WO2014110322A2 - Substituted dioxopiperidinyl phthalimide derivatives - Google Patents
Substituted dioxopiperidinyl phthalimide derivatives Download PDFInfo
- Publication number
- WO2014110322A2 WO2014110322A2 PCT/US2014/010972 US2014010972W WO2014110322A2 WO 2014110322 A2 WO2014110322 A2 WO 2014110322A2 US 2014010972 W US2014010972 W US 2014010972W WO 2014110322 A2 WO2014110322 A2 WO 2014110322A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- compound
- deuterium
- hydrogen
- pharmaceutically acceptable
- acceptable salt
- Prior art date
Links
- MVXMSZXCRFSCOT-UHFFFAOYSA-N 4-(2,3-dioxopiperidin-1-yl)isoindole-1,3-dione Chemical class O=C1NC(=O)C2=C1C=CC=C2N1CCCC(=O)C1=O MVXMSZXCRFSCOT-UHFFFAOYSA-N 0.000 title abstract description 4
- 150000001875 compounds Chemical class 0.000 claims abstract description 239
- 239000000203 mixture Substances 0.000 claims abstract description 74
- 238000000034 method Methods 0.000 claims abstract description 43
- 150000003839 salts Chemical class 0.000 claims abstract description 41
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 34
- 201000010099 disease Diseases 0.000 claims abstract description 27
- 229910052805 deuterium Inorganic materials 0.000 claims description 185
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 claims description 174
- 229910052739 hydrogen Inorganic materials 0.000 claims description 81
- 239000003814 drug Substances 0.000 claims description 79
- 239000001257 hydrogen Substances 0.000 claims description 72
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 68
- 229940124597 therapeutic agent Drugs 0.000 claims description 39
- 230000000155 isotopic effect Effects 0.000 claims description 36
- 239000008194 pharmaceutical composition Substances 0.000 claims description 14
- -1 rituximab Chemical compound 0.000 claims description 14
- 201000001441 melanoma Diseases 0.000 claims description 12
- 206010060862 Prostate cancer Diseases 0.000 claims description 8
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 8
- 206010028537 myelofibrosis Diseases 0.000 claims description 8
- 208000025113 myeloid leukemia Diseases 0.000 claims description 8
- 208000034578 Multiple myelomas Diseases 0.000 claims description 7
- 201000003793 Myelodysplastic syndrome Diseases 0.000 claims description 7
- 206010035226 Plasma cell myeloma Diseases 0.000 claims description 7
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 claims description 6
- 206010028980 Neoplasm Diseases 0.000 claims description 5
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 claims description 5
- 208000011580 syndromic disease Diseases 0.000 claims description 5
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 claims description 4
- 208000003174 Brain Neoplasms Diseases 0.000 claims description 4
- 208000011691 Burkitt lymphomas Diseases 0.000 claims description 4
- 208000023890 Complex Regional Pain Syndromes Diseases 0.000 claims description 4
- 208000004463 Follicular Adenocarcinoma Diseases 0.000 claims description 4
- 206010018338 Glioma Diseases 0.000 claims description 4
- 208000017604 Hodgkin disease Diseases 0.000 claims description 4
- 208000021519 Hodgkin lymphoma Diseases 0.000 claims description 4
- 208000010747 Hodgkins lymphoma Diseases 0.000 claims description 4
- 206010073086 Iris melanoma Diseases 0.000 claims description 4
- 206010024305 Leukaemia monocytic Diseases 0.000 claims description 4
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 claims description 4
- 206010025323 Lymphomas Diseases 0.000 claims description 4
- 208000025205 Mantle-Cell Lymphoma Diseases 0.000 claims description 4
- 208000033833 Myelomonocytic Chronic Leukemia Diseases 0.000 claims description 4
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 claims description 4
- 206010033128 Ovarian cancer Diseases 0.000 claims description 4
- 206010061535 Ovarian neoplasm Diseases 0.000 claims description 4
- 206010033701 Papillary thyroid cancer Diseases 0.000 claims description 4
- 206010037779 Radiculopathy Diseases 0.000 claims description 4
- 201000001947 Reflex Sympathetic Dystrophy Diseases 0.000 claims description 4
- 208000006265 Renal cell carcinoma Diseases 0.000 claims description 4
- 206010042971 T-cell lymphoma Diseases 0.000 claims description 4
- 208000027585 T-cell non-Hodgkin lymphoma Diseases 0.000 claims description 4
- 208000024770 Thyroid neoplasm Diseases 0.000 claims description 4
- 201000005969 Uveal melanoma Diseases 0.000 claims description 4
- 208000033559 Waldenström macroglobulinemia Diseases 0.000 claims description 4
- 206010002022 amyloidosis Diseases 0.000 claims description 4
- 230000001684 chronic effect Effects 0.000 claims description 4
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 claims description 4
- 201000010902 chronic myelomonocytic leukemia Diseases 0.000 claims description 4
- 210000004240 ciliary body Anatomy 0.000 claims description 4
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 claims description 4
- 239000003937 drug carrier Substances 0.000 claims description 4
- 230000000925 erythroid effect Effects 0.000 claims description 4
- 201000003444 follicular lymphoma Diseases 0.000 claims description 4
- 208000005017 glioblastoma Diseases 0.000 claims description 4
- 208000002409 gliosarcoma Diseases 0.000 claims description 4
- 206010027191 meningioma Diseases 0.000 claims description 4
- 201000006894 monocytic leukemia Diseases 0.000 claims description 4
- 208000002154 non-small cell lung carcinoma Diseases 0.000 claims description 4
- 208000016432 ocular melanoma with extraocular extension Diseases 0.000 claims description 4
- 230000000306 recurrent effect Effects 0.000 claims description 4
- 201000009238 refractory plasma cell neoplasm Diseases 0.000 claims description 4
- 208000015347 renal cell adenocarcinoma Diseases 0.000 claims description 4
- 201000006845 reticulosarcoma Diseases 0.000 claims description 4
- 208000029922 reticulum cell sarcoma Diseases 0.000 claims description 4
- 206010062261 spinal cord neoplasm Diseases 0.000 claims description 4
- 208000030901 thyroid gland follicular carcinoma Diseases 0.000 claims description 4
- 208000030045 thyroid gland papillary carcinoma Diseases 0.000 claims description 4
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 claims description 4
- 229960003957 dexamethasone Drugs 0.000 claims description 3
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 claims description 2
- WFOVEDJTASPCIR-UHFFFAOYSA-N 3-[(4-methyl-5-pyridin-4-yl-1,2,4-triazol-3-yl)methylamino]-n-[[2-(trifluoromethyl)phenyl]methyl]benzamide Chemical compound N=1N=C(C=2C=CN=CC=2)N(C)C=1CNC(C=1)=CC=CC=1C(=O)NCC1=CC=CC=C1C(F)(F)F WFOVEDJTASPCIR-UHFFFAOYSA-N 0.000 claims description 2
- NMUSYJAQQFHJEW-KVTDHHQDSA-N 5-azacytidine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-KVTDHHQDSA-N 0.000 claims description 2
- UHNRLQRZRNKOKU-UHFFFAOYSA-N CCN(CC1=NC2=C(N1)C1=CC=C(C=C1N=C2N)C1=NNC=C1)C(C)=O Chemical compound CCN(CC1=NC2=C(N1)C1=CC=C(C=C1N=C2N)C1=NNC=C1)C(C)=O UHNRLQRZRNKOKU-UHFFFAOYSA-N 0.000 claims description 2
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 claims description 2
- 229960002756 azacitidine Drugs 0.000 claims description 2
- 229940087430 biaxin Drugs 0.000 claims description 2
- GXJABQQUPOEUTA-RDJZCZTQSA-N bortezomib Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)B(O)O)NC(=O)C=1N=CC=NC=1)C1=CC=CC=C1 GXJABQQUPOEUTA-RDJZCZTQSA-N 0.000 claims description 2
- 229960001467 bortezomib Drugs 0.000 claims description 2
- AGOYDEPGAOXOCK-KCBOHYOISA-N clarithromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@](C)([C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)OC)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 AGOYDEPGAOXOCK-KCBOHYOISA-N 0.000 claims description 2
- 229960003901 dacarbazine Drugs 0.000 claims description 2
- 229940026692 decadron Drugs 0.000 claims description 2
- 229960003668 docetaxel Drugs 0.000 claims description 2
- 229940115080 doxil Drugs 0.000 claims description 2
- 229960004679 doxorubicin Drugs 0.000 claims description 2
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 claims description 2
- 229960005277 gemcitabine Drugs 0.000 claims description 2
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 claims description 2
- 229960001924 melphalan Drugs 0.000 claims description 2
- QOFFJEBXNKRSPX-ZDUSSCGKSA-N pemetrexed Chemical compound C1=N[C]2NC(N)=NC(=O)C2=C1CCC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 QOFFJEBXNKRSPX-ZDUSSCGKSA-N 0.000 claims description 2
- 229960005079 pemetrexed Drugs 0.000 claims description 2
- 229960004618 prednisone Drugs 0.000 claims description 2
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 claims description 2
- 229960004641 rituximab Drugs 0.000 claims description 2
- KSAVQLQVUXSOCR-UHFFFAOYSA-M sodium lauroyl sarcosinate Chemical compound [Na+].CCCCCCCCCCCC(=O)N(C)CC([O-])=O KSAVQLQVUXSOCR-UHFFFAOYSA-M 0.000 claims description 2
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 claims description 2
- 229960000303 topotecan Drugs 0.000 claims description 2
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 claims description 2
- 229960004528 vincristine Drugs 0.000 claims description 2
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 claims description 2
- 239000003795 chemical substances by application Substances 0.000 abstract description 11
- 239000002253 acid Substances 0.000 abstract description 8
- 230000002519 immonomodulatory effect Effects 0.000 abstract description 3
- 238000010348 incorporation Methods 0.000 description 58
- 239000007787 solid Substances 0.000 description 42
- 229940079593 drug Drugs 0.000 description 34
- 230000015572 biosynthetic process Effects 0.000 description 33
- 238000003786 synthesis reaction Methods 0.000 description 33
- 235000002639 sodium chloride Nutrition 0.000 description 32
- 125000004429 atom Chemical group 0.000 description 27
- 230000002829 reductive effect Effects 0.000 description 23
- 238000011282 treatment Methods 0.000 description 23
- 229910052799 carbon Inorganic materials 0.000 description 22
- 239000000243 solution Substances 0.000 description 22
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 21
- 238000002360 preparation method Methods 0.000 description 19
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 18
- 239000000725 suspension Substances 0.000 description 17
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 16
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 16
- 239000000543 intermediate Substances 0.000 description 16
- GOTYRUGSSMKFNF-UHFFFAOYSA-N lenalidomide Chemical compound C1C=2C(N)=CC=CC=2C(=O)N1C1CCC(=O)NC1=O GOTYRUGSSMKFNF-UHFFFAOYSA-N 0.000 description 15
- 229960004942 lenalidomide Drugs 0.000 description 15
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 14
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 14
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical class CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 14
- 0 C*(C(C(C([C@@]1(*(C(c(c2c(c(O)c3O)O)c3I)(O)O)C2=O)O)(O)O)(O)O)=O)C1=O Chemical compound C*(C(C(C([C@@]1(*(C(c(c2c(c(O)c3O)O)c3I)(O)O)C2=O)O)(O)O)(O)O)=O)C1=O 0.000 description 13
- 239000011541 reaction mixture Substances 0.000 description 13
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 13
- XLYOFNOQVPJJNP-ZSJDYOACSA-N Heavy water Chemical compound [2H]O[2H] XLYOFNOQVPJJNP-ZSJDYOACSA-N 0.000 description 12
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 12
- 238000004296 chiral HPLC Methods 0.000 description 12
- 150000001975 deuterium Chemical group 0.000 description 12
- 230000004060 metabolic process Effects 0.000 description 12
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 11
- 238000009472 formulation Methods 0.000 description 11
- 238000005160 1H NMR spectroscopy Methods 0.000 description 10
- POFVJRKJJBFPII-UHFFFAOYSA-N N-cyclopentyl-5-[2-[[5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl]amino]-5-fluoropyrimidin-4-yl]-4-methyl-1,3-thiazol-2-amine Chemical compound C1(CCCC1)NC=1SC(=C(N=1)C)C1=NC(=NC=C1F)NC1=NC=C(C=C1)CN1CCN(CC1)CC POFVJRKJJBFPII-UHFFFAOYSA-N 0.000 description 10
- 125000004431 deuterium atom Chemical group 0.000 description 10
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 9
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 9
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 9
- PFKFTWBEEFSNDU-UHFFFAOYSA-N carbonyldiimidazole Chemical compound C1=CN=CN1C(=O)N1C=CN=C1 PFKFTWBEEFSNDU-UHFFFAOYSA-N 0.000 description 9
- 238000006243 chemical reaction Methods 0.000 description 9
- 230000000694 effects Effects 0.000 description 9
- 238000000926 separation method Methods 0.000 description 9
- IAZDPXIOMUYVGZ-WFGJKAKNSA-N Dimethyl sulfoxide Chemical compound [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H] IAZDPXIOMUYVGZ-WFGJKAKNSA-N 0.000 description 8
- 238000002347 injection Methods 0.000 description 8
- 239000007924 injection Substances 0.000 description 8
- 239000007788 liquid Substances 0.000 description 8
- 238000010992 reflux Methods 0.000 description 8
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 7
- 238000000576 coating method Methods 0.000 description 7
- 208000035475 disorder Diseases 0.000 description 7
- 239000002552 dosage form Substances 0.000 description 7
- OKKJLVBELUTLKV-VMNATFBRSA-N methanol-d1 Chemical compound [2H]OC OKKJLVBELUTLKV-VMNATFBRSA-N 0.000 description 7
- 239000002904 solvent Substances 0.000 description 7
- 239000000126 substance Substances 0.000 description 7
- 230000001225 therapeutic effect Effects 0.000 description 7
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 6
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 6
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 6
- PCLIMKBDDGJMGD-UHFFFAOYSA-N N-bromosuccinimide Chemical compound BrN1C(=O)CCC1=O PCLIMKBDDGJMGD-UHFFFAOYSA-N 0.000 description 6
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 6
- 125000001584 benzyloxycarbonyl group Chemical group C(=O)(OCC1=CC=CC=C1)* 0.000 description 6
- 150000001721 carbon Chemical group 0.000 description 6
- 239000003153 chemical reaction reagent Substances 0.000 description 6
- 238000004128 high performance liquid chromatography Methods 0.000 description 6
- 230000014759 maintenance of location Effects 0.000 description 6
- 239000000463 material Substances 0.000 description 6
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 6
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 6
- LOUPRKONTZGTKE-LHHVKLHASA-N quinidine Chemical compound C([C@H]([C@H](C1)C=C)C2)C[N@@]1[C@H]2[C@@H](O)C1=CC=NC2=CC=C(OC)C=C21 LOUPRKONTZGTKE-LHHVKLHASA-N 0.000 description 6
- 125000000217 alkyl group Chemical group 0.000 description 5
- 239000002775 capsule Substances 0.000 description 5
- 239000000969 carrier Substances 0.000 description 5
- 239000012043 crude product Substances 0.000 description 5
- 239000000706 filtrate Substances 0.000 description 5
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 5
- 230000002503 metabolic effect Effects 0.000 description 5
- 239000003921 oil Substances 0.000 description 5
- 210000000056 organ Anatomy 0.000 description 5
- 229920001223 polyethylene glycol Polymers 0.000 description 5
- 239000000047 product Substances 0.000 description 5
- 125000006239 protecting group Chemical group 0.000 description 5
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 5
- 238000005406 washing Methods 0.000 description 5
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical class OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 4
- 239000002202 Polyethylene glycol Substances 0.000 description 4
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 239000004480 active ingredient Substances 0.000 description 4
- 125000003118 aryl group Chemical group 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- MJSHDCCLFGOEIK-UHFFFAOYSA-N benzyl (2,5-dioxopyrrolidin-1-yl) carbonate Chemical compound O=C1CCC(=O)N1OC(=O)OCC1=CC=CC=C1 MJSHDCCLFGOEIK-UHFFFAOYSA-N 0.000 description 4
- 239000012267 brine Substances 0.000 description 4
- 239000004615 ingredient Substances 0.000 description 4
- PXZQEOJJUGGUIB-UHFFFAOYSA-N isoindolin-1-one Chemical group C1=CC=C2C(=O)NCC2=C1 PXZQEOJJUGGUIB-UHFFFAOYSA-N 0.000 description 4
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- 229910052757 nitrogen Inorganic materials 0.000 description 4
- 235000019198 oils Nutrition 0.000 description 4
- 239000012074 organic phase Substances 0.000 description 4
- 239000000546 pharmaceutical excipient Substances 0.000 description 4
- UYWQUFXKFGHYNT-UHFFFAOYSA-N phenylmethyl ester of formic acid Natural products O=COCC1=CC=CC=C1 UYWQUFXKFGHYNT-UHFFFAOYSA-N 0.000 description 4
- CNMOHEDUVVUVPP-UHFFFAOYSA-N piperidine-2,3-dione Chemical group O=C1CCCNC1=O CNMOHEDUVVUVPP-UHFFFAOYSA-N 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 230000002265 prevention Effects 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- NPWMTBZSRRLQNJ-UHFFFAOYSA-N pyroglutamine Chemical class NC1CCC(=O)NC1=O NPWMTBZSRRLQNJ-UHFFFAOYSA-N 0.000 description 4
- 229910052938 sodium sulfate Inorganic materials 0.000 description 4
- 235000011152 sodium sulphate Nutrition 0.000 description 4
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 4
- 208000024891 symptom Diseases 0.000 description 4
- 230000002194 synthesizing effect Effects 0.000 description 4
- 239000003826 tablet Substances 0.000 description 4
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 4
- 210000001519 tissue Anatomy 0.000 description 4
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 4
- 125000006552 (C3-C8) cycloalkyl group Chemical group 0.000 description 3
- SNTWKPAKVQFCCF-UHFFFAOYSA-N 2,3-dihydro-1h-triazole Chemical compound N1NC=CN1 SNTWKPAKVQFCCF-UHFFFAOYSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical class N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 3
- WPYMKLBDIGXBTP-UHFFFAOYSA-N Benzoic acid Natural products OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 3
- 101710088194 Dehydrogenase Proteins 0.000 description 3
- VEXZGXHMUGYJMC-DYCDLGHISA-N Deuterium chloride Chemical class [2H]Cl VEXZGXHMUGYJMC-DYCDLGHISA-N 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- BAWFJGJZGIEFAR-NNYOXOHSSA-N NAD zwitterion Chemical compound NC(=O)C1=CC=C[N+]([C@H]2[C@@H]([C@H](O)[C@@H](COP([O-])(=O)OP(O)(=O)OC[C@@H]3[C@H]([C@@H](O)[C@@H](O3)N3C4=NC=NC(N)=C4N=C3)O)O2)O)=C1 BAWFJGJZGIEFAR-NNYOXOHSSA-N 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 3
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 3
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 3
- 150000001408 amides Chemical class 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 238000013459 approach Methods 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- LOUPRKONTZGTKE-UHFFFAOYSA-N cinchonine Natural products C1C(C(C2)C=C)CCN2C1C(O)C1=CC=NC2=CC=C(OC)C=C21 LOUPRKONTZGTKE-UHFFFAOYSA-N 0.000 description 3
- 239000011248 coating agent Substances 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 239000002270 dispersing agent Substances 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- 235000019253 formic acid Nutrition 0.000 description 3
- 239000004220 glutamic acid Substances 0.000 description 3
- 150000004678 hydrides Chemical class 0.000 description 3
- 150000002431 hydrogen Chemical class 0.000 description 3
- 238000005984 hydrogenation reaction Methods 0.000 description 3
- 239000003112 inhibitor Substances 0.000 description 3
- 229910052500 inorganic mineral Inorganic materials 0.000 description 3
- 239000002502 liposome Substances 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 235000010755 mineral Nutrition 0.000 description 3
- 239000011707 mineral Substances 0.000 description 3
- 229950006238 nadide Drugs 0.000 description 3
- 229930027945 nicotinamide-adenine dinucleotide Natural products 0.000 description 3
- 150000007524 organic acids Chemical class 0.000 description 3
- 235000005985 organic acids Nutrition 0.000 description 3
- 238000010791 quenching Methods 0.000 description 3
- 230000000171 quenching effect Effects 0.000 description 3
- 229960001404 quinidine Drugs 0.000 description 3
- 239000011734 sodium Substances 0.000 description 3
- 229910000029 sodium carbonate Inorganic materials 0.000 description 3
- 238000006467 substitution reaction Methods 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- 239000000375 suspending agent Substances 0.000 description 3
- 231100000331 toxic Toxicity 0.000 description 3
- 230000002588 toxic effect Effects 0.000 description 3
- 230000009466 transformation Effects 0.000 description 3
- 239000003981 vehicle Substances 0.000 description 3
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 2
- BHKKSKOHRFHHIN-MRVPVSSYSA-N 1-[[2-[(1R)-1-aminoethyl]-4-chlorophenyl]methyl]-2-sulfanylidene-5H-pyrrolo[3,2-d]pyrimidin-4-one Chemical compound N[C@H](C)C1=C(CN2C(NC(C3=C2C=CN3)=O)=S)C=CC(=C1)Cl BHKKSKOHRFHHIN-MRVPVSSYSA-N 0.000 description 2
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 2
- 239000004342 Benzoyl peroxide Substances 0.000 description 2
- OMPJBNCRMGITSC-UHFFFAOYSA-N Benzoylperoxide Chemical compound C=1C=CC=CC=1C(=O)OOC(=O)C1=CC=CC=C1 OMPJBNCRMGITSC-UHFFFAOYSA-N 0.000 description 2
- 229920002261 Corn starch Polymers 0.000 description 2
- 102000004127 Cytokines Human genes 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N Glutamine Chemical class OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- MKXZASYAUGDDCJ-SZMVWBNQSA-N LSM-2525 Chemical compound C1CCC[C@H]2[C@@]3([H])N(C)CC[C@]21C1=CC(OC)=CC=C1C3 MKXZASYAUGDDCJ-SZMVWBNQSA-N 0.000 description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 2
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 2
- 240000007472 Leucaena leucocephala Species 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 2
- MZRVEZGGRBJDDB-UHFFFAOYSA-N N-Butyllithium Chemical compound [Li]CCCC MZRVEZGGRBJDDB-UHFFFAOYSA-N 0.000 description 2
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 2
- NCDNCNXCDXHOMX-UHFFFAOYSA-N Ritonavir Natural products C=1C=CC=CC=1CC(NC(=O)OCC=1SC=NC=1)C(O)CC(CC=1C=CC=CC=1)NC(=O)C(C(C)C)NC(=O)N(C)CC1=CSC(C(C)C)=N1 NCDNCNXCDXHOMX-UHFFFAOYSA-N 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 208000007502 anemia Diseases 0.000 description 2
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 2
- 235000019400 benzoyl peroxide Nutrition 0.000 description 2
- 235000019445 benzyl alcohol Nutrition 0.000 description 2
- 229920002988 biodegradable polymer Polymers 0.000 description 2
- 239000004621 biodegradable polymer Substances 0.000 description 2
- 239000003054 catalyst Substances 0.000 description 2
- 125000003636 chemical group Chemical group 0.000 description 2
- 238000004587 chromatography analysis Methods 0.000 description 2
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Natural products OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 2
- 238000002648 combination therapy Methods 0.000 description 2
- 239000008120 corn starch Substances 0.000 description 2
- 229940099112 cornstarch Drugs 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- VHUUQVKOLVNVRT-NSPFYZSMSA-N de170a Chemical compound [2H]O[2H].[2H]N([2H])[2H] VHUUQVKOLVNVRT-NSPFYZSMSA-N 0.000 description 2
- 238000010511 deprotection reaction Methods 0.000 description 2
- 229960001985 dextromethorphan Drugs 0.000 description 2
- AQEFLFZSWDEAIP-UHFFFAOYSA-N di-tert-butyl ether Chemical compound CC(C)(C)OC(C)(C)C AQEFLFZSWDEAIP-UHFFFAOYSA-N 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- 229940000406 drug candidate Drugs 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 238000003821 enantio-separation Methods 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 230000002708 enhancing effect Effects 0.000 description 2
- 238000006345 epimerization reaction Methods 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 150000004665 fatty acids Chemical class 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- 235000013922 glutamic acid Nutrition 0.000 description 2
- 125000005456 glyceride group Chemical group 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 239000000017 hydrogel Substances 0.000 description 2
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 239000008101 lactose Substances 0.000 description 2
- 150000002632 lipids Chemical class 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- QPJVMBTYPHYUOC-UHFFFAOYSA-N methyl benzoate Chemical compound COC(=O)C1=CC=CC=C1 QPJVMBTYPHYUOC-UHFFFAOYSA-N 0.000 description 2
- 239000002480 mineral oil Substances 0.000 description 2
- 235000010446 mineral oil Nutrition 0.000 description 2
- 150000007522 mineralic acids Chemical class 0.000 description 2
- 238000011294 monotherapeutic Methods 0.000 description 2
- SYSQUGFVNFXIIT-UHFFFAOYSA-N n-[4-(1,3-benzoxazol-2-yl)phenyl]-4-nitrobenzenesulfonamide Chemical class C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)NC1=CC=C(C=2OC3=CC=CC=C3N=2)C=C1 SYSQUGFVNFXIIT-UHFFFAOYSA-N 0.000 description 2
- 150000002828 nitro derivatives Chemical class 0.000 description 2
- 231100000252 nontoxic Toxicity 0.000 description 2
- 230000003000 nontoxic effect Effects 0.000 description 2
- 239000000346 nonvolatile oil Substances 0.000 description 2
- 239000003208 petroleum Substances 0.000 description 2
- 238000001050 pharmacotherapy Methods 0.000 description 2
- 235000021317 phosphate Nutrition 0.000 description 2
