KR101576921B1 - Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same - Google Patents
Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same Download PDFInfo
- Publication number
- KR101576921B1 KR101576921B1 KR1020130102993A KR20130102993A KR101576921B1 KR 101576921 B1 KR101576921 B1 KR 101576921B1 KR 1020130102993 A KR1020130102993 A KR 1020130102993A KR 20130102993 A KR20130102993 A KR 20130102993A KR 101576921 B1 KR101576921 B1 KR 101576921B1
- Authority
- KR
- South Korea
- Prior art keywords
- group
- compound
- substituted
- unsubstituted
- organic electroluminescent
- Prior art date
Links
Images
Classifications
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6572—Polycyclic condensed heteroaromatic hydrocarbons comprising only nitrogen in the heteroaromatic polycondensed ring system, e.g. phenanthroline or carbazole
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/22—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed systems contains four or more hetero rings
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
- H10K50/12—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers comprising dopants
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
Landscapes
- Chemical & Material Sciences (AREA)
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Optics & Photonics (AREA)
- Electroluminescent Light Sources (AREA)
Abstract
유기전계발광소자용 화합물 및 그를 포함하는 유기전계발광소자가 제공된다. 이에 의하여, 전기적 안정성 및 전자와 정공 수송능력이 우수하며, 삼중항 상태 에너지가 높아 인광발광재료의 발광효율을 향상 시킬 수 있는 호스트, 정공수송 물질 및 전자수송 물질로 사용할 수 있는 유기전계발광소자용 화합물 및 유기전계발광소자를 제공할 수 있다.An organic electroluminescent device compound and an organic electroluminescent device comprising the same are provided. As a result, it can be used as a host, a hole transporting material, and an organic light emitting device which can be used as an electron transporting material, which can improve the luminous efficiency of a phosphorescent light emitting material because of its excellent electrical stability, electron and hole transporting ability, A compound and an organic electroluminescent device can be provided.
Description
본 발명은 유기전계발광소자용 화합물 및 그를 포함하는 유기전계발광소자에 관한 것으로, 보다 상세하게는 유기전계발광소자의 발광효율을 향상시킬 수 있는 유기전계발광소자용 화합물 및 그를 포함하는 유기전계발광소자에 관한 것이다.BACKGROUND OF THE INVENTION 1. Field of the Invention The present invention relates to a compound for an organic electroluminescent device and an organic electroluminescent device including the same, and more particularly to a compound for an organic electroluminescent device capable of improving the luminous efficiency of the organic electroluminescent device, Device.
정보화 사회로의 움직임이 가속화되면서 평판 디스플레이의 비중이 점차 증가하고 있다. 그 중 LCD(liquid crystal display)가 현재 가장 많이 쓰이고 있지만 액정에 전압을 가해 백라이트로부터의 빛을 컬러필터로 통과시켜 삼원색을 얻음으로써 화면을 만드는 방식이며, 유기EL(OLED; Organic Light Emitting Diodes)은 자체발광 소자로써 시야각 및 대조비 등이 우수하고, 경량 및 박형이 가능하며 휘는 성질의 기판에도 사용할 수 있어, 투명, 플렉서블 디스플레이가 가능하여 차세대 표시소자로서 주목을 받고 있다.As the move to the information society accelerates, the proportion of flat panel displays is gradually increasing. LCD (liquid crystal display) is the most widely used method, but it is a method to make a screen by applying voltage to liquid crystal and passing light from backlight through color filter to obtain three primary colors. Organic light emitting diodes (OLED) As a self-luminous element, it has excellent viewing angle and contrast ratio, is lightweight and thin, can be used for a substrate having a bending property, and is capable of transparent and flexible display, and has attracted attention as a next generation display element.
유기EL은 유기물 박막에 음극과 양극을 통하여 주입된 전자와 정공이 재결합을 통해 여기자를 형성하고, 형성된 그 여기자로부터 특정한 파장의 빛이 발생하는 현상으로 1963년 Pope 등에 의해 안트라센(anthracene)의 단결정으로부터 처음으로 발견되었고 이후 이스트만 코닥사의 탕(C.W Tang)등에 의해 적층형의 유기EL 소자의 보고(C.W Tang, S.A Vanslyke, Applied physics Letters. 51권 913p, 1987) 된 이후 활발히 연구 되고 있다.Organic EL is a phenomenon in which excitons are formed by recombination of electrons and holes injected through a cathode and an anode into an organic thin film, and light of a specific wavelength is generated from the excitons formed. In 1963, Pope et al. Reported that an anthracene single crystal (CW Tang, SA Vanslyke, Applied Physics Letters, Vol. 51, No. 913p, 1987) by Eastman Kodak Co., Ltd. (CW Tang) et al.
유기전계발광소자에 사용되는 유기물질은 크게 고분자와 저분자 형태로 나누어 지며 저분자는 순 유기물질과 메탈과 킬레이트를 형성한 메탈 콤플렉스로 나뉘어 질 수 있다.Organic materials used in organic electroluminescent devices are classified into polymer and small molecule, and small molecules can be divided into pure organic material and metal complex which forms metal and chelate.
고분자 물질은 다양한 기능의 유닛을 고분자 체인에 결합하여 다 기능의 물질을 만들어 낼 수 있으나, 합성물 정제시나 소자 형성 시 어려움이 있고, 저분자 물질은 각 특성의 물질을 합성할 수 있으나 다 기능의 특성을 나타내는 물질 합성에는 한계가 있다 하겠다. Polymers can combine diverse functional units into polymer chains to produce multifunctional materials. However, it is difficult to purify compounds or to form devices, while low-molecular materials can synthesize materials of various properties. There is a limit to the synthesis of the substances.
유기전계발광소자를 적층구조로 형성할 수 있다. 적층구조의 장점으로는 각 기능에 맞게끔 물질을 선택하여 사용할 수 있는 것인데, 일반적으로 소자구조는 양극과 음극 사이에 정공주입층, 정공수송층, 발광층, 전자수송층, 전자주입층을 형성하여 발광층에서 여기자 형성을 쉽게 하게 하고, 발광 효율을 높일 수 있다.The organic electroluminescent device can be formed in a laminated structure. In general, the device structure is formed by forming a hole injecting layer, a hole transporting layer, a light emitting layer, an electron transporting layer, and an electron injecting layer between an anode and a cathode to form a light emitting layer The exciton formation can be facilitated and the emission efficiency can be enhanced.
발광물질은 호스트물질과 발광물질(도펀트)물질로 크게 나눌 수 있고, 발광물질은 발광 기작에 따라 형광과 인광으로 구별된다. The luminescent material can be roughly divided into a host material and a luminescent material (dopant) material, and the luminescent material is distinguished by fluorescence and phosphorescence depending on the luminescence mechanism.
화합물 내 전자의 여기 상태는 일중항 대 삼중항의 비율이 1:3으로 삼중항 상태가 3배 정도 더 생성된다. 따라서, 일중항 상태에서 기저상태로 떨어지는 형광의 내부양자효율이 25%에 그치는 반면 삼중항 상태에서 기저상태로 떨어지는 인광의 내부양자효율은 75%이다. 또한 일중항 상태에서 삼중항 상태로 계간전이가 일어날 경우 내부양자효율의 이론적 한계치는 100%에 달한다. 이러한 점을 이용해 발광효율을 개선한 발광재료가 인광 발광재료이다.The excited state of the electrons in the compound is 1: 3 ratio of singlet to triplet, and triplet state is generated about 3 times more. Thus, the internal quantum efficiency of phosphorescence falling from the triplet state to the base state is 75% while the internal quantum efficiency of the fluorescence falling from the singlet state to the ground state is only 25%. In addition, the theoretical limit of internal quantum efficiency reaches 100% when the interplanar transition from singlet state to triplet state occurs. A light emitting material that improves the light emitting efficiency by using this point is a phosphorescent material.
유기물의 특성상 인광 발광은 어려움이 있어, 인광 발광재료로는 전이금속(이리듐)을 이용환 유기금속화합물이 개발되고 있으며, 이를 보조하는 호스트 물질로 유기물질이 이용되고 있다. 인광발광물질을 보조하는 물질(호스트)는 밴드갭이 넓고 삼중항 상태 에너지가 높아야 한다. 전류효율과 발광효율이 우수한 인광물질이 각광을 받고 있으나 전자 수송능력과 홀 수송능력, 열적, 전기적으로 안정한 호스트 물질과 정공이 여기자를 형성할 때까지 유지되고, 전자 수송능력이 뛰어난 유기전계발광소자용 화합물의 개발이 필요한 실정이다.Due to the nature of organic materials, it is difficult to emit phosphorescence. As a phosphorescent material, transition metal (iridium) is utilized as an organometallic compound, and an organic material is used as a host material to assist it. The material that assists the phosphorescent material (host) should have a wide bandgap and a high triplet state energy. A phosphorescent material having excellent current efficiency and luminous efficiency is received in the spotlight, but the electron transporting ability, the hole transporting ability, the host material which is thermally and electrically stable, and the organic electroluminescent device It is necessary to develop a compound for use.
본 발명은 전기적 안정성 및 전자와 정공 수송능력이 우수하며, 삼중항 상태 에너지가 높아 인광발광재료의 발광효율을 향상시킬 수 있는 호스트로서 발광층에 사용될 수 있는 유기발광소자용 화합물 및 이를 포함하는 유기전계발광소자를 제공할 수 있다.The present invention relates to a compound capable of being used for a light emitting layer as a host which is excellent in electrical stability, electron and hole transporting ability, and has high triplet state energy and can improve the luminous efficiency of a phosphorescent light emitting material, A light emitting element can be provided.
또한 본 발명은 유기전계발광소자의 전자수송재료나, 정공수송재료에 사용될 수 있는 유기발광소자용 화합물 및 이를 포함하는 유기전기 발광소자를 제공할 수 있다.In addition, the present invention can provide an organic electroluminescent compound that can be used for an electron transporting material and a hole transporting material of an organic electroluminescent device, and an organic electroluminescent device including the same.
그러나, 본원이 해결하고자 하는 과제는 이상에서 언급한 과제로 제한되지 않으며, 언급되지 않은 또 다른 과제들은 아래의 기재로부터 당업자에게 명확하게 이해될 수 있을 것이다.However, the problems to be solved by the present invention are not limited to the above-mentioned problems, and other problems not mentioned can be clearly understood by those skilled in the art from the following description.
본 발명의 일 측면에 따르면, 하기 구조식 1로 표시되는 유기전계발광소자용 화합물이 제공될 수 있다. According to an aspect of the present invention, a compound for an organic electroluminescence device represented by the following structural formula 1 may be provided.
[구조식 1][Structural formula 1]
상기 구조식 1에서,In the above formula 1,
X1 및 X2는 서로 같거나 다를 수 있고, X1 및 X2는 각각 독립적으로 질소원자 또는 이고, X 1 and X 2 may be the same or different from each other, X 1 and X 2 are each independently a nitrogen atom or ego,
R5는 수소원자, 치환 또는 비치환된 C1 내지 C30 알킬기, 치환 또는 비치환된 C3 내지 C30 시클로알킬기, 치환 또는 비치환된 C1 내지 C30 헤테로시클로알킬기, 치환 또는 비치환된 C6 내지 C30 아릴기, 또는 치환 또는 비치환된 C1 내지 C30 헤테로아릴기이거나, 또는 R5는 R5가 결합된 탄소원자의 이웃한 탄소원자와 추가로 결합하여 치환 또는 비치환된 융합된 C3 내지 C30 시클로알킬기, 치환 또는 비치환된 융합된 C1 내지 C30 헤테로시클로알킬기, 치환 또는 비치환된 융합된 C6 내지 C30 아릴기, 또는 치환 또는 비치환된 융합된 C1 내지 C30 헤테로 아릴기를 형성할 수 있고,R 5 represents a hydrogen atom, a substituted or unsubstituted C1 to C30 alkyl group, a substituted or unsubstituted C3 to C30 cycloalkyl group, a substituted or unsubstituted C1 to C30 heterocycloalkyl group, a substituted or unsubstituted C6 to C30 aryl group, or a substituted or unsubstituted or C1 to C30 heteroaryl ring, or R 5 is R 5 is the combination with the adjacent carbon atoms and more carbon atoms substituted or unsubstituted fused bonding a C3 to C30 cycloalkyl group, a substituted or unsubstituted A substituted or unsubstituted fused C1 to C30 heterocycloalkyl group, a substituted or unsubstituted fused C6 to C30 aryl group, or a substituted or unsubstituted fused C1 to C30 heteroaryl group,
R1 내지 R4는 서로 같거나 다를 수 있고, R1 내지 R4는 각각 독립적으로 수소원자, 치환 또는 비치환된 C1 내지 C30 알킬기, 치환 또는 비치환된 C3 내지 C30 시클로알킬기, 치환 또는 비치환된 C1 내지 C30 헤테로시클로알킬기, 치환 또는 비치환된 C6 내지 C30 아릴기, 또는 치환 또는 비치환된 C1 내지 C30 헤테로아릴기이거나, 또는 R1 내지 R4 중 적어도 어느 하나는 그 어느 하나가 결합된 탄소원자의 이웃한 탄소원자와 추가로 결합하여 치환 또는 비치환된 융합된 C3 내지 C30 시클로알킬기, 치환 또는 비치환된 융합된 C1 내지 C30 헤테로시클로알킬기, 치환 또는 비치환된 융합된 C6 내지 C30 아릴기, 또는 치환 또는 비치환된 융합된 C1 내지 C30 헤테로 아릴기를 형성할 수 있다.R 1 to R 4 may be the same or different from one another and each of R 1 to R 4 independently represents a hydrogen atom, a substituted or unsubstituted C1 to C30 alkyl group, a substituted or unsubstituted C3 to C30 cycloalkyl group, A substituted or unsubstituted C6 to C30 aryl group, or a substituted or unsubstituted C1 to C30 heteroaryl group, or R < 1 > to R < 4 > At least one of them is a fused C3 to C30 cycloalkyl group, a substituted or unsubstituted fused C1 to C30 heterocycloalkyl group, a substituted or unsubstituted fused C3 to C30 heterocycloalkyl group, Or an unsubstituted fused C6 to C30 aryl group, or a substituted or unsubstituted fused C1 to C30 heteroaryl group.
