WO2018055526A1 - Trpv4 antagonists - Google Patents
Trpv4 antagonists Download PDFInfo
- Publication number
- WO2018055526A1 WO2018055526A1 PCT/IB2017/055702 IB2017055702W WO2018055526A1 WO 2018055526 A1 WO2018055526 A1 WO 2018055526A1 IB 2017055702 W IB2017055702 W IB 2017055702W WO 2018055526 A1 WO2018055526 A1 WO 2018055526A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- sulfonyl
- pyrrolidin
- hydroxymethyl
- hydroxy
- chloro
- Prior art date
Links
- 0 *C(CN(C1)S(*)(=O)=O)(C1S(*)(=O)=O)O Chemical compound *C(CN(C1)S(*)(=O)=O)(C1S(*)(=O)=O)O 0.000 description 10
- AICKIBFBGSYUCB-LLVKDONJSA-N C=C(CN(C1)S(c(ccc(C#N)c2)c2Cl)(=O)=O)[C@@H]1O Chemical compound C=C(CN(C1)S(c(ccc(C#N)c2)c2Cl)(=O)=O)[C@@H]1O AICKIBFBGSYUCB-LLVKDONJSA-N 0.000 description 1
- BGIONJICBFLFPS-AVRDEDQJSA-N CC(C)(C)OC(NC[C@](CN(C1)S(c(ccc(C#N)c2)c2Cl)(=O)=O)([C@H]1S(c(cc1)ncc1Cl)(=O)=O)O)=O Chemical compound CC(C)(C)OC(NC[C@](CN(C1)S(c(ccc(C#N)c2)c2Cl)(=O)=O)([C@H]1S(c(cc1)ncc1Cl)(=O)=O)O)=O BGIONJICBFLFPS-AVRDEDQJSA-N 0.000 description 1
- GXMIVFYYHMOGQO-AVRDEDQJSA-N CC(C)(C)OC(NC[C@](CN(C1)S(c(ccc(C#N)c2)c2Cl)(=O)=O)([C@H]1Sc(cc1)ncc1Cl)O)=O Chemical compound CC(C)(C)OC(NC[C@](CN(C1)S(c(ccc(C#N)c2)c2Cl)(=O)=O)([C@H]1Sc(cc1)ncc1Cl)O)=O GXMIVFYYHMOGQO-AVRDEDQJSA-N 0.000 description 1
- VZPPBNLTCBXOHL-DPZKZMLUSA-N C[C@H]([C@](CN(C1)S(c(ccc(C#N)c2)c2Cl)(=O)=O)([C@H]1S(c(cc1)ncc1Cl)(=O)=O)O)O Chemical compound C[C@H]([C@](CN(C1)S(c(ccc(C#N)c2)c2Cl)(=O)=O)([C@H]1S(c(cc1)ncc1Cl)(=O)=O)O)O VZPPBNLTCBXOHL-DPZKZMLUSA-N 0.000 description 1
- GQFHDOABVASHHD-UHFFFAOYSA-N N#Cc(cc(cc1)S)c1Cl Chemical compound N#Cc(cc(cc1)S)c1Cl GQFHDOABVASHHD-UHFFFAOYSA-N 0.000 description 1
- BQDZCYURKGXLCM-ZWKOTPCHSA-N N#Cc(cc1)cc(Cl)c1S(N(C[C@@H]1Sc(cc2)ccc2Br)C[C@]1(CO)O)(=O)=O Chemical compound N#Cc(cc1)cc(Cl)c1S(N(C[C@@H]1Sc(cc2)ccc2Br)C[C@]1(CO)O)(=O)=O BQDZCYURKGXLCM-ZWKOTPCHSA-N 0.000 description 1
- BRDMQWUUMVNAPS-RDTXWAMCSA-N N#Cc(cc1)cc(Cl)c1S(N(C[C@H]1Cc2ncc(C(F)(F)F)cc2)C[C@]1(CO)O)(=O)=O Chemical compound N#Cc(cc1)cc(Cl)c1S(N(C[C@H]1Cc2ncc(C(F)(F)F)cc2)C[C@]1(CO)O)(=O)=O BRDMQWUUMVNAPS-RDTXWAMCSA-N 0.000 description 1
- XFPOFQCBDWTJTL-ZWKOTPCHSA-N N#Cc(cc1)ccc1S([C@@H](CN(C1)S(c(ccc(F)c2)c2Br)(=O)=O)[C@@]1(CO)O)(=O)=O Chemical compound N#Cc(cc1)ccc1S([C@@H](CN(C1)S(c(ccc(F)c2)c2Br)(=O)=O)[C@@]1(CO)O)(=O)=O XFPOFQCBDWTJTL-ZWKOTPCHSA-N 0.000 description 1
- FSEMIGNCPYORBY-NWDGAFQWSA-N N#Cc(cc1)ccc1S([C@@H](CNC1)[C@@]1(CO)O)(=O)=O Chemical compound N#Cc(cc1)ccc1S([C@@H](CNC1)[C@@]1(CO)O)(=O)=O FSEMIGNCPYORBY-NWDGAFQWSA-N 0.000 description 1
- WLVZWRUVPREQAW-DOTOQJQBSA-N NC[C@@](CN(C1)S(c(ccc(C#N)c2)c2Cl)(=O)=O)([C@H]1S(c(cc1)ncc1Cl)(=O)=O)O Chemical compound NC[C@@](CN(C1)S(c(ccc(C#N)c2)c2Cl)(=O)=O)([C@H]1S(c(cc1)ncc1Cl)(=O)=O)O WLVZWRUVPREQAW-DOTOQJQBSA-N 0.000 description 1
- GKQYZHHGIHJDGL-ZVAWYAOSSA-N NC[C@](CN(C1)S(c(cc2)ccc2C#N)(O)O)(C1S(c(cc1)ccc1Cl)(=O)=O)O Chemical compound NC[C@](CN(C1)S(c(cc2)ccc2C#N)(O)O)(C1S(c(cc1)ccc1Cl)(=O)=O)O GKQYZHHGIHJDGL-ZVAWYAOSSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/425—Thiazoles
- A61K31/426—1,3-Thiazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/425—Thiazoles
- A61K31/427—Thiazoles not condensed and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/4439—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a five-membered ring with nitrogen as a ring hetero atom, e.g. omeprazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/506—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim not condensed and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/5375—1,4-Oxazines, e.g. morpholine
- A61K31/5386—1,4-Oxazines, e.g. morpholine spiro-condensed or forming part of bridged ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/10—Laxatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/14—Prodigestives, e.g. acids, enzymes, appetite stimulants, antidyspeptics, tonics, antiflatulents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/18—Drugs for disorders of the alimentary tract or the digestive system for pancreatic disorders, e.g. pancreatic enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/02—Nasal agents, e.g. decongestants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/14—Antitussive agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/16—Central respiratory analeptics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/10—Drugs for disorders of the urinary system of the bladder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/04—Drugs for genital or sexual disorders; Contraceptives for inducing labour or abortion; Uterotonics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/04—Antipruritics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/02—Muscle relaxants, e.g. for tetanus or cramps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/06—Antimigraine agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
- A61P27/06—Antiglaucoma agents or miotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/10—Antioedematous agents; Diuretics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/04—Inotropic agents, i.e. stimulants of cardiac contraction; Drugs for heart failure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/46—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with hetero atoms directly attached to the ring nitrogen atom
- C07D207/48—Sulfur atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D498/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D498/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms in which the condensed system contains two hetero rings
- C07D498/10—Spiro-condensed systems
Definitions
- the present invention relates to pyrrolidine sulfonamide analogs, pharmaceutical compositions containing them and their use as TRPV4 antagonists.
- TRPV4 is a member of the Transient Receptor Potential (TRP) superfamily of cation channels and is activated by heat, demonstrating spontaneous activity at physiological temperatures (Guler et al., 2002. J Neurosci 22: 6408-6414). Consistent with its polymodal activation property TRPV4 is also activated by hypotonicity and physical cell stress/pressure (Strotmann et al., 2000. Nat Cell Biol 2: 695-702), through a mechanism involving phospholipase A2 activation, arachidonic acid and
- tyrosine kinase activity may also regulate TRPV4 (Wegierski et al., 2009. J Biol Chem. 284: 2923-33; Fan et al., 2009. J Biol Chem 284: 27884-91).
- Heart failure results in the decreased ability of the left ventricle to pump blood into the peripheral circulation as indicated by a reduced ejection fraction and/or left ventricular dilation. This increases the left ventricular end diastolic pressure resulting in enhanced pulmonary blood pressures. This places the septal barrier, which separates the circulatory aqueous environment and the alveolar airspaces of the lung, at risk.
- Increased pulmonary pressure results in the flow of fluid from the pulmonary circulation into the alveolar space resulting in lung edema/congestion, as is observed in patients with congestive heart failure.
- TRPV4 is expressed in the lung (Delany et al., 2001 . Physiol. Genomics 4: 165- 174) and its level of expression is up-regulated in individuals with congestive heart failure (Thorneloe et al., 2012. Sci Transl Med 4: 159ra148). TRPV4 has been shown to mediate Ca 2+ entry in isolated endothelial cells and in intact lungs (Jian et al., 2009. Am J Respir Cell Mol Biol 38: 386-92). Endothelial cells are responsible for forming the capillary vessels that mediate oxygen/carbon dioxide exchange and contribute to the septal barrier in the lung.
- TRPV4 channels Activation of TRPV4 channels results in contraction of endothelial cells in culture and cardiovascular collapse in vivo (Willette et al., 2008. J Pharmacol Exp Ther 325: 466-74), at least partially due to the enhanced filtration at the septal barrier evoking lung edema and hemorrage (Alvarez et al., 2006. Circ Res 99: 988-95). Indeed, filtration at the septal barrier is increased in response to increased vascular and/or airway pressures and this response is dependent on the activity of TRPV4 channels (Jian et al., 2008. Am J Respir Cell Mol Biol 38:386-92).
- TRPV4 antagonists prevent and resolve pulmonary edema in heart failure models (Thorneloe et al., 2012. Sci Transl Med 4: 159ra148). Overall this suggests a clinical benefit of inhibiting TRPV4 function in the treatment of acute and/or chronic heart failure associated lung congestion.
- TRPV4 function in pulmonary-based pathologies presenting with symptoms including lung edema/congestion, infection, inflammation, pulmonary remodeling and/or altered airway reactivity.
- a genetic link between TRPV4 and chronic obstructive pulmonary disorder (COPD) has recently been identified (Zhu et al., 2009. Hum Mol Genetics, 18: 2053-62) suggesting potential efficacy for TRPV4 modulation in treatment of COPD with or without coincident emphysema.
- Enhanced TRPV4 activity is also a key driver in ventilator-induced lung injury (Hamanaka et al., 2007.
- TRPV4 activation may underlie pathologies involved in acute respiratory distress syndrome (ARDS), pulmonary fibrosis (Rahaman et al., 2014. J Clin Invest 124: 5225-38), cough (Bonvini et al., 2016 J Allergy Clin Immunol 138: 249-61) and asthma (Liedtke & Simon, 2004. Am J Physiol 287: 269-71).
- ARDS acute respiratory distress syndrome
- pulmonary fibrosis Rahaman et al., 2014. J Clin Invest 124: 5225-38
- cough Bonvini et al., 2016 J Allergy Clin Immunol 138: 249-61
- asthma Liedtke & Simon, 2004.
- Am J Physiol 287: 269-71 A potential clinical benefit for TRPV4 blockers in the treatment of sinusitis, as well as allergic and non-allergic rhinitis is also supported (Bhargave et al., 2008. Am J Rh
- TRPV4 has been shown to be involved in acute lung injury (ALI). Chemical activation of TRPV4 disrupts the alvelor septal blood barrier potentially leading to pulmonary edema (Alvarez et al, Circ Res. 2006 Oct 27;99(9):988-95). In animal models, TRPV4 antagonism attenuates lung damage induced by chemical agents and biological toxins such as HCI, chlorine gas, and platelet activating factor (Balakrishna et al., 2014. Am J Physiol Lung Cell Mol Physiol 307: L158-72; Morty et al., 2014. Am J Physiol Lung Cell Mol Physiol 307: L817-21 ; Yin et al., 2016.
- TRPV4 is necessary in a process known to cause or worsen ALI in humans (Hamanaka et al, Am J Physiol Lung Cell Mol Physiol. 2007 Oct;293(4):L923-32). Overall this suggests a clinical benefit of inhibiting TRPV4 function in the treatment of ARDS and ALL
- TRPV4 has in recent years been implicated in a number of other physiological/pathophysiological processes in which TRPV4 antagonists are likely to provide significant clinical benefit. These include various aspects of pain (Todaka et al., 2004. J Biol Chem 279: 35133-35138; Grant et al., 2007. J Physiol 578: 715-733;
- Chronic cough is highly prevalent worldwide and is highly impactful on the quality of life for suffers, with typical cough rates of 10-50 coughs per hour, during waking hours. It is hypothesized that chronic cough reflects a state of neuronal hypersensitivity involving exaggerated spinal and cortical responses to afferent sensory signals in a manner similar to chronic pain.
