WO2019123066A1 - Process for the preparation of opicapone and intermediates thereof - Google Patents
Process for the preparation of opicapone and intermediates thereof Download PDFInfo
- Publication number
- WO2019123066A1 WO2019123066A1 PCT/IB2018/059598 IB2018059598W WO2019123066A1 WO 2019123066 A1 WO2019123066 A1 WO 2019123066A1 IB 2018059598 W IB2018059598 W IB 2018059598W WO 2019123066 A1 WO2019123066 A1 WO 2019123066A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- formula
- compound
- opicapone
- preparation
- process according
- Prior art date
Links
- 0 *c1cc(C=O)cc([N+]([O-])=O)c1O Chemical compound *c1cc(C=O)cc([N+]([O-])=O)c1O 0.000 description 5
- WRFGQLDAKOYZHS-UHFFFAOYSA-N Cc1c(/C(/N)=N/O)c(Cl)nc(C)c1Cl Chemical compound Cc1c(/C(/N)=N/O)c(Cl)nc(C)c1Cl WRFGQLDAKOYZHS-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/04—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/4439—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a five-membered ring with nitrogen as a ring hetero atom, e.g. omeprazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/78—Carbon atoms having three bonds to hetero atoms, with at the most one bond to halogen, e.g. ester or nitrile radicals
Definitions
- the present invention is relates to a process for the preparation of opicapone and a process to prepare intermediates to be used therein.
- Opicapone is a selective and reversible catechol-O-methyltransferase (COMT) inhibitor, use as adjunctive therapy for parkinson’s disease.
- Opicapone was approved by European Medicine Agency (EMA) on June 24, 2016 and it is developed and marketed as ONGENTYS ® by Bial-Portela in Europe.
- Opicapone is chemically described as 2,5-dichloro-3-(5-(3,4-dihydroxy-5-nitrophenyl)-l,2,4- oxadiazol-3-yl)-4,6-dimethylpyridine-l-oxide and depicted below as compound of formula (I).
- Opicapone and a process for preparation of it is disclosed in US 8,168,793.
- the process disclosescondensation of 3, 4-dibenzyloxy-5-nitrobenzoic acid with (Z)-2, 5-dichloro-N'-hydroxy-4, 6-dimethylnicotinimidamide in presence of N, N’- Carbonyl diimidazole in N, N’-dimethylformamide.
- the crude condensation intermediate was subjected to tetrabutylammonium fluoride (TBAF) mediated cyclization in tetrahydrofuran to give l,2,4-oxadiazole derivative, purifying it by precipitating in 1:1 mixture of dichloromethane: diethyl ether and recrystallized it in isopropyl alcohol.
- Oxidation of l,2,4-oxadiazole compound is carried out using 10 fold excess of urea hydrogen peroxide complex and trifluoroacetic anhydride in dichloromethane and was purified by column chromatography.
- US 9,126,988 also disclose process for the preparation of opicapone, whichinvolves several chemical steps: 1) nitrating vanillic acid in presence of nitric acid in acetic acidfollowed by recrystallization with acetic acid to get nitro compound with yield 40-46%; 2)which converted into acid chloride compoundby treating it with thionyl chloride in presence of catalytic amount of N, N- dimethylformamide in dichloromethane or l,4-dioxane; 3) condensing acid chloride compound with (Z)-2, 5-dichloro-N'-hydroxy-4, 6- dimethylnicotinimidamide in presence of excess amount of pyridine in N,N- dimethyl acetamide/ tetrahydrofuran/ dichloromethane or l,4-dioxane at 5-10 °C and then heating the reaction mixture at H0-l l5°C for 5-6 hours to get 1,2,4- oxadiazole compound; 4) which
- US 9,126,988 also disclosed process for the preparation of 2,5-dichloro-N'- hydroxy-4,6-dimethylnicotinimidamide compound of formula (IV), in which 2,5- dichloro-4,6-dimethylnicotinonitrile compound of formula (VIII) was reacted with hydroxyl amine solution in the presence of catalytic amount of 1,10- phenanthroline in methanokwater at 70-80°C for 6 hrs. After completion reaction mixture was cooled, filtered and dried to get 2,5-dichloro-N'-hydroxy-4,6- dimethylnicotinimidamide of formula (IV) (88%).
- US 5,358, 948 also disclosed process for preparation of 3,4-dimethoxy-5-nitro benzoic acid compound of formula (Ilia).
- a solution of potassium permanganate was added to a solution of 3,4-dimethoxy-5-nitro benzaldehyde in acetone.
