SUBSTITUTED HETEROCYCLIC COMPOUNDS AS MODULATORS OF
THE CCR5 RECEPTOR FIELD OF THE INVENTION This invention relates to substituted heterocyclic compounds which are modulators, agonists or antagonists, of the CC chemokine receptor CC-CKR5 now designated as CCR5 (Nature Medicine 1996, 2, 1174-8). In addition, this invention relates to the treatment and prevention of disease states mediated by CCR5.
BACKGROUND OF THE INVENTION
T cells are not only key regulators of the immune response to infectious agents but are believed critical for the initiation and maintenance of the inflammatory reaction in a variety of chronic diseases. Increased numbers or enhanced activation state of T cells, especially CD4+ T cells, have been demonstrated in the synovium of individuals with rheumatoid arthritis (M. J. Elliott and R. N. Maini, Int. Arch. Allergy Immunol. 104: 112-1125, 1994), in the bronchial mucosa of asthmatics (C.J. Corrigan and A.B. Kay, Immunol. Today 13:501-506, 1992), in the lesions of multiple sclerosis (R. Martin and H. F. McFarland, Crit. Rev. Clin. Lab. Sci. 32: 121-182, 1995), in psoriatic lesions (J.L. Jones, J. Berth- Jone, A. Fletcher and P.E. Hutchinson, J. Pathol. 174: 77-82, 1994) and in the fatty streaks of atherosclerosis (R. Ross, Annu. Rev. Physiol. 57: 791- 804, 1995).
T cells, as well as other inflammatory cells, will migrate into tissues in response to the production of a variety of chemotactic factors. Among these factors are a superfamily of 8-12 kDa proteins known as the chemokines. These proteins share structural features such as the presence of 3-4 conserved cysteine residues. RANTES, which stands for Regulated upon Activation Normal T cell Expressed and Secreted, is an 8 kDa protein member of CC branch of the chemokine family. These proteins recruit and activate immune and inflammatory cells through an interaction with G-protein coupled receptors. The CC branch is defined by the absence of an intervening amino acid residue between the first two cysteine residues and members of this family predominately elicit the migration of mononuclear cells, eosinophils and basophils (M. Baggiolini, B. Dewald, and B. Moser, Adv. Immunol. 55: 97-179, 1994; and J.J. Oppenheim, C.O.C. Zachariae, N. Mukaida, and K. Matsushima, Annu. Rev. Immunol. 9: 617-648, 1991).
RANTES potently produces chemotaxis of T cells, basophils, eosinophils, monocytes and mast cells. RANTES was originally identified as gene product
induced late after antigen activation of T-cells (TJ. Schall, J. Jongstra, B.J. Dyer, J. Jorgensen, et al., J. Immunol. 141:1018-1025, 1988), however, RANTES has been shown to be synthesized and secreted by a diverse group of cells that include epithelial and endothelial cells (C. Stellato, L.A. Beck, G.A. Gorgone, D. Proud, et al., J. Immunol. 155: 410-418, 1995; and A. Marfaing-Koka, O. Devergne, G. Gorgone, A. Portier, et al., J. Immunol. 154: 1870-1878, 1994), synovial fibroblasts (P. Rathanaswami, M. Hachicha, M. Sadick, TJ. Schall, et al., J. Biol. Chem. 268: 5834-5839, 1993) and dermal fibroblasts (M. Sticherling, M. Kupper, F. Koltrowitz, E. Bornscheuer, et al., (J. Invest. Dermatol. 105: 585-591, 1995), mesangial cells (G. Wolf, S. Aberle, F. Thaiss, et al., Kidney Int. 44: 795-804,
1994) and platelets (Y. Koameyoshi, A. Dorschner, A.I. Mallet, E. Christophers, et al., J. Exp. Med. 176: 587-592, 1992). In these cells, RANTES mRNA is rapidly upregulated in response to IL-1 or TNF . Although RANTES mRNA is not usually detected in normal tissues (J.M. Pattison, P J. Nelson, and A.M. Krensky, Clin. Immunother. 4: 1-8, 1995), increased mRNA or protein has been found in diseases characterized by a mononuclear infiltrate. For example, RANTES mRNA was visualized using in situ hybridization in renal allografts undergoing rejection (J.M. Pattison, P.J. Nelson, and A.M. Krensky, Clin. Immunother. 4: 1-8, 1995; and K.C. Nadeau, H. Azuma and N.I. Tilney, Proc. Natl. Acad. USA 92: 8729- 8733, 1995) in the skin of atopic dermatitis patients after exposure to antigen (S. Ying, L. Taborda-Barata, Q. Meng, M. Humbert, et al., J. Exp. Med. 181: 2153- 2159, 1995), and in endothelial cells of coronary arteries undergoing accelerated atherosclerosis after cardiac transplant (J.M. Pattison, P.J. Nelson, and A.M. Krensky, Clin. Immunother. 4: 1-8, 1995). Further, increased immunoreactive protein for RANTES has been detected in bronchoalveolar lavage fluid (R. Alam, J. York, M. Boyers, et al., Am. J. Resp. Crit. Care Med. 149: A951, 1994) and sputum from asthmatic individuals (CM. Gelder, P.S. Thomas, D.H. Yates, I.M. Adcock, et al., Thorax 50: 1033-1037, 1995).
Several receptors have been identified that bind RANTES. In particular, CCR5, when expressed in either HEK 293 cells or CHO cells, binds RANTES . This receptor is expressed in T-cells and in monocytes and macrophages, immune/inflammatory cells that are important in the maintenance of a chronic inflammatory reaction. Pharmacological characterization of CCR5 indicates similarities to the RANTES binding site observed on isolated T cells. Therefore, antagonism of RANTES' action on CCR5, as well as antagonism of other natural modulators of CCR5, should inhibit the recruitment and activation of T cells and macrophages into inflammatory lesions and provide a novel therapeutic approach for the treatment of atopic and autoimmune disorders.
Since T cells express CCR5, selective receptor modulators of CCR5, particularly antagonists, are likely to provide beneficial effects in diseases including, but not limited to, asthma and atopic disorders (for example, atopic dermatitis and allergies), rheumatoid arthritis, sarcoidosis, or idiopathic pulmonary fibrosis and other fibrotic diseases, atherosclerosis, psoriasis, autoimmune diseases such as multiple sclerosis, treating and/or preventing rejection of transplanted organs, and inflammatory bowel disease, all in mammals, preferably humans. Furthermore, since CD8+ T cells have been implicated in chronic obstructive pulmonary disease (COPD), CCR5 may play a role in their recruitment and therefore antagonists to CCR5 could provide potential therapeutic in the treatment of COPD. Also, since CCR5 is a co-receptor for the entry of HIV into cells, selective receptor modulators may be useful in the treatment of HIV infection. Surprisingly, it has now been discovered that this class of non-peptide compounds, in particular substituted heterocyclic compounds of formula (I), function as CCR5 receptor modulators, and therefore, have utility in the treatment and prevention of disease states mediated by CCR5 receptor mechanisms.
SUMMARY OF THE INVENTION
The present invention is to novel compounds of formula (I) and their use as CCR5 modulators for the treatment of certain disease states, including, but not limited to, COPD, asthma and atopic disorders (for example, atopic dermatitis and allergies), rheumatoid arthritis, sarcoidosis, or idiopathic pulmonary fibrosis and other fibrotic diseases, atherosclerosis, psoriasis, autoimmune diseases such as multiple sclerosis, treating and/or preventing rejection of transplanted organs, inflammatory bowel disease, and HIV infection, all in mammals, preferably humans. The preferred compounds for use as CCR5 modulators are those compounds of Formula (I) as noted herein.
Further, the present invention is directed to methods for making and using the compounds of formula (I), as well as pharmaceutical compositions of formula (I) and pharmaceutically acceptable salts or solvates thereof.
Yet further, the present invention is directed to the use of a CCR5 receptor ligand in the manufacture of a medicament for the prophylaxis or treatment of certain disease states, including, but not limited to, COPD, asthma and atopic disorders (for example, atopic dermatitis and allergies), rheumatoid arthritis, sarcoidosis, or idiopathic pulmonary fibrosis and other fibrotic diseases, atherosclerosis, psoriasis, autoimmune diseases such as multiple sclerosis, treating and/or preventing rejection of transplanted organs, inflammatory bowel disease, and HIV infection, for example in a mammal such as a human.
Still further, the present invention is directed to a CCR5 receptor ligand, or a pharmaceutically acceptable salt, or solvate thereof, for use in the prophylaxis or treatment of certain disease states, including, but not limited to, COPD, asthma and atopic disorders (for example, atopic dermatitis and allergies), rheumatoid arthritis, sarcoidosis, or idiopathic pulmonary fibrosis and other fibrotic diseases, atherosclerosis, psoriasis, autoimmune diseases such as multiple sclerosis, treating and/or preventing rejection of transplanted organs, inflammatory bowel disease, and HJN infection, for example in a mammal such as a human.
The present invention is also directed to combined therapy to prevent and treat inflammatory and immunoregulatory disorders or diseases, including asthma and allergic diseases, as well as rheumatoid arthritis and atherosclerosis, and those pathologies noted above, and is illustrated by the combination of the compounds of this invention and other compounds which are know for such utilities.
The present invention is further directed to combinations of the present compounds of formula (I) with one or more agents useful in the prevention or treatment of AIDS. For example, the compounds of this invention may be effectively administered, whether at periods of pre-exposure and/or post-exposure, in combination with effective amounts of the AIDS antivirals, immunomodulators, anti-infectives, or vaccines known to the skilled artisan.
DETAILED DESCRIPTION OF THE INVENTION
It has now been discovered that substituted heterocycles of formula (I) are CCR5 receptor modulators. It has also now been discovered that selective inhibition of CCR5 receptor mechanisms by treatment with the receptor modulators of formula (I), or a pharmaceutically acceptable salt thereof, represents a novel therapeutic and preventative approach to the treatment of a variety of disease states, including, but not limited to, asthma and atopic disorders (for example, atopic dermatitis and allergies), rheumatoid arthritis, sarcoidosis, or idiopathic pulmonary fibrosis and other fibrotic diseases, atherosclerosis, psoriasis, autoimmune diseases such as multiple sclerosis, treating and/or preventing rejection of transplanted organs, and inflammatory bowel disease, all in mammals, preferably humans. Furthermore, since CD8+ T cells have been implicated in COPD, CCR5 may play a role in their recruitment and therefore antagonists to CCR5 could provide potential therapeutic in the treatment of COPD. Also, since CCR5 is a co-receptor for entry into cells, selective receptor modulators may be useful in the treatment of HIV infection.
Preferred compounds for use as CCR5 modulators are those compounds of formula (I) as noted herein.
