WO2021162554A1 - Means and methods for determining mesenchymal stromal cell properties. - Google Patents
Means and methods for determining mesenchymal stromal cell properties. Download PDFInfo
- Publication number
- WO2021162554A1 WO2021162554A1 PCT/NL2021/050096 NL2021050096W WO2021162554A1 WO 2021162554 A1 WO2021162554 A1 WO 2021162554A1 NL 2021050096 W NL2021050096 W NL 2021050096W WO 2021162554 A1 WO2021162554 A1 WO 2021162554A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- msc
- cells
- fold
- markers
- hla
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0662—Stem cells
- C12N5/0663—Bone marrow mesenchymal stem cells (BM-MSC)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/20—Cytokines; Chemokines
- C12N2501/23—Interleukins [IL]
- C12N2501/2301—Interleukin-1 (IL-1)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/20—Cytokines; Chemokines
- C12N2501/24—Interferons [IFN]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/20—Cytokines; Chemokines
- C12N2501/25—Tumour necrosing factors [TNF]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2502/00—Coculture with; Conditioned medium produced by
- C12N2502/11—Coculture with; Conditioned medium produced by blood or immune system cells
- C12N2502/1114—T cells
Definitions
- the invention relates to methods of and kits for determining mesenchymal stromal cell (MSC) properties.
- MSC mesenchymal stromal cell
- Such properties include, the capacity to inhibit an immune response. For example proliferation of target cells such as T-cells, B- cells, NK cells and other immune cells and/or monocyte/ macrophage polarization resulting in immune inhibition through the production of soluble mediators and induction of regulatory T -cells (T Regs) through various mechanisms, including soluble factors such as CCL-18 and other factors.
- T Regs regulatory T -cells
- the invention also relates to a cell bank comprising MSC having such properties and to use of MSC having such properties,
- MSC are a heterogeneous population of cells present in various tissues which can differentiate into a variety of cell types, including osteoblasts, chondrocytes, and adipocytes. In addition to being important for the physiological repair of various mesenchymal tissues, including cartilage and bone, MSC play a substantial role in the regulation of blood cell development in the bone marrow.
- MSC graft- versus -host disease
- MSC intra-and extracellular molecules
- Many of these molecules are not expressed at steady state, but are upregulated in response to stimulation with proinflammatory molecules, such as cytokines. Not all inflammatory stimuli result in the same type and level of response. Whereas stimulation with some proinflammatory molecules Induce MSC with anti-inflammatory function, stimulation with others results in the generation of MSC with proinflammatory function.
- the present invention provides such methods and means to perform such test.
- the tests as disclosed herein allow for quantitative measurement of the biological activity and as such links to relevant biological properties of the cell therapy product.
- the means and methods of the invention may serve as a release test in future MSC manufacturing. Presently, no such standard test or assay is available.
- the invention provides a method comprising:
- cytokines selected from; interleukin (IL)17A, IL-1 ⁇ , oneostatin M (OSM), interferon g (IFN- ⁇ ), tumor necrosis factor a (TNF- ⁇ ), IL-13 and IL-4;
- MSC binding molecules that bind to an MSC -specific marker and one or more selected from: the extracellular markers CD 54, CD274, human leukocyte antigen (HLA)-DR, the intracellular markers COX-2 and indoleamine-pyrrole 2,3-dioxygenase (IDO); said method further comprising determining the presence of said MSC-specific marker and the fold increase of the expression of said one or more of CD54, CD274, HLA-DR, COX-2 and IDO markers in response to said one or more cytokines.
- HLA human leukocyte antigen
- IDO indoleamine-pyrrole 2,3-dioxygenase
- the invention also provides a kit for determining the capacity and/or potency of MSC to inhibit an immune response. For example proliferation of target cells such as T-ceils, B-eells, NK cells and other immune cells and/or monocyte/macrophage polarization resulting in immune inhibition through the production of soluble mediators and induction of T Regs, comprising binding molecules that hind to an MSC- specific marker and one or more of CD54, CD274, HLA-DR, COX-2 and IDO.
- target cells such as T-ceils, B-eells, NK cells and other immune cells and/or monocyte/macrophage polarization resulting in immune inhibition through the production of soluble mediators and induction of T Regs, comprising binding molecules that hind to an MSC- specific marker and one or more of CD54, CD274, HLA-DR, COX-2 and IDO.
- a cell bank comprising MSC of at least 10 different donors, wherein an MSC-specific marker is present on the MSC of each of said donors, and wherein each of said MSC exhibit a fold increase of the expression of one or more of the markers CD54, CD274, HLA-DR, COX-2 and IDO in response to culture with one or more cytokines selected from; IL-17A, IL-18, OSM, ⁇ RN-g, TNF- ⁇ , IL-13 and IL-4.
- a method of preventing and/or inhibiting an immune response in a subject comprising administering to said subject MSC of which the presence of an MSC- specific marker and fold increase of the expression of one or more of CD54, CD274, HLA-DR, COX-2 and IDO was determined using a method or a kit as described herein.
- a method for preventing and/or inhibiting graft versus host disease in a subject comprising administering to said subject MSC of which the presence of an MSC- specific marker and fold increase of the expression of one or more of CD54, CD274, HLA-DR, COX-2 and IDO has been determined using a method and/or kit as described herein.
- a method for determining the capacity and/or potency of MSC to inhibit an immune response for example proliferation of target ceils such as T-cells, B-ceils.
- target ceils such as T-cells, B-ceils.
- cytokines selected from: IL-17A, IL-16, OSM, lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4: - contacting said MSC with binding molecules that bind to an MSC-specific marker and one or more markers selected from:
- MSC mesenchymal stromal cells
- cytokines selected from: interleukin (IL)17A, IL-18, oncostatin M (OSM), interferon y ( lFN- ⁇ ), tumor necrosis factor a (TNF- ⁇ ), IL-13 and IL-4; contacting said cultured MSC with binding molecules that bind to an MSC-specific marker and one or more selected from: the extracellular markers CD54, CD274, human leukocyte antigen (HLA)-DR, and the intracellular markers cyclo-oxygenase-2 (COX- 2) and in doleamlne -pyrrole 2,3-dioxygenase (IDO); and determining from the presence of said MSC-specific marker and the expression of said one or more of CD54, CD274, HLA-DR and IDO markers the capacity' and
- a method for treating a subject in need of MSC based therapy with a population of MSC cells having therapeutic potential comprising: a) validating therapeutic potential of the cell population bye providing MSC; - culturing said MSC in the presence and in the absence of one or more cytokines selected from: interleukin (IL)17A, IL-16, oncostatin M (OSM), interferon g (IFN- g), tumor necrosis factor a ( TNF- ⁇ ), IL-13 and IL-4; contacting said MSC with binding molecules that bind to an MSC-specific marker and one or more selected from: the extracellular markers CD 54, CD274, human leukocyte antigen (HLA)-DR, and the intracellular markers cyclooxygenase' 2 (COX-2) and indoleamine- pyrrole 2, 3 -dioxygenase (IDO); b) determining the presence of said MSC-speeifie marker and the fold increase of the expression of said
- a method for determining a therapeutic potential of mesenchymal stromal cells (MSC) to inhibit an immune response comprising:
- cytokines selected from: interleukin (IL)17A, IL-1 ⁇ , oneostatin M (OSM). interferon g (IFN- g), tumor necrosis factor a (TNF- ⁇ ), IL-13 and IL-4;
- MSC-specific marker binding molecules that bind to an MSC- specific marker and one or more selected from: the extracellular markers CD 54, CD274, human leukocyte antigen (HLA)-DR, and the intracellular markers cyclo- oxygenase-2 (COX-2) and indoleamine-pyrrole 2,3-dioxygenase (IDO); said method further comprising determining the presence of said MSC -specific marker and the fold increase of the expression of said one or more of CD 64, CD274, HLA- DR and IDO markers in response to said one or more cytokines and determining from said presence and said fold-increase the therapeutic potential.
- HLA human leukocyte antigen
- COX-2 cyclo- oxygenase-2
- IDO indoleamine-pyrrole 2,3-dioxygenase
- MSC cells for use in a method of treatment of a subject that: is exhibiting or is at risk of exhibiting an undesired immune response wherein said MSC cells have been determined to have therapeutic potential with a method as described herein,
- Described herein is a method comprising: - providing MSC; culturing said MSC in the presence and in the absence of one or more cytokines selected from: IL-17A, EL- IB, OSM, lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4; contacting said MSC with binding mol etudes that bind to an MSC- speeifie marker and one or more selected from: the extracellular markers CD 64, CD274, HLA-DR, the intracellular markers COX-2 and IDO; said method further comprising determining the presence of said MSC-specific marker and the fold increase of the expression of said one or more of CD64, CD274, HLA-DR, COX-2 and IDO markers in response to said one or more cytokines.
- cytokines selected from: IL-17A, EL- IB, OSM, lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4
- the disclosure describes a method for determining if MSC have a capacity to inhibit an immune response, for example inhibition of proliferation of target cells such as T-cells, B-cells, NK cells and other immune cells and/or monocyte/macrophage polarization resulting in immune suppression through the production of soluble mediators and induction of regulatory T-cells (T Regs), comprising: providing MSC; - culturing said MSC in the presence and in the absence of one or more cytokines selected from: IL-17A, IL-lB, GSM, lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4; contacting said MSC with binding molecules that bind to an MSC- speeifie marker and one or more selected from: the extracellular markers CD 54, CD274, HLA-DR, the intracellular markers COX- 2 and IDO; said method further comprising determining the presence of said MSC 'Specific marker and the fold increase of the expression of said one or more of CD54, CD274, HLA-DR
- the invention also provides a method for determining the capacity anti/or potency of mesenchymal stromal cells (MSC) to inhibit an immune response comprising providing MSC; culturing said MSC in the presence of one or more cytokines selected from: interleukin (IL)17A, 1L-18, oncostafcin M (OSM), interferon g ( lFN- ⁇ ), tumor necrosis factor a (TNF- ⁇ ), IL-13 and IL-4; contacting said cultured MSC with binding molecules that hind to an MSC -specific marker and one or more selected from: the extracellular markers CD54, CD274, human leukocyte antigen (HLA)-DR, and the intracellular markers cyclo-oxygenase- 2 (COX- 2) and indoleamine-pyrrole 2,3-dioxygenase (IDO); and determining from the presence of said MSC-specific marker and the expression of said one or more of CD 54, CD274, HLA-DR and IDO markers the
- a method for testing MSC as described herein comprises the method described herein above directed towards determining if MSC have a capacity to inhibit an immune response.
- Inhibition of an immune response can be inhibition of proliferation of target cells such as T-cells, B-cells, NK cells and other immune cells and/or monocyte/maerophage polarization resulting in immune suppression through the production of soluble mediators and induction of T Regs,
- target cells such as T-cells, B-cells, NK cells and/or monocyte/maerophage polarization.
- inhibition of proliferation of target cells such as T-cells and B-cells and/or monocyte/maerophage polarization.
- a method for testing MSC as described herein also refers to a method for determining the potency of MSC to inhibit an immune response.
- Inhibition of an immune response can be inhibition of proliferation of target cells such as T-cells, B-ceils, NK cells and other immune cells and/or monocyte/macrophage polarization resulting in immune suppression through the production of soluble mediators and induction of T Regs.
- target cells such as T-cells, B-ceils, NK cells and other immune cells and/or monocyte/macrophage polarization resulting in immune suppression through the production of soluble mediators and induction of T Regs.
- target ceils such as T-eells, B- cells, NK cells and/or monocyte/macrophage polarization.
- inhibition of proliferation of target cells such as T-cells and B-cells and/or monocyte/macrophage polarization.
- the capacity and the potency are measures for the quality of MSC for use in anti-inflammatory and regenerative settings, MSC batches that meet the criteria set herein are suitable for administration to a human with the purpose of reducing an undesired inflammatory immune response and/or enhancing regeneration in the thus transplanted human host.
- HLA-DR, COX- 2 and IDO markers in response to culture with one or more cytokines as disclosed herein indicate that such MSC have a high potency and/or capacity to inhibit an immune response when administered to a subject.
- MSC with such potency or capacity are very suitable for administration to subjects in need thereof,
- MSC mesenchymal stromal cells. These stromal cells are sometimes also referred to as mesenchymal stem cells, marrow stromal cells or multipotent mesenchymal stromal cells etc, MSC are adherent multipotent stromal cells that as a population can differentiate into a variety of cell types, including osteoblasts ⁇ hone cells), chondrocytes ⁇ cartilage cells), myocytes (muscle cells) and adipocytes (fat cells) . MSC have a capacity for self- renewal while maintaining their multipotency, MSC have an effect on innate and specific immune cells, MSC can produce a range of molecules having immunomodulatory effects.
- the cells can be derived from various regions of the body such as for instance, bone marrow, umbilical cord, adipose tissue, amniotic fluid, and molar cells.
- MSC from different sources typically exhibit similar functional properties, although the magnitude of the effects may vary.
- MSC have an effect on immune cells such as macrophages, neutrophils, NK cells, mast cells and dendritic cells in innate immunity. MSC are able to migrate to the site of injury where they can exhibit an anti-inflammatory effect or modulate immune responses using intermediate cells such a macrophages.
- an anti-inflammatory effect is preferably estimated on the basis of a capacity to inhibit immune responses, such as inhibition of proliferation of target cells such as T-cells, R- eelis, NK cells and other immune cells and/or monoeyte/macrophage polarization resulting in immune suppression through the production of soluble mediators and induction of T Regs,
- MSC-specific marker such a marker can be: CD73, CD90, CD 105, CD29, HLA-ABC, CD 166, CD146, CD44, CD 140a,
- CD 140b These markers are well known in the art. Antibodies and other binding molecules with which these markers can be visualized are commercially' available.
- CD 140b is used as an MSC- specific marker.
- CD 90, or CD 105 is used as an MSC- specific marker.
- CD 73 is used as an MSC-specifle marker.
- MSC -specific markers are also markers that specifically detect subsets of MSCs. Examples of such subset markers are CD 13, MSCA-1, CD 56, CD271, CD362 and Stro-1. Other MSC markers will likely be developed further and such are also included in the invention.
- the expression is of course measured and/or fold increase determined for the same binding molecules that the MSC are contacted with.
- MSC contacted with binding molecules for one or more markers selected from: the extracellular markers CD64, CD274, HLA-DE, the intracellular markers COX-2 and IDO it is preferred that the MSC are contacted with binding molecules for two or more of the markers, preferably 3 or more of the markers, more preferably 4 or more of the markers. Increasing the number of markers increases the accuracy of the determination.
- the markers are selected from CD54, CD274, HLA-DR, COX-2 and IDO.
- markers CD54, CD274, HLA-DE and IDO Preferably from markers CD54, CD274, HLA-DE and IDO. It is preferred that the markers at least comprise CD54 and IDO, preferably at least CD 54, IDO and CD274, more preferably at least CD54, IDO, CD274 and HLA-DR,
- the MSC are contacted with binding molecules for two or more markers selected from: the extracellular markers CD54, CD274, HLA-DR.
- the intracellular markers COX-2 and IDO Preferably with binding molecules for three or more markers selected from: the extracellular markers CD54, CD274, HLA-DE, the intracellular markers COX-2 and IDO.
- binding molecules for all of the markers CD54, CD274, HLA-DR, COX-2 and IDO are contacted with binding molecules for two or more markers selected from: the extracellular markers CD54, CD274, HLA-DR.
- the MSC are contacted with binding molecules for two or more markers selected from: the extracellular markers CD54, CD274, HLA-DR, the intracellular marker IDO.
- binding molecules for three or more markers selected from: the extracellular markers CD54, CD274, HLA-DR, the intracellular marker IDO Preferably with binding molecules for the four markers selected from: the extracellular markers CD54, CD274, HLA-DE, and the intracellular marker IDO.
- the MSC-speeifie marker is present on at least 90 % of the cells and the fold increase of expression of CD54 is at least 10- fold; of CD274 is at least 8-fold; of HLA-DR is at least 8-fold preferably at least 25-fold, of COX-2 is at least 3-fold and/or of IDO is at least 5-faid.
- Biological activities of MSC include, but are not limited to, inhibition of T-cell proliferation and differentiation of monocytes to DC, Where herein reference is made to the terra “inhibit” in relation to MSC, the term refers to the capacity of MSC to prevent or decrease the extent of a process or action.
- T- ceils are a type of lymphocyte which develop in the thymus gland and play a central role in the adaptive immune response, T-cells have an important role in controlling and shaping the immune response by providing a variety of immune- related functions. Proliferation of T-cells is associated with increased immune-related activity . Methods of inducing T-eell proliferation are known in the art. An exemplary method is described in the examples, Regulatory T-cells, or T Regs are also known as suppressor T-cells, They are a subpopulation of T-cells that modulate the immune system, prevent auto-immune disease, and maintain tolerance to self-antigens.
- B-cel!s are a type of lymphocyte that secretes antibodies. They are part of the adaptive immune response. In addition, B-eelis can also present antigens and secrete cytokines.
- NK-eelis are a type of cytotoxic lymphocytes, that is importan t for the immune system. They derive from the same progenitor cell as T- and B-cells, but are part of the innate immune system. NK-cells are best known for killing viral!y infected cells, and detecting and controlling early signs of cancer.
- Monocytes are a type of leukocyte which can polarize towards DC or macrophages under inflammatory conditions.
- An exemplary method for polarizing monocytes to DC is described in the examples.
- DC are antigen-presenting cells of the mammalian immune system. Their main function is to process antigen material and present it on the cell surface to T-cells, resulting in T-cell activation and/or proliferation.
- Macrophages are specialized cells involved in the detection, phagocytosis and destruction of e.g. microorganisms and dead cells,
- MSC can he provided freshly isolated from tissue.
- MSC can be provided from an existing culture.
- MSC are preferably obtained from frozen/eryopreserved storage. After thawing and washing such cells can be used directly or first be cultured.
- a method as described herein comprises providing MSC; culturing said MSC in the presence and in the absence of one or more cytokines selected from: IL-17A, IL-lB, OSM, 1FN-Y, TNF- ⁇ , IL-13 and IL-4; contacting said MSC with binding molecules that bind to an MSC-sspecific marker and one or more selected from: the extracellular markers CD54, CD274, HLA-DR, the intracellular markers COX-2, and IDO; and determining the presence of said MSC- specific marker and the fold increase of the expression of said one or more of CD54, C-D274, HLA-DR, COX-2 and IDO markers in response to said one or more cytokines.
- cytokines selected from: IL-17A, IL-lB, OSM, 1FN-Y, TNF- ⁇ , IL-13 and IL-4
- Cytokines are a category of small proteins that are important in cell signaling. Cytokines include chemokines, interferons, interleukins, lymphokines, and tumor necrosis factors. Cytokines have been shown to be involved in autocrine, paracrine and endocrine signaling as immune-modulating agents. They act through receptors, and have an effect on the immune system; cytokines can modulate the balance between humoral and cell-based immune responses, and can regulate the maturation, growth, and responsiveness of particular cell populations. They are important in health and disease, specifically In host responses to infection, immune responses, inflammation, trauma, sepsis, cancer, and reproduction.
- a proinflammatory cytokine is a cytokine with an inflammatory stimulatory effect
- a proinflammatory cytokine is often secreted from cells that promote inflammation, like (helper) T-cells
- Inflammatory cytokines have pleiotropic effects that together have an upregulating effect on inflammatory reactions.
- Proinflammatory cytokines include, but are not limited to: IL-1 ⁇ , IL-4, IL-5, IL-6, IL-8, IL-12, IL-13, 1L17A, IL-18, TNF- ⁇ , lFN- ⁇ , granulocyte- macrophage colony stimulating factor (GM-CSF), GSM, MCP-1, monocyte chemoattractant protein- 1
- MCP-1 Chemokine C-C motif ligand 5 (CCL5)/regulated on activation, normal T-eeil expressed and secreted (RANTES), macrophage inflammatory protein (MIP-lu),
- the cytokines IL-17A, IL-1 ⁇ , OSM, lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4 are well known in the art and suitable sources of cytokines are available to the skilled person.
- the cytokines are typically used in saturating amounts which for the indicated cytokines amounts to a concentration in the range of 0.1 ng/ml to 100 ng/uil. Suitable concentrations for the cytokines are indicated in the examples and are typically in the range of 5-60 ng/ml.
- TNF- ⁇ is preferably present in a concentration of at least lng/ml.
- lFN- ⁇ is preferably present in a concentration of at least 0.33 ng/ml.
- IL-17A is preferably present in a concentration of at least 50 ng/ml.
- IL-1 ⁇ is preferably present in a concentration of at least 1 ng/ml.
- OSM is preferably present in a concentration of at least 20 ng/ml.
- IL-13 is preferably present in a concentration of at least 20 ng/ml.
- IL-4 is preferably present in a concentration of at least 20 ng/ml.
- the amount of it used can be adjusted as is typically done by the person skilled in the art.
- the particular activity (quality) of a certain batch of cytokine, antibody or other binding molecule can suitably be determined on arrival of the batch.
- IL-17A, IL-18, OSM, lFN- ⁇ , TNF-o, IL-13 and IL-4 alone or combinations thereof can be used in the culture of MSC as described herein.
- the eytokine(s) used in the culture are one or more of IL-17A, IL-1 ⁇ , OSM, IFN-Y, TNF- ⁇ and IL-13; preferably one or more of IL-17A, IL-1 ⁇ and OSM; or preferably one or more of lFN- ⁇ , TNF- ⁇ or IL-13.
- a combination of two or more of the mentioned cytokines is used, preferably 3, 4, 5, 6 or all 7 of said cytokines.
- a combination of 6 is used, preferably of the cytokines L-17A, IL-1 ⁇ , OSM, lFN- ⁇ , TNF- ⁇ and IL-13.
- a combination of 3 is used.
- the combination of three is IL- G7A, IL-1 ⁇ and GSM,
- the combination of three is lFN- ⁇ , TNF- ⁇ or IL-13.
- the combination of two is IFN-Y and TNF- ⁇ .
- Suitable cultures with one cytokine are cultures with lFN- ⁇ or with IL-16,
- the cytoklneis) used in a method of the invention is/are lFN- ⁇ , IL-1 ⁇ , or the combination of lFN- ⁇ and TNF- ⁇ .
- the one cytokine or combination of cytokines is used that routinely gives a suitable and preferably the highest difference in expression of CD54, CD274, HLA-DE, COX-2 and/or IDO between the two cultures of a typical MSC preparation.
- This typical preparation can be a standardized preparation of MSC or model cells or model cell lines that are a good tell-tale for the effect of the cytokines on MSC.
- the combination of cytokines is determined by measuring the cytokines present in the MSC recipient.
- the markers CD54, CD274, HLA-DR, COX-2 and IDO are well known in the aid. Antibodies and other binding molecules with which these markers can be visualized are commercially available.
- the MSC that are to be tested are preferably a representative sample of a larger batch of MSC of which the functionality is to be predicted.
- the culturing in the presence or absence of certain cytokine(s) is typically done by culturing two samples of the same batch of MSC separately in different cultures, one with and one without the mentioned eytokine(s).
- the cultures are typically performed in parallel but not necessarily so.
- the culture without a cytokine can also be a historical reference. For instance of a different batch of MSC cultured in the absence of said cytokine(s).
- Cultures with and without cytokines are typically essentially the same hut for the presence of the cytokine.
- the representative MSC sample is split into two or more aliquots and one aliquot is cultured in the absence of the indicated eytokine(s) and another aliquot is cultured in the presence of the indicated cytokine(s), A difference in staining of the MSC of the two cultures is indicative for the functionality of the cells.
- MSC are preferably ⁇ cultured at 37°C and 5% C02. Methods for culturing MSC are described in the examples section. Effects of stimuli, such as cytokines, on MSC may be risible directly after culture or after an incubation period.
- Cytokines are a broad category of small proteins that are important in inducing signaling in cells. Cytokines have been shown to he involved in autocrine, paracrine and endocrine signaling as immune- modulating agents. Cytokines include ehemokines, interferons, interleukins, lymphokines, and tumor necrosis factors. They act through cell surface receptors, and are especially important in the immune system ; cytokines modulate the balance between humoral immunity and cell based immune responses, and regulate the maturation, growth, and responsiveness of particular cell populations.