- the mixture was then stirred at 20°C for 18 hrs togives 3,4-dimethoxy-5- nitro benzoic acid compound of formula (Ilia) with 72% yield.
- Oxidation of aldehydes to the corresponding carboxylic acids are commonly carried out using KMn04 in acidic or basic media, or K2Cr207 in acidic medium or chromic acid. These heavy metal-based reagents are hazardous and the protocols produce metal wastes that require special handling owing to their toxicities.
- An object of the invention is to provide process for the preparation of opicapone, overcoming the defects and deficiencies in the prior literatures.
- Yet another object of the invention is to provide intermediates and process thereof for the preparation of opicapone.
- Yet another object of the invention is to provide process for the preparation of compound of formula (IV).
- Yet another object of the invention is to provide process for the preparation of compound of formula (III).
- Anobject of the present invention relates to process for the preparation of opicapone compound of formula (I) comprising;
- Ri and R 2 independently from each other represent hydrogen or a suitable protecting groups for aromatic hydroxyl groups.
- the present invention also relates to provide intermediates of compound of formula (Va), (Via) and (Vila) for the preparation of opicapone compound of formula (I).
- Yet another object of the invention is to control formation of impurities like impurity A, impurity B and impurity C during preparation of opicapone.
- Yet another object of the invention is to provide process for the preparation of compound of formula (IV) wherein process comprises reacting compound of formula (VIII) with hydroxyl amine or salt thereof in the presence of base.
- Yet another object of the invention is to provide process for the preparation of compound of formula (III) by oxidizing compound of formula (II) using oxone.
- Yet another object of the invention is to provide process for the purification of compound of formula (VII) or (Vila) using Bronsted acid in presence of organic solvent.
- Yet another object of the invention is to provide method for purification of opicapone in presence of organic solvent.
- the present invention provides a process for the preparation of opicapone compound of formula (I) comprising;
- Ri and R 2 independently from each other represent hydrogen or a suitable protecting groups for aromatic hydroxyl groups.
- Suitable protective groups for aromatic hydroxyl groups are well known in the art.
- suitable protective groups for aromatic hydroxyl groups include but not limited to methyl, ethyl, isopropyl, butyl, benzyl, 4-methoxybenzyl, methoxymethyl, benzyloxymethyl, methoxyethoxymethyl, tetrahydropyranyl, phenacyl, allyl, trimethylsilyl, tert-butyldimethylsilyl, benzyloxycarbonyl, tert- butoxycarbonyl, ester, sulphonate, carbamate, phosphinate, acetal, ketal derivatives and the like.
- the oxidation of compound of formula (II) or (Ila) with oxone is carried out in presence of solvent selected from group consisting of N,N-Dimethylmethanamide (DMF), acetone, acetonitrile, N-methylpyrrolidone (NMP), Hexamethylphosphoramide (HMPA), pyrrolidinone, Tetrahydrofuran, water, ethyl acetate, l,4-dioxane, acetonitrile, propionitrile, acetone, ethyl methyl ketone, formamide, chlorinated hydrocarbons such as dichloromethane, ethylene dichloride, chloroform, dimethyl acetamide, propionamide, nitromethane, 1,2- dimethoxyethane, 2-methoxyethanol, 2-ethoxy ethanol, aliphatic and alicyclic hydrocarbons such as hexane, heptane, pentane, cyclohexane,
- reaction is carried out for about 1 to 10 hrs at 0° to 50°C, Obtained product is isolated by addition of excess of water followed by filtration and used in the next step with or without purification.
- Purification of crude acid derivative is done by giving water slurry to remove excess of peroxide content followed by filtration and drying.
- Oxone commercially available from Aldrich Chemical Company, is a 2:1:1 molar mixture of KHS05, KHS04, and K2S04 and is readily soluble in water. Considering the water- solubility and the environmentally safe and benign nature of Oxone, we recently used this reagent in conjunction with another oxidant for the oxidation of alcohols to carboxylic acids.
