively, are absent.
"Insertion" or "addition" - is that change in a nucleotide or amino acid sequence which has resulted in the addition of one or more nucleotides or amino acid residues, respectively, as compared to the naturally occurring sequence.
"Substitution" - replacement of one or more nucleotides or amino acids by different nucleotides or amino acids, respectively. As regards amino acid sequences the substitution may be conservative or non- conservative.
"Detection" - refers to a method of detection of a disease. This term may refer to detection of a predisposition to a disease, or to the detection of the severity of the disease.
By "homolog/homology", as utilized in the present invention, is meant at least about 70%, preferably at least about 75% homology, advantageously at least about 80% homology, more advantageously at least about 90% homology, even more advantageously at least about 95%, e.g., at least about 97%, about 98%, about 99% or even about 100% homology. The invention also comprehends that these polynucleotides and polypeptides can be used in the same fashion as the herein or aforementioned polynucleotides and polypeptides.
Alternatively or additionally, "homology", with respect to sequences, can refer to the number of positions with identical nucleotides or amino acid residues, divided by the number of nucleotides or amino acid residues in the shorter of the two sequences, wherein alignment of the two sequences can be determined in accordance with the Wilbur and Lipman algorithm ((1983) Proc. Natl. Acad. Sci. USA 80:726); for instance, using a window size of 20 nucleotides, a word length of 4 nucleotides, and a gap penalty of 4, computer-assisted analysis and interpretation of the sequence data, including alignment, can be conveniently performed using commercially available programs (e.g., Intelligenetics™ Suite, Intelligenetics Inc., CA). When RNA sequences are said to be similar, or to have a degree of sequence identity or homology with DNA sequences, thymidine (T) in the DNA sequence is considered equal to uracil (U) in the RNA sequence. RNA sequences within the scope of the invention can be derived from DNA sequences or their complements, by substituting thymidine (T) in the DNA sequence with uracil (U).
Additionally or alternatively, amino acid sequence similarity or homology can be determined, for instance, using the BlastP program (Altschul et al, Nucl. Acids Res. 25:3389-3402) and available
at NCBI. The following references provide algorithms for comparing the relative identity or homology of amino acid residues of two polypeptides, and additionally, or alternatively, with respect to the foregoing, the teachings in these references can be used for determining percent homology: Smith et al, (1981) Adv. Appl. Math. 2:482-489; Smith et al, (1983) Nucl. Acids Res. 11:2205-2220; Devereux et al, (1984) Nucl. Acids Res. 12:387-395; Feng et al, (1987) J. Molec. Evol. 25:351-360; Higgins et al, (1989) CABIOS 5:151-153; and Thompson et al, (1994) Nucl. Acids Res. 22:4673-4680.
"Having at least X% homology" - with respect to two amino acid or nucleotide sequences, refers to the percentage of residues that are identical in the two sequences when the sequences are optimally aligned. Thus, 90% amino acid sequence identity means that 90% of the amino acids in two or more optimally aligned polypeptide sequences are identical.
By the term "modulates" is meant either increases (promotes, enhances) or decreases (prevents, inhibits).
The invention has been described in an illustrative manner, and it is to be understood that the terminology which has been used is intended to be in the nature of words of description rather than of limitation.
Obviously, many modifications and variations of the present invention are possible in light of the above teachings. It is, therefore, to be understood that within the scope of the appended claims, the invention can be practiced otherwise than as specifically described.
Throughout this application, various publications, including United States patents, are referenced by author and year and patents by number. The disclosures of these publications and patents and patent applications in their entireties are hereby incorporated by reference into this application in order to more fully describe the state of the art to which this invention pertains.
EXAMPLES
Without further elaboration, it is believed that one skilled in the art can, using the preceding description, utilize the present invention to its fullest extent. The following preferred specific embodiments are, therefore, to be construed as merely illustrative, and not limitative of the claimed invention in any way.
Standard molecular biology protocols known in the art not specifically described herein are generally followed essentially as in Sambrook et al., Molecular cloning: A laboratory manual, Cold Springs Harbor Laboratory, New-York (1989, 1992), and in Ausubel et al., Current Protocols in Molecular Biology, John Wiley and Sons, Baltimore, Maryland (1988).
Standard organic synthesis protocols known in the art not specifically described herein are generally followed essentially as in Organic syntheses: Vol.1- 79, editors vary, J. Wiley, New York, (1941 - 2003); Gewert et al., Organic synthesis workbook, Wiley- VCH, Weinheim (2000); Smith & March, Advanced Organic Chemistry, Wiley-Interscience; 5th edition (2001).
Standard medicinal chemistry methods known in the art not specifically described herein are generally followed essentially as in the series "Comprehensive Medicinal Chemistry", by various authors and editors, published by Pergamon Press.
Example 1
Identification of genes involved in depressive conditions
As a first step to the novel drug discovery, key genes involved in depression were identified, as provided by the following.
CMS (chronic mild stress) chip The CMS chip is composed of:
1 -library of brain region of treated and non treated animals (Amygdala, Nucleus Accumbens, Hypothalamus, Hippocampus, Pons and Frontal cortex)
2 -library of the whole brain of treated and non treated animals
3 - clones from the STR chip that were differential in hybridization with CMS samples (-350) 4- ESTs representing major families of receptors and transporters in the brain that are expressed in very low amounts.
The probes are prepared from following animals:
1- Control animals (4 time groups, 6 different brain structures: Amygdala, Nucleus Accumbens, Hypothalamus, Hippocampus, Pons and Frontal cortex)
2- Stressed animals (4 time groups, 6 different brain structures: Amygdala, Nucleus Accumbens, Hypothalamus, Hippocampus, Pons and Frontal cortex)
3- Stressed animals treated with imipramine (4 time groups, 2 treatment groups -responders and not responders- and 6 different brain structures: Amygdala, Nucleus Accumbens, Hypothalamus, Hippocampus, Pons and Frontal cortex)
4- Stressed animals treated with venlafaxine (4 time groups, 2 treatment groups -responders and not responders- and 6 different brain structures: Amygdala, Nucleus Accumbens,
Hypothalamus, Hippocampus, Pons and Frontal cortex)
5- Stressed animals treated with fluoxetine (4 time groups, 2 treatment groups -responders and not responders- and 6 different brain structures: Amygdala, Nucleus Accumbens, Hypothalamus, Hippocampus, Pons and Frontal cortex)
Experiment 1
CMS animals
Behavioral Test (days 0, 7,14, 21, 28, 35, 42, 49, 56, 63) Drug treatment: (started on day 26) :
Venlafaxine: mixed mechanism
SRI: serotonin re-upatke inhibitor
NaRI : noradrenergic reuptake inhibitor
Imipramine: tricyclic class (Positive control in the experiment)
Chip / hybridizations: Hybridization points (days 22, 29, 36, 64)
Experiment 2
Same as 2 except animals were also subjected to stress prior to drug treatment. Also used different drugs:
•Mocbemide-
-Monoamine oxidase A inhibitor»Elevate levels of NE and serotonin -•Citalopram-
-Serotonin reuptake inhibitor (SSRI) ••Amphetamine-
-Inhibit dopamine reuptake»Negative control ••Imipramine - cyclic antidepressant •Positive control
Experiment 3: same as 2 but with the following drugs
•Moclobemide -
-Monoamine oxidase A inhibitor
-Elevates levels of NE and serotonin
•Imipramine - -Cyclic antidepressant
-Positive control
CMS Microarray Hybridizations - Summary
•Three behavioral experiments »Six brain regions (Hypothalamus, Frontal cortex, Amygdala, Hippocampus, Pons, Nucleus
Accumbens)
•Four time points (day 22, 29, 36, 64)
•Individual hybridizations
-Probes from Frontal Cortex (experiment 2) -Probes from Hippocampus (experiment 3 and 4)
•Totally 1,610 hybridizations were performed on CMS microarray in the course of project
Differentially Expressed Genes - Summary •Three conditions inducing differential gene expression: -Stress effect -Drug effect
-Drug response
-•Gene expression was differentially expressed according to at list one of these conditions in at least one brain region, at least in one time point, at least in one experiment ••There were about 1,074 genes which answer to the above criteria
Summary of drugs used:
Venlafaxine: mixed mechanism
SRI: serotonin re-upatke inhibitor NaRI : noradrenergic reuptake inhibitor
Mocbemide-
Monoamine oxidase A inhibitor»Elevate levels of NE and serotonin Citalopram- Serotonin reuptake inhibitor (SSRI)
Amphetamine- Inhibit dopamine reuptake»Negative control
•Moclobemide - -Monoamine oxidase A inhibitor
-Elevates levels of NE and serotonin
-Imipramine: tricyclic class
-Cyclic antidepressant -Positive control in all experiments
Following the above experiments, the genes presented in Table 1 were found to be targets for the treatment of depression / mood disorders. Of these, the genes in Table 2 are preferential targets.
Example 2
General methods
General methods in molecular biology
Standard molecular biology techniques known in the art and not specifically described were generally followed as in Sambrook et al., Molecular Cloning: A Laboratory Manual, Cold Springs Harbor Laboratory, New York (1989, 1992), and in Ausubel et al., Current Protocols in Molecular Biology, John Wiley and Sons, Baltimore, Maryland (1989).
Polymerase chain reaction (PCR) was carried out generally as in PCR Protocols: A Guide To Methods And Applications, Academic Press, San Diego, CA (1990). Reactions and manipulations involving other nucleic acid techniques, unless stated otherwise, were performed as generally described in Sambrook et al., 1989, Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, and methodology as set forth in United States Patent Nos. 4,666,828; 4,683,202; 4,801,531; 5,192,659 and 5,272,057 and incorporated herein by reference.
Protein Purification is performed as described below in Example 5.
Vectors are constructed containing the cDNA of the present invention by those skilled in the art and can contain all expression elements necessary to achieve the desired transcription of the sequences, should transcription be required (see below in specific methods for a more detailed description). Other beneficial characteristics can also be contained within the vectors such as mechanisms for recovery of the nucleic acids in a different form. Phagemids are a specific example of such beneficial vectors because they can be used either as plasmids or as bacteriophage vectors. Examples of other vectors include viruses such as bacteriophages, baculoviruses and retroviruses, DNA viruses, cosmids, plasmids, liposomes and other recombination vectors. The vectors can also contain elements for use in either procaryotic or eucaryotic host systems. One of ordinary skill in the art knows which host systems are compatible with a particular vector.
The vectors are introduced into cells or tissues by any one of a variety of known methods within the art (calcium phosphate transfection; electroporation; lipofection; protoplast fusion; polybrene transfection). The host cell can be any eucaryotic and procaryotic cells, which can be transformed with the vector and which supports the production of the polypeptide. Methods for transformation can be found generally described in Sambrook et al., Molecular Cloning: A Laboratory Manual, Cold Springs Harbor Laboratory, New York (1992), in Ausubel et al., Current Protocols in Molecular Biology, John Wiley and Sons, Baltimore, Maryland (1989), Chang et al., Somatic Gene Therapy, CRC Press, Ann Arbor, MI (1995), Vega et al., Gene Targeting, CRC Press, Ann Arbor, MI (1995) and Gilboa, et al. (1986) and include, for example, stable or transient transfection, lipofection, electroporation and infection with recombinant viral vectors. In addition, see United States Patent No. 4,866,042 for vectors involving the central nervous system and also United States Patent Nos. 5,464,764 and 5,487,992 for positive-negative selection methods.
General methods in immunology
Standard methods in immunology known in the art and not specifically described were generally followed as in Stites et al.(eds), Basic and Clinical Immunology (8th Edition), Appleton & Lange, Norwalk, CT (1994) and Mishell and Shiigi (eds), Selected Methods in Cellular Immunology, W.H. Freeman and Co., New York (1980).
Immunoassays
In general, ELISAs are the preferred immunoassays employed to assess a specimen. ELISA assays are well known to those skilled in the art. Both polyclonal and monoclonal antibodies can be used in the assays. Where appropriate other immunoassays, such as radioimmunoassays (RIA) can be used as are known to those in the art. Available immunoassays are extensively described in the patent and scientific literature. See, for example, United States Patent Nos. 3,791,932; 3,839,153; 3,850,752;
3,850,578; 3,853,987; 3,867,517; 3,879,262; 3,901,654; 3,935,074; 3,984,533; 3,996,345; 4,034,074; 4,098,876; 4,879,219; 5,01 1,771 and 5,281,521 as well as Sambrook et al, Molecular
Cloning: A Laboratory Manual, Cold Springs Harbor, New York, 1989.
Example 3
Preparation of siRNAs
Using proprietary algorithms and the known sequence of any of the genes disclosed herein, the sequences of many potential siRNAs may be generated. siRNA molecules according to the above specifications may be prepared essentially as described herein.
The siRNAs of the present invention can be synthesized by any of the methods which are well- known in the art for synthesis of ribonucleic (or deoxyribonucleic) oligonucleotides. For example, a commercially available machine (available, inter alia, from Applied Biosystems) can be used; the oligonucleotides are prepared according to the sequences disclosed herein. Overlapping pairs of chemically synthesized fragments can be ligated using methods well known in the art (e.g., see U.S. Patent No. 6,121,426). The strands are synthesized separately and then are annealed to each other in the tube. Then, the double-stranded siRNAs are separated from the single- stranded oligonucleotides that were not annealed (e.g. because of the excess of one of them) by HPLC. In relation to the siRNAs or siRNA fragments of the present invention, two or more such sequences can be synthesized and linked together for use in the present invention.
The siRNA molecules of the invention may be synthesized by procedures known in the art e.g. the procedures as described in Usman et al., 1987, J. Am. Chem. Soc, 109, 7845; Scaringe et al., 1990, Nucleic Acids Res., 18, 5433; Wincott et al., 1995, Nucleic Acids Res. 23, 2677-2684; and Wincott et al., 1997, Methods MoI. Bio., 74, 59, and may make use of common nucleic acid protecting and coupling groups, such as dimethoxytrityl at the 5 '-end, and phosphoramidites at the 3'-end. The modified (e.g. 2'-O-methylated) nucleotides and unmodified nucleotides are incorporated as desired.
Alternatively, the nucleic acid molecules of the present invention can be synthesized separately and joined together post-synthetically, for example, by ligation (Moore et al., 1992, Science 256, 9923; Draper et al., International PCT publication No. WO93/23569; Shabarova et al., 1991, Nucleic Acids Research 19, 4247; Bellon et al., 1997, Nucleosides & Nucleotides, 16, 951; Bellon et al., 1997, Bioconjugate Chem. 8, 204), or by hybridization following synthesis and/or deprotection.
The siRNA molecules of the invention can also be synthesized via a tandem synthesis methodology, as described in US patent application publication No. US2004/0019001 (McSwiggen) wherein both siRNA strands are synthesized as a single contiguous oligonucleotide fragment or strand separated by a cleavable linker which is subsequently cleaved to provide
separate siRNA fragments or strands that hybridize and permit purification of the siRNA duplex. The linker can be a polynucleotide linker or a non-nucleotide linker.
For further information, see PCT publication No. WO 2004/015107 (Atugen).
The siRNAs used in the experiments described herein are all 19-mers, having alternating ribonucleotides modified in both the antisense and the sense strands of the compound. The modification is such that a 2'-O-methyl (Me) group is present on the first, third, fifth, seventh, ninth, eleventh, thirteenth, fifteenth, seventeenth and nineteenth nucleotide of the antisense strand, whereby the very same modification, i. e. a 2'-0-Me group, is present at the second, fourth, sixth, eighth, tenth, twelfth, fourteenth, sixteenth and eighteenth nucleotide of the sense strand. These particular siRNA compounds are also blunt ended and are non-phosphorylated at the termini; however, comparative experiments have shown that siRNA compounds phosphorylated at one or both of the 3'-termini have similar activity.
Example 4
Preparation of antibodies
Antibodies may be prepared using an intact polypeptide or fragments containing smaller polypeptides as the immunizing antigen. For example, it may be desirable to produce antibodies that specifically bind to the N- or C- terminal or any other suitable domains. The polypeptide used to immunize an animal can be derived from translated cDNA or chemical synthesis which can be conjugated to a carrier protein, if desired. Such commonly used carriers which are chemically coupled to the polypeptide include keyhole limpet hemocyanin (KLH), thyroglobulin, bovine serum albumin (BSA) and tetanus toxoid. The coupled polypeptide is then used to immunize the animal.
If desired, polyclonal or monoclonal antibodies can be further purified, for example by binding to and elution from a matrix to which the polypeptide or a peptide to which the antibodies were raised is bound. Those skilled in the art know various techniques common in immunology for
purification and/or concentration of polyclonal as well as monoclonal antibodies (Coligan et al, Unit 9, Current Protocols in Immunology, Wiley Interscience, 1994).
Methods for making antibodies of all types, including fragments, are known in the art (See for example, Harlow and Lane, Antibodies: A Laboratory Manual, Cold Spring Harbor Laboratory, New York (1988)). Methods of immunization, including all necessary steps of preparing the immunogen in a suitable adjuvant, determining antibody binding, isolation of antibodies, methods for obtaining monoclonal antibodies, and humanization of monoclonal antibodies are all known to the skilled artisan
The antibodies may be humanized antibodies or human antibodies. Antibodies can be humanized using a variety of techniques known in the art including CDR- grafting (EP239,400: PCT publication WO.91/09967; U.S. patent Nos.5,225,539;5,530,101; and 5,585,089, veneering or resurfacing (EP 592,106; EP 519,596; Padlan, Molecular Immunology 28(4/5):489-498 (1991); Studnicka et al., Protein Engineering 7(6):805-814 (1994); Roguska et al., PNAS 91 :969-973 (1994)), and chain shuffling (U.S. Patent No. 5,565,332).
The monoclonal antibodies as defined include antibodies derived from one species (such as murine, rabbit, goat, rat, human, etc.) as well as antibodies derived from two (or more) species, such as chimeric and humanized antibodies.
Completely human antibodies are particularly desirable for therapeutic treatment of human patients. Human antibodies can be made by a variety of methods known in the art including phage display methods using antibody libraries derived from human immunoglobulin sequences. See also U.S. Patent Nos. 4,444,887 and 4,716,111; and PCT publications WO 98/46645, WO 98/50433, WO 98/24893, WO 98/16654, WO 96/34096, WO 96/33735, and WO 91/10741, each of which is incorporated herein by reference in its entirety.
Additional information regarding all types of antibodies, including humanized antibodies, human antibodies and antibody fragments can be found in WO 01/05998, which is incorporated herein by reference in its entirety.
Example 5
Experimental models
CMS - chronic Mild Stress
Animals Male Wistar rats are singly housed with food and water freely available, and maintained on a 12- h light/dark cycle (lights on at 08.00 am) in a constant temperature (22 ± 0C) and humidity (55 ± 5%).
Procedure The animals are first trained to consume a 1% sucrose solution; training consists of several 1-h baseline tests (twice weekly) in which sucrose solution will is presented, in the home cage, following 14h food and water deprivation. Subsequently, sucrose consumption is monitored, under similar conditions, throughout the duration of the study. On the basis of their sucrose intakes in the final baseline test (Day 0), the animals are divided into two matched groups. One group of animals is subjected to the CMS procedure for a period of 8 consecutive weeks. Each week of the stress regime consists of: two periods of food or water deprivation, two periods of 45-degree cage tilt, two periods of intermittent illumination (light on and off every 2h), two periods of soiled cage (250 ml water in sawdust bedding), two periods of paired housing, two periods of low intensity stroboscopic illumination (150 flashes/min), and two periods of no stress. All the stressors are of 10 - 14 h duration and are applied individually and continuously, day and night. Control non-stressed animals are housed in separate rooms and have no contact with the stressed animals. They are deprived of food and water for 14 h before each sucrose test, but otherwise food and water are available at libitum.
On the basis of their sucrose intake scores following initial 3 weeks of stress, both stressed and control animals are further divided into matched subgroups, and for the subsequent five weeks they receive daily intraperitoneal injections of vehicle (distilled water, 1 ml/kg), imipramine (10 mg/kg), citalopram (10 mg/kg), moclobemide (10 mg/kg) or amphetamine as drug controls. The test animals receive one of the active siRNA inhibitors of the invention. The drugs are
administered at 10.00 and the weekly sucrose tests are carried out 24 h following the last drug injection.
At various time points of the CMS procedure (see below), the control and stressed animals are be decapitated, five brain structures (frontal cortex, hippocampus, Amygdala, nucleus accumbens, hypothalamus, pons) are dissected, frozen and transferred for further molecular analysis. The structures are isolated from the following groups:
- 4 groups of control animals (decapitated on days 22, 29, 36 and 64 of the CMS procedure)
- 4 groups of stressed animals (decapitated on days 22, 29, 36 and 64 of the CMS procedure) - 3 groups of stressed animals receiving imipramine, citalopram, moclobemide , amphetamine or test inhibitor of the invention(decapitated on day 29, 36, and 64 of the CMS procedure). The group of rats killed on day 64 will include both animals responding and non-responding to antidepressant treatments. The animals receiving the active siRNA display less stress than the control animals (receiving water or receiving known drugs).
Model systems for spinal cord injury
Spinal cord injury, or myelopathy, is a disturbance of the spinal cord that results in loss of sensation and/or mobility. The two common types of spinal cord injury are due to trauma and disease. Traumatic injury can be due to automobile accidents, falls, gunshot, diving accidents inter alia, and diseases which can affect the spinal cord include polio, spina bifida, tumors and
Friedreich's ataxia.
Testing the active inhibitors of the invention (such as siRNA) for treating spinal cord injury is done in the rat spinal cord contusion model as described by Young (Prog Brain Res. 2002; 137:231-55). Other predictive animal models of spinal cord injury are described in the following references:
Gruner "A monitored contusion model of spinal cord injury in the rat." J Neurotrauma .1992.
9(2): 123; Hasegawa and Grumet "Trauma-induced tumorigenesis of cells implanted into the rat spinal cord." J Neurosurg 2003. 98(5): 1065-71; and Huang and Young "The effects of arterial blood gas values on lesion volumes in a graded rat spinal cord contusion model." J Neurotrauma
1994, 11(5): 547.
Model systems for CNS injury
1. Closed Head Injury (CHI) - Experimental TBI produces a series of events contributing to neurological and neurometabolic cascades, which are related to the degree and extent of behavioral deficits. CHI is induced under anesthesia, while a weight is allowed to free-fall from a prefixed height (Chen et al, J. Neurotrauma 13, 557, 1996) over the exposed skull covering the left hemisphere in the midcoronal plane.
2. Transient middle cerebral artery occlusion (MCAO) - a 90 to 120 minutes transient focal ischemia is performed in adult, male Sprague Dawley rats, 300-370 gr. The method employed is the intraluminal suture MCAO (Longa et al., Stroke, 30, 84, 1989, and
Dogan et al., J. Neurochem. 72, 765, 1999). Briefly, under halothane anesthesia, a 3-0- nylon suture material coated with Poly-L-Lysine is inserted into the right internal carotid artery (ICA) through a hole in the external carotid artery. The nylon thread is pushed into the ICA to the right MCA origin (20-23 mm). 90-120 minutes later the thread is pulled off, the animal is closed and allowed to recover.
3. Permanent middle cerebral artery occlusion (MCAO) - occlusion is permanent, unilateral- induced by electrocoagulation of MCA. Both methods lead to focal brain ischemia of the ipsilateral side of the brain cortex leaving the contralateral side intact (control). The left MCA is exposed via a temporal craniectomy, as described for rats by Tamura A.et al., J Cereb Blood Flow Metab. 1981 ;1 :53— 60. The MCA and its lenticulostriatal branch are occluded proximally to the medial border of the olfactory tract with microbipolar coagulation. The wound is sutured, and animals returned to their home cage in a room warmed at 26°C to 28°C. The temperature of the animals is maintained all the time with an automatic thermostat.
Evaluation Process
The efficacy of the inhibitor is determined by mortality rate, weight gain, infarct volume, short and long term clinical and neurophysichological and behavioral (including feeding behavior) outcomes in surviving animals. Infarct volumes are assessed histologically (Knight et al., Stroke,
25, 1252, 1994, and Mintorovitch et al., Magn. Reson. Med. 18, 39, 1991). The staircase test ( Montoya et al., J. Neurosci. Methods 36, 219, 1991) or the motor disability scale according to Bederson's method (Bederson et al., Stroke, 17, 472, 1986) is employed to evaluate the functional outcome following MCAO. The animals are followed for different time points, the longest one being two months. At each time point (24h, 1 week, 3, 6, 8 weeks), animals are sacrificed and cardiac perfusion with 4% formaldehyde in PBS is performed. Brains are removed and serial coronal 200 μm sections are prepared for processing and paraffin embedding. The sections are stained with suitable dyes such as TCC. The infarct area is measured in these sections using a computerized image analyzer. The results show that the siRNA inhibitor of the invention is efficacious when compared to the controls.
Example 6
Screening systems ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl ; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 or VIP gene or polypeptide, or any of the genes or polypeptides encoded by the genes presented in Tables 1 or 2, may be used in a screening assay for identifying and isolating compounds which modulate its activity and, in particular, compounds which modulate depression or mood disorders. The compounds to be screened comprise inter alia substances such as small chemical molecules, antibodies, antisense oligonucleotides, antisense DNA or RNA molecules, polypeptides and dominant negatives, and expression vectors.
Many types of screening assays are known to those of ordinary skill in the art. The specific assay which is chosen depends to a great extent on the activity of the candidate gene or the polypeptide expressed thereby. Thus, if it is known that the expression product of a candidate gene has enzymatic activity, then an assay which is based on inhibition (or stimulation) of the enzymatic
activity can be used. If the candidate polypeptide is known to bind to a ligand or other interactor, then the assay can be based on the inhibition of such binding or interaction. When the candidate gene is a known gene, then many of its properties can also be known, and these can be used to determine the best screening assay. If the candidate gene is novel, then some analysis and/or experimentation is appropriate in order to determine the best assay to be used to find inhibitors of the activity of that candidate gene. The analysis can involve a sequence analysis to find domains in the sequence which shed light on its activity.
As is well known in the art, the screening assays can be cell-based or non-cell-based. The cell-based assay is performed using eukaryotic cells such as HeLa cells, and such cell-based systems are particularly relevant in order to directly measure the activity of candidate genes which are anti- apoptotic functional genes, i.e., expression of the gene prevents apoptosis or otherwise prevents cell death in target cells. One way of running such a cell-based assay uses tetracycline-inducible (Tet- inducible) gene expression. Tet-inducible gene expression is well known in the art; see for example, Hofmann et al, 1996, Proc Natl Acad Sci 93( 11 ):5185-5190.
Tet-inducible retroviruses have been designed incorporating the Self-inactivating (SIN) feature of a 3' Ltr enhancer/promoter retroviral deletion mutant. Expression of this vector in cells is virtually undetectable in the presence of tetracycline or other active analogs. However, in the absence of Tet, expression is turned on to maximum within 48 hours after induction, with uniform increased expression of the whole population of cells that harbor the inducible retrovirus, thus indicating that expression is regulated uniformly within the infected cell population.
If the gene product of the candidate gene phosphorylates with a specific target protein, a specific reporter gene construct can be designed such that phosphorylation of this reporter gene product causes its activation, which can be followed by a color reaction. The candidate gene can be specifically induced, using the Tet-inducible system discussed above, and a comparison of induced versus non-induced genes provides a measure of reporter gene activation.
In a similar indirect assay, a reporter system can be designed that responds to changes in protein- protein interaction of the candidate protein. If the reporter responds to actual interaction with the candidate protein, a color reaction occurs.
One can also measure inhibition or stimulation of reporter gene activity by modulation of its expression levels via the specific candidate promoter or other regulatory elements. A specific promoter or regulatory element controlling the activity of a candidate gene is defined by methods well known in the art. A reporter gene is constructed which is controlled by the specific candidate gene promoter or regulatory elements. The DNA containing the specific promoter or regulatory agent is actually linked to the gene encoding the reporter. Reporter activity depends on specific activation of the promoter or regulatory element. Thus, inhibition or stimulation of the reporter is a direct assay of stimulation/inhibition of the reporter gene; see, for example, Komarov et al (1999), Science vol 285,1733-7 and Storz et al (1999) Analytical Biochemistry, 276, 97-104. Various non-cell-based screening assays are also well within the skill of those of ordinary skill in the art. For example, if enzymatic activity is to be measured, such as if the candidate protein has a kinase activity, the target protein can be defined and specific phosphorylation of the target can be followed. The assay can involve either inhibition of target phosphorylation or stimulation of target phosphorylation, both types of assay being well known in the art; for example see Mohney et al (1998) J.Neuroscience 18, 5285 and Tang et al (1997) J Clin. Invest. 100, 1180 for measurement of kinase activity. Specifically, assays for measuring the enzymatic activity of PD2 Synthase, PDE4D, Duspό, Camk2a/2b, Atplal, Cdk5r, NOSl, Chimerinl, MAPKKKK5 and ARHGAP6 are well known in the art. Further, the activity of a transporter / channel / receptor can be assessed by measuring the uptake of a relevant ligand / hormone / neurotransmitter, as the case may be, or a downstream signaling molecule which indicates the activation level of said receptor / transporter. Additionally, there is a possibility that TTR, PD2 Synthase, Adrb3, PDE4D, GABRP, PMCH, Duspό, Camk2a/2b, VIP, Atplal, MEF2C, Cdk5r, NOSl, Neurogranin, Chimerinl, Dendrin, GMF, Cacnb4, SLC17A7, SLC8A1, Drd3, MAPKKKK5 or ARHGAP6 interacts with an enzyme and regulates its enzymatic activity through protein-protein interaction. One can also measure in vitro interaction of a candidate polypeptide with interactors. In this screen, the candidate polypeptide is immobilized on beads. An interactor, such as a receptor ligand, is radioactively labeled and added. When it binds to the candidate polypeptide on the bead, the amount of radioactivity carried on the beads (due to interaction with the candidate polypeptide) can be measured. The assay indicates inhibition of the interaction by measuring the amount of radioactivity on the bead.
Any of the screening assays, according to the present invention, can include a step of identifying the chemical compound (as described above) or other species which tests positive in the assay and can also include the further step of producing as a medicament that which has been so identified. It is considered that medicaments comprising such compounds, or chemical analogs or homologs thereof, are part of the present invention.
Example 7
Gene Therapy
The term "gene therapy" as used herein refers to the transfer of genetic material (e.g DNA or RNA) of interest into a host to treat or prevent a genetic or acquired disease or condition phenotype. The genetic material of interest encodes a product (e.g. a protein, polypeptide, peptide, functional RNA, antisense) the production of which in vivo is desired. For example, the genetic material of interest can encode a hormone, receptor, enzyme, polypeptide or peptide of therapeutic value. Alternatively, the genetic material of interest may encode a suicide gene. For a review see, in general, the text "Gene Therapy" (Advances in Pharmacology 40, Academic Press,
1997).
Gene therapy of the present invention can be carried out in vivo or ex vivo. Ex vivo gene therapy requires the isolation and purification of cells from a patient, the introduction of a therapeutic gene and the introduction of the genetically altered cells back into the patient. A replication-deficient virus such as a modified retrovirus can be used to introduce a therapeutic antisense fragment into such cells. For example, mouse Moloney leukemia virus (MMLV) is a well-known vector in clinical gene therapy trials. See, e.g., Boris-Lauerie et al., Curr. Opin. Genet. Dev., 3, 102-109 (1993).
In contrast, in vivo gene therapy does not require isolation and purification of the cells from a patient. The therapeutic gene or fragment such as an antisense fragment is typically "packaged" for administration to a patient such as in liposomes or in a replication-deficient virus such as adenovirus as described by Berkner, K. L., in Curr. Top. Microbiol. Immunol., 158, 39-66 (1992) or adeno- associated virus (AAV) vectors as described by Muzyczka, N., in Curr. Top. Microbiol. Immunol., 158, 97-129 (1992) and U.S. Pat. No. 5,252,479. Another approach is administration of "naked
DNA" in which the therapeutic gene or fragment such as an antisense fragment is directly injected into the bloodstream or muscle tissue. Still another approach is administration of "naked DNA" in which the therapeutic gene or fragment such as an antisense fragment is introduced into the target tissue by microparticle bombardment using gold particles coated with the DNA.
Gene therapy vectors can be delivered to a subject by, for example, intravenous injection, local administration (see U.S. Pat. No. 5,328,470) or by stereotactic injection (see e.g., Chen et al. (1994) PNAS 91:3054-3057). The pharmaceutical preparation of the gene therapy vector can include the gene therapy vector in an acceptable diluent, or can comprise a slow release matrix in which the gene delivery vehicle is imbedded. Alternatively, where the complete gene delivery vector can be produced intact from recombinant cells, e.g. retroviral vectors, the pharmaceutical preparation can include one or more cells which produce the gene delivery system.
Cell types useful for gene therapy of the present invention include lymphocytes, hepatocytes, myoblasts, fibroblasts, and any cell of the eye such as retinal cells, epithelial and endothelial cells. Preferably the cells are T lymphocytes drawn from the patient to be treated, hepatocytes, any cell of the eye or respiratory or pulmonary epithelial cells. Transfection of pulmonary epithelial cells can occur via inhalation of a neubulized preparation of DNA vectors in liposomes, DNA-protein complexes or replication-deficient adenoviruses. See, e.g., U.S. Patent No. 5,240,846. For a review of the subject of gene therapy, in general, see the text "Gene Therapy" (Advances in Pharmacology 40, Academic Press, 1997).
64
Table 1
Homo sapiens adaptor protein containing pH domain,
2 PTB domain and leucine zipper motif 1 (APPL), mRNA NM. _012096 6912241 APPL
Homo sapiens BMP and activin membrane-bound 3 inhibitor homolog (Xenopus laevis) (BAMBI), mRNA NM. _012342 62953114 BAMBI
Homo sapiens ankyrin repeat domain 17 (ANKRD 17), 4 transcript variant 1 , mRNA NM. _032217 38683806 ANKRD 17
Homo sapiens phosphatidylinositol transfer protein, alpha 5 (PITPNA), mRNA NM. _006224 31377785 PITPNA
Homo sapiens hypothetical protein MGC 12966 6 (MGCl 2966), mRNA NM. _032706 14249303 MGCl 2966
Homo sapiens lipoic acid synthetase (LIAS), nuclear gene encoding mitochondrial protein, transcript variant 1, mRNA NM. _006859 37577165 LIAS
Homo sapiens SPTANl mRNA for non-erythrocytic spectrin alpha, complete cds AB191262 53791224
Homo sapiens transthyretin (prealbumin, amyloidosis type I) (TTR), mRNA NM. _000371 4507724 TTR
Homo sapiens tyrosine 3-monooxygenase/tryptophan 5- monooxygenase activation protein, epsilon polypeptide
10 (YWHAE), mRNA NM. .006761 34304385 YWHAE 11 Homo sapiens KIAAl 463 protein (KIAA1463), mRNA NM. .173602 55749757 KIAA1463 Homo sapiens PRP4 pre-mRNA processing factor 4
12 homolog B (yeast) (PRPF4B), transcript variant 1 , mRNA NNMM_. .000033991133 28872757 PRPF4B Homo sapiens golgi associated PDZ and coiled-coil motif
13 containing (GOPC), transcript variant 2, mRNA [M_001017408 62868212 GOPC
Homo sapiens prostaglandin E receptor 3 (subtype EP3)
14 (PTGER3), transcript variant 6, mRNA NM. .198716 38505181 PTGER3
Homo sapiens adrenergic, beta-3-, receptor (ADRB3),
15 . mRNA NM. .000025 4557266 ADRB3
Homo sapiens similar to 2010300C02Rik protein
16 . (MGC42367), mRNA NM. .207362 46409355 MGC42367
Homo sapiens gamma-aminobutyric acid (GABA) A
17 receptor, delta (GABRD), mRNA NM. .000815 34734070 GABA
Homo sapiens glucose 6 phosphatase, catalytic, 3 18 (G6PC3), mRNA NM_ .138387 46852181 G6PC3
Homo sapiens cysteine dioxygenase, type I (CDOl), 19 mRNA NM_ 001801 56786146 CDOl 20 Homo sapiens sorting nexin 10 (SNXlO), mRNA NM_ .013322 231 1 1022 SNXlO
Homo sapiens synaptosomal-associated protein, 25kDa 21 (SN AP25), transcript variant 2, mRNA NM_ .130811 18765734 SNAP25
Homo sapiens FLYWCH-type zinc finger 1 (FLYWCHl), 22 transcript variant 1 , mRNA NM_ 032296 62953133 FLYWCHl
Homo sapiens ubiquinol-cytochrome c reductase, Rieske 23 iron-sulfur polypeptide 1 (UQCRFSl), mRNA NM_ 006003 5174742 UQCRFSl 24 Homo sapiens KIAA1919 (KIAA1919), mRNA NM 153369 24158475 KIAAl 919
Homo sapiens KIAAOlOO gene product (KIAAOlOO), mRNA NMJH4680 57242773 KIAAOlOO
Homo sapiens gamma-aminobutyric acid (GABA) A receptor, alpha 3 (GABRA3), mRNA NM_000808 34734069 GABA Homo sapiens calsyntenin 2 (CLSTN2), mRNA NM .022131 11545860 CLSTN2 Homo sapiens START domain containing 7 (STARD7), transcript variant 2, mRNA NMJ39267 21450855 STARD7
Homo sapiens glutamate receptor, metabotropic 7 (GRM7), transcript variant 1 , mRNA NM_000844 32528271 GRM7
Homo sapiens protein phosphatase 2C, magnesium- dependent, catalytic subunit (PPM2C), nuclear gene encoding mitochondrial protein, mRNA NMJ) 18444 45439338 PPM2C
Homo sapiens nitric oxide synthase 1 (neuronal) (NOSl), mRNA NM_000620 10835172 NOSl
Homo sapiens dickkopf homolog 3 (Xenopus laevis) (DKK3), transcript variant 2, mRNA NM_013253 66346687 DKK3
Homo sapiens heterogeneous nuclear ribonucleoprotein U (scaffold attachment factor A) (HNRPU), transcript variant 2, mRNA NM_004501 14141160 HNRPU
Homo sapiens fibroblast growth factor 13 (FGF 13), transcript variant IB, mRNA NM_033642 16306542 FGF 13 PREDICTED: Homo sapiens KIAA 1201 protein (KIAA1201), mRNA XM_370660 51468639 K1AA1201
Homo sapiens endosulfine alpha (ENSA), transcript variant 3, mRNA NM_004436 46389548 ENSA
Homo sapiens gamma-aminobutyric acid (GABA) A receptor, alpha 1 (GABRAl), mRNA NM_000806 38327553 GABA Homo sapiens chromobox homolog 6 (CBX6), mRNA NMJ) 14292 46852391 CBX6 Homo sapiens selenoprotein S (SELS), transcript variant 1, mRNA NM_203472 45439348 SELS Homo sapiens kinectin mRNA, complete cds L25616 409465 Homo sapiens THO complex 2 (THOC2), mRNA NM_020449 52486998 THOC2
Homo sapiens cocaine- and amphetamine-regulated transcript (CART), mRNA NM_004291 46852394 CART
Homo sapiens vacuolar protein sorting 41 (yeast) (VPS41 ), transcript variant 1 , mRNA NM_014396 18105059 VPS41
Homo sapiens sorting nexin 6 (SNX6) mRNA, complete cds AF121856 4689251 SNX6
Homo sapiens hypothetical protein FLJ 14624 (FLJ14624), mRNA NM_032813 14249503 FLJ14624
Homo sapiens protein phosphatase 3 (formerly 2B), regulatory subunit B, 19kDa, alpha isoform (calcineurin B, type I) (PPP3R1), mRNA NMJJ00945 45238847 PPP3R1
Homo sapiens cDNA FLJ41288 fis, clone BRAMY2038832, highly similar to Homo sapiens hucep- 10 mRNA for cerebral protein- 10 AK 123282 34528786
PREDICTED: Homo sapiens protein BAP28 (FLJ10359), mRNA XM_375853 51459125 FLJ10359 Homo sapiens Purkinje cell protein 2 (PCP2), mRNA NMJ74895 68299798 PCP2
Homo sapiens glutamate receptor, metabotropic 3 (GRM3), mRNA NM 000840 46358416 GRM3
Homo sapiens beta-l,3-glucuronyltransferase 3 (glucuronosyltransferase I) (B3GAT3), mRNA NM_012200 12408653 B3GAT3 full-length cDNA clone CS0DJ006YB10 of T cells (Jurkat cell line) Cot 10-normalized of Homo sapiens (human) CR610508 50491315
Homo sapiens retinal outer segment membrane protein 1 (ROMl), mRNA NM_000327 19743809 ROMl full-length cDNA clone CS0DI008YL23 of Placenta Cot 25-normalized of Homo sapiens (human) CR607755 50488562
Homo sapiens doublecortin and CaM kinase-like 1 (DCAMKLl), mRNA NM_004734 42544237 DCAMKLl
Homo sapiens RNA binding motif protein 10 (RBMlO), transcript variant 2, mRNA NM_152856 23111017 RBMlO
Homo sapiens nerve growth factor receptor (TNFRSF 16) associated protein 1 (NGFRAPl), transcript variant 3, mRNA NM_014380 7657043 TNFRSF 16
Homo sapiens dynactin 1 (pi 50, glued homolog, Drosophila) (DCTNl), transcript variant 1, mRNA NM_004082 13259509 DCTNl Homo sapiens v-jun sarcoma virus 17 oncogene homolog (avian) (JUN), mRNA NM_002228 44890066 JUN
Homo sapiens brain expressed, X-linked 1 (BEXl), mRNA NMJ) 18476 68533248 BEXl
Homo sapiens scavenger receptor class A, member 3 (SCARA3), transcript variant 1, mRNA NM_016240 33598923 SCARA3
Homo sapiens glutamate receptor, ionotropic, kainate 3 (GRIK3), mRNA NM_000831 28605144 GRJX3 full-length cDNA clone CS0DE009YF20 of Placenta of Homo sapiens (human) CR626719 50507581 Homo sapiens ribosomal protein L39 (RPL39), mRNA NMJ)OlOOO 16306563 RPL39
Homo sapiens sin3-associated polypeptide, 18kDa (S AP 18), mRNA NMJW5870 23510407 SAP 18
Homo sapiens cannabinoid receptor 1 (brain) (CNRl), transcript variant 1, mRNA NMJ) 16083 38683843 CNRl
Homo sapiens glia maturation factor, gamma (GMFG), mRNA NMJJ04877 4758439 GMFG
Homo sapiens CDC42 small effector 1 (CDC42SE1), mRNA NMJ)20239 12965169 CDC42SE1
Homo sapiens SP-A receptor subunit SP-R210 alphaS (MYO 18A) mRNA, complete cds, alternatively spliced AY703984 56159916 MYO 18A
Homo sapiens ubiquitin specific peptidase 19 (USP19), mRNA NMJ)06677 57529245 USP19
Homo sapiens cDNA FLJ46825 fis, clone UTERU2000300 AK128665 34536155 Homo sapiens ribosomal protein L5 (RPL5), mRNA NM_000969 71772259 RPL5 Homo sapiens neuropilin 2 (NRP2), transcript variant 2, mRNA NMJ)03872 41872532 NRP2
Homo sapiens hypothetical protein MGC42105 (MGC42105), mRNA NMJ 53361 37059769 MGC42105
Homo sapiens growth arrest-specific 7 (GAS7), transcript variant b, mRNA NM_201432 41406077 GAS7
Homo sapiens FK506 binding protein 9, 63 kDa (FKBP9), mRNA NMJ)07270 33469984 FKBP9
Homo sapiens NADH dehydrogenase (ubiquinone) 1 beta subcomplex, 11, 17.3kDa, mRNA (cDNA clone BCO 10665 14715010 ubiquinone
MGC:8855 IMAGE:3870660), complete cds
Homo sapiens UPF2 regulator of nonsense transcripts
78 homolog (yeast) (UPF2), transcript variant 2, mRNA NM_015542 18375674 UPF2
Homo sapiens pro-melanin-concentrating hormone
79 (PMCH), mRNA NM_002674 71361683 PMCH
Homo sapiens SUMO-I activating enzyme subunit 1
80 (SAEl), mRNA NM_005500 4885584 SAEl
81 Homo sapiens ribosomal protein L 19 (RPL 19), mRNA NM_000981 68216257 RPL19
82 Homo sapiens neuronal pentraxin I (NPTXl), mRNA NM_002522 55770877 NPTXl Homo sapiens plakophilin 4 (PKP4), transcript variant 2,
83 mRNA NM 001005476 53829375 PKP4
Homo sapiens neuregulin 1 (NRGl), transcript variant
84 HRG-beta2, mRNA NM_013957 7669513 NRGl
85 Homo sapiens cullin 5 (CUL5), mRNA NM_003478 67514034 CUL5
Homo sapiens RAB8B, member RAS oncogene family
86 (RAB8B), mRNA NM_016530 62865646 RAB8B
Homo sapiens synaptosomal-associated protein, 9IkDa
87 homolog (mouse) (SNAP91 ), mRNA NM_014841 7662227 SNAP91
Homo sapiens ubiquinol-cytochrome c reductase core
88 protein I (UQCRCl), mRNA NM_003365 46593006 UQCRCl
Homo sapiens extracellular sulfatase SULF-2 mRNA,
89 complete cds AYlOl 176 27356933
Homo sapiens neural precursor cell expressed,
90 developmentally down-regulated 8 (NEDD8), mRNA NM_006156 5453759 NEDD8 Homo sapiens SPARC-like 1 (mast9, hevin) (SPARCLl),
91 mRNA NM_004684 21359870 SPARCLl
Homo sapiens eukaryotic translation initiation factor 2-
92 alpha kinase 1 (EIF2 AK 1 ), mRNA NM_014413 11125767 EIF2AK1
Homo sapiens zinc finger, RAN-binding domain
93 containing 1 (ZRANBl), mRNA NMJH7580 23943913 ZRANBl
Homo sapiens CDP-diacylglycerol synthase
94 (phosphatidate cytidylyltransferase) 1 (CDSl), mRNA NM_001263 22035623 CDSl
Homo sapiens ribosomal protein L37a, mRNA (cDNA
95 clone MGC26772 IMAGE:4831278), complete cds BC016748 34783289 Homo sapiens CREB binding protein (Rubinstein-Taybi
96 syndrome) (CREBBP), mRNA NM_004380 4758055 CREBBP
97 Homo sapiens KIAA0251 protein (KIAA0251 ), mRNA NM_015027 39930344 KIAA0251 Homo sapiens fragile X mental retardation, autosomal
98 homolog 2 (FXR2), mRNA NM_004860 6598321 FXR2
99 Homo sapiens KIAA0934 (KIAA0934), mRNA NMJH4974 44888817 KIAA0934
100 Homo sapiens NEL-like 2 (chicken) (NELL2), mRNA NM_006159 5453765 NELL2 Homo sapiens vesicle amine transport protein 1 homolog
101 (T californica) (VATl), mRNA NM_006373 18379348 VATl
Homo sapiens cell division cycle 73, Pafl/RNA polymerase II complex component, homolog (S.