A preferred group of compounds for use herein are those compounds of the formula (I) or a pharmaceutically acceptable salt or solvate thereof:
Formula (I) wherein: the basic nitrogen in moiety E may be optionally quaternized with C _ βalkyl or is optionally present as the N-oxide;
A' is aryl or heteroaryl, each of which is substituted with one or more of R! and optionally substituted with one or more of R! '; or A' is aryl or heteroaryl fused to a saturated or partly unsaturated 5-7-membered ring to form a higher order ring moiety, which ring moiety optionally contains 1 or 2 heteroatoms selected from oxygen, nitrogen or sulfur, wherein nitrogen may be optionally substituted with hydrogen, C^galkyl or C3_7cycloalkyl, wherein the higher order ring moiety is substituted with one or more of R!" and optionally substituted with one or more of R1';
Rl' is hydrogen, C^alkyl, C2_6alkenyl, C _6alkynyl, C3_7cycloalkyl, C3_ gcycloalkenyl, CH2CF3, aryl, aralkyl, (CH2)a'NR2'R3', (CH2)a'NR2'COR4', (CH2)a'NR2'CO2R5', (CH2)a>NR2'SO2R6', (CH2)a€ONR7'R8', hydroxyCi. galkyl, Cι_4alkoxy alkyl (optionally substituted by a C^alkoxy or hydroxy group), (CH2)aCO2Cι.6alkyl, (CH2)bOC(O)R9', CR10'=NORH', CNRiO^NOR1 1', COR12', CONR7'R8', CONR7'(CH2)c Cι.4alkyl, CONR7'(CH2)a€O2R13', CONHNR14'R15', CONR7'SO2R16', CO2R17', cyano, trifluoromethyl, NR2'R3', NR2'COR4', NR18'CO(CH2)a>NR18'R19', NRl8'C0NRl8'Rl9', NR2'CO2R5', NR2'SO2R6', N=CNR18'NR18'R19', nitro, hydroxy, C^galkoxy, OCF3, hydroxyC^alkoxy, C^galkoxyCi.galkoxy,
OC(O)NR20'R21', SR22', SOR23', SO2R23', SO2NR20'R21 ' or halogen, or Rris a 5- to 7-membered ring containing 1 to 4 heteroatoms selected from nitrogen, oxygen, or sulfur, optionally substituted with one or more of hydrogen, Cj.galkyl, C3_7cycloalkyl, C3_6cycloalkenyl, hydroxyC^galkyl, (Cι_6alkyl)Cι.6alkyl, CONR7'R8', CO2R17', cyano, aryl, trifluoromethyl, nitro, hydroxy, C^galkoxy, acyloxy, or halogen;
Rl" is hydrogen, (CH2)aGN, (CH2)aCO2H, CR10'=CRH'CO2R12', COCRIO'RH 'OR12', Oaryl, Oaralkyl, O(CH2)aCO2R12', or Saryl; a' is 1, 2, 3 or 4; b' is 0, 1, 2 or 3;
c' is 1, 2 or 3;
R2' and R3' are independently hydrogen or Cj.galkyl, or R2' and R3' together with the nitrogen to which they are attached, form a 5- to 6-membered heterocyclic ring which ring may be optionally substituted by an oxo group, or, when there are 6 ring members, the ring may optionally contain one oxygen or one sulfur atom;
R4'is hydrogen, Ci.βalkyl or Cj^alkoxyalkyl, or, when R* is NR2'COR4', R4' is (CH2)ι_3 and forms a ring with A'; R5'is Cι_6alkyl; R6' is C galkyl or phenyl;
R7' and R8' are independently hydrogen or C galkyl, or R7 and R8' together with the nitrogen to which they are attached form a 5- to 6-membered saturated heterocyclic ring, wherein when there are 6 ring members, the ring may optionally contain one oxygen or one sulfur atom; R9' is Cι_4alkyl, optionally substituted by a C^alkoxy;
RIO' and R! 1 ' are independently hydrogen or C^alkyl; R 2' is hydrogen or C^.galkyl; Rl 'is hydrogen or Cι_6alkyl;
R!4' and R^' are independently hydrogen or C^galkyl; Rl6' 1S hydrogen or C^galkyl;
R 7' is hydrogen or C^alkyl optionally substituted with one or more substituents selected from Cι_6alkyl, C^galkoxy, hydroxy, or NR2'R3'; R! ' and Rl9' are independently hydrogen or C^galkyl; R2^' and R2* ' are independently hydrogen or Ci.g lkyl, or R2^' and R ' together with the nitrogen to which they are attached form a 5- to 6-membered saturated heterocyclic ring which, when the ring is 6-membered, may optionally contain in the ring one oxygen or one sulfur atom. R22' is hydrogen or Chalk !; R23'is Cι_6alkyl; D' is either a bond or represents [C(R24')2]a», [C(R24')2]a»CO, CO, SO2,
CO[C(R24')2]a», O[C(R24')2]a », S[C(R2 ')2]a-, O[C(R24')2]a»CO, [C(R24')2]c»OCO, NR25'[C(R 4')2]a», NR25'[C(R24')2]a»CO, [C(R24')2]C»NR25'C0, NR 5"C0[C(R 4)2]a-, NR 5'sO2[C(R ')2]a», [C(R24')2]C>NR25'S02, CR24'=CR2 'CO, Gέ CCO, (C(R24')2)c»SO2, SO2[C(R24')2]a", NR25'[C(R24')2]a"SO2, NR25'sO2[C(R24')2]a-SO2,
O[C(R24')2]a"SO2, SO2NR 5'[C(R ')2]1_2, [C(R24')2]b »COO[C(R24')2]2, [C(R24')2]b"CONR25'[C(R24')2] i_2; and when E' and G' together are CR27 - C(R26')2, then D' may further be O, NR25', CONR25', SO2NR25', OCONR25',
NR25'C00, NR25'CONR 5', [C(R24')2]a"NR25'[C(R24')2]b.., [C(R 4')2]a»O[C(R24')2]b--, CO[C(R24')2]a--NR25', NR 5'[C(R24')2]a»O, NR25'[C(R24')2]a--NR25', O[C(R2 ')2)]a-NR25', O[C(R24')2]a-O, CO[C(R2 ')2]a» , SO2[C(R24')2]a»NR25', SO2[C(R2 ')2]a"O, [C(R2 ')2]a»SO2NR25', [C(R24')2]a"€ONR25', O[C(R 4')2]a"SO2NR25', O[C(R24')2]a"CONR25', NR25'[C(R2 ')2]a"SO2NR25', NR25'[C(R24')2]a--C0NR 5', NR25'C0[C(R24')2]a"NR25', NR25'SO2[C(R24')2]a"NR25', (C(R24')2)a»S(C(R24')2)b» COO, CR2 'θH, C(R24')a»CR24'OH; and when E' and G' together are CR27'-C(R26')2 or C=CR26', D' may further be CR24'=CR24' or C£ C; and a«' is 1-6, b» is 0-1, c« is 0-2; R24' is hydrogen or Cχ_6alkyl; R2^' is hydrogen or Cχ_6alkyl;
E' and G' together are NC(R26')2, NC(R26')2C(R26')2, CR27'C(R26')2 or C=CR 6'; R2^ ' is hydrogen or C \ _galkyl ;
R27'is hydrogen, OR28', NHR28', CN, NO2, R28', SR29', COR28', CHOHR28', CO2R28', NHCOR28', NHCO2R29', NHSO2R29', or OCONHR28'; R28' is hydrogen, C^alkyl, aryl or aralkyl; R29'is Cχ_5 alkyl, aryl or aralkyl; R' is one or more of hydrogen or Cχ_galkyl, or R' is oxo;
J' is CO or SO2; L'is NR30', O or C(R30')2; R3^'is hydrogen or Cχ_6alkyl; E represents a group (a):
B is oxygen, Cg C, S(O)c, CR7=CR8, or CR7R8, or B is NR9; R! and R2 are independently hydrogen or Cx.galkyl; alternatively B(CR!R2)a is OCR1R2CR1(OH)CR1R2 or OCR1R2CR1(OCOCH3)CR1R2;
R3 and R4 are independently hydrogen, Cχ_6alkyl, C3_7cycloalkyl, aralkyl, C5_7cycloalkenyl, a C5_7heterocyclic ring, or together with the nitrogen atom to which they are attached form an optionally substituted 5- to 7-membered heterocyclic ring which may contain an additional heteroatom selected from oxygen, nitrogen or sulfur, where optional substituents include Cx.galkyl, aryl,
CONRIORI I, NR10Rπ, hydroxy, OCOR12, NHCOCF3, NHSO2R13, NHCO2R14, or NHCOC0-6alkyl wherein the alkyl of NHCOC0-6alkyl is optionally substituted by OH;
R5 is hydrogen, C^alkyl, aryl, CN, CONR15R16, CO2R17, trifluoromethyl, NHCO2R18, hydroxy, C^alkoxy, benzyloxy, OCH2CO2Cχ_ 6alkyl, OCF3, S(O)dR19, SO2NR2θR2l or halogen;
R6 is hydrogen, Cχ_6alkyl, aryl, trifluoromethyl, hydroxy, Cχ_6alkoxy or halogen, or R^ taken together with R3^' forms a group D where D is (CR2 R )e or D is (CR22R23)f-G where G is oxygen, sulfur or CR22=CR23, CR22=N, =CR22O, =CR22S, or =CR22-NR23;
R7, R8, RlO, Rl 1, Rl , Rl5, R16, R17, R20, R21; R22, md R23 ^ independently hydrogen or C _galkyl;
R9 is hydrogen, Cχ_galkyl, or phenylCχ_6alkyl; R13, R14, R18, and R19 are independently Ci^alkyl; a is 1, 2, 3, or 4; b is 1 or 2; c and d are independently 0, 1 or 2; e is 2, 3 or 4; fis O, 1, 2 or 3; alternatively, E represents a group (b):
R24, R25, R26, R27, R28, R29, R31, and R32 are independently hydrogen or Cχ_6alkyl;
R ^ is hydrogen, Cj.galkyl, C3_7cycloalkyl, C5_7cycloalkenyl, or a C5_ 7heterocyclic ring;
R33 is hydrogen, Ci.galkyl, trifluoromethyl, hydroxy or halogen, or R33 and R30' together form a group -K- where K is (CR34R35)i or K is (CR34R35)j - M and M is oxygen, sulfur, CR 4=CR35, CR34=N, or N=N; J is oxygen, CR36R37, or NR38, or J is a group S(O)k; R34, R3^, R36, R37, 3 d R38 are independently hydrogen or Cχ_6alkyl; g is 1, 2 or 3; h is 1, 2 or 3; i is 2, 3, or 4;
j is O, 1, 2, or 3; k is 0, 1 or 2; alternatively, E represents a group (c):
Q is oxygen, S(O)n, CR44=CR45, CR44R45, or Q is NR46; R39 and R ^ are independently hydrogen or Cχ_galkyl; R 1 is a group of formula (d):
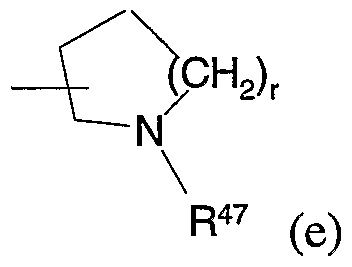
or R41 is a group of formula (e):
R42 is hydrogen, C^ancyl, aryl, CN, CONR 8R49, CO2R50, trifluoromethyl, NHCO2R51 , hydroxy, C \ _6alkoxy , benzyloxy, OCH2CO2C \ _ 6alkyl, OCF3, S(O)sR52, SO2NR53R54, or halogen;
R43 is hydrogen or R43 together with R3^' forms a group R where R is CR55=CR56, CR55=CR56CR55R56, or (CR55R56)t;
R44, R45, R46, R48, R49, R50; R53^ R545 R55? and R56 are independently hydrogen or Cι_galkyl;
R47 is hydrogen, Cχ_galkyl, C3_7 cycloalkyl, C5_7cycloalkenyl, or a C5. 7heterocyclic ring;
R^l and R^2 are independently Cχ_6alkyl; l is O, 1, 2, or 3; m is 1 or 2; n is O, 1, or 2 o, p, and q are independently integers having the value 1, 2, or 3; r is 0,1, 2, or 3;
s is O, 1, or 2; t is 2 or 3; alternatively, E represents a group (f):
R5 and R^8 are independently hydrogen or Cχ_6alkyl; R59 and R^O are independently hydrogen, Cχ_6alkyl, C3_7cycloalkyl, aralkyl, C5_7cycloalkenyl, a C5_7heterocyclic ring, or together with the nitrogen atom to which they are attached form an optionally substituted 5- to 7-membered heterocyclic ring which may contain an additional heteroatom selected from oxygen, nitrogen or sulfur, where optional substituents include C^alkyl, aryl, CONR61R62, NR61R62, hydroxy, OCOR63, NHCOCF3, NHSO2R64, NHCO2R65, or NHCOC0_6alkyl wherein the alkyl of NHCOC0-6alkyl is optionally substituted by OH;
