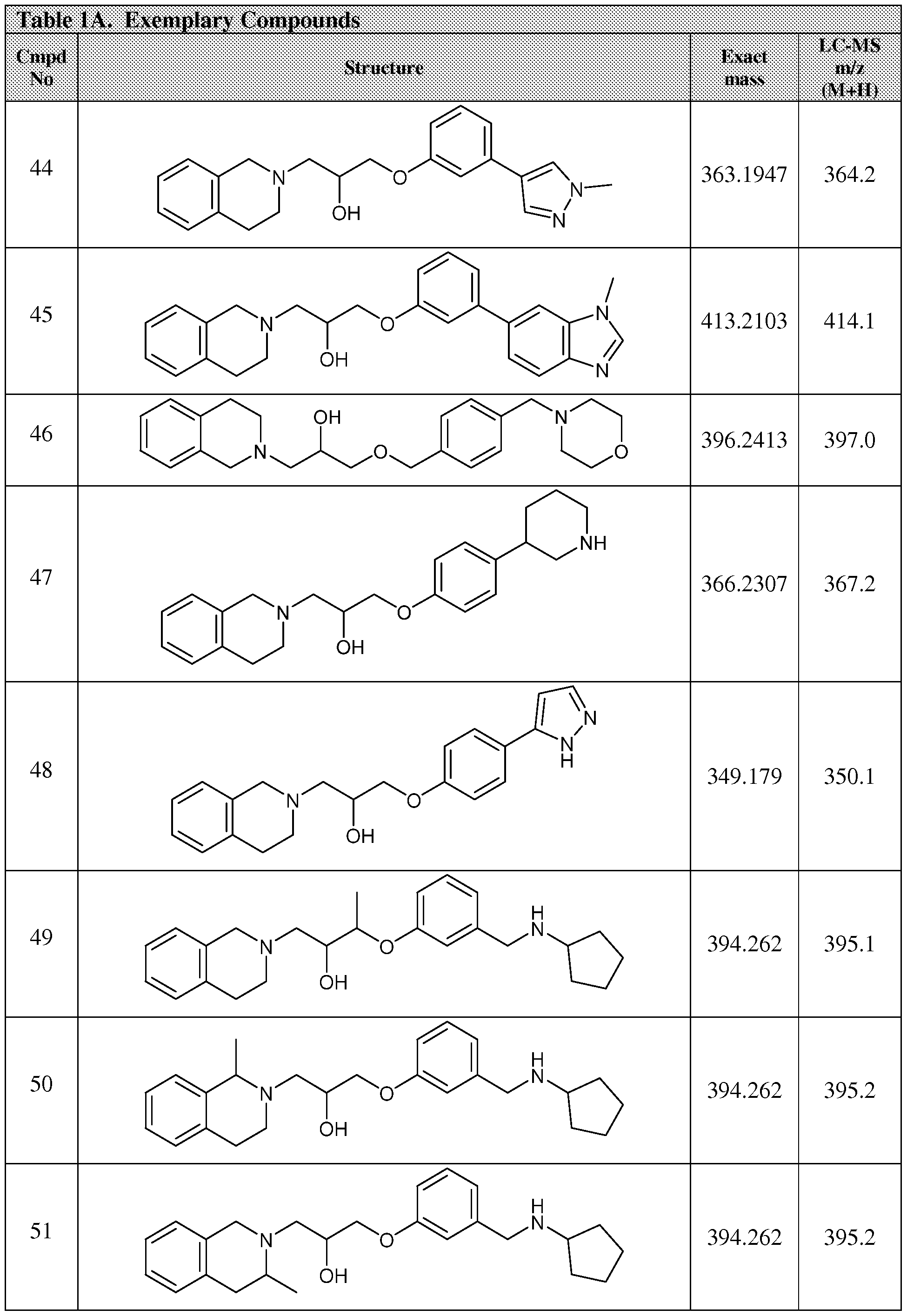
WO2014100695A1 - Prmt5 inhibitors and uses thereof - Google Patents
Prmt5 inhibitors and uses thereof Download PDFInfo
- Publication number
- WO2014100695A1 WO2014100695A1 PCT/US2013/077151 US2013077151W WO2014100695A1 WO 2014100695 A1 WO2014100695 A1 WO 2014100695A1 US 2013077151 W US2013077151 W US 2013077151W WO 2014100695 A1 WO2014100695 A1 WO 2014100695A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- compound
- optionally substituted
- pharmaceutically acceptable
- acceptable salt
- formula
- Prior art date
Links
- 0 C*(C)C(C)(C(C)(*)*1)C1(*)N(CC1)Cc2c1cccc2 Chemical compound C*(C)C(C)(C(C)(*)*1)C1(*)N(CC1)Cc2c1cccc2 0.000 description 34
- OBDIDGKANKNXDV-UHFFFAOYSA-N C(C1OC1)N1Cc2ccccc2CC1 Chemical compound C(C1OC1)N1Cc2ccccc2CC1 OBDIDGKANKNXDV-UHFFFAOYSA-N 0.000 description 1
- JEGMWWXJUXDNJN-UHFFFAOYSA-N CC1CNCCC1 Chemical compound CC1CNCCC1 JEGMWWXJUXDNJN-UHFFFAOYSA-N 0.000 description 1
- MJDIURPBHVJDBM-UHFFFAOYSA-N C[n]1c(cc(cc2)-c3cccc(NCC(CN(CC4)Cc5c4cccc5)O)c3)c2nc1 Chemical compound C[n]1c(cc(cc2)-c3cccc(NCC(CN(CC4)Cc5c4cccc5)O)c3)c2nc1 MJDIURPBHVJDBM-UHFFFAOYSA-N 0.000 description 1
- MEMSMUXNPZZHAZ-UHFFFAOYSA-N C[n]1c(nc(cc2)Cl)c2nc1 Chemical compound C[n]1c(nc(cc2)Cl)c2nc1 MEMSMUXNPZZHAZ-UHFFFAOYSA-N 0.000 description 1
- BTFQKIATRPGRBS-UHFFFAOYSA-N Cc1c(C=O)cccc1 Chemical compound Cc1c(C=O)cccc1 BTFQKIATRPGRBS-UHFFFAOYSA-N 0.000 description 1
- KGRHFKRJDVCGMB-UHFFFAOYSA-N Cc1cc([n](C)nc2)c2cc1 Chemical compound Cc1cc([n](C)nc2)c2cc1 KGRHFKRJDVCGMB-UHFFFAOYSA-N 0.000 description 1
- TYLPQEIDYXVIPY-UHFFFAOYSA-N Cc1ccc2[n](C)ncc2c1 Chemical compound Cc1ccc2[n](C)ncc2c1 TYLPQEIDYXVIPY-UHFFFAOYSA-N 0.000 description 1
- FARVSSLSGAIQMM-UHFFFAOYSA-N Cc1ccc2nc[n](C)c2c1 Chemical compound Cc1ccc2nc[n](C)c2c1 FARVSSLSGAIQMM-UHFFFAOYSA-N 0.000 description 1
- KXXZKVYXBFLEEU-UHFFFAOYSA-N Cc1ncc(cc2)[n]1cc2-c1cccc(OCC(CN(CC2)Cc3c2cccc3)O)c1 Chemical compound Cc1ncc(cc2)[n]1cc2-c1cccc(OCC(CN(CC2)Cc3c2cccc3)O)c1 KXXZKVYXBFLEEU-UHFFFAOYSA-N 0.000 description 1
- VXAVXVARCYERMY-UHFFFAOYSA-N Cc1ncc2[n]1cc(C)cc2 Chemical compound Cc1ncc2[n]1cc(C)cc2 VXAVXVARCYERMY-UHFFFAOYSA-N 0.000 description 1
- NWQTUDBTUDGCAJ-UHFFFAOYSA-N Nc(ccc(Br)c1)c1NCc1ccccc1 Chemical compound Nc(ccc(Br)c1)c1NCc1ccccc1 NWQTUDBTUDGCAJ-UHFFFAOYSA-N 0.000 description 1
- KOEAIQNONMKQID-UHFFFAOYSA-N Nc1cc(OCC(CN(CC2)Cc3c2cccc3)O)ccc1 Chemical compound Nc1cc(OCC(CN(CC2)Cc3c2cccc3)O)ccc1 KOEAIQNONMKQID-UHFFFAOYSA-N 0.000 description 1
- ARGAUQJHHDVCOA-UHFFFAOYSA-N OC(CN(CC1)Cc2c1cccc2)COc1cc(-c2cccc3cccnc23)ccc1 Chemical compound OC(CN(CC1)Cc2c1cccc2)COc1cc(-c2cccc3cccnc23)ccc1 ARGAUQJHHDVCOA-UHFFFAOYSA-N 0.000 description 1
- ROOPQSRQWKXPRM-UHFFFAOYSA-N Oc(cc1)ccc1-c1ccn[n]1Cc(cc1)ccc1C(N1CCCC1)=O Chemical compound Oc(cc1)ccc1-c1ccn[n]1Cc(cc1)ccc1C(N1CCCC1)=O ROOPQSRQWKXPRM-UHFFFAOYSA-N 0.000 description 1
- PZRDBQHVPAXREX-UHFFFAOYSA-N [O-][N+](c(c(NC1CCOCC1)c1)ccc1Br)=O Chemical compound [O-][N+](c(c(NC1CCOCC1)c1)ccc1Br)=O PZRDBQHVPAXREX-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D217/00—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems
- C07D217/02—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems with only hydrogen atoms or radicals containing only carbon and hydrogen atoms, directly attached to carbon atoms of the nitrogen-containing ring; Alkylene-bis-isoquinolines
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D217/00—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems
- C07D217/02—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems with only hydrogen atoms or radicals containing only carbon and hydrogen atoms, directly attached to carbon atoms of the nitrogen-containing ring; Alkylene-bis-isoquinolines
- C07D217/04—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems with only hydrogen atoms or radicals containing only carbon and hydrogen atoms, directly attached to carbon atoms of the nitrogen-containing ring; Alkylene-bis-isoquinolines with hydrocarbon or substituted hydrocarbon radicals attached to the ring nitrogen atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/10—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a carbon chain containing aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
Definitions
- Epigenetic regulation involves heritable modification of genetic material without changing its nucleotide sequence.
- epigenetic regulation is mediated by selective and reversible modification (e.g., methylation) of DNA and proteins (e.g., histones) that control the conformational transition between transcriptionally active and inactive states of chromatin.
- methylation e.g., methylation
- proteins e.g., histones
- methyltransferases e.g., PRMT5
- PRMT5 methyltransferases
- Protein arginine methyltransferase 5 catalyzes the addition of two methyl groups to the two ⁇ -guanidino nitrogen atoms of arginine, resulting in ⁇ -NG, N'G symmetric dimethylation of arginine (sDMA) of the target protein.
- PRMT5 functions in the nucleus as well as in the cytoplasm, and its substrates include histones, spliceosomal proteins, transcription factors (See e.g., Sun et al, PNAS (2011) 108: 20538-20543).
- PRMT5 generally functions as part of a molecule weight protein complex. While the protein complexes of PRMT5 can have a variety of components, they generally include the protein MEP50 (methylosome protein 50). In addition, PRMT5 acts in conjunction with cofactor SAM (S-adenosyl methionine).
- PRMT5 is an attractive target for modulation given its role in the regulation of diverse biological processes. It has now been found that compounds described herein, and pharmaceutically acceptable salts and compositions thereof, are effective as inhibitors of PRMT5.
- Ring A, L, R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , R 12 , R 13 , and n are as defined herein.
- inhibitors of PRMT5 have the general Formula (I):
- Ring A, L, R 1 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , and n are as defined herein.
- compositions which comprise a compound described herein (e.g., a compound of Formula (A), e.g., Formula (I)), or a pharmaceutically acceptable salt thereof, and optionally a pharmaceutically acceptable excipient.
- compounds described herein inhibit activity of PRMT5.
- methods of inhibiting PRMT5 comprise contacting PRMT5 with an effective amount of a compound of Formula (A), e.g., Formula (I), or a pharmaceutically acceptable salt thereof.
- the PRMT5 may be purified or crude, and may be present in a cell, tissue, or a subject. Thus, such methods encompass inhibition of PRMT5 activity both in vitro and in vivo.
- the PRMT5 is wild-type PRMT5.
- the PRMT5 is overexpressed.
- the PRMT5 is a mutant.
- the PRMT5 is in a cell. In certain
- the PRMT5 is in an animal, e.g., a human. In some embodiments, the PRMT5 is in a subject that is susceptible to normal levels of PRMT5 activity due to one or more mutations associated with a PRMT5 substrate. In some embodiments, the PRMT5 is in a subject known or identified as having abnormal PRMT5 activity (e.g., overexpression). In some embodiments, a provided compound is selective for PRMT5 over other
- a provided compound is at least about 10-fold selective, at least about 20-fold selective, at least about 30-fold selective, at least about 40- fold selective, at least about 50-fold selective, at least about 60-fold selective, at least about 70-fold selective, at least about 80-fold selective, at least about 90-fold selective, or at least about 100-fold selective relative to one or more other methyltransferases.
- methods of altering gene expression in a cell comprise contacting a cell with an effective amount of a compound of Formula (A), e.g., Formula (I), or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition thereof.
- the cell in culture in vitro.
- cell is in an animal, e.g., a human.
- methods of altering transcription in a cell comprise contacting a cell with an effective amount of a compound of Formula (A), e.g., Formula (I), or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition thereof.
- the cell in culture in vitro.
- the cell is in an animal, e.g., a human.
- methods of treating a PRMT5-mediated disorder comprise administering to a subject suffering from a PRMT5-mediated disorder an effective amount of a compound described herein (e.g., a compound of Formula (A), e.g., Formula (I)), or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition thereof.
- the PRMT5 -mediated disorder is a proliferative disorder, a metabolic disorder, or a blood disorder.
- compounds described herein are useful for treating cancer.
- compounds described herein are useful for treating hematopoietic cancer, lung cancer, prostate cancer, melanoma, or pancreatic cancer.
- compounds described herein are useful for treating a hemoglobinopathy.
- compounds described herein are useful for treating sickle cell anemia.
- compounds described herein are useful for treating diabetes or obesity.
- a provided compound is useful in treating inflammatory and autoimmune disease.
- Compounds described herein are also useful for the study of PRMT5 in biological and pathological phenomena, the study of intracellular signal transduction pathways mediated by PRMT5, and the comparative evaluation of new PRMT5 inhibitors.
- Compounds described herein can comprise one or more asymmetric centers, and thus can exist in various isomeric forms, e.g., enantiomers and/or diastereomers.
- the compounds described herein can be in the form of an individual enantiomer, diastereomer or geometric isomer, or can be in the form of a mixture of stereoisomers, including racemic mixtures and mixtures enriched in one or more stereoisomer.
- Isomers can be isolated from mixtures by methods known to those skilled in the art, including chiral high pressure liquid chromatography (HPLC) and the formation and crystallization of chiral salts; or preferred isomers can be prepared by asymmetric syntheses.
- HPLC high pressure liquid chromatography
- structures depicted herein are also meant to include compounds that differ only in the presence of one or more isotopically enriched atoms.
- compounds having the present structures except for the replacement of hydrogen by deuterium or tritium, replacement of 19 F with 18 F, or the replacement of a carbon by a 13
- C- or 14 C-enriched carbon are within the scope of the disclosure. Such compounds are useful, for example, as analytical tools or probes in biological assays.
- aliphatic includes both saturated and unsaturated, nonaromatic, straight chain (i.e., unbranched), branched, acyclic, and cyclic (i.e., carbocyclic) hydrocarbons.
- an aliphatic group is optionally substituted with one or more functional groups.
- "aliphatic” is intended herein to include alkyl, alkenyl, alkynyl, cycloalkyl, and cycloalkenyl moieties.
- Ci_ 6 alkyl is intended to encompass, C 1 ; C 2 , C 3 , C 4 , C 5 , C 6 , Ci_6, Ci_5, Cn, Ci-3, Ci-2, C 2 -6, C 2 _5, C-2-A, C 2 _ 3 , C 3 _6, C 3 _5, C 3 ⁇ , C S, C 4 _ 5 , and C 5 _6 alkyl.
- Alkyl refers to a radical of a straight-chain or branched saturated hydrocarbon group having from 1 to 20 carbon atoms (“C ⁇ o alkyl”). In some embodiments, an alkyl group has 1 to 10 carbon atoms (“C ⁇ o alkyl”). In some embodiments, an alkyl group has 1 to 9 carbon atoms ("Ci-9 alkyl”). In some embodiments, an alkyl group has 1 to 8 carbon atoms ("Q- 8 alkyl”). In some embodiments, an alkyl group has 1 to 7 carbon atoms (“C ⁇ alkyl”). In some embodiments, an alkyl group has 1 to 6 carbon atoms (“C ⁇ alkyl”).
- an alkyl group has 1 to 5 carbon atoms ("Q-s alkyl”). In some embodiments, an alkyl group has 1 to 4 carbon atoms ("C ⁇ alkyl”). In some embodiments, an alkyl group has 1 to 3 carbon atoms (“Ci_ 3 alkyl”). In some embodiments, an alkyl group has 1 to 2 carbon atoms (“C ⁇ alkyl”). In some embodiments, an alkyl group has 1 carbon atom (“Ci alkyl”). In some embodiments, an alkyl group has 2 to 6 carbon atoms (“C 2 - 6 alkyl").
- Ci_ 6 alkyl groups include methyl (CO, ethyl (C 2 ), n-propyl (C 3 ), isopropyl (C 3 ), n-butyl (C 4 ), tert-butyl (C 4 ), sec-butyl (C 4 ), iso-butyl (C 4 ), n-pentyl (C 5 ), 3- pentanyl (C 5 ), amyl (C 5 ), neopentyl (C 5 ), 3-methyl-2-butanyl (C 5 ), tertiary amyl (C 5 ), and n- hexyl (C 6 ).
- alkyl groups include n-heptyl (C 7 ), n-octyl (C 8 ) and the like.
- each instance of an alkyl group is independently optionally substituted, e.g. , unsubstituted (an "unsubstituted alkyl") or substituted (a "substituted alkyl") with one or more substituents.
- the alkyl group is unsubstituted Ci-w alkyl (e.g., -CH 3 ).
- the alkyl group is substituted C ⁇ o alkyl.
- an alkyl group is substituted with one or more halogens.
- Perhaloalkyl is a substituted alkyl group as defined herein wherein all of the hydrogen atoms are independently replaced by a halogen, e.g., fluoro, bromo, chloro, or iodo.
- the alkyl moiety has 1 to 8 carbon atoms ("C ⁇ perhaloalkyl”).
- the alkyl moiety has 1 to 6 carbon atoms (“C ⁇ perhaloalkyl”).
- the alkyl moiety has 1 to 4 carbon atoms ("C ⁇ perhaloalkyl").
- the alkyl moiety has 1 to 3 carbon atoms ("C ⁇ perhaloalkyl”). In some embodiments, the alkyl moiety has 1 to 2 carbon atoms ("C ⁇ perhaloalkyl”). In some embodiments, all of the hydrogen atoms are replaced with fluoro. In some embodiments, all of the hydrogen atoms are replaced with chloro. Examples of perhaloalkyl groups include - CF 3 , -CF 2 CF 3 , -CF 2 CF 2 CF 3 , -CC1 3 , -CFC1 2 , -CF 2 C1, and the like.
- alkenyl refers to a radical of a straight-chain or branched hydrocarbon group having from 2 to 20 carbon atoms, one or more carbon-carbon double bonds, and no triple bonds (“C 2 _ 2 o alkenyl”).
- an alkenyl group has 2 to 10 carbon atoms ("C ⁇ io alkenyl”).
- an alkenyl group has 2 to 9 carbon atoms ("C 2 _9 alkenyl”).
- an alkenyl group has 2 to 8 carbon atoms (“C 2 _ 8 alkenyl”).
- an alkenyl group has 2 to 7 carbon atoms (“C 2 _ 7 alkenyl”).
- an alkenyl group has 2 to 6 carbon atoms ("C 2 _ 6 alkenyl”). In some embodiments, an alkenyl group has 2 to 5 carbon atoms (“C 2 _5 alkenyl”). In some embodiments, an alkenyl group has 2 to 4 carbon atoms ("C 2 _4 alkenyl”). In some embodiments, an alkenyl group has 2 to 3 carbon atoms (“C 2 _ 3 alkenyl”). In some embodiments, an alkenyl group has 2 carbon atoms (“C 2 alkenyl”). The one or more carbon-carbon double bonds can be internal (such as in 2-butenyl) or terminal (such as in 1- butenyl).
- Examples of C 2 _ alkenyl groups include ethenyl (C 2 ), 1-propenyl (C 3 ), 2-propenyl (C 3 ), 1-butenyl (C 4 ), 2-butenyl (C 4 ), butadienyl (C 4 ), and the like.
- Examples of C 2 _ 6 alkenyl groups include the aforementioned C 2 _ alkenyl groups as well as pentenyl (C 5 ), pentadienyl (C 5 ), hexenyl (C 6 ), and the like. Additional examples of alkenyl include heptenyl (C 7 ), octenyl (C 8 ), octatrienyl (C 8 ), and the like.
- each instance of an alkenyl group is independently optionally substituted, e.g. , unsubstituted (an "unsubstituted alkenyl") or substituted (a "substituted alkenyl") with one or more substituents.
- the alkenyl group is unsubstituted C 2 _ 10 alkenyl.
- the alkenyl group is substituted C 2 _ 10 alkenyl.
- Alkynyl refers to a radical of a straight-chain or branched hydrocarbon group having from 2 to 20 carbon atoms, one or more carbon-carbon triple bonds, and optionally one or more double bonds ("C2-20 alkynyl”). In some embodiments, an alkynyl group has 2 to 10 carbon atoms ("C2-10 alkynyl”). In some embodiments, an alkynyl group has 2 to 9 carbon atoms (“C2-9 alkynyl”). In some embodiments, an alkynyl group has 2 to 8 carbon atoms (“C2-8 alkynyl”).
- an alkynyl group has 2 to 7 carbon atoms ("C2-7 alkynyl”). In some embodiments, an alkynyl group has 2 to 6 carbon atoms ("C 2 -6 alkynyl”). In some embodiments, an alkynyl group has 2 to 5 carbon atoms (“C 2 _5 alkynyl”). In some embodiments, an alkynyl group has 2 to 4 carbon atoms ("C 2 ⁇ alkynyl”). In some embodiments, an alkynyl group has 2 to 3 carbon atoms (“C2-3 alkynyl”). In some embodiments, an alkynyl group has 2 carbon atoms ("C 2 alkynyl”).
- the one or more carbon- carbon triple bonds can be internal (such as in 2-butynyl) or terminal (such as in 1-butynyl).
- C2- alkynyl groups include, without limitation, ethynyl (C 2 ), 1-propynyl (C 3 ), 2-propynyl (C 3 ), 1-butynyl (C 4 ), 2-butynyl (C 4 ), and the like.
- Examples of C2-6 alkenyl groups include the aforementioned C2- alkynyl groups as well as pentynyl (C 5 ), hexynyl (C 6 ), and the like.
- alkynyl examples include heptynyl (C 7 ), octynyl (Cg), and the like.
- each instance of an alkynyl group is independently optionally substituted, e.g., unsubstituted (an "unsubstituted alkynyl") or substituted (a "substituted alkynyl") with one or more substituents.
- the alkynyl group is unsubstituted C 2 _ 10 alkynyl.
- the alkynyl group is substituted C2-10 alkynyl.
- Carbocyclyl or “carbocyclic” refers to a radical of a non-aromatic cyclic hydrocarbon group having from 3 to 14 ring carbon atoms ("C 3 _ 14 carbocyclyl") and zero heteroatoms in the non-aromatic ring system.
- a carbocyclyl group has 3 to 10 ring carbon atoms ("Cs-io carbocyclyl”).
- a carbocyclyl group has 3 to 8 ring carbon atoms (“C 3 _8 carbocyclyl”).
- a carbocyclyl group has 3 to 6 ring carbon atoms ("C ⁇ carbocyclyl”).
- a carbocyclyl group has 3 to 6 ring carbon atoms ("C 3 _ 6 carbocyclyl”). In some embodiments, a carbocyclyl group has 5 to 10 ring carbon atoms ("Cs-io carbocyclyl").
- Exemplary C 3 _ 6 carbocyclyl groups include, without limitation, cyclopropyl (C 3 ), cyclopropenyl (C 3 ), cyclobutyl (C 4 ), cyclobutenyl (C 4 ), cyclopentyl (C 5 ), cyclopentenyl (C 5 ), cyclohexyl (C 6 ), cyclohexenyl (C 6 ), cyclohexadienyl (C 6 ), and the like.
- Exemplary C 3 _g carbocyclyl groups include, without limitation, the aforementioned C 3 _ 6 carbocyclyl groups as well as cycloheptyl (C 7 ), cycloheptenyl (C 7 ), cycloheptadienyl (C 7 ), cycloheptatrienyl (C 7 ), cyclooctyl (C 8 ), cyclooctenyl (C 8 ), bicyclo[2.2.1]heptanyl (C 7 ), bicyclo[2.2.2]octanyl (C 8 ), and the like.
- Exemplary C 3 _ 10 carbocyclyl groups include, without limitation, the
- the carbocyclyl group is either monocyclic ("monocyclic carbocyclyl") or contain a fused, bridged or spiro ring system such as a bicyclic system ("bicyclic
- Carbocyclyl also includes ring systems wherein the carbocyclyl ring, as defined above, is fused with one or more aryl or heteroaryl groups wherein the point of attachment is on the carbocyclyl ring, and in such instances, the number of carbons continue to designate the number of carbons in the carbocyclic ring system.
- each instance of a carbocyclyl group is independently optionally substituted, e.g. , unsubstituted (an "unsubstituted carbocyclyl") or substituted (a "substituted carbocyclyl") with one or more substituents.
- the carbocyclyl group is unsubstituted C 3 _io carbocyclyl.
- the carbocyclyl group is a substituted C 3 _io carbocyclyl.
- “carbocyclyl” is a monocyclic, saturated carbocyclyl group having from 3 to 14 ring carbon atoms ("C3_ 14 cycloalkyl”). In some embodiments, a cycloalkyl group has 3 to 10 ring carbon atoms ("C 3 _io cycloalkyl”). In some embodiments, a cycloalkyl group has 3 to 8 ring carbon atoms ("C 3 _ 8 cycloalkyl”). In some embodiments, a cycloalkyl group has 3 to 6 ring carbon atoms ("C 3 _6 cycloalkyl").
- a cycloalkyl group has 5 to 6 ring carbon atoms ("Cs_6 cycloalkyl”). In some embodiments, a cycloalkyl group has 5 to 10 ring carbon atoms ("Cs-io cycloalkyl”). Examples of C 5 _6 cycloalkyl groups include cyclopentyl (C 5 ) and cyclohexyl (C 5 ). Examples of C 3 _6 cycloalkyl groups include the aforementioned C 5 _6 cycloalkyl groups as well as cyclopropyl (C 3 ) and cyclobutyl (C 4 ).
- C 3 _ 8 cycloalkyl groups include the aforementioned C 3 _ 6 cycloalkyl groups as well as cycloheptyl (C 7 ) and cyclooctyl (C 8 ).
- each instance of a cycloalkyl group is independently unsubstituted (an "unsubstituted cycloalkyl") or substituted (a "substituted cycloalkyl”) with one or more substituents.
- the cycloalkyl group is unsubstituted C 3 _ 10 cycloalkyl.
- the cycloalkyl group is substituted C 3 _io cycloalkyl.
- Heterocyclyl refers to a radical of a 3- to 14-membered non- aromatic ring system having ring carbon atoms and 1 to 4 ring heteroatoms, wherein each heteroatom is independently selected from nitrogen, oxygen, and sulfur ("3-14 membered heterocyclyl”).
- heterocyclyl or heterocyclic refers to a radical of a 3-10 membered non-aromatic ring system having ring carbon atoms and 1-4 ring
- heterocyclyl groups wherein each heteroatom is independently selected from nitrogen, oxygen, and sulfur (“3-10 membered heterocyclyl”).
- heterocyclyl groups that contain one or more nitrogen atoms, the point of attachment can be a carbon or nitrogen atom, as valency permits.
- a heterocyclyl group can either be monocyclic ("monocyclic heterocyclyl") or a fused, bridged or spiro ring system such as a bicyclic system (“bicyclic heterocyclyl”), and can be saturated or can be partially unsaturated.
- Heterocyclyl bicyclic ring systems can include one or more heteroatoms in one or both rings.
- Heterocyclyl also includes ring systems wherein the heterocyclyl ring, as defined above, is fused with one or more carbocyclyl groups wherein the point of attachment is either on the carbocyclyl or heterocyclyl ring, or ring systems wherein the heterocyclyl ring, as defined above, is fused with one or more aryl or heteroaryl groups, wherein the point of attachment is on the heterocyclyl ring, and in such instances, the number of ring members continue to designate the number of ring members in the
- heterocyclyl ring system each instance of heterocyclyl is independently optionally substituted, e.g., unsubstituted (an "unsubstituted heterocyclyl") or substituted (a "substituted heterocyclyl") with one or more substituents.
- the heterocyclyl group is unsubstituted 3-10 membered heterocyclyl. In certain embodiments, the heterocyclyl group is substituted 3-10 membered heterocyclyl.
- a heterocyclyl group is a 5-10 membered non-aromatic ring system having ring carbon atoms and 1-4 ring heteroatoms, wherein each heteroatom is independently selected from nitrogen, oxygen, and sulfur ("5-10 membered heterocyclyl").
- a heterocyclyl group is a 5-8 membered non-aromatic ring system having ring carbon atoms and 1-4 ring heteroatoms, wherein each heteroatom is
- a heterocyclyl group is a 5-6 membered non-aromatic ring system having ring carbon atoms and 1-4 ring heteroatoms, wherein each heteroatom is
- the 5-6 membered heterocyclyl has 1-3 ring heteroatoms independently selected from nitrogen, oxygen, and sulfur. In some embodiments, the 5-6 membered heterocyclyl has 1-2 ring heteroatoms independently selected from nitrogen, oxygen, and sulfur. In some embodiments, the 5-6 membered heterocyclyl has one ring heteroatom selected from nitrogen, oxygen, and sulfur.
