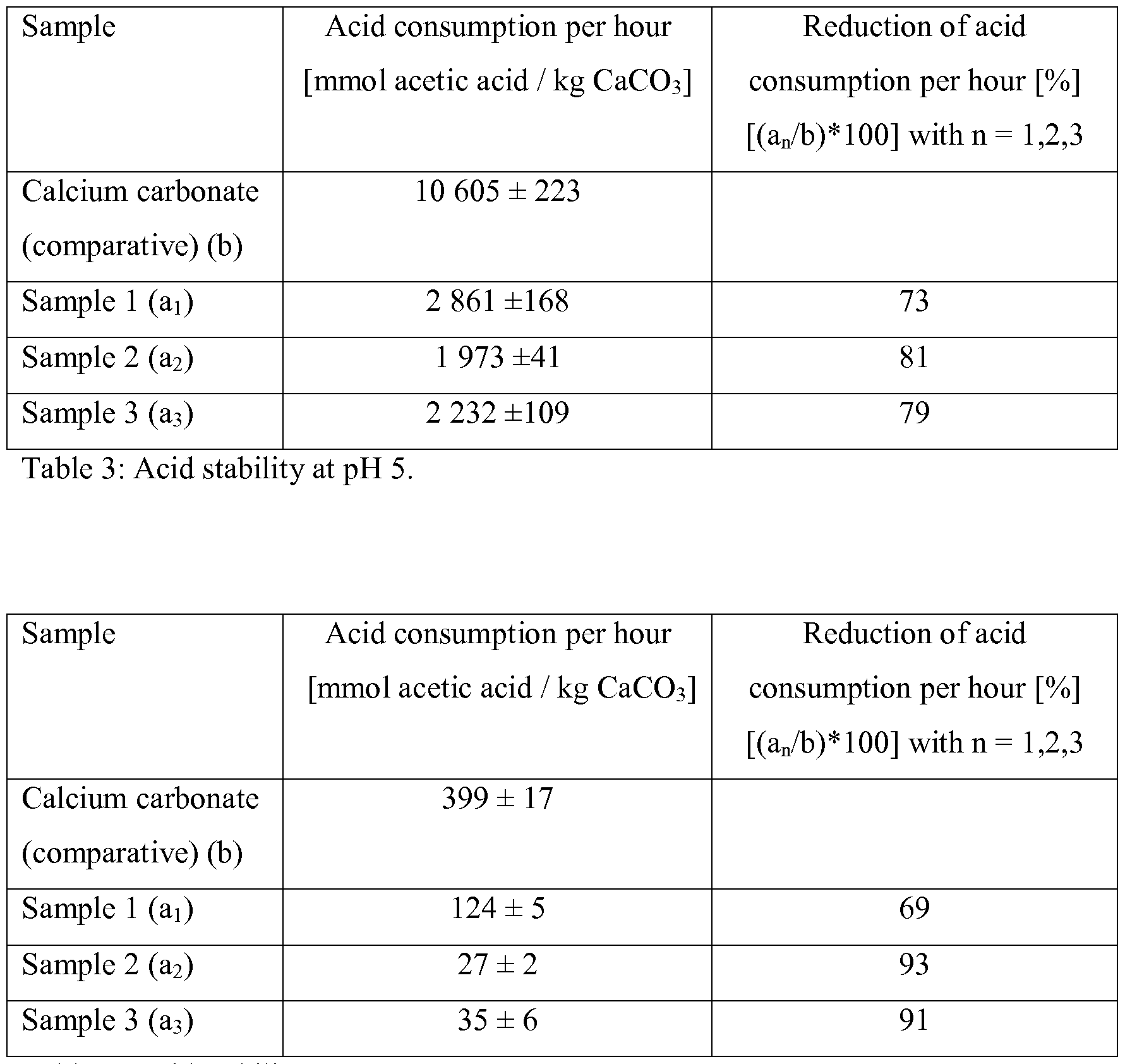
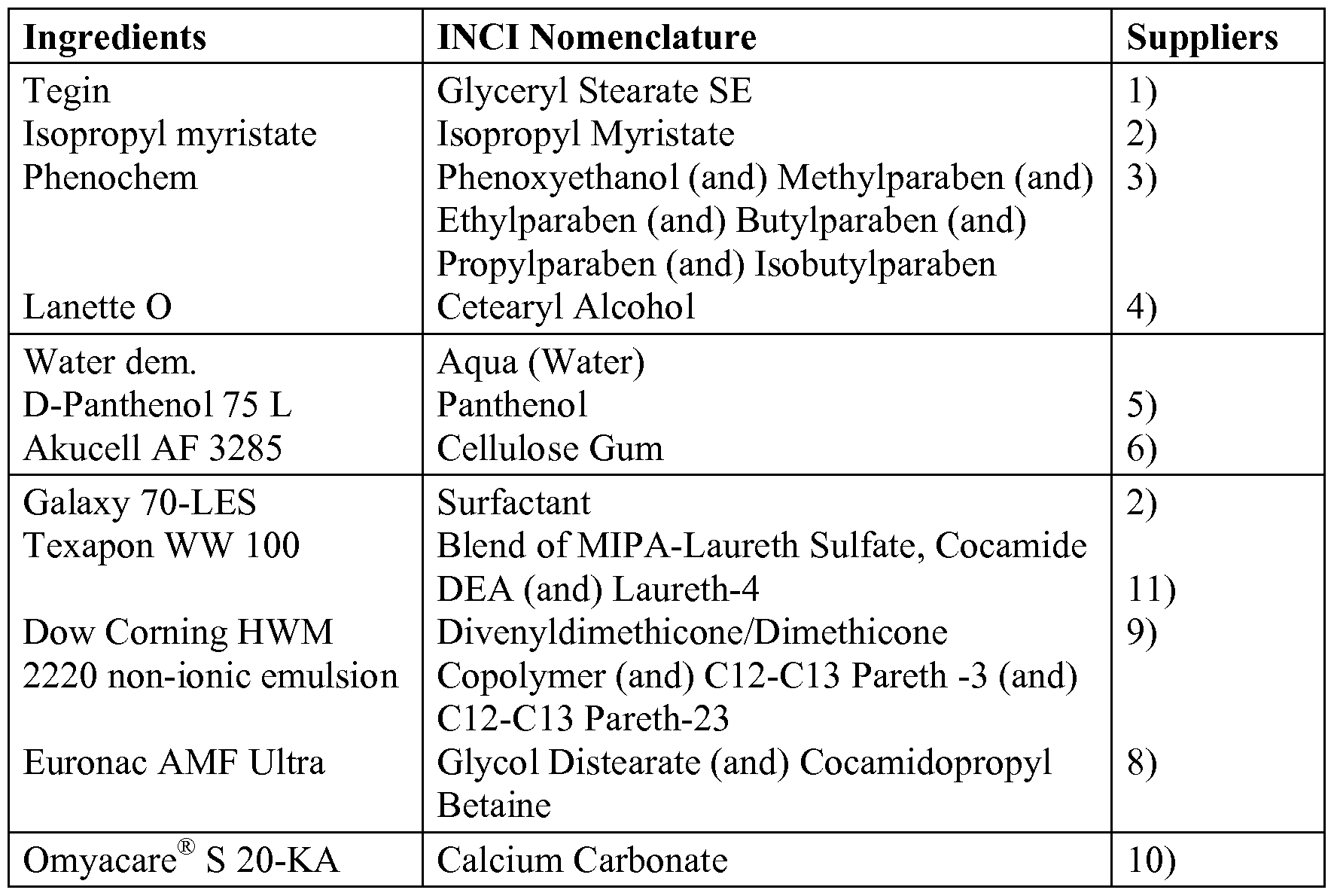
WO2016113285A1 - Surface-treated calcium carbonate with improved stability in environments with a ph of 4.5 to 7 - Google Patents
Surface-treated calcium carbonate with improved stability in environments with a ph of 4.5 to 7 Download PDFInfo
- Publication number
- WO2016113285A1 WO2016113285A1 PCT/EP2016/050536 EP2016050536W WO2016113285A1 WO 2016113285 A1 WO2016113285 A1 WO 2016113285A1 EP 2016050536 W EP2016050536 W EP 2016050536W WO 2016113285 A1 WO2016113285 A1 WO 2016113285A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- calcium carbonate
- acid
- treated calcium
- conjugate base
- treated
- Prior art date
Links
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 title claims abstract description 569
- 229910000019 calcium carbonate Inorganic materials 0.000 title claims abstract description 271
- 239000002253 acid Substances 0.000 claims abstract description 131
- 238000000034 method Methods 0.000 claims abstract description 83
- 239000000203 mixture Substances 0.000 claims description 158
- 239000002585 base Substances 0.000 claims description 87
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 claims description 85
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 claims description 75
- 239000000243 solution Substances 0.000 claims description 69
- 238000004140 cleaning Methods 0.000 claims description 65
- 239000002245 particle Substances 0.000 claims description 56
- 239000003082 abrasive agent Substances 0.000 claims description 47
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 claims description 46
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 40
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 claims description 39
- 229910001868 water Inorganic materials 0.000 claims description 38
- -1 alkali metal salt Chemical class 0.000 claims description 33
- 239000000725 suspension Substances 0.000 claims description 27
- 239000012062 aqueous buffer Substances 0.000 claims description 25
- 238000001035 drying Methods 0.000 claims description 24
- 229910000147 aluminium phosphate Inorganic materials 0.000 claims description 23
- 235000011054 acetic acid Nutrition 0.000 claims description 22
- 239000007900 aqueous suspension Substances 0.000 claims description 22
- 239000007864 aqueous solution Substances 0.000 claims description 21
- 239000002537 cosmetic Substances 0.000 claims description 19
- 238000002156 mixing Methods 0.000 claims description 19
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 claims description 18
- 229940088417 precipitated calcium carbonate Drugs 0.000 claims description 17
- 150000008043 acidic salts Chemical class 0.000 claims description 16
- 239000000463 material Substances 0.000 claims description 16
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 claims description 15
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 claims description 13
- 235000006408 oxalic acid Nutrition 0.000 claims description 13
- 239000011975 tartaric acid Substances 0.000 claims description 13
- 235000002906 tartaric acid Nutrition 0.000 claims description 13
- 229910052783 alkali metal Inorganic materials 0.000 claims description 11
- 229910052784 alkaline earth metal Inorganic materials 0.000 claims description 10
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 claims description 9
- 241001465754 Metazoa Species 0.000 claims description 9
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical compound OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 claims description 9
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 claims description 9
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 claims description 9
- 239000004327 boric acid Substances 0.000 claims description 9
- 235000010338 boric acid Nutrition 0.000 claims description 9
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 claims description 9
- 229910000514 dolomite Inorganic materials 0.000 claims description 9
- 235000019253 formic acid Nutrition 0.000 claims description 9
- DUWWHGPELOTTOE-UHFFFAOYSA-N n-(5-chloro-2,4-dimethoxyphenyl)-3-oxobutanamide Chemical compound COC1=CC(OC)=C(NC(=O)CC(C)=O)C=C1Cl DUWWHGPELOTTOE-UHFFFAOYSA-N 0.000 claims description 9
- 235000019260 propionic acid Nutrition 0.000 claims description 9
- 239000011734 sodium Substances 0.000 claims description 9
- 239000010459 dolomite Substances 0.000 claims description 8
- 235000015165 citric acid Nutrition 0.000 claims description 7
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 claims description 6
- 229910052708 sodium Inorganic materials 0.000 claims description 6
- 230000000699 topical effect Effects 0.000 claims description 5
- 210000000214 mouth Anatomy 0.000 claims description 4
- 235000011007 phosphoric acid Nutrition 0.000 claims description 4
- XAEFZNCEHLXOMS-UHFFFAOYSA-M potassium benzoate Chemical compound [K+].[O-]C(=O)C1=CC=CC=C1 XAEFZNCEHLXOMS-UHFFFAOYSA-M 0.000 claims description 4
- 229910052799 carbon Inorganic materials 0.000 abstract description 3
- 235000010216 calcium carbonate Nutrition 0.000 description 232
- 239000000047 product Substances 0.000 description 35
- 210000003811 finger Anatomy 0.000 description 24
- 238000009472 formulation Methods 0.000 description 23
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 21
- 239000007787 solid Substances 0.000 description 16
- 239000007788 liquid Substances 0.000 description 14
- 210000003813 thumb Anatomy 0.000 description 12
- 239000013078 crystal Substances 0.000 description 11
- 238000002360 preparation method Methods 0.000 description 11
- 239000000872 buffer Substances 0.000 description 10
- 239000007981 phosphate-citrate buffer Substances 0.000 description 10
- 239000007853 buffer solution Substances 0.000 description 9
- 150000001875 compounds Chemical class 0.000 description 9
- 239000011325 microbead Substances 0.000 description 9
- 150000003839 salts Chemical class 0.000 description 9
- 230000001953 sensory effect Effects 0.000 description 9
- 238000013019 agitation Methods 0.000 description 8
- 239000007979 citrate buffer Substances 0.000 description 8
- 239000002270 dispersing agent Substances 0.000 description 8
- 239000002002 slurry Substances 0.000 description 8
- 239000000126 substance Substances 0.000 description 8
- KWIUHFFTVRNATP-UHFFFAOYSA-N Betaine Natural products C[N+](C)(C)CC([O-])=O KWIUHFFTVRNATP-UHFFFAOYSA-N 0.000 description 7
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 7
- 229960003237 betaine Drugs 0.000 description 7
- 238000006243 chemical reaction Methods 0.000 description 7
- 238000004299 exfoliation Methods 0.000 description 7
- 238000005187 foaming Methods 0.000 description 7
- HWPKGOGLCKPRLZ-UHFFFAOYSA-M monosodium citrate Chemical compound [Na+].OC(=O)CC(O)(C([O-])=O)CC(O)=O HWPKGOGLCKPRLZ-UHFFFAOYSA-M 0.000 description 7
- 239000000843 powder Substances 0.000 description 7
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 6
- KWIUHFFTVRNATP-UHFFFAOYSA-O N,N,N-trimethylglycinium Chemical compound C[N+](C)(C)CC(O)=O KWIUHFFTVRNATP-UHFFFAOYSA-O 0.000 description 6
- 239000012615 aggregate Substances 0.000 description 6
- 125000001931 aliphatic group Chemical group 0.000 description 6
- 239000012530 fluid Substances 0.000 description 6
- 239000002524 monosodium citrate Substances 0.000 description 6
- 235000018342 monosodium citrate Nutrition 0.000 description 6
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 6
- 239000001509 sodium citrate Substances 0.000 description 6
- 239000002904 solvent Substances 0.000 description 6
- 238000004448 titration Methods 0.000 description 6
- HRXKRNGNAMMEHJ-UHFFFAOYSA-K trisodium citrate Chemical compound [Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O HRXKRNGNAMMEHJ-UHFFFAOYSA-K 0.000 description 6
- 235000019263 trisodium citrate Nutrition 0.000 description 6
- 229940038773 trisodium citrate Drugs 0.000 description 6
- 125000004432 carbon atom Chemical group C* 0.000 description 5
- ZPWVASYFFYYZEW-UHFFFAOYSA-L dipotassium hydrogen phosphate Chemical compound [K+].[K+].OP([O-])([O-])=O ZPWVASYFFYYZEW-UHFFFAOYSA-L 0.000 description 5
- BNIILDVGGAEEIG-UHFFFAOYSA-L disodium hydrogen phosphate Chemical compound [Na+].[Na+].OP([O-])([O-])=O BNIILDVGGAEEIG-UHFFFAOYSA-L 0.000 description 5
- 239000004615 ingredient Substances 0.000 description 5
- 229910000403 monosodium phosphate Inorganic materials 0.000 description 5
- 235000019799 monosodium phosphate Nutrition 0.000 description 5
- 230000000149 penetrating effect Effects 0.000 description 5
- 239000008363 phosphate buffer Substances 0.000 description 5
- 238000011020 pilot scale process Methods 0.000 description 5
- 239000001508 potassium citrate Substances 0.000 description 5
- QEEAPRPFLLJWCF-UHFFFAOYSA-K potassium citrate (anhydrous) Chemical compound [K+].[K+].[K+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O QEEAPRPFLLJWCF-UHFFFAOYSA-K 0.000 description 5
- 238000001556 precipitation Methods 0.000 description 5
- AJPJDKMHJJGVTQ-UHFFFAOYSA-M sodium dihydrogen phosphate Chemical compound [Na+].OP(O)([O-])=O AJPJDKMHJJGVTQ-UHFFFAOYSA-M 0.000 description 5
- 239000004094 surface-active agent Substances 0.000 description 5
- 235000015870 tripotassium citrate Nutrition 0.000 description 5
- 238000005406 washing Methods 0.000 description 5
- RQALKBLYTUKBFV-UHFFFAOYSA-N 1,4-dioxa-7-thiaspiro[4.4]nonane Chemical compound O1CCOC11CSCC1 RQALKBLYTUKBFV-UHFFFAOYSA-N 0.000 description 4
- 229910021532 Calcite Inorganic materials 0.000 description 4
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 4
- SNPLKNRPJHDVJA-ZETCQYMHSA-N D-panthenol Chemical compound OCC(C)(C)[C@@H](O)C(=O)NCCCO SNPLKNRPJHDVJA-ZETCQYMHSA-N 0.000 description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- 230000002378 acidificating effect Effects 0.000 description 4
- 125000000217 alkyl group Chemical group 0.000 description 4
- 229940001468 citrate Drugs 0.000 description 4
- 239000008367 deionised water Substances 0.000 description 4
- 238000009826 distribution Methods 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- 239000000945 filler Substances 0.000 description 4
- 239000008187 granular material Substances 0.000 description 4
- 229910052500 inorganic mineral Inorganic materials 0.000 description 4
- 150000002500 ions Chemical class 0.000 description 4
- 239000004579 marble Substances 0.000 description 4
- 235000010755 mineral Nutrition 0.000 description 4
- 239000011707 mineral Substances 0.000 description 4
- 229910000402 monopotassium phosphate Inorganic materials 0.000 description 4
- 235000019796 monopotassium phosphate Nutrition 0.000 description 4
- 229940085991 phosphate ion Drugs 0.000 description 4
- PJNZPQUBCPKICU-UHFFFAOYSA-N phosphoric acid;potassium Chemical compound [K].OP(O)(O)=O PJNZPQUBCPKICU-UHFFFAOYSA-N 0.000 description 4
- 239000004033 plastic Substances 0.000 description 4
- 229920003023 plastic Polymers 0.000 description 4
- 238000000926 separation method Methods 0.000 description 4
- 238000005507 spraying Methods 0.000 description 4
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 3
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 3
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 3
- 235000019738 Limestone Nutrition 0.000 description 3
- 229910019142 PO4 Inorganic materials 0.000 description 3
- 239000004698 Polyethylene Substances 0.000 description 3
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 3
- 239000013543 active substance Substances 0.000 description 3
- 125000000129 anionic group Chemical group 0.000 description 3
- 239000011324 bead Substances 0.000 description 3
- 239000011575 calcium Substances 0.000 description 3
- 229910052791 calcium Inorganic materials 0.000 description 3
- 239000001569 carbon dioxide Substances 0.000 description 3
- 229910002092 carbon dioxide Inorganic materials 0.000 description 3
- 150000001768 cations Chemical class 0.000 description 3
- 238000005119 centrifugation Methods 0.000 description 3
- 230000000052 comparative effect Effects 0.000 description 3
- 229920001577 copolymer Polymers 0.000 description 3
- 239000006071 cream Substances 0.000 description 3
- 229910021641 deionized water Inorganic materials 0.000 description 3
- 239000003599 detergent Substances 0.000 description 3
- UZLGHNUASUZUOR-UHFFFAOYSA-L dipotassium;3-carboxy-3-hydroxypentanedioate Chemical compound [K+].[K+].OC(=O)CC(O)(C([O-])=O)CC([O-])=O UZLGHNUASUZUOR-UHFFFAOYSA-L 0.000 description 3
- 239000002526 disodium citrate Substances 0.000 description 3
- 235000019262 disodium citrate Nutrition 0.000 description 3
- 229940079896 disodium hydrogen citrate Drugs 0.000 description 3
- 229910000397 disodium phosphate Inorganic materials 0.000 description 3
- CEYULKASIQJZGP-UHFFFAOYSA-L disodium;2-(carboxymethyl)-2-hydroxybutanedioate Chemical compound [Na+].[Na+].[O-]C(=O)CC(O)(C(=O)O)CC([O-])=O CEYULKASIQJZGP-UHFFFAOYSA-L 0.000 description 3
- 238000001914 filtration Methods 0.000 description 3
- 239000011521 glass Substances 0.000 description 3
- 239000006028 limestone Substances 0.000 description 3
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 239000003921 oil Substances 0.000 description 3
- 229940101267 panthenol Drugs 0.000 description 3
- 235000020957 pantothenol Nutrition 0.000 description 3
- 239000011619 pantothenol Substances 0.000 description 3
- 239000011236 particulate material Substances 0.000 description 3
- 239000000049 pigment Substances 0.000 description 3
- 229920000573 polyethylene Polymers 0.000 description 3
- 238000001694 spray drying Methods 0.000 description 3
- 238000003860 storage Methods 0.000 description 3
- 239000001993 wax Substances 0.000 description 3
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 2
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 2
