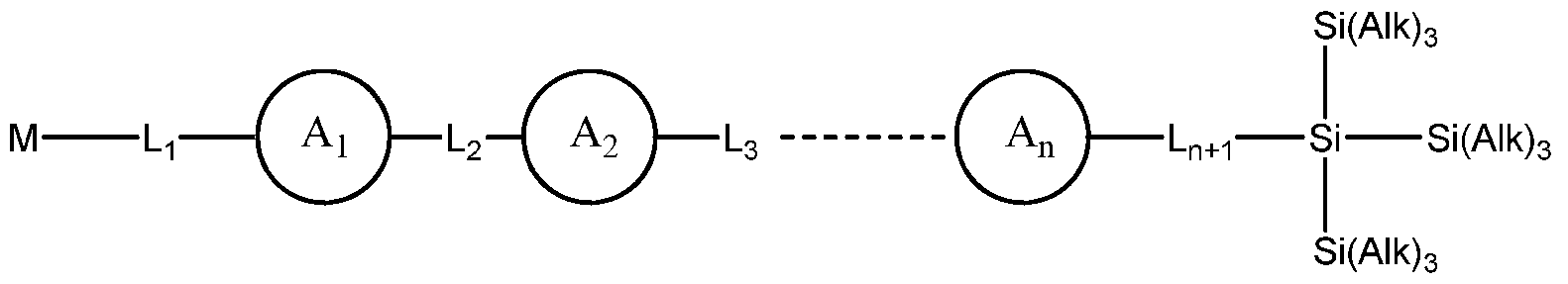WO2014061019A1 - Soluble conjugated oligomers - Google Patents
Soluble conjugated oligomers Download PDFInfo
- Publication number
- WO2014061019A1 WO2014061019A1 PCT/IL2013/050835 IL2013050835W WO2014061019A1 WO 2014061019 A1 WO2014061019 A1 WO 2014061019A1 IL 2013050835 W IL2013050835 W IL 2013050835W WO 2014061019 A1 WO2014061019 A1 WO 2014061019A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- oligomer
- conjugated
- formula
- another embodiment
- independently
- Prior art date
Links
- 0 C[Si](C)(C)[Si](c(cc1)ccc1-c(cc1)ccc1-c(cc1)ccc1-c(cc1)ccc1-c(cc1)ccc1-c(cc1)ccc1[Si](*)(*)[*-])([Si](C)(C)C)[Si](C)(C)C Chemical compound C[Si](C)(C)[Si](c(cc1)ccc1-c(cc1)ccc1-c(cc1)ccc1-c(cc1)ccc1-c(cc1)ccc1-c(cc1)ccc1[Si](*)(*)[*-])([Si](C)(C)C)[Si](C)(C)C 0.000 description 2
- YRSZTDISRFXLJT-UHFFFAOYSA-N CC(CCCC1)CCC1N Chemical compound CC(CCCC1)CCC1N YRSZTDISRFXLJT-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F7/00—Compounds containing elements of Groups 4 or 14 of the Periodic System
- C07F7/02—Silicon compounds
- C07F7/08—Compounds having one or more C—Si linkages
- C07F7/0803—Compounds with Si-C or Si-Si linkages
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/40—Organosilicon compounds, e.g. TIPS pentacene
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/653—Aromatic compounds comprising a hetero atom comprising only oxygen as heteroatom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/30—Organic light-emitting transistors
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E10/00—Energy generation through renewable energy sources
- Y02E10/50—Photovoltaic [PV] energy
- Y02E10/549—Organic PV cells
Definitions
- This invention is directed to conjugated oligomers with terminal tris(trialkyklsilyl)silyl group, process of preparation and uses thereof
- the conjugated oligomers of this invention are well soluble in organic solvents.
- Organic molecules with long ⁇ -conjugation have received much attention as advanced materials and as the building blocks of nano-scale devices for use in solar cells, organic light emitting diodes (OLEDs), organic field effect transistors (OFETs), organic light emitting transistors (OLETs), batteries, electro-luminescent material and sensors.
- OLEDs organic light emitting diodes
- OFETs organic field effect transistors
- OLETs organic light emitting transistors
- batteries electro-luminescent material and sensors.
- Unsubstituted conjugated oligomers are insoluble and difficult to process.
- organic semiconductors are based on small molecules which are insoluble in organic solvents and processed by thermal evaporation. Processing of insoluble materials is expensive and in many cases not possible.
- the object of this invention is directed to the development of compounds which have good semiconducting properties and soluble in organic solvents.
