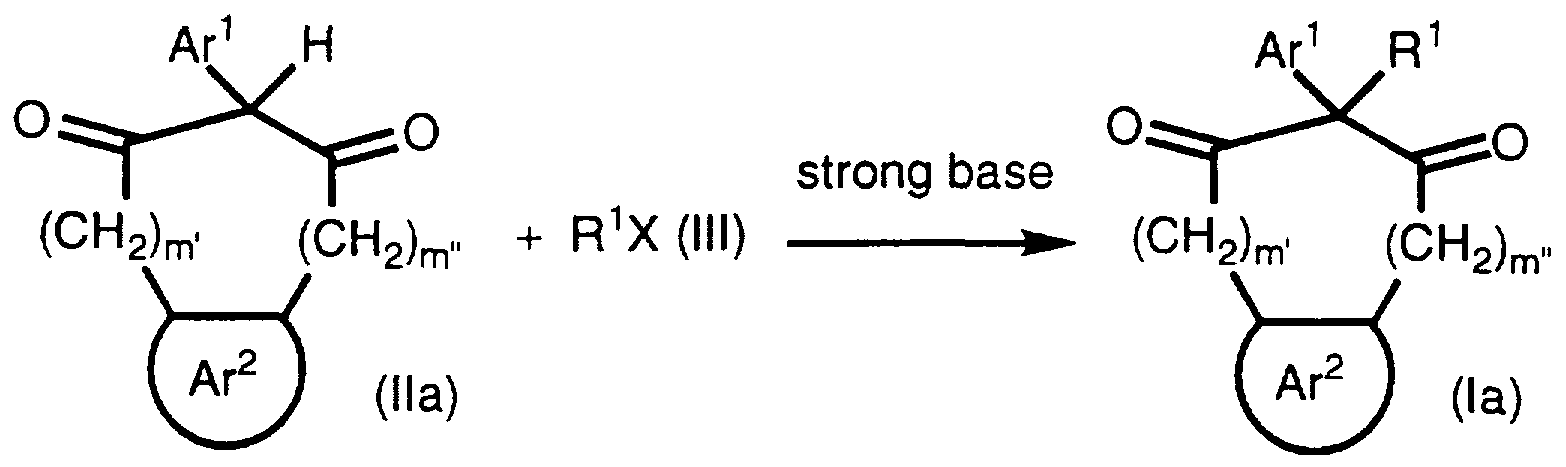SUBSTITUTED AROMATIC COMPOUNDS
1. Field of the Invention
This invention is directed to substituted aromatic compounds, their preparation, pharmaceutical compositions containing these compounds, and their pharmaceutical use in the treatment of disease states associated with proteins that mediate cellular activity.
Disease states associated with abnormally high physiological levels of cytokines such as TNF are treatable according to the invention. TNF is an important pro-inflammatory cytokine which causes hemorrhagic necrosis of tumors and possesses other important biological activities. TNF is released by activated macrophages, activated T-lymphocytes, natural killer cells, mast cells and basophils, flbroblasts, endothelial cells and brain astrocytes among other cells.
The principal in vivo actions of TNF can be classified broadly as inflammatory and catabolic. It has been implicated as a mediator of endotoxic shock, inflammation of joints and of the airways, immune deficiency states, allograft rejection, and in the cachexia associated with malignant disease and some parasitic infections. In view of the association of high serum levels of TNF with poor prognosis in sepsis, graft versus host disease and acute respiratory distress syndrome, and its role in many other immunologic processes, this factor is regarded as an important mediator of general inflammation.
TNF primes or activates neutrophils, eosinophils, flbroblasts and endothelial cells to release tissue damaging mediators. TNF also activates raonocytes. macrophages and T-lymphocytes to cause the production of colony stimulating factors and other pro-inflammatory cytokines such ILi, I 6. IL8 and GM-CSF, which in some cases mediate the end effects of TNF. The ability of TNF to activate T-lymphocytes, monocytes. macrophages and related cells has been implicated in the progression of Human Immunodeficiency Virus (HIV) infection. In order for these cells to become infected with HIV and for HIV replication to take place, the cells must be maintained in an activated state. Cytokines such as TNF have been shown to activate HIV replication in monocytes and macrophages. Features of endotoxic shock such as fever, metabolic acidosis, hypotension and intravascular coagulation are thought to be mediated through the actions of TNF on the hypothalamus and in reducing the anti-coagulant activity of vascular endothelial cells. The cachexia associated with certain disease states is mediated through indirect effects on protein catabolism. TNF also promotes bone resorption and acute phase protein synthesis.
The discussion herein relates to disease states associated with TNF and includes those disease states related to the production of TNF itself, and disease states associated with other cytokines, such as but not limited to IL-1, or IL-6. that are modulated by associated with TNF. For example, a IL-1 associated disease state, where IL- 1 production or action is exacerbated or secreted in response to TNF, would therefore be considered a disease state associated with TNF. TNF-a and TNF-b are also herein referred to collectively as "TNF" unless specifically delineated otherwise, since there is a close structural homology between TNF-a (cachectin) and TNF-b (lymphotoxin) and each of them has a capacity to induce similar biologic responses and bind to the same cellular receptor.
Disease states associated with pathological conditions that are modulated by inhibiting enzymes, which are associated with secondary cellular messengers, such as cyclic AMP phosphodiesterase are also treatable according to the invention. Cyclic AMP phosphodiesterase is an important enzyme which regulates cyclic AMP levels and in turn thereby regulates other important biological reactions. The ability to regulate cyclic AMP phosphodiesterase, including type IV cyclic AMP phosphodiesterase, therefore, has been implicated as being capable of treating assorted biological conditions. In particular, inhibitors of type IV cyclic AMP phosphodiesterase have been implicated as being bronchodilalors and asthma-prophylactic agents and as agents for inhibiting eosinophil accumulation and of the function of eosinophils. and for treating other diseases and conditions characterized by, or having an etiology involving, morbid eosinophil accumulation. Inhibitors of cyclic AMP phosphodiesterase are also implicated in treating inflammatory diseases, proliferative skin diseases and conditions associated with cerebral metabolic inhibition.
2. Reported Developments
Certain substituted mυnocyclic aromatic compounds are reported to have valuable pharmaceutical properties lor the ability to regulate proteins that mediate cellular activity, for example, type IV cyclic AMP phosphodiesterase and/or TNF. as described, for example, the following references: International Patent Application Publication Nos. 95/04045, 95/01338, 94/12461, 94/02465, 93/25517, 93/19750. 93/071 11. 92/19594, 92/12961 , 92/07567, 92/02220, 92/00968 and 91/15451; European Patent Application Publication No. 0470805A1 ; and United States Patent Nos. 5,362,915 and 5.340,827. None of the references disclose a cycloalkyK fused phenyl or monocyclic heteroaryl) compound substituted on the cycloalkyl moiety thereof by a substituted aromatic moiety, which compound has valuable pharmaceutical properties for its ability to regulate proteins that mediate cellular activity, for example, type IV cyclic AMP phosphodiesterase and/or TNF.
SUMMARY OF THE INVENTION
This invention is directed to a compound of formula 1
wherein
is selected from the group consisting ol
Ar1 is selected from the group consisting of
is optionally substituted fused phenyl or optionally substituted fused monocyclic heteroaryl;
R is hydrogen, optionally substituted alkyl or optionally substituted acyl;
R' is optionally substituted alkyl, cycloalkylalkyl, heterocyclylalkyl. optionally substituted aralkyl, optionally substituted heteroaralkyl. arylsulphonyl, heteroarylsulphonyl, R'CO- or R**0C0-;
R* is alkyl, cycloalkylalkyl. heterocyclylalkyl, optionally substituted aralkyl, optionally substituted heteroaralkyl, dialkylamino or diarylamino
Rb is alkyl, cycloalkylalkyl, heterocyclylalkyl, optionally substituted aralkyl or optionally substituted heteroaralkyl;
R' is carboxyalkyl. alkoxycarbonylalkyl, Y*Y2NCO-alkyl, cyanoalkyl. optionally substituted aralkyl or optionally substituted heteroaralkyl;
R: is optionally substituted lower alkyl:
R* is optionally substituted alkyl. optionally substituted alkenyl. optionally substituted alkynyl. optionally substituted cydoalkyl. optionally substituted cycloalkenyl. optionally substituted oxaahphatic, optionally substituted or optionally oxidi/ed cyclothioalkyl. or optionally substituted or optionally oxidized cyclothioalkenyl;
R4 and R6 is hydrogen or optionally substituted lower alkyl:
R? is optionally substituted alkyl. optionally substituted alkoxy. optionally substituted cydoalkyl, optionally substituted heterocyclyl, alkoxycarbonyl. cyano. YJY"NCO-, optionally substituted aryl, optionally substituted heteroaryl. or carboxy where m is other than 0;
R7 is hydrogen, alkoxy, optionally substituted cydoalkyl, optionally substituted cycloalkenyl, optionally substituted cycloalkyloxy, optionally substituted cycloalkenyloxy, optionally substituted aryl, optionally substituted heteroaryl, optionally substituted aryloxy, optionally substituted heteroaryloxy, optionally substituted aralkyloxy, optionally substituted heteroaralkyloxy, optionally substituted alkylthio, optionally substituted alkylsulfmyl, optionally substituted alkylsulphonyl, optionally substituted arylthio, optionally substituted arylsulfinyl, optionally substituted aryl sulphonyl, optionally substituted aralkylthio, optionally substituted aralkylsulphinyl, optionally substituted aralkylsulphonyl, Y3Y NS02-, Y4S02NY3-, Y3Y N-, Y4C(=0)-, Y4C(=0)C(=0)-,
YVNC^O)-, Y4OC(=0 , Y3Y4N(C=0)0-, or Y4C(=0)NY3-;
R8 is hydrogen, optionally substituted lower alkyl, optionally substituted aryl, optionally substituted aralkyl, optionally substituted heteroaryl, optionally substituted heteroaralkyl;
Y1 and Y2 are independently hydrogen, optionally substituted alkyl, optionally substituted aryl, optionally substituted heteroaryl, optionally substituted aralkyl or optionally substituted heteroaralkyl, or one of Y1 and Y2 is hydroxyl and the other of Y1 and Y2 is hydrogen;
Y3 and Y4 are independently hydrogen, optionally substituted alkyl, optionally substituted aryl, optionally substituted heteroaryl, optionally substituted aralkyl or optionally substituted heteroaralkyl;
Q1 and Q- are independently CH2, CHOR or CO;
Q Q4. 5 and Q" are independently nitrogen. CX or CH;
Q'' is nitrogen or CH;
X is halo;
7' and 7" are hydrogen, or 7' and 7" taken together are oxygen or sulfur:
7' and 7: are independentlv direct bond, oxygen or sullur:
7' is sulphonyl or direct bond;
7α is direct bond, oxygen, sulphur, or NH ;
75 is direct bond or optionally substituted lower alkylenyl;
m' and m" are independently 0 or 1 ;
m is 0 to 5: and
p is 1 to 3:
or hydrate thereof, solvate thereof, N-oxide (hereof, prodrug thereof or a pharmaceutically acceptable salt thereof.
Compounds within the scope of the present invention possess useful properties, more particularly pharmaceutical properties. They are especially useful for inhibiting the production or physiological effects of TNF in the treatment of a patient suffering from a disease state associated with a physiologically detrimental excess of tumor necrosis factor (TNF). Compounds within the scope of the present invention also inhibit cyclic AMP phosphodiesterase, and are useful in treating a disease state associated with pathological conditions that are modulated by inhibiting cyclic AMP phosphodiesterase, such disease states including inflammatory and autoimmune diseases, in particular type IV cyclic AMP phosphodiesterase. The present invention is therefore also directed to their pharmaceutical use. pharmaceutical compositions containing the compounds, and methods for their preparation.
DETAILED DESCRIPTION OF THE INVENTION
As used above, and throughout the description of the invention, the following terms, unless otherwise indicated, shall be understood to have the following meanings:
Definitions
"Patient" includes both human and other mammals.
"Alkyl" means an aliphatic hydrocarbon group which may be straight or branched having about 1 to about 15 carbon atoms in the chain. Preferred alkyl groups have 1 to about 12 carbon atoms in the chain. Branched means ti at one or more lower alkyl groups such as methyl, ethyl or propyl are attached to a linear alkyl chain. "Lower alkyl" means about 1 to about 4 carbon atoms in the chain which may be straight or branched. The alkyl group may be substituted by one or more halo, cydoalkyl or cycloalkenyl. The alkyl group may also be substituted by one or more hydroxy when R1 is alkyl. Exemplary alkyl groups include methyl, fluoromethyl, difluoromethyl, trifluoromethyl. cyclopropylmethyl. cyclopentylmethyl, ethyl, /--propyl, i-propyl, i-butyl, t-butyl, n- pentyl. 3-pentyl, heptyl, octyl, nonyl, decyl and dodecyl.
"Alkenyl" means an aliphatic hydrocarbon group containing a carbon-carbon double bond and which may be straight or branched having about 2 to about 15 carbon atoms in the chain. Preferred alkenyl groups have 2 to about 12 carbon atoms in the chain; and more preferably about 2 to about 4 carbon atoms in the chain. Branched means that one or more lower alkyl groups such as methyl, ethyl or propyl are attached to a linear alkenyl chain. "Lower alkenyl" means about 2 to about 4 carbon atoms in the chain which may be straight or branched. The alkenyl group may be
substituted by one or more halo. Exemplary alkenyl groups include ethenyl, propenyl. w-butenyl, i- butenyl. 3-methylbut-2-enyl, n-pentenyl, heptenyl, octenyl and decenyl.
"Alkynyl" means an aliphatic hydrocarbon group containing a carbon-carbon triple bond and which may be straight or branched having about 2 to about 15 carbon atoms in the chain.
Preferred alkynyl groups have 2 to about 12 carbon atoms in the chain; and more preferably about 2 to about 4 carbon atoms in the chain. Branched means that one or more lower alkyl groups such as methyl, ethyl or propyl are attached to a linear alkynyl chain. "Lower alkynyl" means about 2 to about 4 carbon atoms in the chain which may be straight or branched. The alkynyl group may be substituted by one or more halo. Exemplary alkynyl groups include ethynyl, propynyl, /.-butynyl, 2-butynyl, 3-methylbutynyl, w-pentynyl, heptynyl. octynyl and decynyl.
"Alkylenyl" means a bivalent aliphatic hydrocarbon group (-alkyl-) which may be straight or branched and consist of about 1 to about 6 carbon atoms. Preferred alkylenyl groups, i.e.. "Lower alkylenyl", consist of 1 to about 4 carbon atoms. Branched means that one or more lower alkyl groups such as methyl, ethyl or propyl are attached to a linear alkylenyl chain. The alkylenyl group may be substituted by hydroxy, oxo, aryl or heteroaryl; preferably the alkylenyl may be substituted by aryl. Exemplary alkylenyl groups include methylenyl, cthylenyl, methylethylenyl, propylenyl, phenylmethylenyl, phenylethylenyl and phenylpropylenyl.
"Oxaaliphatic" means a straight- or branched chain or non-aromatic monocyclic or multicyclic ring compound of about 2 to about 30 atoms wherein at least one of the atoms is oxygen and the others are carbon. The chain or the ring of the oxaaliphatic group may be saturated or unsaturated. The oxaaliphatic group may be optionally substituted by one or more halo. Exemplary oxaaliphatic groups include alkoxyalkyl, alkenyloxyalkyl, alkynyloxyalkyl, oxacycloalkyl or oxacycloalkenyl groups as described herein.
"Cydoalkyl" means a non-aromatic mono- or multicyclic ring system of about 3 to about 10 carbon atoms. Preferred monocyclic cydoalkyl rings include cyclopentyl, fluorocyclopentyl, cyclohexyl and cycloheptyl; more preferred is cyclopentyl. The cydoalkyl group may be substituted by one or more halo, methylene (H2C=), alkyl or hydroxy. Exemplary multicyclic cydoalkyl rings include 1-decalin, adamant-(l- or 2-)yl and norbornyl.
"Heterocyclyl" means a non-aromatic monocyclic or multicyclic ring system of about 3 to about 10 ring atoms. Preferred rings include about 5 to about 6 ring atoms wherein one of the ring atoms is oxygen, nitrogen or sulfur. The heterocyclyl is optionally partially unsaturated or optionally substituted by one or more halo, methylene (H2C=), alkyl or heterocyclyl; more preferred is halo. The nitrogen or thio moiety of the heterocyclyl is also optionally oxidized to the
corresponding N-oxide, S-oxide (sulfmyl) or S,S-dioxide (sulfonyl). Examples of heterocyclyl include oxacycloalkyl, oxacycloalkenyl, cyclothioalkyl and cyclothioalkenyl as defined herein, and species exemplified include pyrrolidyl, piperidyl, tetrahydrofuranyl, tetrahydrothienyl and tetrahydrothiopyrany 1.
"Oxacycloalkyl" means a non-aromatic mono- or multicyclic ring system of about 3 to about 10 ring atoms wherein at least one of the ring atoms is oxygen and the other ring atoms are carbon. Preferred rings include about 5 to about 8 ring atoms. Preferred rings have one ring atom which is oxygen. The oxacycloalkyl group may be substituted by one or more halo, methylene (H2C=) or alkyl; more preferred is halo. Exemplary monocyclic rings include tetrahydrofuryl. fluorotetrahydrofuryl and tetrahydropyranyl. Preferred is tetrahydrofuryl. Exemplary multicyclic cydoalkyl rings include 7-oxabicyclo[2.2.1]heptanyl and oxatricyclane. The oxacycloalkyl is attached to a oxy group by a direct bond or lower alkyl.
"Oxacycloalkenyl" means a non-aromatic monocyclic or multicyclic ring system of about 3 to about 10 ring atoms wherein at least one of the ring atoms is oxygen and the other ring atoms are carbon and the ring system contains a carbon-carbon double bond. Preferred rings include about 5 to about 8 ring atoms. Preferred rings have one ring atom which is oxygen. The oxacycloalkenyl group may be substituted by one or more halo, methylene (H2C=) or alkyl; more preferred is halo. Exemplary monocyclic rings include dihydrofuryl, fluorodihydrofuryl and dihydropyranyl. Preferred is dihydrofuryl. An exemplary multicyclic cydoalkyl ring is 7-oxabicyclo[2.2.1]- heptenyl. The oxacycloalkenyl is attached to a oxy group by a direct bond or lower alkyl.
"Cyclothioalkyl" means a non-aromatic monocyclic or multicyclic ring system of about 3 to about 10 ring atoms wherein at least one of the ring atoms is sullur and the otiier ring atoms are carbon. Preferred rings include about 5 to about 6 ring atoms. Preferred rings have one ring atom which is sulfur. The cyclothioalkyl may be optionally substituted by one or more halo. Preferred monocyclic cyclothioalkyl rings include tetrahydrothiophenyl and tetrahydrothiopyrany 1; more preferred is tetrahydrothiophenyl. The thio moiety of the cyclothioalkyl ring may also be optionally oxidized to the corresponding S-oxide or S,S-dioxide, i.e.. cyclosulfinylalkyl or cyclosulfonylalkyl.
"Cyclothioalkenyl" means a non-aromatic monocyclic or multicyclic ring system having about 3 to about 10 ring atoms wherein at least one of the ring atoms is sulfur and the other ring atoms are carbon and the ring system contains a carbon-carbon double bond. Preferred rings include about 5 to about 6 ring atoms and wherein one of the ring atoms is sulfur. The cyclothioalkenyl may be optionally substituted by one or more halo. Preferred monocyclic cyclothioalkenyl rings include dihydrothiophenyl and dihydrothiopyranyl; more preferred is
dihydrothiophenyl. The thio moiety of the cyclothioalkenyl may also be optionally oxidized to the corresponding S-oxide or S,S-dioxide. i.e., cyclosulfinylalkenyl or cyclosulfonylalkenyl.
"Aromatic" means aryl or heteroaryl as defined below. Preferred aromatic groups include phenyl, halo substituted phenyl and azaheteroaryl.
"Aryl" means aromatic carbocyclic radical containing about 6 to about 10 carbon atoms. Exemplary aryl include phenyl or naphthyl, or phenyl or naphthyl substituted with one or more aryl group substituents which may be the same or different, where "aryl group substituent" includes alkyl, cycloalkylalkyl. cycloalkyloxy. heterocyclylalkyl, heterocyclyloxy. aryl, heteroaryl. aralkyl, heteroaralkyl. hydroxy. hydroxyalkyl. alkoxy, carboxyalkoxy, alkoxycarbonylalkoxy, cycloalkylalkoxy, heterocyclylalkoxy. aryloxy, aralkoxy, acyl, aroyl. halo, nitro, cyano, carboxy, alkoxycarbonyl. aryloxycarbonyl. aralkoxycarbonyl, acylamino, aroylamino, alkylsulfonyl, arylsulfonyl. heteroarylsulfonyl. alkylsulfmyl, arylsulfinyl. hcteroarylsultinyl. alkylthio. arylthio, heteroarylthio. aralkylthio, heteroaralkylthio, fused cydoalkyl, fused heterocyclyl, H03S-. Y5Y6N-, Y5Y6Nalkoxy. Y5 Y6'Nalkyl. Y5Y6NCO-, Y5Y*NC02- or Y5Y6NSO:-, where Y5 and Y6 are independently hydrogen, alkyl. aryl, aralkyl or heteroaralkyl, or Y5, Y6 and N taken together form a heterocyclyl. The aryl group substituents are as defined herein. Preferred aryl groups are optionally substituted phenyl or optionally substituted naphthyl. Preferred aryl group substituents include alkyl, hydroxy, acyl, aroyl, halo, nitro, alkoxy, cyano. alkoxycarbonyl, acylamino. alkylthio. Y5'Y6N-, Y5 Y6'NCO- or Y Y6'NSO-;-, where Y5 and Y6 are independently hydrogen or alkyl; preferred phenyl group substituents are aryloxy and aryl; and preferred naphthyl group substituents are nitro, alkoxy and
amino. Where
is optionally substituted fused phenyl. preferred aryl group substituents include alkyl. cycloalkyloxy. heterocyclylalkyl. heterocyclyloxy. hydroxy. alkoxy. carboxyalkoxy, alkoxycarbonylalkoxy, heterocyclylalkoxy, aralkoxy. Y
5Y
6Nalkoxy, Y
5 Y
6 NaIkyl or Y
5Y
6NC0
2-.
"Heteroaryl" means about a 5- to about a 10- membered aromatic monocyclic or multicyclic hydrocarbon ring system in which one or more of the carbon atoms in the ring system is/are element(s) other than carbon, for example nitrogen, oxygen or sulfur. The "heteroaryl" may also be substituted by one or more of the above-mentioned "aryl group substituents". Exemplary heteroaryl groups include pyrazinyl, furanyl, thienyl, pyridyl, pyrimidinyl, isoxazolyl, isothiazolyl. oxazolyl. thiazolyl, pyrazolyl, furazanyl, pyrrolyl, imidazo[2.1-b]thiazolyl. benzofurazanyl, indolyl, azaindolyl, benzimidazolyl, benzothienyl. 1,2,4-triazinyl, quinolinyl, imidazolyl and isoquinolinyl. Preferred heteroaryl groups include pyrazinyl, thienyl, pyridyl, pyrimidinyl, isoxazolyl and isothiazolyl. Preferred azaheteroaryl groups include pyridyl, pyrimidinyl, pyridazinyl, pyrazinyl or 1,2,4-triazinyl.
"Aralkyl" means an aryl-alkyl- group in which the aryl and alkyl are as previously described. Preferred aralkyls contain a lower alkyl moiety. Exemplary aralkyl groups include benzyl. 2-phenethyl and naphthienemethyl.
"Hydroxyalkyl" means a HO-alkyl- group in which alkyl is as previously defined.
Preferred hydroxyalkyls contain lower alkyl. Exemplary hydroxyalkyl groups include hydroxymethyl and 2-hydroxyethyl.
"Acyl" means an H-CO- or alkyl-CO- group in which the alkyl group is as previously described. Preferred acyls contain a lower alkyl. Exemplary acyl groups include formyl, acetyl, propanoyl, 2-methylpropanoyl, butanoyl and palmitoyl.
"Aroyl" means an aryl-CO- group in which the alkyl group is as previously described. Exemplary groups include benzoyl and 1 - and 2-naphthoyl.
"Alkoxy" means an alkyl-O- group in which the alkyl group is as previously described. Exemplary alkoxy groups include methoxy, ethoxy, H-propoxy, i-propoxy. /--butoxy and heptoxy.
"Alkenyloxy" means an alkenyl-O- group in which the alkenyl group is as previously described. Exemplary alkenyloxy groups include allyloxy or 3-butenyloxy.
"Alkynyloxy" means an alkynyl-O- group in which the alkynyl group is as previously described. Exemplary alkynyloxy groups include propynyloxy or 3-butynyloxy.
"Alkoxyalkyl" means alkyl-O-alkyl group wherein the alkyl groups are as described previously.
"Alkenyloxyalkyl" means alkenyl-O-alkyl group wherein the alkyl and alkenyl groups are as described previously.
"Alkynyloxyalkyl" means alkynyl-O-alkyl group wherein the alkyl and alkenyl groups are as described previously.
"Aryloxy" means an aryl-O- group in which the aryl group is as previously described.
Exemplary aryloxy groups include phenoxy and naphthoxy.
"Aralkyloxy" means an aralkyl-O- group in which the aralkyl groups is as previously described. Exemplary aralkyloxy groups include benzyloxy and 1- or 2-naphthalenemethoxy.
"Alkylthio" means an alkyl-S- group in which the alkyl group is as previously described.
Exemplary alkylthio groups include methylthio, ethylthio. i-propylthio and heptylthio.
"Arylthio" means an aryl-S- group in which the aryl group is as previously described. Exemplary arylthio groups include phenylthio and naphthylthio.
"Aralkylthio" means an aralkyl-S- group in which the aralkyl group is as previously described. An exemplary aralkylthio group is benzylthio.
"Y'Y2N-" means a subsututed or unsubstituted amino group, wherein Y' and Y2 are as previously described. Exemplary groups include amino (H--N-), mcthylanuno, ethylmethylamino, dimethylamino and dicthylamino.
"Alkoxycarbonyl" means an alkyl-O-CO- group. Exemplary alkoxycarbonyl groups include methoxy- and ethoxycarbonyl
"Aryloxycarbonyl" means an aryl-O-CO- group. Exemplary aryloxycarbonyl groups include phenoxy- and naphthoxycarbonyl.
"Aralkoxycarbon> r means an aralkyl-O-CO- group. An exemplary aralkoxycarbonyl group is benzyloxycarbonyl
"Y'Y2NCO-" means a subsututed or unsubstituted carbamoyl group, wherein Y1 and Y: are as previously described. Exemplary groups are carbamoyl (H,NCO-) and dimethylcarbamoyl (Me,NCO-).
"Y'Y"*NS02-" means a substituted or unsubstituted sulfamoyl group, wherein Y1 and Y2 are as previously described. Exemplary groups are sulfamoyl (H.NS02-) and dimethylsulfamoyl (Me2NS02-).
"Acylamino" is an acyl-NH- group wherein acyl is as defined herein.
"Aroyla ino" is an aroyl-NH- group wherein aroyl is as defined herein.
"Alkylsulfonyl" means an alkyl-SO,- group. Preferred groups are those in which the alkyl group is lower alkyl.
"Alkylsulfmyl" means an alkyl-SO- group. Preferred groups are those in which the alkyl group is lower alkyl.
"Arylsulfonyl" means an aryl-S02- group.
"Arylsulfinyl" means an aryl-SO- group.
"Halo" means fluoro. chloro, bromo. or iodo. Preferred are fluoro, chloro or bromo; more preferred are fluoro or chloro, and further preferred is fluoro.
"Prodrug" means a compound, for example an ester, which is convertible in vivo by metabolic means (e.g.. by hydrolysis) to a compound of formula (I), including N-oxides thereof. Suitable esters are of many different types, for example acetates, citrates, lactates, tartrates. malonates, oxalates, salicylates, propionates. succinates, fumarates. maleates. methylcne-bis-b- hydroxynaphthoates, gentisates, mesylates. isethionates, di-p-toluoyltartrates, methane-sulphonates, ethanesulphonates, benzenesulphonates, p-toluenesulphonates. cydohexylsulphamates and quinates and, especially, 2,2-dimethylpropanoates. An especially useful class of esters may be formed from acid moieties selected from those described by Bundgaard et al., J. ed. Chem.. 32(12). 2503 (1989), and include substituted (aminomethyl)benzoates, for example dialkylamino-methylbenzoates in which the two alkyl groups may be joined together and or interrupted by an oxygen atom or by an optionally substituted nitrogen atom, e.g. an alkylated nitrogen atom, more especially
(morpholinomethyl)benzoates, e.g. 3- or 4-(morpholinomethyπbenzoates. and (4-alkylpiperazin-l- yl)benzoates, e.g. 3- or 4-(4-alkylpiperazin-l-yl)benzoates.
Preferred Embodiments
It is to be understood that this invention covers all appropriate combinations of the particular and preferred groupings referred to herein. With reference to formula (I) above, the following are particular and preferred groupings.
A special embodiment according to the invention is directed to the use of a compound of formula I in treating a disease state associated with a physiologically detrimental excess of tumor necrosis factor.
Another special embodiment according to the invention is directed to the use of a compound of formula I in treating a disease state associated with pathological condition that is modulated by inhibiting cyclic AMP phosphodiesterase.
Another special embodiment according to the invention is a compound of formula I wherein
R' is cycloalkylalkyl, optionally substituted aralkyl, optionally substituted heteroaralkyl or RbOCO-;
Rb is lower alkyl;
R1 is cyanoalkyl. carboxyalkyl. alkoxycarbonylalkyl, optionally substituted aralkyl or optionally substituted heteroaralkyl:
R' is optionally substituted alkyl. optionally substituted cydoalkyl. optionally substituted oxaaliphatic or optionally substituted or optionally oxidized c yclothioalkyl;
R' is optionally substituted alkyl. optionally substituted cydoalkyl. optionally substituted heterocyclyl. optionally substituted aryl or optionally substituted heteroaryl:
R is hydrogen, alkoxy, optionally substituted cydoalkyl, optionally substituted aryl, optionally substituted heteroaryl. optionally substituted aryloxy. optionally substituted heteroaryloxy,;
R8 is hydrogen, or optionally substituted lower alkyl;
Q' and Q: are both CO, or at last one of Q1 and Q is CH: and the other of Q1 and Q2 is CO;
Q3. Q and Q5 are CH, or at least one of QJ and Q4 are independently nitrogen and N-oxides thereof, or CX. and Q' is CH:
Q" is CH;
Q' is CH;
X is fluoro;
Z' and Z" are hydrogen, or Z' and Z" taken together are oxygen;
Z1 and Z** are both oxygen;
Z1 is a direct bond:
Z* is oxygen;
Z3 is optionally substituted lower alkylenyl;
m' and m" are 0;
is 1 to 3; and
p is 1;
or hydrate thereol. solvate thereof, N-oxide thereol. prodrug thcrcot or a pharmaceutically acceptable salt thereof.
Another special embodiment according to the invention is a compound of formula I wherein
Another special embodiment according to the invention is a compound of formula I wherein
Ar' is R (CH2)rnZ>:
Another special embodiment according to the invention is a compound of formula I wherein
Another special embodiment according to the invention is a compound of formula I wherein
Another special embodiment according to the invention is a compound of formula I wherein
Another special embodiment according to the invention is a compound of formula 1 wherein
7.3 and Z" taken together are oxygen.
According to another aspect of the invention, a compound of formula I wherein 7.1 is oxygen is preferred.
According to another aspect of the invention, a compound of formula I wherein Z2 is oxygen is preferred.
According to another aspect of the invention, a compound of formula I wherein R' is optionally substituted cycloalkylalkyl, optionally substituted aralkyl. optionally substituted heteroaralkyl or RbOCO; preferably R' is cyclohexylmethyl, benzyl, naphth-2-ylmethyl or /- butyloxycarbonyl .
According to another aspect of the invention, a compound of formula I wherein R*- is /-alkyl
(alkyl moiety that is tertiary substituted) at its point of attachment to the oxygen of the RbOCO moiety.; more preferably Rb is /-butyl.
According to another aspect of the invention that is preferred is a compound of formula I wherein R: is unsubstituted or substituted by halo, preferably fluoro. It is further preferred that a halo substituted R: is substituted on a position of R** that is attached respectively to Z'. R- is more preferably methyl or difluoromethyl.
According to another aspect of the invention, a compound of formula I wherein R1 is optionally substituted alkyl. optionally substituted cydoalkyl. optionally substituted oxaaliphatic or optionally substituted or optionally oxidi/ed cyclofhioalkvl.
According to another aspect of the invention, a compound of formula I wherein Rl is oxacycloalkyl is preferred; more preferably R is tetrahydrofuryl or tetrahydropyranyl; still more preferably R is tetrahydro-3-furyl.
According to another aspect of the invention, a compound of formula I wherein R3 is optionally oxidized tetrahydrothiophene or optionally oxidized perhydrothiopyran is preferred; more preferably tetrahydrothiophene-S-oxide, especially 3-tetrahydrothiophene-S-oxide.
According to another aspect of the invention, a compound of formula I wherein R3 is a C3.7 alkyl optionally substituted by one or two hydroxy groups is preferred; more preferably R3 is 1 -hydroxyprop-2-yl .
According to another aspect of the invention, a compound of formula I wherein R3 is a cydoalkyl optionally substituted by one or two hydroxy groups is preferred; more preferably R3 is C4.g monocyclic optionally substituted by one or two hydroxy groups; and still more preferably R3 is 3-hydroxycyclopentyl.
According to another aspect of the invention, a compound of formula 1 wherein R3 is hydroxycycloalkyl and the bond connecting the hydroxy to the cydoalkyl is in a trans-configuration with respect to the bond connecting the cydoalkyl to the rest of the molecule is preferred.
According to another aspect of the invention, a compound of formula I wherein R3 is hydroxycycloalkyl and the bond connecting the hydroxy to the cydoalkyl is in a cis-configuration with respect to the bond connecting the cydoalkyl to the rest of the molecule is preferred.
According to a further aspect of the invention, a preferred compound of formula I is described wherein Q\ J and (_)5 are CH. In another aspect of the invention, a preferred compound of formula I is described wherein at least one of Q" and Q4 are independently nitrogen and N-oxides thereof, or CX. and ' is CH. CX is preferably CF. Further preterred are compounds wherein Q14 is CF, or nitrogen and N-oxides thereof.
According to a further aspect of the invention, a preferred compound of formula I is described wherein R" is C-_- alkyl optionally substituted by one or more halo (e.g. fluoro). R*' is more preferably methyl or di fluoro methyl.
According to a further aspect of the invention, a preferred compound of formula I is described wherein is a direct bond.
According to a further aspect of the invention, a preterred compound of formula 1 is described wherein 7/ is alkylenyl; more preferred 7/ is methylenyl. methylmethylenyl. ethylenyl, methylethylenyl. propylenyl, or propylmethylenyl.
According to a further aspect of the invention, a preferred compound of formula I is described wherein Z3 is alkylenyl substituted by alkoxy; more preferred 7." is methoxymethylenyl or methoxypropylmethylenyl.
According to a further aspect of the invention, a preferred compound of formula I is described wherein R is hydrogen, optionally substituted cydoalkyl, alkoxy, optionally substituted aryl, optionally substituted heteroaryl or optionally substituted aryloxy. In addition, when R is a heteroaryl containing at least one nitrogen atom, then R7 may be encompasses the corresponding N-oxide of that heteroaryl. Thus, R may be an N-oxido-pyridinio.
According to a further aspect of the invention, a preferred compound of formula I is described wherein Rτ is cydoalkyl; more preferred R" is cyclopentyl.
According to a further aspect of the invention, a preferred compound of formula I is described wherein R7 is aryl.
According to a further aspect of the invention, a preferred compound of formula I is described wherein R7 is aryloxy.
According to a further aspect of the invention, a preferred compound of formula I is described wherein R" is pyridyl.