T is -(CR66R67)V- or -O(CR66R67)w-; W is oxygen, S(O)x, NR68, or W is CR69=CR70 or CR69R70; R615 R625 R63; R66S R67 685 R695 and R70 are independently hydrogen or Cχ_6alkyl; R64 and R65 are independently C 1 _6alkyl; u is 1 to 4; v is 2 or 3; w is 1, 2, or 3; x is 0, 1 or 2; alternatively, E represents a group (g):
R7* is a 5- to 7-membered saturated or partially saturated heterocyclic ring containing a basic nitrogen atom and optionally a further 1 or 2 heteroatoms selected from nitrogen, oxygen or sulfur, or R7^ is an optionally substituted 6,6 or 6,5 bicyclic ring containing a nitrogen atom and optionally a further heteroatom selected from oxygen, nitrogen or sulfur, which ring systems may be optionally substituted with one
or more of Cχ_6alkyl and optionally substituted on nitrogen with hydrogen, Cχ_ galkyl C3_7cycloalkyl, C5_7cycloalkenyl, or a C5_7heterocyclic ring; and wherein R7 is substituted with one or more of R7!' , wherein R71" is hydrogen, CRlaR2"NR3"R4", CRlaR2"θR3", COR5", CONR6"R7", CO2R8", cyano, NR3"R4", nitro, hydroxy, C^alkoxy, SR9", SOR10", SO2R10", SO2NR6"R7", or SO3H, provided that R71" is not a substituent on the basic nitrogen of R71 ; and wherein R a and R2" are independently hydrogen or Cχ_6alkyl; R3" and R4" are independently hydrogen or Cx.galkyl, or together with the nitrogen atom to which they are attached form a 5- to 6-membered saturated heterocyclic ring, wherein, when the ring is 6-membered, may optionally contain one oxygen or one sulfur atom; or, R4" is CORll", CONR12"R13", CO2Rl4", SO2R15", SO2NRl2"Rl *', or S020R16"; wherein, RU" is hydrogen, Cj.galkyl, aryl, or trifluoromethyl; R^2" and Rl3" are independently hydrogen or Cχ_6alkyl, or together with the nitrogen atom to which they are attached form a 5- to 6-membered saturated heterocyclic ring, wherein, when the ring is 6-membered, may optionally contain one oxygen or one sulfur atom; Rl " is Ci.galkyl or aryl; Rl5" is Cj.galkyl, aryl, or trifluoromethyl; and Rl6" is aryl; R5" is hydrogen, Cχ_6alkyl, aryl, or trifluoromethyl; R6" and R7" are each independently hydrogen or Cx.galkyl, or together with the nitrogen atom to which they are attached form a 5- to 6-membered saturated heterocyclic ring, wherein, when the ring is 6-membered, may optionally contain one oxygen or one sulfur atom; R8" is hydrogen or C^.^alkyl; R9' is hydrogen, Cχ_6alkyl, aryl, or trifluoromethyl; and
RIO ' is Cχ_6alkyl, aryl, or trifluoromethyl; R72 is hydrogen, Cμgalkyl, aryl, CN, CONR74R75, CO2R76, trifluoromethyl, NHCO2R77, hydroxy, Cχ_galkoxy, benzyloxy, OCH2CO2Cχ_ 6alkyl, OCF3, S(O)zR78, SO2NR79R80, or halogen; R73 is hydrogen, Cχ_galkyl, hydroxy, Cχ_galkoxy or halogen, or R73 and
R ^' taken together from a group -X- where X is (CR 1R 2)aa or X is (CR81R 2)ab-Y and Y is oxygen, sulfur or CR81=CR82;
R74, R75, R76, R79, R80, Rδl, and R82 are independently hydrogen or Cι_6alkyl; R77 and R78 are independently Cx.galkyl; y is 1 or 2; z is O, 1, or 2; aa is 2, 3 or 4;
ab is 0, 1, 2 or 3; alternatively, E represents a group (h):
R87 (h); wherein:
R83 and R84 are independently hydrogen or Cx.galkyl; R85 and R8^ are independently hydrogen, C^alkyl, C3_7cycloalkyl, aralkyl, C5_7cycloalkenyl, a C5_7heterocyclic ring, or together with the nitrogen atom to which they are attached form an optionally substituted 5- to 7-membered heterocyclic ring which may contain an additional heteroatom selected from oxygen, nitrogen or sulfur, where optional substituents include C^alkyl, aryl, CONR88R89, NR90R915 hydroxy, OCOR92, NHCOCF3, NHSO2R93, NHCO2R94, or NHCOC0-6alkyl wherein the alkyl of NHCOC0-6alkyl is optionally substituted by OH; R87 is hydrogen or Cχ_6alkyl, C^.^alkoxy, or halogen, or R87 together with R3^' forms a group -AA- where AA is (CR95R 6)a(j or AA is (CR95=CR96)ae-AB and AB is oxygen, sulfur, CR95=CR96, CR95=N, CR95NR96 OΓ N=N;
Z is an optionally substituted 5 to 7-membered heterocyclic ring containing 1 to 3 heteroatoms selected from oxygen, nitrogen or sulfur;
R88, R89, R 0S R91; R92; R95? ancχ R96 are independently hydrogen or Cι_6alkyl;
R9 and R94 are independently C^alkyl; ac is 0 to 4; ad is 1, 2 or 3; ae is 0, 1 or 2; alternatively, E represents a group (i):
R97 and R9 are independently hydrogen, C^ lkyl, C3_7cycloalkyl,
aralkyl, C5_7cycloalkenyl, a C5_7heterocyclic ring, or together with the nitrogen atom to which they are attached form an optionally substituted 5- to 7-membered heterocyclic ring which may contain an additional heteroatom selected from oxygen, nitrogen or sulfur, where optional substituents include Cχ_6alkyl, aryl, CONR102R103, NR104R105, hydroxy, OCOR106, NHCOCF3, NHSO2 Rl07, NHCO2Rl08, or NHCOC0-6alkyl wherein the alkyl of NHCOC0_6alkyl is optionally substituted by OH;
R9 and RIOO are independently hydrogen or Cl-6alkyl; RIOI is hydrogen or C^alkyl or R^Ol and R3^' together form a group - AD- where AD is (CR1Q9R110)ai or AD is (CR1Q9R110)aj-AE and AE is oxygen, sulfur or CR109=CR110;
AC is oxygen, CR11 ΪR112 or NR1 13 or AC is a group S(O)a ;
R102 RIOS, R104 R1055 R106 R109, Rl 10, Rl 11 , Rl 12 and Rl I3 are independently hydrogen or Cj.galkyl; R107 and R108 are independently Cl-6alkyl; af is O, 1, 2, 3, or 4; ag is 1, 2, or 3; ah is 1, 2, 3 or 4; ai is 2, 3 or 4; aj is 0, 1, 2, or 3; and ak is 0, 1 or 2, provided that when Rl ' is hydrogen and E is a group (a), (f) (h) or (i), then one or both of R3 or R4; R59 or R60; R85 or R86; or R97 or R98 is C5_7cycloalkenyl, or a C5_7heterocyclic ring; or when R* ' is hydrogen and E is a group (b) or (c), then R3^ and R47 are C5_7cycloalkenyl, or a C5_7heterocyclic ring; or when Rl" is hydrogen and E is group (g), then either R7 " is not hydrogen and/or R ^ is substituted on nitrogen with C5_7cycloalkenyl or a C5_7heterocyclic ring.
For compounds of formula (I) various embodiments are as follows. It will be understood that the basic nitrogen in moiety E may be optionally quaternized with Cx-.galkyl or is optionally present as the N-oxide.
Suitably, A' is aryl or heteroaryl, each of which is substituted by one or more of R^ ' and each of which is optionally substituted with one or more of Rl '. Alternatively, A' is suitably aryl or heteroaryl fused to a saturated or partly unsaturated 5-7-membered ring to form a higher order ring moiety, which ring moiety optionally contains 1 or 2 heteroatoms selected from oxygen, nitrogen or sulfur, wherein nitrogen may be optionally substituted with hydrogen, Cx.galkyl or C3_7cycloalkyl, wherein the higher order ring moiety is substituted with one or more of Rl " and optionally substituted with one or more of R '. Preferably A' is
phenyl, 5,6,7,8-tetrahydro-l-naphthalenyl, lH-indol-4-yl, or 2-benzothiazolyl. Suitably, R^'is hydrogen, Cj.galkyl, C2_6alkenyl, C2_6alkynyl, C3_ 7cycloalkyl, Cs.gcycloalkenyl, CH CF3, aryl, aralkyl, (CH2)a'NR2'R3', (CH2)a'NR2'COR4', (CH2)aNR2'CO2R5', (CH2)a>NR2'SO2R6', (CH2)aCONR7'R8', hydroxy Ci.galkyl, C^alkoxy alkyl (optionally substituted by a Cχ_4alkoxy or hydroxy group), (CH2)aCO2Cι_6alkyl, (CH2)bOC(O)R9', CRiO^NOR11', CNR10'=NORH ', COR*2', CONR7'R8', CONR7'(CH2)C C1. 4alkyl, CONR7'(CH2)a€O2R13', CONHNR14'R15', CONR7'SO2R16', CO2R17', cyano, trifluoromethyl, NR2'R3', NR2'COR4', NR18'CO(CH2)a>NR18'R19', NR18'CONR18'R19', NR2'CO2R5', NR2'SO2R6', N=CNR18'NR18'R19', nitro, hydroxy, Cχ_6alkoxy, OCF3, hydroxyC1_0'alkoxy, Ci_6alkoxyCI_6alkoxy, OC(O)NR20'R21', SR22', SOR23', SO2R23', SO2NR20'R21' or halogen, or suitably Rl' is a 5- to 7-membered heterocyclic ring containing 1 to 4 heteroatoms selected from oxygen, nitrogen, or sulfur. Suitable heterocyclic rings include aromatic groups such as thienyl, furyl, pyrrolyl, triazolyl, diazolyl, imidazolyl, oxazolyl, thiazolyl, oxadiazolyl, isothiazolyl, isoxazolyl, thiadiazolyl, pyridyl, pyrimidyl, pyrazinyl, and dioxanyl. Saturated and partially saturated rings are also within the scope of the invention, in particular rings including an oxo or thioxo moiety such as lactams and thiolactams. Suitably, the heterocyclic ring can be linked to the remainder of the molecule via a carbon atom, or, when present, a nitrogen atom. Suitably these rings may be optionally substituted with one or more of hydrogen, C^alkyl, C3_7cycloalkyl, C3_6cycloalkenyl, hydroxyC j.galkyl, (Cι_6alkyl)Cι_6alkyl, CONR7'R8', CO2R17', cyano, aryl, trifluoromethyl, nitro, hydroxy, C^alkoxy, acyloxy, or halogen. Preferably, Rl' is one or more of Cι_6alkyl, (CH2)a'NR2'COR4', CF3, CO2R17', wherein R17' is C galkyl, C _galkoxy, halogen, or cyano.
Suitably, R1" is hydrogen, (CH2)a>CN, (CH2)aCO2H, CRiO^CRϋ'CO^13', COCRlO'Ri i'OR13', Oaryl, Oaralkyl, O(CH2)aCO2Rl ', and Saryl. Suitably, R2' and R3' are independently hydrogen or Ci^alkyl, or suitably,
R2' and R3' together with the nitrogen to which they are attached, form a 5- to 6- membered heterocyclic ring. Suitably, the ring may be optionally substituted by an oxo group, or, when R2' and R3' form a 6-membered ring, the ring may optionally contain one oxygen or one sulfur atom. When the ring is a 6-membered ring substituted by an oxygen or sulfur atom, the oxygen or sulfur atom are preferably in the 4-position.