102 cerevisiae) (CDC73), mRNA NM_024529 40018639 CDC73
Homo sapiens SNF8, ESCRT-II complex subunit,
103 homolog (S. cerevisiae) (SNF8), mRNA NM 007241 21361379 SNF8
Homo sapiens prostaglandin F receptor (FP) (PTGFR),
104 mRNA NM. _000959 39995094 FP
Homo sapiens histamine N-methyltransferase (HNMT),
105 transcript variant 1, mRNA NM _006895 66932961 HNMT
Homo sapiens hypothetical protein MGC26694
106 (MGC26694), mRNA NM. _178526 60460886 MGC26694
Homo sapiens adrenergic, alpha- ID-, receptor
107 (ADRAlD), mRNA NM. _000678 15451784 ADRAlD
Homo sapiens CD276 antigen (CD276), transcript variant
108 1, mRNA 1 IM_001024736 67188442 CD276 Homo sapiens CD81 antigen (target of antiproliferative
109 antibody 1 ) (CD81 ), mRNA NM. _004356 62240999 CD81
Homo sapiens CD34 antigen (CD34), transcript variant 1,
110 mRNA ] M_001025109 68342037 CD34
Homo sapiens CD63 antigen (melanoma 1 antigen)
111 (CD63), mRNA NM. .001780 34328936 CD63
112 Homo sapiens tubulin, beta 2 (TUBB2), mRNA NM. _001069 68299771 TUBB2
Homo sapiens tubulin, gamma complex associated protein
113 2 (TUBGCP2), mRNA NM_ .006659 5729839 TUBGCP2
Homo sapiens tubulin, alpha 1 (testis specific) (TUBAl),
114 mRNA NM. .006000 17921988 TUBAl
115 Homo sapiens tubulin, alpha 3 (TUBA3), mRNA NM. .006009 17986282 TUBA3 Homo sapiens adenylate cyclase 2 (brain) (ADCY2),
116 mRNA NM. .020546 25952130 ADCY2
Homo sapiens calcium/calmodulin-dependent protein kinase kinase 2, beta (CAMKK2), transcript variant 1,
117 mRNA NM_ .006549 27437014 CAMKK2
Homo sapiens calcium/calmodulin-dependent protein kinase kinase 1, alpha (CAMKKl), transcript variant 1,
118 mRNA NM_ .032294 27437009 CAMKKl
Homo sapiens calcium/calmodulin-dependent protein kinase (CaM kinase) II beta (CAMK2B), transcript
119 variant 2, mRNA NM_ 172078 26051205 CAMK2B
Homo sapiens calcium/calmodulin-dependent protein
120 kinase II inhibitor 1 (C AMK2N 1 ), mRNA NM_ .018584 31324542 CAMK2N1
Homo sapiens calcium/calmodulin-dependent protein kinase (CaM kinase) II alpha (CAMK2A), transcript
121 variant 2, mRNA NM. .171825 25952117 CAMK2A
Homo sapiens calcium/calmodulin-dependent protein kinase (CaM kinase) II delta (CAMK2D), transcript
122 variant 3, mRNA NM_ .001221 26667179 CAMK2D
Homo sapiens dynein, cytoplasmic, intermediate
123 polypeptide 1 (DNCI 1 ), mRNA NM_ 004411 4758177 DNCIl
Homo sapiens cyclin-dependent kinase 5, regulatory
124 subunit 1 (p35) (CDK5R1), mRNA NM_ .003885 34304373 CDK5R1
Homo sapiens cyclin-dependent kinase 5, regulatory
125 subunit 2 (p39) (CDK5R2), mRNA NM_ .003936 42741664 CDK5R2
Homo sapiens ATP-binding cassette, sub- family G
126 (WHITE), member 2 (ABCG2), mRNA NM_ .004827 62526032 ABCG2
Homo sapiens kinesin family member 2OA (KIF20A),
127 mRNA NM_ .005733 5032012 KIF20A
Homo sapiens kinesin family member 5A (KIF5A),
128 mRNA NM. .004984 45446748 KIF5A
Homo sapiens kinesin heavy chain member 2 (KIF2),
129 mRNA NM 004520 4758643 KIF2
Homo sapiens ceroid-lipofuscinosis, neuronal 8 (epilepsy,
130 progressive with mental retardation) (CLN8), mRNA NM_018941 31083052 CLN8
Homo sapiens actin related protein 2/3 complex, subunit
131 5, 16kDa (ARPC5), mRNA NMJW5717 23238212 ARPC5 Homo sapiens phosphatase and actin regulator 1
132 (PHACTRl), mRNA NM_030948 54144630 PHACTRl Homo sapiens ARP2 actin-related protein 2 homolog
133 (yeast) (ACTR2), transcript variant 2, mRNA NM_005722 53692185 ACTR2 Homo sapiens actin related protein 2/3 complex, subunit
134 5-like (ARPC5L), mRNA NM_030978 13569955 ARPC5L Homo sapiens anillin, actin binding protein (scraps
135 homolog, Drosophila) (ANLN), mRNA NM_018685 31657093 ANLN
Homo sapiens actin binding LIM protein 1 (ABLIMl),
136 transcript variant 3, mRNA NM_001003408 51173714 ABLIMl
137 Homo sapiens actin, beta (ACTB), mRNA NMJ)OI lOl 5016088 ACTB Homo sapiens SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily c, member 1
138 (SMARCCl), mRNA NM_003074 21237801 SMARCCl
Homo sapiens ARP3 actin-related protein 3 homolog
139 (yeast) (ACTR3), mRNA NM_005721 34452698 ACTR3 Homo sapiens mitochondrial ribosomal protein L51
(MRPL51), nuclear gene encoding mitochondrial protein,
140 mRNA NMJH6497 16950606 MRPL51
141 Homo sapiens MARCKS-like 1 (MARCKSLl), mRNA NM_023009 32401423 MARCKSLl
Homo sapiens chaperonin containing TCPl, subunit 5
142 (epsilon) (CCT5), mRNA NM_012073 58331232 CCT5
Homo sapiens cathepsin D (lysosomal aspartyl peptidase)
143 (CTSD), mRNA NMJX) 1909 23110949 CTSD
Homo sapiens annexin A2 (ANXA2), transcript variant 3,
144 mRNA NM_004039 50845389 ANXA2
Homo sapiens wingless-type MMTV integration site
145 family member 2 (WNT2), mRNA NMJ303391 4507926 WNT2
Homo sapiens chromosome 1 open reading frame 58
146 (Clorf58), mRNA NMJ44695 21389600 Clorf58 Homo sapiens adaptor-related protein complex 1 , sigma 2
147 subunit (AP1S2), mRNA NMJ303916 22027654 AP1S2 cr: gi|34527648|dbj|AKl 30772.11 Homo sapiens cDNA
FLJ27262 fis, clone TMSOO 164, highly similar to Histone
148 H3.3 AK130772 34527648
149 Homo sapiens WD repeat domain 50 (WDR50), mRNA NMJ) 16001 7705764 WDR50 Homo sapiens heat shock 6OkDa protein 1 (chaperonin)
(HSPDl), nuclear gene encoding mitochondrial protein,
150 transcript variant 1, mRNA NM_002156 41399283 HSPDl
Homo sapiens protein tyrosine kinase non catalytic form
151 (NTRK2) mRNA, complete cds AF508964 21886727 NTRK2 Homo sapiens A-kinase anchoring protein AKAP350
152 mRNA, partial cds AF083037 4558861 Homo sapiens A kinase (PRKA) anchor protein 5
153 (AKAP5), mRNA NMJJ04857 21493042 AKAP5
Homo sapiens casein kinase 1, gamma 3 (CSNK 1G3),
154 transcript variant 2, mRNA NMJ301031812 73532779 CSNK1G3
155 Homo sapiens ephrin-B3 (EFNB3), mRNA NM_001406 38201712 EFNB3
Homo sapiens poly(A) polymerase beta (testis specific)
156 (PAPOLB), mRNA NM_020144 37202113 PAPOLB
Homo sapiens phospholipase C, beta 4 (PLCB4),
157 transcript variant 2, mRNA NM 182797 33469938 PLCB4
Homo sapiens poly(A) binding protein interacting protein
158 2 (PAIP2), mRNA NM_016480 19923458 PAIP2 Homo sapiens potassium voltage-gated channel, Shab-
159 related subfamily, member 1 (KCNBl), mRNA NM_004975 27436972 KCNBl Homo sapiens potassium voltage-gated channel, shaker- related subfamily, beta member 1 (KCNABl), transcript
160 variant 3, mRNA NMJ72159 46255024 KCNABl
Homo sapiens potassium inwardly-rectifying channel,
161 subfamily J, member 8 (KCNJ8), mRNA NM_004982 25121968 KCNJ8 Homo sapiens potassium inwardly-rectifying channel,
162 subfamily J, member 10 (KCNJ 10), mRNA NM_002241 25121965 KCNJlO Homo sapiens potassium inwardly-rectifying channel, subfamily J, member 4 (KCNJ4), transcript variant 2,
163 mRNA NM_004981 4826797 KCNJ4
Homo sapiens potassium voltage-gated channel, Shaw- related subfamily, member 4 (KCNC4), transcript variant
164 1, mRNA NM 004978 24497461 KCNC4
Homo sapiens potassium voltage-gated channel, shaker-
165 related subfamily, member 6 (KCNA6), mRNA NM_002235 25952089 KCNA6 Homo sapiens potassium voltage-gated channel, Shaw-
166 related subfamily, member 3 (KCNC3), mRNA NM_004977 24497459 KCNC3 Homo sapiens potassium voltage-gated channel, shaker- related subfamily, beta member 2 (KCNAB2), transcript
167 variant 1, mRNA NM_003636 27436967 KCNAB2 Homo sapiens potassium voltage-gated channel, shaker-
168 related subfamily, beta member 3 (KCNAB3), mRNA NM_004732 27436970 KCNAB3 Homo sapiens potassium inwardly-rectifying channel,
169 subfamily J, member 5 (KCNJ5), mRNA NM__000890 24797140 KCNJ5 Homo sapiens potassium voltage-gated channel, Shal- related subfamily, member 3 (KCND3), transcript variant
170 2, mRNA NM_172198 27436985 KCND3 Homo sapiens potassium voltage-gated channel, shaker-
171 related subfamily, member 4 (KCN A4), mRNA NM_002233 25952084 KCNA4 Homo sapiens potassium voltage-gated channel, Shaw- related subfamily, member 2 (KCNC2), transcript variant
172 1, mRNA NMJ39136 24497456 KCNC2
Homo sapiens potassium voltage-gated channel, shaker- related subfamily, member 1 (episodic ataxia with
173 myokymia) (KCNA 1 ), mRNA NM_000217 4557684 KCNAl
Homo sapiens potassium inwardly-rectifying channel,
174 subfamily J, member 2 (KCNJ2), mRNA NM_000891 22095339 KCNJ2 Homo sapiens potassium voltage-gated channel, shaker-
175 related subfamily, member 2 (KCNA2), mRNA NM_004974 25952079 KCNA2 Homo sapiens cytochrome c oxidase subunit VIIb
(COX7B), nuclear gene encoding mitochondrial protein,
176 mRNA NM__001866 18105038 COX7B
Homo sapiens cytochrome c oxidase subunit Via polypeptide 1 (COX6A1), nuclear gene encoding
177 mitochondrial protein, mRNA NM 004373 17999527 COX6A1 Homo sapiens phosphodiesterase 7A (PDE7A), transcript
178 variant 1, mRNA NM 002603 24429565 PDE7A
Homo sapiens 2NULL,3NULL-cyclic nucleotide 3NULL
179 phosphodiesterase (CNP), mRNA NM_033133 38570090 CNP
Homo sapiens ectonucleotidβ pyrophosphatase/phosphodiesterase 2 (autotaxin)
180 (ENPP2), mRNA NM_006209 20070229 ENPP2
Homo sapiens phosphodiesterase 4D, cAMP-specific (phosphodiesterase E3 dunce homolog, Drosophila)
181 (PDE4D), mRNA NM_006203 46361981 PDE4D
Homo sapiens sphingomyelin phosphodiesterase 2, neutral membrane (neutral sphingomyelinase) (SMPD2),
182 mRNA NM_003080 4507094 SMPD2
Homo sapiens phospholipase D3, mRNA (cDNA clone
183 IMAGE:3163199), partial cds BC000553 12653558
Homo sapiens pleckstrin homology domain containing, family B (evectins) member 2 (PLEKHB2), transcript
184 variant 2, mRNA NMJ) 17958 8923679 PLEKHB2
Homo sapiens pleckstrin homology domain containing, family G (with RhoGef domain) member 5 (PLEKHG5),
185 transcript variant 1, mRNA NM_020631 38373681 PLEKHG5
Homo sapiens nuclear receptor subfamily 1, group D,
186 member 2 (NR1D2), mRNA NM_005126 40254809 NRl D2
Homo sapiens myosin, light polypeptide 6, alkali, smooth muscle and non-muscle (MYL6), transcript variant 2,
187 mRNA NM_079423 17986259 MYL6
188 Homo sapiens creatine kinase, brain (CKB), mRNA NMJ)01823 34335231 CKB Homo sapiens angiotensinogen (serpin peptidase
189 inhibitor, clade A, member 8) (AGT), mRNA NM_000029 73622269 AGT
Homo sapiens glucosidase, alpha; acid (Pompe disease,
190 glycogen storage disease type II) (GAA), mRNA NMJ300152 11496988 GAA Homo sapiens solute carrier family 13 (sodium-dependent dicarboxylate transporter), member 3 (SLC 13 A3),
191 transcript variant 1, mRNA NM_022829 58761539 SLC 13 A3
Homo sapiens solute carrier family 28 (sodium-coupled
192 nucleoside transporter), member 1 (SLC28A1), mRNA NMJW4213 42542380 SLC28A1
Homo sapiens solute carrier family 8 (sodium/calcium
193 exchanger), member 1 (SLC8A1), mRNA NM_021097 10863912 SLC8A1
Homo sapiens solute carrier family 34 (sodium
194 phosphate), member 2 (SLC34A2), mRNA NM_006424 5453751 SLC34A2
Homo sapiens solute carrier family 17 (sodium-dependent inorganic phosphate cotransporter), member 7
195 (SLC 17 A7), mRNA NM_020309 46255058 SLCl 7 A7
Homo sapiens solute carrier family 23 (nucleobase transporters), member 2 (SLC23A2), transcript variant 1,
196 mRNA NM_005116 44680146 SLC23A2
Homo sapiens solute carrier family 25 (mitochondrial carrier; adenine nucleotide translocator), member 4 (SLC25A4), nuclear gene encoding mitochondrial protein,
197 mRNA NMJ)Ol 151 55749576 SLC25A4
Homo sapiens solute carrier family 6 (neurotransmitter
198 transporter, glycine), member 5 (SLC6A5), mRNA NM_004211 4759135 SLC6A5 Homo sapiens solute carrier family 6, member 15
199 (SLC6A15), transcript variant 1, mRNA NMJ82767 60115819 SLC6A15
Homo sapiens solute carrier family 1 (high affinity aspartate/glutamate transporter), member 6 (SLCl IA6),
200 mRNA NM_005071 4827011 SLC 1A6
Homo sapiens solute carrier family 6 (proline IMINO transporter), member 20 (SLC6A20), transcript variant 1,
201 mRNA NM_020208 46249401 SLC6A20
Homo sapiens solute carrier family 22 (organic cation
202 transporter), member 17 (SLC22A17), transcript variant NM 016609 63999575 SLC22A17
2, mRNA
Homo sapiens solute carrier family 6 (neurotransmitter transporter, betaine/GABA), member 12 (SLC6A12),
203 mRNA NM. _003044 19923156 SLC6A12
Homo sapiens solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system Xag), member
204 1 (SLClAl), mRNA NM. _004170 66773029 SLClAl
Homo sapiens solute carrier family 6, member 17
205 (SLC6A17), mRNA NM_001010898 58219013 SLC6A17
Homo sapiens solute carrier family 1 (glial high affinity
206 glutamate transporter), member 2 (SLC 1A2), mRNA NM. _004171 40254477 SLC1A2 Homo sapiens solute carrier family 12 (sodium/potassium/chloride transporters), member 1
207 (SLC 12Al), mRNA NM. .000338 4557848 SLC 12Al
Homo sapiens solute carrier family 32 (GABA vesicular
208 transporter), member 1 (SLC32A1), mRNA NM. .080552 40806213 SLC32A1
Homo sapiens solute carrier family 6 (neurotransmitter transporter, glycine), member 9 (SLC6A9), transcript
209 variant 3, mRNA NM OO 1024845 67782316 SLC6A9
Homo sapiens solute carrier family 27 (fatty acid
210 transporter), member 2 (SLC27 A2), mRNA NM. .003645 23111065 SLC27A2 Homo sapiens solute carrier family 18 (vesicular
211 monoamine), member 2 (SLC 18 A2), mRNA NM. .003054 42476324 SLC18A2
Homo sapiens solute carrier family 9 (sodium/hydrogen
212 exchanger), isoform 5 (SLC9A5), mRNA NM. .004594 4759143 SLC9A5 Homo sapiens solute carrier family 24 (sodium/potassium/calcium exchanger), member 2
213 (SLC24A2), mRNA NM. .020344 9966786 SLC24A2
Homo sapiens solute carrier family 1 (glial high affinity
214 glutamate transporter), member 3 (SLC 1 A3), mRNA NM. .004172 34222301 SLC1A3
Homo sapiens solute carrier family 38, member 2
215 (SLC38A2), mRNA NM. 018976 31543637 SLC38A2
Homo sapiens solute carrier family 28 (sodium-coupled
216 nucleoside transporter), member 2 (SLC28 A2), mRNA NM. 004212 4759131 SLC28A2 Homo sapiens solute carrier family 23 (nucleobase transporters), member 1 (SLC23A1), transcript variant 1,
217 mRNA NM. .005847 44680144 SLC23A1
Homo sapiens solute carrier family 9 (sodium/hydrogen
218 exchanger), isoform 4 (SLC9A4), mRNA NM 001011552 58531220 SLC9A4
Homo sapiens solute carrier family 25, member 34
219 (SLC25A34), mRNA NM. .207348 46409329 SLC25A34
Homo sapiens solute carrier family 12 (sodium/potassium/chloride transporters), member 2
220 (SLC 12A2), mRNA NM. .001046 38569461 SLC12A2
Homo sapiens solute carrier family 8 (sodium-calcium
221 exchanger), member 2 (SLC8A2), mRNA NM. .015063 57163986 SLC8A2
Homo sapiens dedicator of cytokinesis 9 (DOCK9),
222 mRNA NM_ .015296 24308028 DOCKS
223 Homo sapiens ribosomal protein L27 (RPL27), mRNA NM. .000988 17017972 RPL27
Homo sapiens UDP glycosyltransferase 8 (UDP-galactose
224 ceramide galactosyltransferase) (UGT8), mRNA NM. 003360 40254470 UGT8
Homo sapiens calcium channel, voltage-dependent, beta 4
225 subunit (CACNB4), transcript variant 2, mRNA NM. .000726 54607063 CACNB4 Homo sapiens calcium channel, voltage-dependent,
226 gamma subunit 5 (CACNG5), transcript variant 1 , mRNA NNMMJ 14455881111 22027550 CACNG5
Homo sapiens calcium channel, voltage-dependent, alpha
227 IH subunit (CACNAlH), transcript variant 2, mRNA NMJ)01005407 53832010 CACNAlH Homo sapiens calcium channel, voltage-dependent, P/Q type, alpha IA subunit (CACNAlA), transcript variant 1,
228 mRNA NM_000068 13386499 CACNAlA
Homo sapiens calcium channel, voltage-dependent, alpha
229 2/delta 3 subunit (C ACNA2D3), mRNA NM_018398 54112396 CACNA2D3
Homo sapiens calcium channel, voltage-dependent, alpha
230 2/delta subunit l (CACNA2Dl), mRNA NM_000722 54112389 CACNA2D1
Homo sapiens glutamate receptor, ionotropic, AMPA 2
231 (GRIA2), mRNA NM_000826 4758479 GRIA2
Homo sapiens glutamate receptor, ionotrophic, AMPA 3
232 (GRIA3), transcript variant flop, mRNA NM_000828 32528274 GRIA3
Homo sapiens glutamate receptor, ionotropic, AMPA 1
233 (GRIAl), mRNA NM_000827 6552333 GRIAl
Homo sapiens cadherin 2, type 1, N-cadherin (neuronal)
234 (CDH2), mRNA NM_001792 14589888 CDH2
Homo sapiens protocadherin gamma subfamily A, 7
235 (PCDHGA7), transcript variant 2, mRNA NMJD2087 14196476 PCDHGA7
236 Homo sapiens protocadherin 19 (PCDH19), mRNA NM_020766 58037112 PCDH19 full-length cDNA clone CS0DF026YA20 of Fetal brain of
237 Homo sapiens (human) CR607419 50488226
Homo sapiens purinergic receptor P2X, ligand-gated ion
238 channel, 7 (P2RX7), transcript variant 1, mRNA NM_002562 34335273 P2RX7 Homo sapiens purinergic receptor P2Y, G-protein
239 coupled, 1 (P2RY1), mRNA NM_002563 28872741 P2RY1 Homo sapiens purinergic receptor P2Y, G-protein
240 coupled, 12 (P2RY 12), transcript variant 1, mRNA NM_022788 29029603 P2RY12 Homo sapiens purinergic receptor P2X, ligand-gated ion
241 channel, 1 (P2RX1), mRNA NM_002558 27894283 P2RX1 Homo sapiens calmodulin 3 (phosphorylase kinase, delta)
242 (CALM3), mRNA NMJW5184 58218967 CALM3 Homo sapiens calmodulin 1 (phosphorylase kinase, delta)
243 (CALMl), mRNA NM_006888 31377794 CALMl
Homo sapiens calmodulin 2 (phosphorylase kinase, delta)
244 (CALM2), mRNA NM_001743 20428653 CALM2
Homo sapiens hypothetical protein HSPC 196 (HSPC 196),
245 mRNA NMJH6464 24475981 HSPC196 Homo sapiens diacylglycerol kinase, gamma 9OkDa
246 (DGKG), mRNA NMJJ01346 4503314 DGKG Homo sapiens 5-hydroxytryptamine (serotonin) receptor
247 2C (HTR2C), mRNA NMJJ00868 4504540 HTR2C
Homo sapiens 5-hydroxytryptamine (serotonin) receptor
248 3A (HTR3A), transcript variant 2, mRNA NMJJ00869 47519823 HTR3A Homo sapiens 5-hydroxytryptamine (serotonin) receptor 7
(adenylate cyclase-coupled) (HTR7), transcript variant b,
249 mRNA NMJ) 19860 30795199 HTR7 Homo sapiens 5-hydroxytryptamine (serotonin) receptor
250 2B (HTR2B), mRNA NM_000867 40254430 HTR2B Homo sapiens transient receptor potential cation channel, subfamily C, member 4 associated protein (TRPC4AP),
251 transcript variant 2, mRNA NMJ99368 41872442 TRPC4AP Homo sapiens transient receptor potential cation channel,
252 subfamily C, member 6 (TRPC6), mRNA NMJW4621 19923256 TRPC6
Homo sapiens transient receptor potential cation channel, subfamily V, member 1 (TRPVl), transcript variant 3,
253 mRNA NM_080706 18375670 TRPVl
Homo sapiens aldehyde dehydrogenase 1 family, member
254 Al (ALDHlAl), mRNA NM_000689 25777722 ALDHlAl Homo sapiens aldehyde dehydrogenase 3 family, member
255 Bl (ALDH3B1), transcript variant 1, mRNA NM_000694 71773289 ALDH3B1
256 Homo sapiens centaurin, gamma 1 (CENTGl), mRNA NM_014770 41281493 CENTGl
257 Homo sapiens centaurin, gamma 3 (CENTG3), mRNA NM_031946 32307157 CENTG3
Homo sapiens glutathione peroxidase 4 (phospholipid
258 hydroperoxidase) (GPX4), mRNA NM_002085 4504106 GPX4
Homo sapiens tripartite motif-containing 2 (TRIM2),
259 mRNA NM_015271 15011942 TRIM2
Homo sapiens p21 (CDKN lA)-activated kinase 3
260 (PAK3), mRNA NM .002578 46249379 CDKNlA Homo sapiens cDNA FLJ41312 fis, clone
BRAMY2042804, highly similar to Homo sapiens M-
261 phase phosphoprotein mRNA AK 123306 34528815 Homo sapiens ATPase, Na+/K+ transporting, alpha 1
262 polypeptide (ATP IAl), transcript variant 1 , mRNA NM 000701 48762680 ATP 1 A 1 Homo sapiens proteasome (prosome, macropain) 26S subunit, non- ATPase, 12 (PSMD 12), transcript variant 1,
263 mRNA NM_002816 28872726 PSMD 12
Homo sapiens ATPase, H+ transporting, lysosomal
264 34kDa, Vl subunit D (ATP6V1 D), mRNA NMJ) 15994 19913437 ATP6V1D
Homo sapiens ATPase, Na+/K+ transporting, beta 1
265 polypeptide (ATPlBl), transcript variant 1, mRNA NM_001677 49574487 ATPlBl
Homo sapiens ATPase, Ca++ transporting, ubiquitous
266 (ATP2A3), transcript variant 6, mRNA NMJ74957 28373112 ATP2A3
Homo sapiens ATPase, Class II, type 9A (ATP9A),
267 mRNA NM_006045 65301138 ATP9A Homo sapiens ATPase, Ca++ transporting, cardiac muscle, slow twitch 2 (ATP2A2), transcript variant 1,
268 mRNA NMJ70665 27886537 ATP2A2 Homo sapiens ATP synthase, H+ transporting, mitochondrial FO complex, subunit c (subunit 9), isoform 1 (ATP5G1), nuclear gene encoding mitochondrial
269 protein, transcript variant 1, mRNA NM_005175 50659067 ATP5G1
Homo sapiens ATPase, H+ transporting, lysosomal
270 42kDa, Vl subunit C isoform 2 (ATP6V1C2), mRNA NMJ44583 47717097 ATP6V1C2 Homo sapiens ATPase, H+ transporting, lysosomal
271 7OkDa5 Vl subunit A (ATPOVlA), mRNA NM_001690 19913423 ATP6V1A Homo sapiens ATP synthase, H+ transporting, mitochondrial Fl complex, beta polypeptide (ATP5B),
272 nuclear gene encoding mitochondrial protein, mRNA NM OO 1686 50345985 ATP5B
Homo sapiens metallothionein 3 (growth inhibitory factor
273 (neurotrophic)) (MT3), mRNA NM_005954 45580728 MT3 Homo sapiens glutathione S-transferase Ml (GSTMl),
274 transcript variant l, mRNA NM_000561 23065543 GSTMl Homo sapiens glutathione S-transferase pi (GSTPl),
275 mRNA NM_000852 6552334 GSTPl Homo sapiens sodium channel, voltage-gated, type II,
276 beta (SCN2B), mRNA NM_004588 56699490 SCN2B Homo sapiens sodium channel, voltage-gated, type II,
277 alpha 2 (SCN2A2), mRNA NM_021007 10337596 SCN2A2
Homo sapiens chloride channel 3 (CLCN3), transcript
278 variant e, mRNA NMJ73872 55770839 CLCN3
Homo sapiens chloride channel 6 (CLCN6), transcript
279 variant ClC-6a, mRNA NMJ)01286 4502872 CLCN6
Homo sapiens chloride channel, calcium activated, family
280 member 1 (CLCAl), mRNA NM_001285 4502864 CLCAl
Homo sapiens nucleoporin 155kDa (NUP 155), transcript
281 variant 1, mRNA NMJ53485 24430148 NUP 155
Homo sapiens nudix (nucleoside diphosphate linked
282 moiety X)-type motif 3 (NUDT3), mRNA NM_006703 37622350 NUDT3
Homo sapiens ribosomal protein S4, X-linked (RPS4X),
283 mRNA NM_001007 39812410 RPS4X
284 Homo sapiens ribosomal protein S21 (RPS21), mRNA NMJ)01024 71483115 RPS21
285 Homo sapiens ribosomal protein S3A (RPS3A), mRNA NMJ)01006 70609888 RPS3A
286 Homo sapiens ribosomal protein S25 (RPS25), mRNA NM_001028 14591916 RPS25
Homo sapiens ras homolog gene family, member N
287 mRNA, complete cds BT019394 54695657
288 Homo sapiens EPH receptor A4 (EPHA4), mRNA NMJJ04438 45439363 EPHA4
Homo sapiens mitogen-activated protein kinase kinase
289 kinase 13 (MAP3K13), mRNA NMJ)04721 32130538 MAP3K13
Homo sapiens mitogen-activated protein kinase kinase
290 kinase kinase 5 (MAP4K5), transcript variant 1, mRNA NMJ)06575 38570133 MAP4K5
Homo sapiens mitogen-activated protein kinase 1
291 (MAPKl), transcript variant 1, mRNA NM_002745 66932915 MAPKl
Homo sapiens mitogen-activated protein kinase 10
292 (MAPKlO), transcript variant 3, mRNA NMJ38980 20986505 MAPKlO
Homo sapiens chloride intracellular channel 4 (CLIC4),
293 mRNA NMJ) 13943 7330334 CLIC4
Homo sapiens cDNA FLJ 14534 fis, clone
NT2RM2000599, weakly similar to Homo sapiens F-box
294 protein Lilina (LILINA) mRNA AK027440 14042115 LILINA
Homo sapiens F-box protein 11 (FBXOl 1), transcript
295 variant 1, mRNA NM_025133 30089925 FBXOI l
PREDICTED: Homo sapiens F-box protein 41
296 (FBXO41), mRNA XM_377742 51460632 FBXO41
Homo sapiens ubiquitin-conjugating enzyme E2B (RAD6
297 homolog) (UBE2B), mRNA NMJ)03337 32967281 UBE2B
Homo sapiens phosphoinositide-3-kinase, catalytic,
298 gamma polypeptide (PIK3CG), mRNA NMJW2649 21237724 PIK3CG
Homo sapiens melanoma antigen family D, 1
299 (MAGEDl), transcript variant 2, mRNA NMJ)06986 52632376 MAGEDl
300 Homo sapiens zinc finger protein 677 (ZNF677), mRNA NMJ 82609 33438597 ZNF677
301 Homo sapiens zinc finger protein 654 (ZNF654), mRNA NMJH8293 8922809 ZNF654
Homo sapiens zinc finger protein 143 (clone pHZ-1)
302 (ZNF 143), mRNA NM_003442 24475652 ZNF143
Homo sapiens zinc finger protein 655 (ZNF655),
303 transcript variant 6, mRNA NMJJ01009956 58331255 ZNF655
304 Homo sapiens zinc fineer nrotein 341 (ZNF341), mRNA NM 032819 40807464 ZNF341
305 Homo sapiens zinc finger protein 513 (ZNF513), mRNA NM_144631 47419890 ZNF513 Homo sapiens zinc finger protein 21 (KOX 14) (ZNF21),
306 transcript variant 1, mRNA NM_006962 55769558 ZNF21 Homo sapiens zinc finger protein 238 (ZNF238),
307 transcript variant 2, mRNA NM_006352 45439300 ZNF238
308 Homo sapiens zinc finger protein 692 (ZNF692), mRNA NM_017865 21361765 ZNF692
309 Homo sapiens zinc finger protein 330 (ZNF330), mRNA NM_014487 13384595 ZNF330
310 Homo sapiens zinc finger protein 189 (ZNF 189), mRNA NM_197977 37574607 ZNF189
Homo sapiens cholinergic receptor, nicotinic, alpha
311 polypeptide 3 (CHRN A3), mRNA NM_000743 19923121 CHRNA3
Homo sapiens cholinergic receptor, nicotinic, alpha
312 polypeptide 2 (neuronal) (CHRNA2), mRNA NM_000742 4502822 CHRNA2
313 Homo sapiens interleukin l l (ILl l), mRNA NM_000641 24430217 ILI l cr: gi|60812206|gb|AY893164.1| Synthetic construct
Homo sapiens clone FLH147927.01L ubiquitin specific
314 protease 10 (USPlO) mRNA, partial cds AY893164 60812206 USPlO cr: gi|60812121 |gb|A Y893160.1| Synthetic construct
Homo sapiens clone FLH147923.01L ubiquitin specific
315 protease 30 (MGC 10702) mRNA, partial cds AY893160 60812121 MGC 10702 PREDICTED: Homo sapiens similar to Ubiquitin carboxyl-terminal hydrolase 7 (Ubiquitin thiolesterase 7) (Ubiquitin-specific processing protease 7) (Deubiquitinating enzyme 7) (Herpesvirus associated
316 ubiquitin-specific protease) (LOC345576), mRNA XM_293886 41147015 LOC345576
Homo sapiens ubiquitin specific protease 16 (USP 16),
317 transcript variant 2, mRNA NM_001001992 50312663 USP 16
Homo sapiens forkhead box Pl (FOXPl), transcript
318 variant 1, mRNA NM_032682 60498985 FOXPl Homo sapiens mRNA for metalloprotease disintegrin 15
319 (ADAMTS 15 gene) AJ315733 19171175
Homo sapiens E74-like factor 2 (ets domain transcription
320 factor) (ELF2), transcript variant 2, mRNA NM_006874 42544175 ELF2
321 Homo sapiens protein kinase C, epsilon (PRKCE), mRNA NM_005400 47157326 PRKCE Homo sapiens protein kinase C, beta 1 (PRKCBl),
322 transcript variant 1, mRNA NM_212535 47157321 PRKCBl Homo sapiens neurogranin (protein kinase C substrate,
323 RC3) (NRGN), mRNA NM_006176 5453799 NRGN Homo sapiens protein kinase C and casein kinase
324 substrate in neurons 1 (PACSINl), mRNA NM_020804 47834327 PACSINl Homo sapiens proteasome (prosome, macropain) subunit,
325 betatype, 4 (PSMB4), mRNA NM_002796 22538466 PSMB4 Homo sapiens MADS box transcription enhancer factor 2, polypeptide C (myocyte enhancer factor 2C) (MEF2C),
326 mRNA NM_002397 19923214 MEF2C
Homo sapiens Rho GTPase activating protein 15
327 (ARHGAP 15), mRNA NM_018460 28466978 ARHGAP 15
328 Homo sapiens basic transcription factor 3 (BTF3), mRNA NM_001207 29126237 BTF3 Homo sapiens myelin transcription factor 1 (MYTl),
329 mRNA NM 004535 41352713 MYTl
Homo sapiens Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain, 2 (CITED2),
330 mRNA NM_006079 51807294 CITED2
Homo sapiens WAS protein family, member 1 (WASFl),
331 transcript variant 1, mRNA NM_003931 68161486 WASFl
332 Homo sapiens crystallin, gamma D (CRYGD), mRNA NM_006891 13377001 CRYGD Homo sapiens chromodomain helicase DNA binding
333 protein 3 (CHD3), transcript variant 1, mRNA NM_001005273 52630325 CHD3
Homo sapiens protein tyrosine phosphatase, receptor type,
334 R (PTPRR), transcript variant 1 , mRNA NM_002849 19743915 PTPRR full-length cDNA clone CSODFOl IYP 12 of Fetal brain of
335 Homo sapiens (human) CR617323 50498130
Homo sapiens RAB36 full length open reading frame (ORF) cDNA clone (cDNA clone
336 C22ORF:pGEM.RAB36.V8) CR456553 47678636 ORF
Homo sapiens amyloid beta (A4) precursor protein (peptidase nexin-II, Alzheimer disease) (APP), transcript
337 variant 3, mRNA NM_201414 41406056 APP
Homo sapiens splicing factor 3b, subunit 4, 49kDa
338 (SF3B4), mRNA NM_005850 23111059 SF3B4
339 Homo sapiens, clone IMAGE:4155302, mRNA BC034454 21675130
Homo sapiens heart muscle inducible nitric oxide
340 synthase (iNOS) mRNA, complete cds AF051164 2944098 iNOS Homo sapiens cDNA FLJ42948 fis, clone BRSTN2006306, highly similar to Mus musculus BM88
341 antigen mRNA AK124938 34530868
Homo sapiens Williams-Beuren syndrome chromosome
342 region 1 (WBSCRl), transcript variant 2, mRNA NM_031992 14702179 WBSCRl
343 Homo sapiens stannin (SNN), mRNA NM_003498 29893560 SNN
Homo sapiens RasGEF domain family, member 1C
344 (RASGEF 1 C), transcript variant 1 , mRNA NM_175062 73088875 RASGEFlC
Homo sapiens tachykinin, precursor 1 (substance K, substance P, neurokinin 1, neurokinin 2, neuromedin L, neurokinin alpha, neuropeptide K, neuropeptide gamma)