본 발명의 일 실시예에 따르면, 바람직하게는,According to an embodiment of the present invention, preferably,
상기 유기전계발광소자용 화합물은 하기 구조식 2로 표시되고,The organic electroluminescent device compound is represented by the following structural formula 2,
[구조식 2][Structural formula 2]
상기 구조식 2에서,In the above formula 2,
R1 내지 R4는 서로 같거나 다를 수 있고, R1 내지 R4는 각각 독립적으로 수소원자, 치환 또는 비치환된 C1 내지 C30 알킬기, 치환 또는 비치환된 C3 내지 C30 시클로알킬기, 치환 또는 비치환된 C1 내지 C30 헤테로시클로알킬기, 치환 또는 비치환된 C6 내지 C30 아릴기, 또는 치환 또는 비치환된 C1 내지 C30 헤테로아릴기일 수 있다.R 1 to R 4 may be the same or different from one another and each of R 1 to R 4 independently represents a hydrogen atom, a substituted or unsubstituted C1 to C30 alkyl group, a substituted or unsubstituted C3 to C30 cycloalkyl group, Substituted C1 to C30 heterocycloalkyl groups, substituted or unsubstituted C6 to C30 aryl groups, or substituted or unsubstituted C1 to C30 heteroaryl groups.
본 발명의 다른 실시예에 따르면, 바람직하게는,According to another embodiment of the present invention, preferably,
R1 내지 R4는 서로 같거나 다를 수 있고, R1 내지 R4는 각각 독립적으로 수소원자, , , , , , , , , 치환 또는 비치환된 C1 내지 C30 알킬기, 치환 또는 비치환된 C3 내지 C30 시클로알킬기, 또는 치환 또는 비치환된 C1 내지 C30 헤테로시클로알킬기이고,R 1 to R 4 may be the same or different from each other, and R 1 to R 4 are each independently a hydrogen atom, , , , , , , , , A substituted or unsubstituted C1 to C30 alkyl group, a substituted or unsubstituted C3 to C30 cycloalkyl group, or a substituted or unsubstituted C1 to C30 heterocycloalkyl group,
X3 내지 X19는 서로 같거나 다를 수 있고, X3 내지 X19는 각각 독립적으로 질소원자 또는 이고, X 3 to X 19 may be the same or different from each other, and each of X 3 to X 19 independently represents a nitrogen atom or ego,
R27은 수소원자, 치환 또는 비치환된 C1 내지 C30 알킬기, 치환 또는 비치환된 C3 내지 C30 시클로알킬기, 치환 또는 비치환된 C1 내지 C30 헤테로시클로알킬기, 치환 또는 비치환된 C6 내지 C30 아릴기, 또는 치환 또는 비치환된 C1 내지 C30 헤테로아릴기이고,R 27 represents a hydrogen atom, a substituted or unsubstituted C1 to C30 alkyl group, a substituted or unsubstituted C3 to C30 cycloalkyl group, a substituted or unsubstituted C1 to C30 heterocycloalkyl group, a substituted or unsubstituted C6 to C30 aryl group, Or a substituted or unsubstituted C1 to C30 heteroaryl group,
Y1 및 Y2는 서로 같거나 다를 수 있고, Y1 및 Y2는 각각 독립적으로 산소원자, 황원자, , 또는 이고,Y 1 And Y 2 may be the same or different from each other, and Y 1 And Y 2 each independently represent an oxygen atom, a sulfur atom, , or ego,
Ar1은 치환 또는 비치환된 C1 내지 C30 알킬기, 치환 또는 비치환된 C3 내지 C30 시클로알킬기, 치환 또는 비치환된 C1 내지 C30 헤테로시클로알킬기, 치환 또는 비치환된 C6 내지 C30 아릴기, 또는 치환 또는 비치환된 C1 내지 C30 헤테로아릴기이고,Ar 1 represents a substituted or unsubstituted C1 to C30 alkyl group, a substituted or unsubstituted C3 to C30 cycloalkyl group, a substituted or unsubstituted C1 to C30 heterocycloalkyl group, a substituted or unsubstituted C6 to C30 aryl group, An unsubstituted C1 to C30 heteroaryl group,
R28 및 R29는 서로 같거나 다를 수 있고, R28 및 R29는 각각 독립적으로 수소원자, 치환 또는 비치환된 C1 내지 C30 알킬기, 치환 또는 비치환된 C3 내지 C30 시클로알킬기, 치환 또는 비치환된 C1 내지 C30 헤테로시클로알킬기, 치환 또는 비치환된 C6 내지 C30 아릴기, 또는 치환 또는 비치환된 C1 내지 C30 헤테로아릴기이고,R 28 and R 29 may be the same or different from each other, and R 28 and R 29 are each independently a hydrogen atom, a substituted or unsubstituted C1 to C30 alkyl group, a substituted or unsubstituted C3 to C30 cycloalkyl group, A substituted or unsubstituted C6 to C30 aryl group, or a substituted or unsubstituted C1 to C30 heteroaryl group,
R6 내지 R26은 서로 같거나 다를 수 있고, R6 내지 R26은 각각 독립적으로 수소원자, 치환 또는 비치환된 C1 내지 C30 알킬기, 치환 또는 비치환된 C3 내지 C30 시클로알킬기, 치환 또는 비치환된 C1 내지 C30 헤테로시클로알킬기, 치환 또는 비치환된 C6 내지 C30 아릴기, 또는 치환 또는 비치환된 C1 내지 C30 헤테로아릴기일 수 있다.R 6 to R 26 may be the same or different from each other and each of R 6 to R 26 independently represents a hydrogen atom, a substituted or unsubstituted C1 to C30 alkyl group, a substituted or unsubstituted C3 to C30 cycloalkyl group, Substituted C1 to C30 heterocycloalkyl groups, substituted or unsubstituted C6 to C30 aryl groups, or substituted or unsubstituted C1 to C30 heteroaryl groups.
본 발명의 다른 또 하나의 실시예에 따르면, 바람직하게는,According to another embodiment of the present invention, preferably,
Y1 및 Y2는 서로 같거나 다를 수 있고, Y1 및 Y2는 각각 독립적으로 산소원자 또는 황원자일 수 있다.Y 1 And Y 2 may be the same or different from each other, and Y 1 And Y < 2 > may each independently be an oxygen atom or a sulfur atom.
상기 치환 또는 비치환된 C6 내지 C30 아릴기의 예는 치환 또는 비치환된 페닐기, 치환 또는 비치환된 바이페닐기, 치환 또는 비치환된 터페닐기, 치환 또는 비치환된 나프탈레닐기, 치환 또는 비치환된 안트라세닐기, 치환 또는 비치환된 페난트레닐기, 치환 또는 비치환된 플루오레닐기, 치환 또는 비치환된 스파이로플루오레닐기, 치환 또는 비치환된 파이레닐기, 또는 치환 또는 비치환된 페릴레닐기일 수 있다.Examples of the substituted or unsubstituted C6 to C30 aryl group include a substituted or unsubstituted phenyl group, a substituted or unsubstituted biphenyl group, a substituted or unsubstituted terphenyl group, a substituted or unsubstituted naphthalenyl group, a substituted or unsubstituted naphthalenyl group, A substituted or unsubstituted phenanthrenyl group, a substituted or unsubstituted fluorenyl group, a substituted or unsubstituted spirobifluorenyl group, a substituted or unsubstituted pyrenyl group, or a substituted or unsubstituted furanyl group, Lt; / RTI >
상기 치환 또는 비치환된 C2 내지 C30 헤테로아릴기의 예는 치환 또는 비치환된 피리디닐기, 치환 또는 비치환된 피리미디닐기, 치환 또는 비치환된 트리아지닐기, 치환 또는 비치환된 싸이오페닐기, 치환 또는 비치환된 피롤릴기, 치환 또는 비치환된 벤조싸이오페닐기, 치환 또는 비치환된 인돌릴기, 치환 또는 비치환된 이미다조[1,2-a]피리디닐기, 치환 또는 비치환된 벤지이미다졸릴기, 치환 또는 비치환된 인다졸릴기, 치환 또는 비치환된 페노티아지닐기, 치환 또는 비치환된 페나지닐기, 치환 또는 비치환된 카바졸릴기, 치환 또는 비치환된 디벤조싸이오페닐기, 치환 또는 비치환된 이미다졸릴기, 치환 또는 비치환된 트리아졸릴기, 치환 또는 비치환된 테트라졸릴기, 치환 또는 비치환된 옥사다이아졸릴기, 치환 또는 비치환된 옥사트리아졸릴기, 치환 또는 비치환된 싸이아트리아졸릴기, 치환 또는 비치환된 벤조트리아졸릴기, 치환 또는 비치환된 피라지닐기, 치환 또는 비치환된 피리다지닐기, 치환 또는 비치환된 퓨리닐기, 치환 또는 비치환된 퀴놀리닐기, 치환 또는 비치환된 이소퀴놀리닐기, 치환 또는 비치환된 프탈라지닐기, 치환 또는 비치환된 나프피리디닐기, 치환 또는 비치환된 퀴녹살리닐기, 치환 또는 비치환된 퀴나졸리닐기, 치환 또는 비치환된 아크리디닐기, 또는 치환 또는 비치환된 페난트롤리닐기, 바람직하게는 치환 또는 비치환된 피리디닐기, 치환 또는 비치환된 피리미디닐기, 치환 또는 비치환된 트리아지닐기, 치환 또는 비치환된 싸이오페닐기, 치환 또는 비치환된 피롤릴기, 치환 또는 비치환된 벤조싸이오페닐기, 치환 또는 비치환된 인돌릴기, 치환 또는 비치환된 이미다조[1,2-a]피리디닐기, 치환 또는 비치환된 벤지이미다졸릴기, 치환 또는 비치환된 인다졸릴기, 치환 또는 비치환된 페노티아지닐기, 치환 또는 비치환된 페나지닐기, 치환 또는 비치환된 카바졸릴기, 또는 치환 또는 비치환된 디벤조싸이오페닐기일 수 있다.Examples of the substituted or unsubstituted C2 to C30 heteroaryl group include a substituted or unsubstituted pyridinyl group, a substituted or unsubstituted pyrimidinyl group, a substituted or unsubstituted thiazinyl group, a substituted or unsubstituted thiophenyl group , A substituted or unsubstituted pyrrolyl group, a substituted or unsubstituted benzothiophenyl group, a substituted or unsubstituted indolyl group, a substituted or unsubstituted imidazo [1,2-a] pyridinyl group, a substituted or unsubstituted A substituted or unsubstituted indazolyl group, a substituted or unsubstituted phenothiazinyl group, a substituted or unsubstituted phenazinyl group, a substituted or unsubstituted carbazolyl group, a substituted or unsubstituted dibenzoyl group, Substituted or unsubstituted imidazolyl groups, substituted or unsubstituted thiazolyl groups, substituted or unsubstituted tetrazolyl groups, substituted or unsubstituted oxadiazolyl groups, substituted or unsubstituted oxatriazolyl groups, However, A substituted or unsubstituted thiazolyl group, a substituted or unsubstituted benzothiazolyl group, a substituted or unsubstituted pyrazinyl group, a substituted or unsubstituted pyridazinyl group, a substituted or unsubstituted furyl group, a substituted or unsubstituted thiazolyl group, A substituted or unsubstituted quinoxalinyl group, a substituted or unsubstituted quinolinyl group, a substituted or unsubstituted quinolinyl group, a substituted or unsubstituted quinoxalinyl group, a substituted or unsubstituted phthalazinyl group, a substituted or unsubstituted naphthyridinyl group, Or a substituted or unsubstituted phenanthrolinyl group, preferably a substituted or unsubstituted pyridinyl group, a substituted or unsubstituted pyrimidinyl group, a substituted or unsubstituted acridinyl group, a substituted or unsubstituted pyrazolinyl group, A substituted or unsubstituted thiazolyl group, a substituted or unsubstituted thiophenyl group, a substituted or unsubstituted pyrrolyl group, a substituted or unsubstituted benzothiophenyl group, a substituted or unsubstituted indolyl group, A substituted or unsubstituted benzothiazolyl group, a substituted or unsubstituted phenothiazyl group, a substituted or unsubstituted phenazinyl group, a substituted or unsubstituted benzothiazolyl group, , A substituted or unsubstituted carbazolyl group, or a substituted or unsubstituted dibenzothiophenyl group.
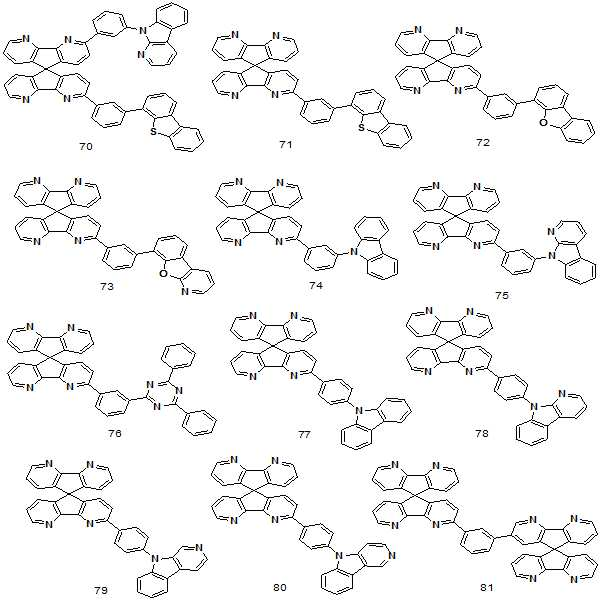
상기 유기전계발광소자용 화합물은 하기 화학식으로 표시되는 화합물 1 내지 100 중에서 선택된 어느 하나일 수 있다. The compound for an organic electroluminescence device may be any one selected from compounds 1 to 100 represented by the following formulas.
