WO2017096274A1 - Cancer treatment using 2-deoxy-2-fluoro-l-fucose in combination with a checkpoint inhibitor - Google Patents
Cancer treatment using 2-deoxy-2-fluoro-l-fucose in combination with a checkpoint inhibitor Download PDFInfo
- Publication number
- WO2017096274A1 WO2017096274A1 PCT/US2016/064783 US2016064783W WO2017096274A1 WO 2017096274 A1 WO2017096274 A1 WO 2017096274A1 US 2016064783 W US2016064783 W US 2016064783W WO 2017096274 A1 WO2017096274 A1 WO 2017096274A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cancer
- fucose
- deoxy
- fluoro
- checkpoint inhibitor
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/39541—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against normal tissues, cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/39558—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against tumor tissues, cells, antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2818—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD28 or CD152
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
Definitions
- L-fucose also referred to as 6-deoxy-L-galactose
- 6-deoxy-L-galactose is a monosaccharide that is a component of some N- and O-linked glycans and glycolipids in animals.
- Fucose is typically added as a terminal modification to glycans, including glycans attached to blood group antigens, selectins and antibodies. Fucose can be attached to glycans via oc(l,2)-, oc(l,3)-, oc(l,4)- and oc(l,6)- linkages by specific fucosyltransferases.
- oc(l,2)-fucose linkages are typically associated with the H-blood group antigens.
- oc(l,3)- and oc(l,4)-fucose linkages are associated with modification of Lewis x antigens.
- oc(l,6)-fucose linkages are associated with N-linked GlcNAc molecules, such as those on antibodies.
- peptidomimetic compounds which are capable of suppressing and/or inhibiting the programmed cell death 1 (PD-1) signaling pathway.
- ipilimumab (Yervoy ® ), a monoclonal antibody that targets cytotoxic T- lymphocyte-associated antigen 4 (CTLA-4) and nivolumab (Opdivo ® ), a monoclonal antibody that targets the programmed cell death protein 1 pathway (PD-1) on the surface of T-cells, have been approved by the U.S. Food and Drug Administration for the treatment of advanced melanoma, advanced renal cell carcinoma, and non-small cell lung cancer.
- Current checkpoint inhibitor therapies are effective at treating cancer in a relatively small population of cancer subject population which is in part due to pre-existing immune activation and presence of the inhibitory receptors. Accordingly, there is a need to develop methods and combination therapies to initiate or enhance the effectiveness of the checkpoint inhibitors in both the nonresponding subject population and the responding subject population.
- the present invention discloses a combination treatment using
- 2-deoxy-2-fluoro-L-fucose also referred to herein as "2FF"
- a checkpoint inhibitor that are effective in treating cancer or inhibiting the proliferation of tumor cells in a subject and/or that can initiate, enhance or prolong the immune response to tumor cells.
- Figure 1A shows in vivo effects of 2-deoxy-2-fluoro-L-fucose on the growth of IV implanted A20 mouse lymphoma cells in BALB/c mice with or without vaccination with KLH-conjugated A20 Id Fab (20 mM 2-deoxy-2-fluoro-L-fucose in drinking water).
- Figure IB shows effect of immune cell subset depletion on the in vivo effects of 2-deoxy-2-fluoro-L-fucose on the growth of IV implanted A20 mouse lymphoma cells in BALB/c mice in combination with vaccination with KLH-conjugated A20 Id Fab (20 mM 2-deoxy-2-fluoro-L-fucose in drinking water).
- Figure 2A shows measurement of dendritic cell (also referred to as "DC") markers on DCs co-cultured with T-cells in serum-containing medium (MFI of 2-deoxy-2-fluoro-L-fucose-treated co-culture compared to control co-culture).
- Figure 2B shows measurement of DC markers on DCs co-cultured with T-cells in Serum-free medium (MFI of 2-deoxy-2-fluoro-L-fucose-treated serum-free co-culture compared to control serum-fee co-culture).
- FIG 3 shows comparison of tetramer binding to T-cells (MFI of binding to 2-deoxy-2-fluoro-L-fucose-treated T-cells compared to control T-cells) for three different tetramers (EBV, Ml, and CMV).
- Figure 4 shows TCR-mediated phosphorylation of ZAP in CD3/CD28 activated T-cells (with or without 2-deoxy-2-fluoro-L-fucose treatment).
- Figure 5 shows expression of galectin-3 in T-cells with or without
- Figure 7A and 7B show comparison of the FOXp3 expression and percentage of regulatory T-cells in 2-deoxy-2-fluoro-L-fucose-treated vs. control T-cells.
- Figures 8A, 8B and 8C show T-cells/DC co-culture cytokines in (A) INFy, (B) IL12p40, and (C) CD40L, respectively.
- Figure 9 shows A20 vaccine model cytokine evaluation, KLH-Idiotype (control) vs. 2-deoxy-2-fluoro-L-fucose + KLH-Idiotype (2-deoxy-2-fluoro-L-fucose) (BALB/c mice).
- Figures 10A and 10B show in vitro production of IFNy and IL-12p70, respectively, in human co-cultures.
- Figure 11 shows in vivo effects of 2-deoxy-2-fluoro-L-fucose, anti-PDl antibody and the combination of 2FF with anti-PDl antibody on the growth of IV implanted A20 mouse lymphoma cells in BALB/c mice (20 mM 2FF in drinking water, 5 mg/kg anti-PDl q3x3).
- Figures 12A-C show active immune response at the tumor microenvironment.
- the present invention describes a novel combination treatment based on activating or enhancing the adaptive immune response.
- the adaptive immune response mechanism implies that a checkpoint inhibitor will only work or work more effectively when there is a pre-existing anti-tumor immune response (i.e., activated T-cells). For example, in patients who do not have preexisting anti-tumor responses, the checkpoint inhibitors alone will potentially not be effective. Accordingly, a combination treatment that can activate anti-tumor activity (e.g., an anti-tumor immune response) and inhibit the checkpoint blockade is preferable because it would allow subjects who do not respond to treatment by checkpoint inhibitors alone to benefit from this combined treatment.
- an anti-tumor immune response e.g., an anti-tumor immune response
- the present invention provides, inter alia, methods for treating cancers, comprising administering to a subject in need thereof an effective amount of
- the present invention also provides, inter alia, methods for inhibiting the proliferation of a tumor in a subject in need thereof comprising administering to the subject an effective amount of
- the present invention further provides, inter alia, methods for enhancing or prolonging the effects of a checkpoint inhibitor, or enabling a subject to respond to a checkpoint inhibitor in a subject in need thereof comprising administering to the subject an effective amount of
- the combination of 2-deoxy-2-fluoro-L-fucose or a prodrug thereof, or a pharmaceutically acceptable salt thereof, and the checkpoint inhibitor administered provides an additive or synergistic effect in the treatment of the cancer or in the inhibition of the proliferation of tumor cells.
- the combination of 2-deoxy-2-fluoro-L-fucose or a prodrug thereof, or a pharmaceutically acceptable salt thereof, and the checkpoint inhibitor administered provides a synergistic effect in the treatment of the cancer or in the inhibition of the proliferation of tumor cells.
- 2-deoxy-2-fluoro-L-fucose or a pharmaceutically acceptable salt thereof is administered in combination with a checkpoint inhibitor.
- the prodrug of 2-deoxy-2-fluoro-L-fucose or a pharmaceutically acceptable salt thereof is administered in combination with a checkpoint inhibitor.
- carboxylic ester prodrug of 2-deoxy-2-fluoro-L-fucose or a pharmaceutically acceptable salt thereof is administered in combination with a checkpoint inhibitor.
- the checkpoint inhibitor is a biologic therapeutic or a small molecule.
- the checkpoint inhibitor is selected from the group consisting of a monoclonal antibody, a humanized antibody, a fully human antibody and a fusion protein or a combination thereof.
- the checkpoint inhibitor inhibits or interact with a ligand of a checkpoint protein selected from the group consisting of CTLA-4, PD-1, PD-L1, PD-L2, B7-H3, B7-H4, BMA, HVEM, TIM3, GAL9, LAG3, VISTA, KIR, 2B4, CD160, CGEN -15049, CHK 1, CHK2, A2aR, and B-7 family ligands or a combination thereof.
- the checkpoint inhibitor is a PD-L1, PD-L2, or PD-1 inhibitor.
- the checkpoint inhibitor is a PD-1 inhibitor.
