WO2020043325A1 - Pharmaceutical composition comprising an immunomodulatory agent and method for the preparation thereof - Google Patents
Pharmaceutical composition comprising an immunomodulatory agent and method for the preparation thereof Download PDFInfo
- Publication number
- WO2020043325A1 WO2020043325A1 PCT/EP2019/025285 EP2019025285W WO2020043325A1 WO 2020043325 A1 WO2020043325 A1 WO 2020043325A1 EP 2019025285 W EP2019025285 W EP 2019025285W WO 2020043325 A1 WO2020043325 A1 WO 2020043325A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- pharmaceutical composition
- fingolimod
- tricalcium phosphate
- composition according
- weight
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/13—Amines
- A61K31/135—Amines having aromatic rings, e.g. ketamine, nortriptyline
- A61K31/137—Arylalkylamines, e.g. amphetamine, epinephrine, salbutamol, ephedrine or methadone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4841—Filling excipients; Inactive ingredients
- A61K9/485—Inorganic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4841—Filling excipients; Inactive ingredients
- A61K9/4858—Organic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4816—Wall or shell material
- A61K9/4825—Proteins, e.g. gelatin
Definitions
- the present invention relates to a stable pharmaceutical formulation for oral administration containing a therapeutically effective quantity of an immunomodulatory agent such as Fingolimod and a method for the preparation thereof.
- MS Multiple sclerosis
- CNS central nervous system
- MS represents the leading cause of non-traumatic neurologic disability in young and middle-aged adults and has a major physical, psychological, social and financial impact on patients and their families, friends and institutions responsible for health care.
- Relapsing MS is the most frequent clinical presentation of the disease. The majority of patients are females (2:1 female to male ratio) diagnosed between the ages of 20 and 40. At diagnosis, approximately 85% of patients have relapsing remitting MS (RRMS), characterized by recurrent acute exacerbations (relapses) of neurological dysfunction followed by recovery. A significant proportion (42 - 57%) of relapses may result in incomplete recovery of function and leave permanent disability and impairment. After 6 - 10 years, 30 - 40% of patients with RRMS have progressed to secondary progressive MS (SPMS), in which a less inflammatory and more neurodegenerative course of the disease takes over. SPMS presents with steady progression in disability with or without superimposed relapses.
- SPMS secondary progressive MS
- Fingolimod is a sphingosine 1 -phosphate receptor modulator. It is metabolized by sphingosine kinase to the active metabolite Fingolimod phosphate. Fingolimod phosphate, binds at low nanomolar concentrations to sphingosine l-phosphate (S1P) receptors 1, 3, and 4 located on lymphocytes, and readily crosses the blood-brain barrier to bind to S1P receptors 1, 3, and 5 located on neural cells in the central nervous system.
- S1P sphingosine l-phosphate
- Fingolimod phosphate By acting as a functional antagonist of S1P receptors on lymphocytes, Fingolimod phosphate blocks the capacity of lymphocytes to egress from lymph nodes, causing a redistribution, rather than depletion, of lymphocytes. This redistribution is claimed to reduce the infiltration of pathogenic lymphocyte cells into the central nervous system, where they would be involved in nerve inflammation and nervous tissue damage.
- Fingolimod hydrochloride became the first oral drug approved to reduce relapses and delay disability progression in patients with relapsing forms of multiple sclerosis (MS).
- MS drugs on the markets were all delivered by frequent injections varying from once-per-day to once-per-week depending on the drug.
- Fingolimod hydrochloride 2-amino-2-(2-(4- octylphenyl)ethyl)propan-l,3-diol hydrochloride. Its molecular formula is C1 9 H33NO2.HCI corresponding to a molecular weight of 343.93. It is a white to practically white powder.
- the salt form of Fingolimod is freely soluble in water and pH 1.0 buffer, very slightly soluble in pH 4.0 buffer and practically insoluble in pH 6.8 buffer.
- Fingolimod is not a chiral molecule and therefore does not show any specific rotation.
- Fingolimod hydrochloride exhibits polymorphism.
- polymorphs are mentioned in literature.
- the polymorphic form of the active substance used in the preferred composition of the present invention is form I which is stable and is also disclosed in WO-A- 2010/055028.
- Fingolimod is instable in presence of many excipients. Especially at high temperatures or humidity conditions many pharmaceutically acceptable excipients are not compatible with Fingolimod because when mixed thereto induce impurities or degradation products at a level above the acceptable level for a pharmaceutical composition according to the Regulatory Health authorities.
- EP 1613288 Bl discloses a solid pharmaceutical composition suitable for oral administration comprising Fingolimod and a sugar alcohol.
- US 6277888 Bl discloses a pharmaceutical composition comprising Fingolimod and a lecithin.
- WO 2011/131370 Al discloses a method for preparing an intermediate comprising melt processing Fingolimod with a matrix former.
- an object of the present invention to provide an improved stable solid dosage formulation for oral administration containing Fingolimod or pharmaceutical acceptable salt thereof as an active ingredient, which overcomes the deficiencies of the prior art.