- Activation of TRPV4 channels in vivo causes ATP release and triggers afferent sensory signals from the lung through binding of ATP to P2X3 channels, resulting in cough (Bonvini SJ, et al., J Allergy Clin Immunol. 2016 Jul;138(1):249-261 .e12).
- ATP levels are increased in exhaled breath of patients with diseases associated with cough, for example COPD (Basoglu OK, et al., Chest. 2015 Aug;148(2):430-5).
- COPD Basoglu OK, et al., Chest. 2015 Aug;148(2):430-5.
- P2X3 anatagonist has demonstrated high level efficacy in reducing chronic cough and improving quality of life scores in a phase 2 clinical trial (Abdulqawi R, et al. Lancet. 2015 Mar 28; 385(9974):1 198-1205).
- TRPV4 receptors are expressed in airway smooth muscle cells (McAlexander MA, et al., J Pharmacol Exp Ther.
- this invention provides for pyrrolidine sulfonamide compounds of Formula (I), pharmaceutically acceptable salts thereof, and pharmaceutical compositions containing them.
- this invention provides for the use of the compounds of
- this invention provides for compounds of Formula (I) for use in therapy.
- this invention provides for the use of the compounds of Formula (I) for treating conditions associated with TRPV4 imbalance.
- this invention provides for a method of treatment of atherosclerosis, disorders related to vasogenic edema, postsurgical abdominal edema, ocular edema, cerebral edema, local and systemic edema, fluid retention, sepsis, hypertension, inflammation, bone related dysfunctions and congestive heart failure, pulmonary disorders, chronic obstructive pulmonary disorder, ventilator induced lung injury, high altitude induced pulmonary edema, acute respiratory distress syndrome, acute lung injury, pulmonary fibrosis and other fibrosis-related disorders, sinusitis/rhinitis, asthma, COPD, cough; including acute cough, sub-acute cough and chronic cough, pulmonary hypertension, overactive bladder, cystitis, pain, motor neuron disorders, genetic gain of function disorders, amyotrophic lateral sclerosis, multiple sclerosis, cardiovascular disease, acute, chronic and polycystic kidney disease, stroke, hydrocephalus, glaucoma, retinopathy, endometriosis,
- this invention provides for the use of the compounds of Formula (I), and pharmaceutically acceptable salts thereof, for the treatment of atherosclerosis, disorders related to vasogenic edema, postsurgical abdominal edema, ocular edema, cerebral edema, local and systemic edema, fluid retention, sepsis, hypertension, inflammation, bone related dysfunctions and congestive heart failure, pulmonary disorders, chronic obstructive pulmonary disorder, ventilator induced lung injury, high altitude induced pulmonary edema, acute respiratory distress syndrome, acute lung injury, pulmonary fibrosis, sinusitis/rhinitis, asthma, COPD, cough; including acute cough, sub-acute cough and chronic cough, pulmonary hypertension, overactive bladder, cystitis, pain, motor neuron disorders, genetic gain of function disorders, cardiovascular disease, acute, chronic and polycystic kidney disease, stroke, glaucoma, retinopathy, endometriosis, pre-term labor, dermatitis, pruritus
- this invention provides for compounds of Formula (I), and pharmaceutically acceptable salts thereof, for use in the treatment of atherosclerosis, disorders related to vasogenic edema, postsurgical abdominal edema, ocular edema, cerebral edema, local and systemic edema, fluid retention, sepsis, hypertension, inflammation, bone related dysfunctions and congestive heart failure, pulmonary disorders, chronic obstructive pulmonary disorder, ventilator induced lung injury, high altitude induced pulmonary edema, acute respiratory distress syndrome, acute lung injury, pulmonary fibrosis, sinusitis/rhinitis, asthma, COPD, cough; including acute cough, sub-acute cough and chronic cough, pulmonary hypertension, overactive bladder, cystitis, pain, motor neuron disorders, genetic gain of function disorders, cardiovascular disease, acute, chronic and polycystic kidney disease, stroke, glaucoma, retinopathy,
- this invention provides for the use of the compounds of Formula (I), and pharmaceutically acceptable salts thereof, in the manufacture of a medicament for the treatment of atherosclerosis, disorders related to vasogenic edema, postsurgical abdominal edema, ocular edema, cerebral edema, local and systemic edema, fluid retention, sepsis, hypertension, inflammation, bone related dysfunctions and congestive heart failure, pulmonary disorders, chronic obstructive pulmonary disorder, ventilator induced lung injury, high altitude induced pulmonary edema, acute respiratory distress syndrome, acute lung injury, pulmonary fibrosis, sinusitis/rhinitis, asthma, COPD, cough; including acute cough, sub-acute cough and chronic cough, pulmonary hypertension, overactive bladder, cystitis, pain, motor neuron disorders, genetic gain of function disorders, cardiovascular disease, acute, chronic and polycystic kidney disease, stroke, glaucoma, retinopathy, endometriosis, pre-term labor, dermatitisis,
- the TRPV4 antagonist may be administered alone or in conjunction with one or more other therapeutic agents, eg. agents selected from the group consisting of endothelin receptor antagonists, angiotensin converting enzyme (ACE) inhibitors, angiotension II receptor antagonists, vasopeptidase inhibitors, vasopressin receptor modulators, diuretics, digoxin, beta blockers, aldosterone antagonists, inotropes, NSAIDS, nitric oxide donors, calcium channel modulators, muscarinic antagonists, steroidal anti-inflammatory drugs, bronchodilators, antihistamines, leukotriene antagonist, HMG-CoA reductase inhibitors, dual non-selective Padrenoceptor and nq-adrenoceptor antagonists, type-5 phosphodiesterase inhibitors, and renin inhibitors.
- ACE angiotensin converting enzyme
- This invention relates to compounds of Formula (I) and to the use of compounds of Formula (I) in the methods of the invention:
- R is selected from:
- heteroaryl heteroaryl substituted from 1 to 4 times by R a ,
- bicycloheteroaryl and bicycloheteroaryl substituted from 1 to 4 times by R a ; lected from: aryl,
- R is selected from Ci-6alkyl, and Ci-6alkyl substituted with from 1 to 6 substituents independently selected from: fluoro, oxo, -OH, -COOH, -NH2, -CN, -OCl -5alkyl,
- R and R are each independently selected from Ci -6alkyl, and Ci-6alkyl substituted with from 1 to 6 substituents independently selected from: fluoro, oxo, -OH, -COOH, -NH2, and -CN, -C(0)OH,
- Y is taken together with the adjacent -OH to form a heterocyclic ring selected from:
- each R a is independently selected from: fluoro,
- Ci -6alkyl substituted with from 1 to 5 substituents independently selected from: fluoro, chloro, bromo, iodo, Ci -4alkyloxy, -OH, Cl -4alkyl, phenyl, oxo, -COOH, -NO2, -NH2 and -CN, cyano,
- -OCi -6alkyl substituted with from 1 to 5 substituents independently selected from: fluoro, chloro, bromo, iodo, Ci -4alkyloxy, -OH, Cl-4alkyl, phenyl, oxo, -COOH, -NO2, -NH2 and
- each R is independently selected from: fluoro
- Ci-6alkyl substituted with from 1 to 5 substituents independently selected from: fluoro, chloro, bromo, iodo, Ci-4alkyloxy, -OH, Cl-4alkyl, phenyl, oxo, -COOH, -NO2, -NH2 and -CN, cyano,
- -OCi -6alkyl substituted with from 1 to 5 substituents independently selected from: fluoro, chloro, bromo, iodo, Ci-4alkyloxy, -OH, Cl -4alkyl, phenyl, oxo, -COOH, -NO2, -NH2 and -CN,
- R is selected from:
- heteroaryl heteroaryl substituted from 1 to 4 times by R a ,
- bicycloheteroaryl and bicycloheteroaryl substituted from 1 to 4 times by R a .
- R 2 is selected from:
- heteroaryl heteroaryl substituted from 1 to 4 times by R b ,
- Y is selected from:
- R is selected from Ci-6alkyl, and Ci-6alkyl substituted with from 1 to 6 substituents independently selected from: fluoro, oxo, -OH, -COOH, -NH2, -CN, -OCl -5alkyl, -OCi-5alkyl substituted from 1 to 6 times by fluoro and -NH2,
- R and R are each independently selected from Ci -6alkyl, and Ci-6alkyl substituted with from 1 to 6 substituents independently selected from: fluoro, oxo, -OH, -COOH, -NH2, and -CN, -C(0)OH,
- Y' is taken together with the adjacent -OH to form a heterocyclic ring selected from:
- R 2 is selected from:
- R is selected from:
- Y is selected from:
- -OCi -6alkyl substituted with from 1 to 6 substituents independently selected from: fluoro, oxo, -OH, -COOH, -NH2, and -CN,
- R is selected from Ci-5alkyl, and Ci-5alkyl substituted with from 1 to 6 substituents independently selected from: fluoro, oxo, -OH, -COOH, -NH2, and -CN, -C(0)OH,
- each R is independently selected from: fluoro,
- Ci-6alkyl substituted with from 1 to 5 substituents independently selected from: fluoro, chloro, bromo, iodo, Ci-4alkyloxy, -OH, Cl-4alkyl, phenyl, oxo, -COOH, -NO2, -NH2 and -CN, cyano,
- -OCi -6alkyl substituted with from 1 to 5 substituents independently selected from: fluoro, chloro, bromo, iodo, Ci-4alkyloxy,
- -Ocycloalkyl and independently selected from: fluoro, chloro,
- Ci-6alkyl substituted with from 1 to 5 substituents independently selected from: fluoro, chloro, bromo, iodo, Ci-4alkyloxy, -OH, Cl-4alkyl, phenyl, oxo, -COOH, -NO2, -NH2 and -CN, cyano,
- -OCi -6alkyl substituted with from 1 to 5 substituents independently selected from: fluoro, chloro, bromo, iodo, Ci-4alkyloxy,
- R 2 is selected from:
- heteroaryl substituted from 1 to 3 times by R , bicycloheteroaryl, and
- R is selected from:
- Y 2 is selected from:
- R is selected from Ci-5alkyl, and Ci -5alkyl substituted with from 1 to 6
- R 3 is selected from:
- R is selected from:
- Y is selected from:
- each R is independently selected from: Ci -6alkyl
- each R is independently selected from: fluoro,
- each R is independently selected from: fluoro
- R 3 is selected from:
- R is selected from:
- Y is selected from:
- Y 3 is taken together with the adjacent -OH to form morpholinyl, where each R is independently selected from: Ci -6alkyl, and Ci -6alkyl substituted with from 1 to 6 substituents independently selected from: fluoro, oxo, -OH, -COOH, -NH2, and -CN.
- R 4 is selected from:
- R 42 is selected from:
- each R is independently selected from: Ci -6alkyl
- Ci -6alkyl substituted with from 1 to 6 substituents independently selected from: fluoro, oxo, -OH, -COOH, -NH2, and -CN;
- each R is independently selected from: fluoro,
- R is selected from:
- R is selected from:
- Y 4 is selected from:
- each R is independently selected from: Ci -6alkyl, and Ci -6alkyl substituted with from 1 to 6 substituents independently selected from: fluoro, oxo, -OH, -COOH, -NH2, and -CN.
- R is phenyl or pyridyl independently substituted from 1 to 3 times by cyano, bromo, chloro and/or fluoro.
- R 2 is a substituted phenyl.
- Y is selected from: -CH2OH
- Representative compounds of the invention include the specific compounds described herein, e.g., the compounds of Formula (I) of the Examples, as well as any alternative stereoisomeric forms, free acid/base forms, salt forms, and alternative salt forms thereof (particularly pharmaceutically acceptable salt or alternative salt forms thereof), as applicable.
- the compound of the invention is a compound of Formula (I) selected from: 3-chloro-4-(((3f?,4S)-4-((5-chloropyridin-2-yl)sulfonyl)-3-hydroxy-3- (hydroxymethyl)pyrrolidin-1 -yl)sulfonyl)benzonitrile;
- salts, including pharmaceutically acceptable salts, of the compounds according to Formula (I) may be prepared. Indeed, in certain embodiments of the invention, salts including pharmaceutically-acceptable salts of the compounds according to Formula (I) may be preferred over the respective free or unsalted compound. Accordingly, the invention is further directed to salts, including pharmaceutically-acceptable salts, of the compounds according to Formula (I).
- salts including pharmaceutically acceptable salts, of the compounds of the invention are readily prepared by those of skill in the art.