- a proinflammatory cytokine is a type of signaling molecule that is secreted from immune cells that promote inflammation, like (helper) T 'Cells
- Inflammatory cytokines play an important role in mediating the innate immune response and are involved in the upregulation of inflammatory reactions
- Proinflammatory cytokines include, but are not limited to: IL-1 ⁇ , IL-4, IL-5, IL-6, IL-8, IL-12, IL-13, IL17A, IL-18, TNF- ⁇ , lFN- ⁇ , granulocyte-macrophage colony stimulating factor (GM-CSF), OSM, MCP-1, CCLo/RANTES, MIP-la, MIPlB, CD40L.
- a method for testing MSC as described herein preferably comprises providing MSC: optionally thawing said MSC when said MSC are retrieved from storage; and culturing said MSC in presence and in the absence of one or more of the mentioned cytokines IL-17A, IL-18, OSM, lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4.
- the method preferably further comprises contacting said MSC with binding molecules that bind to an MSC- speeific marker and one or more selected from; the extracellular markers CD 64, CD274, HLA-DR, the intracellular markers COX- 2 and IDO.
- Contacting said MSC with binding molecules preferably comprises: contacting said MSC with binding molecules that bind to an MSC-speelfie marker, and one or more selected from: the extracellular markers CD 54, CD274, HLA-DR; washing and permeabilizing said MSC; and contacting said MSC with a binding molecule that hinds said intracellular markers COX- 2 and/or IDO,
- the capacity and/or potency of said MSC is determined on the basis of the expression of an MSC-speciflc marker on said cells, and the fold Increase in expression of the markers CD54, CD274, HLA-DR, COX-2 and/or IDO of MSC when cultured in the presence of the cytokine(s) when compared to the absence thereof.
- the MSC are said to have the capacity when said MSC- specific marker is present on at least 90 % of the ceils and the fold increase of expression of CD54 is at least 3-fold, preferably at least 5 fold and more preferably at least 10-fold for CD54; is preferably at least 2-fold, preferably at least 4-fold and more preferably at least 8-fold for CD274; is preferably at least 5-fold, preferably at least 8 fold and more preferably at least 16-fold and more preferably at least 25-fold for HLA-DR, is preferably at least 2-fold, and more preferably at least 3-fold for COX-2, and/or is preferably at least 3- fold and more preferably at least 5-fold for IDO.
- MSC are said to have the capacity when said MSC -specific marker Is present on at least 90 % of the cells and the fold increase of expression of CD54 is at least 10-fold; of CD274 is at least 8-fold; of HLA-DR is at least 8-fold preferably at least 25-fold, of COX-2 is at least 3-fold and/or of IDO is at least 5-fold.
- the MSC are said to have the potency when said MSC-specific marker is present on at least 90 % of the ceils and the fold increase of expression of CD54 is at least 3-fold, preferably at least 5 fold and more preferably at least 10-fold for CD54; is preferably at least 2-fold, preferably at least 4-fold and more preferably at least 8- fold for CD274; is preferably at least 5-fold, preferably at least 8 fold and more preferably at least 16-fold and more preferably at least 25-fold for HLA-DR, is preferably at least 2-fold, and more preferably at least 3-fold for COX-2, and/or is preferably at least 3- fold, and more preferably at least d-fold for IDO.
- the MSC are said to have the potency when said MSC-specific marker is present on at least 90 % of the cells and the fold increase of expression of CD54 is at least 10-fold; of CD274 is at least 8-fold; of HLA-DR is at least 8-fold preferably at least 25-fold, of COX-2 is at least 3-fold and/or of IDO is at least 5-fold,
- the invention provides a method for determining the capacity and/or potency of mesenchymal stromal cells (MSC) to inhibit an immune response comprising providing MSC; culturing said MSC in the presence of one or more cytokines selected from : interleukin (IL)17A, lL-18, oneostatin M (OSM), interferon g ( lFN- ⁇ ), tumor necrosis factor a (TNF- ⁇ ), IL-13 and IL- 4; contacting said cultured MSC with binding molecules that hind to an MSC- specific marker and one or more selected from: the extracellular markers CD54, CD274, human leukocyte antigen (HLA)-DR, and the intracellular markers cyclo-oxygenase- 2 (COX- 2) and indoleamine-pyrroie 2,3-dioxygenase (IDO); and determining from the presence of said MSC-specific marker and the expression of said one or more of CD 54, CD274, HLA-DR and I
- the reference is a parallel sample of the same MSC culture in the absence of said one or more cytokines.
- the comparison of the reference and the cytokine cultured sample preferably yields a score for the relative increase of the expression of said one or more of CD 54, CD274, HLA-DR and IDO markers in said cytokine cultured sample.
- the score is preferably a fold-increase of expression of one or more of said markers in the cytokine cultured sample when compared to a reference MSC population cultured in the absence of said cytokines.
- Said MSC-specific marker is preferably present on at least 90 % of the cells and the fold increase of expression of CD 54 is at least 3 -fold, preferably at least 5 fold and more preferably at least 10-fold for CD 54; is preferably at least 2 -fold, preferably at least 4-fold and more preferably at least 8-fold for CD274; is preferably at least 5 -fold, preferably at least 8 fold and more preferably at least 16-fold and more preferably at least 25-fold for HLA-DR, Is preferably at least 2-fold, and more preferably at least 3- fold for COX-2, and/or is preferably at least 3-fold, and more preferably at least 5-fold for IDO.
- the Invention further provides a kit comprising binding molecules that bind to an MSC-specific marker and one or more markers selected from: CD 54, CD274, HLA- DR, COX-2 and IDO,
- the kit comprises binding molecules that hind to an MSC-specific marker, CD54, CD274, HLA-DR, COX-2 and IDO.
- the kit comprises binding molecules that bind to CD73, CD54, CD274, HLA-DR, COX-2 and IDO.
- the kit is useful in a method as described herein. Such a kit is useful in methods for determining if MSC have a capacity to inhibit an immune response.
- the kit Is also useful in a method for determining the potency of MSC to Inhibit an immune response.
- inhibition of proliferation of target cells such as T-cells, B-eelis, NK cells and other immune cells and/or monocyte/macrophage polarization resulting in immune suppression through the production of soluble mediators and induction of T Regs.
- the kit is also useful in a method to predict recipient response to administration of said MSC.
- the kit preferably further comprises one or more cytokines selected from: IL17A, IL-18, OSM, IFN-y, TNF- ⁇ , IL-13, IL-4.
- cytokines selected from: IL17A, IL-18, OSM, IFN-y, TNF- ⁇ , IL-13, IL-4.
- the preferred number of cytokines and the preferred single or combinations of cytokines are as described elsewhere herein.
- a binding molecule as defined herein is a proteinaceous binding molecule.
- the binding molecule is typically a peptide, a cyclic or bieyclic peptide of up to and including 20 amino acids or a polypeptide having more than 20 amino acid residues.
- Proteinaceous binding molecules Often these include one or more complete or derivative antibody variable domains.
- Non-limiting examples are single chain Fv-fragments, monobodies, VHH, Fab -fragments.
- Derivative variable domains can be artificial or naturally evolved derivatives both belonging to the class of proteins that have the immunoglobulin fold. Examples of non-immunoglobulin fold containing proteinaceous binding moieties are the avimers initially developed by Amgen.
- a binding molecule can be any type of molecule that is capable of specifically binding to the indicated markers.
- Binding molecules as disclosed herein are preferably bivalent monospecific binding molecules. Binding molecules preferably comprise at least a variable domain of an antibody.
- the binding molecules are antibodies, preferably monoclonal antibodies, i.e. bivalent monospecific antibodies.
- Antibodies are well known, well defined molecules of which the production and the use in labelling of cells has matured to the extent that such use has become routine and robust, a characteristic that is highly desired in a method for testing MSC as described herein, A feature of such a test is that it is robust and easily incorporated in the procedure of a new lab without typically requiring extensive optimization. Such robustness is especially important when quantitative results are desired.
- HLA-DR, COX-2 and IDO markers is detected using antibodies.
- the presence of a MSC-specific marker can be detected using any specific binding molecule.
- the presence of said MSC-specific marker is detected with an antibody against an MSC-specific marker
- Binding molecules such as antibodies are preferably labelled with fluo.rochro.mos that are suitable for parallel flow cytometric analysis.
- the parallel flow cytometric analysis is preferably one wherein the signals of said fiuoroehromes can be acquired independently .
- the invention further provides a cell bank comprising MSC of at least 10 different donors, wherein an MSC -specific marker is present on the MSC of each of said donors, and wherein each of said MSC exhibit a fold increase of the expression of one or more of the markers CD54, CD274, HLA-DE, COX-2 and IDO in response to one or more cytokines selected from: 1L-17A, IL-18, QSM, lFN-g, TNF- ⁇ , IL-13 and 1L- 4,
- the cell bank is preferably one wherein the presence and/or the fold increase in expression of said markers of said MSC was determined using a method of testing MSC as described herein or a kit as described herein.
- the invention further provides a method of preventing and/or inhibiting an immune response in a subject, the method comprising administering to said subject MSC of which the expression of and MSC- specific marker and fold increase of the expression of one or more of the markers CD54, CD274, HLA-DE, COX-2 and IDO was determined using a method and/or a kit as described herein.
- the subject is exhibiting or is at risk of exhibiting an undesired immune response.
- the subject is exhibiting inflammatory bowel disease.
- the subject is the recipient of an allogeneic cell or organ transplant.
- the subject is diagnosed with GvHD.
- a method for preventing and/or inhibiting graft versus host disease in a subject comprising administering to said subject MSC of which the presence and fold increase of the expression of said markers has been determined using a method and/or a kit as described herein.
- Also provided is a method comprising: providing MSC; culturing said MSC in the presence and in the absence of one or more cytokines selected from: 1L-17A, IL-1B, OSM, lFN-g, TNF- ⁇ , IL-13 and 1L-4; contacting said MSC with binding molecules that bind to an MSC- specific marker and one or more selected from: the extracellular markers CD54, CD274, H LA-DR, the intracellular marker COX-2 and IDO; said method further comprising determining the expression of said MSC- specific marker and the fold increase of the expression of said one or more of CD 54, CD274, HLA-DE. COX-2 and IDO markers in response to said one or more cytokines.
- the fold increase as referred to in a method of treatment or population for use in a method of treatment as described herein is preferably at least 3-fold, preferably at least 5 fold and more preferably at least 10-fold for CD54; is preferably at least 2-fold, preferably at least 4-fold and more preferably at least 8-fold for CD274; is preferably at least 5-fold, preferably at least 8 fold and more preferably at least 16-fold and more preferably at least 25-fold for HLA-DR, is preferably at least 2-fold, and more preferably at least 3-fold for COX-2, and/or is preferably at least 3-fold, and more preferably at least 5-fold for IDO.
- the fold increase of expression is preferably at least: 10-fold for CD54; 8-fold for CD274; 8-fold and preferably 26-fold for HLA-DR, 3-fold for COX-2, and/or 5-fold for IDO.
- the disclosure provides a method comprising: providing MSC; optionally thawing said MSC, when said MSC are provided frozen; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, IL-1 ⁇ , OSM, lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4; contacting said MSC with binding molecules that bind to an MSC- specific marker and one or more selected from: the extracellular markers CD54, CD274, HLA-DR, the intracellular markers COX-2 and IDO; said method further comprising determining the presence of said MSC-specific marker and the fold increase of the expression of said one or more of CD54, CD274, HLA-DR, COX-2 and IDO in response to said one or more cytokines.
- the disclosure provides a method comprising: providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from:
- cytokines are present in a saturating amount, preferably in a concentration ranging from Q.lng/ml to lOOng/ml, contacting said MSC with binding molecules that bind to an MSC- specific marker and one or more selected from: the extracellular markers CD54, CD274, HLA-DR, the intracellular markers COX-2 and IDO; said method further comprising determining the presence of said MSC-specific marker and the fold increase of the expression of said one or more of CD54, CD274, HLA-DR, COX- 2 and IDO in response to said one or more cytokines.
- the disclosure provides a method comprising: providing MSC; culturing said MSC in presence and in the absence of IL-lti lFN- ⁇ , or IFN-Y and TNF- ⁇ ; wherein said cytokines are present in a saturating amount, preferably in a concentration ranging from Q.lng/ml to lOOng/ml, more preferably IL- 1B in a concentration of Ing/ml, IFN-y in a concentration of lOng/ml, or lFN- ⁇ in a concentration of lOng/ml and TNF- ⁇ in a concentration of Ing/ml.
- MSC-specific marker binds to an MSC- specific marker and one or more selected from: the extracellular markers CD54, CD274, HLA-DR, the intracellular markers COX- 2 and IDO; said .method further comprising determining the presence of said MSC- specific marker and fold increase of the expression of said one or more of CDS4, CD274, HLA- DR, COX-2 and IDO in response to said one or more cytokines.
- antibody refers to an immunoglobulin molecule that is typically composed of two identical parrs of polypeptide chains, each pair having one "heavy” (H) chain and one "light” (L) chain.
- Human light chains are classified as kappa (K) and lambda (l).
- Heavy chains are classified as mu. delta, gamma, alpha, or epsilon, and define the antibody's isotype as IgM, IgD, IgG, IgA, and IgE, respectively.
- Each heavy chain is comprised of a heavy chain variable region (abbreviated herein as HCVR or VH) and a heavy chain constant region.
- HCVR heavy chain variable region
- the heavy chain constant regions of IgD, IgG, and IgA are comprised of three domains, CHI, CH2 and CHS, and the heavy chain constant regions of IgM and IgE are comprised of four domains, CHI, CH2, CH3, and CH4.
- Each light chain is comprised of a light chain variable region ⁇ abbreviated herein as LCVS or VL) and a light chain constant region.
- the light chain constant region is comprised of one domain, CL.
- the constant regions of the antibodies may mediate the binding of the immunoglobulin to host tissues or factors, including various cells of the immune system (e.g., effector cells).
- VH and VL regions can he further subdivided into regions of hypervariability, termed complementarity determining regions (CDR), interspersed with regions that are more conserved, termed framework regions (FR),
- CDR complementarity determining regions
- FR framework regions
- Each VH and VL is composed of three CDEs and four FRs, arranged from the amino- terminus to carboxy-terminus in the following order: FR1, CDR1, FR2, CDR2, FR3, CDR3, FR 4.
- the variable regions of the light and heavy chain together form the antibody binding site and defines the specificity for the epitope.
- binding molecules are labeled with fiuoroehromes.
- Fiuoroehromes suitable for labeling binding molecules and detecting markers bound by labeled binding molecules are known in the art.
- fiuoroehromes used in a method or present in a kit as disclosed herein enable flow cytometric detection of binding molecules bound to their target.
- binding molecules of a method anti/or kit as disclosed herein are fluorescent! ⁇ 7 labeled.
- binding molecules that bind to the same marker are labeled with the same fluoroehrome.
- groups of binding molecule that bind different markers are labeled with distinct fiuoroehromes.
- fiuorescently labeled binding molecules of a method and/or kit as disclosed herein are fiuorescently labeled antibodies.
- Fiuorescently labeled antibodies can be commercially obtained from various companies, including, but not limited to: Bee ton Dickinson (BD), eBiosciences, Bio legend, Dako, Beckman Coulter, CYTOGNOS, Cal tag, Pharmlngen, Exbio, Sanquin, R&D systems, and Invitrogen,
- MSC are contacted with a saturating amount of fiuorescently labeled binding molecules or antibodies.
- the fiuoroehromes are selected for brightness, limited spectral overlap and limited need for compensation, stability, etc. (see: Kalina et al. Leukemia 2012; 26: 1986).
- all fluorochromes of a method and/or kit as disclosed herein can he acquired independently from each other.
- Fluorochromes suitable for a method and/or kit of the disclosure include, but are not limited to: pacific blue (PacB), brilliant violet 421 (BV421), Horizon V450, pacific orange (PacO), Horizon V60Q (HVoQQ), BV6I0, Khrome orange (KO), 00515, Horizon BB515, fluorescein isothiocyanate (FITC), Alexa488, phyeoerythrin (PE), peridinin chlorophyl protein/eyanine 5.6 (PerCP-CyS.5), PerCP, PE Texas Red, phycoerythrin/cyanine?
- PE-Cy7 allophycocyanine
- API allophycocyanine
- AFC-H7 allophycocyanine/hilite 7
- fluorescently labeled binding molecules of a method and/or kit as described herein comprise the fluorochromes PE, APC, APC-H7, BV421, PE- Cy7.
- a method and/or kit as described herein comprises PE- labeled antibodies that bind to CD54, APC-laheled antibodies that bind to CD274,
- the procedures and methods for testing MSC as described herein are preferably performed using standardized flow -cytometer settings, above the electronic noise and target values for the photomultiplier tube (PMT) set using rainbow beads. Multicolor immunostaining and flow -cytometry is preferably performed according to the so-called Euro Flow protocols as described by Van Dongen et al. (Leukemia 2012; 26: 1908) and by Kalina et al.
- a method as described herein comprises the use of software for data integration and multidimensional analysis of flow cytometry files.
- the combinations of fluorescently labeled binding molecules as disclosed can be used in combination with commercially available software tools.
- a method and/or kit of the disclosure is suitable for acquisition of fluorescence according to the standards of the Euro Flow consortium.
- standards include, but are not limited to: flow cytometry acquisition settings, antibody concentration, antibodies, fluorochromes, and combinations of fluorochromes.
- the disclosure provides a method comprising: providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, IL-16, OSM, IEN-g, TNF- ⁇ , IL-13 and IL-4: - contacting said MSC with antibodies that bind to an MSC -specific marker and one or more selected from: the extracellular markers CDS4, CD274, HLA- DR, the intracellular markers COX-2 and IDO; said method further comprising determining the presence of said MSC-specific marker and fold increase of the expression of said one or more of CD54, CD274, HLA- DR, COX-2 and IDO in response to said one or more cytokines.
- marker refers to proteins expressed by ceils, which can serve to help identify and classify cells, Extracellular .markers are present on the outside of the cell, intracellular markers are present on the inside of the cell. Some markers are unique for cells types, others are more widely expressed. Specific combinations of markers can predict certain capacities of cells.
- Markers suitable for, or that can he added to the method include, but are not limited to: CD la, CD2, CDS, CD4, CDS, CD6, CD7, CD8a, CD9, GDI la, CD lib, CD 11c, CD 14, CD16, CD 17, GDIS, CD19, CD20, CD21, CD22, CD23, CD24, CD2o, CD27, CD28, CD29, CD30, CD31, CD33, CD34, CD3S, CD36, CD37, CD38, CD40, CD41, CD42b, CD43, CD44, CD46, CD47, CD48, CD49d, CD50, CD51, CDS2, CDS3, CDo4, CD65, CD56, CD58, CD59, CD61, CD62L, CD62P, CD63, CD66a, CD 66b, CD66c, CD66d/e, CD69, CD71, CD72, CD73, CD79a, CD80, CD90, CD9S, CD97, CD98, CD99, CD 105,
- the disclosure provides a method comprising: providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-I7A, IL-1 ⁇ , OSM, lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4; contacting said MSC with antibodies that bind to an MSC- specific marker and two or more selected from: the extracellular markers CD54, CD274, HLA- DR, the intracellular markers COX-2 and IDO; said method further comprising determining the presence of said MSC-specific marker and fold increase of the expression of said two or more of CD54, CD274, HLA- DR, COX-2 and IDO in response to said one or more cytokines.
- cytokines selected from: IL-I7A, IL-1 ⁇ , OSM, lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4
- the disclosure provides a method comprising: providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, IL-1 ⁇ , OSM, IFN-y, TNF- ⁇ , IL-13 and IL-4; contacting said MSC with binding molecules that bind to an MSC specific extracellular markers and three or more of the extracellular markers CD 54, CD274, HLA-DR and the intracellular markers COX-2 and IDO said method further comprising determining the presence of said MSC-specific marker and fold increase of the expression of said three or more of CD54, CD274,
- HLA-DR HLA-DR
- COX-2 IDO in response to said one or more cytokines.
- the disclosure provides a method comprising: providing MSC; - culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-I7A, IL-16, OSM, lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4; contacting said MSC with binding molecules that bind to an MSC specific extracellular markers and CD54, CD274, HLA-DR and the intracellular markers COX- 2 and/or IDO said method further comprising determining the presence of said MSC-specific marker and fold increase of the expression of CD54, CD274, HLA-DR, COX-2 and/or IDO in response to said one or more cytokines.
- cytokines selected from: IL-I7A, IL-16, OSM, lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4
- a method wherein an intracellular marker is contacted with binding molecules comprises additional steps of washing, fixing, and permeabilizing the ceils.
- the binding molecules that bind to extracellular markers are washed away first.
- MSC are fixed.
- the MSC are permeabiiized, where after the cells are contacted with binding molecules that bind to intracellular markers.
- the disclosure provides a method comprising: providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, IL-lfi OSM, lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4; - contacting said MSC with binding molecules that bind to an MSC- specific marker and one or more selected from: the extracellular markers CD54, CD274, HLA-DR washing said MSC permeabilizing said MSC - contacting said MSC with binding molecules that bind to the intracellular markers COX-2 and/or IDO; said method further comprising determining the presence of said MSC-specific marker and fold increase of the expression of said two or more of CD54, CD274, HLA- DR, COX-2 and IDO in response to said one or more cytokines.
- cytokines selected from: IL-17A, IL-lfi OSM, lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4
- the disclosure provides a method comprising: providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, IL-1 ⁇ , OSM, lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4; contacting said MSC with binding molecules that bind to an MSC- specifie marker and two or more selected from: the extracellular markers CD 54, CD274, HLA-DR, and: washing said MSC; - permeabilizing said MSC; contacting said MSC with binding molecules that bind to the intracellular markers COX-2 and/or IDO; said method further comprising determining the presence of said MSC-specific marker and fold increase of the expression of three or more of CD54, CD274, HLA-DR, COX-2 and IDO in response to said one or more cytokines.
- cytokines selected from: IL-17A, IL-1 ⁇ , OSM, lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4
- the disclosure provides a method comprising: providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, 1L-1B, OSM, IRN-g, TNR-a, IL-13 and 1L-4; contacting said MSC with binding molecules that bind to an MSC- specific marker and extracellular markers CD 51.
- determining refers to the action of ascertaining or establishing by research or calculation.
- a capacity of MSC to inhibit an immune response For example inhibit proliferation of target cells such as T-ceils, B-eelis, NK cells and other immune cells and/or monoeyte/macrophage polarization resulting in immune suppression through the production of soluble mediators and induction of T Regs is determined on basis of the presence of an MSC-specific marker on the MSC and the fold increase of the expression of one or more other markers in response to one or more cytokines.
- presence of a marker
- fold increase the term refers to a ratio.
- the fold increase indicates the number of times the presence of a marker on MSC has increased as a result of contacting MSC with one or more cytokines.
- presence of a marker is deducted from the presence of a binding molecule bound to that marker.
- binding .molecule is labelled with a iluorochrome.
- the fold increase in presence of a marker is typically calculated by dividing the fluorescent signal of the marker on MSC after culturing in presence of one or more cytokines, by the fluorescent signal of the marker on MSC after culturing in absence of cytokines.
- Fold increase can also be referred to as fold induction.
- expression of a .marker
- the reference is to the amount of the marker protein present in or on the cell at the time of analysis. This is also referred to as the level of the m arker or protein.
- Fold increase in the expression and/or the level refers to the ratio of the expression and/or level determined using one condition and the expression anchor level determined in another condition.
- a capacity of MSC to inhibit an immune response For example inhibition of proliferation of target cells such as T-cells, B-cells, NK cells and other immune cells and/or monocyte/maerophage polarization resulting in immune suppression through the production of soluble mediators and induction of regulatory ⁇ -cells is determined on basis of the presence of an MBC-specIfic marker and the fold increase of one or more of CD.54, CD274, BLA-DR, COX-2 and IDO.
- the fold increase is at least 2, preferably at least 3-fold, more preferably at least 5.
- the fold increase of CD54 is at least 2-fold, preferably at least 3-fold, preferably at least 5-fold, more preferably at least 10-fold.
- the fold increase of CD274 is at least 2-fold, preferably at least 3-fold, preferably at least 5-fold, more preferably at least 8-fold.