- 229920001983 poloxamer Polymers 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- NCDNCNXCDXHOMX-XGKFQTDJSA-N ritonavir Chemical compound N([C@@H](C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](CC=1C=CC=CC=1)NC(=O)OCC=1SC=NC=1)CC=1C=CC=CC=1)C(=O)N(C)CC1=CSC(C(C)C)=N1 NCDNCNXCDXHOMX-XGKFQTDJSA-N 0.000 description 2
- 229960000311 ritonavir Drugs 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- 230000028327 secretion Effects 0.000 description 2
- 239000000741 silica gel Substances 0.000 description 2
- 229910002027 silica gel Inorganic materials 0.000 description 2
- 238000009097 single-agent therapy Methods 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 239000001384 succinic acid Substances 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- VZGDMQKNWNREIO-UHFFFAOYSA-N tetrachloromethane Chemical compound ClC(Cl)(Cl)Cl VZGDMQKNWNREIO-UHFFFAOYSA-N 0.000 description 2
- 238000011200 topical administration Methods 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 238000000844 transformation Methods 0.000 description 2
- 238000003828 vacuum filtration Methods 0.000 description 2
- QYYZXEPEVBXNNA-QGZVFWFLSA-N (1R)-2-acetyl-N-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]-5-methylsulfonyl-1,3-dihydroisoindole-1-carboxamide Chemical compound C(C)(=O)N1[C@H](C2=CC=C(C=C2C1)S(=O)(=O)C)C(=O)NC1=CC=C(C=C1)C(C(F)(F)F)(C(F)(F)F)O QYYZXEPEVBXNNA-QGZVFWFLSA-N 0.000 description 1
- HFVMEOPYDLEHBR-UHFFFAOYSA-N (2-fluorophenyl)-phenylmethanol Chemical compound C=1C=CC=C(F)C=1C(O)C1=CC=CC=C1 HFVMEOPYDLEHBR-UHFFFAOYSA-N 0.000 description 1
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 1
- SCYULBFZEHDVBN-UHFFFAOYSA-N 1,1-Dichloroethane Chemical compound CC(Cl)Cl SCYULBFZEHDVBN-UHFFFAOYSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- 238000001644 13C nuclear magnetic resonance spectroscopy Methods 0.000 description 1
- HCSBTDBGTNZOAB-UHFFFAOYSA-N 2,3-dinitrobenzoic acid Chemical compound OC(=O)C1=CC=CC([N+]([O-])=O)=C1[N+]([O-])=O HCSBTDBGTNZOAB-UHFFFAOYSA-N 0.000 description 1
- CHHHXKFHOYLYRE-UHFFFAOYSA-M 2,4-Hexadienoic acid, potassium salt (1:1), (2E,4E)- Chemical compound [K+].CC=CC=CC([O-])=O CHHHXKFHOYLYRE-UHFFFAOYSA-M 0.000 description 1
- DPZHKLJPVMYFCU-UHFFFAOYSA-N 2-(5-bromopyridin-2-yl)acetonitrile Chemical compound BrC1=CC=C(CC#N)N=C1 DPZHKLJPVMYFCU-UHFFFAOYSA-N 0.000 description 1
- OZDAOHVKBFBBMZ-UHFFFAOYSA-N 2-aminopentanedioic acid;hydrate Chemical compound O.OC(=O)C(N)CCC(O)=O OZDAOHVKBFBBMZ-UHFFFAOYSA-N 0.000 description 1
- LEACJMVNYZDSKR-UHFFFAOYSA-N 2-octyldodecan-1-ol Chemical compound CCCCCCCCCCC(CO)CCCCCCCC LEACJMVNYZDSKR-UHFFFAOYSA-N 0.000 description 1
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 1
- 229940123106 24-hydroxylase inhibitor Drugs 0.000 description 1
- QBWKPGNFQQJGFY-QLFBSQMISA-N 3-[(1r)-1-[(2r,6s)-2,6-dimethylmorpholin-4-yl]ethyl]-n-[6-methyl-3-(1h-pyrazol-4-yl)imidazo[1,2-a]pyrazin-8-yl]-1,2-thiazol-5-amine Chemical compound N1([C@H](C)C2=NSC(NC=3C4=NC=C(N4C=C(C)N=3)C3=CNN=C3)=C2)C[C@H](C)O[C@H](C)C1 QBWKPGNFQQJGFY-QLFBSQMISA-N 0.000 description 1
- WHBMMWSBFZVSSR-UHFFFAOYSA-N 3-hydroxybutyric acid Chemical compound CC(O)CC(O)=O WHBMMWSBFZVSSR-UHFFFAOYSA-N 0.000 description 1
- ZYLMCRLXTUAWGM-UHFFFAOYSA-N 4-amino-3h-pteridin-2-one Chemical compound N1=CC=NC2=C(N)NC(=O)N=C21 ZYLMCRLXTUAWGM-UHFFFAOYSA-N 0.000 description 1
- QISOBCMNUJQOJU-UHFFFAOYSA-N 4-bromo-1h-pyrazole-5-carboxylic acid Chemical compound OC(=O)C=1NN=CC=1Br QISOBCMNUJQOJU-UHFFFAOYSA-N 0.000 description 1
- PXACTUVBBMDKRW-UHFFFAOYSA-N 4-bromobenzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=C(Br)C=C1 PXACTUVBBMDKRW-UHFFFAOYSA-N 0.000 description 1
- OBKXEAXTFZPCHS-UHFFFAOYSA-N 4-phenylbutyric acid Chemical compound OC(=O)CCCC1=CC=CC=C1 OBKXEAXTFZPCHS-UHFFFAOYSA-N 0.000 description 1
- PXRKCOCTEMYUEG-UHFFFAOYSA-N 5-aminoisoindole-1,3-dione Chemical compound NC1=CC=C2C(=O)NC(=O)C2=C1 PXRKCOCTEMYUEG-UHFFFAOYSA-N 0.000 description 1
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 1
- 206010054196 Affect lability Diseases 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 201000001320 Atherosclerosis Diseases 0.000 description 1
- IYHHRZBKXXKDDY-UHFFFAOYSA-N BI-605906 Chemical compound N=1C=2SC(C(N)=O)=C(N)C=2C(C(F)(F)CC)=CC=1N1CCC(S(C)(=O)=O)CC1 IYHHRZBKXXKDDY-UHFFFAOYSA-N 0.000 description 1
- 101000950981 Bacillus subtilis (strain 168) Catabolic NAD-specific glutamate dehydrogenase RocG Proteins 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- 102000004506 Blood Proteins Human genes 0.000 description 1
- 108010017384 Blood Proteins Proteins 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- 125000000041 C6-C10 aryl group Chemical group 0.000 description 1
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 1
- KXDHJXZQYSOELW-UHFFFAOYSA-N Carbamic acid Chemical group NC(O)=O KXDHJXZQYSOELW-UHFFFAOYSA-N 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 229940126657 Compound 17 Drugs 0.000 description 1
- 208000032170 Congenital Abnormalities Diseases 0.000 description 1
- 206010010356 Congenital anomaly Diseases 0.000 description 1
- 108010001237 Cytochrome P-450 CYP2D6 Proteins 0.000 description 1
- 208000007220 Cytochrome P-450 CYP2D6 Inhibitors Diseases 0.000 description 1
- 208000003311 Cytochrome P-450 Enzyme Inhibitors Diseases 0.000 description 1
- 108010015742 Cytochrome P-450 Enzyme System Proteins 0.000 description 1
- 102000002004 Cytochrome P-450 Enzyme System Human genes 0.000 description 1
- 102100021704 Cytochrome P450 2D6 Human genes 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- PHOQVHQSTUBQQK-SQOUGZDYSA-N D-glucono-1,5-lactone Chemical compound OC[C@H]1OC(=O)[C@H](O)[C@@H](O)[C@@H]1O PHOQVHQSTUBQQK-SQOUGZDYSA-N 0.000 description 1
- AEMOLEFTQBMNLQ-AQKNRBDQSA-N D-glucopyranuronic acid Chemical compound OC1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O AEMOLEFTQBMNLQ-AQKNRBDQSA-N 0.000 description 1
- 206010051055 Deep vein thrombosis Diseases 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 229920005682 EO-PO block copolymer Polymers 0.000 description 1
- 241000792859 Enema Species 0.000 description 1
- 208000010228 Erectile Dysfunction Diseases 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 description 1
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 102000016901 Glutamate dehydrogenase Human genes 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- AEMRFAOFKBGASW-UHFFFAOYSA-M Glycolate Chemical compound OCC([O-])=O AEMRFAOFKBGASW-UHFFFAOYSA-M 0.000 description 1
- 208000031886 HIV Infections Diseases 0.000 description 1
- 208000037357 HIV infectious disease Diseases 0.000 description 1
- 102000008100 Human Serum Albumin Human genes 0.000 description 1
- 108091006905 Human Serum Albumin Proteins 0.000 description 1
- 206010061598 Immunodeficiency Diseases 0.000 description 1
- 208000029462 Immunodeficiency disease Diseases 0.000 description 1
- OWYWGLHRNBIFJP-UHFFFAOYSA-N Ipazine Chemical compound CCN(CC)C1=NC(Cl)=NC(NC(C)C)=N1 OWYWGLHRNBIFJP-UHFFFAOYSA-N 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- 229940122696 MAP kinase inhibitor Drugs 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-L Malonate Chemical compound [O-]C(=O)CC([O-])=O OFOBLEOULBTSOW-UHFFFAOYSA-L 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 1
- BZLVMXJERCGZMT-UHFFFAOYSA-N Methyl tert-butyl ether Chemical compound COC(C)(C)C BZLVMXJERCGZMT-UHFFFAOYSA-N 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 239000005642 Oleic acid Substances 0.000 description 1
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 1
- 208000002193 Pain Diseases 0.000 description 1
- 208000030852 Parasitic disease Diseases 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-L Phosphate ion(2-) Chemical compound OP([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-L 0.000 description 1
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 description 1
- 229920001214 Polysorbate 60 Polymers 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 102000007327 Protamines Human genes 0.000 description 1
- 108010007568 Protamines Proteins 0.000 description 1
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 1
- 208000010378 Pulmonary Embolism Diseases 0.000 description 1
- 229910006124 SOCl2 Inorganic materials 0.000 description 1
- HVUMOYIDDBPOLL-XWVZOOPGSA-N Sorbitan monostearate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O HVUMOYIDDBPOLL-XWVZOOPGSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N Valeric acid Natural products CCCCC(O)=O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 1
- 206010047249 Venous thrombosis Diseases 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- ZZXDRXVIRVJQBT-UHFFFAOYSA-M Xylenesulfonate Chemical compound CC1=CC=CC(S([O-])(=O)=O)=C1C ZZXDRXVIRVJQBT-UHFFFAOYSA-M 0.000 description 1
- XJLXINKUBYWONI-DQQFMEOOSA-N [[(2r,3r,4r,5r)-5-(6-aminopurin-9-yl)-3-hydroxy-4-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(2s,3r,4s,5s)-5-(3-carbamoylpyridin-1-ium-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphate Chemical group NC(=O)C1=CC=C[N+]([C@@H]2[C@H]([C@@H](O)[C@H](COP([O-])(=O)OP(O)(=O)OC[C@@H]3[C@H]([C@@H](OP(O)(O)=O)[C@@H](O3)N3C4=NC=NC(N)=C4N=C3)O)O2)O)=C1 XJLXINKUBYWONI-DQQFMEOOSA-N 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 229940124532 absorption promoter Drugs 0.000 description 1
- IPBVNPXQWQGGJP-UHFFFAOYSA-N acetic acid phenyl ester Natural products CC(=O)OC1=CC=CC=C1 IPBVNPXQWQGGJP-UHFFFAOYSA-N 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- GZCGUPFRVQAUEE-SLPGGIOYSA-N aldehydo-D-glucose Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O GZCGUPFRVQAUEE-SLPGGIOYSA-N 0.000 description 1
- 229960005310 aldesleukin Drugs 0.000 description 1
- 108700025316 aldesleukin Proteins 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- CEGOLXSVJUTHNZ-UHFFFAOYSA-K aluminium tristearate Chemical compound [Al+3].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CEGOLXSVJUTHNZ-UHFFFAOYSA-K 0.000 description 1
- 229940063655 aluminum stearate Drugs 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 150000003863 ammonium salts Chemical class 0.000 description 1
- 239000003708 ampul Substances 0.000 description 1
- 230000001772 anti-angiogenic effect Effects 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 229940034982 antineoplastic agent Drugs 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 239000011260 aqueous acid Substances 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- 239000010425 asbestos Substances 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 235000013871 bee wax Nutrition 0.000 description 1
- 239000012166 beeswax Substances 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- HSDAJNMJOMSNEV-UHFFFAOYSA-N benzyl chloroformate Chemical compound ClC(=O)OCC1=CC=CC=C1 HSDAJNMJOMSNEV-UHFFFAOYSA-N 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 230000007698 birth defect Effects 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 125000001246 bromo group Chemical group Br* 0.000 description 1
- DQXBYHZEEUGOBF-UHFFFAOYSA-N but-3-enoic acid;ethene Chemical compound C=C.OC(=O)CC=C DQXBYHZEEUGOBF-UHFFFAOYSA-N 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-N carbonic acid Chemical compound OC(O)=O BVKZGUZCCUSVTD-UHFFFAOYSA-N 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 210000004027 cell Anatomy 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 210000003169 central nervous system Anatomy 0.000 description 1
- 208000015114 central nervous system disease Diseases 0.000 description 1
- 229940081733 cetearyl alcohol Drugs 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 150000005829 chemical entities Chemical class 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- KVSASDOGYIBWTA-UHFFFAOYSA-N chloro benzoate Chemical compound ClOC(=O)C1=CC=CC=C1 KVSASDOGYIBWTA-UHFFFAOYSA-N 0.000 description 1
- 125000001309 chloro group Chemical group Cl* 0.000 description 1
- IJOOHPMOJXWVHK-UHFFFAOYSA-N chlorotrimethylsilane Chemical group C[Si](C)(C)Cl IJOOHPMOJXWVHK-UHFFFAOYSA-N 0.000 description 1
- 229960001076 chlorpromazine Drugs 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- 239000008199 coating composition Substances 0.000 description 1
- 229940110456 cocoa butter Drugs 0.000 description 1
- 235000019868 cocoa butter Nutrition 0.000 description 1
- 239000008119 colloidal silica Substances 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 239000012230 colorless oil Substances 0.000 description 1
- 229940125810 compound 20 Drugs 0.000 description 1
- 229940125846 compound 25 Drugs 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229940111134 coxibs Drugs 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 239000003255 cyclooxygenase 2 inhibitor Substances 0.000 description 1
- 230000002559 cytogenic effect Effects 0.000 description 1
- GHVNFZFCNZKVNT-UHFFFAOYSA-M decanoate Chemical compound CCCCCCCCCC([O-])=O GHVNFZFCNZKVNT-UHFFFAOYSA-M 0.000 description 1
- GHVNFZFCNZKVNT-UHFFFAOYSA-N decanoic acid Chemical compound CCCCCCCCCC(O)=O GHVNFZFCNZKVNT-UHFFFAOYSA-N 0.000 description 1
- 230000002939 deleterious effect Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- WYACBZDAHNBPPB-UHFFFAOYSA-N diethyl oxalate Chemical compound CCOC(=O)C(=O)OCC WYACBZDAHNBPPB-UHFFFAOYSA-N 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-M dihydrogenphosphate Chemical compound OP(O)([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-M 0.000 description 1
- GXGAKHNRMVGRPK-UHFFFAOYSA-N dimagnesium;dioxido-bis[[oxido(oxo)silyl]oxy]silane Chemical compound [Mg+2].[Mg+2].[O-][Si](=O)O[Si]([O-])([O-])O[Si]([O-])=O GXGAKHNRMVGRPK-UHFFFAOYSA-N 0.000 description 1
- XPPKVPWEQAFLFU-UHFFFAOYSA-J diphosphate(4-) Chemical compound [O-]P([O-])(=O)OP([O-])([O-])=O XPPKVPWEQAFLFU-UHFFFAOYSA-J 0.000 description 1
- 235000011180 diphosphates Nutrition 0.000 description 1
- ZPWVASYFFYYZEW-UHFFFAOYSA-L dipotassium hydrogen phosphate Chemical compound [K+].[K+].OP([O-])([O-])=O ZPWVASYFFYYZEW-UHFFFAOYSA-L 0.000 description 1
- 229910000396 dipotassium phosphate Inorganic materials 0.000 description 1
- 235000019797 dipotassium phosphate Nutrition 0.000 description 1
- BNIILDVGGAEEIG-UHFFFAOYSA-L disodium hydrogen phosphate Chemical compound [Na+].[Na+].OP([O-])([O-])=O BNIILDVGGAEEIG-UHFFFAOYSA-L 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 238000009510 drug design Methods 0.000 description 1
- 230000001729 effect on metabolism Effects 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 239000008387 emulsifying waxe Substances 0.000 description 1
- 239000007920 enema Substances 0.000 description 1
- 229940095399 enema Drugs 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- 239000005038 ethylene vinyl acetate Substances 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 235000019197 fats Nutrition 0.000 description 1
- 239000012065 filter cake Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 125000001153 fluoro group Chemical group F* 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 150000002303 glucose derivatives Chemical class 0.000 description 1
- 150000002307 glutamic acids Chemical class 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 1
- 229960002449 glycine Drugs 0.000 description 1
- JAXFJECJQZDFJS-XHEPKHHKSA-N gtpl8555 Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@@H]1C(=O)N[C@H](B1O[C@@]2(C)[C@H]3C[C@H](C3(C)C)C[C@H]2O1)CCC1=CC=C(F)C=C1 JAXFJECJQZDFJS-XHEPKHHKSA-N 0.000 description 1
- 231100000226 haematotoxicity Toxicity 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 208000034737 hemoglobinopathy Diseases 0.000 description 1
- MNWFXJYAOYHMED-UHFFFAOYSA-N heptanoic acid Chemical compound CCCCCCC(O)=O MNWFXJYAOYHMED-UHFFFAOYSA-N 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 208000033519 human immunodeficiency virus infectious disease Diseases 0.000 description 1
- 238000007327 hydrogenolysis reaction Methods 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-M hydrogensulfate Chemical compound OS([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-M 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000007813 immunodeficiency Effects 0.000 description 1
- 239000002955 immunomodulating agent Substances 0.000 description 1
- 229940121354 immunomodulator Drugs 0.000 description 1
- 230000002584 immunomodulator Effects 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 201000001881 impotence Diseases 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 229910052738 indium Inorganic materials 0.000 description 1
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 229940102223 injectable solution Drugs 0.000 description 1
- 229940102213 injectable suspension Drugs 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 125000002346 iodo group Chemical group I* 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- KQNPFQTWMSNSAP-UHFFFAOYSA-N isobutyric acid Chemical compound CC(C)C(O)=O KQNPFQTWMSNSAP-UHFFFAOYSA-N 0.000 description 1
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 238000004811 liquid chromatography Methods 0.000 description 1
- 239000006210 lotion Substances 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 208000002780 macular degeneration Diseases 0.000 description 1
- 239000000391 magnesium silicate Substances 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 229910000386 magnesium trisilicate Inorganic materials 0.000 description 1
- 229940099273 magnesium trisilicate Drugs 0.000 description 1
- 235000019793 magnesium trisilicate Nutrition 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-M mandelate Chemical compound [O-]C(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-M 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 125000005341 metaphosphate group Chemical group 0.000 description 1
- FCGIVHSBEKGQMZ-BFWBPSQCSA-N methyl 2-[bromo(dideuterio)methyl]-3-nitrobenzoate Chemical compound [2H]C([2H])(Br)C1=C(C(=O)OC)C=CC=C1[N+]([O-])=O FCGIVHSBEKGQMZ-BFWBPSQCSA-N 0.000 description 1
- CRZGFIMLHZTLGT-UHFFFAOYSA-N methyl 2-methyl-3-nitrobenzoate Chemical compound COC(=O)C1=CC=CC([N+]([O-])=O)=C1C CRZGFIMLHZTLGT-UHFFFAOYSA-N 0.000 description 1
- CRZGFIMLHZTLGT-FIBGUPNXSA-N methyl 3-nitro-2-(trideuteriomethyl)benzoate Chemical compound [2H]C([2H])([2H])C1=C(C(=O)OC)C=CC=C1[N+]([O-])=O CRZGFIMLHZTLGT-FIBGUPNXSA-N 0.000 description 1
- IZYBEMGNIUSSAX-UHFFFAOYSA-N methyl benzenecarboperoxoate Chemical compound COOC(=O)C1=CC=CC=C1 IZYBEMGNIUSSAX-UHFFFAOYSA-N 0.000 description 1
- 229940095102 methyl benzoate Drugs 0.000 description 1
- XMJHPCRAQCTCFT-UHFFFAOYSA-N methyl chloroformate Chemical compound COC(Cl)=O XMJHPCRAQCTCFT-UHFFFAOYSA-N 0.000 description 1
- 229940042472 mineral oil Drugs 0.000 description 1
- 239000002829 mitogen activated protein kinase inhibitor Substances 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- PSZYNBSKGUBXEH-UHFFFAOYSA-N naphthalene-1-sulfonic acid Chemical compound C1=CC=C2C(S(=O)(=O)O)=CC=CC2=C1 PSZYNBSKGUBXEH-UHFFFAOYSA-N 0.000 description 1
- KVBGVZZKJNLNJU-UHFFFAOYSA-N naphthalene-2-sulfonic acid Chemical compound C1=CC=CC2=CC(S(=O)(=O)O)=CC=C21 KVBGVZZKJNLNJU-UHFFFAOYSA-N 0.000 description 1
- 239000007922 nasal spray Substances 0.000 description 1
- 208000004235 neutropenia Diseases 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 1
- 231100000344 non-irritating Toxicity 0.000 description 1
- ZWLPBLYKEWSWPD-UHFFFAOYSA-N o-toluic acid Chemical class CC1=CC=CC=C1C(O)=O ZWLPBLYKEWSWPD-UHFFFAOYSA-N 0.000 description 1
- GLDOVTGHNKAZLK-UHFFFAOYSA-N octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCO GLDOVTGHNKAZLK-UHFFFAOYSA-N 0.000 description 1
- WWZKQHOCKIZLMA-UHFFFAOYSA-M octanoate Chemical compound CCCCCCCC([O-])=O WWZKQHOCKIZLMA-UHFFFAOYSA-M 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 239000012044 organic layer Substances 0.000 description 1
- 102000002574 p38 Mitogen-Activated Protein Kinases Human genes 0.000 description 1
- 108010068338 p38 Mitogen-Activated Protein Kinases Proteins 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 235000010603 pastilles Nutrition 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- DYUMLJSJISTVPV-UHFFFAOYSA-N phenyl propanoate Chemical compound CCC(=O)OC1=CC=CC=C1 DYUMLJSJISTVPV-UHFFFAOYSA-N 0.000 description 1
- 229940049953 phenylacetate Drugs 0.000 description 1
- WLJVXDMOQOGPHL-UHFFFAOYSA-N phenylacetic acid Chemical compound OC(=O)CC1=CC=CC=C1 WLJVXDMOQOGPHL-UHFFFAOYSA-N 0.000 description 1
- 229950009215 phenylbutanoic acid Drugs 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- XNGIFLGASWRNHJ-UHFFFAOYSA-L phthalate(2-) Chemical compound [O-]C(=O)C1=CC=CC=C1C([O-])=O XNGIFLGASWRNHJ-UHFFFAOYSA-L 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- KNCYXPMJDCCGSJ-UHFFFAOYSA-N piperidine-2,6-dione Chemical compound O=C1CCCC(=O)N1 KNCYXPMJDCCGSJ-UHFFFAOYSA-N 0.000 description 1
- 230000036470 plasma concentration Effects 0.000 description 1
- 229960000502 poloxamer Drugs 0.000 description 1
- 229920001200 poly(ethylene-vinyl acetate) Polymers 0.000 description 1
- 229920000747 poly(lactic acid) Polymers 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 239000004632 polycaprolactone Substances 0.000 description 1
- 229920001610 polycaprolactone Polymers 0.000 description 1
- 239000004626 polylactic acid Substances 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 239000001818 polyoxyethylene sorbitan monostearate Substances 0.000 description 1
- 235000010989 polyoxyethylene sorbitan monostearate Nutrition 0.000 description 1
- 229920002503 polyoxyethylene-polyoxypropylene Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229940113124 polysorbate 60 Drugs 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 231100000683 possible toxicity Toxicity 0.000 description 1
- 239000004302 potassium sorbate Substances 0.000 description 1
- 235000010241 potassium sorbate Nutrition 0.000 description 1
- 229940069338 potassium sorbate Drugs 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 238000002953 preparative HPLC Methods 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 230000000770 proinflammatory effect Effects 0.000 description 1
- KCXFHTAICRTXLI-UHFFFAOYSA-N propane-1-sulfonic acid Chemical compound CCCS(O)(=O)=O KCXFHTAICRTXLI-UHFFFAOYSA-N 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- UORVCLMRJXCDCP-UHFFFAOYSA-M propynoate Chemical compound [O-]C(=O)C#C UORVCLMRJXCDCP-UHFFFAOYSA-M 0.000 description 1
- 229950008679 protamine sulfate Drugs 0.000 description 1
- 238000000425 proton nuclear magnetic resonance spectrum Methods 0.000 description 1
- 150000003214 pyranose derivatives Chemical group 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 229940100618 rectal suppository Drugs 0.000 description 1
- 239000006215 rectal suppository Substances 0.000 description 1
- 210000000664 rectum Anatomy 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 208000037803 restenosis Diseases 0.000 description 1
- 229910052895 riebeckite Inorganic materials 0.000 description 1
- YGSDEFSMJLZEOE-UHFFFAOYSA-M salicylate Chemical compound OC1=CC=CC=C1C([O-])=O YGSDEFSMJLZEOE-UHFFFAOYSA-M 0.000 description 1
- 229940116351 sebacate Drugs 0.000 description 1
- CXMXRPHRNRROMY-UHFFFAOYSA-L sebacate(2-) Chemical compound [O-]C(=O)CCCCCCCCC([O-])=O CXMXRPHRNRROMY-UHFFFAOYSA-L 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 239000012354 sodium borodeuteride Substances 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- AKHNMLFCWUSKQB-UHFFFAOYSA-L sodium thiosulfate Chemical class [Na+].[Na+].[O-]S([O-])(=O)=S AKHNMLFCWUSKQB-UHFFFAOYSA-L 0.000 description 1
- 230000003381 solubilizing effect Effects 0.000 description 1
- 238000000527 sonication Methods 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 239000001587 sorbitan monostearate Substances 0.000 description 1
- 235000011076 sorbitan monostearate Nutrition 0.000 description 1
- 229940035048 sorbitan monostearate Drugs 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- TYFQFVWCELRYAO-UHFFFAOYSA-L suberate(2-) Chemical compound [O-]C(=O)CCCCCCC([O-])=O TYFQFVWCELRYAO-UHFFFAOYSA-L 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 1
- YBBRCQOCSYXUOC-UHFFFAOYSA-N sulfuryl dichloride Chemical compound ClS(Cl)(=O)=O YBBRCQOCSYXUOC-UHFFFAOYSA-N 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 238000013269 sustained drug release Methods 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- KKEYFWRCBNTPAC-UHFFFAOYSA-L terephthalate(2-) Chemical compound [O-]C(=O)C1=CC=C(C([O-])=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-L 0.000 description 1
- RKSOPLXZQNSWAS-UHFFFAOYSA-N tert-butyl bromide Chemical compound CC(C)(C)Br RKSOPLXZQNSWAS-UHFFFAOYSA-N 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- MHXBHWLGRWOABW-UHFFFAOYSA-N tetradecyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCCCCCCCCCCCCCC MHXBHWLGRWOABW-UHFFFAOYSA-N 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 206010043554 thrombocytopenia Diseases 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- 235000012431 wafers Nutrition 0.000 description 1
- 238000010792 warming Methods 0.000 description 1
- 239000008215 water for injection Substances 0.000 description 1
- 239000001993 wax Substances 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 210000002268 wool Anatomy 0.000 description 1
- 229940071104 xylenesulfonate Drugs 0.000 description 1
- 150000003751 zinc Chemical class 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
Definitions
- ADME absorption, distribution, metabolism and/or excretion
- a metabolic inhibitor will be co-administered with a drug that is cleared too rapidly.