- HIQIXEFWDLTDED-UHFFFAOYSA-N 4-hydroxy-1-piperidin-4-ylpyrrolidin-2-one Chemical compound O=C1CC(O)CN1C1CCNCC1 HIQIXEFWDLTDED-UHFFFAOYSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 2
- QFOHBWFCKVYLES-UHFFFAOYSA-N Butylparaben Chemical compound CCCCOC(=O)C1=CC=C(O)C=C1 QFOHBWFCKVYLES-UHFFFAOYSA-N 0.000 description 2
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 2
- 235000008733 Citrus aurantifolia Nutrition 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- 244000018633 Prunus armeniaca Species 0.000 description 2
- 235000009827 Prunus armeniaca Nutrition 0.000 description 2
- 235000011941 Tilia x europaea Nutrition 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- 229910000318 alkali metal phosphate Inorganic materials 0.000 description 2
- 150000001340 alkali metals Chemical class 0.000 description 2
- 229910000316 alkaline earth metal phosphate Inorganic materials 0.000 description 2
- 229910000323 aluminium silicate Inorganic materials 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 238000000149 argon plasma sintering Methods 0.000 description 2
- 238000005422 blasting Methods 0.000 description 2
- 239000001110 calcium chloride Substances 0.000 description 2
- 229910001628 calcium chloride Inorganic materials 0.000 description 2
- AXCZMVOFGPJBDE-UHFFFAOYSA-L calcium dihydroxide Chemical compound [OH-].[OH-].[Ca+2] AXCZMVOFGPJBDE-UHFFFAOYSA-L 0.000 description 2
- 239000000920 calcium hydroxide Substances 0.000 description 2
- 235000011116 calcium hydroxide Nutrition 0.000 description 2
- 229910001861 calcium hydroxide Inorganic materials 0.000 description 2
- 239000003093 cationic surfactant Substances 0.000 description 2
- 239000000919 ceramic Substances 0.000 description 2
- 235000013339 cereals Nutrition 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- 238000010494 dissociation reaction Methods 0.000 description 2
- 230000005593 dissociations Effects 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 230000001815 facial effect Effects 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 150000004665 fatty acids Chemical class 0.000 description 2
- 239000012467 final product Substances 0.000 description 2
- 239000004088 foaming agent Substances 0.000 description 2
- 150000002334 glycols Chemical class 0.000 description 2
- UBHWBODXJBSFLH-UHFFFAOYSA-N hexadecan-1-ol;octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO.CCCCCCCCCCCCCCCCCCO UBHWBODXJBSFLH-UHFFFAOYSA-N 0.000 description 2
- 239000008240 homogeneous mixture Substances 0.000 description 2
- 229920001519 homopolymer Polymers 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 125000004435 hydrogen atom Chemical class [H]* 0.000 description 2
- 239000010985 leather Substances 0.000 description 2
- 239000004571 lime Substances 0.000 description 2
- 238000000691 measurement method Methods 0.000 description 2
- LXCFILQKKLGQFO-UHFFFAOYSA-N methylparaben Chemical compound COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 description 2
- 239000004005 microsphere Substances 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 238000001139 pH measurement Methods 0.000 description 2
- 239000006072 paste Substances 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 2
- 239000011435 rock Substances 0.000 description 2
- 238000004062 sedimentation Methods 0.000 description 2
- 239000000377 silicon dioxide Substances 0.000 description 2
- 229910000162 sodium phosphate Inorganic materials 0.000 description 2
- 235000019832 sodium triphosphate Nutrition 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 238000004381 surface treatment Methods 0.000 description 2
- 229940095064 tartrate Drugs 0.000 description 2
- 239000000606 toothpaste Substances 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 239000003981 vehicle Substances 0.000 description 2
- HXKKHQJGJAFBHI-UHFFFAOYSA-N 1-aminopropan-2-ol Chemical compound CC(O)CN HXKKHQJGJAFBHI-UHFFFAOYSA-N 0.000 description 1
- JAHNSTQSQJOJLO-UHFFFAOYSA-N 2-(3-fluorophenyl)-1h-imidazole Chemical compound FC1=CC=CC(C=2NC=CN=2)=C1 JAHNSTQSQJOJLO-UHFFFAOYSA-N 0.000 description 1
- OSCJHTSDLYVCQC-UHFFFAOYSA-N 2-ethylhexyl 4-[[4-[4-(tert-butylcarbamoyl)anilino]-6-[4-(2-ethylhexoxycarbonyl)anilino]-1,3,5-triazin-2-yl]amino]benzoate Chemical compound C1=CC(C(=O)OCC(CC)CCCC)=CC=C1NC1=NC(NC=2C=CC(=CC=2)C(=O)NC(C)(C)C)=NC(NC=2C=CC(=CC=2)C(=O)OCC(CC)CCCC)=N1 OSCJHTSDLYVCQC-UHFFFAOYSA-N 0.000 description 1
- QCDWFXQBSFUVSP-UHFFFAOYSA-N 2-phenoxyethanol Chemical compound OCCOC1=CC=CC=C1 QCDWFXQBSFUVSP-UHFFFAOYSA-N 0.000 description 1
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 244000144725 Amygdalus communis Species 0.000 description 1
- 235000011437 Amygdalus communis Nutrition 0.000 description 1
- 235000019737 Animal fat Nutrition 0.000 description 1
- 235000017166 Bambusa arundinacea Nutrition 0.000 description 1
- 235000017491 Bambusa tulda Nutrition 0.000 description 1
- BHPQYMZQTOCNFJ-UHFFFAOYSA-N Calcium cation Chemical compound [Ca+2] BHPQYMZQTOCNFJ-UHFFFAOYSA-N 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 235000014653 Carica parviflora Nutrition 0.000 description 1
- 244000132059 Carica parviflora Species 0.000 description 1
- GHXZTYHSJHQHIJ-UHFFFAOYSA-N Chlorhexidine Chemical compound C=1C=C(Cl)C=CC=1NC(N)=NC(N)=NCCCCCCN=C(N)N=C(N)NC1=CC=C(Cl)C=C1 GHXZTYHSJHQHIJ-UHFFFAOYSA-N 0.000 description 1
- 235000004866 D-panthenol Nutrition 0.000 description 1
- 239000011703 D-panthenol Substances 0.000 description 1
- FPVVYTCTZKCSOJ-UHFFFAOYSA-N Ethylene glycol distearate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCCOC(=O)CCCCCCCCCCCCCCCCC FPVVYTCTZKCSOJ-UHFFFAOYSA-N 0.000 description 1
- KRHYYFGTRYWZRS-UHFFFAOYSA-M Fluoride anion Chemical compound [F-] KRHYYFGTRYWZRS-UHFFFAOYSA-M 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- XPJVKCRENWUEJH-UHFFFAOYSA-N Isobutylparaben Chemical compound CC(C)COC(=O)C1=CC=C(O)C=C1 XPJVKCRENWUEJH-UHFFFAOYSA-N 0.000 description 1
- 229920002884 Laureth 4 Polymers 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 235000003956 Luffa Nutrition 0.000 description 1
- 244000050983 Luffa operculata Species 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 229920000426 Microplastic Polymers 0.000 description 1
- 240000007594 Oryza sativa Species 0.000 description 1
- 235000007164 Oryza sativa Nutrition 0.000 description 1
- 244000082204 Phyllostachys viridis Species 0.000 description 1
- 235000015334 Phyllostachys viridis Nutrition 0.000 description 1
- 229920000388 Polyphosphate Polymers 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 1
- 238000009621 Solvay process Methods 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 1
- 244000299461 Theobroma cacao Species 0.000 description 1
- 235000009470 Theobroma cacao Nutrition 0.000 description 1
- 238000002441 X-ray diffraction Methods 0.000 description 1
- 238000005299 abrasion Methods 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 229910000288 alkali metal carbonate Inorganic materials 0.000 description 1
- 150000008041 alkali metal carbonates Chemical class 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- 125000002947 alkylene group Chemical group 0.000 description 1
- 235000020224 almond Nutrition 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- 229910021502 aluminium hydroxide Inorganic materials 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 229940115440 aluminum sodium silicate Drugs 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 239000002280 amphoteric surfactant Substances 0.000 description 1
- 239000003945 anionic surfactant Substances 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 239000012736 aqueous medium Substances 0.000 description 1
- 125000003710 aryl alkyl group Chemical group 0.000 description 1
- 239000011425 bamboo Substances 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- 235000012216 bentonite Nutrition 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- 239000003139 biocide Substances 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000011449 brick Substances 0.000 description 1
- 150000007516 brønsted-lowry acids Chemical class 0.000 description 1
- 230000003139 buffering effect Effects 0.000 description 1
- 229940067596 butylparaben Drugs 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- JUNWLZAGQLJVLR-UHFFFAOYSA-J calcium diphosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])(=O)OP([O-])([O-])=O JUNWLZAGQLJVLR-UHFFFAOYSA-J 0.000 description 1
- FUFJGUQYACFECW-UHFFFAOYSA-L calcium hydrogenphosphate Chemical compound [Ca+2].OP([O-])([O-])=O FUFJGUQYACFECW-UHFFFAOYSA-L 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229940043256 calcium pyrophosphate Drugs 0.000 description 1
- 150000005323 carbonate salts Chemical class 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 229940081733 cetearyl alcohol Drugs 0.000 description 1
- WOWHHFRSBJGXCM-UHFFFAOYSA-M cetyltrimethylammonium chloride Chemical compound [Cl-].CCCCCCCCCCCCCCCC[N+](C)(C)C WOWHHFRSBJGXCM-UHFFFAOYSA-M 0.000 description 1
- 230000001427 coherent effect Effects 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 230000003750 conditioning effect Effects 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 239000000001 dental powder Substances 0.000 description 1
- 239000000645 desinfectant Substances 0.000 description 1
- 229960003949 dexpanthenol Drugs 0.000 description 1
- 235000019821 dicalcium diphosphate Nutrition 0.000 description 1
- CGMRCMMOCQYHAD-UHFFFAOYSA-J dicalcium hydroxide phosphate Chemical compound [OH-].[Ca++].[Ca++].[O-]P([O-])([O-])=O CGMRCMMOCQYHAD-UHFFFAOYSA-J 0.000 description 1
- 235000019700 dicalcium phosphate Nutrition 0.000 description 1
- 229940008099 dimethicone Drugs 0.000 description 1
- 239000004205 dimethyl polysiloxane Substances 0.000 description 1
- 235000013870 dimethyl polysiloxane Nutrition 0.000 description 1
- 229910001873 dinitrogen Inorganic materials 0.000 description 1
- 235000021186 dishes Nutrition 0.000 description 1
- KDQPSPMLNJTZAL-UHFFFAOYSA-L disodium hydrogenphosphate dihydrate Chemical compound O.O.[Na+].[Na+].OP([O-])([O-])=O KDQPSPMLNJTZAL-UHFFFAOYSA-L 0.000 description 1
- 238000005108 dry cleaning Methods 0.000 description 1
- 238000009837 dry grinding Methods 0.000 description 1
- 210000003278 egg shell Anatomy 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 229960001617 ethyl hydroxybenzoate Drugs 0.000 description 1
- 239000004403 ethyl p-hydroxybenzoate Substances 0.000 description 1
- 235000010228 ethyl p-hydroxybenzoate Nutrition 0.000 description 1
- LYCAIKOWRPUZTN-UHFFFAOYSA-N ethylene glycol Natural products OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 1
- SFNALCNOMXIBKG-UHFFFAOYSA-N ethylene glycol monododecyl ether Chemical compound CCCCCCCCCCCCOCCO SFNALCNOMXIBKG-UHFFFAOYSA-N 0.000 description 1
- NUVBSKCKDOMJSU-UHFFFAOYSA-N ethylparaben Chemical compound CCOC(=O)C1=CC=C(O)C=C1 NUVBSKCKDOMJSU-UHFFFAOYSA-N 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- 239000012065 filter cake Substances 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 238000005194 fractionation Methods 0.000 description 1
- 239000003205 fragrance Substances 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 229940083124 ganglion-blocking antiadrenergic secondary and tertiary amines Drugs 0.000 description 1
- 229940049294 glyceryl stearate se Drugs 0.000 description 1
- 229940100608 glycol distearate Drugs 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 239000003906 humectant Substances 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 150000008040 ionic compounds Chemical class 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 229940061515 laureth-4 Drugs 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 1
- 239000001095 magnesium carbonate Substances 0.000 description 1
- 229910001607 magnesium mineral Inorganic materials 0.000 description 1
- 238000003760 magnetic stirring Methods 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 125000005341 metaphosphate group Chemical group 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- LVHBHZANLOWSRM-UHFFFAOYSA-N methylenebutanedioic acid Natural products OC(=O)CC(=C)C(O)=O LVHBHZANLOWSRM-UHFFFAOYSA-N 0.000 description 1
- 229960002216 methylparaben Drugs 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- DVEKCXOJTLDBFE-UHFFFAOYSA-N n-dodecyl-n,n-dimethylglycinate Chemical compound CCCCCCCCCCCC[N+](C)(C)CC([O-])=O DVEKCXOJTLDBFE-UHFFFAOYSA-N 0.000 description 1
- 238000001728 nano-filtration Methods 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 239000002736 nonionic surfactant Substances 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 229960005323 phenoxyethanol Drugs 0.000 description 1
- 239000008055 phosphate buffer solution Substances 0.000 description 1
- 150000004714 phosphonium salts Chemical class 0.000 description 1
- 238000005498 polishing Methods 0.000 description 1
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 description 1
- 229920000747 poly(lactic acid) Polymers 0.000 description 1
- 229920001495 poly(sodium acrylate) polymer Polymers 0.000 description 1
- 239000004626 polylactic acid Substances 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 239000001205 polyphosphate Substances 0.000 description 1
- 235000011176 polyphosphates Nutrition 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- JDQURBODCNMVIO-UHFFFAOYSA-N propan-2-yl tetradecanoate Chemical compound CCCCCCCCCCCCCC(=O)OC(C)C.CCCCCCCCCCCCCC(=O)OC(C)C JDQURBODCNMVIO-UHFFFAOYSA-N 0.000 description 1
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- 229960003415 propylparaben Drugs 0.000 description 1
- 239000011241 protective layer Substances 0.000 description 1
- 239000008262 pumice Substances 0.000 description 1
- 150000003242 quaternary ammonium salts Chemical class 0.000 description 1
- 235000009566 rice Nutrition 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 230000035807 sensation Effects 0.000 description 1