- the checkpoint inhibitor is a PD-1 inhibitor is nivolumab or pembrolizumab.
- the checkpoint inhibitor is a PD-1 inhibitor is nivolumab.
- the cancer is a solid tumor. In some embodiments, the cancer is a liquid tumor. In some embodiments, the cancer is selected from the group consisting of urogenital, gynecological, lung, gastrointestinal, head and neck cancer, brain cancers including malignant gliomas and brain metastases, malignant mesothelioma, non-metastatic or metastatic breast cancer, malignant melanoma, Merkel Cell Carcinoma or bone and soft tissue sarcomas, haematologic neoplasias, multiple myeloma, lymphomas such as Hodgkin's disease, non-Hodgkin's lymphoma, acute myelogenous leukemia, chronic myelogenous leukemia, myelodysplastic syndrome and acute lymphoblastic leukemia, non-small cell lung cancer (NSCLC), breast cancer, metastatic colorectal cancers, hormone sensitive or hormone refractory prostate cancer, colorectal cancer
- NSCLC non-small
- the cancer is non-small cell lung cancer (NSCLC), breast cancer, or a colorectal cancer. In some embodiments, the cancer is non-small cell lung cancer (NSCLC).
- NSCLC non-small cell lung cancer
- a composition comprising 2-deoxy-2-fluoro-L-fucose or a prodrug thereof, or a pharmaceutically acceptable salt thereof, is administered to the subject. In some embodiments, the composition is a solid or a liquid formulation. In some embodiments, the checkpoint inhibitor and
- 2-deoxy-2-fluoro-L-fucose are administered simultaneously or sequentially, in either order.
- the subject is a mammal.
- the mammal is a human.
- the terms “inhibit” or “inhibition of” means to reduce by a measurable amount, or to prevent entirely.
- the term inhibition as used herein can refer to an inhibition or reduction of at least about 10%, at least about 15%, at least about 20%, at least about 25%, at least about 30%, at least about 35%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80%, at least about 90%, at least about 95%, or at least about 99%.
- treatment or “treat” refer to slowing, stopping, or reversing the progression of the disease or condition in a patient, as evidenced by a decrease or elimination of a clinical or diagnostic symptom of the disease or condition.
- Treatment can include, for example, a decrease in the severity of a symptom, the number of symptoms, or frequency of relapse.
- pharmaceutically acceptable means approved by a regulatory agency of the Federal or a state government or listed in the U.S. Pharmacopeia or other generally recognized pharmacopeia for use in animals, and more particularly in humans.
- pharmaceutically compatible ingredient refers to a
- prodrug refers to a compound that is converted into the active form of the compound upon administration in vivo.
- a prodrug form of an active compound can be, but not limited to, acylated (acetylated or other) and ether derivatives, carboxylic esters or phosphate esters and various salt forms of the active compound.
- acylated acetylated or other
- ether derivatives carboxylic esters or phosphate esters and various salt forms of the active compound.
- 2-deoxy-2-fluoro-L-fucose or a prodrug thereof, or a pharmaceutically acceptable salt thereof is typically substantially pure from undesired contaminant.
- 2-deoxy-2-fluoro-L-fucose or a prodrug thereof, or a pharmaceutically acceptable salt thereof is typically at least about 50% w/w (weight/weight) or about 80% w/w purity, more preferably at least about 90% or about 95% w/w purity, as well as being substantially free from impurities and other contaminants.
- homogeneous product of at least 99% w/w can be obtained.
- subject or "patient” for purposes of treatment refers to any animal, particularly an animal classified as a mammal, including humans, domesticated and farm animals, and zoo, sports, or pet animals, such as dogs, horses, cats, cows, and the like.
- the subject is human.
- therapeutically effective amount refers to the amount of one or more agents or compositions as described herein that is sufficient to slow, stop, or reverse the progression of cancer in a subject or increase survival of the patient.
- the therapeutically effective amount may refer to a target serum concentration that has been shown to be effective in, for example, slowing disease progression.
- therapeutically effective amount refers to combination therapy, it refers to the amount of the combination of agents taken together so that the combined effect elicits the desired biological or medicinal response.
- Efficacy can be measured in conventional ways, depending on the condition to be treated. For example, in neoplastic diseases, efficacy can be measured by assessing the time to disease progression (TTP), or determining the response rates (RR).
- the term “synergy” or “synergistic effect” when used in connection with a description of the efficacy of a combination of agents means any measured effect of the combination which is greater than the effect predicted from a sum of the effects of the individual agents.
- the term “additive” or “additive effect” when used in connection with a description of the efficacy of a combination of agents means any measured effect of the combination which is similar to the effect predicted from a sum of the effects of the individual agents.
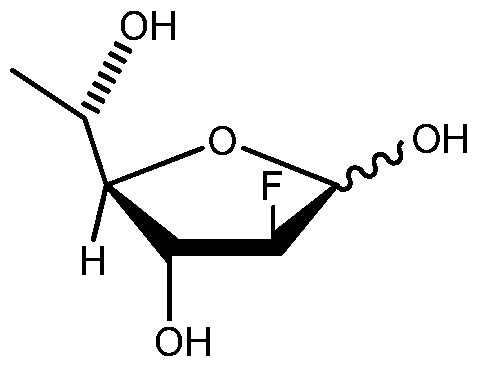
- 2-deoxy-2-fluoro-L-fucose can interconvert via the corresponding aldose form to a compound having a chemical structure (II) as the following:
- 2-deoxy-2-fluoro-L- fucose refers to a compound of formula (I), (II) or the corresponding aldose form, or a mixture thereof, wherein each of formula (I) or (II) can independently be alpha or beta anomer.
- 2-deoxy-2-fluoro-L- fucose reduces regulatory T-cells.
- 2-deoxy-2-fluoro-L-fucoses can be used as an immuno-modulatory agent to treat cancer due to the changes in T-cell activity induced when they are afucosylated by treatment with
- 2-deoxy-2-fluoro-L-fucose Changes that occur include reduction in regulatory T-cell populations, increases in T-cell activation of antigen presenting cells, as well as increased T-cell receptor signaling which can in turn result in the increased activation of APCs.
- the result of this modulation of T-cell activity would be to reduce the immunosuppressive tumor microenvironment as well as increasing T-cell and APC activation.
- treatment with 2-deoxy-2-fluoro-L-fucose would lead to an increased host-mediated anti-tumor immune response resulting in delay of tumor progression or delay to tumor onset.
- the anti-tumor activity may also occur with direct, adoptive transfer of 2-deoxy-2-fluoro-L-fucoses treated T-cells.
- 2-deoxy-2-fluoro-L-fucose for the methods provided herein can be safely administered in combination with a checkpoint inhibitor to a subject in an amount effective to treat cancer in the subject, such as a human in need thereof.
- a checkpoint inhibitor to a subject in an amount effective to treat cancer in the subject, such as a human in need thereof.
- 2-deoxy-2-fluoro-L-fucose in combination with a checkpoint inhibitor also provides a better safety profile than the existing PD-1 combination treatments such as the combination of a PD-1 inhibitor and a CTLA-4 inhibitor.
- 2-deoxy-2-fluoro-L-fucose increases humoral and cellular immune responses when administered with a cancer vaccine.
- 2-deoxy-2-fluoro-L-fucose or an intracellular metabolite or product of
- 2-deoxy-2-fluoro-L-fucose increases CD45R01+ T-cell population (memory T-cell phenotype).
- 2-deoxy-2-fluoro-L-fucose (or an intracellular metabolite or product of 2-deoxy-2-fluoro-L-fucose) treated T-cells activate dendritic cells more than non-treated (control) T-cells.
- control control
- 2-deoxy-2-fluoro-L-fucose increases antigen-specific (for example, EBV-specific) tetramer binding.
- 2-deoxy-2-fluoro-L-fucose or an intracellular metabolite or product of 2-deoxy-2-fluoro-L-fucose
- Anti-CTLA4 checkpoint inhibitor has been shown to decrease regulatory T-cell populations and has been successfully combined with other checkpoint inhibitors with different mechanistic targets such as anti-PDl and anti-PD-Ll antibodies.
- 2-deoxy-2-fluoro-L-fucose or an intracellular metabolite or product of
- 2-deoxy-2-fluoro-L-fucose is administered in combination with another checkpoint inhibitors such as an anti-PDl or anti-PD-Ll antibody.