- a drug that is cleared too rapidly.
- the FDA recommends that these drugs be co-dosed with ritonavir, an inhibitor of cytochrome P450 enzyme 3A4 (CYP3A4), the enzyme typically responsible for their metabolism (see Kempf, D.J. et al., Antimicrobial agents and chemotherapy, 1997, 41(3): 654-60).
- CYP3A4 cytochrome P450 enzyme 3A4
- Ritonavir causes adverse effects and adds to the pill burden for HIV patients who must already take a combination of different drugs.
- the FDA recommends that these drugs be co-dosed with ritonavir, an inhibitor of cytochrome P450 enzyme 3A4 (CYP3A4), the enzyme typically responsible for their metabolism (see Kempf, D.J. et al., Antimicrobial agents and chemotherapy, 1997, 41(3): 654-60).
- Ritonavir causes adverse effects and adds
- CYP2D6 inhibitor quinidine has been added to dextromethorphan for the purpose of reducing rapid CYP2D6 metabolism of dextromethorphan in a treatment of pseudobulbar affect.
- a potentially attractive strategy for improving a drug's metabolic properties is deuterium modification.
- Deuterium is a safe, stable, non-radioactive isotope of hydrogen. Compared to hydrogen, deuterium forms stronger bonds with carbon. In select cases, the increased bond strength imparted by deuterium can positively impact the ADME properties of a drug, creating the potential for improved drug efficacy, safety, and/or tolerability. At the same time, because the size and shape of deuterium are essentially identical to those of hydrogen, replacement of hydrogen by deuterium would not be expected to affect the biochemical potency and selectivity of the drug as compared to the original chemical entity that contains only hydrogen.
- This invention relates to novel substituted dioxopiperidinyl phthalimide derivatives and pharmaceutically acceptable salts thereof.
- the invention also provides compositions comprising a compound of this invention and the use of such compositions in methods of treating diseases and conditions beneficially treated by an immunomodulatory agent.
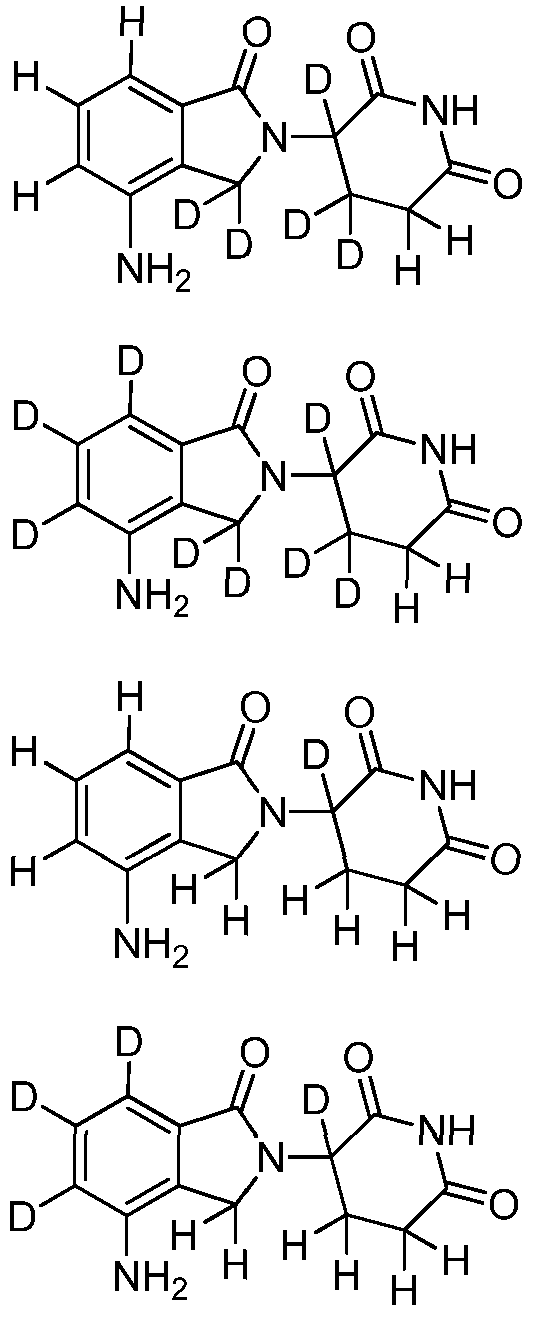
- Lenalidomide chemically known as either 3-(4-amino-l,3-dihydro-l-oxo-2H- isoindol-2-yl)-2,6-piperidinedione or 3-(4-amino-l-oxo l,3-dihydro-2H-isoindol-2- yl)piperidine-2,6-dione, and its pharmaceutically acceptable salts thereof are disclosed as immunomodulatory agents.
- Lenalidomide has been shown to inhibit the secretion of pro- inflammatory cytokines such as tumor necrosis factor alpha (TNF-a) and to increase the secretion of anti-inflammatory cytokines in animals and humans.
- TNF-a tumor necrosis factor alpha
- TNF-a levels is a valuable therapeutic strategy for the treatment of many inflammatory, infectious, immunological, and malignant diseases (PCT publication WO 98/03502).
- Lenalidomide has been demonstrated to be useful in the treatment of anemia due to myelodysplastic syndromes associated with a deletion 5q cytogenic abnormality, as well as in the treatment of multiple myeloma when used in combination with dexamethasone.
- Lenalidomide is also in clinical trials, alone or in combination with other therapeutic agents, for the treatment of Non-Hodgkins lymphoma; papillary and follicular thyroid carcinoma; prostate cancer; chronic lymphocytic leukemia, amyloidosis, complex regional pain syndrome Type I, malignant melanoma, radiculopathy, myelofibrosis, glioblastoma, gliosarcoma, malignant gliomas, myelogenous leukemia, refractory plasma cell neoplasm, chronic myelomonocytic leukemia, follicular lymphoma, ciliary body and chronic melanoma, iris melanoma, recurrent interocular melanoma, extraocular extension melanoma, solid tumors, T-cell lymphoma, erythroid lymphoma, monoblastic and monocytic leukemia;
- myeloid leukemia brain tumor, meningioma, spinal cord tumors, thyroid cancers, mantle cell lymphoma, non-small cell lung cancer, ovarian cancer, prostate cancer, renal cell cancer, myelofibrosis, Burkitt's lymphoma, Hodgkin's lymphoma, large cell lymphoma, and
- Lenalidomide is associated with significant potential toxicities, which include human birth defects; neutropenia; thrombocytopenia; deep vein thrombosis; and pulmonary embolism. See (http://www.fda.gov/cder/foi/label/2006/021880s001.pdf). A majority of patients taking lenalidomide required a dose delay or reduction during clinical trials due to hematologic toxicities. No clinical studies were performed to assess the relationship between exposure and safety.
- ameliorate and “treat” are used interchangeably and include both therapeutic and prophylactic treatment. Both terms mean decrease, suppress, attenuate, diminish, arrest, or stabilize the development or progression of a disease (e.g., a disease or disorder delineated herein), lessen the severity of the disease or improve the symptoms associated with the disease.
- a disease e.g., a disease or disorder delineated herein
- Disease is meant any condition or disorder that damage or interferes with the normal function of a cell, tissue, or organ.
- any atom not specifically designated as a particular isotope is meant to represent any stable isotope of that atom.
- a position is designated specifically as “H” or “hydrogen”
- the position is understood to have hydrogen at its natural abundance isotopic composition.
- a position is designated specifically as “D” or “deuterium”
- the position is understood to have deuterium at an abundance that is at least 3340 times greater than the natural abundance of deuterium, which is 0.015% (i.e., at least 50.1% incorporation of deuterium).
- isotopic enrichment factor means the ratio between the isotopic abundance and the natural abundance of a specified isotope.
- a compound of this invention has an isotopic enrichment factor for each designated deuterium atom of at least 3500 (52.5% deuterium incorporation at each designated deuterium atom), at least 4000 (60% deuterium incorporation), at least 4500 (67.5% deuterium incorporation), at least 5000 (75% deuterium), at least 5500 (82.5% deuterium incorporation), at least 6000 (90% deuterium incorporation), at least 6333.3 (95% deuterium incorporation), at least 6466.7 (97% deuterium incorporation), at least 6600 (99% deuterium incorporation), or at least 6633.3 (99.5% deuterium incorporation).
- isotopologue refers to a species that differs from a specific compound of this invention only in the isotopic composition thereof.
- a compound represented by a particular chemical structure containing indicated deuterium atoms will also contain lesser amounts of isotopologues having hydrogen atoms at one or more of the designated deuterium positions in that structure.
- the relative amount of such isotopologues in a compound of this invention will depend upon a number of factors including the isotopic purity of deuterated reagents used to make the compound and the efficiency of incorporation of deuterium in the various synthesis steps used to prepare the compound.
- the relative amount of such isotopologues in toto will be less than 49.9% of the compound. In other embodiments, the relative amount of such isotopologues in toto will be less than 47.5%, less than 40%, less than 32.5%, less than 25%, less than 17.5%, less than 10%, less than 5%, less than 3%, less than 1%, or less than 0.5% of the compound.
- the invention also provides salts of the compounds of the invention.
- a salt of a compound of this invention is formed between an acid and a basic group of the compound, such as an amino functional group, or a base and an acidic group of the compound, such as a carboxyl functional group. According to another preferred
- the compound is a pharmaceutically acceptable acid addition salt.
- pharmaceutically acceptable refers to a component that is, within the scope of sound medical judgment, suitable for use in contact with the tissues of humans and other mammals without undue toxicity, irritation, allergic response and the like, and are commensurate with a reasonable benefit/risk ratio.
- pharmaceutically acceptable salt means any non-toxic salt that, upon administration to a recipient, is capable of providing, either directly or indirectly, a compound of this invention.
- pharmaceutically acceptable counterion is an ionic portion of a salt that is not toxic when released from the salt upon administration to a recipient.
- Acids commonly employed to form pharmaceutically acceptable salts include inorganic acids such as hydrogen bisulfide, hydrochloric, hydrobromic, hydroiodic, sulfuric and phosphoric acid, as well as organic acids such as para-toluenesulfonic, salicylic, tartaric, bitartaric, ascorbic, maleic, besylic, fumaric, gluconic, glucuronic, formic, glutamic, methanesulfonic, ethanesulfonic, benzenesulfonic, lactic, oxalic, para-bromophenylsulfonic, carbonic, succinic, citric, benzoic and acetic acid, and related inorganic and organic acids.
- inorganic acids such as hydrogen bisulfide, hydrochloric, hydrobromic, hydroiodic, sulfuric and phosphoric acid
- organic acids such as para-toluenesulfonic, salicylic, tartaric, bitartaric, as
- Such pharmaceutically acceptable salts thus include sulfate, pyrosulfate, bisulfate, sulfite, bisulfite, phosphate, monohydrogenphosphate, dihydrogenphosphate, metaphosphate, pyrophosphate, chloride, bromide, iodide, acetate, propionate, decanoate, caprylate, acrylate, formate, isobutyrate, caprate, heptanoate, propiolate, oxalate, malonate, succinate, suberate, sebacate, fumarate, maleate, butyne-l,4-dioate, hexyne-l,6-dioate, benzoate, chlorobenzoate, methylbenzoate, dinitrobenzoate, hydroxybenzoate, methoxybenzoate, phthalate,
- terephthalate sulfonate, xylenesulfonate, phenylacetate, phenylpropionate, phenylbutyrate, citrate, lactate, ⁇ -hydroxybutyrate, glycolate, maleate, tartrate, methanesulfonate,
- Preferred pharmaceutically acceptable acid addition salts include those formed with mineral acids such as hydrochloric acid and hydrobromic acid, and especially those formed with organic acids such as maleic acid.
- the compounds of the present invention contain one or more asymmetric carbon atoms.
- a compound of this invention can exist as the individual enantiomers as well a mixture of enantiomers. Accordingly, a compound of the present invention will include not only a racemic mixture, but also individual respective enantiomers substantially free of other enantiomers.
- the term "substantially free of other enantiomers” as used herein means less than 25% of other enantiomers, preferably less than 10% of other enantiomers, more preferably less than 5% of other enantiomers and most preferably less than 2% of other enantiomers are present.
- stable compounds refers to compounds which possess stability sufficient to allow manufacture and which maintain the integrity of the compound for a sufficient period of time to be useful for the purposes detailed herein (e.g., formulation into therapeutic products, intermediates for use in production of therapeutic compounds, isolatable or storable intermediate compounds, treating a disease or condition responsive to therapeutic agents).
- D refers to deuterium.
- Stepoisomer refers to both enantiomers and diastereomers.
- Tet " £ ", and "t-” each refer to tertiary.
- US refers to the United States of America.
- each Y means, all “Y” groups (e.g., Y 1 and Y 2 ), all “Z” groups (e.g., Z 1 , Z 2 , Z 3 , Z 4 and Z 5 ), and all “W” groups (e.g., W 1 , W 2 , W 3 and W 4 ), respectively.
- the present invention provides a compound of Formula
- each W is independently selected from hydrogen and deuterium
- each Y is independently selected from hydrogen and deuterium
- each Z is independently selected from hydrogen and deuterium
- At least one W, one Y, or one Z is deuterium.
- Z 5 is deuterium.
- Z 3 and Z 4 are each hydrogen.
- Z 3 and Z 4 are each deuterium.
- Z 1 and Z 2 are each hydrogen.
- Z 1 and Z 2 are each deuterium.
- Z 3 and Z 4 are each hydrogen.
- Z 5 is deuterium
- Z 3 and Z 4 are each deuterium
- Z 1 and Z2 are each hydrogen
- W 1 , W2 and W 3 are each hydrogen or each deuterium.
- Z 5 is deuterium
- Z 3 and Z 4 are each deuterium
- Z 1 and Z2 are each deuterium
- W 1 , W2 and W 3 are each hydrogen or each deuterium.
- Z 1 , Z 2 , Z 3 and Z 4 are each hydrogen; and W 1 , W2 and W 3 are each hydrogen or each deuterium.
- W 1 , W 2 and W 3 are the same. In one aspect of this embodiment and W 3 are simultaneously deuterium. In another aspect of this embodiment W 1 , W2 and W are simultaneously hydrogen.
- Z 2 , Z 3 , Z 4 and Z 5 are simultaneously deuteriums and W 1 , W 2 and W 3 are simultaneously hydrogen.
- any atom not designated as deuterium in any of the embodiments set forth above is present at its natural isotopic abundance.
- Z 5 is deuterium.
- Z 3 and Z 4 are deuterium; W 1 , W2 and W 3 are simultaneously hydrogen or simultaneously deuterium; Z 1 and
- Z 2 are simultaneously hydrogen; and Y 1 and Y2 are simultaneously hydrogen.
- Z 5 is deuterium.
- Z 3 and Z 4 are deuterium; W 1 , W2 and W 3 are simultaneously hydrogen or simultaneously deuterium; Z 1 and Z 2 are simultaneously hydrogen; and Y 1 and Y2 are simultaneously deuterium.
- Z 5 is deuterium.
- Z 1 , Z2 , Z 3 and Z 4 are simultaneously hydrogen; W 1 , W 2 and W 3 are simultaneously hydrogen or
- W 1 , W 2 and W 3 are the same. In one aspect of this embodiment W 1 , W2 and W 3 are simultaneously deuterium. In another aspect of this embodiment W 1 , W2 and W 3 are simultaneously hydrogen.
- each Z attached to a common carbon atom (e.g., Z 1 and Z 2 ; or Z 3 and Z 4 ) is the same.
- Z attached to a common carbon atom is deuterium (e.g., at least Z 1 and Z 2 are deuterium; or at least Z 3 and Z 4 are deuterium).
- Z 1 , Z 2 , Z 3 and Z 4 are simultaneously deuterium.
- Z 1 , Z 2 , Z 3 , Z 4 and Z 5 are simultaneously deuterium.
- Z 1 , Z 2 , Z 3 , Z 4 and Z 5 are simultaneously deuterium; and W 1 , W2 and W 3 are simultaneously hydrogen.
- each Y is simultaneously deuterium.
- the compound is selected from any one of the compounds set forth below:
- the compound is selected from any one of the compounds set forth below:
- the compound is selected from any one of the compounds set forth below:
- the invention provides a compound of Formula I which is a compound of Formula la or lb:
- Z 3 and Z 4 are each hydrogen. In another aspect of this embodiment, Z 3 and Z 4 are each deuterium. In one aspect of this embodiment, Z 1 and Z 2 are each hydrogen. In one aspect of this embodiment, Z 1 and Z 2 are each deuterium. In one example of this aspect, Z 3 and Z 4 are each hydrogen.
- Z 3 and Z 4 are each deuterium; Z 1 and Z 2 are each hydrogen; and W 1 , W2 and W 3 are each hydrogen or each deuterium.
- Z 3 and Z 4 are each deuterium; Z 1 and Z 2 are each deuterium; and W 1 , W2 and W 3 are each hydrogen or each deuterium.
- Z 1 , Z 2 , Z 3 and Z 4 are each hydrogen; and W 1 , W 2 and W are each hydrogen or each deuterium.
- W 1 , W 2 and W 3 are the same.
- W 1 , W2 and W 3 are simultaneously deuterium.
- W 1 , W2 and W 3 are simultaneously hydrogen.
- each Z attached to a common carbon atom (that is, either Z 1 and Z 2 or Z 3 and Z 4 ) is the same.
- each member of at least one pair of Z attached to a common carbon atom is deuterium.
- Z 1 , Z 2 , Z 3 , and Z 4 are simultaneously deuterium and W 1 1 , W2" and W 3 J are simultaneously hydrogen.
- the rate of epimerization for a compound of Formula la or lb, as compared to the corresponding enantiomer of lenalidomide, can be readily measured using techniques well known to the skilled artisan. For example, pure samples of compounds of Formula la and lb as well as pure samples of each enantiomer of lenalidomide can be isolated and analyzed using chiral HPLC. These pure samples can be dissolved to an appropriate concentration in an appropriate physiological buffer or bodily fluid or simulant thereof and monitored over time (for example, approximately every 5 minutes) using chiral HPLC, to assess the rate of epimerization.
- Z 3 and Z 4 are deuterium; W 1 , W2 and W 3 are simultaneously hydrogen or simultaneously deuterium; Z 1 and Z 2 are simultaneously hydrogen; and Y 1 and Y 2 are simultaneously hydrogen.
- the compound is selected from any one of the compounds set forth below:
- the compound is selected from any one of the compounds set forth below:
- the compound is selected from any one of the compounds set forth below:
- the compound of formula 102a may be in a form such as the forms disclosed in US provisional application serial no. 61/716,826, filed October 22, 2012, such forms being prepared, for example, as disclosed in paragraphs [0085-0114] of US provisional application serial no. 61/716,826, which are incorporated by reference herein in their entirety.
- the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with “C a " in the figure below (shown for 102a) is at least 5500 (82.5% deuterium
- the isotopic enrichment factor for the deuterium atoms bonded to the carbon indicated with “C b " in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000
- the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with “C c " in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000 (90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation):
- any atom not designated as deuterium is present at its natural isotopic abundance.
- the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with "Ca" in the figure below is at least 5500 (82.5% deuterium
- the isotopic enrichment factor for the deuterium atoms bonded to the carbon indicated with "C b " in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000 (90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
- the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with “C c " in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000 (90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
- the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with “C d " in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000 (90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
- any atom not designated as deuterium is present at its natural isotopic abundance.
- the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with “C a " in the figure below (shown for 110a) is at least 5500 (82.5% deuterium
- the isotopic enrichment factor for the deuterium atoms bonded to the carbon indicated with “C b " in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000 90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation):
- any atom not designated as deuterium is present at its natural isotopic abundance.
- the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with “C a " in the figure below (shown for 111a) is at least 5500 (82.5% deuterium
- the isotopic enrichment factor for the deuterium atoms bonded to the carbon indicated with "C b " in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000 (90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
- the isotopic enrichment factor for the deuterium atom bonded to each carbon indicated with “C e " in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000 90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
- any atom not designated as deuterium is present at its natural isotopic abundance.
- the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with “C a " in the figure below (shown for 114a) is at least 5500 (82.5% deuterium
- any atom not designated as deuterium is present at its natural isotopic abundance.
- the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with “C a " in the figure below (shown for 116a) is at least 5500 (82.5% deuterium
- the isotopic enrichment factor for the deuterium atoms bonded to the carbon indicated with "C c " in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000 90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
- the invention is directed to a hemihydrate of a compound of formula I, such as a crystalline hemihydrate, including a hemihydrate or crystalline hemihydrate of compounds 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116 and 117.
- a compound of formula I such as a crystalline hemihydrate, including a hemihydrate or crystalline hemihydrate of compounds 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116 and 117.
- the invention is directed at a compound of the Formula II
- Y 3 is hydrogen or deuterium
- Y 5 is hydrogen or deuterium
- P 1 is a protecting group.
- Y 3 is hydrogen.
- Y 3 is deuterium.
- Y 5 is hydrogen.
- Y 5 is deuterium.
- P 1 is a group of the formula -C(0)-Q-R 2 , wherein Q is O or NH, and R 2 is (a) C C 6 alkyl optionally substituted with C 6 -Q 0 aryl; (b) C3-C8 cycloalkyl; or (c) C 6 -Cio aryl.
- P 1 is benzyloxycarbonyl.
- the invention is directed at a compound of the Formula III
- Z l -Z 5 are as defined as for Formula I, Y 4 is hydrogen or deuterium and P 2 is a protecting group. In one embodiment, Y 4 is hydrogen. In one embodiment, Y 4 is deuterium. In one embodiment, Z 5 is deuterium. In one aspect of this embodiment, Z 3 and Z 4 are each hydrogen. In another aspect of this embodiment, Z 3 and Z 4 are each deuterium. In one aspect of this embodiment, Z 1 and Z2 are each hydrogen. In one aspect of this embodiment, Z 1 and
- each Z is deuterium. In one more particular aspect, each Z is deuterium.
- Z 5 is deuterium
- Z 3 and Z 4 are each deuterium
- Z 1 and Z 2 are each hydrogen.
- Z 5 is deuterium
- Z 3 and Z 4 are each deuterium
- Z 1 and Z 2 are each deuterium
- P 2 is a group of the formula -C(0)-Q-R 2 , wherein Q is O or NH, and R 2 is (a) C -C alkyl optionally substituted with C 6 -Cio aryl; (b) C 3 -C8 cycloalkyl; or (c) C 6 -Cio aryl.
- P is benzyloxycarbonyl.
- the invention is directed to a compound of Formula IV
- R 12 is CrC 6 alkyl and P 2 is a group of the formula -C(0)-Q-R 2 , wherein Q is O or NH, and R is (a) C -C alkyl optionally substituted with C 6 -C 10 aryl; (b) C 3 -C8 cycloalkyl; or
- C 6 -Cio ary is methyl.
- CDT l, -carbonyl-di-(l,2,4-triazole)
- methanol may be used instead of MeOD in the first step; and NH 4 OH instead of ND 4 OD.
- the invention is directed to a process comprising treating a com ound of formula 20
- the silyl halide may be a compound of formula (R u )3Si-Hal, wherein R 11 is CrC 6 alkyl and Hal is fluoro, chloro, bromo or iodo. In one aspect of this embodiment, the silyl halide is trimethylsilyl chloride.
- the invention is directed to a process comprising treating a com ound of formula IV
- the hydrogen atoms bound to O or N are exchangeable with deuterium and vice versa.
- the invention is directed to compounds differing from Compounds of Formula II- IV or from structures 20, 21, 21', 21", 22, and 12b, only in the presence of deuterium atoms instead of hydrogen atoms, or hydrogen atoms instead of deuterium atoms, that are bound to O or N.
- any atom not designated as deuterium in any of the foregoing embodiments or aspects or examples is present at its natural isotopic abundance.
- Such methods can be carried out utilizing corresponding deuterated and optionally, other isotope-containing reagents and/or intermediates to synthesize the compounds delineated herein, or invoking standard synthetic protocols known in the art for introducing isotopic atoms to a chemical structure.
- D,L-glutamine 10 for use in Scheme 1 above may be prepared, for example, from the corresponding commercially available deuterated glutamic acids (D,L)-2,3,3,4,4-d5-glutamic acid, (D,L)-2,4,4-d3-glutamic acid, or (D,L)-3,3-d 2 - glutamic acid by methods analogous to those employed by Ogrel, A. et al., Russian Journal of Organic Chemistry, 2001, 37(4): 475-479. 85] Scheme 2. Synthesis of a Compound of Formula I.
- ester 16 can be obtained by treating an appropriately deuterated 2-methylbenzoic acid with sulfonyl chloride in methanol. The ester 16 is nitrated with nitric acid in dichloroethane with an Indium catalyst to provide the nitro compound 17, which is then converted to the benzylic halide 18 by treatment with N-bromosuccinimide.
- the R and S enantiomers of a compound of Formula I can then be separated by chiral HPLC in a manner similar to that known for related compounds in the IMiD class of drugs. Examples of this type of chiral HPLC enantiomer separation are found in Sembongi, K. et al., Biological and Pharmaceutical Bulletin, 2008, 31(3): 497-500; Murphy-Poulton, S.F. et al., Journal of Chromatography, B: Analytical Technologies in the Biomedical and Life Sciences, 2006, 831(1-2): 48-56;
- Scheme 3a depicts a preparation of the protected deuterated 3-Aminopiperidine-2,6- dione 12b.
- Deuterated glutamic acid 20 an exemplary preparation of which is shown in Scheme 6 below, is treated with SOCl 2 and CH 3 OD followed by N- (benzyloxycarbonyloxy)succinimide to provide 21.
- Reaction of 21 with ammonium-d4 deuteroxide in D 2 0 gave amide 22 which upon treatment with carbonyldiimidazole (CD I) cyclized to 12b.
- CD I carbonyldiimidazole
- l, -carbonyl-di-(l,2,4-triazole) (CDT) may be used in place of CDI.