According to a further aspect of the invention, a preferred compound of formula I is described wherein is cyclopentyl, aryl, aryloxy or pyridyl.
According to a further aspect of the invention, a preferred compound of formula I is described wherein R is hydrogen and Z' is C,. alkylenyl; more preferred R is hydrogen and 7? is methylenyl.
According to a further aspect of the invention, a preferred compound of formula I is described wherein R^ is hydrogen and 7. is alkylenyl substituted by alkoxy.
According to a further aspect of the invention, a preferred compound of formula I is described wherein R is hydrogen and Z' is isopropylenyl.
According to a further aspect of the invention, a preferred compound of formula I is
According to a further aspect of the invention, a preferred compound of formula I is described wherein R8 is hydrogen or methyl. It should be appreciated that when R8 is hydrogen and
the then the structural isomers so defined are tautomers.
According to a further aspect of the invention, a preferred compound of formula I is described wherein Z4 is preferably oxygen.
According to a further aspect of the invention, a preferred compound of formula I is described wherein Q} is CH.
According to a further aspect of the invention, a preferred compound of formula 1 is described wherein R4 is hydrogen or methyl; more preferably methyl.
According to a further aspect of the invention, a preferred compound of formula I is described wherein R5 is optionally substituted alkyl. optionally substituted cydoalkyl. optionally substituted heterocyclyl, optionally substituted aryl or optionally substituted heteroaryl; more preferably optionally substimted alkyl. optionally substituted cydoalkyl or optionally substituted aryl.
According to a further aspect of the invention, a preferred compound of formula I is described wherein /J is a direct bond.
According to a further aspect of the invention, a preferred compound of formula I is described wherein Q' is CH.
According to a further aspect of the invention, a preferred compound of formula I is described wherein R1 is cyanoalkyl. carboxyalkyl, optionalh substituted aralkyl or optionally substituted heteroaralkyl; more preferably cyanomethyl, carboxybutyl, pyrid-4-yl ethyl. N-oxide of pyrid-4-ylmethyl, 4-hydroxybenzyl, 4-acetoxybenzyl.
According to a further aspect of the invention, a preferred compound of formula I is described wherein m is 1 to 3.
According to a further aspect of the invention, a preferred compound of formula I is described wherein m' and m" are 0.
According to a further aspect of the invention, a preferred compound of formula I is described wherein p is 1.
According to a further aspect of the invention, a preferred compound of formula I is described wherein at least one of Q' and Q: Is CO and the other of Q1 and Q: is CO or CH2; more preferably Q' and Q2are both CO.
According to a further aspect of the invention, a preferred compound of formula I is
described wherein
is optionally substituted fused phenyl; more preferably selected from the group of formulae consisting of
Preferred compounds for use according to the invention are selected from the following:
6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-ylmethylindan- l,3-dione;
t-Butyl 4-[2-(3-Cyclopentyloxy-4-methoxyphenyl)-l,3-dioxoindan-2-yl]butyrate;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-ylmethylindan-l,3-dione;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-2-(3,5-dichloropyrid-4-ylmethyl)indan-l ,3-dione;
4-[2-(3-cyclopentyloxy-4-methoxyphenyl)-l ,3-dioxoindan-2-ylmethyl]phenyl Acetate;
2-Benzyl-(3-cyclopentyloxy-4-methoxyphenyl)indan- 1 ,3-dione;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-2-pyrid-3-ylmethylindan- I ,3-dione;
6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-l ,3-dioxoindan-2-yl- acetonitrile;
6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-(3.5-dimethylisoazol-4- ylmethyl)indan-l ,3-dione;
6-Benzyl-6-(3-cyclopentyloxy-4-methoxyphenyl)-[2]-pyrindine-5.7-dione;
6-(3-Cyclopentyloxy-4-methoxyphenyl)-6-pyrid-4-ylmethyI-[2]-pyrindine-5.7-dione;
6-Benzyloxy-2-(7-methoxy-2-methoxymethyl-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan- 1,3-dione;
6-Benzyloxy-2-(7-methoxy-2-phenyl-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethyl- indan- 1.3-dione;
6-Benzyloxy-2-(7-methoxy-2-phenethyl-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethyl-indan- l ,3- dione;
6-Benzyloxy-2-(2-benzyl-7-methoxy-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethyl- indan- l,3-dione;
6-Benzyloxy-2-((RS)-7-methoxy-2-(l-phenylethyl)-3H-benzimidazol-4-yl)-2-pyrid-4- ylmethylindan- 1 ,3-dione;
6-Benzyloxy-2-(7-methoxy-2-(4-methoxybenzyl)-3H-benzimidazol-4-yl) -2-pyrid-4-ylmethyl indan- l ,3-dione;
6-Benzyloxy-2-((RS)-2-(cyclohexyl-phenyl-methyl)-7-methoxy-3H-benzimidazol-4-yI) -2-pyrid-4- ylmethyl indan-1.3-dione;
6-Benzyloxy-2-((RS)-2-( l,2-diphenylethyl)-7-methoxy-3H-benzimidazol-4-yl) -2 -pyrid-4-yl methyl indan-l ,3-dione;
6-Benzyloxy-2-((RS)-7-methoxy-2-(2-phenylpropyl)-3H-benzimidazol-4-yl) -2-pyrid-4-ylmethyl indan- l ,3-dione;
6-Benzyloxy-2-(7-methoxy-2-(4-methoxyphenoxymethyl)-3H-benzimidazol-4-yl) -2-pyrid-4- ylmethyl indan-l,3-dione;
6-Benzyloxy-2-((RS)-7-methoxy-2-( 1 -phenylbutyl)-3H-benzimidazol-4-yl) -2-pyrid-4-ylmethyl indan- l ,3-dione;
6-Benzyloxy-2-(2-(4-bromobenzyl)-7-methoxy-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan- 1.3-dione;
6-Benzyloxy-2-((RS)-7-methoxy-2-(3-methoxy- l -phenylpropyl)-3H-benzimidazol-4-yl)-2-pyrid- 4-ylmethylindan- l,3-dione;
6-Benzyloxy-2-(2-(4-cyanobenzyl)-7-methoxy-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan- 1 ,3-dione;
6-Benzyloxy-2-(7-methoxy-2-(4-{ 3-pyridyl }benzyl)-3H-benzimidazol-4-yl)-2-pyrid-4- ylmethylindan- 1 ,3-dione;
6-Benzyloxy-2-(7-methoxy-2-(2-methoxybenzyl)-3H-benzimidazol-4-yl)-2-pyrid-4- ylmethylindan- 1 ,3-dione;
6-Benzyloxy-2-((RS)-7-methoxy-2-(methoxyphenylmethyl)-3H-benzimidazol-4- yl)-2-pyrid-4- ylmethylindan-1.3-dione;
6-Benzyloxy-2-(7-methoxy-2-(2-methoxyphenoxy)methyl-3H-benzimidazol-4-yl)-2-pyrid-4- ylmethylindan-l,3-dione;
6-Benzyloxy-2-(7-methoxy-2-(3-pyridyl)-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan- l ,3- dione;
SUBSΗTUTE SHEET (RULE 26)
6-Benzyloxy-2-(2-isopropyl-7-methoxy-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan-1.3- dione;
6-Benzyloxy-2-(7-methoxy-2-methyl-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan- 1 ,3-dione;
6-Benzyloxy-2-(7-methoxy-2-phenoxymethyl-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan- 1.3-dione;
6-Benzyloxy-2-(2-cyclopentyl-7-methoxy-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan-l,3- dione;
6-Benzyloxy-2-(2-ben?.yl-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan-l ,3-dione;
6-Benzyloxy-2-(2-cyclopentyl-7-methoxy-l-methyl-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan- 1.3-dione;
6-Benzyloxy-2-(2-cyclopentyl-7-methoxy-3-methyl-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan- 1,3-dione;
6-Benzyloxy-2-(2,7-dimethoxy-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan- l,3-dione;
6-Benzyloxy-2-(2-cyclopropyl-7-methoxy-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan-l ,3- dione;
6-Benzyloxy-2-( l -cyclohexylmethyl-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan- l ,3-dione;
6-Benzyloxy-2-( l-(2-cyclohexyl)ethyl-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan-l,3- dione;
6-Benzyloxy-2-( l-(3-cyclohexyl)propyl-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan- l,3- dione;
6-Benzyloxy-2-(l-heptyl-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan- l,3-dione;
6-Benzyloxy-2-(l-isobutyl-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan-l ,3-dione;
6-Benzyloxy-2-(l-cyclopcntylmethyl-3-methyl-l H-indol-6-yl)-2-pyrid-4-ylmethylindan-1.3-dione;
6-Benzyloxy-2-(l-benzyl-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan-1.3-dione;
6-Benzyloxy-2-(l-(3-phenyl)propyl-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan- 1.3-dione;
6-Benzyloxy-2-(l-(3-phenyl)butyl-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan- 1.3-dione;
6-Benzyloxy-2-(l-(4-fluorobenzyl)-3-methyl- lH-indol-6-yl)-2-pyrid-4-ylmethylindan- 1.3-dione;
6-Benzyloxy-2-( 1 -(4-chlorobenzyl)-3-methyl- 1 H-indol-6-yl)-2-pyrid-4-ylmethylindan- 1 ,3-dione;
6-Benzyloxy-2-( 1 -(4-methoxybenzyl)-3-methyl- 1 H-indol-6-yl )-2-pyrid-4-ylmethylindan- 1,3- dione;
6-Benzyloxy-2-( l -(4-triπuoromethylbenzyl)-3-methyl- l H-indoi-6-yl)-2-pyrid-4-ylmethylindan- 1,3-dione;
6-Benzyloxy-2-( l-(4-methylsulphonylbenzyl)-3-methyl- l H-indol-6-yl)-2-pyrid-4-ylmethylindan- 1,3-dione;
6-Benzyloxy-2-( l-(1.3-benzodioxol-5-yl)methyl-3-methyl- l H-indol-6-yl)-2-pyrid-4- ylmethylindan- 1 ,3-dione;
6-Benzyloxy-2-( l -(naphthalen-2-yl)methyl-3-methyl- l H-indol-6-yl)-2-pyrid-4-ylmethylindan- l,3- dione;
6-Benzyloxy-2-(l -(tetrahydro-2H-pyran-2-yl)methyl-3-methyl- lH-indol-6-yl)-2-pyrid-4- ylmethy lindan- 1.3-dione ;
6-Benzyloxy-2-(3-methyl-l-(tetrahydrofurfuryl)-lH-indol-6-yl)-2-pyrid-4-ylmethylindan- l ,3- dione;
6-Benzyloxy-2-(3-methyl- 1 -(toluene-4-sulphonyl)- 1 H-indol-6-y l)-2-pyrid-4-ylmethylindan- 1,3- dione;
6-Benzyloxy-2-(3-methyl-l-(tetrahydrofuran-3-yl)-lH-indol-6-yl)-2-pyrid-4-ylmethylindan-l ,3- dione;
6-Benzyloxy-2-(3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan-l ,3-dione;
6-Benzyloxy-2-(l -benzyl-3-methylindolin-6-yl)-2-pyrid-4-ylmethylindan-l ,3-dione;
6-Benzyloxy-2-( l-benzyl-3-methylindazol-6-yI)-2-pyrid-4-ylmethylindan-1.3-dione;
(+) 6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-ylmethylindan-l,3-dione;
(-) 6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-yl-methylindan-l,3-dione;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-2-( l-oxo-pyrid-4-ylmethyl)indan-1.3-dione;
6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-( l-oxopyrid-4-yl-methyl)indan- 1.3-dione;
4-[6-Benzyioxy-2-(3-cyclopentyloxy-4-methoxyphenyl)indan-2-ylmethyl]pyridine;
4-[2-(3-Cyclopentyloxy-4-methoxyphenyl)indan-2-ylmethyl ]pyridine;
2-(3-Cyclopentyioxy-4-methoxyphenyl)-2-pyrid-3-ylmethylindan- l ,3-diol;
5-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-ylmethylindan- l-one;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-hydroxy-2-pyrid-4-ylmethylindan- 1 .3- dione;
Methyl [2-(3-Cyclopentyloxy-4-methoxyphenyl)- 1.3-dioxo-2-pyrid-4-ylmethylindan-5-yl]acetate;
5-(4-Bromobenzyloxy)-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4- ylmethylindan- 1 ,3-dione;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-(2-morpholin-4-ylethoxy)-2-pyrid-4- ylmethylindan-1 ,3-dione;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-ethoxy-2-pyrid-4-ylmethylindan- l ,3- dione;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-pyrid-4-ylmethoxy-2-pyrid-4-ylmethyl-indan- l,3-dione;
5-Cyclopentyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-ylmethylindan- 1.3-dione;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-pyrid-3-yl-2-pyrid-4-ylmethylindan- l,3-dione;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-l,3-dioxo-2-pyrid-4-ylmethylindan-5-yl Moφholine-4-carboxylate;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-1.3-dioxo-2-pyrid-4-ylmethylindan-5-yl [1, 4' ]-Bipiperidinyl-l ' -carboxylate;
4-[2-(3-Cyclopentyloxy-4-methoxyphenyl)- l ,3-dioxoindan-2-yl]butyric acid;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-2-(4-hydroxybenzyl)indan- l ,3-dione;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-methyl-2-pyrid-4-ylmethylindan- 1.3- dione;
2-(3-cyclopentyloxy-4-methoxyphenyl)-5-hydroxymethyl-2-pyrid-4-ylmethylindan- 1.3-dione;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-(4-methylpiperazinyl- l-methyl)-2-pyrid-4- ylmethylindan- l ,3-dione;
3-(3-Cyclopentyloxy-4-methoxyphcnyl)-2-oxo-3-pyrid-4-ylmethylpyrrolidine- l -carboxylic acid tert-butyl ester;
3-(7-methoxy-2-methoxymelhyl-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidinc-2-one-l - carboxylic acid tert-butyl ester;
3-(7-methoxy-2-phenyl-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l-carboxylic acid tert-butyl ester;
3-(7-methoxy-2-phenethyl-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l- carboxylic acid tert-butyl ester;
3-C2-benzyl-7-methoxy-3H-benzimidazol-4-yl) pyrrolidine-2-one- 1 -carboxy lie acid tert-butyl ester;
3-((RS)-7-methoxy-2-(l -phenylethyl)-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2- one-1 -carboxy lie acid tert-butyl ester;
3-(7-methoxy-2-(4-methoxybenzyl)-3H-benzimidazol-4-yl) pyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-((RS)-2-(cyclohexyl-phenyl-methyl)-7-methoxy-3H-benzimidazol-4-yl)-3-pyrid-4- ylmethylpyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-((RS)-2-(l,2-diphenylethyl)-7-methoxy-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2- one-1 -carboxylic acid tert-butyl ester;
3-((RS)-7-methoxy-2-(2-phenylpropyl)-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2- one-1 -carboxylic acid tert-butyl ester;
3-(7-methoxy-2-(4-methoxyphenoxymethyl)-3H-benzimidazol-4-yl)-3-pyrid-4- ylmethylpyrrolidine-2-one- 1 -carboxylic acid tert-butyl ester;
3-((RS)-7-methoxy-2-(l -phenylbutyl)-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2- one-1 -carboxylic acid tert-butyl ester;
3-(2-(4-bromobenzyl)-7-methoxy-3H-benzimidazol-4-yl)-pyrrolidine-2-one- l -carboxylic acid tert- butyl ester;
3-((RS)-7-methoxy-2-(3-methoxy- 1 -phenylpropyl)-3H-benzimidazol-4-yl)-pyrrolidine-2-one- 1 - carboxylic acid tert-butyl ester;
3-(2-(4-cyanobenzyl)-7-methoxy-3H-benzimidazol-4-yl)-pyrrolidine-2-one-l-carboxylic acid tert- butyl ester;
3-(7-methoxy-2-(4-{ 3-pyridyl } benzyl)-3H-benzimidazol-4-yl)-pyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-(7-methoxy-2-(2-methoxybenzyl)-3H-benzimidazol-4-yl)-pyrrolidine-2-one- 1 -carboxylic acid tert-butyl ester;
3-((RS)-7-methoxy-2-(methoxyphenylmethyl)-3H-benz-imidazol-4- yl)pyrrolidine-2-one- 1 - carboxylic acid tert-butyl ester;
3-(7-methoxy-2-(2-methoxyphenoxy)methyl-3H-benzimidazol-4-yI)-3-pyrid-4- ylmethylpyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-(7-methoxy-2-(3-pyridyl)-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(2-isopropyl-7-methoxy-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l - carboxylic acid tert-butyl ester;
3-(7-methoxy-2-methyl-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-(7-methoxy-2-phenoxymethyl-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(2-cyclopentyl-7-methoxy-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(2-benzyl-3H-benzimidazol-4-yI)-3-pyrid-4-ylmethylpyrrolidine-2-one- l-carboxylic acid tert- butyl ester;
3-(2-cyclopentyl-7-methoxy- l-methyl-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(2-cyclopentyl-7-methoxy-3-methyl-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l- carboxylic acid tert-butyl ester;
3-(2,7-dimethoxy-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-(2-cyclopropyl-7-methoxy-3H-benzimidazol-4-yl)pyrrolidine-2-one- 1 -carboxylic acid tert-butyl ester;
3-(l-cyclohexylmethyl-3-methyl-lH-indol-6-yl)pyrrolidine-2-one-l-carboxylic acid tert-butyl ester;
3-(l-(2-cyclohexyl)ethyl-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(l -(3-cyclohexyl)propyl-3-methyl- l H-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(l-heptyl-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l -carboxylic acid tert- butyl ester;
3-(l-isobutyl-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l-carboxylic acid tert- butyl ester;
3-( 1 -cyclopentylmethyl-3-methyl- 1 H-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- 1 - carboxylic acid tert-butyl ester;
3-(l-benzyl-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l -carboxylic acid tert- butyl ester;
3-( l -(3-phenyl)propyl-3-methyl- l H-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l -carboxylic acid tert-butyl ester;
3-(l -(3-phenyl)butyl-3-methyl- l H-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l -carboxylic acid tert-butyl ester;
3-(l -(4-fluorobenzyl)-3-methyl- l H-indol-6-yl)-3-pyrid-4-ylrnctl"iylpyrrolidine-2-one- i -carboxylic acid tert-butyl ester;
3-(l -(4-chlorobenzyl)-3-methyl- l H-indol-6-yI)-3-pyrid-4-ylmethylpyrrolidine-2-one- l-carboxylic acid tert-butyl ester;
3-(l-(4-methoxybenzyl)-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l - carboxylic acid tert-butyl ester;
3-(l-(4-trifluoromethylbenzyl)-3-methyl- lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l- carboxylic acid tert-butyl ester;
3-(l-(4-methylsulphonylbenzyl)-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l- carboxylic acid tert-butyl ester;
3-(l-(l,3-benzodioxol-5-yl)methyl-3-methyl-l H-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- 1 -carboxylic acid tert-butyl ester;
3-(l-(naphthalen-2-yl)methyl-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(l-(tetrahydro-2H-pyran-2-yl)methyl-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2- one-1 -carboxylic acid tert-butyl ester;
3-(3-methyl-l -(tetrahydrofurfuryl)-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l- carboxylic acid tert-butyl ester;
3-(3-methyl- 1 -(toluene-4-sulphony 1)- 1 H-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- 1 - carboxylic acid tert-butyl ester;
3-(3-methyl-l -(tetrahydrofuran-3-yI H-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l- carboxylic acid tert-butyl ester;
3-(3-methyl- lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l -carboxylic acid tert-butyl ester;
3-(l-benzyl-3-methylindolin-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l -carboxylic acid tert- butyl ester;
3-( l-benzyl-3-methylindazol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l -carboxylic acid tert- butyl ester;
3-(3-Cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4-ylmethyl-pyrrolidin-2-one;
l-Benzyl-3-(3-cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4-ylmethyl-pyrrolidin-2-one;
3-(3-Cyclopentyloxy-4-methoxyphenyl)- l-(3-methylbutyl)-3-pyrid-4-ylmethyl-pyrrolidin-2-one;
l-Cyclohexylmethyl-3-(3-cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4-ylmethyl-pyrrolidin-2-one;
3-(3-Cyclopentyloxy-4-methoxyphenyl)-l,3-bis-pyridin-4-ylmethyl-pyrrolidin-2-one;
l-Acetyl-3-(3-cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4-ylmethyl-pyrrolidin-2-one;
l-Butyl-3-(3-cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4-ylmethyl-pyrrolidin-2-one;
3-(3-Cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4-ylmethyl-l-(toluene-4-sulfonyl)-pyrrolidin-2- one;
3-(3-Cyclopentyloxy-4-methoxyphenyl)-naphthalen-2-ylmethyl-3-pyrid-4-ylmethyl-pyrrolidin-2- one;
3-(3-Cyclopentyloxy-4-methoxyphenyl)-2-oxo-3-pyrid-4-ylmethyl-pyrrolidin-l-carboxylic acid isopropyl ester;
3-(3-Cyclopentyloxy-4-methoxyphenyl)-2-oxo-3-pyrid-4-ylmethyl-pyrrolidin-l-carboxylic acid ethyl ester;
3-(3-Cyclopentyloxy-4-methoxyphenyl)-2-oxo-3-pyrid-4-ylmethylpyrrolidJn-l -carboxylic acid methyl ester;
3-(3-Cyclopentyloxy-4-methoxyphenyl)-naphthalen- l -ylmethyl-3-pyrid-4-ylmethylpyrrolidin-2- one;
3-(3-Cyclopentyloxy-4-methoxyphenyl)-2-oxo-3-pyrid-4-ylmelhyl-pyrrolidine- 1 -carboxylic acid dimethyl amide;
3-(3-Cyclopentyloxy-4-methoxyphenyl)-3-pyrid-N-oxide-4-ylmethyl-2-oxopyrrolidine- l- carboxylic acid tert-butyl ester
3-(3-Cyclopentyloxy-4-methoxyphenyl)-3-(l-pyrid-N-oxide-4-yimethyl)-pyrrolidine-2-one;
3-Benzyl-3-(3-cyclopentyloxy-4-methoxyphenyl)-2-oxo-pyrrolidine-l-carboxyIic acid tert-butyl ester;
3-Benzyl-3-(3-cyclopentyloxy-4-methoxyphenyl)-pyrrolidine-2-one;
3-Cyanomethyl-3-(3-Cyclopentyloxy-4-methoxyphenyl)-2-oxo-pyrrolidine-l-carboxylic acid tert- butyl ester;
3-(3-Cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4-ylmethylpyrrolidine; and
3-(3-Cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4-ylmethyl-pyrrolidine-l -carboxylic acid tert- butyl ester.
A particularly preferred compound is:
(±) 6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-ylmethylindan- l,3-dione;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-ylmethylindan-1.3-dione;
(+) 6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-ylmethylindan-1.3-dione;
(-) 6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-yl-methylindan-1.3-dione;
5-Benzyioxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-yl-methylindan-l-one;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-(2-morpholin-4-ylcthoxy)-2-pyrid-4-ylmethylindan- l,3- dione;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-l ,3-dioxo-2-pyrid-4-ylmelhylindan-5-yl [ 1.4']- Bipiperidinyl- 1 '-carboxylate;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-(4-methylpiperazinyl- 1 -n.ethyl)-2-pyrid-4- ylmethylindan- 1 ,3-dione;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-2-( l-oxopyrid-4-ylmethyl)indan- l ,3-dione;
6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-( l-oxopyrid-4-yl-methyl)indan- 1.3-dione;
3-(3-Cyclopentyloxy-4-methoxyphenyl)-2-oxo-3-pyrid-4-ylmethyl-pyrroiidine-l-carboxylic acid tert-butyl ester;
l-Benzyl-3-(3-cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4-ylmethyl-pyrrolidin-2-one;
l-Cyclohexylmethyl-3-(3-cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4-ylmethyl-pyrrolidin-2-one; or
3-(3-Cyclopentyloxy-4-methoxyphenyl)-l-naphthalen-2-yImethyl-3-pyrid-4-ylmethyl-pyrrolidin- 2-one.
Compounds of formula I may be prepared by the application or adaptation of known methods, by which is meant methods used heretofore or described in the literature.
For example, a compound of formula la may be prepared according to Scheme la below wherein an indanedione of formula Ha, wherein Ar1, Ar, ' and m" are as defined above is alkylated with an appropriate organic halide compound of formula III (R'X. wherein R1 is as defined above and X is halo, preferably chloro or bromo) in the presence of a strong base such as an alkali or alkaline earth hydroxide or carbonate hydroxide, e.g., sodium carbonate, potassium hydroxide or calcium carbonate. The reaction preferably takes place in an aprotic polar organic solvent such as DMSO. DMF, acetone or THF at about room temperature to about reflux. In addition, it is preferable that the reaction take place in the presence of a catalytic amount of an alkali iodide such as sodium iodide.
Scheme la
According to another feature of the present invention, a compound of formula I(b-f) may be prepared according to Steps (A-E) in Scheme Ib below.
Scheme Ib
In Step A of Scheme Ib where a compound of formula lib. wherein Ar1 and p are as defined above, and P' is a protecting group for the nitrogen of the amide moiety thereof, for example BOC, is alkylated with an appropriate organic halide compound of formula III as define above under the conditions as defined above for the preparation of the compound of formula la.
In Step B of Scheme Ib, the compound of formula Ib is deprotected using standard deprotection techniques to yield a compound of formula lc. For example , the deprotection is carried out using TFA. at about room temperature.
In Step C of Scheme Ib, the amide compound of formula lc is reduced to the corresponding amine compound of formula Id using an appropriate reducing agent such as Red-Al in an inert solvent such as toluene at about 80°C.
In Step D of Scheme Ib, the compound of formula lc is converted to the corresponding thioamide compound of formula Ie by reaction with phosphorus pentasulfide or Lawesson's reagent (2,4-bis(4-methoxyphenyl)-l,3-dithia-2,4-diphosphetane-2,4-disulfide), preferably in a solvent such as pyridine or toluene, and preferably at about 0°C to about the reflux temperature.
In Step E of Scheme Ib, the compounds of formula I(c-e) are converted to the compound of formula If by alkylation with a compound of formula IV (R'X, wherein R' is optionally substituted alkyl, cycloalkylalkyl, heterocyclylalkyl, optionally substituted aralkyl, optionally substituted heteroaralkyl, arylsulphonyl, heteroarylsulphonyl, R"CO- or RbOCO-, wherein Ra is alkyl, cycloalkylalkyl. heterocyclylalkyl, optionally substituted aralkyl, optionally substituted heteroaralkyl, dialkylamino or diarylamino. and Rb is alkyl, cycloalkylalkyl, heterocyclylalkyl, optionally substituted aralkyl or optionally substituted heteroaralkyl, and X is as defined above). The alkylation is carried out in an aprotic polar solvent such DMF, DMSO or acetone at about room temperature to about 90°C using a strong base such as an alkali or alkaline earth base, such as potassium carbonate.
According to further features of the present invention, a compound of formula I is prepared by an interconversion from one compound of formula I to another compound of formula I.
For example, according to a feature of the present invenϋon. a compound of formula Ig
wherein Ar'. Ar2, R'. m' and m" are as defined above, and at least one of Q' and Q2 is CH, or CHOH is prepared by a selective reduction of a compound of formula la. Reduction agents include <H2> and a catalyst selected from Pt, Pd, Rh and Ru, Li and NH3, Al-Hg, a metal hydride selected from lithium aluminum hydride, lithium aluminum tributoxidehydride, sodium borohydride and borane, an organo tinhydride, a hydrazine and strong base, Zn-Hg and strong acid. The reductions are prepared in inert and/or polar organic solvents, or in a mixed organic-aqueous solvent depending on the reducing agent used and extent of reduction desired. The reductions are effected from about room temperature to about reflux depending on the reducing agent used and extent of reduction desired. To influence the extent of the reduction, one may also adjust the time of the reduction.
According to a further feature of the present invention, a compound of formula Ig. wherein Ar1, Ar, R1. m' and m" are as defined above, and Q1 and Q2 are CH2, is oxidized to a compound of formula Ig wherein Ar1, Ar2, R', m' and m" are as defined above, and one of Q1 and Q2 is CO. 2,2'- Bipyridium chlorochromate is a particularly useful oxidizing agent. The oxidation is effected in a polar aprotic organic solvent such as acetone at about reflux.
As another example, a compound of formula I having a carboxyalkyl group is prepared by hydrolyzing a corresponding compound of formula I having a cyanoalkyl, alkoxycarbonylalkyl or Y'Y**NCO-alkyl group, under acidic or basic conditions, e.g., using acids such as H:S04, HC1 or H3P04 or using bases such as KOH or NaOH, in aqueous or aqueous-alcoholic solutions, at about or above room temperature. Alternatively, a corresponding compound of formula I having a methoxycarbonylalkyl group may be converted to a compound of formula I having a carboxyalkyl group by reacting with lithium iodide in a solvent such as DMF or collidine. A corresponding compound of formula I having an bcnzyloxycarbonylalkyl group may be converted to a compound of formula I having a carboxyalkyl group by hydrogenating using lor example <H,> with a catalyst such as Pd.
As another example, a compound of formula I having a Y'Y2NCO-. Y3Y4NCO- or Y5Y6NCO- group is prepared from a corresponding compound of formula I having a carboxyl group, for example by activating the carboxy group via treatment with thionyl chloride, PC1„ acetic anhydride, TsCl or a coupling agent, such as DCC, followed by reaction with an amine of the formulae
Y'Y2NH, YYNH or YYNH
at about or above room temperature.
As another example, a compound of formula I having an alkoxycarbonyl, aryloxycarbonyl or aralkoxycarbonyl group is prepared from a corresponding compound of formula I having a carboxyl group, for example, by activating the carboxy group via treatment with thionyl chloride. PClj, acetic anhydride, TsCl or a coupling agent, such as DCC. followed by reaction with an alcohol group such as alkylOH. arylOH or aralkylOH, at about or above room temperature.
As another example, a compound of formula I having a cyclothioalkyl, cyclothioalkenyl, alkylthio. arylthio or aralkylthio moiety are converted by oxidation to a compound of formula I having the corresponding cyclosulphinylalkyl. cyclosulphinylalkenyl, alkylsulfinyl, arylsulfinyl and aralkylsulphinyl moieties. The oxidation may be carried out by means of potassium hydrogen peroxomonosulfate in a medium such as aqueous methanol. buffered to about pH 5, at about 0°C to about room temperature. This latter method is preferred for compounds containing an acid-labile
group. such as those wherein R1- contains a carbon-carbon double or triple bond, e.g., a cyclopent-2- enyloxy group.
As another example, a compound of formula I having a cyclothioalkyl. cyclothioalkenyl. alkylthio, arylthio or aralkylthio moiety are converted by oxidation to a compound of formula I having the corresponding cyclosulphonylalkyl, cy osulphonylalkenyl. alkylsulphonyl, aryl sulphonyl and aralkylsulphonyl moieties. The oxidation may be carried out by means of sodium iodate in a medium such as aqueous methanol, or peroxyacid. e.g.. 3-chloroperbenzoic acid, preferably in an inert solvent, e.g., dichloromethane, preferably at about room temperature.
As another example, a compound of formula I having a hydroxymethyl group is prepared by the reduction of a corresponding compound of formula I having an aryloxycarbonyl or, preferably, alkoxycarbonyl group therein, preferably by means of reaction with an alkali metal borohydride. preferably in an inert solvent, e.g.. tetrahydrofuran, preferably at about room temperature.
As another example, a compound of formula I having a formyl group is prepared by the oxidation of a corresponding compound of formula I having a hydroxymethyl group therein. For example, the oxidation is effected by a reaction with oxalyl chloride and dimethyl sulfoxide, in a solvent such as dichloromethane, and preferably at a temperature lower than about -65°C, or, preferably, by reaction with a complex of sulfur trioxide with an amine such as pyridine, preferably in the presence of an amine such as triethylamine, preferably at about room temperature.
As another example, a compound of formula I having an amino group is prepared by the reduction of a corresponding compound of formula I having a nitro group, preferably by means of reaction with iron under acidic conditions, e.g., in acetic acid, preferably at about or above room temperature, more especially at the reflux temperature. Alternatively the reduction is carried out by reaction with hydrazine hydrate in the presence of ferric chloride and activated carbon, conveniently in a solvent such as methanol, at about 25°C to about 80°C. The latter reduction conditions are preferred for compounds containing an acid-labile group, such as those wherein R3 contains a carbon-carbon double or triple bond, e.g., a cyclopent-2-enyloxy group.
As another example, a compound of formula I having an alkanoylamino or aroylamino group is prepared from a compound of formula I having an amino group, preferably by reacting an appropriate acid halide or acid anhydride in the presence of a tertiary base such as triethylamine, optionally in an inert solvent, and preferably at about 0°C to about reflux temperature.
WO 98/05327 PCT/US97/13343 """
- 38 -
As another example, a compound of formula I having an aryl moiety substituted by a cycloalkyloxy, heterocyclyloxy, hydroxy, alkoxy, carboxyalkoxy, cycloalkylalkoxy, heterocyclylalkoxy, aryloxy, aralkoxy, halo, cyano, alkylthio, arylthio, heteroarylthio. aralkylthio or heteroaralkylthio group can be prepared from a compound of formula I having an aryl moiety substituted by an amino group, via diazotization of the amine group and reaction with one of the following reactants: H20 and acid; cycloalkyloxide; heterocyclyloxide; alkoxide; carboxyalkoxide; cycloalkylalkoxide; heterocyclylalkoxide; aryloxide; aralkoxide; halide such as KI. HBF4, CuBr or CuCl; CuCN; alkylsulfide (alkylS '); arylsulfide; heteroarylsulfide; aralkylsulfide; or heteroaralkylsulfide, at about 0°C to about reflux temperature.
As another example, a compound of formula I having an aryl moiety substituted by an acyl or aroyl group can be prepared from a compound of formula I having an aryl moiety substituted by an alkyl or arylmethyl group, under oxidation conditions such as in the presence of DDQ, argentic picolinate. or HN03 and (NH ):Ce(NO,)6.
Alternatively, a compound of formula I having an aryl moiety substituted by an acyl or aroyl group can be prepared from a compound of formula I having an aryl moiety by reaction with an acylhalide or aroylhalide in the presence of a Lewis acid such as A1C13, FeCl3, or BF3, i.e.. a Friedel- Crafts reaction, at about -20°C to about 80°C.
As another example, a compound of formula I having an aryl moiety substituted by an alkyl group can be prepared Jrom a compound of formula I having an aryl moiety by reaction with an alkylhalide in the presence of a Lewis acid such as A1C13. FeCl5, or BF3, i.e., a Friedel-Crafts reaction, at about -20°C to about 80°C.
As another example, a compound of formula I having an aryl moiety substituted by an nitro group can be prepared Irom a compound of formula I having an aryl moiety by reaction with a mixture of nitric acid and sulfuric acid.
As another example, a compound of formula I having an aryl moiety substituted by a Y'Y N-.
Y3Y4N- or Y5Y"*N- group can be prepared from a compound of formula I having an aryl moiety substituted H2N- by alkylation or arylation with Y'X, Y2X, Y3X, Y X, Y5X, or Y^, wherein X is halo and Y', Y\ Y\ Y\ Y5 or Y* are as defined above.
Alternatively, a compound of formula I having an aryl moiety substituted by a Y'Y N-.
Y3Y N- or Y5Y6N- group can be prepared from a compound of formula I having an aryl moiety substituted halo, preferably chloro or fluoro, by reaction with an amine of the formula: YlY2NH, Y3Y NH orY5Y*NH.
As another example, a compound of formula I having an aryl moiety substituted by a H03S- or Y5Y6NS02- group can be prepared from a compound of formula I having an aryl moiety having a diazonium moiety as prepared above reacted with S02 in the presence of copper(II) chloride to prepare the corresponding compound of formula I having an aryl moiety substituted by C1S02- which is then reacted with H20 or an amine of formula Y5Y*NH.
Alternatively, a compound of formula I having an aryl moiety substituted by a H03S- group can be prepared by the action of chlorosulfonic acid on the aryl moiety.
