WO2017040937A1 - Solid soluble ferric pyrophosphate formulations, kits, and methods using the same - Google Patents
Solid soluble ferric pyrophosphate formulations, kits, and methods using the same Download PDFInfo
- Publication number
- WO2017040937A1 WO2017040937A1 PCT/US2016/050120 US2016050120W WO2017040937A1 WO 2017040937 A1 WO2017040937 A1 WO 2017040937A1 US 2016050120 W US2016050120 W US 2016050120W WO 2017040937 A1 WO2017040937 A1 WO 2017040937A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- amount
- solid particulate
- weight
- sfp
- dialysate
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1611—Inorganic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/28—Compounds containing heavy metals
- A61K31/295—Iron group metal compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1617—Organic compounds, e.g. phospholipids, fats
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1617—Organic compounds, e.g. phospholipids, fats
- A61K9/1623—Sugars or sugar alcohols, e.g. lactose; Derivatives thereof; Homeopathic globules
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1682—Processes
- A61K9/1688—Processes resulting in pure drug agglomerate optionally containing up to 5% of excipient
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/14—Dialysis systems; Artificial kidneys; Blood oxygenators ; Reciprocating systems for treatment of body fluids, e.g. single needle systems for hemofiltration or pheresis
- A61M1/16—Dialysis systems; Artificial kidneys; Blood oxygenators ; Reciprocating systems for treatment of body fluids, e.g. single needle systems for hemofiltration or pheresis with membranes
- A61M1/1654—Dialysates therefor
- A61M1/1656—Apparatus for preparing dialysates
- A61M1/1666—Apparatus for preparing dialysates by dissolving solids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/14—Dialysis systems; Artificial kidneys; Blood oxygenators ; Reciprocating systems for treatment of body fluids, e.g. single needle systems for hemofiltration or pheresis
- A61M1/28—Peritoneal dialysis ; Other peritoneal treatment, e.g. oxygenation
- A61M1/287—Dialysates therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/08—Plasma substitutes; Perfusion solutions; Dialytics or haemodialytics; Drugs for electrolytic or acid-base disorders, e.g. hypovolemic shock
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/06—Solids
- A61M2202/064—Powder
Definitions
- the present disclosure relates to a solid particulate formulation comprising soluble ferric pyrophosphate that can be mixed with dialysis solution to form a dialysate and administered to patients.
- Iron deficiency is the most common micronutrient deficiency in the world. Iron has several vital physiological functions, including: (1) carrier of oxygen from lung to tissues; (2) transporter of electrons within cells; and (3) co-factor of essential enzymatic reactions in neurotransmission, synthesis of steroid hormones, synthesis of bile salts, and detoxification processes in the liver. Severe iron deficiency, i.e., iron deficiency anemia, is therefore particularly debilitating.
- iron deficiency anemia Among the consequences of iron deficiency anemia are increased maternal and fetal mortality, an increased risk of premature delivery and low birth weight, learning disabilities and delayed psychomotor development, reduced work capacity, impaired immunity (high risk of infection), an inability to maintain body temperature, and an associated risk of lead poisoning.
- Iron deficiency anemia commonly affects patients having chronic diseases, such as kidney disease, inflammatory bowel disease, cancer, HIV, and diabetes.
- patients receiving regular dialysis treatments for chronic renal failure very frequently are also afflicted with anemia.
- Dialysis is a procedure for removing waste products from the blood of a patient when the kidneys are unable to do so on their own, for example, patients with chronic renal failure.
- Hemodialysis is a form of dialysis in which waste products are removed from the blood by passing the blood along one side of a semi-permeable membrane and passing a specially formulated dialysis solution (i.e., dialysate) along the other side of the semi-permeable membrane.
- Hemodiafiltration is another method for removing waste products from blood, wherein waste products are removed by convection and dialysate is infused into the patient as a replacement fluid.
- the dialysate is an aqueous solution containing various electrolytes.
- the dialysate generally comprises dissolved sodium chloride, potassium chloride, calcium chloride, acetate ions, dextrose and other constituents, in about the same concentration as normal plasma.
- Urea, creatinine, uric acid, phosphate and other metabolites normally eliminated by the kidneys diffuse from the blood of the patient into the dialysate until equivalent concentrations of the compounds are in the blood and dialysate.
- the volume of dialysate fluid used is much greater than the blood volume. The great disparity in volume and the replenishment of dialysate with fresh dialysate ensure that metabolites and excess electrolytes are removed almost completely from the blood.
- the dialysate is generally prepared from a dialysis concentrate formulation, which contains, for example, sodium ions, potassium ions, calcium ions, magnesium ions, chloride ions, acetate ions, citrate, and dextrose; a bicarbonate solution; and water.
- the dialysis concentrate, bicarbonate solution and water are generally combined at, or in close proximity to, the dialysis machine.
- Parenterally administered formulations are, in general, aqueous solutions of specific formulation components, in which the solution pH is in the range from pH 4 to pH 8. Parenteral administration encompasses administration by intravenous injection, intramuscular injection, or dialysis.
- iron-containing compositions for parenteral administration is particularly difficult.
- the solubility of iron compounds in water is strongly dependent on the pH of the solution and the presence of other formulation components.
- iron salts are soluble in acidic solutions.
- iron ions will form insoluble oxides and precipitate from the formulation, unless a chelating agent, such as EDTA is present.
- Soluble ferric pyrophosphate is a complex iron salt that has a molecular mass of about 1000 Da and is highly soluble in aqueous solutions, allowing its infusion via aqueous solutions, e.g., dialysate.
- the administration of SFP overcomes both absolute and functional iron deficiencies in patients, including hemodialysis-dependent CKD (HDD-CKD) patients, and could significantly reduce the amount of erythropoiesis- stimulating agents needed to treat these patients.
- HDD-CKD hemodialysis-dependent CKD
- the present disclosure is directed to a solid particulate formulation of soluble ferric pyrophosphate (SFP).
- the solid particulate formulation of SFP is a powder formulation.
- the solid particulate formulation of SFP is a granule formulation.
- the dialysis concentrate formulation is an acid.
- the dialysis concentrate formulation is a base.
- the present disclosure further provides a kit comprising a solid particulate formulation of SFP and a dialysis concentrate formulation in a solid or liquid form.
- the present disclosure provides a solid particulate formulation of SFP comprising SFP having a particle size less than about 5 microns, wherein the SFP dissolves in aqueous solution, e.g., dialysis solution or intravenous solution or intravenous fluids, in less than 1 minute.
- aqueous solution e.g., dialysis solution or intravenous solution or intravenous fluids
- the solubility of the SFP in aqueous solution is greater than 1 gram per milliliter.
- the SFP has an angle of repose less than 45 degrees, optionally less than 42 degrees.
- the solid particulate formulation is stable in aqueous solution at ambient temperature for at least 24 months.
- the solid particulate formulation of SFP comprises SFP comprising iron chelated with citrate and pyrophosphate.