- Scheme 3b depicts an alternative preparation of 12b.
- Deuterated glutamic acid 20 is treated with TMSC1 (2.2 equivalents) in CH 3 OD to give 21' which is treated with
- Scheme 4 depicts a preparation of deuterated glutamic acid 20.
- Succinic acid 23 was treated with DC1 in D 2 0 to provide, after quenching with a mineral acid such as HC1, 24, which was treated with D-glucose-Di NAD (Nicotinamide adenine dinucleotide).
- D-glucose- Di is the following compound (shown below in its open chain and pyranose forms):
- Z x -Z 4 may be treated with D-glucose-Di NAD, or D-glucose-Di, in the presence of glutamate dehydrogenase and a source of ammonia, such as an ammonium salt, such as NH 4 C1, to afford a compound 20':
- 24 may be treated with a deuteride source to provide 20 (or 20') or a hydride source to provide 20-H, where the deuteride or hydride source is a compound or mixture capable of providing a deuteride or hydride anion, respectively, or the synthetic equivalent thereof.
- a deuteride source to provide 20 (or 20') or a hydride source to provide 20-H
- the deuteride or hydride source is a compound or mixture capable of providing a deuteride or hydride anion, respectively, or the synthetic equivalent thereof.
- Such mixture may comprise a co-factor, an example of which is NAD as illustrated in Scheme 4.
- Another example of a co-factor is NADP.
- the mixture may also comprise a co-factor regeneration system, which may comprise, as an example, a dehydrogenase and a substrate for the dehydrogenase.
- the mixture comprises GDH as the dehydrogenase; D-Glucose-Di (to produce 20) or D- glucose (to produce 20-H) as the substrate; and NAD as the co-factor.
- the D-glucose-Di is generated in situ from inexpensive D-glucono-5-lactone and NaBD 4 .
- This embodiment is advantageous in that an otherwise expensive deuterated glucose substrate is generated from relatively inexpensive reagents.
- Other embodiments of the deuteride or hydride source are disclosed in paragraphs [43[-[53] of application PCT/US2011/050138, and in the corresponding paragraphs of U.S. provisional application 61/379,182, incorporated by reference herein in their entirety.
- the isotopic enrichment factor in 20 and 20-H is over 98% at each of the positions designated with deuterium in the two structures.
- compound 25 may be treated with D 2 over Pd/C to provide 26.
- Reaction of 26 with diethyl oxalate affords 27, which is then treated with DCI to give, after quenching with a mineral acid such as HC1, 24.
- the invention also provides pyrogen-free pharmaceutical compositions comprising an effective amount of a compound of Formula I (e.g., including any of the formulae herein), or a pharmaceutically acceptable salt thereof; and an acceptable carrier.
- a compound of Formula I e.g., including any of the formulae herein
- an acceptable carrier e.g., an acceptable carrier
- Pharmaceutically acceptable carriers, adjuvants and vehicles that may be used in the pharmaceutical compositions of this invention include, but are not limited to, ion exchangers, alumina, aluminum stearate, lecithin, serum proteins, such as human serum albumin, buffer substances such as phosphates, glycine, sorbic acid, potassium sorbate, partial glyceride mixtures of saturated vegetable fatty acids, water, salts or electrolytes, such as protamine sulfate, disodium hydrogen phosphate, potassium hydrogen phosphate, sodium chloride, zinc salts, colloidal silica, magnesium trisilicate, polyvinyl pyrrolidone, cellulose-based substances, polyethylene glycol, sodium carboxymethylcellulose, polyacrylates, waxes, polyethylene-polyoxypropylene-block polymers, polyethylene glycol and wool fat.
- ion exchangers alumina, aluminum stearate, lecithin
- serum proteins such as human serum albumin
- buffer substances such as phosphat
- the solubility and bioavailability of the compounds of the present invention in pharmaceutical compositions may be enhanced by methods well-known in the art.
- One method includes the use of lipid excipients in the formulation. See “Oral Lipid- Based Formulations: Enhancing the Bioavailability of Poorly Water-Soluble Drugs (Drugs and the Pharmaceutical Sciences),” David J. Hauss, ed. Informa Healthcare, 2007; and “Role of Lipid Excipients in Modifying Oral and Parenteral Drug Delivery: Basic Principles and Biological Examples," Kishor M. Wasan, ed. Wiley-Interscience, 2006.
- Another known method of enhancing bioavailability is the use of an amorphous form of a compound of this invention optionally formulated with a poloxamer, such as LUTROLTM and PLURONICTM (BASF Corporation), or block copolymers of ethylene oxide and propylene oxide. See United States patent 7,014,866; and United States patent publications 20060094744 and 20060079502.
- compositions of the invention include those suitable for oral, rectal, nasal, topical (including buccal and sublingual), vaginal or parenteral (including subcutaneous, intramuscular, intravenous and intradermal) administration.
- the compound of the formulae herein is administered transdermally (e.g., using a transdermal patch or iontophoretic techniques).
- Other formulations may conveniently be presented in unit dosage form, e.g., tablets and sustained release capsules, and in liposomes, and may be prepared by any methods well known in the art of pharmacy. See, for example, Remington: The Science and Practice of Pharmacy, Lippincott Williams and Wilkins, Baltimore, MD (20th ed. 2000).
- Such preparative methods include the step of bringing into association with the molecule to be administered ingredients such as the carrier that constitutes one or more accessory ingredients.
- ingredients such as the carrier that constitutes one or more accessory ingredients.
- the compositions are prepared by uniformly and intimately bringing into association the active ingredients with liquid carriers, liposomes or finely divided solid carriers or both, and then if necessary shaping the product.
- the compound is administered orally.
- compositions of the present invention suitable for oral administration may be presented as discrete units such as capsules, sachets or tablets each containing a predetermined amount of the active ingredient; as a powder or granules; as a solution or a suspension in an aqueous liquid or a non-aqueous liquid; or as an oil-in- water liquid emulsion or a water-in-oil liquid emulsion, or packed in liposomes and as a bolus, etc.
- Soft gelatin capsules can be useful for containing such suspensions, which may beneficially increase the rate of compound absorption.
- carriers that are commonly used include lactose and corn starch.
- Lubricating agents such as magnesium stearate, are also typically added.
- useful diluents include lactose and dried cornstarch.
- aqueous suspensions are administered orally, the active ingredient is combined with emulsifying and suspending agents. If desired, certain sweetening and/or flavoring and/or coloring agents may be added.
- compositions suitable for oral administration include lozenges comprising the ingredients in a flavored basis, usually sucrose and acacia or tragacanth; and pastilles comprising the active ingredient in an inert basis such as gelatin and glycerin, or sucrose and acacia.
- Compositions suitable for parenteral administration include aqueous and non-aqueous sterile injection solutions which may contain anti- oxidants, buffers, bacteriostats and solutes which render the formulation isotonic with the blood of the intended recipient; and aqueous and non-aqueous sterile suspensions which may include suspending agents and thickening agents.
- the formulations may be presented in unit-dose or multi-dose containers, for example, sealed ampules and vials, and may be stored in a freeze dried (lyophilized) condition requiring only the addition of the sterile liquid carrier, for example water for injections, immediately prior to use.
- sterile liquid carrier for example water for injections, immediately prior to use.
- Extemporaneous injection solutions and suspensions may be prepared from sterile powders, granules and tablets.
- Such injection solutions may be in the form, for example, of a sterile injectable aqueous or oleaginous suspension.
- This suspension may be formulated according to techniques known in the art using suitable dispersing or wetting agents (such as, for example, Tween 80) and suspending agents.
- the sterile injectable preparation may also be a sterile injectable solution or suspension in a non-toxic parenterally-acceptable diluent or solvent, for example, as a solution in 1,3-butanediol.
- the acceptable vehicles and solvents that may be employed are mannitol, water, Ringer's solution and isotonic sodium chloride solution.
- sterile, fixed oils are conventionally employed as a solvent or suspending medium.
- any bland fixed oil may be employed including synthetic mono- or diglycerides.
- Fatty acids, such as oleic acid and its glyceride derivatives are useful in the preparation of injectables, as are natural pharmaceutically-acceptable oils, such as olive oil or castor oil, especially in their polyoxyethylated versions.
- These oil solutions or suspensions may also contain a long-chain alcohol diluent or dispersant.
- compositions of this invention may be administered in the form of suppositories for rectal administration.
- These compositions can be prepared by mixing a compound of this invention with a suitable non-irritating excipient which is solid at room temperature but liquid at the rectal temperature and therefore will melt in the rectum to release the active components.
- suitable non-irritating excipient include, but are not limited to, cocoa butter, beeswax and polyethylene glycols.
- compositions of this invention may be administered by nasal aerosol or inhalation.
- Such compositions are prepared according to techniques well-known in the art of pharmaceutical formulation and may be prepared as solutions in saline, employing benzyl alcohol or other suitable preservatives, absorption promoters to enhance
- Topical administration of the pharmaceutical compositions of this invention is especially useful when the desired treatment involves areas or organs readily accessible by topical application.
- the pharmaceutical composition should be formulated with a suitable ointment containing the active components suspended or dissolved in a carrier.
- Carriers for topical administration of the compounds of this invention include, but are not limited to, mineral oil, liquid petroleum, white petroleum, propylene glycol, polyoxyethylene polyoxypropylene compound, emulsifying wax and water.
- the pharmaceutical composition can be formulated with a suitable lotion or cream containing the active compound suspended or dissolved in a carrier.
- suitable carriers include, but are not limited to, mineral oil, sorbitan monostearate, polysorbate 60, cetyl esters wax, cetearyl alcohol, 2-octyldodecanol, benzyl alcohol and water.
- the pharmaceutical compositions of this invention may also be topically applied to the lower intestinal tract by rectal suppository formulation or in a suitable enema formulation. Topically-transdermal patches and iontophoretic administration are also included in this invention.
- Application of the subject therapeutics may be local, so as to be administered at the site of interest.
- Various techniques can be used for providing the subject compositions at the site of interest, such as injection, use of catheters, trocars, projectiles, pluronic gel, stents, sustained drug release polymers or other device which provides for internal access.
- the compounds of this invention may be incorporated into compositions for coating an implantable medical device, such as prostheses, artificial valves, vascular grafts, stents, or catheters.
- an implantable medical device such as prostheses, artificial valves, vascular grafts, stents, or catheters.
- Suitable coatings and the general preparation of coated implantable devices are known in the art and are exemplified in US Patents 6,099,562; 5,886,026; and 5,304,121.
- the coatings are typically biocompatible polymeric materials such as a hydrogel polymer, polymethyldisiloxane, polycaprolactone, polyethylene glycol, polylactic acid, ethylene vinyl acetate, and mixtures thereof.
- the coatings may optionally be further covered by a suitable topcoat of fluorosilicone, polysaccharides, polyethylene glycol, phospholipids or combinations thereof to impart controlled release characteristics in the composition.
- Coatings for invasive devices are to be included within the definition of pharmaceutically acceptable carrier, adjuvant or vehicle, as those terms are used herein.
- a compound of Formula I is formulated into a hydrogel for delivery to the eye as described in United States Patent Publication US2005074497.
- the invention provides a method of coating an implantable medical device comprising the step of contacting said device with the coating composition described above. It will be obvious to those skilled in the art that the coating of the device will occur prior to implantation into a mammal.
- the invention provides a method of impregnating an implantable drug release device comprising the step of contacting said drug release device with a compound or composition of this invention.
- Implantable drug release devices include, but are not limited to, biodegradable polymer capsules or bullets, non-degradable, diffusible polymer capsules and biodegradable polymer wafers.
- the invention provides an implantable medical device coated with a compound or a composition comprising a compound of this invention, such that said compound is therapeutically active.
- the invention provides an implantable drug release device impregnated with or containing a compound or a composition comprising a compound of this invention, such that said compound is released from said device and is therapeutically active.
- composition of this invention may be painted onto the organ, or a composition of this invention may be applied in any other convenient way.
- a composition of the present invention further comprises a second therapeutic agent.
- the second therapeutic agent includes any compound or therapeutic agent known to have or that demonstrates advantageous properties when administered with an immunomodulator, an anti- angiogenic or an anti-neoplastic agent. Such agents are described in detail in United States Patent 5,635,517, as well as in PCT patent publications WO2005097125, WO2005055929, WO2004041190, WO2006060507,
- WO2006058008 WO2006053160, WO2005044178, WO2004100953, WO2006089150, WO2006036892, WO2006018182, WO2005082415, WO2005048942, WO2005042558, WO2005035714 and WO2005027842; and in United States Patent publications
- the second therapeutic agent is an agent useful in the treatment or prevention of a disease or condition selected from myelodysplasia syndromes, multiple myeloma, Non-Hodgkins lymphoma; papillary and follicular thyroid carcinoma; prostate cancer; chronic lymphocytic leukemia, amyloidosis, complex regional pain syndrome Type I, malignant melanoma, radiculopathy, myelofibrosis, glioblastoma, gliosarcoma, malignant gliomas, myelogenous leukemia, refractory plasma cell neoplasm, chronic myelomonocytic leukemia, follicular lymphoma, ciliary body and chronic melanoma, iris melanoma, recurrent interocular melanoma, extraocular extension melanoma, solid tumors, T-cell lymphoma, erythroid lymphoma, monoblastic and monocy
- the second therapeutic agent is an agent useful in the treatment or prevention of a disease or condition selected from dysfunctional sleep, hemoglobinopathy, anemia, macular degeneration, atherosclerosis, restenosis, pain, immunodeficiencies, CNS injury and related symptoms, CNS disorders, parasitic disease, or asbestos-related disease.
- the second therapeutic agent co-formulated with a compound of this invention is an agent useful in the treatment of myelodysplasia syndromes or multiple myeloma.
- the second therapeutic agent is selected from aldesleukin; a p38 MAP kinase inhibitor such as disclosed in US2006079461; a 24- hydroxylase inhibitor such as disclosed in WO2006036892; an aminopteridinone such as disclosed in WO2006018182; an IGF-R inhibitor such as disclosed in WO2005082415; a COX-2 inhibitor such as disclosed in WO2005048942; a nucleobase oligomer such as disclosed in WO2005042558; a chlorpromazine compound such as disclosed in
- the second therapeutic agent is selected from pemetrexed, topotecan, doxorubicin, bortezomib, gemcitabine, dacarbazine, dexamethasone, biaxin, doxil, vincristine, decadron, azacitidine, rituximab, prednisone, docetaxel, melphalan, or combinations thereof.
- the invention provides separate dosage forms of a compound of this invention and a second therapeutic agent that are associated with one another.
- association with one another means that the separate dosage forms are packaged together or otherwise attached to one another such that it is readily apparent that the separate dosage forms are intended to be sold and administered together (within less than 24 hours of one another, consecutively or simultaneously).
- the compound of the present invention is present in an effective amount.
- the term "effective amount” refers to an amount which, when administered in a proper dosing regimen, is sufficient to reduce or ameliorate the severity, duration or progression of the disorder being treated, prevent the advancement of the disorder being treated, cause the regression of the disorder being treated, or enhance or improve the prophylactic or therapeutic effect(s) of another therapy.
- Effective doses will also vary, as recognized by those skilled in the art, depending on the diseases treated, the severity of the disease, the route of administration, the sex, age and general health condition of the patient, excipient usage, the possibility of co-usage with other therapeutic treatments such as use of other agents and the judgment of the treating physician. For example, guidance for selecting an effective dose can be determined by reference to the prescribing information for lenalidomide.
- an effective amount of the second therapeutic agent is between about 20% and 100% of the dosage normally utilized in a monotherapy regime using just that agent.
- an effective amount is between about 70% and 100% of the normal monotherapeutic dose.
- the normal monotherapeutic dosages of these second therapeutic agents are well known in the art. See, e.g., Wells et al, eds., Pharmacotherapy Handbook, 2nd Edition, Appleton and Lange, Stamford, Conn. (2000); PDR Pharmacopoeia, Tarascon Pocket Pharmacopoeia 2000, Deluxe Edition, Tarascon Publishing, Loma Linda, Calif. (2000), each of which references are entirely incorporated herein by reference.
- the invention provides a method of treating a disease that is beneficially treated by lenalidomide in a patient in need thereof, comprising the step of administering to the patient an effective amount of a compound or a composition of this invention.
- diseases are well known in the art and are disclosed in United States Patent 5,635,517, as well as in PCT patent publications WO2005097125, WO2005055929, WO2004041190, WO2006060507, WO2006058008, WO2006053160, WO2005044178, WO2004100953, WO2006089150, WO2006036892, WO2006018182, WO2005082415, WO2005048942, WO2005042558, WO2005035714 and WO2005027842; and in United States Patent publications US2005100529, US2006030594, US2005143344 and
- the disease or condition is selected from
- myelodysplasia syndromes multiple myeloma, Non-Hodgkins lymphoma; papillary and follicular thyroid carcinoma; prostate cancer; chronic lymphocytic leukemia, amyloidosis, complex regional pain syndrome Type I, malignant melanoma, radiculopathy, myelofibrosis, glioblastoma, gliosarcoma, malignant gliomas, myelogenous leukemia, refractory plasma cell neoplasm, chronic myelomonocytic leukemia, follicular lymphoma, ciliary body and chronic melanoma, iris melanoma, recurrent interocular melanoma, extraocular extension melanoma, solid tumors, T-cell lymphoma, erythroid lymphoma, monoblastic and monocytic leukemia; myeloid leukemia, brain tumor, meningioma, spinal cord tumors, thyroid cancer
- the disease is selected from myelodysplasia syndromes or multiple myeloma.
- Identifying a patient in need of such treatment can be in the judgment of a patient or a health care professional and can be subjective (e.g. opinion) or objective (e.g. measurable by a test or diagnostic method).
- the above method of treatment comprises the further step of co-administering to the patient one or more second therapeutic agents.
- the choice of second therapeutic agent may be made from any second therapeutic agent known to be useful for co- administration with lenalidomide.
- the choice of second therapeutic agent is also dependent upon the particular disease or condition to be treated. Examples of second therapeutic agents that may be employed in the methods of this invention are those set forth above for use in combination compositions comprising a compound of this invention and a second therapeutic agent.
- the second therapeutic agent and the corresponding disease for which the second therapeutic agent is co-administered with a compound of this invention is set forth in Table 1 below.
- co-administered means that the second therapeutic agent may be administered together with a compound of this invention as part of a single dosage form (such as a composition of this invention comprising a compound of the invention and an second therapeutic agent as described above) or as separate, multiple dosage forms. Alternatively, the additional agent may be administered prior to, consecutively with, or following the administration of a compound of this invention. In such combination therapy treatment, both the compounds of this invention and the second therapeutic agent(s) are administered by conventional methods.
- composition of this invention comprising both a compound of the invention and a second therapeutic agent to a patient does not preclude the separate administration of that same therapeutic agent, any other second therapeutic agent or any compound of this invention to the patient at another time during a course of treatment.
- Effective amounts of these second therapeutic agents are well known to those skilled in the art and guidance for dosing may be found in patents and published patent applications referenced herein, as well as in Wells et al, eds., Pharmacotherapy Handbook, 2nd Edition, Appleton and Lange, Stamford, Conn. (2000); PDR Pharmacopoeia, Tarascon Pocket Pharmacopoeia 2000, Deluxe Edition, Tarascon Publishing, Loma Linda, Calif. (2000), and other medical texts. However, it is well within the skilled artisan's purview to determine the second therapeutic agent's optimal effective-amount range.
- the effective amount of the compound of this invention is less than its effective amount would be where the second therapeutic agent is not administered. In another embodiment, the effective amount of the second therapeutic agent is less than its effective amount would be where the compound of this invention is not administered. In this way, undesired side effects associated with high doses of either agent may be minimized. Other potential advantages (including without limitation improved dosing regimens and/or reduced drug cost) will be apparent to those of skill in the art.
- the invention provides the use of a compound of Formula I alone or together with one or more of the above-described second therapeutic agents in the manufacture of a medicament, either as a single composition or as separate dosage forms, for treatment or prevention in a patient of a disease, disorder or symptom set forth above.
- Another aspect of the invention is a compound of Formula I for use in the treatment or prevention in a patient of a disease, disorder or symptom thereof delineated herein.
- N-(Benzyloxycarbonyloxy)succinimide 91 (8.93 g, 35.88 mmol, 2.1 equiv) was added in one portion and the resulting mixture was stirred at room temperature for 42 hours.
- the mixture was concentrated under reduced pressure to remove most of the tetrahydrofuran, and saturated aqueous sodium bicarbonate solution (30 mL) was added to the residual oily solid.
- the mixture was diluted with water (10 mL) and washed with ethyl acetate (50 mL). The organic phase was discarded.
- the aqueous phase was acidified to pH 1-2 with a mixture of concentrated hydrochloric acid and ice.
- the mixture was extracted with ethyl acetate (5 x 50 mL).
- the reaction mixture was cooled to room temperature and stirred overnight.
- the reaction mixture was concentrated under reduced pressure to remove most of the tetrahydrofuran and the residual yellow oil was partitioned between ethyl acetate (150 mL) and IN hydrochloric acid (100 mL).
- the organic phase was washed with brine (75 mL), dried over sodium sulfate, filtered and concentrated under reduced pressure to give a colorless oil that slowly crystallized.
- the crude product was purified on an Analogix automated chromatography system eluting with a gradient of 25-67% ethyl acetate/heptanes. Product-containing fractions were concentrated under reduced pressure to give 2.41 g (79%) of 12b' as a white solid.
- N-Bromosuccinimide 5.3 g, 30 mmol, 0.33 equiv
- benzoyl peroxide 25% water
- the yellow organic suspension was washed with saturated sodium thiosulfate solution (250 mL), water (200 mL), brine (200 mL), dried over sodium sulfate, filtered, and concentrated under reduced pressure to give 27.9 g of crude product which partially crystallized.
- the crude product was dissolved in a minimum volume of dichloromethane and adsorbed onto silica gel.
- the adsorbed material was dry-loaded onto a column of silica gel (400 g) packed in heptanes.
- the column was eluted with heptanes (2 L), 5% methyl iert-butyl ether/heptanes (2 L), 10% methyl iert-butyl ether/heptanes (2 L) and 20% methyl ie/t-butyl ether/heptanes (3.5 L).
- Product-containing fractions were concentrated under reduced pressure and the resulting solid was triturated with hexanes (approximately 100 mL), filtered and dried to give 20.2 g (81%) of 18a as a pale yellow solid.
- Example 3 Synthesis of 3-(4-Amino-l-oxo-3 -d2-isoindolin-2-yl)(piperidine- 3,4,4,5, 5-dQ-2,6-dione (104).
- Compound 104 was prepared as outlined in Scheme 12 below. Details of the synthesis are set forth below.
- reaction mixture became dark blue upon heating and a suspension reformed.
- the reaction mixture was cooled to room temperature and deuterium oxide (Cambridge Isotopes, 99 atom% D, 10 mL) was added slowly to the reaction mixture.
- the mixture was stirred for 10 minutes, then the solid was filtered, washed with deuterium oxide (20 mL) and then with methanol-di (Cambridge Isotopes, 99 atom% D, 20 mL), and dried to give 1.01 g of 19a as a pale gray solid.
- 1H NMR showed that 19a contained approximately 7-8% H at the 3-position of the piperidinedione ring and approximately 6-7% H at the 3-position of the isoindolinone ring.
- Example 4 Chiral Separation of Compound 104 to Compounds 104a and 104b.
- the enantiomers of Compound 104 were separated via chiral chromatography as described below.
- [12a may be prepared in a manner analogous to the methods disclosed in patent application WO2012/079022A1, except that deuterated reagents and solvents are not utilized in the synthesis.]
- the reaction was heated to reflux for 2.5 days.
- the mixture was cooled to room temperature, diluted with dichloromethane (100 mL), and washed with IN DC1 in D 2 0 (1 x 100 mL).
- the organic layer was dried over Na 2 S0 4 , filtered, and evaporated yielding 12c (2.6 g) as a white solid.
- Proton NMR showed 5% H remaining at the 5 position.
- a second cycle was carried out using the above conditions to yield 12c (2.5 g, 92% yield) as a white solid with less than 1% H at each the 3 and 5 positions.
- the reaction mixture was cooled to 0 °C and D 2 0 (50 mL; Cambridge Isotopes, 99 atom% D) was added dropwise. The mixture was stirred at 0 °C for 30 minutes and the solids were then collected by filtration, washing with D 2 0 (100 mL) and MTBE (100 mL). The off-white solid was dried in a vacuum oven at 40 °C for 3 hours, yielding 450 mg (81% yield) of 19b.
- Example 6 Chiral Separation of Compound 116 to Compounds 116a and 116b. The enantiomers of Compound 116 were separated via chiral chromatography as described below.
- Chiral HPLC analytical method Chiralpak AD 4.6 x 250 mm, 10 um, 100% MeOH isocratic for 40 minutes at 1.0 mL/min; retention times: 4.52 min (116a) and 6.07 min (116b).
Abstract
This invention relates to novel substituted dioxopiperidinyl phthalimide derivatives and pharmaceutically acceptable acid addition salts thereof. The invention also provides compositions comprising a compound of this invention and the use of such compositions in methods of treating diseases and conditions beneficially treated by an immunomodulatory agent.
Description
SUBSTITUTED DIOXOPIPERIDINYL PHTHALIMIDE DERIVATIVES
Related Application
[1] This application claims the benefit of U.S. Provisional Patent Application No.
61/751,512 , filed January 11, 2013, the entire contents of which are hereby incorporated by reference.
Background
[2] Many current medicines suffer from poor absorption, distribution, metabolism and/or excretion (ADME) properties that prevent their wider use. Poor ADME properties are also a major reason for the failure of drug candidates in clinical trials. While formulation technologies and prodrug strategies can be employed in some cases to improve certain ADME properties, these approaches often fail to address the underlying ADME problems that exist for many drugs and drug candidates. One such problem is rapid metabolism that causes a number of drugs, which otherwise would be highly effective in treating a disease, to be cleared too rapidly from the body. A possible solution to rapid drug clearance is frequent or high dosing to attain a sufficiently high plasma level of drug. This, however, introduces a number of potential treatment problems such as poor patient compliance with the dosing regimen, side effects that become more acute with higher doses, and increased cost of treatment.
[3] In some select cases, a metabolic inhibitor will be co-administered with a drug that is cleared too rapidly. Such is the case with the protease inhibitor class of drugs that are used to treat HIV infection. The FDA recommends that these drugs be co-dosed with ritonavir, an inhibitor of cytochrome P450 enzyme 3A4 (CYP3A4), the enzyme typically responsible for their metabolism (see Kempf, D.J. et al., Antimicrobial agents and chemotherapy, 1997, 41(3): 654-60). Ritonavir, however, causes adverse effects and adds to the pill burden for HIV patients who must already take a combination of different drugs. Similarly, the
CYP2D6 inhibitor quinidine has been added to dextromethorphan for the purpose of reducing rapid CYP2D6 metabolism of dextromethorphan in a treatment of pseudobulbar affect.
