JP4736943B2 - Positive electrode active material for lithium secondary battery and method for producing the same - Google Patents
Positive electrode active material for lithium secondary battery and method for producing the same Download PDFInfo
- Publication number
- JP4736943B2 JP4736943B2 JP2006137302A JP2006137302A JP4736943B2 JP 4736943 B2 JP4736943 B2 JP 4736943B2 JP 2006137302 A JP2006137302 A JP 2006137302A JP 2006137302 A JP2006137302 A JP 2006137302A JP 4736943 B2 JP4736943 B2 JP 4736943B2
- Authority
- JP
- Japan
- Prior art keywords
- positive electrode
- active material
- electrode active
- lithium
- composite oxide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Description
本願発明は、リチウム二次電池に使用されるコバルト酸リチウム複合酸化物を主成分とする正極活物質に係り、特に熱安定性及びサイクル特性に優れた正極活物質およびその製造方法に関する。 The present invention relates to a positive electrode active material mainly composed of a lithium cobaltate composite oxide used in a lithium secondary battery, and more particularly to a positive electrode active material excellent in thermal stability and cycle characteristics and a method for producing the same.
リチウムイオン二次電池用正極活物質は、放電容量を向上する目的で充電電圧を上昇させる傾向にある。しかしながら、充電電圧を4.2V付近まで上昇すると、正極活物質の結晶の転移、あるいは正極活物質の分解が起こり、結果としてサイクル劣化を低下する。また、正極活物質の結晶転移あるいは分解に伴い、コバルト酸からの酸素が放出され、この酸素は非水系電解液を酸化燃焼し、その結果、電池自体の熱安定性を低下する。この熱安定性の低下は、電池の安全性に重大な影響を及ぼし、正極活物質のより一層の熱安定性の向上が望まれている。 The positive electrode active material for a lithium ion secondary battery tends to increase the charging voltage for the purpose of improving the discharge capacity. However, when the charging voltage is increased to around 4.2 V, crystal transition of the positive electrode active material or decomposition of the positive electrode active material occurs, resulting in a decrease in cycle deterioration. Further, along with the crystal transition or decomposition of the positive electrode active material, oxygen from the cobalt acid is released, and this oxygen oxidizes and burns the non-aqueous electrolyte, resulting in a decrease in the thermal stability of the battery itself. This reduction in thermal stability has a significant effect on battery safety, and further improvement in thermal stability of the positive electrode active material is desired.
また、正極活物質のコバルト酸リチウム複合酸化物は導電性が低く、そのため導電性のあるカーボンを被覆することで導電性を改善しているが、カーボンとの接触が悪い場合、サイクル劣化を引き起こす原因となっていた。 In addition, the lithium cobaltate composite oxide of the positive electrode active material has low conductivity, and therefore the conductivity is improved by coating the conductive carbon. However, when the contact with the carbon is poor, cycle deterioration is caused. It was the cause.
このような問題を解決する目的で、例えば特許文献1には正極活物質に特定の低価数の遷移金属酸化物を添加した正極を作製することで満充電時の高温保存の安定性の改良がなされるとしている。 In order to solve such a problem, for example, Patent Document 1 discloses an improvement in the stability of high-temperature storage at full charge by preparing a positive electrode in which a specific low-valent transition metal oxide is added to the positive electrode active material. Is going to be made.
また、サイクル特性の改善の目的で特許文献2にはAlあるいはFeをコバルト酸リチウム複合酸化物に固溶させた正極活物質について記載されている。 For the purpose of improving cycle characteristics, Patent Document 2 describes a positive electrode active material in which Al or Fe is dissolved in a lithium cobalt oxide composite oxide.
しかしながら、これらの技術に示される正極活物質の熱安定性、サイクル特性は未だ不十分であり、また、遷移金属の固溶量は1〜20重量%と多く、結果として放電容量の低下を免れない。 However, the thermal stability and cycle characteristics of the positive electrode active material shown in these technologies are still insufficient, and the solid solution amount of the transition metal is as high as 1 to 20% by weight. Absent.
従って、本願発明の目的は上記した問題点を解決することであり、リチウム二次電池の放電容量及び充電容量を低下することなく、充電時における熱安定性、及びサイクル特性を向上できる正極活活物質のコバルト酸リチウム複合酸化物を提供することにある。 Accordingly, an object of the present invention is to solve the above-described problems, and a positive electrode active material that can improve thermal stability and cycle characteristics during charging without reducing the discharge capacity and charge capacity of the lithium secondary battery. An object of the present invention is to provide a lithium cobalt oxide composite oxide.
本発明者等は、リチウムイオン電池のサイクル特性、熱安定性を改善する目的で正極活物質についての数多くの試作を行い鋭意検討した結果、意外にも正極活物質の粒子表面に特定のAl2O3を被覆することに効果があることを見いだし本発明を完成した。 The inventors of the present invention have made a number of prototypes of the positive electrode active material for the purpose of improving the cycle characteristics and thermal stability of the lithium ion battery, and as a result of intensive investigations, unexpectedly, specific Al 2 was added to the particle surface of the positive electrode active material. It was found that there is an effect in coating O 3 and the present invention was completed.
すなわち、本発明のリチウム二次電池用正極活物質の製造方法は、コバルト酸リチウム複合酸化物を製造する工程と、前記コバルト酸リチウム複合酸化物とAl2O3とを混合する工程とを有する。 That is, the method for producing a positive electrode active material for a lithium secondary battery according to the present invention includes a step of producing a lithium cobaltate composite oxide, and a step of mixing the lithium cobaltate composite oxide and Al 2 O 3. .