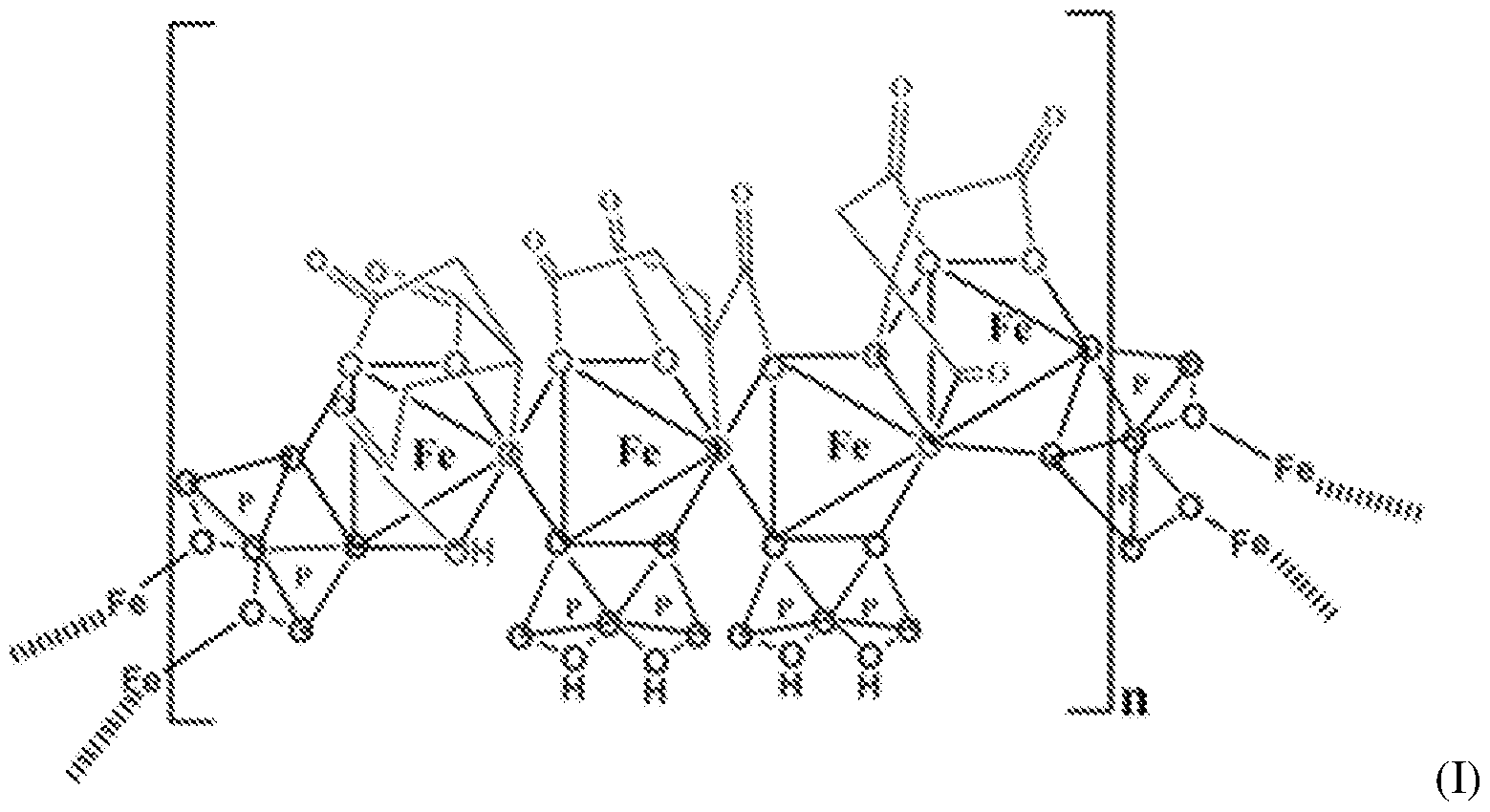
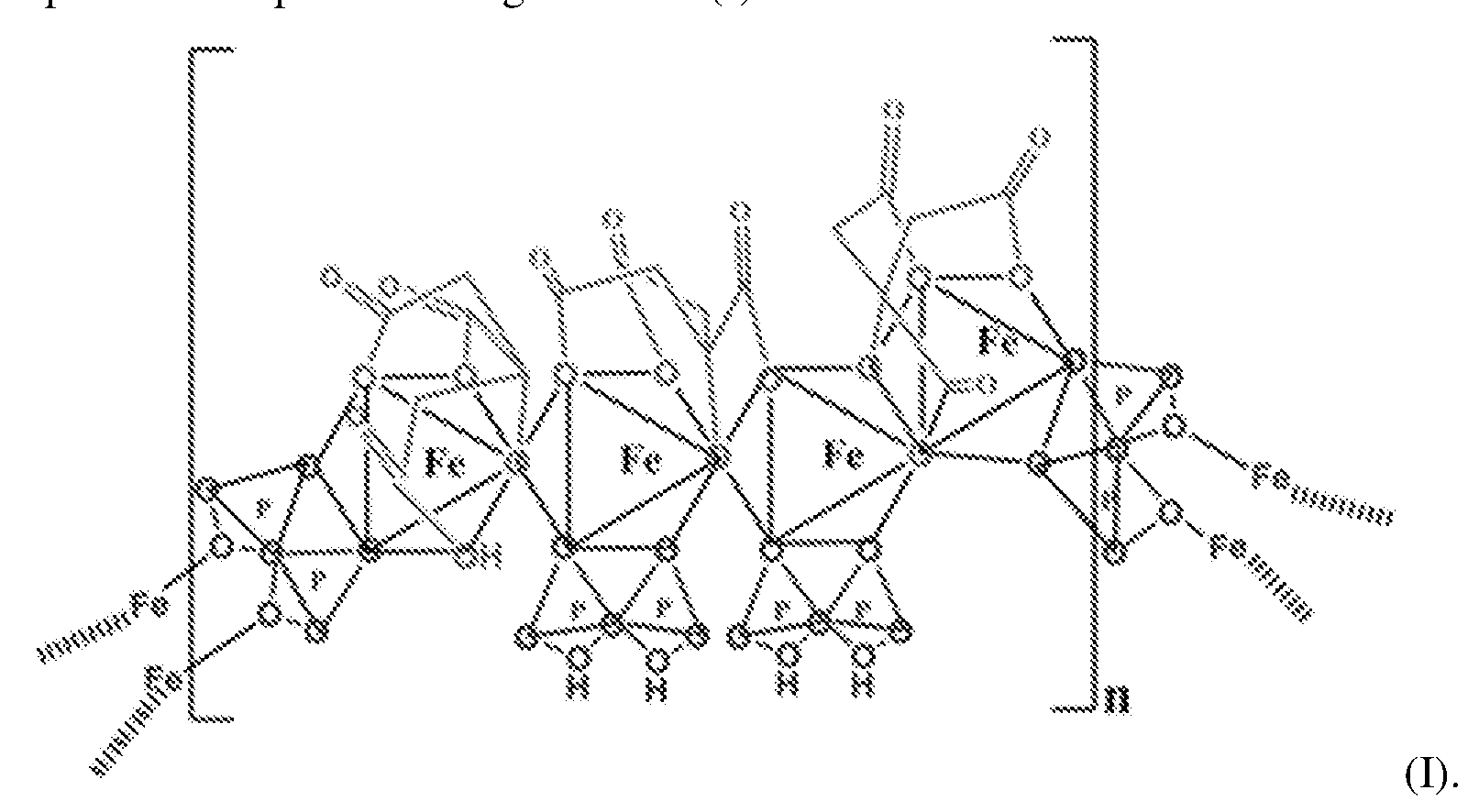
- the SFP comprising iron chelated with citrate and pyrophosphate is ferric pyrophosphate citrate (FPC) having structure (I):
- the solid particulate formulation comprises SFP comprising iron in an amount of 7% to 11% by weight, citrate in an amount of at least 14% by weight, and
- pyrophosphate in an amount of at least 10% by weight.
- the present disclosure also provides a sachet (e.g., a packet) comprising a solid particulate formulation of SFP described herein.
- the sachet comprises a dose or multiple doses of SFP that will be added to a dialysis solution to form a dialysate and result in a final iron concentration of about 110 mcg/L (about 2 ⁇ ) in the dialysate to be administered to a patient e.g., during hemodialysis or hemodiafiltration.
- the sachet comprises a dose or multiple doses of SFP that will be added to an intravenous solution to form an intravenous fluid with a final iron concentration of 1 mg per liter to 1 mg per mL, to be administered to a patient via intravenous injection or infusion.
- the sachet comprises a dose or multiple doses of SFP that can be mixed with food or drink to provide a nutritional supplement.