- the salts of the present invention are pharmaceutically acceptable salts.
- Salts encompassed within the term “pharmaceutically acceptable salts” refer to non-toxic salts of the compounds of this invention.
- Representative pharmaceutically acceptable acid addition salts include, but are not limited to, 4-acetamidobenzoate, acetate, adipate, alginate, ascorbate, aspartate, benzenesulfonate (besylate), benzoate, bisulfate, bitartrate, butyrate, calcium edetate, camphorate, camphorsulfonate (camsylate), caprate (decanoate), caproate (hexanoate), caprylate (octanoate), cinnamate, citrate, cyclamate, digluconate, 2,5-dihydroxybenzoate, disuccinate, dodecylsulfate (estolate), edetate (ethylenediaminetetraacetate), estolate (lauryl sulfate), ethane-1 ,2-disulfonate (edisylate), ethanesulfonate (esylate), formate, fumarate, galactarate
- Representative pharmaceutically acceptable base addition salts include, but are not limited to, aluminium, 2-amino-2-(hydroxymethyl)-1 ,3-propanediol (TRIS, tromethamine), arginine, benethamine (/V-benzylphenethylamine), benzathine ( ⁇ /, ⁇ /'- dibenzylethylenediamine), £>/ ' s-(2-hydroxyethyl)amine, bismuth, calcium, chloroprocaine, choline, clemizole (1 -p chlorobenzyl-2-pyrrolildine-1 '-ylmethylbenzimidazole), cyclohexylamine, dibenzylethylenediamine, diethylamine, diethyltriamine, dimethylamine, dimethylethanolamine, dopamine, ethanolamine, ethylenediamine, L-histidine, iron, isoquinoline, lepidine, lithium, lysine, magnesium, meglumine (/V-methylgluc
- the compounds according to Formula I may contain one or more asymmetric centers (also referred to as a chiral center) and may, therefore, exist as individual enantiomers, diastereomers, or other stereoisomeric forms, or as mixtures thereof.
- Chiral centers such as chiral carbon atoms, may be present in a substituent such as an alkyl group.
- compounds according to Formula I containing one or more chiral centers may be used as racemic mixtures, enantiomerically or diastereomerically enriched mixtures, or as enantiomerically or diastereomerically pure individual stereoisomers.
- the compounds according to Formula (I) and pharmaceutically acceptable salts thereof may be in the form of isotopically-labelled compounds, wherein one or more atoms of Formula (I) are replaced by an atom having an atomic mass or mass number different from the atomic mass or mass number usually found in nature.
- isotopes include isotopes of hydrogen, carbon, nitrogen, oxygen, phosphorous, sulphur, fluorine, iodine, and chlorine, such as 2 H, 3 H, 11 C, 13 C, 14 C, 15 N, 17 0, 18 0, 31 P, 32 P, 35 S, 18 F, 36 CI, 123 l and 125 l.
- Isotopically-labelled compounds for example those into which radioactive isotopes such as 3 H or 14 C are incorporated, are useful in drug and/or substrate tissue distribution assays.
- Tritium, i.e., 3 H, and carbon-14, i.e., 14 C, isotopes are particularly preferred for their ease of preparation and detectability.
- 11 C and 18 F isotopes are particularly useful in PET (positron emission tomography), and 125 l isotopes are particularly useful in SPECT (single photon emission computerized tomography), both are useful in brain imaging.
- Isotopically labelled compounds can generally be prepared by substituting a readily available isotopically labelled reagent for a non-isotopically labelled reagent.
- the compounds according to Formula (I) may also contain double bonds or other centers of geometric asymmetry. Where the stereochemistry of a center of geometric asymmetry present in Formula (I), or in any chemical structure illustrated herein, is not specified, the structure is intended to encompass the trans (E) geometric isomer, the cis (Z) geometric isomer, and all mixtures thereof. Likewise, all tautomeric forms are also included in Formula (I) whether such tautomers exist in equilibrium or predominately in one form.
- the compounds of the invention may exist in solid or liquid form. In solid form, compound of the invention may exist in a continuum of solid states ranging from fully amorphous to fully crystalline.
- 'amorphous' refers to a state in which the material lacks long range order at the molecular level and, depending upon the temperature, may exhibit the physical properties of a solid or a liquid. Typically such materials do not give distinctive X-ray diffraction patterns and, while exhibiting the properties of a solid, are more formally described as a liquid. Upon heating, a change from solid to liquid properties occurs which is characterized by a change of state, typically second order ('glass transition').
- 'crystalline' refers to a solid phase in which the material has a regular ordered internal structure at the molecular level and gives a distinctive X-ray diffraction pattern with defined peaks. Such materials when heated sufficiently will also exhibit the properties of a liquid, but the change from solid to liquid is characterized by a phase change, typically first order ('melting point').
- the compounds of the invention may have the ability to crystallize in more than one form, a characteristic, which is known as polymorphism ("polymorphs").
- Polymorphism generally can occur as a response to changes in temperature or pressure or both and can also result from variations in the crystallization process.
- Polymorphs can be distinguished by various physical characteristics known in the art such as x-ray diffraction patterns, solubility and melting point.
- the compounds of Formula (I) may exist in solvated and unsolvated forms.
- solvate refers to a complex of variable stoichiometry formed by a solute (in this invention, a compound of Formula (I) or a salt) and a solvent. Such solvents, for the purpose of the invention, may not interfere with the biological activity of the solute.
- pharmaceutically acceptable solvates may be formed for crystalline compounds wherein solvent molecules are incorporated into the crystalline lattice during crystallization.
- the incorporated solvent molecules may be water molecules or non-aqueous such as ethanol, isopropanol, DMSO, acetic acid, ethanolamine, and ethyl acetate molecules.
- Crystalline lattice structures incorporated with water molecules are typically referred to as "hydrates". Hydrates include stoichiometric hydrates as well as compositions containing variable amounts of water. It is also noted that the compounds of Formula (I) may form tautomers. Tautomers' refer to compounds that are interchangeable forms of a particular compound structure, and that vary in the displacement of hydrogen atoms and electrons. Thus, two structures may be in equilibrium through the movement of ⁇ electrons and an atom (usually H). For example, enols and ketones are tautomers because they are rapidly interconverted by treatment with either acid or base. It is understood that all tautomers and mixtures of tautomers of the compounds of the present invention are included within the scope of the compounds of the present invention.
- Alkyl refers to a hydrocarbon chain having the specified number of "member atoms".
- C-1 -C5 alkyl refers to an alkyl group having from 1 to 6 member atoms.
- Alkyl groups may be saturated, unsaturated, straight or branched. Representative branched alkyl groups have one, two, or three branches.
- Alkyl includes but is not limited to: methyl, ethyl, ethylene, ethynyl, propyl (n-propyl and isopropyl), butene, butyl (n-butyl, isobutyl, and t-butyl), pentyl and hexyl.
- Alkoxy refers to an -O-alkyl group wherein “alkyl” is as defined herein.
- -C4alkoxy refers to an alkoxy group having from 1 to 4 carbon member atoms.
- Examples of such groups include but is not limited to: methoxy, ethoxy, propoxy, isopropoxy, butoxy, and t-butoxy.
- Aryl refers to an aromatic hydrocarbon ring system.
- Aryl groups are monocyclic, bicyclic, and tricyclic ring systems having a total of five to fourteen ring member atoms, wherein at least one ring system is aromatic and wherein each ring in the system contains 3 to 7 member atoms, such as but no limited to: phenyl, dihydroindene, naphthalene, tetrahydronaphthalene and biphenyl.
- aryl is phenyl.
- Cycloalkyl unless otherwise defined, refers to a saturated or unsaturated non aromatic hydrocarbon ring having from three to seven carbon atoms. Cycloalkyl groups are monocyclic ring systems.
- C3-C7 cycloalkyl refers to a cycloalkyl group having from 3 to 7 member atoms.
- cycloalkyl as used herein include but is not limited to: cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclobutenyl, cyclopentenyl, cyclohexenyl and cycloheptyl.
- cycloalkyl is selected from: cyclopropyl, cyclopentyl and cyclohexyl.
- Heteroaryl refers to a monocyclic aromatic 4 to 8 member ring containing from 1 to 7 carbon atoms and containing from 1 to 4 heteroatoms, provided that when the number of carbon atoms is 3, the aromatic ring contains at least two heteroatoms. Heteroaryl groups containing more than one heteroatom may contain different heteroatoms.
- Heteroaryl includes: pyrrolyl, pyrazolyl, imidazolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, furanyl, furazanyl, thienyl, triazolyl, pyridinyl, pyrimidinyl, pyridazinyl, pyrazinyl, triazinyl, tetrazinyl.
- heteroaryl includes: pyrazole, pyrrole, isoxazole, pyridine, pyrimidine, pyridazine, and imidazole.
- Bicycloheteroaryl refers to two fused rings, at least one of which is aromatic, containing from 1 to 6 heteroatoms as member atoms. Bicycloheteroaryl groups containing more than one heteroatom may contain different heteroatoms. Bicycloheteroaryl rings have from 6 to 1 1 member atoms.
- Bicycloheteroaryl includes: 1 /-/-pyrrolo[3,2-c]pyridine, 1 H-pyrazolo[4,3- c] pyridine, 1 H-pyrazolo[3,4-d]pyrimidine, 1 H-pyrrolo[2,3-d]pyrimidine, 7H-pyrrolo[2,3- d] pyrimidine, thieno[3,2-c]pyridine, thieno[2,3-d]pyrimidine, furo[2,3-c]pyridine, furo[2,3- d]pyrimidine, indolyl, isoindolyl, indolizinyl, indazolyl, purinyl, quinolinyl, isoquinolinyl, quinoxalinyl, quinazolinyl, pteridinyl, cinnolinyl, azabenzimidazolyl, tetrahydrobenzimidazolyl, benzoxadiazole, imid
- Heteroatom refers to a nitrogen, sulphur or oxygen atom.
- Halogen and halo refers to a fluorine, chlorine, bromine, or iodine atom.
- mercapto refers to the group -SH.
- hydroxy refers to the group -OH.
- amino refers to the group -NH2.
- carboxy refers to the group -C(0)OH.
- cyano refers to the group -CN.
- nitro refers to the group -NO2.
- a substituent may be specifically selected to be reactive under the reaction conditions used. Under these circumstances, the reaction conditions convert the selected substituent into another substituent that is either useful as an intermediate compound or is a desired substituent in a target compound.
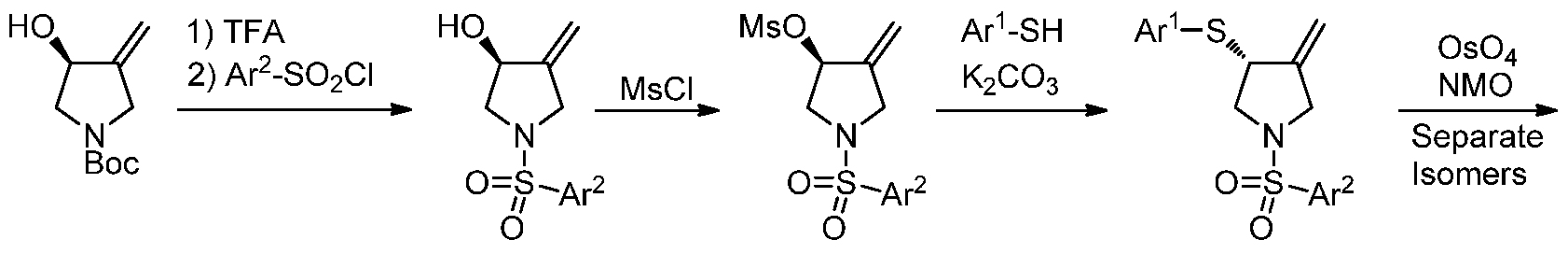
- the compounds of Formula (I) can be prepared by a multi- step sequence.
- the mesylate of the Boc-protected pyrrolidine can undergo displacement with an appropriately substituted thiophenol in the prescence of a base such as K 2 C0 3 to give the corresponding sulfide.
- the sulfide can be oxidized to the sulfone by treatment with m-CPBA.
- the exocyclic olefin of the pyrrolidine ring can be dihydroxylated with catalytic OSC using NMO as a cooxidant and the irans-diastereomer can be obtained by separation techniques such as silica gel column chromatography either in this step or in subsequent steps.
- Removal of the Boc protecting group with an acid such as TFA followed by treatment of the deprotected pyrrolidine with an appropriately substituted arylsulfonyl chloride and base such as NaHC0 3 provides compounds of Formula (I).
- compounds of Formula (I) can be prepared as shown in Scheme 2.
- the mesylate of the Boc protected pyrrolidine can undergo displacement with an appropriately substituted thiophenol and base such as K2CO3 to give the corresponding sulfide.