Suitably, R4'is hydrogen, Ci.galkyl or Cχ_4alkoxyalkyl, or, when Rl'is NR2'COR4', R4' is (CH2)ι_3 and forms a ring with A'.
Suitably R5' is Cμgalkyl. Suitably, R >' is Cχ_galkyl or phenyl. Suitably, R7' and R8 are independently hydrogen or Cj.galkyl, or suitably, R7' and R8' together with the nitrogen to which they are attached form a 5- to 6- membered saturated heterocyclic ring. Suitably, when the ring is 6-membered, the ring may optionally contain one oxygen or one sulfur atom.
Suitably, R ' is C^alkyl, wherein the Cx.galkyl is optionally substituted by a Cx.galkoxy.
Suitably, RlO' and Rl 1 ' are independently hydrogen or
Suitably, R^
2' is hydrogen or C^.^alkyl.
Suitably, R 3' is hydrogen or Cx.galkyl.
Suitably, R^4' and R 5' are independently hydrogen or C^alkyl. Suitably, R^' is hydrogen or Cj.galkyl.
Suitably, R^7' is hydrogen or Cj.galkyl, wherein the Cj.galkyl is optionally substituted with one or more substituents selected from Ci.galkyl, Cχ_ galkoxy, hydroxy, or NR2R3'. Preferably, when there is more than one substituent, there are two substituents.
Suitably, R ' and R^9' are independently hydrogen or Cχ_galkyL Suitably, R2^' and R2 ' are independently hydrogen or C^alkyl, or suitably, R2^' and R21 ' together with the nitrogen to which they are attached form a 5- to 6-membered saturated heterocyclic ring which, when there are 6 ring members, may optionally contain in the ring one oxygen or one sulfur atom. Suitably, R22'is hydrogen or C^.^alkyl. Suitably, R23'is Ci.galkyl. Suitably, D' is either a bond or represents [C(R24')2]a", [C(R24')2]a»CO,
SO2, CO, CO[C(R24')2]a", O[C(R24')2]a», S[C(R24')2]a", O[C(R2 ')2]a"CO, [C(R24')2]c»OCO, NR25'[C(R24')2ja", NR25'[C(R24')2]a»CO, [C(R ')2]c"NR25'CO, NR25'CO[C(R24')2]a.., NR2 'SO2[C(R2 ')2]a", [C(R24')2]c"NR25'SO2, CR24'=CR24'CO, Cg CCO, (C(R24')2)c"SO2, SO2[C(R 4')2]a", NR25'[C(R24')2]a"SO2, NR25'SO2rC(R24')2]a »SO2,
O[C(R24')2]a"SO2, SO2NR25'[C(R24')2]1.2, [C(R24')2]b »COO[C(R24')2]2, [C(R24')2]b»CONR25'[C(R24')2]1_2; and when E' and G' together are CR2 - C(R26') , then D' may further be O, NR25', CONR25', SO2NR25', OCONR25', NR25"COO, NR25CONR25', [C(R24')2]a»NR25'[C(R24')2]b», [C(R24')2]a"O[C(R24')2]b", CO[C(R24')2]a"NR25', NR25 rC(R24')2]a„O, NR25'[C(R24')2]a »NR25', O[C(R24')2)]a-.NR25', O[C(R24')2]a»O, CO[C(R24')2]a"O, SO2[C(R24')2]a"NR25', SO2[C(R24')2]a »O, [C(R24')2]a »SO2NR25', [C(R24')2]a-CONR25', O[C(R24')2]a-SO2NR25',
O[C(R24')2]a"CONR25', NR25'[C(R24')2]a»SO2NR25', NR25'[C(R24')2]a"CONR25', NR25'CO[C(R24')2]a-NR25', NR25'SO2[C(R24')2]a"NR25', (C(R24')2)a»S(C(R24')2)b», COO, CR24'θH, C(R24')a»CR2 'θH; and when E' and G' together are CR27'-C(R26')2 or C=CR26', D' may further be CR24=CR24 or C C; and a» is 1-6, b- is 0-1, c» is 0-2. Preferably, D' is a bond, CO or SO2.
Suitably, R24'is hydrogen or Cx.galkyl. Suitably, R25' is hydrogen or Ci-.galkyl.
Suitably, E' and G' together are NC(R26')2, NC(R26')2C(R26')2, CR27'C(R26')2 or C=CR26'. Preferably, E' and G' together are NC(R26')2.
Suitably, R2"' is hydrogen or C^alkyl. Preferably, R2"' is hydrogen. Suitably, R27'is hydrogen, OR28', NHR28', CN, NO2, R28', SR29', COR29', CHOHR29', CO2R29', NHCOR29', NHCO2R29', NHSO2R29', or OCONHR29'. Suitably, R28' is hydrogen, Ci^alkyl, aryl or aralkyl.
Suitably, R29'is Cχ_5alkyl, aryl or aralkyl. Suitably, R' is one or more of hydrogen or Cχ_galkyl, or R' is oxo. Preferably, R' is hydrogen.
Suitably, J' is CO or SO2. Preferably, J' is CO. Suitably, L' is NR30', O, or C(R30')2. Preferably, L' is NR30'.
Suitably, R30' is hydrogen or Cx.galkyl. Preferably, R30' is hydrogen. Suitably, substituent E is selected from the following groups:
E suitably represents a group (a):
R6 (a). B is suitably oxygen, Cg C, S(O)c, CR7=CR8> or CR7R8, or B is NR9. B is preferably CR7R8, or oxygen.
Rl and R2 are suitably independently hydrogen or C^alkyl. Preferably, R1 and R2 are hydrogen. Alternatively, B(CR!R2)a is OCR^CR^OH^RiR2 or OCR1R2CR1(OCOCH3)CR1R2. Preferably, when B(CR!R2)a is OCR^CR^OH^RiR2 or OCR1R2CR1(OCOCH3)CR1R2, R1 and R2 are hydrogen.
R3 and R4 are suitably independently hydrogen, Cχ_galkyl, C3_ 7cycloalkyl, aralkyl, C5_7cycloalkenyl, a C5_7heterocyclic ring, or together with the nitrogen atom to which they are attached form an optionally substituted 5- to 7- membered heterocyclic ring which may contain an additional heteroatom selected from oxygen, nitrogen or sulfur, where optional substituents include Cχ_6alkyl, aryl, CONR10Rn, NR10RH, hydroxy, OCOR12, NHCOCF3, NHSO2 R13, NHCO2R14, or NHCOC0-6alkyl wherein the alkyl of NHCOC()-6alkyl is optionally substituted by OH. Preferably R3 and R4 are both C^alkyl, C5. 7cycloalkenyl, a C5_7heterocyclic ring, or together with the nitrogen atom to which they are attached form an optionally substituted 5- to 7-membered heterocyclic ring which may contain an additional heteroatom selected from oxygen, nitrogen or sulfur.
Preferably, B-(CR1R2)a-NR3R4 is ortho to R5, meta to L' and para to R6, and R5 is para to L\
R5 is suitably hydrogen, C^alkyl, aryl, CN, CONR15R16, CO2R17, trifluoromethyl, NHCO2R18, hydroxy, Ci.galkoxy, benzyloxy, OCH2CO2Cχ_ 6alkyl, OCF3, S(O)dR19, SO2NR20R21, or halogen. R5 is preferably Cχ_ galkoxy, SC^alkyl or halogen. R is suitably hydrogen, C^alkyl, aryl, trifluoromethyl, hydroxy, Cχ_ galkoxy, or halogen, or R6 taken together with R30' forms a group D where D is
(CR22R23)e or D is (CR22R23)f-G where G is oxygen, sulfur, or CR22=CR23, CR22=N, =CR22O, =CR22S, or =CR22-NR23. Preferably, R6 is hydrogen. R7, R8, RlO, RU, Rl2, R15, R16 R17, R20, R21; R22 and R23 are suitably independently hydrogen or Cχ_6alkyl. R9 is suitably hydrogen, Cχ_6alkyl, or phenylCx.galkyl.
R13, R14, R18, and R19 are suitably independently Ci.galkyl. a is suitably 1, 2, 3, or 4. Preferably, a is 2 or 3. b is suitably 1 or 2. Preferably, b is 1. c and d are suitably independently 0, 1, or 2. e is suitably 2, 3, or 4. f is suitably 0, 1, 2, or 3. Alternatively, E suitably represents a group (b):
Suitably, R
24, R
25, R
26,
R27
; R28
; R29
s R31
; and R
32 are independently hydrogen or Ci^alkyl. R
24, R
25, R
26, R
27, R
28, R
29, R31, and R
32 are preferably hydrogen.
R30 is suitably hydrogen, Cχ_6alkyl, C3_7cycloalkyl, C5_7cycloalkenyl, or a C5_7heterocyclic ring. Preferably, R30 is Cχ_5alkyl, C3_7cycloalkyl, C5_ 7cycloalkenyl, or a C5_7heterocyclic ring.
R33 is suitably hydrogen, Cχ_6alkyl, trifluoromethyl, hydroxy or halogen, or R33 and R30' together form a group -K- where K is (CR34R35)i or K is (CR34R35)j -M and M is oxygen, sulfur, CR 4=CR35, CR34=N, or N=N. Preferably, R33 is hydrogen. J is suitably oxygen, CR36R37, or NR38, or J is a group S(O)k. Preferably,
J is oxygen. Preferably, J is para to L'.
R
34, R
35, R
36, R
37, R
38 are suitably independently hydrogen or Cχ_
6alkyl. g is suitably 1, 2, or 3. Preferably, g is 2 or 3. h is suitably 1, 2, or 3. Preferably, h is 1. i is suitably 2, 3, or 4. j is suitably 0, 1, 2, or 3. k is suitably 0, 1 or 2. Alternatively, E suitably represents a group (c):
Suitably, Q is oxygen, S(O)n, CR =CR45, C=C , or CR44R45, wherein n is 0, 1 or 2, and R44 and R45 are independently hydrogen or C^an yl, or suitably, Q is NR ^ wherein R4^ is hydrogen or alkyl. Suitably, R39 and R40 are independently hydrogen or Cj^alkyl.
Suitably, R42 is hydrogen, Cι_6alkyl, aryl, CN, CONR48R49, CO2R50, trifluoromethyl, NHCO2R51, hydroxy, C galkoxy, benzyloxy, OCH2CO2Cχ_ galkyl, OCF , S(O)sR52, SO2NR53R54, or halogen, wherein R48, R49, R50, R53, and R54 are hydrogen or Cχ_galkyl, and R5 and R52 are Cχ_6alkyl. Suitably, R43 is hydrogen or R43 together with R30' forms a group R where R is CR55=CR56, CR55=CR56CR55R56, or (CR55R56)t wherein R55 and R5^ are independently hydrogen or Cx.galkyl and t is 2 or 3. Suitably, R is selected from a group of formula (d) or (e). Suitably R47 is hydrogen, Cχ_6alkyl, 03.7 cycloalkyl, C5_7cycloalkenyl, or a C5_7heterocyclic ring.
Suitably, 1 is 0, 1, 2 or 3, m is 1 or 2, n and s are independently 0, 1 or 2, o, p and q are independently 1, 2 or 3, and r is 0, 1, 2 or 3. Alternatively, E suitably represents a group (f):
Suitably, R57 and R58 are independently hydrogen or C .galkyl.
Suitably R59 and R^° are independently hydrogen, Ci.galkyl, C3. 7cycloalkyl, aralkyl, C5_7cycloalkenyl, a C5_7heterocyclic ring, or together with the nitrogen atom to which they are attached form an optionally substituted 5- to 7- membered heterocyclic ring which may contain an additional heteroatom selected from oxygen, nitrogen or sulfur, wherein optional substituents include Cχ_6alkyl, aryl, CONR61R62, NR61R62, hydroxy, OCOR63, NHCOCF3, NHSO2R64 NHCO2R65 or NHCOC0_6alkyl, wherein the alkyl of NHCOC0.6alkyl is optionally substituted by OH, and wherein R^l, R62, m R^3 are independently hydrogen or Cχ_6alkyl, and R^ and R^5 are independently Cj.galkyl
Suitably, T is -(CR66R67)V- or -O(CR66R67)w-, wherein R66 an R67 are independently hydrogen or Cx.galkyl, wherein v is 2 or 3, and w is 1, 2 or 3.