- the present disclosure further provides an improved method of administering SFP comprising (a) mixing a solid particulate formulation of SFP with a dialysis solution to form a dialysate and (b) administering the dialysate to a subject in need.
- the dialysis solution is an acid solution.
- the acid solution comprises citrate or lactate.
- the dialysis solution is a base solution.
- the base solution comprises bicarbonate.
- the solubility of the solid particulate formulation in the base solution is greater than 1 gram per mL.
- the mixing of the solid particulate formulation of SFP with the dialysis solution results in an iron concentration of about 100 meg per L to about 150 meg per L in the dialysate.
- the improved method of administering SFP comprises administering a solid particulate formulation of SFP comprising iron chelated with citrate and pyrophosphate.
- the improved method of administering SFP comprises administering a solid particulate formulation of SFP comprising iron in an amount of 7% to 11% by weight, citrate in an amount of at least 14% by weight, and pyrophosphate in an amount of at least 10% by weight.
- the improved method of administering SFP comprises administering a solid particulate formulation of FPC comprising iron chelated with citrate and pyrophosphate having structure (I).
- the present disclosure provides a solid particulate formulation of soluble ferric pyrophosphate (SFP) and a kit comprising a solid particulate formulation of SFP and a dialysis concentrate formulation.
- the disclosure also provides an improved method of administering SFP comprising mixing a solid particulate formulation of SFP described herein with a dialysis solution to form a dialysate and administering the dialysate to a subject in need.
- the solid particulate formulation of SFP described herein and methods of using the same are superior to conventional forms of SFP.
- the solid particulate formulation according to the present disclosure dissolves rapidly and completely in aqueous solutions and thus can be added directly to a dialysis concentrate formulation, e.g., a liquid bicarbonate concentrate, or a dialysate formulation.
- a dialysis concentrate formulation e.g., a liquid bicarbonate concentrate, or a dialysate formulation.
- the solid particulate formulation thus provides many advantages compared to a liquid concentrate formulation of SFP.
- the solid particulate formulation does not need to be sterile, whereas the liquid concentrate formulation must be kept sterile because microbial growth is possible in a liquid formulation, but not in a solid particulate formulation.
- the solid particulate formulation of the present disclosure can be packaged in a sachet and the flowability characteristics of the solid particulate formulation allow for ease of addition by opening the sachet and allowing the solid particulate formulation to simply flow completely into the dialysis concentrate, with very little or no residual SFP remaining in the sachet.
- the solid particulate formulation also minimizes the volume and weight of packaging compared to a liquid formulation, resulting in less required storage space at the manufacturing site, during transport, at the distribution site and at the final site of use (e.g., a dialysis center).
- dialysis refers to the movement of solute and water through a
- dialyzer which separates a solution to be filtered, e.g., blood, from a cleansing solution (the dialysate).
- Dialysis is a clinical treatment procedure by which metabolic by-products, toxins, and excess fluid are removed from the blood of a patient by transfer across a dialysis membrane.
- Dialysis includes hemodialysis, in which a synthetic membrane constitutes the dialysis membrane, and peritoneal dialysis, in which a patient's peritoneal membrane constitutes the dialysis membrane.
- dialysate solution and dialysate” refer to the solution on the opposite side of the dialysis membrane from the patient's blood during dialysis or diafiltration.
- Hemodialysate is generally prepared from two dry powder concentrates, including acid (“A”) and base (“B”) concentrates, which are reconstituted in treated water before use, or from two aqueous concentrates.
- the A concentrate containing an organic acid and electrolytes and osmotic agents other than bicarbonate, is mixed with the B concentrate containing bicarbonate and treated water in a dialysis machine to make the final hemodialysate.
- hemodialysis machines utilize an automated proportioning system to mix salts in deionized water in specific proportions to generate the final dialysate solution.
- the dialysate concentrates are usually supplied by the manufacturer either as a liquid solution ready to use or as a premixed powder that is added to purified water in large reservoirs.
- the concentrates are pumped into a chamber in the dialysis machine, where they are mixed with purified water to make the final dialysate solution.
- the methods of the present disclosure may be used to treat patients undergoing dialysis, such as hemodialysis, or diafiltration, such as hemodiafiltration.
- Hemodialysis uses a hemodialyzer to remove certain solutes from blood by virtue of their concentration gradients across a semipermeable membrane.
- the hemodialyzer also referred to as an artificial kidney, is an apparatus by which hemodialysis is performed, blood being separated by the semipermeable membrane from a solution of such composition as to secure diffusion of certain solutes from the blood.
- the hemodialyzer can be used for ultrafiltration, e.g., during hemodiafiltration, by which differences in fluid pressure bring about filtration of a protein-free fluid from the blood.
- Hemodialysis includes acute hemodialysis and maintenance hemodialysis.
- Maintenance hemodialysis refers to long-term hemodialysis therapy for treatment of end stage renal failure. Patients on maintenance hemodialysis have been estimated to lose about 2 to 3 grams of iron per year, corresponding to approximately 6 ml per day (2 liters per year) blood loss from all sources (Eschbach et al. Ann. Intern Med. 1977, 87(6): 710-3).
- the ionic composition of the final dialysate solution for hemodialysis is as follows: Na + 132 mmol/L to 145 mmol/L, K + 0 mmol/L to 4.0 mmol/L, CI " 99 mmol/L to 112 mmol/L, Ca ++ 2.0 mEq/L to 3.5 mEq/L, Mg +2 0.25 mmol/L to 0.75 mmol/L, dextrose 100 mg/dL to 200 mg/dL, and acetate 4.0 mEq/L to 9.0 mEq/L or citrate 2.0 to 5.0 mEq/L.
- a solid particulate formulation comprising SFP of the present disclosure is compatible with both acetate or citrate and bicarbonate based hemodialysis solutions. In another aspect, a solid particulate formulation comprising SFP of the present disclosure is compatible with only a bicarbonate based hemodialysis solution.
- pill formulation refers to a formulation comprising a population of solid separate particles, optionally in a mixture of sizes, and includes both powder formulations and granular formulations.
- binder formulation refers to a dry mixture of solid particles comprising primary particles having a size range (e.g., diameter) of about 1 ⁇ to aboutlO ⁇ , for example, about 1 ⁇ to about 4 ⁇ , about 3 ⁇ to about 5 ⁇ , about 2 ⁇ to about 6 ⁇ , about 5 ⁇ to about 10 ⁇ , about 1 ⁇ to about 5 ⁇ , about 3 ⁇ to about 4 ⁇ , or about 1 ⁇ , about 2 ⁇ , about 3 ⁇ , about 4 ⁇ , about 5 ⁇ , about 6 ⁇ , about 7 ⁇ , about 8 ⁇ , about 9 ⁇ , or about 10 ⁇ .
- a powder formulation comprises primary particles, aggregates of primary particles (e.g., loose and/or durable aggregates), or combinations thereof.
- Particle size can be determined using methods known in the art, e.g., analytical sieving as described in U.S. Pharmacopeia 29 (USP29), Chapter 786.
- granule formulation refers to a dry mixture of solid particles comprising particles having a size range of about 10 ⁇ to about 50 ⁇ , for example, about 12 ⁇ to about 40 ⁇ , about 15 ⁇ to about 45 ⁇ , about 10 ⁇ to about 40 ⁇ , about 12 ⁇ to about 50 ⁇ , about 20 ⁇ to about 40 ⁇ , or about 10 ⁇ , about 20 ⁇ , about 30 ⁇ , about 40 ⁇ , or about 50 ⁇ .
- a granule formulation may comprise aggregates of powders. Aggregates may be formed using a wet granulation or dry granulation process.
- powder flow can be affected by the pressure on the powder, the environmental conditions (e.g., temperature, humidity), and the testing equipment. Higher flowability or more rapid powder flow indicate a more free-flowing formulation.
- chelate refers to a metal cation and anions that surround the metal cation and are joined to it by electrostatic bonds, for example, a ferric iron cation surrounded by and joined by electrostatic bonds to both citrate and pyrophosphate anions.