- the exocyclic olefin of the pyrrolidine ring can be di hydroxy lated with catalytic Os0 4 using NMO as a cooxidant, and the mixture of cis/trans diasteromer intermediates is carried through the subsequent reactions. Oxidation of the sulfide to the sulfone can be accomplished by m-CPBA.
- compounds of Formula (I) can be prepared as shown in Scheme 3.
- the mesylate of the Boc protected pyrrolidine can undergo displacement with an appropriately substituted thiophenol and base such as K 2 C0 3 to give the corresponding sulfide.
- the exocyclic olefin of the pyrrolidine ring can be di hydroxy lated with catalytic Os0 4 using NMO as a cooxidant, and the mixture of cis/trans diasteromers produced can be carried through the next two steps. Oxidation of the sulfide to the sulfone can be accomplished by m-CPBA.
- compounds of Formula (I) can be prepared as shown in Scheme 4.
- the Boc group of the protected hydroxypyrrolidine can be removed with an acid such as TFA and the unprotected pyrrolidine can be treated with an appropriately substituted arylsulfonyl chloride to give the sulfonamide.
- Mesylation of the hydroxyl group with mesyl chloride followed by displacement with an appropriately substituted thiophenol and base such as K2CO3 can provide the sulfide.
- exocyclic olefin of the pyrrolidine ring can be dihydroxylated with catalytic Os0 4 using NMO as a cooxidant, and the individual enantiopure trans and cis isomers can be obtained by separation techniques such as silica gel column chromatography. Either the cis or trans diastereomer can be oxidized with m-CPBA to give compounds of Formula (I).
- Diastereomer 1 Diastereomer 2
- compounds of Formula (I) can be prepared by a multi- step sequence from substituted sulfonyl chlorides.
- the appropriate sulfonyl chloride can be substituted with an alkyl amine using a base such as K 2 C0 3 to give a secondary sulfonamide which can then be treated with an alkyl halide to give the tertiary
- the tertiary sulfonamide can be cyclized by olefin metathesis using a catalyst, such as Grubbs Catalyst, 2 nd Generation, to give the pyrroline.
- the pyrroline can be epoxidized with m-CPBA and the epoxide ring opened with an appropriately
- the compounds of Formula (I) can be prepared by a multi- step sequence.
- Mesylation of the hydroxyl group of the Boc protected pyrrolidine with mesyl chloride followed by displacement with an appropriately substituted thiophenol with a base such as K 2 C0 3 provide the sulfide.
- the exocyclic olefin of the pyrrolidine ring can be dihydroxylated with catalytic Os0 4 using NMO as a cooxidant, and the mixture of cis/trans diasteromers produced can be carried into the next step.
- Protection of the dihydroxy compound as the cyclic ketal can be accomplished with 2,2-dimethoxypropane and tosic acid in water and the mixture of cis/trans diastereomers can be separated by techniques such as silica gel column chromatography. Either the individual cis or trans diastereomer can be carried through the following steps to provide compounds of Formula (I).
- the Boc and cyclic ketal protecting groups can be hydrolyzed with an acid such as TFA and the deprotected pyrrolidine treated with an appropriately substituted arylsulfonyl chloride and base such as NaHC0 3 to give the sulfonamide.
- the pyrrolidine hydroxyl group can be mesylated with mesyl chloride, the mesylate displaced with sodium azide and the azide reduced to the amine with polymer supported triphenylphosphine. Protection of the amine with Boc anyhydride, oxidation of the sulfide to sulfone with m- CPBA and removal of the Boc protecting group with TFA provide compounds of Formula (I).
- compounds of Formula (I) can be prepared by a multi-step sequence as shown in scheme 7.
- Mesylation of the hydroxyl group of the Boc protected pyrrolidine with mesyl chloride followed by displacement with an appropriately substituted thiophenol gives the corresponding sulfide.
- the exocyclic olefin of the pyrrolidine ring can be dihydroxylated with catalytic Os0 4 using NMO as a cooxidant, and the mixture of cis/trans diasteromers produced can be carried through subsequent steps until separated.
- the pyrrolidine hydroxyl group can be mesylated with mesyl chloride, the mesylate displaced with sodium azide and the azide reduced to the amine with trimethylphosphine.
- Conversion to the morpholin-3-one can be carried out by acylation of the amine with 2- chloroacetyl chloride and TEA followed by cyclization with f-BuOK. Removal of the Boc protecting group with an acid such as TFA, and treatment with an appropriately substituted arylsulfonyl chloride provide the sulfonamide. Oxidation of the sulfide with m- CPBA provide the sulfone as a mixture of cis/trans diastereomers which can now be separated to individual enantiomers by techniques such as silica gel column
- the compounds according to Formula I are TRPV4 antagonists.
- the biological activity of the compounds according to Formula I can be determined using any suitable assay for determining the activity of a candidate compound as a TRPV4 antagonist, as well as tissue and in vivo models.
- the biological activity of the compounds of Formula (I) are demonstrated by the following tests.
- TRPV4 channel activation results in an influx of divalent and monovalent cations including calcium.
- the resulting changes in intracellular calcium were monitored using a calcium specific fluorescent dye Fluo-4 (MDS Analytical Technologies).
- BHK/AC9 cells transduced with BacMam virus expressing the human TRPV4 gene at a MOI of 78 were plated in a 384 well poly-D lysine coated plate (15,000 cells/well in 50 ⁇ _ culture medium containing DMEM/F12 with 15 mM HEPES, 10% FBS, 1 % Penicillin-Streptomycin and 1 % L-glutamine). Cells were incubated for 24 hours at 37 °C and 5% C0 2 .
- Agonist Compound was added to have a final concentration equals to the agonist EC80.
- Calcium signals were measured using FLIPRTETRA (MDS Analytical Technologies) or FLIPR384 (MDS Analytical Technologies) and the inhibition of Agonist Compound-induced calcium signal by the test compound was determined. All examples described herein possessed TRPV4 biological activity with IC 5 o ranges from 0.1 nM - 1 ⁇ (see table below).
- Example 1 The compound of Example 1 was tested generally according to the above TRPV4 assay and in at least one set of experimental runs exhibited an average IC50 (nM) value of 5.
- Example 22 The compound of Example 22 was tested generally according to the above TRPV4 assay and in at least one set of experimental runs exhibited an average IC 5 o (nM) value of 13.
- ICso Ranges 0.1 -10 nM (+++), >10-100 nM (++), >100-1000 nM (+).
- this invention provides a compound of Formula (I) or a pharmaceutically acceptable salt thereof in the treatment of a disease state selected from: atherosclerosis, disorders related to vasogenic edema, postsurgical abdominal edema, ocular edema, cerebral edema, local and systemic edema, fluid retention, sepsis, hypertension, inflammation, bone related dysfunctions and congestive heart failure, pulmonary disorders, chronic obstructive pulmonary disorder, ventilator induced lung injury, high altitude induced pulmonary edema, acute respiratory distress syndrome, acute lung injury, pulmonary fibrosis and other fibrosis-related disorders, sinusitis/rhinitis, asthma, COPD, cough; including acute cough, sub-acute cough and chronic cough, pulmonary hypertension, overactive bladder, cystitis, pain, motor neuron disorders, genetic gain of function disorders, amyotrophic lateral sclerosis, multiple sclerosis, cardiovascular disease, acute, chronic and polycystic kidney disease, stroke, hydrocephal
- the compounds of the invention are used in the treatment of acute lung injury.
- the compounds of the invention are used in the treatment of cerebral edema.
- the compounds of the invention are used in the treatment of heart failure.
- the compounds of the invention are used in the treatment of cough; including acute cough, sub-acute cough and chronic cough.
- the compounds of the invention are used in the treatment of acute respiratory distress syndrome. Accordingly, in another aspect the invention is directed to methods of treating such conditions.
- the compounds of Formula (I) are tested for their ability to treat cough in in vivo in pre-clinical models in which cough is induced, for example the guinea pig model cited in Bonvini SJ, et al., J Allergy Clin Immunol. 2016 Jul;138(1):249-261 .e12.
- the efficacy of compounds of Formula (I) are tested for their ability to treat cough; including acute cough, sub-acute cough and chronic cough, in people using the objective cough monitoring and specific quality of life instruments as cited in Abdulqawi R, et al. Lancet. 2015 Mar 28; 385(9974):1 198-1205.
- the methods of treatment of the invention comprise administering a safe and effective amount of a compound according to Formula I or a pharmaceutically-acceptable salt thereof to a patient in need thereof.
- treat in reference to a condition means: (1) to ameliorate the condition or one or more of the biological manifestations of the condition, (2) to interfere with (a) one or more points in the biological cascade that leads to or is responsible for the condition or (b) one or more of the biological manifestations of the condition, (3) to alleviate one or more of the symptoms or effects associated with the condition, or (4) to slow the progression of the condition or one or more of the biological manifestations of the condition.
- treating and derivatives thereof refers to therapeutic therapy.
- therapeutic therapy is appropriate to alleviate symptoms or to treat at early signs of disease or its progression.
- prevention is not an absolute term.
- prevention is understood to refer to the prophylactic administration of a drug to substantially diminish the likelihood or severity of a condition or biological manifestation thereof, or to delay the onset of such condition or biological manifestation thereof.
- safe and effective amount in reference to a compound of the invention or other pharmaceutically-active agent means an amount of the compound sufficient to treat the patient's condition but low enough to avoid serious side effects (at a reasonable benefit/risk ratio) within the scope of sound medical judgment.
- a safe and effective amount of a compound will vary with the particular compound chosen (e.g.
- patient or “subject” refers to a human or other mammal.
- the invention provides for a compound of Formula (I) or a pharmaceutically acceptable salt thereof for use in the treatment of atherosclerosis, disorders related to vasogenic edema, postsurgical abdominal edema, ocular edema, cerebral edema, local and systemic edema, fluid retention, sepsis, hypertension, inflammation, bone related dysfunctions and congestive heart failure, pulmonary disorders, chronic obstructive pulmonary disorder, ventilator induced lung injury, high altitude induced pulmonary edema, acute respiratory distress syndrome, acute lung injury, pulmonary fibrosis and other fibrosis-related disorders, sinusitis/rhinitis, asthma, COPD, cough; including acute cough, sub-acute cough and chronic cough, pulmonary hypertension, overactive bladder, cystitis, pain, motor neuron disorders, genetic gain of function disorders, amyotrophic lateral sclerosis, multiple sclerosis, cardiovascular disease, acute, chronic and polycystic kidney disease, stroke, hydrocephalus,
- the invention provides for a compound of Formula (I) or a pharmaceutically acceptable salt thereof for use in the treatment cerebral edema.
- the invention provides for a compound of Formula (I) or a pharmaceutically acceptable salt thereof for use in the treatment of heart failure.
- the invention provides for a compound of Formula (I) or a pharmaceutically acceptable salt thereof for use in the treatment of cough; including acute cough, sub-acute cough and chronic cough.
- the invention provides for a compound of Formula (I) or a pharmaceutically acceptable salt thereof for use in the treatment of acute respiratory distress syndrome.
- the invention provides for the use of a compound of Formula (I) or a pharmaceutically acceptable salt thereof in the manufacture of a medicament for the treatment of atherosclerosis, disorders related to vasogenic edema, postsurgical abdominal edema, ocular edema, cerebral edema, local and systemic edema, fluid retention, sepsis, hypertension, inflammation, bone related dysfunctions and congestive heart failure, pulmonary disorders, chronic obstructive pulmonary disorder, ventilator induced lung injury, high altitude induced pulmonary edema, acute respiratory distress syndrome, acute lung injury, pulmonary fibrosis and other fibrosis-related disorders, sinusitis/rhinitis, asthma, COPD, cough; including acute cough, sub-acute cough and chronic cough, pulmonary hypertension, overactive bladder, cystitis, pain, motor neuron disorders, genetic gain of function disorders, amyotrophic lateral sclerosis, multiple sclerosis, cardiovascular disease, acute, chronic and polycystic kidney disease, stroke,
- the invention provides for the use of a compound of Formula (I) or a pharmaceutically acceptable salt thereof in the manufacture of a medicament for the treatment of congestive heart failure.
- the invention provides for the use of a compound of Formula (I) or a pharmaceutically acceptable salt thereof in the manufacture of a medicament for the treatment of acute lung injury.
- the invention provides for the use of a compound of Formula (I) or a
- the invention provides for a compound of Formula (I) or a pharmaceutically acceptable salt thereof in the manufacture of a medicament for the treatment of cerebral edema.
- the invention provides for a compound of Formula (I) or a pharmaceutically acceptable salt thereof in the manufacture of a medicament for the treatment of heart failure.
- the invention provides for a compound of Formula (I) or a pharmaceutically acceptable salt thereof in the manufacture of a medicament for the treatment of cough; including acute cough, sub-acute cough and chronic cough.
