WO2020214946A1 - Cystine cationic lipids - Google Patents
Cystine cationic lipids Download PDFInfo
- Publication number
- WO2020214946A1 WO2020214946A1 PCT/US2020/028755 US2020028755W WO2020214946A1 WO 2020214946 A1 WO2020214946 A1 WO 2020214946A1 US 2020028755 W US2020028755 W US 2020028755W WO 2020214946 A1 WO2020214946 A1 WO 2020214946A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- protein
- cationic lipid
- independently
- mrna
- composition
- Prior art date
Links
- 0 CCC(C)*(CC(CC)C*C*CCNC(C)C(C)C)=* Chemical compound CCC(C)*(CC(CC)C*C*CCNC(C)C(C)C)=* 0.000 description 5
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C323/00—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups
- C07C323/50—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups containing thio groups and carboxyl groups bound to the same carbon skeleton
- C07C323/51—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups containing thio groups and carboxyl groups bound to the same carbon skeleton having the sulfur atoms of the thio groups bound to acyclic carbon atoms of the carbon skeleton
- C07C323/57—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups containing thio groups and carboxyl groups bound to the same carbon skeleton having the sulfur atoms of the thio groups bound to acyclic carbon atoms of the carbon skeleton the carbon skeleton being further substituted by nitrogen atoms, not being part of nitro or nitroso groups
- C07C323/58—Thiols, sulfides, hydropolysulfides or polysulfides substituted by halogen, oxygen or nitrogen atoms, or by sulfur atoms not being part of thio groups containing thio groups and carboxyl groups bound to the same carbon skeleton having the sulfur atoms of the thio groups bound to acyclic carbon atoms of the carbon skeleton the carbon skeleton being further substituted by nitrogen atoms, not being part of nitro or nitroso groups with amino groups bound to the carbon skeleton
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/177—Receptors; Cell surface antigens; Cell surface determinants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/18—Growth factors; Growth regulators
- A61K38/1816—Erythropoietin [EPO]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/45—Transferases (2)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0014—Skin, i.e. galenical aspects of topical compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
- A61K9/0056—Mouth soluble or dispersible forms; Suckable, eatable, chewable coherent forms; Forms rapidly disintegrating in the mouth; Lozenges; Lollipops; Bite capsules; Baked products; Baits or other oral forms for animals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/007—Pulmonary tract; Aromatherapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/127—Liposomes
- A61K9/1271—Non-conventional liposomes, e.g. PEGylated liposomes, liposomes coated with polymers
- A61K9/1272—Non-conventional liposomes, e.g. PEGylated liposomes, liposomes coated with polymers with substantial amounts of non-phosphatidyl, i.e. non-acylglycerophosphate, surfactants as bilayer-forming substances, e.g. cationic lipids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
Definitions
- mRNA messenger RNA
- the present invention provides, among other things, a novel class of cystine cationic lipid compounds for improved in vivo delivery of therapeutic agents, such as nucleic acids, it is contemplated that the compounds provided herein are capable of highly effective in vivo delivery while maintaining favorable toxicity profile due to the biodegradable nature.
- cationic lipids having a structure according to Formula (A),
- each R la is independently hydrogen, R ic , or R 3d ;
- each R lB Is independently R lc or R ld ;
- each R lc is independently -[CH 2 ) 2 C(0)X 1 R 3 ;
- each R ld is independently ⁇ € ⁇ 0)R 4 ;
- each R 2 is independently -[C(R 2a ) 2 ] c R 2b ;
- each R 2a is independently hydrogen or Ci-C 6 alkyl
- R 2b is -N(L I -B) 2 , -(OCH 2 CH 2 ) 6 OH; or -iOCH 2 CH 2 ) b OCH 3 ;
- each R 3 and R 4 is independently C 6 -C 3 o aliphatic
- each Li is independently Ci-Cio alkylene
- each a is independently an integer of 1-10;
- each b is independently an integer of 1-10;
- each c is independently an integer of 1-10.
- each a is 1 or 2.
- each R la and R lb is ⁇ eH 2 ⁇ C[0)C3 ⁇ 4 3 .
- a cationic iipid has a structure according to formula (I):
- each R 3 is C 6 -C alkyl, C 6 -C alkenyl, or C S C 2 a!kyny!.
- each R 3 is unsubstituted C 6 -C 2 alkyl.
- each R la is hydrogen and each R lb is -C(0)R 4 .
- a cationic iipid has a structure according to formula [I I):
- each R 4 is C f ,-C 30 alkyl.
- each R 4 Is unsubstituted Ce-C alkyl.
- each R 4 is C 6 -Ci 0 alkyl substituted with -GC(0)R 5 or
- R !> is unsubstituted C 6 -Ci 6 alkyl.
- each R 2 is— [CH 2 ]c— N(U-B) 2 or - CH(CH 3 )CH 2 -N(Li-B) 2 ;
- each Li is unsubstituted Ci-C-. 0 alkylene.
- each R 2 is -[CH 2 ] c -N(CH 3 ) 2 .
- each R 2 is -[CH 2 ],;-N(CH 2 CH 2 B) 2 , -[CH 2 ] c -N(CH 2 CH 2 CH 2 B) 2 -CH(CH 3 )CH 2 -N(CH 2 CH 2 B) 2 , or -CH(CH 3 )CH 2 -N(CH 2 CH 2 CH 2 B) 2 ; and
- each B is an ionizable nitrogen-containing group.
- each B is NH 2 , guanidine, amidine, a mono- or dialkylamine, 5- to 6- membered heterocycloalkyi, or 5- to 6-membered nitrogen-containing heteroary!.
- each B is NH 2 , guanidine, amidine, NHCH 3 , N(CH 3 ) 2 , or imidazole.
- each B is N(CH 3 ) 2 .
- each c is 2, 3, or 4.
- a cationic lipid is any one of Compounds 1-12.
- the invention features a composition comprising any liposome [e.g., a liposome encapsulating an mRNA encoding a protein) described herein, !n embodiments, the liposome comprises a cationic lipid described herein.
- an mRNA encodes for cystic fibrosis transmembrane conductance
- CTR CTR regulator
- an mRNA encodes for ornithine transcarbamy!ase (OTC) protein.
- the invention features a composition comprising a nucleic acid
- the liposome comprises a cationic lipid described herein.
- a composition further comprises one more lipids selected from the group consisting of one or more cationic lipids, one or more non-cationic lipids, and one or more PEG- ⁇ modified lipids.
- a nucleic acid is an mRNA encoding a peptide or protein.
- an mRNA encodes a peptide or protein for use in the delivery to or
- an mRNA encodes a peptide or protein for use in the delivery to or
- an mRNA encodes for cystic fibrosis transmembrane conductance regulator (CFTR) protein.
- CFTR cystic fibrosis transmembrane conductance regulator
- an mRNA encodes a peptide or protein for use in the delivery to or
- liver ceil Treatment of the liver of a subject or a liver ceil.
- an mRNA encodes for ornithine transcarbamylase (OTC) protein.
- an mRNA encodes a peptide or protein for use in vaccine.
- an mRNA encodes an antigen
- the present invention provides methods of treating a disease in a subject comprising administering to the subject a composition as described herein.
- FIG, 1 depicts in vivo protein production resulting from the delivery of mRNA using lipid nanoparticies comprising Compound 3 or Compound 4 as described herein. As shown in this Figure, use of these compounds allows high levels of in vivo protein production even 24 hours after administration.
- amino acid in its broadest sense, refers to any compound and/or substance that can be incorporated into a polypeptide chain, in some embodiments, an amino acid has the general structure H 2 --C ⁇ H ⁇ (R ⁇ --COOH.
- an amino acid is a naturally occurring amino acid.
- an amino acid is a synthetic amino acid; in some embodiments, an amino acid is a d-amino acid; in some embodiments, an amino acid is an l-amino acid, "Standard amino acid” refers to any of the twenty standard l-amino acids commonly found in naturally occurring peptides.
- Nonstandard amino acid refers to any amino acid, other than the standard amino acids, regardless of whether it is prepared synthetically or obtained from a natural source.
- synthetic amino acid encompasses chemically modified amino acids, including but not limited to salts, amino acid derivatives (such as amides), and/or substitutions.
- Amino acids, including carboxy- and/or amino-terminal amino acids in peptides can be modified by methylation, amidation, acetylation, protecting groups, and/or substitution with other chemical groups that can change the peptide's circulating half-life without adversely affecting their activity. Amino acids may participate in a disulfide bond.
- Amino acids may comprise one or posttranslational modifications, such as association with one or more chemical entitles (e.g., methyl groups, acetate groups, acetyl groups, phosphate groups, formyl moieties, isoprenoid groups, sulfate groups, polyethylene glycol moieties, lipid moieties, carbohydrate moieties, biotin moieties, etc. ⁇ .
- chemical entitles e.g., methyl groups, acetate groups, acetyl groups, phosphate groups, formyl moieties, isoprenoid groups, sulfate groups, polyethylene glycol moieties, lipid moieties, carbohydrate moieties, biotin moieties, etc. ⁇ .
- amino acid is used interchangeably with "amino acid residue,” and may refer to a free amino acid and/or to an amino acid residue of a peptide, it will be apparent from the context in which the term is used whether it refers to a free
- animal refers to any member of the animal kingdom. In some embodiments, “animal” refers to humans, at any stage of development, in some embodiments, “animal” refers to non-human animals, at any stage of development. In certain embodiments, the non-human animal is a mammal (e.g., a rodent, a mouse, a rat, a rabbit, a monkey, a dog, a cat, a sheep, a bovine, a primate, and/or a pig). In some embodiments, animals include, but are not limited to, mammals, birds, reptiles, amphibians, fish, insects, and/or worms. In some embodiments, an animal may be a transgenic animal, genetically- engineered animal, and/or a clone.
- mammal e.g., a rodent, a mouse, a rat, a rabbit, a monkey, a dog, a cat, a sheep, a bovine, a primate, and/
- biologically active refers to a characteristic of any agent that has activity in a biological system, and particularly in an organism. For instance, an agent that, when administered to an organism, has a biological effect on that organism, is considered to be biologically active.
- Cystine refers to the oxidized dimer form of the amino acid cysteine and has the formula (SCH CH(NH )CQ 2 H) .
- Delivery encompasses both local and systemic delivery.
- delivery of rnRIMA encompasses situations in which an mRNA is delivered to a target tissue and the encoded protein is expressed and retained within the target tissue (also referred to as “local distribution” or “local delivery”), and situations in which an mRNA is delivered to a target tissue and the encoded protein Is expressed and secreted into patient's circulation system (e.g., serum) and systematically distributed and taken up by other tissues (also referred to as “systemic distribution” or “systemic delivery”),
- expression refers to translation of an mRNA into a polypeptide, assemble multiple polypeptides into an intact protein (e.g., enzyme) and/or post-iransiationai modification of a polypeptide or fully assembled protein (e.g., enzyme).
- intact protein e.g., enzyme
- post-iransiationai modification of a polypeptide or fully assembled protein e.g., enzyme
- a "functional" biological molecule is a biological molecule in a form in which it exhibits a property and/or activity by which it is characterized.
- Half-life As used herein, the term "half-life" is the time required for a quantity such as nucleic acid or protein concentration or activity to fall to half of its value as measured at the beginning of a time period.
- Helper iipid refers to any neutral or zwitterionic lipid material including cholesterol. Without wishing to be held to a particular theory, helper lipids may add stability, rigidity, and/or fluidity within Iipid biiayers/nanoparticles.
- improve , increase, or reduce As used herein, the terms“improve,” “Increase,” or“reduce,” or grammatical equivalents, indicate values that are relative to a baseline measurement, such as a measurement in the same individual prior to initiation of the treatment described herein, or a measurement in a control subject [or multiple control subject) in the absence of the treatment described herein.
- a "control subject” is a subject afflicted with the same form of disease as the subject being treated, who is about the same age as the subject being treated.
- in vitro refers to events that occur in an artificial
- Isolated refers to a substance and/or entity that has been (1) separated from at least some of the components with which it was associated when initially produced (whether in nature and/or in an experimental setting), and/or (2) produced, prepared, and/or manufactured by the hand of man.
- isolated substances and/or entities may be separated from about 10%, about 20%, about 30%, about 40%, about 50%, about 60%, about 70%, about 80%, about 90%, about 91%, about 92%, about 93%, about 94%, about 95%, about 96%, about 97%, about 98%, about 99%, or more than about 99% of the other components with which they were initially associated.
- isolated agents are about 80%, about 85%, about 90%, about 91%, about 92%, about 93%, about 94%, about 95%, about 96%, about 97%, about 98%, about 99%, or more than about 99% pure.
- a substance is “pure” if it is substantially free of other components.
- calculation of percent purity of isolated substances and/or entities should not include excipients ⁇ e.g., buffer, solvent, water, etc.).
- Liposome refers to any lamellar, multilame!lar, or solid nanoparticle vesicle.
- a liposome as used herein can be formed by mixing one or more lipids or by mixing one or more lipids and polymer(s).
- a liposome suitable for the present invention contains a cationic iipids(s) and optionally non-cationic lipid(s), optionally cholesterol-based lipid(s), and/or optionally PEG-modified lipid(s).
- messenger RNA As used herein, the term "messenger RNA (mRNA)" or “mRNA” refers to a polynucleotide that encodes at least one polypeptide. RNA as used herein encompasses both modified and unmodified RNA. The term “modified mRNA” related to mRNA comprising at least one chemically modified nucleotide. mRNA may contain one or more coding and non-coding regions. mRNA can be purified from natural sources, produced using recombinant expression systems and optionally purified, chemically synthesized, etc.
- mRNA can comprise nucleoside analogs such as analogs having chemically modified bases or sugars, backbone modifications, etc.
- An mRNA sequence is presented in the 5' to 3' direction unless otherwise indicated, in some embodiments, an mRNA is or comprises natural nucleosides ⁇ e.g., adenosine, guanosine, cytidine, uridine); nucleoside analogs (e.g., 2-aminoadenosine, 2-thiothymidine, inosine, pyrrolo-pyrimidine, 3-methyl adenosine, 5-methyicytidine, C-5 propynyi-cytidine, C-5 propynyl-uridine, 2-aminoadenosine, C5-bromouridine, C5-fluorouridine, C5-iodouridine, C5- propynyi-uridine, C5-propy
- nucleic acid refers to any compound and/or substance that is or can be incorporated into a polynucleotide chain
- a nucleic add is a compound and/or substance that is or can be incorporated into a poiynucieotide chain via a phosphodiester linkage
- nucleic acid refers to individual nucleic acid residues (e.g., nucleotides and/or nucleosides)
- nucleic acid refers to a polynucleotide chain comprising individual nucleic acid residues.
- nucleic acid encompasses RNA as well as single and/or double-stranded DNA and/or cDNA. in some embodiments, “nucleic acid” encompasses ribonucleic acids (RNA), including but not limited to any one or more of interference RNAs (RNAi), small interfering RNA (siRNA), short hairpin RNA (shRNA), antisense RNA (aRNA), messenger RNA (mRNA), modified messenger RNA (mmRNA), long non-coding RNA (IncRNA), micro-RNA (miRNA) multimeric coding nucleic acid (MCNA), polymeric coding nucleic acid (PCNA), guide RNA (gRNA) and CRISPR RNA (crRNA).
- RNAi interference RNAs
- siRNA small interfering RNA
- shRNA short hairpin RNA
- aRNA antisense RNA
- mRNA messenger RNA
- mmRNA modified messenger RNA
- IncRNA micro-RNA
- miRNA multimeric coding nucleic acid
- nucleic acid encompasses deoxyribonucleic acid (DNA), including but not limited to any one or more of single-stranded DNA (ssDNA), double-stranded DNA (dsDNA) and complementary DNA (cDNA).
- ssDNA single-stranded DNA
- dsDNA double-stranded DNA
- cDNA complementary DNA
- nucleic acid encompasses both RNA and DNA
- DNA may be in the form of antisense DNA, plasmid DNA, parts of a plasmid DNA, pre-condensed DNA, a product of a polymerase chain reaction (PCR), vectors (e.g., PI, PAC, BAC, YAC, artificial chromosomes), expression cassettes, chimeric sequences, chromosomal DNA, or derivatives of these groups.
- RNA may be in the form of messenger RNA (mRNA), ribosomal RNA (rRNA), signal recognition particle RNA (7 SL RNA or SRP RNA), transfer RNA (tRNA), transfer-messenger RNA (tmRNA), small nuclear RNA (snRNA), small nucleolar RNA (snoRNA), SmY RNA, small Cajal body-specific RNA (scaRNA), guide RNA (gRNA), ribonuclease P (RNase P), Y RNA, telomerase RNA component (TERC), spliced ieader RNA (SL RNA), antisense RNA (aRNA or asRNA), cis-natural antisense transcript (cis-NAT), CRiSPR RNA (crRNA), long noncoding RNA (IncRNA), micro-RNA (miRNA), piwi-interacting RNA (piRNA), small interfering RNA (siRNA), transacting siRNA (tasiRNA), repeat associated siRNA
- patient refers to any organism to which a provided composition may be administered, e.g., for experimental, diagnostic, prophylactic. cosmetic, and/or therapeutic purposes.
- Typical patients include animals [e.g., mammals such as mice, rats, rabbits, non-human primates, and/or humans), in some embodiments, a patient is a human.
- a human includes pre- and post-natal forms,
- Pharmaceutically acceptable salts are well known in the art. For example, S. M Berge et a!., describes pharmaceutically acceptable salts in detail in J. Pharmaceutical Sciences (1977) 66:1-19. Pharmaceutically acceptable salts of the compounds of this invention include those derived from suitable inorganic and organic acids and bases.
- Examples of pharmaceutically acceptable, nontoxic acid addition salts are salts of an amino group formed with inorganic acids such as hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid, and perchloric acid or with organic acids such as acetic acid, oxalic acid, maleic acid, tartaric acid, citric acid, succinic acid, or maionic acid, or by using other methods used in the art such as ion exchange.
- inorganic acids such as hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid, and perchloric acid
- organic acids such as acetic acid, oxalic acid, maleic acid, tartaric acid, citric acid, succinic acid, or maionic acid, or by using other methods used in the art such as ion exchange.
- salts include adipate, alginate, ascorbate, aspartate, benzenesuifonate, benzoate, bisulfate, borate, butyrate, camphorate, camphorsulfonate, citrate, cyciopentanepropionate, digluconate, dodecy!su!fate,
- ethanesulfonate formate, fumarate, glucoheptonate, glycerophosphate, gluconate, hemisulfate, heptanoate, hexanoate, hydroiodide, 2-hydroxy-ethanesulfonate, lactobionate, lactate, laurate, lauryi sulfate, maiate, maleate, malonate, methanesulfonate, 2-naphihaienesuifonate, nicotinate, nitrate, oleate, oxalate, pa!mitate, pamoate, pectinate, persulfate, 3- phenylpropionate, phosphate, picrate, pivalate, propionate, stearate, succinate, sulfate, tartrate, thiocyanate, p-toluenesu!fonate, undecanoate, valerate salts, and the like.
- Salts derived from appropriate bases include alkali metal, alkaline earth metal, ammonium and N + (C I alkyl) 4 salts.
- Representative alkali or alkaline earth metal salts include sodium, lithium, potassium, calcium, magnesium, and the like.
- Further pharmaceutically acceptable salts include, when appropriate, nontoxic ammonium, quaternary ammonium, and amine cations formed using counterions such as halide, hydroxide, carboxylate, sulfate, phosphate, nitrate, sulfonate, and aryl sulfonate.
- Further pharmaceutically acceptable salts include salts formed from the quarternization of an amine using an appropriate electrophile, e.g., an alkyl halide, to form a quarternized alkylated amino salt.
- Systemic distribution or delivery As used herein, the terms “systemic distribution” or
- systemic delivery refers to a delivery or distribution mechanism or approach that affect the entire body or an entire organism. Typically, systemic distribution or delivery is accomplished via body's circulation system, e.g., blood stream.
- Subject refers to a human or any non-human animal ⁇ e.g., mouse, rat, rabbit, dog, cat, cattle, swine, sheep, horse or primate).
- a human includes pre- and post-natal forms.
- a subject is a human being.
- a subject can be a patient, which refers to a human presenting to a medical provider for diagnosis or treatment of a disease.
- the term "subject” is used herein interchangeably with “individual” or "patient,” A subject can be afflicted with or is susceptible to a disease or disorder but may or may not display symptoms of the disease or disorder.
- the term “substantially” refers to the qualitative condition of exhibiting total or near-total extent or degree of a characteristic or property of interest.
- One of ordinary skill in the biological arts will understand that biological and chemical phenomena rarely, if ever, go to completion and/or proceed to completeness or achieve or avoid an absolute result.
- the term “substantially” is therefore used herein to capture the potential lack of completeness inherent in many biological and chemical phenomena.
- Target tissues refers to any tissue that is affected by a disease to be treated, in some embodiments, target tissues include those tissues that display disease-associated pathology, symptom, or feature,
- Therapeuticai!y effective amount As used herein, the term "therapeutically effective
- a therapeutically effective amount means an amount that is sufficient, when administered to a subject suffering from or susceptible to a disease, disorder, and/or condition, to treat, diagnose, prevent, and/or delay the onset of the symptom(s) of the disease, disorder, and/or condition. It will be appreciated by those of ordinary skill in the art that a therapeutically effective amount is typically administered via a dosing regimen comprising at least one unit dose.
- Treating refers to any method used to partially or completely alleviate, ameliorate, relieve, inhibit, prevent, delay onset of, reduce severity of and/or reduce incidence of one or more symptoms or features of a particular disease, disorder, and/or condition. Treatment may be administered to a subject who does not exhibit signs of a disease and/or exhibits only early signs of the disease for the purpose of decreasing the risk of developing pathology associated with the disease.
- Aliphatic refers to Ci-Cso hydrocarbons and includes both saturated and unsaturated hydrocarbons.
- An aliphatic may be linear, branched, or cyclic, for example, C -C aliphatics can include C -C alkyls ⁇ e.g., linear or branched C -C saturated alkyls) / C 2 -C 20 alkenyls (e.g., linear or branched -C 20 dienyis, linear or branched C -C trienyls, and the like), and C 2 -C 20 aikynyls [e.g., linear or branched C 2 --C 2 o alkynyls).
- Ci-C aliphatics can include C 3 -C 20 cyclic aliphatics (e.g., C -C cycloalkyls, Q-C 20 cycloalkenyls, or Cs-C
- the aliphatic may comprise one or more cyclic aliphatic and/or one or more heteroatoms such as oxygen, nitrogen, or sulfur and may optionally be substituted with one or more substituents such as alkyl, halo, aikoxyi, hydroxy, amino, aryl, ether, ester or amide.
- An aliphatic group is unsubstituted or substituted with one or more substituent groups as described herein.
- an aliphatic may be substituted with one or more (e.g., 1, 2, 3, 4, 5, or 6 independently selected substituents) of halogen, -COR', -C0 2 H, - C0 2 R', -CM, -OH, -OR', -OCOR', -0C0 2 R', -NH 2 ,
- R' independently is Ci-C aliphatic [e.g., Ci-C 2 o alkyl, C-.-C alkyl, Ci-Cio alkyl, or C-.-C alkyl).
- R' independently is an unsubstituted alkyl (e.g., unsubstituted Ci-C alkyl, C -C alkyl, C 1 -C 10 alkyl, or C C alkyl).
- R' independently is unsubstituted C 1 -C 3 aikyi.
- the aliphatic is unsubstituted.
- the aliphatic does not include any heteroatoms.
- alkyl means acyclic linear and branched hydrocarbon groups, e.g. "Ci-C 20 alkyl” refers to aikyi groups having 1-20 carbons.
- An alkyl group may be linear or branched. Examples of alkyl groups include, but are not limited to, methyl, ethyl, n- propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl tert-pentylhexyl, isohexy!efc.
- lower alkyl means an alkyl group straight chain or branched alkyl having 1 to 6 carbon atoms.
- Other alkyl groups will be readily apparent to those of skill in the art given the benefit of the present disclosure.
- An alkyl group may be unsubstituted or substituted with one or more substituent groups as described herein.
- an alkyl group may be substituted with one or more (e.g., 1, 2, 3, 4, 5, or 6 independently selected substituents) of halogen, -COR', -CG 2 H, -C0 2 R', -CM, -OH, -OR', -OCOR', -OCO,R', -NH 2 , -NHR', -N(R') 2 , -SR' or- S0 2 R', wherein each instance of R' independently is Ci-C 20 aliphatic (e.g., C 1 -C 20 alkyl, Ci-C-. 5 alkyl, C 1 -C 10 alkyl, or Ci-C 3 alkyl).
- substituents e.g., 1, 2, 3, 4, 5, or 6 independently selected substituents
- R' independently is an unsubstituted alkyl (e.g., unsubstituted C 1 -C 20 alkyl, C 1 -C 15 alkyl, Cj-Cio alkyl, or C 1 -C 3 aikyi).
- R' independently is unsubstituted Ci-C 3 alkyl, in embodiments, the alkyl is substituted [e.g., with 1, 2, 3, 4, 5, or 6 substituent groups as described herein).
- an alkyl group is substituted with a-OH group and may also be referred to herein as a "hydroxyaikyi" group, where the prefix denotes the -OH group and "aikyi" is as described herein.
- Alkylene represents a saturated divalent straight or branched chain hydrocarbon group and is exemplified by methylene, ethylene, isopropylene and the like.
- alkenylene as used herein represents an unsaturated divalent straight or branched chain hydrocarbon group having one or more unsaturated carbon-carbon double bonds that may occur in any stable point along the chain
- alkynylene herein represents an unsaturated divalent straight or branched chain hydrocarbon group having one or more unsaturated carbon-carbon triple bonds that may occur in any stable point along the chain
- an alkylene, alkenylene, or aikynylene group may comprise one or more cyclic aliphatic and/or one or more heteroatoms such as oxygen, nitrogen, or sulfur and may optionally be substituted with one or more substituents such as alkyl, halo, aikoxyi, hydroxy, amino, aryl, ether, ester or amide.
- an alkylene, alkenylene, or aikynylene may be substituted with one or more (e.g., 1, 2, 3, 4, 5, or 6 independently selected substituents) of halogen, -COR', -C0 2 H, -CO R',
- R f independently Is an unsubstituted alkyl (e.g., unsubstituted C-.-C 20 alkyl, C 1 -C 15 aikyi, C 1 -C 10 alkyl, or C-.-C 3 alkyl).
- R' independently is unsubstituted C-.-C 3 alkyl.
- an alkylene, alkenylene, or aikynylene is unsubstituted.
- an alkylene, alkenylene, or aikynylene does not include any heteroatoms.
- alkenyl means any linear or branched hydrocarbon chains having one or more unsaturaied carbon-carbon double bonds that may occur in any stable point along the chain, e.g. "C 2 -C 20 aikeny! refers to an alkenyl group having 2-2.0 carbons.
- an alkenyl group includes prop-2-enyl, but-2-enyl, but-3-enyi, 2-methylprop-2-enyl, hex-2-enyl, hex- 5-enyi, 2,3-dimethylbut-2-enyl, and the like.
- the alkenyl comprises 1, 2, or 3 carbon-carbon double bond, in embodiments, the alkenyl comprises a single carbon-carbon double bond, in embodiments, multiple double bonds (e.g., 2 or 3) are conjugated.
- An alkenyl group may be unsubstituted or substituted with one or more substituent groups as described herein.
- an alkenyl group may be substituted with one or more (e.g., 1, 2, 3, 4, 5, or 6 independently selected substituents) of halogen, -COR', -C0 2 H, -C0 2 R', -CN, -OH, -OR', -OCQR', -OC0 2 R', -NH 2 , -NHR', -N(R') 2 , -SR' or-S0 2 R', wherein each instance of R’ independently is Ci-C 20 aliphatic (e.g., Ci-C 20 alkyl, C 1 -C 15 alkyl, C 1 -C 10 alkyl, or C 1 -C 3 alkyl).
- R' e.g., 1, 2, 3, 4, 5, or 6 independently selected substituents
- R' independently is unsubstituted C 1 -C 3 aikyi.
- the alkenyl is unsubstituted.
- the alkenyl is substituted (e.g., with 1, 2, 3, 4, 5, or 6 substituent groups as described herein), in embodiments, an alkenyl group is substituted with a-QH group and may also be referred to herein as a "hydroxyalkeny! group, where the prefix denotes the -OH group and "alkenyl" is as described herein,
- alkynyi means any hydrocarbon chain of either linear or branched configuration, having one or more carbon-carbon triple bonds occurring in any stable point along the chain, e.g. "C 2 -C 2 o alkynyi” refers to an alkynyi group having 2-20 carbons. Examples of an alkynyi group include prop-2-ynyl, but-2-ynyl, but-3-ynyl, pent-2-ynyl, 3-methylpent-4-ynyl, hex-2-ynyl, hex-5-ynyl, etc. in embodiments, an alkynyi comprises one carbon-carbon triple bond.
- An aikynyi group may be unsubstituted or substituted with one or more substituent groups as described herein.
- an alkynyi group may be substituted with one or more (e.g., 1, 2, 3, 4, 5, or 6 independently selected substituents) of halogen, -COR' ' , -C0 2 H, -C0 2 R', - CN, -OH, -OR', -OCOR',
- R' independently is C C 20 aliphatic ⁇ e.g., Ci-C 20 alkyl, Ci-Cis alkyi, Ci-Cio alkyl, or C 1 -C 3 alkyl), in embodiments, R' independently is an unsubstituted alkyl (e.g., unsubstituted Ci-C 20 alkyl, C 1 -C 15 alkyl, Cj-Cio alkyi, or C 1 -C 3 alkyi).
- R' independently is an unsubstituted alkyl (e.g., unsubstituted Ci-C 20 alkyl, C 1 -C 15 alkyl, Cj-Cio alkyi, or C 1 -C 3 alkyi).
- R' independently is unsubstituted C 1 -C 3 alkyl.
- the alkynyi is unsubstituted.
- the alkynyi is substituted (e.g., with 1, 2, 3, 4, 5, or 6 substituent groups as described herein).
- Aryl refers to a monocyclic, bicyclic, or tricyclic carbocyclic ring system having a total of six to fourteen ring members, wherein said ring system has a single point of attachment to the rest of the molecule, at least one ring in the system is aromatic and wherein each ring in the system contains 4 to 7 ring members.
- an aryl group has 6 ring carbon atoms ("C 6 aryl,” e.g., phenyl), in some embodiments, an ary!
- Aryl also Includes ring systems wherein the aryl ring, as defined above, is fused with one or more carbocydyi or heterocyclyl groups wherein the radical or point of attachment is on the aryl ring, and in such instances, the number of carbon atoms continue to designate the number of carbon atoms in the aryl ring system.
- Exemplary aryls include phenyl, naphthyl, and anthracene.
- aryiene refers to an aryl group that is divalent (that is, having two points of attachment to the molecule).
- exemplary arylenes include phenylene (e.g., unsubstituted phenylene or substituted phenylene).
- Halogen means fluorine, chlorine, bromine, or iodine.
- the term “heteroalkyl” is meant a branched or unbranched alkyl, alkenyl, or alkynyl group having from 1 to 14 carbon atoms In addition to 1, 2, 3 or 4 heteroatoms independently selected from the group consisting of N, O, S, and P, Heteroalkyls include tertiary amines, secondary amines, ethers, thioethers, amides, thioa ides, carbamates, thiocarba ates, hydrazones, imines, phosphodiesters, phosphoramidates, sulfonamides, and disulfides.
- a heteroalkyl group may optionally include monocyclic, bicycllc, or tricyclic rings, in which each ring desirably has three to six members.
- heteroalkyls include polyethers, such as methoxymethyl and ethoxyethyl.
- Heteroa!ky!ene represents a divalent form of a heteroalkyl group as described herein.
- Heteroaryl The term "heteroary!,” as used herein, is fully unsaturaied heteroatom- containing ring wherein at least one ring atom is a heteroatom such as, but not limited to, nitrogen and oxygen.
- Heterocycloa!ky is a non-aromatic ring
- heterocycloalkyl group wherein at least one atom Is a heteroatom such as, but not limited to, nitrogen, oxygen, sulfur, or phosphorus, and the remaining atoms are carbon.
- the heterocycloalkyl group can be substituted or unsubstituted.
- Liposomal-based vehicles are considered an attractive carrier for therapeutic agents and remain subject to continued development efforts. While liposomal-based vehicles that comprise certain lipid components have shown promising results with regard to encapsulation, stability and site localization, there remains a great need for improvement of liposomal-based delivery systems. For example, a significant drawback of liposomal delivery systems relates to the construction of liposomes that have sufficient cell culture or in vivo stability to reach desired target cells and/or intracellular compartments, and the ability of such liposomal delivery systems to efficiently reiease their encapsulated materials to such target ceils.
- cystine cationic lipid compounds for improved in vivo delivery of therapeutic agents, such as nucleic acids
- a cystine cationic lipid described herein may be used as a cationic lipid, optionally with other lipids, to formulate a lipid- based nanoparticle (e.g., liposome) for encapsulating therapeutic agents, such as nucleic acids (e.g., DMA, siRNA, mRNA, microRNA) for therapeutic use.
- nucleic acids e.g., DMA, siRNA, mRNA, microRNA
- compounds described herein can provide one or more desired
- compounds described herein can be characterized as having one or more properties that afford such compounds advantages relative to other similarly classified lipids.
- compounds disclosed herein can allow for the control and tailoring of the properties of liposomal compositions (e.g., lipid
- compounds disclosed herein can be characterized by enhanced transfection efficiencies and their ability to provoke specific biological outcomes. Such outcomes can include, for example enhanced cellular uptake, endosomal/iysosomal disruption capabilities and/or promoting the release of encapsulated materials (e.g., polynucleotides) intraceilulariy. Additionally, the compounds disclosed herein have advantageous pharmacokinetic properties, biodistribution, and efficiency (e.g., due to the different disassociate rates of the polymer group used).
- the cationic lipids of the present invention include compounds having a structure according to Formula (A),
- each R la is independently hydrogen, R ic , or R 3d ;
- each R lB is independently R lc or R ld ;
- each R lc is Independently - ⁇ CM ) 2 C(Q)X 1 R 3 ;
- each R ld is independently -C(0)R 4 ;
- each R 2 is independently -[C(R 2a ) 2 ],;R 2b ;
- each R 2a is independently hydrogen or lower alkyl (e.g., C-.-C & alkyl);
- R 2b is -N(L I -B) 2 , -(OCH 2 CH 2 ) 6 OH; or -(OCH 2 CH 2 ) 6 0CH 3 ; each R 3 and R 4 is independently aliphatic ⁇ e.g., C 6 -C 30 aliphatic); each Li is independently alkylene (e.g., Ci-Cio a!ky!ene);
- each B is independently hydrogen or an ionizable nitrogen-containing group
- each X 1 is independently a covalent bond or O;
- each a is independently an integer (e.g., 1-10);
- each b is independently an integer (e.g., 1-10).
- each c. is independently an integer (e.g., 1-10).
- the cationic lipid has a structure according to Formula (A), wherein each R la is independently hydrogen, R lc , or R 3d ;
- each R lc is independently R lc or R ld ;
- each R lc is independently -(CH 2 ) 2 C(0)X 1 R 3 ;
- each R ld is independently ⁇ C(0)R 4 ;
- each R / is independently -[C ⁇ R 2a ) 2 ] c R 2b ;
- each R 2a is independently hydrogen or CrCs alkyl
- R 2b is -N[L I -B) 2 , -(OCH 2 CH 2 ) / ,OH; or - ⁇ OCH 2 CH 2 ) b OCH 3 ;
- each R 3 and R 4 is independently C 6 -C 3 o aliphatic
- each Li is independently Ci-Cio alkylene
- each B is independently hydrogen or an ionizable nitrogen-containing group
- each X 3 is independently a covalent bond or O;
- each a is Independently an integer of 1-10;
- each b is independently an integer of 1-10;
- each c is independently an integer of 1-10.
- each a is independently 1 or 2. In embodiments, each a is 1. In embodiments, each a is 2,
- each R la and R lb is Independently HCH 2 ) 2 C(0)X 1 R 3 .
- a cationic Iipid has a structure according to formula (I):
- each R 3 is the same, in embodiments, each R 3 is different.
- each R 3 is independently C 6 -C M alkyl, C 6 -C 2 alkenyl, or C 6 -C a!kynyi.
- each R 3 is independently Cs-C 30 alkyl, in embodiments, each R 3 is
- each R 3 is independently unsubstituted C 6 -C 3 o alkyl, in embodiments, each R 3 is independently substituted C 6 -C 3 o alkyl. In embodiments, each R 3 Is independently C 6 -C alkyl. In embodiments, each R 3 Is independently unsubstituted C 6 -C 2 alkyl in embodiments, each R 3 is independently substituted C 6 -C alkyl.
- each R 3 is independently C 6 -C 30 alkenyl. In embodiments, each R 3 is
- each R 3 is independently unsubstituted C 6 -C 30 alkenyl, in embodiments, each R 3 is independently substituted C 6 -C 30 alkenyl. In embodiments, each R 3 is C 6 -C 2 alkenyl. In embodiments, each R 3 is independently unsubstituted C 6 -C 2 alkenyl. In embodiments, each R 3 is independently substituted Ce-C 24 alkenyl.
- each R 3 is independently Cs-C 30 a!kynyl. In embodiments, each R 3 is
- each R 3 is independently unsubstituted C 6 -C 30 alkynyl. In embodiments, each R 3 is independently substituted C 6 -C 30 alkynyl. In embodiments, each R 3 is Independently C 3 -C alkynyl.
- each R 3 is independently unsubstituted C 6 -C 2 alkynyl. In embodiments, each R 3 is independently substituted CK-C M alkynyl.
- each R la is hydrogen and each R lb is -CiOIR c
- a cationic iipid has a structure according to formula ⁇ li):
- each R 4 is the same, in embodiments, each R 4 is different.
- each R 4 is independently C 6 -C 24 alkyl, C 6 C 24 alkenyl, or C 6 -C 24 alkynyl.
- each R 4 is independently C 6 -C 3 o alkyl. In embodiments, each R 4 is
- each R 4 is independently unsubstituted Ce-Cso alkyl, in embodiments, each R 4 is independently substituted CK-C SO alkyl. In embodiments, each R 4 is independently C & -C 2 alkyl, in embodiments, each R 4 is independently unsubstituted Cs-C 24 alkyl, in embodiments, each R 4 is independently substituted C 6 -C 24 alkyl.
- each R 4 is independently C 6 -C 3 o alkenyl. In embodiments, each R 4 is
- each R 4 is independently unsubstituted C 6 -C o alkenyl.
- each R 4 is independently substituted Cs-C 3 o alkenyl, in embodiments, each R 4 is independently C 6 -C 24 alkenyl, in embodiments, each R 4 is independently unsubstituted C 6 ⁇ C alkenyl, in embodiments, each R 4 is independently substituted C 6 -C 2 alkenyl.