- sachet refers to a package, e.g., a bag, pouch, or packet, containing a solid particulate formulation.
- a sachet may be made from any of a number of materials, including paper, plastic, foil, and combinations thereof.
- SFP is an iron preparation of uncertain composition. No definite formula for its constitution is known.
- the term "SFP” refers to a compositions comprising a mixture of ferric pyrophosphate and other salts that has been rendered soluble.
- SFP is mixture of ferric pyrophosphate and sodium citrate and SFP is a mixture of four salts (ferric and sodium pyrophosphates and ferric and sodium citrates)" or SPFP is "ferric pyrophosphate that has been rendered soluble by sodium citrate.”
- SFP single type of SFP
- Conventional SFP is known to have the properties described in Table 1.
- Conventional SFP may be obtained commercially.
- An example of conventional SFP is food grade SFP (FCC-SFP).
- FCC-SFP food grade SFP
- Analysis of FCC-SFP samples has shown that typical preparations contain iron, pyrophosphate anion, citrate anion, phosphate anion, sulfate anion, and sodium (Table 2).
- SFP is the composition is the chelate composition described in US Patent Nos. 7,816,404 and 8,178,709.
- the SFP may be a ferric pyrophosphate citrate (FPC) comprising a mixed-ligand iron compound comprising iron chelated with citrate and
- FPC has the following formula: Fe4(C 6 H 4 0 7 ) 3 (H 2 P 2 0 7 )2(P 2 0 7 ) (relative MW 1313 daltons), e.g., structure (I):
- the present disclosure provides a solid particulate formulation of SFP comprising SFP having a primary particle size less than about 5 ⁇ .
- the SFP can have a particle size of about 5 ⁇ , about 4.5 ⁇ , about 4 ⁇ , about 3.5 ⁇ , about 3 ⁇ , about 2.5 ⁇ , about 2 ⁇ , about 1.5 ⁇ , about 1 ⁇ , or about 0.5 ⁇ .
- a solid particulate formulation of SFP according to the disclosure can be prepared by first forming SFP, e.g., as described in U.S. Patent Nos. 7,816,404 and 8,178,709.
- the desired particle size of the solid particulate formulation may be achieved by using milling techniques and equipment, including, but not limited to, hammer mills, screen mills, pin mills, spiral jet mills, loop jet mills, and fluidized bed jet mills.
- the solid particulate formulation has a median particle size less than about 15 ⁇ , for example, about 15 ⁇ , about 14 ⁇ , about 13 ⁇ , about 12 ⁇ , about 11 ⁇ , or about 10 ⁇ .
- the solid particulate formulation is a granule formulation wherein 90% of the particles have a particle size between about 1 ⁇ and about 50 ⁇ , for example, between about 5 ⁇ and about 50 ⁇ , between about 3 ⁇ and about 40 ⁇ , between about 10 ⁇ and about 30 ⁇ , or between about 1 ⁇ and about 25 ⁇ .
- the particles in the granule formulation may be obtained by using an appropriate milling technique to produce larger particles, or by forming aggregates of powders, for example, by compressing or otherwise agglomerating powder particles.
- the SFP exhibits rapid and complete dissolution in an aqueous solution, such as dialysate, dissolving in aqueous solution in less than about one minute.
- the SFP according to the present disclosure has a crystal structure distinct from FCC-SFP and improved properties including increased aqueous solubility, increased flowability, and faster iron transfer kinetics.
- a solid particulate formulation of SFP is a ferric pyrophosphate citrate (FPC) comprising any SFP composition described herein.
- FPC ferric pyrophosphate citrate
- a solid particulate formulation of the invention comprises a mixed-ligand iron compound comprising iron chelated with citrate and pyrophosphate, optionally FPC has the following formula: Fe 4 (C 6 H 4 07)3(H 2 P 2 07)2(P 2 07) (relative MW 1313 daltons), e.g., structure (I).
- a solid particulate formulation of SFP comprises Fe +3 bound to O as the nearest neighbor (2.00 A) in the primary coordination shell and P (3.20 A) and C (2.98 A) as the next- nearest neighbors in secondary coordination, as determined by X-ray Absorption Fine Structure spectroscopy (EXAFS) and shown below in structure (II) (dotted lines represent first and second coordination shells):
- a solid particulate formulation of SFP comprises a ferric ion covalently bound with one pyrophosphate molecule and two citrate molecules, wherein the coordination environment of iron in the SFP-iron chelate is the same as solid state structure and remains stable for at least months, indicating greater thermodynamic stability, in aqueous solution.
- a solid particulate formulation of SFP according to the disclosure comprises iron in an amount of 7% to 11% by weight, citrate in an amount of at least 14% by weight, and pyrophosphate in an amount of at least 10% by weight.
- a solid particulate formulation of SFP comprises iron in an amount from 7.5-9.0% by weight, citrate in an amount from 15-22% by weight, pyrophosphate in an amount from 15-22% by weight, phosphate in an amount less than 2%, sodium in an amount from 18-25% by weight, and sulfate in an amount from 20-35% by weight.
- a solid particulate formulation of SFP according to the disclosure comprises iron in an amount of 9% to 14% by weight, citrate in an amount of 30% to 60% by weight, and pyrophosphate in an amount of 5% to 20% by weight.
- the SFP according to the present disclosure exhibits significantly higher water solubility compared to FCC-SFP and has a solubility in aqueous solution greater than 1 g per mL.
- a solid particulate formulation of the present disclosure comprises SFP having a high flowability, e.g., as measured in seconds per grams.
- the SFP has improved flowability compared to FCC-SFP, for example, an improvement of at least about 10%, at least about 20%, at least about 30%, at least about 40%, or more.
- Methods of characterizing powder flow are known in the art (see, e.g., USP29, Chapter 1174 and European Pharmacopoeia 8 th Edition, Chapter 2.9.36, incorporated herein by reference). Commonly used methods include measurement of the angle of repose, compressibility (Carr) index, Hausner ratio, and/or flow rate through an orifice.
- Additional methods of analyzing powder flow include determination of cohesivity or avalanching, dielectric imaging, atomic force microscopy, penetrometry, and shear cell (see, e.g., Shah et al., AAPS PharmSciTech, 2008, 9(1): 250-258).
- the angle of repose is related to resistance to movement between particles (interparticulate friction) and is the constant, three-dimensional angle relative to the horizontal base assumed by a cone-shaped pile of powder.
- a symmetrical cone-shaped pile of powder is built by passing a solid particulate formulation through a funnel onto a vibration-free fixed base.
- the height of the funnel is maintained approximately 2 cm to 4 cm above the pile to minimize the effect of falling particles on the top of the pile.
- the SFP in the solid particulate formulation of the present disclosure has an angle of repose less than about 45 degrees, optionally less than about 42 degrees.
- the SFP may have an angle of repose between about 41 degrees and 45 degrees.
- the SFP has an angle of repose of about 41 degrees.
- the SFP in the solid particulate formulation of the present disclosure exhibits significantly faster iron transfer kinetics to apotransferrin compared to FCC-SFP.
- the fast binding kinetics allow Fe 3+ to be sequestered by transferrin for transport in the blood to the bone marrow for hemoglobin synthesis in a physiological manner, while minimizing the release of labile and non-transferrin bound iron.
- a solid particulate formulation of SFP according to the disclosure may optionally comprise one or more pharmaceutically acceptable excipients.
- excipients include, but are not limited to, saccharides (mono-, di-, oligo-, poly-, etc.), alcohols, bulking agents, carriers, disintegrants, diluents, binders, preservatives, salts, additives to improve flowability, and mixtures thereof.
- the excipient(s) may be combined with the SFP in the solid particulate formulation using any conventional technique, optionally using a blender or mixer, e.g., a V- blender, bin blender, static/dynamic continuous blender, planetary blender, high intensity mixer, drum mixer, or tumble mixer.
