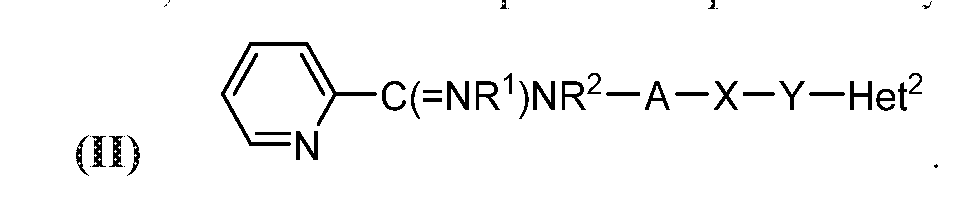WO2018045106A1 - Anti-fungal treatment - Google Patents
Anti-fungal treatment Download PDFInfo
- Publication number
- WO2018045106A1 WO2018045106A1 PCT/US2017/049493 US2017049493W WO2018045106A1 WO 2018045106 A1 WO2018045106 A1 WO 2018045106A1 US 2017049493 W US2017049493 W US 2017049493W WO 2018045106 A1 WO2018045106 A1 WO 2018045106A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- compound
- optionally substituted
- pharmaceutical composition
- alkyl
- represented
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 0 CN1C(C(Nc(cc2)ccc2-c2ccc(-c(cc3)ccc3OC*[n]3cncc3)[o]2)=N)=CC=CC1 Chemical compound CN1C(C(Nc(cc2)ccc2-c2ccc(-c(cc3)ccc3OC*[n]3cncc3)[o]2)=N)=CC=CC1 0.000 description 5
- ZZUYHOUSSOYGHT-VWLOTQADSA-N CC(C)Oc(cc(cc1)NC(C2=CC=CCN2C)=N)c1C(N[C@@H](C[n]1cncc1)c(cc1)c(C)cc1Cl)=O Chemical compound CC(C)Oc(cc(cc1)NC(C2=CC=CCN2C)=N)c1C(N[C@@H](C[n]1cncc1)c(cc1)c(C)cc1Cl)=O ZZUYHOUSSOYGHT-VWLOTQADSA-N 0.000 description 1
- VICFAPVFQMLRCV-NMBPHSMGSA-N CC(C)Oc(cc(cc1)NC(c2ccccn2)=N)c1C(N[C@@H](CN1C=NCC1C)c(ccc(-c(cc1)ccc1F)c1)c1F)=O Chemical compound CC(C)Oc(cc(cc1)NC(c2ccccn2)=N)c1C(N[C@@H](CN1C=NCC1C)c(ccc(-c(cc1)ccc1F)c1)c1F)=O VICFAPVFQMLRCV-NMBPHSMGSA-N 0.000 description 1
- GQIHWOUMKRJWOV-DEOSSOPVSA-N CC(C)Oc1cc(NC(c2ccccn2)=N)ccc1C(N[C@@H](C[n]1cncc1)c(ccc(Cl)c1)c1Cl)=O Chemical compound CC(C)Oc1cc(NC(c2ccccn2)=N)ccc1C(N[C@@H](C[n]1cncc1)c(ccc(Cl)c1)c1Cl)=O GQIHWOUMKRJWOV-DEOSSOPVSA-N 0.000 description 1
- GHDLDPQFLFRZED-MBCWZBCWSA-N CN1C(C(N)Nc(cc2)ccc2C(N[C@@H](C[n]2cncc2)c(ccc(-c(cc2)ccc2F)c2)c2F)=O)=CC=CC1 Chemical compound CN1C(C(N)Nc(cc2)ccc2C(N[C@@H](C[n]2cncc2)c(ccc(-c(cc2)ccc2F)c2)c2F)=O)=CC=CC1 GHDLDPQFLFRZED-MBCWZBCWSA-N 0.000 description 1
- GCEMAEINHUQXGX-LJAQVGFWSA-N C[F]c(cc1)ccc1-c1cc(F)c([C@H](C[n]2cncc2)NC(c(cc2)ccc2NC(c2ncccc2)=N)=O)cc1 Chemical compound C[F]c(cc1)ccc1-c1cc(F)c([C@H](C[n]2cncc2)NC(c(cc2)ccc2NC(c2ncccc2)=N)=O)cc1 GCEMAEINHUQXGX-LJAQVGFWSA-N 0.000 description 1
- DEGVLAIUYQJHCV-QHCPKHFHSA-N Cc1cc(Cl)c([C@H](C[n]2cncc2)NC(c(cc2)ccc2NC(c2ccccn2)=N)=O)cc1 Chemical compound Cc1cc(Cl)c([C@H](C[n]2cncc2)NC(c(cc2)ccc2NC(c2ccccn2)=N)=O)cc1 DEGVLAIUYQJHCV-QHCPKHFHSA-N 0.000 description 1
- DZNZLQOOBKAGGG-UHFFFAOYSA-N N=C(c1ccccn1)Nc(cc1)ccc1-c1ccc(-c(cc2)ccc2OCC[n]2cncc2)[o]1 Chemical compound N=C(c1ccccn1)Nc(cc1)ccc1-c1ccc(-c(cc2)ccc2OCC[n]2cncc2)[o]1 DZNZLQOOBKAGGG-UHFFFAOYSA-N 0.000 description 1
- HJFUIBXTQRWMOC-QFIPXVFZSA-N N=C(c1ncccc1)Nc(cc1)ccc1C(N[C@@H](C[n]1cncc1)c(ccc(Cl)c1)c1Cl)=O Chemical compound N=C(c1ncccc1)Nc(cc1)ccc1C(N[C@@H](C[n]1cncc1)c(ccc(Cl)c1)c1Cl)=O HJFUIBXTQRWMOC-QFIPXVFZSA-N 0.000 description 1
- DPTOQXCDGFDWOW-UHFFFAOYSA-N N=C(c1ncccc1)Nc(cc1)ccc1N1CCN(CCCCCC[n]2cncc2)CC1 Chemical compound N=C(c1ncccc1)Nc(cc1)ccc1N1CCN(CCCCCC[n]2cncc2)CC1 DPTOQXCDGFDWOW-UHFFFAOYSA-N 0.000 description 1
- DBRBCFJUEVSKGZ-UHFFFAOYSA-N [O-][N+](c(cc1)ccc1OCCCCBr)=O Chemical compound [O-][N+](c(cc1)ccc1OCCCCBr)=O DBRBCFJUEVSKGZ-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/4439—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a five-membered ring with nitrogen as a ring hetero atom, e.g. omeprazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene or sparfloxacin
Definitions
- Fungal infections continue to be major causes of morbidity and mortality, particularly for vulnerable subjects with compromised or suppressed immune systems.
- Most fungal infections e.g., aspergillosis, cryptococcosis, and histoplasmosis
- Candidiasis often occurs in hospital settings since at-risk patients are often under inpatient care.
- Subjects at significant risk of fungal infection may include, for example, chemotherapy and other oncology patients with immune suppression, transplant recipients receiving immunosuppressant anti-rejection therapy, subjects with HIV infection or other immune-compromised diseases, individuals receiving anti-inflammatory therapeutics such as TNF-alpha blockers (e.g., Enbrel), burn patients, and the like.
- TNF-alpha blockers e.g., Enbrel
- Treatments exist for systemic fungal infections such as candidiasis and aspergillosis, but are limited by low efficacy, side effects, expense, and drug resistance.
- Known antifungal drug classes include: azoles, such as fluconazole, itraconazole, and voriconazole; polyenes, such as Amphotericin B, nystatin, and natamycin; echinocandins such as caspofungin; and the like.
- azoles such as fluconazole, itraconazole, and voriconazole
- polyenes such as Amphotericin B, nystatin, and natamycin
- echinocandins such as caspofungin
- Each of the currently approved antifungal drugs have one or more significant drawbacks, such as a lack of broad-spectrum activity, low activity, poor oral bioavailability, undesirable side-effects, expense, long treatment durations that impact patient compliance, and drug-drug interactions. Further, systemic administration of many antifungal drugs in effective concentrations, such as amphotericin B, may be toxic, damaging the liver and other organs.
- a method of anti-fungal treatment in a subject in need thereof may include providing the subject, the subject being infected by a fungus or at risk of infection by the fungus.
- the method may include administering a compound to the subject in an amount effective to mitigate infection by the fungus in the subject.
- the compound may be represented by Structural Formula (Ia):
- Ar may be an optionally substituted aryl or nitrogen-containing heteroaryl.
- R 1 and R 2 may independently be H, optionally substituted C 1 -C 6 alkyl, or optionally substituted C 3 -C 6 cycloalkyl.
- A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl.
- X may be O, S, amide, or a bond.
- Y may be optionally substituted C 1 -C 14 alkyl or optionally substituted C 2 -C 14 alkenyl, e.g., optionally substituted C 1 -C 10 alkyl or optionally substituted C 2 - C 10 alkenyl, or optionally substituted C 1 -C 8 alkyl or optionally substituted C 2 -C 8 alkenyl.
- Het 2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
- a method of anti-fungal treatment in a subject in need thereof may include providing the subject, the subject being infected by a fungus or at risk of infection by the fungus.
- the method may include administering a compound to the subject in an amount effective to mitigate infection by the fungus in the subject.
- the compound may be represented by Structural Formula (I):
- Het 1 may be an optionally substituted, nitrogen- containing heteroaryl.
- R 1 and R 2 may independently be H, optionally substituted C 1 -C 6 alkyl, or optionally substituted C 3 -C 6 cycloalkyl.
- A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings.
- Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl.
- X may be O, S, amide, or a bond.
- Y may be optionally substituted C 1 -C 10 alkyl or optionally substituted C 2 -C 10 alkenyl.
- Het 2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
- a pharmaceutical composition may include a pharmaceutically acceptable carrier or excipient.
- the pharmaceutical composition may include a compound represented by Structural Formula (Ia):
- Ar may be an optionally substituted aryl or nitrogen-containing heteroaryl.
- R 1 and R 2 may independently be H, optionally substituted C 1 -C 6 alkyl, or optionally substituted C 3 -C 6 cycloalkyl.
- A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl.
- X may be O, S, amide, or a bond.
- Y may be optionally substituted C 1 -C 14 alkyl or optionally substituted C 2 -C 14 alkenyl, e.g., optionally substituted C 1 -C 10 alkyl or optionally substituted C 2 -C 10 alkenyl, or optionally substituted C 1 -C 8 alkyl or optionally substituted C 2 -C 8 alkenyl.
- Het 2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
- a pharmaceutical composition may include a pharmaceutically acceptable carrier or excipient.
- the pharmaceutical composition may include a compound represented by Structural Formula (I):
- Het 1 may be an optionally substituted, nitrogen- containing heteroaryl.
- R 1 and R 2 may independently be H, optionally substituted C 1 -C 6 alkyl, or optionally substituted C 3 -C 6 cycloalkyl.
- A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings.
- Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl.
- X may be O, S, amide, or a bond.
- Y may be optionally substituted C 1 -C 10 alkyl or optionally substituted C 2 -C 10 alkenyl.
- Het 2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
- kits for anti-fungal treatment of a subject in need thereof may include any anti-fungal compound described herein, for example, the compound represented by Structural Formula (Ia):
- Ar may be an optionally substituted aryl or nitrogen-containing heteroaryl.
- R 1 and R 2 may independently be H, optionally substituted C 1 -C 6 alkyl, or optionally substituted C 3 -C 6 cycloalkyl.
- A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl.
- X may be O, S, amide, or a bond.
- Y may be optionally substituted C 1 -C 14 alkyl or optionally substituted C 2 -C 14 alkenyl, e.g., optionally substituted C 1 -C 10 alkyl or optionally substituted C 2 -C 10 alkenyl, or optionally substituted C 1 -C 8 alkyl or optionally substituted C 2 -C 8 alkenyl.
- Het 2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
- the kit may include instructions. The instructions may direct a user to provide the subject, the subject being infected by a fungus or at risk of infection by the fungus. The instructions may direct a user to administer the compound or the pharmaceutical composition to the subject in an amount effective to mitigate infection by the fungus in the subject.
- kits for anti-fungal treatment of a subject in need thereof may include any anti-fungal compound described herein, for example, the compound represented by Structural Formula (I):
- Het 1 may be an optionally substituted, nitrogen-containing heteroaryl.
- R 1 and R 2 may independently be H, optionally substituted C 1 -C 6 alkyl, or optionally substituted C 3 -C 6 cycloalkyl.
- A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl.
- X may be O, S, amide, or a bond.
- the kit may include instructions.
- the instructions may direct a user to provide the subject, the subject being infected by a fungus or at risk of infection by the fungus.
- the instructions may direct a user to administer the compound or the pharmaceutical composition to the subject in an amount effective to mitigate infection by the fungus in the subject.
- FIG. 1A is a synthetic scheme for various phenoxyalkyl linker anti-fungal compounds.
- FIG.1B is a synthetic scheme for various phenoxyalkyl hybrid target compounds substituted meta to the arylimidamide group.
- FIG. 1C is a synthetic scheme for various phenoxyalkyl hybrid target compounds substituted ortho to the arylimidamide group.
- FIG. 1D is a synthetic scheme for various phenoxyalkyl hybrid target compounds substituted with pyrrole.
- FIG. 2A is a synthetic scheme for various phenyl-unsubstituted diphenylfuran alkyloxy linker hybrid compounds.
- FIG. 2B is a synthetic scheme for various phenyl substituted diphenylfuran alkyloxy linker hybrid compounds.
- FIG. 2C is a synthetic scheme for various phenyl substituted diphenylfuran alkyloxy linker hybrid compounds.
- FIG. 2D is a prophetic synthetic scheme for various diphenylfuran alkyloxy linker hybrid compounds.
- FIG. 3 is a prophetic synthetic scheme for various alkylamide linker hybrid compounds.
- FIG. 4A is a prophetic synthetic scheme for various phenylalkyl linker hybrid compounds 8a and 8b.
- FIG.4B is a synthetic scheme for various phenylalkyl linker hybrid compounds.
- FIG. 5A is a synthetic scheme for various unsubstituted and substituted biphenyl linker anti-fungal compounds.
- FIG. 5B is a synthetic scheme for various substituted biphenyl linker anti- fungal compounds.
- FIG. 5C is a synthetic scheme for various substituted biphenyl linker anti- fungal compounds.
- FIG. 5D is a synthetic scheme for various substituted biphenyl linker anti- fungal compounds.
- FIG. 6A is a synthetic scheme for various phenyl-piperazinyl-phenyl linker anti-fungal compounds.
- FIG. 6B is a synthetic scheme for various phenyl-piperazinyl linker anti-fungal compounds.
- FIG. 7 is a table depicting IC 50 values of various compounds against Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, and Histoplasma capsulatum.
- FIG. 8A is a graph depicting relative fungal growth of Aspergillus fumigatus versus concentration in ⁇ M of various compounds.
- FIG. 8B is a graph depicting relative fungal growth of Candida albicans versus concentration in ⁇ M of various compounds.
- FIG. 8C is a graph depicting relative fungal growth of Cryptococcus neoformans versus concentration in ⁇ M of various compounds.
- FIG. 8D is a graph depicting relative fungal growth of Histoplasma capsulatum versus concentration in ⁇ M of various compounds.
- a method of anti-fungal treatment in a subject in need thereof may include providing the subject, the subject being infected by a fungus or at risk of infection by the fungus.
- the method may include administering a compound to the subject in an amount effective to mitigate infection by the fungus in the subject.
- the compound may be represented by Structural Formula (Ia):
- Ar may be an optionally substituted aryl or nitrogen-containing heteroaryl.
- R 1 and R 2 may independently be H, optionally substituted C 1 -C 6 alkyl, or optionally substituted C 3 -C 6 cycloalkyl.
- A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl.
- X may be O, S, amide, or a bond.
- Y may be optionally substituted C 1 -C 14 alkyl or optionally substituted C 2 -C 14 alkenyl, e.g., optionally substituted C 1 -C 10 alkyl or optionally substituted C 2 -C 10 alkenyl, or optionally substituted C 1 -C 8 alkyl or optionally substituted C 2 -C 8 alkenyl.
- Het 2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
- a method of anti-fungal treatment in a subject in need thereof may include providing the subject, the subject being infected by a fungus or at risk of infection by the fungus.
- the method may include administering a compound to the subject in an amount effective to mitigate infection by the fungus in the subject.
- the compound may be represented by Structural Formula (I):
- Het 1 may be an optionally substituted, nitrogen- containing heteroaryl.
- R 1 and R 2 may independently be H, optionally substituted C 1 -C 6 alkyl, or optionally substituted C 3 -C 6 cycloalkyl.
- A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl.
- X may be O, S, amide, or a bond.
- Y may be optionally substituted C 1 -C 10 alkyl or optionally substituted C 2 -C 10 alkenyl.
- Het 2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
- the method may include administering the compound represented by Structural Formula (I) or (Ia) in the form of any pharmaceutical composition described herein.
- the subject may be infected by the fungus.
- the method may include administering the compound to the subject in an amount effective to mitigate one or more symptoms of infection by the fungus in the subject. Additionally or alternatively, the subject may be at risk of infection by the fungus.
- the method may include administering the compound to the subject in an amount effective to mitigate infection or re-infection of the subject by the fungus.
- the fungus may include CYP51.
- the fungus may include at least two distinct cytochrome P450 mediated biosterol synthesis pathways, e.g., one of which may include CYP51.
- the fungus may belong to a genus that is one of: Aspergillus, Candida, Cryptococcus, Histoplasma, and the like.
- the fungus may be one of: Aspergillus fumigatus, Candida albicans, Candida glabrata, Cryptococcus neoformans, Histoplasma capsulatum, and the like.
- the subject may have, or be at risk of aspergillosis, candidiasis, cryptococcosis, histoplasmosis, and the like.
- the subject may suffer from or be at risk of suffering from an infection by the fungus of one or more of: skin, nail, hoof, hair, fur, scale, mucosal membrane, blood, lymph, brain, lung, heart, liver, pancreas, spleen, kidney, bladder, stomach, and intestine.
- the subject may suffer from a systemic infection by the fungus or be at risk of suffering from a systemic infection by the fungus.
- the subject may be one of: suffering from cancer; undergoing chemotherapy; undergoing immune suppression therapy; a transplant recipient, e.g., one undergoing immune suppression therapy; suffering from an immuno- deficiency disease or condition, such as HIV infection; a burn patient; and the like.
- the subject may be a human, dog, cat, cow, horse, sheep, pig, bird, amphibian, or fish.
- the compound represented by Structural Formula (Ia) or (I) may exclude certain compounds.
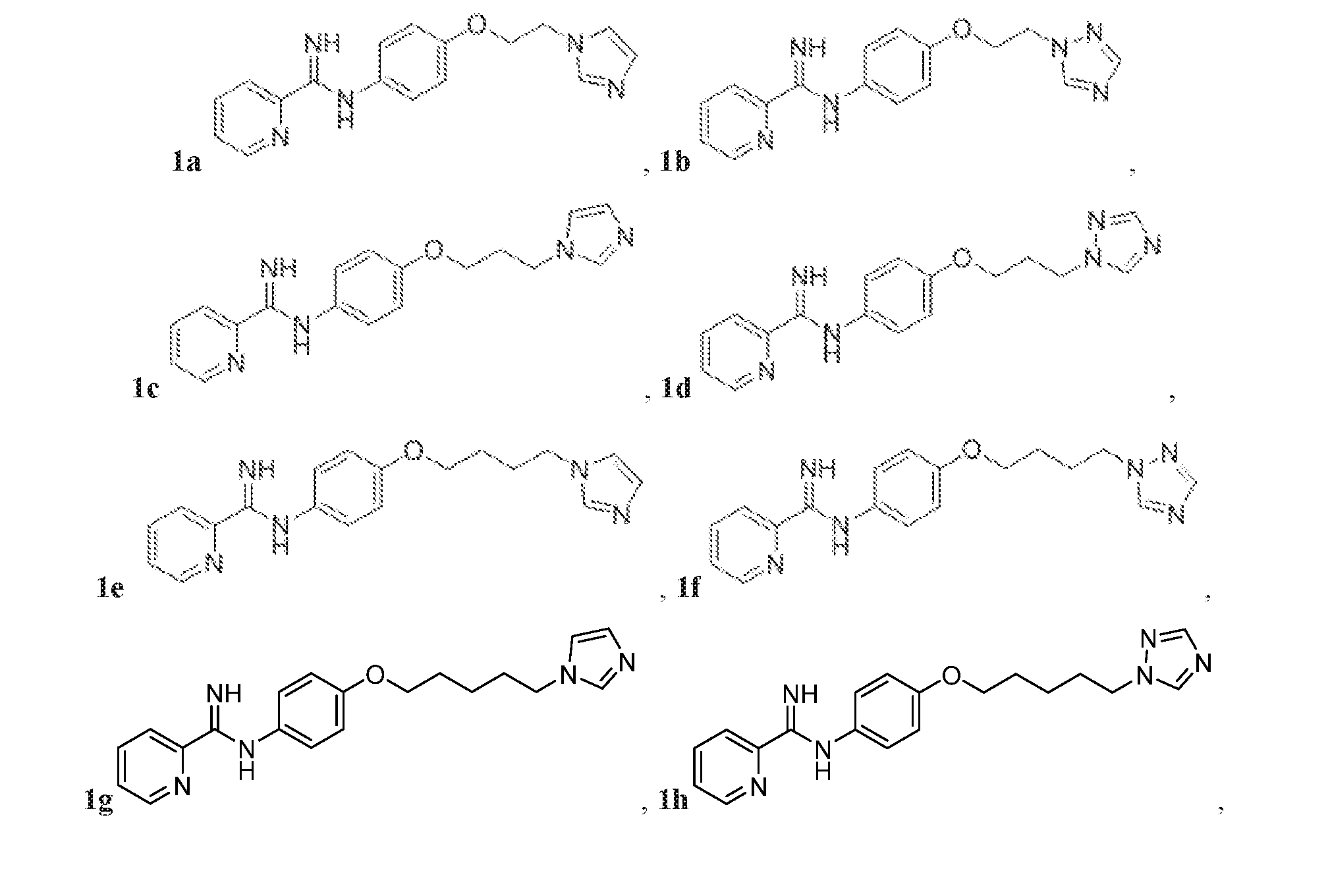
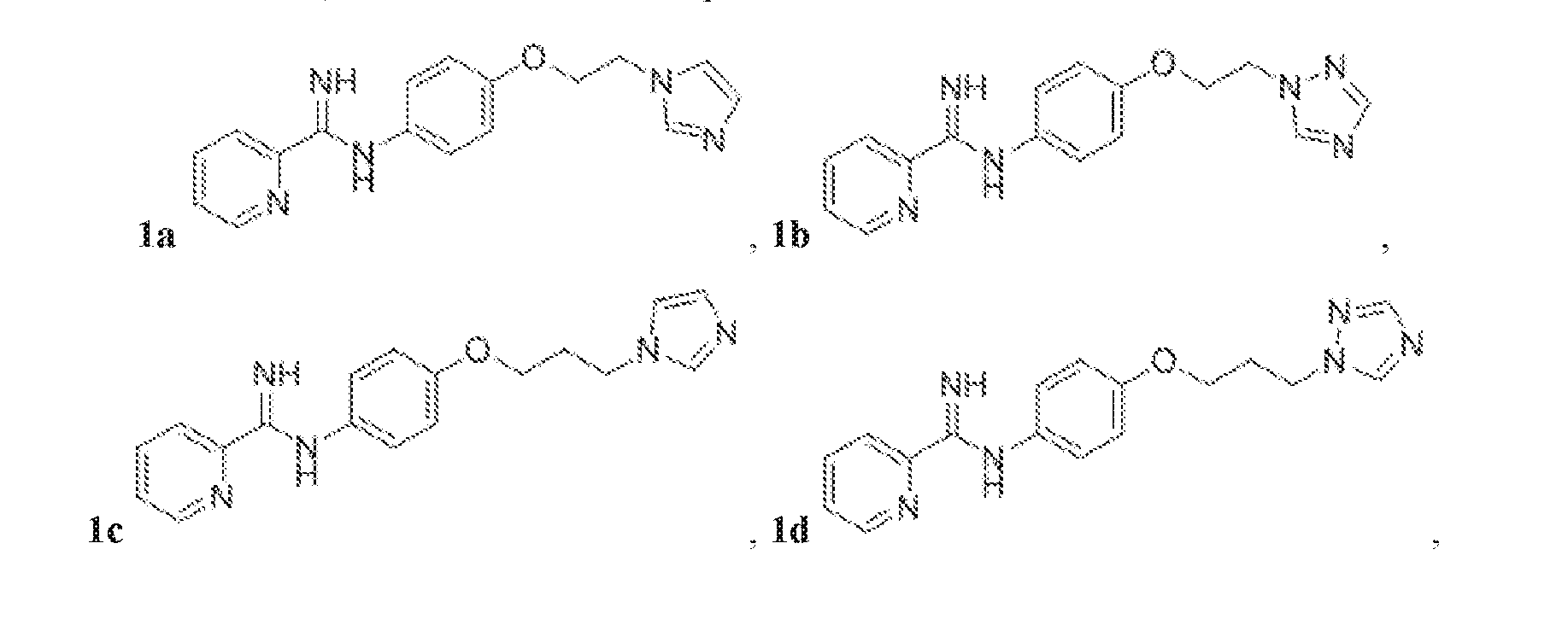
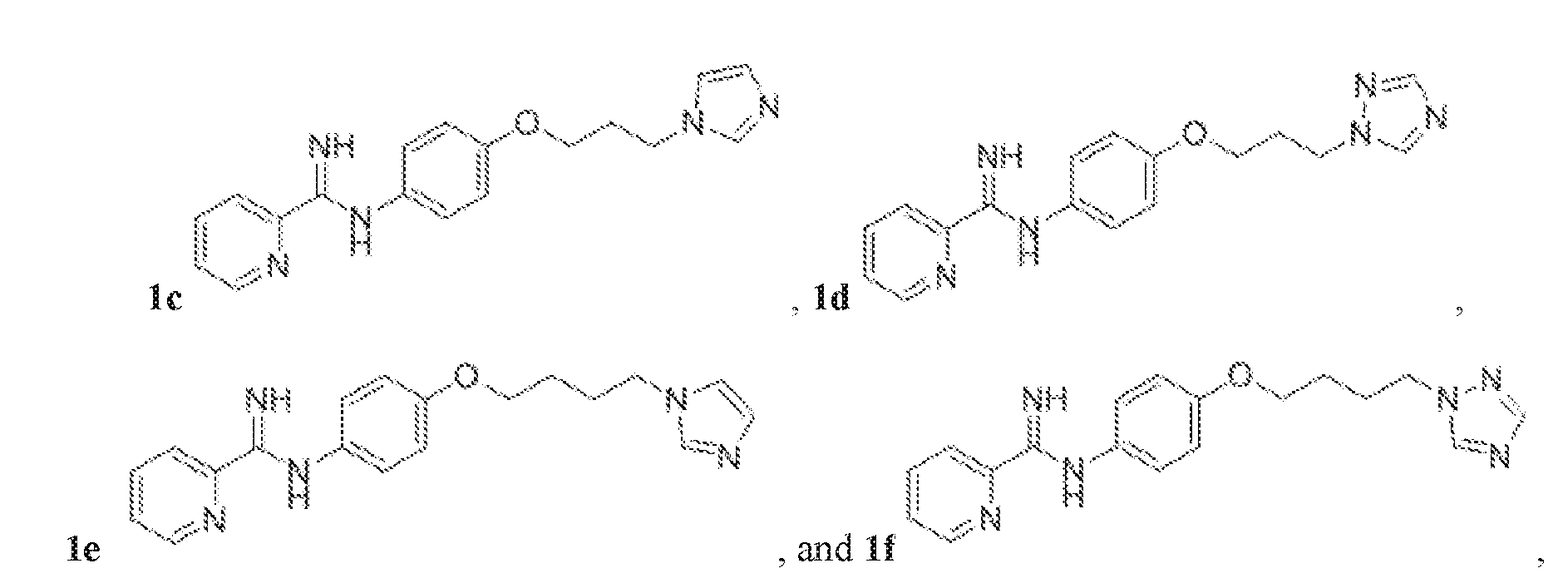
- the compound represented by Structural Formula (Ia) or (I) may exclude free-base (neutral or non-salt) forms of Compounds 1a-f, wherein: Het 1 is unsubstituted pyrid-2-yl; R 1 and R 2 are each H; X is O; Y is unbranched, unsubstituted C 2 -C 4 alkyl; and Het 2 is unsubstituted imidazole-1-yl or unsubstituted 1, 2, 4 triazol-2-yl:
- the compound represented by Structural Formula (Ia) or (I) may include pharmaceutically acceptable salts of Compounds 1a-f.
- the compound represented by Structural Formula (Ia) or (I) may exclude free-base forms of Compounds 1a-f and pharmaceutically acceptable salts of Compounds 1a-f.
- the compound represented by Structural Formula (Ia) or (I) may exclude protonated forms of Compounds 1a-f.
- the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein Het 1 is one of unsubstituted pyridyl and pyrid-2-yl substituted with methyl or ethyl, for example, when: R 1 and R 2 are each H; X is O; Y is unbranched, unsubstituted C 2 -C 4 alkyl; and Het 2 is unsubstituted imidazole-1-yl or unsubstituted 1,2,4-triazol-2-yl.
- the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein one of R 1 and R 2 is H and the other is methyl or ethyl, for example, when: Het 1 is unsubstituted pyrid-2-yl; X is O; Y is unbranched, unsubstituted C 2 - C 4 alkyl; and Het 2 is unsubstituted imidazole-1-yl or unsubstituted 1,2,4-triazol-2-yl.
- the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein X is S, for example, when: Het 1 is unsubstituted pyrid-2-yl; R 1 and R 2 are each H; Y is unbranched, unsubstituted C 2 -C 4 alkyl; and Het 2 is unsubstituted imidazole-1-yl or unsubstituted 1,2,4-triazol-2-yl.
- the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein Y is C 2 -C 4 alkyl substituted with methyl or ethyl, unsubstituted or methyl substituted C 1 -C 5 alkyl, or unsubstituted C 1 -C 6 alkyl, for example, when: Het 1 is unsubstituted pyrid-2-yl; R 1 and R 2 are each H; X is O; and Het 2 is unsubstituted imidazole-1-yl or unsubstituted 1,2,4-triazol-2-yl.
- the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein Het 2 is one of: methyl or ethyl substituted imidazole-1-yl; unsubstituted imidazolyl; methyl or ethyl substituted imidazolyl; methyl or ethyl substituted 1,2,4-triazol-2-yl; unsubstituted 1,2,4-triazolyl; methyl or ethyl substituted 1,2,4-triazolyl; unsubstituted triazolyl; and methyl or ethyl substituted triazolyl; for example, when: Het 1 is unsubstituted pyrid-2-yl; R 1 and R 2 are each H; X is O; and Y is unbranched, unsubstituted C 2 -C 4 alkyl.
- Het 1 may be optionally substituted pyridyl, pyrazinyl, pyrimidinyl, or pyridizinyl.
- Het 1 may be optionally substituted pyridyl.
- the compound may be represented by Structural Formula (II):
- the compound may be represented by Structural Formula (III):
- each ring in the optionally substituted linking moiety represented by A may be independently and optionally substituted by one or more of: hydroxy, halo, and C 1 -C 6 alkoxy.