- Exemplary 3-membered heterocyclyl groups containing one heteroatom include, without limitation, azirdinyl, oxiranyl, and thiorenyl.
- Exemplary 4-membered heterocyclyl groups containing one heteroatom include, without limitation, azetidinyl, oxetanyl, and thietanyl.
- Exemplary 5-membered heterocyclyl groups containing one heteroatom include, without limitation, tetrahydrofuranyl, dihydrofuranyl, tetrahydrothiophenyl,
- Exemplary 5- membered heterocyclyl groups containing two heteroatoms include, without limitation, dioxolanyl, oxasulfuranyl, disulfuranyl, and oxazolidin-2-one.
- Exemplary 5-membered heterocyclyl groups containing three heteroatoms include, without limitation, triazolinyl, oxadiazolinyl, and thiadiazolinyl.
- Exemplary 6-membered heterocyclyl groups containing one heteroatom include, without limitation, piperidinyl, tetrahydropyranyl, dihydropyridinyl, and thianyl.
- Exemplary 6-membered heterocyclyl groups containing two heteroatoms include, without limitation, piperazinyl, morpholinyl, dithianyl, and dioxanyl.
- Exemplary 6- membered heterocyclyl groups containing two heteroatoms include, without limitation, triazinanyl.
- Exemplary 7-membered heterocyclyl groups containing one heteroatom include, without limitation, azepanyl, oxepanyl and thiepanyl.
- Exemplary 8-membered heterocyclyl groups containing one heteroatom include, without limitation, azocanyl, oxecanyl, and thiocanyl.
- Exemplary 5-membered heterocyclyl groups fused to a C 6 aryl ring include, without limitation, indolinyl, isoindolinyl, dihydrobenzofuranyl, dihydrobenzothienyl, benzoxazolinonyl, and the like.
- Exemplary 6-membered heterocyclyl groups fused to an aryl ring include, without limitation, tetrahydroquinolinyl,
- Aryl refers to a radical of a monocyclic or polycyclic (e.g., bicyclic or tricyclic) 4n+2 aromatic ring system (e.g., having 6, 10, or 14 ⁇ electrons shared in a cyclic array) having 6-14 ring carbon atoms and zero heteroatoms provided in the aromatic ring system ("C6-14 aryl").
- an aryl group has six ring carbon atoms ("C 6 aryl”; e.g., phenyl).
- an aryl group has ten ring carbon atoms ("Cio aryl”; e.g., naphthyl such as 1-naphthyl and 2-naphthyl). In some embodiments, an aryl group has fourteen ring carbon atoms ("G ⁇ aryl”; e.g., anthracyl).
- Aryl also includes ring systems wherein the aryl ring, as defined above, is fused with one or more carbocyclyl or heterocyclyl groups wherein the radical or point of attachment is on the aryl ring, and in such instances, the number of carbon atoms continue to designate the number of carbon atoms in the aryl ring system.
- each instance of an aryl group is independently optionally substituted, e.g. , unsubstituted (an "unsubstituted aryl") or substituted (a "substituted aryl") with one or more substituents.
- the aryl group is unsubstituted Ce_ 14 aryl. In certain embodiments, the aryl group is substituted Ce_ 14 aryl.
- Heteroaryl refers to a radical of a 5-14 membered monocyclic or polycyclic (e.g., bicyclic or tricyclic) 4n+2 aromatic ring system (e.g., having 6 or 10 ⁇ electrons shared in a cyclic array) having ring carbon atoms and 1-4 ring heteroatoms provided in the aromatic ring system, wherein each heteroatom is independently selected from nitrogen, oxygen and sulfur ("5-14 membered heteroaryl").
- heteroaryl refers to a radical of a 5-10 membered monocyclic or bicyclic 4n+2 aromatic ring system having ring carbon atoms and 1-4 ring heteroatoms provided in the aromatic ring system, wherein each heteroatom is independently selected from nitrogen, oxygen and sulfur ("5-10 membered heteroaryl").
- heteroaryl groups that contain one or more nitrogen atoms the point of attachment can be a carbon or nitrogen atom, as valency permits.
- Heteroaryl bicyclic ring systems can include one or more heteroatoms in one or both rings.
- Heteroaryl includes ring systems wherein the heteroaryl ring, as defined above, is fused with one or more carbocyclyl or heterocyclyl groups wherein the point of attachment is on the heteroaryl ring, and in such instances, the number of ring members continue to designate the number of ring members in the heteroaryl ring system.
- Heteroaryl also includes ring systems wherein the heteroaryl ring, as defined above, is fused with one or more aryl groups wherein the point of attachment is either on the aryl or heteroaryl ring, and in such instances, the number of ring members designates the number of ring members in the fused (aryl/heteroaryl) ring system.
- Bicyclic heteroaryl groups wherein one ring does not contain a heteroatom e.g., indolyl, quinolinyl, carbazolyl, and the like
- the point of attachment can be on either ring, e.g., either the ring bearing a heteroatom (e.g., 2-indolyl) or the ring that does not contain a heteroatom (e.g., 5-indolyl).
- a heteroaryl group is a 5-14 membered aromatic ring system having ring carbon atoms and 1-4 ring heteroatoms provided in the aromatic ring system, wherein each heteroatom is independently selected from nitrogen, oxygen, and sulfur ("5-14 membered heteroaryl").
- a heteroaryl group is a 5-10 membered aromatic ring system having ring carbon atoms and 1-4 ring heteroatoms provided in the aromatic ring system, wherein each heteroatom is independently selected from nitrogen, oxygen, and sulfur ("5-10 membered heteroaryl").
- a heteroaryl group is a 5-8 membered aromatic ring system having ring carbon atoms and 1-4 ring heteroatoms provided in the aromatic ring system, wherein each heteroatom is independently selected from nitrogen, oxygen, and sulfur ("5-8 membered heteroaryl").
- a heteroaryl group is a 5-6 membered aromatic ring system having ring carbon atoms and 1-4 ring heteroatoms provided in the aromatic ring system, wherein each heteroatom is independently selected from nitrogen, oxygen, and sulfur ("5-6 membered heteroaryl”).
- the 5-6 membered heteroaryl has 1-3 ring heteroatoms independently selected from nitrogen, oxygen, and sulfur.
- the 5-6 membered heteroaryl has 1-2 ring heteroatoms independently selected from nitrogen, oxygen, and sulfur. In some embodiments, the 5-6 membered heteroaryl has 1 ring heteroatom selected from nitrogen, oxygen, and sulfur. In certain embodiments, each instance of a heteroaryl group is independently optionally substituted, e.g., unsubstituted ("unsubstituted heteroaryl") or substituted ("substituted heteroaryl”) with one or more substituents. In certain embodiments, the heteroaryl group is unsubstituted 5-14 membered heteroaryl. In certain embodiments, the heteroaryl group is substituted 5-14 membered heteroaryl.
- Exemplary 5-membered heteroaryl groups containing one heteroatom include, without limitation, pyrrolyl, furanyl and thiophenyl.
- Exemplary 5-membered heteroaryl groups containing two heteroatoms include, without limitation, imidazolyl, pyrazolyl, oxazolyl, isoxazolyl, thiazolyl, and isothiazolyl.
- Exemplary 5-membered heteroaryl groups containing three heteroatoms include, without limitation, triazolyl, oxadiazolyl, and thiadiazolyl.
- Exemplary 5-membered heteroaryl groups containing four heteroatoms include, without limitation, tetrazolyl.
- Exemplary 6-membered heteroaryl groups containing one heteroatom include, without limitation, pyridinyl.
- Exemplary 6-membered heteroaryl groups containing two heteroatoms include, without limitation, pyridazinyl, pyrimidinyl, and pyrazinyl.
- Exemplary 6-membered heteroaryl groups containing three or four heteroatoms include, without limitation, triazinyl and tetrazinyl, respectively.
- Exemplary 7-membered heteroaryl groups containing one heteroatom include, without limitation, azepinyl, oxepinyl, and thiepinyl.
- Exemplary 5,6-bicyclic heteroaryl groups include, without limitation, indolyl, isoindolyl, indazolyl, benzotriazolyl, benzothiophenyl, isobenzothiophenyl, benzofuranyl, benzoisofuranyl, benzimidazolyl, benzoxazolyl, benzisoxazolyl, benzoxadiazolyl, benzthiazolyl, benzisothiazolyl, benzthiadiazolyl, indolizinyl, and purinyl.
- Exemplary 6,6- bicyclic heteroaryl groups include, without limitation, naphthyridinyl, pteridinyl, quinolinyl, isoquinolinyl, cinnolinyl, quinoxalinyl, phthalazinyl, and quinazolinyl.
- Fused or “ortho-fused” are used interchangeably herein, and refer to two rings that have two atoms and one bond in common, e.g.,
- Bridged refers to a ring system containing (1) a bridgehead atom or group of atoms which connect two or more non-adjacent positions of the same ring; or (2) a bridgehead atom or group of atoms which connect two or more positions of different rings of a ring system and does not thereby form an ortho-fused ring, e.g.,
- Spiro or “Spiro-fused” refers to a group of atoms which connect to the same atom of a carbocyclic or heterocyclic ring system (geminal attachment), thereby forming a ring, e.g.,
- Spiro-fusion at a bridgehead atom is also contemplated.
- Partially unsaturated refers to a group that includes at least one double or triple bond.
- the term “partially unsaturated” is intended to encompass rings having multiple sites of unsaturation, but is not intended to include aromatic groups (e.g., aryl or heteroaryl groups) as herein defined.
- saturated refers to a group that does not contain a double or triple bond, i.e. , contains all single bonds.
- aliphatic, alkyl, alkenyl, alkynyl, carbocyclyl, heterocyclyl, aryl, and heteroaryl groups, as defined herein, are optionally substituted (e.g., "substituted” or “unsubstituted” aliphatic, "substituted” or “unsubstituted” alkyl, "substituted” or
- substituted carbocyclyl, "substituted” or “unsubstituted” heterocyclyl, "substituted” or “unsubstituted” aryl or “substituted” or “unsubstituted” heteroaryl group).
- substituted whether preceded by the term “optionally” or not, means that at least one hydrogen present on a group (e.g., a carbon or nitrogen atom) is replaced with a permissible substituent, e.g., a substituent which upon substitution results in a stable compound, e.g., a compound which does not spontaneously undergo transformation such as by rearrangement, cyclization, elimination, or other reaction.
- a “substituted” group has a substituent at one or more substitutable positions of the group, and when more than one position in any given structure is substituted, the substituent is either the same or different at each position.
- substituted is contemplated to include substitution with all permissible substituents of organic compounds, including any of the substituents described herein that results in the formation of a stable compound.
- heteroatoms such as nitrogen may have hydrogen substituents and/or any suitable substituent as described herein which satisfy the valencies of the heteroatoms and results in the formation of a stable moiety.
- R ⁇ is, independently, selected from Ci-io alkyl, Ci-io perhaloalkyl, C 2 _io alkenyl, C 2 _ 10 alkynyl, C 3 _ 10 carbocyclyl, 3-14 membered heterocyclyl, C6-14 aryl, and 5-14 membered heteroaryl, or two R ⁇ groups are joined to form a 3-14 membered heterocyclyl or 5-14 membered heteroaryl ring, wherein each alkyl, alkenyl, alkynyl, carbocyclyl, heterocyclyl, ary
- each instance of R cc is, independently, selected from hydrogen, Ci-io alkyl, Ci-io perhaloalkyl, C 2 _io alkenyl, C 2 _io alkynyl, C 3 _io carbocyclyl, 3-14 membered heterocyclyl, C 6 -14 aryl, and 5-14 membered heteroaryl, or two R cc groups are joined to form a 3-14 membered heterocyclyl or 5-14 membered heteroaryl ring, wherein each alkyl, alkenyl, alkynyl, carbocyclyl, heterocyclyl, aryl, and heteroaryl is independently substituted with 0, 1, 2, 3, 4, or 5 R dd groups;
- each instance of R ee is, independently, selected from Q_6 alkyl, C ⁇ perhaloalkyl, C 2 6 alkenyl, C 2 _ 6 alkynyl, C 3 _ 10 carbocyclyl, C 6 -io aryl, 3-10 membered heterocyclyl, and 3-10 membered heteroaryl, wherein each alkyl, alkenyl, alkynyl, carbocyclyl, heterocyclyl, aryl, and heteroaryl is independently substituted with 0, 1, 2, 3, 4, or 5 R gg groups; each instance of R is, independently, selected from hydrogen, C ⁇ alkyl, C ⁇ perhaloalkyl, C 2 _ 6 alkenyl, C 2 _ 6 alkynyl, C 3 _io carbocyclyl, 3-10 membered heterocyclyl, C6- ff
- io aryl and 5-10 membered heteroaryl, or two R groups are joined to form a 3-14 membered heterocyclyl or 5-14 membered heteroaryl ring, wherein each alkyl, alkenyl, alkynyl, carbocyclyl, heterocyclyl, aryl, and heteroaryl is independently substituted with 0, 1, 2, 3, 4, or 5 R gg groups; and
- a "counterion” or “anionic counterion” is a negatively charged group associated with a cationic quaternary amino group in order to maintain electronic neutrality.
- exemplary counterions include halide ions (e.g., F “ , CI “ , Br “ , ⁇ ), N0 3 " , C10 4 " , OFT, H 2 P0 4 " , HS0 4 " , sulfonate ions (e.g., methansulfonate, trifluoromethanesulfonate, p-toluenesulfonate, benzenesulfonate, 10-camphor sulfonate, naphthalene-2-sulfonate, naphthalene-l-sulfonic acid-5-sulfonate, ethan-l-sulfonic acid-2-sulfonate, and the like), and carboxylate ions (e.g., acetate, ethanoate,
- Halo or "halogen” refers to fluorine (fluoro, -F), chlorine (chloro, -CI), bromine (bromo, -Br), or iodine (iodo, -I).
- Nitrogen atoms can be substituted or unsubstituted as valency permits, and include primary, secondary, tertiary, and quarternary nitrogen atoms.
- the substituent present on a nitrogen atom is a nitrogen protecting group (also referred to as an amino protecting group).
- Amide nitrogen protecting groups include, but are not limited to, formamide, acetamide, chloroacetamide, trichloroacetamide, trifluoroacetamide,
- Carbamate nitrogen protecting groups include, but are not limited to, methyl carbamate, ethyl carbamante, 9-fluorenylmethyl carbamate (Fmoc), 9-(2- sulfo)fluorenylmethyl carbamate, 9-(2,7-dibromo)fluoroenylmethyl carbamate, 2,7-di-i- butyl-[9-( 10,10-dioxo-l 0, 10,10, 10-tetrahydrothioxanthyl)] methyl carbamate (DBD-Tmoc), 4-methoxyphenacyl carbamate (Phenoc), 2,2,2-trichloroethyl carbamate (Troc), 2- trimethylsilylethyl carbamate (Teoc), 2-phenylethyl carbamate (hZ), l-(l-adamantyl)-l- methylethoxyphenacyl carbamate (Phenoc), 2,2,2-trichloroethy
- TBOC l-methyl-l-(4-biphenylyl)ethyl carbamate
- Bpoc l-(3,5-di-i-butylphenyl)-l- methylethyl carbamate
- Pyoc 2-(2'- and 4'-pyridyl)ethyl carbamate
- 2-(N,N- dicyclohexylcarboxamido)ethyl carbamate i-butyl carbamate (BOC), 1-adamantyl carbamate (Adoc), vinyl carbamate (Voc), allyl carbamate (Alloc), 1-isopropylallyl carbamate (Ipaoc), cinnamyl carbamate (Coc), 4-nitrocinnamyl carbamate (Noc), 8-quinolyl carbamate, N-hydroxypiperidinyl carbamate, alkyldithio carbamate, benzyl carb
- Sulfonamide nitrogen protecting groups include, but are not limited to, p-toluenesulfonamide (Ts), benzenesulfonamide, 2,3,6,-trimethyl-4- methoxybenzenesulfonamide (Mtr), 2,4,6-trimethoxybenzenesulfonamide (Mtb), 2,6- dimethyl-4-methoxybenzenesulfonamide (Pme), 2,3,5, 6-tetramethyl-4- methoxybenzenesulfonamide (Mte), 4-methoxybenzenesulfonamide (Mbs), 2,4,6- trimethylbenzenesulfonamide (Mts), 2,6-dimethoxy-4-methylbenzenesulfonamide (iMds), 2,2,5,7, 8-pentamethylchroman-6-sulfonamide (Pmc), methanesulfonamide (M
- nitrogen protecting groups include, but are not limited to, phenothiazinyl- (10)-acyl derivative, N'-p-toluenesulfonylaminoacyl derivative, N'-phenylaminothioacyl derivative, N-benzoylphenylalanyl derivative, N-acetylmethionine derivative, 4,5-diphenyl- 3-oxazolin-2-one, N-phthalimide, N-dithiasuccinimide (Dts), N-2,3-diphenylmaleimide, N-2,5-dimethylpyrrole, N-l, l,4,4-tetramethyldisilylazacyclopentane adduct (STABASE), 5-substituted l,3-dimethyl-l,3,5-triazacyclohexan-2-one, 5-substituted 1,3-dibenzyl- l,3,5-triazacyclohexan-2-one,
- benzenesulfenamide o-nitrobenzenesulfenamide (Nps), 2,4-dinitrobenzenesulfenamide, pentachlorobenzenesulfenamide, 2-nitro-4-methoxybenzenesulfenamide,
- triphenylmethylsulfenamide triphenylmethylsulfenamide
- 3-nitropyridinesulfenamide Npys
- the substituent present on an oxygen atom is an oxygen protecting group (also referred to as a hydroxyl protecting group).
- Oxygen protecting groups are well known in the art and include those described in detail in Protecting Groups in Organic Synthesis, T. W. Greene and P. G. M. Wuts, 3 rd edition, John Wiley & Sons, 1999, incorporated herein by reference.
- oxygen protecting groups include, but are not limited to, methyl, methoxylmethyl (MOM), methylthiomethyl (MTM), i-butylthiomethyl,
- DPMS diphenylmethylsilyl
- TMPS i-butylmethoxyphenylsilyl
- the substituent present on a sulfur atom is a sulfur protecting group (also referred to as a thiol protecting group).
- Sulfur protecting groups are well known in the art and include those described in detail in Protecting Groups in Organic Synthesis, T. W. Greene and P. G. M. Wuts, 3 rd edition, John Wiley & Sons, 1999, incorporated herein by reference.
- LG is a term understood in the art to refere to a molecular fragment that departs with a pair of electrons upon heterolytic bond cleavage, wherein the molecular fragment is an anion or neutral molecule.
- suitable leaving groups include, but are not limited to, halides (such as chloride, bromide, or iodide),
- the leaving group is a sulfonic acid ester.
- the sulfonic acid ester comprises the formula -OS0 2 R LG1 wherein R LG1 is selected from the group consisting alkyl optionally, alkenyl optionally substituted, heteroalkyl optionally substituted, aryl optionally substituted, heteroaryl optionally substituted, arylalkyl optionally substituted, and heterarylalkyl optionally substituted.
- R LG1 is substituted or unsubstituted C ⁇ -Ce alkyl.
- R LG1 is selected from the group consisting alkyl optionally, alkenyl optionally substituted, heteroalkyl optionally substituted, aryl optionally substituted, heteroaryl optionally substituted, arylalkyl optionally substituted, and heterarylalkyl optionally substituted.
- R LG1 is substituted or unsubstituted C ⁇ -Ce alkyl.
- R L"G U 1 1 is methyl.
- R LG 1 is -CF .
- R LG 1 is substituted or unsubstituted aryl.
- R LG1 is substituted or unsubstituted phenyl. In so
- “Pharmaceutically acceptable salt” refers to those salts which are, within the scope of sound medical judgment, suitable for use in contact with the tissues of humans and other animals without undue toxicity, irritation, allergic response, and the like, and are
- Pharmaceutically acceptable salts are well known in the art. For example, Berge et al. describe pharmaceutically acceptable salts in detail in J. Pharmaceutical Sciences (1977) 66: 1-19. Pharmaceutically acceptable salts of the compounds describe herein include those derived from suitable inorganic and organic acids and bases. Examples of pharmaceutically acceptable, nontoxic acid addition salts are salts of an amino group formed with inorganic acids such as hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid and perchloric acid or with organic acids such as acetic acid, oxalic acid, maleic acid, tartaric acid, citric acid, succinic acid, or malonic acid or by using other methods used in the art such as ion exchange.
- inorganic acids such as hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid and perchloric acid
- organic acids such as acetic acid, oxalic acid, maleic acid, tartaric acid, citric acid, succinic acid, or malonic acid or by using
- salts include adipate, alginate, ascorbate, aspartate, benzenesulfonate, benzoate, bisulfate, borate, butyrate, camphorate, camphorsulfonate, citrate, cyclopentanepropionate, digluconate, dodecylsulfate, ethanesulfonate, formate, fumarate, glucoheptonate, glycerophosphate, gluconate, hemisulfate, heptanoate, hexanoate, hydroiodide, 2-hydroxy-ethanesulfonate, lactobionate, lactate, laurate, lauryl sulfate, malate, maleate, malonate, methanesulfonate, 2- naphthalenesulfonate, nicotinate, nitrate, oleate, oxalate, palmitate, pamoate, pectinate
- Salts derived from appropriate bases include alkali metal, alkaline earth metal, ammonium and N + (C 1 ⁇ alkyl) 4 salts.
- Representative alkali or alkaline earth metal salts include sodium, lithium, potassium, calcium, magnesium, and the like.
- Further pharmaceutically acceptable salts include, when appropriate, quaternary salts.
- a "subject" to which administration is contemplated includes, but is not limited to, humans (e.g., a male or female of any age group, e.g., a pediatric subject (e.g, infant, child, adolescent) or adult subject (e.g., young adult, middle-aged adult or senior adult)) and/or other non-human animals, for example, non-human mammals (e.g., primates (e.g., cynomolgus monkeys, rhesus monkeys); commercially relevant mammals such as cattle, pigs, horses, sheep, goats, cats, and/or dogs), birds (e.g., commercially relevant birds such as chickens, ducks, geese, and/or turkeys), rodents (e.g., rats and/or mice), reptiles, amphibians, and fish.
- the non-human animal is a mammal.
- the non-human animal may be a male or female at any stage of development.
- Treating encompasses an action that occurs while a subject is suffering from a condition which reduces the severity of the condition or retards or slows the progression of the condition ("therapeutic treatment”).
- Treating also encompasses an action that occurs before a subject begins to suffer from the condition and which inhibits or reduces the severity of the condition (“prophylactic treatment”).
- an "effective amount" of a compound refers to an amount sufficient to elicit the desired biological response, e.g. , treat the condition.
- the effective amount of a compound described herein may vary depending on such factors as the desired biological endpoint, the pharmacokinetics of the compound, the condition being treated, the mode of administration, and the age and health of the subject.
- An effective amount encompasses therapeutic and prophylactic treatment.
- a “therapeutically effective amount” of a compound is an amount sufficient to provide a therapeutic benefit in the treatment of a condition or to delay or minimize one or more symptoms associated with the condition.
- a therapeutically effective amount of a compound means an amount of therapeutic agent, alone or in combination with other therapies, which provides a therapeutic benefit in the treatment of the condition.
- the term "therapeutically effective amount” can encompass an amount that improves overall therapy, reduces or avoids symptoms or causes of the condition, or enhances the therapeutic efficacy of another therapeutic agent.
- a prophylactically effective amount of a compound is an amount sufficient to prevent a condition, or one or more symptoms associated with the condition or prevent its recurrence.
- a prophylactically effective amount of a compound means an amount of a therapeutic agent, alone or in combination with other agents, which provides a prophylactic benefit in the prevention of the condition.
- the term “prophylactically effective amount” can encompass an amount that improves overall prophylaxis or enhances the prophylactic efficacy of another prophylactic agent.
- methyltransferase represents transferase class enzymes that are able to transfer a methyl group from a donor molecule to an acceptor molecule, e.g. , an amino acid residue of a protein or a nucleic base of a DNA molecule.
- Methytransferases typically use a reactive methyl group bound to sulfur in S-adenosyl methionine (SAM) as the methyl donor.
- SAM S-adenosyl methionine
- a methyltransferase described herein is a protein methyltransferase.
- a methyltransferase described herein is a histone methyltransferase.
- Histone methyltransferases are histone-modifying enzymes, (including histone-lysine N-methyltransf erase and histone-arginine N-methyl transferase), that catalyze the transfer of one or more methyl groups to lysine and arginine residues of histone proteins.
- a methyltransferase described herein is a histone-arginine N-methyltransferase.
- R 12 is hydrogen, halogen, or optionally substituted C 1-3 alkyl
- R 13 is hydrogen, halogen, optionally substituted C 1-3 alkyl, -NR A1 R A2 , or -OR 1 ;
- R A1 and R ⁇ are each independently hydrogen, optionally substituted C 1-3 alkyl, a nitrogen protecting group, or R A1 and R ⁇ are taken together with the intervening nitrogen atom to form an optionally substituted 3-6 membered heterocyclic ring;
- R 1 is hydrogen, R z , or -C(0)R z , wherein R z is optionally substituted Ci_6 alkyl;
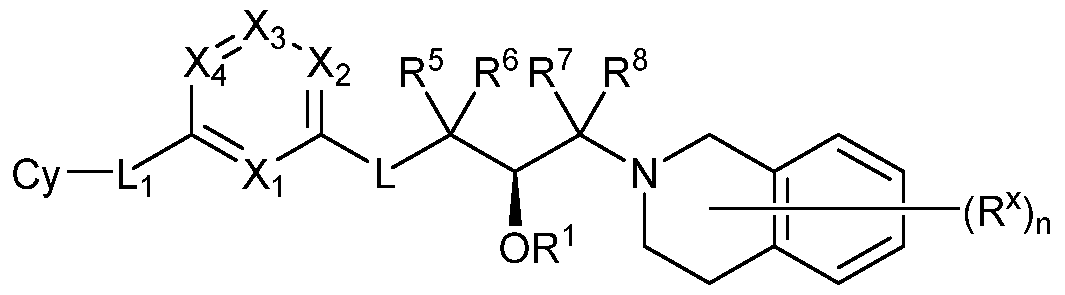
- L is -0-, -N(R)-,-C(R 2 )(R 3 )-, -0-CR 2 R 3 , -N(R)-CR 2 R 3 -, -0-CR 2 R 3 -0-, -N(R)-CR 2 R 3 -0, -N(R)-CR 2 R 3 -N(R)-, -0-CR 2 R 3 -N(R)-, -CR 2 R 3 -0-, -CR 2 R 3 -N(R)-, -0-CR 2 R 3 -CR 9 R 10 -, -N(R)-CR 2 R 3 -CR 9 R 10 -, -CR 2 R 3 -CR 9 R 10 -O-, -CR 2 R 3 -CR 9 R 10 -N(R)-, or -CR 2 R 3 - CR 9 R 10 -;
- each R is independently hydrogen or optionally substituted C 1-6 aliphatic
- R 2 and R 3 are taken together with their intervening atoms to form an optionally substituted carbocyclic or heterocyclic ring; or R 2" and R 3 J are taken together with their intervening atoms to form an optionally substituted carbocyclic or heterocyclic ring; each R is independently selected from the group consisting of hydrogen, optionally substituted aliphatic, optionally substituted carbocyclyl, optionally substituted heterocyclyl, optionally substituted aryl, and optionally substituted heteroaryl;
- each R is independently selected from the group consisting of hydrogen, optionally substituted aliphatic, optionally substituted carbocyclyl, optionally substituted heterocyclyl, optionally substituted aryl, and optionally substituted heteroaryl, or two R groups are taken together with their intervening atoms to form an optionally substituted heterocyclic ring;
- Ring A is a monocyclic or bicyclic, saturated, partially unsaturated, or aromatic ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, and sulfur;
- R 4 is -L Cy
- U is a bond, -0-, -S-, -N(R)-, -C(O)-, -C(0)N(R)-, -N(R)C(0)N(R)-, - N(R)C(0)-, -N(R)C(0)0- -OC(0)N(R)-, -S0 2 - -S0 2 N(R)-, -N(R)S0 2 - -OC(O)-, - C(0)0-, or an optionally substituted, straight or branched, Ci_6 aliphatic chain wherein one, two, or three methylene units of hi are optionally and independently replaced by -0-, -S-, - N(R)-, -C(O)-, -C(0)N(R)-, -N(R)C(0)N(R)-, -N(R)C(0)-, -N(R)C(0)0- - OC(0)N(R)-, -S0 2
- Cy is an optionally substituted, monocyclic, bicyclic or tricyclic, saturated, partially unsaturated, or aromatic ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, and sulfur;
- R 5 , R 6 , R 7 , and R 8 are each independently hydrogen, halo, or optionally substituted aliphatic;
- R 9 and R 10 are each independently selected from the group consisting of hydrogen, halo, -CN, -N0 2 , optionally substituted aliphatic, optionally substituted carbocyclyl;
- each R x is independently selected from the group consisting of halo, -CN, optionally substituted aliphatic, -OR', and -N(R") 2 ;
- R' is hydrogen or optionally substituted aliphatic
- each R" is independently hydrogen or optionally substituted aliphatic, or two R" are taken together with their intervening atoms to form a heterocyclic ring;
- n 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10, as valency permits;
- n 0, 1, 2, 3, 4, 5, 6, 7, or 8, as valency permits
- p 0 or 1.