Quinidine, however, has unwanted side effects that greatly limit its use in potential combination therapy (see Wang, L et al., Clinical Pharmacology and Therapeutics, 1994, 56(6 Pt 1): 659-67; and FDA label for quinidine at www.accessdata.fda.gov).
[4] In general, combining drugs with cytochrome P450 inhibitors is not a satisfactory strategy for decreasing drug clearance. The inhibition of a CYP enzyme's activity can affect
the metabolism and clearance of other drugs metabolized by that same enzyme. CYP inhibition can cause other drugs to accumulate in the body to toxic levels.
[5] A potentially attractive strategy for improving a drug's metabolic properties is deuterium modification. In this approach, one attempts to slow the CYP-mediated metabolism of a drug by replacing one or more hydrogen atoms with deuterium atoms.
Deuterium is a safe, stable, non-radioactive isotope of hydrogen. Compared to hydrogen, deuterium forms stronger bonds with carbon. In select cases, the increased bond strength imparted by deuterium can positively impact the ADME properties of a drug, creating the potential for improved drug efficacy, safety, and/or tolerability. At the same time, because the size and shape of deuterium are essentially identical to those of hydrogen, replacement of hydrogen by deuterium would not be expected to affect the biochemical potency and selectivity of the drug as compared to the original chemical entity that contains only hydrogen.
[6] Over the past 35 years, the effects of deuterium substitution on the rate of metabolism have been reported for a very small percentage of approved drugs (see, e.g., Blake, MI et al, J Pharm Sci, 1975, 64:367-91; Foster, AB, Adv Drug Res 1985, 14: 1-40 ("Foster"); Kushner, DJ et al, Can J Physiol Pharmacol 1999, 79-88; Fisher, MB et al, Curr Opin Drug Discov Devel, 2006, 9: 101-09 ("Fisher")). The results have been variable and unpredictable. For some compounds deuteration caused decreased metabolic clearance in vivo. For others, there was no change in metabolism. Still others demonstrated increased metabolic clearance. The variability in deuterium effects has also led experts to question or dismiss deuterium modification as a viable drug design strategy for inhibiting adverse metabolism (see Foster at p. 35 and Fisher at p. 101).
[7] The effects of deuterium modification on a drug's metabolic properties are not predictable even when deuterium atoms are incorporated at known sites of metabolism. Only by actually preparing and testing a deuterated drug can one determine if and how the rate of metabolism will differ from that of its non-deuterated counterpart. See, for example, Fukuto et al. (J. Med. Chem. 1991, 34, 2871-76). Many drugs have multiple sites where metabolism is possible. The site(s) where deuterium substitution is required and the extent of deuteration necessary to see an effect on metabolism, if any, will be different for each drug.
[8] This invention relates to novel substituted dioxopiperidinyl phthalimide derivatives and pharmaceutically acceptable salts thereof. The invention also provides compositions comprising a compound of this invention and the use of such compositions in methods of treating diseases and conditions beneficially treated by an immunomodulatory agent.
[9] Lenalidomide, chemically known as either 3-(4-amino-l,3-dihydro-l-oxo-2H- isoindol-2-yl)-2,6-piperidinedione or 3-(4-amino-l-oxo l,3-dihydro-2H-isoindol-2- yl)piperidine-2,6-dione, and its pharmaceutically acceptable salts thereof are disclosed as immunomodulatory agents. Lenalidomide has been shown to inhibit the secretion of pro- inflammatory cytokines such as tumor necrosis factor alpha (TNF-a) and to increase the secretion of anti-inflammatory cytokines in animals and humans. Decreasing TNF-a levels is a valuable therapeutic strategy for the treatment of many inflammatory, infectious, immunological, and malignant diseases (PCT publication WO 98/03502). Lenalidomide has been demonstrated to be useful in the treatment of anemia due to myelodysplastic syndromes associated with a deletion 5q cytogenic abnormality, as well as in the treatment of multiple myeloma when used in combination with dexamethasone.
(http://www.fda.gov/cder/foi/label/2006/021880s001.pdf).
[10] Lenalidomide is also in clinical trials, alone or in combination with other therapeutic agents, for the treatment of Non-Hodgkins lymphoma; papillary and follicular thyroid carcinoma; prostate cancer; chronic lymphocytic leukemia, amyloidosis, complex regional pain syndrome Type I, malignant melanoma, radiculopathy, myelofibrosis, glioblastoma, gliosarcoma, malignant gliomas, myelogenous leukemia, refractory plasma cell neoplasm, chronic myelomonocytic leukemia, follicular lymphoma, ciliary body and chronic melanoma, iris melanoma, recurrent interocular melanoma, extraocular extension melanoma, solid tumors, T-cell lymphoma, erythroid lymphoma, monoblastic and monocytic leukemia;
myeloid leukemia, brain tumor, meningioma, spinal cord tumors, thyroid cancers, mantle cell lymphoma, non-small cell lung cancer, ovarian cancer, prostate cancer, renal cell cancer, myelofibrosis, Burkitt's lymphoma, Hodgkin's lymphoma, large cell lymphoma, and
Waldenstrom's macroglobulinemia.
[11] Lenalidomide is associated with significant potential toxicities, which include human birth defects; neutropenia; thrombocytopenia; deep vein thrombosis; and pulmonary embolism. See (http://www.fda.gov/cder/foi/label/2006/021880s001.pdf). A majority of patients taking lenalidomide required a dose delay or reduction during clinical trials due to hematologic toxicities. No clinical studies were performed to assess the relationship between exposure and safety.
[12] Despite the beneficial activities of lenalidomide, there is a continuing need for new compounds to treat the aforementioned diseases and conditions.
Definitions
[13] The terms "ameliorate" and "treat" are used interchangeably and include both therapeutic and prophylactic treatment. Both terms mean decrease, suppress, attenuate, diminish, arrest, or stabilize the development or progression of a disease (e.g., a disease or disorder delineated herein), lessen the severity of the disease or improve the symptoms associated with the disease.
[14] "Disease" is meant any condition or disorder that damage or interferes with the normal function of a cell, tissue, or organ.
[15] It will be recognized that some variation of natural isotopic abundance occurs in a synthesized compound depending upon the origin of chemical materials used in the synthesis. Thus, a preparation of lenalidomide will inherently contain small amounts of deuterated isotopologues. The concentration of naturally abundant stable hydrogen isotopes, notwithstanding this variation, is small and immaterial with respect to the degree of stable isotopic substitution of compounds of this invention. See for instance Wada, E and Hanba, Y, Seikagaku, 1994, 66: 15; Gannes, LZ et al, Comp Biochem Physiol A Mol Integr Physiol, 1998, 119: 725.
[16] In the compounds of this invention any atom not specifically designated as a particular isotope is meant to represent any stable isotope of that atom. Unless otherwise stated, when a position is designated specifically as "H" or "hydrogen", the position is understood to have hydrogen at its natural abundance isotopic composition. Also unless otherwise stated, when a position is designated specifically as "D" or "deuterium", the position is understood to have deuterium at an abundance that is at least 3340 times greater than the natural abundance of deuterium, which is 0.015% (i.e., at least 50.1% incorporation of deuterium).
[17] The term "isotopic enrichment factor" as used herein means the ratio between the isotopic abundance and the natural abundance of a specified isotope.
[18] In other embodiments, a compound of this invention has an isotopic enrichment factor for each designated deuterium atom of at least 3500 (52.5% deuterium incorporation at each designated deuterium atom), at least 4000 (60% deuterium incorporation), at least 4500 (67.5% deuterium incorporation), at least 5000 (75% deuterium), at least 5500 (82.5% deuterium incorporation), at least 6000 (90% deuterium incorporation), at least 6333.3 (95% deuterium incorporation), at least 6466.7 (97% deuterium incorporation), at least 6600 (99% deuterium incorporation), or at least 6633.3 (99.5% deuterium incorporation).
[19] The term "isotopologue" refers to a species that differs from a specific compound of this invention only in the isotopic composition thereof.
[20] The term "compound," when referring to a compound of this invention, refers to a collection of molecules having an identical chemical structure, except that there may be isotopic variation among the constituent atoms of the molecules. Thus, it will be clear to those of skill in the art that a compound represented by a particular chemical structure containing indicated deuterium atoms, will also contain lesser amounts of isotopologues having hydrogen atoms at one or more of the designated deuterium positions in that structure. The relative amount of such isotopologues in a compound of this invention will depend upon a number of factors including the isotopic purity of deuterated reagents used to make the compound and the efficiency of incorporation of deuterium in the various synthesis steps used to prepare the compound. However, as set forth above the relative amount of such isotopologues in toto will be less than 49.9% of the compound. In other embodiments, the relative amount of such isotopologues in toto will be less than 47.5%, less than 40%, less than 32.5%, less than 25%, less than 17.5%, less than 10%, less than 5%, less than 3%, less than 1%, or less than 0.5% of the compound.
[21] The invention also provides salts of the compounds of the invention.
[22] A salt of a compound of this invention is formed between an acid and a basic group of the compound, such as an amino functional group, or a base and an acidic group of the compound, such as a carboxyl functional group. According to another preferred
embodiment, the compound is a pharmaceutically acceptable acid addition salt.
[23] The term "pharmaceutically acceptable," as used herein, refers to a component that is, within the scope of sound medical judgment, suitable for use in contact with the tissues of humans and other mammals without undue toxicity, irritation, allergic response and the like, and are commensurate with a reasonable benefit/risk ratio. A "pharmaceutically acceptable salt" means any non-toxic salt that, upon administration to a recipient, is capable of providing, either directly or indirectly, a compound of this invention. A "pharmaceutically acceptable counterion" is an ionic portion of a salt that is not toxic when released from the salt upon administration to a recipient.
[24] Acids commonly employed to form pharmaceutically acceptable salts include inorganic acids such as hydrogen bisulfide, hydrochloric, hydrobromic, hydroiodic, sulfuric and phosphoric acid, as well as organic acids such as para-toluenesulfonic, salicylic, tartaric, bitartaric, ascorbic, maleic, besylic, fumaric, gluconic, glucuronic, formic, glutamic, methanesulfonic, ethanesulfonic, benzenesulfonic, lactic, oxalic, para-bromophenylsulfonic,
carbonic, succinic, citric, benzoic and acetic acid, and related inorganic and organic acids. Such pharmaceutically acceptable salts thus include sulfate, pyrosulfate, bisulfate, sulfite, bisulfite, phosphate, monohydrogenphosphate, dihydrogenphosphate, metaphosphate, pyrophosphate, chloride, bromide, iodide, acetate, propionate, decanoate, caprylate, acrylate, formate, isobutyrate, caprate, heptanoate, propiolate, oxalate, malonate, succinate, suberate, sebacate, fumarate, maleate, butyne-l,4-dioate, hexyne-l,6-dioate, benzoate, chlorobenzoate, methylbenzoate, dinitrobenzoate, hydroxybenzoate, methoxybenzoate, phthalate,
terephthalate, sulfonate, xylenesulfonate, phenylacetate, phenylpropionate, phenylbutyrate, citrate, lactate, β-hydroxybutyrate, glycolate, maleate, tartrate, methanesulfonate,
propanesulfonate, naphthalene- 1- sulfonate, naphthalene-2- sulfonate, mandelate and the like salts. Preferred pharmaceutically acceptable acid addition salts include those formed with mineral acids such as hydrochloric acid and hydrobromic acid, and especially those formed with organic acids such as maleic acid.
[25] The compounds of the present invention contain one or more asymmetric carbon atoms. As such, a compound of this invention can exist as the individual enantiomers as well a mixture of enantiomers. Accordingly, a compound of the present invention will include not only a racemic mixture, but also individual respective enantiomers substantially free of other enantiomers. The term "substantially free of other enantiomers" as used herein means less than 25% of other enantiomers, preferably less than 10% of other enantiomers, more preferably less than 5% of other enantiomers and most preferably less than 2% of other enantiomers are present. Methods of obtaining or synthesizing enantiomers are well known in the art and may be applied as practicable to final compounds or to starting material or intermediates.
[26] Unless otherwise indicated when a disclosed compound is named or depicted by a structure without specifying the stereochemistry and has one or more chiral centers, it is understood to represent all possible stereoisomers of the compound.
[27] The term "stable compounds", as used herein, refers to compounds which possess stability sufficient to allow manufacture and which maintain the integrity of the compound for a sufficient period of time to be useful for the purposes detailed herein (e.g., formulation into therapeutic products, intermediates for use in production of therapeutic compounds, isolatable or storable intermediate compounds, treating a disease or condition responsive to therapeutic agents).
[28] "D" refers to deuterium. "Stereoisomer" refers to both enantiomers and diastereomers. "Tert", " £ ", and "t-" each refer to tertiary. "US" refers to the United States of America.
[29] Throughout this specification, the terms "each Y," "each Z," and "each W" means, all "Y" groups (e.g., Y1 and Y2), all "Z" groups (e.g., Z1, Z2, Z3, Z4 and Z5), and all "W" groups (e.g., W1, W2, W3 and W4), respectively.
Therapeutic Compounds
[30] According to one embodiment, the present invention provides a compound of Formula
or a salt thereof, wherein:
each W is independently selected from hydrogen and deuterium;
each Y is independently selected from hydrogen and deuterium;
each Z is independently selected from hydrogen and deuterium; and
at least one W, one Y, or one Z is deuterium.
[30] In one embodiment, Z5 is deuterium. In one aspect of this embodiment, Z3 and Z4 are each hydrogen. In another aspect of this embodiment, Z3 and Z4 are each deuterium. In one aspect of this embodiment, Z 1 and Z 2 are each hydrogen. In one aspect of this embodiment, Z1 and Z2 are each deuterium. In one example of this aspect, Z3 and Z4 are each hydrogen.
[31] In one aspect of the embodiment wherein Z5 is deuterium, Z3 and Z4 are each deuterium; Z 1 and Z2 are each hydrogen; and W 1 , W2 and W 3 are each hydrogen or each deuterium.
[32] In one aspect of the embodiment wherein Z5 is deuterium, Z3 and Z4 are each deuterium; Z 1 and Z2 are each deuterium; and W 1 , W2 and W 3 are each hydrogen or each deuterium.
[33] In one aspect of the embodiment wherein Z5 is deuterium, Z1, Z2, Z3 and Z4 are each hydrogen; and W 1 , W2 and W 3 are each hydrogen or each deuterium.
[34] In one embodiment, W1, W2 and W3 are the same. In one aspect of this embodiment and W 3 are simultaneously deuterium. In another aspect of this embodiment W 1 , W2 and W are simultaneously hydrogen.
[35] In another embodiment, each Z attached to a common carbon atom (that is, either=Z and Z 2 or Z 3 and Z 4 ) is the same. In one aspect of this embodiment, each member of at least one pair of Z attached to a common carbon atom is deuterium. In another aspect of this embodiment, Z1, Z2, Z3 and Z4 are simultaneously deuterium. In another aspect of this embodiment, Z1, Z2, Z3, Z4 and Z5 are simultaneously deuterium. In still another aspect, Z1,
Z 2 , Z 3 , Z 4 and Z 5 are simultaneously deuteriums and W 1 , W 2 and W 3 are simultaneously hydrogen.
[36] In another set of embodiments, any atom not designated as deuterium in any of the embodiments set forth above is present at its natural isotopic abundance.
[37] In one embodiment, Z5 is deuterium. In one aspect of this embodiment, Z3 and Z4 are deuterium; W 1 , W2 and W 3 are simultaneously hydrogen or simultaneously deuterium; Z 1 and
Z 2 are simultaneously hydrogen; and Y 1 and Y2 are simultaneously hydrogen.
[38] In one embodiment, Z5 is deuterium. In one aspect of this embodiment, Z3 and Z4 are deuterium; W 1 , W2 and W 3 are simultaneously hydrogen or simultaneously deuterium; Z 1 and Z 2 are simultaneously hydrogen; and Y 1 and Y2 are simultaneously deuterium.
[39] In one embodiment, Z 5 is deuterium. In one aspect of this embodiment, Z 1 , Z2 , Z 3 and Z4 are simultaneously hydrogen; W1, W2 and W3 are simultaneously hydrogen or
simultaneously deuterium; and Y 1 and Y 2 are simultaneously hydrogen.
[40] In another embodiment, W1, W2 and W3 are the same. In one aspect of this embodiment W 1 , W2 and W 3 are simultaneously deuterium. In another aspect of this embodiment W 1 , W2 and W 3 are simultaneously hydrogen.
[41] In another embodiment, each Z attached to a common carbon atom (e.g., Z 1 and Z 2 ; or Z3 and Z4) is the same. In one aspect of this embodiment, each member of at least one pair of
Z attached to a common carbon atom is deuterium (e.g., at least Z 1 and Z 2 are deuterium; or at least Z3 and Z4 are deuterium). In another aspect of this embodiment, Z1, Z2, Z3 and Z4 are simultaneously deuterium. In another aspect of this embodiment, Z1, Z2, Z3, Z4 and Z5 are simultaneously deuterium. In still another aspect, Z1, Z2, Z3, Z4 and Z5 are simultaneously deuterium; and W 1 , W2 and W 3 are simultaneously hydrogen.
[42] In yet another embodiment, each Y is simultaneously deuterium.
[43] In another embodiment, the compound is selected from any one of the compounds set forth below:
Compound 100,
Compound 101,
Compound 102,
Compound 103,
Compound 104,
Compound 105,
Compound 106,
Compound 109,
Compound 110, and
or a pharmaceutically acceptable salt thereof.
[44] In another embodiment, the compound is selected from any one of the compounds set forth below:
Compound 112,
Compound 113,
Compound 114, and
[45] In another embodiment, the compound is selected from any one of the compounds set forth below:
Compound 117, and-a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[46] In another embodiment, the invention provides a compound of Formula I which is a compound of Formula la or lb:
[47] In one aspect of this embodiment, Z3 and Z4 are each hydrogen. In another aspect of this embodiment, Z3 and Z4 are each deuterium. In one aspect of this embodiment, Z1 and Z2 are each hydrogen. In one aspect of this embodiment, Z 1 and Z 2 are each deuterium. In one example of this aspect, Z3 and Z4 are each hydrogen.
[48] In one aspect of this embodiment, Z3 and Z4 are each deuterium; Z1 and Z2 are each hydrogen; and W 1 , W2 and W 3 are each hydrogen or each deuterium.
[49] In one aspect of this embodiment, Z3 and Z4 are each deuterium; Z1 and Z2 are each deuterium; and W 1 , W2 and W 3 are each hydrogen or each deuterium.
[50] In one aspect of this embodiment, Z1, Z2, Z3 and Z4 are each hydrogen; and W1, W2 and W are each hydrogen or each deuterium.
[51] In one aspect of this embodiment, W1, W2 and W3 are the same. In one aspect of this embodiment W 1 , W2 and W 3 are simultaneously deuterium. In another aspect of this embodiment W 1 , W2 and W 3 are simultaneously hydrogen.
[52] In another aspect of this embodiment, each Z attached to a common carbon atom (that is, either Z1 and Z2 or Z3 and Z4) is the same. In one aspect of this embodiment, each member of at least one pair of Z attached to a common carbon atom is deuterium. In one more particular aspect of this embodiment, Z1, Z2, Z3, and Z4 are simultaneously deuterium and W 11, W2" and W 3J are simultaneously hydrogen.
[53] Compounds of Formula la and lb may be obtained from compounds of Formula I, for example, by chiral HPLC separation.
[54] The rate of epimerization for a compound of Formula la or lb, as compared to the corresponding enantiomer of lenalidomide, can be readily measured using techniques well known to the skilled artisan. For example, pure samples of compounds of Formula la and lb as well as pure samples of each enantiomer of lenalidomide can be isolated and analyzed using chiral HPLC. These pure samples can be dissolved to an appropriate concentration in an appropriate physiological buffer or bodily fluid or simulant thereof and monitored over time (for example, approximately every 5 minutes) using chiral HPLC, to assess the rate of epimerization.
[55] In one embodiment of Formula la or lb, Z 3 and Z 4 are deuterium; W 1 , W2 and W 3 are simultaneously hydrogen or simultaneously deuterium; Z 1 and Z 2 are simultaneously hydrogen; and Y 1 and Y 2 are simultaneously hydrogen.
[56] In a further embodiment, the compound is selected from any one of the compounds set forth below:
Compound 102a, Compound 102b,
Compound 105a, Compound 105b,
Compound 110a, Compound 110b,
Compound 111a, Compound 111b;
and a pharmaceutically acceptable salt thereof.
[57] In another further embodiment, the compound is selected from any one of the compounds set forth below:
Compound 113b,
Compound 114a, Compound 114b,
Compound 115a, and Compound 115b;
and a pharmaceutically acceptable salt thereof.
[58] In another embodiment, the compound is selected from any one of the compounds set forth below:
Compound 117a,
and a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[59] In one embodiment, the compound of formula 102a may be in a form such as the forms disclosed in US provisional application serial no. 61/716,826, filed October 22, 2012, such forms being prepared, for example, as disclosed in paragraphs [0085-0114] of US provisional application serial no. 61/716,826, which are incorporated by reference herein in their entirety.
[60] In one embodiment of Compound 102a or 102b, or a pharmaceutically acceptable salt thereof, the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with "Ca" in the figure below (shown for 102a) is at least 5500 (82.5% deuterium
incorporation), at least 6000 (90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
the isotopic enrichment factor for the deuterium atoms bonded to the carbon indicated with "Cb" in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000
(90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
and the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with "Cc" in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000 (90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation):
wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[61] In one embodiment of Compound 104a or 104b, or a pharmaceutically acceptable salt thereof, the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with "Ca" in the figure below (shown for 104a) is at least 5500 (82.5% deuterium
incorporation), at least 6000 (90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
the isotopic enrichment factor for the deuterium atoms bonded to the carbon indicated with "Cb" in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000 (90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with "Cc" in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000 (90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
and the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with "Cd" in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000 (90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[62] In one embodiment of Compound 110a or 110b, or a pharmaceutically acceptable salt thereof, the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with "Ca" in the figure below (shown for 110a) is at least 5500 (82.5% deuterium
incorporation), at least 6000 (90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
and the isotopic enrichment factor for the deuterium atoms bonded to the carbon indicated with "Cb" in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000 90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation):
wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[63] In one embodiment of Compound 111a or 111b, or a pharmaceutically acceptable salt thereof, the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with "Ca" in the figure below (shown for 111a) is at least 5500 (82.5% deuterium
incorporation), at least 6000 (90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
the isotopic enrichment factor for the deuterium atoms bonded to the carbon indicated with "Cb" in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000 (90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
and the isotopic enrichment factor for the deuterium atom bonded to each carbon indicated with "Ce" in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000 90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[64] In one embodiment of Compound 114a or 114b or a pharmaceutically acceptable salt thereof, the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with "Ca" in the figure below (shown for 114a) is at least 5500 (82.5% deuterium
incorporation), at least 6000 (90% deuterium incorporation), or at least 6333.3 (95% deuterium incor oration):
[65] In one embodiment of Compound 116a or 116b, or a pharmaceutically acceptable salt thereof, the isotopic enrichment factor for the deuterium atom bonded to the carbon indicated with "Ca" in the figure below (shown for 116a) is at least 5500 (82.5% deuterium
incorporation), at least 6000 (90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
and the isotopic enrichment factor for the deuterium atoms bonded to the carbon indicated with "Cc" in the figure below is at least 5500 (82.5% deuterium incorporation), at least 6000 90% deuterium incorporation), or at least 6333.3 (95% deuterium incorporation);
[66] A hemihydrate of lenalidomide has been described in US Patent No. 7,465,800. Accordingly, in one embodiment, the invention is directed to a hemihydrate of a compound of formula I, such as a crystalline hemihydrate, including a hemihydrate or crystalline hemihydrate of compounds 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116 and 117.
[67] In one embodiment the invention is directed at a compound of the Formula II
wherein Y 3 is hydrogen or deuterium, Y 5 is hydrogen or deuterium, and P 1 is a protecting group. In one embodiment, Y 3 is hydrogen. In one embodiment, Y 3 is deuterium. In one embodiment, Y5 is hydrogen. In one embodiment, Y5 is deuterium. In one embodiment, P1 is a group of the formula -C(0)-Q-R 2 , wherein Q is O or NH, and R 2 is (a) C C6 alkyl optionally substituted with C6-Q0 aryl; (b) C3-C8 cycloalkyl; or (c) C6-Cio aryl. In one more particular embodiment, P1 is benzyloxycarbonyl.
[68] In one embodiment the invention is directed at a compound of the Formula III
wherein Zl-Z5 are as defined as for Formula I, Y4 is hydrogen or deuterium and P2 is a protecting group. In one embodiment, Y4 is hydrogen. In one embodiment, Y4 is deuterium. In one embodiment, Z5 is deuterium. In one aspect of this embodiment, Z3 and Z4 are each
hydrogen. In another aspect of this embodiment, Z3 and Z4 are each deuterium. In one aspect of this embodiment, Z 1 and Z2 are each hydrogen. In one aspect of this embodiment, Z 1 and
Z are each deuterium. In one more particular aspect, each Z is deuterium.
[69] In one aspect of the embodiment wherein Z5 is deuterium, Z3 and Z4 are each deuterium; and Z 1 and Z 2 are each hydrogen.
[70] In one aspect of the embodiment wherein Z5 is deuterium, Z3 and Z4 are each deuterium; and Z1 and Z2 are each deuterium.
[71] In one aspect of any of the foregoing embodiments or aspects, P2 is a group of the formula -C(0)-Q-R 2 , wherein Q is O or NH, and R 2 is (a) C -C alkyl optionally substituted with C6-Cio aryl; (b) C3-C8 cycloalkyl; or (c) C6-Cio aryl. In one more particular aspect, P is benzyloxycarbonyl.
[72] In one embodiment, the invention is directed to a compound of Formula IV
wherein R 12 is CrC6 alkyl and P 2 is a group of the formula -C(0)-Q-R 2 , wherein Q is O or NH, and R is (a) C -C alkyl optionally substituted with C6-C10 aryl; (b) C3-C8 cycloalkyl; or
(c) l. In one aspect, P 2 is benzyloxycarbonyl. In one aspect R 12
C6-Cio ary is methyl.
[73] The following scheme illustrates the preparation of such a compound of Formula IV and its use in reparing intermediates of Formula 12b.
[74] In the foregoing scheme, l, -carbonyl-di-(l,2,4-triazole) (CDT ) may be used in place of CDI to convert 22 to 12b.
