KR100691543B1 - New material for transporting electron and organic electroluminescent display using the same - Google Patents
New material for transporting electron and organic electroluminescent display using the same Download PDFInfo
- Publication number
- KR100691543B1 KR100691543B1 KR1020020003025A KR20020003025A KR100691543B1 KR 100691543 B1 KR100691543 B1 KR 100691543B1 KR 1020020003025 A KR1020020003025 A KR 1020020003025A KR 20020003025 A KR20020003025 A KR 20020003025A KR 100691543 B1 KR100691543 B1 KR 100691543B1
- Authority
- KR
- South Korea
- Prior art keywords
- formula
- light emitting
- biphenyl
- phenyl
- naphthyl
- Prior art date
Links
Images
Classifications
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6572—Polycyclic condensed heteroaromatic hydrocarbons comprising only nitrogen in the heteroaromatic polycondensed ring system, e.g. phenanthroline or carbazole
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D235/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings
- C07D235/02—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings condensed with carbocyclic rings or ring systems
- C07D235/04—Benzimidazoles; Hydrogenated benzimidazoles
- C07D235/06—Benzimidazoles; Hydrogenated benzimidazoles with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached in position 2
- C07D235/08—Radicals containing only hydrogen and carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D235/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings
- C07D235/02—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings condensed with carbocyclic rings or ring systems
- C07D235/04—Benzimidazoles; Hydrogenated benzimidazoles
- C07D235/18—Benzimidazoles; Hydrogenated benzimidazoles with aryl radicals directly attached in position 2
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/615—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene
- H10K85/626—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene containing more than one polycyclic condensed aromatic rings, e.g. bis-anthracene
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/14—Carrier transporting layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/615—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene
- H10K85/622—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene containing four rings, e.g. pyrene
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
- H10K85/633—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine comprising polycyclic condensed aromatic hydrocarbons as substituents on the nitrogen atom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/654—Aromatic compounds comprising a hetero atom comprising only nitrogen as heteroatom
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S428/00—Stock material or miscellaneous articles
- Y10S428/917—Electroluminescent
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Electroluminescent Light Sources (AREA)
- Luminescent Compositions (AREA)
Abstract
본 발명은 유기 발광 소자의 수명과 효율을 크게 향상시킬 수 있는 새로운 전자 주입 수송층의 물질 및 이를 이용한 유기 발광 소자에 관한 것으로, 안트라센의 4 개의 치환장소에 1 개 내지 2 개의 헤테로 작용기가 도입된 신규의 물질, 및 이를 함유하는 유기화합물층을 포함하는 유기 발광 소자를 제공한다.The present invention relates to a material of a novel electron injection transport layer and an organic light emitting device using the same, which can greatly improve the lifespan and efficiency of the organic light emitting device, and a novel in which one or two hetero functional groups are introduced at four substitution sites of anthracene. It provides an organic light emitting device comprising a material of, and an organic compound layer containing the same.
유기 발광 소자, 전자 주입 수송층, 안트라센, 헤테로 작용기, 유기화합물층Organic light emitting device, electron injection transport layer, anthracene, hetero functional group, organic compound layer
Description
도 1은 유기 전기발광 소자의 한 예를 나타내는 단면도이다.1 is a cross-sectional view showing an example of an organic electroluminescent device.
도면부호 1은 기판, 2는 양극, 3은 정공주입층, 4는 정공수송층, 5는 유기발광층, 6은 전자수송층, 7은 음극이다.Reference numeral 1 denotes a substrate, 2 an anode, 3 a hole injection layer, 4 a hole transport layer, 5 an organic light emitting layer, 6 an electron transport layer, and 7 a cathode.
본 발명은 유기 발광 소자의 수명과 효율을 크게 향상시킬 수 있는 새로운 전자 주입 수송층의 물질 및 이를 이용한 유기 발광 소자에 관한 것이다.The present invention relates to a material of a novel electron injection transport layer and an organic light emitting device using the same, which can greatly improve the lifetime and efficiency of the organic light emitting device.
전기 발광 소자는 고체 형광성 물질의 전기 발광을 이용한 발광 디바이스로 정의되며, 현재 무기계 재료를 발광체로 이용한 무기물 전기 발광 소자가 실용화되고 있다. 그러나 무기물 전기 발광 소자는 발광시키기 위한 필요한 전압이 100 V 이상으로 높고 청색 발광이 어렵기 때문에 RGB(red, green, blue)의 삼원색에 의한 풀 컬러화가 곤란하다.An electroluminescent device is defined as a light emitting device using electroluminescence of a solid fluorescent material, and inorganic electroluminescent devices using inorganic materials as light emitters have been put to practical use. However, the inorganic electroluminescent device has a high voltage of more than 100 V and is difficult to emit blue light, making it difficult to make full colors by three primary colors of RGB (red, green, blue).
한편, 유기 재료를 이용한 전기 발광 소자에 관한 연구도 오래 전부터 주목받고 검토가 진행되어 왔지만 발광 효율이 낮아 실용화 연구는 크게 진전되지는 않 고 있다. 유기 발광 소자는 단분자 또는 고분자로 이루어진 유기물 박막에 음극과 양극을 통하여 주입된 전자와 정공이 여기자를 형성하고, 형성된 여기자로부터 특정한 파장의 빛이 발생되는 원리를 이용한 것으로 1965년에 포프(Pope) 등에 의해 안트라센의 단결정으로부터 처음 발견되었다. 이어 1987년에 코닥사의 탕(Tang) 에 의하여 유기 재료를 정공 수송층과 발광층의 2 층으로 나눈 기능 분리형의 적층 구조를 갖는 유기 전기 발광 소자가 제안되고, 10 V 이하의 저 전압에도 불구하고 1000 cd/㎡ 이상의 높은 발광휘도가 얻어지는 것이 확인되었다(Tang, C. W.; VanSlyke, S. A. Appl. Phys. Lett. 1987, 51, 913.). 이후에, 유기 전기발광 소자가 갑자기 주목받기 시작하고 현재도 동일한 기능분리형의 적층 구조를 갖는 유기 전기발광 소자에 관한 연구가 활발하게 진행되고있다.On the other hand, research on the electroluminescent device using organic materials has also been focused and reviewed for a long time, but due to the low luminous efficiency, practical research has not progressed much. The organic light emitting device uses a principle that electrons and holes injected through a cathode and an anode form excitons in an organic thin film made of a single molecule or a polymer, and light of a specific wavelength is generated from the formed excitons. It was first discovered from single crystals of anthracene. Then, in 1987, Kodak's Tang proposed an organic electroluminescent device having a laminated structure of a functional separation type in which an organic material was divided into two layers, a hole transport layer and a light emitting layer. It was confirmed that a high luminous luminance of more than /
일반적인 유기 전기발광 소자의 구조는 도 1에서 보여주는 바와 같이 기판(1), 양극(2), 정공을 양극으로부터 받아들이는 정공주입층(3), 정공을 이송하는 정공 수송층(4), 정공과 전자가 결합하여 빛을 내는 발광층(5), 전자를 음극으로부터 받아들여 발광층으로 전달하는 전자 수송층(6), 및 음극(7)으로 구성되어 있다. 경우에 따라서는 별도의 발광층(5) 없이 전자 수송층(6)이나 정공 이송층(4)에 소량의 형광 또는 인광성 염료를 도핑하여 발광층을 구성할 수도 있으며, 고분자를 사용할 경우에는 일반적으로 정공 수송층(4)과 발광층(5), 및 전자 수송층(6)의 역할을 하나의 고분자가 동시에 수행할 수 있다. 두 전극 사이의 유기물 박막층들은 진공증착법 또는 스핀코팅, 잉크젯프린팅, 롤코팅 등의 방법으로 형성되며, 음극으로부터 전자의 효율적인 주입을 위해 별도의 전자주입층을 삽입하는 경우도 있다. The structure of a general organic electroluminescent device is a substrate (1), an anode (2), a hole injection layer (3) for receiving holes from the anode, a hole transport layer (4) for transporting holes, holes and electrons as shown in FIG. Is composed of a
이렇게 유기 전기발광 소자를 다층 박막 구조로 제작하는 이유는 전극과 유기물사이의 계면 안정화를 위해서 또는 유기물질의 경우 정공과 전자의 이동속도가 크게 차이가 나므로 적절한 정공 수송층과 전자 수송층을 사용하면 정공과 전자가 발광층으로 효과적으로 전달될 수 있고 발광층에서 정공과 전자의 밀도가 균형을 이루도록 하면 발광효율을 높일 수 있기 때문이다.The reason why the organic electroluminescent device is manufactured in a multi-layered thin film structure is to stabilize the interface between the electrode and the organic material or in the case of organic materials, because the movement speed of the holes and the electrons varies greatly. This is because the electrons can be effectively transferred to the light emitting layer and the light emission efficiency can be increased by balancing the density of holes and electrons in the light emitting layer.
정공을 주입시키는 정공 주입 물질로 양극과 안정된 계면을 유지하면서 열적 안정성이 우수한 화합물을 사용하는데, 대표적인 예로는 미국의 코닥사에서 연구되어 미국특허 제4,356,429호에 개시된 포피린계 구리 착화합물인 구리 프탈로시아닌 (copper phthalocyanine, 이하 CuPc라 함)이 있다. 정공주입층으로 CuPc가 가장 안정하기 때문에 널리 사용되어 왔으나 적색영역에서의 흡수가 강하기 때문에 풀컬러(full color)의 디스플레이를 제작할 때에는 문제가 된다.As a hole injection material for injecting holes, a compound having excellent thermal stability while maintaining a stable interface with the positive electrode is used. A representative example is copper phthalocyanine, a porphyrin-based copper complex disclosed in US Pat. No. 4,356,429. phthalocyanine, hereinafter referred to as CuPc). Since CuPc is the most stable hole injecting layer, it has been widely used, but it is a problem when manufacturing a full color display due to its strong absorption in the red region.
정공수송층용 유기 단분자 물질은 높은 정공 이동 속도와 전기적 안정성이 뛰어난 아릴아민계가 대표적 물질이다. 아릴아민계 물질의 열적 안정성을 높이기 위해 나프틸 치환체나 스피로 그룹을 도입한 정공수송물질이 보고되었다(미국특허 제5,554,459호 및 미국특허 제5,840,217호).The organic monomolecular material for the hole transport layer is typically an arylamine based material having high hole transport speed and excellent electrical stability. In order to increase the thermal stability of the arylamine-based material, a hole transport material introducing a naphthyl substituent or a spiro group has been reported (US Pat. Nos. 5,554,459 and 5,840,217).
초기의 정공수송층 유기 물질로는 N,N'-디페닐-N,N'-비스(3-메틸페닐)-1,1'-디페닐-4,4'-디아민(이하 TPD라 함)이 흔히 사용되었으나, 60 ℃ 이상에서 불안정하기 때문에 95 ℃까지 안정한 N-나프틸-N-페닐-1,1'-디페닐-4,4'-디아민(이하 NPD라 함)계열 또는 좀 더 많은 방향족기가 치환된 아민류를 사용하고 있다. 이외에 코비온(Covion)사에서 발표한 스피로(spiro) 화합물을 함유한 아릴아민계화합물, 모토롤라사에서 발표한 페릴렌을 함유한 아릴아민계화합물(미국특허 제6,013,383호), 미국특허 제6,150,043호에 개시된 아자시클로헵타트리엔화합물, 코닥사에서 발표하고 한국공개특허공보 제2000-48006호에 개시된 비스(디페닐비닐페닐)안트라센, TDK에서 발표하고 미국특허 제6,249,085호에 개시된 실리콘게르마늄옥사이드화합물, 일본공개특허공보 제2001-172284호, 제2001-172280호에 개시된 실리콘계아릴아민화합물, 질소원자를 함유한 퓨즈드(fused)된 화합물 등의 정공수송층용 물질들이 보고되었다. 특히 정공수송층 유기 단분자 물질은 정공 이동속도가 빨라야 하며 발광층과 접하여 계면을 형성하기 때문에 정공수송층-발광층 계면여기자의 발생을 억제하기 위해서 이온화포텐셜이 정공주입층과 발광층 사이의 적절한 값을 가지는 것이 매우 중요하다. 또 하나의 기능은 발광층에서 이동되어 오는 전자를 적절히 제어하는 능력이 필요하다. 따라서 전자친화도가 작은 것일수록 특성이 좋다. Early hole transport layer organic materials are commonly referred to as N, N'-diphenyl-N, N'-bis (3-methylphenyl) -1,1'-diphenyl-4,4'-diamine (hereinafter referred to as TPD). N-naphthyl-N-phenyl-1,1'-diphenyl-4,4'-diamine (hereinafter referred to as NPD) series or more aromatic groups, which have been used but are unstable at temperatures above Substituted amines are used. In addition, an arylamine compound containing a spiro compound published by Covion, an arylamine compound containing a perylene released by Motorola (US Pat. No. 6,013,383), US Pat. No. 6,150,043 Azacycloheptatriene compound disclosed in the present invention, bis (diphenylvinylphenyl) anthracene disclosed by Kodak Corporation and published in Korean Patent Publication No. 2000-48006, silicon germanium oxide compound disclosed in US Pat. No. 6,249,085, Materials for hole transport layers, such as silicon-based arylamine compounds and fused compounds containing nitrogen atoms, disclosed in Japanese Patent Application Laid-Open Nos. 2001-172284 and 2001-172280 have been reported. In particular, since the hole transport layer organic monomolecular material has to have a fast hole moving speed and forms an interface in contact with the light emitting layer, it is very important that the ionization potential has an appropriate value between the hole injection layer and the light emitting layer in order to suppress the generation of the excitons of the hole transport layer and the light emitting layer. It is important. Another function requires the ability to properly control the electrons moving in the light emitting layer. Therefore, the smaller the electron affinity, the better the characteristics.
정공과 전자가 결합하여 발광을 하는 발광층용 유기 단분자는 기능적인 측면에서 크게 호스트(host) 물질과 게스트(guest)용 물질로 나누어진다. 일반적으로 호스트용 또는 게스트용 물질 단독으로 빛을 낼 수 있으나 효율 및 휘도가 낮고 같은 분자들끼리 셀프패킹(self-packing) 현상 때문에 각 분자 고유의 특성 아닌 엑사머(excimer) 특성이 함께 나타내기 때문에 바람직하지 않다. 따라서 호스트에 게스트를 소량 도핑(doping)하여 이러한 문제점들을 보완할 수 있다. The organic monomolecule for the light emitting layer, in which holes and electrons combine to emit light, is largely divided into a host material and a guest material in terms of function. In general, the host or guest material can emit light alone, but the efficiency and brightness are low, and because of the self-packing phenomenon of the same molecules, each of the molecules exhibits the characteristics of the excimer rather than the characteristics of each molecule. Not desirable Therefore, a small amount of doping of the guest to the host can compensate for these problems.
먼저 녹색 발광층으로는 8-히드록시퀴놀린 알루미늄염(8-hydroxyquinoline aluminum salt, 이하 Alq3라 함)이 가장 보편적으로 사용되고 있으며 효율을 높이 기 위해 퀴나클리돈(quinacridone)의 유도체, 큐마린(coumarine) 등의 유도체와 같은 양자효율이 높은 물질를 도핑하여 사용한다.First, 8-hydroxyquinoline aluminum salt (Alq3) is most commonly used as a green light emitting layer, and derivatives of quinacridone, coumarine, etc. are used to increase efficiency. Doped materials with high quantum efficiency, such as derivatives, are used.
청색 발광층용 유기 물질들은 녹색 발광체인 Alq3에 비해 융점과 초기 상태의 발광 안정성이 낮고 수명이 짧은 문제점들을 가지고 있다. 또한 대부분의 청색 발광층용 물질들은 순수한 청색이 아닌 하늘색(light blue)이기 때문에 여전히 풀컬러화 디스플레이에 부적합하며, 녹색의 경우처럼 발광효율를 높이기 위해 페릴렌 (perylene)등을 도핑하여 사용한다. 대표적인 청색 발광층용 유기 물질들은 방향족 탄화수소, 스피로(spiro)형 물질, 알루미늄을 포함한 유기금속 화합물, 이미다졸기를 가진 이형고리 화합물, 퓨즈드(fused)된 방향족 화합물 등이 있는데, 이들은 미국특허 제5,516,577호, 제5,366,811호, 제5,840,217호, 제5,150,006호 및 제5,645,948호에 개시되었다.The organic materials for the blue light emitting layer have problems of low melting point and light emission stability at the initial state and short lifespan, compared to Alq3, which is a green light emitter. In addition, since most of the materials for the blue light emitting layer are light blue rather than pure blue, they are still unsuitable for full-color display, and doped with perylene to improve luminous efficiency as in the case of green. Representative organic materials for blue light emitting layers include aromatic hydrocarbons, spiro-type materials, organometallic compounds including aluminum, heterocyclic compounds with imidazole groups, and fused aromatic compounds, which are described in US Patent No. 5,516,577. 5,366,811, 5,840,217, 5,150,006 and 5,645,948.
적색 발광층의 경우 밴드갭(band gap)이 작은 적색 발광의 특성상 다량의 녹색 발광 물질인 Alq3에 적색 발광 물질를 소량 도핑하여 사용하고 있으나 소자의 안정성과 높은 구동전압이 문제점으로 제기되고 있다. 다른 접근 방법으로는 별도의 정공 수송층 없이, 정공 수송과 발광의 역할을 동시에 하는 형광성 고분자를 사용하여 소자를 제작할 수 있다.In the case of the red light emitting layer, a small amount of the red light emitting material is doped into Alq3, which is a large amount of green light emitting material due to the characteristics of the red light having a small band gap, but device stability and high driving voltage have been raised as problems. As another approach, a device may be manufactured using a fluorescent polymer that simultaneously plays a role of hole transport and light emission without a separate hole transport layer.
전자 수송층용 유기 단분자 물질로는 전자에 대한 안정도와 전자이동 속도가 상대적으로 우수한 유기금속착제들이 좋은 후보들이다. 그 중에서 안정성이 우수하고 전자 친화도가 큰 Alq3가 가장 우수한 것으로 보고되었으나 청색 발광소자에 사용할 경우 엑시톤 디퓨젼(exciton diffusion)에 의한 발광 때문에 색순도가 떨어 지는 문제점이 있다. 또한 종래에 공지된 전자 수송용 물질로는 산요(Sanyo)사에서 발표한 플라본(Flavon) 유도체 또는 치소(Chisso)사의 게르마늄 및 실리콘시클로페타디엔 유도체 등이 알려져 있다(일본공개특허공보 제1998-017860호, 일본공개특허공보 제1999-087067호).As organic monomolecular materials for the electron transport layer, organometallic complexes having excellent electron stability and electron transfer speed are good candidates. Among them, Alq3 having excellent stability and high electron affinity has been reported to be the most excellent. However, when used in a blue light emitting device, color purity is degraded due to emission due to exciton diffusion. In addition, conventionally known materials for electron transport include flavon derivatives published by Sanyo or germanium and silicon cyclopetadiene derivatives from Chisso (Japanese Patent Publication No. 1998-017860). Japanese Patent Application Laid-Open No. 1999-087067).
한편, 미국 남가주대학의 톰슨 교수와 프린스톤 대학의 포리스트 교수는 전자 친화도가 크고 전자수송능력이 우수한 PBD(full name), TAZ(full name)나 TPBI(full name)와 같은 호스트물질에 페닐피리딘 이리듐과 같은 유기 인광물질을 도핑하여 고효율의 소자를 얻었다(C. Adachi, M. A. Baldo, and S. R. Forrest, Applied Physics Letter, 77, 904, 2000., C. Adachi, M. A. Baldo, S. R. Forrest, S. Lamansky, M. E. Thompsom, and R. C. Kwong, Applied Physics Letter, 78, 1622, 2001). 이러한 결과는 현재까지의 싱글렛-싱글렛 전이(singlet-singlet transition)에 의한 한계효율을 트리플렛-싱글렛 전이(triplet-singlet transition)로 극복한 예로, 본 발명에서 개시되는 새로운 전자주입 및 수송용 물질에 적용할 경우 훨씬 높은 발광효율과 긴 수명을 갖는 유기 전기발광 소자를 얻는 것이 가능할 것이다.Meanwhile, Professor Thomson of the University of Southern California and Professor Forest of the University of Princeton have phenylpyridine iridium in host materials such as PBD (full name), TAZ (full name) or TPBI (full name), which have high electron affinity and excellent electron transport ability. Doping organic phosphors such as to obtain a high efficiency device (C. Adachi, MA Baldo, and SR Forrest, Applied Physics Letter , 77 , 904, 2000., C. Adachi, MA Baldo, SR Forrest, S. Lamansky, ME Thompsom, and RC Kwong, Applied Physics Letter , 78 , 1622, 2001). This result is an example of overcoming the limit efficiency caused by the singlelet-singlet transition to the triplet-singlet transition, and for the new electron injection and transport disclosed in the present invention. When applied to the material it will be possible to obtain an organic electroluminescent device having a much higher luminous efficiency and long life.
상기에서 각각의 층에 대한 적절한 유기 단분자 물질들를 이용한 유기 발광 소자는 발광수명이 짧고, 보존내구성 및 신뢰성이 낮은 문제점들를 가지고 있다. 이러한 원인들로서는 유기물질의 물리, 화학적인 변화, 광화학적, 전기화학적인 변화, 음극의 산화, 박리현상 및 유기화합물의 용융, 결정화, 열분해 현상들이 있다.The organic light emitting device using the appropriate organic monomolecular materials for each layer has problems of short light emitting life, low durability, and low reliability. These causes include physical, chemical changes, photochemical and electrochemical changes of organic materials, oxidation of cathodes, delamination and melting, crystallization and pyrolysis of organic compounds.
따라서 유기 전기발광 소자는 적절한 유기 단분자 물질의 구조를 변화시켜 임의의 발광색을 얻는 것이 가능하며 호스트 게스트 시스템에 의한 여러가지의 고효율의 유기 전기발광 소자들이 제안되고 있으나, 실용화 수준에서의 사용할 때 만족스러운 휘도 특성, 수명 및 내구성이 결여되어 있다. Therefore, the organic electroluminescent device can obtain an arbitrary emission color by changing the structure of an appropriate organic monomolecular material and various high efficiency organic electroluminescent devices have been proposed by the host guest system, but it is satisfactory when used at the practical level. It lacks luminance characteristics, lifetime and durability.
본 발명은 상기 종래기술의 문제점을 고려하여, 발광효율, 안정성 및 소자 수명을 크게 향상시킬 수 있는 신규한 전자주입 및 수송층용 물질과 이를 이용한 유기 전기 발광 소자를 제공하는 것을 목적으로 한다.Disclosure of Invention It is an object of the present invention to provide a novel electron injection and transport layer material and an organic electroluminescent device using the same, which can considerably improve the luminous efficiency, stability and device life.
본 발명의 다른 목적은 열적 안정성을 가지고 있으면서 진공 증착 공정에 필요한 승화성을 갖는 물질 및 이를 이용한 유기 전기발광 소자를 제공하는 것이다.Another object of the present invention is to provide a material having thermal stability and sublimation necessary for a vacuum deposition process and an organic electroluminescent device using the same.
본 발명은 상기 목적을 달성하기 위하여, 하기 화학식 1로 표시되는 화합물, 하기 화학식 2로 표시되는 화합물, 하기 화학식 3으로 표시되는 화합물, 하기 화학식 4로 표시되는 화합물, 및 하기 화학식 5로 표시되는 화합물을 제공한다:The present invention, in order to achieve the above object, a compound represented by the following formula (1), a compound represented by the formula (2), a compound represented by the formula (3), a compound represented by the formula (4), and a compound represented by the formula (5) Provides:
[화학식 1][Formula 1]
[화학식 2][Formula 2]
[화학식 3][Formula 3]
[화학식 4][Formula 4]
[화학식 5][Formula 5]
상기 화학식 1, 2, 3, 4, 및 5의 식에서,In the
R1, 및 R2 는 각각 독립적으로 또는 동시에, 수소원자, 탄소수 1 내지 20의 지방족 탄화수소, 페닐, 나프틸, 바이페닐, 안트라세닐, 방향족 복소환 혹은 방향족환 집합으로부터 유도된 기이고, 상기 R1, 및 R2 중 적어도 하나는 페닐, 나프틸, 바이페닐, 방향족 복소환 혹은 방향족 환 집합으로부터 유도된 기이며,R 1 and R 2 are each independently or simultaneously a group derived from a hydrogen atom, an aliphatic hydrocarbon having 1 to 20 carbon atoms, phenyl, naphthyl, biphenyl, anthracenyl, aromatic heterocycle, or aromatic ring assembly, wherein R At least one of 1 , and R 2 is a group derived from phenyl, naphthyl, biphenyl, aromatic heterocycle or aromatic ring assembly,
Ar은 페닐, 나프틸, 바이페닐, 안트라세닐, 또는 방향족 복소환 혹은 방향족환 집합으로부터 유도된 기이며,Ar is a group derived from phenyl, naphthyl, biphenyl, anthracenyl, or an aromatic heterocycle or an aromatic ring assembly,
R3는 수소원자, 탄소수 1 내지 6의 알킬기 또는 지방족 탄화수소, 치환된 페닐, 나프틸, 바이페닐, 안트라세닐, 또는 방향족 복소환 혹은 방향족환 집합이며,R 3 is a hydrogen atom, an alkyl group having 1 to 6 carbon atoms or an aliphatic hydrocarbon, substituted phenyl, naphthyl, biphenyl, anthracenyl, or an aromatic heterocyclic or aromatic ring assembly,
X는 NR4, 유황원자, 또는 산소원자이며,X is NR 4 , sulfur atom or oxygen atom,
상기 R4는 수소원자, 탄소수 1 내지 7 의 알킬기 또는 지방족 탄화수소, 페닐, 나프틸, 바이페닐, 안트라세닐, 또는 방향족 복소환 혹은 방향족환 집합으로부터 유도된 기이다.R 4 is a hydrogen atom, an alkyl group having 1 to 7 carbon atoms or an aliphatic hydrocarbon, phenyl, naphthyl, biphenyl, anthracenyl, or a group derived from an aromatic heterocycle or an aromatic ring assembly.
또한 본 발명은 상기 화학식 1로 표시되는 화합물, 상기 화학식 2로 표시되는 화합물, 상기 화학식 3으로 표시되는 화합물, 상기 화학식 4로 표시되는 화합물, 및 상기 화학식 5로 표시되는 화합물로 이루어진 군으로부터 1 종 이상 선택되는 유기화합물을 함유하는 유기화합물층을 포함하는 유기 발광 소자를 제공한다.In addition, the present invention is a compound represented by Formula 1, a compound represented by
이하에서 본 발명을 상세하게 설명한다.Hereinafter, the present invention will be described in detail.
본 발명은 전계 발광 소자인 유기 박막 전계 발광(유기 EL; organic electroluminescence) 소자의 유기화합물층에 함유되어 발광 효율과 수명의 향상 및 저전압구동을 실현할 수 있는 안트라센의 4 개의 치환장소에 1 개 내지 2 개의 헤테로 작용기가 도입된 신규의 물질 및 이 물질을 유기화합물층에 함유하는 유기 발광 소자를 제공하는 것이다.The present invention is contained in the organic compound layer of the organic electroluminescence (organic EL) device, an electroluminescent device, one to two at four substitution sites of anthracene, which can improve luminous efficiency, lifetime, and low voltage driving. A novel material into which a hetero functional group is introduced and an organic light emitting device containing the material in an organic compound layer are provided.
먼저 본 발명의 신규의 물질이 적용될 수 있는 유기발광소자의 구조에 대하여 설명한다.First, the structure of the organic light emitting device to which the novel material of the present invention can be applied will be described.
도 1은 본 발명의 유기 전기발광 소자에 적용될 수 있는 구조의 한 예를 나타내는 단면도로, 도면부호 1은 기판, 2는 양극, 3은 정공주입층, 4는 정공수송층, 5는 유기발광층, 6은 전자수송층, 7은 음극을 각각 나타낸다.1 is a cross-sectional view showing an example of a structure that can be applied to the organic electroluminescent device of the present invention, 1 is a substrate, 2 is an anode, 3 is a hole injection layer, 4 is a hole transport layer, 5 is an organic light emitting layer, 6 Silver electron transport layer, 7 represents a cathode, respectively.
기판(1)은 유기 전기발광 소자의 지지체이며, 실리콘 웨이퍼, 석영 또는 유리판, 금속판, 플라스틱 필름이나 시트 등이 사용될 수 있다.The substrate 1 is a support of an organic electroluminescent device, and a silicon wafer, quartz or glass plate, metal plate, plastic film or sheet, or the like can be used.