345 (TAC 1), transcript variant alpha, mRNA NM_013996 7770074 TACl
346 Homo sapiens ribosomal protein L41 (RPL41), mRNA NM_021104 10863874 RPL41 Homo sapiens myelin basic protein (MBP), transcript
347 variant 6, mRNA NM_001025098 68509935 MBP
Homo sapiens transmembrane and coiled-coil domains 3
348 (TMCO3), mRNA NM_017905 46358357 TMCO3
Homo sapiens spastic paraplegia 7, paraplegin (pure and complicated autosomal recessive) (SPG7), nuclear gene encoding mitochondrial protein, transcript variant 1,
349 mRNA NM_003119 40806171 SPG7
Homo sapiens hypothetical protein MGC33962
350 (MGC33962), mRNA NMJ 52479 46391095 MGC33962
351 Human mRNA for KIAA0143 gene, partial cds D63477 1469867 Homo sapiens death-associated protein 6 (DAXX),
352 mRNA NM_001350 53828721 DAXX
Homo sapiens cDNA FLJ30498 fis, clone
353 BRAWH2000417, highly similar to N-CHIMAERIN AK055060 16549710
354 Homo sapiens catenin, beta like 1 (CTNNBLl), mRNA NM_030877 29570786 CTNNBLl
355 Homo sapiens clone 23688 mRNA sequence AF052115 3360422 Homo sapiens mal, T-cell differentiation protein 2
356 (MAL2), mRNA NM_052886 16418396 MAL2
PREDICTED: Homo sapiens similar to contains
357 transmembrane (TM) region (LOC342865), mRNA XM_292785 51474647 TM Homo sapiens dopamine receptor D3 (DRD3), transcript
358 variant e, mRNA NM_033663 56549126 DRD3
Homo sapiens mRNA; cDNA DKFZp686L07113 (from
359 clone DKFZp686L07113) CR627394 50949881
Homo sapiens thromboxane A2 receptor (TBXA2R),
360 transcript variant 2, mRNA NMJ)01060 42518083 TBXA2R
Homo sapiens glial fibrillary acidic protein (GFAP),
361 mRNA NM_002055 24430142 GFAP
Homo sapiens neurofilament, heavy polypeptide 20OkDa
362 (NEFH), mRNA NM_021076 32483415 NEFH
Homo sapiens hypothetical protein FLJ90013, mRNA (cDNA clone MGC:87011 IMAGE:4825556), complete
363 cds BC066899 45219875
Homo sapiens heterogeneous nuclear ribonucleoprotein
364 A2/B1 (HNRPA2B1), transcript variant Bl, mRNA NM_031243 14043071 HNRPA2B1 Homo sapiens DnaJ (Hsp40) related, subfamily B,
365 member 13 (DNAJB 13), mRNA NMJ53614 39204546 Hsp40
Homo sapiens KIAAl 102 protein, mRNA (cDNA clone
366 IMAGE:4538099) BC053639 31565553
367 Homo sapiens Ras-like without CAAX 1 (RITl), mRNA NM_006912 52856419 RITl
368 Homo sapiens synaptotagmin I (SYTl), mRNA NM_005639 5032138 SYTl 369 Homo sapiens mRNA for KIAA 1096 protein, partial cds AB029019 20521763 full-length cDNA clone CSODIOl 6 YH22 of Placenta Cot
370 25-normalized of Homo sapiens (human) CR621158 50501965
371 Homo sapiens dopamine receptor D4 (DRD4), mRNA NM_000797 32483396 DRD4 Homo sapiens TGF beta-inducible nuclear protein 1
372 (TINPl), mRNA NM 014886 21359901 TINPl
Homo sapiens API gamma subunit binding protein 1, mRNA (cDNA clone MGC: 104959 IMAGE:3077104),
373 complete cds BC090930 60688296
Homo sapiens eukaryotic translation initiation factor 4A
374 isoform 2-like protein mRNA, partial cds AY207392 29169169
Homo sapiens mRNA; cDNA DKFZp686C20269 (from
375 clone DKFZp686C20269) BX648458 34367620
Homo sapiens tumor differentially expressed 2 (TDE2),
376 mRNA NM_020755 34222115 TDE2
Homo sapiens ryanodine receptor 1 (skeletal) (RYRl),
377 mRNA NM 000540 10863870 RYRl
Homo sapiens presenilin enhancer 2 homolog (C. elegans), mRNA (cDNA clone MGC: 14572
378 IMAGE:4096271), complete cds BC009575 16306999
Homo sapiens dual specificity phosphatase 6 (DUSP6),
379 transcript variant 1, mRNA NM 001946 42764682 DUSP6
380 Homo sapiens reticulon 4a mKNA, complete cds AF148537 10039550 Homo sapiens microtubule-associated protein tau
381 (MAPT), transcript variant l, mRNA NM 016835 8400712 MAPT Homo sapiens synaptotagmin III, mRNA (cDNA clone
382 MGC:33677 IMAGE:5275216), complete cds BC031067 21410306
Homo sapiens thioredoxin 1 (TRXl) pseudogene 5
383 sequence AF357532 13448894 TRXl
Homo sapiens tumor rejection antigen (gp96) 1 (TRAl),
384 mRNA NM_003299 4507676 TRAl
Homo sapiens cDNA FLJ35271 fis, clone
385 PROST2005886 AK092590 21751218 full-length cDNA clone CS0DK002YG07 of HeLa cells
386 Cot 25-normalized ofHomo sapiens (human) CR591956 50472763 Homo sapiens mRNA; cDNA DKFZp566B064 (from
387 clone DKFZp566B064) AL713702 19584419
Homo sapiens succinate-CoA ligase, ADP-forming, beta
388 subunit (SUCLA2), mRNA NM_003850 11321582 SUCLA2
389 Homo sapiens KIAAl 862 protein (KIAAl 862), mRNA NM_032534 51468087 KIAA 1862 Homo sapiens erbb2 interacting protein (ERBB2IP),
390 transcript variant 7, mRNA NM_001006600 55770894 ERBB2IP Homo sapiens septin 7 (SEPT7), transcript variant 1,
391 mRNA NM_001788 58535458 SEPT7
Homo sapiens tumor differentially expressed 1 (TDEl),
392 transcript variant 2, mRNA NM 198941 39812105 TDEl
Homo sapiens scavenger receptor class F, member 2
393 (SCARF2), transcript variant 2, mRNA NM_182895 33598936 SCARF2
Homo sapiens copine family member (LOC151835),
394 mRNA NMJ53635 25121973 LOC151835
395 Homo sapiens cDN A FLJ40429 fis, clone TESTI2039177 AK097748 21757613 Homo sapiens cDNA FLJ39507 fis, clone
PROST2017617, highly similar to Homo sapiens
396 ENIGMA protein mRNA AK096826 21756403 full-length cDNA clone CSODCOO 1YO02 of Neuroblastoma Cot 25-normalized of Homo sapiens
397 (human) CR623629 50504436
Homo sapiens cDNA FLJ36793 fis, clone
398 ADRGL2006124, highly similar to SCGlO-like-protein AK094112 21753106 Homo sapiens mitofusin 1 (MFNl), nuclear gene encoding mitochondrial protein, transcript variant 1,
399 mRNA NM_033540 45269136 MFNl
Homo sapiens tweety homolog 1 (Drosophila), mRNA
400 (cDNA clone IMAGE:5735116), partial cds BC065919 42406317
401 Homo sapiens mRNA for KIAA0379 protein, partial cds AB002377 6634024 402 Homo sapiens calumenin (CALU), mRNA NM OO 1219 6005991 CALU full-length cDNA clone CL0BA009ZC04 of Placenta of
403 Homo sapiens (human) CR614775 50495582
404 Homo sapiens cDN A FLJ46114 fis, clone TESTI2036822 AK 127996 34535149 PREDICTED: Homo sapiens similar to Gamma- aminobutyric-acid receptor rho-3 subunit precursor
405 (GABA(A) receptor) (LOC200959), mRNA XM_116036 51492723 GABA(A
Homo sapiens oral- facial-digital syndrome 1, mRNA (cDNA clone MGC: 117038 IMAGE:40008704),
406 complete cds BC096344 64654924
Homo sapiens mitochondrial tumor suppressor 1 (MTUSl), nuclear gene encoding mitochondrial protein,
407 transcript variant 4, mRNA NM_001001931 50348625 MTUSl
Homo sapiens mRNA; cDNA DKFZp686P07111 (from
408 clone DKFZp686P07111) AL832150 21732694
Homo sapiens adrenergic, alpha-2B-, receptor
409 (ADRA2B), mRNA NM_000682 55743081 ADRA2B
Homo sapiens mRNA for RaP2 interacting protein 8
410 variant protein AB209802 62089189
Homo sapiens cDNA clone MGC:88735
411 IMAGE:6263100, complete cds BC072022 47938399
Homo sapiens glutamate receptor, ionotropic, kainate 1
412 (GRIK 1 ), transcript variant 1 , mRNA NM_000830 59710094 GRIKl
413 Homo sapiens ribosomal protein L4 (RPL4), mRNA NM_000968 16579884 RPL4 Homo sapiens peptidylprolyl isomerase A (cyclophilin A)
414 (PPIA), transcript variant 1 , mRNA NM_021130 45439309 PPIA
Homo sapiens glutamate receptor, ionotropic, kainate 2
415 (GRIK2), transcript variant 1 , mRNA NM_021956 28558991 GRIK2 full-length cDNA clone CS0DB008YA09 of Neuroblastoma Cot 10-normalized of Homo sapiens
416 (human) CR614918 50495725
Homo sapiens APH-Ib mRNA for anterior pharynx
417 defective 1 b splice variant-2, complete cds AB197122 68158701
418 Human mRNA for TPRDI, complete cds D84294 1632761
Homo sapiens heterogeneous nuclear ribonucleoprotein
419 Hl (H) (HNRPHl), mRNA NM_005520 5031752 HNRPHl
420 Homo sapiens hemoglobin, alpha 2 (HBA2), mRNA NM_000517 14043068 HBA2 Homo sapiens zinc finger, C3HC-type containing 1
421 (ZC3HCl), mRNA NM_016478 66348044 ZC3HC1
Homo sapiens mRNA; cDNA DKFZp686El 1103 (from
422 clone DKFZp686E 11103) AL832116 21732659
Homo sapiens syndecan binding protein (syntenin)
423 (SDCBP), transcript variant 3, mRNA NM_001007068 55749503 SDCBP
Homo sapiens signal peptidase complex subunit 3
424 homolog (S. cerevisiae) (SPCS3), mRNA NM_021928 11345461 SPCS3 Homo sapiens cDNA PSEC0239 fis, clone OVARC 1000363, highly similar to Homo sapiens
425 aspartyl protease mRNA AK075539 22761749
Homo sapiens cDNA clone IMAGE:5277710, containing
426 frame-shift errors BC042364 27503450
Homo sapiens SRY (sex determining region Y)-box 9 (campomelic dysplasia, autosomal sex-reversal) (SOX9),
427 mRNA NM_000346 37704387 SOX9
Homo sapiens CUG triplet repeat, RNA binding protein 2
428 (CUGBP2), transcript variant 1 , mRNA NM_001025076 68303644 CUGBP2 Homo sapiens cDNA FLJ30643 fis, clone CTONG2003782, highly similar to GAMMA- AMINOBUTYRIC-ACID RECEPTOR PI SUBUNIT
429 PRECURSOR AK055205 16549881
Homo sapiens, Similar to cask-interacting protein 2, clone
430 IMAGE:5222780, mRNA BC032359 21619625
Homo sapiens mRNA; cDNA DKFZp761I141 (from
431 clone DKFZp76 II 141) AL 136564 13276634
Homo sapiens adipocyte-specific adhesion molecule
432 (ASAM), mRNA NM_024769 41393588 ASAM
Homo sapiens adducin 1 (alpha) (ADDl), transcript
433 variant 1, mRNA NMJ)Ol 119 29826318 ADDl
Homo sapiens cDNA clone MGC: 102952
434 IMAGE:30529503, complete cds BC094690 63101198
Homo sapiens olfactomedin 1 (OLFMl), transcript
435 variant 2, mRNA NM_006334 34335283 OLFMl
Homo sapiens brevican (BCAN), transcript variant 2,
436 mRNA NMJ98427 38372930 BCAN full-length cDNA clone CS0DJ008YB17 of T cells (Jurkat cell line) Cot 10-normalized of Homo sapiens
437 (human) CR609550 50490357 full-length cDNA clone CS0DF034YK23 of Fetal brain of
438 Homo sapiens (human) CR626484 50507291
Homo sapiens mortality factor 4 like 1 (MORF4L1),
439 transcript variant 1, mRNA NM_006791 45643136 MORF4L1
440 Homo sapiens synaptophysin (SYP), mRNA NM_003179 56699493 SYP
Homo sapiens uridine-cytidine kinase 1-like 1 (UCKLl),
441 mRNA NMJH7859 57863311 UCKLl
Homo sapiens potassium channel, subfamily K, member 1
442 (KCNKl), mRNA NM_002245 15451900 KCNKl full-length cDNA clone CSODBOOl YA17 of Neuroblastoma Cot 10-normalized of Homo sapiens
443 (human) CR607507 50488314
Homo sapiens WIG-1/PAG608 protein mRNA, complete
444 cds AF355465 15077640
Homo sapiens prostaglandin D2 receptor (DP) (PTGDR),
445 mRNA NM_000953 38505191 PTGDR
Homo sapiens cisplatin resistance-associated overexpressed protein (CROP), transcript variant 2,
446 mRNA NM_006107 52426742 CROP
447 Homo sapiens KIAA 1049 protein (KIAA 1049), mRNA NM_014972 14149656 KIAA 1049 Homo sapiens DNA fragmentation factor DFF35 (DFF35)
448 mRNA, complete cds AF087573 4926917 DFF35 full-length cDNA clone CS0DI034YB17 of Placenta Cot
449 25-normalized of Homo sapiens (human) CR618353 50499160 full-length cDNA clone CS0DK009YH19 of HeLa cells
450 Cot 25-normalized of Homo sapiens (human) CR624892 50505699 full-length cDNA clone CS0DF007YC14 of Fetal brain of
451 Homo sapiens (human) CR619603 50500410
452 Homo sapiens cDNA clone IMAGE:4692052, partial cds BC065716 41389046 Homo sapiens hypothetical protein MGC21688, mRNA (cDNA clone MGC:33603 IMAGE:4825140), complete
453 cds BC037299 22713421
Homo sapiens cholinergic receptor, nicotinic, alpha
454 polypeptide 7 (CHRNA7), mRNA NM_000746 21536283 CHRNA7
Homo sapiens mRNA for translocation protein- 1,
455 complete cds D87127 1817551
Homo sapiens zinc finger, SWIM-type containing 6,
456 mRNA (cDNA clone IMAGE:5303767) BC039438 24659895
457 Homo sapiens mRNA for KIAA 1043 protein, partial cds AB028966 58257738 Human somatic cytochrome c (HCl) processed
458 pseudogene, complete cds M22878 181256 HCl Homo sapiens microtubule-associated protein IA
459 (MAPlA), mRNA NM_002373 45580726 MAPlA
460 Homo sapiens TSP Y-like 4 (TSPYL4), mRNA NM_021648 46094050 TSPYL4
461 Homo sapiens signal peptide peptidase 3 (SPPL3), mRNA NM 139015 37537690 SPPL3
462 Homo sapiens CGI-51 protein (CGI-51 ), mRNA NM_015380 34222323 CGI-51 Homo sapiens eukaryotic translation initiation factor 2C,
463 2 (EIF2C2), mRNA NM_012154 29171733 EIF2C2
464 Homo sapiens secernin 1 (SCRNl), mRNA NMJ) 14766 28461170 SCRNl
Homo sapiens zinc finger, DHHC-type containing 12
465 (ZDHHC 12), mRNA NMJB2799 42516559 ZDHHC 12
466 Homo sapiens junctophilin 3 (JPH3), mRNA NM_020655 21704282 JPH3
467 Homo sapiens plexin A2 (PLXNA2), mRNA NM_025179 46275829 PLXN A2
Homo sapiens leucine rich repeat and sterile alpha motif
468 containing 1 (LRSAMl), transcript variant 1, mRNA NM_138361 53729358 LRSAMl
469 Homo sapiens syntaphilin (SNPH), mRNA NM_014723 38202245 SNPH PREDICTED: Homo sapiens discs, large (Drosophila)
470 homolog-associated protein 3 (DLGAP3), mRNA XM_035601 51458944 DLGAP3 Homo sapiens dynamin 1 (DNMl), transcript variant 2,
471 mRNA NM_001005336 56549116 DNMl
Homo sapiens hUNC18a alternatively-spliced mRNA,
472 complete cds AF004562 3041872
Homo sapiens glycine amidinotransferase (L-
473 arginine:glycine amidinotransferase) (GATM), mRNA NM_001482 4503932 GATM Homo sapiens secretory carrier membrane protein 4
474 (SCAMP4), mRNA NMJ)79834 50593003 SCAMP4
Homo sapiens tropomodulin 3 (ubiquitous) (TMOD3),
475 mRNA NMJH4547 34222315 TMOD3
Homo sapiens chromosome 2 open reading frame 23
476 (C2orf23), mRNA NMJ)22912 12597656 C2orf23
Homo sapiens seizure related 6 homolog (mouse)-like
477 (SEZ6L), mRNA NMJ)21115 55956782 SEZ6L
478 Homo sapiens glypican 1 (GPCl), mRNA NMJ102081 4504080 GPCl Homo sapiens programmed cell death 2 (PDCD2),
479 transcript variant 1, mRNA NMJJ02598 21735591 PDCD2
480 Homo sapiens neurotensin (NTS), mRNA NMJTO6183 31563516 NTS 481 Homo sapiens carboxypeptidase E (CPE), mRNA NMJTO1873 4503008 CPE Homo sapiens heat shock 7OkDa protein 4-like
482 (HSPA4L), mRNA NM 014278 31541940 HSPA4L
Homo sapiens carnitine palmitoyltransferase IA (liver) (CPTlA), nuclear gene encoding mitochondrial protein,
483 transcript variant 1, mRNA NM 001876 73623029 CPTlA
Homo sapiens mal, T-cell differentiation protein (MAL),
484 transcript variant a, mRNA NM_002371 12408666 MAL
Homo sapiens sirtuin (silent mating type information regulation 2 homolog) 2 (S. cerevisiae) (SIRT2),
485 transcript variant 1, mRNA NM_012237 13775599 SIRT2
Homo sapiens fasciculation and elongation protein zeta 1
486 (zygin I) (FEZl), transcript variant 1, mRNA NM_005103 17105402 FEZl
Homo sapiens transmembrane protein 30A (TMEM30A),
487 mRNA NM_018247 52694667 TMEM30A
Homo sapiens calcineurin inhibitor ZAKI-4 beta splice
488 variant 1 mRNA, complete cds, alternatively spliced AY034085 21307622 Homo sapiens echinoderm microtubule associated protein
489 like 1 (EMLl), transcript variant 2, mRNA NM_004434 56790929 EMLl
Homo sapiens NADH dehydrogenase (ubiquinone) Fe-S protein 2, 49kDa (NADH-coenzyme Q reductase)
490 (NDUFS2), mRNA NM_004550 34147556 NDUFS2
Homo sapiens inositol polyphosphate-5-phosphatase,
491 145kDa (INPP5D), transcript variant 2, mRNA NM_005541 64085176 INPP5D
Homo sapiens coiled-coil and C2 domain containing IB,
492 mRNA (cDNA clone IMAGE:4299066), partial cds BC007912 39644789 Homo sapiens hypothetical protein MGC33600
493 (MGC33600), mRNA NM_182539 32698909 MGC33600
494 Homo sapiens nischarin (NISCH), mRNA NM_007184 66472381 NISCH
Homo sapiens chromosome 1 open reading frame 21
495 (C lorΩl ), mRNA NM_030806 58761542 Clorf21
Homo sapiens UDP-Gal:betaGlcNAc beta 1,4-
496 galactosyltransferase, polypeptide 1 (B4GALT1), mRNA NM_001497 13929461 B4GALT1 Homo sapiens neural precursor cell expressed, developmentally down-regulated 4 (NEDD4), transcript
497 variant 1, mRNA NM_006154 38257154 NEDD4
Homo sapiens SUMO/sentrin specific peptidase family
498 member 8 (SENP8), mRNA NM_145204 33942065 SENP8
Homo sapiens hypothetical protein FLJ 10700
499 (FLJ 10700), mRNA NMJH8182 8922595 FLJ 10700
Homo sapiens Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain, 1 (CITEDl),
500 mRNA NM_004143 20127458 CITEDl
501 Homo sapiens latrophilin 3 (LPHN3), mRNA NM_015236 59814434 LPHN3 Homo sapiens adrenergic, alpha- IB-, receptor
502 (ADRAlB), mRNA NM_000679 73747802 ADRAlB
Homo sapiens NADH dehydrogenase (ubiquinone) 1 beta
503 subcomplex, 8, 19kDa (NDUFB8), mRNA NM_005004 56676316 NDUFB8
Homo sapiens AT rich interactive domain 2 (ARID, RFX-
504 like) (ARID2), mRNA NMJ52641 56549667 ARID2
505 Homo sapiens clone 23887 mRNA sequence AF052114 3360421
506 Homo sapiens ETAA 16 protein (ETAA 16), mRNA NM_019002 37059813 ETAA16 Homo sapiens chromosome 1 open reading frame 63
507 (C 1 orf63), transcript variant 1 , mRNA NM_207035 46309850 Clorf63 full-length cDNA clone CS0DI036YF07 of Placenta Cot
508 25-normalized of Homo sapiens (human) CR604907 50485714
Homo sapiens hypothetical protein IMPACT (IMPACT),
509 mRNA NM 018439 8923818 IMPACT
Homo sapiens neurochondrin (NCDN), transcript variant
510 3, mRNA NM_014284 62526027 NCDN
Homo sapiens frequenin homolog (Drosophila) (FREQ),
511 mRNA NMJH4286 17738307 FREQ
Homo sapiens Rap guanine nucleotide exchange factor
512 (GEF)-like 1 (RAPGEFLl), mRNA NMJH6339 7705938 GEF
Homo sapiens glycoprotein M6B (GPM6B), transcript
513 variant 3, mRNA NM_005278 50263051 GPM6B
Homo sapiens protein inhibitor of activated STAT, 3
514 (PIAS3), mRNA NM_006099 31543399 PIAS3
Homo sapiens eukaryotic translation elongation factor 2
515 (EEF2), mRNA NM_001961 25453476 EEF2
Homo sapiens glutamate receptor, metabotropic 2
516 (GRM2), mRNA NM_000839 66529099 GRM2
Homo sapiens Fas apoptotic inhibitory molecule 2
517 (FAIM2), mRNA NMJH2306 34101289 FAIM2
Homo sapiens SUMOl/sentrin specific peptidase 7
518 (SENP7), mRNA NM_020654 19923563 SENP7
519 Homo sapiens archain 1 (ARCN 1 ), mRNA NM_001655 21626463 ARCNl Homo sapiens modulator of apoptosis 1 (MOAPl),
520 mRNA NM_022151 73747827 MOAPl
Homo sapiens kelch-like 2, Mayven (Drosophila)
521 (KLHL2), mRNA NM_007246 21359895 KLHL2
Homo sapiens methyl CpG binding protein 2 (Rett
522 syndrome) (MECP2), mRNA NM_004992 7710148 MECP2
Homo sapiens protein tyrosine phosphatase, receptor type,
523 D (PTPRD), transcript variant 1, mRNA NM_002839 4506308 PTPRD
Homo sapiens secreted protein, acidic, cysteine-rich
524 (osteonectin) (SPARC), mRNA NM_003118 48675809 SPARC
Homo sapiens unc-5 homolog A (C. elegans) (UNC5A),
525 mRNA NM_133369 62243566 UNC5A
Homo sapiens peptidylglycine alpha-amidating
526 monooxygenase (PAM), transcript variant 1, mRNA NM_000919 21070983 PAM
Homo sapiens growth hormone inducible transmembrane
527 protein (GHITM), mRNA NMJH4394 7657479 GHITM
Homo sapiens sparc/osteonectin, cwcv and kazal-like
528 domains proteoglycan (testican) (SPOCK), mRNA NM_004598 15451924 SPOCK Homo sapiens vasoactive intestinal peptide (VIP),
529 transcript variant 1, mRNA NM_003381 37588851 VIP
530 Homo sapiens mRNA for KIAA0735 protein, partial cds ABO 18278 3882190 Homo sapiens armadillo repeat containing 8 (ARMC8),
531 mRNA NMJH5396 47458046 ARMC8
Homo sapiens zinc finger and BTB domain containing 11
532 (ZBTBI l), mRNA NMJ)14415 7657702 ZBTBI l
Homo sapiens membrane-associated ring finger (C3HC4)
533 6 (MARCH6), mRNA NM_005885 33589845 C3HC4
Homo sapiens bruno-like 4, RNA binding protein
534 (Drosophila) (BRUNOL4), mRNA NM_020180 68303807 BRUNOL4
Homo sapiens ankyrin repeat and SOCS box-containing 3
535 (ASB3), transcript variant 1, mRNA NM_016115 22208952 ASB3
Homo sapiens hypothetical protein CG003 (13CDNA73),
536 mRNA NM 023037 12957487 13CDNA73
Homo sapiens membrane interacting protein of RGS 16
537 (MIR16), mRNA NMJ) 16641 39753954 MIR16
Homo sapiens dihydropyrimidinase-like 2 (DPYSL2),
538 mRNA NM_001386 50811906 DPYSL2
Homo sapiens mRNA for ets variant gene 1 variant
539 protein AB209202 62087983
Homo sapiens clathrin, heavy polypeptide (Hc) (CLTC),
540 mRNA NM_004859 41327727 CLTC
541 Homo sapiens KIAA0523 protein (KIAA0523), mRNA NM_015253 54792093 KIAA0523 Homo sapiens hypothetical protein MGC20235
542 (MGC20235), mRNA NMJ45041 21450795 MGC20235
Homo sapiens Smad nuclear interacting protein 1
543 (SNIPl), mRNA NM_024700 21314719 SNIPl
544 Homo sapiens dopamine receptor Dl (DRDl), mRNA NM_000794 16445404 DRDl Homo sapiens PABPl -dependent poly A-specific
545 ribonuclease subunit PAN3 (PAN3), mRNA NMJ 75854 68303557 PAN3 Homo sapiens prosaposin (variant Gaucher disease and
546 variant metachromatic leukodystrophy) (PSAP), mRNA NM_002778 11386146 PSAP Homo sapiens KIAA0256 gene product, mRNA (cDNA
547 clone MGC:26224 IMAGE:4825784), complete cds BC033001 34194204
Homo sapiens gelsolin (amyloidosis, Finnish type)
548 (GSN), transcript variant 1, mRNA NM_000177 38016941 GSN
PREDICTED: Homo sapiens KIAA0342 gene product
549 (KIAA0342), mRNA XM_047357 37550037 KIAA0342
Homo sapiens DNA-damage-inducible transcript 4-like
550 (DDIT4L), mRNA NMJ45244 34222182 DDIT4L
Homo sapiens aldolase C, fructose-bisphosphate
551 (ALDOC), mRNA NMJJ05165 68303552 ALDOC
Homo sapiens gamma-aminobutyric acid (GABA)
552 receptor, theta (GABRQ), mRNA NMJ)18558 8924257 GABA
553 Homo sapiens LIM domain only 4 (LMO4), mRNA NM_006769 7108354 LMO4
Homo sapiens RAPlB, member of RAS oncogene family
554 (RAPlB), transcript variant 1, mRNA NMJM5646 58219793 RAPlB
Homo sapiens LEM domain containing 3 (LEMD3),
555 mRNA NM_014319 36287116 LEMD3
Homo sapiens chromosome 6 open reading frame 111
556 (C6orfl l l), mRNA NMJB2870 18699723 C6orfl l l
Homo sapiens gamma-aminobutyric acid (GABA) A
557 receptor, alpha 2 (GABRA2), mRNA NMJJ00807 4557600 GABRA2
Homo sapiens arrestin, beta 1 (ARRBl), transcript variant
558 l, mRNA NM_004041 58219795 ARRBl
Homo sapiens FUN14 domain containing 1 (FUNDCl),
559 mRNA NM_173794 31341096 FUNDCl
Homo sapiens synapsin II (SYN2), transcript variant Ua,
560 mRNA NMJ33625 46399195 SYN2
561 Homo sapiens transferrin (TF), mRNA NM_001063 21536430 TF
Homo sapiens cholinergic receptor, nicotinic, alpha
562 polypeptide 9 (CHRNA9), mRNA NM_017581 70995129 CHRNA9
Homo sapiens opioid receptor, sigma 1 (OPRSl),
563 transcript variant 4, mRNA NM 147159 22212937 OPRSl
PREDICTED: Homo sapiens KIAA0674 (KIAA0674),
564 mRNA XM_376903 51467600 KIAA0674
Homo sapiens peroxisomal biogenesis factor 3 (PEX3),
565 mRNA NM_003630 4505726 PEX3
Homo sapiens tyrosine 3-monooxygenase/tryptophan 5- monooxygenase activation protein, gamma polypeptide
566 (YWHAG), mRNA NM_012479 21464100 YWHAG
Homo sapiens Mof4 family associated protein 1
567 (MRFAPl), mRNA NM_033296 15193293 MRFAPl
Homo sapiens amyotrophic lateral sclerosis 2 (juvenile)
568 chromosome region, candidate 3 (ALS2CR3), mRNA NM_015049 13027379 ALS2CR3
Homo sapiens achaete-scute complex-like 1 (Drosophila)
569 (ASCLl), mRNA NM_004316 55743093 ASCLl
Homo sapiens 3-oxoacid CoA transferase 1 (OXCTl),
570 nuclear gene encoding mitochondrial protein, mRNA NM_000436 47458829 OXCTl
Homo sapiens nucleosome assembly protein 1-like 5
571 (NAP 1L5), mRNA NM_153757 24371267 NAP1L5
PREDICTED: Homo sapiens plexin B2 (PLXNB2),
572 mRNA XM_371474 51476105 PLXNB2
573 Homo sapiens histone acetyltransferase 1 (HATl), mRNA NNMM_000033664422 4504340 HATl Homo sapiens praja 2, RING-H2 motif containing (PJA2),
574 mRNA NM_014819 41281511 PJA2
Homo sapiens protein kinase, cAMP-dependent, regulatory, type I, alpha (tissue specific extinguisher 1), transcript variant 2, mRNA (cDNA clone MGC: 111057
575 IMAGE:6514677), complete cds BC093042 62205277 full-length cDNA clone CLOBAO 12ZA08 of Placenta of
576 Homo sapiens (human) CR609237 50490044
Homo sapiens DNA segment on chromosome 4 (unique)
577 234 expressed sequence (D4S234E), mRNA NM_014392 36951161 D4S234E Homo sapiens xenotropic and polytropic retrovirus
578 receptor (XPRl), mRNA NM_004736 19923271 XPRl
Homo sapiens nucleolar protein 7, 27kDa (NOL7),
579 mRNA NMJH6167 15743546 NOL7
Homo sapiens leucine rich repeat containing 42
580 (LRRC42), mRNA NM_052940 31543202 LRRC42
Homo sapiens eukaryotic translation initiation factor (elF)
581 2A (eIF2A), mRNA NM_032025 54873623 eIF2A
Homo sapiens protein kinase (cAMP-dependent, catalytic) inhibitor gamma (PKIG), transcript variant 2,
582 mRNA NM_007066 32483384 PKIG
Homo sapiens hypothetical protein, clone 2746033
583 (HSA272196), mRNA NM_018405 24475639 HSA272196
Homo sapiens growth factor receptor-bound protein 10
584 (GRBlO), transcript variant 4, mRNA NM_001001555 48762696 GRBlO
Homo sapiens gamma-aminobutyric acid (GABA) A
585 receptor, beta 1 (GABRBl), mRNA NM_000812 12548775 GABA
Homo sapiens fracture callus 1 homolog (rat) (FXCl),
586 mRNA NM_012192 29837656 FXCl
Homo sapiens NCK-associated protein 1 (NCKAPl),
587 transcript variant 2, mRNA NM_205842 45545410 NCKAPl
Homo sapiens proteolipid protein 1 (Pelizaeus- Merzbacher disease, spastic paraplegia 2, uncomplicated)
588 (PLPl), transcript variant 1, mRNA NM_000533 41349498 PLPl
589 Homo sapiens calsyntenin 3 (CLSTN3), mRNA NM 014718 42475533 CLSTN3
Homo sapiens topoisomerase (DNA) II beta 18OkDa
590 (TOP2B), mRNA NMJ)01068 19913407 TOP2B
Homo sapiens ADP-ribosylation factor-like 4 (ARL4),
591 transcript variant 1, mRNA NM_005738 47078225 ARL4
Homo sapiens aquaporin 4 (AQP4), transcript variant a,
592 mRNA NM_001650 50659061 AQP4
Homo sapiens microtubule-associated protein IB
593 (MAP IB), transcript variant 1 , mRNA NM_005909 14165457 MAPlB
594 Homo sapiens opioid receptor, kappa 1 (OPRKl), mRNA NM_000912 39725939 OPRKl Homo sapiens hypothetical protein MGC 13125
595 (MGC 13125), mRNA NM_032725 39725657 MGC13125
Homo sapiens 5NULL-nucleotidase, cytosolic II-like 1
596 (NT5C2L1), mRNA NM_152729 38570155 NT5C2L1
Homo sapiens endothelial differentiation, lysophosphatidic acid G-protein-coupled receptor, 2
597 (EDG2), transcript variant 2, mRNA NM_057159 45580698 EDG2
Homo sapiens malate dehydrogenase 1, NAD (soluble)
598 (MDHl), mRNA NM_005917 21735619 MDHl
599 Homo sapiens trophoblast glycoprotein (TPBG), mRNA NM_006670 34222307 TPBG Homo sapiens chromosome 20 open reading frame 152
600 (C20orfl52), mRNA NM_080834 18201889 C20orfl52
Homo sapiens protein phosphatase 3 (formerly 2B), catalytic subunit, beta isoform (calcineurin A beta)
601 (PPP3CB), mRNA NM_021132 11036639 PPP3CB
Homo sapiens prostaglandin 12 (prostacyclin) receptor
602 (IP) (PTGIR), mRNA NM_000960 39995095 PTGIR
Homo sapiens protein phosphatase IE (PP2C domain
603 containing) (PPM 1 E), mRNA NM_014906 30089947 PPMlE
Homo sapiens glycoprotein M6A (GPM6A), transcript
604 variant 2, mRNA NM_201591 42476107 GPM6A
605 Homo sapiens mRNA for NDRG4-Bvar, complete cds AB044945 12083724 Homo sapiens dystonin (DST), transcript variant IeA,
606 mRNA NMJH5548 34577048 DST
Homo sapiens Rho GDP dissociation inhibitor (GDI)
607 alpha (ARHGDIA), mRNA NM_004309 34147601 GDI
Homo sapiens chromosome 10 open reading frame 70
608 (C10orf70), mRNA NM_018464 50355973 ClOorHO
Homo sapiens armadillo repeat containing 1 (ARMCl),
609 mRNA NM_018120 34222195 ARMCl
610 Homo sapiens transportin2 mRNA, complete cds AF019039 2589203 Homo sapiens arginine vasopressin (neurophysin II, antidiuretic hormone, diabetes insipidus,
611 neurohypophyseal) (AVP), mRNA NM_000490 50959227 AVP
Homo sapiens PDZ domain containing 10 (PDZKlO),
612 mRNA NM_014728 55741444 PDZKlO full-length cDNA clone CL0BA006ZC10 of Placenta of
613 Homo sapiens (human) CR602328 50483135
614 Homo sapiens synaptogyrin 3 (SYNGR3), mRNA NM_004209 22091456 SYNGR3
Homo sapiens inositol 1,4,5-trisphosphate receptor type 1
615 (ITPRl) mRNA, complete cds L38019 46107961 ITPRl
Homo sapiens spectrin repeat containing, nuclear
616 envelope 1 (SYNEl), transcript variant longer, mRNA NM_033071 23097307 SYNEl
Homo sapiens ankyrin repeat and MYND domain
617 containing 2 (ANKMY2), mRNA NM_020319 28461128 ANKMY2
618 Homo sapiens proenkephalin (PENK)5 mRNA NM_006211 40254835 PENK
Homo sapiens chromosome 22 open reading frame 1
619 (C22orfl), mRNA NM_001585 31542268 C22orfl
620 Homo sapiens cyclin D2 (CCND2), mRNA NM_001759 16950656 CCND2
Homo sapiens methionyl aminopeptidase 2 (METAP2),
621 mRNA NM_006838 27597083 METAP2
Homo sapiens acyl-CoA synthetase long-chain family
622 member 5 (ACSL5), transcript variant 2, mRNA NM_203379 42794757 ACSL5
Homo sapiens gamma-aminobutyric acid (GABA) A
623 receptor, alpha 4 (GABRA4), mRNA NM_000809 34452722 GABA
Homo sapiens cytoplasmic linker associated protein 1
624 (CLASPl), mRNA NM_015282 31563536 CLASPl
Homo sapiens nuclear distribution gene C homolog (A.