- 239000002453 shampoo Substances 0.000 description 1
- 239000000344 soap Substances 0.000 description 1
- 239000000429 sodium aluminium silicate Substances 0.000 description 1
- 235000012217 sodium aluminium silicate Nutrition 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- BBMHARZCALWXSL-UHFFFAOYSA-M sodium dihydrogenphosphate monohydrate Chemical compound O.[Na+].OP(O)([O-])=O BBMHARZCALWXSL-UHFFFAOYSA-M 0.000 description 1
- 229940057950 sodium laureth sulfate Drugs 0.000 description 1
- NNMHYFLPFNGQFZ-UHFFFAOYSA-M sodium polyacrylate Chemical group [Na+].[O-]C(=O)C=C NNMHYFLPFNGQFZ-UHFFFAOYSA-M 0.000 description 1
- SXHLENDCVBIJFO-UHFFFAOYSA-M sodium;2-[2-(2-dodecoxyethoxy)ethoxy]ethyl sulfate Chemical compound [Na+].CCCCCCCCCCCCOCCOCCOCCOS([O-])(=O)=O SXHLENDCVBIJFO-UHFFFAOYSA-M 0.000 description 1
- IWMMSZLFZZPTJY-UHFFFAOYSA-M sodium;3-(dodecylamino)propane-1-sulfonate Chemical compound [Na+].CCCCCCCCCCCCNCCCS([O-])(=O)=O IWMMSZLFZZPTJY-UHFFFAOYSA-M 0.000 description 1
- HWCHICTXVOMIIF-UHFFFAOYSA-M sodium;3-(dodecylamino)propanoate Chemical compound [Na+].CCCCCCCCCCCCNCCC([O-])=O HWCHICTXVOMIIF-UHFFFAOYSA-M 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- RWSOTUBLDIXVET-UHFFFAOYSA-O sulfonium Chemical compound [SH3+] RWSOTUBLDIXVET-UHFFFAOYSA-O 0.000 description 1
- 239000001117 sulphuric acid Substances 0.000 description 1
- 235000011149 sulphuric acid Nutrition 0.000 description 1
- 239000002335 surface treatment layer Substances 0.000 description 1
- 239000008399 tap water Substances 0.000 description 1
- 235000020679 tap water Nutrition 0.000 description 1
- 150000003505 terpenes Chemical class 0.000 description 1
- 235000007586 terpenes Nutrition 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 229940034610 toothpaste Drugs 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 229940078499 tricalcium phosphate Drugs 0.000 description 1
- 229910000391 tricalcium phosphate Inorganic materials 0.000 description 1
- 235000019731 tricalcium phosphate Nutrition 0.000 description 1
- UNXRWKVEANCORM-UHFFFAOYSA-I triphosphate(5-) Chemical compound [O-]P([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O UNXRWKVEANCORM-UHFFFAOYSA-I 0.000 description 1
- 235000019871 vegetable fat Nutrition 0.000 description 1
- 238000003911 water pollution Methods 0.000 description 1
- 238000001238 wet grinding Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 239000002888 zwitterionic surfactant Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01F—COMPOUNDS OF THE METALS BERYLLIUM, MAGNESIUM, ALUMINIUM, CALCIUM, STRONTIUM, BARIUM, RADIUM, THORIUM, OR OF THE RARE-EARTH METALS
- C01F11/00—Compounds of calcium, strontium, or barium
- C01F11/18—Carbonates
- C01F11/185—After-treatment, e.g. grinding, purification, conversion of crystal morphology
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09C—TREATMENT OF INORGANIC MATERIALS, OTHER THAN FIBROUS FILLERS, TO ENHANCE THEIR PIGMENTING OR FILLING PROPERTIES ; PREPARATION OF CARBON BLACK ; PREPARATION OF INORGANIC MATERIALS WHICH ARE NO SINGLE CHEMICAL COMPOUNDS AND WHICH ARE MAINLY USED AS PIGMENTS OR FILLERS
- C09C1/00—Treatment of specific inorganic materials other than fibrous fillers; Preparation of carbon black
- C09C1/02—Compounds of alkaline earth metals or magnesium
- C09C1/021—Calcium carbonates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/02—Cosmetics or similar toiletry preparations characterised by special physical form
- A61K8/0241—Containing particulates characterized by their shape and/or structure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/19—Cosmetics or similar toiletry preparations characterised by the composition containing inorganic ingredients
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q11/00—Preparations for care of the teeth, of the oral cavity or of dentures; Dentifrices, e.g. toothpastes; Mouth rinses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/10—Washing or bathing preparations
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q5/00—Preparations for care of the hair
- A61Q5/02—Preparations for cleaning the hair
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D17/00—Detergent materials or soaps characterised by their shape or physical properties
- C11D17/0008—Detergent materials or soaps characterised by their shape or physical properties aqueous liquid non soap compositions
- C11D17/0013—Liquid compositions with insoluble particles in suspension
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/02—Inorganic compounds ; Elemental compounds
- C11D3/12—Water-insoluble compounds
- C11D3/1233—Carbonates, e.g. calcite or dolomite
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/02—Inorganic compounds ; Elemental compounds
- C11D3/12—Water-insoluble compounds
- C11D3/14—Fillers; Abrasives ; Abrasive compositions; Suspending or absorbing agents not provided for in one single group of C11D3/12; Specific features concerning abrasives, e.g. granulometry or mixtures
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/20—Chemical, physico-chemical or functional or structural properties of the composition as a whole
- A61K2800/28—Rubbing or scrubbing compositions; Peeling or abrasive compositions; Containing exfoliants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/40—Chemical, physico-chemical or functional or structural properties of particular ingredients
- A61K2800/41—Particular ingredients further characterized by their size
- A61K2800/412—Microsized, i.e. having sizes between 0.1 and 100 microns
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/40—Chemical, physico-chemical or functional or structural properties of particular ingredients
- A61K2800/60—Particulates further characterized by their structure or composition
- A61K2800/61—Surface treated
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/40—Chemical, physico-chemical or functional or structural properties of particular ingredients
- A61K2800/60—Particulates further characterized by their structure or composition
- A61K2800/61—Surface treated
- A61K2800/612—By organic compounds
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/51—Particles with a specific particle size distribution
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/60—Particles characterised by their size
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/60—Particles characterised by their size
- C01P2004/61—Micrometer sized, i.e. from 1-100 micrometer
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2006/00—Physical properties of inorganic compounds
- C01P2006/12—Surface area
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2006/00—Physical properties of inorganic compounds
- C01P2006/22—Rheological behaviour as dispersion, e.g. viscosity, sedimentation stability
Definitions
- the present invention relates to a surface-treated calcium carbonate, dry or in a suspension, with improved stability in environments with a pH of 4.5 to 7, a process for producing the same, and its use, as well as products containing a calcium carbonate with improved stability in environments with a pH of 4.5 to 7.
- Abrasive cleaners are well-known in the art and are widely used for cleaning all kinds of surfaces, especially surfaces which become soiled with difficult to remove stains.
- abrasive cleaners may be used for cleaning household surfaces such as tables, sinks or dish surfaces.
- Abrasive materials can also be used in personal care products such as toothpastes, as exfoliants in facial and body scrubs, or soaps.
- personal care products such as toothpastes, as exfoliants in facial and body scrubs, or soaps.
- polymer beads such as polyethylene microspheres are widely used in personal care products as abrasive materials.
- microbeads polyethylene microspheres
- microbeads from cosmetics and personal care products can be washed down the drain after use, entering the sewerage system before making their way into rivers and canals. From there, they can easily end up in seas and oceans. As a consequence, microbeads have already been banned in some states of the United States and in the Netherlands. Illinois became the first U.S. state to enact legislation banning the manufacture and sale of products containing microbeads: the two-part ban goes into effect in 2018 and 2019.
- a liquid cleaning composition comprising calcium carbonate as abrasive material is, for example, described in WO 2013/078949.
- a dry blasting process for the cleaning of solid surfaces involving the use of natural alkaline earth carbonate particles is disclosed in WO 2009/133173.
- EP 1 666 203, and EP 1 738 872 also disclose a process for dry cleaning of solid surfaces involving the use of a precipitate or agglomerate of an insoluble alkali metal carbonate.
- US 5,827,114, US 5,531,634, and WO 94/07658 disclose the use of calcium carbonate for blast cleaning.
- a microdermabrasion procedure is described in US 2001/0023351. The rounded particles used in this procedure can be glass beads.
- an object of the present invention to provide an abrasive material, which avoids at least some of the foregoing disadvantages.
- abrasive material which is derivable from natural sources. It is also desirable to provide an abrasive agent which is easily biodegradable.
- a process for producing a surface- treated calcium carbonate with improved stability in environments with a pH of 4.5 to 7 comprises the steps of:
- step iv) contacting the calcium carbonate of step i), the at least one acid of step ii), and the at least one conjugate base of step iii) to form surface- treated calcium carbonate,
- step ii) and/or the at least one conjugate base of step iii) are provided in form of an aqueous solution.
- a suspension of a surface-treated calcium carbonate with improved stability in environments with a pH of 4.5 to 7, and obtainable by a process according to the present invention is provided.
- a dried surface-treated calcium carbonate with improved stability in environments with a pH of 4.5 to 7, and obtainable by a process according to the present invention is provided.
- an abrasive cleaning composition comprising a suspension of a surface-treated calcium carbonate or a dried surface-treated calcium carbonate according to the present invention is provided.
- a suspension of a surface-treated calcium carbonate according to the present invention as abrasive material is provided, preferably as abrasive material for cleaning application.
- a dried surface-treated calcium carbonate according to the present invention as abrasive material is provided, preferably as abrasive material for cosmetic application and/or cleaning application, more preferably as abrasive material for cosmetic application, and most preferably as abrasive material for topical skin application.
- an abrasive cleaning composition according to the present invention for cleaning a surface is provided, preferably for cleaning an animate surface, and more preferably for cleaning an animate surface selected from the group consisting of human skin, animal skin, human hair, animal hair, and tissues of the oral cavity such as teeth, gums, tongue or buccal surfaces.
- an abrasive cleaning composition according to the present invention for cleaning a surface is provided, preferably for cleaning an animate surface, and more preferably for cleaning an animate surface selected from the group consisting of human skin, animal skin, human hair, animal hair, and tissues of the oral cavity such as teeth, gums, tongue or buccal surfaces.
- step iv) comprises the steps of: (al) contacting the calcium carbonate of step i) and the at least one acid of step ii) to form a pre-treated calcium carbonate, and (a2) contacting the pre-treated calcium carbonate of step al) with the at least one conjugate base of step iii) to form surface-treated calcium carbonate, wherein at least the at least one acid of step ii) is provided in form of an aqueous solution.
- step iv) comprises the steps of: (bl) contacting the calcium carbonate of step i) and the at least one conjugate base of step iii) to form a pre-treated calcium carbonate, and (b2) contacting the pre-treated calcium carbonate of step bl) with the at least one acid of step ii) to form surface-treated calcium carbonate, wherein at least the at least one conjugate base of step iii) is provided in form of an aqueous solution.
- step iv) comprises the steps of: (cl) mixing the calcium carbonate of step i), the aqueous buffer solution, and optionally water, to form an aqueous suspension of surface-treated calcium carbonate, and (c2) separating the surface-treated calcium carbonate from the aqueous suspension obtained from step cl).
- the calcium carbonate of step i) is natural ground calcium carbonate, precipitated calcium carbonate, dolomite, agglomerates or aggregates thereof, or a mixture of the aforementioned materials, and preferably natural ground calcium carbonate.
- the calcium carbonate of step i) is in form of particles having a volume determined median particle size dso from 1 to 1500 ⁇ , preferably from 25 to 1 400 ⁇ , more preferably from 100 from 1 200 ⁇ , even more preferably from 200 to 1 000 ⁇ , and most preferably from 300 to 800 ⁇ .
- the at least one acid of step ii) is selected from the group consisting of phosphoric acid, citric acid, sulphurous acid, boric acid, acetic acid, tartaric acid, formic acid, propanoic acid, oxalic acid, phosphonic acid, acidic salt, and mixtures thereof.
- the at least one acid of step ii) is selected from the group consisting of phosphoric acid, citric acid, tartaric acid, oxalic acid, their acidic salts, and mixtures thereof.
- the at least one acid of step ii) is selected from the group consisting of phosphoric acid, citric acid, their acidic salts, and mixtures thereof.