- each R 4 is independently C 6 -C 3 o alkynyl. In embodiments, each R 4 is
- each R 4 is independently unsubstituted C 6 -C o alkynyl. In embodiments, each R 4 is independently substituted Cs-C 3 o alkynyl. In embodiments, each R 4 is independently C 8 -C 24 alkynyl.
- each R 4 is independently unsubstituted C 6 ⁇ C alkynyl. in embodiments, each R 4 is independently substituted C 6 -C 24 alkynyl,
- each R 4 is independently C 6 -Cio alkyl substituted with -GC(Q)R 5 or-C0 2 R 5 , wherein R 5 is unsubstituted Cg-Cie alkyl, in embodiments, each R 4 is independently C 3 -Ci 0 alkyl substituted with ⁇ OC ⁇ Q)R : '. in embodiments, each R 4 is independently C 6 -Cio alkyl substituted with -C0 2 R r> .
- R 5 is independently unsubstituted linear C 6 -Cis alkyl, in embodiments, R 5 is independently unsubstituted branched C 6 -C 36 alkyl.
- each R 2 is independently -[CH 2 3 !; -iM(Li-B) 2 or ⁇ CH(CH 3 )CH 2 --N(LrB) 2 .
- each Li is unsubstituted Ci-Cio aikyiene.
- each R 2 is independently -[CH 2jc -N ⁇ CH 3 ) 2 .
- each R 2 is independently -[CH 2 ] c -N ⁇ CH 2 CH 2 B) 2 , -[CH 2 ] c -N(CH 2 CH 2 CH 2 B) 2 , - CH(CH 3 )CH 2 --i ⁇ i ⁇ CH 2 CH 2 B) 2 , or ⁇ CH ⁇ CH 3 )CH 2 -N(CH 2 CH 2 CH 2 B) 2 ; and each B is an ionizabie nitrogen- containing group.
- each B is independently NH 2 , guanidine, amidine, a mono- or dialkylamine, 5- to 6-membered heterocycloaikyl, or 5- to 6-membered nitrogen-containing heteroaryl.
- each B is NH 2 .
- each B is guanidine, in embodiments, each B is amidine.
- each B is a monoalkylamine.
- each B is a diaikyiamine.
- each B is a 5- to 6-membered heterocycloalkyl.
- each B is a 5- to 6- membered nitrogen -containing heteroaryi.
- each B is independently NH 2 , guanidine, amidine, NHCH 3 , N(CH 3 ) 2 , or imidazole, in embodiments, each B is N(CH 3 ) 2 .
- each c is independently 2, 3, or 4. in embodiments, each c is 2. in
- each c is 3. in embodiments, each c is 4.
- a C S ⁇ C 30 alky! is a C 8-26 a!ky!.
- a C 6 -C 3 o alkyl is a straight- chain Cg 26 alkyl
- a C 6 -C 30 alkyl is 6H 3 (6H 2 ⁇ 6 OH 2 -, CH 3 (CH 2 ) 7 CH 2 -, CH 3 (CH 2 )gCH 2 -, CH 3 ⁇ CH 2 )gCH 2 -, CHJ,(CH 2 )IOCH 2 -, CH 3 (CH 2 )UCH 2 -, CH 3 (CH 2 ) 12 CH 2 -, CH 3 (CH 2 ) I; ,CH 2 -, CH 3 (CH 2 ) M CH 2 -, CHB ⁇ CH 2 )I 5 CH 2 -, CHB(CH 2 )I6CH 2 ⁇ , CH 3 (CH 2 )I 7 CH 2 -, CH 3 (CH 2 )I 8 CH 2 -
- a Ce-C 3 o alkenyl is a Cs-ze alkenyl having one or two carbon-carbon double bonds
- a C 6 -C 30 alkenyl is C 8-26 aliphatic having three, four, five or six carbon-carbon double bonds.
- a C -C alkenyl is:
- a Cs-C 30 alkenyl is:
- a C s ⁇ C 3 o alkenyl is:
- an aliphatic group described herein is the aliphatic chain of a saturated or unsaturated fatty acid.
- an aliphatic group is the aliphatic chain of capry!ic, pelargonic, capric, undecylic, iauric, tridecyclic, myristic, pentadecylic, margaric, stearic, nonadecylic, arachidic, heneicosylic, behenic, triosylic, lignoceric, oleic, linoleic, pentacosylic or cerotic acid, in embodiments, an aliphatic group is the aliphatic chain of caprylic, pelargonic, capric, undecylic,
- an aliphatic group is the aliphatic chain of Iauric, tridecyclic, myristic, peniadecyiic, or margaric acid.
- an aliphatic group is the aliphatic chain of Iauric, tridecyclic, myristic, or pentadecylic acid.
- an aliphatic group aliphatic chain of Iauric or myristic acid.
- an aliphatic group is the aliphatic chain of stearic, nonadecylic, arachidic, heneicosylic, behenic, triosylic, lignoceric, oleic, linoleic, pentacosylic or cerotic add.
- an aliphatic group is the aliphatic chain of lignoceric, oleic, linoleic, pentacosylic or cerotic acid. In embodiments, an aliphatic group is the aliphatic chain of oleic, linoleic or pentacosylic acid. In embodiments, R 2 is the aliphatic chain of oleic or linoleic acid. In embodiments, an aliphatic group is the aliphatic chain of oleic acid. In some embodiments, an aliphatic group is the aliphatic chain of linoleic acid.
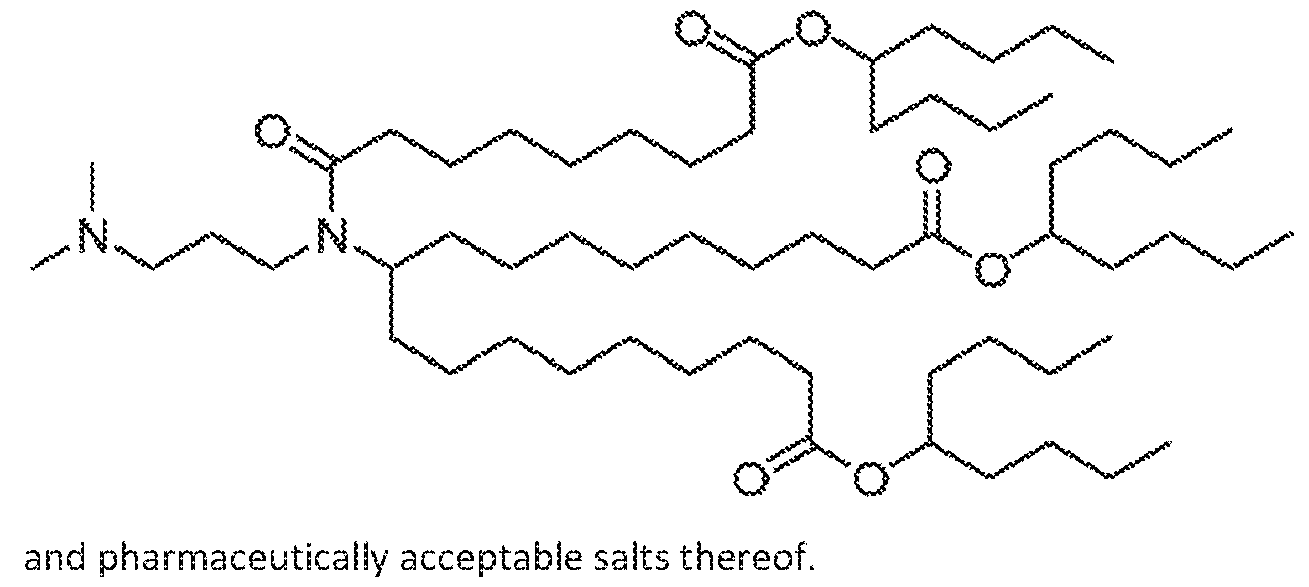
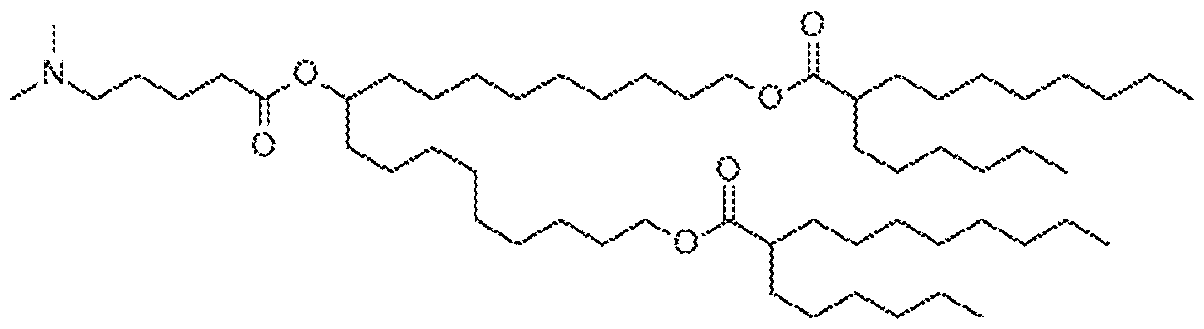
- a cationic lipid is any one of Compounds 1-12.
- a cationic lipid is Compound 1. !n embodiments, a cationic lipid is
- a cationic lipid in embodiments, a cationic lipid is Compound 3, In embodiments, a cationic lipid is Compound 4. in embodiments, a cationic lipid is Compound 5, In embodiments, a cationic lipid is Compound 6, in embodiments, a cationic lipid is Compound 7. in embodiments, a cationic lipid is Compound 8, In embodiments, a cationic lipid is Compound 9. In embodiments, a cationic lipid is Compound 10. in embodiments, a cationic lipid is Compound 11. In embodiments, a cationic lipid is Compound 12.
- a compound of Formula (A), such as Formulae (! or (il) or any one of Compounds 1-12) can be used to prepare compositions useful for the delivery of nucleic acids.
- Nucleic acids according to the present invention may be synthesized according to any known methods.
- mRIMAs according to the present invention may be synthesized via in vitro transcription (!VT), Briefly, IVT is typically performed with a linear or circular DNA template containing a promoter, a pool of ribonucleotide triphosphates, a buffer system that may include DTT and magnesium ions, and an appropriate RINA polymerase ⁇ e.g., T3, T7, mutated T7 or SP6 RNA polymerase), DNAse I, pyrophosphatase, and/or RNAse inhibitor. The exact conditions will vary according to the specific application.
- a DNA template is transcribed in vitro.
- a suitable DNA template typically has a promoter, for example a 13, 17, mutated 17 or SP6 promoter, for in vitro transcription, followed by desired nucleotide sequence for desired mRNA and a termination signal.
- Desired mRNA sequence(s) according to the invention may be determined and incorporated into a DNA template using standard methods. For example, starting from a desired amino acid sequence ⁇ e.g., an enzyme sequence), a virtual reverse translation is carried out based on the degenerated genetic code. Optimization algorithms may then be used for selection of suitable codons. Typically, the G/C content can be optimized to achieve the highest possible G/C content on one hand, taking into the best possible account the frequency of the tRNAs according to codon usage on the other hand. The optimized RNA sequence can be established and displayed, for example, with the aid of an appropriate display device and compared with the original (wild- type) sequence. A secondary structure can also be analyzed to calculate stabilizing and destabilizing properties or, respectively, regions of the RNA.
- a desired amino acid sequence e.g., an enzyme sequence
- Optimization algorithms may then be used for selection of suitable codons.
- the G/C content can be optimized to achieve the highest possible G/C content on one hand, taking into the best possible account the
- nucleic acid in its broadest sense, refers to any compound and/or substance that is or can be incorporated into a polynucleotide chain.
- DNA may be in the form of antisense DNA, plasmid DNA, parts of a plasmid DNA, pre-condensed DNA, a product of a polymerase chain reaction (PCR), vectors (e.g., PI, PAC, BAG, YAC, artificial chromosomes), expression cassettes, chimeric sequences, chromosomal DNA, or derivatives of these groups.
- PCR polymerase chain reaction
- vectors e.g., PI, PAC, BAG, YAC, artificial chromosomes
- expression cassettes e.g., chimeric sequences, chromosomal DNA, or derivatives of these groups.
- RNA may be in the form of messenger RNA (mRNA), ribosomal RNA (rRNA), signal recognition particle RNA (7 SL RNA or SRP RNA), transfer RNA (tRNA), transfer-messenger RNA (tmRNA), small nuclear RNA (snRNA), small nucleolar RNA (snoRNA), SmY RNA, small Cajal body-specific RNA (scaRNA), guide RNA (gRNA), ribonuclease P (RNase P), Y RNA, telomerase RNA component (TERC), spliced leader RNA (SL RNA), antisense RNA (aRNA or asRNA), cis-natural antisense transcript (cis-NAT), CR!SPR RNA (crRNA), iong noncoding RNA (incRNA), microRNA ( lRN.A), piwi-interacting RNA (pi RNA), small interfering RNA (siRNA), transacting siRNA (tasiRNA), repeat associated siRNA (rasi
- RNAs according to the present invention may be synthesized according to any of a variety of known methods.
- mRNAs according to the present invention may be synthesized via in vitro transcription (!VT).
- !VT is typically performed with a linear or circular DNA template containing a promoter, a pool of ribonucleotide triphosphates, a buffer system that may include DTT and magnesium ions, and an appropriate RNA polymerase ⁇ e.g., T3, T7 or SP6 RNA polymerase), DNAse I, pyrophosphatase, and/or RNAse Inhibitor.
- RNA polymerase e.g., T3, T7 or SP6 RNA polymerase
- the in vitro transcribing occurs in a single batch.
- a DNA template is transcribed in vitro
- a suitable DNA template typically has a promoter, for example a T3, T7 or SP6 promoter, for in vitro transcription, followed by desired nucleotide sequence for desired mRNA and a termination signal.
- Desired mRNA sequence(s) according to the invention may be determined and incorporated into a DNA template using standard methods. For example, starting from a desired amino acid sequence ⁇ e.g., an enzyme sequence), a virtual reverse translation is carried out based on the degenerated genetic code. Optimization algorithms may then be used for selection of suitable codons. Typically, the G/C content can be optimized to achieve the highest possible G/C content on one hand, taking into the best possible account the frequency of the tRNAs according to codon usage on the other hand. The optimized RNA sequence can be established and displayed, for example, with the aid of an appropriate display device and compared with the original (wild- type) sequence. A secondary structure can also be analyzed to calculate stabilizing and destabilizing properties or, respectively, regions of the RNA.
- a desired amino acid sequence e.g., an enzyme sequence
- Optimization algorithms may then be used for selection of suitable codons.
- the G/C content can be optimized to achieve the highest possible G/C content on one hand, taking into the best possible account the
- mRNA according to the present invention may be synthesized as unmodified or modified mRNA, Modified mRNA comprise nucleotide modifications in the RNA.
- a modified mRNA according to the invention can thus include nucleotide modification that are, for example, backbone modifications, sugar modifications or base modifications.
- mRNAs may be synthesized from naturally occurring nucleotides and/or nucleotide analogues (modified nucleotides) including, but not limited to, purines (adenine (A), guanine (G)) or pyrimidines (thymine (T), cytosine (C), uracil (U)), and as modified nucleotides analogues or derivatives of purines and pyrimidines, such as e.g., 1-methyl-adenine, 2-methyl- adenine, 2-methyithio-N-6-isopentenyi-adenine, N6-methyi-adenine, N6-isopentenyl-adenine, 2- thio-cytosine, 3-methyi-cyiosine, 4-acetyl-cytosine,
- mRNAs may contain RNA backbone modifications.
- a RNA backbone modifications typically, a RNA backbone modifications.
- backbone modification is a modification in which the phosphates of the backbone of the nucleotides contained in the RNA are modified ehemiealiy.
- Exemplary backbone modifications typically include, but are not limited to, modifications from the group consisting of
- methylphosphonates methylphosphoramidates, phosphoramidates, phosphorothioates (e.g., cytidine 5'-0-(l-thiophosphate)), boranophosphates, positively charged guanidlnlum groups etc., which means by replacing the phosphodiester linkage by other anionic, cationic or neutral groups.
- RNAs may contain sugar modifications.
- modification is a chemical modification of the sugar of the nucleotides it contains including, but not limited to, sugar modifications chosen from the group consisting of 4'-thio-ribonucleotide (see, e.g., US Patent Application Publication No. US 2016/0031928, incorporated by reference herein), 2'-deoxy-2'-fluoro-oligoribonucleotide (2'-fluoro-2'-deoxycytidine 5'-triphosphate, 2'- fluoro-2'-deoxyuridine 5'-triphosphate), 2'-deoxy-2'-deamine-oligoribonucleotide (2'-amino-2'- deoxycytidine 5'-triphosphate, 2'-amino-2'-deoxyuridine S'-triphosphaie), 2'-0- alkyloligorlbonucieotide, 2'-deoxy-2 !
- -C-alkyioiigoribonudeotide (2'-0-methylcytidine 5'- triphosphate, 2'-methyluridine 5'-triphosphate), 2'-C-alkyloligoribonucleotide, and isomers thereof (2'-aracytidine 5'-triphosphate, 2'-arauridine 5'-triphosphate), or azidotriphosphates (2'- azido-2'-deoxycytidine 5'-triphosphate, 2'-azido-2'-deoxyuridine 5'-triphosphate).
- mRNAs may contain modifications of the bases of the nucleotides (base modifications).
- a modified nucleotide which contains a base modification is also called a base-modified nucleotide.
- base-modified nucleotides include, but are not limited to, 2-amino-6-chloropurine riboside S'-triphosphate, 2-aminoadenosine S'-triphosphate, 2-thiocytidine 5'-triphosphate, 2-thiouridine 5'-triphosphate, 4-thiouridjne 5'-triphosphate, 5- aminoallylcytidine 5'-triphosphate, 5-aminoally!uridine 5'-triphosphate, 5-bromocytidine 5'- triphosphate, 5-bromouridine S'-triphosphate, 5-iodocytidine S'-triphosphate, 5-iodouridine S'- triphosphate, 5-methylcytidine S'-triphosphate, S-
- mRNA synthesis includes the addition of a“cap” on the N-terminal (5') end, and a “tail” on the C-terminai (3') end.
- a“cap” on the N-terminal (5') end
- a “tail” on the C-terminai (3') end.
- the presence of the cap is important in providing resistance to nucleases found in most eukaryotic cells.
- the presence of a "tali” serves to protect the mRNA from exonuclease degradation.
- mRNAs include a 5' cap structure.
- a 5' cap is typically added as follows: first, an RNA terminal phosphatase removes one of the terminal phosphate groups from the 5' nucleotide, leaving two terminal phosphates; guanosine triphosphate (GTP) is then added to the terminal phosphates via a guanyly! transferase, producing a 5'5'5 triphosphate linkage; and the 7-nitrogen of guanine is then methylated by a methyltransferase.
- GTP guanosine triphosphate
- cap structures include, but are not limited to, m7G(5')ppp (5'(A,G(5')ppp(5')A and G(5')ppp ⁇ 5')G.
- mRNAs include a 3' poly(A) tail structure.
- a poly-A tail on the 3' terminus of mRNA typically inciudes about 10 to 300 adenosine nucleotides (e.g., about 10 to 200 adenosine nucleotides, about 10 to 150 adenosine nucleotides, about 10 to 100 adenosine nucleotides, about 20 to 70 adenosine nucleotides, or about 20 to 60 adenosine nucleotides).
- mRNAs include a 3' poly(C) tail structure.
- a suitable po!y-C tail on the 3’ terminus of mRNA typically include about 10 to 200 cytosine nucleotides ⁇ e.g., about 10 to 150 cytosine nucleotides, about 10 to 100 cytosine nucleotides, about 20 to 70 cytosine nucleotides, about 20 to 60 cytosine nucleotides, or about 10 to 40 cytosine nucleotides).
- the poly-C tail may be added to the poly-A tail or may substitute the po!y-A tail.
- mRNAs include a 5' and/or 3' untranslated region.
- a 5' untranslated region includes one or more elements that affect an mRNA's stability or translation, for example, an iron responsive element, in some embodiments, a 5' untranslated region may be between about 50 and 500 nucleotides in length.
- a 3' untranslated region includes one or more of a polyadenylation signal, a binding site for proteins that affect an mRNA's stability of location in a cell, or one or more binding sites for miRNAs, In some embodiments, a 3' untranslated region may be between 50 and 500 nucleotides in length or longer.
- R!MAs include a 5' cap structure.
- a 5' cap is typically added as follows: first, an RNA terminal phosphatase removes one of the terminal phosphate groups from the 5' nucleotide, leaving two terminal phosphates; guanosine triphosphate (GTP) is then added to the terminal phosphates via a guanylyl transferase, producing a 5'-5' triphosphate linkage; and the 7-nitrogen of guanine is then methylated by a methyltransferase.
- GTP guanosine triphosphate
- the nucleotide forming the cap is further methylated at the 3'position. In some embodiments, the nucleotide directly adjacent to the cap Is further methylated at the 2' position.
- cap structures include, but are not limited to, m7G(5')ppp(5')(2'OMeG), m7G(5')ppp(5')(2'OMeA),
- the cap structure is m7G(5')ppp(5')(2OMeG).
- Naturally occurring cap structures comprise a 7-methyl guanosine that is linked via a
- the cap is added enzymatically.
- the cap is added in the nucleus and is catalyzed by the enzyme guanylyl transferase.
- the addition of the cap to the 5‘ terminal end of RNA occurs immediately after initiation of transcription.
- the terminal nucleoside is typically a guanosine, and is in the reverse orientation to all the other nucleotides, i.e., G(5')ppp(5')GpNpNp.
- the cap for mRNA produced by in vitro transcription is
- RNAs having a cap structure in their 5'-termini The prevailing method for the in vitro synthesis of capped RNA employs a pre-formed dinucleotide of the form m''G(5')ppp(5 ! )G ("m'GpppG") as an Initiator of transcription.
- a form of a synthetic dinucleotide cap used in in vitro translation experiments is the Anti-Reverse Cap Analog ("ARCA") or modified ARCA, which is generally a modified cap analog In which the 2' or 3' OH group is replaced with -OCH 3 .
- ARCA Anti-Reverse Cap Analog
- modified ARCA which is generally a modified cap analog In which the 2' or 3' OH group is replaced with -OCH 3 .
- Additional cap analogs include, but are not limited to, a chemical structures selected from the group consisting of m 7 GpppG, m'GpppA, m 7 GpppC; unmethylated cap analogs ⁇ e.g., GpppG); dimethylated cap analog (e.g., m 2,7 GpppG), trimethylated cap analog (e.g., m 2 ' 2,7 GpppG), dimethylated symmetrical cap analogs ⁇ e.g., m 7 Gpppm 7 G), or anti reverse cap analogs ( e.g., ARCA; rn 7 '0me GpppG, m 72'd GpppG, rrs 7 ’ 3'0me GpppG, m 7,3'd GpppG and their tetraphosphate derivatives) (see, e.g,, Jemieiity, J. ef a!. , "Novel 'anti-reverse' cap analogs with superior translationa chemical structures selected
- a suitable cap is a 7-methyi guanylate (“m 7 G") linked via a
- m 7 G(5')ppp(5')N where N is any nucleoside.
- a preferred embodiment of a m ; G cap utilized in embodiments of the invention is m 7 G(5')ppp(5')G.
- the cap is a CapO structure.
- CapO structures lack a 2'-0-methyl residue of the ribose attached to bases 1 and 2.
- the cap is a Capl structure.
- Capl structures have a 2'-0-methy! residue at base 2.
- the cap is a Cap2 structure.
- Cap2 structures have a 2'-0-methyl residue attached to both bases 2 and 3,
- cap analogs for use in embodiments of the invention include N7-benzyiated dinucleoside tetraphosphate analogs (described in Grudzien, E. et al. , RNA, 10: 1479-1487 (2004)), phosphorothioate cap analogs (described in Grudzien-Nogalska, E,, et ai. , RNA, 13: 1745-1755 (2007)), and cap analogs (including biotinylated cap analogs) described in U.S. Patent Nos. 8,093,367 and 8,304,529, incorporated by reference herein.
- a "tail” serves to protect the mRNA from exonuciease degradation.
- the poly A tail is thought to stabilize natural messengers and synthetic sense RNA. Therefore, in certain embodiments a long poly A tail can be added to an RNA molecule thus rendering the RNA more stable.
- Poly A tails can be added using a variety of art-recognized techniques. For example, long poly A tails can be added to synthetic or in vitro transcribed RNA using poly A polymerase (Yokoe, et ai. Nature Biotechnology. 1996; 14: 1252-1256).
- a transcription vector can also encode long poly A tails, in addition, poly A tails can be added by transcription directly from PCR products.
- Poly A may also be ligated to the 3' end of a sense RNA with RNA ligase (see, e.g., Molecular Cloning A Laboratory Manual, 2nd Ed,, ed, by Sambrook, Fritsch and Maniatis (Cold Spring Harbor Laboratory Press: 1991 edition)).
- mRNAs include a 3' poly(A) tail structure.
- the length of the poly A tail can be at least about 10, 50, 100, 200, 300, 400 at least 500 nucleotides
- a poly-A tail on the 3' terminus of mRNA typically includes about 10 to 300 adenosine nucleotides ⁇ e.g., about 10 to 200 adenosine nucleotides, about 10 to 150 adenosine nucleotides, about 10 to 100 adenosine nucleotides, about 20 to 70 adenosine nucleotides, or about 20 to 60 adenosine nucleotides), in some embodiments, RNAs include a 3' poly(C) tail structure.
- a suitable poiy-C tall on the 3' terminus of mRNA typically include about 10 to 200 cytosine nucleotides (e.g., about 10 to 150 cytosine nucleotides, about 10 to 100 cytosine nucleotides, about 20 to 70 cytosine nucleotides, about 20 to 60 cytosine nucleotides, or about 10 to 40 cytosine nucleotides).
- the poly-C tail may be added to the poly-A tail or may substitute the po!y-A tail.
- the length of the poly A or poly C tail is adjusted to control the
- the length of the poly A tail can influence the half-life of a sense mRNA molecule, the length of the poly A tail can be adjusted to modify the level of resistance of the mRNA to nucleases and thereby control the time course of polynucleotide expression and/or polypeptide production in a target cell.
- mRNAs include a 5' and/or 3' untranslated region.
- a 5' untranslated region includes one or more elements that affect an mRNA's stability or translation, for example, an iron responsive element.
- a 5' untranslated region may be between about 50 and 500 nucleotides in length.
- a 3' untranslated region includes one or more of a polyadenyiation signal, a binding site for proteins that affect an mRNA's stability of iocaiion in a cell, or one or more binding sites for miRNAs.
- a 3' untranslated region may be between 50 and 500 nucleotides in length or longer.
- Exemplary 3' and/or 5' UTR sequences can be derived from mRNA molecules which are stable (e.g., globin, actin, GAPDH, tubulin, histone, or citric acid cycle enzymes) to increase the stability of the sense mRNA molecule.
- a 5' UTR sequence may include a partial sequence of a CMV immediate-early 1 (IE1) gene, or a fragment thereof to improve the nuclease resistance and/or improve the haif-life of the polynucleotide.
- IE1 immediate-early 1
- hGH human growth hormone
- modifications improve the stability and/or pharmacokinetic properties ⁇ e.g., half-life) of the polynucleotide relative to their unmodified counterparts, and include, for example modifications made to improve such polynucleotides' resistance to in vivo nuclease digestion.
- the compounds described herein e.g., a compound of Formula (A), such as Formulae (I) or (li) or any one of Compounds 1-12
- a compound of Formula (A) such as Formulae (I) or (li) or any one of Compounds 1-12
- pharmaceutical and liposomal compositions comprising such lipids
- encapsulated materials e.g., one or more polynucleotides such as mRNA
- cationic lipids described herein are characterized as resulting in one or more of receptor-mediated endocytosis, clathrin-mediated and caveolae-mediated endocytosis, phagocytosis and macropinocytosis, fusogenicity, endosomai or lysosomal disruption and/or releasable properties that afford such compounds advantages relative other similarly classified lipids.
- a nucleic acid e.g., mRNA encoding a protein ⁇ e.g., a full length, fragment or portion of a protein
- a delivery vehicle comprising a compound as described herein ⁇ e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12).
- delivery vehicle As used herein, the terms “delivery vehicle,” “transfer vehicle,” “nanoparticle,” or
- the present invention provides a composition (e.g., a pharmaceutical
- compositions comprising a compound described herein (e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) and one or more polynucleotides.
- a composition e.g., a pharmaceutical composition
- a composition exhibits an enhanced (e.g., increased) ability to transfect one or more target cells.
- methods of transfecting one or more target ceils generally comprise the step of contacting the one or more target cells with the cationic lipids and/or pharmaceutical compositions disclosed herein (e.g., a liposomal formulation comprising a compound described herein (e.g., a compound of Formula (A), such as Formulae (! or (li) or any one of Compounds 1-12) encapsulating one or more polynucleotides) such that the one or more target cells are transfected with the materials encapsulated therein ⁇ e.g., one or more polynucleotides).
- a liposomal formulation comprising a compound described herein (e.g., a compound of Formula (A), such as Formulae (! or (li) or any one of Compounds 1-12) encapsulating one or more polynucleotides
- transfect or “transfection'' refer to the intracellular introduction of one or more encapsulated materials [e.g., nucleic acids and/or polynucleotides) into a cell, or preferably into a target ceil.
- the introduced polynucleotide may be stably or transiently maintained in the target ceil.
- transfection efficiency refers to the relative amount of such encapsulated material [e.g., polynucleotides) up-taken by, introduced into, and/or expressed by the target cell which is subject to transfection.
- transfection efficiency may be estimated by the amount of a reporter polynucleotide product produced by the target ceils following transfection, in certain embodiments, the compounds and pharmaceutical compositions described herein demonstrate high transfection efficiencies thereby improving the likelihood that appropriate dosages of the encapsulated materials ⁇ e.g., one or more polynucleotides) will be delivered to the site of pathology and subsequently expressed, while at the same time minimizing potential systemic adverse effects or toxicity associated with the compound or their encapsulated contents.
- the encapsulated materials e.g., one or more polynucleotides
- the production of the product ⁇ e.g., a polypeptide or protein) encoded by such polynucleotide may be preferably stimulated and the capability of such target cells to express the polynucleotide and produce, for example, a polypeptide or protein of interest is enhanced.
- transfection of a target cell by one or more compounds or pharmaceutical compositions encapsulating mRNA will enhance (i.e., increase) the production of the protein or enzyme encoded by such mRNA.
- delivery vehicles described herein ⁇ e.g., liposomal delivery vehicles
- the lipid nanoparticies of the present invention may be prepared to achieve enhanced delivery to the target ceils and tissues.
- polynucleotides e.g., mRNA
- encapsulated polynucleotides e.g., mRNA
- the encapsulated polynucleotides are capable of being expressed and functional polypeptide products produced (and in some instances excreted) by the target cell, thereby conferring a beneficial property to, for example the target cells or tissues.
- Such encapsulated polynucleotides may encode, for example, a hormone, enzyme, receptor, polypeptide, peptide or other protein of interest.
- a composition is a suitable delivery vehicle, in embodiments, a composition is a liposomal delivery vehicle, e.g., a lipid nanoparticle.
- Enriching liposomal compositions with one or more of the cationic lipids disclosed herein may be used as a means of improving (e.g., reducing) the toxicity or otherwise conferring one or more desired properties to such enriched liposomal composition (e.g., improved delivery of the encapsulated polynucleotides to one or more target cells and/or reduced In vivo toxicity of a liposomal composition).
- the compounds described herein e.g., a compound of
- Formula (A), such as Formulae (! or (II) or any one of Compounds 1-12) may be used as a component of a liposomal composition to facilitate or enhance the delivery and release of encapsulated materials (e.g., one or more therapeutic agents) to one or more target cells (e.g., by permea ting or fusing with the lipid membranes of such target ceils).
- encapsulated materials e.g., one or more therapeutic agents
- liposomal delivery vehicles e.g., lipid nanoparticles
- lipid nanoparticles are usually
- Bilayer membranes of liposomes are typically formed by amphiphilic molecules, such as lipids of synthetic or natural origin that comprise spatially separated hydrophilic and hydrophobic domains (Lasic, Trends Biotechnoi., 16: 307-321, 1998), Bilayer membranes of the liposomes can also be formed by amphophilic polymers and surfactants (e.g., polymerosomes, niosomes, etc.), in the context of the present invention, a liposomal delivery vehicle typically serves to transport a desired mRNA to a target cell or tissue.
- compositions e.g., liposomal compositions
- encapsulate materials such as for example, one or more biological!y-active polynucleotides (e.g., mRIMA).
- a composition (e.g., a pharmaceutical composition) comprises an mRNA encoding a protein, encapsulated within a liposome in embodiments, a liposome comprises one or more cationic lipids, one or more non-cationic lipids, one or more cholesterol-based lipids and one or more PEG-modified lipids, and wherein at least one PEG-modified lipid is a compound as described herein (e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12).
- a compound of Formula (A) such as Formulae (I) or (II) or any one of Compounds 1-12.
- a composition comprises an mRNA encoding for a protein (e.g., any protein described herein), in embodiments, a composition comprises an mRNA encoding for cystic fibrosis transmembrane conductance regulator (CFTR) protein, in embodiments, a composition comprises an mRNA encoding for ornithine transcarbamyiase (OTC) protein.
- CFTR cystic fibrosis transmembrane conductance regulator
- OTC ornithine transcarbamyiase
- a composition (e.g., a pharmaceutical composition) comprises a nucleic acid encapsulated within a liposome, wherein the liposome comprises any compound described herein (e.g., a compound of Formula (A), such as Formulae (! or ⁇ II) or any one of Compounds 1- 12) as described herein,
- a nucleic acid is an mRNA encoding a peptide or protein.
- an mRNA encodes a peptide or protein for use in the delivery to or treatment of the lung of a subject or a lung ceil (e.g., an mRNA encodes cystic fibrosis transmembrane conductance regulator (CFTR) protein).
- CFTR cystic fibrosis transmembrane conductance regulator
- an mRNA encodes a peptide or protein for use in the delivery to or treatment of the liver of a subject or a liver cell (e.g., an mRNA encodes ornithine transcarbamyiase (OTC) protein).
- OTC ornithine transcarbamyiase
- a liposomal delivery vehicle e.g., a lipid nanoparticle
- a net positive charge e.g., a lipid nanoparticle
- a liposomal delivery vehicle e.g., a lipid nanoparticle
- a net negative charge e.g., a net negative charge
- a liposomal delivery vehicle e.g., a lipid nanoparticle
- a net neutral charge e.g., a lipid nanoparticle
- a lipid nanoparticle that encapsulates a nucleic acid comprises one or more compounds described herein ( ⁇ e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12).
- the amount of a compound as described herein e.g., a compound of Formula (A), such as Formulae (I) or (I! or any one of Compounds 1-12
- a percentage wt% of the combined dry weight of all lipids of a composition (e.g., the combined dry weight of all lipids present in a liposomal composition).
- a compound as described herein e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12
- a compound as described herein is present in an amount that is about 0.5 wt% to about 30 wt% (e.g., about 0.5 wt% to about 20 wt%) of the combined dry weight of all lipids present in a composition (e.g., a liposomal composition).
- a compound as described herein e.g., a compound of Formula (A), such as Formulae (i) or (II) or any one of Compounds 1-12
- a compound as described herein is present in an amount that Is about 1 wt% to about 30 wt%, about 1 wt% to about 20 wt%, about 1 wt% to about 15 wt%, about 1 wt% to about 10 wt%, or about 5 wt% to about 25 wt% of the combined dry weight of a!i lipids presen t in a composition (e.g., a liposomal composition), in embodiments, a compound as described herein (e.g., a compound of Formula (A), such as Formulae (i) or (II) or any one of Compounds 1- 12) is present in an amount that is about 0.5 wt% to about 5 wt%, about 1 wt% to about 10 wt%, about 5 wt% to
- the amount of a compound as described herein ⁇ e.g., a compound of Formula (A), such as Formulae (! or (II) or any one of Compounds 1-12) is present in an amount that is at least about 5 wt%, about 10 wt%, about 15 wt%, about 20 wt%, about 25 wt%, about 30 wt%, about 35 wt%, about 40 wt%, about 45 wt%, about 50 wt%, about 55 wt%, about 60 wt%, about 65 wt%, about 70 wt%, about 75 wt%, about 80 wt%, about 85 wt%, about 90 wt%, about 95 wt%, about 96 wt%, about 97 wt%, about 98 wt%, or about 99 wt% of the combined dry weight of total lipids in a composition ⁇ e.g., a liposomal composition
- the amount of a compound as described herein ⁇ e.g., a compound of Formula (A), such as Formulae (! or (II) or any one of Compounds 1-12) is present in an amount that is no more than about 5 wt%, about 10 wt%, about 15 wt%, about 20 wt%, about 25 wt%, about 30 wt%, about 35 wt%, about 40 wt%, about 45 wt%, about 50 wt%, about 55 wt%, about 60 wt%, about 65 wt%, about 70 wt%, about 75 wt%, about 80 wt%, about 85 wt%, about 90 wt%, about 95 wt%, about 96 wt%, about 97 wi%, about 98 wt%, or about 99 wt% of the combined dry weight of total lipids in a composition ⁇ e.g., a liposomal composition).
- compositions ⁇ e.g., a liposomal delivery vehicle such as a lipid
- nanoparticle comprises about 0.1 wt% to about 20 wt% (e.g., about 0.1 wt% to about 15 wt%) of a compound described herein (e.g., a compound of Formula (A), such as Formulae (I) or (!l) or any one of Compounds 1-12).
- a compound described herein e.g., a compound of Formula (A), such as Formulae (I) or (!l) or any one of Compounds 1-12.
- a delivery vehicle ⁇ e.g., a liposomal delivery vehicle such as a lipid nanoparticle
- a delivery vehicle comprises about 0.5 wi%, about 1 wt%, about 3 wt%, about 5 wt%, or about 10 wt% of a compound described herein ⁇ e.g., a compound of Formula (A), such as Formulae (! or (II) or any one of Compounds 1-12).
- a delivery vehicle ⁇ e.g., a liposomal delivery vehicle such as a lipid nanoparticle
- a delivery vehicle comprises up to about 0,5 wt%, about 1 wt%, about 3 wt%, about 5 wt%, about 10 wt%, about 15 wt%, or about 20 wt% of a compound described herein ⁇ e.g., a compound of Formula (A), such as Formulae (i) or (I! or any one of Compounds 1-12).
- the percentage results in an improved beneficial effect ⁇ e.g., improved delivery to targeted tissues such as the liver or the lung).