- R 12 is hydrogen, halogen, or optionally substituted Ci_
- R 12 is hydrogen. In certain embodiments, R 12 is optionally substituted C 1-3 alkyl, e.g., optionally substituted with halogen. In certain embodiments, R 12 is optionally substituted Cialkyl, e.g., methyl or trifluoromethyl. In certain embodiments, R 12 is optionally substituted C 2 alkyl, e.g., ethyl. In certain embodiments, R 12 is optionally substituted C 3 alkyl, e.g., propyl. In certain embodiments, R 12 is fluoro, provided that R 13 is not -OR 1. In certain embodiments, R 12 is chloro, provided that R 13 is not -OR 1. In certain embodiments, R 12 is bromo, provided that R 13 is not -OR 1. In certain embodiments, R 12 is iodo, provided that R 13 is not -OR 1.
- R 13 is hydrogen, halogen, optionally substituted Ci_ 3 alkyl, -NR A1 R A2 or -OR 1 . In certain embodiments, R 13 is hydrogen. In certain
- R 13 is optionally substituted C ⁇ alkyl, e.g., optionally substituted with halogen.
- R 13 is optionally substituted Cialkyl, e.g., methyl or trifluoromethyl.
- R 13 is optionally substituted C 2 alkyl, e.g., ethyl. In certain embodiments, R 13 is optionally substituted C 3 alkyl, e.g., propyl. In certain embodiments, R 13 is fluoro. In certain embodiments, R 13 is chloro. In certain embodiments, R 13 is bromo. In certain embodiments, R 13 is iodo. In certain embodiments, R 13 is -NR A1 R A2 . [0065] For example, in some embodiments of Formula (A), wherein R is hydrogen, the present disclosure provides a com ound of Formula (A-l):
- Ring A, L, R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , R 12 , and n are as defined herein.
- Ring A, L, R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , R 13 , and n are as defined herein.
- Ring A, L, R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , and n are as defined herein.
- R 12 , and n are as defined herein.
- R 12 , and n are as defined herein.
- the present disclosure provides a compound of Formula (A-5):
- R 12 , R 13 , and n are as defined herein.
- the present disclosure provides a compound of Formula (I):
- Ring A, L, R 1 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , and n are as defined herein.
- the com ound is not one of the followin :
- a rovided compound is of Formula (I-a):
- Ring A, L, R 1 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , and n are as defined herein.
- a provided compound is of Formula (I-b):
- Ring A, L, R 1 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , and n are as defined herein.
- a provided compound is of Formula (I-c):
- Ring A, L, R 1 , R 4 , R y , m, p, R x , and n are as defined herein.
- a provided compound is of Formula (I-d):
- a provided compound is of Formula (I'):
- Ring A, L, R 1 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , and n are as defined herein.
- a provided compound is of Formula (I'-a):
- Ring A, L, R 1 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , and n are as defined herein.
- a provided compound is of Formula (I'-b):
- Ring A, L, R 1 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , and n are as defined herein.
- a provided compound is of Formula (I'-c):
- a provided compound is of Formula (I'-d):
- Ring A, L, R 1 , R 4 , R 5 , R y , m, p, R x , and n are as defined herein.
- a provided compound is of Formula (A-6):
- R 13 , and n are as defined herein.
- a provided compound is of Formula (A-7):
- Ring A, R, R 1 , R x , R 12 , R 13 , and n are as defined herein.
- a provided compound is of Formula (A-8):
- Ring A, R, R 1 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , R 12 , R 13 , and n are as defined herein.
- a provided compound is of Formula (A-9):
- Ring A, R, R 1 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , R 12 , R 13 , and n are as defined herein.
- a provided compound is of Formula (A-10):
- R 13 , and n are as defined herein.
- a provided compound is of Formula (II):
- Ring A, R, R 1 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , and n are as defined herein.
- a provided compound is of Formula (Il-a):
- Ring A, R, R 1 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , and n are as defined herein.
- a rovided compound is of Formula (Il-b):
- a provided compound is of Formula (II-c):
- Ring A, R, R 1 , R 4 , R y , m, p, R x , and n are as defined herein.
- a provided compound is of Formula (Il-d):
- Ring A, R, R 1 , R 4 , R 5 , R y , m, p, R x , and n are as defined herein.
- a provided compound is of Formula (A-ll):
- Ring A, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , R 12 , R 13 and n are as defined herein.
- a provided compound is of Formula (A-12):
- R 12 , R 1 1 3 J and n are as defined herein.
- a provided compound is of Formula (III):
- Ring A, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , and n are as defined herein.
- a rovided compound is of Formula (Ill-a):
- Ring A, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , and n are as defined herein.
- a rovided compound is of Formula (Ill-b):
- Ring A, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, p, R x , and n are as defined herein.
- a provided compound is of Formula (III-c):
- Ring A, R 1 , R 2 , R 3 , R 4 , R y , m, p, R x , and n are as defined herein.
- a provided compound is of Formula (Ill-d):
- Ring A, R 1 , R 2 , R 3 , R 4 , R 5 , R y , m, p, R x , and n are as defined herein.
- a provided compound is of Formula (A-13):
- Ring A, R 1 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, R x , R 12 , R 13 , and n are as defined herein.
- a provided compound is of Formula (A-14):
- Ring A, R 1 , R 4 , R y , m, R x , R 12 , R 13 , and n are as defined herein.
- a provided compound is of Formula (IV):
- Ring A, R 1 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, R x , and n are as defined herein.
- a provided compound is of Formula (IV-a):
- a provided compound is of Formula (IV-b):
- Ring A, R 1 , R 4 , R 5 , R 6 , R 7 , R 8 , R y , m, R x , and n are as defined herein.
- a provided compound is of Formula (IV-c):
- a provided compound is of Formula (IV-d):
- Ring A, R 1 , R 4 , R 5 , R y , m, R x , and n are as defined herein.
- a provided compound is of Formula (A-15):
- R 7 , R 8 , R x , R 12 , R 13 and n are as defined herein.
- a provided compound is of Formula (A-16):
- R 1J and n are as defined herein.
- a provided compound is of Formula (V): or a pharmaceutically acceptable salt thereof, wherein X 1 ; X 2 , X 3 , X 4 , L, L 1 ; Cy, R 1 , R 5 , R 6 ,
- R', R°, R ⁇ and n are as defined herein.
- a provided compound is of Formula (V-a):
- R', R°, R A , and n are as defined herein.
- a provided compound is of Formula (V-b):
- a provided compound is of Formula (V-c):
- R 7', R 8°, R ⁇ and n are as defined herein.
- a provided compound is of Formula (V-d):
- R 7', R 8°, R ⁇ and n are as defined herein.
- a provided compound is of Formula (A-17):
- R 1 1 3 J and n are as defined herein.
- a provided compound is of Formula (A-18):
- R and n are as defined herein.
- a provided compound is of Formula (A-19):
- a provided compound is of Formula (A-20):
- a provided compound is of Formula (VI):
- a provided compound is of Formula (Vl-a):
- a provided compound is of Formula (Vl-b):
- a provided compound is of Formula (VI-c):
- a provided compound is of Formula (Vl-d):
- a provided compound is of Formula (A-21):
- R , and n are as defined herein.
- a provided compound is of Formula (A-22):
- R 13 , and n are as defined herein.
- a provided compound is of Formula (A-23):
- a provided compound is of Formula (A-24):
- a provided compound is of Formula (VII):
- a provided compound is of Formula (Vll-a):
- a provided compound is of Formula (Vll-b):
- a provided compound is of Formula (VII-c):
- a provided compound is of Formula (Vll-d):
- a provided compound is of Formula (A-25):
- R 13 , and n are as defined herein.
- a provided compound is of Formula (A-26):
- R 13 , and n are as defined herein.
- a provided compound is of Formula (A-27):
- L, L 1; Cy, R 1 , R 5 , R 6 , R V , R 8 , R x , R 13 , and n are as defined herein.
- a provided compound is of Formula (A-28):
- a provided compound is of Formula (VIII):
- a provided compound is of Formula (VHI-a):
- a provided compound is of Formula (VHI-b):
- a provided compound is of Formula (VIII-c):
- a provided compound is of Formula (VUI-d):
- a provided compound is of Formula (A-29):
- R 13 , and n are as defined herein.
- a provided compound is of Formula (A-30):
- R 13 , and n are as defined herein.
- a provided compound is of Formula (A-31):
- a provided compound is of Formula (A-32):
- a provided compound is of Formula (A-33):
- a provided compound is of Formula (A-34):
- a provided compound is of Formula (A-35):
- a provided compound is of Formula (A-36):
- a provided compound is of Formula (A-37):
- a provided compound is of Formula (A-38):
- R 13 , and n are as defined herein.
- a provided compound is of Formula (A-39):
- a provided compound is of Formula (A-40):
- a provided compound is of Formula (A-41):
- a provided compound is of Formula (A-42):
- a provided compound is of Formula (A-43):
- a provided compound is of Formula (A-44):
- a provided compound is of Formula (A-45):
- a provided compound is of Formula (A-46):
- a provided compound is of Formula (A-47):
- R 13 , and n are as defined herein.
- a provided compound is of Formula (A-48):
- a provided compound is of Formula (A-49):
- a provided compound is of Formula (A-50):
- a provided compound is of Formula (IX):
- a provided compound is of Formula (IX-a):
- a provided compound is of Formula (IX-b):
- a provided compound is of Formula (IX-c):
- a provided compound is of Formula (IX-d):
- a provided compound is of Formula (A-51):
- a provided compound is of Formula A-52):
- a provided compound is of Formula (A-53):
- a provided compound is of Formula (A-54):
- a provided compound is of Formula (X):
- a provided compound is of Formula (X-a):
- a provided compound is of Formula (X-b):
- a provided compound is of Formula (X-c):
- a provided compound is of Formula (X-d):
- R 1 is hydrogen, R z , or -C(0)R z , wherein R z is optionally substituted C 1-6 alkyl.
- R 1 is hydrogen.
- R 1 is optionally substituted Ci_6 alkyl.
- R 1 is unsubstituted Ci_6 alkyl.
- R 1 is methyl, ethyl, or propyl.
- R 1 is -C(0)R z , wherein R z is optionally substituted C 1-6 alkyl.
- R 1 is -C(0)R z , wherein R z is unsubstituted C 1-6 alkyl.
- R 1 is acetyl.
- L is -0-, -N(R)-,-C(R 2 )(R 3 )-, -0-CR 2 R 3 , -N(R)- CR 2 R 3 -, -0-CR 2 R 3 -0-, -N(R)-CR 2 R 3 -0, -N(R)-CR 2 R 3 -N(R)-, -0-CR 2 R 3 -N(R)-, -CR 2 R 3 -0-, - CR 2 R 3 -N(R)-, -0-CR 2 R 3 -CR 9 R 10 -, -N(R)-CR 2 R 3 -CR 9 R 10 -, -CR 2 R 3 -CR 9 R 10 -O-, -CR 2 R 3 - CR 9 R 10 -N(R)-, or -CR 2 R 3 -CR 9 R 10 -.
- L is -0-, -N(R)-, or -CR 2 R 3 -, wherein R, R 2 , and R 3 are as described herein.
- L is -0-.
- L is -N(R)-.
- L is -NH-.
- L is -N(R)-, wherein R is optionally substituted C 1-6 aliphatic.
- L is - N(R)-, wherein R is optionally substituted C 1-6 alkyl.
- L is -N(R)-, wherein R is unsubstituted C 1-6 alkyl.
- L is -N(R)-, wherein R is acetyl. In certain embodiments, L is -CH 2 -0-. In certain embodiments, L is -CR 2 R 3 -0-. In certain embodiments, L is -CR 2 R 3 -N(R)-. In certain embodiments, L is -CH 2 -NH-.
- each R is independently hydrogen or optionally substituted C 1-6 aliphatic.
- R is hydrogen.
- R is optionally substituted C 1-6 aliphatic.
- R is substituted Ci_6 aliphatic.
- R is unsubstituted C 1-6 aliphatic.
- R is optionally substituted C 1-6 alkyl.
- R is substituted C 1-6 alkyl.
- R is unsubstituted Ci_6 alkyl.
- R is methyl, ethyl, or propyl.
- R is substituted with an oxo to give an acyl group.
- R 2 and R 3 are independently selected from the group consisting of hydrogen, halo, -CN, -N0 2 , optionally substituted aliphatic, optionally substituted
- R 2 is hydrogen. In some embodiments, R 2 is not hydrogen. In some embodiments, R 2 is halo. In certain embodiments, R 2 is fluoro. In some embodiments, R 2 is optionally substituted aliphatic. In certain embodiments, R 2 is optionally substituted Ci_6 aliphatic. In certain embodiments, R is optionally substituted C 1-6 alkyl. In certain embodiments, R 2 is substituted C 1-6 alkyl. In certain embodiments, R 2 is -CF 3 , CHF 2 , or CH 2 F. In certain embodiments, R 2 is unsubstituted C 1-6 alkyl. In certain embodiments, R 2 is methyl, ethyl, or propyl. In some embodiments, R is -CN or -N0 2 . In some embodiments, R is -CN or -N0 2 . In some embodiments,
- R is optionally substituted carbocyclyl, optionally substituted phenyl, optionally substituted heterocyclyl, or optionally substituted heteroaryl.
- R 2 is -N(R B ) 2 ,
- R 2 is -NH 2 . In certain embodimetns, R 2 is -OR A . In certain embodiments, R is -OH.
- R 3 is hydrogen. In some embodiments, R 3 is not hydrogen. In some embodiments, R 3 is halo. In certain embodiments, R 3 is fluoro. In some embodiments, R 3 is optionally substituted aliphatic. In certain embodiments, R 3 is optionally substituted Ci_6 aliphatic. In certain embodiments, R is optionally substituted C 1-6 alkyl. In certain embodiments, R 3 is substituted C 1-6 alkyl. In certain embodiments, R 3 is -CF 3 , CHF 2 , or CH 2 F. In certain embodiments, R 3 is unsubstituted C 1-6 alkyl. In certain embodiments, R 3 is methyl, ethyl, or propyl. In some embodiments, R is -CN or -N0 2 . In some embodiments, R is -CN or -N0 2 . In some embodiments,
- R is optionally substituted carbocyclyl, optionally substituted phenyl, optionally substituted heterocyclyl, or optionally substituted heteroaryl.
- R 3 is -N(R B
- R 3 is -NH 2 . In certain embodimetns, R 3 is -OR A . In certain embodiments, R is -OH.
- R 2 and R 3 are the same. In some embodiments, R 2 and R 3 are different. In some embodiments, R 2 and R 3 are each hydrogen. In some embodiments, R 2 is hydrogen and R 3 is not hydrogen. In some embodiments, R 2 is hydrogen and R 3 is optionally substituted aliphatic. In some embodiments, R 2 is hydrogen and R 3 is Ci_6 alkyl.
- R 2 is hydrogen and R 3 is methyl. In some embodiments, R 2 is hydrogen and R 3 is ethyl or propyl. In some embodiments, R 2 is hydrogen and R 3 is -CF 3 ,
- R 2 is hydrogen and R 3 is -N(R B ) 2 or -OR A .
- R 2 is hydrogen and R 3 is -NH 2 .
- R 2 is hydrogen and R 3 is -OH.
- R 2 and R 3 are not hydrogen.
- R 2 and R 3 are not hydrogen.
- R 3 are independently optionally substituted aliphatic. In some embodiments, R 2 and R 3 are methyl. In some embodiments, R 2 and R 3 are taken together with their intervening atoms to form an optionally substituted carbocyclic or heterocyclic ring.
- Ring A is a monocyclic or bicyclic, saturated, partially unsaturated, or aromatic ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, and sulfur.
- Ring A is aromatic.
- Ring A is saturated.
- Ring A is partially unsaturated.
- Ring A is monocyclic.
- Ring A is bicyclic.
- Ring A is phenyl. In certain embodiments, Ring A is a monocyclic heteroaryl having 1-3 heteroatoms independently selected from nitrogen, oxygen, and sulfur. In certain embodiments, Ring A is a 5- to 6-membered heteroaryl having 1-3 heteroatoms independently selected from nitrogen, oxygen, and sulfur. In certain embodiments, Ring A is a 5-membered heteroaryl having 1-3 heteroatoms independently selected from nitrogen, oxygen, and sulfur (e.g.
- Ring A is a 6-membered heteroaryl having 1-3 nitrogens (e.g. , pyridyl, pyrimidyl, pyridazinyl, pyrazinyl, triazinyl).
- Ring A is pyridyl.
- Ring A is pyrimidyl.
- Ring A is pyridazinyl.
- Ring A is a carbocyclic ring. In some embodiments, Ring A is a 3- to 8-membered saturated carbocyclic ring. In some embodiments, Ring A is a 3- to 8-membered heterocyclic ring having 1-2 heteroatoms independently selected from nitrogen, oxygen, and sulfur.
- Ring A is a bicyclic saturated, partially unsaturated, or aromatic ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, and sulfur.
- Ring A is an 8- to 12-membered bicyclic saturated, partially unsaturated, or aromatic ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, and sulfur.
- Ring A is an 8- to 10-membered bicyclic heteroaryl having 1-4 heteroatoms independently selected from nitrogen, oxygen, and sulfur.
- Ring A is a 9-membered bicyclic heteroaryl having 1-3 heteroatoms independently selected from nitrogen, oxygen, and sulfur (e.g.
- indolyl isoindolyl, indazolyl, benzotriazolyl, benzothiophenyl, isobenzothiophenyl, benzofuranyl, benzoisofuranyl, benzimidazolyl, benzoxazolyl, benzisoxazolyl, benzoxadiazolyl, benzthiazolyl,
- Ring A is a 10- membered bicyclic heteroaryl having 1-3 heteroatoms independently selected from nitrogen, oxygen, and sulfur (e.g. , naphthyridinyl, quinolinyl, isoquinolinyl, quinoxalinyl, quinazolinyl.
- Ring A is selected from the group consisting of quinoline, benzimidazole, benzopyrazole, quinoxaline, tetrahydroquinoline, tetrahydroisoquinoline, naphthalene, tetrahydronaphthalene, 2,3-dihydrobenzo[b][l,4]dioxine, isoindole, 2H- benzo[b][l,4]oxazin-3(4H)-one, 3,4-dihydro-2H-benzo[b][l,4]oxazine, and quinoxalin- 2(lH)-one.
- hi is a bond. In some embodiments, hi is -0-, -S-, or - N(R)-.
- hi is a Ci_6 aliphatic chain wherein one, two, or three methylene units of hi are optionally and independently replaced by -0-, -S-, -N(R)-, -C(O)-, -C(0)N(R)-, - N(R)C(0)N(R)-, -N(R)C(0)-, -N(R)C(0)0- -OC(0)N(R)-, -S0 2 - -S0 2 N(R)-, - N(R)S0 2 -, -OC(O)-, or -C(0)0-.
- hi is a C 1-3 aliphatic chain wherein one methylene unit of hi is optionally replaced by -0-, -S-, -N(R)-, -C(O)-, - C(0)N(R)-, -N(R)C(0)N(R)-, -N(R)C(0)-, -N(R)C(0)0-, -OC(0)N(R)-, -S0 2 - - S0 2 N(R)-, -N(R)S0 2 -, -OC(O)-, or -C(0)0-.
- Cy is an optionally substituted, monocyclic, bicyclic or tricyclic, saturated, partially unsaturated, or aromatic ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, and sulfur.
- Cy is aromatic.
- Cy is saturated.
- Cy is partially unsaturated.
- Cy is monocyclic.
- Cy is bicyclic.
- Cy is tricyclic.
- Cy is optionally substituted phenyl.
- Cy is an optionally substituted 5- to 6-membered heteroaryl having 1-3 heteroatoms independently selected from nitrogen, oxygen, and sulfur.
- Cy is an optionally substituted 5-membered heteroaryl having 1-3 heteroatoms independently selected from nitrogen, oxygen, and sulfur (e.g., furanyl, thienyl, pyrrolyl, oxazolyl, isoxazolyl, thiazolyl, imidazolyl, pyrazolyl, isothiazolyl, triazolyl, oxadiazolyl, thiadiazolyl.
- Cy is an optionally substituted 6-membered heteroaryl having 1-3 nitrogens (e.g., pyridyl, pyrimidyl, pyridazinyl, pyrazinyl, triazinyl).
- Cy is optionally substituted pyrazole, optionally substituted pyridyl, or optionally substituted pyrimidyl. In some embodiments, Cy is an optionally substituted carbocyclic ring. In some embodiments, Cy is an optionally substituted 3- to 8-membered saturated carbocyclic ring. In some embodiments, Cy is an optionally substituted 3- to 8- membered heterocyclic ring having 1-2 heteroatoms independently selected from nitrogen, oxygen, and sulfur.
- Cy is an optionally substituted bicyclic saturated, partially unsaturated, or aromatic ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, and sulfur. In certain embodiments, Cy is an optionally substituted 8- to 12- membered bicyclic saturated, partially unsaturated, or aromatic ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, and sulfur. In certain embodiments, Cy is an optionally substituted 8- to 10-membered bicyclic heteroaryl having 1-4 heteroatoms independently selected from nitrogen, oxygen, and sulfur. In certain embodiments, Cy is an optionally substituted 9- to 10-membered bicyclic heteroaryl having 1-4 heteroatoms independently selected from nitrogen, oxygen, and sulfur.
- Cy is an optionally substituted 9-membered bicyclic heteroaryl having 1-3 heteroatoms independently selected from nitrogen, oxygen, and sulfur (e.g. , indolyl, isoindolyl, indazolyl, benzotriazolyl, benzothiophenyl, isobenzothiophenyl, benzofuranyl, benzoisofuranyl, benzimidazolyl, benzoxazolyl, benzisoxazolyl, benzoxadiazolyl, benzthiazolyl, benzisothiazolyl,
- indolyl isoindolyl, indazolyl, benzotriazolyl, benzothiophenyl, isobenzothiophenyl, benzofuranyl, benzoisofuranyl, benzimidazolyl, benzoxazolyl, benzisoxazolyl, benzoxadiazolyl, benzthiazolyl, benzisothiazolyl
- Cy is an optionally substituted 10- membered bicyclic heteroaryl having 1-3 heteroatoms independently selected from nitrogen, oxygen, and sulfur (e.g. , naphthyridinyl, quinolinyl, isoquinolinyl, quinoxalinyl, quinazolinyl.
- Cy is optionally substituted indazole, optionally substituted quinoline, optionally substituted benzimidazole, optionally substituted benzothiazole, optionally substituted deazapurine, optionally substituted indole, optionally substituted purine, optionally substituted pyrazolopyridine, optionally substituted pyrrolopyridine, optionally substituted pyrroloprimidine, optionally substituted imidazopyridine, or optionally substituted imidazopyridine.
- R 5 , R 6 , R 7 , and R 8 are each independently hydrogen, halo, or optionally substituted aliphatic. In some embodiments, R 5 , R 6 , R 7 , and R 8 are hydrogen. In some embodiments, R 6 , R 7 , and R 8 are hydrogen, and R 5 is optionally substituted aliphatic. In some embodiments, R 6 , R 7 , and R 8 are hydrogen, and R 5 is
- R , R , and R are hydrogen, and R 5 is optionally substituted Ci_3 aliphatic.
- R 6 , R 7 , and R 8 are hydrogen, and R 5 is methyl.
- R 6 , R 7 , and R 5 are hydrogen, and R 8 is optionally substituted aliphatic.
- R 6 , R 7 , and R 5 are hydrogen, and R 8 is optionally substituted C 1-6 aliphatic.
- R 6 , R 7 , and R 5 are hydrogen, and R 8 is optionally substituted Ci_3 aliphatic.
- R 6 , R 7 , and R 5 are
- R is hydrogen, and R is methyl.
- R is hydrogen.
- R 5 is halo.
- R 5 is fluoro.
- R 5 is optionally substituted Ci_6 aliphatic.
- R 5 is optionally substituted Ci_3 alkyl.
- R 5 is methyl.
- R 6 is hydrogen.
- R 6 is halo.
- R 6 is fluoro.
- R 6 is optionally substituted C 1-6 aliphatic.
- R 6 is optionally substituted C 1-3 alkyl.
- R 6 is methyl.
- R 7 is hydrogen.
- R 7 is halo. In certain embodiments, R 7 is fluoro. In some embodiments, R 7 is optionally substituted C 1-6 aliphatic. In some embodiments, R is optionally substituted C 1-3 alkyl. In certain embodiments, R 7 is methyl. In some embodiments, R 8 is hydrogen. In some embodiments, R 8 is halo. In certain embodiments, R 8 is fluoro. In some embodiments, R 8 is optionally substituted C 1-6 aliphatic. In some embodiments, R is optionally substituted C 1-3 alkyl. In certain embodiments, R is methyl.
- R 9 is hydrogen. In some embodiments, R 9 is not hydrogen. In some embodiments, R 9 is halo. In certain embodiments, R 9 is fluoro. In some embodiments, R 9 is optionally substituted aliphatic. In certain embodiments, R 9 is optionally substituted C 1-6 aliphatic. In certain embodiments, R 9 is optionally substituted C 1-6 alkyl. In certain embodiments, R 9 is substituted C 1-6 alkyl. In certain embodiments, R 9 is -CF 3 , CHF 2 , or CH 2 F. In certain embodiments, R 9 is unsubstituted Ci_6 alkyl. In certain embodiments, R 9 is methyl, ethyl, or propyl. In some embodiments, R 9 is -CN or -N0 2 . In some embodiments,
- R 9 is optionally substituted carbocyclyl, optionally substituted phenyl, optionally substituted heterocyclyl, or optionally substituted heteroaryl.
- R is -N(R ) 2 .
- R is - NHR B .
- R 9 is -NH 2 .
- R 9 is -OR A .
- R 9 is -OH.
- R 10 is hydrogen. In some embodiments, R 10 is not hydrogen. In some embodiments, R 10 is halo. In certain embodiments, R 10 is fluoro. In some embodiments, R 10 is optionally substituted aliphatic. In certain embodiments, R 10 is optionally substituted C 1-6 aliphatic. In certain embodiments, R 10 is optionally substituted Ci_ 6 alkyl. In certain embodiments, R 10 is substituted Ci_6 alkyl. In certain embodiments, R 10 is -CF 3 , CHF 2 , or CH 2 F. In certain embodiments, R 10 is unsubstituted Ci_6 alkyl. In certain embodiments, R 10 is methyl, ethyl, or propyl.
- R 10 is -CN or -N0 2 . In some embodiments, R 10 is optionally substituted carbocyclyl, optionally substituted phenyl, optionally substituted heterocyclyl, or optionally substituted heteroaryl. In some
- R is -N(R ) 2 .
- R is - NHR B .
- R 10 is -NH 2 .
- R 10 is -OR A .
- R 10 is -OH.
- R 9 and R 10 are the same. In some embodiments, R 9 and R 10 are different. In some embodiments, R 9 and R 10 are each hydrogen. In some embodiments, R 9 is hydrogen and R 10 is not hydrogen. In some embodiments, R 9 is hydrogen and R 10 is optionally substituted aliphatic. In some embodiments, R 9 is hydrogen and R 10 is C 1-6 alkyl. In some embodiments, R 9 is hydrogen and R 10 is methyl. In some embodiments, R 9 is hydrogen and R 10 is ethyl or propyl. In certain embodiments, R 9 and hydrogen and R 10 is - CF 3 , CHF 2 , or CH 2 F.
- R 9 is hydrogen and R 10 is -N(R B ) 2 or -OR A . In some embodiments, R 9 is hydrogen and R 10 is -NH 2 . In some embodiments, R 9 is hydrogen and R 10 is -OH. In some embodiments, R 9 and R 10 are not hydrogen. In some embodiments, R 9 and R 10 are independently optionally substituted aliphatic. In some embodiments, R 9 and R 10 are methyl. In some embodiments, R 9 and R 10 are taken together with their intervening atoms to form an optionally substituted carbocyclic or heterocyclic ring.
- At least one R y is halo. In certain embodiments, at least one R y is fluoro. In certain embodiments, at least one R y is chloro. In some embodiments, at least one R y is -CN. In some embodiments, at least one R y is -OR A , wherein R A is optionally substituted aliphatic. In some embodiments, at least one R y is -OR A , wherein R A is unsubstituted C 1-6 alkyl. In certain embodiments, at least one R y is methoxy, ethoxy, or propoxy. In certain embodiments, at least one R y is methoxy.
- At least one R y is -OR A , wherein R A is substituted Ci_6 alkyl. In certain embodiments, at least one R y is -OCH 2 CH 2 N(CH 3 ) 2 . In some embodiments, at least one R y is -OR A , wherein R A is optionally substituted heterocyclyl. In some embodiments, at least one R y is -OR A , wherein R A is an optionally subsituted 4- to 7-membered heterocyclyl having 1-2 heteroatoms independently selected from nitrogen, oxygen, and sulfur.
- At least one R y is -OR A , wherein R A is oxetanyl, tetrahydrofuranyl, or tetrahydropyranyl. In some embodiments, at least one R y is -N(R B ) 2 . In some embodiments, at least one R y is -N(R B ) 2 , wherein each R is independently hydrogen or Ci_6 alkyl. In some embodiments, at least one R y is -NHR B . In some embodiments, at least one R y is -N(C 1-6 alkyl) 2 , -NH(C 1-6 alkyl), or - NH 2 .
- At least one R y is -NH 2 . In certain embodiments, at least one R y is -NHCH 3 . In certain embodiments, at least one R y is -N(CH 3 ) 2 . In some embodiments, at least one R v y is -N(R B ) 2 or -NHR B , wherein at least one R B is optionally substituted heterocyclyl. In some embodiments, at least one R v y is -N(R B ) 2 or -NHR B , wherein at least one R is an optionally subsituted 4- to 7-membered heterocyclyl having 1-2 heteroatoms independently selected from nitrogen, oxygen, and sulfur. In some embodiments, at least one
- R v y is -N(R B ) 2 or -NHR B , wherein at least one R B is oxetanyl, tetrahydropyranyl, or tetrahydrofuranyl. In some embodiments, at least one R v y is -N(R B ) 2 or -NHR B , wherein at least one R is optionally substituted piperidinyl or optionally substituted piperazinyl.