- Further object of the present invention is to provide hard gelatin capsules comprising Fingolimod HC1 as an active ingredient, which are bioavailable and with sufficient self-life.
- a major object of the present invention is the selection of the optimal combination of pharmaceutical acceptable excipients and method of preparation in order to achieve the appropriate dissolution profile and stability for the finished dosage form.
- Said dosage form affords predictable and reproducible drug release rates in order to achieve better treatment to a patient.
- a further approach of the present invention is to provide a capsule composition for oral administration comprising Fingolimod HC1 which is manufactured through a fast, simple and cost-effective process.
- a pharmaceutical composition for oral administration comprising Fingolimod as an active ingredient in combination with inorganic salts in order to avoid any interaction with the active ingredient and formation of degradation products compromising the dissolution characteristics.
- a process for the preparation of a stable, solid dosage form for oral administration containing Fingolimod or pharmaceutical acceptable salt thereof as an active ingredient in combination with inorganic salts in order to avoid any interaction with the active ingredient and formation of degradation products compromising the dissolution characteristics is provided, which comprises the following steps:
- a pharmaceutical composition comprising an active ingredient is considered to be“stable” if said ingredient degrades less or more slowly than it does on its own and/or in known pharmaceutical compositions.
- the main object of the present invention is to provide a stable pharmaceutical composition of Fingolimod for oral administration that is simple to manufacture, bioavailable, cost effective and possesses good pharmacotechnical properties.
- Oral administration is a route of administration where a substance is taken through the mouth. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration is the most frequently used route of administration because of its simplicity and convenience, which improve patient compliance.
- Capsules are solid dosage forms in which the drug is enclosed within either a hard or soft soluble container or“shell”.
- the shells are usually formed from gelatin; however, they also may be made from starch or other suitable substances.
- Hard gelatin capsules consist of two, telescoping cap and body pieces. Generally, there are unique grooves or indentations molded into the cap and body portions to provide a positive closure when fully engaged, which helps prevent the accidental separation of the filled capsules during shipping and handling.
- Hard shell capsules typically are filled with powder, beads, or granules but semisolids or liquids may be also filled into them. Mixing or blending of powder ingredients is a very common industrial process practiced in many industries including pharmaceuticals. The main advantage of dry mixing process is that less equipment and space is required. Further, said process eliminates the need for binder solution, heavy mixing equipment and time consuming drying step required for wet granulation.
- hard gelatin capsules are filled with powder blend.
- the first step in that process is the disintegration of the dosage form followed by dissolution of the active ingredient. Since the invention relates to an immediate release dosage form the dissolution properties are critical for its performance.
- excipients are known to facilitate administration and modulate release of the active component. They can also stabilize it against degradation from the environment. Most excipients have no direct pharmacological action but they can impart useful properties to the formulation. However, they can also give rise to inadvertent and/or unintended effects such as increased degradation of the drug. Physical and chemical interactions between drugs and excipients can affect the chemical nature, the stability and bioavailability of drug products, and consequently, their therapeutic efficacy and safety.
- Fingolimod is an amino-diol which may undergo unwanted reactions with several excipients. Acid-base interactions and Maillard reactions are probably the most common API-excipient interactions reported.
- the excipients used in the present invention were selected with the perspective to enhance dissolution, physicochemical properties and stability of drug substances in the finished dosage form and thus were subjected to compatibility study with the API.
- Diluents or fillers increase the bulk of a solid pharmaceutical composition, and may make a pharmaceutical dosage form easier for the patient and care giver to handle. They also improve flowability and permit use of direct compression manufacturing.
- Diluents for solid compositions include, for example, microcrystalline cellulose (MCC), dextrose, dextrates, fructose, mannitol, maltodextrin, maltitol, lactose, sucrose, calcium phosphates, maltose.
- Fingolimod undergoes Maillard reaction in the presence of reducing sugars and thus inorganic salts are used preferably as fillers in the present invention.
- Inorganic salts in general void of functional groups that can react with the drug substance leading to the formation of unwanted by-products. Also they are stable during storage maintaining their physical properties. Calcium salts like calcium phosphates, tricalcium citrate are widely used as fillers in solid dosage forms providing very good flow properties.
- Calcium phosphates here including, the dihydrate and anhydrous form of dibasic calcium phosphate and tribasic calcium phosphate are granular insoluble materials. They are widely used as capsule fillers as well as wet granulation and direct compression diluents in tablet formulation. They are also used in pharmaceutical products because of their compaction properties, and the good flow properties of the coarse-grade material. Bulk density of calcium phosphates is higher than that of organic fillers. They are used extensively in vitamin and mineral preparations. Calcium phosphate is used both as an excipient and as a source of calcium in nutritional supplements. They are chemically stable and compatible with a great number of drug substances.