- the amount of a compound as described herein ⁇ e.g., a compound of Formula (A), such as Formulae (! or (II) or any one of Compounds 1-12) in a composition also can be described as a percentage ("mol%") of the combined molar amounts of total lipids of a composition ⁇ e.g., the combined molar amounts of all lipids present in a liposomal delivery vehicle).
- a compound as described herein ⁇ e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) is present in an amount that is about 0.5 mol% to about 5 mol%, about 1 moi% to about 10 moi%, about 5 moi% to about 20 moi%, or about 10 mol% to about 20 mol% of the combined molar amounts of all lipids present In a composition such as a liposomal delivery vehicle.
- a compound of Formula (A) such as Formulae (I) or (II) or any one of Compounds 1-12
- a compound as described herein ⁇ e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1- 12) is present in an amount that is about 1 mol% to about 30 mol%, about 1 mol% to about 20 mo!%, about .1 mo!% to about 15 mol%, about .1 mol% to about 10 mol%, or about 5 moi% to about 25 mol% of the combined dry weight of ail lipids present in a composition such as a liposomal delivery vehicle
- a compound as described herein can comprise from about 0.1 mo!% to about 50 moi%, or from 0.5 mo!% to about 50 mo!%, or from about 1 mol% to about 25 mol%, or from abou t 1 mol% to about 10 mol% of the total amount of lipids in a composition (e.g., a liposomal delivery vehicle).
- a compound as described herein e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12
- a compound as described herein can comprise greater than about 0.1 mol%, or greater than about 0.5 mol%, or greater than about .1 mol%, or greater than about 5 mol% of the total amount of lipids in the lipid nanoparticle.
- a compound as described ⁇ e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) can comprise less than about 25 mol%, or less than about 10 moi%, or less than about 5 mol%, or less than about 1 mo!% of the total amount of lipids in a composition ⁇ e.g., a liposomal delivery vehicle).
- the amount of a compound as described herein ⁇ e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) is present in an amount that is at least about 5 ma!%, about 10 mol%, about 15 moi%, about 20 mol%, about 25 mol%, about 30 mof%, about 35 mol%, about 40 mo!%, about 45 mol%, about 50 mol%, about 55 mol%, about 60 mol%, about 65 mol%, about 70 mo!%, about 75 mol%, about 80 mol%, about 85 mol%, about 90 mol%, about 95 mol%, about 96 mol%, about 97 mol%, about 98 mol%, or about 99 mol% of the combined dry weight of total iipids in a composition (e.g., a liposomal composition).
- a composition e.g., a liposomal composition
- the amount of a compound as described herein ⁇ e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) is present in an amount that is no more than about 5 moi%, about 10 mol%, about 15 mol%, about 20 moi%, about 25 mol%, about 30 moi%, about 35 mo!%, about 40 mol%, about 45 moi%, about 50 mol%, about 55 mol%, about 60 moi%, about 65 mo!%, about 70 mol%, about 75 moi%, about 80 mol%, about 85 mol%, about 90 mo!%, about 95 mol%, about 96 mol%, about 97 mol%, about 98 mol%, or about 99 mol% of the combined dry weight of total lipids in a composition ⁇ e.g., a liposomal composition).
- the percentage results in an improved beneficial effect ⁇ e.g., improved delivery to targeted tissues such as the liver or the lung).
- a composition further comprises one more iipids ⁇ e.g., one more iipids selected from the group consisting of one or more cationic Iipids, one or more non-cationic iipids, one or more cholesterol-based Iipids, and one or more PEG-modified Iipids).
- one more iipids e.g., one more iipids selected from the group consisting of one or more cationic Iipids, one or more non-cationic iipids, one or more cholesterol-based Iipids, and one or more PEG-modified Iipids.
- such pharmaceutical ⁇ e.g., liposomal) compositions comprise one or more of a PEG-modified lipid, a non-cationic lipid and a cholesterol lipid.
- such pharmaceutical ⁇ e.g., liposomal) compositions comprise: one or more PEG-modified Iipids; one or more non-cationic Iipids; and one or more cholesterol lipids
- such pharmaceutical (e.g., liposomal) compositions comprise: one or more PEG-modified Iipids and one or more cholesterol Iipids.
- a composition e.g., lipid nanoparticie that encapsulates a nucleic acid ⁇ e.g., rnRNA encoding a peptide or protein
- a composition comprises one or more compounds as described herein ⁇ e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1- 12) and one or more lipids selected from the group consisting of a cationic lipid, a non-cationic lipid, and a PEGyiated lipid.
- a composition ⁇ e.g., lipid nanoparticie that encapsulates a nucleic acid ⁇ e.g., mRNA encoding a peptide or protein) comprises one or more compound as described herein ⁇ e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds .1- 12); one or more lipids selected from the group consisting of a cationic lipid, a non-cationic lipid, and a PEGylated lipid; and further comprises a cholesterol- based lipid,
- a lipid nanoparticle that encapsulates a nucleic acid comprises one or more compound as described herein ( ⁇ e.g., a compound of Formula (A), such as Formulae (! or (II) or any one of Compounds 1-12), as well as one or more lipids selected from the group consisting of a cationic lipid, a non-cationic lipid, a PEGylated lipid, and a cholesterol-based lipid.
- the selection of cationic lipids, non-cationic lipids and/or PEG-modified lipids which comprise the lipid nanoparticle, as well as the relative molar ratio of such lipids to each other is based upon the characteristics of the selected lipid(s), the nature of the intended target ceils, the characteristics of the mRNA to be delivered. Additional considerations include, for example, the saturation of the alkyl chain, as well as the size, charge, pH, pKa, fusogenicity and toxicity of the selected iipid(s). Thus, the molar ratios may be adjusted accordingly.
- composition may comprise one or more additional cationic lipids,
- liposomes may comprise one or more additional cationic lipids.
- cationic lipid refers to any of a number of lipid species that have a net positive charge at a selected pH, such as physiological pH. Several cationic lipids have been described in the literature, many of which are commercially available,
- Suitable additional cationic lipids for use in the compositions include the cationic lipids as described in International Patent Publication WO 2010/144740, which is incorporated herein by reference.
- the compositions include a cationic lipid, (6Z,9Z,28Z,31Z)- heptatriaconta-6,9,28,31-tetraen-19-yl 4-(dimethylamino) butanoate, having a compound structure of:
- compositions include ionizable cationic lipids as described in international Patent Publication WO 2013/149140, which is incorporated herein by reference.
- compositions include a cationic lipid of one of the following formulas:
- Ri and R 2 are each Independently selected from the group consisting of hydrogen, an optionally substituted, variably saturated or unsaturated alkyl and an optionally substituted, variably saturated or unsaturated C 6 -C 20 acyl; wherein U and l. 2 are each independently selected from the group consisting of hydrogen, an optionally substituted alkyl, an optionally substituted variably unsaturated alkenyl, and an optionally substituted alkynyl; wherein m and o are each independently selected from the group consisting of zero and any positive integer [e.g., where m is three); and wherein n is zero or any positive integer ⁇ e.g., where n is one).
- compositions include the cationic lipid (15Z, 18Z)-N,N-dimethyl-6-(9Z,12Z)-octadeca-9,12-dien-l - yi) tetracosa- 15,18-dien-l-amine ("HGT5000”), having a compound structure of:
- compositions include the cationic lipid (15Z, 18Z)-N,N-dimethyl-6-((9Z,12Z)-octadeca-9,12-dien-l-yl) tetracosa ⁇ 4,15,18-trien-! -amine ("HGT5001”), having a compound structure of:
- the include the cationic lipid and (15Z,18Z)-N,N-dimethyi-6-((9Z,12Z)-octadeca-9,12-dien-l-yi) tetracosa-5,15,18-trien- 1 -amine ("HGT5002”), having a compound structure of:
- compositions include cationic lipids described as aminoalcohol lipidoids in International Patent Publication WO 2010/053572, which is incorporated herein by reference.
- compositions include a cationic lipid having a compound structure of:
- compositions include the cationic lipids as described in International Patent Publication WO 2016/118725, which is incorporated herein by reference, in certain embodiments, the compositions include a cationic lipid having a compound structure of:
- compositions include the cationic lipids as described in International Patent Publication WO 2016/118724, which is incorporated herein by reference.
- the compositions include a cationic lipid having a compound structure of:
- Suitable cationic lipids for use in the compositions include a cationic lipid having the formula of 14,25-ditridecyl 15, 18,21, 24-tetraaza-octatriacontane, and pharmaceutically acceptable salts thereof.
- compositions include the cationic lipids as described In International Patent Publications WO 2013/063468 and WO 2016/205691, each of which are incorporated herein by reference, in some embodiments, the compositions include a cationic lipid of the following formula:
- compositions include a cationic lipid having a compound structure of:
- compositions include a cationic lipid having a compound structure of:
- compositions include a cationic lipid having a compound structure of:
- compositions include a cationic lipid having a compound structure of:
- compositions include the cationic lipids as described in International Patent Publication WO 2015/184256, which is incorporated herein by reference.
- compositions include a cationic lipid of the following formula:
- compositions include the cationic lipids as described in International Patent Publication WO 2016/004202, which is incorporated herein by reference, in some embodiments, the compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound
- compositions include a cationic lipid having the compound structure:
- Suitable additional cationic lipids for use in the compositions include the cationic lipids as described in J. McClellan, M. C. King, Cell 2010, 141, 210-217 and in Whitehead et a!., Nature Communications (2014) 5:4277, which is incorporated herein by reference, in certain embodiments, the cationic lipids of the compositions include a cationic iipid having a compound structure of:
- compositions include the cationic lipids as described in International Patent Publication WO 2015/199952, which is incorporated herein by reference, in some embodiments, the compositions inciude a cationic iipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound structure:
- compositions include the cationic lipids as described in International Patent Publication WO 2017/075531, which is incorporated herein by reference.
- compositions include a cationic lipid of the following formula:
- compositions include the cationic lipids as described in International Patent Publication WO 2017/117528, which is incorporated herein by reference.
- compositions include a cationic lipid having the compound structure:
- compositions include a cationic lipid having the compound
- compositions include a cationic lipid having the compound structure:
- Suitable additional cationic lipids for use in the compositions include the cationic lipids as described in International Patent Publication WO 2017/049245, which is incorporated herein by reference.
- the cationic lipids of the compositions and methods of the present invention include a compound of one of the following formulas:
- R I Independently selected from -(CH 2 ) n Q and -(CH 2 ) n CHQR;
- Q is selected from the group consisting of -OR, -OH, ⁇ OiCH 2 ) n N ⁇ R) 2 , -OC ⁇ 0)R, -CX 3 , -CN, -N(R)C(0)R, -N(H)C(0)R, - (R)S ⁇ 0) 2 R, -N(H)S(0) 2 R, -N(R)C(0)N(R) 2 , -N(H)C(0)N(R) 2 , - (H)C[0)N[H)(R), -N(R)C(S)N(R) 2 , -N[H)C(S)N(R) 2 , - N(H)C(S)N(H)(R), and a heterocycle;
- R is independently selected from the group consisting of -OR, -OH, ⁇ O
- compositions include a cationic lipid having a compound
- compositions include a cationic lipid having a compound
- compositions include a cationic lipid having a compound
- compositions include a cationic lipid having a compound
- compositions include a cationic lipid having a compound structure of:
- compositions include a cationic lipid having a compound structure of:
- compositions include a cationic lipid having a compound structure of:
- compositions include a cationic lipid having a compound structure of:
- compositions include cholesterol- based cationic lipids, in certain embodiments, the compositions include imidazole cholesterol ester or "ICE", having a compound structure of:
- compositions include a cationic lipid of the following formula:
- Ri is selected from the group consisting of Imidazole, guanidinium, amino, imine, enamine, an optionally-substituted alkyl amino ⁇ e.g ., an alkyl amino such as di ethylamino) and pyridyl; wherein R 2 is selected from the group consisting of one of the following two formulas:
- R and R 4 are each independently selected from the group consisting of an optionally substituted, variably saturated or unsaturated C 6 -C 2 o alkyl and an optionally substituted, variably saturated or unsaturated C 6 -C 2 o acyl; and wherein n is zero or any positive integer [e.g., one, two, three, four, five, six, seven, eight, nine, ten, eleven, twelve, thirteen, fourteen, fifteen, sixteen, seventeen, eighteen, nineteen, twenty or more),
- compositions include a cationic lipid, "H6T4001", having a compound structure of:
- compositions include a cationic lipid, "HGT4002", having a compound structure of:
- compositions include a cationic lipid, "HGT4003", having a compound structure of:
- compositions include a cationic lipid, "HGT4004", having a compound structure of: and pharmaceutically acceptable salts thereof.
- compositions include a cationic lipid "HGT400S", having a compound structure of:
- the compositions include the cationic lipid, N-[l-(2,3- dioleyloxy)propyl]-N,N,N-trimethylammonium chloride ("DOTMA").
- DOTMA N-[l-(2,3- dioleyloxy)propyl]-N,N,N-trimethylammonium chloride
- Feigner et ai. Proc. Nat'l Acad, Sci, 84, 7413 (1987); U.S. Pat. No. 4,897,355, each of which is incorporated herein by reference.
- DOTMA can be formulated alone or can be combined with a neutral lipid (e.g., dioieoyiphosphatidyi-ethanoiamlne or "DOPE") or still other cationic or non-cationic lipids into a liposomal transfer vehicle or a lipid nanoparticle, and such liposomes can be used to enhance the delivery of nucleic acids into target cells.
- a neutral lipid e.g., dioieoyiphosphatidyi-ethanoiamlne or "DOPE”
- DOPE dioieoyiphosphatidyi-ethanoiamlne
- cationic lipids suitable for the compositions include, for example, 5-carboxyspermylgiycinedioctadecylamide (“DOGS”); 2,3-dioley!oxy-N- [2(spermine-carboxamido)ethyl]-N,N-dimethyl-l-propanaminium (“DOSPA”) (Behr et al. Proc. Nat.'l Acad. Sci. 86, 6982 (1989), U.S, Pat. No, 5,171,678; U.S. Pat, No. 5,334,761); 1,2-Dioieoyi 3- Dimethylammonium-Propane ("DO DAP”); l,2-Dioleoyl-3-Trimethylammonium-Propane
- DOGS 5-carboxyspermylgiycinedioctadecylamide
- DOSPA 2,3-dioley!oxy-N- [2(spermine-carboxamido)eth
- DOcarbDAP 1,2-N,N'-dioleylcarbamyl-3-dimethylaminopropane
- DLinDAP 2,3-Dilinoleoyloxy-N,N- dimethylpropylamine
- DLincarbDAP 1 ,2-Dilinoleoylcarbamyl-3-dimethylaminopropane
- DLin-K-DMA 2,2-dilinoleyl- 4-dimethylaminomethy!-[i,3]-dioxolane
- Octyl-CLinDMA (2R)-2-((8-[(3beta)-cholest-5-en-3-yloxy]octyl)oxy)-N, N-dimethyl-3-[(9Z, 12Z)-octadeca-9, 12-dien-l-yloxy]propan-l -amine
- one or more of the cationic lipids comprise at least one of an imidazole, dialkylamino, or guanidinium moiety.
- one or more cationic lipids suitable for the compositions include 2,2- Dilinoieyl-4-dimethylaminoethyl-[l,3]-dioxo!ane ("XTC"); (3aR,5s,6aS)-N,N-dimethyl-2,2- di((9Z,12Z)-octadeca-9,12-dienyl)tetrahydro-3aH-cyclopenta[d] [1 ,3]dioxoi-5-amine (“ALNY- 100”) and/or 4,7,13-tris(3-oxo-3-(undecylamino)propyl)-Nl,N16-diundecyl-4,7,10,13- tetraazahexadecane- 1,16-diamide (“NC98-5").
- XTC 2,2- Dilinoieyl-4-dimethylaminoethyl-[l,3]-diox
- the compositions include one or more cationic lipids that constitute at least about 5%, 10%, 20%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, or 70%, measured by weight, of the total lipid content in the composition, e.g., a lipid nanoparticle.
- the compositions include one or more cationic lipids that constitute at least about 5%, 10%, 20%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, or 70%, measured as a mol%, of the total lipid content in the composition, e.g., a lipid nanoparticle.
- the compositions include one or more cationic lipids that constitute about 30-70 % (e.g., about 30- 65%, about 30-60%, about 30-55%, about 30-50%, about 30-45%, about 30-40%, about 35-50%, about 35-45%, or about 35-40%), measured by weight, of the total lipid content in the composition, e.g., a lipid nanoparticle.
- the compositions include one or more cationic !ipids that constitute about 30-70 % (e.g,, about 30-65%, about 30-60%, about 30- 55%, about 30-50%, about 30-45%, about 30-40%, about 35-50%, about 35-45%, or about 35- 40%), measured as mol %, of the total lipid content in the composition, e.g., a lipid nanoparticle.
- compositions ⁇ e.g., liposomal compositions
- helper lipids include non-cationic lipids.
- non-cationic lipid refers to any neutral, zwitterionic or anionic lipid.
- anionic lipid refers to any of a number of lipid species that carry a net negative charge at a selected pH, such as physiological pH.
- Non-cationic lipids include, but are not limited to,
- DSPC distearoylphosphatidylcholine
- DOPC dio!eoy!phosphatidylcholine
- DPPC dipaimitoylphosphatidyichoiine
- DOPG dioleoylphosphatidylglycero!
- DPPG dipa!mitoy!phosphatidy!glycerol
- DOPE dioleoylphosphatidylethanolamine
- palmitoyloleoylphosphatidylcholine POPC
- palmitoyloleoyl-phosphatidylethanolamine POPE
- dioleoyl-phosphatidylethanolamine 4-(N-maleimidomethyl)-cyclohexane-l-carboxylate DOPE- ma!
- dipaimitoyi phosphatidyl ethanolamine DPPE
- dimyristoylphosphoethanolamine DMPE
- distearoyl-phosphatidyl-ethanolamine DSPE
- 16-O-monomethyl PE 16-O-dimethyl PE
- 18-1- trans PE l-stearoyl-2-oleoyl-phosphatidyethanolamine
- SORE l-stearoyl-2-oleoyl-phosphatidyethanolamine
- a non-cationic or helper iipid is dioleoylphosphatidylethanolamine (DOPE).
- a non-cationic Iipid is a neutral Iipid, i.e., a Iipid that does not carry a net charge in the conditions under which the composition is formulated and/or administered.
- a non-cationic iipid may be present in a molar ratio (mol%) of about 5% to about 90%, about 5% to about 70%, about 5% to about 50%, about 5% to about 40%, about 5% to about 30%, about 10 % to about 70%, about 10% to about 50%, or about 10% to about 40% of the total lipids present in a composition.
- total non-cationic lipids may be present in a molar ratio (moi%) of about 5% to about 90%, about 5% to about 70%, about 5% to about 50%, about 5% to about 40%, about 5% to about 30%, about 10 % to about 70%, about 10% to about 50%, or about 10% to about 40% of the total lipids present in a composition, in some embodiments, the percentage of non-cationic Iipid in a liposome may be greater than about 5 mol%, greater than about 10 moi%, greater than about 20 mol%, greater than about 30 moi%, or greater than about 40 mo!%.
- the percentage total non-cationic lipids in a liposome may be greater than about 5 mol%, greater than about 10 mol%, greater than about 20 mol%, greater than about 30 mo!%, or greater than about 40 mol%. in some embodiments, the percentage of non-cationic Iipid in a liposome is no more than about 5 mo!%, no more than about 10 mof%, no more than about 20 moi%, no more than about 30 moi%, or no more than about 40 mol%.
- the percentage total non- cationic lipids in a liposome may be no more than about 5 mo!%, no more than about 10 moi%, no more than about 20 mol%, no more than about 30 moi%, or no more than about 40 mol%.
- a non-cationic Iipid may be present in a weight ratio (wt%) of about
- total non-cationic lipids may be present in a weight ratio (wt%) of about 5% to about 90%, about 5% to about 70%, about 5% to about 50%, about 5% to about 40%, about 5% to about 30%, about 10 % to about 70%, about 10% to about 50%, or about 10% to about 40% of the total iipids present in a composition, in some embodiments, the percentage of non -cationic iipid in a liposome may be greater than about 5 wt%, greater than about 10 wt%, greater than about 20 wt%, greater than about 30 wt%, or greater than about 40 wt%.
- the percentage total non- cationic lipids in a liposome may be greater than about 5 wt%, greater than about 10 wt%, greater than about 20 wt%, greater than about 30 wt%, or greater than about 40 wt%. In some embodiments, the percentage of non-cationlc lipid in a liposome is no more than about 5 wt%, no more than about 10 wt%, no more than about 20 wt%, no more than about 30 wi%, or no more than about 40 wt%.
- the percentage total non-cationic lipids in a liposome may be no more than about 5 wt%, no more than about 10 wt%, no more than about 20 wt%, no more than about 30 wt%, or no more than about 40 wt%.
- a composition (e.g., a liposomal composition) comprises one or more cholesterol-based lipids.
- suitable cholesterol-based lipids include cholesterol and, for example, DC-Chol (N,N-dimethyl-N-ethylcarboxamidocholesterol), l,4-bis(3-N-oleylamino- propyl)piperazine [Gao, et ai. Biochem. Biophys. Res. Comm, 179, 280 (1991); Wolf et a/.
- a cholesterol-based lipid may be present in a molar ratio (mol%) of about 1% to about 30%, or about 5% to about 20% of the total lipids present in a liposome.
- the percentage of cholesterol-based lipid in the lipid nanoparticle may be greater than about 5 mo!%, greater than about 10 mol%, greater than about 20 mol%, greater than about 30 mol%, or greater than about 40 mo!%.
- the percentage of cholesterol-based lipid in the lipid nanoparticle may be no more than about 5 mol%, no more than about 10 mol%, no more than about 20 mol%, no more than about 30 mo!%, or no more than about 40 mol%.
- a cholesterol-based lipid may be present in a weight ratio (wt%) of about 1% to about 30%, or about 5% to about 20% of the total lipids present in a liposome, in some embodiments, the percentage of cholesterol-based lipid in the lipid nanoparticle may be greater than about 5 wt%, greater than about 10 wt%, greater than about 20 wt%, greater than about 30 wt%, or greater than about 40 wt%.
- the percentage of cholesterol-based lipid in the lipid nanoparticle may be no more than about 5 wt%, no more than about 10 wt%, no more than about 20 wt%, no more than about 30 wt%, or no more than about 40 wt%.
- a composition (e.g., a liposomal composition) comprises one or more further PEGyiated lipids.
- polyethylene glycol (PEG)-modified phospholipids and derivatized lipids such as derivatized ceramides (PEG-CER), including N-octanoyi-sphingosine-1- [succinyi(methoxy polyethylene giycol)-2000] (C8 PEG-2000 ceramide) is also contemplated by the present invention in combination with one or more of compounds described herein (e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) and, in some embodiments, other lipids together which comprise the liposome.
- particularly useful exchangeable lipids are PEG-cera ides having shorter acyl chains ⁇ e.g., C or C 18 ).
- Contemplated further PEG-modified lipids ⁇ also referred to herein as a PEGyiated lipid, which term is interchangeable with PEG-modified lipid) include, but are not limited to, a polyethylene glycol chain of up to 5 kDa in length covalently attached to a lipid with alkyl chain(s) of C 6 -C 2 o length.
- a PEG-modified or PEGyiated lipid is PEGyiated cholesterol or PEG-2K.
- the addition of such components may prevent complex aggregation and may also provide a means for increasing circulation lifetime and increasing the delivery of the lipid-nucleic acid composition to the target ceil, (Klibanov et a!. (1990) FEBS Letters, 268 (1): 235-237), or they may be selected to rapidly exchange out of the formulation in vivo ⁇ see U.S. Pat. No. 5,885,613).
- PEG-modified phospholipid and derivatized lipids of the present invention may be present in a molar ratio (moi%) from about 0% to about 15%, about 0.5% to about 15%, about 1% to about 15%, about 4% to about 10%, or about 2% of the total lipid present in the composition (e.g., a liposomal composition).
- PEG-modified phospholipid and derivatized lipids of the present invention may be present in a weight ratio (wt%) from about 0% to about 15%, about 0.5% to about 15%, about 1% to about 15%, about 4% to about 10%, or about 2% of the total lipid present in the composition (e.g., a liposomal composition).
- wt% weight ratio from about 0% to about 15%, about 0.5% to about 15%, about 1% to about 15%, about 4% to about 10%, or about 2% of the total lipid present in the composition (e.g., a liposomal composition).
- Compounds described herein ⁇ e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) may be used in the preparation of compositions (e.g., to construct liposomal compositions) that facilitate or enhance the delivery and release of encapsulated materials ⁇ e.g., one or more therapeutic polynucleotides) to one or more target cells ⁇ e.g., by permeating or fusing with the lipid membranes of such target cells).
- compositions e.g., to construct liposomal compositions
- encapsulated materials e.g., one or more therapeutic polynucleotides
- target cells e.g., by permeating or fusing with the lipid membranes of such target cells.
- a liposomal composition ⁇ e.g., a lipid nanoparticle
- a liposomal composition comprises or is
- the phase transition in the lipid bilayer of the one or more target ceils may facilitate the delivery of the encapsulated materials ⁇ e.g., one or more therapeutic polynucleotides encapsulated In a lipid nanoparticie) into the one or more target cells.
- Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) may be used to prepare liposomal vehicles that are characterized by their reduced toxicity in vivo, in certain embodiments, the reduced toxicity is a function of the high transfection efficiencies associated with the compositions disclosed herein, such that a reduced quantity of such composition may administered to the subject to achieve a desired therapeutic response or outcome.
- pharmaceu tical formulations comprising a compound described (e.g., a compound of Formula (A), such as Formulae (I) or (li) or any one of Compounds 1-12) and nucleic adds provided by the present invention may be used for various therapeutic purposes.
- a compound described herein ⁇ e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12
- nucleic acids can be formulated in combination with one or more additional pharmaceutical carriers, targeting ligands or stabilizing reagents.
- a compound described herein e.g., a compound of Formula (A), such as Formulae (! or (II) or any one of Compounds 1-12
- a composition comprising a compound described herein ⁇ e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) can be formulated using post-insertion techniques into the lipid membrane of the nanoparticles. Techniques for formulation and administration of drugs may be found in "Remington's Pharmaceutical Sciences,” Mack Publishing Co., Easton, Pa., latest edition.
- Suitable routes of administration include, for example, oral, rectal, vaginal, transmucosai, pulmonary including intratracheal or inhaled, or intestinal administration; parenteral delivery, including intradermal, transderma! (topical), intramuscular, subcutaneous, intramedullary injections, as well as intrathecal, direct intraventricular, intravenous, intraperitoneal, or intranasal.
- the intramuscular administration is to a muscle selected from the group consisting of skeletal muscle, smooth muscle and cardiac muscle.
- the administration results in delivery of the nucleic acids to a muscle cell
- the administration results in delivery of the nucleic acids to a hepatocyte (i.e., liver cell)
- compositions of the invention may be any suitable pharmaceutical formulations of the invention.
- tissue to be targeted preferably in a sustained release formulation.
- Local delivery can be affected in various ways, depending on the tissue to be targeted.
- Exemplary tissues in which delivered mRNA may be delivered and/or expressed include, but are not limited to the liver, kidney, heart, spleen, serum, brain, skeletal muscle, lymph nodes, skin, and/or cerebrospinal fluid.
- the tissue to be targeted in the liver include, but are not limited to the liver, kidney, heart, spleen, serum, brain, skeletal muscle, lymph nodes, skin, and/or cerebrospinal fluid.
- compositions of the present invention can be inhaled (for nasal, tracheal, or bronchial delivery); compositions of the present invention can be injected into the site of injury, disease manifestation, or pain, for example; compositions can be provided in lozenges for oral, tracheal, or esophageal application; can be supplied In liquid, tablet or capsule form for administration to the stomach or intestines, can be supplied in suppository form for rectal or vaginal application; or can even be delivered to the eye by use of creams, drops, or even Injection.
- compositions described herein can comprise RNA encoding peptides including those described herein (e.g., a polypeptide such as a protein).
- a mRNA encodes a polypeptide.
- a mRNA encodes a protein.
- exemplary peptides encoded by mRNA ⁇ e.g., exemplary proteins encoded by mRNA are described herein.
- the present invention provides methods for delivering a composition having full-length mRNA molecules encoding a peptide or protein of interest for use in the treatment of a subject, e.g., a human subject or a cell of a human subject or a cell that is treated and delivered to a human subject.
- the present invention provides a method for producing a therapeutic composition comprising full-length mRNA that encodes a peptide or protein for use in the delivery to or treatment of the lung of a subject or a lung cell, in certain embodiments the present invention provides a method for producing a therapeutic composition having full- length mRNA that encodes for cystic fibrosis transmembrane conductance regulator (CFTR) protein.
- CFTR cystic fibrosis transmembrane conductance regulator
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for ATP-binding cassette sub family A member 3 protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for dynein axonemai intermediate chain 1 protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for dynein axonemai heavy chain 5 (DNAH5) protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for alpha-l-antitrypsin protein.
- the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for forkhead box P3 (FOXP3) protein.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes one or more surfactant protein, e.g., one or more of surfactant A protein, surfactant B protein, surfactant C protein, and surfactant D protein.
- the present invention provides a method for producing a
- peptides and polypeptides can Include those associated with a urea cycle disorder, associated with a lysosomal storage disorder, with a glycogen storage disorder, associated with an amino acid metabolism disorder, associated with a lipid metabolism or fibrotic disorder, associated with methylmalonic acidemia, or associated with any other metabolic disorder for which delivery to or treatment of the liver or a liver ceil with enriched full-length mRNA provides therapeutic benefit,
- the present invention provides a method for producing a
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for ornithine transcarbamylase (OTC) protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for arginosuccinate synthetase 1 protein.
- OTC ornithine transcarbamylase
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for carbamoyl phosphate synthetase I protein in certain embodiments the present invention provides a method for producing a therapeutic composition having fuil-iength mRNA that encodes for arginosuccinate lyase protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for arginase protein.
- the present invention provides a method for producing a
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a protein associated with a lysosomal storage disorder. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for alpha gaiactosidase protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for
- the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for iduronaie-2- sulfatase protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for iduronidase protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for N-acetyi-alpha-D- giucosaminidase protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for heparan N- sulfatase protein.
- the present invention provides a method for producing a therapeutic composition having full-length rnRNA that encodes for galactosamine-6 sulfatase protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for beta- gaiactosidase protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for lysosomal lipase protein.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for arylsulfatase B (N- acetyigaiactosamine-4-sulfatasej protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for transcription factor EB (TFEB).
- TFEB transcription factor EB
- the present invention provides a method for producing a
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a protein associated with a glycogen storage disorder.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for acid alpha- giucosidase protein.
- the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for glucose-6- phosphatase (G6PC) protein.
- G6PC glucose-6- phosphatase
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for liver glycogen phosphorylase protein.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for muscle phosphoglycerate mutase protein, !n certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for glycogen debranching enzyme.
- the present invention provides a method for producing a
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a protein associated with amino acid metabolism.
- the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for phenylalanine hydroxylase enzyme
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for glutaryl-CoA dehydrogenase enzyme.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for propionyl-CoA caboxylase enzyme.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for oxalase a!anine- glyoxylate aminotransferase enzyme.
- the present invention provides a method for producing a
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a mTOR inhibitor.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for ATPase phospholipid transporting 8B1 (ATP8B1) protein
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for one or more NF-kappa B inhibitors , such as one or more of i-kappa B alpha, interferon-reiated development regulator 1 (IFRD1), and Sirtuin 1 (S!RTlj.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for PPAR-gamma protein or an active variant.
- the present invention provides a method for producing a
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a protein associated with methylmalonic acidemia.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for methylmalonyl CoA mutase protein.
- the present Invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for methylmalonyl CoA epimerase protein
- the present invention provides a method for producing a
- the present invention provides a method for producing a therapeutic composition having full-length mRNA for which delivery to or treatment of the liver can provide therapeutic benefit
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for ATP7B protein, also known as Wilson disease protein.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for porphobilinogen deaminase enzyme.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for one or clotting enzymes, such as Factor VIII, Factor IX, Factor VII, and Factor X.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for human hemochromatosis (HFE) protein.
- HFE human hemochromatosis
- the present invention provides a method for producing a
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes a peptide or protein for use in the delivery to or treatment of the cardiovasculature of a subject or a cardiovascular cell.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for vascular endothelial growth factor A protein.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for reiaxin protein.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for bone morphogenetic protein-9 protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for bone morphogenetic protein-2 receptor protein.
- the present invention provides a method for producing a
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes a peptide or protein for use in the delivery to or treatment of the muscle of a subject or a muscle cell.
- the present invention provides a method for producing a therapeutic composition having full- length RNA that encodes for dystrophin protein
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for frataxin protein.
- the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes a peptide or protein for use in the delivery to or treatment of the cardiac muscle of a subject or a cardiac muscle cell, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a protein that modulates one or both of a potassium channel and a sodium channel in muscle tissue or in a muscle cell.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a protein that modulates a Kv7.1 channel in muscle tissue or in a muscle ceil in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a protein that modulates a Navi.5 channel in muscle tissue or in a muscle cell.
- the present invention provides a method for producing a
- the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for survival motor neuron 1 protein.
- the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for survival motor neuron 2 protein.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for frataxin protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for ATP binding cassette subfamily D member 1 (ABCD1) protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for CLN3 protein.
- the present invention provides a method for producing a
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes a peptide or protein for use in the delivery to or treatment of the blood or bone marrow of a subject or a blood or bone marrow cell, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for beta globin protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for Bruton's tyrosine kinase protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for one or cloting enzymes, such as Factor VIII, Factor IX, Factor VII, and Factor X.
- one or cloting enzymes such as Factor VIII, Factor IX, Factor VII, and Factor X.
- the present invention provides a method for producing a
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for collagen type IV alpha 5 chain (COL4A5) protein,
- the present invention provides a method for producing a
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes a peptide or protein for use in the delivery to or treatment of the eye of a subject or an eye cell.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for ATP-binding cassette sub-family A member 4 (ABCA4) protein.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for retinoschisin protein.
- the present invention provides a method for producing a therapeutic composition having fuli-iength mRNA that encodes for retinal pigment epithelium-specific 65 kDa (RPE65) protein in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for centrosomal protein of 290 kDa (CEP290),
- the present invention provides a method for producing a
- the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes a peptide or protein for use in the delivery of or treatment with a vaccine for a subject or a ceil of a subject.
- the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for an antigen from an Infectious agent, such as a virus
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from influenza virus.
- the present Invention provides a method for producing a therapeutic composition having full-length RNA that encodes for an antigen from respiratory syncytial virus.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from rabies virus, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from cytomegalovirus, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from rotavirus, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from a hepatitis virus, such as hepatitis A virus, hepatitis B virus, or hepaiis C virus, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from human papillomavirus.
- a hepatitis virus such as hepatitis A virus, hepatitis B virus, or hepaiis C virus
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from a herpes simplex virus, such as herpes simplex virus 1 or herpes simplex virus 2. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from a human
- immunodeficiency virus such as human immunodeficiency virus type 1 or human
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from a human metapneumovirus. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from a human parainfluenza virus, such as human parainfluenza virus type 1, human parainfluenza virus type 2, or human parainfluenza virus type 3.
- the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for an antigen from malaria virus, in certain embodiments the present invention provides a method for producing a therapeutic composition having fuli- iength mRNA that encodes for an antigen from zika virus. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNi ⁇ that encodes for an antigen from chikungunya virus.
- the present invention provides a method for producing a
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen associated with a cancer of a subject or identified from a cancer cel! of a subject, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen determined from a subject's own cancer cell, i.e., to provide a personalized cancer vaccine. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen expressed from a mutant KRAS gene.
- the present invention provides a method for producing a
- the antibody can be a bi-specific antibody.
- the antibody can be part of a fusion protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antibody to 0X40. in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antibody to VEGF.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antibody to tissue necrosis factor alpha, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antibody to CDS. in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antibody to CD19.
- the present invention provides a method for producing a
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an immunomodulator.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for Interleukin 12.
- the present invention provides a method for producing a therapeutic composition having full- length RNA that encodes for Interleukin 23.
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for Interleukin 36 gamma
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a constitutively active variant of one or more stimulator of interferon genes (STING) proteins,
- the present invention provides a method for producing a
- the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an endonuclease, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for an RNA-guided DNA endonuclease protein, such as Cas 9 protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a meganuclease protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a transcription activator-like effector nuclease protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a zinc finger nuclease protein,
- compositions and methods of the invention provide for delivery of mRNA encoding a secreted protein.
- the compositions and methods of the invention provide for delivery of mRNA encoding one or more secreted proteins listed in Table 1; thus, compositions of the invention may comprise an mRNA encoding a protein listed in Table 1 (or a homolog thereof) along with other components set out herein, and methods of the invention may comprise preparing and/or administering a composition comprising an mRNA encoding a protein listed In Table 1 (or a homolog thereof) along with other components set out herein. Table 1. Secreted Proteins
- compositions and methods of the invention provide for the delivery of one or more mRNAs encoding one or more additional exemplary proteins listed in Table 2; thus, compositions of the invention may comprise an mRNA encoding a protein listed in Table 2 (or a homolog thereof) along with other components set out herein, and methods of the invention may comprise preparing and/or administering a composition comprising an mR!MA encoding a protein chosen from the proteins listed in Table 2 (or a homolog thereof) along with other components set out herein.
- the Uniprot !Ds set forth in Tab!e 1 and Table 2 refer to the human versions the listed proteins and the sequences of each are available from the Uniprot database. Sequences of the listed proteins are also generally available for various animals, including various mammals and animals of veterinary or industrial interest.
- compositions and methods of the invention provide for the delivery of one or more mRNAs encoding one or more proteins chosen from mammalian homo!ogs or homoiogs from an animal of veterinary or industrial interest of the secreted proteins listed in Table 1 and Table 2; thus, compositions of the invention may comprise an mRNA encoding a protein chosen from mammalian homoiogs or homologs from an animal of veterinary or industrial interest of a protein listed in Table 1 and Table 2 along with other components set out herein, and methods of the invention may comprise preparing and/or administering a composition comprising an mRNA encoding a protein chosen from mammalian homoiogs or homoiogs from an animal of veterinary or industrial interest of a protein listed in Table 1 and Table 2 along with other components set out herein.
- mammalian homoiogs are chosen from mouse, rat, hamster, gerbi!, horse, pig, cow, llama, alpaca, mink, dog, cat, ferret, sheep, goat, or camel homoiogs.
- the animal of veterinary or industrial interest is chosen from the mammals listed above and/or chicken, duck, turkey, salmon, catfish, or tiiapia.