- At least one R y is optionally substituted aliphatic. In certain embodiments, at least one R y is substituted aliphatic. In certain embodiments, at least one R y is unsubstituted aliphatic. In some embodiments, at least one R y is optionally substituted Ci_6 alkyl. In certain embodiments, at least one R y is unsubstituted Ci_6 alkyl. In certain embodiments, at least one R y is substituted Ci_6 alkyl. In certain embodiments, at least one R y is methyl, ethyl, or propyl. In certain embodiments, at least one R y is methyl.
- At least one R y is -CF 3 , CHF 2 , or CH 2 F. In certain embodiments, at least one R y is Ci_6 alkyl substituted with aryl, heteroaryl, or heterocyclyl. In certain embodiments, at least one R y is benzyl. In certain embodiments, at least one R y is -(C 1-6 alkyl)-aryl. In certain embodiments, at least one R y is -(C 1-6 alkyl)-heteroaryl. In certain embodiments, at least one R y is -(C 1-6 alkyl)-heterocyclyl. In certain embodiments, at least one R y is -CH 2 - aryl. In certain embodiments, at least one R y is -CH 2 -heteroaryl. In certain embodiments, at least one R y is -CH 2 -heterocyclyl.
- At least one R y is -C(0)N(R B ) 2 . In certain embodiments, at least one R y is -C(0)NHR B . In certain embodiments, at least one R y is -C(0)NH 2 . In certain embodiments, at least one R v y is -C(0)N(R B ) 2 , wherein the R B groups are taken together with their intervening atoms to form an optionally substituted 5- to 6-membered heterocyclyl. In certain embodiments, at least one R v y is -C(0)N(R B ) 2 , wherein the R B groups are taken together with their intervening atoms to form an optionally substituted morpholinyl.
- At least one R y is -S0 2 N(R B ) 2 . In certain embodiments, at least one R y is -S0 2 NHR B . In certain embodiments, at least one R y is -S0 2 NH 2 . In certain embodiments, at least one R v y is -S0 2 N(R B ) 2 , wherein neither R B is hydrogen. In certain embodiments, at least one R y is -S0 2 NH(C 1 _6 alkyl) or -S0 2 N(C 1 _6 alkyl) 2 . In certain embodiments, at least one R y is -S0 2 N(CH ) 2 . In certain embodiments, at least one R y is -
- R B is taken together with their intervening atoms to form an optionally substituted 5- to 6-membered heterocyclyl.
- at least one R y is -S0 2 -morpholinyl.
- at least one R y is -S0 2 -piperidinyl, -S0 2 - piperazinyl, or -S0 2 -piperidinyl.
- At least one R y is -S0 2 R A . In some embodiments, at least one R y is -S0 2 R A , wherein R A is optionally substituted aliphatic. In some embodiments, at least one R y is -S0 2 (C 1-6 alkyl). In some embodiments, at least one R y is -S0 2 CH 3 . In some embodiments, at least one R y is -C(0)R A . In some embodiments, at least one R y is -C(0)R , wherein R A is optionally substituted aliphatic. In some embodiments, at least one R y is - C(0)(Ci-6 alkyl). In some embodiments, at least one R y is -C(0)CH 3 .
- At least one R y is -N(R B )C(0)R A . In certain embodiments, at least one R y is -NHC(0)R A . In certain embodiments, at least one R y is -NHC(0)(C 1-6 alkyl). In certain embodiments, at least one R y is -NHC(0)CH . [00203] In some embodiments, at least one R y is -N(R B )S0 2 R A In some embodiments, at least one R y is -NHS0 2 R A . In some embodiments, at least one R y is -N(C 1-6 alkyl)S0 2 R .
- At least one R y is -NHS0 2 (Ci-6 alkyl) or -N(C 1-6 alkyl)S0 2 (C 1 _6 alkyl). In certain embodiments, at least one R y is -NHS0 2 CH 3 . In certain embodiments, at least one R y is -N(CH 3 )S0 2 CH 3 .
- At least one R y is optionally substituted heterocyclyl, optionally substituted carbocyclyl, optionally substituted aryl, or optionally substituted heteroaryl. In certain embodiments, at least one R y is an optionally substituted 5- to 6- membered heterocyclyl having 1-2 heteroatoms independently selected from nitrogen, oxygen, and sulfur. In certain embodiments, at least one R y is an optionally substituted 5- membered heterocyclyl having one heteroatom selected from nitrogen, oxygen, and sulfur. In certain embodiments, at least one R y is optionally substituted pyrrolidinyl.
- At least one R y is pyrroldinyl, hydroxypyrrolidinyl, or methylpyrrolidinyl. In certain embodiments, at least one R y is an optionally substituted 6-membered heterocyclyl having 1-2 heteroatoms independently selected from nitrogen, oxygen, and sulfur. In certain embodiments, at least one R y is an optionally substituted 6-membered heterocyclyl having one heteroatom selected from nitrogen, oxygen, and sulfur. In certain embodiments, at least one R y is optionally substituted piperidinyl. In certain embodiments, at least one R y is an optionally substituted 6-membered heterocyclyl having two heteroatoms independently selected from nitrogen, oxygen, and sulfur.
- At least one R y is optionally substituted piperdinyl, optionally substituted piperazinyl, or optionally substituted morpholinyl. In certain embodiments, at least one R y is morpholinyl, tetrahydropyranyl, piperidinyl, methylpiperidinyl, piperazinyl, methylpiperazinyl, acetylpiperazinyl, methylsulfonylpiperazinyl, aziridinyl, or methylaziridinyl. In some embodiments, at least one R y is an optionally substituted 5- to 6-membered heteroaryl having 1-3 heteroatoms independently selected from nitrogen, oxygen, and sulfur.
- At least one R y is an optionally substituted 5-membered heteroaryl having 1-3 heteroatoms independently selected from nitrogen, oxygen, and sulfur. In certain embodiments, at least one R y is an optionally substituted 5-membered heteroaryl having one heteroatom selected from nitrogen, oxygen, and sulfur. In certain embodiments, at least one R y is an optionally substituted 5-membered heteroaryl having two heteroatoms independently selected from nitrogen, oxygen, and sulfur. In certain embodiments, at least one R y is an optionally substituted 6-membered heteroaryl having 1-3 nitrogens. In certain embodiments, at least one R y is an optionally substituted pyrazolyl. In certain embodiments, at least one R y is an optionally substituted imidazolyl.
- At least one R y is an optionally substituted pyridyl. In certain embodiments, at least one R y is an optionally substituted pyrimidyl. In certain embodiments, at least one R y is pyrazolyl, methylpyrazolyl, imidazolyl, or methylimidazolyl.
- R y is -OR A . In some embodiments, R y is -OR A , wherein R A is optionally substituted heterocyclyl. In some embodiments, R y is -OR A , wherein R A is optionally substituted heteroaryl. In some embodiments, R y is -OR A , wherein R A is optionally substituted cycloalkyl. In some embodiments, R v y is -N(R B ) 2 . In some
- R v y is -NHR B . In some embodiments, R v y is -NHR B , wherein R B is optionally substituted heterocyclyl. In some embodiments, R v y is -NHR B , wherein R B is optionally substituted heteroaryl. In some embodiments, R v y is -NHR B , wherein R B is optionally substituted cycloalkyl. In some embodiments, R v y is -N(R B ) 2 , wherein one R B is optionally substituted heterocyclyl, and the other R B is Ci_4 alkyl.
- R v y is -N(R B ) 2 , wherein one R B is optionally substituted heteroaryl, and the other R B is Ci_4 alkyl. In some embodiments, R v y is -N(R B ) 2 , wherein one R B is optionally substituted cycloalkyl, and the other R is C 1-4 alkyl.
- Cy is selected from the group consisting of:
- each R x is independently selected from the group consisting of halo, -CN, optionally substituted aliphatic, -OR', and -N(R") 2 .
- at least one R x is halo.
- at least one R x is fluoro.
- at least one R x is -CN.
- at least one R x is optionally substituted aliphatic.
- at least one R x is optionally substituted Ci_6 alkyl.
- at least one R x is methyl.
- at least one R x is -CF 3 .
- At least one R x is -OR' or - N(R") 2 . In certain embodiments, R x is not -OR' or -N(R") 2 . In certain embodiments, at least one R x is -OCH . In certain embodiments, R x is not -OCH .
- n and R x are as defined herein.
- each of the atoms of the phenyl ring and the nitrogen-containing ring can be independently optionally substituted with R x , as valency permits.
- the nitrogen-containing ring does not comprise an Rx substituent.
- only atoms of the phenyl ring are optionally substituted with one or more R x .
- the nitrogen-containing ring is optionally substituted and the fused bicyclic ring system is of the formula:
- R x is as defined above, and nl is 0, 1, 2, 3, or 4.
- an R x group can be attached anywhere on the tetrahydroisoquinoline or dihydroisoquinoline ring.
- an R x group is attached to the benzene portion of the tetrahydroisoquinoline or dihydroisoquinoline ring.
- an R x group is attached to the tetrahydropyridine or dihydropyridine portion of the tetrahydroisoquinoline or dihydroisoquinoline ring.
- R x groups are attached to both the benzene portion and the tetrahydropyridine (or dihydropyridine) portion of the
- a provided compound is of Formula (XI):
- n is 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10, as valency permits. In certain embodiments, n is 0. In certain embodiments, n is 1. In certain embodiments, n is 2.
- R A1 and R ⁇ are independently hydrogen, substituted or unsubstituted C 1-3 alkyl, substituted or unsubstituted acyl, or a nitrogen protecting group.
- R A1 is hydrogen.
- R A1 is substituted or unsubstituted C 1-3 alkyl.
- R A1 is unsubstituted C 1-3 alkyl.
- R A1 is methyl, ethyl, n-propyl, or isopropyl.
- R A1 is substituted C 1-3 alkyl.
- R A1 is -CF 3 , -CHF 2 , -CH 2 F, or -CH(CF 3 )CH 3 .
- R A1 is substituted or unsubstituted acyl.
- R A1 is acetyl.
- R A1 is a nitrogen protecting group.
- R A1 is CH 3 S0 2 -.
- R ⁇ is hydrogen.
- R ⁇ is substituted or unsubstituted C 1-3 alkyl.
- R ⁇ is unsubstituted C 1-3 alkyl.
- R ⁇ is methyl, ethyl, n-propyl, or isopropyl. In some embodiments, R ⁇ is substituted C 1-3 alkyl. In some embodiments, R ⁇ is -CF 3 , -CHF 2 , -CH 2 F, or - CH(CF 3 )CH 3 . In some embodiments, R is substituted or unsubstituted acyl. In some embodiments, R ⁇ is acetyl. In some embodiments, R ⁇ is a nitrogen protecting group. In some embodiments, R ⁇ is CH 3 S0 2 -. In some embodiments, R A1 is hydrogen, and R ⁇ is hydrogen.
- R A1 is hydrogen, and R ⁇ is substituted or unsubstituted Ci- 3 alkyl. In some embodiments, R A1 is hydrogen, and R ⁇ is methyl, ethyl, n-propyl, or isopropyl. In some embodiments, R A1 is hydrogen, and R ⁇ is -CF 3 , -CHF 2 , -CH 2 F, or - CH(CF 3 )CH 3 . In some embodiments, R A1 is hydrogen, and R ⁇ is substituted or
- R A1 is hydrogen, and R ⁇ is acetyl. In some embodiments, R A1 is hydrogen, and R ⁇ is a nitrogen protecting group. In some
- R A1 is hydrogen and R ⁇ is CH S0 2 -. In some embodiments, R A1 is substituted or unsubstituted C 1-3 alkyl, and R ⁇ is substituted or unsubstituted C 1-3 alkyl. In some embodiments, R A1 is substituted or unsubstituted C 1-3 alkyl, and R ⁇ is methyl. In some embodiments, R A1 is substituted or unsubstituted C 1-3 alkyl, and R ⁇ is ethyl. In some embodiments, R A1 is substituted or unsubstituted C 1-3 alkyl, and R ⁇ is n-propyl.
- R A1 is substituted or unsubstituted C 1-3 alkyl, and R ⁇ is isopropyl. In some embodiments, R A1 is substituted or unsubstituted C 1-3 alkyl, and R ⁇ is substituted or unsubstituted acyl. In some embodiments, R A1 is substituted or unsubstituted C 1-3 alkyl, and R ⁇ is a nitrogen protecting group. In some embodiments, R A1 is methyl, and R ⁇ is substituted or unsubstituted C 1-3 alkyl. In some embodiments, R A1 is methyl, and R ⁇ is methyl. In some embodiments, R A1 is methyl, and R ⁇ is ethyl.
- R A1 is methyl, and R ⁇ is n-propyl. In some embodiments, R A1 is methyl, and R A2 is isopropyl. In some embodiments, R A1 is methyl, and R ⁇ is substituted or unsubstituted acyl. In some embodiments, R A1 is methyl, and R ⁇ is a nitrogen protecting group. In some embodiments, R A1 is ethyl, and R ⁇ is substituted or unsubstituted C 1-3 alkyl. In some embodiments, R A1 is ethyl, and R ⁇ is methyl. In some embodiments, R A1 is ethyl, and R ⁇ is ethyl.
- R A1 is ethyl, and R ⁇ is n-propyl. In some embodiments, R A1 is ethyl, and R ⁇ is isopropyl. In some embodiments, R A1 is ethyl, and R ⁇ is substituted or unsubstituted acyl. In some embodiments, R A1 is ethyl, and R ⁇ is a nitrogen protecting group. In some embodiments, R A1 is n-propyl, and R ⁇ is substituted or unsubstituted C 1-3 alkyl. In some embodiments, R A1 is n-propyl, and R ⁇ is methyl.
- R A1 is n-propyl, and R ⁇ is ethyl. In some embodiments, R A1 is n-propyl, and R ⁇ is n-propyl. In some embodiments, R A1 is n-propyl and R ⁇ is isopropyl. In some embodiments, R A1 is n-propyl, and R ⁇ is substituted or unsubstituted acyl. In some embodiments, R A1 is n-propyl and R A2 is a nitrogen protecting group. In some embodiments, R A1 is isopropyl and R A2 is substituted or unsubstituted C 1-3 alkyl. In some embodiments, R is isopropyl and R is methyl.
- R A1 is isopropyl and R ⁇ is ethyl. In some embodiments, R A1 is isopropyl, and R ⁇ is n-propyl. In some embodiments, R A1 is isopropyl, and R ⁇ is isopropyl. In some embodiments, R A1 is isopropyl, and R ⁇ is substituted or unsubstituted acyl. In some embodiments, R A1 is isopropyl, and R ⁇ is a nitrogen protecting group. In some embodiments,
- R A1 is substituted or unsubstituted acyl, and R ⁇ is substituted or unsubstituted Ci-3 alkyl.
- R A1 is a nitrogen protecting group, and R ⁇ is substituted or unsubstituted C 1-3 alkyl.
- R A1 is a nitrogen protecting group and R ⁇ is methyl.
- R A1 is a nitrogen protecting group, and R ⁇ is ethyl.
- R A1 is a nitrogen protecting group, and R ⁇ is n-propyl.
- R A1 is a nitrogen protecting group, and R ⁇ is isopropyl.
- R A1 is a nitrogen protecting group
- R ⁇ is a nitrogen protecting group
- R A1 and R ⁇ can be taken together with the
- R A1 and R ⁇ can be taken together with the intervening nitrogen atom to form a substituted or unsubstituted 3-6 membered heterocyclic ring.
- R A1 and R ⁇ can be taken together with the intervening nitrogen atom to form a substituted or unsubstituted azetidine.
- R A1 and R ⁇ can be taken together with the intervening nitrogen atom to form a substituted or unsubstituted pyrrolidine.
- R A1 and R ⁇ can be taken together with the intervening nitrogen atom to form a substituted or unsubstituted piperidine.
- R A1 and R ⁇ can be taken together with the intervening nitrogen atom to form a substituted or unsubstituted piperazine.
- R A1 and R ⁇ can be taken together with the intervening nitrogen atom to form a substituted or unsubstituted morpholine.
- the provided compound is of a free base form. In some embodiments, e.g. for Formula (A), Formula (I), or any subgenera thereof, the provided compound is in the form of a
- a provided compound is a hydrochloride salt thereof. In some embodiments, the provided compound is a tartrate salt thereof. In some embodiments, the provided compound is a monotartrate salt thereof. In some embodiments, the provided compound is a bitartrate salt thereof. [00217] In certain embodiments, a provided compound is a compound listed in Table 1A, or a pharmaceutically acceptable salt thereof.
- a provided compound is a compound listed in Table IB, or a pharmaceutically acceptable salt thereof.
- a provided compound inhibits PRMT5. In certain embodiments, a provided compound inhibits wild- type PRMT5. In certain embodiments, a provided compound inhibits a mutant PRMT5. In certain embodiments, a provided compound inhibits PRMT5, e.g., as measured in an assay described herein. In certain embodiments, the PRMT5 is from a human. In certain embodiments, a provided compound inhibits PRMT5 at an IC 50 less than or equal to 10 ⁇ . In certain embodiments, a provided compound inhibits PRMT5 at an IC 50 less than or equal to 1 ⁇ . In certain embodiments, a provided compound inhibits PRMT5 at an IC 50 less than or equal to 0.1 ⁇ .
- a provided compound inhibits PRMT5 in a cell at an EC 50 less than or equal to 10 ⁇ . In certain embodiments, a provided compound inhibits PRMT5 in a cell at an EC 50 less than or equal to 1 ⁇ . In certain embodiments, a provided compound inhibits PRMT5 in a cell at an EC 50 less than or equal to 0.1 ⁇ . In certain embodiments, a provided compound inhibits cell proliferation at an EC 50 less than or equal to 10 ⁇ . In certain embodiments, a provided compound inhibits cell proliferation at an EC 50 less than or equal to 1 ⁇ . In certain embodiments, a provided compound inhibits cell proliferation at an EC 50 less than or equal to 0.1 ⁇ .
- a provided compound is selective for PRMT5 over other methyltransferases. In certain embodiments, a provided compound is at least about 10- fold selective, at least about 20-fold selective, at least about 30-fold selective, at least about 40-fold selective, at least about 50-fold selective, at least about 60-fold selective, at least about 70-fold selective, at least about 80-fold selective, at least about 90-fold selective, or at least about 100-fold selective for PRMT5 relative to one or more other methyltransferases.
- the PRMT5 can be wild- type PRMT5, or any mutant or variant of PRMT5.
- the mutant or variant of PRMT5 contains one or more mutations (e.g., conservative substitutions).
- a PRMT5 point mutant In some embodiments, provided herein is a PRMT5 point mutant.
- the PRMT point mutant has an amino acid sequence that a degree of homology to the amino acid sequence of SEQ ID NO: 1 of at least about 80%, e.g., at least about 85%, at least about 90%, at least about 95% , or at least about 97%.
- a protein that has a degree of homology to the amino acid sequence of SEQ ID NO: 2 of at least about 80%, e.g., at least about 85%, at least about 90%, at least about 95% , or at least about 97%.
- the PRMT5 is isoform A (GenBank accession no.
- NP006100 (SEQ ID NO.: 1):
- the PRMT5 is isoform B (GenBank accession no.
- the PRMT5 is transcript variant 1 (GenBank accession no. NM_006109).
- compositions comprising a compound described herein, e.g., a compound of Formula (A), e.g., Formula (I), or a pharmaceutically acceptable salt thereof, as described herein, and optionally a
- a provided composition comprises two or more compounds described herein.
- a compound described herein, or a pharmaceutically acceptable salt thereof is provided in an effective amount in the pharmaceutical composition.
- the effective amount is a therapeutically effective amount.
- the effective amount is an amount effective for inhibiting PRMT5.
- the effective amount is an amount effective for treating a PRMT5-mediated disorder.
- the effective amount is a prophylactically effective amount.
- the effective amount is an amount effective to prevent a PRMT5 -mediated disorder.
- the provided pharmaceutical compositions comprise a compound described herein, e.g., a compound of Formula (A), e.g., Formula (I), or any subgenera thereof, and optionally a pharmaceutically acceptable excipient, wherein the compound is of a free base form.
- the provided pharmaceutical compositions comprise a compound described herein, e.g., a compound of Formula (A), e.g., Formula (I), or any subgenera thereof, and optionally a pharmaceutically acceptable excipient, wherein the compound is in the form of a pharmaceutically acceptable salt as generally defined herein.
- the provided pharmaceutical compositions comprise a hydrochloride salt of a compound described herein and optionally a pharmaceutically acceptable excipient. In certain embodiments, the provided pharmaceutical compositions comprise a tartrate salt of a compound described herein and optionally a pharmaceutically acceptable excipient. In certain embodiments, the provided pharmaceutical compositions comprise a monotartrate salt of a compound described herein and optionally a
- the provided pharmaceutical compositions comprise a bitartrate salt of a compound described herein and optionally a pharmaceutically acceptable excipient. In certain embodiments, the provided pharmaceutical compositions comprise a monotartrate salt and a bitartrate salt of a compound described herein and optionally a pharmaceutically acceptable excipient. In certain embodiments, the provided pharmaceutical compositions comprise a compound described herein in a form of free base, and a pharmaceutically acceptable salt thereof, and optionally a pharmaceutically acceptable excipient.
- compositions agents include any and all solvents, diluents, or other liquid vehicles, dispersions, suspension aids, surface active agents, isotonic agents, thickening or emulsifying agents, preservatives, solid binders, lubricants, and the like, as suited to the particular dosage form desired.
- solvents diluents, or other liquid vehicles, dispersions, suspension aids, surface active agents, isotonic agents, thickening or emulsifying agents, preservatives, solid binders, lubricants, and the like.
- compositions described herein can be prepared by any method known in the art of pharmacology. In general, such preparatory methods include the steps of bringing a compound described herein (the "active ingredient") into association with a carrier and/or one or more other accessory ingredients, and then, if necessary and/or desirable, shaping and/or packaging the product into a desired single- or multi-dose unit.
- Pharmaceutical compositions can be prepared, packaged, and/or sold in bulk, as a single unit dose, and/or as a plurality of single unit doses.
- a "unit dose” is discrete amount of the pharmaceutical composition comprising a predetermined amount of the active ingredient. The amount of the active ingredient is generally equal to the dosage of the active ingredient which would be administered to a subject and/or a convenient fraction of such a dosage such as, for example, one-half or one-third of such a dosage.
- compositions of the present disclosure will vary, depending upon the identity, size, and/or condition of the subject treated and further depending upon the route by which the composition is to be administered.
- the composition may comprise between 0.1% and 100% (w/w) active ingredient.
- compositions used in the manufacture of provided pharmaceutical compositions include inert diluents, dispersing and/or granulating agents, surface active agents and/or emulsifiers, disintegrating agents, binding agents, preservatives, buffering agents, lubricating agents, and/or oils. Excipients such as cocoa butter and suppository waxes, coloring agents, coating agents, sweetening, flavoring, and perfuming agents may also be present in the composition.
- Exemplary diluents include calcium carbonate, sodium carbonate, calcium phosphate, dicalcium phosphate, calcium sulfate, calcium hydrogen phosphate, sodium phosphate lactose, sucrose, cellulose, microcrystalline cellulose, kaolin, mannitol, sorbitol, inositol, sodium chloride, dry starch, cornstarch, powdered sugar, and mixtures thereof.
- Exemplary granulating and/or dispersing agents include potato starch, corn starch, tapioca starch, sodium starch glycolate, clays, alginic acid, guar gum, citrus pulp, agar, bentonite, cellulose and wood products, natural sponge, cation-exchange resins, calcium carbonate, silicates, sodium carbonate, cross-linked poly(vinyl-pyrrolidone) (crospovidone), sodium carboxymethyl starch (sodium starch glycolate), carboxymethyl cellulose, cross- linked sodium carboxymethyl cellulose (croscarmellose), methylcellulose, pregelatinized starch (starch 1500), microcrystalline starch, water insoluble starch, calcium carboxymethyl cellulose, magnesium aluminum silicate (Veegum), sodium lauryl sulfate, quaternary ammonium compounds, and mixtures thereof.
- crospovidone cross-linked poly(vinyl-pyrrolidone)
- sodium carboxymethyl starch sodium starch glycolate
- Exemplary surface active agents and/or emulsifiers include natural emulsifiers (e.g., acacia, agar, alginic acid, sodium alginate, tragacanth, chondrux, cholesterol, xanthan, pectin, gelatin, egg yolk, casein, wool fat, cholesterol, wax, and lecithin), colloidal clays (e.g., bentonite (aluminum silicate) and Veegum (magnesium aluminum silicate)), long chain amino acid derivatives, high molecular weight alcohols (e.g., stearyl alcohol, cetyl alcohol, oleyl alcohol, triacetin monostearate, ethylene glycol distearate, glyceryl monostearate, and propylene glycol monostearate, polyvinyl alcohol), carbomers (e.g., carboxy polymethylene, polyacrylic acid, acrylic acid polymer, and carboxyvinyl polymer), carrageenan, cell
- Exemplary binding agents include starch (e.g., cornstarch and starch paste), gelatin, sugars (e.g., sucrose, glucose, dextrose, dextrin, molasses, lactose, lactitol, mannitol, etc.), natural and synthetic gums (e.g., acacia, sodium alginate, extract of Irish moss, panwar gum, ghatti gum, mucilage of isapol husks, carboxymethylcellulose, methylcellulose, ethylcellulose, hydroxyethylcellulose, hydroxypropyl cellulose, hydroxypropyl
- methylcellulose methylcellulose, microcrystalline cellulose, cellulose acetate, poly(vinyl-pyrrolidone), magnesium aluminum silicate (Veegum), and larch arabogalactan), alginates, polyethylene oxide, polyethylene glycol, inorganic calcium salts, silicic acid, polymethacrylates, waxes, water, alcohol, and/or mixtures thereof.
- Exemplary preservatives include antioxidants, chelating agents, antimicrobial preservatives, antifungal preservatives, alcohol preservatives, acidic preservatives, and other preservatives.
- antioxidants include alpha tocopherol, ascorbic acid, acorbyl palmitate, butylated hydroxyanisole, butylated hydroxytoluene, monothioglycerol, potassium
- Exemplary chelating agents include ethylenediaminetetraacetic acid (EDTA) and salts and hydrates thereof (e.g. , sodium edetate, disodium edetate, trisodium edetate, calcium disodium edetate, dipotassium edetate, and the like), citric acid and salts and hydrates thereof (e.g.
- antimicrobial preservatives include benzalkonium chloride, benzethonium chloride, benzyl alcohol, bronopol, cetrimide, cetylpyridinium chloride, chlorhexidine, chlorobutanol, chlorocresol, chloroxylenol, cresol, ethyl alcohol, glycerin, hexetidine, imidurea, phenol, phenoxyethanol, phenylethyl alcohol, phenylmercuric nitrate, propylene glycol, and thimerosal.
- Exemplary antifungal preservatives include butyl paraben, methyl paraben, ethyl paraben, propyl paraben, benzoic acid, hydroxybenzoic acid, potassium benzoate, potassium sorbate, sodium benzoate, sodium propionate, and sorbic acid.
- Exemplary alcohol preservatives include ethanol, polyethylene glycol, phenol, phenolic compounds, bisphenol, chlorobutanol, hydroxybenzoate, and phenylethyl alcohol.
- Exemplary acidic preservatives include vitamin A, vitamin C, vitamin E, beta- carotene, citric acid, acetic acid, dehydroacetic acid, ascorbic acid, sorbic acid, and phytic acid.
- preservatives include tocopherol, tocopherol acetate, deteroxime mesylate, cetrimide, butylated hydroxyanisol (BHA), butylated hydroxytoluened (BHT),
- the preservative is an anti-oxidant. In other embodiments, the preservative is a chelating agent.
- Exemplary buffering agents include citrate buffer solutions, acetate buffer solutions, phosphate buffer solutions, ammonium chloride, calcium carbonate, calcium chloride, calcium citrate, calcium glubionate, calcium gluceptate, calcium gluconate, D- gluconic acid, calcium glycerophosphate, calcium lactate, propanoic acid, calcium levulinate, pentanoic acid, dibasic calcium phosphate, phosphoric acid, tribasic calcium phosphate, calcium hydroxide phosphate, potassium acetate, potassium chloride, potassium gluconate, potassium mixtures, dibasic potassium phosphate, monobasic potassium phosphate, potassium phosphate mixtures, sodium acetate, sodium bicarbonate, sodium chloride, sodium citrate, sodium lactate, dibasic sodium phosphate, monobasic sodium phosphate, sodium phosphate mixtures, tromethamine, magnesium hydroxide, aluminum hydroxide, alginic acid, pyrogen-free water, isotonic s
- Exemplary lubricating agents include magnesium stearate, calcium stearate, stearic acid, silica, talc, malt, glyceryl behanate, hydrogenated vegetable oils, polyethylene glycol, sodium benzoate, sodium acetate, sodium chloride, leucine, magnesium lauryl sulfate, sodium lauryl sulfate, and mixtures thereof.
- Exemplary natural oils include almond, apricot kernel, avocado, babassu, bergamot, black current seed, borage, cade, camomile, canola, caraway, carnauba, castor, cinnamon, cocoa butter, coconut, cod liver, coffee, corn, cotton seed, emu, eucalyptus, evening primrose, fish, flaxseed, geraniol, gourd, grape seed, hazel nut, hyssop, isopropyl myristate, jojoba, kukui nut, lavandin, lavender, lemon, litsea cubeba, macademia nut, mallow, mango seed, meadowfoam seed, mink, nutmeg, olive, orange, orange roughy, palm, palm kernel, peach kernel, peanut, poppy seed, pumpkin seed, rapeseed, rice bran, rosemary, safflower, sandalwood, sasquana, savoury,
- Exemplary synthetic oils include, but are not limited to, butyl stearate, caprylic triglyceride, capric triglyceride, cyclomethicone, diethyl sebacate, dimethicone 360, isopropyl myristate, mineral oil, octyldodecanol, oleyl alcohol, silicone oil, and mixtures thereof.