前記コバルト酸リチウム複合酸化物と前記Al2O3との混合物を加熱処理することが好ましい。
It is preferable to heat-treat the mixture of the lithium cobaltate composite oxide and the Al 2 O 3 .
前記Al2O3の混合量は、前記コバルト酸リチウム複合酸化物100重量部に対し、0.05〜10重量部であることが好ましい。 The mixing amount of the Al 2 O 3 is preferably 0.05 to 10 parts by weight with respect to 100 parts by weight of the lithium cobalt oxide composite oxide.
前記Al2O3の平均粒子径は、0.01μm〜0.25μmであることが好ましい。 The average particle diameter of the Al 2 O 3 is preferably 0.01 μm to 0.25 μm.
前記コバルト酸リチウム複合酸化物を製造する工程において、前記コバルト酸リチウム複合酸化物は、組成式がLixCoO2(但し、xは、0.98<x≦1.10を満たす)で表され、Co以外の遷移金属をさらに含有することが好ましい。 In the step of producing the lithium cobaltate composite oxide, the lithium cobaltate composite oxide has a composition formula represented by Li x CoO 2 (where x satisfies 0.98 <x ≦ 1.10.). It is preferable to further contain a transition metal other than Co.
また、本発明のリチウム二次電池用正極活物質は、コバルト酸リチウム複合酸化物の粒子表面がAl2O3によって被覆されており、前記コバルト酸リチウム複合酸化物は、組成式がLixCoO2で表され、xの値が、0.98<x≦1.10を満たす。
Further, in the positive electrode active material for a lithium secondary battery of the present invention, the particle surface of the lithium cobalt oxide composite oxide is coated with Al 2 O 3 , and the lithium cobalt oxide composite oxide has a composition formula of LixCoO 2 . And the value of x satisfies 0.98 <x ≦ 1.10.
以下に示すように、正極活物質を本願発明の構成とすることで、正電極の単極の放電容量を低下することなく発熱量を低下することができる。 As shown below, when the positive electrode active material has the configuration of the present invention, the calorific value can be reduced without reducing the single electrode discharge capacity of the positive electrode.
また、Coに対するLiの比率を高くできる範囲を拡大することが可能となり、その結果、Liの仕込み比率を大きくでき、放電容量を大きくすることができた。さらにまた、Li比率を高くしてもハイレート時の放電容量の低下が少ない正極活物質を得ることができる。 In addition, the range in which the ratio of Li to Co can be increased can be expanded. As a result, the charging ratio of Li can be increased and the discharge capacity can be increased. Furthermore, even if the Li ratio is increased, a positive electrode active material with little reduction in discharge capacity at the high rate can be obtained.
以下、本発明に係るリチウム二次電池用正極活物質およびその製造方法を具体的に説明する。ただし、本発明は、この実施の形態に限定されない。 Hereinafter, a positive electrode active material for a lithium secondary battery and a method for producing the same according to the present invention will be specifically described. However, the present invention is not limited to this embodiment.
本発明における正極活物質の熱安定性の評価は次のようにして測定試料を作製し、示差熱分析により反応熱を測定して行った。 The thermal stability of the positive electrode active material in the present invention was evaluated by preparing a measurement sample as follows and measuring the heat of reaction by differential thermal analysis.
(1)先ず、測定試料の正極活物質粉末90重量部と導電剤としてのアセチレンブラック粉末2.5重量部と、カーボン2.5重量部と、PVDF(ポリフッ化ビニリデン)5重量部とを混練してペーストを調製する。 (1) First, 90 parts by weight of a positive electrode active material powder as a measurement sample, 2.5 parts by weight of acetylene black powder as a conductive agent, 2.5 parts by weight of carbon, and 5 parts by weight of PVDF (polyvinylidene fluoride) are kneaded. To prepare a paste.
(2)得られたペーストを単極評価可能なデマンタブル式のセル正極集電体に塗布し、二次電池を作製し、定電流による充放電を行いなじませる。なじませた電池を一定電流の下で電池電圧が4.3Vになるまで充電を行う。 (2) The obtained paste is applied to a detachable cell positive electrode current collector capable of single electrode evaluation to produce a secondary battery, and charging and discharging with a constant current are performed. The battery that has been conditioned is charged under a constant current until the battery voltage reaches 4.3V.
(3)充電が完了すると、デマンタブル式の二次電池から正極を取り出し、洗浄して乾燥し、正極から正極活物質を削り取る。 (3) When charging is completed, the positive electrode is taken out from the detachable secondary battery, washed and dried, and the positive electrode active material is scraped off from the positive electrode.
(4)電解液に使用するエチレンカーボネートをAlセルに約2.0mgと、正極から削り取った活物質を約5mgを秤量し、示差熱分析を室温〜400℃の範囲で行う。 (4) About 2.0 mg of ethylene carbonate used for the electrolytic solution in an Al cell and about 5 mg of the active material scraped from the positive electrode are weighed, and differential thermal analysis is performed in the range of room temperature to 400 ° C.
このようにして測定した示差熱分析チャートは図1に示すように、低温部では温度が上昇しても示差熱は変化しないが、ある温度以上では示差熱が大きく増大する。このときの温度Aを発熱開始温度とし、この示差熱曲線のピークにおける高さを発熱量a(ベースラインからの高さ)と定義する。満充電を行った正極活物質を測定試料とし、示差熱分析装置により試料温度を上昇させると、前述したように正極活物質からの酸素の脱離が起こり、この酸素が試料中に共存する非水電解質のエチレンカーボネートを酸化し、その酸化反応の反応熱を測定することができる。 In the differential thermal analysis chart thus measured, as shown in FIG. 1, the differential heat does not change even if the temperature rises in the low temperature part, but the differential heat greatly increases above a certain temperature. The temperature A at this time is defined as the heat generation start temperature, and the height at the peak of this differential heat curve is defined as the heat generation amount a (height from the baseline). When the fully charged positive electrode active material is used as a measurement sample and the sample temperature is raised by a differential thermal analyzer, desorption of oxygen from the positive electrode active material occurs as described above, and this oxygen coexists in the sample. It is possible to oxidize ethylene carbonate of the water electrolyte and measure the reaction heat of the oxidation reaction.