As another example, a compound of formula I wherein Z'. 7: and 7/ each are preferably oxygen atoms, Q\ Q4, Qs or Q" is a nitrogen atom and/or having a heteroaryl group containing one or more nitrogen ring atoms, are converted to a compound of formula I wherein the aforesaid nitrogen atoms are oxidized to the corresponding N-oxides. The oxidation is effected by reacting a mixture of hydrogen peroxide and an organic acid, e.g., acetic acid, preferably at about or above room temperature, more preferably at about 60°C to about 90°C. Alternatively, the oxidation is carried out by reacting a peracid, for example, -chloroperoxybenzoic acid in an inert solvent such as dichloromethane, at about room temperature to about reflux, preferably at elevated temperature. Alternatively, the oxidation is carried out by reaction with hydrogen peroxide in the presence of sodium tungstatc at about room temperature to about 60°C. This last method is preferred for compounds containing an acid-labile group, such as those wherein the moiety R: contains a carbon-carbon double or triple bond, e.g., a cyclopent-2- enyloxy group.
As another example, an N-oxide group in a compound of formula I can be reduced to the corresponding nitrogen atom. The reduction of an N-oxide group may be carried out by reaction with diphosphorus tetraiodide in an inert solvent, such as dichloromethane. preferably at about room temperature, or by reaction with a chlorotrialkylsilane, preferably chlorotrimethylsilane, in the presence of zinc and an alkali metal iodide, e.g., potassium iodide, in an inert solvent, e.g., acetonitrile, at about 0°C to about room temperature, preferably below room temperature.
As another example, a compound of formula I wherein R' and Rf'is fluoro substituted on its alpha-carbon is prepared by the reacting xenon difluoride with a corresponding compound of formula I wherein said alpha-carbon atoms of R: and Rf bear hydrogen atoms instead of fluoro atoms. The reaction is carried out in a solvent, such as dichloromethane, in the presence of a molecular sieve, and in an inert atmosphere, at a low temperature, e.g., at about 0°C.
As another example, a compound of formula I having a trans-alkenyl group are convertible to a compound of formula I having a cis-alkenyl group by the action of ultraviolet radiation.
The compounds of the present invention are useful in the form of the free base or acid or in the form of a pharmaceutically acceptable salt thereof. All forms are within the scope of the invention.
Where the compound of the present invention is substituted with a basic moiety, acid addition salts are formed and are simply a more convenient form for use; and in practice, use of the salt form inherently amounts to use of the free base form. The acids which can be used to prepare the acid addition salts include preferably those which produce, when combined with the free base, pharmaceutically acceptable salts, that is, salts whose anions are non-toxic to the patient in pharmaceutical doses of the salts, so that the beneficial inhibitory effects on PDE and TNF inherent in the free base are not vitiated by side effects ascribable to the anions. Although pharmaceutically acceptable salts of said basic compounds are preferred, all acid addition salts are useful as sources of the free base form even if the particular salt, per se, is desired only as an intermediate product as, for example, when the salt is formed only for purposes of purification, and identification, or when it is used as intermediate in preparing a pharmaceutically acceptable salt by ion exchange procedures. Pharmaceutically acceptable salts within the scope of the invention are those derived from the following acids: mineral acids such as hydrochloric acid, sulfuric acid, phosphoric acid and sulfamic acid; and organic acids such as acetic acid, citric acid, lactic acid, tartaric acid, malonic acid, methanesufonic acid, ethanesulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid, cyclohexylsulfamic acid, quinic acid, and the like. The corresponding acid addition salts comprise the following: hydrohalides. e.g.. hydrochloride and hydrobromide. sulfate, phosphate, nitrate, sulfamate, acetate, citrate, lactate. tartarate. malonate, oxalate. salicylate, propionate. succinate, fumarate, maleate. methylene-bis-B-hydroxynaphthoates, gentisates. mesylates. isethionates and di-p- toluoyltartratesmethanesulfonate, ethanesulfonate, benzenesulfonate, p-toluenesulfonate, cyclohexylsulfamate and quinate, respectively.
According to a further feature of the invention, acid addition salts of the compounds of this invention are prepared by reaction of the free base with the appropriate acid, by the application or adaptation of known methods. For example, the acid addition salts of the compounds of this invention are prepared either by dissolving the free base in aqueous or aqueous-alcohol solution or other suitable solvents containing the appropriate acid and isolating the salt by evaporating the solution, or by reacting the free base and acid in an organic solvent, in which case the salt separates directly or can be obtained by concentration of the solution.
The acid addition salts of the compounds of this invention can be regenerated from the salts by the application or adaptation of known methods. For example, parent compounds of the invention can be regenerated from their acid addition salts by treatment with an alkali, e.g.. aqueous sodium bicarbonate solution or aqueous ammonia solution.
Where the compound of the invention is substituted with an acidic moiety, base addition salts may be formed and are simply a more convenient form for use; and in practice, use of the salt form inherently amounts to use of the free acid form. The bases which can be used to prepare the base addition salts include preferably those which produce, when combined with the free acid, pharmaceutically acceptable salts, that is, salts whose cations are non-toxic to the animal organism in pharmaceutical doses of the salts, so that the beneficial inhibitory effects on TNF inherent in the free acid are not vitiated by side effects ascribable to the cations. Pharmaceutically acceptable salts, including for example alkali and alkaline earth metal salts, within the scope of the invention are those derived from the following bases: sodium hydride, sodium hydroxide, potassium hydroxide, calcium hydroxide, aluminum hydroxide, lithium hydroxide, magnesium hydroxide, zinc hydroxide, ammonia, ethylenediamine. N-methyl-glucamine, lysine. arginine. ornithine. choline, N.N'- dibenzylethylene-diamine. chloroprocaine, diethanolamine. procaine. N-benzylphenethylamine, diethylamine, piperazine, tris(hydroxyrnethyl)-aminomethane, tetramethylammonium hydroxide, and the like.
Metal salts of compounds of the present invention may be obtained by contacting a hydride, hydroxide, carbonate or similar reactive compound of the chosen metal in an aqueous or organic solvent with the free acid form of the compound. The aqueous solvent employed may be water or it may be a mixture of water with an organic solvent, preferably an alcohol such as methanol or ethanol. a ketone such as acetone, an aliphatic ether such as tetrahydrol'uran. or an ester such as ethyl acetate. Such reactions are normally conducted at ambient temperature but they may, if desired, be conducted with heating.
Amine salts of compounds of the present invention may be obtained by contacting an amine in an aqueous or organic solvent with the free acid form of the compound. Suitable aqueous solvents include water and mixtures of water with alcohols such as methanol or ethanol. ethers such as tetrahydrofuran. nitriles such as acetonitrile. or ketones such as acetone. Amino acid salts may be similarly prepared.
The base addition salts of the compounds of this invention can be regenerated from the salts by the application or adaptation of known methods. For example, parent compounds of the invention can be regenerated from their base addition salts by treatment with an acid, e.g., hydrochloric acid.
As well as being useful in themselves as active compounds, salts of compounds of the invention are useful for the purposes of purification of the compounds, for example by exploitation of the solubility differences between the salts and the parent compounds, side products and/or starting materials by techniques well known to those skilled in the art.
It will be apparent to those skilled in the art that certain compounds of formula I can exhibit isomerism, for example geometrical isomerism, e.g., E or Z isomerism, and optical isomerism. e.g., R or S configurations. Geometrical isomers include the cis and trans forms of compounds of the invention having alkenyl moieties. Individual geometrical isomers and stereoisomers within formula I, and their mixtures, are within the scope of the invention.
Such isomers can be separated from their mixtures, by the application or adaptation of known methods, for example chromatographic techniques and recrystallization techniques, or they are separately prepared from the appropriate isomers of their intermediates, for example by the application or adaptation of methods described herein.
The starting materials and intermediates are either commercially available or prepared by the application or adaptation of known methods, for example as in the methods as described herein or their obvious chemical equivalents.
A compound of formula Ila above is prepared according to Scheme Ila below wherein an aromatic acetic acid of formula V and aromatic anhydride of formula VI are reacted under thermal conditions via a Gabriel-modified Perkin Reaction.
Scheme Ila
Alternatively, a compound of formula Ila may be prepared according to Scheme lib below wherein an aromatic acetic acid of formula V and aromatic diacid of formula VII are reacted under thermal conditions involving acetic anhydride and a tertiary amine such as triethylamine.
Scheme lib
-*"
- 43 -
A compound of formula lib above is prepared according to Scheme III below. In the first step, an aromatic acetic acid of formula VIII, wherein R"' is a lower alkyl. and Ar1 is as defined above (ester of compound V), is alkylated by a cyanoalkylhalide of formula IX, wherein p and X are as defined above, under basic conditions, preferably using a non-nucleophilic base such as potassium hexamethyl disilazane. in an apolar aprotic organic solvent such as dimethoxyethane, at reduced temperatures such as about -78°C. The resultant alkylated compound X wherein Ar' and p are as defined above, is subjected to conditions to reduce the cyano moiety thereof to the corresponding amine ester compound that undergoes an intramolecular amidation reaction to form the corresponding lactam compound of formula XI. The reduction is carried out preferably under catalytic conditions such as using Raney-nickel, with H2 in an organic solvent such as an alcohol such as methanol at about room temperature. The lactam is then N-protected with a hindered protecting group such as tert-butoxycarbonyl using (BOC)20 in a non-nucleophilic basic solvent such as Et3N or pyridine preferably with a catalyst such as DMAP at room temperature.
Scheme III
A substituted aromatic acetic acid of formula V above is prepared according to Scheme IV below from a corresponding substituted aromatic acyl halide compound of formula XII, wherein Ar1 is as defined above and X' is chloro or bromo, preferably chloro, through the Arndt-Eistert Synthesis, e.g., using diazomethane and a finely divided metal such as silver, copper, or platinum,
"WO 98/05327 PCT/US97/13343 "*
- 44 - e.g., silver oxide or silver nitrate, in a solvent such as water, alcohol, ammonia or an amine. The reaction is carried out at about 50°C to about 80°C. Where the solvent is an alcohol the product is an ester which is hydrolyzed to the desired corresponding acid, and where the solvent is ammonia or an amine, the product is an amide which is also hydrolyzed to the desired corresponding acid.
Scheme IV
Alternately, a substituted aromatic acetic acid of formula Va is also prepared according to
Scheme V below from an alkyl 3-hydroxy-4-lower alkoxyphenyl-acetate compound of formula XIII wherein R' is as defined above and R"' is lower alkyl through an alkylation reaction with either a halide of formula R3X. wherein R' and X are as hereinbefore defined, and X is preferably bromo. preferably in the presence of a base, for example an alkali metal hydride, e.g., sodium hydride, an alkali metal hydroxide or carbonate, e.g., sodium hydroxide or carbonate, or an amine, preferably a tertiary amine, e.g., triethylamine or pyridine, optionally in an inert solvent, for example dichloromethane, dimethylformamide, or an ether, e.g., diethyl ether or tetrahydrofuran. preferably at a temperature from 0°C to the reflux temperature, followed by hydrolysis of die ester to the corresponding desired acid, or alternatively under Mitsunobu conditions by reaction with an alcohol of formula compounds of the formula R'OH, wherein ' is as hereinbefore defined, preferably in the presence of a compound such as diisopropyl azodicarboxylate. followed by hydrolysis of the ester to d e corresponding desired acid.
Scheme V
An aromatic acyl halide compound of formula XII above, is prepared from a corresponding aromatic acid compound of formula XIV. wherein Ar' is
as hereinbefore defined, by reaction witfi PC13, PC1„ PBr3, thionyl chloride or. preferably, oxalyl chloride in the presence of triethylamine.
An aromatic acid compound of formula XIV wherein Ar1 is
is prepared as described in United States Patent No. 4,894,386, which is incoφorated herein by reference, or by A. Kasahara et al. Bull. Chem. Soc. Jpn, 59, 927 (1986). which is incoφorated herein by reference.
An aromatic acid compound of formula XIV above is prepared by the oxidation of a compound of formula XV. wherein Ar' is as
hereinbefore defined, e.g., by means of reaction with potassium permanganate, or with a mixture of sulfamic acid and sodium clilorite in acetic acid, or with sodium chlorite in the presence of sodium dihydrogen phosphate.
An aromatic aldehyde compound of formula XVa, wherein R\ R
J, Q
*\ Q\
→ and Z' are as hereinbefore defined, is prepared from a compound of formula XVI wherein R
:. Q \ Q
4, Q' and Z
1 are as hereinbefore defined, by reaction with
a compound of the formula R3X above in die presence of a base, for example an alkali metal hydride, e.g., sodium hydride, an alkali metal hydroxide or carbonate, e.g., sodium hydroxide or carbonate, or an amine, preferably a tertiary amine. e.g., triethylamine or pyridine, optionally in an inert solvent, for example dichloromethane. dimethylformamide. or an ether, e.g., diethyl ether or tetrahydrofuran, preferably at a temperature from 0°C to the reflux temperature, or alternatively by reaction with compounds of the formula R3OH above, preferably in the presence of a compound such as diisopropyl azodicarboxylate.
Alternatively, a compound of formula XlVa. wherein R\ R*, Q'. 4, Q1 and Z1 are as hereinbefore defined, are prepared by the hydrolysis of a compound of formula XVII wherein R'", R**, R . Q\ Q4. Q* and Z' are as hereinbefore
defined, e.g., by reaction with a base, such as an alkali metal carbonate or bicarbonate in the presence of water, followed by reaction with an aqueous acid such as dilute hydrochloric acid.
A compound of formula XVII above, is prepared from a compound of formula XVIII wherein R"\ R\ Q', . Q1 and Z1 are as hereinbefore defined, by
(XVIII) (XVII)
reaction with compounds of the formula R3OH above, preferably in the presence of diisopropyl azodicarboxylate and triphenylphosphine.
Alternatively. a compound of formula XVIIa. wherein Z1, R'", R' and R: are as hereinbefore defined, and Q3 and Q4 are CH, and Q' is nitrogen, are prepared by reacting a compound of formula XIX. wherein X, Z1, R"'" and R** are
as hereinbefore defined, with a compound of formula XX, wherein R ' is as
ROM (XX)
hereinbefore defined and M is an alkali metal atom, e.g.. sodium, preferably in the presence of a base, for example alkali metal or alkali metal hydride, such as sodium or sodium hydride, without co- solvent or in the presence of an inert solvent, such as tetrahydrofuran, at about room temperature to about reflux, preferably at about room temperature.
A compound of formula XIX above, is prepared by the reaction of a compound of formula XXI, wherein X, Z1 and R are as hereinbefore defined.
witii a compound of formula XXII, wherein R'" is as hereinbefore defined.
R'"OH XXII
preferably using the compound of formula XXII as the solvent, at about room temperature to about reflux.
A compound of formula XXI above, is prepared from a compound of formula XXIII, wherein X, R
2 and Z' are as hereinbefore defined, by adaptation
XXIII
of procedures described by K.R. Reistad et al., Acta. Chemica. Scandanavica B, 28, 667-72 (1974), incoφorated herein by reference.
A compound of formula XXIII above, is prepared by the reaction of a compound of formula XXIV, wherein X and Z' are as hereinbefore defined, with
a compound of formula XXV. wherein R: is as hereinbefore defined, by
(R:)2S04 XXV
adaptation of procedures described by K.R. Reistad el al. Acta. Chemica. Scandanavica B. 28. 667- 72 (1974). incorporated herein by reference.
AlternaUvely. a compound of formula XlVa, wherein k* and R ' are as hereinbefore defined, Z' and Z' arc oxygen, Q' and Q' are CH. and Q4 is nitrogen, is prepared by the deprotecϋon and then oxidation of a compound of formula XXVI, wherein R and R ' are as hereinbefore defined, and P is a
protecting group, such as a silyl group, for example r-butyldimethylsilyl. or a trityl group, by deprotecting with an excess or a catalytic quantity of aqueous acid, for example formic acid, trifluoroacetic acid or acetic acid, neat or in the presence of a co-solvent, for example ethyl acetate, at about room temperature to about 100°C. and oxidizing the resultant alcohol with an oxidant such as KMn04.
A compound of formula XXVI above is prepared by the reaction of a compound of formula XXVII, wherein Y is P1 or R2. P' is a protecting group, such as
a benzyl group, and R: and P are as hereinbefore defined, (1) when Y in a compound of formula XXVII is R\ with an alcohol compound of formula RJOH, wherein R* is as hereinbefore defined, in the presence of a dialkyl diazodiarboxylate, for example diisopropyl di azodicarboxylate, and a phosphine. preferably a iriarylphosphine. such as triphenyl-phosphine. in an inert solvent, for example toluene or an ether, such as tetrahydrofuran or diethyl ether, at a temperature from about - 20 C to reflux, or (2) when Y in a compound of formula XXVII is P'. as hereinbefore defined, as treated in die prior procedure ( 1 ) to yield a compound of formula XXVIII. wherein Y is P!. and
P. P' and R5 are as hereinbefore defined, that is then selectively deprotected to remove P1 as a benzyl group, for example by hydrocenolysis in the presence of a supported metal catalyst, such as 5% palladium on charcoal, in an men solvent, for example ethyl acetaie. or preferably eϋianol to yield a compound of formula XXIX. wherein P and R' are as hereinbetorc
defined, that is then alkylated with compounds of formula XXX, wherein k" and
R2X XXX
X are as hereinbefore defined, in the presence of a base, such an amine. such as 1.8- diazabicyclo[5.4.0]undec-7-ene, or preferably an alkali metal carbonate, such as potassium carbonate, in an inert solvent, for example dimethylformamide, at about 0°C to about 120°C.
A compound of formula XXVII above, is prepared by the reaction of a compound of formula XXXI, wherein Y is as hereinbefore defined, with a
compound of formula XXXII, wherein P and X are as hereinbefore defined,
PX XXXII
preferably in the presence of base, for example an amine. preferably a tertiary amine, for example triethylamine or preferably 4-dimethylanιinopyridine. in an inert solvent, for example dimethyl formamide or tetrahydrofuran. at about room temperature to about 120 C. preferably from about 60°C to about 100°C.
Compounds of formula XXXI above is prepared from a compound of formula XXXIII, wherein Y is as hereinbefore defined, by adaptation of the procedures
described by H.C. Beyerman. Receueil. 77, 249-57, (1958) and European Patent 204207 (20/05/86), incorporated herein by reference.
Compounds of formula XXXIII is prepared by the reaction of compounds of formula XXXIV
with compounds of formula XVIII above by adaptation of the alkylation procedure described by H.C. Beyerman. Receueil. 77. 249-57, (1958) and European Patent 204207 (20/05/86), incoφorated herein by reference.
A compound of formula XVII above, wherein Z' and Z2 are oxygen, Q3 and Q ' are CH, and Q4 is nitrogen, and R'", R: and R3 are as hereinbefore defined, is prepared by die reaction of a compound of formula XXXV. wherein
R: and R'" are as hereinbefore defined, with an alcohol compound of formula R3OH, as hereinbefore defined, in the presence of a dialkyl diazodiarboxylate, for example diisopropyl diazodicarboxylate, and a phosphine, preferably a triarylphosphine. such as triphenylphosphine. in an inert solvent, for example toluene or an ether, such as tetrahydrofuran or diediyl ether, at about 20°C to reflux.
A compound of formula XXXV above is prepared by the reaction of a compound of formula XXXVI. wherein R1 is as hereinbefore defined, with a
compound of formula XXXVII. wherein R"* is as hereinbefore defined, in the
R"'OH (XXXVII)
presence of an acid, preferably a mineral acid, for example sulfuric acid, or preferably hydrogen chloride, at about 0°C to reflux, preferably at an elevated temperature.
A compound of formula XXXVI above is prepared from a compound of formula XXXI above, wherein Y is R\ and R~ is as hereinbefore defined, by adaptation of the procedure described by H.C. Beyerman. Receueil, 77, 249-57, (1958), incoφorated herein by reference.
A compound of formula XlVa above, wherein Z1 is oxygen, Q? and QJ are nitrogen, and Q5 is
CH, and R'" is as hereinbefore defined, is prepared from a compound of formula XXXVIII. wherein R"\ R
:, and R
5 are as hereinbefore
XXXVIII
defined, by reaction with aqueous alkali metal hydroxide or carbonate, such as potassium hydroxide or preferably potassium carbonate, in an inert co-solvent, such as medianol. at about room temperature to about reflux, followed by the oxidation of die resultant alcohol to the corresponding acid.
A compound of formula XXXVIII above, is prepared from a compound of formula XXXIX, wherein Z1 is oxygen, and, R: and R3 are as hereinbefore
defined (prepared by adaptation of d e method of M. Ogata and H. ano. J. Heterocyclic Chem., 1 1, 29-35. (1963), incorporated herein by reference), by reaction with a compound of the formula XXXX. wherein R"* is as hereinbefore
(R'"CO)2θ (XXXX)
defined, preferably a methyl group, using compound XXXX as solvent, al about room temperature to about reflux, preferably al elevated temperature.
The starting matcnals and intermediates are prepared by the application or adaptation of known methods, for example methods as described in the Reference Examples or their obvious chemical equivalents.
The present invention is further exemplified but not limited by the following examples which illustrate the preparation of the compounds according to the invention.
In the nuclear magnetic resonance spectra (NMR) the chemical shifts are expressed in ppm relative to tetramethylsilane. Abbreviations have the following significance: s=singlet; d=doublet; t=triplet; m=multiplet; dd=doublet of doublets; ddd=doublet of doublets of doublets; dt=doublet of triplets. b=broad, bs=broad singlet, q=quartet, AB=AB pattern.
Example 1 6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-yl- methylindan- 1 , 3-dione
A suspension of 6-benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)indan-l ,3-dione (210 mg, 0.48 mmole). potassium carbonate (552 mg, 4 mmole), sodium iodide (100 mg) and 4-picoyl chloride hydrochloride salt (90 mg, 0.54 mmole) in 20 mL of acetone is refluxed for one hour and then diluted with ether (50 mL) at room temperature. The residue after filtration and concentration is purified by chromatography on silica gel (50% ether hexane) to give 202 mg (85%) of the desired product. A yellow solid, m.p. 65-68 °C; MS m/z: 533 (M*); Anal. calc. for Cpp,H31N05: C 76.53, H 5.86, N 3.2.62; Found C 76.04, H 5.88. N 2.46
Following the same procedure, but using the appropriate indandiones and alkyl halides the following are prepared:
t-Butyl 4-[2-(3-Cyclopentyloxy-4-methoxyphenyl)- l ,3-dioxo-indan-2-yI]butyrate;
2-(3-Cyclopentyioxy-4-methoxyphenyl)-2-pyrid-4-ylmethylindan-l ,3-dione as a yellow solid, m.p. 154-155 °C; Yield 73%; MS m/z: 427 (NT); Anal. calc. for C:7H:5NO. C 75.86, H 5.89. N 3.28; Found: C75.46. H 5.85. N3.35;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-2-(3,5-dichloropyrid-4-ylmethyl)indan- l,3-dione as a yellow solid, m.p. 124-126 °C; Yield 987c; MS m/z 496 (M*+ l ); Anal. calc. for C27H23C NO,: C 65.33, H 4.67, N 2.82; Found: C 65.28. H 4.59, N 2.61 ;
4-[2-(3-cyclopentyloxy-4-methoxyphenyl)- l ,3-dioxoindan-2-ylmeϋ.yl]phenyl Acetate as a yellow solid, m.p. 130-132 °C; Yield 747^; MS m/z: 484 (M+); Anal. calc. tor C30H28Cv C 74.36, H 5.82; Found C 74.01. H 5.95;
2-Benzyl-(3-cyclopentyloxy-4-methoxyphenyl)indan-l,3-dione as a yellow solid, m.p. 116- 1 19 °C; Yield 53%; MS m/z: 426 (M*); Anal. calc. for C28H,60< * C 78.85. H 6.14; Found C 78.51, H 6.28;
2-(3-Cyclopentyloxy-4-methoxyphenyl)-2-pyrid-3-ylmethylindan-l,3-dione as a yellow solid, m.p. 45-48 °C; Yield 82%; MS m/z: 427 (M*); Anal. calc. for C27H,5NO,: C 75.86. H 5.89, N 3.28; Found C 75.17, H 5.69, N 3.29;
6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-l,3-dioxoindan-2-yl-acetonitrile as a yellow solid, m.p. 55-59 °C; MS m/z: 481 (NT); Anal. calc. for C30H27NO5: C 74.83, H5.65, N 2.91; Found C 74.2, H 5.5. N 2.8;
6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyI)-2-(3,5-dimethylisoazol-4-ylmethyl)indan-l,3- dione as a tan solid. MS m/z: 551 (M*); Anal. calc. for C34H33N06: C 74.03, H 6.03. N 2.54; Found C 74.08. H 6.03, N 2.39; and
6-Benzyl-6-(3-cyclopentyloxy-4-methoxyphenyl)-[2]-pyrindine-5.7-dione as a yellow solid. Yield 6%>; MS m/z: 427 (NT); Anal. calc. for C27H25N04: C 75.86, H 5.89, N 3.28; Found C 75.14, H 5.80, N 2.97;
6-(3-Cyclopentyloxy-4-methoxyphenyl)-6-pyrid-4-ylmethyl-[2]-pyrindine-5.7-dione as a yellow solid, m.p. 150-153 °C; Yield 257c; MS m/z: 428 (M*);
6-Benzyloxy-2-(7-methoxy-2-methoxymethyl-3H-benzimidazol-4-yl)-2-pyrid-4-yl-methylindan- 1.3-dione;
6-Benzyloxy-2-(7-methoxy-2-phenyl-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethyl- indan-l,3-dione;
6-Benzyloxy-2-(7-methoxy-2-phenethyl-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethyl-indan-l,3- dione;
6-Benzyloxy-2-(2-benzyl-7-methoxy-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethyl- indan-l,3-dione;
6-Benzyloxy-2-((RS)-7-methoxy-2-(l-phenylethyl)-3H-benzimidazol-4-yl)-2-pyrid-4- ylmethylindan-l,3-dione;
6-Benzyloxy-2-(7-methoxy-2-(4-methoxybenzyl)-3H-benzimidazol-4-yl) -2-pyrid-4-ylmethyl indan-l,3-dione;
6-Benzyloxy-2-((RS)-2-(cyclohexyl-phenyl-methyl)-7-methoxy-3H-benzimidazol-4-yl) -2-pyrid-4- ylmethyl indan-l,3-dione;
6-Benzyloxy-2-((RS)-2-(l,2-diphenylethyl)-7-methoxy-3H-benzimidazol-4-yl) -2-pyrid-4-ylmethyl indan-l,3-dione;
6-Benzyloxy-2-((RS)-7-methoxy-2-(2-phenylpropyl)-3H-benzimidazol-4-yl) -2-pyrid-4-ylmethyl indan- 1.3 -dione;
6-Benzyloxy-2-(7-methoxy-2-(4-methoxyphenoxymethyl)-3H-benzimidazol-4-yl) -2-pyrid-4- ylmethyl indan-l,3-dione;
6-Benzyloxy-2-((RS)-7-methoxy-2-(l-phenylbutyl)-3H-benzimidazol-4-yl) -2-pyrid-4-ylmethyl indan-1.3-dione;
6-Benzyloxy-2-(2-(4-bromobenzyl)-7-methoxy-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan- 1,3-dione;
6-Benzyloxy-2-((RS)-7-methoxy-2-(3-methoxy- l-phenylpropyl)-3H-benzimidazol-4-yl)-2-pyrid- 4-ylmethylindan-l ,3-dione;
6-Benzyloxy-2-(2-(4-cyanobenzyl)-7-methoxy-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan- 1,3-dione;
6-Benzyloxy-2-(7-methoxy-2-(4- { 3-pyridyl }benzyl)-3H-bcnzimidazol-4-yl)-2-pyrid-4- ylmethylindan- 1 ,3-dione;
6-Benzyloxy-2-(7-methoxy-2-(2-methoxybenzyl)-3H-benzimidazol-4-yl)-2-pyrid-4- ylmethylindan-1.3-dione;
6-Benzyloxy-2-((RS)-7-methoxy-2-(methoxyphenylmethyl)-3H-benzimidazol-4- yl)-2-pyrid-4- ylmelhylindan- 1 ,3-dione;
6-Benzyloxy-2-(7-methoxy-2-(2-methoxyphenoxy)methyl-3H-benzimidazol-4-yl)-2-pyrid-4- ylmethylindan- 1 ,3-dione;
6-Benzyloxy-2-(7-methoxy-2-(3-pyridyl)-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan- 1.3- dione;
6-Benzyloxy-2-(2-isopropyI-7-methoxy-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan-l,3- dione;
6-Benzyloxy-2-(7-methoxy-2-methyl-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan-l,3-dione;
6-Benzyloxy-2-(7-methoxy-2-phenoxymethyl-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan- 1,3-dione;
6-Benzyloxy-2-(2-cyclopentyl-7-methoxy-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan-l,3- dione;
6-Benzyloxy-2-(2-benzyl-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethyIindan- l,3-dione;
6-Benzyloxy-2-(2-cyclopentyl-7-methoxy-l-methyl-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan- 1,3-dione;
6-Benzyloxy-2-(2-cyclopentyl-7-methoxy-3-methyl-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan- 1,3-dione;
6-Benzyloxy-2-(2.7-dimethoxy-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan- 1.3-dione;
6-Benzyloxy-2-(2-cyclopropyl-7-methoxy-3H-benzimidazol-4-yl)-2-pyrid-4-ylmethylindan- l ,3- dione;
6-Benzyloxy-2-(l-cyclohexylmethyl-3-methyl- lH-indol-6-yl)-2-pyrid-4-ylmethylindan- l,3-dione;
6-Benzyloxy-2-(l-(2-cyclohexyl)ethyl-3-methyl- l H-indol-6-yl)-2-pyrid-4-ylmethylindan-1.3- dione;
6-Benzyloxy-2-(l-(3-cyclohexyl)propyl-3-methyl-l H-indυl-6-yl)-2-pyrid-4-ylmethylindan-l,3- dione;
6-Benzyloxy-2-(l-heptyl-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan-1.3-dione;
6-Benzyloxy-2-(l-isobutyl-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan-1.3-dione:
6-Benzyloxy-2-(l-cyclopentylmethyl-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan- 1.3-dione;
6-Benzyloxy-2-(l-benzyl-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan-1.3-dione;
6-Benzyloxy-2-(l-(3-phenyl)propyl-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan- l,3-dione;
6-Benzyloxy-2-(l-(3-phenyl)butyl-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethyIindan- l,3-dione;
6-Benzyloxy-2-(l-(4-fluorobenzyl)-3-methyl- lH-indol-6-yl)-2-pyrid-4-ylmethylindan- 1.3-dione;
6-Benzyloxy-2-(l-(4-chlorobenzyl)-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan-l,3-dione;
6-Benzyloxy-2-(l-(4-methoxybenzyl)-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan- l,3- dione;
6-Benzyloxy-2-(l-(4-trifluoromethylbenzyl)-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan- 1,3-dione;
6-Benzyloxy-2-(l-(4-methylsulphonylbenzyI)-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan- 1,3-dione;
6-Benzyloxy-2-(l-(1.3-benzodioxol-5-yl)methyl-3-methyl- lH-indol-6-yl)-2-pyrid-4- ylmethylindan- 1 ,3-dione;
6-Benzyloxy-2-( l-(naphthalen-2-yl)methyl-3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmethylindan-l,3- dione;
6-Benzyloxy-2-(l-(tetrahydro-2H-pyran-2-yl)methyl-3-methyl- lH-indol-6-yl)-2-pyrid-4- ylmethylindan- 1 ,3-dione;
6-Benzyloxy-2-(3-methyl-l-(tetrahydrofurfuryl)-lH-indol-6-yl)-2-pyrid-4-ylmethylindan- l,3- dione;
6-Benzyloxy-2-(3-methyl-l-(toluene-4-sulphonyl)-lH-indol-6-yl)-2-pyrid-4-ylmethylindan-1.3- dione;
6-Benzyloxy-2-(3-methyl-l-(tetrahydrofuran-3-yl)-lH-indol-6-yl)-2-pyrid-4-ylmethylindan-l,3- dione;
6-Benzyloxy-2-(3-methyl-lH-indol-6-yl)-2-pyrid-4-ylmedιylindan-l ,3-dione;
6-Benzyloxy-2-(l-benzyl-3-methylindolin-6-yl)-2-pyrid-4-ylmethylindan-1.3-dione; and
6-Benzyloxy-2-(l-benzyl-3-methylindazol-6-yl)-2-pyrid-4-ylmethylindan-1.3-dione.
Example 2 (+) 6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4- yl- methylindan-1.3-dione and (-) 6-Benzyloxy-2-(3- cyclopentyloxy-4- methoxyphenyl)-2-pyrid-4-yl-methylindan-l,3- dione
The titled two enandomers are resolved on chiral HPLC from race ic 6-benzyloxy-2-(3- cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-yl-methylindan-l,3-dione. CHIRACEL OD® (Daicel Chem. Ind. Ltd.) eluted with heptane/ethanol containing 0.1% diethylamine (1 :3, v/v) detected at UV 230 nm at flow rate of 1 mL/minute.
(+) 6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-ylmethylindan-l,3-dione as a yellow solid, m.p. 55-60 °C; MS /z 533 (NT); [a]D:+10 ° (c=0.17, CHC13). Retention time is 9.292 minutes.
(-) 6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-yl-methylindan-1.3-dione as a yellow solid, m.p. 58-61 °C; MS m/z: 533 (M+); [a]D:-8.8 ° (c=0.17, CHC13). Retenϋon time is 6.467 minutes. 6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-( l-oxopyrid-4-yl-methyl)indan- 1.3-dione Example 3 2-(3-Cyclopentyloxy-4-methoxyphenyl)-2-( l -oxopyrid-4- ylmethyl)indan-l,3-dione
To a solution of 2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-ylmethylindan-l,3- dione (400 mg. 0.94 mmole) in 4 mL of dichloromethane at room temperature is added MCPBA (74%, 300 mg, 1.3 mmole). The resulting suspension is stirred at room temperature for two hours before being quenched with a solution of sodium bisulfite. After washing with 1 N sodium hydroxide
(10 mL), die organic solution is concentrated and the residue is purified by chromatography on silica gel using ethyl acetate to yield d e desired product (400 mg; 96%) as a yellow solid, m.p. 215-217 UC; MS m/z: 443 (M+); Anal. calc. for C:7H25N05: C 73.12, H 5.68, N 3.16; Found C 72.95, H 5.59. N 3.05
Following the same procedure, but using 6-benzyloxy-2-(3-cyclopentyloxy-4- methoxyphenyl)-2-pyrid-4-yl-methylindan-l,3-dione as the indandione to be oxidized ϋiere is prepared 6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-( l-oxopyrid-4-yl-methyl)indan- 1,3-dione as a yellow solid, m.p. 72-75 °C; Yield 95%; MS m/z: 550 (M'+l)
Example 4 4-[6-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)indan-2- ylmethyl]pyridine
To a solution of 6-benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4- ylmethylindan-l,3-dione (347 mg, 0.65 mmole) in methanol at room temperature is added sodium borohydride (76 mg, 2 mmole). The bubbling suspension is stirred at room temperature for 1 hour before being concentrated to dryness. The residue is treated with trifluoroacetic acid (4 mL) and triethvlsilane (4 mL). The resulting mixture is stirred vigorously overnight before being diluted with
dichloromethane (100 L) and washed with saturated sodium bicarbonate (50 mL). The organic solution is dried over magnesium sulfate and then filtered. The residue after being concentrated is purified by chromatography on silica gel (ether/hexane). A white solid The desired product (100 mg, 30%) is obtained as a white solid, m.p. 45-48 °C; MS /z: 505 (M*).