상기 기판(1) 위에는 양극(2)이 위치된다. 이러한 양극(2)은 그 위에 위치되는 정공주입층(3)으로 정공을 주입하며, 알루미늄, 금, 은, 니켈, 팔라듐, 백금 등의 금속, 인듐-주석 산화물, 인듐-아연 산화물 등의 일함수가 큰 물질이 사용될 수 있으며, 카본블랙, 폴리티오펜, 폴리피롤, 또는 폴리아닐린 등의 전도성 고분자도 사용될 수 있다.The
상기 양극(2) 위에는 정공주입층(3)이 위치된다. 이러한 정공주입층(3)의 물질로 요구되는 조건은 양극으로부터의 정공주입 효율이 높으며, 주입된 정공을 효율적으로 수송할 수 있어야 한다. 이를 위해서는 이온화 포텐셜이 작고 가시광선에 대한 투명성이 높으며, 정공에 대한 안정성이 우수해야 한다.
The
상기 정공주입층(3) 위에는 정공수송층(4)이 위치된다. 이러한 정공수송층(4)은 정공주입층(3)으로부터 정공을 전달받아 그 위에 위치되는 유기발광층(5)으로 수송하는 역할을 하며, 높은 정공 이동도와 정공에 대한 안정성 및 전자를 막아주는 역할를 한다. 이러한 일반적 요구 이외에 차체 표시용으로 응용할 경우 소자에 대한 내열성이 요구되며, 유리 전이 온도(Tg)가 70 ℃ 이상의 값을 갖는 재료가 바람직하다. 이와 같은 조건을 만족하는 물질들로는 NPD(혹은 NPB라 함), 스피로-아릴아민계화합물, 페릴렌-아릴아민계화합물, 아자시클로헵타트리엔화합물, 비스(디페닐비닐페닐)안트라센, 실리콘게르마늄옥사이드화합물, 실리콘계아릴아민화합물 등이 있다.The
상기 정공수송층(4) 위에는 유기발광층(5)이 위치된다. 이러한 유기발광층(5)는 양극(2)과 음극(7)으로부터 각각 주입된 정공과 전자가 재결합하여 발광을 하는 층이며, 양자효율이 높은 물질로 이루어져 있다.The organic
이와 같은 조건을 만족하는 물질로는 녹색의 경우 Alq3가, 청색의 경우 Balq(8-hydroxyquinoline beryllium salt), DPVBi(4,4'-bis(2,2-diphenylethenyl)-1,1'-biphenyl) 계열, 스피로(Spiro) 물질, 스피로-DPVBi(Spiro-4,4'-bis(2,2-diphenylethenyl)-1,1'-biphenyl), LiPBO(2-(2-benzoxazoyl)-phenol lithium salt), 비스(디페닐비닐페닐비닐)벤젠, 알루미늄-퀴놀린 금속착체, 이미다졸, 티아졸 및 옥사졸의 금속착체 등이 있으며, 청색 발광 효율을 높이기 위해 페릴렌, 및 BczVBi(3,3'[(1,1'-biphenyl)-4,4'-diyldi-2,1-ethenediyl]bis(9-ethyl)-9H-carbazole; DSA(distrylamine)류)를 소량 도핑하여 사용할 수 있다. 적색의 경우 는 녹색 발광 물질에 DCJTB([2-(1,1-dimethylethyl)-6-[2-(2,3,6,7-tetrahydro-1,1,7,7-tetramethyl-1H,5H-benzo(ij)quinolizin-9-yl)ethenyl]-4H-pyran-4-ylidene]-propanedinitrile)와 같은 물질을 소량 도핑하여 사용한다. 잉크젯프린팅, 롤코팅, 스핀코팅 등의 공정을 사용하여 발광층을 형성할 경우에, 폴리페닐렌비닐렌(PPV) 계통의 고분자나 폴리 플로렌(poly fluorene) 등의 고분자를 유기발광층(5)에 사용할 수 있다.Alq3 for green, Balq (8-hydroxyquinoline beryllium salt), DPVBi (4,4'-bis (2,2-diphenylethenyl) -1,1'-biphenyl) for green Series, Spiro substance, Spiro-DPVBi (Spiro-4,4'-bis (2,2-diphenylethenyl) -1,1'-biphenyl), LiPBO (2- (2-benzoxazoyl) -phenol lithium salt) , Bis (diphenylvinylphenylvinyl) benzene, aluminum-quinoline metal complexes, metal complexes of imidazole, thiazole and oxazole, and the like, perylene, and BczVBi (3,3 '[( 1,1'-biphenyl) -4,4'-diyldi-2,1-ethenediyl] bis (9-ethyl) -9H-carbazole; DSA (distrylamine) can be used by doping in small amounts. In the case of red, DCJTB ([2- (1,1-dimethylethyl) -6- [2- (2,3,6,7-tetrahydro-1,1,7,7-tetramethyl-1H, 5H A small amount of doping such as -benzo (ij) quinolizin-9-yl) ethenyl] -4H-pyran-4-ylidene] -propanedinitrile) is used. When forming a light emitting layer using a process such as inkjet printing, roll coating, or spin coating, a polymer such as polyphenylene vinylene (PPV) -based polymer or poly fluorene may be added to the organic
상기 유기발광층(5) 위에는 전자수송층(6)이 위치된다. 이러한 전자수송층(6)은 그 위에 위치되는 음극(7)으로부터 전자주입 효율이 높고 주입된 전자를 효율적으로 수송할 수 있는 물질이 필요하다. 이를 위해서는 전자 친화력과 전자 이동속도가 크고 전자에 대한 안정성이 우수한 물질로 이루어져야 한다. 이와 같은 조건을 충족시키는 재료로는, 테트라 페닐 부타디엔 등의 방향족 화합물(일본공개특허공보 소57-51781호), 8-히드록시 퀴놀린의 알루미늄 등의 금속착체(일본공개특허공보 소59-194393호), 10-히드록시 벤조[h] 퀴놀린의 금속 착체(일본공개특허공보 평6-322362호), 시클로 펜타디엔 유도체(일본공개특허공보 평2-289675호), 비스 스티릴 벤젠 유도체(일본공개특허공보 평1-245087호, 및 일본공개특허공보 평2-222484호), 페릴렌 유도체(일본공개특허공보 평2-189890호, 및 일본공개특허공보 평3-791호), p-페닐렌 유도체(일본공개특허공보 평3-33183호), 및 옥사졸 유도체(일본공개특허공보 평11-345686호) 등이 사용될 수 있다.The electron transport layer 6 is positioned on the organic
상기 전자수송층(6) 위에는 음극(7)이 위치된다. 이러한 음극(7)은 전자수송층(6)에 전자를 주입하는 역할을 한다. 음극으로 사용하는 재료는 상기 양극(2) 에 사용된 재료를 이용하는 것이 가능하며, 효율적인 전자주입을 위해서는 일 함수가 낮은 금속이 보다 바람직하다. 특히 주석, 마그네슘, 인듐, 칼슘, 나트륨, 리튬, 알루미늄, 은 등의 적당한 금속, 또는 그들의 적절한 합금이 사용될 수 있다. 또한 100 ㎛ 이하 두께의 리튬플루오라이드와 알루미늄, 산화리튬과 알루미늄, 스트론튬산화물과 알루미늄 등의 2 층 구조의 전극도 사용될 수 있다.The
따라서 본 발명에 의한 상기 화학식 1 내지 5로 표시되는 유기 화합물을 함유하는 유기화합물층이 정공을 주입하는 양극과 전자를 주입하는 음극 사이에 적어도 하나가 위치되도록 한다. 이와 같이 양극과 음극 사이의 유기화합물층, 바람직하게는 발광층과 음극사이의 전자주입 및 수송층에 본 발명의 신규 물질을 함유시큼으로써 적용되는 유기 전기발광 소자의 효율과 수명을 크게 향상시킬 수 있다. 또한 구동전압을 낮추며 안정성이 우수한 유기 전기발광 소자를 제공할 수 있다. 또한 별도의 발광층 없이 본 발명에서 사용되는 물질에 적절한 도판트(dopant)를 첨가하여, 또는 단독적으로 전자수송층 역할과 발광층 역할을 동시에 수행할 수 있다.Therefore, at least one organic compound layer containing the organic compound represented by Chemical Formulas 1 to 5 according to the present invention is positioned between the anode for injecting holes and the cathode for injecting electrons. As such, by containing the novel material of the present invention in the organic compound layer between the anode and the cathode, preferably in the electron injection and transport layer between the light emitting layer and the cathode, the efficiency and lifespan of the organic electroluminescent device applied can be greatly improved. In addition, it is possible to provide an organic electroluminescent device having low driving voltage and excellent stability. In addition, by adding a suitable dopant (dopant) to the material used in the present invention without a separate light emitting layer, it can be performed at the same time as the electron transport layer and the light emitting layer alone.
본 발명의 상기 화학식 1 내지 5로 표시되는 화합물을 함유하는 유기화합물층은 특히 전자주입 및 수송기능을 가지는 전자 주입 및 수송층, 전자 주입 및 발광 기능을 가지는 전자 주입 및 발광 층, 또는 전자 수송 및 발광 기능을 가지는 전자 수송 및 발광 층이 되는 것이 바람직하다. 이를테면 본 발명의 신규의 물질들이 전자 주입 및 수송층을 형성하고 다른 물질이 발광층을 형성할 수 있으며, 또는 본 발명의 신규의 물질들이 전자 주입 및 수송층과 발광층을 동시에 형성할 수 도 있다.The organic compound layer containing the compound represented by Chemical Formulas 1 to 5 of the present invention is particularly an electron injection and transport layer having an electron injection and transport function, an electron injection and light emitting layer having an electron injection and light emission function, or an electron transport and light emission function It is preferable to be an electron transporting and emitting layer having For example, the novel materials of the present invention may form an electron injection and transport layer and other materials may form an emission layer, or the novel materials of the present invention may simultaneously form an electron injection and transport layer and an emission layer.
본 발명의 유기화합물층에 함유되는 상기 화학식 1 내지 5의 화합물 중에서 보다 바람직한 화합물은 하기 화학식 1a, 또는 화학식 3a의 화합물이다.Among the compounds of Formulas 1 to 5 contained in the organic compound layer of the present invention, a more preferable compound is a compound of Formula 1a or Formula 3a.
[화학식 1a][Formula 1a]
[화학식 3a][Formula 3a]
상기 화학식 1a, 및 화학식 3a의 식에서,In the formula of Formula 1a and Formula 3a,
Ar1, Ar2, 및 Ar3는 각각 독립적으로 또는 동시에, 벤젠, 나프탈렌, 바이페닐등의 방향족 탄화수소를 나타내고, R5는 수소원자 및 탄소수 1 내지 4의 알킬기, 방향족 탄화수소 또는 이형고리화합물를 나타낸다.Ar 1 , Ar 2 , and Ar 3 each independently or simultaneously represent an aromatic hydrocarbon such as benzene, naphthalene, biphenyl, and R 5 represents a hydrogen atom and an alkyl group having 1 to 4 carbon atoms, an aromatic hydrocarbon or a heterocyclic compound.
종래의 전자 주입 및 수송층용 물질로는 이미다졸기, 옥사졸기, 티아졸기를 가진 유기단분자 물질들이 많이 보고되었다. 그러나 이러한 물질들이 전자수송용 물질로 보고되기 이전에 모토롤라(Motorola)사의 유럽공개특허공보 제0700917 A2호에 이러한 물질들의 금속착체화합물들이 청색 발광층 또는 청록색 발광층의 유기 전기발광 소자로 적용된 것이 이미 보고되었다. 이어 미국특허 제5,766,779호, 및 제5,645,948호에 이미다졸, 티아졸 또는 옥사졸기를 가진 유기 물질들이 전자수송층 및 발광층으로 사용된 유기 전기발광 소자가 발표되었는데, 미국특허 제5,766,779호에 게재된 내용에는 이러한 헤테로 작용기들이 한 분자 내에 2 개 내지 8 개까지 포함되었으며, 이를 이용해 유기 전기발광 소자의 전자수송층에 적용되었다. 또한 미국특허 제5,645,948호에는 같은 헤테로작용기들이 한 분자내에 3 개 내지 8 개까지 포함된 유기물질를 이용하여 발광층에 응용하었으나 본 발명에서는 안트라센의 4 개의 치환장소에 1 개 내지 2 개의 헤테로작용기를 도입하여 전자주입 및 수송용 물질로 사용한 것이 큰 특징이라 할 수 있다.As a conventional material for electron injection and transport layer, many organic monomer materials having imidazole group, oxazole group, and thiazole group have been reported. However, before these materials were reported as electron transporting materials, European Patent Publication No. 0700917 A2 of Motorola had already reported application of metal complex compounds of such materials as organic electroluminescent devices of a blue light emitting layer or a cyan light emitting layer. . Subsequently, U.S. Patent Nos. 5,766,779 and 5,645,948 disclose organic electroluminescent devices in which organic materials having imidazole, thiazole, or oxazole groups are used as the electron transporting layer and the emitting layer, which are disclosed in U.S. Patent No. 5,766,779. Two to eight such hetero functional groups were included in one molecule, and were applied to the electron transport layer of the organic electroluminescent device. In addition, US Pat. No. 5,645,948 applies the same heterofunctional groups to the light emitting layer by using an organic material containing 3 to 8 molecules in one molecule, but in the present invention, 1 to 2 heterofunctional groups are introduced at 4 substitution sites of anthracene. Therefore, it can be said that it is used as a material for electron injection and transport.
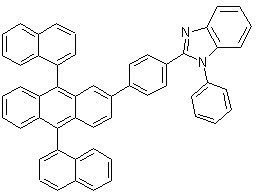
1996년도에 코닥사에서 발표하고 미국특허 제5,645,948호에 개시된 TPBI(화학식 6)는 이미다졸기를 가진 대표적인 전자 수송층용 물질로 알려져 있으며, 하기 화학식 6에서 볼 수 있듯이 벤젠의 1,3,5-치환 위치에 세 개의 N-페닐 벤즈이미다졸기를 함유하고 기능적으로는 전자를 전달하는 능력뿐 아니라 발광층에서 넘어오는 정공을 차단하는 기능도 있으나 실제 소자에 적용하기에는 안정성이 낮은 문제점을 가지고 있다.
TPBI (Formula 6), published by Kodak Corporation in 1996 and disclosed in US Pat. No. 5,645,948, is known as a representative electron transport layer material having an imidazole group, and as shown in the following
[화학식 6][Formula 6]
또한 일본공개특허공보 평11-345686호에 개시된 전자수송용 물질들은 하기 화학식 7 내지 10에서 알 수 있듯이 옥사졸기, 티아졸기를 함유하고 있고 발광층에도 적용할 수 있다고 보고하였으나 실용화하기에는 구동전압, 휘도 및 소자의 수명측면에서 충분하지 않음을 알 수 있다.In addition, the electron transporting materials disclosed in Japanese Patent Application Laid-Open No. 11-345686 reported that they can be applied to the light emitting layer containing oxazole group and thiazole group as shown in the following
[화학식 7][Formula 7]
[화학식 8][Formula 8]
[화학식 9][Formula 9]
[화학식 10][Formula 10]
종래의 기술에서 볼 수 있듯이 Alq3와 같은 유기금속 착체를 제외한 어떠한 유기물질도 디스플레이의 실용화가 어렵다는 인식아래 본 발명자들은 상기 화학식 1 내지 5로 표시되는 신규한 유기물질들을 합성하였고, 적절한 정공주입층, 정공수송층 및 발광층을 선택한 후, 이들을 전자주입 및 수송층에 적용해 본 결과 종래의 Alq3보다 구동전압, 효율, 소자의 수명 및 열적 안정성 측면에서 훨씬 우수한 결과를 얻는데 성공하였다.As can be seen from the prior art, the present inventors synthesized the novel organic materials represented by Chemical Formulas 1 to 5 under the recognition that any organic material except an organometallic complex such as Alq3 was difficult to be used, and the appropriate hole injection layer, After selecting the hole transport layer and the light emitting layer, and applying them to the electron injection and transport layer, the results were much better than the conventional Alq3 in terms of driving voltage, efficiency, device life and thermal stability.
본 발명자들이 새로운 전자 주입 및 수송층에 사용한 안트라센의 유도체들은 미국의 코닥(Kodak)사나 티디케이(TDK) 등에서 상당한 수준의 연구가 진행되었고 여러 특허에도 개시되었지만, 안트라센의 역사적인 유래는 이미 1960년대 초반부터 시작되었다. 1965년에 헬프리치(Helfrich)와 포프(Pope)는 안트라센의 단결정을 이용한 유기 전기발광 현상을 처음으로 발표하였으나 발광효율이 낮고 높은 전압이 필요했기 때문에 실용화하기에는 많은 문제점을 가지고 있었다(W. Helfrich, W. G. Schneider, Phys. Rev. Lett. 14, 229, 1965. M. Pope, H. Kallmann, J. Giachino, J. Chem. Phys., 42,2540, 1965).Derivatives of anthracene used by the inventors for the new electron injection and transport layer have been studied in the US, Kodak, TDK, and the like, and have been disclosed in various patents, but the historical origin of anthracene is from the early 1960s. Started. In 1965, Helrich and Pope first announced organic electroluminescence using anthracene single crystals, but they had many problems in practical use because of their low luminous efficiency and high voltage (W. Helfrich, WG Schneider, Phys. Rev. Lett . 14 , 229, 1965. M. Pope, H. Kallmann, J. Giachino, J. Chem. Phys ., 42 , 2540, 1965).
따라서 성능이 우수한 유기 전기발광 소자를 개발하기 위해서는 안트라센의 10 개의 반응치환 장소 중에서 어느 위치에 어떤 작용기를 도입하느냐가 매우 중요 하다. 코닥사 및 티디케이 등에서 발표한 안트라센 유도체들은 모두 유기 전기발광 소자의 발광층용으로만 사용한 것이 특징이다. 일본공개특허공보 평11-345686호에 개시된 안트라센 유도체는 발광층 및 전자수송물질로 사용할 수 있다고 주장하였으나 그 특허의 광의의 청구범위에 포함되었을 뿐 합성예나 실시예 등에서 더 이상의 언급이 없고 현재까지 전자주입 및 수송용 물질로 실용화 한 예는 찾아볼 수 없다.Therefore, in order to develop an excellent organic electroluminescent device, it is very important which functional group is introduced at which position among the 10 reaction substitution sites of anthracene. The anthracene derivatives published by Kodak Corp. and Tidike Corp. are all used for the light emitting layer of the organic electroluminescent device. The anthracene derivative disclosed in Japanese Patent Application Laid-open No. Hei 11-345686 claims that it can be used as a light emitting layer and an electron transporting material, but it is included in the broad claims of the patent, but there is no further mention in the synthesis examples or examples and electron injection has been made so far. And examples of practical use as transporting materials are not found.
이러한 문제점을 극복하기 위해 본 발명자들은 안트라센의 2,6,9,10의 치환장소를 이용한 반면에 일본공개특허공보 평11-345686호는 1,5, 1,8 또는 2,6의 두 개의 치환장소만을 이용하고 9,10 위치는 수소원자로만 치환된 것이 특징이다.In order to overcome this problem, the present inventors use the place of substitution of
먼저 본 발명자들이 합성한 상기 화학식 1 내지 5로 표시되는 화합물의 구조적인 특징을 살펴보면 다음과 같다. 안트라센은 10 개의 치환장소가 있는데 본 발명자들은 안트라센의 2,6,9,10의 4 개의 치환장소를 자유롭게 변환시키면서 최적의 화합물을 찾아내는데 주력하였고 이러한 개념이 가장 큰 특징이라 할수 있다. 즉, 안트라센의 9,10 위치에 페닐, 나프틸, 바이페닐 등의 방향족 탄화수소를 각각 또는 동시에 치환하고, 2,6 위치에는 이미다졸, 옥사졸 및 티아졸기를 각각 또는 동시에 직접 치환하거나, 페닐 등의 방향족 탄화수소를 치환한후에 이미다졸, 옥사졸 및 티아졸기를 각각 또는 동시에 치환하여 물질들을 합성하였다.First, the structural characteristics of the compound represented by Chemical Formulas 1 to 5 synthesized by the present inventors are as follows. Anthracene has 10 substitution sites, and the present inventors focused on finding an optimal compound while freely converting 4 substitution sites of 2,6,9,10 of anthracene, and this concept is the biggest feature. That is, at 9,10 positions of anthracene, aromatic hydrocarbons such as phenyl, naphthyl, and biphenyl are substituted, respectively or simultaneously, and at 2,6 positions, imidazole, oxazole and thiazole groups are respectively or simultaneously directly substituted, or phenyl, etc. Substances were synthesized by substituting the imidazole, oxazole and thiazole groups, respectively, or simultaneously after substituting the aromatic hydrocarbons.
이들은 유기 발광 소자에서 전자수송능력을 함유하는 층으로 사용하였을 때 뛰어난 구동전압과 소자의 수명이 동시에 향상됨을 알 수 있었다. 대표적인 물질들은 하기 실시예에서 보다 구체적으로 설명하였다. 본 발명의 대표적인 전자주입 및 수송능력을 가진 물질들은 하기 화학식 1-1 내지 1-16, 화학식 2-1 내지 2-5, 화학식 3-1 내지 3-9, 화학식 4-1 내지 화학식 4-5, 화학식 5-1 내지 5-5의 화합물들이다.When the organic light emitting device is used as a layer containing the electron transporting ability, it was found that the excellent driving voltage and the lifetime of the device are simultaneously improved. Representative materials are described in more detail in the Examples below. Representative materials having electron injection and transport ability of the present invention are the following formula 1-1 to 1-16, formula 2-1 to 2-5, formula 3-1 to 3-9, formula 4-1 to formula 4-5 , Compounds of the formulas 5-1 to 5-5.
[화학식 1-1] [화학식 1-2][Formula 1-1] [Formula 1-2]
[화학식 1-3] [화학식 1-4][Formula 1-3] [Formula 1-4]
[화학식 1-5] [화학식 1-6] [Formula 1-5] [Formula 1-6]
[화학식 1-7] [화학식 1-8][Formula 1-7] [Formula 1-8]
[화학식 1-9] [화학식 1-10][Formula 1-9] [Formula 1-10]
[화학식 1-11] [화학식 1-12] [Formula 1-11] [Formula 1-12]
[화학식 1-13] [Formula 1-13]
[화학식 1-14][Formula 1-14]
[화학식 1-15][Formula 1-15]
[화학식 1-16][Formula 1-16]
[화학식 2-1][Formula 2-1]
[화학식 2-2][Formula 2-2]
[화학식 2-3][Formula 2-3]
[화학식 2-4][Formula 2-4]
[화학식 2-5][Formula 2-5]
[화학식 3-1] [화학식 3-2] [Formula 3-1] [Formula 3-2]
[화학식 3-3] [화학식 3-4][Formula 3-3] [Formula 3-4]
[화학식 3-5] [화학식 3-6][Formula 3-5] [Formula 3-6]
[화학식 3-7] [화학식 3-8][Formula 3-7] [Formula 3-8]
[화학식 3-9][Formula 3-9]
[화학식 4-1] [화학식 4-2] [Formula 4-1] [Formula 4-2]
[화학식 4-3] [화학식 4-4][Formula 4-3] [Formula 4-4]
[화학식 4-5][Formula 4-5]
[화학식 5-1][Formula 5-1]
[화학식 5-2][Formula 5-2]
[화학식 5-3][Formula 5-3]
[화학식 5-4][Formula 5-4]
[화학식 5-5][Formula 5-5]
상기에서 제시한 화합물의 예는 단지 본 발명의 이해를 돕는 목적으로 일부를 제시한 것일 뿐 본 발명이 제공하는 화합물은 이들만으로 국한되지 않는다.Examples of the compounds presented above are only a part for the purpose of understanding the present invention, and the compounds provided by the present invention are not limited thereto.
상기 화학식 1, 2, 3, 4, 및 5를 만족하는 화합물의 합성방법과 이를 이용한 유기 전기발광 소자는 이하의 실시예 및 비교예에 의하여 더욱 구체적으로 설명한다. 단, 실시예는 본 발명을 예시하기 위한 것이지 이들만으로 한정되지 않으며, 실시예에 기재되지 않은 상기 화학식 1, 2, 3, 4, 및 5를 만족하는 화합물도 같은 범주 내에서 제조되고, 유기발광 소자의 유기화합물층에 함유되어 적용될 수 있다.Synthesis method of a
[실시예]EXAMPLE
상기 화학식 1 내지 5로 표시되는 화합물의 합성을 위해서 하기 화학식 a 내지 i의 화합물들 중에서 출발물질을 선택하였으며, 이들의 제조는 하기 제조예 1 내지 9에 나타내었다.Starting materials were selected from the compounds of Formulas a to i for the synthesis of the compounds represented by Formulas 1 to 5, and their preparation is shown in Preparation Examples 1 to 9 below.
[화학식 a] [화학식 b] [화학식 c][Formula a] [Formula b] [Formula c]
[화학식 d] [화학식 e] [화학식 f][Formula d] [Formula e] [Formula f]
[화학식 g] [화학식 h] [화학식 i] [Formula g] [Formula h] [Formula i]
[화학식 j] [화학식 k][Formula j] [Formula k]
[화학식 ℓ][Formula 1]
제조예 1Preparation Example 1
(화학식 a로 표시되는 출발물질의 제조)(Preparation of starting material represented by Formula a)
2,6-디아미노안트라퀴논(23.8 g, 100 mmol)을 48 중량% 농도의 하이드로겐 브로마이드 수용액에 분산시킨 후, 20 ℃에서 나트륨 나이트라이트(NaNO2, 14.1 g; 204 mmol)을 천천히 가했다. 기체 발생이 끝난 후 48 중량% 농도의 하이드로겐 브로마이드 수용액(63 ㎖)에 브롬화구리(CuBr, 29.5 g; 206 mmol)을 녹인 용액을 소량의 에탄올(50 ㎖)와 함께 천천히 가하였다. 반응물의 온도를 상온까지 서서히 올린 후 한 시간 동안 환류하였다. 상온으로 냉각 후 물을 가해 생성된 침전물을 여과하고 물로 씻은 후 진공 건조하였다. 얻어진 고체를 클로로포름에 녹여 실리카켈을 통과시킨 후 감압하여 용매를 제거하였다. 컬럼크로마토그래피로 분리한 후 클로로포름으로 재결정하여 연한 노란색의 출발물질인 화학식 a의 화합물(10 g, 수율 27 %)을 얻었다.2,6-diaminoanthraquinone (23.8 g, 100 mmol) was dispersed in an aqueous solution of hydrogen bromide at a concentration of 48 wt%, and sodium nitrite (NaNO 2 , 14.1 g; 204 mmol) was slowly added at 20 ° C. After gas evolution, a solution of copper bromide (CuBr, 29.5 g; 206 mmol) in 48 wt% aqueous hydrogen bromide solution (63 mL) was slowly added with a small amount of ethanol (50 mL). The temperature of the reaction was slowly raised to room temperature and then refluxed for one hour. After cooling to room temperature, water was added, and the resulting precipitate was filtered, washed with water, and dried in vacuo. The obtained solid was dissolved in chloroform, passed through silica gel, and the solvent was removed under reduced pressure. After separation by column chromatography, the mixture was recrystallized with chloroform to obtain a compound of formula a (10 g, yield 27%) as a light yellow starting material.
이 화합물의 분석결과는 다음과 같다.The analysis results of this compound are as follows.
1H NMR (300 MHz, CDCl3), 8.44 (d, J = 2.1 Hz, 2H), 8.18 (d, J = 8.0 Hz, 2H), 7.95 (dd, J = 2.1, 8.0 Hz, 2H.)1 H NMR (300 MHz, CDCl 3 ), 8.44 (d, J = 2.1 Hz, 2H), 8.18 (d, J = 8.0 Hz, 2H), 7.95 (dd, J = 2.1, 8.0 Hz, 2H.)
제조예 2Preparation Example 2
(화학식 b로 표시되는 출발물질의 제조)(Preparation of starting material represented by Formula b)
질소분위기 하에서 2-브로모 비페닐(8.83 ㎖, 51.2 mmol)을 정제된 THF(200 ㎖)에 녹이고 -78 ℃로 냉각한 후 터셔리-부틸리튬(60 ㎖, 1.7 M 펜탄용액)을 천천 히 가했다. 동일온도에서 40 분간 교반한 후 상기 제조예 1에서 제조된 화학식 a의 화합물(7.50 g; 20.5 mmol)을 동일온도에서 가하였다. 냉각용기를 제거한 후 반응물을 상온에서 15 시간동안 교반하였다. 반응 용액을 디에틸 에테르(200 ㎖)와 2 N 염산용액(200 ㎖)의 혼합용매에 천천히 가하여주고 40 분간 상온에서 교반하였다. 생성된 침전물을 여과한 후 물과 에틸 에테르로 충분히 씻어주었다. 이 물질을 진공 건조하여 화합물 b로 표시되는 화합물(11.8 g, 수율 85 %)를 얻었다.In a nitrogen atmosphere, 2-bromo biphenyl (8.83 ml, 51.2 mmol) was dissolved in purified THF (200 ml), cooled to -78 ° C and tertiary-butyllithium (60 ml, 1.7 M pentane solution) was slowly added. Added. After stirring at the same temperature for 40 minutes, the compound of Formula a prepared in Preparation Example 1 (7.50 g; 20.5 mmol) was added at the same temperature. After removing the cooling vessel, the reaction was stirred for 15 hours at room temperature. The reaction solution was slowly added to a mixed solvent of diethyl ether (200 mL) and 2N hydrochloric acid solution (200 mL) and stirred at room temperature for 40 minutes. The resulting precipitate was filtered and washed well with water and ethyl ether. This material was dried in vacuo to give the compound represented by compound b (11.8 g, yield 85%).
