WO2014201409A1 - Phosphatidylinositol 3-kinase inhibitors - Google Patents
Phosphatidylinositol 3-kinase inhibitors Download PDFInfo
- Publication number
- WO2014201409A1 WO2014201409A1 PCT/US2014/042392 US2014042392W WO2014201409A1 WO 2014201409 A1 WO2014201409 A1 WO 2014201409A1 US 2014042392 W US2014042392 W US 2014042392W WO 2014201409 A1 WO2014201409 A1 WO 2014201409A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- diamino
- oxo
- chloro
- dihydroquinazolin
- carbonitrile
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- NHZVHHAMKTUMFU-INIZCTEOSA-N Nc1nc(N)nc(N[C@@H](C2CC2)C(N(c2cccnc2)C(c2c(cc3)C#N)=O)=Nc2c3F)c1Cl Chemical compound Nc1nc(N)nc(N[C@@H](C2CC2)C(N(c2cccnc2)C(c2c(cc3)C#N)=O)=Nc2c3F)c1Cl NHZVHHAMKTUMFU-INIZCTEOSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/517—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with carbocyclic ring systems, e.g. quinazoline, perimidine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/04—Drugs for disorders of the muscular or neuromuscular system for myasthenia gravis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
Definitions
- the present disclosure relates generally to inhibitors of phosphatidylinositol 3- kinase (PI3K) activity and to novel compounds that are selective inhibitors of PI3K delta activity.
- PI3K phosphatidylinositol 3- kinase
- PI3K originally was identified as a protein associated with viral oncoproteins and growth factor receptor tyrosine kinases that phosphorylate phosphatidylinositol (PI) and its phosphorylated derivatives at the 3 '-hydroxyl of the inositol ring. See Panayotou et al , Trends Cell Biol 2:358-60 (1992).
- Class I PI3Ks phosphorylate phosphatidylinositol (PI), phosphatidylinositol-4- phosphate, and phosphatidylinositol-4,5-biphosphate (PIP 2 ) to produce phosphatidylinositol- 3-phosphate (PIP), phosphatidylinositol-3,4-biphosphate, and phosphatidylinositol-3,4,5- triphosphate, respectively.
- Class II PI3Ks phosphorylate PI and phosphatidylinositol-4- phosphate
- Class III PI3Ks phosphorylate PI.
- PI 3-kinase The initial purification and molecular cloning of PI 3-kinase revealed that it was a heterodimer consisting of p85 and pi 10 subunits. See Otsu et al , Cell, 65:91-104 (1991); Hiles et al , Cell, 70:419-29 (1992). Since then, four distinct Class I PI3Ks have been identified, designated as PI3K ⁇ , ⁇ , ⁇ , and ⁇ isomers, each consisting of a distinct 110 kDa catalytic subunit and a regulatory subunit. More specifically, three of the catalytic subunits, i.e.
- pi 10a, pi 10 ⁇ , and pi 105 each interact with the same regulatory subunit, i.e. , p85, whereas pi 10 ⁇ interacts with a distinct pi 01 regulatory subunit.
- the patterns of expression of each of these PI3Ks in human cells and tissues also are distinct.
- the present application provides novel compounds that are inhibitors of PI3K isoforms, such as PI3K5.
- the application also provides compositions, including
- compositions, kits that include the compounds, and methods of using and making the compounds.
- the compounds provided herein are useful in treating diseases, disorders, or conditions that are mediated by PI3K isoforms, such as PI3K5.
- the application also provides the compounds for use in therapy.
- the application further provides compounds for use in a method of treating a disease, disorder, or condition that is mediated by PI3K isoforms.
- the application provides uses of the compounds in the manufacture of a medicament for the treatment of a disease, disorder or condition that is mediated by PI3K isoforms.
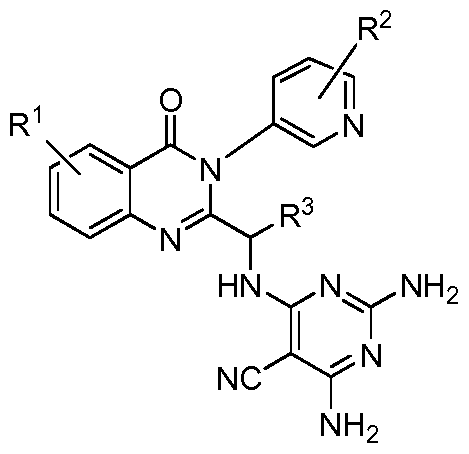
- the PI3K inhibitor is a compound having the structure of formula
- n 0, 1, 2, or 3;
- each R 2 is independently halo, optionally substituted alkoxy, optionally substituted alkyl, optionally substituted cycloalkyl, or optionally substituted heterocycloalkyl;
- R 3 is hydrogen, optionally substituted alkyl, optionally substituted cycloalkyl, optionally substituted alkoxy, or optionally substituted heterocycloalkyl;
- R 4 is cyano
- n 0, 1, 2 or 3;
- each R 1 is independently selected from halogen, cyano, alkyl, or alkylsulfonyl, wherein the alkyl moiety may be optionally substituted with 1 to 3 halogen;
- n 0, 1, 2 or 3;
- each R 2 is independently selected from halogen, alkoxy, alkyl, or cycloalkyl, wherein the alkyl moiety may be optionally substituted with 1 to 3 halogen;
- R 3 is hydrogen, alkyl, or cycloalkyl, wherein the alkyl moiety may be optionally substituted with cycloalkyl;
- R 4 is cyano
- the compounds have the structure of formula (I) wherein each R 1 is independently selected from halogen, cyano, Ci ⁇ alkyl, Ci-4 haloalkyl, or C1-4 alkylsulfonyl.
- each R 1 is independently selected from fluoro, chloro, iodo, bromo, cyano, methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, trifluoroethyl, methylsulfonyl, ethylsulfonyl, or
- each R 2 is independently selected from halogen, C1-4 alkoxy, C1-4 alkyl, Ci-4 haloalkyl, or C3-6 cycloalkyl.
- each R 2 is independently selected from fluoro, chloro, iodo, bromo, methoxy, ethoxy, propoxy, butoxy, pentoxy, hexoxy, methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl.
- the compounds have the structure of formula (I) wherein R 3 is selected from hydrogen, alkyl, cycloalkyl, or cycloalkylalkyl.
- R 3 is selected from hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C3-6 cycloalkylCi-4 alkyl.
- R 3 is selected from hydrogen, methyl, ethyl, propyl, butyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclopropylmethyl, cyclopropylethyl, cyclobutylmethyl, or cyclopropylbutyl.
- n 0, 1, 2, 3, or 4;
- each R 1 is independently halo, cyano, optionally substituted alkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, optional substituted alkoxy, or S0 2 R lx wherein R lx is optionally substituted alkyl;
- n 0, 1, 2, or 3;
- each R 2 is independently halo, -NH 2 , optionally substituted alkoxyalkyl, optionally substituted alkyl, optionally substituted cycloalkyl, or optionally substituted heterocycloalkyl;
- R 3 is hydrogen, optionally substituted alkyl, optionally substituted cycloalkyl, optionally substituted alkoxyalkyl, or optionally substituted heterocycloalkyl ;
- R 4 is selected from halo, cyano, and -CONH 2 .
- n 0, 1, 2, 3, or 4;
- each R 1 is independently selected from halo, cyano, alkyl, or alkylsulfonyl, wherein the alkyl moiety may be optionally substituted with 1 to 3 halogen;
- n 0, 1, 2, or 3;
- each R 2 is independently selected from halo, -NH 2 , alkoxy, alkyl, or cycloalkyl, wherein the alkyl moiety may be optionally substituted with 1 to 3 halogen;
- R 3 is hydrogen, alkyl, or cycloalkyl, wherein the alkyl moiety may be optionally substituted with cycloalkyl;
- R 4 is cyano, halo, or -CONH 2 .
- the compounds having the structure of formula (I) wherein R 4 is selected from the group consisting of fluoro, chloro, bromo, iodo, cyano, and -CONH 2 .
- the compounds have the structure of formula (I) wherein n is 1 or 2. In another embodiment, n is 1. In yet another embodiment, n is 2.
- the compounds have the structure of formula (I) wherein m is 0, 1 , or 2. In another embodiment, m is 0. In other embodiment, m is 1. In yet another embodiment, m is 2.
- the compounds have the structure of formula (I), wherein:
- n 1 or 2;
- each R 1 is independently selected from halogen, cyano, alkylsulfonyl, or alkyl, wherein the alkyl moiety is optionally substituted with halogen;
- n 0, 1, or 2;
- each R 2 is independently selected from halo, alkoxy, alkyl, or cycloalkyl, wherein the alkoxy, alkyl or cycloalkyl moieties are optionally substituted with halogen, alkyl, or cycloalkyl;
- R 3 is hydrogen, alkyl, or cycloalkyl, wherein the alkyl or cycloalkyl moieties are optionally substituted with halogen or cycloalkyl;
- R 4 is cyano
- the compounds have the structure of formula (I), wherein: n is 1 or 2;
- each R 1 is independently selected from halogen, cyano, Ci ⁇ alkyl, Ci-4 haloalkyl, or C 1-4 alkylsulfonyl;
- n 0, 1, or 2;
- each R 2 is independently selected from halogen, C 1-4 alkoxy, d ⁇ alkyl, Ci-4 haloalkyl, or C3-6 cycloalkyl.
- R 3 is selected from hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C3_6cycloalkylCi_4alkyl;
- R 4 is cyano
- the compounds have the structure of formula (I), wherein: n is 1 or 2; each R 1 is independently selected from fluoro, chloro, iodo, bromo, cyano, methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, trifluoroethyl, methylsulfonyl, ethylsulfonyl, or propylsulfonyl;
- n 0, 1, or 2;
- each R 2 is independently selected from fluoro, chloro, iodo, bromo, methoxy, ethoxy, propoxy, butoxy, pentoxy, hexoxy, methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl;
- R 3 is selected from hydrogen, methyl, ethyl, propyl, butyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclopropylmethyl, cyclopropylethyl, cyclobutylmethyl, or cyclopropylbutyl;
- R 4 is cyano
- the PI3K inhibitor is a compound having the structure of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, prodrug, or solvate thereof, wherein:
- n 2 and m is 2;
- each R 1 is independently selected from halogen, cyano, C 1-4 alkylsulfonyl, C 1-4 alkyl, or Ci_4 haloalkyl;
- each R 2 is independently selected from halogen, C 1-4 alkoxy, C 1-4 alkyl, Ci-4 haloalkyl, or C3-6 cycloalkyl;
- R 3 is selected from hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C 1-4 cycloalkylCi-4 alkyl;
- R 4 is cyano
- the PI3K inhibitor is a compound having the structure of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, prodrug, or solvate thereof, wherein:
- R 1 is independently selected from halogen, cyano, C 1-4 alkylsulfonyl, C 1-4 alkyl, or Ci_ 4 haloalkyl;
- each R 2 is independently selected from halogen, C 1-4 alkoxy, C 1-4 alkyl, Ci_ 4 haloalkyl, or C3-6 cycloalkyl;
- R 3 is selected from hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C 1-4 cycloalkylCi_ 4 alkyl; and R 4 is cyano.
- the PI3K inhibitor is a compound having the structure of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, prodrug, or solvate thereof, wherein:
- n 2 and m is 1 ;
- each R 1 is independently selected from halogen, cyano, C 1-4 alkylsulfonyl, C 1-4 alkyl, or Ci_ 4 haloalkyl;
- R 2 is selected from halogen, C 1-4 alkoxy, C 1-4 alkyl, C 1-4 haloalkyl, or C3-6 cycloalkyl;
- R 3 is selected from hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C 1-4 cycloalkylCi-4 alkyl;
- R 4 is cyano
- the PI3K inhibitor is a compound having the structure of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, prodrug, or solvate thereof, wherein:
- n i and m is 1 ;
- R 1 is selected from halogen, cyano, Ci_ 4 alkylsulfonyl, C 1-4 alkyl, or Ci_ 4 haloalkyl
- R 2 is selected from halogen, C 1-4 alkoxy, C 1-4 alkyl, C 1-4 haloalkyl, or C3-6 cycloalkyl
- R 3 is selected from hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C 1-4 cycloalkylCi_ 4 alkyl;
- R 4 is cyano
- the PI3K inhibitor is a compound having the structure of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, prodrug, or solvate thereof, wherein:
- R 1 is selected from halogen, cyano, C 1-4 alkylsulfonyl, C 1-4 alkyl, or C 1-4 haloalkyl
- R 3 is selected from hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C 1-4 cycloalkylCi_ 4 alkyl
- the PI3K inhibitor is a compound having the structure of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, prodrug, or solvate thereof, wherein:
- n 2 and m is 0;
- each R 1 is independently selected from halogen, cyano, C 1-4 alkylsulfonyl, C 1-4 alkyl, or Ci_4 haloalkyl;
- R 3 is selected from hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C 1-4 cycloalkylCi-4 alkyl;
- R 4 is cyano
- the compounds have the structure of formula (I), wherein:
- n 1 or 2;
- each R 1 is independently selected from halo, cyano, alkylsulfonyl, or alkyl, wherein the alkyl moiety is optionally substituted with halogen;
- n 0, 1, or 2;
- each R 2 is independently selected from halo, -NH 2 , alkoxyalkyl, alkyl, or cycloalkyl, wherein the alkyl or cycloalkyl moieties are optionally substituted with halogen, alkyl, or cycloalkyl;
- R 3 is hydrogen, alkyl, or cycloalkyl, wherein the alkyl or cycloalkyl moieties are optionally substituted with halogen or cycloalkyl;
- R 4 is cyano, halo, or -CONH 2 .
- the compounds have the structure of formula (I), wherein: n is 1 or 2;
- each R 1 is independently selected from halo, cyano, C 1-4 alkyl, Ci-4 haloalkyl, or Ci_ 4 alkylsulfonyl;
- n 0, 1, or 2;
- each R 2 is independently selected from halo, -NH 2 , C ⁇ alkyl, d ⁇ haloalkyl, or C3-6 cycloalkyl.
- R 3 is selected from hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C3_6cycloalkylCi_4alkyl;
- R 4 is cyano, halo, or -CONH 2 .
- the compounds have the structure of formula (I), wherein: n is 1 or 2;
- each R 1 is independently selected from fluoro, chloro, iodo, bromo, cyano, methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, trifluoroethyl, methylsulfonyl, ethylsulfonyl, or propylsulfonyl;
- n 0, 1, or 2;
- each R 2 is independently selected from fluoro, chloro, iodo, -NH 2 , bromo, methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl;
- R 3 is selected from hydrogen, methyl, ethyl, propyl, butyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclopropylmethyl, cyclopropylethyl, cyclobutylmethyl, or cyclopropylbutyl;
- R 4 is cyano, halo, or -CONH 2 .
- the PI3K inhibitor is a compound having the structure of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, prodrug, or solvate thereof, wherein:
- n 2 and m is 2;
- each R 1 is independently selected from halo, cyano, C 1-4 alkylsulfonyl, C 1-4 alkyl, or Ci_ 4 haloalkyl;
- each R 2 is independently selected from halo, -NH 2 , C 1-4 alkyl, Ci ⁇ haloalkyl, or C3-6 cycloalkyl;
- R 3 is selected from hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C 1-4 cycloalkylCi-4 alkyl;
- R 4 is cyano, halo, or -CONH 2 .
- the PI3K inhibitor is a compound having the structure of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, prodrug, or solvate thereof, wherein:
- R 1 is independently selected from halo, cyano, Ci_ 4 alkylsulfonyl, Ci_ 4 alkyl, or Ci_ 4 haloalkyl;
- each R 2 is independently selected from halo, -NH 2 , C 1-4 alkoxy, C 1-4 alkyl, C 1-4 haloalkyl, or C3-6 cycloalkyl;
- R 3 is selected from hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C 1-4 cycloalkylCi-4 alkyl;
- R 4 is cyano, halo, or -CONH 2 .
- the PI3K inhibitor is a compound having the structure of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, prodrug, or solvate thereof, wherein:
- n 2 and m is 1 ;
- each R 1 is independently selected from halo, cyano, C 1-4 alkylsulfonyl, C 1-4 alkyl, or Ci_ 4 haloalkyl;
- R 2 is selected from halo, -NH 2 , C 1-4 alkyl, Ci ⁇ haloalkyl, or C 3-6 cycloalkyl;
- R 3 is selected from hydrogen, C 1-4 alkyl, C 3 _6 cycloalkyl, or C 1-4 cycloalkylCi-4 alkyl;
- R 4 is cyano, halo, or -CONH 2 .
- the PI3K inhibitor is a compound having the structure of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, prodrug, or solvate thereof, wherein:
- n i and m is 1 ;
- R 1 is selected from halo, cyano, C 1-4 alkylsulfonyl, C 1-4 alkyl, or C 1-4 haloalkyl;
- R 2 is selected from halo, -NH 2 , C 1-4 alkyl, C 1-4 haloalkyl, or C 3-6 cycloalkyl;
- R 3 is selected from hydrogen, C 1-4 alkyl, C 3 _6 cycloalkyl, or C 1-4 cycloalkylCi-4 alkyl;
- R 4 is cyano, halo, or -CONH 2 .
- the PI3K inhibitor is a compound having the structure of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, prodrug, or solvate thereof, wherein:
- R 1 is selected from halo, cyano, C 1-4 alkylsulfonyl, C 1-4 alkyl, or C 1-4 haloalkyl;
- R 3 is selected from hydrogen, C 1-4 alkyl, C 3 _6 cycloalkyl, or C 1-4 cycloalkylCi-4 alkyl;
- the PI3K inhibitor is a compound having the structure of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, prodrug, or solvate thereof, wherein:
- n 2 and m is 0;
- each R 1 is independently selected from halo, cyano, C 1-4 alkylsulfonyl, C 1-4 alkyl, or Ci_ 4 haloalkyl;
- R 3 is selected from hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C 1-4 cycloalkylCi-4 alkyl;
- R 4 is cyano, halo, or -CONH 2 .
- the PI3K inhibitor is a compound having the structure of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, prodrug, or solvate thereof, wherein:
- n 1 ;
- m 1 ;
- R 1 is selected from halo, cyano, C 1-4 alkylsulfonyl, C 1-4 alkyl, or C 1-4 haloalkyl;
- R 2 is -NH 2 ;
- R 3 is selected from hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C 1-4 cycloalkylCi-4 alkyl;
- R 4 is cyano, halo, or -CONH 2 .
- the PI3K inhibitor is a compound having the structure of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, prodrug, or solvate thereof, wherein:
- n 2;
- m 1 ;
- each R 1 is independently selected from fluoro, chloro, iodo, bromo, cyano, methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, trifluoroethyl, methylsulfonyl, ethylsulfonyl, or propylsulfonyl;
- each R 2 is -NH 2 ;
- R 3 is selected from hydrogen, methyl, ethyl, propyl, butyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclopropylmethyl, cyclopropylethyl, cyclobutylmethyl, or cyclopropylbutyl;
- R 4 is cyano, halo, or -CONH 2 .
- the PI3K inhibitors are the compounds selected from Table 1, or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, or prodrug thereof.
- the compounds are the atropisomers.
- the compounds are the (S)-enantiomer.
- the compounds are the (R)-enantiomer.
- the compounds are atropisomers.
- the application also provides a pharmaceutical composition that includes a compound of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, or prodrug thereof, together with at least one pharmaceutically acceptable vehicle.
- pharmaceutically acceptable vehicle may be selected from carriers, adjuvants, and excipients.
- Also provided herein is a method of treating a disease or condition in a human in need thereof by administering to the human a therapeutically effective amount of a compound of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, or prodrug thereof.
- a compound of formula (I) for use in a method of treating a disease, disorder or condition that is mediated by PI3K isoforms is also provides the use of a compounds of formula (I) in the manufacture of a medicament for the treatment of a disease, disorder or condition that is mediated by PI3K isoforms.
- the disease or condition is associated or mediated by PI3K.
- the disease or condition is an inflammatory disorder, an autoimmune disease, or a cancer. In certain other embodiments, the disease or condition is an inflammatory disorder. In other embodiments, the disease or condition is an autoimmune disease. In additional embodiments, the disease or condition is a cancer.