- Liquid dosage forms for oral and parenteral administration include
- liquid dosage forms may comprise inert diluents commonly used in the art such as, for example, water or other solvents, solubilizing agents and emulsifiers such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol, 1,3-butylene glycol, dimethylformamide, oils (e.g.
- the oral compositions can include adjuvants such as wetting agents, emulsifying and suspending agents, sweetening, flavoring, and perfuming agents.
- adjuvants such as wetting agents, emulsifying and suspending agents, sweetening, flavoring, and perfuming agents.
- solubilizing agents such as CremophorTM, alcohols, oils, modified oils, glycols, polysorbates, cyclodextrins, polymers, and mixtures thereof.
- Injectable preparations for example, sterile injectable aqueous or oleaginous suspensions can be formulated according to the known art using suitable dispersing or wetting agents and suspending agents.
- the sterile injectable preparation can be a sterile injectable solution, suspension or emulsion in a nontoxic parenterally acceptable diluent or solvent, for example, as a solution in 1,3-butanediol.
- acceptable vehicles and solvents that can be employed are water, Ringer's solution, U.S. P. and isotonic sodium chloride solution.
- sterile, fixed oils are conventionally employed as a solvent or suspending medium.
- any bland fixed oil can be employed including synthetic mono- or diglycerides.
- fatty acids such as oleic acid are used in the preparation of injectables.
- the injectable formulations can be sterilized, for example, by filtration through a bacterial-retaining filter, or by incorporating sterilizing agents in the form of sterile solid compositions which can be dissolved or dispersed in sterile water or other sterile injectable medium prior to use.
- compositions for rectal or vaginal administration are typically suppositories which can be prepared by mixing the compounds described herein with suitable non-irritating excipients or carriers such as cocoa butter, polyethylene glycol or a suppository wax which are solid at ambient temperature but liquid at body temperature and therefore melt in the rectum or vaginal cavity and release the active ingredient.
- suitable non-irritating excipients or carriers such as cocoa butter, polyethylene glycol or a suppository wax which are solid at ambient temperature but liquid at body temperature and therefore melt in the rectum or vaginal cavity and release the active ingredient.
- Solid dosage forms for oral administration include capsules, tablets, pills, powders, and granules.
- the active ingredient is mixed with at least one inert, pharmaceutically acceptable excipient or carrier such as sodium citrate or dicalcium phosphate and/or a) fillers or extenders such as starches, lactose, sucrose, glucose, mannitol, and silicic acid, b) binders such as, for example, carboxymethylcellulose, alginates, gelatin, polyvinylpyrrolidinone, sucrose, and acacia, c) humectants such as glycerol, d) disintegrating agents such as agar, calcium carbonate, potato or tapioca starch, alginic acid, certain silicates, and sodium carbonate, e) solution retarding agents such as paraffin, f) absorption accelerators such as quaternary ammonium compounds, g) wetting agents such as, for example, cetyl alcohol and g
- Solid compositions of a similar type can be employed as fillers in soft and hard- filled gelatin capsules using such excipients as lactose or milk sugar as well as high molecular weight polyethylene glycols and the like.
- the solid dosage forms of tablets, dragees, capsules, pills, and granules can be prepared with coatings and shells such as enteric coatings and other coatings well known in the pharmaceutical formulating art. They may optionally comprise opacifying agents and can be of a composition that they release the active ingredient(s) only, or preferentially, in a certain part of the intestinal tract, optionally, in a delayed manner. Examples of embedding compositions which can be used include polymeric substances and waxes.
- Solid compositions of a similar type can be employed as fillers in soft and hard-filled gelatin capsules using such excipients as lactose or milk sugar as well as high molecular weight polyethylene glycols and the like.
- the active ingredient can be in micro-encapsulated form with one or more excipients as noted above.
- the solid dosage forms of tablets, dragees, capsules, pills, and granules can be prepared with coatings and shells such as enteric coatings, release controlling coatings and other coatings well known in the pharmaceutical formulating art.
- the active ingredient can be admixed with at least one inert diluent such as sucrose, lactose, or starch.
- Such dosage forms may comprise, as is normal practice, additional substances other than inert diluents, e.g. , tableting lubricants and other tableting aids such a magnesium stearate and microcrystalline cellulose.
- the dosage forms may comprise buffering agents. They may optionally comprise opacifying agents and can be of a composition that they release the active ingredient(s) only, or preferentially, in a certain part of the intestinal tract, optionally, in a delayed manner.
- opacifying agents include polymeric substances and waxes.
- Dosage forms for topical and/or transdermal administration of a provided compound may include ointments, pastes, creams, lotions, gels, powders, solutions, sprays, inhalants and/or patches.
- the active ingredient is admixed under sterile conditions with a pharmaceutically acceptable carrier and/or any desired preservatives and/or buffers as can be required.
- the present disclosure encompasses the use of transdermal patches, which often have the added advantage of providing controlled delivery of an active ingredient to the body.
- Such dosage forms can be prepared, for example, by dissolving and/or dispensing the active ingredient in the proper medium.
- the rate can be controlled by either providing a rate controlling membrane and/or by dispersing the active ingredient in a polymer matrix and/or gel.
- Suitable devices for use in delivering intradermal pharmaceutical compositions described herein include short needle devices such as those described in U.S. Patents 4,886,499; 5,190,521; 5,328,483; 5,527,288; 4,270,537; 5,015,235; 5,141,496; and
- Intradermal compositions can be administered by devices which limit the effective penetration length of a needle into the skin, such as those described in PCT publication WO 99/34850 and functional equivalents thereof. Jet injection devices which deliver liquid vaccines to the dermis via a liquid jet injector and/or via a needle which pierces the stratum corneum and produces a jet which reaches the dermis are suitable. Jet injection devices are described, for example, in U.S. Patents 5,480,381; 5,599,302; 5,334,144;
- Ballistic powder/particle delivery devices which use compressed gas to accelerate vaccine in powder form through the outer layers of the skin to the dermis are suitable.
- conventional syringes can be used in the classical mantoux method of intradermal administration.
- Formulations suitable for topical administration include, but are not limited to, liquid and/or semi liquid preparations such as liniments, lotions, oil in water and/or water in oil emulsions such as creams, ointments and/or pastes, and/or solutions and/or suspensions.
- Topically-administrable formulations may, for example, comprise from about 1% to about 10% (w/w) active ingredient, although the concentration of the active ingredient can be as high as the solubility limit of the active ingredient in the solvent.
- Formulations for topical administration may further comprise one or more of the additional ingredients described herein.
- a provided pharmaceutical composition can be prepared, packaged, and/or sold in a formulation suitable for pulmonary administration via the buccal cavity.
- a formulation may comprise dry particles which comprise the active ingredient and which have a diameter in the range from about 0.5 to about 7 nanometers or from about 1 to about 6 nanometers.
- Such compositions are conveniently in the form of dry powders for
- a device comprising a dry powder reservoir to which a stream of propellant can be directed to disperse the powder and/or using a self propelling
- solvent/powder dispensing container such as a device comprising the active ingredient dissolved and/or suspended in a low-boiling propellant in a sealed container.
- Such powders comprise particles wherein at least 98% of the particles by weight have a diameter greater than 0.5 nanometers and at least 95% of the particles by number have a diameter less than 7 nanometers. Alternatively, at least 95% of the particles by weight have a diameter greater than 1 nanometer and at least 90% of the particles by number have a diameter less than 6 nanometers.
- Dry powder compositions may include a solid fine powder diluent such as sugar and are conveniently provided in a unit dose form.
- Low boiling propellants generally include liquid propellants having a boiling point of below 65 °F at atmospheric pressure.
- the propellant may constitute 50 to 99.9% (w/w) of the composition, and the active ingredient may constitute 0.1 to 20% (w/w) of the composition.
- the propellant may further comprise additional ingredients such as a liquid non-ionic and/or solid anionic surfactant and/or a solid diluent (which may have a particle size of the same order as particles comprising the active ingredient).
- compositions formulated for pulmonary delivery may provide the active ingredient in the form of droplets of a solution and/or suspension.
- Such formulations can be prepared, packaged, and/or sold as aqueous and/or dilute alcoholic solutions and/or suspensions, optionally sterile, comprising the active ingredient, and may conveniently be administered using any nebulization and/or atomization device.
- Such formulations may further comprise one or more additional ingredients including, but not limited to, a flavoring agent such as saccharin sodium, a volatile oil, a buffering agent, a surface active agent, and/or a preservative such as methylhydroxybenzoate.
- the droplets provided by this route of administration may have an average diameter in the range from about 0.1 to about 200 nanometers.
- Formulations described herein as being useful for pulmonary delivery are useful for intranasal delivery of a pharmaceutical composition.
- Another formulation suitable for intranasal administration is a coarse powder comprising the active ingredient and having an average particle from about 0.2 to 500 micrometers. Such a formulation is administered by rapid inhalation through the nasal passage from a container of the powder held close to the nares.
- Formulations for nasal administration may, for example, comprise from about as little as 0.1% (w/w) and as much as 100% (w/w) of the active ingredient, and may comprise one or more of the additional ingredients described herein.
- a provided pharmaceutical composition can be prepared, packaged, and/or sold in a formulation for buccal
- Such formulations may, for example, be in the form of tablets and/or lozenges made using conventional methods, and may contain, for example, 0.1 to 20% (w/w) active ingredient, the balance comprising an orally dissolvable and/or degradable
- formulations for buccal administration may comprise a powder and/or an aerosolized and/or atomized solution and/or suspension comprising the active ingredient.
- Such powdered, aerosolized, and/or aerosolized formulations, when dispersed, may have an average particle and/or droplet size in the range from about 0.1 to about 200 nanometers, and may further comprise one or more of the additional ingredients described herein.
- a provided pharmaceutical composition can be prepared, packaged, and/or sold in a formulation for ophthalmic administration.
- Such formulations may, for example, be in the form of eye drops including, for example, a 0.1/1.0% (w/w) solution and/or suspension of the active ingredient in an aqueous or oily liquid carrier.
- Such drops may further comprise buffering agents, salts, and/or one or more other of the additional ingredients described herein.
- Other opthalmically-administrable formulations which are useful include those which comprise the active ingredient in microcrystalline form and/or in a liposomal preparation. Ear drops and/or eye drops are contemplated as being within the scope of this disclosure.
- compositions suitable for administration to humans are principally directed to pharmaceutical compositions which are suitable for administration to humans, it will be understood by the skilled artisan that such compositions are generally suitable for administration to animals of all sorts. Modification of pharmaceutical compositions suitable for administration to humans in order to render the compositions suitable for administration to various animals is well understood, and the ordinarily skilled veterinary pharmacologist can design and/or perform such modification with ordinary experimentation .
- Compounds provided herein are typically formulated in dosage unit form for ease of administration and uniformity of dosage. It will be understood, however, that the total daily usage of provided compositions will be decided by the attending physician within the scope of sound medical judgment.
- the specific therapeutically effective dose level for any particular subject or organism will depend upon a variety of factors including the disease, disorder, or condition being treated and the severity of the disorder; the activity of the specific active ingredient employed; the specific composition employed; the age, body weight, general health, sex and diet of the subject; the time of administration, route of administration, and rate of excretion of the specific active ingredient employed; the duration of the treatment; drugs used in combination or coincidental with the specific active ingredient employed; and like factors well known in the medical arts.
- the compounds and compositions provided herein can be administered by any route, including enteral (e.g. , oral), parenteral, intravenous, intramuscular, intra-arterial, intramedullary, intrathecal, subcutaneous, intraventricular, transdermal, interdermal, rectal, intravaginal, intraperitoneal, topical (as by powders, ointments, creams, and/or drops), mucosal, nasal, bucal, sublingual; by intratracheal instillation, bronchial instillation, and/or inhalation; and/or as an oral spray, nasal spray, and/or aerosol.
- enteral e.g. , oral
- parenteral intravenous
- intramuscular intra-arterial
- intramedullary intrathecal
- subcutaneous intraventricular
- transdermal transdermal
- interdermal interdermal
- rectal intravaginal
- topical as by powders, ointments, creams, and/or drops
- the most appropriate route of administration will depend upon a variety of factors including the nature of the agent (e.g. , its stability in the environment of the gastrointestinal tract), and/or the condition of the subject (e.g. , whether the subject is able to tolerate oral administration).
- the exact amount of a compound required to achieve an effective amount will vary from subject to subject, depending, for example, on species, age, and general condition of a subject, severity of the side effects or disorder, identity of the particular compound(s), mode of administration, and the like.
- the desired dosage can be delivered three times a day, two times a day, once a day, every other day, every third day, every week, every two weeks, every three weeks, or every four weeks.
- the desired dosage can be delivered using multiple administrations (e.g. , two, three, four, five, six, seven, eight, nine, ten, eleven, twelve, thirteen, fourteen, or more administrations).
- an effective amount of a compound for administration one or more times a day to a 70 kg adult human may comprise about 0.0001 mg to about 3000 mg, about 0.0001 mg to about 2000 mg, about 0.0001 mg to about 1000 mg, about 0.001 mg to about 1000 mg, about 0.01 mg to about 1000 mg, about 0.1 mg to about 1000 mg, about 1 mg to about 1000 mg, about 1 mg to about 100 mg, about 10 mg to about 1000 mg, or about 100 mg to about 1000 mg, of a compound per unit dosage form.
- a compound described herein may be administered at dosage levels sufficient to deliver from about 0.001 mg/kg to about 1000 mg/kg, from about 0.01 mg/kg to about mg/kg, from about 0.1 mg/kg to about 40 mg/kg, from about 0.5 mg/kg to about 30 mg/kg, from about 0.01 mg/kg to about 10 mg/kg, from about 0.1 mg/kg to about 10 mg/kg, or from about 1 mg/kg to about 25 mg/kg, of subject body weight per day, one or more times a day, to obtain the desired therapeutic effect.
- a compound described herein is administered one or more times per day, for multiple days. In some embodiments, the dosing regimen is continued for days, weeks, months, or years.
- dose ranges as described herein provide guidance for the administration of provided pharmaceutical compositions to an adult.
- the amount to be administered to, for example, a child or an adolescent can be determined by a medical practitioner or person skilled in the art and can be lower or the same as that administered to an adult.
- a compound or composition, as described herein can be administered in combination with one or more additional therapeutically active agents.
- a compound or composition provided herein is administered in combination with one or more additional therapeutically active agents that improve its bioavailability, reduce and/or modify its metabolism, inhibit its excretion, and/or modify its distribution within the body.
- the therapy employed may achieve a desired effect for the same disorder, and/or it may achieve different effects.
- the compound or composition can be administered concurrently with, prior to, or subsequent to, one or more additional therapeutically active agents.
- the additional therapeutically active agent is a compound of Formula (A), e.g. , Formula (I).
- the additional therapeutically active agent is not a compound of Formula (A), e.g. , Formula (I).
- each agent will be administered at a dose and/or on a time schedule determined for that agent.
- the additional therapeutically active agent utilized in this combination can be administered together in a single composition or administered separately in different compositions.
- the particular combination to employ in a regimen will take into account compatibility of a provided compound with the additional therapeutically active agent and/or the desired therapeutic effect to be achieved.
- it is expected that additional therapeutically active agents utilized in combination be utilized at levels that do not exceed the levels at which they are utilized individually.
- Exemplary additional therapeutically active agents include, but are not limited to, small organic molecules such as drug compounds (e.g. , compounds approved by the U.S. Food and Drug Administration as provided in the Code of Federal Regulations (CFR)), peptides, proteins, carbohydrates, monosaccharides, oligosaccharides, polysaccharides, nucleoproteins, mucoproteins, lipoproteins, synthetic polypeptides or proteins, small molecules linked to proteins, glycoproteins, steroids, nucleic acids, DNAs, RNAs, nucleotides, nucleosides, oligonucleotides, antisense oligonucleotides, lipids, hormones, vitamins, and cells.
- drug compounds e.g. , compounds approved by the U.S. Food and Drug Administration as provided in the Code of Federal Regulations (CFR)
- CFR Code of Federal Regulations
- peptides e.g., compounds approved by the U.S. Food and Drug Administration as provided in the Code of Federal Regulation
- kits e.g., pharmaceutical packs
- the kits provided may comprise a provided pharmaceutical composition or compound and a container (e.g., a vial, ampule, bottle, syringe, and/or dispenser package, or other suitable container).
- a container e.g., a vial, ampule, bottle, syringe, and/or dispenser package, or other suitable container.
- provided kits may optionally further include a second container comprising a pharmaceutical excipient for dilution or suspension of a provided pharmaceutical composition or compound.
- a provided pharmaceutical composition or compound provided in the container and the second container are combined to form one unit dosage form.
- a provided kits further includes instructions for use.
- compositions described herein are generally useful for the inhibition of PRMT5.
- methods of treating PRMT5 -mediated disorder in a subject comprise administering an effective amount of a compound described herein (e.g., a compound of Formula (A), e.g., Formula (I)), or a pharmaceutically acceptable salt thereof), to a subject in need of treatment.
- the effective amount is a therapeutically effective amount.
- the effective amount is a prophylactically effective amount.
- the subject is suffering from a PRMT5-mediated disorder.
- the subject is susceptible to a PRMT5 -mediated disorder.
- PRMT5-mediated disorder means any disease, disorder, or other pathological condition in which PRMT5 is known to play a role. Accordingly, in some embodiments, the present disclosure relates to treating or lessening the severity of one or more diseases in which PRMT5 is known to play a role.
- the present disclosure provides a method of inhibiting PRMT5 comprising contacting PRMT5with an effective amount of a compound described herein (e.g., a compound of Formula (A), e.g., Formula (I)), or a pharmaceutically acceptable salt thereof.
- a compound described herein e.g., a compound of Formula (A), e.g., Formula (I)
- the PRMT5 may be purified or crude, and may be present in a cell, tissue, or subject.
- the method is an in vitro method, e.g., such as an assay method. It will be understood by one of ordinary skill in the art that inhibition of PRMT5 does not necessarily require that all of the PRMT5 be occupied by an inhibitor at once.
- Exemplary levels of inhibition of PRMT5 include at least 10% inhibition, about 10% to about 25% inhibition, about 25% to about 50% inhibition, about 50% to about 75% inhibition, at least 50% inhibition, at least 75% inhibition, about 80% inhibition, about 90% inhibition, and greater than 90% inhibition.
- a method of inhibiting PRMT5 activity in a subject in need thereof comprising administering to the subject an effective amount of a compound described herein (e.g., a compound of Formula (A), e.g., Formula (I)), or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition thereof.
- a compound described herein e.g., a compound of Formula (A), e.g., Formula (I)
- a pharmaceutically acceptable salt thereof e.g., a pharmaceutically acceptable salt thereof, or a pharmaceutical composition thereof.
- a method of altering gene expression in a cell which comprises contacting a cell with an effective amount of a compound of Formula (A), e.g., Formula (I), or a pharmaceutically acceptable salt thereof.
- the cell in culture in vitro.
- the cell is in an animal, e.g., a human.
- the cell is in a subject in need of treatment.
- a method of altering transcription in a cell which comprises contacting a cell with an effective amount of a compound of Formula (A), e.g., Formula (I), or a pharmaceutically acceptable salt thereof.
- the cell in culture in vitro.
- the cell is in an animal, e.g., a human.
- the cell is in a subject in need of treatment.
- a method is provided of selecting a therapy for a subject having a disease associated with PRMT5 -mediated disorder or mutation comprising the steps of determining the presence of PRMT5 -mediated disorder or gene mutation in the PRMT5 gene or and selecting, based on the presence of PRMT5-mediated disorder a gene mutation in the PRMT5 gene a therapy that includes the administration of a provided compound.
- the disease is cancer.
- a method of treatment for a subject in need thereof comprising the steps of determining the presence of PRMT5-mediated disorder or a gene mutation in the PRMT5 gene and treating the subject in need thereof, based on the presence of a PRMT5 -mediated disorder or gene mutation in the PRMT5 gene with a therapy that includes the administration of a provided compound.
- the subject is a cancer patient.
- a provided compound is useful in treating a proliferative disorder, such as cancer, a benign neoplasm, an autoimmune disease, or an inflammatory disease.
- a proliferative disorder such as cancer, a benign neoplasm, an autoimmune disease, or an inflammatory disease.
- PRMT5 has been shown to be involved in cyclin Dl dysregulated cancers. Increased PRMT5 activity mediates key events associated with cyclin Dl -dependent neoplastic growth including CUL4 repression, CDT1 overexpression, and DNA re-replication.
- human cancers harboring mutations in Fbx4, the cyclin Dl E3 ligase exhibit nuclear cyclin Dl accumulation and increased PRMT5 activity. See, e.g., Aggarwal et al., Cancer Cell.
- PRMT5 has also been implicated in accelerating cell cycle progression through Gl phase and modulating regulators of Gl; for example, PRMT5 may upregulate cyclin- dependent kinase (CDK) 4, CDK6, and cyclins Dl, D2 and El. Moreover, PRMT5 may activate phosphoinositide 3-kinase (PI3K)/AKT signaling. See, e.g., Wei et al., Cancer Sci. (2012) 103(9): 1640-50. PRMT5 has been reported to play a role in apoptosis through methylation of E2F-1. See, e.g., Cho et al, EMBO J.
- PRMT5 has been reported to be an essential regulator of splicing and affect the alternative splicing of 'sensor' mRNAs that can then lead to defects in downstream events such as apoptosis. See, e.g., Bezzi et al., Genes Dev. (2013) 27: 1903-1916. PRMT5 has been reported to play a role in the RAS-ERK pathway. See, e.g., Andrew-Perez et al., Sci Signal. (2011) Sep 13;4(190)ra58 doi: 10.1126/scisignal.2001936.
- PRMT5 has been reported to affect C/EBPb target genes through interaction with the Mediator complex and hence affect cellular differentiation and inflammatory response. See, e.g.,Tsutsui et al., J. Biol. Chem. (2013) 288:20955-20965. PRMT5 has been shown to methylate HOXA9 essential for ELAM expression during the EC inflammatory response. See, e.g., Bandyopadhyay et al., Mol. Cell. Biol. (2012) 32: 1202-1203.
- the inhibition of PRMT5 by a provided compound is useful in treating the following non-limiting list of cancers: breast cancer, esophageal cancer, bladder cancer, lung cancer, hematopoietic cancer, lymphoma, medulloblastoma, rectum adenocarcinoma, colon adenocarcinoma, gastric cancer, pancreatic cancer, liver cancer, adenoid cystic carcinoma, lung adenocarcinoma, head and neck squamous cell carcinoma, brain tumors, hepatocellular carcinoma, renal cell carcinoma, melanoma, oligodendroglioma, ovarian clear cell carcinoma, and ovarian serous
- cystadenocarcinoma See, e.g., Pal et al., EMBO J. (2007) 26:3558-3569 (mantle cell lymphoma); Wang et al., Mol. Cell Biol. (2008) 28:6262-77 (chronic lymphocytic leukemia (CLL)); and Tae et al, Nucleic Acids Res. (2011) 39:5424-5438.
- CLL chronic lymphocytic leukemia
- the inhibition of PRMT5 by a provided compound is useful in treating prostate cancer and lung cancer, in which PRMT5 has been shown to play a role. See, e.g., Gu et al, PLoS One 2012;7(8):e44033; Gu et al, Biochem. J. (2012) 446:235-241.
- a provided compound is useful to delay the onset of, slow the progression of, or ameliorate the symptoms of cancer.
- a provided compound is administered in combination with other compounds, drugs, or therapeutics to treat cancer.
- compounds described herein are useful for treating a cancer including, but not limited to, acoustic neuroma, adenocarcinoma, adrenal gland cancer, anal cancer, angiosarcoma (e.g., lymphangio sarcoma, lymphangioendothelio sarcoma,
- hemangio sarcoma hemangio sarcoma
- appendix cancer benign monoclonal gammopathy
- biliary cancer e.g., cholangiocarcinoma
- bladder cancer e.g., adenocarcinoma of the breast, papillary carcinoma of the breast, mammary cancer, medullary carcinoma of the breast
- brain cancer e.g., meningioma
- glioma e.g., astrocytoma, oligodendroglioma
- glioma e.g., astrocytoma, oligodendroglioma
- medulloblastoma bronchus cancer
- carcinoid tumor cervical cancer (e.g., cervical adenocarcinoma), choriocarcinoma, chordoma, craniopharyngioma, colorectal cancer (e.g., colon cancer, rectal cancer, colorectal adenocarcinoma), epithelial carcinoma, ependymoma, endothelio sarcoma (e.g., Kaposi's sarcoma, multiple idiopathic hemorrhagic sarcoma), endometrial cancer (e.g.
- uterine cancer uterine sarcoma
- esophageal cancer e.g., adenocarcinoma of the esophagus, Barrett' s adenocarinoma
- eye cancer e.g., intraocular melanoma, retinoblastoma
- gastric cancer e.g., stomach adenocarcinoma
- gastrointestinal stromal tumor GIST
- head and neck cancer e.g., head and neck squamous cell carcinoma
- oral cancer e.g., oral squamous cell carcinoma (OSCC)
- throat cancer e.g., laryngeal cancer, pharyngeal cancer, nasopharyngeal cancer, oropharyngeal cancer
- hematopoietic cancers e.g., leukemia such as acute lymphocytic leukemia (ALL)
- ALL acute lymphocytic leukemia
- B-cell NHL such as diffuse large cell lymphoma (DLCL) (e.g., diffuse large B-cell lymphoma (DLBCL)), follicular lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), mantle cell lymphoma (MCL), marginal zone B-cell lymphomas (e.g., mucosa-associated lymphoid tissue (MALT) lymphomas, nodal marginal zone B-cell lymphoma, splenic marginal zone B-cell
- DLCL diffuse large cell lymphoma
- follicular lymphoma e.g., chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), mantle cell lymphoma (MCL)
- marginal zone B-cell lymphomas e.g., mucosa-associated lymphoid tissue (MALT) lymphomas, nodal marginal zone B-cell lymphom
- T-cell NHL such as precursor T-lymphoblastic lymphoma/leukemia, peripheral T-cell lymphoma (PTCL) (e.g., cutaneous T-cell lymphoma (CTCL) (e.g., mycosis fungiodes, Sezary syndrome), angioimmunoblastic T-cell lymphoma, extranodal natural killer T-cell lymphoma, enteropathy type T-cell lymphoma, subcutaneous panniculitis-like T-cell lymphoma, anaplastic large cell lymphoma); a mixture of one or more leukemia
- alpha chain disease e.g., alpha chain disease, gamma chain disease, mu chain disease
- hemangioblastoma e.g., hemangioblastoma a.k.a.
- HCC hepatocellular cancer
- lung cancer e.g., bronchogenic carcinoma, small cell lung cancer (SCLC), non-small cell lung cancer (NSCLC), adenocarcinoma of the lung
- myelofibrosis MF
- chronic idiopathic myelofibrosis CML
- chronic neutrophilic leukemia CML
- hypereosinophilic syndrome HES
- neuroblastoma e.g., neurofibromatosis (NF) type 1 or type 2, schwannomatosis
- NF neurofibromatosis
- neuroendocrine cancer e.g., gastroenteropancreatic neuroendoctrine tumor (GEP-NET), carcinoid tumor), osteosarcoma, ovarian cancer (e.g., cystadenocarcinoma, ovarian embryonal carcinoma, ovarian adenocarcinoma), papillary adenocarcinoma, pancreatic cancer (e.g., pancreatic andenocarcinoma, intraductal papillary mucinous neoplasm (IPMN), Islet cell tumors), penile cancer (e.g., Paget' s disease of the penis and scrotum), pinealoma, primitive neuroectodermal tumor (PNT), prostate cancer (e.g., prostate adenocarcinoma), rectal cancer, rhabdomyosarcoma, salivary gland cancer, skin cancer (e.g., squamous cell carcinoma (SCC), keratoacanthoma (KA), mel
- MMH histiocytoma
- liposarcoma malignant peripheral nerve sheath tumor (MPNST), chondrosarcoma, fibrosarcoma, myxosarcoma), sebaceous gland carcinoma, sweat gland carcinoma, synovioma
- testicular cancer e.g., seminoma, testicular embryonal carcinoma
- thyroid cancer e.g., papillary carcinoma of the thyroid, papillary thyroid carcinoma (PTC), medullary thyroid cancer
- urethral cancer e.g. , Paget' s disease of the vulva
- vulvar cancer e.g. , Paget' s disease of the vulva
- a provided compound is useful in treating a metabolic disorder, such as diabetes or obesity.
- a metabolic disorder such as diabetes or obesity.
- a role for PRMT5 has been recognized in adipogenesis. Inhibition of PRMT5 expression in multiple cell culture models for adipogenesis prevented the activation of adipogenic genes, while overexpression of PRMT5 enhanced adipogenic gene expression and differentiation. See, e.g., LeBlanc et al., Mol Endocrinol. (2012) 26:583-597. Additionally, it has been shown that adipogenesis plays a pivotal role in the etiology and progression of diabetes and obesity. See, e.g., Camp et al., Trends Mol Med. (2002) 8:442-447. Thus in some embodiments, the inhibition of PRMT5 by a provided compound is useful in treating diabetes and/or obesity.
- a provided compound is useful to delay the onset of, slow the progression of, or ameliorate the symptoms of, diabetes.
- the diabetes is Type 1 diabetes.
- the diabetes is Type 2 diabetes.
- a provided compound is useful to delay the onset of, slow the progression of, or ameliorate the symptoms of, obesity.
- a provided compound is useful to help a subject lose weight.
- a provided compound could be used in combination with other compounds, drugs, or therapeutics, such as metformin and insulin, to treat diabetes and/or obesity.
- a provided compound is useful in treating a blood disorder, e.g., a hemoglobinopathy, such as sickle cell disease or ⁇ -thalassemia.
- a blood disorder e.g., a hemoglobinopathy, such as sickle cell disease or ⁇ -thalassemia.
- PRMT5 is a known repressor of ⁇ -globin gene expression, and increased fetal ⁇ -globin (HbF) levels in adulthood are associated with symptomatic amelioration in sickle cell disease and ⁇ -thalassemia.