제조예 3Preparation Example 3
(화학식 c로 표시되는 출발물질의 제조)(Preparation of starting material represented by Formula c)
상기 제조예 2에서 제조된 화학식 b로 표시되는 화합물(4.00 g; 5.93 mmol), 칼륨요오드(9.85 g; 59.3 mmol)과 나트륨 하이포포스파이트 하이드레이트(10.44 g, 98.0 mmol)의 혼합물을 오르소-디클로로벤젠(600 ㎖)와 아세트산(80 ㎖)의 혼합용액에서 24 시간동안 환류하였다. 상온으로 냉각한 후 혼합물을 클로로포름으로 추출하고 무수황산마그테슘으로 수분을 제거한 후 감압하여 용매를 제거하였다. 얻어진 고체를 클로로포름에 녹이고 짧은 실리카겔 컬럼을 통과시킨 후 감압하여 용매를 제거하였다. 이를 노르말-헥산에 분산시켜 교반하고 여과한 후 진공 건조하여 연한 노랑색의 화학식 c로 표시되는 화합물(3.3 g, 수율 87 %)을 얻었다.A compound of the compound represented by Formula b prepared in Preparation Example 2 (4.00 g; 5.93 mmol), potassium iodine (9.85 g; 59.3 mmol) and sodium hypophosphite hydrate (10.44 g, 98.0 mmol) was prepared by ortho-dichloro It was refluxed for 24 hours in a mixed solution of benzene (600 mL) and acetic acid (80 mL). After cooling to room temperature, the mixture was extracted with chloroform, water was removed with anhydrous magnesium sulfate, and the solvent was removed under reduced pressure. The obtained solid was dissolved in chloroform, passed through a short silica gel column, and the solvent was removed under reduced pressure. The mixture was dispersed in normal-hexane, stirred, filtered, and dried in vacuo to obtain a compound (3.3 g, yield 87%) represented by light yellow (C).
이 화합물의 분석결과는 다음과 같다.The analysis results of this compound are as follows.
녹는점 478.1 C; 1H NMR (300 MHz, CDCl3) 7.92 (d, J = 7.6 Hz, 4H), 7.46 (t, J = 8.0 Hz, 4H), 7.33 (t, J = 7.4 Hz, 4H), 7.21 (d, J = 7.6 Hz, 4H), 6.88 (dd, J = 2.1, 8.6 Hz, 2H), 6.47 (d, J = 2.1 Hz, 2H), 6.22 (d, J = 8.6 Hz, 2H); MS (M+) 636; Anal. Calcd for C38H22Br2: C, 71.50; H, 3.47; Br, 25.03. Found: C, 71.90; H, 3.40; Br, 25.7.Melting point 478.1 C; 1 H NMR (300 MHz, CDCl 3) 7.92 (d, J = 7.6 Hz, 4H), 7.46 (t, J = 8.0 Hz, 4H), 7.33 (t, J = 7.4 Hz, 4H), 7.21 (d, J = 7.6 Hz, 4H), 6.88 (dd, J = 2.1, 8.6 Hz, 2H), 6.47 (d, J = 2.1 Hz, 2H), 6.22 (d, J = 8.6 Hz, 2H); MS (M +) 636; Anal. Calcd for C 38 H 22 Br 2 : C, 71.50; H, 3.47; Br, 25.03. Found: C, 71.90; H, 3. 40; Br, 25.7.
제조예 4Preparation Example 4
(화학식 d로 표시되는 출발물질의 제조) (Preparation of starting material represented by Formula d)
65 ℃에서 아세토니트릴(250 ㎖)에 브롬화구리(CuBr2, 18.0 g, 80.0 mmol)과 터셔리-부틸 나이트라이트(12 ㎖, 101 mmol)을 분산시키고 교반한 후, 여기에 2-아미노안트라퀴논(15.0 g, 67.2 mmol)을 5 분에 걸쳐 천천히 적가하였다. 기체 발생이 끝나면 반응용액을 상온으로 냉각하고 반응용액을 20 중량% 농도의 염산수용액(1000 ㎖)에 가하고 디클로로메탄으로 추출하였다. 유기층을 무수황산마그네슘으로 잔류수분을 제거한 후 감압하여 건조하였다. 컬럼크로마토그래피로 분리(디클로로메탄 / n-헥산=4/1)하여 연한 노란색의 화학식 d로 표시되는 화합물(14.5 g, 수율 75 %)을 얻었다.Copper bromide (CuBr 2 , 18.0 g, 80.0 mmol) and tertiary-butyl nitrite (12 mL, 101 mmol) were dispersed in acetonitrile (250 mL) at 65 ° C. and stirred, followed by 2-aminoanthraquinone (15.0 g, 67.2 mmol) was added slowly dropwise over 5 minutes. After the generation of gas, the reaction solution was cooled to room temperature, and the reaction solution was added to 20 wt% aqueous hydrochloric acid solution (1000 mL) and extracted with dichloromethane. The organic layer was dried over anhydrous magnesium sulfate, followed by removal of residual moisture. Separation by column chromatography (dichloromethane / n-hexane = 4/1) to give a compound of the light yellow formula (14.5 g, yield 75%).
이 화합물의 분석결과는 다음과 같다.The analysis results of this compound are as follows.
녹는점 207.5 C; 1H NMR (500 MHz, CDCl3) 8.43 (d, J = 1.8 Hz, 1H), 8.30 (m, 2H), 8.17 (d, J = 8.3 Hz, 1H), 7.91 (dd, J = 1.8, 8.3 Hz, 1H), 7.82 (m, 2H); MS (M+) 286; Anal. Calcd for C14H7BrO2: C, 58.57; H, 2.46; Br, 27.83; O, 11.14. Found: C, 58.88; H, 2.39; Br, 27.80; O, 10.93.Melting point 207.5 C; 1 H NMR (500 MHz, CDCl 3) 8.43 (d, J = 1.8 Hz, 1H), 8.30 (m, 2H), 8.17 (d, J = 8.3 Hz, 1H), 7.91 (dd, J = 1.8, 8.3 Hz, 1H), 7.82 (m, 2 H); MS (M +) 286; Anal. Calcd for C 14 H 7 BrO 2: C, 58.57; H, 2. 46; Br, 27.83; O, 11.14. Found: C, 58.88; H, 2.39; Br, 27.80; O, 10.93.
제조예 5Preparation Example 5
(화학식 e로 표시되는 출발물질의 제조) (Preparation of starting material represented by Formula e)
질소분위기 하에서 건조된 테트라하이드로 퓨란(100 ㎖)에 2-브로모비페닐 (9.0 ㎖, 52 mmol)을 녹이고 -78 ℃에서 터셔리-부틸리튬(40 ㎖, 1.7 M 펜탄용액) 을 천천히 가하였다. 동일온도에서 한 시간 동안 교반한 후, 상기 제조예 4에서 제조된 화학식 d로 표시되는 화합물(4.9 g, 17 mmol)을 가하였다. 냉각용기를 제거한 후 상온에서 3 시간동안 교반하였다. 반응 혼합물에 염화암모늄수용액을 가한 후 메틸렌클로라이드로 추출하였다. 유기층을 무수황산 마그테슘으로 건조하고 용매를 제거하였다. 얻어진 고체를 에탄올에 분산시켜 한 시간 동안 교반한 후 여과하고 깨끗한 에탄올로 씻었다. 건조 후 화학식 e로 표시되는 화합물(9.50 g, 수율 94 %)를 얻었다. 2-bromobiphenyl (9.0 mL, 52 mmol) was dissolved in tetrahydrofuran (100 mL) dried under nitrogen atmosphere, and tertiary-butyllithium (40 mL, 1.7 M pentane solution) was slowly added at -78 ° C. After stirring at the same temperature for one hour, the compound represented by Chemical Formula d prepared in Preparation Example 4 (4.9 g, 17 mmol) was added thereto. After removing the cooling vessel and stirred for 3 hours at room temperature. An aqueous ammonium chloride solution was added to the reaction mixture, followed by extraction with methylene chloride. The organic layer was dried over anhydrous magnesium sulfate and the solvent was removed. The resulting solid was dispersed in ethanol, stirred for one hour, filtered and washed with clean ethanol. After drying, a compound represented by the formula (e) (9.50 g, yield 94%) was obtained.
제조예 6Preparation Example 6
(화학식 f로 표시되는 출발물질의 제조)(Preparation of starting material represented by Formula f)
질소분위기 하에서 상기 제조예 5에서 제조된 화학식 e로 표시되는 화합물(6.0 g, 10.1 mmol)를 아세트산 300 ㎖에 분산시킨 후, 칼륨요오드(16.8 g, 101 mmol), 나트륨 하이포 포스파이트 하이드레이트(17.7 g, 167 mmol)을 가하고 3 시간 동안 끓이면서 교반하였다. 상온으로 냉각한 후 여과하고 물과 메탄올로 씻은 후 진공 건조하여 연한 황색의 화학식 f로 표시되는 화합물(5.0 g, 수율 88 %)을 얻었다.After dispersing the compound represented by Chemical Formula e (6.0 g, 10.1 mmol) prepared in Preparation Example 5 in 300 ml of acetic acid under nitrogen atmosphere, potassium iodine (16.8 g, 101 mmol) and sodium hypophosphite hydrate (17.7 g) 167 mmol) was added and stirred with boiling for 3 hours. After cooling to room temperature, the mixture was filtered, washed with water and methanol and dried in vacuo to obtain a compound (5.0 g, yield 88%) represented by the light yellow formula f.
제조예 7Preparation Example 7
(화학식 g로 표시되는 출발물질의 제조)(Preparation of starting material represented by Chemical Formula g)
아세트산 100 ㎖에 상기 제조예 5에서 제조된 화학식 e로 표시되는 화합물(9.5 g, 16 mmol)을 분산시키고, 여기에 진한 황산 5 방울을 가하여 3 시간 동안 환류하였다. 상온으로 냉각하고 생성된 고체를 여과하고 깨끗한 아세트산으로 씻은 후 물과 에탄올로 차례로 씻었다. 건조한 후 승화법으로 정제하여 흰색 고체인 화학식 g로 표시되는 화합물(8.0 g, 수율 89 %)를 얻었다. To 100 ml of acetic acid was dispersed the compound represented by the formula (e) prepared in Preparation Example 5 (9.5 g, 16 mmol), and 5 drops of concentrated sulfuric acid was added thereto and refluxed for 3 hours. After cooling to room temperature, the resulting solid was filtered, washed with clean acetic acid, and then washed with water and ethanol. After drying, the product was purified by sublimation to obtain a compound represented by the formula g as a white solid (8.0 g, 89% yield).
제조예 8Preparation Example 8
(화학식 h로 표시되는 출발물질의 제조)(Preparation of starting material represented by Formula h)
질소분위기 하에서 150 ㎖의 정제된 THF에 상기 제조예 7에서 제조된 화학식 g로 표시되는 화합물(10 g, 17.9 mmol)을 완전히 녹인 후, -78 ℃에서 터셔리-부틸리튬(31.5 ㎖, 1.7 M 펜탄용액)을 천천히 가하였다. 동일 온도에서 한 시간 동안 교반한 후 트리메틸보레이트(8 ㎖ ,71.5 mmol)을 가하였다. 냉각 용기를 제거하고 반응 혼합물을 3 시간 동안 상온에서 교반하였다. 반응 혼합물에 2 N 염산수용액(100 ㎖)를 가하고 1.5 시간 동안 상온에서 교반하였다. 생성된 침전물을 거르고 물과 에틸에테르로 차례로 씻은 후 진공 건조하였다. 건조후 에틸에테르에 분산시켜 두 시간 동안 교반한 후 여과하고 건조하여 흰색의 화학식 h로 표시되는 화합물(7.6 g, 수율 81 %)를 얻었다.After completely dissolving the compound represented by Chemical Formula g (10 g, 17.9 mmol) prepared in Preparation Example 7 in 150 mL of purified THF under a nitrogen atmosphere, tertiary-butyllithium (31.5 mL, 1.7 M at -78 ° C) was dissolved. Pentane solution) was added slowly. After stirring for one hour at the same temperature, trimethylborate (8 mL, 71.5 mmol) was added. The cold vessel was removed and the reaction mixture was stirred at room temperature for 3 hours. 2N aqueous hydrochloric acid solution (100 mL) was added to the reaction mixture, which was stirred for 1.5 hours at room temperature. The resulting precipitate was filtered off, washed successively with water and ethyl ether, and dried in vacuo. After drying, the mixture was dispersed in ethyl ether, stirred for 2 hours, filtered, and dried to obtain a white compound (7.6 g, yield 81%).
제조예 9Preparation Example 9
(화학식 i로 표시되는 출발물질의 제조)(Preparation of starting material represented by Formula i)
질소분위기 하에서 건조된 테트라하이드로 퓨란(100 ㎖)에 2-브로모나프탈렌 (11g, 53.12 mmol)을 녹이고, -78 ℃에서 터셔리-부틸리튬(47.0 ㎖, 1.7 M 펜탄용액)을 천천히 가하였다. 동일온도에서 한 시간 동안 교반한 후 상기 제조예 4에서 제조된 화학식 d로 표시되는 화합물(6.31 g, 22 mmol)을 가하였다. 냉각용기를 제거한 후 상온에서 3 시간 동안 교반하였다. 반응 혼합물에 염화암모늄수용액을 가한 후 메틸렌클로라이드로 추출하였다. 유기 층을 무수황산 마그테슘으로 건조하고 용매를 제거하였다. 얻어진 혼합물에 에틸에테르 소량으로 녹인 후 석유에테르를 가하여 수 시간 동안 교반시켜 고체화합물을 얻었다. 여과 한 후 진공 건조하여 순수하지 않은 디나프틸디알콜(11.2 g, 수율 94 %)를 얻었다.2-bromonaphthalene (11 g, 53.12 mmol) was dissolved in tetrahydrofuran (100 mL) dried under a nitrogen atmosphere, and tertiary-butyllithium (47.0 mL, 1.7 M pentane solution) was slowly added at -78 ° C. After stirring at the same temperature for one hour, Compound (6.31 g, 22 mmol) represented by Chemical Formula d prepared in Preparation Example 4 was added thereto. After removing the cooling vessel and stirred for 3 hours at room temperature. An aqueous ammonium chloride solution was added to the reaction mixture, followed by extraction with methylene chloride. The organic layer was dried over anhydrous magnesium sulfate and the solvent was removed. A small amount of ethyl ether was dissolved in the obtained mixture, and petroleum ether was added thereto, followed by stirring for several hours to obtain a solid compound. After filtration and drying in vacuo, pure naphthyldialcohol (11.2 g, yield 94%) was obtained.
질소분위기 하에서 디나프틸디알콜(11.2g, 20.5 mmol)를 아세트산 200 ㎖에 분산시킨 후, 칼륨요오드(34.2 g, 210 mmol), 나트륨 하이포 포스파이트 하이드레이트(37 g, 340 mmol)을 가한 후 3 시간 동안 끓이면서 교반하였다. 상온으로 냉각한 후 여과하고 물과 메탄올로 씻은 후 진공 건조하여 연한 황색의 화학식 i로 표시되는 화합물(7.2 g, 수율 64 %)을 얻었다. Dinaphthyldialcohol (11.2 g, 20.5 mmol) was dispersed in 200 ml of acetic acid under nitrogen atmosphere, and then potassium iodine (34.2 g, 210 mmol) and sodium hypophosphite hydrate (37 g, 340 mmol) were added for 3 hours. Stir while boiling. After cooling to room temperature, the mixture was filtered, washed with water and methanol and dried in vacuo to obtain a compound (7.2 g, yield 64%) represented by the formula (I) in light yellow color.
실시예 1Example 1
(화학식 2-3으로 표시되는 화합물의 제조)(Production of Compound Represented by Formula 2-3)
4-브로모페닐알데하이드(41.6 g, 225 mmol)와 1,3-프로판디올(16.31 ㎖, 225 mmol)을 톨루엔 500 ㎖에 녹인 후, 파라-톨루엔술폰산 1 g을 넣고 물을 제거하면서 이틀동안 환류시켰다. 반응물에 에테르 100 ㎖를 가해 묽힌 후, 물 100 ㎖를 붓고 추출하였다. 유기 용매를 제거하여 얻은 액체를 컬럼 크로마토그래피로 분리하고 얻어진 액체에 석유에테르로 결정을 형성시켜 4-브로모페닐아세탈을 흰색고체로 얻었다(45 g, 수율 82 %). Dissolve 4-bromophenylaldehyde (41.6 g, 225 mmol) and 1,3-propanediol (16.31 ml, 225 mmol) in 500 ml of toluene, add 1 g of para-toluenesulfonic acid and remove water to reflux for two days. I was. 100 ml of ether was added to the reaction mixture and diluted, and then 100 ml of water was poured out and extracted. The liquid obtained by removing the organic solvent was separated by column chromatography, and crystals were formed by petroleum ether in the obtained liquid to obtain 4-bromophenylacetal as a white solid (45 g, yield 82%).
4-브로모페닐아세탈(5.00 g, 20.6 mmol)에 정제한 THF 100 ㎖를 넣고 완전히 녹인 후 -78 ℃로 반응온도를 낮추었다. 여기에 터셔리-부틸리튬(1.7 M 펜탄용액, 24.2 ㎖, 41.1 mmol)를 천천히 주입한 후 1 시간 동안 교반하였다. 트라이메틸보레이트(7 ㎖, 62.4 mmol)을 -78 ℃에서 천천히 주입한 후, 반응 온도를 서서히 올려 3 시간 동안 실온에서 교반하였다.100 mL of purified THF was added to 4-bromophenyl acetal (5.00 g, 20.6 mmol), and completely dissolved, and the reaction temperature was lowered to -78 ° C. Tertiary-butyllithium (1.7 M pentane solution, 24.2 mL, 41.1 mmol) was slowly added thereto, followed by stirring for 1 hour. Trimethylborate (7 mL, 62.4 mmol) was slowly injected at −78 ° C., and then the reaction temperature was slowly raised and stirred at room temperature for 3 hours.
2 N 염산 수용액 200 ㎖에 상기 반응물을 붓고 1 시간 동안 교반한 후 여과 하고, 물, 및 석유에테르로 씻어준 후 건조시켜 흰색 4-포밀페닐보로닉산(2.5 g, 수율 81 %)을 얻었다.The reaction was poured into 200 mL of 2N hydrochloric acid aqueous solution, stirred for 1 hour, filtered, washed with water and petroleum ether, and dried to obtain white 4-formylphenylboronic acid (2.5 g, 81% yield).
상기 제조예 3에서 제조된 화학식 c로 표시되는 화합물(0.4 g, 0.62 mmol)과 4-포밀페닐보로닉산(1.1 g, 7.34 mmol)을 2 N 탄산칼륨 수용액 10 ㎖와 톨루엔 30 ㎖의 용액에 넣고 교반하면서 Pd(PPh3)4 (0.16 g, 0.14 mmol)을 넣고 3 일 동안 환류하였다. 톨루엔층을 추출한 후 물로 씻고 무수 마그네슘황산으로 건조시킨 후 실리카겔 층을 통과시키고 용매를 제거하여 고체 화합물을 얻었다. 고체화합물을 에탄올 40 ㎖로 씻어 준 후 여과하고 클로로포름으로 재결정시켜 디알데하이드(155 mg, 수율 36 %)를 얻었다.The compound represented by Formula c prepared in Preparation Example 3 (0.4 g, 0.62 mmol) and 4-formylphenylboronic acid (1.1 g, 7.34 mmol) were added to a solution of 10 ml of 2 N potassium carbonate solution and 30 ml of toluene. Pd (PPh 3 ) 4 (0.16 g, 0.14 mmol) was added thereto while stirring, and the mixture was refluxed for 3 days. The toluene layer was extracted, washed with water, dried over anhydrous magnesium sulfate, passed through a silica gel layer, and the solvent was removed to obtain a solid compound. The solid compound was washed with 40 ml of ethanol, filtered and recrystallized with chloroform to obtain dialdehyde (155 mg, yield 36%).
디알데하이드(120 mg, 0.17 mmol)와 N-페닐-1,2-페닐렌디아민(82 mg, 0.45 mmol)을 톨루엔 20 ㎖와 초산 10 ㎖에 넣고 2 일간 환류하고 실온으로 온도를 내려 형성된 고체를 여과한 후 에탄올로 씻어 주었다. 얻어진 고체를 클로로포름 100 ㎖로 씻고 여과하여 깨끗한 화학식 2-3으로 표시되는 화합물(120 mg, 수율 68 %)을 얻었다.Dialdehyde (120 mg, 0.17 mmol) and N-phenyl-1,2-phenylenediamine (82 mg, 0.45 mmol) were added to 20 ml of toluene and 10 ml of acetic acid, refluxed for 2 days, and the temperature was lowered to room temperature. After filtration and washed with ethanol. The obtained solid was washed with 100 ml of chloroform and filtered to obtain a clean compound (120 mg, yield 68%) represented by Chemical Formula 2-3.
이 화합물의 분석결과는 다음과 같다.The analysis results of this compound are as follows.
녹는점 395.0 ℃; 1H NMR (300 MHz, CDCl3) 7.88(2H), 7.62(2H), 7.66-7.60 (10H), 7.55-7.44(15H), 7.40(2H), 7.38-30(9H), 6.95(6H), 6.83(4H); MS [M+H] 1019.Melting point 395.0 ° C .; 1 H NMR (300 MHz, CDCl 3 ) 7.88 (2H), 7.62 (2H), 7.66-7.60 (10H), 7.55-7.44 (15H), 7.40 (2H), 7.38-30 (9H), 6.95 (6H), 6.83 (4 H); MS [M + H] 1019.
실시예 2Example 2
(화학식 1-2로 표시되는 화합물의 제조)(Production of Compound Represented by Formula 1-2)
상기 제조예 6에서 제조된 화학식 f로 표시되는 화합물(1.00 g, 1.80 mmol)과 4-포밀페닐보로닉산(0.74 g, 4.93 mmol)을 2 N 탄산칼륨 수용액 20 ㎖와 톨루엔 40 ㎖의 용액에 넣고 교반하면서 Pd(PPh3)4 (0.20 g, 0.17 mmol)을 넣고 3 일 동안 환류하였다. 톨루엔층을 추출한 후 물로 씻고 무수 마그네슘황산으로 건조시킨 후 실리카겔 층을 통과시키고 용매를 제거하여 고체 화합물을 얻었다. 에탄올 100 ㎖로 씻어준 후 여과하고 에틸 아세테이트로 재결정시켜 안트라센 페닐알데하이드 (330 mg, 수율 31 %)를 얻었다.Compound (1.00 g, 1.80 mmol) and 4-formylphenylboronic acid (0.74 g, 4.93 mmol) represented by Chemical Formula f prepared in Preparation Example 6 were added to a solution of 20 ml of 2N aqueous potassium carbonate solution and 40 ml of toluene. Pd (PPh 3 ) 4 (0.20 g, 0.17 mmol) was added thereto while stirring and refluxed for 3 days. The toluene layer was extracted, washed with water, dried over anhydrous magnesium sulfate, passed through a silica gel layer, and the solvent was removed to obtain a solid compound. Washed with 100 ml of ethanol, filtered and recrystallized with ethyl acetate to obtain anthracene phenylaldehyde (330 mg, 31% yield).
안트라센 페닐알데하이드(0.33 g, 0.56 mmol)과 N-페닐-1,2-페닐렌디아민 (0.11 g, 0.60 mmol)에 톨루엔 40 ㎖와 초산 10 ㎖을 넣고 환류하였다. 2 일간 반응시킨 후 실온으로 온도을 내려 형성된 고체를 여과하고 에탄올과 클로로포름으로 씻고 여과하여 화학식 1-2로 표시되는 화합물(120 mg, 수율 28 %)을 얻었다.40 ml of toluene and 10 ml of acetic acid were refluxed in anthracene phenylaldehyde (0.33 g, 0.56 mmol) and N-phenyl-1,2-phenylenediamine (0.11 g, 0.60 mmol). After reacting for 2 days, the mixture was cooled to room temperature, the solid formed was filtered, washed with ethanol and chloroform, and filtered to obtain a compound represented by Chemical Formula 1-2 (120 mg, yield 28%).
이 화합물의 분석결과는 다음과 같다.The analysis results of this compound are as follows.
1H NMR (300 MHz, CDCl3) 7.89(1H), 7.75(1H), 7.62-7.29 (24H), 6.98- 6.76(12H); MS [M+H] 751.1 H NMR (300 MHz, CDCl 3 ) 7.89 (1 H), 7.75 (1 H), 7.62-7.29 (24 H), 6.98-6.76 (12H); MS [M + H] 751.
실시예 3Example 3
(화학식 1-4로 표시되는 화합물의 제조)(Production of Compound Represented by Formula 1-4)
상기 제조예 9에서 제조된 화학식 i로 표시되는 화합물(4.00 g, 7.85 mmol)와 4-포밀페닐보로닉산(3.53 g, 23.5 mmol)을 2 N 탄산칼륨 수용액 20 ㎖와 톨루엔 60 ㎖의 용액에 넣고 교반하면서 Pd(PPh3)4 (0.27 g, 0.23 mmol)을 넣고 3 일 동안 환류하였다. 톨루엔층을 추출한 후 물로 씻고 무수 마그네슘황산으로 건조시킨 후 실리카겔 층을 통과시키고 용매를 제거하여 고체 화합물을 얻었다. 에탄올 100 ㎖로 씻어준 후 여과하고 에틸 아세테이트로 재결정시켜 안트라센 페닐알데하이드 (2.00 g, 수율 47.6 %)를 얻었다.Compound (4.00 g, 7.85 mmol) and 4-formylphenylboronic acid (3.53 g, 23.5 mmol) represented by Formula (I) prepared in Preparation Example 9 were added to a solution of 20 ml of 2N potassium carbonate aqueous solution and 60 ml of toluene. Pd (PPh 3 ) 4 (0.27 g, 0.23 mmol) was added thereto while stirring and refluxed for 3 days. The toluene layer was extracted, washed with water, dried over anhydrous magnesium sulfate, passed through a silica gel layer, and the solvent was removed to obtain a solid compound. Washed with 100 ml of ethanol, filtered and recrystallized with ethyl acetate to give anthracene phenylaldehyde (2.00 g, yield 47.6%).
안트라센 페닐알데하이드(2 g, 3.74 mmol)과 N-페닐-1,2-페닐렌디아민 (0.69 g, 3.74 mmol)에 톨루엔 40 ㎖와 초산 10 ㎖을 넣고 환류하였다. 2 일간 반응시킨 후 실온으로 온도를 내려 형성된 고체를 여과하고 에탄올과 클로로포름으로 씻고 여과하여 화학식 1-4로 표시되는 화합물(1.3g, 수율 49.7 %)을 얻었다.To anthracene phenylaldehyde (2 g, 3.74 mmol) and N-phenyl-1,2-phenylenediamine (0.69 g, 3.74 mmol), 40 ml of toluene and 10 ml of acetic acid were added to reflux. After reacting for 2 days, the mixture was cooled to room temperature and the solid formed was filtered, washed with ethanol and chloroform, and filtered to obtain a compound represented by Chemical Formula 1-4 (1.3 g, yield 49.7%).
이 화합물의 분석결과는 다음과 같다.The analysis results of this compound are as follows.
녹는점 352.0 ℃; 1H NMR (300 MHz, CDCl3) 8.28(s, 2H), 8.14(d, 2H), 7.99(t, 4H), 7.81(t, 4H), 7.62(m, 4H), 7.53(d, 2H), 7.45(m, 4H), 7.32-7.26(m, 6H), 7.22(s, 6H); MS [M+H] 699.Melting point 352.0 ° C .; 1 H NMR (300 MHz, CDCl 3 ) 8.28 (s, 2H), 8.14 (d, 2H), 7.99 (t, 4H), 7.81 (t, 4H), 7.62 (m, 4H), 7.53 (d, 2H) , 7.45 (m, 4H), 7.32-7.26 (m, 6H), 7.22 (s, 6H); MS [M + H] 699.