- 2-deoxy-2-fluoro-L-fucose provided herein is useful for treating cancer in a subject.
- Administration of 2-deoxy-2-fluoro-L-fucose to an animal (e.g., a mammal, such as a human) in need thereof can result in inhibition of the multiplication of a tumor cell(s) or cancer cell(s), or treatment of cancer in an animal (e.g. , a human patient).
- 2-deoxy-2-fluoro-L-fucose can be used accordingly in a variety of settings for the treatment of animal cancers.
- cancers that can be treated with 2-deoxy-2-fluoro-L-fucose include, solid tumors and hematologic malignancies.
- Such cancers include, but are not limited to: (1) solid tumors, including but not limited to fibrosarcoma, myxosarcoma, liposarcoma, chondrosarcoma, osteogenic sarcoma, chordoma, angiosarcoma, endotheliosarcoma, lymphangiosarcoma, lymphangioendotheliosarcoma, synovioma, mesothelioma, Ewing's tumor, leiomyosarcoma, rhabdomyosarcoma, colon cancer, colorectal cancer, kidney cancer, pancreatic cancer, bone cancer, breast cancer, ovarian cancer, prostate cancer, esophogeal cancer, stomach cancer, oral cancer, nasal cancer, throat cancer, squamous cell carcinoma, basal cell carcinoma, aden
- 2-deoxy-2-fluoro-L-fucose is soluble in formulation buffer (e.g. aqueous formulation buffer) at a concentration of at least 10 mM. In some embodiments, 2-deoxy-2-fluoro-L-fucose is soluble in formulation buffer at a concentration of at least 100 mM. In some aspects, 2-deoxy-2-fluoro-L-fucose is soluble in formulation buffer (e.g. aqueous formulation buffer) at a concentration of at least 100 ⁇ g/ml, at least 1 mg/ml, at least 50 mg/ml, at least about 100 mg/ml, at least about 200 mg/ml, or at least about 300 mg/ml.
- formulation buffer e.g. aqueous formulation buffer
- 2-deoxy-2-fluoro-L-fucose or its prodrug, or a pharmaceutically acceptable salt thereof can be formulated as pharmaceutical compositions comprising a
- compositions of 2-deoxy-2-fluoro-L-fucose and pharmaceutical excipients are provided in which an effective amount of 2-deoxy-2-fluoro-L-fucose(s) is in admixture with the excipients, suitable for administration to a mammal.
- 2-deoxy-2-fluoro-L-fucose is formulated for administration to a human.
- the present invention provides a pharmaceutical composition comprising 2-deoxy-2-fluoro-L-fucose formulated for administration to a human.
- the formulated 2-deoxy-2-fluoro-L-fucose will generally comprise one or more pharmaceutically compatible (acceptable) ingredients.
- Exemplary pharmaceutical or non-pharmaceutical compositions typically include one or more carriers (e.g. , sterile liquids, such as water and oils, including those of petroleum, animal, vegetable or synthetic origin, such as peanut oil, soybean oil, mineral oil, sesame oil and the like). Water is a more typical carrier when the pharmaceutical composition is administered intravenously. Saline solutions and aqueous dextrose and glycerol solutions can also be employed as liquid carriers, particularly for injectable solutions.
- carriers e.g. , sterile liquids, such as water and oils, including those of petroleum, animal, vegetable or synthetic origin, such as peanut oil, soybean oil, mineral oil, sesame oil and the like.
- Water is a more typical carrier when the pharmaceutical composition is administered intravenously.
- Saline solutions and aqueous dextrose and glycerol solutions can also be employed as liquid carriers, particularly for injectable solutions.
- Suitable excipients include, for example, amino acids, starch, glucose, lactose, sucrose, gelatin, malt, rice, flour, chalk, silica gel, sodium stearate, glycerol monostearate, talc, sodium chloride, dried skim milk, glycerol, propylene, glycol, water, ethanol, and the like.
- the composition if desired, can also contain minor amounts of wetting or emulsifying agents, or pH buffering agents. These compositions can take the form of solutions, suspensions, emulsion, tablets, pills, capsules, powders, sustained-release formulations and the like. Examples of suitable pharmaceutical carriers are described in "Remington's Pharmaceutical Sciences" by E.W. Martin.
- compositions will typically contain a therapeutically effective amount of 2-deoxy-2-fluoro-L-fucose, typically in purified form, together with a suitable amount of carrier so as to provide the form for proper administration to the subject.
- suitable amount of carrier so as to provide the form for proper administration to the subject.
- the formulations correspond to the mode of administration.
- compositions described herein can be in any form that allows for the composition to be administered to an animal (e.g. , a mammal).
- the compositions can be in the form of a solid or liquid.
- Typical routes of administration include, without limitation, oral, parenteral, and sublingual.
- Parenteral administration includes subcutaneous injections, intraperitoneal injections, intravenous,
- compositions are administered orally.
- These pharmaceutical compositions can be formulated so as to allow 2-deoxy-2-fluoro-L-fucose to be bioavailable upon administration of the composition to an animal.
- Compositions can also take the form of one or more dosage units, where for example, a tablet can be a single dosage unit, and a container of 2-deoxy-2-fluoro-L-fucose in solid form can hold a plurality of dosage units.
- compositions can be non-toxic in the amounts used. It will be evident to those of ordinary skill in the art that the optimal dosage of the active ingredient(s) in the pharmaceutical composition will depend on a variety of factors. Relevant factors include, without limitation, the type of animal (e.g., human), the particular form of 2-deoxy-2-fluoro-L-fucose, the manner of administration, the composition employed, and the severity of the disease or condition being treated.
- the pharmaceutically acceptable carrier or vehicle can be particulate, so that the compositions are, for example, in tablet or powder form.
- the carrier(s) can be liquid, with the compositions being, for example, an oral syrup, flavored water, or injectable liquid.
- composition When intended for oral administration, the composition is preferably in solid or liquid form, where semi-solid, semi-liquid, suspension and gel forms are included within the forms considered herein as either solid or liquid.
- the composition can be formulated into a powder, granule, compressed tablet, pill, capsule, chewing gum, wafer or the like form.
- a solid composition typically contains one or more inert diluents.
- binders such as carboxymethylcellulose, ethyl cellulose, microcrystalline cellulose, or gelatin;
- excipients such as starch, lactose or dextrins, disintegrating agents such as alginic acid, sodium alginate, Primogel, corn starch and the like; lubricants such as magnesium stearate or Sterotex; glidants such as colloidal silicon dioxide; sweetening agents such as sucrose or saccharin, a flavoring agent such as peppermint, methyl salicylate or orange flavoring, and a coloring agent.
- composition when in the form of a capsule, e.g., a gelatin capsule, it can contain, in addition to materials of the above type, a liquid carrier such as polyethylene glycol, cyclodextrin or fatty oil.
- a liquid carrier such as polyethylene glycol, cyclodextrin or fatty oil.
- the composition can be in the form of a liquid, e.g., an elixir, syrup, solution, emulsion or suspension.
- the liquid can be useful for oral administration or for delivery by injection.
- a composition can comprise one or more of a sweetening agent, preservatives, dye/colorant, and flavor enhancer.
- the composition is formulated into a powder and the end user mixes the power in an aqueous solution for oral administration.
- a surfactant, preservative, wetting agent, dispersing agent, suspending agent, buffer, stabilizer and isotonic agent can also be included.
- the amount of 2-deoxy-2-fluoro-L-fucose that is effective in the methods described herein will depend on the nature of the disorder or condition, and can be determined by standard clinical techniques.
- in vitro or in vivo assays can optionally be employed to help identify optimal dosage ranges.
- the precise dose to be employed in the compositions will also depend on the route of administration, and the seriousness of the disease or disorder, and should be decided according to the judgment of the practitioner and each patient's circumstances.
- compositions comprise an effective amount of 2-deoxy-2-fluoro-L-fucose such that a suitable dosage will be obtained. Typically, this amount is at least about 0.01% of 2-deoxy-2-fluoro-L-fucose by weight of the composition. In some aspects, when intended for oral administration, this amount can be varied to range from about 0.1% to about 100% by weight of the composition. Preferred oral compositions can comprise, for example, from about 4% to 100%, 4% to 75% or from 4% to about 50% of 2-deoxy-2-fluoro-L-fucose by weight of the composition. [0058] In some aspects, for intravenous administration, the amount administered will be in the range from about 1 to about 500 mg/kg of body weight of
- the oral dosage of 2-deoxy-2-fluoro-L-fucose administered to an animal is about 1 mg/kg to about 1 g/kg of the animal's body weight, more typically about 5 mg/kg, 10 mg/kg, 25 mg/kg, 50 mg/kg, 100 mg/kg, 150 mg/kg, 200 mg/kg, 250 mg/kg, or 300 mg/kg to about 500 mg/kg of the animal's body weight.