- HbF fetal ⁇ -globin
- the inhibition of PRMT5 by a provided compound is useful in treating a blood disorder, such as a hemoglobinopathy such as sickle cell disease or ⁇ -thalassemia.
- a provided compound is useful to delay the onset of, slow the progression of, or ameliorate the symptoms of, sickle cell disease. In some embodiments, a provided compound is useful to delay the onset of, slow the progression of, or ameliorate the symptoms of, ⁇ -thalassemia. In some embodiments, a provided compound could be used in combination with other compounds, drugs, or therapeutics, to treat a hemoglobinopathy such as sickle cell disease or ⁇ -thalassemia.
- a provided compound is useful in treating inflammatory and autoimmune disease.
- PRMT5 is reported to activate NFkB signaling pathway through the methylation of p65.
- PRMT5 is reported to interact with Death receptor 4 and Death receptor 5 contributing to TRAIL- induced activation of inhibitor or kB kinase (IKK) and nuclear factor-kB (NF-kB).
- IKK inhibitor or kB kinase
- NF-kB nuclear factor-kB
- inflammatory disease refers to those diseases, disorders or conditions that are characterized by signs of pain (dolor, from the generation of noxious substances and the stimulation of nerves), heat (calor, from vasodilatation), redness (rubor, from
- Inflammation takes on many forms and includes, but is not limited to, acute, adhesive, atrophic, catarrhal, chronic, cirrhotic, diffuse, disseminated, exudative, fibrinous, fibrosing, focal, granulomatous, hyperplastic, hypertrophic, interstitial, metastatic, necrotic, obliterative, parenchymatous, plastic, productive, proliferous, pseudomembranous, purulent, sclerosing,
- Exemplary inflammatory diseases include, but are not limited to, inflammation associated with acne, anemia (e.g., aplastic anemia, haemolytic autoimmune anaemia), asthma, arteritis (e.g., polyarteritis, temporal arteritis, periarteritis nodosa, Takayasu's arteritis), arthritis (e.g., crystalline arthritis, osteoarthritis, psoriatic arthritis, gouty arthritis, reactive arthritis, rheumatoid arthritis and Reiter's arthritis), ankylosing spondylitis, amylosis, amyotrophic lateral sclerosis, autoimmune diseases, allergies or allergic reactions, atherosclerosis, bronchitis, bursitis, chronic prostatitis, conjunctivitis, Chagas disease, chronic obstructive pulmonary disease, cermatomyositis, diverticulitis, diabetes (e.g., type I diabetes mellitus, type
- ileus e.g. , postoperative ileus and ileus during sepsis
- idiopathic thrombocytopenic purpura interstitial cystitis (painful bladder syndrome)
- gastrointestinal disorder e.g. , selected from peptic ulcers, regional enteritis, diverticulitis, gastrointestinal bleeding, eosinophilic gastrointestinal disorders (e.g.
- eosinophilic esophagitis eosinophilic gastritis, eosinophilic gastroenteritis, eosinophilic colitis
- gastritis diarrhea, gastroesophageal reflux disease (GORD, or its synonym GERD
- IBD inflammatory bowel disease
- IBS inflammatory bowel syndrome
- lupus multiple sclerosis, morphea, myeasthenia gravis, myocardial ischemia, nephrotic syndrome, pemphigus vulgaris, pernicious aneaemia, peptic ulcers, polymyositis, primary biliary cirrhosis, neuroinflammation associated with brain disorders (e.g.
- prostatitis chronic inflammation associated with cranial radiation injury, pelvic inflammatory disease, reperfusion injury, regional enteritis, rheumatic fever, systemic lupus erythematosus, schleroderma, scierodoma, sarcoidosis, spondyloarthopathies, Sjogren's syndrome, thyroiditis, transplantation rejection, tendonitis, trauma or injury (e.g. , frostbite, chemical irritants, toxins, scarring, burns, physical injury), vasculitis, vitiligo and Wegener's granulomatosis.
- trauma or injury e.g. , frostbite, chemical irritants, toxins, scarring, burns, physical injury
- vasculitis vitiligo and Wegener's granulomatosis.
- the inflammatory disease is an acute inflammatory disease (e.g. , for example, inflammation resulting from infection).
- the inflammatory disease is a chronic inflammatory disease (e.g. , conditions resulting from asthma, arthritis and inflammatory bowel disease).
- the compounds may also be useful in treating inflammation associated with trauma and non-inflammatory myalgia.
- the compounds may also be useful in treating inflammation associated with cancer.
- Exemplary autoimmune diseases include, but are not limited to, arthritis
- rheumatoid arthritis including rheumatoid arthritis, spondyloarthopathies, gouty arthritis, degenerative joint diseases such as osteoarthritis, systemic lupus erythematosus, Sjogren's syndrome, ankylosing spondylitis, undifferentiated spondylitis, Behcet's disease, haemolytic autoimmune anaemias, multiple sclerosis, amyotrophic lateral sclerosis, amylosis, acute painful shoulder, psoriatic, and juvenile arthritis), asthma, atherosclerosis, osteoporosis, bronchitis, tendonitis, bursitis, skin condition (e.g.
- eosinophilic disease e.g. , selected from peptic ulcers, regional enteritis, diverticulitis, gastrointestinal bleeding, eosinophilic gastrointestinal disorders (e.g. , eosinophilic esophagitis, eosinophilic gastritis, eosinophilic gastroenteritis, eosinophilic colitis), gastritis, diarrhea, gastroesophageal reflux disease (GORD, or its synonym GERD), inflammatory bowel disease (IBD) (e.g.
- a gastroprokinetic agent e.g. , ileus, postoperative ileus and ileus during sepsis;
- GORD gastroesophageal reflux disease
- GERD gastroesophageal reflux disease
- eosinophilic esophagitis gastroparesis such as diabetic gastroparesis
- NUD non-ulcerative dyspepsia
- NCCP non-cardiac chest pain
- a provided compound is useful in somatic cell
- reprogramming such as reprogramming somatic cells into stem cells.
- a provided compound is useful in germ cell development, and are thus envisioned useful in the areas of reproductive technology and regenerative medicine. See, e.g., Ancelin et al., Nat. Cell. Biol. (2006) 8:623- 630.
- compounds described herein can prepared using methods shown in general Scheme 1, comprising a ring opening of a chiral or racemic epoxide group. Further substitution of the tetrahydroisoquinoline ring and/or the phenyl ring can be carried out before or after the coupling with the epoxide.
- the epoxide opening is the final step in the synthesis, as shown in exemplary Scheme 2.
- R or R is an amine.
- a non-limiting example of the synthetic sequence used to prepare such analogs is provided herein (see Scheme 7).
- an alcohol of Formula (Z-l) is oxidized under suitable conditions SI to affect transformation into an intermediate ketone of Formula (Z-2).
- a ketone of Formula (Z-2) can be contacted with a primary or secondary amine under suitable conditions S2 to affect a reductive amination which can afford an amino compound of Formula (Z-3).
- the oxidation reaction SI is carried out directly with a stoichiometeric oxidant.
- the stoichiometric oxidant is pyridinium chlorochromate.
- the stoichiometric oxidant is pyridinium dichromate.
- the stoichiometric oxidant is Dess-Martin periodinane.
- the stoichiometric oxidant is prepared in situ.
- the stoichiometric oxidant is prepared in situ using sulfur trioxide pyridine complex and dimethylsulfoxide.
- the stoichiometric oxidant is prepared in situ using oxallyl chloride and dimethylsulfoxide. In some embodiments, the stoichiometric oxidant is prepared in situ using a carbodiimide and dimethylsulfoxide. In some embodiments, the stoichiometric oxidant is prepared in situ using N-chlorosuccinimide and dimethylsulfide. In some embodiments, the oxidation reaction SI is catalyzed. In some embodiments, the catalyst is (2,2,6, 6-tetramethyl-piperidin-l-yl)oxyl. In some embodiments, the catalyst is a ruthenium complex. In some embodiments, the catalyst is a palladium complex.
- the catalyst is a copper complex.
- standard methods and conditions for alcohol oxidation see Epstein et al., Chem. Rev. (1967) 67(3):247-260 and B.M. Trost ed. "Comprehensive Organic Synthesis", (1991), Vol. 7, p 281-305.
- both the oxidation step SI and reductive amination step S2 occur in one pot. In some embodiments, both the oxidation step SI and the reductive amination step S2 are carried out using the same catalyst.
- the catalyst is a rhodium complex. In some embodiments, the catalyst is a ruthenium complex. In some embodiments, the catalyst is an iridium complex.
- the reductive amination reaction S2 is carried out using a borohydride. In some embodiments, the reductive amination reaction S2 is carried out using sodium borohydride. In some embodiments, the reductive amination reaction S2 is carried out using sodium cyanoborohydride. In some embodiments, the reductive amination reaction S2 is carried out using sodium triacetoxyborohydride. In some embodiments, the reductive amination reaction S2 is carried out using a borane. In some embodiments, the reductive amination reaction S2 is carried out using a silyl hydride. In some embodiments, the reductive amination reaction S2 is carried out using hydrogen.
- the reductive amination reaction S2 is carried out in two steps, by first contacting a ketone of (Z- 2) with an amine to form an intermediate imine, and then reducing the intermediate imine under sufficient conditions to afford a compound of Formula (Z-3).
- the reaction conditions S2 comprise addition of a protic acid.
- the reaction conditions S2 comprise addition of an aprotic acid.
- the reaction conditions S2 comprise in situ formation of the reducing agent.
- the reaction conditions S2 comprise a catalyst. In some embodiments, the reaction conditions S2 comprise a transition metal catalyst. In some embodiments, the reaction conditions S2 comprise a palladium or nickel catalyst. In some embodiments, the reductive amination reaction S2 is stereoselective. In some embodiments, the stereoselective reductive amination reaction S2 is carried out in the presence of a chiral catalyst. For examples of standard methods and conditions for reductive aminations, see Gomez et ah, Adv. Synth. Catal. (2002) 344(10): 1037-1057 and Abdel-Magid et al, J. Org. Chem. (1996), 61:3849.
- LG of Formula (Z-5) is a halide. In some embodiments, LG of Formula (Z-5) is bromine. In some embodiments, LG of Formula (Z-5) is iodine. In some embodiments, LG of Formula (Z-5) is a substituted or unsubstituted alkyl sulfonate. In some embodiments, LG of Formula (Z-5) is a substituted or unsubstituted aryl sulfonate. In some embodiments, LG of Formula (Z-5) is methyl sulfonate. In some embodiments, LG of Formula (A-5) is trifluoromethane sulfonate.
- LG of Formula (Z-5) is a toluene sulfonate. In some embodiments, LG of Formula (Z-5) is a nitrobenzene sulfonate. In some embodiments, when LG of Formula (Z-5) is halide, conditions S3 comprise a phosphoryl halide. In some embodiments, when LG of Formula (Z-5) is halide, conditions S3 comprise a sulfuryl halide. In some embodiments, when LG of Formula (Z-5) is sulfonate, conditions S3 comprise a sulfonyl halide. In some embodiments, when LG of Formula (Z-5) is sulfonate, conditions S3 comprise a sulfonyl anhydride.
- conditions S4 are neutral. In some embodiments, conditions S4 comprise addition of a base. In certain embodiments of conditions S4, the base is either inorganic or organic. In certain embodiments of conditions S4, the base is inorganic. In certain embodiments of conditions S4, the base is organic. In certain embodiments of conditions S4, the base is a metal acetate, alkoxide, amide, amidine, carbonate, hydroxide, phenoxide, or phosphate. In certain embodiments of conditions S4, the base is sodium, potassium, or caesium carbonate. In certain embodiments of conditions S4, the base is sodium, potassium, or caesium bicarbonate.
- the base is 1,1,3,3-tetramethylguanidine, l,4-diazabicyclo[2.2.2]octane, 1,8- bis(dimethylamino)naphthalene, l,8-diazabicycloundec-7-ene, ammonia, diisopropylamine, imidazole, ⁇ , ⁇ -diisopropylethylamine, piperidine, pyridine, pyrrolidine, or triethylamine.
- the solvent is a polar protic solvent.
- conditions S4 the solvent is a polar aprotic solvent. In some embodiments of conditions S4, the reaction is performed in the absence of solvent. In some embodiments, conditions S4 comprise a catalyst. In some embodiments of conditions S4, the catalyst is an iodide salt. In some embodiments, both step S3 and the displacement step S4 occur in one pot.
- the hydroxyl moiety of a compound of Formula (Z-4) is converted into a leaving group in situ. In some embodiments, the hydroxyl moiety of a compound of Formula (Z-4) is converted into a leaving group in situ using an
- Step 3 l-(3,4-dihydroisoquinolin-2(lH)-yl)-3-(3-(((tetrahydro-2H-pyran-4- yl)amino)methyl)phenoxy)propan-2-ol
- Step 1 methyl 4-((5-bromo-lH-pyrazol-l-yl)methyl)benzoate
- methyl 4-(bromomethyl)benzoate (242 mg, 2 mmol) and K 2 C0 3 (276 mg, 2 mmol) were placed in a 100-mL flask with 2-butanone (20 mL) and heated at reflux temperature for 17 h. The mixture was cooled, filtered and evaporated in vacuuo leaving a residue which was dissolved in ethyl acetate and washed with water. The separated organic layer was dried and concentrated to give desired product as an oil (188mg, 100% yield). This crude material was used without further purification.
- Step 3 (4-((5-bromo-lH-pyrazol-l-yl)methyl)phenyl)(pyrrolidin-l-yl)methanone
- Step 5 (4-((5-(4-(oxiran-2-ylmethoxy)phenyl)-lH-pyrazol-l- yl)methyl)phenyl)(pyrrolidin-l-yl)methanone
- Step 2 tert-Butyl cyclopentyl(3-((3-(3,4-dihydroisoquinolin-2(lH)-yl)-2- hydroxypropyl)amino)benzyl)carbamate
- Step 3 l-((3-((cyclopentylamino)methyl)phenyl)amino)-3-(3,4-dihydroisoquinolin- -yl)propan-2-ol
- Step 2 l-(3-bromophenoxy)- -(3, 4-dihydroisoquinolin-2(lH)-yl)propan-2-ol
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Diabetes (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Hematology (AREA)
- Obesity (AREA)
- Emergency Medicine (AREA)
- Endocrinology (AREA)
- Child & Adolescent Psychology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Plural Heterocyclic Compounds (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Other In-Based Heterocyclic Compounds (AREA)
Abstract
Description
Claims
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/654,303 US9908887B2 (en) | 2012-12-21 | 2013-12-20 | PRMT5 inhibitors and uses thereof |
| EP13824243.3A EP2935240A1 (en) | 2012-12-21 | 2013-12-20 | Prmt5 inhibitors and uses thereof |
| JP2015549813A JP2016505000A (en) | 2012-12-21 | 2013-12-20 | PRMT5 inhibitors and uses thereof |
| CA2894126A CA2894126A1 (en) | 2012-12-21 | 2013-12-20 | Prmt5 inhibitors and uses thereof |
| US15/866,045 US20180298010A1 (en) | 2012-12-21 | 2018-01-09 | Prmt5 inhibitors and uses thereof |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261745393P | 2012-12-21 | 2012-12-21 | |
| US61/745,393 | 2012-12-21 | ||
| US201361784958P | 2013-03-14 | 2013-03-14 | |
| US61/784,958 | 2013-03-14 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/654,303 A-371-Of-International US9908887B2 (en) | 2012-12-21 | 2013-12-20 | PRMT5 inhibitors and uses thereof |
| US15/866,045 Continuation US20180298010A1 (en) | 2012-12-21 | 2018-01-09 | Prmt5 inhibitors and uses thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014100695A1 true WO2014100695A1 (en) | 2014-06-26 |
Family
ID=50001259
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2013/077151 WO2014100695A1 (en) | 2012-12-21 | 2013-12-20 | Prmt5 inhibitors and uses thereof |
Country Status (5)
| Country | Link |
|---|---|
| US (6) | US8906900B2 (en) |
| EP (1) | EP2935240A1 (en) |
| JP (1) | JP2016505000A (en) |
| CA (1) | CA2894126A1 (en) |
| WO (1) | WO2014100695A1 (en) |
Cited By (72)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8940726B2 (en) | 2012-12-21 | 2015-01-27 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US8993555B2 (en) | 2012-12-21 | 2015-03-31 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US9221794B2 (en) | 2012-12-21 | 2015-12-29 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| WO2016034671A1 (en) * | 2014-09-03 | 2016-03-10 | Ctxt Pty Ltd | Aminoindane-, aminotetrahydronaphthalene- and aminobenzocyclobutane-derived prmt5-inhibitors |
| WO2016034675A1 (en) * | 2014-09-03 | 2016-03-10 | Ctxt Pty Ltd | Tetrahydroisoquinoline derived prmt5-inhibitors |
| WO2016038550A1 (en) | 2014-09-11 | 2016-03-17 | Novartis Ag | Inhibition of prmt5 to treat mtap-deficiency-related diseases |
| WO2016044446A2 (en) | 2014-09-17 | 2016-03-24 | Ironwood Pharmaceuticals, Inc. | Sgc stimulators |
| US9346802B2 (en) | 2013-03-15 | 2016-05-24 | Epizyme, Inc. | CARM1 inhibitors and uses thereof |
| WO2016089883A1 (en) | 2014-12-01 | 2016-06-09 | Novartis Ag | Compositions and methods for diagnosis and treatment of prostate cancer |
| US9365555B2 (en) | 2012-12-21 | 2016-06-14 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| WO2016117647A1 (en) * | 2015-01-21 | 2016-07-28 | 大日本住友製薬株式会社 | New benzimidazole derivative and pharmaceutical use thereof |
| US9440950B2 (en) | 2013-03-14 | 2016-09-13 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| US9447079B2 (en) | 2013-03-14 | 2016-09-20 | Epizyme, Inc. | PRMT1 inhibitors and uses thereof |
| US9475776B2 (en) | 2013-03-14 | 2016-10-25 | Epizyme, Inc. | PRMT1 inhibitors and uses thereof |
| WO2016178870A1 (en) | 2015-05-04 | 2016-11-10 | Eli Lilly And Company | 5'-substituted nucleoside analogs |
| WO2017032840A1 (en) | 2015-08-26 | 2017-03-02 | Janssen Pharmaceutica Nv | Novel 6-6 bicyclic aromatic ring substituted nucleoside analogues for use as prmt5 inhibitors |
| US9598374B2 (en) | 2013-03-14 | 2017-03-21 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| US9630961B2 (en) | 2013-03-14 | 2017-04-25 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| WO2017096165A1 (en) | 2015-12-03 | 2017-06-08 | Agios Pharmaceuticals, Inc. | Mat2a inhibitors for treating mtap null cancer |
| US9718816B2 (en) | 2013-03-15 | 2017-08-01 | Epizyme, Inc. | 1-phenoxy-3-(alkylamino)-propan-2-ol derivatives as CARM1 inhibitors and uses thereof |
| US9724332B2 (en) | 2013-03-14 | 2017-08-08 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| US9732041B2 (en) | 2013-03-14 | 2017-08-15 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| US9738651B2 (en) | 2013-03-15 | 2017-08-22 | Epizyme, Inc. | CARM1 inhibitors and uses thereof |
| WO2017153186A1 (en) | 2016-03-10 | 2017-09-14 | Janssen Pharmaceutica Nv | Substituted nucleoside analogues for use as prmt5 inhibitors |
| CN107207497A (en) * | 2014-12-02 | 2017-09-26 | 拜耳作物科学股份公司 | It is used as the dicyclic compound of pest control agent |
| CN107286083A (en) * | 2017-06-16 | 2017-10-24 | 康化(上海)新药研发有限公司 | A kind of synthetic method of the pyridine carboxaldehyde of 2 chlorine, 6 bromine 3 |
| US9908887B2 (en) | 2012-12-21 | 2018-03-06 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| WO2018065365A1 (en) | 2016-10-03 | 2018-04-12 | Janssen Pharmaceutica Nv | Novel monocyclic and bicyclic ring system substituted carbanucleoside analogues for use as prmt5 inhibitors |
| WO2018100532A1 (en) | 2016-12-01 | 2018-06-07 | Glaxosmithkline Intellectual Property Development Limited | Combination therapy |
| WO2018100535A1 (en) | 2016-12-01 | 2018-06-07 | Glaxosmithkline Intellectual Property Development Limited | Combination therapy |
| EP3160477A4 (en) * | 2014-06-25 | 2018-07-04 | Epizyme, Inc. | Prmt5 inhibitors and uses thereof |
| WO2018154104A1 (en) | 2017-02-27 | 2018-08-30 | Janssen Pharmaceutica Nv | Use of biomarkers in identifying cancer patients that will be responsive to treatment with a prmt5 inhibitor |
| WO2019094311A1 (en) | 2017-11-08 | 2019-05-16 | Merck Sharp & Dohme Corp. | Prmt5 inhibitors |
| WO2019110734A1 (en) | 2017-12-08 | 2019-06-13 | Janssen Pharmaceutica Nv | Novel spirobicyclic analogues |
| WO2019116302A1 (en) | 2017-12-13 | 2019-06-20 | Lupin Limited | Substituted bicyclic heterocyclic compounds as prmt5 inhibitors |
| US10421743B2 (en) | 2016-03-09 | 2019-09-24 | Ctxt Pty Ltd | Tetrahydroisoquinolines as PRMT5 inhibitors |
| KR20190129936A (en) * | 2017-03-17 | 2019-11-20 | 아르고너트 테라퓨틱스 리미티드 | Compounds Useful for the Treatment or Prevention of PRMT5-Mediated Diseases |
| WO2019219805A1 (en) | 2018-05-16 | 2019-11-21 | Ctxone Pty Ltd | Combination therapy |
| US10494376B2 (en) | 2014-09-03 | 2019-12-03 | Ctxt Pty. Ltd. | Tetrahydroisoquinoline derived PRMT5-inhibitors |
| WO2019229614A1 (en) | 2018-05-31 | 2019-12-05 | Glaxosmithkline Intellectual Property Development Limited | Combination of a type ii protein arginine methyltransferase inhibitor and an icos binding protein to treat cancer |
| US10519167B2 (en) | 2016-03-09 | 2019-12-31 | Ctxt Pty Ltd | 3-oxa-8-azabicyclo[3.2.1]octane derivatives and their use in the treatment of cancer and hemoglobinopathies |
| US10550096B2 (en) | 2016-03-09 | 2020-02-04 | Ctxt Pty Ltd | Tetrahydroisoquinolines as PRMT5 inhibitors |
| CN110950801A (en) * | 2019-11-13 | 2020-04-03 | 济南大学 | Preparation and application of sulfanilamide and benzothiazole compounds containing tetrahydroisoquinoline |
| CN111039934A (en) * | 2018-10-15 | 2020-04-21 | 中国石油化工股份有限公司 | Amino compound, preparation method thereof and application of amino compound as flame retardant |
| US10653693B2 (en) | 2014-08-04 | 2020-05-19 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US10745409B2 (en) | 2016-12-15 | 2020-08-18 | Janssen Pharmaceutica Nv | Azepane inhibitors of menin-MLL interaction |
| US10745380B2 (en) | 2016-03-09 | 2020-08-18 | Ctxt Pty Ltd | Pyridine derivatives and their use in the treatment of cancer and hemoglobinopathies |
| CN111592522A (en) * | 2020-06-17 | 2020-08-28 | 郑州大学 | Arginine methylation transferase 5 small-molecule inhibitor and preparation method and application thereof |
| US10787434B2 (en) | 2016-03-09 | 2020-09-29 | Ctxt Pty, Ltd | Benzopiperdine derivatives and their use in the treatment of cancer and hemoglobinopathies |
| WO2020250123A1 (en) | 2019-06-10 | 2020-12-17 | Lupin Limited | Prmt5 inhibitors |
| WO2020249663A1 (en) | 2019-06-12 | 2020-12-17 | Janssen Pharmaceutica Nv | Novel spirobicyclic intermediates |
| CN112125886A (en) * | 2019-06-24 | 2020-12-25 | 南京圣和药物研发有限公司 | Tricyclic compound serving as PRMT5 inhibitor and application thereof |
| US10898504B2 (en) | 2016-03-10 | 2021-01-26 | Janssen Pharmaceutica Nv | Substituted nucleoside analogues for use as PRMT5 inhibitors |
| US10961256B2 (en) | 2016-03-09 | 2021-03-30 | Ctxt Pty Ltd | PRMT5 inhibitors |
| US10975100B2 (en) | 2016-09-14 | 2021-04-13 | Janssen Pharmaceutica Nv | Fused bicyclic inhibitors of menin-MLL interaction |
| WO2021068953A1 (en) * | 2019-10-12 | 2021-04-15 | 南京圣和药业股份有限公司 | Substituted tricyclic compound as prmt5 inhibitor and use thereof |
| WO2021079302A1 (en) | 2019-10-22 | 2021-04-29 | Lupin Limited | Pharmaceutical combination of prmt5 inhibitors |
| WO2021088992A1 (en) * | 2019-11-07 | 2021-05-14 | 江苏先声药业有限公司 | Tetrahydroisoquinoline spiro compound as prmt5 inhibitor |
| WO2021111322A1 (en) | 2019-12-03 | 2021-06-10 | Lupin Limited | Substituted nucleoside analogs as prmt5 inhibitors |
| US11098062B2 (en) | 2016-10-03 | 2021-08-24 | Janssen Pharmaceutica Nv | Monocyclic and bicyclic ring system substituted carbanucleoside analogues for use as PRMT5 inhibitors |
| US11111237B2 (en) | 2019-10-02 | 2021-09-07 | Sk Biopharmaceuticals Co., Ltd. | Bicyclic compound and use thereof |
| WO2021254493A1 (en) * | 2020-06-18 | 2021-12-23 | 上海翊石医药科技有限公司 | Cyclic compound having anti-tumor activity and use thereof |
| US11220517B2 (en) | 2016-09-14 | 2022-01-11 | Janssen Pharmaceutica Nv | Spiro bicyclic inhibitors of menin-MLL interaction |
| WO2022048631A1 (en) | 2020-09-04 | 2022-03-10 | 上海翊石医药科技有限公司 | Compound having antitumor activity and use thereof |
| WO2022067082A1 (en) * | 2020-09-28 | 2022-03-31 | Cardurion Pharmaceuticals, Llc | Fused heteroaryl compounds and their use as camkii inhibitors |
| WO2022063094A1 (en) * | 2020-09-22 | 2022-03-31 | 南京明德新药研发有限公司 | Tetrahydroisoquinoline derivative and use thereof |
| US11396517B1 (en) | 2017-12-20 | 2022-07-26 | Janssen Pharmaceutica Nv | Exo-aza spiro inhibitors of menin-MLL interaction |
| CN115124534A (en) * | 2021-11-23 | 2022-09-30 | 中山大学 | Non-nucleotide PRMT5 small molecule inhibitor, preparation method and application |
| WO2022237858A1 (en) | 2021-05-13 | 2022-11-17 | 上海翊石医药科技有限公司 | Compound having anti-tumor activity and use thereof |
| US11571437B2 (en) | 2019-06-06 | 2023-02-07 | Janssen Pharmaceutica Nv | Methods of treating cancer using PRMT5 inhibitors |
| US12084462B2 (en) | 2016-09-14 | 2024-09-10 | Janssen Pharmaceutica Nv | Spiro bicyclic inhibitors of menin-MLL interaction |
| KR102716092B1 (en) | 2015-08-26 | 2024-10-10 | 얀센 파마슈티카 엔브이 | Novel 6-6 bicyclic aromatic ring substituted nucleoside analogues for use as PRMT5 inhibitors |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2970220A2 (en) | 2013-03-14 | 2016-01-20 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| AR107512A1 (en) | 2016-02-04 | 2018-05-09 | VIIV HEALTHCARE UK Nº 5 LTD | TRITERPENOIDS MODIFIED IN C-3 AND C-17 AS HIV-1 INHIBITORS |
| WO2019152731A1 (en) * | 2018-01-31 | 2019-08-08 | Alpine Androsciences, Inc. | Androgen receptor antagonists |
| KR102522856B1 (en) * | 2018-03-09 | 2023-04-19 | 파마블럭 사이언시스 (난징), 인코포레이티드 | Inhibitors of protein arginine methyltransferase 5 (PRMT 5), pharmaceutical products thereof and methods thereof |