625 nidulans) (NUDC), mRNA NM_006600 31543300 NUDC
Homo sapiens cofactor required for SpI transcriptional
626 activation, subunit 7, 7OkDa (CRSP7), mRNA NM_004831 73088830 CRSP7
Homo sapiens insulin induced gene 1 (INSIGl), transcript
627 variant 1, mRNA NM_005542 38327527 INSIGl
Homo sapiens DNA-damage-inducible transcript 3
628 (DDIT3), mRNA NM_004083 50345277 DDIT3
Homo sapiens glutamate receptor, ionotropic, N-methyl
629 D-aspartate 2B (GRIN2B), mRNA NM_000834 6006003 GRIN2B
Homo sapiens chromosome 10 open reading frame 93
630 (C10orf93), mRNA NM_173572 27734836 C10orf93
Homo sapiens tropomyosin 1 (alpha) (TPMl), transcript
631 variant 1, mRNA NMJ)OlO 18005 63252897 TPMl
Homo sapiens cDNA FLJ 14864 fis, clone
PLACE 1001845, weakly similar to Mus musculus cyclin
632 ania-6a mRNA AK027770 14042694
Homo sapiens calcium binding protein 7 (CABP7),
633 mRNA NM_182527 32698883 CABP7
Homo sapiens milk fat globule-EGF factor 8 protein
634 (MFGE8), mRNA NMJJ05928 5174556 MFGE8
Homo sapiens protein predicted by clone 23733
635 (HSU79274), mRNA NMJ) 13300 9558740 HSU79274
Homo sapiens ectodermal-neural cortex (with BTB-like
636 domain) (ENCl), mRNA NMJ)03633 4505460 ENCl
Homo sapiens nasal embryonic LHRH factor variant 3
637 mRNA, complete cds, alternatively spliced AY255131 32478637
Homo sapiens succinate dehydrogenase complex, subunit A, flavoprotein (Fp) (SDHA), nuclear gene encoding
638 mitochondrial protein, mRNA NMJ)04168 4759079 SDHA
Homo sapiens SM-11044 binding protein (SMBP),
639 mRNA NMJ)20123 33859832 SMBP
Homo sapiens cofactor required for SpI transcriptional activation, subunit 3, 13OkDa (CRSP3), transcript variant
640 1, mRNA NMJW4830 28558970 CRSP3
Homo sapiens cadherin, EGF LAG seven-pass G-type receptor 2 (flamingo homolog, Drosophila) (CELSR2),
641 mRNA NM 001408 13325063 CELSR2
Homo sapiens GNAS complex locus (GNAS), transcript
642 variant 4, mRNA NM_016592 7706588 GNAS
643 Homo sapiens KIAA1600 (KIAA1600), mRNA NM_020940 61098051 KIAA1600 Homo sapiens fragile X mental retardation, autosomal
644 homolog 1 (FXRl), transcript variant 2, mRNA NM_001013438 61835163 FXRl Homo sapiens glutamate decarboxylase 1 (brain, 67kDa)
645 (GAD 1 ), transcript variant GAD67, mRNA NM_000817 58331245 GADl
Homo sapiens insulin-like growth factor binding protein
646 2, 36kDa (IGFBP2), mRNA NM_000597 55925575 IGFBP2
Homo sapiens preimplantation protein 3 (PREI3),
647 transcript variant 1, mRNA NMJH5387 41349448 PREI3
Homo sapiens p8 protein (candidate of metastasis 1) (P8),
648 mRNA NM_012385 6912569 P8
Homo sapiens glutamate receptor, metabotropic 4
649 (GRM4), mRNA NM_000841 4504140 GRM4
Homo sapiens spectrin, beta, non-erythrocytic 1
650 (SPTBN 1 ), transcript variant 1 , mRNA NM_003128 4507194 SPTBNl
Homo sapiens hypothetical transmembrane protein
651 SBBI54 (SBBI54), mRNA NM_138334 19923878 SBBI54
Homo sapiens GTP_binding protein, mRNA (cDNA
652 clone MGC:39306 IMAGE:5444745), complete cds BC026039 45708795 human full-length cDNA clone CS0DC014YA22 of
653 Neuroblastoma of Homo sapiens (human) BX248025 28193209
654 Homo sapiens oxidation resistance 1 (OXRl), mRNA NMJ81354 63055068 OXRl
Homo sapiens tRNA isopentenyltransferase 1 (TRITl),
655 mRNA NM_017646 32306540 TRITl
656 Homo sapiens ADP-ribosylation factor 3 (ARF3), mRNA NM_001659 4502202 ARF3
Homo sapiens adenosine monophosphate deaminase 2
657 (isoform L) (AMPD2), transcript variant 2, mRNA NMJ39156 55929936 AMPD2
658 Homo sapiens glypican 3 (GPC3), mRNA NM_004484 5360213 GPC3
659 Homo sapiens importin 7 (IPO7), mRNA NM_006391 5453997 IPO7
Homo sapiens peroxisomal biogenesis factor 5-like
660 (PEX5L), mRNA NM_016559 7706670 PEX5L
Homo sapiens protein kinase, cAMP-dependent,
661 regulatory, type I, beta (PRKAR 1 B), mRNA NM_002735 38257138 PRKARlB
Homo sapiens KIAAl 189 (KIAAl 189), transcript variant
662 2, mRNA NM_020711 58331174 KIAAl 189
Homo sapiens RAS-related on chromosome 22 (RRP22),
663 transcript variant 1, mRNA NM_006477 55953118 RRP22
Homo sapiens gamma-aminobutyric acid (GABA) A
664 receptor, alpha 5 (GABRA5), mRNA NM_000810 6031207 GABA
Homo sapiens G protein-coupled receptor 23 (GPR23),
665 mRNA NM_005296 4885310 GPR23
666 Homo sapiens cerebellin 1 precursor (CBLNl), mRNA NM_004352 4757921 CBLNl Homo sapiens fibroblast growth factor receptor 3 (achondroplasia, thanatophoric dwarfism) (FGFR3),
667 transcript variant 1, mRNA NM_000142 13112046 FGFR3
Homo sapiens pyridoxal (pyridoxine, vitamin B6) kinase
668 (PDXK), mRNA NM 003681 48928052 PDXK
Homo sapiens APG16 autophagy 16-like (S. cerevisiae)
669 (APG16L), transcript variant 2, mRNA NM_017974 38683867 APG16L
Homo sapiens gamma-aminobutyric acid (GABA) A
670 receptor, beta 2 (GABRB2), transcript variant 2, mRNA NM_000813 4503864 GABA
671 Homo sapiens septin 11 (SEPTl 1), mRNA NM_018243 38605734 SEPTI l
Homo sapiens gamma-aminobutyric acid (GABA) A
672 receptor, alpha 6 (GABRA6), mRNA NM_000811 4557606 GABA
Homo sapiens galactose- 1 -phosphate uridylyltransferase
673 (GALT), transcript variant 1, mRNA NM_000155 22165415 GALT
Homo sapiens complexin 2 (CPLX2), transcript variant 2,
674 mRNA NM_001008220 56550064 CPLX2
Homo sapiens vesicle-associated membrane protein 1
675 (synaptobrevin I) (VAMPl), transcript variant 2, mRNA NM_199245 40549445 VAMPl Homo sapiens F-box and leucine-rich repeat protein 18
676 (FBXL18), mRNA NM_024963 21361980 FBXL18
677 Homo sapiens c-myc binding protein (MYCBP), mRNA NM_012333 57242776 MYCBP
678 Homo sapiens fibulin 5 (FBLN5), mRNA NM_006329 19743802 FBLN5
679 Homo sapiens phytoceramidase, alkaline (PHCA), mRNA NM O 18367 31543398 PHCA
Homo sapiens tumor protein p53 binding protein, 2
680 (TP53BP2), transcript variant 2, mRNA NM 005426 4885642 TP53BP2 Homo sapiens peroxiredoxin 2 (PRDX2), nuclear gene encoding mitochondrial protein, transcript variant 1,
681 mRNA NM_005809 33188450 PRDX2
682 Homo sapiens importin 9 (IPO9), mRNA NM_018085 21361658 IPO9 Homo sapiens eukaryotic translation initiation factor 3,
683 subunit 10 theta, 150/17OkDa (EIF3S10), mRNA NM_OO375O 4503508 EIF3S10 Homo sapiens FK506 binding protein IA, 12kDa
684 (FKBPlA), transcript variant 12B, mRNA NM_000801 17149837 FKBPlA
685 Homo sapiens arginase, liver (ARGl), mRNA NM_000045 10947138 ARGl Homo sapiens likely ortholog of mouse hypoxia induced
686 gene 1 (HIGl), mRNA NM_014056 7661619 HIGl
Homo sapiens RABlO, member RAS oncogene family
687 (RABlO), mRNA NM_016131 33695094 RABlO
Homo sapiens DnaJ (Hsp40) homolog, subfamily B,
688 member 1 (DNAJBl), mRNA NM_006145 5453689 Hsp40
Homo sapiens nucleolar protein 5A (56kDa with KKE/D
689 repeat) (NOL5A), mRNA NM_006392 32483373 NOL5A Homo sapiens carboxylesterase 1 (monocyte/macrophage
690 serine esterase I) (CESl), transcript variant 1, mRNA NM_001025195 68508966 CESl Homo sapiens microtubule-associated protein 2 (MAP2),
691 transcript variant 4, mRNA NMJDl 847 14195621 MAP2
692 Homo sapiens klotho (KL), transcript variant 1, mRNA NMJ104795 24497613 KL Homo sapiens glycerol-3-phosphate dehydrogenase 1
693 (soluble) (GPDl), mRNA NMJW5276 33695087 GPDl full-length cDNA clone CS0DI013YG09 of Placenta Cot
694 25-normalized ofHomo sapiens (human) CR599620 50480427 Homo sapiens zinc fingers and homeoboxes 1 (ZHXl),
695 transcript variant 2, mRNA NM_007222 63079679 ZHXl
696 Homo sapiens complexin 1 (CPLXl), mRNA NM 006651 34335235 CPLXl Homo sapiens regulator of chromosome condensation 2
697 (RCC2), mRNA NM_018715 29789089 RCC2 Homo sapiens ryanodine receptor 2 (cardiac) (RYR2),
698 mRNA NM_001035 4506756 RYR2 Homo sapiens hypothetical protein FLJ20607 (TSC),
699 mRNA NM_017899 8923562 TSC Homo sapiens muscleblind-like 2 (Drosophila) (MBNL2),
700 transcript variant 1, mRNA NM_144778 46411178 MBNL2
Homo sapiens transmembrane, prostate androgen induced
701 RNA (TMEPAI), transcript variant 3, mRNA NMJ99170 40317617 TMEPAI
Homo sapiens inositol 1,4,5-triphosphate receptor, type 3
702 (ITPR3), mRNA NM_002224 4504794 ITPR3 Homo sapiens protein phosphatase 3 (formerly 2B), catalytic subunit, alpha isoform (calcineurin A alpha)
703 (PPP3CA), mRNA NM_000944 19923130 PPP3CA
Homo sapiens chromosome 10 open reading frame 4
704 (C10orf4), transcript variant FRAl OAC 1-3.3, mRNA NM 203438 50409606 C10orf4 Homo sapiens cholinergic receptor, muscarinic 5
705 (CHRM5), mRNA NM_012125 38176159 CHRM5
Homo sapiens DNA segment, Chr 15, Wayne State
706 University 75, expressed (D15Wsu75e), mRNA NMJH5704 63055039 D15Wsu75e
707 Homo sapiens cholecystokinin (CCK), mRNA NM 000729 50962774 CCK
Homo sapiens pleckstrin and Sec7 domain containing
708 (PSD), mRNA NM_002779 56790298 PSD
Homo sapiens glutamate-ammonia ligase (glutamine
709 synthase) (GLUL), mRNA NM_002065 21361767 GLUL Homo sapiens chromosome 18 open reading frame 55
710 (C18orf55), mRNA NM_014177 7661809 C18orf55 Homo sapiens cDNA FLJ34580 fis, clone
KIDNE2008473, moderately similar to Homo sapiens FH1/FH2 domain-containing protein FHOS (FHOS)
711 mRNA AK091899 21750373 FHOS
Homo sapiens Rho GTPase-activating protein (RICS),
712 mRNA NM_014715 29469070 RICS
Homo sapiens transcript expressed during hematopoiesis
713 2 (MGC33894), mRNA NMJ52914 23097269 MGC33894 Homo sapiens G protein-coupled receptor 22 (GPR22),
714 mRNA NM_005295 4885308 GPR22 Homo sapiens discs, large (Drosophila) homolog- associated protein 1 (DLGAPl), transcript variant alpha,
715 mRNA NMJ)04746 19923273 DLGAPl
Homo sapiens cDNA FLJ37260 fis, clone BRAMY2010441, highly similar to PEANUT-LIKE
716 PROTEIN 2 AK094579 21753666 Homo sapiens GDP dissociation inhibitor 2 (GDI2),
717 mRNA NMJ)01494 6598322 GDI2 Homo sapiens glutamate receptor, ionotropic, N-methyl
718 D-aspartate 2 A (GRIN2A), mRNA NM _000833 6006002 GRIN2A Homo sapiens NADH dehydrogenase (ubiquinone) Fe-S protein 4, 18kDa (NADH-coenzyme Q reductase)
719 (NDUFS4), mRNA NMJ)02495 4505368 NDUFS4
Homo sapiens RIM binding protein 2 (KIAA0318),
720 mRNA NM 015347 61316483 KIAA0318
Homo sapiens upregulated during skeletal muscle growth 5, mRNA (cDNA clone MGC:87882 IMAGE:6056764),
721 complete cds BC072683 49116143
722 Homo sapiens follistatin-like 1 (FSTLl), mRNA NM_007085 34304366 FSTLl
Homo sapiens heat shock 9OkDa protein 1, alpha
723 (HSPCA), transcript variant 2, mRNA NM_005348 40254815 HSPCA
Homo sapiens neural cell adhesion molecule 1 (NCAMl),
724 transcript variant 2, mRNA NM_181351 41281936 NCAMl Homo sapiens gap junction protein, alpha 1, 43kDa
725 (connexin 43) (GJAl), mRNA NM_000165 4755136 GJAl
Homo sapiens muscle RAS oncogene homolog (MRAS),
726 mRNA NM_012219 32189356 MRAS
727 Homo sapiens ring finger protein 103 (RNF 103), mRNA NM_005667 37595534 RNF103 Homo sapiens fibronectin 1 (FNl), transcript variant 1,
728 mRNA NM_212482 47132556 FNl
729 Homo sapiens hippocalcin like 4 (HPCAL4), mRNA NM_016257 19913444 HPCAL4 Homo sapiens v-rel reticuloendotheliosis viral oncogene homolog B, nuclear factor of kappa light polypeptide gene
730 enhancer in B-cells 3 (avian) (RELB), mRNA NM_006509 35493877 RELB Homo sapiens mevalonate (diphospho) decarboxylase
731 (MVD), mRNA NM_002461 4505288 MVD
Homo sapiens TAF5-like RNA polymerase II, p300/CBP- associated factor (PCAF)-associated factor, 65kDa
732 (TAF5L), transcript variant 1 , mRNA NM_014409 69122714 PCAF
733 Homo sapiens hippocalcin (HPCA), mRNA NM 002143 19913445 HPCA
Homo sapiens RecQ protein-like (DNA helicase Ql -like)
734 (RECQL), transcript variant 2, mRNA NM_032941 14591901 RECQL Homo sapiens potassium large conductance calcium- activated channel, subfamily M, beta member 1
735 (KCNMBl), mRNA NM_004137 25777641 KCNMBl
Homo sapiens v-crk sarcoma virus CTlO oncogene
736 homolog (avian) (CRK), transcript variant II, mRNA NM_016823 41327711 CRK Homo sapiens internexin neuronal intermediate filament
737 protein, alpha (INA), mRNA NM_032727 39725658 INA Homo sapiens opiate receptor-like 1 (OPRLl), transcript
738 variant 2, mRNA NM_000913 33286425 OPRLl Homo sapiens DEAD/H (Asp-Glu-Ala- Asp/His) box
739 polypeptide 26 (DDX26), mRNA NM_012141 11024693 DDX26
740 Homo sapiens paralbumin (PVALB), mRNA NM_002854 55925656 PVALB
Homo sapiens septin 5 (SEPT5), transcript variant 2,
741 mRNA NM_001009939 58331273 SEPT5 Homo sapiens gamma-aminobutyric acid (GABA) B
742 receptor, 1 (GABBR 1 ), transcript variant 2, mRNA NM_021903 11497611 GABA Homo sapiens polycomb group ring finger 5, mRNA
(cDNA clone MGC60159 IMAGE:5199133), complete
743 cds BC051845 30353996
744 Homo sapiens prostatic binding protein (PBP), mRNA NM_002567 38016928 PBP
Table 2
Homo sapiens transthyretin (prealbumin, amyloidosis type I) (TTR), mRNA NM 000371 4507724 TTR
Homo sapiens prostaglandin E receptor 3 (subtype
14 EP3) (PTGER3), transcript variant 6, mRNA NM 198716 38505181 PTGER3
27 Homo sapiens calsyntenin 2 (CLSTN2), mRNA NM_022131 11545860 CLSTN2
Homo sapiens START domain containing 7 28 (STARD7), transcript variant 2, mRNA NM 139267 21450855 STARD7
Homo sapiens nitric oxide synthase 1 (neuronal)
31 (NOSl), mRNA NM 000620 10835172 NOSl
Homo sapiens dickkopf homolog 3 (Xenopus
32 laevis) (DKK3), transcript variant 2, mRNA NM 013253 66346687 DKK3
40 Homo sapiens kinectin mRNA, complete cds L25616 409465
Homo sapiens cocaine- and amphetamine-regulated 42 transcript (CART), mRNA NM_004291 46852394 CART
Homo sapiens scavenger receptor class A, member 61 3 (SCARA3), transcript variant 1 , mRNA NM_016240 33598923 SCARA3
Homo sapiens glia maturation factor, gamma 67 (GMFG), mRNA NM_004877 4758439 GMFG
Homo sapiens neuropilin 2 (NRP2), transcript
73 variant 2, mRNA NM_003872 41872532 NRP2
Homo sapiens hypothetical protein MGC42105
74 (MGC42105), mRNA NM_153361 37059769 MGC42105
Homo sapiens pro-melanin-concentrating hormone 79 (PMCH), mRNA NM_002674 71361683 PMCH
Homo sapiens neuregulin 1 (NRGl), transcript 84 variant HRG-beta2, mRNA NM_013957 7669513 NRGl
Homo sapiens NEL-like 2 (chicken) (NELL2), 100 mRNA NM_006159 5453765 NELL2
Homo sapiens CD276 antigen (CD276), transcript 108 variant U mRNA NM 001024736 67188442 CD276
Homo sapiens CD81 antigen (target of 109 antiproliferative antibody 1 ) (CD81 ), mRN A NM 004356 62240999 CD81 Homo sapiens calcium/calmodulin-dependent protein kinase (CaM kinase) II alpha (CAMK2A), 121 transcript variant 2, mRNA NM 171825 25952117 CAMK2A
Homo sapiens protein tyrosine kinase non catalytic 151 form (NTRK2) mRNA, complete cds AF508964 21886727 NTRK2
Homo sapiens A kinase (PRKA) anchor protein 5 153 (AKAP5), mRNA NM_004857 21493042 AKAP5
155 Homo sapiens ephrin-B3 (EFNB3), mRNA NM 001406 38201712 EFNB3
Homo sapiens 2NULL,3NULL-cyclic nucleotide
179 3NULL phosphodiesterase (CNP), mRNA NM 033133 38570090 CNP Homo sapiens ectonucleotide pyrophosphatase/phosphodiesterase 2 (autotaxin)
180 (ENPP2), mRNA NM 006209 20070229 ENPP2
Homo sapiens sphingomyelin phosphodiesterase 2, neutral membrane (neutral sphingomyelinase) 182 (SMPD2), mRNA NM 003080 4507094 SMPD2
Homo sapiens creatine kinase, brain (CKB), 188 mRNA NM 001823 34335231 CKB
Homo sapiens solute carrier family 6, member 15 199 (SLC6A 15), transcript variant 1 , mRNA NM 182767 60115819 SLC6A15
Homo sapiens solute carrier family 6 (proline IMINO transporter), member 20 (SLC6A20),
201 transcript variant 1, mRNA NM 020208 46249401 SLC6A20
Homo sapiens solute carrier family 22 (organic cation transporter), member 17 (SLC22A17),
202 transcript variant 2, mRNA NM 016609 63999575 SLC22A17
Homo sapiens solute carrier family 6, member 17
205 (SLC6A17), mRNA NM 001010898 58219013 SLC6A17
Homo sapiens solute carrier family 6 (neurotransmitter transporter, glycine), member 9
209 (SLC6A9), transcript variant 3, mRNA NM 001024845 67782316 SLC6A9
Homo sapiens transient receptor potential cation 252 channel, subfamily C, member 6 (TRPC6), mRNA NM 004621 19923256 TRPC6
Homo sapiens chloride channel 3 (CLCN3), 278 transcript variant e, mRNA NM 173872 55770839 CLCN3
288 Homo sapiens EPH receptor A4 (EPHA4), mRNA NM_004438 45439363 EPHA4
Homo sapiens mitogen-activated protein kinase 1 291 (MAPK 1 ), transcript variant 1 , mRNA NM 002745 66932915 MAPKl
Homo sapiens neurogranin (protein kinase C
323 substrate, RC3) (NRGN), mRNA NM 006176 5453799 NRGN
Homo sapiens protein tyrosine phosphatase, receptor type, R (PTPRR), transcript variant 1,
334 mRNA NM 002849 19743915 PTPRR
full-length cDNA clone CSODFOl IYP 12 of Fetal 335 brain of Homo sapiens (human) CR617323 50498130
Homo sapiens cDNA FLJ42948 fis, clone
BRSTN2006306, highly similar to Mus musculus 341 BM88 antigen mRNA AK124938 34530868
Homo sapiens tachykinin, precursor 1 (substance
K, substance P, neurokinin 1, neurokinin 2, neuromedin L, neurokinin alpha, neuropeptide K, neuropeptide gamma) (TACl), transcript variant 345 alpha, mRNA NM_013996 7770074 TACl
351 Human mRNA for KIAAO 143 gene, partial cds D63477 1469867
PREDICTED: Homo sapiens similar to contains 357 transmembrane (TM) region (LOC342865), mRNA XM_292785 51474647 TM
Homo sapiens thromboxane A2 receptor 360 (TBXA2R), transcript variant 2, mRNA NM OO 1060 42518083 TBXA2R
Homo sapiens hypothetical protein FLJ90013, mRNA (cDNA clone MGC:87011 363 IMAGE:4825556), complete cds BC066899 45219875
Homo sapiens tumor differentially expressed 2 376 (TDE2), mRNA NM_020755 34222115 TDE2
Homo sapiens dual specificity phosphatase 6 379 (DUSP6), transcript variant 1, mRNA NM_001946 42764682 DUSP6 full-length cDNA clone CS0DK002YG07 of HeLa
386 cells Cot 25-normalized of Homo sapiens (human) CR591956 50472763
Homo sapiens mRNA; cDNA DKFZp566B064
387 (from clone DKFZp566B064) AL713702 19584419
Homo sapiens tumor differentially expressed 1 392 (TDE 1 ), transcript variant 2, mRNA NM l 98941 39812105 TDEl
Homo sapiens cDNA FLJ46114 fis, clone
404 TESTI2036822 AK127996 34535149 PREDICTED: Homo sapiens similar to Gamma- aminobutyric-acid receptor rho-3 subunit precursor
405 (GABA(A) receptor) (LOC200959), mRNA XM_116036 51492723 GABA(A)
Homo sapiens cDNA clone MGC:88735 411 IMAGE:6263100, complete cds BC072022 47938399 full-length cDNA clone CS0DB008YA09 of
Neuroblastoma Cot 10-normalized of Homo 416 sapiens (human) CR614918 50495725
Homo sapiens cDNA FLJ30643 fis, clone
CTONG2003782, highly similar to GAMMA-
AMINOBUTYRIC-ACID RECEPTOR PI 429 SUBUNIT PRECURSOR AK055205 16549881
Homo sapiens brevican (BCAN), transcript variant 436 2, mRNA NM_198427 38372930 BCAN full-length cDNA clone CS0DB001YA17 of
Neuroblastoma Cot 10-normalized of Homo 443 sapiens (human) CR607507 50488314
Homo sapiens prostaglandin D2 receptor (DP) (PTGDR), mRNA NM 000953 38505191 PTGDR
Homo sapiens hypothetical protein MGC21688, mRNA (cDNA clone MGC:33603 IMAGE:4825140), complete cds BC037299 22713421
Homo sapiens zinc finger, DHHC-type containing 12 (ZDHHC 12), mRNA NM 032799 42516559 ZDHHC12
Homo sapiens plexin A2 (PLXNA2), mRNA NM 025179 46275829 PLXNA2
Homo sapiens syntaphilin (SNPH), mRNA NM 014723 38202245 SNPH PREDICTED: Homo sapiens discs, large (Drosophila) homolog-associated protein 3 (DLGAP3), mRNA XM 035601 51458944 DLGAP3
Homo sapiens secretory carrier membrane protein 4 (SCAMP4), mRNA NM 079834 50593003 SCAMP4
Homo sapiens seizure related 6 homolog (mouse)- like (SEZ6L), mRNA NM 021115 55956782 SEZ6L
Homo sapiens neurotensin (NTS), mRNA NM 006183 31563516 NTS
Homo sapiens carboxypeptidase E (CPE), mRNA NM OO 1873 4503008 CPE
Homo sapiens transmembrane protein 30A (TMEM30A), mRNA NM 018247 52694667 TMEM30A
Homo sapiens hypothetical protein FLJ 10700 (FLJ10700), mRNA NM 018182 8922595 FLJ 10700
Homo sapiens latrophilin 3 (LPHN3), mRNA NM 015236 59814434 LPHN3
Homo sapiens glycoprotein M6B (GPM6B), transcript variant 3, mRNA NM 005278 50263051 GPM6B
Homo sapiens protein tyrosine phosphatase, receptor type, D (PTPRD), transcript variant 1, mRNA NM 002839 4506308 PTPRD
Homo sapiens unc-5 homolog A (C. elegans) (UNC5A), mRNA NM 133369 62243566 UNC5A
Homo sapiens peptidylglycine alpha-amidating monooxygenase (PAM), transcript variant 1, mRNA NM 000919 21070983 PAM
Homo sapiens growth hormone inducible transmembrane protein (GHITM), mRNA NM 014394 7657479 GHITM Homo sapiens sparc/osteonectin, cwcv and kazal- like domains proteoglycan (testican) (SPOCK), mRNA NM 004598 15451924 SPOCK
Homo sapiens vasoactive intestinal peptide (VIP), transcript variant 1, mRNA NM 003381 37588851 VIP
Homo sapiens hypothetical protein MGC20235 542 (MGC20235), mRNA NM 145041 21450795 MGC20235
Homo sapiens FUN 14 domain containing 1
559 (FUNDCl), mRNA NM 173794 31341096 FUNDCl
Homo sapiens protein kinase (cAMP-dependent, catalytic) inhibitor gamma (PKIG), transcript
582 variant 2, mRNA NM 007066 32483384 PKIG
589 Homo sapiens calsyntenin 3 (CLSTN3), mRNA NM_014718 42475533 CLSTN3
Homo sapiens aquaporin 4 (AQP4), transcript
592 variant a, mRNA NM_001650 50659061 AQP4
Homo sapiens endothelial differentiation, lysophosphatidic acid G-protein-coupled receptor,
597 2 (EDG2), transcript variant 2, mRNA NM 057159 45580698 EDG2
Homo sapiens protein phosphatase 3 (formerly 2B), catalytic subunit, beta isoform (calcineurin A
601 beta) (PPP3CB), mRNA NM 021132 11036639 PPP3CB
Homo sapiens protein phosphatase IE (PP2C
603 domain containing) (PPM 1 E), mRNA NM 014906 30089947 PPMlE
Homo sapiens glycoprotein M6A (GPM6A),
604 transcript variant 2, mRNA NM 201591 42476107 GPM6A
Homo sapiens armadillo repeat containing 1
609 (ARMCl), mRNA NM 018120 34222195 ARMCl
Homo sapiens arginine vasopressin (neurophysin II, antidiuretic hormone, diabetes insipidus,
611 neurohypophyseal) (AVP), mRNA NM 000490 50959227 AVP
614 Homo sapiens synaptogyrin 3 (SYNGR3), mRNA NM_004209 22091456 SYNGR3
618 Homo sapiens proenkephalin (PENK), mRNA NM 006211 40254835 PENK
Homo sapiens chromosome 10 open reading frame 630 93 (C10orf93), mRNA NMJ73572 27734836 C10ori93
Homo sapiens SM-11044 binding protein (SMBP), 639 mRNA NM 020123 33859832 SMBP
Homo sapiens insulin-like growth factor binding 646 protein 2, 36kDa (IGFBP2), mRNA NM_000597 55925575 IGFBP2
Homo sapiens G protein-coupled receptor 23 665 (GPR23), mRNA NM 005296 4885310 GPR23
Homo sapiens cerebellin 1 precursor (CBLNl), 666 mRNA NM 004352 4757921 CBLNl
Homo sapiens phytoceramidase, alkaline (PHCA), 679 mRNA NM 018367 31543398 PHCA
Homo sapiens likely ortholog of mouse hypoxia 686 induced gene 1 (HIGl), mRNA NM_014056 7661619 HIGl
Homo sapiens G protein-coupled receptor 22 (GPR22), mRNA NM 005295 4885308 GPR22
Homo sapiens discs, large (Drosophila) homolog- associated protein 1 (DLGAPl), transcript variant alpha, mRNA NM 004746 19923273 DLGAPl
Homo sapiens follistatin-like 1 (FSTLl), mRNA NM_007085 34304366 FSTLl
Homo sapiens ring finger protein 103 (RNF103), mRNA NM 005667 37595534 RNF103
Table 3
Table Λ: ABΛT - 4-aminobutyrate aminotransferase 19mers
Sense si UNA ΛntiSense siRNΛ um;ιn-%304477 ORI": 184-1686
1 CAGACUCUUAAACUAACAA UUGUUAGUUUAAGAGUCUG [3578-3596](19/19) 31UTR
2 GGGUGCAAAUAUUCAGUAU AUACUGAAUAUUUGCACCC [3960-3978](19/19) 31UTR
3 ACAGACUCUUAAACUAACA UGUUAGUUUAAGAGUCUGU [3577-3595](19/19) 3'UTR
4 CGACCUAUUUUUGUUGAAG CUUCAACAAAAAUAGGUCG [3232-3250](19/19) 3'UTR
5 AGUACAUCCUCACUUUUAU AUAAAAGUGAGGAUGUACU [4498-4516](19/19) 3'UTR
6 UCGACCUAUUUUUGUUGAA UUCAACAAAAAUAGGUCGA [3231-3249](19/19) 3'UTR
7 GCAAAAUUGUGUAUAGCAU AUGCUAUACACAAUUUUGC [4564-4582](19/19) 3'UTR
8 GUACAUCCUCACUUUUAUU AAUAAAAGUGAGGAUGUAC [4499-4517](19/19) 31UTR
9 UGCAAACCAAACCAUUAUU AAUAAUGGUUUGGUUUGCA [3897-3915](19/19) 3'UTR
10 CCCAGCAAUUUUUCCAAAA UUUUGGAAAAAUUGCUGGG [1995-2013](19/19) 3'UTR
Table B: ABAT - 4-aminobutyrate aminotransferase 21 mers
Other I Human-%304477 ORF: 184-
Numbvrl Sense siRNΛ AntiScnsc siRNΛ Sp I 1686
1 CGACCUAUUUUUGUUGAAGAA UUCUUCAACAAAAAUAGGUCG [3232-32521(21/20 31UTR
2 CAACAGACUCUUAAACUAACA UGUUAGUUUAAGAGUCUGUUG [3575-3595](21/21) 31UTR
3 GAAACAACAGACUCUUAAACU AGUUUAAGAGUCUGUUGUUUC [3571-3591](21/21) 3'UTR
4 CGGGAAUACAAAGAUGAGAUA UAUCUCAUCUUUGUAUUCCCG [3512-3532](21/21) 31UTR
5 GGAGUUAAUGAAACAGCUGAA UUCAGCUGUUUCAUUAACUCC [351-371](21/21) ORF
6 GGAAACAACAGACUCUUAAAC GUUUAAGAGUCUGUUGUUUCC [3570-35901(21/20 31UTR
7 GGGAAUACAAAGAUGAGAUAA UUAUCUCAUCUUUGUAUUCCC [3513-3533](21/21) 31UTR
8 GGGAGCAUUUUUGGUGGUCUU AAGACCACCAAAAAUGCUCCC [1815-1835](21/21) 3UTR
9 GCUCAUUUUAAUUGCCAGAAA UUUCUGGCAAUUAAAAUGAGC [1539-1559](21/21) ORF
10 GGAGGAUCUGAUUGUGAAAUA UAUUUCACAAUCAGAUCCUCC [1002-1022](21/21) ORF
Table C: ADRBl - adrenergic, beta-1-, receptor 19mers
Sense siRNΛ ΛntiSense siRNΛ lOthcr Spll l uniiin-l 10349783 ORI :87-1520
1 GCAGAUAGAAAGACUUGUU AACAAGUCUUUCUAUCUGC [2249-2267](19/19) 31UTR
2 CAGAUAGAAAGACUUGUUU AAACAAGUCUUUCUAUCUG [2250-2268](19/19) 31UTR
3 CUACUUCUGUUGUCUAGUA UACUAGACAACAGAAGUAG [2570-2588](19/19) 31UTR
4 GCAAUAAAUACCAAUGAAG CUUCAUUGGUAUUUAUUGC [2835-2853](19/19) 3TJTR
5 GGGCAGAUCUUAAAUAAAA UUUUAUUUAAGAUCUGCCC [2543-2561](19/19) 3'UTR
6 GUGUGAUGCAUCUUUAGAU AUCUAAAGAUGCAUCACAC [1809-1827](19/19) 3'UTR
7 CCGUUGCACAAAAAGGAAA UUUCCUUUUUGUGCAACGG [1661-1679](19/19) 3'UTR
8 GCACAGUGUUAGGAAUUAC GUAAUUCCUAACACUGUGC [2460-2478](19/19) 3'UTR
9 CAAUUGAAGACAGGACAUU AAUGUCCUGUCUUCAAUUG [2349-2367](19/19) 31UTR
10 GGUUCAAAAUGCCAUUUUU AAAAAUGGCAUUUUGAACC [2441-2459](19/19) 31UTR
Table D: ADRBl - adrenergic, beta-1-, receptor 21 mers
Other I H unian-1 10349783 ORF:87-
Sense si RNA ftTTiCTrroϋiKfl^ Sn I 1520 GCAGAUAGAAAGACUUGUUUA UAAACAAGUCUUUCUAUCUGC [2249-2269](21/21) 3'UTR GCACAGUGUUAGGAAUUACAA UUGUAAUUCCUAACACUGUGC [2460-2480](21/21) 31UTR AGCAGAUAGAAAGACUUGUUU AAACAAGUCUUUCUAUCUGCU [2248-2268](21/21) 31UTR CAGCAGAUAGAAAGACUUGUU AACAAGUCUUUCUAUCUGCUG [2247-2267](21/21) 31UTR GCACAGCAGAUAGAAAGACUU AAGUCUUUCUAUCUGCUGUGC [2244-2264](21/21) 31UTR GGAUGGAGGCAAAAUAAAAAA UUUUUUAUUUUGCCUCCAUCC [2089-2109](21/21) 3'UTR CCACGGACCGUUGCACAAAAA UUUUUGUGCAACGGUCCGUGG [1654-1674](21/21) 3'UTR UCAAUUGAAGACAGGACAUUA UAAUGUCCUGUCUUCAAUUGA [2348-23681(21/20 31UTR GAGUGGAAGAUGGGUGGGUUA UAACCCACCCAUCUUCCACUC [1873-18931(21/21) 31UTR GCGUGUGAUGCAUCUUUAGAU AUCUAAAGAUGCAUCACACGC [1807-1827](21/21) 3'UTR
Table E: ADRB3 - adrenergic, beta-3-, receptor 19mers
Sense s RNA RTIlIMUlMJa I Eu Other Snl l luman-4557266 OUr : 198-I 42; CCUAAUCUUCAUCAAACAA UUGUUUGAUGAAGAUUAGG [2200-2218](19/19) 3'UTR ACCUUCCUCUUCUCGUGAU AUCACGAGAAGAGGAAGGU [838-856](19/19) ORF UCUUCCUAAUCUUCAUCAA UUGAUGAAGAUUAGGAAGA [2196-2214](19/19) 3'UTR CUCUUUUCUUCCUAAUCUU AAGAUUAGGAAGAAAAGAG [2190-2208](19/19) 3'UTR GCAGUAAAUUAGGCCUAAU AUUAGGCCUAAUUUACUGC [2166-2184](19/19) 31UTR CUCCUUCUACCUUCCUCUU AAGAGGAAGGUAGAAGGAG [830-8481(19/19) ORF CCUUUUGCAAUCAGAUAAA UUUAUCUGAUUGCAAAAGG [2038-2056](19/19) 31UTR CUCUUCUCGUGAUGCUCUU AAGAGCAUCACGAGAAGAG [844-862](19/19) ORF CCUUCCUGAAACUCUUGAA UUCAAGAGUUUCAGGAAGG [2283-23011(19/19) 31UTR CUACCUUCCUCUUCUCGUG CACGAGAAGAGGAAGGUAG [836-854](19/19) ORF
Table F: ADRB3 - adrenergic, beta-3-, receptor 21 mers I 98-
GUCUCCUUCUACCUUCCUCUU AAGAGGAAGGUAGAAGGAGAC [828-848](21/21) ORF GGCUUUUGACAGAGGCAGUAA UUACUGCCUCUGUCAAAAGCC [2152-2172](21/21) 31UTR CACUCUUUUCUUCCUAAUCUU AAGAUUAGGAAGAAAAGAGUG [2188-2208](21/21) 3'UTR ACCCUUCCUGAAACUCUUGAA UUCAAGAGUUUCAGGAAGGGU [2281-2301](21/21) 31UTR CCUCACUCUUUUCUUCCUAAU AUUAGGAAGAAAAGAGUGAGG [2185-2205](21/21) 3'UTR CUACCUUCCUCUUCUCGUGAU AUCACGAGAAGAGGAAGGUAG [836-856](21/21) ORF CCUCUUCUCGUGAUGCUCUUC GAAGAGCAUCACGAGAAGAGG [843-863](21/21) ORF UCCUCUUCUCGUGAUGCUCUU AAGAGCAUCACGAGAAGAGGA [842-862](21/21) ORF CUUUUGACAGAGGCAGUAAAU AUUUACUGCCUCUGUCAAAAG [2154-2174](21/21) 3'UTR GCUUGAGCCUUUGAUAUCUUG CAAGAUAUCAAAGGCUCAAGC [2253-22731(21/21) 3'UTR