본 발명의 다른 측면에 따르면, 상기 유기전계발광소자용 화합물을 포함하는 유기전계발광소자가 제공될 수 있다.According to another aspect of the present invention, there is provided an organic electroluminescent device comprising the compound for an organic electroluminescent device.
본 발명의 또 다른 측면에 따르면, 제1전극, 제2전극 및 상기 제1전극과 제2전극 사이에 단수 또는 복수의 유기물층을 포함하는 유기전계발광소자에 있어서, 상기 단수 또는 복수의 유기물층 중에서 선택된 1종 이상의 유기물층은 상기 유기전계발광소자용 화합물을 포함하는 것을 특징으로 하는 유기전계발광소자가 제공될 수 있다.According to another aspect of the present invention, there is provided an organic electroluminescent device comprising a first electrode, a second electrode, and a single or a plurality of organic layers between the first electrode and the second electrode, The organic electroluminescent device may further include at least one organic compound layer including the organic electroluminescent compound.
상기 단수 또는 복수의 유기물층은 발광층을 포함할 수 있다.The singular or plural organic layers may include a light emitting layer.
상기 복수의 유기물층은 발광층을 포함하고, 상기 복수의 유기물층은 전자주입층, 전자수송층, 정공차단층, 전자차단층, 정공수송층 및 정공주입층 중에서 선택된 1종 이상을 추가로 포함할 수 있다.The plurality of organic layers may include a light emitting layer, and the plurality of organic layers may further include at least one selected from an electron injecting layer, an electron transporting layer, a hole blocking layer, an electron blocking layer, a hole transporting layer, and a hole injecting layer.
상기 발광층은 호스트와 도펀트를 포함할 수 있다.The light emitting layer may include a host and a dopant.
본 발명은 전기적 안정성 및 전자와 홀 수송능력이 우수하며, 삼중항 상태 에너지가 높아 인광발광재료의 발광효율을 향상 시킬 수 있는 호스트로서 발광층에 사용 될 수 있는 유기전계발광소자용 화합물 및 이를 포함하는 유기전기발광소자를 제공할 수 있다.The present invention relates to a compound for an organic electroluminescent device which can be used for a light emitting layer as a host which is excellent in electrical stability and electron and hole transporting ability and has high triplet state energy and can improve the luminous efficiency of a phosphorescent light emitting material, An organic electroluminescent device can be provided.
또한 본 발명은 유기전계발광소자의 전자수송재료나, 정공수송재료에 사용될 수 있는 유기전계발광소자용 화합물 및 이를 포함하는 유기전계발광소자를 제공할 수 있다.In addition, the present invention can provide an organic electroluminescent compound which can be used for an electron transporting material and a hole transporting material of an organic electroluminescent device, and an organic electroluminescent device including the same.
도 1은 본 발명의 일 실시예에 따른 유기전계발광소자의 단면을 나타낸 단면도이다.
도 2는 본 발명의 다른 일 실시예에 따른 유기전계발광소자의 단면을 나타낸 단면도이다.1 is a cross-sectional view illustrating an organic electroluminescent device according to an embodiment of the present invention.
2 is a cross-sectional view illustrating an organic electroluminescent device according to another embodiment of the present invention.
본 발명은 다양한 변환을 가할 수 있고 여러 가지 실시예를 가질 수 있는 바, 특정 실시예들을 예시하고 상세한 설명에 상세하게 설명하고자 한다. 그러나, 이는 본 발명을 특정한 실시 형태에 대해 한정하려는 것이 아니며, 본 발명의 사상 및 기술 범위에 포함되는 모든 변환, 균등물 내지 대체물을 포함하는 것으로 이해되어야 한다. 본 발명을 설명함에 있어서 관련된 공지 기술에 대한 구체적인 설명이 본 발명의 요지를 흐릴 수 있다고 판단되는 경우 그 상세한 설명을 생략한다.The invention is capable of various modifications and may have various embodiments, and particular embodiments are exemplified and will be described in detail in the detailed description. It is to be understood, however, that the invention is not to be limited to the specific embodiments, but includes all modifications, equivalents, and alternatives falling within the spirit and scope of the invention. DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS Hereinafter, the present invention will be described in detail with reference to the accompanying drawings.
또한, 이하에서 사용될 제1, 제2 등과 같이 서수를 포함하는 용어는 다양한 구성요소들을 설명하는데 사용될 수 있지만, 상기 구성요소들은 상기 용어들에 의해 한정되지는 않는다. 상기 용어들은 하나의 구성요소를 다른 구성요소로부터 구별하는 목적으로만 사용된다. 예를 들어, 본 발명의 권리 범위를 벗어나지 않으면서 제1 구성요소는 제2 구성요소로 명명될 수 있고, 유사하게 제2 구성요소도 제1 구성요소로 명명될 수 있다. Furthermore, terms including an ordinal number such as first, second, etc. to be used below can be used to describe various elements, but the constituent elements are not limited by the terms. The terms are used only for the purpose of distinguishing one component from another. For example, without departing from the scope of the present invention, the first component may be referred to as a second component, and similarly, the second component may also be referred to as a first component.
또한, 어떤 구성요소가 다른 구성요소 상에 "형성되어" 있다거나 "적층되어" 있다고 언급된 때에는, 그 다른 구성요소의 표면 상의 전면 또는 일면에 직접 부착되어 형성되어 있거나 적층되어 있을 수도 있지만, 중간에 다른 구성요소가 더 존재할 수도 있다고 이해되어야 할 것이다.Also, when an element is referred to as being "formed" or "laminated" on another element, it may be directly attached or laminated to the front surface or one surface of the other element, It will be appreciated that other components may be present in the < / RTI >
단수의 표현은 문맥상 명백하게 다르게 뜻하지 않는 한, 복수의 표현을 포함한다. 본 출원에서, "포함하다" 또는 "가지다" 등의 용어는 명세서상에 기재된 특징, 숫자, 단계, 동작, 구성요소, 부품 또는 이들을 조합한 것이 존재함을 지정하려는 것이지, 하나 또는 그 이상의 다른 특징들이나 숫자, 단계, 동작, 구성요소, 부품 또는 이들을 조합한 것들의 존재 또는 부가 가능성을 미리 배제하지 않는 것으로 이해되어야 한다.The singular expressions include plural expressions unless the context clearly dictates otherwise. In the present application, the terms "comprises" or "having" and the like are used to specify that there is a feature, a number, a step, an operation, an element, a component or a combination thereof described in the specification, But do not preclude the presence or addition of one or more other features, integers, steps, operations, elements, components, or combinations thereof.
본 명세서에서 "원자가결합"이란 별도의 정의가 없는 한, 단일결합, 이중결합 또는 삼중결합을 의미한다.As used herein, "atomic bond" means a single bond, a double bond or a triple bond, unless otherwise defined.
본 명세서에서 "치환"이란 별도의 정의가 없는 한, 치환기 또는 화합물 중의 적어도 하나의 수소가 중수소, 할로겐기, 히드록시기, 아미노기, C1 내지 C30 아민기, 니트로기, C1 내지 C30 실릴기, C1 내지 C30 알킬기, C1 내지 C30 알킬실릴기, C3 내지 C30 시클로알킬기, C1 내지 C30 헤테로시클로알킬기, C6 내지 C30 아릴기, C1 내지 C30 헤테로 아릴기, C1 내지 C20 알콕시기, C1 내지 C10 트리플루오로알킬기 또는 시아노기로 치환된 것을 의미한다.As used herein, unless otherwise defined, "substituent" means that at least one hydrogen in the substituent or compound is substituted with one or more substituents selected from the group consisting of deuterium, a halogen group, a hydroxy group, an amino group, a C1 to C30 amine group, a nitro group, a C1 to C30 silyl group, An alkyl group, a C1 to C30 alkylsilyl group, a C3 to C30 cycloalkyl group, a C1 to C30 heterocycloalkyl group, a C6 to C30 aryl group, a C1 to C30 heteroaryl group, a C1 to C20 alkoxy group, a C1 to C10 trifluoroalkyl group, It means that it has been replaced by anger.
또한 상기 치환된 할로겐기, 히드록시기, 아미노기, C1 내지 C30 아민기, C3 내지 C30 실릴기, C1 내지 C30 알킬기, C1 내지 C30 알킬실릴기, C3 내지 C30 시클로알킬기, C6 내지 C30 아릴기, C1 내지 C20 알콕시기, C1 내지 C10 트리플루오로알킬기 또는 시아노기 중 인접한 두 개의 치환기가 융합되어 고리를 형성할 수도 있다.A C1 to C30 alkyl group, a C1 to C30 alkylsilyl group, a C3 to C30 cycloalkyl group, a C6 to C30 aryl group, a C1 to C20 aryl group, a C1 to C30 aryl group, a C1 to C30 aryl group, An alkoxy group, a C1 to C10 trifluoroalkyl group or a cyano group may be fused to form a ring.
본 명세서에서 "헤테로"란 별도의 정의가 없는 한, 하나의 작용기 내에 N, O, S 및 P로 이루어진 군에서 선택되는 헤테로 원자를 1 내지 4개 함유하고, 나머지는 탄소인 것을 의미한다.As used herein, unless otherwise defined, it is meant that one functional group contains 1 to 4 heteroatoms selected from the group consisting of N, O, S, and P, and the remainder is carbon.
본 명세서에서 "이들의 조합"이란 별도의 정의가 없는 한, 둘 이상의 치환기가 연결기로 결합되어 있거나, 둘 이상의 치환기가 축합하여 결합되어 있는 것을 의미한다.In the present specification, the term "combination thereof" means that two or more substituents are bonded to each other via a linking group or two or more substituents are condensed and bonded.
본 명세서에서 "수소"란 별도의 정의가 없는 한, 일중수소, 이중수소, 또는 삼중수소를 의미한다. As used herein, "hydrogen" means monohydrogen, double hydrogen, or tritium, unless otherwise defined.
본 명세서에서 "알킬(alkyl)기"란 별도의 정의가 없는 한, 지방족 탄화수소기를 의미한다. As used herein, unless otherwise defined, the term "alkyl group" means an aliphatic hydrocarbon group.
알킬기는 어떠한 이중결합이나 삼중결합을 포함하고 있지 않은 "포화 알킬(saturated alkyl)기" 일 수 있다. The alkyl group may be a "saturated alkyl group" which does not contain any double or triple bonds.
알킬기는 적어도 하나의 이중결합 또는 삼중결합을 포함하고 있는 "불포화 알킬(unsaturated alkyl)기"일 수도 있다. The alkyl group may be an "unsaturated alkyl group" comprising at least one double bond or triple bond.
"알케닐렌(alkenylene)기"는 적어도 두 개의 탄소원자가 적어도 하나의 탄소-탄소 이중 결합으로 이루어진 작용기를 의미하며, "알키닐렌(alkynylene)기" 는 적어도 두 개의 탄소원자가 적어도 하나의 탄소-탄소 삼중 결합으로 이루어진 작용기를 의미한다. 포화이든 불포화이든 간에 알킬기는 분지형, 직쇄형 또는 환형일 수 있다. "Alkynylene group" means a functional group in which at least two carbon atoms are composed of at least one carbon-carbon double bond, and "alkynylene group" means that at least two carbon atoms have at least one carbon- Quot; means a functional group formed by bonding. The alkyl group, whether saturated or unsaturated, can be branched, straight chain or cyclic.
알킬기는 C1 내지 C30 알킬기일 수 있다. 보다 구체적으로 알킬기는 C1 내지 C20 알킬기, C1 내지 C10 알킬기 또는 C1 내지 C6 알킬기일 수도 있다.The alkyl group may be a C1 to C30 alkyl group. More specifically, the alkyl group may be a C1 to C20 alkyl group, a C1 to C10 alkyl group or a C1 to C6 alkyl group.
예를 들어, C1 내지 C4 알킬기는 알킬쇄에 1 내지 4 개의 탄소원자, 즉, 알킬쇄는 메틸, 에틸, 프로필, 이소-프로필, n-부틸, 이소-부틸, sec-부틸 및 t-부틸로 이루어진 군에서 선택됨을 나타낸다.For example, the C1 to C4 alkyl groups may have 1 to 4 carbon atoms in the alkyl chain, i.e., the alkyl chain may be optionally substituted with one or more substituents selected from the group consisting of methyl, ethyl, propyl, iso-propyl, n-butyl, Indicating that they are selected from the group.
구체적인 예를 들어 상기 알킬기는 메틸기, 에틸기, 프로필기, 이소프로필기, 부틸기, 이소부틸기, t-부틸기, 펜틸기, 헥실기, 에테닐기, 프로페닐기, 부테닐기, 시클로프로필기, 시클로부틸기, 시클로펜틸기, 시클로헥실기 등을 의미한다.Specific examples of the alkyl group include a methyl group, ethyl group, propyl group, isopropyl group, butyl group, isobutyl group, t-butyl group, pentyl group, hexyl group, ethenyl group, Butyl group, cyclopentyl group, cyclohexyl group, and the like.
"아민기"는 아릴아민기, 알킬아민기, 아릴알킬아민기, 또는 알킬아릴아민기를 포함한다.The "amine group" includes an arylamine group, an alkylamine group, an arylalkylamine group, or an alkylarylamine group.
"시클로알킬(cycloalkyl)기"는 모노시클릭 또는 융합고리 폴리시클릭(즉, 탄소원자들의 인접한 쌍들을 나눠 가지는 고리) 작용기를 포함한다."Cycloalkyl group" includes monocyclic or fused-ring polycyclic (i. E., Rings that divide adjacent pairs of carbon atoms) functional groups.