- Lubricants prevent adherence of granule/powder to die wall and promote injection from the die after compaction. They also reduce inter particle friction and improve the rate of flow of the powder. Lubricants tend to be hydrophobic, so their level needs to be optimized. Under lubricated blends tend to flow poorly and show compression sticking problems. Over lubricated blends can adversely affect dissolution rate as well as strength. Common lubricants used in pharmaceutical preparations are talc, magnesium stearate, calcium stearate, sodium stearyl fumarate, glyceryl behenate, stearic acid. Most preferably stearic acid is used as lubricant in the present invention in an amount 0.2-2% by weight. A number of immediate release compositions comprising different excipients were tested as presented in the following examples to achieve the optimal properties with respect to the objectives of the present invention.
- Compositions 1-5 exhibited acceptable dissolution profiles and they were further stored under accelerated conditions to study the chemical stability. In the table below are depicted the pre-stability data of Compositions 1-5 after 6 months storage at 40°C/75% RH. Table 2: Pre-stability data after storage for 6 months at 40°C/75%
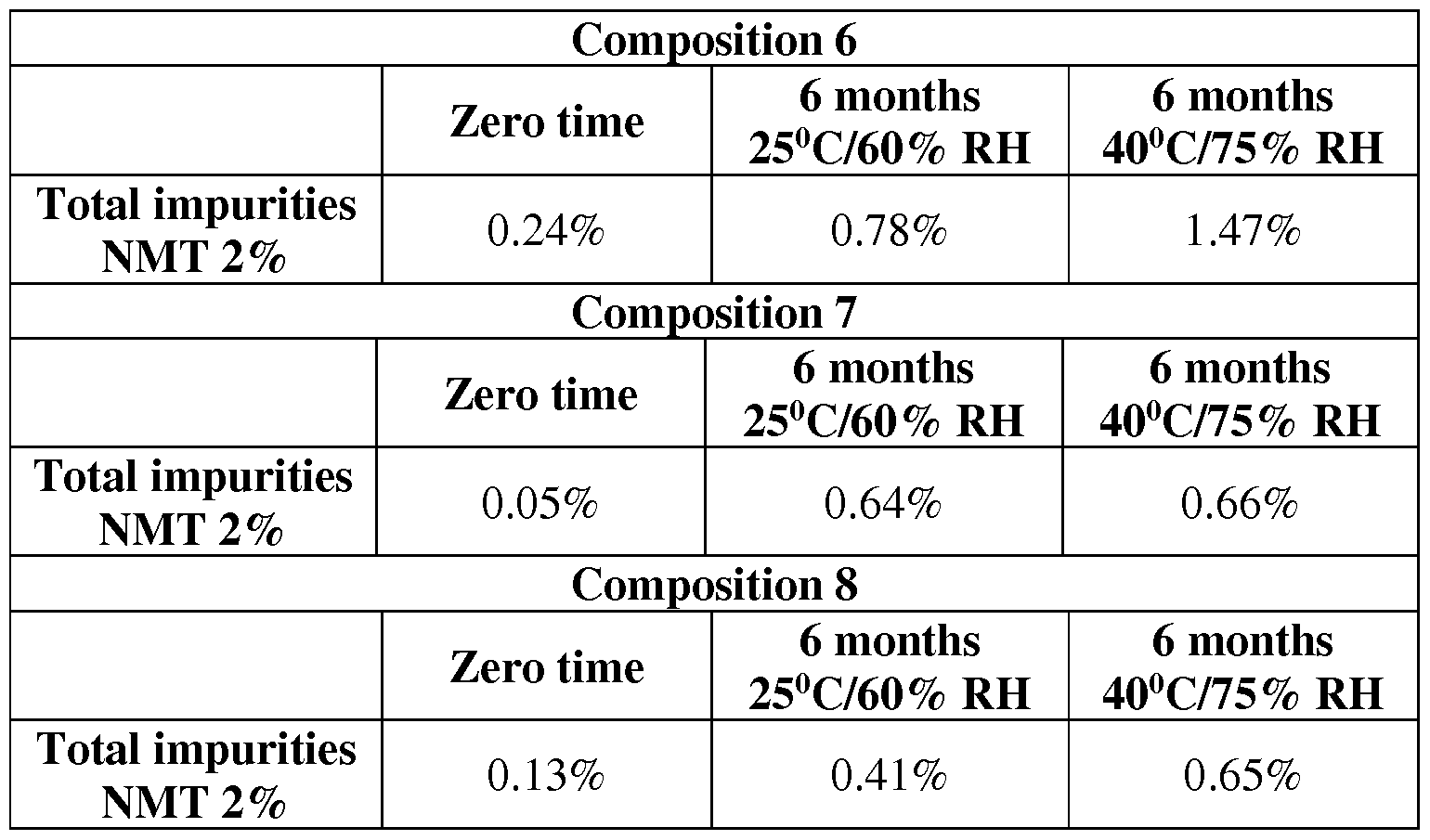
- Table 5 Stability data of trials 6-8 under normal and accelerated conditions for 6 months
- Composition 8 in which tricalcium phosphate was used as filler provided lower level of degradation products. Since it exhibited also faster dissolution profile it was selected to be used in the formulation of Fingolimod hard gelatin capsules.