- the at least one conjugate base of step iii) is an alkali metal salt and/or alkaline earth metal salt of an acid having a pK a value from 0 to 8, when measured at 20°C, preferably the at least one conjugate base of step iii) is an alkali metal salt and/or alkaline earth metal salt of an acid selected from the group consisting of phosphoric acid, citric acid, sulphurous acid, boric acid, acetic acid, tartaric acid, formic acid, propanoic acid, oxalic acid, phosphonic acid, and mixtures thereof, more preferably the at least one conjugate base of step iii) is a sodium and/or potassium salt of an acid selected from the group consisting of phosphoric acid, citric acid, sulphurous acid, boric acid, acetic acid, tartaric acid, formic acid, propanoic acid, oxalic acid, phosphonic acid, and mixtures thereof, and most preferably the at least one
- the alkali metal of the above alkali metal salt can be selected from the group consisting of Li + , Na + , K + , and is preferably selected from the group consisting of Na + and K + .
- the metal of the above alkaline earth metal salt is Mg 2+ .
- the aqueous buffer solution has a pH value from 4.0 to 7.5, preferably from 4.5 to 6.0, and most preferably of 4.8 to 5.5.
- the aqueous buffer solution has a concentration from 0.01 mol/kg to 2 mol/kg, preferably from 0.04 to 1.0 mol/kg, more preferably from 0.06 to 0.5 mol/kg, even more preferably from 0.07 to 0.4 mol/kg, and most preferably from 0.08 to 0.2 mol kg.
- abrasive material in the meaning of the present invention refers to a particulate substance that is capable of polishing or cleaning surfaces by rubbing or exfoliating.
- an “acid” is defined as Bronsted-Lowry acid, that is to say, it is an H 3 0 + ion provider.
- the acid can also be an acid salt, generating H 3 0 + ions under the preparation conditions.
- An “acid salt” is defined as an H 3 0 + ion-provider that is partially neutralized by an electropositive element.
- a “salt” is defined as an electrically neutral ionic compound formed from anions and cations.
- a “partially crystalline salt” is defined as a salt that, on XRD analysis, presents an essentially discrete diffraction pattern.
- pK a is the symbol representing the acid dissociation constant associated with a given ionisable hydrogen in a given acid, and is indicative of the natural degree of dissociation of this hydrogen from this acid at equilibrium in water at a given temperature.
- pK a values may be found in reference textbooks such as Harris, D. C. "Quantitative Chemical Analysis: 3 rd Edition", 1991, W.H. Freeman & Co. (USA), ISBN 0-7167-2170-8, or CRC Handbook of Chemistry and Physics, 1994-1995 75 m edition, 8-43 to 8-55, CRC Press Inc., 1995.
- conjugate base refers to a substance that is formed by removal of a proton from an acid. By gaining a proton the conjugate base is reformed into the acid.
- Natural ground calcium carbonate in the meaning of the present invention is a calcium carbonate obtained from natural sources, such as limestone, marble, or chalk, and processed through a wet and/or dry treatment such as grinding, screening and/or fractionation, for example, by a cyclone or classifier.
- Precipitated calcium carbonate in the meaning of the present invention is a synthesized material, generally obtained by precipitation following a reaction of carbon dioxide and calcium hydroxide (hydrated lime) in an aqueous environment or by precipitation of a calcium- and a carbonate source in water. Additionally, precipitated calcium carbonate can also be the product of introducing calcium and carbonate salts, calcium chloride and sodium carbonate for example, in an aqueous environment.
- PCC may have a vateritic, calcitic or aragonitic crystalline form. PCCs are described, for example, in EP 2 447 213 Al, EP 2 524 898 Al, EP 2 371 766 Al, or WO 2013/142473 Al .
- an “agglomerate” and “aggregate” shall have the definition as set out in ISO 14887:2000(E).
- an “agglomerate” is defined as an assemblage of particles which are loosely coherent
- an “aggregate” is defined as an assemblage of particles rigidly joined together.
- the "particle size" of a filler material or other particulate material is described by its distribution of particle sizes.
- the value d x represents the diameter relative to which x % by volume of the particles have diameters less than d x .
- the ⁇ 5 o value is thus the volume determined median particle size, i.e. 50 % of the total volume of all particles results from particles bigger and 50 % of the total volume of all particles results from particles smaller than this particle size.
- the particle size is specified as volume determined median particle size ⁇ i 5 o unless indicated otherwise.
- a Mastersizer 2000 or Mastersizer 3000 from the company Malvern Instruments Ltd., Great Britain, using the Fraunhofer light scattering model may be used.
- the weight determined particle size distribution may correspond to the volume determined particle size if the density of all the particles is equal.
- the method and the instrument are known to the skilled person and are commonly used to determine grain size of fillers and pigments.
- the samples are dispersed using a high speed stirrer and supersonics.
- the "solids content" of a liquid composition is a measure of the amount of material remaining after all the solvent or water has been evaporated.
- a “specific surface area (SSA)" of a calcium carbonate in the meaning of the present invention is defined as the surface area of the mineral pigment divided by the mass of the calcium carbonate. As used herein, the specific surface area is measured by nitrogen gas adsorption using the BET isotherm (ISO 9277:2010) and is specified in m 2 /g.
- surface-treated calcium carbonate in the meaning of the present invention refers to a calcium carbonate which has been contacted with at least one acid and at least one conjugate base as defined in claim 1 , such as to obtain a surface-treatment on at least a part of the surface of the calcium carbonate.
- surfactant refers to a compound that lowers the surface tension or interfacial tension between two liquids or between a liquid and a solid, and may act as detergent, wetting agent, emulsifier, foaming agent, or dispersant.
- viscosity or “Brookfield viscosity” refers to Brookfield viscosity.
- the Brookfield viscosity is for this purpose measured by a Brookfield DV-II+ Pro viscometer at 25°C ⁇ 1°C at 100 rpm using an appropriate spindle of the Brookfield RV-spindle set and is specified in mPa-s. Based on his technical knowledge, the skilled person will select a spindle from the Brookfield DV-II+ Pro viscometer at 25°C ⁇ 1°C at 100 rpm using an appropriate spindle of the Brookfield RV-spindle set and is specified in mPa-s. Based on his technical knowledge, the skilled person will select a spindle from the Brookfield DV-II+ Pro viscometer at 25°C ⁇ 1°C at 100 rpm using an appropriate spindle of the Brookfield RV-spindle set and is specified in mPa-s. Based on his technical knowledge, the skilled person will select a spind
- Brookfield RV-spindle set which is suitable for the viscosity range to be measured.
- the spindle number 3 may be used, for a viscosity range between 400 and 1 600 mPa-s the spindle number 4 may be used, for a viscosity range between 800 and 3 200 mPa-s the spindle number 5 may be used, for a viscosity range between 1 000 and 2 000 000 mPa-s the spindle number 6 may be used, and for a viscosity range between 4 000 and
- the spindle number 7 may be used.
- a “suspension” or “slurry” in the meaning of the present invention comprises insoluble solids and a solvent or liquid, preferably water, and optionally further additives, and usually contains large amounts of solids and, thus, is more viscous and can be of higher density than the liquid from which it is formed.
- the inventive process for producing a surface-treated calcium carbonate with improved stability in environments with a pH of 4.5 to 7 comprises the steps of: (i) providing calcium carbonate, (ii) providing at least one acid having a pKa value from 0 to 8, when measured at 20°C, (iii) providing at least one conjugate base,
- step (iv) contacting the calcium carbonate of step (i), the at least one acid of step (ii), and the at least one conjugate base of step (iii) to form surface-treated calcium carbonate.
- the at least one acid of step (ii) and/or the at least one conjugate base of step (iii) are provided in form of an aqueous solution.
- step i) of the process of the present invention calcium carbonate is provided.
- the calcium carbonate of step i) is natural ground calcium carbonate, precipitated calcium carbonate, dolomite, agglomerates or aggregates thereof, or a mixture of the aforementioned materials.
- the calcium carbonate of step i) is natural ground calcium carbonate.
- Natural ground calcium carbonate is understood to be manufactured from a naturally occurring form of calcium carbonate, mined from sedimentary rocks such as limestone or chalk, or from metamorphic marble rocks, eggshells or seashells.
- Calcium carbonate is known to exist as three types of crystal polymorphs: calcite, aragonite and vaterite.
- Calcite the most common crystal polymorph, is considered to be the most stable crystal form of calcium carbonate.
- Less common is aragonite, which has a discrete or clustered needle orthorhombic crystal structure.
- Vaterite is the rarest calcium carbonate polymorph and is generally unstable.
- Ground calcium carbonate is almost exclusively of the calcitic polymorph, which is said to be trigonal-rhombohedral and represents the most stable of the calcium carbonate polymorphs.
- the term "source" of the calcium carbonate in the meaning of the present application refers to the naturally occurring mineral material from which the calcium carbonate is obtained.
- the source of the calcium carbonate may comprise further naturally occurring components such as magnesium carbonate, alumino silicate etc.
- the source of natural ground calcium carbonate (GCC) is selected from marble, chalk, limestone, or mixtures thereof.
- the source of natural ground calcium carbonate is marble.
- the GCC is obtained by dry grinding. According to another embodiment of the present invention the GCC is obtained by wet grinding and subsequent drying.
- Dolomite in the meaning of the present invention is a calcium carbonate containing mineral, namely a carbonic calcium-magnesium-mineral, having the chemical composition of CaMg(C0 3 ) 2 ("CaC0 3 ⁇ MgCCV').
- a dolomite mineral may contain at least 30.0 wt.-% MgC0 3 , based on the total weight of dolomite, preferably more than 35.0 wt.-%, and more preferably more than 40.0 wt.-% MgC0 3 .
- the calcium carbonate comprises one type of natural ground calcium carbonate. According to another embodiment of the present invention, the calcium carbonate comprises a mixture of two or more types of natural ground calcium carbonates selected from different sources.
- Precipitated calcium carbonate in the meaning of the present invention is a synthesized material, generally obtained by precipitation following reaction of carbon dioxide and lime in an aqueous environment or by precipitation of a calcium and carbonate ion source in water or by precipitation by combining calcium and carbonate ions, for example CaCl 2 and Na 2 CC"3, out of solution.
- Further possible ways of producing PCC are the lime soda process, or the Solvay process in which PCC is a by-product of ammonia production.
- Precipitated calcium carbonate exists in three primary crystalline forms: calcite, aragonite and vaterite, and there are many different polymorphs (crystal habits) for each of these crystalline forms.
- Calcite has a trigonal structure with typical crystal habits such as scalenohedral (S-PCC), rhombohedral (R-PCC), hexagonal prismatic, pinacoidal, colloidal (C-PCC), cubic, and prismatic (P-PCC).
- Aragonite is an orthorhombic structure with typical crystal habits of twinned hexagonal prismatic crystals, as well as a diverse assortment of thin elongated prismatic, curved bladed, steep pyramidal, chisel shaped crystals, branching tree, and coral or worm-like form.
- Vaterite belongs to the hexagonal crystal system.
- the obtained PCC slurry can be mechanically dewatered and dried.
- the calcium carbonate comprises one type of precipitated calcium carbonate.
- the calcium carbonate comprises a mixture of two or more precipitated calcium carbonates selected from different crystalline forms and different polymorphs of precipitated calcium carbonate.
- the at least one precipitated calcium carbonate may comprise one PCC selected from S-PCC and one PCC selected from R-PCC.
- the calcium carbonate comprises agglomerates or aggregates of precipitated calcium carbonate having a volume determined median particle size dso from 25 to 1 500 ⁇ .
- the calcium carbonate is a natural ground calcium carbonate.
- the calcium carbonate is a precipitated calcium carbonate.
- the calcium carbonate is a mixture of natural ground calcium carbonate and precipitated calcium carbonate.
- the calcium carbonate is dolomite, optionally in mixture with natural ground calcium carbonate and/or precipitated calcium carbonate.
- the calcium carbonate of step i) is in form of particles having a volume determined median particle size dso from 1 to 1 500 ⁇ , preferably from 25 to 1 400 ⁇ , more preferably from 100 from 1 200 ⁇ , even more preferably from 200 to 1 000 ⁇ , and most preferably from 300 to 800 ⁇ .
- the calcium carbonate of step i) is selected from the group consisting of natural ground calcium carbonate, dolomite, aggregates or agglomerates of PCC, or mixtures of the aforementioned, and is in form of particles having a volume determined median particle size dso from 25 to 1 500 ⁇ , preferably from 25 to 1 400 ⁇ , more preferably from 100 to 1 200 ⁇ , even more preferably from 150 from 1 100 ⁇ , still more preferably from 200 to 1 000 ⁇ , and most preferably from 300 to 800 ⁇ .
- the calcium carbonate of step i) is in form of particles having a volume determined top cut particle size ( ⁇ 3 ⁇ 4 8 ) from 40 to 2 000 ⁇ , preferably from 200 to 1 800 ⁇ , more preferably from 300 to 1 500 ⁇ , even more preferably from 400 to 1 200 ⁇ , and most preferably from 500 to 1 000 ⁇ .
- the calcium carbonate of step i) is in form of particles having a specific BET surface area in the range of > 0 to 5 m 2 /g, preferably in the range of > 0 to 2 m 2 /g, and more preferably in the range of > 0 to 1.6 m 2 /g.
- the calcium carbonate may be used in any suitable liquid or dry form.
- the calcium carbonate may be in form of a powder and/or a suspension.
- the suspension can be obtained by mixing particles of calcium carbonate with a solvent, preferably water.
- the calcium carbonate to be mixed with a solvent, and preferably water may be provided in any form, for example, as suspension, slurry, dispersion, paste, powder, a moist filter cake or in pressed or granulated form.
- the suspension can be undispersed or dispersed, i.e. the suspension includes a dispersant, and thus, forms an aqueous dispersion.
- the calcium carbonate is used in form of an aqueous suspension, which does not contain a dispersant.
- calcium carbonate is used in form of an aqueous suspension, which contains a dispersant.
- a suitable dispersant may be selected from polyphosphates, and is in particular a tripolyphosphate.
- Another suitable dispersant may be selected from the group comprising homopolymers or copolymers of polycarboxylic acid salts based on, for example, acrylic acid, methacrylic acid, maleic acid, fumaric acid or itaconic acid and acrylamide or mixtures thereof. Homopolymers or copolymers of acrylic acid are especially preferred.
- the weight average molecular weight M w of such products is preferably in the range from 2 000 to 15 000 g/mol, with a weight average molecular weight M w from 3 000 to 7 000 g/mol or 3 500 to 6 000 g/mol being especially preferred.
- the dispersant is sodium polyacrylate having a weight average molecular weight M w from 2 000 to 15 000 g/mol, preferably from 3 000 to 7 000 g/mol, and most preferably from 3 500 to 6 000 g/mol.
- the solids content of the suspension of calcium carbonate can be adjusted by the methods known to the skilled person.
- the aqueous suspension may be partially dewatered by a settling, filtration, centrifugation or thermal separation process.
- the solids content of the aqueous suspension of calcium carbonate is from 1 to 85 wt.-%, more preferably from 5 to 75 wt.-%, and most preferably from 10 to 65 wt.-%, based on the total weight of the aqueous suspension.
- the at least one acid and the at least one conjugate base are provided.
- At least one acid is provided having a pIQ value from 0 to 8, when measured at 20°C.
- the at least one acid of step ii) can be any medium-strong acid, or weak acid, or mixtures thereof, generating H 3 0 + ions under the preparation conditions.
- the at least one acid can also be an acidic salt, generating H 3 0 + ions under the preparation conditions.
- the at least one acid of step ii) and the at least one conjugate base of step iii), when available in dry form, may include crystalline water.
- the at least one acid of step ii) is a medium-strong acid having a pK a value from 0 to 2.5, when measured at 20°C. If the pK a at 20°C is from 0 to 2.5, the acid is preferably selected from sulphurous acid, phosphoric acid, oxalic acid, or mixtures thereof, more preferably from phosphoric acid, oxalic acid, or mixtures thereof, and most preferably from phosphoric acid.
- the at least one acid can also be an acidic salt, for example, H 2 PO 4 " , being at least partially neutralised by a corresponding cation such as Li + , Na + or K + , or HPO 4 2" , being at least partially neutralised by a corresponding cation such as Li + , Na + ' K + , Mg 2+ or Ca 2+ .
- the at least one acid can also be a mixture of one or more acids and one or more acidic salts.
- the at least one acid is a weak acid having a pK a value of greater than 2.5 and less than or equal to 7, when measured at 20°C.