- Also provided herein is a method of inhibiting kinase activity of a
- phosphatidylinositol 3-kinase delta polypeptide by contacting the polypeptide with a compound of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, or prodrug thereof.
- a method of inhibiting kinase activity of a phosphatidylinositol 3-kinase beta polypeptide by contacting the polypeptide with a compound of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, or prodrug thereof.
- a method of inhibiting excessive or destructive immune reactions comprising administering an effective amount of a compound of formula (I) described herein or a pharmaceutically acceptable salt, isomer, a mixture of isomers, or prodrug thereof such as asthma, rheumatoid arthritis, multiple sclerosis, and lupus.
- a method of inhibiting excess or destructive immune reactions comprising administering an effective amount of a compound of formula (I) described herein or a pharmaceutically acceptable salt, isomer, a mixture of isomers, or prodrug thereof such as psoriasis, or chronic obstructive pulmonary disease (COPD).
- a compound of formula (I) described herein or a pharmaceutically acceptable salt, isomer, a mixture of isomers, or prodrug thereof such as psoriasis, or chronic obstructive pulmonary disease (COPD).
- COPD chronic obstructive pulmonary disease
- Also provided is a method of disrupting leukocyte function comprising contacting the leukocytes with an effective amount of a compound of formula (I) described herein or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, or prodrug thereof.
- a method of inhibiting a growth or a proliferation of cancer cells comprising contacting the cancer cells with an effective amount of a compound of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, or prodrug thereof.
- the cancer cells are of hematopoietic origin.
- the cancer is lymphoma, leukemia, or solid tumor.
- kits that includes a compound of formula (I) or a
- kits may further comprise a label and/or instructions for use of the compound in the treatment of a disease or condition in a human in need thereof.
- the disease or condition may be associated or mediated by PI3K5 activity.
- the disease or condition may be associated or mediated by PI3K5 and/or ⁇ 3 ⁇ activity.
- articles of manufacture that include a compound of formula (I) or a pharmaceutically acceptable salt, tautomer, isomer, a mixture of isomers, or prodrug thereof, and a container.
- the container may be a vial, jar, ampoule, preloaded syringe, or an intravenous bag.
- a dash (“-") that is not between two letters or symbols is used to indicate a point of attachment for a substituent.
- -CONH2 is attached through the carbon atom.
- Reference to "about” a value or parameter herein includes (and describes) embodiments that are directed to that value or parameter per se.
- description referring to "about X” includes description of "X”.
- the term “about” includes the indicated amount + 10%.
- the term “about” includes the indicated amount ⁇ 5%.
- alkyl refers to a monoradicai unbranched or branched saturated hydrocarbon chain. As used herein, alkyl has i to 20 carbon atoms (i.e. , Ci- ? .o alkyl), i to 8 carbon atoms (? ' . ⁇ ;. , Ci-8 alkyl), 1 to 6 carbon atoms (i. e. , Ci-e alkyl), or 1 to 4 carbon atoms (i.e. , C1.4 alkyl).
- alkyl groups include methyl, ethyl, propyl, isopropyl, n-butyi, sec-butyl, iso- butyl, tert-butyl, pentyl, 2-pentyl, isopentyl, neopentyl, hexyl, 2-hexyl, 3-hexyl, and 3- methylpentyl.
- butyl can include n-butyl, sec -butyl, isobutyl and t-butyl
- propyl can include n-propyi and isopropyl
- Cycloalkyl refers to a cyclic alkyl group.
- cycloalkyl has from 3 to 20 ring carbon atoms (i.e. , C3-20 cycloalkyl), or 3 to 12 ring carbon atoms (i.e. , C3..12 cycloalkyl), 3 to 8 ring carbon atoms (i.e. , C3.8 cycloalkyl), or 3 to 6 ring carbon atoms (i.e. , ( ' ! , ⁇ , cycloalkyl).
- Examples of cycloalkyl groups include cyclopropyl, cyclobutyl, cyclopentyl, and cyclohexyl.
- Heterocycloalkyl refers to a cyclic alkyl group, with one or more ring heteroatoms independently selected from nitrogen, oxygen and sulfur.
- heterocycloalkyl has 2 to 20 ring carbon atoms (i.e. , C2-2 0 heterocycloalkyl), 2 to 12 ring carbon atoms (i.e. , C 2-12 heterocycloalkyl), or 2 to 8 ring carbon atoms (i. e. , C2-8 heterocycloalkyl); and 1 to 5 ring heteroatoms, 1 to 4 ring heteroatoms, 1 to 3 ring heteroatoms, 1 or 2 ring heteroatoms, or 1 ring heteroatom independently selected from nitrogen, sulfur or oxygen.
- heterocycloalkyl groups may include pyrrolidinyl, piperidinyl, piperazinyl, oxetanyl, dioxolanyl, azetidinyl, and morpholinyl.
- Alkoxy refers to the group “alkyl-O-”. Examples of alkoxy groups may include methoxy, ethoxy, n-propoxy, iso-propoxy, n-butoxy, tert-butoxy, sec-butoxy, n-pentoxy, n- hexoxy, and 1 ,2-dimethylbutoxy.
- Aryl refers to an aromatic carbocyclic group having a single ring (e.g. , phenyl), multiple rings (e.g. , biphenyl), or multiple fused rings (e.g. , naphthyl, fluorenyl, and anthryl).
- aryl groups may include 6 to 20 ring carbon atoms (i.e. , C & . 2 o aryl), or 6 to 12 carbon ring atoms (i.e. , Ce-iz ary )- Aryl, however, does not encompass or overlap in any way with heteroaryl, separately defined below. If one or more aryl groups are fused with a heteroaryi ring, the resulting ring system is heteroaryl.
- Heteroaryl refers to an aromatic group having a single ring, multiple rings, or multiple fused rings, with one or more ring heteroatoms independently selected from nitrogen, oxygen, and sulfur. Heteroaryl may be an aromatic, monocyclic or bicyclic ring containing one or more heteroatoms independently selected from nitrogen, oxygen and sulfur with the remaining ring atoms being carbon. As used herein, heteroaryi include 1 to 20 ring carbon atoms (i.e. , ⁇ ? ⁇ heteroaryl), 1 to 12 ring carbon atoms (i.e. , C .n heteroaryl), or 1 to 8 carbon ring atoms (i.e.
- heteroaryl groups include pyridyl, pyridazinyi, pyrimidinyl, benzothiazolyi, and pyrazoiyl. Heteroaryi does not encompass or overlap with aryl as defined above.
- substituted means that any one or more hydrogen atoms on the designated atom or group is replaced with a moiety other than hydrogen, provided that the designated atom's normal valence is not exceeded.
- Substituted alky refers to an alkyl group having one or more substituents including, for example, hydroxy], haloaikyl, alkoxy, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, cyano, halo, carboxyl, and NR 2» where each R is independently hydrogen, alkyl, haloaikyl, alkylC(O)-, aikylOC(O)-, or H 2 NC(0)-.
- a substituted alkyl may have 1 to 5 substituents, 1 to 3 substituents, 1 to 2 substituents, or 1 substituent.
- a substituted alkyl may have 1 to 4 substituents.
- a substituted alkyl is haloaikyl, cycloalkylalkyl, or heterocycloalkylalkyl.
- a substituted alkyl is Ci- haloaikyl, d-e cycloalkyl, C . heterocycloalkyl, C1- haloaikyl. CM cycloalkyl, or C 1-4 heterocycloalkyl.
- a substituted alkyl is Ci_e haloaikyl, Ci-6 cycloalkylalkyl, Ci-6 heterocycloalkylalkyl, C haloaikyl, C M.
- cycloalkylalkyl or C heterocycloalkylalkyl.
- substituted alkyl group may include - O N ' . -CHF 2 , CF 3 , -CH 2 FCH 3 , -CHF 2 CH 3 , -CH 2 CH 2 F, -CH 2 CF 3 ,
- cyclopropylmethyi cyclopropylethyl , cyclopropylpropyl, cyclobuty knetbyl, cyciobutyiethyl , or cyclobutylpropyl.
- Substituted cycloalkyl refers to a cycloalkyl group having one or more substituents including, for example, alkyl, haloaikyl, heterocycloalkyl, aryl, heteroaryl, alkoxy, cyano, halo, carboxyl, hydroxyl, and -N 2 , where each R is independently hydrogen, alkyl, haloaikyl, alkylC(O)-, alkylOC(O)-, or H 2 NC(0)-.
- a substituted cycloalkyl may have 1 to 5 substituents, 1 to 3 substituents, 1 to 2 substituents, or 1 substituent.
- a substituted cycloalkyl may have 1 to 4 substituents.
- a substituted alkyl is haloaikyl, cycloalkylalkyl, or heterocycloalkylalkyl.
- a substituted alkyl is Ci-e haloaikyl, C3-6 cycloalkyl, Ci-6 heterocycloalkyl, C haloaikyl, C3.4 cycloalkyl, or C 1-4 heterocycloalkyl .
- a substituted cycloalkyl is halocycloalkyl, or alkylcycloalkyl,.
- a substituted cycloalkyl is C 3 -j halocycloalkyl , Cs-iocycloalkyl, C2-10 heterocycloalkyl, C3-6 halocycloalkyl, C3..6 cycloalkyl, or C2-5 heterocycloalkyl .
- Substituted heterocycloalkyl refers to a heterocycloalkyl group having one or more substituents including, for example, alkyl, haloaikyl, cycloalkyl, aryl, heteroaryl, alkoxy, cyano, halo, carboxyl, hydroxy!, and -NR 2 , where each R is independently hydrogen, alkyl, haloaikyl, alkylC(O)-, alkylOC(O)-, or H 2 NC(0) ⁇ .
- a substituted heterocycloalkyl may have 1 to 5 substituents, 1 to 3 substituents, 1 to 2 substituents, or 1 substituent.
- a substituted heterocycloalkyl may have 1 to 4
- a substituted heterocycloalkyl may contain 1, 2 or 3 heteroatoms independently selected from nitrogen, oxygen, and sulfur.
- Substituted aryl refers to an aryl group having one or more substituents including, for example, halo, -OR . -NR 2 , -C(0)NR' 2 , -SO > ⁇ ' k ⁇ >. alkyl, haloalkyl, heterocycloalkyl, heteroaryl, alkoxy, amino, cyano, and carboxyl, where each R is independently hydrogen, alkyl, haloalkyl, alkylC(O)-, a!kvK K ' ; ( ⁇ ;-. or H 2 NC(0)- and each R' is independently hydrogen, alkyl, haloalkyl.
- a substituted aryl may have 1 to 5 substituents, 1 to 3 substituents, 1 to 2 substituents, or 1 substituent.
- Substituted heteroaryl refers to a heteroaryl group having one or more substituents including, for example, alkyl, haloalkyl, halo, -NR 2 , -OR, -C(0)OR,
- heterocycloalkyl, aryl, and cyano where each R is independently hydrogen, alky], haloalkyl, alkyiCYO)-, alkylOC(O)-, or H 2 NC(0)-.
- a substituted heteroaryl may have 1 to 5 substituents, 1 to 3 substituents, 1 to 2 substituents, or 1 substituent.
- a substituted heteroaryl may have 1 to 4 substituents.
- a substituted heteroaryl may contain 1 , 2 or 3 heteroatoms independently selected from nitrogen, oxygen, and sulfur.
- Substituted sulfonyl refers to the group “S0 2 R” where R refers to a substituent including, for example, alkyl, haloalkyl, cycloalkyl, heterocycloalkyl, heteroaryl, and aryl and R is further substituted with alkyl, haloalkyl, cycloalkyl, heterocycloalkyl, heteroaryl, or aryl.
- Sulfonyl refers to the group “-S0 2 R” where R refers to a substituent including, for example, alkyl, haloalkyl, cycloalkyl, heterocycloalkyl, heteroaryl, and aryl.
- the sulfonyl group is alkylsulfonyl, in which R is alkyl.
- examples of the sulfonyl groups may include S0 2 CH 3 , S0 2 CH 2 CH 3 , or S0 2 Ph.
- halogen or “halo” includes fluoro, chio.ro, bromo, and iodo, and the term “halogen” includes fluorine, chlorine, bromine, and iodine.
- Haloalkyl refers to an unbranched or branched chain alkyl group as defined above, wherein one or more hydrogen atoms are replaced by a halogen. For example, where a residue is substituted with more than one halogen, it may be referred to by using a prefix corresponding to the number of halogen moieties attached.
- dihaloaryl, dihaloalkyl, and trihaloaryl refer to aryl and alkyl substituted with two ("di") or three ("tri") halo groups, which may be, but are not necessarily, the same halogen; thus, for example, 3,5-difluorophenyl, 3-chloro-5-fluorophenyl, 4-chloro- 3-fluorophenyl, and 3,5-difluoro-4-chlorophenyl is within the scope of dihaloaryl.
- a haloalkyl group include difluoromethyl (-CHF 2 ) and trifluoromethyl (-CF 3 ).
- a divalent group such as a divalent "alkyl” group, a divalent “aryl” group, etc., may also be referred to as an "alkylene” group or an "alkylenyl” group, an "arylene” group or an
- arylenyl group, respectively. Also, unless defined otherwise, where combinations of groups are referred to herein as one moiety, e.g. arylalkyl, the last mentioned group contains the atom by which the moiety is attached to the rest of the molecule.
- PI3K inhibitors are the compound having formula (II):
- A is N or CH
- n 0, 1, 2, 3, or 4;
- R lx is independently hydrogen, optionally substituted alkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, alkylene-N(R lx ) 2 , optionally substituted aryl, arylalkyl, alkylenearyl, optionally substituted heteroaryl, optionally substituted heteroarylalkyl, or alkyleneheteroaryl, or two R lx groups are taken together to form a 5- or 6-membered ring, optionally containing at least one heteroatom,
- R ly is hydrogen, optionally substituted alkyl, optionally substituted aryl, optionally substituted heteroaryl, arylalkyl, heteroarylalkyl, alkylenearyl, and
- n 0, 1, 2, 3, or 4;
- each R 2 is independently selected from hydrogen, halo, cyano, optionally substituted alkyl, optionally substituted haloalkyl, optionally substituted alkoxy, optionally substituted cycloalkyl, or NR 2x R 2y , wherein each R 2x and R 2y is independently hydrogen, C(0)R 2s or C(0)OR 2s , wherein R 2s is optionally substituted alkyl;
- R 3 is hydrogen, optionally substituted cycloalkyl, or optionally substituted alkyl; and R 4 is hydrogen, cyano, CON(R 4a ) 2 , S0 2 -alkyl, halo, or haloalkyl, where each R 4a is independently hydrogen or optionally substituted alkyl.
- the application provides the compounds having the structure of formula (I) that function as inhibitors of PI3K isoforms, such as PI3K5.
- the structure of formula (I) is shown below:
- n 0, 1, 2, 3, or 4;
- each R 1 is independently halo, cyano, optionally substituted alkylsulfonyl, optionally substituted alkyl, optionally substituted cycloalkyl, optionally substituted heterocycloalkyl, or optionally substituted alkoxy;
- n 0, 1, 2, 3, or 4;
- each R 2 is independently halo, optionally substituted alkoxy, optionally substituted alkyl, optionally substituted cycloalkyl, or optionally substituted heterocycloalkyl;
- R 3 is hydrogen, optionally substituted alkyl, optionally substituted cycloalkyl, optionally substituted alkoxy, or optionally substituted heterocycloalkyl;
- R 4 is cyano
- n 1, 2, or 3;
- each R 1 is independently halo, cyano, Ci_ 6 alkylsulfonyl, or Ci_ 6 alkyl, wherein the alkyl moiety is optionally substituted with halogen or cycloalkyl;
- n 0, 1, 2, or 3;
- each R 2 is independently halo, Ci_ 6 alkoxy, Ci_ 6 alkyl, or C3_ 6 cycloalkyl, wherein the alkoxy, alkyl, or cycloalkyl moieties are optionally substituted with halogen, alkyl, or cycloalkyl;
- R 3 is hydrogen, Ci_6 alkyl, or C3-6 cycloalkyl, wherein the alkyl or cycloalkyl moieties are optionally substituted with halogen, alkyl, or cycloalkyl;
- R 4 is cyano
- n 1 or 2;
- each R 1 is independently selected from halogen, cyano, C 1-4 alkyl, Ci-4 haloalkyl, C 1-4 alkylsulfonyl, or C3_6 cycloalkylCi_4alkyl;
- n 0, 1, or 2;
- each R 2 is independently selected from halogen, C1-4 alkoxy, C 1-4 alkyl, C 1-4 haloalkyl, C3-6 cycloalkyl, or C3-6 cycloalkylC ⁇ alkyl;
- R 3 is selected from hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C3_6 cycloalkylCi_4alkyl;
- R 4 is cyano
- n 1 or 2;
- each R 1 is independently selected from fluoro, chloro, iodo, bromo, cyano, methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, trifluoroethyl, methylsulfonyl, ethylsulfonyl, or propylsulfonyl;
- n 0, 1, or 2;
- each R 2 is independently selected from fluoro, chloro, iodo, bromo, methoxy, ethoxy, propoxy, butoxy, pentoxy, hexoxy, methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl;
- R 3 is selected from hydrogen, methyl, ethyl, propyl, butyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclopropylmethyl, cyclopropylethyl, cyclobutylmethyl, or cyclopropylbutyl;
- R 4 is cyano
- n 0, 1, 2, 3, or 4;
- each R 2 is independently halo, -NH 2 , optionally substituted alkoxyalkyl, optionally substituted alkyl, optionally substituted cycloalkyl, or optionally substituted heterocycloalkyl;
- R 3 is hydrogen, optionally substituted alkyl, optionally substituted cycloalkyl, optionally substituted alkoxyalkyl, or optionally substituted heterocycloalkyl;
- R 4 is cyano, halo or -CONH 2 .
- n 1, 2, or 3;
- each R 1 is independently halo, cyano, Ci_ 6 alkylsulfonyl, or Ci_ 6 alkyl, wherein the alkyl moiety is optionally substituted with halogen or cycloalkyl;
- n 0, 1, 2, or 3;
- each R 2 is independently halo, -NH 2 , Ci_ 6 alkoxy, Ci_ 6 alkyl, or C3_ 6 cycloalkyl, wherein the alkoxy, alkyl, or cycloalkyl moieties are optionally substituted with halogen, alkyl, or cycloalkyl;
- R 3 is hydrogen, Ci_6 alkyl, or C3-6 cycloalkyl, wherein the alkyl or cycloalkyl moieties are optionally substituted with halogen, alkyl, or cycloalkyl;
- R 4 is cyano, halo or -CONH 2 .
- n 1 or 2;
- each R 1 is independently selected from halo, cyano, C1-4 alkyl, Ci ⁇ haloalkyl, C1-4 alkylsulfonyl, or C3_6 cycloalkylCi_4alkyl;
- n 0, 1, or 2;
- each R 2 is independently selected from halo, -NH 2 , C1-4 alkoxy, C1-4 alkyl, C1-4 haloalkyl, C3-6 cycloalkyl, or C3_6 cycloalkylCi_4alkyl;
- R 3 is selected from hydrogen, C1-4 alkyl, C3-6 cycloalkyl, or C3_6 cycloalkylCi_4alkyl;
- R 4 is cyano, halo or -CONH 2 .
- n 1 or 2;
- each R 1 is independently selected from fluoro, chloro, iodo, bromo, cyano, methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, trifluoroethyl, methylsulfonyl, ethylsulfonyl, or propylsulfonyl;
- n 0, 1, or 2;
- each R 2 is independently selected from fluoro, chloro, iodo, bromo, -NH 2 , methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl;
- R 3 is selected from hydrogen, methyl, ethyl, propyl, butyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclopropylmethyl, cyclopropylethyl, cyclobutylmethyl, or cyclopropylbutyl;
- R 4 is cyano, halo or -CONH 2 .