- the invention provides for a compound of Formula (I) or a pharmaceutically acceptable salt thereof in the manufacture of a medicament for the treatment of acute respiratory distress syndrome.
- the compounds of the invention may be administered by any suitable route of administration, including both systemic administration and topical administration.
- Systemic administration includes oral administration, parenteral administration, transdermal administration, rectal administration, and administration by inhalation.
- Parenteral administration refers to routes of administration other than enteral, transdermal, or by inhalation, and is typically by injection or infusion.
- Parenteral administration includes intravenous, intramuscular, and subcutaneous injection or infusion.
- Inhalation refers to administration into the patient's lungs whether inhaled through the mouth or through the nasal passages.
- Topical administration includes application to the skin as well as intraocular, otic, intravaginal, and intranasal administration.
- the administration is oral.
- the administration is intravenous.
- the administration is by inhalation.
- the compounds of the invention may be administered once or according to a dosing regimen wherein a number of doses are administered at varying intervals of time for a given period of time.
- doses may be administered one, two, three, or four times per day. Doses may be administered until the desired therapeutic effect is achieved or indefinitely to maintain the desired therapeutic effect.
- Suitable dosing regimens for a compound of the invention depend on the pharmacokinetic properties of that compound, such as absorption, distribution, and half-life, which can be determined by the skilled artisan.
- suitable dosing regimens, including the duration such regimens are administered, for a compound of the invention depend on the condition being treated, the severity of the condition being treated, the age and physical condition of the patient being treated, the medical history of the patient to be treated, the nature of concurrent therapy, the desired therapeutic effect, and like factors within the knowledge and expertise of the skilled artisan. It will be further understood by such skilled artisans that suitable dosing regimens may require adjustment given an individual patient's response to the dosing regimen or over time as individual patient needs change.
- Typical daily dosages may vary depending upon the particular route of administration chosen. Typical dosages for oral administration range from 1 mg to 1000 mg per person per dose. Preferred dosages are 1— 500 mg once daily or BID per person. Additionally, the compounds of the invention may be administered as prodrugs.
- a prodrug of a compound of the invention is a functional derivative of the compound which, upon administration to a patient, eventually liberates the compound of the invention in vivo.
- Administration of a compound of the invention as a prodrug may enable the skilled artisan to do one or more of the following: (a) modify the onset of the compound in vivo; (b) modify the duration of action of the compound in vivo; (c) modify the transportation or distribution of the compound in vivo; (d) modify the solubility of the compound in vivo; and (e) overcome or overcome a side effect or other difficulty encountered with the compound.
- Typical functional derivatives used to prepare prodrugs include modifications of the compound that are chemically or enzymatically cleaved in vivo. Such modifications, which include the preparation of phosphates, amides, ethers, esters, thioesters, carbonates, and carbamates, are well known to those skilled in the art.
- the compounds of Formula (I) and pharmaceutically acceptable salts thereof may be used in combination with one or more other agents which may be useful in the prevention or treatment of respiratory disease for example; antigen immunotherapy, anti- histamines, corticosteroids, (e.g., fluticasone propionate, fluticasone furoate, beclomethasone dipropionate, budesonide, ciclesonide, mometasone
- antigen immunotherapy e.g., anti- histamines, corticosteroids, (e.g., fluticasone propionate, fluticasone furoate, beclomethasone dipropionate, budesonide, ciclesonide, mometasone
- leukotriene modulators e.g., montelukast, zafirlukast, pranlukast
- tryptase inhibitors IKK2 inhibitors, p38 inhibitors, Syk inhibitors
- protease inhibitors such as elastase inhibitors
- integrin antagonists e.g., beta-2 integrin antagonists
- mediator release inhibitors such as sodium chromoglycate, 5-lipoxygenase inhibitors (zyflo), DP1 antagonists, DP2 antagonists, PI3K delta inhibitors, ITK inhibitors, LP (lysophosphatidic) inhibitors or FLAP (5-lipoxygenase activating protein) inhibitors (e.g., sodium 3-(3-(tert-butylthio)-1 -(4-(6-ethoxypyridin-3- yl) inhibitors or FLAP (5-lipoxygenase activating protein) inhibitors (e.g., sodium 3-(
- bronchodilators e.g..muscarinic antagonists, beta-2 agonists), methotrexate, and similar agents
- monoclonal antibody therapy such as anti-lgE, anti-TNF, anti-IL-5, anti-IL-6, anti- IL-12, anti-IL-1 and similar agents
- cytokine receptor therapies e.g. etanercept and similar agents
- antigen non-specific immunotherapies e.g. interferon or other
- cytokines/chemokines chemokine receptor modulators such as CCR3, CCR4 or CXCR2 antagonists, other cytokine/chemokine agonists or antagonists, TLR agonists and similar agents).
- chemokine receptor modulators such as CCR3, CCR4 or CXCR2 antagonists, other cytokine/chemokine agonists or antagonists, TLR agonists and similar agents.
- compounds or pharmaceutical formulations of the invention may be administered together with an anti-inflammatory agent such as, for example, a corticosteroid, or a pharmaceutical formulation thereof.
- an anti-inflammatory agent such as, for example, a corticosteroid, or a pharmaceutical formulation thereof.
- a compound of the invention may be formulated together with an antiinflammatory agent, such as a corticosteroid, in a single formulation, such as a dry powder formulation for inhalation.
- a pharmaceutical formulation comprising a compound of the invention may be administered in conjunction with a pharmaceutical formulation comprising an anti-inflammatory agent, such as a corticosteroid, either simultaneously or sequentially.
- a pharmaceutical formulation comprising a compound of the invention and a pharmaceutical formulation comprising an anti-inflammatory agent, such as a corticosteroid may each be held in device suitable for the simultaneous administration of both formulations via inhalation.
- Suitable corticosteroids for administration together with a compound of the invention include, but are not limited to, fluticasone furoate, fluticasone propionate, beclomethasone diproprionate, budesonide, ciclesonide, mometasone furoate, triamcinolone, flunisolide and prednisilone.
- a corticosteroids for administration together with a compound of the invention via inhalation includes fluticasone furoate, fluticasone propionate, beclomethasone diproprionate, budesonide, ciclesonide, mometasone furoate, and, flunisolide.
- compounds or pharmaceutical formulations of the invention may be administered together with one or more bronchodilators, or pharmaceutical formulations thereof.
- a compound of the invention may be formulated together with one or more bronchodilators in a single formulation, such as a dry powder formulation for inhalation.
- a pharmaceutical formulation comprising a compound of the invention may be administered in conjunction with a pharmaceutical formulation comprising one or more bronchodilators, either
- a formulation comprising a compound of the invention and a bronchodilator may be administered in conjunction with a pharmaceutical formulation comprising a further bronchodilator.
- a pharmaceutical formulation comprising one or more bronchodilators may each be held in device suitable for the simultaneous administration of both formulations via inhalation.
- a pharmaceutical formulation comprising a compound of the invention together with a bronchodilator and a pharmaceutical formulation comprising a further bronchodilator may each be held in one or more devices suitable for the simultaneous administration of both formulations via inhalation.
- Suitable bronchodilators for administration together with a compound of the invention include, but are not limited to, p2-adrenoreceptor agonists and anticholinergic agents.
- p2-adrenoreceptor agonists include, for example, vilanterol, salmeterol, salbutamol, formoterol, salmefamol, fenoterol carmoterol, etanterol, naminterol, clenbuterol, pirbuterol, flerbuterol, reproterol, bambuterol, indacaterol, terbutaline and salts thereof, for example the xinafoate (1 -hydroxy-2- naphthalenecarboxylate) salt of salmeterol, the sulphate salt of salbutamol or the fumarate salt of formoterol.
- Suitable anticholinergic agents include umeclidinium (for example, as the bromide), ipratropium (for example, as the bromide), oxitropium (for example, as the bromide) and tiotropium (for example, as the bromide).
- a compound of the invention may be administered together with a p2-adrenoreceptor agonist, such as vilanterol, and an anticholinergic agent, such as, umeclidinium.
- the compounds of the invention will normally, but not necessarily, be formulated into pharmaceutical compositions prior to administration to a patient. Accordingly, in another aspect the invention is directed to pharmaceutical compositions comprising a compound of the invention and a pharmaceutically-acceptable excipient.
- the pharmaceutical compositions of the invention may be prepared and packaged in bulk form wherein a safe and effective amount of a compound of the invention can be extracted and then given to the patient such as with powders or syrups.
- the pharmaceutical compositions of the invention may be prepared and packaged in unit dosage form wherein each physically discrete unit contains a safe and effective amount of a compound of the invention.
- the pharmaceutical compositions of the invention typically contain from 1 mg to 1000 mg.
- compositions of the invention typically contain one compound of the invention. However, in certain embodiments, the pharmaceutical compositions of the invention contain more than one compound of the invention. For example, in certain embodiments the pharmaceutical compositions of the invention contain two compounds of the invention. In addition, the pharmaceutical compositions of the invention may optionally further comprise one or more additional pharmaceutically active compounds.
- pharmaceutically-acceptable excipient means a
- each excipient must be compatible with the other ingredients of the pharmaceutical composition when commingled such that interactions which would substantially reduce the efficacy of the compound of the invention when administered to a patient and interactions which would result in pharmaceutical compositions that are not pharmaceutically acceptable are avoided.
- each excipient must of course be of sufficiently high purity to render it pharmaceutically-acceptable.
- dosage forms include those adapted for (1) oral administration such as tablets, capsules, caplets, pills, troches, powders, syrups, elixers, suspensions, solutions, emulsions, sachets, and cachets; (2) parenteral administration such as sterile solutions, suspensions, and powders for reconstitution; (3) transdermal administration such as transdermal patches; (4) rectal administration such as suppositories; (5) inhalation such as dry powders, aerosols, suspensions, and solutions; and (6) topical administration such as creams, ointments, lotions, solutions, pastes, sprays, foams, and gels.
- oral administration such as tablets, capsules, caplets, pills, troches, powders, syrups, elixers, suspensions, solutions, emulsions, sachets, and cachets
- parenteral administration such as sterile solutions, suspensions, and powders for reconstitution
- transdermal administration such as transdermal patches
- rectal administration such as
- Suitable pharmaceutically-acceptable excipients will vary depending upon the particular dosage form chosen.
- suitable pharmaceutically-acceptable excipients may be chosen for a particular function that they may serve in the composition.
- certain pharmaceutically-acceptable excipients may be chosen for their ability to facilitate the production of uniform dosage forms.
- Certain pharmaceutically- acceptable excipients may be chosen for their ability to facilitate the production of stable dosage forms.
- Certain pharmaceutically-acceptable excipients may be chosen for their ability to facilitate the carrying or transporting of the compound or compounds of the invention once administered to the patient from one organ, or portion of the body, to another organ, or portion of the body.
- Certain pharmaceutically-acceptable excipients may be chosen for their ability to enhance patient compliance.
- Suitable pharmaceutically-acceptable excipients include the following types of excipients: diluents, fillers, binders, disintegrants, lubricants, glidants, granulating agents, coating agents, wetting agents, solvents, co-solvents, suspending agents, emulsifiers, sweetners, flavoring agents, flavor masking agents, coloring agents, anticaking agents, hemectants, chelating agents, plasticizers, viscosity increasing agents, antioxidants, preservatives, stabilizers, surfactants, and buffering agents.
- excipients include the following types of excipients: diluents, fillers, binders, disintegrants, lubricants, glidants, granulating agents, coating agents, wetting agents, solvents, co-solvents, suspending agents, emulsifiers, sweetners, flavoring agents, flavor masking agents, coloring agents, anticaking agents, hemectants, chel
- the invention is directed to a solid oral dosage form such as a tablet or capsule comprising a safe and effective amount of a compound of the invention and a diluent or filler.
- Suitable diluents and fillers include lactose, sucrose, dextrose, mannitol, sorbitol, starch (e.g. corn starch, potato starch, and pre-gelatinized starch), cellulose and its derivatives (e.g. microcrystalline cellulose), calcium sulfate, and dibasic calcium phosphate.
- the oral solid dosage form may further comprise a binder. Suitable binders include starch (e.g.
- the oral solid dosage form may further comprise a disintegrant. Suitable disintegrants include crospovidone, sodium starch glycolate, croscarmelose, alginic acid, and sodium carboxymethyl cellulose.
- the oral solid dosage form may further comprise a lubricant. Suitable lubricants include stearic acid, magnesuim stearate, calcium stearate, and talc.