- the weak acid has a pK a value from 2.6 to 5 at 20°C, and more preferably the weak acid is selected from the group consisting of acetic acid, formic acid, propanoic acid, citric acid, boric acid, tartaric acid, phosphonic acid, and mixtures thereof, preferably from the group consisting of citric acid, tartaric acid, and mixtures thereof, and is most preferably citric acid.
- the at least one acid of step ii) is selected from the group consisting of phosphoric acid, citric acid, sulphurous acid, boric acid, acetic acid, tartaric acid, formic acid, propanoic acid, oxalic acid, phosphonic acid, acidic salt, and mixtures thereof, preferably selected from the group consisting of phosphoric acid, citric acid, tartaric acid, oxalic acid, their acidic salts, and mixtures thereof, and most preferably selected from the group consisting of phosphoric acid, citric acid, their acidic salts, and mixtures thereof.
- the at least one acid of step ii) may be a phosphoric acid or acidic salt thereof, a citric acid or acidic salt thereof, or a mixture thereof.
- the at least one acid is selected from the group consisting of phosphoric acid, citric acid, sodium dihydrogen phosphate, potassium dihydrogen phosphate, potassium dihydrogen citrate, sodium dihydrogen citrate, disodium hydrogen citrate, dipotassium hydrogen citrate and mixtures thereof.
- the used acid(s) is an organic acid
- it is preferably not an aliphatic monocarboxylic acid derived from an animal or vegetable fat, oil or wax, and having a chain of 4 to 28 carbons (usually branched and even-numbered), which may be saturated or unsaturated. It has to be noted that this is the IUPAC definition of fatty acids.
- an organic acid is used in the process of the present invention, then it is preferably not a fatty acid.
- the aforementioned also applies to the conjugate base.
- the at least one acid of step ii) can be provided in liquid or dry form.
- the at least one acid may be in form of a solid such as granules or a powder and/or a solution.
- the at least one acid of step ii) is provided in form of an aqueous solution.
- the acid may include crystalline water.
- the at least one acid of step ii) can be provided as a concentrated solution or a more diluted solution.
- the molar ratio of the at least one acid to the calcium carbonate is from 1 : 10 to 1 : 100, preferably from 1 :20 to 1 :80, and more preferably from 1 :50 to 1 :60.
- the aqueous solution of the at least one acid of step ii) has a concentration from 0.01 mol/kg to 2 mol/kg, preferably from 0.04 to 1.0 mol/kg, more preferably from 0.06 to 0.5 mol/kg, even more preferably from 0.07 to 0.4 mol/kg, and most preferably from 0.08 to 0.2 mol kg.
- a conjugate base according to the present invention is a substance that is formed by removal of a proton from an acid. By gaining a proton the conjugate base is reformed into the corresponding acid.
- the at least one conjugate base of step iii) can be an alkali metal salt and/or alkaline earth metal salt of an acid.
- the at least one conjugate base is an alkali metal salt and/or alkaline earth metal salt of an acid being selected from the group consisting of phosphoric acid, citric acid, sulphurous acid, boric acid, acetic acid, tartaric acid, formic acid, propanoic acid, oxalic acid, phosphonic acid, and mixtures thereof.
- the alkali metal may be selected from lithium, sodium, or potassium.
- the alkaline earth metal may be magnesium.
- the at least one conjugate base of step iii) may be an alkali metal salt and/or alkaline earth metal salt of an acid being selected from a phosphoric acid or acidic salt thereof, a citric acid or acidic salt thereof, or a mixture thereof.
- the at least one conjugate base of step iii) is disodium hydrogen phosphate, dipotassium hydrogen phosphate, tripotassium citrate and/or trisodium citrate.
- the at least one conjugate base of step iii) is a conjugate base of the at least one acid of step ii).
- the at least one acid of step ii) is phosphoric acid
- the at least one conjugate base of step iii) is an alkali metal phosphate and/or alkaline earth metal phosphate.
- the at least one acid of step ii) is citric acid
- the at least one conjugate base of step iii) is an alkali metal citrate and/or alkaline earth metal citrate.
- the at least one conjugate base of step iii) can also be a conjugate base of an acid different from the at least one acid of step ii).
- the at least one acid of step ii) can be citric acid and the at least one conjugate base of step iii) can be an alkali metal phosphate and/or alkaline earth metal phosphate.
- the at least one conjugate base of step iii) can be provided in liquid or dry form.
- the at least one conjugate base may be in form of a solid such as granules or a powder and/or a solution.
- the at least one conjugate base of step iii) is provided in form of an aqueous solution.
- the at least one conjugate base of step iii) can be provided as a concentrated solution or a more diluted solution.
- the molar ratio of the at least one conjugate base to the calcium carbonate is from 1 : 10 to 1 : 100, preferably from 1 :20 to 1 :80, and more preferably from 1 :50 to 1 :60.
- the aqueous solution of the at least one conjugate base of step iii) has a concentration from 0.01 mol/kg to 2 mol/kg, preferably from 0.04 to 1.0 mol/kg, more preferably from 0.06 to 0.5 mol/kg, even more preferably from 0.07 to 0.4 mol/kg, and most preferably from 0.08 to 0.2 mol kg.
- the at least one acid of process step ii) and the at least one conjugate base of process step iii) can be provided separately or in combination.
- the at least one acid of step ii) and/or the at least one conjugate base of step iii) are provided in form of an aqueous solution.
- the at least one acid of step ii) as well as the at least one conjugate base of step iii) are provided as separate aqueous solutions.
- the at least one acid of step ii) and the at least one conjugate base of step iii) are provided together in form of an aqueous buffer solution.
- the aqueous buffer solution is selected from the group consisting of phosphate buffer, phosphate-citrate buffer, citrate buffer, tartrate buffer, borate buffer, preferably the aqueous buffer solution is a phosphate buffer, a phosphate-citrate buffer, or a citrate buffer, and more preferably a phosphate-citrate buffer, or a citrate buffer.
- the at least one acid of step ii) and the at least one conjugate base of step iii) are provided together in form of an aqueous buffer solution, wherein the at least one acid of step ii) is selected from sodium dihydrogen phosphate, potassium dihydrogen phosphate, citric acid, sodium dihydrogen citrate, potassium dihydrogen citrate, disodium hydrogen citrate, dipotassium hydrogen citrate, or mixtures thereof, and the at least one conjugate base of step iii) is disodium hydrogen phosphate, dipotassium hydrogen phosphate, tripotassium citrate and/or trisodium citrate.
- the at least one acid of step ii) is selected from sodium dihydrogen phosphate, potassium dihydrogen phosphate, citric acid, sodium dihydrogen citrate, potassium dihydrogen citrate, disodium hydrogen citrate, dipotassium hydrogen citrate, or mixtures thereof
- the at least one conjugate base of step iii) is disodium hydrogen phosphate,
- the at least one acid of step ii) may be selected from sodium dihydrogen phosphate, sodium dihydrogen citrate, or mixtures thereof, and the at least one conjugate base of step iii) may be disodium hydrogen phosphate, dipotassium hydrogen phosphate, tripotassium citrate and/or trisodium citrate.
- the aqueous buffer solution has a pH value from 4.0 to 7.5, preferably from 4.5 to 6.0, and most preferably from 4.8 to 5.5.
- the aqueous buffer solution of step has a concentration from 0.01 mol/kg to 2 mol/kg, preferably from 0.04 to 1.0 mol/kg, more preferably from 0.06 to 0.5 mol/kg, even more preferably from 0.07 to 0.4 mol/kg, and most preferably from 0.08 to 0.2 mol/kg.
- the aqueous buffer solution is provided in an amount from 0.1 to 99 wt.-%, based on the total amount of the aqueous suspension of calcium carbonate, preferably in an amount from 1 to 90 wt.-%, more preferably in an amount from 10 to 80 wt.-%, and most preferably from 60 to 70 wt.-%.
- a process for producing a surface- treated calcium carbonate with improved stability in environments with a pH of 4.5 to 7 comprises the steps of:
- step iv) contacting the calcium carbonate of step i), the at least one acid of step ii), and the at least one conjugate base of step iii) to form surface- treated calcium carbonate,
- step ii) wherein the at least one acid of step ii) and/or the at least one conjugate base of step iii) are provided in form of an aqueous solution.
- step iv) of the inventive process the calcium carbonate of step i), the at least one acid of step ii), and the at least one conjugate base of step iii) are contacted.
- the calcium carbonate of step i) may be contacted with the at least one acid of step ii) and the at least one conjugate base of step iii) in one step or in several steps.
- step iv) comprises the steps of:
- step al) contacting the pre-treated calcium carbonate of step al) with the at least one conjugate base of step iii) to form surface-treated calcium carbonate,
- step iv) comprises the steps of:
- step bl contacting the calcium carbonate of step i) and the at least one conjugate base of step iii) to form a pre-treated calcium carbonate
- step b2) contacting the pre-treated calcium carbonate of step bl) with the at least one acid of step ii) to form surface-treated calcium carbonate,
- step iii) wherein at least the at least one conjugate base of step iii) is provided in form of an aqueous solution.
- the calcium carbonate of step i), the at least one acid of step ii) and the at least one conjugate base of step iii) are contacted simultaneously.
- the at least one acid of step ii) and the at least one conjugate base of step iii) may be provided separately or as a mixture.
- the at least one acid of step ii) and the at least one conjugate base of step iii) may be provided as a mixture, for example, in form of an aqueous buffer solution comprising the at least acid of step ii) and the at least one conjugate base of step iii).
- the at least one acid of step ii) and the at least one conjugate base of step iii) are provided together in form of an aqueous buffer solution, and in step iv) the calcium carbonate of step i) is contacted with the aqueous buffer solution to form surface-treated calcium carbonate.
- the contacting step iv) can be carried out by any means known in the art.
- the calcium carbonate of step i), the at least one acid of step ii), and the at least one conjugate base of step iii) can be brought into contact by spraying and/or mixing. Suitable process equipment for spraying or mixing is known to the skilled person.
- process step iv) is carried out by spraying.
- process step iv) is carried out by mixing.
- the skilled person will adapt the mixing conditions such as the mixing speed and temperature according to his process equipment.
- the mixing may take place by means of a ploughshare mixer.
- Ploughshare mixers function by the principle of a fluidized bed produced mechanically.
- Ploughshare blades rotate close to the inside wall of a horizontal cylindrical drum and convey the components of the mixture out of the product bed and into the open mixing space.
- the fluidized bed produced mechanically ensures intense mixing of even large batches in a very short time.
- Choppers and/or dispersers are used to disperse lumps in a dry operation.
- Equipment that may be used in the inventive process is available, for example, from Gebruder Lodige Maschinenbau GmbH,
- tubular mixing apparatus for example, from Ystral GmbH, Ballrechten-Dottingen, Germany may be used.
- Process step iv) may be carried out at room temperature, i.e. at a temperature of 20°C ⁇ 2°C, or at other temperatures. According to one embodiment of the present invention, process step iv) is carried out for at least 1 s, preferably for at least one min, e.g. for at least 2 min. According to one embodiment, process step iv) is carried out by mixing for at least 1 min, preferably at least 5 min, more preferably at least 10 min, and most preferably at least 20 min.
- the at least one acid of step ii) and/or the at least one conjugate base of step iii) are provided in form of an aqueous solution.
- the surface-treated calcium carbonate is obtained in form of a wet or moist surface-treated calcium carbonate or in form of an aqueous suspension of surface-treated calcium carbonate. It is also within the confines of the present invention that additional water may be introduced during process step iv), for example, in order to control and/or maintain and/or achieve the desired solids content or Brookfield viscosity of the obtained wet or moist surface-treated calcium carbonate or aqueous suspension of surface-treated calcium carbonate.
- a suspension of surface-treated calcium carbonate is obtained, the suspension having a solid content in the range from 1 to 80 wt.-%, more preferably in the range from 10 to 60 wt.-%, and most preferably in the range from 20 to 50 wt.-%, based on the weight of the surface-treated calcium carbonate in the suspension.
- the process can further comprise a step v) of drying the surface-treated calcium carbonate.
- the drying step v) may take place using any suitable drying equipment and can, for example, include thermal drying and/or drying at reduced pressure using equipment such as an evaporator, a flash drier, an oven, a spray drier and/or drying in a vacuum chamber.
- suitable drying equipment such as an evaporator, a flash drier, an oven, a spray drier and/or drying in a vacuum chamber.
- drying step v) is a spray drying step, preferably said spray drying step is carried out with an inlet temperature of 100°C - 1000°C and preferably from 200°C to 600°C.
- Spray drying may be preferred in case of small particle sizes, e.g., at a volume determined median particle size ⁇ i 5 o from 1 to 20 ⁇ .
- a volume determined median particle size dso of more than 20 ⁇ other drying methods, e.g., drying in an oven, fluid bed dryer or flash drying may be preferred.
- a dried surface-treated calcium carbonate is obtained, preferably having a low total moisture content.
- the surface-treated calcium carbonate obtained by step v) has a total moisture content which is less than or equal to 1.0 wt.-%, based on the total weight of the dried surface-treated calcium carbonate.
- the dried surface-treated calcium carbonate obtained by step v) has a total moisture content of less than or equal to 0.5 wt.-%, and preferably less than or equal to 0.2 wt.-%, based on the total weight of the dried surface-treated calcium carbonate.
- the dried surface-treated calcium carbonate obtained by step v) has a total moisture content of between 0.01 and 0.15 wt.-%, preferably between 0.02 and 0.10 wt.-%, and more preferably between 0.03 and 0.07 wt.-%, based on the total weight of the dried surface-treated calcium carbonate.
- the surface-treated calcium carbonate is left to stand for at least 6 h, preferably at least 12 h, more preferably at least 24 h, and most preferably at least 48 h. This prolonged contacting time may improve the efficiency of the surface treatment and may lead to the formation of a more homogeneous surface-treatment layer.
- step iv) the calcium carbonate of step i), the at least one acid of step ii), the at least one conjugate base of step iii), and optionally water, are contacted to form an aqueous suspension of surface-treated calcium carbonate, and after step iv) and before step v) the surface-treated calcium carbonate is separated from the aqueous suspension obtained from step iv).
- the calcium carbonate of step i) is provided in an amount from 10 to 99 wt.-%, based on the total amount of the aqueous suspension of surface-treated calcium carbonate, preferably in an amount from 10 to 95 wt.-%, more preferably in an amount from 20 to 90 wt.-%, and most preferably in an amount from 30 to 40 wt.-%.
- the at least one acid of step ii) and the at least one conjugate base of step iii) are provided together in form of an aqueous buffer solution, and step iv) comprises the steps of:
- step i mixing the calcium carbonate of step i), the aqueous buffer solution, and optionally water, to form an aqueous suspension of surface-treated calcium carbonate, and
- the expression "separating” means that the surface-treated calcium carbonate is removed or isolated from the aqueous suspension obtained from step cl) of the inventive process.
- the surface-treated calcium carbonate obtained from step cl) may be separated from the aqueous suspension by any conventional means of separation known to the skilled person.
- the surface-treated calcium carbonate is separated mechanically and/or thermally.
- the surface-treated calcium carbonate is separated mechanically, preferably by filtration and/or centrifugation.
- the surface-treated calcium carbonate is washed with a washing solution, preferably water.
- the surface-treated calcium carbonate can be washed one time, two times or three times with a washing solution, preferably water. Suitable washing solutions may be selected from water, ethanol, or mixtures thereof.
- aqueous buffer solution comprising at least one acid having a pK a value from 0 to 8, when measured at 20°C, and at least one conjugate base
- step III contacting the calcium carbonate of step I), the buffer solution of step II), and optionally water, to form surface-treated calcium carbonate.
- the aqueous buffer solution is selected from the group consisting of phosphate buffer, phosphate-citrate buffer, citrate buffer, tartrate buffer, borate buffer, preferably the aqueous buffer solution is a phosphate buffer, a phosphate-citrate buffer, or a citrate buffer, and more preferably a phosphate-citrate buffer, or a citrate buffer.
- the at least one acid is selected from sodium dihydrogen phosphate, potassium dihydrogen phosphate, citric acid, sodium dihydrogen citrate, potassium dihydrogen citrate, disodium hydrogen citrate, dipotassium hydrogen citrate, or mixtures thereof, and the at least one conjugate base is disodium hydrogen phosphate, dipotassium hydrogen phosphate, tripotassium citrate and/or trisodium citrate.