- kits comprising a solid particulate formulation of SFP and a dialysis concentrate formulation.
- the dialysis concentrate formulation is a solid form.
- a dialysis concentrate formulation in solid form may comprise 100% sodium bicarbonate or 73.7% sodium bicarbonate and 26.3% sodium chloride.
- the dialysis concentrate formulation is a liquid form.
- a dialysis concentrate formulation in liquid form may comprise 60-70 w/v% water, 19-21 w/v% sodium chloride, up to 0.5 w/v% potassium chloride, up to 0.6 w/v% calcium chloride, 0.2-0.3 w/v% magnesium chloride, up to 7 w/v% dextrose, and 10.3-10.9 w/v% sodium acetate.
- a dialysis concentrate formulation in liquid form may comprise 8% sodium bicarbonate in water or 6.6% sodium bicarbonate and 2.3% sodium chloride in water.
- the dialysis concentrate formulation may be an acid or base.
- an acid dialysis concentrate formulation in a solid or liquid form may comprise 75-80 w/v% water, 17-26 w/v% sodium chloride, up to 1.3 w/v% potassium chloride, up to 1.0 w/v% calcium chloride, 0.1-0.6 w/v% magnesium chloride, up to 10 w/v% dextrose, and 0.6-0.7% citric acid.
- an acid dialysis concentrate formulation in solid or liquid form may comprise 75-80 w/v% water, 17-26 w/v% sodium chloride, up to 1.3 w/v% potassium chloride, up to 1.0 w/v% calcium chloride, 0.2-0.6 w/v% magnesium chloride, up to 10 w/v% dextrose, and 0.6-0.7 w/v% acetic acid.
- Dialysate may be prepared from a dialysis concentrate formulation using a mixing system, e.g., as described in U.S. Patent No. 6,395,180, incorporated herein by reference.
- the solid particulate formulation of SFP in the kit is a powder formulation.
- the solid particulate formulation of SFP in the kit is a granule formulation.
- the kit includes written instructions for mixing the solid particulate formulation of SFP with the dialysis concentrate formulation, and optionally diluting the mixture with water to form a dialysis solution to be administered to a patient.
- Solid particulate formulations of SFP of the present disclosure may be stored in packages of various types.
- a solid particulate formulation may be stored in a capsule that is broken, a blister pack that is pierced or peeled, or a sachet that are opened, to allow for the solid particulate formulation contained therein to be added to an aqueous solution, e.g., a dialysis concentrate formulation.
- the solid particulate formulation is formed into a single mass, e.g., a tablet or wafer, that can be added directly to an aqueous solution, or the solid particulate formulation is stored within a dissolvable package that is soluble in an aqueous solution.
- the present disclosure provides a sachet, e.g., a packet, comprising a solid particulate formulation described herein, optionally in a kit.
- the sachet comprises a dose or multiple doses of SFP to be added to a dialysis solution to form a dialysate, optionally to result in a final iron concentration of about 110 mcg/L (about 2 ⁇ ) in the dialysate to be administered to a patient e.g., during hemodialysis or hemodiafiltration.
- the sachet comprises a dose or multiple doses of SFP that will be added to an intravenous solution [to form an intravenous fluid with a final iron concentration of 1 mg per liter to 1 mg per mL, to be administered to a patient via intravenous injection or infusion.
- the sachet comprises a dose of SFP that can be mixed with food or drink to provide a nutritional supplement.
- Table 4 shows the amount of iron derived from SFP present in a sachet according to the present disclosure and the corresponding volume of a bicarbonate dialysis concentrate formulation for mixing with the contents of the sachet.
- the present disclosure provides an improved method of administering SFP comprising
- the dialysis solution is an acid solution.
- the acid solution comprises citrate.
- the solubility of the solid particulate formulation in the acid solution is greater than 1 g/mL.
- the dialysis solution is a base solution.
- the base solution comprises bicarbonate.
- the solubility of the solid particulate formulation in the base solution is greater than 1 g/mL.
- the mixing of the solid particulate formulation of SFP with the dialysis solution results in an iron concentration of about 100 ⁇ g/L to about 150 ⁇ g/L in the dialysate, for example, about 110 ⁇ g/L or about 2 ⁇ .
- the improved method of administering SFP comprises administering a solid particulate formulation of SFP comprising iron chelated with citrate and pyrophosphate, for example, iron (III) covalently bound to pyrophosphate and citrate.
- a solid particulate formulation of SFP comprising iron chelated with citrate and pyrophosphate, for example, iron (III) covalently bound to pyrophosphate and citrate.
- the improved method of administering SFP comprises a solid particulate formulation of SFP comprising iron in an amount of 7% to 11% by weight, citrate in an amount of at least 14% by weight, and pyrophosphate in an amount of at least 10% by weight, for example, iron in an amount from 7.5-9.0% by weight, citrate in an amount from 15-22% by weight, and
- the improved method of administering SFP comprising a solid particulate formulation of SFP comprising iron in an amount of 9% to 14% by weight, citrate in an amount of 30% to 60% by weight, and pyrophosphate in an amount of 5% to 20% by weight, optionally further comprising sodium in an amount from 1% to 15% by weight, and essentially no sulfate.
- the improved method of administering SFP comprises a solid particulate formulation of SFP comprising iron chelated with citrate and pyrophosphate, for example, FPC having the following formula: Fe 4 (C 6 H 4 0 7 )3(H 2 P 2 0 7 ) 2 (P 2 0 7 ) ( relative MW 1313 daltons, e.g., structure (I)..
- the dose of SFP for any of the preceding methods is administered via dialysate at an iron dose ranging from 90 ⁇ g/L dialysate to 150 ⁇ g/L dialysate, or at a dose ranging from 90 ⁇ g/L dialysate to 140 ⁇ g/L dialysate, or at a dose ranging from 90 ⁇ g/L dialysate to 130 ⁇ g/L dialysate, or at a dose ranging from 90 ⁇ g/L dialysate to 120 ⁇ g/L dialysate, or at a dose ranging from 90 ⁇ g/L dialysate to 110 ⁇ g/L dialysate, or at a dose ranging from 90 ⁇ g/L dialysate to 105 ⁇ g/L dialysate, or at a dose ranging from 105 ⁇ g/L dialysate to
- the dose of SFP is administered at a dose of 110 ⁇ g or 2 ⁇ ⁇ SFP-iron per liter of hemodialysate.
- the invention provides for methods wherein the dose of SFP iron is administered via dialysate at a dose of about 105 ⁇ g/L dialysate, about 106 ⁇ g/L dialysate, about 107 ⁇ g/L dialysate, about 108 ⁇ g/L dialysate, about 109 ⁇ g/L dialysate, about 110 ⁇ g/L dialysate, about 111 ⁇ g/L dialysate or about 112 ⁇ g/L dialysate.
- the SFP crosses the dialyzer membrane from the hemodialysate to the blood compartment and the SFP-derived Fe(III) binds rapidly to apotransferrin, for example, to the N-lobe in conjunction with pyrophosphate and to the C lobe in conjunction with carbonate.
- the SFP raises serum ion levels, decreases unsaturated iron binding capacity (UIBC) of plasma by direct binding of SPF-iron to apotransferrin and monoferric transferrin, and/or maintains reticulocyte hemoglobin and whole blood hemoglobin, demonstrating that SFP- derived iron is delivered to the erythron for hemoglobin generation and erythropoiesis.
- UIBC unsaturated iron binding capacity
- the direct iron transfer from SFP to iron binding sites on plasma apotransferrin mimics the physiological handling of dietary iron after absorption, which is a unique mode of action distinct from the mode of action of iron-carbohydrate complexes currently approved for intravenous administration, which are typically nanoparticles that are removed from the circulation, stored, and processed by macrophages prior to release of iron in the circulation for binding to apotransferrin.