- A may include an optionally substituted heteroaryl or optionally substituted heterocycloalkyl ring.
- A may include an optionally substituted, oxygen-containing, heteroaryl or heterocycloalkyl ring.
- A may include an optionally substituted furanyl or optionally substituted tetrahydrofuranyl ring.
- A may include optionally substituted 2,5-furanyl.
- A may include one or two optionally substituted phenyl rings.
- A may include optionally substituted 1,4-phenyl.
- A may be optionally substituted 1,4-phenyl.
- A may be optionally substituted phenyl-heteroaryl-phenyl.
- the compound of Structural Formula III may be represented by one of Structural Formulas (IIIa)-(IIIf):
- Z, Z 1 , and Z 2 are each independently CH or N, n may be 1-14, e.g., 1-10, and R 3 may represent H, halogen, optionally halogenated C 1 -C 6 alkyl, or optionally halogenated C 1 -C 6 alkoxy.
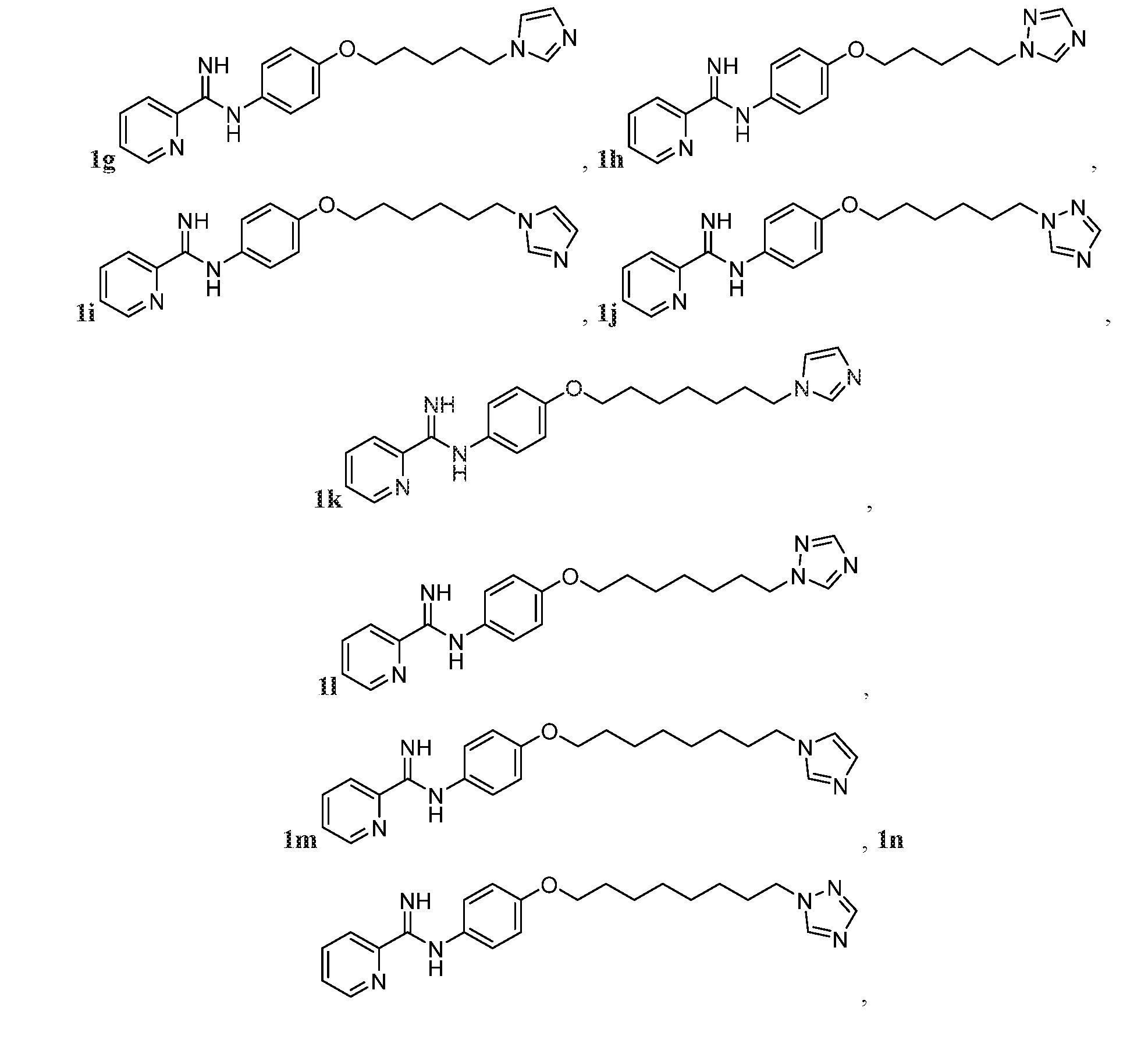
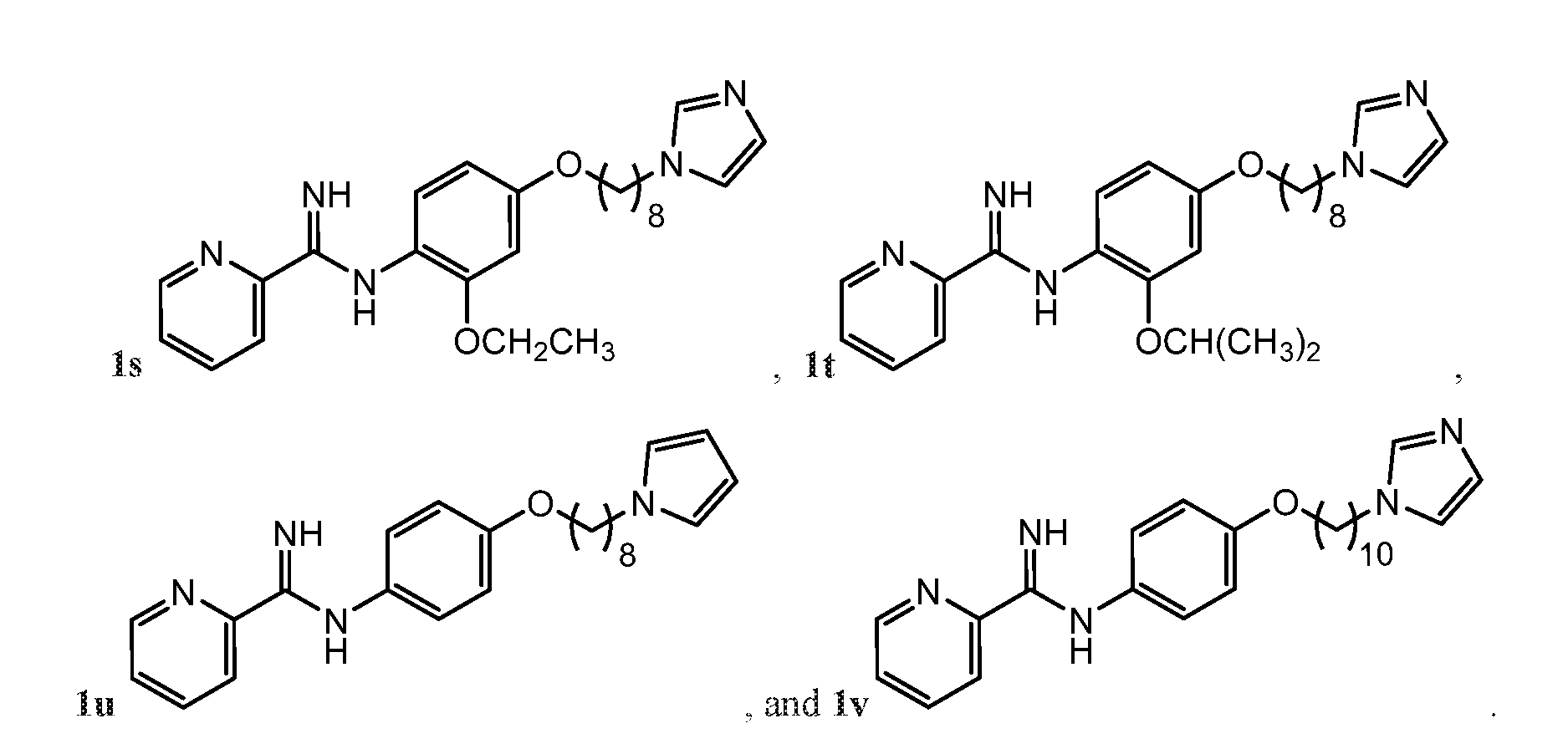
- the compound may be represented by one of Compounds 1g-1v, e.g., 1g-1n:
- the compound may be, for example, represented by Structural Formula (IIIf) above, e.g., compound 14a:
- the compound may be represented by Structural Formula (IV):
- each R 3 may independently represent H, halogen, optionally halogenated C 1 -C 6 alkyl, or optionally halogenated C 1 -C 6 alkoxy.
- the compound may be represented by Structural Formula (V):
- Y may include at least 4 linking atoms between X and Het 2 .
- X may be O or a bond and Y may be C 1 -C 14 , e.g., C 1 -C 10 alkyl optionally substituted with one or more of: optionally halogenated C 1 -C 8 alkyl and optionally halogenated aryl.
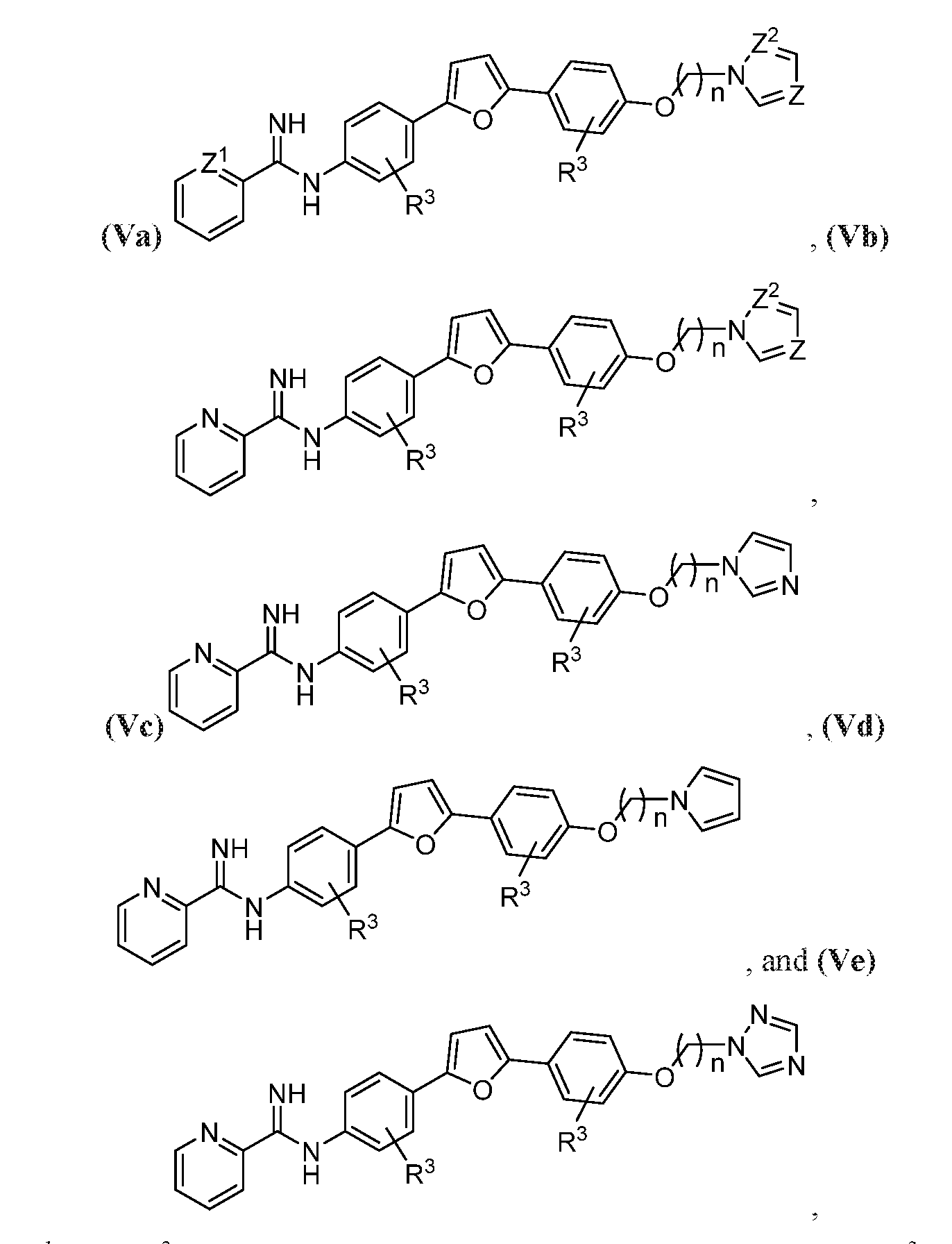
- the compound may be represented by one of Structural Formulas (Va)-(Ve), wherein each Z, Z 1 , and Z 2 are independently CH or N:
- Z, Z 1 , and Z 2 are each independently CH or N, n may be 1-14, e.g., 1-10, and R 3 may represent H, halogen, optionally halogenated C 1 -C 6 alkyl, or optionally halogenated C 1 -C 6 alkoxy.
- the compound may be represented by Structural Formula (VII):
- Z may be CH or N; each R 3 may independently be H, halogen, optionally halogenated C 1 -C 10 alkyl, or optionally halogenated C 1 -C 10 alkoxy; and n may be an integer from 1 to 10.
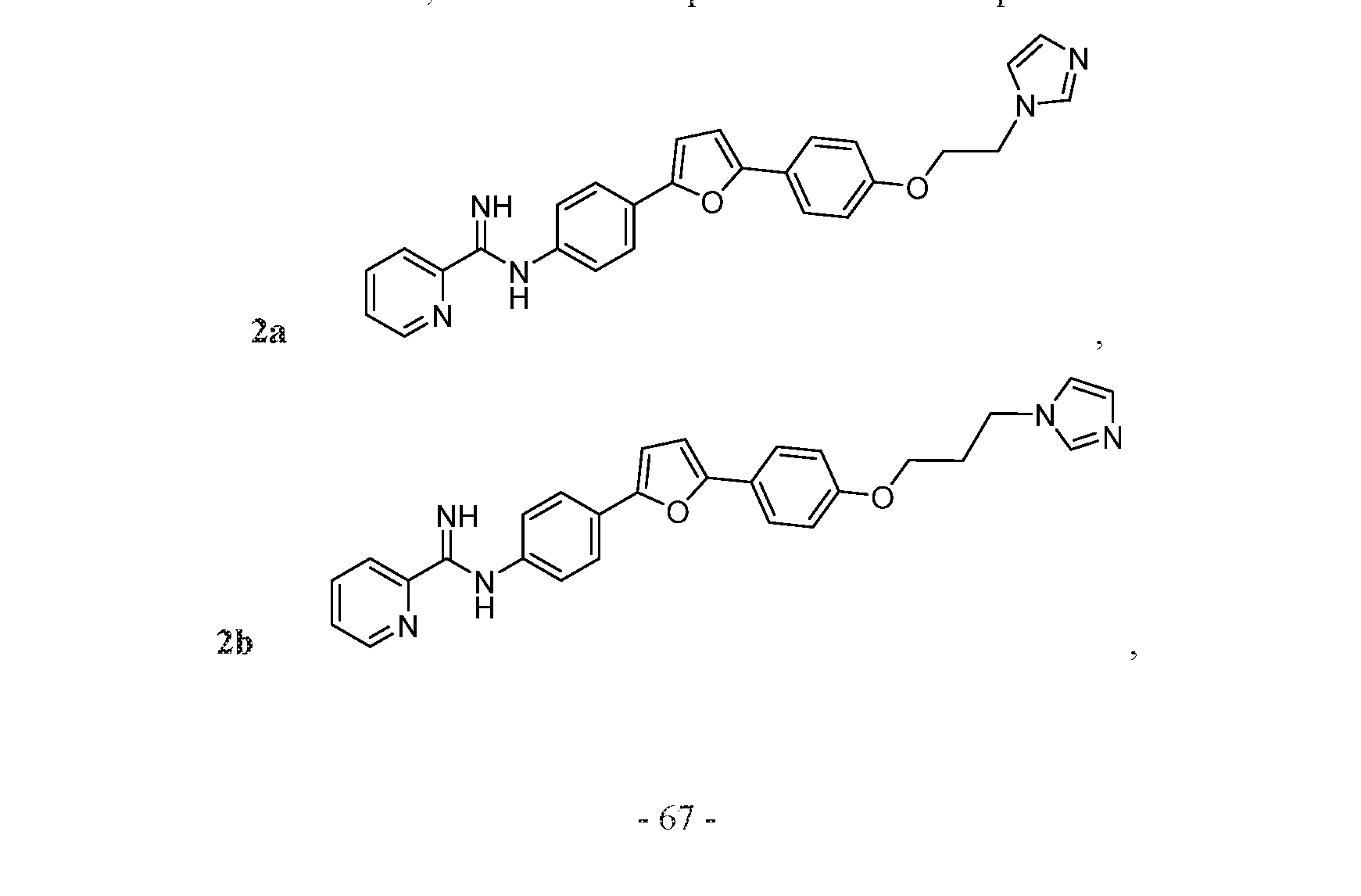
- the compound may be one of Compounds 2a-2h:
- the compound may be, for example, one of Compounds 3a-3g:
- the compound may be, for example, one of Compounds 4a-4m, e.g., 4a-4i:
- the compound may be, for example, represented by Structural Formula (Vd) above, e.g., one of compounds 13a-13c:
- the compound may be represented by Structural Formula (VIa):
- R 4 may be H, optionally halogenated C 1 -C 10 alkyl, or optionally halogenated aryl.
- the compound may be represented by Structural Formula (VIb):
- Het 2 may include an optionally substituted one of: pyrrole, diazole, thiadiazole, oxadiazole, and triazole.
- Het 2 may be optionally substituted imidazole or optionally substituted 1, 2, 4 triazole.
- the compound may be represented by Structural Formula (VIII):
- each R 5 may independently be H, halogen, C 1 -C 4 alkyl, or C 1 -C 4 alkoxy; and R 6 may be H, optionally halogenated C 1 -C 6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
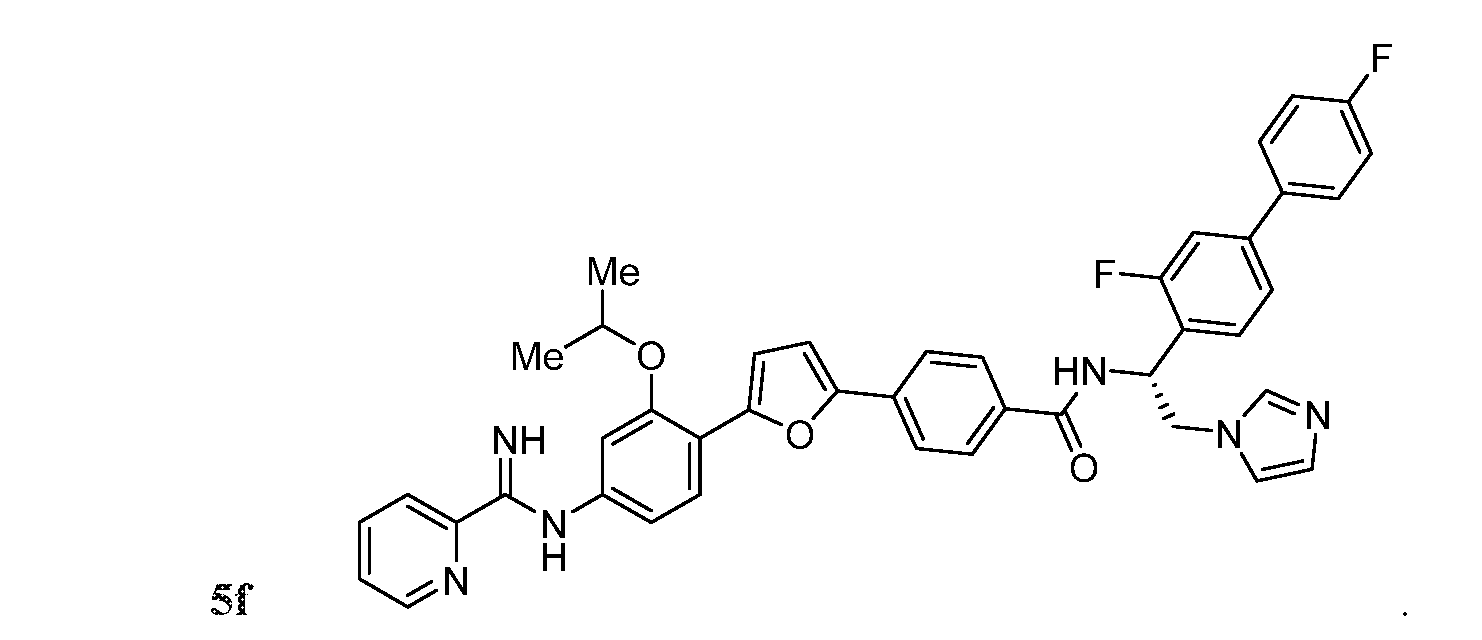
- the compound may be one of Compounds 5a-5f:
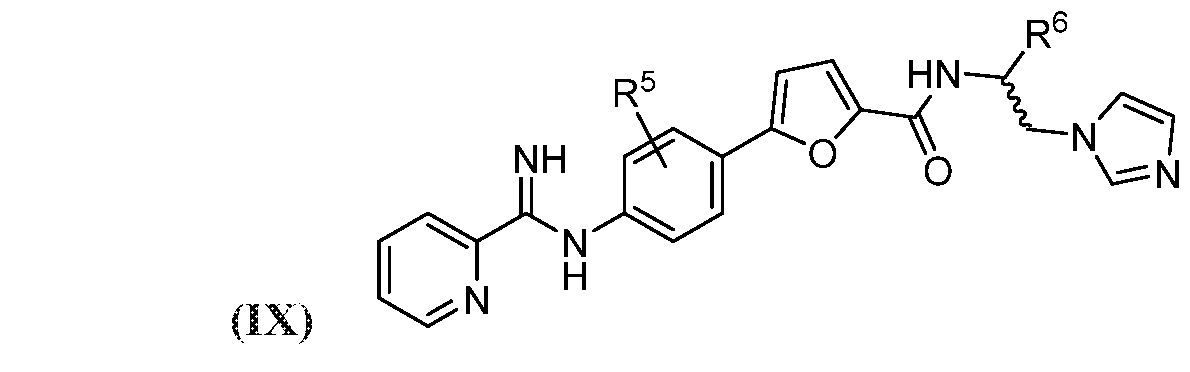
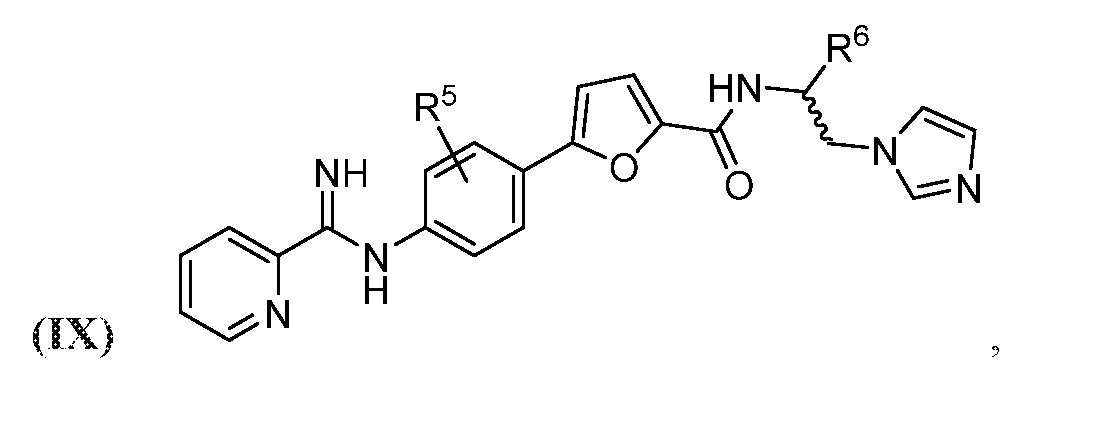
- the compound may be represented by Structural Formula (IX):
- each R 5 may independently be H, halogen, C 6
- R may be H, optionally halogenated C 1 -C 6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
- the compound may be one of Compounds 6a-6f:
- the compound may be represented by Structural Formula (X):
- each R 5 may independently be H, halogen, C 1 -C 4 alkyl, or C 1 -C 4 alkoxy; and R 6 may be H, optionally halogenated C 1 -C 6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
- the compound may be one of Compounds 7a-7f:
- the compound may be represented by Structural Formula (XI):
- each R 5 may independently be H, halogen, C 1 -C 4 alkyl, or C 1 -C 4 alkoxy; and R 6 may be H, optionally halogenated C 1 -C 6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
- the compound may be represented by Structural Formula (XII):
- R 5 may independently be H, halogen, C 1 -C 4 alkyl, or C 1 -C 4 alkoxy; and R 6 may be H, optionally halogenated C 1 -C 6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
- A may be phenyl and X may be a bond.
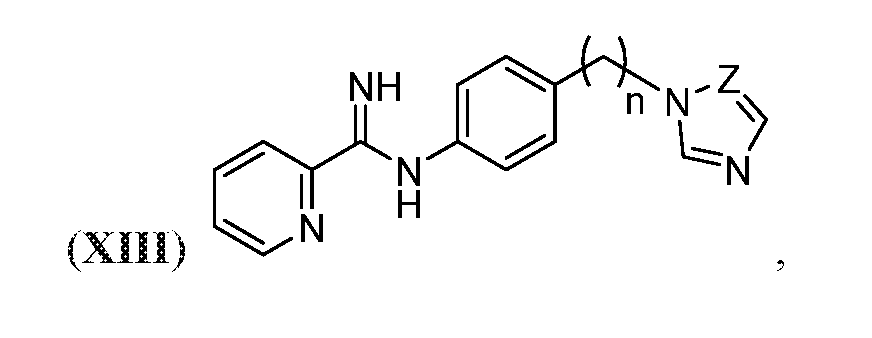
- the compound may be represented by Structural Formula (XIII):
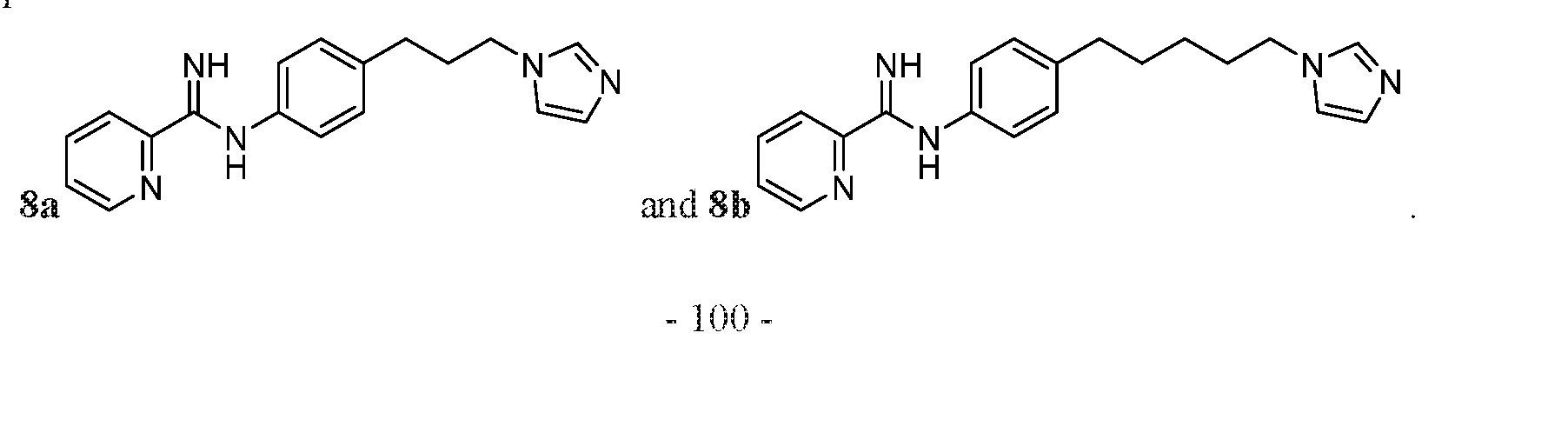
- Z may be CH or N; and n may be an integer from 1 to 10.
- the compound may be one of Compounds 8a-8b: .
- A may be a bond.
- the compound may be represented by Structural Formula (XIV):
- Z may be CH or N; and n may be an integer from 1 to 10.
- the compound may be one of Compounds 9a-9d, e.g., 9a-9c:
- A may be optionally substituted biphenyl.
- the compound may be represented by Structural Formula (XV):
- n may be 1-14, e.g., 1-10, and R 3 may represent H, halogen, optionally halogenated C 1 -C 6 alkyl, or optionally halogenated C 1 -C 6 alkoxy.
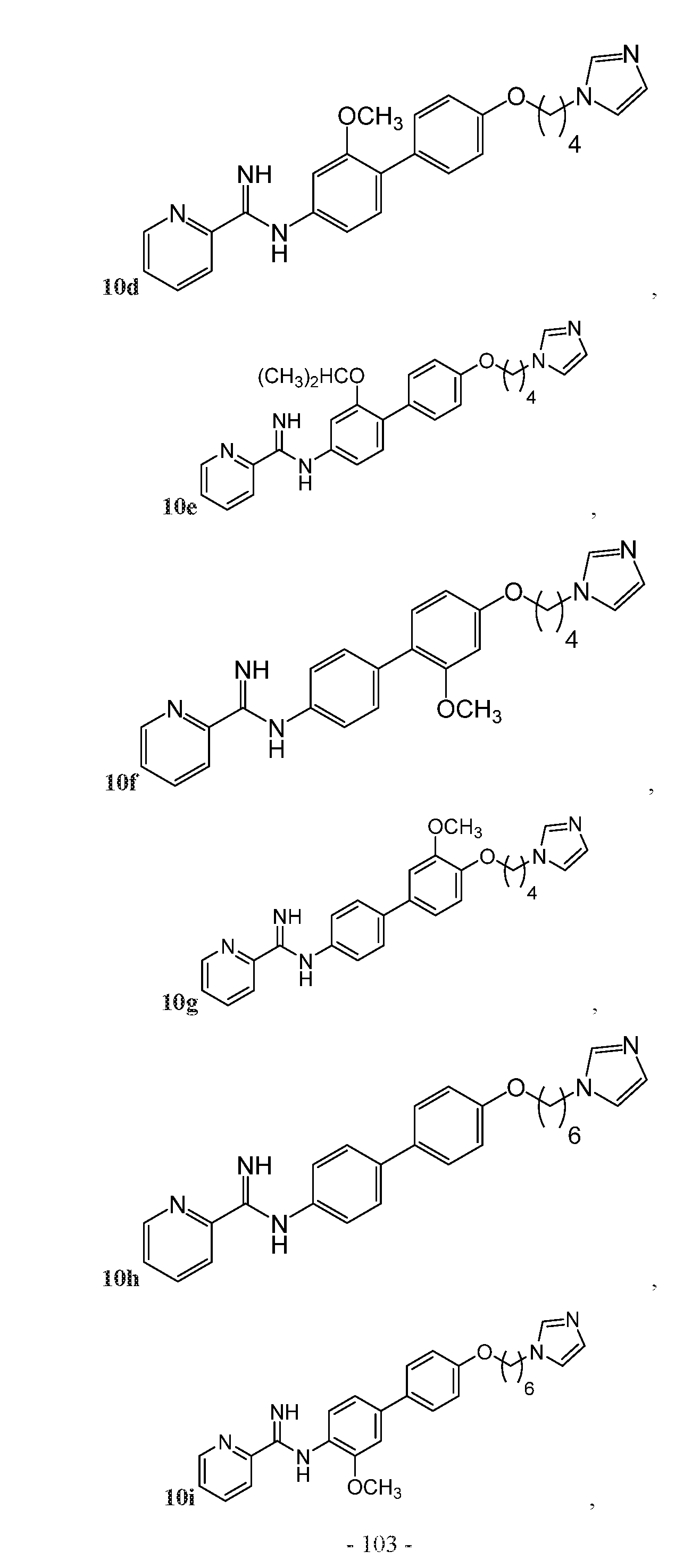
- the compound may be one of Compounds 10a-10n:
- A may be optionally substituted phenyl-piperazinyl-phenyl.