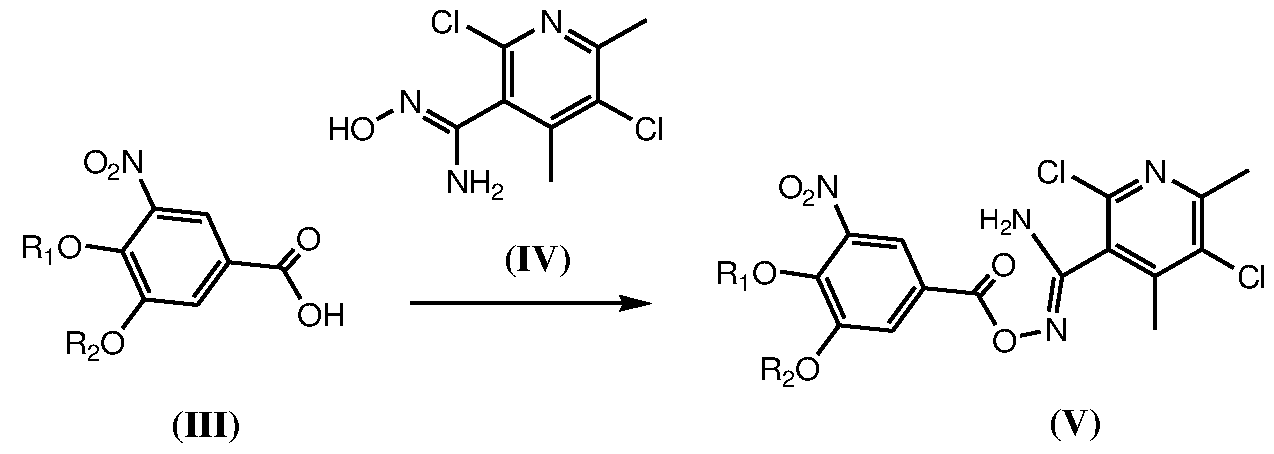
- the condensation of compound of formula (III) or (Ilia) with compound of formula (IV) is carried out in presence of condensing agent and suitable solvent at temperature 0°C to 30°Cdepending on boiling point of the utilized solvent system.
- the reaction temperature preferably at room temperature.
- the reaction time is in the range of 30 minutes to 24 hours.
- the condensing agents used for reaction are selected from group consisting of N, N’-Carbonyldiimidazole, thionyl chloride, sulfonyl chloride, N,N'- dicyclohexylcarbodiimide, l-hydroxybenzotriazole and N-(3- dimethylaminopropyl)-N'-ethylcarbodiimide, phosgene, PC13, POC13, PC15, anhydrides, trichlorotriazine and chlorodimethoxytriazine and the like.
- step (b) is performed in an organic solvent selected from dimethylformamide, dimethylsulfoxide, dimethylacetamide and N- methylpyrrolidinone, acetonitrile, Tetrahydrofuran, ethyl acetate, l,4-dioxane, acetonitrile, propionitrile, acetone, ethyl methyl ketone, formamide, chlorinated hydrocarbons such as dichloromethane, ethylene dichloride, chloroform, dimethyl sulphoxide, sulpholane, acetamide, propionamide, nitromethane, anisole, aliphatic and alicyclic hydrocarbons such as hexane, heptane, pentane, cyclohexane, methyl cyclohexane, aliphatic esters and aromatic hydrocarbons such as toluene, mixture of xylenes and mixture(s) thereof.
- organic solvent selected from dimethylformamide,
- the compound of formula (V) or (Va) is isolated by addition of excess of water followed by filtration and drying.
- Cyclization of compound of formula (V) or (Va) is carried out atroom temperature in presence of base and organic solvent to obtain compound of formula (VI) or (Via).
- the reaction time is in the range of 30 minutes to 24 hours.
- Cyclization is carried out in presence of organic solvent selected from methanol, ethanol, propanol, isopropanol, n-butanol, tert-butanol, tetrahydrofuran, water, l,4-dioxane, acetonitrile, propionitrile, acetone, ethyl methyl ketone, formamide, N,N, dimethylformamide, chlorinated hydrocarbons such as dichloromethane (MDC), ethylene dichloride, chloroform, dimethyl sulphoxide, sulpholane, acetamide, propionamide, nitromethane, l,2-dimethoxyethane, 2-methoxyethanol, 2-ethoxy ethanol, anisole, aliphatic and alicyclic hydrocarbons such as hexane, heptane, pentane, cyclohexane, methyl cyclohexane, aliphatic esters, aromatic hydro
- the base used in cyclization reaction are organic bases such as triethyl amine, diisopropylethylamine, DMAP, and/oraqueous solution(s) and mixture(s) thereof and inorganic bases such as sodium hydroxide (NaOH), potassium hydroxide (KOH), potassium carbonate, sodium carbonate, sodiumbicarbonate, potassium bicarbonate, sodium hydride and/or and aqueous(s) solution and mixture(s) thereof; other bases such as potassium tert-butoxide, sodium tert-butoxide and/or and aqueous(s) solution and mixture(s) thereof.