[75] In one alternative to the foregoing scheme, methanol (MeOH) may be used instead of MeOD in the first step; and NH4OH instead of ND4OD.
[76] In one embodiment, the invention is directed to a process comprising treating a com ound of formula 20
20
silyl halide and a compound of formula R OD to provide a compound of formula
or salt thereof, wherein R is CrC6 alkyl. The silyl halide may be a compound of formula (Ru)3Si-Hal, wherein R11 is CrC6 alkyl and Hal is fluoro, chloro, bromo or iodo. In one aspect of this embodiment, the silyl halide is trimethylsilyl chloride.
[77] In one embodiment, the invention is directed to a process comprising treating a com ound of formula IV
22
In Compounds of Formula II- IV and in compounds 20, 21, 21', 21", 22, and 12b, the hydrogen atoms bound to O or N are exchangeable with deuterium and vice versa. In one embodiment, the invention is directed to compounds differing from Compounds of Formula II- IV or from structures 20, 21, 21', 21", 22, and 12b, only in the presence of deuterium atoms instead of hydrogen atoms, or hydrogen atoms instead of deuterium atoms, that are bound to O or N.
[78] In another set of embodiments, any atom not designated as deuterium in any of the foregoing embodiments or aspects or examples is present at its natural isotopic abundance.
[79] The synthesis of compounds disclosed herein can be readily achieved by synthetic chemists of ordinary skill by reference to the Exemplary Synthesis and Examples disclosed herein. Relevant procedures and intermediates are disclosed, for instance, in US Patent No. 5,635,517 and US Patent Application 2006052609, in addition to MuUer, GW et al., Bioorg Med Chem Lett, 1999, 9(11): 1625.
[80] Such methods can be carried out utilizing corresponding deuterated and optionally, other isotope-containing reagents and/or intermediates to synthesize the compounds delineated herein, or invoking standard synthetic protocols known in the art for introducing isotopic atoms to a chemical structure.
Exemplary Synthesis
[81] A convenient method for synthesizing compounds of Formula I is depicted in Schemes 1 and 2.
[82] Scheme 1. Synthesis of an Appropriately Deuterated 3-Aminopiperidine-2,6-dione 03}·
12 13
[83] As shown in Scheme 1, an appropriately deuterated d,l-glutamine 10 is reacted with Cbz-chloride to yield the carbamate 11, which is then cyclized with Ι,Γ-carbonyldiimidazole (CDI) to yield 12. The carbamate protecting group is then removed from 12 by
hydrogenolysis to provide the appropriately deuterated 3-aminopiperidine-2,6-dione 13. This amine is then used as shown in Scheme 2 to produce a compound of Formula I.
[84] Appropriately deuterated D,L-glutamine 10 for use in Scheme 1 above may be prepared, for example, from the corresponding commercially available deuterated glutamic acids (D,L)-2,3,3,4,4-d5-glutamic acid, (D,L)-2,4,4-d3-glutamic acid, or (D,L)-3,3-d2- glutamic acid by methods analogous to those employed by Ogrel, A. et al., Russian Journal of Organic Chemistry, 2001, 37(4): 475-479. 85] Scheme 2. Synthesis of a Compound of Formula I.
Formula I
[86] As depicted in Scheme 2 for the preparation of a compound of Formula I, an appropriately deuterated l-bromo-2-methylbenzene 14 is lithiated with n-butyl lithium followed by reaction with methyl chloroformate to provide ester 16. Alternatively, ester 16 can be obtained by treating an appropriately deuterated 2-methylbenzoic acid with sulfonyl chloride in methanol. The ester 16 is nitrated with nitric acid in dichloroethane with an Indium catalyst to provide the nitro compound 17, which is then converted to the benzylic halide 18 by treatment with N-bromosuccinimide. Reaction of the benzylic halide 18 with an appropriately deuterated 3-aminopiperidine-2,6-dione 13 in the presence of triethylamine and heat yields the cyclized nitro compound 19, which is then converted to a compound of Formula I by hydrogenation using a Pd/C catalyst. If desired, the R and S enantiomers of a compound of Formula I can then be separated by chiral HPLC in a manner similar to that known for related compounds in the IMiD class of drugs. Examples of this type of chiral HPLC enantiomer separation are found in Sembongi, K. et al., Biological and Pharmaceutical Bulletin, 2008, 31(3): 497-500; Murphy-Poulton, S.F. et al., Journal of Chromatography, B: Analytical Technologies in the Biomedical and Life Sciences, 2006, 831(1-2): 48-56;
Eriksson, T. et al., Journal of Pharmacy and Pharmacology, 2000, 52(7): 807-817; Eriksson, T. et al., Chirality, 1998, 10(3): 223-228; Reepmeyer, J.C. et al., Chirality, 1996, 8(1): 11- 17; Aboul-Enein, H.Y. et al., Journal of Liquid Chromatography, 1991, 14(4): 667-73; and Teo, S.K. et al., Chirality, 2003, 15(4): 348-351.
[87] Scheme 3a. Preparation of Intermediate 12b.
[88] Scheme 3a depicts a preparation of the protected deuterated 3-Aminopiperidine-2,6- dione 12b. Deuterated glutamic acid 20, an exemplary preparation of which is shown in Scheme 6 below, is treated with SOCl2 and CH3OD followed by N- (benzyloxycarbonyloxy)succinimide to provide 21. Reaction of 21 with ammonium-d4 deuteroxide in D20 gave amide 22 which upon treatment with carbonyldiimidazole (CD I) cyclized to 12b. Alternatively, l, -carbonyl-di-(l,2,4-triazole) (CDT) may be used in place of CDI.
[89] Scheme 3b. Alternative Preparation of Intermediate 12b.
[90] Scheme 3b depicts an alternative preparation of 12b. Deuterated glutamic acid 20 is treated with TMSC1 (2.2 equivalents) in CH3OD to give 21' which is treated with
N-(benzyloxycarbonyloxy)succinimide and sodium carbonate (2 equivalents) to provide 21. Reaction of 21 with deuterated ammonia in D20 gave amide 22 which upon treatment with carbonyldiimidazole (CDI) cyclized to 12b. Alternatively, l, -carbonyl-di-(l,2,4-triazole) (CDT ) may be used in place of CDI. [91] Scheme 4. Preparation of Deuterated Glutamic acid 20
quenching with H+
[92] Scheme 4 depicts a preparation of deuterated glutamic acid 20. Succinic acid 23 was treated with DC1 in D20 to provide, after quenching with a mineral acid such as HC1, 24, which was treated with D-glucose-Di NAD (Nicotinamide adenine dinucleotide). D-glucose- Di is the following compound (shown below in its open chain and pyranose forms):
Similarly, a compound of formula 24'
where Zx-Z4 are as defined herein, may be treated with D-glucose-Di NAD, or D-glucose-Di, in the presence of glutamate dehydrogenase and a source of ammonia, such as an ammonium salt, such as NH4C1, to afford a compound 20':
[94] More generally, 24 (or 24') may be treated with a deuteride source to provide 20 (or 20') or a hydride source to provide 20-H, where the deuteride or hydride source is a compound or mixture capable of providing a deuteride or hydride anion, respectively, or the synthetic equivalent thereof. Such mixture may comprise a co-factor, an example of which is NAD as illustrated in Scheme 4. Another example of a co-factor is NADP. The mixture may also comprise a co-factor regeneration system, which may comprise, as an example, a dehydrogenase and a substrate for the dehydrogenase. In the example shown in Scheme 4, the mixture comprises GDH as the dehydrogenase; D-Glucose-Di (to produce 20) or D- glucose (to produce 20-H) as the substrate; and NAD as the co-factor. In one embodiment,
the D-glucose-Di is generated in situ from inexpensive D-glucono-5-lactone and NaBD4. This embodiment is advantageous in that an otherwise expensive deuterated glucose substrate is generated from relatively inexpensive reagents. Other embodiments of the deuteride or hydride source are disclosed in paragraphs [43[-[53] of application PCT/US2011/050138, and in the corresponding paragraphs of U.S. provisional application 61/379,182, incorporated by reference herein in their entirety. The isotopic enrichment factor in 20 and 20-H is over 98% at each of the positions designated with deuterium in the two structures.
[95] Intermediate 24, disclosed in Scheme 4, also may be prepared as shown in Scheme 4' below:
[96] Scheme 4' . Preparation of Intermediate 24.
[97] As shown in Scheme 4', compound 25 may be treated with D2 over Pd/C to provide 26. Reaction of 26 with diethyl oxalate affords 27, which is then treated with DCI to give, after quenching with a mineral acid such as HC1, 24.
[98] Scheme 5. Synthesis of a Compound of Formula I wherein Z 1 , Z2 and Z 5 are deuterium.
Formula I
chiral S-enantiomer
separation
Formula I
Formula I
[99] As shown in Scheme 5, 41 may be treated with 2,3,4,6,7, 8-hexahydro-lH- pyrimido[l,2-a]pyrimidine, in the presence of CDC13, to provide 42. The Cbz
(benzyloxycarbonyl) protecting group of 42 is reductively cleaved to provide 43, which is treated with 18' to provide 19'. Reduction of the nitro group of 19' provides a compound of Formula I, which may be separated into its enantiomers by chiral HPLC. Scheme 5 may be used to provide a compound of formula I wherein Z3 and Z4 are hydrogen or, in an analogous fashion, a compound of formula I wherein Z3 and Z4 are deuterium by using, for example, previously described 12b as the starting material 12b may be converted to 43b in a manner similar to that used to convert 42 to 43, as shown in Scheme 6. 100] Scheme 6. Preparation of intermediate 43b.
12b 43b
43b is then used in the process leading to a compound of formula I, such as 102 (which may then be separate in its enantiomers 102a or 102b) that is similar to the process shown in Scheme 5 to obtain a compound of Formula I starting withjntermediate 43.
[101] Scheme 7. Preparation of intermediate 53a or 53b.
O D D O BnEt3NCI o D D O H2 O D D O
D20 Z DN-CBZ TBUBR Z2 DN.CBZ Z Z2 ND2
64 65 53a - Z1=Z2=H
53b - Z =Z2=D
[102] As shown in Scheme 7, 20 or 61 (obtained from 20, disclosed in Scheme 4, by treatment with aqueous acid) is treated with thionyl chloride and CH3OD to afford 62, followed by N-(benzyloxycarbonyloxy)succinimide and sodium carbonate to provide 63. Alternatively, TMSC1 in CH3OD is used in the transformation of 61 to 62. Reaction of 63 with ammonium-d4 deuteroxide in D20 gives amide 64 which is protected with t-butyl bromide in the presence of BnEt3NCl and K2C03 to afford t-butyl ester 65. Deprotection of the benzyloxycarbonyl group of 65 via hydrogenation gives compound 53a wherein Z1 and Z2 are each hydrogen, or 53b wherein Z1 and Z2 are each deuterium.
[103] The specific approaches and compounds shown above are not intended to be limiting. The chemical structures in the schemes herein depict variables that are hereby defined commensurately with chemical group definitions (moieties, atoms, etc.) of the corresponding position in the compound formulae herein, whether identified by the same variable name (i.e.,
1 2 3
R , R , R , etc.) or not. The suitability of a chemical group in a compound structure for use in the synthesis of another compound is within the knowledge of one of ordinary skill in the art.
[104] Additional methods of synthesizing compounds of the formulae herein and their synthetic precursors, including those within routes not explicitly shown in Schemes herein, are within the means of chemists of ordinary skill in the art. Synthetic chemistry
transformations and protecting group methodologies (protection and deprotection) useful in synthesizing the applicable compounds are known in the art and include, for example, those described in R. Larock, Comprehensive Organic Transformations, VCH Publishers (1989);
T.W. Greene and P.G.M. Wuts, Protective Groups in Organic Synthesis, 3r Ed., John Wiley and Sons (1999); L. Fieser and M. Fieser, Fieser and Fieser's Reagents for Organic
Synthesis, John Wiley and Sons (1994); and L. Paquette, ed., Encyclopedia of Reagents for Organic Synthesis, John Wiley and Sons (1995) and subsequent editions thereof.
[105] Combinations of substituents and variables envisioned by this invention are only those that result in the formation of stable compounds.
Compositions
[106] The invention also provides pyrogen-free pharmaceutical compositions comprising an effective amount of a compound of Formula I (e.g., including any of the formulae herein), or a pharmaceutically acceptable salt thereof; and an acceptable carrier. The carrier(s) must be "acceptable" in the sense of being compatible with the other ingredients of the formulation and, in the case of a pharmaceutically acceptable carrier, not deleterious to the recipient thereof in amounts typically used in medicaments.
[107] Pharmaceutically acceptable carriers, adjuvants and vehicles that may be used in the pharmaceutical compositions of this invention include, but are not limited to, ion exchangers, alumina, aluminum stearate, lecithin, serum proteins, such as human serum albumin, buffer substances such as phosphates, glycine, sorbic acid, potassium sorbate, partial glyceride mixtures of saturated vegetable fatty acids, water, salts or electrolytes, such as protamine sulfate, disodium hydrogen phosphate, potassium hydrogen phosphate, sodium chloride, zinc salts, colloidal silica, magnesium trisilicate, polyvinyl pyrrolidone, cellulose-based substances, polyethylene glycol, sodium carboxymethylcellulose, polyacrylates, waxes, polyethylene-polyoxypropylene-block polymers, polyethylene glycol and wool fat.
[108] If required, the solubility and bioavailability of the compounds of the present invention in pharmaceutical compositions may be enhanced by methods well-known in the art. One method includes the use of lipid excipients in the formulation. See "Oral Lipid- Based Formulations: Enhancing the Bioavailability of Poorly Water-Soluble Drugs (Drugs and the Pharmaceutical Sciences)," David J. Hauss, ed. Informa Healthcare, 2007; and "Role of Lipid Excipients in Modifying Oral and Parenteral Drug Delivery: Basic Principles and Biological Examples," Kishor M. Wasan, ed. Wiley-Interscience, 2006.
[109] Another known method of enhancing bioavailability is the use of an amorphous form of a compound of this invention optionally formulated with a poloxamer, such as LUTROL™ and PLURONIC™ (BASF Corporation), or block copolymers of ethylene oxide and
propylene oxide. See United States patent 7,014,866; and United States patent publications 20060094744 and 20060079502.
[110] The pharmaceutical compositions of the invention include those suitable for oral, rectal, nasal, topical (including buccal and sublingual), vaginal or parenteral (including subcutaneous, intramuscular, intravenous and intradermal) administration. In certain embodiments, the compound of the formulae herein is administered transdermally (e.g., using a transdermal patch or iontophoretic techniques). Other formulations may conveniently be presented in unit dosage form, e.g., tablets and sustained release capsules, and in liposomes, and may be prepared by any methods well known in the art of pharmacy. See, for example, Remington: The Science and Practice of Pharmacy, Lippincott Williams and Wilkins, Baltimore, MD (20th ed. 2000).
[Ill] Such preparative methods include the step of bringing into association with the molecule to be administered ingredients such as the carrier that constitutes one or more accessory ingredients. In general, the compositions are prepared by uniformly and intimately bringing into association the active ingredients with liquid carriers, liposomes or finely divided solid carriers or both, and then if necessary shaping the product.
[112] In certain preferred embodiments, the compound is administered orally.
Compositions of the present invention suitable for oral administration may be presented as discrete units such as capsules, sachets or tablets each containing a predetermined amount of the active ingredient; as a powder or granules; as a solution or a suspension in an aqueous liquid or a non-aqueous liquid; or as an oil-in- water liquid emulsion or a water-in-oil liquid emulsion, or packed in liposomes and as a bolus, etc. Soft gelatin capsules can be useful for containing such suspensions, which may beneficially increase the rate of compound absorption.
[113] In the case of tablets for oral use, carriers that are commonly used include lactose and corn starch. Lubricating agents, such as magnesium stearate, are also typically added. For oral administration in a capsule form, useful diluents include lactose and dried cornstarch. When aqueous suspensions are administered orally, the active ingredient is combined with emulsifying and suspending agents. If desired, certain sweetening and/or flavoring and/or coloring agents may be added.
[114] Compositions suitable for oral administration include lozenges comprising the ingredients in a flavored basis, usually sucrose and acacia or tragacanth; and pastilles comprising the active ingredient in an inert basis such as gelatin and glycerin, or sucrose and acacia.
[115] Compositions suitable for parenteral administration include aqueous and non-aqueous sterile injection solutions which may contain anti- oxidants, buffers, bacteriostats and solutes which render the formulation isotonic with the blood of the intended recipient; and aqueous and non-aqueous sterile suspensions which may include suspending agents and thickening agents. The formulations may be presented in unit-dose or multi-dose containers, for example, sealed ampules and vials, and may be stored in a freeze dried (lyophilized) condition requiring only the addition of the sterile liquid carrier, for example water for injections, immediately prior to use. Extemporaneous injection solutions and suspensions may be prepared from sterile powders, granules and tablets.
[116] Such injection solutions may be in the form, for example, of a sterile injectable aqueous or oleaginous suspension. This suspension may be formulated according to techniques known in the art using suitable dispersing or wetting agents (such as, for example, Tween 80) and suspending agents. The sterile injectable preparation may also be a sterile injectable solution or suspension in a non-toxic parenterally-acceptable diluent or solvent, for example, as a solution in 1,3-butanediol. Among the acceptable vehicles and solvents that may be employed are mannitol, water, Ringer's solution and isotonic sodium chloride solution. In addition, sterile, fixed oils are conventionally employed as a solvent or suspending medium. For this purpose, any bland fixed oil may be employed including synthetic mono- or diglycerides. Fatty acids, such as oleic acid and its glyceride derivatives are useful in the preparation of injectables, as are natural pharmaceutically-acceptable oils, such as olive oil or castor oil, especially in their polyoxyethylated versions. These oil solutions or suspensions may also contain a long-chain alcohol diluent or dispersant.
[117] The pharmaceutical compositions of this invention may be administered in the form of suppositories for rectal administration. These compositions can be prepared by mixing a compound of this invention with a suitable non-irritating excipient which is solid at room temperature but liquid at the rectal temperature and therefore will melt in the rectum to release the active components. Such materials include, but are not limited to, cocoa butter, beeswax and polyethylene glycols.
[118] The pharmaceutical compositions of this invention may be administered by nasal aerosol or inhalation. Such compositions are prepared according to techniques well-known in the art of pharmaceutical formulation and may be prepared as solutions in saline, employing benzyl alcohol or other suitable preservatives, absorption promoters to enhance
bioavailability, fluorocarbons, and/or other solubilizing or dispersing agents known in the art.
Such administration is known to be effective with erectile dysfunction drugs: Rabinowitz JD and Zaffaroni AC, US Patent 6,803,031, assigned to Alexza Molecular Delivery Corporation.
[119] Topical administration of the pharmaceutical compositions of this invention is especially useful when the desired treatment involves areas or organs readily accessible by topical application. For application topically to the skin, the pharmaceutical composition should be formulated with a suitable ointment containing the active components suspended or dissolved in a carrier. Carriers for topical administration of the compounds of this invention include, but are not limited to, mineral oil, liquid petroleum, white petroleum, propylene glycol, polyoxyethylene polyoxypropylene compound, emulsifying wax and water.
Alternatively, the pharmaceutical composition can be formulated with a suitable lotion or cream containing the active compound suspended or dissolved in a carrier. Suitable carriers include, but are not limited to, mineral oil, sorbitan monostearate, polysorbate 60, cetyl esters wax, cetearyl alcohol, 2-octyldodecanol, benzyl alcohol and water. The pharmaceutical compositions of this invention may also be topically applied to the lower intestinal tract by rectal suppository formulation or in a suitable enema formulation. Topically-transdermal patches and iontophoretic administration are also included in this invention.
[120] Application of the subject therapeutics may be local, so as to be administered at the site of interest. Various techniques can be used for providing the subject compositions at the site of interest, such as injection, use of catheters, trocars, projectiles, pluronic gel, stents, sustained drug release polymers or other device which provides for internal access.
[121] Thus, according to yet another embodiment, the compounds of this invention may be incorporated into compositions for coating an implantable medical device, such as prostheses, artificial valves, vascular grafts, stents, or catheters. Suitable coatings and the general preparation of coated implantable devices are known in the art and are exemplified in US Patents 6,099,562; 5,886,026; and 5,304,121. The coatings are typically biocompatible polymeric materials such as a hydrogel polymer, polymethyldisiloxane, polycaprolactone, polyethylene glycol, polylactic acid, ethylene vinyl acetate, and mixtures thereof. The coatings may optionally be further covered by a suitable topcoat of fluorosilicone, polysaccharides, polyethylene glycol, phospholipids or combinations thereof to impart controlled release characteristics in the composition. Coatings for invasive devices are to be included within the definition of pharmaceutically acceptable carrier, adjuvant or vehicle, as those terms are used herein. In one preferred embodiment, a compound of Formula I is formulated into a hydrogel for delivery to the eye as described in United States Patent Publication US2005074497.
[122] According to another embodiment, the invention provides a method of coating an implantable medical device comprising the step of contacting said device with the coating composition described above. It will be obvious to those skilled in the art that the coating of the device will occur prior to implantation into a mammal.
[123] According to another embodiment, the invention provides a method of impregnating an implantable drug release device comprising the step of contacting said drug release device with a compound or composition of this invention. Implantable drug release devices include, but are not limited to, biodegradable polymer capsules or bullets, non-degradable, diffusible polymer capsules and biodegradable polymer wafers.
[124] According to another embodiment, the invention provides an implantable medical device coated with a compound or a composition comprising a compound of this invention, such that said compound is therapeutically active.
[125] According to another embodiment, the invention provides an implantable drug release device impregnated with or containing a compound or a composition comprising a compound of this invention, such that said compound is released from said device and is therapeutically active.
[126] Where an organ or tissue is accessible because of removal from the patient, such organ or tissue may be bathed in a medium containing a composition of this invention, a composition of this invention may be painted onto the organ, or a composition of this invention may be applied in any other convenient way.
[127] In another embodiment, a composition of the present invention further comprises a second therapeutic agent. The second therapeutic agent includes any compound or therapeutic agent known to have or that demonstrates advantageous properties when administered with an immunomodulator, an anti- angiogenic or an anti-neoplastic agent. Such agents are described in detail in United States Patent 5,635,517, as well as in PCT patent publications WO2005097125, WO2005055929, WO2004041190, WO2006060507,
WO2006058008, WO2006053160, WO2005044178, WO2004100953, WO2006089150, WO2006036892, WO2006018182, WO2005082415, WO2005048942, WO2005042558, WO2005035714 and WO2005027842; and in United States Patent publications
US2005100529, US2006030594, US2005143344 and US2006079461, each of the foregoing of which describes second therapeutic agents that may be combined with lenalidomide.
[128] In one embodiment, the second therapeutic agent is an agent useful in the treatment or prevention of a disease or condition selected from myelodysplasia syndromes, multiple myeloma, Non-Hodgkins lymphoma; papillary and follicular thyroid carcinoma; prostate
cancer; chronic lymphocytic leukemia, amyloidosis, complex regional pain syndrome Type I, malignant melanoma, radiculopathy, myelofibrosis, glioblastoma, gliosarcoma, malignant gliomas, myelogenous leukemia, refractory plasma cell neoplasm, chronic myelomonocytic leukemia, follicular lymphoma, ciliary body and chronic melanoma, iris melanoma, recurrent interocular melanoma, extraocular extension melanoma, solid tumors, T-cell lymphoma, erythroid lymphoma, monoblastic and monocytic leukemia; myeloid leukemia, brain tumor, meningioma, spinal cord tumors, thyroid cancers, mantle cell lymphoma, non-small cell lung cancer, ovarian cancer, prostate cancer, renal cell cancer, myelofibrosis, Burkitt's lymphoma, Hodgkin's lymphoma, large cell lymphoma, or Waldenstrom's macroglobulinemia.
[129] In another embodiment, the second therapeutic agent is an agent useful in the treatment or prevention of a disease or condition selected from dysfunctional sleep, hemoglobinopathy, anemia, macular degeneration, atherosclerosis, restenosis, pain, immunodeficiencies, CNS injury and related symptoms, CNS disorders, parasitic disease, or asbestos-related disease.
[130] Even more preferably the second therapeutic agent co-formulated with a compound of this invention is an agent useful in the treatment of myelodysplasia syndromes or multiple myeloma.
[131] In another preferred embodiment, the second therapeutic agent is selected from aldesleukin; a p38 MAP kinase inhibitor such as disclosed in US2006079461; a 24- hydroxylase inhibitor such as disclosed in WO2006036892; an aminopteridinone such as disclosed in WO2006018182; an IGF-R inhibitor such as disclosed in WO2005082415; a COX-2 inhibitor such as disclosed in WO2005048942; a nucleobase oligomer such as disclosed in WO2005042558; a chlorpromazine compound such as disclosed in
WO2005027842.
[132] In yet another preferred embodiment, the second therapeutic agent is selected from pemetrexed, topotecan, doxorubicin, bortezomib, gemcitabine, dacarbazine, dexamethasone, biaxin, doxil, vincristine, decadron, azacitidine, rituximab, prednisone, docetaxel, melphalan, or combinations thereof.
[133] In another embodiment, the invention provides separate dosage forms of a compound of this invention and a second therapeutic agent that are associated with one another. The term "associated with one another" as used herein means that the separate dosage forms are packaged together or otherwise attached to one another such that it is readily apparent that the separate dosage forms are intended to be sold and administered together (within less than 24 hours of one another, consecutively or simultaneously).
[134] In the pharmaceutical compositions of the invention, the compound of the present invention is present in an effective amount. As used herein, the term "effective amount" refers to an amount which, when administered in a proper dosing regimen, is sufficient to reduce or ameliorate the severity, duration or progression of the disorder being treated, prevent the advancement of the disorder being treated, cause the regression of the disorder being treated, or enhance or improve the prophylactic or therapeutic effect(s) of another therapy.
[135] The interrelationship of dosages for animals and humans (based on milligrams per meter squared of body surface) is described in Freireich et al., 1966, Cancer Chemother Rep, 50: 219. Body surface area may be approximately determined from height and weight of the patient. See, e.g., Scientific Tables, Geigy Pharmaceuticals, Ardsley, N.Y., 1970, 537. An effective amount of a compound of this invention can range from about 0.005 mg/kg to about 200 mg/kg, more preferably 0.01 mg/kg to about 100 mg/kg, more preferably 0.05 mg/kg to about 60 mg/kg.
[136] Effective doses will also vary, as recognized by those skilled in the art, depending on the diseases treated, the severity of the disease, the route of administration, the sex, age and general health condition of the patient, excipient usage, the possibility of co-usage with other therapeutic treatments such as use of other agents and the judgment of the treating physician. For example, guidance for selecting an effective dose can be determined by reference to the prescribing information for lenalidomide.