"헤테로시클로알킬(heterocycloalkyl)기"는 시클로알킬기 내에 N, O, S 및 P로 이루어진 군에서 선택되는 헤테로원자를 1 내지 4개 함유하고, 나머지는 탄소인 것을 의미한다. 상기 헤테로시클로알킬기가 융합고리인 경우, 적어도 하나의 고리가 상기 헤테로 원자를 1 내지 4개 포함할 수 있다."Heterocycloalkyl group" means that the cycloalkyl group contains 1 to 4 hetero atoms selected from the group consisting of N, O, S and P in the cycloalkyl group, and the remainder is carbon. When the heterocycloalkyl group is a fused ring, at least one ring may contain 1 to 4 heteroatoms.
"방향족(aromatic)기"는 고리 형태인 작용기의 모든 원소가 p-오비탈을 가지고 있으며, 이들 p-오비탈이 공액(conjugation)을 형성하고 있는 작용기를 의미한다. 구체적인 예로 아릴기와 헤테로아릴기가 있다. "An aromatic group" means a functional group in which all elements of a functional group in the form of a ring have a p-orbital, and these p-orbital forms a conjugation. Specific examples thereof include an aryl group and a heteroaryl group.
"아릴(aryl)기"는 모노시클릭 또는 융합 고리 폴리시클릭(즉, 탄소원자들의 인접한 쌍들을 나눠 가지는 고리) 작용기를 포함한다. An "aryl group" includes a monocyclic or fused ring polycyclic (i. E., A ring that divides adjacent pairs of carbon atoms) functional groups.
"헤테로아릴(heteroaryl)기"는 아릴기 내에 N, O, S 및 P로 이루어진 군에서 선택되는 헤테로원자를 1 내지 4개 함유하고, 나머지는 탄소인 것을 의미한다. 상기 헤테로아릴기가 융합고리인 경우, 적어도 하나의 고리가 상기 헤테로 원자를 1 내지 4개 포함할 수 있다. "Heteroaryl group" means that the aryl group contains 1 to 4 hetero atoms selected from the group consisting of N, O, S and P, and the remainder is carbon. When the heteroaryl group is a fused ring, at least one ring may contain 1 to 4 heteroatoms.
아릴기 및 헤테로아릴기에서 고리의 원자수는 탄소수 및 비탄소원자수의 합이다.In the aryl group and the heteroaryl group, the number of atoms in the ring is the sum of carbon number and non-carbon atom number.
"알킬아릴기" 또는 "아릴알킬기"와 같이 조합하여 사용할 때, 상기에 든 각각의 알킬 및 아릴의 용어는 상기 나타낸 의미와 내용을 가진다.When used in combination, such as "alkylaryl" or "arylalkyl group ", the terms alkyl and aryl of each of the above have the meanings and contents indicated above.
"아릴알킬기"이란 용어는 벤질과 같은 아릴 치환된 알킬 라디칼을 의미하며 알킬기에 포함된다.The term "arylalkyl group " means an aryl substituted alkyl radical, such as benzyl, and is included in the alkyl group.
"알킬아릴기"이란 용어는 알킬 치환된 아릴 라디칼을 의미하며 아릴기에 포함된다.The term "alkylaryl group " means an alkyl substituted aryl radical and is included in the aryl group.
이하, 본 발명의 실시예를 첨부도면을 참조하여 설명하기로 하며, 첨부 도면을 참조하여 설명함에 있어, 동일하거나 대응하는 구성 요소는 동일한 도면번호를 부여하고 이에 대한 중복되는 설명은 생략하기로 한다.DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT Hereinafter, embodiments of the present invention will be described with reference to the accompanying drawings. Referring to the accompanying drawings, the same or corresponding components are denoted by the same reference numerals, .
도 1 및 2를 참고하면, 본 발명의 실시예에 따르면 본 발명에 따른 유기전계발광소자용 화합물을 포함하는 유기전계발광소자(1)가 제공될 수 있다.Referring to FIGS. 1 and 2, an organic electroluminescent device 1 including the compound for an organic electroluminescent device according to the present invention can be provided according to an embodiment of the present invention.
본 발명의 다른 실시예에 따르면, 상기 유기전계발광소자는 제1전극(110); 제2전극(150); 및 상기 제1전극과 제2전극 사이에 단수 또는 복수의 유기물층(130)을 포함하며, 상기 단수 또는 복수의 유기물층(130) 중에서 선택된 1종 이상의 유기물층은 본 발명에 따른 유기발광소자용 화합물을 포함할 수 있다.According to another embodiment of the present invention, the organic electroluminescent device includes a
여기서, 상기 단수 또는 복수의 유기물층(130)은 발광층(134)을 포함할 수 있다. Here, the single or plural
또한 상기 복수의 유기물층(130)은 발광층(134)을 포함하고, 상기 복수의 유기물층은 전자주입층(131), 전자수송층(132), 정공차단층(133), 전자차단층(135), 정공수송층(136) 및 정공주입층(137) 중에서 선택된 1종 이상을 추가로 포함할 수 있다.The plurality of
상기 발광층(134)은 호스트와 도펀트를 포함할 수 있다.The light emitting layer 134 may include a host and a dopant.
상기 유기전계발광소자는 바람직하게는 투명기판에 의하여 지지된다. 투명기판의 재료로는 양호한 기계적 강도, 열안정성 및 투명성을 갖는 한 특별한 제한은 없다. 구체적인 예를 들면, 유리, 투명 플라스틱 필름 등을 사용할 수 있다.The organic electroluminescent device is preferably supported by a transparent substrate. The material of the transparent substrate is not particularly limited as long as it has good mechanical strength, thermal stability and transparency. Specific examples thereof include glass, transparent plastic film, and the like.
본 발명의 유기전계발광소자의 양극재료로서는 4eV 이상의 일함수를 갖는 금속, 합금, 전기전도성 화합물 또는 이의 혼합물을 사용할 수 있다. 구체적으로는 금속인 Au 또는 CuI, ITO(인듐 주석 산화물), SnO2 및 ZnO와 같은 투명 전도성 재료를 들 수 있다. 양극 필름의 두께는 10 내지 200nm 가 바람직하다.As the cathode material of the organic electroluminescent device of the present invention, a metal, an alloy, an electroconductive compound or a mixture thereof having a work function of 4 eV or more can be used. Specifically, transparent conductive materials such as Au or CuI, ITO (indium tin oxide), SnO 2 and ZnO, which are metals, can be mentioned. The thickness of the positive electrode film is preferably 10 to 200 nm.
본 발명의 유기전계발광소자의 음극 재료로서는 4eV 미만의 일함수를 갖는 금속, 합금, 전기 전도성 화합물 또는 이의 혼합물을 사용할 수 있다. 구체적으로는, Na, Na-K 합금, 칼슘, 마그네슘, 리튬, 리튬 합금, 인듐, 알루미늄, 마그네슘 합금, 알루미늄 합금을 들 수 있다. 이외에, 알루미늄/AlO2, 알루미늄/리튬, 마그네슘/은 또는 마그네슘/인듐 등도 사용될 수 있다. 음극필름의 두께는 10 내지 200nm 가 바람직하다.As the anode material of the organic electroluminescent device of the present invention, a metal, an alloy, an electrically conductive compound or a mixture thereof having a work function of less than 4 eV may be used. Specifically, Na, Na-K alloy, calcium, magnesium, lithium, lithium alloy, indium, aluminum, magnesium alloy and aluminum alloy can be mentioned. In addition, aluminum / AlO 2 , aluminum / lithium, magnesium / silver or magnesium / indium may be used. The thickness of the negative electrode film is preferably 10 to 200 nm.
유기 EL 소자의 발광효율을 높이기 위해서는 하나 이상의 전극은 바람직하게는 10% 이상의 광투과율을 가지는 것이 바람직하다. 전극의 쉬트저항은 바람직하게는 수백 Ω/mm 이하이다. 전극의 두께는 10nm 내지 1㎛, 보다 바람직하게는 10 내지 400nm 이다. 이러한 전극은 화학적 기상증착(CVD), 물리적 기상증착(PVD) 등의 기상증착법 또는 스퍼터링법을 통하여 상기한 전극 재료를 박막으로 형성하여 제조할 수 있다.In order to increase the luminous efficiency of the organic EL device, at least one electrode preferably has a light transmittance of 10% or more. The sheet resistance of the electrode is preferably several hundreds? / Mm or less. The thickness of the electrode is 10 nm to 1 탆, more preferably 10 to 400 nm. Such an electrode can be manufactured by forming the electrode material into a thin film by a vapor deposition method such as chemical vapor deposition (CVD) or physical vapor deposition (PVD) or a sputtering method.
또한 본 발명의 목적에 적합하게 본 발명의 유기전계발광소자용 화합물이 사용될 때, 공지된 정공수송 물질, 정공주입 물질, 발광층 물질, 발광층의 호스트 물질, 전자수송 물질, 및 전자주입 물질이 상기 각각의 유기물층에서 단독으로 사용되거나 또는 본 발명의 유기전계발광소자용 화합물과 선택적으로 병행하여 사용될 수 있다.When the compound for an organic electroluminescence device of the present invention is used for the purpose of the present invention, the known hole transporting material, hole injecting material, light emitting layer material, host material of the light emitting layer, electron transporting material, Or may be used in combination with the organic electroluminescent device compound of the present invention selectively.
정공 수송 물질로서 N,N-dicarbazolyl-3,5-benzene(mCP), poly(3,4-ethylenedioxythiophene):polystyrenesulfonate (PEDOT:PSS), N, N’-di(1-naphthyl)-N,N’-diphenylbenzidine(NPD), N,N'-디페닐-N,N'-디(3-메틸페닐)-4,4'-디아미노비페닐(TPD), N,N'-디페닐-N,N'-디나프틸-4,4'-디아미노비페닐, N,N,N'N'-테트라-p-톨릴-4,4'-디아미노비페닐, N,N,N'N'-테트라페닐-4,4'-디아미노비페닐, 코퍼(II)1,10,15,20-테트라페닐-21H,23H-포피린 등과 같은 포피린(porphyrin)화합물 유도체, 주쇄 또는 측쇄내에 방향족 3차아민을 갖는 중합체, 1,1-비스(4-디-p-톨릴아미노페닐)시클로헥산, N,N,N-트리(p-톨릴)아민, 4, 4', 4'-트리스[N-(3-메틸페닐)-N-페닐아미노]트리페닐아민과 같은 트리아릴아민 유도체, N-페닐카르바졸 및 폴리비닐카르바졸과 같은 카르바졸 유도체, 무금속 프탈로시아닌, 구리프탈로시아닌과 같은 프탈로시아닌 유도체, 스타버스트 아민 유도체, 엔아민스틸벤계 유도체, 방향족 삼급아민과 스티릴 아민 화합물의 유도체, 및 폴리실란 등을 들 수 있다.N-dicarbazolyl-3,5-benzene (mCP), poly (3,4-ethylenedioxythiophene): polystyrenesulfonate (PEDOT: PSS), N, N'- (NPD), N, N'-diphenyl-N, N'-di (3-methylphenyl) -4,4'- diaminobiphenyl (TPD) N, N'N'-tetra-p-tolyl-4,4'-diaminobiphenyl, N, N'N'N'N'- Porphyrin compound derivatives such as tetraphenyl-4,4'-diaminobiphenyl, copper (II) 1,10,15,20-tetraphenyl-21H, 23H-porphyrin and the like, aromatic tertiary (4-di-p-tolylaminophenyl) cyclohexane, N, N, N-tri (3-methylphenyl) -N-phenylamino] triphenylamine, carbazole derivatives such as N-phenylcarbazole and polyvinylcarbazole, phthalocyanine derivatives such as nonmetal phthalocyanine and copper phthalocyanine, An aminostilbene derivative, a derivative of an aromatic tertiary amine and a styrylamine compound, and polysilane.
전자 수송 물질로서 diphenylphosphine oxide-4-(triphenylsilyl)phenyl (TSPO1), Alq3, 2,5-디아릴 실롤 유도체(PyPySPyPy), 퍼플루오리네이티드 화합물(PF-6P), Octasubstituted cyclooctatetraene 화합물(COTs)을 들 수 있다.The diphenylphosphine oxide-4- (triphenylsilyl) phenyl (TSPO1), Alq 3, 2,5- diaryl silole derivatives (PyPySPyPy), perfluoro rineyi suited compound (PF-6P), Octasubstituted cyclooctatetraene compound (COTs) as an electron transport material .
본 발명의 유기전계발광소자에 있어서, 전자 주입층, 전자 수송층, 정공 수송층 및 정공 주입층은 상기한 화합물의 하나 이상의 종류를 함유하는 단일 층으로 형성되거나, 또는 상호 적층된, 상이한 종류의 화합물을 함유하는 복수의 층으로 구성될 수 있다.In the organic electroluminescent device of the present invention, the electron injecting layer, the electron transporting layer, the hole transporting layer, and the hole injecting layer may be formed of a single layer containing at least one kind of the above-mentioned compounds, And the like.