- compositions and methods of the invention provide for the delivery of mRNA encoding a lysosomal protein chosen from Table 3, in some embodiments, the compositions and methods of the invention provide for the delivery of one or more mRNAs encoding one or more lysosomal and/or related proteins listed in Table 3; thus, compositions of the invention may comprise an mRNA encoding a protein listed in Table 3 ⁇ or a homolog thereof) along with other components set out herein, and methods of the invention may comprise preparing and/or administering a composition comprising an RNA encoding a protein chosen from the proteins listed in Table 3 (or a homolog thereof) along with other components set out herein.
- the protein listed in Table 3 and encoded by mRNA in the compositions and methods of the invention is a human protein. Sequences of the listed proteins are also available for various animals, including various mammals and animals of veterinary or industrial interest as described above.
- compositions and methods of the invention provide for the delivery of RNA encoding a therapeutic protein ⁇ e.g., cytosolic, transmembrane or secreted) such as those listed in Table 4.
- the compositions and methods of the invention provide for the delivery of an mRNA encoding a therapeutic protein useful in treating a disease or disorder (i.e., indication) listed in Table 4; thus, compositions of the invention may comprise an rrsRNA encoding a therapeutic protein listed or not listed In Table 4 [or a homoiog thereof, as discussed below) along with other components set out herein for treating a disease or disorder (i.e., Indication) listed in Table 4, and methods of the Invention may comprise preparing and/or administering a composition comprising an mRNA encoding a such a protein (or a homolog thereof, as discussed below) along with other components set out herein for treatment of a disease or disorder listed in Table 4.
- the present invention is used to prevent, treat, and/or cure a subject affected with a disease or disorder listed or associated with the proteins listed in Tables 1, 2, 3, or 4.
- an mRNA encodes one or more of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR), argininosuccinate synthetase (ASSlj, Factor IX, survival motor neuron 1 (SMN1), or phenylalanine hydroxylase (PAH), Delivery Methods
- CFTR Cystic Fibrosis Transmembrane Conductance Regulator
- ASSlj argininosuccinate synthetase
- SNS1 survival motor neuron 1
- PAH phenylalanine hydroxylase
- the route of delivery used in the methods of the invention allows for non-invasive, self administration of the compounds of the invention ⁇ e.g., a compound of Formula (A), such as Formulae (! or (II) or any one of Compounds 1-12).
- the methods involve intratracheal or pulmonary administration by aerosolization, nebulization, or instillation of a compositions comprising mRNA encoding a therapeutic protein in a suitable transfection or lipid carrier vehicles as described above.
- the protein is encapsulated with a liposome.
- the liposome comprises a lipid, which is a compound of the invention [e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12).
- administration of a compound of the invention includes administration of a composition comprising a compound of the invention.
- nanoparticle compositions of the invention pass, through the lung airway-blood barrier, resulting in translation of the intact nanoparticle to non-lung cells and tissues, such as, e.g., the heart, the liver, the spleen, where it results in the production of the encoded protein in these non-lung tissues.
- the utility of the compounds of the invention ⁇ e.g., a compound of Formula (A), such as Formulae (l) or (II) or any one of Compounds 1-12) and methods of the invention extend beyond production of therapeutic protein in lung ceils and tissues of the lung and can be used to delivery to non-lung target cells and/or tissues They are useful in the management and treatment of a large number of diseases, and in particular peripheral diseases which result from both secreted and non-secreted protein and/or enzyme deficiencies (e.g., one or more lysosomal storage disorders).
- a compound of Formula (A) such as Formulae (l) or (II) or any one of Compounds 1-12
- methods of the invention extend beyond production of therapeutic protein in lung ceils and tissues of the lung and can be used to delivery to non-lung target cells and/or tissues They are useful in the management and treatment of a large number of diseases, and in particular peripheral diseases which result from both secreted and non-secreted protein and/or enzyme deficiencies (e.g., one
- the compounds of the invention ⁇ e.g., a compound of Formula (A), such as Formulae (! or (II) or any one of Compounds 1-12), used in the methods of the invention result in the distribution of the mRNA encapsulated nanoparticles and production of the encoded protein in the liver, spleen, heart, and/or other non-lung cells.
- a compound of Formula (A) such as Formulae (! or (II) or any one of Compounds 1-12
- a compound of Formula (A), such as Formulae (I) or (I! or any one of Compounds 1-12 by aerosolization, nebulization, or instillation to the lung will result in the composition itself and its protein product (e.g., functional beta galactosidase protein) will be detectable in both the local cells and tissues of the lung, as well as in peripheral target cells, tissues and organs as a result of translocation of the mRIMA and delivery vehicle to non-lung ceils,
- protein product e.g., functional beta galactosidase protein
- the compounds of the invention ⁇ e.g., a compound of Formula (A), such as Formulae (! or (!!) or any one of Compounds 1-12
- a compound of Formula (A) such as Formulae (! or (!!) or any one of Compounds 1-12
- the compounds of the invention may be employed in the methods of the invention to specifically target peripheral cells or tissues.
- the compounds of the invention ⁇ e.g., a compound of Formula (A), such as Formulae (! or (II) or any one of Compounds 1-12) cross the lung airway-blood barrier and distribute into cells other than the local lung cells.
- the compounds disclosed herein may be administered to a subject by way of the pulmonary route of administration, using a variety of approach known by those skilled in the art ⁇ e.g., by inhalation), and distribute to both the local target cells and tissues of the lung, as well as in peripheral non-lung cells and tissues (e.g., cells of the liver, spleen, kidneys, heart, skeletal muscle, lymph nodes, brain, cerebrospinal fluid, and plasma).
- pulmonary route of administration using a variety of approach known by those skilled in the art ⁇ e.g., by inhalation
- peripheral non-lung cells and tissues e.g., cells of the liver, spleen, kidneys, heart, skeletal muscle, lymph nodes, brain, cerebrospinal fluid, and plasma.
- both the local ceils of the lung and the peripheral non-lung cells can serve as biological reservoirs or depots capable of producing and/or secreting a translation product encoded by one or more polynucleotides.
- the present invention is not limited to the treatment of lung diseases or conditions, but rather can be used as a non-invasive means of facilitating the delivery of polynucleotides, or the production of enzymes and proteins encoded thereby, in peripheral organs, tissues and cells (e.g., hepatocytes) which would otherwise be achieved only by systemic administration.
- Exemplary peripheral non-lung ceils include, but are not limited to, hepatocytes, epithelial cells, hematopoietic cells, epithelial cells, endothelial cells, bone cells, stem cells, mesenchymal cells, neural cells, cardiac ceils, adipocytes, vascular smooth muscle cells, cardiomyocytes, skeletal muscle ceils, beta cells, pituitary cells, synovial lining ceils, ovarian ceils, testicular cells, fibroblasts, 3 cells, T ceils, reticulocytes, leukocytes, granulocytes and tumor cells.
- the protein product encoded by the mRIMA (e.g., a functional protein or enzyme) is detectable in the peripheral target tissues for at least about one to seven days or longer following administration of the compound to the subject.
- the amount of protein product necessary to achieve a therapeutic effect will vary depending on the condition being treated, the protein encoded, and the condition of the patient.
- the protein product may be detectable In the peripheral target tissues at a concentration (e.g., a therapeutic concentration) of at least 0.025-1.5 pg/ml (e.g., at least 0.050 pg/ml, at least 0.075 pg/ml, at least 0.1 pg/ml, at least 0.2 pg/ml, at least 0.3 pg/ml, at least 0,4 pg/ml, at least 0,5 pg/ml, at least 0.6 pg/ml, at least 0.7 pg/ml, at least 0.8 pg/ml, at least 0.9 pg/mi, at least 1.0 pg/ml, at least 1.1 pg/mi, at least 1.2 pg/ml, at least 1.3 pg/ml, at least 1.4 pg/ml, or at least 1.5 pg/ml), for at least about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11,12
- nucleic acids can be delivered to the lungs by intratracheal administration of a liquid suspension of the compound and inhalation of an aerosol mist produced by a liquid nebulizer or the use of a dry powder apparatus such as that described in U.S. patent 5,780,014, incorporated herein by reference.
- the compounds of the invention may be formulated such that they may be aerosolized or otherwise delivered as a particulate liquid or solid prior to or upon administration to the subject.
- Such compounds may be administered with the assistance of one or more suitable devices for administering such solid or liquid particulate compositions (such as, e.g., an aerosolized aqueous solution or suspension) to generate particles that are easily respirable or inhalable by the subject.
- such devices facilitate the administration of a predetermined mass, volume or dose of the compositions ⁇ e.g., about 0.5 mg/kg of mRNA per dose) to the subject.
- a predetermined mass, volume or dose of the compositions e.g., about 0.5 mg/kg of mRNA per dose
- the compounds of the invention ⁇ e.g., a compound of Formula (A), such as Formulae (I) or (11) or any one of Compounds 1-12) are administered to a subject using a metered dose inhaler containing a suspension or solution comprising the compound and a suitable propellant.
- the compounds of the invention may be formulated as a particulate powder (e.g., respirable dry particles) intended for inhalation
- a particulate powder e.g., respirable dry particles
- compositions of the invention formulated as respirable particles are appropriately sized such that they may be respirable by the subject or delivered using a suitable device (e.g., a mean D50 or D90 particle size less than about 500pm, 400pm, 300pm, 250pm, 200pm, 150pm, lOOpm, 75pm, 50pm, 25pm, 20pm, 15pm, 12.5pm, 10pm, 5pm, 2.5pm or smaller).
- the compounds of the invention are formulated to include one or more pulmonary surfactants ⁇ e.g., lamellar bodies).
- the compounds of the invention ⁇ e.g., a compound of Formula (A), such as Formulae (I) or (I! or any one of Compounds 1-12) are administered to a subject such that a concentration of at least 0.05 mg/kg, at least 0.1 g/kg, at least 0.5 mg/kg, at least 1.0 mg/kg, at least 2.0 mg/kg, at least 3,0 mg/kg, at least 4.0 g/kg, at least 5.0 mg/kg, at least 6.0 g/kg, at least 7.0 mg/kg, at least 8.0 mg/kg, at least 9.0 g/kg, at least 10 mg/kg, at least 15 mg/kg, at least 20 rng/'kg, at least 25 mg/kg, at least 30 mg/kg, at least 35 mg/kg, at least 40 rng/'kg, at least 45 g/kg, at least 50 mg/kg, at least 55 mg/kg, at least 60 g/kg, at least 65 mg/kg, at least 70 mg/kg
- the compounds of the invention are administered to a subject such that a total amount of at least 0,1 mg, at least 0.5 g, at least 1.0 mg, at least 2.0 g, at least 3.0 mg, at least 4.0 mg, at least 5.0 mg, at least 6.0 g, at least 7.0 mg, at least 8.0 g, at least 9.0 mg, at least 10 mg, at least 15 g, at least 20 mg, at least 25 mg, at least 30 g, at least 35 mg, at least 40 mg, at least 45 mg, at least 50 mg, at least 55 g, at least 60 g, at least 65 mg, at least 70 g, at least 75 mg, at least 80 mg, at least 85 mg, at least 90 g, at least 95 g or at least 100 g mRNA is administered in one or more doses.
- a compound of Formula (A) such as Formulae (!) or (II) or any one of Compounds 1-12
- a cationic lipid described herein can be prepared using a cystine starting material.
- the cystine can be treated with an alkylating or acylating agent to functionalize the amino groups, which in turn can be esterified to afford certain cationic lipids described herein.
- alkylating or acylating agents are described in Table A, and exemplary alcohols for esterification are described in Table B.
- cationic lipids herein include those resulting from any combination of the precursors described in Table A and Table B.
- cationic lipids described herein can be used in the preparation of lipid nanoparticles according to methods known in the art.
- suitable methods include methods described in international Publication No. WO 2018/089801, which is hereby incorporated by reference in its entirety.
- Lipid nanoparticle formulations comprising human erythropoietin (hEPQ) mRNA, Cationic Lipid, DMG-PEG2000, Cholesterol and DOPE were administered intramuscularly to study mRNA delivery and resultant hEPQ expression.
- Male BALB/c mice at 6-8 weeks old are given a single injection of the LNP formulations into the gastrocnemius muscle at a dosage level of 0.1 ug. Blood samples were collected at 6 and 24 hours post-dose.
- hEPQ protein expression levels were measured in the sera samples by ELISA and presented in Figure 1.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Zoology (AREA)
- Dispersion Chemistry (AREA)
- Biophysics (AREA)
- Molecular Biology (AREA)
- Dermatology (AREA)
- Pulmonology (AREA)
- Cell Biology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Nutrition Science (AREA)
- Physiology (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
The present invention provides, in part cystine cationic lipid compounds of formula A, and sub-formulas thereof: or a pharmaceutically acceptable salt thereof. The compounds provided herein can be useful for delivery and expression of mRNA and encoded protein, e.g., as a component of liposomal delivery vehicle, and accordingly can be useful for treating various diseases, disorders and conditions, such as those associated with deficiency of one or more proteins.
Description
CYSTINE CATIONIC LIPIDS
RELATED APPLICATIONS
[001] This application claims benefit of U.S. Provisional Application Number 62/835,814, filed on April 18, 2019, the entire disclosure of which is hereby incorporated by reference.
BACKGROUND
[002] Delivery of nucleic acids has been explored extensively as a potential therapeutic option for certain disease states, in particular, messenger RNA (mRNA) therapy has become an increasingly important option for treatment of various diseases, including for those associated with deficiency of one or more proteins.
SUMMARY
[003] The present invention provides, among other things, a novel class of cystine cationic lipid compounds for improved in vivo delivery of therapeutic agents, such as nucleic acids, it is contemplated that the compounds provided herein are capable of highly effective in vivo delivery while maintaining favorable toxicity profile due to the biodegradable nature.
[004] In an aspect, provided herein are cationic lipids having a structure according to Formula (A),
or a pharmaceutically acceptable salt thereof, wherein
wherein
each Rla is independently hydrogen, Ric, or R3d;
each RlB Is independently Rlc or Rld;
each Rlc is independently -[CH2)2C(0)X1R3;
each Rld is independently ~€{0)R4;
each R2 is independently -[C(R2a)2]cR2b;
each R2a is independently hydrogen or Ci-C6 alkyl;
R2b is -N(LI-B)2, -(OCH2CH2)6OH; or -iOCH2CH2)bOCH3;
each R3 and R4 is independently C6-C3o aliphatic;
each Li is independently Ci-Cio alkylene;
each B is independently hydrogen or an ionizable nitrogen-containing group;
each X1 is independently a covalent bond or O;
each a is independently an integer of 1-10;
each b is independently an integer of 1-10; and
each c is independently an integer of 1-10.
[005] in embodiments, each a is 1 or 2.
[006] In embodiments, each Rla and Rlb is ~ίeH2}C[0)C¾3.
[007] In embodiments, a cationic iipid has a structure according to formula (I):
[008] in embodiments, each R3 is C6-C alkyl, C6-C alkenyl, or CS C2 a!kyny!.
[009] In embodiments, each R3 is unsubstituted C6-C2 alkyl.
[010] In embodiments, each Rla is hydrogen and each Rlb is -C(0)R4.
[Oil] in embodiments, a cationic iipid has a structure according to formula [I I):
[012] In embodiments, each R4 is Cf,-C30 alkyl.
[013] In embodiments, each R4 Is unsubstituted Ce-C alkyl.
[014] in embodiments, each R4 is C6-Ci0 alkyl substituted with -GC(0)R5 or
-C02Rs, wherein R!> is unsubstituted C6-Ci6 alkyl.
[015] In embodiments,
each R2 is— [CH2]c— N(U-B)2 or - CH(CH3)CH2-N(Li-B)2; and
each Li is unsubstituted Ci-C-.0 alkylene.
[016] In embodiments, each R2 is -[CH2]c-N(CH3)2.
[017] In embodiments,
each R2 is -[CH2],;-N(CH2CH2B)2, -[CH2]c-N(CH2CH2CH2B)2 -CH(CH3)CH2-N(CH2CH2B)2, or -CH(CH3)CH2-N(CH2CH2CH2B)2; and
each B is an ionizable nitrogen-containing group.
[018] In embodiments, each B is NH2, guanidine, amidine, a mono- or dialkylamine, 5- to 6- membered heterocycloalkyi, or 5- to 6-membered nitrogen-containing heteroary!.
[019] In embodiments, each B is NH2, guanidine, amidine, NHCH3, N(CH3)2, or imidazole.
[020] in embodiments, each B is N(CH3)2.
[021] In embodiments, each c is 2, 3, or 4.
[022] In embodiments, a cationic lipid is any one of Compounds 1-12.
[023] in another aspect, the invention features a composition comprising any liposome [e.g., a liposome encapsulating an mRNA encoding a protein) described herein, !n embodiments, the liposome comprises a cationic lipid described herein.
[024] in embodiments, an mRNA encodes for cystic fibrosis transmembrane conductance
regulator (CFTR) protein.
[025] In embodiments, an mRNA encodes for ornithine transcarbamy!ase (OTC) protein.
[026] In another aspect, the invention features a composition comprising a nucleic acid
encapsulated within a liposome as described herein, in embodiments, the liposome comprises a cationic lipid described herein.
[027] In embodiments, a composition further comprises one more lipids selected from the group consisting of one or more cationic lipids, one or more non-cationic lipids, and one or more PEG-· modified lipids.
[028] In embodiments, a nucleic acid is an mRNA encoding a peptide or protein.
[029] in embodiments, an mRNA encodes a peptide or protein for use in the delivery to or
treatment of the lung of a subject or a lung ceil.
[030] In embodiments, an mRNA encodes a peptide or protein for use in the delivery to or
treatment of the lung of a subject or a lung cell.
[031] In embodiments, an mRNA encodes for cystic fibrosis transmembrane conductance regulator (CFTR) protein.
[032] in embodiments, an mRNA encodes a peptide or protein for use in the delivery to or
treatment of the liver of a subject or a liver ceil.
[033] In embodiments, an mRNA encodes for ornithine transcarbamylase (OTC) protein.
[034] In embodiments, an mRNA encodes a peptide or protein for use in vaccine.
[035] in embodiments, an mRNA encodes an antigen.
[036] In some aspects, the present invention provides methods of treating a disease in a subject comprising administering to the subject a composition as described herein.
BRIEF DESCRIPTION OF DRAWINGS
[037] FIG, 1 depicts in vivo protein production resulting from the delivery of mRNA using lipid nanoparticies comprising Compound 3 or Compound 4 as described herein. As shown in this Figure, use of these compounds allows high levels of in vivo protein production even 24 hours after administration.
DETAILED DESCRIPTION OF CERTAIN EMBODIMENTS
Definitions
[038] In order for the present invention to be more readily understood, certain terms are first defined below. Additional definitions for the following terms and other terms are set forth throughout the specification. The publications and other reference materials referenced herein to describe the background of the invention and to provide additional detail regarding its practice are hereby incorporated by reference.
[039] Amino acid: As used herein, the term "amino acid," in its broadest sense, refers to any compound and/or substance that can be incorporated into a polypeptide chain, in some embodiments, an amino acid has the general structure H2 --C{H}(R}--COOH. In some embodiments, an amino acid is a naturally occurring amino acid. In some embodiments, an amino acid is a synthetic amino acid; in some embodiments, an amino acid is a d-amino acid; in some embodiments, an amino acid is an l-amino acid, "Standard amino acid" refers to any of the twenty standard l-amino acids commonly found in naturally occurring peptides.
“Nonstandard amino acid" refers to any amino acid, other than the standard amino acids, regardless of whether it is prepared synthetically or obtained from a natural source. As used
herein, "synthetic amino acid" encompasses chemically modified amino acids, including but not limited to salts, amino acid derivatives (such as amides), and/or substitutions. Amino acids, including carboxy- and/or amino-terminal amino acids in peptides, can be modified by methylation, amidation, acetylation, protecting groups, and/or substitution with other chemical groups that can change the peptide's circulating half-life without adversely affecting their activity. Amino acids may participate in a disulfide bond. Amino acids may comprise one or posttranslational modifications, such as association with one or more chemical entitles (e.g., methyl groups, acetate groups, acetyl groups, phosphate groups, formyl moieties, isoprenoid groups, sulfate groups, polyethylene glycol moieties, lipid moieties, carbohydrate moieties, biotin moieties, etc.}. The term "amino acid" is used interchangeably with "amino acid residue," and may refer to a free amino acid and/or to an amino acid residue of a peptide, it will be apparent from the context in which the term is used whether it refers to a free amino acid or a residue of a peptide.
[040] Animal : As used herein, the term "animal" refers to any member of the animal kingdom. In some embodiments, "animal" refers to humans, at any stage of development, in some embodiments, "animal" refers to non-human animals, at any stage of development. In certain embodiments, the non-human animal is a mammal (e.g., a rodent, a mouse, a rat, a rabbit, a monkey, a dog, a cat, a sheep, a bovine, a primate, and/or a pig). In some embodiments, animals include, but are not limited to, mammals, birds, reptiles, amphibians, fish, insects, and/or worms. In some embodiments, an animal may be a transgenic animal, genetically- engineered animal, and/or a clone.
[041] Approximately or about: As used herein, the term "approximately" or "about," as applied to one or more values of interest, refers to a value that is similar to a stated reference value. In certain embodiments, the term "approximately" or "about" refers to a range of values that fall within 25%, 20%, 19%, 18%, 17%, 16%, 15%, 14%, 13%, 12%, 11%, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%, or less in either direction (greater than or less than) of the stated reference value unless otherwise stated or otherwise evident from the context (except where such number would exceed .100% of a possible value),
[042] Biologically active: As used herein, the term "biologically active" refers to a characteristic of any agent that has activity in a biological system, and particularly in an organism. For instance, an agent that, when administered to an organism, has a biological effect on that organism, is considered to be biologically active.
[043] Cystine: The term "cystine," as used herein, refers to the oxidized dimer form of the amino acid cysteine and has the formula (SCH CH(NH )CQ2H) .
[044] Delivery. As used herein, the term "delivery" encompasses both local and systemic delivery. For example, delivery of rnRIMA encompasses situations in which an mRNA is delivered to a target tissue and the encoded protein is expressed and retained within the target tissue (also referred to as "local distribution" or "local delivery"), and situations in which an mRNA is delivered to a target tissue and the encoded protein Is expressed and secreted into patient's circulation system (e.g., serum) and systematically distributed and taken up by other tissues (also referred to as "systemic distribution" or "systemic delivery"),
[045] Expression : As used herein, "expression" of a nucleic acid sequence refers to translation of an mRNA into a polypeptide, assemble multiple polypeptides into an intact protein (e.g., enzyme) and/or post-iransiationai modification of a polypeptide or fully assembled protein (e.g., enzyme). In this application, the terms "expression" and "production," and grammatical equivalents thereof, are used interchangeably,
[046] Functional·. As used herein, a "functional" biological molecule is a biological molecule in a form in which it exhibits a property and/or activity by which it is characterized.
[047] Half-life: As used herein, the term "half-life" is the time required for a quantity such as nucleic acid or protein concentration or activity to fall to half of its value as measured at the beginning of a time period.
[048] Helper iipid: The term "helper lipid" as used herein refers to any neutral or zwitterionic lipid material including cholesterol. Without wishing to be held to a particular theory, helper lipids may add stability, rigidity, and/or fluidity within Iipid biiayers/nanoparticles.
[049] improve , increase, or reduce: As used herein, the terms“improve," "Increase," or“reduce," or grammatical equivalents, indicate values that are relative to a baseline measurement, such as a measurement in the same individual prior to initiation of the treatment described herein, or a measurement in a control subject [or multiple control subject) in the absence of the treatment described herein. A "control subject" is a subject afflicted with the same form of disease as the subject being treated, who is about the same age as the subject being treated.
[050] In Vitro: As used herein, the term“in vitro" refers to events that occur in an artificial
environment, e.g., in a test tube or reaction vessel, in cell culture, etc., rather than within a multi-cellular organism.
[051] In Vivo: As used herein, the term "in vivo" refers to events that occur within a multi-cellular organism, such as a human and a non-human animal, in the context of cell-based systems, the term may be used to refer to events that occur within a living cell (as opposed to, for example, in vitro systems).
[052] Isolated: As used herein, the term "isolated" refers to a substance and/or entity that has been (1) separated from at least some of the components with which it was associated when initially produced (whether in nature and/or in an experimental setting), and/or (2) produced, prepared, and/or manufactured by the hand of man. isolated substances and/or entities may be separated from about 10%, about 20%, about 30%, about 40%, about 50%, about 60%, about 70%, about 80%, about 90%, about 91%, about 92%, about 93%, about 94%, about 95%, about 96%, about 97%, about 98%, about 99%, or more than about 99% of the other components with which they were initially associated. In some embodiments, isolated agents are about 80%, about 85%, about 90%, about 91%, about 92%, about 93%, about 94%, about 95%, about 96%, about 97%, about 98%, about 99%, or more than about 99% pure. As used herein, a substance is “pure" if it is substantially free of other components. As used herein, calculation of percent purity of isolated substances and/or entities should not include excipients {e.g., buffer, solvent, water, etc.).
[053] Liposome: As used herein, the term "liposome" refers to any lamellar, multilame!lar, or solid nanoparticle vesicle. Typically, a liposome as used herein can be formed by mixing one or more lipids or by mixing one or more lipids and polymer(s). In some embodiments, a liposome suitable for the present invention contains a cationic iipids(s) and optionally non-cationic lipid(s), optionally cholesterol-based lipid(s), and/or optionally PEG-modified lipid(s).
[054] messenger RNA ( mRNA ): As used herein, the term "messenger RNA (mRNA)" or "mRNA" refers to a polynucleotide that encodes at least one polypeptide. RNA as used herein encompasses both modified and unmodified RNA. The term "modified mRNA" related to mRNA comprising at least one chemically modified nucleotide. mRNA may contain one or more coding and non-coding regions. mRNA can be purified from natural sources, produced using recombinant expression systems and optionally purified, chemically synthesized, etc. Where appropriate, e.g., in the case of chemically synthesized molecules, mRNA can comprise nucleoside analogs such as analogs having chemically modified bases or sugars, backbone modifications, etc. An mRNA sequence is presented in the 5' to 3' direction unless otherwise indicated, in some embodiments, an mRNA is or comprises natural nucleosides {e.g., adenosine, guanosine, cytidine, uridine); nucleoside analogs (e.g., 2-aminoadenosine, 2-thiothymidine, inosine, pyrrolo-pyrimidine, 3-methyl adenosine, 5-methyicytidine, C-5 propynyi-cytidine, C-5 propynyl-uridine, 2-aminoadenosine, C5-bromouridine, C5-fluorouridine, C5-iodouridine, C5- propynyi-uridine, C5-propynyi-cytidine, C5-methylcytidine, 2-aminoadenosine, 7- deazaadenosine, 7-deazaguanosine, 8-oxoadenosine, 8-oxoguanosine, 0(6)-methylguanine, and 2-thiocytidine); chemically modified bases; biologically modified bases {e.g., methylated bases);
intercalated bases; modified sugars (e.g., 2'-fluororibose, ribose, 2'-deoxyribose, arabinose, and hexose); and/or modified phosphate groups (e.g., phosphorothioates and 5'-/V-phosphoramidite linkages).
[055] Nucleic acid: As used herein, the term "nucleic acid/' in its broadest sense, refers to any compound and/or substance that is or can be incorporated into a polynucleotide chain, in some embodiments, a nucleic add is a compound and/or substance that is or can be incorporated into a poiynucieotide chain via a phosphodiester linkage, in some embodiments, "nucleic acid" refers to individual nucleic acid residues (e.g., nucleotides and/or nucleosides), in some embodiments, "nucleic acid" refers to a polynucleotide chain comprising individual nucleic acid residues. In some embodiments, "nucleic acid" encompasses RNA as well as single and/or double-stranded DNA and/or cDNA. in some embodiments, "nucleic acid" encompasses ribonucleic acids (RNA), including but not limited to any one or more of interference RNAs (RNAi), small interfering RNA (siRNA), short hairpin RNA (shRNA), antisense RNA (aRNA), messenger RNA (mRNA), modified messenger RNA (mmRNA), long non-coding RNA (IncRNA), micro-RNA (miRNA) multimeric coding nucleic acid (MCNA), polymeric coding nucleic acid (PCNA), guide RNA (gRNA) and CRISPR RNA (crRNA). in some embodiments, "nucleic acid" encompasses deoxyribonucleic acid (DNA), including but not limited to any one or more of single-stranded DNA (ssDNA), double-stranded DNA (dsDNA) and complementary DNA (cDNA). in some embodiments, "nucieic acid" encompasses both RNA and DNA, in embodiments, DNA may be in the form of antisense DNA, plasmid DNA, parts of a plasmid DNA, pre-condensed DNA, a product of a polymerase chain reaction (PCR), vectors (e.g., PI, PAC, BAC, YAC, artificial chromosomes), expression cassettes, chimeric sequences, chromosomal DNA, or derivatives of these groups. In embodiments, RNA may be in the form of messenger RNA (mRNA), ribosomal RNA (rRNA), signal recognition particle RNA (7 SL RNA or SRP RNA), transfer RNA (tRNA), transfer-messenger RNA (tmRNA), small nuclear RNA (snRNA), small nucleolar RNA (snoRNA), SmY RNA, small Cajal body-specific RNA (scaRNA), guide RNA (gRNA), ribonuclease P (RNase P), Y RNA, telomerase RNA component (TERC), spliced ieader RNA (SL RNA), antisense RNA (aRNA or asRNA), cis-natural antisense transcript (cis-NAT), CRiSPR RNA (crRNA), long noncoding RNA (IncRNA), micro-RNA (miRNA), piwi-interacting RNA (piRNA), small interfering RNA (siRNA), transacting siRNA (tasiRNA), repeat associated siRNA (rasiRNA), 73K RNA, retrotransposons, a viral genome, a viroid, satellite RNA, or derivatives of these groups. In some embodiments, a nucleic acid is a mRNA encoding a protein such as an enzyme.
[056] Patient: As used herein, the term "patient" or "subject" refers to any organism to which a provided composition may be administered, e.g., for experimental, diagnostic, prophylactic.
cosmetic, and/or therapeutic purposes. Typical patients include animals [e.g., mammals such as mice, rats, rabbits, non-human primates, and/or humans), in some embodiments, a patient is a human. A human includes pre- and post-natal forms,
[057] Pharmaceutical!)/ acceptable·. The term "pharmaceutically acceptable," as used herein, refers to substances that, within the scope of sound medical judgment, are suitable for use in contact with the tissues of human beings and animals without excessive toxicity, irritation, allergic response, or other problem or complication, commensurate with a reasonable benefit/risk ratio.
[058] Pharmaceutically acceptable salt: Pharmaceutically acceptable salts are well known in the art. For example, S. M Berge et a!., describes pharmaceutically acceptable salts in detail in J. Pharmaceutical Sciences (1977) 66:1-19. Pharmaceutically acceptable salts of the compounds of this invention include those derived from suitable inorganic and organic acids and bases.
Examples of pharmaceutically acceptable, nontoxic acid addition salts are salts of an amino group formed with inorganic acids such as hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid, and perchloric acid or with organic acids such as acetic acid, oxalic acid, maleic acid, tartaric acid, citric acid, succinic acid, or maionic acid, or by using other methods used in the art such as ion exchange. Other pharmaceutically acceptable salts include adipate, alginate, ascorbate, aspartate, benzenesuifonate, benzoate, bisulfate, borate, butyrate, camphorate, camphorsulfonate, citrate, cyciopentanepropionate, digluconate, dodecy!su!fate,
ethanesulfonate, formate, fumarate, glucoheptonate, glycerophosphate, gluconate, hemisulfate, heptanoate, hexanoate, hydroiodide, 2-hydroxy-ethanesulfonate, lactobionate, lactate, laurate, lauryi sulfate, maiate, maleate, malonate, methanesulfonate, 2-naphihaienesuifonate, nicotinate, nitrate, oleate, oxalate, pa!mitate, pamoate, pectinate, persulfate, 3- phenylpropionate, phosphate, picrate, pivalate, propionate, stearate, succinate, sulfate, tartrate, thiocyanate, p-toluenesu!fonate, undecanoate, valerate salts, and the like. Salts derived from appropriate bases include alkali metal, alkaline earth metal, ammonium and N+(CI alkyl)4 salts. Representative alkali or alkaline earth metal salts include sodium, lithium, potassium, calcium, magnesium, and the like. Further pharmaceutically acceptable salts include, when appropriate, nontoxic ammonium, quaternary ammonium, and amine cations formed using counterions such as halide, hydroxide, carboxylate, sulfate, phosphate, nitrate, sulfonate, and aryl sulfonate. Further pharmaceutically acceptable salts include salts formed from the quarternization of an amine using an appropriate electrophile, e.g., an alkyl halide, to form a quarternized alkylated amino salt.
[059] Systemic distribution or delivery: As used herein, the terms "systemic distribution" or
"systemic delivery," or grammatical equivalents thereof, refer to a delivery or distribution
mechanism or approach that affect the entire body or an entire organism. Typically, systemic distribution or delivery is accomplished via body's circulation system, e.g., blood stream.
Compared to the definition of "local distribution or delivery."
[060] Subject: As used herein, the term "subject" refers to a human or any non-human animal {e.g., mouse, rat, rabbit, dog, cat, cattle, swine, sheep, horse or primate). A human includes pre- and post-natal forms. In many embodiments, a subject is a human being. A subject can be a patient, which refers to a human presenting to a medical provider for diagnosis or treatment of a disease. The term "subject" is used herein interchangeably with "individual" or "patient," A subject can be afflicted with or is susceptible to a disease or disorder but may or may not display symptoms of the disease or disorder.
[061] Substantially: As used herein, the term "substantially" refers to the qualitative condition of exhibiting total or near-total extent or degree of a characteristic or property of interest. One of ordinary skill in the biological arts will understand that biological and chemical phenomena rarely, if ever, go to completion and/or proceed to completeness or achieve or avoid an absolute result. The term "substantially" is therefore used herein to capture the potential lack of completeness inherent in many biological and chemical phenomena.
[062] Target tissues: As used herein, the term "target tissues" refers to any tissue that is affected by a disease to be treated, in some embodiments, target tissues include those tissues that display disease-associated pathology, symptom, or feature,
[063] Therapeuticai!y effective amount: As used herein, the term "therapeutically effective
amount" of a therapeutic agent means an amount that is sufficient, when administered to a subject suffering from or susceptible to a disease, disorder, and/or condition, to treat, diagnose, prevent, and/or delay the onset of the symptom(s) of the disease, disorder, and/or condition. It will be appreciated by those of ordinary skill in the art that a therapeutically effective amount is typically administered via a dosing regimen comprising at least one unit dose.
[064] Treating: As used herein, the term "treat," "treatment," or "treating" refers to any method used to partially or completely alleviate, ameliorate, relieve, inhibit, prevent, delay onset of, reduce severity of and/or reduce incidence of one or more symptoms or features of a particular disease, disorder, and/or condition. Treatment may be administered to a subject who does not exhibit signs of a disease and/or exhibits only early signs of the disease for the purpose of decreasing the risk of developing pathology associated with the disease.
[065] Aliphatic: As used herein, the term aliphatic refers to Ci-Cso hydrocarbons and includes both saturated and unsaturated hydrocarbons. An aliphatic may be linear, branched, or cyclic, for example, C -C aliphatics can include C -C alkyls {e.g., linear or branched C -C saturated
alkyls)/ C2-C20 alkenyls (e.g., linear or branched -C20 dienyis, linear or branched C -C trienyls, and the like), and C2-C20 aikynyls [e.g., linear or branched C2--C2o alkynyls). Ci-C aliphatics can include C3-C20 cyclic aliphatics (e.g., C -C cycloalkyls, Q-C20 cycloalkenyls, or Cs-C
cycloalkynyls). In certain embodiments, the aliphatic may comprise one or more cyclic aliphatic and/or one or more heteroatoms such as oxygen, nitrogen, or sulfur and may optionally be substituted with one or more substituents such as alkyl, halo, aikoxyi, hydroxy, amino, aryl, ether, ester or amide. An aliphatic group is unsubstituted or substituted with one or more substituent groups as described herein. For example, an aliphatic may be substituted with one or more (e.g., 1, 2, 3, 4, 5, or 6 independently selected substituents) of halogen, -COR', -C02H, - C02R', -CM, -OH, -OR', -OCOR', -0C02R', -NH2,
-NHR', -IM(R')2, -SR' or-S02R', wherein each instance of R' independently is Ci-C aliphatic [e.g., Ci-C2o alkyl, C-.-C alkyl, Ci-Cio alkyl, or C-.-C alkyl). In embodiments, R' independently is an unsubstituted alkyl (e.g., unsubstituted Ci-C alkyl, C -C alkyl, C1-C10 alkyl, or C C alkyl). In embodiments, R' independently is unsubstituted C1-C3 aikyi. In embodiments, the aliphatic is unsubstituted. In embodiments, the aliphatic does not include any heteroatoms.
[066] Alkyl: As used herein, the term "alkyl" means acyclic linear and branched hydrocarbon groups, e.g. "Ci-C20 alkyl" refers to aikyi groups having 1-20 carbons. An alkyl group may be linear or branched. Examples of alkyl groups include, but are not limited to, methyl, ethyl, n- propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl tert-pentylhexyl, isohexy!efc. The term "lower alkyl" means an alkyl group straight chain or branched alkyl having 1 to 6 carbon atoms. Other alkyl groups will be readily apparent to those of skill in the art given the benefit of the present disclosure. An alkyl group may be unsubstituted or substituted with one or more substituent groups as described herein. For example, an alkyl group may be substituted with one or more (e.g., 1, 2, 3, 4, 5, or 6 independently selected substituents) of halogen, -COR', -CG2H, -C02R', -CM, -OH, -OR', -OCOR', -OCO,R', -NH2, -NHR', -N(R')2, -SR' or- S02R', wherein each instance of R' independently is Ci-C20 aliphatic (e.g., C1-C20 alkyl, Ci-C-.5 alkyl, C1-C10 alkyl, or Ci-C3 alkyl). In embodiments, R' independently is an unsubstituted alkyl (e.g., unsubstituted C1-C20 alkyl, C1-C15 alkyl, Cj-Cio alkyl, or C1-C3 aikyi). In embodiments, R' independently is unsubstituted Ci-C3 alkyl, in embodiments, the alkyl is substituted [e.g., with 1, 2, 3, 4, 5, or 6 substituent groups as described herein). In embodiments, an alkyl group is substituted with a-OH group and may also be referred to herein as a "hydroxyaikyi" group, where the prefix denotes the -OH group and "aikyi" is as described herein.
[067] Affixing the suffix "-ene" to a group indicates the group is a divalent moiety, e.g., arylene is the divalent moiety of aryl, and heteroarylene is the divalent moiety of heteroaryl.