- organic bases such as triethyl amine, diisopropylethylamine, DMAP, and/oraqueous solution(s) and mixture(s) thereof
- inorganic bases such as sodium hydroxide (NaOH), potassium hydroxide (KOH), potassium carbonate, sodium carbonate, sodiumbicarbonate, potassium bicarbonate, sodium hydride and/or and
- object of the present invention is to provide one pot process to prepare compound of formula (VI) or (Via) by condensation and cyclization reaction is carried out in the same reaction vessel.
- condensation of compound of formula (III) or (Ilia) with compound of formula (IV) is carried out in presence of condensing agent as described in above (step (b)), followed by cyclization of compound of formula (V) or (Va) in presence of base as described in above.
- condensation and cyclization are conducted sequentially in the same reaction vessel without isolation of compound of formula (V) or (Va).
- Yet another objective of the present invention is to use alkyl group protection for both phenolic hydroxy group in compound of formula (Ilia).
- the alkyl protection of both phenolic hydroxy group in compound of formula (Ilia) becomes very advantageous because one can remove all acidic impurities present in the compound of formula of (Via) or (Vila) by simple washing with basic aqueous solution during work up procedure.
- Cyclization step in present invention for preparation of l,2,4-oxadiazole derivative involves simple reaction conditions such as ambient reaction temperature and use of cheaper inorganic base such as KOH or NaOH making process simple and cost effective.
- Oxidation reaction of compound of formula (VI) or (Via) is performed with oxidising agent.
- N-oxide group can be introduced to compound of formula (VI) or (Via) by using oxidizing agent such as hydrogen peroxide, Mn0 2 , peracetic acid, trifluoroperacetic acid, t-butylhydroperoxide, m-chloroperoxybenzoic acid, persulfuric acids, Oxone®, urea hydrogen peroxide complex and trifluoroacetic anhydride, pyridinium chlorochromate and permanganate ions.
- Preferred oxidation is performed with urea hydrogen peroxide complex in presence of organic acid anhydride such as trifluoroacetic anhydride.
- the solvent used in oxidation step (d) is selected from halogenated solvents, such as dichloromethane, chloroform, chlorobenzene and carbon tetrachloride, aromatic solvents such as benzene and toluene, alkanes such as cyclohexane and hexane, and ethers such as THF, l,4-dioxane diisopropyl ethyl ether, cyclopentyl methyl ether and tert-butylmethylether and mixture(s) thereof and other solvents are formic acid, acetic acid, trifluoroacetic acid, DMF, N,N-dimethylacetamide (DMA), and mixture(s) thereof.
- halogenated solvents such as dichloromethane, chloroform, chlorobenzene and carbon tetrachloride
- aromatic solvents such as benzene and toluene
- alkanes such as cyclohex
- step (d) is performed at temperature is in range from 5°C-l00°C, during reaction and workup process for about 1 to 24 hours.
- Obtained N-oxide derivative compound of formula (VII) or (Vila) is purified by using organic solvent selected from acetone, toluene, ethyl acetate, dichloromethane, chloroform, carbontetrachloride, methanol, ethanol, isopropyl alcohol, n-propanol, n-butanol, DMF, dimethyl sulfoxide (DMSO), DMA, NMP, Bronsted acids such as HC1, H 2 S0 4 , HBr, acetic acid, formic acid, trifluoroacetic acid and/or mixture(s) thereof. More preferably N-oxide derivative was purified by using cone hydrochloric acid and ethyl acetate.
- N-oxide derivative is treated with cone hydrochloric acid in ethyl acetate at 25- 80°C followed by cooling to room temperature and filtration to obtain compound of formula (VII) or (Vila)
- the deprotection is carried out in the presence of Lewis acid, Bronsted acids such as HC1, H2SO4, HBr, acetic acid, formic acid, trifluoroacetic acid, Pd/c, AICI3.
- deprotection of hydroxy protecting group of compound of formula (VII) or (Vila) is carried out in presence of aluminium chloride (A1C13) in organic solvent such as N, N- dimethyl formamide at temperature is in range temperature range from 5-l20°C.
- aluminium chloride (A1C13) in organic solvent such as N, N- dimethyl formamide at temperature is in range temperature range from 5-l20°C.
- the present invention developed cost effective process by avoiding use of pyridine which creates complications during work up procedure.
- the organic solvent selected from toluene, ethyl acetate, xylenes, DMF, DMSO, MDC, NMP and/or mixture(s) thereof.
- Opicapone compound of formula (I) is further purified by using organic solvent selected from methanol, ethanol, isopropyl alcohol, DMF, DMSO, DMA, NMP, acetic acid, and/or mixture(s) thereof.