- n 1 or 2;
- each R 1 is independently selected from fluoro, chloro, iodo, bromo, cyano, methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, trifluoroethyl, methylsulfonyl, ethylsulfonyl, or propylsulfonyl;
- n 0, 1, or 2;
- each R 2 is independently selected from fluoro, chloro, iodo, bromo, -NH 2 , methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl;
- R 3 is selected from hydrogen, methyl, ethyl, propyl, butyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclopropylmethyl, cyclopropylethyl, cyclobutylmethyl, or cyclopropylbutyl;
- R 4 is cyano
- n is 1 or 2; each R 1 is independently selected from fluoro, chloro, iodo, bromo, cyano, methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, trifluoroethyl, methylsulfonyl, ethylsulfonyl, or propylsulfonyl;
- n 0, 1, or 2;
- each R 2 is independently selected from fluoro, chloro, iodo, bromo, -NH 2 , methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl;
- R 3 is selected from hydrogen, methyl, ethyl, propyl, butyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclopropylmethyl, cyclopropylethyl, cyclobutylmethyl, or cyclopropylbutyl;
- R 4 is fluoro, chloro, or bromo.
- n 1 or 2;
- each R 1 is independently selected from fluoro, chloro, iodo, bromo, cyano, methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, trifluoroethyl, methylsulfonyl, ethylsulfonyl, or propylsulfonyl;
- n 0, 1, or 2;
- each R 2 is independently selected from fluoro, chloro, iodo, bromo, -NH 2 , methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl;
- R 3 is selected from hydrogen, methyl, ethyl, propyl, butyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclopropylmethyl, cyclopropylethyl, cyclobutylmethyl, or cyclopropylbutyl;
- R 4 is -CONH 2 .
- n is 0. In some embodiments, n is 1, 2, 3, or 4. In other embodiments, n is 1, 2 or 3. In certain embodiments, n is 1 or 2. In one
- n is 1.
- the R 1 moiety may be located on any position of the quinazolinone ring, as depicted below.
- n is 2. In embodiments where n is 2, both R may be the same or different. Two R 1 moieties may be located on any two positions of the
- R 1 moieties may be in para-, meta- or ortho-positio
- n is 3.
- all R 1 may be the same or different, or two R 1 may be the same and different from the third R 1 .
- Three R 1 moieties may be located on any three positions of the quinazolinone ring as depicted below.
- the first R 1 may be ortho to the second R 1
- the first R 1 may be para to the third R 1 .
- n is 4. In embodiments where n is 4, all R 1 may be the same or different, three R 1 may be the same and different from the fourth R 1 , two R 1 may be the same and different from the third and the fourth R 1 .
- each R 1 is independently halo, cyano, optionally substituted alkyl, optionally substituted haloalkyl, optionally substituted alkoxy, optionally substituted cycloalkyl, or optionally substituted alkylsulfonyl. In certain embodiments, each R 1 is independently halo, cyano, optionally substituted alkyl, or optionally substituted alkylsulfonyl.
- each R 1 is independently halo, cyano, optionally substituted C 1-4 alkyl, optionally substituted C 1-4 haloalkyl, optionally substituted Ci-4 alkoxy, hydroxy, optionally substituted C3-6 cycloalkyl, or optionally substituted Ci_6 alkylsulfonyl.
- each R 1 is independently halo, cyano, optionally substituted C 1-4 alkyl, optionally substituted C 3 -6 alkoxy, optionally substituted C3-6 cycloalkyl, or optionally substituted C 1-4 alkylsulfonyl.
- each R 1 is independently halo, cyano, C 1-4 haloalkyl, C 1-4 alkyl, or C 1-4 alkylsulfonyl.
- each R 1 is independently selected from fluoro, chloro, iodo, bromo, cyano, methyl, ethyl, propyl, butyl, fluoromethyl, fluoroethyl, difluoromethyl, difluoroethyl, trifluoromethyl, trifluoroethyl, methylsulfonyl, ethylsulfonyl, or propylsulfonyl.
- each R 1 is independently fluoro, chloro, iodo, cyano, methyl, difluoromethyl (-CHF 2 ), trifluoromethyl (-CF 3 ), ethyl, methoxy,
- each R 1 is independently fluoro, chloro, cyano, methylsulfonyl, methyl, or trifluoromethyl.
- R 1 is halo, cyano, optionally substituted alkyl, optionally substituted haloalkyl, optionally substituted alkoxy, hydroxy, optionally substituted alkylsulfonyl, or optionally substituted cycloalkyl.
- R 1 is independently halo, cyano, C 1-4 haloalkyl, Ci-4 alkyl, or C 1-4 alkylsulfonyl.
- R 1 is fluoro, chloro, iodo, bromo, cyano, methyl, ethyl, propyl, butyl, -CHF2, -CF 3 , fluoroethyl, fluoropropyl, methylsulfonyl, or ethylsulfonyl.
- R 1 is fluoro, chloro, cyano, methyl, trifluoromethyl (-CF 3 ), or methylsulfonyl (-SO2CH 3 ).
- R 1 is fluoro, chloro, bromo, cyano, methyl, trifluoromethyl (-CF 3 ), or methylsulfonyl (-SO2CH 3 ).
- the R 1 moiety may be located on any position of the quinazolinone ring.
- both R 1 are independently halo, which may be the same (e.g. , both R 1 are fluoro, chloro, or iodo) or different (e.g. , one R 1 is fluoro and the other R 1 is chloro).
- n 2
- one R 1 is halo and the other R 1 is optionally substituted alkyl.
- one R 1 is halo and the other R is optionally substituted cycloalkyl.
- each R 1 is independently selected from fluoro, chloro, bromo, cyano, methyl, trifluoromethyl (-CF 3 ), or methylsulfonyl (-SO 2 CH 3 ).
- one R 1 is bromo and the other R 1 is fluoro.
- both R 1 are chloro, or both R 1 are fluoro.
- one R 1 is chloro and the other R 1 is fluoro; one R 1 is chloro and the other R 1 is methyl; one R 1 is fluoro and the other R 1 is methyl; one R 1 is fluoro and the other R 1 is cyano; one R 1 is chloro and the other R 1 is cyano.
- n 2; one R 1 is bromo and the other R 1 is fluoro.
- the two R 1 moieties may be located at any two positions of the quinazolinone ring as depicted below.
- n and R 1 may be combined with each and every variation of m, R 2 and R 3 as described for formula (I), as if each and every combination is individually described.
- m is 0. In certain embodiments, m is 1 , 2, 3, or 4. In other embodiments, m is 1, 2 or 3. In yet other embodiments, m is 1 or 2. In one embodiment, m is 1.
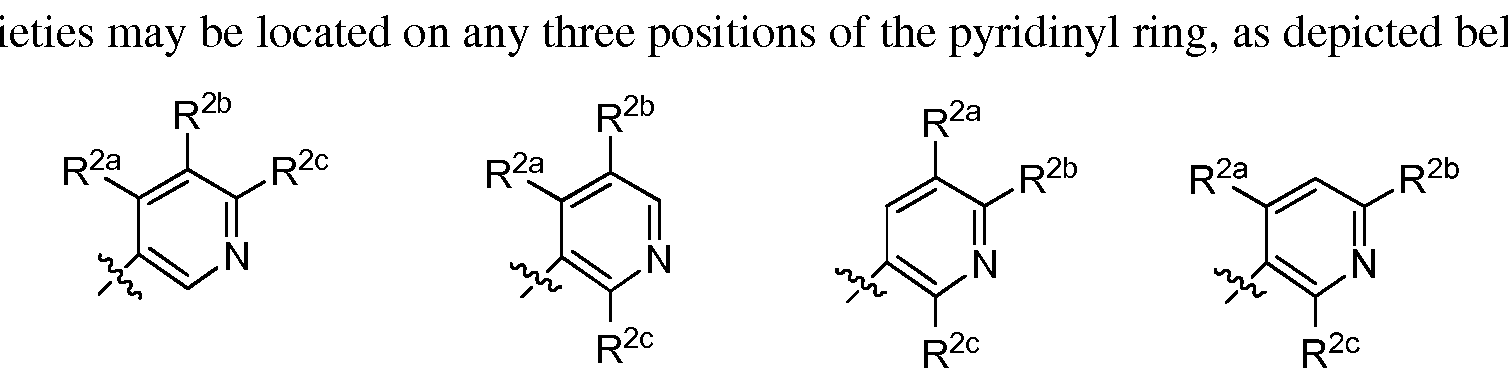
- the R 2 moiety may be located on any position of the pyridinyl ring, as depicted below.
- n is 2.
- both R 2 may be the same or different.
- the two R 2 moieties may be located on any two positions of the pyridinyl ring, as depicted below.
- m is 3. In embodiments where m is 3, all R 2 may be the same or different, or two R 2 may be the same and different from the third R 2 . The three R 2 moi low.
- m is 4. In embodiments where m is 4, all R 2 may be the same or different, three R 2 may be the same and different from the fourth R 2 , or two R 2 may be the same and different from the third and the fourth R 2 .
- each R 2 is independently halo, cyano, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted haloalkyl, optionally substituted alkoxy, optionally substituted heterocycloalkyl, or optionally substituted cycloalkyl.
- each R 2 is independently halo, cyano, optionally substituted alkyl, optionally substituted alkoxy, optionally substituted haloalkyl, or optionally substituted cycloalkyl.
- each R 2 is independently halo, cyano, optionally substituted Ci_6 alkyl, optionally substituted Ci_6 haloalkyl, optionally substituted Ci_6 alkoxy, or optionally substituted C3-8 cycloalkyl.
- each R 2 is independently halo, -NH 2 , cyano, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted haloalkyl, optionally substituted alkoxy, optionally substituted heterocycloalkyl, or optionally substituted cycloalkyl.
- each R 2 is independently halo, -NH 2 , cyano, optionally substituted Ci_6 alkyl, optionally substituted Ci_6 haloalkyl, optionally substituted Ci_6 alkoxy, or optionally substituted C3-8 cycloalkyl.
- each R 2 is independently halo, -NH 2 , cyano, optionally substituted C1-4 alkyl, optionally substituted C1-4 haloalkyl, optionally substituted C1-4 alkoxy, or optionally substituted C3-6 cycloalkyl.
- each R 2 is independently fluoro, chloro, iodo, bromo, -NH 2 , methoxy, ethoxy, propoxy, butoxy, pentoxy, hexoxy, - CHF 2 , -CF 3 , fluoroethyl, difluoroethyl, methyl, ethyl, propyl, butyl, cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl.
- each R 2 is independently fluoro, chloro, methoxy, methyl, -NH 2 , -CHF 2 , -CF 3 , or cyclopropyl.
- each R 2 is independently halo, cyano, optionally substituted Ci_ 4 alkyl, optionally substituted Ci_ 4 haloalkyl, optionally substituted Ci-4 alkoxy, or optionally substituted C3-6 cycloalkyl.
- each R 2 is independently fluoro, chloro, iodo, methoxy, ethoxy, propoxy, butoxy, pentoxy, hexoxy, - CHF 2 , -CF 3 , fluoroethyl, difluoroethyl, methyl, ethyl, propyl, butyl, cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl.
- each R 2 is independently fluoro, chloro, methoxy, methyl, -CHF 2 , -CF 3 , or cyclopropyl.
- R 2 is halo, cyano, optionally substituted haloalkyl, optionally substituted alkyl, optionally substituted cycloalkyl, or optionally substituted alkoxy.
- R 2 is fluoro, chloro, iodo, cyano, Ci_ 4 alkyl, Ci_ 4 haloalkyl, Ci_ 4 alkoxy, or C3-6 cycloalkyl.
- R 2 is fluoro, chloro, methoxy, methyl, ethoxy, propoxy, butoxy, pentoxy, hexoxy, -CHF 2 , -CF 3 , fluoroethyl, difluoroethyl, ethyl, propyl, cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl.
- R 2 is fluoro, chloro, methoxy, methyl, difluoromethyl (-CHF 2 ), trifluoromethyl (-CF 3 ), or cyclopropyl.
- R 2 may be located on any position of the pyridinyl ring.
- R 2 is fluoro, chloro, iodo, bromo, -NH 2 , cyano, Ci_ 4 alkyl, Ci_ 4 haloalkyl, Ci ⁇ alkoxy, or C3-6 cycloalkyl.
- R 2 is fluoro, chloro, methoxy, methyl, ethoxy, propoxy, butoxy, pentoxy, hexoxy, -NH 2 , -CHF 2 , -CF 3 , fluoroethyl, difluoroethyl, ethyl, propyl, cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl.
- R 2 is fluoro, chloro, -NH 2 , methoxy, methyl, difluoromethyl (-CHF 2 ), trifluoromethyl (-CF 3 ), or cyclopropyl.
- the R 2 moiety may be located on any position of the pyridinyl ring.
- both R 2 are independently halo, which may be the same (e.g. , both R 2 are fluoro or chloro) or different (e.g. , one R 2 is fluoro and the other R 2 is chloro).
- both R 2 are independently optionally substituted alkyl, which may be the same (e.g. , both R 2 are methyl) or which may be different (e.g. , one R 2 is methyl and the other R 2 is ethyl).
- both R 2 are independently optionally substituted haloalkyl, which may the same (e.g.
- both R are -CF 3 ) or which may be different (e.g. , one R 2 is -CF 3 and the other R 2 is -CHF 2 ).
- both R 2 are independently optionally substituted alkoxy, which may be the same (e.g. , both R 2 are methoxy) or which may be different (e.g. , one R 2 is methoxy and the other R 2 is ethoxy).
- both R 2 are independently optionally substituted cycloalkyl, which may the same (e.g. , both R 2 are cyclopropyl) or which may be different (e.g.
- one R 2 is methylcyclopropyl and the other R 1 is cyclopropyl).
- one R 2 is halo and the other R 2 is cyano
- one R 2 is halo and the other R 2 is optionally substituted haloalkyl
- one R 2 is halo and the other R 2 is optionally substituted alkyl
- one R 2 is halo and the other R 2 is optionally substituted alkoxy
- one R 2 is halo and the other R 2 is optionally substituted cycloalkyl
- one R 2 is optionally substituted alkyl and the other R 2 is optionally substituted cycloalkyl
- one R 2 is optionally substituted alkyl and the other R 2 is optionally substituted cycloalkyl
- one R 2 is optionally substituted alkyl and the other R 2 is optionally substituted alkoxy.
- each R 2 is independently selected from fluoro, chloro, -NH 2 , methoxy, methyl, difluoromethyl (-CHF 2 ), trifluoromethyl (-CF 3 ), or cyclopropyl.
- m is 2
- one R 2 is -NH 2 and the other R 2 is optionally substituted alkyl.
- both R 2 are fluoro, both R 2 are chloro, both R 2 are methoxy, both R 2 is methyl, or both R 2 is cyclopropyl.
- one R 2 is fluoro and the other R 2 is chloro
- one R 2 is fluoro and the other R 2 is cyano
- one R 2 is chloro and the other R 2 is cyano
- one R 2 is fluoro and the other R 2 is -CF 3
- one R 2 is fluoro and the other R 2 is -CHF 2
- one R 2 is chloro and the other R 2 is -CF 3
- one R 2 is chloro and the other R 2 is -CHF 2
- one R 2 is cyano and the other R 2 is -CF 3
- one R 2 is cyano and the other R 2 is -CHF 2i one R 2 is fluoro and the other R 2 is methyl, one R 2 is chloro
- R 2 are independently halo, which may be the same (e.g. , two R 2 are fluoro) or which may be different (e.g. , one R 2 is fluoro and another R 2 is chloro), the third R 2 is optionally substituted alkoxy (e.g. , third R 2 is methoxy).
- the third R 2 is halo.
- the three R 2 moieties may be located on any three positions of the pyridinyl ring.
- Each and every variation of m and R 2 may be combined with each and every variation of n, R 1 and R 3 as described for formula (I), as if each and every combination is individually described.
- R 3 is hydrogen, optionally substituted alkyl, optionally substituted heteroalkyl, optionally substituted heterocycloalkyl, or optionally substituted cycloalkyl.
- R 3 is hydrogen, optionally substituted Ci_6 alkyl, optionally substituted C3-8 cycloalkylCi-6 alkyl or optionally substituted C3-8 cycloalkyl.
- R 3 is hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C3-6 cycloalkylCi-4 alkyl.
- R 3 is hydrogen, methyl, ethyl, propyl, butyl, cyclopropylmethyl, cyclopropylbutyl, cyclobutylmethyl, or cyclopropylethyl, cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl.
- R 3 is methyl, ethyl, cyclopropylmethyl, or cyclopropyl.
- R 3 is methyl, ethyl, propyl, butyl, cyclopropylmethyl, or cyclopropyl.
- R 3 may be combined with each and every variation of n, R 1 , m and R 2 as described for formula (I), as if each and every combination is individually described.
- R 4 is hydrogen, cyano, -C(0)N(R 4a ) 2 , or halo, wherein each R 4a is independently hydrogen or optionally substituted Ci_6 alkyl.
- R 4 is cyano, halo, -C(0)N(R 4a ) 2 wherein each R 4a is independently hydrogen or Ci-4 alkyl.
- R 4 is cyano, fluoro, bromo, chloro, or - C(0)NH 2 .
- R 4 is cyano, chloro, or -C(0)NH 2 .
- n 1 or 2;
- each R 1 is independently halo, cyano, C3-6 alkylsulfonyl, C1-4 haloalkyl, or Ci ⁇ alkyl; m is 0, 1 or 2;
- each R 2 is independently halo, C1-4 alkoxy, C1-4 alkyl, C 1-4 haloalkyl, or C3-6 cycloalkyl;
- R 3 is hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C3-6 cycloalkylCi-4 alkyl;
- R 4 is cyano
- n 1 or 2;
- each R 1 is independently selected from fluoro, chloro, methylsulfonyl, cyano, methyl, trifluoromethyl;
- n 0, 1 or 2;
- each R 2 is independently selected from fluoro, chloro, methoxy, methyl,
- R 3 is methyl, ethyl, cyclopropyl, or cyclopropylmethyl
- R 4 is cyano
- n 1 or 2;
- each R 1 is independently halo, cyano, C3-6 alkylsulfonyl, C 1-4 haloalkyl, or C 1-4 alkyl; m is 0, 1 or 2;
- each R 2 is independently halo, -NH 2 , Ci_ 4 alkoxy, Ci_ 4 alkyl, Ci_ 4 haloalkyl, or C3_ 6 cycloalkyl;
- R 3 is hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C3-6 cycloalkylCi_ 4 alkyl;
- R 4 is cyano, halo or -CONH 2
- n 1 or 2;
- each R 1 is independently selected from fluoro, chloro, methylsulfonyl, cyano, methyl, trifluoromethyl;
- n 0, 1 or 2;
- each R 2 is independently selected from fluoro, chloro, -NH 2 , methoxy, methyl, difluoromethyl, trifluoromethyl, or cyclopropyl;
- R 3 is methyl, ethyl, cyclopropyl, or cyclopropylmethyl
- R 4 is cyano, halo or -CONH 2 .
- n 1 or 2;
- each R 1 is independently halo, cyano, C3-6 alkylsulfonyl, C 1-4 haloalkyl, or C 1-4 alkyl; m is 0, 1 or 2;
- each R 2 is independently halo, -NH 2 , Ci_ 4 alkoxy, Ci_ 4 alkyl, Ci_ 4 haloalkyl, or C 3 _ 6 cycloalkyl;
- R 3 is hydrogen, C 1-4 alkyl, C3-6 cycloalkyl, or C3-6 cycloalkylCi_ 4 alkyl;
- R 4 is halo or -CONH 2 .
- n 1 or 2;
- each R 1 is independently selected from fluoro, chloro, bromo, cyano, methyl, trifluoromethyl, and methylsulfonyl;
- n 0, 1, or 2;
- each R 2 is independently selected from fluoro, chloro, bromo, methoxy, methyl, difluoromethyl, trifluoromethyl, and cyclopropyl;
- R 3 is selected from methyl, ethyl, propyl, butyl, cyclopropyl, and cyclopropylmethyl;
- R 4 is chloro, fluoro, bromo, or -CONH 2 .
- each R la and R lb can be independently selected from the moieties defined for R 1 of formula (I);
- each R 2a and R 2b can be independently selected from the moieties defined for R 2 of formula (I);
- R 3 is as defined for formula (I). [00115] In other embodiments of formula (I) where n is 1, m is 2, and R 4 is cyano, the compounds have the structure of formula (IB):
- R 1 is as defined for formula (I);
- each R 2a and R 2b can be independently selected from the moieties defined for R 2 of formula (I);
- R 3 is as defined for formula (I).