[068] Alkylene: The term "a!kylene," as used herein, represents a saturated divalent straight or branched chain hydrocarbon group and is exemplified by methylene, ethylene, isopropylene and the like. Likewise, the term "alkenylene" as used herein represents an unsaturated divalent straight or branched chain hydrocarbon group having one or more unsaturated carbon-carbon double bonds that may occur in any stable point along the chain, and the term "aikynylene" herein represents an unsaturated divalent straight or branched chain hydrocarbon group having one or more unsaturated carbon-carbon triple bonds that may occur in any stable point along the chain, in certain embodiments, an alkylene, alkenylene, or aikynylene group may comprise one or more cyclic aliphatic and/or one or more heteroatoms such as oxygen, nitrogen, or sulfur and may optionally be substituted with one or more substituents such as alkyl, halo, aikoxyi, hydroxy, amino, aryl, ether, ester or amide. For example, an alkylene, alkenylene, or aikynylene may be substituted with one or more (e.g., 1, 2, 3, 4, 5, or 6 independently selected substituents) of halogen, -COR', -C02H, -CO R',
-CN, -OH, -OR', -QCOR', -OCO,R', -NH2, -NH R', -N(R')2, -SR' or -S02R\ wherein each instance of R' independently is Ci-C20 aliphatic {e.g., Ci-C2o alkyl, Ci-Cis alkyl, Ci-Cio alkyl, or C -C6, alkyl), in embodiments, Rf independently Is an unsubstituted alkyl (e.g., unsubstituted C-.-C20 alkyl, C1-C15 aikyi, C1-C10 alkyl, or C-.-C3 alkyl). In embodiments, R' independently is unsubstituted C-.-C3 alkyl. In certain embodiments, an alkylene, alkenylene, or aikynylene is unsubstituted. In certain embodiments, an alkylene, alkenylene, or aikynylene does not include any heteroatoms.
[069] Alkenyl·. As used herein, "alkenyl" means any linear or branched hydrocarbon chains having one or more unsaturaied carbon-carbon double bonds that may occur in any stable point along the chain, e.g. "C2-C20 aikeny!" refers to an alkenyl group having 2-2.0 carbons. For example, an alkenyl group includes prop-2-enyl, but-2-enyl, but-3-enyi, 2-methylprop-2-enyl, hex-2-enyl, hex- 5-enyi, 2,3-dimethylbut-2-enyl, and the like. In embodiments, the alkenyl comprises 1, 2, or 3 carbon-carbon double bond, in embodiments, the alkenyl comprises a single carbon-carbon double bond, in embodiments, multiple double bonds (e.g., 2 or 3) are conjugated. An alkenyl group may be unsubstituted or substituted with one or more substituent groups as described herein. For exampie, an alkenyl group may be substituted with one or more (e.g., 1, 2, 3, 4, 5, or 6 independently selected substituents) of halogen, -COR', -C02H, -C02R', -CN, -OH, -OR', -OCQR', -OC02R', -NH2, -NHR', -N(R')2, -SR' or-S02R', wherein each instance of R’ independently is Ci-C20 aliphatic (e.g., Ci-C20 alkyl, C1-C15 alkyl, C1-C10 alkyl, or C1-C3 alkyl). In embodiments, R'
independently is an unsubstituted alkyl (e.g., unsubstituted Ci-C20 alkyl, C1-C15 alkyl, C -Cio aikyi, or C1-C3 aikyi). in embodiments, R' independently is unsubstituted C1-C3 aikyi. In embodiments, the alkenyl is unsubstituted. In embodiments, the alkenyl is substituted (e.g., with 1, 2, 3, 4, 5,
or 6 substituent groups as described herein), in embodiments, an alkenyl group is substituted with a-QH group and may also be referred to herein as a "hydroxyalkeny!" group, where the prefix denotes the -OH group and "alkenyl" is as described herein,
[070] Aikynyi: As used herein, "alkynyi" means any hydrocarbon chain of either linear or branched configuration, having one or more carbon-carbon triple bonds occurring in any stable point along the chain, e.g. "C2-C2o alkynyi" refers to an alkynyi group having 2-20 carbons. Examples of an alkynyi group include prop-2-ynyl, but-2-ynyl, but-3-ynyl, pent-2-ynyl, 3-methylpent-4-ynyl, hex-2-ynyl, hex-5-ynyl, etc. in embodiments, an alkynyi comprises one carbon-carbon triple bond. An aikynyi group may be unsubstituted or substituted with one or more substituent groups as described herein. For example, an alkynyi group may be substituted with one or more (e.g., 1, 2, 3, 4, 5, or 6 independently selected substituents) of halogen, -COR'', -C02H, -C02R', - CN, -OH, -OR', -OCOR',
-OC02R', -NH2, -NHR', -N(R')2, -SR' or-S02R', wherein each instance of R’ independently is C C20 aliphatic {e.g., Ci-C20 alkyl, Ci-Cis alkyi, Ci-Cio alkyl, or C1-C3 alkyl), in embodiments, R' independently is an unsubstituted alkyl (e.g., unsubstituted Ci-C20 alkyl, C1-C15 alkyl, Cj-Cio alkyi, or C1-C3 alkyi). in embodiments, R' independently is unsubstituted C1-C3 alkyl. In embodiments, the alkynyi is unsubstituted. In embodiments, the alkynyi is substituted (e.g., with 1, 2, 3, 4, 5, or 6 substituent groups as described herein).
[071] Aryl: The term "ary!" used alone or as part of a larger moiety as in "aralkyl," refers to a monocyclic, bicyclic, or tricyclic carbocyclic ring system having a total of six to fourteen ring members, wherein said ring system has a single point of attachment to the rest of the molecule, at least one ring in the system is aromatic and wherein each ring in the system contains 4 to 7 ring members. In embodiments, an aryl group has 6 ring carbon atoms ("C6 aryl," e.g., phenyl), in some embodiments, an ary! group has 10 ring carbon atoms ("C10 aryl," e.g., naphthyl such as 1-naphthyl and 2-naphthyl). In some embodiments, an aryl group has 14 ring carbon atoms ("C aryl," e.g., anthracyi). "Aryl" also Includes ring systems wherein the aryl ring, as defined above, is fused with one or more carbocydyi or heterocyclyl groups wherein the radical or point of attachment is on the aryl ring, and in such instances, the number of carbon atoms continue to designate the number of carbon atoms in the aryl ring system. Exemplary aryls include phenyl, naphthyl, and anthracene.
[072] Ary!ene: The term "aryiene" as used herein refers to an aryl group that is divalent (that is, having two points of attachment to the molecule). Exemplary arylenes include phenylene (e.g., unsubstituted phenylene or substituted phenylene).
[073] Halogen: As used herein, the term "halogen" means fluorine, chlorine, bromine, or iodine.
[074] Heteroalkyl·. The term "heteroalkyl" is meant a branched or unbranched alkyl, alkenyl, or alkynyl group having from 1 to 14 carbon atoms In addition to 1, 2, 3 or 4 heteroatoms independently selected from the group consisting of N, O, S, and P, Heteroalkyls include tertiary amines, secondary amines, ethers, thioethers, amides, thioa ides, carbamates, thiocarba ates, hydrazones, imines, phosphodiesters, phosphoramidates, sulfonamides, and disulfides. A heteroalkyl group may optionally include monocyclic, bicycllc, or tricyclic rings, in which each ring desirably has three to six members. Examples of heteroalkyls include polyethers, such as methoxymethyl and ethoxyethyl.
[075] Heteroa!ky!ene: The term "heteroalkylene," as used herein, represents a divalent form of a heteroalkyl group as described herein.
[076] Heteroaryl: The term "heteroary!," as used herein, is fully unsaturaied heteroatom- containing ring wherein at least one ring atom is a heteroatom such as, but not limited to, nitrogen and oxygen.
[077] Heterocycloa!ky!: The term "heterocycloalkyl," as used herein, is a non-aromatic ring
wherein at least one atom Is a heteroatom such as, but not limited to, nitrogen, oxygen, sulfur, or phosphorus, and the remaining atoms are carbon. The heterocycloalkyl group can be substituted or unsubstituted.
Compounds of the Invention
[078] Liposomal-based vehicles are considered an attractive carrier for therapeutic agents and remain subject to continued development efforts. While liposomal-based vehicles that comprise certain lipid components have shown promising results with regard to encapsulation, stability and site localization, there remains a great need for improvement of liposomal-based delivery systems. For example, a significant drawback of liposomal delivery systems relates to the construction of liposomes that have sufficient cell culture or in vivo stability to reach desired target cells and/or intracellular compartments, and the ability of such liposomal delivery systems to efficiently reiease their encapsulated materials to such target ceils.
[079] In particular, there remains a need for improved lipids compounds that demonstrate
improved pharmacokinetic properties and which are capable of delivering macromolecules, such as nucleic acids to a wide variety cell types and tissues with enhanced efficiency. Importantly, there also remains a particular need for novel lipid compounds that are characterized as having reduced toxicity and are capable of efficiently delivering encapsulated nucleic acids and polynucleotides to targeted cells, tissues and organs.
[080] Described herein a novel class of cystine cationic lipid compounds for improved in vivo delivery of therapeutic agents, such as nucleic acids, in particular, a cystine cationic lipid described herein may be used as a cationic lipid, optionally with other lipids, to formulate a lipid- based nanoparticle (e.g., liposome) for encapsulating therapeutic agents, such as nucleic acids (e.g., DMA, siRNA, mRNA, microRNA) for therapeutic use.
[081] In embodiments, compounds described herein can provide one or more desired
characteristics or properties. That is, in certain embodiments, compounds described herein can be characterized as having one or more properties that afford such compounds advantages relative to other similarly classified lipids. For example, compounds disclosed herein can allow for the control and tailoring of the properties of liposomal compositions (e.g., lipid
nanoparticles) of which they are a component. In particular, compounds disclosed herein can be characterized by enhanced transfection efficiencies and their ability to provoke specific biological outcomes. Such outcomes can include, for example enhanced cellular uptake, endosomal/iysosomal disruption capabilities and/or promoting the release of encapsulated materials (e.g., polynucleotides) intraceilulariy. Additionally, the compounds disclosed herein have advantageous pharmacokinetic properties, biodistribution, and efficiency (e.g., due to the different disassociate rates of the polymer group used).
Compounds of Formula (A)
[082] Provided herein are compounds which are cationic lipids. For example, the cationic lipids of the present invention include compounds having a structure according to Formula (A),
or a pharmaceutically acceptable salt thereof, wherein
wherein
each Rla is independently hydrogen, Ric, or R3d;
each RlB is independently Rlc or Rld;
each Rlc is Independently -{CM )2C(Q)X1R3;
each Rld is independently -C(0)R4;
each R2 is independently -[C(R2a)2],;R2b;
each R2a is independently hydrogen or lower alkyl (e.g., C-.-C& alkyl);
R2b is -N(LI-B)2, -(OCH2CH2)6OH; or -(OCH2CH2)60CH3;
each R3 and R4 is independently aliphatic {e.g., C6-C30 aliphatic); each Li is independently alkylene (e.g., Ci-Cio a!ky!ene);
each B is independently hydrogen or an ionizable nitrogen-containing group;
each X1 is independently a covalent bond or O;
each a is independently an integer (e.g., 1-10);
each b is independently an integer (e.g., 1-10); and
each c. is independently an integer (e.g., 1-10).
[083] in embodiments, the cationic lipid has a structure according to Formula (A), wherein each Rla is independently hydrogen, Rlc, or R3d;
each Rlc is independently Rlc or Rld;
each Rlc is independently -(CH2)2C(0)X1R3;
each Rld is independently ~C(0)R4;
each R/: is independently -[C{R2a)2]cR2b;
each R2a is independently hydrogen or CrCs alkyl;
R2b is -N[LI-B)2, -(OCH2CH2)/,OH; or -{OCH2CH2)bOCH3;
each R3 and R4 is independently C6-C3o aliphatic;
each Li is independently Ci-Cio alkylene;
each B is independently hydrogen or an ionizable nitrogen-containing group;
each X3 is independently a covalent bond or O;
each a is Independently an integer of 1-10;
each b is independently an integer of 1-10; and
each c is independently an integer of 1-10.
[084] In embodiments, each a is independently 1 or 2. In embodiments, each a is 1. In embodiments, each a is 2,
[085] In embodiments, each Rla and Rlb is Independently HCH2)2C(0)X1R3.
[086] In embodiments, a cationic Iipid has a structure according to formula (I):
[087] In embodiments, each R3 is the same, in embodiments, each R3 is different.
[088] in embodiments, each R3 is independently C6-CM alkyl, C6-C2 alkenyl, or C6-C a!kynyi.
[089] In embodiments, each R3 is independently Cs-C30 alkyl, in embodiments, each R3 is
independently unsubstituted C6-C3o alkyl, in embodiments, each R3 is independently substituted C6-C3o alkyl. In embodiments, each R3 Is independently C6-C alkyl. In embodiments, each R3 Is independently unsubstituted C6-C2 alkyl in embodiments, each R3 is independently substituted C6-C alkyl.
[090] In embodiments, each R3 is independently C6-C30 alkenyl. In embodiments, each R3 is
independently unsubstituted C6-C30 alkenyl, in embodiments, each R3 is independently substituted C6-C30 alkenyl. In embodiments, each R3 is C6-C2 alkenyl. In embodiments, each R3 is independently unsubstituted C6-C2 alkenyl. In embodiments, each R3 is independently substituted Ce-C24 alkenyl.
[091] In embodiments, each R3 is independently Cs-C30 a!kynyl. In embodiments, each R3 is
independently unsubstituted C6-C30 alkynyl. In embodiments, each R3 is independently substituted C6-C30 alkynyl. In embodiments, each R3 is Independently C3-C alkynyl.
In embodiments, each R3 is independently unsubstituted C6-C2 alkynyl. In embodiments, each R3 is independently substituted CK-CM alkynyl.
[092] In embodiments, each Rla is hydrogen and each Rlb is -CiOIR c
[093] in embodiments, a cationic iipid has a structure according to formula {li):
pharmaceutically acceptable salt thereof.
[094] In embodiments, each R4 is the same, in embodiments, each R4 is different.
[095] In embodiments, each R4 is independently C6-C24 alkyl, C6 C24 alkenyl, or C6-C24 alkynyl.
[096] in embodiments, each R4 is independently C6-C3o alkyl. In embodiments, each R4 is
independently unsubstituted Ce-Cso alkyl, in embodiments, each R4 is independently substituted CK-CSO alkyl. In embodiments, each R4 is independently C&-C2 alkyl, in embodiments, each R4 is independently unsubstituted Cs-C24 alkyl, in embodiments, each R4 is independently substituted C6-C24 alkyl.
[097] In embodiments, each R4 is independently C6-C3o alkenyl. In embodiments, each R4 is
independently unsubstituted C6-C o alkenyl. In embodiments, each R4 is independently substituted Cs-C3o alkenyl, in embodiments, each R4 is independently C6-C24 alkenyl, in embodiments, each R4 is independently unsubstituted C6~C alkenyl, in embodiments, each R4 is independently substituted C6-C2 alkenyl.
[098] in embodiments, each R4 is independently C6-C3o alkynyl. In embodiments, each R4 is
independently unsubstituted C6-C o alkynyl. In embodiments, each R4 is independently substituted Cs-C3o alkynyl. In embodiments, each R4 is independently C8-C24 alkynyl.
in embodiments, each R4 is independently unsubstituted C6~C alkynyl. in embodiments, each R4 is independently substituted C6-C24 alkynyl,
[099] in embodiments, each R4 is independently C6-Cio alkyl substituted with -GC(Q)R5 or-C02R5, wherein R5 is unsubstituted Cg-Cie alkyl, in embodiments, each R4 is independently C3-Ci0 alkyl substituted with ~OC{Q)R:'. in embodiments, each R4 is independently C6-Cio alkyl substituted with -C02Rr>. In embodiments, R5 is independently unsubstituted linear C6-Cis alkyl, in embodiments, R5 is independently unsubstituted branched C6-C36 alkyl.
[0100] In embodiments, each R2 is independently -[CH23!;-iM(Li-B)2 or ~ CH(CH3)CH2--N(LrB)2. in embodiments, each Li is unsubstituted Ci-Cio aikyiene.
[0101] in embodiments, each R2 is independently -[CH2jc-N{CH3)2.
[0102] in embodiments, each R2 is independently -[CH2]c-N{CH2CH2B)2, -[CH2]c-N(CH2CH2CH2B)2, - CH(CH3)CH2--i\i{CH2CH2B)2, or ~CH{CH3)CH2-N(CH2CH2CH2B)2; and each B is an ionizabie nitrogen- containing group.
[0103] In embodiments, each B is independently NH2, guanidine, amidine, a mono- or dialkylamine, 5- to 6-membered heterocycloaikyl, or 5- to 6-membered nitrogen-containing heteroaryl.
[0104] In embodiments, each B is NH2. In embodiments, each B is guanidine, in embodiments, each B is amidine. in embodiments, each B is a monoalkylamine. in embodiments, each B is a
diaikyiamine. In embodiments, each B is a 5- to 6-membered heterocycloalkyl. In embodiments, each B is a 5- to 6- membered nitrogen -containing heteroaryi.
[0105] in embodiments, each B is independently NH2, guanidine, amidine, NHCH3, N(CH3)2, or imidazole, in embodiments, each B is N(CH3)2.
[0106] In embodiments, each c is independently 2, 3, or 4. in embodiments, each c is 2. in
embodiments, each c is 3. in embodiments, each c is 4.
[0107] In embodiments, a CS~C30 alky! is a C8-26 a!ky!. In embodiments, a C6-C3o alkyl is a straight- chain Cg 26 alkyl, in embodiments, a C6-C30 alkyl is 6H3(6H2}6OH2-, CH3(CH2)7CH2-, CH3(CH2)gCH2-, CH3{CH2)gCH2-, CHJ,(CH2)IOCH2-, CH3(CH2)UCH2-, CH3(CH2)12CH2-, CH3(CH2)I;,CH2-, CH3(CH2)MCH2-, CHB}CH2)I5CH2-, CHB(CH2)I6CH2~, CH3(CH2)I7CH2-, CH3(CH2)I8CH2-, CH3(CH2)19CH2-, CH3(CH2)20CH2-, CH3(CH2)2ICH2-, CHB(CH2)22CH3-, CH3(CH2)23CH2- or CH3(CH2)2 CH2-.ln embodiments, a C6-C30 alkyl is CH3(CH2)I3CH2-, CH3(CH2)I4CH2-, CH3(CH2)ISCH2-, CH3[CH2)16CH2-, CH3(CH2)17CH2- or CH3(CH2)I8CH2-. In embodiments, a C6-C30 alkyl is CH3[CH2)I CH2-, CH3(CH2)I5CH2- or CH3(CH2)I6CH2-.
[0108] In embodiments, a Ce-C3o alkenyl is a Cs-ze alkenyl having one or two carbon-carbon double bonds, in embodiments, a C6-C30 alkenyl is c/s-CH3(CH2)3CH=CH(CH2)7CH2-, cis- CH3(CH2)sCH=CH(CH2)7CH2-, c/s-CH3(CH2)aCH=CH(CH2)4CHr, c/s-CH3(CH2)7CH=CH(CH2)7CH2-, cis- CH3(CH2)9CH=CH(CH2)7CH2-, C/S-CH3(CH2)7CH=CH(CH2)9CH2-, trans-CH3(CH2)7CH=CH(CH2)7CH3-, ir a ns- C H { C H 3 ) s C H = C H ( C H 2 ) g C H 2- , C/S-CH3(CH2)9CH=CH(CH2)7CH3-, C/S-CH3(CH2)7CH=CH(CH2)HCH2-, cis- CH3(CH2)7CH=CH[CH2)i3CH2-, c¾c/s-CH3(CH2)4CH=CHCH2CH=CH(CH2)7CH2- C/S,C/S-CH3(CH2)4CH=CHCH2CH=CH[CH2)9CH2- or cis, C/S-CH3(CH2)4CH=CHCH2CH=CH(CH2)HCH2-.
in embodiments, a C3-C30 alkenyl is c/s-CH3(CH2)3CH=CH(CH2)7CH2-, c/s-CH3(CH2)5CH=CH(CH2)7CH2-, c/'s- C H B { C H 2 ) a C H = C H ( C H ) C H - , c/s-CH3(CH2)7CH=CH(CH2)7CH2-, cis-CH3(CH2) CH=CH(CH2)7CH2-, trans- CH3(CH2)7CH=CH(CH2)7CH2-, CIS, c/s-CH3(CH2)4CH=CHCH2CH=CH(CH2)7CH2- or
C/5,C/S-CH3(CH2)4CH=CHCH2CH=CH{CH2)9CH2-. In embodiments, a C6-C3o alkenyl is c/s- CH3(CH2)7CH=CH(CH2)7CH2-, C/S-CH3(CH2)9CH=CH(CH2)7CH2-,
C/S,C/S-CH3(CH2)4CH=CHCH2CH=CH(CH2)7CH2- or c/s,c/s-CH3(CH2) CH=CHCH2CH=CH(CH )9CH -. in embodiments, a C6-C3o alkenyl is c/s-CH3(CH2)7CH=CH(CH )7CH - or
c/s,c/s-CH3(CH2)4CH=CH-CH2CH=CH(CH2)7CH2-. In embodiments, a C6-C30 alkenyl is C8-26 aliphatic having three, four, five or six carbon-carbon double bonds.
[0109] In embodiments, a C -C alkenyl is:
cis, cis, cis- C H 3 C H 2 C H = C H C H 2CH=CHCH2C H = C H [ C H 2 )7 C H 3 -,
c/s,c/s,c/s-CH3[CH2)4CH=CHCH2CH=CHCH2CH=CH(CH2)4CH2-,
c/s,c/s,c/s-CH3(CH2)4CH=CHCH2CH=CHCH2CH2CH=CH(CH2)3CH2-,
irons, ira/is/irons-CH3(CH2)7CH=CHCH2CH=CHCH2CH=CH(CH2)3CH2-,
C/S,C/S,C/S-CH3(CH2)4CH=CHCH2CH=CHCH2CH=CH(CH2)6CH2-,
C/S,C/S/C/S-CH3CH2CH=CHCH2CH=CHCH2CH=CH{CH2)9CH2-/
s c/s,c/s,c/s-CH CH2CH==CHCH2CH= HCH2CH==CHCH2CH=CH(CH2) CH2-,
ci¾c/s,c/s/c/s-CH (CH2) CH=CHCH2CH=CHCH2CH=CHCH2CH=CHiCH2) CH2-,
C/S/C/S C/S,C/5-CH3CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CH{CH2)6CH2-,
c/¾C7¾fra 7¾fraris s-CH3iCH2)4CH=CHCH=CHCH=CHCH=CHCH2CH=CH(CH2)3CHr, c/s,ds/c/s c/¾c/s-CH CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHiCH2) CH2··, ds ds,ds,ds/ds-CH3(CH2} CH=:CHCH2CH==CHCH2CH=:CHCH2CH==CHCH2CH= H(CH2)2CH2-, ds,ds,ds/ds ds-CH3CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)5CH2-,.
a5^/5^/5^/5,a5-eH3GH2GH=GHGH2€H=GHGH2GH=€HGH2eH=GHGH2GH=€H(GH2)7GH2-,
CIS , cis, CIS, cis, cis, cis-
CH CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)2CH / or
CIS , cis, cis , cis, cis, cis-
CH3CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CH{CH2)4CH2-.
[0110] In embodiments, a Cs-C30 alkenyl is:
ds,ds,ds-CH3CH2CH=CHCH2CH=CHCH2CH=CH{CH2)7CH2-,
ds,ds,ds,c/s-CH3CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2} CH2-,
ds,d.s,fra/7S,iraris ds-CH3iCH2)4CH=CHCH=CHCH=CHCH=CHCH2CH=CH(CH2)3CHr, or cis, cis, cis, cis, cis, cis-
CH3CH2CH=:CHCH2CH==CHCH2CH;=CHCH2CH==CHCH2CH;=CHCH2CH==CH(CH2)2CH2-.
[0111] in embodiments, a Cs~C3o alkenyl) is:
ds, d.s, c/s - C H 3 C H C H = C H C H C H = C H C H C H = C H ( C H ) 7 C H 2- or
c/£,c/s,ds,ds-CH CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHfCH2) CH2··,
[0112] In embodiments, an aliphatic group described herein is the aliphatic chain of a saturated or unsaturated fatty acid. In embodiments, an aliphatic group is the aliphatic chain of capry!ic, pelargonic, capric, undecylic, iauric, tridecyclic, myristic, pentadecylic, margaric, stearic, nonadecylic, arachidic, heneicosylic, behenic, triosylic, lignoceric, oleic, linoleic, pentacosylic or cerotic acid, in embodiments, an aliphatic group is the aliphatic chain of caprylic, pelargonic, capric, undecylic,
Iauric, tridecyclic, myristic, peniadecyiic, or margaric acid, in embodiments, an aliphatic group is the aliphatic chain of Iauric, tridecyclic, myristic, or pentadecylic acid. In some embodiments, an aliphatic group aliphatic chain of Iauric or myristic acid. In embodiments, an aliphatic group is the aliphatic chain of stearic, nonadecylic, arachidic, heneicosylic, behenic, triosylic, lignoceric, oleic, linoleic,
pentacosylic or cerotic add. in embodiments, an aliphatic group is the aliphatic chain of lignoceric, oleic, linoleic, pentacosylic or cerotic acid. In embodiments, an aliphatic group is the aliphatic chain of oleic, linoleic or pentacosylic acid. In embodiments, R2 is the aliphatic chain of oleic or linoleic acid. In embodiments, an aliphatic group is the aliphatic chain of oleic acid. In some embodiments, an aliphatic group is the aliphatic chain of linoleic acid.
Exemplary Compounds
[0113] In embodiments, a cationic lipid is any one of Compounds 1-12.
[0114] In embodiments, a cationic lipid is Compound 1. !n embodiments, a cationic lipid is
Compound 2, in embodiments, a cationic lipid is Compound 3, In embodiments, a cationic lipid is Compound 4. in embodiments, a cationic lipid is Compound 5, In embodiments, a cationic lipid is Compound 6, in embodiments, a cationic lipid is Compound 7. in embodiments, a cationic lipid is Compound 8, In embodiments, a cationic lipid is Compound 9. In embodiments, a cationic lipid is Compound 10. in embodiments, a cationic lipid is Compound 11. In embodiments, a cationic lipid is Compound 12.
Synthesis of Compounds of the Invention
[0115] The compounds described herein (e.g., a compound of Formula (A), such as Formulae (!) or (il) or any one of Compounds 1-12) can be prepared according to methods known in the art, including the exemplary syntheses of the Examples provided herein.
Nucleic Acids
[0116] The compounds described herein {e.g , a compound of Formula (A), such as Formulae (!) or (il) or any one of Compounds 1-12) can be used to prepare compositions useful for the delivery of nucleic acids.
Synthesis of Nucleic Acids
[0117] Nucleic acids according to the present invention may be synthesized according to any known methods. For example, mRIMAs according to the present invention may be synthesized via in vitro transcription (!VT), Briefly, IVT is typically performed with a linear or circular DNA template containing a promoter, a pool of ribonucleotide triphosphates, a buffer system that may include DTT and magnesium ions, and an appropriate RINA polymerase {e.g., T3, T7, mutated T7 or SP6 RNA polymerase), DNAse I, pyrophosphatase, and/or RNAse inhibitor. The exact conditions will vary according to the specific application.
[0118] In some embodiments, for the preparation of mRNA according to the invention, a DNA template is transcribed in vitro. A suitable DNA template typically has a promoter, for example a 13, 17, mutated 17 or SP6 promoter, for in vitro transcription, followed by desired nucleotide sequence for desired mRNA and a termination signal.
[0119] Desired mRNA sequence(s) according to the invention may be determined and incorporated into a DNA template using standard methods. For example, starting from a desired amino acid sequence {e.g., an enzyme sequence), a virtual reverse translation is carried out based on the degenerated genetic code. Optimization algorithms may then be used for selection of suitable codons. Typically, the G/C content can be optimized to achieve the highest possible G/C content on one hand, taking into the best possible account the frequency of the tRNAs according to codon usage on the other hand. The optimized RNA sequence can be established and displayed, for example, with the aid of an appropriate display device and compared with the original (wild- type) sequence. A secondary structure can also be analyzed to calculate stabilizing and destabilizing properties or, respectively, regions of the RNA.
[0120] As described above, the term "nucleic acid," in its broadest sense, refers to any compound and/or substance that is or can be incorporated into a polynucleotide chain. DNA may be in the form of antisense DNA, plasmid DNA, parts of a plasmid DNA, pre-condensed DNA, a product of a polymerase chain reaction (PCR), vectors (e.g., PI, PAC, BAG, YAC, artificial chromosomes), expression cassettes, chimeric sequences, chromosomal DNA, or derivatives of these groups. RNA may be in the form of messenger RNA (mRNA), ribosomal RNA (rRNA), signal recognition particle RNA (7 SL RNA or SRP RNA), transfer RNA (tRNA), transfer-messenger RNA (tmRNA), small nuclear RNA (snRNA), small nucleolar RNA (snoRNA), SmY RNA, small Cajal body-specific RNA (scaRNA), guide RNA (gRNA), ribonuclease P (RNase P), Y RNA, telomerase RNA component (TERC), spliced leader RNA (SL RNA), antisense RNA (aRNA or asRNA), cis-natural antisense transcript (cis-NAT), CR!SPR RNA (crRNA), iong noncoding RNA (incRNA), microRNA ( lRN.A), piwi-interacting RNA (pi RNA), small interfering RNA (siRNA), transacting siRNA (tasiRNA), repeat associated siRNA (rasi RNA), 73K RNA, retrotransposons, a viral genome, a virold, satellite RNA, or derivatives of these groups. In some embodiments, a nucleic acid is a mRNA encoding a protein.
Synthesis of mRNA
[0121] RNAs according to the present invention may be synthesized according to any of a variety of known methods. For example, mRNAs according to the present invention may be synthesized via in vitro transcription (!VT). Briefly, !VT is typically performed with a linear or circular DNA template containing a promoter, a pool of ribonucleotide triphosphates, a buffer system that
may include DTT and magnesium ions, and an appropriate RNA polymerase {e.g., T3, T7 or SP6 RNA polymerase), DNAse I, pyrophosphatase, and/or RNAse Inhibitor. The exact conditions will vary according to the specific application. The presence of these reagents is undesirable in the final product according to several embodiments and may thus be referred to as impurities and a preparation containing one or more of these impurities may be referred to as an impure preparation, in some embodiments, the in vitro transcribing occurs in a single batch.
[0122] in some embodiments, for the preparation of mRNA according to the invention, a DNA template is transcribed in vitro A suitable DNA template typically has a promoter, for example a T3, T7 or SP6 promoter, for in vitro transcription, followed by desired nucleotide sequence for desired mRNA and a termination signal.
[0123] Desired mRNA sequence(s) according to the invention may be determined and incorporated into a DNA template using standard methods. For example, starting from a desired amino acid sequence {e.g., an enzyme sequence), a virtual reverse translation is carried out based on the degenerated genetic code. Optimization algorithms may then be used for selection of suitable codons. Typically, the G/C content can be optimized to achieve the highest possible G/C content on one hand, taking into the best possible account the frequency of the tRNAs according to codon usage on the other hand. The optimized RNA sequence can be established and displayed, for example, with the aid of an appropriate display device and compared with the original (wild- type) sequence. A secondary structure can also be analyzed to calculate stabilizing and destabilizing properties or, respectively, regions of the RNA.
Modified
[0124] in some embodiments, mRNA according to the present invention may be synthesized as unmodified or modified mRNA, Modified mRNA comprise nucleotide modifications in the RNA.
A modified mRNA according to the invention can thus include nucleotide modification that are, for example, backbone modifications, sugar modifications or base modifications. In some embodiments, mRNAs may be synthesized from naturally occurring nucleotides and/or nucleotide analogues (modified nucleotides) including, but not limited to, purines (adenine (A), guanine (G)) or pyrimidines (thymine (T), cytosine (C), uracil (U)), and as modified nucleotides analogues or derivatives of purines and pyrimidines, such as e.g., 1-methyl-adenine, 2-methyl- adenine, 2-methyithio-N-6-isopentenyi-adenine, N6-methyi-adenine, N6-isopentenyl-adenine, 2- thio-cytosine, 3-methyi-cyiosine, 4-acetyl-cytosine, 5-methyl-cytosine, 2,6-diaminapurine, 1- methyi-guanine, 2-meihyl-guanlne, 2,2-dimethyl-guanine, 7-methyl-guanine, inosine, 1-methyl- inosine, pseudouracil (5-uracil), dihydro-uracil, 2-th io-uracil, 4-thio- racii, 5- carboxymethylaminomethyl-2-thio-uracil, 5-(carboxyhydroxymethyl)-uracil, 5-fluoro-uracil, 5-
bromo-uracil, 5-carboxymethylaminomethyl-uracil, 5-methyl-2-th!o-uracil, 5-methyl-uracil, N- uracil-5-oxyacetic acid methyl ester, 5-methylaminomethyl-uracil, 5-methoxyaminomethyl-2- thio-uracil, 5'-methoxycarbonylmethyl-uracil, 5-methoxy-uradl, uracil-5-oxyacetlc acid methyl ester, uracil-5-oxyacetic add (v), 1-methyl-pseudouracil, queuosine, beta-D-mannosyl- queuoslne, wybutoxosine, and phosphoramidates, phosphorothloates, peptide nucleotides, methylphosphonates, 7-deazaguanosine, 5-methylcytosine and inosine. The preparation of such analogues is known to a person skilled in the art e.g., from the U.S. Pat. No, 4,373,071, U.S. Pat. No. 4,401,796, U.S. Pat. No. 4,415,732, U.S. Pat, No. 4,458,066, U.S, Pat. No. 4,500,707, U.S. Pat. No. 4,668,777, U.S. Pat. No. 4,973,679, U.S. Pat. No. 5,047,524, U.S. Pat. No. 5,132,418, U.S. Pat. No. 5,153,319, U.S. Pat. Nos. 5,262,530 and 5,700,642, the disclosures of which are incorporated by reference in their entirety.
[0125] in some embodiments, mRNAs may contain RNA backbone modifications. Typically, a
backbone modification is a modification in which the phosphates of the backbone of the nucleotides contained in the RNA are modified ehemiealiy. Exemplary backbone modifications typically include, but are not limited to, modifications from the group consisting of
methylphosphonates, methylphosphoramidates, phosphoramidates, phosphorothioates (e.g., cytidine 5'-0-(l-thiophosphate)), boranophosphates, positively charged guanidlnlum groups etc., which means by replacing the phosphodiester linkage by other anionic, cationic or neutral groups.
[0126] In some embodiments, RNAs may contain sugar modifications. A typical sugar
modification is a chemical modification of the sugar of the nucleotides it contains including, but not limited to, sugar modifications chosen from the group consisting of 4'-thio-ribonucleotide (see, e.g., US Patent Application Publication No. US 2016/0031928, incorporated by reference herein), 2'-deoxy-2'-fluoro-oligoribonucleotide (2'-fluoro-2'-deoxycytidine 5'-triphosphate, 2'- fluoro-2'-deoxyuridine 5'-triphosphate), 2'-deoxy-2'-deamine-oligoribonucleotide (2'-amino-2'- deoxycytidine 5'-triphosphate, 2'-amino-2'-deoxyuridine S'-triphosphaie), 2'-0- alkyloligorlbonucieotide, 2'-deoxy-2!-C-alkyioiigoribonudeotide (2'-0-methylcytidine 5'- triphosphate, 2'-methyluridine 5'-triphosphate), 2'-C-alkyloligoribonucleotide, and isomers thereof (2'-aracytidine 5'-triphosphate, 2'-arauridine 5'-triphosphate), or azidotriphosphates (2'- azido-2'-deoxycytidine 5'-triphosphate, 2'-azido-2'-deoxyuridine 5'-triphosphate).
[0127] in some embodiments, mRNAs may contain modifications of the bases of the nucleotides (base modifications). A modified nucleotide which contains a base modification is also called a base-modified nucleotide. Examples of such base-modified nucleotides include, but are not limited to, 2-amino-6-chloropurine riboside S'-triphosphate, 2-aminoadenosine S'-triphosphate,
2-thiocytidine 5'-triphosphate, 2-thiouridine 5'-triphosphate, 4-thiouridjne 5'-triphosphate, 5- aminoallylcytidine 5'-triphosphate, 5-aminoally!uridine 5'-triphosphate, 5-bromocytidine 5'- triphosphate, 5-bromouridine S'-triphosphate, 5-iodocytidine S'-triphosphate, 5-iodouridine S'- triphosphate, 5-methylcytidine S'-triphosphate, S-methyluridine 5'-triphosphate, 6-azacytidine S'-triphosphate, 6-azauridine S'-triphosphate, 6-chloropurine riboside S'-triphosphate, 7- deazaadenosine S'-triphosphaie, 7-deazaguanosine 5'-triphosphate, 8-azaadenosine 5'- triphosphate, 8-azidoadenosine 5'-triphosphate, benzimidazole riboside 5'-triphosphate, Nl- methy!adenosine 5'-triphosphate, Nl-methylguanosine 5'-triphosphate, N6-methyladenosine S'- triphosphaie, 06-methylguanosine S'-triphosphate, pseudouridine 5'-triphosphate, puromycin S'-triphosphaie or xanthosine S'-triphosphate.
[0128] Typically, mRNA synthesis includes the addition of a“cap" on the N-terminal (5') end, and a "tail" on the C-terminai (3') end. The presence of the cap is important in providing resistance to nucleases found in most eukaryotic cells. The presence of a "tali" serves to protect the mRNA from exonuclease degradation.
[0129] Thus, in some embodiments, mRNAs include a 5' cap structure. A 5' cap is typically added as follows: first, an RNA terminal phosphatase removes one of the terminal phosphate groups from the 5' nucleotide, leaving two terminal phosphates; guanosine triphosphate (GTP) is then added to the terminal phosphates via a guanyly! transferase, producing a 5'5'5 triphosphate linkage; and the 7-nitrogen of guanine is then methylated by a methyltransferase. Examples of cap structures include, but are not limited to, m7G(5')ppp (5'(A,G(5')ppp(5')A and G(5')ppp{5')G.
[0130] In some embodiments, mRNAs include a 3' poly(A) tail structure. A poly-A tail on the 3' terminus of mRNA typically inciudes about 10 to 300 adenosine nucleotides (e.g., about 10 to 200 adenosine nucleotides, about 10 to 150 adenosine nucleotides, about 10 to 100 adenosine nucleotides, about 20 to 70 adenosine nucleotides, or about 20 to 60 adenosine nucleotides). In some embodiments, mRNAs include a 3' poly(C) tail structure. A suitable po!y-C tail on the 3’ terminus of mRNA typically include about 10 to 200 cytosine nucleotides {e.g., about 10 to 150 cytosine nucleotides, about 10 to 100 cytosine nucleotides, about 20 to 70 cytosine nucleotides, about 20 to 60 cytosine nucleotides, or about 10 to 40 cytosine nucleotides). The poly-C tail may be added to the poly-A tail or may substitute the po!y-A tail.