발광재료로서 예를 들면 축광 형광재료, 형광증백제, 레이저 색소, 유기 신틸레이터 및 형광 분석용 시약을 들 수 있다. 구체적으로는, 카바졸계 화합물, 포스핀옥사이드계 화합물, 카바졸계 포스핀옥사이드 화합물, bis((3,5-difluoro-4-cyanophenyl)pyridine) iridium picolinate(FCNIrpic), tris(8-hydroxyquinoline) aluminum(Alq3), 안트라센, 페난트렌, 피렌, 크리센, 페릴렌, 코로넨, 루브렌 및 퀴나크리돈과 같은 폴리아로마틱 화합물, 퀴터페닐과 같은 올리고페닐렌 화합물, 1,4-비스 (2-메틸스티릴)벤젠, 1,4-비스(4-메틸스티릴)벤젠, 1,4-비스(4-메틸-5-페닐-2-옥사졸릴)벤젠, 1,4-비스(5-페닐-2-옥사졸릴)벤젠, 2,5-비스(5-t-부틸-2-벤즈옥사졸릴)사이오펜, 1,4-디페닐-1,3-부타디엔, 1,6-디페닐-1,3,5-헥사트리엔,1,1,4,4-테트라페닐-1,3-부타디엔과 같은 액체신틸레이션용 신틸레이터, 옥신 유도체의 금속착체, 쿠마린 색소, 디시아노메틸렌피란 색소, 디시아노메틸렌사이오피란 색소, 폴리메틴 색소, 옥소벤즈안트라센 색소, 크산텐 색소, 카르보스티릴 색소, 페릴렌 색소, 옥사진 화합물, 스틸벤 유도체, 스피로 화합물, 옥사디아졸 화합물 등을 들 수 있다.Examples of the light emitting material include a phosphorescent fluorescent material, a fluorescent whitening agent, a laser dye, an organic scintillator, and a reagent for fluorescence analysis. Specifically, a carbazole compound, a phosphine oxide compound, a carbazole-based phosphine oxide compound, bis (3,5-difluoro-4-cyanophenyl) pyridine, iridium picolinate (FCNIrpic), tris (8-hydroxyquinoline) aluminum Alq 3 ), polyaromatic compounds such as anthracene, phenanthrene, pyrene, chrysene, perylene, coronene, rubrene and quinacridone, oligophenylene compounds such as quaterphenyl, 1,4- Bis (4-methylstyryl) benzene, 1,4-bis (4-methyl- Bis (5-t-butyl-2-benzoxazolyl) thiophene, 1,4-diphenyl-1,3-butadiene, 1,6- Liquid scintillation scintillators such as 3,5-hexatriene and 1,1,4,4-tetraphenyl-1,3-butadiene, metal complexes of oxine derivatives, coumarin dyes, dicyanomethylenepyran dyes, dicyanomethylene Cyopyran pigment, polymethine pigment, oxobenzanthracene There may be mentioned a colorant, a xanthene colorant, a carbostyryl colorant, a perylene colorant, an oxazine compound, a stilbene derivative, a spiro compound, and an oxadiazole compound.
본 발명의 유기 EL 소자를 구성하는 각 층은 진공 증착, 스핀 코팅 또는 캐스팅과 같은 공지된 방법을 통하여 박막으로 형성시키거나, 각 층에서 사용되는 재료를 이용하여 제조할 수 있다. 이들 각층의 막 두께에 대해서는 특별한 제한은 없으며, 재료의 특성에 따라 알맞게 선택할 수 있으나, 보통 2nm 내지 5,000nm의 범위에서 결정될 수 있다.Each layer constituting the organic EL device of the present invention can be formed into a thin film through a known method such as vacuum deposition, spin coating or casting, or can be manufactured using a material used in each layer. The thickness of each of these layers is not particularly limited and may be appropriately selected according to the characteristics of the material, but may be determined usually in the range of 2 nm to 5,000 nm.
본 발명의 따른 유기전계발광소자용 화합물은 진공 증착법에 의하여 형성될 수 있으므로, 박막 형성 공정이 간편하고, 핀홀(pin hole)이 거의 없는 균질한 박막으로 용이하게 얻을 수 있는 장점이 있다.
The compound for an organic electroluminescence device according to the present invention can be formed by a vacuum deposition method, so that it is advantageous that a thin film forming process is simple and a homogeneous thin film having almost no pinhole can be easily obtained.
[실시예][Example]
이하, 실시예를 통하여 본 발명에 따른 유기전계발광소자용 화합물 및 이를 포함하는 유기전계발광소자의 제조방법을 더욱 구체적으로 설명한다. 그러나 이는 예시를 위한 것으로서 이에 의하여 본 발명의 범위가 한정되는 것이 아니다.Hereinafter, the compound for an organic electroluminescent device according to the present invention and the method for manufacturing the organic electroluminescent device including the same will be described in more detail with reference to the following examples. However, this is for the purpose of illustration only and is not intended to limit the scope of the invention.
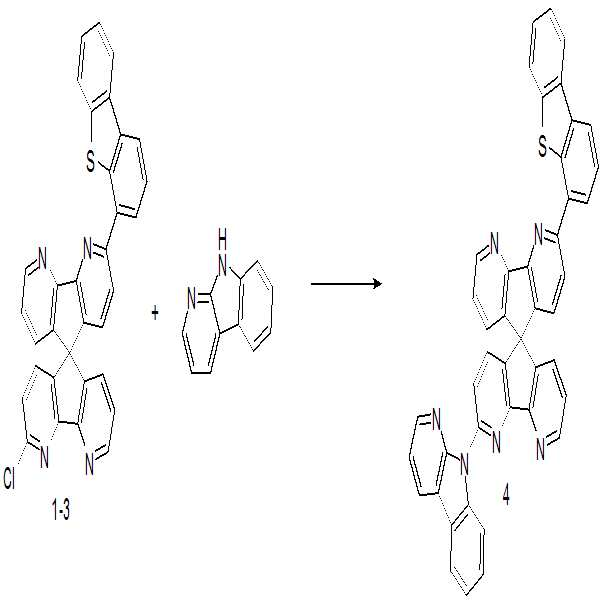
실시예Example 1: 화합물 1 합성 1: Synthesis of compound 1
(1) (One) 제조예Manufacturing example 1-1: 중간체 1-1 합성 1-1: Intermediate 1-1 Synthesis
5H-benzo-indeno[1,2-b]pyridin-5-one(10.0g, 0.055mol/중국산)에 3-bromo-2,2'-bipyridine(25.9g, 0.110mol/중국산), Mg(2.7g, 0.110mol/sigma Aldrich)에 acetic aicd 300ml를 넣고 25℃에서 24시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 중간체 1-1을 13.2g(수율 75%) 수득하였다.3-bromo-2,2'-bipyridine (25.9 g, 0.110 mol / Chinese acid) and Mg (2.7 mmol) were added to 5H-benzo-indeno [1,2- b] pyridin- g, 0.110 mol / sigma Aldrich), and the mixture was reacted at 25 ° C for 24 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC and subjected to column purification (n-hexane: MC) to obtain 13.2 g (yield 75%) of Intermediate 1-1.
LC/MS: m/z=320 [(M+1)+]LC / MS: m / z = 320 [(M + 1) < + &
(2) (2) 제조예Manufacturing example 1-2: 중간체 1-2 합성 1-2: Intermediate 1-2 Synthesis
중간체 1-1 (10.0g, 0.031mol), Pd(OAc)2(0.6g, 0.003mol/sigma Aldrich), t-BuOK(33g, 0.295mol /sigma Aldrich), trihexylphosphine(0.84g, 0.003mol /sigma Aldrich)에 acetic acid 150ml를 넣고 25℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 중간체 1-2를 3.0g(수율 25%) 수득하였다.(0.3 g, 0.003 mol / sigma Aldrich), t-BuOK (33 g, 0.295 mol / sigma Aldrich), trihexylphosphine (0.84 g, 0.003 mol / sigma Aldrich) were added 150 ml of acetic acid and reacted at 25 ° C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC and subjected to column purification (n-hexane: MC) to obtain 3.0 g (yield: 25%) of Intermediate 1-2.
LC/MS: m/z=389 [(M+1)+]LC / MS: m / z = 389 [(M + 1) < + &
(3) (3) 제조예Manufacturing example 1-3: 중간체 1-3 합성 1-3: Synthesis of intermediate 1-3
중간체 1-2(10.0g, 0.026mol)에 dibenzo[b,d]thiophen-4-ylboronic acid (6.8g, 0.030mol/sigma Aldrich), Pd(PPh3)4(1.5g, 0.0013mol/p&h tech), potassium carbonate(7.2g, 0.052mol/sigma aldrich)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 중간체 1-3을 10.1g(수율 72%) 수득하였다.Dibenzo [b, d] thiophen-4-ylboronic acid (6.8g, 0.030mol / Sigma Aldrich), Pd (PPh3) 4 (1.5g, 0.0013mol / p & h tech) was added to Intermediate 1-2 (10.0g, 0.026mol) , potassium carbonate (7.2 g, 0.052 mol / sigma aldrich), and the mixture was reacted at 65 ° C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC, and subjected to column purification (n-hexane: MC) to obtain 10.1 g (yield: 72%) of Intermediate 1-3.
LC/MS: m/z=537 [(M+1)+]LC / MS: m / z = 537 [(M + 1) < + &
(4) (4) 제조예Manufacturing example 1-4: 화합물 1 합성 1-4: Synthesis of compound 1
중간체 1-3(10.0g, 0.019mol)에 dibenzo[b,d]thiophen-4-ylboronic acid (5.1g, 0.022mol), Pd(PPh3)4(1.2g, 0.0010mol), potassium carbonate(5.3g, 0.038mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 1을 9.2g(수율 71%) 수득하였다.Dibenzo [b, d] thiophen-4-ylboronic acid (5.1 g, 0.022 mol), Pd (PPh3) 4 (1.2 g, 0.0010 mol), potassium carbonate (5.3 g, , 0.038 mol), 400 ml of THF was added, and the mixture was reacted at 65 ° C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC and subjected to column purification (n-hexane: MC) to obtain 9.2 g of Compound 1 (71% yield).
H-NMR (200MHz, CDCl3):δ ppm, 2H(7.98/D, 7.50/M, 7.52/M, 8.45/D, 8.47/D, 7.61/M, 8.31/D, 7.62/D, 7.48/D, 7.20/D, 6.81/M, 8.51/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 2H (7.98 / D, 7.50 / M, 7.52 / M, 8.45 / D, 8.47 / D, 7.61 / M, 8.31 / D, 7.62 / D, 7.48 / D , 7.20 / D, 6.81 / M, 8.51 / D)
LC/MS: m/z=685 [(M+1)+] LC / MS: m / z = 685 [(M + 1) < + &
실시예Example 2: 화합물 2 합성 2: Synthesis of compound 2
중간체 1-3(10.0g, 0.019mol)에 dibenzo[b,d]furan-4-ylboronic acid (4.7g, 0.022mol/중국산), Pd(PPh3)4(1.2g, 0.0010mol), potassium carbonate(5.3g, 0.038mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 2를 9.4g(수율 74%) 수득하였다.Dibenzo [b, d] furan-4-ylboronic acid (4.7g, 0.022mol / Chinese acid), Pd (PPh3) 4 (1.2g, 0.0010mol), potassium carbonate 5.3 g, 0.038 mol), 400 ml of THF was added, and the mixture was reacted at 65 DEG C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC, and subjected to column purification (n-hexane: MC) to obtain 9.4 g (yield: 74%) of Compound 2.
H-NMR (200MHz, CDCl3):δ ppm, 1H(8.31/D, 7.61/M, 8.47/D, 8.45/D, 7.52/M, 7.50/M, 7.98/D, 8.13/D, 7.41/M, 7.91/D, 7.89/D, 7.32/M, 7.38/M, 7.66/D) 2H(7.20/D, 7.48/D, 6.81/M, 8.51/D, 7.62/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 1H (8.31 / D, 7.61 / M, 8.47 / D, 8.45 / D, 7.52 / M, 7.50 / M, 7.98 / D, 8.13 / D, 7.41 / M D, 7.91 / D, 7.89 / D, 7.32 / M, 7.38 / M, 7.66 / D) 2H (7.20 / D, 7.48 / D, 6.81 / M, 8.51 / D, 7.62 / D)
LC/MS: m/z=669 [(M+1)+] LC / MS: m / z = 669 [(M + 1) < + &
실시예Example 3: 화합물 3 합성 3: Synthesis of compound 3
중간체 1-3(10.0g, 0.019mol)에 9H-carbazole (3.7g, 0.022mol/sigma aldrich), Pd(PPh3)4(1.2g, 0.0010mol), potassium carbonate(5.3g, 0.038mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 3을 9.3g(수율 73%) 수득하였다.Pd (PPh3) 4 (1.2g, 0.0010mol) and potassium carbonate (5.3g, 0.038mol) were added to 9H-carbazole (3.7g, 0.022mol / sigma aldrich), Intermediate 1-3 (10.0g, 0.019mol) And the mixture was reacted at 65 ° C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, and subjected to column separation on H 2 O: MC, followed by column purification (n-hexane: MC) to obtain 9.3 g (yield: 73%) of Compound 3.
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.62/D, 7.20/D, 7.94/D, 7.33/M, 7.25/M, 8.55/D, 8.12/D, 7.29/M, 7.50/M, 7.63/D, 7.55/D, 8.31/D, 7.61/M, 8.47/D, 8.45/D, 7.52/M, 7.50/M, 7.98/D) 2H(6.81/M, 8.51/D) 3H(7.48/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 1H (7.62 / D, 7.20 / D, 7.94 / D, 7.33 / M, 7.25 / M, 8.55 / D, 8.12 / D, 7.29 / M, 7.50 / M M, 8.51 / D) 3H (7.48 / D, 7.63 / D, 7.55 / D, 8.31 / D, 7.61 / M, 8.47 / D, 8.45 / D, 7.52 / / D)
LC/MS: m/z=668 [(M+1)+]LC / MS: m / z = 668 [(M + 1) < + &
실시예Example 4: 화합물 4 합성 4: Synthesis of compound 4
중간체 1-3(10.0g, 0.019mol)에 9H-pyrido[2,3-b]indole (3.7g, 0.022mol/중국산), Pd(PPh3)4(1.2g, 0.0010mol), potassium carbonate(5.3g, 0.038mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 4를 9.7g(수율 76%) 수득하였다.Pd (PPh3) 4 (1.2g, 0.0010mol), potassium carbonate (5.3g, 0.022mol / Chinese acid), 9H- g, 0.038 mol), 400 ml of THF was added, and the mixture was reacted at 65 DEG C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC and subjected to column purification (n-hexane: MC) to obtain 9.7 g (yield 76%) of compound 4.