- two different grades of tricalcium phosphate were used in the formulation, so that blend uniformity is achieved with optimum flow properties of the blend.
- tricalcium phosphate with bulk density 0,25g/ml was first mixed with Fingolimod HC1 since their physical properties (e.g. particle size, bulk density) are similar and thus result in good blend uniformity.
- the rest amount of tricalcium phosphate that was added in the blend had higher bulk density (0,5g/ml) providing very good flow properties to the final blend.
- the formula is presented in the following table (Composition 9).
- compositions 10-13 were prepared according to the following manufacturing process:
- composition 12 Comparing Compositions 9-12 containing different types of lubricant as well as Composition 13 without lubricant it was concluded that stearic acid was the most suitable in terms of blend uniformity and chemical stability. Slight differences were also observed in dissolution properties. Consequently, the preferred composition of the present invention is Composition 12
Abstract
The present invention relates to a stable pharmaceutical formulation of solid dosage forms for oral administration containing a therapeutically effective amount of Lingolimod HCl in combination with inorganic salts in order to avoid any interaction with the active ingredient and formation of degradation products. It also relates to a process for the preparation thereof.
Description
PHARMACEUTICAL COMPOSITION COMPRISING AN IMMUNOMODULATORY AGENT AND METHOD FOR THE PREPARATION THEREOF
TECHNICAL FIELD OF THE INVENTION
The present invention relates to a stable pharmaceutical formulation for oral administration containing a therapeutically effective quantity of an immunomodulatory agent such as Fingolimod and a method for the preparation thereof.
BACKGROUND OF THE INVENTION
Multiple sclerosis (MS) is a chronic, autoimmune and neurodegenerative disorder of the central nervous system (CNS), characterized by inflammation, demyelination, oligodendrocyte and neuronal loss. MS represents the leading cause of non-traumatic neurologic disability in young and middle-aged adults and has a major physical, psychological, social and financial impact on patients and their families, friends and institutions responsible for health care.
Relapsing MS is the most frequent clinical presentation of the disease. The majority of patients are females (2:1 female to male ratio) diagnosed between the ages of 20 and 40. At diagnosis, approximately 85% of patients have relapsing remitting MS (RRMS), characterized by recurrent acute exacerbations (relapses) of neurological dysfunction followed by recovery. A significant proportion (42 - 57%) of relapses may result in incomplete recovery of function and leave permanent disability and impairment. After 6 - 10 years, 30 - 40% of patients with RRMS have progressed to secondary progressive MS (SPMS), in which a less inflammatory and more neurodegenerative course of the disease takes over. SPMS presents with steady progression in disability with or without superimposed relapses.
Fingolimod is a sphingosine 1 -phosphate receptor modulator. It is metabolized by sphingosine kinase to the active metabolite Fingolimod phosphate. Fingolimod phosphate, binds at low nanomolar concentrations to sphingosine l-phosphate (S1P) receptors 1, 3, and 4 located on lymphocytes, and readily crosses the blood-brain barrier to bind to S1P receptors 1, 3, and 5 located on neural cells in the central nervous system. By acting as a
functional antagonist of S1P receptors on lymphocytes, Fingolimod phosphate blocks the capacity of lymphocytes to egress from lymph nodes, causing a redistribution, rather than depletion, of lymphocytes. This redistribution is claimed to reduce the infiltration of pathogenic lymphocyte cells into the central nervous system, where they would be involved in nerve inflammation and nervous tissue damage.
Fingolimod hydrochloride became the first oral drug approved to reduce relapses and delay disability progression in patients with relapsing forms of multiple sclerosis (MS). Before, the MS drugs on the markets were all delivered by frequent injections varying from once-per-day to once-per-week depending on the drug.
The chemical name of Fingolimod hydrochloride is 2-amino-2-(2-(4- octylphenyl)ethyl)propan-l,3-diol hydrochloride. Its molecular formula is C19H33NO2.HCI corresponding to a molecular weight of 343.93. It is a white to practically white powder. The salt form of Fingolimod is freely soluble in water and pH 1.0 buffer, very slightly soluble in pH 4.0 buffer and practically insoluble in pH 6.8 buffer.
Fingolimod is not a chiral molecule and therefore does not show any specific rotation. However, Fingolimod hydrochloride exhibits polymorphism. Several polymorphs are mentioned in literature. The polymorphic form of the active substance used in the preferred composition of the present invention is form I which is stable and is also disclosed in WO-A- 2010/055028.
Fingolimod is instable in presence of many excipients. Especially at high temperatures or humidity conditions many pharmaceutically acceptable excipients are not compatible with Fingolimod because when mixed thereto induce impurities or degradation products at a level above the acceptable level for a pharmaceutical composition according to the Regulatory Health Authorities.
EP 1613288 Bl discloses a solid pharmaceutical composition suitable for oral administration comprising Fingolimod and a sugar alcohol.
US 6277888 Bl discloses a pharmaceutical composition comprising Fingolimod and a lecithin.