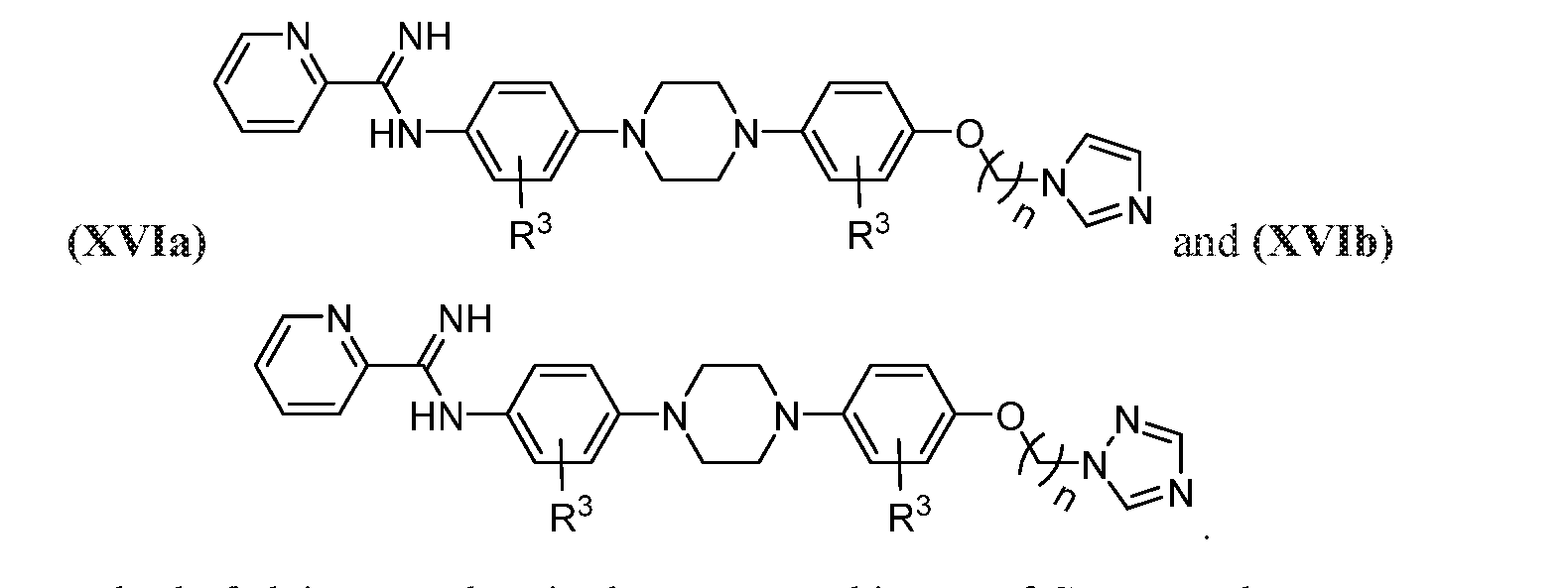
- the compound may be represented by one of Structural Formulas (XVI) and (XVII):
- the compounds may be represented by one of (XVIa)-(XVIb):
- Z, Z 1 , and Z 2 are each independently CH or N, n may be 1-14, e.g., 1-10, and R 3 may represent H, halogen, optionally halogenated C 1 -C 6 alkyl, or optionally halogenated C 1 -C 6 alkoxy.
- the compound may be one of Compounds 11a-11d:
- the compounds may be represented by one of (XVIIa)-(XVIIb):
- Z, Z 1 , and Z 2 are each independently CH or N, n may be 1-14, e.g., 1-10, and R 3 may represent H, halogen, optionally halogenated C 1 -C 6 alkyl, or optionally halogenated C 1 -C 6 alkoxy.
- the compound may be Compound 12a:
- a pharmaceutical composition may include a pharmaceutically acceptable carrier or excipient.
- the pharmaceutical composition may include a compound represented by Structural Formula (Ia):
- Ar may be an optionally substituted aryl or nitrogen-containing heteroaryl.
- R 1 and R 2 may independently be H, optionally substituted C 1 -C 6 alkyl, or optionally substituted C 3 -C 6 cycloalkyl.
- A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl.
- X may be O, S, amide, or a bond.
- Y may be optionally substituted C 1 -C 14 alkyl or optionally substituted C 2 -C 14 alkenyl, e.g., optionally substituted C 1 -C 10 alkyl or optionally substituted C 2 -C 10 alkenyl, or optionally substituted C 1 -C 8 alkyl or optionally substituted C 2 -C 8 alkenyl.
- Het 2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
- a pharmaceutical composition may include a pharmaceutically acceptable carrier or excipient.
- the pharmaceutical composition may include a compound represented by Structural Formula (I):
- Het 1 may be an optionally substituted, nitrogen- containing heteroaryl.
- R 1 and R 2 may independently be H, optionally substituted C 1 -C 6 alkyl, or optionally substituted C 3 -C 6 cycloalkyl.
- A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl.
- X may be O, S, amide, or a bond.
- Y may be optionally substituted C 1 -C 10 alkyl or optionally substituted C 2 -C 10 alkenyl.
- Het 2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
- the compound of the pharmaceutical composition may include any selection of variables or any compound described or encompassed by Structural Formula (I) or (Ia) of the method described herein.
- the compound represented by Structural Formula (Ia) or (I) may include one of Compounds 1a-f.
- each of Compounds 1a-f may be in the form of a solid.
- each of Compounds 1a-f may be in the form of a pharmaceutically acceptable salt.
- at least a portion of the pharmaceutically acceptable carrier or excipient may be in the form of a solid or gel.
- the pharmaceutical composition may be configured for administration in unit dosage form.
- the pharmaceutical composition may be configured for administration in the form of one of: a tablet; a capsule; a lozenge; a cream, a spray, a transdermal patch, an aerosol, a suppository, a depot preparation; a suture that is coated or impregnated with one of Compounds 1a-f; a bandage that is coated or impregnated with one of Compounds 1a-f; a medical device that is coated or impregnated with one of Compounds 1a-f; and the like.
- pharmaceutically acceptable salts of Compounds 1a-1f are included.
- free-base (neutral) Compounds 1a-f may be excluded.
- protonated forms of Compounds 1a-1f may be excluded.
- the pharmaceutical composition may incorporate any of the various embodiments described herein for excluding certain compounds represented by Structural Formula (Ia) or (I).
- the compound represented by Structural Formula (Ia) or (I) may exclude free-base (neutral or non-salt) forms of Compounds 1a-f.
- the compound represented by Structural Formula (Ia) or (I) may include pharmaceutically acceptable salts of Compounds 1a-f.
- the compound represented by Structural Formula (Ia) or (I) may exclude free-base forms of Compounds 1a-f and pharmaceutically acceptable salts of Compounds 1a-f.
- the compound represented by Structural Formula (Ia) or (I) may exclude protonated forms of Compounds 1a-f.
- the compound may include any aspect of the compounds represented by Structural Formulas (Ia) or (I) as described herein.
- the compounds of the pharmaceutical composition may be represented by, as described herein, any one of, or any group of, Structural Formulas: (I), (Ia), (II), (III), (IIIa), (IIIb), (IIIc), (IIId), (IIIe), (IIIf), (IV), (V), (Va), (Vb), (Vc), (Vd), (Ve), (Via), (VIb), (VII), (VIII), (IX), (X), (XI), (XII), (XIII), (XIV), (XV), (XVa), (XVb), (XVc), (XVI), (XVIa), (XVIb), (XVII), (XVIIa), and (XVIIb).
- the compound may be any one of, or any group of, as described herein, Compounds: 1a-1v; 2a-2h; 3a-3g; 4a-4m; 5a-5f; 6a-6f; 7a-7f; 8a-8b; 9a-9d; 10a-10n; 11a-11d; 12a; 13a-13c; and 14a.
- the compounds represented by Structural Formulas (Ia) or (I) may exclude compounds wherein Het 1 is one of unsubstituted pyridyl and pyrid-2-yl substituted with methyl or ethyl, for example, when: R 1 and R 2 are each H; X is O; Y is unbranched, unsubstituted C 2 -C 10 alkyl; and Het 2 is unsubstituted imidazole-1-yl or unsubstituted 1, 2, 4 triazol-2-yl.
- the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein one of R 1 and R 2 is H and the other is methyl or ethyl, for example, when: Het 1 is unsubstituted pyrid- 2-yl; X is O; Y is unbranched, unsubstituted C 2 -C 10 alkyl; and Het 2 is unsubstituted imidazole-1- yl or unsubstituted 1, 2, 4 triazol-2-yl.
- the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein X is S, for example, when: Het 1 is unsubstituted pyrid-2-yl; R 1 and R 2 are each H; Y is unbranched, unsubstituted C 2 -C 10 alkyl; and Het 2 is unsubstituted imidazole-1-yl or unsubstituted 1, 2, 4 triazol-2-yl.
- the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein Y is C 2 -C 4 alkyl substituted with methyl or ethyl, unsubstituted or methyl substituted C 1 -C 5 alkyl, or unsubstituted C 1 -C 6 alkyl, for example, when: Het 1 is unsubstituted pyrid-2-yl; R 1 and R 2 are each H; X is O; and Het 2 is unsubstituted imidazole-1-yl or unsubstituted 1, 2, 4 triazol-2-yl.
- the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein Het 2 is one of: methyl or ethyl substituted imidazole-1-yl; unsubstituted imidazolyl; methyl or ethyl substituted imidazolyl; methyl or ethyl substituted 1,-2,-4-triazol-2-yl; unsubstituted 1,-2,-4-triazolyl; methyl or ethyl substituted 1,-2,- 4-triazolyl; unsubstituted triazolyl; and methyl or ethyl substituted triazolyl; for example, when: Het 1 is unsubstituted pyrid-2-yl; R 1 and R 2 are each H; X is O; and Y is unbranched, unsubstituted C 2 -C 4 alkyl.
- Het 1 may be optionally substituted pyridyl, pyrazinyl, pyrimidinyl, or pyridizinyl.
- Het 1 may be optionally substituted pyridyl.
- the compound of the pharmaceutical composition may be represented by Structural Formula (II).
- the compound of the pharmaceutical composition may be represented by Structural Formula (III).
- Y may include at least 4 linking atoms between X and Het 2 .
- X may be O or a bond and Y may be C 1 -C 14 , e.g., C 1 -C 10 alkyl optionally substituted with one or more of: optionally halogenated C 1 -C 8 alkyl and optionally halogenated aryl.
- the compound may be one of Compounds 1a-n.
- the compound of the pharmaceutical composition may be represented by any of Structural Formulas (IIIa), (IIIb), (IIIc), (IIId), (IIIe), and (IIIf), wherein Z, Z 1 , and Z 2 may be each independently CH or N, n may be 1-14, and R 3 may be H, halogen, optionally halogenated C 1 -C 6 alkyl, or optionally halogenated C 1 -C 6 alkoxy.
- the compound of the pharmaceutical composition may be any one of Compounds 1a-1v.
- the compound of the pharmaceutical composition may be any one of Compounds 1a-1n.
- each ring in the optionally substituted linking moiety represented by A may be independently and optionally substituted by one or more of: hydroxy, halo, and C 1 -C 10 , e.g., C 1 -C 6 alkoxy.
- A may include an optionally substituted heteroaryl or optionally substituted heterocycloalkyl ring.
- A may include an optionally substituted, oxygen-containing, heteroaryl or heterocycloalkyl ring.
- A may include an optionally substituted furanyl or optionally substituted tetrahydrofuranyl ring.
- A may include optionally substituted 2,5-furanyl.
- A may include one or two optionally substituted phenyl rings.
- A may include optionally substituted 1,4-phenyl.
- A may be optionally substituted 1,4-phenyl.
- A may be optionally substituted phenyl-heteroaryl-phenyl.
- the compound may be represented by Structural Formula (IV), wherein each R 3 may independently represent H, halogen, optionally halogenated C 1 -C 6 alkyl, or optionally halogenated C 1 -C 6 alkoxy.
- the compound may be represented by Structural Formula (V).
- the compound of the pharmaceutical composition may be represented by any of Structural Formulas (V), (Va), (Vb), (Vc), (Vd), and (Ve), wherein Z, Z 1 , and Z 2 may be each independently CH or N, n may be 1-14, and R 3 may be H, halogen, optionally halogenated C 1 -C 6 alkyl, or optionally halogenated C 1 -C 6 alkoxy.
- the compound may be represented by Structural Formula (VIa) wherein R 4 may be H, optionally halogenated C 1 -C 8 alkyl, or optionally halogenated aryl.
- the compound may be represented by Structural Formula (VIb).
- Het 2 may include an optionally substituted one of: pyrrole, diazole, thiadiazole, oxadiazole, and triazole.
- Het 2 may be optionally substituted imidazole or optionally substituted 1, 2, 4 triazole.
- the compound may be represented by Structural Formula (VII), wherein: Z may be CH or N; each R 3 may independently be H, halogen, optionally halogenated C 1 -C 6 alkyl, or optionally halogenated C 1 -C 6 alkoxy; and n may be an integer from 1 to 10.
- the compound may be one of Compounds 2a-2g.
- the compound may be, for example, one of Compounds 3a-3g.
- the compound may be, for example, one of Compounds 4a-4m, e.g., 4a-4i.
- the compound may be, for example, one of Compounds 13a-13c.
- the compound may be represented by Structural Formula (VIII), wherein each R 5 may independently be H, halogen, C 1 -C 4 alkyl, or C 1 -C 4 alkoxy, and R 6 may be H, optionally halogenated C 1 -C 6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
- R 5 may independently be H, halogen, C 1 -C 4 alkyl, or C 1 -C 4 alkoxy
- R 6 may be H, optionally halogenated C 1 -C 6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
- the compound may be one of Compounds 5a-5f.
- the compound may be represented by Structural Formula (IX), wherein: each R 5 may independently be H, halogen, C 1 -C 4 alkyl, or C 1 -C 4 alkoxy; and R 6 may be H, optionally halogenated C 1 -C 6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
- R 5 may independently be H, halogen, C 1 -C 4 alkyl, or C 1 -C 4 alkoxy
- R 6 may be H, optionally halogenated C 1 -C 6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
- the compound may be one of Compounds 6a-5f.
- the compound may be represented by Structural Formula (X) wherein: each R 5 may independently be H, halogen, C 1 -C 4 alkyl, or C 1 -C 4 alkoxy; and R 6 may be H, optionally halogenated C 1 -C 6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
- R 5 may independently be H, halogen, C 1 -C 4 alkyl, or C 1 -C 4 alkoxy
- R 6 may be H, optionally halogenated C 1 -C 6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
- the compound may be one of Compounds 7a-7f.
- the compound may be represented by Structural Formula (XI), wherein each R 5 may independently be H, halogen, C 1 -C 4 alkyl, or C 1 -C 4 alkoxy; and R 6 may be H, optionally halogenated C 1 -C 6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
- R 5 may independently be H, halogen, C 1 -C 4 alkyl, or C 1 -C 4 alkoxy
- R 6 may be H, optionally halogenated C 1 -C 6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
- the compound may be represented by Structural Formula (XII), wherein each R 5 may independently be H, halogen, C 1 -C 4 alkyl, or C 1 -C 4 alkoxy; and R 6 may be H, optionally halogenated C 1 -C 6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
- R 5 may independently be H, halogen, C 1 -C 4 alkyl, or C 1 -C 4 alkoxy
- R 6 may be H, optionally halogenated C 1 -C 6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
- the compound may be represented by Structural Formula (Ia) or (I), wherein A may be phenyl and X may be a bond.
- the compound may be represented by Structural Formula (XIII), wherein Z may be CH or N; and n may be an integer from 1 to 10.
- the compound may be one of Compounds 8a-8b.
- the compound may be represented by Structural Formula (Ia) or (I), wherein A may be a bond.
- the compound may be represented by Structural Formula (XIV), wherein Z may be CH or N; and n may be an integer from 1 to 10.
- the compound may be one of Compounds 9a-9d, for example, 9a-9c.
- the compound may be represented by Structural Formula (XV), wherein Z, Z 1 , and Z 2 may be each independently CH or N, n may be 1-14, and R 3 may be H, halogen, optionally halogenated C 1 -C 6 alkyl, or optionally halogenated C 1 -C 6 alkoxy.
- the compound may be represented by one of Structural Formulas (XVa)-(XVc).
- the compound may be one of Compounds 10a- 10n.
- the compound may be represented by one of Structural Formulas (XVI) and (XVII), wherein Z, Z 1 , and Z 2 may be each independently CH or N, n may be 1-14, and R 3 may be H, halogen, optionally halogenated C 1 -C 6 alkyl, or optionally halogenated C 1 -C 6 alkoxy.
- the compound may be represented by one of Structural Formulas (XVIa)-(XVIb).
- the compound may be one of Compounds 11a-11d.
- the compound may be represented by one of Structural Formulas (XVIIa)-(XVIIb), e.g., the compound may be Compound 12a.
- a kit for anti-fungal treatment of a subject in need thereof is provided.
- the kit may include any anti-fungal compound described herein, for example, the compound represented by Structural Formula (Ia):
- Ar may be an optionally substituted aryl or nitrogen-containing heteroaryl.
- R 1 and R 2 may independently be H, optionally substituted C 1 -C 6 alkyl, or optionally substituted C 3 -C 6 cycloalkyl.
- A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl.
- X may be O, S, amide, or a bond.
- Y may be optionally substituted C 1 -C 14 alkyl or optionally substituted C 2 -C 14 alkenyl, e.g., optionally substituted C 1 -C 10 alkyl or optionally substituted C 2 -C 10 alkenyl, or optionally substituted C 1 -C 8 alkyl or optionally substituted C 2 -C 8 alkenyl.
- Het 2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
- the kit may include instructions. The instructions may direct a user to provide the subject, the subject being infected by a fungus or at risk of infection by the fungus. The instructions may direct a user to administer the compound or the pharmaceutical composition to the subject in an amount effective to mitigate infection by the fungus in the subject.
- kits for anti-fungal treatment of a subject in need thereof may include any anti-fungal compound described herein, for example, the compound represented by Structural Formula (Ia):
- Ar may be an optionally substituted aryl or nitrogen-containing heteroaryl.
- R 1 and R 2 may independently be H, optionally substituted C 1 -C 6 alkyl, or optionally substituted C 3 -C 6 cycloalkyl.
- A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl.
- X may be O, S, amide, or a bond.
- Y may be optionally substituted C 1 -C 14 alkyl or optionally substituted C 2 -C 14 alkenyl, e.g., optionally substituted C 1 -C 10 alkyl or optionally substituted C 2 -C 10 alkenyl, or optionally substituted C 1 -C 8 alkyl or optionally substituted C 2 -C 8 alkenyl.
- Het 2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
- the kit may include instructions. The instructions may direct a user to provide the subject, the subject being infected by a fungus or at risk of infection by the fungus. The instructions may direct a user to administer the compound or the pharmaceutical composition to the subject in an amount effective to mitigate infection by the fungus in the subject.
- kits for anti-fungal treatment of a subject in need thereof may include any anti-fungal compound described herein, for example, the compound represented by Structural Formula (I):
- Het 1 may be an optionally substituted, nitrogen-containing heteroaryl.
- R 1 and R 2 may independently be H, optionally substituted C 1 -C 6 alkyl, or optionally substituted C 3 -C 6 cycloalkyl.
- A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl.
- X may be O, S, amide, or a bond.
- the kit may include instructions.
- the instructions may direct a user to provide the subject, the subject being infected by a fungus or at risk of infection by the fungus.
- the instructions may direct a user to administer the compound or the pharmaceutical composition to the subject in an amount effective to mitigate infection by the fungus in the subject.
- the compound represented by Structural Formula (I) or (Ia) may include any aspect of the anti-fungal compounds described herein, either alone or as encompassed by any of the methods or anti-fungal pharmaceutical compositions described herein.
- the instructions may direct a user to conduct any step or combination of steps described herein for the method.
- FIG. 1A is a synthetic scheme various phenoxyalkyl linker anti-fungal Compounds 1a-1n and 1v.
- Compounds 1a, 1b, 1c, 1e, 1f, 1g, 1i, 1m, and 1v were made according to FIG.1A.
- reagents and conditions for synthesis of compounds according to FIG. 1A were made according to FIG.1A.
- 1A included: a) ⁇ , ⁇ -dibromoalkane, K 2 CO 3 , acetone; b) imidazole or 1,2,4-triazole, K 2 CO 3 , CH 3 CN; c) SnCl 2 .2H 2 O, EtOAc; d) S-(2-naphthylmethyl)-2-pyridylthioimidate hydrobromide, CH 3 CN/EtOH (1:3), rt.
- FIG. 1B is a synthetic scheme for various phenoxyalkyl hybrid target compounds substituted meta to the arylimidamide group, e.g., Compounds 1o-1q.
- reagents and conditions for synthesis of compounds according to FIG.2B included: a) MOMCl, K 2 CO 3 , acetone, 30oC; b) RI, K 2 CO 3 , sealed tube, 80oC; c) HCl, MeOH/CH 2 Cl 2 , rt; d) 1,8- Dibromooctane, K 2 CO 3 , CH 3 CN, reflux; e) imidazole, K 2 CO 3 , CH 3 CN, reflux; f) SnCl .
- FIG. 1C is a synthetic scheme for various phenoxyalkyl hybrid target compounds substituted ortho to the arylimidamide group, e.g., Compounds 1r-1t.
- reagents and conditions for synthesis of compounds according to FIG. 2C included: a) RI, K 2 CO 3 , sealed tube, 80oC; b) NaOH, DMSO, reflux; c) 1,8-Dibromooctane, K 2 CO 3 , CH 3 CN, reflux; d) imidazole, K 2 CO 3 , CH 3 CN, reflux; e) SnCl .
- FIG. 1D is a synthetic scheme for various phenoxyalkyl hybrid target compounds substituted with pyrrole, e.g., Compound 1u.
- reagents and conditions for synthesis of Compound 1u according to FIG. 2D included: a) 1,8-dibromooctane, K 2 CO 3 , acetone; b) pyrrole, K 2 CO 3 , CH 3 CN; c) SnCl 2 .2H 2 O, EtOAc; d) S-(2-naphthylmethyl)-2- pyridylthioimidate hydrobromide, CH 3 CN/EtOH (1:3), rt.
- FIG. 2A is a synthetic scheme for various phenyl-unsubstituted diphenylfuran alkyloxy linker hybrid Compounds 2a-2g and 3a-g.
- Compounds 2a-e, 2g, 3a-e, and 3g were made according to FIG.2A.
- Compound 2h was made according to FIG.2A, but starting with 1- bromo-2-oxy(prop-2-yl)-4-nitrobenzene.
- 2A included: a) Pd(PPh 3 ) 4 , dioxane, 90°C; b) NBS, DMF, rt; c) K 2 CO 3 , acetone, reflux; d) Pd(PPh 3 ) 4 , K 2 CO 3 , MeOH, toluene, 80°C; e) azole, NaH, DMF, rt; f) H 2 , Pd(C), EtOH-EtOAc; g) (i) S-(2-naphthylmethyl-2-pyridylthioimidate hydrobromide, EtOH; (ii) NaOH; (iii) ethanolic HCl.
- Tetrakistriphenylphosphine palladium (0.5 mmol) was added to a stirred mixture of the 2-(tributylstannyl) furan (11 mmol) and 1-bromo-4-nitrobenzene (10 mmol) in deaerated dioxane (25 mL) under a nitrogen atmosphere. The vigorously stirred mixture was heated at 90- 100 °C for 24 h. The solvent was evaporated under reduced pressure, the resulting solid was partitioned between ethyl acetate (200 mL) and 5 mL of concentrated ammonia to remove the palladium complex, washed with water, passed through celite to remove the catalyst, dried over sodium sulfate and evaporated.
- N-bromosuccinimide (2.13 gm, 12 mmol) was added portion-wise to a stirred solution of the previous nitro compound (10 mmol) in dimethylformamide (20 mL). The reaction mixture was stirred at room temperature for 12 h then poured onto cold water, the precipitate was collected and dried. Purification was conducted by column chromatography on silica gel, using hexanes/ethyl acetate (95/5, v/v). Yellow solid, yield 96 %; m.p.
- 1,4-dibromobutane (36 mmol) was added to a solution of the p- hydroxyphenylboronic acid ester (1.32 gm, 6 mmol), dry K 2 CO 3 (1.65 gm, 12 mmol) and Cs 2 CO 3 (0.39 gm, 1.2 mmol) in anhydrous dimethylacetamide (15 mL) under a nitrogen atmosphere. Stirring was continued for 12 h and ice water was added and the reaction mixture was filtered and air dried. Purification was performed by column chromatography on silica gel, using hexanes/ethyl acetate (93/7, v/v). White solid (73%) m.p.
- the solid was dissolved in ethanol (2 mL); the solution was cooled to 0 o C in an ice bath and 10% NaOH was added until pH reached approximately 10.
- the free base was extracted with ethyl acetate (3 ⁇ 50 mL). The organic layer was washed with distilled water, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The resulting suspension was crystallized by adding dry hexanes and then filtered.
- the free base was suspended in dry ethanol (20 mL) and cooled to 0 o C in an ice bath. Freshly prepared ethanolic HCl solution (2 mL) was added to the suspension and the mixture was stirred at room temperature for overnight. The resulting red solution was concentrated under reduced pressure.
- FIG. 3B is a synthetic scheme for various phenyl substituted diphenylfuran alkyloxy linker hybrid compounds, each of which may be prepared, for example, from a suitably substituted aryl boronate according to FIG. 3A.
- compounds may be prepared from the corresponding phenol and bromoalkyl imidazole, as shown in FIG. 3B.
- the phenol may be prepared from a phenol protected aryl boronate according to FIG. 3A, followed by subsequent deprotection.
- Compound 4j was made according to FIG. 3B.
- 3B included: a) Pd(PPh 3 ) 4 , dioxane, 90°C; b) NBS, DMF, rt; c) K 2 CO 3 , Cs 2 CO 3 , DMA, rt; d) Pd(PPh 3 ) 4 , K 2 CO 3 , MeOH, toluene, 80°C; e) imidazole, NaH, DMF, rt; f) H 2 , Pd(C), EtOH-EtOAc; g) (i) S-(2-naphthylmethyl)-2- pyridylthioimidate hydrobromide, EtOH; (ii) NaOH.
- FIG. 2C is a synthetic scheme for various phenyl substituted diphenylfuran alkyloxy linker hybrid compounds.
- Compounds 2h, 4k, 13b, and 13c were made according to FIG.2C.
- 2C included: aa) Pd(PPh 3 ) 4 , dioxane, 90°C; b) NBS, DMF, rt; c) K 2 CO 3 , Cs 2 CO 3 , DMA, rt; d) Pd(PPh 3 ) 4 , K 2 CO 3 , MeOH, toluene, 80°C; e) imidazole, NaH, DMF, rt; f) H 2 , Pd(C), EtOH-EtOAc; g) (i) S-(2-naphthylmethyl-2-pyridylthioimidate hydrobromide, EtOH; (ii) NaOH.
- Example 2D Prophetic
- FIG. 2D is a prophetic synthetic scheme for various diphenylfuran alkyloxy linker hybrid Compounds 4a-i, each of which may be prepared, for example, from a suitably substituted aryl boronate according to FIG. 2A.
- Compounds 4a-i may be prepared from the corresponding phenol and bromoalkyl imidazole, as shown in FIG. 2D.
- the phenol may be prepared from a phenol protected aryl boronate according to FIG. 2A, followed by subsequent deprotection.
- FIG.3 is a synthetic scheme for various alkylamide linker hybrid Compounds 5a-f, 6a-f, and 7a-f.
- precursor carboxylic acids were prepared similarly to FIG. 2A by a cross-coupling of a benzylalcohol boronate ester with 2-(4-nitrophenyl)furan, nitro reduction, and imidamide formation.
- Oxidation of the benzyl alcohol provided the carboxylic acid compound shown in FIG.3, and subsequent peptide coupling with the illustrated imidazoyl amine is proposed to afford Compounds 5a and 5c.
- Compound 6a may be prepared from 2-(4- nitrophenyl)furan, followed by nitro reduction and imidamide formation. Bromination of the resulting furanyl compound followed by a metal-mediated carboxylation may provide the carboxylic acid shown in FIG. 3. Subsequent peptide coupling with the illustrated imidazoyl amine may afford Compound 6a.
- Compound 7a may be prepared from a suitable 4-aminobenzoic acid derivative. Imidamide formation may provide the carboxylic acid compound shown in FIG. 3, and subsequent peptide coupling with the illustrated imidazoyl amine may provide Compound 6a.
- FIG.4A is a prophetic synthetic scheme for various phenylalkyl linker hybrid Compounds 8a and 8b.
- Compound 8a and 8b may be prepared from phenyllithium and the corresponding dibromoalkane, as shown in FIG. 4A. Nitration of the resulting alkylaryl bromide followed by displacement of the bromide with imidazole can provide the nitrophenyl alkylimidazole. Subsequent reduction of the nitro group to the amine, followed by amidine synthesis with naphthalene-2-ylmethylpyridine-2-carbimidothioate may provide Compounds 8a and 8b.
- FIG. 4B is a synthetic scheme for various phenylalkyl linker hybrid Compounds 9a-9d.
- the synthesis of Compound 9d is representative, as follows.
- Benzyl (8-aminooctyl)car m [00119] Benzyl chloroformate (1 g, 5.86 mmol) was added to 10 equivalents of 1,8- diaminooctane (8.45 g, 58.6 mmol) dissolved in 60 mL dry DCM/EtOH (1:1) in an ice bath. The mixture was allowed to stir for 3 hours at 0 oC and left to stir overnight at room temperature. The mixture was then filtered and partitioned between DCM (100 mL) and water (50 mL) and brine (50 mL) then dried over sodium sulfate.
- Aqueous glyoxal (40%, 0.88 mL, 6.19 mmol), ammonium acetate (0.48 g, 6.19 mmol), and aqueous formaldehyde solution (37% w/v, 0.51 mL, 6.19 mmol) were added to benzyl (8-aminooctyl)carbamate (0.87 g, 3.12 mmol) in methanol (8 mL).
- the reaction was heated to reflux overnight then the solution was evaporated under reduced pressure.
- the pH was rendered alkaline by the addition of 2N NaOH and the mixture was extracted with DCM (100 mL). The organic phase was dried over sodium sulfate and the liquid was evaporated under reduced pressure.