- the dose of SFP for any of the preceding methods is administered via infusion at a dose ranging from 2.4 mg to 48 mg per day at a rate of 0.1 to 2 mg per hour.
- the dose of SFP is administered via intravenous injection at a dose ranging from 2.4 mg to 48 mg per day at a rate of 0.1 to 2 mg per hour.
- the present disclosure provides for any of the preceding methods wherein, the dose of SFP is administered into the circulation at a dose ranging from 2.4 mg to 48 mg per day at a rate of 0.1 to 2 mg per hour.
- the dose administered to the subject is based on the bioavailability of SFP using the specific route of administration.
- intravenous injection or delivery into the circulation include a dose ranging from 5 mg to 48 mg per day at a rate of 0.1 to 2 mg per hour, or at a dose ranging from 10 mg to 48 mg per day at a rate of 0.01 to 2 mg per hour, or at a dose ranging from 20 mg to 48 mg per day at a rate of 0.01 to 2 mg per hour, or at a dose ranging from 30 mg to 48 mg per day at a rate of 0.01 to 2 mg per hour, or at a dose ranging from 40 mg to 48 mg per day at a rate of 0.01 to 2 mg per hour, or a dose ranging from 2.4 mg to 48 mg per day at a rate of 1 to 2 mg per hour, or a dose ranging from 5 mg to 48 mg per day at a rate of 1 to 2 mg per hour, or at a dose ranging from 10 mg to
- the methods of the invention may be used to treat a subject in need.
- the invention also provides for using the solid particulate formulation of SFP comprising iron chelated with citrate and pyrophosphate for the preparation of a medicament to treat a subject in need, and provides for compositions comprising the solid particulate formulation of SFP comprising iron chelated with citrate and pyrophosphate for the use in treating a subject in need.
- Suitable subjects are those that would benefit from iron supplementation, including subjects suffering from iron deficiency including anemia and/or subjects undergoing dialysis or diafiltration.
- Anemia is a condition when the number of red blood cells and/or the amount of Hgb found in the red blood cells is below normal, and may be acute or chronic.
- Examples of anemia that may be treated using the formulations, kits, and methods of the present disclosure include, but are not limited to, iron deficiency anemia, renal anemia, anemia of chronic diseases/inflammation, cancer-related anemia, chemotherapy-related anemia, anemia caused by impaired production of ESA, hypochromic anemia, and microcytic anemia,.
- Anemia may cause serious symptoms, including hypoxia, chronic fatigue, lack of concentration, pale skin, low blood pressure, dizziness and heart failure.
- the subject may be suffering from chronic kidney disease (CKD), optionally stage II, III, IV or V.
- CKD chronic kidney disease
- stage II, III, IV or V optionally stage II, III, IV or V.
- the present disclosure provides for any of the preceding methods, uses, or compositions, wherein the subject is undergoing hemodialysis or hemodiafiltration.
- the subject is a CKD patient on hemodialysis (e.g., having HDD-CKD) with iron- restricted erythropoiesis.
- the method comprises administering small regular doses of SFP to replace concurrent CKD and hemodialysis related iron losses to maintain the iron balance in a subject having inadequate iron stores.
- the present disclosure also provides for any of the preceding methods, uses, or compositions, wherein the subject is suffering from anemia of inflammation. [0062] The present disclosure also provides for any of the preceding methods, uses, or compositions, wherein the subject is suffering from infection, optionally chronic infection.
- the present disclosure provides for any of the preceding methods, uses, or compositions, wherein the subject is suffering from cancer, heart failure, autoimmune disease, sickle cell disease, thalassemia, blood loss, transfusion reaction, diabetes, vitamin B 12 deficiency, collagen vascular disease, Shwachman syndrome, thrombocytopenic purpura, Celiac disease, endocrine deficiency state such as hypothyroidism or Addison's disease, autoimmune disease such as Crohn's Disease, systemic lupus erythematosis, rheumatoid arthritis or juvenile rheumatoid arthritis, ulcerative colitis immune disorders such as eosinophilic fasciitis, hypoimmunoglobulinemia, or thymoma/thymic carcinoma, graft vs. host disease, preleukemia, Nonhematologic syndrome (Down's, Dubowwitz, Seckel), Felty syndrome, hemolytic uremic syndrome, myel
- osteomyelofibrosis pancytopenia, pure red-cell aplasia, Schoenlein-Henoch purpura, malaria, protein starvation, menorrhagia, systemic sclerosis, liver cirrhosis, hypometabolic states, congestive heart failure, chronic infections such as HIV/AIDS, tuberculosis, oseomyelitis, hepatitis B, hepatitis C, Epstein-bar virus or parvovirus, T cell leukemia virus, bacterial overgrowth syndrome, fungal or parasitic infections, and/or red cell membrane disorders such as hereditary spherocytosis, hereditary elliptocytosis, heriditray pyrpoikilocytosis, hereditary stomatocytosis, red cell enzyme defects, hypersplenism, immune hemolysis or paroxysmal nocturnal hemoglobinuria.
- chronic infections such as HIV/AIDS, tuberculosis, oseomyelitis,
- the present disclosure provides for any of the preceding methods, uses, or compositions, wherein the anemia is due to overt iron deficiency with depleted iron stores or a functional iron deficiency.
- compositions wherein SFP is administered during hemodialysis or hemodiafiltration within the hemodialysate solution.
- present disclosure provides for any of the preceding methods wherein SFP is administered with oral or parenteral (e.g., intravenous injection or infusion) nutrition within a nutrition admixture.
- the invention also provides for any of the preceding methods, uses, or compositions, wherein SFP is administered at a therapeutically effective dose that i) increases at least one marker of iron status selected from the group consisting of serum iron, transferrin saturation, reticulocyte hemoglobin, serum ferritin, reticulocyte count, and whole blood hemoglobin and ii) decreases or eliminates the need for ESA administration to achieve or maintain target hemoglobin levels, or the need for transfusion of whole blood, packed red blood cell or blood substitutes.
- any of the preceding methods are carried out in a subject is suffering from non-anemic iron deficiency and administration of the therapeutically effective dose of SFP reduces fatigue, increases physical and cognitive ability, or improves exercise tolerance in the subject.
- the present disclosure provides for any of the preceding methods, uses, or compositions wherein SFP is administered in a therapeutically effective dose that will reduce or abolish the clinical manifestations of restless leg syndrome associated with iron deficiency.
- the solid particulate formulations of the present disclosure can be prepared by the methods described in U.S. Patent Nos. 7,816,404 and 8,178,709, incorporated herein by reference.
- the active components of solid particulate formulation may be ordinarily combined with one or more excipients appropriate to the indicated route of administration.
- a solid particulate formulation of SFP comprising iron chelated with citrate and pyrophosphate (FPC having structure (I)) was prepared as described above. Structural characterization of the compounds was carried out using Fe K edge X-ray Absorption Near Edge Structure (XANES) and Extended X-Ray Absorption Fine Structure (EXAFS) spectroscopy to ascertain differences in the coordination environment of Fe in a solid particulate formulation of the present disclosure compared to FCC-SFP. Linear combination fitting of Fe XANES data showed that Iron was in the ferric (Fe 3+ ) state and did not complex with sulfate in the food grade as well as in the solid particulate formulation.
- XANES Fe K edge X-ray Absorption Near Edge Structure
- EXAFS Extended X-Ray Absorption Fine Structure
- Particle morphology of a solid particulate formulation comprising iron chelated with citrate and pyrophosphate prepared as described in Example 1 was examined via light microscopy as dry powder dispersions and dispersions of powder in mineral oil under various objective lenses. The gross visual appearance of the material was a light green cohesive powder. Microscopically, the formulation appeared as loose aggregates and durable agglomerates comprised of primary particles typically > 5 ⁇ . The particle size distribution of the formulation was measured via an in-house particle size method using a Cilas 1180LD laser diffraction particle sizer in liquid dispersion mode with mineral oil as the dispersion media. Three replicate measurements were collected and the results are given in Table 6.