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.20/D, 7.62/D, 8.31/D, 7.61/M, 7.52/M, 8.47/D, 8.45/D, 7.50/M, 7.98/D, 7.55/D, 7.94/D, 7.33/M, 7.25/M, 8.55/D, 8.51/D, 7.36/M, 8.43/D) 2H(6.81/M, 8.51/D) 3H(7.48/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 1H (7.20 / D, 7.62 / D, 8.31 / D, 7.61 / M, 7.52 / M, 8.47 / D, 8.45 / D, 7.50 / M, 7.98 / D (7.58 / D, 7.94 / D, 7.33 / M, 7.25 / M, 8.55 / D, 8.51 / D, 7.36 / M, 8.43 / D)
LC/MS: m/z=669 [(M+1)+]LC / MS: m / z = 669 [(M + 1) < + &
실시예Example 5: 화합물 8 합성 5: Compound 8 Synthesis
중간체 1-3(10.0g, 0.019mol)에 benzofuro[3,2-b]pyridin-4-ylboronic acid (4.7g, 0.022mol/중국산), Pd(PPh3)4(1.2g, 0.0010mol), potassium carbonate(5.3g, 0.038mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 8을 9.3g(수율 73%) 수득하였다.Benzofuro [3,2-b] pyridin-4-ylboronic acid (4.7g, 0.022mol / Chinese acid), Pd (PPh3) 4 (1.2g, 0.0010mol), potassium (5.3 g, 0.038 mol), 400 ml of THF was added, and the mixture was reacted at 65 DEG C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, and subjected to column separation on H 2 O: MC, followed by column purification (n-hexane: MC) to obtain 9.3 g (yield 73%) of Compound 8.
H-NMR (200MHz, CDCl3):δ ppm, 1H(8.31/D, 7.61/M, 8.47/D, 8.45/D, 7.52/M, 7.50/M, 7.98/D, 8.13/D, 7.41/M, 7.52/M, 8.51/D, 7.36/M, 8.43/D) 2H(7.62/D, 7.20/D, 7.48/D, 6.81/M, 8.51/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 1H (8.31 / D, 7.61 / M, 8.47 / D, 8.45 / D, 7.52 / M, 7.50 / M, 7.98 / D, 8.13 / D, 7.41 / M D, 7.42 / D, 6.81 / M, 8.51 / D), 7.52 / M, 8.51 / D, 7.36 /
LC/MS: m/z=6370 [(M+1)+] LC / MS: m / z = 6370 [(M + 1) < + &
실시예Example 6: 6: 화합물21합성Compound 21 Synthesis
(1) (One) 제조예Manufacturing example 6-1: 중간체 21-1 합성 6-1: Synthesis of Intermediate 21-1
중간체 1-2(10.0g, 0.026mol)에 dibenzo[b,d]furan-1-ylboronic acid (6.4g, 0.030mol), Pd(PPh3)4(1.5g, 0.0013mol), potassium carbonate(7.2g, 0.052mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 중간체 21-1을 9.5g(수율 70%)수득하였다.Dibenzo [b, d] furan-1-ylboronic acid (6.4 g, 0.030 mol), Pd (PPh3) 4 (1.5 g, 0.0013 mol), potassium carbonate (7.2 g, , 0.052 mol), 400 ml of THF was added, and the mixture was reacted at 65 ° C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC and subjected to column purification (n-hexane: MC) to obtain 9.5 g of Intermediate 21-1 (yield: 70%).
LC/MS: m/z=521 [(M+1)+]LC / MS: m / z = 521 [(M + 1) < + &
(2)(2) 제조예Manufacturing example 6-2: 화합물 21 합성 6-2: Compound 21 Synthesis
중간체 21-1(10.0g, 0.019mol)에 dibenzo[b,d]furan-1-ylboronic acid (4.9g, 0.023mol), Pd(PPh3)4(1.2g, 0.0010mol), potassium carbonate(5.3g, 0.038mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 21을 8.4g(수율68%) 수득하였다.Dibenzo [b, d] furan-1-ylboronic acid (4.9 g, 0.023 mol), Pd (PPh3) 4 (1.2 g, 0.0010 mol) and potassium carbonate (5.3 g, 0.019 mol) , 0.038 mol), 400 ml of THF was added, and the mixture was reacted at 65 ° C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC and subjected to column purification (n-hexane: MC) to obtain 8.4 g (yield: 68%) of Compound 21.
H-NMR (200MHz, CDCl3):δ ppm, 1H(8.22/S, 7.75/D, 8.32/D, 8.13/D, 7.41/M, 7.91/D) 2H(7.62/D, 7.20/D, 7.48/D, 7.81/M, 8.51/D, 7.66/D, 7.38/M, 7.32/M, 7.89/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 1H (8.22 / S, 7.75 / D, 8.32 / D, 8.13 / D, 7.41 / M, 7.91 / D) 2H (7.62 / D, 7.20 / D, 7.48 / D, 7.81 / M, 8.51 / D, 7.66 / D, 7.38 / M, 7.32 /
LC/MS: m/z=653 [(M+1)+] LC / MS: m / z = 653 [(M + 1) < + &
실시예Example 7: 화합물 24 합성 7: Compound 24 Synthesis
중간체 21-1(10.0g, 0.019mol)에 benzofuro[2,3-b]pyridin-4-ylboronic acid (4.9g, 0.023mol), Pd(PPh3)4(1.2g, 0.0010mol), potassium carbonate(5.3g, 0.038mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 24를 8.6g(수율 69%) 수득하였다Benzofuro [2,3-b] pyridin-4-ylboronic acid (4.9 g, 0.023 mol), Pd (PPh3) 4 (1.2 g, 0.0010 mol), potassium carbonate (10.0 g, 0.019 mol) 5.3 g, 0.038 mol), 400 ml of THF was added, and the mixture was reacted at 65 DEG C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC and subjected to column purification (n-hexane: MC) to obtain 8.6 g (yield: 69%
H-NMR (200MHz, CDCl3):δ ppm, 1H(8.22/S, 8.32/D, 7.75/D, 8.13/D, 7.41/M, 7.91/D, 7.89/D, 7.32/M, 7.38/M, 7.66/D, 8.51/D, 7.36/M, 8.43/D) 2H(7.62/D, 7.20/D, 7.48/D, 6.81/M, 8.51/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 1H (8.22 / S, 8.32 / D, 7.75 / D, 8.13 / D, 7.41 / M, 7.91 / D, 7.89 / D, 7.32 / M, 7.38 / M D, 7.36 / M, 8.43 / D) 2H (7.62 / D, 7.20 / D, 7.48 / D, 6.81 / M, 8.51 / D)
LC/MS: m/z=654 [(M+1)+]LC / MS: m / z = 654 [(M + 1) < + &
실시예Example 8: 화합물 25 합성 8: Compound 25 Synthesis
중간체 21-1(10.0g, 0.019mol)에 9H-carbazole(3.8g, 0.023mol), Pd(PPh3)4(1.2g, 0.0010mol), potassium carbonate(5.3g, 0.038mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 25를 8.1g(수율 65%) 수득하였다400 ml of THF was added to 9.0 g-carbazole (3.8 g, 0.023 mol), Pd (PPh3) 4 (1.2 g, 0.0010 mol) and potassium carbonate (5.3 g, 0.038 mol) The mixture was stirred at 65 占 폚 for 18 hours to react. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC and subjected to column purification (n-hexane: MC) to obtain 8.1 g (yield: 65%
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.55/D, 7.94/D, 7.33/M, 7.25/M, 8.55/D, 8.12/D, 7.29/M, 7.50/M, 7.63/D, 7.20/D, 7.62/D, 8.32/D, 7.75/D, 8.22/S, 7.89/D, 7.32/M, 7.38/M, 7.66/D) 2H(6.81/D, 8.51/D) 3H(7.48/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 1H (7.55 / D, 7.94 / D, 7.33 / M, 7.25 / M, 8.55 / D, 8.12 / D, 7.29 / M, 7.50 / M, 7.63 / D , 7.20 / D, 7.62 / D, 8.32 / D, 7.75 / D, 8.22 / S, 7.89 / D, 7.32 / M, 7.38 / / D)
LC/MS: m/z=652 [(M+1)+] LC / MS: m / z = 652 [(M + 1) < + &
실시예Example 9: 화합물 26 합성 9: Compound 26 Synthesis
중간체 21-1(10.0g, 0.019mol)에 9H-pyrido[2,3-b]indole(4.9g, 0.023mol), Pd(PPh3)4(1.2g, 0.0010mol), potassium carbonate(5.3g, 0.038mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 26을 8.4g(수율 68%) 수득하였다.Pyrido [2,3-b] indole (4.9 g, 0.023 mol), Pd (PPh3) 4 (1.2 g, 0.0010 mol), potassium carbonate (5.3 g, 0.038 mol), 400 ml of THF was added, and the mixture was reacted at 65 DEG C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, and subjected to column separation on H 2 O: MC, followed by column purification (n-hexane: MC) to obtain 8.4 g (yield: 68%) of Compound 26.
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.20/D, 7.62/D, 7.55/D, 8.43/D, 7.36/M, 8.51/D, 8.55/D, 7.25/M, 7.33/M, 7.94/D, 7.75/D, 8.32/D, 8.22/S, 7.89/D, 7.32/M, 7.38/M, 7.66/D) 2H(6.81/D, 8.51/D) 3H(7.48/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 1H (7.20 / D, 7.62 / D, 7.55 / D, 8.43 / D, 7.36 / M, 8.51 / D, 8.55 / D, 7.25 / M, 7.33 / M D, 7.75 / D, 8.32 / D, 8.22 / S, 7.89 / D, 7.32 / M, 7.38 / M, 7.66 /
LC/MS: m/z=653 [(M+1)+]LC / MS: m / z = 653 [(M + 1) < + &
실시예Example 10: 화합물 30 합성 10: Compound 30 Synthesis
중간체 21-1(10.0g, 0.019mol)에 pyridin-2-ylboronic acid (2.9g, 0.023mol/중국산), Pd(PPh3)4(1.2g, 0.0010mol), potassium carbonate(5.3g, 0.038mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 30을 8.4g(수율 68%) 수득하였다Pyridine-2-ylboronic acid (2.9g, 0.023mol / Chinese acid), Pd (PPh3) 4 (1.2g, 0.0010mol) and potassium carbonate (5.3g, 0.038mol) were added to Intermediate 21-1 (10.0g, 0.019mol) , 400 ml of THF was added, and the mixture was reacted at 65 占 폚 for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC and subjected to column purification (n-hexane: MC) to obtain 8.4 g (yield: 68%
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.62/D, 7.20/D, 7.39/D, 8.58/D, 9.30/D, 7.70/M, 7.14/M, 8.53/D, 8.32/D, 7.75/D, 7.66/D, 7.38/M, 7.32/M, 7.89/D, 8.22/S) 2H(7.48/D, 6.81/D, 8.51/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 1H (7.62 / D, 7.20 / D, 7.39 / D, 8.58 / D, 9.30 / D, 7.70 / M, 7.14 / M, 8.53 / D, 8.32 / D , 7.75 / D, 7.66 / D, 7.38 / M, 7.32 / M, 7.89 / D, 8.22 /
LC/MS: m/z=564 [(M+1)+]LC / MS: m / z = 564 [(M + 1) < + &
실시예Example 11: 화합물 74 합성 11: Compound 74 Synthesis
(1) (One) 제조예Manufacturing example 11-1: 중간체 74-1 합성 11-1: Synthesis of Intermediate 74-1
중간체 1-1(10.0g, 0.031mol), Pd(OAc)2(0.6g, 0.003mol/sigma Aldrich), t-BuOK(33g, 0.295mol /sigma Aldrich), trihexylphosphine(0.84g, 0.003mol /sigma Aldrich)에 acetic acid 150ml를 넣고 25℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 중간체 74-1을 3.3g(수율 30%) 수득하였다.(0.3 g, 0.003 mol / sigma Aldrich), t-BuOK (33 g, 0.295 mol / sigma Aldrich), trihexylphosphine (0.84 g, 0.003 mol / sigma Aldrich) were added 150 ml of acetic acid and reacted at 25 ° C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC and subjected to column purification (n-hexane: MC) to obtain 3.3 g (yield: 30%) of Intermediate 74-1.
LC/MS: m/z=355 [(M+1)+]LC / MS: m / z = 355 [(M + 1) < + &
(2) (2) 제조예Manufacturing example 11-2: 화합물 74 합성 11-2: Compound 74 Synthesis
중간체 74-1(10.0g, 0.028mol)에 3-(9H-carbazol-9-yl)phenylboronic acid (9.6g, 0.033mol/중국산), Pd(PPh3)4(1.6g, 0.0014mol), potassium carbonate(7.6g, 0.056mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 74를 11.6g(수율 74%) 수득하였다.3- (9H-carbazol-9-yl) phenylboronic acid (9.6g, 0.033mol / Chinese acid), Pd (PPh3) 4 (1.6g, 0.0014mol), potassium carbonate (7.6 g, 0.056 mol), 400 ml of THF was added, and the mixture was reacted at 65 ° C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC and column-purified (n-hexane: MC) to obtain 11.6 g (yield: 74%) of Compound 74.