- the product was obtained after column chromatography purification over neutralized silica gel with DCM/MeOH (100:1) as a brown oil of the protected aminoalkyl imidazole, 0.48 g.
- a dry 25 mL two-necked flask was charged with Pd/C (10%, 0.15 g) and the protected amine (0.48 g, 1.45 mmol) in 20 mL absolute ethanol under nitrogen. The mixture was stirred under 1 atmosphere of hydrogen for 24 hours.
- the solid was dissolved in ethanol (2 mL); the solution was cooled to 0 o C in an ice bath and 10% NaOH was added until the pH reached approximately 10.
- the free base was extracted with ethyl acetate (3 ⁇ 25 mL).
- the organic layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure.
- the resulting suspension was purified by column chromatography over silica gel using DCM/MeOH/TEA (9.5:1.2:0.6) as eluent then further purified by 5 mL hexanes/diethyl ether (1:1) to yield a buff powder, 0.085 g, 55%.
- FIG. 5A is a synthetic scheme for various unsubstituted and substituted biphenyl linker anti-fungal compounds, which was used to produce Compounds 10a, 10d, 10e, 10h, 10k, and 10l. Biphenyl linkers in these compounds were either unsubstituted or were substituted meta to the amidine group. Generally, reagents and conditions for synthesis of compounds according to FIG.
- 5A included: dibromoalkane, K 2 CO 3 , CH 3 CN, reflux; b) imidazole, K 2 CO 3 , CH 3 CN, reflux; c) 2-alkoxy-4-nitroiodobenzene, Pd(dppf)Cl 2 , K 2 CO 3 , DMSO, 100°C; d) SnCl 2 .2H 2 O, EtOAc, reflux; e) S-(2-naphthylmethyl)-2-pyridylthioimidate hydrobromide, CH 3 CN/EtOH (1:3), rt.
- the resulting suspension was purified by column chromatography over silica gel (neutralized by washing with trimethylamine) using DCM:MeOH (200:1 to 50:1) as eluent then further purified by crystallization from hexanes/ethyl acetate to yield yellow crystals, 0.135 g (68%).
- FIG. 5B is a synthetic scheme for various substituted biphenyl linker anti- fungal compounds, which was used to produce Compounds 10b, 10c, 10i, and 10j. Biphenyl linkers in these compounds were substituted ortho to the amidine group. Generally, reagents and conditions for synthesis of compounds according to FIG.
- 5B included: a) dibromoalkane, K 2 CO 3 , CH 3 CN, reflux; b) imidazole, K 2 CO 3 , CH 3 CN, reflux; c) 23a,b, Pd[P(Ph) 3 ] 4 , K 2 CO 3 , DMF, 100°C; d) SnCl 2 .2H 2 O, EtOAc, reflux; e) S-(2-naphthylmethyl)-2-pyridylthioimidate hydrobromide, CH 3 CN/EtOH (1:3), rt.
- FIG. 5C is a synthetic scheme for various substituted biphenyl linker anti- fungal compounds, which was used to produce Compounds 10f and 10m. Biphenyl linkers in these compounds were substituted meta to the alkoxy linking group.
- reagents and conditions for synthesis of compounds according to FIG.5C included: a) TsCl, K 2 CO 3 , acetone, reflux; b) MeI, K 2 CO 3 , sealed tube, CH 3 CN, reflux; c) bis(pinocolato)diboron, Pd(dppf)Cl 2 , AcOK; d) 4-nitro-iodobenzene, Pd(dppf)Cl 2 , dimethoxyethane/DMF/water (7:3:1), reflux; e) aq NaOH, EtOH/DMSO; f) 1,4-dibromobutane or 1,6-dibromohexane, K 2 CO 3 , CH 3 CN, reflux; g) imidazole, K 2 CO 3 , CH 3 CN, reflux; h) SnCl 2 .2H 2 O, EtOAc, reflux; i) S-(2-naphthylmethyl)-2
- FIG. 5D is a synthetic scheme for various substituted biphenyl linker anti- fungal compounds, which was used to produce Compounds 10g and 10n. Biphenyl linkers in these compounds were substituted ortho to the alkoxy linking group. Generally, reagents and conditions for synthesis of compounds according to FIG.
- 5D included: a) TsCl, acetonitrile, K 2 CO 3 , reflux ; b) Bis(pinacolato)diboron, Pd(dppf)Cl 2 , AcOK, dioxane, 100°C; c) 1-Iodo-4- nitrobenzene, Pd[P(Ph) 3 ] 4 or Pd(dppf)Cl 2 , K 2 CO 3 , DME:DMF:H 2 O (7:3:1),100°C; d) aq NaOH, EtOH/DMSO; e) dibromoalkane, acetonitrile, K 2 CO 3 , reflux; f) Imidazole, K 2 CO 3 .
- FIG. 6A is a synthetic scheme for various phenyl-piperazinyl-phenyl linker anti-fungal compounds, which was used to produce Compounds 11a-11d. Generally, reagents and conditions for synthesis of compounds according to FIG.
- 6A included: a) NMP, DIPEA, 4- chloronitrobenzene; b) ⁇ , ⁇ -dihaloalkane, K 2 CO 3 , acetone or Cs 2 CO 3 , DMF; c) imidazole or 1,2,4-triazole, Cs 2 CO 3 , DMF; d) Pd/C, H 2 , EtOAc/MeOH; e) S-(2-naphthylmethyl-2- pyridylthioimidate hydrobromide, CH 3 CN/EtOH.
- FIG. 6B is a synthetic scheme for various phenyl-piperazinyl linker anti- fungal compounds, e.g., corresponding to Structural Formula (XVII). Generally, reagents and conditions for synthesis of compounds according to FIG.
- 6B included: a) K 2 CO 3 /DMSO; b) ⁇ , ⁇ -dihaloalkane, e.g., 1,6-dibromohexane, Cs 2 CO 3 , DMF; c) imidazole, Cs 2 CO 3 , DMF; d) Pd/C, H 2 , MeOH; e) S-(2-naphthylmethyl-2-pyridylthioimidate hydrobromide, CH 3 CN/EtOH.
- the synthesis of Compound 12a, N-(4-(4-(6-(1H-imidazol-1-yl)hexyl)piperazin-1- yl)phenyl)picolinimidamide is representative, as follows.
- reaction mixture was cooled, diluted with ice water (40 mL), neutralized with 2N hydrochloric acid and then extracted with ethyl acetate (30 mL x 3). The combined organic layers were washed with water and brine, dried over Na 2 SO 4 , and concentrated under reduced pressure to yield 1-(4-nitrophenyl)piperazine as a yellow solid.
- Step 5 Synthesis of N-(4-(4-(6-(1H-imidazol-1-yl)hexyl)piperazin-1-yl)phenyl) picolinimidamide
- a two-fold concentration dilution series of each compound was added to 96-well microtiter plates in RPMI medium buffered to pH 7.0 with 20 mM HEPES.
- fungal cells were added as follows: Aspergillus fumigatus conidia (final concentration 5000 per mL), Candida albicans yeasts (final concentration 2500 per mL), Cryptococcus neoformans (final concentration 10,000 per mL), Histoplasma capsulatum (final concentration 2,000,000 per mL).
- FIG.7 is a table demonstrating IC 50 values in ⁇ M in Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, and Histoplasma capsulatum for Compounds 1c, 1e, 1f, 1i, and 1m.. All tested compounds displayed excellent activity, in most cases demonstrating IC 50 values at or less than 10 ⁇ M.
- FIGs.8A, 8B, 8C, and 8D are graphs showing concentration-dependent inhibition of fungal growth by the indicated compounds in ⁇ M for Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, and Histoplasma capsulatum, respectively.
- the graphs in FIGs. 8A, 8B, 8C, and 8D were used to determine the IC 50 values in ⁇ M shown in the table in FIG.7.
- the disclosed anti-fungal compounds may also be counter-screened for toxicity to J774 macrophages and HepG2 hepatocellular carcinoma cells.
- J774 cells (10 3 in 100 ⁇ L) or HepG2 cells (5 ⁇ 10 3 in 100 ⁇ L) may be incubated for 72 h with serial dilutions of compounds in DMEM (for J774 cells) or RPMI medium (for HepG2 cells) supplemented with 10% fetal bovine serum. MTT may then be added and absorbance at 570 nm may provide an assessment of cell proliferation.
- Determination of IC 50 values in each assay may permit the calculation of a selectivity index (SI, e.g., target fungus IC 50 versus mammalian cell line/IC 50 versus, e.g., target fungi in genera such as Aspergillus, Candida, Cryptococcus, Histoplasma, and the like) for each target compound.
- SI selectivity index
- an“alkyl” group includes straight chain and branched chain alkyl groups having a number of carbon atoms, for example, from 1 to 12, 1 to 10, 1 to 8, 1 to 6, or 1 to 4.
- straight chain alkyl groups include groups such as methyl, ethyl, n- propyl, n-butyl, n-pentyl, n-hexyl, n-heptyl, and n-octyl groups.
- branched alkyl groups include, e.g., isopropyl, iso-butyl, sec-butyl, tert-butyl, neopentyl, isopentyl, and 2,2- dimethylpropyl groups.
- Representative substituted alkyl groups may be substituted one or more times with substituents such as those listed above and include, without limitation, haloalkyl (e.g., trifluoromethyl), hydroxyalkyl, thioalkyl, aminoalkyl, alkylaminoalkyl, dialkylaminoalkyl, alkoxyalkyl, or carboxyalkyl.
- an“alkoxy” group means a hydroxyl group (-OH) in which the bond to the hydrogen atom is replaced by a bond to a carbon atom of a substituted or unsubstituted alkyl group.
- linear alkoxy groups include, e.g., methoxy, ethoxy, propoxy, butoxy, pentoxy, or hexoxy.
- branched alkoxy groups include, e.g., isopropoxy, sec-butoxy, tert-butoxy, isopentoxy, or isohexoxy.
- cycloalkoxy groups include, e.g., cyclopropyloxy, cyclobutyloxy, cyclopentyloxy, or cyclohexyloxy.
- Representative substituted alkoxy groups may be substituted one or more times.
- a“cycloalkyl” group includes mono-, bi- or tricyclic alkyl groups having from 3 to 12 carbon atoms in each ring, for example, 3 to 10, 3 to 8, or 3 to 4, 5, or 6 carbon atoms.
- Exemplary monocyclic cycloalkyl groups include, for example, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, and the like.
- a cycloalkyl group may have a number of ring carbons of from 3 to 8, 3 to 7, 3 to 6, or 3 to 5.
- Bi- and tricyclic ring systems may include both bridged cycloalkyl groups and fused rings, e.g., bicyclo[2.1.1]hexane, adamantyl, decalinyl, and the like.
- Substituted cycloalkyl groups may be substituted one or more times with non-hydrogen and non-carbon groups as defined above.
- Substituted cycloalkyl groups may include rings that may be substituted with straight or branched chain alkyl groups.
- Representative substituted cycloalkyl groups may be mono-substituted or substituted more than once, for example, 2,2-, 2,3-, 2,4- 2,5- or 2,6-disubstituted cyclohexyl groups.
- a“heterocycloalkyl” ring means an aromatic carbocyclic ring having one or more ring carbon atoms replaced by a heteroatom (e.g., N, S, or O).
- Non-aromatic heterocyclic rings may have 4, 5, 6, 7, or 8 ring atoms. Examples include oxazolinyl, thiazolinyl, oxazolidinyl, thiazolidinyl, tetrahydrofuranyl, tetrahyrothiophenyl, morpholino, thiomorpholino, pyrrolidinyl, piperazinyl, piperidinyl, thiazolidinyl, and the like.
- an“aryl” group means a carbocyclic aromatic hydrocarbon.
- Aryl groups herein include monocyclic, bicyclic and tricyclic ring systems.
- Aryl groups include, e.g., phenyl, azulenyl, heptalenyl, biphenyl, fluorenyl, phenanthrenyl, anthracenyl, indenyl, indanyl, pentalenyl, naphthyl, and the like, for example, phenyl, biphenyl, and naphthyl.
- Aryl groups may contain, for example, 6 to 14, 6 to 12, or 6 to 10 ring carbons.
- the aryl groups may be phenyl or naphthyl.
- the phrase“aryl groups” may include groups containing fused rings, such as fused aromatic-aliphatic ring systems (e.g., indanyl or tetrahydronaphthyl), an“aryl” group, unless stated to be substituted or optionally substituted, does not include aryl groups that have other groups, such as alkyl or halo groups, bonded to one of the ring members. Rather, groups such as tolyl may be referred to as substituted aryl groups.
- Representative substituted aryl groups may be mono-substituted or substituted more than once.
- monosubstituted aryl groups include, but are not limited to, 2-, 3-, 4-, 5-, or 6-substituted phenyl or naphthyl, which may be substituted with substituents such as those above.
- an“aralkyl” group means an alkyl group in which a hydrogen or carbon bond of an alkyl group is replaced with a bond to an aryl group.
- aralkyl groups contain 7 to 16 carbon atoms, 7 to 14 carbon atoms, or 7 to 10 carbon atoms.
- Substituted aralkyl groups may be substituted at the alkyl, the aryl or both the alkyl and aryl portions of the group.
- Representative aralkyl groups include, e.g., benzyl and phenethyl groups and fused (cycloalkylaryl)alkyl groups such as 4-indanylethyl.
- a“heteroaryl” group means a carbocyclic aromatic ring having one or more ring carbon atoms replaced by a heteroatom (e.g., N, S, or O).
- Heteroaryl groups may include, for example, imidazolyl, isoimidazolyl, thienyl, furanyl, pyridyl, pyrimidyl, pyranyl, pyrazolyl, pyrrolyl, pyrazinyl, thiazoyl, isothiazolyl, oxazolyl, isooxazolyl, 1,2,3- trizaolyl, 1,2,4-triazolyl, and tetrazolyl.
- Heteroaryl groups also include fused polycyclic aromatic ring systems in which a carbocyclic aromatic ring or heteroaryl ring is fused to one or more other heteroaryl rings.
- heteroaryl groups may include benzothienyl, benzofuranyl, indolyl, quinolinyl, benzothiazolyl, benzoisothiazolyl, benzooxazolyl, benzoisooxazolyl, benzimidazolyl, quinolinyl, isoquinolinyl and isoindolyl.
- Groups described herein having two or more points of attachment may be designated by use of the suffix,“ene.”
- divalent alkyl groups may be alkylene groups
- divalent aryl groups may be arylene groups
- divalent heteroaryl groups may be heteroarylene groups
- certain polymers may be described by use of the suffix“ene” in conjunction with a term describing the polymer repeat unit.
- “optionally substituted” means a compound or group that may be substituted or unsubstituted.
- the term“substituted” refers to an organic group (e.g., an alkyl group) in which one or more bonds to a hydrogen atom contained therein may be replaced by a bond to non-hydrogen or non-carbon atoms.
- Substituted groups also include groups in which one or more bonds to a carbon or hydrogen atom may be replaced by one or more bonds, including double or triple bonds, to a heteroatom.
- a substituted group may be substituted with one or more substituents, unless otherwise specified. In some embodiments, a substituted group may be substituted with 1, 2, 3, 4, 5, or 6 substituents.
- substituent groups include: halogens (F, Cl, Br, and I); hydroxyl; alkoxy, alkenoxy, aryloxy, aralkyloxy, heterocyclooxy, and heterocycloalkoxy groups; carbonyls (oxo); carboxyls; esters; urethanes; oximes; hydroxylamines; alkoxyamines; aralkoxyamines; thiols; sulfides; sulfoxides; sulfones; sulfonyls; sulfonamides; amines; N-oxides; hydrazines; hydrazides; hydrazones; azides; amides; ureas; amidines; guanidines; enamines; imides; isocyanates; isothiocyanates; cyanates; thiocyanates; imines; nitro groups; or nitriles.
- halogens F, Cl, Br, and I
- A“per”- substituted compound or group is a compound or group having all or substantially all substitutable positions substituted with the indicated substituent.
- 1,6-diiodo perfluoro hexane indicates a compound of formula C 6 F 12 I 2 , where all the substitutable hydrogens have been replaced with fluorine atoms.
- suitable substituents for an alkyl group, cycloalkyl group, heterocycloalkyl group, or an aryl group ring carbon are those which do not substantially interfere with the activity of the disclosed compounds.
- Each of R A -R D may independently be an aliphatic, substituted aliphatic, benzyl, substituted benzyl, aryl or substituted aryl group, for example, an alkyl, benzylic or aryl group. Further,–NR A R D , taken together, may form a substituted or unsubstituted non-aromatic heterocyclic group. A non-aromatic heterocyclic group, benzylic group or aryl group may also have an aliphatic or substituted aliphatic group as a substituent.
- a substituted aliphatic group may also have a non-aromatic heterocyclic ring, a substituted a non- aromatic heterocyclic ring, benzyl, substituted benzyl, aryl or substituted aryl group as a substituent.
- a substituted aliphatic, non-aromatic heterocyclic group, substituted aryl, or substituted benzyl group may have more than one substituent.
- Suitable substituents for heteroaryl ring nitrogen atoms having three covalent bonds to other heteroaryl ring atoms may include–OH and C 1 to C 10 alkoxy.
- Substituted heteroaryl ring nitrogen atoms that have three covalent bonds to other heteroaryl ring atoms are positively charged, which may be balanced by counteranions such as chloride, bromide, formate, acetate and the like. Examples of other suitable counteranions may include counteranions found in the described pharmacologically acceptable salts.
- Suitable substituents for heteroaryl ring nitrogen atoms having two covalent bonds to other heteroaryl ring atoms include alkyl, substituted alkyl (including haloalkyl), phenyl, substituted phenyl,–S(O) 2 -(alkyl),–S(O) 2 –NH(alkyl),–S(O) 2 –NH(alkyl) 2 , and the like.
- compositions described herein may react with any of a number of organic or inorganic acids to form a salt.
- compounds disclosed herein that possess a sufficiently acidic functional group may react with any of a number of organic or inorganic bases to form a salt.
- Acids commonly employed to form acid addition salts from compounds with basic groups may include inorganic acids such as hydrochloric acid, hydrobromic acid, hydroiodic acid, sulfuric acid, phosphoric acid, and the like, and organic acids such as p-toluenesulfonic acid, methanesulfonic acid, oxalic acid, p- bromophenyl-sulfonic acid, carbonic acid, succinic acid, citric acid, benzoic acid, acetic acid, and the like.
- inorganic acids such as hydrochloric acid, hydrobromic acid, hydroiodic acid, sulfuric acid, phosphoric acid, and the like
- organic acids such as p-toluenesulfonic acid, methanesulfonic acid, oxalic acid, p- bromophenyl-sulfonic acid, carbonic acid, succinic acid, citric acid, benzoic acid, acetic acid, and the like.
- salts may include the sulfate, pyrosulfate, bisulfate, sulfite, bisulfite, phosphate, monohydrogenphosphate, dihydrogenphosphate, metaphosphate, pyrophosphate, chloride, bromide, iodide, acetate, propionate, decanoate, caprylate, acrylate, formate, isobutyrate, caproate, heptanoate, propiolate, oxalate, malonate, succinate, suberate, sebacate, fumarate, maleate, butyne-1,4-dioate, hexyne-1,6-dioate, benzoate, chlorobenzoate, methylbenzoate, dinitrobenzoate, hydroxybenzoate, methoxybenzoate, phthalate, sulfonate, xylenesulfonate, phenylacetate, phenylpropionate, phenylbuty
- Base addition salts include those derived from inorganic bases, such as ammonium or alkali or alkaline earth metal hydroxides, carbonates, bicarbonates, and the like.
- bases useful in preparing the salts of the described compounds may include sodium hydroxide, potassium hydroxide, ammonium hydroxide, potassium carbonate, and the like.
- An“effective amount” is the quantity of compound in which a beneficial clinical outcome may be achieved when the compound is administered to a subject suffering from the described fungus.
- A“beneficial clinical outcome” may include one or more of: a reduction in number of fungal spores in a subject; a reduction in the rate of fungus growth in a subject; a reduction in fungus consumption of a subject’s bodily resources; a reduction in biomarkers, toxins, proteins, peptides, and other biomolecules associated with infection of the subject by the fungus; a reduction in inflammatory, allergic, toxic, disfigurement, or other effects on the subject by the fungus; a reduction in the severity of the symptoms associated with the fungus and/or an increase in the longevity or health of the subject compared with the absence of the treatment.
- the precise amount of compound administered to a subject may depend on the species, lifecycle, of the fungal infection.
- the precise amount of compound administered to a subject may also depend on the characteristics of the subject, such as general health, age, sex, body weight and tolerance to drugs. A skilled artisan may determine appropriate dosages depending on these and other factors. Effective amounts of the disclosed compounds typically range between about 1 mg/mm 2 per day and about 10 grams/mm 2 per day, and preferably between 10 mg/mm 2 per day and about 5 grams/mm 2 .
- the disclosed compounds and pharmaceutical compositions may be administered by any suitable route, including, for example, orally in tablets, pills, gelcaps, lozenges, or suspensions; by parenteral administration.
- Parenteral administration can include, for example, systemic administration, such as by intramuscular, intravenous, subcutaneous, or intraperitoneal injection.
- the compounds may also be administered, for example, orally (e.g., dietary); topically, in the form of creams, sprays, patches, and the like; by inhalation (e.g., intrabronchial, intranasal, or oral inhalation of an aerosol formulation, by intranasal drops, and the like); via absorption through mucus membranes (e.g., tissues such as oral, nasal, rectal, vaginal, and the like) via, for example, creams, lozenges, sprays, drops, suppositories, and the like); depot preparations; coatings on sutures, bandages, medical devices, and the like.
- oral or parenteral administration are exemplary modes of administration.
- the disclosed compounds may be administered to the subject in conjunction with an acceptable pharmaceutical carrier as part of a pharmaceutical composition for treatment of infection by the described fungus.
- Formulation of the compound to be administered may vary according to the route and vehicle of administration selected (e.g., solution for injection, capsule or tablet for ingestion, and the like).
- Suitable pharmaceutical carriers may contain inert ingredients that do not interact with the described compound. Standard pharmaceutical formulation techniques may be employed, such as those described in Remington's Pharmaceutical Sciences, 22 nd ed., Mack Publishing Company, Easton, PA, 2012.
- Suitable pharmaceutical carriers for parenteral administration may include, for example, sterile water, physiological saline, bacteriostatic saline (e.g., saline containing about 0.9% mg/mL benzyl alcohol, and the like), phosphate-buffered saline, Hank's solution, Ringer's-lactate and the like.
- Methods for encapsulating compositions (such as in a coating of hard gelatin or cyclodextrin) or tableting compositions are known in the art (Baker, et al.,“Controlled Release of Biological Active Agents,” John Wiley and Sons, New York, 1986).
- A“subject” may be any animal subject to infection by the described funguss, e.g., the subject may be a mammal, bird, marsupial, fish, or amphibian.
- the subject may be a mammal, such as a human.
- the subject may also be a domestic or wild animal in need of veterinary treatment, e.g., companion animals (e.g., dogs, cats, and the like), farm animals (e.g., cows, sheep, pigs, horses, and the like), laboratory animals (e.g., rats, mice, guinea pigs, and the like), birds, fish, marsupials, and the like.
- the terms“in” or“into” are used in the specification or the claims, it is intended to additionally mean“on” or“onto.”
- selective is used in the specification or the claims, it is intended to refer to a condition of a component wherein a user of the apparatus may activate or deactivate the feature or function of the component as is necessary or desired in use of the apparatus.
- operatively connected is used in the specification or the claims, it is intended to mean that the identified components are connected in a way to perform a designated function.
- the term“substantially” is used in the specification or the claims, it is intended to mean that the identified components have the relation or qualities indicated with degree of error as would be acceptable in the subject industry.
- the term“about” in conjunction with a number is intended to include ⁇ 10% of the number.
- “about 10” may mean from 9 to 11.
- the term“about” may mean ⁇ 10% of the number, or ⁇ 5, ⁇ 4, ⁇ 3, ⁇ 2, or ⁇ 1 of the number.
Landscapes
- Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Provided are compounds, methods, and pharmaceutical compositions useful for treatment of fungal infections, e.g., aspergillosis, candidiasis, cryptococcosis, histoplasmosis, and the like. For example, the pharmaceutical composition may include a pharmaceutically acceptable carrier or excipient, and a compound represented by Ar— C(=NR1)NR2— A---X— Y— Het2 and pharmaceutically acceptable salts thereof. Ar may be an optionally substituted aryl or nitrogen- containing heteroaryl. R1 and R2 may independently be H, optionally substituted C1-C6 aikyi, or optionally substituted C3-C6 cyeloalkyi. A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cyeloalkyi, heterocycloalkyl, and heteroaryl. X may be O, S, amide, or a bond. Y may be optionally substituted C1-C10 alkyi or optionally substituted C2-C10 alkenyl. Het2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
Description
ANTI-FUNGAL TREATMENT
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority from U.S. Provisional Patent Application Nos.: 62/381,090 filed on August 30, 2016, the entire contents of which are incorporated herein by reference.
BACKGROUND
[0002] Fungal infections continue to be major causes of morbidity and mortality, particularly for vulnerable subjects with compromised or suppressed immune systems. Most fungal infections (e.g., aspergillosis, cryptococcosis, and histoplasmosis) result from inhalation of fungal cells in the environment making exposure nearly unavoidable. Candidiasis often occurs in hospital settings since at-risk patients are often under inpatient care. Subjects at significant risk of fungal infection may include, for example, chemotherapy and other oncology patients with immune suppression, transplant recipients receiving immunosuppressant anti-rejection therapy, subjects with HIV infection or other immune-compromised diseases, individuals receiving anti-inflammatory therapeutics such as TNF-alpha blockers (e.g., Enbrel), burn patients, and the like.
[0003] Treatments exist for systemic fungal infections, such as candidiasis and aspergillosis, but are limited by low efficacy, side effects, expense, and drug resistance. Known antifungal drug classes include: azoles, such as fluconazole, itraconazole, and voriconazole; polyenes, such as Amphotericin B, nystatin, and natamycin; echinocandins such as caspofungin; and the like. Currently, only certain azoles, polyenes, and echinocandins are approved to treat systemic fungal infections. Each of the currently approved antifungal drugs have one or more significant drawbacks, such as a lack of broad-spectrum activity, low activity, poor oral bioavailability, undesirable side-effects, expense, long treatment durations that impact patient compliance, and drug-drug interactions. Further, systemic administration of many antifungal drugs in effective concentrations, such as amphotericin B, may be toxic, damaging the liver and other organs.
[0004] The present application appreciates that developing treatments for fungal infections may be a challenging endeavor.
SUMMARY
[0005] In one embodiment, a method of anti-fungal treatment in a subject in need thereof is provided. The method may include providing the subject, the subject being infected by a fungus or at risk of infection by the fungus. The method may include administering a compound to the subject in an amount effective to mitigate infection by the fungus in the subject. The compound may be represented by Structural Formula (Ia):
[0006] and pharmaceutically acceptable salts thereof. Ar may be an optionally substituted aryl or nitrogen-containing heteroaryl. R1 and R2 may independently be H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl. A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl. X may be O, S, amide, or a bond. Y may be optionally substituted C1-C14 alkyl or optionally substituted C2-C14 alkenyl, e.g., optionally substituted C1-C10 alkyl or optionally substituted C2- C10 alkenyl, or optionally substituted C1-C8 alkyl or optionally substituted C2-C8 alkenyl. Het2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
[0007] In one embodiment, a method of anti-fungal treatment in a subject in need thereof is provided. The method may include providing the subject, the subject being infected by a fungus or at risk of infection by the fungus. The method may include administering a compound to the subject in an amount effective to mitigate infection by the fungus in the subject. The compound may be represented by Structural Formula (I):
and pharmaceutically acceptable salts thereof. Het1 may be an optionally substituted, nitrogen- containing heteroaryl. R1 and R2 may independently be H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl. A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl. X may be O, S, amide, or a bond. Y may be optionally substituted C1-C10 alkyl or optionally substituted C2-C10 alkenyl. Het2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
[0008] In one embodiment, a pharmaceutical composition is provided. The pharmaceutical composition may include a pharmaceutically acceptable carrier or excipient. The pharmaceutical composition may include a compound represented by Structural Formula (Ia):
and pharmaceutically acceptable salts thereof. Ar may be an optionally substituted aryl or nitrogen-containing heteroaryl. R1 and R2 may independently be H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl. A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl. X may be O, S,
amide, or a bond. Y may be optionally substituted C1-C14 alkyl or optionally substituted C2-C14 alkenyl, e.g., optionally substituted C1-C10 alkyl or optionally substituted C2-C10 alkenyl, or optionally substituted C1-C8 alkyl or optionally substituted C2-C8 alkenyl. Het2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
[0009] In one embodiment, a pharmaceutical composition is provided. The pharmaceutical composition may include a pharmaceutically acceptable carrier or excipient. The pharmaceutical composition may include a compound represented by Structural Formula (I):
and pharmaceutically acceptable salts thereof. Het1 may be an optionally substituted, nitrogen- containing heteroaryl. R1 and R2 may independently be H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl. A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl. X may be O, S, amide, or a bond. Y may be optionally substituted C1-C10 alkyl or optionally substituted C2-C10 alkenyl. Het2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
[0010] In another embodiment, a kit for anti-fungal treatment of a subject in need thereof is provided. The kit may include any anti-fungal compound described herein, for example, the compound represented by Structural Formula (Ia):
[0011] pharmaceutically acceptable salts thereof; and mixtures thereof with a pharmaceutically acceptable carrier or excipient to form a pharmaceutical composition, e.g., any pharmaceutical composition described herein. Ar may be an optionally substituted aryl or nitrogen-containing heteroaryl. R1 and R2 may independently be H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl. A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl. X may be O, S, amide, or a bond. Y may be optionally substituted C1-C14 alkyl or optionally substituted C2-C14 alkenyl, e.g., optionally substituted C1-C10 alkyl or optionally substituted C2-C10 alkenyl, or optionally substituted C1-C8 alkyl or optionally substituted C2-C8 alkenyl. Het2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms. The kit may include instructions. The instructions may direct a user to provide the subject, the subject being infected by a fungus or at risk of infection by the fungus.
The instructions may direct a user to administer the compound or the pharmaceutical composition to the subject in an amount effective to mitigate infection by the fungus in the subject.
[0012] In another embodiment, a kit for anti-fungal treatment of a subject in need thereof is provided. The kit may include any anti-fungal compound described herein, for example, the compound represented by Structural Formula (I):
pharmaceutically acceptable salts thereof; and mixtures thereof with a pharmaceutically acceptable carrier or excipient to form a pharmaceutical composition, e.g., any pharmaceutical composition described herein. Het1 may be an optionally substituted, nitrogen-containing heteroaryl. R1 and R2 may independently be H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl. A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl. X may be O, S, amide, or a bond. Y may be optionally substituted C1-C10 alkyl or optionally substituted C2-C10 alkenyl. Het2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms. The kit may include instructions. The instructions may direct a user to provide the subject, the subject being infected by a fungus or at risk of infection by the fungus. The instructions may direct a user to administer the compound or the pharmaceutical composition to the subject in an amount effective to mitigate infection by the fungus in the subject.
BRIEF DESCRIPTION OF THE DRAWINGS
[0013] The accompanying figures, which are incorporated in and constitute a part of the specification, illustrate example methods and compositions, and are used merely to illustrate example embodiments.