WO 2011/131370 Al discloses a method for preparing an intermediate comprising melt processing Fingolimod with a matrix former.
Although each of the patents above represents an attempt to overcome the stability problems associated with pharmaceutical compositions comprising Fingolimod, there still exists a need for an improved pharmaceutical composition which avoids such problems and provides increased patient compliance.
SUMMARY OF THE INVENTION
It is, therefore, an object of the present invention to provide an improved stable solid dosage formulation for oral administration containing Fingolimod or pharmaceutical acceptable salt thereof as an active ingredient, which overcomes the deficiencies of the prior art.
Further object of the present invention is to provide hard gelatin capsules comprising Fingolimod HC1 as an active ingredient, which are bioavailable and with sufficient self-life.
A major object of the present invention is the selection of the optimal combination of pharmaceutical acceptable excipients and method of preparation in order to achieve the appropriate dissolution profile and stability for the finished dosage form. Said dosage form affords predictable and reproducible drug release rates in order to achieve better treatment to a patient.
A further approach of the present invention is to provide a capsule composition for oral administration comprising Fingolimod HC1 which is manufactured through a fast, simple and cost-effective process.
In accordance with the above aspects of the present invention, a pharmaceutical composition for oral administration is provided comprising Fingolimod as an active ingredient in combination with inorganic salts in order to avoid any interaction with the active ingredient and formation of degradation products compromising the dissolution characteristics.
According to another embodiment of the present invention, a process for the preparation of a stable, solid dosage form for oral administration, containing Fingolimod or pharmaceutical acceptable salt thereof as an active ingredient in combination with inorganic salts in order to avoid any interaction with the active ingredient and formation of degradation products
compromising the dissolution characteristics is provided, which comprises the following steps:
-Mixing Fingolimod HC1 with the fillers;
-Lubrication of the powder blend with the lubricant;
-Filling in capsules.
Other objects and advantages of the present invention will become apparent to those skilled in the art in view of the following detailed description.
DETAILED DESCRIPTION OF THE INVENTION
For the purposes of the present invention, a pharmaceutical composition comprising an active ingredient is considered to be“stable” if said ingredient degrades less or more slowly than it does on its own and/or in known pharmaceutical compositions.
As already mentioned the main object of the present invention is to provide a stable pharmaceutical composition of Fingolimod for oral administration that is simple to manufacture, bioavailable, cost effective and possesses good pharmacotechnical properties.
Oral administration is a route of administration where a substance is taken through the mouth. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration is the most frequently used route of administration because of its simplicity and convenience, which improve patient compliance.
Capsules are solid dosage forms in which the drug is enclosed within either a hard or soft soluble container or“shell”. The shells are usually formed from gelatin; however, they also may be made from starch or other suitable substances. Hard gelatin capsules consist of two, telescoping cap and body pieces. Generally, there are unique grooves or indentations molded into the cap and body portions to provide a positive closure when fully engaged, which helps prevent the accidental separation of the filled capsules during shipping and handling. Hard shell capsules typically are filled with powder, beads, or granules but semisolids or liquids may be also filled into them.
Mixing or blending of powder ingredients is a very common industrial process practiced in many industries including pharmaceuticals. The main advantage of dry mixing process is that less equipment and space is required. Further, said process eliminates the need for binder solution, heavy mixing equipment and time consuming drying step required for wet granulation.
According to embodiments of the present invention hard gelatin capsules are filled with powder blend.
In order for a drug to have its effect after oral administration it must go into solution and then diffuse through the gut wall into the body. The first step in that process is the disintegration of the dosage form followed by dissolution of the active ingredient. Since the invention relates to an immediate release dosage form the dissolution properties are critical for its performance.
The formulation of a drug substance frequently involves it being blended with different excipients to improve manufacturability, and to maximize the product’s ability to administer the drug dose effectively. Excipients are known to facilitate administration and modulate release of the active component. They can also stabilize it against degradation from the environment. Most excipients have no direct pharmacological action but they can impart useful properties to the formulation. However, they can also give rise to inadvertent and/or unintended effects such as increased degradation of the drug. Physical and chemical interactions between drugs and excipients can affect the chemical nature, the stability and bioavailability of drug products, and consequently, their therapeutic efficacy and safety.
Fingolimod is an amino-diol which may undergo unwanted reactions with several excipients. Acid-base interactions and Maillard reactions are probably the most common API-excipient interactions reported.
The excipients used in the present invention were selected with the perspective to enhance dissolution, physicochemical properties and stability of drug substances in the finished dosage form and thus were subjected to compatibility study with the API.
Diluents or fillers increase the bulk of a solid pharmaceutical composition, and may make a pharmaceutical dosage form easier for the patient and care giver to handle. They also improve flowability and permit use of direct compression manufacturing. Diluents for solid compositions include, for example, microcrystalline cellulose (MCC), dextrose, dextrates, fructose, mannitol, maltodextrin, maltitol, lactose, sucrose, calcium phosphates, maltose.