| CN111825656B (en) * | 2019-04-15 | 2023-03-31 | 南京药石科技股份有限公司 | Inhibitors of protein arginine methyltransferase 5 (PRMT 5), pharmaceutical products thereof, and methods thereof |
| WO2020263550A1 (en) | 2019-06-28 | 2020-12-30 | Als Therapy Development Institute | Inhibition of dipeptide repeat proteins |
| CN112778275B (en) * | 2019-11-11 | 2023-05-12 | 石药集团中奇制药技术(石家庄)有限公司 | Adamantyl PRMT5 inhibitor and application thereof |
| CN113045543B (en) * | 2019-12-26 | 2023-05-12 | 石药集团中奇制药技术(石家庄)有限公司 | PRMT5 inhibitor and application thereof |
| EP4228632A2 (en) * | 2020-10-16 | 2023-08-23 | Saint Louis University | Rev-erb agonists |
Citations (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4270537A (en) | 1979-11-19 | 1981-06-02 | Romaine Richard A | Automatic hypodermic syringe |
| US4596556A (en) | 1985-03-25 | 1986-06-24 | Bioject, Inc. | Hypodermic injection apparatus |
| US4790824A (en) | 1987-06-19 | 1988-12-13 | Bioject, Inc. | Non-invasive hypodermic injection device |
| US4886499A (en) | 1986-12-18 | 1989-12-12 | Hoffmann-La Roche Inc. | Portable injection appliance |
| US4940460A (en) | 1987-06-19 | 1990-07-10 | Bioject, Inc. | Patient-fillable and non-invasive hypodermic injection device assembly |
| US4941880A (en) | 1987-06-19 | 1990-07-17 | Bioject, Inc. | Pre-filled ampule and non-invasive hypodermic injection device assembly |
| US5015235A (en) | 1987-02-20 | 1991-05-14 | National Carpet Equipment, Inc. | Syringe needle combination |
| WO1991013865A1 (en) * | 1990-03-09 | 1991-09-19 | Isis Innovation Limited | Antiarrhythmic agents |
| US5064413A (en) | 1989-11-09 | 1991-11-12 | Bioject, Inc. | Needleless hypodermic injection device |
| US5141496A (en) | 1988-11-03 | 1992-08-25 | Tino Dalto | Spring impelled syringe guide with skin penetration depth adjustment |
| US5190521A (en) | 1990-08-22 | 1993-03-02 | Tecnol Medical Products, Inc. | Apparatus and method for raising a skin wheal and anesthetizing skin |
| WO1994001408A1 (en) * | 1992-07-10 | 1994-01-20 | Laboratoires Glaxo S.A. | Anilide derivatives |
| US5312335A (en) | 1989-11-09 | 1994-05-17 | Bioject Inc. | Needleless hypodermic injection device |
| US5328483A (en) | 1992-02-27 | 1994-07-12 | Jacoby Richard M | Intradermal injection device with medication and needle guard |
| US5334144A (en) | 1992-10-30 | 1994-08-02 | Becton, Dickinson And Company | Single use disposable needleless injector |
| US5339163A (en) | 1988-03-16 | 1994-08-16 | Canon Kabushiki Kaisha | Automatic exposure control device using plural image plane detection areas |
| US5383851A (en) | 1992-07-24 | 1995-01-24 | Bioject Inc. | Needleless hypodermic injection device |
| US5417662A (en) | 1991-09-13 | 1995-05-23 | Pharmacia Ab | Injection needle arrangement |
| US5466220A (en) | 1994-03-08 | 1995-11-14 | Bioject, Inc. | Drug vial mixing and transfer device |
| US5480381A (en) | 1991-08-23 | 1996-01-02 | Weston Medical Limited | Needle-less injector |
| US5527288A (en) | 1990-12-13 | 1996-06-18 | Elan Medical Technologies Limited | Intradermal drug delivery device and method for intradermal delivery of drugs |
| US5569189A (en) | 1992-09-28 | 1996-10-29 | Equidyne Systems, Inc. | hypodermic jet injector |
| US5599302A (en) | 1995-01-09 | 1997-02-04 | Medi-Ject Corporation | Medical injection system and method, gas spring thereof and launching device using gas spring |
| WO1997013537A1 (en) | 1995-10-10 | 1997-04-17 | Visionary Medical Products Corporation | Gas pressured needle-less injection device |
| US5649912A (en) | 1994-03-07 | 1997-07-22 | Bioject, Inc. | Ampule filling device |
| WO1997037705A1 (en) | 1996-04-11 | 1997-10-16 | Weston Medical Limited | Spring-powered dispensing device for medical purposes |
| US5893397A (en) | 1996-01-12 | 1999-04-13 | Bioject Inc. | Medication vial/syringe liquid-transfer apparatus |
| WO1999034850A1 (en) | 1998-01-08 | 1999-07-15 | Fiderm S.R.L. | Device for controlling the penetration depth of a needle, for application to an injection syringe |
| US5993412A (en) | 1997-05-19 | 1999-11-30 | Bioject, Inc. | Injection apparatus |
| US6130217A (en) * | 1995-09-20 | 2000-10-10 | Pfizer Inc | Compounds enhancing antitumor activity of other cytotoxic agents |
| WO2001019821A1 (en) * | 1999-09-14 | 2001-03-22 | Aventis Pharmaceuticals Inc. | Benzisoxazolyl-, pyridoisoxazolyl- and benzthienyl-phenoxy derivatives useful as d4 antagonists |
| WO2001019833A1 (en) * | 1999-09-14 | 2001-03-22 | Aventis Pharmaceuticals, Inc. | Thienoisoxazolyl- and thienylpyrrazolyl-phenoxy substituted propyl derivatives useful as d4 antagonists |
| US6218393B1 (en) * | 1996-10-18 | 2001-04-17 | Xenova Limited | Anthranilic acid derivatives as multi drug resistance modulators |
| US20090093493A1 (en) * | 2007-10-09 | 2009-04-09 | Francesco Berardi | 1-phenylalcoxy-2-beta-phenylethyl derivatives as p-glycoprotein (p-gp) inhibitors useful in drug resistance events |
| WO2011079236A1 (en) * | 2009-12-22 | 2011-06-30 | The Ohio State University Research Foundation | Compositions and methods for cancer detection and treatment |
Family Cites Families (96)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DD68906A (en) | ||||
| GB1374366A (en) | 1972-07-21 | 1974-11-20 | Science Union & Cie | Propanol derivatives and a process for their preparation |
| US4022776A (en) | 1974-01-31 | 1977-05-10 | Otsuka Pharmaceutical Company Limited | 5-[1-Hydroxy-2-(substituted-amino)]ethyl-8-hydroxycarbostyril derivatives |
| CA1072359A (en) | 1974-10-08 | 1980-02-26 | Hiroyuki Konishi | Method for controlling the growth of plants |
| HU179951B (en) | 1979-10-11 | 1983-01-28 | Chinoin Gyogyszer Es Vegyeszet | Process for preparing 1,2,4-oxadiazolin-5-one derivatives and pharmaceutical compositions containing thereof |
| DK275083A (en) | 1982-06-16 | 1983-12-17 | May & Baker Ltd | PROCEDURE FOR PREPARING A PYRAZOLOPYRIDINE DERIVATIVE |
| US4684459A (en) | 1985-11-29 | 1987-08-04 | The Dow Chemical Company | Collector compositions for the froth flotation of mineral values |
| US4746655A (en) | 1987-06-10 | 1988-05-24 | A. H. Robins Company, Incorporated | Fused aromatic-spiropiperidine oxazepinones(and thiones) |
| DE3804793A1 (en) | 1988-02-16 | 1989-08-24 | Hoechst Ag | RENIN-INHIBITING AMINOSAUTE DERIVATIVES |
| DE3839126A1 (en) | 1988-11-19 | 1990-05-23 | Hoechst Ag | RENIN-INHIBITING UREA DERIVATIVES OF DIPEPTIDES, METHOD FOR THE PRODUCTION THEREOF, THESE AGENTS AND THEIR USE |
| US5776963A (en) | 1989-05-19 | 1998-07-07 | Hoechst Marion Roussel, Inc. | 3-(heteroaryl)-1- (2,3-dihydro-1h-isoindol-2-yl)alkyl!pyrrolidines and 3-(heteroaryl)-1- (2,3-dihydro-1h-indol-1-yl)alkyl!pyrrolidines and related compounds and their therapeutic untility |
| WO1993001174A1 (en) | 1991-07-08 | 1993-01-21 | Glaxo Group Limited | Thiazolidine derivatives and their use as anti-viral compounds |
| US5294621A (en) | 1992-10-07 | 1994-03-15 | Ortho Pharmaceutical Corporation | Thieno tetrahydropyridines useful as class III antiarrhythmic agents |
| US5693847A (en) | 1995-04-19 | 1997-12-02 | Vertex Pharmaceuticals Incorporated | Heteroatom functionalized α-methyl ketones |
| SE9600769D0 (en) | 1996-02-28 | 1996-02-28 | Astra Ab | Compounds useful as analgesic |
| US20020169101A1 (en) | 1999-05-10 | 2002-11-14 | Gonzalez Maria Isabel | Treatment of sexual dysfunction |
| US7125903B1 (en) | 1999-09-14 | 2006-10-24 | Aventis Pharmaceuticals Inc. | Thienoisoxazolyl-and thienylpyrrazolyl-phenoxy substituted propyl derivatives useful as D4 antagonists |
| US7253165B2 (en) | 1999-09-14 | 2007-08-07 | Aventis Pharmaceuticals Inc. | Benzisoxazolyl-, pyridoisoxazolyl-and benzthienyl-phenoxy derivatives useful as D4 antagonists |
| WO2002014277A1 (en) | 2000-08-10 | 2002-02-21 | Tanabe Seiyaku Co., Ltd. | Biphenylcarboxamidoisoindoline compounds, processes for the preparation of the same and intermediates for the synthesis thereof |
| RS13004A (en) | 2001-07-11 | 2006-10-27 | Elan Pharmaceuticals Inc. | N-(3-amino-2-hydroxy-propyl)substituted alkylamide compounds |
| TWI259081B (en) | 2001-10-26 | 2006-08-01 | Sugen Inc | Treatment of acute myeloid leukemia with indolinone compounds |
| SE0103644D0 (en) | 2001-11-01 | 2001-11-01 | Astrazeneca Ab | Therapeutic isoquinoline compounds |
| US7176242B2 (en) | 2001-11-08 | 2007-02-13 | Elan Pharmaceuticals, Inc. | N,N′-substituted-1,3-diamino-2-hydroxypropane derivatives |
| US7338969B2 (en) | 2002-03-08 | 2008-03-04 | Quonova, Llc | Modulation of pathogenicity |
| US7335779B2 (en) | 2002-03-08 | 2008-02-26 | Quonova, Llc | Modulation of pathogenicity |
| JP2005528366A (en) | 2002-03-26 | 2005-09-22 | メルク エンド カムパニー インコーポレーテッド | Spirocyclic amides as cannabinoid receptor modulators |
| CA2480856A1 (en) | 2002-04-05 | 2003-10-23 | Merck & Co., Inc. | Substituted aryl amides |
| AU2003268512A1 (en) | 2002-09-09 | 2004-03-29 | Janssen Pharmaceutica N.V. | Hydroxy alkyl substituted 1,3,8-triazaspiro[4.5]decan-4-one derivatives useful for the treatment of ORL-1 receptor mediated disorders |
| SE0300010D0 (en) | 2003-01-07 | 2003-01-07 | Astrazeneca Ab | Novel Compounds |
| WO2004078114A2 (en) | 2003-02-28 | 2004-09-16 | Encysive Pharmaceuticals Inc. | Pyridine, pyrimidine, quinoline, quinazoline, and naphthalene urotensin-ii receptor antagonists. |
| CN100572360C (en) * | 2003-07-02 | 2009-12-23 | 上海医药工业研究院 | The preparation method of Isoquinolinium compound and salt thereof and application |
| US7408008B2 (en) | 2003-12-09 | 2008-08-05 | Janssen Pharmaceutica, N.V. | Method of producing highly functionalized 1,3-diamino-propan-2-ols from solid support |
| EP1720855A4 (en) | 2004-03-02 | 2008-12-17 | Smithkline Beecham Corp | Inhibitors of akt activity |
| WO2005087752A2 (en) | 2004-03-09 | 2005-09-22 | Elan Pharmaceuticals, Inc. | Substituted hydroxyethylamine aspartyl protease inhibitors |
| WO2005118543A1 (en) | 2004-06-03 | 2005-12-15 | Ono Pharmaceutical Co., Ltd. | Kinase inhibitor and use thereof |
| MXPA06014574A (en) | 2004-06-24 | 2007-03-12 | Incyte Corp | N-substituted piperidines and their use as pharmaceuticals. |
| US20060009510A1 (en) | 2004-07-09 | 2006-01-12 | Pharmacia & Upjohn Company Llc | Method of synthesizing indolinone compounds |
| ES2426345T3 (en) | 2005-07-20 | 2013-10-22 | Eli Lilly And Company | Compound bound in 1-amino position |
| JP2009512716A (en) | 2005-10-21 | 2009-03-26 | ユニバーシティ・オブ・アラバマ・アット・バーミンガム | Small molecule inhibitors of HIV-1 capsid construction |
| ES2462240T3 (en) | 2006-02-28 | 2014-05-22 | Dart Neuroscience (Cayman) Ltd | Therapeutic piperazines as PDE4 inhibitors |
| EP2394647A1 (en) | 2006-11-02 | 2011-12-14 | Aestus Therapeutics, Inc. | Methods of treating neuropathic pain by modulation of glycogenolysis or glycolysis pathways |
| CN101012223A (en) | 2006-11-13 | 2007-08-08 | 西安新安医药科技有限公司 | Ornidazole derivative for treatment, preparing method and use |
| EP2123649B1 (en) | 2006-12-04 | 2012-02-29 | Jiangsu Simcere Pharmaceutical R&D Co., Ltd. | 3-pyrrolo-cyclohexylene-2-dihydro-indolinone derivatives and uses thereof |
| US20090099157A1 (en) | 2007-02-15 | 2009-04-16 | Ameriks Michael K | Tetrahydro-pyrazolo-pyridine thioether modulators of cathepsin s |
| US8338437B2 (en) | 2007-02-28 | 2012-12-25 | Methylgene Inc. | Amines as small molecule inhibitors |
| DE102007020492A1 (en) | 2007-04-30 | 2008-11-06 | Grünenthal GmbH | Substituted sulfonamide derivatives |
| WO2008145398A1 (en) | 2007-06-01 | 2008-12-04 | Pfizer Italia S.R.L. | 4-arylpyrrole substituted 2-indoline derivatives active as protein kinase inhibitors |
| CA2704199C (en) | 2007-11-01 | 2016-01-19 | Acucela Inc. | Amine derivative compounds for treating ophthalmic diseases and disorders |
| WO2009059225A2 (en) | 2007-11-02 | 2009-05-07 | Pain Therapeutics, Inc. | Analgesia with minimal tolerance and dependence by a mu opioid receptor agonist that also binds filamin a |
| WO2009086303A2 (en) * | 2007-12-21 | 2009-07-09 | University Of Rochester | Method for altering the lifespan of eukaryotic organisms |
| WO2009126782A1 (en) | 2008-04-11 | 2009-10-15 | High Point Pharmaceuticals, Llc | Histamine h3 receptor ligands |
| US8791135B2 (en) | 2008-07-01 | 2014-07-29 | Purdue Research Foundation | Nonpeptide HIV-1 protease inhibitors |
| CA2736229C (en) | 2008-09-05 | 2015-06-09 | Acucela Inc. | Sulfur-linked compounds for treating opthalmic diseases and disorders |
| WO2010048332A2 (en) | 2008-10-22 | 2010-04-29 | Acucela, Inc. | Compounds for treating ophthalmic diseases and disorders |
| WO2010057101A2 (en) | 2008-11-17 | 2010-05-20 | Schering Corporation | Compounds useful as hiv blockers |
| US8309547B2 (en) | 2009-04-28 | 2012-11-13 | Apotex Pharmachem Inc. | Processes for the preparation of rivaroxaban and intermediates thereof |
| GB0922332D0 (en) | 2009-12-22 | 2010-02-03 | Isis Innovation | Method of treatment and screening method |
| US8247436B2 (en) | 2010-03-19 | 2012-08-21 | Novartis Ag | Pyridine and pyrazine derivative for the treatment of CF |
| TWI513694B (en) | 2010-05-11 | 2015-12-21 | Amgen Inc | Pyrimidine compounds that inhibit anaplastic lymphoma kinase |
| JOP20190250A1 (en) | 2010-07-14 | 2017-06-16 | Regeneron Pharma | Stabilized formulations containing anti-ngf antibodies |
| AU2010362639B2 (en) | 2010-10-18 | 2016-10-27 | Apotex Pharmachem Inc. | Processes for the preparation of Rivaroxaban and intermediates thereof |
| WO2013038378A1 (en) | 2011-09-16 | 2013-03-21 | Novartis Ag | Pyridine amide derivatives |
| WO2013038381A1 (en) | 2011-09-16 | 2013-03-21 | Novartis Ag | Pyridine/pyrazine amide derivatives |
| AR092289A1 (en) | 2011-11-14 | 2015-04-15 | Sunshine Lake Pharma Co Ltd | DERIVATIVES OF AMINOQUINAZOLINE AND ITS SALTS AND METHODS OF USE |
| EP2797886A2 (en) | 2011-12-30 | 2014-11-05 | Ecole Nationale Superieure de Chimie de Clermont Ferrand | Pain relief compounds |
| EP2872899B1 (en) | 2012-07-13 | 2018-07-11 | Pain Therapeutics, Inc. | Alzheimer's disease assay in a living patient |
| CA2894126A1 (en) | 2012-12-21 | 2014-06-26 | Epizyme, Inc. | Prmt5 inhibitors and uses thereof |
| WO2014100716A1 (en) | 2012-12-21 | 2014-06-26 | Epizyme, Inc. | Prmt5 inhibitors and uses thereof |
| CA2894157A1 (en) | 2012-12-21 | 2014-06-26 | Epizyme, Inc. | Prmt5 inhibitors and uses thereof |
| US8940726B2 (en) | 2012-12-21 | 2015-01-27 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| EP2935242A2 (en) | 2012-12-21 | 2015-10-28 | Epizyme, Inc. | Methods of inhibiting prmt5 |
| US9604930B2 (en) | 2012-12-21 | 2017-03-28 | Epizyme, Inc. | Tetrahydro- and dihydro-isoquinoline PRMT5 inhibitors and uses thereof |
| US9120757B2 (en) | 2013-03-14 | 2015-09-01 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| EP2970220A2 (en) | 2013-03-14 | 2016-01-20 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| EP3363434A1 (en) | 2013-03-14 | 2018-08-22 | Epizyme Inc | Arginine methyltransferase inhibitors and uses thereof |
| WO2014144659A1 (en) | 2013-03-14 | 2014-09-18 | Epizyme, Inc. | Pyrazole derivatives as prmt1 inhibitors and uses thereof |
| EP2970133B1 (en) | 2013-03-14 | 2018-10-24 | Epizyme, Inc. | Pyrazole derivatives as prmt1 inhibitors and uses thereof |
| BR112015022785A2 (en) | 2013-03-14 | 2017-07-18 | Epizyme Inc | compound; pharmaceutical composition; packaged pharmaceutical kit or article; method of inhibiting an arginine methyl transferase (rmt); method of modulating gene expression; transcription modulation method; and method of treating an rmt-mediated disorder |
| EP2970134B1 (en) | 2013-03-14 | 2018-02-28 | Epizyme, Inc. | Pyrazole derivatives as prmt1 inhibitors and uses thereof |
| US9045455B2 (en) | 2013-03-14 | 2015-06-02 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| EP2970137A1 (en) | 2013-03-14 | 2016-01-20 | Epizyme, Inc. | Pyrazole derivatives as arginine methyltransferase inhibitors and uses thereof |
| WO2014153214A1 (en) | 2013-03-14 | 2014-09-25 | Epizyme, Inc. | Arginine methyl transferase inhibtors and uses thereof |
| MX2015012803A (en) | 2013-03-15 | 2016-08-19 | Epizyme Inc | Carm1 inhibitors and uses thereof. |
| US9718816B2 (en) | 2013-03-15 | 2017-08-01 | Epizyme, Inc. | 1-phenoxy-3-(alkylamino)-propan-2-ol derivatives as CARM1 inhibitors and uses thereof |
| US9346802B2 (en) | 2013-03-15 | 2016-05-24 | Epizyme, Inc. | CARM1 inhibitors and uses thereof |
| WO2015200680A2 (en) | 2014-06-25 | 2015-12-30 | Epizyme, Inc. | Prmt5 inhibitors and uses thereof |
| US20170210751A1 (en) | 2014-06-25 | 2017-07-27 | Epizyme, Inc. | Prmt5 inhibitors and uses thereof |
| AU2015301196A1 (en) | 2014-08-04 | 2017-01-12 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US20170283440A1 (en) | 2014-09-17 | 2017-10-05 | Epizyme, Inc. | Carm1 inhibitors and uses thereof |
| WO2016044626A1 (en) | 2014-09-17 | 2016-03-24 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| JP2017527594A (en) | 2014-09-17 | 2017-09-21 | エピザイム,インコーポレイティド | Salts, co-crystals, amorphous forms, and crystalline forms of coactivator-related arginine methyltransferase 1 (CARM1) inhibitors |
| WO2016044576A1 (en) | 2014-09-17 | 2016-03-24 | Epizyme, Inc. | Salts, co-crystals, amorphous forms, and crystalline forms of an arginine methyltransferase inhibitor |
| US20170305922A1 (en) | 2014-09-17 | 2017-10-26 | Epizyme, Inc. | Carm1 inhibitors and uses thereof |
| WO2016044641A2 (en) | 2014-09-17 | 2016-03-24 | Epizyme, Inc. | Carm1 inhibitors and uses thereof |
| US20170283400A1 (en) | 2014-09-17 | 2017-10-05 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| WO2016044585A1 (en) | 2014-09-17 | 2016-03-24 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
-
2013
- 2013-12-20 CA CA2894126A patent/CA2894126A1/en not_active Abandoned
- 2013-12-20 WO PCT/US2013/077151 patent/WO2014100695A1/en active Application Filing
- 2013-12-20 US US14/136,551 patent/US8906900B2/en active Active
- 2013-12-20 EP EP13824243.3A patent/EP2935240A1/en not_active Withdrawn
- 2013-12-20 US US14/654,303 patent/US9908887B2/en active Active
- 2013-12-20 JP JP2015549813A patent/JP2016505000A/en active Pending
-
2014
- 2014-10-15 US US14/515,154 patent/US9388173B2/en active Active
-
2016
- 2016-06-08 US US15/177,056 patent/US9777008B2/en active Active
-
2017
- 2017-08-17 US US15/679,655 patent/US20180186798A1/en not_active Abandoned
-
2018
- 2018-01-09 US US15/866,045 patent/US20180298010A1/en not_active Abandoned
Patent Citations (38)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4270537A (en) | 1979-11-19 | 1981-06-02 | Romaine Richard A | Automatic hypodermic syringe |
| US4596556A (en) | 1985-03-25 | 1986-06-24 | Bioject, Inc. | Hypodermic injection apparatus |
| US4886499A (en) | 1986-12-18 | 1989-12-12 | Hoffmann-La Roche Inc. | Portable injection appliance |
| US5015235A (en) | 1987-02-20 | 1991-05-14 | National Carpet Equipment, Inc. | Syringe needle combination |
| US4941880A (en) | 1987-06-19 | 1990-07-17 | Bioject, Inc. | Pre-filled ampule and non-invasive hypodermic injection device assembly |
| US4940460A (en) | 1987-06-19 | 1990-07-10 | Bioject, Inc. | Patient-fillable and non-invasive hypodermic injection device assembly |
| US4790824A (en) | 1987-06-19 | 1988-12-13 | Bioject, Inc. | Non-invasive hypodermic injection device |
| US5339163A (en) | 1988-03-16 | 1994-08-16 | Canon Kabushiki Kaisha | Automatic exposure control device using plural image plane detection areas |
| US5141496A (en) | 1988-11-03 | 1992-08-25 | Tino Dalto | Spring impelled syringe guide with skin penetration depth adjustment |
| US5064413A (en) | 1989-11-09 | 1991-11-12 | Bioject, Inc. | Needleless hypodermic injection device |
| US5503627A (en) | 1989-11-09 | 1996-04-02 | Bioject, Inc. | Ampule for needleless injection |
| US5312335A (en) | 1989-11-09 | 1994-05-17 | Bioject Inc. | Needleless hypodermic injection device |
| WO1991013865A1 (en) * | 1990-03-09 | 1991-09-19 | Isis Innovation Limited | Antiarrhythmic agents |
| US5190521A (en) | 1990-08-22 | 1993-03-02 | Tecnol Medical Products, Inc. | Apparatus and method for raising a skin wheal and anesthetizing skin |
| US5527288A (en) | 1990-12-13 | 1996-06-18 | Elan Medical Technologies Limited | Intradermal drug delivery device and method for intradermal delivery of drugs |
| US5480381A (en) | 1991-08-23 | 1996-01-02 | Weston Medical Limited | Needle-less injector |
| US5417662A (en) | 1991-09-13 | 1995-05-23 | Pharmacia Ab | Injection needle arrangement |
| US5328483A (en) | 1992-02-27 | 1994-07-12 | Jacoby Richard M | Intradermal injection device with medication and needle guard |
| WO1994001408A1 (en) * | 1992-07-10 | 1994-01-20 | Laboratoires Glaxo S.A. | Anilide derivatives |
| US5383851A (en) | 1992-07-24 | 1995-01-24 | Bioject Inc. | Needleless hypodermic injection device |
| US5520639A (en) | 1992-07-24 | 1996-05-28 | Bioject, Inc. | Needleless hypodermic injection methods and device |
| US5569189A (en) | 1992-09-28 | 1996-10-29 | Equidyne Systems, Inc. | hypodermic jet injector |
| US5704911A (en) | 1992-09-28 | 1998-01-06 | Equidyne Systems, Inc. | Needleless hypodermic jet injector |
| US5334144A (en) | 1992-10-30 | 1994-08-02 | Becton, Dickinson And Company | Single use disposable needleless injector |
| US5649912A (en) | 1994-03-07 | 1997-07-22 | Bioject, Inc. | Ampule filling device |
| US5466220A (en) | 1994-03-08 | 1995-11-14 | Bioject, Inc. | Drug vial mixing and transfer device |
| US5599302A (en) | 1995-01-09 | 1997-02-04 | Medi-Ject Corporation | Medical injection system and method, gas spring thereof and launching device using gas spring |
| US6130217A (en) * | 1995-09-20 | 2000-10-10 | Pfizer Inc | Compounds enhancing antitumor activity of other cytotoxic agents |
| WO1997013537A1 (en) | 1995-10-10 | 1997-04-17 | Visionary Medical Products Corporation | Gas pressured needle-less injection device |
| US5893397A (en) | 1996-01-12 | 1999-04-13 | Bioject Inc. | Medication vial/syringe liquid-transfer apparatus |
| WO1997037705A1 (en) | 1996-04-11 | 1997-10-16 | Weston Medical Limited | Spring-powered dispensing device for medical purposes |
| US6218393B1 (en) * | 1996-10-18 | 2001-04-17 | Xenova Limited | Anthranilic acid derivatives as multi drug resistance modulators |
| US5993412A (en) | 1997-05-19 | 1999-11-30 | Bioject, Inc. | Injection apparatus |
| WO1999034850A1 (en) | 1998-01-08 | 1999-07-15 | Fiderm S.R.L. | Device for controlling the penetration depth of a needle, for application to an injection syringe |
| WO2001019821A1 (en) * | 1999-09-14 | 2001-03-22 | Aventis Pharmaceuticals Inc. | Benzisoxazolyl-, pyridoisoxazolyl- and benzthienyl-phenoxy derivatives useful as d4 antagonists |
| WO2001019833A1 (en) * | 1999-09-14 | 2001-03-22 | Aventis Pharmaceuticals, Inc. | Thienoisoxazolyl- and thienylpyrrazolyl-phenoxy substituted propyl derivatives useful as d4 antagonists |
| US20090093493A1 (en) * | 2007-10-09 | 2009-04-09 | Francesco Berardi | 1-phenylalcoxy-2-beta-phenylethyl derivatives as p-glycoprotein (p-gp) inhibitors useful in drug resistance events |
| WO2011079236A1 (en) * | 2009-12-22 | 2011-06-30 | The Ohio State University Research Foundation | Compositions and methods for cancer detection and treatment |
Non-Patent Citations (82)
| Title |
|---|
| "Comprehensive Organic Synthesis", vol. 7, 1991, pages: 281 - 305 |
| "Handbook of Chemistry and Physics" |
| "Remington: The Science and Practice of Pharmacy", 2005, LIPPINCOTT WILLIAMS & WILKINS |
| ABDEL-MAGID ET AL., J. ORG. CHEM., vol. 61, 1996, pages 3849 |
| AGGARWAL ET AL., CANCER CELL, vol. 18, no. 4, 2010, pages 329 - 40 |
| ANCELIN ET AL., NAT. CELL. BIOL., vol. 8, 2006, pages 623 - 630 |
| ANDREW-PEREZ ET AL., SCI SIGNAL., vol. 4, no. 190, 13 September 2011 (2011-09-13), pages RA58 |
| BANDYOPADHYAY ET AL., MOL. CELL. BIOL., vol. 32, 2012, pages 1202 - 1203 |
| BEZZI, GENES DEV., vol. 27, 2013, pages 1903 - 1916 |
| CAMP ET AL., TRENDS MOL MED., vol. 8, 2002, pages 442 - 447 |
| CARRUTHERS: "Some Modem Methods of Organic Synthesis", 1987, CAMBRIDGE UNIVERSITY PRESS |
