WO2015149345A1 - Lithium-ion batteries and preparation method thereof - Google Patents
Lithium-ion batteries and preparation method thereof Download PDFInfo
- Publication number
- WO2015149345A1 WO2015149345A1 PCT/CN2014/074786 CN2014074786W WO2015149345A1 WO 2015149345 A1 WO2015149345 A1 WO 2015149345A1 CN 2014074786 W CN2014074786 W CN 2014074786W WO 2015149345 A1 WO2015149345 A1 WO 2015149345A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- lithium
- carbonate
- ion battery
- battery according
- electrolyte
- Prior art date
Links
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0564—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of organic materials only
- H01M10/0566—Liquid materials
- H01M10/0567—Liquid materials characterised by the additives
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
- H01M10/0525—Rocking-chair batteries, i.e. batteries with lithium insertion or intercalation in both electrodes; Lithium-ion batteries
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0564—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of organic materials only
- H01M10/0566—Liquid materials
- H01M10/0568—Liquid materials characterised by the solutes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0564—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of organic materials only
- H01M10/0566—Liquid materials
- H01M10/0569—Liquid materials characterised by the solvents
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/38—Selection of substances as active materials, active masses, active liquids of elements or alloys
- H01M4/386—Silicon or alloys based on silicon
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/38—Selection of substances as active materials, active masses, active liquids of elements or alloys
- H01M4/387—Tin or alloys based on tin
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/485—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of mixed oxides or hydroxides for inserting or intercalating light metals, e.g. LiTi2O4 or LiTi2OxFy
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/58—Selection of substances as active materials, active masses, active liquids of inorganic compounds other than oxides or hydroxides, e.g. sulfides, selenides, tellurides, halogenides or LiCoFy; of polyanionic structures, e.g. phosphates, silicates or borates
- H01M4/5825—Oxygenated metallic salts or polyanionic structures, e.g. borates, phosphates, silicates, olivines
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/58—Selection of substances as active materials, active masses, active liquids of inorganic compounds other than oxides or hydroxides, e.g. sulfides, selenides, tellurides, halogenides or LiCoFy; of polyanionic structures, e.g. phosphates, silicates or borates
- H01M4/583—Carbonaceous material, e.g. graphite-intercalation compounds or CFx
- H01M4/587—Carbonaceous material, e.g. graphite-intercalation compounds or CFx for inserting or intercalating light metals
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M2300/00—Electrolytes
- H01M2300/0017—Non-aqueous electrolytes
- H01M2300/0025—Organic electrolyte