- Yet another object of the invention is to provide the intermediate compound of the formula (Via), (Vila) and (Villa) and the process for the preparation of same as described above.
- Yet another object of the invention is to provide process for the preparation of compound of formula (IV) comprising reacting compound of formula (VIII) with hydroxyl amine or salt thereof in the presence of base.
- reaction of compound of formula (VIII) was carried out using hydroxylamine in the presence of catalytic or stoichiometric amount of organic base such as pyridine, triethyl amine, N,N,N',N'-tetramethylethylenediamine, diisopropylethyl amine, 4-dimethylaminopyridine, N-methyl morpholine, pyrazine or its derivatives (2-methyl pyrazine, 2,5- dimethyl pyrazine)and/or aqueous thereof.
- organic base such as pyridine, triethyl amine, N,N,N',N'-tetramethylethylenediamine, diisopropylethyl amine, 4-dimethylaminopyridine, N-methyl morpholine, pyrazine or its derivatives (2-methyl pyrazine, 2,5- dimethyl pyrazine)and/or aqueous thereof.
- reaction of compound of formula (VIII) was carried out using hydroxylamine salts in the presence of inorganic base such as LiOH, KOH, NaOH, K 2 C0 3 , Na 2 C0 3 , Li 2 C0 3 , NaHC0 3 , KHC0 3 and catalytic amount of organic base such as pyridine, triethyl amine, N,N,N',N'-tetramethylethylenediamine, diisopropylethyl amine, 4-dimethylaminopyridine, N-methyl morpholine, pyrazine or its derivatives and/or aqueous thereof.
- inorganic base such as LiOH, KOH, NaOH, K 2 C0 3 , Na 2 C0 3 , Li 2 C0 3 , NaHC0 3 , KHC0 3 and catalytic amount of organic base such as pyridine, triethyl amine, N,N,N',N'-tetramethylethylenediamine, diisopropyleth
- the solvent used in the above reaction is selected from methanol, ethanol, isopropanol, n-propanol, n-butanol, tert-butanol, DMF, DMSO, NMP, acetonitrile, tetrahydrofuran, l,4-dioxane, water and/or mixture(s) thereof .
- the reaction is carried out at temperature range from 25°-90°C.
- the reaction time is in the range of 5-10 hours.
- hydroxylamine salt is selected from hydrochloride, hydrobromide, sulfate salts.
- the present invention use of commercially cheap hydroxyl amine salts for preparation of compound of formula (IV) to make cost effective process.
- Yet another object of the invention is to provide process for the preparation of compound of formula (III)as described in step (a) as above.
- Opicapone Purification of Opicapone is carried out in presence of organic solvent selected from methanol, ethanol, isopropyl alcohol, dichloromethane, Tetrahydrofuran, toluene, N,N-dimethylformamide, Dimethylsulfoxide, N,N-dimethylacetamide, N- methyl-2-pyrrolidone, acetic acid, ethylacetate, acetone, and mixture(s) thereof. More preferably Opicapone of formula (I)was purified using mixture of N,N- dimethylformamide andmethanol to obtain compound of formula (I).
- organic solvent selected from methanol, ethanol, isopropyl alcohol, dichloromethane, Tetrahydrofuran, toluene, N,N-dimethylformamide, Dimethylsulfoxide, N,N-dimethylacetamide, N- methyl-2-pyrrolidone, acetic acid, ethylacetate, acetone, and mixture(s)
- reaction mixture was washed with 1000 ml of water, 1N aqueous HC1 solution (500ml x 2) followed by 500 ml of 5% aqueous sodium bicarbonate solution. Solvent was distilled out at atmospheric pressure at 40°C. To the residue was added 1200 ml of methanol and the suspension was stirred at 55-60°C for 2 hours. The reaction mixture was allowed to cool to room temperature, maintained for 2 hours and filtered.
- the reaction mixture was washed with water (300 ml x 2), 300ml of 5% aqueous sodium sulphite solution to quench residual peroxides and finally with 300 ml of water.
- Dichloromethane layer was distilled out at atmospheric pressure.
- the obtained solid was suspended in 250 ml of ethyl acetate and 12.5 ml of cone. HC1 was added at room temperature. The resulting suspension was then stirred at 65-70°C for 1 hour and allowed to cool to room temperature.
- reaction mixture was filtered, solid washed with water (100 ml X 3) followed by methanol (50 ml x2) and dried at 50°C under vacuum to obtain 5-[3-(2,5- Dichloro-4, 6-dimethyl- l-oxido-3-pyridinyl)- 1,2, 4-oxadiazol-5-yl]-3-nitro- 1,2- benzenediol of formula (I) (22 g, 94%).