Following the same procedure, but using 2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4- ylmethylindan-l,3-dione as the indandione to be reduced there is prepared 4-[2-(3-Cyclopentyloxy- 4-methoxyphenyl)indan-2-ylmethyl]pyridine (90%) is produced as a colorless oil. MS m/z 399 (M+); Anal. calc. for C,7H29N02: C 81.17, H 7.32. N 3.51 ; Found C 81.03. H 7.43, N 3.41
Example 5 2-(3-Cyclopentyloxy-4-methoxyphenyl)-2-pyrid-3-ylmethylindan- 1 ,3- diol
To a solution of 2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-ylmethylindan- 1.3- dione (370 mg. 0.87 mmole) in 10 mL of THF at 0 °C is added a 1 M LAH/THF solution ( 1.7 mL. 1.7 mmole) and die mixture is stirred for 3 hours before being quenched with 1 N hydrochloric acid and ϋ en neutralized to pH 7. The mixture is extracted witii dichloromed ane (2 x 50 mL), and tiien rotary evaporated to remove die dichloromethane and yield a residue which is purified by chromatography on silica gel (60% ethyl acetate/hexane). The titled diol (277 mg; 74%) is obtained as a white solid, m.p. 99-101 °C; MS /z 432 (M*)
Example 6 5-Benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-yl- methylindan- 1-one
A suspension of 4-[6-benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)indan-2- ylmethyl]pyridine ( 100 mg, 0.2 mmole) and 2.2'-bipyridinium chlorochromate (584 mg. 2 mmole) in 20 mL of acetone is refluxed overnight before being diluted wilh ether (100 mL) at room temperature. The solution is filtered, washed with a saturated solution of copper (II) sulfate and then concentrated. The residue is purified by chromatography on silica gel (50 % ether/hexane) to yield the titled compound (25 mg, 25%) as a white solid, m.p. 52-55 °C; MS m/z: 520 (M
Example 7 2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-hydroxy-2-pyrid-4- ylmethylindan- 1 ,3-dione
A suspension of 6-benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-yl- metiιylindan-l,3-dione (200 mg, 0.38 mmole) in 10 mL of ethyl acetate containing 200 mg of Pd/C (10%) is stirred at room temperature with a hydrogen balloon for two hours before the filtration. The solvent is concentrated to yield the titled compound (165 mg; 100%) as a yellow solid, m.p. 106-109 °C; MS m/z: 443 (M*)
Example 8 Methyl [2-(3-Cyclopentyloxy-4-methoxyphenyl)-1.3-dioxo-2-ρyrid- ylmethylindan-5-yl]acetate
A suspension of 2-(3-cyclopentyloxy-4-methoxyphenyl)-5-hydroxy-2-pyrid-4- yl ethylindan- 1.3 -dione (44 mg, 0.1 mmole), methyl bromoacetate (18.5 mg, 0.1 mmole) and potassium carbonate (138 mg, 1 mmole) is refluxed for one hour. The residue, after being filtered and concentrated, is purified by chromatography on silica gel (ether) to give 40 mg (78%) of the desired product as a yellow solid, m.p. 51-54 °C; MS m z: 516 (M*)
Example 9 5-(4-Bromobenzyloxy)-2-(3-cyclopentyloxy-4-methoxyphenyl)-2- pyrid-
4-ylmethylindan-l,3-dione
2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-hydroxy-2-pyrid-4-ylmethylindan-l ,3-dione (150 mg, 0.34 mmole) is dissolved in 10 mL of THF and treated with 4-bromobenzyl alcohol (190 mg, 1 mmole), triphenylphosphine (267 mg, 1 mmole) and DEAD (0.16 mL. 1 mmole) and d e resulting brown solution is sϋrred at room temperature overnight. After being concentrated to dryness. the residue is diluted with 50 mL of ether and the solid is removed by filtradon. The solution is concentrated and the residue purified by chromatography on silica gel (30% ethyl acetate/hexane) to give 42 mg (20%) of the desired product as a yellow solid, m.p. 60-62 °C; MS /z: 611 (M+)
Following the same procedure, but using the appropriate alcohol in place of the 4- bromobenzyl alcohol the following are prepared:
2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-(2-morpholin-4-ylethoxy)-2-pyrid-4- ylmethylindan-l,3-dione (41%) as a yellow solid, m.p. 58-62 °C: MS m z: 557 (M +l);
2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-ethoxy-2-pyrid-4-ylmethylindan-l ,3-dione (33%) as a yellow solid, m.p. 60-65 ϋC. MS m/z: All (M*+l);
2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-pyrid-4-ylmethoxy-2-pyrid-4-ylmethylindan- 1,3-dione (39%) as a yellow solid, m.p. 67-70 °C; MS m/z: 534 (M*)", and
5-Cyclopentyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrid-4-ylmethylindan-l,3- dione (28%) as a yellow solid, m.p. 65-68 °C; MS m/z: 511 (M*)
Example 10 2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-pyrid-3-yl-2-pyrid-4- ylmethylindan- 1 ,3-dione
A suspension of 2-(3-cyclopentyloxy-4-methoxyphenyl)-5-hydroxy-2-pyrid-4- ylmethylindan-l,3-dione (350 mg, 0.79 mmole), N-phenyltrifluoro-memanesulfonimide (339 mg, 0.95 mmole) and potassium carbonate (138 mg, 1 mmole) in 10 mL of THF is refluxed for two
hours. After filtration and concentration, the residue is purified by chromatography on silica gel (ether) to give 420 mg (92%) of the corresponding triflate.
A mixture of the triflate (140 mg. 0.24 mmole), Pd(PPh3)4 (56 mg. 0.048 mmole). diethyl(3- pyridino)borane (88 mg, 0.6 mmole) and potassium carbonate (138 mg, 1 mmole) in 10 mL of toluene is refluxed for six hours before being filtered. The residue after concentration is purified by chromatography on silica gel (ethyl acetate) to give 40 mg (33%) of the desired product as a brown solid, m.p. 55-58 °C; MS m/z: 504 (M+) Example 11 2-(3-Cyclopentyloxy-4-methoxyphenyl)-1.3-dioxo-2-pyrid-4- ylmethylindan-5-yl Morpholine-4-carboxylate
A mixture of 2-(3-cyclopentyloxy-4-methoxyphenyl)-5-hydroxy-2-pyrid-4-ylmethylindan- 1,3-dione (48 mg, 0.14 mmole). chlorocarbonylmorpholine (25 mg. 0.17 mmole) and pyridine (0.1 mL) in 5 mL of THF is stirred overnight at room temperature before being diluted with ether (20 mL) and filtered. The residue after concentration is purified by chromatography on silica gel (eϋier) to give 55 mg (70%) of the desired product as a yellow solid, m.p. 55-59 °C; MS m/z: 556 (M*)
Following the same procedure, but using [l,4']-Bipiperidinyl-r-carbonylchloride in place of the chlorocarbonylmorpholine the following is prepared:
2-(3-Cyclopentyloxy-4-methoxyphenyl)-l,3-dioxo-2-pyrid-4-ylmethylindan-5-yl [1,4']- Bipiperidinyl-1 '-carboxylate (32%) as a yellow solid, m.p. 75-78 UC; MS m/z: 638 (M*+ l)
Example 12 4-f2-(3-Cyclopentyloxy-4-methoxyphenyl)- 1.3-dioxoindan-2-yl]- butyπc acid
To a solution ol l-butyl 4-[2-(3-cyclopentyloxy-4-metlιoxyphenyl)-1.3-dioxo-indan-2- yljbutyrate (300 mg. 0.63 mmole) in 10 mL of dichloromethane at room temperature is added trifluoroacetic acid (5 mL). The mixture is vigorously stirred at room temperature for four hours before being washed with water (2 x mL). The residue after removal of dicholoromethane is purified by chromatography on silica gel (ethyl acetate) to give 212 mg (80%) of the desired acid as a yellow oil. MS m/z: All (M+) Example 13 2-(3-Cyclopentyloxy-4-methoxyphenyl)-2-(4- hydroxybenzyl)indan- 1 ,3-dione
To a solution of 4-[2-(3-cyclopentyloxy-4-methoxyphenyl)-l,3-dioxoindan-2- ylmethyljphenyl acetate (280 mg, 0.58 mmole) in 10 mL of methanol at 0 °C is added a 25% sodium methoxide/medianol solution (0.15 L, 0.67 mmole). The resulting reddish solution is stirred at room temperature for two hours before being concentrated to dryness and tiien acidified with 1 N
hydrochloric acid. The acidified mixture is extracted with dichloromethane (2 x 50 mL) and then concentrated by rotary evaporation. The residue is purified by chromatography on silica gel (50% ethyl acetate/hexane) to give 192 mg (15%) of the desired product as a yellow solid, m.p. 172-173
°C; MS m/z: 443 (M*+l)
Example 14 2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-methyl-2-pyrid-4- ylmethylindan- 1 ,3-dione
2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-methylindan- l,3-dione (2.2 g. 6.29 mmole) is taken-up in acetone. Potassium carbonate (3.53 g, 25.2 mmole). sodium iodide (940 mg, 6.29 mmole) and 4-picolyl chloride (1.03 g, 6.29 mmole) are added to the reaction flask. The mixture is heated to reflux for two hours before the reaction mixture is filtered through a fritted funnel containing Celite. The mother liquor is concentrated under vacuum. The crude material is purified via flash column to give the desired product (2.16 g. 78 %) as a yellow solid. MS m/z: 441 (M'); Anal. calc. for C;βH37NO-: C 76.17. H 6.16, N 3.17; Found C 75.88, H 6.31. N 3.08
Example 15 2-(3-cyclopentyloxy-4-methoxyphenyl)-5-hydroxymethyl-2-pyrid- 4- ylmethylindan- 1 ,3-dione
2-(3-cyclopentyloxy-4-methoxyphenyl)-5-hydroxymethylindan-1.3-dione (1.17 g, 2.77 mmole) is taken-up in acetone. Potassium carbonate (1.53 g, 1 1.1 mmole), sodium iodide (415.5 mg. 2.77 mmole) and 4-picolyl chloride (454 mg, 2.77 mmole) are added to the reaction flask. The mixture is heated to reflux for two hours. The reaction mixture is filtered dirough a fritted funnel containing Celite and the mother liquor is concentrated by rotary evaporation. The crude material is purified via flash column to give 2-(3-cyclopentyloxy-4-methoxyphenyl)-5-hydroxymethyl-2- pyrid-4-ylmethylindan- l ,3-dione ( 1.09 g, 86 %). MS m/z: 457.
Example 16 2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-(4-methylpiperazinyl-l- methyl)-2-pyrid-4-ylmethylindan- 1 ,3-dione
2-(3-Cyclopentyloxy-4-methoxyphenyl)-5-hydroxymethyl-2-pyrid-4-ylmethylindan-l,3- dione (280 mg. 0.613 mmole) is dissolved in THF and treated with sodium hydride (36.8 mg. 0.92 mmole, 60%). followed by die addition of a-toluene sulfonyl chloride at 0°C. The reaction is allowed to warm up to room temperature and stirred for 2 hours. Another portion of α-toluene chloride (116.8 mg, 0.613 mmole) is added and the mixture is stirred at room temperature overnight. The solvent is removed and the residue dissolved in dichloromethane, washed with water and then dried over MgS04. The solution after filtration is concentrated to dryness and die resulting residue is dissolved in 10 mL of acetone before the additions of potassium carbonate (364 mg, 2.64 mmole) and 1-methylpiperazine (73 mL, 0.66 mmole). The reaction is stirred for four days at room
temperature. The residue after concentration is purified by chromatography on silica gel to yield 123 mg (34%) of die titled compound as a tan solid, m.p. 55-59 °C; MS m/z 540 (M*+l )
Example 17 3-(3-Cyclopentyloxy-4-methoxyphenyl)-2-oxo-3- pyridin-4-ylmethylpyrrolidine-l -carboxylic acid tert- butyl ester
NaHC03 (50 L) is added to a suspension of the hydrochloride salt of 4-picolyl chloride (65 mg, 0.39 mmol) in ether (50 mL). The organic layer is then separated and washed witii H,0 (50 mL). The organic layer is dried (Na2S04) and concentrated to approximately 1 mL. DMF (5 mL), 3-(3-cyclopentyloxy-4-methoxyphenyl)-2-oxopyrrolidine-l -carboxylic acid tert-butyl ester (50 mg, 0.13 mmol) and NaH (10 mg, 0.39 mmol) are added and stirred under nitrogen at room temperature for 20 hours. The reaction is quenched with sat NH.C1 (30 mL) and extracted with EtOAc (3 x 15 L). The combined organic layers are dried (Na;S04) and evaporated in vacuo. The crude product is purified by flash column chromatography (eluant EtOAc:hexane. 1 :4) to yield the desired product 3-(3-Cyclopentyloxy-4-methoxyphenyl)-2-oxo-3-pyrid-4-ylmethyl-pyrrolidine- l-carboxylic acid tert-butyl ester (30 mg) as a white solid, mp 148- 149°C. For C:7H3405N2: Calculated C 69.51 H 7.34 N 6.0; Found C 69.37 H 7.33 N 5.86 M.spec (FAB). Exact mass measurement- Theoretical (M+H*) 467.2546, measured 467.2560. Η(CDC13) 1.5(9H, s, 3x CH,), 1.55-1.60, 1.75-1.95(8H, 2xm. cyclopentyl), 2.0-2.1, 2.3-2.4, 3.35-3.45, 3.6-3.7(4H, 3xm, CILCH,), 3.05-3.25(2H. s. CljUpy), 3.8(3H, s, OCH3). 4.7-4.75(1 H, . CH). 6.75-6.80. 7.0(5H. . s. H3, 2 from C5H4N), 8.35-8.40(2H, br m, 2 from C5H4N).
Proceeding in a similar manner, but starting with the appropriate starting material there are prepared:
3-(7-methoxy-2-methoxymethy]-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l - carboxylic acid tert-butyl ester;
3-(7-methoxy-2-phenyl-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l-carboxylic acid tert-butyl ester;
3-(7-methoxy-2-phenethyI-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l- carboxylic acid tert-butyl ester;
3-(2-benzyl-7-methoxy-3H-benzimidazol-4-yl) pyrrolidine-2-one- 1 -carboxylic acid tert-butyl ester;
3-((RS)-7-methoxy-2-(l -phenylethyl)-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2- one-1 -carboxylic acid tert-butyl ester;
3-(7-methoxy-2-(4-methoxybenzyl)-3H-benzimidazol-4-yl) pyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-((RS)-2-(cyclohexyl-phenyl-methyl)-7-methoxy-3H-benzimidazol-4-yl)-3-pyrid-4- ylmethylpyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-((RS)-2-(l,2-diphenylethyl)-7-methoxy-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2- one-1 -carboxylic acid tert-butyl ester,
3-((RS)-7-methoxy-2-(2-phenylpropyl)-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2- one-1 -carboxylic acid tert-butyl ester;
3-(7-methoxy-2-(4-methoxyphenoxymethyl)-3H-benzimidazol-4-yl)-3-pyrid-4- ylmethylpyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-((RS)-7-methoxy-2-(l -phenylbutyl)-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2- one-1 -carboxylic acid tert-butyl ester;
3-(2-(4-bromobenzyl)-7-methoxy-3H-benzimidazol-4-yl)-pyrrolidine-2-one-l -carboxylic acid tert- butyl ester;
3-((RS)-7-methoxy-2-(3-methoxy-l-phenylpropyl)-3H-benzimidazol-4-yl)-pyrrolidine-2-one- l- carboxylic acid tert-butyl ester;
3-(2-(4-cyanobenzyl)-7-methoxy-3H-benzimidazol-4-yl)-pyrrolidine-2-one-l-carboxylic acid tert- butyl ester;
3-(7-methoxy-2-(4-{ 3-pyridyl }benzyl)-3H-benzimidazol-4-yl)-pyrrolidine-2-one-l-carboxylic acid tert-butyl ester;
3-(7-methoxy-2-(2-methoxybenzyl)-3H-benzimidazol-4-yl)-pyrrolidine-2-one- l -carboxylic acid tert-butyl ester;
3-((RS)-7-methoxy-2-(methoxyphenylmethyl)-3H-benz-imidazol-4- yl)pyrrolidine-2-one- 1 - carboxylic acid tert-butyl ester;
3-(7-methoxy-2-(2-methoxyphenoxy)methyl-3H-benzimidazol-4-yl)-3-pyrid-4- ylmethylpyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-(7-methoxy-2-(3-pyridyl)-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l- carboxylic acid tert-butyl ester;
3-(2-isopropyl-7-methoxy-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyπ*olidine-2-one-l- carboxylic acid tert-butyl ester;
3-(7-methoxy-2-methyl-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-(7-methoxy-2-phenoxymethyl-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(2-cyclopentyl-7-methoxy-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l - carboxylic acid tert-butyl ester;
3-(2-benzyl-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l -carboxylic acid tert- butyl ester;
3-(2-cyclopentyl-7-methoxy- l -methyl-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(2-cyclopentyl-7-methoxy-3-methyl-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l- carboxylic acid tert-butyl ester;
3-(2,7-dimethoxy-3H-benzimidazol-4-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- 1 -carboxylic acid tert-butyl ester;
3-(2-cyclopropyl-7-methoxy-3H-benzimidazol-4-yl)pyrrolidine-2-one- 1 -carboxylic acid tert-butyl ester;
3-(l-cyclohexylmethyl-3-methyl-lH-indol-6-yl)pyrrolidine-2-one-l-carboxylic acid tert-butyl ester;
3-(l-(2-cyclohexyl)ethyl-3-methyI-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(l-(3-cyclohexyl)propyl-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(l-heptyl-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l-carboxylic acid tert- butyl ester;
3-(l-isobutyl-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l-carboxylic acid tert- butyl ester;
3-(l-cyclopentylmethyl-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(l-benzyl-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l-carboxylic acid tert- butyl ester;
3-(l-(3-phenyl)propyl-3-methyl- lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-(l-(3-phenyl)butyl-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l-carboxylic acid tert-butyl ester;
3-(l-(4-fluorobenzyl)-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l -carboxylic acid tert-butyl ester;
3-(l-(4-chlorobenzyl)-3-methyl- lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l -carboxylic acid tert-butyl ester;
3-(l-(4-methoxybenzyl)-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(l-(4-trifluoromethylbenzyl)-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l- carboxylic acid tert-butyl ester;
3-(l-(4-methylsulphonylbenzyl)-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(l-(l,3-benzodioxol-5-yl)methyl-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- 1-carboxylic acid tert-butyl ester;
3-(l-(naphthalen-2-yl)methyl-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(l-(tetrahydro-2H-pyran-2-yl)methyl-3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2- one- 1 -carboxylic acid tert-butyl ester;
3-(3-methyl-l-(tetrahydrofurfuryl)-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(3-methyl- 1 -(toluene -4-sulphony 1)- 1 H-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- 1 - carboxylic acid tert-butyl ester;
3-(3-methyl-l-(tetrahydrofuran-3-yl)- lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one- l- carboxylic acid tert-butyl ester;
3-(3-methyl-lH-indol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-(l-benzyl-3-methylindolin-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l -carboxylic acid tert- butyl ester; and
3-(l-benzyl-3-methylindazol-6-yl)-3-pyrid-4-ylmethylpyrrolidine-2-one-l-carboxylic acid tert- butyl ester.
Example 18 3-(3-Cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4- ylmethyl-pyrrolidin-2-one
Trifluoroacetic acid (0.3 mL) is added to a stirring solution of 3-(3-Cyclopentyloxy-4- methoxyphenyl)-2-oxo-3-pyrid-4-ylmethyl-pyrrolidine-l-carboxylic acid tert-butyl ester (334 mg) in dichloromethane (20 mL). The reaction is stirred at room temperature for 2 hours, then more TFA (0.2 mL) is added. The reaction is stirred for a further 2 hours, then quenched with sat NaHC03. The organic layer is dried (NajSO,,) and evaporated in vacuo. The crude material is preabsorbed onto flash silica and purified by flash column chromatography (gradient elution, EtOAc to EtOAc:MeOH, 20:1) to yield the desired product 3-(3-Cyclopentyloxy-4-methoxyphenyl)-3- pyrid-4-ylmethyl-pyrrolidin-2-one (216 mg) as a white foam. Η(CDC13) 1.55-1.6, 1.75-1.95(8H,
2xm. cyclopentyl), 2.2-2.3, 2.4-2.5. 3.1-3.2(4H, 3xm, CHjCH,). 3.15(2H. s, CH^py). 3.8(3H, s. OCHj), 4.7-4.75(lH. m, CH), 6.0(1H, br s, NH). 6.75-6.80, 6.85-6.90, 6.90-6.95. 7.1-7.15(5H, 4xm, C^, 2 from C5H4N).8.4-8.45(2H. br , 2 from C5H„N)
Example 19 l-Benzyl-3-(3-cyclopentyloxy-4-methoxyphenyl)-3- pyridin-4-ylmethyl-pyrrolidin-2-one
Sodium Hydride (6 mg) and benzyl bromide(18 μl, 0.15 mmol) are added to a stirring solution of 3-(3-Cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4-ylmethyl-pyrrolidin-2-one at -30°C under nitrogen in DMF (3 mL) and stirred for 30 minutes. The temperature is increased to -5°C and stirred for a further 30 minutes. The temperature is increased to 0°C and stirred for a further 30 minutes. The reaction is quenched witii H20, then partitioned between EtOAc (25 mL) and H20 (50 mL). The organic layer is dried (Na,S04) and evaporated in vacuo. The crude material is pre- absorbed onto flash silica and the product purified by flash column chromatography (eluant : EtOAc) to yield the desired product l-Benzyl-3-(3-cyclopentyloxy-4-methoxyphenyI)-3-pyrid-4- ylmethyl-pyrrolidin-2-one (36 mg) as a white foam. M.spec (EI) 456(M+), 388, 364. 296, 149, 91. Exact mass measurement (FAB)- Theoretical (M+H*) 457.2491, measured 457.2460. Η(CDC13) 1.55- 1.65, 1.8-2.0(8H. 2xm. cyclopentyl). 2.1-2.2, 2.3-2.4, 2.9-3.0, 3.0-3.1(4H. . CH-CIL). 3.1- 3.3(2H, m, CI py), 3.8(3H, s, OCH3). 4.4-4.5(2H. m, CH:CβH4), 4.7-4.8(lH. m, CH). 6.75-6.8. 6.8- 6.85, 6.9-7.0, 7.0-7.1 , 7.1-7.15, 7.2-7.3(10H, 6xm. C6H„ C&. 2 from C,H4N), 8.4(2H, br s. 2 from C5H4N).
Proceeding in a similar manner but starting with the appropriate starting materials there are prepared:
3-(3-Cyclopentyloxy-4-methoxyphenyl)-2-oxo-3-pyrid-4-ylmethyl-pyrrolidine- l -carboxylic acid tert-butyl ester.
3-(3-Cyclopentyloxy-4-methoxyphenyl)-l-(3-methylbutyl)-3-pyrid-4-ylmethyl-pyrrolidin-2-one, an oil. M.spec (EI) 436(M*), 368, 344. Exact mass measurement(FAB)- Theoretical (M+H*) 437.2804. measured 437.2764. Η(CDC13) 1.25-1.35(2H, m, CH H), 1.35-1.45(1H. m, CHCH3), 1.55-1.65. 1.75-1.95(8H. 2x br m. cyclopentyl). 1.85-1.90(6H, m, CH, x2), 2.1-2.2. 2.3-2.4. 2.95- 3.05, 3.1-3.25, 3.3-3.4(6H. 5xm, CH^O NCH^, 3.1-3.2(2H. , CILpy), 3.8(3H, s, OCH>), 4.7-
4.8(1H, m. CH). 6.75-6.8, 6.85-6.9, 6.95-7.0. 7.1-7.15(5H, 4xm.
2 from C
5H
4N), 8.35-8.5(2H, br m, 2 from C
5H
4N)
l-Cyclohexylmethyl-3-(3-cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4-ylmethyl-pyrrolidin-2-one, as an oil. M.spec (FAB) 463(MH+), 395. 370. Exact mass measurement(FAB)- Theoretical (M+H*) 463.2961, measured 463.2948. *H(CDC13) 0.8-0.95, 1.05-1.20. 1.2-1.25, 1.4-1.7. 1.75-2.0(18H. 5xm, cyclohexyl, cyclopentyl), 2.1-2.2. 2.3-2.4, 2.9-3.0, 3.1-3.2(8H, 6xm. Cϊ CHjNC ,, CH^py),
3.8(3H, s, OCH3). 4.7-4.75(1H, m, CH), 6.7-6.75, 6.8-6.85, 6.9-6.95. 7.1-7.15(5H. 4xm, C6H,. 2 from C5H4N). 8.35-8.45(2H, br m, 2 from C5H„N)
3-(3-Cyclopentyloxy-4-met oxyp enyl)-l,3-bis-pyridin-4-ylmethyl-pyrrolidin-2-one. as an oil. M.spec (EI) 457(M+), 389, 365, 297. Exact mass measurement(FAB)- Theoredcal (M+H+) 458.2444, measured 458.2422. *H(CDC13), 1.55-1.65, 1.8-2.0(8H, m, cyclopentyl), 2.15-2.25, 2.4-2.5, 2.9-3.0, 3.1-3.2 (4H, 4xm. CHjCH-), 3.15-3.25 (2H, m, CHj-y), 3.8(3H. s, OCHj), 4.3-4.5(2H, m, CH^y), 4.7- 4.8(1H, m, CH). 6.75-6.8. 6.8-6.85. 6.9-6.95, 7.05-7.1 (7H, 4xm, H-, 4 from 2x C5H4N), 8.3-8.5 (4H. br m, 4 from 2x C5H4N).
l-Acetyl-3-(3-cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4-ylmethyl-pyrrolidin-2-one, clear oil. M.spec (EI) 408(M*), 340, 316, 248. Η(CDC13) 1.55-1.65, 1.75-1.95(8H, 2xm. cyclopentyl). 2.05- 2.15. 2.40-2.45. 3.45-3.55, 3.7-3.85(4H, m. CH,CH,). 3.05-3.30(2H. m. CH,py). 3.8(3H, s. OCH3), 4.65-4.70(lH. m. CH), 6.75, 7.0(5H, 2xs, H3. 2 from C5H4N), 8.4(2H. br s. 2 from C3H.N).
1 -Butyl- 3-(3-cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4-ylmethyl-pyrrolidin-2-one, oil. M.spec (EI) 422(M*), 354, 330, 262. Exact mass measurement (FAB)- Theoredcal (M+H+) 423.2648, measured 423.2633. *H(CDC13) 0.8-0.9(3H, m. CH3CH,), 1.15-1.25. 1.35-1.45(2H, m. CH^CHj), 1.55-1.70, 1.8-2.0(8H. 2xm, cyclopentyl), 2.05-2.2(2H, m. NCH,). 2.3-2.4, 3.0-3.05. 3.1-3.2, 3.3- 3.4(4H, 4xm. CWCl ring), 3.1-3.25(2H, m. CHjpy). 3.8(3H, s. OCH3), 4.7-4.8(lH, m. CH), 6.75- 6.80, 6.8-6.85. 6.85-6.9, 7.05-7.10(5H. 4xm, C6R,, 1 from C5H4N), 8.4-8.45(2H.m, 2 from C5H4N).
3-(3-Cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4-ylmethyl- l-(toluene-4-sulfonyl)-pyrrolidin-2- one. clear foam. M.spec (FAB) 5 1(MH+). Exact mass measurement (FAB)- Theoredcal (M+H+) 521.2110, measured 521.2108. Η(CDC13) 1.55-1.65. 1.7-1.9(8H, 3xm, cyclopentyl), 2.1-2.2, 2.3- 2.4, 3.45-3.55, 3.65-3.7(4H, m, CH,CH,), 2.5(3H. s, C6H4CH,), 3.0(2H. s. CHj>y). 3.8(3H, s, OCH,), 4.5-4.6(lH, m, CH). 6.45-6.55. 6.6-6,65, 6.7-6.75, 7.2-7.3. 7.7-7.8(9H. 5xm. C-&. C6H4, 2 from C5H4N), 8.3(2H, br m, 2 from C5H4N).
3-(3-Cyclopentyloxy-4-methoxyphenyl)-l-naphthalen-2-ylmethyl-3-pyrid-4-ylmethyl-pyrrolidin- 2-one, as an oil. M.spec (FAB) 507 (MH*), 414. Exact mass measurement- Theoredcal (M+H*) 507.2648. measured 507.2679. Η(CDC13)1.55-1.65. 1.75-2.0(8H. 3xm, cyclopentyl). 2.05-2.2. 2.25-2.4. 2.9-3.0, 3.05-3.1(4H, m, CHjCH,), 3.15-3.30(2H, m, CH,py), 3.7(3H, s, OCH,). 4.75- 4.80(1H. m. CH), 4.5-4.7(2H. m, CH^napth), 6.75-6.8. 6.85-6.9, 6.9-6.95, 7.1-7.2, 7.4-7.5, 7.6-7.65. 7.65-7.7, 7.75-7.8(12H, 8xm, H,, 2 from C5H4N, C1QH7), 8.35-8.45(2H, br m, 2 from C5H4N).
3-(3-Cyclopentyloxy-4-methoxyphenyl)-2-oxo-3-pyrid-4-ylmethyl-pyrrolidin- 1 -carboxylic acid isopropyl ester, oil. M.spec (FAB). Exact mass measurement- Theoredcal (M+H+) 453.2389,
measured 453.2402. *H(CDC13) 1.29- 1.31(6H, 2xs, 2x CHj), 1.55-1.6. 1.65-2.0(8H, 3xm, cyclopentyl), 2.1-2.2, 2.35-2.45, 3.4-3.5, 3.65-3.75(4H. 4xm. CU-CE,), 3.05-3.25(2H, m. CHjpy), 3.7(3H, s, OCHj), 4.7-4.8(1 H, m. CH), 5.0-5.1 (1H, m, CHCH3), 6.75-6.8(4H. m. 2 from C5H4N, 2 from H,), 7.0(1H, s, 1 from H,). 8.35-8.4(2H, br m, 2 from C5H4N).
3-(3-Cyclopentyloxy-4-methoxyphenyl)-2-oxo-3-pyrid-4-ylmethyl-pyrrolidin- 1 -carboxylic acid ethyl ester, oil. M.spec (FAB). Exact mass measurement- Theoredcal (M+H
*) 439.2212. measured 439.2212.
*H(CDC1
3) 1.25-1.35(3H. m, CH^CH,), 1.5-1.6, 1.7-2.0 (8H, 2xm. cyclopentyl), 2.05- 2.15, 2.35-2.45, 3.4-3.5, 3.7-3.8(4H, 4xm.
3.05-3.2(2H. m, CH,py), 3.7(3H. s, OCH,), 4.25- 4.35QH. m, CH,CH
3), 4.7-4.8(lH. m, CH), 6.75-6.8(4H. m, 2 from C
5H
4N. 2 from C- L.), 7.0(1H, s, 1 from C
6Hj), 8.35-8.45(2H. br m. 2 from C
5H
4N).
3-(3-Cyclopentyloxy-4-methoxyphenyl)-2-oxo-3-pyrid-4-ylmethyl-pyrrolidin- 1 -carboxylic acid methyl ester, oil. M.spec (FAB) 425(MH*). Exact mass measurement- Theoredcal (M+H*) 425.2076. measured 425.21 15. 'H (CDCl,) 1.5-1.7, 1.7-1.9 (8H, 2x m, cyclopentyl). 2.1-2.2, 2.35- 2.45, 3.4-3.5, 3.7-3.8 (4H, 2xm, CH,CH:). 3.05-3.25 (2H, m. CHopyr), 3.8 (2 x 3H, 2xs, 2xCHj), 4.7- 4.75(1H, m, CH). 6.75-6.85(4H, m. 2H from C6H,, 2H from C5H.N), 7.0(1H, s. 1H from OJJ,), 8.5 (2H, br s, C5H.N).
3-(3-Cyclopentyloxy-4-methoxyphenyl)-naphthalen- l-ylmethyl-3-pyrid-4-ylmethyl-pyrrolidin-2- one, mp 48-51°C , clear semi-solid. M.spec (FAB). Exact mass measurement- Theoredcal (M+H*) 507.2648, measured 507.2635. Η(CDC13) 1.55-1.65, 1.75-1.95 (8H, 3xm. cyclopentyl). 2.0-2.1, 2.2-2.3. 2.75-2.85. 2.9-3.0(4H, m. CH^CH,), 3.1-3.3 (2H, m, C _py). 3.7(3H, s, OCH3). 4.65-4.7(lH, m. CH). 4.7-4.95(2H, m. CH^napth). 6.7-6.75. 6.8-6.85, 6.9-6.95. 7.1-7.15. 7.15-7.2, 7.3-7.35. 7.4- 7.5, 7.7-7.8, 7.95-8.0 (12H, 9xm. H,, 2 from C5H,N. CI0H7), 8.4 (2H, br s. 2 from C5H4N).
3-(3-Cyclopentyloxy-4-methoxyphenyl)-2-oxo-3-pyrid-4-ylmethyl-pyrrolidine- l -carboxylic acid dimethyl amide, white foam. M.spec (FAB) 438 (MHr), 345. Exact mass measurement- Theoretical (M+H*) 438.2393, measured 438.2384. Η(CDC1}) 1.55-1.65, 1.7-1.9(8H. s, cyclopentyl), 2.15-2.25, 2.4-2.5, 3.4-3.5, 3.6-3.7(4H, 4xm. CH.CH^), 2.8-3.0(6H, br m, N(CH3)2), 3.05-3.2(2H, m, CH^y), 3.8(3H. s, OCH3), 4.7-4.8(lH, m. CH), 6.7-6.8. 6.8-6.85, 7.0-7.05(5H, 3xm, C6H,. 2 from C5H4N), 8.2-8.6(2H, br m, 2 from C5H4N).
Example 20 3-(3-Cyclopentyloxy-4-methoxyphenyl)-3-pyrid-N- oxide- 4-ylmethyl-2-oxopyrrolidine-l -carboxylic acid tert-butyl ester
To a stirring soludon of the 3-(3-Cyclopentyloxy-4-medιoxyphenyl)-2-oxo-3-pyrid-4- ylmethyl-pyrrolidine-1 -carboxylic acid tert-butyl ester (50 mg, 0.1 mmol) in CH,C1
2 (3 mL) is added
m-c loroperbenzoic acid (18 mg, 0.1 mmol). The reaction is stirred at room temperature for 4 hours then quenched with sat NaHC0
3 solution. The organic layer is washed with H
20 twice, dried (Na_S0
4) and evaporated in vacuo. The crude material is purified by flash column chromatography (gradient elution 50-100% EtOAc in hexane, then 10% MeOH in EtOAc) to yield 3-(3- cyclopentyloxy-4-methoxyphenyl)-3-pyrid-N-oxide-4-ylmethyl-2-oxo-pyrrolidine-l -carboxylic acid tert-butyl ester (50 mg) as a white solid, mp 153-155°C. M.spec (Ion Spray) 483(MH
+). 500(M+NH
4 +). Exact mass measurement (FAB)- Theoredcal (M+H
*) 483.2495, measured 483.2493.
1H(CDC1
3)
*H(CDC1
3) 1.5(9H, s, 3xCH
3), 1.6-1.65, 1.7-1.9(8H. s, cyclopentyl), 2.0-2.1, 2.4-2.5, 3.4- 3.5, 3.65-3.75(4H, 4xm,
3.0-3.25(2H, m, CHjpy), 3.85(3H, s, OCHj), 4.7-4.8(lH. m, CH), 6.7-6.8, 7.0(5H, m, s, QH„ 2 from C
5H
4N), 7.95-8.0(2H, m, 2 from C
5H
4N).
Proceeding in a similar manner but starting with the appropriate starting material there is prepared:
3-(3-Cyclopentyloxy-4-methoxyphenyl)-3-(l-pyridin-N-oxide-4-ylmethyl)-pyrrolidine-2-one. mp 166-167°C, white solid. M.spec (FAB) 383(MH+). 367. 274. 206. Exact mass measurement (FAB)- Theoretical (M+H*) 383.1971 , measured 383.1947. Η(CDC13) 1.55-1.65(8H, s, cyclopentyl). 2.15- 2.3, 2.4-2.5, 3.1-3.2. 3.2-3.25(6H, 4xm. CI py, CftCH,), 3.8(3H, s, OCH,), 4.7-4.8(lH. m. CH), 6.4(1H. br s, NH), 6.75-6.85, 6.85-6.90, 7.05-7.1 (5H, 3xm, C6H,. 2 from C5H4N), 8.0-8.05(2H, m, 2 from C5H4N).