- the fold increase of HLA-DR is at least 2-fold, preferably at least 3-fold, more preferably at least 5-fold, at least 10-fold, at least 20-fold, more preferably at least 25- fold.
- the fold increase of COX-2 is at least 2-fold, preferably at least 3-fold, in a further embodiment the fold increase of IDO is at least 2 -fold, preferably at least 3-fold, preferably at least 6-fold.
- the disclosure provides a method suitable for determining if MSC have a capacity to inhibit an immune response, for example inhibition of proliferation of target cells such as T-cells, B-cells, NK cells and other immune cells and/or monocyte/maerophage polarization resulting in immune suppression through the production of soluble mediators and induction of regulatory T-cells comprising: providing MSC; - culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, IL-1 ⁇ , OSM, IFN-y, TNF- ⁇ , IL-13 and IL-4; contacting said MSC with binding molecules that bind to an MSC- specific marker and one or more selected from: the extracellular markers CD 54,
- the disclosure provides a method suitable for determining if MSC have a capacity to inhibit an immune response, for example inhibition of proliferation of target cells such as T-cells, B-cells, NK cells and other immune cells and/or monocyte/maerophage polarization resulting in immune suppression through the production of soluble mediators and induction of regulatory T-cells comprising: - providing MSC: culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, 1L-1B, OSM, IRN-g, TNF- ⁇ , IL-13 and IL-4; contacting
- the disclosure provides a method suitable for determining if MSC have a capacity to inhibit an immune response, for example inhibition of proliferation of target cells such as T-cells, B-cells, NK cells and other immune cells and/or monocyte/maerophage polarization resulting in immune suppression through the production of soluble mediators and induction of regulatory T-cells comprising: providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, IL-1 ⁇ , OSM, lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4; - contacting said MSC with binding molecules that bind to an MSC- specific marker and one or more selected from: the extracellular markers CD 54.
- target cells such as T-cells, B-cells, NK cells and other immune cells and/or monocyte/maerophage polarization resulting in immune suppression through the production of soluble mediators and induction of regulatory T-cells
- cytokines selected from: IL
- CD274, HLA-DR the intracellular markers COX-2 and IDO; said method further comprising determining the presence of said MSC-specifie marker and fold increase of the expression of said one or more of CD54, CD274, HLA- DR, COX-2 and IDO in response to said one or more cytokines, wherein said MSC have said capacity if said one or more markers are increased at least 5-fold,
- the term ‘'ceil death” refers to the event of a biological cell ceasing to carry out its functions. Cell death may result from various causes, for example apoptosis, programmed cell death, mitotic catastrophe, necrosis, ischemic cell death and/or immunogenic ceil death.
- the term “cell viability” relates to the capacity of the cell to perform certain functions, such as metabolism, growth, reproduction, some form of responsiveness, and adaptability. Cell death and cell viability can be evaluated by a number of suitable assay’s known to the skilled person. Dye exclusion methods are frequently used as a measure to determine dead cells. Dyes as trypan blue do not easily pass the membrane of living cells hut will enter dead cells as these are not able to maintain the integrity of their cell membrane.
- a method suitable for determining if MSC have a capacity to inhibit proliferation of T- cells and/or inhibit differentiation of monocytes to DC further comprises contacting MSC with a viability stain and determining viability- of the MSC.
- MSC are contacted with a dye for exclusion together with binding mol etudes binding to extracellular markers.
- MSC preparations comprise preferably at least 80%, preferably at least 90%, and more preferably at least 96% viable cells.
- the disclosure provides a method comprising: - providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, IL-1 ⁇ , OSM, lFN- ⁇ , TNF- ⁇ , EL- 13 and IL-4; contacting said MSC with binding molecules that bind to an MSC- specific marker and one or more selected from: the extracellular markers CD 54, CD274, HLA-DR and a dye for exclusion of dead cells, and the intracellular markers
- COX-2 and IDO said method further comprising determining the presence of said MSC-specific marker and fold increase of the expression of said one or more of CD54, CD274, HLA- DR, COX-2 and IDO in response to said one or more cytokines and the viability of said MSC.
- kit refers to a set of articles or equipment needed for a specific purpose.
- a kit comprises binding molecules that bind to an MSC-specific marker and one or more of CD 54, CD274, HLA-DR, COX-2 and IDO.
- binding molecules are antibodies.
- a kit further comprises one or more proinflammatory cytokines, more preferably one or more cytokines selected from: IL17A, IL-18, OSM, IFN-y, TNF- ⁇ , IL-13, IL-4. More preferably lFN- ⁇ ; IL-1 ⁇ ; lFN- ⁇ and TNF- ⁇ ; IL17A,IL-1B and OSM; IL17A, IL-18, OSM, lFN- ⁇ , TNF- ⁇ and IL-13; IL17A, IL-18, OSM, lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4, or; lFN- ⁇ , TNF- ⁇ , IL-13 and IL-4.
- cytokines more preferably one or more cytokines selected from: IL17A, IL-18, OSM, IFN-y, TNF- ⁇ , IL-13, IL-4. More preferably lFN- ⁇ ; IL-1 ⁇ ; lFN- ⁇ and TNF- ⁇ ; IL17A,IL
- the term “ceil bank” refers to a facility that stores cells for the purpose of future use in a product or medicinal needs .
- a cell bank stores cryopreserved cells, Cryopreserved cells are typically stored in vials or freezing bags.
- the term “vial” refers to a small container, typically cylindrical, suitable for storing cells, including cryopreserved cells.
- one vial contains MSC of one donor.
- one vial contains MSC of more than one donor.
- the term “freezing bag” refers to a bag suitable for storing cells, including cryopreserved cells,
- one freezing contains MSC of one donor. In a further embodiment, one freezing bag contains MSC of more than one donor.
- presence and fold increase of markers is determined using a method and/or kit as disclosed herein.
- the disclosure provides a method for preventing and/or inhibiting an immune response in a subject, comprising administering to a subject
- MSC of which the presence of an MSC-specific marker and the fold increase of one or more of the markers CD54, CD274, HLA-DR, COX-2 and IDO was determined using a method and/or kit as disclosed herein.
- the subject is exhibiting or at risk of exhibiting an undesired immune response.
- Undesired immune responses include, but are not limited to: autoimmune disorders including inflammatory bowel disease, Type 1 diabetes, rheumatoid arthritis and multiple sclerosis, disorders associated with ado-immune responses, including solid organ transplantation and graft versus host disease, and (chronic or acute) inflammatory disorders including pulmonary emphysema, chronic kidney disease osteoarthritis, inflamed skin, brain lesions such as stroke, etc.
- the subject is exhibiting inflammatory bowel disease.
- the subject is the recipient of an allogeneic ceil or organ transplant
- the present disclosure provides a method for preventing and/or inhibiting graft versus host or host versus graft disease in a subject comprising administering to a subject MSC of which the presence of an MSC- specific marker and the fold increase of one or more of the markers CD54, CD274, HLA-DR, COX-2 and IDO was determined using a method and/or kit as disclosed herein.
- a “subject” is an human or an animal.
- Subjects include, but are not limited to, mammals such as humans, pig's, ferrets, seals, rabbits, cats, dogs, cows and horses, and birds such as chickens, ducks, geese and turkeys.
- a subject is a mammal.
- the subject is a human.
- the disclosure provides a cell therapy product for use comprising MSC of which the presence of an MSC-specific marker and the fold increase of one or more of the markers CD 54, CD274, HLA-DR, COX- 2 and IDO was determined using a method and/or kit as disclosed herein.
- a “cell therapy product'’ is a product suitable for cell therapy.
- ceil therapy this term is intended to refer to grafting, injecting, applying or implanting a cell therapy product into a subject
- to comprise and its conjugations is used in its non-limiting sense to mean that items following the word are included, but items not specifically mentioned are not excluded.
- verb “to consist” may be replaced by “to consist essentially of’ meaning that a compound or adjunct compound as defined herein may comprise additional components) than the ones specifically identified, said additional components) not altering the unique characteristic of the invention.
- treatment refers to reversing, alleviating, delaying the onset of, or inhibiting the progress of a disease or disorder, or one oi' more symptoms thereof, as described herein.
- treatment may be administered after one or more symptoms have developed.
- treatment may be administered in the absence of symptoms.
- treatment may be administered to a susceptible individual prior to the onset of symptoms (e.g., in light of a history of symptoms and/or in light of genetic or other susceptibility factors). Treatment may also be continued after symptoms have resolved, for example to prevent or delay their recurrence.
- lFN- ⁇ stimulation results in an increased capacity of MSC to inhibit T-eell proliferation
- IE- 18 stimulation enhances the capacity of MSC to inhibit DC differentiation.
- Antibody array-based analysis on culture supernatants (left) or cell pellets (right) of unstimulated and IFN-y-stimulaied MSC (n 3 MSC per stimulus). Significantly differentially expressed proteins showing a log2 (fold change) of ⁇ -0.5 or > 0.5 in the supernatant of unstimulated or IFN-y-treated stimulated MSC are depicted.
- Targets screened with different techniques (as indicated). Arrows indicate downregulated (white) and upregulated (black) genes as identified by mKNA sequencing. Numbers indicate the amount of antibodies used to identify the target. Numbers highlighted in white and black indicate downregulated and upregulated targets, respectively. The first number indicates the amount of antibodies identifying the target as a hit, followed by the number of antibodies that this target was screened for with the indicated technique. The column indicated with “FACS” shows the targets either analyzed (highlighted in grey) or confirmed (highlighted in black) by flow cytometry.
- Figure 7 Venn-diagram of the overlap in targets that were identified using the different IL-1 ⁇ -based screenings, as well as the FACS-eonfirmed hits. For mRNA hits; only those hits are shown that were also screened for with at least one of the other screening methods.
- Figure 8
- Targets and hits observed in IL-16 screening Targets that are screened using different approaches are indicated. Numbers indicate the amount of antibodies that identify the target using each technique. Numbers highlighted in black indicate upregulated targets, in which the first number indicates the amount of antibodies that identified the target as a hit, followed by the number of antibodies that this target was screened for with the indicated technique.
- the column indicated with "FACS” show the targets that were either screened (highlighted in grey) or confirmed (highlighted in black) by flow cytometry.
- MSC peripheral blood mononuclear cells
- PRMC peripheral blood mononuclear cells
- Y- cell proliferation is presented as percentage proliferation as compared to anti- CD3/CD28 bead stimulated PBMC.
- IDO and COX-2 inhibitors used were Cay 1058.1. and NS398, respectively.
- Blockade of IDO and COX-2 with enzyme- inhibiting drugs and their impact on the capacity of MSC to inhibit DC proliferation Purified CD14 + cells were co -cultured with MSC in the presence of GM-CSF and IL-4. Following 6 days of cultures, the ceils were analysed for CD la and CD 14 expression by flow cytometry. This assay was performed in the presence or absence DMSO, IDO inhibitor (INCB-024360) or COX-2 inhibitor (NS398). The percentage of CDla+CDl4- ceils after 6 days of co-culture with CD14+ monocytes is shown.
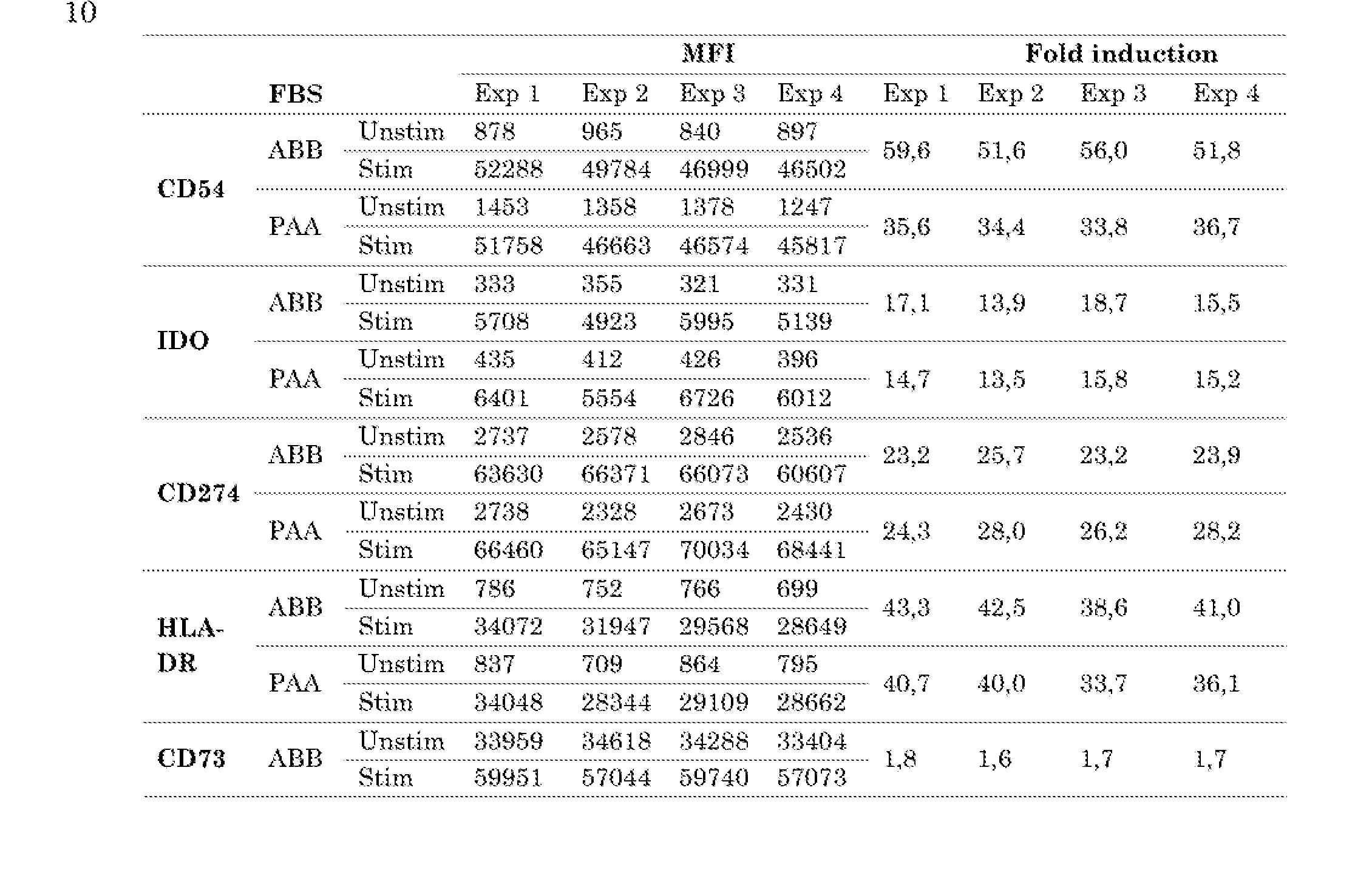
- Figure 14 The ratio of fold induction and of the mean fluorescence intensity (MFI) of MSC tested in the potency assay.
- the numbers refer to the different culture regimens. Each dot represents the ratio of fold induction by dividing the fold induction of experiment 1 by the fold induction obtained in experiment 2 (i.e. the duplo of experiment 1).
- N 4 MSC tested in 4 experiments.
- Upregulation of COX-2 was measured by flow cytometry using a PE -labelled anti- COX-2 antibody (clone AS67, BD Pharmingen).
- MSC mesenchymal cells
- pro-inflammatory molecules such as cytokines
- cytokines the effect of different cytokines on two anti-inflammatory capacities of MSC were tested, These two anti-inflammatory capacities were the capacity to inhibit T-cell proliferation, and the capacity to inhibit dendritic ceil (DC) differentiation.
- MSC were stimulated for different periods of time (as indicated in Figure 1) in the presence or absence of different pro-inflammatory molecules at the following concentrations' Poly (1:C) (20 pg/rnL; from Sigma Aldrich Chernie), IL-18 (1 ng/mL) (R&D Systems Inc.), lFN- ⁇ (10 ng/mL; Peprotech), TNF- ⁇ (1 ng/mL; Peprotech).
- MSC were plated at 16. ox 10 3 cells/cm 2 and cultured overnight in culture medium after which the culture medium was replaced by medium containing pro- inflammatory molecules.
- PBMC Human peripheral blood mononuclear cells
- T-cell-enrlehed PBMC Approximately' Ib.dcIO 3 MSC (either un- or pre-stimulated) and 1.0x10 5 freshly isolated T-cell-enrlehed PBMC were plated in a 96- wells V-shaped plate (Corning Inc.) In a MSCiPBMC ratio of 1:6 in RPMI 1640 culture medium (Gibco #22409-015) supplemented with Glutamax (lx, Gibco #35050-38), T-cell- enriched PBMC were stimulated with human T-cell-activator CD3+/CD2S+ Dynabeads (Thermo Fisher Scientific Inc.) in a celbhead ratio of 5:1.
- Approximately 3x10 3 MSC ⁇ either un- or pre-stimulated) and 1.0x10 6 freshly isolated monocyte- enriched PBMC were eo -cultured in 6- well plates in RPMI culture medium supplemented with IL-4 (10 ng/mL) and granulocyte-monocyte colony- stimulating factor (GM-CSF) (o ng/mL) (both from Invitrogen Corp.). After 2 days, half of the medium was replaced by cytokine-containing RPMI culture medium (IL-4 and GM-CSF at 20 ng/mL and 10 ng/mL, respectively). After 6 days, cells were analyzed by flow cytometry for expression of CD 14 and CD la.
- IL-4 ng/mL
- GM-CSF granulocyte-monocyte colony- stimulating factor
- MSC pre-stimulated with lFN- ⁇ were found to be most potent in inhibiting T- eell proliferation, followed by MSC pre-stimulated with TNF- ⁇ or IL-16 ( Figure 1, upper panel). Inhibition of dendritic cell differentiation was most potently induced by pre-stimulation with IL-16 ( Figure 1, lower panel).
- IL-l ⁇ most strongly induces the capacity of MSC to inhibit differentiation from monocytes to DCs.
- infra -and extracellular molecules have been identified to be implicated in the immunomodulatory action of MSC. Many of these molecules are not expressed at steady state, but tire upregulated in response to stimulation with proinflamraatory molecules, such as cytokines.
- hmMSC Human hone marrow MSC
- MSC were stimulated for different periods of time (as indicated) in the presence or absence of different pro-inflammatory molecules at the following concentrations: LPS (200 ng/niL), Poly (I:C) (20 pg/mL; both from Sigma Aldrich Chemie), IL-16 (1 ng/mL) (E&D Systems Inc.), lFN- ⁇ (10 ng/mL is default, 1 and 100 ng/ml when indicated), TNF- ⁇ (1 ng/.mL) (both Peprotech) or both iFN-g and TNF- ⁇ (10 ng/ml IFN- Y and 1 ng/ml TNF- ⁇ ).
- LPS 200 ng/niL
- Poly (I:C) 20 pg/mL; both from Sigma Aldrich Chemie
- IL-16 (1 ng/mL)
- lFN- ⁇ 10 ng/mL is default, 1 and 100 ng/ml when indicated
- TNF- ⁇ (1 ng/.mL) (both Peprotech
- RNA analysis by sequencing MSC were detached using trypsin/EDTA or TrypLE Select, resuspended in RNAlater and stored at -80°C.
- MSC were detached using trypsin/EDTA or TrypLE Select, resuspended in 350 uL RLT-buffer (Qlagen) containing Rib 6-mereaptoethanol (Sigma Aldrich Cheraie) and stored at -80°C.
- RLT-buffer Qlagen
- Rib 6-mereaptoethanol Sigma Aldrich Cheraie
- antibody array analysis MSC were cult ured in phenol -red free Optimum supplemented with penicillin/ streptomycin (Gibeo).
- MSC were detached using TrypLE Select and used for co-cultures.
- RNA Total RNA (mRNA, miRNA and other small RNAs) was isolated using a QIAsymphony using the miRNA CT 400 protocol and an elution volume of 100 m ⁇ The samples were processed using the NEBNext Ultra RNA Library Prep Kit and the protocol “NEBNext Ultra Directional RNA Library Prep Kit for IUumina”(NEB#E7420S/L). Briefly, mRNA was Isolated from total RNA using the oligo-dT magnetic beads. After fragmentation of the mRNA, a cDNA synthesis was performed. This was used for ligation with the sequencing adapters and PCR amplification of the resulting product. The quality' and yield after sample preparation was measured with a Fragment analyzer.
- the size of the resulting products was consistent with the expected size distribution, i.e. a broad peak between 300-500 bp.
- Clustering and DNA sequencing using the Illumina cBOT and HiSeqy 4000 was performed according to the manufacturer’s protocols. A concentration of 3.0 nM of DNA was used. Image analysis, base calling, and quality check was performed with the Illumina data analysis pipeline RTA v2,7.7 and Bel2fastq v2.17.
- the antibody microarrays (ScioCD version 1) were produced and hybridized by Sciomics (Sciomics, Germany). For hybridization, samples were labelled with NHS- esters of the fluorescence dye Dy-649 (Dyomies, Jena, Germany), Samples of unstimulated cells were used as reference sample and labelled with the fluorescent dye Dy- 549 (Dyomies) for competitive dual-color incubations. Scanning of the slides was performed with a PowerSeanner (Tecan) at a resolution of 10 pm, maintaining laser power and the photomultiplier constant. Spot segmentation was performed with GenePix Pro 6.0 software package (Molecular Devices, Sunnyvale, CA, USA).
- the spot signals were analysed with R-Bioeonductor using a one-factorial linear model fitted with LIMMA package, which resulted in a t-tesfc based on moderated statistics.
- a specialized invariant Lowess method was applied as described before (C, Schroder et al. Proteoraics Clin, Appl. 2013), In the analyses, duplicate spots were taken into consideration.
- a one-factorial linear model was fitted with LIMMA resulting in a two-sided t-iesfc based on moderated statistics. Negative fold change values indicate a lower abundance, positive values indicate a higher abundance of the given protein in the sample as compared to the unstimulated condition.
- MSC were washed with staining buffer (CSM, lx PBS with 0.5% bovine serum albumin and 0.02%» sodium azide, Fluidigm Sciences) and incubated with 1 ml CSM containing 1:500 diluted 500 pM rhodium DNA intercalator (Fluidigm Sciences) for 15 min at room temperature (RT) to stain dead cells.
- CSM staining buffer
- lx PBS with 0.5% bovine serum albumin and 0.02%» sodium azide
- Fluidigm Sciences 1 ml CSM containing 1:500 diluted 500 pM rhodium DNA intercalator (Fluidigm Sciences) for 15 min at room temperature (RT) to stain dead cells.
- RT room temperature
- Antibody staining reactions were performed in 100 pi final volume.
- MSC flow cytometry analysis
- MSC were incubated for 30 minutes at 4°C with antibodies against extracellular markers.
- the MSC were fixed (fixation/permeabilization buffer and diluent, eBioseience) for 20 minutes at room temperature (RT) and thereafter permeabilized (permeabilization buffer, eBioseience) and stained for the intracellular markers for 25 minutes at RT.
- eBioseience fixation/permeabilization buffer and diluent
- eBioseience permeabilized permeabilization buffer
- eBioseience permeabilization buffer
- MFI median fluorescent intensity
- CD9, C-D29 and CD39 were downregulated and CD 140b and CD273 were upregulated by MSC at the protein level following ⁇ RN-g stimulation.
- CyTOF- experiments identified up regulation of CD54 and downregulation of CD9, CD 103, CD 120a and CD 140a upon IL-1 ⁇ -stimulation ( Figure 6A).
- qRT PCR we identified upregulation of CNCL9, CXCLIO, CXCL11, TSG-6, IDO, COX-2,
- the MSC showed a different slope, suggesting that there might be differences between MSC products in the extent to which they respond to cytokine- stimulation with regards to upregulation of molecules and the capacity to modulate T-cell proliferation ⁇ figure 9).
- Example 3 Determine which of these molecules can be measured by flow cytometry in a reproducible and standardized fashion
- the target values for the PMTss were set using rainbow beads.
- the optimal fluorescent channels for visualizing the surface markers and intracellular stains were determined, taking into account the level of auto-fluorescenee of the MSC in a given channel and the expected expression level for a given target molecule.
- all antibodies were titrated till saturating levels. If chosen antibodies did not reach saturation and/or went out of the linearity range of the flow cytometer, additional antibody clones that recognized the same target and/or other iluoroehrome- conjugates were tested. In total 25 different antibodies and 10 different clones have been evaluated to come to the most optimal antibody panel.