- a process for the preparation of opicapone compound of formula (I) comprising steps of;
- Ri and R 2 independently from each other represent hydrogen or a suitable protecting groups for aromatic hydroxyl groups.
- suitable protective groups for aromatic hydroxyl groups are selected from methyl, ethyl, isopropyl, butyl, benzyl, 4-methoxybenzyl, methoxymethyl, benzyloxymethyl, methoxyethoxymethyl, tetrahydropyranyl, phenacyl, allyl, trimethylsilyl, tert- butyldimethylsilyl, benzyloxycarbonyl, tert-butoxycarbonyl, ester, sulphonate, carbamate, phosphinate, acetal, ketal derivatives.
- step (a) is performed in solvent selected from N, N-Dimethylmethanamide (DMF), acetone, acetonitrile, N- methylpyrrolidone (NMP), Hexamethylphosphoramide (HMPA), pyrrolidinone, tetrahydrofuran, water, thyl acetate, l,4-dioxane, acetonitrile, propionitrile, acetone, ethyl methyl ketone, formamide, dichloromethane, ethylene dichloride, chloroform, dimethyl acetamide, propionamide, nitromethane, l,2-dimethoxyethane, 2-methoxyethanol, 2-ethoxy ethanol, aliphatic, hexane, heptane, pentane, cyclohexane, methyl cyclohexane, aliphatic esters and mixture(s) thereof.
- solvent selected from N, N-Dimethylmethanamide (
- base is selected from group consisting of triethyl amine, diisopropylethylamine, DMAP, sodium hydroxide (NaOH), potassium hydroxide (KOH), potassium carbonate, sodium carbonate, sodiumbicarbonate, potassium bicarbonate, sodium hydride, potassium ter- butoxide, sodium ter-butoxide and/or and aqueous(s) solution and mixture(s) thereof.
- oxidizing agent is selected from the group consisting of peroxide, Mn0 2 , peracetic acid, trifluoroperacetic acid, t-butylhydroperoxide, m-chloroperoxybenzoic acid, persulfuric acids, Oxone®, urea hydrogen peroxide complex and trifluoroacetic anhydride, pyridinium chlorochromate and permanganate ions.
- oxidizing agent is selected from the group consisting of peroxide, Mn0 2 , peracetic acid, trifluoroperacetic acid, t-butylhydroperoxide, m-chloroperoxybenzoic acid, persulfuric acids, Oxone®, urea hydrogen peroxide complex and trifluoroacetic anhydride, pyridinium chlorochromate and permanganate ions.
- deprotection is carried out in presence of aluminium chloride (A1C13) in organic solvent.
- opicapone further comprising a purification step of opicapone compound of formula (I) with a organic solvent selected from methanol, ethanol, isopropyl alcohol, dichloromethane, tetrahydrofuran, toluene, N,N-dimethylformamide, dimethylsulfoxide, N,N- dimethylacetamide, N-methyl-2-pyrrolidone, acetic acid, ethylacetate, acetone, and mixture(s) thereof.
- a process for the preparation of compound of formula (IV) comprising; a. reacting compound of formula (VIII) with hydroxyl amine in the presence of catalytic amount of pyrazine or pyrazine derivative.