- each R la and R lb can be independently selected from the moieties defined for R 1 of formula (I);
- R 2 is as defined for formula (I);
- R 3 is as defined for formula (I). [00117] In another embodiments where n is 1, m is 1, and R 4 is cyano, the compounds have the structure of formula (ID):
- R 1 is as defined for formula (I);
- R 2 is as defined for formula (I);
- R 3 is as defined for formula (I).
- R 1 is as defined for formula (I);
- R 3 is as defined for formula (I). [00119] In another embodiments where n is 2, m is 0, and R 4 is cyano, the compounds have the structure of formula (IF):
- each R la and R lb can be selected from the moieties defined for R 1 of formula (I); and R 3 is as defined for formula (I).
- each unique stereoisomer has an unique compound number.
- the structure below bearing one chiral center can be resolved into the (S) and (R) enantiomer.
- the compound according to any of the formulae described herein or a pharmaceutically acceptable salt thereof is the (S)- enantiomer.
- the compound according to any of the formulae described herein or a pharmaceutically acceptable salt thereof is the (R)- enantiomer.
- the application also provides a composition containing a mixture of enantiomers of the compound according to any of the formulae described herein or a pharmaceutically acceptable salt thereof.
- the composition contains the (S)-enantiomer of the compound and is substantially free of its corresponding (R)-enantiomer.
- a composition substantially free of the (R)-enantiomer has less than or about 40%, 35%, 30%, 25%, 20%, 15%, 10%, 5%, 1 %, 0.05%, or 0.01 % of the (R)-enantiomer.
- the composition containing the (S)-enantiomer of a compound according to any of formulae described herein or a pharmaceutically acceptable salt thereof predominates over its corresponding ( ?)-enantiomer by a molar ratio of at least or about 9: 1 , at least or about 19: 1 , at least or about 40: 1 , at least or about 80: 1 , at least or about 160: 1 , or at least or about 320: 1.
- composition containing a compound according to any of the formulae described herein or a pharmaceutically acceptable salt thereof may also contain the compound in enantiomeric excess (e.e.).
- a compound with 95% ( ⁇ -isomer and 5% ( ?)-isomer will have an e.e. of 90%.
- the compound has an e.e. of at least or about 60%, 75%, 80%, 85%, 90%, 95%, 98% or 99%.
- the compound is enantiomerically-enriched in the (S) -isomer of compound according to any of the formula described herein.
- composition comprising a mixture of the (S)-enantiomer and the (R)-enantiomer of a compound according to any of the formulae described herein or a pharmaceutically acceptable salt thereof.
- the mixture is a racemic mixture.
- the composition comprises the (S)-enantiomer of a compound according to any of formulae described herein or a pharmaceutically acceptable salt thereof, wherein the (S)-enantiomer of the compound is present in excess of over the corresponding the (R)-enantiomer of the compound, or a pharmaceutically acceptable salt thereof.
- the compound according to any of the formulae described herein or a pharmaceutically acceptable salt thereof is an atropisomer.
- a composition containing a mixture of atropisomers of the compound of any of the formulae described herein or a pharmaceutically acceptable salt thereof, is also provided herein.
- Atropisomers refers to conformational stereoisomers which occur when rotation about a single bond in the molecule is prevented, or greatly hindered, as a result of steric interactions with other parts of the molecule and the substituents at both ends of the single bond are asymmetrical, i.e. , they do not require a stereocenter. Where the rotational barrier about the single bond is high enough, and interconversion between conformations is slow enough, separation and isolation of the isomeric species may be permitted. Atropisomers are enantiomers without a single asymmetric atom. In some embodiments, the compounds described herein may contain a mixture of diastereomers.
- the compound according to any of the formulae described herein or a pharmaceutically acceptable salt thereof is the atropisomer.
- the atropisomer is the atropisomer.
- Table 1 Representative compounds of the present application are listed in Table 1 below.
- the compounds in Table 1 are named using ChemBioDraw Ultra 12.0 and it should be understood that other names may be used to identify compounds of the same structure.
- Other compounds or radicals may be named with common names, or systematic or non-systematic names.
- the compounds may also be named using other nomenclature systems and symbols that are commonly recognized in the art of chemistry including, for example, Chemical Abstract Service (CAS) and International Union of Pure and Applied Chemistry (IIJPAC).
- CAS Chemical Abstract Service
- IIJPAC International Union of Pure and Applied Chemistry Table 1 shows the naming and numbering of the compounds that represent the formulae described herein.
- the compounds provided in Table 1 may be a single enantiomer (e.g.
- the present application provides the compounds according to any of the formulae described herein or pharmaceutically acceptable salts, tautomers, isomers, prodrugs, or solvates thereof, in which from 1 to n hydrogen atoms attached to a carbon atom may be replaced by a deuterium atom or D, in which n is the number of hydrogen atoms in the molecule. It is known that the deuterium atom is a non-radioactive isotope of the hydrogen atom. Such compounds may increase resistance to metabolism, and thus may be useful for increasing the half-life of the compounds of any of the formulae described herein or pharmaceutically acceptable salts, isomers, prodrugs, or solvates thereof, when administered to a mammal.
- the present application also provides pharmaceutically acceptable salts, hydrates, solvates, tautomeric forms, polymorphs, and prodrugs of the compounds of any of the formulae described herein.
- “Pharmaceutically acceptable” or “physiologically acceptable” refer to compounds, salts, compositions, dosage forms and other materials which are useful in preparing a pharmaceutical composition that is suitable for veterinary or human pharmaceutical use.
- “Pharmaceutically acceptable salts” or “physiologically acceptable salts” refer to salts of pharmaceutical compounds that retain the biological effectiveness and properties of the underlying compound, and which are not biologically or otherwise undesirable.
- Pharmaceutically acceptable acid addition salts may be prepared from inorganic and organic acids. Acids and bases useful for reaction with an underlying compound to form pharmaceutically acceptable salts (acid addition or base addition salts respectively) are known to one of skill in the art.
- “Pharmaceutically acceptable salts” include, for example, salts with inorganic acids and salts with an organic acid.
- the free base can be obtained by basifying a solution of the acid salt.
- an addition salt, particularly a pharmaceutically acceptable addition salt may be produced by dissolving the free base in a suitable organic solvent and treating the solution with an acid, in accordance with conventional procedures for preparing acid addition salts from base compounds.
- salts include, but are not limited to salts with inorganic acids, such as hydrochlorate, phosphate, diphosphate, hydrobromate, sulfate, sulfinate, nitrate; as well as salts with an organic acid, such as malate, maleate, fumarate, tartrate, succinate, citrate, acetate, lactate, methanesulfonate, p-toluenesulfonate, 2-hydroxyethylsulfonate, benzoate, salicylate, stearate, and alkanoate such as acetate, HOOC-(CH 2 ) n -COOH where n is 0-4.
- inorganic acids such as hydrochlorate, phosphate, diphosphate, hydrobromate, sulfate, sulfinate, nitrate
- organic acid such as malate, maleate, fumarate, tartrate, succinate, citrate, acetate, lactate, methanesulfonate,
- pharmaceutically acceptable cations include, but are not limited to sodium, potassium, calcium, aluminum, lithium, and ammonium.
- the free base can be obtained by basifying a solution of the acid salt.
- a "solvate” is formed by the interaction of a solvent and a compound. Solvates of salts of the compounds of any of the formulae described herein are also provided. Hydrates of the compounds of any of the formulae are also provided.
- a "prodrug” includes any compound that becomes a compound of the formulae described herein when administered to a subject, e.g. , upon metabolic processing of the prodrug.
- prodrug includes any compound that becomes a compound of the formulae described herein when administered to a subject, e.g. , upon metabolic processing of the prodrug.
- the single enantiomer or diastereomer, i. e. , optically active form can be obtained by asymmetric synthesis or by resolution of the racemate. Resolution of racemates can be accomplished, for example, by conventional methods such as crystallization in the presence of a resolving agent, or chromatography, using, for example a chiral high pressure liquid chromatography (HPLC) column.
- HPLC high pressure liquid chromatography
- Z- and E- forms (or cis- and trans- forms) of the compounds of the formulae described herein or pharmaceutically acceptable salts, prodrugs, or solvates thereof with carbon-carbon double bonds Provided are also all tautomeric forms of the compounds of any of the formulae or pharmaceutically acceptable salts, isomers, prodrugs, or solvates thereof.
- provided herein are the free base forms of the compounds of the formulae described herein or pharmaceutically acceptable salts, isomers, or mixture of isomers, prodrugs or solvates thereof.
- provided herein are the (S)- enantiomers of the compounds of the formulae described herein or pharmaceutically acceptable salts, isomers, or mixture of isomers, prodrugs or solvates thereof.
- provided herein are the (R)-enantiomers of the compounds of the formulae described herein or pharmaceutically acceptable salts, isomers, or mixture of isomers, prodrugs or solvates thereof.
- provided herein are the atropisomers of the compounds of the formulae described herein or pharmaceutically acceptable salts, isomers, or mixture of isomers, prodrugs or solvates thereof.
- compositions provided herein that include a compound of the formulae described herein or a pharmaceutically acceptable salt, prodrug, or solvate thereof may include racemic mixtures, or mixtures containing an enantiomeric excess of one enantiomer or single diastereomers or diastereomeric mixtures. All such isomeric forms of these compounds are expressly included herein the same as if each and every isomeric form were specifically and individually listed.
- provided herein are also crystalline and amorphous forms of the compounds of the formulae described herein or pharmaceutically acceptable salts, isomer, a mixture of isomers, prodrugs, or solvates thereof.
- provided are also chelates, non-covalent complexes, and mixtures thereof, of the compounds of the formula described herein or pharmaceutically acceptable salts, prodrugs, or solvates thereof.
- a "chelate” is formed by the coordination of a compound to a metal ion at two (or more) points.
- a “non-covalent complex” is formed by the interaction of a compound and another molecule wherein a covalent bond is not formed between the compound and the molecule. For example, complexation can occur through van der Waals interactions, hydrogen bonding, and electrostatic interactions (also called ionic bonding).
- the compounds of the formulae described herein or a pharmaceutically acceptable salt, isomers, prodrug, or solvate thereof may be used for the treatment of diseases and/or conditions mediated by PI3K isomers, such as PI3K5.
- PI3K isomers such as PI3K5.
- methods for inhibiting one or more PI3K isomers such as PI3K ⁇ , ⁇ , ⁇ , and ⁇ .
- 'ITie PI3K isomers may be selectively or specifically inhibited.
- the compounds may be used to inhibit PI3K activity therapeutically or prophylactically.
- the compounds according to the present application may be used in combination with other therapeutic agents.
- the therapeutic agents may be in the forms of compounds, antibodies, polypeptides, or polynucleotides.
- the application provides a product comprising a compound described herein and an additional therapeutic agent as a combined preparation for simultaneous, separate or sequential use in therapy, e.g. a method of treating a disease, disorder, or condition that is mediated by PI3K isoforms.
- the therapeutic agents may be those that inhibit or modulate the activities of Bruton's tyrosine kinase, spleen tyrosine kinase, apoptosis signal-regulating kinase, Janus kinase, lysyl oxidase, lysyl oxidase-like proteins, or matrix metallopeptidase.
- the therapeutic agents may be those that inhibit or modulate the activites of bromodomain-containing protein, adenosine A2B receptor, isocitrate dehydrogenase, serine/threonine kinase TPL2, discoidin domain receptor, serine/threonine-protein kinase, IKK, MEK, EGFR, histone deacetylase, protein kinase C, or any combination thereof.
- the methods include administering a compound of the formula described herein or a pharmaceutically acceptable salt, isomers, prodrug, or solvate thereof, in a therapeutically effective amount to a human in need thereof.
- the method can be employed to treat a patient who has or is believed to have a disease or condition whose symptoms or pathology is mediated by PI3K5 expression or activity. Additionally, the method can be employed to treat a patient who has or is believed to have a disease or condition whose symptoms or pathology is mediated by ⁇ 3 ⁇ expression or activity.
- the patient may be a mammal or a human.
- certain compounds of any of the formulae described herein or a pharmaceutically acceptable salt, isomers, prodrug, or solvate thereof have one or more properties selected from: (i) selectivity to any PI3K isoforms, such as PI3K5; (ii) hepatocyte stability; and (iii) potency in a cellular assay.
- certain compounds of the formulae or a pharmaceutically acceptable salt, prodrug, isomers, or solvate thereof have selectivity to any PI3K isoforms, such as PI3K5.
- certain compounds of the formulae described herein or a pharmaceutically acceptable salt, prodrug, or solvate thereof have selectivity to at least PI3K5.
- certain compounds of the formulae or a pharmaceutically acceptable salt, prodrug, isomers, or solvate thereof have selectivity to PI3K5 and/or ⁇ 3 ⁇ .
- certain compounds of the formulae or a pharmaceutically acceptable salt, prodrug, or solvate thereof have one of the properties selected from: (i) selectivity to PI3K5; (ii) hepatocyte stability; and (iii) potency in a cellular assay.
- certain compounds of the formulas according to the present application or a pharmaceutically acceptable salt, prodrug, or solvate thereof have: selectivity to PI3K5 and hepatocyte stability; or selectivity to PI3K5 and potency in a cellular assay; or hepatocyte stability and potency in a cellular assay.
- certain compounds of the formulae or a pharmaceutically acceptable salt, prodrug, or solvate thereof have selectivity to PI3K5, hepatocyte stability, and potency in a cellular assay.
- certain compounds of the formulae described herein or a pharmaceutically acceptable salt, isomers, prodrug, or solvate thereof have hepatocyte stability.
- Hepatocyte stability of a compound can be determined using any methods currently known in the art, including the methods described in the Examples below.
- hepatocyte stability may be characterized based on half-life. In some embodiments, the half- life is greater than or about 3 hours, 4 hours, 5 hours, 6 hours, 7 hours, 8 hours, 9 hours, 10 hours, 1 1 hours, 12 hours, or 15 hours when incubated in human hepatocytes.
- certain compounds of the formulae described herein or a pharmaceutically acceptable salt, prodrug, or solvate thereof have potency in a cellular assay.
- Potency in a cellular assay can be determined using any methods currently known in the art, including the methods described in the Examples below.
- the activity in the cellular assay is less than 10 nM, 9 nM, 8 nM, 7 nM, 6 nM, 5 nM, 4 nM, 3 nM, 2 nM, InM, 0.1 nM, or 0.01 nM.
- certain compounds inhibit at least one PI3K isomer, including PI3K5.
- the compounds have an EC 50 in the described cellular assay less than 10 nM, and have a half life in hepatocytes of greater than 3 hours.
- Treatment is an approach for obtaining beneficial or desired results including clinical results.
- beneficial or desired clinical results may include one or more of the following: a) inhibiting the disease or condition (e.g. , decreasing one or more symptoms resulting from the disease or condition, and/or diminishing the extent of the disease or condition); b) slowing or arresting the development of one or more clinical symptoms associated with the disease or condition (e.g. , stabilizing the disease or condition, preventing or delaying the worsening or progression of the disease or condition, and/or preventing or delaying the spread (e.g. , metastasis) of the disease or condition); and/or
- clinical symptoms e.g. , ameliorating the disease state, providing partial or total remission of the disease or condition, enhancing effect of another medication, delaying the progression of the disease, increasing the quality of life, and/or prolonging survival.
- Prevention means any treatment of a disease or condition that causes the clinical symptoms of the disease or condition not to develop.
- Compounds may, in some embodiments, be administered to a subject (including a human) who is at risk or has a family history of the disease or condition.
- Subject refers to an animal, such as a mammal (including a human), that has been or will be the object of treatment, observation or experiment.
- the methods described herein may be useful in human therapy and/or veterinary applications.
- the subject is a mammal.
- the subject is a human.
- Human in need thereof refers to a human who may have or is suspect to have diseases or conditions that would benefit from certain treatment; for example, being treated with the PI3K inhibitor of the compounds according to the present application.
- a therapeutically effective amount of a compound of the present application or a pharmaceutically acceptable salt, isomers, prodrug, or solvate thereof means an amount sufficient to effect treatment when administered to a subject, to provide a therapeutic benefit such as amelioration of symptoms or slowing of disease progression.
- a therapeutically effective amount may be an amount sufficient to decrease a symptom of a disease or condition responsive to inhibition of PI3K5 activity.
- the therapeutically effective amount may vary depending on the subject, and disease or condition being treated, the weight and age of the subject, the severity of the disease or condition, and the manner of administering, which can readily be determined by one or ordinary skill in the art.
- inhibitortion indicates a decrease in the baseline activity of a biological activity or process.
- inhibitor of activity of PI3K isomers or variants thereof refer to a decrease in activity in any PI3K isomer (e.g.
- alpha, beta, gamma, or delta as a direct or indirect response to the presence of a compound of any of the formula (I), (IA), (IB), (IC), (ID), (IE), (IF), or (II) or a pharmaceutically acceptable salt, isomer, a mixture of isomers, prodrug, or solvent thereof, relative to the activity of PI3K isomer in the absence of such compound or a pharmaceutically acceptable salt, isomer, a mixture of isomers, prodrug, or solvent thereof.
- “Inhibition of PI3K5 activity” or variants thereof refer to a decrease in PI3K5 activity as a direct or indirect response to the presence of a compound of formula (I), (IA), (IB), (IC), (ID), (IE), (IF) or (II) or a pharmaceutically acceptable salt, isomer, a mixture of isomers, prodrug, or solvate thereof, relative to the activity of PI3K5 in the absence of such compound.
- the inhibition of PI3K5 activity may be compared in the same subject prior to treatment, or other subjects not receiving the treatment.
- the compounds inhibit PI3K5 activity also inhibit PI3K ⁇ activity.
- the decrease in PI3K5 activity may be due to the direct interaction of the compound with PI3K5, or due to the interaction of the compounds described herein with one or more other factors that in turn affect PI3K5 activity.
- the presence of the compounds of the formulae described herein or a pharmaceutically acceptable salt, prodrug, or solvate thereof may decrease PI3K5 activity by directly binding to the PI3K5, by causing (directly or indirectly) another factor to decrease PI3K5 activity, or by (directly or indirectly) decreasing the amount of PI3K5 present in the cell or organism.
- a compound of the present application refers to a compound having the structure of any of the formula (I), (IA), (IB), (IC), (ID), (IE), (IF), or (II).
- the PI3K inhibitors are the compounds having the structure of the formula (I).
- the PI3K inhibitors are the compounds having the structure of the formula (IA).
- the PI3K inhibitors are the compounds having the structure of the formula (IB).
- the PI3K inhibitors are the compounds having the structure of the formula (IC).
- the PI3K inhibitors are the compound having the structure of formula (ID).
- the PI3K inhibitors are the compounds having the structure of formula (IE).
- the PI3K inhibitors are the compound having the structure of formula (IF).
- PI3K inhibitor or variant thereof refers to a compound that inhibits the activity of PI3K.
- PI3K isoform selective inhibitor or variant thereof refers to a compound that inhibits the activity of one or more PI3K isoforms more effectively than the other remaining PI3K isoforms.
- PI3K5 selective inhibitor generally refers to a compound that inhibits the activity of the PI3K5 isoform more effectively than other isoforms of the PI3K family (e.g. , PI3K ⁇ , ⁇ , or ⁇ ).
- PI3Ka selective inhibitor generally refers to a compound that inhibits the activity of the PI3Ka isoform more effectively than other isoforms of the PI3K family (e.g. , PI3K ⁇ , ⁇ , or ⁇ ).
- ⁇ 3 ⁇ selective inhibitor generally refers to a compound that inhibits the activity of the ⁇ 3 ⁇ isoform more effectively than other isoforms of the PI3K family (e.g. , PI3K ⁇ , ⁇ , or ⁇ ).
- the term "dual ⁇ 3 ⁇ / ⁇ selective inhibitor generally refers to a compound that inhibits the activity of the PI3Ka and ⁇ 3 ⁇ isoforms more effectively than other isoforms of the PI3K family (e.g. , PI3K ⁇ or ⁇ ).
- the relative efficacies of compounds as inhibitors of an enzyme activity can be established by determining the concentrations at which each compound inhibits the activity to a predefined extent and then comparing the results.
- the efficacy of a compound as an inhibitor of one or more PI3K isoforms can be measured by the concentration that inhibits 50% of the activity in a biochemical assay, i.e. , the 50% inhibitory concentration or "IC 50 ".