[0014] FIG. 1A is a synthetic scheme for various phenoxyalkyl linker anti-fungal compounds.
[0015] FIG.1B is a synthetic scheme for various phenoxyalkyl hybrid target compounds substituted meta to the arylimidamide group.
[0016] FIG. 1C is a synthetic scheme for various phenoxyalkyl hybrid target compounds substituted ortho to the arylimidamide group.
[0017] FIG. 1D is a synthetic scheme for various phenoxyalkyl hybrid target compounds substituted with pyrrole.
[0018] FIG. 2A is a synthetic scheme for various phenyl-unsubstituted diphenylfuran alkyloxy linker hybrid compounds.
[0019] FIG. 2B is a synthetic scheme for various phenyl substituted diphenylfuran alkyloxy linker hybrid compounds.
[0020] FIG. 2C is a synthetic scheme for various phenyl substituted diphenylfuran alkyloxy linker hybrid compounds.
[0021] FIG. 2D is a prophetic synthetic scheme for various diphenylfuran alkyloxy linker hybrid compounds.
[0022] FIG. 3 is a prophetic synthetic scheme for various alkylamide linker hybrid compounds.
[0023] FIG. 4A is a prophetic synthetic scheme for various phenylalkyl linker hybrid compounds 8a and 8b.
[0024] FIG.4B is a synthetic scheme for various phenylalkyl linker hybrid compounds.
[0025] FIG. 5A is a synthetic scheme for various unsubstituted and substituted biphenyl linker anti-fungal compounds.
[0026] FIG. 5B is a synthetic scheme for various substituted biphenyl linker anti- fungal compounds.
[0027] FIG. 5C is a synthetic scheme for various substituted biphenyl linker anti- fungal compounds.
[0028] FIG. 5D is a synthetic scheme for various substituted biphenyl linker anti- fungal compounds.
[0029] FIG. 6A is a synthetic scheme for various phenyl-piperazinyl-phenyl linker anti-fungal compounds.
[0030] FIG. 6B is a synthetic scheme for various phenyl-piperazinyl linker anti-fungal compounds.
[0031] FIG. 7 is a table depicting IC50 values of various compounds against Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, and Histoplasma capsulatum.
[0032] FIG. 8A is a graph depicting relative fungal growth of Aspergillus fumigatus versus concentration in μM of various compounds.
[0033] FIG. 8B is a graph depicting relative fungal growth of Candida albicans versus concentration in μM of various compounds.
[0034] FIG. 8C is a graph depicting relative fungal growth of Cryptococcus neoformans versus concentration in μM of various compounds.
[0035] FIG. 8D is a graph depicting relative fungal growth of Histoplasma capsulatum versus concentration in μM of various compounds.
DETAILED DESCRIPTION
[0036] In various embodiments, a method of anti-fungal treatment in a subject in need thereof is provided. The method may include providing the subject, the subject being infected by a fungus or at risk of infection by the fungus. The method may include administering a compound to the subject in an amount effective to mitigate infection by the fungus in the subject. The compound may be represented by Structural Formula (Ia):
and pharmaceutically acceptable salts thereof. Ar may be an optionally substituted aryl or nitrogen-containing heteroaryl. R1 and R2 may independently be H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl. A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl. X may be O, S, amide, or a bond. Y may be optionally substituted C1-C14 alkyl or optionally substituted C2-C14 alkenyl, e.g., optionally substituted C1-C10 alkyl or optionally substituted C2-C10 alkenyl, or optionally substituted C1-C8 alkyl or optionally substituted C2-C8 alkenyl. Het2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
[0037] In some embodiments, a method of anti-fungal treatment in a subject in need thereof is provided. The method may include providing the subject, the subject being infected by a fungus or at risk of infection by the fungus. The method may include administering a compound to the subject in an amount effective to mitigate infection by the fungus in the subject. The compound may be represented by Structural Formula (I):
and pharmaceutically acceptable salts thereof. Het1 may be an optionally substituted, nitrogen- containing heteroaryl. R1 and R2 may independently be H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl. A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl. X may be O, S, amide, or a bond. Y may be optionally substituted C1-C10 alkyl or optionally substituted C2-C10 alkenyl. Het2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
[0038] In several embodiments, the method may include administering the compound represented by Structural Formula (I) or (Ia) in the form of any pharmaceutical composition described herein.
[0039] In some embodiments, the subject may be infected by the fungus. The method may include administering the compound to the subject in an amount effective to mitigate one or more symptoms of infection by the fungus in the subject. Additionally or alternatively, the subject may be at risk of infection by the fungus. The method may include administering the compound to the subject in an amount effective to mitigate infection or re-infection of the subject by the fungus.
[0040] In several embodiments, the fungus may include CYP51. The fungus may include at least two distinct cytochrome P450 mediated biosterol synthesis pathways, e.g., one of which may include CYP51. The fungus may belong to a genus that is one of: Aspergillus, Candida, Cryptococcus, Histoplasma, and the like. For example, the fungus may be one of: Aspergillus fumigatus, Candida albicans, Candida glabrata, Cryptococcus neoformans, Histoplasma capsulatum, and the like. The subject may have, or be at risk of aspergillosis, candidiasis, cryptococcosis, histoplasmosis, and the like. The subject may suffer from or be at risk of suffering from an infection by the fungus of one or more of: skin, nail, hoof, hair, fur, scale, mucosal membrane, blood, lymph, brain, lung, heart, liver, pancreas, spleen, kidney, bladder, stomach, and intestine. The subject may suffer from a systemic infection by the fungus or be at risk of suffering from a systemic infection by the fungus. The subject may be one of: suffering from cancer; undergoing chemotherapy; undergoing immune suppression therapy; a transplant recipient, e.g., one undergoing immune suppression therapy; suffering from an immuno- deficiency disease or condition, such as HIV infection; a burn patient; and the like. The subject may be a human, dog, cat, cow, horse, sheep, pig, bird, amphibian, or fish.
[0041] In several embodiments, the compound represented by Structural Formula (Ia) or (I) may exclude certain compounds. For example, the compound represented by Structural Formula (Ia) or (I) may exclude free-base (neutral or non-salt) forms of Compounds 1a-f, wherein: Het1 is unsubstituted pyrid-2-yl; R1 and R2 are each H; X is O; Y is unbranched, unsubstituted C2-C4 alkyl; and Het2 is unsubstituted imidazole-1-yl or unsubstituted 1, 2, 4 triazol-2-yl:
[0042] In several embodiments, the compound represented by Structural Formula (Ia) or (I) may include pharmaceutically acceptable salts of Compounds 1a-f. The compound represented by Structural Formula (Ia) or (I) may exclude free-base forms of Compounds 1a-f and pharmaceutically acceptable salts of Compounds 1a-f. The compound represented by Structural Formula (Ia) or (I) may exclude protonated forms of Compounds 1a-f.
[0043] In some embodiments, the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein Het1 is one of unsubstituted pyridyl and pyrid-2-yl substituted with methyl or ethyl, for example, when: R1 and R2 are each H; X is O; Y is unbranched, unsubstituted C2-C4 alkyl; and Het2 is unsubstituted imidazole-1-yl or unsubstituted 1,2,4-triazol-2-yl. In some embodiments, the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein one of R1 and R2 is H and the other is methyl or ethyl, for example, when: Het1 is unsubstituted pyrid-2-yl; X is O; Y is unbranched, unsubstituted C2- C4 alkyl; and Het2 is unsubstituted imidazole-1-yl or unsubstituted 1,2,4-triazol-2-yl. In some embodiments, the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein X is S, for example, when: Het1 is unsubstituted pyrid-2-yl; R1 and R2 are each H; Y is unbranched, unsubstituted C2-C4 alkyl; and Het2 is unsubstituted imidazole-1-yl or unsubstituted 1,2,4-triazol-2-yl. In some embodiments, the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein Y is C2-C4 alkyl substituted with methyl or ethyl, unsubstituted or methyl substituted C1-C5 alkyl, or unsubstituted C1-C6 alkyl, for example, when: Het1 is unsubstituted pyrid-2-yl; R1 and R2 are each H; X is O; and Het2 is unsubstituted imidazole-1-yl or unsubstituted 1,2,4-triazol-2-yl. In some embodiments, the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein Het2 is one of: methyl or ethyl substituted imidazole-1-yl; unsubstituted imidazolyl; methyl or ethyl substituted imidazolyl; methyl or ethyl substituted 1,2,4-triazol-2-yl; unsubstituted 1,2,4-triazolyl; methyl or ethyl substituted 1,2,4-triazolyl; unsubstituted triazolyl; and methyl or ethyl substituted triazolyl;
for example, when: Het1 is unsubstituted pyrid-2-yl; R1 and R2 are each H; X is O; and Y is unbranched, unsubstituted C2-C4 alkyl.
[0044] In several embodiments, Het1 may be optionally substituted pyridyl, pyrazinyl, pyrimidinyl, or pyridizinyl. For example, Het1 may be optionally substituted pyridyl. The compound may be represented by Structural Formula (II):
[0046] In various embodiments, each ring in the optionally substituted linking moiety represented by A may be independently and optionally substituted by one or more of: hydroxy, halo, and C1-C6 alkoxy. A may include an optionally substituted heteroaryl or optionally substituted heterocycloalkyl ring. A may include an optionally substituted, oxygen-containing, heteroaryl or heterocycloalkyl ring. A may include an optionally substituted furanyl or optionally substituted tetrahydrofuranyl ring. A may include optionally substituted 2,5-furanyl. A may include one or two optionally substituted phenyl rings. A may include optionally substituted 1,4-phenyl. A may be optionally substituted 1,4-phenyl. A may be optionally substituted phenyl-heteroaryl-phenyl.
[0047] For example, the compound of Structural Formula III may be represented by one of Structural Formulas (IIIa)-(IIIf):
wherein Z, Z1, and Z2 are each independently CH or N, n may be 1-14, e.g., 1-10, and R3 may represent H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy.
[0048] In some embodiments, the compound may be represented by one of Compounds 1g-1v, e.g., 1g-1n:
[0049] The compound may be, for example, represented by Structural Formula (IIIf) above, e.g., compound 14a:
[0050] In some embodiments, the compound may be represented by Structural Formula (IV):
wherein each R3 may independently represent H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy. For example, the compound may be represented by Structural Formula (V):
[0051] In several embodiments, Y may include at least 4 linking atoms between X and Het2. X may be O or a bond and Y may be C1-C14, e.g., C1-C10 alkyl optionally substituted with one or more of: optionally halogenated C1-C8 alkyl and optionally halogenated aryl.
[0052] For example, the compound may be represented by one of Structural Formulas (Va)-(Ve), wherein each Z, Z1, and Z2 are independently CH or N:
wherein Z, Z1, and Z2 are each independently CH or N, n may be 1-14, e.g., 1-10, and R3 may represent H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy.
[0053] For example, in some embodiments, the compound may be represented by Structural Formula (VII):
wherein: Z may be CH or N; each R3 may independently be H, halogen, optionally halogenated C1-C10 alkyl, or optionally halogenated C1-C10 alkoxy; and n may be an integer from 1 to 10. For example, the compound may be one of Compounds 2a-2h:
[0054] The compound may be, for example, one of Compounds 3a-3g:
[0055] The compound may be, for example, one of Compounds 4a-4m, e.g., 4a-4i:
[0056] The compound may be, for example, represented by Structural Formula (Vd) above, e.g., one of compounds 13a-13c:
[0057] In various embodiments, the compound may be represented by Structural Formula (VIa):
(VIa) ,
wherein R4 may be H, optionally halogenated C1-C10 alkyl, or optionally halogenated aryl. The compound may be represented by Structural Formula (VIb):
(VIb) .
[0058] In some embodiments, Het2 may include an optionally substituted one of: pyrrole, diazole, thiadiazole, oxadiazole, and triazole. For example, Het2 may be optionally substituted imidazole or optionally substituted 1, 2, 4 triazole.
[0059] In several embodiments, the compound may be represented by Structural Formula (VIII):
wherein: each R5 may independently be H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and R6 may be H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl. For example, the compound may be one of Compounds 5a-5f:
[0060] In various embodiments, the compound may be represented by Structural Formula (IX):
wherein: each R5 may independently be H, halogen, C 6
1-C4 alkyl, or C1-C4 alkoxy; and R may be H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl. For example, the compound may be one of Compounds 6a-6f:
wherein: each R5 may independently be H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and R6 may be H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl. For example, the compound may be one of Compounds 7a-7f:
wherein: each R5 may independently be H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and R6 may be H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
[0063] In several embodiments, the compound may be represented by Structural Formula (XII):
wherein: R5 may independently be H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and R6 may be H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
[0064] In several embodiments of the compound represented by Structural Formula (Ia) or (I), A may be phenyl and X may be a bond. For example, the compound may be represented by Structural Formula (XIII):
wherein Z may be CH or N; and n may be an integer from 1 to 10. For example, the compound may be one of Compounds 8a-8b:
.
[0065] In several embodiments of the compound represented by Structural Formula (Ia) or (I), A may be a bond. For example, the compound may be represented by Structural Formula (XIV):
wherein Z may be CH or N; and n may be an integer from 1 to 10. For example, the compound may be one of Compounds 9a-9d, e.g., 9a-9c:
[0066] In several embodiments of the compound represented by Structural Formula (Ia) or (I), A may be optionally substituted biphenyl. For example, the compound may be represented by Structural Formula (XV):
e.g., one of (XVa)-(XVc):
wherein Z, Z1, and Z2 are each independently CH or N, n may be 1-14, e.g., 1-10, and R3 may represent H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy.
[0067] For example, the compound may be one of Compounds 10a-10n:
[0068] In several embodiments of the compound represented by Structural Formula (Ia) or (I), A may be optionally substituted phenyl-piperazinyl-phenyl. For example, the compound may be represented by one of Structural Formulas (XVI) and (XVII):
For example, the compounds may be represented by one of (XVIa)-(XVIb):
wherein Z, Z1, and Z2 are each independently CH or N, n may be 1-14, e.g., 1-10, and R3 may represent H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy. For example, the compound may be one of Compounds 11a-11d:
Further, for example, the compounds may be represented by one of (XVIIa)-(XVIIb):
wherein Z, Z1, and Z2 are each independently CH or N, n may be 1-14, e.g., 1-10, and R3 may represent H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy. For example, the compound may be Compound 12a:
[0069] In various embodiments, a pharmaceutical composition is provided. The pharmaceutical composition may include a pharmaceutically acceptable carrier or excipient. The pharmaceutical composition may include a compound represented by Structural Formula (Ia):
and pharmaceutically acceptable salts thereof. Ar may be an optionally substituted aryl or nitrogen-containing heteroaryl. R1 and R2 may independently be H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl. A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl. X may be O, S, amide, or a bond. Y may be optionally substituted C1-C14 alkyl or optionally substituted C2-C14 alkenyl, e.g., optionally substituted C1-C10 alkyl or optionally substituted C2-C10 alkenyl, or optionally substituted C1-C8 alkyl or optionally substituted C2-C8 alkenyl. Het2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
[0070]
[0071] In some embodiments, a pharmaceutical composition is provided. The pharmaceutical composition may include a pharmaceutically acceptable carrier or excipient. The pharmaceutical composition may include a compound represented by Structural Formula (I):
and pharmaceutically acceptable salts thereof. Het1 may be an optionally substituted, nitrogen- containing heteroaryl. R1 and R2 may independently be H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl. A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl. X may be O, S, amide, or a bond. Y may be optionally substituted C1-C10 alkyl or optionally substituted C2-C10 alkenyl. Het2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms. In various embodiments, the compound of the pharmaceutical composition may include any selection of variables or any compound described or encompassed by Structural Formula (I) or (Ia) of the method described herein.
[0072] In several embodiments of the pharmaceutical composition, the compound represented by Structural Formula (Ia) or (I) may include one of Compounds 1a-f. In some embodiments, each of Compounds 1a-f may be in the form of a solid. Additionally or alternatively, each of Compounds 1a-f may be in the form of a pharmaceutically acceptable salt. Additionally or alternatively, at least a portion of the pharmaceutically acceptable carrier or excipient may be in the form of a solid or gel. Additionally or alternatively, the pharmaceutical composition may be configured for administration in unit dosage form. Additionally or
alternatively, the pharmaceutical composition may be configured for administration in the form of one of: a tablet; a capsule; a lozenge; a cream, a spray, a transdermal patch, an aerosol, a suppository, a depot preparation; a suture that is coated or impregnated with one of Compounds 1a-f; a bandage that is coated or impregnated with one of Compounds 1a-f; a medical device that is coated or impregnated with one of Compounds 1a-f; and the like.
[0073] In some embodiments of the pharmaceutical composition, pharmaceutically acceptable salts of Compounds 1a-1f are included. In some embodiments of the pharmaceutical composition, free-base (neutral) Compounds 1a-f may be excluded. In some embodiments of the pharmaceutical composition, protonated forms of Compounds 1a-1f may be excluded. Alternatively or in addition, the pharmaceutical composition may incorporate any of the various embodiments described herein for excluding certain compounds represented by Structural Formula (Ia) or (I). For example, the compound represented by Structural Formula (Ia) or (I) may exclude free-base (neutral or non-salt) forms of Compounds 1a-f. The compound represented by Structural Formula (Ia) or (I) may include pharmaceutically acceptable salts of Compounds 1a-f. The compound represented by Structural Formula (Ia) or (I) may exclude free-base forms of Compounds 1a-f and pharmaceutically acceptable salts of Compounds 1a-f. The compound represented by Structural Formula (Ia) or (I) may exclude protonated forms of Compounds 1a-f.
[0074] In several embodiments of the pharmaceutical composition, the compound may include any aspect of the compounds represented by Structural Formulas (Ia) or (I) as described herein. For example, the compounds of the pharmaceutical composition may be represented by, as described herein, any one of, or any group of, Structural Formulas: (I), (Ia), (II), (III), (IIIa), (IIIb), (IIIc), (IIId), (IIIe), (IIIf), (IV), (V), (Va), (Vb), (Vc), (Vd), (Ve), (Via), (VIb), (VII), (VIII), (IX), (X), (XI), (XII), (XIII), (XIV), (XV), (XVa), (XVb), (XVc), (XVI), (XVIa), (XVIb), (XVII), (XVIIa), and (XVIIb). Further, for example, the compound may be any one of, or any group of, as described herein, Compounds: 1a-1v; 2a-2h; 3a-3g; 4a-4m; 5a-5f; 6a-6f; 7a-7f; 8a-8b; 9a-9d; 10a-10n; 11a-11d; 12a; 13a-13c; and 14a.
[0075] For example, in some embodiments of the pharmaceutical composition, the compounds represented by Structural Formulas (Ia) or (I) may exclude compounds wherein Het1 is one of unsubstituted pyridyl and pyrid-2-yl substituted with methyl or ethyl, for example, when: R1 and R2 are each H; X is O; Y is unbranched, unsubstituted C2-C10 alkyl; and Het2 is unsubstituted imidazole-1-yl or unsubstituted 1, 2, 4 triazol-2-yl. In some embodiments, the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein one of R1 and R2 is H and the other is methyl or ethyl, for example, when: Het1 is unsubstituted pyrid-
2-yl; X is O; Y is unbranched, unsubstituted C2-C10 alkyl; and Het2 is unsubstituted imidazole-1- yl or unsubstituted 1, 2, 4 triazol-2-yl. In some embodiments, the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein X is S, for example, when: Het1 is unsubstituted pyrid-2-yl; R1 and R2 are each H; Y is unbranched, unsubstituted C2-C10 alkyl; and Het2 is unsubstituted imidazole-1-yl or unsubstituted 1, 2, 4 triazol-2-yl. In some embodiments, the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein Y is C2-C4 alkyl substituted with methyl or ethyl, unsubstituted or methyl substituted C1-C5 alkyl, or unsubstituted C1-C6 alkyl, for example, when: Het1 is unsubstituted pyrid-2-yl; R1 and R2 are each H; X is O; and Het2 is unsubstituted imidazole-1-yl or unsubstituted 1, 2, 4 triazol-2-yl. In some embodiments, the compound represented by Structural Formula (Ia) or (I) may exclude compounds wherein Het2 is one of: methyl or ethyl substituted imidazole-1-yl; unsubstituted imidazolyl; methyl or ethyl substituted imidazolyl; methyl or ethyl substituted 1,-2,-4-triazol-2-yl; unsubstituted 1,-2,-4-triazolyl; methyl or ethyl substituted 1,-2,- 4-triazolyl; unsubstituted triazolyl; and methyl or ethyl substituted triazolyl; for example, when: Het1 is unsubstituted pyrid-2-yl; R1 and R2 are each H; X is O; and Y is unbranched, unsubstituted C2-C4 alkyl.
[0076] In several embodiments of the pharmaceutical composition, Het1 may be optionally substituted pyridyl, pyrazinyl, pyrimidinyl, or pyridizinyl. For example, Het1 may be optionally substituted pyridyl. The compound of the pharmaceutical composition may be represented by Structural Formula (II). The compound of the pharmaceutical composition may be represented by Structural Formula (III). In several embodiments of the pharmaceutical composition, Y may include at least 4 linking atoms between X and Het2. X may be O or a bond and Y may be C1-C14, e.g., C1-C10 alkyl optionally substituted with one or more of: optionally halogenated C1-C8 alkyl and optionally halogenated aryl. For example, the compound may be one of Compounds 1a-n. The compound of the pharmaceutical composition may be represented by any of Structural Formulas (IIIa), (IIIb), (IIIc), (IIId), (IIIe), and (IIIf), wherein Z, Z1, and Z2 may be each independently CH or N, n may be 1-14, and R3 may be H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy. The compound of the pharmaceutical composition may be any one of Compounds 1a-1v. The compound of the pharmaceutical composition may be any one of Compounds 1a-1n.
[0077] In various embodiments of the pharmaceutical composition, each ring in the optionally substituted linking moiety represented by A may be independently and optionally substituted by one or more of: hydroxy, halo, and C1-C10 , e.g., C1-C6 alkoxy. A may include an optionally substituted heteroaryl or optionally substituted heterocycloalkyl ring. A may include
an optionally substituted, oxygen-containing, heteroaryl or heterocycloalkyl ring. A may include an optionally substituted furanyl or optionally substituted tetrahydrofuranyl ring. A may include optionally substituted 2,5-furanyl. A may include one or two optionally substituted phenyl rings. A may include optionally substituted 1,4-phenyl. A may be optionally substituted 1,4-phenyl. A may be optionally substituted phenyl-heteroaryl-phenyl.
[0078] In some embodiments of the pharmaceutical composition, the compound may be represented by Structural Formula (IV), wherein each R3 may independently represent H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy. The compound may be represented by Structural Formula (V). The compound of the pharmaceutical composition may be represented by any of Structural Formulas (V), (Va), (Vb), (Vc), (Vd), and (Ve), wherein Z, Z1, and Z2 may be each independently CH or N, n may be 1-14, and R3 may be H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy.
[0079] In various embodiments of the pharmaceutical composition, the compound may be represented by Structural Formula (VIa) wherein R4 may be H, optionally halogenated C1-C8 alkyl, or optionally halogenated aryl. The compound may be represented by Structural Formula (VIb).
[0080] In some embodiments of the pharmaceutical composition, Het2 may include an optionally substituted one of: pyrrole, diazole, thiadiazole, oxadiazole, and triazole. For example, Het2 may be optionally substituted imidazole or optionally substituted 1, 2, 4 triazole. For example, the compound may be represented by Structural Formula (VII), wherein: Z may be CH or N; each R3 may independently be H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy; and n may be an integer from 1 to 10. For example, the compound may be one of Compounds 2a-2g. The compound may be, for example, one of Compounds 3a-3g. The compound may be, for example, one of Compounds 4a-4m, e.g., 4a-4i. The compound may be, for example, one of Compounds 13a-13c.
[0081] In several embodiments of the pharmaceutical composition, the compound may be represented by Structural Formula (VIII), wherein each R5 may independently be H, halogen, C1-C4 alkyl, or C1-C4 alkoxy, and R6 may be H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl. For example, the compound may be one of Compounds 5a-5f. The compound may be represented by Structural Formula (IX), wherein: each R5 may independently be H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and R6 may be H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl. For example, the compound may be one of Compounds 6a-5f. The compound may be represented by Structural Formula (X) wherein: each R5 may independently
be H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and R6 may be H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl. For example, the compound may be one of Compounds 7a-7f.
[0082] In several embodiments of the pharmaceutical composition, the compound may be represented by Structural Formula (XI), wherein each R5 may independently be H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and R6 may be H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
[0083] In several embodiments of the pharmaceutical composition, the compound may be represented by Structural Formula (XII), wherein each R5 may independently be H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and R6 may be H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
[0084] In several embodiments of the pharmaceutical composition, the compound may be represented by Structural Formula (Ia) or (I), wherein A may be phenyl and X may be a bond. For example, the compound may be represented by Structural Formula (XIII), wherein Z may be CH or N; and n may be an integer from 1 to 10. For example, the compound may be one of Compounds 8a-8b.
[0085] In several embodiments of the pharmaceutical composition, the compound may be represented by Structural Formula (Ia) or (I), wherein A may be a bond. For example, the compound may be represented by Structural Formula (XIV), wherein Z may be CH or N; and n may be an integer from 1 to 10. For example, the compound may be one of Compounds 9a-9d, for example, 9a-9c.
[0086] In several embodiments of the pharmaceutical composition, the compound may be represented by Structural Formula (XV), wherein Z, Z1, and Z2 may be each independently CH or N, n may be 1-14, and R3 may be H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy. For example, the compound may be represented by one of Structural Formulas (XVa)-(XVc). For example, the compound may be one of Compounds 10a- 10n.
[0087] In several embodiments of the pharmaceutical composition, the compound may be represented by one of Structural Formulas (XVI) and (XVII), wherein Z, Z1, and Z2 may be each independently CH or N, n may be 1-14, and R3 may be H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy. For example, the compound may be represented by one of Structural Formulas (XVIa)-(XVIb). For example, the compound may be one of Compounds 11a-11d. Further, for example, the compound may be represented by one of Structural Formulas (XVIIa)-(XVIIb), e.g., the compound may be Compound 12a.
[0088] In various embodiments, a kit for anti-fungal treatment of a subject in need thereof is provided. The kit may include any anti-fungal compound described herein, for example, the compound represented by Structural Formula (Ia):
(Ia) Ar—C(=NR1)NR2—A—X—Y—Het2
[0089] pharmaceutically acceptable salts thereof; and mixtures thereof with a pharmaceutically acceptable carrier or excipient to form a pharmaceutical composition, e.g., any pharmaceutical composition described herein. Ar may be an optionally substituted aryl or nitrogen-containing heteroaryl. R1 and R2 may independently be H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl. A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl. X may be O, S, amide, or a bond. Y may be optionally substituted C1-C14 alkyl or optionally substituted C2-C14 alkenyl, e.g., optionally substituted C1-C10 alkyl or optionally substituted C2-C10 alkenyl, or optionally substituted C1-C8 alkyl or optionally substituted C2-C8 alkenyl. Het2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms. The kit may include instructions. The instructions may direct a user to provide the subject, the subject being infected by a fungus or at risk of infection by the fungus. The instructions may direct a user to administer the compound or the pharmaceutical composition to the subject in an amount effective to mitigate infection by the fungus in the subject.
[0090] In various embodiments, a kit for anti-fungal treatment of a subject in need thereof is provided. The kit may include any anti-fungal compound described herein, for example, the compound represented by Structural Formula (Ia):
(Ia) Ar—C(=NR1)NR2—A—X—Y—Het2
[0091] and pharmaceutically acceptable salts thereof. Ar may be an optionally substituted aryl or nitrogen-containing heteroaryl. R1 and R2 may independently be H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl. A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl. X may be O, S, amide, or a bond. Y may be optionally substituted C1-C14 alkyl or optionally substituted C2-C14 alkenyl, e.g., optionally substituted C1-C10 alkyl or optionally substituted C2-C10 alkenyl, or optionally substituted C1-C8 alkyl or optionally substituted C2-C8 alkenyl. Het2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms. The kit may include instructions.
The instructions may direct a user to provide the subject, the subject being infected by a fungus or at risk of infection by the fungus. The instructions may direct a user to administer the compound or the pharmaceutical composition to the subject in an amount effective to mitigate infection by the fungus in the subject.
[0092] In various embodiments, a kit for anti-fungal treatment of a subject in need thereof is provided. The kit may include any anti-fungal compound described herein, for example, the compound represented by Structural Formula (I):
(I) Het1-(CNR1NR2)-A-X-Y-Het2,
pharmaceutically acceptable salts thereof; and mixtures thereof with a pharmaceutically acceptable carrier or excipient to form a pharmaceutical composition, e.g., any pharmaceutical composition described herein. Het1 may be an optionally substituted, nitrogen-containing heteroaryl. R1 and R2 may independently be H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl. A may be a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings. Each ring in the optionally substituted linking moiety may independently be one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl. X may be O, S, amide, or a bond. Y may be optionally substituted C1-C10 alkyl or optionally substituted C2-C10 alkenyl. Het2 may be an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms. The kit may include instructions. The instructions may direct a user to provide the subject, the subject being infected by a fungus or at risk of infection by the fungus. The instructions may direct a user to administer the compound or the pharmaceutical composition to the subject in an amount effective to mitigate infection by the fungus in the subject.
[0093] In some embodiments of the kit, the compound represented by Structural Formula (I) or (Ia) may include any aspect of the anti-fungal compounds described herein, either alone or as encompassed by any of the methods or anti-fungal pharmaceutical compositions described herein. In several embodiments of the kit, the instructions may direct a user to conduct any step or combination of steps described herein for the method.
EXAMPLES
Example 2A: Synthesis of Phenoxyalkyl Linker Anti-Fungal Compounds
[0094] FIG. 1A is a synthetic scheme various phenoxyalkyl linker anti-fungal Compounds 1a-1n and 1v. Compounds 1a, 1b, 1c, 1e, 1f, 1g, 1i, 1m, and 1v were made according to FIG.1A. Generally, reagents and conditions for synthesis of compounds according to FIG. 1A included: a) Į,^-dibromoalkane, K2CO3, acetone; b) imidazole or 1,2,4-triazole,
K2CO3, CH3CN; c) SnCl2.2H2O, EtOAc; d) S-(2-naphthylmethyl)-2-pyridylthioimidate hydrobromide, CH3CN/EtOH (1:3), rt.
[0095] Details of the representative synthesis of Compound 1e are as follows.
[0096] Reaction of 4-nitrophenol (2.00 g, 14.4 mmol), 1,4-dibromobutane (3.5 mL, 6.33 g, 29.3 mmol), and potassium carbonate (5.96 g, 43.2 mmol) in acetone at reflux afforded 1-(4- bromobutoxy)-4-nitrobenzene as white crystals, yield 1.69 g, 6.15 mmol, 43%; m.p. 39-42 °C. The 1H NMR spectrum of this material was consistent with that reported in Narlawar et al., Journal of Medicinal Chemistry, 2010, 53, 3028-3037.