- the present invention i s relates to a process for the preparation of opicapone and a process to prepare intermediates to be used therein.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Psychology (AREA)
- Epidemiology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Nitrogen And Oxygen As The Only Ring Hetero Atoms (AREA)
- Plural Heterocyclic Compounds (AREA)
- Pyridine Compounds (AREA)
Abstract
Description
Claims
Priority Applications (11)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020207017502A KR20200100075A (en) | 2017-12-18 | 2018-12-04 | Opicaphone and its intermediate manufacturing method |
| BR112020011888-5A BR112020011888A2 (en) | 2017-12-18 | 2018-12-04 | process of preparing opicapone and its intermediates |
| EA202091259A EA202091259A1 (en) | 2017-12-18 | 2018-12-04 | METHOD FOR OBTAINING OPICAPONE AND ITS INTERMEDIATES |
| AU2018392845A AU2018392845A1 (en) | 2017-12-18 | 2018-12-04 | Process for the preparation of opicapone and intermediates thereof |
| US16/954,501 US20210087183A1 (en) | 2017-12-18 | 2018-12-04 | Process for the preparation of opicapone and intermediates thereof |
| EP18892354.4A EP3728241A4 (en) | 2017-12-18 | 2018-12-04 | Process for the preparation of opicapone and intermediates thereof |
| CN201880082145.7A CN111511735A (en) | 2017-12-18 | 2018-12-04 | Method for preparing ompapone and intermediate thereof |
| JP2020531598A JP2021506762A (en) | 2017-12-18 | 2018-12-04 | How to make Opikapon and its intermediates |
| MX2020006283A MX2020006283A (en) | 2017-12-18 | 2018-12-04 | Process for the preparation of opicapone and intermediates thereof. |
| PH12020550871A PH12020550871A1 (en) | 2017-12-18 | 2020-06-11 | Process for the preparation of opicapone and intermediates thereof |
| ZA2020/03590A ZA202003590B (en) | 2017-12-18 | 2020-06-15 | Process for the preparation of opicapone and intermediates thereof |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN201721045330 | 2017-12-18 | ||
| IN201721045330 | 2017-12-18 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019123066A1 true WO2019123066A1 (en) | 2019-06-27 |
Family
ID=66993196
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IB2018/059598 WO2019123066A1 (en) | 2017-12-18 | 2018-12-04 | Process for the preparation of opicapone and intermediates thereof |
Country Status (12)
| Country | Link |
|---|---|
| US (1) | US20210087183A1 (en) |
| EP (1) | EP3728241A4 (en) |
| JP (1) | JP2021506762A (en) |
| KR (1) | KR20200100075A (en) |
| CN (1) | CN111511735A (en) |
| AU (1) | AU2018392845A1 (en) |
| BR (1) | BR112020011888A2 (en) |
| EA (1) | EA202091259A1 (en) |
| MX (1) | MX2020006283A (en) |
| PH (1) | PH12020550871A1 (en) |
| WO (1) | WO2019123066A1 (en) |
| ZA (1) | ZA202003590B (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022180649A1 (en) * | 2021-02-26 | 2022-09-01 | Msn Laboratories Private Limited, R&D Center | Novel process for the preparation of 2,5-dichloro-3-(5-(3,4-dihydroxy-5-nitrophenyl)-1,2,4-oxadiazol-3-yl)-4,6-dimethylpyridine-1-oxide |
| WO2023191648A1 (en) * | 2022-04-01 | 2023-10-05 | Bial - Portela & Ca, S.A. | Prodrugs of opicapone |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112375014A (en) * | 2020-12-17 | 2021-02-19 | 重庆柳江医药科技有限公司 | Oppicapone process impurity, preparation method and application |
| CN114015332A (en) * | 2021-11-26 | 2022-02-08 | 广州双隆文化发展有限公司 | Tinplate printing process |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100256194A1 (en) * | 2009-04-01 | 2010-10-07 | Bial - Portela & Ca, S.A. | Pharmaceutical formulations comprising nitrocatechol derivatives and methods of making the same |
| US8168793B2 (en) * | 2005-07-26 | 2012-05-01 | Portela & Ca., S.A. | Nitrocatechol derivatives as COMT inhibitors |
| US9126988B2 (en) * | 2011-12-13 | 2015-09-08 | Bial—Portela & Ca, S.A. | Intermediate for preparing a catechol-O-methyltransferase inhibitor |
-
2018
- 2018-12-04 MX MX2020006283A patent/MX2020006283A/en unknown
- 2018-12-04 AU AU2018392845A patent/AU2018392845A1/en not_active Abandoned
- 2018-12-04 US US16/954,501 patent/US20210087183A1/en not_active Abandoned
- 2018-12-04 WO PCT/IB2018/059598 patent/WO2019123066A1/en unknown
- 2018-12-04 EA EA202091259A patent/EA202091259A1/en unknown
- 2018-12-04 CN CN201880082145.7A patent/CN111511735A/en not_active Withdrawn
- 2018-12-04 KR KR1020207017502A patent/KR20200100075A/en unknown
- 2018-12-04 EP EP18892354.4A patent/EP3728241A4/en not_active Withdrawn
- 2018-12-04 JP JP2020531598A patent/JP2021506762A/en active Pending
- 2018-12-04 BR BR112020011888-5A patent/BR112020011888A2/en not_active Application Discontinuation