- the dosage administered to an animal is about lg, about 5 g, or about 10 g to about 150 g per day, or from about 1 g, about 5 g, about 10 g, about 15 g or about 20 g to about 60 g per day.
- 2-deoxy-2-fluoro-L-fucose or a pharmaceutical composition thereof can be administered on a daily, weekly, biweekly or monthly schedule, according to the desired effect.
- 2-deoxy-2-fluoro-L-fucose or a pharmaceutical composition thereof can be administered from about 1 to 5, about 1 to about 10, about 1 to about 15, or more cycles, wherein each cycle is a month in duration.
- the doses within each cycle can be given on daily (including once daily, twice daily, or more than twice daily), every other day, twice weekly, weekly, bi-weekly, once every three weeks or monthly basis.
- a cycle may optionally include a resting period. Alternatively, a resting period can be included between cycles.
- administration will be for the duration of the disease.
- the preferred mode of administration of 2-deoxy-2-fluoro-L-fucose, or a pharmaceutical composition thereof, is left to the discretion of the practitioner, and will depend in-part upon the site of the medical condition.
- 2-deoxy-2-fluoro-L-fucose or compositions are administered parenterally.
- 2-deoxy-2-fluoro-L-fucose or compositions are administered orally.
- 2-deoxy-2-fluoro-L-fucose can be delivered in a vesicle, in particular a liposome (see Langer, Science 249: 1527-1533 (1990); Treat et al., in LIPOSOMES IN THE THERAPY OF INFECTIOUS DISEASE AND CANCER,
- 2-deoxy-2-fluoro-L-fucose or compositions can be delivered in a controlled release system.
- a pump can be used (see Langer, supra; Sefton, CRC Crit. Ref. Biomed. Eng. 14:201 (1987); Buchwald et al.,
- polymeric materials can be used (see MEDICAL APPLICATIONS OF
- carrier refers to a diluent, adjuvant or excipient, with which 2-deoxy-2-fluoro-L-fucose is administered.
- Such pharmaceutical carriers can be liquids, such as water and oils, including those of petroleum, animal, vegetable or synthetic origin, such as peanut oil, soybean oil, mineral oil, sesame oil and the like.
- the carriers can be saline, gum acacia, gelatin, starch paste, talc, keratin, colloidal silica, urea, and the like.
- auxiliary, stabilizing, thickening, lubricating and coloring agents can be used.
- 2-deoxy-2-fluoro-L-fucose or compositions and pharmaceutically acceptable carriers when administered to an animal, are sterile.
- Water is a preferred carrier when 2-deoxy-2-fluoro-L-fucose are administered intravenously.
- Saline solutions and aqueous dextrose and glycerol solutions can also be employed as liquid carriers, particularly for injectable solutions.
- Suitable pharmaceutical carriers also include excipients such as starch, glucose, lactose, sucrose, gelatin, malt, rice, flour, chalk, silica gel, sodium stearate, glycerol monostearate, talc, sodium chloride, dried skim milk, glycerol, propylene, glycol, water, ethanol and the like.
- excipients such as starch, glucose, lactose, sucrose, gelatin, malt, rice, flour, chalk, silica gel, sodium stearate, glycerol monostearate, talc, sodium chloride, dried skim milk, glycerol, propylene, glycol, water, ethanol and the like.
- the present compositions if desired, can also contain minor amounts of wetting or emulsifying agents, or pH buffering agents.
- Immune checkpoints refer to inhibitory pathways in the immune system that are responsible for maintaining self-tolerance and modulating the degree of immune system response to minimize peripheral tissue damage.
- tumor cells can also activate immune system checkpoints to decrease the effectiveness of immune response ('block' the immune response) against tumor tissues.
- checkpoint inhibitors do not target tumor cells directly, but rather target lymphocyte receptors or their ligands in order to enhance the endogenous antitumor activity of the immune system.
- Therapy with antagonistic checkpoint blocking antibodies against immune system checkpoints such as CTLA4, PD1 and PD-L1 are one of the most promising new avenues of immunotherapy for cancer and other diseases.
- checkpoint targets such as TIM-3, LAG-3, various B-7 ligands, CHK 1 and CHK2 kinases, BTLA, A2aR, and others.
- three checkpoint inhibitors have received rapid approval from the U.S. Food and Drug Administration for cancer treatment, including ipilimumab (Yervoy®), a CTLA-4 inhibitor, and pembrolizumab (Keytruda®) and nivolumab (Opdivo®), both PD-1 inhibitors.
- ipilimumab Yervoy®
- CTLA-4 inhibitor a CTLA-4 inhibitor
- pembrolizumab Keytruda®
- nivolumab Opdivo®
- CTLA-4 has been found to be expressed in tumors at higher levels on regulatory T-cells (also referred to herein as "Treg cells”) as compared with intra-tumoral effector T-cells (also referred to herein as "Teff cells”), resulting in the hypothesis of anti-CTLA-4 preferentially impacting the Treg cell.
- Treg cells regulatory T-cells
- Teff cells intra-tumoral effector T-cells
- WO 2015/069770 discloses a combination treatment based on activating the adaptive immune response, in particular the combination of CTLA-4 and PD-1 inhibitors, for the treatment of cancer.
- the disclosure of WO 2015/069770 is incorporated by reference in its entirety in the disclosure of this application.
- checkpoint blockade anti-CTLA-4 antibodies mediate anti-tumor effect is by decreasing regulatory T-cells. Due to the distinct mechanism of action of anti-CTLA-4 antibodies, they can successfully combine with the anti-PDl checkpoint blockade antibodies which work to release the suppressive signaling conferred to effector T-cells. Dual blockade with these antibodies combine to improve anti-tumor response both preclinically (Proc Natl Acad Sci USA 2010, 107, 4275-4280) and in the clinic (N Engl J Med 2013, 369, 122-133; N Engl J Med 2015, 372, 2006-2017).
- the optimal amount of 2-deoxy-2-fluoro-L-fucose and the checkpoint inhibitor that is effective in the treatment of cancer can be determined by standard clinical techniques.
- in vitro assays may optionally be employed to help identify optimal dosage ranges.
- the precise dose to be employed in the formulation will also depend on the route of administration, and the stage of malignancy, and should be decided according to the judgment of the practitioner and each patient's circumstances. Effective doses may be extrapolated from dose-response curves derived from in vitro or animal model test systems.
- the checkpoint inhibitor and 2-deoxy-2-fluoro-L-fucose are administered simultaneously or sequentially, in either order.
- the checkpoint inhibitor is a PD-1 inhibitor or CTLA-4 inhibitor.
- the checkpoint inhibitor is a PD-1 inhibitor.
- 2-deoxy-2-fluoro-L-fucose and the checkpoint inhibitor will be administered to a subject at the Maximal Tolerable Dose (MTD) or the Optimal Biological Dose (OBD). It is within the art to determine MTD or OBD.
- MTD Maximal Tolerable Dose
- OBD Optimal Biological Dose
- 2-deoxy-2-fluoro-L-fucose will be provided at its MTD or OBD and the checkpoint inhibitor will be dosed at 50%-100%, preferably at 50% to 90% of the MTD or OBD.
- the checkpoint inhibitor will be dosed at its MTD or OBD and 2-deoxy-2-fluoro-L-fucose will be dosed at at 50%- 100%, preferably at 50% to 90% of the MTD or OBD. In some aspects, both 2-deoxy-2-fluoro-L-fucose and the checkpoint inhibitor will be dosed at 60% to 90% of the MTD or OBD.
- the combination regimen can be given simultaneously or can be given in a staggered regimen, with the checkpoint inhibitor being given at a different time during the course of therapy than 2-deoxy-2-fluoro-L-fucose.
- This time differential may range from several minutes, hours, days, weeks, or longer between administration of the two agents. Therefore, the term combination does not necessarily mean administered at the same time or as a unitary dose, but that each of the components are administered during a desired treatment period.