- H01M2300/0028—Organic electrolyte characterised by the solvent
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M2300/00—Electrolytes
- H01M2300/0017—Non-aqueous electrolytes
- H01M2300/0025—Organic electrolyte
- H01M2300/0028—Organic electrolyte characterised by the solvent
- H01M2300/0037—Mixture of solvents
- H01M2300/004—Three solvents
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02T—CLIMATE CHANGE MITIGATION TECHNOLOGIES RELATED TO TRANSPORTATION
- Y02T10/00—Road transport of goods or passengers
- Y02T10/60—Other road transportation technologies with climate change mitigation effect
- Y02T10/70—Energy storage systems for electromobility, e.g. batteries
Landscapes
- Chemical & Material Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Inorganic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- General Physics & Mathematics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- Physics & Mathematics (AREA)
- Crystallography & Structural Chemistry (AREA)
- Materials Engineering (AREA)
- Secondary Cells (AREA)
Abstract
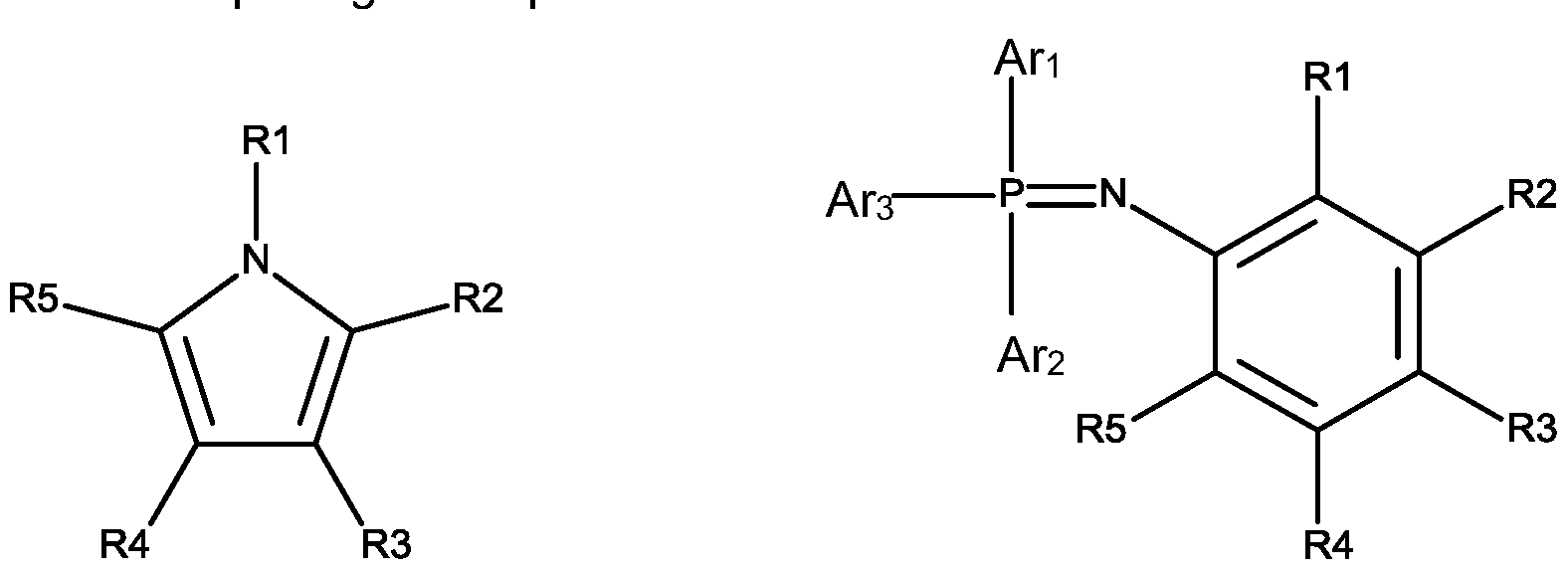
The invention relates to a lithium-ion battery comprising: (1)a cathode comprising LiFePO4; (2) an anode; and (3) an electrolyte comprising: (A) a lithium salt; (B) a non-aqueous organic solvent; and (C) an additive comprising a compound of formula I or formula II, wherein each R1 to R5 is independently H or C1-C10 alkyl, each Ar1to Ar3is independently aryl.
Description
Lithium-ion Batteries and Preparation Method thereof
Field of the Invention
The invention relates to lithium-ion batteries and a method of producing the lithium-ion batteries.
Description of Related Arts
Recently, lithium-ion batteries have been widely used as high energy density sources in many consumer electronics as well as electric vehicles.
Impedance growth and capacity loss of lithium-ion batteries at elevated temperatures are still problems for obtaining high energy density sources. It is well known that impedance growth and capacity loss of lithium-ion batteries are mainly attributed to continued chemical and/or electrochemical reactions among electrolyte components, anode and cathode materials.
US2005/0019670 discloses non-aqueous electrolytes comprising stabilization additives, showing improved capacity retention at a temperature of 55°C. However, after 100 cycles, its cell system only shows capacity retention of no more than 75%, even no more than 60% (vide figure 3 and figure 4 of US2005/0019670).
Thus, there is a need to provide lithium-ion batteries having higher capacity retention at elevated temperature. The present invention provides a solution for excellent capacity retention after 1 ,000 cycles.
Summary of the Invention
For the purpose of the invention, the invention provides a lithium-ion battery comprising:
(1 ) a cathode comprising LiFeP04;
(2) an anode; and
(3) an electrolyte comprising:
(A) a lithium salt;
(B) a non-aqueous organic solvent; and
(C) an additive comprising a compound of formula I or formula I I of:
(I) (I I)
wherein each R1 to R5 is independently H or C1 -C10 alkyl, each Ari to Ar3 is independently aryl.