실시예 4Example 4
(화학식 2-4로 표시되는 화합물의 제조)(Production of Compound Represented by Formula 2-4)
질소분위기 하에서 2-브로모 나프탈렌(5.78 g, 28.0 mmol)을 정제된 THF(40 ㎖)에 녹이고 -78 ℃로 냉각한 후 터셔리-부틸리튬(21 ㎖, 1.7 M 펜탄용액)을 천천히 가하였다. 동일온도에서 40 분간 교반한 후 상기 제조예 1에서 제조된 화학식 a로 표시되는 화합물(2.93 g, 8.00 mmol)을 동일온도에서 가하였다. 냉각용기를 제거한 후 반응물을 상온에서 3 시간 동안 교반하였다. 반응 용액에 암모늄클로라이드용액(40 ㎖)를 천천히 가하여 주고 40 분간 상온에서 교반하였다. 생성된 침전물을 여과한 후 물과 석유에테르로 충분히 씻어주었다. 이 물질을 진공 건조하여 디알콜(4.10 g, 수율 82 %)를 얻었다.2-bromo naphthalene (5.78 g, 28.0 mmol) was dissolved in purified THF (40 mL) under nitrogen atmosphere, cooled to -78 ° C, and tert-butyllithium (21 mL, 1.7 M pentane solution) was added slowly. . After stirring at the same temperature for 40 minutes, the compound represented by Chemical Formula a (2.93 g, 8.00 mmol) prepared in Preparation Example 1 was added at the same temperature. After removing the cooling vessel, the reaction was stirred at room temperature for 3 hours. Ammonium chloride solution (40 mL) was slowly added to the reaction solution, and the mixture was stirred at room temperature for 40 minutes. The resulting precipitate was filtered and washed well with water and petroleum ether. This material was dried in vacuo to give a dialcohol (4.10 g, yield 82%).
디알콜(4.10 g, 6.59 mmol), 칼륨요오드(10.94 g, 65.9 mmol)과 나트륨 하이포포스파이트 하이드레이트(11.6 g, 109 mmol)의 혼합물을 아세트산(200 ㎖)에서 24 시간 동안 환류하였다. 상온으로 내각하고 여과한 다음 아세트산, 물, 석유에테르로 차례로 씻은 후 건조하여 2,6-디브로모-9,10-디나프탈렌-2-일-안트라센 (3.15 g, 수율 81 %)을 얻었다.A mixture of dialcohol (4.10 g, 6.59 mmol), potassium iodine (10.94 g, 65.9 mmol) and sodium hypophosphite hydrate (11.6 g, 109 mmol) was refluxed in acetic acid (200 mL) for 24 hours. Cabinet, filtered and washed with acetic acid, water, petroleum ether in turn and dried to give 2,6-dibromo-9,10- dinaphthalen-2-yl-anthracene (3.15 g, 81% yield).
2,6-디브로모-9,10-디나프탈렌-2-일-안트라센 (3.15 g, 5.35 mmol)와 4-포밀페닐보로닉산(2.81 g, 18.7 mmol)을 2 N 탄산칼륨 수용액 20 ㎖와 톨루엔 100 ㎖ 의 용액에 넣고 교반하면서 Pd(PPh3)4 (0.25 g, 0.22 mmol)을 넣고 15 시간 동안 환류하였다. 톨루엔층을 추출한 후 물로 씻고 무수 마그네슘황산으로 건조시킨 후 관 크로마토그래피로 분리하여 디나프틸 안트라센 페닐디알데하이드(2.77 g, 수율 81 %)를 얻었다.2 ml of 2 N aqueous potassium carbonate solution with 2,6-dibromo-9,10-dinaphthalen-2-yl-anthracene (3.15 g, 5.35 mmol) and 4-formylphenylboronic acid (2.81 g, 18.7 mmol) Pd (PPh 3 ) 4 (0.25 g, 0.22 mmol) was added thereto under stirring and 100 mL of toluene, and the mixture was refluxed for 15 hours. The toluene layer was extracted, washed with water, dried over anhydrous magnesium sulfate, and separated by column chromatography to obtain dinaphthyl anthracene phenyldialdehyde (2.77 g, yield 81%).
상기 디나프틸 안트라센 페닐디알데하이드(2.77 g, 4.34 mmol)과 N-페닐-1,2-페닐렌디아민(2.00 g, 10.9 mmol)에 톨루엔 120 ㎖와 초산 60 ㎖을 넣고 환류하였다. 15 시간 동안 반응시킨 후 망간옥사이드(1.51 g, 17.4 mmol)를 넣고 2 시간 동안 반응을 더 시킨 후 실온으로 온도를 내려 형성된 고체를 여과하고 에탄올과 클로로포름으로 씻고 여과하여 화학식 2-4로 표시되는 화합물(1.52 g, 수율 36 %)을 얻었다. 이 화합물의 녹는점은 487.4 ℃이다.120 mL of toluene and 60 mL of acetic acid were refluxed in the dinaphthyl anthracene phenyldialdehyde (2.77 g, 4.34 mmol) and N-phenyl-1,2-phenylenediamine (2.00 g, 10.9 mmol). After reacting for 15 hours, manganese oxide (1.51 g, 17.4 mmol) was added thereto, followed by further reaction for 2 hours, and then the temperature was reduced to room temperature. The solid formed was filtered, washed with ethanol and chloroform, and filtered to obtain a compound represented by Chemical Formula 2-4. (1.52 g, yield 36%) was obtained. The melting point of this compound is 487.4 ° C.
실시예 5Example 5
(화학식 1-14로 표시되는 화합물의 제조)(Preparation of a compound represented by Formula 1-14)
9,10-비스-비페닐-2-일-2,6-디브로모안트라센(1.00 g, 3.55 mmol)과 4-포밀페닐보로닉산(0.81 g, 3.90 mmol)을 2 N 탄산칼륨 10 ㎖와 톨루엔 30 ㎖의 용액에 넣고 교반하면서 Pd(PPh3)4 (0.10 g, 0.09 mmol)을 넣고 2 일 동안 환류하였다.10 mL of 2 N potassium carbonate with 9,10-bis-biphenyl-2-yl-2,6-dibromoanthracene (1.00 g, 3.55 mmol) and 4-formylphenylboronic acid (0.81 g, 3.90 mmol) Pd (PPh 3 ) 4 (0.10 g, 0.09 mmol) was added thereto under stirring and 30 mL of toluene, followed by reflux for 2 days.
톨루엔층을 추출한 후 물로 씻고 무수 마그네슘황산으로 건조시킨 후 용매를 제거하고 관 크로마토그래피로(CHCl3/HEX=1/2) 분리하여 원하는 4-(9,10-비스-비페닐-2-일-6-브로모-안트라센-2-일)-벤즈알데하이드을 0.8 g 합성하였다(수율 34 %).The toluene layer was extracted, washed with water, dried over anhydrous magnesium sulfate, the solvent was removed and the resultant was separated by column chromatography (CHCl 3 / HEX = 1/2) to obtain the desired 4- (9,10-bis-biphenyl-2-yl. 0.8 g of -6-bromo-anthracen-2-yl) -benzaldehyde was synthesized (yield 34%).
상기에서 4-(9,10-비스-비페닐-2-일-6-브로모-안트라센-2-일)-벤즈알데하이드(0.80 g, 1.20 mmol)과 N-페닐-1,2-페닐렌디아민(0.22 g, 1.20 mmol)에 톨루엔 50 ㎖와 초산 10 ㎖을 넣고 환류하였다. 2 일간 반응시킨 후 실온으로 온도를 내려 형성된 고체를 여과하고 관 크로마토그래피로 분리한 후 에탄올과 클로로포름으 로 재결정하여 화학식 1-14로 표시되는 화합물(0.45 g, 수율 45 %)을 합성하였다.4- (9,10-bis-biphenyl-2-yl-6-bromo-anthracen-2-yl) -benzaldehyde (0.80 g, 1.20 mmol) and N-phenyl-1,2-phenylene To diamine (0.22 g, 1.20 mmol), 50 ml of toluene and 10 ml of acetic acid were added and refluxed. After reacting for 2 days, the mixture was cooled to room temperature, the solid formed was filtered, separated by column chromatography, and recrystallized with ethanol and chloroform to synthesize the compound represented by Chemical Formula 1-14 (0.45 g, yield 45%).
이 화합물의 분석결과는 다음과 같다.The analysis results of this compound are as follows.
녹는점 417.7 ℃; MS [M+H] 1231.Melting point 417.7 ° C .; MS [M + H] 1231.
실시예 6Example 6
(화학식 3-2로 표시되는 화합물의 제조)(Production of Compound Represented by Formula 3-2)
9,10-디옥소-9,10-디하이드로-안트라센-2-카바알데하이드(1.63 g, 6.9 mmol)에 1.27 g(6.9 mmol)의 N-페닐-1,2-페닐렌디아민(1.27 g, 6.90 mmol)을 톨루엔 80 ㎖과 아세트산 10 ㎖를 넣고 12 시간 동안 환류시켰다. 용매를 제거시키고 에탄올을 부어 결정화시킨 후 여과하여 벤조이미다졸 화합물(2-(1-페닐-1H-벤조이미다졸-2-일)-안트라퀴논, 1.14 g, 수율 41 %)을 얻었다.In 9,10-dioxo-9,10-dihydro-anthracene-2-carbaaldehyde (1.63 g, 6.9 mmol), 1.27 g (6.9 mmol) of N-phenyl-1,2-phenylenediamine (1.27 g, 6.90 mmol) was added to 80 ml of toluene and 10 ml of acetic acid and refluxed for 12 hours. The solvent was removed, ethanol was poured and crystallized, followed by filtration to obtain a benzimidazole compound (2- (1-phenyl-1H-benzoimidazol-2-yl) -anthraquinone, 1.14 g, yield 41%).
2-브로모비페닐(1.46 g, 1.1 ㎖, 6.25 mmol)을 테트라하이드로퓨란(50 ㎖)에 녹이고 터셔리-부틸리튬(8.3 ㎖, 1.5 M 펜탄용액)을 -78 ℃에서 천천히 가하여 반응시켰다. 이 반응물에 상기에서 합성한 2-(1-페닐-1H-벤조이미다졸-2-일)-안트라퀴논(1.00 g, 2.50 mmol)을 천천히 가한 후 실온에서 4 시간 동안 교반시켰다. 2 N 염산 용액과 에틸에테르 혼합물에 반응물을 부어 1 시간 동안 교반시켰다. 고체 화합물을 여과시킨 후 건조시켜 9,10-비스[비페닐-2-일-2(1-페닐-1H-벤조이미다졸-2-일)]-9,10-디하이드로-안트라센-9,10-디알올(1.00 g, 수율 57 %)을 얻었다. 2-bromobiphenyl (1.46 g, 1.1 ml, 6.25 mmol) was dissolved in tetrahydrofuran (50 ml) and tertiary-butyllithium (8.3 ml, 1.5 M pentane solution) was added slowly at -78 deg. 2- (1-phenyl-1H-benzoimidazol-2-yl) -anthraquinone (1.00 g, 2.50 mmol) synthesized above was slowly added to the reaction, followed by stirring at room temperature for 4 hours. The reaction was poured into 2N hydrochloric acid solution and ethyl ether mixture and stirred for 1 hour. The solid compound was filtered off and dried to give 9,10-bis [biphenyl-2-yl-2 (1-phenyl-1H-benzoimidazol-2-yl)]-9,10-dihydro-anthracene-9, 10-dialol (1.00 g, yield 57%) was obtained.
질소분위기 하에서 디알콜(0.70 g, 1.00 mmol)를 아세트산 60 ㎖에 분산시킨 후 칼륨요오드(1.66 g, 10 mmol), 나트륨 하이포 포스파이트 하이드레이트(1.66 g, 15.7 mmol)을 가한 후 3 시간 동안 끓이면서 교반하였다. 상온으로 냉각한 후 여과하고 물과 메탄올로 씻은 후 진공 건조하여 연한 황색의 화학식 3-2로 표시되는 화합물(0.45 g, 수율 67 %)을 얻었다. Dialcohol (0.70 g, 1.00 mmol) was dispersed in 60 mL of acetic acid under a nitrogen atmosphere, followed by addition of potassium iodine (1.66 g, 10 mmol) and sodium hypophosphite hydrate (1.66 g, 15.7 mmol), followed by stirring for 3 hours. It was. After cooling to room temperature, the mixture was filtered, washed with water and methanol, and dried in vacuo to obtain a compound (0.45 g, yield 67%) as a pale yellow chemical formula 3-2.
이 화합물의 분석결과는 다음과 같다.The analysis results of this compound are as follows.
녹는점 270.0 ℃; 1H NMR (300 MHz, CDCl3) 7.86(d, 1H), 7.75(dd, 1H), 7.70(s, 1H), 7.63-7.48(m, 8H), 7.42-7.0(m, 12H), 6.92-6.81(m, 9H), 6.63(d, 2H); MS [M+H] 675.Melting point 270.0 ° C .; 1 H NMR (300 MHz, CDCl 3 ) 7.86 (d, 1H), 7.75 (dd, 1H), 7.70 (s, 1H), 7.63-7.48 (m, 8H), 7.42-7.0 (m, 12H), 6.92- 6.81 (m, 9 H), 6.63 (d, 2 H); MS [M + H] 675.
실시예 7Example 7
(유기 발광 소자의 제조)(Manufacture of organic light emitting element)
ITO(indium tin oxide)가 1500 Å의 두께로 박막 코팅된 유리 기판을 세제를 녹인 증류수에 넣고 초음파로 세척하였다. 사용한 세제는 피셔(Fischer Co.)사의 제품을 사용하였으며, 밀리포어(Millipore Co.)사 제품의 필터(Filter)로 2 차로 걸러진 증류수를 사용하였다. ITO를 30 분간 세척한 후 증류수로 2 회 반복하여 초음파 세척을 10 분간 진행하였다. 증류수 세척이 끝나면 이소프로필알콜, 아세톤, 메탄올 등의 용제로 초음파 세척을 하고 건조시킨 후 플라즈마 세정기로 이송시켰다. 또한 산소플라즈마를 이용하여 상기 기판을 5 분간 세정한 후 진공 증착기로 기판을 이송시켰다.A glass substrate coated with a thin film of ITO (indium tin oxide) at a thickness of 1500 Å was placed in distilled water in which detergent was dissolved and ultrasonically cleaned. Fischer Co., Ltd. product was used as a detergent, and distilled water filtered secondly was used as a filter of Millipore Co., Ltd. product. After washing ITO for 30 minutes, ultrasonic washing was repeated 10 times with distilled water twice. After the distilled water was washed, ultrasonic cleaning with a solvent such as isopropyl alcohol, acetone, methanol, dried and then transferred to a plasma cleaner. In addition, the substrate was cleaned for 5 minutes using an oxygen plasma, and then the substrate was transferred to a vacuum evaporator.
이렇게 준비된 ITO 투명 전극 위에 헥사니트릴 헥사아자트리페닐렌 (hexanitrile hexaazatriphenylene)를 500 Å의 두께로 열 진공 증착하여 정공주입층을 형성하였다. 그 위에 정공을 이송하는 물질인 NPB(600 Å)를 진공 증착한 후 발광층역할을 하는 상기 화학식 j로 표시되는 화합물을 100 Å의 두께로 진공 증착 하였다. 발광층 위에 전자의 주입 및 이송역할를 하는 상기의 화학식 1-2의 화합물을 200 Å의 두께로 진공 증착 하여 유기물층의 박막 형성을 완료하였다. 상기 전자주입 및 수송층위에 순차적으로 5 Å의 두께로 리튬 플루라이드(LiF)와 2500 Å의 두께의 알루미늄을 증착하여 음극을 형성하였다. 상기의 과정에서 유기물의 증착속도는 1 Å/sec를 유지하였고 리튬플루라이드는 0.2 Å/sec, 알루미늄은 3~7 Å/sec의 증착속도를 유지하였다.Hexanitrile hexaazatriphenylene was thermally vacuum deposited to a thickness of 500 kPa on the prepared ITO transparent electrode to form a hole injection layer. NPB (600 kV), which is a material for transporting holes, was vacuum deposited thereon, and the compound represented by Chemical Formula j serving as a light emitting layer was vacuum deposited to a thickness of 100 kPa. The compound of Chemical Formula 1-2, which serves to inject and transport electrons on the light emitting layer, was vacuum deposited to a thickness of 200 kPa to complete the formation of a thin film of the organic material layer. Lithium fluoride (LiF) and aluminum having a thickness of 2500 kPa were deposited on the electron injection and transport layer sequentially to form a cathode. In the above process, the deposition rate of the organic material was maintained at 1 Å / sec, the lithium fluoride was 0.2 Å / sec, and the aluminum was maintained at the deposition rate of 3-7 Å / sec.
상기에서 제조된 전기 유기발광 소자에 4.04 V의 순방향 전계를 가한 결과, 10 mA/㎠의 전류밀도에서 1931 CIE color coordinate 기준으로 x = 0.16, y = 0.11 에 해당하는 184 nit 밝기의 청색 스펙트럼이 관찰되었다.As a result of applying a forward electric field of 4.04 V to the electroluminescent device manufactured above, a blue spectrum of 184 nit brightness corresponding to x = 0.16 and y = 0.11 based on 1931 CIE color coordinate at a current density of 10 mA /
실시예 8Example 8
(유기 발광 소자의 제조)(Manufacture of organic light emitting element)
상기 실시예 7에서와 같이 준비된 ITO 투명 전극위에 헥사니트릴 헥사아자트리페닐렌 (hexanitrile hexaazatriphenylene)를 500 Å의 두께로 열 진공 증착하여 정공주입층을 형성하였다. 그 위에 정공을 이송하는 물질인 NPB(600 Å)를 진공증착한 후 발광층역할을 하는 상기 화학식 ℓ로 표시되는 화합물을 200 Å의 두께로 진공 증착하였다. 발광층 위에 전자의 주입 및 이송역할를 하는 상기 화학식 2-2로 표시되는 화합물을 200 Å의 두께로 진공 증착하여 유기물층의 박막 형성을 완료하였다. 상기 전자주입 및 수송층위에 순차적으로 5 Å의 두께로 리튬 플루라이드(LiF)와 2500 Å의 두께의 알루미늄을 증착하여 음극을 형성하였다. 상기의 과 정에서 유기물의 증착속도는 1 Å/sec를 유지하였고 리튬플루라이드는 0.2 Å/sec, 알루미늄은 3~7 Å/sec의 증착속도를 유지하였다.Hexitrile hexaazatriphenylene was thermally vacuum deposited to a thickness of 500 kPa on the prepared ITO transparent electrode as in Example 7 to form a hole injection layer. NPB (600 kPa), which is a material for transferring holes, was vacuum-deposited thereon, and the compound represented by Chemical Formula 1 serving as a light emitting layer was vacuum deposited to a thickness of 200 kPa. The compound represented by Chemical Formula 2-2, which serves to inject and transport electrons on the light emitting layer, was vacuum deposited to a thickness of 200 kPa to complete the formation of a thin film of the organic material layer. Lithium fluoride (LiF) and aluminum having a thickness of 2500 kPa were deposited on the electron injection and transport layer sequentially to form a cathode. In the above process, the deposition rate of organic material was maintained at 1 Å / sec, the lithium fluoride was 0.2 Å / sec, and the aluminum was maintained at a deposition rate of 3-7 Å / sec.
상기에서 제조된 전기 유기발광 소자에 4.63 V의 순방향 전계를 가한 결과, 10 mA/㎠의 전류밀도에서 1931 CIE color coordinate 기준으로 x = 0.16, y = 0.19 에 해당하는 226 nit 밝기의 청색 스펙트럼이 관찰되었다.As a result of applying a forward electric field of 4.63 V to the electroluminescent device manufactured above, a blue spectrum of 226 nit brightness corresponding to x = 0.16 and y = 0.19 based on 1931 CIE color coordinate at a current density of 10 mA /
실시예 9Example 9
(유기 발광 소자의 제조)(Manufacture of organic light emitting element)
상기 실시예 7에서와 같이 준비된 ITO 투명 전극 위에 헥사니트릴 헥사아자트리페닐렌(hexanitrile hexaazatriphenylene)를 500 Å의 두께로 열 진공 증착하여 정공주입층을 형성하였다. 그 위에 정공을 이송하는 물질인 NPB(600 Å)를 진공 증착한 후 발광층역할을 하는 상기 화학식 j로 표시되는 화합물을 200 Å의 두께로 진공 증착하였다. 발광층 위에 전자의 주입 및 이송역할를 하는 상기 화학식 1-4로 표시되는 화합물을 200 Å의 두께로 진공 증착하여 유기물층의 박막 형성을 완료하였다. 상기 전자주입 및 수송층위에 순차적으로 5 Å의 두께로 리튬 플루라이드(LiF)와 2500 Å의 두께의 알루미늄을 증착하여 음극을 형성하였다. 상기의 과정에서 유기물의 증착속도는 1 Å/sec를 유지하였고 리튬플루라이드는 0.2 Å/sec, 알루미늄은 3~7 /sec의 증착속도를 유지하였다.Hexitrile hexaazatriphenylene was thermally vacuum deposited to a thickness of 500 kPa on the prepared ITO transparent electrode as in Example 7 to form a hole injection layer. NPB (600 kV), which is a material for transferring holes, was vacuum deposited thereon, and the compound represented by Chemical Formula j serving as a light emitting layer was vacuum deposited to a thickness of 200 kPa. The compound represented by Chemical Formula 1-4, which serves to inject and transport electrons on the light emitting layer, was vacuum deposited to a thickness of 200 kPa to complete the formation of a thin film of the organic material layer. Lithium fluoride (LiF) and aluminum having a thickness of 2500 kPa were deposited on the electron injection and transport layer sequentially to form a cathode. In the above process, the deposition rate of the organic material was maintained at 1 Å / sec, the lithium fluoride was 0.2 Å / sec, and the aluminum was maintained at the deposition rate of 3-7 / sec.
상기에서 제조된 전기 유기발광 소자에 6.16 V의 순방향 전계를 가한 결과, 10 mA/㎠의 전류밀도에서 1931 CIE color coordinate 기준으로 x = 0.16, y = 0.12 에 해당하는 175 nit 밝기의 청색 스펙트럼이 관찰되었다.
As a result of applying a forward electric field of 6.16 V to the electroluminescent device manufactured above, a blue spectrum of 175 nit brightness corresponding to x = 0.16 and y = 0.12 was observed based on 1931 CIE color coordinate at a current density of 10 mA /
실시예 10Example 10
(유기 발광 소자의 제조)(Manufacture of organic light emitting element)
상기 실시예 7에서와 같이 준비된 ITO 투명 전극위에 헥사니트릴 헥사아자트리페닐렌(hexanitrile hexaazatriphenylene)를 500 Å의 두께로 열 진공 증착하여 정공주입층을 형성하였다. 그 위에 정공을 이송하는 물질인 NPB(600 Å)를 진공증착한 후 발광층역할을 하는 상기 화학식 k로 표시되는 화합물을 200 Å의 두께로 진공 증착하였다. 발광층 위에 전자의 주입 및 이송역할를 하는 상기 화학식 3-2로 표시되는 화합물을 200 Å의 두께로 진공 증착하여 유기물층의 박막 형성을 완료하였다. 상기 전자주입 및 수송층위에 순차적으로 5 Å의 두께로 리튬 플루라이드(LiF)와 2500 Å의 두께의 알루미늄을 증착하여 음극을 형성하였다. 상기의 과정에서 유기물의 증착속도는 1 Å/sec를 유지하였고 리튬플루라이드는 0.2 Å/sec, 알루미늄은 3~7 Å/sec의 증착속도를 유지하였다.Hexitrile hexaazatriphenylene was thermally vacuum deposited to a thickness of 500 kPa on the prepared ITO transparent electrode as in Example 7 to form a hole injection layer. NPB (600 kPa), which is a material for transporting holes, was vacuum-deposited thereon, and the compound represented by Chemical Formula k serving as a light emitting layer was vacuum deposited to a thickness of 200 kPa. The compound represented by Chemical Formula 3-2, which serves to inject and transport electrons on the emission layer, was vacuum deposited to a thickness of 200 kPa to complete the formation of a thin film of the organic layer. Lithium fluoride (LiF) and aluminum having a thickness of 2500 kPa were deposited on the electron injection and transport layer sequentially to form a cathode. In the above process, the deposition rate of the organic material was maintained at 1 Å / sec, the lithium fluoride was 0.2 Å / sec, and the aluminum was maintained at the deposition rate of 3-7 Å / sec.
상기에서 제조된 전기 유기발광 소자에 5.17 V의 순방향 전계를 가한 결과, 10 mA/㎠의 전류밀도에서 1931 CIE color coordinate 기준으로 x = 0.16, y = 0.17 에 해당하는 124 nit 밝기의 청색 스펙트럼이 관찰되었다.As a result of applying a forward electric field of 5.17 V to the electroluminescent device manufactured above, a blue spectrum of 124 nit brightness corresponding to x = 0.16 and y = 0.17 based on 1931 CIE color coordinate at a current density of 10 mA /
본 발명의 신규의 물질은 전계 발광 소자인 유기 박막 전계 발광(유기 EL; organic electroluminescence) 소자의 유기화합물층에 함유되어 발광 효율과 수명의 향상 및 저전압구동을 실현할 수 있다.The novel material of the present invention is contained in an organic compound layer of an organic thin-film electroluminescence (organic EL) device which is an electroluminescent device, thereby improving luminous efficiency, lifetime, and low voltage driving.