- the at least one acid may be selected from sodium dihydrogen phosphate, potassium dihydrogen phosphate, sodium dihydrogen citrate, potassium dihydrogen citrate, or mixtures thereof, and the at least one conjugate base may be disodium hydrogen phosphate, dipotassium hydrogen phosphate, tripotassium citrate and/or trisodium citrate.
- this process can further comprise a step IV) of drying the surface-treated calcium carbonate as described above with respect to drying step v).
- a suspension of a surface- treated calcium carbonate with improved stability in environments with a pH of 4.5 to 7 is provided, which is obtainable by a process comprising the steps of:
- step iv) contacting the calcium carbonate of step i), the at least one acid of step ii), and the at least one conjugate base of step iii) to form surface- treated calcium carbonate,
- step ii) wherein the at least one acid of step ii) and/or the at least one conjugate base of step iii) are provided in form of an aqueous solution.
- a dried surface-treated calcium carbonate with improved stability in environments with a pH of 4.5 to 7 is provided, which is obtainable by a process comprising the steps of:
- step iv) contacting the calcium carbonate of step i), the at least one acid of step ii), and the at least one conjugate base of step iii) to form surface- treated calcium carbonate, and
- step ii) and/or the at least one conjugate base of step iii) are provided in form of an aqueous solution.
- a surface-treated calcium carbonate can be prepared that shows a similar BET surface area like the calcium carbonate that has been used for its preparation.
- the treatment of the present invention does not lead to or only leads to a minor increase of the BET surface of the treated product.
- the surface-treated calcium carbonate is in form of particles having a volume determined median particle size dso from 1 to 1 500 ⁇ , preferably from 25 to 1 400 ⁇ , more preferably from 100 to 1 200 ⁇ , even more preferably from 200 to 1 000 ⁇ , and most preferably from 300 to 800 ⁇ .
- the surface-treated calcium carbonate may be in form of particles having a volume determined top cut particle size from 40 to 2 000 ⁇ , preferably from 200 to 1 800 ⁇ , more preferably from 300 to 1 500 ⁇ , even more preferably from 400 to 1 200 ⁇ , and most preferably from 500 to 1 000 ⁇ .
- the surface-treated calcium carbonate has a reduced acid consumption per hour at pH 5 and pH 6 when treated with acetic acid, the reduction being at least 25 %, more preferably at least 50 %, and most preferably at least 70 %.
- the surface-treated calcium carbonate is in form of particles having a specific BET surface area in the range of > 0 to 5 m 2 /g, preferably in the range of > 0 to 2 m 2 /g, and more preferably in the range of > 0 to 1.6 m 2 /g.
- the surface-treated calcium carbonate obtainable by the process of the present invention may be used for various applications.
- the surface-treated calcium carbonate is used as abrasive material. The inventors of the present invention found that the surface-treated calcium carbonate obtained by the process of the present invention may provide a very gentle abrasiveness, which makes it suitable for delicate surfaces and especially cosmetic applications.
- the dried surface-treated calcium carbonate obtainable by the process of the present invention is used as abrasive material for cosmetic application and/or cleaning application, more preferably as abrasive material for cosmetic application, and most preferably as abrasive material for topical skin application.
- the suspension of the surface-treated calcium carbonate obtainable by the process of the present invention is used as abrasive material for cleaning application.
- the inventors of the present invention surprisingly found that the surface-treated calcium carbonate obtainable by the inventive process exhibits an improved stability at environments with a pH of 4.5 to 7. Therefore, the surface-treated calcium carbonate is especially suitable for applications in such environments.
- the inventive surface-treated calcium carbonate, in suspension or in dry from can also be used in applications requiring pH values of > 7, for example pH values in the range from > 7 to 10 or 11.
- the surface-treated calcium carbonate obtained by the inventive process can replace conventional abrasive materials such as microbeads in abrasive cleaning compositions without affecting the sensory properties of the composition.
- the dried surface-treated calcium carbonate is a non-toxic material and biologically degradable, which makes it, for example, most suitable for cosmetic applications and eco-friendly household cleaners.
- an abrasive cleaning composition comprising a surface-treated calcium carbonate obtainable by a process according to the present invention is provided.
- the abrasive cleaning composition comprises at least 1 wt.-%, based on the total weight of the composition, of the surface-treated calcium carbonate as an abrasive material.
- the abrasive cleaning composition can comprise at least 2 wt.-%, at least 5 wt.-%, or at least 8 wt.-%, based on the total weight of the composition, of the surface-treated calcium carbonate as an abrasive material.
- the composition comprises from 10 to 80 wt.-%, preferably from 15 to 70 wt.-%, more preferably from 17 to 60 wt.-%, even more preferably from 19 to 50 wt.-%, and most preferably about 20 wt.-% of the surface-treated calcium carbonate, based on the total weight of the composition.
- the surface-treated calcium carbonate can consist of only one type of surface-treated calcium carbonate or can be a mixture of two or more types of surface-treated calcium carbonate.
- the abrasive cleaning composition of the present invention may contain the surface-treated calcium carbonate as the only abrasive material.
- the abrasive cleaning composition of the present invention may contain the surface-reacted calcium carbonate in combination with at least one additional abrasive material.
- the further abrasive material can be made from plastic, hard waxes, inorganic and organic abrasives, or natural materials.
- the abrasive cleaning composition further comprises at least one additional abrasive material, preferably selected from the group consisting of silica, precipitated silica, alumina, aluminosilicate, metaphosphate, tricalcium phosphate, calcium pyrophosphate, natural ground calcium carbonate, precipitated calcium carbonate, sodium bicarbonate, bentonite, kaolin, aluminium hydroxide, calcium hydrogen phosphate, hydroxylapatite, apricot kernels, plum kernels, salt, sugar, polyethylene beads, luffa, rice, bamboo, microcrystalline cellulose, basalt, waxes, coffee, polylactic acid, cocoa exfoliator and mixtures thereof.
- additional abrasive material preferably selected from the group consisting of silica, precipitated silica, alumina, aluminosilicate, metaphosphate, tricalcium phosphate, calcium pyrophosphate, natural ground calcium carbonate, precipitated calcium carbonate, sodium bicarbonate, bentonite, ka
- the additional abrasive material can have the same volume determined median particle size dso as the surface-treated calcium carbonate, namely a dso from 1 to 1 500 ⁇ , preferably from 25 to 1 400 ⁇ , more preferably from 100 from 1 200 ⁇ , even more preferably from 200 to 1 000 ⁇ , and most preferably from 300 to 800 ⁇ .
- This abrasive material is suitable for topical applications.
- the additional abrasive material has volume determined median particle size dso from 0.1 to 100 ⁇ , preferably from 0.5 to 50 ⁇ , more preferably from 1 to 20 ⁇ , and most preferably from 2 to 10 ⁇ .
- dso volume determined median particle size from 0.1 to 100 ⁇ , preferably from 0.5 to 50 ⁇ , more preferably from 1 to 20 ⁇ , and most preferably from 2 to 10 ⁇ .
- Such an abrasive material is suitable for oral applications.
- the at least one additional abrasive material can be present in the abrasive cleaning composition in an amount from 1 to 80 wt.-%, based on the total weight of the composition, preferably from 5 to 70 wt.-%, more preferably from 10 to 60 wt.-%, and most preferably from 20 to 50 wt.-%.
- the abrasive cleaning composition of the present invention may be in form of a solid or in form of a liquid.
- the abrasive cleaning composition is in form of a solid, preferably a powder, a granulate, a tablet, a stick, a chunk, a block, a sponge or a brick.
- the abrasive cleaning composition is in form of a liquid, preferably a solution, a suspension, a paste, an emulsion, a cream, an oil, or a gel.
- the abrasive cleaning composition is a liquid, aqueous composition.
- the aqueous composition comprises from 1 to 90 wt.-% water, based on the total weight of the composition, preferably from 5 to 80 wt.-%, more preferably from 10 to 70, and most preferably from 20 to 60 wt.-%.
- the abrasive cleaning composition is a liquid, non-aqueous composition.
- the aqueous composition comprises from 1 to 90 wt.-% of a solvent, based on the total weight of the composition, preferably from 5 to 80 wt.-%, more preferably from 10 to 70, and most preferably from 20 to 60 wt.-%.
- Suitable solvents are known to the skilled person and are, for example, aliphatic alcohols, ethers and diethers having from 4 to 14 carbon atoms, glycols, alkoxylated glycols, glycol ethers, alkoxylated aromatic alcohols, aromatic alcohols, terpenes, natural oils, or mixtures thereof.
- the abrasive cleaning composition has a pH from 5.0 to 7.0, measured at 20°C, preferably from 5.5 to 6.5, and most preferably of about 6.
- the abrasive cleaning composition has a pH above 5, measured at 20°C.
- the composition has a pH above 7.0, measured at 20°C.
- the abrasive cleaning composition may have a pH from 7.5 to 11 or from 7.5 to 10. Means for adjusting the pH are known to the skilled person.
- the abrasive cleaning composition is in liquid form, it may be a thickened composition having a Brookfield viscosity from 4 000 to 50 000 rnPa-s at 20° C.
- the abrasive cleaning composition may further comprise a surfactant.
- the abrasive cleaning composition comprises from 0.1 to 30 wt.-% of a surfactant, preferably from 0.5 to 25 wt.-%, and most preferably from 2 to 20 wt.-%, based on the total amount of the composition.
- Suitable surfactants are known to the skilled person and may be selected from non- ionic, anionic, zwitterionic, amphoteric, cationic surfactants, and mixtures thereof.
- nonionic surfactants are compounds produced by the condensation of simple alkylene oxides, which are hydrophilic in nature, with an aliphatic or alkyl- aromatic hydrophobic compound having a reactive hydrogen atom.
- Suitable anionic surfactants are, for example, water-soluble salts of organic sulphuric acid mono-esters and sulphonic acids which have in the molecular structure a branched or straight chain alkyl group containing from 6 to 22 carbon atoms in the alkyl part.
- Suitable zwitterionic surfactants are of aliphatic quaternary ammonium, sulphonium and phosphonium compounds having an aliphatic group of from 8 to 18 carbon atoms and an aliphatic group substituted by an anionic water- solubilising group, for instance betaine and betaine derivatives such as alkyl betaine, in particular C 12 -C 16 alkyl betaine, 3-(N,N-dimethyl-N- hexadecylammonium)- propane- 1-sulphonate betaine, 3 -(dodecylmethyl- sulphonium)- propane- 1-sulphonate betaine, 3 -(cetylmethyl-phosphonium)-propane- 1-sulphonate betaine and
- betaine and betaine derivatives such as alkyl betaine, in particular C 12 -C 16 alkyl betaine, 3-(N,N-dimethyl-N- hexadecylammonium)- propane- 1-sul
- Suitable amphoteric surfactants are, for example, derivatives of aliphatic secondary and tertiary amines containing an alkyl group of 8 to 20 carbon atoms and an aliphatic group substituted by an anionic
- water-solubilising group for instance sodium 3-dodecylamino-propionate, sodium 3-dodecylaminopropane-sulphonate and sodium N-2-hydroxy-dodecyl-N- methyltaurate.
- suitable cationic surfactants are quaternary ammonium salts having one or two alkyl or aralkyl groups of from 8 to 20 carbon atoms and two or three small aliphatic (e.g. methyl) groups, for instance, cetyltrimethylammonium chloride. Further examples are given in well-known text books such as "Household Cleaning, Care and Maintenance Products", Edts. Herrmann G. Hauthal, G. Wagner, 1 st Edition, Verlag fur Chem.
- the abrasive composition of the present invention may further comprise ingredients which aid in its cleaning performance.
- the composition may contain detergent builders and mixtures of such builders in an amount of up to 25 wt.-%, based on the total amount of the composition.
- Suitable inorganic or organic detergent builders are known to the skilled person and may be selected, for example, from sodium tripolyphosphate or sodium aluminium silicate.
- the abrasive cleaning composition further comprises dispersing agents, thickeners, preservatives, humectants, foaming agents, fluoride sources (e.g., in case the abrasive cleaning composition is an oral care product), flavouring agents, fragrances, colorants, active agents, and/or buffering systems.
- active agents are pharmaceutically, biologically or cosmetically active agents, biocides, or disinfecting agents.
- the abrasive cleaning composition of the present invention may also comprise further optional ingredients which are not mentioned here but known to skilled person.
- the abrasive cleaning composition is a cosmetic composition, preferably a cosmetic composition for topical application, and more preferably a facial cleanser, a body wash, a body scrub, a scrub shampoo, a massage cream, a body polisher, an exfoliator, a peeling cream, a food scrub, a microderm abrasion product, or an oral care composition.
- an oral care composition are a toothpaste, a toothpowder, a powder for dental powder blasting, or a chewable gum.
- the abrasive cleaning composition is a cosmetic composition having a pH value from 5.0 to 7.0, preferably from 5.5 to 6.5, and most preferably of about 6.
- a cosmetic composition comprising a surface-treated calcium carbonate with improved stability in environments with a pH of 4.5 to 7 is provided, wherein the cosmetic composition has a pH value from 5.0 to 7.0 and the surface-treated calcium carbonate is obtainable by a process comprising the steps of:
- step iv) contacting the calcium carbonate of step i), the at least one acid of step ii), and the at least one conjugate base of step iii) to form surface-treated calcium carbonate, and
- the at least one acid of step ii) and/or the at least one conjugate base of step iii) are provided in form of an aqueous solution.
- the abrasive cleaning composition of the present invention is used for cleaning a surface.
- a method for cleaning a surface wherein the surface is contacted with an abrasive cleaning composition according to the present invention.
- the surface may be contacted with the inventive composition by applying the composition to the surface, for example, by spraying, pouring or squeezing.
- the abrasive cleaning composition may be applied to the surface by using an appropriate means such as a mop, a paper towel, a brush, or a cloth, soaked in the composition, which can be in neat or in diluted form.
- the surface is an inanimate surface, preferably selected from the group consisting of household hard surfaces, dish surfaces, leather surfaces, and automotive vehicle surfaces.
- household hard surfaces are working surfaces, ceramic surfaces, refrigerators, freezers, washing machines, automatic dryers, ovens, microwave ovens, ceramic cook tops, bathroom fittings or dishwashers.
- dish surfaces are dishes, cutlery, cutting boards, or pans.
- the surface is an animate surface, preferably selected from the group consisting of human skin, animal skin, human hair, animal hair, and tissues of the oral cavity such as teeth, gums, tongue or buccal surfaces.
- the method of the present invention further comprises a step of rinsing the composition.
- the abrasive cleaning composition of the present invention is used for cleaning an animate surface, and more preferably for cleaning an animate surface selected from the group consisting of human skin, animal skin, human hair, animal hair, and tissues of the oral cavity such as teeth, gums, tongue or buccal surfaces.
- the surface to be cleaned is an inanimate surface, preferably selected from the group consisting of household hard surfaces, dish surfaces, leather surfaces, and automotive vehicle surfaces.
- the pH of a suspension or solution was measured at 25°C using a Mettler Toledo Seven Easy pH meter and a Mettler Toledo InLab® Expert Pro pH electrode.
- a three point calibration (according to the segment method) of the instrument was first made using commercially available buffer solutions having pH values of 4, 7 and 10 at 20°C (from Sigma- Aldrich Corp., USA). The reported pH values are the endpoint values detected by the instrument (the endpoint was when the measured signal differed by less than 0.1 mV from the average over the last 6 seconds).
- the pH of a suspension or solution was measured at 25°C using a Mettler Toledo DL58 Titrator and a Mettler Toledo DG 115-SC electrode.
- a three point calibration (according to the segment method) of the instrument was first made using commercially available buffer solutions having pH values of 4.01, 7.00 and 9.21 at 20°C (Duracal Buffers from Hamilton, Switzerland).
- the reported pH values are the endpoint values detected by the instrument (the endpoint was when the measured signal differed by less than 0.1 mV from the average over the last 2.5 seconds).