Suitably, W is oxygen, S(O)x, wherein x is 0, 1 or 2, or W is NR^ , wherein R68 is hydrogen or C^alkyl, or W is CR69=CR70, C=C, or CR69R70, wherein R^9 and R70 are independently hydrogen or Cj.galkyl. Suitably, u is an integer from 1-4. Alternatively, E suitably represents a group (g):
Suitably, R7^ is an optionally substituted 5- to 7-membered saturated or partially saturated heterocyclic ring containing a basic nitrogen atom, and optionally containing one or two heteroatoms selected from nitrogen, oxygen or sulfur, or R7 is an optionally substituted 6,6 or 6,5-bicyclic ring system containing a nitrogen atom, and optionally containing a heteroatom selected from oxygen, nitrogen or sulfur, which ring systems may be optionally substituted with one or more of Cχ_6alkyl, and optionally substituted on nitrogen with hydrogen, Cχ_galkyl C3_7cycloalkyl, C5_7cycloalkenyl, or a C5_7heterocyclic ring. Examples of such ring systems include, but are not limited to, pyrrolidine, piperidine, piperazine, morpholine, imidazolidine, pyrazolidine, 1,2,3,6- tetrahydropyridine, hexahydroazepine, tropane, isoquinuclidine and granatane rings. Preferably, R7^ is an optionally substituted 5- or 6-membered saturated or partially saturated heterocyclic ring containing a nitrogen atom and substituted on nitrogen with Cx.galkyl, C3„7cycloalkyl, C5_7cycloalkenyl, or a C5_7heterocyclic ring.
Suitably, R7* is substituted with one or more of R7*", wherein R71" is hydrogen, CRlaR2"NR3"R4", CRlaR2"OR 3", COR5", CONR6"R7", CO2R8", cyano, NR3"R4", nitro, hydroxy, C^alkoxy, SR9", SOR10", SO2R10", SO2NR6"R7", or SO3H, provided that R71" is not a substituent on the basic nitrogen of R7 .
Suitably, R*a and R2" are independently hydrogen or C^galkyl. Suitably, R3 and R4" are independently hydrogen or Cχ_galkyl, or taken together with the nitrogen to which they are attached form a 5- to 6-membered saturated heterocyclic ring, wherein, when the ring is 6-membered, may optionally contain one oxygen or one sulfur atom; or, R4" is CORl l", CONR12"R13", CO2R14", SO2R15", SO2NR12"R13", or SO2ORl6", wherein Rl 1" is hydrogen, C^alkyl, aryl, or trifluoromethyl; R^2" ^d Rl3 are independently hydrogen or Cj.galkyl, or taken together with the nitrogen to which they are attached form a 5- to 6-membered saturated heterocyclic ring, wherein,
when the ring is 6-membered, may optionally contain one oxygen or one sulfur atom; Rl4" is Cχ_galkyl or aryl; R^5" is Cχ_galkyl, aryl, or trifluoromethyl; and Rl°"" is aryl. Suitably, R5" is hydrogen, Cχ_galkyl, aryl, or trifluoromethyl. Suitably, R^" and R7 are independently hydrogen or Cχ_galkyl, or taken together with the nitrogen to which they are attached form a 5- to 6-membered saturated heterocyclic ring, wherein, when the ring is 6-membered, may optionally contain one oxygen or one sulfur atom.
Suitably, R8 ' is hydrogen or Cχ_galkyl. Suitably, R9" is hydrogen, Cχ_galkyl, aryl, or trifluoromethyl. Suitably, Rl° ' is Cχ_6alkyl, aryl, or trifluoromethyl.
Preferably, R7 ' is hydrogen or cyano.
R71 is preferably located meta to L', ortho to R72 and para to R73, and R72 is located para to L\
Suitably, R72 is hydrogen, C _galkyl, aryl, CN, CONR74R75, CO2R76, trifluoromethyl, NHCO2R77, hydroxy, C .galkoxy , benzyloxy, OCH2CO2C i _ 6alkyl, OCF3, S(O)zR78, SO2NR79R80; or halogen wherein R74, R75, R76, R79 and R80 are independently hydrogen or Cx.galkyl, R77 and R78 are Cχ_galkyl, and z is 0, 1, or 2. R72 is preferably Cχ_6alkoxy, SCχ_galkyl or halogen.
Suitably, R73 is hydrogen, Cx.galkyl, hydroxy, Oχ .galkoxy or halogen, or R73 and R4^' taken together from a group -X- where X is (CR8 lR82)aa> wherein aa is 2, 3 or 4, and R ^ and R82 are independently hydrogen or Cx.galkyl, or X is (CR iR 2)ab-Y, wherein ab is 0, 1, 2 or 3, and Y is oxygen, sulfur or CR81=CR82 wherein R8^ and R82 are independently hydrogen or Cχ_galkyl. Preferably, R73 is hydrogen. Suitably, y is an integer from 1-2. Preferably, y is 1.
Alternatively, E suitably represents a group (h):
R87 ' (h).
Suitably, R87 is hydrogen, Cχ_galkyl, Cχ_galkoxy or halogen, or R87 together with R30' form a group -AA-, wherein AA is (CR95R88)ad, wherein ad is 1, 2 or 3, and R95 and R88 are independently hydrogen or Cχ_galkyl, or AA is (CR95CR96)ae-AB, wherein ae is 0, 1 or 2, and AB is oxygen, sulfur, CR9 =CR96, CR95=N, CR 5NR96 or N=N wherein R95 and R 6 are independently hydrogen or Cχ_galkyl. Suitably, R83 and R84 are independently hydrogen or Cχ_galkyl.
Suitably, R85 and R ^ are independently hydrogen, Cχ_galkyl, C3.
7cycloalkyl, aralkyl, C5_7cycloalkenyl, a C5_7heterocyclic ring, or together with the nitrogen atom to which they are attached form an optionally substituted 5- to 7-membered heterocyclic ring which may contain an additional heteroatom selected from oxygen, nitrogen or sulfur, where optional substituents include Cχ_ galkyl, aryl, CONR 8R 9, NR90R91, hydroxy, OCOR92, NHCOCF3,
NHSO2R93, NHCO2R94, or NHCOC0_galkyl wherein the alkyl of the NHCOCQ. galkyl is optionally substituted by OH, and wherein R88, R89, R90, R91 and R92 are independently hydrogen or C .galkyl, and R93 and R94 are independently Cχ_ galkyl. Suitably Z is an optionally substituted 5 to 7-membered heterocyclic ring containing 1 to 3 heteroatoms selected from oxygen, nitrogen or sulfur; suitably ac is 0-4.
Alternatively, E suitably represents a group (i):
Suitably, R!°1 is hydrogen or Cχ.galkyl or R!°1 and R30' together form a group -AD- wherein AD is (CR1°9R1 *°)ai wherein ai is 2, 3 or 4 or AD is
(CR1°9R1 l°)aj-AE wherein aj is 0, 1, 2 or 3 and AE is oxygen, sulfur or
CR109=CRi 10, ^ R109 and R110 are independently hydrogen or Cχ.galkyl. Suitably, R97 and R98 are independently hydrogen, Cχ.galkyl, C%_
7cycloalkyl, aralkyl, C5_7cycloalkenyl, a C5_7heterocyclic ring, or together with the nitrogen atom to which they are attached form an optionally substituted 5- to 7- membered heterocyclic ring, wherein optionally an additional heteroatom is selected from oxygen, nitrogen or sulfur, and wherein optional substituents include Cχ.galkyl, aryl, CONR102Rl03, NR104R105, hydroxy, OCOR106, NHCOCF3, NHSO2 R107, NHCO2R108, or NHCOC0_galkyl, wherein the alkyl of NHCOCQ. galkyl is optionally substituted by OH, and wherein R102, R103, R104, R105 and R106 are independently hydrogen or Cχ.galkyl, and R^07 and Rl08 are independently Cl-6alkyl. Suitably, R99 and R1°° are independently hydrogen or C .galkyl; suitably,
AC is oxygen, CR ιRll2 or NR113, wherein R11 1, R112 and RH3 are independently hydrogen or C .galkyl, or AC is a group S(O)ak wherein ak is 0, 1 or 2; suitably, ag is an integer from 1-3, ah is an integer from 1-4, and af is 0-4. Preferably, E is selected from group (a), (b) and (g).
Suitably, when A' is phenyl, 5,6,7,8-tetrahydro-l-naphthalenyl, lH-indol- 4-yl, or 2-benzothiazolyl, and R^'is hydrogen, (CH2)aCN, (CH )aCO2H, CR10'=CRH 'CO2R13', COCRIO'RH 'ORI ', Oaryl, Oaralkyl, O(CH2)aCO2Rl3', or Saryl and optionally, Rl'is one or more of Cχ.galkyl, (CH2)aNR2COR4, CF3, CO2Cχ_galkyl, Cχ_galkoxy, halogen, or cyano, D' is a bond, E' and G' together are NC(R26)2, R' is hydrogen, J' is CO, L' is NR30, and E is group (a), (b), (c), (f), (g), (h), or (i), provided that when E is group (g), Rl" and R7 " are not both hydrogen. More preferably, A' is phenyl, 5,6,7,8-tetrahydro-l-napthalenyl, lH-indol- 4-yl, or 6-chloro-2-benzothiazolyl; and when A' is phenyl, Rl' is one or more of Cχ_galkyl, CF3, CO2CH2CH3, Cχ_galkoxy, halogen, or cyano substituted at the 2,3-, 2,4-, 2,5-, 2-, 3-, 4-, 3,4-, and 3,5- positions, D' is a bond, E' and G' together are NCH2, R' is hydrogen, J' is CO, L' is NH, and E is group (a), (b), or (g).
Most preferably, A' is phenyl, 5,6,7, 8-tetrahydro-l-napthalenyl, lH-indol- 4-yl, or 6-chloro-2-benzothiazolyl; and when A' is phenyl, Rl' is one or more of methyl, chloro or trifluoromethyl substituted at the 2,3-positions, 2,4-dimethyl, 2- methoxy-5-chloro, 2-methyl, 3-ethoxycarbonyl, or 3,5-dichloro, D' is a bond, E' and G' together are NCH2, R' is hydrogen, J' is CO, L' is NH, and E is group (g).
More preferably, when E is group (a), L' is attached to group (a) meta to B- (CR!R2)a-NR3R4 and para to (R5)b, wherein B is oxygen or CR7R8, R1 and R 2 are hydrogen, R5 is methoxy, methylthio or iodo, R3 and R4 are independently C%_ galkyl, or R3 and R4 taken together with the nitrogen to which they are attached form a 5- or 6-membered heterocyclic ring optionally substituted with one or more of Cx .galkyl and acetamido or hydroxyl, R^ is hydrogen, a is 2 or 3 when B is oxygen and a is 2 when B is CR R8, and b is 1. Most preferably, when E is group (a), L' is attached to group (a) meta to B-
(CR1R2)a-NR3R4 and para to (R5)b, wherein B is oxygen or CH2, R1 and R 2 are hydrogen, R5 is methoxy, R3 and R4 are independently isopropyl or tert-butyl, or R3 and R4 taken together with the nitrogen to which they are attached are 1 -(2,2,6,6- tetramethylpiperidinyl), l-(4-acetamido-2,2,6,6-tetramethyl piperidinyl), l-(4-hydroxy- 2,2,6,6-tetramethyl piperidinyl) or l-(4-hydroxy-2,2,4,6,6-pentamethyl piperidinyl), R6 is hydrogen, a is 2 when B is oxygen, and b is 1.