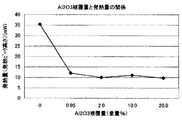
本発明において、正極活物質の粒子表面にAl2O3を被覆することにより熱安定性向上に効果がある。Al2O3の被覆量と示差熱分析による発熱量の関係を図2に示す。Al2O3は平均粒子径が0.01μmのAl2O3を用いた。図2に示すように、Al2O3の被覆量が正極活物質100重量部に対して、0.05重量部以上である場合、発熱量は10mW付近まで低下している。 In the present invention, coating of Al 2 O 3 on the surface of the positive electrode active material particles is effective in improving the thermal stability. FIG. 2 shows the relationship between the coating amount of Al 2 O 3 and the calorific value by differential thermal analysis. Al 2 O 3 has an average particle diameter of Al 2 O 3 was used in 0.01 [mu] m. As shown in FIG. 2, when the coating amount of Al 2 O 3 is 0.05 parts by weight or more with respect to 100 parts by weight of the positive electrode active material, the heat generation amount is reduced to about 10 mW.
さらに正極の単極の放電容量を上述のデマンタブル式のセルを用い、電池電圧4.3Vまで充電し、次に2.75Vまで放電させ、そのときに取り出した電気量を測定した。Al2O3の被覆量と放電容量の関係を図3にプロットした。Al2O3の被覆量を増加すると10重量部までは放電容量に変化を及ぼさないが、20重量部被覆すると大きく低下している。これはAl2O3の被覆により正極活物質の抵抗が増大する結果と推定される。従ってAl2O3の被覆量は20重量部が上限である。これよりも多くなると放電容量の低下が大きくなりすぎて実用的でない。 Furthermore, the single-electrode discharge capacity of the positive electrode was charged to a battery voltage of 4.3 V and then discharged to 2.75 V using the above-described detachable cell, and the amount of electricity taken out at that time was measured. The relationship between the coating amount of Al 2 O 3 and the discharge capacity is plotted in FIG. When the coating amount of Al 2 O 3 is increased, the discharge capacity is not changed up to 10 parts by weight, but when the coating amount is 20 parts by weight, it is greatly reduced. This is presumed to be a result of the resistance of the positive electrode active material being increased by the coating of Al 2 O 3 . Therefore, the upper limit of the coating amount of Al 2 O 3 is 20 parts by weight. If it exceeds this, the decrease in discharge capacity becomes too large, which is not practical.
従って、本発明の正極活物質は、示差熱分析と放電容量の測定からAl2O3の被覆量は0.05〜20重量部の範囲であることが必要とされ、0.05〜10重量部の範囲がより好ましい。 Therefore, the positive electrode active material of the present invention is required to have a coating amount of Al 2 O 3 in the range of 0.05 to 20 parts by weight based on differential thermal analysis and discharge capacity measurement, and 0.05 to 10 parts by weight. A range of parts is more preferred.
また、種々の平均粒子径のAl2O3を正極活物質に対し0.1重量部被覆した正極活物質について、上記した示差熱分析による反応熱と、放電容量を測定した結果を表1に示す。 Table 1 shows the results of measurement of reaction heat and discharge capacity by the differential thermal analysis described above for the positive electrode active material in which 0.1 part by weight of Al 2 O 3 having various average particle diameters was coated on the positive electrode active material. Show.
表1より試料5のAl2O3を付着した場合、発熱量の低下はほとんどなく効果がない。また、平均粒子径が大きくなるに従い放電容量も低下傾向にある。従ってAl2O3の平均粒子径は好ましくは0.25μm以下であり、好ましくは0.10μm以下である。
From Table 1, when Al 2 O 3 of
本発明に使用する正極活物質は、組成式がLixCoO2で表現されるリチウム−遷移金属複合酸化物であり、Coの一部を、Fe、Mn、Cr、Ni等の不可避的な遷移金属を含有するか、あるいは混入しているようなものに対しても適用可能である。 The positive electrode active material used in the present invention is a lithium-transition metal composite oxide whose composition formula is expressed by Li x CoO 2 , and a part of Co is unavoidable transition such as Fe, Mn, Cr, Ni, etc. The present invention is also applicable to a metal containing or mixed metal.
x値はコバルトに対するリチウムの比であり、充放電はLiの量が多い方が有利である。従って理想的にはx値は化学量論比(x=1)よりも通常大きく設定される。しかしあまり大きくすると、ハイレート時(放電時に大電流を流す場合)、放電容量が低下する傾向があり、実際にはx値はあまり大きくすることができない。 The x value is the ratio of lithium to cobalt, and it is advantageous for charge / discharge to have a large amount of Li. Therefore, ideally, the x value is usually set larger than the stoichiometric ratio (x = 1). However, if it is too large, the discharge capacity tends to decrease at the high rate (when a large current flows at the time of discharge), and the x value cannot actually be increased too much.