Claims (17)
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020020003025A KR100691543B1 (en) | 2002-01-18 | 2002-01-18 | New material for transporting electron and organic electroluminescent display using the same |
| US10/345,310 US6878469B2 (en) | 2002-01-18 | 2003-01-16 | Material for transporting electrons and organic electroluminescent display using the same |
| AU2003215914A AU2003215914A1 (en) | 2002-01-18 | 2003-01-17 | New material for transporting electrons and organic electroluminescent display using the same |
| EP03729561.5A EP1465874B1 (en) | 2002-01-18 | 2003-01-17 | New material for transporting electrons and organic electroluminescent display using the same |
| CN038001551A CN1625552B (en) | 2002-01-18 | 2003-01-17 | New material for transporting electrons and organic electroluminescent display using the same |
| JP2003560958A JP4308663B2 (en) | 2002-01-18 | 2003-01-17 | New electron transport material and organic light emitting device using the same |
| PCT/KR2003/000112 WO2003060956A2 (en) | 2002-01-18 | 2003-01-17 | New material for transporting electrons and organic electroluminescent display using the same |
| TW092121428A TWI227655B (en) | 2002-01-18 | 2003-08-05 | New material for transporting electrons and organic electroluminescent display using the same |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020020003025A KR100691543B1 (en) | 2002-01-18 | 2002-01-18 | New material for transporting electron and organic electroluminescent display using the same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20030067773A KR20030067773A (en) | 2003-08-19 |
| KR100691543B1 true KR100691543B1 (en) | 2007-03-09 |
Family
ID=19718612
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020020003025A KR100691543B1 (en) | 2002-01-18 | 2002-01-18 | New material for transporting electron and organic electroluminescent display using the same |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US6878469B2 (en) |
| EP (1) | EP1465874B1 (en) |
| JP (1) | JP4308663B2 (en) |
| KR (1) | KR100691543B1 (en) |
| CN (1) | CN1625552B (en) |
| AU (1) | AU2003215914A1 (en) |
| TW (1) | TWI227655B (en) |
| WO (1) | WO2003060956A2 (en) |
Cited By (39)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20140082437A (en) | 2012-12-24 | 2014-07-02 | 에스에프씨 주식회사 | Antracene derivatives having heteroaryl substituted phenyl group and organic light-emitting diode including the same |
| KR20140082345A (en) | 2012-12-24 | 2014-07-02 | 에스에프씨 주식회사 | Antracene derivatives having heteroaryl substituted naphthyl group and organic light-emitting diode including the same |
| KR20140090037A (en) | 2013-01-08 | 2014-07-16 | 에스에프씨 주식회사 | Asymmetric antracene derivatives having two naphthyl groups and organic light-emitting diode including the same |
| KR20140090035A (en) | 2013-01-08 | 2014-07-16 | 에스에프씨 주식회사 | Antracene derivatives having heteroaryl substituted naphthyl group and organic light-emitting diode including the same |
| KR20140090036A (en) | 2013-01-08 | 2014-07-16 | 에스에프씨 주식회사 | Antracene derivatives having heteroaryl substituted phenyl group and organic light-emitting diode including the same |
| KR20140090411A (en) | 2013-01-09 | 2014-07-17 | 에스에프씨 주식회사 | Asymmetric antracene derivatives having two naphthyl groups and organic light-emitting diode including the same |
| KR20140090410A (en) | 2013-01-09 | 2014-07-17 | 에스에프씨 주식회사 | Asymmetric antracene derivatives having two phenyl groups and organic light-emitting diode including the same |
| US9181474B2 (en) | 2012-02-07 | 2015-11-10 | Samsung Display Co., Ltd. | Amine-based compound and organic light-emitting diode including the same |
| US9397300B2 (en) | 2013-02-14 | 2016-07-19 | Samsung Display Co., Ltd. | Compound and organic light-emitting diode including the same |
| US9587172B2 (en) | 2008-10-01 | 2017-03-07 | Lg Display Co., Ltd. | Organic light-emitting diode and method of manufacturing the same |
| US9601699B2 (en) | 2013-10-31 | 2017-03-21 | Samsung Display Co., Ltd. | Chrysene-based compound and organic light-emitting device including the same |
| US9680106B2 (en) | 2013-10-01 | 2017-06-13 | Samsung Display Co., Ltd. | Pyrene-based compound and organic light-emitting device including the same |
| US9722187B2 (en) | 2014-10-22 | 2017-08-01 | Samsung Display Co., Ltd. | Compound and organic light-emitting device including the same |
| US9825231B2 (en) | 2013-09-06 | 2017-11-21 | Samsung Display Co., Ltd. | Condensed cyclic compound and organic light-emitting device including the same |
| US9831443B2 (en) | 2014-03-19 | 2017-11-28 | Samsung Display Co., Ltd. | Condensed cyclic compound and organic light-emitting device comprising the same |
| US9837614B2 (en) | 2013-09-06 | 2017-12-05 | Samsung Display Co., Ltd. | Condensed-cyclic compound and organic light-emitting device comprising the same |
| US9871208B2 (en) | 2014-02-26 | 2018-01-16 | Samsung Display Co., Ltd. | Condensed cyclic compound and organic light-emitting device including the same |
| US9882143B2 (en) | 2012-02-07 | 2018-01-30 | Samsung Display Co., Ltd. | Amine-based compound and organic light-emitting diode including the same |
| CN108264486A (en) * | 2016-12-30 | 2018-07-10 | 吉林奥来德光电材料股份有限公司 | A kind of novel glyoxaline compound and its preparation and electroluminescent device |
| US10069085B2 (en) | 2016-07-18 | 2018-09-04 | Samsung Display Co., Ltd. | Heterocyclic compound and organic light-emitting device including the same |
| US10069083B2 (en) | 2014-06-02 | 2018-09-04 | Samsung Display Co., Ltd. | Condensed cyclic compounds and organic light-emitting devices including the same |
| US10205102B2 (en) | 2015-06-17 | 2019-02-12 | Samsung Display Co., Ltd. | Material for organic electroluminescent device and organic electroluminescent device including the same |
| US10211407B2 (en) | 2015-03-16 | 2019-02-19 | Samsung Display Co., Ltd. | Condensed cyclic compound and organic light-emitting device including the same |
| US10236452B2 (en) | 2015-08-04 | 2019-03-19 | Samsung Display Co., Ltd. | Compound and organic light-emitting device including the same |
| US10367150B2 (en) | 2015-02-05 | 2019-07-30 | Samsung Display Co., Ltd. | Compound and organic light-emitting device including the same |
| US10461262B2 (en) | 2015-12-22 | 2019-10-29 | Samsung Display Co., Ltd. | Condensed cyclic compound and an organic light-emitting device including the same |
| US10580994B2 (en) | 2015-09-16 | 2020-03-03 | Samsung Display Co., Ltd. | Compound and organic light-emitting device including the same |
| US10693079B2 (en) | 2015-06-17 | 2020-06-23 | Samsung Display Co., Ltd. | Mono amine derivatives and organic electroluminescent device including the same |
| US10693083B2 (en) | 2016-12-09 | 2020-06-23 | Samsung Display Co., Ltd. | Condensed cyclic compound and organic light-emitting device including the same |
| US10998503B2 (en) | 2017-10-27 | 2021-05-04 | Samsung Display Co., Ltd. | Condensed cyclic compound and organic light-emitting device including the same |
| US11251376B2 (en) | 2017-10-31 | 2022-02-15 | Samsung Display Co., Ltd. | Heterocyclic compound and organic light-emitting device including the same |
| US11279693B2 (en) | 2015-11-13 | 2022-03-22 | Sfc Co., Ltd. | Organic heterocyclic compound and light-emitting diode comprising same |
| US11563183B2 (en) | 2017-06-21 | 2023-01-24 | Samsung Display Co., Ltd. | Heterocyclic compound and organic light-emitting device including the same |
| US11581487B2 (en) | 2017-04-26 | 2023-02-14 | Oti Lumionics Inc. | Patterned conductive coating for surface of an opto-electronic device |
| US11588111B2 (en) | 2017-08-04 | 2023-02-21 | Samsung Display Co., Ltd. | Condensed-cyclic compound and organic light-emitting device including the same |
| US11730012B2 (en) | 2019-03-07 | 2023-08-15 | Oti Lumionics Inc. | Materials for forming a nucleation-inhibiting coating and devices incorporating same |
| US11751415B2 (en) | 2018-02-02 | 2023-09-05 | Oti Lumionics Inc. | Materials for forming a nucleation-inhibiting coating and devices incorporating same |
| KR20240034107A (en) | 2022-09-05 | 2024-03-13 | 롬엔드하스전자재료코리아유한회사 | Organic Electroluminescent Device |
| US11985841B2 (en) | 2021-12-07 | 2024-05-14 | Oti Lumionics Inc. | Patterning a conductive deposited layer using a nucleation inhibiting coating and an underlying metallic coating |
Families Citing this family (854)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100377321B1 (en) | 1999-12-31 | 2003-03-26 | 주식회사 엘지화학 | Electronic device comprising organic compound having p-type semiconducting characteristics |
| US7012364B2 (en) * | 2002-10-01 | 2006-03-14 | Dai Nippon Printing Co., Ltd. | Organic electroluminescent display |
| JP4045932B2 (en) * | 2002-11-21 | 2008-02-13 | コニカミノルタホールディングス株式会社 | Organic electroluminescence element and display device |
| KR100998838B1 (en) * | 2003-03-13 | 2010-12-06 | 이데미쓰 고산 가부시키가이샤 | Nitrogen-containing heterocycle derivative and organic electroluminescent element using the same |
| JP4481930B2 (en) * | 2003-07-11 | 2010-06-16 | 出光興産株式会社 | White organic electroluminescence device |
| US20050136289A1 (en) * | 2003-12-22 | 2005-06-23 | Chu Hye Y. | White organic light emitting device |
| US8048536B2 (en) * | 2003-12-24 | 2011-11-01 | Mitsubishi Heavy Industries Ltd. | Single-layer organic EL device |
| US7252893B2 (en) | 2004-02-17 | 2007-08-07 | Eastman Kodak Company | Anthracene derivative host having ranges of dopants |
| JP2011249349A (en) * | 2004-02-18 | 2011-12-08 | Sony Corp | Display element |
| US7326371B2 (en) | 2004-03-25 | 2008-02-05 | Eastman Kodak Company | Electroluminescent device with anthracene derivative host |
| KR20120101558A (en) * | 2004-04-07 | 2012-09-13 | 이데미쓰 고산 가부시키가이샤 | Nitrogenous heterocycle derivative and organic electroluminescent element employing the same |
| KR101192512B1 (en) | 2004-04-07 | 2012-10-17 | 이데미쓰 고산 가부시키가이샤 | Nitrogenous heterocycle derivative and organic electroluminescent element employing the same |
| WO2005109542A1 (en) | 2004-05-11 | 2005-11-17 | Lg Chem. Ltd. | Organic electronic device |
| KR100669718B1 (en) | 2004-07-29 | 2007-01-16 | 삼성에스디아이 주식회사 | Organic electroluminescence device |
| JP4829486B2 (en) * | 2004-08-04 | 2011-12-07 | Jnc株式会社 | Organic electroluminescence device |
| EP1794255B1 (en) * | 2004-08-19 | 2016-11-16 | LG Chem, Ltd. | Organic light-emitting device comprising buffer layer and method for fabricating the same |
| US7427301B2 (en) * | 2004-09-13 | 2008-09-23 | L'ORéAL S.A. | Composition comprising at least one substituted carbocyanin derivative, process for treating keratin fibers using it, device therefor and use thereof |
| KR100637177B1 (en) * | 2004-10-11 | 2006-10-23 | 삼성에스디아이 주식회사 | Organic electroluminescent device |
| US8026510B2 (en) * | 2004-10-20 | 2011-09-27 | Dai Nippon Printing Co., Ltd. | Organic electronic device and method for producing the same |
| JP4790260B2 (en) * | 2004-12-22 | 2011-10-12 | 出光興産株式会社 | Organic electroluminescence device using anthracene derivative |
| US7517595B2 (en) | 2005-03-10 | 2009-04-14 | Eastman Kodak Company | Electroluminescent devices with mixed electron transport materials |
| US20060204783A1 (en) * | 2005-03-10 | 2006-09-14 | Conley Scott R | Organic electroluminescent device |
| US9070884B2 (en) | 2005-04-13 | 2015-06-30 | Universal Display Corporation | Hybrid OLED having phosphorescent and fluorescent emitters |
| US20060240281A1 (en) * | 2005-04-21 | 2006-10-26 | Eastman Kodak Company | Contaminant-scavenging layer on OLED anodes |
| US8007927B2 (en) | 2007-12-28 | 2011-08-30 | Universal Display Corporation | Dibenzothiophene-containing materials in phosphorescent light emitting diodes |
| US8586204B2 (en) | 2007-12-28 | 2013-11-19 | Universal Display Corporation | Phosphorescent emitters and host materials with improved stability |
| US20060269782A1 (en) * | 2005-05-25 | 2006-11-30 | Eastman Kodak Company | OLED electron-transporting layer |
| TWI321968B (en) | 2005-07-15 | 2010-03-11 | Lg Chemical Ltd | Organic light meitting device and method for manufacturing the same |
| US8766023B2 (en) * | 2005-07-20 | 2014-07-01 | Lg Display Co., Ltd. | Synthesis process |
| US8187727B2 (en) * | 2005-07-22 | 2012-05-29 | Lg Chem, Ltd. | Imidazole derivatives, preparation method thereof and organic electronic device using the same |
| JP4785509B2 (en) * | 2005-11-30 | 2011-10-05 | 三洋電機株式会社 | Organic electroluminescent device and organic electroluminescent display device |
| US7385095B2 (en) * | 2005-10-21 | 2008-06-10 | Lg Chem, Ltd. | Indene derivatives and organic light emitting diode using the same |
| KR100890862B1 (en) * | 2005-11-07 | 2009-03-27 | 주식회사 엘지화학 | Organic electroluminescent device and method for preparing the same |
| DE502005009802D1 (en) * | 2005-11-10 | 2010-08-05 | Novaled Ag | Doped organic semiconductor material |
| US9666826B2 (en) * | 2005-11-30 | 2017-05-30 | Global Oled Technology Llc | Electroluminescent device including an anthracene derivative |
| KR100830332B1 (en) * | 2005-11-30 | 2008-05-19 | 삼성에스디아이 주식회사 | Organic light-emitting device |
| US7919010B2 (en) * | 2005-12-22 | 2011-04-05 | Novaled Ag | Doped organic semiconductor material |
| KR101252083B1 (en) | 2005-12-22 | 2013-04-12 | 엘지디스플레이 주식회사 | Organic electro-luminescence display device and fabricating method thereof |
| KR100845694B1 (en) | 2006-01-18 | 2008-07-11 | 주식회사 엘지화학 | Oled having stacked organic light-emitting units |
| KR102103062B1 (en) | 2006-02-10 | 2020-04-22 | 유니버셜 디스플레이 코포레이션 | METAL COMPLEXES OF CYCLOMETALLATED IMIDAZO[1,2-f]PHENANTHRIDINE AND DIIMIDAZO[1,2-A:1',2'-C]QUINAZOLINE LIGANDS AND ISOELECTRONIC AND BENZANNULATED ANALOGS THEREOF |
| KR20080103056A (en) * | 2006-02-23 | 2008-11-26 | 이데미쓰 고산 가부시키가이샤 | Material for organic electroluminescent device, method for producing same and organic electroluminescent device |
| US20070196688A1 (en) * | 2006-02-23 | 2007-08-23 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device |
| CN101395107A (en) * | 2006-02-28 | 2009-03-25 | 出光兴产株式会社 | Naphthacene derivative and organic electroluminescent element using same |
| TWI348463B (en) * | 2006-03-06 | 2011-09-11 | Lg Chemical Ltd | Novel anthracene derivative and organic electronic device using the same |
| EP1837927A1 (en) * | 2006-03-22 | 2007-09-26 | Novaled AG | Use of heterocyclic radicals for doping of organic semiconductors |
| ES2310380T3 (en) | 2006-03-21 | 2009-01-01 | Novaled Ag | RADICAL OR DIRRADICAL HETEROCICLIC, ITS DIMEROS, OLIGOMEROS, POLYMERS, DIESPIRO COMPOUNDS AND POLICICLOS, ITS USE, ORGANIC SEMICONDUCTOR MATERIAL AS WELL AS ELECTRONIC COMPONENT. |
| DE102006013802A1 (en) * | 2006-03-24 | 2007-09-27 | Merck Patent Gmbh | New anthracene compounds useful in organic electronic devices, preferably organic electroluminescent device e.g. integrated organic electroluminescent devices and organic field-effect-transistors |
| US20070252517A1 (en) * | 2006-04-27 | 2007-11-01 | Eastman Kodak Company | Electroluminescent device including an anthracene derivative |
| WO2007127069A1 (en) * | 2006-04-27 | 2007-11-08 | Eastman Kodak Company | Electroluminescent device including an anthracene derivative |
| US7733009B2 (en) | 2006-04-27 | 2010-06-08 | Global Oled Technology Llc | Electroluminescent device including an anthracene derivative |
| EP2018090A4 (en) * | 2006-05-11 | 2010-12-01 | Idemitsu Kosan Co | Organic electroluminescent device |
| GB2441354B (en) * | 2006-08-31 | 2009-07-29 | Cambridge Display Tech Ltd | Display drive systems |
| KR101064260B1 (en) * | 2006-11-09 | 2011-09-14 | 주식회사 엘지화학 | Magnetic Toner Composition Having Superior Uniform Electrification |
| KR100989467B1 (en) * | 2006-12-06 | 2010-10-22 | 주식회사 엘지화학 | New fluorene derivatives and organic electronic device using the same |
| US8778508B2 (en) | 2006-12-08 | 2014-07-15 | Universal Display Corporation | Light-emitting organometallic complexes |
| TWI481089B (en) | 2006-12-28 | 2015-04-11 | Universal Display Corp | Long lifetime phosphorescent organic light emitting device (oled) structures |
| TWI437075B (en) * | 2006-12-28 | 2014-05-11 | Semiconductor Energy Lab | Anthracene derivatives and light-emitting devices using the anthracene derivatives |
| WO2008102740A1 (en) * | 2007-02-19 | 2008-08-28 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent device |
| TWI373987B (en) * | 2007-03-07 | 2012-10-01 | Sony Corp | Organic electroluminescent device and display device |
| US20130032785A1 (en) | 2011-08-01 | 2013-02-07 | Universal Display Corporation | Materials for organic light emitting diode |
| US9130177B2 (en) | 2011-01-13 | 2015-09-08 | Universal Display Corporation | 5-substituted 2 phenylquinoline complexes materials for light emitting diode |
| EP2489716B1 (en) | 2007-03-08 | 2017-12-27 | Universal Display Corporation | Phosphorescent materials |
| US20080241588A1 (en) * | 2007-03-09 | 2008-10-02 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device and display |
| DE102007012794B3 (en) * | 2007-03-16 | 2008-06-19 | Novaled Ag | New pyrido(3,2-h)quinazoline compounds useful to prepare doped organic semi-conductor, which is useful in an organic light-emitting diode, preferably organic solar cells, and modules for an electronic circuits, preferably displays |
| JP2008244012A (en) * | 2007-03-26 | 2008-10-09 | Fujifilm Corp | Organic electroluminescent element |
| KR100857026B1 (en) * | 2007-03-28 | 2008-09-05 | (주)그라쎌 | Thiazole system organic electroluminescent compounds and organic light emitting diode using the same |
| DE102007018456B4 (en) * | 2007-04-19 | 2022-02-24 | Novaled Gmbh | Use of main group element halides and/or pseudohalides, organic semiconducting matrix material, electronic and optoelectronic components |
| EP1988587B1 (en) | 2007-04-30 | 2016-12-07 | Novaled GmbH | Oxocarbon, pseudo oxocarbon and radialene compounds and their use |
| US20080278072A1 (en) * | 2007-04-30 | 2008-11-13 | Lg Chem, Ltd. | Organic light emitting device and method of producing the same |
| EP1990847B1 (en) * | 2007-05-10 | 2018-06-20 | Novaled GmbH | Use of quinoid bisimidazoles and their derivatives as dopant for doping an organic semi-conductor matrix material |
| DE102007031220B4 (en) | 2007-07-04 | 2022-04-28 | Novaled Gmbh | Quinoid compounds and their use in semiconducting matrix materials, electronic and optoelectronic components |
| KR100910134B1 (en) * | 2007-07-13 | 2009-08-03 | (주)그라쎌 | Organic luminescent material and organic light emitting device using the same |
| WO2009016964A1 (en) * | 2007-07-27 | 2009-02-05 | Toray Industries, Inc. | Light-emitting device material and light-emitting device |
| KR20160104752A (en) | 2007-08-08 | 2016-09-05 | 유니버셜 디스플레이 코포레이션 | Single triphenylene chromophores in phosphorescent light emitting diodes |
| JP6009144B2 (en) | 2007-08-08 | 2016-10-19 | ユニバーサル ディスプレイ コーポレイション | Benzo-fused thiophene or benzo-fused furan compounds containing a triphenylene group |
| TW200932872A (en) * | 2007-10-17 | 2009-08-01 | Cheil Ind Inc | Novel compound for organic photoelectric device and organic photoelectric device including the same |
| EP2107062A1 (en) | 2008-04-03 | 2009-10-07 | SOLVAY (Société Anonyme) | Naphthyl-substituted anthracene derivatives and their use in organic light-emitting diodes |
| US8221905B2 (en) | 2007-12-28 | 2012-07-17 | Universal Display Corporation | Carbazole-containing materials in phosphorescent light emitting diodes |
| KR101317531B1 (en) * | 2008-01-16 | 2013-10-15 | 주식회사 엘지화학 | New imidazole derivatives and organic electronic device using the same |
| KR101003267B1 (en) | 2008-01-18 | 2010-12-21 | 주식회사 엘지화학 | Organic Light Emitting Device and Method for Manufacturing the Same |
| KR20090082777A (en) * | 2008-01-28 | 2009-07-31 | 삼성모바일디스플레이주식회사 | Organic light emitting diode and manufacturing thereof |
| KR20090082778A (en) | 2008-01-28 | 2009-07-31 | 삼성모바일디스플레이주식회사 | Organic light emitting diode and manufacturing thereof |
| US8040053B2 (en) | 2008-02-09 | 2011-10-18 | Universal Display Corporation | Organic light emitting device architecture for reducing the number of organic materials |
| US8057712B2 (en) * | 2008-04-29 | 2011-11-15 | Novaled Ag | Radialene compounds and their use |
| EP2299786B1 (en) | 2008-05-16 | 2014-03-26 | LG Chem, Ltd. | Stacked organic light-emitting diode |
| KR100924144B1 (en) | 2008-06-05 | 2009-10-28 | 삼성모바일디스플레이주식회사 | OLED and Method for fabricating the Same |
| US8440326B2 (en) | 2008-06-30 | 2013-05-14 | Universal Display Corporation | Hole transport materials containing triphenylene |
| KR100882199B1 (en) * | 2008-07-23 | 2009-02-10 | (주)그라쎌 | Thiazole system Organic Electroluminescent Compounds and Organic Light Emitting Diode using the same |
| KR100857027B1 (en) * | 2008-07-23 | 2008-09-05 | (주)그라쎌 | Thiazole system organic electroluminescent compounds and organic light emitting diode using the same |
| US20110156016A1 (en) * | 2008-07-28 | 2011-06-30 | Masahiro Kawamura | Organic light-emitting medium and organic el element |
| WO2010013675A1 (en) * | 2008-07-28 | 2010-02-04 | 出光興産株式会社 | Organic light-emitting medium and organic el element |
| TW201012293A (en) * | 2008-09-03 | 2010-03-16 | Jwo-Huei Jou | Organic light-emitting diode and method of fabricating the same |
| WO2010027583A1 (en) | 2008-09-03 | 2010-03-11 | Universal Display Corporation | Phosphorescent materials |
| TW201012294A (en) * | 2008-09-03 | 2010-03-16 | Jwo-Huei Jou | Organic light-emitting diode and method of fabricating the same |
| US9034483B2 (en) | 2008-09-16 | 2015-05-19 | Universal Display Corporation | Phosphorescent materials |
| JP5676454B2 (en) | 2008-09-25 | 2015-02-25 | ユニバーサル ディスプレイ コーポレイション | Organic selenium materials and their use in organic light emitting devices |
| US8053770B2 (en) | 2008-10-14 | 2011-11-08 | Universal Display Corporation | Emissive layer patterning for OLED |
| EP2570412B1 (en) | 2008-11-03 | 2017-01-04 | LG Chem, Ltd. | Novel nitrogen-containing heterocyclic compound and organic electronic device using the same |
| JP5854839B2 (en) | 2008-11-11 | 2016-02-09 | ユニバーサル ディスプレイ コーポレイション | Phosphorescent emitter |
| JP5629970B2 (en) * | 2008-11-14 | 2014-11-26 | コニカミノルタ株式会社 | Organic electroluminescence element, display device and lighting device |
| US8778511B2 (en) | 2008-12-12 | 2014-07-15 | Universal Display Corporation | OLED stability via doped hole transport layer |
| US8815415B2 (en) | 2008-12-12 | 2014-08-26 | Universal Display Corporation | Blue emitter with high efficiency based on imidazo[1,2-f] phenanthridine iridium complexes |
| DE102008064200A1 (en) | 2008-12-22 | 2010-07-01 | Merck Patent Gmbh | Organic electroluminescent device |
| CN102272262B (en) | 2008-12-30 | 2014-09-10 | 第一毛织株式会社 | Novel compounds for an organic photoelectric device, and organic photoelectric device comprising same |
| US9067947B2 (en) | 2009-01-16 | 2015-06-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| DE102009005746A1 (en) | 2009-01-23 | 2010-07-29 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102009009277B4 (en) | 2009-02-17 | 2023-12-07 | Merck Patent Gmbh | Organic electronic device, process for its production and use of compounds |
| DE102009012346B4 (en) | 2009-03-09 | 2024-02-15 | Merck Patent Gmbh | Organic electroluminescent device and method for producing the same |
| US8709615B2 (en) | 2011-07-28 | 2014-04-29 | Universal Display Corporation | Heteroleptic iridium complexes as dopants |
| DE102009014513A1 (en) | 2009-03-23 | 2010-09-30 | Merck Patent Gmbh | Organic electroluminescent device |
| US8722205B2 (en) | 2009-03-23 | 2014-05-13 | Universal Display Corporation | Heteroleptic iridium complex |
| US11910700B2 (en) | 2009-03-23 | 2024-02-20 | Universal Display Corporation | Heteroleptic iridium complexes as dopants |
| JP5347662B2 (en) * | 2009-04-03 | 2013-11-20 | ソニー株式会社 | Organic electroluminescence device and display device |
| CN105820192B (en) | 2009-04-06 | 2020-04-07 | 通用显示公司 | Metal complexes comprising novel ligand structures |
| DE102009017064A1 (en) | 2009-04-09 | 2010-10-14 | Merck Patent Gmbh | Organic electroluminescent device |
| TWI638807B (en) | 2009-04-28 | 2018-10-21 | 環球展覽公司 | Iridium complex with methyl-d3 substitution |
| TWI541234B (en) | 2009-05-12 | 2016-07-11 | 環球展覽公司 | 2-azatriphenylene materials for organic light emitting diodes |
| US8586203B2 (en) | 2009-05-20 | 2013-11-19 | Universal Display Corporation | Metal complexes with boron-nitrogen heterocycle containing ligands |
| US9153790B2 (en) | 2009-05-22 | 2015-10-06 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent device |
| WO2010149259A2 (en) | 2009-06-22 | 2010-12-29 | Merck Patent Gmbh | Conducting formulation |
| WO2011015265A2 (en) | 2009-08-04 | 2011-02-10 | Merck Patent Gmbh | Electronic devices comprising multi cyclic hydrocarbons |
| DE102009042680A1 (en) | 2009-09-23 | 2011-03-24 | Merck Patent Gmbh | Organic electroluminescent device |
| US8545996B2 (en) | 2009-11-02 | 2013-10-01 | The University Of Southern California | Ion-pairing soft salts based on organometallic complexes and their applications in organic light emitting diodes |
| US8748014B2 (en) | 2009-11-12 | 2014-06-10 | Hodogaya Chemical Co., Ltd. | Compound having a substituted anthracene ring structure and pyridoindole ring structure, and organic electroluminescent device |
| KR101231931B1 (en) | 2009-11-13 | 2013-02-08 | 주식회사 엘지화학 | New fused cyclic compound and organic electronic device |
| US8580394B2 (en) | 2009-11-19 | 2013-11-12 | Universal Display Corporation | 3-coordinate copper(I)-carbene complexes |
| JP5968786B2 (en) | 2009-12-22 | 2016-08-10 | メルク パテント ゲーエムベーハー | Electroluminescence formulation |
| WO2011076326A1 (en) | 2009-12-22 | 2011-06-30 | Merck Patent Gmbh | Electroluminescent functional surfactants |
| JP5836970B2 (en) | 2009-12-22 | 2015-12-24 | メルク パテント ゲーエムベーハー | Formulations containing functional materials |
| WO2011076324A1 (en) | 2009-12-23 | 2011-06-30 | Merck Patent Gmbh | Compositions comprising organic semiconducting compounds |
| CN102668152A (en) | 2009-12-23 | 2012-09-12 | 默克专利有限公司 | Compositions comprising polymeric binders |
| US8288187B2 (en) | 2010-01-20 | 2012-10-16 | Universal Display Corporation | Electroluminescent devices for lighting applications |
| DE102010006121B4 (en) | 2010-01-29 | 2022-08-11 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US9156870B2 (en) | 2010-02-25 | 2015-10-13 | Universal Display Corporation | Phosphorescent emitters |
| US9175211B2 (en) | 2010-03-03 | 2015-11-03 | Universal Display Corporation | Phosphorescent materials |
| DE102010010481A1 (en) | 2010-03-06 | 2011-09-08 | Merck Patent Gmbh | Organic electroluminescent device |
| JP6246468B2 (en) | 2010-03-11 | 2017-12-13 | メルク パテント ゲーエムベーハー | Fiber in therapy and cosmetics |
| EP2545600A2 (en) | 2010-03-11 | 2013-01-16 | Merck Patent GmbH | Radiative fibers |
| WO2011119162A1 (en) | 2010-03-25 | 2011-09-29 | Universal Display Corporation | Solution processable doped triarylamine hole injection materials |
| KR101182446B1 (en) * | 2010-04-02 | 2012-09-12 | 삼성디스플레이 주식회사 | Organic light emitting device |
| EP2559078A1 (en) | 2010-04-12 | 2013-02-20 | Merck Patent GmbH | Composition having improved performance |
| JP2011222831A (en) | 2010-04-12 | 2011-11-04 | Idemitsu Kosan Co Ltd | Organic electroluminescent element |
| EP2559079B1 (en) | 2010-04-12 | 2020-04-01 | Merck Patent GmbH | Composition and method for preparation of organic electronic devices |
| US8968887B2 (en) | 2010-04-28 | 2015-03-03 | Universal Display Corporation | Triphenylene-benzofuran/benzothiophene/benzoselenophene compounds with substituents joining to form fused rings |
| US9040962B2 (en) | 2010-04-28 | 2015-05-26 | Universal Display Corporation | Depositing premixed materials |
| DE102010020044A1 (en) | 2010-05-11 | 2011-11-17 | Merck Patent Gmbh | Organic electroluminescent device |
| RU2012156386A (en) | 2010-05-27 | 2014-07-10 | Мерк Патент Гмбх | COMPOSITION AND METHOD FOR PRODUCING ORGANIC ELECTRONIC DEVICES |
| EP2576723B1 (en) | 2010-05-27 | 2017-09-20 | Merck Patent GmbH | Compositions comprising quantum dots |
| US8742657B2 (en) | 2010-06-11 | 2014-06-03 | Universal Display Corporation | Triplet-Triplet annihilation up conversion (TTA-UC) for display and lighting applications |
| US8673458B2 (en) | 2010-06-11 | 2014-03-18 | Universal Display Corporation | Delayed fluorescence OLED |
| JP4680322B1 (en) * | 2010-07-09 | 2011-05-11 | 富士フイルム株式会社 | Organic electroluminescence device |
| JP4729643B1 (en) * | 2010-07-09 | 2011-07-20 | 富士フイルム株式会社 | Organic electroluminescence device |
| JP2013539584A (en) | 2010-07-26 | 2013-10-24 | メルク パテント ゲーエムベーハー | Quantum dots and hosts |
| CN106887522B (en) | 2010-07-26 | 2018-09-18 | 默克专利有限公司 | Include the device of nanocrystal |
| US9435021B2 (en) | 2010-07-29 | 2016-09-06 | University Of Southern California | Co-deposition methods for the fabrication of organic optoelectronic devices |
| US9512137B2 (en) | 2010-08-05 | 2016-12-06 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence device |
| US9954180B2 (en) | 2010-08-20 | 2018-04-24 | Universal Display Corporation | Bicarbazole compounds for OLEDs |
| US20120049168A1 (en) | 2010-08-31 | 2012-03-01 | Universal Display Corporation | Cross-Linked Charge Transport Layer Containing an Additive Compound |
| US8932734B2 (en) | 2010-10-08 | 2015-01-13 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US8269317B2 (en) | 2010-11-11 | 2012-09-18 | Universal Display Corporation | Phosphorescent materials |
| US20120138906A1 (en) | 2010-12-07 | 2012-06-07 | The University of Southern California USC Stevens Institute for Innovation | Capture agents for unsaturated metal complexes |
| US9349964B2 (en) * | 2010-12-24 | 2016-05-24 | Lg Chem, Ltd. | Organic light emitting diode and manufacturing method thereof |