More preferably, when E is group (b), L' is attached to group (b) para to J, J is oxygen, R33 is hydrogen, R24, R25, R26, R27, R28, R29, R31 and R32 are hydrogen, R30 is C3_galkyl, g is 2, and h is 1. Most preferably, when E is group (b), L' is attached to group (b) para to J, J is oxygen, R33 is hydrogen, R24, R25, R26, R27, R28, R29, R31 and R32 are hydrogen, R30 is isopropyl, g is 2 and h is 1.
More preferably, when E is group (g), L' is attached to group (g) meta to
R71 and para to R72, and R7 is an optionally substituted 5- or 6-membered saturated or partially saturated heterocyclic ring containing a nitrogen atom, and substituted on nitrogen with C3_galkyl or C3_7cycloalkyl, R71" is hydrogen or cyano and is attached to the benzylic carbon of R71, R72 is methoxy, methylthio or iodo, y is 1, and R73 is hydrogen.
Most preferably, when E is group (g), L' is attached to group (g) meta to R71 and para to R72, and R7 is piperidin-4-yl substituted on nitrogen with isopropyl, R " is hydrogen or 4-cyano, R72 is methoxy, y is 1, and R73 is hydrogen. A particularly effective subgenus of compounds of formula (I) is wherein
A' is phenyl, 5,6,7, 8-tetrahydro-l-naphthalenyl, or lH-indol-4-yl, and when A' is phenyl, Rl' is methyl, chloro or trifluoromethyl substituted at the 2,3-positions, 2,4-dimethyl, 2-methoxy-5-chloro, 2-methyl, 3-ethoxycarbonyl, or 3,5-dichloro, D' is a bond, E' and G' together are NC(R2^')2, wherein R2^' is hydrogen, R' is hydrogen, J' is CO, L' is NR30', wherein R30' is hydrogen, and E is group (g), wherein L' is attached to group (g) meta to R7^ and para to R72, and R7* is piperidin-4-yl substituted on nitrogen with isopropyl, R7 ' is hydrogen or 4-cyano, R72 is methoxy, y is 1, and R73 is hydrogen.
The term "acyloxy" is used herein at all occurrences to mean a moiety -O-C(O)-R, wherein R is hydrogen or C\ .galkyl as defined below.
The term " Cχ_4alkanoyl " is used herein at all occurrences to mean a - C(O)Cχ-4alkyl group wherein the alkyl portion is as defined below.
The term "alkenyl" is used herein at all occurrences to mean a straight or branched chain radical of 2 to 6 carbon atoms, unless the length is limited thereto, wherein there is at least one double bond between two of the carbon atoms in the chain, including, but not limited to, ethenyl, 1-propenyl, 2-propenyl, 2-methyl- 1- propenyl, 1-butenyl, 2-butenyl, and the like.
The term "alkoxy" is used herein at all occurrences to mean a straight or branched chain radical of 1 to 6 carbon atoms, unless the chain length is limited thereto, bonded to an oxygen atom, including, but not limited to, methoxy, ethoxy, n- propoxy, isopropoxy, and the like.
The term "Cχ_galkoxyCχ_galkoxy" is used herein at all occurrences to mean an alkoxy group as defined above, substituted with an alkoxy group as defined above. The term "Cχ_4alkoxyalkyl" is used herein at all occurrences to mean a Cχ_
4alkoxy group as defined above bonded to an alkyl group as defined below, including, but not limited to, -CH2-CH2-O-CH2-CH2-CH3 and the like.
The term "Cχ_galkyl" is used herein at all occurrences to mean a straight or
branched chain radical of 1 to 6 carbon atoms, unless the chain length is limited thereto, including, but not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, and the like.
The term "alkynyl" is used herein at all occurrences to mean a straight or branched chain radical of 2 to 8 carbon atoms, unless the chain length is limited thereto, wherein there is at least one triple bond between two of the carbon atoms in the chain, including, but not limited to, acetylene, 1- propylene, 2-propylene, and the like.
The term "aralkyl" is used herein at all occurrences to mean an aryl moiety as defined above, which is connected to an alkyl moiety as defined below including, but not limited to, benzyl or phenethyl, and the like.
The term "aryl" is used herein at all occurrences to mean a 6-14-membered substituted or unsubstituted aromatic ring(s) or ring systems which may include bi- or tri-cyclic systems, including, but not limited to, phenyl, naphthalenyl, biphenyl, phenanthryl, anthracenyl, and the like.
The term "6,6 or 6,5 bicyclic ring" is used herein at all occurrences to mean a 6,6 or 6,5-bicyclic ring system containing a nitrogen atom and optionally a further heteroatom selected from nitrogen, oxygen, or sulfur, which ring system may be optionally substituted with Cχ_galkyl. Examples of such ring systems include, but are not limited to, tropane, isoquinuclidine and granatane rings.
The term "cycloalkenyl" is used herein at all occurrences to mean cyclic radicals, preferably of 5 to 8 carbons, which have at least one double bond between two of the carbon atoms in the ring, including but not limited to, cyclopentenyl, cyclohexenyl, and the like. The terms "cycloalkyl" and "cyclic alkyl" are used herein at all occurrences to mean cyclic radicals, preferably comprising 3 to 7 carbon atoms which may be mono- or bicyclo- fused ring systems which may additionally include unsaturation, including, but not limited to, cyclopropyl, cyclopentyl, cyclohexyl, 1,2,3,4- tetrahydronaphthalenyl, and the like. The terms "halo" or "halogen" are used interchangeably herein at all occurrences to mean radicals derived from the elements chlorine, fluorine, iodine and bromine.
The term "heteroaryl" is used herein at all occurrences to mean a 5-14- membered substituted or unsubstituted aromatic ring(s) or ring systems which may include bi- or tri-cyclic systems, which ring or ring systems contain 1 to 4 heteroatoms selected from nitrogen, oxygen, and sulfur, including, but not limited to, indolyl, benzofuranyl, thianaphthenyl, quinolyl, isoquinolyl, pyrrolyl, furanyl, thienyl, pyridyl, and the like.
The term "hydroxyCχ_galkoxy" is used herein at all occurrences to mean an hydroxyl group bonded to an alkoxy group as defined above including, but not limited to, -O-CH2-CH(OH)CH3 and the like.
The terms "hydroxyCχ .galkyl" and "hydroxyalkyl" are used herein interchangeably to mean an hydroxyl group bonded to a C\ .galkyl group as defined above, including, but not limited to, methanol, ethanol, n-propanol, isopropanol, n-butanol, sec-butanol, isobutanol, tert-butanol, and the like.
The term "heterocyclic ring" is used herein at all occurrences to mean a saturated or partially saturated 5-10-membered ring system (unless the cyclic ring system is otherwise limited) in which the ring system contains one to 3 heteroatoms selected from oxygen, sulfur, or nitrogen, which ring system may be optionally substituted with Cχ_ galkyl. Examples of such rings include, but are not limited to, piperidine, tetrahydropyridine, piperazine, pyrrolidine, morpholine, imidazolidine, pyrazolidine, hexahydroazepine, and the like. When the heterocyclic ring is fused to a phenyl group, as when E is the group (h), the term "heterocyclic ring", together with the phenyl ring to which it is fused, forms a ring which includes, but is not limited to, dihydro-1,4- benzoxazine and 1,2,3,4-tetrahydroquinoline, which may be optionally substituted by Cχ_ galkyl or oxo.
The term "heteroatom" is used herein at all occurrences to mean an oxygen atom, a sulfur atom or a nitrogen atom. It will be recognized that when the heteroatom is nitrogen, it may form an NRa or NRaRb moiety, wherein Ra and Rb are, independently, hydrogen or Cj to C alkyl, or together with the nitrogen to which they are bound, form a saturated or unsaturated 5-, 6- or 7-membered ring, including, but not limited to, pyrrolidine, piperidine, piperazine, morpholine, pyridine, and the like. It will be recognized that the saturated or unsaturated 5-, 6- or 7-membered ring may optionally have one or more additional heteroatoms in the ring.
The term "optionally substituted" is used herein at all occurrences to mean an optionally substituted 5- to 7-membered heterocyclic ring wherein the optional substituents are one or more of Cχ_galkyl. The term "oxo" is used herein at all occurrences to mean a double bonded oxygen atom attached to a chemical moiety as a substituent.
The term "CCR5 mediated disease state" is used herein at all occurrences to mean any disease state which is mediated (or modulated) by CCR5.
Suitably, pharmaceutically acceptable salts of formula (I) include, but are not limited to, salts with inorganic acids such as hydrochloride, sulfate, phosphate, diphosphate, hydrobromide, and nitrate, or salts with an organic acid such as malate, maleate, fumarate, tartrate, succinate, citrate, acetate, lactate, methanesulfonate, p-toluenesulfonate, palmitate, salicylate, and stearate.
The compounds of the invention can exist in unsolvated as well as solvated forms, including hydrated forms. In general, the solvated forms, with pharmaceutically acceptable solvents such as water, ethanol, and the like, are equivalent to the unsolvated forms for purposes of this invention. The compounds of the present invention may contain one or more asymmetric carbon atoms and may exist in racemic and optically active forms. The stereocenters may be of any combination of R and S configuration, for example, (R,R), (R,S), (S,S) or (S,R). All of these compounds are within the scope of the present invention. Novel intermediates falling within the scope of this invention that are useful in making compounds of formula (I), are compounds of formula (II)
Formula (II) wherein: R is hydrogen; and
R71, R72 and y are as defined above.
A particularly useful intermediate herein is 3- [4-cyano- 1-(1 -methylethyl)-4- piperidinyl]-4-methoxy-benzenamine.
Among the preferred compounds of the invention are the following compounds:
N- [3- [4-Cy ano- 1 -( 1 -methylethyl)-4-piperidinyl] -4-methoxyphenyl] -4- (5,6,7,8-tetrahydro- 1 -naphthalenyl)- 1 -piperazinecarboxamide; and
N-[3-[4-Cyano-l-(l-methylethyl)-4-piperidinyl]-4-methoxyphenyl]-4-[3- (ethoxycarbonyl)phenyl] - 1 -piperazinecarboxamide.
Among the most preferred compounds of the invention is the following compound:
N- [3- [4-Cy ano- 1 -( 1 -methylethyl)-4-piperidinyl] -4-methoxyphenyl] -4- (5,6,7,8-tetrahydro-l-naphthalenyl)-l-piperazinecarboxamide.
Formulation of Pharmaceutical Compositions
The pharmaceutically effective compounds of this invention (and the pharmaceutically acceptable salts thereof) are administered in conventional dosage forms prepared by combining a compound of formula (I) ("active ingredient") in an amount sufficient to treat COPD, asthma and atopic disorders (for example, atopic dermatitis and allergies), rheumatoid arthritis, sarcoidosis, or idiopathic pulmonary
fibrosis and other fibrotic diseases, atherosclerosis, psoriasis, autoimmune diseases such as multiple sclerosis, treating and/or preventing rejection of transplanted organs, inflammatory bowel disease, and HJN infection, ("CCR5- mediated disease states") with standard pharmaceutical carriers or diluents according to conventional procedures well known in the art. These procedures may involve mixing, granulating and compressing or dissolving the ingredients as appropriate to the desired preparation.
The pharmaceutical carrier employed may be, for example, either a solid or liquid. Exemplary of solid carriers are lactose, terra alba, sucrose, talc, gelatin, agar, pectin, acacia, magnesium stearate, stearic acid and the like. Exemplary of liquid carriers are syrup, peanut oil, olive oil, water and the like. Similarly, the carrier or diluent may include time delay material well known to the art, such as glyceryl monostearate or glyceryl distearate alone or with a wax.