The invention also provides a method for preparing such lithium-ion battery, the method comprises the steps of:
(1 ) injecting an electrolyte into a cell; and
(2) performing initial formation charge below a voltage of 3.9V.
It is found that the present lithium-ion batteries have good capacity retention at relative high temperature after 1 ,000 cycles.
Brief Description of Drawings
Figure 1 shows cycle curves of lithium-ion batteries according to example 1 and comparison example 1 after 1 ,000 cycles.
Figure 2 shows cycle curves of lithium-ion batteries according to example 1 , example 6 and comparison example 3 at 60°C and 1 C.
Figure 3 shows cycle curves of lithium-ion batteries according to example 7 and comparison example 1 after 1 ,000 cycles.
Embodiments of the Invention
In one aspect, the invention provides a lithium-ion battery comprising:
(1 ) a cathode comprising LiFeP04;
(2) an anode; and
(3) an electrolyte comprising:
(A) a lithium salt;
(B) a non-aqueous organic solvent; and
(C) an additive comprising a compound of formula I or formula II of:
(I) (II)
wherein each R1 to R5 is independently H or C1 -C10 alkyl, each Ari to Ar3 is independently aryl.
Halo represents fluorine, chlorine, bromine, and iodine.
Preferably, the alkyl is C1 -C8 alkyl, preferably C1 -C4 alkyl. Alkyl includes linear or branched alkyl, and its non-limited examples include methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl, nonyl, decyl. Non-limited examples of branched alkyl include -CH(CH3)2 -CH(CH3)(CH2CH3) -CH(CH2CH3)2 -C(CH3)3 -C(CH2CH3)3 -CH2CH(CH3)2 -CH2CH(CH3)(CH2CH3) -CH2CH(CH2CH3)2 -CH2C(CH3)3 -CH2C(CH2CH3)3 -CH(CH3)CH(CH3)CH2CH3) -CH2CH2CH(CH3)2 -CH2CH2CH(CH3)(CH2CH3) -CH2CH2CH(CH2CH3)2 -CH2CH2C(CH3)3 -CH2CH2C(CH2CH3)3 -CH(CH3)CH2CH(CH3)2 -CH(CH3)CH(CH3)CH(CH3)2 -CH(CH2CH3)CH(CH3)CH(CH3)(CH2CH3).
In one preferred embodiment of the invention, each R1 to R5 is independently H or C1 -C8 alkyl, preferably C1 -C4 alkyl, more preferably methyl, ethyl, propyl, butyl.
Preferably, the aryl is substituted or unsubstituted C6-C10 aryl, such as phenyl, naphthyl, etc, more preferably phenyl. Examples of substituted aryl are C1 -C4 alkyl substituted phenyl; C1 -C4 alkoxy substituted phenyl; hydroxy, halogen or nitro substituted phenyl. Preferably, examples for alkyl substituted phenyl are ethylbenzene, toluene, xylene and its isomers, mesitylene or isopropylbenzene. Halogen substituted phenyl is for example
chlorobenzene, bromobenzene, chlorotoluene or bromotoluene.
In one further embodiment of the invention, the content of the additive is 0.2-10wt%, preferably 0.2-7wt%, more preferably 0.2-5wt% based on the total weight of the electrolyte.
In one embodiment of the invention, the additive (C) is formula (I), preferably N-methylpyrrole.
In one embodiment of the invention, the additive (C) is formula (I I), wherein each Ari to Ar3 is independently C6-C10 aryl, preferably phenyl. Preferably, the compound of formulae I I is N-(Triphenylphosphoranylidene)aniline.
In one further embodiment of the invention, a mixture of compounds of formulae I and formula I I can be used.
In one preferred embodiment of the invention, the content of the compound of formula I or formula I I is 0.2-10wt%, preferably 1 -10wt%, more preferably 2-7wt% based on the total weight of the electrolyte.
Preferably, the additive can further comprise vinylene carbonate (VC), 1 ,3-propane sultone (PS) or combination thereof. Preferably, the content of vinylene carbonate is 1 -5wt% based on the total weight of the electrolyte.