-
2020
- 2020-06-11 PH PH12020550871A patent/PH12020550871A1/en unknown
- 2020-06-15 ZA ZA2020/03590A patent/ZA202003590B/en unknown
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8168793B2 (en) * | 2005-07-26 | 2012-05-01 | Portela & Ca., S.A. | Nitrocatechol derivatives as COMT inhibitors |
| US20100256194A1 (en) * | 2009-04-01 | 2010-10-07 | Bial - Portela & Ca, S.A. | Pharmaceutical formulations comprising nitrocatechol derivatives and methods of making the same |
| US9126988B2 (en) * | 2011-12-13 | 2015-09-08 | Bial—Portela & Ca, S.A. | Intermediate for preparing a catechol-O-methyltransferase inhibitor |
| US20160009700A1 (en) * | 2011-12-13 | 2016-01-14 | BIAL - Portela & Cª., S.A | Chemical compound useful as intermediate for preparing a catechol-o-methyltransferase inhibitor |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3728241A4 * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022180649A1 (en) * | 2021-02-26 | 2022-09-01 | Msn Laboratories Private Limited, R&D Center | Novel process for the preparation of 2,5-dichloro-3-(5-(3,4-dihydroxy-5-nitrophenyl)-1,2,4-oxadiazol-3-yl)-4,6-dimethylpyridine-1-oxide |
| WO2023191648A1 (en) * | 2022-04-01 | 2023-10-05 | Bial - Portela & Ca, S.A. | Prodrugs of opicapone |
Also Published As
| Publication number | Publication date |
|---|---|
| ZA202003590B (en) | 2022-01-26 |
| MX2020006283A (en) | 2020-12-09 |
| KR20200100075A (en) | 2020-08-25 |
| BR112020011888A2 (en) | 2020-11-24 |
| PH12020550871A1 (en) | 2021-04-05 |
| US20210087183A1 (en) | 2021-03-25 |
| EA202091259A1 (en) | 2020-09-22 |
| EP3728241A1 (en) | 2020-10-28 |
| EP3728241A4 (en) | 2021-02-24 |
| AU2018392845A1 (en) | 2020-06-18 |
| CN111511735A (en) | 2020-08-07 |
| JP2021506762A (en) | 2021-02-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3728241A1 (en) | Process for the preparation of opicapone and intermediates thereof | |
| EP2190804B1 (en) | Process and intermediates for preparing integrase inhibitors | |
| US8969561B2 (en) | Apixaban preparation process | |
| JP5881691B2 (en) | Process for producing quinoline-3-carboxamide | |
| CN110105193B (en) | Synthetic method of 2-halogen-5-bromobenzoic acid | |
| DE69907056T2 (en) | Process for the preparation of benzoic acids | |
| JPS626718B2 (en) | ||
| KR20020033617A (en) | Salts of 2,2-dimethyl-1,3-dioxane intermediates and process for the preparation thereof | |
| CA2127945C (en) | Process of producing 2-cyano-4-oxo-4h-benzopyran compounds | |
| EP2714691B1 (en) | Process for the preparation of 2-amino-9-((2-phenyl-1,3-dioxan-5-yloxy)methyl)-1h-purin-6(9h)-one compound useful in the preparation of valganciclovir | |
| US20050096352A1 (en) | Process for the preparation of pantoprazole and salts thereof | |
| WO2016135616A1 (en) | An improved process for the preparation of bisoprolol and its intermediate | |
| CN108530384B (en) | Preparation method of prochlorperazine | |
| AU2016280243B2 (en) | A novel process for the preparation of teriflunomide | |
| WO2017004964A1 (en) | Preparation method of 3-[5-(2-fluorophenyl)-1,2,4-oxadiazol-3-yl]benzoic acid | |
| EP1981885A2 (en) | A method of preparing a 4-oxo-1-(3-substituted phenyl)-1,4-dihydro-1,8-naphthyridine-3-carboxamide phosphodiesterase-4 inhibitor | |
| WO1997027171A1 (en) | Process for preparing n-(3-amino-4-chlorophenyl) acylamides | |
| CN107207435B (en) | Process for preparing 4-cyanopiperidine hydrochloride | |
| JP3486922B2 (en) | Method for producing acid amide | |
| KR960005828B1 (en) | Process for preparation of pyridine derivatives | |
| CN116134021A (en) | Process for producing oxazoles | |
| WO2014067219A1 (en) | Preparation method of (1s)-1-phenyl-3,4-dihydro-2(1h)-isoquinoline carboxylate | |
| CN118613470A (en) | Synthesis method of 7, 8-dihydro-2H-cyclopenta-pyrrolopyrazinone compound | |
| CN111825583A (en) | Preparation method of isoxazole herbicide intermediate | |
| JP2007277101A (en) | Manufacturing method of 2,3,6,7,10,11-hexahydroxytriphenylene |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18892354 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2020531598 Country of ref document: JP Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2018392845 Country of ref document: AU Date of ref document: 20181204 Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2018892354 Country of ref document: EP Effective date: 20200720 |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112020011888 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112020011888 Country of ref document: BR Kind code of ref document: A2 Effective date: 20200612 |