- IC 50 determinations can be accomplished using conventional techniques known in the art, including the techniques describes in the Examples below. In general, an IC 50 can be determined by measuring the activity of a given enzyme in the presence of a range of concentrations of the compound under study.
- the experimentally obtained values of enzyme activity may then be plotted against the compound concentrations used.
- concentration of the inhibitor that shows 50% enzyme activity is taken as the IC 50 value.
- other inhibitory concentrations can be defined through appropriate determinations of activity. For example, in some settings it may be desirable to establish a 90% inhibitory concentration, i.e. ,
- a PI3K5 selective inhibitor is a compound that exhibits a 50% inhibitory concentration (IC 50 ) with respect to PI3K5 that is at least 10-fold, in another aspect at least 20-fold, and in another aspect at least 30-fold, lower than the IC 50 value with respect to any or all of the other Class I PI3K family members.
- a PI3K5 selective inhibitor is a compound that exhibits an IC 50 with respect to PI3K5 that is at least 50-fold, in another aspect at least 100-fold, in an additional aspect at least 200-fold, and in yet another aspect at least 500-fold, lower than the IC 50 with respect to any or all of the other PI3K Class I family members.
- a PI3K5 selective inhibitor is typically administered in an amount such that it selectively inhibits PI3K5 activity, as described above.
- a PI3Ka selective inhibitor is a compound that exhibits a 50% inhibitory concentration (IC 50 ) with respect to PI3Kot that is at least 10-fold, in another aspect at least 20-fold, and in another aspect at least 30-fold, lower than the IC 50 value with respect to any or all of the other Class I PI3K family members.
- a PI3Ka selective inhibitor is a compound that exhibits an IC 50 with respect to PI3Ka that is at least 50-fold, in another aspect at least 100-fold, in an additional aspect at least 200-fold, and in yet another aspect at least 500-fold, lower than the IC 50 with respect to any or all of the other PI3K Class I family members.
- a PI3Ka selective inhibitor is typically administered in an amount such that it selectively inhibits PI3Ka activity, as described above [0159]
- a ⁇ 3 ⁇ selective inhibitor is a compound that exhibits a 50% inhibitory concentration (IC 50 ) with respect to ⁇ 3 ⁇ that is at least 10-fold, in another aspect at least 20-fold, and in another aspect at least 30-fold, lower than the IC 50 value with respect to any or all of the other Class I PI3K family members.
- a ⁇ 3 ⁇ selective inhibitor is a compound that exhibits an IC 50 with respect to ⁇ 3 ⁇ that is at least 50-fold, in another aspect at least 100-fold, in an additional aspect at least 200-fold, and in yet another aspect at least 500-fold, lower than the IC 50 with respect to any or all of the other PI3K Class I family members.
- a ⁇ 3 ⁇ selective inhibitor is typically administered in an amount such that it selectively inhibits ⁇ 3 ⁇ activity, as described above.
- a dual ⁇ 3 ⁇ / ⁇ selective inhibitor is a compound that exhibits a 50% inhibitory concentration (IC 50 ) with respect to PI3Ka and ⁇ 3 ⁇ that is at least 10- fold, in another aspect at least 20-fold, and in another aspect at least 30-fold, lower than the IC 50 value with respect to any or all of the other Class I PI3K family members.
- IC 50 inhibitory concentration
- a dual ⁇ 3 ⁇ / ⁇ selective inhibitor is a compound that exhibits an IC 50 with respect to PI3Ka and ⁇ 3 ⁇ that is at least 50-fold, in another aspect at least 100-fold, in an additional aspect at least 200-fold, and in yet another aspect at least 500-fold, lower than the IC 50 with respect to any or all of the other PI3K Class I family members.
- a ⁇ 3 ⁇ / ⁇ selective inhibitor is a compound that exhibits an IC 50 with respect to ⁇ 3 ⁇ and PI3K5 that is at least 10-fold, at least 20-fold, at least 50-fold, at least 100-fold, at least 200-fold, at least 300-fold, at least 400-fold, and at least 500-fold, lower than the IC 50 with respect to either PI3Ka or ⁇ 3 ⁇ or to both PI3Ka and ⁇ 3 ⁇ .
- a dual ⁇ 3 ⁇ / ⁇ selective inhibitor is typically administered in an amount such that it selectively inhibits PI3Ka and ⁇ 3 ⁇ activity, as described above.
- the methods described herein may be applied to cell populations in vivo or ex vivo.
- “In vivo” means within a living individual, as within an animal or human. In this context, the methods described herein may be used therapeutically in an individual.
- "Ex vivo” means outside of a living individual. Examples of ex vivo cell populations include in vitro cell cultures and biological samples including fluid or tissue samples obtained from individuals. Such samples may be obtained by methods well known in the art. Exemplary biological fluid samples include blood, cerebrospinal fluid, urine, and saliva. Exemplary tissue samples include tumors and biopsies thereof. In this context, the invention may be used for a variety of purposes, including therapeutic and experimental purposes.
- the invention may be used ex vivo to determine the optimal schedule and/or dosing of administration of a PI3K5 selective inhibitor for a given indication, cell type, individual, and other parameters. Information gleaned from such use may be used for experimental purposes or in the clinic to set protocols for in vivo treatment. Other ex vivo uses for which the invention may be suited are described below or will become apparent to those skilled in the art.
- the selected compounds of the formula described herein or a pharmaceutically acceptable salt, prodrug, or solvate thereof may be further characterized to examine the safety or tolerance dosage in human or non-human subjects. Such properties may be examined using commonly known methods to those skilled in the art.
- PI3K5 is generally expressed in hematopoietic cells. Consequently, the direct effects of selective inhibitors of PI3K5 can be observed in hematopoietic cells.
- Hematopoietic cells typically differentiate into either lymphoid progenitor cells or myeloid progenitor cells, both of which ultimately differentiate into various mature cell types including leukocytes. Aberrant proliferation of hematopoietic cells of one type often interferes with the production or survival of other hematopoietic cell types, which can result in compromised immunity, anemia, and/or thrombocytopenia.
- the methods described herein may treat aberrant proliferation of hematopoietic cells by inhibiting aberrant proliferation of hematopoietic cells. As a result, these methods may also ameliorate the symptoms and secondary conditions that result from a primary effect such as excessive system or localized levels of leukocytes or lymphocytes.
- the compounds described herein may be used to treat subjects having various disease states, disorders, and conditions (also collectively referred to as "indications") involving aberrant proliferation of hematopoietic cells (including excessive production of lymphoid progenitor cell-derived cells and/or myeloid progenitor cell-derived cells).
- indications may include, for example, leukemias, lymphomas,
- the compounds described herein may be used to treat hematologic malignancies, inflammation, autoimmune disorders, allergic conditions, cardiovascular disease, and autoimmune diseases.
- allergic conditions may include all forms of hypersensitivity.
- the compounds described herein may be used to treat cancers that are mediated by, dependent on or associated with PI3K activity, such as PI3K5 activity.
- the disease is a hematologic malignancy.
- the hematologic malignancy is leukemia or lymphoma.
- the disease is acute lymphocytic leukemia (ALL), acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), myelodysplastic syndrome (MDS), myeloproliferative disease (MPD), chronic myeloid leukemia (CML), multiple myeloma (MM), indolent non-Hodgkin' s lymphoma (iNHL), refractory iNHL, non-Hodgkin' s lymphoma (NHL), mantle cell lymphoma (MCL), follicular lymphoma, Waldestrom' s macroglobulinemia (WM), T-cell lymphoma, B-cell lymphoma, and diffuse large B-cell lymphoma (DLBCL).
- ALL acute lymphocytic leukemia
- AML acute myeloid leukemia
- CLL chronic lymphocytic leukemia
- SLL small lympho
- the disease is T-cell acute lymphoblastic leukemia (T-ALL), or B-cell acute lymphoblastic leukemia (B-ALL).
- T-ALL T-cell acute lymphoblastic leukemia
- B-ALL B-cell acute lymphoblastic leukemia
- the non- Hodgkin lymphoma encompasses the indolent B-cell diseases that include, for example, follicular lymphoma, lymphoplasmacytic lymphoma, Waldenstrom macroglobulinemia, and marginal zone lymphoma, as well as the aggressive lymphomas that include, for example, Burkitt lymphoma, diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL).
- follicular lymphoma follicular lymphoma
- lymphoplasmacytic lymphoma Waldenstrom macroglobulinemia
- MCL mantle cell lymphoma
- the disease is a solid tumor.
- the solid tumor is from pancreatic cancer, bladder cancer, colorectal cancer, breast cancer, prostate cancer, renal cancer, hepatocellular cancer, lung cancer, ovarian cancer, cervical cancer, gastric cancer, esophageal cancer, head and neck cancer, melanoma, neuroendocrine cancers, CNS cancers, brain tumors (e.g. , glioma, anaplastic oligodendroglioma, adult glioblastoma multiforme, and adult anaplastic astrocytoma), bone cancer, or soft tissue sarcoma.
- the solid tumor is from non-small cell lung cancer, small- cell lung cancer, colon cancer, CNS cancer, melanoma, ovarian cancer, renal cancer, prostate cancer, or breast cancer.
- the disease is an autoimmune disease.
- the autoimmune disease is systemic lupus erythematosus (SLE), myestenia gravis, rheumatoid arthritis (RA), acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiple sclerosis (MS), Sjoegren' s syndrome, or autoimmune hemolytic anemia.
- the disease is inflammation.
- the disease is excessive or destructive immune reactions, such as asthma, rheumatoid arthritis, multiple sclerosis, and lupus.
- the disease is excessive or destructive immune reactions, such as psoriasis or chronic obstructive pulmonary disease (COPD).
- COPD chronic obstructive pulmonary disease
- the present application also provides a method for treating a subject, who has or is suspected of having a disease or condition responsive or believed to be responsive to the inhibition of PI3K5 activity by administering to the subject a compound of the formulae described herein or a pharmaceutically acceptable salt, prodrug, or solvate thereof.
- the application provides a method of inhibiting kinase activity of a phosphatidylinositol 3-kinase delta polypeptide by contacting the polypeptide with a compound of the formulae described herein or a pharmaceutically acceptable salt, prodrug, or solvate thereof.
- a method of disrupting leukocyte function comprising contacting the leukocytes with an effective amount of a compound of any of the formulae described herein or a pharmaceutically acceptable salt, prodrug, or solvate thereof, in a subject in need thereof (e.g. , a human).
- a method of inhibiting a growth or a proliferation of cancer cells of hematopoietic origin comprising contacting the cancer cells with an effective amount of a compound of the formulae described herein or a pharmaceutically acceptable salt, prodrug, or solvate thereof.
- kits that include a compound of the formulae of the present application or a pharmaceutically acceptable salt, prodrug, or solvate thereof, and suitable packaging.
- a kit further includes instructions for use.
- a kit includes a compound of the formulae described herein or a pharmaceutically acceptable salt, prodrug, or solvate thereof, and a label and/or instructions for use of the compounds in the treatment of the indications, including the diseases or conditions, described herein.
- articles of manufacture that include a compound of any of the formulae described herein or a pharmaceutically acceptable salt, prodrug, or solvate thereof, in a suitable container.
- the container may be a vial, jar, ampoule, preloaded syringe, and intravenous bag.
- compositions that contain one or more of the compounds of any of the formulae, including (I), (IA), (IB), (IC), (ID), (IE), (IF), or (II), or a pharmaceutically acceptable salt, isomers, prodrug, or solvate thereof, and one or more pharmaceutically acceptable vehicles selected from carriers, adjuvants and excipients.
- suitable pharmaceutically acceptable vehicles may include, for example, inert solid diluents and fillers, diluents, including sterile aqueous solution and various organic solvents, permeation enhancers, solubilizers and adjuvants.
- compositions are prepared in a manner well known in the pharmaceutical art. See, e.g. , Remington's Pharmaceutical Sciences, Mace Publishing Co., Philadelphia, Pa. 17th Ed. (1985); and Modern Pharmaceutics, Marcel Dekker, Inc. 3rd Ed. (G.S. Banker & C.T.
- the pharmaceutical compositions may be administered in either single or multiple doses.
- the pharmaceutical composition may be administered by various methods including, for example, rectal, buccal, intranasal and transdermal routes.
- the pharmaceutical composition may be administered by intra- arterial injection, intravenously, intraperitoneally, parenterally, intramuscularly, subcutaneously, orally, topically, or as an inhalant.
- Oral administration may be another route for administration of the compounds described herein. Administration may be via, for example, capsule or enteric coated tablets.
- the active ingredient is usually diluted by an excipient and/or enclosed within such a carrier that can be in the form of a capsule, sachet, paper or other container.
- the excipient serves as a diluent, it can be in the form of a solid, semi-solid, or liquid material, which acts as a vehicle, carrier or medium for the active ingredient.
- compositions can be in the form of tablets, pills, powders, lozenges, sachets, cachets, elixirs, suspensions, emulsions, solutions, syrups, aerosols (as a solid or in a liquid medium), ointments containing, for example, up to 10% by weight of the active compound, soft and hard gelatin capsules, sterile injectable solutions, and sterile packaged powders.
- excipients include lactose, dextrose, sucrose, sorbitol, mannitol, starches, gum acacia, calcium phosphate, alginates, tragacanth, gelatin, calcium silicate, microcrystalline cellulose, polyvinylpyrrolidone, cellulose, sterile water, syrup, and methyl cellulose.
- the formulations can additionally include lubricating agents such as talc, magnesium stearate, and mineral oil; wetting agents; emulsifying and suspending agents; preserving agents such as methyl and propylhydroxy-benzoates; sweetening agents; and flavoring agents.
- compositions that include at least one compound of any of the formulae described herein or a pharmaceutically acceptable salt, prodrug, or solvate thereof, can be formulated so as to provide quick, sustained or delayed release of the active ingredient after administration to the subject by employing procedures known in the art.
- Controlled release drug delivery systems for oral administration include osmotic pump systems and
- transdermal delivery devices Such transdermal patches may be used to provide continuous or discontinuous infusion of the compounds described herein in controlled amounts.
- the construction and use of transdermal patches for the delivery of pharmaceutical agents is well known in the art. See, e.g., U.S. Patent Nos. 5,023,252, 4,992,445 and 5,001,139.
- patches may be constructed for continuous, pulsatile, or on demand delivery of pharmaceutical agents.
- the principal active ingredient may be mixed with a pharmaceutical excipient to form a solid preformulation composition containing a homogeneous mixture of a compound of any of the above formulae or a pharmaceutically acceptable salt, prodrug, or solvate thereof.
- a pharmaceutical excipient When referring to these preformulation compositions as homogeneous, the active ingredient may be dispersed evenly throughout the composition so that the composition may be readily subdivided into equally effective unit dosage forms such as tablets, pills and capsules.
- the tablets or pills of the compounds described herein may be coated or otherwise compounded to provide a dosage form affording the advantage of prolonged action, or to protect from the acid conditions of the stomach.
- the tablet or pill can include an inner dosage and an outer dosage component, the latter being in the form of an envelope over the former.
- the two components can be separated by an enteric layer that serves to resist disintegration in the stomach and permit the inner component to pass intact into the duodenum or to be delayed in release.
- enteric layers or coatings such materials including a number of polymeric acids and mixtures of polymeric acids with such materials as shellac, cetyl alcohol, and cellulose acetate.
- compositions for inhalation or insufflation may include solutions and suspensions in pharmaceutically acceptable, aqueous or organic solvents, or mixtures thereof, and powders.
- the liquid or solid compositions may contain suitable pharmaceutically acceptable excipients as described supra.
- the compositions are administered by the oral or nasal respiratory route for local or systemic effect.
- compositions in pharmaceutically acceptable solvents may be nebulized by use of inert gases. Nebulized solutions may be inhaled directly from the nebulizing device or the nebulizing device may be attached to a facemask tent, or intermittent positive pressure breathing machine. Solution, suspension, or powder compositions may be administered, preferably orally or nasally, from devices that deliver the formulation in an appropriate manner.
- a dosage may be expressed as a number of milligrams of a compound of the formula per kilogram of the subject's body weight (mg/kg). Dosages of between about 0.01 and 150 mg/kg may be appropriate. Dosages of between about 0.001 and 10 mg/kg, between about 0.005 and 5mg/kg, and between about 0.01 and 50 mg/kg may be appropriate.
- a dosage of between 0.05 and 60 mg/kg may be appropriate. Normalizing according to the subject's body weight is particularly useful when adjusting dosages between subjects of widely disparate size, such as occurs when using the drug in both children and adult humans or when converting an effective dosage in a non-human subject such as dog to a dosage suitable for a human subject.
- the daily dosage may also be described as a total amount of a compound of the formulae administered per dose or per day.
- Daily dosage of a compound may be between about 1 mg and 2,000 mg, between about 1,000 to 2,000 mg/day, between about 1 to 1,000 mg/day, between about 1 to 500 mg/day, between about 5 to 400 mg/day, between about 10 to 300 mg/day, between about 25 to 250 mg/day, between about 50 to 225 mg/day, between about 75 to 200 mg/day, between about 100 to 150 mg/day, between about 1 to 100 mg/day between about between about 1 to 75 mg/day, between about 1 to 50 mg/day, between about 1 to 25 mg/day, between about 1 to 20 mg/day, between about 1 to 15 mg/day, between about 1 to 10 mg/day, between about 1 to 5 mg/day, between about 5 to 100 mg/day, between about between about 5 to 75 mg/day, between about 5 to 50 mg/day, between about 5 to 25 mg/day, between about 5 to 15 mg/day, between about 5 to
- daily dosage of a compound may be between about 0.01 mg and 1,000 mg, between about 0.05 mg and 500 mg, between about 0.075 mg and 250 mg, between about 0.1 mg and 100 mg, between about 0.5 mg and 50 mg, between about 0.75 mg and 25mg, between about 1 mg and 10 mg, [0184]
- the total daily dosage for a human subject may be between 1 mg and 1,000 mg, between about 1 to 100 mg/day, between about 1 to 50 mg/day, between about 5 to 50 mg/day, between 5 to 25 mg/day, between about 5 to 75 mg/day, between about 10-500 mg/day, between about 10 to 150 mg/day, between about 10 to 200 mg/day, between about 50-300 mg/day, between about 75-200 mg/day, between about 75 to 150 mg/day, or between about 100-150 mg/day.
- daily dosage for a human may be administered between about 0.01 mg and 1,000 mg, between about 0.05 mg and 500 mg, between about 0.075 mg and 250 mg, between about 0.1 mg and 100 mg, between about 0.5 mg and 50 mg, between about 0.75 mg and 25mg, between about 1 mg and 10 mg,
- the compounds of the present application or the compositions thereof may be administered once, twice, three, or four times daily, using any suitable mode described above. Also, administration or treatment with the compounds according to any of the formulae described herein may be continued for a number of days; for example, commonly treatment would continue for at least 7 days, 14 days, or 28 days, for one cycle of treatment. In some embodiments, the compounds or the composition thereof may be administered continuously, i.e. every day. Treatment cycles are well known in cancer chemotherapy, and are frequently alternated with resting periods of about 1 to 28 days, commonly about 7 days or about 14 days, between cycles. The treatment cycles, in other embodiments, may also be continuous.
- the method comprises administering to the subject an initial daily dose of about 1 to 500 mg of a compound of the above formula and increasing the dose by increments until clinical efficacy is achieved. Increments of about 5, 10, 25, 50, or 100 mg can be used to increase the dose.
- the methods comprising administering to the subject an initial daily dose of about 0.01 to 100 mg of the compound described herein and increasing the dose by increments of about 0.01, 0.05, 0.1, 0.5, 1, 2, 2.5, 5, 10, 25, 50, or 100 mg.
- the dosage can be increased daily, every other day, twice per week, or once per week.
- the compounds of formula (I), (IA), (IB), (IC), (ID), (IE), (IF), or (II) may be prepared using the methods disclosed herein and routine modifications thereof, which will be apparent given the disclosure herein and methods well known in the art. Conventional and well-known synthetic methods may be used in addition to the teachings herein. The synthesis of typical compounds described herein may be accomplished as described in the following examples. If available, reagents may be purchased commercially, e.g. , from Sigma Aldrich or other chemical suppliers.