[0097] Reaction of imidazole (0.44 g, 6.43 mmol), 1-(4-bromobutoxy)-4-nitrobenzene (1.20 g, 4.28 mmol), and potassium carbonate (0.89 g, 6.4 mmol) in acetonitrile at reflux afforded 1-[4-(4-nitrophenoxy)butyl]-1H-imidazole as a dark brown solid, yield: 0.67 g, 2.6 mmol, 60%; m.p. 71-74 °C, lit m.p. 67-69 °C. The 1H NMR spectrum of this material was consistent with that reported in Salerno et. al, Bioorganic and Medicinal Chemistry, 2013, 21, 5145-5153. Reaction of 1-[4-(4-nitrophenoxy)butyl]-1H-imidazole (0.10 g, 0.38 mmol) and stannous chloride dihydrate (0.43 g, 1.9 mmol) in ethyl acetate at reflux afforded 4-(4-(1H- imidazol-1-yl)butoxy)aniline as a bright orange powder, yield: 0.046 g, 0.20 mmol, 52%. 1H NMR (300 MHz, DMSO-d6): į 1.57 (quint, J= 7.0, 2H), 1.82 (quint, J= 7.3, 2H), 3.81 (t, J= 6.4, 2H), 4.00 (t, J= 7.0, 2H), 4.60 (br, 2H) 6.48 (m, 2H), 6.62 (m, 2H), 6.9 (s, 1H), 7.2 (s, 1H), 7.6 (s, 1H).
[0098] Reaction of 4-(4-(1H-imidazol-1-yl)butoxy)aniline (0.15 g, 0.64 mmol) and S-(2- naphthylmethyl)-2-pyridyl thioimidate hydrobromide (0.34 g, 0.95 mmol) in anhydrous acetonitrile (6 mL) and ethanol (20 mL) at room temperature afforded N-(4-(4-(1H-imidazol-1- yl)butoxy)phenyl)picolinimidamide (1e) as a white crystal, yield: 0.0178 g, 0.053 mmol, 9%;
m.p. 99-101 °C. 1H NMR (300 MHz, DMSO-d6): į 1.65 (m, 2H), 1.88 (m, 2H), 3.95 (t, J= 6.3, 2H), 4.04 (t, J= 7.0, 2H), 6.40 (br, 2H), 6.88 (m, 5H), 7.19 (s, 1H), 7.54 (m, 1H), 7.64 (s, 1H), 7.93 (td, J1=7.7, J2=1.68, 1H), 8.30 (d, J=7.9, 1H), 8.62 (sd, J=4.2, 1H) ppm; 13C NMR (300 MHz, CDCl3): į 26.4, 28.1, 46.8, 67.4, 115.5, 118.7, 121.5, 122.6, 125.1, 129.5, 136.8, 137.1, 143.2, 147.8, 151.7, 153.0, 154.8 ppm.
Example 1B: Synthesis of meta-Substituted Phenoxyalkyl Linker Anti-Fungal Compounds
[0099] FIG. 1B is a synthetic scheme for various phenoxyalkyl hybrid target compounds substituted meta to the arylimidamide group, e.g., Compounds 1o-1q. Generally, reagents and conditions for synthesis of compounds according to FIG.2B included: a) MOMCl, K2CO3, acetone, 30ºC; b) RI, K2CO3, sealed tube, 80ºC; c) HCl, MeOH/CH2Cl2, rt; d) 1,8- Dibromooctane, K2CO3, CH3CN, reflux; e) imidazole, K2CO3, CH3CN, reflux; f) SnCl .
22H2O, EtOAc, reflux; g) S-(2-naphthylmethyl)-2-pyridylthioimidate hydrobromide, CH3CN/EtOH (1:3), rt.
Example 1C: Synthesis of ortho-Substituted Phenoxyalkyl Linker Anti-Fungal Compounds
[00100] FIG. 1C is a synthetic scheme for various phenoxyalkyl hybrid target compounds substituted ortho to the arylimidamide group, e.g., Compounds 1r-1t. Generally, reagents and conditions for synthesis of compounds according to FIG. 2C included: a) RI, K2CO3, sealed tube, 80ºC; b) NaOH, DMSO, reflux; c) 1,8-Dibromooctane, K2CO3, CH3CN, reflux; d) imidazole, K2CO3, CH3CN, reflux; e) SnCl .
22H2O, EtOAc, reflux; f) S-(2- naphthylmethyl)thioimidate hydrobromide, CH3CN/EtOH, rt..
Example 1D: Synthesis of Pyrrole-Substituted Phenoxyalkyl Linker Anti-Fungal Compounds
[00101] FIG. 1D is a synthetic scheme for various phenoxyalkyl hybrid target compounds substituted with pyrrole, e.g., Compound 1u. Generally, reagents and conditions for synthesis of Compound 1u according to FIG. 2D included: a) 1,8-dibromooctane, K2CO3, acetone; b) pyrrole, K2CO3, CH3CN; c) SnCl2.2H2O, EtOAc; d) S-(2-naphthylmethyl)-2- pyridylthioimidate hydrobromide, CH3CN/EtOH (1:3), rt.
Example 2A: Synthesis of Diphenylfuran Alkyloxy Linker Anti-Fungal Compounds
[00102] FIG. 2A is a synthetic scheme for various phenyl-unsubstituted diphenylfuran alkyloxy linker hybrid Compounds 2a-2g and 3a-g. Compounds 2a-e, 2g, 3a-e, and 3g were made according to FIG.2A. Compound 2h was made according to FIG.2A, but starting with 1- bromo-2-oxy(prop-2-yl)-4-nitrobenzene. Generally, reagents and conditions for synthesis of compounds according to FIG. 2A included: a) Pd(PPh3)4, dioxane, 90°C; b) NBS, DMF, rt; c)
K2CO3, acetone, reflux; d) Pd(PPh3)4, K2CO3, MeOH, toluene, 80°C; e) azole, NaH, DMF, rt; f) H2, Pd(C), EtOH-EtOAc; g) (i) S-(2-naphthylmethyl-2-pyridylthioimidate hydrobromide, EtOH; (ii) NaOH; (iii) ethanolic HCl.
[00103] The synthesis of Compound 2c is representative, as follows.
[00104] Tetrakistriphenylphosphine palladium (0.5 mmol) was added to a stirred mixture of the 2-(tributylstannyl) furan (11 mmol) and 1-bromo-4-nitrobenzene (10 mmol) in deaerated dioxane (25 mL) under a nitrogen atmosphere. The vigorously stirred mixture was heated at 90- 100 °C for 24 h. The solvent was evaporated under reduced pressure, the resulting solid was partitioned between ethyl acetate (200 mL) and 5 mL of concentrated ammonia to remove the palladium complex, washed with water, passed through celite to remove the catalyst, dried over sodium sulfate and evaporated. Purification by column chromatography on silica gel, using hexanes/ethyl acetate (93/7, v/v) followed by recrystallization from hexanes/ethyl acetate to afford a yellow solid, yield (68 %); m.p. 134-135 oC, (Lit Molander et al.“Scope of the SuzukiíMiyaura Cross-Coupling Reactions of Potassium Heteroaryltrifluoroborates,” J. Org. Chem.2009, 74(3), 973–980, the entire teachings of which are incorporated herein by reference), m.p. 131-132 oC; 1H NMR (DMSO-d6) ^ 6.71 (d, J = 3.6 Hz, 1H), 7.02 (br s, 1H), 7.23 (d, J = 3.6 Hz, 1H), 7.90 (d, J = 8.8 Hz, 2H), 7.98 (d, J = 8.8 Hz, 2H).
[00105] N-bromosuccinimide (2.13 gm, 12 mmol) was added portion-wise to a stirred solution of the previous nitro compound (10 mmol) in dimethylformamide (20 mL). The reaction mixture was stirred at room temperature for 12 h then poured onto cold water, the precipitate was collected and dried. Purification was conducted by column chromatography on silica gel, using hexanes/ethyl acetate (95/5, v/v). Yellow solid, yield 96 %; m.p. 141-143 oC (see Ismail et al.,“An efficient synthesis of 5,5ː-diaryl-2,2ː-bichalcophenes,” Tet Lett 2006, 47(5), 795-797, the entire teachings of which are incorporated herein by reference); 1H NMR (DMSO-d6) ^ 6.83 (d, J = 3.6 Hz, 1H), 7.35 (d, J = 3.6 Hz, 1H), 7.90 (d, J = 8.8 Hz, 2H), 8.26
(d, J = 8.8 Hz, 2H); HRMS: m/z calculated for C10H7BrNO3: 267.9609, found: 267.9602 (M+ +1).
2-(4-(4-(bromobutoxy)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
[00106] 1,4-dibromobutane (36 mmol) was added to a solution of the p- hydroxyphenylboronic acid ester (1.32 gm, 6 mmol), dry K2CO3 (1.65 gm, 12 mmol) and Cs2CO3 (0.39 gm, 1.2 mmol) in anhydrous dimethylacetamide (15 mL) under a nitrogen atmosphere. Stirring was continued for 12 h and ice water was added and the reaction mixture was filtered and air dried. Purification was performed by column chromatography on silica gel, using hexanes/ethyl acetate (93/7, v/v). White solid (73%) m.p. 112-113 oC, 1H NMR (DMSO-d6) ^ 1.27 (s, 12 H), 1.82-1.85 (m, 2 H), 1.94-1.98 (m, 2 H), 3.61 (t, J =5.2 Hz, 2 H), 4.01 (t, J =5.2 Hz, 2 H), 6.93 (d, J = 8.4 Hz, 2 H), 7.68 (d, J =8.4 Hz, 2 H).
[00107] 2.3 mL deaerated 2 M aqueous solution of K2CO3 and 2-(4- (bromobutoxy)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (2.73 mmol) in 5 mL deaerated methanol were added to a stirred solution of 2-bromo-5-(4-nitrophenyl)furan (2.28 mmol), and tetrakistriphenylphosphine palladium (0.114 mmol) in deaerated toluene (25 mL) under a nitrogen atmosphere. The vigorously stirred mixture was warmed to 80 °C for 24 h. The solvent was evaporated under reduced pressure. Purification was carried out by column chromatography on silica gel, using hexanes/ethyl acetate (90/10, v/v). Orange solid, yield (71 %); m.p. 81-83 oC; 1HNMR (DMSO-d6) ^ 1.84-1.88 (m, 2 H), 1.97-2.00 (m, 2 H), 3.62 (t, J = 6.4 Hz, 2 H), 4.06 (t, J = 6.4 Hz, 2 H), 7.03-7.05 (m, 3 H), 7.40 (d, J = 3.6 Hz, 1 H), 7.80 (d, J =8.4 Hz, 2H), 8.02 (d, J =8.8 Hz, 2H), 8.29 (d, J = 8.8 Hz, 2H).
[00108] 2-(4-(4-(bromobutoxy)phenyl)-5-(4-nitrophenyl)furan (2 mmol) was added to a solution of the azole (2 mmol) and NaH (2.5 mmol) in dry DMF (10 mL) under a nitrogen atmosphere. The reaction mixture was stirred at room temperature for 12 h, then poured on ice- water (50 mL), filtered and dried. Purification was conducted by recrystallization from ethyl acetate/hexanes. Yellow solid, yield (71 %); m.p. 75-78 oC; 1HNMR (DMSO-d6) ^ 1.66 (br s, 2H), 1.85 (br s, 2H), 4.02-4.05 (m, 4 H), 6.90 (br s, 1H), 7.01-7.04 (m, 3 H), 7.20 (br s, 1H), 7.41 (d, J = 3.6 Hz, 1 H), 7.66 (br s, 1H), 7.79 (d, J = 8.4 Hz, 2 H), 8.02 (d, J = 8.8 Hz, 2H), 8.27 (d, J = 8.4 Hz, 2H); HRMS: m/z calculated for C23H22N3O4: 404.1610, found: 404.1595 (M+ +1). 4-(5-(4-(4-(1H-azol-1-yl)bu
[00109] Pd/C (10 %) (0.2 gm) was added to a de-aerated solution of 1-(4-(4-(5-(4- nitrophenyl)furan-2-yl)phenoxy)butyl)-1H-imidazole (5 mmol) in ethyl acetate/ethanol (60 mL: 20 mL) mixture. Stirring in a Parr hydrogenator under 50 atmosphere until the uptake of hydrogen ceased, the consumption of hydrogen gave a clear solution. The solution was filtrated through celite, and the filtrate was removed under reduced pressure, the residue formed was used directly in the next step without further purification (the amine is easily oxidized and decomposes on standing). White solid, yield (94 %); m.p.62-63 oC; 1HNMR (CDCl3) ^ 1.80 (br s, 2 H), 202-204 (m, 2 H), 3.77 (s, 2 H), 4.00-4.06 (m, 4 H), 6.52 (d, J = 3.6 Hz, 1 H), 6.57 (d, J = 3.6 Hz, 1 H), 6.74 (d, J = 8.4 Hz, 2 H), 6.91 (d, J = 8.4 Hz, 2 H), 6.96 (br s, 1 H), 7.09 (br s, 1H), 7.53 (br s, 1H), 7.56 (d, J = 8.8 Hz, 2 H), 7.66 (d, J = 8.8 Hz, 2 H); HRMS: m/z calculated for C23H24N3O2: 374.1869, found: 374.1857 (M+ +1).
N-(4-(5-(4-(4-(1H-imidazol-1-yl)butoxy)phenyl)furan-2-yl)phenyl)picolinimidamide hydrochloride
[00110] S-(2-Naphthylmethyl)-2-pyridyl thioimidate hydrobromide (1.87 mmol) was added to a cooled solution of 4-(5-(4-(4-(1H-azol-1-yl)butoxy)phenyl)furan-2-yl)aniline (1.7 mmol) in dry ethanol (30 mL) in an ice bath. The reaction mixture was stirred at room temperature for overnight. After the disappearance of the starting material, the organic solvent was evaporated under reduced pressure to yield a crude oil product. Dry ether (100 mL) was added to the crude material and the mixture was stirred at room temperature for 1 h. The precipitate was filtered and washed with dry ether. The solid was dissolved in ethanol (2 mL); the solution was cooled to 0 oC in an ice bath and 10% NaOH was added until pH reached approximately 10. The free base was extracted with ethyl acetate (3 × 50 mL). The organic layer was washed with distilled water, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The resulting suspension was crystallized by adding dry hexanes and then filtered. The free base was suspended in dry ethanol (20 mL) and cooled to 0 oC in an ice bath. Freshly prepared ethanolic HCl solution (2 mL) was added to the suspension and the mixture was stirred at room temperature for overnight. The resulting red solution was concentrated under reduced pressure. The red crude solid was recrystallized twice from dry ethanol and dry ether and filtered. Yellow solid, yield (57 %); m.p. 83-85 oC Dec.; 1HNMR (DMSO-d6) ^ 1.71 (t, J = 6 Hz, 2 H), 1.97-2 (m, 2 H), 4.06 (t, J = 6 Hz, 2 H), 4.30 (t, J = 6 Hz, 2 H), 6.98 (d, J = 3.2 Hz, 1 H), 7.02 (d, J = 8.8 Hz, 2 H), 7.19 (d, J = 3.2 Hz, 1 H), 7.56 (d, J = 8.4 Hz, 2 H), 7.72 (br s, 2 H), 7.78 (d, J = 8.4 Hz, 2 H), 7.86 (d, J = 8.8 Hz, 2 H), 7.98 (d, J = 8 Hz, 1 H), 8.21-8.25 (m, 1 H), 8.51(d, J = 7.6 Hz, 1 H), 8.91 (br s, 1H), 9.26 (br s, 1H), 9.37 (s, 1H), 10.15 (s, 1H), 11.91 (s, 1 H); 13CNMR (DMSO-d6) ^ 24.9, 25.9, 47.7, 66.4, 106.2, 108.8, 114.4, 119.3, 121.5, 122.4, 122.9, 123.6, 124.6, 124.8, 125.9, 128, 129.7, 132.6, 134.7, 137.8, 144, 150.5, 157.8, 158.9; HRMS: m/z calculated for C29H28N5O2: 478.2238, found: 478.2216 (base M+ +1); Anal. Calcd. For C29H27N5O2. 2 HCl. 1.75 H2O: C, 59.85; H, 5.63; N, 12.03; Found: C, 59.35; H, 5.77; N, 11.83.
Example 3B: Synthesis of Phenyl-Substituted Diphenylfuran Alkyloxy Linker Anti-Fungal Compounds
[00111] FIG. 3B is a synthetic scheme for various phenyl substituted diphenylfuran alkyloxy linker hybrid compounds, each of which may be prepared, for example, from a suitably substituted aryl boronate according to FIG. 3A. Alternatively, compounds may be prepared from the corresponding phenol and bromoalkyl imidazole, as shown in FIG. 3B. The phenol may be prepared from a phenol protected aryl boronate according to FIG. 3A, followed by subsequent deprotection.
[00112] Compound 4j was made according to FIG. 3B. Generally, reagents and conditions for synthesis of compounds according to FIG. 3B included: a) Pd(PPh3)4, dioxane, 90°C; b) NBS, DMF, rt; c) K2CO3, Cs2CO3, DMA, rt; d) Pd(PPh3)4, K2CO3, MeOH, toluene, 80°C; e) imidazole, NaH, DMF, rt; f) H2, Pd(C), EtOH-EtOAc; g) (i) S-(2-naphthylmethyl)-2- pyridylthioimidate hydrobromide, EtOH; (ii) NaOH.
Example 2C: Synthesis of Phenyl-Substituted Diphenylfuran Alkyloxy Linker Anti-Fungal Compounds
[00113] FIG. 2C is a synthetic scheme for various phenyl substituted diphenylfuran alkyloxy linker hybrid compounds. For example, Compounds 2h, 4k, 13b, and 13c were made according to FIG.2C. Generally, reagents and conditions for synthesis of compounds according to FIG. 2C included: aa) Pd(PPh3)4, dioxane, 90°C; b) NBS, DMF, rt; c) K2CO3, Cs2CO3, DMA, rt; d) Pd(PPh3)4, K2CO3, MeOH, toluene, 80°C; e) imidazole, NaH, DMF, rt; f) H2, Pd(C), EtOH-EtOAc; g) (i) S-(2-naphthylmethyl-2-pyridylthioimidate hydrobromide, EtOH; (ii) NaOH. Example 2D (Prophetic): Synthesis of Diphenylfuran Alkyloxy Linker Anti-Fungal Compounds
[00114] FIG. 2D is a prophetic synthetic scheme for various diphenylfuran alkyloxy linker hybrid Compounds 4a-i, each of which may be prepared, for example, from a suitably substituted aryl boronate according to FIG. 2A. Alternatively, Compounds 4a-i may be prepared from the corresponding phenol and bromoalkyl imidazole, as shown in FIG. 2D. The phenol may be prepared from a phenol protected aryl boronate according to FIG. 2A, followed by subsequent deprotection.
Example 3: Synthesis of Alkylamide Linker Anti-Fungal Compounds
[00115] FIG.3 is a synthetic scheme for various alkylamide linker hybrid Compounds 5a-f, 6a-f, and 7a-f. For example, precursor carboxylic acids were prepared similarly to FIG. 2A by a cross-coupling of a benzylalcohol boronate ester with 2-(4-nitrophenyl)furan, nitro reduction, and imidamide formation. Oxidation of the benzyl alcohol provided the carboxylic acid compound shown in FIG.3, and subsequent peptide coupling with the illustrated imidazoyl amine is proposed to afford Compounds 5a and 5c.
[00116] Similarly, for example, Compound 6a may be prepared from 2-(4- nitrophenyl)furan, followed by nitro reduction and imidamide formation. Bromination of the resulting furanyl compound followed by a metal-mediated carboxylation may provide the carboxylic acid shown in FIG. 3. Subsequent peptide coupling with the illustrated imidazoyl amine may afford Compound 6a. Similarly, for example, Compound 7a may be prepared from a suitable 4-aminobenzoic acid derivative. Imidamide formation may provide the carboxylic acid
compound shown in FIG. 3, and subsequent peptide coupling with the illustrated imidazoyl amine may provide Compound 6a.
Example 4A (Prophetic): Synthesis of Phenylalkyl Linker Anti-Fungal Compounds
[00117] FIG.4A is a prophetic synthetic scheme for various phenylalkyl linker hybrid Compounds 8a and 8b. For example, Compound 8a and 8b may be prepared from phenyllithium and the corresponding dibromoalkane, as shown in FIG. 4A. Nitration of the resulting alkylaryl bromide followed by displacement of the bromide with imidazole can provide the nitrophenyl alkylimidazole. Subsequent reduction of the nitro group to the amine, followed by amidine synthesis with naphthalene-2-ylmethylpyridine-2-carbimidothioate may provide Compounds 8a and 8b.
Example 4B: Synthesis of Alkyl Linker Anti-Fungal Compounds
[00118] FIG. 4B is a synthetic scheme for various phenylalkyl linker hybrid Compounds 9a-9d. The synthesis of Compound 9d is representative, as follows.
Benzyl (8-aminooctyl)car m
[00119] Benzyl chloroformate (1 g, 5.86 mmol) was added to 10 equivalents of 1,8- diaminooctane (8.45 g, 58.6 mmol) dissolved in 60 mL dry DCM/EtOH (1:1) in an ice bath. The mixture was allowed to stir for 3 hours at 0 ºC and left to stir overnight at room temperature. The mixture was then filtered and partitioned between DCM (100 mL) and water (50 mL) and brine (50 mL) then dried over sodium sulfate. The solution was evaporated under reduced pressure and the crude product was purified by column chromatography using DCM/MeOH/TEA (100:6:0.7) as eluent to obtain benzyl (8-aminooctyl)carbamate as a yellow oil, 0.95 g, yield 58%. 1H NMR (300 MHz, CDCl3) ^ 1.31-1.50 (m, 12 H), 2.68 (t, J = 6.8 Hz, 2H), 3.16-3.22 (m, 2H), 4.78 (br s, 1H), 5.10 (s, 2H), 7.31-7.37 (m, 5H).
[00120] Aqueous glyoxal (40%, 0.88 mL, 6.19 mmol), ammonium acetate (0.48 g, 6.19 mmol), and aqueous formaldehyde solution (37% w/v, 0.51 mL, 6.19 mmol) were added to benzyl (8-aminooctyl)carbamate (0.87 g, 3.12 mmol) in methanol (8 mL). The reaction was heated to reflux overnight then the solution was evaporated under reduced pressure. The pH was rendered alkaline by the addition of 2N NaOH and the mixture was extracted with DCM (100
mL). The organic phase was dried over sodium sulfate and the liquid was evaporated under reduced pressure. The product was obtained after column chromatography purification over neutralized silica gel with DCM/MeOH (100:1) as a brown oil of the protected aminoalkyl imidazole, 0.48 g. A dry 25 mL two-necked flask was charged with Pd/C (10%, 0.15 g) and the protected amine (0.48 g, 1.45 mmol) in 20 mL absolute ethanol under nitrogen. The mixture was stirred under 1 atmosphere of hydrogen for 24 hours. The mixture was filtered and ethanol was removed under reduced pressure to obtain the crude product which was purified by column chromatography over silica gel with DCM/MeOH/TEA (100:15:0.1) to obtain 8-(1H-imidazol-1- yl)octan-1-amine as a yellow oil, 0.15 g, overall yield 25% from benzyl (8- aminooctyl)carbamate. 1H NMR (300 MHz, CDCl3) ^ 1.31 (br s, 8H), 1.42-1.46 (m, 2H), 1.76- 1.85 (m, 4H), 2.69 (t, J = 6.7 Hz, 2H), 3.93 (t, J = 7 Hz, 2H), 6.91 (t, J = 1.1 Hz, 1H), 7.06 (br t, J = 0.9 Hz, 1H), 7.47 (s, 1H).
[00121] S-(2-Naphthylmethyl)-2-pyridyl thioimidate hydrobromide (0.40 g, 1.11 mmol) was added to a cooled solution of 8-(1H-imidazol-1-yl)octan-1-amine (0.1 g, 0.51 mmol) in dry ethanol:acetonitrile (7:3) (10 mL) in an ice bath. The reaction mixture was stirred at room temperature for 48 hours. After the disappearance of the starting material, the organic solvent was evaporated under reduced pressure to yield a crude oil. Dry ether (50 mL) was added to the crude material and the mixture was stirred at room temperature overnight. The precipitate was filtered and washed with dry ether. The solid was dissolved in ethanol (2 mL); the solution was cooled to 0 oC in an ice bath and 10% NaOH was added until the pH reached approximately 10. The free base was extracted with ethyl acetate (3 × 25 mL). The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The resulting suspension was purified by column chromatography over silica gel using DCM/MeOH/TEA (9.5:1.2:0.6) as eluent then further purified by 5 mL hexanes/diethyl ether (1:1) to yield a buff powder, 0.085 g, 55%. 1H NMR (400 MHz, CDCl3) ^ 1.28-1.45 (m, 8H), 1.67-1.82 (m, 4H), 3.29 (t, J = 6.9 Hz, 2H), 3.93 (t, J = 7 Hz, 2H), 6.04 (br s, 2H), 6.91 (t, J = 1.2 Hz, 1H), 7.06 (t, J = 1 Hz, 1H), 7.36 (ddd, J1 = 7.6 Hz, J2 = 4.8 Hz, J3 = 1.2 Hz, 1H), 7.48 (br s, 1H), 7.79 (td, J1 = 7.8 Hz, J2 = 1.7, 1H), 8.23 (d, J = 7.8, 1H) 8.55 (ddd, J1 = 4.8 Hz, J2 = 1.7 Hz, J3 = 0.95 Hz, 1H). 13C NMR (100 MHz, CDCl3) ^ 26.48, 27.19, 28.97, 29.17, 29.44, 31.04, 42.55, 47.00, 118.74, 120.83, 124.85, 129.40, 136.89, 137.06, 147.82, 151.49. HRMS: 300.21707 [M+ H+].
Example 5A: Synthesis of Unsubstituted and Certain Substituted Biphenyl Linker Anti- Fungal Compounds
[00122] FIG. 5A is a synthetic scheme for various unsubstituted and substituted biphenyl linker anti-fungal compounds, which was used to produce Compounds 10a, 10d, 10e, 10h, 10k, and 10l. Biphenyl linkers in these compounds were either unsubstituted or were substituted meta to the amidine group. Generally, reagents and conditions for synthesis of compounds according to FIG. 5A included: dibromoalkane, K2CO3, CH3CN, reflux; b) imidazole, K2CO3, CH3CN, reflux; c) 2-alkoxy-4-nitroiodobenzene, Pd(dppf)Cl2, K2CO3, DMSO, 100°C; d) SnCl2.2H2O, EtOAc, reflux; e) S-(2-naphthylmethyl)-2-pyridylthioimidate hydrobromide, CH3CN/EtOH (1:3), rt.
Synthesis of 2-(4-(4-bromobutoxy)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane.
[00123] Reaction of 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenol (2.00 g, 9.1 mmol), 1,4-dibromobutane (5.42 mL, 9.80 g, 45.4 mmol), and potassium carbonate (2.5 g, 18.2 mmol) was performed in dry acetonitrile under reflux overnight followed by filtration and evaporation of the filtrate under reduced pressure. Purification of the crude product was performed by column chromatography on silica gel using hexane to hexane/ethyl acetate (10:1) to afford a white solid which was further crystallized from methanol to yield the product as a white crystalline solid. Yield 2.42 g (75%), mp = 62-64 °C, lit mp 64-66 °C.1 The 1H NMR spectrum of this material was consistent with the literature report.
Synthesis of 1-(4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenoxy)butyl)-1H-imidazole.
[00124] Reaction of 2-(4-(4-bromobutoxy)phenyl)-4,4,5,5-tetramethyl-1,3,2- dioxaborolane (1.38 g, 3.9 mmol), imidazole (0.52 g, 7.8 mmol), and potassium carbonate (1.07 g, 7.8 mmol) was performed in dry acetonitrile under reflux overnight followed by filtration and evaporation of the filtrate under reduced pressure. Purification of the crude product was performed by column chromatography on silica gel using DCM: MeOH (25:1) to afford a white solid which was further crystallized from the ethyl acetate/hexane to yield the product as white crystalline solid. Yield 0.75 g (56%), mp=113-115 °C. 1H NMR (400 MHz, CDCl3) ^1.35 (s, 12H), 1.78-1.83 (m, 2H), 1.97-2.04 (m, 2H), 4.00-4.06 (m, 4H), 6.88 (d, J=6.8 Hz, 2H), 6.95 (s, 1H), 7.09 (s, 1H), 7.51 (s, 1H), 7.76 (d, J= 6.8 Hz, 2H).
Synthesis of 1-(4-((4'-nitro-[1,1'-biphenyl]-4-yl)oxy)butyl)-1H-imidazole.
[00125] 1-(4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenoxy)butyl)-1H- imidazole (0.35 g, 1.02 mmol), 4-iodonitrobenzene (0.35 g, 1.4 mmol), and tetrakis(triphenylphosphine)palladium(0) (59 mg, 0.05 mmol) were added to a three necked flask. Degassed dry DMF (10 ml) was added and the flask was purged with nitrogen. Degassed potassium carbonate (1 mL of a 2M aqueous solution) was added and the flask was heated to reflux overnight. The reaction mixture was filtered over celite, then the filtrate was evaporated under reduced pressure and purified by column chromatography on silica gel using hexanes/ethyl acetate (1:3) as the eluent to afford the pure product as a yellow powder, yield 0.25 g (73%), mp=113-115 °C.1H NMR (400 MHz, CDCl3) ^1.82-1.88 (m, 2H), 2.01-2.08 (m, 2H), 4.03-4.09 (m, 4H), 6.97-7.01 (m, 3H), 7.10 (s, 1H), 7.53-7.60 (m, 3H), 7.70 (d, J= 8.8 Hz, 2H), 8.28 (d, J=8.8 Hz, 2H).
Synthesis of 4'-(4-(1H-imidazol-1-yl)butoxy)-[1,1'-biphenyl]-4-amine.
[00126] A mixture of 1-(4-((4'-nitro-[1,1'-biphenyl]-4-yl)oxy)butyl)-1H-imidazole (0.23 g, 0.68 mmol) and tin chloride dihydrate (0.77 g, 3.40 mmol) in ethyl acetate was heated at reflux overnight. The reaction mixture was cooled, then aqueous sodium hydroxide (2N) was added until the pH reached 11. The product was then extracted with ethyl acetate (25 mL × 3), dried over anhydrous sodium sulfate, filtered over celite, and evaporated under reduced pressure to yield the product as a buff powder which was taken directly to the next step without further purification, yield 0.19 g (91%). 1H NMR (300 MHz, CDCl3) ^ 1.76-1.85 (m, 2H), 1.97-2.07 (m, 2H), 3.68 (br s, 2H), 3.99-4.07 (m, 4H), 6.74-6.77 (m, 2H), 6.91-6.95 (m, 3H), 7.09 (s, 1H), 7.35-7.38 (m, 2H), 7.45-7.47 (m, 2H), 7.52 (s, 1H).
Synthesis of N-(4'-(4-(1H-imidazol-1-yl)butoxy)-[1,1'-biphenyl]-4-yl)picolinimidamide.