- this invention is directed to a conjugated oligomer comprising at least 3 conjugated monomers; wherein said oligomer is terminated with at least one tris(trialkyllsilyl)silyl group.
- this invention is directed to a conjugated oligomer represented by the structure of formula I:
- Ai to A n and Li to Ln + i are conjugated monomers and said oligomer comprises at least 3 conjugated monomers;
- Aj to A n is independently an aryl or a bond
- Lj to Ln +1 is independently a bond, linear or branched C2-C2 0 alkenyl, linear or branched
- n is an integer of at least 3;
- M is optionally Si[Si(Aik) 3 ] 3 ;
- Alk is independently the same or a different alkyl group.
- this invention is directed to a conjugated oligomer represented
- this invention provides a process for the preparation of a con ugated oligomer represented by the structure of formula II:
- Aj to A n and Lj to Ln +1 are conjugated monomers and said oligomer comprises at conjugated monomers;
- Aj to A n is independently an aryl or a bond
- Lj to Ln +1 is independently a bond, linear or branched C2-C2 0 alkenyl, linera or branched C2-C2 0 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
- Alk is independently the same or a different alkyl group
- n is an integer of at least 3; comprising reacting
- A is independently an aryl or a bond
- L is independently a bond, linear or branched C2-C2 0 alkenyl, linear or branched C2-C2 0 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
- At least one of A or L is not a bond
- x is an integer; wherein said integer is at least 1 ;
- Alk is independently the same or a different alkyl group
- n is an integer of at least 3.
- the conjugated oligomers of this invention are fluorescent.
- this invention provides a fluorescent marker comprising the conjugated oligomer of this invention.
- this invention provides a field effect transistor device comprising the conjugated oligomer of this invention.
- this invention provides a light emitting transistor device comprising the conjugated oligomer of this invention.
- this invention provides a blue light emitting diodes comprising the conjugated oligomer of this invention.
- this invention provides a solar cell comprising the conjugated oligomer of this invention.
- Fig. 1A and IB depict spectroscopic characterization of oligomers of this invention.
- Figure 1A depicts normalized absorption and
- Figure IB depicts fluorescence spectra of (Me 3 Si) 3 Si substituted oligofurans of this invention, measured in dioxane.
- this invention is directed to conjugated oligomers, synthesis, characterization and uses thereof.
- the conjugated oligomers of this invention are soluble in organic solvents, have improved crystallinity, increased fluorescence, and lower HOMO- LUMO gap compared to non silylated oligomer.
- this invention provides a conjugated oligomer comprising at least 3 conjugated monomers; wherein said oligomer is terminated with at least one tris(trialkylsilyl)silyl group.
- the oligomer of this invention is terminated with tris(trimethylsilyl)silyl.
- the oligomer of this invention is terminated with one tris(trialkylsilyl)silyl group.
- the oligomer of this invention is terminated with two tris(trialkylsilyl)silyl group.
- this invention provides a conjugated oligomer represented by the structure of formula I:
- Aj to A n and Lj to Ln +1 are conjugated monomers and said oligomer comprises at least 3 conjugated monomers;
- Aj to A n is independently an aryl or a bond
- Lj to Ln +1 is independently a bond, linear or branched C2-C2 0 alkenyl, linear or branched C2-C2 0 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
- n is an integer of at least 3;
- M is optionally Si[Si(Alk) 3 ]3;
- Alk is independently the same or a different alkyl group.
- the oligomer of formula I comprises at least three aryl groups.
- this invention provides a conjugated oligomer represented by the structure of formula la:
- Aj to A n and Lj to Ln +1 are as defined for the structure of formula I;
- M is optionally Si(SiMe 3 ) 3 .
- the oligomer of formula la comprises at least three aryl groups.
- M of formula I or formula la is nothing. In another embodiment, M of formula I is Si[Si(Alk)3]3. In another embodiment, M of formula I or formula la is Si(SiMe 3 )3
- this invention provides a conjugated oligomer represented by the structure of formula II:
- Aj to A n and Lj to Ln +1 are conjugated monomers and said oligomer comprises at least 3 conjugated monomers;
- Aj to A n is independently an aryl or a bond
- Li to Ln + i is independently a bond, linear or branched C2-C2 0 alkenyl, linear or branched C2-C2 0 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
- Alk is independently the same or a different alkyl group
- n is an integer of at least 3.
- the oligomer of formula II comprises at least three aryl groups.
- this invention provides a conjugated oligomer represented by the structure of formula Ila:
- Aj to A n and Lj to Ln +1 are as defined for the structure of formula II.