- the BET specific surface area is measured via the BET process according to ISO 9277 using nitrogen, following conditioning of the sample by heating at 250°C for a period of 30 minutes. Prior to such measurements, the sample is filtered within a Buchner funnel, rinsed with deionised water and dried at 1 10°C in an oven for at least 12 hours.
- the weight median particle size of all particulate materials was determined by the sedimentation method, which is an analysis of sedimentation behaviour in a gravimetric field.
- the measurement was made with a SedigraphTM 5100, Micromeritics Instrument Corporation. The method and the instrument are known to the skilled person and are commonly used to determine grain size of fillers and pigments in the range from 0.1 to 5 ⁇ .
- the measurement was carried out in an aqueous solution of 0.1 wt.-% Na 4 P 2 07. The samples are dispersed using a high speed stirrer and supersonicated.
- the volume determined median particle size dso of calcium carbonate and surface- treated calcium carbonate was evaluated using a Malvern Mastersizer 2000 Laser Diffraction System (Malvern Instruments Pic, Great Britain), using the Fraunhofer light scattering approximation.
- the method and instrument are known to the skilled person are commonly used to determine particle sizes of fillers and other particulate materials of > 5 ⁇ .
- it is preferable to use the Sedigraph method for particle sizes of less than 5 ⁇ it is also possible to do volume based particles size determination using the Malvern Mastersizer for particle sizes as low as 1 ⁇ .
- the samples were dispersed using a high speed stirrer and in the presence of supersonics.
- a first solution was prepared by placing 1 000 g sodium dihydrogen citrate solution (0.1 mol/kg) in a beaker, and adding 48.2 g trisodium citrate to the beaker while stirring with a magnetic stirring bar.
- the total citrate concentration of said first solution was 0.27 mol/kg.
- Table 1 lists the amounts of the used compounds as well as the resulting ionic concentrations. All compounds were purchased from Sigma Aldrich.
- Table 1 Compounds used for preparation of different buffers and resulting ionic concentrations and pH.
- the calcium carbonate used was a natural ground calcium carbonate from Karabiga, Turkey, having a (volume determined) of 200 ⁇ , a ⁇ i 2 o (volume determined) of 243 ⁇ , a ⁇ i 50 (volume determined) of 362 ⁇ , a dgo (volume determined) of 640 ⁇ , and a dgs (volume determined) of 81 ⁇ ⁇ ), and a BET specific surface area less than 1.0. 3. Examples
- Example 1 A - Preparation of surface-treated calcium carbonate - lab-scale Three samples of surface-treated calcium carbonate were prepared by treating the calcium carbonate with any one of the three prepared buffer solutions as follows:
- the pH of the mixture was measured. The measured pH values are given in Table 2 below. Then, the mixture was filtered through a 100 ⁇ sieve. The obtained residue on the sieve was washed with approximately 500 g of tap water in a beaker for about half a minute and the mixture was filtered again. Then the residue obtained on the sieve was dried in an oven at 90°C over night.
- Table 2 pH values of solution after preparation of surface-treated calcium carbonate.
- Example IB Preparation of surface-treated calcium carbonate - pilot-scale
- phosphate citrate buffer solution D 30 kg was used. This solution was then added to 15 kg of the above-mentioned dry calcium carbonate in a ploughshare mixer. Concentrations of the solution were such that the dose of citrate ion on calcium carbonate was 2.0 % and the dose of phosphate ion on calcium carbonate was 1.9 %. This slurry was then mixed for 10 minutes and emptied into a drum. One sample, TO, taken immediately after mixing was sieved at 106 ⁇ and was dried at 115°C, and a second sample, Tl, was left overnight to mature, after which it was sieved at 106 ⁇ and was dried at 115°C.
- Example 1 A The samples prepared according to Example 1 A were analyzed with regard to their stability under acidic conditions at pH 5 and pH 6 by titrating the samples with acetic acid (2.0 mo 1/1).
- the untreated calcium carbonate was also analyzed as comparative sample.
- the titration of the sample was performed in triplicate with the prepared acetic acid solution over time in order to reach a pH value of 5.0 with a control band of 0.5. The titration was performed over a time period of 60 minutes. Stability at pH 6
- a 0.1 mo 1/1 solution of acetic acid was prepared from concentrated acetic acid (from Sigma- Aldrich, CAS No. 64-19-7, puriss. 99-100%).
- Example 2B Stability of surface-treated calcium carbonate - pilot-scale
- Example IB The samples prepared according to Example IB were analyzed with regard to their stability under acidic conditions at pH 5 by titrating the samples with acetic acid (1.0 mo 1/1). The untreated calcium carbonate was also analyzed as comparative sample.
- a 1.0 mo 1/1 solution of acetic acid was prepared from concentrated acetic acid (from Sigma- Aldrich, CAS No. 64-19-7, puriss. 99-100%).
- the titration of the sample was performed in triplicate with the prepared acetic acid solution over time in order to reach a pH value of 5.0 with a control band of 0.5. The titration was performed over a time period of 60 minutes.
- Example 3A Preparation of body scrub formulations - from abrasive material prepared in lab-scale
- the abrasive material used in part D is listed in Table 6 below.
- Heidolph RZR 2041 mixer 300 to 350 rpm.
- Phenochem Phenoxyethanol (and) Methylparaben (and) 3)
- Example 3B Preparation of body scrub formulations - from abrasive material prepared in pilot-scale (Sample 6 (TO and Tl))
- the abrasive material used in part D is listed in Table 8 below.
- Table 8 Composition of prepared body scrub formulations.
- Heidolph RZR 2041 mixer 300 to 350 rpm.
- the sensory properties of the prepared body scrub formulations 1 to 5 were tested by applying approx. 0.075 ml of the body scrub on the hand or the finger by using a small spoon.
- the sensory properties of the formulations mentioned below were marked with a rating from 0 to 10, wherein 0 means “not good” and 10 means “very good”.
- Non fluid When the product is placed between the thumb and index finger by applying a pressure, there is no flow between the both fingers and it moves not away from the pressure zone. A resistance is felt. Stringy When the product is placed between the thumb and index finger and a distance is slowly applied between the both fingers, it is a continuous string. It breaks when the distance between the fingers becomes too large.
- Non foaming No foaming is visible between 10 and 15 rotations on the hand.
- Non Soft The particles give no pleasant sensation during the application (exfoliation) on the skin.
- the products When the product is placed between the thumb and index finger and a movement of friction is applied, the products have an oily aspect.
- Penetrating The product is disappearing and no residue is detected when touching skin after two minutes.
- Non penetrating There can still a residue be detected on the skin after touching the skin after two minutes.
- Non slippery Under water by performing a pressure with index finger on the hand, there is a resistance and no sliding on the hand.
- Table 11 Sensory properties after 6 weeks storag e at 5°C.
- Table 12 Sensory properties after 6 weeks storage at 40°C.
- inventive formulation 2 showed the best "slippery" effect on the skin. Foaming is similar for all, but better at 5°C and 40°C. Spread is improved for the samples stored at 40°C, because the viscosity decreased at the higher storage temperature. The best spread remains the same for the formulations 2 and 4.
- formulation 5 cannot be compared with formulations 1 to 4 since different components has been used as ingredient C, however, this formulation also shows a good performance.
- all inventive body scrub formulations showed good sensory performance, confirming that the surface-treated calcium carbonate obtained by the process of the present invention is suitable for cosmetic products such as body scrub.
Abstract
Description
Claims
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| MX2017008937A MX2017008937A (en) | 2015-01-15 | 2016-01-13 | Surface-treated calcium carbonate with improved stability in environments with a ph of 4.5 to 7. |
| CN201680005822.6A CN107108252B (en) | 2015-01-15 | 2016-01-13 | Surface in the environment that pH value is 4.5-7 with improved stability is through handling calcium carbonate |
| CA2971958A CA2971958A1 (en) | 2015-01-15 | 2016-01-13 | Surface-treated calcium carbonate with improved stability in environments with a ph of 4.5 to 7 |
| KR1020177022691A KR20170105068A (en) | 2015-01-15 | 2016-01-13 | a surface treated calcium carbonate having improved stability in an environment having a pH of 4.5 to 7 |
| JP2017537393A JP6490819B2 (en) | 2015-01-15 | 2016-01-13 | Surface treated calcium carbonate with improved stability in pH 4.5 to 7 environments |
| EP16700449.8A EP3245257B2 (en) | 2015-01-15 | 2016-01-13 | Surface-treated calcium carbonate with improved stability in environments with a ph of 4.5 to 7 |
| US15/538,804 US20180258288A1 (en) | 2015-01-15 | 2016-01-13 | Surface-treated calcium carbonate with improved stability in environments with a ph of 4.5 to 7 |
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP15151341.3 | 2015-01-15 | ||
| EP15151341.3A EP3045503A1 (en) | 2015-01-15 | 2015-01-15 | Surface-treated calcium carbonate with improved stability in environments with a pH of 4.5 to 7 |
| US201562106243P | 2015-01-22 | 2015-01-22 | |
| US62/106,243 | 2015-01-22 | ||
| US201562114135P | 2015-02-10 | 2015-02-10 | |
| US62/114,135 | 2015-02-10 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016113285A1 true WO2016113285A1 (en) | 2016-07-21 |
Family
ID=52354805
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2016/050536 WO2016113285A1 (en) | 2015-01-15 | 2016-01-13 | Surface-treated calcium carbonate with improved stability in environments with a ph of 4.5 to 7 |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US20180258288A1 (en) |
| EP (2) | EP3045503A1 (en) |
| JP (1) | JP6490819B2 (en) |
| KR (1) | KR20170105068A (en) |
| CN (1) | CN107108252B (en) |
| CA (1) | CA2971958A1 (en) |
| MX (1) | MX2017008937A (en) |
| TW (1) | TWI588218B (en) |
| WO (1) | WO2016113285A1 (en) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107555460A (en) * | 2017-09-18 | 2018-01-09 | 广西合山东来化工科技有限公司 | A kind of Nano calcium carbonate dedicated preparation method of rubber tyre |
| WO2018046127A1 (en) | 2016-09-08 | 2018-03-15 | Schaefer Kalk Gmbh & Co. Kg | Inhibiting calcium carbonate additive |
| CN109789247A (en) * | 2016-09-08 | 2019-05-21 | 卡尔莱布宁医疗技术有限公司 | Contain the implantation material for inhibiting calcium carbonate |
| JP2019527666A (en) * | 2016-07-25 | 2019-10-03 | オムヤ インターナショナル アーゲー | Method for producing surface-reacted calcium carbonate |
| JP2020508575A (en) * | 2017-02-24 | 2020-03-19 | イラミーナ インコーポレーテッド | Calcium carbonate slurry |
| EP3725851A1 (en) | 2019-04-16 | 2020-10-21 | Omya International AG | Process for preparing surface-reacted calcium carbonate |
| US11352491B2 (en) | 2016-09-08 | 2022-06-07 | Schaefer Kalk Gmbh & Co. Kg | Calcium-salt-containing composite powder having microstructured particles |
| US11441008B2 (en) | 2016-09-08 | 2022-09-13 | Schaefer Kalk Gmbh & Co. Kg | Composite powder containing calcium carbonate and having microstructured particles |
| US11760874B2 (en) | 2016-09-08 | 2023-09-19 | Schaefer Kalk Gmbh & Co. Kg | Composite powder containing calcium carbonate and having microstructured particles having inhibiting calcium carbonate |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN108348408A (en) * | 2015-11-13 | 2018-07-31 | 宝洁公司 | Dentifrice composition with improved fluoride stability |
| CN108348414A (en) | 2015-11-13 | 2018-07-31 | 宝洁公司 | The dentifrice composition absorbed with Difluoride source and improved fluoride |
| CN108348429A (en) | 2015-11-13 | 2018-07-31 | 宝洁公司 | The dentifrice composition absorbed with improved fluoride |
| US11124654B2 (en) * | 2016-09-08 | 2021-09-21 | Karl Leibinger Medizintechnik Gmbh & Co. Kg | Method for producing an implant comprising calcium carbonate-containing composite powder having microstructured particles having inhibiting calcium carbonate |
| WO2018157087A1 (en) * | 2017-02-24 | 2018-08-30 | California Institute Of Technology | Microabrasive compositions containing oöids |
| EP3517178A1 (en) * | 2018-01-26 | 2019-07-31 | Omya International AG | Surface-reacted calcium carbonate for modifying the biomechanical properties of the skin |
| EP3517176A1 (en) | 2018-01-26 | 2019-07-31 | Omya International AG | Surface-reacted calcium carbonate for the use as skin appearance modifier |
Citations (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5043017A (en) | 1990-03-09 | 1991-08-27 | Pfizer Inc. | Acid-stabilized calcium carbonate, process for its production and method for its use in the manufacture of acidic paper |
| US5156719A (en) | 1990-03-09 | 1992-10-20 | Pfizer Inc. | Acid-stabilized calcium carbonate, process for its production and method for its use in the manufacture of acidic paper |
| WO1994007658A1 (en) | 1992-09-25 | 1994-04-14 | Norsk Hydro A.S. | Blasting agent and a process for removing coatings |
| US5531634A (en) | 1995-02-03 | 1996-07-02 | Schott; Paul | Method of using an abrasive material for blast cleaning of solid surfaces |
| WO1997008247A1 (en) | 1995-08-24 | 1997-03-06 | Ecc International Inc. | Acid resistant calcium carbonate composition and uses therefor |
| WO1997008248A1 (en) | 1995-08-24 | 1997-03-06 | Ecc International Inc. | Surface modified calcium carbonate composition and uses therefor |
| WO1997014651A1 (en) | 1995-10-20 | 1997-04-24 | Ecc International Inc. | Stabilized calcium carbonate composition using sodium carbonate and mixtures of acids and uses therefor |
| US5827114A (en) | 1996-09-25 | 1998-10-27 | Church & Dwight Co., Inc. | Slurry blasting process |
| WO2000039222A1 (en) | 1998-12-24 | 2000-07-06 | Plüss-Staufer Ag | NOVEL TREATED FILLER OR PIGMENT OR MINERAL FOR PAPER, IN PARTICULAR PIGMENT CONTAINING NATURAL CaCO3, METHOD FOR MAKING SAME, COMPOSITIONS CONTAINING THEM AND USES |
| US6228161B1 (en) | 1996-12-30 | 2001-05-08 | Minerals Technologies Inc. | Use of calcium carbonate in an acidic aqueous media |
| US20010023351A1 (en) | 1999-12-01 | 2001-09-20 | Eilers George J. | Skin abrasion system and method |
| US6540878B1 (en) | 1999-02-08 | 2003-04-01 | Aga Aktiebolag | Stabilized filler, its production and use |
| WO2003061908A1 (en) | 2002-01-24 | 2003-07-31 | Exa Sa | A process for treating a surface |
| WO2004083316A1 (en) | 2003-03-18 | 2004-09-30 | Omya Development Ag | Novel inorganic pigment containing calcium carbonate, aqueous suspension containing same, and uses thereof |
| WO2005121257A2 (en) | 2004-06-11 | 2005-12-22 | Omya Development Ag | Novel dry mineral pigment containing calcium carbonate, aqueous suspension containing said pigment, and the uses thereof |