これに対しAl2O3の被覆によりx値の増加によるハイレート時の放電容量の低下を改善することができる。図4にAl2O3を0.1重量部被覆した本発明の正極活物質と、Al2O3を被覆しない比較例の正極活物質について、上述したデマンタブルセルに適用した場合についてハイレート時の放電容量とx値の関係をプロットした。この場合、充放電負荷は1.5C、充電電位4.2V、放電電位2.75V迄で行った。(1Cは1時間で充電又は放電が終了する電流負荷)図4より、本発明の正極活物質は放電容量はx=1.10まで低下していない。これに対し比較例はx値が1.00より大きくなると低下している。 On the other hand, the reduction of the discharge capacity at the high rate due to the increase of the x value can be improved by coating with Al 2 O 3 . A positive electrode active material of the present invention that the Al 2 O 3 coated 0.1 part by weight in Fig. 4, the positive electrode active material of Comparative Example without coating the Al 2 O 3, high rate when the case of application to Demantaburuseru described above The relationship between the discharge capacity and the x value was plotted. In this case, the charge / discharge load was 1.5 C, the charge potential was 4.2 V, and the discharge potential was 2.75 V. (1C is a current load that completes charging or discharging in one hour.) From FIG. 4, the discharge capacity of the positive electrode active material of the present invention does not decrease to x = 1.10. On the other hand, the comparative example decreases when the x value is larger than 1.00.
また、通常の電流密度で放電させる場合(0.25C)について、x値と放電容量の関係について図5にプロットした。この図より、x値の増加とともに放電容量は増大している。x値が1.00より小さくなると、放電容量は大幅に低下する。従ってx値は0.98を超えることが必要とされる。 Further, in the case of discharging at a normal current density (0.25 C), the relationship between the x value and the discharge capacity is plotted in FIG. From this figure, the discharge capacity increases as the x value increases. When the x value is smaller than 1.00, the discharge capacity is greatly reduced. Therefore, the x value needs to exceed 0.98.
上記したハイレート時の放電容量と、通常時の放電容量のいずれも考慮すると、xは0.98を超え1.1以下の範囲に設定する必要がある。 Considering both the discharge rate at the high rate and the discharge capacity at the normal time, x needs to be set in the range of more than 0.98 and not more than 1.1.
電極を電解質液に浸漬し、電極を外部回路に電気的に接続した場合、電極界面近傍の電解質液中に電気二重層が形成される。この電気二重層内において、イオンは電子の授受が行われる。本発明の対象の正極活物質の表面においても同じ現象が生じ、具体的には、放電時においてLiイオンは正極活物質内部の結晶格子内を移動し、電極界面まで到達して電子を電極に残して電解質中に離脱する。この電極界面においてはLiイオンは電気二重層中を拡散し、電解質バルク中へ泳動する。この場合、大電流の放電の際、比較的な容易な電気二重層中の電気泳動は迅速に起こるが、移動の抵抗が大きい結晶格子中のLiの移動は遅くなる。その結果、電極界面近傍にはLiイオンは不足状態となり、結果的にキャリヤー濃度の低下により電気伝導性が低下し、放電容量の低下を引き起こす要因になっている。本発明において微粒子のAl2O3を特定量被覆することにより、電気二重層中の移動速度を多少低下することができ、その結果、結晶格子中のLiイオンの移動速度とバランスが保たれ電圧降下を改善することができたと推定している。 When the electrode is immersed in the electrolyte solution and the electrode is electrically connected to an external circuit, an electric double layer is formed in the electrolyte solution near the electrode interface. In this electric double layer, ions exchange electrons. The same phenomenon occurs on the surface of the positive electrode active material of the present invention. Specifically, during discharge, Li ions move in the crystal lattice inside the positive electrode active material, reach the electrode interface, and electrons are transferred to the electrode. Leave it in the electrolyte. At this electrode interface, Li ions diffuse in the electric double layer and migrate into the electrolyte bulk. In this case, during the discharge of a large current, relatively easy electrophoresis in the electric double layer occurs rapidly, but the movement of Li in the crystal lattice having a large movement resistance is delayed. As a result, Li ions are insufficient in the vicinity of the electrode interface, and as a result, the electrical conductivity is lowered due to a decrease in the carrier concentration, which causes a decrease in the discharge capacity. By coating a specific amount of fine Al 2 O 3 in the present invention, the moving speed in the electric double layer can be somewhat reduced, and as a result, the moving speed of Li ions in the crystal lattice is balanced with the voltage. It is estimated that the descent could be improved.
以下に実施例を示して本発明を具体的に説明するが、本発明はこれらに限られるものではない。 EXAMPLES The present invention will be specifically described below with reference to examples, but the present invention is not limited to these examples.
本発明に使用する正極活物質基体は、通常の製造方法により得られるものなら制限なく適用することができる。例えば、Co、及びLiのそれぞれの酸化物、若しくは高温で加熱すると酸化物を生成するそれぞれの化合物を所定の割合に混合して、次にこの混合物を大気雰囲気下、500〜1000℃の温度で焼成することで得られる。高温で加熱すると酸化物を生成する化合物とは、炭酸塩、蓚酸塩、硝酸塩、硫酸塩、水酸化物等を意味する。また、高温で加熱するとは、後の工程において焼成する程度の温度の意味である。 The positive electrode active material substrate used in the present invention can be applied without limitation as long as it is obtained by a normal production method. For example, the respective oxides of Co and Li, or the respective compounds that generate oxides when heated at a high temperature are mixed in a predetermined ratio, and then the mixture is heated at a temperature of 500 to 1000 ° C. in an air atmosphere. Obtained by firing. A compound that generates an oxide when heated at a high temperature means a carbonate, oxalate, nitrate, sulfate, hydroxide, or the like. Moreover, heating at a high temperature means a temperature at which firing is performed in a later step.