- MSC flow cytometry analysis to validate rnENA and proteomics based screenings
- MSC were harvested and stained for flow cytometry analysis, MSC were incubated for 30 minutes at 4°C with antibodies against T-cell surface markers.
- the MSC were fixed (ilxatiuii/permeabilization buffer and diluent, eBioscience) for 20 minutes at room temperature (RT) and thereafter permeabilized (permeabilizaiion buffer, eBioscience) and stained for the intracellular markers for 25 minutes at RT, When cells were stained for intracellular IL-6, cells were incubated with Golgistop (RD Biosciences) overnight the morning before harvesting the cells.
- MSC The MSC were analysed by flow cytometry with the BD FACSTM Canto II cytometer (BD Bioseiences). Analysis of the data was performed with FlowJo software version 8.7.1 (Tree Star Inc., Ashland, OR, USA). For single cells the median fluorescent intensity (MFI) was obtained and fold induction was calculated by dividing the MFI of stimulated MSC by the MFI of unstimulated MSC,
- CD 106 was added to serve as an identifying marker for MSC.
- Alternatives for CD73 are CD90, CDIGo, CD29, HLA-ABC, CD 166, CD 146, CD44, CD 140a, CD 140b. All details on the antibodies that were evaluated and selected for the final panel are depicted in table 5.
- Example 4 Optimization of the in vitro MSC stimulation to obtain a robust and standardised assay
- MSC 63, MSC 67 and MSC 120 were used for both experiments. The experiments were performed on different days.
- potency of a single MSC was evaluated when cultured in 2 different batches of fetal bovine serum (batches ABB and PAA), The test was performed in quadruplicate; experiments I and 2, and experiments 3 and 4 are duplicates performed on the same day. The experiments 1-2 and 3-4 were performed on different days.
- MSC are thawed and plated in 2 wells of a 6-well plate, at a density of 160 x10 3 viable cells per well, in 2.5 ml culture medium (DMEM, supplemented with 10% fetal bovine serum, penicillin and streptomycin) and cultured overnight at 37°C, 7% COg.
- DMEM fetal bovine serum
- Preprotech 1 ng/ml TNF- ⁇
- cells are harvested using TrypLE Select and a total of 45,000 cells are stained for 30 minutes (4°C, protected from light) with antibodies directed against CD54, CD274, HLA-DR and CD73 using the clones and fiuoroehromes shown in table 5.
- the antibodies are diluted in PBS supplemented with 2% (v/v) human albumin.
- cells are washed in PBS and dead cells stained using the fixable Aqua dead cell stain kit (Thermo Fisher) according to the manufacturer ’ s recommendation.
- the cells Prior to staining the intracellular molecule IDO, the cells are fixated and permeabilised using the fixation/per meabilization kit obtained from eBioseience according to the manufacturer’s recommendation. Cells are acquired in a Canto II using pre- defined Euroflow MSC- settings.
- CDS4 expression is dependent on the fetal bovine serum batch used.
- COX-2 is consistently upregulated upon incubation with JL- Ib for 24h
- Nine individual MSC products were incubated with IL-1 ⁇ for 24 hours. All MSC products showed an increase in expression of COX-2 in response to IL-1 ⁇ (Figure 17), supporting that expression of COX-2 is consistently increased in MSC in response to IL-1 ⁇ .
- FIG. 16 shows the results of 81 MSC in total (79 bone marrow-derived MSC and 2 iPSC-derived MSC). Differences in FBS and/or TNF- ⁇ have limited to no influence on the reproducibility. This number of assays allows us to obtain an overview of the variability in fold- induction values that can be obtained per cell marker.
- iMSC P5 and iMSC P9 Two MSC-lines derived from induced Pluripotent Stem Cells (iPSC) were evaluated in the potency assay: iMSC P5 and iMSC P9, The same standardized protocol was used as for the BM-derived MSC. In table 9, the result of the potency assay is depicted.
- the markers CD54, IDO. CD274, HLA-DR and CD 73 were measured. COX- 2 was not analyzed in these experiments.
- CD73 expression was upregulated compared to bone marrow-derived MSC.
- this marker serves as an MSC -identifying marker and it’s expression is not related to MSC potency.
- the potency assay can also be used to evaluate iPSC-derlved MSC.
- MSC soluble pro inflammatory mediators
- IRNg soluble pro inflammatory mediators
- IL-1 ⁇ soluble pro inflammatory mediators
- TNFa soluble pro inflammatory mediators
- GMP Good Manufacturing Practice
- MSC products that have been administered to patients with steroid refractory aGvHD after allogeneic stem cell transplantation in the context of a clinical trial, exhibit enhanced expression of the surface- markers CD54, CD274 and HLA-DE and of the intracellular markers IDO and/or COX-2, when exposed in-vitro to pro-inflammatory mediators, including IFNy, IL-18 and TNFa.
- Results are shown in the table below: Testing MSC products from all 1.4 healthy donors, including multiple products derived from a single donor where applicable; our results show a significant increase in the expression of the intracellular marker IDO and of the extracellular markers CD 64, CD274 and HLA-DR, showing the reproducible and robust immune suppressive potential of these cells,
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Immunology (AREA)
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Biomedical Technology (AREA)
- Biotechnology (AREA)
- General Health & Medical Sciences (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Genetics & Genomics (AREA)
- Developmental Biology & Embryology (AREA)
- Rheumatology (AREA)
- General Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Hematology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Transplantation (AREA)
- Cell Biology (AREA)
- Public Health (AREA)
- Microbiology (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Abstract
The invention relates to means and method for testing mesenchymal stromal cells (MSC) to determine the quality, potency and/or capacity to inhibit an immune response, such as inhibit proliferation of target cells such as T-cells, B-cells, NK cells and other immune cells and/or monocyte/macrophage polarization resulting in direct immune suppression through the production of soluble mediators and induction of T Regs. Such methods may comprise providing MSC; culturing said MSC in the presence and in the absence of one or more cytokines selected from: interleukin (IL)17A, lL-1β, oncostatin M (OSM), interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), IL-13 and IL-4; contacting said MSC with binding molecules that hind to an MSC -specific marker and one or more selected from: the extracellular markers CD54, CD274, human leukocyte antigen (HLA)-DR, the intracellular markers cyclo oxygenase-2 (COX-2) and indoleamine-pyrrole 2,3-dioxygenase (IDO). Such method may further comprise determining said quality, potency and/or capacity to inhibit on the basis of the expression of said MSC- specific marker and the fold increase of the expression of said one or more of CD54, CD274, HLA-DR, COX-2 and IDO markers in response to said one or more cytokínes. The invention further provides cell banks, kits useful in and for methods of treatment using the tested MSC.
Description
Title: Means and methods for determining mesenchymal stromal cell properties.
FIELD OF THE INVENTION
The invention relates to methods of and kits for determining mesenchymal stromal cell (MSC) properties. Such properties include, the capacity to inhibit an immune response. For example proliferation of target cells such as T-cells, B- cells, NK cells and other immune cells and/or monocyte/ macrophage polarization resulting in immune inhibition through the production of soluble mediators and induction of regulatory T -cells (T Regs) through various mechanisms, including soluble factors such as CCL-18 and other factors. The invention also relates to a cell bank comprising MSC having such properties and to use of MSC having such properties,
BACKGROUND OF THE INVENTION
MSC are a heterogeneous population of cells present in various tissues which can differentiate into a variety of cell types, including osteoblasts, chondrocytes, and adipocytes. In addition to being important for the physiological repair of various mesenchymal tissues, including cartilage and bone, MSC play a substantial role in the regulation of blood cell development in the bone marrow.
In vitro co-cultures of MSC with immune cells, as well as animal studies, indicate that MSC not only support maintenance of hematopoietic cells, but also modulate Immune responses by acting on effector cells of the immune system. Based on these properties, MSC are now extensively tested in various diseases associated with auto -and allo-immunity and chronic inflammation, especially conditions characterized by the presence of an inflammatory component, accompanied by severe tissue damage, such as graft- versus -host disease (GvHD).
In pre-clinieal research, intra -and extracellular molecules have been identified to he implicated in the immunomodulatory action of MSC. Many of these molecules are not expressed at steady state, but are upregulated in response to stimulation with proinflammatory molecules, such as cytokines. Not all inflammatory stimuli result in the same type and level of response. Whereas stimulation with some proinflammatory molecules Induce MSC with anti-inflammatory function, stimulation with others results in the generation of MSC with proinflammatory function.
Multiple clinical trials employing MSC in steroid-refractory acute GvHD (aGvHB) indicate that allogeneic MSC can be an effective therapy. Systematic reviews and meta-analyses assessing the response to and survival after MSC treatment in patients with steroid-refractory aGvHD revealed that 72% of the patients had an overall response, of which 28% were complete responses. In patients who responded to MSC treatment, 83% were alive at 6 months as opposed to 16% in non -responders. Evidence from these studies implies that intravenous administration of MSC could be an effective treatment for steroid-refractory aGvHD patients.
Given the nature of the biological MSC cell product and the increasing utility thereof there is an increasing need for an accurate and robust method for determining the therapeutic suitability of a produced batch of MSC . Standardization of such methods further improves the implementation and utility of MSC in vivo. The present invention provides such methods and means to perform such test. The tests as disclosed herein allow for quantitative measurement of the biological activity and as such links to relevant biological properties of the cell therapy product. The means and methods of the invention may serve as a release test in future MSC manufacturing. Presently, no such standard test or assay is available.
SUMMARY OF THE INVENTION
In one aspect the invention provides a method comprising:
- providing MSC; - culturing said MSC in the presence and in the absence of one or more cytokines selected from; interleukin (IL)17A, IL-1β, oneostatin M (OSM), interferon g (IFN-γ), tumor necrosis factor a (TNF-α), IL-13 and IL-4;
- contacting said MSC with binding molecules that bind to an MSC -specific marker and one or more selected from: the extracellular markers CD 54, CD274, human leukocyte antigen (HLA)-DR, the intracellular markers COX-2 and indoleamine-pyrrole 2,3-dioxygenase (IDO); said method further comprising determining the presence of said MSC-specific marker and the fold increase of the expression of said one or more of CD54, CD274, HLA-DR, COX-2 and IDO markers in response to said one or more cytokines.
The invention also provides a kit for determining the capacity and/or potency of MSC to inhibit an immune response. For example proliferation of target cells such as T-ceils, B-eells, NK cells and other immune cells and/or monocyte/macrophage polarization resulting in immune inhibition through the production of soluble mediators and induction of T Regs, comprising binding molecules that hind to an MSC- specific marker and one or more of CD54, CD274, HLA-DR, COX-2 and IDO.
Also provided is a cell bank comprising MSC of at least 10 different donors, wherein an MSC-specific marker is present on the MSC of each of said donors, and wherein each of said MSC exhibit a fold increase of the expression of one or more of the markers CD54, CD274, HLA-DR, COX-2 and IDO in response to culture with one or more cytokines selected from; IL-17A, IL-18, OSM, ΪRN-g, TNF-α, IL-13 and IL-4.
Further provided is a method of preventing and/or inhibiting an immune response in a subject, the method comprising administering to said subject MSC of which the presence of an MSC- specific marker and fold increase of the expression of one or more of CD54, CD274, HLA-DR, COX-2 and IDO was determined using a method or a kit as described herein.
Further provided is a method for preventing and/or inhibiting graft versus host disease in a subject comprising administering to said subject MSC of which the presence of an MSC- specific marker and fold increase of the expression of one or more of CD54, CD274, HLA-DR, COX-2 and IDO has been determined using a method and/or kit as described herein.
Further provided is a method for determining the capacity and/or potency of MSC to inhibit an immune response, for example proliferation of target ceils such as T-cells, B-ceils. NK ceils and other immune cells and/or rnonoeyte/macrophage polarization resulting in immune inhibition through the production of soluble mediators and induction of T Regs, comprising:
- providing MSC;
- culturing said MSC in the presence and in the absence of one or more cytokines selected from: IL-17A, IL-16, OSM, lFN-γ, TNF-α, IL-13 and IL-4: - contacting said MSC with binding molecules that bind to an MSC-specific marker and one or more markers selected from: the extracellular markers CD54, CD274, H LA-DR, the intracellular markers COX-2 and IDO; said method further comprising determining said capacity and/or potency of said MSC on the basis of the expression of said MSC-specific marker and the fold increase of the expression of said one or more of CD54, CD274, HLA-DR, COX-2 and IDO markers in response to said one or more cytokines .
Further provided is a method for determining the capacity and/or potency of mesenchymal stromal cells (MSC) to inhibit an immune response comprising providing MSC; culturing said MSC in the presence of one or more cytokines selected from: interleukin (IL)17A, IL-18, oncostatin M (OSM), interferon y ( lFN-γ), tumor necrosis factor a (TNF-α), IL-13 and IL-4; contacting said cultured MSC with binding molecules that bind to an MSC-specific marker and one or more selected from: the extracellular markers CD54, CD274, human leukocyte antigen (HLA)-DR, and the intracellular markers cyclo-oxygenase-2 (COX- 2) and in doleamlne -pyrrole 2,3-dioxygenase (IDO); and determining from the presence of said MSC-specific marker and the expression of said one or more of CD54, CD274, HLA-DR and IDO markers the capacity' and/or potency to inhibit said immune response.
Provided is also a method for treating a subject in need of MSC based therapy with a population of MSC cells having therapeutic potential comprising: a) validating therapeutic potential of the cell population bye providing MSC; - culturing said MSC in the presence and in the absence of one or more cytokines selected from: interleukin (IL)17A, IL-16, oncostatin M (OSM), interferon g (IFN- g), tumor necrosis factor a ( TNF-α), IL-13 and IL-4; contacting said MSC with binding molecules that bind to an MSC-specific marker and one or more selected from: the extracellular markers CD 54, CD274,
human leukocyte antigen (HLA)-DR, and the intracellular markers cyclooxygenase' 2 (COX-2) and indoleamine- pyrrole 2, 3 -dioxygenase (IDO); b) determining the presence of said MSC-speeifie marker and the fold increase of the expression of said one or more of CD54, CD274, HLA-DR and IDO markers in response to said one or more cytokines; and c) identifying the MSC population as having therapeutic potential if the score is greater than or equal to a predetermined cut-off value, and b) administering a therapeutically effective amount of the MSC population having therapeutic potential to the subject.
Further provided is a method for determining a therapeutic potential of mesenchymal stromal cells (MSC) to inhibit an immune response comprising:
- providing MSC;
- culturing said MSC in the presence and in the absence of one or more cytokines selected from: interleukin (IL)17A, IL-1β, oneostatin M (OSM). interferon g (IFN- g), tumor necrosis factor a (TNF-α), IL-13 and IL-4;
- contacting said MSC with binding molecules that bind to an MSC- specific marker and one or more selected from: the extracellular markers CD 54, CD274, human leukocyte antigen (HLA)-DR, and the intracellular markers cyclo- oxygenase-2 (COX-2) and indoleamine-pyrrole 2,3-dioxygenase (IDO); said method further comprising determining the presence of said MSC -specific marker and the fold increase of the expression of said one or more of CD 64, CD274, HLA- DR and IDO markers in response to said one or more cytokines and determining from said presence and said fold-increase the therapeutic potential.
Further provided is a population of MSC cells for use in a method of treatment of a subject that: is exhibiting or is at risk of exhibiting an undesired immune response wherein said MSC cells have been determined to have therapeutic potential with a method as described herein,
DETAILED DESCRIPTION OF THE DISCLOSED EMBODIMENTS
Described herein is a method comprising: - providing MSC; culturing said MSC in the presence and in the absence of one or more cytokines selected from: IL-17A, EL- IB, OSM, lFN-γ, TNF-α, IL-13 and IL-4; contacting said MSC with binding mol etudes that bind to an MSC- speeifie marker and one or more selected from: the extracellular markers CD 64, CD274, HLA-DR, the intracellular markers COX-2 and IDO; said method further comprising determining the presence of said MSC-specific marker and the fold increase of the expression of said one or more of CD64, CD274, HLA-DR, COX-2 and IDO markers in response to said one or more cytokines. In the present invention it was shown that the method is robust, reliable, reproducible, easy
to use and easily implementable in clinical laboratories. The method is further a suitable predictor for biological activity of a cell therapy product, such as MSC.
The disclosure describes a method for determining if MSC have a capacity to inhibit an immune response, for example inhibition of proliferation of target cells such as T-cells, B-cells, NK cells and other immune cells and/or monocyte/macrophage polarization resulting in immune suppression through the production of soluble mediators and induction of regulatory T-cells (T Regs), comprising: providing MSC; - culturing said MSC in the presence and in the absence of one or more cytokines selected from: IL-17A, IL-lB, GSM, lFN-γ, TNF-α, IL-13 and IL-4; contacting said MSC with binding molecules that bind to an MSC- speeifie marker and one or more selected from: the extracellular markers CD 54, CD274, HLA-DR, the intracellular markers COX- 2 and IDO; said method further comprising determining the presence of said MSC 'Specific marker and the fold increase of the expression of said one or more of CD54, CD274, HLA-DR, COX-2 and IDO markers in response to said one or more cytokines. Said capacity can be exerted through various mechanisms, including soluble factors such as CCL-I8 and other factors,
The invention also provides a method for determining the capacity anti/or potency of mesenchymal stromal cells (MSC) to inhibit an immune response comprising providing MSC; culturing said MSC in the presence of one or more cytokines selected from: interleukin (IL)17A, 1L-18, oncostafcin M (OSM), interferon g ( lFN-γ), tumor necrosis factor a (TNF-α), IL-13 and IL-4; contacting said cultured MSC with binding molecules that hind to an MSC -specific marker and one or more selected from: the extracellular markers CD54, CD274, human leukocyte antigen (HLA)-DR, and the intracellular markers cyclo-oxygenase- 2 (COX- 2) and indoleamine-pyrrole 2,3-dioxygenase (IDO); and determining from the presence of said MSC-specific marker and the expression of said one or more of CD 54, CD274, HLA-DR and IDO markers the capacity and/or patency to inhibit said immune response.
A method for testing MSC as described herein comprises the method described herein above directed towards determining if MSC have a capacity to inhibit an immune response. Inhibition of an immune response can be inhibition of proliferation of target cells such as T-cells, B-cells, NK cells and other immune cells and/or monocyte/maerophage polarization resulting in immune suppression through the production of soluble mediators and induction of T Regs, Preferably, inhibition of proliferation of target cells such as T-cells, B-cells, NK cells and/or monocyte/maerophage polarization. Preferably inhibition of proliferation of target cells such as T-cells and B-cells and/or monocyte/maerophage polarization. Preferably inhibition of proliferation of T-cells and/or monocyte/maerophage polarization. More preferably inhibition of proliferation of T-cells and/or monocyte polarization. A method for testing MSC as described herein also refers to a method for determining the
potency of MSC to inhibit an immune response. Inhibition of an immune response can be inhibition of proliferation of target cells such as T-cells, B-ceils, NK cells and other immune cells and/or monocyte/macrophage polarization resulting in immune suppression through the production of soluble mediators and induction of T Regs. Preferably, inhibition of proliferation of target ceils such as T-eells, B- cells, NK cells and/or monocyte/macrophage polarization. Preferably inhibition of proliferation of target cells such as T-cells and B-cells and/or monocyte/macrophage polarization. Preferably Inhibition of proliferation of T-cells and/or monoeyte/macrophage polarization. More preferably inhibition of proliferation of T-cells and/or monocyte polarization. The capacity and the potency are measures for the quality of MSC for use in anti-inflammatory and regenerative settings, MSC batches that meet the criteria set herein are suitable for administration to a human with the purpose of reducing an undesired inflammatory immune response and/or enhancing regeneration in the thus transplanted human host. The presence of an MSC-specific marker on MSC and the fold increase of the expression level of one or more of CD 54. CD274. HLA-DR, COX- 2 and IDO markers in response to culture with one or more cytokines as disclosed herein indicate that such MSC have a high potency and/or capacity to inhibit an immune response when administered to a subject. MSC with such potency or capacity are very suitable for administration to subjects in need thereof,
Where herein reference is made to “MSC”, this term refers to mesenchymal stromal cells. These stromal cells are sometimes also referred to as mesenchymal stem cells, marrow stromal cells or multipotent mesenchymal stromal cells etc, MSC are adherent multipotent stromal cells that as a population can differentiate into a variety of cell types, including osteoblasts {hone cells), chondrocytes {cartilage cells), myocytes (muscle cells) and adipocytes (fat cells) . MSC have a capacity for self- renewal while maintaining their multipotency, MSC have an effect on innate and specific immune cells, MSC can produce a range of molecules having immunomodulatory effects. The cells can be derived from various regions of the body such as for instance, bone marrow, umbilical cord, adipose tissue, amniotic fluid, and molar cells. MSC from different sources typically exhibit similar functional properties, although the magnitude of the effects may vary.
MSC have an effect on immune cells such as macrophages, neutrophils, NK cells, mast cells and dendritic cells in innate immunity. MSC are able to migrate to the site of injury where they can exhibit an anti-inflammatory effect or modulate immune responses using intermediate cells such a macrophages. In the present invention an anti-inflammatory effect is preferably estimated on the basis of a capacity to inhibit immune responses, such as inhibition of proliferation of target cells such as T-cells, R- eelis, NK cells and other immune cells and/or monoeyte/macrophage polarization resulting in immune suppression through the production of soluble mediators and induction of T Regs,
Wherein herein reference is made to an “MSC- specific marker”, such a marker can be: CD73, CD90, CD 105, CD29, HLA-ABC, CD 166, CD146, CD44, CD 140a,
CD 140b), These markers are well known in the art. Antibodies and other binding molecules with which these markers can be visualized are commercially' available. In a
preferred embodiment CD73, CD 29, CD90, CD 105, HLA-ABC, CD166, CD 146, CD44, CD 140a. CD 140b is used as an MSC- specific marker. In a preferred embodiment CD 73, CD29. CD 90, or CD 105 is used as an MSC- specific marker. Preferably CD 73 is used as an MSC-specifle marker. MSC -specific markers are also markers that specifically detect subsets of MSCs. Examples of such subset markers are CD 13, MSCA-1, CD 56, CD271, CD362 and Stro-1. Other MSC markers will likely be developed further and such are also included in the invention.
The expression is of course measured and/or fold increase determined for the same binding molecules that the MSC are contacted with. When herein reference is made to MSC contacted with binding molecules for one or more markers selected from: the extracellular markers CD64, CD274, HLA-DE, the intracellular markers COX-2 and IDO it is preferred that the MSC are contacted with binding molecules for two or more of the markers, preferably 3 or more of the markers, more preferably 4 or more of the markers. Increasing the number of markers increases the accuracy of the determination. When two or more of the markers are used it is preferred that the markers are selected from CD54, CD274, HLA-DR, COX-2 and IDO. Preferably from markers CD54, CD274, HLA-DE and IDO. It is preferred that the markers at least comprise CD54 and IDO, preferably at least CD 54, IDO and CD274, more preferably at least CD54, IDO, CD274 and HLA-DR,
In a preferred embodiment the MSC are contacted with binding molecules for two or more markers selected from: the extracellular markers CD54, CD274, HLA-DR. the intracellular markers COX-2 and IDO, Preferably with binding molecules for three or more markers selected from: the extracellular markers CD54, CD274, HLA-DE, the intracellular markers COX-2 and IDO. Preferably with binding molecules for four or more markers selected from: the extracellular markers CD 54, CD274, HLA-DR, the intracellular markers COX-2 and IDO. Preferably with binding molecules for all of the markers CD54, CD274, HLA-DR, COX-2 and IDO.
In a preferred embodiment the MSC are contacted with binding molecules for two or more markers selected from: the extracellular markers CD54, CD274, HLA-DR, the intracellular marker IDO. Preferably with binding molecules for three or more markers selected from: the extracellular markers CD54, CD274, HLA-DR, the intracellular marker IDO, Preferably with binding molecules for the four markers selected from: the extracellular markers CD54, CD274, HLA-DE, and the intracellular marker IDO.
In these embodiments it is preferred that the MSC-speeifie marker is present on at least 90 % of the cells and the fold increase of expression of CD54 is at least 10- fold; of CD274 is at least 8-fold; of HLA-DR is at least 8-fold preferably at least 25-fold, of COX-2 is at least 3-fold and/or of IDO is at least 5-faid.