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.62/D, 7.20/D, 8.30/D, 8.60/S, 7.54/M, 7.52/D, 7.94/D, 7.33/M, 7.25/M, 8.55/D, 8.12/D, 7.29/M, 7.50/M, 7.63/D) 3H97.48/D ,6.81/M ,8.51/D ) H-NMR (200MHz, CDCl 3 ): δ ppm, 1H (7.62 / D, 7.20 / D, 8.30 / D, 8.60 / S, 7.54 / M, 7.52 / D, 7.94 / D, 7.33 / M, 7.25 / M D, 8.12 / D, 7.29 / M, 7.50 / M, 7.63 / D) 3H97.48 / D, 6.81 / M, 8.51 / D)
LC/MS: m/z=562 [(M+1)+] LC / MS: m / z = 562 [(M + 1) < + &
실시예Example 12: 화합물 75 합성 12: Compound 75 Synthesis
중간체 74-1(10.0g, 0.028mol)에 3-(9H-pyrido[2,3-b]indol-9-yl)phenylboronic acid(9.6g, 0.033mol/중국산), Pd(PPh3)4(1.6g, 0.0014mol), potassium carbonate(7.6g, 0.056mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 75를 11.7g(수율 75%) 수득하였다.Pd (PPh3) 4 (1.6 g, 0.033 mol) was added to Intermediate 74-1 (10.0 g, 0.028 mol) g, 0.0014 mol) and potassium carbonate (7.6 g, 0.056 mol) were added 400 ml of THF and the mixture was reacted at 65 ° C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC, and subjected to column purification (n-hexane: MC) to obtain 11.7 g (yield 75%) of Compound 75.
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.20/D, 7.62/D, 8.60/S, 8.30/D, 7.54/M, 7.52/D, 7.94/D, 7.33/M, 7.25/M, 8.55/D, 8.51/D, 7.36/M, 8.43/D) 3H(7.48/D, 6.81/M, 8.51/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 1H (7.20 / D, 7.62 / D, 8.60 / S, 8.30 / D, 7.54 / M, 7.52 / D, 7.94 / D, 7.33 / M, 7.25 / M , 8.55 / D, 8.51 / D, 7.36 / M, 8.43 / D) 3H (7.48 / D, 6.81 /
LC/MS: m/z=563[(M+1)+] LC / MS: m / z = 563 [(M + 1) < + &
실시예Example 13: 화합물 76 합성 13: Compound 76 Synthesis
중간체 74-1(10.0g, 0.028mol)에 3-(4,6-diphenyl-1,3,5-triazin-2-yl)phenylboronic acid(11.7g, 0.033mol/중국산), Pd(PPh3)4(1.6g, 0.0014mol), potassium carbonate(7.6g, 0.056mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 76을 13.5g(수율 77%) 수득하였다.3- (4,6-diphenyl-1,3,5-triazin-2-yl) phenylboronic acid (11.7 g, 0.033 mol / Chinese acid) and Pd (PPh3) 4 (10.0 g, 0.028 mol) (1.6 g, 0.0014 mol) and potassium carbonate (7.6 g, 0.056 mol) were added 400 ml of THF and the mixture was reacted at 65 ° C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, and subjected to column separation on H 2 O: MC, followed by column purification (n-hexane: MC) to obtain 13.5 g (yield: 77%) of 76.
H-NMR (200MHz, CDCl3):δ ppm, 1H(7.20/D, 7.62/D, 8.21/S, 8.26/D, 7.60/M, 8.30/D) 2H(7.41/M) 3H(6.81/M, 7.48/D, 8.51/D) 4H(8.28/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 1H (7.20 / D, 7.62 / D, 8.21 / S, 8.26 / D, 7.60 / M, 8.30 / D) 2H (7.41 / M) 3H (6.81 / M , 7.48 / D, 8.51 / D) 4H (8.28 / D)
LC/MS: m/z=628 [(M+1)+] LC / MS: m / z = 628 [(M + 1) < + &
실시예Example 14: 화합물 82 합성 14: Compound 82 Synthesis
(1) (One) 제조예Manufacturing example 14-1: 중간체 82-1 합성 14-1: Synthesis of Intermediate 82-1
중간체 1-1(10.0g, 0.031mol), Pd(OAc)2(0.6g, 0.003mol/sigma Aldrich), t-BuOK(33g, 0.295mol), trihexylphosphine(0.84g, 0.003mol)에 acetic acid 150ml를 넣고 25℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 중간체 82-1을 3.3g(수율 23%) 수득하였다.Acetic acid (150 ml) was added to Intermediate 1-1 (10.0 g, 0.031 mol), Pd (OAc) 2 (0.6 g, 0.003 mol / sigma Aldrich), t- BuOK (33 g, 0.295 mol), trihexylphosphine Followed by stirring at 25 ° C for 18 hours. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC and subjected to column purification (n-hexane: MC) to obtain 3.3 g (yield: 23%) of Intermediate 82-1.
LC/MS: m/z=458 [(M+1)+]LC / MS: m / z = 458 [(M + 1) < + &
(2) (2) 제조예Manufacturing example 14-2: 중간체 82-2 합성 14-2: Intermediate 82-2 Synthesis
중간체 82-1(10.0g, 0.022mol)에 9H-carbazole(8.6g, 0.052mol), Pd(PPh3)4(1.3g, 0.0011mol), potassium carbonate(6.1g, 0.044mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 중간체 82-2를 11.9g(수율 75%) 수득하였다.400 ml of THF was added to 9H-carbazole (8.6 g, 0.052 mol), Pd (PPh3) 4 (1.3 g, 0.0011 mol) and potassium carbonate (6.1 g, 0.044 mol) The mixture was stirred at 65 占 폚 for 18 hours to react. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC and subjected to column purification (n-hexane: MC) to obtain 11.9 g (yield 75%) of Intermediate 82-2.
LC/MS: m/z=720 [(M+1)+]LC / MS: m / z = 720 [(M + 1) < + &
(3) (3) 제조예Manufacturing example 14-3: 화합물 82 합성 14-3: Compound 82 Synthesis
중간체 82-2(10.0g, 0.014mol)에 9H-carbazole(5.6g, 0.033mol), Pd(PPh3)4(0.8g, 0.0007mol), potassium carbonate(3.9g, 0.028mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 82를 10.3g(수율 75%) 수득하였다.400 ml of THF was added to 9H-carbazole (5.6 g, 0.033 mol), Pd (PPh3) 4 (0.8 g, 0.0007 mol) and potassium carbonate (3.9 g, 0.028 mol) The mixture was stirred at 65 占 폚 for 18 hours to react. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC, and subjected to column purification (n-hexane: MC) to obtain 10.3 g (yield 75%) of Compound 82.
H-NMR (200MHz, CDCl3):δ ppm, 4H(7.48/D, 7.55/D, 7.94/D, 7.33/M, 7.25/M, 8.55/D, 8.12/D, 7.29/M, 7.50/M, 7.63/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 4H (7.48 / D, 7.55 / D, 7.94 / D, 7.33 / M, 7.25 / M, 8.55 / D, 8.12 / D, 7.29 / M, 7.50 / M , 7.63 / D)
LC/MS: m/z=982 [(M+1)+] LC / MS: m / z = 982 [(M + 1) < + &
실시예Example 15: 화합물 83 합성 15: Compound 83 Synthesis
중간체 82-2(10.0g, 0.014mol)에 9H-pyrido[2,3-b]indole(5.6g, 0.033mol), Pd(PPh3)4(0.8g, 0.0007mol), potassium carbonate(3.9g, 0.028mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 83을 5.8g(수율 73%) 수득하였다.Pyrido [2,3-b] indole (5.6g, 0.033mol), Pd (PPh3) 4 (0.8g, 0.0007mol), potassium carbonate (3.9g, 0.014mol) 0.028 mol), 400 ml of THF was added, and the mixture was reacted at 65 캜 for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC, and subjected to column purification (n-hexane: MC) to obtain 5.8 g (yield: 73%) of Compound 83.
H-NMR (200MHz, CDCl3):δ ppm, 2H(8.51/D, 7.36/M, 7.63/D, 8.43/D, 7.50/M, 7.21/M, 8.12/D) 4H(7.48/D, 7.55/D, 7.94/D, 7.33/M, 7.25/M, 8.55/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 2H (8.51 / D, 7.36 / M, 7.63 / D, 8.43 / D, 7.50 / M, 7.21 / M, 8.12 / D) 4H (7.48 / D, 7.55 / D, 7.94 / D, 7.33 / M, 7.25 / M, 8.55 / D)
LC/MS: m/z= 563[(M+1)+]LC / MS: m / z = 563 [(M + 1) < + &
실시예Example 16: 화합물 85 합성 16: Compound 85 Synthesis
중간체 82-2(10.0g, 0.014mol)에 dibenzo[b,d]furan-2-ylboronic acid(7.0g, 0.033mol), Pd(PPh3)4(0.8g, 0.0007mol), potassium carbonate(3.9g, 0.028mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 85를 9.8g(수율 71%) 수득하였다.Dibenzo [b, d] furan-2-ylboronic acid (7.0 g, 0.033 mol), Pd (PPh3) 4 (0.8 g, 0.0007 mol), potassium carbonate (3.9 g, , 0.028 mol) was added 400 ml of THF, and the mixture was reacted at 65 ° C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC and subjected to column purification (n-hexane: MC) to obtain Compound (85) (9.8 g, 71%).
H-NMR (200MHz, CDCl3):δ ppm, 2H(7.94/D, 7.33/M, 7.25/M, 7.29/M, 8.55/D, 8.12/D, 7.50/M, 7.63/D, 7.20/D, 7.48/D, 7.62/D, 7.55/D, 8.22/S, 8.32/D, 7.75/D, 7.89/D, 7.32/M, 7.38/M, 7.66/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 2H (7.94 / D, 7.33 / M, 7.25 / M, 7.29 / M, 8.55 / D, 8.12 / D, 7.50 / M, 7.63 / D, 7.20 / D , 7.48 / D, 7.62 / D, 7.55 / D, 8.22 / S, 8.32 / D, 7.75 / D, 7.89 / D, 7.32 / M, 7.38 /
LC/MS: m/z= 984[(M+1)+]LC / MS: m / z = 984 [(M + 1) < + &
실시예Example 17: 화합물 90 합성 17: Compound 90 Synthesis
중간체 82-2(10.0g, 0.014mol)에 dibenzo[b,d]furan-4-ylboronic acid (7.0g, 0.033mol), Pd(PPh3)4(0.8g, 0.0007mol), potassium carbonate(3.9g, 0.028mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 90을 10.0g(수율 72%) 수득하였다.Dibenzo [b, d] furan-4-ylboronic acid (7.0 g, 0.033 mol), Pd (PPh3) 4 (0.8 g, 0.0007 mol), potassium carbonate (3.9 g, , 0.028 mol) was added 400 ml of THF, and the mixture was reacted at 65 ° C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC, and subjected to column purification (n-hexane: MC) to obtain 10.0 g (yield 72%) of Compound 90.
H-NMR (200MHz, CDCl3):δ ppm, 2H(7.94/D, 7.33/M, 7.25/M, 8.55/D, 7.20/D, 7.29/M, 8.12/D, 7.50/M, 7.63/D, 7.48/D, 7.62/D, 8.13/D, 7.41/M, 7.91/D, 7.89/D, 7.32/M, 7.38/M, 7.66/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 2H (7.94 / D, 7.33 / M, 7.25 / M, 8.55 / D, 7.20 / D, 7.29 / M, 8.12 / D, 7.50 / M, 7.63 / D , 7.48 / D, 7.62 / D, 8.13 / D, 7.41 / M, 7.91 / D, 7.89 / D, 7.32 / M, 7.38 /
LC/MS: m/z= 984[(M+1)+] LC / MS: m / z = 984 [(M + 1) < + &
실시예Example 18: 화합물 91 합성 18: Compound 91 Synthesis
중간체 82-2(10.0g, 0.014mol)에 dibenzo[b,d]thiophen-4-ylboronic acid (7.5g, 0.033mol), Pd(PPh3)4(0.8g, 0.0007mol), potassium carbonate(3.9g, 0.028mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 91을 10.7g(수율 75%) 수득하였다.Dibenzo [b, d] thiophen-4-ylboronic acid (7.5g, 0.033mol), Pd (PPh3) 4 (0.8g, 0.0007mol), potassium carbonate (3.9g , 0.028 mol) was added 400 ml of THF, and the mixture was reacted at 65 ° C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC and subjected to column purification (n-hexane: MC) to obtain 10.7 g (yield: 75%) of Compound 91.
H-NMR (200MHz, CDCl3):δ ppm, 2H(8.31/D, 7.61/M, 8.47/D, 8.45/D, 7.52/M, 7.50/M, 7.98/D, 7.62/D, 7.20/D, 7.48/D, 7.55/D, 7.94/D, 7.33/M, 7.25/M, 8.55/D, 8.12/D, 7.29/M, 7.50/M, 7.63/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 2H (8.31 / D, 7.61 / M, 8.47 / D, 8.45 / D, 7.52 / M, 7.50 / M, 7.98 / D, 7.62 / D, 7.20 / D , 7.48 / D, 7.55 / D, 7.94 / D, 7.33 / M, 7.25 / M, 8.55 / D, 8.12 / D, 7.29 / M, 7.50 /
LC/MS: m/z= 1013[(M+1)+]LC / MS: m / z = 1013 [(M + 1) < + &
실시예Example 19: 화합물 96 합성 19: Compound 96 Synthesis
중간체 82-2(10.0g, 0.014mol)에 pyridin-3-ylboronic acid (4.1g, 0.033mol), Pd(PPh3)4(0.8g, 0.0007mol), potassium carbonate(3.9g, 0.028mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 96을 7.9g(수율 70%) 수득하였다.To a solution of pyridin-3-ylboronic acid (4.1g, 0.033mol), Pd (PPh3) 4 (0.8g, 0.0007mol) and potassium carbonate (3.9g, 0.028mol) in THF And the mixture was reacted at 65 ° C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC, and subjected to column purification (n-hexane: MC) to obtain 7.9 g (yield 70%) of Compound 96.