焼成温度は500℃〜1000℃の範囲の温度で、1〜24時間加熱する、好ましくは700〜1000℃の温度範囲で6〜12時間焼成することにより得ることができる。
焼成温度が500℃以下の場合、未反応の原料が正極活物質に残留し正極活物質の本来の特徴を生かせない。逆に、1000℃以上になると、正極活物質の粒径が大きくなり過ぎて電池特性を低下する。
The calcination temperature can be obtained by heating at a temperature in the range of 500 ° C. to 1000 ° C. for 1 to 24 hours, preferably in the temperature range of 700 to 1000 ° C. for 6 to 12 hours.
When the firing temperature is 500 ° C. or lower, unreacted raw materials remain in the positive electrode active material, and the original characteristics of the positive electrode active material cannot be utilized. On the other hand, when the temperature is 1000 ° C. or higher, the particle size of the positive electrode active material becomes too large and the battery characteristics deteriorate.
焼成時間は、1時間未満では原料粒子間の拡散反応が進行せず、24時間経過すると拡散反応はほとんど完了しているため、それ以上焼成する必要がない。 When the firing time is less than 1 hour, the diffusion reaction between the raw material particles does not proceed. When 24 hours have elapsed, the diffusion reaction is almost completed, and therefore no further firing is necessary.
焼成時の雰囲気は大気中の中性雰囲気あるいは弱酸化雰囲気が好ましい。還元性雰囲気で行うと、目的の酸化物組成を得にくくなり、炭素あるいは硫黄等の不要物が残留するので避けなければならない。
[実施例1]
コバルト原料として四三酸化コバルト(Co3O4)と、リチウム原料として炭酸リチウム(Li2CO3)をx=1.02になるように計量し、乾式混合した。得られた混合粉体を大気雰囲気中900℃で10時間焼成して、Li1.02CoO2で表されるリチウム−遷移金属複合酸化物を得た。次いで、これをらいかい乳鉢を用いて粉砕して、平均粒子径約5μmの正極活物質粉末を得た。
The atmosphere during firing is preferably a neutral atmosphere or a weak oxidizing atmosphere in the air. If it is carried out in a reducing atmosphere, it becomes difficult to obtain the target oxide composition, and unnecessary substances such as carbon or sulfur remain, so this must be avoided.
[Example 1]
Cobalt tetroxide (Co 3 O 4 ) as a cobalt raw material and lithium carbonate (Li 2 CO 3 ) as a lithium raw material were weighed so that x = 1.02, and dry mixed. The obtained mixed powder was baked at 900 ° C. for 10 hours in an air atmosphere to obtain a lithium-transition metal composite oxide represented by Li 1.02 CoO 2 . Next, this was pulverized using a rough mortar to obtain a positive electrode active material powder having an average particle size of about 5 μm.
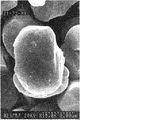
得られた正極活物質粉末を10kgと平均粒子径0.05μmのAl2O3(デグサ社製アロンC)200gをヘンシェルミキサーに充填し、回転速度1000rpmで6分間混合した。最後に200メッシュのフルイを通して本発明の正極活物質を得た。得られた正極活物質を化学分析した結果、Al2O3がLiCoO2の100重量部に対し2重量部被覆されており、粒子表面のSEM写真によると、図6に示すように正極活物質の粒子表面をAl2O3で被覆されている。
[実施例2、3]
Al2O3の混合量をそれぞれ5g、1000gとする以外、実施例1と同様にしてAl2O3を0.05重量部、10重量部被覆した正極活物質を得た。
[比較例1、2]
Al2O3の混合量をそれぞれ0g、2000gとする以外、実施例1と同様にしてAl2O3を0重量部、20重量部被覆した正極活物質を得た。図7に比較例1で得られた正極活物質の顕微鏡写真のSEM写真を示す。尚、これは図6に示す本発明品との対比のために示した。
[比較例3]
Al2O3を平均粒子径が0.3μmの住友化学工業社製のAl2O3に変更する以外実施例1と同様にした。
[比較例4]
Al2O3を平均粒子径が0.50μmの住友化学工業社製のAl2O3に変更する以外実施例1と同様にした。
[比較例5]
Al2O3を平均粒子径が1.25μmの住友化学工業社製社製のAl2O3に変更する以外実施例1と同様にした。
[比較例6]
Al2O3を平均粒子径が2.60μmの住友化学工業社製のAl2O3に変更する以外実施例1と同様にした。
[比較例7]
Al2O3を平均粒子径が4.90μmの住友化学工業社製のAl2O3に変更する以外実施例1と同様にした。
[実施例4]
実施例1のAl2O3被覆された正極活物質を大気雰囲気中300℃で10時間加熱処理を行った。この加熱によりAl2O3の被覆はより強固になる。この処理によりAl2O3がLiCoO2と反応したかどうか確認する目的で、X線回折、XPS(X線光電子分光法)による解析を適用した結果、Al、あるいはAl2O3のLiCoO2中への固溶は確認できなかった。
[比較例8]
実施例1のAl2O3被覆された正極活物質を大気雰囲気中800℃で10時間加熱処理を行った。実施例9と同様にしてX線回折、XPSによる解析をした結果、粒子表面のAl2O3のピークは消失し、しかも焼成物表面の格子定数は小さくなり、Al、あるいはAl2O3のLiCoO2中への固溶は確認された。
A Henschel mixer was charged with 10 kg of the obtained positive electrode active material powder and 200 g of Al 2 O 3 (Aron C, manufactured by Degussa) having an average particle diameter of 0.05 μm, and mixed for 6 minutes at a rotation speed of 1000 rpm. Finally, the positive electrode active material of the present invention was obtained through a 200-mesh sieve. As a result of chemical analysis of the obtained positive electrode active material, 2 parts by weight of Al 2 O 3 was coated on 100 parts by weight of LiCoO 2 , and according to the SEM photograph of the particle surface, as shown in FIG. The particle surface is coated with Al 2 O 3 .