The term “capacity” as used herein in relation to MSC, refers to a biological activity. Biological activities of MSC include, but are not limited to, inhibition of T-cell proliferation and differentiation of monocytes to DC,
Where herein reference is made to the terra “inhibit” in relation to MSC, the term refers to the capacity of MSC to prevent or decrease the extent of a process or action.
T- ceils are a type of lymphocyte which develop in the thymus gland and play a central role in the adaptive immune response, T-cells have an important role in controlling and shaping the immune response by providing a variety of immune- related functions. Proliferation of T-cells is associated with increased immune-related activity . Methods of inducing T-eell proliferation are known in the art. An exemplary method is described in the examples, Regulatory T-cells, or T Regs are also known as suppressor T-cells, They are a subpopulation of T-cells that modulate the immune system, prevent auto-immune disease, and maintain tolerance to self-antigens.
B-cel!s are a type of lymphocyte that secretes antibodies. They are part of the adaptive immune response. In addition, B-eelis can also present antigens and secrete cytokines.
NK-eelis are a type of cytotoxic lymphocytes, that is importan t for the immune system. They derive from the same progenitor cell as T- and B-cells, but are part of the innate immune system. NK-cells are best known for killing viral!y infected cells, and detecting and controlling early signs of cancer.
Monocytes are a type of leukocyte which can polarize towards DC or macrophages under inflammatory conditions. An exemplary method for polarizing monocytes to DC is described in the examples. DC are antigen-presenting cells of the mammalian immune system. Their main function is to process antigen material and present it on the cell surface to T-cells, resulting in T-cell activation and/or proliferation. Macrophages are specialized cells involved in the detection, phagocytosis and destruction of e.g. microorganisms and dead cells,
In one embodiment of a method of the invention. MSC can he provided freshly isolated from tissue. In a further embodiment, MSC can be provided from an existing culture. MSC are preferably obtained from frozen/eryopreserved storage. After thawing and washing such cells can be used directly or first be cultured.
A method as described herein comprises providing MSC; culturing said MSC in the presence and in the absence of one or more cytokines selected from: IL-17A, IL-lB, OSM, 1FN-Y, TNF-α, IL-13 and IL-4; contacting said MSC with binding molecules that bind to an MSC-sspecific marker and one or more selected from: the extracellular markers CD54, CD274, HLA-DR, the intracellular markers COX-2, and IDO; and determining the presence of said MSC- specific marker and the fold increase of the expression of said one or more of CD54, C-D274, HLA-DR, COX-2 and IDO markers in response to said one or more cytokines.
Cytokines are a category of small proteins that are important in cell signaling. Cytokines include chemokines, interferons, interleukins, lymphokines, and tumor necrosis factors. Cytokines have been shown to be involved in autocrine, paracrine and endocrine signaling as immune-modulating agents. They act through receptors, and have an effect on the immune system; cytokines can modulate the balance between humoral and cell-based immune responses, and can regulate the maturation, growth, and responsiveness of particular cell populations. They are important in health and disease, specifically In host responses to infection, immune responses, inflammation, trauma, sepsis, cancer, and reproduction. A proinflammatory cytokine is a cytokine with an inflammatory stimulatory effect, A proinflammatory cytokine is often secreted from cells that promote inflammation, like (helper) T-cells, Inflammatory cytokines have pleiotropic effects that together have an upregulating effect on inflammatory reactions. Proinflammatory cytokines include, but are not limited to: IL-1β, IL-4, IL-5, IL-6, IL-8, IL-12, IL-13, 1L17A, IL-18, TNF-α, lFN-γ, granulocyte- macrophage colony stimulating factor (GM-CSF), GSM, MCP-1, monocyte chemoattractant protein- 1
(MCP-1), Chemokine C-C motif ligand 5 (CCL5)/regulated on activation, normal T-eeil expressed and secreted (RANTES), macrophage inflammatory protein (MIP-lu),
MIP1B, CD40Ligand.
The cytokines IL-17A, IL-1β, OSM, lFN-γ, TNF-α, IL-13 and IL-4 are well known in the art and suitable sources of cytokines are available to the skilled person. The cytokines are typically used in saturating amounts which for the indicated cytokines amounts to a concentration in the range of 0.1 ng/ml to 100 ng/uil. Suitable concentrations for the cytokines are indicated in the examples and are typically in the range of 5-60 ng/ml. When used, TNF-α is preferably present in a concentration of at least lng/ml. When used, lFN-γ is preferably present in a concentration of at least 0.33 ng/ml. When used, IL-17A is preferably present in a concentration of at least 50 ng/ml. When used, IL-1β is preferably present in a concentration of at least 1 ng/ml. When used, OSM is preferably present in a concentration of at least 20 ng/ml. When used, IL-13 is preferably present in a concentration of at least 20 ng/ml. When used, IL-4 is preferably present in a concentration of at least 20 ng/ml.
Depending on the activity of a certain batch of cytokine, antibody or other binding molecule the amount of it used can be adjusted as is typically done by the person skilled in the art. The particular activity (quality) of a certain batch of cytokine, antibody or other binding molecule can suitably be determined on arrival of the batch.
Each of IL-17A, IL-18, OSM, lFN-γ, TNF-o, IL-13 and IL-4 alone or combinations thereof can be used in the culture of MSC as described herein. In a preferred embodiment the eytokine(s) used in the culture are one or more of IL-17A, IL-1β, OSM, IFN-Y, TNF-α and IL-13; preferably one or more of IL-17A, IL-1β and OSM; or preferably one or more of lFN-γ, TNF-α or IL-13. In a preferred embodiment a combination of two or more of the mentioned cytokines is used, preferably 3, 4, 5, 6 or all 7 of said cytokines. In one embodiment a combination of 6 is used, preferably of the cytokines L-17A, IL-1β, OSM, lFN-γ, TNF-α and IL-13. In another embodiment a
combination of 3 is used. In a preferred embodiment the combination of three is IL- G7A, IL-1β and GSM, In another preferred embodiment the combination of three is lFN-γ, TNF-α or IL-13. In another preferred embodiment the combination of two is IFN-Y and TNF-α. Suitable cultures with one cytokine are cultures with lFN-γ or with IL-16, In a particularly preferred embodiment the cytoklneis) used in a method of the invention is/are lFN-γ, IL-1β, or the combination of lFN-γ and TNF-α.
In a preferred embodiment the one cytokine or combination of cytokines is used that routinely gives a suitable and preferably the highest difference in expression of CD54, CD274, HLA-DE, COX-2 and/or IDO between the two cultures of a typical MSC preparation. This typical preparation can be a standardized preparation of MSC or model cells or model cell lines that are a good tell-tale for the effect of the cytokines on MSC. In one preferred embodiment, the combination of cytokines is determined by measuring the cytokines present in the MSC recipient. The markers CD54, CD274, HLA-DR, COX-2 and IDO are well known in the aid. Antibodies and other binding molecules with which these markers can be visualized are commercially available.
The MSC that are to be tested are preferably a representative sample of a larger batch of MSC of which the functionality is to be predicted. The culturing in the presence or absence of certain cytokine(s) is typically done by culturing two samples of the same batch of MSC separately in different cultures, one with and one without the mentioned eytokine(s). The cultures are typically performed in parallel but not necessarily so. The culture without a cytokine can also be a historical reference. For instance of a different batch of MSC cultured in the absence of said cytokine(s).
Cultures with and without cytokines are typically essentially the same hut for the presence of the cytokine. In one embodiment of the method of testing MSC as described herein the representative MSC sample is split into two or more aliquots and one aliquot is cultured in the absence of the indicated eytokine(s) and another aliquot is cultured in the presence of the indicated cytokine(s), A difference in staining of the MSC of the two cultures is indicative for the functionality of the cells. MSC are preferably· cultured at 37°C and 5% C02. Methods for culturing MSC are described in the examples section. Effects of stimuli, such as cytokines, on MSC may be risible directly after culture or after an incubation period.
Cytokines are a broad category of small proteins that are important in inducing signaling in cells. Cytokines have been shown to he involved in autocrine, paracrine and endocrine signaling as immune- modulating agents. Cytokines include ehemokines, interferons, interleukins, lymphokines, and tumor necrosis factors. They act through cell surface receptors, and are especially important in the immune system ; cytokines modulate the balance between humoral immunity and cell based immune responses, and regulate the maturation, growth, and responsiveness of particular cell populations. They are important in health and disease, specifically in host responses to infection, immune responses, inflammation, trauma, sepsis, cancer, and reproduction, A proinflammatory cytokine is a type of signaling molecule that is
secreted from immune cells that promote inflammation, like (helper) T 'Cells, Inflammatory cytokines play an important role in mediating the innate immune response and are involved in the upregulation of inflammatory reactions, Proinflammatory cytokines include, but are not limited to: IL-1β, IL-4, IL-5, IL-6, IL-8, IL-12, IL-13, IL17A, IL-18, TNF-α, lFN-γ, granulocyte-macrophage colony stimulating factor (GM-CSF), OSM, MCP-1, CCLo/RANTES, MIP-la, MIPlB, CD40L.
A method for testing MSC as described herein preferably comprises providing MSC: optionally thawing said MSC when said MSC are retrieved from storage; and culturing said MSC in presence and in the absence of one or more of the mentioned cytokines IL-17A, IL-18, OSM, lFN-γ, TNF-α, IL-13 and IL-4. The method preferably further comprises contacting said MSC with binding molecules that bind to an MSC- speeific marker and one or more selected from; the extracellular markers CD 64, CD274, HLA-DR, the intracellular markers COX- 2 and IDO.
Contacting said MSC with binding molecules preferably comprises: contacting said MSC with binding molecules that bind to an MSC-speelfie marker, and one or more selected from: the extracellular markers CD 54, CD274, HLA-DR; washing and permeabilizing said MSC; and contacting said MSC with a binding molecule that hinds said intracellular markers COX- 2 and/or IDO,
The capacity and/or potency of said MSC is determined on the basis of the expression of an MSC-speciflc marker on said cells, and the fold Increase in expression of the markers CD54, CD274, HLA-DR, COX-2 and/or IDO of MSC when cultured in the presence of the cytokine(s) when compared to the absence thereof.
The MSC are said to have the capacity when said MSC- specific marker is present on at least 90 % of the ceils and the fold increase of expression of CD54 is at least 3-fold, preferably at least 5 fold and more preferably at least 10-fold for CD54; is preferably at least 2-fold, preferably at least 4-fold and more preferably at least 8-fold for CD274; is preferably at least 5-fold, preferably at least 8 fold and more preferably at least 16-fold and more preferably at least 25-fold for HLA-DR, is preferably at least 2-fold, and more preferably at least 3-fold for COX-2, and/or is preferably at least 3- fold and more preferably at least 5-fold for IDO. In one aspect MSC are said to have the capacity when said MSC -specific marker Is present on at least 90 % of the cells and the fold increase of expression of CD54 is at least 10-fold; of CD274 is at least 8-fold; of HLA-DR is at least 8-fold preferably at least 25-fold, of COX-2 is at least 3-fold and/or of IDO is at least 5-fold. The MSC are said to have the potency when said MSC-specific marker is present on at least 90 % of the ceils and the fold increase of expression of CD54 is at least 3-fold, preferably at least 5 fold and more preferably at least 10-fold for CD54; is preferably at least 2-fold, preferably at least 4-fold and more preferably at least 8- fold for CD274; is preferably at least 5-fold, preferably at least 8 fold and more preferably at least 16-fold and more preferably at least 25-fold for HLA-DR, is preferably at least
2-fold, and more preferably at least 3-fold for COX-2, and/or is preferably at least 3- fold, and more preferably at least d-fold for IDO.
The MSC are said to have the potency when said MSC-specific marker is present on at least 90 % of the cells and the fold increase of expression of CD54 is at least 10-fold; of CD274 is at least 8-fold; of HLA-DR is at least 8-fold preferably at least 25-fold, of COX-2 is at least 3-fold and/or of IDO is at least 5-fold,
In one aspect the invention provides a method for determining the capacity and/or potency of mesenchymal stromal cells (MSC) to inhibit an immune response comprising providing MSC; culturing said MSC in the presence of one or more cytokines selected from : interleukin (IL)17A, lL-18, oneostatin M (OSM), interferon g ( lFN-γ), tumor necrosis factor a (TNF-α), IL-13 and IL- 4; contacting said cultured MSC with binding molecules that hind to an MSC- specific marker and one or more selected from: the extracellular markers CD54, CD274, human leukocyte antigen (HLA)-DR, and the intracellular markers cyclo-oxygenase- 2 (COX- 2) and indoleamine-pyrroie 2,3-dioxygenase (IDO); and determining from the presence of said MSC-specific marker and the expression of said one or more of CD 54, CD274, HLA-DR and IDO markers the capacity and/or patency to inhibit said immune response. It is preferred that the presence of the MSC- specific marker and the expression of said one or more of CD54, CD274, HLA-DR and IDO markers is compared with a reference. The reference can be a historic reference.
In one embodiment the reference is a parallel sample of the same MSC culture in the absence of said one or more cytokines. The comparison of the reference and the cytokine cultured sample preferably yields a score for the relative increase of the expression of said one or more of CD 54, CD274, HLA-DR and IDO markers in said cytokine cultured sample. The score is preferably a fold-increase of expression of one or more of said markers in the cytokine cultured sample when compared to a reference MSC population cultured in the absence of said cytokines.
Said MSC-specific marker is preferably present on at least 90 % of the cells and the fold increase of expression of CD 54 is at least 3 -fold, preferably at least 5 fold and more preferably at least 10-fold for CD 54; is preferably at least 2 -fold, preferably at least 4-fold and more preferably at least 8-fold for CD274; is preferably at least 5 -fold, preferably at least 8 fold and more preferably at least 16-fold and more preferably at least 25-fold for HLA-DR, Is preferably at least 2-fold, and more preferably at least 3- fold for COX-2, and/or is preferably at least 3-fold, and more preferably at least 5-fold for IDO.
The Invention further provides a kit comprising binding molecules that bind to an MSC-specific marker and one or more markers selected from: CD 54, CD274, HLA- DR, COX-2 and IDO, In a preferred embodiment the kit comprises binding molecules that hind to an MSC-specific marker, CD54, CD274, HLA-DR, COX-2 and IDO. In a preferred embodiment the kit comprises binding molecules that bind to CD73, CD54, CD274, HLA-DR, COX-2 and IDO. The kit is useful in a method as described herein. Such a kit is useful in methods for determining if MSC have a capacity to inhibit an immune response. For example inhibition of proliferation of target cells such as T- cells, B-cells, NK cells and other immune cells and/or monocyte/macrophage
polarization resulting in immune suppression through the production of soluble mediators and induction of T Regs. The kit Is also useful in a method for determining the potency of MSC to Inhibit an immune response. For example inhibition of proliferation of target cells such as T-cells, B-eelis, NK cells and other immune cells and/or monocyte/macrophage polarization resulting in immune suppression through the production of soluble mediators and induction of T Regs. The kit is also useful in a method to predict recipient response to administration of said MSC.
The kit preferably further comprises one or more cytokines selected from: IL17A, IL-18, OSM, IFN-y, TNF-α, IL-13, IL-4. The preferred number of cytokines and the preferred single or combinations of cytokines are as described elsewhere herein.
A binding molecule as defined herein is a proteinaceous binding molecule. The binding molecule is typically a peptide, a cyclic or bieyclic peptide of up to and including 20 amino acids or a polypeptide having more than 20 amino acid residues.
The art knows many proteinaceous binding molecules. Often these include one or more complete or derivative antibody variable domains. Non-limiting examples are single chain Fv-fragments, monobodies, VHH, Fab -fragments. Derivative variable domains can be artificial or naturally evolved derivatives both belonging to the class of proteins that have the immunoglobulin fold. Examples of non-immunoglobulin fold containing proteinaceous binding moieties are the avimers initially developed by Amgen.
A binding molecule can be any type of molecule that is capable of specifically binding to the indicated markers. Binding molecules as disclosed herein are preferably bivalent monospecific binding molecules. Binding molecules preferably comprise at least a variable domain of an antibody. In a preferred embodiment the binding molecules are antibodies, preferably monoclonal antibodies, i.e. bivalent monospecific antibodies. Antibodies are well known, well defined molecules of which the production and the use in labelling of cells has matured to the extent that such use has become routine and robust, a characteristic that is highly desired in a method for testing MSC as described herein, A feature of such a test is that it is robust and easily incorporated in the procedure of a new lab without typically requiring extensive optimization. Such robustness is especially important when quantitative results are desired. In a preferred embodiment the fold Increase in expression of one or more of CD54, CD274. HLA-DR, COX-2 and IDO markers is detected using antibodies. In such an embodiment the presence of a MSC-specific marker can be detected using any specific binding molecule. In a preferred embodiment the presence of said MSC-specific marker is detected with an antibody against an MSC-specific marker Binding molecules such as antibodies are preferably labelled with fluo.rochro.mos that are suitable for parallel flow cytometric analysis. The parallel flow cytometric analysis is preferably one wherein the signals of said fiuoroehromes can be acquired independently .
The invention further provides a cell bank comprising MSC of at least 10 different donors, wherein an MSC -specific marker is present on the MSC of each of said donors, and wherein each of said MSC exhibit a fold increase of the expression of one or more of the markers CD54, CD274, HLA-DE, COX-2 and IDO in response to one or more cytokines selected from: 1L-17A, IL-18, QSM, lFN-g, TNF-α, IL-13 and 1L- 4, The cell bank is preferably one wherein the presence and/or the fold increase in expression of said markers of said MSC was determined using a method of testing MSC as described herein or a kit as described herein. The invention further provides a method of preventing and/or inhibiting an immune response in a subject, the method comprising administering to said subject MSC of which the expression of and MSC- specific marker and fold increase of the expression of one or more of the markers CD54, CD274, HLA-DE, COX-2 and IDO was determined using a method and/or a kit as described herein. In a preferred embodiment the subject is exhibiting or is at risk of exhibiting an undesired immune response. In one embodiment the subject is exhibiting inflammatory bowel disease. In another embodiment the subject is the recipient of an allogeneic cell or organ transplant. In a further embodiment the subject is diagnosed with GvHD. Further provided is a method for preventing and/or inhibiting graft versus host disease in a subject comprising administering to said subject MSC of which the presence and fold increase of the expression of said markers has been determined using a method and/or a kit as described herein. Also provided is a method comprising: providing MSC; culturing said MSC in the presence and in the absence of one or more cytokines selected from: 1L-17A, IL-1B, OSM, lFN-g, TNF-α, IL-13 and 1L-4; contacting said MSC with binding molecules that bind to an MSC- specific marker and one or more selected from: the extracellular markers CD54, CD274, H LA-DR, the intracellular marker COX-2 and IDO; said method further comprising determining the expression of said MSC- specific marker and the fold increase of the expression of said one or more of CD 54, CD274, HLA-DE. COX-2 and IDO markers in response to said one or more cytokines.
The fold increase as referred to in a method of treatment or population for use in a method of treatment as described herein is preferably at least 3-fold, preferably at least 5 fold and more preferably at least 10-fold for CD54; is preferably at least 2-fold, preferably at least 4-fold and more preferably at least 8-fold for CD274; is preferably at least 5-fold, preferably at least 8 fold and more preferably at least 16-fold and more preferably at least 25-fold for HLA-DR, is preferably at least 2-fold, and more preferably at least 3-fold for COX-2, and/or is preferably at least 3-fold, and more preferably at least 5-fold for IDO.
The fold increase of expression is preferably at least:
10-fold for CD54; 8-fold for CD274; 8-fold and preferably 26-fold for HLA-DR, 3-fold for COX-2, and/or 5-fold for IDO.
In one aspect the disclosure provides a method comprising: providing MSC; optionally thawing said MSC, when said MSC are provided frozen; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, IL-1β, OSM, lFN-γ, TNF-α, IL-13 and IL-4; contacting said MSC with binding molecules that bind to an MSC- specific marker and one or more selected from: the extracellular markers CD54, CD274, HLA-DR, the intracellular markers COX-2 and IDO; said method further comprising determining the presence of said MSC-specific marker and the fold increase of the expression of said one or more of CD54, CD274, HLA-DR, COX-2 and IDO in response to said one or more cytokines.
In a further aspect the disclosure provides a method comprising: providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from:
- lFN-γ;
- IL-1B;
- lFN-γ and TNF-α;
- IL17A, IL-lS and OSM:
- IL17A, IL- 18, OSM, lFN-γ, TNF-α and IL-13;
- IL17A, IL- 18, OSM, lFN-γ, TNF-α, IL-13 and IL-4, or;
- IFN-y, TNF-α, IL-13 and IL-4 wherein said one or more cytokines are present in a saturating amount, preferably in a concentration ranging from Q.lng/ml to lOOng/ml, contacting said MSC with binding molecules that bind to an MSC- specific marker and one or more selected from: the extracellular markers CD54, CD274, HLA-DR, the intracellular markers COX-2 and IDO; said method further comprising determining the presence of said MSC-specific marker and the fold increase of the expression of said one or more of CD54, CD274, HLA-DR, COX- 2 and IDO in response to said one or more cytokines.
In a further aspect the disclosure provides a method comprising: providing MSC; culturing said MSC in presence and in the absence of IL-lti lFN-γ, or IFN-Y and TNF-α; wherein said cytokines are present in a saturating amount, preferably in a concentration ranging from Q.lng/ml to lOOng/ml, more preferably IL- 1B in a concentration of Ing/ml, IFN-y in a concentration of lOng/ml, or lFN-γ in a concentration of lOng/ml and TNF-α in a concentration of Ing/ml. contacting said MSC with binding molecules that bind to an MSC- specific marker and one or more selected from: the extracellular markers CD54, CD274, HLA-DR, the intracellular markers COX- 2 and IDO;
said .method further comprising determining the presence of said MSC- specific marker and fold increase of the expression of said one or more of CDS4, CD274, HLA- DR, COX-2 and IDO in response to said one or more cytokines.
The term "antibody" refers to an immunoglobulin molecule that is typically composed of two identical parrs of polypeptide chains, each pair having one "heavy" (H) chain and one "light" (L) chain. Human light chains are classified as kappa (K) and lambda (l). Heavy chains are classified as mu. delta, gamma, alpha, or epsilon, and define the antibody's isotype as IgM, IgD, IgG, IgA, and IgE, respectively. Each heavy chain is comprised of a heavy chain variable region (abbreviated herein as HCVR or VH) and a heavy chain constant region. The heavy chain constant regions of IgD, IgG, and IgA are comprised of three domains, CHI, CH2 and CHS, and the heavy chain constant regions of IgM and IgE are comprised of four domains, CHI, CH2, CH3, and CH4. Each light chain is comprised of a light chain variable region {abbreviated herein as LCVS or VL) and a light chain constant region. The light chain constant region is comprised of one domain, CL. The constant regions of the antibodies may mediate the binding of the immunoglobulin to host tissues or factors, including various cells of the immune system (e.g., effector cells). The VH and VL regions can he further subdivided into regions of hypervariability, termed complementarity determining regions (CDR), interspersed with regions that are more conserved, termed framework regions (FR), Each VH and VL is composed of three CDEs and four FRs, arranged from the amino- terminus to carboxy-terminus in the following order: FR1, CDR1, FR2, CDR2, FR3, CDR3, FR 4. The variable regions of the light and heavy chain together form the antibody binding site and defines the specificity for the epitope.
Where herein reference is made to ‘Ttuorochraraes”, it is intended to refer to chemicals that have the capacity to fluoresce. In a preferred embodiment binding molecules are labeled with fiuoroehromes. Fiuoroehromes suitable for labeling binding molecules and detecting markers bound by labeled binding molecules are known in the art. In a preferred embodiment, fiuoroehromes used in a method or present in a kit as disclosed herein enable flow cytometric detection of binding molecules bound to their target.