[0131] In some embodiments, mRNAs include a 5' and/or 3' untranslated region. In some
embodiments, a 5' untranslated region includes one or more elements that affect an mRNA's stability or translation, for example, an iron responsive element, in some embodiments, a 5' untranslated region may be between about 50 and 500 nucleotides in length.
[0132] In some embodiments, a 3' untranslated region includes one or more of a polyadenylation signal, a binding site for proteins that affect an mRNA's stability of location in a cell, or one or more binding sites for miRNAs, In some embodiments, a 3' untranslated region may be between 50 and 500 nucleotides in length or longer.
Cap structure
[0133] In some embodiments, in some embodiments, R!MAs (e.g., mRNAs encoding CFTR) include a 5' cap structure. A 5' cap is typically added as follows: first, an RNA terminal phosphatase removes one of the terminal phosphate groups from the 5' nucleotide, leaving two terminal phosphates; guanosine triphosphate (GTP) is then added to the terminal phosphates via a guanylyl transferase, producing a 5'-5' triphosphate linkage; and the 7-nitrogen of guanine is then methylated by a methyltransferase. In some embodiments, the nucleotide forming the cap is further methylated at the 3'position. In some embodiments, the nucleotide directly adjacent to the cap Is further methylated at the 2' position. Examples of cap structures include, but are not limited to, m7G(5')ppp(5')(2'OMeG), m7G(5')ppp(5')(2'OMeA),
m7(3'OMeG)(5')ppp(5')(2OMeG), m7(3'OMeG)(5')ppp(5')(2'OMeA); m7G(5')ppp
(5!{A,6(5')rrr(5')A and G(5')ppp(5')G. In a specific embodiment, the cap structure is m7G(5')ppp(5')(2OMeG).
[0134] Naturally occurring cap structures comprise a 7-methyl guanosine that is linked via a
triphosphate bridge to the 5'-end of the first transcribed nucleotide, resulting in a dinucleotide cap of m;G(5')ppp(5')N, where N is any nucleoside. In vivo, the cap is added enzymatically. The cap is added in the nucleus and is catalyzed by the enzyme guanylyl transferase. The addition of the cap to the 5‘ terminal end of RNA occurs immediately after initiation of transcription. The terminal nucleoside is typically a guanosine, and is in the reverse orientation to all the other nucleotides, i.e., G(5')ppp(5')GpNpNp.
[0135] In some embodiments, the cap for mRNA produced by in vitro transcription is
m7G{5')ppp(5')G, which has been used as the dinucleotide cap in transcription with T7 or SP6 RNA polymerase in vitro to obtain RNAs having a cap structure in their 5'-termini. The prevailing method for the in vitro synthesis of capped RNA employs a pre-formed dinucleotide of the form m''G(5')ppp(5!)G ("m'GpppG") as an Initiator of transcription.
[0136] In some embodiments, a form of a synthetic dinucleotide cap used in in vitro translation experiments is the Anti-Reverse Cap Analog ("ARCA") or modified ARCA, which is generally a modified cap analog In which the 2' or 3' OH group is replaced with -OCH3.
[0137] Additional cap analogs include, but are not limited to, a chemical structures selected from the group consisting of m7GpppG, m'GpppA, m7GpppC; unmethylated cap analogs {e.g., GpppG);
dimethylated cap analog (e.g., m2,7GpppG), trimethylated cap analog (e.g., m2'2,7GpppG), dimethylated symmetrical cap analogs {e.g., m7Gpppm7G), or anti reverse cap analogs ( e.g., ARCA; rn7 '0meGpppG, m72'dGpppG, rrs7’3'0meGpppG, m7,3'dGpppG and their tetraphosphate derivatives) (see, e.g,, Jemieiity, J. ef a!. , "Novel 'anti-reverse' cap analogs with superior translationai properties”, RNA, 9: 1108-1122 (2003)).
[0138] In some embodiments, a suitable cap is a 7-methyi guanylate ("m7G") linked via a
triphosphate bridge to the 5'-end of the first transcribed nucleotide, resulting in
m7G(5')ppp(5')N, where N is any nucleoside. A preferred embodiment of a m;G cap utilized in embodiments of the invention is m7G(5')ppp(5')G.
[0139] In some embodiments, the cap is a CapO structure. CapO structures lack a 2'-0-methyl residue of the ribose attached to bases 1 and 2. in some embodiments, the cap is a Capl structure. Capl structures have a 2'-0-methy! residue at base 2. In some embodiments, the cap is a Cap2 structure. Cap2 structures have a 2'-0-methyl residue attached to both bases 2 and 3,
[0140] A variety of m7G cap analogs are known in the art, many of which are commercially
available. These include the m'GpppG described above, as well as the ARCA 3'-OCH3 and 2'- OCH3 cap analogs {Jemieiity, J. et ai. , RNA, 9: 1108-1122 (2003)). Additional cap analogs for use in embodiments of the invention include N7-benzyiated dinucleoside tetraphosphate analogs (described in Grudzien, E. et al. , RNA, 10: 1479-1487 (2004)), phosphorothioate cap analogs (described in Grudzien-Nogalska, E,, et ai. , RNA, 13: 1745-1755 (2007)), and cap analogs (including biotinylated cap analogs) described in U.S. Patent Nos. 8,093,367 and 8,304,529, incorporated by reference herein.
[0141] Additional cap structures are also described in published US Application No. US
2016/0032356 and U.S. Provisional Application 62/464,327, filed February 27, 2017, which are incorporated herein by reference,
Taii structure
[0142] Typically, the presence of a "tail" serves to protect the mRNA from exonuciease degradation.
The poly A tail is thought to stabilize natural messengers and synthetic sense RNA. Therefore, in certain embodiments a long poly A tail can be added to an RNA molecule thus rendering the RNA more stable. Poly A tails can be added using a variety of art-recognized techniques. For example, long poly A tails can be added to synthetic or in vitro transcribed RNA using poly A polymerase (Yokoe, et ai. Nature Biotechnology. 1996; 14: 1252-1256). A transcription vector can also encode long poly A tails, in addition, poly A tails can be added by transcription directly from PCR products. Poly A may also be ligated to the 3' end of a sense RNA with RNA ligase (see,
e.g., Molecular Cloning A Laboratory Manual, 2nd Ed,, ed, by Sambrook, Fritsch and Maniatis (Cold Spring Harbor Laboratory Press: 1991 edition)).
[0143] in some embodiments, mRNAs include a 3' poly(A) tail structure. Typically, the length of the poly A tail can be at least about 10, 50, 100, 200, 300, 400 at least 500 nucleotides, in some embodiments, a poly-A tail on the 3' terminus of mRNA typically includes about 10 to 300 adenosine nucleotides {e.g., about 10 to 200 adenosine nucleotides, about 10 to 150 adenosine nucleotides, about 10 to 100 adenosine nucleotides, about 20 to 70 adenosine nucleotides, or about 20 to 60 adenosine nucleotides), in some embodiments, RNAs include a 3' poly(C) tail structure. A suitable poiy-C tall on the 3' terminus of mRNA typically include about 10 to 200 cytosine nucleotides (e.g., about 10 to 150 cytosine nucleotides, about 10 to 100 cytosine nucleotides, about 20 to 70 cytosine nucleotides, about 20 to 60 cytosine nucleotides, or about 10 to 40 cytosine nucleotides). The poly-C tail may be added to the poly-A tail or may substitute the po!y-A tail.
[0144] In some embodiments, the length of the poly A or poly C tail is adjusted to control the
stability of a modified sense mRNA molecule of the invention and, thus, the transcription of protein. For example, since the length of the poly A tail can influence the half-life of a sense mRNA molecule, the length of the poly A tail can be adjusted to modify the level of resistance of the mRNA to nucleases and thereby control the time course of polynucleotide expression and/or polypeptide production in a target cell.
5' and 3 ' Untranslated Region
[0145] In some embodiments, mRNAs include a 5' and/or 3' untranslated region. In some
embodiments, a 5' untranslated region includes one or more elements that affect an mRNA's stability or translation, for example, an iron responsive element. In some embodiments, a 5' untranslated region may be between about 50 and 500 nucleotides in length.
[0146] In some embodiments, a 3' untranslated region includes one or more of a polyadenyiation signal, a binding site for proteins that affect an mRNA's stability of iocaiion in a cell, or one or more binding sites for miRNAs. in some embodiments, a 3' untranslated region may be between 50 and 500 nucleotides in length or longer.
[0147] Exemplary 3' and/or 5' UTR sequences can be derived from mRNA molecules which are stable (e.g., globin, actin, GAPDH, tubulin, histone, or citric acid cycle enzymes) to increase the stability of the sense mRNA molecule. For example, a 5' UTR sequence may include a partial sequence of a CMV immediate-early 1 (IE1) gene, or a fragment thereof to improve the nuclease resistance and/or improve the haif-life of the polynucleotide. Also contemplated is the inclusion of a sequence encoding human growth hormone (hGH), or a fragment thereof to the 3' end or
untranslated region of the polynucleotide {e.g., mRIMA) to further stabilize the polynucleotide. Generally, these modifications improve the stability and/or pharmacokinetic properties {e.g., half-life) of the polynucleotide relative to their unmodified counterparts, and include, for example modifications made to improve such polynucleotides' resistance to in vivo nuclease digestion.
Pharmaceutical Formulations of Cationic Lipids and Nudeic Adds
[0148] in certain embodiments, the compounds described herein (e.g., a compound of Formula (A), such as Formulae (I) or (li) or any one of Compounds 1-12), as well as pharmaceutical and liposomal compositions comprising such lipids, can be used in formulations to facilitate the delivery of encapsulated materials {e.g., one or more polynucleotides such as mRNA) to, and subsequent transfection of one or more target ceils. For example, in certain embodiments cationic lipids described herein (and compositions such as liposomal compositions comprising such lipids) are characterized as resulting in one or more of receptor-mediated endocytosis, clathrin-mediated and caveolae-mediated endocytosis, phagocytosis and macropinocytosis, fusogenicity, endosomai or lysosomal disruption and/or releasable properties that afford such compounds advantages relative other similarly classified lipids.
[0149] According to the present invention, a nucleic acid, e.g., mRNA encoding a protein {e.g., a full length, fragment or portion of a protein) as described herein may be delivered via a delivery vehicle comprising a compound as described herein {e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12).
[0150] As used herein, the terms "delivery vehicle," "transfer vehicle," "nanoparticle," or
grammatical equivalents thereof, are used interchangeably.
[0151] For example, the present invention provides a composition (e.g., a pharmaceutical
composition) comprising a compound described herein (e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) and one or more polynucleotides. A composition (e.g., a pharmaceutical composition) may further comprise one or more cationic lipids, one or more non-cationic lipids, one or more cholesterol-based lipids and/or one or more PEG-modified lipids.
[0152] In certain embodiments a composition exhibits an enhanced (e.g., increased) ability to transfect one or more target cells. Accordingly, also provided herein are methods of transfecting one or more target ceils. Such methods generally comprise the step of contacting the one or more target cells with the cationic lipids and/or pharmaceutical compositions disclosed herein (e.g., a liposomal formulation comprising a compound described herein (e.g., a compound of Formula (A), such as Formulae (!) or (li) or any one of Compounds 1-12) encapsulating one or
more polynucleotides) such that the one or more target cells are transfected with the materials encapsulated therein {e.g., one or more polynucleotides). As used herein, the terms "transfect" or "transfection'' refer to the intracellular introduction of one or more encapsulated materials [e.g., nucleic acids and/or polynucleotides) into a cell, or preferably into a target ceil. The introduced polynucleotide may be stably or transiently maintained in the target ceil. The term “transfection efficiency" refers to the relative amount of such encapsulated material [e.g., polynucleotides) up-taken by, introduced into, and/or expressed by the target cell which is subject to transfection. In practice, transfection efficiency may be estimated by the amount of a reporter polynucleotide product produced by the target ceils following transfection, in certain embodiments, the compounds and pharmaceutical compositions described herein demonstrate high transfection efficiencies thereby improving the likelihood that appropriate dosages of the encapsulated materials {e.g., one or more polynucleotides) will be delivered to the site of pathology and subsequently expressed, while at the same time minimizing potential systemic adverse effects or toxicity associated with the compound or their encapsulated contents.
[0153] Following transfection of one or more target cells by, for example, the polynucleotides encapsulated in the one or more lipid nanoparticies comprising the pharmaceutical or liposomal compositions disclosed herein, the production of the product {e.g., a polypeptide or protein) encoded by such polynucleotide may be preferably stimulated and the capability of such target cells to express the polynucleotide and produce, for example, a polypeptide or protein of interest is enhanced. For example, transfection of a target cell by one or more compounds or pharmaceutical compositions encapsulating mRNA will enhance (i.e., increase) the production of the protein or enzyme encoded by such mRNA.
[0154] Further, delivery vehicles described herein {e.g., liposomal delivery vehicles) may be
prepared to preferentially distribute to other target tissues, cells or organs, such as the heart, lungs, kidneys, spleen, in embodiments, the lipid nanoparticies of the present invention may be prepared to achieve enhanced delivery to the target ceils and tissues. For example, polynucleotides (e.g., mRNA) encapsulated in one or more of the compounds or pharmaceutical and liposomal compositions described herein can be delivered to and/or transfect targeted cells or tissues, in some embodiments, the encapsulated polynucleotides (e.g., mRNA) are capable of being expressed and functional polypeptide products produced (and in some instances excreted) by the target cell, thereby conferring a beneficial property to, for example the target cells or tissues. Such encapsulated polynucleotides (e.g., mRNA) may encode, for example, a hormone, enzyme, receptor, polypeptide, peptide or other protein of interest.
Lipo omal Delivery Veh ides
[0155] In some embodiments, a composition is a suitable delivery vehicle, in embodiments, a composition is a liposomal delivery vehicle, e.g., a lipid nanoparticle.
[0156] The terms "liposomal delivery vehicle" and "liposomal composition" are used
interchangeably.
[0157] Enriching liposomal compositions with one or more of the cationic lipids disclosed herein may be used as a means of improving (e.g., reducing) the toxicity or otherwise conferring one or more desired properties to such enriched liposomal composition (e.g., improved delivery of the encapsulated polynucleotides to one or more target cells and/or reduced In vivo toxicity of a liposomal composition). Accordingly, also contemplated are pharmaceutical compositions, and in particular liposomal compositions, that comprise one or more of the cationic lipids disclosed herein.
[0158] Thus, in certain embodiments, the compounds described herein (e.g., a compound of
Formula (A), such as Formulae (!) or (II) or any one of Compounds 1-12) may be used as a component of a liposomal composition to facilitate or enhance the delivery and release of encapsulated materials (e.g., one or more therapeutic agents) to one or more target cells (e.g., by permea ting or fusing with the lipid membranes of such target ceils).
[0159] As used herein, liposomal delivery vehicles, e.g., lipid nanoparticles, are usually
characterized as microscopic vesicles having an interior aqua space sequestered from an outer medium by a membrane of one or more bilayers. Bilayer membranes of liposomes are typically formed by amphiphilic molecules, such as lipids of synthetic or natural origin that comprise spatially separated hydrophilic and hydrophobic domains (Lasic, Trends Biotechnoi., 16: 307-321, 1998), Bilayer membranes of the liposomes can also be formed by amphophilic polymers and surfactants (e.g., polymerosomes, niosomes, etc.), in the context of the present invention, a liposomal delivery vehicle typically serves to transport a desired mRNA to a target cell or tissue.
[0160] In certain embodiments, such compositions (e.g., liposomal compositions) are loaded with or otherwise encapsulate materials, such as for example, one or more biological!y-active polynucleotides (e.g., mRIMA).
[0161] In embodiments, a composition (e.g., a pharmaceutical composition) comprises an mRNA encoding a protein, encapsulated within a liposome in embodiments, a liposome comprises one or more cationic lipids, one or more non-cationic lipids, one or more cholesterol-based lipids and one or more PEG-modified lipids, and wherein at least one PEG-modified lipid is a compound as described herein (e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12). In embodiments, a composition comprises an mRNA encoding for
a protein (e.g., any protein described herein), in embodiments, a composition comprises an mRNA encoding for cystic fibrosis transmembrane conductance regulator (CFTR) protein, in embodiments, a composition comprises an mRNA encoding for ornithine transcarbamyiase (OTC) protein.
[0162] in embodiments, a composition (e.g., a pharmaceutical composition) comprises a nucleic acid encapsulated within a liposome, wherein the liposome comprises any compound described herein (e.g., a compound of Formula (A), such as Formulae (!) or {II) or any one of Compounds 1- 12) as described herein,
[0163] in embodiments, a nucleic acid is an mRNA encoding a peptide or protein. In embodiments, an mRNA encodes a peptide or protein for use in the delivery to or treatment of the lung of a subject or a lung ceil (e.g., an mRNA encodes cystic fibrosis transmembrane conductance regulator (CFTR) protein). In embodiments, an mRNA encodes a peptide or protein for use in the delivery to or treatment of the liver of a subject or a liver cell (e.g., an mRNA encodes ornithine transcarbamyiase (OTC) protein). Still other exemplary mRNAs are described herein.
[0164] In embodiments, a liposomal delivery vehicle (e.g., a lipid nanoparticle) can have a net positive charge.
[0165] in embodiments, a liposomal delivery vehicle (e.g., a lipid nanoparticle) can have a net negative charge.
[0166] In embodiments, a liposomal delivery vehicle (e.g., a lipid nanoparticle) can have a net neutral charge.
[0167] In embodiments, a lipid nanoparticle that encapsulates a nucleic acid (e.g., mRNA encoding a peptide or protein) comprises one or more compounds described herein ({e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12).
[0168] For example, the amount of a compound as described herein (e.g., a compound of Formula (A), such as Formulae (I) or (I!) or any one of Compounds 1-12) in a composition can be described as a percentage ("wt%") of the combined dry weight of all lipids of a composition (e.g., the combined dry weight of all lipids present in a liposomal composition).
[0169] In embodiments of the pharmaceutical compositions described herein, a compound as described herein (e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) is present in an amount that is about 0.5 wt% to about 30 wt% (e.g., about 0.5 wt% to about 20 wt%) of the combined dry weight of all lipids present in a composition (e.g., a liposomal composition).
[0170] In embodiments, a compound as described herein (e.g., a compound of Formula (A), such as Formulae (i) or (II) or any one of Compounds 1-12) is present in an amount that Is about 1 wt%
to about 30 wt%, about 1 wt% to about 20 wt%, about 1 wt% to about 15 wt%, about 1 wt% to about 10 wt%, or about 5 wt% to about 25 wt% of the combined dry weight of a!i lipids presen t in a composition (e.g., a liposomal composition), in embodiments, a compound as described herein (e.g., a compound of Formula (A), such as Formulae (i) or (II) or any one of Compounds 1- 12) is present in an amount that is about 0.5 wt% to about 5 wt%, about 1 wt% to about 10 wt%, about 5 wt% to about 20 wt%, or about 10 wt% to about 20 wt% of the combined molar amounts of all lipids present in a composition such as a liposomal delivery vehicle.
[0171] in embodiments, the amount of a compound as described herein {e.g., a compound of Formula (A), such as Formulae (!) or (II) or any one of Compounds 1-12) is present in an amount that is at least about 5 wt%, about 10 wt%, about 15 wt%, about 20 wt%, about 25 wt%, about 30 wt%, about 35 wt%, about 40 wt%, about 45 wt%, about 50 wt%, about 55 wt%, about 60 wt%, about 65 wt%, about 70 wt%, about 75 wt%, about 80 wt%, about 85 wt%, about 90 wt%, about 95 wt%, about 96 wt%, about 97 wt%, about 98 wt%, or about 99 wt% of the combined dry weight of total lipids in a composition {e.g., a liposomal composition).
[0172] In embodiments, the amount of a compound as described herein {{e.g., a compound of Formula (A), such as Formulae (!) or (II) or any one of Compounds 1-12) is present in an amount that is no more than about 5 wt%, about 10 wt%, about 15 wt%, about 20 wt%, about 25 wt%, about 30 wt%, about 35 wt%, about 40 wt%, about 45 wt%, about 50 wt%, about 55 wt%, about 60 wt%, about 65 wt%, about 70 wt%, about 75 wt%, about 80 wt%, about 85 wt%, about 90 wt%, about 95 wt%, about 96 wt%, about 97 wi%, about 98 wt%, or about 99 wt% of the combined dry weight of total lipids in a composition {e.g., a liposomal composition).
[0173] in embodiments, a composition {e.g., a liposomal delivery vehicle such as a lipid
nanoparticle) comprises about 0.1 wt% to about 20 wt% (e.g., about 0.1 wt% to about 15 wt%) of a compound described herein (e.g., a compound of Formula (A), such as Formulae (I) or (!l) or any one of Compounds 1-12). in embodiments, a delivery vehicle {e.g., a liposomal delivery vehicle such as a lipid nanoparticle) comprises about 0.5 wi%, about 1 wt%, about 3 wt%, about 5 wt%, or about 10 wt% of a compound described herein {e.g., a compound of Formula (A), such as Formulae (!) or (II) or any one of Compounds 1-12). in embodiments, a delivery vehicle {e.g., a liposomal delivery vehicle such as a lipid nanoparticle) comprises up to about 0,5 wt%, about 1 wt%, about 3 wt%, about 5 wt%, about 10 wt%, about 15 wt%, or about 20 wt% of a compound described herein {e.g., a compound of Formula (A), such as Formulae (i) or (I!) or any one of Compounds 1-12). In embodiments, the percentage results in an improved beneficial effect {e.g., improved delivery to targeted tissues such as the liver or the lung).
[0174] The amount of a compound as described herein {e.g., a compound of Formula (A), such as Formulae (!) or (II) or any one of Compounds 1-12) in a composition also can be described as a percentage ("mol%") of the combined molar amounts of total lipids of a composition {e.g., the combined molar amounts of all lipids present in a liposomal delivery vehicle).
[0175] In embodiments of pharmaceutical compositions described herein, a compound as
described herein {e.g., a compound of Formula (A), such as Formulae (I) or (I!) or any one of Compounds 1-12) Is present In an amount that is about 0.5 mol% to about 30 mol% {e.g., about 0.5 mo!% to about 20 mo!%) of the combined molar amounts of all lipids present in a composition such as a liposomal delivery vehicle.
[0176] In embodiments, a compound as described herein {e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) is present in an amount that is about 0.5 mol% to about 5 mol%, about 1 moi% to about 10 moi%, about 5 moi% to about 20 moi%, or about 10 mol% to about 20 mol% of the combined molar amounts of all lipids present In a composition such as a liposomal delivery vehicle. In embodiments, a compound as described herein {e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1- 12) is present in an amount that is about 1 mol% to about 30 mol%, about 1 mol% to about 20 mo!%, about .1 mo!% to about 15 mol%, about .1 mol% to about 10 mol%, or about 5 moi% to about 25 mol% of the combined dry weight of ail lipids present in a composition such as a liposomal delivery vehicle
[0177] In certain embodiments, a compound as described herein (e.g., a compound of Formula (A), such as Formulae (I) or (!i) or any one of Compounds 1-12) can comprise from about 0.1 mo!% to about 50 moi%, or from 0.5 mo!% to about 50 mo!%, or from about 1 mol% to about 25 mol%, or from abou t 1 mol% to about 10 mol% of the total amount of lipids in a composition (e.g., a liposomal delivery vehicle).
[0178] In certain embodiments, a compound as described herein (e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) can comprise greater than about 0.1 mol%, or greater than about 0.5 mol%, or greater than about .1 mol%, or greater than about 5 mol% of the total amount of lipids in the lipid nanoparticle.
[0179] In certain embodiments, a compound as described {e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) can comprise less than about 25 mol%, or less than about 10 moi%, or less than about 5 mol%, or less than about 1 mo!% of the total amount of lipids in a composition {e.g., a liposomal delivery vehicle).
[0180] In embodiments, the amount of a compound as described herein {e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) is present in an amount
that is at least about 5 ma!%, about 10 mol%, about 15 moi%, about 20 mol%, about 25 mol%, about 30 mof%, about 35 mol%, about 40 mo!%, about 45 mol%, about 50 mol%, about 55 mol%, about 60 mol%, about 65 mol%, about 70 mo!%, about 75 mol%, about 80 mol%, about 85 mol%, about 90 mol%, about 95 mol%, about 96 mol%, about 97 mol%, about 98 mol%, or about 99 mol% of the combined dry weight of total iipids in a composition (e.g., a liposomal composition).
[0181] in embodiments, the amount of a compound as described herein {e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) is present in an amount that is no more than about 5 moi%, about 10 mol%, about 15 mol%, about 20 moi%, about 25 mol%, about 30 moi%, about 35 mo!%, about 40 mol%, about 45 moi%, about 50 mol%, about 55 mol%, about 60 moi%, about 65 mo!%, about 70 mol%, about 75 moi%, about 80 mol%, about 85 mol%, about 90 mo!%, about 95 mol%, about 96 mol%, about 97 mol%, about 98 mol%, or about 99 mol% of the combined dry weight of total lipids in a composition {e.g., a liposomal composition).
[0182] In embodiments, the percentage results in an improved beneficial effect {e.g., improved delivery to targeted tissues such as the liver or the lung).
[0183] in embodiments, a composition further comprises one more iipids {e.g., one more iipids selected from the group consisting of one or more cationic Iipids, one or more non-cationic iipids, one or more cholesterol-based Iipids, and one or more PEG-modified Iipids).
[0184] In certain embodiments, such pharmaceutical {e.g., liposomal) compositions comprise one or more of a PEG-modified lipid, a non-cationic lipid and a cholesterol lipid. In embodiments, such pharmaceutical {e.g., liposomal) compositions comprise: one or more PEG-modified Iipids; one or more non-cationic Iipids; and one or more cholesterol lipids, in embodiments, such pharmaceutical (e.g., liposomal) compositions comprise: one or more PEG-modified Iipids and one or more cholesterol Iipids.
[0185] In embodiments, a composition (e.g., lipid nanoparticie) that encapsulates a nucleic acid {e.g., rnRNA encoding a peptide or protein) comprises one or more compounds as described herein {e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1- 12) and one or more lipids selected from the group consisting of a cationic lipid, a non-cationic lipid, and a PEGyiated lipid.
[0186] In embodiments, a composition {e.g., lipid nanoparticie) that encapsulates a nucleic acid {e.g., mRNA encoding a peptide or protein) comprises one or more compound as described herein {e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds .1-
12); one or more lipids selected from the group consisting of a cationic lipid, a non-cationic lipid, and a PEGylated lipid; and further comprises a cholesterol- based lipid,
[0187] in embodiments, a lipid nanoparticle that encapsulates a nucleic acid (e.g., RNA encoding a peptide or protein) comprises one or more compound as described herein ({e.g., a compound of Formula (A), such as Formulae (!) or (II) or any one of Compounds 1-12), as well as one or more lipids selected from the group consisting of a cationic lipid, a non-cationic lipid, a PEGylated lipid, and a cholesterol-based lipid.
[0188] According to various embodiments, the selection of cationic lipids, non-cationic lipids and/or PEG-modified lipids which comprise the lipid nanoparticle, as well as the relative molar ratio of such lipids to each other, is based upon the characteristics of the selected lipid(s), the nature of the intended target ceils, the characteristics of the mRNA to be delivered. Additional considerations include, for example, the saturation of the alkyl chain, as well as the size, charge, pH, pKa, fusogenicity and toxicity of the selected iipid(s). Thus, the molar ratios may be adjusted accordingly.
Cationic Lipids
[0189] In addition to any of the compounds as described herein (e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12), a composition may comprise one or more additional cationic lipids,
[0190] In some embodiments, liposomes may comprise one or more additional cationic lipids. As used herein, the phrase "cationic lipid" refers to any of a number of lipid species that have a net positive charge at a selected pH, such as physiological pH. Several cationic lipids have been described in the literature, many of which are commercially available,
[0191] Suitable additional cationic lipids for use in the compositions include the cationic lipids as described in International Patent Publication WO 2010/144740, which is incorporated herein by reference. In certain embodiments, the compositions include a cationic lipid, (6Z,9Z,28Z,31Z)- heptatriaconta-6,9,28,31-tetraen-19-yl 4-(dimethylamino) butanoate, having a compound structure of:
and pharmaceutically acceptable salts thereof.
[0192] Other suitable additional cationic lipids for use in the compositions include ionizable cationic lipids as described in international Patent Publication WO 2013/149140, which is incorporated herein by reference. In some embodiments, the compositions include a cationic lipid of one of the following formulas:
or a pharmaceutically acceptable salt thereof, wherein Ri and R2 are each Independently selected from the group consisting of hydrogen, an optionally substituted, variably saturated or unsaturated
alkyl and an optionally substituted, variably saturated or unsaturated C6-C20 acyl; wherein U and l.2 are each independently selected from the group consisting of hydrogen, an optionally substituted
alkyl, an optionally substituted variably unsaturated
alkenyl, and an optionally substituted
alkynyl; wherein m and o are each independently selected from the group consisting of zero and any positive integer [e.g., where m is three); and wherein n is zero or any positive integer {e.g., where n is one). In certain embodiments, the compositions include the cationic lipid (15Z, 18Z)-N,N-dimethyl-6-(9Z,12Z)-octadeca-9,12-dien-l - yi) tetracosa- 15,18-dien-l-amine ("HGT5000"), having a compound structure of:
and pharmaceutically acceptable salts thereof, in certain embodiments, the compositions include the cationic lipid (15Z, 18Z)-N,N-dimethyl-6-((9Z,12Z)-octadeca-9,12-dien-l-yl) tetracosa·· 4,15,18-trien-! -amine ("HGT5001"), having a compound structure of:
and pharmaceutically acceptable salts thereof. In certain embodiments, the include the cationic lipid and (15Z,18Z)-N,N-dimethyi-6-((9Z,12Z)-octadeca-9,12-dien-l-yi) tetracosa-5,15,18-trien- 1 -amine ("HGT5002"), having a compound structure of:
and pharmaceutically acceptable salts thereof.
[0193] Other suitable additional cationic lipids for use in the compositions include cationic lipids described as aminoalcohol lipidoids in International Patent Publication WO 2010/053572, which
is incorporated herein by reference. In certain embodiments, the compositions include a cationic lipid having a compound structure of:
and pharmaceutically acceptable salts thereof.
[0194] Other suitable additional cationic lipids for use in the compositions include the cationic lipids as described in International Patent Publication WO 2016/118725, which is incorporated herein by reference, in certain embodiments, the compositions include a cationic lipid having a compound structure of:
and pharmaceutically acceptable salts thereof.
[0195] Other suitable additional cationic lipids for use in the compositions include the cationic lipids as described in International Patent Publication WO 2016/118724, which is incorporated herein by reference. In certain embodiments, the compositions include a cationic lipid having a compound structure of:
and pharmaceutically acceptable salts thereof.
[0196] Other suitable cationic lipids for use in the compositions include a cationic lipid having the formula of 14,25-ditridecyl 15, 18,21, 24-tetraaza-octatriacontane, and pharmaceutically acceptable salts thereof.
[0197] Other suitable additional cationic lipids for use in the compositions include the cationic lipids as described In International Patent Publications WO 2013/063468 and WO 2016/205691, each of which are incorporated herein by reference, in some embodiments, the compositions include a cationic lipid of the following formula:
or pharmaceutically acceptable salts thereof, wherein each instance of RL is independently optionally substituted Ce-C® alkenyl. In certain embodiments, the compositions include a cationic lipid having a compound structure of:
and pharmaceutically acceptable salts thereof.
[0198] In certain embodiments, the compositions include a cationic lipid having a compound structure of:
and pharmaceutically acceptable salts thereof.
[0199] In certain embodiments, the compositions include a cationic lipid having a compound structure of:
[0200] In certain embodiments, the compositions include a cationic lipid having a compound structure of:
and pharmaceutically acceptable salts thereof,
[0201] Other suitable additional cationic lipids for use in the compositions include the cationic lipids as described in International Patent Publication WO 2015/184256, which is incorporated herein by reference. In some embodiments, the compositions include a cationic lipid of the following formula:
or a pharmaceutically acceptable salt thereof, wherein each X independently is O or S; each Y Independently is O or S; each m independently is 0 to 20; each n independently is 1 to 6; each RA is independently hydrogen, optionally substituted Cl-50 alkyl, optionally substituted C2-50 alkenyl, optionally substituted C2-50 alkynyl, optionally substituted C3-10 carbocyclyl, optionally
substituted 3-14 membered heterocycly!, optionally substituted C6-14 aryi, optionally substituted 5-14 membered heteroaryl or halogen; and each Rs is independently hydrogen, optionally substituted Cl-50 alkyl, optionally substituted C2-50 alkenyl, optionally substituted C2-50 alkynyi, optionally substituted C3-10 carbocyciy!, optionally substituted 3-14 membered heterocydyi, optionally substituted C6-14 aryl, optionally substituted 5-14 membered heteroaryl or halogen, in certain embodiments, the compositions include a cationic lipid, "Target 23", having a compound structure of:
and pharmaceutically acceptable salts thereof.
[0202] Other suitable additional cationic lipids for use in the compositions include the cationic lipids as described in International Patent Publication WO 2016/004202, which is incorporated herein by reference, in some embodiments, the compositions include a cationic lipid having the compound structure:
or a pharmaceutically acceptable salt thereof.
[0203] In some embodiments, the compositions include a cationic lipid having the compound
or a pharmaceutically acceptable salt thereof.
[0204] in some embodiments, the compositions include a cationic lipid having the compound structure:
or a pharmaceutically acceptable salt thereof.
[0205] Other suitable additional cationic lipids for use in the compositions include the cationic lipids as described in J. McClellan, M. C. King, Cell 2010, 141, 210-217 and in Whitehead et a!., Nature Communications (2014) 5:4277, which is incorporated herein by reference, in certain embodiments, the cationic lipids of the compositions include a cationic iipid having a compound structure of:
[0206] Other suitable additional cationic lipids for use in the compositions include the cationic lipids as described in International Patent Publication WO 2015/199952, which is incorporated herein by reference, in some embodiments, the compositions inciude a cationic iipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0207] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0208] in some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0209] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0210] in some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0211] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0212] In some embodiments, the compositions include a cationic lipid having the compound structure:
[0213] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0214] in some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof,
[0215] In some embodiments, the compositions include a cationic lipid having the compound structure:
[0216] and pharmaceutically acceptable salts thereof,
[0217] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0218] In some embodiments, the compositions Include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0219] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0220] Other suitable additional cationic lipids for use In the compositions Include the cationic lipids as described in International Patent Publication WO 2017/004143, which is incorporated herein by reference.
[0221] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0222] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0223] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0224] in some embodiments, the compositions include a cationic lipid having the compound structure:
[0225] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0226] in some embodiments, the compositions include a cationic lipid having the compound structure:
[0227] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof,
[0228] in some embodiments, the compositions include a cationic lipid having the compound structure:
[0229] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0230] In some embodiments, the compositions include a cationic lipid having the compound structure:
[0231] In some embodiments, the compositions include a cationic lipid having the compound structure:
[0232] in some embodiments, the compositions include a cationic lipid having the compound structure:
[0233] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0234] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0235] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0236] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0237] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0238] Other suitable additional cationic lipids for use in the compositions include the cationic lipids as described in International Patent Publication WO 2017/075531, which is incorporated herein
by reference. In some embodiments, the compositions include a cationic lipid of the following formula:
or a pharmaceutically acceptable salt thereof, wherein one of L1 or L2 is -0(C=0)-, -(C=0)0-, - C[=G)-, -0-, -S(0)x, -S-S-, -C(=0)S-, -SC(=0)-, -NRaC(=0)-, -C(=0)NR% NRaC(=0)NRa-, -0C(=0)NRa-, or -NRaC(=0)0-; and the other of L1 or L2 is -0(C=0)-, -(C=0)0-, -C(=0)-, -0-, -S(O) s, -S-S-, -C(=0)S- , SC(=0)-, -NRaC{=:0)-, -C(=0)NR3-, ,NRaC(==0)NR -0C(=O)NRa- or -NRaC(=0)0- or a direct bond; G1 and G2 are each independently unsubstituted C -C alkylene or C -C alkenylene; G;i is C -C alkylene, C-.-C alkenylene, Cg-Cg cycioalkylene, Cg-Cg cycloalkenylene; Ra is H or C -C alkyl; R1 and R are each independently C -C alkyl or C -C alkenyl; R Is H, OR3, CN, -C(=0)0R4, - 0C(=0)R4 or - R5 C(=0)R4; R4 is C Ci2 alkyl; R5 is H or C C6 alkyl; and x is 0, 1 or 2.
[0239] Other suitable additional cationic lipids for use in the compositions include the cationic lipids as described in International Patent Publication WO 2017/117528, which is incorporated herein by reference. In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0240] In some embodiments, the compositions include a cationic lipid having the compound
structure:
and pharmaceutically acceptable salts thereof.
[0241] In some embodiments, the compositions include a cationic lipid having the compound structure:
and pharmaceutically acceptable salts thereof.
[0242] Other suitable additional cationic lipids for use in the compositions include the cationic lipids as described in International Patent Publication WO 2017/049245, which is incorporated herein by reference. In some embodiments, the cationic lipids of the compositions and methods of the present invention include a compound of one of the following formulas:
and pharmaceutically acceptable salts thereof. For any one of these four formulas, R Is Independently selected from -(CH2)nQ and -(CH2) nCHQR; Q is selected from the group consisting of -OR, -OH, ~OiCH2)nN{R)2, -OC{0)R, -CX3, -CN, -N(R)C(0)R, -N(H)C(0)R, - (R)S{0)2R, -N(H)S(0)2R, -N(R)C(0)N(R)2, -N(H)C(0)N(R)2, - (H)C[0)N[H)(R), -N(R)C(S)N(R)2, -N[H)C(S)N(R)2, -
N(H)C(S)N(H)(R), and a heterocycle; R is independently selected from the group consisting of C-.-3 alkyl, C alkenyl, and H; and n is 1, 2, or 3.
[0243] in certain embodiments, the compositions include a cationic lipid having a compound
structure of:
and pharmaceutically acceptable salts thereof.
[0244] in certain embodiments, the compositions include a cationic lipid having a compound
structure of:
and pharmaceutically acceptable salts thereof.
[0245] In certain embodiments, the compositions include a cationic lipid having a compound
structure of:
and pharmaceutically acceptable salts thereof.
[0246] In certain embodiments, the compositions include a cationic lipid having a compound
structure of:
and pharmaceutically acceptable salts thereof.
[0247] Other suitable additional cationic lipids for use in the compositions include the cationic lipids as described in International Patent Publication WO 2017/173054 and WO 2015/095340, each of which is incorporated herein by reference.
[0248] In certain embodiments, the compositions include a cationic lipid having a compound structure of:
and pharmaceutically acceptable salts thereof.