Example 21 3-benzyl-3-(3-cyclopentyloxy-4-methoxyphenyl)-2- oxo- pyrrolidine-1 -carboxylic acid tert-butyl ester
To a stirring solution of 3-(3-Cyclopentyloxy-4-methoxyphenyl)-2-oxo-3-pyrid-4- ylmethyl-pyrrolidine- 1 -carboxylic acid tert-butyl ester (216 mg, 0.58 mmol) in DMF (5 mL) at room temperature is added benzyl chloride (0.198 L, 1.7 mmol) and NaH (40 mg, 1.7 mmol). The reaction is stirred under nitrogen for 1 hour at room temperature then partitioned between sat NH4C1 (30 mL) and EtOAc ( x 15 mL). The combined organic layers are dried (NajSO and evaporated in vacuo. The product is purified by flash column chromatography (eluant 5-10%
EtOAc in hexane) to yield 3-benzyl-3-(3-cyclopentyloxy-4-methoxyphenyl)-2-oxo-pyrrolidine-l- carboxylic acid tert-butyl ester (35 mg) as an oil. M.spec (EI) 465(M+), 374, 364, 274. Exact mass measurement (FAB)- Theoretical (M+) 465.2515, measured 465.2551. *H(CDC13) 1.45(9H. s. 3xCH3). 1.55-1.6, 1.75-1.9(8H, 2xm, cyclopentyl), 2.1-2.2, 2.3-2.4, 3.35-3.40, 3.45-3.55(4H, 4xm, CHX.F ), 3.1(2H, 2H, s. CI Ar), 3.8(3H. s, OCH3), 4.7-4.75(lH, m, CH). 6.7-6.8, 6.85-6.9, 6.95-7.0, 7.1-7.2(8H, 4xm, C^ C-f -)
Example 22 3-Benzyl-3-(3-cyclopentyloxy-4-methoxyphenyl)- pyrrolidine-2-one
TFA (0.1 mL) is added to a stirring solution of 3-benzyl-(3-cyclopentyl-oxy-4- methoxyphenyl)-2-oxo-3-pyrid-4-ylmethyl-pyrrolidine-l -carboxylic acid tert-butyl ester (40 mg) in CH2C1-, (3 mL) and stirred at room temperature for
3 hours. A further 0.1 mL of TFA is added and the solution stirred for 15 hours. The reaction is quenched with sat. NaHCO, (10 mL) and extracted with CH2C1, (3 x 10 mL). The combined organic layers are dried (Na2SO.) and evaporated in vacuo. The product is purified by flash column chromatography (eluant 10-40% EtOAc in hexane) to yield 3-benzyl-3-(3-cyclopentyloxy-4- methoxy-phenyl)-pyrrolidine-2-one (20 mg). mp 103-105°C, white foam. M.spec (EI) 365(M+), 303, 297. 274. Exact mass measurement(FAB)- Theoretical (M+H*) 366.2069, measured 366.2063. Η(CDC13) 1.6-1.7, 1.8-2.0(8H, 2xm, cyclopentyl), 2.3-2.5. 2.9-3.0. 3.05-3.15(4H. 3xm. CR.CIL), 3.1-3.25(2H. 2H. s, Q Ar). 3.85(3H. s. OCH,), 4.7-4.8(lH, . CH). 6.1( 1H. br s. NH). 6.75-6.8. 6.9- 7.0, 7.0-7.05, 7.1-7.15, 7.2-7.25(8H. 5xm. QHj. QH,).
Proceeding in a similar manner but starting with the appropriate starting material there is prepared:
3-Cyanomethyl-3-(3-Cyclopentyloxy-4-methoxyphenyl)-2-oxo-pyrrolidine- l -carboxylic acid tert- butyl ester, oil. M.spec. (EI) 415(MH*). 346. *H(CDC1,) 1.5(9H. s. 3x CH,), 1.55- 1.60. 1.8-2.0(8H, m, cyclopentyl). 2.25-2.35, 2.7-2.8, 3.4-3.5, 3.7-3.75(4H, 4xm. Cjr Cϊ ). 2.8-2.95(2H. m, CH,CN), 3.8(3H. s. OCHj), 4.7-4.8(lH, m, CH), 6.8-6.85, 6.9-6.95. 6.95-7.0(3H. 3xm. Jr J.
Example 23 3-(3-cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4- ylmethoxylpyrrolidine
To a stirring solution of 3-(3-cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4-ylmethyl- pyrrolidin-2-one (180 mg. 0.49 mmol) in toluene (15 mL) is added Red-Al (0.3 mL). The reaction is stirred at 80°C for 2 hours. The reaction is quenched with 1 N NaOH (50 mL) and extracted with EtOAc (3 x 50 mL).The combined organic layers are washed with brine (50 mL). H20 (50 mL). dried (Na,S04) and evaporated in vacuo. The crude product 3-(3-cyclopentyloxy-4- methoxyphenyl)-3-pyrid-4-ylmethoxylpyrrolidine (130 mg) is used without any further purification.
Example 24 3-(3-CyclopentyIoxy-4-methoxyphenyl)-3-pyrid-4- ylmethyl-pyrrolidine-1 -carboxylic acid tert-butyl ester
To a stirring solution of the crude 3-(3-cyclopentyloxy-4-methoxyphenyl)-3-pyrid-4- ylmethyl-pyrrolidin-2-one (130 mg. 0.37 mmol) in CH2C1, (10 mL) is added BOC -anhydride (120 mg, 0.55 mmol), Et3N (0.1 mL, 0 74 mmol) and DMAP (cat.). The reacuon is stirred at room temperature for 3 hours, then quenched with H20 (10 mL) and extracted with CH,C12 (2 x 10 mL). The combined organic layers are dried and evaporated in vacuo. The product is purified by flash column chromatography (gradient eluϋon 10-50% EtOAc in hexane) to yield 3-(3-cyclopentyloxy- 4-methoxyphenyl)-3-pyrid-4-ylmethyl-pyrrolidine-l -carboxylic acid tert-butyl ester (50 mg) as an oil. M.spec (FAB) 453(MH*), 397, 304. Exact mass measurement (FAB):- Theoretical (M+H+) 453.2753, measured 453.2711. Η(CDC1,) 1.5(9H. s, 3x CH3), 1.55-1.65, 1.75-1.85(2H. 2xm, cyclopentyl), 2.05-2.2. 3.35-3.70(6H. 2xm, CKUNCH.CΪL), 2.85-2.90(2H. m, CHjpy), 3.8(3H. s. OCH3), 4.55-4.65(1 H, m, CH). 6.40-6.45, 6.45-6.55, 6.55-6.6. 6.75-6.80(5H. 4xm. C6H3, 2 from C5H N), 8.30-8.35(2H, , C5H,N)
Reference Example 1 4-Methoxy-3-cyclopentyloxyphenylacetιc acid
Method A.
To a solution of 3-hydroxy-4-methoxyphenylacetιc acid (10 g. 55 mmole) in 50 mL of anhydrous methanol is added 1 mL of concentrated sulfuric acid and the resulting mixture is refluxed for two hours before being concentrated to dryness. The residue is redissolved in 100 mL of ether, the organic layer washed with saturated sodium bicarbonate and then dried over magnesium sulfate. 10.6 g (98%) of methyl 3-hydroxy-4-methoxyphenylacctate is obtained as an oil after filtration and concentration.
The above ester is dissoKed in 20 mL ot THF and ueated with cyclopentyl alcohol (5 g. 58 mmole). tnphenylphosphine ( 17 c. 65 mmole) and DEAD (1 1 3 g, 65 mmole). and the resulting brown solution is stirred ai room temperature overnight. Alter being concentrated to dryness. the residue is diluted with 100 mL of ether and the solid is removed by filtration. The residue after concentration of ether is puπfied by chromatography on silica gel (30% ether/hexane) to give 12.6 g (89%) of methyl 3-cyclopentyloxy-4-methoxy-phenylacetate as a liquid.
The above liquid is treated with 60 mL of methanol and 60 mL of 1 N sodium hydroxide and the mixture is vigorously stirred at room temperature overnight. The aqueous solution after removal of methanol is acidified with 1 N hydrochloric acid. The solid is collected by filtration and then dried under high vacuum to afford 11.4 g (95%) of 3-cyclopentyloxy-4-methoxy-phenylacetic acid.
Method B.
HC1 gas is bubbled through a solution of 3 -hydroxy phenyl acetic acid (30 g) dissolved in MeOH (250 mL) for 10 minutes. The solvent is evaporated in vacuo and the crude material partitioned between EtOAc (3 x 200 mL) and sat NaHC03 (200 mL). The combined organic layers are dried (Na-SO„) and evaporated in vacuo. The product is purified by flash column chromatography (eluant EtOAc :Hexane, 1 :1) to yield 3-hydroxy-4-methoxyphenylacetate (32 g) as an oil.
The ester (50 g, 0.26 mol), cyclopentylbromide (39 mL, 0.39 mol) and K,C03 (53 g) in DMF (100 mL) are stirred for 20 hours at 70°C. A further amount of cyclopentyl bromide (25 mL) and K,C03 (30 g) are added and the reaction stirred is stirred for a further 15 hours at 70°C. The solution is partitioned between EtOAc (200 mL) and H20 (400 mL). The organic layer is washed with H,0 (3 x 300 mL), dried (Na:S04) and evaporated in vacuo. The product is purified by flash column chromatography (eluant EtOAc:Hexane. 1 :3) to yield methyl 3-cycIopentyloxy-4- methoxyphenylacetate (23 g) as an oil.
Reference Example 2 4-Benzyloxyphthalic acid
A suspension of 4-hydroxyphthalic acid dimethyl ester (8.4 g. 40 mmole). potassium carbonate (6.9 g. 50 mmole) and benzyl bromide (7.7 g. 45 mmole) in 50 mL of acetone is refluxed overnight. The solution after filtration is concentrated to dryness. The residue is dissolved in 30 mL of methanol before being treated with 2 N sodium hydroxide (50 mL). The mixture is vigorously stirred overnight and concentrated by rotary evaporation. The aqueous solution is acidified with concentrated hydrochloric acid, the solid is collected by filtration and dried under high vacuum to give 9.5 g (877c) of 4-benzyloxyphthalic acid.
Reference Example 3 2-(3-Cyclopentyloxy-4-methoxyphenyl)indan- l,3- dione
A mixture of 4-methoxy-3-cyclopentyloxyphenylacetic acid (2.5 g. 10 mmole), phthalic anhydride (4.5 g, 30 mmole) and sodium acetate (1 g, 12 mmole) is heated at 200 °C for four hours and then cooled down to room temperature before being dissolved in 100 mL of dichloromethane. The organic solution is washed with saturated sodium bicarbonate (100 mL) and dried over magnesium sulfate. The residue after filtration and concentration is redissolved in 20 mL of methanol before being treated with a 25% sodium methoxide/methanol solution (6.9 mL). The reddish suspension is refluxed for 0.5 hour and then concentrated to dryness. The residue is acidified with concentrated hydrochloric acid at 0 °C, extracted with dichoromethane (3 x 50 mL), and then dried over magnesium sulfate. The organic solution after filtration is concentrated to give
the desired indan-l,3-dione (2.62 g, 78%) as a yellow solid, m.p. 128-130 °C; MS m/z: 336 (M*); Anal. calc. for C21H20O4: C 74.98, H 5.99; Found: C 74.75, H 6.01
Following the same procedure, but using 3.4-pyridinedicarboxylic anhydride instead of phthalic anhydride, is prepared 6-(3-cyclopentyloxy-4-methoxyphenyl)-[2]-pyrindine-5.7-dione.
Reference Example 4 6-Benzyloxy-2-(3-cyclopentyloxy-4- methoxyphenyl)indan- 1 ,3-dione
4-Benzyloxy phthalic acid (5 g, 18.5 mmole) is placed in a 250 mL round bottom flask equipped with Dean-Stark trap and reflux condenser. Acetic anhydride (30 mL) is added, and the reaction mixture is heated to an oil bath temperature of 180 °C. A mixture of acetic anhydride and acetic acid ( 15 mL) is collected. The reaction mixture is cooled to room temperature before the addition of triethylamine (13 mL, 93.3 mmole). 3-cyclopentyloxy-4-methoxyphenylacetic acid (4.63 g. 18.5 mmole) and acetic anhydride (10 mL). The reaction mixture is heated to reflux for a total of four hours. The mixture is rotary evaporated to yield a thick black tar which is dissolved in dichloromethane (100 mL) and then poured into ice water (200 mL). The organic phase, after washing with water, is dried with magnesium sulfate. filtered and concentrated. The resulting crude reddish brown tar is dissolved in methanol before the addition of 25 % sodium methoxide in methanol (30 mL). The resulting reaction mixture is refluxed for one hour, and then worked-up as above to yield 3.7 g (45%) of 6-benzyloxy-2-(3-cyclopentyloxy-4-methoxyphenyl)indan-l,3-dione as a yellow solid.
Following the same procedure, but using the appropriate derivatizcd acetic acid there are prepared:
6-Benzyloxy-2-(7-methoxy-2-melhoxymethyl-3H-benzimidazol-4-yl)indan-l ,3-dione;
6-Benzyloxy-2-(7-methoxy-2-phenyl-3H-benzimidazol-4-yl)indan-1.3-dione;
6-Benzyloxy-2-(7-methoxy-2-phenethyl-3H-benzimidazol-4-yl)indan-1.3-dione;
6-Benzyloxy-2-(2-benzyl-7-methoxy-3H-benzimidazol-4-yl)indan-l,3-dione;
6-Benzyloxy-2-((RS)-7-methoxy-2-(l-phenylethyl)-3H-benzimidazol-4-yl)indan- l,3-dione;
6-Benzyloxy-2-(7-methoxy-2-(4-methoxybenzyl)-3H-benzimidazol-4-yl)indan-l,3-dione;
6-Benzyloxy-2-((RS)-2-(cyclohexyl-phenyl-methyl)-7-methoxy-3H-benzimidazol-4-yl)indan-l,3- dione;
6-Benzyloxy-2-((RS)-2-(1.2-diphenylethyl)-7-methoxy-3H-benzimidazol-4-yl)indan-1.3-dione;
6-Benzyloxy-2-((RS)-7-methoxy-2-(2-phenylpropyl)-3H-benzimidazol-4-yl)indan-l,3-dione;
6-Benzyloxy-2-(7-methoxy-2-(4-methoxyphenoxymethyl)-3H-benzimidazol-4-yl)indan-1.3-dione;
6-Benzyloxy-2-((RS)-7-methoxy-2-(l-phenylbutyl)-3H-benzimidazol-4-yl)indan- l,3-dione;
6-Benzyloxy-2-(2-(4-bromobenzyl)-7-methoxy-3H-bcnzimidazol-4-yl)indan- l ,3-dione;
6-Benzyloxy-2-((RS)-7-methoxy-2-(3-methoxy-l -phenylpropyl)-3H-benzimidazol-4-yl)indan-1.3- dione;
6-Benzyloxy-2-(2-(4-cyanobenzyl)-7-methoxy-3H-benzimidazol-4-yl)indan- 1.3-dione;
6-Benzyloxy-2-(7-methoxy-2-(4-{ 3-pyridyl }benzyl)-3H-benzimidazol-4-yl)indan- l ,3-dione;
6-Benzyloxy-2-(7-methoxy-2-(2-methoxybenzyl)-3H-benzimidazol-4-yl)indan- 1.3-dione;
6-Benzyloxy-2-((RS)-7-methoxy-2-(methoxyphenylmethyl)-3H-benzimidazol-4- yl)indan- 1.3- dione;
6-Benzyloxy-2-(7-methoxy-2-(2-methoxyphenoxy)methyl-3H-benzimidazol-4-yl)indan- l ,3-dione;
6-Benzyloxy-2-(7-mcthυxy-2-(3-pyridyl)-3H-benzimidazol-4-yl)indan- l,3-dione;
6-Benzyloxy-2-(2-isopropyl-7-methoxy-3H-benzimidazol-4-yl)indan-1.3-dione;
6-Benzyloxy-2-(7-methoxy-2-methyl-3H-benzimidazol-4-yl)indan- l ,3-dione;
6-Benzyloxy-2-(7-methoxy-2-phenoxymethyl-3H-benzimidazol-4-yl)indan- 1.3-dione;
6-Benzyloxy-2-(2-cyclopentyl-7-methoxy-3H-benzimidazol-4-yl)indan-l ,3-dione;
6-Benzyloxy-2-(2-benzyl-3H-benzimidazol-4-yl)indan-l ,3-dione;
6-Benzyloxy-2-(2-cyclopentyl-7-methoxy-l-methyl-benzimidazol-4-yl)indan-l,3-dione;
6-Benzyloxy-2-(2-cyclopentyl-7-methoxy-3-methyl-benzimidazol-4-yl)indan-l,3-dione;
6-Benzyloxy-2-(2,7-dimethoxy-3H-benzimidazol-4-yl)indan-l,3-dione;
6-Benzyloxy-2-(2-cyclopropyl-7-methoxy-3H-benzimidazol-4-yl)indan-l,3-dione;
6-Benzyloxy-2-(l-cyclohexylmethyl-3-methyl-lH-indol-6-yl)indan-1.3-dione;
6-Benzyloxy-2-(l-(2-cyclohexyl)ethyl-3-methyl-lH-indol-6-yl)indan-l,3-dione;
6-Benzyloxy-2-(l-(3-cyclohexyl)propyl-3-methyl-lH-indol-6-yl)indan-1.3-dione;
6-Benzyloxy-2-(l-heptyl-3-methyl-lH-indol-6-yl)indan-1.3-dione;
6-Benzyloxy-2-(l-isobutyl-3-methyl-lH-indol-6-yl)indan-l,3-dione;
6-Benzyloxy-2-(l-cyclopentylmethyl-3-methyl-lH-indol-6-yl)indan-1.3-dione;
6-Benzyloxy-2-(l-benzyl-3-methyl-lH-indol-6-yl)indan-1.3-dione;
6-Benzyloxy-2-(l-(3-phenyl)propyl-3-methyl-lH-indol-6-yl)indan-1.3-dione;
6-Benzyloxy-2-(l-(3-phenyl)butyl-3-methyl-lH-indol-6-yl)indan-1.3-dione;
6-Benzyloxy-2-(l-(4-fluorobenzyl)-3-methyl-lH-indol-6-yl)indan-1.3-dione;
6-Benzyloxy-2-(l-(4-chlorobenzyl)-3-methyl-lH-indol-6-yl)indan-1.3-dione;
6-Benzyloxy-2-(l-(4-methoxybenzyl)-3-methyl-lH-indol-6-yl)indan-1.3-dione;
6-Benzyloxy-2-(l-(4-trifluoromethylbenzyl)-3-methyl-lH-indol-6-yl)indan-l,3-dione;
6-Benzyloxy-2-(l-(4-methylsulphonylbenzyl)-3-methyl-lH-indol-6-yl)indan-l,3-dione;
6-Benzyloxy-2-(l-(l,3-benzodioxol-5-yl)methyl-3-methyl-lH-indol-6-yl)indan-l,3-dione;
6-Benzyloxy-2-(l-(naphthalen-2-yl)methyl-3-methyl-lH-indol-6-yl)indan-l,3-dione;
6-Benzyloxy-2-(l-(tetrahydro-2H-pyran-2-yl)methyl-3-methyl-lH-indol-6-yl)indan-1.3-dione;
5 6-Benzyloxy-2-(3-methyl-l-(tetrahydrofurfuryl)-lH-indol-6-yl)indan-l,3-dione;
6-Benzyloxy-2-(3-methyl-l-(toluene-4-sulphonyl)- lH-indol-6-yl)indan-1.3-dione;
10 6-Benzyloxy-2-(3-methyl-l-(tetrahydrofuran-3-yl)-lH-indol-6-yl)indan-1.3-dione;
6-Benzyloxy-2-(3-methyl- 1 H-indol-6-yl)indan- 1 ,3-dione;
6-Benzyloxy-2-(l-benzyl-3-methylindolin-6-yl)indan- 1.3-dione; and
15
6-Benzyloxy-2-(l -benzyl-3-methylindazol-6-yl)indan- l,3-dione.
Reference Example 5 2-(3-cyclopentyloxy-4-methoxyphenyl)-5- methylindan- 1.3-dione 0
5-Methylphthalic acid (4.86 g, 27 mmole) is refluxed in Ac20 (40 mL) for ten minutes and then 30 mL of liquid is collected by distillation at 185 °C. Fresh Ac:0 ( 15 mL) is added as well as Et3N (14 mL ) and 3-cyclopentyl-4-methoxy phenyl acetic acid (6.76 g. 27 mmole). The reaction is refluxed under argon for two hours before the Et,N is removed by rotary evaporation. The crude 5 mixture is poured into ice/H 0 mixture and stirred for several minules. The aqueous mixture is extracted with methylene chloride (3 x 100 mL). The organic extracts are combined and dried with MgSO,. The solution after filtration is concentrated by rotary evaporation to yield a brown oil. The crude oil is dissolved in methanol (30 mL) and then treated with 25% sodium methoxide/methanol (25 mL. 111 mmole). Enough sodium methoxide is added to make the reaction mixture basic (pH
30 10). The mixture is refluxed for one hour before being concentrated by rotary evaporation. The crude mixture is acidified to a pH of 4 using 1 N HC1 solution. The aqueous mixture is extracted with methylene chloride (3 x 100 mL). The crude brown solid after removal of methylene chloride is then redissolved in a minimum amount of methylene chloride, and ether is added to facilitate precipitation of the product out of the crude mixture. The solid is collected by filtration and dried
35 under high vacuum to give 2-(3-cyclopentyloxy-4-methoxyphenyl)-5-methylindan-l,3-dione (2.2 g, 23 % ). MS m/z 350
Reference Example 6 2-(3-cyclopentyloxy-4-methoxyphenyl)-5- hydroxymethylindan- 1.3-dione
-tn
A mixture of dimethyl 4-bromomethylphthalate (8.27 g, 28.8 mmole) in 150 mL of THF and 60 mL of 2 N sodium hydroxide (120 mmole) is refluxed over a period of four hours. After removal of THF, the aqueous solution is extracted with ether (2 x 100 mL). The aqueous solution is acidified to pH 3 and then extracted with ethyl acetate (3 x 100 mL). The combined ethyl acetate is dried over MgS04 and filtered. The removal of solvent yields the corresponding diacid quantitatively.
The above diacid (4.8 g, 24.5 mmole) is refluxed in acetic anhydride (40 mL) for ten minutes and then 30 mL of liquid is collected by distillation at 185 °C. Fresh Ac20 (15 mL) is added as well as triethyl amine (14 mL) and
3-cyclopentyloxy-4-methoxy phenyl acetic acid (6.12 g, 24.5 mmole ). The reaction is heated under argon at reflux for two hours before being concentrated by rotary evaporation. The crude mixture is poured into an ice/H:0 mixture and stirred for several minutes. The aqueous mixture is extracted with methylene chloride (3 x 100 mL). The organic extracts are combined and dried with magnesium sulfate. The solution is filtered and then concentrated by rotary evaporation to yield a brown oil. The crude oil is dissolved in methanol (30 mL) and then treated with 25% sodium methoxide methanol (25 mL. 1 1 1 mmole). The mixture is refluxed for one hour before being concentrated by rotary evaporation. The crude mixture is acidified to pH 4 using 1 N HC1 solution and the aqueous mixture is extracted with methylene chloride (3 x 100 mL). A crude brown solid results after the solvent is removed. The resultant solid is redissolved in a minimum amount of methylene chloride, and ether is added to facilitate the precipitation of the product out of the crude mixture. The solid is collected by filtration and dried under high vacuum to give 2-(3- cyclopentyloxy-4-methoxyphenyI)-5-hydroxymethylindan- 1.3-dione (2.28 g, 26 %). MS m/z 366.
Reference Example 7 (7-Methoxy-2-methoxymethyl-3H-benzimidazol-4- yl)-acetic acid
A solution of 7-methoxy-2-methoxymethyl-3H-benzimidazole-4-carboxylic acid chloride (1.9 g; 0.01 mole) in 60 mL of absolute ether is added at 5 °C to a solution of diazomethane that is prepared from 3.5 g of nitrosomethylurea in 50 mL of absolute ether. The mixture is stirred for eight hours and then the ether is removed by rotary evaporation to yield the crude corresponding diazoketone.
The crude diazoketone is dissolved in 10 mL of dioxane. and that solution is added dropwise with stirring to a 30 L mixture of silver oxide (0.26 g; 2.1 mmole), anhydrous sodium carbonate (1.05 g; 10 mmole) and sodium thiosulfate (0.63 g; 4 mmole) in water at 55 °C. The stirring is continued for two hours following the completion of the addition, and then the mixture is heated to 90 °C. The solution is then cooled, diluted with water, and acidified with dilute nitric acid to yield titled compound.
Following the same procedure, but using the appropriate carboxylic acid chloride there are prepared:
7-methoxy-2-phenyl-3H-benzimidazol-4-ylacetic acid;
7-methoxy-2-phenethyl-3H-benzimidazol-4-ylacetic acid;
2-benzyl-7-methoxy-3H-benzimidazol-4-ylacetic acid;
(RS)-7-methoxy-2-(l-phenylethyl)-3H-benzimidazol-4-ylacetic acid;
7-methoxy-2-(4-methoxybenzyl)-3H-benzimidazol-4-ylacetic acid;
(RS)-2-(cyclohexyl-phenyl-methyl)-7-methoxy-3H-benzimidazol-4-ylacetic acid;
(RS)-2-(l ,2-diphenylethyl)-7-methoxy-3H-benzimidazol-4-ylacetic acid;
(RS)-7-methoxy-2-(2-phenylpropyl)-3H-benzimidazol-4-ylacetic acid;
7-methoxy-2-(4-methoxyphenoxymethyl)-3H-benzimidazol-4-ylacetic acid;
(RS)-7-methoxy-2-(l-phenylbutyl)-3H-benzimidazol-4-ylacetic acid;
2-(4-bromobenzyl)-7-methoxy-3H-benzimidazol-4-ylacetic acid;
(RS)-7-methoxy-2-(3-methoxy- 1 -phenyl -propyl)-3H-benzimidazol-4-ylacetic acid;
2-(4-cyanobenzyl)-7-methoxy-3H-benzimidazol-4-ylacetic acid;
7-methoxy-2-(4-{ 3-pyridyl }benzyl)-3H-benzimidazol-4-ylacetic acid;
7-methoxy-2-(2-methoxybenzyl)-3H-benzimidazol-4-ylacetic acid;
(RS)-7-methoxy-2-(methoxy-phenyl-methyl)-3H-benzimidazole-4-carboxylic acid chloride;
7-methoxy-2-(2-methoxyphenoxy)methyl-3H-benzimidazol-4-ylacetic acid;
7-methoxy-2-(3-pyridyl)-3H-benzimidazol-4-ylacetic acid;
2-isopropyl-7-methoxy-3H-benzimidazol-4-ylacetic acid;
7-methoxy-2-methyl-3H-benzimidazol-4-ylacetic acid;
7-methoxy-2-phenoxymethyl-3H-benzimidazol-4-ylacetic acid;
2-cyclopentyl-7-methoxy-3H-benzimidazol-4-ylacetic acid;
2-benzyl-3H-benzimidazol-4-ylacetic acid;
2-cyclopentyl-7-methoxy- 1 -methyl-benzimidazoi-4-ylacetic acid:
2-cyclopentyl-7-methoxy-3-methyl-benzimidazol-4-ylacetic acid;
2.7-dimethoxy-3H-benzimidazol-4-ylacetic acid;
2-cyclopropyl-7-methoxy-3H-benzimidazol-4-ylacetic acid;
l-Cyclohexylmethyl-3-methyl- l H-indol-6-ylacetic acid;
1 -(2-cyclohexyl)ethyl-3-methyl- 1 H-indol-6-ylacetic acid;
l-(3-cyclohexyl)propyl-3-methyl- lH-indol-6-ylacetic acid;
l-heptyl-3-methyl-lH-indol-6-ylacetic acid;
l-isobutyl-3-methyl-lH-indol-6-ylacetic acid;
l-cyclopentylmethyl-3-methyl- lH-indol-6-ylacetic acid;
l-benzyl-3-methyl-lH-indol-6-ylacetic acid;
l-(3-phenyl)propyl-3-methyl-lH-indol-6-ylacetic acid;
l-(3-phenyl)butyl-3-methyl-lH-indol-6-ylacetic acid;
1 -(4-fluorobenzyl)-3-methyl- 1 H-indol-6-ylacetic acid;
l-(4-chlorobenzyl)-3-methyl- lH-indol-6-ylacetic acid;
l-(4-methoxybenzyl)-3-methyl- lH-indol-6-ylacetic acid;
l-(4-trifluoromethylbenzyl)-3-methyl- lH-indol-6-ylacetic acid;
l-(4-methylsulphonylbenzyl)-3-methyl-lH-indol-6-ylacetic acid;
l-(l ,3-benzodioxol-5-yl)methyl-3-methyl- lH-indol-6-ylacetic acid;
l-(naphthalen-2-yl)methyl-3-methyl- l H-indol-6-ylacetic acid;
l-(tetrahydro-2H-pyran-2-yl)methyl-3-methyl- l H-indol-6-ylaceιic acid:
3-methyl- l -(tetrahydrofurfuryl)- l H-indol-6-ylacetic acid;
3-methyl- l-(toluene-4-sulphonyl)- lH-indol-6-ylacetic acid;
3-methyl-l -(tetrahydrofuran- -yl)- l H-indol-6-ylacetic acid;
3-methyl-l H-indol-6-ylacetic acid;
l-benzyl-3-methylindolin-6-ylacetic acid; and
l-benzyl-3-methylindazol-6-ylacetic acid.
Reference Example 8 7-Methoxy-2-methoxymethyl-3H-benzimidazole-4- carboxylic acid chloride
7-Methoxy-2-methoxymethyl-3H-benzimidazole-4-carboxylic acid (2.35 g; 0.01 mole) is added dropwise to thionyl chloride (5.7 g; 0.048 mole). The mixture is heated to about 80 °C until the evolution of SO; and HC1 stops, and then excess thionyl chloride is removed by rotary evaporation to yield the crude titled compound.
Following the same procedure, but using the appropriate carboxylic acid there are prepared:
7-methoxv-2-ohenvl-3H-benzimidazole-4-carboxylic acid chloride;
7-methoxy-2-phenethyl-3H-benzimidazole-4-carboxylic acid chloride;
2-benzyl-7-methoxy-3H-benzimidazole-4-carboxylic acid chloride;
(RS)-7-methoxy-2-(l-phenylethyl)-3H-benzimidazole-4-carboxylic acid chloride;
7-methoxy-2-(4-methoxybenzyl)-3H-benzimidazole-4-carboxylic acid chloride;
(RS)-2-(cyclohexyl-phenyi-methyl)-7-methoxy-3H-benzimidazole-4-carboxylic acid chloride;
(RS)-2-(1.2-diphenylethyl)-7-methoxy-3H-benzimidazole-4-carboxylic acid chloride;
(RS)-7-methoxy-2-(2-phenylpropyl)-3H-benzimidazole-4-carboxylic acid chloride:
7-methoxy-2-(4-methoxyphenoxymethyl)-3H-benzimidazole-4-carboxylic acid chloride;
(RS)-7-methoxy-2-( l-phenylbutyl)-3H-benzimidazole-4-carboxylic acid chloride;
2-(4-bromobenzyl)-7-methoxy-3H-benzimidazole-4-carboxylic acid chloride;
(RS)-7-methoxy-2-(3-methoxy- l-phenyl-propyl)-3H-benzimidazole-4-carboxylic acid chloride;
2-(4-cyanobenzyl)-7-methoxy-3H-benzimidazole-4-carbox lic acid chloride;
7-methoxy-2-(4-{ 3-pyridyI }benzyl)-3H-benzimidazole-4-carboxylic acid chloride;
7-methoxy-2-(2-methoxybenzyl)-3H-benzimidazole-4-carboxylic acid chloride;
(RS)-7-methoxy-2-(methoxy-phenyl-methyl)-3H-benzimidazole-4-carboxylic acid chloride;
7-methoxy-2-(2-methoxyphenoxy)methyl-3H-benzimidazole-4-carboxylic acid chloride;
7-methoxy-2-(3-pyridyl)-3H-benzimidazole-4-carboxylic acid chloride;
2-isopropyl-7-methoxy-3H-benzimidazole-4-carboxylic acid chloride;
7-methoxy-2-methyl-3H-benzimidazole-4-carboxylic acid chloride;
7-methoxy-2-phenoxymethyl-3H-benzimidazole-4-carboxylic acid chloride;
2-cyclopentyl-7-methoxy-3H-benzimidazole-4-carboxylic acid chloride;
2-benzyl-3H-benzimidazole-4-carboxylic acid chloride;
2-cyclopentyl-7-methoxy- 1 -methyl-benzimidazole-4-carboxylic acid chloride;
2-cyclopentyl-7-methoxy-3-methyl-benzimidazole-4-carboxylic acid chloride;
2.7-dimethoxy-3H-benzimidazole-4-carboxylic acid chloride;
2-cyclopropyl-7-methoxy-3H-benzimidazole-4-carboxylic acid chloride:
l-Cyclohexylmethyl-3-methyl-lH-indole-6-carboxylic acid chloride;
1 -(2-cyclohexyl)ethyl-3-methyl- 1 H-indole-6-carboxylic acid chloride;
l-(3-cyclohexyl)propyl-3-methyl-lH-indole-6-carboxylic acid chloride;
l-heptyl-3-methyl-lH-indole-6-carboxylic acid chloride;
l-isobutyl-3-methyl-lH-indole-6-carboxylic acid chloride;
l-cyclopentylmethyl-3-methyl-lH-indole-6-carboxylic acid chloride;
l-benzyl-3-methyl-lH-indole-6-carboxylic acid chloride;
l -(3-phenyl)propyl-3-methyl-lH-indole-6-carboxylic acid chloride;
l-(3-phenyl)butyl-3-methyl-lH-indole-6-carboxylic acid chloride;
l-(4-fluorobenzyl)-3-methyl-lH-indole-6-carboxylic acid chloride;
l-(4-chlorobenzyl)-3-methyl-lH-indole-6-carboxylic acid chloride;
l-(4-methoxybenzyl)-3-methyl-lH-indole-6-carboxylic acid chloride;
l-(4-trifluoromethylbenzyl)-3-methyl-lH-indole-6-carboxylic acid chloride;
l-(4-methylsulphonylbenzyl)-3-methyl-lH-indole-6-carboxylic acid chloride;
l-(l,3-benzodioxol-5-yl)methyl-3-methyl-lH-indole-6-carboxylic acid chloride;
l-(naphthalen-2-yl)methyl-3-methyl-lH-indole-6-carboxylic acid chloride;
l-(tetrahydro-2H-pyran-2-yl)methyl-3-methyl-lH-indole-6-carboxylic acid chloride;
3-methyl-l-(tetrahydrofurfuryD- 1 H-indole-6-carboxylic acid chloride:
3-methyl-l-(toluene-4-sulphonyl)- lH-indole-6-carboxylic acid chloride;
3-methyl- l-(tetrahydrofuran-3-yl)-lH-indole-6-carboxylic acid chloride;
3-methyl-lH-indole-6-carboxylic acid chloride;
l-benzyl-3-methylindoline-6-carboxylic acid chloride; and
l-benzyl-3-methylindazole-6-carboxylic acid chloride.
Reference Example 9 -2-mcthoxymethyl-3H-bcnzimidazole-4-
A solution ol
ethoxy-2-methoxymethyl-3H-benzimidazole-4-carboxylate (12.12 g) in methanol (100 ml.) is treated with 2 M sodium hydroxide (48 mL). The resulting mixture is heated to 50°C and then stirred at this temperature for 6 hours. The reaction mixture is concentrated to half its original volume and then treated with 1M hydrochloric acid (98 mL). The solution is cooled in an ice bath and the resulting solid filtered then dried under high vacuum overnight to give the title compound (11 g) as a solid. M+236. This material is used without further purification.