In one preferred embodiment of the invention, the additive consists of the compound of formulae I and vinylene carbonate, wherein the content of the compound of formulae I is 5-20wt% based on the weight of the additive.
In one embodiment of the invention, the concentration of the lithium salt in the electrolyte is 0.5-2 mol/L, preferably 0.5-1 .5 mol/L, more preferably 0.8-1 .5 mol/L.
Preferably, the lithium salt is selected from the group consisting of lithium hexafluorophosphate (LiPFe), lithium bis(oxalate)borate (LiBOB), lithium difluoro(oxalato)borate (LiODFB), lithium tetrafluoroborate (LiBF4), lithium perchlorate (LiCI04), lithium trifluoromethanesulfonate (UCF3SO3), bis(trifluoromethane)sulfonimide lithium (LiTFSI) and combination thereof.
In more preferred embodiment of the invention, the lithium salt is selected from the group consisting of lithium hexafluorophosphate, lithium bis(oxalate)borate, lithium difluoro(oxalato)borate, lithium tetrafluoroborate and combination thereof. Particularly, the
concentration of the lithium salt in the electrolyte is 0.8-1 .5 mol/L.
In even more preferred embodiment of the invention, the lithium salt is selected from the group consisting of lithium hexafluorophosphate (LiPF6), lithium tetrafluoroborate (LiBF4) and combination thereof. More preferably, the lithium salt is a mixture of lithium hexafluorophosphate (LiPFe) and lithium tetrafluoroborate (LiBF4), and the total concentration of both is 0.5-2 mol/L, preferably 0.8-1 .5 mol/L.
In one embodiment of the invention, the non-aqueous organic solvent is selected from the group consisting of ethylene carbonate (EC), propylene carbonate (PC), ethylmethyl carbonate (EMC), dimethyl carbonate (DMC), diethyl carbonate (DEC), γ-butyrolactone (GBL), methyl propyl carbonate (MPC), methyl formate (MF), ethyl formate (EF), methyl acetate (MA), ethyl acetate (EA), ethyl propionate (EP), ethyl butyrate (EB), acetonitrile (AN), Ν,Ν-dimethyllformamide (DMF) and combination thereof. The combination of two, three or more non-aqueous organic solvents above is preferred.
In one preferred embodiment of the invention, the non-aqueous organic solvent is a mixture of two or three solvents selected from the group consisting of ethylene carbonate (EC), propylene carbonate (PC), ethylmethyl carbonate (EMC), dimethyl carbonate (DMC), and diethyl carbonate (DEC). Preferably, the non-aqueous organic solvent comprises 5-20wt% ethylene carbonate, 20-50wt% ethylmethyl carbonate, and 20-60wt% dimethyl carbonate.
In one embodiment of the invention, the cathode can also further comprise L1C0O2, LiNi02, LiNii-xCOyMetz02, LiMno.5Nio.5O2, LiMno.3Coo.3Nio.3O2, LiMn2O4, LiFeO2, LiMeto.5Mni.5O4, vanadium oxide, or mixtures of any two or more thereof, wherein Met is Al, Mg, Ti, B, Ga, Si, Ni, or Co, and wherein 0<x<0.3, 0<y<0.5, and 0<z<0.5.
In one embodiment of the invention, the anode comprises graphite, carbon, Li4Ti5Oi2, tin alloys, silica alloys, intermetallic compounds, lithium metal, or mixtures of any two or more thereof.
In one preferred embodiment of the invention, the lithium-ion battery is lithium iron phosphate (LFP) battery.
The invention also provides a method for preparing such lithium-ion battery, the
method comprises the steps of:
(1 ) injecting an electrolyte into a cell; and
(2) performing initial formation charge below a voltage of 3.9V.
In one preferred embodiment of the invention, the initial formation charge is performed in a range of 2.0 - 3.8V.
All percentages are mentioned by weight unless otherwise indicated .
Examples
The present invention is illustrated by reference to the following examples, however, the examples are used for the purpose of explanation and not intended to limit the scopes of the invention.