- Typical embodiments of compounds described herein may be synthesized using the general reaction schemes described below. It will be apparent given the description herein that the general schemes may be altered by substitution of the starting materials with other materials having similar structures to result in products that are correspondingly different. Descriptions of syntheses follow to provide numerous examples of how the starting materials may vary to provide corresponding products. Given a desired product for which the substituent groups are defined, the necessary starting materials generally may be determined by inspection. Starting materials are typically obtained from commercial sources or synthesized using published methods. For synthesizing compounds which are embodiments described in the present disclosure, inspection of the structure of the compound to be synthesized will provide the identity of each substituent group. The identity of the final product will generally render apparent the identity of the necessary starting materials by a simple process of inspection, given the examples herein. In general, compounds described herein are typically stable and isolatable at room temperature and pressure.
- solvent refers to a solvent inert under the conditions of the reaction being described in conjunction therewith (including, for example, benzene, toluene, acetonitrile, tetrahydrofuran (“THF”), dimethylformamide (“DMF”), chloroform, methylene chloride (or dichloromethane), diethyl ether, methanol, pyridine and the like).
- solvents used in the reactions of the present invention are inert organic solvents, and the reactions are carried out under an inert gas, preferably nitrogen.
- the compound of formula (1) can be made by combining compounds (A), (B) and (C) in the presence of a dehydrating agent.
- Compounds (A), (B) and (C) are commercially available or can be made by the methods that are commonly known or used by one skilled in the art.
- R 1 , R 2 , and R 3 are defined as in the formula (I).
- Compound (A) is mixed with Compound (B) in the presence of a coupling agent such as diphenyl phosphite in a solvent such as pyridine. After stirring at a temperature between ambient and 100°C for 1 to 5 hours, compound (C) is added. The mixture is further stirred at a temperature between ambient and 100°C for 5 to 24 hours and cooled to room temperature.
- an organic solvent such as ethyl acetate (EtOAc) is added. Then the reaction is washed with mild acid, water, and brine. The organic phase is concentrated to obtain the compound of formula (1).
- the compound of formula (1) is purified by any suitable methods known in the art, such as chromatography on silica gel. Alternatively, the compound of formula (1) is used in the next step without purification. In some instance, the compound of formula (1) is purified directly without an aqueous work-up. In cases where an R 1 is a cyano, the corresponding bromide can be converted to a nitrile by methods known in the art.
- the compound of formula (2) can be made by removing the protecting group(s) from the compound of formula (1).
- the compound of formula (1) is dissolved in a suitable solvent and treated with a suitable acid.
- suitable solvents include dichloromethane or dioxane
- suitable acids include trifluoroacetic acid, hydrochloric acid, or boron tribromide (BBr 3 ).
- the reaction is carried out at temperatures between -78°C to ambient temperature. After the reaction is complete, solvent is removed to obtain the compound of formula (2).
- the compound of formula (3) can be made by treating 5-substituted-2,4,6- trihalopyrimidine with ammonium hydroxide in a suitable solvent such as dioxane, where the halo is either chloro or fluoro.
- a suitable solvent such as dioxane, where the halo is either chloro or fluoro.
- the reaction is carried out at an elevated temperature between 30 and 80°C between 2 and 8 hours or when the reaction was complete. Then, water is added to the cooled solution, and the precipitate is collected by filtration. If necessary, separation of regiosiomers is accomplished by standard methods, such as chromatography. The nitrile can be converted to the carboxamide under standard conditions.
- Step 4 Preparation of a compound of formula (I)
- the compound of formula (I) can generally be prepared by coupling compound of formula (2) and compound of formula (3) in the presence of a suitable base in a suitable solvent.
- a suitable base is diisopropylethylamine.
- An example of a suitable solvent is N-methylpyrrolidone (NMP), DMF, DMSO, or isopropanol.
- NMP N-methylpyrrolidone
- DMF DMF
- DMSO dimethylpyrrolidone
- an additive such as potassium fluoride may be used.
- the reaction is typically performed at a temperature between 50°C to 150°C for about 30 minutes to 24 hours. Alternatively the reaction can be performed in a microwave at a temperature between 100°C to 150°C for about 30 minutes to 24 hours.
- the product can be isolated by methods known in the art, for example by removal of solvent under reduced pressure.
- the product can be purified using any suitable methods known in the art, for example, chromatography of the residue on a silica column. In certain instance, the product can be purified using recrystallization or precipitation.
- Enzymatic activity of PI3K isoforms was measured to determine the inhibitory activities to the PI3K isoforms selectivity of the compounds tested, including the activities against PI3K5.
- a cellular assay measuring the inhibition of basophil activation was used to assess the potency of the compounds in a cellular assay. Stability in human hepatocytes was also measured to assess the half-life of the tested compounds in human subjects. i. Enzymatic activity ofPI3K isoforms
- Enzymatic activity of the class I PI3K isoforms in the presence of the compounds of Table 1 and Compounds X, Y and Z was measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay.
- TR-FRET time-resolved fluorescence resonance energy transfer
- the TR-FRET assay was used to monitor formation of the product 3,4,5-inositol triphosphate molecule (PIP3) as it competed with fluorescently labeled PIP3 for binding to the GRP- 1 pleckstrin homology domain protein.
- An increase in phosphatidylinositide 3-phosphate product results in a decrease in TR-FRET signal as the labeled fluorophore is displaced from the GRP- 1 protein binding site.
- Class I PI3K isoforms were expressed and purified as heterodimeric recombinant proteins. All assay reagents and buffers for the TR-FRET assay were purchased from Millipore. PI3K isoforms were assayed under initial rate conditions in the presence of 25 mM Hepes (pH 7.4), and 2 X Km ATP (75-500 ⁇ ), 2 ⁇ PIP2, 5% glycerol, 5 mM MgC12, 50 mM NaCl, 0.05% (v/v) Chaps, 1 mM dithiothreitol, 1 % (v/v) DMSO at the following concentrations for each isoforms: ⁇ 3 ⁇ , ⁇ 3 ⁇ , and PI3K5 between 25 and 50 pM, and ⁇ 3 ⁇ at 2 nM.
- Table 2 summarizes the IC 50 values (nM) that were collected for PI3K5 for compounds in the Example.
- Table 2a summarizes the IC 50 (nM) values for ⁇ 3 ⁇ and half life (hours) values in cryopreserved human hepatocytes.
- Table 2 The PI3K5 IC 50 Values for Representive Compounds
- erythrocytes were lysed and fixed by the addition of 2 mL of lysing solution (B-BAT-LYR) for 10 minutes at room temperature. Cells were pelleted by centrifugation at 1200 rpm for 10 minutes at room temperature in a swing- out rotor. Supernatant was aspirated and cell pellet resuspended in 400 ⁇ L ⁇ of wash buffer. Flow cytometric analysis of the basophil activation was performed on a FC500MPL flow cytometer (Beckman Coulter Inc., Fullerton, CA).
- CCR3-staining and side scatter were applied to gate at least 200 basophils that expressed a high density of CCR3.
- the percent CD63 positive cells within the gated basophil population were determined in different treatment groups and normalized to the vehicle control (0.3% DMSO) with anti-FceRI mAb of fMLP stimulus as 100%. Final compound concentration was adjusted to correct for dilution effect of added reagents.
- the EC 50 values were calculated from the analysis of the dose-response curves to a four-parameter equation. All EC 50 values represented geometric mean values and were reported in units of nM. Table 3 below summarizes the EC 50 data collected in the Example.
- This assay was used to evaluate the metabolic stability of test articles (TA) following incubation in cryopreserved hepatocytes by monitoring parent drug disappearance via LC/MC.
- the TA was incubated with 1 million hepatocytes/mL at 2 ⁇ substrate in duplicate. The incubation was carried out at 37°C with 5% C0 2 and saturating humidity. Samples were taken at 0, 1 , 2, and 4 hours to monitor the disappearance of TA and a half-life (t 2 ) was determined.
- Table 3 summarizes the human hepatocyte t 2 values (hours) collected in the Example. [0470] The symbols used in Table 3 below are as follows:
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Pulmonology (AREA)
- Epidemiology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Neurology (AREA)
- Physical Education & Sports Medicine (AREA)
- Hematology (AREA)
- Rheumatology (AREA)
- Diabetes (AREA)
- Neurosurgery (AREA)
- Biomedical Technology (AREA)
- Pain & Pain Management (AREA)
- Dermatology (AREA)
- Urology & Nephrology (AREA)
- Oncology (AREA)
- Transplantation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
Abstract
Description
Claims
Priority Applications (18)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2014277950A AU2014277950B2 (en) | 2013-06-14 | 2014-06-13 | Phosphatidylinositol 3-kinase inhibitors |
| BR112015031475A BR112015031475A2 (en) | 2013-06-14 | 2014-06-13 | compound, pharmaceutical composition, method for treating a disease or condition, kit, and use of compound. |
| EP14738957.1A EP3008053B1 (en) | 2013-06-14 | 2014-06-13 | Phosphatidylinositol 3-kinase inhibitors |
| KR1020157035901A KR101709436B1 (en) | 2013-06-14 | 2014-06-13 | Phosphatidylinositol 3-kinase inhibitors |
| PL14738957T PL3008053T3 (en) | 2013-06-14 | 2014-06-13 | Phosphatidylinositol 3-kinase inhibitors |
| JP2015563142A JP6030783B2 (en) | 2013-06-14 | 2014-06-13 | Phosphatidylinositol 3-kinase inhibitor |
| HK16108969.2A HK1220968B (en) | 2013-06-14 | 2014-06-13 | Phosphatidylinositol 3-kinase inhibitors |
| MX2015017192A MX347988B (en) | 2013-06-14 | 2014-06-13 | Phosphatidylinositol 3-kinase inhibitors. |
| ES14738957.1T ES2667173T3 (en) | 2013-06-14 | 2014-06-13 | Phosphatidylinositol 3-kinase inhibitors |
| NZ714710A NZ714710A (en) | 2013-06-14 | 2014-06-13 | Phosphatidylinositol 3-kinase inhibitors |
| CN201480033119.7A CN105308034B (en) | 2013-06-14 | 2014-06-13 | Phosphatidylinositol 3‑kinase inhibitors |
| SI201430672T SI3008053T1 (en) | 2013-06-14 | 2014-06-13 | Phosphatidylinositol 3-kinase inhibitors |
| CA2915129A CA2915129C (en) | 2013-06-14 | 2014-06-13 | Phosphatidylinositol 3-kinase inhibitors |
| SG11201509910YA SG11201509910YA (en) | 2013-06-14 | 2014-06-13 | Phosphatidylinositol 3-kinase inhibitors |
| HK16110710.0A HK1223911A1 (en) | 2013-06-14 | 2014-06-13 | Phosphatidylinositol 3-kinase inhibitors |
| EA201592182A EA028319B1 (en) | 2013-06-14 | 2014-06-13 | Phosphatidylinositol 3-kinase inhibitors |
| IL242677A IL242677B (en) | 2013-06-14 | 2015-11-19 | Phosphatidylinositol 3-kinase inhibitors |
| AU2016201584A AU2016201584B2 (en) | 2013-06-14 | 2016-03-11 | Phosphatidylinositol 3-kinase inhibitors |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361835333P | 2013-06-14 | 2013-06-14 | |
| US61/835,333 | 2013-06-14 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014201409A1 true WO2014201409A1 (en) | 2014-12-18 |
Family
ID=51177160
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2014/042392 Ceased WO2014201409A1 (en) | 2013-06-14 | 2014-06-13 | Phosphatidylinositol 3-kinase inhibitors |
Country Status (24)
| Country | Link |
|---|---|
| US (2) | US9221795B2 (en) |
| EP (1) | EP3008053B1 (en) |
| JP (1) | JP6030783B2 (en) |
| KR (1) | KR101709436B1 (en) |
| CN (1) | CN105308034B (en) |
| AR (1) | AR096621A1 (en) |
| AU (2) | AU2014277950B2 (en) |
| BR (1) | BR112015031475A2 (en) |
| CA (1) | CA2915129C (en) |
| EA (1) | EA028319B1 (en) |
| ES (1) | ES2667173T3 (en) |
| HK (1) | HK1223911A1 (en) |
| IL (1) | IL242677B (en) |
| MX (1) | MX347988B (en) |
| NO (1) | NO2994608T3 (en) |
| NZ (1) | NZ714710A (en) |
| PL (1) | PL3008053T3 (en) |
| PT (1) | PT3008053T (en) |
| SG (1) | SG11201509910YA (en) |
| SI (1) | SI3008053T1 (en) |
| TR (1) | TR201806822T4 (en) |
| TW (1) | TWI644909B (en) |
| UY (1) | UY35617A (en) |
| WO (1) | WO2014201409A1 (en) |
Cited By (83)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9115141B2 (en) | 2011-08-29 | 2015-08-25 | Infinity Pharmaceuticals, Inc. | Substituted isoquinolinones and methods of treatment thereof |
| US9181221B2 (en) | 2010-05-21 | 2015-11-10 | Infinity Pharmaceuticals, Inc. | Chemical compounds, compositions and methods for kinase modulation |
| WO2016126552A1 (en) | 2015-02-03 | 2016-08-11 | Gilead Sciences, Inc. | Combination therapies for treating cancers |
| US9481667B2 (en) | 2013-03-15 | 2016-11-01 | Infinity Pharmaceuticals, Inc. | Salts and solid forms of isoquinolinones and composition comprising and methods of using the same |
| US9512114B2 (en) | 2013-09-22 | 2016-12-06 | Calitor Sciences, Llc | Substituted aminopyrimidine compounds and methods of use |
| WO2017059224A2 (en) | 2015-10-01 | 2017-04-06 | Gilead Sciences, Inc. | Combination of a btk inhibitor and a checkpoint inhibitor for treating cancers |
| US9637488B2 (en) | 2015-01-29 | 2017-05-02 | Fuqiang Ruan | Heterocyclic compounds as inhibitors of class I PI3KS |
| WO2017106556A1 (en) | 2015-12-17 | 2017-06-22 | Gilead Sciences, Inc. | Tank-binding kinase inhibitor compounds |
| WO2017152062A1 (en) | 2016-03-04 | 2017-09-08 | Gilead Sciences, Inc. | Compositions and combinations of autotaxin inhibitors |
| WO2017177179A1 (en) | 2016-04-08 | 2017-10-12 | Gilead Sciences, Inc. | Compositions and methods for treating cancer, inflammatory diseases and autoimmune diseases |
| WO2017215590A1 (en) | 2016-06-13 | 2017-12-21 | I-Mab | Anti-pd-l1 antibodies and uses thereof |
| WO2018026835A1 (en) | 2016-08-04 | 2018-02-08 | Gilead Sciences, Inc. | Cobicistat for use in cancer treatments |
| US9944639B2 (en) | 2014-07-04 | 2018-04-17 | Lupin Limited | Quinolizinone derivatives as PI3K inhibitors |
| WO2018085069A1 (en) | 2016-11-03 | 2018-05-11 | Gilead Sciences, Inc. | Combination of a bcl-2 inhibitor and a bromodomain inhibitor for treating cancer |
| WO2018097977A1 (en) | 2016-11-22 | 2018-05-31 | Gilead Sciences, Inc. | Crystalline forms of a phosphate complex of a bet inhibitor |
| WO2018137598A1 (en) | 2017-01-24 | 2018-08-02 | I-Mab | Anti-cd73 antibodies and uses thereof |
| WO2018156901A1 (en) | 2017-02-24 | 2018-08-30 | Gilead Sciences, Inc. | Inhibitors of bruton's tyrosine kinase |
| WO2018156895A1 (en) | 2017-02-24 | 2018-08-30 | Gilead Sciences, Inc. | Inhibitors of bruton's tyrosine kinase |
| WO2018195321A1 (en) | 2017-04-20 | 2018-10-25 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| JP2019511452A (en) * | 2016-03-05 | 2019-04-25 | ハンチョウ ヂョンシャン ファーマシューティカルズ カンパニー リミテッドHangzhou Zhengxiang Pharmaceuticals Co., Ltd. | Quinoline analogues as phosphatidylinositol 3-kinase inhibitors |
| WO2019160882A1 (en) | 2018-02-13 | 2019-08-22 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2019193543A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotides |
| WO2019193533A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'2'-cyclic dinucleotides |
| WO2019193542A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotides |
| US10442799B1 (en) | 2018-04-07 | 2019-10-15 | Fuqiang Ruan | Heterocyclic compounds and uses thereof |
| WO2019204609A1 (en) | 2018-04-19 | 2019-10-24 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2019211799A1 (en) | 2018-05-03 | 2019-11-07 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide |
| WO2019217780A1 (en) | 2018-05-11 | 2019-11-14 | Phosphorex, Inc. | Microparticles and nanoparticles having negative surface charges |
| WO2019222112A1 (en) | 2018-05-14 | 2019-11-21 | Gilead Sciences, Inc. | Mcl-1 inhibitors |
| WO2020014643A1 (en) | 2018-07-13 | 2020-01-16 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2020072656A1 (en) | 2018-10-03 | 2020-04-09 | Gilead Sciences, Inc. | Imidozopyrimidine derivatives |
| WO2020086556A1 (en) | 2018-10-24 | 2020-04-30 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2020092621A1 (en) | 2018-10-31 | 2020-05-07 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds as hpk1 inhibitors |
| WO2020092528A1 (en) | 2018-10-31 | 2020-05-07 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds having hpk1 inhibitory activity |
| WO2020178770A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotides and prodrugs thereof |
| WO2020178769A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotides and prodrugs thereof |
| WO2020178768A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide as sting modulator |
| WO2020214663A1 (en) | 2019-04-17 | 2020-10-22 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| WO2020214652A1 (en) | 2019-04-17 | 2020-10-22 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| WO2020237025A1 (en) | 2019-05-23 | 2020-11-26 | Gilead Sciences, Inc. | Substituted exo-methylene-oxindoles which are hpk1/map4k1 inhibitors |