| US10008677B2 (en) | 2011-01-13 | 2018-06-26 | Universal Display Corporation | Materials for organic light emitting diode |
| CN103328475A (en) | 2011-01-18 | 2013-09-25 | 保土谷化学工业株式会社 | Compound having pyridoindole ring structure and substituted bipyridyl group, and organic electroluminescent element |
| US8415031B2 (en) | 2011-01-24 | 2013-04-09 | Universal Display Corporation | Electron transporting compounds |
| JP6351974B2 (en) | 2011-02-14 | 2018-07-04 | メルク パテント ゲーエムベーハー | Devices and methods for treatment of cells and cellular tissues |
| US8748011B2 (en) | 2011-02-23 | 2014-06-10 | Universal Display Corporation | Ruthenium carbene complexes for OLED material |
| US8563737B2 (en) | 2011-02-23 | 2013-10-22 | Universal Display Corporation | Methods of making bis-tridentate carbene complexes of ruthenium and osmium |
| KR101975067B1 (en) | 2011-02-23 | 2019-05-03 | 호도가야 가가쿠 고교 가부시키가이샤 | Compound containing substituted triphenyle ring structure, and organic electroluminescent element |
| CN103476781B (en) | 2011-02-23 | 2017-02-15 | 通用显示公司 | Novel tetradentate platinum complexes |
| US9005772B2 (en) | 2011-02-23 | 2015-04-14 | Universal Display Corporation | Thioazole and oxazole carbene metal complexes as phosphorescent OLED materials |
| US8492006B2 (en) | 2011-02-24 | 2013-07-23 | Universal Display Corporation | Germanium-containing red emitter materials for organic light emitting diode |
| US8883322B2 (en) | 2011-03-08 | 2014-11-11 | Universal Display Corporation | Pyridyl carbene phosphorescent emitters |
| EP2688120B1 (en) | 2011-03-14 | 2017-08-23 | Toray Industries, Inc. | Light-emitting element material and light-emitting element |
| JP6356060B2 (en) | 2011-03-24 | 2018-07-11 | メルク パテント ゲーエムベーハー | Organic ionic functional materials |
| US8580399B2 (en) | 2011-04-08 | 2013-11-12 | Universal Display Corporation | Substituted oligoazacarbazoles for light emitting diodes |
| US8927308B2 (en) | 2011-05-12 | 2015-01-06 | Universal Display Corporation | Method of forming bus line designs for large-area OLED lighting |
| US8432095B2 (en) | 2011-05-11 | 2013-04-30 | Universal Display Corporation | Process for fabricating metal bus lines for OLED lighting panels |
| US9391288B2 (en) | 2011-05-12 | 2016-07-12 | Toray Industries, Inc. | Light emitting device material and light emitting device |
| EP2707911B1 (en) | 2011-05-12 | 2017-07-05 | Merck Patent GmbH | Compositions and electronic devices |
| US9212197B2 (en) | 2011-05-19 | 2015-12-15 | Universal Display Corporation | Phosphorescent heteroleptic phenylbenzimidazole dopants |
| US8795850B2 (en) | 2011-05-19 | 2014-08-05 | Universal Display Corporation | Phosphorescent heteroleptic phenylbenzimidazole dopants and new synthetic methodology |
| CN102795983B (en) * | 2011-05-25 | 2016-01-20 | 海洋王照明科技股份有限公司 | Anthraquinone derivative material and its preparation method and application |
| US8748012B2 (en) | 2011-05-25 | 2014-06-10 | Universal Display Corporation | Host materials for OLED |
| US10158089B2 (en) | 2011-05-27 | 2018-12-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10079349B2 (en) | 2011-05-27 | 2018-09-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP2715821A1 (en) | 2011-06-01 | 2014-04-09 | Merck Patent GmbH | Hybrid ambipolar tfts |
| CN103596967B (en) | 2011-06-08 | 2016-12-21 | 环球展览公司 | Heteroleptic iridium carbene complex and use its light-emitting device |
| JP5858653B2 (en) * | 2011-06-08 | 2016-02-10 | キヤノン株式会社 | 6,12-Dinaphthylchrysene derivative and organic light-emitting device using the same |
| US8884316B2 (en) | 2011-06-17 | 2014-11-11 | Universal Display Corporation | Non-common capping layer on an organic device |
| US8659036B2 (en) | 2011-06-17 | 2014-02-25 | Universal Display Corporation | Fine tuning of emission spectra by combination of multiple emitter spectra |
| US9023420B2 (en) | 2011-07-14 | 2015-05-05 | Universal Display Corporation | Composite organic/inorganic layer for organic light-emitting devices |
| WO2013009708A1 (en) | 2011-07-14 | 2013-01-17 | Universal Display Corporation | Inorganic hosts in oleds |
| US9397310B2 (en) | 2011-07-14 | 2016-07-19 | Universal Display Corporation | Organice electroluminescent materials and devices |
| EP2737553A1 (en) | 2011-07-25 | 2014-06-04 | Merck Patent GmbH | Copolymers with functionalized side chains |
| US9783564B2 (en) | 2011-07-25 | 2017-10-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US8409729B2 (en) | 2011-07-28 | 2013-04-02 | Universal Display Corporation | Host materials for phosphorescent OLEDs |
| US8926119B2 (en) | 2011-08-04 | 2015-01-06 | Universal Display Corporation | Extendable light source with variable light emitting area |
| TWI465441B (en) | 2011-08-12 | 2014-12-21 | Ind Tech Res Inst | Imidazole derivatives having vinyl group and its use in electroluminescent element |
| DE102012016192A1 (en) | 2011-08-19 | 2013-02-21 | Merck Patent Gmbh | New compounds capable of forming hydrogen bonds are useful in electronic device, e.g. organic electroluminescent device, organic light-emitting transistor and organic light-emitting electrochemical cell |
| US9493698B2 (en) | 2011-08-31 | 2016-11-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP2013093541A (en) | 2011-10-06 | 2013-05-16 | Udc Ireland Ltd | Organic electroluminescent element and compound and material for organic electroluminescent element usable therefor, and luminescent device, display device and lighting device using the element |
| KR101992506B1 (en) | 2011-10-06 | 2019-06-24 | 메르크 파텐트 게엠베하 | Organic electroluminescent device |
| JP2013084732A (en) | 2011-10-07 | 2013-05-09 | Udc Ireland Ltd | Organic field light-emitting element and light-emitting material for the same, and light-emitting device, display device and illuminating device |
| KR102083691B1 (en) | 2011-10-14 | 2020-03-02 | 호도가야 가가쿠 고교 가부시키가이샤 | New benzotriazole derivative and organic electroluminescent element in which said derivative is used |
| JP2013118349A (en) | 2011-11-02 | 2013-06-13 | Udc Ireland Ltd | Organic electroluminescent element, material for organic electroluminescent element, and light emitting device, display device and illumination device which employ said organic electroluminescent element |
| US8652656B2 (en) | 2011-11-14 | 2014-02-18 | Universal Display Corporation | Triphenylene silane hosts |
| US9193745B2 (en) | 2011-11-15 | 2015-11-24 | Universal Display Corporation | Heteroleptic iridium complex |
| JP2013103918A (en) | 2011-11-15 | 2013-05-30 | Udc Ireland Ltd | Charge-transporting material, organic electroluminescent element, and light-emitting device, display device and illumination device characterized by using the element |
| US9217004B2 (en) | 2011-11-21 | 2015-12-22 | Universal Display Corporation | Organic light emitting materials |
| CN104247070B (en) | 2011-11-30 | 2017-04-12 | 诺瓦尔德股份有限公司 | Display |
| US9512355B2 (en) | 2011-12-09 | 2016-12-06 | Universal Display Corporation | Organic light emitting materials |
| US20130146875A1 (en) | 2011-12-13 | 2013-06-13 | Universal Display Corporation | Split electrode for organic devices |
| US8987451B2 (en) | 2012-01-03 | 2015-03-24 | Universal Display Corporation | Synthesis of cyclometallated platinum(II) complexes |
| US9461254B2 (en) | 2012-01-03 | 2016-10-04 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9163174B2 (en) | 2012-01-04 | 2015-10-20 | Universal Display Corporation | Highly efficient phosphorescent materials |
| KR102012047B1 (en) | 2012-01-06 | 2019-08-19 | 유니버셜 디스플레이 코포레이션 | Highly efficient phosphorescent materials |
| US8969592B2 (en) | 2012-01-10 | 2015-03-03 | Universal Display Corporation | Heterocyclic host materials |
| US10211413B2 (en) | 2012-01-17 | 2019-02-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP5978843B2 (en) | 2012-02-02 | 2016-08-24 | コニカミノルタ株式会社 | Iridium complex compound, organic electroluminescence device material, organic electroluminescence device, lighting device and display device |
| JP6118034B2 (en) | 2012-02-06 | 2017-04-19 | ユー・ディー・シー アイルランド リミテッド | ORGANIC ELECTROLUMINESCENT ELEMENT, COMPOUND USABLE FOR THE SAME, ORGANIC ELECTROLUMINESCENT ELEMENT MATERIAL, AND LIGHT EMITTING DEVICE, DISPLAY DEVICE AND LIGHTING DEVICE USING THE ELEMENT |
| US9118017B2 (en) | 2012-02-27 | 2015-08-25 | Universal Display Corporation | Host compounds for red phosphorescent OLEDs |
| KR101596547B1 (en) * | 2012-02-27 | 2016-02-23 | 주식회사 엘지화학 | Organic light emitting diode |
| US9386657B2 (en) | 2012-03-15 | 2016-07-05 | Universal Display Corporation | Organic Electroluminescent materials and devices |
| US9871201B2 (en) | 2012-03-15 | 2018-01-16 | Merck Patent Gmbh | Electronic devices |
| US9054323B2 (en) | 2012-03-15 | 2015-06-09 | Universal Display Corporation | Secondary hole transporting layer with diarylamino-phenyl-carbazole compounds |
| WO2013149958A1 (en) | 2012-04-02 | 2013-10-10 | Novaled Ag | Use of a semiconducting compound in an organic light emitting device |
| EP2749560B1 (en) * | 2012-04-13 | 2019-10-02 | LG Chem, Ltd. | Novel nitrogen-containing heterocyclic compounds and organic electronic device using same |
| US8723209B2 (en) | 2012-04-27 | 2014-05-13 | Universal Display Corporation | Out coupling layer containing particle polymer composite |
| US9184399B2 (en) | 2012-05-04 | 2015-11-10 | Universal Display Corporation | Asymmetric hosts with triaryl silane side chains |
| US9773985B2 (en) | 2012-05-21 | 2017-09-26 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2013180539A1 (en) | 2012-05-31 | 2013-12-05 | 주식회사 엘지화학 | Organic light emitting diode |
| US9508950B2 (en) | 2012-05-31 | 2016-11-29 | Lg Display Co., Ltd. | Organic light emitting diode |
| KR101640637B1 (en) | 2012-05-31 | 2016-07-18 | 주식회사 엘지화학 | New heterocyclic compounds, production process thereof and organic electronic device using the same |
| CN104321896B (en) | 2012-05-31 | 2017-07-14 | 乐金显示有限公司 | Stack-based organic light emitting diode |
| CN104335378B (en) | 2012-05-31 | 2017-08-29 | 乐金显示有限公司 | Organnic electroluminescent device |
| KR101640285B1 (en) | 2012-05-31 | 2016-07-15 | 주식회사 엘지화학 | New compound and organic light emitting device using the same |
| KR101640636B1 (en) | 2012-05-31 | 2016-07-18 | 주식회사 엘지화학 | New heterocyclic compounds and organic electronic device using the same |
| JP6134786B2 (en) | 2012-05-31 | 2017-05-24 | エルジー ディスプレイ カンパニー リミテッド | Organic electroluminescence device |
| US9670404B2 (en) | 2012-06-06 | 2017-06-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN104350627B (en) | 2012-06-12 | 2016-05-18 | 东丽株式会社 | Light emitting element material and light-emitting component |
| US9502672B2 (en) | 2012-06-21 | 2016-11-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP2871686B1 (en) | 2012-07-05 | 2019-08-21 | Toray Industries, Inc. | Light emitting elment material and light emitting element |
| US9725476B2 (en) | 2012-07-09 | 2017-08-08 | Universal Display Corporation | Silylated metal complexes |
| US9231218B2 (en) | 2012-07-10 | 2016-01-05 | Universal Display Corporation | Phosphorescent emitters containing dibenzo[1,4]azaborinine structure |
| US9059412B2 (en) | 2012-07-19 | 2015-06-16 | Universal Display Corporation | Transition metal complexes containing substituted imidazole carbene as ligands and their application in OLEDs |
| US9540329B2 (en) | 2012-07-19 | 2017-01-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN104488105B (en) | 2012-07-25 | 2017-03-22 | 东丽株式会社 | Light emitting element material and light emitting element |
| US9663544B2 (en) | 2012-07-25 | 2017-05-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9318710B2 (en) | 2012-07-30 | 2016-04-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20150207080A1 (en) | 2012-08-24 | 2015-07-23 | Konica Minolta Inc. | Transparent electrode, electronic device, and method for manufacturing transparent electrode |
| US9978958B2 (en) | 2012-08-24 | 2018-05-22 | Universal Display Corporation | Phosphorescent emitters with phenylimidazole ligands |
| US8952362B2 (en) | 2012-08-31 | 2015-02-10 | The Regents Of The University Of Michigan | High efficiency and brightness fluorescent organic light emitting diode by triplet-triplet fusion |
| US10957870B2 (en) | 2012-09-07 | 2021-03-23 | Universal Display Corporation | Organic light emitting device |
| US9287513B2 (en) | 2012-09-24 | 2016-03-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9312505B2 (en) | 2012-09-25 | 2016-04-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9252363B2 (en) | 2012-10-04 | 2016-02-02 | Universal Display Corporation | Aryloxyalkylcarboxylate solvent compositions for inkjet printing of organic layers |
| CN104768926B (en) | 2012-10-12 | 2017-09-22 | 东丽株式会社 | 1,2 benzo acenaphthene derivative, light emitting element material and light-emitting component containing it |
| KR101963104B1 (en) | 2012-10-31 | 2019-03-28 | 메르크 파텐트 게엠베하 | Electronic device |
| US8692241B1 (en) | 2012-11-08 | 2014-04-08 | Universal Display Corporation | Transition metal complexes containing triazole and tetrazole carbene ligands |
| US9748500B2 (en) | 2015-01-15 | 2017-08-29 | Universal Display Corporation | Organic light emitting materials |
| US8946697B1 (en) | 2012-11-09 | 2015-02-03 | Universal Display Corporation | Iridium complexes with aza-benzo fused ligands |
| US9634264B2 (en) | 2012-11-09 | 2017-04-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9685617B2 (en) | 2012-11-09 | 2017-06-20 | Universal Display Corporation | Organic electronuminescent materials and devices |
| CN104781247B (en) | 2012-11-12 | 2017-08-15 | 默克专利有限公司 | Material for electronic device |
| US10069090B2 (en) | 2012-11-20 | 2018-09-04 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9190623B2 (en) | 2012-11-20 | 2015-11-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9512136B2 (en) | 2012-11-26 | 2016-12-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9166175B2 (en) | 2012-11-27 | 2015-10-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP6363090B2 (en) | 2012-11-30 | 2018-07-25 | メルク パテント ゲーエムベーハー | Electronic element |
| US9209411B2 (en) | 2012-12-07 | 2015-12-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP6367230B2 (en) | 2013-01-03 | 2018-08-01 | メルク パテント ゲーエムベーハー | Electronic element |
| US20150329772A1 (en) | 2013-01-03 | 2015-11-19 | Merck Patent Gmbh | Materials for Electronic Devices |
| WO2014106524A2 (en) | 2013-01-03 | 2014-07-10 | Merck Patent Gmbh | Materials for electronic devices |
| US10400163B2 (en) | 2013-02-08 | 2019-09-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10367154B2 (en) | 2013-02-21 | 2019-07-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN107963972B (en) * | 2013-02-22 | 2021-09-03 | 保土谷化学工业株式会社 | Organic electroluminescent device |
| CN105143198A (en) | 2013-02-26 | 2015-12-09 | 保土谷化学工业株式会社 | Novel naphthotriazole derivative and organic electroluminescence element |
| US8927749B2 (en) | 2013-03-07 | 2015-01-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9419225B2 (en) | 2013-03-14 | 2016-08-16 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20140284580A1 (en) * | 2013-03-22 | 2014-09-25 | E-Ray Optoelectronics Techonology Co., Ltd. | Electron transporting compounds and organic electroluminescent devices using the same |
| US9997712B2 (en) | 2013-03-27 | 2018-06-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2014157610A1 (en) | 2013-03-29 | 2014-10-02 | コニカミノルタ株式会社 | Organic electroluminescent element, lighting device, display device, light-emitting thin film and composition for organic electroluminescent element, and light-emitting method |
| US20160043334A1 (en) | 2013-03-29 | 2016-02-11 | Konica Minolta, Inc. | Material for organic electroluminescent elements, organic electroluminescent element, display device and lighting device |
| JP6350518B2 (en) | 2013-03-29 | 2018-07-04 | コニカミノルタ株式会社 | ORGANIC ELECTROLUMINESCENCE ELEMENT, LIGHTING DEVICE AND DISPLAY DEVICE HAVING THE SAME |
| US9537106B2 (en) | 2013-05-09 | 2017-01-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2014188947A1 (en) | 2013-05-20 | 2014-11-27 | 保土谷化学工業株式会社 | Novel pyrimidine derivative and organic electroluminescent element |
| JP6301729B2 (en) * | 2013-05-21 | 2018-03-28 | 保土谷化学工業株式会社 | Compound having indoloquinoxaline ring structure and organic electroluminescence device |
| US10033007B2 (en) | 2013-06-07 | 2018-07-24 | Lg Display Co., Ltd. | Organic light emitting diode |
| US9735373B2 (en) | 2013-06-10 | 2017-08-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2014199567A1 (en) * | 2013-06-12 | 2014-12-18 | 保土谷化学工業株式会社 | Organic electroluminescence element |
| US9673401B2 (en) | 2013-06-28 | 2017-06-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10199581B2 (en) | 2013-07-01 | 2019-02-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10121975B2 (en) | 2013-07-03 | 2018-11-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20160118591A1 (en) * | 2013-07-12 | 2016-04-28 | Hodogaya Chemical Co., Ltd. | Organic electroluminescent device |
| US9761807B2 (en) | 2013-07-15 | 2017-09-12 | Universal Display Corporation | Organic light emitting diode materials |
| US9553274B2 (en) | 2013-07-16 | 2017-01-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9324949B2 (en) | 2013-07-16 | 2016-04-26 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9224958B2 (en) | 2013-07-19 | 2015-12-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20150028290A1 (en) | 2013-07-25 | 2015-01-29 | Universal Display Corporation | Heteroleptic osmium complex and method of making the same |
| CN105409021B (en) | 2013-07-29 | 2018-07-13 | 默克专利有限公司 | Electroluminescent device |
| JP2016525781A (en) | 2013-07-29 | 2016-08-25 | メルク、パテント、ゲゼルシャフト、ミット、ベシュレンクテル、ハフツングMerck Patent GmbH | Electro-optic element and use thereof |
| CN112079814B (en) | 2013-07-29 | 2023-11-07 | 保土谷化学工业株式会社 | Benzotriazole derivative and organic electroluminescent device |
| US20160164002A1 (en) | 2013-07-30 | 2016-06-09 | Merck Patent Gmbh | Materials for electronic devices |
| EP3027707B1 (en) | 2013-07-30 | 2019-12-11 | Merck Patent GmbH | Materials for electronic devices |
| EP2840622B1 (en) | 2013-08-19 | 2019-02-13 | Novaled GmbH | Electronic or optoelectronic device comprising an anchored thin molecular layer, process for its preparation and compound used therein |
| US9831437B2 (en) | 2013-08-20 | 2017-11-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10074806B2 (en) | 2013-08-20 | 2018-09-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9932359B2 (en) | 2013-08-30 | 2018-04-03 | University Of Southern California | Organic electroluminescent materials and devices |
| US10199582B2 (en) | 2013-09-03 | 2019-02-05 | University Of Southern California | Organic electroluminescent materials and devices |
| US9735378B2 (en) | 2013-09-09 | 2017-08-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP5683763B1 (en) | 2013-09-09 | 2015-03-11 | 保土谷化学工業株式会社 | Benzopyridindole derivatives and organic electroluminescence devices |
| US9748503B2 (en) | 2013-09-13 | 2017-08-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102191996B1 (en) | 2013-09-27 | 2020-12-17 | 삼성디스플레이 주식회사 | Herocyclic compound and organic light emitting device comprising same |
| US10003034B2 (en) | 2013-09-30 | 2018-06-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102191993B1 (en) | 2013-10-02 | 2020-12-17 | 삼성디스플레이 주식회사 | Organic compounds and organic light emitting device comprising the same |
| US9831447B2 (en) | 2013-10-08 | 2017-11-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9293712B2 (en) | 2013-10-11 | 2016-03-22 | Universal Display Corporation | Disubstituted pyrene compounds with amino group containing ortho aryl group and devices containing the same |
| US9853229B2 (en) | 2013-10-23 | 2017-12-26 | University Of Southern California | Organic electroluminescent materials and devices |
| US20150115250A1 (en) | 2013-10-29 | 2015-04-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9306179B2 (en) | 2013-11-08 | 2016-04-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9647218B2 (en) | 2013-11-14 | 2017-05-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9905784B2 (en) | 2013-11-15 | 2018-02-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10056565B2 (en) | 2013-11-20 | 2018-08-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10644251B2 (en) | 2013-12-04 | 2020-05-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3077475B1 (en) | 2013-12-06 | 2018-07-04 | Merck Patent GmbH | Compositions containing a polymeric binder which comprises acrylic and/or methacrylic acid ester units |
| KR102380808B1 (en) | 2013-12-06 | 2022-03-30 | 메르크 파텐트 게엠베하 | Substituted oxepines |
| US9876173B2 (en) | 2013-12-09 | 2018-01-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10355227B2 (en) | 2013-12-16 | 2019-07-16 | Universal Display Corporation | Metal complex for phosphorescent OLED |
| US9847496B2 (en) | 2013-12-23 | 2017-12-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10135008B2 (en) | 2014-01-07 | 2018-11-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9978961B2 (en) | 2014-01-08 | 2018-05-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9755159B2 (en) | 2014-01-23 | 2017-09-05 | Universal Display Corporation | Organic materials for OLEDs |
| US9935277B2 (en) | 2014-01-30 | 2018-04-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9590194B2 (en) | 2014-02-14 | 2017-03-07 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10003033B2 (en) | 2014-02-18 | 2018-06-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9847497B2 (en) | 2014-02-18 | 2017-12-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10707423B2 (en) | 2014-02-21 | 2020-07-07 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9647217B2 (en) | 2014-02-24 | 2017-05-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9502656B2 (en) | 2014-02-24 | 2016-11-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10403825B2 (en) | 2014-02-27 | 2019-09-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9181270B2 (en) | 2014-02-28 | 2015-11-10 | Universal Display Corporation | Method of making sulfide compounds |
| US9673407B2 (en) | 2014-02-28 | 2017-06-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9590195B2 (en) | 2014-02-28 | 2017-03-07 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9190620B2 (en) | 2014-03-01 | 2015-11-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9397309B2 (en) | 2014-03-13 | 2016-07-19 | Universal Display Corporation | Organic electroluminescent devices |
| US10208026B2 (en) | 2014-03-18 | 2019-02-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN104926732B (en) * | 2014-03-21 | 2017-06-13 | 昱镭光电科技股份有限公司 | Compound for organic illuminating element and the organic illuminating element with the compound |
| US9748504B2 (en) | 2014-03-25 | 2017-08-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9929353B2 (en) | 2014-04-02 | 2018-03-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR101537500B1 (en) * | 2014-04-04 | 2015-07-20 | 주식회사 엘지화학 | Organic light emitting diode |
| KR20150115622A (en) | 2014-04-04 | 2015-10-14 | 주식회사 엘지화학 | Hetero-cyclic compound and organic light emitting device comprising the same |
| KR101542714B1 (en) | 2014-04-04 | 2015-08-12 | 주식회사 엘지화학 | Hetero-cyclic compound and organic light emitting device comprising the same |
| US9691993B2 (en) | 2014-04-09 | 2017-06-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9905785B2 (en) | 2014-04-14 | 2018-02-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10008679B2 (en) | 2014-04-14 | 2018-06-26 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9450198B2 (en) | 2014-04-15 | 2016-09-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10256427B2 (en) | 2014-04-15 | 2019-04-09 | Universal Display Corporation | Efficient organic electroluminescent devices |
| US9741941B2 (en) | 2014-04-29 | 2017-08-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN106255687B (en) | 2014-04-30 | 2020-06-05 | 默克专利有限公司 | Material for electronic devices |
| US10457699B2 (en) | 2014-05-02 | 2019-10-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3140871B1 (en) | 2014-05-08 | 2018-12-26 | Universal Display Corporation | Stabilized imidazophenanthridine materials |
| US10636983B2 (en) | 2014-05-08 | 2020-04-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10301338B2 (en) | 2014-05-08 | 2019-05-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10403830B2 (en) | 2014-05-08 | 2019-09-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9997716B2 (en) | 2014-05-27 | 2018-06-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10461260B2 (en) | 2014-06-03 | 2019-10-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3156402B1 (en) | 2014-06-11 | 2021-11-10 | Hodogaya Chemical Co., Ltd. | Pyrimidine derivative and organic electroluminescent element |
| DE102014008722A1 (en) | 2014-06-18 | 2015-12-24 | Merck Patent Gmbh | Compositions for electronic devices |
| JP6648014B2 (en) | 2014-06-26 | 2020-02-14 | 保土谷化学工業株式会社 | Organic electroluminescence device |
| US9911931B2 (en) | 2014-06-26 | 2018-03-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP2963696A1 (en) | 2014-07-04 | 2016-01-06 | Novaled GmbH | Organic light-emitting diode (OLED) including an electron transport layer stack comprising different lithium compounds |
| US10297762B2 (en) | 2014-07-09 | 2019-05-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10566546B2 (en) | 2014-07-14 | 2020-02-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9929357B2 (en) | 2014-07-22 | 2018-03-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN106716666B (en) | 2014-07-25 | 2019-01-08 | 保土谷化学工业株式会社 | Organic electroluminescence device |
| KR102319951B1 (en) | 2014-07-29 | 2021-10-29 | 호도가야 가가쿠 고교 가부시키가이샤 | Organic electroluminescent element |
| US11108000B2 (en) | 2014-08-07 | 2021-08-31 | Unniversal Display Corporation | Organic electroluminescent materials and devices |
| US10411200B2 (en) | 2014-08-07 | 2019-09-10 | Universal Display Corporation | Electroluminescent (2-phenylpyridine)iridium complexes and devices |
| WO2016027687A1 (en) * | 2014-08-20 | 2016-02-25 | 保土谷化学工業株式会社 | Organic electroluminescent element |
| EP2999019B1 (en) | 2014-09-19 | 2019-06-12 | Novaled GmbH | Organic light-emitting diode including an electron transport layer stack comprising different lithium compounds and elemental metal |
| US10135007B2 (en) | 2014-09-29 | 2018-11-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10043987B2 (en) | 2014-09-29 | 2018-08-07 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10749113B2 (en) | 2014-09-29 | 2020-08-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3002796A1 (en) | 2014-10-01 | 2016-04-06 | Novaled GmbH | Organic light-emitting diode including an electron transport layer comprising a three component blend of a matrix compound and two lithium compounds |
| US10361375B2 (en) | 2014-10-06 | 2019-07-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP2016074623A (en) * | 2014-10-06 | 2016-05-12 | Jnc株式会社 | Compound having alkyl-substituted azole, electron transport material including the compound and organic electroluminescent element using the same |
| US10854826B2 (en) | 2014-10-08 | 2020-12-01 | Universal Display Corporation | Organic electroluminescent compounds, compositions and devices |
| US9397302B2 (en) | 2014-10-08 | 2016-07-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10950803B2 (en) | 2014-10-13 | 2021-03-16 | Universal Display Corporation | Compounds and uses in devices |
| US9484541B2 (en) | 2014-10-20 | 2016-11-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP5848480B1 (en) * | 2014-10-28 | 2016-01-27 | 三星ディスプレイ株式會社Samsung Display Co.,Ltd. | Material for organic electroluminescence device and organic electroluminescence device using the same |
| US10868261B2 (en) | 2014-11-10 | 2020-12-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10411201B2 (en) | 2014-11-12 | 2019-09-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10038151B2 (en) | 2014-11-12 | 2018-07-31 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9871212B2 (en) | 2014-11-14 | 2018-01-16 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9882151B2 (en) | 2014-11-14 | 2018-01-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN107112427B (en) | 2014-11-14 | 2019-09-24 | 保土谷化学工业株式会社 | Organic electroluminescence device |
| US9761814B2 (en) | 2014-11-18 | 2017-09-12 | Universal Display Corporation | Organic light-emitting materials and devices |
| US10892420B2 (en) | 2014-11-18 | 2021-01-12 | Hodogaya Chemical Co., Ltd. | Organic electroluminescent device |
| US9444075B2 (en) | 2014-11-26 | 2016-09-13 | Universal Display Corporation | Emissive display with photo-switchable polarization |
| KR102493763B1 (en) | 2014-12-05 | 2023-01-30 | 호도가야 가가쿠 고교 가부시키가이샤 | Organic electroluminescent element |
| US9450195B2 (en) | 2014-12-17 | 2016-09-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3035400B1 (en) | 2014-12-17 | 2019-10-23 | Novaled GmbH | Organic light-emitting diode comprising electron transport layers with different matrix compounds |
| CN107112425B (en) | 2014-12-24 | 2019-05-17 | 保土谷化学工业株式会社 | Organic electroluminescence device |
| US10636978B2 (en) | 2014-12-30 | 2020-04-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10253252B2 (en) | 2014-12-30 | 2019-04-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102490882B1 (en) * | 2014-12-31 | 2023-01-25 | 삼성디스플레이 주식회사 | Organic light-emitting device |
| KR101580429B1 (en) | 2015-01-02 | 2015-12-24 | 주식회사 엘지화학 | Organic light emitting diode |
| US9312499B1 (en) | 2015-01-05 | 2016-04-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10593884B2 (en) | 2015-01-06 | 2020-03-17 | Hodogaya Chemical Co., Ltd. | Organic electroluminescent device |