[Examples 2 and 3]
A positive electrode active material coated with 0.05 part by weight and 10 parts by weight of Al 2 O 3 was obtained in the same manner as in Example 1 except that the mixed amounts of Al 2 O 3 were 5 g and 1000 g, respectively.
[Comparative Examples 1 and 2]
A positive electrode active material coated with 0 to 20 parts by weight of Al 2 O 3 was obtained in the same manner as in Example 1 except that the mixed amount of Al 2 O 3 was 0 g and 2000 g, respectively. FIG. 7 shows an SEM photograph of a micrograph of the positive electrode active material obtained in Comparative Example 1. This is shown for comparison with the product of the present invention shown in FIG.
[Comparative Example 3]
The average particle diameter of the Al 2 O 3 was the same manner as in Example 1 except for changing the Al 2 O 3 of 0.3μm of Sumitomo Chemical Co., Ltd..
[Comparative Example 4]
The average particle diameter of the Al 2 O 3 was the same manner as in Example 1 except for changing the Al 2 O 3 of Sumitomo Chemical Co., Ltd. 0.50 .mu.m.
[Comparative Example 5]
The average particle diameter of the Al 2 O 3 was the same manner as in Example 1 except for changing the Al 2 O 3 of Sumitomo Chemical Co., Inc. of 1.25 .mu.m.
[Comparative Example 6]
The average particle diameter of the Al 2 O 3 was the same manner as in Example 1 except for changing the Al 2 O 3 of Sumitomo Chemical Co. 2.60Myuemu.
[Comparative Example 7]
The average particle diameter of the Al 2 O 3 was the same manner as in Example 1 except for changing the Al 2 O 3 of Sumitomo Chemical Co. 4.90Myuemu.
[Example 4]
The positive electrode active material coated with Al 2 O 3 of Example 1 was subjected to heat treatment at 300 ° C. for 10 hours in the air atmosphere. This heating strengthens the coating of Al 2 O 3 . As a result of applying analysis by X-ray diffraction and XPS (X-ray photoelectron spectroscopy) for the purpose of confirming whether Al 2 O 3 reacted with LiCoO 2 by this treatment, Al or Al 2 O 3 in LiCoO 2 The solid solution was not confirmed.
[Comparative Example 8]
The positive electrode active material coated with Al 2 O 3 of Example 1 was subjected to heat treatment at 800 ° C. for 10 hours in the air atmosphere. As a result of analysis by X-ray diffraction and XPS in the same manner as in Example 9, the peak of Al 2 O 3 on the particle surface disappears, and the lattice constant on the surface of the fired product decreases, and Al or Al 2 O 3 Solid solution in LiCoO 2 was confirmed.
図8にAl2O3被覆後の加熱の温度と発熱量(ピーク高さ)の関係をプロットした。これらの点は各温度で10時間加熱した結果である。加熱による発熱ピーク高さは変化がない。図9に放電容量と加熱温度の関係をプロットした。加熱温度は発熱量には影響しないが、300℃を超えて加熱した場合放電容量を低下する傾向にある。従って、加熱温度は好ましくは300℃以下である。 FIG. 8 plots the relationship between the heating temperature and the calorific value (peak height) after Al 2 O 3 coating. These points are the results of heating at each temperature for 10 hours. The exothermic peak height due to heating is not changed. FIG. 9 plots the relationship between the discharge capacity and the heating temperature. The heating temperature does not affect the calorific value, but tends to lower the discharge capacity when heated above 300 ° C. Accordingly, the heating temperature is preferably 300 ° C. or lower.
Claims (2)
前記コバルト酸リチウム複合酸化物とAl2O3とをヘンシェルミキサーで混合する工程と、
前記コバルト酸リチウム複合酸化物と前記Al2O3との混合物を300℃以下で加熱処理する工程と、を有するリチウム二次電池用正極活物質の製造方法であって、
前記コバルト酸リチウム複合酸化物は、組成式がLixCoO2(但し、xは、0.98<x≦1.10を満たす)で表され、
前記Al2O3の混合量は、前記コバルト酸リチウム複合酸化物100重量部に対し、0.05〜10重量部であり、
前記Al2O3の平均粒子径は、0.01μm〜0.25μmであることを特徴とするリチウム二次電池用正極活物質の製造方法。 Producing a lithium cobaltate composite oxide;
Mixing the lithium cobaltate composite oxide and Al 2 O 3 with a Henschel mixer;
A step of heat-treating a mixture of the lithium cobaltate composite oxide and the Al 2 O 3 at 300 ° C. or less, and a method for producing a positive electrode active material for a lithium secondary battery,
The lithium cobaltate composite oxide is represented by the composition formula Li x CoO 2 (where x satisfies 0.98 <x ≦ 1.10.)