Example 1
The electrolyte solution is prepared in BRAUN glove box with argon gas of 99.999% purity and water content of ≤ 5ppm at room temperature, wherein 12.77g ethylene carbonate, 40.85g ethylmethyl carbonate, 31 .49g dimethyl carbonate, 2g vinylene carbonate and 0.2g N-methylpyrrole are mixed evenly, and then LiPF6 is added and mixed sufficiently to obtain 1 .Omol/L of LiPF6 solution. The resulting electrolyte solution is injected into dried cell of lithium iron phosphate battery and placed for 18 to 24 hours, and then performing initial formation charge below a voltage of 3.9V in the battery cabinet.
Example 2
The procedure of example 2 is similar to example 1 , except that 12.73g ethylene carbonate, 40.73g ethylmethyl carbonate, 31 .4g dimethyl carbonate, 2g vinylene carbonate and 0.5g N-methylpyrrole are mixed evenly, and then LiPF6 is added and mixed sufficiently to obtain 1 .Omol/L of LiPF6 solution.
Example 3
The procedure of example 3 is similar to example 1 , except that 12.53g ethylene carbonate, 40.10g ethylmethyl carbonate, 30.91 g dimethyl carbonate, 2g vinylene carbonate and 2g N-methylpyrrole are mixed evenly, and then LiPF6 is added and mixed sufficiently to obtain 1 .Omol/L of LiPF6 solution.
Example 4
The procedure of example 4 is similar to example 1 , except that 12.66g ethylene carbonate, 40.52g ethylmethyl carbonate, 31 .23g dimethyl carbonate, 2g vinylene carbonate and 1 g N-methylpyrrole are mixed evenly, and then LiPF6 is added and mixed sufficiently to obtain 1 .Omol/L of LiPF6 solution.
Example 5
The procedure of example 5 is similar to example 1 , except that 12.64g ethylene carbonate, 40.44g ethylmethyl carbonate, 31 .17g dimethyl carbonate, 2g vinylene carbonate, 2g N-methylpyrrole and 1 g 1 ,3-propane sultone are mixed evenly, and then LiPF6 is added and mixed sufficiently to obtain 1 .Omol/L of LiPF6 solution.
Example 6
The procedure of example 6 is similar to example 1 , except that 12.77g ethylene carbonate, 40.85g ethylmethyl carbonate, 31 .49g dimethyl carbonate, 2g vinylene carbonate and 0.2g N-methylpyrrole are mixed evenly, and then LiPF6 is added and mixed sufficiently to obtain 1 .Omol/L of LiPF6 solution. The resulting electrolyte solution is injected into dried cell of lithium iron phosphate battery and placed for 18 to 24 hours, and then performing initial formation charge below a voltage of 3.8V in the battery cabinet.
Example 7
The procedure of example 7 is similar to example 1 , except that 12.73g ethylene carbonate, 40.73g ethylmethyl carbonate, 31 .4g dimethyl carbonate, 2g vinylene carbonate and 0.5g N-(Triphenylphosphoranylidene)aniline are mixed evenly, and then LiPF6 is added and mixed sufficiently to obtain 1 .Omol/L of LiPF6 solution. The resulting electrolyte solution is injected into dried cell of lithium iron phosphate battery and placed for 18 to 24 hours, and then performing initial formation charge below a voltage of 3.9V in the battery cabinet.
Example 8
The procedure of example 8 is similar to example 7, except that 12.66g ethylene carbonate, 40.52g ethylmethyl carbonate, 31 .23g dimethyl carbonate, 2g vinylene carbonate and 1 g N-(Triphenylphosphoranylidene)aniline are mixed evenly, and then LiPF6 is added and mixed sufficiently to obtain 1 .Omol/L of LiPF6 solution.
Comparison example 1
The electrolyte solution is prepared in BRAUN glove box with argon gas of 99.999% purity and water content of ≤ 5ppm at room temperature, wherein 12.79g ethylene carbonate, 40.94g ethylmethyl carbonate, 31 .56g dimethyl carbonate and 2g vinylene carbonate are mixed evenly, and then LiPF6 is added and mixed sufficiently to obtain 1 .0mol/L of LiPF6 solution. The resulting electrolyte solution is injected into dried cell of lithium iron phosphate battery and placed for 18 to 24 hours, and then performing initial formation charge below a voltage of 3.9V in the battery cabinet.