| US9406892B2 (en) | 2015-01-07 | 2016-08-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102459260B1 (en) * | 2015-01-08 | 2022-10-25 | 호도가야 가가쿠 고교 가부시키가이샤 | Organic electroluminescent element |
| CN107406415B (en) | 2015-01-20 | 2021-02-19 | 保土谷化学工业株式会社 | Pyrimidine derivative and organic electroluminescent device |
| US10418569B2 (en) | 2015-01-25 | 2019-09-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9711730B2 (en) | 2015-01-25 | 2017-07-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP7030519B2 (en) | 2015-01-30 | 2022-03-07 | メルク パテント ゲーエムベーハー | Formulations with low particle content |
| WO2016119992A1 (en) | 2015-01-30 | 2016-08-04 | Merck Patent Gmbh | Materials for electronic devices |
| JP6715781B2 (en) * | 2015-02-03 | 2020-07-01 | 保土谷化学工業株式会社 | Organic electroluminescent device |
| US10644247B2 (en) | 2015-02-06 | 2020-05-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10355222B2 (en) | 2015-02-06 | 2019-07-16 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10418562B2 (en) | 2015-02-06 | 2019-09-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10177316B2 (en) | 2015-02-09 | 2019-01-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP5831654B1 (en) | 2015-02-13 | 2015-12-09 | コニカミノルタ株式会社 | Aromatic heterocycle derivative, organic electroluminescence device using the same, illumination device and display device |
| US10144867B2 (en) | 2015-02-13 | 2018-12-04 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10680183B2 (en) | 2015-02-15 | 2020-06-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9929361B2 (en) | 2015-02-16 | 2018-03-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3093288A1 (en) | 2015-05-12 | 2016-11-16 | Novaled GmbH | Organic light-emitting diode comprising different matrix compounds in the first and second electron transport layer |
| US10600966B2 (en) | 2015-02-27 | 2020-03-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11056657B2 (en) | 2015-02-27 | 2021-07-06 | University Display Corporation | Organic electroluminescent materials and devices |
| US10686143B2 (en) | 2015-03-05 | 2020-06-16 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10270046B2 (en) | 2015-03-06 | 2019-04-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9780316B2 (en) | 2015-03-16 | 2017-10-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9911928B2 (en) | 2015-03-19 | 2018-03-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9871214B2 (en) | 2015-03-23 | 2018-01-16 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10529931B2 (en) | 2015-03-24 | 2020-01-07 | Universal Display Corporation | Organic Electroluminescent materials and devices |
| US10297770B2 (en) | 2015-03-27 | 2019-05-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11818949B2 (en) | 2015-04-06 | 2023-11-14 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11495749B2 (en) | 2015-04-06 | 2022-11-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10693082B2 (en) | 2015-04-06 | 2020-06-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10403826B2 (en) | 2015-05-07 | 2019-09-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10777749B2 (en) | 2015-05-07 | 2020-09-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9478758B1 (en) | 2015-05-08 | 2016-10-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9859510B2 (en) | 2015-05-15 | 2018-01-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10256411B2 (en) | 2015-05-21 | 2019-04-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10109799B2 (en) | 2015-05-21 | 2018-10-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP6433852B2 (en) | 2015-05-28 | 2018-12-05 | 株式会社東芝 | Compound, organic photoelectric conversion device, and solid-state imaging device |
| US10033004B2 (en) | 2015-06-01 | 2018-07-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10418568B2 (en) | 2015-06-01 | 2019-09-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11925102B2 (en) | 2015-06-04 | 2024-03-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3109916B1 (en) | 2015-06-23 | 2021-08-25 | Novaled GmbH | Organic light emitting device comprising polar matrix, metal dopant and silver cathode |
| EP3109919B1 (en) | 2015-06-23 | 2021-06-23 | Novaled GmbH | N-doped semiconducting material comprising polar matrix and metal dopant |
| EP3109915B1 (en) | 2015-06-23 | 2021-07-21 | Novaled GmbH | Organic light emitting device comprising polar matrix and metal dopant |
| US10749115B2 (en) | 2015-06-23 | 2020-08-18 | Novaled Gmbh | N-doped semiconducting material comprising polar matrix and metal dopant |
| US10825997B2 (en) | 2015-06-25 | 2020-11-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10873036B2 (en) | 2015-07-07 | 2020-12-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102002023B1 (en) * | 2015-07-14 | 2019-07-22 | 에스에프씨주식회사 | An organic light emitting diode for high efficiency |
| US9978956B2 (en) | 2015-07-15 | 2018-05-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102643183B1 (en) | 2015-07-15 | 2024-03-04 | 메르크 파텐트 게엠베하 | Compositions Comprising Organic Semiconducting Compounds |
| TWI697485B (en) | 2015-07-21 | 2020-07-01 | 日商捷恩智股份有限公司 | Compound containing oxazoline ring, material for electron transport/injection layer containing the same, organic electroluminescent element using the same, display device and lighting device |
| GB201513037D0 (en) | 2015-07-23 | 2015-09-09 | Merck Patent Gmbh | Phenyl-derived compound for use in organic electronic devices |
| US11127905B2 (en) | 2015-07-29 | 2021-09-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11018309B2 (en) | 2015-08-03 | 2021-05-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10696664B2 (en) | 2015-08-14 | 2020-06-30 | Merck Patent Gmbh | Phenoxazine derivatives for organic electroluminescent devices |
| US11522140B2 (en) | 2015-08-17 | 2022-12-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3133664A1 (en) | 2015-08-18 | 2017-02-22 | Novaled GmbH | Triaryl amine thick layer doped with metal amides for use as hole injection layer for an organic light-emitting diode (oled) |
| US10522769B2 (en) | 2015-08-18 | 2019-12-31 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3133663B1 (en) | 2015-08-18 | 2022-06-15 | Novaled GmbH | Metal amides for use as hole injection layer for an organic light-emitting diode (oled) |
| US10181564B2 (en) | 2015-08-26 | 2019-01-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3343657A4 (en) * | 2015-08-27 | 2019-04-24 | Hodogaya Chemical Co., Ltd. | Organic electroluminescent element |
| US10361381B2 (en) | 2015-09-03 | 2019-07-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11706972B2 (en) | 2015-09-08 | 2023-07-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11302872B2 (en) | 2015-09-09 | 2022-04-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR20170032532A (en) * | 2015-09-14 | 2017-03-23 | 삼성디스플레이 주식회사 | Condensed-cyclic compound and organic light-emitting device comprising the same |
| US10770664B2 (en) | 2015-09-21 | 2020-09-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20170092880A1 (en) | 2015-09-25 | 2017-03-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3147961A1 (en) | 2015-09-28 | 2017-03-29 | Novaled GmbH | Organic electroluminescent device |
| EP3147958B1 (en) | 2015-09-28 | 2019-10-23 | Novaled GmbH | Organic electroluminescent devices comprising borane compounds |
| US10593892B2 (en) | 2015-10-01 | 2020-03-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10847728B2 (en) | 2015-10-01 | 2020-11-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10991895B2 (en) | 2015-10-06 | 2021-04-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10388892B2 (en) | 2015-10-29 | 2019-08-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP6815326B2 (en) | 2015-10-29 | 2021-01-20 | 保土谷化学工業株式会社 | Organic electroluminescence device |
| US10177318B2 (en) | 2015-10-29 | 2019-01-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10388893B2 (en) | 2015-10-29 | 2019-08-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2017086357A1 (en) | 2015-11-17 | 2017-05-26 | 保土谷化学工業株式会社 | Organic electroluminescence element |
| EP3171418A1 (en) | 2015-11-23 | 2017-05-24 | Novaled GmbH | Organic semiconductive layer comprising phosphine oxide compounds |
| US10998507B2 (en) | 2015-11-23 | 2021-05-04 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10476010B2 (en) | 2015-11-30 | 2019-11-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP6752226B2 (en) | 2015-12-08 | 2020-09-09 | 保土谷化学工業株式会社 | Organic electroluminescence element |
| EP3182478B1 (en) | 2015-12-18 | 2018-11-28 | Novaled GmbH | Electron injection layer for an organic light-emitting diode (oled) |
| US10957861B2 (en) | 2015-12-29 | 2021-03-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11024808B2 (en) | 2015-12-29 | 2021-06-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10135006B2 (en) | 2016-01-04 | 2018-11-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP6788314B2 (en) | 2016-01-06 | 2020-11-25 | コニカミノルタ株式会社 | Organic electroluminescence element, manufacturing method of organic electroluminescence element, display device and lighting device |
| EP3411455B1 (en) | 2016-02-05 | 2020-10-21 | Merck Patent GmbH | Materials for electronic devices |
| US10457864B2 (en) | 2016-02-09 | 2019-10-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10707427B2 (en) | 2016-02-09 | 2020-07-07 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20170229663A1 (en) | 2016-02-09 | 2017-08-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10600967B2 (en) | 2016-02-18 | 2020-03-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11094891B2 (en) | 2016-03-16 | 2021-08-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10276809B2 (en) | 2016-04-05 | 2019-04-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10236456B2 (en) | 2016-04-11 | 2019-03-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10566552B2 (en) | 2016-04-13 | 2020-02-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11081647B2 (en) | 2016-04-22 | 2021-08-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11228003B2 (en) | 2016-04-22 | 2022-01-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11228002B2 (en) | 2016-04-22 | 2022-01-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10840458B2 (en) | 2016-05-25 | 2020-11-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3252837B1 (en) | 2016-05-30 | 2021-05-05 | Novaled GmbH | Organic light emitting diode comprising an organic semiconductor layer |
| EP3252841A1 (en) | 2016-05-30 | 2017-12-06 | Novaled GmbH | Organic light emitting diode comprising an organic semiconductor layer |
| US10468609B2 (en) | 2016-06-02 | 2019-11-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10672997B2 (en) | 2016-06-20 | 2020-06-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10686140B2 (en) | 2016-06-20 | 2020-06-16 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10862054B2 (en) | 2016-06-20 | 2020-12-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10727423B2 (en) | 2016-06-20 | 2020-07-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10651403B2 (en) | 2016-06-20 | 2020-05-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11482683B2 (en) | 2016-06-20 | 2022-10-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10957866B2 (en) | 2016-06-30 | 2021-03-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9929360B2 (en) | 2016-07-08 | 2018-03-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10680184B2 (en) | 2016-07-11 | 2020-06-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10153443B2 (en) | 2016-07-19 | 2018-12-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10720587B2 (en) | 2016-07-19 | 2020-07-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10205105B2 (en) | 2016-08-15 | 2019-02-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3291319B1 (en) | 2016-08-30 | 2019-01-23 | Novaled GmbH | Method for preparing an organic semiconductor layer |
| US10608186B2 (en) | 2016-09-14 | 2020-03-31 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10505127B2 (en) | 2016-09-19 | 2019-12-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10680187B2 (en) * | 2016-09-23 | 2020-06-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11196010B2 (en) | 2016-10-03 | 2021-12-07 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11183642B2 (en) | 2016-10-03 | 2021-11-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11127906B2 (en) | 2016-10-03 | 2021-09-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11189804B2 (en) | 2016-10-03 | 2021-11-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11081658B2 (en) | 2016-10-03 | 2021-08-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11011709B2 (en) | 2016-10-07 | 2021-05-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TWI764942B (en) | 2016-10-10 | 2022-05-21 | 德商麥克專利有限公司 | Electronic device |
| US11239432B2 (en) | 2016-10-14 | 2022-02-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10608185B2 (en) | 2016-10-17 | 2020-03-31 | Univeral Display Corporation | Organic electroluminescent materials and devices |
| DE102017008794A1 (en) | 2016-10-17 | 2018-04-19 | Merck Patent Gmbh | Materials for use in electronic devices |
| US10236458B2 (en) | 2016-10-24 | 2019-03-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11302870B2 (en) | 2016-11-02 | 2022-04-12 | Merck Patent Gmbh | Materials for electronic devices |
| KR102564613B1 (en) | 2016-11-08 | 2023-08-07 | 메르크 파텐트 게엠베하 | Compounds for Electronic Devices |
| US20180130956A1 (en) | 2016-11-09 | 2018-05-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10340464B2 (en) | 2016-11-10 | 2019-07-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10680188B2 (en) | 2016-11-11 | 2020-06-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10897016B2 (en) | 2016-11-14 | 2021-01-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10662196B2 (en) | 2016-11-17 | 2020-05-26 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10964893B2 (en) | 2016-11-17 | 2021-03-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10833276B2 (en) | 2016-11-21 | 2020-11-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10153445B2 (en) | 2016-11-21 | 2018-12-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TW201833118A (en) | 2016-11-22 | 2018-09-16 | 德商麥克專利有限公司 | Materials for electronic devices |
| US11555048B2 (en) | 2016-12-01 | 2023-01-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11548905B2 (en) | 2016-12-15 | 2023-01-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10490753B2 (en) | 2016-12-15 | 2019-11-26 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11545636B2 (en) | 2016-12-15 | 2023-01-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10811618B2 (en) | 2016-12-19 | 2020-10-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TWI761406B (en) | 2016-12-22 | 2022-04-21 | 德商麥克專利有限公司 | Materials for electronic devices |
| US11152579B2 (en) | 2016-12-28 | 2021-10-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11201298B2 (en) | 2017-01-09 | 2021-12-14 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11780865B2 (en) | 2017-01-09 | 2023-10-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10804475B2 (en) | 2017-01-11 | 2020-10-13 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11545637B2 (en) | 2017-01-13 | 2023-01-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10629820B2 (en) | 2017-01-18 | 2020-04-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10964904B2 (en) | 2017-01-20 | 2021-03-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11053268B2 (en) | 2017-01-20 | 2021-07-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11765968B2 (en) | 2017-01-23 | 2023-09-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11050028B2 (en) | 2017-01-24 | 2021-06-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20200013960A1 (en) | 2017-02-02 | 2020-01-09 | Merck Patent Gmbh | Materials for electronic devices |
| US10978647B2 (en) | 2017-02-15 | 2021-04-13 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10844084B2 (en) | 2017-02-22 | 2020-11-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11393987B2 (en) | 2017-03-01 | 2022-07-19 | Merck Patent Gmbh | Organic electroluminescent device |
| KR102557516B1 (en) | 2017-03-02 | 2023-07-20 | 메르크 파텐트 게엠베하 | Materials for Organic Electronic Devices |
| US10745431B2 (en) | 2017-03-08 | 2020-08-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10741780B2 (en) | 2017-03-10 | 2020-08-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2018168674A1 (en) | 2017-03-15 | 2018-09-20 | 保土谷化学工業株式会社 | Organic electroluminescent element |
| US10672998B2 (en) | 2017-03-23 | 2020-06-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10873037B2 (en) | 2017-03-28 | 2020-12-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10910577B2 (en) | 2017-03-28 | 2021-02-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11158820B2 (en) | 2017-03-29 | 2021-10-26 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10844085B2 (en) | 2017-03-29 | 2020-11-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11056658B2 (en) | 2017-03-29 | 2021-07-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10862046B2 (en) | 2017-03-30 | 2020-12-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11139443B2 (en) | 2017-03-31 | 2021-10-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11276829B2 (en) | 2017-03-31 | 2022-03-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102090364B1 (en) | 2017-04-10 | 2020-03-17 | 주식회사 엘지화학 | Anthracene derivatives and organic light emitting device using the same |
| US11038117B2 (en) | 2017-04-11 | 2021-06-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10777754B2 (en) | 2017-04-11 | 2020-09-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN110520503A (en) | 2017-04-13 | 2019-11-29 | 默克专利有限公司 | Composition for organic electronic device |
| US11101434B2 (en) | 2017-04-21 | 2021-08-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11084838B2 (en) | 2017-04-21 | 2021-08-10 | Universal Display Corporation | Organic electroluminescent materials and device |
| US10975113B2 (en) | 2017-04-21 | 2021-04-13 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11649249B2 (en) | 2017-04-25 | 2023-05-16 | Merck Patent Gmbh | Compounds for electronic devices |
| US10910570B2 (en) | 2017-04-28 | 2021-02-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11038137B2 (en) | 2017-04-28 | 2021-06-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11117897B2 (en) | 2017-05-01 | 2021-09-14 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10941170B2 (en) | 2017-05-03 | 2021-03-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11201299B2 (en) | 2017-05-04 | 2021-12-14 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10862055B2 (en) | 2017-05-05 | 2020-12-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10870668B2 (en) | 2017-05-05 | 2020-12-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10930864B2 (en) | 2017-05-10 | 2021-02-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10822362B2 (en) | 2017-05-11 | 2020-11-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10944060B2 (en) | 2017-05-11 | 2021-03-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11038115B2 (en) | 2017-05-18 | 2021-06-15 | Universal Display Corporation | Organic electroluminescent materials and device |
| US10840459B2 (en) | 2017-05-18 | 2020-11-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10790455B2 (en) | 2017-05-18 | 2020-09-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10944062B2 (en) | 2017-05-18 | 2021-03-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10934293B2 (en) | 2017-05-18 | 2021-03-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3406617B1 (en) | 2017-05-23 | 2021-03-24 | Novaled GmbH | Use of phosphine oxide compounds in a semiconducting layer comprised in an electronic device |
| US10930862B2 (en) | 2017-06-01 | 2021-02-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TW201920343A (en) | 2017-06-21 | 2019-06-01 | 德商麥克專利有限公司 | Materials for electronic devices |
| US11725022B2 (en) | 2017-06-23 | 2023-08-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11814403B2 (en) | 2017-06-23 | 2023-11-14 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11495757B2 (en) | 2017-06-23 | 2022-11-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11678565B2 (en) | 2017-06-23 | 2023-06-13 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11832510B2 (en) | 2017-06-23 | 2023-11-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11174259B2 (en) | 2017-06-23 | 2021-11-16 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11552261B2 (en) | 2017-06-23 | 2023-01-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10968226B2 (en) | 2017-06-23 | 2021-04-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11802136B2 (en) | 2017-06-23 | 2023-10-31 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11758804B2 (en) | 2017-06-23 | 2023-09-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20180370999A1 (en) | 2017-06-23 | 2018-12-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN110753685A (en) | 2017-06-23 | 2020-02-04 | 默克专利有限公司 | Material for organic electroluminescent device |
| US11608321B2 (en) | 2017-06-23 | 2023-03-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR20200022010A (en) | 2017-06-26 | 2020-03-02 | 메르크 파텐트 게엠베하 | Homogeneous mixture |
| CN110799484B (en) | 2017-06-28 | 2023-09-26 | 默克专利有限公司 | Material for electronic devices |
| TWI813576B (en) | 2017-07-03 | 2023-09-01 | 德商麥克專利有限公司 | Formulations with a low content of phenol type impurities |
| TWI786143B (en) | 2017-07-03 | 2022-12-11 | 德商麥克專利有限公司 | Organic electroluminescent device and method for producing the same |
| EP3649213B1 (en) | 2017-07-05 | 2021-06-23 | Merck Patent GmbH | Composition for organic electronic devices |
| KR102594782B1 (en) | 2017-07-05 | 2023-10-27 | 메르크 파텐트 게엠베하 | Compositions for organic electronic devices |
| US11469382B2 (en) | 2017-07-12 | 2022-10-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11765970B2 (en) | 2017-07-26 | 2023-09-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11917843B2 (en) | 2017-07-26 | 2024-02-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11239433B2 (en) | 2017-07-26 | 2022-02-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11228010B2 (en) | 2017-07-26 | 2022-01-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11322691B2 (en) | 2017-07-26 | 2022-05-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11968883B2 (en) | 2017-07-26 | 2024-04-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3658541A1 (en) | 2017-07-28 | 2020-06-03 | Merck Patent GmbH | Spirobifluorene derivatives for use in electronic devices |
| CN109309166B (en) * | 2017-07-28 | 2022-05-20 | 北京鼎材科技有限公司 | Organic electroluminescent device and anthracene compound containing 2,6,9, 10-tetra-substitution |
| JP7245777B2 (en) | 2017-08-04 | 2023-03-24 | 保土谷化学工業株式会社 | Organic electroluminescence device containing compound having benzazole ring structure |
| US11744141B2 (en) | 2017-08-09 | 2023-08-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11508913B2 (en) | 2017-08-10 | 2022-11-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11349083B2 (en) | 2017-08-10 | 2022-05-31 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11910699B2 (en) | 2017-08-10 | 2024-02-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11744142B2 (en) | 2017-08-10 | 2023-08-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11462697B2 (en) | 2017-08-22 | 2022-10-04 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11723269B2 (en) | 2017-08-22 | 2023-08-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11437591B2 (en) | 2017-08-24 | 2022-09-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11605791B2 (en) | 2017-09-01 | 2023-03-14 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11444249B2 (en) | 2017-09-07 | 2022-09-13 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11424420B2 (en) | 2017-09-07 | 2022-08-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11696492B2 (en) | 2017-09-07 | 2023-07-04 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TW201923028A (en) | 2017-09-08 | 2019-06-16 | 德商麥克專利有限公司 | Materials for electronic devices |
| WO2019049965A1 (en) | 2017-09-11 | 2019-03-14 | 保土谷化学工業株式会社 | Compound having pyrimidine ring structure and organic electroluminescent element |
| US10608188B2 (en) | 2017-09-11 | 2020-03-31 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11778897B2 (en) | 2017-09-20 | 2023-10-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN108675975A (en) | 2017-10-17 | 2018-10-19 | 默克专利有限公司 | Material for organic electroluminescence device |
| US11214587B2 (en) | 2017-11-07 | 2022-01-04 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11910702B2 (en) | 2017-11-07 | 2024-02-20 | Universal Display Corporation | Organic electroluminescent devices |
| US11183646B2 (en) | 2017-11-07 | 2021-11-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TWI785142B (en) | 2017-11-14 | 2022-12-01 | 德商麥克專利有限公司 | Composition for organic electronic devices |
| US11168103B2 (en) | 2017-11-17 | 2021-11-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP3714022B1 (en) | 2017-11-23 | 2023-06-07 | Merck Patent GmbH | Materials for electronic devices |
| TWI820057B (en) | 2017-11-24 | 2023-11-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| US11639339B2 (en) | 2017-11-24 | 2023-05-02 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US11825735B2 (en) | 2017-11-28 | 2023-11-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20190161504A1 (en) | 2017-11-28 | 2019-05-30 | University Of Southern California | Carbene compounds and organic electroluminescent devices |
| EP3492480B1 (en) | 2017-11-29 | 2021-10-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11937503B2 (en) | 2017-11-30 | 2024-03-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11233204B2 (en) | 2017-12-14 | 2022-01-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11233205B2 (en) | 2017-12-14 | 2022-01-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US10971687B2 (en) | 2017-12-14 | 2021-04-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2019115577A1 (en) | 2017-12-15 | 2019-06-20 | Merck Patent Gmbh | Substituted aromatic amines for use in organic electroluminescent devices |
| US20210036245A1 (en) | 2017-12-20 | 2021-02-04 | Merck Patent Gmbh | Heteroaromatic compounds |
| US11081659B2 (en) | 2018-01-10 | 2021-08-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11700765B2 (en) | 2018-01-10 | 2023-07-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11271177B2 (en) | 2018-01-11 | 2022-03-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11515493B2 (en) | 2018-01-11 | 2022-11-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11367840B2 (en) | 2018-01-26 | 2022-06-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11845764B2 (en) | 2018-01-26 | 2023-12-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11542289B2 (en) | 2018-01-26 | 2023-01-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11180519B2 (en) | 2018-02-09 | 2021-11-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11239434B2 (en) | 2018-02-09 | 2022-02-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11957050B2 (en) | 2018-02-09 | 2024-04-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11342509B2 (en) | 2018-02-09 | 2022-05-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TW201938761A (en) | 2018-03-06 | 2019-10-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| TWI802656B (en) | 2018-03-06 | 2023-05-21 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| US11279722B2 (en) | 2018-03-12 | 2022-03-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11165028B2 (en) | 2018-03-12 | 2021-11-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11142538B2 (en) | 2018-03-12 | 2021-10-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11557733B2 (en) | 2018-03-12 | 2023-01-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11217757B2 (en) | 2018-03-12 | 2022-01-04 | Universal Display Corporation | Host materials for electroluminescent devices |
| CN111819167A (en) | 2018-03-16 | 2020-10-23 | 默克专利有限公司 | Material for organic electroluminescent device |
| US11882759B2 (en) | 2018-04-13 | 2024-01-23 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11390639B2 (en) | 2018-04-13 | 2022-07-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11616203B2 (en) | 2018-04-17 | 2023-03-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11515494B2 (en) | 2018-05-04 | 2022-11-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11753427B2 (en) | 2018-05-04 | 2023-09-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11342513B2 (en) | 2018-05-04 | 2022-05-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11793073B2 (en) | 2018-05-06 | 2023-10-17 | Universal Display Corporation | Host materials for electroluminescent devices |
| US11459349B2 (en) | 2018-05-25 | 2022-10-04 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11450822B2 (en) | 2018-05-25 | 2022-09-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11716900B2 (en) | 2018-05-30 | 2023-08-01 | Universal Display Corporation | Host materials for electroluminescent devices |
| US20220332724A1 (en) | 2018-05-30 | 2022-10-20 | Merck Patent Gmbh | Composition for organic electronic devices |