Table G: ARHGEF9 - Cdc42 guanine nucleotide exchange factor (GEF) 9 19mcrs ju H.1UKI I t«n^ mm fc.TUina.il E« Other Snl Hιιιiiiin-76d2 l ()7 ORI- :802-2352
GGAGCCCAAUUAAAUACCU AGGUAUUUAAUUGGGCUCC [4867-4885](19/19) 31UTR
GGCUUUGAAAUUUCUGAAA UUUCAGAAAUUUCAAAGCC [2101-2119](19/19) ORF UGGAGAAACAGUAUAACAA UUGUUAUACUGUUUCUCCA [1298-1316](19/19) ORF GGAAAACGAGCGAAGUAAA UUUACUUCGCUCGUUUUCC [82-100](19/19) 5'UTR CACAAAGCCUUAUGAAAAU AUUUUCAUAAGGCUUUGUG [5362-5380](19/19) 31UTR GCCAAAUAGUGAUCUUUUA UAAAAGAUCACUAUUUGGC [5167-5185](19/19) 3'UTR CCCUCUCUAUGUGUAUAUU AAUAUACACAUAGAGAGGG [5094-5112](19/19) 31UTR AGGAAAACGAGCGAAGUAA UUACUUCGCUCGUUUUCCU [81-99](19/19) 51UTR UCAUCAGCUACCUUCAAAU AUUUGAAGGUAGCUGAUGA [3524-3542](19/19) 31UTR CAGUAUUCCUAUUGCUUUU AAAAGCAAUAGGAAUACUG [3041-3059](19/19) 31UTR
Table H: ARHGEF9 - Cdc42 guanine nucleotide exchange factor (GEF) 921mers
Human-76621 U7 ORF:8()2-
Sense siRNA AntiSense siRNA
2352 GGAGAAACAGUAUAACAAUGA UCAUUGUUAUACUGUUUCUCC [1299-13191(21/2I) ORF CCCUUCAGUGUACCUCUUUUU AAAAAGAGGUACACUGAAGGG [4525-4545](21/21) 31UTR GUGUACCUCUUUUUCCAACUU AAGUUGGAAAAAGAGGUACAC [4532-4552](21/21) 31UTR CUCUUUUUCCAACUUCUCACU AGUGAGAAGUUGGAAAAAGAG [4538-4558](21/21) 31UTR UCAGUGUACCUCUUUUUCCAA UUGGAAAAAGAGGUACACUGA [4529-45491(21/2031UTR UGGGAAUCAAAUUACAAGCUA UAGCUUGUAAUUUGAUUCCCA [4295-43151(21/2I) S1UTR GGAUAGACCUUGAACAACAAA UUUGUUGUUCAAGGUCUAUCC [4038-4058](21/21) 31UTR GGAGGAUUUUUGGUAUUGAUA UAUCAAUACCAAAAAUCCUCC [3764-3784](21/21) 31UTR GGCUUUGAAAUUUCUGAAAAC GUUUUCAGAAAUUUCAAAGCC [2101-2121](21/21) ORF CCGGAAGAGAAGGGACAUGUU AACAUGUCCCUUCUCUUCCGG [1200-1220](21/21) ORF
Table I: ARRBl - arrestin, beta 1 19mers
Sense siRNA AntiSense si R.N Λ lOther Spl ll ii ni an-58219795 ORI- :223-1479 AGAUUCUCAAUGCCUUUUU AAAAAGGCAUUGAGAAUCU [1973-1991](19/19) 31UTR UCAAUGCCUUUUUGAAGUU AACUUCAAAAAGGCAUUGA [1979-1997](19/19) 3'UTR CACAGCUCAACAACAGAUA UAUCUGUUGUUGAGCUGUG [1460-14781(19/19) ORF GGAUCAUUGUUUCCUACAA UUGUAGGAAACAAUGAUCC [1169-1187](19/19) ORF CCAAGUAUAUUUCAGUACU AGUACUGAAAUAUACUUGG [2165-2183](19/19) 31UTR GGAGUAGAGAAAAGCAACU AGUUGCUUUUCUCUACUCC [2091-2109](19/19) 31UTR ACAUUGUAUUUGAGGACUU AAGUCCUCAAAUACAAUGU Mouse [1376-1394](19/19) ORF CCCAAGUAUAUUUCAGUAC GUACUGAAAUAUACUUGGG [2164-2182](19/19) 31UTR GCCUUUUUGAAGUUCUGAC GUCAGAACUUCAAAAAGGC [1984-2002](19/19) 3'UTR CACAAAUGAUGACGACAUU AAUGUCGUCAUCAUUUGUG [1362-1380](19/19) ORF
Table J: ARRBl - arrestin, beta 1 21mers
Sense si UNA ΛntiSense siRNA Other I lluman-5821979S ORF:223- Sp I 1479 CGACAUUGUAUUUGAGGACUU AAGUCCUCAAAUACAAUGUCG Mouse [1374-1394](21/21) ORF
2 ACAGAUUCUCAAUGCCUUUUU AAAAAGGCAUUGAGAAUCUGU [1971-1991](21/21) 3'UTR
3 GGAACUGCCCUUCACCCUAAU AUUAGGGUGAAGGGCAGUUCC [1257-1277](21/21) ORF
4 GGAUAAGGAGAUCUAUUACCA UGGUAAUAGAUCUCCUUAUCC Mouse [831-851](21/21) ORF
5 ACAAAUGAUGACGACAUUGUA UACAAUGUCGUCAUCAUUUGU [1363-1383](21/21) ORF
6 GGGAUCAUUGUUUCCUACAAA UUUGUAGGAAACAAUGAUCCC [1168-1188](21/21) ORF
7 CCCAAGUAUAUUUCAGUACUG CAGUACUGAAAUAUACUUGGG [2164-21841(21/20 31UTR
8 CCAGGAGUAGAGAAAAGCAAC GUUGCUUUUCUCUACUCCUGG [2088-2108](21/21) 31UTR
9 GACAUUGUAUUUGAGGACUUU AAAGUCCUCAAAUACAAUGUC Mouse [1375-13951(21/21) ORF
10 GACACAAAUGAUGACGACAUU AAUGUCGUCAUCAUUUGUGUC [1360-13801(21/21) ORF
Table K: ATPlAl - ATPase, Na+/K+ transporting, alpha 1 polypeptide 19mers
Number! Sense siKNΛ ΛntiSense siKNΛ lOthcr SpI 11 uiiiii n-48762680 ()RI :299-3370
1 GGAAAACCUACCUAUUCUU AAGAAUAGGUAGGUUUUCC [1609-1627](19/19) ORF
2 GGAACCUCAAAACGAUAAU AUUAUCGUUUUGAGGUUCC [667-685](19/19) ORF
3 CGAUAAUCUGUGCUUUGUU AACAAAGCACAGAUUAUCG [2038-2056](19/19) ORF
4 UCGGUAUCAUCGUAGCCAA UUGGCUACGAUGAUACCGA [1272-1290](19/19) ORF
5 GGUGAGAAAAUGAGCAUAA UUAUGCUCAUUUUCUCACC [821-839](19/19) ORF
6 GCAAAAAAGACAGGGACAU AUGUCCCUGUCUUUUUUGC [375-393](19/19) ORF
7 GCCUUCUUUUCAACCAAUU AAUUGGUUGAAAAGAAGGC [1025-1043](19/19) ORF
8 GUGAGAAAAUGAGCAUAAA UUUAUGCUCAUUUUCUCAC [822-840](19/19) ORF
9 GCCCUUGUGAUUCGAAAUG CAUUUCGAAUCACAAGGGC Mouse [803-821](19/19) ORF
10 UCAUAAACUUAGCCUUGAU AUCAAGGCUAAGUUUAUGA [427-445](19/19) ORF
Table L: ATPlAl - ATPase, Na+/K+ transporting, alpha 1 polypeptide 21mers
Other I l lιιnian-487(.2680 ORF:299-
Number Sense siKNA ΛntiSense si KNA Sp I 3370
1 GGAAAACCUACCUAUUCUUAA UUAAGAAUAGGUAGGUUUUCC [1609-1629](21/21) ORF
2 CCAGAAUUGCAGGUCUUUGUA UACAAAGACCUGCAAUUCUGG [1563-1583](21/21) ORF
3 GAAAAACUGCUUAGUGAAGAA UUCUUCACUAAGCAGUUUUUC [1357-1377](21/21) ORF
4 UGGUGAGAAAAUGAGCAUAAA UUUAUGCUCAUUUUCUCACCA [820-840](21/21) ORF
5 GCCCUUGUGAUUCGAAAUGGU ACCAUUUCGAAUCACAAGGGC [803-823](21/21) ORF
6 GGAACCUCAAAACGAUAAUCU AGAUUAUCGUUUUGAGGUUCC [667-687](21/21) ORF
7 CCCUAUCGAUAAUCUGUGCUU AAGCACAGAUUAUCGAUAGGG [2032-2052](21/21) ORF
8 UGCUUAGUGAAGAACUUAGAA UUCUAAGUUCUUCACUAAGCA [1364-1384](21/21) ORF
9 CUGGAAUGGGUGUUGCUCUUA UAAGAGCAACACCCAUUCCAG [3213-3233](21/21) ORF
10 GAAAACCUACCUAUUCUUAAG CUUAAGAAUAGGUAGGUUUUC [1610-1630](21/21) ORF
Table M: CACNB4 - calcium channel, voltage-dependent, beta 4 subunit 19mers
Number Sense siKNΛ ΛntiSense siKNΛ Other Sp l l lιιmanό4(>070(>3 OKI :20-1582
1 GGAAGCUAAAUUUACACUU AAGUGUAAAUUUAGCUUCC Rat,Mouse [2095-21131(19/19) 3'UTR
2 CAAUAAUUGAACGUUCGAA UUCGAACGUUCAAUUAUUG [852-870](19/19) ORF
3 AGAGCAAUAAUUGAACGUU AACGUUCAAUUAUUGCUCU Rat,Mouse [848-866](19/19) ORF
4 GCAAUAAUUGAACGUUCGA UCGAACGUUCAAUUAUUGC [851-869](19/19) ORF
5 GAGCAAUAAUUGAACGUUC GAACGUUCAAUUAUUGCUC Rat,Mouse [849-867](19/19) ORF
6 GGCUAUAAUUAUGGAAUUG CAAUUCCAUAAUUAUAGCC [2964-2982](19/19) 3'UTR
7 GCAGAAAUUAUAUAGUCUC GAGACUAUAUAAUUUCUGC [2223-2241](19/19) 3'UTR
8 AGCUGAUAAACUUGCACAA UUGUGCAAGUUUAUCAGCU [1108-1126](19/19) ORF
9 CAAAUUUUUUUCAGCAGUG CACUGCUGAAAAAAAUUUG [2371-2389](19/19) 31UTR
10 CUCAAAUUUUUUUCAGCAG CUGCUGAAAAAAAUUUGAG [2369-2387](19/19) 31UTR
Table N: CACNB4 - calcium channel, voltage-dependent, beta 4 subunit 21 mers
Hunian-546(l7063 ORI-:20-
Sense siRNA ΛnliScnse siRNA Other Sp
1 GCUGUCAUUUUAUGCUAAGUA UACUUAGCAUAAAAUGACAGC [1696-1716](21/21) 3'UTR
2 GCAAUAAUUGAACGUUCGAAC GUUCGAACGUUCAAUUAUUGC [851-8711(21/21) ORF
3 GGAAUUGGUGACCUAUGUUUU AAAACAUAGGUCACCAAUUCC [2976-2996](21/21) 3'UTR
4 GCGGAAGUACAAAGUGAAAUU AAUUUCACUUUGUACUUCCGC Mouse [887-907](21/21) ORF
5 GGUAACAGACAUGAUGCAGAA UUCUGCAUCAUGUCUGUUACC Rat,Mouse [718-738](21/21) ORF
6 CCCUUUAAUCAAUCUGAGCAC GUGCUCAGAUUGAUUAAAGGG [2457-2477](21/21) 3'UTR
7 GAGCAAUAAUUGAACGUUCGA UCGAACGUUCAAUUAUUGCUC [849-8691(21/21) ORF
8 GGCUAUAAUUAUGGAAUUGGU ACCAAUUCCAUAAUUAUAGCC [2964-2984](21/21 ) 3'UTR
9 CUCAAAUUUUUUUCAGCAGUG CACUGCUGAAAAAAAUUUGAG [2369-2389](21/21) 3'UTR
10 UCAUACCAGGACACUUACAAA UUUGUAAGUGUCCUGGUAUGA Rat [1502-1522](21/21) ORF
Table O: CAMK2A - calcium/calmodulin-dependent protein kinase (CaM kinase) II alpha 19mers
Sense siRNA ΛntiSense siRNΛ Other Sp l l luman-259521 17 ORI-": 149-1585
1 AGAUCAUCAACACAAAGAA UUCUUUGUGUUGAUGAUCU [273-291](19/19) ORF
2 GACCAUUAACCCAUCCAAA UUUGGAUGGGUUAAUGGUC [904-9221(19/19) ORF
3 GGAAAUACCUGGACUUUUC GAAAAGUCCAGGUAUUUCC [2747-27651(19/19J S1UTR
4 CCAAGGAUCUGAUCAAUAA UUAUUGAUCAGAUCCUUGG Rat,Mouse [879-8971(19/19) ORF
5 CGGGUUCGCAUUCUCUCUU AAGAGAGAAUGCGAACCCG [2969-29871(19/19) 31UTR
6 CCACCUCCAACAUUAACCU AGGUUAAUGUUGGAGGUGG [2234-2252](19/19) 31UTR
7 GGAUUUUGCUGGAAUUCUC GAGAAUUCCAGCAAAAUCC Mouse [1667-1685](19/19) 31UTR
8 GGAAACUGAAGGGAGCCAU AUGGCUCCCUUCAGUUUCC Rat,Mouse [1038-1056](19/19) ORF
9 UGAAGCCUGAGAAUCUGUU AACAGAUUCUCAGGCUUCA Rat,Mouse [555-5731(19/19) ORF
10 GGAUUUCAUCUCACCAUAU AUAUGGUGAGAUGAAAUCC [2810-2828](19/19) 31UTR
1 CCAUUAACCCAUCCAAACGCA UGCGUUUGGAUGGGUUAAUGG [906-9261(21/21) ORF
2 CAAGAUCAUCAACACAAAGAA UUCUUUGUGUUGAUGAUCUUG [271-291](21/21) ORF
3 CCAGGAUUUCAUCUCACCAUA UAUGGUGAGAUGAAAUCCUGG [2807-28271(21/21) 31UTR
4 GGAAAUACCUGGACUUUUCUU AAGAAAAGUCCAGGUAUUUCC [2747-27671(21/21) 31UTR
5 GGGUAGUUUUUUUCUGUUCUA UAGAACAGAAAAAAACUACCC [2507-25271(21/2I) S1UTR
6 GGAUUUCGCGUUAAUGGAUAU AUAUCCAUUAACGCGAAAUCC [2413-2433](21/21) 3'UTR
7 CAAUAAGAUGCUGACCAUUAA UUAAUGGUCAGCAUCUUAUUG [892-912](21/21) ORF
8 GCGGAAACAGGAAAUUAUAAA UUUAUAAUUUCCUGUUUCCGC [1183-12031(21/21) ORF
9 GGAGCAAACGUCUGGCUAUGU ACAUAGCCAGACGUUUGCUCC [3827-38471(21/21) 3UTR
10 GGAUUUCAUCUCACCAUAUCU AGAUAUGGUGAGAUGAAAUCC [2810-28301(21/21) 31UTR
Table Q: CAMK2D - calcium/calmodulin-dependent protein kinase (CaM kinase) II delta 19mers
Sense si RN A ΛntiSensc siRNΛ .Other Spllluniun-26667185 OKI':
1 CCAGAAGUUUUACGUAAAG CUUUACGUAAAACUUCUGG [1051-10691(19/19) ORF
2 CCCUAAUAUUGUGCGACUU AAGUCGCACAAUAUUAGGG [714-732](19/19) ORF
3 AGAUAUUAUUGCUAGUGGA UCCACUAGCAAUAAUAUCU [3589-3607](19/19) 31UTR
4 GGCAAAAUCACUUAUGAAA UUUCAUAAGUGAUUUUGCC [2519-2537](19/19) 31UTR
5 CCAAGAUUUAACAGACUUA UAAGUCUGUUAAAUCUUGG [2289-23071(19/19) 31UTR
6 CCAAAAGCAAUAAACCAAU AUUGGUUUAUUGCUUUUGG Mouse [1715-1733](19/19) ORF
7 UCCAGAAGUUUUACGUAAA UUUACGUAAAACUUCUGGA [1050-10681(19/19) ORF
8 GCCAAGAUUUAACAGACUU AAGUCUGUUAAAUCUUGGC [2288-23061(19/19) 31UTR
9 GCAUAUAUUAGGCUCACAC GUGUGAGCCUAAUAUAUGC [1792-18101(19/19) ORF
10 CUCCAGAAGUUUUACGUAA UUACGUAAAACUUCUGGAG [1049-1067](19/19) ORF
1 CCAGAAGUUUUACGUAAAGAU AUCUUUACGUAAAACUUCUGG [1051-1071](21/21) ORF
2 CCUAAUAUUGUGCGACUUCAU AUGAAGUCGCACAAUAUUAGG [715-7351(21/21) ORF
3 GCCAAUGAGAUCUGUGAAAAA UUUUUCACAGAUCUCAUUGGC Mouse [3715-3735](21/21) 3XJTR
4 CCACACAAACUUAAAUUACUG CAGUAAUUUAAGUUUGUGUGG [2182-2202](21/21) 31UTR
5 GCCAAAGACCUCAUCAAUAAA UUUAUUGAUGAGGUCUUUGGC [1237-1257](21/21) ORF
6 GGAGUUAGAAUUUCCCAGUUU AAACUGGGAAAUUCUAACUCC [3308-3328](21/21) 3'UTR
7 GCCAAGAUUUAACAGACUUAA UUAAGUCUGUUAAAUCUUGGC [2288-23081(21/20 31UTR
8 GCAAUAAACCAAUCCACACUA UAGUGUGGAUUGGUUUAUUGC [1721-1741](21/21) ORF
9 CUCCAGAAGUUUUACGUAAAG CUUUACGUAAAACUUCUGGAG [1049-10691(21/2I) ORF
10 UCUCCAGAAGUUUUACGUAAA UUUACGUAAAACUUCUGGAGA [1048-10681(21/21) ORF
Table S: CBLNl - cerebellin 1 precursor 19mers
N u m her Sense siRNΛ ΛntiScnsc siRNΛ Other Sp 11 lιιman-4757921 ORT:315-8%
1 GCCUACUGAAAUCUGAUAU AUAUCAGAUUUCAGUAGGC [1286-1304](19/19) 31UTR
2 CCCUGCAAUUUAUUUAAAG CUUUAAAUAAAUUGCAGGG [1205-1223](19/19) 31UTR
3 GCACUCCCAUUUCCAAAAA UUUUUGGAAAUGGGAGUGC [1104-11221(19/19) 31UTR
4 GGAUCUACAGUUUUAACUU AAGUUAAAACUGUAGAUCC [643-661](19/19) ORF
5 CCAUGAUCAUCUACUUCGA UCGAAGUAGAUGAUCAUGG Rat,Mouse [556-574](19/19) ORF
6 GGGAUCUACAGUUUUAACU AGUUAAAACUGUAGAUCCC [642-660](19/19) ORF
7 CCUUGUCAUUGUUUCUUAU AUAAGAAACAAUGACAAGG [1170-1188](19/19) 3'UTR
8 CGACUUGAAACUUCCUACA UGUAGGAAGUUUCAAGUCG [988-1006](19/19) 3'UTR
9 CUACAGUUUUAACUUCCAC GUGGAAGUUAAAACUGUAG Rat,Mouse [647-665](19/19) ORF
10 UGCCUACUGAAAUCUGAUA UAUCAGAUUUCAGUAGGCA [1285-1303](19/19) 3'UTR
1 AGAAAGCAGCACGACUUGAAA UUUCAAGUCGUGCUGCUUUCU [977-997](21/21) 31UTR
2 CAAAGGGAUCUACAGUUUUAA UUAAAACUGUAGAUCCCUUUG [638-658](21/21) ORF
3 AGGGAUCUACAGUUUUAACUU AAGUUAAAACUGUAGAUCCCU [641-661](21/21) ORF
4 CCAACCUUUUUUUUUCGAGUU AACUCGAAAAAAAAAGGUUGG [1317-1337](21/21) 31UTR
5 UGCCUACUGAAAUCUGAUAUA UAUAUCAGAUUUCAGUAGGCA [1285-13051(21/20 31UTR
6 CAAGUGUUGCUCUUCUUAAAA UUUUAAGAAGAGCAACACUUG [1405-14251(21/2I) S1UTR
7 GAGAAAGCAGCACGACUUGAA UUCAAGUCGUGCUGCUUUCUC [976-996](21/21) 31UTR
8 CUGCCUACUGAAAUCUGAUAU AUAUCAGAUUUCAGUAGGCAG [1284-13041(21/2031UTR
9 GGAAUCCUUCUGCCUACUGAA UUCAGUAGGCAGAAGGAUUCC [1275-1295](21/21) 3'UTR
10 GCCUUGUCAUUGUUUCUUAUC GAUAAGAAACAAUGACAAGGC [1169-1189](21/21) 3'UTR
Table U: CDH22 - cadherin-like 22 19mers
CTHiWl Sense siRNΛ ΛntiScnsc siRNΛ Other Sp ll luinan-16753220 ORI :402-2888
1 CCAUCAAGUACACCAUCUC GAGAUGGUGUACUUGAUGG Rat,Mouse [688-706](19/19) ORF
2 GCCAUCAAGUACACCAUCU AGAUGGUGUACUUGAUGGC [687-705](19/19) ORF
3 AGAGUUGUGAGGCUAAAAA UUUUUAGCCUCACAACUCU [3560-35781(19/19) 31UTR
4 GGCCAUCAAGUACACCAUC GAUGGUGUACUUGAUGGCC [686-704](19/19) ORF
5 GCAACCCUCAUUUCUCUCU AGAGAGAAAUGAGGGUUGC Mouse [2032-2050](19/19) ORF
6 CGGGACAACGUCAUCAAAU AUUUGAUGACGUUGUCCCG [2391-2409](19/19) ORF
7 CCAUCAUCGUAGUGCAGAA UUCUGCACUACGAUGAUGG [1414-1432](19/19) ORF
8 GGACAUCAAUGACAGUGAG CUCACUGUCAUUGAUGUCC [878-896](19/19) ORF
9 GGCUAAAAAGGGUUCAUAU AUAUGAACCCUUUUUAGCC [3570-3588](19/19) 3'UTR
10 CAACCCUCAUUUCUCUCUG CAGAGAGAAAUGAGGGUUG Mouse [2033-2051](19/19) ORF
Table V: CDH22 - cadherin-like 22 2 liners
IIum;ιn-16753220 ORI :402-
Sense siRNΛ ΛntiSense siRNΛ
2S.S8
1 GGACAACGUCAUCAAAUACAA UUGUAUUUGAUGACGUUGUCC [2393-2413](21/21) ORF
2 GGCCAUCAAGUACACCAUCUC GAGAUGGUGUACUUGAUGGCC [686-706](21/21) ORF
3 GCCAUCAAGUACACCAUCUCA UGAGAUGGUGUACUUGAUGGC [687-707](21/21) ORF
4 CGGGACAACGUCAUCAAAUAC GUAUUUGAUGACGUUGUCCCG [2391-2411](21/21) ORF
5 GCGGGACAACGUCAUCAAAUA UAUUUGAUGACGUUGUCCCGC [2390-24101(21/2I) ORF
6 CCAGCAACCCUCAUUUCUCUC GAGAGAAAUGAGGGUUGCUGG [2029-20491(21/21) ORF
7 CCAUCAAGUACACCAUCUCAG CUGAGAUGGUGUACUUGAUGG [688-7081(21/21) ORF
8 CUAAAAAGGGUUCAUAUGUGU ACACAUAUGAACCCUUUUUAG [3572-35921(21/20 31UTR
9 GAAGAGUUGUGAGGCUAAAAA UUUUUAGCCUCACAACUCUUC [3558-3578](21/21) 3'UTR
10 GCAUUAAAACUGAGGCGAGAC GUCUCGCCUCAGUUUUAAUGC [3105-3125](21/21) 3'UTR
Table W: CDKSRl - cyclin-dependent kinase 5, regulatory subunit 1 19mers
OtBnBn iaBHHnsii Λtπ IVIUIHt-HIMkIM Other Snllluman-34304373 ORF:222-I 14
1 CCUUCUCUUUCUCCGUAUU AAUACGGAGAAAGAGAAGG [3044-3062](19/19) 31UTR
2 GGAGAACAAAGACCAAUGU ACAUUGGUCUUUGUUCUCC [2446-2464](19/19) 31UTR 3 CUCUUUCUCCGUAUUUAUU AAUAAAUACGGAGAAAGAG [3048-30661(19/19) 31UTR 4 AGAAAGCUGUGAAAUUGAA UUCAAUUUCACAGCUUUCU [2620-2638](19/19) 3'UTR 5 CAAAAAGAAAGCUGUGAAA UUUCACAGCUUUCUUUUUG [2615-2633](19/19) 3'UTR 6 UCCUUCUCUUUCUCCGUAU AUACGGAGAAAGAGAAGGA [3043-3061](19/19) 3'UTR 7 GCAAUUUCUUCUGUAGCUA UAGCUACAGAAGAAAUUGC [3635-3653](19/19) 31UTR 8 UUCUCUUUCUCCGUAUUUA UAAAUACGGAGAAAGAGAA [3046-3064](19/19) 31UTR 9 ACAAAAAGAAAGCUGUGAA UUCACAGCUUUCUUUUUGU [2614-26321(19/19) 31UTR 10 GCACAUGCACUACUGAAAU AUUUCAGUAGUGCAUGUGC [2591-2609](19/19) 3'UTR
Table X: CDKSRl - cyclin-dependent kinase S, regulatory subunit 1 21mers iflWIMtlNkH KTmaiinaaafHara fRπnsffl Huniiin-34304373 ORI :222-
1 GCACAUGCACUACUGAAAUUU AAAUUUCAGUAGUGCAUGUGC [2591-2611](21/21) 31UTR
2 CUUCUCUUUCUCCGUAUUUAU AUAAAUACGGAGAAAGAGAAG [3045-30651(21/21) 3'UTR 3 UCCUUCUCUUUCUCCGUAUUU AAAUACGGAGAAAGAGAAGGA [3043-30631(21/21) 31UTR 4 CAACAAUGAGAACCUGAAGAA UUCUUCAGGUUCUCAUUGUUG Rat,Mouse [464-484](21/21) ORF 5 GAGAACAAAGACCAAUGUGAA UUCACAUUGGUCUUUGUUCUC [2447-2467](21/21) 31UTR 6 ACACUACUUCACACAGGUCUU AAGACCUGUGUGAAGUAGUGU [1058-10781(21/21) ORF 7 CCAGGAGCAUUUUGUGUCUUA UAAGACACAAAAUGCUCCUGG [3341-3361](21/21) 3'UTR 8 CCUUCUCUUUCUCCGUAUUUA UAAAUACGGAGAAAGAGAAGG [3044-3064](21/21) 3'UTR 9 GAACAACAUCACGCACCUCAA UUGAGGUGCGUGAUGUUGUUC [446-466](21/21) ORF 10 CCCGUUAAUUUAAAGCUGUUU AAACAGCUUUAAAUUAACGGG [2133-21531(21/21) 3'UTR
Table Y: CHNl - chimerin (chimaerin) 1 19mers
WTBIMUKIIMkI-I aτπn-τuιLta-i. ιt<-»i Other SnlHuιnan-68533258 ORF:476-18
1 CGAGCAAAUUCCAAAAUAU AUAUUUUGGAAUUUGCUCG [1063-1081](19/19) ORF
2 CCAGCAUACAAAAAACAUA UAUGUUUUUUGUAUGCUGG [938-956](19/19) ORF 3 ACGAGCAAAUUCCAAAAUA UAUUUUGGAAUUUGCUCGU [1062-1080](19/19) ORF 4 CCAUACAGAAGAAACAACA UGUUGUUUCUUCUGUAUGG [2129-2147](19/19) 31UTR 5 UGAAAGAAAACGAGCAAAU AUUUGCUCGUUUUCUUUCA [1053-10711(19/19) ORF 6 GGAGAAUCUUAUGAAUGCA UGCAUUCAUAAGAUUCUCC [1702-1720](19/19) ORF 7 CGAAGAAUUACCUGUACUU AAGUACAGGUAAUUCUUCG [566-584](19/19) ORF 8 GGAUACACAACCUUAAACA UGUUUAAGGUUGUGUAUCC [914-932](19/19) ORF 9 GCGUGAAUAACUUUUUCUU AAGAAAAAGUUAUUCACGC [285-3031(19/19) 51UTR 10 CCAUAUCACAUGCCUACAU AUGUAGGCAUGUGAUAUGG [2328-23461(19/19) 31UTR
Table Z: CHNl - chimerin (chimaerin) 1 21mers
Other I l luman-68533258 ORr :476
3553353 BWSKϊIHSl 1-TΠIMUIHH.II t*a&» Sp I 1855
1 CCCAUACAGAAGAAACAACAA UUGUUGUUUCUUCUGUAUGGG [2128-2148](21/21) 3'UTR
2 CCAUACAGAAGAAACAACAAA UUUGUUGUUUCUUCUGUAUGG [2129-2149](21/21) 3'UTR
3 GCCAGCAUACAAAAAACAUAU AUAUGUUUUUUGUAUGCUGGC [937-957](21/21) ORF
4 GGAGAAUCUUAUGAAUGCAGA UCUGCAUUCAUAAGAUUCUCC [1702-1722](21/21) ORF
5 CGAAAAGGAGAAUCUUAUGAA UUCAUAAGAUUCUCCUUUUCG [1696-17161(21/21) ORF
6 CAAUAACGCGUGAAUAACUUU AAAGUUAUUCACGCGUUAUUG [278-298](21/21) 5'UTR
7 GGAGAAACGCUUUGAGUCCAU AUGGACUCAAAGCGUUUCUCC [793-813](21/21) ORF
8 CCAAUUCCACUCAUUACAUAU AUAUGUAAUGAGUGGAAUUGG [1526-15461(21/2I) ORF
9 CUGAAAGAAAACGAGCAAAUU AAUUUGCUCGUUUUCUUUCAG [1052-1072](21/21) ORF
10 CCAGCAUGUUUCACAGAGUAA UUACUCUGUGAAACAUGCUGG [2197-2217](21/21) 3'UTR
Table AA: CTSD - cathepsin D 19mers
Hffiπffπ πu i KIUKΠ ;i«i BTΠIKTMIKIUKIIM»&I Other Sn l l luniun-231 10949 OKI : 134-137
1 CUGUGUUUGACCGUGACAA UUGUCACGGUCAAACACAG [1317-1335](19/19) ORF
2 GCCGCUACUACACUGUGUU AACACAGUGUAGUAGCGGC [1305-1323](19/19) ORF 3 ACAAGUACAACAGCGACAA UUGUCGCUGUUGUACUUGU [495-513](19/19) ORF 4 GACAUGGAAAUACAGUUGU ACAACUGUAUUUCCAUGUC [2083-2101](19/19) 3'UTR 5 CCGCUACUACACUGUGUUU AAACACAGUGUAGUAGCGG [1306-1324](19/19) ORF 6 CAGACUCCAAGUAUUACAA UUGUAAUACUUGGAGUCUG [882-900](19/19) ORF 7 AGAACAUCUUCUCCUUCUA UAGAAGGAGAAGAUGUUCU Rat,Mouse [810-828](19/19) ORF 8 CGCUACUACACUGUGUUUG CAAACACAGUGUAGUAGCG [1307-13251(19/19) ORF 9 GAAAUACAGUUGUUGGCCU AGGCCAACAACUGUAUUUC [2089-2107](19/19) 3'UTR 10 CUACUACACUGUGUUUGAC GUCAAACACAGUGUAGUAG Mouse [1309-1327](19/19) ORF
Table AB: CTSD - cathepsin D 2 liners
RfljHBπrai ιιman-23 (1949 O R : 34- i-1 Il IHII I KIUKI I [MM
1 UGACAUGGAAAUACAGUUGUU AACAACUGUAUUUCCAUGUCA [2082-2102](21/21) 3'UTR 2 GGAAAUACAGUUGUUGGCCUC GAGGCCAACAACUGUAUUUCC [2088-21081(21/20 31UTR 3 GCUGUUUUGUUCUGUGGUUUU AAAACCACAGAACAAAACAGC [1521-1541](21/21) 31UTR 4 CCGCUACUACACUGUGUUUGA UCAAACACAGUGUAGUAGCGG [1306-13261(21/21) ORF 5 GGCCGCUACUACACUGUGUUU AAACACAGUGUAGUAGCGGCC [1304-1324](21/21) ORF 6 UGACAACAACAGGGUGGGCUU AAGCCCACCCUGUUGUUGUCA [1330-1350](21/21) ORF 7 CACAGACUCCAAGUAUUACAA UUGUAAUACUUGGAGUCUGUG [880-900](21/21) ORF 8 CGAGGUGCUCAAGAACUACAU AUGUAGUUCUUGAGCACCUCG [337-357](21/21) ORF 9 GACAUGGAAAUACAGUUGUUG CAACAACUGUAUUUCCAUGUC [2083-21031(21/20 31UTR 10 GCUGAUUCAGGGCGAGUACAU AUGUACUCGCCCUGAAUCAGC [1090-1110](21/21) ORF
Table AC: DDN - dendrin 19mers
IaSiHElBHl Other Spl l lιιman-62079298 OKI- : 181 -2154
1 CGAUCCCAAUAUGUUCCAA UUGGAACAUAUUGGGAUCG [1405-1423](19/19) ORF
2 GGCCCAUCUUUGUACUCAU AUGAGUACAAAGAUGGGCC [3455-3473](19/19) 31UTR
3 GCCCAUCUUUGUACUCAUC GAUGAGUACAAAGAUGGGC [3456-34741(19/19) 31UTR
4 GGGAGCAAGAGAAAAGGAA UUCCUUUUCUCUUGCUCCC [341-3591(19/19) ORF
5 CCAUUUAUUAGCCAUGUGA UCACAUGGCUAAUAAAUGG Mouse [3552-3570](19/19) 3'UTR
6 GGGAAUAAGUGAACAGAGU ACUCUGUUCACUUAUUCCC [2843-2861](19/19) 3'UTR
7 GGGCCCAUCUUUGUACUCA UGAGUACAAAGAUGGGCCC [3454-3472](19/19) 3'UTR
8 GGGUGUGAUACUCUCUAUA UAUAGAGAGUAUCACACCC [3271-3289](19/19) 3'UTR
9 GAAUAAGUGAACAGAGUUU AAACUCUGUUCACUUAUUC [2845-2863](19/19) 31UTR
10 GGAAUAAGUGAACAGAGUU AACUCUGUUCACUUAUUCC [2844-2862](19/19) 31UTR
Table AD: DDN - dendrin 21 mers
Other I I l ιιman-62079298 Oltl : I 8I -
Sense si UNA AntiScn.se siltNΛ Sn I 2154
1 CGGGAGCAAGAGAAAAGGAAA UUUCCUUUUCUCUUGCUCCCG [340-360](21/21) ORF
2 GCAACAACCAACAAAGCAGAA UUCUGCUUUGUUGGUUGUUGC [2602-2622](21/21) 31UTR
3 GCAUAUGCCCAUUAAUGAUUU AAAUCAUUAAUGGGCAUAUGC [2305-23251(21/20 31UTR
4 CCCAUCUUUGUACUCAUCCAC GUGGAUGAGUACAAAGAUGGG [3457-3477](21/21) 31UTR
5 GGGCCCAUCUUUGUACUCAUC GAUGAGUACAAAGAUGGGCCC [3454-3474](21/21) 3'UTR
6 UGGGAAUAAGUGAACAGAGUU AACUCUGUUCACUUAUUCCCA [2842-28621(21/2031UTR
7 CCUCCUGGUAUAUAAAAUGAU AUCAUUUUAUAUACCAGGAGG [2727-27471(21/2031UTR
8 CGAUCCCAAUAUGUUCCAACC GGUUGGAACAUAUUGGGAUCG [1405-14251(21/21) ORF
9 CCAAGGAGACCGAGCGAAAAA UUUUUCGCUCGGUCUCCUUGG [383-403](21/21) ORF
10 GGAAUAAGUGAACAGAGUUUG CAAACUCUGUUCACUUAUUCC [2844-2864](21/21) 31UTR
Table AE: DRD3 - dopamine receptor D3 19mers
PΠΠTWΠ Sense siKNΛ ΛntiSense siltNΛ Other Sn l l l u m ;ιn-89l 9I S60 Oltl;:432-l 634
1 CCAUCUCCAACCCUGAUUU AAAUCAGGGUUGGAGAUGG Rat,Mouse [976-994](19/19) ORF
2 CCCUCCCAAAUCCUCUAAA UUUAGAGGAUUUGGGAGGG [129-147](19/19) 51UTR
3 CCAACCCUGAUUUUGUCAU AUGACAAAAUCAGGGUUGG Rat,Mouse [982-1000](19/19) ORF
4 CUCUUCUGUUUGGCUUUAA UUAAAGCCAAACAGAAGAG [931-9491(19/19) ORF
5 CUAAAGAAAACGGAUACAU AUGUAUCCGUUUUCUUUAG [143-161](19/19) 51UTR
6 UCCAUCUCCAACCCUGAUU AAUCAGGGUUGGAGAUGGA Rat,Mouse [975-993](19/19) ORF
7 CCACCUUCAAUAUCGAGUU AACUCGAUAUUGAAGGUGG [1582-1600](19/19) ORF
8 CCUCUAAAGAAAACGGAUA UAUCCGUUUUCUUUAGAGG [140-158](19/19) 5'UTR
9 GAUUUUGUCAUCUACUCUU AAGAGUAGAUGACAAAAUC [990-1008](19/19) ORF
10 UCUUCUGUUUGGCUUUAAU AUUAAAGCCAAACAGAAGA [932-950](19/19) ORF
Table AF: DRD3 - dopamine receptor D3 21 mers l lunian-89191860 OltF:432-
Sense siltNΛ ΛntiScnsc siltNΛ Other Sp
1 CCCUCUUCUGUUUGGCUUUAA UUAAAGCCAAACAGAAGAGGG [929-949](21/21) ORF
2 CCUCUUCUGUUUGGCUUUAAU AUUAAAGCCAAACAGAAGAGG [930-950](21/21) ORF
3 CUCUUCUGUUUGGCUUUAAUA UAUUAAAGCCAAACAGAAGAG [931-951](21/21) ORF
4 CUCCAUCUCCAACCCUGAUUU AAAUCAGGGUUGGAGAUGGAG Rat,Mouse [974-994](21/21) ORF
5 CUCUAAAGAAAACGGAUACAU AUGUAUCCGUUUUCUUUAGAG [141-161](21/21) 51UTR
6 UCCAUCUCCAACCCUGAUUUU AAAAUCAGGGUUGGAGAUGGA Rat,Mouse [975-995](21/21) ORF
7 CCCUCCCAAAUCCUCUAAAGA UCUUUAGAGGAUUUGGGAGGG [129-149](21/21) 5'UTR
8 GCUCCAUCUCCAACCCUGAUU AAUCAGGGUUGGAGAUGGAGC Rat,Mouse [973-993](21/21) ORF
9 CCUCUAAAGAAAACGGAUACA UGUAUCCGUUUUCUUUAGAGG [140-160](21/21) 51UTR
10 GCAAUGGCAGAUUAUCGACAU AUGUCGAUAAUCUGCCAUUGC [1336-1356](21/21) ORF
Table AG: DUSP6 - dual specificity phosphatase 6 19mers
Number Sense siRNΛ ΛntiSense siRNA Other Sn ll lunian-42764682 OUF:48I -I626
1 CCAACCAGAAUGUAUACCA UGGUAUACAUUCUGGUUGG [1583-1601](19/19) ORF
2 CCCAUUUGAUAAGAGAAAU AUUUCUCUUAUCAAAUGGG [2675-2693](19/19) 3'UTR 3 GGGCUUAAAAGAAAUUAUC GAUAAUUUCUUUUAAGCCC [2187-22051(19/19) 31UTR 4 ACCCAUUUGAUAAGAGAAA UUUCUCUUAUCAAAUGGGU [2674-2692](19/19) 31UTR 5 GGGCUCACUUUAAAAAAAG CUUUUUUUAAAGUGAGCCC [2501-2519](19/19) 31UTR 6 UGGGCUCACUUUAAAAAAA UUUUUUUAAAGUGAGCCCA [2500-25181(19/19) 31UTR 7 CCAUUUCUUUCAUAGAUGA UCAUCUAUGAAAGAAAUGG Rat,Mouse [1304-13221(19/19) ORF 8 CCAUUAUGUUCGUGGUGUA UACACCACGAACAUAAUGG [2728-2746](19/19) 3'UTR 9 CCCUAACUUCAACUUCAUG CAUGAAGUUGAAGUUAGGG [1473-1491](19/19) ORF 10 GGGCUGCUUUCUUUGUGUG CACACAAAGAAAGCAGCCC [1695-17131(19/19) 31UTR
Table AH: DUSP6 - dual specificity phosphatase 621mers
Human-42764682 ORI :481-
Number Sense si RNA AntiSensc siRNΛ Other Sp
1 UGACAUCUUUACGGACACUAU AUAGUGUCCGUAAAGAUGUCA [2324-2344](21/21) 31UTR
2 CCAAUUCUGAUGACAUCUUUA UAAAGAUGUCAUCAGAAUUGG [2314-2334](21/21) 31UTR
3 ACAUCUUUACGGACACUAUUA UAAUAGUGUCCGUAAAGAUGU [2326-2346](21/21) 31UTR
4 CGCUCGCUGUUUGUAUCCAUU AAUGGAUACAAACAGCGAGCG [5-25](21/21) 5'UTR
5 UCAUGGGCUCACUUUAAAAAA UUUUUUAAAGUGAGCCCAUGA [2497-2517](21/21) 3'UTR
6 GGCUGCUUUCUUUGUGUGUGG CCACACACAAAGAAAGCAGCC Rat [1696-1716](21/21) 3'UTR
7 GCUGGGCUGCUUUCUUUGUGU ACACAAAGAAAGCAGCCCAGC [1692-1712](21/21) 31UTR
8 CCCUGAGGCCAUUUCUUUCAU AUGAAAGAAAUGGCCUCAGGG Rat,Mouse [1296-1316](21/21) ORF
9 CCCAAUUUGCCGAAUCUCUUU AAAGAGAUUCGGCAAAUUGGG [1201-1221](21/21) ORF
10 GACAUCUUUACGGACACUAUU AAUAGUGUCCGUAAAGAUGUC [2325-2345](21/21) 31UTR
Table AI: ENPPl - ectonucleotide pyrophosphatase/phosphodiesterase 1 19mers
Number Sense siRNΛ ΛntiSense siUNΛ lOther Spllluman-13324676 ORI- : 173-2794
1 GGCAGAAUAUUUACACACU AGUGUGUAAAUAUUCUGCC [679-697](19/19) ORF
2 GGAAACUGCUGUUUAGAUU AAUCUAAACAGCAGUUUCC [404-422](19/19) ORF
3 GCCAAAGAAGUUAAAAGUU AACUUUUAACUUCUUUGGC [320-338](19/19) ORF
4 GGGCAGAAUAUUUACACAC GUGUGUAAAUAUUCUGCCC [678-696](19/19) ORF
5 CCACCACAACUAAAUAAAA UUUUAUUUAGUUGUGGUGG [2240-22581(19/19) ORF
6 CCCACCACAACUAAAUAAA UUUAUUUAGUUGUGGUGGG [2239-2257](19/19) ORF
7 CCAUGAAUCUUUUUGAGAG CUCUCAAAAAGAUUCAUGG [2816-2834](19/19) 3'UTR
8 CAAACACCAUGAAUCUUUU AAAAGAUUCAUGGUGUUUG [2810-2828](19/19) 3'UTR
9 UGCCAAAGAAGUUAAAAGU ACUUUUAACUUCUUUGGCA [319-337](19/19) ORF
10 GGGUUUGAAACGCCUCCUA UAGGAGGCGUUUCAAACCC [632-650](19/19) ORF
Table AJ: ENPPl - ectonucleotide pyrophosphatase/phosphodiesterase 1 21mers
Other I lluman-13324676 OUF: 173-
Number! KπiHKmwn mil IMg iKVMΛi ;>iii Sp I 2794
1 CCAAACACCAUGAAUCUUUUU AAAAAGAUUCAUGGUGUUUGG [2809-2829](21/21) 31UTR
2 CCAUGAAUCUUUUUGAGAGAA UUCUCUCAAAAAGAUUCAUGG [2816-28361(21/2I) S1UTR
3 CCCAAACACCAUGAAUCUUUU AAAAGAUUCAUGGUGUUUGGG [2808-28281(21/21) 3'UTR
4 CCAAAGAAGUUAAAAGUUGCA UGCAACUUUUAACUUCUUUGG [321-341](21/21) ORF
5 UGAAUUUGACACCGGCUCCUA UAGGAGCCGGUGUCAAAUUCA [1746-1766](21/21) ORF
6 GUGCCAAAGAAGUUAAAAGUU AACUUUUAACUUCUUUGGCAC [318-3381(21/21) ORF
7 UGGAAACUGCUGUUUAGAUUA UAAUCUAAACAGCAGUUUCCA [403-4231(21/21) ORF
8 GCAUGAAACUUUACCCUAUGG CCAUAGGGUAAAGUUUCAUGC [1975-19951(21/21) ORF
9 CCUGAAACAUUUCUUACCUAA UUAGGUAAGAAAUGUUUCAGG [1486-15061(21/2I) ORF
10 GCCAAAGAAGUUAAAAGUUGC GCAACUUUUAACUUCUUUGGC [320-340](21/21) ORF
Table AK: ENPP2 - ectonucleotide pyrophosphatase/phosphodiesterase 2 I9mcrs
■kUJrlMiϋkil l&Til IKTU I H MM 1 MKIM Other Sπll luιiiiin-91823273 ORI- :87-283-l
1 CCAAAGAAAAGAAGAAGAA UUCUUCUUCUUUUCUUUGG [1149-1167](19/19) ORF
2 CCAACUAAAACCUUUCCUA UAGGAAAGGUUUUAGUUGG [705-723](19/19) ORF
3 GCUCAACAUUUAAGUACAA UUGUACUUAAAUGUUGAGC [1717-1735](19/19) ORF
4 CGGAGAAUAUUAACCAUAU AUAUGGUUAAUAUUCUCCG [936-954](19/19) ORF
5 GGGAAAUCGACAAAAUUGU ACAAUUUUGUCGAUUUCCC [1231-1249](19/19) ORF
6 UGAGGGAAAUCGACAAAAU AUUUUGUCGAUUUCCCUCA [1228-1246](19/19) ORF
7 GCGGAGAAUAUUAACCAUA UAUGGUUAAUAUUCUCCGC [935-953](19/19) ORF
8 GGCUCAACAUUUAAGUACA UGUACUUAAAUGUUGAGCC [1716-17341(19/19) ORF
9 GGUUAUGGCUCAACAUUUA UAAAUGUUGAGCCAUAACC [1710-1728](19/19) ORF
10 CUAAAACCUUUCCUAACUU AAGUUAGGAAAGGUUUUAG [709-727](19/19) ORF
1 GCGGAGAAUAUUAACCAUAUU AAUAUGGUUAAUAUUCUCCGC [935-9551(21/21) ORF
2 CCCAACUAAAACCUUUCCUAA UUAGGAAAGGUUUUAGUUGGG [704-724](21/21) ORF
3 CGAGGGCGAGAGAAAUUUAAU AUUAAAUUUCUCUCGCCCUCG [819-8391(21/21) ORF
4 AGGUUAUGGCUCAACAUUUAA UUAAAUGUUGAGCCAUAACCU [1709-1729](21/21) ORF
5 CAACUAAAACUGCAUCGGUGU ACACCGAUGCAGUUUUAGUUG [1275-12951(21/21) ORF
6 CUGAGGGAAAUCGACAAAAUU AAUUUUGUCGAUUUCCCUCAG [1227-12471(21/21) ORF
7 CGAGCGGAGAAUAUUAACCAU AUGGUUAAUAUUCUCCGCUCG [932-952](21/21) ORF
8 UGGCUCAACAUUUAAGUACAA UUGUACUUAAAUGUUGAGCCA [1715-1735](21/21) ORF
9 GGAGUCAAUAUCUGCUUAGGA UCCUAAGCAGAUAUUGACUCC [147-167](21/21) ORF
10 GGCGAGAGAAAUUUAAUCAUA UAUGAUUAAAUUUCUCUCGCC [823-843](21/21) ORF
Table AM: EPHA4 - EPH receptor A4 19mers
[3073-
1 GCACUUUUUUUACUUCGUC GACGAAGUAAAAAAAGUGC Monkey,Chimpanzee,Rat 3091](19/19)
3'UTR
[4121-
2 CCAGACUCUUUGCGGUUUU AAAACCGCAAAGAGUCUGG 4139](19/19) 31UTR
[865-
3 GCUUGCAAAAUUGGAUAUU AAUAUCCAAUUUUGCAAGC Monkey,Chimpanzee,Rat,Mouse 883](19/19) ORF
[1526-
4 CCAGGAACACAGAUAUCAA UUGAUAUCUGUGUUCCUGG Monkey,Chimpanzee,Rat,Mouse 1544](19/19)
ORF
[4321-
5 CCAUUGCAGUGAUAUAAAU AUUUAUAUCACUGCAAUGG Mouse 4339](19/19)
3'UTR
[3977-
6 CCAUACCUGAAAAGAAUAA UUAUUCUUUUCAGGUAUGG 3995](19/19)
31UTR
[3442-
7 GAACAUUUUUUAAGGACUC GAGUCCUUAAAAAAUGUUC Chimpanzee 3460](19/19) 31UTR
[3074-
8 CACUUUUUUUACUUCGUCU AGACGAAGUAAAAAAAGUG Monkey,Chimpanzee,Rat 3092](19/19) 31UTR
[3072-
9 UGCACUUUUUUUACUUCGU ACGAAGUAAAAAAAGUGCA Monkey,Chimpanzee,Rat 3090](19/19) 31UTR
[2177-
10 GGAAAAAUGAUGGCAGAUU AAUCUGCCAUCAUUUUUCC Monkey,Chimpanzee 2195](19/19)
ORF
Table AN: EPHA4 - EPH receptor A421mers
[3073-
1 GCACUUUUUUUACUUCGUCUU AAGACGAAGUAAAAAAAGUGC Monkey,Chimpanzee,Rat 3093](21/21)
31UTR
[3251-
2 CCAGUCUGUAAAAUACAUGUA UACAUGUAUUUUACAGACUGG Monkey.Chimpanzee 32711(21/21)
31UTR
[2177-
3 GGAAAAAUGAUGGCAGAUUUA UAAAUCUGCCAUCAUUUUUCC Monkey.Chimpanzee 2197](21/21)
ORF
[869-
4 GCAAAAUUGGAUAUUACAAGG CCUUGUAAUAUCCAAUUUUGC Monkey.Chimpanzee 889](21/21) ORF
[5912-
5 CCAUAAAGGAAAGUUCUAGAA UUCUAGAACUUUCCUUUAUGG 5932](21/21) 3'UTR
[3074-
6 CACUUUUUUUACUUCGUCUUC GAAGACGAAGUAAAAAAAGUG Monkey,Chimpanzee,Rat 3094](21/21) 3'UTR
[4320-
7 GCCAUUGCAGUGAUAUAAAUA UAUUUAUAUCACUGCAAUGGC Mouse 4340](21/21)