The mixing amount of the Al 2 O 3 is 0.05 to 10 parts by weight with respect to 100 parts by weight of the lithium cobalt oxide composite oxide,
The average particle diameter of the Al 2 O 3 The manufacturing method of a lithium secondary battery positive electrode active material for which is a 0.01Myuemu~0.25Myuemu.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2006137302A JP4736943B2 (en) | 2006-05-17 | 2006-05-17 | Positive electrode active material for lithium secondary battery and method for producing the same |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2006137302A JP4736943B2 (en) | 2006-05-17 | 2006-05-17 | Positive electrode active material for lithium secondary battery and method for producing the same |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP32060299A Division JP2001143703A (en) | 1999-11-11 | 1999-11-11 | Positive electrode active substance for use in lithium secondary battery |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2006261132A JP2006261132A (en) | 2006-09-28 |
| JP2006261132A5 JP2006261132A5 (en) | 2007-07-05 |
| JP4736943B2 true JP4736943B2 (en) | 2011-07-27 |
Family
ID=37100093
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2006137302A Expired - Lifetime JP4736943B2 (en) | 2006-05-17 | 2006-05-17 | Positive electrode active material for lithium secondary battery and method for producing the same |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP4736943B2 (en) |
Families Citing this family (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101017079B1 (en) * | 2007-11-07 | 2011-02-25 | 한국과학기술연구원 | Fabrication method for electrode active material and lithium battery comprising electrode active material fabricated therefrom |
| JP5189384B2 (en) * | 2008-02-29 | 2013-04-24 | 株式会社日立製作所 | Lithium secondary battery |
| EP2471133A4 (en) * | 2009-08-27 | 2014-02-12 | Envia Systems Inc | Metal oxide coated positive electrode materials for lithium-based batteries |
| US9843041B2 (en) | 2009-11-11 | 2017-12-12 | Zenlabs Energy, Inc. | Coated positive electrode materials for lithium ion batteries |
| CN103081189B (en) * | 2010-08-17 | 2017-02-15 | 尤米科尔公司 | Aluminum dry-coated and heat treated cathode material precursors |
| KR20140038884A (en) * | 2012-09-21 | 2014-03-31 | 가부시키가이샤 한도오따이 에네루기 켄큐쇼 | Electrode material for power storage device, electrode for power storage device, and power storage device |
| US10115962B2 (en) | 2012-12-20 | 2018-10-30 | Envia Systems, Inc. | High capacity cathode material with stabilizing nanocoatings |
| TWI600202B (en) * | 2014-03-06 | 2017-09-21 | 烏明克公司 | Doped and coated lithium transition metal oxide cathode materials for batteries in automotive applications |
| KR101758992B1 (en) * | 2014-10-02 | 2017-07-17 | 주식회사 엘지화학 | Positive electrode active material for lithium secondary battery, method for preparing the same, and lithium secondary battery comprising the same |
| US10193135B2 (en) | 2015-01-15 | 2019-01-29 | Zenlabs Energy, Inc. | Positive electrode active materials with composite coatings for high energy density secondary batteries and corresponding processes |
| CN116565297A (en) | 2016-07-05 | 2023-08-08 | 株式会社半导体能源研究所 | Lithium ion secondary battery |
| KR20230066123A (en) | 2016-10-12 | 2023-05-12 | 가부시키가이샤 한도오따이 에네루기 켄큐쇼 | Positive electrode active material particle and manufacturing method of positive electrode active material particle |
| CN112201778A (en) | 2017-05-12 | 2021-01-08 | 株式会社半导体能源研究所 | Positive electrode active material particles |
| CN117038957A (en) | 2017-05-19 | 2023-11-10 | 株式会社半导体能源研究所 | Lithium ion secondary battery |
| CN111933906A (en) | 2017-06-26 | 2020-11-13 | 株式会社半导体能源研究所 | Method for producing positive electrode active material |
| KR20210021976A (en) | 2018-06-22 | 2021-03-02 | 가부시키가이샤 한도오따이 에네루기 켄큐쇼 | Positive electrode active material, positive electrode, secondary battery, and manufacturing method of positive electrode |
| KR20220082091A (en) | 2018-08-03 | 2022-06-16 | 가부시키가이샤 한도오따이 에네루기 켄큐쇼 | Positive electrode active material and manufacturing method of positive electrode active material |
| KR20210092234A (en) | 2018-11-16 | 2021-07-23 | 가부시키가이샤 한도오따이 에네루기 켄큐쇼 | Positive electrode active material, secondary battery, electronic device, and vehicle |
| KR20210092247A (en) | 2018-11-16 | 2021-07-23 | 가부시키가이샤 한도오따이 에네루기 켄큐쇼 | Positive electrode active material, secondary battery, electronic device, and vehicle |
| JPWO2020104881A1 (en) | 2018-11-21 | 2021-10-14 | 株式会社半導体エネルギー研究所 | Positive electrode active material and secondary battery |
| KR20210100130A (en) | 2018-12-13 | 2021-08-13 | 가부시키가이샤 한도오따이 에네루기 켄큐쇼 | Method of manufacturing a cathode active material |
| DE112019006253T5 (en) | 2018-12-17 | 2021-09-09 | Semiconductor Energy Laboratory Co., Ltd. | Positive electrode active material and secondary battery |
| CN109994729B (en) | 2019-03-19 | 2021-03-05 | 宁德新能源科技有限公司 | Positive electrode material and electrochemical device using same |
| KR20210143215A (en) | 2019-03-29 | 2021-11-26 | 가부시키가이샤 한도오따이 에네루기 켄큐쇼 | Cathode active material and secondary battery |
| WO2020201916A1 (en) | 2019-04-05 | 2020-10-08 | 株式会社半導体エネルギー研究所 | Method for producing positive electrode active material, method for producing secondary battery, and secondary battery |
| WO2020208459A1 (en) | 2019-04-12 | 2020-10-15 | 株式会社半導体エネルギー研究所 | Method for preparing positive electrode active material |
| CN113994506A (en) | 2019-06-07 | 2022-01-28 | 株式会社半导体能源研究所 | Secondary battery, electronic device, and vehicle |
| WO2022090843A1 (en) | 2020-10-26 | 2022-05-05 | 株式会社半導体エネルギー研究所 | Secondary battery, electronic device and vehicle |
| KR20240000578A (en) | 2021-04-29 | 2024-01-02 | 가부시키가이샤 한도오따이 에네루기 켄큐쇼 | Secondary batteries and electronic devices |
| JP2023152003A (en) * | 2022-04-01 | 2023-10-16 | 住友金属鉱山株式会社 | Positive electrode active material for lithium ion secondary battery and method of producing positive electrode active material for lithium ion secondary battery |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH04355057A (en) * | 1991-05-30 | 1992-12-09 | Matsushita Electric Ind Co Ltd | Nonaqueous electrolyte secondary battery |