| US11404653B2 (en) | 2018-06-04 | 2022-08-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11925103B2 (en) | 2018-06-05 | 2024-03-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11339182B2 (en) | 2018-06-07 | 2022-05-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11228004B2 (en) | 2018-06-22 | 2022-01-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11261207B2 (en) | 2018-06-25 | 2022-03-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11753425B2 (en) | 2018-07-11 | 2023-09-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20200075870A1 (en) | 2018-08-22 | 2020-03-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TWI823993B (en) | 2018-08-28 | 2023-12-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| KR20210052487A (en) | 2018-08-28 | 2021-05-10 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| EP3844244B1 (en) | 2018-08-28 | 2022-08-03 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| US11233203B2 (en) | 2018-09-06 | 2022-01-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11485706B2 (en) | 2018-09-11 | 2022-11-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| CN112639052A (en) | 2018-09-12 | 2021-04-09 | 默克专利有限公司 | Material for organic electroluminescent device |
| US11718634B2 (en) | 2018-09-14 | 2023-08-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11903305B2 (en) | 2018-09-24 | 2024-02-13 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR20200038061A (en) * | 2018-10-02 | 2020-04-10 | 엘지디스플레이 주식회사 | Organic light emitting diode device |
| US11469383B2 (en) | 2018-10-08 | 2022-10-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11495752B2 (en) | 2018-10-08 | 2022-11-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11476430B2 (en) | 2018-10-15 | 2022-10-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR20210079267A (en) | 2018-10-17 | 2021-06-29 | 호도가야 가가쿠 고교 가부시키가이샤 | A compound having a pyrimidine ring structure and an organic electroluminescent device |
| US11515482B2 (en) | 2018-10-23 | 2022-11-29 | Universal Display Corporation | Deep HOMO (highest occupied molecular orbital) emitter device structures |
| JP2022509407A (en) | 2018-10-31 | 2022-01-20 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Materials for organic electroluminescence devices |
| US11469384B2 (en) | 2018-11-02 | 2022-10-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11825736B2 (en) | 2018-11-19 | 2023-11-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11963441B2 (en) | 2018-11-26 | 2024-04-16 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11706980B2 (en) | 2018-11-28 | 2023-07-18 | Universal Display Corporation | Host materials for electroluminescent devices |
| US11690285B2 (en) | 2018-11-28 | 2023-06-27 | Universal Display Corporation | Electroluminescent devices |
| US11672176B2 (en) | 2018-11-28 | 2023-06-06 | Universal Display Corporation | Host materials for electroluminescent devices |
| US11716899B2 (en) | 2018-11-28 | 2023-08-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11672165B2 (en) | 2018-11-28 | 2023-06-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR20210097096A (en) | 2018-11-29 | 2021-08-06 | 호도가야 가가쿠 고교 가부시키가이샤 | A compound having an azabenzooxazole ring structure and an organic electroluminescence device |
| US11623936B2 (en) | 2018-12-11 | 2023-04-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11737349B2 (en) | 2018-12-12 | 2023-08-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11834459B2 (en) | 2018-12-12 | 2023-12-05 | Universal Display Corporation | Host materials for electroluminescent devices |
| US11780829B2 (en) | 2019-01-30 | 2023-10-10 | The University Of Southern California | Organic electroluminescent materials and devices |
| US11812624B2 (en) | 2019-01-30 | 2023-11-07 | The University Of Southern California | Organic electroluminescent materials and devices |
| US20200251664A1 (en) | 2019-02-01 | 2020-08-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11370809B2 (en) | 2019-02-08 | 2022-06-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11325932B2 (en) | 2019-02-08 | 2022-05-10 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20220052273A1 (en) | 2019-02-13 | 2022-02-17 | Hodogaya Chemical Co., Ltd. | Compound having benzazole ring structure, and organic electroluminescent device |
| CN113424332A (en) | 2019-02-18 | 2021-09-21 | 默克专利有限公司 | Composition for organic electronic device |
| US11773320B2 (en) | 2019-02-21 | 2023-10-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11557738B2 (en) | 2019-02-22 | 2023-01-17 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11871653B2 (en) | 2019-02-22 | 2024-01-09 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20220127286A1 (en) | 2019-03-04 | 2022-04-28 | Merck Patent Gmbh | Ligands for nano-sized materials |
| US11512093B2 (en) | 2019-03-04 | 2022-11-29 | Universal Display Corporation | Compound used for organic light emitting device (OLED), consumer product and formulation |
| US11739081B2 (en) | 2019-03-11 | 2023-08-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11569480B2 (en) | 2019-03-12 | 2023-01-31 | Universal Display Corporation | Plasmonic OLEDs and vertical dipole emitters |
| US11637261B2 (en) | 2019-03-12 | 2023-04-25 | Universal Display Corporation | Nanopatch antenna outcoupling structure for use in OLEDs |
| JP2020158491A (en) | 2019-03-26 | 2020-10-01 | ユニバーサル ディスプレイ コーポレイション | Organic electroluminescent materials and devices |
| US11963438B2 (en) | 2019-03-26 | 2024-04-16 | The University Of Southern California | Organic electroluminescent materials and devices |
| US20220181552A1 (en) | 2019-04-11 | 2022-06-09 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US11639363B2 (en) | 2019-04-22 | 2023-05-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11613550B2 (en) | 2019-04-30 | 2023-03-28 | Universal Display Corporation | Organic electroluminescent materials and devices comprising benzimidazole-containing metal complexes |
| WO2020225826A1 (en) * | 2019-05-07 | 2020-11-12 | Council Of Scientific & Industrial Research | Fluorescent polymer for the dual mode selective detection of tnt at nanomolar level |
| US11560398B2 (en) | 2019-05-07 | 2023-01-24 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11495756B2 (en) | 2019-05-07 | 2022-11-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR20200129304A (en) | 2019-05-08 | 2020-11-18 | 주식회사 엘지화학 | Organic light emitting device |
| US11827651B2 (en) | 2019-05-13 | 2023-11-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP2020188121A (en) * | 2019-05-14 | 2020-11-19 | 出光興産株式会社 | Organic electroluminescense device and electronic apparatus |
| US11634445B2 (en) | 2019-05-21 | 2023-04-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JPWO2020246484A1 (en) | 2019-06-06 | 2020-12-10 | ||
| US11647667B2 (en) | 2019-06-14 | 2023-05-09 | Universal Display Corporation | Organic electroluminescent compounds and organic light emitting devices using the same |
| US11920070B2 (en) | 2019-07-12 | 2024-03-05 | The University Of Southern California | Luminescent janus-type, two-coordinated metal complexes |
| US11926638B2 (en) | 2019-07-22 | 2024-03-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11685754B2 (en) | 2019-07-22 | 2023-06-27 | Universal Display Corporation | Heteroleptic organic electroluminescent materials |
| US20210032278A1 (en) | 2019-07-30 | 2021-02-04 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11708355B2 (en) | 2019-08-01 | 2023-07-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11374181B2 (en) | 2019-08-14 | 2022-06-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11930699B2 (en) | 2019-08-15 | 2024-03-12 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20210047354A1 (en) | 2019-08-16 | 2021-02-18 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11925105B2 (en) | 2019-08-26 | 2024-03-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11937494B2 (en) | 2019-08-28 | 2024-03-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11600787B2 (en) | 2019-08-30 | 2023-03-07 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11820783B2 (en) | 2019-09-06 | 2023-11-21 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR20220065801A (en) | 2019-09-19 | 2022-05-20 | 메르크 파텐트 게엠베하 | organic electroluminescent device |
| EP3798225B1 (en) * | 2019-09-26 | 2022-11-02 | Samsung Electronics Co., Ltd. | Organometallic compound, organic light-emitting device including the same and electronic apparatus including the organic light-emitting device |
| US11864458B2 (en) | 2019-10-08 | 2024-01-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11950493B2 (en) | 2019-10-15 | 2024-04-02 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11697653B2 (en) | 2019-10-21 | 2023-07-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11919914B2 (en) | 2019-10-25 | 2024-03-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11765965B2 (en) | 2019-10-30 | 2023-09-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TW202130783A (en) | 2019-11-04 | 2021-08-16 | 德商麥克專利有限公司 | Organic electroluminescent device |
| US20210135130A1 (en) | 2019-11-04 | 2021-05-06 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP4055642A1 (en) | 2019-11-04 | 2022-09-14 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| TW202134252A (en) | 2019-11-12 | 2021-09-16 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| US11889708B2 (en) | 2019-11-14 | 2024-01-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TW202136181A (en) * | 2019-12-04 | 2021-10-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| US20210217969A1 (en) | 2020-01-06 | 2021-07-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11778895B2 (en) | 2020-01-13 | 2023-10-03 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11917900B2 (en) | 2020-01-28 | 2024-02-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20220336759A1 (en) | 2020-01-28 | 2022-10-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US11932660B2 (en) | 2020-01-29 | 2024-03-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR102509034B1 (en) * | 2020-02-13 | 2023-03-10 | 삼성디스플레이 주식회사 | Organic light emitting device and apparatus including the same |
| CN115280538A (en) | 2020-03-11 | 2022-11-01 | 默克专利有限公司 | Organic electroluminescent device |
| EP4118695B1 (en) | 2020-03-11 | 2024-05-01 | Merck Patent GmbH | Organic electroluminescent apparatus |
| TW202200529A (en) | 2020-03-13 | 2022-01-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| KR20220157456A (en) | 2020-03-23 | 2022-11-29 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| EP4139971A1 (en) | 2020-04-21 | 2023-03-01 | Merck Patent GmbH | Emulsions comprising organic functional materials |
| US11970508B2 (en) | 2020-04-22 | 2024-04-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR20230017816A (en) | 2020-05-29 | 2023-02-06 | 메르크 파텐트 게엠베하 | organic electroluminescent device |
| EP3937268A1 (en) | 2020-07-10 | 2022-01-12 | Universal Display Corporation | Plasmonic oleds and vertical dipole emitters |
| KR20230043106A (en) | 2020-07-22 | 2023-03-30 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| CN115885599A (en) | 2020-07-22 | 2023-03-31 | 默克专利有限公司 | Material for organic electroluminescent device |
| CN112694394B (en) * | 2020-08-25 | 2022-05-27 | 天津大学 | Ultrasensitive multi-output signal biosensor and preparation method and application thereof |
| US20220158096A1 (en) | 2020-11-16 | 2022-05-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20220165967A1 (en) | 2020-11-24 | 2022-05-26 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20220162243A1 (en) | 2020-11-24 | 2022-05-26 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TW202237797A (en) | 2020-11-30 | 2022-10-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| US20220271241A1 (en) | 2021-02-03 | 2022-08-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP4060758A3 (en) | 2021-02-26 | 2023-03-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP4059915A3 (en) | 2021-02-26 | 2022-12-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20220298192A1 (en) | 2021-03-05 | 2022-09-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20220298190A1 (en) | 2021-03-12 | 2022-09-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20220298193A1 (en) | 2021-03-15 | 2022-09-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| KR20220134125A (en) * | 2021-03-26 | 2022-10-05 | 주식회사 엘지화학 | Compound and organic light emitting device comprising same |
| US20220340607A1 (en) | 2021-04-05 | 2022-10-27 | Universal Display Corporation | Organic electroluminescent materials and devices |
| TW202309243A (en) | 2021-04-09 | 2023-03-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| TW202305091A (en) | 2021-04-09 | 2023-02-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| KR20230169215A (en) | 2021-04-09 | 2023-12-15 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| EP4075531A1 (en) | 2021-04-13 | 2022-10-19 | Universal Display Corporation | Plasmonic oleds and vertical dipole emitters |
| US20220352478A1 (en) | 2021-04-14 | 2022-11-03 | Universal Display Corporation | Organic eletroluminescent materials and devices |
| US20230006149A1 (en) | 2021-04-23 | 2023-01-05 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20220407020A1 (en) | 2021-04-23 | 2022-12-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20230133787A1 (en) | 2021-06-08 | 2023-05-04 | University Of Southern California | Molecular Alignment of Homoleptic Iridium Phosphors |
| WO2023012084A1 (en) | 2021-08-02 | 2023-02-09 | Merck Patent Gmbh | A printing method by combining inks |
| CN117917983A (en) | 2021-09-13 | 2024-04-23 | 默克专利有限公司 | Material for organic electroluminescent device |
| EP4151699A1 (en) | 2021-09-17 | 2023-03-22 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2023052272A1 (en) | 2021-09-28 | 2023-04-06 | Merck Patent Gmbh | Materials for electronic devices |
| WO2023052314A1 (en) | 2021-09-28 | 2023-04-06 | Merck Patent Gmbh | Materials for electronic devices |
| WO2023052275A1 (en) | 2021-09-28 | 2023-04-06 | Merck Patent Gmbh | Materials for electronic devices |
| WO2023052313A1 (en) | 2021-09-28 | 2023-04-06 | Merck Patent Gmbh | Materials for electronic devices |
| WO2023094412A1 (en) | 2021-11-25 | 2023-06-01 | Merck Patent Gmbh | Materials for electronic devices |
| EP4212539A1 (en) | 2021-12-16 | 2023-07-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2023117837A1 (en) | 2021-12-21 | 2023-06-29 | Merck Patent Gmbh | Process for preparing deuterated organic compounds |
| WO2023152346A1 (en) | 2022-02-14 | 2023-08-17 | Merck Patent Gmbh | Materials for electronic devices |
| EP4231804A3 (en) | 2022-02-16 | 2023-09-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20230292592A1 (en) | 2022-03-09 | 2023-09-14 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US20230337516A1 (en) | 2022-04-18 | 2023-10-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2023208899A1 (en) | 2022-04-28 | 2023-11-02 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2023222559A1 (en) | 2022-05-18 | 2023-11-23 | Merck Patent Gmbh | Process for preparing deuterated organic compounds |
| US20230389421A1 (en) | 2022-05-24 | 2023-11-30 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2023237458A1 (en) | 2022-06-07 | 2023-12-14 | Merck Patent Gmbh | Method of printing a functional layer of an electronic device by combining inks |
| EP4293001A1 (en) | 2022-06-08 | 2023-12-20 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2023247663A1 (en) | 2022-06-24 | 2023-12-28 | Merck Patent Gmbh | Composition for organic electronic devices |
| WO2023247662A1 (en) | 2022-06-24 | 2023-12-28 | Merck Patent Gmbh | Composition for organic electronic devices |
| US20240016051A1 (en) | 2022-06-28 | 2024-01-11 | Universal Display Corporation | Organic electroluminescent materials and devices |
| WO2024013004A1 (en) | 2022-07-11 | 2024-01-18 | Merck Patent Gmbh | Materials for electronic devices |
| WO2024033282A1 (en) | 2022-08-09 | 2024-02-15 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US20240107880A1 (en) | 2022-08-17 | 2024-03-28 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP4362645A3 (en) | 2022-10-27 | 2024-05-15 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP4362630A2 (en) | 2022-10-27 | 2024-05-01 | Universal Display Corporation | Organic electroluminescent materials and devices |
| EP4362631A3 (en) | 2022-10-27 | 2024-05-08 | Universal Display Corporation | Organic electroluminescent materials and devices |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2000113985A (en) * | 1998-08-07 | 2000-04-21 | Fuji Photo Film Co Ltd | Organic electroluminescent element |
| US6171715B1 (en) * | 1997-08-07 | 2001-01-09 | Fuji Photo Film Co., Ltd. | Organic electroluminescent element |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4356429A (en) | 1980-07-17 | 1982-10-26 | Eastman Kodak Company | Organic electroluminescent cell |
| US4539507A (en) * | 1983-03-25 | 1985-09-03 | Eastman Kodak Company | Organic electroluminescent devices having improved power conversion efficiencies |
| JPH01245087A (en) | 1987-12-11 | 1989-09-29 | Idemitsu Kosan Co Ltd | Organic electroluminescence element |
| JPH02289675A (en) | 1989-01-13 | 1990-11-29 | Ricoh Co Ltd | Electric field light emitting element |
| JP2665788B2 (en) | 1989-01-17 | 1997-10-22 | 旭化成工業株式会社 | Organic electroluminescent device |
| JP2749407B2 (en) | 1989-02-20 | 1998-05-13 | パイオニア株式会社 | EL device |
| JP2731216B2 (en) | 1989-02-23 | 1998-03-25 | パイオニア株式会社 | EL device |
| JP2790353B2 (en) | 1989-03-15 | 1998-08-27 | 出光興産株式会社 | Organic thin-film electroluminescence device |
| JP3071091B2 (en) | 1993-03-17 | 2000-07-31 | 三洋電機株式会社 | EL device |
| EP0700917B1 (en) | 1994-09-12 | 2002-05-08 | Motorola, Inc. | Light emitting devices comprising organometallic complexes |
| US5533817A (en) * | 1995-05-19 | 1996-07-09 | International Business Machines Corporation | Biaxial printer |
| US5645948A (en) | 1996-08-20 | 1997-07-08 | Eastman Kodak Company | Blue organic electroluminescent devices |
| US5766779A (en) * | 1996-08-20 | 1998-06-16 | Eastman Kodak Company | Electron transporting materials for organic electroluminescent devices |
| JPH11345686A (en) | 1997-08-07 | 1999-12-14 | Fuji Photo Film Co Ltd | Organic electroluminescence element |
| US6436558B1 (en) * | 1998-08-07 | 2002-08-20 | Fuji Photo Film Co., Ltd. | Organic electroluminescence element |
| US6998487B2 (en) * | 2001-04-27 | 2006-02-14 | Lg Chem, Ltd. | Double-spiro organic compounds and organic electroluminescent devices using the same |
-
2002
- 2002-01-18 KR KR1020020003025A patent/KR100691543B1/en active IP Right Grant
-
2003
- 2003-01-16 US US10/345,310 patent/US6878469B2/en not_active Expired - Lifetime
- 2003-01-17 CN CN038001551A patent/CN1625552B/en not_active Expired - Lifetime
- 2003-01-17 EP EP03729561.5A patent/EP1465874B1/en not_active Expired - Lifetime
- 2003-01-17 JP JP2003560958A patent/JP4308663B2/en not_active Expired - Lifetime
- 2003-01-17 AU AU2003215914A patent/AU2003215914A1/en not_active Abandoned
- 2003-01-17 WO PCT/KR2003/000112 patent/WO2003060956A2/en active Application Filing
- 2003-08-05 TW TW092121428A patent/TWI227655B/en not_active IP Right Cessation
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6171715B1 (en) * | 1997-08-07 | 2001-01-09 | Fuji Photo Film Co., Ltd. | Organic electroluminescent element |
| JP2000113985A (en) * | 1998-08-07 | 2000-04-21 | Fuji Photo Film Co Ltd | Organic electroluminescent element |
Cited By (42)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9587172B2 (en) | 2008-10-01 | 2017-03-07 | Lg Display Co., Ltd. | Organic light-emitting diode and method of manufacturing the same |
| US9181474B2 (en) | 2012-02-07 | 2015-11-10 | Samsung Display Co., Ltd. | Amine-based compound and organic light-emitting diode including the same |
| US9882143B2 (en) | 2012-02-07 | 2018-01-30 | Samsung Display Co., Ltd. | Amine-based compound and organic light-emitting diode including the same |
| KR20140082345A (en) | 2012-12-24 | 2014-07-02 | 에스에프씨 주식회사 | Antracene derivatives having heteroaryl substituted naphthyl group and organic light-emitting diode including the same |
| KR20140082437A (en) | 2012-12-24 | 2014-07-02 | 에스에프씨 주식회사 | Antracene derivatives having heteroaryl substituted phenyl group and organic light-emitting diode including the same |
| KR20140090036A (en) | 2013-01-08 | 2014-07-16 | 에스에프씨 주식회사 | Antracene derivatives having heteroaryl substituted phenyl group and organic light-emitting diode including the same |
| KR20140090035A (en) | 2013-01-08 | 2014-07-16 | 에스에프씨 주식회사 | Antracene derivatives having heteroaryl substituted naphthyl group and organic light-emitting diode including the same |
| KR20140090037A (en) | 2013-01-08 | 2014-07-16 | 에스에프씨 주식회사 | Asymmetric antracene derivatives having two naphthyl groups and organic light-emitting diode including the same |
| KR20140090410A (en) | 2013-01-09 | 2014-07-17 | 에스에프씨 주식회사 | Asymmetric antracene derivatives having two phenyl groups and organic light-emitting diode including the same |
| KR20140090411A (en) | 2013-01-09 | 2014-07-17 | 에스에프씨 주식회사 | Asymmetric antracene derivatives having two naphthyl groups and organic light-emitting diode including the same |
| US9397300B2 (en) | 2013-02-14 | 2016-07-19 | Samsung Display Co., Ltd. | Compound and organic light-emitting diode including the same |
| US9825231B2 (en) | 2013-09-06 | 2017-11-21 | Samsung Display Co., Ltd. | Condensed cyclic compound and organic light-emitting device including the same |
| US9837614B2 (en) | 2013-09-06 | 2017-12-05 | Samsung Display Co., Ltd. | Condensed-cyclic compound and organic light-emitting device comprising the same |
| US9680106B2 (en) | 2013-10-01 | 2017-06-13 | Samsung Display Co., Ltd. | Pyrene-based compound and organic light-emitting device including the same |
| US9601699B2 (en) | 2013-10-31 | 2017-03-21 | Samsung Display Co., Ltd. | Chrysene-based compound and organic light-emitting device including the same |
| US9871208B2 (en) | 2014-02-26 | 2018-01-16 | Samsung Display Co., Ltd. | Condensed cyclic compound and organic light-emitting device including the same |
| US9831443B2 (en) | 2014-03-19 | 2017-11-28 | Samsung Display Co., Ltd. | Condensed cyclic compound and organic light-emitting device comprising the same |
| US10069083B2 (en) | 2014-06-02 | 2018-09-04 | Samsung Display Co., Ltd. | Condensed cyclic compounds and organic light-emitting devices including the same |
| US10608189B2 (en) | 2014-06-02 | 2020-03-31 | Samsung Display Co., Ltd. | Condensed cyclic compounds and organic light-emitting devices including the same |
| US9722187B2 (en) | 2014-10-22 | 2017-08-01 | Samsung Display Co., Ltd. | Compound and organic light-emitting device including the same |
| US10367150B2 (en) | 2015-02-05 | 2019-07-30 | Samsung Display Co., Ltd. | Compound and organic light-emitting device including the same |
| US10211407B2 (en) | 2015-03-16 | 2019-02-19 | Samsung Display Co., Ltd. | Condensed cyclic compound and organic light-emitting device including the same |
| US10205102B2 (en) | 2015-06-17 | 2019-02-12 | Samsung Display Co., Ltd. | Material for organic electroluminescent device and organic electroluminescent device including the same |
| US10693079B2 (en) | 2015-06-17 | 2020-06-23 | Samsung Display Co., Ltd. | Mono amine derivatives and organic electroluminescent device including the same |
| US10236452B2 (en) | 2015-08-04 | 2019-03-19 | Samsung Display Co., Ltd. | Compound and organic light-emitting device including the same |
| US10580994B2 (en) | 2015-09-16 | 2020-03-03 | Samsung Display Co., Ltd. | Compound and organic light-emitting device including the same |
| US11279693B2 (en) | 2015-11-13 | 2022-03-22 | Sfc Co., Ltd. | Organic heterocyclic compound and light-emitting diode comprising same |
| US10461262B2 (en) | 2015-12-22 | 2019-10-29 | Samsung Display Co., Ltd. | Condensed cyclic compound and an organic light-emitting device including the same |
| US10069085B2 (en) | 2016-07-18 | 2018-09-04 | Samsung Display Co., Ltd. | Heterocyclic compound and organic light-emitting device including the same |
| US10693083B2 (en) | 2016-12-09 | 2020-06-23 | Samsung Display Co., Ltd. | Condensed cyclic compound and organic light-emitting device including the same |
| CN108264486B (en) * | 2016-12-30 | 2020-11-13 | 吉林奥来德光电材料股份有限公司 | Novel imidazole compound, preparation thereof and electroluminescent device |
| CN108264486A (en) * | 2016-12-30 | 2018-07-10 | 吉林奥来德光电材料股份有限公司 | A kind of novel glyoxaline compound and its preparation and electroluminescent device |
| US11581487B2 (en) | 2017-04-26 | 2023-02-14 | Oti Lumionics Inc. | Patterned conductive coating for surface of an opto-electronic device |
| US11563183B2 (en) | 2017-06-21 | 2023-01-24 | Samsung Display Co., Ltd. | Heterocyclic compound and organic light-emitting device including the same |
| US11844273B2 (en) | 2017-06-21 | 2023-12-12 | Samsung Display Co., Ltd. | Heterocyclic compound and organic light-emitting device including the same |
| US11588111B2 (en) | 2017-08-04 | 2023-02-21 | Samsung Display Co., Ltd. | Condensed-cyclic compound and organic light-emitting device including the same |
| US10998503B2 (en) | 2017-10-27 | 2021-05-04 | Samsung Display Co., Ltd. | Condensed cyclic compound and organic light-emitting device including the same |
| US11251376B2 (en) | 2017-10-31 | 2022-02-15 | Samsung Display Co., Ltd. | Heterocyclic compound and organic light-emitting device including the same |
| US11751415B2 (en) | 2018-02-02 | 2023-09-05 | Oti Lumionics Inc. | Materials for forming a nucleation-inhibiting coating and devices incorporating same |
| US11730012B2 (en) | 2019-03-07 | 2023-08-15 | Oti Lumionics Inc. | Materials for forming a nucleation-inhibiting coating and devices incorporating same |
| US11985841B2 (en) | 2021-12-07 | 2024-05-14 | Oti Lumionics Inc. | Patterning a conductive deposited layer using a nucleation inhibiting coating and an underlying metallic coating |
| KR20240034107A (en) | 2022-09-05 | 2024-03-13 | 롬엔드하스전자재료코리아유한회사 | Organic Electroluminescent Device |
Also Published As
| Publication number | Publication date |
|---|---|
| US20030165715A1 (en) | 2003-09-04 |
| TWI227655B (en) | 2005-02-01 |
| WO2003060956A3 (en) | 2003-11-13 |
| JP2005515233A (en) | 2005-05-26 |
| AU2003215914A8 (en) | 2003-07-30 |
| US6878469B2 (en) | 2005-04-12 |
| AU2003215914A1 (en) | 2003-07-30 |
| EP1465874B1 (en) | 2013-06-19 |
| CN1625552B (en) | 2011-09-28 |
| KR20030067773A (en) | 2003-08-19 |
| CN1625552A (en) | 2005-06-08 |
| WO2003060956A2 (en) | 2003-07-24 |
| JP4308663B2 (en) | 2009-08-05 |
| EP1465874A2 (en) | 2004-10-13 |
| TW200507696A (en) | 2005-02-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR100691543B1 (en) | New material for transporting electron and organic electroluminescent display using the same | |
| KR100664390B1 (en) | New materials for injecting or transporting holes and organic electroluminescence devices using the same | |
| US6984462B2 (en) | Organic electroluminescent devices using double-spiro organic compounds | |
| EP1902013B1 (en) | Novel anthracene derivatives, process for preparation thereof, and organic electronic light emitting device using the same | |
| EP1341403B1 (en) | Luminescent element material and luminescent element comprising the same | |
| JP3838816B2 (en) | Compound for organic EL device and organic EL device | |
| EP2316905B1 (en) | Organic compound and organic light emitting device using the same | |
| KR101460361B1 (en) | New imidazole derivatives and organic electronic device using the same | |
| EP2371812A1 (en) | Indenofluorenedione derivative, material for organic electroluminescent element, and organic electroluminescent element | |
| US6838194B2 (en) | Efficient organic electroluminescent devices with red fluorescent dopants | |
| KR20090114008A (en) | New imidazole derivatives and organic electronic device using the same | |
| KR101053466B1 (en) | Chemical and organic electronic element using the same, electronic device thereof | |
| JPH0812600A (en) | Phenylanthracene derivative and organic el element | |
| KR101321988B1 (en) | Aromatic derivatives and organic electroluminescent device comprising same | |
| JP3642606B2 (en) | Organic EL device | |
| KR101950255B1 (en) | Compound for organic electronic element, organic electronic element using the same, and a electronic device thereof | |
| KR20130102669A (en) | Compound for organic electronic element, organic electronic element using the same, and a electronic device thereof | |
| KR20050118098A (en) | New materials for injecting or transporting holes and organic electroluminescence devices using the same | |
| KR20150082156A (en) | New compounds and organic light emitting device comprising the same | |
| JP4612846B2 (en) | Biskinoxaline compound and organic light-emitting device | |
| US6649089B2 (en) | Red organic electroluminescent materials | |
| KR20130083887A (en) | New imidazole derivatives and organic electronic device using the same | |
| US6506505B1 (en) | Cyclooctatetraenes as electron transporters in organic light emitting diodes | |
| KR20110065415A (en) | Aromatic derivatives and organic electroluminescent device comprising same | |
| KR102026861B1 (en) | Compound for organic electronic element, organic electronic element using the same, and a electronic device thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination | ||
| E902 | Notification of reason for refusal | ||
| E701 | Decision to grant or registration of patent right | ||
| GRNT | Written decision to grant | ||
| FPAY | Annual fee payment |
Payment date: 20130111 Year of fee payment: 7 |
|
| FPAY | Annual fee payment |
Payment date: 20140103 Year of fee payment: 8 |
|
| FPAY | Annual fee payment |
Payment date: 20150119 Year of fee payment: 9 |
|
| FPAY | Annual fee payment |
Payment date: 20160216 Year of fee payment: 10 |
|
| FPAY | Annual fee payment |
Payment date: 20170216 Year of fee payment: 11 |
|
| FPAY | Annual fee payment |
Payment date: 20180116 Year of fee payment: 12 |
|
| FPAY | Annual fee payment |
Payment date: 20190116 Year of fee payment: 13 |
|
| FPAY | Annual fee payment |
Payment date: 20200116 Year of fee payment: 14 |