- the oligomer of formula Ila comprises at least three aryl groups.
- this invention provides a conjugated oligomer represented by the structure of formula III:
- Ai to A n and Li to Ln + i are conjugated monomers and said oligomer comprises at least 3 conjugated monomers;
- Aj to A n is independently an aryl or a bond
- Lj to Ln +1 is independently a bond, linear or branched C2-C2 0 alkenyl, linear or branched C2-C2 0 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
- Alk is independently the same or a different alkyl group
- n is an integer of at least 3.
- the oligomer of formula III comprises at least three aryl groups.
- this invention provides a conjugated oligomer represented by the structure of formula Ilia:
- Ai to A n and Li to Ln + i are as defined for the structure of formula III.
- the oligomer of formula Ilia comprises at least three aryl groups.
- this invention provides a conjugated oligomer represented by the structure of formula IV:
- Ai to A n is a conjugated monomer and said oligomer comprises at least 3 conjugated monomers;
- Ai to A n is independently an aryl
- Alk is independently the same or a different alkyl group
- n is an integer of at least 3.
- the oligomer of formula IV comprises at least three aryl groups.
- this invention provides a conjugated oligomer represented by the structure of formula IVa:
- Ai to A n are as defined for the structure of formula IV.
- the oligomer of formula IVa comprises at least three aryl groups.
- this invention provides a conjugated oligomer represented by the structure of formula V:
- Ai to A n is a conjugated monomer and said oligomer comprises at least 3 conjugated monomers; wherein Ai to A n is independently an aryl;
- Alk is independently the same or a different alkyl group
- n is an integer of at least 3.
- the oligomer of formula V comprises at least three aryl groups. [0037] In one embodiment, this invention provides a conjugated oligomer represented by the structure of formula Va:
- Aj to A n are as defined for the structure of formula IV.
- the oligomer of formula Va comprises at least three aryl groups.
- this invention is directed to oligomers comprising at least three conjugated monomers.
- three conjugated monomers are selected from an aryl, a linear or branched alkenyl, a linear or branched alkynyl, or combination thereof, wherein one or more carbons of the alkenyl or alkynyl are optionally replaced by a heteroatom.
- the oligomer of this invention comprises at least three aryl groups.
- Ai to A n of formula I-V and la-Va is independently an aryl. In another embodiment, Aj to A n of formula I-V and la-Va is independently a phenyl. In another embodiment, Aj to A n of formula I-V and la-Va is independently a furan. In another embodiment, Aj to A n of formula I-V and la-Va is independently a selenophene. In another embodiment, Aj to A n of formula I-V and la-Va is independently a thiophene. In another embodiment, Aj to A n of formula I-V and la-Va is independently a naphthalene.
- Ai to A n of formula I-V and la-Va is independently an anthracene. In another embodiment, Aj to A n of formula I-V and la-Va is independently a perylene. In another embodiment, Aj to A n of formula I-V and la-Va is independently a fiuorene. In another embodiment, Aj to A n of formula I-V and la-Va is independently an indole. In another embodiment, Aj to A n of formula I-V and la-Va is independently a pyridyl.
- Aj to A n of formula I-V and la-Va is independently a phenyl, furan, selenophene, thiophene, naphthalene, anthracene, indole, pyridyl, perylene, fiuorene or combination thereof.
- Aj to A n of formula I-V and la-Va is the same.
- Aj to A n of formula I-V and la-Va is different.
- Ai to A n of formula I-V and la-Va is substituted or unsubstituted.
- the substituents include an alkyl, halide, alkoxy, hydroxyl, thiol, cyano, nitro, amino, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or combination thereof.
- Li to Ln+i of formula I-V and Ia-Va is independently a bond, linear or branched alkenyl, linear or branched alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom.
- Lj to Ln +1 of formula I-V and Ia-Va is a linear or branched C2-C20 alkenyl, linear or branched C2-C20 alkynyl or combination thereof.
- Lj to Ln +1 of formula I-V and Ia- Va is a linear or branched C2-C10 alkenyl, linear or branched C2-C10 alkynyl or combination thereof.
- Lj to Ln +1 of formula I-V and Ia-Va is independently a linear or branched C2-C6 alkenyl, linear or branched C2-C6 alkynyl or combination thereof.
- Lj to Ln +1 of formula I-V and Ia-Va is independently an alkenyl or alkynyl, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom.
- the heteroatom is S, P, N, O and/or Se.
- Li to Ln+i of formula I-V and Ia-Va is independently a bond.
- Lj to Ln +1 of formula I-V and Ia-Va is independently an acetylene (-C ⁇ C-).