| WO2020263830A1 (en) | 2019-06-25 | 2020-12-30 | Gilead Sciences, Inc. | Flt3l-fc fusion proteins and methods of use |
| WO2021011544A1 (en) | 2019-07-16 | 2021-01-21 | Gilead Sciences, Inc. | Hiv vaccines and methods of making and using |
| US10966999B2 (en) | 2017-12-20 | 2021-04-06 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3′3′ cyclic dinucleotides with phosphonate bond activating the sting adaptor protein |
| WO2021067181A1 (en) | 2019-09-30 | 2021-04-08 | Gilead Sciences, Inc. | Hbv vaccines and methods treating hbv |
| WO2021076908A1 (en) | 2019-10-18 | 2021-04-22 | Forty Seven, Inc. | Combination therapies for treating myelodysplastic syndromes and acute myeloid leukemia |
| WO2021087064A1 (en) | 2019-10-31 | 2021-05-06 | Forty Seven, Inc. | Anti-cd47 and anti-cd20 based treatment of blood cancer |
| WO2021096860A1 (en) | 2019-11-12 | 2021-05-20 | Gilead Sciences, Inc. | Mcl1 inhibitors |
| WO2021130638A1 (en) | 2019-12-24 | 2021-07-01 | Carna Biosciences, Inc. | Diacylglycerol kinase modulating compounds |
| WO2021163064A2 (en) | 2020-02-14 | 2021-08-19 | Jounce Therapeutics, Inc. | Antibodies and fusion proteins that bind to ccr8 and uses thereof |
| WO2021222522A1 (en) | 2020-05-01 | 2021-11-04 | Gilead Sciences, Inc. | Cd73 inhibiting 2,4-dioxopyrimidine compounds |
| US11203610B2 (en) | 2017-12-20 | 2021-12-21 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2′3′ cyclic dinucleotides with phosphonate bond activating the sting adaptor protein |
| WO2022221304A1 (en) | 2021-04-14 | 2022-10-20 | Gilead Sciences, Inc. | CO-INHIBITION OF CD47/SIRPα BINDING AND NEDD8-ACTIVATING ENZYME E1 REGULATORY SUBUNIT FOR THE TREATMENT OF CANCER |
| WO2022245671A1 (en) | 2021-05-18 | 2022-11-24 | Gilead Sciences, Inc. | Methods of using flt3l-fc fusion proteins |
| WO2022271684A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271677A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271650A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271659A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2023076983A1 (en) | 2021-10-28 | 2023-05-04 | Gilead Sciences, Inc. | Pyridizin-3(2h)-one derivatives |
| WO2023077030A1 (en) | 2021-10-29 | 2023-05-04 | Gilead Sciences, Inc. | Cd73 compounds |
| WO2023107954A1 (en) | 2021-12-08 | 2023-06-15 | Dragonfly Therapeutics, Inc. | Antibodies targeting 5t4 and uses thereof |
| WO2023107956A1 (en) | 2021-12-08 | 2023-06-15 | Dragonfly Therapeutics, Inc. | Proteins binding nkg2d, cd16 and 5t4 |
| WO2023122581A2 (en) | 2021-12-22 | 2023-06-29 | Gilead Sciences, Inc. | Ikaros zinc finger family degraders and uses thereof |
| WO2023122615A1 (en) | 2021-12-22 | 2023-06-29 | Gilead Sciences, Inc. | Ikaros zinc finger family degraders and uses thereof |
| WO2023147418A1 (en) | 2022-01-28 | 2023-08-03 | Gilead Sciences, Inc. | Parp7 inhibitors |
| EP4245756A1 (en) | 2022-03-17 | 2023-09-20 | Gilead Sciences, Inc. | Ikaros zinc finger family degraders and uses thereof |
| WO2023183817A1 (en) | 2022-03-24 | 2023-09-28 | Gilead Sciences, Inc. | Combination therapy for treating trop-2 expressing cancers |
| WO2023196784A1 (en) | 2022-04-05 | 2023-10-12 | Gilead Sciences, Inc. | Combinations of antibody therapies for treating colorectal cancer |
| WO2023205719A1 (en) | 2022-04-21 | 2023-10-26 | Gilead Sciences, Inc. | Kras g12d modulating compounds |
| WO2024006929A1 (en) | 2022-07-01 | 2024-01-04 | Gilead Sciences, Inc. | Cd73 compounds |
| WO2024015741A1 (en) | 2022-07-12 | 2024-01-18 | Gilead Sciences, Inc. | Hiv immunogenic polypeptides and vaccines and uses thereof |
| WO2024064668A1 (en) | 2022-09-21 | 2024-03-28 | Gilead Sciences, Inc. | FOCAL IONIZING RADIATION AND CD47/SIRPα DISRUPTION ANTICANCER COMBINATION THERAPY |
| WO2024137852A1 (en) | 2022-12-22 | 2024-06-27 | Gilead Sciences, Inc. | Prmt5 inhibitors and uses thereof |
| WO2024215754A1 (en) | 2023-04-11 | 2024-10-17 | Gilead Sciences, Inc. | Kras modulating compounds |
| WO2024220917A1 (en) | 2023-04-21 | 2024-10-24 | Gilead Sciences, Inc. | Prmt5 inhibitors and uses thereof |
| WO2025006720A1 (en) | 2023-06-30 | 2025-01-02 | Gilead Sciences, Inc. | Kras modulating compounds |
| WO2025024811A1 (en) | 2023-07-26 | 2025-01-30 | Gilead Sciences, Inc. | Parp7 inhibitors |
| WO2025024663A1 (en) | 2023-07-26 | 2025-01-30 | Gilead Sciences, Inc. | Parp7 inhibitors |
| US12213983B2 (en) | 2012-11-01 | 2025-02-04 | Infinity Pharmaceuticals, Inc. | Treatment of cancers using PI3 kinase isoform modulators |
| WO2025054347A1 (en) | 2023-09-08 | 2025-03-13 | Gilead Sciences, Inc. | Kras g12d modulating compounds |
| WO2025054530A1 (en) | 2023-09-08 | 2025-03-13 | Gilead Sciences, Inc. | Pyrimidine-containing polycyclic derivatives as kras g12d modulating compounds |
| WO2025096589A1 (en) | 2023-11-03 | 2025-05-08 | Gilead Sciences, Inc. | Prmt5 inhibitors and uses thereof |
| WO2025137640A1 (en) | 2023-12-22 | 2025-06-26 | Gilead Sciences, Inc. | Azaspiro wrn inhibitors |
| WO2025245003A1 (en) | 2024-05-21 | 2025-11-27 | Gilead Sciences, Inc. | Prmt5 inhibitors and uses thereof |
Families Citing this family (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2673701B2 (en) | 1988-06-29 | 1997-11-05 | 東洋紡績株式会社 | Textile processing equipment |
| US8193182B2 (en) | 2008-01-04 | 2012-06-05 | Intellikine, Inc. | Substituted isoquinolin-1(2H)-ones, and methods of use thereof |
| DK2240451T3 (en) | 2008-01-04 | 2017-11-20 | Intellikine Llc | ISOQUINOLINON DERIVATIVES SUBSTITUTED WITH A PURIN USED AS PI3K INHIBITORS |
| PE20180318A1 (en) | 2011-01-10 | 2018-02-09 | Infinity Pharmaceuticals Inc | PROCEDURE FOR PREPARING ISOQUINOLINONES AND SOLID FORMS OF ISOQUINOLINONES |
| US8828998B2 (en) | 2012-06-25 | 2014-09-09 | Infinity Pharmaceuticals, Inc. | Treatment of lupus, fibrotic conditions, and inflammatory myopathies and other disorders using PI3 kinase inhibitors |
| NZ714710A (en) * | 2013-06-14 | 2016-11-25 | Gilead Sciences Inc | Phosphatidylinositol 3-kinase inhibitors |
| US20150320755A1 (en) | 2014-04-16 | 2015-11-12 | Infinity Pharmaceuticals, Inc. | Combination therapies |
| EA201692266A1 (en) | 2014-06-13 | 2017-06-30 | Джилид Сайэнс, Инк. | PHOSPHATIDYLINOSITOL-3-KINASE INHIBITORS |
| HK1231477A1 (en) | 2014-06-13 | 2017-12-22 | 吉利德科学公司 | Phosphatidylinositol 3-kinase inhibitors |
| EA201692267A1 (en) | 2014-06-13 | 2017-06-30 | Джилид Сайэнс, Инк. | PHOSPHATIDYLINOSITOL-3-KINASE INHIBITORS |
| MX2016016530A (en) | 2014-06-13 | 2017-03-27 | Gilead Sciences Inc | Phosphatidylinositol 3-kinase inhibitors. |
| GB201506786D0 (en) * | 2015-04-21 | 2015-06-03 | Ucb Biopharma Sprl | Therapeutic use |
| HK1255034A1 (en) | 2015-07-01 | 2019-08-02 | Crinetics Pharmaceuticals, Inc. | Somatostatin modulators and uses thereof |
| UA125216C2 (en) | 2016-06-24 | 2022-02-02 | Інфініті Фармасьютікалз, Інк. | COMBINED THERAPY |
| TW201813963A (en) | 2016-09-23 | 2018-04-16 | 美商基利科學股份有限公司 | Phosphatidylinositol 3-kinase inhibitors |
| TW201815787A (en) * | 2016-09-23 | 2018-05-01 | 美商基利科學股份有限公司 | Phosphatidylinositol 3-kinase inhibitors |
| TW201825465A (en) | 2016-09-23 | 2018-07-16 | 美商基利科學股份有限公司 | Phosphatidylinositol 3-kinase inhibitors |
| CN108017641B (en) * | 2016-11-02 | 2021-01-05 | 深圳铂立健医药有限公司 | Pyrazolopyrimidine compound as PI3K inhibitor and application thereof |
| US11028068B2 (en) | 2017-07-25 | 2021-06-08 | Crinetics Pharmaceuticals, Inc. | Somatostatin modulators and uses thereof |
| AU2019209960B2 (en) | 2018-01-20 | 2023-11-23 | Sunshine Lake Pharma Co., Ltd. | Substituted aminopyrimidine compounds and methods of use |
| WO2025040167A1 (en) * | 2023-08-24 | 2025-02-27 | 上海艾力斯医药科技股份有限公司 | Phosphoinositide 3-kinase allosteric inhibitor for treating disease related to pi3k regulation |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003035075A1 (en) * | 2001-10-19 | 2003-05-01 | Icos Corporation | Inhibitors of human phosphatidyl-inositol 3-kinase delta |
| WO2011146882A1 (en) * | 2010-05-21 | 2011-11-24 | Intellikine, Inc. | Chemical compounds, compositions and methods for kinase modulation |
Family Cites Families (65)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3845770A (en) | 1972-06-05 | 1974-11-05 | Alza Corp | Osmatic dispensing device for releasing beneficial agent |
| US4326525A (en) | 1980-10-14 | 1982-04-27 | Alza Corporation | Osmotic device that improves delivery properties of agent in situ |
| US5364620A (en) | 1983-12-22 | 1994-11-15 | Elan Corporation, Plc | Controlled absorption diltiazem formulation for once daily administration |
| US5023252A (en) | 1985-12-04 | 1991-06-11 | Conrex Pharmaceutical Corporation | Transdermal and trans-membrane delivery of drugs |
| US5001139A (en) | 1987-06-12 | 1991-03-19 | American Cyanamid Company | Enchancers for the transdermal flux of nivadipine |
| US4992445A (en) | 1987-06-12 | 1991-02-12 | American Cyanamid Co. | Transdermal delivery of pharmaceuticals |
| US5182297A (en) | 1988-02-25 | 1993-01-26 | Merrell Dow Pharmaceuticals Inc. | Inhibitors of lysyl oxidase |
| US4965288A (en) | 1988-02-25 | 1990-10-23 | Merrell Dow Pharmaceuticals Inc. | Inhibitors of lysyl oxidase |
| US5021456A (en) | 1988-02-25 | 1991-06-04 | Merrell Dow Pharmaceuticals Inc. | Inhibitors of lysyl oxidase |
| US5252608A (en) | 1988-02-25 | 1993-10-12 | Merrell Dow Pharmaceuticals Inc. | Inhibitors of lysyl oxidase |
| US4943593A (en) | 1988-02-25 | 1990-07-24 | Merrell Dow Pharmaceuticals Inc. | Inhibitors of lysyl oxidase |
| US5059714A (en) | 1988-02-25 | 1991-10-22 | Merrell Dow Pharmaceuticals Inc. | Inhibitors of lysyl oxidase |
| US4902514A (en) | 1988-07-21 | 1990-02-20 | Alza Corporation | Dosage form for administering nilvadipine for treating cardiovascular symptoms |
| US5120764A (en) | 1988-11-01 | 1992-06-09 | Merrell Dow Pharmaceuticals Inc. | Inhibitors of lysyl oxidase |
| US4997854A (en) | 1989-08-25 | 1991-03-05 | Trustees Of Boston University | Anti-fibrotic agents and methods for inhibiting the activity of lysyl oxidase in-situ using adjacently positioned diamine analogue substrates |
| GB9611460D0 (en) | 1996-06-01 | 1996-08-07 | Ludwig Inst Cancer Res | Novel lipid kinase |
| US5858753A (en) | 1996-11-25 | 1999-01-12 | Icos Corporation | Lipid kinase |
| ATE303997T1 (en) | 1997-06-09 | 2005-09-15 | Pfizer Prod Inc | CHINAZOLIN-4-ON AMPA ANTAGONISTS |
| DE60017157T2 (en) | 1999-09-16 | 2005-12-22 | Curis, Inc., Cambridge | MEDIATOR OF IGEL SIGNALWAYS, ITS COMPOSITIONS AND USES |
| US6545004B1 (en) | 1999-10-27 | 2003-04-08 | Cytokinetics, Inc. | Methods and compositions utilizing quinazolinones |
| EP1686120A3 (en) | 1999-10-27 | 2007-05-30 | Cytokinetics, Inc. | Methods and compositions utilizing quinazolinones |
| EP1278748B1 (en) | 2000-04-25 | 2011-03-23 | ICOS Corporation | Inhibitors of human phosphatidyl-inositol 3-kinase delta |
| FR2828206B1 (en) | 2001-08-03 | 2004-09-24 | Centre Nat Rech Scient | USE OF LYSYL OXIDASE INHIBITORS FOR CELL CULTURE AND TISSUE ENGINEERING |
| AU2003228283A1 (en) | 2002-03-07 | 2003-09-22 | X-Ceptor Therapeutics, Inc. | Quinazolinone modulators of nuclear receptors |
| AU2003301662A1 (en) | 2002-10-21 | 2004-05-13 | Bristol-Myers Squibb Company | Quinazolinones and derivatives thereof as factor xa inhibitors |
| US7939539B2 (en) | 2003-11-25 | 2011-05-10 | Novartis Vaccines And Diagnostics, Inc. | Quinazolinone compounds as anticancer agents |
| RS55546B1 (en) | 2004-05-13 | 2017-05-31 | Icos Corp | HINAZOLINONS AS HUMAN PHOSPHATIDYLONOSITOL 3-DELTA KINASE INHIBITORS |
| WO2005123696A1 (en) | 2004-06-15 | 2005-12-29 | Astrazeneca Ab | Substituted quinazolones as anti-cancer agents |
| US7939538B2 (en) | 2004-06-28 | 2011-05-10 | Amgen Inc. | Compounds, compositions and methods for prevention and treatment of inflammatory and immunoregulatory disorders and diseases |
| DE102005024017A1 (en) | 2005-05-25 | 2006-11-30 | Merck Patent Gmbh | quinazolinones |
| HRP20140147T1 (en) | 2007-03-23 | 2014-03-28 | Amgen Inc. | 3-SUBSTITUTED QUINOLINE OR QUINOXALINE DERIVATIVES AND THEIR USE AS PHOSPHATIDYLINOSITOL 3-KINASE INHIBITOR (PI3K) |
| US20110217300A1 (en) | 2007-06-22 | 2011-09-08 | Arqule, Inc. | Quinazolinone Compounds and Methods of Use Thereof |
| US8193182B2 (en) | 2008-01-04 | 2012-06-05 | Intellikine, Inc. | Substituted isoquinolin-1(2H)-ones, and methods of use thereof |
| DK2240451T3 (en) | 2008-01-04 | 2017-11-20 | Intellikine Llc | ISOQUINOLINON DERIVATIVES SUBSTITUTED WITH A PURIN USED AS PI3K INHIBITORS |
| CA2738429C (en) | 2008-09-26 | 2016-10-25 | Intellikine, Inc. | Heterocyclic kinase inhibitors |
| WO2010059593A1 (en) | 2008-11-18 | 2010-05-27 | Intellikine, Inc. | Methods and compositions for treatment of ophthalmic conditions |
| EP2396315B1 (en) | 2009-02-13 | 2016-08-31 | UCB Biopharma SPRL | Quinoline derivatives as pi3k kinase inhibitors |
| EP2427195B1 (en) | 2009-05-07 | 2019-05-01 | Intellikine, LLC | Heterocyclic compounds and uses thereof |
| AU2010266064A1 (en) | 2009-06-25 | 2012-01-19 | Amgen Inc. | 4H - pyrido [1, 2 - a] pyrimidin - 4 - one derivatives as PI3 K inhibitors |
| EA201270013A1 (en) | 2009-06-25 | 2012-06-29 | Амген Инк. | HETEROCYCLIC COMPOUNDS AND THEIR APPLICATION |
| JP2013504325A (en) | 2009-09-09 | 2013-02-07 | アビラ セラピューティクス, インコーポレイテッド | PI3 kinase inhibitors and uses thereof |
| US8957090B2 (en) | 2009-11-12 | 2015-02-17 | Ucb Pharma S.A. | Fused bicyclic pyridine and pyrazine derivatives as kinase inhibitors |
| CA2784830C (en) | 2009-12-18 | 2018-03-27 | Sunovion Pharmaceuticals Inc. | Compounds for treating disorders mediated by metabotropic glutamate receptor 5, and methods of use thereof |
| AU2010330875B2 (en) | 2009-12-18 | 2013-08-01 | Amgen Inc. | Heterocyclic compounds and their uses |
| UY33304A (en) | 2010-04-02 | 2011-10-31 | Amgen Inc | HETEROCYCLIC COMPOUNDS AND THEIR USES |
| NZ603789A (en) | 2010-05-26 | 2015-03-27 | Sunovion Pharmaceuticals Inc | Heteroaryl compounds and methods of use thereof |
| EP2588467A1 (en) | 2010-07-01 | 2013-05-08 | Amgen Inc. | Heterocyclic compounds and their use as inhibitors of pi3k activity |
| WO2012003271A1 (en) | 2010-07-02 | 2012-01-05 | Amgen Inc. | Heterocyclic compounds and their use as inhibitors of pi3k activity |
| AU2011302196B2 (en) | 2010-09-14 | 2016-04-28 | Exelixis, Inc. | Inhibitors of PI3K-delta and methods of their use and manufacture |
| US8765773B2 (en) | 2010-10-18 | 2014-07-01 | Millennium Pharmaceuticals, Inc. | Substituted hydroxamic acids and uses thereof |
| AU2011323243A1 (en) | 2010-11-04 | 2013-05-23 | Amgen Inc. | Heterocyclic compounds and their uses |
| AU2011326427B2 (en) | 2010-11-10 | 2016-01-07 | Infinity Pharmaceuticals Inc. | Heterocyclic compounds and uses thereof |
| MX2013005567A (en) | 2010-11-17 | 2013-10-30 | Amgen Inc | Quinoline derivatives as pik3 inhibitors. |
| JP2014501261A (en) | 2010-12-23 | 2014-01-20 | アムジエン・インコーポレーテツド | Heterocyclic compounds and their use |
| PE20180318A1 (en) | 2011-01-10 | 2018-02-09 | Infinity Pharmaceuticals Inc | PROCEDURE FOR PREPARING ISOQUINOLINONES AND SOLID FORMS OF ISOQUINOLINONES |
| BR112013020329A2 (en) | 2011-02-09 | 2016-08-02 | Hoffmann La Roche | heterocyclic compounds as pi3 kinase inhibitors |
| US9108984B2 (en) | 2011-03-14 | 2015-08-18 | Incyte Corporation | Substituted diamino-pyrimidine and diamino-pyridine derivatives as PI3K inhibitors |
| US9126948B2 (en) | 2011-03-25 | 2015-09-08 | Incyte Holdings Corporation | Pyrimidine-4,6-diamine derivatives as PI3K inhibitors |
| EP2518070A1 (en) | 2011-04-29 | 2012-10-31 | Almirall, S.A. | Pyrrolotriazinone derivatives as PI3K inhibitors |
| EP2518071A1 (en) | 2011-04-29 | 2012-10-31 | Almirall, S.A. | Imidazopyridine derivatives as PI3K inhibitors |
| AR091790A1 (en) * | 2011-08-29 | 2015-03-04 | Infinity Pharmaceuticals Inc | DERIVATIVES OF ISOQUINOLIN-1-ONA AND ITS USES |
| US9202145B2 (en) | 2012-11-30 | 2015-12-01 | Safety Management Services, Inc. | System and method of determining material reaction or sensitivity using high-speed video frames |
| JP6125663B2 (en) * | 2012-12-21 | 2017-05-10 | ギリアード カリストガ エルエルシー | Substituted pyrimidine aminoalkyl-quinazolones as phosphatidylinositol 3-kinase inhibitors |
| NZ708870A (en) | 2012-12-21 | 2016-09-30 | Gilead Calistoga Llc | Isoquinolinone or quinazolinone phosphatidylinositol 3-kinase inhibitors |
| NZ714710A (en) * | 2013-06-14 | 2016-11-25 | Gilead Sciences Inc | Phosphatidylinositol 3-kinase inhibitors |
-
2014
- 2014-06-13 NZ NZ714710A patent/NZ714710A/en not_active IP Right Cessation
- 2014-06-13 US US14/304,539 patent/US9221795B2/en active Active
- 2014-06-13 MX MX2015017192A patent/MX347988B/en active IP Right Grant
- 2014-06-13 PT PT147389571T patent/PT3008053T/en unknown
- 2014-06-13 SG SG11201509910YA patent/SG11201509910YA/en unknown
- 2014-06-13 SI SI201430672T patent/SI3008053T1/en unknown
- 2014-06-13 CA CA2915129A patent/CA2915129C/en active Active
- 2014-06-13 EA EA201592182A patent/EA028319B1/en not_active IP Right Cessation
- 2014-06-13 TR TR2018/06822T patent/TR201806822T4/en unknown
- 2014-06-13 AR ARP140102277A patent/AR096621A1/en unknown
- 2014-06-13 ES ES14738957.1T patent/ES2667173T3/en active Active
- 2014-06-13 TW TW103120507A patent/TWI644909B/en active
- 2014-06-13 PL PL14738957T patent/PL3008053T3/en unknown
- 2014-06-13 KR KR1020157035901A patent/KR101709436B1/en active Active
- 2014-06-13 AU AU2014277950A patent/AU2014277950B2/en active Active