Following the same procedure, but using the appropriate carboxylate there are prepared:
7-methoxy-2-phenyl-3H-benzimidazole-4-carboxylic acid as a white solid. M+268;
7-methoxy-2-phenethyl-3H-benzimidazole-4-carboxylic acid as a white solid. NMR {(CD3),SO}: δ 3.10 (m,2H), 3.25 (m,2H), 4.05 (s.3H), 6.90 (d.J=8Hz,lH), 7.25 (m,5H). 7.83 (d,.I=8Hz.lH);
2-benzyl-7-methoxy-3H-benzimidazole-4-carboxylic acid as a solid. NMR {(CD3)2SO}: δ 4.00 (s,3H), 4.28 (s,2H), 6.92 (d.J=8Hz,lH). 7.30 (m,5H), 7.78 (d.J=8Hz,lH);
(RS)-7-methoxy-2-(l-phenylethyl)-3H-benzimidazole-4-carboxylic acid. M+296;
7-methoxy-2-(4-methoxybenzyl)-3H-benzimidazole-4-carboxylic acid. M+312;
(RS)-2-(cyclohexyl-phenyl-methyl)-7-methoxy-3H-benzimidazole-4-carboxylic acid as a tan colored solid;
(RS)-2-(1.2-diphenylethyl)-7-methoxy-3H-benzimidazole-4-carboxylic acid. M+372:
(RS)-7-methoxy-2-(2-phenylpropyl)-3H-benzimidazole-4-carboxylic acid. NMR { (CD3):SO) : δ 1.20 (d,3H), 3.50 (m.3H). 3.95 (s,3H), 7.15 (m. lH). 7.15-7.20 (m. lH), 7.23-7.36 (m.4H). 7.69 (d.lH). 12.10 (bs.lH);
7-methoxy-2-(4-methoxyphenox methyl)- 3H-benzimidazole-4-carboxylic acid. M+328;
(RS)-7-methoxy-2-(l-phenylbutyl)-3H-benzimidazole-4-carboxylic acid. M+324;
2-(4-bromobenzyl)-7-methoxy-3H-benzimidazole-4-carboxylic acid. NMR {(CD3)-,SO} : δ 3.90 (s.3H), 4.30 (s,2H), 6.80 (d. lH). 7.20 (d,2H), 7.40 (d,2H). 7.75 (d. lH):
(RS)-7-methoxy-2-(3-methoxy- l-phenyl-propyl)-3H-benzimida/.ole-4-carboxylic acid. M+340;
2-(4-cyanobenzyl)-7-methoxy-3H-benzimidazole-4-carboxylic acid. NMR { (CD3)2SO} : 6 4.00 (s,3H), 4.35 (s,2H), 6.80 (d.lH), 7.35 (d,2H). 7.50 (d,2H). 7.75 (d. lH);
7-methoxy-2-(4-{ 3-pyridyl}benzyl)-3H-benzimidazole-4-carboxylic acid. NMR {(CD3),SO} : δ 3.95 (S.3H). 4.30 (s.2H). 6.75 (d.lH), 7.45 (d,3H), 7.70 (d.3H). 8.05 (dd.lH), 8.55 (d.lH), 8.85 (d.lH);
7-methoxy-2-(2-methoxybenzyl)-3H-benzimidazole-4-carboxylic acid. M+312;
(RS)-7-methoxy-2-(methoxy-phenyl-methyl)-3H-benzimidazole-4-carboxylic acid. M+312;
7-methoxy-2-(2-methoxyphenoχy)methyl-3H-benzimidazole-4-carboxylic acid;
7-methoxy-2-(3-pyridyl)-3H-benzimidazole-4-carboxylic acid;
2-isopropyl-7-methoxy-3H-benzimidazole-4-carboxylic acid as a solid. NMR {(CD3)2SO}: δ 1.36 (d.J=6Hz.6H), 3.50 (m,lH), 4.05 (s.3H), 6.95 (d.J=8Hz.lH). 7.85 (d,J=8Hz.lH);
7-methoxy-2-methyl-3H-benzimidazole-4-carboxylic acid as a white solid. M+206;
7-methoxy-2-phenoxymethyl-3H-benzimidazole-4-carboxylic acid as a solid. M+298;
2-cyclopentyl-7-methoxy-3H-benzimidazole-4-carboxylic acid as a solid. NMR {(CD-^SO) : δ 1.68 (m.2H). 1.82 (m,2H), 1.94 (m.2H), 2.09 (m,2H), 3.56 (m,lH). 4.04 (s,3H). 7.00 (d.J=8Hz.lH), 7.86 (d,.T=8Hz,lH);
2-benzyl-3H-benzimidazole-4-carboxylic acid. M+252;
2-cyclopentyl-7-methoxy- l-methyl-benzimidazole-4-carboxylic acid. M+274;
2-cyclopentyl-7-methoxy-3-methyl-benzimidazole-4-carboxylic acid;
2,7-dimethoxy-3H-benzimidazole-4-carboxylic acid. M+222; and
2-cyclopropyl-7-methoxy-3H-benzimidazole-4-carboxylic acid. [Elemental analysis:- C.62.06; H.5.21; N.12.059"-. Calculated:- C.62.07; H.5.17; N.12.07<7rl.
Reference Example 10 Methyl 7-methoxy-2-methoxymethyl-3H- benzimidazole-4-carboxylate
A solution of methyl 3-(l-imino-2-methoxyethylamino)-4-methoxy-benzoate (15.7 g) in methanol (150 mL) is treated with 1 M hydrochloric acid (62.6 ml.) and then with sodium hypochlorite solution (32.3 mL. 13%). Further aliquots of sodium hypochlorite solution are added until all the starting material is consumed. At this point a saturated solution of sodium carbonate
(8.62 g) in water is added. The reaction mixture is then refluxed for 1 hour, then cooled to room temperature, then diluted with water and then extracted with chloroform. The chloroform extract is washed with brine, dried over magnesium sulphate and then evaporated. The residue is subjected to flash chromatography on silica eluting with a mixture of ethyl acetate and hexane (1:1. v/v) then with a mixture of ethyl acetate and hexane (6:1, v/v) to give the title compound (13 g) as a solid. M+250. NMR (CDC13): δ 3.48 (s.3H). 3.98 (s,3H), 4.10 (S.3H), 4.78 (s,2H). 6.70 (d.J=8Hz.lH), 7.87
(d,J=8Hz,lH).
By proceeding in a similar manner but using methyl 3-(iminophenyl-methylamino)-
4-methoxybenzoate. there is prepared methyl 7-methoxy-2-phenyl-3H-benzimidazole-4-carboxylate as a solid. NMR (CDCI3): δ 4.00 (s.3H), 4.11 (s,3H), 6.74 (d,J=8Hz,lH), 7.5 (m,3H), 7.88 (d,J=8Hz.lH), 8.12 (m.2H). 10.69 (bs.lH).
By proceeding in a similar manner but using methyl 3-(l-imino-3-phenylpropylamino)- 4-methoxybenzoate, there is prepared methyl 7-methoxy-2-phenethyl-3H-benzimidazole- 4-carboxylate as a white solid. NMR (CDCI3): δ 3.20 ( . 4H), 3.90 (s,3H), 4.08 (s,3H). 6.70 (d,J=8Hz,lH), 7.25 (m.5H), 7.83 (d.J=8Hz,lH). 9.95 (bs.lH).
By proceeding in a similar manner but using methyl 3-(l-imino-2-phenylethylamino)- 4-methoxybenzoate, there is prepared methyl 2-benzyl-7-methoxy-3H-benzimidazole- 4-carboxylate. NMR (CDCI3): δ 3.90 (s.3H). 4.10 (s,3H). 4.35 (s.2H), 6.70 (d.J=8Hz.lH). 7.30 (m,5H), 7.80 (d.J=8Hz. lH), 9.97 (bs.lH).
By proceeding in a similar manner but using (RS)-methyl
3-(l-imino-2-phenylpropylamino)-4-methoxybenzoate. there is prepared (RS)- ethyl 7-methoxy-2-
(l-phenylethyl)-3H-benzimidazole-4-carboxylate. NMR (CDCI3): δ 1.88 (d.J=7.5Hz.3H), 3.90 (s,3H). 4.10 (s,3H), 4.44 (q,J=7.5Hz.lH), 6.70
(d,J=8Hz.lH). 7.30 (m.5H). 7.82 (d.J=8Hz,lH).
By proceeding in a similar manner but using methyl 3-( l -imino-2-
{4-methoxyphenyl }ethylamιno)-4-methoxybenzoate. there is prepared methyl 7-methoxy- 2-(4-methoxybenzyl)-3H-benzimidazole-4-carboxylate. NMR (CDCI3): δ 3.80 (s,3H). 3.90 (s.3H),
4.08 (s,3H), 4.27 (s.2H). 6.69 (d.J=8Hz,lH), 6.88 (m,2H), 7.25 (m.2H). 7.90 (d.J=8Hz.lH), 9.90 (bs.lH).
By proceeding in a similar manner but using (RS)-methyl 3-(2-cyclohexyl-l-imino-2- phenylethylamino)-4-methoxybenzoate, there is prepared (RS)-methyl 2-(cyclohexyl- phenyl-methyl)-7-methoxy-3H-benzimidazole-4-carboxylate. NMR (CDCI3): δ 0.80-1.40 (m,5H),
1.6 (m,5H), 2.4 (m.lH). 3.86 (d,lH). 3.90 (s,3H). 4.07 (s.3H). 6.65 (d.J=8Hz,lH), 7.20 (m.lH). 7.3 (m.2H), 7.45 (m,2H), 7.78 (d.J=8Hz,lH), 10.1 (bs.lH).
By proceeding in a similar manner but using (RS)-methyl 3-(2,3-diphenyl-l- iminopropylamino)-4-methoxybenzoate, there is prepared (RS)-methyl 2-(1.2-diphenylethyl)-7- methoxy-3H-benzimidazole-4-carboxylate as a solid. NMR (CDCI3): δ 3.40 (ddj=15 and
8.5Hz.3H). 3.87 (s,3H), 3.94 (dd, J= 15 and 7Hz,lH), 4.10 (s.3H). 4.43 (dd, J=8.5 and 7Hz,lH). 6.70 (d,J=8Hz.lH). 7.00-7.30 (m.lOH), 7.33 (d,J=8Hz.lH), 9.93 (bs.lH).
By proceeding in a similar manner but using (RS)-methyl 3-(l-imino-3-phenylbutylamino)-4-methoxybenzoate, there is prepared (RS)-methyl 7-methoxy-2-
(2-phenylpropyl)-3H-benzimidazole-4-carboxylate.
NMR (CDCI3): δ 1.38 (d,3H). 3.22 (d,2H), 3.36-3.49 (m.lH). 3.90 (s.3H), 4.08 (s,3H), 6.70 (d,lH),
7.22-7.39 (m,5H), 7.81 (d.lH), 9.65 (bs.lH).
By proceeding in a similar manner but using methyl 3-(l-imino-2-
{4-methoxyphenoxy}ethylamino)-4-methoxybenzoate, there is prepared methyl 7-methoxy-2-(4-methoxyphenoxymethyl)-3H-benzimidazole-4-carboxylate. NMR (CDCI3): d 3.79
(s.3H), 3.94 (s,3H). 4.10 (s.3H). 5.32 (s,2H), 6.71 (d,J=8Hz.lH). 6.84 (m.2H). 6.97 (m,2H), 7.90 (d,J=8Hz,lH).
By proceeding in a similar manner but using (RS)- ethyl 3-(l-imino-2-phenylpentylamino)-4-methoxybenzoate, there is prepared (RS)-methyl
7-methoxy-2-( l-phenylbutyl)-3H-benzimidazole-4-carboxylate.
NMR (CDCI3): δ 0.93 (l,J=7.5Hz,3H), 1.3 (m.2H), 2.06 (m. l H). 2.46 (m. lH), 3.90 (bs.3H), 4.10 (s,3H). 4.23 (dd, .1=9 and 7Hz.lH). 6.69 (d,J=8Hz,lH), 7.30 (m.5H), 7.79 (d,J=8Hz.lH). 9.90 (bs.lH).
By proceeding in a similar manner but using methyl 3-(2-{4-bromophenyl }-l- iminoethylamino)-4-methoxybenzoate. there is prepared methyl 2-(4-bromo-benzyl)-7-methoxy- 3H-benzimidazole-4-carboxylate as a solid. NMR (CDCI3): δ 3.90 (s.3H). 4.06 (s,3H). 4.25 (s,2H), 6.70 (d.J=8Hz.lH), 7.19 (d.J=8Hz. lH), 7.45 (d,J=8Hz.2H), 7.83 (d.J=8Hz.lH). 10.04 (bs.lH).
By proceeding in a similar manner but using (RS)-methyl 3-(l-imino-4-methoxy-2- phenylbutylamino)-4-methoxybenzoate, there is prepared (RS)-methyl 7-methoxy-2- (3-methoxy-l-phenylpropyl)-3H-benzimidazole-4-carboxylate. NMR (CDCI3): δ 2.39 (m,lH), 2.73 (m,lH), 3.31 (s,3H), 3.39 (s.2H), 3.91 (s,3H), 4.10 (s,3H). 4.50 (t,J=8Hz.lH). 6.70 (d.J=8Hz,lH), 7.30 (m.5H). 7.84 (d.J=8Hz,lH).
By proceeding in a similar manner but using methyl 3-(l-imino-2-{2-methoxyphenyl}- ethylamino)-4-methoxybenzoate, there is prepared methyl 7-methoxy-2-(2-methoxybenzyl)-3H-benzimidazole-4-carboxylate. NMR (CDCl 3): δ 3.92 (s,3H),
4.02 (s,3H), 4.03 (s,3H), 4.79 (s,2H). 6.62 (d,J=9Hz,lH), 6.92 (m,2H), 7.24 (m,lH), 7.30 (m.lH). 7.78 (d,J=9Hz,lH), 10.58 (bs.lH).
By proceeding in a similar manner but using methyl 3-(l-imino-2-methoxy-2- phenyl-ethylamino)-4-methoxybenzoate. there is prepared methyl 7-methoxy-2-(methoxy- phenyl-methyl)-3H-benzimidazole-4-carboxylate.
By proceeding in a similar manner but using methyl 3-(l-imino-2- {2-methoxyphenoxy}-ethylamino)-4-methoxybenzoate, there is prepared methyl 7-methoxy-2-(2-methoxyphenoxy)methyl-3H-benzimidazole-4-carboxylate. NMR (CDCI3): δ 3.95
(s,3H). 3.96 (s,3H), 4.07 (s,3H), 5.47 (s,2H), 6.71 (d,J=8Hz,lH), 6.82-7.05 (m.3H), 7.10 (m.lH), 7.90 (d,J=8Hz,lH).
By proceeding in a similar manner but using methyl 3-(l-imino-2-methylpropylamino)-4-methoxybenzoatc. there is prepared methyl 2-isopropyl-7- methoxy-3H-benzimidazole-4-carboxylate as a tan colored solid.
By proceeding in a similar manner but using methyl 3-(l-imino-ethylamino)-
4-methoxybenzoate. there is prepared methyl 7-methoxy-2-mctiιyl-3H-benzimidazole-4- carboxylate. NMR (CDCI3): δ 2.65 (S.3H). 3.96 (s,3H), 4.07 (s.3H). 6.68 (d.J=8Hz,lH). 7.80
(d,J=SHz,lH).
By proceeding in a similar manner but using methyl 3-( l-imino-2-phenoxy- ethylamino)-4-methoxybenzoate. there is prepared methyl 7-methoxy-2-phenoxymethyl-3H- benzimidazole-4-carboxylate. NMR (CDCI3): δ 3.95 (s.3H). 4.10 (s.3H). 5.40 (s,2H), 6.73
(d,J=8Hz,lH), 7.05 (m,3H), 7.73 (m,2H), 7.90 (d.J=8Hz.lH).
By proceeding in a similar manner but using methyl 3-(cyclopentyl-imino- methylamino)-4-methoxybenzoate. there is prepared methyl 2-cyclopentyl-7-methoxy-3H- benzimidazole-4-carboxylate. as a solid. NMR (CDCI3): δ 1.73 (m,2H). 1.85 (m,2H), 2.00 (m,2H),
2.16 (m,2H), 3.31 (m.lH), 3.98 (s,3H), 4.08 (s.3H), 6.70 (d.J=8Hz,lH). 7.80 (d.J=8Hz,lH). 10.10 (bs.lH).
By proceeding in a similar manner but using methyl 3-(2-phenyl-l-imino- ethylamino)benzoate. there is prepared methyl 2-benzyl-3H-benzimidazole-4-carboxylate. NMR (CDCI3): δ 3.90 (s,3H), 4.33 (s,2H), 7.20-7.40 (m,5H), 7.82 (d,J=7.6Hz.lH), 7.93 (d.J=7.6Hz.lH).
10.02 (bs,lH).
By proceeding in a similar manner but using methyl 3-(cyclopropyl-imino- methylamino)-4-methoxybenzoate. there is prepared methyl 2-cyclopropyl-7-methoxy-3H-
benzimidazole-4-carboxylate, m.p. 124-126°C. [Elemental analysis:- C.53.89; H.5.11; N.9.62%. Calculated:- C.55.21 ; H.5.35; N,9.90%].
Reference Example 1 1 Methyl 3-(l-imino-2-methoxy-ethylamino)-4- methoxybenzoate
Method A: 4-Toluenesulphonic acid monohydrate (17.8 g) is heated under vacuum at 100°C for 4 hours, allowed to cool to room temperature and then treated with methoxy-acetonitrile (7.4 g) and methyl 3-amino-4-methoxybenzoate (17.5 g). The resulting mixture is heated to 180°C and then stirred at this temperature for 4 hours. The reaction mixture is allowed to cool to room temperature, diluted with chloroform and then washed sequentially with 1 M sodium hydroxide solution, saturated sodium bicarbonate and brine. The organic phase is dried over magnesium sulphate and then evaporated. The residue is subjected to flash chromatography on silica, eluting initially with a mixture of hexane and ethyl acetate (4: 1, v/v), then with a mixture of hexane and ethyl acetate (1: 1, v/v) and finally with a mixture of ethyl acetate and triethylamine (50: 1, v/v) to give methyl
3-(l-imino-2-methoxy-ethylamino)-4-methoxybenzoate (15.79 g) as a solid. M+252. NMR (CDCI3): δ 3.48 (bs,3H), 3.90 (bs.6H), 4.20 (bs,2H). 4.95 (bs.l H). 6.92 (d.J=8Hz.lH), 7.60 (bs. lH),
7.77 (d.J=8Hz,lH).
Method B: A solution of methyl 2-methoxyacetimidate (36.5 g. prepared by treating the corresponding hydrochloride [prepared according to the procedure of C.G. Bakker et. al., Rec.Trav.Chim.Pavs-Bas, 100. 13 ( 1981)] with aqueous sodium hydroxide) and methyl 3-amino-4- methoxybenzoate (64.1 g) in butan-2-one (256 mL) is heated at reflux with stirring under a nitrogen atmosphere for 3.5 hours and then a further quantity of methyl 2-methoxyacetimidate (36.5 g) is added. After heating at reflux for a further 4 hours the reaction mixture is left at ambient temperature for 18 hours and then concentrated under reduced pressure. The residual brown oil is treated with cyclohexane (100 mL) and then evaporated. The residual oil is dissolved in a mixture of cyclohexane and ethyl acetate ( 150 mL, 7:3, v/v) and heated to 50°C. Some seed crystals of methyl 3-(l-imino-2-methoxy-ethylamino)-4-methoxybenzoate are added and then mixture is allowed to cool to ambient temperature with stirring. The resulting solid is collected by filtration, then washed with a small amount of a mixture of cyclohexane and ethyl acetate (7:3, v/v), and then dried to give methyl 3-(l-imino-2-methoxy-ethylamino)-4-methoxybenzoate (62.72 g).
By proceeding in a similar manner to method A. but using benzonitrile, there is prepared methyl 3-(iminophenylmethylamino)-4-methoxybenzoate as a tan colored solid. NMR (CDCI3): δ
3.85 (s,3H), 3.86 (s,3H), 6.94 (bd,J=8.8Hz,lH), 7.45 (m,3H), 7.65 (s,lH), 7.75 (m,2H), 7.90 (bs.lH)]
By proceeding in a similar manner to method A. but using hydrocinnamonitrile. there is prepared ethvl 3-( l-imino-3-ρhenγlpropyl-amino)-4-methoxybenzoate as a tan colored solid.
NMR (CDCI3) δ 2.65 (bt,2H), 3.10 (bt,2H), 3.90 (s,6H), 4.34 (bs.lH). 6.90 (d.J=8Hz,lH), 7.30 (m,5H), 7.52 (bs,lH), 7.74 (dd.J=8 and 1 HZ,1H)1
By proceeding in a similar manner to method A, but using phenylacetonitrile, there is prepared methyl 3-(l-imino-2-phenylethylamino)-4-nιethoxybenzoate as a solid. M+298.
By proceeding in a similar manner to method A. but using α-methylbenzyl cyanide, there is prepared (RS)-methyl 3-(l-imino-2-phenylpropylamino)-4-methoxybenzoate. M+312.
By proceeding in a similar manner to method A, but using 4-methoxy-phenylacetonitrile, there is prepared methyl 3-( l-imino-2-{4-methoxyphenyl}-ethylamino)-4-methoxybenzoate. M+328.
By proceeding in a similar manner to method A. but using α-cyclohexyl-benzyl cyanide, there is prepared (RS)-methyl 3-(2-cyclohexyl- l-imino-2-phenyl-ethylamino)-4-methoxybenzoate as an orange solid. M+H 381.
By proceeding in a similar manner to method A. but using 2.3-diphenyl-proprionitrile. there is prepared (RS)-methyl 3-(2,3-diphenyl-l-iminopropyl-amino)-4-methoxybenzoate as a solid.
By proceeding in a similar manner to method A, but using 3-phenyl-butyronitrile. there is prepared (RS)-methyl 3-(l-imino-3-phenylbutylamino)-4-methoxybenzoate. NMR (CDCI3): δ 1.43
(d.3H), 2.60 (d.2H). 3.26-3.39 (m. lH). 3.85 (s.3H). 3.87 (s,3H), 4.20 (bs.2H). 6.89 (d,l H). 7.25-7.35 (m.5H,. 7.42 (bs,lH), 7.75 (dd.lH).
By proceeding in a similar manner to method A. but using 4-methoxy-phenoxyacetonitrile. there is prepared methyl 3-( l-imino-2-{4-methoxy-phenoxy}ethylamino)-4-methoxybenzoate. NMR (CDCI3): δ 3.79 (s,3H), 3.88 (s.3H), 3.99 (s,3H), 4.74 (bs.2H). 5.00 (bs.lH). 6.80-7.00 (m.5H),
7.60 (bs, lH), 7.78 (dd,J=8 and lHz.lH).
By proceeding in a similar manner to method A, but using α-propylphenylacetonitrile, there is prepared (RS)-methyl 3-(l-imino-2-phenylpentylamino)-4-methoxybenzoate. M+H 341.
By proceeding in a similar manner to method A. but using 4-bromophenyl-acetonitrile, there is prepared methyl 3-(2-{4-bromophenyl }-l-iminoethylamino)-4-methoxybenzoate as a tan colored solid. M+H 378. NMR (CDCI3): δ 3.70 (s.2H). 3.90 (d,6H), 4.35 (s,lH). 6.90 (d,lH), 7.30 (d.2H),
7.50 (m,3H), 7.75 (d.lH).
By proceeding in a similar manner to method A, but using 4-methoxy-2- phenylbutyronitrile, there is prepared (RS)- ethyl 3-( l-imino-4-methoxy-2-phenylbutylamino)-4- methoxybenzoate. NMR (CDCI3): δ 2.10 (m,lH), 2.54 (m,lH), 3.35 (bs,3H), 3.40 (m.lH). 3.60
(m.lH). 3.74 (m.lH), 3.85 (bs,6H). 4.25 (bs,2H), 6.90 (bd,J=8Hz,lH). 7.30 (m.lH), 7.38 (m.2H). 7.50 (m.2H), 7.75 (m.lH).
By proceeding in a similar manner to method A, but using 2-methoxyphenyl-acetonitrile, there is prepared methyl 3-(l-imino-2-{2-methoxyphenyl }ethylamino)-4-methoxybenzoate.
By proceeding in a similar manner to method A. but using methoxyphenyl-acetonitrile. there is prepared methyl 3-( l-imino-2-methoxy-2-phenylethylamino)-4-methoxybenzoate.
By proceeding in a similar manner to method A. but using (2-methoxy- phenoxy)acetonitrile. there is prepared methyl 3-(l-inιino-2- { 2-methoxyphenoxy}-ethylamino)-4- methoxybenzoate. M+344.
By proceeding in a similar manner to method A. but using i-butyronitrile, there is prepared methyl 3-( l-imino-2-methyipropylamino)-4-methoxy-benzoate. NMR (CDCl 3): δ 1.29
(d.J=6Hz,6H), 2.60 (m.lH). 3.88 (bs.6H). 4.33 (bs.lH), 6.89 (d.J=8Hz. lH), 7.50 (bs.lH), 7.72 (dd.J=8Hz.lH).
By proceeding in a similar manner to method A. but using acetonitrile, there is prepared methyl 3-( l-imino-ethylamino)-4-methoxybenzoate. M+222.
By proceeding in a similar manner to method A. but using phenoxy-acetonitrile. there is prepared methyl 3-(l-imino-2-phenoxyethylamino)-4-methoxybenzoate. M+314.
By proceeding in a similar manner to method A, but using cyclopentanecarbonitrile. there is prepared methyl 3-(cyclopentyliminomethylamino)-4-methoxybenzoate as a solid. NMR (CDCI3): δ 1.54-2.10 (m,8H), 2.75 (m.lH), 3.86 (bs,6H), 4.30 (bs.lH), 6.88 (bd.J=8Hz.lH), 7.53 (bs.lH), 7.73 (d,J=8Hz.lH).
By proceeding in a similar manner to method A, but using phenylacetonitrile and methyl 3-aminobenzoate. there is prepared methyl 3-(2-phenyl-l-iminoethylamino)benzoate as a tan colored solid. M+312.
By proceeding in a similar manner to method A, but using cyclopropyl cyanide and methyl 3-amιno-4-methoxybenzoate, there is prepared methyl 3-(cyclopropylιπunomethylamιno)benzoate as a colorless solid.
Reference Example 12 Methyl 2-cyclopentyl-7-methoxy-l-methylbenz- ιmιdazolyl-4-carboxylate and methyl 2-(cyclopentyl)- 7-methoxy-3-methyl-benzιmιdazolyl-4-carboxylate
A suspension of sodium hydride (0.55 g, 60% dispersion in mineral oil) in dimethyl formamide (1 mL), cooled to 0°C, is treated with a solution ol methyl
2-cyclopentyl-7-methoxy-3H-benzιmιdazole-4-carboxylate (3 61 g) in dimethylformamide (34 mL). The resulung mixture is stirred for 40 minutes then treated with lodomethane (0 82 mL). The reaction mixture is allowed to stand at 4°C for 2 days then diluted with diethyl ether, then washed with bπne. then dried over magnesium sulphate and then evaporated The residue is subjected to flash chromatography on silica to give methyl
benzιmιdazolyl-4-carboxylate (3 18 g). [NMR (CDC1
3) δ 1 70 (m.2H) 1 90 (m.2H). 2.16 (m.4H),
3.25 (m.lH), 3 95 (s,6H), 4 00 (s.3H). 6 64 (d,T=8Hz,lH), 7 89 (d.l=8Hz.lH)]. and methyl
2-(cyclopentyl)-7-methoxy-3-meth\ lbenzιmιdazolyl-4-carboxylate (0 "".7 g). [M+288, NMR (CDCI3): δ 1 70 (m,2H), 1 90 (m.2H), 2.14 (m.4H), 3 25 (m. lH). 3 92 (s,6H). 4 02 (s,3H), 6 64 (d,J=8Hz,lH), 7 75 (d.J=8Hz, lH)]
Reference Example 13 Methyl 2.7-dιmethoxy-3H-benzιmιdazole 4-carboxylate
A mixture of methyl 2.3 dιamιno-4-methoxybenzoate (0 5 g) acetic acid (0 15 mL) and tetramethoxymethane (0 53 mL) is stirred at 80°C for 40 minutes Alter cooling to room temperature, the reaction mixture is diluted with a mixture ot methanol (3 6 mL). 1 N sodium hydroxide (2 55 mL) and water (8 mL) The resulting precipitate is liltered and then passed through a short filtrauon silica gel column to give the title compound (049 g) as a tan colored solid M+236. NMR (CDCI3) δ 3.93 (s.3H). 4 05 (s.3H), 4 23 (s,3H). 6 69 (d.J=8Hz. lH), 7 74 (d,J=8Hz,lH) 948
(bs,lH)]
Reference Example 14 Methyl 2.3-dιanuno-4-methoxybenzoate
A solution of methyl 2-amιno-4-methoxy-3-nιtrobenzoate (1 84 g) in ethanol (100 mL) is treated with 107r palladium on carbon (0.2 g). The resulting suspension is surred under 3 atmospheres of hydrogen tor 3 hours The catalyst is then removed by filtrauon and the filtrate evaporated to give the Utle compound (1 6 g) as a black solid which is used without further purification. M+196
Reference Example 15 Methyl 2-amino-4-methoxy-3-nitrobenzoate
A solution of methyl 2-carboxy-4-methoxy-3-nitrobenzoate (3.43 g) is dissolved in toluene (20 mL) is treated with thionyl chloride (1.5 mL) and then with dimethylformamide (0.015 mL). The resulting solution is stirred at reflux for 1 hour, cooled to room temperature and then evaporated. The residue is dissolved in acetone (20 mL) and added to a solution of sodium azide (1.3 g) in water (20 mL) cooled in an ice bath. The mixture is stirred for 1 hour and then diluted with water. The resulting precipitate is collected by filtration. This solid is dissolved in a mixture of t-butanol and water (20 mL. 9: 1) and gradually warmed to reflux and held at this temperature for 1 hour. The solution is cooled to room temperature and then evaporated. The residue is subjected to flash chromatography on silica to give the title compound ( 1.8 g). M+H 227. NMR ((CD3),SO): δ 3.82 (s.3H), 3.90 (s,3H). 6.53 (d,J=8Hz.lH). 7.1 (bs.2H). 7.96 (d.J=8Hz.l H).
Reference Example 16 Methyl 2-carboxy-4-methoxy-3-nitrobenzoate
A solution of 3-nitro-4-methoxyphthalic acid (25.1 g) in methanol ( 160 mL). cooled to 0°C, is saturated with hydrogen chloride gas then allowed to stand at 4°C for 2 days. The reaction mixmre is then diluted with water and then extracted with ether. The ether extract is washed with saturated sodium bicarbonate solution. The bicarbonate washings are acidified and then extracted with ether. These ether extracts are dried over magnesium sulphate and then evaporated. The residue is recrystallized from a mixture of chloroform and methanol to give the title compound (3.42 g). M+255. NMR {(CD3):SO} δ 3.85 (s.3H), 4.00 (s,3H). 7.55 (d.J=8.5Hz.lH). 8.07 (d.J=8.5Hz.lH). A further quantity of the title compound (3.54 g) is obtained alter subjecting the mother liquors from the recrystallization to flash chromatography on silica.
Reference Example 17 3-Nitro-4-methoxyphthalic acid
4-Methoxyphthalic acid (21.5 g) is treated dropwise with fuming nitric acid (75 mL). The resulting mixture is heated to 60°C and stirred for 15 minutes whereupon the reaction mixture becomes homogenous. This solution is cooled to room temperature and then diluted with water.
The mixture is extracted with diethyl ether. The combined extracts are washed with brine, dried over magnesium sulphate and then evaporated to give the title compound (25.1 g) as a tan colored solid. M+241.
Reference Example 18 Methyl 7-methoxy-2-(α-methoxybenzyl)-3H-benz- imidazole-4-carboxylate
A solution of α-methoxy phenyl acetic acid (0.596 g) in chloroform (10 mL) is treated with dimethvlformamide CIO uLi then with thionyl chloride (0.52 mL). The reaction mixture is stirred at
ambient temperature for 2 hours and then evaporated. The residue is dissolved in chloroform (4 mL) and the solution added to a stirred solution of methyl 2,3-dianιino-4-methoxybenzoate (0.352 g) in a mixture of chloroform (6 mL) and triethylamine (1 mL). After stirring for 1 hour, the mixmre is treated with ether and then with water. The organic phase is washed with sodium bicarbonate solution and then with brine before being dried over magnesium sulphate. The solvent is evaporated and the residue dissolved in acetic acid (8 mL). The solution is heated at 80°C for 1.5 hours, and then cooled to ambient temperature before being diluted with ether. The mixture is washed sequentially with water, sodium bicarbonate solution and brine before being dried over magnesium sulphate. The ethereal solution is evaporated and the residue subjected to flash chromatography on silica eluting with a mixture of ethyl acetate and hexane (1:1, v/v) to give the title compound (0.36 g). NMR (CDCI3): δ 3.50 (s,3H), 3.96 (s.3H). 4.05 (s.3H). 5.17 (s,lH), 6.70
(d.J=8Hz,lH), 7.24-7.40 (m.3H), 7.46 (m.2H). 7.85 (d.J=8Hz,lH)].
Reference Example 19 Methyl 7-methoxy-2-(3-pyridyl)-3H-benzimidazole- 4-carboxylate
A solution of methyl 2.3-diamino-4-methoxybenzoate (0.73 g) and triethylamine (0.94 g) in dry dichloromethane (20 mL), at 0°C, is treated with nicotine chloride (0.53 g). The reaction mixture is stirred at ambient temperature for 2 hours and then evaporated. The residue is dissolved in acetic acid (8 mL) and the solution heated at 80°C for 2 hours. After cooling to room temperature, the reaction mixture is treated with water. The resultant insoluble material is subjected to flash chromatography on silica to give the title compound (0.46 g). NMR (CDCI3): δ 4.00 (s.3H).
4.15 (s,3H), 6.70 (d.lH). 7.40 (m.lH), 7.90 (d.lH). 8.45 (m.lH). 8.75 (d.lH). 9.30 (d,lH), 10.80 (s,lH)].
Reference Example 20 Methyl 2-(4-cyanobenzyl)-7-methoxy-3H-benz- imidazole-4-carboxylate
A solution of methyl 2-(4-bromobenzyl)-7-methoxy-3H-benzimidazole-4-carboxylate (1.4 g) in dry dimethylformamide is treated with tetrakis(triphenyl-phosphine) palladium (0) (0.266 g) and zinc cyanide (0.275 g). The reaction mixmre is heated at 100°C for 12 hours then cooled to room temperature. The mixture is diluted with ethyl acetate, and then washed sequentially with ammonium hydroxide (2 N). water and brine. The organic solution is dried over magnesium sulphate and then evaporated. The residue is subjected to flash chromatography on silica to give the Utle compound (0.88 g). NMR (CDCI3): δ 3.85 (s,3H), 4.00 (s,3H), 4.40 (s,2H), 6.70 (d,lH), 7.40
(d.2H). 7.65 (d.2H), 7.85 (d.lH).
Reference Example 21 Methyl 7-methoxy-2-(4-{pyrid-3-yl }benzyl)-3H- benzimidazole-4-carboxylate
A solution of methyl 2-(4-bromobenzyl)-7-methoxy-3H-benzimidazole-4-carboxylate (0.268 g) in toluene (8 mL) is treated with tetrakis(tτiphenylphosphine) palladium (0) (0.266 g), aqueous sodium carbonate solution (0.5 mL, 2 M) and diethyl (3-pyridyl)borane (0.085 g). The mixmre is heated at reflux for 12 hours, cooled to room temperature and then subjected to an aqueous work-up. the resultant residue is subjected to flash chromatography on silica to give the title compound (0.128 g). NMR {(CDλ)2SO}: δ 3.90 (s,3H), 4.10 (s,3H), 4.40 (s,2H), 6.70 (d.lH), 7.45 (d,3H), 7.60 (d,3H), 7.90 (dd,lH), 8.60 (d.lH), 8.85 (d.lH), 10.10 (s,lH).