Fingolimod undergoes Maillard reaction in the presence of reducing sugars and thus inorganic salts are used preferably as fillers in the present invention.
Inorganic salts in general void of functional groups that can react with the drug substance leading to the formation of unwanted by-products. Also they are stable during storage maintaining their physical properties. Calcium salts like calcium phosphates, tricalcium citrate are widely used as fillers in solid dosage forms providing very good flow properties.
Calcium phosphates, here including, the dihydrate and anhydrous form of dibasic calcium phosphate and tribasic calcium phosphate are granular insoluble materials. They are widely used as capsule fillers as well as wet granulation and direct compression diluents in tablet formulation. They are also used in pharmaceutical products because of their compaction properties, and the good flow properties of the coarse-grade material. Bulk density of calcium phosphates is higher than that of organic fillers. They are used extensively in vitamin and mineral preparations. Calcium phosphate is used both as an excipient and as a source of calcium in nutritional supplements. They are chemically stable and compatible with a great number of drug substances.
Most preferably two different grades of tricalcium phosphate (bulk density 0.25g/ml & 0.5g/ml) are used as fillers in the present invention in an amount 90-99.5 % by weight.
Lubricants prevent adherence of granule/powder to die wall and promote injection from the die after compaction. They also reduce inter particle friction and improve the rate of flow of the powder. Lubricants tend to be hydrophobic, so their level needs to be optimized. Under lubricated blends tend to flow poorly and show compression sticking problems. Over lubricated blends can adversely affect dissolution rate as well as strength. Common lubricants used in pharmaceutical preparations are talc, magnesium stearate, calcium stearate, sodium stearyl fumarate, glyceryl behenate, stearic acid.
Most preferably stearic acid is used as lubricant in the present invention in an amount 0.2-2% by weight. A number of immediate release compositions comprising different excipients were tested as presented in the following examples to achieve the optimal properties with respect to the objectives of the present invention.
EXAMPLES
In a first approach, several soluble diluents were tested in order to study their effect on the dissolution properties of Fingolimod final dosage form as well as their effect on its chemical stability. In Table 1 below Fingolimod HC1 and filler were sieved and geometrically mixed due to the very low percentage of the API in the final mixture. The blend was lubricated with magnesium stearate and finally filled into capsules.
Table 1: Compositions 1-5
Compositions 1-5 exhibited acceptable dissolution profiles and they were further stored under accelerated conditions to study the chemical stability. In the table below are depicted the pre-stability data of Compositions 1-5 after 6 months storage at 40°C/75% RH. Table 2: Pre-stability data after storage for 6 months at 40°C/75%
Based on the results presented above, it can be concluded that the use of saccharides with Fingolimod hydrochloride leads to the formation of high level of degradation products after storage for 6 months at 40°C/75% RH. Thus, further trials were prepared using inorganic salts as fillers in order to avoid any interaction with the active ingredient.
In table 3 below Fingolimod HC1 and inorganic salts used as fillers were mixed. Magnesium stearate was used for lubrication purposes in the final blends. The powder was filled in capsules.
Table 3: Composition 6-8
The next table presents the dissolution profiles of the trials 6-8
Table 4: Dissolution profiles in 0.1N HCL + 0.2% SLS, Baskets lOOrpm , 500ml
Comparing the dissolution profiles of Compositions 6-8 a faster drug release is achieved in Composition 8 at the initial time points.
Also the trials were tested for their chemical stability. Capsules from trials 6-8 were stored under normal and accelerated conditions for 6 months and were analyzed in terms of degradation products.
Table 5: Stability data of trials 6-8 under normal and accelerated conditions for 6 months
According to the results, Composition 8 in which tricalcium phosphate was used as filler provided lower level of degradation products. Since it exhibited also faster dissolution profile it was selected to be used in the formulation of Fingolimod hard gelatin capsules.
As a further optimization of the manufacturing process, two different grades of tricalcium phosphate were used in the formulation, so that blend uniformity is achieved with optimum flow properties of the blend. In particular, tricalcium phosphate with bulk density 0,25g/ml was first mixed with Fingolimod HC1 since their physical properties (e.g. particle size, bulk density) are similar and thus result in good blend uniformity. The rest amount of tricalcium phosphate that was added in the blend had higher bulk density (0,5g/ml) providing very good flow properties to the final blend. The formula is presented in the following table (Composition 9).
Table 6: Composition 9
Based on Composition 9, different types of lubricants were also tested in order to study their effect on blend uniformity of the powder blend as well as their effect on chemical stability.
Apart from magnesium stearate, which is the most common lubricant used in solid dosage forms, other lubricants were also tested such as talc, stearic acid, sodium stearyl fumarate. The respective formulas are presented in the following table.
Table 7: Compositions 10-13
-Mixing of Fingolimod HC1 with Tricalcium phosphate (bulk dens. 0,25);
-Mixing with Tricalcium phosphate (bulk dens. 0,5);
-Lubrication of the powder blend with the respective lubricant (only in Compositions 10-12); -Filling in capsules.