[00127] S-(2-Naphthylmethyl)-2-pyidyl thioimidate hydrobromide (0.40 g, 1.1 mmol) was added to a cooled solution of 4'-(4-(1H-imidazol-1-yl)butoxy)-[1,1'-biphenyl]-4-amine (0.15 g, 0.48 mmol) in dry ethanol: acetonitrile (7:3, 10 mL) in an ice bath. The reaction mixture was stirred at room temperature for 48 hours. After the disappearance of the starting material, the
organic solvent was evaporated under reduced pressure to yield the product as an oil. Dry diethyl ether (100 mL) was added to the crude material and the mixture was stirred at room temperature overnight. The precipitate was filtered and washed with dry diethyl ether. The solid was dissolved in ethanol (2 mL), then the solution was cooled to 0 oC in an ice bath and 10% NaOH was added until pH reached approximately 10. The free base was extracted with ethyl acetate (3 × 25 mL). The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The resulting suspension was purified by column chromatography over silica gel (neutralized by washing with trimethylamine) using DCM:MeOH (200:1 to 50:1) as eluent then further purified by crystallization from hexanes/ethyl acetate to yield yellow crystals, 0.135 g (68%). 1H NMR (300 MHz, CDCl3) ^ 1.80-1.87 (m, 2H), 1.99-2.06 (m, 2H), 4.02-4.09 (m, 4H), 5.91 (brs, 2H), 6.95-6.98 (m, 3H), 7.08-7.10 (m, 3H), 7.42 (ddd, J1=1.1 Hz, J2=4.9 Hz, J3=6.8 Hz, 1H), 7.53-7.60 (m, 5H), 7.84 (td, J1=1.7 Hz, J2=7.7 Hz, 1H), 8.46 (d, J=7.7 Hz, 1H), 8.59-8.61 (m, 1H).
Example 5B: Synthesis of Certain Substituted Biphenyl Linker Anti-Fungal Compounds
[00128] FIG. 5B is a synthetic scheme for various substituted biphenyl linker anti- fungal compounds, which was used to produce Compounds 10b, 10c, 10i, and 10j. Biphenyl linkers in these compounds were substituted ortho to the amidine group. Generally, reagents and conditions for synthesis of compounds according to FIG. 5B included: a) dibromoalkane, K2CO3, CH3CN, reflux; b) imidazole, K2CO3, CH3CN, reflux; c) 23a,b, Pd[P(Ph)3]4, K2CO3, DMF, 100°C; d) SnCl2.2H2O, EtOAc, reflux; e) S-(2-naphthylmethyl)-2-pyridylthioimidate hydrobromide, CH3CN/EtOH (1:3), rt.
Example 5C: Synthesis of Certain Substituted Biphenyl Linker Anti-Fungal Compounds
[00129] FIG. 5C is a synthetic scheme for various substituted biphenyl linker anti- fungal compounds, which was used to produce Compounds 10f and 10m. Biphenyl linkers in these compounds were substituted meta to the alkoxy linking group. Generally, reagents and conditions for synthesis of compounds according to FIG.5C included: a) TsCl, K2CO3, acetone, reflux; b) MeI, K2CO3, sealed tube, CH3CN, reflux; c) bis(pinocolato)diboron, Pd(dppf)Cl2, AcOK; d) 4-nitro-iodobenzene, Pd(dppf)Cl2, dimethoxyethane/DMF/water (7:3:1), reflux; e) aq NaOH, EtOH/DMSO; f) 1,4-dibromobutane or 1,6-dibromohexane, K2CO3, CH3CN, reflux; g) imidazole, K2CO3, CH3CN, reflux; h) SnCl2.2H2O, EtOAc, reflux; i) S-(2-naphthylmethyl)-2- pyridylthioimidate hydrobromide, CH3CN/EtOH (1:3), rt.
Example 5D: Synthesis of Certain Substituted Biphenyl Linker Anti-Fungal Compounds
[00130] FIG. 5D is a synthetic scheme for various substituted biphenyl linker anti- fungal compounds, which was used to produce Compounds 10g and 10n. Biphenyl linkers in
these compounds were substituted ortho to the alkoxy linking group. Generally, reagents and conditions for synthesis of compounds according to FIG. 5D included: a) TsCl, acetonitrile, K2CO3, reflux ; b) Bis(pinacolato)diboron, Pd(dppf)Cl2, AcOK, dioxane, 100°C; c) 1-Iodo-4- nitrobenzene, Pd[P(Ph)3]4 or Pd(dppf)Cl2, K2CO3, DME:DMF:H2O (7:3:1),100°C; d) aq NaOH, EtOH/DMSO; e) dibromoalkane, acetonitrile, K2CO3, reflux; f) Imidazole, K2CO3. reflux; g) SnCl2.2H2O, ethyl acetate, reflux; h) S-(2-naphthylmethyl)-2-pyridylthioimidate hydrobromide, CH3CN/EtOH (1:3), rt.
Example 6A: Synthesis of Phenyl-Piperazinyl-Phenyl Linker Anti-Fungal Compounds
[00131] FIG. 6A is a synthetic scheme for various phenyl-piperazinyl-phenyl linker anti-fungal compounds, which was used to produce Compounds 11a-11d. Generally, reagents and conditions for synthesis of compounds according to FIG. 6A included: a) NMP, DIPEA, 4- chloronitrobenzene; b) Į,^-dihaloalkane, K2CO3, acetone or Cs2CO3, DMF; c) imidazole or 1,2,4-triazole, Cs2CO3, DMF; d) Pd/C, H2, EtOAc/MeOH; e) S-(2-naphthylmethyl-2- pyridylthioimidate hydrobromide, CH3CN/EtOH.
Example 6B: Synthesis of Phenyl-Piperazinyl Linker Anti-Fungal Compounds
[00132] FIG. 6B is a synthetic scheme for various phenyl-piperazinyl linker anti- fungal compounds, e.g., corresponding to Structural Formula (XVII). Generally, reagents and conditions for synthesis of compounds according to FIG. 6B included: a) K2CO3/DMSO; b) Į,^-dihaloalkane, e.g., 1,6-dibromohexane, Cs2CO3, DMF; c) imidazole, Cs2CO3, DMF; d) Pd/C, H2, MeOH; e) S-(2-naphthylmethyl-2-pyridylthioimidate hydrobromide, CH3CN/EtOH. The synthesis of Compound 12a, N-(4-(4-(6-(1H-imidazol-1-yl)hexyl)piperazin-1- yl)phenyl)picolinimidamide, is representative, as follows.
[00133] 4-Chloro nitrobenzene (1.57 g, 10 mmol, 1 eq), piperazine (1.12 g, 13 mmol, 1.3 eq), potassium carbonate (2.07 g, 15 mmol, 1.5 eq) and tetra-N-butyl ammonium iodide (37 mg, 0.1 mmol, 0.01 eq) were added to a reaction vessel, then dimethyl sulfoxide (25 mL) was added under an inert atmosphere. The resulting suspension was heated at 120 oC overnight. The reaction mixture was cooled, diluted with ice water (40 mL), neutralized with 2N hydrochloric acid and then extracted with ethyl acetate (30 mL x 3). The combined organic layers were washed with water and brine, dried over Na2SO4, and concentrated under reduced pressure to yield 1-(4-nitrophenyl)piperazine as a yellow solid. Yield = 1.65 g, 80%; 1H NMR (CDCl3, 500
MHz) į 3.01-3.03 (t, 4H, J = 5.15 Hz), 3.37-3.39 (t, 4H, 4.9 Hz), 6.81-6.82 (d, 2H, J = 9.3 Hz), 8.11-8.13 (d, 2H, J = 9.35 Hz).
[00134] 1 -(4-nitrophenyl)piperazine (1.03 g, 5 mmol, 1 eq), cesium carbonate (25 mmol, 8.14 g, 5 eq) were added to dry dimethylformamide (25 mL) under an inert atmosphere. To the resulting suspension was added 1,6-dibromohexane (1.02 mL, 6.6 mmol, 1.3 eq) and the mixture was stirred at room temperature for 24 hours. On completion, the reaction mixture was extracted with ethyl acetate (25 mL x 2). The combined organic layers were evaporated under reduced pressure and the resulting liquid was purified by column chromatography using ethyl acetate/hexane (1:1) as an eluent. Fractions containing the main product were combined and evaporated to dryness to give 1-(6-bromohexyl)-4-(4-nitrophenyl)piperazine as a yellow solid. Yield = 550 mg, 30%; 1H NMR (CDCl3, 500 MHz) į 1.37-1.42 (m, 2H), 1.47-1.59 (m, 4H), 1.87-1.93 (m, 2H), 2.40-2.43 (t, 2H, J= 7.45 Hz), 2.59-2.61 (t, 4H, J = 5.2 Hz), 3.43-3.46 (m, 6H), 6.83-6.86 (d, 2H, J = 9.4 Hz), 8.13-8.15 (d, 2H, J = 9.4 Hz).
Step 3: Synthesis of 1-(6-(1H-imidazol-1-yl)hexyl)-4-(4-nitrophenyl)piperazine
[00135] 1-(6-bromohexyl)-4-(4-nitrophenyl)piperazine (518 mg, 1.4 mmol, 1 eq), 1H- imidazole (143 mg, 2.1 mmol, 1.5 eq) and cesium carbonate (2.28 g, 7 mmol, 5 eq) were added to a reaction vessel, then dry dimethylformamide (10 mL) was added. The reaction was stirred at 50 °C for 6 hours, then the reaction mixture was cooled to rt, diluted with water and extracted with ethyl acetate. The combined organic layers were concentrated under reduced pressure and purified by column chromatography using dichloromethane/methanol (95:5) as an eluent. Fractions containing the product were combined and evaporated to dryness to give 1-(6-(1H- imidazol-1-yl)hexyl)-4-(4-nitrophenyl)piperazine as a brown solid. Yield = 365 mg, 73%; 1H NMR (CDCl3, 500 MHZ) į 1.31-1.37 (m, 4H), 1.48-1.54 (m, 2H), 1.76-1.82 (m, 2H), 2.34-2.37 (t, 2H, J = 7.4 Hz), 2.55-2.57 (t, 4H, J = 5.0 Hz), 3.41-3.43 (t, 4H, J = 4.9 Hz), 3.92-3.95(t, 2H, J = 7.0 Hz), 6.80-6.82 (d, 2H, J = 9.3 Hz), 6.9 (s, 1H), 7.05 (s, 1H), 7.46 (s, 1H), 8.10-8.12 (d, 2H, J = 9.25 Hz).
Step 4: 4-(4-(6-(1H-imidazol-1-yl)hexyl)piperazin-1-yl)aniline
[00136] 1-(6-(1H-imidazol-1-yl)hexyl)-4-(4-nitrophenyl)piperazine (322 mg, 0.9 mmol) was dissolved in methanol (30 mL). This mixture was hydrogenated at 40-45 psi using 10% Pd/C as catalyst for 30 minutes in a Parr hydrogenator. The reaction mixture was filtered through a bed of celite and the filtrate was concentrated to give 4-(4-(6-(1H-imidazol-1- yl)hexyl)piperazin-1-yl)aniline as a brown solid. Yield = 275 mg, 93%; 1H NMR (DMSO-d6, 500 MHz) į 1.18-1.24 (m, 2H), 1.26-1.32 (m, 2H), 1.39-1.43 (m, 2H), 1.67-1.73 (m, 2H), 2.25- 2.27 (t, 2H, J = 7.2 Hz), 2.44 (s, 4H), 2.88 (s, 4H), 3.92-3.95 (t, 2H, J = 7.0 Hz), 4.53 (s, 2H), 6.47-6.49 (d, 2H, J = 8.55 Hz), 6.66-6.68 (d, 2H, J = 8.65 Hz), 6.87 (s, 1H), 7.15 (s, 1H), 7.6 (s, 1H).
Step 5: Synthesis of N-(4-(4-(6-(1H-imidazol-1-yl)hexyl)piperazin-1-yl)phenyl) picolinimidamide
[00137] S-(2-Naphthylmethyl)-2-pyridyl thioimidate hydrobromide (216 mg, 6 mmol, 1.2 eq) was added to an ice cooled solution of 4-(4-(6-(1H-imidazol-1-yl)hexyl)piperazin-1- yl)aniline (164 mg, 0.5 mmol, 1 eq) in a mixture of absolute ethanol/acetonitrile (2:1, 15mL). The resulting mixture was stirred at room temperature for 24 hours. Upon completion of the reaction indicated by TLC, the solvent was evaporated under reduced pressure. To the crude residue was then added dry diethyl ether (50 mL) and the resulting suspension was stirred at room temperature for 6 hours. The separated solid was filtered and washed with dry diethyl ether. The solid was suspended in cold ethanol (5 mL); the mixture was placed in an ice bath and 10% NaOH was added until a pH of approximately 10 was reached. The free base was extracted with ethyl acetate (3 × 20 mL). The organic layer was washed with distilled water, dried over anhydrous K2CO3, filtered and concentrated under reduced pressure. Dry hexane was added to the resulting suspension, which was then filtered to provide the product as the free base (light yellow solid). Yield = 108 mg, 50%; 1H NMR (DMSO-d6, 500 MHZ) į 1.20-1.26 (m, 2H), 1.28-1.34 (m, 2H), 1.41-1.46 (m, 2H), 1.68-1.74 (m, 2H), 2.27-2.30 (t, 2H, J = 7.2 Hz), 2.49 (s, 4H), 3.07 (s, 4H), 3.93-3.96 (t, 2H, J = 7.1 Hz), 6.36-6.66 (br, 2H), 6.83-6.84 (d, 2H, J = 8.7 Hz), 6.88 (s, 1H), 6.92-6.94 (d, 2H, J = 8.8 Hz), 7.16 (s, 1H), 7.52-7.54 (dd, 1H, J = 5.5, 7.1 Hz),
7.61 (s, 1H), 7.91-7.94 (td, 1H, J = 1.6, 7.8 Hz), 8.29-8.30 (d, 1H, J = 7.9 Hz), 8.61-8.62 (d, 1H, J = 4.5 Hz); 13C NMR (DMSO-d6, 125 MHz) į 26.32, 26.63, 26.87, 31.01, 46.35, 49.49, 53.40, 58.25, 117.10, 119.70, 121.57, 122.60, 125.66, 128.74, 137.48, 137.66, 142.34, 147.15, 148.41, 151.94, 152.23; HRMS exact mass of (M+H)+, 432.2876 amu; observed mass of (M+H)+, 432.2878 amu.
Example 7: Biological Evaluation of Phenoxyalkyl Linker Anti-Fungal Compounds
[00138] Selected compounds were tested to determine IC50 values in an assay using one of Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, and Histoplasma capsulatum following procedures established by the Clinical & Laboratory Standards Institute (CLSI protocols M38-A2 and M27-A3) for antifungal susceptibility testing or modifications for microdilution-based testing of Histoplasma capsulatum (Goughenour, et al.“Quantitative Microplate-Based Growth Assay for Determination of Antifungal Susceptibility of Histoplasma capsulatum Yeasts,” J Clin Microbiol 2015, 53, 3286–3295, the entire teachings of which are incorporated herein by reference). A two-fold concentration dilution series of each compound was added to 96-well microtiter plates in RPMI medium buffered to pH 7.0 with 20 mM HEPES. To the drug concentration series, fungal cells were added as follows: Aspergillus fumigatus conidia (final concentration 5000 per mL), Candida albicans yeasts (final concentration 2500 per mL), Cryptococcus neoformans (final concentration 10,000 per mL), Histoplasma capsulatum (final concentration 2,000,000 per mL). Plates were mixed and incubated at 37 degrees Celsius for 24 hours (Candida albicans), 48 hours (Aspergillus fumigatus and Cryptococcus neoformans), or 96 hours (Histoplasma capsulatum). Fungal growth was determined by reading the optical density at 595 nm (Cryptococcus neoformans) or by addition of 0.1 M resazurin (7- hydroxy-10-oxidophenooxazin-10-ium-3-one) and measurement of the conversion to resorufin (7-hydroxyphenoxazin-3-one) by measurement of resorufin fluorescence (530 nm excitation, 590 nm emission wavelengths) for Candida albicans, Aspergillus fumigatus, and Histoplasma capsulatum. Relative fungal growth was determined by normalization to growth in the absence of any drug compound.
[00139] FIG.7 is a table demonstrating IC50 values in μM in Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, and Histoplasma capsulatum for Compounds 1c, 1e, 1f, 1i, and 1m.. All tested compounds displayed excellent activity, in most cases demonstrating IC50 values at or less than 10^M.
[00140] FIGs.8A, 8B, 8C, and 8D are graphs showing concentration-dependent inhibition of fungal growth by the indicated compounds in μM for Aspergillus fumigatus, Candida albicans,
Cryptococcus neoformans, and Histoplasma capsulatum, respectively. The graphs in FIGs. 8A, 8B, 8C, and 8D were used to determine the IC50 values in μM shown in the table in FIG.7. Example 8 (Prophetic): Counter-screening The Disclosed Anti-Fungal Compounds For Toxicity
[00141] The disclosed anti-fungal compounds may also be counter-screened for toxicity to J774 macrophages and HepG2 hepatocellular carcinoma cells. J774 cells (103 in 100 ^L) or HepG2 cells (5×103 in 100 ^L) may be incubated for 72 h with serial dilutions of compounds in DMEM (for J774 cells) or RPMI medium (for HepG2 cells) supplemented with 10% fetal bovine serum. MTT may then be added and absorbance at 570 nm may provide an assessment of cell proliferation. Determination of IC50 values in each assay may permit the calculation of a selectivity index (SI, e.g., target fungus IC50 versus mammalian cell line/IC50 versus, e.g., target fungi in genera such as Aspergillus, Candida, Cryptococcus, Histoplasma, and the like) for each target compound.
DEFINITIONS
[00142] As used herein, an“alkyl” group includes straight chain and branched chain alkyl groups having a number of carbon atoms, for example, from 1 to 12, 1 to 10, 1 to 8, 1 to 6, or 1 to 4. Examples of straight chain alkyl groups include groups such as methyl, ethyl, n- propyl, n-butyl, n-pentyl, n-hexyl, n-heptyl, and n-octyl groups. Examples of branched alkyl groups include, e.g., isopropyl, iso-butyl, sec-butyl, tert-butyl, neopentyl, isopentyl, and 2,2- dimethylpropyl groups. Representative substituted alkyl groups may be substituted one or more times with substituents such as those listed above and include, without limitation, haloalkyl (e.g., trifluoromethyl), hydroxyalkyl, thioalkyl, aminoalkyl, alkylaminoalkyl, dialkylaminoalkyl, alkoxyalkyl, or carboxyalkyl.
[00143] As used herein, an“alkoxy” group means a hydroxyl group (-OH) in which the bond to the hydrogen atom is replaced by a bond to a carbon atom of a substituted or unsubstituted alkyl group. Examples of linear alkoxy groups include, e.g., methoxy, ethoxy, propoxy, butoxy, pentoxy, or hexoxy. Examples of branched alkoxy groups include, e.g., isopropoxy, sec-butoxy, tert-butoxy, isopentoxy, or isohexoxy. Examples of cycloalkoxy groups include, e.g., cyclopropyloxy, cyclobutyloxy, cyclopentyloxy, or cyclohexyloxy. Representative substituted alkoxy groups may be substituted one or more times.
[00144] As used herein, a“cycloalkyl” group includes mono-, bi- or tricyclic alkyl groups having from 3 to 12 carbon atoms in each ring, for example, 3 to 10, 3 to 8, or 3 to 4, 5, or 6 carbon atoms. Exemplary monocyclic cycloalkyl groups include, for example, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, and the like. A cycloalkyl group
may have a number of ring carbons of from 3 to 8, 3 to 7, 3 to 6, or 3 to 5. Bi- and tricyclic ring systems may include both bridged cycloalkyl groups and fused rings, e.g., bicyclo[2.1.1]hexane, adamantyl, decalinyl, and the like. Substituted cycloalkyl groups may be substituted one or more times with non-hydrogen and non-carbon groups as defined above. Substituted cycloalkyl groups may include rings that may be substituted with straight or branched chain alkyl groups. Representative substituted cycloalkyl groups may be mono-substituted or substituted more than once, for example, 2,2-, 2,3-, 2,4- 2,5- or 2,6-disubstituted cyclohexyl groups.
[00145] As used herein, a“heterocycloalkyl” ring means an aromatic carbocyclic ring having one or more ring carbon atoms replaced by a heteroatom (e.g., N, S, or O). Non-aromatic heterocyclic rings may have 4, 5, 6, 7, or 8 ring atoms. Examples include oxazolinyl, thiazolinyl, oxazolidinyl, thiazolidinyl, tetrahydrofuranyl, tetrahyrothiophenyl, morpholino, thiomorpholino, pyrrolidinyl, piperazinyl, piperidinyl, thiazolidinyl, and the like.
[00146] As used herein, an“aryl” group means a carbocyclic aromatic hydrocarbon. Aryl groups herein include monocyclic, bicyclic and tricyclic ring systems. Aryl groups include, e.g., phenyl, azulenyl, heptalenyl, biphenyl, fluorenyl, phenanthrenyl, anthracenyl, indenyl, indanyl, pentalenyl, naphthyl, and the like, for example, phenyl, biphenyl, and naphthyl. Aryl groups may contain, for example, 6 to 14, 6 to 12, or 6 to 10 ring carbons. In some embodiments, the aryl groups may be phenyl or naphthyl. Although the phrase“aryl groups” may include groups containing fused rings, such as fused aromatic-aliphatic ring systems (e.g., indanyl or tetrahydronaphthyl), an“aryl” group, unless stated to be substituted or optionally substituted, does not include aryl groups that have other groups, such as alkyl or halo groups, bonded to one of the ring members. Rather, groups such as tolyl may be referred to as substituted aryl groups. Representative substituted aryl groups may be mono-substituted or substituted more than once. For example, monosubstituted aryl groups include, but are not limited to, 2-, 3-, 4-, 5-, or 6-substituted phenyl or naphthyl, which may be substituted with substituents such as those above.
[00147] As used herein, an“aralkyl” group means an alkyl group in which a hydrogen or carbon bond of an alkyl group is replaced with a bond to an aryl group. In some embodiments, aralkyl groups contain 7 to 16 carbon atoms, 7 to 14 carbon atoms, or 7 to 10 carbon atoms. Substituted aralkyl groups may be substituted at the alkyl, the aryl or both the alkyl and aryl portions of the group. Representative aralkyl groups include, e.g., benzyl and phenethyl groups and fused (cycloalkylaryl)alkyl groups such as 4-indanylethyl. Substituted aralkyls may be substituted one or more times.
[00148] As used herein, a“heteroaryl” group means a carbocyclic aromatic ring having one or more ring carbon atoms replaced by a heteroatom (e.g., N, S, or O). Heteroaryl groups may include, for example, imidazolyl, isoimidazolyl, thienyl, furanyl, pyridyl, pyrimidyl, pyranyl, pyrazolyl, pyrrolyl, pyrazinyl, thiazoyl, isothiazolyl, oxazolyl, isooxazolyl, 1,2,3- trizaolyl, 1,2,4-triazolyl, and tetrazolyl. Heteroaryl groups also include fused polycyclic aromatic ring systems in which a carbocyclic aromatic ring or heteroaryl ring is fused to one or more other heteroaryl rings. Examples of heteroaryl groups may include benzothienyl, benzofuranyl, indolyl, quinolinyl, benzothiazolyl, benzoisothiazolyl, benzooxazolyl, benzoisooxazolyl, benzimidazolyl, quinolinyl, isoquinolinyl and isoindolyl.
[00149] Groups described herein having two or more points of attachment (e.g., divalent, trivalent, or polyvalent) within the compound of the technology may be designated by use of the suffix,“ene.” For example, divalent alkyl groups may be alkylene groups, divalent aryl groups may be arylene groups, divalent heteroaryl groups may be heteroarylene groups, and so forth. In particular, certain polymers may be described by use of the suffix“ene” in conjunction with a term describing the polymer repeat unit.
[00150] As used herein,“optionally substituted” means a compound or group that may be substituted or unsubstituted. The term“substituted” refers to an organic group (e.g., an alkyl group) in which one or more bonds to a hydrogen atom contained therein may be replaced by a bond to non-hydrogen or non-carbon atoms. Substituted groups also include groups in which one or more bonds to a carbon or hydrogen atom may be replaced by one or more bonds, including double or triple bonds, to a heteroatom. A substituted group may be substituted with one or more substituents, unless otherwise specified. In some embodiments, a substituted group may be substituted with 1, 2, 3, 4, 5, or 6 substituents.
[00151] Examples of substituent groups include: halogens (F, Cl, Br, and I); hydroxyl; alkoxy, alkenoxy, aryloxy, aralkyloxy, heterocyclooxy, and heterocycloalkoxy groups; carbonyls (oxo); carboxyls; esters; urethanes; oximes; hydroxylamines; alkoxyamines; aralkoxyamines; thiols; sulfides; sulfoxides; sulfones; sulfonyls; sulfonamides; amines; N-oxides; hydrazines; hydrazides; hydrazones; azides; amides; ureas; amidines; guanidines; enamines; imides; isocyanates; isothiocyanates; cyanates; thiocyanates; imines; nitro groups; or nitriles. A“per”- substituted compound or group is a compound or group having all or substantially all substitutable positions substituted with the indicated substituent. For example, 1,6-diiodo perfluoro hexane indicates a compound of formula C6F12I2, where all the substitutable hydrogens have been replaced with fluorine atoms.
[00152] In particular, suitable substituents for an alkyl group, cycloalkyl group, heterocycloalkyl group, or an aryl group ring carbon are those which do not substantially interfere with the activity of the disclosed compounds. Examples include -OH, halogen (-Br, -, - I and -F), -ORA, -O(CO)RA, -(CO)RA, -CN, -NO2, -CO2H, -SO3H, -NH2, -NHRA, -N(RARB), - (CO)ORA, -(CO)H, -CONH2, -CONHRA, -CON(RARB), -NHCORA, -NRCORA, -NHCONH2, - NHCONRAH, -NHCON(RARB), -NRCCONH2, -NRCCONRAH, -NRCCON(RARB), -C(=NH)– NH2, -C(=NH)–NHRA, -C(=NH)–N(RARB), -C(=NRC)–NH2, -C(=NRC)–NHRA, -C(=NRC)– N(RARB), -NH–C(=NH)–NH2, -NH–C(=NH)–NHRA, -NH–C(=NH)–N(RARB), -NH–C(=NRC)– NH2, -NH–C(=NRC)–NHRA, -NH–C(=NRC)–N(RARB), NRDH–C(=NH)–NH2, -NRD–C(=NH)– NHRA, -NRD–C(=NH)–N(RARB), -NRD–C(=NRC)NH2, -NRD–C(=NRC)–NHRA, -NRD– C(=NRC)–N(RARB), -NHNH2, -NHNHRA, -NHRARB, -SO2NH2, -SO2NHRA, -SO2NRARB, - CH=CHRA, -CH=CRARB, -CRC=CRARB, -CRC=CHRA, -CRC=CRB, -CCRA, -SH, -SOkRA (k is 0, 1 or 2) and -NH–C(=NH)–NH2. Each of RA-RD may independently be an aliphatic, substituted aliphatic, benzyl, substituted benzyl, aryl or substituted aryl group, for example, an alkyl, benzylic or aryl group. Further,–NRARD, taken together, may form a substituted or unsubstituted non-aromatic heterocyclic group. A non-aromatic heterocyclic group, benzylic group or aryl group may also have an aliphatic or substituted aliphatic group as a substituent. A substituted aliphatic group may also have a non-aromatic heterocyclic ring, a substituted a non- aromatic heterocyclic ring, benzyl, substituted benzyl, aryl or substituted aryl group as a substituent. A substituted aliphatic, non-aromatic heterocyclic group, substituted aryl, or substituted benzyl group may have more than one substituent.
[00153] Suitable substituents for heteroaryl ring nitrogen atoms having three covalent bonds to other heteroaryl ring atoms may include–OH and C1 to C10 alkoxy. Substituted heteroaryl ring nitrogen atoms that have three covalent bonds to other heteroaryl ring atoms are positively charged, which may be balanced by counteranions such as chloride, bromide, formate, acetate and the like. Examples of other suitable counteranions may include counteranions found in the described pharmacologically acceptable salts.
[00154] Suitable substituents for heteroaryl ring nitrogen atoms having two covalent bonds to other heteroaryl ring atoms include alkyl, substituted alkyl (including haloalkyl), phenyl, substituted phenyl,–S(O)2-(alkyl),–S(O)2–NH(alkyl),–S(O)2–NH(alkyl)2, and the like.
[00155] Also included are pharmaceutically acceptable salts of the compounds described herein. Compounds disclosed herein that possess a sufficiently basic functional group may react with any of a number of organic or inorganic acids to form a salt. Likewise, compounds disclosed herein that possess a sufficiently acidic functional group may react with
any of a number of organic or inorganic bases to form a salt. Acids commonly employed to form acid addition salts from compounds with basic groups may include inorganic acids such as hydrochloric acid, hydrobromic acid, hydroiodic acid, sulfuric acid, phosphoric acid, and the like, and organic acids such as p-toluenesulfonic acid, methanesulfonic acid, oxalic acid, p- bromophenyl-sulfonic acid, carbonic acid, succinic acid, citric acid, benzoic acid, acetic acid, and the like. Examples of such salts may include the sulfate, pyrosulfate, bisulfate, sulfite, bisulfite, phosphate, monohydrogenphosphate, dihydrogenphosphate, metaphosphate, pyrophosphate, chloride, bromide, iodide, acetate, propionate, decanoate, caprylate, acrylate, formate, isobutyrate, caproate, heptanoate, propiolate, oxalate, malonate, succinate, suberate, sebacate, fumarate, maleate, butyne-1,4-dioate, hexyne-1,6-dioate, benzoate, chlorobenzoate, methylbenzoate, dinitrobenzoate, hydroxybenzoate, methoxybenzoate, phthalate, sulfonate, xylenesulfonate, phenylacetate, phenylpropionate, phenylbutyrate, citrate, lactate, gamma- hydroxybutyrate, glycolate, tartrate, methanesulfonate, propanesulfonate, naphthalene-1- sulfonate, naphthalene-2-sulfonate, mandelate, and the like. Base addition salts include those derived from inorganic bases, such as ammonium or alkali or alkaline earth metal hydroxides, carbonates, bicarbonates, and the like. Such bases useful in preparing the salts of the described compounds may include sodium hydroxide, potassium hydroxide, ammonium hydroxide, potassium carbonate, and the like.
[00156] An“effective amount” is the quantity of compound in which a beneficial clinical outcome may be achieved when the compound is administered to a subject suffering from the described fungus. A“beneficial clinical outcome” may include one or more of: a reduction in number of fungal spores in a subject; a reduction in the rate of fungus growth in a subject; a reduction in fungus consumption of a subject’s bodily resources; a reduction in biomarkers, toxins, proteins, peptides, and other biomolecules associated with infection of the subject by the fungus; a reduction in inflammatory, allergic, toxic, disfigurement, or other effects on the subject by the fungus; a reduction in the severity of the symptoms associated with the fungus and/or an increase in the longevity or health of the subject compared with the absence of the treatment.
[00157] The precise amount of compound administered to a subject may depend on the species, lifecycle, of the fungal infection. The precise amount of compound administered to a subject may also depend on the characteristics of the subject, such as general health, age, sex, body weight and tolerance to drugs. A skilled artisan may determine appropriate dosages depending on these and other factors. Effective amounts of the disclosed compounds typically
range between about 1 mg/mm2 per day and about 10 grams/mm2 per day, and preferably between 10 mg/mm2 per day and about 5 grams/mm2.