| CHO ET AL., EMBO J., vol. 31, 2012, pages 1785 - 1797 |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 12 August 2012 (2012-08-12), XP002723529, retrieved from STN Database accession no. 1390079-59-0 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 12 August 2012 (2012-08-12), XP002723530, retrieved from STN Database accession no. 1389775-53-4 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 13 August 2012 (2012-08-13), XP002723527, retrieved from STN Database accession no. 1390485-95-6 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 13 October 2008 (2008-10-13), XP002723550, retrieved from STN Database accession no. 1060542-64-4 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 13 October 2008 (2008-10-13), XP002723551, retrieved from STN Database accession no. 1060402-35-8 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 14 October 2008 (2008-10-14), XP002723549, retrieved from STN Database accession no. 1061124-54-6 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 17 November 2006 (2006-11-17), XP002723557, retrieved from STN Database accession no. 913503-11-4 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 17 September 2008 (2008-09-17), XP002723552, retrieved from STN Database accession no. 1049761-31-0 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 17 September 2009 (2009-09-17), XP002723541, retrieved from STN Database accession no. 1185407-05-9 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 18 September 2012 (2012-09-18), XP002723528, retrieved from STN Database accession no. 1394703-31-1 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 2 August 2012 (2012-08-02), XP002723533, retrieved from STN Database accession no. 1385588-44-2 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 2 December 2011 (2011-12-02), XP002723537, retrieved from STN Database accession no. 1347256-93-2 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 2 November 2008 (2008-11-02), XP002723543, retrieved from STN Database accession no. 1069906-08-6 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 2 November 2008 (2008-11-02), XP002723544, retrieved from STN Database accession no. 1069895-07-3 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 2 November 2008 (2008-11-02), XP002723545, retrieved from STN Database accession no. 1069781-86-7 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 20 December 2007 (2007-12-20), XP002723555, retrieved from STN Database accession no. 958982-78-0 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 21 March 2010 (2010-03-21), XP002723539, retrieved from STN Database accession no. 1212367-31-1 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 22 February 2008 (2008-02-22), XP002723554, retrieved from STN Database accession no. 1005082-40-5 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 24 January 2010 (2010-01-24), XP002723540, retrieved from STN Database accession no. 1203413-85-7 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 24 October 2008 (2008-10-24), XP002723548, retrieved from STN Database accession no. 1065507-26-7 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 26 February 2007 (2007-02-26), XP002723556, retrieved from STN Database accession no. 923141-67-7 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 26 May 2008 (2008-05-26), XP002723553, retrieved from STN Database accession no. 1022648-78-7 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 27 July 2004 (2004-07-27), XP002723560, retrieved from STN Database accession no. 717121-35-2 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 27 October 2008 (2008-10-27), XP002723546, retrieved from STN Database accession no. 1067029-15-5 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 27 October 2008 (2008-10-27), XP002723547, retrieved from STN Database accession no. 1066959-19-0 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 3 June 2003 (2003-06-03), XP002723561, retrieved from STN Database accession no. 524721-03-7 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 3 November 2008 (2008-11-03), XP002723542, retrieved from STN Database accession no. 1070344-15-8 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 31 May 2006 (2006-05-31), XP002723558, retrieved from STN Database accession no. 886136-98-7 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 6 December 2011 (2011-12-06), XP002723536, retrieved from STN Database accession no. 1349512-40-8 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 7 April 2005 (2005-04-07), XP002723559, retrieved from STN Database accession no. 848051-57-0 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 7 August 2012 (2012-08-07), XP002723532, retrieved from STN Database accession no. 1387169-01-8 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 7 December 2011 (2011-12-07), XP002723535, retrieved from STN Database accession no. 1350276-64-0 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 7 February 2012 (2012-02-07), XP002723534, retrieved from STN Database accession no. 1355607-64-5 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 7 June 2012 (2012-06-07), XP002723526, retrieved from STN Database accession no. 1376006-97-1 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 8 February 2012 (2012-02-08), XP002723525, retrieved from STN Database accession no. 1355914-92-9 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 9 April 2010 (2010-04-09), XP002723538, retrieved from STN Database accession no. 1217840-37-3 * |
| DATABASE REGISTRY [Online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 9 August 2012 (2012-08-09), XP002723531, retrieved from STN Database accession no. 1388392-84-9 * |
| E. W. MARTIN: "Remington's Pharmaceutical Sciences", 1980, MACK PUBLISHING CO. |
| ELIEL: "Stereochemistry of Carbon Compounds", 1962, MCGRAW-HILL |
| EPSTEIN, CHEM. REV., vol. 67, no. 3, 1967, pages 247 - 260 |
| GOMEZ ET AL., ADV. ,SYNTH. CATAL., vol. 344, no. 10, 2002, pages 1037 - 1057 |
| GU ET AL., BIOCHEM. J., vol. 446, 2012, pages 235 - 241 |
| GU ET AL., PLOS ONE, vol. 7, no. 8, 2012, pages E44033 |
| JACQUES ET AL.: "Enantiomers, Racemates and Resolutions", 1981, WILEY INTERSCIENCE |
| LAROCK: "Comprehensive Organic Transformations", 1989, VCH PUBLISHERS, INC. |
| LAUTENS, SYNTHESIS, vol. 2, 2011, pages 342 - 346 |
| LEBLANC ET AL., MOL ERADOCRINOL., vol. 26, 2012, pages 583 - 597 |
| MARCOTULLIO ET AL., SYNTHESIS, vol. 16, 2006, pages 2760 - 2766 |
| NAGAMATSU ET AL., J BIOL CHEM., vol. 286, 2011, pages 10641 - 10648 |
| PAL ET AL., EMBO J., vol. 26, 2007, pages 3558 - 3569 |
| PHARMACEUTICAL SCIENCES, vol. 66, 1977, pages 1 - 19 |
| RANK, BLOOD, vol. 116, 2010, pages 1585 - 1592 |
| SALVATORE, TETRAHEDRON, vol. 57, 2001, pages 7785 - 7811 |
| See also references of EP2935240A1 * |
| SMITH; MARCH: "Advanced Organic Chemistry", pages: 501 - 502 |
| SMITH; MARCH: "March's Advanced Organic Chemistry", 2001, JOHN WILEY & SONS, INC. |
| SUN ET AL., PNAS, vol. 108, 2011, pages 20538 - 20543 |
| T. W. GREENE; P. G. M. WUTS: "Protecting Groups in Organic Synthesis", 1999, JOHN WILEY & SONS |
| TAE ET AL., NUCLEIC ACIDS RES., vol. 39, 2011, pages 5424 - 5438 |
| TANAKA ET AL., MOL. CANCER. RES., vol. 7, 2009, pages 557 - 569 |
| THOMAS SORRELL: "Organic Chemistry", 1999, UNIVERSITY SCIENCE BOOKS |
| TSUTSUI ET AL., J. BIOL. CHEM., vol. 288, 2013, pages 20955 - 20965 |
| WANG ET AL., MOL. CELL BIOL., vol. 28, 2008, pages 6262 - 77 |
| WASILKO, D.J.; S.E. LEE: "T1PS: titerless infected-cells preservation and scale-up", BIOPROCESS J., vol. 5, 2006, pages 29 - 32 |
| WEI ET AL., CANCER SCI., vol. 103, no. 9, 2012, pages 1640 - 50 |
| WEI ET AL., PROC. NAT'L. ACAD. SCI. USA, vol. 110, 2013, pages 13516 - 21 |
| WILEN ET AL., TETRAHEDRON, vol. 33, 1977, pages 2725 |
| WILEN: "Tables of Resolving Agents and Optical Resolutions", 1972, UNIV. OF NOTRE DAME PRESS, pages: 268 |
| XU, HAEMATOLOGICA, vol. 97, 2012, pages 1632 - 1640 |
| ZHENG ET AL., MOL. CELL, vol. 52, 2013, pages 37 - 51 |
Cited By (134)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9604930B2 (en) | 2012-12-21 | 2017-03-28 | Epizyme, Inc. | Tetrahydro- and dihydro-isoquinoline PRMT5 inhibitors and uses thereof |
| US8993555B2 (en) | 2012-12-21 | 2015-03-31 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US9221794B2 (en) | 2012-12-21 | 2015-12-29 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US10307413B2 (en) | 2012-12-21 | 2019-06-04 | Epizyme, Inc. | Tetrahydro- and dihydro-isoquinoline PRMT5 inhibitors and uses thereof |
| US10150758B2 (en) | 2012-12-21 | 2018-12-11 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US10118918B2 (en) | 2012-12-21 | 2018-11-06 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US8940726B2 (en) | 2012-12-21 | 2015-01-27 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US9908887B2 (en) | 2012-12-21 | 2018-03-06 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US10980794B2 (en) | 2012-12-21 | 2021-04-20 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US9365519B2 (en) | 2012-12-21 | 2016-06-14 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US9365555B2 (en) | 2012-12-21 | 2016-06-14 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US9765068B2 (en) | 2012-12-21 | 2017-09-19 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US9745291B2 (en) | 2012-12-21 | 2017-08-29 | Epizyme, Inc. | PRMT5 inhibitors containing a dihydro- or tetrahydroisoquinoline and uses thereof |
| US9732072B2 (en) | 2012-12-21 | 2017-08-15 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US10391089B2 (en) | 2012-12-21 | 2019-08-27 | Epizyme, Inc. | PRMT5 inhibitors and uses therof |
| US9675614B2 (en) | 2012-12-21 | 2017-06-13 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US9611257B2 (en) | 2012-12-21 | 2017-04-04 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US9724332B2 (en) | 2013-03-14 | 2017-08-08 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| US11185531B2 (en) | 2013-03-14 | 2021-11-30 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| US10632103B2 (en) | 2013-03-14 | 2020-04-28 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| US9630961B2 (en) | 2013-03-14 | 2017-04-25 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| US11512053B2 (en) | 2013-03-14 | 2022-11-29 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| US10227307B2 (en) | 2013-03-14 | 2019-03-12 | Epizyme, Inc. | PRMT1 inhibitors and uses thereof |
| US10800743B2 (en) | 2013-03-14 | 2020-10-13 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| US9475776B2 (en) | 2013-03-14 | 2016-10-25 | Epizyme, Inc. | PRMT1 inhibitors and uses thereof |
| US9447079B2 (en) | 2013-03-14 | 2016-09-20 | Epizyme, Inc. | PRMT1 inhibitors and uses thereof |
| US9732041B2 (en) | 2013-03-14 | 2017-08-15 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| US9598374B2 (en) | 2013-03-14 | 2017-03-21 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| US9440950B2 (en) | 2013-03-14 | 2016-09-13 | Epizyme, Inc. | Arginine methyltransferase inhibitors and uses thereof |
| US9856267B2 (en) | 2013-03-15 | 2018-01-02 | Epizyme, Inc. | CARM1 inhibitors and uses thereof |
| US9718816B2 (en) | 2013-03-15 | 2017-08-01 | Epizyme, Inc. | 1-phenoxy-3-(alkylamino)-propan-2-ol derivatives as CARM1 inhibitors and uses thereof |
| US11834455B2 (en) | 2013-03-15 | 2023-12-05 | Epizyme, Inc. | Carm1 inhibitors and uses thereof |
| US10118931B2 (en) | 2013-03-15 | 2018-11-06 | Epizyme, Inc. | CARM1 inhibitors and uses thereof |
| US10633389B2 (en) | 2013-03-15 | 2020-04-28 | Epizyme, Inc. | CARM1 inhibitors and uses thereof |
| US9738651B2 (en) | 2013-03-15 | 2017-08-22 | Epizyme, Inc. | CARM1 inhibitors and uses thereof |
| US9346802B2 (en) | 2013-03-15 | 2016-05-24 | Epizyme, Inc. | CARM1 inhibitors and uses thereof |
| EP3160477A4 (en) * | 2014-06-25 | 2018-07-04 | Epizyme, Inc. | Prmt5 inhibitors and uses thereof |
| US10653693B2 (en) | 2014-08-04 | 2020-05-19 | Epizyme, Inc. | PRMT5 inhibitors and uses thereof |
| US10005792B2 (en) | 2014-09-03 | 2018-06-26 | Ctxt Pty. Ltd. | Aminoindane-, aminotetrahydronaphthalene- and aminobenzocyclobutane-derived PRMT5-inhibitors |
| US10647708B2 (en) | 2014-09-03 | 2020-05-12 | Ctxt Pty. Ltd | Tetrahydroisoquinoline derived PRMT5-inhibitors |
| US10494376B2 (en) | 2014-09-03 | 2019-12-03 | Ctxt Pty. Ltd. | Tetrahydroisoquinoline derived PRMT5-inhibitors |
| WO2016034675A1 (en) * | 2014-09-03 | 2016-03-10 | Ctxt Pty Ltd | Tetrahydroisoquinoline derived prmt5-inhibitors |
| WO2016034671A1 (en) * | 2014-09-03 | 2016-03-10 | Ctxt Pty Ltd | Aminoindane-, aminotetrahydronaphthalene- and aminobenzocyclobutane-derived prmt5-inhibitors |
| WO2016038550A1 (en) | 2014-09-11 | 2016-03-17 | Novartis Ag | Inhibition of prmt5 to treat mtap-deficiency-related diseases |
| WO2016044446A2 (en) | 2014-09-17 | 2016-03-24 | Ironwood Pharmaceuticals, Inc. | Sgc stimulators |
| WO2016089883A1 (en) | 2014-12-01 | 2016-06-09 | Novartis Ag | Compositions and methods for diagnosis and treatment of prostate cancer |
| CN107207497A (en) * | 2014-12-02 | 2017-09-26 | 拜耳作物科学股份公司 | It is used as the dicyclic compound of pest control agent |
| WO2016117647A1 (en) * | 2015-01-21 | 2016-07-28 | 大日本住友製薬株式会社 | New benzimidazole derivative and pharmaceutical use thereof |
| WO2016178870A1 (en) | 2015-05-04 | 2016-11-10 | Eli Lilly And Company | 5'-substituted nucleoside analogs |
| US9840532B2 (en) | 2015-05-04 | 2017-12-12 | Eli Lilly And Company | 5′-substituted nucleoside compounds |
| US10653711B2 (en) | 2015-08-26 | 2020-05-19 | Janssen Pharmaceutica Nv | 6-6 bicyclic aromatic ring substituted nucleoside analogues for use as PRMT5 inhibitors |
| US11318157B2 (en) | 2015-08-26 | 2022-05-03 | Janssen Pharmaceutica Nv | 6-6 bicyclic aromatic ring substituted nucleoside analogues for use as PRMT5 inhibitors |
| JP2021169520A (en) * | 2015-08-26 | 2021-10-28 | ヤンセン ファーマシューティカ エヌ.ベー. | Novel 6-6 bicyclic aromatic ring substituted nucleoside analogues for use as prmt5 inhibitors |
| KR102716092B1 (en) | 2015-08-26 | 2024-10-10 | 얀센 파마슈티카 엔브이 | Novel 6-6 bicyclic aromatic ring substituted nucleoside analogues for use as PRMT5 inhibitors |
| CN114057815A (en) * | 2015-08-26 | 2022-02-18 | 詹森药业有限公司 | Novel 6-6 bicyclic aromatic ring substituted nucleosides as PRMT5 inhibitors |
| US11883367B2 (en) | 2015-08-26 | 2024-01-30 | Janssen Pharmaceutica Nv | 6-6 bicyclic aromatic ring substituted nucleoside analogues for use as PRMT5 inhibitors |
| WO2017032840A1 (en) | 2015-08-26 | 2017-03-02 | Janssen Pharmaceutica Nv | Novel 6-6 bicyclic aromatic ring substituted nucleoside analogues for use as prmt5 inhibitors |
| JP2018528946A (en) * | 2015-08-26 | 2018-10-04 | ヤンセン ファーマシューティカ エヌ.ベー. | Novel 6-6 bicyclic aromatic ring substituted nucleoside analogs for use as PRMT5 inhibitors |
| EP4219496A1 (en) * | 2015-08-26 | 2023-08-02 | JANSSEN Pharmaceutica NV | Novel 6-6 bicyclic aromatic ring substituted nucleoside analogues for use as prmt5 inhibitors |
| EP3974428A1 (en) * | 2015-08-26 | 2022-03-30 | Janssen Pharmaceutica NV | Novel 6-6 bicyclic aromatic ring substituted nucleoside analogues for use as prmt5 inhibitors |
| WO2017096165A1 (en) | 2015-12-03 | 2017-06-08 | Agios Pharmaceuticals, Inc. | Mat2a inhibitors for treating mtap null cancer |
| US11028101B2 (en) | 2016-03-09 | 2021-06-08 | Ctxt Pty Ltd | 3-oxa-8-azabicyclo[3.2.1]octane derivatives and their use in the treatment of cancer and hemoglobinopathies |
| US10550096B2 (en) | 2016-03-09 | 2020-02-04 | Ctxt Pty Ltd | Tetrahydroisoquinolines as PRMT5 inhibitors |
| US10519167B2 (en) | 2016-03-09 | 2019-12-31 | Ctxt Pty Ltd | 3-oxa-8-azabicyclo[3.2.1]octane derivatives and their use in the treatment of cancer and hemoglobinopathies |
| US10421743B2 (en) | 2016-03-09 | 2019-09-24 | Ctxt Pty Ltd | Tetrahydroisoquinolines as PRMT5 inhibitors |
| US10745380B2 (en) | 2016-03-09 | 2020-08-18 | Ctxt Pty Ltd | Pyridine derivatives and their use in the treatment of cancer and hemoglobinopathies |
| US10787434B2 (en) | 2016-03-09 | 2020-09-29 | Ctxt Pty, Ltd | Benzopiperdine derivatives and their use in the treatment of cancer and hemoglobinopathies |
| US10961256B2 (en) | 2016-03-09 | 2021-03-30 | Ctxt Pty Ltd | PRMT5 inhibitors |
| WO2017153186A1 (en) | 2016-03-10 | 2017-09-14 | Janssen Pharmaceutica Nv | Substituted nucleoside analogues for use as prmt5 inhibitors |
| US10898504B2 (en) | 2016-03-10 | 2021-01-26 | Janssen Pharmaceutica Nv | Substituted nucleoside analogues for use as PRMT5 inhibitors |
| US12084462B2 (en) | 2016-09-14 | 2024-09-10 | Janssen Pharmaceutica Nv | Spiro bicyclic inhibitors of menin-MLL interaction |
| US11220517B2 (en) | 2016-09-14 | 2022-01-11 | Janssen Pharmaceutica Nv | Spiro bicyclic inhibitors of menin-MLL interaction |
| US10975100B2 (en) | 2016-09-14 | 2021-04-13 | Janssen Pharmaceutica Nv | Fused bicyclic inhibitors of menin-MLL interaction |
| WO2018065365A1 (en) | 2016-10-03 | 2018-04-12 | Janssen Pharmaceutica Nv | Novel monocyclic and bicyclic ring system substituted carbanucleoside analogues for use as prmt5 inhibitors |
| US11098062B2 (en) | 2016-10-03 | 2021-08-24 | Janssen Pharmaceutica Nv | Monocyclic and bicyclic ring system substituted carbanucleoside analogues for use as PRMT5 inhibitors |
| US11993614B2 (en) | 2016-10-03 | 2024-05-28 | Janssen Pharmaceutica Nv | Monocyclic and bicyclic ring system substituted carbanucleoside analogues for use as PRMT5 inhibitors |
| WO2018100532A1 (en) | 2016-12-01 | 2018-06-07 | Glaxosmithkline Intellectual Property Development Limited | Combination therapy |
| WO2018100535A1 (en) | 2016-12-01 | 2018-06-07 | Glaxosmithkline Intellectual Property Development Limited | Combination therapy |
| US11530226B2 (en) | 2016-12-15 | 2022-12-20 | Janssen Pharmaceutica Nv | Azepane inhibitors of menin-MLL interaction |
| US10745409B2 (en) | 2016-12-15 | 2020-08-18 | Janssen Pharmaceutica Nv | Azepane inhibitors of menin-MLL interaction |
| US11279970B2 (en) | 2017-02-27 | 2022-03-22 | Janssen Pharmaceutica Nv | Use of biomarkers in identifying cancer patients that will be responsive to treatment with a PRMT5 inhibitor |
| WO2018154104A1 (en) | 2017-02-27 | 2018-08-30 | Janssen Pharmaceutica Nv | Use of biomarkers in identifying cancer patients that will be responsive to treatment with a prmt5 inhibitor |
| US11999993B2 (en) | 2017-02-27 | 2024-06-04 | Janssen Pharmaceutica Nv | Use of biomarkers in identifying cancer patients that will be responsive to treatment with a PRMT5 inhibitor |
| KR102616970B1 (en) | 2017-03-17 | 2023-12-22 | 아르고너트 테라퓨틱스 리미티드 | Compounds useful for the treatment or prevention of PRMT5-mediated diseases |
| KR20190129936A (en) * | 2017-03-17 | 2019-11-20 | 아르고너트 테라퓨틱스 리미티드 | Compounds Useful for the Treatment or Prevention of PRMT5-Mediated Diseases |
| CN110650950B (en) * | 2017-03-17 | 2023-09-19 | 阿古诺治疗有限公司 | Compounds for the treatment or prophylaxis of PRMT5 mediated diseases |
| CN110650950A (en) * | 2017-03-17 | 2020-01-03 | 阿古诺治疗有限公司 | Compounds for treating or preventing PRMT5 mediated diseases |
| US11485731B2 (en) | 2017-03-17 | 2022-11-01 | Argonaut Therapeutics Limited | Compounds and methods useful in the treatment of a PRMT5-mediated disorder |
| CN107286083A (en) * | 2017-06-16 | 2017-10-24 | 康化(上海)新药研发有限公司 | A kind of synthetic method of the pyridine carboxaldehyde of 2 chlorine, 6 bromine 3 |
| EP3706742A4 (en) * | 2017-11-08 | 2021-05-05 | Merck Sharp & Dohme Corp. | Prmt5 inhibitors |
| WO2019094311A1 (en) | 2017-11-08 | 2019-05-16 | Merck Sharp & Dohme Corp. | Prmt5 inhibitors |
| EP4385996A2 (en) | 2017-12-08 | 2024-06-19 | JANSSEN Pharmaceutica NV | Novel spirobicyclic analogues |
| US11059850B2 (en) | 2017-12-08 | 2021-07-13 | Janssen Pharmaceutica Nv | Spirobicyclic analogues |
| WO2019110734A1 (en) | 2017-12-08 | 2019-06-13 | Janssen Pharmaceutica Nv | Novel spirobicyclic analogues |
| US11702441B2 (en) | 2017-12-08 | 2023-07-18 | Janssen Pharmaceutica Nv | Spirobicyclic analogues |
| WO2019116302A1 (en) | 2017-12-13 | 2019-06-20 | Lupin Limited | Substituted bicyclic heterocyclic compounds as prmt5 inhibitors |
| US11952380B2 (en) | 2017-12-13 | 2024-04-09 | Lupin Limited | Substituted bicyclic heterocyclic compounds as PRMT5 inhibitors |
| IL274936B1 (en) * | 2017-12-13 | 2023-05-01 | Lupin Ltd | Substituted bicyclic heterocyclic compounds as prmt5 inhibitors |
| CN111712498B (en) * | 2017-12-13 | 2023-09-29 | 鲁平有限公司 | Substituted bicyclic heterocyclic compounds as PRMT5 inhibitors |
| CN111712498A (en) * | 2017-12-13 | 2020-09-25 | 鲁平有限公司 | Substituted bicyclic heterocyclic compounds as PRMT5 inhibitors |
| IL274936B2 (en) * | 2017-12-13 | 2023-09-01 | Lupin Ltd | Substituted bicyclic heterocyclic compounds as prmt5 inhibitors |
| US11459330B2 (en) | 2017-12-13 | 2022-10-04 | Lupin Limited | Substituted bicyclic heterocyclic compounds as PRMT5 inhibitors |
| US11396517B1 (en) | 2017-12-20 | 2022-07-26 | Janssen Pharmaceutica Nv | Exo-aza spiro inhibitors of menin-MLL interaction |
| WO2019219805A1 (en) | 2018-05-16 | 2019-11-21 | Ctxone Pty Ltd | Combination therapy |
| WO2019229614A1 (en) | 2018-05-31 | 2019-12-05 | Glaxosmithkline Intellectual Property Development Limited | Combination of a type ii protein arginine methyltransferase inhibitor and an icos binding protein to treat cancer |
| CN111039934B (en) * | 2018-10-15 | 2022-08-09 | 中国石油化工股份有限公司 | Amino compound, preparation method thereof and application of amino compound as flame retardant |
| CN111039934A (en) * | 2018-10-15 | 2020-04-21 | 中国石油化工股份有限公司 | Amino compound, preparation method thereof and application of amino compound as flame retardant |
| US11571437B2 (en) | 2019-06-06 | 2023-02-07 | Janssen Pharmaceutica Nv | Methods of treating cancer using PRMT5 inhibitors |
| WO2020250123A1 (en) | 2019-06-10 | 2020-12-17 | Lupin Limited | Prmt5 inhibitors |
| WO2020249663A1 (en) | 2019-06-12 | 2020-12-17 | Janssen Pharmaceutica Nv | Novel spirobicyclic intermediates |
| CN112125886B (en) * | 2019-06-24 | 2023-06-23 | 南京圣和药物研发有限公司 | Tricyclic compounds as PRMT5 inhibitors and application thereof |
| CN112125886A (en) * | 2019-06-24 | 2020-12-25 | 南京圣和药物研发有限公司 | Tricyclic compound serving as PRMT5 inhibitor and application thereof |
| US11725001B2 (en) | 2019-10-02 | 2023-08-15 | Sk Biopharmaceuticals Co., Ltd. | Bicyclic compound and use thereof |
| US11111237B2 (en) | 2019-10-02 | 2021-09-07 | Sk Biopharmaceuticals Co., Ltd. | Bicyclic compound and use thereof |
| WO2021068953A1 (en) * | 2019-10-12 | 2021-04-15 | 南京圣和药业股份有限公司 | Substituted tricyclic compound as prmt5 inhibitor and use thereof |
| CN114466846A (en) * | 2019-10-12 | 2022-05-10 | 南京圣和药业股份有限公司 | Substituted tricyclic compound as PRMT5 inhibitor and application thereof |
| WO2021079302A1 (en) | 2019-10-22 | 2021-04-29 | Lupin Limited | Pharmaceutical combination of prmt5 inhibitors |
| WO2021088992A1 (en) * | 2019-11-07 | 2021-05-14 | 江苏先声药业有限公司 | Tetrahydroisoquinoline spiro compound as prmt5 inhibitor |
| CN114728938A (en) * | 2019-11-07 | 2022-07-08 | 江苏先声药业有限公司 | Tetrahydroisoquinoline spiro compounds as PRMT5 inhibitors |
| CN114728938B (en) * | 2019-11-07 | 2024-05-24 | 南京再明医药有限公司 | Tetrahydroisoquinoline spiro compounds as PRMT5 inhibitors |
| CN110950801A (en) * | 2019-11-13 | 2020-04-03 | 济南大学 | Preparation and application of sulfanilamide and benzothiazole compounds containing tetrahydroisoquinoline |
| WO2021111322A1 (en) | 2019-12-03 | 2021-06-10 | Lupin Limited | Substituted nucleoside analogs as prmt5 inhibitors |
| CN111592522B (en) * | 2020-06-17 | 2022-09-09 | 郑州大学 | Arginine methylation transferase 5 small-molecule inhibitor and preparation method and application thereof |
| CN111592522A (en) * | 2020-06-17 | 2020-08-28 | 郑州大学 | Arginine methylation transferase 5 small-molecule inhibitor and preparation method and application thereof |
| WO2021254493A1 (en) * | 2020-06-18 | 2021-12-23 | 上海翊石医药科技有限公司 | Cyclic compound having anti-tumor activity and use thereof |
| CN115768764A (en) * | 2020-06-18 | 2023-03-07 | 上海翊石医药科技有限公司 | Fused ring compound with anti-tumor activity and application thereof |
| WO2022048631A1 (en) | 2020-09-04 | 2022-03-10 | 上海翊石医药科技有限公司 | Compound having antitumor activity and use thereof |
| CN116113626A (en) * | 2020-09-04 | 2023-05-12 | 上海翊石医药科技有限公司 | Compound with anti-tumor activity and application thereof |
| WO2022063094A1 (en) * | 2020-09-22 | 2022-03-31 | 南京明德新药研发有限公司 | Tetrahydroisoquinoline derivative and use thereof |
| US11795172B2 (en) | 2020-09-28 | 2023-10-24 | Cardurion Pharmaceuticals, Inc. | Substituted imidazo[1,2-b]pyridazines and [1,2,4]triazolo[4,3-b]pyridazines as CaMKII inhibitors |
| WO2022067082A1 (en) * | 2020-09-28 | 2022-03-31 | Cardurion Pharmaceuticals, Llc | Fused heteroaryl compounds and their use as camkii inhibitors |
| WO2022237858A1 (en) | 2021-05-13 | 2022-11-17 | 上海翊石医药科技有限公司 | Compound having anti-tumor activity and use thereof |
| CN115124534B (en) * | 2021-11-23 | 2023-09-15 | 中山大学 | Non-nucleotide PRMT5 small molecule inhibitor, preparation method and application |
| CN115124534A (en) * | 2021-11-23 | 2022-09-30 | 中山大学 | Non-nucleotide PRMT5 small molecule inhibitor, preparation method and application |
Also Published As
| Publication number | Publication date |
|---|---|
| US20140228343A1 (en) | 2014-08-14 |
| US20150133427A1 (en) | 2015-05-14 |
| US20170114061A1 (en) | 2017-04-27 |
| EP2935240A1 (en) | 2015-10-28 |
| US20180186798A1 (en) | 2018-07-05 |
| US20160214985A1 (en) | 2016-07-28 |
| US9908887B2 (en) | 2018-03-06 |
| JP2016505000A (en) | 2016-02-18 |
| US8906900B2 (en) | 2014-12-09 |
| US9388173B2 (en) | 2016-07-12 |
| US20180298010A1 (en) | 2018-10-18 |
| US9777008B2 (en) | 2017-10-03 |
| CA2894126A1 (en) | 2014-06-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2018202150B2 (en) | Teatrahydro-and dihydro-isoquinoline prmt5 inhibitors and uses thereof | |
| WO2014100695A1 (en) | Prmt5 inhibitors and uses thereof | |
| EP2935243B1 (en) | Prmt5 inhibitors containing a dihydro- or tetrahydroisoquinoline and uses thereof | |
| EP2935247B1 (en) | Prmt5 inhibitors and uses thereof | |
| EP2935241A1 (en) | Prmt5 inhibitors and uses thereof | |
| EP2970266A1 (en) | 1 -phenoxy-3-(alkylamino)-propan-2-ol derivatives as carm1 inhibitors and uses thereof | |
| WO2015200680A2 (en) | Prmt5 inhibitors and uses thereof | |
| AU2013364139A1 (en) | PRMT5 inhibitors and uses thereof | |
| AU2013364033A1 (en) | PRMT5 inhibitors containing a dihydro- or tetrahydroisoquinoline and uses thereof | |
| AU2013364160A1 (en) | PRMT5 inhibitors and uses thereof | |
| AU2013364037A1 (en) | PRMT5 inhibitors and uses thereof | |
| AU2013364163A1 (en) | Teatrahydro- and dihydro-isoquinoline PRMT5 inhibitors and uses thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13824243 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2894126 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2013824243 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14654303 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: 2015549813 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2013364139 Country of ref document: AU Date of ref document: 20131220 Kind code of ref document: A |