Table 8: Results of Compositions 9-13
Comparing Compositions 9-12 containing different types of lubricant as well as Composition 13 without lubricant it was concluded that stearic acid was the most suitable in terms of blend uniformity and chemical stability. Slight differences were also observed in dissolution properties. Consequently, the preferred composition of the present invention is Composition 12
While the present invention has been described with respect to the particular embodiments, it will be apparent to those skilled in the art that various changes and modifications may be made in the invention without departing from the spirit and scope thereof, as defined in the appended claims.
Claims
1. A pharmaceutical composition for oral administration comprising Fingolimod HC1 in combination with inorganic salts as fillers in order to avoid any interaction with the active ingredient and formation of impurities and/or degradation products.
2. A pharmaceutical composition according to claim 1, wherein the fillers are selected from dibasic calcium phosphate anhydrous, tricalcium citrtate, tricalcium phosphate.
3. A pharmaceutical composition according to claim 1, wherein the filler is preferably tricalcium phosphate.
4. A pharmaceutical composition according to claim 3, comprising two different grades of tricalcium phosphate.
5 A pharmaceutical composition according to claim 4, comprising tricalcium phosphate with bulk density 0.25g/ml and tricalcium phosphate with bulk density 0.5g/ml.
6. A pharmaceutical composition according to any preceding claim, comprising tricalcium phosphate in an amount of from 90 to 99.5% by weight of the total weight of the
composition.
7. A pharmaceutical composition according to any preceding claim, wherein it further comprises lubricants.
8. A pharmaceutical composition according to claim 7, wherein the lubricant is preferably stearic acid.
9. A pharmaceutical composition according to claim 8, comprising stearic acid in an amount of from 0.2 to 2% by weight of the total weight of the composition.
10. A pharmaceutical composition according to any preceding claim, wherein it is in the form of hard gelatin capsules.
11. A process for the preparation of a pharmaceutical composition for oral administration comprising Fingolimod HC1 in combination with inorganic salts as fillers in order to avoid any interaction with the active ingredient and formation of impurities and/or degradation products is provided, which comprises the following steps:
- Mixing of Fingolimod HC1 with Tricalcium phosphate (bulk dens. 0,25);
-Mixing with Tricalcium phosphate (bulk dens. 0,5);
-Lubrication of the powder blend with stearic acid;
-Filling in capsules.
12. The process according to claim 11, comprising tricalcium phosphate in an amount of from 90 to 99.5% by weight of the total weight of the composition.
13. The process according to claim 11, comprising stearic acid in an amount of from 0.2 to 2% by weight of the total weight of the composition.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP19765411.4A EP3843707A1 (en) | 2018-08-31 | 2019-08-28 | Pharmaceutical composition comprising an immunomodulatory agent and method for the preparation thereof |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GR20180100397A GR1009654B (en) | 2018-08-31 | 2018-08-31 | Pharmaceutical composition comprising an immunomodulatory agent and method for the preparation thereof |
| GR20180100397 | 2018-08-31 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2020043325A1 true WO2020043325A1 (en) | 2020-03-05 |
Family
ID=67875422
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2019/025285 WO2020043325A1 (en) | 2018-08-31 | 2019-08-28 | Pharmaceutical composition comprising an immunomodulatory agent and method for the preparation thereof |
Country Status (3)
| Country | Link |
|---|---|
| EP (1) | EP3843707A1 (en) |
| GR (1) | GR1009654B (en) |
| WO (1) | WO2020043325A1 (en) |
Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6277888B1 (en) | 1997-02-27 | 2001-08-21 | Welfide Corporation | Drug composition |
| EP1613288A1 (en) | 2003-04-08 | 2006-01-11 | Novartis AG | Solid pharmaceutical compositions comprising a s1p receptor agonist and a sugar alcohol |
| CN1891212A (en) * | 2005-07-07 | 2007-01-10 | 马启明 | Oral preparation and its preparing method |
| WO2010055028A2 (en) | 2008-11-11 | 2010-05-20 | Novartis Ag | Organic compounds |
| WO2011131370A1 (en) | 2010-04-22 | 2011-10-27 | Ratiopharm Gmbh | Melt-granulated fingolimod |
| US20130034603A1 (en) * | 2011-08-01 | 2013-02-07 | Julia Hrakovsky | Process for preparing pharmaceutical compositions of fingolimod |
| IN2011CH01844A (en) * | 2011-05-30 | 2013-06-14 | ||
| US20140199382A1 (en) * | 2013-01-11 | 2014-07-17 | Cadila Healthcare Limited | Stable pharmaceutical compositions of an s1p receptor agonist |