- a sachet comprising Paper/7.5#LDPA/0.000285 ga Foil/13#EAA:LDPE (3.0" x 2.5") containing SFP in a ferric pyrophosphate citrate complex, as described in Example 1, corresponding to 272 mg iron was prepared.
- the SPF was administered to patients undergoing hemodialysis, as described in Gupta et al., Kidney Int. 2015 Nov; 88(5): 1187-94, advance online publication 8 Jul 2015, and Fishbane et al., Nephrol Dial Transplant 2015 Dec; 30(12):2019-26, advance online publication 13 Jul 2015, incorporated herein by reference.
- a total of 292 patients were administered the SFP for periods of up to 48 weeks.
- the SFP having a final concentration of 110 meg iron/L in the dialysate was administered 3 or 4 times per week during hemodialysis. Patients were receiving stable dose of erythropoiesis stimulating agents (ESAs) at baseline, and the ESA doses were not to be changed 6 weeks prior to randomization.
- ESAs erythropoiesis stimulating agents
- the primary endpoint of the studies was the mean change in hemoglobin from baseline to the end-of-treatment period (average hemoglobin of the last one- sixth (l/6th) of the time in the randomized treatment period).
- Table 7 shows the mean changes in hemoglobin (Hgb) and iron parameters in each treatment group from baseline to the end-of- treatment period for the ITT population.
- Table 7 Changes from Baseline to End of Treatment in Hemoglobin, Ferritin, Reticulocyte Hgb (CHr), and Transferrin Saturation (TSAT).
- the pharmacokinetics of serum iron was investigated in healthy volunteers administered the following doses of SFP according to the disclosure: dose of 2.5, 5, 7.5 and 10 mg SFP were intravenously administered over 4 hours, or dose of 15 mg and 20 mg SFP were administered intravenously over 12 hours. After correcting for the basal iron levels, the AUC and Cmax of baseline-corrected serum iron increased in a dose proportional manner. The half-life of serum iron was approximately 1.48 hours, the mean clearance (CL) ranged from 0.406 to 0.556 L/hour, the mean apparent volume of distribution (Vz) ranged from 0.765 to 0.859 L after a 4 hour intravenous administration of SFP according to the disclosure.
Abstract
Description
Claims
Priority Applications (13)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/756,286 US11517555B2 (en) | 2015-09-04 | 2016-09-02 | Solid soluble ferric pyrophosphate formulations, kits, and methods using the same |
| EP16766192.5A EP3344235A1 (en) | 2015-09-04 | 2016-09-02 | Solid soluble ferric pyrophosphate formulations, kits, and methods using the same |
| KR1020187009195A KR20180050346A (en) | 2015-09-04 | 2016-09-02 | Soluble solid ferric pyrophosphate formulation, kit and method of using same |
| JP2018511682A JP7055738B2 (en) | 2015-09-04 | 2016-09-02 | Solid soluble ferric pyrophosphate preparations, kits, and methods of using them |
| AU2016315877A AU2016315877B2 (en) | 2015-09-04 | 2016-09-02 | Solid soluble ferric pyrophosphate formulations, kits, and methods using the same |
| EA201890642A EA201890642A1 (en) | 2015-09-04 | 2016-09-02 | SOLID SOLUBLE IRON PYROPHOSPHATE COMPOSITIONS, KITS AND METHODS OF THEIR APPLICATION |
| MX2018002633A MX2018002633A (en) | 2015-09-04 | 2016-09-02 | Solid soluble ferric pyrophosphate formulations, kits, and methods using the same. |
| CA2997328A CA2997328A1 (en) | 2015-09-04 | 2016-09-02 | Solid soluble ferric pyrophosphate formulations, kits, and methods using the same |
| BR112018004244A BR112018004244A2 (en) | 2015-09-04 | 2016-09-02 | soluble ferric pyrophosphate solid formulations, kits and their methods of use |
| CN201680064674.5A CN108601738A (en) | 2015-09-04 | 2016-09-02 | Solid solubility ferric pyrophosphate preparation, medicine box and its application method |
| IL257796A IL257796B (en) | 2015-09-04 | 2018-02-28 | Solid soluble ferric pyrophosphate formulations, kits, and methods using the same |
| PH12018500463A PH12018500463A1 (en) | 2015-09-04 | 2018-03-02 | Solid soluble ferric pyrophosphate formulations, kits, and methods using the same |
| CONC2018/0002421A CO2018002421A2 (en) | 2015-09-04 | 2018-03-02 | Soluble solid ferric pyrophosphate formulations |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562214908P | 2015-09-04 | 2015-09-04 | |
| US62/214,908 | 2015-09-04 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017040937A1 true WO2017040937A1 (en) | 2017-03-09 |
Family
ID=56926337
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2016/050120 WO2017040937A1 (en) | 2015-09-04 | 2016-09-02 | Solid soluble ferric pyrophosphate formulations, kits, and methods using the same |
Country Status (17)
| Country | Link |
|---|---|
| US (1) | US11517555B2 (en) |
| EP (1) | EP3344235A1 (en) |
| JP (1) | JP7055738B2 (en) |
| KR (1) | KR20180050346A (en) |
| CN (1) | CN108601738A (en) |
| AU (1) | AU2016315877B2 (en) |
| BR (1) | BR112018004244A2 (en) |
| CA (1) | CA2997328A1 (en) |
| CL (1) | CL2018000566A1 (en) |
| CO (1) | CO2018002421A2 (en) |
| EA (1) | EA201890642A1 (en) |
| IL (1) | IL257796B (en) |
| MX (1) | MX2018002633A (en) |
| PE (1) | PE20181162A1 (en) |
| PH (1) | PH12018500463A1 (en) |
| SG (1) | SG10202107245UA (en) |
| WO (1) | WO2017040937A1 (en) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11278651B2 (en) * | 2018-10-17 | 2022-03-22 | Gambro Lundia Ab | Membrane and device for treating restless leg syndrome |
| CN110063965A (en) * | 2019-06-04 | 2019-07-30 | 吉林省富生医疗器械有限公司 | A kind of haemodialysis concentrate |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6395180B2 (en) | 1998-09-18 | 2002-05-28 | Rockwell Medical Technologies, Inc. | Method and apparatus for preparing liquid dialysate |
| US6689275B1 (en) | 1996-12-31 | 2004-02-10 | Ajay Gupta | Method and pharmaceutical composition for replacing iron losses in dialysis patients |
| US6779468B1 (en) | 1997-08-07 | 2004-08-24 | Ajay Gupta | Method and pharmaceutical composition for iron delivery in hemodialysis and peritoneal dialysis patients |
| US7816404B2 (en) | 2007-07-20 | 2010-10-19 | Rockwell Medical Technologies, Inc. | Methods for the preparation and use of ferric pyrophosphate citrate chelate compositions |
| US7857977B2 (en) | 2005-07-12 | 2010-12-28 | Rockwell Medical Technologies, Inc. | Packaging of ferric pyrophosphate for dialysis |
| US8178709B2 (en) | 2009-07-21 | 2012-05-15 | Biolink Life Sciences, Inc. | Iron preparation suitable for pharmaceutical formulation and process for the preparation thereof |
| WO2012092305A2 (en) * | 2010-12-27 | 2012-07-05 | Incube Labs, Llc | Nanonized iron compositions and methods of use thereof |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5177068A (en) * | 1984-04-19 | 1993-01-05 | National Research Development Corporation | Pharmaceutical compositions |
| EP0855906B1 (en) * | 1995-10-17 | 2008-02-20 | Jagotec AG | Insoluble drug delivery |
| JP3778240B2 (en) | 1998-06-22 | 2006-05-24 | ライオン株式会社 | Granulated composition masked by unpleasant taste and method for producing the same |
| NZ522239A (en) * | 2000-04-20 | 2004-03-26 | Skyepharma Canada Inc | Improved water-insoluble drug particle process |