[00158] The disclosed compounds and pharmaceutical compositions may be administered by any suitable route, including, for example, orally in tablets, pills, gelcaps, lozenges, or suspensions; by parenteral administration. Parenteral administration can include, for example, systemic administration, such as by intramuscular, intravenous, subcutaneous, or intraperitoneal injection. The compounds may also be administered, for example, orally (e.g., dietary); topically, in the form of creams, sprays, patches, and the like; by inhalation (e.g., intrabronchial, intranasal, or oral inhalation of an aerosol formulation, by intranasal drops, and the like); via absorption through mucus membranes (e.g., tissues such as oral, nasal, rectal, vaginal, and the like) via, for example, creams, lozenges, sprays, drops, suppositories, and the like); depot preparations; coatings on sutures, bandages, medical devices, and the like.. In some embodiments, oral or parenteral administration are exemplary modes of administration. The disclosed compounds may be administered to the subject in conjunction with an acceptable pharmaceutical carrier as part of a pharmaceutical composition for treatment of infection by the described fungus. Formulation of the compound to be administered may vary according to the route and vehicle of administration selected (e.g., solution for injection, capsule or tablet for ingestion, and the like). Suitable pharmaceutical carriers may contain inert ingredients that do not interact with the described compound. Standard pharmaceutical formulation techniques may be employed, such as those described in Remington's Pharmaceutical Sciences, 22nd ed., Mack Publishing Company, Easton, PA, 2012. Suitable pharmaceutical carriers for parenteral administration may include, for example, sterile water, physiological saline, bacteriostatic saline (e.g., saline containing about 0.9% mg/mL benzyl alcohol, and the like), phosphate-buffered saline, Hank's solution, Ringer's-lactate and the like. Methods for encapsulating compositions (such as in a coating of hard gelatin or cyclodextrin) or tableting compositions are known in the art (Baker, et al.,“Controlled Release of Biological Active Agents,” John Wiley and Sons, New York, 1986).
[00159] A“subject” may be any animal subject to infection by the described funguss, e.g., the subject may be a mammal, bird, marsupial, fish, or amphibian. For example, the subject may be a mammal, such as a human. The subject may also be a domestic or wild animal in need of veterinary treatment, e.g., companion animals (e.g., dogs, cats, and the like), farm animals (e.g., cows, sheep, pigs, horses, and the like), laboratory animals (e.g., rats, mice, guinea pigs, and the like), birds, fish, marsupials, and the like.
[00160] To the extent that the term“includes” or“including” is used in the specification or the claims, it is intended to be inclusive in a manner similar to the term “comprising” as that term is interpreted when employed as a transitional word in a claim. Furthermore, to the extent that the term“or” is employed (e.g., A or B) it is intended to mean“A or B or both.” When the applicants intend to indicate“only A or B but not both” then the term “only A or B but not both” will be employed. Thus, use of the term“or” herein is the inclusive, and not the exclusive use. See Bryan A. Garner, A Dictionary of Modern Legal Usage 624 (2d. Ed. 1995). Also, to the extent that the terms“in” or“into” are used in the specification or the claims, it is intended to additionally mean“on” or“onto.” To the extent that the term “selectively” is used in the specification or the claims, it is intended to refer to a condition of a component wherein a user of the apparatus may activate or deactivate the feature or function of the component as is necessary or desired in use of the apparatus. To the extent that the term “operatively connected” is used in the specification or the claims, it is intended to mean that the identified components are connected in a way to perform a designated function. To the extent that the term“substantially” is used in the specification or the claims, it is intended to mean that the identified components have the relation or qualities indicated with degree of error as would be acceptable in the subject industry.
[00161] As used in the specification and the claims, the singular forms“a,”“an,” and “the” include the plural unless the singular is expressly specified. For example, reference to“a compound” may include a mixture of two or more compounds, as well as a single compound.
[00162] As used herein, the term“about” in conjunction with a number is intended to include ± 10% of the number. In other words,“about 10” may mean from 9 to 11. Where the term“about” is used with respect to a number that is an integer, the term“about” may mean ± 10% of the number, or ± 5, ± 4, ± 3, ± 2, or ± 1 of the number.
[00163] As used herein, the terms “optional” and“optionally” mean that the subsequently described circumstance may or may not occur, so that the description includes instances where the circumstance occurs and instances where it does not.
[00164] In addition, where features or aspects of the disclosure are described in terms of Markush groups, those skilled in the art will recognize that the disclosure is also thereby described in terms of any individual member or subgroup of members of the Markush group. As will be understood by one skilled in the art, for any and all purposes, such as in terms of providing a written description, all ranges disclosed herein also encompass any and all possible sub-ranges and combinations of sub-ranges thereof. Any listed range can be easily recognized as sufficiently describing and enabling the same range being broken down into at least equal halves,
thirds, quarters, fifths, tenths, and the like. As a non-limiting example, each range discussed herein can be readily broken down into a lower third, middle third and upper third, and the like. As will also be understood by one skilled in the art all language such as“up to,”“at least,” “greater than,”“less than,” include the number recited and refer to ranges which can be subsequently broken down into sub-ranges as discussed above. Finally, as will be understood by one skilled in the art, a range includes each individual member. For example, a group having 1-3 members refers to groups having 1, 2, or 3 members . Similarly, a group having 1-5 members refers to groups having 1, 2, 3, 4, or 5 members, and so forth. While various aspects and embodiments have been disclosed herein, other aspects and embodiments will be apparent to those skilled in the art.
[00165] As stated above, while the present application has been illustrated by the description of embodiments thereof, and while the embodiments have been described in considerable detail, it is not the intention of the applicants to restrict or in any way limit the scope of the appended claims to such detail. Additional advantages and modifications will readily appear to those skilled in the art, having the benefit of the present application. Therefore, the application, in its broader aspects, is not limited to the specific details, illustrative examples shown, or any apparatus referred to. Departures may be made from such details, examples, and apparatuses without departing from the spirit or scope of the general inventive concept.
Claims
1. A method of anti-fungal treatment, comprising:
providing a subject that is infected by or at risk of infection by a fungus;
administering a compound to the subject in an amount effective to mitigate infection by the fungus in the subject, the compound being represented by Structural Formula (I):
and pharmaceutically acceptable salts thereof, wherein:
Ar is an optionally substituted aryl or nitrogen-containing heteroaryl;
R1 and R2 are independently H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl;
A is a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings, each ring in the optionally substituted linking moiety independently being one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl;
X is O, S, amide, or a bond;
Y is optionally substituted C1-C14 alkyl or optionally substituted C2-C14 alkenyl; and Het2 is an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
2. The method of claim 2, comprising:
providing a subject that is infected by or at risk of infection by a fungus;
administering a compound to the subject in an amount effective to mitigate infection by the fungus in the subject, the compound being represented by Structural Formula (I):
(I) Het1—C(=NR1)NR2—A—X—Y—Het2 and pharmaceutically acceptable salts thereof, wherein:
Het1 is an optionally substituted, nitrogen-containing heteroaryl;
R1 and R2 are independently H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl;
A is a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings, each ring in the optionally substituted linking moiety independently being one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl;
X is O, S, amide, or a bond;
Y is optionally substituted C1-C10 alkyl or optionally substituted C2-C10 alkenyl; and Het2 is an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
3. The method of claim 1, the subject being infected by the fungus, the method comprising administering the compound to the subject in an amount effective to mitigate one or more symptoms of infection by the fungus in the subject.
4. The method of claim 1, the subject being at risk of infection by the fungus, the method comprising administering the compound to the subject in an amount effective to mitigate infection or re-infection of the subject by the fungus.
5. The method of claim 1, the fungus comprising CYP51.
6. The method of claim 1, the fungus comprising at least two distinct cytochrome P450 mediated biosterol synthesis pathways.
7. The method of claim 2, the fungus comprising CYP51.
8. The method of claim 1, the fungus belonging to a genus that is one of: Aspergillus, Candida, Cryptococcus, and Histoplasma.
9. The method of claim 8, the fungus being one of: Aspergillus fumigatus, Candida albicans, Candida glabrata, Cryptococcus neoformans, and Histoplasma capsulatum.
10. The method of claim 1, wherein the subject suffers from infection by the fungus or is at risk of suffering from infection by the fungus, the infection being of one or more of: skin, nail, hoof, hair, fur, scale, mucosal membrane, blood, lymph, brain, lung, heart, liver, pancreas, spleen, kidney, bladder, stomach, and intestine.
11. The method of claim 1, wherein the subject suffers from or is at risk of suffering from a systemic infection by the fungus.
12. The method of claim 1, wherein the subject is one or more of: suffering from cancer; undergoing chemotherapy; undergoing immune suppression therapy; a transplant recipient; suffering from an immuno-deficiency, and a burn patient.
13. The method of claim 1, wherein the subject is a human, dog, cat, cow, horse, sheep, pig, bird, amphibian, or fish.
14. The method of claim 1, provided that the compound represented by Structural Formula (Ia) is not one of free-base Compounds 1a-f:
15. The method of claim 2, wherein Het1 is optionally substituted pyridyl, pyrazinyl, pyrimidinyl, or pyridizinyl.
16. The method of claim 2, wherein Het1 is optionally substituted pyridyl.
19. The method of claim 1, wherein each ring in the optionally substituted linking moiety represented by A is independently and optionally substituted by one or more of: hydroxy, halo, and C1-C6 alkoxy.
20. The method of claim 1, wherein A comprises an optionally substituted heteroaryl or optionally substituted heterocycloalkyl ring.
21. The method of claim 1, wherein A comprises an optionally substituted, oxygen- containing, heteroaryl or heterocycloalkyl ring.
22. The method of claim 1, wherein A comprises an optionally substituted furanyl or optionally substituted tetrahydrofuranyl ring.
23. The method of claim 1, wherein A comprises optionally substituted 2,5-furanyl.
24. The method of claim 1, wherein A comprises one or two optionally substituted phenyl rings.
25. The method of claim 1, wherein A comprises optionally substituted 1,4-phenyl.
26. The method of claim 1, wherein A is optionally substituted 1,4-phenyl.
27. The method of claim 1, wherein A is optionally substituted phenyl-heteroaryl-phenyl.
28. The method of claim 1, wherein the compound is represented by one of Structural Formulas (IIIa)-(IIIf):
wherein Z, Z1, and Z2 are each independently CH or N, n is 1-14, and R3 is H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy.
29. The method of claim 28, wherein the compound is one of Compounds 1a-1v:
30. The method of claim 28 wherein the com ound is Com ound 14a:
31. The method of claim 1, wherein the compound is represented by Structural Formula (IV):
wherein each R3 is independently H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy.
32. The method of claim 1, wherein the compound is represented by Structural Formula (V):
wherein each R3 is independently H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy.
33. The method of claim 1, wherein the compound is represented by one of Structural Formulas (Va)-(Ve):
wherein Z, Z1, and Z2 are each independently CH or N, n is 1-14, and R3 is H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy.
34. The compound of claim 1, wherein Y comprises at least 4 linking atoms between X and Het2.
35. The method of claim 1, wherein X is O or a bond and Y is C1-C12 alkyl optionally substituted with one or more of: optionally halogenated C1-C8 alkyl and optionally halogenated aryl.
wherein R4 is H, optionally halogenated C1-C8 alkyl, or optionally halogenated aryl.
wherein R4 is H, optionally halogenated C1-C8 alkyl, or optionally halogenated aryl.
38. The method of claim 2, wherein Het2 comprises an optionally substituted one of: pyrrole, diazole, thiadiazole, oxadiazole, and triazole.
39. The method of claim 2, wherein Het2 is optionally substituted imidazole or optionally substituted 1, 2, 4 triazole.
40. The method of claim 1, wherein the compound is represented by Structural Formula (VII):
Z = CH or N;
each R3 is independently H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy; and
n is an integer from 1 to 10.
41. The method of claim 40 wherein the com ound is one of Com ounds 2a-2h:
43. The method of claim 40, wherein the compound is one of Compounds 4a-4m:
44. The method of claim 1, wherein the compound is one of Compounds 13a-13c:
45. The method of claim 1, wherein the compound is represented by Structural Formula (VIII):
each R5 is independently H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and
R6 is H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
47. The method of claim 1, wherein the compound is represented by Structural Formula (IX):
each R5 is independently H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and
R6 is H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
48. The method of claim 47, wherein the compound is one of Compounds 6a-6f:
49. The method of claim 1 wherein the com ound is re resented by Structural Formula (X):
wherein:
each R5 is independently H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and
R6 is H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
51. The method of claim 1, wherein the compound is represented by Structural Formula (XI):
wherein:
each R5 is independently H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and
R6 is H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
wherein:
each R5 is independently H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and
R6 is H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
53. The method of claim 1, wherein A is phenyl and X is a bond.
54. The method of claim 1, wherein the compound is represented by Structural Formula (XIII):
Z = CH or N; and
n is an integer from 1 to 10.
55. The method of claim 54 wherein the com ound is one of Com ounds 8a-8b:
56. The method of claim 2, wherein A is a bond.
57. The pharmaceutical composition of claim 68, wherein the compound is represented by Structural Formula (XIV):
wherein:
n is an integer from 1 to 10.
59. The method of claim 1, wherein the compound is represented by Structural Formula (XV):
wherein Z, Z1, and Z2 are each independently CH or N, n is 1-14, and R3 is H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy.
60. The method of claim 59, wherein the compound is represented by one of Structural Formulas (XVa)-(XVc):
62. The method of claim 1, wherein the compound is represented by one of Structural Formulas (XVI) and (XVII):
wherein Z, Z1, and Z2 are each independently CH or N, n is 1-14, and R3 is H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy.
63. The method of claim 62, wherein the compound is represented by one of Structural Formulas (XVIa)-(XVIb):
64. The method of claim 63, wherein the compound is one of Compounds 11a-11d:
65. The method of claim 1, wherein the compound is represented by one of Structural Formulas (XVIIa)-(XVIIb):
66. The method of claim 65, wherein the compound is Compound 12a:
67. A pharmaceutical composition, comprising:
a pharmaceutically acceptable carrier or excipient; and
and pharmaceutically acceptable salts thereof, wherein:
Ar is an optionally substituted aryl or nitrogen-containing heteroaryl;
R1 and R2 are independently H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl;
A is a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings, each ring in the optionally substituted linking moiety independently being one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl;
X is O, S, amide, or a bond;
Y is optionally substituted C1-C10 alkyl or optionally substituted C2-C10 alkenyl; and Het2 is an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
68. The pharmaceutical composition of claim 67, comprising:
the pharmaceutically acceptable carrier or excipient; and
the compound, represented by Structural Formula (I): and pharmaceu
tically acceptable salts thereof, wherein:
Het1 is an optionally substituted, nitrogen-containing heteroaryl;
R1 and R2 are independently H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl;
A is a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings, each ring in the optionally substituted linking moiety independently being one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl;
X is O, S, amide, or a bond;
Y is optionally substituted C1-C10 alkyl or optionally substituted C2-C10 alkenyl; and Het2 is an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms.
69. The pharmaceutical composition of claim 67, wherein when the compound represented by Structural Formula (I) is one of Compounds 1a-f:
one of:
each of Compounds 1a-f is in the form of a solid;
each of Compounds 1a-f is in the form of a pharmaceutically acceptable salt;
the pharmaceutical composition excludes mixtures consisting of one of Compounds 1a-f in a solution of one or more of: distilled water, dimethyl sulfoxide, ethanol, aqueous saline, and aqueous phosphate buffer;
at least a portion of the pharmaceutically acceptable carrier or excipient is in the form of a solid or gel;
the pharmaceutical composition is configured for administration in unit dosage form; and the pharmaceutical composition is configured for administration in the form of one of: a tablet; a capsule; a lozenge; a cream, a spray, a transdermal patches, an aerosol, a suppository, a depot preparation; a coated or impregnated suture; a coated or impregnated bandage; and a coated or impregnated medical device.
70. The pharmaceutical composition of claim 67, provided that the compound represented by Structural Formula (I) excludes each of Compounds 1a-f:
71. The pharmaceutical composition of claim 68, wherein Het1 is optionally substituted pyridyl, pyrazinyl, pyrimidinyl, or pyridizinyl.
72. The pharmaceutical composition of claim 68, wherein Het1 is optionally substituted pyridyl.
75. The pharmaceutical composition of claim 67, wherein each ring in the optionally substituted linking moiety represented by A is independently and optionally substituted by one or more of: hydroxy, halo, and C1-C6 alkoxy.
76. The pharmaceutical composition of claim 67, wherein A comprises an optionally substituted heteroaryl or optionally substituted heterocycloalkyl ring.
77. The pharmaceutical composition of claim 67, wherein A comprises an optionally substituted, oxygen-containing, heteroaryl or heterocycloalkyl ring.
78. The pharmaceutical composition of claim 67, wherein A comprises an optionally substituted furanyl or optionally substituted tetrahydrofuranyl ring.
79. The pharmaceutical composition of claim 67, wherein A comprises optionally substituted 2,5-furanyl.
80. The pharmaceutical composition of claim 67, wherein A comprises one or two optionally substituted phenyl rings.
81. The pharmaceutical composition of claim 67, wherein A comprises optionally substituted 1,4-phenyl.
82. The pharmaceutical composition of claim 67, wherein A is optionally substituted 1,4- phenyl.
83. The pharmaceutical composition of claim 67, wherein A is optionally substituted phenyl- heteroaryl-phenyl.
84. The pharmaceutical composition of claim 67, wherein the compound is represented by one of Structural Formulas (IIIa)-(IIIf):
wherein Z, Z1, and Z2 are each independently CH or N, n is 1-14, and R3 is H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy.
85. The pharmaceutical composition of claim 84, wherein the compound is one of
Compounds 1a-1v:
86. The pharmaceutical com osition of claim 84 wherein the com ound is Compound 14a:
87. The pharmaceutical composition of claim 67, wherein the compound is represented by Structural Formula (IV):
wherein each R3 is independently H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy.
88. The pharmaceutical composition of claim 67, wherein the compound is represented by Structural Formula (V):
wherein each R3 is independently H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy.
89. The pharmaceutical composition of claim 67, wherein the compound is represented by one of Structural Formulas (Va)-(Ve):
90. The compound of claim 1, wherein Y comprises at least 4 linking atoms between X and Het2.
91. The pharmaceutical composition of claim 67, wherein X is O or a bond and Y is C1-C12 alkyl optionally substituted with one or more of: optionally halogenated C1-C8 alkyl and optionally halogenated aryl.
92. The pharmaceutical composition of claim 67, wherein the compound is represented by Structural Formula (VIa):
wherein R4 is H, optionally halogenated C1-C8 alkyl, or optionally halogenated aryl.
93. The pharmaceutical composition of claim 67, wherein the compound is represented by Structural Formula (VIb):
wherein R4 is H, optionally halogenated C1-C8 alkyl, or optionally halogenated aryl.
94. The pharmaceutical composition of claim 68, wherein Het2 comprises an optionally substituted one of: pyrrole, diazole, thiadiazole, oxadiazole, and triazole.
95. The pharmaceutical composition of claim 68, wherein Het2 is optionally substituted imidazole or optionally substituted 1, 2, 4 triazole.
96. The pharmaceutical composition of claim 67, wherein the compound is represented by Structural Formula (VII):
wherein:
Z = CH or N;
each R3 is independently H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy; and
n is an integer from 1 to 10.
97. The pharmaceutical composition of claim 96, wherein the compound is one of Compounds 2a-2h:
99. The pharmaceutical composition of claim 96, wherein the compound is one of Compounds 4a-4m:
100. The pharmaceutical composition of claim 67, wherein the compound is one of
Compounds 13a-13c:
101. The pharmaceutical composition of claim 67, wherein the compound is represented by Structural Formula (VIII):
wherein:
each R5 is independently H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and
R6 is H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
102. The pharmaceutical composition of claim 101, wherein the compound is one of Compounds 5a-5f:
103. The pharmaceutical composition of claim 67, wherein the compound is represented by Structural Formula (IX):
wherein:
each R5 is independently H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and
R6 is H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
104. The pharmaceutical composition of claim 103, wherein the compound is one of Compounds 6a-6f:
105. The pharmaceutical composition of claim 67, wherein the compound is represented by Structural Formula (X):
wherein:
each R5 is independently H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and
R6 is H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
106. The pharmaceutical composition of claim 105, wherein the compound is one of Com ounds 7a-7f:
107. The pharmaceutical composition of claim 67, wherein the compound is represented by Structural Formula (XI):
wherein:
each R5 is independently H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and
R6 is H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
108. The pharmaceutical composition of claim 67, wherein the compound is represented by Structural Formula (XII):
wherein:
each R5 is independently H, halogen, C1-C4 alkyl, or C1-C4 alkoxy; and
R6 is H, optionally halogenated C1-C6 alkyl, optionally halogenated phenyl, or optionally halogenated biphenyl.
109. The pharmaceutical composition of claim 67, wherein A is phenyl and X is a bond.
110. The pharmaceutical composition of claim 67, wherein the compound is represented by Structural Formula (XIII):
Z = CH or N; and
n is an integer from 1 to 10.
111. The pharmaceutical composition of claim 110, wherein the compound is one of Compounds 8a-8b:
112. The pharmaceutical composition of claim 67, wherein A is a bond.
113. The pharmaceutical composition of claim 112, wherein the compound is represented by Structural Formula (XIV):
Z = CH or N; and
n is an integer from 1 to 10.
114. The pharmaceutical composition of claim 113, wherein the compound is one of
Compounds 9a-9d:
115. The pharmaceutical composition of claim 67, wherein the compound is represented by Structural Formula (XV):
wherein Z, Z1, and Z2 are each independently CH or N, n is 1-14, and R3 is H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy.
116. The pharmaceutical composition of claim 115, wherein the compound is represented by one of Structural Formulas (XVa)-(XVc):
117. The pharmaceutical composition of claim 116, wherein the compound is one of Compounds 10a-10m:
118. The pharmaceutical composition of claim 67, wherein the compound is represented by one of Structural Formulas (XVI) and (XVII):
wherein Z, Z1, and Z2 are each independently CH or N, n is 1-14, and R3 is H, halogen, optionally halogenated C1-C6 alkyl, or optionally halogenated C1-C6 alkoxy.
119. The pharmaceutical composition of claim 118, wherein the compound is represented by one of Structural Formulas (XVIa)-(XVIb):
120. The pharmaceutical composition of claim 119, wherein the compound is one of Compounds 11a-11d:
121. The pharmaceutical composition of claim 67, wherein the compound is represented by one of Structural Formulas (XVIIa)-(XVIIb):
122. The pharmaceutical com osition of claim 123 wherein the com ound is Compound 12a:
123. A kit for anti-fungal treatment of a subject in need thereof, comprising:
a compound represented by Structural Formula (I):
pharmaceutically acceptable salts thereof, and mixtures thereof with a pharmaceutically acceptable carrier or excipient, wherein:
Ar is an optionally substituted aryl or nitrogen-containing heteroaryl; R1 and R2 are independently H, optionally substituted C1-C6 alkyl, or optionally substituted C3-C6 cycloalkyl;
A is a bond or an optionally substituted linking moiety comprising 1, 2, or 3 rings, each ring in the optionally substituted linking moiety independently being one of: aryl, cycloalkyl, heterocycloalkyl, and heteroaryl;
X is O, S, amide, or a bond;
Y is optionally substituted C1-C14 alkyl or optionally substituted C2-C14 alkenyl; and
Het2 is an optionally substituted five-membered nitrogen-containing heteroaromatic ring comprising 1, 2, or 3 ring heteroatoms; and
instructions, the instructions directing a user to:
provide the subject, the subject being infected by a fungus or at risk of infection by the fungus; and
administer the compound or the pharmaceutical composition to the subject in an amount effective to mitigate infection by the fungus in the subject.
124. The kit for anti-fungal treatment of 123, wherein when the compound represented by Structural Formula (I) is one of Compounds 1a-f:
and one of:
each of Compounds 1a-f is in the form of a solid;
each of Compounds 1a-f is in the form of a pharmaceutically acceptable salt; the pharmaceutical composition excludes mixtures consisting of one of Compounds 1a-f in a solution of one or more of: distilled water, dimethyl sulfoxide, ethanol, aqueous saline, and aqueous phosphate buffer;
at least a portion of the pharmaceutically acceptable carrier or excipient is in the form of a solid or gel;
the pharmaceutical composition is configured for administration in unit dosage form; and the pharmaceutical composition is configured for administration in the form of one of: a tablet; a capsule; a lozenge; a cream, a spray, a transdermal patches, an aerosol, a suppository, a depot preparation; a coated or impregnated suture; a coated or impregnated bandage; and a coated or impregnated medical device.
125. The kit for anti-fungal treatment of claim 123, provided that the compound represented by Structural Formula (I) excludes each of Compounds 1a-f:
126. The kit for anti-fungal treatment of claim 123, the instructions directing the user to conduct the method of any of claims 1-66.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662381090P | 2016-08-30 | 2016-08-30 | |
| US62/381,090 | 2016-08-30 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018045106A1 true WO2018045106A1 (en) | 2018-03-08 |
Family
ID=61301670
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2017/049493 Ceased WO2018045106A1 (en) | 2016-08-30 | 2017-08-30 | Anti-fungal treatment |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2018045106A1 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023069613A1 (en) * | 2021-10-20 | 2023-04-27 | Ohio State Innovation Foundation | Arylimidamides for use in treatment of cancers |
| US11827627B2 (en) | 2021-06-04 | 2023-11-28 | Vertex Pharmaceuticals Incorporated | N-(hydroxyalkyl (hetero)aryl) tetrahydrofuran carboxamides as modulators of sodium channels |
| US11834441B2 (en) | 2019-12-06 | 2023-12-05 | Vertex Pharmaceuticals Incorporated | Substituted tetrahydrofurans as modulators of sodium channels |
| EP4603484A2 (en) | 2019-12-19 | 2025-08-20 | Georgia State University Research Foundation, Inc. | Compounds for the treatment of bacterial infections and potentiation of antibiotics |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6319944B1 (en) * | 1999-05-10 | 2001-11-20 | Merck & Co., Inc. | Aryl amidines, compositions containing such compounds and methods of use |
| US20020156098A1 (en) * | 2000-11-06 | 2002-10-24 | Werbovetz Karl A. | Reversed amidines and methods of using for treating, preventing, or inhibiting leishmaniasis |
| US20050182118A1 (en) * | 2004-01-23 | 2005-08-18 | Neurochem (International) Limited | Amidine derivatives for treating amyloidosis |
| US20060025458A1 (en) * | 2004-07-30 | 2006-02-02 | University Of Connecticut | Alkylammonium compounds as antifungal and antitrypanosomal agents |
| US20100331368A1 (en) * | 2007-10-17 | 2010-12-30 | Tidwell Richard R | 2,5-diaryl selenophene compounds, aza 2,5-diaryl thiophene compounds, and their prodrugs as antiprotozoal agents |
-
2017
- 2017-08-30 WO PCT/US2017/049493 patent/WO2018045106A1/en not_active Ceased
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6319944B1 (en) * | 1999-05-10 | 2001-11-20 | Merck & Co., Inc. | Aryl amidines, compositions containing such compounds and methods of use |
| US20020156098A1 (en) * | 2000-11-06 | 2002-10-24 | Werbovetz Karl A. | Reversed amidines and methods of using for treating, preventing, or inhibiting leishmaniasis |
| US20050182118A1 (en) * | 2004-01-23 | 2005-08-18 | Neurochem (International) Limited | Amidine derivatives for treating amyloidosis |
| US20060025458A1 (en) * | 2004-07-30 | 2006-02-02 | University Of Connecticut | Alkylammonium compounds as antifungal and antitrypanosomal agents |
| US20100331368A1 (en) * | 2007-10-17 | 2010-12-30 | Tidwell Richard R | 2,5-diaryl selenophene compounds, aza 2,5-diaryl thiophene compounds, and their prodrugs as antiprotozoal agents |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11834441B2 (en) | 2019-12-06 | 2023-12-05 | Vertex Pharmaceuticals Incorporated | Substituted tetrahydrofurans as modulators of sodium channels |
| US11919887B2 (en) | 2019-12-06 | 2024-03-05 | Vertex Pharmaceuticals Incorporated | Substituted tetrahydrofurans as modulators of sodium channels |
| US12247021B2 (en) | 2019-12-06 | 2025-03-11 | Vertex Pharmaceuticals Incorporated | Substituted tetrahydrofurans as modulators of sodium channels |
| EP4603484A2 (en) | 2019-12-19 | 2025-08-20 | Georgia State University Research Foundation, Inc. | Compounds for the treatment of bacterial infections and potentiation of antibiotics |
| US11827627B2 (en) | 2021-06-04 | 2023-11-28 | Vertex Pharmaceuticals Incorporated | N-(hydroxyalkyl (hetero)aryl) tetrahydrofuran carboxamides as modulators of sodium channels |
| US12258333B2 (en) | 2021-06-04 | 2025-03-25 | Vertex Pharmaceuticals Incorporated | N-(hydroxyalkyl (hetero)aryl) tetrahydrofuran carboxamides as modulators of sodium channels |
| WO2023069613A1 (en) * | 2021-10-20 | 2023-04-27 | Ohio State Innovation Foundation | Arylimidamides for use in treatment of cancers |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| ES2266212T3 (en) | DERIVATIVES OF PIRAZOL. | |
| RU2476427C2 (en) | Dual-acting antihypertensive compounds, methods for preparing them, based pharmaceutical compositions and intermediate compounds | |
| JPH06506003A (en) | Novel imidazole derivatives, their production and their therapeutic applications | |
| US20160362392A1 (en) | Glucagon receptor modulators | |
| WO2018045106A1 (en) | Anti-fungal treatment | |
| JP7469522B2 (en) | Benzothiazole derivatives and their uses | |
| US20090018150A1 (en) | 5-Ht2b Receptor Antagonists | |
| JP2005510476A (en) | Spiro-hydantoin compounds useful as anti-inflammatory agents | |
| CZ20033361A3 (en) | Anthranilic acid amides with heteroarylsulfonyl side chain, their use as medicaments or diagnostic agents and pharmaceutical compositions in which the amides are comprised | |
| KR20030085565A (en) | Thiohydantoins and use thereof for treating diabetes | |
| JP6509238B2 (en) | Pyrazolo [1,5-a] pyrimidin-7-amine derivatives useful for therapy | |
| SK112893A3 (en) | Benzofuran derivatives, method of their preparation and pharmaceutical compositions containing them | |
| AU2004229466A1 (en) | Imidazole derivatives for treatment of allergic and hyperproliferative disorders | |
| US20080167371A1 (en) | Diaryl, Dipyridinyl and Aryl-Pyridinyl Derivatives and Uses Thereof | |
| JPH0233031B2 (en) | ||
| WO2019173790A1 (en) | Pyridinone- and pyridazinone-based compounds and medical uses thereof | |
| US10961202B2 (en) | Bis-benzimidazole compounds and methods of using the same | |
| US9499490B2 (en) | Fluorophenyl pyrazol compounds | |
| WO2018045104A1 (en) | Anti-parasitic compounds | |
| US20250049806A1 (en) | Pharmaceutical composition for providing treatment for or preventing alport syndrome | |
| WO2008080938A1 (en) | Use 2-substituted pyridines for cancer treatment | |
| KR101112992B1 (en) | Nitroimidazole compounds, process for the preparation thereof, and pharmaceutical composition for treating tuberculosis comprising the same | |
| ZA200207969B (en) | Therapeutically useful new salts of CCK inhibitors, process for the preparation thereof and pharmaceutical preparations containing them. | |
| Farwa et al. | Triazoles-A paradigm shift in drug discovery: A review on synthesis and therapeutic potential | |
| US20090275753A1 (en) | Ortho-substituted aniline derivative and antioxidant drug |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17847516 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 17847516 Country of ref document: EP Kind code of ref document: A1 |