- the compounds may be administered alone or in conjunction with one or more other therapeutic agents, said agents being selected from the group consisting of endothelin receptor antagonists, angiotensin converting enzyme (ACE) inhibitors, angiotension II receptor antagonists, vasopeptidase inhibitors, vasopressin receptor modulators, diuretics, digoxin, beta blockers, aldosterone antagonists, inotropes, NSAIDS, nitric oxide donors, calcium channel modulators, muscarinic antagonists, steroidal anti-inflammatory drugs, bronchodilators, antihistamines, leukotriene antagonists, HMG-CoA reductase inhibitors, dual non-selective Padrenoceptor and n1 - adrenoceptor antagonists, type-5 phosphodiesterase inhibitors, and renin inhibitors.
- ACE angiotensin converting enzyme
- the invention is directed to a dosage form adapted for administration to a patient by inhalation.
- the compound of the invention may be inhaled into the lungs as a dry powder, an aerosol, a suspension, or a solution.
- Dry powder compositions for delivery to the lung by inhalation typically comprise a compound of the invention as a finely divided powder together with one or more pharmaceutically acceptable excipients as finely divided powders.
- Pharmaceutically acceptable excipients particularly suited for use in dry powders are known to those skilled in the art and include lactose, starch, mannitol, and mono-, di-, and polysaccharides.
- compositions for use in accordance with the present invention are administered via inhalation devices.
- inhalation devices can encompass capsules and cartridges of for example gelatin, or blisters of, for example, laminated aluminum foil.
- each capsule, cartridge or blister may contain doses of composition according to the teachings presented herein.
- inhalation devices can include those intended for unit dose or multi-dose delivery of composition, including all of the devices set forth herein.
- the formulation can be pre-metered (e.g., as in Diskus, see GB2242134, U.S. Patent Nos.
- the Diskus inhalation device comprises an elongate strip formed from a base sheet having a plurality of recesses spaced along its length and a lid sheet peelably sealed thereto to define a plurality of containers, each container having therein an inhalable formulation containing the compound optionally with other excipients and additive taught herein.
- the peelable seal is an engineered seal, and in one embodiment the engineered seal is a hermetic seal.
- the strip is sufficiently flexible to be wound into a roll.
- the lid sheet and base sheet will preferably have leading end portions which are not sealed to one another and at least one of the leading end portions is constructed to be attached to a winding means.
- the engineered seal between the base and lid sheets extends over their whole width.
- the lid sheet may preferably be peeled from the base sheet in a longitudinal direction from a first end of the base sheet.
- a dry powder composition may also be presented in an inhalation device which permits separate containment of two different components of the composition.
- these components are administrable simultaneously but are stored separately, e.g., in separate pharmaceutical compositions, for example as described in WO
- an inhalation device permitting separate containment of components is an inhaler device having two peelable blister strips, each strip containing pre-metered doses in blister pockets arranged along its length, e.g., multiple containers within each blister strip, e.g., as found in ELLIPTA®.
- Said device has an internal indexing mechanism which, each time the device is actuated, peels opens a pocket of each strip and positions the blisters so that each newly exposed dose of each strip is adjacent to the manifold which communicates with the mouthpiece of the device.
- each dose is simultaneously drawn out of its associated pocket into the manifold and entrained via the mouthpiece into the patient's respiratory tract.
- Aerosols may be formed by suspending or dissolving a compound of the invention in a liquefied propellant.
- propellants include halocarbons, hydrocarbons, and other liquefied gases.
- propellants include: trichlorofluoromethane (propellant 1 1 ), dichlorofluoromethane (propellant 12), dichlorotetrafluoroethane (propellant 1 14), tetrafluoroethane (HFA-134a), 1 ,1 -difluoroethane (HFA-152a), difluoromethane (HFA-32), pentafluoroethane (HFA-12), heptafluoropropane (HFA- 227a), perfluoropropane, perfluorobutane, perfluoropentane, butane, isobutane, and pentane.
- Aerosols comprising a compound of the invention will typically be administered to a patient via a metered dose inhaler (MDI). Such devices are known to those skilled in the art.
- MDI metered dose inhaler
- the aerosol may contain additional pharmaceutically acceptable excipients typically used with multiple dose inhalers such as surfactants, lubricants, cosolvents and other excipients to improve the physical stability of the formulation, to improve valve performance, to improve solubility, or to improve taste.
- Suspensions and solutions comprising a compound of the invention may also be administered to a patient via a nebulizer.
- the solvent or suspension agent utilized for nebulization may be any pharmaceutically acceptable liquid such as water, aqueous saline, alcohols or glycols, e.g., ethanol, isopropyl alcohol, glycerol, propylene glycol, polyethylene glycol, etc. or mixtures thereof.
- Saline solutions utilize salts which display little or no pharmacological activity after administration.
- organic salts such as alkali metal or ammonium halogen salts, e.g., sodium chloride, potassium chloride or organic salts, such as potassium, sodium and ammonium salts or organic acids, e.g., ascorbic acid, citric acid, acetic acid, tartaric acid, etc. may be used for this purpose.
- organic acids e.g., ascorbic acid, citric acid, acetic acid, tartaric acid, etc.
- Other pharmaceutically acceptable excipients may be added to the suspension or solution.
- the compound of the invention may be stabilized by the addition of an inorganic acid, e.g., hydrochloric acid, nitric acid, sulfuric acid and/or phosphoric acid; an organic acid, e.g., ascorbic acid, citric acid, acetic acid, and tartaric acid, etc., a complexing agent such as EDTA or citric acid and salts thereof; or an antioxidant such as antioxidant such as vitamin E or ascorbic acid.
- an inorganic acid e.g., hydrochloric acid, nitric acid, sulfuric acid and/or phosphoric acid
- an organic acid e.g., ascorbic acid, citric acid, acetic acid, and tartaric acid, etc.
- a complexing agent such as EDTA or citric acid and salts thereof
- an antioxidant such as antioxidant such as vitamin E or ascorbic acid.
- Preservatives may be added such as benzalkonium chloride or benzoic acid and salts thereof.
- Surfactant
- LCMS data was generated using electrospray positive [ES+ve to give M+H + ion]equipped with a C18 column eluting with a gradient of 10% - 100% acetonitrile/water containing either 0.05% or 0.1 % TFA.
- the naming program used is ACD Name Pro 6.02 or the naming functionality of Chem Draw Ultra 12.0.
- Step 1 fe/f-butyl 3-(benzoyloxy -methylenepyrrolidine-1 -carboxylate
- Step 2 Chiral Resolution: (R) and (S)-fe/f-butyl 3-(benzoyloxy)-4-methylenepyrrolidine-1 - carboxylate
- Racemic fe/ -butyl 3-hydroxy-4-methylenepyrrolidine-1 -carboxylate (800 g) was resolved in 12.5 g batches at a 10 min cycle time via preparative HPLC (Chiralpak IC, 100 x 250 mm) eluting with heptanes/IPA (75/25) at a flowrate of 500 mL/min.
- the respective enantiomer fractions were combined, concentrated under reduced pressure, and reconcentrated from Et 2 0 to give each enantiomer as a faint yellow liquid.
- Step 1 (/?)-fe/f-butyl 3-hydroxy-4-methylenepyrrolidine-1 -carboxylate
- Step 2 (SHe/f-butyl 3-(benzoyloxy -methylenepyrrolidine-1 -carboxylate
- Step 1 0-(4-chloro-3-cvanophenyl) dimethylcarbamothioate
- INTERMEDIATES 5-9 were prepared from the appropriate phenol by the three step method analogous to that described for intermediate 4.
- Step 1 O-ethyl S-(6-(trifluoromethyl)pyridin-3-yl) carbonodithioate
- Step 1 5-(benzylthio)-2-(difluoromethoxy)pyridine
- INTERMEDIATES 14-16 were prepared by the 2 step method analogous to that described for intermediate 13.
- Conversion of the thioether to the sulfonyl chloride in the second step can alternatively be accomplished by bubbling Cl 2 gas into a solution of the thioether in formic acid.
- INTERMEDIATE 18 was prepared from the appropriate aniline by the one step method analogous to that described for intermediate 17.
- Step 1 0-(4-cvano-2-cvclopropoxyphenyl) dimethylcarbamothioate
- Step 3 4-cyano-2-cvclopropoxybenzene-1 -sulfonyl chloride S-(4-cyano-2-cyclopropoxyphenyl) dimethylcarbamothioate (0.89 g, 3.40 mmol) was dissolved in MeOH (5 ml) and THF (5 ml). NCS (1 .4 g, 10 mmol) was added and the reaction mixture was stirred at rt for 30 min. The mixture was concentrated under reduced pressure to near dryness and purified by flash column chromatography (S1O2) eluting with a gradient of 0-50% MTBE in hexanes. The desired fractions were pooled and concentrated under reduced pressure to give the title compound as a clear oil that became a white solid on standing (466 mg, 53 % yield). MS (m/z) 258.1 (M+H + ).
- INTERMEDIATE 20 was prepared from the appropriate phenol by the 3 step method analogous to that described for intermediate 19.
- Step 1 4-bromo-3-(difluoromethyl ' )benzonitrile
- Step 3 4-cyano-2-(difluoromethyl)benzene-1 -sulfonyl chloride 4-(benzylthio)-3-(difluoromethyl)benzonitrile (1 .1 g, 4.2 mmol) was dissolved in
- INTERMEDIATE 22 was prepared from the appropriate benzaldehyde by the 3 step method analogous to that described for intermediate 21 .
- Step 1 ( ?)-fe/?-butyl S-CCS-chloropyridin ⁇ -vDthio ' t ⁇ -methylenepyrrolidine-l -carboxylate
- Step 2 (R)-tert-but ⁇ 3-((5-chloropyridin-2-yl)sulfonyl)-4-methylenepyrrolidine-1 - carboxylate
- Step 4 3-chloro-4-(((3f?,4SV4-((5-chloropyridin-2-yl)sulfonyl)-3-hvdroxy-3- (hvdroxymethyl)pyrrolidin-1-yl)sulfonyl)benzonitrile
- Step 1 ( ?)-fe/?-butyl S- ⁇ -chlorophenvOthioV ⁇ methylenepyrrolidine-l -carboxylate
- Step 3 (3f?,4S)-fe/f-butyl 4-((4-chlorophenyl)sulfonyl)-3-hvdroxy-3- (hvdroxymethyl)pyrrolidine-l -carboxylate and (3S,4S)-fe/f-butyl 4-((4- chlorophenyl)sulfonyl)-3-hvdroxy-3-(hvdroxymethyl)pyrrolidine-1 -carboxylate
- Step 5 3-Chloro-4-(((3f?,4S)-4-((4-chlorophenyl)sulfonyl)-3-hydroxy-3- (hvdroxymethyl)pyrrolidin-1 -yl)sulfonyl)benzonitrile
- Step 1 (ffl-fe/f-butyl S- ⁇ -cvanophenvDthio ' i ⁇ -methylenepyrrolidine-l -carboxylate
- the reaction was quenched with H 2 0 (500 mL) and extracted with hexanes/EtOAc (1 :1 , 2 x 500 mL). The combined extracts were washed with H2O (4 x 500 mL), brine (1 x500 mL), dried over Na 2 S0 4 , filtered and concentrated under reduced pressure.
- the crude product was purified by flash column chromatography (Si0 2 ) eluting with a gradient of 0-60% EtOAc in hexanes. The product fractions were pooled, concentrated and triturated with DCM/hexane (100 mL/300 mL).
- Step 3 (3f?,4S)-fe/f-butyl 4-((4-cyanophenyl)sulfonyl)-3-hvdroxy-3- (hvdroxymethyl)pyrrolidine-l -carboxylate and (3S,4S)-fe/f-butyl 4-((4- cvanophenyl)sulfonyl)-3-hvdroxy-3-(hvdroxymethyl)pyrrolidine-1 -carboxylate
- Step 4 (5f?,9S)-fe/f-butyl 9-((4-cyanophenyl)sulfonyl)-2,2-dimethyl-1 ,3-dioxa-7- azaspiro[4.41nonane-7-carboxylate or (5S,9S)-fe/f-butyl 9-((4-cyanophenyl)sulfonyl)- 2,2-dimethyl-1 ,3-dioxa-7-azaspiro[4.41nonane-7-carboxylate
- the reaction mixture was diluted with DCM and quenched with sat'd NaHC0 3 (aq). The water layer was removed and the DCM layer was washed with H 2 0, dried over Na 2 S0 4 , filtered and concentrated under reduced pressure.
- the crude isomer mixture was purified and separated by flash column chromatography (Si0 2 ) eluting with a gradient of 0-50% EtOAc in hexanes to give the title compounds as the individual trans and cis isomers.