Comparison example 2
The procedure of comparison example 2 is similar to comparison example 1 , except that 12.66g ethylene carbonate, 40.52g ethylmethyl carbonate, 31 .23g dimethyl carbonate, 2g vinylene carbonate, and 1 g 1 ,3-propane sultone are mixed evenly, and then LiPF6 is added and mixed sufficiently to obtain 1 .Omol/L of LiPF6 solution.
Comparison example 3
The procedure of comparison example 3 is similar to comparison example 1 , except that 12.77g ethylene carbonate, 40.85g ethylmethyl carbonate, 31 .49g dimethyl carbonate, 2g vinylene carbonate, and 0.2g N-methylpyrrole are mixed evenly, and then LiPF6 is added and mixed sufficiently to obtain 1 . Omol/L of LiPF6 solution. The resulting electrolyte solution is injected into dried cell of lithium iron phosphate battery and placed for 18 to 24 hours, and then performing initial formation charge below a voltage of 3.6V in the battery cabinet.
test and results
The dry cell comprises LiFeP04 as cathode and artificial graphite (AG) as anode, purchased from WANXIANG EV CO., LTD and the design capacity of the lithium-ion battery is 4500mAh. Dry cell is placed in the oven of 80-85°C for 48 hours and then transferred to glove box for use.
The electrolyte solutions prepared according to examples and comparison examples are injected into dried cell, then remained for 24 hours, and are subjected to initial charging, vacuum sealing and formation to obtain the lithium ion batteries.
Performance test
The lithium ion batteries are measured at 60°C / 1 C cycle with cut-off voltage range of 2.0V-3.65V by using capacity test cabinet for lithium ion batteries (NEWARE CT-3008W-5V-6A). The results are shown in figures 1 and 2.
Figure 1 shows the comparison of cycling performances of LFP batteries at 60°C, and it indicates that the cycle capacity retention of the present LFP batteries is more than 80% after 1 ,000 cycles, while the cycle capacity retention of comparison example is less than 70% after 1 ,000 cycles.
Figures 2 and 3 show that the cycle capacity retention according to the present invention is significantly greater than that of the lithium ion battery prepared according to the prior art.
It will be apparent to those skilled in the art that various modifications and variations can be made in the present invention without departing from the scope or spirit of the invention. Thus, it is intended that the present invention cover such modifications and variations as come within the scope of the appended claims and their equivalents.
Claims
1 . A lithium-ion battery comprising:
(1 ) a cathode comprising LiFeP04;
(2) an anode; and
(3) an electrolyte comprising:
(A) a lithium salt;
(B) a non-aqueous organic solvent; and
(C) an additive comprising a compound of formula I or formula II of:
(I) (II)
wherein each R1 to R5 is independently H or C1 -C10 alkyi, each An to Ar3 is independently aryl.
2. The lithium-ion battery according to claim 1 , wherein the electrolyte further comprises additives of vinylene carbonate (VC), 1 ,3-propane sultone (PS) or a combination thereof.
3. The lithium-ion battery according to any one of claims 1 to 2, wherein the additive (C) is formula (I).
4. The lithium-ion battery according to any one of claims 1 to 3, wherein each R1 to R5 is independently H or C1 -C8 alkyi, preferably C1 -C4 alkyi, more preferably methyl, ethyl, propyl, butyl.
5. The lithium-ion battery according to claim 4, wherein the compound of formula (I) is N-methylpyrrole.
6. The lithium-ion battery according to any one of claims 1 to 2, wherein the additive (C) is formula (II), wherein each A to Ar3 is independently C6-C10 aryl, preferably phenyl.
7. The lithium-ion battery according to claim 6, wherein the compound of formulae I I is
N-(Triphenylphosphoranylidene)aniline.