- 2014-06-13 JP JP2015563142A patent/JP6030783B2/en active Active
- 2014-06-13 EP EP14738957.1A patent/EP3008053B1/en active Active
- 2014-06-13 WO PCT/US2014/042392 patent/WO2014201409A1/en not_active Ceased
- 2014-06-13 BR BR112015031475A patent/BR112015031475A2/en not_active IP Right Cessation
- 2014-06-13 HK HK16110710.0A patent/HK1223911A1/en unknown
- 2014-06-13 CN CN201480033119.7A patent/CN105308034B/en active Active
- 2014-06-16 UY UY0001035617A patent/UY35617A/en not_active Application Discontinuation
- 2014-07-31 NO NO14832802A patent/NO2994608T3/no unknown
-
2015
- 2015-10-21 US US14/919,261 patent/US20160039793A1/en not_active Abandoned
- 2015-11-19 IL IL242677A patent/IL242677B/en not_active IP Right Cessation
-
2016
- 2016-03-11 AU AU2016201584A patent/AU2016201584B2/en not_active Ceased
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003035075A1 (en) * | 2001-10-19 | 2003-05-01 | Icos Corporation | Inhibitors of human phosphatidyl-inositol 3-kinase delta |
| WO2011146882A1 (en) * | 2010-05-21 | 2011-11-24 | Intellikine, Inc. | Chemical compounds, compositions and methods for kinase modulation |
Non-Patent Citations (1)
| Title |
|---|
| AMERIKS M K ET AL: "Small Molecule Inhibitors of Phosphoinositide 3-Kinase (PI3K) .delta. and .gamma", CURRENT TOPICS IN MEDICINAL CHEMISTRY, BENTHAM SCIENCE PUBLISHERS LTD, HILVERSUM; NL, vol. 9, no. 8, 1 May 2009 (2009-05-01), pages 738 - 753, XP002556393, ISSN: 1568-0266, DOI: 10.2174/156802609789044434 * |
Cited By (111)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9738644B2 (en) | 2010-05-21 | 2017-08-22 | Infinity Pharmaceuticals, Inc. | Chemical compounds, compositions and methods for kinase modulation |
| US9181221B2 (en) | 2010-05-21 | 2015-11-10 | Infinity Pharmaceuticals, Inc. | Chemical compounds, compositions and methods for kinase modulation |
| US9115141B2 (en) | 2011-08-29 | 2015-08-25 | Infinity Pharmaceuticals, Inc. | Substituted isoquinolinones and methods of treatment thereof |
| US9546180B2 (en) | 2011-08-29 | 2017-01-17 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US12213983B2 (en) | 2012-11-01 | 2025-02-04 | Infinity Pharmaceuticals, Inc. | Treatment of cancers using PI3 kinase isoform modulators |
| US9481667B2 (en) | 2013-03-15 | 2016-11-01 | Infinity Pharmaceuticals, Inc. | Salts and solid forms of isoquinolinones and composition comprising and methods of using the same |
| US9657007B2 (en) | 2013-09-22 | 2017-05-23 | Calitor Sciences, Llc | Substituted aminopyrimidine compounds and methods of use |
| US9670194B2 (en) | 2013-09-22 | 2017-06-06 | Calitor Sciences, Llc | Substituted aminopyrimidine compounds and methods of use |
| US9518046B2 (en) | 2013-09-22 | 2016-12-13 | Calitor Sciences, Llc | Substituted aminopyrimidine compounds and methods of use |
| US9512114B2 (en) | 2013-09-22 | 2016-12-06 | Calitor Sciences, Llc | Substituted aminopyrimidine compounds and methods of use |
| US9944639B2 (en) | 2014-07-04 | 2018-04-17 | Lupin Limited | Quinolizinone derivatives as PI3K inhibitors |
| US9637488B2 (en) | 2015-01-29 | 2017-05-02 | Fuqiang Ruan | Heterocyclic compounds as inhibitors of class I PI3KS |
| WO2016126552A1 (en) | 2015-02-03 | 2016-08-11 | Gilead Sciences, Inc. | Combination therapies for treating cancers |
| WO2017059224A2 (en) | 2015-10-01 | 2017-04-06 | Gilead Sciences, Inc. | Combination of a btk inhibitor and a checkpoint inhibitor for treating cancers |
| WO2017106556A1 (en) | 2015-12-17 | 2017-06-22 | Gilead Sciences, Inc. | Tank-binding kinase inhibitor compounds |
| WO2017152062A1 (en) | 2016-03-04 | 2017-09-08 | Gilead Sciences, Inc. | Compositions and combinations of autotaxin inhibitors |
| JP7201992B2 (en) | 2016-03-05 | 2023-01-11 | ナンキン ヂョンシャン ファーマシューティカルズ カンパニー リミテッド | Quinoline analogues as phosphatidylinositol 3-kinase inhibitors |
| JP2019511452A (en) * | 2016-03-05 | 2019-04-25 | ハンチョウ ヂョンシャン ファーマシューティカルズ カンパニー リミテッドHangzhou Zhengxiang Pharmaceuticals Co., Ltd. | Quinoline analogues as phosphatidylinositol 3-kinase inhibitors |
| US11446301B2 (en) | 2016-03-05 | 2022-09-20 | Nanjing Zhengxiang Pharmaceuticals Co., Ltd. | Quinoline analogs as phosphatidylinositol 3-kinase inhibitors |
| WO2017177179A1 (en) | 2016-04-08 | 2017-10-12 | Gilead Sciences, Inc. | Compositions and methods for treating cancer, inflammatory diseases and autoimmune diseases |
| WO2017215590A1 (en) | 2016-06-13 | 2017-12-21 | I-Mab | Anti-pd-l1 antibodies and uses thereof |
| WO2018026835A1 (en) | 2016-08-04 | 2018-02-08 | Gilead Sciences, Inc. | Cobicistat for use in cancer treatments |
| WO2018085069A1 (en) | 2016-11-03 | 2018-05-11 | Gilead Sciences, Inc. | Combination of a bcl-2 inhibitor and a bromodomain inhibitor for treating cancer |
| WO2018097977A1 (en) | 2016-11-22 | 2018-05-31 | Gilead Sciences, Inc. | Crystalline forms of a phosphate complex of a bet inhibitor |
| WO2018137598A1 (en) | 2017-01-24 | 2018-08-02 | I-Mab | Anti-cd73 antibodies and uses thereof |
| EP4050030A1 (en) | 2017-01-24 | 2022-08-31 | I-Mab Biopharma US Limited | Anti-cd73 antibodies and uses thereof |
| WO2018156895A1 (en) | 2017-02-24 | 2018-08-30 | Gilead Sciences, Inc. | Inhibitors of bruton's tyrosine kinase |
| US10370381B2 (en) | 2017-02-24 | 2019-08-06 | Gilead Sciences, Inc. | Inhibitors of bruton'S tyrosine kinase |
| WO2018156901A1 (en) | 2017-02-24 | 2018-08-30 | Gilead Sciences, Inc. | Inhibitors of bruton's tyrosine kinase |
| US10314844B2 (en) | 2017-02-24 | 2019-06-11 | Gilead Sciences, Inc. | Inhibitors of Bruton's tyrosine kinase |
| EP4026835A2 (en) | 2017-04-20 | 2022-07-13 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2018195321A1 (en) | 2017-04-20 | 2018-10-25 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| US11203610B2 (en) | 2017-12-20 | 2021-12-21 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2′3′ cyclic dinucleotides with phosphonate bond activating the sting adaptor protein |
| US10966999B2 (en) | 2017-12-20 | 2021-04-06 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3′3′ cyclic dinucleotides with phosphonate bond activating the sting adaptor protein |
| EP4227302A1 (en) | 2018-02-13 | 2023-08-16 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2019160882A1 (en) | 2018-02-13 | 2019-08-22 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2019193542A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotides |
| WO2019193533A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'2'-cyclic dinucleotides |
| US11292812B2 (en) | 2018-04-06 | 2022-04-05 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3′3′-cyclic dinucleotides |
| US11149052B2 (en) | 2018-04-06 | 2021-10-19 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2′3′-cyclic dinucleotides |
| WO2019193543A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotides |
| US10442799B1 (en) | 2018-04-07 | 2019-10-15 | Fuqiang Ruan | Heterocyclic compounds and uses thereof |
| WO2019204609A1 (en) | 2018-04-19 | 2019-10-24 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| EP4600247A2 (en) | 2018-04-19 | 2025-08-13 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2019211799A1 (en) | 2018-05-03 | 2019-11-07 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide |
| WO2019217780A1 (en) | 2018-05-11 | 2019-11-14 | Phosphorex, Inc. | Microparticles and nanoparticles having negative surface charges |
| WO2019222112A1 (en) | 2018-05-14 | 2019-11-21 | Gilead Sciences, Inc. | Mcl-1 inhibitors |
| EP4029868A1 (en) | 2018-05-14 | 2022-07-20 | Gilead Sciences, Inc. | Mcl-1 inhibitors |
| EP4234030A2 (en) | 2018-07-13 | 2023-08-30 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2020014643A1 (en) | 2018-07-13 | 2020-01-16 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2020072656A1 (en) | 2018-10-03 | 2020-04-09 | Gilead Sciences, Inc. | Imidozopyrimidine derivatives |
| WO2020086556A1 (en) | 2018-10-24 | 2020-04-30 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2020092528A1 (en) | 2018-10-31 | 2020-05-07 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds having hpk1 inhibitory activity |
| EP4371987A1 (en) | 2018-10-31 | 2024-05-22 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds as hpk1 inhibitors |
| WO2020092621A1 (en) | 2018-10-31 | 2020-05-07 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds as hpk1 inhibitors |
| WO2020178770A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotides and prodrugs thereof |
| WO2020178769A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotides and prodrugs thereof |
| US12318403B2 (en) | 2019-03-07 | 2025-06-03 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2′3′-cyclic dinucleotides and prodrugs thereof |
| US11766447B2 (en) | 2019-03-07 | 2023-09-26 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3′3′-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide as sting modulator |
| WO2020178768A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide as sting modulator |
| EP4458416A2 (en) | 2019-04-17 | 2024-11-06 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| WO2020214663A1 (en) | 2019-04-17 | 2020-10-22 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| WO2020214652A1 (en) | 2019-04-17 | 2020-10-22 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| WO2020237025A1 (en) | 2019-05-23 | 2020-11-26 | Gilead Sciences, Inc. | Substituted exo-methylene-oxindoles which are hpk1/map4k1 inhibitors |
| WO2020263830A1 (en) | 2019-06-25 | 2020-12-30 | Gilead Sciences, Inc. | Flt3l-fc fusion proteins and methods of use |
| WO2021011544A1 (en) | 2019-07-16 | 2021-01-21 | Gilead Sciences, Inc. | Hiv vaccines and methods of making and using |
| WO2021067181A1 (en) | 2019-09-30 | 2021-04-08 | Gilead Sciences, Inc. | Hbv vaccines and methods treating hbv |
| EP4458975A2 (en) | 2019-09-30 | 2024-11-06 | Gilead Sciences, Inc. | Hbv vaccines and methods treating hbv |
| WO2021076908A1 (en) | 2019-10-18 | 2021-04-22 | Forty Seven, Inc. | Combination therapies for treating myelodysplastic syndromes and acute myeloid leukemia |
| WO2021087064A1 (en) | 2019-10-31 | 2021-05-06 | Forty Seven, Inc. | Anti-cd47 and anti-cd20 based treatment of blood cancer |
| WO2021096860A1 (en) | 2019-11-12 | 2021-05-20 | Gilead Sciences, Inc. | Mcl1 inhibitors |
| WO2021130638A1 (en) | 2019-12-24 | 2021-07-01 | Carna Biosciences, Inc. | Diacylglycerol kinase modulating compounds |
| EP4445902A2 (en) | 2019-12-24 | 2024-10-16 | Carna Biosciences, Inc. | Diacylglycerol kinase modulating compounds |
| US12297282B2 (en) | 2020-02-14 | 2025-05-13 | Gilead Sciences, Inc. | Nucleic acids encoding, and methods of producing, antibodies that bind human chemokine (C—C motif) receptor 8 (CCR8) |
| US11692038B2 (en) | 2020-02-14 | 2023-07-04 | Gilead Sciences, Inc. | Antibodies that bind chemokine (C-C motif) receptor 8 (CCR8) |
| WO2021163064A2 (en) | 2020-02-14 | 2021-08-19 | Jounce Therapeutics, Inc. | Antibodies and fusion proteins that bind to ccr8 and uses thereof |
| WO2021222522A1 (en) | 2020-05-01 | 2021-11-04 | Gilead Sciences, Inc. | Cd73 inhibiting 2,4-dioxopyrimidine compounds |
| WO2022221304A1 (en) | 2021-04-14 | 2022-10-20 | Gilead Sciences, Inc. | CO-INHIBITION OF CD47/SIRPα BINDING AND NEDD8-ACTIVATING ENZYME E1 REGULATORY SUBUNIT FOR THE TREATMENT OF CANCER |
| WO2022245671A1 (en) | 2021-05-18 | 2022-11-24 | Gilead Sciences, Inc. | Methods of using flt3l-fc fusion proteins |
| WO2022271659A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271650A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271684A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271677A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2023076983A1 (en) | 2021-10-28 | 2023-05-04 | Gilead Sciences, Inc. | Pyridizin-3(2h)-one derivatives |
| WO2023077030A1 (en) | 2021-10-29 | 2023-05-04 | Gilead Sciences, Inc. | Cd73 compounds |
| WO2023107956A1 (en) | 2021-12-08 | 2023-06-15 | Dragonfly Therapeutics, Inc. | Proteins binding nkg2d, cd16 and 5t4 |
| WO2023107954A1 (en) | 2021-12-08 | 2023-06-15 | Dragonfly Therapeutics, Inc. | Antibodies targeting 5t4 and uses thereof |
| EP4667056A1 (en) | 2021-12-22 | 2025-12-24 | Gilead Sciences, Inc. | Ikaros zinc finger family degraders and uses thereof |
| WO2023122615A1 (en) | 2021-12-22 | 2023-06-29 | Gilead Sciences, Inc. | Ikaros zinc finger family degraders and uses thereof |
| WO2023122581A2 (en) | 2021-12-22 | 2023-06-29 | Gilead Sciences, Inc. | Ikaros zinc finger family degraders and uses thereof |
| WO2023147418A1 (en) | 2022-01-28 | 2023-08-03 | Gilead Sciences, Inc. | Parp7 inhibitors |
| EP4245756A1 (en) | 2022-03-17 | 2023-09-20 | Gilead Sciences, Inc. | Ikaros zinc finger family degraders and uses thereof |
| EP4464703A2 (en) | 2022-03-17 | 2024-11-20 | Gilead Sciences, Inc. | Ikaros zinc finger family degraders and uses thereof |
| WO2023178181A1 (en) | 2022-03-17 | 2023-09-21 | Gilead Sciences, Inc. | Ikaros zinc finger family degraders and uses thereof |
| WO2023183817A1 (en) | 2022-03-24 | 2023-09-28 | Gilead Sciences, Inc. | Combination therapy for treating trop-2 expressing cancers |
| WO2023196784A1 (en) | 2022-04-05 | 2023-10-12 | Gilead Sciences, Inc. | Combinations of antibody therapies for treating colorectal cancer |
| WO2023205719A1 (en) | 2022-04-21 | 2023-10-26 | Gilead Sciences, Inc. | Kras g12d modulating compounds |
| WO2024006929A1 (en) | 2022-07-01 | 2024-01-04 | Gilead Sciences, Inc. | Cd73 compounds |
| WO2024015741A1 (en) | 2022-07-12 | 2024-01-18 | Gilead Sciences, Inc. | Hiv immunogenic polypeptides and vaccines and uses thereof |
| WO2024064668A1 (en) | 2022-09-21 | 2024-03-28 | Gilead Sciences, Inc. | FOCAL IONIZING RADIATION AND CD47/SIRPα DISRUPTION ANTICANCER COMBINATION THERAPY |
| WO2024137852A1 (en) | 2022-12-22 | 2024-06-27 | Gilead Sciences, Inc. | Prmt5 inhibitors and uses thereof |
| WO2024215754A1 (en) | 2023-04-11 | 2024-10-17 | Gilead Sciences, Inc. | Kras modulating compounds |
| WO2024220917A1 (en) | 2023-04-21 | 2024-10-24 | Gilead Sciences, Inc. | Prmt5 inhibitors and uses thereof |
| WO2025006720A1 (en) | 2023-06-30 | 2025-01-02 | Gilead Sciences, Inc. | Kras modulating compounds |
| WO2025024663A1 (en) | 2023-07-26 | 2025-01-30 | Gilead Sciences, Inc. | Parp7 inhibitors |
| WO2025024811A1 (en) | 2023-07-26 | 2025-01-30 | Gilead Sciences, Inc. | Parp7 inhibitors |
| WO2025054530A1 (en) | 2023-09-08 | 2025-03-13 | Gilead Sciences, Inc. | Pyrimidine-containing polycyclic derivatives as kras g12d modulating compounds |
| WO2025054347A1 (en) | 2023-09-08 | 2025-03-13 | Gilead Sciences, Inc. | Kras g12d modulating compounds |
| WO2025096589A1 (en) | 2023-11-03 | 2025-05-08 | Gilead Sciences, Inc. | Prmt5 inhibitors and uses thereof |
| WO2025137640A1 (en) | 2023-12-22 | 2025-06-26 | Gilead Sciences, Inc. | Azaspiro wrn inhibitors |
| WO2025245003A1 (en) | 2024-05-21 | 2025-11-27 | Gilead Sciences, Inc. | Prmt5 inhibitors and uses thereof |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2016201584B2 (en) | Phosphatidylinositol 3-kinase inhibitors | |
| JP5336606B2 (en) | Antitumor effect enhancer | |
| AU2015274628B2 (en) | Phosphatidylinositol 3-kinase inhibitors | |
| US10611777B2 (en) | Imidazopyridazine compounds and their use | |
| CA2952018A1 (en) | Quinazolinone derivatives as phosphatidylinositol 3-kinase inhibitors | |
| US9388165B2 (en) | Isoquinoline-5-carboxamide derivative having inhibitory activity for protein kinase | |
| TWI523856B (en) | BCR-ABL kinase inhibitor and its application | |
| US20210155604A1 (en) | Quinazoline compound serving as egfr triple mutation inhibitor and applications thereof | |
| KR101684950B1 (en) | Furan-containing fused cyclic compound or its salt and pharmaceutical composition comprising the same | |
| TW202031653A (en) | Novel heteroaromatic amide derivative and medicine containing same | |
| WO2022206583A1 (en) | Indazole compound, preparation method therefor and application thereof | |
| HK1220968B (en) | Phosphatidylinositol 3-kinase inhibitors | |
| KR101796779B1 (en) | Dihydropteridin-one derivatives or pharmaceutically acceptable salts thereof, preparation method thereof and pharmaceutical composition for use in preventing or treating PI3 kinase related diseases | |
| KR20250041906A (en) | Novel compounds having HPK1 and MLK3 inhibitory activities and anti-cancer compositions containing the same | |
| KR20250097910A (en) | A novel inhibitor of phosphatidylinositol 3-kinase |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201480033119.7 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14738957 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 242677 Country of ref document: IL |
|
| ENP | Entry into the national phase |
Ref document number: 2915129 Country of ref document: CA Ref document number: 2015563142 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2015/017192 Country of ref document: MX |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 201592182 Country of ref document: EA |
|
| ENP | Entry into the national phase |
Ref document number: 20157035901 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2014277950 Country of ref document: AU Date of ref document: 20140613 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2014738957 Country of ref document: EP |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112015031475 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112015031475 Country of ref document: BR Kind code of ref document: A2 Effective date: 20151210 |