[137] For pharmaceutical compositions that comprise a second therapeutic agent, an effective amount of the second therapeutic agent is between about 20% and 100% of the dosage normally utilized in a monotherapy regime using just that agent. Preferably, an effective amount is between about 70% and 100% of the normal monotherapeutic dose. The normal monotherapeutic dosages of these second therapeutic agents are well known in the art. See, e.g., Wells et al, eds., Pharmacotherapy Handbook, 2nd Edition, Appleton and Lange, Stamford, Conn. (2000); PDR Pharmacopoeia, Tarascon Pocket Pharmacopoeia 2000, Deluxe Edition, Tarascon Publishing, Loma Linda, Calif. (2000), each of which references are entirely incorporated herein by reference.
[138] It is expected that some of the second therapeutic agents referenced above will act synergistically with the compounds of this invention. When this occurs, its will allow the effective dosage of the second therapeutic agent and/or the compound of this invention to be reduced from that required in a monotherapy. This has the advantage of minimizing toxic side effects of either the second therapeutic agent of a compound of this invention,
synergistic improvements in efficacy, improved ease of administration or use and/or reduced overall expense of compound preparation or formulation.
Methods of Treatment
[139] According to another embodiment, the invention provides a method of treating a disease that is beneficially treated by lenalidomide in a patient in need thereof, comprising the step of administering to the patient an effective amount of a compound or a composition of this invention. Such diseases are well known in the art and are disclosed in United States Patent 5,635,517, as well as in PCT patent publications WO2005097125, WO2005055929, WO2004041190, WO2006060507, WO2006058008, WO2006053160, WO2005044178, WO2004100953, WO2006089150, WO2006036892, WO2006018182, WO2005082415, WO2005048942, WO2005042558, WO2005035714 and WO2005027842; and in United States Patent publications US2005100529, US2006030594, US2005143344 and
US2006079461.
[140] In one preferred embodiment, the disease or condition is selected from
myelodysplasia syndromes, multiple myeloma, Non-Hodgkins lymphoma; papillary and follicular thyroid carcinoma; prostate cancer; chronic lymphocytic leukemia, amyloidosis, complex regional pain syndrome Type I, malignant melanoma, radiculopathy, myelofibrosis, glioblastoma, gliosarcoma, malignant gliomas, myelogenous leukemia, refractory plasma cell neoplasm, chronic myelomonocytic leukemia, follicular lymphoma, ciliary body and chronic melanoma, iris melanoma, recurrent interocular melanoma, extraocular extension melanoma, solid tumors, T-cell lymphoma, erythroid lymphoma, monoblastic and monocytic leukemia; myeloid leukemia, brain tumor, meningioma, spinal cord tumors, thyroid cancers, mantle cell lymphoma, non-small cell lung cancer, ovarian cancer, prostate cancer, renal cell cancer, myelofibrosis, Burkitt's lymphoma, Hodgkin's lymphoma, large cell lymphoma, or
Waldenstrom's macroglobulinemia.
[141] In another embodiment, the disease is selected from myelodysplasia syndromes or multiple myeloma.
[142] Identifying a patient in need of such treatment can be in the judgment of a patient or a health care professional and can be subjective (e.g. opinion) or objective (e.g. measurable by a test or diagnostic method).
[143] In another embodiment, the above method of treatment comprises the further step of co-administering to the patient one or more second therapeutic agents. The choice of second therapeutic agent may be made from any second therapeutic agent known to be useful for co-
administration with lenalidomide. The choice of second therapeutic agent is also dependent upon the particular disease or condition to be treated. Examples of second therapeutic agents that may be employed in the methods of this invention are those set forth above for use in combination compositions comprising a compound of this invention and a second therapeutic agent.
[144] In one embodiment, the second therapeutic agent and the corresponding disease for which the second therapeutic agent is co-administered with a compound of this invention is set forth in Table 1 below.
[145] Table 1. Second Therapeutic Agents for Various Diseases or Conditions
[146] The term "co-administered" as used herein means that the second therapeutic agent may be administered together with a compound of this invention as part of a single dosage form (such as a composition of this invention comprising a compound of the invention and an second therapeutic agent as described above) or as separate, multiple dosage forms.
Alternatively, the additional agent may be administered prior to, consecutively with, or following the administration of a compound of this invention. In such combination therapy treatment, both the compounds of this invention and the second therapeutic agent(s) are administered by conventional methods. The administration of a composition of this invention comprising both a compound of the invention and a second therapeutic agent to a patient does not preclude the separate administration of that same therapeutic agent, any other second therapeutic agent or any compound of this invention to the patient at another time during a course of treatment.
[147] Effective amounts of these second therapeutic agents are well known to those skilled in the art and guidance for dosing may be found in patents and published patent applications referenced herein, as well as in Wells et al, eds., Pharmacotherapy Handbook, 2nd Edition, Appleton and Lange, Stamford, Conn. (2000); PDR Pharmacopoeia, Tarascon Pocket Pharmacopoeia 2000, Deluxe Edition, Tarascon Publishing, Loma Linda, Calif. (2000), and other medical texts. However, it is well within the skilled artisan's purview to determine the second therapeutic agent's optimal effective-amount range.
[148] In one embodiment of the invention where a second therapeutic agent is administered to a patient, the effective amount of the compound of this invention is less than its effective amount would be where the second therapeutic agent is not administered. In another embodiment, the effective amount of the second therapeutic agent is less than its effective amount would be where the compound of this invention is not administered. In this way, undesired side effects associated with high doses of either agent may be minimized. Other potential advantages (including without limitation improved dosing regimens and/or reduced drug cost) will be apparent to those of skill in the art.
[149] In yet another aspect, the invention provides the use of a compound of Formula I alone or together with one or more of the above-described second therapeutic agents in the manufacture of a medicament, either as a single composition or as separate dosage forms, for treatment or prevention in a patient of a disease, disorder or symptom set forth above.
[150] Another aspect of the invention is a compound of Formula I for use in the treatment or prevention in a patient of a disease, disorder or symptom thereof delineated herein.
Examples
[151] Example 1. Synthesis of (^-3-(Amino-d7)(piperidine-l,3,4,4,5,5-dg)-2,6-dione (43b) deuterium chloride salt. The DC1 salt of intermediate 43b was prepared as outlined in Scheme 10 below. Details of the synthesis follow.
152] Scheme 10. Preparation of Intermediate 43b.
12b' 43b «DCI
[153] Synthesis of (5)-5-Amino-(2-benzyloxycarbonylamino)-5-oxo-(2,3,3,4,4-d5)- pentanoic acid (11a). Deuterium oxide (Cambridge Isotopes, 99 atom D, 2.5 mL) was added to a suspension of L-glutamine-2,3,3,4,4-ds 10a (CDN Isotopes, 99.2 atom D, 2.58 g, 17.09 mmol, 1.0 equiv) in tetrahydrofuran (150 mL) and the suspension was stirred for 0.25 hours (h). N-(Benzyloxycarbonyloxy)succinimide 91 (8.93 g, 35.88 mmol, 2.1 equiv) was added in one portion and the resulting mixture was stirred at room temperature for 42 hours. The mixture was concentrated under reduced pressure to remove most of the tetrahydrofuran, and saturated aqueous sodium bicarbonate solution (30 mL) was added to the residual oily solid. The mixture was diluted with water (10 mL) and washed with ethyl acetate (50 mL). The organic phase was discarded. The aqueous phase was acidified to pH 1-2 with a mixture of concentrated hydrochloric acid and ice. The mixture was extracted with ethyl acetate (5 x 50 mL). The combined organic phases were washed with brine (50 mL), dried over sodium sulfate, filtered and concentrated under reduced pressure to give a gel-like residue. The residue was dissolved in methanol (30 mL), the solution was diluted with toluene (30 mL) and the mixture was concentrated under reduced pressure. The residue was redissolved in methanol (30 mL), and the resulting solution was diluted with toluene (30 mL) and then seeded prior to concentration. The mixture was concentrated under reduced pressure at room temperature to give a white solid. The solid was suspended in 1: 1 toluene/heptane (60 mL) and concentrated under reduced pressure. The resulting white solid was dried under high vacuum for 1.75 hours to give 3.80 g (78%) of 11a.
[154] Synthesis of (S)-Benzyl 2,6-dioxo(piperidin-3,4,4,5,5-d5)-3-ylcarbamate (12b'). A mixture of 11a (3.27 g, 11.47 mmol, 1.0 equiv) and Ν,Ν'-carbonyldiimidazole "CDI" (3.60 g, 13.72 mmol, 1.2 equiv) in tetrahydrofuran (75 mL) was heated at reflux for 8.5 hours. A clear solution formed after approximately 0.75 hours and a yellow color developed gradually over the course of the reaction. The reaction mixture was cooled to room temperature and stirred overnight. The reaction mixture was concentrated under reduced pressure to remove most of the tetrahydrofuran and the residual yellow oil was partitioned between ethyl acetate (150 mL) and IN hydrochloric acid (100 mL). The organic phase was washed with brine (75 mL), dried over sodium sulfate, filtered and concentrated under reduced pressure to give a colorless oil that slowly crystallized. The crude product was purified on an Analogix automated chromatography system eluting with a gradient of 25-67% ethyl acetate/heptanes. Product-containing fractions were concentrated under reduced pressure to give 2.41 g (79%) of 12b' as a white solid.
[155] Synthesis of (5)-3-(Amino-d2)(piperidine-l,3,4,4,5,5-d6)-2,6-dione, (43b), deuterium chloride salt. A mixture of 12b' and methanol-di (Cambridge Isotopes, 99 atom% D, 10 mL) was warmed until all solids dissolved, then cooled to room temperature and concentrated under reduced pressure. The residual solid was redissolved in a mixture of methanol-di (10 mL) and tetrahydrofuran (10 mL) and 10% Pd-C (50 mg) was added. The mixture was subjected to hydrogenation at 35-40 psi hydrogen pressure for 2.75 hours. The mixture was filtered through a pad of Celite, which was washed with methanol-di (40 mL). A solution of 35% deuterium chloride in deuterium oxide (Aldrich, 99.5 atom% D, 0.75 mL) was added to the combined filtrates. After several minutes, a small amount of white solid formed. The mixture was then concentrated under reduced pressure to give a wet solid. The wet solid was azeotropically dried by concentrating under reduced pressure with toluene (4 x 25 mL). The resulting white solid was further dried under high vacuum at room temperature for 1.5 hours to give 0.58 g (103%) of the DC1 salt of 43b.
[156] Example 2. Synthesis of Methyl 2-(Bromomethyl-d?)-3-nitrobenzoate (18a).
Intermediate 18a was prepared as outlined in Scheme 11 below. Details of the synthesis are as follows.
[157] Scheme 11. Preparation of Intermediate 18a.
17a 17b 18a
[158] Synthesis of Methyl 2-(Methyl-d3)-3-nitrobenzoate (17b). Sodium (0.27 g, 11.7 mmol, 11.7 mol%) was dissolved in methanol-di (Aldrich, 99.5 atom% D, 250 mL). Methyl 2-methyl-3-nitrobenzoate 17a (19.5 g, 100 mmol) was added and the mixture was heated at reflux for 25 hours. An aliquot of the reaction mixture was withdrawn and concentrated under a stream of nitrogen. 1H NMR of the residual solid showed approximately 88% deuterium incorporation at the 2-methyl group. The mixture was heated at reflux for an additional 18 hours. 1H NMR of an aliquot showed approximately 92% deuterium incorporation. The mixture was cooled to room temperature and concentrated under reduced pressure to give a brown solid. This solid was combined with approximately 1.6 g of material (approximately 95% D) from an earlier batch and all of the solids were dissolved in fresh methanol-di (200 mL). A solution of sodium (0.27 g, 11.7 mmol) in methanol-di (15 mL) was added and the mixture was heated at reflux for 24 hours. 1H NMR of an aliquot showed approximately 99% deuterium incorporation. The mixture was cooled to room temperature and concentrated under reduced pressure to give a brown solid. The solid was dissolved in methyl ie/t-butyl ether (600 mL) and the solution was washed with water (100 mL). The organic phase was separated, washed with water (200 mL), brine (100 mL), dried over sodium sulfate, filtered, and concentrated under reduced pressure to give 19.3 g (88% combined yield) of 17b as an off-white solid.
[159] Synthesis of Methyl 2-(bromomethyl-d2)-3-nitrobenzoate (18a). Benzoyl peroxide (25% water) (1.6 g, 4.5 mmol, 5 mol%) was added to a suspension of 17b (17.8 g, 90 mmol, 1.0 equiv) and N-bromosuccinimide (17.8 g, 99 mmol, 1.1 equiv) in carbon tetrachloride (350 mL). The reaction mixture was heated at reflux for 22.5 hours, and then cooled to room temperature. N-Bromosuccinimide (5.3 g, 30 mmol, 0.33 equiv) and benzoyl peroxide (25% water) (0.5 g) were added and the reaction mixture was heated at reflux for 8 hours, cooled to room temperature and stirred overnight. The yellow organic suspension was washed with saturated sodium thiosulfate solution (250 mL), water (200 mL), brine (200 mL), dried over sodium sulfate, filtered, and concentrated under reduced pressure to give 27.9 g of crude
product which partially crystallized. The crude product was dissolved in a minimum volume of dichloromethane and adsorbed onto silica gel. The adsorbed material was dry-loaded onto a column of silica gel (400 g) packed in heptanes. The column was eluted with heptanes (2 L), 5% methyl iert-butyl ether/heptanes (2 L), 10% methyl iert-butyl ether/heptanes (2 L) and 20% methyl ie/t-butyl ether/heptanes (3.5 L). Product-containing fractions were concentrated under reduced pressure and the resulting solid was triturated with hexanes (approximately 100 mL), filtered and dried to give 20.2 g (81%) of 18a as a pale yellow solid.
[160] Example 3. Synthesis of 3-(4-Amino-l-oxo-3 -d2-isoindolin-2-yl)(piperidine- 3,4,4,5, 5-dQ-2,6-dione (104). Compound 104 was prepared as outlined in Scheme 12 below. Details of the synthesis are set forth below.
[161] Scheme 12. Preparation of Compound 104.
43b 18a
19a 104
[162] Synthesis of 3-(4-Nitro-l-oxo-3,3-d2-isoindolin-2-yl)(piperidine-3,4,4,5,5-d5)-2,6- dione (19a). Triethylamine (1.05 g, 1.45 mL, 10.4 mmol, 2.1 equiv) was added dropwise via syringe to a stirred suspension of 43b (0.86 g, 4.97 mmol, 1.0 equiv) and 18a (1.37 g, 4.37 mmol, 1.0 equiv) in anhydrous N,N-dimethylacetamide (15 mL). The reaction mixture was heated to approximately 85 °C for 1.5 hours. The reaction mixture became dark blue upon heating and a suspension reformed. The reaction mixture was cooled to room temperature and deuterium oxide (Cambridge Isotopes, 99 atom% D, 10 mL) was added slowly to the reaction mixture. The mixture was stirred for 10 minutes, then the solid was filtered, washed with deuterium oxide (20 mL) and then with methanol-di (Cambridge Isotopes, 99 atom% D, 20 mL), and dried to give 1.01 g of 19a as a pale gray solid. 1H NMR showed that 19a contained approximately 7-8% H at the 3-position of the piperidinedione ring and approximately 6-7%
H at the 3-position of the isoindolinone ring. A portion of the crude product 19a (500 mg) was then suspended in acetonitrile (40 mL), and deuterium oxide (Cambridge Isotopes, 99.8 atom% D, 4 mL) was added followed by triethylamine (0.23 mL, 1.68mmol). The suspension was heated at reflux for 8 h, cooled to rt and stirred overnight. The solid was filtered, washed with acetonitrile (5 mL), and dried to give 371 mg of an off-white solid. 1H NMR showed that recovered 19a contained approximately 3% H at the 3-position of the piperidinedione ring and approximately 2% H at the 3-position of the isoindolinone ring.
[163] Synthesis of 3-(4-Amino-l-oxo-3,3-d2-isoindolin-2-yl)(piperidine-3,4,4,5,5-d5)-2,6- dione (104). Approximately 10 mg of 10% palladium on carbon (approximately 50% wet with deuterium oxide) was added to a suspension of 19a (350 mg) in methanol-di
(Cambridge Isotopes, 99 atom% D, 350 mL) and the mixture was subjected to an atmosphere of deuterium gas (approximately 50 psi) for 5 hr. The mixture was filtered through a pad of Celite and the pad was washed with methanol-di (100 mL). The filtrate was concentrated under reduced pressure to give a white solid with some gummy material present. The crude product was triturated with hot ethyl acetate (approximately 20 mL) and filtered while warm to give 261 mg of 104 as a light tan solid. 1H-NMR (300 MHz, DMSO-d6): δ 5.42 (s, 1H), 5.44 (s, 1H), 6.79 (d, J = 7.9, 1H), 6.91 (d, J = 7.0, 1H), 7.19 (dd, J \= 7.6, J 2= 7.6, 1H), 11.02 (s, 1H). 13C-NMR (75 MHz, DMSO-d6): δ 111.08, 117.04, 126.16, 129.55, 133.04, 144.32, 144.38, 169.62, 172.01, 173.69. HPLC (method: Zorbax 4.6x50 mm SB-Aq 3.5 μηι column - gradient method 2-98% ACN + 0.1% formic acid over 6.0 min with MSD in ESI positive mode; 0.63 mL/min; wavelength: 254 nm): retention time: 3.99 min; 98.6% purity; MS (M+H): 267.0.
[164] Example 4. Chiral Separation of Compound 104 to Compounds 104a and 104b. The enantiomers of Compound 104 were separated via chiral chromatography as described below.
104a 104b
[165] Separation of isomers 3-(4-Amino-l-oxo-3,3-d2-isoindolin-2-yl)(piperidine- 3,4,4,5,5-d5)-2,6-dione (104a, S-enantiomer; and 104b, R-enantiomer). Batches of 104 (25 mg/batch) for injection into the HPLC instrument were dissolved in methanol-D
(Cambridge Isotopes, 99 atom% D, 15-17 mL/batch) via sonication. Separation was carried
out in 36 injections on a Daicel ChiralPak AD column (20 x 250 mm, 10 μιη) with
approximately 1400 μΐ^ of 104 solution per injection. Each run was eluted with the isopropanol/hexanes solvent system shown in Table 2 below.
[166] Table 2. Solvent System for Chiral HPLC Separation
Time Flow Rate Hexanes
(min) (mL/min) IPA (%) (%)
0 10 40 60
2 12 40 60
25 12 50 50
27 12 50 50
28 12 40 60
33 12 40 60
Fractions containing the 1st eluting enantiomer were pooled and concentrated to give 34.2 mg as an off-white solid. Chiral HPLC analysis indicated the 1st eluting enantiomer was
>99% ee. HPLC analysis indicated the sample was 95.8% pure. 1H NMR showed that the 1st eluting enantiomer contained approximately 2% H at the 3-position of the piperidinedione ring and approximately 2% H at the 3-position of the isoindolinone ring. 1H-NMR (300 MHz, DMSO-d6): δ 5.41 (s, 2H), 6.79 (d, J = 7.9, 1H), 6.91 (d, J = 7.3, 1H), 7.18 (dd, J i= 7.9, J 2= 7.3, 1H), 10.99 (s, 1H). HPLC (method: Zorbax 4.6x50 mm SB-Aq 3.5 μηι column - gradient method 2-98% ACN + 0.1% formic acid over 6.0 min with MSD in ESI positive mode; 0.63 mL/min; wavelength: 254 nm): retention time: 3.91 min; 95.8% purity; Chiral HPLC (method: Chiralpak AD 25 cm column - isocratic method 50% hexane/ 50% isopropanol for 45 minutes at 0.600 mL/min; Wavelength: 210 nm): retention time: 12.08 min; >99% ee. MS (M+H): 267.3.
[167] Fractions containing the 2nd eluting enantiomer were pooled and concentrated to give 29.1 mg as a light tan solid. Chiral HPLC analysis indicated the 2nd eluting enantiomer was >99% ee. HPLC analysis indicated the sample was >99% pure. 1H NMR showed that the 2nd eluting enantiomer contained approximately 2% H at the 3-position of the piperidinedione ring and approximately 2% H at the 3-position of the isoindolinone ring. 1H-NMR (300 MHz, DMSO-d6): δ 5.41 (s, 2H), 6.79 (d, J = 7.9, 1H), 6.91 (d, J = 7.6, 1H), 7.18 (dd, J 1= 7.9, J 2= 7.3, 1H), 10.98 (s, 1H). HPLC (method: Zorbax 4.6x50 mm SB-Aq 3.5 μηι column - gradient method 2-98% ACN + 0.1% formic acid over 6.0 min with MSD in ESI positive mode; 0.63 mL/min; wavelength: 254 nm): retention time: 3.91 min; 99.6% purity; Chiral HPLC (method: Chiralpak AD 25 cm column - isocratic method 50% hexane/ 50%
isopropanol for 45 minutes at 0.600 mL/min; Wavelength: 210 nm): retention time: 14.75 min; 99.3% ee. MS (M+H): 267.3.
[168] Example 5. Synthesis of (£)-4-Amino-2-(3,5,5-d3-2,6-dioxopiperidin-3- yl)isoindorine- 3-dione (116). Compound 116 was prepared as outlined in Scheme 13 below. Details of the synthesis follow.
[169] Scheme 13. Synthesis of Compound 116.
Compound 116
[170] Synthesis of Benzyl 2,6-dioxo(piperidin-3,5,5-d3)-3-ylcarbamate (12c). To 12a (2.7 g, 10.4 mmol, 1.0 equiv) in CDC13 (100 mL, Cambridge Isotopes, 99.8 atom% D) at room temperature under nitrogen was added 2,3,4,6,7, 8-hexahydro-lH-pyrimido[ 1,2- fl]pyrimidine (150 mg, 1.1 mmol, 0.1 equiv). [12a may be prepared in a manner analogous to the methods disclosed in patent application WO2012/079022A1, except that deuterated reagents and solvents are not utilized in the synthesis.] The reaction was heated to reflux for 2.5 days. The mixture was cooled to room temperature, diluted with dichloromethane (100 mL), and washed with IN DC1 in D20 (1 x 100 mL). The organic layer was dried over
Na2S04, filtered, and evaporated yielding 12c (2.6 g) as a white solid. Proton NMR showed 5% H remaining at the 5 position. A second cycle was carried out using the above conditions to yield 12c (2.5 g, 92% yield) as a white solid with less than 1% H at each the 3 and 5 positions.
[171] Synthesis of 3-Amino(piperidine-3,5,5-d3)-2,6-dione (43a). A 500 mL Parr shaker bottle was charged with 42a (500 mg, 1.9 mmol, 1 equiv), 10% Pd/C (50 mg, dry), MeOD (20 mL, Cambridge Isotopes, 99 atom% D), and THF (20 mL). The reaction was
hydrogenated at 40 psi H2 for 5 hours. The reaction mixture was filtered through a pad of Celite, washing with THF. The filtrate was evaporated, yielding 43a (270 mg, quantitative yield) as a light blue solid which was used immediately.
[172] Synthesis of 3-(4-Nitro-l-oxoisoindolin-2-yl)(piperidine-3,5,5-d3)-2,6-dione (19b). Anhydrous N,N-dimethylacetamide (10 mL) was added to a mixture of 43a (270 mg, 1.9 mmol, 1 equiv), 18b (520 mg, 1.9 mmol, 1 equiv) and sodium carbonate (100 mg, 0.95 mmol, 0.5 equiv) at room temperature. The reaction mixture was stirred for 18 hours, resulting in the formation of a grey solid in a purple solution. The reaction mixture was cooled to 0 °C and D20 (50 mL; Cambridge Isotopes, 99 atom% D) was added dropwise. The mixture was stirred at 0 °C for 30 minutes and the solids were then collected by filtration, washing with D20 (100 mL) and MTBE (100 mL). The off-white solid was dried in a vacuum oven at 40 °C for 3 hours, yielding 450 mg (81% yield) of 19b.
[173] Synthesis of 3-(4-Amino-l-oxoisoindolin-2-yl)(piperidine-3,5,5-d3)-2,6-dione (Compound 116). A suspension of 19b (430 mg, 1.5 mmol) in anhydrous DMF (40 mL) was added to 10% Pd/C (40 mg, dry) and the mixture was reduced at 45 psi pressure of hydrogen for 4 hours. The reaction mixture was then filtered through Celite, washing with DMF, and the filtrate was concentrated under reduced pressure (high vacuum pump) at 45 °C, yielding a brown oil. The residue was cooled to 0 °C and, with vigorous stirring, ethyl acetate (50 mL) was added dropwise to produce an off-white precipitate. The solids were collected by vacuum filtration, washing with ethyl acetate, to yield 360 mg of Compound 116 which contained residual DMF. The solids were dissolved in ACN (150 mL) with gentle warming at 40 °C. The solution was filtered through a pad of Celite to remove insoluble impurities. The filtrate was concentrated under reduced pressure to a final slurry volume of approximately 5 mL, ensuring the residue did not evaporate to dryness. The resulting solids were collected by vacuum filtration, washing with minimal ACN. The solids were dried on the filter cake, yielding 340 mg (88% yield) of Compound 116 as an off-white solid.
[174] Example 6. Chiral Separation of Compound 116 to Compounds 116a and 116b. The enantiomers of Compound 116 were separated via chiral chromatography as described below.
[175] Separation of isomers 3-(4-amino-l-oxoisoindolin-2-yl)(piperidine-3,5,5-d3)-2,6- dione (116a, S-enantiomer; and 116b, R-enantiomer). Racemic compound 116 (280 mg) was dissolved in acetonitrile at a concentration of 2 mg/mL (preparing 10-20 mL of solution at a time) and the product was separated by preparative HPLC on a Daicel ChiralPak AD column (20 x 250 mm, 10 μιη) with approximately 1500 μΐ^ of 116 solution per injection using the following gradient:
Time Flow Rate Hexanes
(min) (mL/min) IPA (%) ( )
0 10 35 65
2 12 35 65
25 12 50 50
27 12 50 50
28 12 35 65
33 12 35 65.
Fractions containing each enantiomer were pooled and concentrated, yielding 116a (93 m: 99.8%ee; [M+H]+ = 263) and 116b (89 mg; 98.5%ee; [M+H]+ = 263) as off-white solids.
Chiral HPLC analytical method: Chiralpak AD 4.6 x 250 mm, 10 um, 100% MeOH isocratic for 40 minutes at 1.0 mL/min; retention times: 4.52 min (116a) and 6.07 min (116b).
[176] Without further description, it is believed that one of ordinary skill in the art can, using the preceding description and the illustrative examples, make and utilize the
compounds of the present invention and practice the claimed methods. It should be understood that the foregoing discussion and examples merely present a detailed description of certain preferred embodiments. It will be apparent to those of ordinary skill in the art that various modifications and equivalents can be made without departing from the spirit and scope of the invention.