3'UTR
[4121-
8 CCAGACUCUUUGCGGUUUUAU AUAAAACCGCAAAGAGUCUGG 4141](21/21)
3'UTR
[3072-
9 UGCACUUUUUUUACUUCGUCU AGACGAAGUAAAAAAAGUGCA Monkey,Chimpanzee,Rat 3092](21/21)
31UTR
[3071-
10 CUGCACUUUUUUUACUUCGUC GACGAAGUAAAAAAAGUGCAG Monkey ,Chimpanzee,Rat 3091](21/21) 3'UTR
Table AO: GABRAl - gamma-aminobutyric acid 19mers
Sense siRNA ΛntiScnsc siRNA Other Sp | ll uman-38327553 ORF:3 I6-1686
1 CCAACUAUUUCAAUAAGUG CACUUAUUGAAAUAGUUGG Rat,Mouse [3059-3077](19/19) 3'UTR
2 CCCAAGAAAACCUUUAACA UGUUAAAGGUUUUCUUGGG Rat,Mouse [1540-1558](19/19) ORF
3 GCAUGAGAUUGUACAUUUU AAAAUGUACAAUCUCAUGC [2698-2716]( 19/19) 31UTR
4 CCACAAGUCACGGUCUAAA UUUAGACCGUGACUUGUGG [3361-3379](19/19) 31UTR
5 AGCUAUGGAUUGGUUUAUU AAUAAACCAAUCCAUAGCU [1248-12661(19/19) ORF
6 UCAAGUCAUUCCACACAUU AAUGUGUGGAAUGACUUGA [2946-2964](19/19) 31UTR
7 GGAAAUACAUUUAGAACCU AGGUUCUAAAUGUAUUUCC [2796-28141(19/19) 31UTR
8 ACACUCACUUAAUUCUCAU AUGAGAAUUAAGUGAGUGU [2529-2547](19/19) 31UTR
9 GCAUAAAUCUGUUAGCAUU AAUGCUAACAGAUUUAUGC [3398-3416](19/19) 31UTR
10 CCACACAUUUCCCUAUUUU AAAAUAGGGAAAUGUGUGG [2956-2974](19/19) 31UTR
Table AP: GABRAl - gamma-aminobutyric acid 21mers
I luman-38327553 OR1 :316-
Number! Sense siRNA AntiSense siRNA Other Sp
1 CAACACUCACUUAAUUCUCAU AUGAGAAUUAAGUGAGUGUUG [2527-2547](21/21) 31UTR
2 CCUUUAACAGUGUCAGCAAAA UUUUGCUGACACUGUUAAAGG [1550-1570](21/21) ORF
3 CAAGAAAACCUUUAACAGUGU ACACUGUUAAAGGUUUUCUUG [1542-1562](21/21) ORF
4 AGAACCCAAGAAAACCUUUAA UUAAAGGUUUUCUUGGGUUCU Rat,Mouse [1536-15561(21/21) ORF
5 ACAGCUAUGGAUUGGUUUAUU AAUAAACCAAUCCAUAGCUGU [1246-12661(21/21) ORF
6 GCAACAGCUAUGGAUUGGUUU AAACCAAUCCAUAGCUGUUGC [1243-1263](21/21) ORF
7 GGAUCACGUCUAAACCAGUAU AUACUGGUUUAGACGUGAUCC [949-9691(21/21) ORF
8 CCAUGCUUGCCCACUAAAAUU AAUUUUAGUGGGCAAGCAUGG [846-866](21/21) ORF
9 CCCAUGCUUGCCCACUAAAAU AUUUUAGUGGGCAAGCAUGGG [845-8651(21/21) ORF
GCCCAUGCUUGCCCACUAAAA UUUUAGUGGGCAAGCAUGGGC [844-864](21/21) ORF
Table AQ: GMFG - glia maturation factor, gamma 19mers
■kffimJmMil AntiSense siRNA GAAUUCCUGAUGUCUGAGU ACUCAGACAUCAGGAAUUC [451-469](19/19) 3'UTR CUCCAAGAAAAGUUGUCUU AAGACAACUUUUCUUGGAG [404-422](19/19) ORF CGAAAUCCGCACCACUGAU AUCAGUGGUGCGGAUUUCG [367-385](19/19) ORF GCAAGCCGGAACAACAGAU AUCUGUUGUUCCGGCUUGC [291-309](19/19) ORF CCCUUUGUGUUUCAUCUUC GAAGAUGAAACACAAAGGG Rat [256-274](19/19) ORF UGGAGGAAGAAUUUCAGAA UUCUGAAAUUCUUCCUCCA [138-156](19/19) ORF GGCUCCAAGAAAAGUUGUC GACAACUUUUCUUGGAGCC [402-420](19/19) ORF CCUACCCUUUGUGUUUCAU AUGAAACACAAAGGGUAGG Rat [252-270](19/19) ORF CAAGUACGUGCAUGACGAU AUCGUCAUGCACGUACUUG [223-241](19/19) ORF ACAAGUACGUGCAUGACGA UCGUCAUGCACGUACUUGU [222-240](19/19) ORF
Table AR: GMFG - glia maturation factor, gamma 21mers mil-JiHkMEl Wm% ii fw jj i Kityπ It her SnlHuman-4758439 ORF:5-43:
GGAGGAAGAAUUUCAGAACAU AUGUUCUGAAAUUCUUCCUCC [139-159](21/21) ORF UGAAUUCCUGAUGUCUGAGUC GACUCAGACAUCAGGAAUUCA [450-470](21/21) 31UTR GAGGAAGAAUUUCAGAACAUU AAUGUUCUGAAAUUCUUCCUC [140-160](21/21) ORF GGCUCCAAGAAAAGUUGUCUU AAGACAACUUUUCUUGGAGCC [402-422](21/21) ORF UGAUGUAUGCAGGGAGUAAAA UUUUACUCCCUGCAUACAUCA [309-329](21/21) ORF CCCUUUGUGUUUCAUCUUCUC GAGAAGAUGAAACACAAAGGG Rat [256-276](21/21) ORF CUACCCUUUGUGUUUCAUCUU AAGAUGAAACACAAAGGGUAG Rat [253-273](21/21) ORF CAAAAUGGAGUUGCCGGAGAG CUCUCCGGCAACUCCAUUUUG [175-195](21/21) ORF GGAAGAAUUUCAGAACAUUUC GAAAUGUUCUGAAAUUCUUCC [142-162](21/21) ORF CCCUAGGACCUGAACAACCAA UUGGUUGUUCAGGUCCUAGGG [494-514](21/21) 31UTR
Table AS: GPM6A - glycoprotein M6A 19mers
■ktø.UJ.-IW.tøP AntiSense siRNA IJllTTJoϊi FSTTmTiTiB KE nil 1IV-TlJ UIHIiIKS lSfl
CCACGCUUCUAUUUCUUUU AAAAGAAAUAGAAGCGUGG [41-591(19/19) 5'UTR GGGUUCAUUUUGCUUCCUU AAGGAAGCAAAAUGAACCC [3225-3243](19/19) 31UTR CACGCUUCUAUUUCUUUUU AAAAAGAAAUAGAAGCGUG [42-601(19/19) 5'UTR CCAAAAUUCUCAGGCUAUU AAUAGCCUGAGAAUUUUGG [485-5031(19/19) 51UTR GCCACGCUUCUAUUUCUUU AAAGAAAUAGAAGCGUGGC [40-58](19/19) 51UTR AGCCACGCUUCUAUUUCUU AAGAAAUAGAAGCGUGGCU [39-57](19/19) 51UTR GGCUUUUAUUUCUGGUAUU AAUACCAGAAAUAAAAGCC [3431-3449](19/19) 31UTR GCACUUAAAUUUCCCAAUU AAUUGGGAAAUUUAAGUGC [1797-1815](19/19) 31UTR GCAGCUUUGAGAAGAAAAA UUUUUCUUCUCAAAGCUGC Mouse [538-556](19/19) 5'UTR CAAACCUCAAUUUCUUUUC GAAAAGAAAUUGAGGUUUG [394-412](19/19) 5'UTR
Table AT: GPM6A - glycoprotein M6A 21mers
CCACGCUUCUAUUUCUUUUUA UAAAAAGAAAUAGAAGCGUGG [41-61](21/21) 5'UTR' GCCACGCUUCUAUUUCUUUUU AAAAAGAAAUAGAAGCGUGGC [40-60](21/21) 5'UTR GGGUUCAUUUUGCUUCCUUUU AAAAGGAAGCAAAAUGAACCC [3225-3245](21/21) 3'UTR AGCCACGCUUCUAUUUCUUUU AAAAGAAAUAGAAGCGUGGCU [39-59](21/21) 5'UTR GGAAAUAACUACUGUAGACUA UAGUCUACAGUAGUUAUUUCC [2431-2451](21/21) 3'UTR GGCAGCUUUGAGAAGAAAAAU AUUUUUCUUCUCAAAGCUGCC Mouse [537-557](21/21) 5'UTR GGGACCAUUGUACAUUAUGAA UUCAUAAUGUACAAUGGUCCC Rat [2077-2097](21/21) 3'UTR UGAUUUUUAACCACUUCCCAU AUGGGAAGUGGUUAAAAAUCA [1944-1964](21/21) 3'UTR ACAAUUGGAGAGGAAAAGAAA UUUCUUUUCCUCUCCAAUUGU Rat,Mouse [1354-1374](21/21) ORF GCGUUCUUUGUGUAUGGCAUU AAUGCCAUACACAAAGAACGC [1075-1095](21/21) ORF
Table AU: GPNMB - glycoprotein (transmembrane) nmb 19mere
GGAGACAUGAGGUGGAAAA UUUUCCACCUCAUGUCUCC [354-372](19/19) ORF CACCUCUGUUUGUAACUAA UUAGUUACAAACAGAGGUG [2673-2691](19/19) 3'UTR UCACCUCUGUUUGUAACUA UAGUUACAAACAGAGGUGA [2672-2690](19/19) 3'UTR UCACAAUUGUAGAGGGAAU AUUCCCUCUACAAUUGUGA [1303-1321](19/19) ORF CCAAAAGGAAGAUGCCAAU AUUGGCAUCUUCCUUUUGG [476-494](19/19) ORF CUGAUGAAAAUGACUGGAA UUCCAGUCAUUUUCAUCAG [307-325](19/19) ORF GGGAGCACAAUCAAUUAAA UUUAAUUGAUUGUGCUCCC [277-295](19/19) ORF GGCCCUGUAUUUAAUUAAA UUUAAUUAAAUACAGGGCC [34-52](19/19) 5'UTR CCGCUUAAUACCAUCACAU AUGUGAUGGUAUUAAGCGG [3-2I](19/19) 51UTR ACCUCUGUUUGUAACUAAA UUUAGUUACAAACAGAGGU [2674-2692](19/19) 3'UTR
Table AV: GPNMB - glycoprotein (transmembrane) nmb 21mers fcWllMHtMLI Human-52694751 ORF: 162- 1880 UGAAAGACCUUCUGCUUACAU AUGUAAGCAGAAGGUCUUUCA [254-274](21/21) ORF CCAGAGGAAUUCAGAGUUAAA UUUAACUCUGAAUUCCUCUGG [110-130](21/21) 51UTR UCACCUCUGUUUGUAACUAAA UUUAGUUACAAACAGAGGUGA [2672-2692](21/21) 31UTR CUACUAUUGAUUAGAGCCUAA UUAGGCUCUAAUCAAUAGUAG [2193-2213](21/21) 31UTR GGGAGACAUGAGGUGGAAAAA UUUUUCCACCUCAUGUCUCCC [353-373](21/21) ORF CACCUCUGUUUGUAACUAAAA UUUUAGUUACAAACAGAGGUG [2673-2693](21/21) 3'UTR CAAUGAAAGACCUUCUGCUUA UAAGCAGAAGGUCUUUCAUUG [251-2713(21/21) ORF GCCAAUGUAGUCCAGUUUCUA UAGAAACUGGACUACAUUGGC [2276-2296](21/21) 3'UTR CCAUGUUGUGAAACUGAUAAA UUUAUCAGUUUCACAACAUGG [2068-20883(21/21) 31UTR GCUGACUGUGAGACGAACCUU AAGGUUCGUCUCACAGUCAGC [1514-1534](21/21) ORF
Table AW: GPR23 - G protein-coupled receptor 23 19mers
■bMWcjjtø.M.1 AntiSense siRNA GGUGAAUUAAUGCUAGAAU AUUCUAGCAUUAAUUCACC [1150-1168](19/19) ORF GCAACACCAACAUAUUUUU AAAAAUAUGUUGGUGUUGC [1940-19583(19/19) 3'UTR
3 UGGUGAAUUAAUGCUAGAA UUCUAGCAUUAAUUCACCA [1149-1167](19/19) ORF
4 GGUUUAUCAUUCCUCUAAU AUUAGAGGAAUGAUAAACC [701-719](19/19) ORF
5 CAAUUAUAAUCACCAGCAG CUGCUGGUGAUUAUAAUUG [1776-1794](19/19) 3'UTR
6 ACAAUUAUAAUCACCAGCA UGCUGGUGAUUAUAAUUGU [1775-1793](19/19) 31UTR
7 GCCAUUGUCUAUCCUUUUC GAAAAGGAUAGACAAUGGC [481-499](19/19) ORF
8 GGCCAUUGUCUAUCCUUUU AAAAGGAUAGACAAUGGCC [480-498](19/19) ORF
9 CCUGAAUUAAUCCUUUUUG CAAAAAGGAUUAAUUCAGG [2147-2165](19/19) 3'UTR
10 GGACUGUCAUUGAGUUUAU AUAAACUCAAUGACAGUCC [1601-16191(19/19) 3'UTR
Table AX: GPR23 - G protein-coupled receptor 2321mers
Other I Muman-48853IO ORr:67
Number! Sense siRNA ΛntiSensc siRNA Sp I 1 179
1 GUGGUGAAUUAAUGCUAGAAU AUUCUAGCAUUAAUUCACCAC [1148-1168](21/21) ORF
2 CCAUUGUCUAUCCUUUUCGAU AUCGAAAAGGAUAGACAAUGG [482-502](21/21) ORF
3 GGUGGUGAAUUAAUGCUAGAA UUCUAGCAUUAAUUCACCACC [1147-1167](21/21) ORF
4 GCCUUGCAACUCUGAACUGUU AACAGUUCAGAGUUGCAAGGC [959-979](21/21) ORF
5 CGAAUUUUGGAGCCUAAUAUA UAUAUUAGGCUCCAAAAUUCG [2214-2234](21/21) 3'UTR
6 ACAAUUAUAAUCACCAGCAGU ACUGCUGGUGAUUAUAAUUGU [1775-1795](21/21) 3'UTR
7 UGGUGAAUUAAUGCUAGAAUC GAUUCUAGCAUUAAUUCACCA [1149-1169](21/21) ORF
8 CACAAUUAUAAUCACCAGCAG CUGCUGGUGAUUAUAAUUGUG [1774-17941(21/2I) S1UTR
9 ACACAAUUAUAAUCACCAGCA UGCUGGUGAUUAUAAUUGUGU [1773-1793](21/21) 3'UTR
10 CAACAAAUAAUGGUGGUGAAU AUUCACCACCAUUAUUUGUUG Mouse [1136-1156](21/21) ORF
Table AY: HAPLN4 - hyaluronan and proteoglycan link protein 4 19mers
Number! Sense siRNΛ ΛntiScnse siRNΛ lOtlicr Spllϊιιman-30794471 ϋRF:20-1228
1 ACACAUUUCUCAUCUCAUU AAUGAGAUGAGAAAUGUGU [2233-2251](19/19) 3'UTR
2 CCACACAUUUCUCAUCUCA UGAGAUGAGAAAUGUGUGG [2231-2249](19/19) 31UTR
3 GGAUACCACACAUUUCUCA UGAGAAAUGUGUGGUAUCC [2226-2244](19/19) 3'UTR
4 GGGCUCAAUAAAUCCUUGU ACAAGGAUUUAUUGAGCCC [3365-33831(19/19) 3'UTR
5 GGCGCUUAUUUUCCCUUAU AUAAGGGAAAAUAAGCGCC [1782-1800](19/19) 31UTR
6 GCGCUUAUUUUCCCUUAUG CAUAAGGGAAAAUAAGCGC [1783-1801](19/19) 3'UTR
7 GUGGUCCCUUACAAACUAA UUAGUUUGUAAGGGACCAC [1336-1354](19/19) 3'UTR
8 ACCACACAUUUCUCAUCUC GAGAUGAGAAAUGUGUGGU [2230-2248](19/19) 3'UTR
9 CACACAUUUCUCAUCUCAU AUGAGAUGAGAAAUGUGUG [2232-2250](19/19) 3'UTR
10 GGCUCAAGUGGACAAAGGU ACCUUUGUCCACUUGAGCC [267-285](19/19) ORF
Table AZ: HAPLN4 - hyaluronan and proteoglycan link protein 421mers
Other I lluman-30794471 OKF:20-
Number Sense si KNA ΛntiSense si KN A Sp I 1228
1 CCACACAUUUCUCAUCUCAUU AAUGAGAUGAGAAAUGUGUGG [2231-2251](21/21) 3'UTR
2 CCCUCUUUACUGUCACGUCAU AUGACGUGACAGUAAAGAGGG [3180-3200](21/21) 3'UTR
3 ACCACACAUUUCUCAUCUCAU AUGAGAUGAGAAAUGUGUGGU [2230-22501(21/21) 31UTR
4 UGGAUACCACACAUUUCUCAU AUGAGAAAUGUGUGGUAUCCA [2225-2245](21/21) 31UTR
CUGUGGUCCCUUACAAACUAA UUAGUUUGUAAGGGACCACAG [1334-1354](21/21) 3'UTR CACACAUUUCUCAUCUCAUUG CAAUGAGAUGAGAAAUGUGUG [2232-2252](21/21) 3'UTR GGAUACCACACAUUUCUCAUC GAUGAGAAAUGUGUGGUAUCC [2226-2246](21/21) 3'UTR ACGUUAACUCUAUGCCGGAUA UAUCCGGCAUAGAGUUAACGU [2754-2774](21/21) 31UTR GCUCAAGUGGACAAAGGUGGU ACCACCUUUGUCCACUUGAGC [268-288](21/21) ORF GGCAUGUUUGUCGUCGUCCUA UAGGACGACGACAAACAUGCC [2808-2828](21/21) 3'UTR
Table BA: IGF2 - insulin-like growth factor 2 19mers
■Ujwnmmgi OthL-r Snl l l uiiiii n-109 4X515 OK :727- 26' CCAUCACUAAAAAUCACAG CUGUGAUUUUUAGUGAUGG [1973-1991](19/19) 3'UTR CCCACACAUAAAAAAUCAA UUGAUUUUUUAUGUGUGGG [1549-15671(19/19) 3'UTR GCAACUACGAUAUCUGUAU AUACAGAUAUCGUAGUUGC [2872-2890](19/19) 31UTR GCAGUGACAAAAGCAAGAA UUCUUGCUUUUGUCACUGC [3741-37591(19/19) 3'UTR CCAAAUUUCAUGUCAAUUG CAAUUGACAUGAAAUUUGG [3596-3614](19/19) 3'UTR CCAGGCCUAAUUCCAUCUU AAGAUGGAAUUAGGCCUGG [3079-30971(19/19) 31UTR CCCGCUAAGAUUCUCCAAU AUUGGAGAAUCUUAGCGGG [2906-2924](19/19) 3'UTR GGCAAAAUAAAGGAAUUUG CAAAUUCCUUUAUUUUGCC [1741-1759](19/19) 3'UTR AGAGCCAAAUUGUCACAAU AUUGUGACAAUUUGGCUCU [4633-4651](19/19) 3'UTR CCGCUAAGAUUCUCCAAUG CAUUGGAGAAUCUUAGCGG [2907-2925](19/19) 3'UTR
Table BB: IGF2 - insulin-like growth factor 22 liners
■killiMmitKH i.iιιικτaικtM.iικ-1.^ Other I Human-l09148515 OKI :72 Sp I 1269 CCGCUAAGAUUCUCCAAUGUU AACAUUGGAGAAUCUUAGCGG [2907-2927](21/21) 3'UTR GCAUAUCUAAGCAACUACGAU AUCGUAGUUGCUUAGAUAUGC [2862-28821(21/21) 31UTR UCACUCCCUUUUCCAUCACUA UAGUGAUGGAAAAGGGAGUGA [1961-19811(21/2I) S1UTR CCCAAAUUUCAUGUCAAUUGA UCAAUUGACAUGAAAUUUGGG [3595-3615](21/21) 31UTR UCACUAAAAAUCACAGAGCAG CUGCUCUGUGAUUUUUAGUGA [1976-19961(21/2I) S1UTR CACUCCCUUUUCCAUCACUAA UUAGUGAUGGAAAAGGGAGUG [1962-1982](21/21) 3'UTR ACAACAACCCUCUUAAAACUA UAGUUUUAAGAGGGUUGUUGU [1599-16191(21/21) 3'UTR GGGCAGUGACAAAAGCAAGAA UUCUUGCUUUUGUCACUGCCC [3739-37591(21/2I) S1UTR CCCGCUAAGAUUCUCCAAUGU ACAUUGGAGAAUCUUAGCGGG [2906-29261(21/2I) S1UTR GGGCAAGUUCUUCCAAUAUGA UCAUAUUGGAAGAACUUGCCC Mouse [1056-10761(21/21) ORF
Table BC: IGFBP2 - insulin-like growth factor binding protein 2 19mers
Si-nsc siKYA CCAGUUCUGACACACGUAU AUACGUGUGUCAGAACUGG [1206-1224](19/19) 3'UTR CCAAACACCGGCAGAAAAC GUUUUCUGCCGGUGUUUGG [U52-1170](19/19) 31UTR UUCUGACACACGUAUUUAU AUAAAUACGUGUGUCAGAA [1210-12281(19/19) 3'UTR GUGUCAUCUCUUCUACAAU AUUGUAGAAGAGAUGACAC [1044-1062](19/19) ORF AGUUCUGACACACGUAUUU AAAUACGUGUGUCAGAACU [1208-1226](19/19) 3'UTR CAACCUCAAACAGUGCAAG CUUGCACUGUUUGAGGUUG Rat [930-948]( 19/19) ORF GCAUAAGAUUAAAGGAAGG CCUUCCUUUAAUCUUAUGC [1412-1430](19/19) 3'UTR
8 CAGUUCUGACACACGUAUU AAUACGUGUGUCAGAACUG [1207-1225](19/19) 3'UTR
9 GGAUUUUCCAGUUCUGACA UGUCAGAACUGGAAAAUCC [1199-1217](19/19) 3'UTR
10 CCCAACUGUGACAAGCAUG CAUGCUUGUCACAGUUGGG Rat,Mouse [904-922](19/19) ORF
Table BD: IGFBP2 - insulin-like growth factor binding protein 2 2 liners
Iluinan-55925575 ORF: I21-
Sense siRNΛ ΛntiScnsc siRNΛ
1 GCAUAAGAUUAAAGGAAGGAA UUCCUUCCUUUAAUCUUAUGC [1412-1432](21/21) 3'UTR
2 CCAGUUCUGACACACGUAUUU AAAUACGUGUGUCAGAACUGG [1206-12261(21/2I) S1UTR
3 GUUCUGACACACGUAUUUAUA UAUAAAUACGUGUGUCAGAAC [1209-1229](21/21) 3'UTR
4 CAGUUCUGACACACGUAUUUA UAAAUACGUGUGUCAGAACUG [1207-1227](21/21) 31UTR
5 UCCAGUUCUGACACACGUAUU AAUACGUGUGUCAGAACUGGA [1205-1225](21/21) 3'UTR
6 CCUCAAACAGUGCAAGAUGUC GACAUCUUGCACUGUUUGAGG Rat [933-953](21/21) ORF
7 UGCAUAAGAUUAAAGGAAGGA UCCUUCCUUUAAUCUUAUGCA [1411-1431](21/21) 31UTR
8 CCCUGUGUCCCUUUUGCAUAA UUAUGCAAAAGGGACACAGGG [1397-1417](21/21) 3'UTR
9 CCUGUGUCCCUUUUGCAUAAG CUUAUGCAAAAGGGACACAGG [1398-1418](21/21) 3'UTR
10 CUGUGUCCCUUUUGCAUAAGA UCUUAUGCAAAAGGGACACAG [1399-1419](21/21) 3'UTR
Table BE: KCNAl - potassium voltage-gated channel, shaker-related subfamily, member 1 19mers
Sense si RNA ΛntiSense siRNΛ lθther Spll luman-1 19395747 ORF: 1 106-2593
1 ACAACAGAAAACCAACAAA UUUGUUGGUUUUCUGUUGU [812-8301(19/19) 51UTR
2 GCAGCUCAAAAGACUUAAA UUUAAGUCUUUUGAGCUGC [2629-26471(19/19) 31UTR
3 GCCCUCUCAUUUAAUAUAG CUAUAUUAAAUGAGAGGGC [6647-6665](19/19) 3'UTR
4 GCACACAGAACUUGAAACA UGUUUCAAGUUCUGUGUGC [5456-5474](19/19) 31UTR
5 CUAAACAACAACAACAGAA UUCUGUUGUUGUUGUUUAG [802-820](19/19) 5'UTR
6 AGACGGACUUCUUCAAAAA UUUUUGAAGAAGUCCGUCU [1842-18601(19/19) ORF
7 GGAUGACUAUUUUUUGGUU AACCAAAAAAUAGUCAUCC [5883-5901](19/19) 3'UTR
8 GCACAGACCUUUUGCUUUU AAAAGCAAAAGGUCUGUGC Mouse [4334-4352](19/19) 3'UTR
9 GCAGAGCUGAUUUUUUUUU AAAAAAAAAUCAGCUCUGC [4133-4151](19/19) 3'UTR
10 AGCCCUCUCAUUUAAUAUA UAUAUUAAAUGAGAGGGCU [6646-6664](19/19) 31UTR
Table BF: KCNAl - potassium voltage-gated channel, shaker-related subfamily, member 1 21mers l l uman-1 19395747
Number Sense siRNΛ ΛntiSense siRNΛ ORF: 1 106-2593
1 CAACAACAGAAAACCAACAAA UUUGUUGGUUUUCUGUUGUUG [810-830](21/21) 51UTR
2 ACAACAACAGAAAACCAACAA UUGUUGGUUUUCUGUUGUUGU [809-829](21/21) 51UTR
3 GCAGCUCAAAAGACUUAAAAA UUUUUAAGUCUUUUGAGCUGC [2629-26491(21/21) 31UTR
4 CCCUGAAGAAACUAAACCAAU AUUGGUUUAGUUUCUUCAGGG [4205-4225](21/21) 3'UTR
5 GCAUGUUCUCUUAUUCAGCAU AUGCUGAAUAAGAGAACAUGC [3685-37051(21/21) 31UTR
6 CAACAACAACAGAAAACCAAC GUUGGUUUUCUGUUGUUGUUG [807-827](21/21) 5'UTR
7 ACAGAAAACCUAGUGACUCAU AUGAGUCACUAGGUUUUCUGU [2655-2675](21/21) 3'UTR
8 CCUAGCCCUCUCAUUUAAUAU AUAUUAAAUGAGAGGGCUAGG [6643-6663](21/21) 3'UTR
9 GGGAUGACUAUUUUUUGGUUU AAACCAAAAAAUAGUCAUCCC [5882-5902](21/21) 3'UTR
10 CGCUCUCUCAUUUCACCACUG CAGUGGUGAAAUGAGAGAGCG [4367-4387](21/21) 3'UTR
Table BG: KlFSA - kinesin family member SA 19mers
KfflHHΪBHl Other SnI I Iu ιniin-454-16748 ORI- :209-330:
1 GGCUGCUAAUUGAGACAUA UAUGUCUCAAUUAGCAGCC [3871-3889](19/19) 31UTR
2 GAAAUUUACCUGGACAAAA UUUUGUCCAGGUAAAUUUC [617-635](19/19) ORF
3 UGAAAUUUACCUGGACAAA UUUGUCCAGGUAAAUUUCA [616-634](19/19) ORF
4 UGCUCACCAUCCAGUUAUA UAUAACUGGAUGGUGAGCA [1118-1136](19/19) ORF
5 UUGAAAUUUACCUGGACAA UUGUCCAGGUAAAUUUCAA [615-633](19/19) ORF
6 GCUGCUAAUUGAGACAUAU AUAUGUCUCAAUUAGCAGC [3872-3890](19/19) 3'UTR
7 AGAGAUGACAAAAGGAAAA UUUUCCUUUUGUCAUCUCU [3673-3691](19/19) 3'UTR
8 ACAACGAAUGUAGCAUCAA UUGAUGCUACAUUCGUUGU [222-240](19/19) ORF
9 CAACAACGAAUGUAGCAUC GAUGCUACAUUCGUUGUUG [220-238](19/19) ORF
10 GGCAAAGAAUAUCAACAAG CUUGUUGAUAUUCUUUGCC Mouse [961-979](19/19) ORF
Table BH: KIF5A - kinesin family member 5A 21 mere
H uman-45446748 ORI :209- miiMUIIMkgl
3307
1 GGCUGCUAAUUGAGACAUAUA UAUAUGUCUCAAUUAGCAGCC [3871-3891](21/21) 3'UTR
2 CAACAACGAAUGUAGCAUCAA UUGAUGCUACAUUCGUUGUUG [220-2401(21/21) ORF
3 CGAGGCAAAGAAUAUCAACAA UUGUUGAUAUUCUUUGCCUCG Mouse [958-9781(21/21) ORF
4 CUUUGAAAUUUACCUGGACAA UUGUCCAGGUAAAUUUCAAAG [613-6331(21/21) ORF
5 GGGCUGCUAAUUGAGACAUAU AUAUGUCUCAAUUAGCAGCCC [3870-3890](21/21) 31UTR
6 CCAUAUGUUUUUGACCGUGUA UACACGGUCAAAAACAUAUGG [344-364](21/21) ORF
7 GCAAAGAAUAUCAACAAGUCA UGACUUGUUGAUAUUCUUUGC [962-9821(21/21) ORF
8 GGACGAGGCAAAGAAUAUCAA UUGAUAUUCUUUGCCUCGUCC Mouse [955-975](21/21) ORF
9 CCGAGACAUCUUCAACCACAU AUGUGGUUGAAGAUGUCUCGG Rat [547-567](21/21) ORF
10 CAGAGAUGACAAAAGGAAAAA UUUUUCCUUUUGUCAUCUCUG [3672-36921(21/2I) S1UTR
Table BI: MAPKlO - mitogen-activated protein kinase 10 19mers
N umber rcroπτ*m*sιi Other Sp llluman-20986504 ORI :224-149
1 CCAUCUCAGAUCUUCACUA UAGUGAAGAUCUGAGAUGG [131-1491(19/19) 5'UTR
2 GACUAAAAAUGGUGUAGUA UACUACACCAUUUUUAGUC [1435-1453](19/19) ORF
3 GCAAAAGCAAAGUUGACAA UUGUCAACUUUGCUUUUGC [342-360](19/19) ORF
4 CCAGAAUCUAAAGCCUAUU AAUAGGCUUUAGAUUCUGG [415-433](19/19) ORF
5 GCCUCCAUUUCUUAUACUA UAGUAUAAGAAAUGGAGGC Rat,Mouse [228-246](19/19) ORF
6 CCAAACAUUACAACAUGAG CUCAUGUUGUAAUGUUUGG Mouse [324-342](19/19) ORF
7 AGACUAAAAAUGGUGUAGU ACUACACCAUUUUUAGUCU [1434-1452](19/19) ORF
8 GGAUGAAAGAGAACACACA UGUGUGUUCUCUUUCAUCC [1363-1381](19/19) ORF
9 GCAAAGUUGACAACCAGUU AACUGGUUGUCAACUUUGC [348-3661(19/19) ORF
10 ACCUGUAACUGCCCUUUUA UAAAAGGGCAGUUACAGGU [1949-1967](19/19) 3'UTR
Table BJ: MAPKlO - mitogen-activated protein kinase 10 21mers
Other I l l uman-2()986504 OR] :224-
Number! Sense siRNΛ AntiSense siRNΛ Sp I 1492
1 CCAAAUGUUGUGUGGCAUUAA UUAAUGCCACACAACAUUUGG [736-756](21/21) ORF
2 CCCAUCUCAGAUCUUCACUAU AUAGUGAAGAUCUGAGAUGGG [130-1501(21/2I) S1UTR
3 AGAUCUUCACUAUGGCAACUU AAGUUGCCAUAGUGAAGAUCU [138-158](21/21) 51UTR
4 CCAUCUCAGAUCUUCACUAUG CAUAGUGAAGAUCUGAGAUGG [131-1511(21/21) 5'UTR
5 CCAAUGAUGCUUACUACAGAA UUCUGUAGUAAGCAUCAUUGG [1861-1881](21/21) 31UTR
6 GGCCCAUCUCAGAUCUUCACU AGUGAAGAUCUGAGAUGGGCC [128-1481(21/2I) S1UTR
7 GGAAGUAAUGAAUUCAGAAGA UCUUCUGAAUUCAUUACUUCC [1411-1431](21/21) ORF
8 GGAUGAAAGAGAACACACAAU AUUGUGUGUUCUCUUUCAUCC [1363-13831(21/21) ORF
9 AGGUAAUUGAACAACUAGGAA UUCCUAGUUGUUCAAUUACCU [1044-1064](21/21) ORF
10 CCAGGAAGGGACUAUAUUGAC GUCAAUAUAGUCCCUUCCUGG [1013-1033](21/21) ORF
Table BK: MEF2C - myocyte enhancer factor 2C 19mers
Sense siRNΛ ΛntiScnse siRN.Λ lθtlier Spl l lιιman- l 9923214 ORI :402- 1823
1 GGACGAAGUAAAGAUUAUU AAUAAUCUUUACUUCGUCC [159-177](19/19) 51UTR
2 CAGGAAAAUUAACGAAGAU AUCUUCGUUAAUUUUCCUG [752-770](19/19) ORF
3 GGAAUGAAUAACCGUAAAC GUUUACGGUUAUUCAUUCC [1140-1158](19/19) ORF
4 CGAAGAUAUUGAUCUAAUG CAUUAGAUCAAUAUCUUCG [764-782](19/19) ORF
5 UCCACAAUUAGACCACAAU AUUGUGGUCUAAUUGUGGA [3999-4017](19/19) 3'UTR
6 UGGACGAAGUAAAGAUUAU AUAAUCUUUACUUCGUCCA [158-176](19/19) 5'UTR
7 AGGAAUGAAUAACCGUAAA UUUACGGUUAUUCAUUCCU [1139-1157](19/19) ORF
8 GCCAUAAGAAAGUCAAUAU AUAUUGACUUUCUUAUGGC [3699-3717](19/19) 3'UTR
9 CCCUUUCUGAUCUCACCAU AUGGUGAGAUCAGAAAGGG [3138-3156](19/19) 3'UTR
10 ACAGGAAAAUUAACGAAGA UCUUCGUUAAUUUUCCUGU [751-769](19/19) ORF
Table BL: MEF2C - myocyte enhancer factor 2C 21 mers
Other I ll uman-199232 l 4 ORF:402-
Sense siRNΛ AntiSense siRNΛ Sp I 1823
1 GGACAAGUACAGGAAAAUUAA UUAAUUUUCCUGUACUUGUCC [743-763](21/21) ORF
2 CGUGGAGACGUUGAGAAAGAA UUCUUUCUCAACGUCUCCACG [653-6731(21/21) ORF
3 CCAUUUCAAGAAAUCCCAUAA UUAUGGGAUUUCUUGAAAUGG [3581-36011(21/20 3TJTR
4 GGACGAAGUAAAGAUUAUUGU ACAAUAAUCUUUACUUCGUCC [159-179](21/21) 51UTR
5 GGGCUGCUAUUGGAUUGACUU AAGUCAAUCCAAUAGCAGCCC Rat [74-94](21/21) 51UTR
6 CCCUUUCUGAUCUCACCAUUU AAAUGGUGAGAUCAGAAAGGG [3138-3158](21/21) 31UTR
7 CUGGACGAAGUAAAGAUUAUU AAUAAUCUUUACUUCGUCCAG [157-1771(21/2I) S1UTR
8 UGCUAUGUGUUUCUCAUUGUA UACAAUGAGAAACACAUAGCA [3904-3924](21/21) 31UTR
9 CUCUUGUCUAAUAUUCGUCUA UAGACGAAUAUUAGACAAGAG [3642-3662](21/21) 3'UTR
10 CCAGAAUAGAAAAGCUAUGUU AACAUAGCUUUUCUAUUCUGG [3265-3285](21/21) 3'UTR
Table BM: NAPB - N-ethylmaleimide-sensitive factor attachment protein, beta 19mers
Number Sense siRNΛ AntiSense siRNΛ [)5 ORI :43-939
1 GGCUUAAUUUUGUUGCAUA UAUGCAACAAAAUUAAGCC [1025-1043](19/19) 31UTR
2 GCAAACUUACUUCCUUUAA UUAAAGGAAGUAAGUUUGC [3233-3251](19/19) 3'UTR
3 ACGUGUUUUUAUGUCCUUU AAAGGACAUAAAAACACGU [1245-1263](19/19) 31UTR
4 CCACGUGUUUUUAUGUCCU AGGACAUAAAAACACGUGG [1243-1261](19/19) 3'UTR
5 CCCUCAUAUUUCAUUGGUA UACCAAUGAAAUAUGAGGG [1199-1217](19/19) 3'UTR
6 CGAGUUGAAUGCCAAGCUU AAGCUUGGCAUUCAACUCG [693-711](19/19) ORF
7 CCAUGUGUAACAAUAAAUC GAUUUAUUGUUACACAUGG [3755-3773](19/19) 3'UTR
8 GGACUUGAUUGUUCUCUUA UAAGAGAACAAUCAAGUCC [1978-19961(19/19) 3'UTR
9 ACAUGUGCAUGGCAAAAAU AUUUUUGCCAUGCACAUGU [1487-1505](19/19) 3'UTR
10 UGGCUUAAUUUUGUUGCAU AUGCAACAAAAUUAAGCCA [1024-1042](19/19) 3'UTR
Table BN NAPB - N-ethylmaleimide-sensitive factor attachment protein, beta 21mers
Human-44917G05 ORF:43-
Sense siRNA ΛntiSense siRNA 939
1 CCACGUGUUUUUAUGUCCUUU AAAGGACAUAAAAACACGUGG [1243-12631(21/2I) S1UTR
2 GCCACGUGUUUUUAUGUCCUU AAGGACAUAAAAACACGUGGC [1242-1262](21/21) 3'UTR
3 CACGUGUUUUUAUGUCCUUUU AAAAGGACAUAAAAACACGUG [1244-1264](21/21) 3'UTR
4 GGCUUAAUUUUGUUGCAUAUG CAUAUGCAACAAAAUUAAGCC [1025-10451(21/2I) S1UTR
5 CCCUCAUAUUUCAUUGGUAUU AAUACCAAUGAAAUAUGAGGG [1199-12191(21/21) 3'UTR
6 GGAGACCUAAAAUGAAAUGUU AACAUUUCAUUUUAGGUCUCC [925-945](21/21) ORF+3'UTR
7 CCAAGUUCUGAGAUUCUUAAA UUUAAGAAUCUCAGAACUUGG [2659-26791(21/20 31UTR
8 GAGACUGAACUUGUAGACAUU AAUGUCUACAAGUUCAGUCUC [436-456](21/21) ORF
9 CAAACCUUUGUGACUUCCUUU AAAGGAAGUCACAAAGGUUUG [3605-3625](21/21) 31UTR
10 ACCUGCCAGAAGAUUAAAAAA UUUUUUAAUCUUCUGGCAGGU [3181-3201](21/21) 3'UTR
Table BO: NOSl - nitric oxide synthase 1 19mers
Sense siKNA ΛntiSense siRNA Other Sn | l lum:in-I HK35172 ORI :6X6~I990
1 GCGAUGCAAUGCUUUUCUA UAGAAAAGCAUUGCAUCGC [5185-52031(19/19) 3'UTR
2 CCAUCUUCCAGGCCUUCAA UUGAAGGCCUGGAAGAUGG Rat,Mouse [3966-3984](19/19) ORF
3 UCAGUCCCUACAUAUUUAU AUAAAUAUGUAGGGACUGA [157-175](19/19) 5'UTR
4 GCCUGGGAUUUCUGGUGAA UUCACCAGAAAUCCCAGGC [765-7831(19/19) ORF
5 GGAAGCUAGUUACCAUGGA UCCAUGGUAACUAGCUUCC [672-6901(19/19) 51UTR-K)RF
6 GCAGUUGAUCUCCAAAGUU AACUUUGGAGAUCAACUGC [6270-6288](19/19) 31UTR
7 CGAUGCAAUGCUUUUCUAA UUAGAAAAGCAUUGCAUCG [5186-5204](19/19) 31UTR
8 GCGUAUUCAUCAGCCGGAU AUCCGGCUGAUGAAUACGC [4833-4851](19/19) ORF
9 CUAUCCAAUGUCCACAAAA UUUUGUGGACAUUGGAUAG [3650-36681(19/19) ORF
10 GUCUAUCCAAUGUCCACAA UUGUGGACAUUGGAUAGAC [3648-3666](19/19) ORF
1 GCAGUUGAUCUCCAAAGUUUU AAAACUUUGGAGAUCAACUGC [6270-6290](21/21) 3'UTR
2 GCAAUUUGAUAUCCAACACAA UUGUGUUGGAUAUCAAAUUGC [4489-4509](21/21) ORF
3 GGAGAUGAUGUCAACAUUGAA UUCAAUGUUGACAUCAUCUCC [3533-35531(21/21) ORF
4 GGAAGAGGUGAACAAAGAGAU AUCUCUUUGUUCACCUCUUCC [1843-1863](21/21) ORF
5 CCCAUUAAAGACAAAUUGAUC GAUCAAUUUGUCUUUAAUGGG [7094-71141(21/2I) S1UTR
6 CUAUCCAAUGUCCACAAAAAG CUUUUUGUGGACAUUGGAUAG [3650-3670](21/21) ORF
7 GUCUAUCCAAUGUCCACAAAA UUUUGUGGACAUUGGAUAGAC [3648-3668](21/21) ORF
8 GGUCUAUCCAAUGUCCACAAA UUUGUGGACAUUGGAUAGACC [3647-3667](21/21) ORF
9 GGAGGGACAUUAAGAAAUAAA UUUAUUUCUUAAUGUCCCUCC [104-124](21/21) 51UTR
10 GCUCAGUCCCUACAUAUUUAU AUAAAUAUGUAGGGACUGAGC [155-1751(21/21) 51UTR
Table BQ: NPTX2 - neuronal pentraxin II 19mers
Sense siRNΛ ΛntiSense siRNΛ Other Sp |lluman-28l 95383 ORI': l66-l461
1 UCACUAGCUAUGGCAUUAA UUAAUGCCAUAGCUAGUGA [2177-2195](19/19) 31UTR
2 CGAGAACUCUUCUGAUGAU AUCAUCAGAAGAGUUCUCG [2653-2671](19/19) 31UTR
3 ACAGAACUCCUCAAGAAAA UUUUCUUGAGGAGUUCUGU [2418-2436](19/19) 31UTR
4 CUCAGCCAGUUCAACAUAU AUAUGUUGAACUGGCUGAG Mouse [1282-1300](19/19) ORF
5 CCCACUUAAACUCUUGUCA UGACAAGAGUUUAAGUGGG [1530-1548](19/19) 31UTR
6 UCCGCACAAACUACCUAUA UAUAGGUAGUUUGUGCGGA [857-875](19/19) ORF
7 ACAAGAAAUUGUCAACAUC GAUGUUGACAAUUUCUUGU [1320-13381(19/19) ORF
8 GCCAGUUCAACAUAUGGGA UCCCAUAUGUUGAACUGGC Rat,Mouse [1286-1304](19/19) ORF
9 AGCUCAGCCAGUUCAACAU AUGUUGAACUGGCUGAGCU Mouse [1280-12981(19/19) ORF
10 UCAUCACUAGCUAUGGCAU AUGCCAUAGCUAGUGAUGA [2174-2192](19/19) 3'UTR
Table BR: NPTX2 - neuronal pentraxin II 21mers
Other I lluιnan-28195383 ORI : 166-
Sense siRNΛ ΛntiSense siRNΛ Sp I 1461
1 UCACUAGCUAUGGCAUUAAAA UUUUAAUGCCAUAGCUAGUGA [2177-21971(21/21) 31UTR
2 AGAAAUUGUCAACAUCGCCAA UUGGCGAUGUUGACAAUUUCU [1323-1343](21/21) ORF
3 CAUCACUAGCUAUGGCAUUAA UUAAUGCCAUAGCUAGUGAUG [2175-21951(21/21) 31UTR
4 UGACAUUGUAUGCUACGAGAA UUCUCGUAGCAUACAAUGUCA [2638-2658](21/21) 31UTR
5 UCAUCACUAGCUAUGGCAUUA UAAUGCCAUAGCUAGUGAUGA [2174-2194](21/21) 31UTR
6 GAAUCAUCACUAGCUAUGGCA UGCCAUAGCUAGUGAUGAUUC [2171-2191](21/21) 31UTR
7 CUACAGAACUCCUCAAGAAAA UUUUCUUGAGGAGUUCUGUAG [2416-2436](21/21) 3XJTR
8 GCUCAGCCAGUUCAACAUAUG CAUAUGUUGAACUGGCUGAGC Mouse [1281-1301](21/21) ORF
9 GAGCUCAGCCAGUUCAACAUA UAUGUUGAACUGGCUGAGCUC Mouse [1279-1299](21/21) ORF
10 GGAGCUCAGCCAGUUCAACAU AUGUUGAACUGGCUGAGCUCC [1278-1298](21/21) ORF
Table BS: NRGN - neurogranin 19mers
N u in her Sense siRNΛ ΛntiSense siRNΛ Other Sp
1 CCAAAAUCCAGGCGAGUUU AAACUCGCCUGGAUUUUGG Rat,Mouse [328-346](19/19) ORF
2 ACGACGACAUUCUAGACAU AUGUCUAGAAUGUCGUCGU [274-292](19/19) ORF
3 GUGAACAAUAAAGAGGAAU AUUCCUCUUUAUUGUUCAC Rat [1271-1289](19/19) 31UTR
4 CCGAAAGGUUUCUGAUCUC GAGAUCAGAAACCUUUCGG [1220-12381(19/19) 31UTR
5 GCCGAAAGGUUUCUGAUCU AGAUCAGAAACCUUUCGGC [1219-1237](19/19) 31UTR
6 GCAUUUUCAAAGUUCCCGA UCGGGAACUUUGAAAAUGC [485-503](19/19) 31UTR
7 CGGACGACGACAUUCUAGA UCUAGAAUGUCGUCGUCCG [271-289](19/19) ORF
8 GACGACGACAUUCUAGACA UGUCUAGAAUGUCGUCGUC [273-291](19/19) ORF
9 CCAGAACUGAGCAUUUUCA UGAAAAUGCUCAGUUCUGG [475-493](19/19) 31UTR
10 AGCACACUCACUUAAAGAA UUCUUUAAGUGAGUGUGCU [855-8731(19/19) 31UTR
Table BT: NRGN - neurogranin 21mcrs
I lum:in-5453799 ORK:237-
Sense siRNΛ ΛntiSense siRNΛ
473
1 GGACGACGACAUUCUAGACAU AUGUCUAGAAUGUCGUCGUCC [272-292](21/21) ORF
2 UGAACAAUAAAGAGGAAUGUC GACAUUCCUCUUUAUUGUUCA Rat [1272-12921(21/20 31UTR
3 GUGAACAAUAAAGAGGAAUGU ACAUUCCUCUUUAUUGUUCAC Rat [1271-12911(21/2I) S1UTR
4 GGCCGAAAGGUUUCUGAUCUC GAGAUCAGAAACCUUUCGGCC [1218-12381(21/2I) S1UTR
5 CCAAAAUCCAGGCGAGUUUUC GAAAACUCGCCUGGAUUUUGG Rat [328-348](21/21) ORF
6 GAAUGUGAACAAUAAAGAGGA UCCUCUUUAUUGUUCACAUUC Rat [1267-1287](21/21) 3'UTR
7 UGGAAUGUGAACAAUAAAGAG CUCUUUAUUGUUCACAUUCCA Rat [1265-12851(21/2I) S1UTR
8 CCAGCUCUCUUGUUUAUGCAA UUGCAUAAACAAGAGAGCUGG [1099-11191(21/2031UTR
9 CCCUUUUAGUUAGUUCUGCAG CUGCAGAACUAACUAAAAGGG [660-680](21/21) 31UTR
10 GCACACUCACUUAAAGAAAAA UUUUUCUUUAAGUGAGUGUGC [856-876](21/21) 3'UTR
Table BU: NTS - neurotensin 19mers
CTWIiWB Sense siRNΛ AntiScnse siR.NA IOthcr Snllluman-3l5635l6 ORF:108-620
1 AGACCCUACAUACUCAAAA UUUUGAGUAUGUAGGGUCU [582-600](19/19) ORF
2 GACCCUACAUACUCAAAAG CUUUUGAGUAUGUAGGGUC [583-601](19/19) ORF
3 ACCCUACAUACUCAAAAGA UCUUUUGAGUAUGUAGGGU [584-602](19/19) ORF
4 GCAUAUCAUUCACCCAAAA UUUUGGGUGAAUGAUAUGC [965-9831(19/19) 31UTR
5 GGAAGAUAUUCUUGAUACU AGUAUCAAGAAUAUCUUCC [476-494](19/19) ORF
6 CAAAAAUGGAAAGGAAGAA UUCUUCCUUUCCAUUUUUG [503-521](19/19) ORF
7 CAGGAAGAUAUUCUUGAUA UAUCAAGAAUAUCUUCCUG [474-492](19/19) ORF
8 AGAAGUUCAUGAAGAGGAG CUCCUCUUCAUGAACUUCU [335-3531(19/19) ORF
9 GAGAAGUUCAUGAAGAGGA UCCUCUUCAUGAACUUCUC [334-352](19/19) ORF
10 GAAAGAUGAUGGCAGGAAU AUUCCUGCCAUCAUCUUUC [103-121](19/19) 5'UTR+ORF
Table BV: NTS — neurotensin 21mers
Other I Ilunian-31563516 ORI-": 1(M- ftumbcrl Sense siRNΛ AntiScnse siR.NA Sp I 620
1 GGAAGAAGUCAUAAAGAGAAA UUUCUCUUUAUGACUUCUUCC [515-5351(21/21) ORF
2 GGAGAAGUUCAUGAAGAGGAG CUCCUCUUCAUGAACUUCUCC [333-353](21/21) ORF
3 GCAGAUUUCUUGACCAAUAUG CAUAUUGGUCAAGAAAUCUGC [210-2301(21/21) ORF
4 AGAAGACCCUACAUACUCAAA UUUGAGUAUGUAGGGUCUUCU [579-5991(21/21) ORF
5 ACAGGAGAAGUUCAUGAAGAG CUCUUCAUGAACUUCUCCUGU [330-3501(21/2I) ORF
6 AGCAGAUUUCUUGACCAAUAU AUAUUGGUCAAGAAAUCUGCU [209-2291(21/21) ORF
7 AGACCCUACAUACUCAAAAGA UCUUUUGAGUAUGUAGGGUCU [582-602](21/21) ORF
8 CAGAAGACCCUACAUACUCAA UUGAGUAUGUAGGGUCUUCUG [578-5981(21/21) ORF
GAAGACCCUACAUACUCAAAA UUUUGAGUAUGUAGGGUCUUC [580-600](21/21) ORF CCAGAAGACCCUACAUACUCA UGAGUAUGUAGGGUCUUCUGG [577-597](21/21) ORF
Table BW: NUCBl - nucleobindin 1 19mers