| US20150141520A1 (en) * | 2013-11-18 | 2015-05-21 | Chandrasekhar Kandi | Stabilized pharmaceutical compositions of fingolimod and process for preparation thereof |
| WO2015104666A2 (en) * | 2014-01-09 | 2015-07-16 | Torrent Pharmaceuticals Limited | Pharmaceutical composition of fingolimod |
| EP3143991A1 (en) * | 2015-09-18 | 2017-03-22 | Sanovel Ilac Sanayi ve Ticaret A.S. | Fingolimod capsule composition |
| WO2017058364A1 (en) * | 2015-10-02 | 2017-04-06 | Mylan Inc. | Stable formulations of fingolimod |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MX370184B (en) * | 2013-05-13 | 2019-12-04 | Synthon Bv | Pharmaceutical composition comprising fingolimod. |
-
2018
- 2018-08-31 GR GR20180100397A patent/GR1009654B/en active IP Right Grant
-
2019
- 2019-08-28 WO PCT/EP2019/025285 patent/WO2020043325A1/en unknown
- 2019-08-28 EP EP19765411.4A patent/EP3843707A1/en active Pending
Patent Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6277888B1 (en) | 1997-02-27 | 2001-08-21 | Welfide Corporation | Drug composition |
| EP1613288A1 (en) | 2003-04-08 | 2006-01-11 | Novartis AG | Solid pharmaceutical compositions comprising a s1p receptor agonist and a sugar alcohol |
| CN1891212A (en) * | 2005-07-07 | 2007-01-10 | 马启明 | Oral preparation and its preparing method |
| WO2010055028A2 (en) | 2008-11-11 | 2010-05-20 | Novartis Ag | Organic compounds |
| WO2011131370A1 (en) | 2010-04-22 | 2011-10-27 | Ratiopharm Gmbh | Melt-granulated fingolimod |
| IN2011CH01844A (en) * | 2011-05-30 | 2013-06-14 | ||
| US20130034603A1 (en) * | 2011-08-01 | 2013-02-07 | Julia Hrakovsky | Process for preparing pharmaceutical compositions of fingolimod |
| US20140199382A1 (en) * | 2013-01-11 | 2014-07-17 | Cadila Healthcare Limited | Stable pharmaceutical compositions of an s1p receptor agonist |
| US20150141520A1 (en) * | 2013-11-18 | 2015-05-21 | Chandrasekhar Kandi | Stabilized pharmaceutical compositions of fingolimod and process for preparation thereof |
| WO2015104666A2 (en) * | 2014-01-09 | 2015-07-16 | Torrent Pharmaceuticals Limited | Pharmaceutical composition of fingolimod |
| EP3143991A1 (en) * | 2015-09-18 | 2017-03-22 | Sanovel Ilac Sanayi ve Ticaret A.S. | Fingolimod capsule composition |
| WO2017058364A1 (en) * | 2015-10-02 | 2017-04-06 | Mylan Inc. | Stable formulations of fingolimod |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3843707A1 (en) | 2021-07-07 |
| GR1009654B (en) | 2019-11-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20200276137A1 (en) | Formulations comprising 2-amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol | |
| CN103002877A (en) | A sustained release formulation of methotrexate as a disease-modifying antirheumatic drug (DMARD) and an anti-cancer agent | |
| HUE031251T2 (en) | Controlled release oral dosage form comprising oxycodone | |
| JP4853818B2 (en) | Solid formulation containing ibuprofen and ambroxol hydrochloride | |
| JPS6036424A (en) | Medicinal composition containing liquid lubricating agent | |
| BR112015014430B1 (en) | TABLET COMPOSITION | |
| WO2020043325A1 (en) | Pharmaceutical composition comprising an immunomodulatory agent and method for the preparation thereof | |
| AU2011379627A1 (en) | Sublingual pharmaceutical composition containing an antihistamine agent and method for the preparation thereof | |
| US20020037899A1 (en) | Compositions containing an inhibitor of dihydrofolate reductase and a folate | |
| AU2020209883A1 (en) | A method of manufacturing a pharmaceutical composition comprising nefopam and acetaminophen, and the pharmaceutical composition obtained thereby | |
| JP2018100259A (en) | Solid preparation | |
| EP3297612A1 (en) | Pharmaceutical composition comprising atomoxetine and method for the preparation thereof | |
| KR101893110B1 (en) | Formulation containing biphenyldimethyldicarboxylate and garlic oil | |
| WO2024028262A1 (en) | Novel formulation | |
| AU2011100878A4 (en) | Oral formulations | |
| AU2011100879B4 (en) | Oral formulations | |
| AU2011100876A4 (en) | Oral formulations | |
| Banupriya | Development and Evaluation of Extended Release Tablets of Repinirole using various Polymes | |
| Dineshchandra | Design and In vitro evaluation of mouth dissolving tablets of olanzapine | |
| Karumanchi | Formulation Development and Evaluation of Deferasirox Dispersible Tablets | |
| Karbhari | Development And Evaluation Of Sustained Release Matrices Of Tramadol Hydrochloride Using Synthetic Polymers | |
| Aravapalli | Formulation and Evaluation of Escitalopram Oxalate Immediate Release Tablets | |
| KR20180089811A (en) | A high swellable sustained-release triple-layer tablet containing pregabalin | |
| JPH0357881B2 (en) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19765411 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2019765411 Country of ref document: EP Effective date: 20210331 |