- the agents may also be administered by different routes.
- prodrug of 2-deoxy-2-fluoro-L-fucose or a pharmaceutically acceptable salt of 2-deoxy-2-fluoro-L-fucose or its prodrug is also provided herein.
- 2-deoxy-2-fluoro-L-fucose or its prodrug can be used.
- Example 1 A20 mouse lymphoma study with immune subset depletion
- KLH-A20 Id Fab was generated as described in Okeley et al PNAS 2012.
- A20 cells (ATCC) were cultured in RPMI 1640 with 10% FBS, 10 mM HEPES, 1 mM sodium pyruvate, 50 ⁇ 2-mercaptoethanol and penicillin (100U/ml)/streptomycin (100 ⁇ g/ml) (PS).
- Immunization groups (BALB/c, Harlan) were injected
- CD4 or CD8 T-cells depletion by anti-CD4 (GK1.5) or -CD8 (53-6.72) antibody was verified in blood at day -1, 7, 14, 21, 29 (FACS analysis). Similar results were seen in spleen at day 0 and 29 from BALB/c mice.
- Figures 1A and IB demonstrate that depletion of CD4 or CD8 T-cells reduces the activity of the A20 idiotype vaccine plus
- CD4 and CD8 T-cells play a role in the combination activity.
- T-cells were isolated from 10 mL of whole blood which was centrifuged first at 1200 rpm (300 x g) for 10 min (without brakes). The top layer containing platelets was removed carefully without disrupting the white blood cell layer. Then RosetteSepTM Human T-Cell Enrichment Cocktail (Pan T-cells from StemCell technologies, Vancouver, BC) was added to the remaining blood (500 ⁇ mL blood). This was incubated for 20 min and then 1 mL FBS was added along with 10 mL PBS. Histopaque (20-25 mL) placed in a 50 mL Falcon the prepared blood/PBS solution was overlaid very slowly.
- T-cells were then resuspended in T-cell media (RPMI media supplemented with 10% Fetal calf serum (FCS), 1% PS and spilt into two T25 flasks (with or without 100-200 ⁇ 2-deoxy-2-fluoro-L-fucose).
- CD3/CD28 antibody coated beads (20 ⁇ 7 ⁇ Miltenyi Biotec) were added to activate the T-cells.
- 24 hr IL2 was added (100 ng ⁇ L, R&D Systems). Each time the cells are passaged new IL2 and 2-deoxy-2-fluoro-L-fucose were added.
- PBMCs were isolated similarly to the T-cells, except that 90 mL of blood were used and after the removal of platelets the RosetteSepTM Human T-Cell Enrichment Cocktail was not added, instead an equal volume of PBS (90 mL) was added before overlaying on Histopaque. Centrifugation over Histopaque as above results in
- the PBMCs were resuspended in dendritic cell (DC) media (30% DMEM 70% X-VIVOTM 15 + 2 mM glutamax + 10% ATB serum + 1% PS), were plated into a 6-well plate and incubated overnight. The following day the supernatant was aspirated and discarded while the adhering cells were washed in media and then replenished with 2 mL media supplemented with IL4 (100 ng ⁇ L, R&D Systems) and GM-CSF (200 ng ⁇ L, R&D Systems) per well to differentiate and proliferate the adhering monocytes into DCs. The plate was incubated for 4-5 days and then cells moved into a T25 flask. Each time the cells were passaged (every 2-3 days) new cytokines were given.
- DC dendritic cell
- Transwell assays were performed in 24-well plates. Co-cultures were plated out as described above with the final volume per well of 500 ⁇ . For the transwell samples DCs were plated in the well and T-cells placed into the insert. The cells were then incubated 24 h and examined in the same manner as co-cultures without trans wells.
- DCs were grown as described until day 8 when there were split into two flasks, one with normal media and the other in serum free media. While no serum was present, X-VIVOTM 15 media contains growth factors which help maintain the health of the cultures. The co-culture experiment was performed as above in 48-well plates.
- Co-cultures were examined using FACS analysis. After incubation, cells were washed in BD stain buffer (BSA) with Human Fc block (EMD Millipore) and incubated on ice for 30 min. Cells were then stained with fluorescently labeled anti-MHCII, anti-CD86, anti-CD83, and anti-CD40 primary antibodies (BD, 1: 100 or 1 :50 in BD stain buffer (BSA)) or with appropriate isotype controls (ice, dark, 40 min). Cells were then washed twice in BD stain buffer (BSA) and analyzed on an LSRII flow cytometer.
- BSA BD stain buffer
- EMD Millipore Human Fc block
- FIG. 2B demonstrates that co-culture experiments of T-cells with autologous DCs revealed that 2-deoxy-2-fluoro-L-fucose-treated T-cells activate DCs more so than control T-cells. This is shown by increases in the DC activation and maturation markers MHCII, CD86, CD83 and CD40. These increases are contact dependent, since they do not occur when transwell inserts are used to separate T-cells and DCs, suggesting that a soluble factor is not likely to be solely responsible. DCs alone do not show changes in these markers either with or without 2-deoxy-2-fluoro-L-fucose treatment. This interaction also requires antigen to be present for the increases in these cell surface activation markers since serum-free medium does not provide the same increases.
- Antigen specific tetramers and negative control tetramer were purchased from MBL (Woburn, MA). T-cells expanded as described above with or without
- 2-deoxy-2-fluoro-L-fucose 100 ⁇ were plated in a round bottom 96- well plate, were centrifuged, and re-suspended in BD stain buffer (BSA) with Human Fc block (EMD Millipore) and incubated at RT for 10 min. Cells were then stained with the desired tetramer 10 ⁇ ⁇ or negative control and incubated for 30 min RT in the dark. Cells were centrifuged and washed in BD stain buffer (BSA) and then resuspened in cold BD BSA stain buffer for analysis with an LSRII flow cytometer.
- BSA BD stain buffer
- EMD Millipore Human Fc block
- Figure 3 demonstrates MFI fold change of binding to 2-deoxy-2-fluoro-L-fucose-treated T-cells compared to control T-cells for three different tetramers (EBV, Ml, and CMV; MBL Bio).
- T-cells were plated in 6-well plates (2.5xl0 6 cells/well, 2.5 mL T-cell medium) and were activated with CD3/CD28 beads (20 beads/well, Miltenyi Biotec, San Diego, CA) at 37 °C for 0-4 h. At the indicated time, samples were then harvested, washed in PBS, lysed with RIPA buffer (Thermo Scientific, containing DNAse and protease/phosphatase inhibitors), and snap frozen on dry ice followed by storage at -80 °C.
- CD3/CD28 beads beads/well, Miltenyi Biotec, San Diego, CA
- Cell lysates ( ⁇ 3 ⁇ g/sample determined by BCA assay) were run on SDS-PAGE and examined by western blot on nitrocellulose membranes with anti-pZAP70 antibody (Cell Signaling Technologies). Briefly, blots were blocked for 1 h at room temperature in 5% milk in TBST (TBST from Cell Signling Technologies), rinsed with TBST and then incubated with primary antibody (anti-pZAP70 1 : 1000 in 5% BSA in TBST) at 4 °C overnight.
- TBST TBST from Cell Signling Technologies
- Blots were washed 3x with TBST and probed with HRP-conjugated secondary antibodies for 1 hr at RT (1:2000 in 5% non-fat milk in TBST) followed by washing (3 x TBST) and detection using Cell Signaling Elite ECL as per manufacturer instructions. Blots were scanned using an AmershamTM 600 imager (GE Healthcare). Once imaged, blots could be stripped and reprobed for additional markers using other primary antibodies. For this method, membranes were stripped for 30 minutes at RT using RestoreTM PLUS Western Blot Stripping Buffer from Thermo Scientific after first washing in PBS. Following this, membranes were washed in TBST and blocking with 5% milk in TBST for 1 hr at RT.
- Figure 4 demonstrates the pZAP70 protein level normalized to the ZAP70 intensity compared to control T-cells with 2-deoxy-2-fluoro-L-fucose-treated T-cells.
- T-cells were plated in 12- well plates (10 6 cells/well, 1-2 mL T-cell medium) and were activated with CD3/CD28 beads (4 ⁇ ⁇ beads/2 mL media, Miltenyi Biotec). Cells were incubated at 37 °C overnight and were then analyzed for Galectin-3 expression after fixation and permeabilization followed by FACS analysis. In brief, cells were pelleted (2xl0 5 and washed twice with BD stain buffer (FBS) followed by resuspension in cold BD CytofixTM fixation buffer (30 min, RT).