In one aspect of the disclosure, binding molecules of a method anti/or kit as disclosed herein are fluorescent!}7 labeled. In a further preferred embodiment, binding molecules that bind to the same marker are labeled with the same fluoroehrome. Preferably, groups of binding molecule that bind different markers are labeled with distinct fiuoroehromes. Preferably, fiuorescently labeled binding molecules of a method and/or kit as disclosed herein are fiuorescently labeled antibodies. Fiuorescently labeled antibodies can be commercially obtained from various companies, including, but not limited to: Bee ton Dickinson (BD), eBiosciences, Bio legend, Dako, Beckman Coulter, CYTOGNOS, Cal tag, Pharmlngen, Exbio, Sanquin, R&D systems, and Invitrogen,
In one aspect of the disclosure, in a method disclosed herein, MSC are contacted with a saturating amount of fiuorescently labeled binding molecules or antibodies. In a preferred embodiment the fiuoroehromes are selected for brightness, limited spectral
overlap and limited need for compensation, stability, etc. (see: Kalina et al. Leukemia 2012; 26: 1986). in a preferred embodiment, all fluorochromes of a method and/or kit as disclosed herein can he acquired independently from each other. Fluorochromes suitable for a method and/or kit of the disclosure include, but are not limited to: pacific blue (PacB), brilliant violet 421 (BV421), Horizon V450, pacific orange (PacO), Horizon V60Q (HVoQQ), BV6I0, Khrome orange (KO), 00515, Horizon BB515, fluorescein isothiocyanate (FITC), Alexa488, phyeoerythrin (PE), peridinin chlorophyl protein/eyanine 5.6 (PerCP-CyS.5), PerCP, PE Texas Red, phycoerythrin/cyanine? (PE-Cy7), allophycocyanine (APC), AIexa647, allophycocyanine/hilite 7 (AFC-H7), APC-Cy7, Alexa680„ APC- A750, APC-C750 and Alexa700,
In one embodiment, fluorescently labeled binding molecules of a method and/or kit as described herein comprise the fluorochromes PE, APC, APC-H7, BV421, PE- Cy7. In a further embodiment, a method and/or kit as described herein comprises PE- labeled antibodies that bind to CD54, APC-laheled antibodies that bind to CD274,
APC-H7 labeled antibodies that bind to HLA-DR, BV421 labeled antibodies that bind to CD73, PE-labeled antibodies that bind to COX-2 and/or PE-Cy7 labeled antibodies that bind to IDO. The procedures and methods for testing MSC as described herein are preferably performed using standardized flow -cytometer settings, above the electronic noise and target values for the photomultiplier tube (PMT) set using rainbow beads. Multicolor immunostaining and flow -cytometry is preferably performed according to the so-called Euro Flow protocols as described by Van Dongen et al. (Leukemia 2012; 26: 1908) and by Kalina et al. (Leukemia 2012; 26: 1986), or that are available via the Euro Flow webpage (www. EuroFiow.org). Preferably, a method as described herein comprises the use of software for data integration and multidimensional analysis of flow cytometry files. In a further preferred embodiment, the combinations of fluorescently labeled binding molecules as disclosed can be used in combination with commercially available software tools.
In one aspect a method and/or kit of the disclosure is suitable for acquisition of fluorescence according to the standards of the Euro Flow consortium. Such standards include, but are not limited to: flow cytometry acquisition settings, antibody concentration, antibodies, fluorochromes, and combinations of fluorochromes.
In one aspect the disclosure provides a method comprising: providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, IL-16, OSM, IEN-g, TNF-α, IL-13 and IL-4: - contacting said MSC with antibodies that bind to an MSC -specific marker and one or more selected from: the extracellular markers CDS4, CD274, HLA- DR, the intracellular markers COX-2 and IDO; said method further comprising determining the presence of said MSC-specific marker and fold increase of the expression of said one or more of CD54, CD274, HLA- DR, COX-2 and IDO in response to said one or more cytokines.
The term “marker" refers to proteins expressed by ceils, which can serve to help identify and classify cells, Extracellular .markers are present on the outside of the cell, intracellular markers are present on the inside of the cell. Some markers are unique for cells types, others are more widely expressed. Specific combinations of markers can predict certain capacities of cells. Markers suitable for, or that can he added to the method include, but are not limited to: CD la, CD2, CDS, CD4, CDS, CD6, CD7, CD8a, CD9, GDI la, CD lib, CD 11c, CD 14, CD16, CD 17, GDIS, CD19, CD20, CD21, CD22, CD23, CD24, CD2o, CD27, CD28, CD29, CD30, CD31, CD33, CD34, CD3S, CD36, CD37, CD38, CD40, CD41, CD42b, CD43, CD44, CD46, CD47, CD48, CD49d, CD50, CD51, CDS2, CDS3, CDo4, CD65, CD56, CD58, CD59, CD61, CD62L, CD62P, CD63, CD66a, CD 66b, CD66c, CD66d/e, CD69, CD71, CD72, CD73, CD79a, CD80, CD90, CD9S, CD97, CD98, CD99, CD 105, CD 106, CD 109, CD 116, CD 117, CD 119, CD 120a, CD 120b, CD 12 lb, CD123, CD 126, CD131, CD137, CD 139, CD 140a, CDl40b, CD 146, CD 147, CD 162, CD 166, CD 177, CD 178, CD222, CD223, CD235a, CD235b, CD253, CD271, CD 273, CD274, CD279, CD362, B7-H4, IL-la, IL-lb, IL-4, JL-6, IL--7, ID-8, ELIO, IL-12B, IL-13, IL-15, ID- 18, IL-18, IL-37, hepatoeyte growth factor (HGF), IFNa, lFN-γ, leukemia inhibitory factor (LIF), transforming growth factor ('1X317)41, TNF-α, tumor necrosis factor -inducible gene (TSG)6, thymic stromal lymphopoetin (TSLP), granulocyte-macrophage colony-stimulating factor (GM-CSF), transglutaminase (TGM)2, chemokine (C-X-C motif) ligand (CXCL)9, CXCL10, CXCLll, IDO, HLA-ABC, HLA--II, HLA-I, HLA-DR, HLA-DPB1, chemokine (CC motif) ligand (CCL)3, CCL5, CCL7, CCL8, CCL11, CCL18, cytokine receptor-like factor (CRLF)2, FCERA, CCR4, CCR5, CCE.7, CCR9, CX3CR1, CXCR4, CXCR5, CXCR6, BDNF, COXl, COX-2, cytokine-like (CY'TL)l, myeloperoxidase (MPO), neurotrophin (NT) -4, mm-T-ceO activation linker (NTAL), P53, proteinase (PRTN)3, Syk, MSCA1, arginase-2 (ARG2). As described herein, MSC- specific markers and markers for MSC subsets include:
CD 73, CD29, CD90, CD105, HLA-ABC, CD 166, CD146, CD44, CD140a, CD140b,
CD 13, MSCAI and Stro-L
In one aspect the disclosure provides a method comprising: providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-I7A, IL-1β, OSM, lFN-γ, TNF-α, IL-13 and IL-4; contacting said MSC with antibodies that bind to an MSC- specific marker and two or more selected from: the extracellular markers CD54, CD274, HLA- DR, the intracellular markers COX-2 and IDO; said method further comprising determining the presence of said MSC-specific marker and fold increase of the expression of said two or more of CD54, CD274, HLA- DR, COX-2 and IDO in response to said one or more cytokines.
In a further aspect the disclosure provides a method comprising: providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, IL-1β, OSM, IFN-y, TNF-α, IL-13 and IL-4;
contacting said MSC with binding molecules that bind to an MSC specific extracellular markers and three or more of the extracellular markers CD 54, CD274, HLA-DR and the intracellular markers COX-2 and IDO said method further comprising determining the presence of said MSC-specific marker and fold increase of the expression of said three or more of CD54, CD274,
HLA-DR, COX-2 and IDO in response to said one or more cytokines.
In a further aspect the disclosure provides a method comprising: providing MSC; - culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-I7A, IL-16, OSM, lFN-γ, TNF-α, IL-13 and IL-4; contacting said MSC with binding molecules that bind to an MSC specific extracellular markers and CD54, CD274, HLA-DR and the intracellular markers COX- 2 and/or IDO said method further comprising determining the presence of said MSC-specific marker and fold increase of the expression of CD54, CD274, HLA-DR, COX-2 and/or IDO in response to said one or more cytokines.
In a preferred embodiment, a method wherein an intracellular marker is contacted with binding molecules comprises additional steps of washing, fixing, and permeabilizing the ceils. Preferably, the binding molecules that bind to extracellular markers are washed away first. Subsequently, MSC are fixed. Thereafter the MSC are permeabiiized, where after the cells are contacted with binding molecules that bind to intracellular markers.
In one aspect the disclosure provides a method comprising: providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, IL-lfi OSM, lFN-γ, TNF-α, IL-13 and IL-4; - contacting said MSC with binding molecules that bind to an MSC- specific marker and one or more selected from: the extracellular markers CD54, CD274, HLA-DR washing said MSC permeabilizing said MSC - contacting said MSC with binding molecules that bind to the intracellular markers COX-2 and/or IDO; said method further comprising determining the presence of said MSC-specific marker and fold increase of the expression of said two or more of CD54, CD274, HLA- DR, COX-2 and IDO in response to said one or more cytokines.
In one aspect the disclosure provides a method comprising: providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, IL-1β, OSM, lFN-γ, TNF-α, IL-13 and IL-4;
contacting said MSC with binding molecules that bind to an MSC- specifie marker and two or more selected from: the extracellular markers CD 54, CD274, HLA-DR, and: washing said MSC; - permeabilizing said MSC; contacting said MSC with binding molecules that bind to the intracellular markers COX-2 and/or IDO; said method further comprising determining the presence of said MSC-specific marker and fold increase of the expression of three or more of CD54, CD274, HLA-DR, COX-2 and IDO in response to said one or more cytokines.
In a further aspect the disclosure provides a method comprising: providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, 1L-1B, OSM, IRN-g, TNR-a, IL-13 and 1L-4; contacting said MSC with binding molecules that bind to an MSC- specific marker and extracellular markers CD 51. CD274, HLA-DR, and; washing said MSC; permeabilizing said MSC; - contacting said MSC with binding molecules that bind to the intracellular markers COX- 2 and/or IDO; said method further comprising determining said capacity of said MSC on basis of presence of said MSC-specific marker and fold increase of the expression of CD54, CD274, HLA-DR, COX-2 and/or IDO in response to said one or more cytokines.
Where herein reference is made to “determining”, this term refers to the action of ascertaining or establishing by research or calculation. In a method of the invention, a capacity of MSC to inhibit an immune response. For example inhibit proliferation of target cells such as T-ceils, B-eelis, NK cells and other immune cells and/or monoeyte/macrophage polarization resulting in immune suppression through the production of soluble mediators and induction of T Regs is determined on basis of the presence of an MSC-specific marker on the MSC and the fold increase of the expression of one or more other markers in response to one or more cytokines. Where herein reference is made to “presence” of a marker, the term refers to the state or fact of a marker being present in or on a cell. Where herein reference is made to “fold increase”, the term refers to a ratio. In an embodiment of the invention, the fold increase indicates the number of times the presence of a marker on MSC has increased as a result of contacting MSC with one or more cytokines. Preferably, presence of a marker is deducted from the presence of a binding molecule bound to that marker. Preferably the binding .molecule is labelled with a iluorochrome. The fold increase in presence of a marker is typically calculated by dividing the fluorescent signal of the marker on MSC after culturing in presence of one or more cytokines, by the fluorescent signal of the marker on MSC after culturing in absence of cytokines. Fold increase can also be referred to as fold induction.
Where herein reference is made to “expression” of a .marker, the reference is to the amount of the marker protein present in or on the cell at the time of analysis. This is also referred to as the level of the m arker or protein. Fold increase in the expression and/or the level refers to the ratio of the expression and/or level determined using one condition and the expression anchor level determined in another condition.
In an embodiment of the invention, a capacity of MSC to inhibit an immune response. For example inhibition of proliferation of target cells such as T-cells, B-cells, NK cells and other immune cells and/or monocyte/maerophage polarization resulting in immune suppression through the production of soluble mediators and induction of regulatory ϊ-cells is determined on basis of the presence of an MBC-specIfic marker and the fold increase of one or more of CD.54, CD274, BLA-DR, COX-2 and IDO. In a preferred embodiment, the fold increase is at least 2, preferably at least 3-fold, more preferably at least 5. In one embodiment the fold increase of CD54 is at least 2-fold, preferably at least 3-fold, preferably at least 5-fold, more preferably at least 10-fold. In a further embodiment the fold increase of CD274 is at least 2-fold, preferably at least 3-fold, preferably at least 5-fold, more preferably at least 8-fold. In one embodiment the fold increase of HLA-DR is at least 2-fold, preferably at least 3-fold, more preferably at least 5-fold, at least 10-fold, at least 20-fold, more preferably at least 25- fold. In a further embodiment the fold increase of COX-2 is at least 2-fold, preferably at least 3-fold, in a further embodiment the fold increase of IDO is at least 2 -fold, preferably at least 3-fold, preferably at least 6-fold.
In one aspect the disclosure provides a method suitable for determining if MSC have a capacity to inhibit an immune response, for example inhibition of proliferation of target cells such as T-cells, B-cells, NK cells and other immune cells and/or monocyte/maerophage polarization resulting in immune suppression through the production of soluble mediators and induction of regulatory T-cells comprising: providing MSC; - culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, IL-1β, OSM, IFN-y, TNF-α, IL-13 and IL-4; contacting said MSC with binding molecules that bind to an MSC- specific marker and one or more selected from: the extracellular markers CD 54,
CD274, HLA-DR, the intracellular markers COX-2 and IDO; said method further comprising determining the presence of said MSC-specific marker and fold increase of the expression of said one or more of CD54, CD274, HLA- DR, COX-2 and IDO in response to said one or more cytokines, wherein said MSC have said capacity if said one or more markers are increased at least 2-fold, In one aspect the disclosure provides a method suitable for determining if MSC have a capacity to inhibit an immune response, for example inhibition of proliferation of target cells such as T-cells, B-cells, NK cells and other immune cells and/or monocyte/maerophage polarization resulting in immune suppression through the production of soluble mediators and induction of regulatory T-cells comprising: - providing MSC:
culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, 1L-1B, OSM, IRN-g, TNF-α, IL-13 and IL-4; contacting said MSC with binding molecules that bind to an MSC- specific marker and one or more selected from; the extracellular markers CD54, CD274, H LA-DR, the intracellular markers COX-2 and IDO; said method further comprising determining the presence of said MSC-specifie marker and fold increase of the expression of said one or more of CD54, CD 274, HLA- DR, COX-2 and IDO in response to said one or more cytokines, wherein said MSC have said capacity if said one or more markers are increased at least 3-fold or preferably at least 4-fold.
In one aspect the disclosure provides a method suitable for determining if MSC have a capacity to inhibit an immune response, for example inhibition of proliferation of target cells such as T-cells, B-cells, NK cells and other immune cells and/or monocyte/maerophage polarization resulting in immune suppression through the production of soluble mediators and induction of regulatory T-cells comprising: providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, IL-1β, OSM, lFN-γ, TNF-α, IL-13 and IL-4; - contacting said MSC with binding molecules that bind to an MSC- specific marker and one or more selected from: the extracellular markers CD 54.
CD274, HLA-DR, the intracellular markers COX-2 and IDO; said method further comprising determining the presence of said MSC-specifie marker and fold increase of the expression of said one or more of CD54, CD274, HLA- DR, COX-2 and IDO in response to said one or more cytokines, wherein said MSC have said capacity if said one or more markers are increased at least 5-fold,
The term ‘'ceil death” refers to the event of a biological cell ceasing to carry out its functions. Cell death may result from various causes, for example apoptosis, programmed cell death, mitotic catastrophe, necrosis, ischemic cell death and/or immunogenic ceil death. The term “cell viability” relates to the capacity of the cell to perform certain functions, such as metabolism, growth, reproduction, some form of responsiveness, and adaptability. Cell death and cell viability can be evaluated by a number of suitable assay’s known to the skilled person. Dye exclusion methods are frequently used as a measure to determine dead cells. Dyes as trypan blue do not easily pass the membrane of living cells hut will enter dead cells as these are not able to maintain the integrity of their cell membrane. A suitable method for determining the viability of cells is described in the example section. In one aspect of the disclosure, a method suitable for determining if MSC have a capacity to inhibit proliferation of T- cells and/or inhibit differentiation of monocytes to DC further comprises contacting MSC with a viability stain and determining viability- of the MSC. In a preferred embodiment MSC are contacted with a dye for exclusion together with binding mol etudes binding to extracellular markers.
MSC preparations comprise preferably at least 80%, preferably at least 90%, and more preferably at least 96% viable cells.
In one aspect the disclosure provides a method comprising: - providing MSC; culturing said MSC in presence and in the absence of one or more cytokines selected from: IL-17A, IL-1β, OSM, lFN-γ, TNF-α, EL- 13 and IL-4; contacting said MSC with binding molecules that bind to an MSC- specific marker and one or more selected from: the extracellular markers CD 54, CD274, HLA-DR and a dye for exclusion of dead cells, and the intracellular markers
COX-2 and IDO; said method further comprising determining the presence of said MSC-specific marker and fold increase of the expression of said one or more of CD54, CD274, HLA- DR, COX-2 and IDO in response to said one or more cytokines and the viability of said MSC.
Where herein reference is made to “kit”, the term refers to a set of articles or equipment needed for a specific purpose. In a preferred embodiment a kit comprises binding molecules that bind to an MSC-specific marker and one or more of CD 54, CD274, HLA-DR, COX-2 and IDO. Preferably said binding molecules are antibodies.
In a preferred embodiment of the Invention a kit further comprises one or more proinflammatory cytokines, more preferably one or more cytokines selected from: IL17A, IL-18, OSM, IFN-y, TNF-α, IL-13, IL-4. More preferably lFN-γ; IL-1β; lFN-γ and TNF-α; IL17A,IL-1B and OSM; IL17A, IL-18, OSM, lFN-γ, TNF-α and IL-13; IL17A, IL-18, OSM, lFN-γ, TNF-α, IL-13 and IL-4, or; lFN-γ, TNF-α, IL-13 and IL-4.
The term “ceil bank” refers to a facility that stores cells for the purpose of future use in a product or medicinal needs , Typically, a cell bank stores cryopreserved cells, Cryopreserved cells are typically stored in vials or freezing bags. The term “vial” refers to a small container, typically cylindrical, suitable for storing cells, including cryopreserved cells. In one embodiment, one vial contains MSC of one donor. In a further embodiment, one vial contains MSC of more than one donor. The term “freezing bag” refers to a bag suitable for storing cells, including cryopreserved cells,
In one embodiment, one freezing contains MSC of one donor. In a further embodiment, one freezing bag contains MSC of more than one donor.
In a preferred embodiment, presence and fold increase of markers is determined using a method and/or kit as disclosed herein.
In one embodiment the disclosure provides a method for preventing and/or inhibiting an immune response in a subject, comprising administering to a subject
MSC of which the presence of an MSC-specific marker and the fold increase of one or more of the markers CD54, CD274, HLA-DR, COX-2 and IDO was determined using a method and/or kit as disclosed herein. In a preferred embodiment the subject is exhibiting or at risk of exhibiting an undesired immune response. Undesired immune responses include, but are not limited to: autoimmune disorders including
inflammatory bowel disease, Type 1 diabetes, rheumatoid arthritis and multiple sclerosis, disorders associated with ado-immune responses, including solid organ transplantation and graft versus host disease, and (chronic or acute) inflammatory disorders including pulmonary emphysema, chronic kidney disease osteoarthritis, inflamed skin, brain lesions such as stroke, etc. In a particular embodiment of this method the subject is exhibiting inflammatory bowel disease. In a further particular embodiment of this method the subject is the recipient of an allogeneic ceil or organ transplant, In a further embodiment the present disclosure provides a method for preventing and/or inhibiting graft versus host or host versus graft disease in a subject comprising administering to a subject MSC of which the presence of an MSC- specific marker and the fold increase of one or more of the markers CD54, CD274, HLA-DR, COX-2 and IDO was determined using a method and/or kit as disclosed herein.
As used herein, a “subject” is an human or an animal. Subjects include, but are not limited to, mammals such as humans, pig's, ferrets, seals, rabbits, cats, dogs, cows and horses, and birds such as chickens, ducks, geese and turkeys. In a preferred embodiment of the invention, a subject is a mammal. In a particularly preferred embodiment, the subject is a human.
In one embodiment the disclosure provides a cell therapy product for use comprising MSC of which the presence of an MSC-specific marker and the fold increase of one or more of the markers CD 54, CD274, HLA-DR, COX- 2 and IDO was determined using a method and/or kit as disclosed herein.
As used herein, a “cell therapy product'’ is a product suitable for cell therapy. Where herein reference is made to “ceil therapy" this term is intended to refer to grafting, injecting, applying or implanting a cell therapy product into a subject,
Where herein reference is made to the term “suitable for" it is intended that a method or kit referred to herein can be used for the stated purpose. The phrase “suitable for" does not exclude other purposes of the claimed method or kit as referred to herein,
As used herein, "to comprise" and its conjugations is used in its non-limiting sense to mean that items following the word are included, but items not specifically mentioned are not excluded. In addition, the verb “to consist” may be replaced by “to consist essentially of’ meaning that a compound or adjunct compound as defined herein may comprise additional components) than the ones specifically identified, said additional components) not altering the unique characteristic of the invention.
The articles “a" and “an” are used herein to refer to one or to more than one (i.e,, to at least one) of the grammatical object of the article. By way of example, “an element” means one element or more than one element.
The word “approximately” or “about” when used in association with a numerical value (approximately 10, about 10) preferably means that the value may be the given value of 10 more or less 1% of the value. As used herein “at least” comprises the number itself and all numbers higher than that number.
As used herein, the terms "treatment," "treat," and "treating" refer to reversing, alleviating, delaying the onset of, or inhibiting the progress of a disease or disorder, or one oi' more symptoms thereof, as described herein. In some embodiments, treatment may be administered after one or more symptoms have developed. In other embodiments, treatment may be administered in the absence of symptoms. For example, treatment may be administered to a susceptible individual prior to the onset of symptoms (e.g., in light of a history of symptoms and/or in light of genetic or other susceptibility factors). Treatment may also be continued after symptoms have resolved, for example to prevent or delay their recurrence.
For the purpose of clarity and a concise description features are described herein as part of the same or separate embodiments. However, it will be appreciated that the scope of the invention may include embodiments having combinations of all or some of the features described,
AO patent and literature references cited in the present specification are hereby incorporated by reference in their entirety,
The invention is further explained in the examples. These examples do not limit the scope of the invention, but merely serve to clarify the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1
Effect of pro-inflammatory molecules on MSC function. lFN-γ stimulation results in an increased capacity of MSC to inhibit T-eell proliferation, IE- 18 stimulation enhances the capacity of MSC to inhibit DC differentiation.
Figure 2
Antibody array-based analysis on culture supernatants (left) or cell pellets (right) of unstimulated and IFN-y-stimulaied MSC (n=3 MSC per stimulus). Significantly differentially expressed proteins showing a log2 (fold change) of < -0.5 or > 0.5 in the supernatant of unstimulated or IFN-y-treated stimulated MSC are depicted.
Figure 3
(a) CyTOF- based screening of IFN-y stimulated cells. Fold induction of the indicated molecules is depicted as based on CyTOF-based surface expression analysis
of unstimulated and li’N-y-stimulated MSC. The graph represents a selection of those molecules, of the tested molecules, that were clearly expressed (i.e having an average signal intensity >6 ) .
(b) qRT PCR-based screening of lFN-γ stimulated cells. Fold induction is depicted of the indicated molecules as based on qRT PCR-based mKNA expression analysis of unstimulated and IFN-y-stimulated MSC.
(c) FACS confirmation of lFN-y-indueed molecules. Fold induction of the indicated molecules as based on FACS -based analysis of unstimulated and IFN-y- stimulated MSC.
Figure 4
Venn-diagram of the overlap in targets identified using different IFN-y-based screenings, as well as the FACS-eonfirraed hits. For mRNA hits; only those hits are shown that were screened for with at least one of the other screening methods
Figure 5
Targets screened with different techniques (as indicated). Arrows indicate downregulated (white) and upregulated (black) genes as identified by mKNA sequencing. Numbers indicate the amount of antibodies used to identify the target. Numbers highlighted in white and black indicate downregulated and upregulated targets, respectively. The first number indicates the amount of antibodies identifying the target as a hit, followed by the number of antibodies that this target was screened for with the indicated technique. The column indicated with “FACS” shows the targets either analyzed (highlighted in grey) or confirmed (highlighted in black) by flow cytometry.