| JPH04355056A (en) * | 1991-05-30 | 1992-12-09 | Matsushita Electric Ind Co Ltd | Nonaqueous electrolyte secondary battery |
| JPH07153495A (en) * | 1993-11-26 | 1995-06-16 | Haibaru:Kk | Secondary battery |
| JPH08102332A (en) * | 1994-09-30 | 1996-04-16 | Hitachi Ltd | Secondary battery |
| JPH08222219A (en) * | 1995-02-14 | 1996-08-30 | Yuasa Corp | Nonaqueous electrolyte battery |
| JPH08236114A (en) * | 1995-02-27 | 1996-09-13 | Sanyo Electric Co Ltd | Lithium secondary battery |
| JP2001143703A (en) * | 1999-11-11 | 2001-05-25 | Nichia Chem Ind Ltd | Positive electrode active substance for use in lithium secondary battery |
-
2006
- 2006-05-17 JP JP2006137302A patent/JP4736943B2/en not_active Expired - Lifetime
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH04355057A (en) * | 1991-05-30 | 1992-12-09 | Matsushita Electric Ind Co Ltd | Nonaqueous electrolyte secondary battery |
| JPH04355056A (en) * | 1991-05-30 | 1992-12-09 | Matsushita Electric Ind Co Ltd | Nonaqueous electrolyte secondary battery |
| JPH07153495A (en) * | 1993-11-26 | 1995-06-16 | Haibaru:Kk | Secondary battery |
| JPH08102332A (en) * | 1994-09-30 | 1996-04-16 | Hitachi Ltd | Secondary battery |
| JPH08222219A (en) * | 1995-02-14 | 1996-08-30 | Yuasa Corp | Nonaqueous electrolyte battery |
| JPH08236114A (en) * | 1995-02-27 | 1996-09-13 | Sanyo Electric Co Ltd | Lithium secondary battery |
| JP2001143703A (en) * | 1999-11-11 | 2001-05-25 | Nichia Chem Ind Ltd | Positive electrode active substance for use in lithium secondary battery |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2006261132A (en) | 2006-09-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4736943B2 (en) | Positive electrode active material for lithium secondary battery and method for producing the same | |
| JP4465264B2 (en) | Lithium metal composite oxide having excellent life characteristics and safety and method for producing the same | |
| JP2001143703A (en) | Positive electrode active substance for use in lithium secondary battery | |
| JP3860542B2 (en) | Positive electrode active material for lithium secondary battery and method for producing the same | |
| JP4954451B2 (en) | Positive electrode material for lithium secondary battery and method for producing the same | |
| JP6611438B2 (en) | Positive electrode material for non-aqueous electrolyte secondary battery, method for producing the same, and non-aqueous electrolyte secondary battery | |
| JP6650956B2 (en) | Positive active material for lithium ion battery, lithium ion battery, and method for producing positive active material for lithium ion battery | |
| JP2012155994A (en) | Electrode for solid-state battery | |
| JP5961911B2 (en) | Mixed cathode active material with improved output characteristics and safety and lithium secondary battery including the same | |
| JP2002175808A (en) | Lithium/transition metal compound oxide for cathode active material of lithium secondary battery, and its manufacturing method | |
| JP4604347B2 (en) | Method for producing positive electrode active material for non-aqueous electrolyte secondary battery | |
| JP6303279B2 (en) | Positive electrode active material particle powder, method for producing the same, and nonaqueous electrolyte secondary battery | |
| WO2017034001A1 (en) | Positive electrode active material for nonaqueous electrolyte secondary cell, method for manufacturing same, and nonaqueous electrolyte secondary cell | |
| JP2014116161A (en) | Method of producing positive electrode active material for lithium ion secondary battery | |
| JP2023508021A (en) | Positive electrode active material, manufacturing method thereof, and lithium secondary battery including the same | |
| JP6068247B2 (en) | Positive electrode material for non-aqueous electrolyte lithium ion secondary battery and non-aqueous electrolyte lithium ion secondary battery using the positive electrode material | |
| JP5300370B2 (en) | Cathode active material for non-aqueous electrolyte secondary battery, method for producing the same, and non-aqueous electrolyte secondary battery | |
| JP2006012616A (en) | Positive electrode material for lithium secondary battery and its manufacturing method | |
| KR102015425B1 (en) | Positive active material for lithium secondary battery with copper-manganese coating thereon, lithium secondary battery having the same, and method for manufacturing thereof | |
| JP2014167873A (en) | Negative electrode active material for lithium ion secondary battery, process of manufacturing the same, and lithium ion secondary battery | |
| JP7333477B2 (en) | Positive electrode active material, manufacturing method thereof, and lithium secondary battery including the same | |
| JP7302826B2 (en) | Lithium-manganese composite oxide and method for producing the same | |
| JP2005067924A (en) | Method for manufacturing polyanion type lithium iron multiple oxide and battery using the same | |
| JP3835235B2 (en) | Cathode active material for non-aqueous electrolyte secondary battery and method for producing the same | |
| JP2000128540A (en) | Manganese oxide, its production, lithium manganese multiple oxide produced with the same and production of the same multiple oxide |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20061110 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20070517 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100323 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100524 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20100720 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20101007 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20101027 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20110405 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20110418 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 Ref document number: 4736943 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140513 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140513 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| EXPY | Cancellation because of completion of term |