H-NMR (200MHz, CDCl3):δ ppm, 2H(7.94/D, 7.33/M, 7.25/M, 8.55/D, 8.12/D, 7.29/M, 7.50/M, 7.63/D, 7.55/D, 7.48/D, 7.54/D, 7.68/D, 9.75/S, 8.93/D, 7.63/M, 8.76/D) H-NMR (200MHz, CDCl 3 ): δ ppm, 2H (7.94 / D, 7.33 / M, 7.25 / M, 8.55 / D, 8.12 / D, 7.29 / M, 7.50 / M, 7.63 / D, 7.55 / D , 7.48 / D, 7.54 / D, 7.68 / D, 9.75 / S, 8.93 / D, 7.63 /
LC/MS: m/z= 805[(M+1)+]LC / MS: m / z = 805 [(M + 1) < + &
실시예Example 20: 화합물 97 합성 20: Compound 97 Synthesis
중간체 82-2(10.0g, 0.014mol)에 biphenyl-3-ylboronic acid (6.5g, 0.033mol), Pd(PPh3)4(0.8g, 0.0007mol), potassium carbonate(3.9g, 0.028mol)에 THF 400ml를 넣고 65℃에서 18시간 교반하여 반응시켰다. 반응 종료 후 냉각하여 H20 : MC에 층분리 후 컬럼정제(n-Hexane :MC)하여 화합물 97을 9.5g(수율 71%) 수득하였다.3-ylboronic acid (6.5g, 0.033mol), Pd (PPh3) 4 (0.8g, 0.0007mol) and potassium carbonate (3.9g, 0.028mol) were added to intermediate 82-2 (10.0g, 0.014mol) And the mixture was reacted at 65 ° C for 18 hours with stirring. After completion of the reaction, the reaction mixture was cooled, separated into H 2 O: MC and subjected to column purification (n-hexane: MC) to obtain 9.5 g (yield: 71%) of 97.
H-NMR (200MHz, CDCl3):δ ppm, 2H(7.94/D, 7.33/M, 7.25/M, 8.55/D, 7.29/M, 8.12/D, 7.50/M, 7.63/D, 7.55/D, 7.48/D, 7.20/D, 7.62/D, 8.21/D, 8.26/D, 7.60/M, 7.54/D, 7.41/M) 4H(7.52/D, 7.51/M) H-NMR (200MHz, CDCl 3 ): δ ppm, 2H (7.94 / D, 7.33 / M, 7.25 / M, 8.55 / D, 7.29 / M, 8.12 / D, 7.50 / M, 7.63 / D, 7.55 / D (7.42 / D, 7.20 / D, 7.62 / D, 8.21 / D, 8.26 / D, 7.60 / M, 7.54 / D, 7.41 /
LC/MS: m/z= 956[(M+1)+]
LC / MS: m / z = 956 [(M + 1) < + &
본 발명의 실시예에서 쓰이는 약어는 아래와 같다.Abbreviations used in the embodiment of the present invention are as follows.
NPB: N,N'-Bis(naphthalen-1-yl)-N,N'-bis(phenyl)benzidineNPB: N, N'-bis (naphthalen-1-yl) -N,
Ir(ppy)3: Iridium, tris(2-phenylpyidine)Ir (ppy) 3 : Iridium, tris (2-phenylpyidine)
Balq: Bis(2-methyl-8-quinolinolato-N1,O8)-(1,1'-Biphenyl-4-olato)aluminumBalq: Bis (2-methyl-8-quinolinolato-N1, O8) - (1,1'-Biphenyl-4-olato) aluminum
Alq3: tris(8-quinolinolato)-aluminium(III)Alq 3 : tris (8-quinolinolato) -aluminium (III)
CBP: (4,4-N,N-dicarbazole)biphenyl
CBP: (4,4-N, N-dicarbazole) biphenyl
소자실시예Device Example 1: 화합물 1을 1: Compound 1 발광층의The light- 호스트 재료로 하여 As a host material 유기전계발광소자Organic electroluminescent device 제조 Produce
ITO로 코팅된 유리기판 위에 NPB를 증착하여 120nm의 정공수송층을 형성하였으며, 이어서 Ir(ppy)3을 도펀트로 하여 화합물 1의 증착속도를 0.1nm/sec, Ir(ppy)3 증착속도를 0.009nm/sec로 증착하고, 증착속도 비율이 9%가 되도록 Ir(ppy)3을 도핑하여 정공수송층 상에 발광층을 30nm 두께로 형성하였다.Depositing NPB on the glass substrate coated with ITO to form a hole transport layer was of 120nm, followed by Ir (ppy) the deposition rate of the compounds 1 to 3 as the dopant 0.1nm / sec, Ir (ppy) 3 deposition rate 0.009nm / sec, and Ir (ppy) 3 was doped so that the deposition rate ratio was 9% to form a light emitting layer with a thickness of 30 nm on the hole transport layer.
그 위에 Balq를 10nm 두께로 증착하여 정공이 발광층을 지나 전자수송층으로 이동하는 것을 방지하는 정공차단층을 형성하고, 그 위에 Alq3를 증착하여 40nm의 전자수송층을 형성하였으며, 그 위에 불화리튬을 증착하여 1nm의 전자주입층을 형성하였다. 전자주입층 상에 알루미늄을 증착하여 120nm의 음극을 형성하여 유기전계발광소자를 제조하였다.Balq was deposited thereon to a thickness of 10 nm to form a hole blocking layer for preventing holes from moving to the electron transporting layer through the light emitting layer, and Alq 3 was deposited thereon to form an electron transporting layer of 40 nm, and lithium fluoride Thereby forming an electron injection layer having a thickness of 1 nm. Aluminum was deposited on the electron injecting layer to form a 120 nm cathode, thereby fabricating an organic electroluminescent device.
이때, 각 물질의 증착속도는 유기물질인, 화합물 1, NPB, Alq3, Balq는 0.1 nm/sec, 불화리튬은 0.01 nm/sec, 알루미늄은 0.5 nm/sec로 하였다.At this time, the deposition rate of each material was set to 0.1 nm / sec for compound 1, NPB, Alq 3 , Balq, 0.01 nm / sec for lithium fluoride, and 0.5 nm / sec for aluminum.
소자실시예Device Example 2 내지 20 2 to 20
상기 화합물 1 대신 하기 표 1에 기재된 발광재료로 사용한 것을 제외하고는 소자실시예 1과 동일한 방법으로 소자실시예 2 내지 20의 유기전계발광소자를 제조하였다.An organic electroluminescent device of each of the device embodiments 2 to 20 was fabricated in the same manner as in the device example 1, except that the compound 1 was used instead of the luminescent material described in the following table 1.
소자비교예Device comparison example 1 One
상기 화합물 1 대신 (4,4-N,N-dicarbazole)biphenyl(CBP)를 발광재료로 사용한 것을 제외하고는 소자실시예 1과 동일한 방법으로 소자비교예 1의 유기전계발광소자를 제조하였다.
An organic electroluminescent device of Comparative Example 1 was prepared in the same manner as in Example 1 except that (4,4-N, N-dicarbazole) biphenyl (CBP)
이하, 상기 소자실시예 1 내지 20과 소자비교예 1에 따라 제조된 유기전계발광소자의 특성을 비교한 결과를 하기 표 1 에 나타내었다.Hereinafter, the characteristics of the organic electroluminescent device manufactured according to the device embodiments 1 to 20 and the device comparison example 1 are compared and the results are shown in Table 1 below.
(at 1000cd/m2)The driving voltage (V)
(at 1000 cd / m 2 )
(cd/A)Luminous efficiency
(cd / A)
(CIE(x,y))Color coordinates
(CIE (x, y))
구동전압 및 발광효율 측정Measurement of driving voltage and luminous efficiency
위에서 만든 유기발광소자(기판크기: 25*25mm2/증착면적: 2*2mm2)를 IVL 측정셋트(CS-2000+지그+IVL프로그램)에 고정 한 후 전류를 1mA/m2씩 상승시키며 증착면의 발광 휘도(cd/m2), 구동전압(V), 전류밀도(A/m2), 발광효율(cd/A)을 측정하여 휘도가 1000cd/m2 일 때 구동전압과 발광효율을 상기 표 1에 나타내었다.The organic light emitting element made from above (substrate size: 25 * 25mm 2 / deposition area: 2 * 2mm 2) an IVL measuring set (CS-2000 + fixture + IVL program) by the current deposition raises by 1mA / m 2 and then fixed to the the brightness of light emitted by the surface (cd / m 2), a driving voltage (V), current density (a / m 2), luminous efficiency (cd / a) drive voltage when measured brightness is 1000cd / m 2 days and luminous efficiency Shown in Table 1 above.
표 1에 따르면, 본 발명에 따른 유기전계발광소자용 화합물이 유기전계발광소자의 발광층의 호스트 물질로 사용될 때 종래의 CBP를 발광재료로 사용할 때보다 구동전압은 상당히 떨어지고, 발광효율은 상당히 향상된 것을 알 수 있다.According to Table 1, when the compound for an organic electroluminescence device according to the present invention is used as a host material of a light emitting layer of an organic electroluminescence device, the driving voltage is considerably lower than that of the conventional CBP as a light emitting material and the luminous efficiency is considerably improved Able to know.
Claims (10)
[구조식 2]
상기 [구조식 2]에서,
R1 내지 R4는 서로 같거나 다를 수 있고, 또는 이고,
상기 Y1은 산소 원자 또는 황 원자이며, 상기 R6 내지 R9는 수소 원자이고, 상기 X3 내지 X7은 서로 같거나 다를 수 있고, 각각 독립적으로 질소원자 또는 이고, 상기 R27은 수소 원자이다.The compound for an organic electroluminescence device represented by the following structural formula 2:
[Structural formula 2]
In the above formula 2,
R 1 to R 4 may be the same or different from each other, or ego,
Wherein Y 1 is an oxygen atom or a sulfur atom, R 6 to R 9 are each a hydrogen atom, X 3 to X 7 may be the same or different from each other, And R < 27 > is a hydrogen atom.
상기 유기전계발광소자용 화합물은 하기 화학식으로 표시되는 화합물 82 내지 93 중에서 선택된 어느 하나인 것을 특징으로 하는 유기전계발광소자용 화합물:
3. The method of claim 2,
Wherein the compound for an organic electroluminescence device is any one selected from compounds 82 to 93 represented by the following formula:
상기 단수 또는 복수의 유기물층 중에서 선택된 1종 이상의 유기물층은 제2항에 따른 유기전계발광소자용 화합물을 포함하는 것을 특징으로 하는 유기전계발광소자.1. An organic electroluminescent device comprising a first electrode, a second electrode, and a single or a plurality of organic layers between the first electrode and the second electrode,
The organic electroluminescent device according to claim 2, wherein the at least one organic compound layer selected from the group consisting of the single or plural organic compound layers comprises the compound for organic electroluminescence devices according to claim 2.
상기 단수 또는 복수의 유기물층은 발광층을 포함하는 것을 특징으로 하는 유기전계발광소자.8. The method of claim 7,
Wherein the single or plural organic layers include a light emitting layer.
상기 복수의 유기물층은 발광층을 포함하고, 상기 복수의 유기물층은 전자주입층, 전자수송층, 정공차단층, 전자차단층, 정공수송층 및 정공주입층 중에서 선택된 1종 이상을 추가로 포함하는 것을 특징으로 하는 유기전계발광소자.8. The method of claim 7,
Wherein the plurality of organic layers include a light emitting layer and the plurality of organic layers further include at least one selected from an electron injecting layer, an electron transporting layer, a hole blocking layer, an electron blocking layer, a hole transporting layer and a hole injecting layer Organic electroluminescent device.
상기 발광층은 호스트와 도펀트를 포함하는 것을 특징으로 하는 유기전계발광소자.9. The method of claim 8,
Wherein the light emitting layer comprises a host and a dopant.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020130102993A KR101576921B1 (en) | 2013-08-29 | 2013-08-29 | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020130102993A KR101576921B1 (en) | 2013-08-29 | 2013-08-29 | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20150025379A KR20150025379A (en) | 2015-03-10 |
| KR101576921B1 true KR101576921B1 (en) | 2015-12-11 |
Family
ID=53021627
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020130102993A KR101576921B1 (en) | 2013-08-29 | 2013-08-29 | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same |
Country Status (1)
| Country | Link |
|---|---|
| KR (1) | KR101576921B1 (en) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10777749B2 (en) * | 2015-05-07 | 2020-09-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102617952B1 (en) * | 2018-03-19 | 2023-12-26 | 솔루스첨단소재 주식회사 | Organic light-emitting compound and organic electroluminescent device comprising the same |
| CN110563727A (en) * | 2019-08-26 | 2019-12-13 | 武汉华星光电半导体显示技术有限公司 | luminescent material having spiroazafluorene compound and luminescent device using the same |
| CN110776496A (en) * | 2019-11-18 | 2020-02-11 | 苏州久显新材料有限公司 | 3, 4-diazaspiro fluorene derivative and synthesis method thereof, and electronic device containing 3, 4-diazaspiro fluorene derivative |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003020847A1 (en) * | 2001-09-03 | 2003-03-13 | Canon Kabushiki Kaisha | Organic luminescence device |
-
2013
- 2013-08-29 KR KR1020130102993A patent/KR101576921B1/en active IP Right Grant
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003020847A1 (en) * | 2001-09-03 | 2003-03-13 | Canon Kabushiki Kaisha | Organic luminescence device |
Non-Patent Citations (1)
| Title |
|---|
| Chemical Abstarct 153:591572* |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20150025379A (en) | 2015-03-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101593465B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR101447961B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR101521790B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR101447959B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR101584753B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR101527181B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR101550429B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR101499102B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR101626523B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR101468493B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR20150012974A (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR20150022615A (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR101666825B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR20140004549A (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR101396552B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR101576921B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR20160041223A (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR20150059680A (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR101562883B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR20130069431A (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR20150037133A (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR101562882B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR101438080B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR101502764B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same | |
| KR101516279B1 (en) | Novel compound for organic electroluminescent device and organic electroluminescent device comprising the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination | ||
| E902 | Notification of reason for refusal | ||
| GRNT | Written decision to grant |