Reference Example 22 l-Cyclohexylmethyl-3-methyl-lH-indole-6- carboxylic acid
A mixture of methyl l-cyclohexylmethyl-3-methyl-lH-indole-6-carboxylate (9 g) and lithium hydroxide (8 g) in aqueous methanol (300 mL, 1:2, v/v) is heated at 70°C for 4 hours. The reaction mixture is cooled to room temperamre. acidified by addition of dilute hydrochloric acid and then extracted with ethyl acetate (3 x 150 mL). The combined extracts are dried over sodium sulphate and then evaporated to give the title compound as a white solid (7.3 g). M*271.
By proceeding in a similar manner but using methyl l-(2-cyclohexyl)ethyl-3-methyl-lH- indole-6-carboxylate. there is prepared l -(2-cyclohexyl)ethyl-3-methyl- lH-indole-6-carboxylic acid as a white solid.
By proceeding in a similar manner but using methyl l -(3-cyclohexyl)propyl-3-methyl-lH- indole-6-carboχylate, there is prepared l-(3-cyclohexyl)propyl-3-methyl- l H-indole-6-carboxylic acid as a white solid. NMR (CDC13): δ 0.80-0.90. 1.00-1.30, 1.60- 1.70 and 1.79-1.80(m,15H); 2.30(s.3H); 4.00-4. lϋ(m,2H); 7.00(s. l H); 7.50-7.60(m, l H): 7.80-7.90(ιn, l H); 8.20(s,lH).
By proceeding in a similar manner but using methyl l -heptyl-3-methyl- lH-indole-6- carboxylate, there is prepared l-heptyl-3-methyl-lH-indole-6-carboxylic acid.
By proceeding in a similar manner but using methyl l-isobutyl-3-methyl-lH-indole-6- carboxylate. there is prepared l -isobutyl-3-methyl-lH-indole-6-carboxylic acid.
By proceeding in a similar manner but using methyl l-cyclopentylmethyl-3-methyl- lH- indole-6-carboxylate, there is prepared l-cyclopentylmethyl-3-methyl-lH-indole-6-carboxylic acid. NMR (CD3OD): δ 1.20-1.30 and 1.40-1.70(m,8H); 2.30(s,3H); 2.30-2.40(m,lH); 3.90-4.09(m.2H);
7.00(s.lH); 7.50-7.60(m.lH); 7.70-7.80(m,lH); 8.00(s.lH).
By proceeding in a similar manner but using methyl l-benzyl-3-methyl-lH-indole-6- carboxylate, there is Dreϋared l-benzyl-3-methyl-lH-indole-6-carboxylic acid as a white solid.
NMR {(CD3)2SO}: δ 2.20(s,3H); 5.50(s,2H); 7.10-7.20(m,2H); 7.20-7.30(m,3H): 7.40(s.lH); 7.50- 7.60 and 7.60-7.70(m,2H); 8.00(s.lH).
By proceeding in a similar manner but using methyl l-(3-phenyl)propyl-3-methyl-lH- indole-6-carboxylate, there is prepared l-(3-phenyl)propyl-3-methyl-lH-indole-6-carboxylic acid. NMR (CDCI3): δ 2.10-2.20(m,2H); 2.30(s,3H); 2.50-2.60(m,2H); 4.00-4.10(m,2H); 7.00(s.lH);
7.10-7.30(m,5H); 7.50-7.60(m,lH); 7.80-7.90(m,lH); 8.10(s, lH); 12.20(s,lH).
By proceeding in a similar manner but using methyl l-(3-phenyl)butyl-3-methyl-lH-indole- 6-carboxylate, there is prepared l-(3-phenyl)butyl-3-methyl-lH-indole-6-carboxylic acid as a white solid. NMR (CDCI3): δ 1.60-1.70(m.2H); 1.80-l ,90(m.2H); 2.30(s.3H); 2.60-2.70(m.2H); 4.10-
4.20(m.2H); 7.00(s,lH); 7.10-7.3ϋ(m.5H); 7.50-7.60(m,lH); 7.80-7.90(m.lH); 8.20(s,lH).
By proceeding in a similar manner but using methyl l-(4-fluorobenzyl)-3-methyl-lH- indole-6-carboxylate. there is prepared l-(4-fluorobenzyl)-3-methyl-lH-indole-6-carboxylic acid as a white solid. NMR (CDCI3): δ 2.30 (s). 5.30 (s), 6.90-7.10 (m), 7.60-7.70 (m). 7.80-7.90 (m), 8.10
(s).
By proceeding in a similar manner but using methyl l-(4-chlorobenzyl)-3-methyl-lH- indole-6-carboxylate. there is prepared l-(4-chlorobenzyl)-3-methyl- lH-indole-6-carboxylic acid.
By proceeding in a similar manner but using methyl l -(4-methoxybenzyl)-3-methyl-lH- indole-6-carboxylate, there is prepared l -(4-methoxybenzyl)-3-methyl- l H-indole-6-carboxylic acid.
By proceeding in a similar manner but using methyl l-(4-tril"luoromethylbenzyl)-3-methyl- lH-indole-6-carboxylate. there is prepared l-(4-trifluoromethylbenzyl)-3-methyl-lH-indole-6- carboxylic acid as a white solid. NMR {(CD3)2SO}: δ 2.30 (s), 5.50 (s), 7.20-7.30 (m). 7.30-7.40 (m). 7.60-7.70 (m), 7.90 (s).
By proceeding in a similar manner but using methyl l-(4-methylsulphonylbenzyl)-3-methyl- lH-indole-6-carboxylate. there is prepared l-(4-methylsulphonylbenzyl)-3-methyl-lH-indole-6- carboxylic acid as a white solid.
By proceeding in a similar manner but using methyl l-(1.3-benzodioxol-5-yl)methyl-3- methyl- lH-indole-6-carboxylate. there is prepared l-(l,3-benzodioxol-5-yl)methyl-3-methyl-lH- indole-6-carboxylic acid.
By proceeding in a similar manner but using methyl l-(naphthalen-2-yl)methyl-3-methyl- lH-indole-6-carboxylate, there is prepared l-(naphthalen-2-yl)methyl-3-methyl-lH-indole-6- carboxylic acid as a white solid. NMR {(CD3)2SO}: δ 2.30 (s), 5.60 (s), 7.30-8.10 (m).
By proceeding in a similar manner but using methyl l-(tetrahydro-2H-pyran-2-yl)methyl-3- methyl-lH-indole-6-carboxylate. there is prepared l-(tetrahydro-2H-pyran-2-yl)methyl-3-methyl- lH-indole-6-carboxylic acid.
By proceeding in a similar manner but using methyl 3-methyl-l-(tetrahydrofurfuryl)-lH- indole-6-carboxylate. there is prepared 3-methyl-l-(tetrahydrofurfuryl)-lH-indole-6-carboxylic acid, as a white solid, m.p. 217-219°C. [Elemental analysis: C.69.3; H,6.6; N.5.27r. Calculated for C15H7Nθ : C.69.48; H.6.61 ; N.5.40%].
By proceeding in a similar manner but using methyl 3-methyl- l -(toluenc-4-sulphonyl)-lH- indole-6-carboxylate. there is prepared 3-methyl-l -(loluene-4-sulphonyl)- l H-indole-6-carboxylic acid.
By proceeding in a similar manner but using methyl 3-methyl- l -(tetrahydrofuran-3-yl)-lH- indole-6-carboxylate. there is prepared 3-methyl- l -(tetrahydrofuran-3-yl)- lH-indole-6-carboxylic acid as a white solid, m.p. 21 1-213°C. [Elemental analysis: C.68.00; H.6.20; N.5.60 . Calculated for C14H 15NO3: C.68.56; H.6.16; N.5.71 %].
By proceeding in a similar manner but using methyl 3-methyl- lH-indole-6-carboxylate. there is prepared 3-methyl-ιndolc-6-carboxylic acid as a white solid. NMR (CD3OD): δ 2.10 (s). 7.10 (s). 7.30-7.40 (m i. 7.50-7.60 (m). 8.00 (s).
By proceeding in a similar manner but using methyl l-benzyl-3-methyl- lH-indolence-6- carboxylate, there is prepared l -bcnzyl-3-methyl-indolence-6-carboxylic acid as a white solid. NMR (CD3OD): δ 2.10 (s). 7.10 (s). 7.30-7.40 (m). 7.50-7.60 (m), 8.00 (s).
By proceeding in a similar manner but using methyl l-benzyl-3-methyl-indazole-6- carboxylate. there is prepared l-benzyl-3-methyl-indazole-6-carboxylic acid.
Reference Example 23 Methyl l-cyclohexylmethyl-3-methyl- lH-indole-6- carboxylate
A mixmre of methyl 3-methyl-lH-indole-6-carboxylate (10 g), cyclohexyl-methylbromide (19 g), potassium hydroxide (12 g) and sodium iodide (0.1 g) in acetone (200 mL) is stirred at room
temperature for 6 hours. The reaction mixture is evaporated. The residue is partitioned between ethyl acetate
(250 mL) and water (250 L). The aqueous layer is extracted with ethyl acetate (3 x 250 mL). The total combined organic phases are dried over sodium sulphate and then evaporated. The residue is subjected to flash column chromatography on silica eluting with a mixmre of ethyl acetate and hexane (1:9, v/v) to yield the title compound (9.5 g). NMR (CDCI3): δ 0.90-1.10 (m), 1.10-1.40
(m), 1.60-1.90 (m), 2.30 (s), 3.90-4.00 (m), 3.90 (s), 7.00 (s), 7.50-7.60 (m), 7.70-7.80 (m), 8.00 (s).
By proceeding in a similar manner but using (2-cyclohexyl)ethyl bromide there is prepared methyl l-(2-cyclohexyl)ethyl-3-methyl-lH-indole-6-carboxyiate. NMR (CDCI3): δ 0.80-1.00 (m).
1.10-1.30 (m). 1.60-1.80 (m), 2.30 (s), 3.90 (s), 4.10-4.20 (t). 7.00 (s). 7.60 (d). 7.80 (d), 8.10 (s).
By proceeding in a similar manner but using (3-cyclohexyl)propyl bromide there is prepared methyl l-(3-cyclohexyl)propyl-3-methyl-lH-indole-6-carboxylate.
By proceeding in a similar manner but using heptyl bromide there is prepared methyl 1- heptyl-3-methyI-lH-indole-6-carboxylate.
By proceeding in a similar manner but using isobutyl bromide there is prepared methyl 1- isobutyl-3-methyl-lH-indole-6-carboxylate. NMR (CDCI3): δ 0.80-0.90 (m). 2.00-2.20 (m), 2.30
(s). 3.80-3.90 (m). 3.90 (s). 7.00 (s), 7.50-7.60 (m). 7.60-7.70 (m), 8.00 (s).
By proceeding in a similar manner but using cyclopentylmethyl bromide there is prepared methyl l-cyclopentylmethyl-3-methyl- lH-indole-6-carboxylate.
By proceeding in a similar manner but using benzyl bromide there is prepared methyl 1- benzyl-3-methyl-lH-indole-6-carboxylate as a white solid. NMR (CDCI3): δ 2.30 (s), 3.80 (s). 5.20
(s), 7.00 (s), 7.00-7.10 (m). 7.10-7.20 (m), 7.50-7.60 (m), 7.70-7.80 (m), 8.00 (s).
By proceeding in a similar manner but using (3-phenyl)propyl bromide there is prepared methyl l-(3-phenyl)propyl-3-methyl-lH-indole-6-carboxylate. NMR (CDCI3): δ 0.80-0.90(m);
1.20-1.30(m); 2.00-2.20(m); 2.30(s); 2.50-2.60(m); 3.90(s); 3.90-4.00(m); 6.90(s); 7.00-7.30(m); 7.50-7.60(m); 7.70-7.80(m); 8.00(s).
By proceeding in a similar manner but using (3-phenyl)butyl bromide there is prepared methyl l-(3-phenyl)butyl-3-methyl-lH-indole-6-carboxylate.
By proceeding in a similar manner but using 4-fluorobenzyl bromide there is prepared methyl 1 -(4-fluorobenzy 1 )- 3 - methyl- 1 H-indole-6-carboxylate.
By proceeding in a similar manner but using 4-chlorobenzyl bromide there is prepared methyl l-(4-chlorobenzyl)-3-methyl-lH-indole-6-carboxylate.
By proceeding in a similar manner but using 4-methoxybenzyl bromide there is prepared methyl l-(4-methoxybenzyl)-3-methyl-lH-indole-6-carboxylate as a white solid, m.p. 116-118°C. [Elemental analysis: C.73.48; H.6.27; N.4.36%. Calculated for C19H19NO3: C.73.77; H.6.19; N.4.53%1.
By proceeding in a similar manner but using 4-trifluoromethylbenzyl bromide there is prepared methyl l-(4-trifluoromethylbenzyl)-3-methyl-l H-indole-6-carboxylate as a white solid. NMR (CDCI3): δ 2.30(s); 3.90(s); 5.40(s); 7.00(s); 7.10-7.2 (m); 7.50-7.60(m); 7.80-7.90(m); 8.00(s).
By proceeding in a similar manner but using 4-methylsulphonylbenzyl bromide there is prepared methyl l -(4-methylsulphonylbenzyl)-3-methyl-lH-indole-6-carboxylate as a white solid. NMR (CDCI3): δ 2.40(s); 3.00(s); 3.90(s); 5.40(s); 7.00(s); 7.20-7.30(m); 7.50-7.70(m); 7.80- 7.90(m); 8.00(s).
By proceeding in a similar manner but using piperonyl chloride there is prepared methyl l-( l ,3-benzodioxol-5-yl)methyl-3-methyl- lH-indole-6-carboxylate.
By proceeding in a similar manner but using (naphthalen-2-yl)methyl chloride there is prepared methyl l-(naphthalen-2-yl)methyl-3-methyl-lH-indole-6-carboxylate.
By proceeding in a similar manner but using (tetrahydro-2H-pyran-2-yl)methyl chloride there is prepared methyl l-(tetrahydro-2H-pyran-2-yl)methyl-3-methyl- lH-indole-6-carboxylate.
By proceeding in a similar manner but using tetrahydrofurfuryl chloride there is prepared methyl 3-Methyl- 1 -(tetrahydrofurfuryl)- 1 H-indole-6-carboxylate.
By proceeding in a similar manner but using toluene-4-sulphonyl chloride there is prepared methyl 3-methyl-l-(toluene-4-sulphonyl)-lH-indole-6-carboxylate.
By proceeding in a similar manner but using tetrahydrofuran- 3 -yl chloride there is prepared methyl 3-methyl-l-(tetrahydrofuran-3-yl)-lH-indole-6-carboxylate.
Reference Example 24 Methyl 3-methyl-lH-indole-6-carboxylate
A mixture of methyl 3-formyl-lH-indole-6-carboxylate (12 g), p-toluene-sulfonic acid (2 g) and p-toluenesulfonylhydrazide (13 g) in a mixture of dimethylformamide ( 100 mL) and sulfolane (50 mL) is heated at 100°C for 15 minutes and then cooled to room temperamre. The mixture is treated with sodium cyanoborohydride (15 g, 5 g portions after 10 minute intervals), and then heated at 100°C for 2 hours. After cooling to ambient temperature the reaction mixture is treated with ice water (500 mL) to yield a white precipitate. Water (1 L) is added and the mixture stirred for 30 minutes before being filtered. The off-white solid is washed with warm water and then toluene is added and removed by rotary evaporation to azeotrope residual water. The title compound ( 10.2 g) is obtained as a white solid.
Reference Example 25 l -(6.6-Dimethylbicyclo[3.1.1 .]hept-3-yimethyl)-3- methyl- lH-indole-6-carboxylic acid
A mixture of 3-methyl-indole-6-carboxylic acid (1.8 g). ( l .2S.5S)-(-)-myrtanol tosylate and potassium hydroxide (3.17 g) in dimethyl sulphoxide
(35 mL) is stirred at room temperature for 18 hours. The reaction mixture is partitioned twice between ethyl acetate (25 mL) and dilute hydrochloric acid
(25 mL. 1 M). The combined organic layers are dried over sodium sulphate and then evaporated. The residue is subjected to flash chromatography on silica to give the title compound (2.45 g) as a white solid. M*325.
By proceeding in a similar manner but using cyclohexanol tosyiate there is prepared 1- cyclohexyl-3-methyl-lH-indole-6-carboxylic acid as a white solid.
By proceeding in a similar manner but using cyclopentanol tosylate there is prepared 1- cyclopentyl-3-methyl-lH-indole-6-carboxylic acid as a white solid. NMR { (CD3)2CO} : δ 0.80- 0.90(m). 1.20-1.30(m). 1.70-1.90(m), 2.10-2.30(m), 2.30(s). 4.90-5.00(m). 7.30(s). 7.50(d), 7.70(d). 8.20(s).
By proceeding in a similar manner but using cycloheptyl methanol tosylate there is prepared l-cycloheptylmethyl-3-methyl-lH-indole-6-carboxylic acid as a white solid. NMR {(CD3)2CO) : δ 1.10-1.80(m), 2.30(s). 3.30-3.40(m), 4.00-4.10(m), 7.30(s). 7.50-7.6ϋ(m), 7.70-7.80(m), 8.10(s).
Reference Example 26 Methyl l-benzyl-3-methyl-lH-indoline-6-carboxylate
A solution of methyl l-benzyl-3-methyl-lH-indole-6-carboxylate (0.8 g) in trifluoroacetic acid at 0°C is treated with a solution of boraπe-tetrahydrofuran complex in tetrahydrofuran (9 mL, 1
M). The solution is kept at 0°C for 24 hours, quenched with methanol. and then evaporated. The residual solid is dried under high vacuum and used without purification.
5
Reference Example 27 3-methyl-l-{3-(phenyl)propyl}-lH-indole-6- carboxylate
A stirred solution of methyl 3-methyl-lH-indole-6-carboxylate (0.5 g) in acetone (35 mL) is treated 10 with l-bromo-3-phenylprυpane (0.577 g) and sodium hydroxide (0.116 g). The mixmre is stirred at room temperamre for 12 hours, poured into water (35 mL) and then extracted with ethyl acetate (3 x 50 mL). The combined extracts are washed sequentially with dilute hydrochloric acid (50 mL), a saturated sodium bicarbonate solution (50 mL) and then dried over magnesium sulphate before being evaporated. The residue is subjected to flash column chromatography on silica eluting with a 15 mixmre of ethyl acetate and hexane (50: 1. v/v) to yield the title compound (0.58 g).
Reference Example 28 l-Benzyl-3-methyl-6-indazoyl chloride
A solution of l-benzyl-3-methyl-indazole-6-carboxylic acid (0.15 g) in dichloromethane is 20 treated with dimethylformamide (2 drops) and then with oxalyl chloride (1.69 mL). After stirring for 2 hours the reaction mixture is evaporated and the residue is dried under high vacuum to give the title compound (0.16 g) which is used without further purification.
(b) By proceeding in a similar manner but using l-(4-methoxybcnzyl)-3-methyl-lH-indole-6- 25 carboxylic acid, there is prepared l-(4-methoxybenzyl)-3-methyl-l H-indole-6-carboxylic acid chloride.
Reference Example 29 Methyl l-benzyl-3-methyl-lH-indazole-6- carboxylate
30
A solution of methyl 3-methyl-indazole-6-carboxylate (0.2 g) in acetone (15 mL) is treated with benzyl bromide (0.898 g) then with potassium carbonate (0.29 g) and a catalytic amount of 18- crown-6. The mixmre is stirred for 12 hours at room temperamre then poured into water (30 mL) and then extracted with ethyl acetate (3 x 30 mL). The combined extracts are dried over sodium 35 sulphate and then evaporated. The residue is subjected to flash chromatography on silica eluting with a mixmre of ethyl acetate and hexane (7:1, v/v) to yield the title compound (0.161 g) and methyl 2-benzyl-3-methylindazole-6-carboxylate (0.069 g).
Reference Example 30 Methyl 3-methyl-lH-indazole-6-carboxylate
AC→
A solution of 3-methyl-indazole-6-carboxylic acid (1.57 g) in methanol (75 m ) is treated with hydrogen chloride gas for 10 minutes. The reaction mixture is stirred for 12 hours at room temperature then evaporated. The residue is partitioned between ethyl acetate (50 mL) and saturated sodium bicarbonate solution (50 mL). The combined extracts are dried over sodium sulphate and then evaporated. The residue is washed with hexane to give the title compound (1.56 g) which is used without further purification.
Reference Example 31 3-methyl-lH-indazole-6-carboxylic acid
A solution of methyl l-triflyl-3-methyl-indazole-6-carboxylate(0.668 g) in a mixture of methanol and water (3:1, 80 mL) is treated with potassium carbonate (1.5 g). The mixture is heated at reflux for 5 hours, cooled to room temperamre and then poured into 1 N hydrochloric acid (50 mL). The mixmre is extracted with ethyl acetate (3 x 50 mL). The combined extracts are dried over sodium sulphate and then evaporated. The residue is washed with a mixmre of hexane and ether to give the title compound (0.36 g).
Reference Example 32 Methyl l-triflyl-3-methyl-lH-indazole-6-carboxylate
The 6-triflyloxy-l-triflyl-3-methyl-indazole (1 g, Reference Example 18) is dissolved in dimethylformamide under argon and the solution is flushed with carbon monoxide for 5 minutes. The solution is treated with palladium acetate (0.11 g), 1.3-bis(diphenylphosphino)propane (0.272 g), triethylamine (0.491 g) and methanol (1.56 g) then stirred at room temperamre for 12 hours under an atmosphere of carbon monoxide. The reaction mixmre is poured into water (150 mL) and the aqueous layer is extracted with ethyl acetate (3 x 35 mL). The combined extracts are dried over sodium sulphate and then evaporated. The residue is subjected to flash chromatography on silica eluting with a mixmre of ethyl acetate and hexane (1 :7. v/v) to yield the title compound.
Reference Example 33 6-triflyloxy-l-triflyl-3-methyl-lH-indazole
A solution of 6-hydroxy-3-methyl-lH-indazole (450 mg) in tetrahydrofuran (30 mL) under argon is treated with sodium hydride (198 mg). After the initial effervescence has subsided, the solution is warmed to 50°C for 1 hour. The reaction mixmre is cooled to room temperamre and N- phenyltri-fluoromethanesulfonimide is added. The mixture is stirred for 2 hours, poured into water (50 mL) and then extracted with ethyl acetate (3 x 50 mL). The combined extracts are dried over sodium sulphate and then evaporated. The residue is subjected to flash chromatography on silica eluting with a mixmre of ethyl acetate and hexane (1:7, v/v) to yield the title compound (LI g).
Reference Example 34 6-hydroxy-3-methyl-lH-indazole
A solution of 6-methoxy-3-methyl-lH-indazole (500 mg) in dichloromethane (75 mL) is cooled to 0°C and then treated with boron tribromide in methylene chloride (54 mL, 1 M). The mixmre is allowed to warm to room temperature and then stirred for 12 hours. The solution is poured into an ice-saturated sodium bicarbonate mixture and the aqueous layer is extracted with ethyl acetate (3 x 50 mL). The combined extracts are dried over sodium sulphate and then the solvent is removed by rotary evaporation. The residue is subjected to flash chromatography on silica eluting with a mixture of ethyl acetate and hexane (2:1, v/v) to yield the title compound (0.45 g).
Reference Example 35 6-methoxy-3-methyl-lH-indazole
2-Fluoro-4-methoxyacetophenone (5 g) is treated with hydrazine (75 mL) under argon and the mixture is heated to reflux for 12 hours. After cooling to room temperamre. the reaction mixmre is poured into water (200 mL). and then extracted with ethyl acetate (3 x 50 mL). The combined extracts are dried over sodium sulphate and then evaporated. The residue is subjected to flash chromatography on silica eluting with a mixmre of ethyl acetate and hexane (1 :3. v/v) to yield the title compound (4.05 g).
Reference Example 36 Diazomethane
The titled compound is prepared as disclosed by W.E. Bachmann and W.S. Struve Org. Reactions, 1, 38 (1942) which is incoφorated herein by reference. The diazomethane is prepared from N-mtrosomethylurea and potassium hydroxide.
Reference Example 37 Methyl 3-hydroxy-4-methoxyphenylacetate
HC1 gas is bubbled through a solution of 3-hydroxy-4-methoxyphenylacetic acid (30 g) dissolved in MeOH (250 mL) for 10 minutes. The solvent is evaporated in vacuo and the crude material partitioned between EtOAc (3 x 200 mL) and sat NaHCO, (200 mL). The combined organic layers are dried (Na2S04) and evaporated in vacuo. The product is purified by flash column chromatography (eluant EtOAc :Hexane. 1:1) to yield the product methyl 3-hydroxy-4-methoxy- phenylacetate (32 g) as an oil.
Reference Example 38 Methyl 3-cyclopentyloxy-4-methoxyphenylacetate
Methyl 3-hydroxy-4-methoxyphenylacetate (50 g. 0.26 mol), cyclopentylbromide (39 mL; 0.39 mol) and K2C03(53 g) in DMF(100 mL) are stirred for 20 hours at 70°C. A further amount of cyclopentyl bromide (25 mL) and K2CO, (30 g) are added and the reaction stirred is stirred for a
further 15 hours at 70°C. The solution is partitioned between EtOAc (200 mL) and H,0 (400 mL). The organic layer is washed with H,0 (3 x 300 mL), dried (Na2S04) and evaporated in vacuo. The product is purified by flash column chromatography (eluant EtOAc:Hexane, 1:3) to yield methyl 3- cyclopentyloxy-4-methoxyphenylacetate (23 g) as an oil.
Reference Example 39 Methyl 3-cyano-2-(3-cyclopentyloxy-4- methoxyphenyl)propanoate
Potassium hexamethyl disilazane (KHMDS) (256 mL of a 0.5 M solution in toluene) is added dropwise to a stirring solution of methyl 3-cyclopentyloxy-4-methoxy phenylacetate (33.8 g, 0.128 mol) in DME (400 mL) at -78°C under nitrogen. The reaction is stirred for one hour and bromoacetonitrile (8.9 mL, 0.128 mmol) is added. The reaction is stirred for a further 3 hours. The reaction is quenched with H:0 and warmed to room temperature. The solution is partitioned between EtOAc and H:0. The organic layer is dried (Na2S04) and evaporated. The product is separated by flash column chromatography (eluant EtOAc:hexane, 1:2) to give the desired product methyl 3- cyano-2-(3-cyclopentyloxy-4-methoxyphenyl)propanoate (6.5 g). M.spec (EI) 303(M*), 235. 195, 176. Η(CDC13) 1.55- 1.65. 1.8-2.0(8H. 2xm. cyclopentyl), 2.75-3.05(2H, . CH,CN), 3.7(3H, s, OCH,), 3.8(3H, s. OCHj), 3.75-3.8UH, m. CHCH2), 4.7-4.8( IH. m. CH ). 6.75-6.9(3H. , C^).
Proceeding in a similar manner, but starting with the appropriate starting material there are prepared:
methyl 3-cyano-2-(7-methoxy-2-methoxymethyl-3H-benzimida7υl-4-yl)propanoate;
methyl 3-cyano-2-(7-methoxy-2-phenyl-3H-benzimidazol-4-yl)propanoate;
methyl 3-cyano-2-(7-methoxy-2-phenethyl-3H-benzimidazol-4-yl)propanoate;
methyl 3-cyano-2-(2-benzyl-7-methoxy-3H-benzimidazol-4-yl) propanoate;
methyl 3-cyano-2-((RS)-7-methoxy-2-(l-phenylethyl)-3H-benzimidazol-4-yl) propanoate;
methyl 3-cyano-2-(7-methoxy-2-(4-methoxybenzyl)-3H-benzimidazol-4-yl) propanoate;
methyl 3-cyano-2-((RS)-2-(cyclohexyl-phenyl-methyl)-7-methoxy-3H-benzimidazol-4- yl)propanoate;
methyl 3-cyano-2-((RS)-2-( 1 ,2-diphenylethyl)-7-methoxy-3H-benzimidazol-4-yl)propanoate;
methyl 3-cyano-2- (RS)-7-methoxy-2-(2-phenylpropyl)-3H-benzimidazol-4-yl)propanoate;
methyl 3-cyano-2- 7-methoxy-2-(4-methoxyphenoxymethyl)-3H-benzimidazol-4-yl)propanoate;
methyl 3-cyano-2- (RS)-7-methoxy-2-(l-phenylbutyl)-3H-benzimidazol-4-yl)propanoate;
methyl 3-cyano-2- 2-(4-bromobenzyl)-7-methoxy-3H-benzimidazol-4-yl)-propanoate;
methyl 3-cyano-2- (RS)-7-methoxy-2-(3-methoxy-l-phenylpropyl)-3H-benzimidazol-4-yl)- propanoate;
methyl 3-cyano-2- 2-(4-cyanobenzyl)-7-methoxy-3H-benzimidazol-4-yl)-propanoate;
methyl 3-cyano-2-' 7-methoxy-2-(4- { -pyridyl }benzyl)-3H-benzimidazoI-4-yl)-propanoate;
methyl 3-cyano-2- 7-methoxy-2-(2-methoxybenzyl)-3H-benzimidazol-4-yl)-propanoate;
methyl 3-cyano-2 (RS)-7-methoxy-2-(methoxyphenylmethyl)-3H-benz-imidazol-4- yl)propanoate;
methyl 3-cyano-2 7-methoxy-2-(2-methoxyphenoxy)methyl-3H-benzimidazol-4-yl)propanoate;
methyl 3-cyano-2- 7-methoxy-2-(3-pyridyl)-3H-benzimidazol-4-yl)propanoate;
methyl 3-cyano-2- 2-isopropyl-7-methoxy-3H-benzimidazol-4-yl)propanoate;
methyl 3-cyano-2- 7-methoxy-2-methyl-3H-benzimidazol-4-yl)propanoate;
methyl 3-cyano-2- 7-methoxy-2-phenoxymethyl-3H-benzimidazol-4-yl)propanoate;
methyl 3-cyano-2 2-cyclopentyl-7-methoxy-3H-benzimidazol-4-yl)propanoate;
methyl 3-cyano-2- 2-benzyl-3H-benzimidazol-4-yl)propanoate;
methyl 3-cyano-2- 2-cyclopentyl-7-methoxy-l-methyl-benzimidazol-4-yl)propanoate;
methyl 3-cyano-2 2-cyclopentyl-7-methoxy-3-methyl-benzimidazol-4-yl)propanoate:
methy 3-cyano-2 2.7-dimethoxy-3H-benzimidazol-4-yl)propanoate;
methy 3-cyano-2- 2-cyclopropyl-7-methoxy-3H-benzimidazol-4-yl)propanoate;
methy 3-cyano-2- l-cyclohexylmethyl-3-methyl-lH-indol-6-yl)propanoate;
methy 3-cyano-2- l-(2-cyclohexyl)ethyl-3-methyl-lH-indol-6-yl)propanoate;
methy 3-cyano-2- l-(3-cyclohexyl)propyl-3-methyl-lH-indol-6-yl)propanoate;
methy 3-cyano-2- l-heptyl-3-methyl-lH-indol-6-yl)propanoate;
methy 3-cyano-2- l-isobutyl-3-methyl-l H-indol-6-yl)propanoate;
methy 3-cyano-2- l-cyclopentylmethyl-3-methyl- lH-indol-6-yl)propanoate;
methy 3-cyano-2- l-benzyl-3-methyl-lH-indol-6-yl)propanoate;
methy 3-cyano-2- l-(3-phenyl)propyl-3-methyl- lH-indol-6-yl)propanoate;
methy 3-cyano-2- l-(3-phenyl)butyl-3-methyl-lH-indol-6-yl)propanoate;
methy 3-cyano-2- l -(4-fluorobenzyl)-3-methyl-lH-indol-6-yl)propanoate;
methy 3-cyano-2- l-(4-chlorobenzyl)-3-methyl- lH-indol-6-yl)propanoate;
methy 3-cyano-2- 1 -(4-methoxy benzyl)-3-methyl- 1 H-indol-6-y propanoate;
methy 3-cvano-2- l-(4-trifluoromethylbenzyl)-3-methyl-lH-indol-6-yl)propanoate;
methy 3-cyano-2- l-(4-methylsulphonylbenzyl)-3-methyl- lH-indol-6-yl)propanoate;
methy 3-cyano-2- l-(l,3-benzodioxol-5-yl)methyl-3-methyl-l H-indol-6-yl)propanoate;
methy 3-cyano-2- l-(naphthalen-2-yl)methyl-3-methyi- lH-indol-6-yl)propanoate;
methy 3-cyano-2 l-(tetrahydro-2H-pyran-2-yl)methyl-3-methyl-lH-indol-6-yl)propanoate;
methyl 3-cyano-2-(3-methyl-l -(tetrahydrofurfuryl)- lH-indol-6-yl)propanoate;
methyl 3-cyano-2-(3-methyl-l-(toluene-4-sulphonyl)-lH-indol-6-yl)propanoate;
methyl 3-cyano-2-(3-methyl-l -(tetrahydrofuran-3-yl)-lH-indol-6-yl)propanoate;
methyl 3-cyano-2-(3-methyl- lH-indol-6-yl)propanoate;
methyl 3-cyano-2-(l -benzyl-3-methylindolin-6-yl)propanoate; and
methyl 3-cyano-2-(l -benzyl-3-methylindazol-6-yl)propanoate;
Reference Example 40 3-(3-cyclopentyloxy-4-methoxyphenyl)pyrrolidine-2- one
Raney-nickel (approx. 2 mL of a 50% slurry in H20) is added to a stirring solution of methyl 3-cyano-2-(3-cyclopentyloxy-4-methoxyphenyl)propanoate (3.1 g) in MeOH (130 mL) and stirred at 50°C under H, for 20 hours. The reaction mixture is cooled to room temperature and filtered through celite. The solvent is evaporated in vacuo and the crude material purified by column chromatography (Eluant EtOAc:Hexane, 1:4 to 100% EtOAc) to yield the product 3-(3- cyclopentyloxy-4-methoxyphenyl)pyrrolidine-2-one 1.6 g) as a white solid, mp 144-145°C. For C.6H:,NO,: Calculated C 69.79 H 7.69 N 5.09; Found C 69.90 H 7.64 N 5.03. M.spec (FAB). Exact mass measurement- Theoretical (M*) 276.1600, measured 276.1610. Η(CDC13) 1.5-1.6, 1.75- 1.95(8H. 2xm. cyclopentyl). 2.15-2.25. 2.5-2.6, 3.4-3.5, 3.5-3.55. 3.6-3.65(5H. 5xm, CH-CFLCR). 3.8(3H,s. OCHj), 4.7-4.75(lH. br s. CH), 6.45(1H, br s, NH), 6.8-6.85(3H. m. QH3).