[0249] In certain embodiments, the compositions include a cationic lipid having a compound structure of:
and pharmaceutically acceptable salts thereof.
[0250] In certain embodiments, the compositions include a cationic lipid having a compound structure of:
and pharmaceutically acceptable salts thereof.
[0251] In certain embodiments, the compositions include a cationic lipid having a compound structure of:
and pharmaceutically acceptable salts thereof.
[0252] Other suitable additional cationic lipids for use in the compositions include cholesterol- based cationic lipids, in certain embodiments, the compositions include imidazole cholesterol ester or "ICE", having a compound structure of:
and pharmaceutically acceptable salts thereof.
[0253] Other suitable additional cationic lipids for use in the compositions include deavable
cationic lipids as described in international Patent Publication WO 2012/170889, which is incorporated herein by reference, in some embodiments, the compositions include a cationic lipid of the following formula:
wherein Ri is selected from the group consisting of Imidazole, guanidinium, amino, imine, enamine, an optionally-substituted alkyl amino { e.g ., an alkyl amino such as di ethylamino) and pyridyl; wherein R2 is selected from the group consisting of one of the following two formulas:
and wherein R and R4 are each independently selected from the group consisting of an optionally substituted, variably saturated or unsaturated C6-C2o alkyl and an optionally substituted, variably saturated or unsaturated C6-C2o acyl; and wherein n is zero or any positive integer [e.g., one, two, three, four, five, six, seven, eight, nine, ten, eleven, twelve, thirteen, fourteen, fifteen, sixteen, seventeen, eighteen, nineteen, twenty or more),
[0254] In certain embodiments, the compositions include a cationic lipid, "H6T4001", having a compound structure of:
and pharmaceutically acceptable salts thereof,
[0255] in certain embodiments, the compositions include a cationic lipid, "HGT4002", having a compound structure of:
and pharmaceutically acceptable salts thereof,
[0256] in certain embodiments, the compositions include a cationic lipid, "HGT4003", having a compound structure of:
(HGT4003)
and pharmaceutically acceptable salts thereof.
[0257] In certain embodiments, the compositions include a cationic lipid, "HGT4004", having a compound structure of:
and pharmaceutically acceptable salts thereof.
[0258] In certain embodiments, the compositions Include a cationic lipid "HGT400S", having a compound structure of:
and pharmaceutically acceptable salts thereof,
[0259] in some embodiments, the compositions include the cationic lipid, N-[l-(2,3- dioleyloxy)propyl]-N,N,N-trimethylammonium chloride ("DOTMA"). Feigner et ai. (Proc. Nat'l Acad, Sci, 84, 7413 (1987); U.S. Pat. No. 4,897,355, each of which is incorporated herein by reference. DOTMA can be formulated alone or can be combined with a neutral lipid (e.g., dioieoyiphosphatidyi-ethanoiamlne or "DOPE") or still other cationic or non-cationic lipids into a liposomal transfer vehicle or a lipid nanoparticle, and such liposomes can be used to enhance the delivery of nucleic acids into target cells. Other cationic lipids suitable for the compositions include, for example, 5-carboxyspermylgiycinedioctadecylamide ("DOGS"); 2,3-dioley!oxy-N- [2(spermine-carboxamido)ethyl]-N,N-dimethyl-l-propanaminium ("DOSPA") (Behr et al. Proc. Nat.'l Acad. Sci. 86, 6982 (1989), U.S, Pat. No, 5,171,678; U.S. Pat, No. 5,334,761); 1,2-Dioieoyi 3- Dimethylammonium-Propane ("DO DAP"); l,2-Dioleoyl-3-Trimethylammonium-Propane
("DGTAP").
[0260] Additional exemplary cationic lipids suitable for the compositions also include: 1,2- distearyioxy-N,N-dimethyl-3-aminopropane ( "DSDMA"); l,2-dioleyloxy-N,N-dimethyl-3- aminopropane ("DODMA"); .1 ,2-dllinoleyloxy-N,N-dimethyl-3-aminopropane ("DLinDMA"); 1,2- dilinolenyloxy-N,N-dimethyl-3-aminopropane ("DLenDMA"); N-dioleyl-N,N-dimethylammonium chloride ("DQDAC"); N,N-distearyl-N,N-dimethylarnrnonium bromide ("DDAB"); N-(l,2- dimyrisiyioxyprop-3-yi)-N,N-dimethyl-N-hydroxyethyl ammonium bromide ("DM RI E"); 3- dimethyiamino-2-(choiest-5-en-3-beta-oxybutan-4-oxy)-l-(cis,cis-9,12-octadecadienoxy)propane ("Clin DMA"); 2-[5’-(choiest-5-en-3-beta-oxy)-3‘-oxapentoxy)-3-dimethy i-l-(cis,cis-9', 1-2'- octadecadienoxy)propane ("CpLinDMA"); N,N-dimethyl-3,4-dioleyloxybenzylamine ("D OBA");
1 ,2-N,N'-dioleylcarbamyl-3-dimethylaminopropane ("DOcarbDAP"); 2,3-Dilinoleoyloxy-N,N- dimethylpropylamine ("DLinDAP"); l,2-N,N'-Dilinoleylcarbamyl-3-dimethylaminopropane
("DLincarbDAP"); 1 ,2-Dilinoleoylcarbamyl-3-dimethylaminopropane ("DLinCDAP"); 2,2-dilinoleyl- 4-dimethylaminomethy!-[i,3]-dioxolane ("DLin-K-DMA"); 2-((8-[(3P)-cholest-5-en-3- yloxy]octyl)oxy)-N, N-dimethyl-3-[(9Z, 12Z)-octadeca-9, 12-dien-l -yloxy]propane-l-amine ("Octyl-CLinDMA"); (2R)-2-((8-[(3beta)-cholest-5-en-3-yloxy]octyl)oxy)-N, N-dimethyl-3-[(9Z, 12Z)-octadeca-9, 12-dien-l-yloxy]propan-l -amine ("Octyl-CLinDMA (2R)"); (2S)-2-((8-[(3P)-
cholest-5-en-B-yloxy]octyl)oxy)-N, fsi-dimethyh3-[(9Z, 12Z)-octadeca-9, 12-dien-l -yloxy]propan- 1 -amine ("Octyl-CLinDMA (25)"}; 2,2-dilinoleyl-4-dimethylaminoethyl-[l,3j-dioxolane ("DLin-K- XTC2-DMA"); and 2-(2,2-di((9Z,12Z)-octadeca-9,l 2-dien- l-yi)-i ,3-dioxolan-4-yl)-N,N- dimethylethanamine ("DLin-KC2-DMA") (see, WO 2010/042877, which is incorporated herein by reference; Semple et a!., Nature Biotech. 28: 172-176 (2010)). (Heyes, J„ et al., J Controlled Release 107: 276-287 (2005); Morrissey, DV., et al., Nat. Biotechnol. 23(8): 1003-1007 (2005); international Patent Publication WO 2005/121348). In some embodiments, one or more of the cationic lipids comprise at least one of an imidazole, dialkylamino, or guanidinium moiety.
[0261] In some embodiments, one or more cationic lipids suitable for the compositions include 2,2- Dilinoieyl-4-dimethylaminoethyl-[l,3]-dioxo!ane ("XTC"); (3aR,5s,6aS)-N,N-dimethyl-2,2- di((9Z,12Z)-octadeca-9,12-dienyl)tetrahydro-3aH-cyclopenta[d] [1 ,3]dioxoi-5-amine ("ALNY- 100") and/or 4,7,13-tris(3-oxo-3-(undecylamino)propyl)-Nl,N16-diundecyl-4,7,10,13- tetraazahexadecane- 1,16-diamide ("NC98-5").
[0262] In some embodiments, the compositions include one or more cationic lipids that constitute at least about 5%, 10%, 20%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, or 70%, measured by weight, of the total lipid content in the composition, e.g., a lipid nanoparticle. In some embodiments, the compositions include one or more cationic lipids that constitute at least about 5%, 10%, 20%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, or 70%, measured as a mol%, of the total lipid content in the composition, e.g., a lipid nanoparticle. In some embodiments, the compositions include one or more cationic lipids that constitute about 30-70 % (e.g., about 30- 65%, about 30-60%, about 30-55%, about 30-50%, about 30-45%, about 30-40%, about 35-50%, about 35-45%, or about 35-40%), measured by weight, of the total lipid content in the composition, e.g., a lipid nanoparticle. In some embodiments, the compositions include one or more cationic !ipids that constitute about 30-70 % (e.g,, about 30-65%, about 30-60%, about 30- 55%, about 30-50%, about 30-45%, about 30-40%, about 35-50%, about 35-45%, or about 35- 40%), measured as mol %, of the total lipid content in the composition, e.g., a lipid nanoparticle.
Helper Lipids
[0263] Compositions {e.g., liposomal compositions) may also comprise one or more helper lipids.
Such helper lipids include non-cationic lipids. As used herein, the phrase "non-cationic lipid” refers to any neutral, zwitterionic or anionic lipid. As used herein, the phrase "anionic lipid" refers to any of a number of lipid species that carry a net negative charge at a selected pH, such as physiological pH. Non-cationic lipids include, but are not limited to,
distearoylphosphatidylcholine (DSPC), dio!eoy!phosphatidylcholine (DOPC),
dipaimitoylphosphatidyichoiine (DPPC), dioleoylphosphatidylglycero! (DOPG),
dipa!mitoy!phosphatidy!glycerol (DPPG), dioleoylphosphatidylethanolamine (DOPE),
palmitoyloleoylphosphatidylcholine (POPC), palmitoyloleoyl-phosphatidylethanolamine (POPE), dioleoyl-phosphatidylethanolamine 4-(N-maleimidomethyl)-cyclohexane-l-carboxylate (DOPE- ma!), dipaimitoyi phosphatidyl ethanolamine (DPPE), dimyristoylphosphoethanolamine (DMPE), distearoyl-phosphatidyl-ethanolamine (DSPE), 16-O-monomethyl PE, 16-O-dimethyl PE, 18-1- trans PE, l-stearoyl-2-oleoyl-phosphatidyethanolamine (SORE), or a mixture thereof, in embodiments, a non-cationic or helper iipid is dioleoylphosphatidylethanolamine (DOPE).
[0264] in some embodiments, a non-cationic Iipid is a neutral Iipid, i.e., a Iipid that does not carry a net charge in the conditions under which the composition is formulated and/or administered.
[0265] In some embodiments, a non-cationic iipid may be present in a molar ratio (mol%) of about 5% to about 90%, about 5% to about 70%, about 5% to about 50%, about 5% to about 40%, about 5% to about 30%, about 10 % to about 70%, about 10% to about 50%, or about 10% to about 40% of the total lipids present in a composition. In some embodiments, total non-cationic lipids may be present in a molar ratio (moi%) of about 5% to about 90%, about 5% to about 70%, about 5% to about 50%, about 5% to about 40%, about 5% to about 30%, about 10 % to about 70%, about 10% to about 50%, or about 10% to about 40% of the total lipids present in a composition, in some embodiments, the percentage of non-cationic Iipid in a liposome may be greater than about 5 mol%, greater than about 10 moi%, greater than about 20 mol%, greater than about 30 moi%, or greater than about 40 mo!%. in some embodiments, the percentage total non-cationic lipids in a liposome may be greater than about 5 mol%, greater than about 10 mol%, greater than about 20 mol%, greater than about 30 mo!%, or greater than about 40 mol%. in some embodiments, the percentage of non-cationic Iipid in a liposome is no more than about 5 mo!%, no more than about 10 mof%, no more than about 20 moi%, no more than about 30 moi%, or no more than about 40 mol%. in some embodiments, the percentage total non- cationic lipids in a liposome may be no more than about 5 mo!%, no more than about 10 moi%, no more than about 20 mol%, no more than about 30 moi%, or no more than about 40 mol%.
[0266] in some embodiments, a non-cationic Iipid may be present in a weight ratio (wt%) of about
5% to about 90%, about 5% to about 70%, about 5% to about 50%, about 5% to about 40%, about 5% to about 30%, about 10 % to about 70%, about 10% to about 50%, or about 10% to about 40% of the total lipids present In a composition. In some embodiments, total non-cationic lipids may be present in a weight ratio (wt%) of about 5% to about 90%, about 5% to about 70%, about 5% to about 50%, about 5% to about 40%, about 5% to about 30%, about 10 % to about 70%, about 10% to about 50%, or about 10% to about 40% of the total iipids present in a composition, in some embodiments, the percentage of non -cationic iipid in a liposome may be
greater than about 5 wt%, greater than about 10 wt%, greater than about 20 wt%, greater than about 30 wt%, or greater than about 40 wt%. In some embodiments, the percentage total non- cationic lipids in a liposome may be greater than about 5 wt%, greater than about 10 wt%, greater than about 20 wt%, greater than about 30 wt%, or greater than about 40 wt%. In some embodiments, the percentage of non-cationlc lipid in a liposome is no more than about 5 wt%, no more than about 10 wt%, no more than about 20 wt%, no more than about 30 wi%, or no more than about 40 wt%. In some embodiments, the percentage total non-cationic lipids in a liposome may be no more than about 5 wt%, no more than about 10 wt%, no more than about 20 wt%, no more than about 30 wt%, or no more than about 40 wt%.
Cho!estero!-hased Lipids
[0267] in some embodiments, a composition (e.g., a liposomal composition) comprises one or more cholesterol-based lipids. For example, suitable cholesterol-based lipids include cholesterol and, for example, DC-Chol (N,N-dimethyl-N-ethylcarboxamidocholesterol), l,4-bis(3-N-oleylamino- propyl)piperazine [Gao, et ai. Biochem. Biophys. Res. Comm, 179, 280 (1991); Wolf et a/.
BioTechniques 23, 139 [1997); U.S. Pat. No, 5,744,335), or imidazole cholesterol ester [ICE), which has the following structure,
[0268] in some embodiments, a cholesterol-based lipid may be present in a molar ratio (mol%) of about 1% to about 30%, or about 5% to about 20% of the total lipids present in a liposome. In some embodiments, the percentage of cholesterol-based lipid in the lipid nanoparticle may be greater than about 5 mo!%, greater than about 10 mol%, greater than about 20 mol%, greater than about 30 mol%, or greater than about 40 mo!%. In some embodiments, the percentage of cholesterol-based lipid in the lipid nanoparticle may be no more than about 5 mol%, no more than about 10 mol%, no more than about 20 mol%, no more than about 30 mo!%, or no more than about 40 mol%.
[0269] In some embodiments, a cholesterol-based lipid may be present in a weight ratio (wt%) of about 1% to about 30%, or about 5% to about 20% of the total lipids present in a liposome, in some embodiments, the percentage of cholesterol-based lipid in the lipid nanoparticle may be greater than about 5 wt%, greater than about 10 wt%, greater than about 20 wt%, greater than
about 30 wt%, or greater than about 40 wt%. In some embodiments, the percentage of cholesterol-based lipid in the lipid nanoparticle may be no more than about 5 wt%, no more than about 10 wt%, no more than about 20 wt%, no more than about 30 wt%, or no more than about 40 wt%.
PEGyiated Lipids
[0270] In some embodiments, a composition (e.g., a liposomal composition) comprises one or more further PEGyiated lipids.
[0271] For example, the use of polyethylene glycol (PEG)-modified phospholipids and derivatized lipids such as derivatized ceramides (PEG-CER), including N-octanoyi-sphingosine-1- [succinyi(methoxy polyethylene giycol)-2000] (C8 PEG-2000 ceramide) is also contemplated by the present invention in combination with one or more of compounds described herein (e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) and, in some embodiments, other lipids together which comprise the liposome. In some embodiments, particularly useful exchangeable lipids are PEG-cera ides having shorter acyl chains {e.g., C or C18).
[0272] Contemplated further PEG-modified lipids {also referred to herein as a PEGyiated lipid, which term is interchangeable with PEG-modified lipid) include, but are not limited to, a polyethylene glycol chain of up to 5 kDa in length covalently attached to a lipid with alkyl chain(s) of C6-C2o length. In some embodiments, a PEG-modified or PEGyiated lipid is PEGyiated cholesterol or PEG-2K. The addition of such components may prevent complex aggregation and may also provide a means for increasing circulation lifetime and increasing the delivery of the lipid-nucleic acid composition to the target ceil, (Klibanov et a!. (1990) FEBS Letters, 268 (1): 235-237), or they may be selected to rapidly exchange out of the formulation in vivo {see U.S. Pat. No. 5,885,613).
[0273] Further PEG-modified phospholipid and derivatized lipids of the present invention may be present in a molar ratio (moi%) from about 0% to about 15%, about 0.5% to about 15%, about 1% to about 15%, about 4% to about 10%, or about 2% of the total lipid present in the composition (e.g., a liposomal composition).
[0274] Further PEG-modified phospholipid and derivatized lipids of the present invention may be present in a weight ratio (wt%) from about 0% to about 15%, about 0.5% to about 15%, about 1% to about 15%, about 4% to about 10%, or about 2% of the total lipid present in the composition (e.g., a liposomal composition).
Pharmaceutical Formulations and Therapeutic Uses
[0275] Compounds described herein {e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) may be used in the preparation of compositions (e.g., to construct liposomal compositions) that facilitate or enhance the delivery and release of encapsulated materials {e.g., one or more therapeutic polynucleotides) to one or more target cells {e.g., by permeating or fusing with the lipid membranes of such target cells).
[0276] For example, when a liposomal composition {e.g., a lipid nanoparticle) comprises or is
otherwise enriched with one or more of the compounds disclosed herein, the phase transition in the lipid bilayer of the one or more target ceils may facilitate the delivery of the encapsulated materials {e.g., one or more therapeutic polynucleotides encapsulated In a lipid nanoparticie) into the one or more target cells.
[0277] Similarly, in certain embodiments compounds described herein {e.g., a compound of
Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) may be used to prepare liposomal vehicles that are characterized by their reduced toxicity in vivo, in certain embodiments, the reduced toxicity is a function of the high transfection efficiencies associated with the compositions disclosed herein, such that a reduced quantity of such composition may administered to the subject to achieve a desired therapeutic response or outcome.
[0278] Thus, pharmaceu tical formulations comprising a compound described (e.g., a compound of Formula (A), such as Formulae (I) or (li) or any one of Compounds 1-12) and nucleic adds provided by the present invention may be used for various therapeutic purposes. To facilitate delivery of nucleic acids in vivo, a compound described herein {e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) and nucleic acids can be formulated in combination with one or more additional pharmaceutical carriers, targeting ligands or stabilizing reagents. In some embodiments, a compound described herein (e.g., a compound of Formula (A), such as Formulae (!) or (II) or any one of Compounds 1-12) can be formulated via pre-mixed lipid solution. In other embodiments, a composition comprising a compound described herein {e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) can be formulated using post-insertion techniques into the lipid membrane of the nanoparticles. Techniques for formulation and administration of drugs may be found in "Remington's Pharmaceutical Sciences," Mack Publishing Co., Easton, Pa., latest edition.
[0279] Suitable routes of administration include, for example, oral, rectal, vaginal, transmucosai, pulmonary including intratracheal or inhaled, or intestinal administration; parenteral delivery, including intradermal, transderma! (topical), intramuscular, subcutaneous, intramedullary injections, as well as intrathecal, direct intraventricular, intravenous, intraperitoneal, or
intranasal. In particular embodiments, the intramuscular administration is to a muscle selected from the group consisting of skeletal muscle, smooth muscle and cardiac muscle. In some embodiments the administration results in delivery of the nucleic acids to a muscle cell, in some embodiments the administration results in delivery of the nucleic acids to a hepatocyte (i.e., liver cell),
[0280] Alternatively or additionally, pharmaceutical formulations of the invention may be
administered in a local rather than systemic manner, for example, via injection of the pharmaceutical formulation directly into a targeted tissue, preferably in a sustained release formulation. Local delivery can be affected in various ways, depending on the tissue to be targeted. Exemplary tissues in which delivered mRNA may be delivered and/or expressed include, but are not limited to the liver, kidney, heart, spleen, serum, brain, skeletal muscle, lymph nodes, skin, and/or cerebrospinal fluid. In embodiments, the tissue to be targeted in the liver. For example, aerosols containing compositions of the present invention can be inhaled (for nasal, tracheal, or bronchial delivery); compositions of the present invention can be injected into the site of injury, disease manifestation, or pain, for example; compositions can be provided in lozenges for oral, tracheal, or esophageal application; can be supplied In liquid, tablet or capsule form for administration to the stomach or intestines, can be supplied in suppository form for rectal or vaginal application; or can even be delivered to the eye by use of creams, drops, or even Injection.
[0281] Compositions described herein can comprise RNA encoding peptides including those described herein (e.g., a polypeptide such as a protein).
[0282] in embodiments, a mRNA encodes a polypeptide.
[0283] in embodiments, a mRNA encodes a protein.
[0284] Exemplary peptides encoded by mRNA {e.g., exemplary proteins encoded by mRNA) are described herein.
[0285] The present invention provides methods for delivering a composition having full-length mRNA molecules encoding a peptide or protein of interest for use in the treatment of a subject, e.g., a human subject or a cell of a human subject or a cell that is treated and delivered to a human subject.
[0286] Accordingly, in certain embodiments the present invention provides a method for producing a therapeutic composition comprising full-length mRNA that encodes a peptide or protein for use in the delivery to or treatment of the lung of a subject or a lung cell, in certain embodiments the present invention provides a method for producing a therapeutic composition having full- length mRNA that encodes for cystic fibrosis transmembrane conductance regulator (CFTR)
protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for ATP-binding cassette sub family A member 3 protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for dynein axonemai intermediate chain 1 protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for dynein axonemai heavy chain 5 (DNAH5) protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for alpha-l-antitrypsin protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for forkhead box P3 (FOXP3) protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes one or more surfactant protein, e.g., one or more of surfactant A protein, surfactant B protein, surfactant C protein, and surfactant D protein.
[0287] In certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes a peptide or protein for use in the delivery to or treatment of the liver of a subject or a liver cell. Such peptides and polypeptides can Include those associated with a urea cycle disorder, associated with a lysosomal storage disorder, with a glycogen storage disorder, associated with an amino acid metabolism disorder, associated with a lipid metabolism or fibrotic disorder, associated with methylmalonic acidemia, or associated with any other metabolic disorder for which delivery to or treatment of the liver or a liver ceil with enriched full-length mRNA provides therapeutic benefit,
[0288] In certain embodiments the present invention provides a method for producing a
therapeutic composition having fuil-iength mRNA that encodes for a protein associated with a urea cycle disorder. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for ornithine transcarbamylase (OTC) protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for arginosuccinate synthetase 1 protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for carbamoyl phosphate synthetase I protein in certain embodiments the present invention provides a method for producing a therapeutic composition having fuil-iength mRNA that encodes for arginosuccinate lyase protein. In certain embodiments the present invention
provides a method for producing a therapeutic composition having full-length mRNA that encodes for arginase protein.
[0289] in certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes for a protein associated with a lysosomal storage disorder. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for alpha gaiactosidase protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for
glucocerebrosidase protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for iduronaie-2- sulfatase protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for iduronidase protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for N-acetyi-alpha-D- giucosaminidase protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for heparan N- sulfatase protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length rnRNA that encodes for galactosamine-6 sulfatase protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for beta- gaiactosidase protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for lysosomal lipase protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for arylsulfatase B (N- acetyigaiactosamine-4-sulfatasej protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for transcription factor EB (TFEB).
[0290] In certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes for a protein associated with a glycogen storage disorder. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for acid alpha- giucosidase protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for glucose-6- phosphatase (G6PC) protein. In certain embodiments the present invention provides a method
for producing a therapeutic composition having full-length mRNA that encodes for liver glycogen phosphorylase protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for muscle phosphoglycerate mutase protein, !n certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for glycogen debranching enzyme.
[0291] in certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes for a protein associated with amino acid metabolism. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for phenylalanine hydroxylase enzyme, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for glutaryl-CoA dehydrogenase enzyme. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for propionyl-CoA caboxylase enzyme. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for oxalase a!anine- glyoxylate aminotransferase enzyme.
[0292] in certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes for a protein associated with a lipid metabolism or fibrotic disorder, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a mTOR inhibitor. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for ATPase phospholipid transporting 8B1 (ATP8B1) protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for one or more NF-kappa B inhibitors,, such as one or more of i-kappa B alpha, interferon-reiated development regulator 1 (IFRD1), and Sirtuin 1 (S!RTlj. in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for PPAR-gamma protein or an active variant.
[0293] In certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes for a protein associated with methylmalonic acidemia. For example, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for methylmalonyl CoA mutase protein. In certain embodiments the present Invention provides a
method for producing a therapeutic composition having full-length mRNA that encodes for methylmalonyl CoA epimerase protein,
[0294] in certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA for which delivery to or treatment of the liver can provide therapeutic benefit, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for ATP7B protein, also known as Wilson disease protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for porphobilinogen deaminase enzyme. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for one or clotting enzymes, such as Factor VIII, Factor IX, Factor VII, and Factor X. in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for human hemochromatosis (HFE) protein.
[0295] In certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes a peptide or protein for use in the delivery to or treatment of the cardiovasculature of a subject or a cardiovascular cell. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for vascular endothelial growth factor A protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for reiaxin protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for bone morphogenetic protein-9 protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for bone morphogenetic protein-2 receptor protein.
[0296] In certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes a peptide or protein for use in the delivery to or treatment of the muscle of a subject or a muscle cell. In certain embodiments the present invention provides a method for producing a therapeutic composition having full- length RNA that encodes for dystrophin protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for frataxin protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes a peptide or protein for use in the delivery to or treatment of the cardiac muscle of a subject or a cardiac muscle cell, in certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes for a protein that modulates one or both of a potassium channel and a sodium channel in muscle tissue or in a muscle cell. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a protein that modulates a Kv7.1 channel in muscle tissue or in a muscle ceil in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a protein that modulates a Navi.5 channel in muscle tissue or in a muscle cell.
[0297] in certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes a peptide or protein for use in the delivery to or treatment of the nervous system of a subject or a nervous system cell. For example,, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for survival motor neuron 1 protein. For example, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for survival motor neuron 2 protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for frataxin protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for ATP binding cassette subfamily D member 1 (ABCD1) protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for CLN3 protein.
[0298] in certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes a peptide or protein for use in the delivery to or treatment of the blood or bone marrow of a subject or a blood or bone marrow cell, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for beta globin protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for Bruton's tyrosine kinase protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for one or cloting enzymes, such as Factor VIII, Factor IX, Factor VII, and Factor X.
[0299] in certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes a peptide or protein for use in the delivery to or treatment of the kidney of a subject or a kidney cell, in certain embodiments
the present invention provides a method for producing a therapeutic composition having full- length mRNA that encodes for collagen type IV alpha 5 chain (COL4A5) protein,
[0300] in certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes a peptide or protein for use in the delivery to or treatment of the eye of a subject or an eye cell, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for ATP-binding cassette sub-family A member 4 (ABCA4) protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for retinoschisin protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having fuli-iength mRNA that encodes for retinal pigment epithelium-specific 65 kDa (RPE65) protein in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for centrosomal protein of 290 kDa (CEP290),
[0301] In certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes a peptide or protein for use in the delivery of or treatment with a vaccine for a subject or a ceil of a subject. For example, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for an antigen from an Infectious agent, such as a virus, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from influenza virus. In certain embodiments the present Invention provides a method for producing a therapeutic composition having full-length RNA that encodes for an antigen from respiratory syncytial virus. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from rabies virus, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from cytomegalovirus, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from rotavirus, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from a hepatitis virus, such as hepatitis A virus, hepatitis B virus, or hepaiis C virus, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from human papillomavirus. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an
antigen from a herpes simplex virus, such as herpes simplex virus 1 or herpes simplex virus 2. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from a human
immunodeficiency virus, such as human immunodeficiency virus type 1 or human
immunodeficiency virus type 2. in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from a human metapneumovirus. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen from a human parainfluenza virus, such as human parainfluenza virus type 1, human parainfluenza virus type 2, or human parainfluenza virus type 3. in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for an antigen from malaria virus, in certain embodiments the present invention provides a method for producing a therapeutic composition having fuli- iength mRNA that encodes for an antigen from zika virus. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNi\ that encodes for an antigen from chikungunya virus.
[0302] in certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes for an antigen associated with a cancer of a subject or identified from a cancer cel! of a subject, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen determined from a subject's own cancer cell, i.e., to provide a personalized cancer vaccine. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antigen expressed from a mutant KRAS gene.
[0303] In certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes for an antibody. In certain embodiments, the antibody can be a bi-specific antibody. In certain embodiments, the antibody can be part of a fusion protein, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antibody to 0X40. in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antibody to VEGF. in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antibody to tissue necrosis factor alpha, in certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes for an antibody to CDS. in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for an antibody to CD19.
[0304] In certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes for an immunomodulator. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for Interleukin 12. In certain embodiments the present invention provides a method for producing a therapeutic composition having full- length RNA that encodes for Interleukin 23. in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for Interleukin 36 gamma, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a constitutively active variant of one or more stimulator of interferon genes (STING) proteins,
[0305] In certain embodiments the present invention provides a method for producing a
therapeutic composition having full-length mRNA that encodes for an endonuclease, in certain embodiments the present invention provides a method for producing a therapeutic composition having full-length RNA that encodes for an RNA-guided DNA endonuclease protein, such as Cas 9 protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a meganuclease protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a transcription activator-like effector nuclease protein. In certain embodiments the present invention provides a method for producing a therapeutic composition having full-length mRNA that encodes for a zinc finger nuclease protein,
[0306] In embodiments, exemplary therapeutic uses result from the delivery of mRNA encoding a secreted protein. Accordingly, in embodiments, the compositions and methods of the invention provide for delivery of mRNA encoding a secreted protein. In some embodiments, the compositions and methods of the invention provide for delivery of mRNA encoding one or more secreted proteins listed in Table 1; thus, compositions of the invention may comprise an mRNA encoding a protein listed in Table 1 (or a homolog thereof) along with other components set out herein, and methods of the invention may comprise preparing and/or administering a composition comprising an mRNA encoding a protein listed In Table 1 (or a homolog thereof) along with other components set out herein.
Table 1. Secreted Proteins
[0307] in some embodiments, the compositions and methods of the invention provide for the delivery of one or more mRNAs encoding one or more additional exemplary proteins listed in Table 2; thus, compositions of the invention may comprise an mRNA encoding a protein listed in Table 2 (or a homolog thereof) along with other components set out herein, and methods of the invention may comprise preparing and/or administering a composition comprising an mR!MA encoding a protein chosen from the proteins listed in Table 2 (or a homolog thereof) along with other components set out herein.
Table 2, Additional Exemplary Proteins
[0308] The Uniprot !Ds set forth in Tab!e 1 and Table 2 refer to the human versions the listed proteins and the sequences of each are available from the Uniprot database. Sequences of the listed proteins are also generally available for various animals, including various mammals and animals of veterinary or industrial interest. Accordingly, in some embodiments, compositions and methods of the invention provide for the delivery of one or more mRNAs encoding one or more proteins chosen from mammalian homo!ogs or homoiogs from an animal of veterinary or industrial interest of the secreted proteins listed in Table 1 and Table 2; thus, compositions of the invention may comprise an mRNA encoding a protein chosen from mammalian homoiogs or homologs from an animal of veterinary or industrial interest of a protein listed in Table 1 and Table 2 along with other components set out herein, and methods of the invention may comprise preparing and/or administering a composition comprising an mRNA encoding a protein chosen from mammalian homoiogs or homoiogs from an animal of veterinary or industrial interest of a protein listed in Table 1 and Table 2 along with other components set out herein.
In some embodiments, mammalian homoiogs are chosen from mouse, rat, hamster, gerbi!, horse, pig, cow, llama, alpaca, mink, dog, cat, ferret, sheep, goat, or camel homoiogs. In some embodiments, the animal of veterinary or industrial interest is chosen from the mammals listed above and/or chicken, duck, turkey, salmon, catfish, or tiiapia.
[0309] In embodiments, the compositions and methods of the invention provide for the delivery of mRNA encoding a lysosomal protein chosen from Table 3, in some embodiments, the compositions and methods of the invention provide for the delivery of one or more mRNAs encoding one or more lysosomal and/or related proteins listed in Table 3; thus, compositions of the invention may comprise an mRNA encoding a protein listed in Table 3 {or a homolog thereof) along with other components set out herein, and methods of the invention may comprise preparing and/or administering a composition comprising an RNA encoding a protein chosen from the proteins listed in Table 3 (or a homolog thereof) along with other components set out herein.
Table 3, Lysosomal arid Related Proteins
[0310] information regarding lysosomal proteins is available from Lubke et a!., "Proteomics of the Lysosome," Biochim Biop ys Acta. (2009) 1793: 625-635. in some embodiments, the protein listed in Table 3 and encoded by mRNA in the compositions and methods of the invention is a human protein. Sequences of the listed proteins are also available for various animals, including various mammals and animals of veterinary or industrial interest as described above.
[0311] In some embodiments, the compositions and methods of the invention provide for the delivery of RNA encoding a therapeutic protein {e.g., cytosolic, transmembrane or secreted) such as those listed in Table 4. In some embodiments, the compositions and methods of the invention provide for the delivery of an mRNA encoding a therapeutic protein useful in treating a disease or disorder (i.e., indication) listed in Table 4; thus, compositions of the invention may comprise an rrsRNA encoding a therapeutic protein listed or not listed In Table 4 [or a homoiog thereof, as discussed below) along with other components set out herein for treating a disease or disorder (i.e., Indication) listed in Table 4, and methods of the Invention may comprise preparing and/or administering a composition comprising an mRNA encoding a such a protein (or a homolog thereof, as discussed below) along with other components set out herein for treatment of a disease or disorder listed in Table 4.
Table 4. Exemplary Indications and Reiated Proteins
[0312] in some embodiments, the present invention is used to prevent, treat, and/or cure a subject affected with a disease or disorder listed or associated with the proteins listed in Tables 1, 2, 3, or 4. In some embodiments, an mRNA encodes one or more of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR), argininosuccinate synthetase (ASSlj, Factor IX, survival motor neuron 1 (SMN1), or phenylalanine hydroxylase (PAH),
Delivery Methods
[0313] The route of delivery used in the methods of the invention allows for non-invasive, self administration of the compounds of the invention {e.g., a compound of Formula (A), such as Formulae (!) or (II) or any one of Compounds 1-12). in some embodiments, the methods involve intratracheal or pulmonary administration by aerosolization, nebulization, or instillation of a compositions comprising mRNA encoding a therapeutic protein in a suitable transfection or lipid carrier vehicles as described above. In some embodiments, the protein is encapsulated with a liposome. In some embodiments, the liposome comprises a lipid, which is a compound of the invention [e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12). As used herein below, administration of a compound of the invention includes administration of a composition comprising a compound of the invention.
[0314] Although the local cells and tissues of the lung represent a potential target capable of functioning as a biological depot or reservoir for production and secretion of the protein encoded by the mRNA, applicants have discovered that administration of the compounds of the invention (e.g., a compound of Formula (A), such as Formulae (I) or (I!) or any one of Compounds 1-12) to the lung via aerosolization, nebulization, or instillation results in the distribution of even non-secreted proteins outside the lung cells. Without wishing to be bound by any particular theory, it is contemplated that nanoparticle compositions of the invention pass, through the lung airway-blood barrier, resulting in translation of the intact nanoparticle to non-lung cells and tissues, such as, e.g., the heart, the liver, the spleen, where it results in the production of the encoded protein in these non-lung tissues. Thus, the utility of the compounds of the invention {e.g., a compound of Formula (A), such as Formulae (l) or (II) or any one of Compounds 1-12) and methods of the invention extend beyond production of therapeutic protein in lung ceils and tissues of the lung and can be used to delivery to non-lung target cells and/or tissues They are useful in the management and treatment of a large number of diseases, and in particular peripheral diseases which result from both secreted and non-secreted protein and/or enzyme deficiencies (e.g., one or more lysosomal storage disorders). In certain embodiments, the compounds of the invention {e.g., a compound of Formula (A), such as Formulae (!) or (II) or any one of Compounds 1-12), used in the methods of the invention result in the distribution of the mRNA encapsulated nanoparticles and production of the encoded protein in the liver, spleen, heart, and/or other non-lung cells. For example, administration of the compounds of the invention (e.g., a compound of Formula (A), such as Formulae (I) or (I!) or any one of Compounds 1-12), by aerosolization, nebulization, or instillation to the lung will result in the composition itself and its protein product (e.g., functional beta galactosidase protein) will be detectable in
both the local cells and tissues of the lung, as well as in peripheral target cells, tissues and organs as a result of translocation of the mRIMA and delivery vehicle to non-lung ceils,
[0315] in certain embodiments, the compounds of the invention {e.g., a compound of Formula (A), such as Formulae (!) or (!!) or any one of Compounds 1-12) may be employed in the methods of the invention to specifically target peripheral cells or tissues. Following the pulmonary delivery, it is contemplated the compounds of the invention {e.g., a compound of Formula (A), such as Formulae (!) or (II) or any one of Compounds 1-12) cross the lung airway-blood barrier and distribute into cells other than the local lung cells. Accordingly, the compounds disclosed herein (e.g., a compound of Formula (A), such as Formulae (!) or (II) or any one of Compounds 1-12) may be administered to a subject by way of the pulmonary route of administration, using a variety of approach known by those skilled in the art {e.g., by inhalation), and distribute to both the local target cells and tissues of the lung, as well as in peripheral non-lung cells and tissues (e.g., cells of the liver, spleen, kidneys, heart, skeletal muscle, lymph nodes, brain, cerebrospinal fluid, and plasma). As a result, both the local ceils of the lung and the peripheral non-lung cells can serve as biological reservoirs or depots capable of producing and/or secreting a translation product encoded by one or more polynucleotides. Accordingly, the present invention is not limited to the treatment of lung diseases or conditions, but rather can be used as a non-invasive means of facilitating the delivery of polynucleotides, or the production of enzymes and proteins encoded thereby, in peripheral organs, tissues and cells (e.g., hepatocytes) which would otherwise be achieved only by systemic administration. Exemplary peripheral non-lung ceils include, but are not limited to, hepatocytes, epithelial cells, hematopoietic cells, epithelial cells, endothelial cells, bone cells, stem cells, mesenchymal cells, neural cells, cardiac ceils, adipocytes, vascular smooth muscle cells, cardiomyocytes, skeletal muscle ceils, beta cells, pituitary cells, synovial lining ceils, ovarian ceils, testicular cells, fibroblasts, 3 cells, T ceils, reticulocytes, leukocytes, granulocytes and tumor cells.