- FBS BD stain buffer
- TCR T-cell receptor
- Regulatory T-cells in cultures were identified using the Human Regulatory T-cell Cocktail (BD PharmingenTM) and analyzed using FACS. Briefly, cells were washed into BD stain buffer (BSA) with human Fc block (EMD Millipore) and incubated on ice for 30 min. Cells were then stained with fluorescently labeled Human Regulatory T-cell Cocktail (BD PharmingenTM, 1: 100 in BD stain buffer (BSA)) or with appropriate isotype controls (ice, dark, 40 min). Cells were then washed twice in BD stain buffer (BSA) and analyzed on an LSRII flow cytometer. Regulatory T-cells were identified as CD4+, CD25+ and CD127 LOW. Figures 7A and 7B demonstrate the percent of cells in this triple gated population.
- T-cells were prepared in the same manner as for intracellular staining as above, where they were fixed and permeabilized and stained with an anti-FOXp3 antibody (BD Biosciences) and analyzed by FACS.
- FBS BD stain buffer
- FBS Human Fc block
- RT dark
- FBS BD stain buffer
- TGF R is fucosylated and literature suggests that this fucosylation can affect TGF binding in tumor cells (British Journal of Cancer (2014) 110, 156-163).
- SMAD-mediated transcription requires phosphorylation following TGF binding to the TGF i receptor.
- Figure 6 demonstrates that 2-deoxy-2-fluoro-L-fucose treatment of T-cells results in reduced SMAD2 phosphorylation compared to control T-cells following TGF stimulation, supporting alterations in TGF binding and signaling on afucosylated T-cells.
- FOXp3 expression is driven by the SMAD transcription factors that are downstream from TGF signaling.
- 2-deoxy-2-fluoro-L-fucose-treated T-cells show decreased expression of FOXp3+ (Figure 7A) which was associated with a decrease in regulatory T-cell populations (percent of total cells, Figure 7B). Table 1 shows that this result was translated in vivo when rats were treated with
- anti-CTLA4 antibodies Part of the activity conferred by anti-CTLA4 antibodies is directed depletion of regulatory T-cells (Simpson et al. (2013) JEM, 210).
- Anti-CTLA4 antibodies preclincially, and recently in the clinic, have been successfully combined with anti-PDl or anti-PDLl checkpoint antibodies (Larkin et al. (2015) NEJM 373).
- the effect of 2-deoxy-2-fluoro-L-fucose on reducing regulatory T-cells would suggest that combining 2-deoxy-2-fluoro-L-fucose affected T-cells with PD1 or PDL1 targeting agents would confer further enhenced anti-tumor immunity.
- Example 7 Analysis of Secreted Cytokines in T-cell/DC co-cultures and observed in vivo
- Tissue culture supernatants from T-cell/DC co-cultures described previously were collected and assessed for cytokine changes by Luminex assay.
- blood samples from an A20 vaccine model study were allowed to clot and serum was collected for analysis of cytokines (MCYTOMAGOlLlX-13 Mouse Cytokine
- FIGS. 8A, 8B and 8C demonstrate that in the co-cultures of DCs and 2-deoxy-2-fluoro-L-fucose-treated T-cells, cytokines important for antigen specific T-cell activation such as INFy, IL12p40, and CD40L were increased in tissue culture supernatants when compared to cultures containing control T-cells.
- Figure 9 demonstrates that in addition to cytokine changes observed in tissue culture co-cultures systems, specific increases in cytokines important for immune responses were observed in an A20 tumor vaccine model (described above, no depletion, samples taken at day -21, -14, 0, and 1; IL15). These data indicate that 2-deoxy-2-fluoro-L-fucose treatment can result in the upregulation of cytokines key for optimal immune responses in vitro and in vivo.
- Example 8 In vitro 2FF and anti-PDl antibody combination in human PBMCs [0093] CMV reactive human PMBCs from a CMV positive donor (AstarteTM
- T-cells in the supernatant were isolated using StemSep human T-cell enrichment kit (Stemcell technologies).
- the T-cells were then resuspended in T-cell media (RPMI + 10% Fetal calf serum, 1% PS) and spilt into two T25 flasks (with or without 100-200 ⁇ 2FF).
- CD3CD28 antibody coated beads (Miltenyi Biotec) (20 ⁇ 7 ⁇ 1 ⁇ 483 ⁇ 4 were added to activate the T-cells. After 24 h IL2 was added (100 ng/ ⁇ ). Each time the cells were passaged new IL2 and 2FF were added.
- the cells were cultured for 10-12 days and co-culture experiments were performed in DC media at a ratio of 10: 1 (T-cells:DCs) in 48 well plates with or without 5 ⁇ g/mL CMV antigen stimulation (Astarte Biologies) and with or without 1 ⁇ g/mL anti-PDl (Pembrolizumab, Keytruda). Co-cultures were incubated for 24 h after which supernatants were assayed for IFNy and IL12p70 using the Human T-cell high sensitivity Lumenix assay (Millipore) following the manufacturer's instructions.
- IFNy and IL12p70 in the supernatants from 5 day co-cultures were measured using a Lumenix assay and the concentration of each cytokine was calculated using an internal standard curve.
- 2FF matured T-cells exposed to CMV antigen have an elevated antigen specific T cell response compared to control cell and the addition of anti-PDl to the combination further enhances the antigen specific response of 2FF matured T-cells.
- A20 cells were cultured in RPMI 1640 with 10% FBS, 10 mM HEPES, 1 mM sodium pyruvate, 50 ⁇ 2-mercaptoethanol and penicillin
- 2FF treatment groups received drinking water containing 20 mM 2FF beginning on the day of tumor implantand continued throughout the study. Mice not receiving 2FF were provided with normal drinking water.
- Anti-PDl antibody (Ebiosciences, clone J43) treatment groups received three doses of 5 mg/kg every three days starting on day 5 post tumor implant. As shown in Figure 11, the 2FF alone treated group had increased survival compared to the untreated group while the anti-PDl antibody alone treated group did not improve survival compared to the untreated group.
- the group treated with the combination of 2FF with anti-PDl antibody showed a durable survival of 50% of the mice by 54 days post tumor implant lasting through the end of the experiment at -90 days post tumor implant. This is compared to only 17% survival in the untreated and the anti-PDl alone treated groups after about 40 days of treatment lasting to the end of the experiment, and also compared to only 17% survival in 2FF alone treated group after 70 days of treatment also lasting to the end of the experiment at ⁇ 90 days. These data show that the combination of 2FF and anti-PDl prolonged survival of tumor implanted mice compared to untreated and either 2FF or anti-PDl single agent treatment.
- 2FF has demonstrated the ability to reduce regulatory T-cell population, similar to what is observed with the anti-CTLA4 antibodies.
- the reduction in regulatory T-cell population is associated with attenuation of TGF-beta signaling and FOXp3 induction which is critical for regulatory T-cell development.
- combinatorial activity of 2FF in the presence of anti-PDl blockade antibodies was evaluated both in vitro and in vivo.
- balb/c mice were implanted with 4T1 cells (2xl0 4 injected sc.) and on the same day started on 20 mM 2FF drinking water.
- DMEM high glucose
- FBS 5% FBS
- 1 M HEPES 2 mg/mL collagenase D
- DNAse I 50 mg/mL stock in 20 mM Tri
- the cells phenotyped for T regulatory cells were first stained with the desired antibodies against cell surface antigens for 30 minutes in the dark on ice. The cells were then washed and fixed, permeabilized and stained with FOXp3 using the eBioscience staining set as per the manufactures instructions (catalog#77-5775).