A wide variety of pharmaceutical forms can be employed. Thus, if a solid carrier is used, the preparation can be tableted, placed in a hard gelatin capsule in powder or pellet form or in the form of a troche or lozenge. The amount of solid carrier will vary widely but preferably will be from about 25 mg to about 1000 mg. When a liquid carrier is used, the preparation will be in the form of a syrup, emulsion, soft gelatin capsule, sterile injectable liquid such as an ampule or nonaqueous liquid suspension.
The active ingredient may also be administered topically to a mammal in need of treatment or prophylaxis of CCR5 mediated disease states. The amount of active ingredient required for therapeutic effect on topical administration will, of course, vary with the compound chosen, the nature and severity of the disease state being treated and the mammal undergoing treatment, and is ultimately at the discretion of the physician. A suitable dose of an active ingredient is 1.5 mg to 500 mg for topical administration, the most preferred dosage being 1 mg to 100 mg, for example 5 to 25 mg administered two or three times daily.
By topical administration is meant non-systemic administration and includes the application of the active ingredient externally to the epidermis, to the buccal cavity and instillation of such a compound into the ear, eye and nose, and where the compound does not significantly enter the blood stream. By systemic administration is meant oral, intravenous, intraperitoneal and intramuscular administration.
While it is possible for an active ingredient to be administered alone as the raw chemical, it is preferable to present it as a pharmaceutical formulation. The active ingredient may comprise, for topical administration, from 0.001% to 10% w/w, e.g. from 1% to 2% by weight of the formulation although it may comprise as much as 10% w/w but preferably not in excess of 5% w/w and more preferably from 0.1%
to 1% w/w of the formulation.
The topical formulations of the present invention, both for veterinary and for human medical use, comprise an active ingredient together with one or more acceptable carrier(s) therefor and optionally any other therapeutic ingredient(s). The carrier(s) must be 'acceptable' in the sense of being compatible with the other ingredients of the formulation and not deleterious to the recipient thereof.
Formulations suitable for topical administration include liquid or semi-liquid preparations suitable for penetration through the skin to the site of inflammation such as liniments, lotions, creams, ointments or pastes, and drops suitable for administration to the eye, ear or nose.
Drops according to the present invention may comprise sterile aqueous or oily solutions or suspensions and may be prepared by dissolving the active ingredient in a suitable aqueous or alcoholic solution of a bactericidal and/or fungicidal agent and/or any other suitable preservative, and preferably including a surface active agent. The resulting solution may then be clarified by filtration, transferred to a suitable container which is then sealed and sterilized by autoclaving or maintaining at 98- 100°C for half an hour. Alternatively, the solution may be sterilized by filtration and transferred to the container by an aseptic technique. Examples of bactericidal and fungicidal agents suitable for inclusion in the drops are phenylmercuric nitrate or acetate (0.002%), benzalkonium chloride (0.01%) and chlorhexidine acetate (0.01%). Suitable solvents for the preparation of an oily solution include glycerol, diluted alcohol and propylene glycol.
Lotions according to the present invention include those suitable for application to the skin or eye. An eye lotion may comprise a sterile aqueous solution optionally containing a bactericide and may be prepared by methods similar to those for the preparation of drops. Lotions or liniments for application to the skin may also include an agent to hasten drying and to cool the skin, such as an alcohol or acetone, and/or a moisturizer such as glycerol or an oil such as castor oil or arachis oil.
Creams, ointments or pastes according to the present invention are semi-solid formulations of the active ingredient for external application. They may be made by mixing the active ingredient in finely divided or powdered form, alone or in solution or suspension in an aqueous or non-aqueous fluid, with the aid of suitable machinery, with a greasy or non-greasy basis. The basis may comprise hydrocarbons such as hard, soft or liquid paraffin, glycerol, beeswax, a metallic soap; a mucilage; an oil of natural origin such as almond, corn, arachis, castor or olive oil; wool fat or its derivatives, or a fatty acid such as stearic or oleic acid together with an alcohol such as propylene glycol. The formulation may incorporate any suitable surface-active agent such as an anionic, cationic or non-ionic surfactant such as esters or
polyoxyethylene derivatives thereof. Suspending agents such as natural gums, cellulose derivatives or inorganic materials such as silicaceous silicas, and other ingredients such as lanolin, may also be included.
The active ingredient may also be administered by inhalation. By "inhalation" is meant intranasal and oral inhalation administration. Appropriate dosage forms for such administration, such as an aerosol formulation or a metered dose inhaler, may be prepared by conventional techniques. The daily dosage amount of the active ingredient administered by inhalation is from about 0.1 mg to about 100 mg per day, preferably about 1 mg to about 10 mg per day. In one aspect, this invention relates to a method of treating COPD, asthma and atopic disorders (for example, atopic dermatitis and allergies), rheumatoid arthritis, sarcoidosis, or idiopathic pulmonary fibrosis and other fibrotic diseases, atherosclerosis, psoriasis, autoimmune diseases such as multiple sclerosis, treating and/or preventing rejection of transplanted organs, inflammatory bowel disease, and HIV infection, all in mammals, preferably humans, which comprises administering to such mammal an effective amount of a CCR5 receptor modulator, in particular, a compound as depicted in formula (I).
By the term "treating" is meant either prophylactic or therapeutic therapy. Such formula (I) compound can be administered to such mammal in a conventional dosage form prepared by combining the formula (I) compound with a conventional pharmaceutically acceptable carrier or diluent according to known techniques. It will be recognized by one of skill in the art that the form and character of the pharmaceutically acceptable carrier or diluent is dictated by the amount of active ingredient with which it is to be combined, the route of administration and other well-known variables. The formula (I) compound is administered to a mammal in need of treatment for COPD, asthma and atopic disorders (for example, atopic dermatitis and allergies), rheumatoid arthritis, sarcoidosis, or idiopathic pulmonary fibrosis and other fibrotic diseases, atherosclerosis, psoriasis, autoimmune diseases such as multiple sclerosis, treating and/or preventing rejection of transplanted organs, inflammatory bowel disease, and HIN infection, in an amount sufficient to decrease symptoms associated with these disease states. The route of administration may be oral or parenteral.
In another aspect, the invention relates to a method for modulating factors which exacerbate the symptoms of the CCR5-mediated diseases described herein. The term parenteral as used herein includes intravenous, intramuscular, subcutaneous, intra-rectal, intravaginal or intraperitoneal administration. The subcutaneous and intramuscular forms of parenteral administration are generally preferred. The daily parenteral dosage regimen will preferably be from about 30
mg to about 300 mg per day of active ingredient. The daily oral dosage regimen will preferably be from about 100 mg to about 2000 mg per day of active ingredient.
It will be recognized by one of skill in the art that the optimal quantity and spacing of individual dosages of a formula (I) compound will be determined by the nature and extent of the condition being treated, the form, route and site of administration, and the particular mammal being treated, and that such optimums can be determined by conventional techniques. It will also be appreciated by one of skill in the art that the optimal course of treatment, i.e., the number of doses of the formula (I) compound given per day for a defined number of days, can be ascertained by those skilled in the art using conventional course of treatment determination tests. Methods of Preparation
The compounds of formula (I) can be prepared by art-recognized procedures from known or commercially available starting materials. If the starting materials are unavailable from a commercial source, their synthesis is described herein, or they can be prepared by procedures known in the art.
For example, as depicted in Scheme 1, wherein compounds of formula (I) where L' is NR30' are prepared by treating a suitably substituted aniline 1-1 with suitable reagent, for example triphosgene, and a suitable base, for example triethylamine, in a suitable solvent, for example dichloromethane, followed by treatment with a suitably substituted amine 1-2, e.g., l-(5,6,7,8-tetrahydro-l- naphthaleny piperazine, ethyl 3-(l-piperazinyl)benzoate, 4-(phenyl)piperidine, 1- (phenyl)piperazine, 4-phenyl-2,3,4,6-tetrahydropyrdine, hexahydro-1 -phenyl- 1H- 1,4-diazepine, etc., to afford the title compound 1-3.
Scheme 1
H
1 -2
Suitably substituted anilines used to prepare compounds of formula (I) where E is a group of formula (a) are prepared according to the methods of international application publication number WO 95/15954, published 15 June 1995, international application publication number WO 95/17398, published 29 June 1995, international application publication number WO 95/26328, published 5 October 1995, and international application publication number WO 96/06079, published 29 February 1996.
Suitably substituted anilines used to prepare compounds of formula (I) where E is a group of formula (b) are prepared according to the methods of international application publication number WO 95/11934, published 25 April
1995, and WO 95/19477, published 27 June 1995. Four other applications relate to the spiro compounds WO 97/17350 published 15 May 1997; WO 97/34900 published 25 September 1997; WO 97/34901 published 25 September 1997; WO 97/35862 published 2 October 1997. Suitably substituted anilines used to prepare compounds of formula (I) where E is a group of formula (c) are prepared according to the methods of international application publication number WO 95/30675, published 16 November 1995.
Suitably substituted anilines used to prepare compounds of formula (I) where E is a group of formula (f) are prepared according to the methods of international application publication number WO 95/17401, published 29 June 1995.
Suitably substituted anilines used to prepare compounds of formula (I) where E is a group or formula (g) are prepared according to the methods of international application publication number WO 96/31508 published 10 October 1996.
Anilines used in the preparation of compounds of formula (I) wherein E is represented by group (g), R72 is, for example, Cχ.galkoxy, R is piperidinyl, and R71 is attached to the piperidinyl ring at the 4-position and is, for example, COR5", CONR6"R7", CO2R8", cyano, SO2Rl°", or SO2 NR6"R7" can be prepared following the general procedures of Cammack and Reeves, J. Heterocyclic Chem., 1986, 23, 73-5; Iorio, et. al., Farmaco, Ed. Sci., 1977, 32, 212-19; Buchi, et. al., Helv. Chim. Acta, 1952, 35, 1527-1536; and DE 735866, and the general procedure shown in Scheme 2. Alternatively, 2-5 may be obtained from 2-4 by reductive amination using an appropriately substituted aldehyde or ketone, an appropriate reducing agent, for example sodium cyanoborohydride, in an appropriate solvent, for example methanol containing acetic acid.
Anilines wherein R7 " is NR3"R4" or SR9" can be prepared following the
general procedures of Chen, et. al., Bioorg. Med. Chem. Lett., 1997, 7, 555-560,
Ong, et. al., J. Med. Chem., 1981, 24, 74-79, Kornblum et al., Tetrahedron, 1989,
45, 1311-1322, and Kornblum et al., J. Org. Chem., 1988, 53, 1475-1481, and as shown in Scheme 3 using 3-1 (WO 9827081). Anilines wherein R71 " is CRlaR2"NR3"R4" or CRlaR2"θR3" can be prepared following the general procedures of Ong, et. al., J. Med. Chem., 1983, 26,
981-986 and lorio, et. al., Farmaco, Ed. Sci., 1977, 32, 212-219 by reduction of 2-5 or 2-6 wherein R71" is COR5", CONR6"R7", CO2R8", or cyano, with a suitable reducing agent, for example lithium aluminum hydride, in a suitable solvent, for example ether or tetrahydrofuran, or, wherein R7^ ' is cyano, by catalytic or diborane hydrogenation. Scheme 2
2-1 2-2 2-3
2-4 2-5
2-6 (a) CH3N(CH2CH2C1)2, NaH, DMF, 50-90°C; (b) HNO3, Ac2O; (c) ClCO2CHClCH3, DDBA, 1,2-dichloroethane; MeOH, •; (d) iPrl, K2CO3, acetone;
70°C, 24 h; (e) H2, Pd/C, ethanol.
Scheme 3
3-1 3-2
(a) NaCN, HOAc, H2SO4; (b) HSR9", H2SO4, H2O.
Suitably substituted anilines used to prepare compounds of formula (I) where E is a group of formula (h) are prepared according to the methods of international application publication number WO 95/32967, published 7 December 1995, WO 97/07120, published 27 February 1997, and WO 97/07120, published 27 February 1997.