SEflEIKIlMKH fcτπιvmιnj.iir*»» Other SnI umun-39725676 OK : S- -40. AGAAAGUGUACGACCCAAA UUUGGGUCGUACACUUUCU [838-856](19/19) ORF GAGAAAGUGUACGACCCAA UUGGGUCGUACACUUUCUC [837-855](19/19) ORF UUCCAUCCAAAUACACUUU AAAGUGUAUUUGGAUGGAA [2222-2240](19/19) 31UTR GGAGAAAGUGUACGACCCA UGGGUCGUACACUUUCUCC [836-854](19/19) ORF AAGUGUACGACCCAAAGAA UUCUUUGGGUCGUACACUU [841-859](19/19) ORF GUUCCAUCCAAAUACACUU AAGUGUAUUUGGAUGGAAC [2221-2239](19/19) 3'UTR AGAAUGAGGAGGACGACAU AUGUCGUCCUCCUCAUUCU [856-874](19/19) ORF GCUGGAGAAAGUGUACGAC GUCGUACACUUUCUCCAGC [833-851](19/19) ORF AGCUGGAGAAAGUGUACGA UCGUACACUUUCUCCAGCU [832-850](19/19) ORF GGAGCUGGAGAAAGUGUAC GUACACUUUCUCCAGCUCC [830-848](19/19) ORF
Table BX: NUCBl - nucleobindin 1 21 mers
I luniiin-39725676 OKI- : 18-
BSSfflSkSl Rττiιvrιiιi.τa.iitt^» 1403 GGAGAAAGUGUACGACCCAAA UUUGGGUCGUACACUUUCUCC [836-8561(21/21) ORF GGUUCCAUCCAAAUACACUUU AAAGUGUAUUUGGAUGGAACC [2220-2240](21/21) 3'UTR AAAGUGUACGACCCAAAGAAU AUUCUUUGGGUCGUACACUUU [840-8601(21/21) ORF GAAAGUGUACGACCCAAAGAA UUCUUUGGGUCGUACACUUUC [839-8591(21/21) ORF GAGAAAGUGUACGACCCAAAG CUUUGGGUCGUACACUUUCUC [837-857](21/21) ORF GGAGCUGGAGAAAGUGUACGA UCGUACACUUUCUCCAGCUCC [830-850](21/21) ORF CCAAAGAAUGAGGAGGACGAC GUCGUCCUCCUCAUUCUUUGG [852-8721(21/2I) ORF UCAUGAAGAGUUCAAGCGCUA UAGCGCUUGAACUCUUCAUGA [533-5531(21/2I) ORF GCAUGUGAUGAAGAAUGUGGA UCCACAUUCUUCAUCACAUGC [911-931](21/21) ORF GCUCAGUGAUCGGCUUAACUU AAGUUAAGCCGAUCACUGAGC [1651-16711(21/21) 31UTR
Table BY: PCP4 - Purkinje cell protein 4 19mers
Sense si KNA ΛntiSense siKNΛ CCAAUGGAAAAGACAAGAC GUCUUGUCUUUUCCAUUGG Rat [110-1281(19/19) ORF GGCAACCAAUGGAAAAGAC GUCUUUUCCAUUGGUUGCC [105-1231(19/19) ORF GGGCAACCAAUGGAAAAGA UCUUUUCCAUUGGUUGCCC [104-122](19/19) ORF CAAUGGAAAAGACAAGACA UGUCUUGUCUUUUCCAUUG Rat [111-129](19/19) ORF GCAACCAAUGGAAAAGACA UGUCUUUUCCAUUGGUUGC [106-124](19/19) ORF CGCAUAGCAAACCUCCAAU AUUGGAGGUUUGCUAUGCG [381-399](19/19) 3'UTR CAACCAAUGGAAAAGACAA UUGUCUUUUCCAUUGGUUG [107-125](19/19) ORF ACCAAUGGAAAAGACAAGA UCUUGUCUUUUCCAUUGGU Rat [109-127](19/19) ORF ACGCAUAGCAAACCUCCAA UUGGAGGUUUGCUAUGCGU [380-398](19/19) 3'UTR GGAAUUGUGAGUAGCUUAA UUAAGCUACUCACAAUUCC [452-470](19/19) 3'UTR
Table BZ: PCP4 - Purkinje cell protein 421mers luman-48762917 OR :82-
■LflllMIHikll iMinι»a«reπ-κm««
1 CCAAUGGAAAAGACAAGACAU AUGUCUUGUCUUUUCCAUUGG Rat [110-130](21/21) ORF
2 GGGCAACCAAUGGAAAAGACA UGUCUUUUCCAUUGGUUGCCC [104-124](21/21) ORF
3 GGCAACCAAUGGAAAAGACAA UUGUCUUUUCCAUUGGUUGCC [105-125](21/21) ORF
4 CCAUCAUCUGUCAAGAAAUUA UAAUUUCUUGACAGAUGAUGG [314-334](21/21) 3'UTR
5 GCAACCAAUGGAAAAGACAAG CUUGUCUUUUCCAUUGGUUGC [106-126](21/21) ORF
6 ACCAAUGGAAAAGACAAGACA UGUCUUGUCUUUUCCAUUGGU Rat [109-129](21/21) ORF
7 CAACCAAUGGAAAAGACAAGA UCUUGUCUUUUCCAUUGGUUG [107-127](21/21) ORF
8 CCUCCAAUGCAUGUACAGAAA UUUCUGUACAUGCAUUGGAGG [392-412](21/21) 31UTR
9 CACGCAUAGCAAACCUCCAAU AUUGGAGGUUUGCUAUGCGUG [379-399](21/21) 31UTR
10 GACAAGACAUCUGGUGAAAAU AUUUUCACCAGAUGUCUUGUC [121-1411(21/21) ORF
Table CA: PDCD2 - programmed cell death 2 19mers
MUMMUIH-Ul ΛntiSense siRNΛ LiIliQmaπ |i
1 CCAGGAAAAACGAUUUUUA UAAAAAUCGUUUUUCCUGG [381-399](19/19) ORF
2 CCCAGGAAAAACGAUUUUU AAAAAUCGUUUUUCCUGGG [380-3981(19/19) ORF
3 GCAUCUUAAAGCCUUGAAA UUUCAAGGCUUUAAGAUGC [1117-1135](19/19) 3'UTR
4 GGAAAAACGAUUUUUACUC GAGUAAAAAUCGUUUUUCC [384-402](19/19) ORF
5 ACCCAGGAAAAACGAUUUU AAAAUCGUUUUUCCUGGGU [379-397](19/19) ORF
6 GCGAGUUUUUAGGAAUCAA UUGAUUCCUAAAAACUCGC [358-376](19/19) ORF
7 CGAGUUUUUAGGAAUCAAC GUUGAUUCCUAAAAACUCG [359-377](19/19) ORF
8 CUACCCAGGAAAAACGAUU AAUCGUUUUUCCUGGGUAG [377-3951(19/19) ORF
9 CCAGAAACAGGAGAAUCAG CUGAUUCUCCUGUUUCUGG [431-449](19/19) ORF
10 AGCUCCUAAACUACCUGAA UUCAGGUAGUUUAGGAGCU [966-984](19/19) ORF
Table CB: PDCD2 - programmed cell death 2 2Imers
KHJHΪHBKI l luman-21735591 ORI :80-
BiIIiMUiKf ».iιnk.-ni 1 1 14
1 ACCCAGGAAAAACGAUUUUUA UAAAAAUCGUUUUUCCUGGGU [379-3991(21/21) ORF
2 CCCAGGAAAAACGAUUUUUAC GUAAAAAUCGUUUUUCCUGGG [380-400](21/21) ORF
3 CUACCCAGGAAAAACGAUUUU AAAAUCGUUUUUCCUGGGUAG [377-397](21/21) ORF
4 GCAUCUUAAAGCCUUGAAAAA UUUUUCAAGGCUUUAAGAUGC [1117-11371(21/20 31UTR
5 CGAGUUUUUAGGAAUCAACUA UAGUUGAUUCCUAAAAACUCG [359-379](21/21) ORF
6 CCAGGAAAAACGAUUUUUACU AGUAAAAAUCGUUUUUCCUGG [381-401](21/21) ORF
7 UCAACUACCCAGGAAAAACGA UCGUUUUUCCUGGGUAGUUGA [373-393](21/21) ORF
8 GCGAGUUUUUAGGAAUCAACU AGUUGAUUCCUAAAAACUCGC [358-378](21/21) ORF
9 CUGCGAGUUUUUAGGAAUCAA UUGAUUCCUAAAAACUCGCAG [356-3761(21/2I) ORF
10 GGCCUGCGAGUUUUUAGGAAU AUUCCUAAAAACUCGCAGGCC [353-3731(21/2I) ORF
Table CC: PDE4D - phosphodiesterase 4D 19mers
QRmS-B Kfltsmisii Bin mgiKireπ ME»^ IOtlicr Spll luniiiιi-46361981 ORF:75-20%
1 GCCUUAACUUUAACCAAUA UAUUGGUUAAAGUUAAGGC [5561-5579](19/19) 3'UTR
2 GGCUUUACAUUUCCUAAAA UUUUAGGAAAUGUAAAGCC [4953-4971](19/19) 3'UTR
3 GGGAACUUGUUUCUUUUAA UUAAAAGAAACAAGUUCCC [4499-4517](19/19) 3'UTR
4 CACAUUUCAUGCUCAUUUU AAAAUGAGCAUGAAAUGUG [3306-3324](19/19) 3'UTR
5 CUCUAAUAAUCCACACAUU AAUGUGUGGAUUAUUAGAG [3293-3311](19/19) 3'UTR
6 GCUCUAAUAAUCCACACAU AUGUGUGGAUUAUUAGAGC [3292-3310](19/19) 3'UTR
7 CACAUUUCAUGCUCUAAUA UAUUAGAGCAUGAAAUGUG [3282-3300](19/19) 31UTR
8 CCACAGAAAUUGCAUAAUU AAUUAUGCAAUUUCUGUGG [2563-2581](19/19) 3'UTR
9 CGAUCCAUAAGCAUAUCUU AAGAUAUGCUUAUGGAUCG [3762-3780](19/19) 3'UTR
10 UGCCUUAACUUUAACCAAU AUUGGUUAAAGUUAAGGCA [5560-5578](19/19) 3'UTR
Table CD: PDE4D - phosphodiesterase 4D 21 mers l lιιman-46361981 ORI :75-
N umber! Sense siRiYΛ ΛntiSensc siRNΛ Other Sp
1 GCUCUAAUAAUCCACACAUUU AAAUGUGUGGAUUAUUAGAGC [3292-3312](21/21) 3'UTR
2 CCUUUAUCACUUAUCCUCGAU AUCGAGGAUAAGUGAUAAAGG [3745-3765](21/21) 3'UTR
3 UGCUCUAAUAAUCCACACAUU AAUGUGUGGAUUAUUAGAGCA [3291-3311](21/21) 3'UTR
4 GGAGUUCUUCUUCUUGAUAAU AUUAUCAAGAAGAAGAACUCC [1458-1478](21/21) ORF
5 GUGCCUUAACUUUAACCAAUA UAUUGGUUAAAGUUAAGGCAC [5559-55791(21/21) 3'UTR
6 CCAUCAACAAAGCCACCAUAA UUAUGGUGGCUUUGUUGAUGG [451-471](21/21) ORF
7 GCCACAGAAAUUGCAUAAUUU AAAUUAUGCAAUUUCUGUGGC [2562-2582](21/21) 3'UTR
8 CAACCAACCAUCCAUCAACAA UUGUUGAUGGAUGGUUGGUUG Rat,Mouse [440-460](21/21) ORF
9 GUGGCUUUACAUUUCCUAAAA UUUUAGGAAAUGUAAAGCCAC [4951-4971](21/21) 3'UTR
10 GCAACCAACCAUCCAUCAACA UGUUGAUGGAUGGUUGGUUGC Rat,Mouse [439-459](21/21) ORF
Table CE: PENK - proenkephalin 19mers
Sense si UNA ΛntiSense siR.NA lθtheι Spl l luninn-4()254835 ORI- :77-88<l
1 GGAGGAUUUAUGAGAUUUU AAAAUCUCAUAAAUCCUCC [860-8781(19/19) ORF
2 GGAAACUGUUGAUGGUGUU AACACCAUCAACAGUUUCC [936-954](19/19) 31UTR
3 CCAGGAUGGCAGUGAUAAU AUUAUCACUGCCAUCCUGG [592-610](19/19) ORF
4 CAGUGAUAAUGAGGAAGAA UUCUUCCUCAUUAUCACUG [601-619](19/19) ORF
5 CCAGCACCCUCAGAGAAAA UUUUCUCUGAGGGUGCUGG [318-336](19/19) ORF
6 GCAGCUAUCUCGUUUUCAU AUGAAAACGAGAUAGCUGC [1079-1097](19/19) 31UTR
7 GGGAAACUGUUGAUGGUGU ACACCAUCAACAGUUUCCC [935-9531(19/19) 31UTR
8 CCUGAAAUGGAAAAAAGAU AUCUUUUUUCCAUUUCAGG [839-857](19/19) ORF
9 AGAAAAUGGAUGAGCUUUA UAAAGCUCAUCCAUUUUCU [411-429](19/19) ORF
10 GCAAACCGGAAGAAAGCCA UGGCUUUCUUCCGGUUUGC [339-357](19/19) ORF
1 GGCAGUGAUAAUGAGGAAGAA UUCUUCCUCAUUAUCACUGCC [599-619](21/21) ORF
2 GGGAAACUGUUGAUGGUGUUU AAACACCAUCAACAGUUUCCC [935-955](21/21) 31UTR
GGAAGAAGUGAGCAAGAGAUA UAUCUCUUGCUCACUUCUUCC [613-633](21/21) ORF GCAAACCGGAAGAAAGCCAUU AAUGGCUUUCUUCCGGUUUGC [339-359](21/21) ORF GAAACUGUUGAUGGUGUUUUA UAAAACACCAUCAACAGUUUC [937-957](21/21) 3'UTR GCGUAAUGGAAUGUGAAGGUA UACCUUCACAUUCCAUUACGC [219-239](21/21) ORF GCAGUGAUAAUGAGGAAGAAG CUUCUUCCUCAUUAUCACUGC [600-620](21/21) ORF CCAGGAUGGCAGUGAUAAUGA UCAUUAUCACUGCCAUCCUGG [592-612](21/21) ORF UGGGAAACUGUUGAUGGUGUU AACACCAUCAACAGUUUCCCA [934-954](21/21) 3'UTR CGUAAUGGAAUGUGAAGGUAA UUACCUUCACAUUCCAUUACG [220-240](21/21) ORF
Table CG: PHCA - phytoceramidase, alkaline 19mers
Sense siRNA ΛntiSense sikNΛ lθther Spl l-luman-l 4 l 802267 ORF:(i9-872 CCAAAACAUUAAGAGAACU AGUUCUCUUAAUGUUUUGG [2482-25001(19/19) 3'UTR GCCAAAACAUUAAGAGAAC GUUCUCUUAAUGUUUUGGC [2481-2499](19/19) 3'UTR CCACCAAGAAAACAAACAA UUGUUUGUUUUCUUGGUGG [882-900](19/19) 3'UTR GGCCGUGAUACAUUUUUUA UAAAAAAUGUAUCACGGCC [2625-26431(19/19) 3'UTR CGGCCGUGAUACAUUUUUU AAAAAAUGUAUCACGGCCG [2624-2642](19/19) 3'UTR GCAGCAUUUUAUGGAAAUC GAUUUCCAUAAAAUGCUGC [3097-3115](19/19) 3'UTR UGCCAAAACAUUAAGAGAA UUCUCUUAAUGUUUUGGCA [2480-2498](19/19) 3'UTR GCAUAUUUUAACUGGCCUU AAGGCCAGUUAAAAUAUGC [728-7461(19/19) ORF UGCAGCAUUUUAUGGAAAU AUUUCCAUAAAAUGCUGCA [3096-3114](19/19) 3'UTR GCCGUGAUACAUUUUUUAA UUAAAAAAUGUAUCACGGC [2626-26441(19/19) 3'UTR
Table CH: PHCA - phytoceramidase, alkaline 21mers
Other I H uman-l 41802267 ORI-':69-
Sense si RNA ΛntiSense si RNA Sn I 872 GCCAAAACAUUAAGAGAACUU AAGUUCUCUUAAUGUUUUGGC [2481-2501](21/21) 31UTR CGGCCGUGAUACAUUUUUUAA UUAAAAAAUGUAUCACGGCCG [2624-2644](21/21) 3'UTR GCAGCAUUUUAUGGAAAUCAC GUGAUUUCCAUAAAAUGCUGC [3097-3117](21/21) 3'UTR GCCAUUACACUCUGAUUUUUA UAAAAAUCAGAGUGUAAUGGC [1745-17651(21/21) 3'UTR GGAAGUGUGUUGCAUUAACUA UAGUUAAUGCAACACACUUCC [1815-18351(21/2I) S1UTR GGGUUAUACAUCAUUGGGUAU AUACCCAAUGAUGUAUAACCC [590-610](21/21) ORF CCAAUAGUGAUUUGUUUGCAU AUGCAAACAAAUCACUAUUGG [3290-3310](21/21) 3'UTR CCCGCUGUCAUUUGUUUUAUU AAUAAAACAAAUGACAGCGGG [3219-32391(21/20 31UTR GGCCGUGAUACAUUUUUUAAU AUUAAAAAAUGUAUCACGGCC [2625-2645](21/21) 31UTR UGCCAAAACAUUAAGAGAACU AGUUCUCUUAAUGUUUUGGCA [2480-25001(21/21) 31UTR
Table CI: PJA2 - praja 2, RING-H2 motif containing 19mers
Sense siRNA ΛntiSense siRNΛ lθther Sp|l lιιman-142350628 ORF: l 16-2242 GGAAACAGAAAAUAACCAA UUGGUUAUUUUCUGUUUCC [1276-1294](19/19) ORF GGCUUUGGAAGUUGAGGAA UUCCUCAACUUCCAAAGCC [1159-1177](19/19) ORF CCACACAUUAUAUUUAGAC GUCUAAAUAUAAUGUGUGG [3978-3996](19/19) 3'UTR GCCACACAUUAUAUUUAGA UCUAAAUAUAAUGUGUGGC [3977-39951(19/19) 31UTR GCAUCCAUUUUCUUUAGUU AACUAAAGAAAAUGGAUGC Rat [2447-2465](19/19) 31UTR
6 GACCUCUUAAUAAAAUGUG CACAUUUUAUUAAGAGGUC [1190-1208](19/19) ORF
7 GCAUGUAAUGUGAUGAAUU AAUUCAUCACAUUACAUGC [3624-3642](19/19) 31UTR
8 CCAACUCUAUGACAAAGAU AUCUUUGUCAUAGAGUUGG [1372-1390](19/19) ORF
9 CCAAAUGACAUCAGAAAGU ACUUUCUGAUGUCAUUUGG [1291-1309](19/19) ORF
10 CACAAAGGGAAACAGAAAA UUUUCUGUUUCCCUUUGUG [1269-1287](19/19) ORF
Table CJ: PJA2 - praja 2, RING-H2 motif containing 21mers
Other I Human-142350628 ORI-": 1 16-
Number! Sense siRiVV ΛntiSense siRNΛ Sp I 2242
1 GCCACACAUUAUAUUUAGACU AGUCUAAAUAUAAUGUGUGGC [3977-3997](21/21) 3'UTR
2 CCCAUCAUUUUCUGUGUAAAU AUUUACACAGAAAAUGAUGGG [4702-4722](21/21) 31UTR
3 GCAUGUAAUGUGAUGAAUUUG CAAAUUCAUCACAUUACAUGC [3624-3644](21/21) 31UTR
4 GAAAUGAACCUGCCAAUGAAU AUUCAUUGGCAGGUUCAUUUC [1662-1682](21/21) ORF
5 GGAAAUGAACCUGCCAAUGAA UUCAUUGGCAGGUUCAUUUCC [1661-1681](21/21) ORF
6 GCAGGAGGGUAUCAGACAAUU AAUUGUCUGAUACCCUCCUGC [188-208](21/21) ORF
7 GGAAUGGCUGUGGAGAUUAUU AAUAAUCUCCACAGCCAUUCC [1350-1370](21/21) ORF
8 GGAAUGCAAGUUUUUCAAAAG CUUUUGAAAAACUUGCAUUCC [4636-4656](21/21) 31UTR
9 GCAUGCAUGUAAUGUGAUGAA UUCAUCACAUUACAUGCAUGC [3620-36401(21/20 31UTR
10 GCACAUAUGAGGCUUAAAUAU AUAUUUAAGCCUCAUAUGUGC [3492-35121(21/21) 31UTR
Table CK: PLPl - proteolipid protein 1 19mers
Number Sense siRNΛ ΛntiSense siRYΛ Other Sp ll lιιman-41349498 ORI- :171-I004
1 CCAACCUUCUGUCCAUCUG CAGAUGGACAGAAGGUUGG Rat,Mouse [835-8531(19/19) ORF
2 ACCAACCCUUCCCUGAAAA UUUUCAGGGAAGGGUUGGU [2823-2841](19/19) 31UTR 3 UAACAAAUAACCAACCCUU AAGGGUUGGUUAUUUGUUA [2814-2832](19/19) 31UTR 4 GGACAAAGAUACUCAGAGA UCUCUGAGUAUCUUUGUCC [35-531(19/19) 51UTR 5 ACAUAACAAAUAACCAACC GGUUGGUUAUUUGUUAUGU [2811-2829](19/19) 31UTR 6 GGGAAAAUGUUUUGUAAGA UCUUACAAAACAUUUUCCC [2731-2749](19/19) 31UTR 7 GGUUUUAAUUGAAGGAAAG CUUUCCUUCAAUUAAAACC [2548-2566](19/19) 31UTR 8 UGGUGUACAAAAUGGAAUU AAUUCCAUUUUGUACACCA [1447-1465](19/19) 3'UTR 9 GCACCAGUCAUCAGCUAUU AAUAGCUGAUGACUGGUGC [2409-2427](19/19) 3'UTR 10 GAAAAACAAUAAGAGCGUU AACGCUCUUAUUGUUUUUC [1515-1533](19/19) 31UTR
Table CL: PLPl - proteolipid protein 1 21 mers
Ilιiman-41349498 ORI'
1 ACAUAACAAAUAACCAACCCU AGGGUUGGUUAUUUGUUAUGU [2811-28311(21/2I) S1UTR
2 CAACCUUCUGUCCAUCUGCAA UUGCAGAUGGACAGAAGGUUG Rat,Mouse [836-856](21/21) ORF
3 ACAAAGAUACUCAGAGAGAAA UUUCUCUCUGAGUAUCUUUGU [37-57](21/21) 5'UTR
4 ACAAAUAACCAACCCUUCCCU AGGGAAGGGUUGGUUAUUUGU [2816-2836](21/21) 3'UTR
5 CAUAACAAAUAACCAACCCUU AAGGGUUGGUUAUUUGUUAUG [2812-2832](21/21) 31UTR
6 GCAGGAGAAGAGGACAAAGAU AUCUUUGUCCUCUUCUCCUGC [24-44](21/21) 51UTR
7 UAACCAACCCUUCCCUGAAAA UUUUCAGGGAAGGGUUGGUUA [2821-2841](21/21) 31UTR
8 GCAGAAACAGAUUUGGUGAAU AUUCACCAAAUCUGUUUCUGC [2249-2269](21/21) 3'UTR
9 GGAAAAUUGGACAUUAAGCAU AUGCUUAAUGUCCAAUUUUCC [2164-2184](21/21) 3'UTR
10 CCAACCUUCUGUCCAUCUGCA UGCAGAUGGACAGAAGGUUGG Rat,Mouse [835-855](21/21) ORF
Table CM: PMCH - pro-melanin-conccntrating hormone 19mers uιiiiin-71361683 OU :67-56-
1 ACCUUAUCUUGCACUAAAA UUUUAGUGCAAGAUAAGGU [375-393](19/19) ORF
2 CACUGAAUCUGGCUAUAAA UUUAUAGCCAGAUUCAGUG [356-374](19/19) ORF
3 CCACUGAAUCUGGCUAUAA UUAUAGCCAGAUUCAGUGG [355-373](19/19) ORF
4 ACAGGCUCCAAACAUAAUU AAUUAUGUUUGGAGCCUGU [319-337](19/19) ORF
5 CCCUGGAACAAUAUAAAAA UUUUUAUAUUGUUCCAGGG [251-269](19/19) ORF
6 CAGGCUCCAAACAUAAUUU AAAUUAUGUUUGGAGCCUG [320-3381(19/19) ORF
7 CGAAGAGGAAAAUAAAGUU AACUUUAUUUUCCUCUUCG [291-309](19/19) ORF
8 AACCUUAUCUUGCACUAAA UUUAGUGCAAGAUAAGGUU [374-392](19/19) ORF
9 GGAAGACACUGCAGAAAAA UUUUUCUGCAGUGUCUUCC [216-234](19/19) ORF
10 GUGCAUUAAACUUUGUGAA UUCACAAAGUUUAAUGCAC [727-7451(19/19) 31UTR
1 CCACUGAAUCUGGCUAUAAAA UUUUAUAGCCAGAUUCAGUGG [355-375](21/21) ORF
2 GGCUCCAAACAUAAUUUCUUA UAAGAAAUUAUGUUUGGAGCC [322-342](21/21) ORF
3 CUAUAAAACCUUAUCUUGCAC GUGCAAGAUAAGGUUUUAUAG [368-3881(21/21) ORF
4 CACAGGCUCCAAACAUAAUUU AAAUUAUGUUUGGAGCCUGUG [318-338](21/21) ORF
5 CCCUGGAACAAUAUAAAAAUG CAUUUUUAUAUUGUUCCAGGG [251-271](21/21) ORF
6 GCUAUAAAACCUUAUCUUGCA UGCAAGAUAAGGUUUUAUAGC [367-3871(21/2I) ORF
7 CCAAGUCCAUAAGAAAUUUAG CUAAAUUUCUUAUGGACUUGG [146-166](21/21) ORF
8 AGCAUCCAAGUCCAUAAGAAA UUUCUUAUGGACUUGGAUGCU [141-161](21/21) ORF
9 ACACAGGCUCCAAACAUAAUU AAUUAUGUUUGGAGCCUGUGU [317-3371(21/21) ORF
10 GCAUCCAAGUCCAUAAGAAAU AUUUCUUAUGGACUUGGAUGC [142-162](21/21) ORF
Table CO: PVALB - parvalbumin 19mers
WJiUMI WAl ΛΨΛ n-πnκπ-Eπ Mm^ uιmιn-5S925(ό6 OKI- :51-38.
1 CUGCUAAAGAAACCAAGAU AUCUUGGUUUCUUUAGCAG [286-3041(19/19) ORF
2 UGCUAAAGAAACCAAGAUG CAUCUUGGUUUCUUUAGCA [287-3051(19/19) ORF
3 CUGUUCAGUUCGUUUAUGU ACAUAAACGAACUGAACAG [464-482](19/19) 3'UTR
4 GGCUGCUGGAGACAAAGAU AUCUUUGUCUCCAGCAGCC [311-329](19/19) ORF
5 AAGAAACCAAGAUGCUGAU AUCAGCAUCUUGGUUUCUU [292-3101(19/19) ORF
6 GCCCUCUAAUGACACCAUU AAUGGUGUCAUUAGAGGGC [509-527](19/19) 3'UTR
7 GAAAAAGAGUGCGGAUGAU AUCAUCCGCACUCUUUUUC Rat [161-1791(19/19) ORF
8 UCUGUUCAGUUCGUUUAUG CAUAAACGAACUGAACAGA [463-4811(19/19) 31UTR
9 GUCUGCUAAAGAAACCAAG CUUGGUUUCUUUAGCAGAC [284-302](19/19) ORF
10 ACAUGCUGGACAAGGACAA UUGUCCUUGUCCAGCAUGU [196-214](19/19) ORF
Table CP: PVALB - paralbumin 21mers -
1 UAAAGAAACCAAGAUGCUGAU AUCAGCAUCUUGGUUUCUUUA [290-310](21/21) ORF
2 UGCUAAAGAAACCAAGAUGCU AGCAUCUUGGUUUCUUUAGCA [287-307](21/21) ORF
3 UCUGUUCAGUUCGUUUAUGUU AACAUAAACGAACUGAACAGA [463-4831(21/2I) S1UTR
4 CUGUUCAGUUCGUUUAUGUUA UAACAUAAACGAACUGAACAG [464-4841(21/20 31UTR
5 GGACAAGGACAAAAGUGGCUU AAGCCACUUUUGUCCUUGUCC [203-223](21/21) ORF
6 GGAGAAGCAAUAAAGGUUGUA UACAACCUUUAUUGCUUCUCC [543-563](21/21) 31UTR
7 UUCUGUUCAGUUCGUUUAUGU ACAUAAACGAACUGAACAGAA [462-482](21/21) 3'UTR
8 GUCUGCUAAAGAAACCAAGAU AUCUUGGUUUCUUUAGCAGAC [284-304](21/21) ORF
9 CACAUGCUGGACAAGGACAAA UUUGUCCUUGUCCAGCAUGUG [195-2151(21/2I) ORF
10 GGUGUUUCACAUGCUGGACAA UUGUCCAGCAUGUGAAACACC [188-208](21/21) ORF
Table CQ: QDPR - quinoid dihydropteridine reductase 19mers
Number! Sense siRNΛ ΛntiSense siRNΛ lOther Spl l luman-4506358 ORI :81 -8 I .
1 GGAGGUCUUUUAAUCUGAU AUCAGAUUAAAAGACCUCC [1484-1502](19/19) 31UTR
2 GGUCUUUUAAUCUGAUGUU AACAUCAGAUUAAAAGACC [1487-1505](19/19) 31UTR 3 GGACAAGUGUAAUACAUUU AAAUGUAUUACACUUGUCC [970-988](19/19) 31UTR 4 GUCUUUUAAUCUGAUGUUC GAACAUCAGAUUAAAAGAC [1488-1506](19/19) 31UTR 5 GAGGUCUUUUAAUCUGAUG CAUCAGAUUAAAAGACCUC [1485-15031(19/19) 31UTR 6 AGGUCUUUUAAUCUGAUGU ACAUCAGAUUAAAAGACCU [1486-1504](19/19) 31UTR 7 GGAUCGCUUUGUGACUGUU AACAGUCACAAAGCGAUCC [1096-1114](19/19) 31UTR 8 GGAUUGCUGGAGGUCUUUU AAAAGACCUCCAGCAAUCC [1476-1494](19/19) 31UTR 9 GUAUACUAGAAUCAUGGAU AUCCAUGAUUCUAGUAUAC [1461-1479](19/19) 31UTR 10 GGCUGAGUUAUUUUUGAAU AUUCAAAAAUAACUCAGCC [1184-1202](19/19) 31UTR
Table CR: QDPR - quinoid dihydropteridine reductase 21mers
Sense siRNΛ ΛntiScnsc siRNΛ .Other Spll luman-4506358 ORI :81 -81 :
1 GGAUUGCUGGAGGUCUUUUAA UUAAAAGACCUCCAGCAAUCC [1476-1496](21/21) 31UTR
2 GAGGUCUUUUAAUCUGAUGUU AACAUCAGAUUAAAAGACCUC [1485-1505](21/21) 31UTR
3 GGAGGUCUUUUAAUCUGAUGU ACAUCAGAUUAAAAGACCUCC [1484-15041(21/2I) S1UTR
4 GCACUUGUUGUCUGUCUUCUU AAGAAGACAGACAACAAGUGC [1402-1422](21/21) 31UTR
5 CGAGAUAAUGAGUCCUAUUUU AAAAUAGGACUCAUUAUCUCG [918-9381(21/2I) S1UTR
6 GGUCUUUUAAUCUGAUGUUCU AGAACAUCAGAUUAAAAGACC [1487-1507](21/21) 3'UTR
7 AGGUCUUUUAAUCUGAUGUUC GAACAUCAGAUUAAAAGACCU [1486-15061(21/20 31UTR
8 CUGGAGGUCUUUUAAUCUGAU AUCAGAUUAAAAGACCUCCAG [1482-15021(21/2I) S1UTR
9 GGACAUUUUAAAGGCUGAGUU AACUCAGCCUUUAAAAUGUCC [1172-1192](21/21) 31UTR
10 GGGACAAGUGUAAUACAUUUA UAAAUGUAUUACACUUGUCCC [969-989](21/21) 31UTR
Table CS: RPM - ribophorin 1 19mers
N u m her Sense siRNΛ ΛntiSensc siRNΛ Other Spllluman-62739176 ORI :77-1900
1 GGAACCAUUAUUUCUACUC GAGUAGAAAUAAUGGUUCC [549-567](19/19) ORF
2 GGGAACCAUUAUUUCUACU AGUAGAAAUAAUGGUUCCC [548-566](19/19) ORF
3 GGACCAUGUGUUUGAUGAA UUCAUCAAACACAUGGUCC [1144-11621(19/19) ORF
4 UGGAAGAAAAUGUGGACUU AAGUCCACAUUUUCUUCCA [807-825](19/19) ORF
5 GGGUAAUAUUGCUGUGGAA UUCCACAGCAAUAUUACCC [793-811](19/19) ORF
6 GCGACAGAGUGAGCGAAAU AUUUCGCUCACUCUGUCGC [1710-1728](19/19) ORF
7 GGAUGAGAUUGGCAAUGUU AACAUUGCCAAUCUCAUCC [958-976](19/19) ORF
8 UCAAGAAAGACACGUACAU AUGUACGUGUCUUUCUUGA [1806-1824](19/19) ORF
9 ACCAUGUGUUUGAUGAACA UGUUCAUCAAACACAUGGU [1146-1164](19/19) ORF
10 GAAGAAAAUGUGGACUUAA UUAAGUCCACAUUUUCUUC [809-827](19/19) ORF
Table CT: RPNl - ribophorin I 21mers
Other I Iluman-62739I76 ORF
Sense si RNΛ AntiSense si RNΛ Sp I 1900
1 CCAUUAUUUCUACUCUCCCUA UAGGGAGAGUAGAAAUAAUGG Mouse [553-573](21/21) ORF
2 GGAAGAAAAUGUGGACUUAAA UUUAAGUCCACAUUUUCUUCC [808-828](21/21) ORF
3 GUGUUUGAUGAACAAGUGAUA UAUCACUUGUUCAUCAAACAC [1151-1171](21/21) ORF
4 GGACCAUGUGUUUGAUGAACA UGUUCAUCAAACACAUGGUCC [1144-1164](21/21) ORF
5 GGGAACCAUUAUUUCUACUCU AGAGUAGAAAUAAUGGUUCCC [548-5681(21/21) ORF
6 GAAGAAAAUGUGGACUUAAAG CUUUAAGUCCACAUUUUCUUC [809-8291(21/21) ORF
7 UGGAAGAAAAUGUGGACUUAA UUAAGUCCACAUUUUCUUCCA [807-827](21/21) ORF
8 GUGGAAGAAAAUGUGGACUUA UAAGUCCACAUUUUCUUCCAC [806-826](21/21) ORF
9 CUAAUUCUGAUGUUUGGGUAU AUACCCAAACAUCAGAAUUAG [2242-22621(21/21) 31UTR
10 CAAGCUCAAGAAAGACACGUA UACGUGUCUUUCUUGAGCUUG [1801-1821](21/21) ORF
Table CU: SLC17A7 - solute carrier family 17, member 7 19mers
Number! Sense siRNA ΛntiScnsc si RNΛ lOther Sp|l lum:ιn-46255()58 ORF: 148-1830
1 CAACCAAAAUCGCAAUUUU AAAAUUGCGAUUUUGGUUG [2491-25091(19/19) 31UTR
2 UUAACAAUAUGCCUCUCUA UAGAGAGGCAUAUUGUUAA [2508-25261(19/19) 31UTR
3 CCAAAUUUCUCAAGACCCU AGGGUCUUGAGAAAUUUGG [2439-2457](19/19) 31UTR
4 CCACUCUAAACAUGCUGAU AUCAGCAUGUUUAGAGUGG [611-629](19/19) ORF
5 CGACCUCUUACUCUCUCUU AAGAGAGAGUAAGAGGUCG [2127-21451(19/19J S1UTR
6 GCUCUGUUUUCUACGUCUA UAGACGUAGAAAACAGAGC [851-869](19/19) ORF
7 GGAGCUCUGUUUUCUACGU ACGUAGAAAACAGAGCUCC [848-866](19/19) ORF
8 GCACUUUAUUCUCCUGGGU ACCCAGGAGAAUAAAGUGC Mouse [2660-26781(19/19) 31UTR
9 GUCAAAAAUUUGCAGCCAA UUGGCUGCAAAUUUUUGAC [560-5781(19/19) ORF
10 GCUACAUUAUCGCCAUCAU AUGAUGGCGAUAAUGUAGC [332-350](19/19) ORF
Table CV: SLC17A7 - solute carrier family 17, member 7 21 mers
Other I llunian-46255058 ORI- : 148-
1 GGCAACAUCCACUCUAAACAU AUGUUUAGAGUGGAUGUUGCC [603-623](21/21) ORF
2 GGAGGAUUUAUCUGUCAAAAA UUUUUGACAGAUAAAUCCUCC [547-5671(21/21) ORF
3 GAAGUUUCAAAUCUCUCCCAA UUGGGAGAGAUUUGAAACUUC [2261-22811(21/20 31UTR
4 GGAGCUCUGUUUUCUACGUCU AGACGUAGAAAACAGAGCUCC [848-868](21/21) ORF
5 CCCAACUCUGUUCUGCAUCUU AAGAUGCAGAACAGAGUUGGG [2277-2297](21/21) 31UTR
6 GAGCUCUGUUUUCUACGUCUA UAGACGUAGAAAACAGAGCUC [849-869](21/21) ORF
7 CCCGUGCACUUUUUCUGACAU AUGUCAGAAAAAGUGCACGGG [2529-2549](21/21) 31UTR
8 GGGUUGUGUCUUUGUGUCUCU AGAGACACAAAGACACAACCC [2868-28881(21/20 31UTR
9 CUCUUCUCCCUAGCUUAGCAU AUGCUAAGCUAGGGAGAAGAG [2456-24761(21/20 31UTR
10 CCUCUAGAAGUUUCAAAUCUC GAGAUUUGAAACUUCUAGAGG [2255-22751(21/20 31UTR
Table CW: SLC28A2 - solute carrier family 28 (sodium-coupled nucleoside transporter), member 2 19mers
Number ■aaiwaaitiasi ETi1IK1UlMJ.il Mt»l Other Si)IIIu nian-142348142 OKI-':6()-203(
1 CCAACCUGAUUGCCUUUUU AAAAAGGCAAUCAGGUUGG [1333-1351](19/19) ORF
2 GCAUGUUCAUCCUUAUCCU AGGAUAAGGAUGAACAUGC [601-6191(19/19) ORF
3 CCAAGAUGUUUAACAGUAA UUACUGUUAAACAUCUUGG [2129-21471(19/19) 31UTR
4 GGAAAAAGAAGUAGAGCCU AGGCUCUACUUCUUUUUCC [140-158](19/19) ORF
5 GGAAACAUCUUUGUGGGUA UACCCACAAAGAUGUUUCC [981-9991(19/19) ORF
6 AGAGCUGAAAUCAUUACAA UUGUAAUGAUUUCAGCUCU [1629-1647](19/19) ORF
7 GCUCAUGGAAAAAGAAGUA UACUUCUUUUUCCAUGAGC [134-152](19/19) ORF
8 CGGCUCCAGUUUUGUCUUU AAAGACAAAACUGGAGCCG [785-8031(19/19) ORF
9 UGUUCAUCCUUAUCCUCUU AAGAGGAUAAGGAUGAACA [604-622](19/19) ORF
10 UCUGCAUGUUCAUCCUUAU AUAAGGAUGAACAUGCAGA [598-616](19/19) ORF
Table CX: SLC28A2 - solute carrier family 28 (sodium-coupled nucleoside transporter), member 2 21mers
1 GGGAAUCAAGUUCUUCAUAAA UUUAUGAAGAACUUGAUUCCC [1511-1531](21/21) ORF
2 GGCUGCUUGAUCUAUUUCUAU AUAGAAAUAGAUCAAGCAGCC [2037-20571(21/21) 31UTR
3 GGAAACAUCUUUGUGGGUAUG CAUACCCACAAAGAUGUUUCC [981-1001](21/21) ORF
4 AUGUUCAUCCUUAUCCUCUUU AAAGAGGAUAAGGAUGAACAU [603-623](21/21) ORF
5 GGAGAACAGGAGAUGGAGAAA UUUCUCCAUCUCCUGUUCUCC [48-68](21/21) 5'UTR+ORF
6 UGAGAGCUGAAAUCAUUACAA UUGUAAUGAUUUCAGCUCUCA [1627-1647](21/21) ORF
7 GGAAAAAGAAGUAGAGCCUGA UCAGGCUCUACUUCUUUUUCC [140-160](21/21) ORF
8 GGAGUCCAAGUUCAAGAGUGA UCACUCUUGAACUUGGACUCC [1214-1234](21/21) ORF
9 GCUCAUGGAAAAAGAAGUAGA UCUACUUCUUUUUCCAUGAGC [134-154](21/21) ORF
10 GGCUGUGGCAGGAAACAUCUU AAGAUGUUUCCUGCCACAGCC [971-991](21/21) ORF
Table CY: SLC8A1 - solute carrier family 8 (sodium/calcium exchanger), member 1 19mers
UUiLIPmK1Kl
1 CCACACUUUACAAUUCUUU AAAGAAUUGUAAAGUGUGG [5479-5497](19/19) 31UTR
2 CCCAUCUUUUAUCUUUGUU AACAAAGAUAAAAGAUGGG [5438-54561(19/19) 31UTR
3 CCCACACUUUACAAUUCUU AAGAAUUGUAAAGUGUGGG [5478-54961(19/19) 3'UTR
4 CCCAAUCACUUUAAAACUU AAGUUUUAAAGUGAUUGGG [3418-3436](19/19) 31UTR
5 GGCUUUGAUUUGUUUUGUU AACAAAACAAAUCAAAGCC [3216-3234](19/19) 31UTR
6 GCAGCAAAAAAGUAGAGCA UGCUCUACUUUUUUGCUGC [1092-1110](19/19) ORF
7 CCAUCUUUUAUCUUUGUUG CAACAAAGAUAAAAGAUGG [5439-5457](19/19) 31UTR
8 GGAUCAUAUUACUGUAAGA UCUUACAGUAAUAUGAUCC [175-193](19/19) ORF
9 CCAUAUAAAACCAUCGAAG CUUCGAUGGUUUUAUAUGG [1741-1759](19/19) ORF
10 CGGUUCUGCACAUUCAUUU AAAUGAAUGUGCAGAACCG [4232-4250](19/19) 31UTR
Table CZ: SLC8A1 - solute carrier family 8 (sodium/calcium exchanger), member 1 21mers
Munian-1 15529447 ORI :25-
Number! Sense siRNΛ ΛntiSense sikN'Λ Other Sp
1 CCAGAUAAAGAAAUAGAGCAA UUGCUCUAUUUCUUUAUCUGG [1036-1056](21/21) ORF
2 GCCCACACUUUACAAUUCUUU AAAGAAUUGUAAAGUGUGGGC [5477-5497](21/21) 3'UTR
3 GGCCCACACUUUACAAUUCUU AAGAAUUGUAAAGUGUGGGCC [5476-54961(21/21) 31UTR
4 GCCCAGUGUAUCCCAUCUUUU AAAAGAUGGGAUACACUGGGC [5427-5447](21/21) 31UTR
5 GGACAAACUCAUUAAGAAGAC GUCUUCUUAAUGAGUUUGUCC Rat,Mouse [2202-2222](21/21) ORF
6 CCACUGUAACUAUUUUUGAUG CAUCAAAAAUAGUUACAGUGG [1604-1624](21/21) ORF
7 GGACUAAGCCAUGAACAAAAC GUUUUGUUCAUGGCUUAGUCC [5086-5106](21/21) 3'UTR
8 GGUUCUGCACAUUCAUUUCUA UAGAAAUGAAUGUGCAGAACC [4233-42531(21/20 31UTR
9 CCAGUGUAUCCCAUCUUUUAU AUAAAAGAUGGGAUACACUGG [5429-5449](21/21) 31UTR
10 GCAAACUACAAAGAUCUUCAU AUGAAGAUCUUUGUAGUUUGC [4307-4327](21/21) 31UTR
Table DA: SNAP91 - synaptosomal-associated protein, 9IkDa homolog 19mcrs
Number! Sense siRNΛ ΛntiSense siRNΛ Other Sn l l luman-7662227 ORF:244-2967
1 CCAAUGUUAAUAUUCCUCA UGAGGAAUAUUAACAUUGG [398-416](19/19) ORF
2 CCGGUUGAUUUAUUUGCAA UUGCAAAUAAAUCAACCGG [1222-1240](19/19) ORF
3 CCCACCGGUUGAUUUAUUU AAAUAAAUCAACCGGUGGG [1218-1236](19/19) ORF
4 CCCUAUUUAUCACUGCUAU AUAGCAGUGAUAAAUAGGG [3640-3658](19/19) 31UTR
5 CCACAACAAAAAAGGGAGA UCUCCCUUUUUUGUUGUGG [2561-2579](19/19) ORF
6 CAAUGUUAAUAUUCCUCAG CUGAGGAAUAUUAACAUUG [399-417](19/19) ORF
7 CCCUACUCUUGAAAUUGUA UACAAUUUCAAGAGUAGGG [3159-3177](19/19) 3'UTR
8 GGCUGCACUUUCCUCUGUU AACAGAGGAAAGUGCAGCC Rat,Mouse [1407-1425](19/19) ORF
9 GCUUGAAUUUGAUGUGCAU AUGCACAUCAAAUUCAAGC [777-795](19/19) ORF
10 CCCUCUGAGAUGCAAUAAA UUUAUUGCAUCUCAGAGGG Mouse [4337-43551(19/19) 3'UTR
1 CCAAUGUUAAUAUUCCUCAGA UCUGAGGAAUAUUAACAUUGG [398-418](21/21) ORF
2 GCAAUAAACACCUUGAACAAA UUUGUUCAAGGUGUUUAUUGC [4348-43681(21/20 31UTR
3 UGCAAUAAACACCUUGAACAA UUGUUCAAGGUGUUUAUUGCA [4347-43671(21/2I) S1UTR
4 CCAAUACUACAGGGACAAAUU AAUUUGUCCCUGUAGUAUUGG [748-768](21/21) ORF
5 ACAACAAAAAAGGGAGAUCUU AAGAUCUCCCUUUUUUGUUGU [2563-2583](21/21) ORF
6 CCACAACAAAAAAGGGAGAUC GAUCUCCCUUUUUUGUUGUGG [2561-2581](21/21) ORF
7 CCUCCACUACUACAACUGUUA UAACAGUUGUAGUAGUGGAGG [1514-1534](21/21) ORF
8 CAACGAGACCAAUGUUAAUAU AUAUUAACAUUGGUCUCGUUG [390-410](21/21) ORF
9 CCACCGGUUGAUUUAUUUGCA UGCAAAUAAAUCAACCGGUGG [1219-1239](21/21) ORF
10 CCCUUGGAAGUGAUCUUGAUU AAUCAAGAUCACUUCCAAGGG [2498-2518](21/21) ORF
Table DC: SYN2 - synapsin II 19mers
Number Sense siRNA ΛntiSense siRNA Other Sp| l-luman-l 19672896 ORI-": 165-1913
1 GCACCAAUAAAAAAUCCAU AUGGAUUUUUUAUUGGUGC [3287-3305](19/19) 3'UTR
2 AGCGUUUCUACCUAUAUUA UAAUAUAGGUAGAAACGCU [2970-29881(19/19) 31UTR
3 CAGCGUUUCUACCUAUAUU AAUAUAGGUAGAAACGCUG [2969-2987](19/19) 3'UTR
4 CCAACUUACAAGAUCUUAG CUAAGAUCUUGUAAGUUGG [2436-2454](19/19) 31UTR
5 UGGAAUCCAUAUACAACUU AAGUUGUAUAUGGAUUCCA [811-829](19/19) ORF
6 UGCCAAGCUUUGUGUUCAU AUGAACACAAAGCUUGGCA [2623-2641](19/19) 31UTR
7 CCCAACUUACAAGAUCUUA UAAGAUCUUGUAAGUUGGG [2435-2453](19/19) 3'UTR
8 GGACUUUGUAACCUUUCAA UUGAAAGGUUACAAAGUCC [2990-3008](19/19) 31UTR
9 GCGUUUCUACCUAUAUUAC GUAAUAUAGGUAGAAACGC [2971-2989](19/19) 31UTR
10 GCAUUCACCAACAUUUGAU AUCAAAUGUUGGUGAAUGC [2684-2702](19/19) 31UTR
Table DD: SYN2 - synapsin II 21 mers
Other I l luιnan-1 19672896 ORK: 165-
Sense siRNA AntiSense siRNA Sp I 1913
1 GGAAUCCAUAUACAACUUCUG CAGAAGUUGUAUAUGGAUUCC [812-832](21/21) ORF
2 GGCAAAAAAGUCCUUGGAGAU AUCUCCAAGGACUUUUUUGCC [558-578](21/21) ORF
3 GGACUUUGUAACCUUUCAAAU AUUUGAAAGGUUACAAAGUCC [2990-30101(21/21) 31UTR
4 CAGCGUUUCUACCUAUAUUAC GUAAUAUAGGUAGAAACGCUG [2969-2989](21/21) 31UTR
5 GCAAAAAAGUCCUUGGAGAUU AAUCUCCAAGGACUUUUUUGC [559-5791(21/21) ORF
6 CCAUAUACAACUUCUGUGACA UGUCACAGAAGUUGUAUAUGG [817-8371(21/2I) ORF
7 GAAUCCAUAUACAACUUCUGU ACAGAAGUUGUAUAUGGAUUC [813-8331(21/21) ORF
8 UGGAAUCCAUAUACAACUUCU AGAAGUUGUAUAUGGAUUCCA [811-831](21/21) ORF
9 CCAACAUUUGAUUCAGCCAUG CAUGGCUGAAUCAAAUGUUGG [2691-2711](21/21) 3'UTR
10 GGGAUUUCACUCUGGCUGCUU AAGCAGCCAGAGUGAAAUCCC [3197-3217](21/21) 3'UTR
Table DE: SYTl - synaptotagmin 1 19mers
N um her Sense si RNA AntiSense siUNA lother Spll luman-5032138 ORF:28-I 296
1 CACCACGUCUUUUCAAAAA UUUUUGAAAAGACGUGGUG [3221-3239](19/19) 31UTR
2 GGAUAUCACACCACGUCUU AAGACGUGGUGUGAUAUCC [3213-3231](19/19) 31UTR
3 CCACCGAAAAACCCUUAAU AUUAAGGGUUUUUCGGUGG [621-6391(19/19) ORF
4 CCAAAGAAGAGGAGAAACU AGUUUCUCCUCUUCUUUGG [437-455](19/19) ORF
5 GCAAAAAUAUCAACUCGUA UACGAGUUGAUAUUUUUGC [1752-1770](19/19) 31UTR
6 AGAAAAAGAAGAACCCAAA UUUGGGUUCUUCUUUUUCU [423-4411(19/19) ORF
7 CACACCACGUCUUUUCAAA UUUGAAAAGACGUGGUGUG [3219-3237](19/19) 3'UTR
8 GCCUGACUAUUUAAAAAGA UCUUUUUAAAUAGUCAGGC [2203-2221](19/19) 3'UTR
9 GGAGAAACUGGGAAAACUU AAGUUUUCCCAGUUUCUCC [447-465](19/19) ORF
10 AGAGGAGAAACUGGGAAAA UUUUCCCAGUUUCUCCUCU [444-462](19/19) ORF
Table DF: SYTl - synaptotagmin I 21mers