- 2FF-treated animals had significantly decreased T regulatory cells and increased dendritic cells which are more activated at day 19 after implantation. The results also show that at day 28 after implantation, tumors from 2FF-treated animals had increased memory and effector T cells.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Immunology (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Microbiology (AREA)
- Biomedical Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Mycology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Oncology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Abstract
Description
Claims
Priority Applications (12)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| SG11201804263PA SG11201804263PA (en) | 2015-12-04 | 2016-12-02 | Cancer treatment using 2-deoxy-2-fluoro-l-fucose in combination with a checkpoint inhibitor |
| JP2018528650A JP6906520B2 (en) | 2015-12-04 | 2016-12-02 | Cancer treatment with 2-deoxy-2-fluoro-L-fucose in combination with checkpoint inhibitors |
| AU2016362993A AU2016362993A1 (en) | 2015-12-04 | 2016-12-02 | Cancer treatment using 2-deoxy-2-fluoro-L-fucose in combination with a checkpoint inhibitor |
| KR1020187017824A KR20180086233A (en) | 2015-12-04 | 2016-12-02 | Cancer treatments using 2-deoxy-2-fluoro-L-fucose in combination with checkpoint inhibitors |
| MX2018006674A MX2018006674A (en) | 2015-12-04 | 2016-12-02 | Cancer treatment using 2-deoxy-2-fluoro-l-fucose in combination with a checkpoint inhibitor. |
| US15/781,256 US20180353524A1 (en) | 2015-12-04 | 2016-12-02 | Cancer treatment using 2-deoxy-2-fluoro-l-fucose in combination with a checkpoint inhibitor |
| EA201891340A EA201891340A1 (en) | 2015-12-04 | 2016-12-02 | CANCER TREATMENT USING 2-DEOXY-2-FLUOR-L-FUKOZA IN COMBINATION WITH CONTROLLER INHIBITOR INHIBITOR |
| CA3005997A CA3005997A1 (en) | 2015-12-04 | 2016-12-02 | Cancer treatment using 2-deoxy-2-fluoro-l-fucose in combination with a checkpoint inhibitor |
| BR112018011261A BR112018011261A2 (en) | 2015-12-04 | 2016-12-02 | Methods for treating cancer, for inhibiting tumor proliferation and for initiating, enhancing or prolonging the effects of a checkpoint inhibitor or allowing an individual to respond to a checkpoint inhibitor. |
| CN201680071076.0A CN108289903B (en) | 2015-12-04 | 2016-12-02 | Cancer treatment using 2-deoxy-2-fluoro-L-fucose in combination with checkpoint inhibitors |
| EP16871645.4A EP3383404A4 (en) | 2015-12-04 | 2016-12-02 | Cancer treatment using 2-deoxy-2-fluoro-l-fucose in combination with a checkpoint inhibitor |
| IL259479A IL259479B (en) | 2015-12-04 | 2018-05-21 | Cancer treatment using 2-deoxy-2-fluoro-l-fucose in combination with a checkpoint inhibitor |
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562263228P | 2015-12-04 | 2015-12-04 | |
| US62/263,228 | 2015-12-04 | ||
| US201662308583P | 2016-03-15 | 2016-03-15 | |
| US62/308,583 | 2016-03-15 | ||
| US201662321857P | 2016-04-13 | 2016-04-13 | |
| US62/321,857 | 2016-04-13 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017096274A1 true WO2017096274A1 (en) | 2017-06-08 |
Family
ID=58797961
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2016/064783 WO2017096274A1 (en) | 2015-12-04 | 2016-12-02 | Cancer treatment using 2-deoxy-2-fluoro-l-fucose in combination with a checkpoint inhibitor |
Country Status (13)
| Country | Link |
|---|---|
| US (1) | US20180353524A1 (en) |
| EP (1) | EP3383404A4 (en) |
| JP (1) | JP6906520B2 (en) |
| KR (1) | KR20180086233A (en) |
| CN (1) | CN108289903B (en) |
| AU (1) | AU2016362993A1 (en) |
| BR (1) | BR112018011261A2 (en) |
| CA (1) | CA3005997A1 (en) |
| EA (1) | EA201891340A1 (en) |
| IL (1) | IL259479B (en) |
| MX (1) | MX2018006674A (en) |
| SG (2) | SG10202005298RA (en) |
| WO (1) | WO2017096274A1 (en) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018226701A1 (en) * | 2017-06-07 | 2018-12-13 | Seattle Genetics, Inc. | T cells with reduced surface fucosylation and methods of making and using the same |
| WO2019075449A1 (en) * | 2017-10-13 | 2019-04-18 | H. Lee Moffitt Cancer Center And Research Institute, Inc. | Fucosylation and immune surveillance in melanoma |
| WO2020041541A2 (en) | 2018-08-23 | 2020-02-27 | Seattle Genetics, Inc. | Anti-tigit antibodies |
| US11033561B2 (en) | 2010-08-05 | 2021-06-15 | Seagen Inc. | Methods of inhibition of protein fucosylation in vivo using fucose analogs |
| US12036286B2 (en) | 2021-03-18 | 2024-07-16 | Seagen Inc. | Selective drug release from internalized conjugates of biologically active compounds |
| WO2024191807A1 (en) | 2023-03-10 | 2024-09-19 | Seagen Inc. | Methods of treating cancer with anti-tigit antibodies |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA3037380A1 (en) | 2016-10-11 | 2018-04-19 | Agenus Inc. | Anti-lag-3 antibodies and methods of use thereof |
| MX2021007327A (en) * | 2018-12-19 | 2021-09-08 | Seagen Inc | Controlled fucosylation of antibodies. |
| WO2021034774A1 (en) * | 2019-08-16 | 2021-02-25 | H. Lee Moffitt Cancer Center And Research Institute, Inc. | Fucosylation and immune modulation in cancer |
| CN111973749B (en) * | 2020-09-07 | 2023-03-21 | 威海人生药业集团股份有限公司 | Pharmaceutical composition for anti-tumor immunotherapy |
| WO2022221766A1 (en) * | 2021-04-16 | 2022-10-20 | H. Lee Moffitt Cancer Center And Research Institute, Inc. | Fucosylation and immune modulation in cancer |
| CN113274502B (en) * | 2021-05-05 | 2023-01-03 | 中山大学肿瘤防治中心(中山大学附属肿瘤医院、中山大学肿瘤研究所) | Compositions for specific type three-negative breast cancer immunotherapy |
| US20240238318A1 (en) * | 2021-05-06 | 2024-07-18 | H. Lee Moffitt Cancer Center And Research Institute, Inc. | L-fucose and anti-androgen receptor therapy for treatment of cancer |
| CN113413465B (en) * | 2021-06-15 | 2022-06-03 | 北京大学 | Application of fucosylation inhibitor in resisting inflammation caused by cancer |
| CN115466297B (en) * | 2022-08-25 | 2023-07-07 | 青岛农业大学 | Application of L-fucose and animal feed |
| WO2024077106A2 (en) * | 2022-10-04 | 2024-04-11 | H. Lee Moffitt Cancer Center And Research Institute, Inc. | Leveraging l-fucose-mediated signaling to induce monocyte-derived dendritic cell polarization |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012019165A2 (en) | 2010-08-05 | 2012-02-09 | Seattle Genetics, Inc. | Methods of inhibition of protein fucosylation in vivo using fucose analogs |
| WO2015069770A1 (en) | 2013-11-05 | 2015-05-14 | Cognate Bioservices, Inc. | Combinations of checkpoint inhibitors and therapeutics to treat cancer |
-
2016
- 2016-12-02 MX MX2018006674A patent/MX2018006674A/en unknown
- 2016-12-02 SG SG10202005298RA patent/SG10202005298RA/en unknown
- 2016-12-02 CN CN201680071076.0A patent/CN108289903B/en not_active Expired - Fee Related
- 2016-12-02 BR BR112018011261A patent/BR112018011261A2/en not_active Application Discontinuation
- 2016-12-02 SG SG11201804263PA patent/SG11201804263PA/en unknown
- 2016-12-02 AU AU2016362993A patent/AU2016362993A1/en not_active Abandoned
- 2016-12-02 JP JP2018528650A patent/JP6906520B2/en active Active
- 2016-12-02 KR KR1020187017824A patent/KR20180086233A/en unknown
- 2016-12-02 CA CA3005997A patent/CA3005997A1/en not_active Abandoned
- 2016-12-02 WO PCT/US2016/064783 patent/WO2017096274A1/en active Application Filing
- 2016-12-02 EA EA201891340A patent/EA201891340A1/en unknown
- 2016-12-02 EP EP16871645.4A patent/EP3383404A4/en not_active Withdrawn
- 2016-12-02 US US15/781,256 patent/US20180353524A1/en not_active Abandoned