Figure 6
(a) CyTOF- based screening of IL-16 stimulated cells. Fold induction of the indicated molecules as based on CyTOF-based surface expression analysis of unstimulated and IL-iB- stimulated MSC. The graph represents a selection of those molecules that were clearly expressed {i.e having an average signal intensity >6 ).
(b) qRT PCR-based screening of IL-18 stimulated cells. Fold induction of the indicated molecules as based on qRT PCR-based mRNA expression analysis of unstimulated and IFN-y-stimulated MSC. (c) FACS confirmation of IL- 18-induced molecules. Fold induction of the indicated molecules as based on FACS-based analysis of unstimulated and IL- IB- stimulated MSC
Figure 7 Venn-diagram of the overlap in targets that were identified using the different IL-1β-based screenings, as well as the FACS-eonfirmed hits. For mRNA hits; only those hits are shown that were also screened for with at least one of the other screening methods.
Figure 8
Targets and hits observed in IL-16 screening. Targets that are screened using different approaches are indicated. Numbers indicate the amount of antibodies that identify the target using each technique. Numbers highlighted in black indicate upregulated targets, in which the first number indicates the amount of antibodies that identified the target as a hit, followed by the number of antibodies that this target was screened for with the indicated technique. The column indicated with "FACS” show the targets that were either screened (highlighted in grey) or confirmed (highlighted in black) by flow cytometry.
Figure 9
Correlation between the fold induction of the indicated molecules and the capacity of MSC to inhibit T-eell proliferation following pre- stimulation with pro- inflammatory mediators. Numbers in the legend indicate the concentration of lFN-γ in ng/ml.
Figure 10
Blockade of the indicated molecules with either antibodies or enzyme-inhibiting drugs and their impact on the capacity of MSC to inhibit T-cell proliferation. MSC were eo-eultured with peripheral blood mononuclear cells (PRMC) that were stimulated with anti-CD3/CD28 beads. Next, tritium thymidine incorporation was analyzed after 7 days (pulsed with 3H thymidine at day 6), This assay was performed in the presence or absence of the indicated neutralizing antibodies and inhibitors. Y- cell proliferation is presented as percentage proliferation as compared to anti- CD3/CD28 bead stimulated PBMC. IDO and COX-2 inhibitors used were Cay 1058.1. and NS398, respectively.
Figure 11
Blockade of IDO and COX-2 with enzyme- inhibiting drugs and their impact on the capacity of MSC to inhibit DC proliferation. Purified CD14+ cells were co -cultured with MSC in the presence of GM-CSF and IL-4. Following 6 days of cultures, the ceils were analysed for CD la and CD 14 expression by flow cytometry. This assay was performed in the presence or absence DMSO, IDO inhibitor (INCB-024360) or COX-2 inhibitor (NS398). The percentage of CDla+CDl4- ceils after 6 days of co-culture with CD14+ monocytes is shown.
Figure 12
Principle and timing of the potency assay. Three different culture regimens were tested. Ceils were either thawed prior to day 0 (condition 1), on day 0 (condition 2) or on day 1 (condition 3),
Figure 13
Calculation of fold induction. Here, the fold induction is calculated by dividing the mean fluorescence intensity of the stimulated cells by the mean fluorescence
intensity of the unstimiUated cells. In this case: 50784/409 = 124.2, which is the fold induction for HLA-DR.
Figure 14 The ratio of fold induction and of the mean fluorescence intensity (MFI) of MSC tested in the potency assay. The numbers refer to the different culture regimens. Each dot represents the ratio of fold induction by dividing the fold induction of experiment 1 by the fold induction obtained in experiment 2 (i.e. the duplo of experiment 1).
N=4 MSC tested in 4 experiments.
Figure 15
Different TNF-α batches yield similar results. Three different MSC were evaluated using 2 different batches of TNF-α {depicted as TNF1 and TNF2). Figure 16
Overview of potency assays performed thus far using culture method 2. The last two bars in each panel are 1MSC5 and iMSC9 respectively.
Figure 17 Upregulation of COX-2 fallowing 24 hours of stimulation with IL-16.
Upregulation of COX-2 was measured by flow cytometry using a PE -labelled anti- COX-2 antibody (clone AS67, BD Pharmingen).
EXAMPLES
Example 1. Effect of pro-inflammatory molecules on MSC function
In pre-clinkal research, the potency of MSC has been shown to be influenced by pro-inflammatory molecules, such as cytokines . Not all inflammatory stimuli result in the same type and level of response. Whereas stimulation with some pro-inflammatory molecules induces MSC with an anti-inflammatory function, stimulation with others results in the generation of MSC with a pro-inflammatory function. To assess which stimuli are most potent in inducing MSC having anti-inflammatory functions, the effect of different cytokines on two anti-inflammatory capacities of MSC were tested, These two anti-inflammatory capacities were the capacity to inhibit T-cell proliferation, and the capacity to inhibit dendritic ceil (DC) differentiation.
Materials and Methods MSC culture
Human bone marrow MSC (bmMSO) were isolated and characterized as described before (Duijvesteijn et ah, Gut (2010) 59:1662-1669) and cultured in complete culture medium consisting of Dulbeeeo’s Modified Eagle Medium (DMEM) with Glutamax (Gibco, Gaithersburg, MD, USA) supplemented with 10% fetal calf serum (FCS, Sigma-Aldrich, Saint Louis, MO, USA) and penicillin/streptomycin
(Gibco), MSC were passaged when 80-90% eonflueney was reached. For passaging and flow cytometry analysis, cells were harvested by TrypLE Select (Invitrogen) incubation for i0 minutes. MSC between passage 4 -7 were used in experiments.
Stimulation of human MSC
MSC were stimulated for different periods of time (as indicated in Figure 1) in the presence or absence of different pro-inflammatory molecules at the following concentrations' Poly (1:C) (20 pg/rnL; from Sigma Aldrich Chernie), IL-18 (1 ng/mL) (R&D Systems Inc.), lFN-γ (10 ng/mL; Peprotech), TNF-α (1 ng/mL; Peprotech). For stimulation, MSC were plated at 16. ox 103 cells/cm2 and cultured overnight in culture medium after which the culture medium was replaced by medium containing pro- inflammatory molecules.
Immune cell isolation
Human peripheral blood mononuclear cells (PBMC) were isolated from huffy coats of healthy donors obtained from Sanquin Blood Supply (Leiden, The Netherlands) using a Ficoll-Amidotrizoate density gradient (LUMC Pharmacy). Monocytes were enriched from PBMC by magnetic activated cell sorting (MACS) using anti-CD14 microbeads, followed by separation on a MACS LS column according to the manufacturer's protocols (Miltenyi Biotec GmbH. Bergisch Gladbach, Germany),
T-cell co-culture
Approximately' Ib.dcIO3 MSC (either un- or pre-stimulated) and 1.0x105 freshly isolated T-cell-enrlehed PBMC were plated in a 96- wells V-shaped plate (Corning Inc.) In a MSCiPBMC ratio of 1:6 in RPMI 1640 culture medium (Gibco #22409-015) supplemented with Glutamax (lx, Gibco #35050-38), T-cell- enriched PBMC were stimulated with human T-cell-activator CD3+/CD2S+ Dynabeads (Thermo Fisher Scientific Inc.) in a celbhead ratio of 5:1. After 1 day of co-culture at 37°C and 5% 002, tritium-labelled thymidine was added. After overnight culture (1.6 hours), the cellswere harvested on day 2 on a glass fiber filter and the tritium thymidine incorporation was measured using a liquid scintillation counter (PerkinEImer).
Monocyte co-culture
Approximately 3x103 MSC {either un- or pre-stimulated) and 1.0x106 freshly isolated monocyte- enriched PBMC were eo -cultured in 6- well plates in RPMI culture medium supplemented with IL-4 (10 ng/mL) and granulocyte-monocyte colony- stimulating factor (GM-CSF) (o ng/mL) (both from Invitrogen Corp.). After 2 days, half of the medium was replaced by cytokine-containing RPMI culture medium (IL-4 and GM-CSF at 20 ng/mL and 10 ng/mL, respectively). After 6 days, cells were analyzed by flow cytometry for expression of CD 14 and CD la.
Results
MSC pre-stimulated with lFN-γ were found to be most potent in inhibiting T- eell proliferation, followed by MSC pre-stimulated with TNF-α or IL-16 (Figure 1, upper panel). Inhibition of dendritic cell differentiation was most potently induced by pre-stimulation with IL-16 (Figure 1, lower panel).
Conclusion
Pre-incubation with lFN-γ most potently affects the capacity of MSC to inhibit T-cell proliferation, IL-lβ most strongly induces the capacity of MSC to inhibit differentiation from monocytes to DCs.
Example 2. Identification of molecules induced upon stimulation with IFN-γ and IL-1β and their correlation with immunomodulatory function of MSC
In p re-clinical research, infra -and extracellular molecules have been identified to be implicated in the immunomodulatory action of MSC. Many of these molecules are not expressed at steady state, but tire upregulated in response to stimulation with proinflamraatory molecules, such as cytokines.
Since pre-incubation with lFN-γ or IL-1β resulted in the largest enhancement of MSC -mediated T-eeli proliferation inhibition and DC differentiation inhibition respectively (figure 1), we performed screens to identify molecules that are differentially expressed in response to these stimuli.
To this end we applied mRNA sequencing of stimulated and unstimulated cells. This is an unbiased method to identify any gene that is regulated by IFN g or IL-1β at the transcriptional level. To assess whether the genes affected by IFN-y or IL-1β stimulation at the transcriptional level were also affected at the protein level, we performed additional screenings using an antibody array and/or mass cytometry (cytometry by time of flight, CyTOF). Some hits that were identified in the mRNA sequencing screening were not included on the antibody array and/or CyTOF panel, therefore we also performed qRT PCR analysis for confirmation of some of the hits identified by mRNA sequencing.
Next, we determined whether the identified molecules can also he identified by flow cytometry, followed by correlating the induction of these molecules with the capacity of pre- stimulated MSC to inhibit T-cell proliferation.
To determine whether molecules upregulated upon lFN-g and/or IL-1β stimulation are directly invol ved in MSC- mediated T-cell proliferation inhibition or DC differentiation, we analyzed the effect of blocking these molecules on the capacity of MSC to modulate these responses.
Materials and Methods
MSC culture
Human hone marrow MSC (hmMSC) were isolated and characterized as described before (Duijvesteijn et al., Gut (2010) 59:1662-1669) and cultured in complete culture medium consisting of Dulbeeco’s Modified Eagle Medium (DMEM) with Glutamax (Gibco, Gaithersburg, MD, USA) supplemented with 10% fetal calf serum (FCS, Sigma-Aldrich, Saint Louis, MO, LISA) and penicillin/streptomycin (Gibco). MSC were passaged when 80-90% confluency was reached. For passaging and flow cytometry analysis, cells were harvested by TrypLE Select (invitrogen) incubation for 10 minutes. MSC between passage 4 - 7 were used in experiments.
Stimulation of human MSC
MSC were stimulated for different periods of time (as indicated) in the presence or absence of different pro-inflammatory molecules at the following concentrations: LPS (200 ng/niL), Poly (I:C) (20 pg/mL; both from Sigma Aldrich Chemie), IL-16 (1 ng/mL) (E&D Systems Inc.), lFN-γ (10 ng/mL is default, 1 and 100 ng/ml when indicated), TNF-α (1 ng/.mL) (both Peprotech) or both iFN-g and TNF-α (10 ng/ml IFN- Y and 1 ng/ml TNF-α). For stimulation, MSC were plated at 16.5x10s cells/cm2 and cultured overnight in cult ure medium after which the culture medium was replaced by medium containing pro-inflammatory molecules.
For RNA analysis by sequencing, MSC were detached using trypsin/EDTA or TrypLE Select, resuspended in RNAlater and stored at -80°C. For RNA analysis by qRT PCR, MSC were detached using trypsin/EDTA or TrypLE Select, resuspended in 350 uL RLT-buffer (Qlagen) containing Rib 6-mereaptoethanol (Sigma Aldrich Cheraie) and stored at -80°C. For antibody array analysis, MSC were cult ured in phenol -red free Optimum supplemented with penicillin/ streptomycin (Gibeo). For antibody array analysis of the cells, MSC were detached using trypsin/EDTA and washed once with PBS. 5 pL lx protease inhibitor cocktail was added to the pellet, which was then frozen at -80°C. For antibody array analysis of the supernatants, 22-30 ml of conditioned medium of± 2,dc10ϋ cells was harvested, spun at 3000 g for 5 minutes to remove debris and concentrated using Vivaspin 20 filtration columns (Sartorius, 3,000 M.WCO, #VS2091). PBS was loaded on the filtration columns to substitute the medium. Concentrated samples were then frozen at -80°C and shipped together with the cell pellets to Sciomics (Seiomics, Germany) for further analysis.
For in vitro co-cultures with immune cells, MSC were detached using TrypLE Select and used for co-cultures. rnENA sequencing
Total RNA (mRNA, miRNA and other small RNAs) was isolated using a QIAsymphony using the miRNA CT 400 protocol and an elution volume of 100 mΐ The samples were processed using the NEBNext Ultra RNA Library Prep Kit and the protocol “NEBNext Ultra Directional RNA Library Prep Kit for IUumina”(NEB#E7420S/L). Briefly, mRNA was Isolated from total RNA using the oligo-dT magnetic beads. After fragmentation of the mRNA, a cDNA synthesis was performed. This was used for ligation with the sequencing adapters and PCR amplification of the resulting product. The quality' and yield after sample preparation was measured with a Fragment analyzer. The size of the resulting products was consistent with the expected size distribution, i.e. a broad peak between 300-500 bp. Clustering and DNA sequencing using the Illumina cBOT and HiSeqy 4000 was performed according to the manufacturer’s protocols. A concentration of 3.0 nM of DNA was used. Image analysis, base calling, and quality check was performed with the Illumina data analysis pipeline RTA v2,7.7 and Bel2fastq v2.17.
Antibody array analysis
The antibody microarrays (ScioCD version 1) were produced and hybridized by Sciomics (Sciomics, Germany). For hybridization, samples were labelled with NHS-
esters of the fluorescence dye Dy-649 (Dyomies, Jena, Germany), Samples of unstimulated cells were used as reference sample and labelled with the fluorescent dye Dy- 549 (Dyomies) for competitive dual-color incubations. Scanning of the slides was performed with a PowerSeanner (Tecan) at a resolution of 10 pm, maintaining laser power and the photomultiplier constant. Spot segmentation was performed with GenePix Pro 6.0 software package (Molecular Devices, Sunnyvale, CA, USA). The spot signals were analysed with R-Bioeonductor using a one-factorial linear model fitted with LIMMA package, which resulted in a t-tesfc based on moderated statistics. For normalization, a specialized invariant Lowess method was applied as described before (C, Schroder et al. Proteoraics Clin, Appl. 2013), In the analyses, duplicate spots were taken into consideration. For differential analysis of protein expression, a one-factorial linear model was fitted with LIMMA resulting in a two-sided t-iesfc based on moderated statistics. Negative fold change values indicate a lower abundance, positive values indicate a higher abundance of the given protein in the sample as compared to the unstimulated condition.
CyTOF
MSC were washed with staining buffer (CSM, lx PBS with 0.5% bovine serum albumin and 0.02%» sodium azide, Fluidigm Sciences) and incubated with 1 ml CSM containing 1:500 diluted 500 pM rhodium DNA intercalator (Fluidigm Sciences) for 15 min at room temperature (RT) to stain dead cells. Cells were washed with CSM and surface stained for 45 min at RT with a mixture of metal isotope-conjugated antibodies using predetermined concentrations. Antibody staining reactions were performed in 100 pi final volume. After staining, cells were washed twice with CSM and then resuspended ίh 1 ml of 1:1,000 diluted 125 pM iridium DNA intercalator (DVS Sciences) in Fix and Perm Buffer (PBS with 1.6% paraformaldehyde, Fluidigm Sciences) for 1 hour at RT (or overnight at 4 X) to allow discrimination of single cells. Finally, cells were washed twice in CSM and once in distilled water at RT, Prior to data acquisition, cell pellets were diluted in distilled water containing 1:10 diluted EQ Four Element Calibration Beads (Fluidigm Sciences) to the concentration of 0.4 x 10R cells/mL and acquired on a CyTOF 2™ mass cytometer (Fluidigm Sciences), CyTOF data were acquired and analyzed on-the-fly, using dual-count mode and noise- reduction on. All other settings were either default settings or optimized with tuning solution, as instructed by Fluidigm Sciences, After data acquisition, the mass bead signal was used to normalize the short-term signal fluctuations with the reference EQ passport P13H2302 during the course of each experiment and the bead events were removed. qETPCR
To determine the expression levels of the mRNA transcripts, total RNA was extracted using the RNeasy- minikit (Qiagen) according to the manufacturer’s protocol, cDNA was synthesized using Superscript III RT (Invitrogen Corp.), qRT-PCR analyses were performed on a StepOnePlus real-time PCR system (Applied Biosystems) using the universal probe library (Roche life sciences). All qRT-PCR data were normalized to GAPDH expression and fold induction was calculated by dividing the gene expression
of stimulated ceils by the expression of unstimulated cells using the delta-deita-Ct method.
Flow cytometry analysis to validate mRNA and proteomics based screenings
After stimulation of 60,000 MSC for 24 or 48 hours, MSC were harvested and stained for flow cytometry analysis. MSC were incubated for 30 minutes at 4°C with antibodies against extracellular markers. For staining of intracellular markers the MSC were fixed (fixation/permeabilization buffer and diluent, eBioseience) for 20 minutes at room temperature (RT) and thereafter permeabilized (permeabilization buffer, eBioseience) and stained for the intracellular markers for 25 minutes at RT. When ceils were stained for intracellular IL-8, cells were incubated with Golgistop (BD Biosciences) overnight the morning before harvesting the cells. The MSC were analysed by flow cytometry with the BD FACS™ Canto II cytometer (BD Biosciences). Analysis of the data was performed with FlowJo software version 8.7.1 (Tree Star Inc., Ashland, OR, USA), For single ceils the median fluorescent intensity (MFI) was obtained and fold induction was calculated by dividing the MFI of stimulated MSC by the MFI of unstimulated MSC.
Results
Screens to identify molecules that are induced by MSC upon stimulation with IFN-y
To identify molecules that are differentially expressed by unstimulated and IFN-y- stimulated MSC, we performed mRNA sequencing of stimulated and unstimulated cells. This analysis revealed 461 differentially regulated mRNAs of which 361 were upregulated and 99 were downregulated in IFN-y treated MSC compared to unstimulated cells. Reaetome analysis (https://reactome.org/) indicated that the most affected process was the “Immune system” and that the pathways “Interferon-g signalling”, “Interferon-a/B signalling” and “Antigen Presentation” were identified as the three most affected pathways.
To assess whether the genes affected by lFN-γ stimulation at the transcriptional level were also affected at the protein level, we performed additional screenings using an antibody array and mass cytometry (cytometry by time of flight, CyTOF). Some hits that were identified in the mRNA sequencing screening were not included on the antibody array anti/or CyTOF panel, therefore we also performed qRT FCR analysis for confirmation of some of the hits identified by mRNA sequencing (Table I) .
Using antibody array- (Figure 2), CyTOF- (Figure 3A) and qRT PCR (Figure 3B), we confirmed several of the hits previously identified using mRNA sequencing. These include IL-6, CD96, HLA-I, HLA-DPB1, CCL6, CCL7, CCL8, CD274, HLA-DR, CD54, CD 1,06, IDO, CXCL9, CXCL10 and CXCL11 .
Using antibody array- and CyTOF, additional markers were identified. Of these markers, CD9, C-D29 and CD39 were downregulated and CD 140b and CD273 were upregulated by MSC at the protein level following ΪRN-g stimulation.
Next, we determined whether these molecules can also be identified by flow cytometry (FACS) (Figure 3C). HLA-1, HLA-DK, CD 54, CD 106, CD274 and IDO were confirmed to be upregulated in response to lFN-g stimulation (Figure 4 and Figure 5).
Screens to identify molecules that are induced by MSC upon stimulation with IL-Ib
To identify molecules that are differentially expressed in IL-1β- stimulated MSC compared to unstimulated cells, we performed CyTOF and qRT PCR screenings.
CyTOF- experiments identified up regulation of CD54 and downregulation of CD9, CD 103, CD 120a and CD 140a upon IL-1β-stimulation (Figure 6A). Using qRT PCR we identified upregulation of CNCL9, CXCLIO, CXCL11, TSG-6, IDO, COX-2,
IL6, and IL-8 following IL-lS- stimulation (Figure 6B).
Subsequent flow cytometry analysis confirmed the hits CD 34, IL6 and COX- 2, whereas CD 105, CD 140a and IDO expression remained unaffected by IL-1β- stimulation (Figure 6C, Figure 7 and Figure 8), Other IL-1β-induced molecules were thus far not evaluated by flow cytometry (Table 2) .
Flow cytometry analysis to identify molecules correlating with the immunomodulatory function of MSC lFN-γ stimulated MSC were most potent to inhibit T-eell proliferation in vitro (Figure 1), After identifying the molecules that are upregulated in response to lFN-γ that can be measured by flow cytometry (Table 1 and Figure 4), we correlated the induction of these molecules with the capacity of pre-stimulated MSC to inhibit T-cell proliferation. To this end, MSC were pre-stimuiated with different concentrations of lFN-γ, the combination of lFN-γ and TNF-et, LPS, Poly(I:C), TNF-α or IL-18.
A correlation was found for lFN-γ- induced upregulation of CD 106, HLA-DR, IDO, CD274 and CD54 and the capacity to inhibit T-cell proliferation. This indicates that the level of stimulus -Induced expression of these molecules can he a predictor of the capacity MSC to inhibit T-cell proliferation (Figure 9).
For some molecules, such as HLA-DR and CD54 the MSC showed a different slope, suggesting that there might be differences between MSC products in the extent to which they respond to cytokine- stimulation with regards to upregulation of molecules and the capacity to modulate T-cell proliferation {figure 9).
Blocking experiment to determine which molecules are responsible for the immunomodulatory function of MSC
To determine whether molecules upregulated upon IFK-g and/or IL-1β stimulation are directly involved in MSC-mediated T-cell proliferation inhibition or DC differentiation, we analyzed the effect of blocking these molecules on the capacity of MSC to modulate these responses. CD54, CD 106, CD274, IDO and COX-2 might all be directly involved in the MSC-mediated inhibition of T-eell proliferation (Figure 10), whereas COX-2, but not IDO, is directly involved in the MSC-mediated inhibition of DC differentiation (Figure 11).
Conclusion
Using mHNA sequencing, antibody array, CyTOF and qRT PCR, several molecules were identified that are differentially expressed by MSC in response to preincubation with IFN-y anchor IL-18, These molecules were confirmed using flow cytometry. For 1FN- g this resulted in the hits: CD274, IDO, HLA-I, HLA-DR, CD54
and CD 106 (Figure 4), all of which were found to be upregulated (Figure 6), Plow cytometry confirmed that pre-incubation with IL-1β resulted upregulation of CD64, IL- 6 and COX-2 (figure 7 and 8), Inhibition of T-cell proliferation was shown to correlate with the fold induction of CD 106, HLA-DR, IDO, CD274 and CD54 (figure 9),
Blocking experiments using antibodies or inhibitors of the upregulated molecules proved direct involvement of CD 64, CD 106, CD274, IDO and COX-2 in inhibition of T- cell proliferation (Figure 10), and COX-2 in inhibition of DC differentiation (Figure 11). These results are summarized in Table 3
Table 3 An overview of the results of the different analysis based on Figure 4. 5, 7, 8» 9, 19 and 11 V: Molecule is upregulated upon cytokine stimulation; X: molecule is not upregulated in response to cytokine stimulation; NT: Not tested.
Example 3. Determine which of these molecules can be measured by flow cytometry in a reproducible and standardized fashion
After generating the optimal settings for the analysis of unstained MSC, taking into account the auto-fluorescence of these cells, the linearity range of the flow cytometer and the electronic noise, the target values for the PMTss were set using rainbow beads.