Suitably substituted anilines used to prepare compounds of formula (I) where E is a group of formula (i) are prepared according to the methods of international application publication number WO 97/19070 published 29 May 1997. The invention will now be described by reference to the following examples which are merely illustrative and are not to be construed as a limitation of the scope of the present invention. In the Examples, mass spectra were performed upon a VG Zab mass spectrometer using fast atom bombardment, unless otherwise indicated.
EXAMPLES
Preparation 1 Preparation of 3-r4-Cvano-l-(l-methylethyl)-4-piperidinvn-4-methoxy- benzenamine a) 4-[2-(methoxy)phenyl]-l-methyl-4-piperidinecarbonitrile
Following the general procedure of Ong, et. al., J. Heterocycl. Chem. 1981, 18, 815-20 and of Patane, et. al., Bioorg. Med. Chem. Lett. 2000, 10, 1621-1624, a solution of (2-methoxyphenyl)acetonitrile (7.4 g, 50 mmol) in anhydrous dimethylformamide (120 mL) was added over 5 minutes to sodium hydride (4.8 g, 200 mmol) with good stirring. The mixture was stirred for 1 h and treated with a solution of N-methylbis(2-chloroethyl)-
amine (7.19 g, 50 mmol) in dimethylformamide (100 mL) at a rate such that the internal temperature remained below 50°C. The resulting mixture was gradually heated to 90°C and stirred at 90°C for 16 h. The mixture was carefully quenched with ice water and extracted with ether three times. The combined organic phase was extracted with 2N hydrochloric acid and the acidic aqueous extract was carefully basified with 10% aqueous sodium hydroxide. The resulting mixture was extracted with ether, dried (MgSO4), and concentrated in vacuo to give the title compound (9.65 g, 84%). MS(ES) m/e 231.2 [M+H]+. b) 4-[2-methoxy-5-(nitro)phenyl]-l-methyl-4-piperidinecarbonitrile 70% Nitric acid (4.9 mL, 76 mmol) was added dropwise to a solution of the compound of Preparation 1(a) (8.7 g, 38 mmol) stirred in acetic anhydride (50 mL) at 0°C and the mixture was stirred for 1 h. The reaction was carefully quenched with ice water, basified with 10% aqueous sodium hydroxide, and extracted with dichloromethane three times. The combined organic phase was dried (MgSO4) and concentrated in vacuo to give a mixture of the title compound, accompanied by a small amount of 4-[2-methoxy-3-(nitro)phenyl]-l-methyl-4- piperidinecarbonitrile, as a yellow oil that solidified on standing (9.2 g). c) 4-[2-methoxy-5-(nitro)phenyl]-4-piperidinecarbonitrile
A solution of the compound of Preparation 1(b). (9.2 g, 33 mmol) and diisopropylethylamine (6.5 g, 50 mmol) in 1,2-dichloroethane (250 mL) was treated with 1-chloroethyl chloroformate (6.5 g, 43 mmol) at RT, stirred for 1 h, heated to reflux for 20 min, cooled, and concentrated in vacuo. The residue was dissolved in methanol, heated to reflux for 2 h, and the mixture was concentrated in vacuo. The residue was partitioned between 5% sodium bicarbonate and dichoromethane, the aqueous phase was extracted with dichloromethane, and the combined organic phase was dried (MgSO4) and concentrated in vacuo to give the title compound as a tan solid (7.88 g). d) 4- [2-methoxy-5-(nitro)phenyl]- 1 -( 1 -methylethyl)-4-piperidinecarbonitrile The compound of Preparation 1(c) (7.9 g, 30 mmol) was dissolved in acetonitrile (150 mL) and acetone (50 mL) and treated with potassium carbonate (16.7 g, 120 mmol) followed by isopropyl iodide (15.3 g, 90 mmol). The resulting mixture was heated to 70°C for 24 h, cooled, filtered, and the filtrate was concentrated in vacuo. The residue was dissolved in dichloromethane and washed with water three times, dried (MgSO4), concentrated in vacuo, and the resulting tan oil was purified by flash chromatography (silica gel, 3:1 hexane/ethyl acetate followed by 1 : 1 hexane/ethyl acetate) to give the title compound as a yellow oil that solidified on standing (2.35 g). MS(ES) m/e 304.2 [M+H]+. e) 3-[4-cyano-l-(l-methylethyl)-4-piperidinyl]-4-methoxybenzenamine
A mixture of the compound of Preparation 1(d) (2.1 g, 7 mmol) and 10% palladium-on-carbon (1 g) in ethanol (70 mL) was shaken in a hydrogen atmosphere (50 psi) for 2 h. The resulting mixture was filtered through Celite®, and the filtrate was concentrated in vacuo to give the title compound as a tan oil (2 g). MS(ES) m e 274.2 [M+H]+.
Preparation 2 Preparation of l-(5,6,7,8-Tetrahydro-l-naphthalenyl)piperazine
Following the general procedure of Kuipers, et. al., J. Med. Chem., 1995, 38, 1942-1954, bis(chloroethyl)amine hydrochloride (2 g, 11.2 mmol) was added to a solution of 5,6,7, 8-tetrahydro-l-naphthylamine (1.65 g, 11.2 mmol) in chlorobenzene (15 mL) and the mixture was heated to 135°C for 2 days. The mixture was cooled, concentrated in vacuo, and the residue was purified by flash chromatography (silica gel, 5% methanol/dichloromethane) to give the title compound as a tan solid which was further purified by HPLC (YMC CombiPrep ODS-A, 50 x 20 mm, 20 mL/min, A:0.1% trifluoroacetic acid in acetonitrile B:0.1% aqueous trifluoroacetic acid, A:10 to 90% during 10 min, UV detection at 254 nm) to give the title compound as a tan solid (0.25 g).
Preparation 3
Preparation of Ethyl 3-(l-piperazinyl)benzoate
Following the general procedure of Kato et. al., WO 9802432 and of Preparation 2, except substituting ethyl 3-aminobenzoate for 5,6,7, 8-tetrahydro-l- naphthylamine, gave the title compound. MS(ES) m/e 235.2 [M+H]+.
Example 1 Preparation of N- \3 - r4-Cvano- HI -methylethyl)-4-piperidinyll -4-methoxyphenyll - 4-(5,6,7,8-tetrahvdro-l-naphthalenyl)-l-piperazinecarboxamide
Triphosgene (18 mg, 0.06 mmol) was added to a solution of the compound of Preparation 1(e) (49 mg, 0.18 mmol) in dichloromethane (1 mL). The mixture was stirred for 30 min and triethylamine (73 mg, 0.72 mmol) was added. The resulting mixture was stirred for 1 h, treated with a solution of the compound of Preparation 2 (32.4 mg, 0.15 mmol) in dichloromethane (0.75 mL), and the mixture was stirred at RT overnight. The resultant mixture was concentrated in vacuo and the residue was purified by preparative HPLC (YMC CombiPrep ODS- A, 50 x 20 mm, 20 mL/min, A:0.1% trifluoroacetic acid in acetonitrile B:0.1% aqueous trifluoroacetic acid, A: 10 to 90% during 10 min, UV detection at 254 nm) to give the title compound (14.3 mg). MS(ES) m/e 516.4 [M+H] +. Also obtained
was N,N'-bis[3-[4-cyano-l-(l-methylethyl)-4-piperidinyl]-4-methoxyphenyl]urea (47.6 mg).
Example 2 Preparation of N- \ - F4-Cyano- 1 -( 1 -methylethyl)-4-piperidinyll-4-methoxy- phenyn-4-r3-(ethoxycarbonyl)phenyll-l-piperazinecarboxamide
Using the general procedure of Example 1, except substituting the compound of Preparation 3 for the compound of Preparation 2, gave the title compound. MS(ES) m/e 534.4 [M+H]+.
Biological Data:
CCR5 Receptor Binding Assay
CHO cell membranes (0.25 xlO6 cell equivalents) derived from CHO cells stably transfected with CCR5 were incubated with 0.3 125I-RANTES in a 96 well plate for 45 min. at room temperature (final reaction volume 200 uL). The reaction was terminated by filtration and the filters (GF/C) were washed twelve times with a solution of phosphate buffered saline containing 0.1 % bovine serum albumin and 0.05 % NaN3. The radioactivity bound to filters was measured by liquid scintillation spectrometry. Non-specific binding was determined in the presence of unlabelled RANTES (10 or 30 nM) and averages 30-50% of total binding.
CCR5 Receptor Functional Assay
The cellular functional assay used to assess antagonist activity of compounds was
RANTES-induced Ca2+ mobilization in RBL 2H3 cells stably expressing the hCCR5 receptor (RBL 2H3 hCCR5). Agonist activity is determined by Ca2+ mobilization in the same cells which is inhibitable by a selective CCR5 antagonist. Cells were grown to 80-100% confluency in T-150 flasks and washed with phosphate-buffered saline. Cells were lifted from the flasks by treating with 3 mL of 1 mM EDTA for 3 min. at room temperature and diluting to 2 X 10^ cells/mL with Krebs Ringer Henseleit buffer (KRH; 118 mM NaCl, 4.6 mM KCl, 25 mM NaHCO3, 1 mM KH2PO4 and 11 mM glucose) containing 5 mM HEPES (pH 7.4), 1 mM CaCl2, 1 mM MgCl2 and 0.1% BSA and centrifuged at 200g for 3 min. Cells were resuspended at 2 X 10^ cells/mL in the same buffer with 2 μM Fura-2AM, and incubated for 35 min. at 37° C. Cells were centrifuged at 200 x g for 3 min. and resuspended in the same buffer without Fura-2AM, then incubated for 15 min. at 37° C to complete the hydrolysis of intracellular Fura-2AM, and then centrifuged as before. Cells (10^ cells/mL) were resuspended in cold KRH with 5 mM HEPES (pH 7.4), 1 mM CaCl2, 1 mM MgCl2 and 0.1% gelatin and
maintained on ice until assayed. For antagonist studies, aliquots (2 mL) of cells were prewarmed at 37° C for 5 min. in 3 mL plastic cuvettes and fluorescence measured in a fluorometer (Johnson Foundation Biomedical Group, Philadelphia, PA, USA) with magnetic stirring and temperature maintained at 37° C. Excitation was set at 340 nm and emission set at 510 nm. Various concentrations of antagonists or vehicle were added and fluorescence monitored for -15 sec to ensure that there was no change in baseline fluorescence, followed by the addition of 33 nM RANTES. Maximal Ca2+ attained after 33 nM RANTES stimulation was calculated as described by Grynkiewicz et al., (1985). The percent of maximal RANTES -induced Ca2+ was determined for each concentration of antagonist and the IC50 defined as the concentration of test compound that inhibits 50% of the maximal 33 nM RANTES response, obtained from the concentration-response curves (5-7 concentrations of antagonists).
The compounds of this invention show CCR5 receptor modulator activity having IC50 values in the range of 0.0001 to 100 μM. The full structure/activity relationship has not yet been established for the compounds of this invention. However, given the disclosure herein, one of ordinary skill in the art can utilize the present assays in order to determine which compounds of formula (I) are modulators of the CCR5 receptor and which bind thereto with an IC50 value in the range of 0.0001 to 100 μM.
All publications, including, but not limited to, patents and patent applications cited in this specification, are herein incorporated by reference as if each individual publication were specifically and individually indicated to be incorporated by reference herein as though fully set forth. The above description fully discloses the invention including preferred embodiments thereof. Modifications and improvements of the embodiments specifically disclosed herein are within the scope of the following claims. Without further elaboration it is believed that one skilled in the art can, given the preceding description, utilize the present invention to its fullest extent. Therefore any examples are to be construed as merely illustrative and not a limitation on the scope of the present invention in any way. The embodiments of the invention in which an exclusive property or privilege is claimed are defined as follows.