-
2018
- 2018-05-21 IL IL259479A patent/IL259479B/en unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012019165A2 (en) | 2010-08-05 | 2012-02-09 | Seattle Genetics, Inc. | Methods of inhibition of protein fucosylation in vivo using fucose analogs |
| WO2015069770A1 (en) | 2013-11-05 | 2015-05-14 | Cognate Bioservices, Inc. | Combinations of checkpoint inhibitors and therapeutics to treat cancer |
| US20150202291A1 (en) | 2013-11-05 | 2015-07-23 | Cognate Bioservices, Inc. | Combinations of checkpoint inhibitors and therapeutics to treat cancer |
Non-Patent Citations (20)
| Title |
|---|
| "CONTROLLED DRUG BIOAVAILABILITY, DRUG PRODUCT DESIGN AND PERFORMANCE", 1984, WILEY |
| "MEDICAL APPLICATIONS OF CONTROLLED RELEASE", 1974, CRC PRES. |
| BUCHWALD ET AL., SURGERY, vol. 88, 1980, pages 507 |
| CHRISTIAN U. BLANKALEXANDER ENK: "Therapeutic use of anti-CTLA-4 antibodies", INTERNATIONAL IMMUNOLOGY, vol. 27, no. 1, pages 3 - 10, XP055422940, DOI: 10.1093/intimm/dxu076 |
| CURRAN ET AL.: "Combination of CTLA-4 and PD-1 blockade expands infiltrating T-cells and reduces regulatory T and myeloid cells within B16 melanoma tumors", PNAS, vol. 107, no. 9, 2 March 2010 (2010-03-02), pages 4275 - 4280, XP055611670, DOI: 10.1073/pnas.0915174107 |
| DURING ET AL., ANN. NEUROL., vol. 25, 1989, pages 351 |
| ELLER, CE ET AL.: "Human Cancer Antigen Globo H Is a Cell -Surface Ligand for Human Ribonuclease 1", ACS CENTRAL SCIENCE ., vol. 1, no. 4, 13 July 2015 (2015-07-13), pages 181 - 190, XP055367234 * |
| HOWARD ET AL., J. NEUROSURG., vol. 71, 1989, pages 105 |
| LANGER, SCIENCE, vol. 249, 1990, pages 1527 - 1533 |
| LARKIN ET AL., THE NEW ENGLAND JOURNAL OF MEDICINE, vol. 373, no. 1, 2 July 2015 (2015-07-02), pages 23 - 34 |
| LEVY ET AL., SCIENCE, vol. 228, 1985, pages 190 |
| N ENGL JMED, vol. 369, 2013, pages 122 - 133 |
| NENGL J MED, vol. 372, 2015, pages 2006 - 2017 |
| OKELEY ET AL., CANCER RESEARCH, vol. 74, no. 19, 30 September 2014 (2014-09-30), pages 2890 - 2890 |
| PARDOLL, NATURE REVIEWS CANCER, vol. 12, 2012, pages 252 - 264 |
| PROC NATL ACAD SCI USA, vol. 107, 2010, pages 4275 - 4280 |
| RANGERPEPPAS, J. MACROMOL. SCI. REV. MACROMOL. CHEM., vol. 23, 1983, pages 61 |
| SAUDEK ET AL., N. ENGL. J. MED., vol. 321, 1989, pages 574 - 365 |
| See also references of EP3383404A4 |
| SEFTON, CRC CRIT. REF BIOMED. ENG., vol. 14, 1987, pages 201 |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11033561B2 (en) | 2010-08-05 | 2021-06-15 | Seagen Inc. | Methods of inhibition of protein fucosylation in vivo using fucose analogs |
| WO2018226701A1 (en) * | 2017-06-07 | 2018-12-13 | Seattle Genetics, Inc. | T cells with reduced surface fucosylation and methods of making and using the same |
| CN110740734A (en) * | 2017-06-07 | 2020-01-31 | 西雅图基因公司 | T cells with reduced surface fucosylation and methods of making and using the same |
| US11891644B2 (en) | 2017-06-07 | 2024-02-06 | Seagen Inc. | T cells with reduced surface fucosylation and methods of making and using the same |
| WO2019075449A1 (en) * | 2017-10-13 | 2019-04-18 | H. Lee Moffitt Cancer Center And Research Institute, Inc. | Fucosylation and immune surveillance in melanoma |
| US20200330494A1 (en) * | 2017-10-13 | 2020-10-22 | H. Lee Moffitt Cancer Center And Research Institute, Inc. | Fucosylation and immune surveillance in melanoma |
| US11911404B2 (en) | 2017-10-13 | 2024-02-27 | H. Lee Moffitt Cancer Center And Research Institute, Inc. | Fucosylation and immune surveillance in melanoma |
| WO2020041541A2 (en) | 2018-08-23 | 2020-02-27 | Seattle Genetics, Inc. | Anti-tigit antibodies |
| US12036286B2 (en) | 2021-03-18 | 2024-07-16 | Seagen Inc. | Selective drug release from internalized conjugates of biologically active compounds |
| WO2024191807A1 (en) | 2023-03-10 | 2024-09-19 | Seagen Inc. | Methods of treating cancer with anti-tigit antibodies |
Also Published As
| Publication number | Publication date |
|---|---|
| SG11201804263PA (en) | 2018-06-28 |
| CN108289903A (en) | 2018-07-17 |
| JP2019501145A (en) | 2019-01-17 |
| US20180353524A1 (en) | 2018-12-13 |
| IL259479A (en) | 2018-07-31 |
| EA201891340A1 (en) | 2018-11-30 |
| KR20180086233A (en) | 2018-07-30 |
| SG10202005298RA (en) | 2020-07-29 |
| EP3383404A4 (en) | 2019-07-31 |
| CA3005997A1 (en) | 2017-06-08 |
| IL259479B (en) | 2022-03-01 |
| EP3383404A1 (en) | 2018-10-10 |
| AU2016362993A1 (en) | 2018-07-12 |
| JP6906520B2 (en) | 2021-07-21 |
| BR112018011261A2 (en) | 2018-11-21 |
| CN108289903B (en) | 2021-08-03 |
| MX2018006674A (en) | 2018-11-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20180353524A1 (en) | Cancer treatment using 2-deoxy-2-fluoro-l-fucose in combination with a checkpoint inhibitor | |
| US11779555B2 (en) | Combination of immunotherapy with local chemotherapy for the treatment of malignancies | |
| JP2024060024A (en) | T cell receptor constructs and uses thereof | |
| JP7360418B2 (en) | Beta-glucan methods and compositions that influence the intratumoral microenvironment | |
| CN117100753A (en) | Use of Plinabulin in combination with immune checkpoint inhibitors | |
| JP6893594B2 (en) | Β-Glucan in combination with antineoplastic agents that affect the tumor microenvironment | |
| EP3132802B1 (en) | Therapeutic agent for solid cancer | |
| JP2018522045A (en) | Modulation of EZH2 inhibitor and regulatory T cell function | |
| JP2022512161A (en) | Compositions and Methods for Immunotherapy | |
| AU2018344001A1 (en) | Immunogenic composition for the treatment of cancer | |
| WO2017214565A1 (en) | Methods of use and pharmaceutical combinations of hdac inhibitors with bet inhibitors | |
| TW202241468A (en) | Treatment of cancer patients with tumor infiltrating lymphocyte therapies in combination with braf inhibitors and/or mek inhibitors | |
| Lee et al. | Venlafaxine inhibits the development and differentiation of dendritic cells through the regulation of P-glycoprotein | |
| WO2019232533A1 (en) | Combination treatments of hsp90 inhibitors for enhancing tumor immunogenicity and methods of use thereof | |
| KR102352127B1 (en) | Composition for inhibiting myeloid-derived suppressor cells comprising MITF inhibitor | |
| WO2018222989A1 (en) | Ceramide nanoliposomes, compositions and methods of using for immunotherapy | |
| CN113316449A (en) | Guanabenz as an adjuvant for immunotherapy |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16871645 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 11201804263P Country of ref document: SG Ref document number: 259479 Country of ref document: IL |
|
| ENP | Entry into the national phase |
Ref document number: 3005997 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2018/006674 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2018528650 Country of ref document: JP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112018011261 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 20187017824 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 201891340 Country of ref document: EA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2016871645 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2016362993 Country of ref document: AU Date of ref document: 20161202 Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2016871645 Country of ref document: EP Effective date: 20180704 |
|
| ENP | Entry into the national phase |
Ref document number: 112018011261 Country of ref document: BR Kind code of ref document: A2 Effective date: 20180601 |