Next, the optimal fluorescent channels for visualizing the surface markers and intracellular stains were determined, taking into account the level of auto-fluorescenee of the MSC in a given channel and the expected expression level for a given target molecule. Next, all antibodies were titrated till saturating levels. If chosen antibodies did not reach saturation and/or went out of the linearity range of the flow cytometer, additional antibody clones that recognized the same target and/or other iluoroehrome- conjugates were tested. In total 25 different antibodies and 10 different clones have been evaluated to come to the most optimal antibody panel.
Materials and Methods
Flow cytometry analysis to validate rnENA and proteomics based screenings After stimulation of 60,000 MSC for 24 or 48 hours, MSC were harvested and stained for flow cytometry analysis, MSC were incubated for 30 minutes at 4°C with antibodies against T-cell surface markers. For staining of intracellular markers the MSC were fixed (ilxatiuii/permeabilization buffer and diluent, eBioscience) for 20 minutes at room temperature (RT) and thereafter permeabilized (permeabilizaiion buffer, eBioscience) and stained for the intracellular markers for 25 minutes at RT, When cells were stained for intracellular IL-6, cells were incubated with Golgistop (RD Biosciences) overnight the morning before harvesting the cells. The MSC were analysed by flow cytometry with the BD FACS™ Canto II cytometer (BD Bioseiences). Analysis of the data was performed with FlowJo software version 8.7.1 (Tree Star Inc., Ashland, OR, USA). For single cells the median fluorescent intensity (MFI) was obtained and fold induction was calculated by dividing the MFI of stimulated MSC by the MFI of unstimulated MSC,
Conclusion
These extensive screens lead to the exclusion of CD 106 from the panel as the expression levels of this marker were highly variable between stains and did not allow standardization. In addition, a dead stain has been added to the panel to allow exclusion of dead cell contamination. Anti-CD73 was added to serve as an identifying marker for MSC. Alternatives for CD73 are CD90, CDIGo, CD29, HLA-ABC, CD 166, CD 146, CD44, CD 140a, CD 140b. All details on the antibodies that were evaluated and selected for the final panel are depicted in table 5.
Example 4, Optimization of the in vitro MSC stimulation to obtain a robust and standardised assay
To maximize the robustness of the assay, we have compared the outcome of 3 different culture regimens (1, 2, 3) (figure 12). Cells were thawed prior to days 0 (condition 1), on day 0 (condition 2) or on day 1 (condition 3). Experiments were performed in duplicates, where MSC were assessed on the same day as well as on different days.
To test the reproducibility of the assay, 3 MSC products were tested in 2 experiments using method 2. The same MSC products (MSC 63, MSC 67 and MSC 120) were used for both experiments. The experiments were performed on different days.
To assess the effect of fetal bovine serum on the potency assay, potency of a single MSC was evaluated when cultured in 2 different batches of fetal bovine serum (batches ABB and PAA), The test was performed in quadruplicate; experiments I and 2, and experiments 3 and 4 are duplicates performed on the same day. The experiments 1-2 and 3-4 were performed on different days.
Materials and Methods
Standardized potency assay
At day 0, MSC are thawed and plated in 2 wells of a 6-well plate, at a density of 160 x103 viable cells per well, in 2.5 ml culture medium (DMEM, supplemented with 10% fetal bovine serum, penicillin and streptomycin) and cultured overnight at 37°C, 7% COg. On day 2, MSC are stimulated with 10 ng/ml lFN-γ Preprotech) and 1 ng/ml TNF-α (Preprotech) for 46-50 hours at 37*0, 5% CO2.
At day 3, cells are harvested using TrypLE Select and a total of 45,000 cells are stained for 30 minutes (4°C, protected from light) with antibodies directed against CD54, CD274, HLA-DR and CD73 using the clones and fiuoroehromes shown in table 5. The antibodies are diluted in PBS supplemented with 2% (v/v) human albumin. Subsequently, cells are washed in PBS and dead cells stained using the fixable Aqua dead cell stain kit (Thermo Fisher) according to the manufacturer’s recommendation. Prior to staining the intracellular molecule IDO, the cells are fixated and permeabilised using the fixation/per meabilization kit obtained from eBioseience according to the manufacturer’s recommendation. Cells are acquired in a Canto II using pre- defined Euroflow MSC- settings.
Results
Culture regimen
On day 3, all cells were evaluated for the expression of extracellular and intracellular markers. Next the fold induction, i.e. for example the mean fluorescence intensity for a specific marker of the stimulated MSC divided by the mean fluorescence intensity for the same marker of the unstimulated MSC, was determined. An example of this calculation is shown in figure 13, an exemplary formula is shown below.
To directly compare the fold induction of the MSC that were tested in duplicates (either on the same day or on different days), we calculated the ratio of fold induction by dividing the fold Induction of experiment 1 by the fold Induction obtained in experiment 2 fi.e. the duplicate of experiment 1). ratio of told induction
If the fold inductions are similar the ratio would be close to 1 (figure 14, left column) , We also compared the mean fluorescence intensities of both stimulated and unstimulated MSC using the same calculation (figure 14, right column).
Testing different culture regiments: These experiments indicated that the highest reproducibility was obtained when MSC were thawed from liquid nitrogen on the day before cytokine stimulation, i.e, culture method 2. This is also apparent from figure 14, where it is demonstrated that the ratio is closest to 1 when using culture method 2.
Reproducibility
The results are shown in table 8. Due to high variability, CD 106 was excluded from the final potency test.
Effect of FBS hatch
The results are shown in table 7 and S, Only CD54 expression is affected by the batch of fetal bovine serum (Table 7). To confirm if the expression of CDS4 alone is affected by the batch of fetal bovine serum used, two batches of fetal bovine serum (PAA and 084) were directly compared in the potency assay testing 3 MSC products (Table 8), This test confirmed that CD 54 expression alone may be influenced by the fetal bovine serum hatch used.
Table 8 CDS4 expression is dependent on the fetal bovine serum batch used.
Effect of TNF-α hatch
Three different MSC products were tested using two different TNF-α batches. No differences could he observed between the different batches (Figure 15).
COX-2 is consistently upregulated upon incubation with JL- Ib for 24h
Nine individual MSC products were incubated with IL-1β for 24 hours. All MSC products showed an increase in expression of COX-2 in response to IL-1β (Figure 17), supporting that expression of COX-2 is consistently increased in MSC in response to IL-1β.
Conclusion
Thus far, over 80 potency assays have been performed using culture method 2 (figure 16), and show that the assay is reproducible. Figure 16 presently shows the results of 81 MSC in total (79 bone marrow-derived MSC and 2 iPSC-derived MSC). Differences in FBS and/or TNF-α have limited to no influence on the reproducibility. This number of assays allows us to obtain an overview of the variability in fold- induction values that can be obtained per cell marker.
Summary of potency testing iPSC-derived MSC
Two MSC-lines derived from induced Pluripotent Stem Cells (iPSC) were evaluated in the potency assay: iMSC P5 and iMSC P9, The same standardized protocol was used as for the BM-derived MSC. In table 9, the result of the potency assay is depicted.
The markers CD54, IDO. CD274, HLA-DR and CD 73 were measured. COX- 2 was not analyzed in these experiments.
In table 10, the averages, lowest and highest values and standard deviation are shown for fold inductions obtained assessing 79 bone marrow-derived MSC that have been assayed.
Table 10. Results of potency testing of bone marrow-derived MSC
FI: Fold Induction Min: lowest value obtained Max: highest values obtained SDi standard deviation
The fold inductions of CD54, IDO and CD274 observed for iMSC are similar to the average values obtained with bone marrow- derived MSC. HLA-DE upregulation was lower compared to the average bone marrow- derived MSC. but within the range observed for bone marrow-derived MSC.
CD73 expression was upregulated compared to bone marrow-derived MSC. However, this marker serves as an MSC -identifying marker and it’s expression is not related to MSC potency.
Conclusion
The potency assay can also be used to evaluate iPSC-derlved MSC.
Potency of culture-expanded MSC administered to patients with aGvHD
The inventors have found that biological properties of MSC relate to the ability of these cells to be induced by soluble pro inflammatory mediators, such as IRNg, IL-1β and TNFa, These mediators activate effector mechanisms including a,o. the upregulation of immune suppressive soluble mediators, such as EL- 10 and the intracellular markers IDO and CQX2, leading to suppression of proliferation of immune cells, including T cells, B cells and NK cells and an increase in immune suppressive M2-type macrophages and T Regs in vivo and in vitro. In addition. GMP (Good Manufacturing Practice)- grade MSC products that have been administered to patients with steroid refractory aGvHD after allogeneic stem cell transplantation in the context of a clinical trial, exhibit enhanced expression of the surface- markers CD54, CD274 and HLA-DE and of the intracellular markers IDO and/or COX-2, when exposed in-vitro to pro-inflammatory mediators, including IFNy, IL-18 and TNFa. This shows the immune suppressive function of the cells and that these MSC were suitable cell therapy products for Immune suppressive functions in aGvHD and other inflammatory diseases associated with an over-active immune system.
In the context of a randomized placebo -controlled multicenter phase III study (HO VON 113 trial, EUDRACT Database #2012-004915-30), we administered two intravenous injections at a seven- day interval, of allogeneic MSC at a dose of 2x106/kg body weight {range 1.5 -2.5 x106 per kg). The MSC were expanded from hone marrow of
healthy adult individuals and administered to children and adults developing steroid- refractory grade II- IV visceral acute Graft- versus-Hosfc disease after allogeneic stem cell transplantation, 21 patients were randomized to the MSC arm and 21 to the placebo arm. We administered MSC products from 14 healthy donors and tested these in our assay. Results are shown in the table below:
Testing MSC products from all 1.4 healthy donors, including multiple products derived from a single donor where applicable; our results show a significant increase in the expression of the intracellular marker IDO and of the extracellular markers CD 64, CD274 and HLA-DR, showing the reproducible and robust immune suppressive potential of these cells,
These results further support the conclusion that the pro-inflammatory induction responses as measured by the indicated marker expression herein, provides a suitable assay system for testing whether MSC grafts are statable for transplantation and performing immune suppressive functions after transplantation.
Claims
1, A method for determining the capacity and/or potency of mesenchymal stromal cells
(MSC) to inhibit an immune response comprising:
- providing MSC;
* culturing said MSC in the presence and in the absence of one or more cytokines selected from: interleukin (IL)17A, IL-1β, oncostatin M (OSM), interferon y (IFN- y), tumor necrosis factor ct (TNF-α), IL-13 and IL-4;
- contacting said MSC with binding molecules that hind to an MSC -specific marker and one or more selected from: the extracellular markers CD 54. CD274, human leukocyte antigen (HLA)-DR, and the intracellular markers cyelo- oxygenase-2 (COX-2) and indoleamine-pyrrole 2,3-dioxygenase (IDO); said method further comprising determining the presence of said MSC-specific marker and the fold increase of the expression of said one or more of CD 54, CD274, HLA- DR and IDO markers in response to said one or more cytokines and determining from said presence and said fold-increase the capacity and/or potency to inhibit said immune response.
2, A method for treating a subject in need of MSC based therapy" with a population of
MSC cells having therapeutic potential comprising: a) validating therapeutic potential of the cell population by: providing MSC; culturing said MSC in the presence and in the absence of one or more cytokines selected from: interleukin (IL)17A, IL-1β. oncostatin M (OSM). interferon g (IFN- g), tumor necrosis factor a (TNF-α), IL-13 and IL-4; contacting said MSC with binding molecules that bind to an MSC-specific marker and one or more selected from: the extracellular markers CD 54, CD274, human leukocyte antigen {HLA)-DR, and the intracellular markers cyclooxygenase-2 (COX-2) and indoleamine-pyrrole 2,3-dioxygenase (IDO): b) determining the presence of said MSC-specific marker and the fold increase of the expression of said one or more of CD54, CD274, HLA-DR and IDO markers in response to said one or more cytokines; and c) identifying the MSC population as having therapeutic potential if the score is greater than or equal to a predetermined cut-off value, and b) administering a therapeutically effective amount of the MSC population having therapeutic potential to the subject.
3, A population of MSC cells for use in a method of treatment of a subject that is exhibiting or is at risk of exhibiting an undesired immune response wherein said MSC cells have been determining to have therapeutic potential with a method according to claim 1.
4, The method of claim 1 or 2 or the population of MSC cells for use of claim 3, wherein said MSC-specific marker is CD73, CD90, CD105, CD29, HLA-ABC, CD 166,
CD 146, CD 44, CD 140a, CD 140b, CD 13 or MSCAl.
5. The method or the population of MSC cells for use of claims 1-4, wherein contacting said MSC with binding molecules comprises:
- contacting said MSC with binding molecules that bind to said MSC- specific marker, and one or more of said extracellular markers;
- washing and permeabiilzing said MSC, and;
- contacting said MSC with binding molecules that bind one or more of said intracellular markers,
6. The method or the population of MSC cells for use of claims 1-5, wherein said fold increase of expression:
- of CD 54 is at least 10-fold;
- of CD274 is at least 8-fold;
- of HLA-DR is at least 8 and preferably at least 25-fold,
- of COX-2 is at least 3-fold and/or;
- of IDO is at least 5 -fold.
7. The method or the population of MSC cells for use of claims 1-6, wherein said one or more cytokines are selected from:
- IFN-Y;
- IL-1β;
- IFN-y and TNF-α;
- IL17A, IL-1β and OSM;
- IL17A, IL-1β, OSM, lFN-γ, TNF-α and IL-13;
- IL17A, IL-1β, OSM, lFN-γ, TNF-α, IL-13 and IL-4, or;
- IFN-y, TNF-α, IL-13 and IL-4.
8. A kit for determining the potency and/or capacity of MSC to inhibit an immune response, such as inhibit proliferation of target cells such as T-cells, B-cells, NK cells and other immune cells and/or monocyte/maerophage polarization resulting in direct immune suppression through the production of soluble mediators and induction of T Regs, comprising binding molecules that bind to an MSC-speeifie marker and one or more of: CD 54, CD274, HLA-DR, COX- 2 and IDO.
9. A kit for determining if MSC have a capacity to inhibit proliferation of T-cells and/or inhibit differentiation of monocytes to DC, comprising binding molecules that bind to an MSC- specific marker and one or more of: CD54, CD274, HLA-DR, COX-2 and IDO.
10. The kit of claim 8 or claim 9, further comprising one or more cytokines selected from: ILT7A, IL-1β, oneostatin M (OSM), LFN-g, TNF-α, IL-13, IL-4,
11. The kit of claim 10, wherein said one or more cytokines are selected from:
- IFN-Y;
- IFN-Y and TNF-α;
- IL17AJL-18 and OSM;
- IL17A,IL-18, OSM, IFN-y, TNF-α and IL-13;
- IL17A,1L-18, OSM, IFN-y, TNF-α, IL-13 and IL-4, or;
- IFN-y, TNF-α, IL-13 and IL-4.
12. The method or the population of MSC cells for use of claims 1-7 or the kit of any one of claims 8-11, wherein said binding molecules are antibodies.
13. The method or the population of MSC cells for use of claims 1-7, 12 or the kit of any one of claims 8-12, wherein said binding molecules or antibodies are labelled with fluoroehromes that are suitable for parallel flow cytometric analysis, and wherein the signals of said fluoroehromes can be acquired independently.
14. A cell bank comprising MSC of at least 10 different donors, wherein an MSC- speeifie marker is present on the MSC of each of said donors, and wherein each of said MSC exhibit a fold increase of the expression of one or more of the markers CD54, CD274, HLA-DR, COX-2 and IDO in response to culture with one or more cytokines selected from: IL-17A, IL-18, OSM, IFN-y, TNF-α, IL-13 and IL-4.
15. The cell bank of claim 14, wherein the expression anti/or the fold increase in expression of said markers of said MSC was determined using the method or the population of MSC cells for use of claims 1.-7, 12, 13 or the kit of any one of claims 8-13,
16. A method of preventing and/or inhibiting an immune response in a subject, the method comprising administering to said subject MSC of which the presence of an MSC -specific marker and fold increase of the expression of one or more of the markers CD54, CD274, HLA-DR, COX-2 and IDO was determined using a method or the population of MSC cells for use of claims 1-7, 12, 13 or the kit of any one of claims 8-13.
17. The method of claim 16, wherein the subject is exhibiting or is at risk of exhibiting an undesired immune response.
18. The method of claim 16 or claim 17, wherein the subject is exhibiting inflammatory bowel disease.
19. The method of claim 16 or claim 17, wherein the subject is the recipient of an allogeneic cell or organ transplant,
20. A method for preventing and/or inhibiting graft versus host or host versus graft disease in a subject comprising administering to said subject MSC of which the presence of an MSC-speeifie marker and fold increase of the expression of one or more of the markers CD54, CD274, HLA-DR, COX-2 and IDO has been determined using a method or the population of MSC cells for use of claims 1-7, 12, 13 or the kit of any one of claims 8-13,
21, A method for determining the potency and/or capacity of MSC to inhibit an immune response,, such as inhibit proliferation of target cells such as T-cells, B- eells, NK cells and other immune cells and/or monocyte/ macrophage polarization resulting in direct immune suppression through the production of soluble mediators and induction of T Regs comprising: providing MSC; culturing said MSC in the presence and in the absence of one or more cytokines selected from: IL-17A, IL-18, GSM, IFN-y, TNF-α, IL-13 and LL-4; - contacting said MSC with binding molecules that bind to an MSC -specific marker and one or more selected from: the extracellular markers CD64, CD274, HLA-DK, the intracellular markers COX- 2 and IDO; said method further comprising determining said capacity and/or of said MSC on the basis of the expression of said MSC -specific marker and the fold increase of the expression of said one or more of CD54, CD274, HLA-DR, COX-2 and IDO markers in response to said one or more cytokines.
22. The method of claim 20 or claim 21, wherein said fold increase of expression: of CD54 is at least 10-fold; - of CD274 is at least 8-fold; of HLA-DR is at least 8 and preferably at least 25 -fold, of COX-2 is at least 3-fold and/or; of IDO is at least 5-fold.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP20157519.8 | 2020-02-14 | ||
| EP20157519 | 2020-02-14 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2021162554A1 true WO2021162554A1 (en) | 2021-08-19 |
Family
ID=69630203
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/NL2021/050096 WO2021162554A1 (en) | 2020-02-14 | 2021-02-12 | Means and methods for determining mesenchymal stromal cell properties. |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2021162554A1 (en) |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014093948A1 (en) * | 2012-12-14 | 2014-06-19 | Rutgers, The State University Of New Jersey | Methods modulating immunoregulatory effect of stem cells |
| EP3527981A2 (en) * | 2016-10-17 | 2019-08-21 | Samsung Life Public Welfare Foundation | Method for sorting highly effective stem cells for treating immune disorder |
-
2021
- 2021-02-12 WO PCT/NL2021/050096 patent/WO2021162554A1/en active Application Filing
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014093948A1 (en) * | 2012-12-14 | 2014-06-19 | Rutgers, The State University Of New Jersey | Methods modulating immunoregulatory effect of stem cells |
| EP3527981A2 (en) * | 2016-10-17 | 2019-08-21 | Samsung Life Public Welfare Foundation | Method for sorting highly effective stem cells for treating immune disorder |
Non-Patent Citations (5)
| Title |
|---|
| ELENA REDONDO-CASTRO ET AL: "Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro", STEM CELL RESEARCH & THERAPY, vol. 8, no. 1, 17 April 2017 (2017-04-17), XP055583458, DOI: 10.1186/s13287-017-0531-4 * |
| GHANNAM S ET AL: "Mesenchymal Stem Cells Inhibit Human Th17 Cell Differentiation and Function and Induce a T Regulatory Cell Phenotype", THE JOURNAL OF IMMUNOLOGY, AMERICAN ASSOCIATION OF IMMUNOLOGISTS, US, vol. 185, no. 1, 1 July 2010 (2010-07-01), pages 302 - 312, XP002612951, ISSN: 0022-1767, [retrieved on 20100528], DOI: 10.4049/JIMMUNOL.0902007 * |
| MARIA ESTER BERNARDO ET AL: "Mesenchymal Stromal Cells: Sensors and Switchers of Inflammation", CELL STEM CELL, vol. 13, no. 4, 1 October 2013 (2013-10-01), AMSTERDAM, NL, pages 392 - 402, XP055763657, ISSN: 1934-5909, DOI: 10.1016/j.stem.2013.09.006 * |
| NÁDIA DE CÁSSIA NORONHA ET AL: "Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies", STEM CELL RESEARCH & THERAPY, vol. 10, no. 1, 2 May 2019 (2019-05-02), XP055763658, DOI: 10.1186/s13287-019-1224-y * |
| NORA G. SINGER ET AL: "Mesenchymal Stem Cells: Mechanisms of Inflammation", ANNUAL REVIEW OF PATHOLOGY: MECHANISMS OF DISEASE, vol. 6, no. 1, 28 February 2011 (2011-02-28), US, pages 457 - 478, XP055763655, ISSN: 1553-4006, DOI: 10.1146/annurev-pathol-011110-130230 * |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Pereira et al. | Sestrins induce natural killer function in senescent-like CD8+ T cells | |
| Saha et al. | Programmed death ligand-1 expression on donor T cells drives graft-versus-host disease lethality | |
| Comte et al. | Signaling lymphocytic activation molecule family member 7 engagement restores defective effector CD8+ T cell function in systemic lupus erythematosus | |
| Cherrier et al. | Notch, Id2, and RORγt sequentially orchestrate the fetal development of lymphoid tissue inducer cells | |
| JP4714767B2 (en) | CD4 + CD25 + regulatory T cells derived from human blood | |
| Xu et al. | Promyelocytic leukemia zinc finger protein regulates interferon-mediated innate immunity | |
| Verstegen et al. | Inborn errors of adaptive immunity in Down syndrome | |
| Xie et al. | Semaphorin 4D induces an imbalance of Th17/Treg cells by activating the aryl hydrocarbon receptor in ankylosing spondylitis | |
| Blijdorp et al. | Expansion of interleukin‐22–and granulocyte–macrophage colony‐stimulating factor–expressing, but not interleukin‐17A–expressing, group 3 innate lymphoid cells in the inflamed joints of patients with spondyloarthritis | |
| De Matteis et al. | Immunosuppressive Treg cells acquire the phenotype of effector-T cells in chronic lymphocytic leukemia patients | |
| Schaafsma et al. | VISTA targeting of T-cell quiescence and myeloid suppression overcomes adaptive resistance | |
| Flinn et al. | Thymopoiesis, alterations in dendritic cells and tregs, and reduced T cell activation in successful extracorporeal photopheresis treatment of GVHD | |
| Hidalgo et al. | The response of T cells to interleukin‐6 is differentially regulated by the microenvironment of the rheumatoid synovial fluid and tissue | |
| Pontes Ferreira et al. | CXCR3 chemokine receptor guides Trypanosoma cruzi-specific T-cells triggered by DNA/adenovirus ASP2 vaccine to heart tissue after challenge | |
| Umland et al. | Induction of various immune modulatory molecules in CD34+ hematopoietic cells | |
| WO2020069000A9 (en) | Mot cells as a therapeutic screening tool for regulatory t-cell activity | |
| WO2021162554A1 (en) | Means and methods for determining mesenchymal stromal cell properties. | |
| Clay et al. | Chemokine networks and in vivo T-lymphocyte trafficking in nonhuman primates | |
| Dawson et al. | Functional effects of chimeric antigen receptor co-receptor signaling domains in human Tregs | |
| Leveque et al. | T helper cell-licensed mast cells promote inflammatory Th17 cells | |
| JP2020115791A (en) | Marker that determines effectiveness of application of cancer therapy including cancer immune therapy to cancer patient, and use thereof | |
| Varela et al. | In vitro differentiation of myeloid suppressor cells (MDSC-like) from an immature myelomonocytic precursor THP-1 | |
| US20180321223A1 (en) | Method for Monitoring the Immunological Profile of a Subject | |
| Piede et al. | Validation of an ICH Q2 Compliant Flow Cytometry-Based Assay for the Assessment of the Inhibitory Potential of Mesenchymal Stromal Cells on T Cell Proliferation., 2023, 12, 850 | |
| Market et al. | A method of assessment of Human Natural Killer Cell Phenotype and function in whole blood |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 21705641 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 21705641 Country of ref document: EP Kind code of ref document: A1 |