Proceeding in a similar manner, but starting with the appropriate starting material there are prepared:
3-(7-methoxy-2-methoxymethyl-3H-benzimidazol-4-yl)pyrroIidine-2-one;
3-(7-methoxy-2-phenyl-3H-benzimidazol-4-yl)pyrrolidine-2-one;
3-(7-methoxy-2-phenethyl-3H-benzimidazol-4-yl)pyrrolidine-2-one;
3-(2-benzyl-7-methoxy-3H-benzimidazol-4-yl) pyrrolidine-2-one;
3-((RS)-7-methoxy-2-(l-phenylethyl)-3H-benzimidazol-4-yl)pyrrolidine-2-one;
3- 7-methoxy-2-(4-methoxybenzyl)-3H-benzimidazol-4-yl) pyrrolidine-2-one;
(RS)-2-(cyclohexyl-ρhenyl-methyl)-7-methoxy-3H-benzimidazol-4-yl)ρyrrolidine-2-one;
3- (RS)-2-(l,2-diphenylethyl)-7-methoxy-3H-benzimidazol-4-yl)pyrrolidine-2-one;
(RS)-7-methoxy-2-(2-phenylproρyl)-3H-benzimidazol-4-yl)pyrrolidine-2-one;
3- 7-methoxy-2-(4-methoxyphenoxymethyl)-3H-benzimidazol-4-yl)pyrrolidine-2-one;
(RS)-7-methoxy-2-(l-phenylbutyl)-3H-benzimidazol-4-yl)pyrrolidine-2-one:
2-(4-bromobenzyl)-7-methoxy-3H-benzimidazol-4-yl)-pyrrolidine-2-one;
(RS)-7-methoxy-2-(3-methoxy- l-phenylpropyl)-3H-benzimidazol-4-yl)-pyrrolidine-2-one;
3- 2-(4-cyanobenzyl)-7-methoxy-3H-benzimidazol-4-yl)-pyrrolidinc-2-one;
3- 7-methoxy-2-(4-{ 3-pyridyl }benzyl)-3H-benzimidazol-4-yl)-pyrrolidine-2-one;
7-methoxy-2-(2-methoxybenzyl)-3H-benzimidazol-4-y])-pyrrolidine-2-one;
(RS)-7-methoxy-2-(methoxyphenylmethyl)-3H-benz-imidazol-4- yl)pyrrolidine-2-one;
3- 7-methoxy-2-(2-methoxyphenoxy)methyl-3H-benzimidazol-4-yl)pyrrolidine-2-one;
7-methoxy-2-(3-pyridyl)-3H-benzimidazol-4-yl)pyrrolidinc-2-one;
3- 2-isopropyl-7-methoxy-3H-benzimidazol-4-yl)pyrrolidine-2-one;
3- 7-methoxy-2-methyl-3H-benzimidazol-4-yl)pyrrolidine-2-one;
3- 7-methoxy-2-phenoxymethyl-3H-benzimidazol-4-yl)pyrrolidine-2-one;
3- 2-cyclopentyl-7-methoxy-3H-benzimidazol-4-yl)pyrrolidine-2-one;
2-benzyl-3H-benzimidazol-4-yl)pyrrolidine-2-one;
- I l l -
3-(2-cyclopentyl-7-methoxy-l-methyl-benzimidazol-4-yl)pyrrolidine-2-one;
3-(2-cyclopentyl-7-methoxy-3-methyl-benzimidazol-4-yl)pyrrolidine-2-one;
3-(2,7-dimethoxy-3H-benzimidazol-4-yl)pyrrolidine-2-one;
3-(2-cyclopropyl-7-methoxy-3H-benzimidazol-4-yl)pyrrolidine-2-one;
3-( l-cyclohexylmethyl-3-methyl-lH-indol-6-yl)pyrrolidine-2-one;
3-(l-(2-cyclohexyl)ethyl-3-methyl-lH-indol-6-yl)pyrrolidine-2-one;
3-(l -(3-cyclohexyl)propyl-3-methy]- l H-indol-6-yl)pyrrolidine-2-one;
3-(l-heptyl-3-methyl- l H-indol-6-yl)pyrrolidine-2-one;
3-(l-isobutyl-3-methyI- l H-indol-6-yl)pyrrolidine-2-one;
3-(l-cyclopentylmethyl-3-methyl- l H-indol-6-yl)pyrrolidine-2-one;
3-(l-benzyl-3-methyl- l H-indol-6-yl)pyrrolidine-2-one;
3-( l -(3-phenyl)propyl-3-methyl- l H-indol-6-yl)pyrrolidinc-2-one;
3-(l -(3-phenyl)butyl-3-mcthyl- l H-indol-6-yl)pyrrolidine-2-one;
3-(l -(4-fluorobenzyl)-3-methyl- l H-indol-6-yl)pyrrolidine-2-one;
3-(l-(4-chlorobenzyl)-3-methyl- lH-indol-6-yl)pyrrolidine-2-one;
3-(l-(4-methoxybenzyl)-3-methyl-lH-indol-6-yl)pyrrolidine-2-one;
3-(l-(4-trifluoromethylbenzyl)-3-methyl-lH-indol-6-yl)pyrrolidine-2-one;
3-(l-(4-methylsulphonylbenzyl)-3-methyl-lH-indol-6-yl)pyrrolidine-2-one;
3-(l-(l ,3-benzodioxol-5-yl)methyl-3-methyl-lH-indol-6-yl)pyrrolidine-2-one;
3-(l-(naphthalen-2-yl)methyl-3-methyl-lH-indol-6-yl)pyrrolidine-2-one;
3-(l-(tetrahydro-2H-pyran-2-yl)methyl-3-methyl-lH-indol-6-yl)pyrrolidine-2-one;
3-(3-methyl-l -(tetrahydrofurfuryl)- lH-indol-6-yl)pyrrolidine-2-one;
3-(3-methyl-l-(toluene-4-sulphonyl)-lH-indol-6-yl)pyrrolidine-2-one;
3-(3-methyl-l-(tetrahydro uran-3-yl)-lH-indol-6-yl)pyrrolidine-2-one;
3-(3-methyl-lH-indol-6-yl)pyrrolidine-2-one;
3-(l-benzyl-3-methylindolin-6-yl)pyrrolidine-2-one; and
3-(l -benzyl- 3-methylindazol-6-yl)pyrrolidine-2-one.
Reference Example 41 3-(3-cyclopentyloxy-4-methoxyphenyl)-2- oxopyrrolidine- 1 -carboxylic acid tert-butyl ester
A mixmre of 3-(3-cyclopentyloxy-4-methoxyphenyl)pyrrolidine-2-one (500 mg), (BOC)20 (433 mg). Et3N (0.5 mL) and DMAP (cat) are stirred at room temperamre for 48 hours. The solution is partitioned between ether (20 mL) and 1 N HC1 (20 mL). The organic layer is dried and evaporated in vacuo. The crude material is preabsorbed onto flash silica and purified by column chromatography (eluant Hexane: EtOAc. 4: 1 ) to yield 3-(3-cyclopentyloxy-4- methoxyphenyl)-2-oxopyrrolidine-l -carboxylic acid tert-butyl ester (430 mg) as a white solid, mp 95-96°C. For C21H,,05N: Calculated C 67.18 H 7.79 N 3.73; Found C 67.31 H 7.76 N 3.58. Η(CDC13) 1.5(9H, s. 3X CR,), 1.55-1.60. 1.75-2.0(8H, 2xm, cyclopentyl). 2.05-2.2, 2.4-2.5. 3.6-3.7, 3.8-3.95(5H, 4xm, CHO CH^, 3.8(3H,s,OCH3), 4.7-4.8(lH, m, CH), 6.7-6.9(3H. m, H,).
Proceeding in a similar manner, but starting with the appropriate starting material there are prepared:
3-(7-methoxy-2-methoxymethyl-3H-benzimidazol-4-yl)pyrrolidine-2-one-l -carboxylic acid tert- butyl ester;
3-(7-methoxy-2-phenyl-3H-benzimidazol-4-yl)pyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-(7-methoxy-2-phenethyl-3H-benzimidazol-4-yl)pyrrolidine-2-one- 1 -carboxylic acid tert-butyl ester;
3-(2-benzyl-7-methoxy-3H-benzimidazol-4-yl) pyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-((RS)-7-methoxy-2-(l-phenylethyl)-3H-benzimidazol-4-yl)pyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-(7-methoxy-2-(4-methoxybenzyl)-3H-benzimidazol-4-yl) pyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-((RS)-2-(cyclohexyl-phenyi-methyl)-7-methoxy-3H-benzimidazol-4-yl)pyrrolidine-2-one- l- carboxylic acid tert-butyl ester;
3-((RS)-2-( 1.2-diphenylethyl)-7-methoxy-3H-benzimidazol-4-yl)pyrrolidine-2-one- l-carboxylic acid tert-butyl ester;
3-((RS)-7-methoxy-2-(2-phenylpropyl)-3H-benzimidazol-4-yl)pyrrolidine-2-one-l-carboxylic acid tert-butyl ester;
3-(7-methoxy-2-(4-methoxyphenoxymethyl)-3H-benzimidazol-4-yl)pyrrolidine-2-one- l- carboxylic acid tert-butyl ester;
3-((RS)-7-methoxy-2-(l-phenylbutyl)-3H-benzimidazol-4-yl)pyrrolidine-2-one- l-carboxylic acid tert-butyl ester;
3-(2-(4-bromobenzyl)-7-methoxy-3H-benzimidazol-4-yl)-pyrrolidine-2-one-l -carboxylic acid tert- butyl ester;
3-((RS)-7-methoxy-2-(3-methoxy-l-phenylpropyl)-3H-benzimidazol-4-yl)-pyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(2-(4-cyanobenzyl)-7-methoxy-3H-benzimidazol-4-yl)-pyrrolidine-2-one- 1 -carboxylic acid tert- butyl ester;
3-(7-methoxy-2-(4-{ 3-pyridyl }benzyl)-3H-benzimidazol-4-yl)-pyrrolidine-2-one- l-carboxylic acid tert-butyl ester;
3-(7-methoxy-2-(2-methoxybenzyl)-3H-benzimidazol-4-yl)-pyrrolidine-2-one- 1 -carboxylic acid tert-butyl ester;
3-((RS)-7-methoxy-2-(methoxyphenylmethyl)-3H-benz-imidazol-4- yl)pyrrolidine-2-one-l- carboxylic acid tert-butyl ester;
3-(7-methoxy-2-(2-methoxyphenoxy)methyl-3H-benzimidazol-4-yl)pyrrolidine-2-one-l - carboxylic acid tert-butyl ester;
3-(7-methoxy-2-(3-pyridyl)-3H-benzimidazol-4-yl)pyrrolidine-2-one-l-carboxylic acid tert-butyl ester;
3-(2-isopropyl-7-methoxy-3H-benzimidazol-4-yl)pyrrolidine-2-one- l -carboxylic acid tert-butyl ester;
3-(7-methoxy-2-methyl-3H-benzimidazol-4-yl)pyrrolidine-2-one- l -carboxylic acid tert-butyl ester;
3-(7-methoxy-2-phenoxymethyl-3H-benzimidazol-4-yl)pyrrolidine-2-υrιe- l -carboxylic acid tert- butyl ester;
3-(2-cyclopentyl-7-methoxy-3H-benzimidazol-4-yl)pyrrolidine-2-one- l -carboxylic acid tert-butyl ester;
3-(2-benzyl-3H-bcnzιmιdazol-4-yl )pyrrolidιne-2-one- l-carb()xylic acid tert-butyl ester;
- l -methyl-benzimidazol-4-yl)pyrrolidιne-2-one- l -carboxylic acid tert- butyl ester;
3-(2-cyclopentyl-7-methoxy-3-melhyl-benzimidazol-4-yl)pyrrolidine-2-one- l -carboxylic acid tert- butyl ester;
3-(2.7-dimethoxy-3H-benzimidazol-4-yl)pyrrolidine-2-one- l-carboxylic acid tert-butyl ester;
3-(2-cyclopropyl-7-methoxy-3H-benzimidazol-4-yl)pyrrolidine-2-one- 1 -carboxylic acid tert-butyl ester;
3-(l-cyclohexylmethyl-3-methyl- lH-indol-6-yl)pyrrolidine-2-one- l-carboxylic acid tert-butyl ester;
3-(l-(2-cyclohexyl)ethyl-3-methyl- l H-indol-6-yl)pyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-(l -(3-cyclohexyl)propyl-3-methyl- 1 H-indol-6-yl)pyrrolidine-2-one- 1 -carboxylic acid tert-butyl ester;
3-(l-heptyl-3-methyl-lH-indol-6-yl)pyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-(l-isobutyl-3-methyl-lH-indol-6-yl)pyrrolidine-2-one- l -carboxylic acid tert-butyl ester;
3-( 1 -cyclopentylmethy 1-3-methyl- 1 H-indol-6-yl)pyrrolidine-2-one- 1 -carboxylic acid tert-butyl ester;
3-(l-benzyl-3-mcthyl- l H-indol-6-yl)pyrrolidine-2-one- I -carboxylic acid tert-butyl ester;
3-(l-(3-phenyl)propyl-3-methyl- l H-indol-6-yl)pyrrolidine-2-one- l-carboxylic acid tert-butyl ester;
3-( l-(3-phenyl)butyl-3-methyl- l H-indol-6-yl)pyrrolidine-2-one- l -carboxylic acid tert-butyl ester;
3-(l-(4-fluorobenzyl)-3-methyl- lH-indol-6-yl)pyrrolidine-2-one- l -carboxylic acid tert-butyl ester;
3-(l-(4-chlorobenzyl)-3-methyl- l H-indol-6-yl)pyrrolidine-2-onc- l -carboxylic acid tert-butyl ester;
3-(l-(4-methoxybenzyl)-3-methyl- l H-indol-6-yl)pyrrolidine-2-one- l -carboxylic acid tert-butyl ester;
3-(l-(4-trifluoromethylbenzyl)-3-methyl-lH-indol-6-yl)pyrrolidine-2-one- l -carboxylic acid tert- butyl ester;
3-(l-(4-methylsulphonylbenzyl)-3-methyl-lH-indol-6-yl)pyrrolidinc-2-one- l-carboxylic acid tert- butyl ester;
3-(l-(l ,3-benzodioxol-5-yl)methyl-3-methyl-lH-indol-6-yl)pyrrolidine-2-one- l-carboxylic acid tert-butyl ester;
3-(l-(naphthalen-2-yl)methyl-3-methyl-lH-indol-6-yl)pyrroiidine-2-one-l -carboxylic acid tert- butyl ester;
3-( 1 -(tetrahydro-2H-pyran-2-yl)methyl-3-methyl- 1 H-indol-6-yl)pyrrolidine-2-one- 1 -carboxylic acid tert-butyl ester;
3-(3-methyl- 1 -(tetrahydrofurfuryl)- lH-indol-6-yl)pyrrolidine-2-one-l -carboxylic acid tert-butyl ester;
3-(3-methyl-l-(toluene-4-sulphonyl)-lH-indol-6-yl)pyrrolidine-2-one-l-carboxylic acid tert-butyl ester;
3-(3-methyl-l-(tetrahydrofuran-3-yl)-lH-indol-6-yl)pyrrolidine-2-one-l-carboxylic acid tert-butyl ester;
3-(3-methyl-lH-indol-6-yl)pyrrolidine-2-one-l-carboxylic acid tert-butyl ester;
3-( l-benzyl-3-methylindolin-6-yl)pyrrolidine-2-one-l -carboxylic acid tert-butyl ester; and
3-(l-benzyl-3-methylindazol-6-yl)pyrrolidine-2-one-l -carboxy lie acid tert-butyl ester.
The compounds of formula I exhibit useful pharmacological activity and accordingly are incorporated into pharmaceutical compositions and used in the treatment of patients suffering from certain medical disorders.
More especially, the compounds of formula I are cyclic AMP phosphodiesterase inhibitors, in particular type IV cyclic AMP phosphodiesterase inhibitors. The present invention provides compounds of formula I, and compositions containing compounds of t'ormula I, which are of use in a method for the treatment of a patient suffering from, or subject to, conditions which can be ameliorated or prevented by the administration of an inhibitor of cyclic AMP phosphodiesterase, especially type IV cyclic AMP phosphodiesterase. For example, compounds within the present invention are useful as bronchodilators and asthma-prophylactic agents and agents for the inhibition of eosinophil accumulation and of the function of eosinophils, e.g., for the treatment of inflammatory airways disease, especially reversible airway obstruction or asthma, and for the treatment of other diseases and conditions characterized by, or having an etiology involving, morbid eosinophil accumulation. As further examples of conditions which may be ameliorated or prevented by the administration of inhibitors of cyclic AMP phosphodiesterase. such as compounds of formula I. there may be mentioned inflammatory diseases, such as atopic dermatitis, urticaria, allergic rhinitis, psoriasis, rheumatic arthritis, ulcerative colitis, Crohn's disease, adult respiratory distress syndrome and diabetes insipidus, other proliferative skin diseases such as keratosis and various types of dermatitis, conditions associated with cerebral metabolic inhibition, such as cerebral senility, multi-
infarct dementia, senile dementia (Alzheimer's disease), and memory impairment associated with Parkinson's disease, and conditions ameliorated by neuroprotectant activity, such as cardiac arrest, stroke, and intermittent claudication.
The compounds are also inhibitors of tumor necrosis factor, especially TNF-a. Thus, the present invention provides compounds of formula I. and compositions containing compounds of formula I. which are of use in a method for treating a patient suffering from, or subject to, conditions which can be ameliorated or prevented by the administration of an inhibitor of TNF-a. For example compounds of the present invention are useful in joint inflammation, including arthritis, rheumatoid arthritis and other arthritic conditions such as rheumatoid spondylitis and osteoarthritis.
Additionally, the compounds are useful in treatment of sepsis, septic shock, gram negative sepsis, toxic shock syndrome, acute respiratory distress syndrome, asthma and other chronic pulmonary diseases, bone resorption diseases, reperfusion injury, graft vs. host reaction, allograft rejection and leprosy. Furthermore, the compounds are useful in the treatment of infections such as viral infections and parasitic infections, for example malaria such as cerebral malaria, fever and myalgias due to infection, HIV, AIDS, cachexia such as cachexia secondary to AIDS or to cancer.
Another group of conditions which may be treated with the compounds of formula I includes diseases and disorders of the central nervous system such as brain trauma, ischaemia, Huntington's disease and tardive dyskinaesia.
Other disease states that may be treated with the compounds of formula I include Crohn's disease, ulcerative colitis, pyresis. systemic lupus erythematosus. multiple sclerosis, type I diabetes mellitus, psoriasis. Beςhet's disease, anaphylactoid purpura nephritis, chronic glomerulonephritis. inflammatory bowel disease and leukemia.
A special embodiment of the therapeutic methods of the present invention is the treating of asthma. Another special embodiment of the therapeutic methods of the present invention is the treating of joint inflammation.
According to a further feature of the invention there is provided a method for the treatment of a human or animal patient suffering from, or subject to, conditions which may be ameliorated or prevented by the administration of an inhibitor of cyclic AMP phosphodiesterase, especially type IV cyclic AMP phosphodiesterase, or of TNF, especially TNF-a, for example conditions as hereinbefore described, which comprises the administration to the patient of an effective amount of compound of formula I or a composition containing a compound of formula I. "Effective amount" is meant to describe an amount of compound of the present invention effective in inhibiting cyclic AMP phosphodiesterase and/or TNF and thus producing the desired therapeutic effect.
Reference herein to treatment should be understood to include prophylactic therapy as well as treatment of established conditions.
The present invention also includes within its scope pharmaceutical compositions which comprise pharmaceutically acceptable amount of at least one of the compounds of formula I in association with a pharmaceutically acceptable carrier or excipient.
In practice compounds or compositions for treating according to the present invention may administered by any suitable means, for example, by inhalation, topically, parenterally, rectally or orally, but they are preferably administered orally.
The compounds of formula 1 may be presented in forms permitting administration by the most suitable route and the invention also relates to pharmaceutical compositions containing at least one compound according to the invenϋon which are suitable lor use in human or veterinary medicine. These compositions may be prepared according to the customary methods, using one or more pharmaceutically acceptable adjuvants or excipients. The adjuvants comprise, inter alia, diluents, sterile aqueous media and the various non-toxic organic solvents. The compositions may be presented in the form of tablets, pills, granules, powders, aqueous solutions or suspensions, injectable solutions, elixirs or syrups, and may contain one or more agents chosen from the group comprising sweeteners, flavorings, colorings, or stabilizers in order to obtain pharmaceutically acceptable preparations.
The choice of vehicle and the content of active substance in the vehicle arc generally determined in accordance with the solubility and chemical propenies of the product, the particular mode of administration and the provisions to be observed in pharmaceutical practice. For example, excipients such as lactose, sodium citrate, calcium carbonate, dicalcium phosphate and disintegrating agents such as starch, alginic acids and certain complex silica gels combined with lubricants such as magnesium stearate. sodium lauryl sulfate and talc may be used for preparing tablets. To prepare a capsule, it is advantageous to use lactose and high molecular weight polyethylene glycols. When aqueous suspensions are used they may contain emulsifying agents or agents which facilitate suspension. Diluents such as sucrose, ethanol, polyethylene glycol. propylene glycol, glycerol and chloroform or mixtures thereof may also be used.
For parenteral administration, emulsions, suspensions or solutions of the compounds according to the invention in vegetable oil, for example sesame oil, groundnut oil or olive oil. or aqueous-organic solutions such as water and propylene glycol, injectable organic esters such as ethyl oleate. as well as sterile aqueous solutions of the pharmaceutically acceptable salts, are used. The
solutions of the salts of the products according to the invenϋon are especially useful for administration by intramuscular or subcutaneous injection. The aqueous solutions, also comprising solutions of the salts in pure distilled water, may be used for intravenous administration with the proviso that their pH is suitably adjusted, that they are judiciously buffered and rendered isotonic with a sufficient quantity of glucose or sodium chloride and that they are sterilized by heating, irradiation and or microfiltration.
Topical administration, gels (water or alcohol based), creams or ointments containing compounds of the invention may be used. Compounds of the invention may be also incoφorated in a gel or matrix base for application in a patch, which would allow a controlled release of compound through transdermal barrier.
For administration by inhalation, compounds of the invention may be dissolved or suspended in a suitable carrier for use in a nebulizer or a suspension or solution aerosol, or may be absorbed or adsorbed onto a suitable solid carrier for use in a dry powder inhaler.
Solid compositions for rectal administration include suppositories formulated in accordance with known methods and containing at least one compound of formula I.
The percentage of active ingredient in the compositions of the invention may be varied, it being necessary that it should constitute a proportion such that a suitable dosage shall be obtained. Obviously, several unit dosage forms may be administered at about the same time. A dose employed may be determined by a physician or qualified medical professional, and depends upon the desired therapeutic effect, the route of administration and the duration of the treatment, and the condition of the patient. In the adult, the doses are generally from about 0.001 to about 50. preferably about 0.001 to about 5. mg/kg body weight per day by inhalation, from about 0.01 to about 100, preferably 0.1 to 70, more especially 0.5 to 10, mg/kg body weight per day by oral administration, and from about 0.001 to about 10, preferably 0.01 to 1, mg/kg body weight per day by intravenous administration. In each particular case, the doses are determined in accordance with the factors distinctive to the patient to be treated, such as age, weight, general state of health and other characteristics which can influence the efficacy of the compound according to the invention.
The compounds according to the invention may be administered as frequently as necessary in order to obtain the desired therapeutic effect. Some patients may respond rapidly to a higher or lower dose and may find much weaker maintenance doses adequate. For other patients, it may be necessary to have long-term treatments at the rate of 1 to 4 doses per day, in accordance with the physiological requirements of each particular patient. Generally, the active product may be
administered orally 1 to 4 times per day. Of course, for other patients, it will be necessary to prescribe not more than one or two doses per day.
The compounds of the present invention may also be formulated for use in conjunction with other therapeutic agents such as agents which increase cyclic AMP production including b-agonists and PGE2. It is understood that the present invention includes combinations of compounds of the present invention with one or more of the aforementioned therapeutic agents
Compounds within the scope of the present invention exhibit marked pharmacological activities according to tests described in the literature which tests results are believed to correlate to pharmacological activity in humans and other mammals. The following pharmacological in vitro and in vivo test results are typical for characterizing compounds of the present invention.
IN VITRO AND IN VIVO TEST PROCEDURES
1. (a) Inhibitory effects of compounds on PDE IV activity.
1.1 Preparation of PDE from guinea pig macrophages.
The method is described in Turner et al. Br. J. Pharmacol., 108, 876 (1993). Briefly, cells are harvested from the peritoneal cavity of horse-serum treated (0.5 mL i.p.) Dunkin Hartley guinea pigs (250-400 g) and the macrophages purified by discontinuous (55%. 6 %, 70% v/v) gradient (Percoll) centrifugation. Washed macrophages are plated out in cell culture flasks and allowed to adhere. The cells are washed with Hank's balanced salt solution, scraped from the flasks and centrifuged (1000 g). The supernatant is removed and the pellets stored at -80°C until use. The pellet is homogenized in 20 mM tris(hydroxymethyl)aminomethane HC1, pH 7.5, 2 mM magnesium chloride. 1 mM dithiothreitol, 5 mM ethylenediaminetetraacetic acid, 0.25 M sucrose, 20 mM p-tosyl-L- lysine chloromethyl ketone, 10 mg/mL leupeptin and 2000 U/mL aprotinin.
1.2 Measurement of PDE activity.
PDE activity is determined in macrophage homogenates by the two-step radioisotopic method of Thompson et al., (Adv. Cyclic Nucl. Res., 10, 69 (1979). The reaction mixmre contains 20 mM tris(hydroxymethyl)aminomethane HC1 (pH 8), 10 mM magnesium chloride, 4 mM 2-mercaptoethanol, 0.2 mM ethylenebis(oxyethylenenitrilo)tetraacetic acid and 0.05 mg of bovine serum albumin/mL. The concentration of substrate is 1 μM. The IC50 values (i.e. concentrations which produce 50% inhibition of substrate hydrolysis) for the compounds examined are determined from concentration-response curves in which concentrations range from 0.1 nM to 40 μM.
1.3 Preparation of PDE from human platelets.
The method is described in R.E. Weishaar et al. (Biochem.Pharmacol., 35, 787
(1986).
1.4 Measurement of PDE activity. PDE activity is determined by the radioisotopic method of Thompson et al., (Adv. Cyclic
Nucl. Res., 10, 69 (1979). Following incubation for 30 minutes at 30°C [3H]-Guanosine 5'-monophosphate is separated from the substrate, guanosine [3H]-guanosine 3':5'-cyclic monophosphate, by elution on cation-exchange columns, and radioactivity is determined using a liquid scintillation counter (LS 1701, Beckman) using a liquid scintillation cocktail (Flow Scint III, Packard). The concentration of substrate is 1 μM. The IC50 values (i.e. concentrations which produce 50% inhibition of substrate hydrolysis) for the compounds examined are determined from concentration-response curves in which concentrations range from 10'9M to 10"5M.
2. In vivo bronchodilator actions of compounds. 2.1 Measurement of bronchodialation.
Bronchorelaxant activity is measured in in vivo tests in the anaesthetized guinea-pig or rat according to the method described in Underwood et al., Pulm. Pharmacol. 5. 203 (1992) in which the effects on bronchospasm induced by bistamine (or other spasmogens such as methacholine or leukotriene D4) is determined. Compounds are administered orally 1 hour prior to administration of spasmogen.
3. In vivo actions of compounds on antigen (ovalbamin)-induced eosinophilia in guinea-pigs.
3.1 Treatment of animals and measurement of eosinophil numbers. Male Dunkin-Hartley guinea-pigs weighing 200-250g are sensitized using 10 μg ovalbumin in 1 mL of a 100 mg/mL suspension of aluminum hydroxide, i.p. 28 days after sensitization guinea-pigs are dosed orally. 23 Hours later this procedure is repeated and 60 minutes later the guinea-pigs are challenged with nebulized saline or ovalbumin (1% in saline) for 15 seconds. 24 Hours after challenge the guinea-pigs are killed and the lungs are lavaged with warm saline. Total and differential cell counts are made.
4. Inhibitory effects of compounds against antigen-induced eosinophilia in the rat in vivo. 4.1. Treatment of rats and measurement of eosinophil numbers.
Male Brown Norway rats weighing 150-250 g are sensitized on days 0, 12 and 21 with ovalbumin (100 μg. i.p.). Rats are challenged on any one day between days 27-32. 24 hours and 1 hour before antigen challenge rats are dosed orally. Rats are challenged by exposure for 30 minutes to Nebulized saline or ovalbumin (1% in saline). 24 hours after challenge, rats are killed and the airways are lavaged with physiological salt solution. Total and differential cell counts are made.
5. In Vitro Inhibitory Effects on TNF-alpha Release by Human Monocytes
The effects of compounds on TNF-alpha production by human peripheral blood monocytes (PBMs) are examined as follows.
5.1. Preparation of blood leukocytes.
Blood is drawn from normal donors, mixed with dextran, and the erythrocytes allowed to sediment for 35 minutes at 37°C. Leukocytes are fractionated by centrifugation through a discontinuous (18, 20 and 22%) metrizamide gradient. The mononuclear cell fraction comprising 30-40% PBMs is suspended in Hank's balanced salt solution and stored at 4°C until use.
5.2. Measurement of TNF-alpha.
Cells from the PBM-rich metrizamide fraction are spun down (200 g for 10 minutes at 20°C), resuspended at 106PBMs/mL of medium; RPMI 1640 containing l%v/v FCS, 50U/mL penicillin and 50 mg mL streptomycin (Gibco. U.K.), then plated out in 96 well plates at 2xl05 cells/well. The medium (200 μL) is changed to remove any non-adherent cells and the remaining, adherent PBMs left in the incubator overnight (18 hours). One hour prior to challenge, the medium is changed to that containing compound for test or drug vehicle. Control treatments and compounds for test are assayed in quadruplicate wells. Compounds are tested within the concentration range of 3X1 " !0M to 3X10""6M. Medium (50 μL) with or without 10 ng/mL LPS (E. Coli. 055 B5 from Sigma. U.K.) is then added. The incubation is then continued for a further 4 hours. Cell supematants are removed for storage at -20°C.
TNF-alpha levels in cell supematants are quantified using a standard sandwich ELISA technique. ELISA plates (Costar, U.K.) are coated overnight at 4°C with 3 mg/mL polyclonal goat anti-human TNF-alpha antibody (British Biotechnology, U.K.) in pH 9.9 bicarbonate buffer. Rabbit polyclonal anti-human TNF-alpha antiserum (Janssen Biochimicha. Belgium) at 1/500 dilution is used as the second antibody and polyclonal goat anti-rabbit IgG horseradish peroxidase (Calbiochem, U.S.A.) at 1/8000 dilution is used as the detection antibody. Color development is measured by absorbance at 450 nm using a Titek plate reader.
TNF-alpha levels are calculated by interpolation from a standard curve using recombinant human TNF-alpha (British Biotechnology U.K.)(0.125-8 ng/mL). Data (log-cone. vs. log-resp) are fitted by linear regression (p > 0.99) using a Multicalc (Wallac Pharmacia, U.K.) software program. Basal TNF-alpha levels are less than 100 pg/mL whilst LPS (lipopoly-saccharide) stimulation of the PBMs increases TNF-alpha levels to 3-10 ng/mL.
6. Inhibitory effects of compounds on antigen-induced bronchoconstriction in the conscious guinea-pig.
6.1 Sensitization of guinea-pigs and measurement of antigen-induced bronchoconstriction.
Male Dunkin-Hartley guinea-pigs (550-700 g) are sensitized as above. Specific airways resistance (SRaw) is measured in conscious animals by whole body plethysmography using a variation of the method of Pennock et al., J. Appl. Physiol., 46,399 (1979). Test compounds or vehicle are administered orally 24 hours and 1 hour before antigen challenge. 30 Minutes before
challenge the animals are injected with mepyramine (30 mg/kg i.p.) to prevent anaphylactic collapse and placed into the plethysmography chambers where SRaw is determined at 1 minute intervals. Resting SRaw is then determined. Animals are challenged with an aerosol of ovalbumin and SRaw is determined every 5 minutes for 15 minutes.
7. Inhibitory effects of compounds against antigen-induced bronchoconstriction in the anaesthetized rat in vivo.
7.1. Treatment of rats and measurement of antigen-induced bronchoconstriction.
Male Brown Norway rats weighing 150-250 g are sensitized on days 0, 12 and 21 with ovalbumin (100 μg, i.p.). Rats are challenged on any one day between days 27-32. 24 hours and 1 hour before antigen challenge rats are dosed orally. Rats are anaesthetized to allow recording of lung function (airway resistance and lung compliance) using respiratory mechanics software. Rats are challenged with ovalbumin i.v. and the peak changes in airway resistance and lung compliance are determined.
8. Inhibitory effects of compounds on serum TNF-alpha levels in LPS-challenged mice. 8.1. Treatment of animals and measurement of murine TNF-alpha.
Female Balb/c mice (age 6-8 weeks, weight 20-22 g from Charles River, U.K.) in groups of five or more animals are dosed p.o. with compounds suspended in 1.5% (w/v) carboxymethyl cellulose then challenged after a minimum period of 30 minutes with 30 mg of LPS i.p. After 90 minutes the animals are killed by carbon dioxide asphyxiation and bled by cardiac puncture. Blood is allowed to clot at 4°C. centrifuged (12,000 g for 5 minutes) and scrum taken for TNF-alpha analysis. TNF-alpha levels are measured using a commercially available murine TNF-alpha ELISA kit. purchased from Genzyme (Cat. no. 1509.00), as recommended by the manufacturer. Values for TNF-alpha are calculated from a recombinant murine TNF-alpha standard curve.
9. Systemic bioavailabiliiy in female Balb/c mouse. Intravenous administration :
Following surgery lo expose the jugular vein for dosing, a solution of test compound in dimethylsulphoxide is added at a dose of 1 mg/kg body weight. Oral administration :
A suspension of test compound in 1.5% aqueous carboxymethylcellulose is introduced into the stomach by gavage at a dose of 1 mg/kg body weight. Following either i.v. or oral dosing, blood is obtained by cardiac puncture following carbon dioxide asphyxiation and is obtained at a single time post-dose for each animal. Three animals are sacrificed at each time point. Blood samples are obtained at the following times after dosing by both the i.v. and oral routes; 5 minutes (i.v. only), 0.25, 0.5, 1, 2. 3, 4, 5.5, 7 and 24 hours. Corresponding plasma is obtained by centrifugation of each blood sample. The drug content in the plasma samples is then determined using conventional methods. 9.1 Metabolism.
(i) Preparation of mouse liver homogenate
Fresh mouse liver is homogenized in sucrose-phosphate buffer. Following centrifugation the resulting supernatant (liver homogenate) is used fresh or frozen in liquid nitrogen for one minute and stored at -30°C to -40°C prior to use. (ii) Incubation of compounds with mouse liver homogenate
To 0.5 mL of mouse liver homogenate is added 0.5 mL taken from a vortexed mixture of 8 mg NADPH added to a mixture of aqueous magnesium chloride (1 mL, 0.15 M) nicotinamide (1 mL, 0.5M) and pH 7.4 tris buffer (8.5 mL, 0.1 M). The compound is added at a concentration of 1 mg mL in 10 mL of solvent. Incubates are maintained at 37°C. Samples are taken at 0 minutes, 5 minutes, 10 minutes. 20 minutes and 30 minutes and the incubation stopped by the addition of 100 mL acetonitrile. The drug content in the incubation samples is determined using conventional methods.
10. Streptococcal Cell Wall-Induced Arthritis in Rats. 10.1 Preparation of S. pyogenes purified cell wall.
Purified S. pyogenes cell wall is prepared from the cell pellet of a log-phase culture of S. pyogenes, group A, strain D-58. The whole bacteria are homogenized by grinding with glass beads and the crude cell wall collected by centrifugation and subsequently washed with 2% sodium dodecyl sulphate in phosphate buffered saline followed by phosphate buffered saline to remove contaminating proteins and nucleic acids. The cell wall is further purified by sonication and differential centrifugation to obtain a purified preparation which pelleted at 100,000 g. This material is suspended in sterile phosphate buffered saline and the quantity of cell wall determined by measuring the rhamnose content of the preparation (purified cell wall contains 28% rhamnose by weight). The material is filtered through a 0.22 mM filter and stored at 4°C until used for arthritis induction.
10.2 Arthritis Induction and measurement of joint diameters.
Female Lewis rats weighing 140-160 g are injected intra-articularly into the left or right tibio-tarsal joint on day 0 with purified S. pyogenes cell wall extract (10 mg in 10 mL sterile saline). On day 20, rats received an intravenous injection of purified cell wall (100 mg in 100 mL sterile saline) via the lateral vein of the tail. Joint diameters are measured with calipers across the lateral and medial malleoli of the previously intra-articularly injected joint immediately prior to the i.v. injection and then daily through day 24. The net joint diameter is determined by subtracting the value for the contralateral joint. Body weights are also measured daily. Compounds or vehicle are administered by oral gavage on days 20-23. Typically. 8-10 animals are used per group. For each dose, the total daily dose is divided into two equal aliquots which are given at approximately 9 a.m. and 3 p.m.
The present invention may be embodied in other specific forms without departing from the spirit or essential attributes thereof.