| EP2070991A1 (en) | 2007-12-12 | 2009-06-17 | Omya Development AG | Surface-reacted precipitated calcium carbonate, process to make same, and uses thereof |
| WO2009133173A1 (en) | 2008-04-30 | 2009-11-05 | Omya Development Ag | Alkaline earth carbonate containing mineral for surface cleaning |
| EP2264109A1 (en) | 2009-06-15 | 2010-12-22 | Omya Development AG | Process for preparing surface-reacted calcium carbonate and its use |
| EP2264108A1 (en) | 2009-06-15 | 2010-12-22 | Omya Development AG | Process to prepare a surface-reacted calcium carbonate implementing a weak acid, resulting products and uses thereof |
| EP2371766A1 (en) | 2010-04-01 | 2011-10-05 | Omya Development Ag | Process for obtaining precipitated calcium carbonate |
| EP2447213A1 (en) | 2010-10-26 | 2012-05-02 | Omya Development AG | Production of high purity precipitated calcium carbonate |
| US20120111520A1 (en) * | 2010-11-04 | 2012-05-10 | Jing Shen | Method for the preparation of acid-tolerant calcium carbonate fillers and filled paper based on high-lignin-content deinked pulp derived from recycled newspaper |
| EP2524898A1 (en) | 2011-05-16 | 2012-11-21 | Omya Development AG | Precipitated calcium carbonate from pulp mill waste having an improved brightness, method for the production and use thereof |
| WO2013078949A1 (en) | 2011-12-01 | 2013-06-06 | Unilever N.V. | Liquid composition for cleaning of head surfaces |
| WO2013142473A1 (en) | 2012-03-23 | 2013-09-26 | Omya Development Ag | Process for preparing scalenohedral precipitated calcium carbonate |
| WO2013191903A1 (en) * | 2012-06-18 | 2013-12-27 | 3M Innovative Properties Company | Powder composition for air polishing the surface of hard dental tissue |
Family Cites Families (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1447566A (en) | 1974-02-14 | 1976-08-25 | Ici Ltd | Production of calcium carbonate |
| US4157379A (en) | 1976-04-16 | 1979-06-05 | Toyo Soda Manufacturing Co., Ltd. | Process for producing chain structured corpuscular calcium carbonate |
| US5164006A (en) | 1991-04-08 | 1992-11-17 | Ecc America Inc. | Method for preparing acid resistant calcium carbonate pigments |
| US5149552A (en) | 1991-05-23 | 1992-09-22 | Kraft General Foods, Inc. | Calcium citrate anticaking agent |
| JP3004852B2 (en) * | 1993-10-18 | 2000-01-31 | 奥多摩工業株式会社 | Method for producing light calcium carbonate-hydroxyapatite composite and light calcium carbonate-apatite composite |
| DE19517498A1 (en) | 1995-05-12 | 1996-11-14 | Budenheim Rud A Oetker Chemie | Enriching solid base substance with trace substance |
| EP0859885A4 (en) | 1995-10-20 | 1999-06-09 | Ecc Int Inc | Acid resistant calcium carbonate filler |
| US5711799A (en) | 1996-03-13 | 1998-01-27 | Ecc International Inc. | Acid tolerant calcium carbonate composition and uses therefor |
| FR2747569B1 (en) * | 1996-04-22 | 1998-05-22 | Rhone Poulenc Chimie | TOOTHPASTE COMPOSITION COMPRISING A FLUORINE COMPATIBLE WITH A SILICA AND CALCIUM CARBONATE ABRASIVE |
| US6039775A (en) | 1997-11-03 | 2000-03-21 | 3M Innovative Properties Company | Abrasive article containing a grinding aid and method of making the same |
| GB9822093D0 (en) * | 1998-10-09 | 1998-12-02 | Unilever Plc | Abrasive of toothpaste |
| FR2785534A1 (en) * | 1998-11-09 | 2000-05-12 | Rhodia Chimie Sa | FLUORINATED TOOTHPASTE COMPOSITION COMPRISING FLUORINE-COMPATIBLE ABRASIVE PARTICLES OF CALCIUM MATERIAL |
| US20030213937A1 (en) | 2001-02-22 | 2003-11-20 | Isaac Yaniv | Precipitated aragonite and a process for producing it |
| FR2826950B1 (en) | 2001-07-04 | 2004-09-10 | Solvay | PROCESS FOR OBTAINING PRECIPITED CALCIUM CARBONATE PARTICLES STRUCTURED ON THE NANOMETRIC SCALE |
| US7135157B2 (en) | 2003-06-06 | 2006-11-14 | Specialty Minerals (Michigan) Inc. | Process for the production of platy precipitated calcium carbonates |
| EP1746073A1 (en) | 2005-07-20 | 2007-01-24 | SOLVAY (Société Anonyme) | Process for making a solid compound by precipitation, suspensions of solid in liquids and solids obtained by the process and their use as additives |
| FR2913427B1 (en) * | 2007-03-05 | 2011-10-07 | Omya Development Ag | DRY GRINDING PROCESS OF ONE OR MORE MATERIALS COMPRISING AT LEAST ONE CALCIUM CARBONATE |
| CA2694980C (en) | 2008-10-31 | 2011-09-20 | Calera Corporation | Non-cementitious compositions comprising co2 sequestering additives |
| US8865195B2 (en) * | 2011-10-13 | 2014-10-21 | Kimberly-Clark Worldwide, Inc. | Foaming formulations and cleansing products including silicone polyesters |
| CN104507869A (en) | 2012-05-03 | 2015-04-08 | 卡勒拉公司 | Non-cementitious compositions comprising vaterite and methods thereof |
| EP2662416B1 (en) * | 2012-05-11 | 2015-07-22 | Omya International AG | Treatment of calcium carbonate containing materials for increased filler load in paper |
| PT2883573T (en) | 2013-12-13 | 2018-01-04 | Omya Int Ag | Abrasive cleaning composition |
| EP2997833B1 (en) | 2014-09-22 | 2018-01-31 | Omya International AG | Surface-reacted calcium carbonate for use as anti-caking agent |
-
2015
- 2015-01-15 EP EP15151341.3A patent/EP3045503A1/en not_active Withdrawn
-
2016
- 2016-01-13 CN CN201680005822.6A patent/CN107108252B/en active Active
- 2016-01-13 CA CA2971958A patent/CA2971958A1/en not_active Abandoned
- 2016-01-13 JP JP2017537393A patent/JP6490819B2/en active Active
- 2016-01-13 US US15/538,804 patent/US20180258288A1/en not_active Abandoned
- 2016-01-13 MX MX2017008937A patent/MX2017008937A/en unknown
- 2016-01-13 EP EP16700449.8A patent/EP3245257B2/en active Active
- 2016-01-13 KR KR1020177022691A patent/KR20170105068A/en not_active Application Discontinuation
- 2016-01-13 WO PCT/EP2016/050536 patent/WO2016113285A1/en active Application Filing
- 2016-01-15 TW TW105101172A patent/TWI588218B/en not_active IP Right Cessation
Patent Citations (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5043017A (en) | 1990-03-09 | 1991-08-27 | Pfizer Inc. | Acid-stabilized calcium carbonate, process for its production and method for its use in the manufacture of acidic paper |
| US5156719A (en) | 1990-03-09 | 1992-10-20 | Pfizer Inc. | Acid-stabilized calcium carbonate, process for its production and method for its use in the manufacture of acidic paper |
| WO1994007658A1 (en) | 1992-09-25 | 1994-04-14 | Norsk Hydro A.S. | Blasting agent and a process for removing coatings |
| US5531634A (en) | 1995-02-03 | 1996-07-02 | Schott; Paul | Method of using an abrasive material for blast cleaning of solid surfaces |
| WO1997008247A1 (en) | 1995-08-24 | 1997-03-06 | Ecc International Inc. | Acid resistant calcium carbonate composition and uses therefor |
| WO1997008248A1 (en) | 1995-08-24 | 1997-03-06 | Ecc International Inc. | Surface modified calcium carbonate composition and uses therefor |
| WO1997014651A1 (en) | 1995-10-20 | 1997-04-24 | Ecc International Inc. | Stabilized calcium carbonate composition using sodium carbonate and mixtures of acids and uses therefor |
| US5827114A (en) | 1996-09-25 | 1998-10-27 | Church & Dwight Co., Inc. | Slurry blasting process |
| US7033428B2 (en) | 1996-12-30 | 2006-04-25 | Minerals Technologies Inc. | Acid stabilized calcium carbonate an method of making it |
| US6228161B1 (en) | 1996-12-30 | 2001-05-08 | Minerals Technologies Inc. | Use of calcium carbonate in an acidic aqueous media |
| WO2000039222A1 (en) | 1998-12-24 | 2000-07-06 | Plüss-Staufer Ag | NOVEL TREATED FILLER OR PIGMENT OR MINERAL FOR PAPER, IN PARTICULAR PIGMENT CONTAINING NATURAL CaCO3, METHOD FOR MAKING SAME, COMPOSITIONS CONTAINING THEM AND USES |
| US20040020410A1 (en) | 1998-12-24 | 2004-02-05 | Omya Ag | Filler or pigment or processed mineral for paper, in particular a pigment containing natural CaCO3, its manufacturing process, preparations containing it and their applications |
| US6540878B1 (en) | 1999-02-08 | 2003-04-01 | Aga Aktiebolag | Stabilized filler, its production and use |
| US20010023351A1 (en) | 1999-12-01 | 2001-09-20 | Eilers George J. | Skin abrasion system and method |
| WO2003061908A1 (en) | 2002-01-24 | 2003-07-31 | Exa Sa | A process for treating a surface |
| EP1738872A1 (en) | 2002-01-24 | 2007-01-03 | Exa SA | Agent for exfoliating skin |
| EP1666203A1 (en) | 2002-01-24 | 2006-06-07 | Exa SA | A process for removing a coating from a surface |
| WO2004083316A1 (en) | 2003-03-18 | 2004-09-30 | Omya Development Ag | Novel inorganic pigment containing calcium carbonate, aqueous suspension containing same, and uses thereof |
| WO2005121257A2 (en) | 2004-06-11 | 2005-12-22 | Omya Development Ag | Novel dry mineral pigment containing calcium carbonate, aqueous suspension containing said pigment, and the uses thereof |
| EP2070991A1 (en) | 2007-12-12 | 2009-06-17 | Omya Development AG | Surface-reacted precipitated calcium carbonate, process to make same, and uses thereof |
| WO2009074492A1 (en) | 2007-12-12 | 2009-06-18 | Omya Development Ag | Surface-reacted precipitated calcium carbonate, process to make same, and uses thereof |
| WO2009133173A1 (en) | 2008-04-30 | 2009-11-05 | Omya Development Ag | Alkaline earth carbonate containing mineral for surface cleaning |
| EP2264109A1 (en) | 2009-06-15 | 2010-12-22 | Omya Development AG | Process for preparing surface-reacted calcium carbonate and its use |
| EP2264108A1 (en) | 2009-06-15 | 2010-12-22 | Omya Development AG | Process to prepare a surface-reacted calcium carbonate implementing a weak acid, resulting products and uses thereof |
| EP2371766A1 (en) | 2010-04-01 | 2011-10-05 | Omya Development Ag | Process for obtaining precipitated calcium carbonate |
| EP2447213A1 (en) | 2010-10-26 | 2012-05-02 | Omya Development AG | Production of high purity precipitated calcium carbonate |
| US20120111520A1 (en) * | 2010-11-04 | 2012-05-10 | Jing Shen | Method for the preparation of acid-tolerant calcium carbonate fillers and filled paper based on high-lignin-content deinked pulp derived from recycled newspaper |
| EP2524898A1 (en) | 2011-05-16 | 2012-11-21 | Omya Development AG | Precipitated calcium carbonate from pulp mill waste having an improved brightness, method for the production and use thereof |
| WO2013078949A1 (en) | 2011-12-01 | 2013-06-06 | Unilever N.V. | Liquid composition for cleaning of head surfaces |
| WO2013142473A1 (en) | 2012-03-23 | 2013-09-26 | Omya Development Ag | Process for preparing scalenohedral precipitated calcium carbonate |
| WO2013191903A1 (en) * | 2012-06-18 | 2013-12-27 | 3M Innovative Properties Company | Powder composition for air polishing the surface of hard dental tissue |
Non-Patent Citations (2)
| Title |
|---|
| "CRC Handbook of Chemistry and Physics", 1994, CRC PRESS INC., pages: 8 - 43,8-55 |
| HARRIS, D. C.: "Quantitative Chemical Analysis", 1991, W.H. FREEMAN & CO. (USA |
Cited By (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2019527666A (en) * | 2016-07-25 | 2019-10-03 | オムヤ インターナショナル アーゲー | Method for producing surface-reacted calcium carbonate |
| RU2738378C2 (en) * | 2016-09-08 | 2020-12-11 | Шефер Кальк Гмбх Унд Ко. Кг | Calcium carbonate additive |
| CN109661419B (en) * | 2016-09-08 | 2022-05-27 | 谢菲尔考克有限两合公司 | Inhibiting calcium carbonate additives |
| CN109789247A (en) * | 2016-09-08 | 2019-05-21 | 卡尔莱布宁医疗技术有限公司 | Contain the implantation material for inhibiting calcium carbonate |
| WO2018046127A1 (en) | 2016-09-08 | 2018-03-15 | Schaefer Kalk Gmbh & Co. Kg | Inhibiting calcium carbonate additive |
| US11760874B2 (en) | 2016-09-08 | 2023-09-19 | Schaefer Kalk Gmbh & Co. Kg | Composite powder containing calcium carbonate and having microstructured particles having inhibiting calcium carbonate |
| US11548998B2 (en) | 2016-09-08 | 2023-01-10 | Schaefer Kalk Gmbh & Co. Kg | Inhibiting calcium carbonate additive |
| CN109661419A (en) * | 2016-09-08 | 2019-04-19 | 谢菲尔考克有限两合公司 | Inhibition calcium carbonate additive |
| US11441008B2 (en) | 2016-09-08 | 2022-09-13 | Schaefer Kalk Gmbh & Co. Kg | Composite powder containing calcium carbonate and having microstructured particles |
| US11352491B2 (en) | 2016-09-08 | 2022-06-07 | Schaefer Kalk Gmbh & Co. Kg | Calcium-salt-containing composite powder having microstructured particles |
| JP2020508575A (en) * | 2017-02-24 | 2020-03-19 | イラミーナ インコーポレーテッド | Calcium carbonate slurry |
| US11214712B2 (en) | 2017-02-24 | 2022-01-04 | Illumina, Inc. | Calcium carbonate slurry |
| US11806836B2 (en) | 2017-02-24 | 2023-11-07 | Illumina, Inc. | Calcium carbonate slurry |
| CN107555460A (en) * | 2017-09-18 | 2018-01-09 | 广西合山东来化工科技有限公司 | A kind of Nano calcium carbonate dedicated preparation method of rubber tyre |
| WO2020212204A1 (en) | 2019-04-16 | 2020-10-22 | Omya International Ag | Process for preparing surface-reacted calcium carbonate |
| EP3725851A1 (en) | 2019-04-16 | 2020-10-21 | Omya International AG | Process for preparing surface-reacted calcium carbonate |
Also Published As
| Publication number | Publication date |
|---|---|
| CN107108252B (en) | 2019-10-18 |
| EP3245257A1 (en) | 2017-11-22 |
| CA2971958A1 (en) | 2016-07-21 |
| KR20170105068A (en) | 2017-09-18 |
| MX2017008937A (en) | 2017-12-07 |
| TWI588218B (en) | 2017-06-21 |
| CN107108252A (en) | 2017-08-29 |
| JP2018508612A (en) | 2018-03-29 |
| JP6490819B2 (en) | 2019-03-27 |
| EP3245257B2 (en) | 2022-11-30 |
| EP3045503A1 (en) | 2016-07-20 |
| US20180258288A1 (en) | 2018-09-13 |
| EP3245257B1 (en) | 2019-11-13 |
| TW201638239A (en) | 2016-11-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3245257B2 (en) | Surface-treated calcium carbonate with improved stability in environments with a ph of 4.5 to 7 | |
| KR101851087B1 (en) | Abrasive cleaning composition | |
| AU2014363407A1 (en) | Abrasive cleaning composition | |
| AU2006325386B8 (en) | Silicas | |
| CN101594846A (en) | Show precipitated silica material with the cetylpyridinium chloride high-compatibility | |
| HU227429B1 (en) | Oral compositions | |
| WO2014082951A2 (en) | Oral care compositions | |
| CN101588784A (en) | High electrolyte additions for precipitated silica material production | |
| JP5768489B2 (en) | Method for producing surface-treated spherical calcium carbonate particles for cosmetics | |
| CN104837469B (en) | Oral care composition | |
| JP2508057B2 (en) | Polishing base material | |
| WO2021172146A1 (en) | Fatty acid calcium salt particles and cosmetics | |
| JP6368639B2 (en) | Process for producing dentifrice granules |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16700449 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2971958 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15538804 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2017/008937 Country of ref document: MX |
|
| ENP | Entry into the national phase |
Ref document number: 2017537393 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REEP | Request for entry into the european phase |
Ref document number: 2016700449 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 20177022691 Country of ref document: KR Kind code of ref document: A |