8. The lithium-ion battery according to any one of claims 1 to 7, wherein the content of the additive (C) is 0.2-10wt% based on the total weight of the electrolyte, preferably 0.2-7wt%, more preferably 0.2-5wt%.
9. The lithium-ion battery according to any one of claims 1 to 8, wherein the lithium salt is selected from the group consisting of lithium hexafluorophosphate, lithium bis(oxalate)borate, lithium difluoro(oxalato)borate, lithium tetrafluoroborate, lithium perchlorate, lithium trifluoromethanesulfonate, bis(trifluoromethane)sulfonimide lithium and combination thereof.
10. The lithium-ion battery according to any one of claims 1 to 9, wherein the concentration of the lithium salt in the electrolyte is 0.5-2 mol/L, preferably 0.5-1 .5 mol/L
11 . The lithium-ion battery according to any one of claims 1 to 10, wherein the non-aqueous organic solvent is selected from the group consisting of dimethyl carbonate (DMC), diethyl carbonate (DEC), dipropyl carbonate (DPC), methylpropyl carbonate (MPC), ethylpropyl carbonate (EPC), ethylmethyl carbonate (EMC), ethylene carbonate (EC), propylene carbonate (PC), butylene carbonate (BC) or mixtures thereof.
12. The lithium-ion battery according to claims 1 to 11 , wherein the non-aqueous organic solvent comprises 5-20wt% ethylene carbonate, 20-50wt% ethylmethyl carbonate, and 20-60wt% dimethyl carbonate.
13. The lithium-ion battery according to any one of claims 1 to 12, wherein the anode comprises graphite, carbon, Li4Ti50i2, tin alloys, silica alloys, intermetallic compounds, lithium metal, or mixtures of any two or more thereof.
14. A method for preparing a lithium-ion battery according to any one of claims 1 to 13, the method comprises the steps of:
(1 ) injecting an electrolyte into a cell; and
(2) performing initial formation charge below a voltage of 3.9V.
15. The method according to claim 14, wherein the initial formation charge is performed in a range of 2.0 - 3.8V.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/301,156 US20170025707A1 (en) | 2014-04-04 | 2014-04-04 | Lithium-ion Batteries and Preparation Method Thereof |
| PCT/CN2014/074786 WO2015149345A1 (en) | 2014-04-04 | 2014-04-04 | Lithium-ion batteries and preparation method thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/CN2014/074786 WO2015149345A1 (en) | 2014-04-04 | 2014-04-04 | Lithium-ion batteries and preparation method thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015149345A1 true WO2015149345A1 (en) | 2015-10-08 |
Family
ID=54239318
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CN2014/074786 WO2015149345A1 (en) | 2014-04-04 | 2014-04-04 | Lithium-ion batteries and preparation method thereof |
Country Status (2)
| Country | Link |
|---|---|
| US (1) | US20170025707A1 (en) |
| WO (1) | WO2015149345A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN110718716A (en) * | 2019-10-25 | 2020-01-21 | 河南省法恩莱特新能源科技有限公司 | Silicon-based negative electrode lithium ion battery electrolyte and preparation method thereof |
| US10991972B2 (en) | 2016-01-29 | 2021-04-27 | Byd Company Limited | Electrolyte solution, positive electrode, and lithium-ion battery comprising the electrolyte solution and/or the positive electrode |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050100786A1 (en) * | 2003-09-19 | 2005-05-12 | Ryu Duk H. | Nonaqueous lithium secondary battery with cyclability and/or high temperature safety improved |
| CN101160684A (en) * | 2005-03-02 | 2008-04-09 | U芝加哥阿谷尼有限公司 | Novel redox shuttles for overcharge protection of lithium batteries |
| CN102569896A (en) * | 2010-12-10 | 2012-07-11 | 比亚迪股份有限公司 | Lithium ion secondary battery and preparation method thereof |
-
2014
- 2014-04-04 US US15/301,156 patent/US20170025707A1/en not_active Abandoned
- 2014-04-04 WO PCT/CN2014/074786 patent/WO2015149345A1/en active Application Filing
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050100786A1 (en) * | 2003-09-19 | 2005-05-12 | Ryu Duk H. | Nonaqueous lithium secondary battery with cyclability and/or high temperature safety improved |