- Step 6 4-(((3S,4f?)-1 -((2-bromo-4-fluorophenyl)sulfonyl)-4-hydroxy-4- (hvdroxymethyl)pyrrolidin-3-yl)sulfonyl)benzonitrile
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Epidemiology (AREA)
- Pulmonology (AREA)
- Cardiology (AREA)
- Neurology (AREA)
- Diabetes (AREA)
- Heart & Thoracic Surgery (AREA)
- Immunology (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Urology & Nephrology (AREA)
- Hematology (AREA)
- Pain & Pain Management (AREA)
- Physical Education & Sports Medicine (AREA)
- Obesity (AREA)
- Endocrinology (AREA)
- Ophthalmology & Optometry (AREA)
- Gastroenterology & Hepatology (AREA)
- Rheumatology (AREA)
- Hospice & Palliative Care (AREA)
- Dermatology (AREA)
- Reproductive Health (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Child & Adolescent Psychology (AREA)
- Gynecology & Obstetrics (AREA)
- Oncology (AREA)
Abstract
Description
Claims
Priority Applications (10)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CA3036933A CA3036933A1 (en) | 2016-09-20 | 2017-09-20 | Trpv4 antagonists |
| BR112019005416A BR112019005416A2 (en) | 2016-09-20 | 2017-09-20 | trpv4 antagonists |
| EP17784401.6A EP3515888B1 (en) | 2016-09-20 | 2017-09-20 | Trpv4 antagonists |
| ES17784401T ES2877763T3 (en) | 2016-09-20 | 2017-09-20 | Trpv4 antagonists |
| KR1020197010953A KR20190049865A (en) | 2016-09-20 | 2017-09-20 | TRPV4 antagonist |
| AU2017330620A AU2017330620A1 (en) | 2016-09-20 | 2017-09-20 | TRPV4 antagonists |
| JP2019515428A JP7106528B2 (en) | 2016-09-20 | 2017-09-20 | TRPV4 antagonist |
| RU2019110821A RU2019110821A (en) | 2016-09-20 | 2017-09-20 | ANTAGONISTS TRPV4 |
| US16/334,400 US11260049B2 (en) | 2016-09-20 | 2017-09-20 | TRPV4 antagonists |
| CN201780057911.XA CN109790116B (en) | 2016-09-20 | 2017-09-20 | TRPV4 antagonists |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662397008P | 2016-09-20 | 2016-09-20 | |
| US62/397,008 | 2016-09-20 | ||
| US201762482300P | 2017-04-06 | 2017-04-06 | |
| US62/482,300 | 2017-04-06 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018055526A1 true WO2018055526A1 (en) | 2018-03-29 |
Family
ID=60084021
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IB2017/055702 WO2018055526A1 (en) | 2016-09-20 | 2017-09-20 | Trpv4 antagonists |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US11260049B2 (en) |
| EP (1) | EP3515888B1 (en) |
| JP (1) | JP7106528B2 (en) |
| KR (1) | KR20190049865A (en) |
| CN (1) | CN109790116B (en) |
| AU (1) | AU2017330620A1 (en) |
| BR (1) | BR112019005416A2 (en) |
| CA (1) | CA3036933A1 (en) |
| ES (1) | ES2877763T3 (en) |
| RU (1) | RU2019110821A (en) |
| WO (1) | WO2018055526A1 (en) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10588891B2 (en) | 2016-09-20 | 2020-03-17 | Glaxosmithkline Intellectual Property Development Limited | TRPV4 antagonists |
| US10590077B2 (en) | 2016-09-20 | 2020-03-17 | Glaxosmithkline Intellectual Property Development Limited | TRPV4 antagonists |
| WO2022014707A1 (en) | 2020-07-16 | 2022-01-20 | ラクオリア創薬株式会社 | Trpv4 inhibitor as therapeutic drug for eye disease |
| US11234982B2 (en) | 2019-02-15 | 2022-02-01 | Novartis Ag | Methods for treating ocular surface pain |
| US11478480B2 (en) | 2019-02-15 | 2022-10-25 | Novartis Ag | Formulations of 4-(7-Hydroxy-2-isopropyl-4-oxo-4H-quinazolin-3-yl)-benzonitrile |
| KR20230005227A (en) | 2020-04-30 | 2023-01-09 | 라퀄리아 파마 인코포레이티드 | Pyrimidin-4(3H)-one derivatives as TRPV4 antagonists |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN110407721A (en) * | 2019-08-13 | 2019-11-05 | 上海毕得医药科技有限公司 | A kind of synthetic method of 4- cyano -3- (trifluoromethyl) benzene -1- sulfonic acid chloride |
| CN112500419A (en) * | 2020-11-23 | 2021-03-16 | 浙大城市学院 | Epoxy fused 2-methylene pyrrolidine compound and preparation method thereof |
| CN114058694A (en) * | 2021-11-29 | 2022-02-18 | 上海市普陀区中心医院 | Application of TRPV1 in screening or preparing medicines for preventing, relieving and/or treating liver diseases |
Citations (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2064336A (en) | 1979-12-06 | 1981-06-17 | Glaxo Group Ltd | Device for dispensing medicaments |
| EP0069715A1 (en) | 1981-07-08 | 1983-01-12 | Aktiebolaget Draco | Powder inhalator |
| GB2129691A (en) | 1982-10-08 | 1984-05-23 | Glaxo Group Ltd | Devices for administering medicaments to patients |
| GB2169265A (en) | 1982-10-08 | 1986-07-09 | Glaxo Group Ltd | Pack for medicament |
| GB2178965A (en) | 1985-07-30 | 1987-02-25 | Glaxo Group Ltd | Devices for administering medicaments to patients |
| US4778054A (en) | 1982-10-08 | 1988-10-18 | Glaxo Group Limited | Pack for administering medicaments to patients |
| GB2242134A (en) | 1990-03-02 | 1991-09-25 | Glaxo Group Ltd | Inhalation device |
| US6321747B1 (en) | 1997-01-08 | 2001-11-27 | Smithkline Beecham Corporation | Inhalation device |
| US6378519B1 (en) | 1990-03-02 | 2002-04-30 | Glaxo Group Limited | Inhalation device |
| US6536427B2 (en) | 1990-03-02 | 2003-03-25 | Glaxo Group Limited | Inhalation device |
| WO2003061743A1 (en) | 2002-01-25 | 2003-07-31 | Glaxo Group Limited | Medicament dispenser |
| WO2007012871A1 (en) | 2005-07-28 | 2007-02-01 | Glaxo Group Limited | Medicament dispenser |
| WO2007068896A1 (en) | 2005-12-12 | 2007-06-21 | Glaxo Group Limited | Manifold for use in medicament dispenser |
| WO2007082262A2 (en) * | 2006-01-11 | 2007-07-19 | Smithkline Beecham Corporation | Novel compounds |
| US8113199B2 (en) | 2004-02-16 | 2012-02-14 | Glaxo Group Limited | Counter for use with a medicament dispenser |
| US8161968B2 (en) | 2003-07-24 | 2012-04-24 | Glaxo Group Limited | Medicament dispenser |
| US9333310B2 (en) | 2004-08-16 | 2016-05-10 | Glaxo Group Limited | Medicament dispenser |
Family Cites Families (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| BR9713465A (en) | 1996-08-28 | 2000-03-28 | Procter & Gamble | Cytically substituted amine metallotprotease inhibitors |
| US6852865B2 (en) | 2001-12-06 | 2005-02-08 | Cornell Research Foundation, Inc. | Catalytic carbonylation of three and four membered heterocycles |
| US7595322B2 (en) | 2003-03-27 | 2009-09-29 | Cytokinetics, Inc. | Heterocyclic sulfonamides as modulators of cardiac sarcomeres |
| AR048523A1 (en) | 2004-04-07 | 2006-05-03 | Kalypsys Inc | COMPOUNDS WITH ARIL SULFONAMIDE AND SULFONYL STRUCTURE AS PPAR MODULATORS AND METHODS TO TREAT METABOLIC DISORDERS |
| CN101103027A (en) | 2004-04-28 | 2008-01-09 | 默克公司 | 3,3-disubstituted tetrahydropyranyl cyclopentyl amide modulators of chemokine receptor activity |
| US20060035884A1 (en) | 2004-05-20 | 2006-02-16 | Elan Pharmaceuticals, Inc. | N-cyclic sulfonamido inhibitors of gamma secretase |
| CN1329374C (en) | 2004-06-09 | 2007-08-01 | 上海靶点药物有限公司 | Compound as CCR5 agonist |
| BRPI0513262A (en) | 2004-07-12 | 2008-04-29 | Bayer Cropscience Ag | substituted heterocycles |
| DOP2006000010A (en) | 2005-01-10 | 2006-07-31 | Arena Pharm Inc | PROCEDURE TO PREPARE AROMATIC ETERES |
| US7879880B2 (en) | 2005-12-21 | 2011-02-01 | Schering Corporation | Substituted aniline derivatives useful as histamine H3 antagonists |
| KR20090042779A (en) | 2006-06-30 | 2009-04-30 | 쉐링 코포레이션 | Substituted piperidines that increase p53 activity and the uses thereof |
| WO2008091863A1 (en) | 2007-01-23 | 2008-07-31 | Kalypsys, Inc. | Sulfonyl-substituted bicyclic compounds as ppar modulators for the treatment of non-alcoholic steatohepatitis |
| EP2244571A4 (en) | 2008-01-17 | 2014-02-19 | Purdue Research Foundation | Small molecule inhibitors of hiv proteases |
| EP2303859A4 (en) | 2008-06-20 | 2012-08-22 | Metabolex Inc | Aryl gpr119 agonists and uses thereof |
| CN104918935B (en) | 2012-11-16 | 2017-07-28 | 百时美施贵宝公司 | Pyrazoline GPR40 conditioning agents |
| CN105209457A (en) | 2013-03-15 | 2015-12-30 | 艾伯维德国有限责任两合公司 | Pyrrolidine derivatives, pharmaceutical compositions containing them, and their use in therapy |
| JP6473133B2 (en) | 2013-03-15 | 2019-02-20 | アラクセス ファーマ エルエルシー | Covalent inhibitor of KRASG12C |
| EP2982666B1 (en) | 2013-04-04 | 2019-08-07 | Takeda Pharmaceutical Company Limited | Heterocyclic compound |
| WO2016016370A1 (en) | 2014-07-31 | 2016-02-04 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Flt3 receptor antagonists |
| TW201825458A (en) | 2016-09-20 | 2018-07-16 | 英商葛蘭素史克智慧財產(第二)有限公司 | TRPV 4 antagonists |
| WO2018055527A1 (en) | 2016-09-20 | 2018-03-29 | Glaxosmithkline Intellectual Property (No.2) Limited | Trpv4 antagonists |
-
2017
- 2017-09-20 CA CA3036933A patent/CA3036933A1/en active Pending
- 2017-09-20 RU RU2019110821A patent/RU2019110821A/en not_active Application Discontinuation
- 2017-09-20 ES ES17784401T patent/ES2877763T3/en active Active
- 2017-09-20 BR BR112019005416A patent/BR112019005416A2/en active Search and Examination
- 2017-09-20 US US16/334,400 patent/US11260049B2/en active Active
- 2017-09-20 AU AU2017330620A patent/AU2017330620A1/en not_active Abandoned
- 2017-09-20 CN CN201780057911.XA patent/CN109790116B/en active Active
- 2017-09-20 JP JP2019515428A patent/JP7106528B2/en active Active
- 2017-09-20 EP EP17784401.6A patent/EP3515888B1/en active Active
- 2017-09-20 KR KR1020197010953A patent/KR20190049865A/en unknown
- 2017-09-20 WO PCT/IB2017/055702 patent/WO2018055526A1/en unknown
Patent Citations (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2064336A (en) | 1979-12-06 | 1981-06-17 | Glaxo Group Ltd | Device for dispensing medicaments |
| EP0069715A1 (en) | 1981-07-08 | 1983-01-12 | Aktiebolaget Draco | Powder inhalator |
| GB2129691A (en) | 1982-10-08 | 1984-05-23 | Glaxo Group Ltd | Devices for administering medicaments to patients |
| GB2169265A (en) | 1982-10-08 | 1986-07-09 | Glaxo Group Ltd | Pack for medicament |
| US4778054A (en) | 1982-10-08 | 1988-10-18 | Glaxo Group Limited | Pack for administering medicaments to patients |