| JP2002302435A (en) | 2000-12-26 | 2002-10-18 | Takeda Chem Ind Ltd | Porous substance and method for producing the same |
| US20040052854A1 (en) | 2000-12-26 | 2004-03-18 | Tomohiro Yoshinari | Porous substances and methods for producing the same |
| US7754243B2 (en) * | 2004-08-03 | 2010-07-13 | Clemson University Research Foundation | Aqueous suspension of nanoscale drug particles from supercritical fluid processing |
| US20060134227A1 (en) * | 2004-12-22 | 2006-06-22 | Bortz Jonathan D | Compositions including iron |
| EP1743530B1 (en) * | 2005-07-15 | 2011-08-31 | Unilever N.V. | Iron fortified food product and additive |
| WO2014121155A1 (en) | 2013-02-01 | 2014-08-07 | Charak Llc | Methods of treating iron deficiency with soluble ferric pyrophosphate |
-
2016
- 2016-09-02 PE PE2018000341A patent/PE20181162A1/en not_active Application Discontinuation
- 2016-09-02 MX MX2018002633A patent/MX2018002633A/en unknown
- 2016-09-02 CA CA2997328A patent/CA2997328A1/en active Pending
- 2016-09-02 EP EP16766192.5A patent/EP3344235A1/en not_active Withdrawn
- 2016-09-02 JP JP2018511682A patent/JP7055738B2/en active Active
- 2016-09-02 EA EA201890642A patent/EA201890642A1/en unknown
- 2016-09-02 US US15/756,286 patent/US11517555B2/en active Active
- 2016-09-02 WO PCT/US2016/050120 patent/WO2017040937A1/en active Application Filing
- 2016-09-02 CN CN201680064674.5A patent/CN108601738A/en active Pending
- 2016-09-02 SG SG10202107245UA patent/SG10202107245UA/en unknown
- 2016-09-02 KR KR1020187009195A patent/KR20180050346A/en not_active Application Discontinuation
- 2016-09-02 AU AU2016315877A patent/AU2016315877B2/en not_active Expired - Fee Related
- 2016-09-02 BR BR112018004244A patent/BR112018004244A2/en not_active IP Right Cessation
-
2018
- 2018-02-28 IL IL257796A patent/IL257796B/en unknown
- 2018-03-02 PH PH12018500463A patent/PH12018500463A1/en unknown
- 2018-03-02 CL CL2018000566A patent/CL2018000566A1/en unknown
- 2018-03-02 CO CONC2018/0002421A patent/CO2018002421A2/en unknown
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6689275B1 (en) | 1996-12-31 | 2004-02-10 | Ajay Gupta | Method and pharmaceutical composition for replacing iron losses in dialysis patients |
| US6779468B1 (en) | 1997-08-07 | 2004-08-24 | Ajay Gupta | Method and pharmaceutical composition for iron delivery in hemodialysis and peritoneal dialysis patients |
| US6395180B2 (en) | 1998-09-18 | 2002-05-28 | Rockwell Medical Technologies, Inc. | Method and apparatus for preparing liquid dialysate |
| US7857977B2 (en) | 2005-07-12 | 2010-12-28 | Rockwell Medical Technologies, Inc. | Packaging of ferric pyrophosphate for dialysis |
| US7816404B2 (en) | 2007-07-20 | 2010-10-19 | Rockwell Medical Technologies, Inc. | Methods for the preparation and use of ferric pyrophosphate citrate chelate compositions |
| US8178709B2 (en) | 2009-07-21 | 2012-05-15 | Biolink Life Sciences, Inc. | Iron preparation suitable for pharmaceutical formulation and process for the preparation thereof |
| WO2012092305A2 (en) * | 2010-12-27 | 2012-07-05 | Incube Labs, Llc | Nanonized iron compositions and methods of use thereof |
Non-Patent Citations (5)
| Title |
|---|
| "European Pharmacopoeia" |
| ESCHBACH ET AL., ANN. INTERN MED, vol. 87, no. 6, 1977, pages 710 - 3 |
| FISHBANE ET AL., NEPHROL DIAL TRANSPLANT, vol. 30, no. 12, 13 July 2015 (2015-07-13), pages 2019 - 26 |
| GUPTA ET AL., KIDNEY INT., vol. 88, no. 5, November 2015 (2015-11-01), pages 1187 - 94 |
| SHAH ET AL., AAPS P7 NRRRISCITEC7, vol. 9, no. 1, 2008, pages 250 - 258 |
Also Published As
| Publication number | Publication date |
|---|---|
| US11517555B2 (en) | 2022-12-06 |
| KR20180050346A (en) | 2018-05-14 |
| MX2018002633A (en) | 2019-02-07 |
| AU2016315877B2 (en) | 2022-05-26 |
| SG10202107245UA (en) | 2021-08-30 |
| IL257796A (en) | 2018-04-30 |
| JP2018529674A (en) | 2018-10-11 |
| CL2018000566A1 (en) | 2018-08-03 |
| BR112018004244A2 (en) | 2018-09-25 |
| PH12018500463A1 (en) | 2018-09-17 |
| PE20181162A1 (en) | 2018-07-19 |
| CN108601738A (en) | 2018-09-28 |
| US20180243256A1 (en) | 2018-08-30 |
| EA201890642A1 (en) | 2018-10-31 |
| JP7055738B2 (en) | 2022-04-18 |
| CO2018002421A2 (en) | 2018-07-19 |
| AU2016315877A1 (en) | 2018-04-05 |
| CA2997328A1 (en) | 2017-03-09 |
| EP3344235A1 (en) | 2018-07-11 |
| IL257796B (en) | 2021-09-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2018203205B2 (en) | Use of ferric citrate in the treatment of chronic kidney disease patients | |
| US7816404B2 (en) | Methods for the preparation and use of ferric pyrophosphate citrate chelate compositions | |
| TWI583378B (en) | Dialysis acid precursor composition, use thereof and method of providing dialysis acid concentrate solution | |
| JP2001513370A (en) | Dialysis solution containing water-soluble vitamins and nutrients | |
| US7857977B2 (en) | Packaging of ferric pyrophosphate for dialysis | |
| KR20140026354A (en) | Pharmaceutical compositions of iron for oral administration | |
| TW201219040A (en) | Dialysis precursor composition | |
| CA2900043A1 (en) | Methods of treating iron deficiency with soluble ferric pyrophosphate | |
| AU2016315877B2 (en) | Solid soluble ferric pyrophosphate formulations, kits, and methods using the same | |
| Zhou et al. | The influence of citrate, maltolate and fluoride on the gastrointestinal absorption of aluminum at a drinking water-relevant concentration: A 26Al and 14C study | |
| EP2934483B1 (en) | Dialysis composition | |
| JP6305156B2 (en) | Iron metabolism improving infusion | |
| CA2638081C (en) | Methods for the preparation and use of ferric pyrophosphate citrate chelate compositions | |
| CN109475519B (en) | HD acid concentrate comprising amino acids | |
| JP6925148B2 (en) | Iron-containing infusion | |
| CA2986095A1 (en) | Liquid pharmaceutical formulations of tetraiodothyronine |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16766192 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2997328 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 11201801689T Country of ref document: SG Ref document number: MX/A/2018/002633 Country of ref document: MX |
|
| ENP | Entry into the national phase |
Ref document number: 2018511682 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2018-000034 I Country of ref document: NI Ref document number: 000341-2018 Country of ref document: PE Ref document number: 12018500463 Country of ref document: PH |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20187009195 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: A201803454 Country of ref document: UA |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112018004244 Country of ref document: BR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 201890642 Country of ref document: EA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2016766192 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2016315877 Country of ref document: AU Date of ref document: 20160902 Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 112018004244 Country of ref document: BR Kind code of ref document: A2 Effective date: 20180302 |