| CN101160684A (en) * | 2005-03-02 | 2008-04-09 | U芝加哥阿谷尼有限公司 | Novel redox shuttles for overcharge protection of lithium batteries |
| CN102569896A (en) * | 2010-12-10 | 2012-07-11 | 比亚迪股份有限公司 | Lithium ion secondary battery and preparation method thereof |
Non-Patent Citations (1)
| Title |
|---|
| JE-NAM LEE ET AL.: "N-(triphenylphosphoranylidene) aniline as a novel electrolyte additive for high voltage LiCo02 operations in lithium ion batteries", ELECTROCHIMICA ACTA, vol. 56, no. Issue 14, pages 5195 - 5200, XP055227949, ISSN: 0013-4686 * |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10991972B2 (en) | 2016-01-29 | 2021-04-27 | Byd Company Limited | Electrolyte solution, positive electrode, and lithium-ion battery comprising the electrolyte solution and/or the positive electrode |
| CN110718716A (en) * | 2019-10-25 | 2020-01-21 | 河南省法恩莱特新能源科技有限公司 | Silicon-based negative electrode lithium ion battery electrolyte and preparation method thereof |
| CN110718716B (en) * | 2019-10-25 | 2021-03-09 | 河南省法恩莱特新能源科技有限公司 | Silicon-based negative electrode lithium ion battery electrolyte and preparation method thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| US20170025707A1 (en) | 2017-01-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101810758B1 (en) | New electrolyte composition for high-energy anodes | |
| EP3972024A1 (en) | Secondary lithium-ion battery electrolyte for reducing battery resistance and secondary lithium-ion battery thereof | |
| EP3879617A1 (en) | Electrolyte for lithium secondary battery, and lithium secondary battery comprising same | |
| US20040091772A1 (en) | Lithium-ion battery electrolytes with improved thermal stability | |
| WO2017138452A1 (en) | Electrolytic solution for nonaqueous electrolytic solution battery, and nonaqueous electrolytic solution battery using same | |
| CN111527636A (en) | Electrolyte for nonaqueous electrolyte battery and nonaqueous electrolyte battery using same | |
| KR20150022657A (en) | Electrolyte and lithium secondary battery with the same | |
| CN102569890A (en) | Lithium ion secondary battery and electrolyte thereof | |
| KR102355697B1 (en) | Electrolyte and lithium secondary battery comprising the same | |
| EP3273519B1 (en) | Non-aqueous electrolyte, and non-aqueous electrolyte secondary cell | |
| EP3618163B1 (en) | Non-aqueous electrolyte solution, and secondary battery comprising the same | |
| CN106785048B (en) | A kind of electrolyte containing pyridine ring sulfimide lithium and the battery using the electrolyte | |
| WO2021047500A1 (en) | Electrolyte, and lithium ion battery, battery module, battery pack and device comprising same | |
| CN111788732A (en) | Lithium secondary battery electrolyte and lithium secondary battery comprising same | |
| WO2013097596A1 (en) | Lithium ion secondary battery and electrolyte therefor and use of amide compound therein | |
| CN110635167B (en) | Nonaqueous electrolyte solution, battery containing same, and electric vehicle | |
| KR20130079833A (en) | Electrolyte and lithium secondary battery comprising same | |
| KR20160002315A (en) | Electrolyte and lithium secondary battery with the same | |
| KR20150019994A (en) | Electrolyte and lithium secondary battery comprising the same | |
| WO2015149345A1 (en) | Lithium-ion batteries and preparation method thereof | |
| WO2022168755A1 (en) | Nonaqueous electrolyte solution, nonaqueous electrolyte battery, and compound | |
| KR20170064191A (en) | Electrolyte and lithium secondary battery comprising the same | |
| KR20160006097A (en) | Electrolyte and lithium secondary battery with the same | |
| KR20160002313A (en) | Electrolyte and lithium secondary battery with the same | |
| KR20150032140A (en) | Electrolyte and lithium secondary battery comprising the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14887967 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15301156 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14887967 Country of ref document: EP Kind code of ref document: A1 |