Claims
1. A compound of Formula I:
or a pharmaceutically acceptable salt thereof, wherein:
each W is independently selected from hydrogen or deuterium;
each Y is independently selected from hydrogen or deuterium;
each Z is independently selected from hydrogen or deuterium; and
at least one W, one Y, or one Z is deuterium.
2. The compound of claim 1, wherein Z5 is deuterium.
3. The compound of claim 2, wherein Z 3 and Z 4 are deuterium; W 1 , W2 and W 3 are simultaneously hydrogen or simultaneously deuterium; Z 1 and Z 2 are simultaneously hydrogen; and Y 1 and Y 2 are simultaneously hydrogen.
4. The compound of claim 2, wherein Z 3 and Z 4 are deuterium; W 1 , W2 and W 3 are simultaneously hydrogen or simultaneously deuterium; Z 1 and Z 2 are simultaneously hydrogen; and Y 1 and Y 2 are simultaneously deuterium
5. The compound of claim 1, wherein W 1 , W2 and W 3 are the same.
6. The compound of claim 5, wherein W 1 , W2 and W 3 are simultaneously hydrogen.
7. The compound of claim 6, wherein Z1, Z2, Z3 and Z4 are the same.
8. The compound of claim 7, wherein Z1, Z2, Z3, Z4 and Z5 are simultaneously deuterium.
The compound of claim 8, wherein each Y is simultaneously deuterium.
10. The compound of claim 1, wherein the compound is selected from the group consisting of:
Compound 100,
Compound 101,
Compound 102,
Compound 103,
Compound 104,
Compound 105,
Compound 106,
11. The compound of claim 1, wherein the compound is selected from the group consisting of:
Compound 110, and
or a pharmaceutically acceptable salt thereof.
12. The compound of claim 1, wherein the compound is selected from the group consisting of:
or a pharmaceutically acceptable salt thereof.
13. The compound of claim 1, which is a compound of Formula la or lb:
or a pharmaceutically acceptable salt thereof.
14. The compound of claim 13, wherein Z 3 and Z 4 are deuterium; W 1 , W2 and W 3 are simultaneously hydrogen or simultaneously deuterium; Z 1 and Z 2 are simultaneously hydrogen; and Y 1 and Y 2 are simultaneously hydrogen.
15. The compound of claim 13, wherein Z 3 and Z 4 are deuterium; W 1 , W2 and W 3 are simultaneously hydrogen or simultaneously deuterium; Z 1 and Z 2 are simultaneously hydrogen; and Y 1 and Y 2 are simultaneously deuterium.
16. The compound of claim 13, wherein the compound is selected from the group consisting of:
Compound 102a,
or a pharmaceutically acceptable salt thereof.
17. The compound of claim 13, wherein the compound is selected from the group consisting of:
Compound 102b,
Compound 106b;
or a pharmaceutically acceptable salt thereof.
18. The compound of claim 13, wherein the compound is selected from the group consisting of:
ompound 110a, Compound 110b,
Compound 111a, and Compound 111b;
or a pharmaceutically acceptable salt thereof.
19. The compound of claim 13, wherein the compound is selected from the group consistin of:
Compound 112a, Compound 112b,
Compound 113a, and Compound 113b;
or a pharmaceutically acceptable salt thereof.
20. The compound of claim 13, wherein the compound is selected from the group consisting of:
Compound 116a Compound 116b
or a pharmaceutically acceptable salt thereof.
21. The compound of any one of claims 1-20, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
22. A pyrogen-free pharmaceutical composition comprising a compound of claim 1, and a pharmaceutically acceptable carrier.
23. The composition of claim 22, additionally comprising a second therapeutic agent selected from pemetrexed, topotecan, doxorubicin, bortezomib, gemcitabine, dacarbazine, dexamethasone, biaxin, doxil, vincristine, decadron, azacitidine, rituximab, prednisone, docetaxel, melphalan, and combinations thereof.
24. A method of treating a disease or condition selected from myelodysplasia syndromes, multiple myeloma, Non-Hodgkins lymphoma; papillary and follicular thyroid carcinoma; prostate cancer; chronic lymphocytic leukemia, amyloidosis, complex regional pain syndrome Type I, malignant melanoma, radiculopathy, myelofibrosis, glioblastoma, gliosarcoma, malignant gliomas, myelogenous leukemia, refractory plasma cell neoplasm, chronic myelomonocytic leukemia, follicular lymphoma, ciliary body and chronic melanoma, iris melanoma, recurrent interocular melanoma, extraocular extension melanoma, solid tumors, T-cell lymphoma, erythroid lymphoma, monoblastic and monocytic leukemia;
myeloid leukemia, brain tumor, meningioma, spinal cord tumors, thyroid cancers, mantle cell lymphoma, non-small cell lung cancer, ovarian cancer, prostate cancer, renal cell cancer, myelofibrosis, Burkitt's lymphoma, Hodgkin's lymphoma, large cell lymphoma, or
Waldenstrom's macro globulinemia in a patient in need thereof, the method comprising the step of administering to the patient a composition of claim 22.
25. The method of claim 24, wherein the disease is selected from myelodysplastic syndromes or multiple myeloma.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361751512P | 2013-01-11 | 2013-01-11 | |
| US61/751,512 | 2013-01-11 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2014110322A2 true WO2014110322A2 (en) | 2014-07-17 |
| WO2014110322A3 WO2014110322A3 (en) | 2014-09-12 |
Family
ID=50031578
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2014/010972 WO2014110322A2 (en) | 2013-01-11 | 2014-01-10 | Substituted dioxopiperidinyl phthalimide derivatives |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2014110322A2 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3407889A4 (en) * | 2016-03-25 | 2019-03-13 | Intra-Cellular Therapies, Inc. | Organic compounds |
| US10597394B2 (en) | 2014-04-04 | 2020-03-24 | Intra-Cellular Therapies, Inc. | Organic compounds |
| US10716786B2 (en) | 2017-03-24 | 2020-07-21 | Intra-Cellular Therapies, Inc. | Transmucosal and subcutaneous compositions |
| US11052084B2 (en) | 2018-08-31 | 2021-07-06 | Intra-Cellular Therapies, Inc. | Pharmaceutical capsule compositions comprising lumateperone mono-tosylate |
Citations (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5304121A (en) | 1990-12-28 | 1994-04-19 | Boston Scientific Corporation | Drug delivery system making use of a hydrogel polymer coating |
| US5635517A (en) | 1996-07-24 | 1997-06-03 | Celgene Corporation | Method of reducing TNFα levels with amino substituted 2-(2,6-dioxopiperidin-3-yl)-1-oxo-and 1,3-dioxoisoindolines |
| WO1998003502A1 (en) | 1996-07-24 | 1998-01-29 | Celgene Corporation | Substituted 2(2,6-dioxopiperidin-3-yl)phthalimides and -1-oxoisoindolines and method of reducing tnf-alpha levels |
| US5886026A (en) | 1993-07-19 | 1999-03-23 | Angiotech Pharmaceuticals Inc. | Anti-angiogenic compositions and methods of use |
| US6099562A (en) | 1996-06-13 | 2000-08-08 | Schneider (Usa) Inc. | Drug coating with topcoat |
| WO2004041190A2 (en) | 2002-10-31 | 2004-05-21 | Celgene Corporation | Composition for the treatment of macular degenration |
| US6803031B2 (en) | 2001-05-24 | 2004-10-12 | Alexza Molecular Delivery Corporation | Delivery of erectile dysfunction drugs through an inhalation route |
| WO2004100953A1 (en) | 2003-05-19 | 2004-11-25 | Pharmacia & Upjohn Company Llc | Combination of irinotecan and revimid for the treating multiple myeloma |
| WO2005027842A2 (en) | 2003-09-18 | 2005-03-31 | Combinatorx, Incorporated | Combinations of drugs for the treatment of neoplasms |
| US20050074497A1 (en) | 2003-04-09 | 2005-04-07 | Schultz Clyde L. | Hydrogels used to deliver medicaments to the eye for the treatment of posterior segment diseases |
| WO2005035714A2 (en) | 2003-08-26 | 2005-04-21 | Board Of Regents, The University Of Texas System | Vaccines for cancer, autoimmune disease and infections |
| WO2005042558A1 (en) | 2003-10-30 | 2005-05-12 | Aegera Therapeutics, Inc. | Iap nucleobase oligomers and oligomeric complexes and uses thereof |
| US20050100529A1 (en) | 2003-11-06 | 2005-05-12 | Zeldis Jerome B. | Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of asbestos-related diseases and disorders |
| WO2005044178A2 (en) | 2003-10-23 | 2005-05-19 | Celgene Corporation | Methods of using and compositions comprising immunomodulatory compounds for treatment, modification and management of pain |
| WO2005048942A2 (en) | 2003-11-13 | 2005-06-02 | Pharmacia Corporation | Combination therapy comprising a cox-2 inhibitor and an antineoplastic agent |
| WO2005055929A2 (en) | 2003-12-02 | 2005-06-23 | Celgene Corporation | Methods and compositions for the treatment and management of hemoglobinopathy and anemia |
| US20050143344A1 (en) | 2003-12-30 | 2005-06-30 | Zeldis Jerome B. | Methods and compositions using immunomodulatory compounds for the treatment and management of central nervous system disorders or diseases |
| WO2005082415A2 (en) | 2004-02-25 | 2005-09-09 | Dana Farber Cancer Institute, Inc. | Inhibitors of insulin-like growth factor receptor-1 for inhibiting tumor cell growth |
| WO2005097125A2 (en) | 2004-04-01 | 2005-10-20 | Celgene Corporation | Methods and compositions for the treatment, prevention or management of diysfunctional sleep and dysfunctional sleep associated with disease |
| US20060030594A1 (en) | 2002-05-17 | 2006-02-09 | Celgene Corporation | Method using 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione for treatment of certain leukemias |
| WO2006018182A1 (en) | 2004-08-14 | 2006-02-23 | Boehringer Ingelheim International Gmbh | Combinations for the treatment of diseases involving cell proliferation |
| US20060052609A1 (en) | 2004-09-03 | 2006-03-09 | Muller George W | Processes for the preparation of substituted 2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolines |
| US7014866B2 (en) | 2001-05-03 | 2006-03-21 | Hoffmann-La Roche Inc. | High dose solid unit oral pharmaceutical dosage form of amorphous nelfinavir mesylate and process for making same |
| WO2006036892A2 (en) | 2004-09-24 | 2006-04-06 | Sapphire Therapeutics, Inc. | Use of inhibitors of 24-hydroxylase in the treatment of cancer |
| US20060079461A1 (en) | 2003-12-24 | 2006-04-13 | Scios, Inc. | Treatment of multiple myeloma by inhibition of p38 MAP kinase |
| US20060079502A1 (en) | 1999-11-02 | 2006-04-13 | Steffen Lang | Pharmaceutical compositions |
| US20060094744A1 (en) | 2004-09-29 | 2006-05-04 | Maryanoff Cynthia A | Pharmaceutical dosage forms of stable amorphous rapamycin like compounds |
| WO2006053160A2 (en) | 2004-11-12 | 2006-05-18 | Celgene Corporation | Methods and compositions using immunomodulatory compounds for treatment and management of parasitic diseases |
| WO2006058008A1 (en) | 2004-11-23 | 2006-06-01 | Celgene Corporation | Methods and compositions using immunomodulatory compounds for treatment and management of central nervous system injury |
| WO2006060507A2 (en) | 2004-12-01 | 2006-06-08 | Celgene Corporation | Compositions comprising immunomodulatory compounds and the use thereof for the treatment of immunodeficiency disorders |
| WO2006089150A2 (en) | 2005-02-18 | 2006-08-24 | Novartis Vaccines And Diagnostics Inc. | Antiangiogenic agents with aldesleukin |
| US7465800B2 (en) | 2003-09-04 | 2008-12-16 | Celgene Corporation | Polymorphic forms of 3-(4-amino-1-oxo-1,3 dihydro-isoindol-2-yl)-piperidine-2,6-dione |
| WO2012079022A1 (en) | 2010-12-10 | 2012-06-14 | Concert Pharmaceuticals, Inc. | Substituted dioxopiperidinyl phthalimide derivatives |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8288414B2 (en) * | 2007-09-12 | 2012-10-16 | Deuteria Pharmaceuticals, Inc. | Deuterium-enriched lenalidomide |
| MX2011005112A (en) * | 2008-11-14 | 2011-06-16 | Concert Pharmaceuticals Inc | Substituted dioxopiperidinyl phthalimide derivaties. |
| US9045453B2 (en) * | 2008-11-14 | 2015-06-02 | Concert Pharmaceuticals, Inc. | Substituted dioxopiperidinyl phthalimide derivatives |
| WO2010093434A1 (en) * | 2009-02-11 | 2010-08-19 | Celgene Corporation | Isotopologues of lenalidomide |
-
2014
- 2014-01-10 WO PCT/US2014/010972 patent/WO2014110322A2/en active Application Filing
Patent Citations (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5304121A (en) | 1990-12-28 | 1994-04-19 | Boston Scientific Corporation | Drug delivery system making use of a hydrogel polymer coating |
| US5886026A (en) | 1993-07-19 | 1999-03-23 | Angiotech Pharmaceuticals Inc. | Anti-angiogenic compositions and methods of use |
| US6099562A (en) | 1996-06-13 | 2000-08-08 | Schneider (Usa) Inc. | Drug coating with topcoat |
| US5635517A (en) | 1996-07-24 | 1997-06-03 | Celgene Corporation | Method of reducing TNFα levels with amino substituted 2-(2,6-dioxopiperidin-3-yl)-1-oxo-and 1,3-dioxoisoindolines |
| WO1998003502A1 (en) | 1996-07-24 | 1998-01-29 | Celgene Corporation | Substituted 2(2,6-dioxopiperidin-3-yl)phthalimides and -1-oxoisoindolines and method of reducing tnf-alpha levels |
| US5635517B1 (en) | 1996-07-24 | 1999-06-29 | Celgene Corp | Method of reducing TNFalpha levels with amino substituted 2-(2,6-dioxopiperidin-3-YL)-1-oxo-and 1,3-dioxoisoindolines |
| US20060079502A1 (en) | 1999-11-02 | 2006-04-13 | Steffen Lang | Pharmaceutical compositions |
| US7014866B2 (en) | 2001-05-03 | 2006-03-21 | Hoffmann-La Roche Inc. | High dose solid unit oral pharmaceutical dosage form of amorphous nelfinavir mesylate and process for making same |
| US6803031B2 (en) | 2001-05-24 | 2004-10-12 | Alexza Molecular Delivery Corporation | Delivery of erectile dysfunction drugs through an inhalation route |
| US20060030594A1 (en) | 2002-05-17 | 2006-02-09 | Celgene Corporation | Method using 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione for treatment of certain leukemias |
| WO2004041190A2 (en) | 2002-10-31 | 2004-05-21 | Celgene Corporation | Composition for the treatment of macular degenration |
| US20050074497A1 (en) | 2003-04-09 | 2005-04-07 | Schultz Clyde L. | Hydrogels used to deliver medicaments to the eye for the treatment of posterior segment diseases |
| WO2004100953A1 (en) | 2003-05-19 | 2004-11-25 | Pharmacia & Upjohn Company Llc | Combination of irinotecan and revimid for the treating multiple myeloma |
| WO2005035714A2 (en) | 2003-08-26 | 2005-04-21 | Board Of Regents, The University Of Texas System | Vaccines for cancer, autoimmune disease and infections |
| US7465800B2 (en) | 2003-09-04 | 2008-12-16 | Celgene Corporation | Polymorphic forms of 3-(4-amino-1-oxo-1,3 dihydro-isoindol-2-yl)-piperidine-2,6-dione |
| WO2005027842A2 (en) | 2003-09-18 | 2005-03-31 | Combinatorx, Incorporated | Combinations of drugs for the treatment of neoplasms |
| WO2005044178A2 (en) | 2003-10-23 | 2005-05-19 | Celgene Corporation | Methods of using and compositions comprising immunomodulatory compounds for treatment, modification and management of pain |
| WO2005042558A1 (en) | 2003-10-30 | 2005-05-12 | Aegera Therapeutics, Inc. | Iap nucleobase oligomers and oligomeric complexes and uses thereof |
| US20050100529A1 (en) | 2003-11-06 | 2005-05-12 | Zeldis Jerome B. | Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of asbestos-related diseases and disorders |
| WO2005048942A2 (en) | 2003-11-13 | 2005-06-02 | Pharmacia Corporation | Combination therapy comprising a cox-2 inhibitor and an antineoplastic agent |
| WO2005055929A2 (en) | 2003-12-02 | 2005-06-23 | Celgene Corporation | Methods and compositions for the treatment and management of hemoglobinopathy and anemia |
| US20060079461A1 (en) | 2003-12-24 | 2006-04-13 | Scios, Inc. | Treatment of multiple myeloma by inhibition of p38 MAP kinase |
| US20050143344A1 (en) | 2003-12-30 | 2005-06-30 | Zeldis Jerome B. | Methods and compositions using immunomodulatory compounds for the treatment and management of central nervous system disorders or diseases |
| WO2005082415A2 (en) | 2004-02-25 | 2005-09-09 | Dana Farber Cancer Institute, Inc. | Inhibitors of insulin-like growth factor receptor-1 for inhibiting tumor cell growth |
| WO2005097125A2 (en) | 2004-04-01 | 2005-10-20 | Celgene Corporation | Methods and compositions for the treatment, prevention or management of diysfunctional sleep and dysfunctional sleep associated with disease |
| WO2006018182A1 (en) | 2004-08-14 | 2006-02-23 | Boehringer Ingelheim International Gmbh | Combinations for the treatment of diseases involving cell proliferation |
| US20060052609A1 (en) | 2004-09-03 | 2006-03-09 | Muller George W | Processes for the preparation of substituted 2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolines |
| WO2006036892A2 (en) | 2004-09-24 | 2006-04-06 | Sapphire Therapeutics, Inc. | Use of inhibitors of 24-hydroxylase in the treatment of cancer |
| US20060094744A1 (en) | 2004-09-29 | 2006-05-04 | Maryanoff Cynthia A | Pharmaceutical dosage forms of stable amorphous rapamycin like compounds |
| WO2006053160A2 (en) | 2004-11-12 | 2006-05-18 | Celgene Corporation | Methods and compositions using immunomodulatory compounds for treatment and management of parasitic diseases |
| WO2006058008A1 (en) | 2004-11-23 | 2006-06-01 | Celgene Corporation | Methods and compositions using immunomodulatory compounds for treatment and management of central nervous system injury |
| WO2006060507A2 (en) | 2004-12-01 | 2006-06-08 | Celgene Corporation | Compositions comprising immunomodulatory compounds and the use thereof for the treatment of immunodeficiency disorders |
| WO2006089150A2 (en) | 2005-02-18 | 2006-08-24 | Novartis Vaccines And Diagnostics Inc. | Antiangiogenic agents with aldesleukin |
| WO2012079022A1 (en) | 2010-12-10 | 2012-06-14 | Concert Pharmaceuticals, Inc. | Substituted dioxopiperidinyl phthalimide derivatives |
Non-Patent Citations (29)
| Title |
|---|
| "Role of Lipid Excipients in Modifying Oral and Parenteral Drug Delivery: Basic Principles and Biological Examples", 2006, WILEY-INTERSCIENCE |
| "Scientific Tables", 1970, ARDSLEY, pages: 537 |
| "Tarascon Pocket Pharmacopoeia 2000", 2000, TARASCON PUBLISHING, article "PDR Pharmacopoeia" |
| ABOUL-ENEIN, H.Y. ET AL., JOURNAL OF LIQUID CHROMATOGRAPHY, vol. 14, no. 4, 1991, pages 667 - 73 |
| BLAKE, MI ET AL., J PHARM SCI, vol. 64, 1975, pages 367 - 91 |
| DAVID J. HAUSS,: "Oral Lipid-Based Formulations: Enhancing the Bioavailability of Poorly Water-Soluble Drugs (Drugs and the Pharmaceutical Sciences", 2007, INFORMA HEALTHCARE |
| ERIKSSON, T. ET AL., CHIRALITY, vol. 10, no. 3, 1998, pages 223 - 228 |
| ERIKSSON, T. ET AL., JOURNAL OF PHARMACY AND PHARMACOLOGY, vol. 52, no. 7, 2000, pages 807 - 817 |
| FISHER, MB ET AL., CURR OPIN DRUG DISCOV DEVEL, vol. 9, 2006, pages 101 - 09 |
| FOSTER, AB, ADV DRUG RES, vol. 14, 1985, pages 1 - 40 |
| FREIREICH ET AL., CANCER CHEMOTHER REP, vol. 50, 1966, pages 219 |
| FUKUTO ET AL., J. MED. CHEM., vol. 34, 1991, pages 2871 - 76 |
| GANNES, LZ ET AL., COMP BIOCHEM PHYSIOL A MOL INTEGR PHYSIOL, vol. 119, 1998, pages 725 |
| KEMPF, D.J. ET AL., ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, vol. 41, no. 3, 1997, pages 654 - 60 |
| KUSHNER, DJ ET AL., CAN J PHYSIOL PHARMACOL, 1999, pages 79 - 88 |
| L. FIESER; M. FIESER: "Fieser and Fieser's Reagents for Organic Synthesis", 1994, JOHN WILEY AND SONS |
| L. PAQUETTE,: "Encyclopedia of Reagents for Organic Synthesis", 1995, JOHN WILEY AND SONS |
| MULLER, GW ET AL., BIOORG MED CHEM LETT, vol. 9, no. 11, 1999, pages 1625 |
| MURPHY-POULTON, S.F. ET AL., JOURNAL OF CHROMATOGRAPHY, B: ANALYTICAL TECHNOLOGIES IN THE BIOMEDICAL AND LIFE SCIENCES, vol. 831, no. 1-2, 2006, pages 48 - 56 |
| OGREL, A. ET AL., RUSSIAN JOURNAL OF ORGANIC CHEMISTRY, vol. 37, no. 4, 2001, pages 475 - 479 |
| R. LAROCK: "Comprehensive Organic Transformations", 1989, VCH PUBLISHERS |
| REEPMEYER, J.C. ET AL., CHIRALITY, vol. 8, no. 1, 1996, pages 11 - 17 |
| REMINGTON: "The Science and Practice of Pharmacy", 2000, LIPPINCOTT WILLIAMS AND WILKINS |
| SEMBONGI, K. ET AL., BIOLOGICAL AND PHARMACEUTICAL BULLETIN, vol. 31, no. 3, 2008, pages 497 - 500 |
| T.W. GREENE; P.G.M. WUTS: "Protective Groups in Organic Synthesis, 3rd Ed.", 1999, JOHN WILEY AND SONS |
| TEO, S.K. ET AL., CHIRALITY, vol. 15, no. 4, 2003, pages 348 - 351 |
| WADA, E; HANBA, Y, SEIKAGAKU, vol. 66, 1994, pages 15 |
| WANG, L ET AL., CLINICAL PHARMACOLOGY AND THERAPEUTICS, vol. 56, 1994, pages 659 - 67 |
| WELLS ET AL,: "Pharmacotherapy Handbook", 2000, APPLETON AND LANGE |
Cited By (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10597394B2 (en) | 2014-04-04 | 2020-03-24 | Intra-Cellular Therapies, Inc. | Organic compounds |
| US10899762B2 (en) | 2014-04-04 | 2021-01-26 | Intra-Cellular Therapies, Inc. | Organic compounds |
| US11560382B2 (en) | 2014-04-04 | 2023-01-24 | Intra-Cellular Therapies, Inc. | Organic compounds |
| EP3888656A1 (en) * | 2016-03-25 | 2021-10-06 | Intra-Cellular Therapies, Inc. | Deuterated heterocyclic gamma-carboline compounds and their use in the treatment or prophylaxis of a central nervous system disorder |
| US10688097B2 (en) | 2016-03-25 | 2020-06-23 | Intra-Cellular Therapies, Inc. | Organic compounds |
| EP3407889A4 (en) * | 2016-03-25 | 2019-03-13 | Intra-Cellular Therapies, Inc. | Organic compounds |
| US11723909B2 (en) | 2016-03-25 | 2023-08-15 | Intra-Cellular Therapies, Inc. | Organic compounds |
| US11096944B2 (en) | 2016-03-25 | 2021-08-24 | Intra-Cellular Therapies, Inc. | Organic compounds |
| US10716786B2 (en) | 2017-03-24 | 2020-07-21 | Intra-Cellular Therapies, Inc. | Transmucosal and subcutaneous compositions |
| US11052083B2 (en) | 2017-03-24 | 2021-07-06 | Intra-Cellular Therapies, Inc. | Transmucosal methods for treating psychiatric and neurological conditions |
| US11806347B2 (en) | 2017-03-24 | 2023-11-07 | Intra-Cellular Therapies, Inc. | Transmucosal methods for treating psychiatric and neurological conditions |
| US11052084B2 (en) | 2018-08-31 | 2021-07-06 | Intra-Cellular Therapies, Inc. | Pharmaceutical capsule compositions comprising lumateperone mono-tosylate |
| US11806348B2 (en) | 2018-08-31 | 2023-11-07 | Intra-Cellular Therapies, Inc. | Methods of treatment using pharmaceutical capsule compositions comprising lumateperone mono-tosylate |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2014110322A3 (en) | 2014-09-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2373165B1 (en) | Substituted dioxopiperidinyl phthalimide derivaties | |
| US9045453B2 (en) | Substituted dioxopiperidinyl phthalimide derivatives | |
| US20130150408A1 (en) | Substituted dioxopiperidinyl phthalimide derivatives | |
| AU2010260249B2 (en) | Deuterated isoindoline-1,3-dione derivatives as PDE4 and TNF-alpha inhibitors | |
| US8575221B2 (en) | Derivatives of dimethylcurcumin | |
| WO2014110322A2 (en) | Substituted dioxopiperidinyl phthalimide derivatives | |
| EP2836492B1 (en) | Substituted xanthine derivatives | |
| US8410082B2 (en) | Fluorinated diaryl urea derivatives | |
| WO2013036434A1 (en) | Tetrahydronaphthalene derivatives as t-type calcium channel blocker | |
| WO2012079075A1 (en) | Deuterated phthalimide derivatives | |
| WO2013130849A1 (en) | Substituted dioxopiperidinyl phthalimide derivatives | |
| EP2542534A1 (en) | Deuterated tetrahydronaphthalene derivatives | |
| WO2018013686A1 (en) | Deuterated idalopirdine |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14702359 Country of ref document: EP Kind code of ref document: A2 |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14702359 Country of ref document: EP Kind code of ref document: A2 |