| GB2178965A (en) | 1985-07-30 | 1987-02-25 | Glaxo Group Ltd | Devices for administering medicaments to patients |
| US4811731A (en) | 1985-07-30 | 1989-03-14 | Glaxo Group Limited | Devices for administering medicaments to patients |
| US5035237A (en) | 1985-07-30 | 1991-07-30 | Newell Robert E | Devices for administering medicaments to patients |
| US6032666A (en) | 1990-03-02 | 2000-03-07 | Glaxo Group Limited | Inhalation device |
| US5590645A (en) | 1990-03-02 | 1997-01-07 | Glaxo Group Limited | Inhalation device |
| US5860419A (en) | 1990-03-02 | 1999-01-19 | Glaxo Group Limited | Inhalation device |
| US5873360A (en) | 1990-03-02 | 1999-02-23 | Glaxo Group Limited | Inhalation device |
| GB2242134A (en) | 1990-03-02 | 1991-09-25 | Glaxo Group Ltd | Inhalation device |
| US6378519B1 (en) | 1990-03-02 | 2002-04-30 | Glaxo Group Limited | Inhalation device |
| US6536427B2 (en) | 1990-03-02 | 2003-03-25 | Glaxo Group Limited | Inhalation device |
| US6321747B1 (en) | 1997-01-08 | 2001-11-27 | Smithkline Beecham Corporation | Inhalation device |
| WO2003061743A1 (en) | 2002-01-25 | 2003-07-31 | Glaxo Group Limited | Medicament dispenser |
| US8511304B2 (en) | 2002-01-25 | 2013-08-20 | Glaxo Group Limited | Medicament dispenser |
| US8161968B2 (en) | 2003-07-24 | 2012-04-24 | Glaxo Group Limited | Medicament dispenser |
| US8113199B2 (en) | 2004-02-16 | 2012-02-14 | Glaxo Group Limited | Counter for use with a medicament dispenser |
| US9333310B2 (en) | 2004-08-16 | 2016-05-10 | Glaxo Group Limited | Medicament dispenser |
| WO2007012871A1 (en) | 2005-07-28 | 2007-02-01 | Glaxo Group Limited | Medicament dispenser |
| US8746242B2 (en) | 2005-07-28 | 2014-06-10 | Glaxo Group Limited | Medicament dispenser |
| WO2007068896A1 (en) | 2005-12-12 | 2007-06-21 | Glaxo Group Limited | Manifold for use in medicament dispenser |
| US8534281B2 (en) | 2005-12-12 | 2013-09-17 | Glaxo Group Limited | Manifold for use in medicament dispenser |
| WO2007082262A2 (en) * | 2006-01-11 | 2007-07-19 | Smithkline Beecham Corporation | Novel compounds |
Non-Patent Citations (60)
| Title |
|---|
| "Remington's Pharmaceutical Sciences", MACK PUBLISHING COMPANY |
| "The Handbook of Pharmaceutical Additives", 1997, GOWER PUBLISHING LIMITED |
| "The Handbook of Pharmaceutical Excipients", AMERICAN PHARMACEUTICAL ASSOCIATION AND THE PHARMACEUTICAL PRESS |
| ABDULQAWI R ET AL., LANCET, vol. 385, no. 9974, 28 March 2015 (2015-03-28), pages 1198 - 1205 |
| AKIYAMA ET AL., J INVEST DERMATOL, vol. 136, 2016, pages 154 - 60 |
| ALCARAZ, L.; CRIDLAND, A.; KINCHIN, E., ORG. LETT., vol. 3, 2001, pages 4051 |
| ALESSANDRI-HABER ET AL., J NEUROSCI, vol. 26, 2006, pages 3864 - 3874 |
| ALVAREZ ET AL., CIRC RES, vol. 99, 2006, pages 988 - 95 |
| ALVAREZ ET AL., CIRC RES., vol. 99, no. 9, 27 October 2006 (2006-10-27), pages 988 - 95 |
| AUER-GRUMBACH ET AL., NAT GENET, 2009 |
| BALAKRISHNA ET AL., AM J PHYSIOL LUNG CELL MOL PHYSIOL, vol. 307, 2014, pages L158 - 72 |
| BALAKRISHNA ET AL., AM J PHYSIOL LUNG CELL MOL PHYSIOL., vol. 307, 2014, pages L158 - L172 |
| BASOGLU OK ET AL., CHEST., vol. 148, no. 2, August 2015 (2015-08-01), pages 430 - 5 |
| BHARGAVE ET AL., AM J RHINOL, vol. 22, 2008, pages 7 - 12 |
| BONVINI ET AL., J ALLERGY CLIN IMMUNOL, vol. 138, 2016, pages 249 - 61 |
| BONVINI SJ ET AL., J ALLERGY CLIN IMMUNOL., vol. 138, no. 1, July 2016 (2016-07-01), pages 249 - 261 |
| CHEN ET AL., J BIOL CHEM, vol. 291, 2016, pages 10252 - 62 |
| DELANY ET AL., PHYSIOL. GENOMICS, vol. 4, 2001, pages 165 - 174 |
| DELANY NS ET AL., PHYSIOL GENOMICS, vol. 4, no. 3, 19 January 2001 (2001-01-19), pages 165 - 74 |
| DENG ET AL., NAT GENET, 2009 |
| DUAN ET AL., MOL GENET GENOMICS, vol. 290, 2015, pages 1357 - 65 |
| EARLEY ET AL., CIRC RES, vol. 97, 2005, pages 1270 - 9 |
| EVERAERTS ET AL., PROC NATL ACAD SCI USA, vol. 107, 2010, pages 19084 - 19089 |
| FAN ET AL., J BIOL CHEM, vol. 284, 2009, pages 27884 - 91 |
| GRANT ET AL., J PHYSIOL, vol. 578, 2007, pages 715 - 733 |
| GULER ET AL., J NEUROSCI, vol. 22, 2002, pages 6408 - 6414 |
| HAMANAKA ET AL., AM J PHYSIOL LUNG CELL MOL PHYSIOL., vol. 293, no. 4, October 2007 (2007-10-01), pages L923 - 32 |
| HAMANAKA ET AL., AM J PHYSIOL, vol. 293, 2007, pages L923 - 32 |
| HILFIKER ET AL., ACS MED. CHEM. LETT., vol. 4, 2013, pages 293 - 296 |
| JIAN ET AL., AM J RESPIR CELL MOL BIOL, vol. 38, 2008, pages 386 - 92 |
| JIAN ET AL., AM J RESPIR CELL MOL BIOL, vol. 38, 2009, pages 386 - 92 |
| JIE ET AL., FRONT CELL NEUROSCI, vol. 9, 2015, pages 141 |
| JO ET AL., PROC NATL ACAD SCI U S A, vol. 113, 2016, pages 3885 - 90 |
| KRAKOW ET AL., AM J HUM GENET, vol. 84, 2009, pages 307 - 15 |
| LANDOURE ET AL., NAT GENET, 2009 |
| LI ET AL., FRONT CELL NEUROSCI, vol. 7, 2013, pages 17 |
| LIEDTKE; SIMON, AM J PHYSIOL, vol. 287, 2004, pages 269 - 71 |
| MAGUIRE, R. J.; MULZER, J.; BATS, J. W., J. ORG. CHEM., vol. 61, 1996, pages 6936 |
| MASUYAMA ET AL., CELL METAB, vol. 8, 2008, pages 257 - 65 |
| MCALEXANDER MA ET AL., J PHARMACOL EXP THER., vol. 349, no. 1, April 2014 (2014-04-01), pages 118 - 25 |
| MONAGHAN ET AL., PLOS ONE, vol. 10, 2015, pages e0128359 |
| MORTY ET AL., AM J PHYSIOL LUNG CELL MOL PHYSIOL, vol. 307, 2014, pages L817 - 21 |
| MURAMATSU ET AL., J. BIOL. CHEM., vol. 282, 2007, pages 32158 - 67 |
| RAHAMAN ET AL., J CLIN INVEST, vol. 124, 2014, pages 5225 - 38 |
| ROCK ET AL., NAT GENET, vol. 40, 2008, pages 999 - 1003 |
| SARAH E. SKERRATT ET AL: "Identification of false positives in "HTS hits to lead": The application of Bayesian models in HTS triage to rapidly deliver a series of selective TRPV4 antagonists", MEDCHEMCOMM, vol. 4, no. 1, 1 January 2013 (2013-01-01), United Kingdom, pages 244 - 251, XP055421415, ISSN: 2040-2503, DOI: 10.1039/C2MD20259J * |
| SKERRATT ET AL., MED. CHEM. COMMUN., vol. 4, 2013, pages 244 - 251 |
| STROTMANN ET AL., NAT CELL BIOL, vol. 2, 2000, pages 695 - 702 |
| THORNELOE ET AL., SCI TRANSL MED, vol. 4, 2012, pages 159ra148 |
| THORNELONE ET AL., SCI TRANS MED, vol. 4, 2012, pages 159ra148 |
| TODAKA ET AL., J BIOL CHEM, vol. 279, 2004, pages 35133 - 35138 |
| VERGNOLLE, BIOCHEM PHARMACOL, vol. 89, 2014, pages 157 - 61 |
| VINCENT ET AL., BIOCHEM BIOPHYS RES COMMUN, vol. 389, 2009, pages 490 - 494 |
| VRIENS ET AL., PROC NATL ACAD SCI U S A, vol. 101, 2004, pages 396 - 401 |
| WEGIERSKI ET AL., J BIOL CHEM., vol. 284, 2009, pages 2923 - 33 |
| WILLETTE ET AL., J PHARMACOL EXP THER, vol. 325, 2008, pages 466 - 74 |
| YANG ET AL., AM. J PHYSIOL., vol. 290, 2006, pages L1267 - L1276 |
| YE ET AL., CELL, vol. 151, 2012, pages 96 - 110 |
| YIN ET AL., AM J RESPIR CELL MOL BIOL, vol. 54, 2016, pages 370 - 83 |
| ZHU ET AL., HUM MOL GENETICS, vol. 18, 2009, pages 2053 - 62 |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10588891B2 (en) | 2016-09-20 | 2020-03-17 | Glaxosmithkline Intellectual Property Development Limited | TRPV4 antagonists |
| US10590077B2 (en) | 2016-09-20 | 2020-03-17 | Glaxosmithkline Intellectual Property Development Limited | TRPV4 antagonists |
| US11229623B2 (en) | 2016-09-20 | 2022-01-25 | Glaxosmithkline Intellectual Property (No. 2) Limited | TRPV4 antagonists |
| US11234982B2 (en) | 2019-02-15 | 2022-02-01 | Novartis Ag | Methods for treating ocular surface pain |
| US11478480B2 (en) | 2019-02-15 | 2022-10-25 | Novartis Ag | Formulations of 4-(7-Hydroxy-2-isopropyl-4-oxo-4H-quinazolin-3-yl)-benzonitrile |
| KR20230005227A (en) | 2020-04-30 | 2023-01-09 | 라퀄리아 파마 인코포레이티드 | Pyrimidin-4(3H)-one derivatives as TRPV4 antagonists |
| WO2022014707A1 (en) | 2020-07-16 | 2022-01-20 | ラクオリア創薬株式会社 | Trpv4 inhibitor as therapeutic drug for eye disease |
Also Published As
| Publication number | Publication date |
|---|---|
| US20210299113A1 (en) | 2021-09-30 |
| CN109790116A (en) | 2019-05-21 |
| KR20190049865A (en) | 2019-05-09 |
| EP3515888B1 (en) | 2021-03-31 |
| CA3036933A1 (en) | 2018-03-29 |
| AU2017330620A1 (en) | 2019-03-28 |
| US11260049B2 (en) | 2022-03-01 |
| EP3515888A1 (en) | 2019-07-31 |
| RU2019110821A (en) | 2020-10-22 |
| BR112019005416A2 (en) | 2019-06-18 |
| ES2877763T3 (en) | 2021-11-17 |
| CN109790116B (en) | 2022-10-28 |
| JP7106528B2 (en) | 2022-07-26 |
| JP2019532052A (en) | 2019-11-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11229623B2 (en) | TRPV4 antagonists | |
| EP3515888B1 (en) | Trpv4 antagonists | |
| AU2018313850B2 (en) | Compounds, compositions and methods | |
| ES2952994T3 (en) | Compounds, compositions and methods | |
| US10590077B2 (en) | TRPV4 antagonists | |
| ES2936517T3 (en) | Triazole azines of cyclohexylic acid as LPA antagonists | |
| ES2942767T3 (en) | Isoxazole azoles of cyclohexylic acid as LPA antagonists | |
| ES2474152T3 (en) | 6,7,8,9-tetrahydro-5H-pyrimido [4,5-d] azepin-4-yl] -amine derivatives as modulators of TRPV1 for the treatment of pain. | |
| WO2018109647A1 (en) | Bisaryl amides as nrf2 regulators | |
| JP2020502123A (en) | Bisaryl heterocycles as NRF2 activators | |
| EP3555082B1 (en) | Ether linked triazoles as nrf2 regulators |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17784401 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 3036933 Country of ref document: CA |
|
| ENP | Entry into the national phase |
Ref document number: 2019515428 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2017330620 Country of ref document: AU Date of ref document: 20170920 Kind code of ref document: A |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112019005416 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 20197010953 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2017784401 Country of ref document: EP Effective date: 20190423 |
|
| ENP | Entry into the national phase |
Ref document number: 112019005416 Country of ref document: BR Kind code of ref document: A2 Effective date: 20190320 |