[0316] Following administration of the composition to the subject, the protein product encoded by the mRIMA (e.g., a functional protein or enzyme) is detectable in the peripheral target tissues for at least about one to seven days or longer following administration of the compound to the subject. The amount of protein product necessary to achieve a therapeutic effect will vary depending on the condition being treated, the protein encoded, and the condition of the patient. For example, the protein product may be detectable In the peripheral target tissues at a concentration (e.g., a therapeutic concentration) of at least 0.025-1.5 pg/ml (e.g., at least 0.050 pg/ml, at least 0.075 pg/ml, at least 0.1 pg/ml, at least 0.2 pg/ml, at least 0.3 pg/ml, at least 0,4 pg/ml, at least 0,5 pg/ml, at least 0.6 pg/ml, at least 0.7 pg/ml, at least 0.8 pg/ml, at least 0.9
pg/mi, at least 1.0 pg/ml, at least 1.1 pg/mi, at least 1.2 pg/ml, at least 1.3 pg/ml, at least 1.4 pg/ml, or at least 1.5 pg/ml), for at least about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11,12, 13, 14, 15, 16,
17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 35, 40, 45 days or longer following administration of the compound to the subject.
[0317] It has been demonstrated that nucleic acids can be delivered to the lungs by intratracheal administration of a liquid suspension of the compound and inhalation of an aerosol mist produced by a liquid nebulizer or the use of a dry powder apparatus such as that described in U.S. patent 5,780,014, incorporated herein by reference.
[0318] In certain embodiments, the compounds of the invention (e.g., a compound of Formula (A), such as Formulae (!) or (I!) or any one of Compounds 1-12) may be formulated such that they may be aerosolized or otherwise delivered as a particulate liquid or solid prior to or upon administration to the subject. Such compounds may be administered with the assistance of one or more suitable devices for administering such solid or liquid particulate compositions (such as, e.g., an aerosolized aqueous solution or suspension) to generate particles that are easily respirable or inhalable by the subject. In some embodiments, such devices {e.g., a metered dose inhaler, jet-nebulizer, ultrasonic nebulizer, dry-powder-inhalers, propellant-based inhaler or an insufflator) facilitate the administration of a predetermined mass, volume or dose of the compositions {e.g., about 0.5 mg/kg of mRNA per dose) to the subject. For example, in certain embodiments, the compounds of the invention {e.g., a compound of Formula (A), such as Formulae (I) or (11) or any one of Compounds 1-12) are administered to a subject using a metered dose inhaler containing a suspension or solution comprising the compound and a suitable propellant. In certain embodiments, the compounds of the invention {e.g., a compound of Formula (A), such as Formulae (I) or (II) or any one of Compounds 1-12) may be formulated as a particulate powder (e.g., respirable dry particles) intended for inhalation, in certain embodiments, compositions of the invention formulated as respirable particles are appropriately sized such that they may be respirable by the subject or delivered using a suitable device (e.g., a mean D50 or D90 particle size less than about 500pm, 400pm, 300pm, 250pm, 200pm, 150pm, lOOpm, 75pm, 50pm, 25pm, 20pm, 15pm, 12.5pm, 10pm, 5pm, 2.5pm or smaller). In yet other embodiments, the compounds of the invention (e.g., a compound of Formula (A), such as Formulae (!) or (II) or any one of Compounds 1-12) are formulated to include one or more pulmonary surfactants {e.g., lamellar bodies). In some embodiments, the compounds of the invention {e.g., a compound of Formula (A), such as Formulae (I) or (I!) or any one of Compounds 1-12) are administered to a subject such that a concentration of at least 0.05 mg/kg, at least 0.1 g/kg, at least 0.5 mg/kg, at least 1.0 mg/kg, at least 2.0 mg/kg, at least 3,0
mg/kg, at least 4.0 g/kg, at least 5.0 mg/kg, at least 6.0 g/kg, at least 7.0 mg/kg, at least 8.0 mg/kg, at least 9.0 g/kg, at least 10 mg/kg, at least 15 mg/kg, at least 20 rng/'kg, at least 25 mg/kg, at least 30 mg/kg, at least 35 mg/kg, at least 40 rng/'kg, at least 45 g/kg, at least 50 mg/kg, at least 55 mg/kg, at least 60 g/kg, at least 65 mg/kg, at least 70 mg/kg, at least 75 mg/kg, at least 80 mg/ kg, at least 85 mg/kg, at least 90 mg/kg, at least 95 g/kg, or at least 100 mg/kg body weight is administered in a single dose. In some embodiments, the compounds of the invention (e.g., a compound of Formula (A), such as Formulae (!) or (II) or any one of Compounds 1-12) are administered to a subject such that a total amount of at least 0,1 mg, at least 0.5 g, at least 1.0 mg, at least 2.0 g, at least 3.0 mg, at least 4.0 mg, at least 5.0 mg, at least 6.0 g, at least 7.0 mg, at least 8.0 g, at least 9.0 mg, at least 10 mg, at least 15 g, at least 20 mg, at least 25 mg, at least 30 g, at least 35 mg, at least 40 mg, at least 45 mg, at least 50 mg, at least 55 g, at least 60 g, at least 65 mg, at least 70 g, at least 75 mg, at least 80 mg, at least 85 mg, at least 90 g, at least 95 g or at least 100 g mRNA is administered in one or more doses.
EXAMPLES
[0319] While certain compounds, compositions and methods of the present invention have been described with specificity in accordance with certain embodiments, the following examples serve only to illustrate the compounds of the invention and are not intended to limit the same.
[0320] In embodiments, a cationic lipid described herein can be prepared using a cystine starting material. The cystine can be treated with an alkylating or acylating agent to functionalize the amino groups, which in turn can be esterified to afford certain cationic lipids described herein. Exemplary N-aikyiating or N-acylating agents are described in Table A, and exemplary alcohols for esterification are described in Table B. Accordingly, cationic lipids herein include those resulting from any combination of the precursors described in Table A and Table B.
Table A. N-AlkySafing or -Acylating Agents
Example 1. Synthesis of Intermediate A:
Intermediate A
[0321] L-Cystine (1.0 g, 4.16 mmol) was dissolved into DMSO/Saturated Brine solution (100 ml, 1:1 v/v ratio), isodecy! Acrylate (6.06 mL, 24.9 mmol) was added into it and the reaction mixture was heated at 90°C for 2 days. The reaction was stopped after 2 days and cooled to room temperature. The organic layer was then separated, diluted with ethyl acetate (100 ml) and washed with brine solution (3 x 50 mL), After drying over anhydrous IMa2S0 , the organic layer was evaporated under reduced pressure, and the residue was purified by silica gel
chromatography (eluent: 100% DCM) to obtain the compound Intermediate A as a colorless oil (2.0 g, 44%), isolation of compound intermediate A was confirmed based on MS analysis.
[0322] Results ESI-MS analysis: Calculated CssHioeNzOizSz, [M-H] = 1086.72, Observed = 1086.70
Example 2. Synthesis of Compound 3:
Compound 3
[0323] To a solution of Intermediate A (0.5 g, 0.46 mmol) and l-(bis(3-
(dimethylamino)propyl)amino)-2-propanol (0.38 ml, 1.38 mmol) in Dfvl F (6 ml) were added HOSt (0.19 g, 1.38 mmol), HBTU (0,52 g, 1.38 mmol), and DMAP (0.17 g, 1.38 mmol) followed by slow addition of D!PEA (0.48 ml, 2.75 mmol). The reaction was heated at 65°C for 1 hour and continued stirring for another 24h at room temperature. Reaction mixture was then diluted with ethyl acetate (100 mL) and washed with brine solution (3 x 50 ml). After drying over anhydrous Na2$04, the organic layer was evaporated under reduced pressure, and the residue was purified
by silica gel chromatography (eluent: 0, 5-1.0% MeOH in DCM) to obtain the Compound 3 as a sticky yellow solid (0,36g, 51%), Isolation of Compound 3 was confirmed based on MS analysis.
[0324] Results: ESI-MS analysis: Calculated C34H167NSO12S2, [M+H÷] = 1544,21, Observed = 1544.20
Example 3. Synthesis of Compound 1:
[0325] To a solution of intermediate A (0.5 g, 0.46 mmol) and 2-dimethylaminoethanol (0.28 ml, 2.76 mmol) in DMF (6 ml) were added HQBt (0.19 g, 1.40 mmol), HBTU (0.52 g, 1.40 mmol), and DMAP (0,17 g, 1.40 mmol) followed by slow addition of DIPEA (0.48 l., 2.75 mmol). The reaction was heated at 65°C for 1 hour and continued stirring for another 24h at room temperature. Reaction mixture was then diluted with ethyl acetate (100 ml) and washed with brine solution (3 x 50 mL). After drying over anhydrous a2SO , the organic layer was evaporated under reduced pressure, and the residue was purified by silica gel chromatography (eluent: 0.5- 1.0% MeOH in DCM) to obtain the Compound 1 as a light yellow oil (0.34 g, 60 %). Isolation of Compound 1 was confirmed based on MS analysis.
Results: ESI-MS analysis: Calculated CeeH UGizSz, [M+H ] = 1231.89, Observed = 1231.90
Example 4. Synthesis of Compound 4:
[0326] To a solution of Intermediate A {0.5 g, 0.46 mmol) and 2-[2-(2- methoxyethoxy)ethoxy]ethanol (0.43 ml, 2.76 mmol) in DMF (6 ml) were added HOBt (0,19 g, 1,38 mmol), HBTU (0.52 g, 1,38 mmol), and D!ViAP (0.17 g, 1.38 mmol) followed by slow addition of DIPEA (0.48 mL, 2,75 mmol). The reaction was heated at 65°C for 1 hour and continued stirring for another 24 h at room temperature. Reaction mixture was then diluted with ethyl acetate (100 ml) and washed with brine solution (3 x 50 L). After drying over anhydrous Na S , the organic layer was evaporated under reduced pressure, and the residue was purified by silica gel chromatography (eluent: 0.5-0, 6% MeOH in DCM) to obtain the Compound 4 as a light brown oil (0,38 g, 60%). isolation of Compound 4 was confirmed based on MS analysis.
[0327] Results: ES!-MS analysis: Calculated C H N O S , [M+hT] = 1381.93, Observed = 1381.90
Example S. In Viva Expression ofhEPQ after IM injection in BALB/c Mice:
[0328] in embodiments, cationic lipids described herein can be used in the preparation of lipid nanoparticles according to methods known in the art. For example, suitable methods Include methods described in international Publication No. WO 2018/089801, which is hereby incorporated by reference in its entirety.
[0329] Lipid nanoparticle formulations comprising human erythropoietin (hEPQ) mRNA, Cationic Lipid, DMG-PEG2000, Cholesterol and DOPE were administered intramuscularly to study mRNA delivery and resultant hEPQ expression. Male BALB/c mice at 6-8 weeks old are given a single injection of the LNP formulations into the gastrocnemius muscle at a dosage level of 0.1 ug. Blood samples were collected at 6 and 24 hours post-dose. hEPQ protein expression levels were measured in the sera samples by ELISA and presented in Figure 1. These studies show that the cationic lipids described herein are highly effective at delivery mRNA in vivo, resulting in high expression of the protein or polypeptide encoded by the delivered mRNA.
[0330] From the foregoing description, one skilled in the art can easily ascertain the essential characteristics of this invention, and without departing from the spirit and scope thereof, can make various changes and modifications of the invention to adapt it to various usages and conditions.
[0331] Ail references, patents or applications, IJ.S. or foreign, cited in the application are hereby incorporated by reference as if written herein in their entireties. Where any inconsistencies arise, material literally disclosed herein controls.
Claims
1. A cationic lipid having the following structure:
wherein
each Rla is independently hydrogen, Rlc, or Rld;
each Rlb is independently Rlc or Rld;
each Ric is independently -{O-l2)2C(0)X3R3;
each Rld Is independently -C(0)R4;
each R2 is independently -[C(R2aj2]cR2b;
each R2a is independently hydrogen or Ci-C6 alkyl;
R2b is -N(Li-B)2/ -iOCH2CH )i OH; or -{OCH2CH2)t,OCH3;
each R3 and R4 is independently C6-C3o aliphatic;
each I.3 is independently C1-C10 alkylene;
each B is independently hydrogen or an ionizab!e nitrogen-containing group; each X1 is independently a covalent bond or O;
each a is independently an integer of 1-10;
each b is independently an integer of 1-10; and
each c is independently an integer of 1-10.
2. The cationic lipid of claim 1, wherein each a is 1 or 2.
3. The cationic lipid of claim 1 or 2, wherein each Rla and Rlb is ~(CH2) CfO)X1R3.
4. The cationic lipid of claim 3, having the following structure:
5. The cationic lipid of claim 4, wherein each R3 is C6-C24 alkyl, C6-C2 alkenyl, or C6-C2 alkynyl.
6 The cationic lipid of claim 5, wherein each R3 is unsubstituted Cs-C24 alkyl.
7. The cationic lipid of claim 1 or 2, wherein each Ria is hydrogen and each Rlb is -C(0)R4.
8, The cationic lipid of claim 7, having the following structure:
9. The cationic lipid of claim 8, wherein each R4 is C6 C3o alkyl.
10 The cationic lipid of claim 9, wherein each R4 is unsubstituted C6-C alkyl.
The cationic lipid of claim 9, wherein each R4 is C6-Ci0 alkyl substituted with ~QC(0)R:5 or -C02R!!, wherein R5 is unsubstituted Cs-C-.g alkyl.
The cationic lipid of any one of claims 1-11, wherein
each R2 is -[CH2]C-N(LI-B)2 or - CH(CH3)CH2-N(Li-B)2; and
each Li is unsubstituted Ci-C-.r, a!ky!ene.
13. The cationic lipid of claim 12, wherein each R2 is [CH2]c-— N{CH
14. The cationic lipid of claim 12, wherein
each R2 is -[CH2]c-NiCH2CH2B)2, -[CH2]cH\l(CH2CH2CH2B)2,-CH(CH3)CHHSI(CH2CH2B)2, or -CH(CH3)CH2-N(CH2CH2CH2B)2; and
each B is an ionizable nitrogen-containing group.
15. The cationic lipid of claim 14, wherein each B is NH2, guanidine, amidine, a mono- or
diaikylamine, 5- to 6-membered heterocycloalkyl, or 5- to 6-membered nitrogen-containing heteroaryl,
16. The cationic lipid of claim 15, wherein each B is H2, guanidine, amidine, HCHs, N{CH3)2, or imidazole.
17. The cationic lipid of claim 16, wherein each B is N(CH3)2.
18. The cationic lipid of any one of claims .12-17, wherein each c is 2, 3, or 4.
19. The cationic lipid of claim 1, wherein the cationic lipid is any one of Compounds 1-12.
20. A composition comprising an mRNA encoding a protein, encapsulated within a liposome, wherein the liposome comprises a cationic lipid according to any one of claims 1-19,
2.1. The composition of claim 20, comprising an mRNA encoding for cystic fibrosis
transmembrane conductance regulator (CFTR) protein.
2.2. The composition of claim 20, comprising an mRNA encoding for ornithine transcarbamylase (OTC) protein.
23. A composition comprising a nucleic acid encapsulated within a liposome, wherein the liposome comprises a cationic lipid according to any one of claims 1-19,
24. The composition of claim 23, wherein the nucleic acid is an mRNA encoding a peptide or protein.
25. The composition of claim 24, wherein the RNA encodes a peptide or protein for use in the delivery to or treatment of the lung of a subject or a lung cell.
26. The composition of claim 25, wherein the RNA encodes cystic fibrosis transmembrane conductance regulator (CFTR) protein.
27. The composition of claim 24, wherein the mRNA encodes a peptide or protein for use in the delivery to or treatment of the liver of a subject or a liver cell.
28. The composition of claim 27, wherein the mRNA encodes ornithine transcarbamylase (OTC) protein.
29. The composition of claim 20 or 23, wherein the mRNA encodes a peptide or protein for use in vaccine.
30. The composition of claim 29, wherein the mRNA encodes an antigen.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP20724352.8A EP3956303A1 (en) | 2019-04-18 | 2020-04-17 | Cystine cationic lipids |
| US17/604,244 US20220177423A1 (en) | 2019-04-18 | 2020-04-17 | Cystine cationic lipids |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201962835814P | 2019-04-18 | 2019-04-18 | |
| US62/835,814 | 2019-04-18 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2020214946A1 true WO2020214946A1 (en) | 2020-10-22 |
Family
ID=70554265
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2020/028755 WO2020214946A1 (en) | 2019-04-18 | 2020-04-17 | Cystine cationic lipids |
Country Status (3)
| Country | Link |
|---|---|
| US (1) | US20220177423A1 (en) |
| EP (1) | EP3956303A1 (en) |
| WO (1) | WO2020214946A1 (en) |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP4046629A1 (en) | 2021-02-19 | 2022-08-24 | ModernaTX, Inc. | Lipid nanoparticle compositions and methods of formulating the same |
| WO2022221736A2 (en) | 2021-04-16 | 2022-10-20 | Genentech, Inc. | Optimized tlr7 ligands and uses thereof |
| WO2023031394A1 (en) | 2021-09-03 | 2023-03-09 | CureVac SE | Novel lipid nanoparticles for delivery of nucleic acids |
| WO2023073228A1 (en) | 2021-10-29 | 2023-05-04 | CureVac SE | Improved circular rna for expressing therapeutic proteins |
| CN116218862A (en) * | 2023-01-11 | 2023-06-06 | 郑州大学 | SELENOO gene mutant and application thereof |
| WO2023144330A1 (en) | 2022-01-28 | 2023-08-03 | CureVac SE | Nucleic acid encoded transcription factor inhibitors |
| WO2023177904A1 (en) | 2022-03-18 | 2023-09-21 | Modernatx, Inc. | Sterile filtration of lipid nanoparticles and filtration analysis thereof for biological applications |
| WO2023227608A1 (en) | 2022-05-25 | 2023-11-30 | Glaxosmithkline Biologicals Sa | Nucleic acid based vaccine encoding an escherichia coli fimh antigenic polypeptide |
| WO2024044147A1 (en) | 2022-08-23 | 2024-02-29 | Modernatx, Inc. | Methods for purification of ionizable lipids |
| DE202023106198U1 (en) | 2022-10-28 | 2024-03-21 | CureVac SE | Nucleic acid-based vaccine |
| US12023376B1 (en) | 2020-04-17 | 2024-07-02 | Triad National Security, Llc | Conserved region T cell vaccines for coronavirus and methods of use |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA3097912A1 (en) * | 2018-05-15 | 2019-11-21 | Translate Bio, Inc. | Subcutaneous delivery of messenger rna |
| KR20240066080A (en) * | 2022-11-01 | 2024-05-14 | 주식회사 삼양홀딩스 | Cationic lipid having amide and ester functional groups and method for preparing the same |
| KR20240072916A (en) * | 2022-11-16 | 2024-05-24 | 주식회사 삼양홀딩스 | Nanoparticle composition for drug delivery |
Citations (42)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4373071A (en) | 1981-04-30 | 1983-02-08 | City Of Hope Research Institute | Solid-phase synthesis of polynucleotides |
| US4401796A (en) | 1981-04-30 | 1983-08-30 | City Of Hope Research Institute | Solid-phase synthesis of polynucleotides |
| US4415732A (en) | 1981-03-27 | 1983-11-15 | University Patents, Inc. | Phosphoramidite compounds and processes |
| US4458066A (en) | 1980-02-29 | 1984-07-03 | University Patents, Inc. | Process for preparing polynucleotides |
| US4500707A (en) | 1980-02-29 | 1985-02-19 | University Patents, Inc. | Nucleosides useful in the preparation of polynucleotides |
| US4668777A (en) | 1981-03-27 | 1987-05-26 | University Patents, Inc. | Phosphoramidite nucleoside compounds |
| US4897355A (en) | 1985-01-07 | 1990-01-30 | Syntex (U.S.A.) Inc. | N[ω,(ω-1)-dialkyloxy]- and N-[ω,(ω-1)-dialkenyloxy]-alk-1-yl-N,N,N-tetrasubstituted ammonium lipids and uses therefor |
| US4973679A (en) | 1981-03-27 | 1990-11-27 | University Patents, Inc. | Process for oligonucleo tide synthesis using phosphormidite intermediates |
| US5047524A (en) | 1988-12-21 | 1991-09-10 | Applied Biosystems, Inc. | Automated system for polynucleotide synthesis and purification |
| US5132418A (en) | 1980-02-29 | 1992-07-21 | University Patents, Inc. | Process for preparing polynucleotides |
| US5153319A (en) | 1986-03-31 | 1992-10-06 | University Patents, Inc. | Process for preparing polynucleotides |
| US5171678A (en) | 1989-04-17 | 1992-12-15 | Centre National De La Recherche Scientifique | Lipopolyamines, their preparation and their use |
| US5262530A (en) | 1988-12-21 | 1993-11-16 | Applied Biosystems, Inc. | Automated system for polynucleotide synthesis and purification |
| US5334761A (en) | 1992-08-28 | 1994-08-02 | Life Technologies, Inc. | Cationic lipids |
| US5700642A (en) | 1995-05-22 | 1997-12-23 | Sri International | Oligonucleotide sizing using immobilized cleavable primers |
| US5744335A (en) | 1995-09-19 | 1998-04-28 | Mirus Corporation | Process of transfecting a cell with a polynucleotide mixed with an amphipathic compound and a DNA-binding protein |
| US5780014A (en) | 1995-04-14 | 1998-07-14 | Inhale Therapeutic Systems | Method and apparatus for pulmonary administration of dry powder alpha 1-antitrypsin |
| US5885613A (en) | 1994-09-30 | 1999-03-23 | The University Of British Columbia | Bilayer stabilizing components and their use in forming programmable fusogenic liposomes |
| WO2005121348A1 (en) | 2004-06-07 | 2005-12-22 | Protiva Biotherapeutics, Inc. | Lipid encapsulated interfering rna |
| WO2010042877A1 (en) | 2008-10-09 | 2010-04-15 | Tekmira Pharmaceuticals Corporation | Improved amino lipids and methods for the delivery of nucleic acids |
| WO2010053572A2 (en) | 2008-11-07 | 2010-05-14 | Massachusetts Institute Of Technology | Aminoalcohol lipidoids and uses thereof |
| WO2010144740A1 (en) | 2009-06-10 | 2010-12-16 | Alnylam Pharmaceuticals, Inc. | Improved lipid formulation |
| US8093367B2 (en) | 2007-10-31 | 2012-01-10 | Applied Biosystems, Llc | Preparation and isolation of 5′ capped mRNA |
| US8304529B2 (en) | 2006-07-28 | 2012-11-06 | Life Technologies Corporation | Dinucleotide MRNA cap analogs |
| WO2012170889A1 (en) | 2011-06-08 | 2012-12-13 | Shire Human Genetic Therapies, Inc. | Cleavable lipids |
| WO2013063468A1 (en) | 2011-10-27 | 2013-05-02 | Massachusetts Institute Of Technology | Amino acid derivates functionalized on the n- terminal capable of forming drug incapsulating microspheres |
| WO2013149140A1 (en) | 2012-03-29 | 2013-10-03 | Shire Human Genetic Therapies, Inc. | Ionizable cationic lipids |
| WO2015095340A1 (en) | 2013-12-19 | 2015-06-25 | Novartis Ag | Lipids and lipid compositions for the delivery of active agents |
| WO2015184256A2 (en) | 2014-05-30 | 2015-12-03 | Shire Human Genetic Therapies, Inc. | Biodegradable lipids for delivery of nucleic acids |
| WO2015199952A1 (en) | 2014-06-25 | 2015-12-30 | Acuitas Therapeutics Inc. | Novel lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2016004202A1 (en) | 2014-07-02 | 2016-01-07 | Massachusetts Institute Of Technology | Polyamine-fatty acid derived lipidoids and uses thereof |
| US20160031928A1 (en) | 2013-03-14 | 2016-02-04 | Shire Human Genetic Therapies, Inc. | RIBONUCLEIC ACIDs WITH 4'-THIO-MODIFIED NUCLEOTIDES AND RELATED METHODS |
| US20160032356A1 (en) | 2013-03-14 | 2016-02-04 | Shire Human Genetic Therapies, Inc. | Quantitative assessment for cap efficiency of messenger rna |
| WO2016118725A1 (en) | 2015-01-23 | 2016-07-28 | Moderna Therapeutics, Inc. | Lipid nanoparticle compositions |
| WO2016118724A1 (en) | 2015-01-21 | 2016-07-28 | Moderna Therapeutics, Inc. | Lipid nanoparticle compositions |
| WO2016205691A1 (en) | 2015-06-19 | 2016-12-22 | Massachusetts Institute Of Technology | Alkenyl substituted 2,5-piperazinediones and their use in compositions for delivering an agent to a subject or cell |
| WO2017004143A1 (en) | 2015-06-29 | 2017-01-05 | Acuitas Therapeutics Inc. | Lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2017049245A2 (en) | 2015-09-17 | 2017-03-23 | Modernatx, Inc. | Compounds and compositions for intracellular delivery of therapeutic agents |
| WO2017075531A1 (en) | 2015-10-28 | 2017-05-04 | Acuitas Therapeutics, Inc. | Novel lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2017117528A1 (en) | 2015-12-30 | 2017-07-06 | Acuitas Therapeutics, Inc. | Lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2017173054A1 (en) | 2016-03-30 | 2017-10-05 | Intellia Therapeutics, Inc. | Lipid nanoparticle formulations for crispr/cas components |
| WO2018089801A1 (en) | 2016-11-10 | 2018-05-17 | Translate Bio, Inc. | Improved process of preparing mrna-loaded lipid nanoparticles |
-
2020
- 2020-04-17 US US17/604,244 patent/US20220177423A1/en active Pending
- 2020-04-17 EP EP20724352.8A patent/EP3956303A1/en active Pending
- 2020-04-17 WO PCT/US2020/028755 patent/WO2020214946A1/en unknown
Patent Citations (42)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5132418A (en) | 1980-02-29 | 1992-07-21 | University Patents, Inc. | Process for preparing polynucleotides |
| US4458066A (en) | 1980-02-29 | 1984-07-03 | University Patents, Inc. | Process for preparing polynucleotides |
| US4500707A (en) | 1980-02-29 | 1985-02-19 | University Patents, Inc. | Nucleosides useful in the preparation of polynucleotides |
| US4415732A (en) | 1981-03-27 | 1983-11-15 | University Patents, Inc. | Phosphoramidite compounds and processes |
| US4668777A (en) | 1981-03-27 | 1987-05-26 | University Patents, Inc. | Phosphoramidite nucleoside compounds |
| US4973679A (en) | 1981-03-27 | 1990-11-27 | University Patents, Inc. | Process for oligonucleo tide synthesis using phosphormidite intermediates |
| US4401796A (en) | 1981-04-30 | 1983-08-30 | City Of Hope Research Institute | Solid-phase synthesis of polynucleotides |
| US4373071A (en) | 1981-04-30 | 1983-02-08 | City Of Hope Research Institute | Solid-phase synthesis of polynucleotides |
| US4897355A (en) | 1985-01-07 | 1990-01-30 | Syntex (U.S.A.) Inc. | N[ω,(ω-1)-dialkyloxy]- and N-[ω,(ω-1)-dialkenyloxy]-alk-1-yl-N,N,N-tetrasubstituted ammonium lipids and uses therefor |
| US5153319A (en) | 1986-03-31 | 1992-10-06 | University Patents, Inc. | Process for preparing polynucleotides |
| US5262530A (en) | 1988-12-21 | 1993-11-16 | Applied Biosystems, Inc. | Automated system for polynucleotide synthesis and purification |
| US5047524A (en) | 1988-12-21 | 1991-09-10 | Applied Biosystems, Inc. | Automated system for polynucleotide synthesis and purification |
| US5171678A (en) | 1989-04-17 | 1992-12-15 | Centre National De La Recherche Scientifique | Lipopolyamines, their preparation and their use |
| US5334761A (en) | 1992-08-28 | 1994-08-02 | Life Technologies, Inc. | Cationic lipids |
| US5885613A (en) | 1994-09-30 | 1999-03-23 | The University Of British Columbia | Bilayer stabilizing components and their use in forming programmable fusogenic liposomes |
| US5780014A (en) | 1995-04-14 | 1998-07-14 | Inhale Therapeutic Systems | Method and apparatus for pulmonary administration of dry powder alpha 1-antitrypsin |
| US5700642A (en) | 1995-05-22 | 1997-12-23 | Sri International | Oligonucleotide sizing using immobilized cleavable primers |
| US5744335A (en) | 1995-09-19 | 1998-04-28 | Mirus Corporation | Process of transfecting a cell with a polynucleotide mixed with an amphipathic compound and a DNA-binding protein |
| WO2005121348A1 (en) | 2004-06-07 | 2005-12-22 | Protiva Biotherapeutics, Inc. | Lipid encapsulated interfering rna |
| US8304529B2 (en) | 2006-07-28 | 2012-11-06 | Life Technologies Corporation | Dinucleotide MRNA cap analogs |
| US8093367B2 (en) | 2007-10-31 | 2012-01-10 | Applied Biosystems, Llc | Preparation and isolation of 5′ capped mRNA |
| WO2010042877A1 (en) | 2008-10-09 | 2010-04-15 | Tekmira Pharmaceuticals Corporation | Improved amino lipids and methods for the delivery of nucleic acids |
| WO2010053572A2 (en) | 2008-11-07 | 2010-05-14 | Massachusetts Institute Of Technology | Aminoalcohol lipidoids and uses thereof |
| WO2010144740A1 (en) | 2009-06-10 | 2010-12-16 | Alnylam Pharmaceuticals, Inc. | Improved lipid formulation |
| WO2012170889A1 (en) | 2011-06-08 | 2012-12-13 | Shire Human Genetic Therapies, Inc. | Cleavable lipids |
| WO2013063468A1 (en) | 2011-10-27 | 2013-05-02 | Massachusetts Institute Of Technology | Amino acid derivates functionalized on the n- terminal capable of forming drug incapsulating microspheres |
| WO2013149140A1 (en) | 2012-03-29 | 2013-10-03 | Shire Human Genetic Therapies, Inc. | Ionizable cationic lipids |
| US20160031928A1 (en) | 2013-03-14 | 2016-02-04 | Shire Human Genetic Therapies, Inc. | RIBONUCLEIC ACIDs WITH 4'-THIO-MODIFIED NUCLEOTIDES AND RELATED METHODS |
| US20160032356A1 (en) | 2013-03-14 | 2016-02-04 | Shire Human Genetic Therapies, Inc. | Quantitative assessment for cap efficiency of messenger rna |
| WO2015095340A1 (en) | 2013-12-19 | 2015-06-25 | Novartis Ag | Lipids and lipid compositions for the delivery of active agents |
| WO2015184256A2 (en) | 2014-05-30 | 2015-12-03 | Shire Human Genetic Therapies, Inc. | Biodegradable lipids for delivery of nucleic acids |
| WO2015199952A1 (en) | 2014-06-25 | 2015-12-30 | Acuitas Therapeutics Inc. | Novel lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2016004202A1 (en) | 2014-07-02 | 2016-01-07 | Massachusetts Institute Of Technology | Polyamine-fatty acid derived lipidoids and uses thereof |
| WO2016118724A1 (en) | 2015-01-21 | 2016-07-28 | Moderna Therapeutics, Inc. | Lipid nanoparticle compositions |
| WO2016118725A1 (en) | 2015-01-23 | 2016-07-28 | Moderna Therapeutics, Inc. | Lipid nanoparticle compositions |
| WO2016205691A1 (en) | 2015-06-19 | 2016-12-22 | Massachusetts Institute Of Technology | Alkenyl substituted 2,5-piperazinediones and their use in compositions for delivering an agent to a subject or cell |
| WO2017004143A1 (en) | 2015-06-29 | 2017-01-05 | Acuitas Therapeutics Inc. | Lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2017049245A2 (en) | 2015-09-17 | 2017-03-23 | Modernatx, Inc. | Compounds and compositions for intracellular delivery of therapeutic agents |
| WO2017075531A1 (en) | 2015-10-28 | 2017-05-04 | Acuitas Therapeutics, Inc. | Novel lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2017117528A1 (en) | 2015-12-30 | 2017-07-06 | Acuitas Therapeutics, Inc. | Lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| WO2017173054A1 (en) | 2016-03-30 | 2017-10-05 | Intellia Therapeutics, Inc. | Lipid nanoparticle formulations for crispr/cas components |
| WO2018089801A1 (en) | 2016-11-10 | 2018-05-17 | Translate Bio, Inc. | Improved process of preparing mrna-loaded lipid nanoparticles |
Non-Patent Citations (21)
| Title |
|---|
| BEHR ET AL., PROC. NAT.'1 ACAD. SCI., vol. 86, 1989, pages 6982 |
| DEFU ZHI ET AL: "A review on cationic lipids with different linkers for gene delivery", ADVANCES IN COLLOID AND INTERFACE SCIENCE, vol. 253, 1 March 2018 (2018-03-01), NL, pages 117 - 140, XP055698824, ISSN: 0001-8686, DOI: 10.1016/j.cis.2017.12.006 * |
| FEIGNER ET AL., PROC. NAT'L ACAD. SCI., vol. 84, 1987, pages 7413 |
| GAO ET AL., BIOCHEM. BIOPHYS. RES. COMM., vol. 179, 1991, pages 280 |
| GRUDZIEN, E. ET AL., RNA, vol. 10, 2004, pages 1479 - 1487 |
| GRUDZIEN-NOGALSKA, E., RNA, vol. 13, 2007, pages 1745 - 1755 |
| HEYES, J. ET AL., J CONTROLLED RELEASE, vol. 107, 2005, pages 276 - 287 |
| J. MCCLELLANM. C. KING, CELL, vol. 141, 2010, pages 210 - 217 |
| J. PHARMACEUTICAL SCIENCES, vol. 66, 1977, pages 1 - 19 |
| JEMIELITY, J. ET AL., RNA, vol. 9, 2003, pages 1108 - 1122 |
| JEMIELITY, J. ET AL.: "Novel 'anti-reverse' cap analogs with superior translational properties", RNA, vol. 9, 2003, pages 1108 - 1122, XP002466761, DOI: 10.1261/rna.5430403 |
| KLIBANOV, FEBS LETTERS, vol. 268, no. 1, 1990, pages 235 - 237 |
| LASIC, TRENDS BIOTECHNOL., vol. 16, 1998, pages 307 - 321 |
| LUBKE ET AL.: "Proteomics of the Lysosome", BIOCHIM BIOPHYS ACTA, vol. 1793, 2009, pages 625 - 635, XP026073291, DOI: 10.1016/j.bbamcr.2008.09.018 |
| MORRISSEY, DV. ET AL., NAT. BIOTECHNOL., vol. 23, no. 8, 2005, pages 1003 - 1007 |
| SEMPLE, NATURE BIOTECH., vol. 28, 2010, pages 172 - 176 |
| WHITEHEAD ET AL., NATURE COMMUNICATIONS, vol. 5, 2014, pages 4277 |
| WOLF, BIOTECHNIQUES, vol. 23, 1997, pages 139 |
| YI ZHENG ET AL: "A novel gemini-like cationic lipid for the efficient delivery of siRNA", NEW JOURNAL OF CHEMISTRY, vol. 38, no. 10, 1 January 2014 (2014-01-01), GB, pages 4952 - 4962, XP055698806, ISSN: 1144-0546, DOI: 10.1039/C4NJ00531G * |
| YI-MEI ZHANG ET AL: "Cationic gemini lipids with cyclen headgroups: interaction with DNA and gene delivery abilities", RSC ADV., vol. 4, no. 83, 1 January 2014 (2014-01-01), pages 44261 - 44268, XP055470447, DOI: 10.1039/C4RA05974C * |
| YOKOE ET AL., NATURE BIOTECHNOLOGY, vol. 14, 1996, pages 1252 - 1256 |
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12023376B1 (en) | 2020-04-17 | 2024-07-02 | Triad National Security, Llc | Conserved region T cell vaccines for coronavirus and methods of use |
| EP4046629A1 (en) | 2021-02-19 | 2022-08-24 | ModernaTX, Inc. | Lipid nanoparticle compositions and methods of formulating the same |
| WO2022221736A2 (en) | 2021-04-16 | 2022-10-20 | Genentech, Inc. | Optimized tlr7 ligands and uses thereof |
| WO2023031394A1 (en) | 2021-09-03 | 2023-03-09 | CureVac SE | Novel lipid nanoparticles for delivery of nucleic acids |
| WO2023073228A1 (en) | 2021-10-29 | 2023-05-04 | CureVac SE | Improved circular rna for expressing therapeutic proteins |
| WO2023144330A1 (en) | 2022-01-28 | 2023-08-03 | CureVac SE | Nucleic acid encoded transcription factor inhibitors |
| WO2023177904A1 (en) | 2022-03-18 | 2023-09-21 | Modernatx, Inc. | Sterile filtration of lipid nanoparticles and filtration analysis thereof for biological applications |
| WO2023227608A1 (en) | 2022-05-25 | 2023-11-30 | Glaxosmithkline Biologicals Sa | Nucleic acid based vaccine encoding an escherichia coli fimh antigenic polypeptide |
| WO2024044147A1 (en) | 2022-08-23 | 2024-02-29 | Modernatx, Inc. | Methods for purification of ionizable lipids |
| DE202023106198U1 (en) | 2022-10-28 | 2024-03-21 | CureVac SE | Nucleic acid-based vaccine |
| CN116218862A (en) * | 2023-01-11 | 2023-06-06 | 郑州大学 | SELENOO gene mutant and application thereof |
| CN116218862B (en) * | 2023-01-11 | 2024-01-30 | 郑州大学 | SELENOO gene mutant and application thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| US20220177423A1 (en) | 2022-06-09 |
| EP3956303A1 (en) | 2022-02-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2020214946A1 (en) | Cystine cationic lipids | |
| AU2019278813B2 (en) | Cationic lipids comprising a steroidal moiety | |
| JP7488193B2 (en) | Thioester Cationic Lipids | |
| EP3962902A1 (en) | Di-thioester cationic lipids | |
| EP3801627A1 (en) | Phosphoester cationic lipids | |
| WO2019232095A1 (en) | Vitamin cationic lipids | |
| CN114401942B (en) | Tris (hydroxymethyl) methylglycine and citrate lipids | |
| WO2020219427A1 (en) | Thioester cationic lipids | |
| WO2020257611A1 (en) | Cationic lipids comprising an hydroxy moiety | |
| ES2971849T3 (en) | Cationic lipid compounds and compositions thereof for use in the delivery of messenger RNA | |
| EP3877368A1 (en) | 2,5-dioxopiperazine lipids with intercalated ester, thioester, disulfide and anhydride moieities | |
| WO2020243540A1 (en) | Macrocyclic lipids | |
| EP3876994A2 (en) | Peg lipidoid compounds | |
| EP3877444A1 (en) | Multi-peg lipid compounds |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 20724352 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2020724352 Country of ref document: EP Effective date: 20211118 |