■LHUimillMk'gl nτπ ιuBiκrreπ ;<wτ-τ l lιιnian-S032138 ORF:28- 12% UCACACCACGUCUUUUCAAAA UUUUGAAAAGACGUGGUGUGA [3218-32381(21/2031UTR GCCAUAGUUCUGAAUGCACUU AAGUGCAUUCAGAACUAUGGC Mouse [2492-2512](21/21) 31UTR GAAAAAGAAGAACCCAAAGAA UUCUUUGGGUUCUUCUUUUUC [424-444](21/21) ORF GCAGGAUAUUUUUAACACAAC GUUGUGUUAAAAAUAUCCUGC [2370-2390](21/21) 3'UTR GCAAGAGAAAUUGGGUGAUAU AUAUCACCCAAUUUCUCUUGC [837-857](21/21) ORF GGAGAAGUUUAUGAAUGAGUU AACUCAUUCAUAAACUUCUCC [159-179](21/21) ORF GAGGAGAAACUGGGAAAACUU AAGUUUUCCCAGUUUCUCCUC [445-465](21/21) ORF GGAUUGACAGAUGGAGAAGAA UUCUUCUCCAUCUGUCAAUCC [406-426](21/21) ORF GGAUAUCACACCACGUCUUUU AAAAGACGUGGUGUGAUAUCC [3213-3233](21/21) 3'UTR GGAGGAGUUUUUAAACGUUUU AAAACGUUUAAAAACUCCUCC [2661-2681](21/21) 3'UTR
Table DG: TKT - transketolase 19mers
BϋHBIIHSl Rττnκτ«ιHnmr«θM iiinmicEKinrewiaiiMmαM CCGGCAAAUACUUCGACAA UUGUCGAAGUAUUUGCCGG [413-431](19/19) ORF GCAAAUACUUCGACAAGGC GCCUUGUCGAAGUAUUUGC [416-434](19/19) ORF GGCAAAUACUUCGACAAGG CCUUGUCGAAGUAUUUGCC [415-433](19/19) ORF CCAGAGCAAAAAGAAGAUC GAUCUUCUUUUUGCUCUGG [834-852](19/19) ORF CCAUCAUCUAUAACAACAA UUGUUGUUAUAGAUGAUGG [1433-1451](19/19) ORF GCCCAGAAAAUGCCAUCAU AUGAUGGCAUUUUCUGGGC [1421-1439](19/19) ORF UCAACUGUCUUUUACCCAA UUGGGUAAAAGACAGUUGA [1327-1345](19/19) ORF UCCAGAGCAAAAAGAAGAU AUCUUCUUUUUGCUCUGGA [833-851](19/19) ORF AGCAGAUCAUCCAGGAGAU AUCUCCUGGAUGAUCUGCU [803-821](19/19) ORF ACCGGCAAAUACUUCGACA UGUCGAAGUAUUUGCCGGU [412-430](19/19) ORF
Table DH: TKT - transketolase 21mers
■LWiMlMHMk.il Other Snl l luιiiun-4507520 ORI : I - 1 S72 CGGCAAAUACUUCGACAAGGC GCCUUGUCGAAGUAUUUGCCG [414-434](21/21) ORF CACCGGCAAAUACUUCGACAA UUGUCGAAGUAUUUGCCGGUG [411-431](21/21) ORF GGAACUAGCCGCCAAUACAAA UUUGUAUUGGCGGCUAGUUCC [1374-1394](21/21) ORF CCAGAUCCAGAGCAAAAAGAA UUCUUUUUGCUCUGGAUCUGG [828-848](21/21) ORF GGAAAGGUGCUCAAAGAUGUA UACAUCUUUGAGCACCUUUCC [1921-19411(21/2I) S1UTR GGGUAGAAGAUAAGGAGUCUU AAGACUCCUUAUCUUCUACCC [749-769](21/21) ORF GCAAAUACUUCGACAAGGCCA UGGCCUUGUCGAAGUAUUUGC [416-436](21/21) ORF ACCGGCAAAUACUUCGACAAG CUUGUCGAAGUAUUUGCCGGU [412-432](21/21) ORF UGAGCAGAUCAUCCAGGAGAU AUCUCCUGGAUGAUCUGCUCA [801-821](21/21) ORF GAAGAUAAGGAGUCUUGGCAU AUGCCAAGACUCCUUAUCUUC [754-774](21/21) ORF
Table DI: TPTl - tumor protein, translationally-controlled 1 19mers
iNumberl KOTHratW-l Other Sn I i luιtiiin-141 <S() 191 1 ORI :94-6l
1 GCUCUUCAUUUAUUUUGAC GUCAAAAUAAAUGAAGAGC [720-738](19/19) 31UTR
2 GAGGCAUUGUUUUUAAGAA UUCUUAAAAACAAUGCCUC [757-775](19/19) 3'UTR
3 UCGCCAUCAUGAUUAUCUA UAGAUAAUCAUGAUGGCGA [86-104](19/19) 5'UTR+ORF
4 UCUCCGACAUCUACAAGAU AUCUUGUAGAUGUCGGAGA Rat,Mouse [134-152](19/19) ORF
5 UGAAAUCAAUCAAAGGGAA UUCCCUUUGAUUGAUUUCA [380-398](19/19) ORF
6 CCAUCAUGAUUAUCUACCG CGGUAGAUAAUCAUGAUGG [89-107](19/19) 5'UTR+ORF
7 UCCCUUCAGUCGCCAUCAU AUGAUGGCGACUGAAGGGA [77-951(19/19) 5'UTR+ORF
8 GUGGAGGCAUUGUUUUUAA UUAAAAACAAUGCCUCCAC [754-772](19/19) 3'UTR
9 UCAGCCACGAUGAGAUGUU AACAUCUCAUCGUGGCUGA [116-134](19/19) ORF
10 GGGCUGCAGAACAAAUCAA UUGAUUUGUUCUGCAGCCC [443-461](19/19) ORF
1 GGAGGCAUUGUUUUUAAGAAA UUUCUUAAAAACAAUGCCUCC [756-776](21/21) 3'UTR
2 GCUCUUCAUUUAUUUUGACUG CAGUCAAAAUAAAUGAAGAGC [720-7401(21/21) 3'UTR
3 GGACAGAAGGUAACAUUGAUG CAUCAAUGUUACCUUCUGUCC [206-226](21/21) ORF
4 GAAAUCAAUCAAAGGGAAACU AGUUUCCCUUUGAUUGAUUUC [381-401](21/21) ORF
5 CGCCAUCAUGAUUAUCUACCG CGGUAGAUAAUCAUGAUGGCG [87-1071(21/21) 5'UTR+ORF
6 GUCGAUAUUGUCAUGAACCAU AUGGUUCAUGACAAUAUCGAC [301-321](21/21) ORF
7 GACUCGCUCAUUGGUGGAAAU AUUUCCACCAAUGAGCGAGUC [226-246](21/21) ORF
8 CCCUUCAGUCGCCAUCAUGAU AUCAUGAUGGCGACUGAAGGG [78-981(21/21) 5'UTR+ORF
9 GACCAGAAAGAGUAAAACCUU AAGGUUUUACUCUUUCUGGUC [413-433](21/21) ORF
10 GCCAUCAUGAUUAUCUACCGG CCGGUAGAUAAUCAUGAUGGC [88-1081(21/21) 5'UTR+ORF
Table DK: UGT8 - UDP glycosyltransferase 8 19mers
EffiHHπSKl BTTHKUlMU.mVt-1 Other Sp l l luman-4<l254470 ORK:516-2141
1 GCCUUGUUAUACUUUCUCU AGAGAAAGUAUAACAAGGC [1971-1989](19/19) ORF
2 CCAGGGAUCUUUAACAGUA UACUGUUAAAGAUCCCUGG [735-7531(19/19) ORF
3 CCUUGUUAUACUUUCUCUU AAGAGAAAGUAUAACAAGG [1972-1990](19/19) ORF
4 GGAAGACAGUUACUGAAAA UUUUCAGUAACUGUCUUCC [1717-17351(19/19) ORF
5 CUAAUGAAAUUCUGUGGAA UUCCACAGAAUUUCAUUAG [2266-2284](19/19) 3'UTR
6 GCCAUAUGUACAUUUUCAA UUGAAAAUGUACAUAUGGC [613-631](19/19) ORF
7 CCUUUUAGAAGCCUUAAUU AAUUAAGGCUUCUAAAAGG [2368-23861(19/19) 3'UTR
8 CCGCCAAUUAUGUUUGAAA UUUCAAACAUAAUUGGCGG Rat,Mouse [594-612](19/19) ORF
9 GCACAGUUAAUGGACAUUA UAAUGUCCAUUAACUGUGC Mouse [2053-20711(19/19) ORF
10 GAAGACAGUUACUGAAAAA UUUUUCAGUAACUGUCUUC [1718-1736](19/19) ORF
Table DL: UGT8 - UDP glycosyltransferase 8 21mers
■kimmuiiKgi Other I lluman-40254470 ORI :516- Sp I 2141
1 GCCUUGUUAUACUUUCUCUUG CAAGAGAAAGUAUAACAAGGC [1971-1991](21/21) ORF
2 UGCCUUGUUAUACUUUCUCUU AAGAGAAAGUAUAACAAGGCA [1970-1990](21/21) ORF
3 GGUGCUGCCUUGUUAUACUUU AAAGUAUAACAAGGCAGCACC [1965-1985](21/21) ORF
4 UGGAAGACAGUUACUGAAAAA UUUUUCAGUAACUGUCUUCCA [1716-1736](21/21) ORF
5 GGAUCACUAUACUAAGAACUG CAGUUCUUAGUAUAGUGAUCC [836-856](21/21) ORF
6 GGAAUUAAGAUGGCUGUAAAA UUUUACAGCCAUCUUAAUUCC [2281-2301](21/21) 31UTR
7 UGCUGCCUUGUUAUACUUUCU AGAAAGUAUAACAAGGCAGCA [1967-1987](21/21) ORF
8 GGAUGAAAAAUACCGGUGUUU AAACACCGGUAUUUUUCAUCC [1093-1113](21/21) ORF
9 CCCAGGGAUCUUUAACAGUAC GUACUGUUAAAGAUCCCUGGG [734-754](21/21) ORF
10 GCUGCCUUGUUAUACUUUCUC GAGAAAGUAUAACAAGGCAGC [1968-1988](21/21) ORF
Table DM: VIP - vasoactive intestinal peptide 19mers
ΠHMΠH ■kffllMJϋtmfl RTnIMBiWl-MIHM-I ( ) t he r S n I u in a n -3758885 ( ) K • : 73-68
1 CCAAAACAAACCAGAACAG CUGUUCUGGUUUGUUUUGG [115-133](19/19) 5'UTR
2 GGAGAGUAGAACAGAUAAU AUUAUCUGUUCUACUCUCC [1087-1105](19/19) 3'UTR
3 GAGUAGUAAUAGAGCAAAA UUUUGCUCUAUUACUACUC [1031-1049](19/19) 3'UTR
4 CCAGAAGAGUUAGAAAAAU AUUUUUCUAACUCUUCUGG [665-683](19/19) ORF
5 AGAAAACAAAUGGCUGUAA UUACAGCCAUUUGUUUUCU [584-602](19/19) ORF
6 GCACAGAAAUGGACACCAG CUGGUGUCCAUUUCUGUGC [165-1831(19/19) 5'UTR-K)RF
7 AGAGCAAAAUUGAUGUGUU AACACAUCAAUUUUGCUCU [1041-1059](19/19) 3'UTR
8 ACCUAAGACAGCUCCAAAA UUUUGGAGCUGUCUUAGGU [102-120](19/19) 5'UTR
9 AGAGUAGUAAUAGAGCAAA UUUGCUCUAUUACUACUCU [1030-10481(19/19) 3'UTR
10 GGAGACACAACUAUUUUUC GAAAAAUAGUUGUGUCUCC [1506-1524](19/19) 31UTR
Table DN: VlP - vasoactive intestinal peptide 21 mers
RfflWl!Ht»_l .MlHKHIlKtI-MIMMM riuιnan-37588851 ORI- : 173-
6S5
1 GGAAAAUGAUACGCAACAUAA UUAUGUUGCGUAUCAUUUUCC [739-759](21/21) 3'UTR
2 GCAAAAUGCAUUAGCUGAAAA UUUUCAGCUAAUGCAUUUUGC [355-375](21/21) ORF
3 ACAGAAAUGGACACCAGAAAU AUUUCUGGUGUCCAUUUCUGU [167-187](21/21) 5'UTR+ORF
4 CUAAGACAGCUCCAAAACAAA UUUGUUUUGGAGCUGUCUUAG [104-124](21/21) 5'UTR
5 GGAAGAGUAGUAAUAGAGCAA UUGCUCUAUUACUACUCUUCC [1027-10471(21/21) 3'UTR
6 AGAAAUGGACACCAGAAAUAA UUAUUUCUGGUGUCCAUUUCU [169-189](21/21) 5'UTR+ORF
7 GCACAGAAAUGGACACCAGAA UUCUGGUGUCCAUUUCUGUGC [165-1851(21/21) 5'UTR+ORF
8 GUGUAAAAUGUGAAGUGAAUG CAUUCACUUCACAUUUUACAC [1393-1413](21/21) 3'UTR
9 CCUAAGACAGCUCCAAAACAA UUGUUUUGGAGCUGUCUUAGG [103-1231(21/20 51UTR
10 GGAGAGUAGAACAGAUAAUCA UGAUUAUCUGUUCUACUCUCC [1087-11071(21/2I) S1UTR
Claims
1. A compound having the structure:
5' (N)x - Z 3' (antisense strand) 3' Z'-(N')y 5' (sense strand)
wherein each of N and N' is a ribonucleotide which may be modified or unmodified in its sugar residue;
wherein each of (N)x and (N')y is an oligomer in which each consecutive N or N' is joined to the next N or N' by a covalent bond;
wherein each of x and y is an integer between 18 and 40;
wherein each of Z and Z' may be present or absent, but if present comprises 1-5 nucleotides and is covalently attached at the 3' terminus of the strand in which it is present; and
wherein the sequence of (N)x comprises an antisense sequence relative to the mRNA transcribed from a mammalian gene selected from the group consisting of ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1;
CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A;
GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB;
NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl;
PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 and VIP.
2. The compound of claim 1, wherein the sequence of (N)x comprises one or more of the antisense sequences present in Tables A-DN.
3. The compound of claim 1, wherein the covalent bond is a phosphodiester bond.
4. The compound of claim 1, wherein x = y.
5. The compound of claim 4, wherein x = y and wherein x is an integer selected from the group consisting of 19 and 21.
6. The compound of claim 5, wherein x = y =19.
7. The compound of claim 1, wherein Z and Z' are both absent.
8. The compound of claim 1, wherein one of Z or Z' is present.
9. The compound of claim 1, wherein all of the ribonucleotides are unmodified in their sugar residues.
10. The compound of claim 1, wherein at least one ribonucleotide is modified in its sugar residue.
11. The compound of claim 10, wherein the modification of the sugar residue comprises a modification at the 2' position.
12. The compound of claim 11, wherein the modification at the 2' position results in the presence of a moiety selected from the group consisting of an amino, a fluoro, a methoxy, an alkoxy and an alkyl group.
13. The compound of claim 12, wherein the moiety at the 2' position is methoxy (2'-O-methyl).
14. The compound of claim 1, wherein alternating ribonucleotides are modified in both the antisense and the sense strands.
15. The compound according to claim 14 wherein the middle ribonucleotide in the antisense strand is unmodified.
16. The compound of claim 14, wherein the ribonucleotides at the 5' and 3' termini of the antisense strand are modified in their sugar residues, and the ribonucleotides at the 5' and 3' termini of the sense strand are unmodified in their sugar residues.
17. The compound of claim 14, wherein the antisense and the sense strands are non- phosphorylated at the 3' and 5' termini or wherein the antisense and the sense strands are phosphorylated at the 3' termini.
18. The compound of claim 1, wherein at least one modified ribonucleotide is selected from a locked nucleic acid, a morpholino, a peptide nucleic acid and a mirror nucleic acid.
19. The compound of claim 15 wherein the sense or the antisense siRNA sequence comprises a sequence present in Tables A-DN.
20. A compound having the structure
5' (N) 3' antisense strand 3' (N') 5' sense strand
wherein each of N and N' =19 and are fully complementary;
wherein alternating ribonucleotides in the antisense and the sense strands are modified to result in a 2'-O-methyl modification in the sugar residue of the ribonucleotides;
wherein the ribonucleotides at the 5' and 3' termini of the antisense strand are modified;
wherein the ribonucleotides at the 5' and 3' termini of the sense strand are unmodified;
wherein the antisense and the sense strands are phosphorylated or non-phosphorylated at the 3' and 5' termini; and wherein each of N and N' are selected from the group of oligomers set forth in Tables A-DN.
21. A pharmaceutical composition comprising one or more compounds according to any one of claims 1-20 in an amount effective to inhibit gene expression of a gene selected from the group consisting of ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 and VIP; and a pharmaceutically acceptable carrier.
22. A method of treating a mood disorder or a neurodegenerative disease in a subject in need thereof, comprising administering to the subject a compound according to claim 1 in an amount effective to treat the disease or condition.
23. The method according to claim 22 wherein the antisense strand comprises a sufficient number of consecutive nucleotides having a sequence of sufficient homology to a nucleic acid sequence present within a gene selected from the group consisting of ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1;
CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A;
GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB;
NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl;
PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 and VIP; to hybridize to the mRNA transcribed from the gene and reduce or inhibit expression of the gene in the subject.
24. A method of treating a patient suffering from a mood disorder, comprising administering to the patient a pharmaceutical composition comprising a therapeutically effective amount of an inhibitor to a gene selected from the group consisting of TTR, PD2 Synthase, Adrb3, PDE4D, GABRP, PMCH, Duspό, Camk2a/2b, VIP, Atplal, MEF2C, Cdk5r, NOSl, Neurogranin, Chimerinl, Dendrin, GMF, Cacnb4, SLC17A7, SLC8A1, Drd3, MAPKKKK5, ARHGAP6 CLU, BAMBI, PTGER3, CLSTN2, STARD7, DKK3, CART, SCARA3, NRP2, MGC42105, NRGl, NELL2, CD276, CD81, NTRK2, AKAP5, EFNB3, CNP, ENPP2, SMPD2, CKB, SLC6A15, SLC6A20, SLC22A17, SLC6A17, SLC6A9, TRPC6, CLCN3, EPHA4, MAPKl, PTPRR, TACl, TM, TBXA2R, TDE2, TDEl, GABA(A), BCAN, PTGDR, ZDHHC12, PLXNA2, SNPH, DLGAP3, SCAMP4, SEZ6L, NTS, CPE, TMEM30A, FLJ10700, LPHN3, GPM6B, PTPRD, UNC5A, PAM, GHITM, SPOCK, MGC20235, FUNDCl, PKIG, CLSTN3, AQP4, EDG2, PPP3CB, PPMlE, GPM6A, ARMCl, AVP, SYNGR3, PENK, ClOorfitf, SMBP, IGFBP2, GPR23, CBLNl, PHCA, HIGl, GPR22, DLGAPl, FSTLl, RNF103, ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 and VIP , so as to thereby treat the patient.
25. The method of claim 24 wherein the mood disorder is a depression.
26. Use of a therapeutically effective amount of an inhibitor to a gene selected from the group consisting of TTR, PD2 Synthase, MAPKKKK5, ARHGAP6, CLU, BAMBI, PTGER3, CLSTN2, STARD7, DKK3, CART, SCARA3, MGC42105, NELL2, CD276, CD81, NTRK2, AKAP5, EFNB3, CNP, SMPD2, CKB, SLC6A15, SLC6A20, SLC22A17, SLC6A17, SLC6A9, TRPC6, CLCN3, MAPKl, PTPRR, TACl, TM, TBXA2R, TDE2, TDEl, GABA(A), BCAN, PTGDR, ZDHHC 12, PLXNA2, SNPH, DLGAP3, SCAMP4, SEZ6L, NTS, CPE, TMEM30A, FLJ 10700, LPHN3, GPM6B, PTPRD, UNC5A, PAM, GHITM, SPOCK, MGC20235, FUNDCl, PKIG, CLSTN3, AQP4, EDG2, PPP3CB, PPMlE, GPM6A, ARMCl, AVP, SYNGR3, PENK, C10orf93, SMBP, IGFBP2, GPR23, CBLNl, PHCA, HIGl, GPR22, DLGAPl, FSTLl, RNF103 ABAT; ADRBl; ADRB3; ARHGEF9; ARRBl; ATPlAl; CACNB4; CAMK2A; CAMK2D; CBLNl; CDH22; CDK5R1; CHNl; CTSD; DDN; DRD3; DUSP6; ENPPl; ENPP2; EPHA4; GABRAl; GMFG; GPM6A; GPNMB; GPR23; HAPLN4; IGF2; IGFBP2; KCNAl; KIF5A; MAPKlO; MEF2C; NAPB; NOSl; NPTX2; NRGN; NTS; NUCBl; PCP4; PDCD2; PDE4D; PENK; PHCA; PJA2; PLPl; PMCH; PVALB; QDPR; RPNl; SLC17A7; SLC28A2; SLC8A1; SNAP91; SYN2; SYTl; TKT; TPTl; UGT8 and VIP for the preparation of a medicament for promoting or enhancing recovery in a patient suffering from a mood disorder.
27. The method of claim 12 wherein the mood disorder is a depression.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US83809706P | 2006-08-15 | 2006-08-15 | |
| US60/838,097 | 2006-08-15 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2008020435A2 true WO2008020435A2 (en) | 2008-02-21 |
| WO2008020435A3 WO2008020435A3 (en) | 2009-05-07 |
Family
ID=39082444
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IL2007/001013 WO2008020435A2 (en) | 2006-08-15 | 2007-08-14 | Compositions and methods for treatment of mood disorders |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2008020435A2 (en) |
Cited By (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009043922A2 (en) * | 2007-10-04 | 2009-04-09 | Vib Vzw | Extracellular targets for alzheimer's disease |
| WO2010142603A1 (en) * | 2009-06-08 | 2010-12-16 | Vib Vzw | Screening for compounds that modulate gpr3-mediated beta-arrestin signaling and amyloid beta peptide generation |
| JP2011523637A (en) * | 2008-05-15 | 2011-08-18 | ジーイー・ヘルスケア・リミテッド | GABA biomarkers for depression |
| JP2011523638A (en) * | 2008-05-15 | 2011-08-18 | ジーイー・ヘルスケア・リミテッド | Glutamine biomarkers for depression |
| JP2011523636A (en) * | 2008-05-15 | 2011-08-18 | ジーイー・ヘルスケア・リミテッド | Biomarkers for depression |
| WO2012080509A1 (en) * | 2010-12-17 | 2012-06-21 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | Nucleic acids targeting tctp for use in the treatment of chemo-or hormone-resistant cancers |
| US8444983B2 (en) | 2009-03-23 | 2013-05-21 | Quark Pharmaceuticals, Inc. | Composition of anti-ENDO180 antibodies and methods of use for the treatment of cancer and fibrotic diseases |
| WO2013098813A1 (en) | 2012-01-01 | 2013-07-04 | Qbi Enterprises Ltd. | Endo180-targeted particles for selective delivery of therapeutic and diagnostic agents |
| US9233102B2 (en) | 2012-03-07 | 2016-01-12 | Mayo Foundation For Medical Education And Research | Methods and materials for treating cancer |
| WO2015148580A3 (en) * | 2014-03-25 | 2016-02-25 | Arcturus Therapeutics, Inc. | Una oligomers having reduced off-target effects in gene silencing |
| WO2016044697A1 (en) * | 2014-09-19 | 2016-03-24 | The Johns Hopkins University | Biomarkers of cognitive dysfunction |
| US9458464B2 (en) | 2014-06-23 | 2016-10-04 | The Johns Hopkins University | Treatment of neuropathic pain |
| US9856475B2 (en) | 2014-03-25 | 2018-01-02 | Arcturus Therapeutics, Inc. | Formulations for treating amyloidosis |
| US9982259B2 (en) | 2014-03-25 | 2018-05-29 | Arcturus Therapeutics, Inc. | Transthyretin allele selective UNA oligomers for gene silencing |
| WO2019016123A1 (en) * | 2017-07-17 | 2019-01-24 | Vib Vzw | Targeting synaptogyrin-3 in tauopathy treatment |
| US10421964B2 (en) | 2015-07-23 | 2019-09-24 | Arcturus Therapeutics, Inc. | UNA oligomers and compositions for treating amyloidosis |
| US10519447B2 (en) | 2015-04-01 | 2019-12-31 | Arcturus Therapeutics, Inc. | Therapeutic UNA oligomers and uses thereof |
| WO2021016474A1 (en) * | 2019-07-23 | 2021-01-28 | Fundación Ciencia Para La Vida (Fcv) | Ibd therapy by inhibition of drd3 in regulatory t cells |
| CN112957453A (en) * | 2021-03-04 | 2021-06-15 | 上海市精神卫生中心(上海市心理咨询培训中心) | Application of insulin-like growth factor binding protein 2 in preparation of medicine for treating mental disorder caused by brain trauma |
| US11058767B2 (en) | 2018-02-21 | 2021-07-13 | Bristol-Myers Squibb Company | CAMK2D antisense oligonucleotides and uses thereof |
| WO2022031847A3 (en) * | 2020-08-04 | 2022-04-14 | Dicerna Pharmaceuticals Inc. | Compositions and methods for inhibiting plp1 expression |
| WO2022109165A1 (en) * | 2020-11-18 | 2022-05-27 | Indiana University Research And Technology Corporation | Methods for objective assessment, risk prediction, matching to existing medications and new methods of using drugs, and monitoring responses to treatments for mood disorders |
| EP4035659A1 (en) | 2016-11-29 | 2022-08-03 | PureTech LYT, Inc. | Exosomes for delivery of therapeutic agents |
| CN116271051A (en) * | 2023-05-16 | 2023-06-23 | 山东大学 | Application of Phactr4 inhibitor in preparation of depression treatment drugs |
| US11732263B2 (en) | 2020-06-29 | 2023-08-22 | Ionis Pharmaceuticals, Inc. | Compounds and methods for modulating PLP1 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050020525A1 (en) * | 2002-02-20 | 2005-01-27 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20050209181A1 (en) * | 2003-11-05 | 2005-09-22 | Huda Akil | Compositions and methods for diagnosing and treating mental disorders |
| US20060141493A1 (en) * | 2001-11-09 | 2006-06-29 | Duke University Office Of Science And Technology | Atherosclerotic phenotype determinative genes and methods for using the same |
-
2007
- 2007-08-14 WO PCT/IL2007/001013 patent/WO2008020435A2/en active Application Filing
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060141493A1 (en) * | 2001-11-09 | 2006-06-29 | Duke University Office Of Science And Technology | Atherosclerotic phenotype determinative genes and methods for using the same |
| US20050020525A1 (en) * | 2002-02-20 | 2005-01-27 | Sirna Therapeutics, Inc. | RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA) |
| US20050209181A1 (en) * | 2003-11-05 | 2005-09-22 | Huda Akil | Compositions and methods for diagnosing and treating mental disorders |
Non-Patent Citations (1)
| Title |
|---|
| REYNOLDS ET AL.: 'Rational siRNA design for RNA interference.' NATURE BIOTECHNOLOGY. vol. 22, no. 3, March 2004, pages 326 - 330 * |
Cited By (38)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009043922A3 (en) * | 2007-10-04 | 2009-08-27 | Vib Vzw | Extracellular targets for alzheimer's disease |
| WO2009043922A2 (en) * | 2007-10-04 | 2009-04-09 | Vib Vzw | Extracellular targets for alzheimer's disease |
| JP2011523637A (en) * | 2008-05-15 | 2011-08-18 | ジーイー・ヘルスケア・リミテッド | GABA biomarkers for depression |
| JP2011523638A (en) * | 2008-05-15 | 2011-08-18 | ジーイー・ヘルスケア・リミテッド | Glutamine biomarkers for depression |
| JP2011523636A (en) * | 2008-05-15 | 2011-08-18 | ジーイー・ヘルスケア・リミテッド | Biomarkers for depression |
| US8562950B2 (en) | 2008-05-15 | 2013-10-22 | Ge Healthcare Limited | GABA biomarkers for depression |
| US9993567B2 (en) | 2009-03-23 | 2018-06-12 | Quark Pharmaceuticals, Inc. | Composition of anti-ENDO180 antibodies and methods of use for the treatment of cancer and fibrotic diseases |
| US8444983B2 (en) | 2009-03-23 | 2013-05-21 | Quark Pharmaceuticals, Inc. | Composition of anti-ENDO180 antibodies and methods of use for the treatment of cancer and fibrotic diseases |
| WO2010142603A1 (en) * | 2009-06-08 | 2010-12-16 | Vib Vzw | Screening for compounds that modulate gpr3-mediated beta-arrestin signaling and amyloid beta peptide generation |
| US8580493B2 (en) | 2009-06-08 | 2013-11-12 | Vib Vzw | Screening for compounds that modulate GPR3-mediated beta-arrestin signaling and amyloid beta peptide generation |
| WO2012080509A1 (en) * | 2010-12-17 | 2012-06-21 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | Nucleic acids targeting tctp for use in the treatment of chemo-or hormone-resistant cancers |
| WO2013098813A1 (en) | 2012-01-01 | 2013-07-04 | Qbi Enterprises Ltd. | Endo180-targeted particles for selective delivery of therapeutic and diagnostic agents |
| US10160972B2 (en) | 2012-03-07 | 2018-12-25 | Mayo Foundation For Medical Education And Research | Methods and materials for treating cancer |
| US9233102B2 (en) | 2012-03-07 | 2016-01-12 | Mayo Foundation For Medical Education And Research | Methods and materials for treating cancer |
| US10604758B2 (en) | 2014-03-25 | 2020-03-31 | Arcturus Therapeutics, Inc. | Therapeutic oligomers for treating amyloidosis |
| US9856475B2 (en) | 2014-03-25 | 2018-01-02 | Arcturus Therapeutics, Inc. | Formulations for treating amyloidosis |
| US9982259B2 (en) | 2014-03-25 | 2018-05-29 | Arcturus Therapeutics, Inc. | Transthyretin allele selective UNA oligomers for gene silencing |
| WO2015148580A3 (en) * | 2014-03-25 | 2016-02-25 | Arcturus Therapeutics, Inc. | Una oligomers having reduced off-target effects in gene silencing |
| US10683500B2 (en) | 2014-03-25 | 2020-06-16 | Arcturus Therapeutics, Inc. | UNA oligomers having reduced off-target effects in gene silencing |
| US9458464B2 (en) | 2014-06-23 | 2016-10-04 | The Johns Hopkins University | Treatment of neuropathic pain |
| WO2016044697A1 (en) * | 2014-09-19 | 2016-03-24 | The Johns Hopkins University | Biomarkers of cognitive dysfunction |
| US10222386B2 (en) | 2014-09-19 | 2019-03-05 | The Johns Hopkins University | Biomarkers of congnitive dysfunction |
| US10914749B2 (en) | 2014-09-19 | 2021-02-09 | The Johns Hopkins University | Biomarkers of cognitive dysfunction |
| US10519447B2 (en) | 2015-04-01 | 2019-12-31 | Arcturus Therapeutics, Inc. | Therapeutic UNA oligomers and uses thereof |
| US10421964B2 (en) | 2015-07-23 | 2019-09-24 | Arcturus Therapeutics, Inc. | UNA oligomers and compositions for treating amyloidosis |
| EP4035659A1 (en) | 2016-11-29 | 2022-08-03 | PureTech LYT, Inc. | Exosomes for delivery of therapeutic agents |
| US11332526B2 (en) | 2017-07-17 | 2022-05-17 | Vib Vzw | Targeting synaptogyrin-3 in tauopathy treatment |
| WO2019016123A1 (en) * | 2017-07-17 | 2019-01-24 | Vib Vzw | Targeting synaptogyrin-3 in tauopathy treatment |
| EP4036234A1 (en) * | 2017-07-17 | 2022-08-03 | Vib Vzw | Screening method for synaptogyrin-3 inhibitors |
| US11058767B2 (en) | 2018-02-21 | 2021-07-13 | Bristol-Myers Squibb Company | CAMK2D antisense oligonucleotides and uses thereof |
| WO2021016474A1 (en) * | 2019-07-23 | 2021-01-28 | Fundación Ciencia Para La Vida (Fcv) | Ibd therapy by inhibition of drd3 in regulatory t cells |
| US11732263B2 (en) | 2020-06-29 | 2023-08-22 | Ionis Pharmaceuticals, Inc. | Compounds and methods for modulating PLP1 |
| WO2022031847A3 (en) * | 2020-08-04 | 2022-04-14 | Dicerna Pharmaceuticals Inc. | Compositions and methods for inhibiting plp1 expression |
| WO2022109165A1 (en) * | 2020-11-18 | 2022-05-27 | Indiana University Research And Technology Corporation | Methods for objective assessment, risk prediction, matching to existing medications and new methods of using drugs, and monitoring responses to treatments for mood disorders |
| CN112957453A (en) * | 2021-03-04 | 2021-06-15 | 上海市精神卫生中心(上海市心理咨询培训中心) | Application of insulin-like growth factor binding protein 2 in preparation of medicine for treating mental disorder caused by brain trauma |
| CN113144173A (en) * | 2021-03-04 | 2021-07-23 | 上海市精神卫生中心(上海市心理咨询培训中心) | Application of insulin-like growth factor binding protein 2 in preparation of medicine for treating mental disorder caused by stress in adult period |
| CN116271051A (en) * | 2023-05-16 | 2023-06-23 | 山东大学 | Application of Phactr4 inhibitor in preparation of depression treatment drugs |
| CN116271051B (en) * | 2023-05-16 | 2023-08-11 | 山东大学 | Application of Phactr4 inhibitor in preparation of depression treatment drugs |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2008020435A3 (en) | 2009-05-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2008020435A2 (en) | Compositions and methods for treatment of mood disorders | |
| CA2880290C (en) | Double-stranded oligonucleotide molecules targeting p53 and methods of use thereof | |
| TWI465238B (en) | Modulation of hsp47 expression | |
| AU2007228570B2 (en) | Treatment of CNS conditions | |
| KR102002771B1 (en) | Retinoid-liposomes for enhancing modulation of hsp47 expression | |
| US20100273854A1 (en) | Compositions and methods for inhibiting nadph oxidase expression | |
| US20090214575A1 (en) | Annexin II and uses thereof | |
| CA2599524C (en) | Materials and methods for treatment of allergic disease | |
| WO2013020097A1 (en) | Double-stranded oligonucleotide compounds for treating hearing and balance disorders | |
| WO2013106494A1 (en) | Combination therapy for treating hearing and balance disorders | |
| US7795233B2 (en) | Composition comprising double stranded RNA that inhibits expression of NEU3 and method for treating cancer | |
| DK2895608T3 (en) | DOUBLE-STRENGTHED OIGONUCLEOTIDE MOLECULES FOR P53 AND PROCEDURES FOR USING IT | |
| WO2023171587A1 (en) | MODIFIED siRNA FOR SELECTIVELY INHIBITING EXPRESSION OF MUTANT FUS | |
| US20230407297A1 (en) | Bioengineered wnt5a therapeutics for advanced cancers | |
| TW202400193A (en) | Compositions and methods for inhibiting transmembrane serine protease 6 (tmprss6) expression |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 07790065 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase in: |
Ref country code: DE |
|
| NENP | Non-entry into the national phase in: |
Ref country code: RU |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 07790065 Country of ref document: EP Kind code of ref document: A2 |