- Lj to Ln +1 of formula I-V and Ia-Va is the same.
- Li to Ln+i of formula I-V is different.
- Li to Ln+i of formula I-V and Ia-Va is substituted or unsubstituted.
- the substituents include alkyl, halide, hydroxyl, thiol, alkoxy, cyano, nitro, amino, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or combination thereof.
- n of formula I-V and Ia-Va is an integer of at least 3. In one embodiment, n of formula I-V and Ia-Va is an integer of at least 4. In another embodiment, n of formula I-V and Ia-Va is an integer between 3 to 100. In another embodiment, n of formula I-V and Ia-Va is an integer between 3 to 50. In another embodiment, n of formula I-V and Ia-Va is an integer between 4 to 100. In another embodiment, n of formula I-V and Ia-Va is an integer between 4 to 50. In another embodiment, n of formula I-V and Ia-Va is an integer between 5 to 100.
- n of formula I-V and Ia-Va is an integer between 5 to 50. In another embodiment, n of formula I-V and Ia-Va is an integer between 3 to 20. In another embodiment, n of formula I-V and Ia-Va is an integer between 4 to 20. In another embodiment, n of formula I-V and Ia-Va is an integer between 5 to 20. In another embodiment, n of formula I-V and Ia-Va is an integer between 3 to 10. In another embodiment, n of formula I-V and Ia-Va is an integer between 4 to 10. In another embodiment, n of formula I-V and Ia-Va is an integer between 5 to 10.
- the oligomer of formula I-V is terminated with a Si[Si(Alk)3]3 group, wherein Alk is independently the same or a different alkyl group.
- the oligomer of formula I-V is terminated with a Si(SiMe 3 )3 group.
- the alkyl is independently a linear or a branched alkyl.
- the alkyl is independently a linear or a branched C ⁇ -Ce alkyl.
- the alkyl is independently a linear or a branched C 1 -C4 alkyl.
- the alkyl is independently a linear or a branched C 1 -C3 alkyl.
- the alkyl group is methyl, ethyl, propyl, isopropyl, butyl, tert-butyl, pentyl or combination thereof.
- this invention is directed to a hypersilylated conjugated oligomer represented
- this invention is directed to a hypersilylated conjugated oligomer represe
- this invention is directed to a hypersilylated conjugated oligomer
- this invention is directed to a hypersilylated conjugated oligomer
- this invention is directed to a hypersilylated conjugated oligomer represented by the structure of 2Si-3F: Me 3 Si SiMe 3
- this invention is directed to a hypersilylated conjugated oligomer represented by the structure of 2Si-4F:
- this invention is directed to a hypersilylated conjugated oligomer represented by the structure of 2Si-6F:
- this invention is directed to a hypersilylated conjugated oligomer represented by the structure of 2Si-9F:
- this invention is directed to a hypersilylated conjugated gomer represented by the structure of Si-3F:
- this invention is directed to a hypersilylated conjugated oligomer represented by the structure of Si-4F:
- this invention provides a process for the preparation of a
- Ai to A n and Li to Ln + i are conjugated monomers and said oligomer comprises at least 3 conjugated monomers;
- Aj to A n is independently an aryl or a bond
- Lj to Ln +1 is independently a bond, linear or branched C2-C2 0 alkenyl, linear or branched C2-C2 0 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
- Alk is independently the same or a different alkyl group
- n is an integer of at least 3;
- A is independently an aryl or a bond
- L is independently a bond, linear or branched C2-C2 0 alkenyl, linear or branched C2-C2 0 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
- At least one of A or L is not a bond
- x is an integer; wherein said integer is at least 1 ;
- Alk is independently the same or a different alkyl group
- n is an integer of at least 3.
- this invention provides a process for the preparation of a conjugated oligomer of formula Ila, wherein said process comprises the same steps as disclosed for the process of preparing a structure of formula II, wherein the Alk group is a methyl group.
- this invention provides a process for the preparation of a conjugated oligomer of formula IV:
- Aj to A n are conjugated monomers and said oligomer comprises at least 3 conjugated monomers;
- Aj to A n is independently an aryl
- Alk is independently the same or a different alkyl group
- n is an integer of at least 3;
- A is independently an aryl
- x is an integer; wherein said integer is at least 1 ;
- n is an integer of at least 3;
- Alk is independently the same or a different alkyl group.
- this invention provides a process for the preparation of a conjugated oligomer of formula IVa, wherein said process comprises the same steps as disclosed for the process of preparing a structure of formula IV, wherein the Alk group is a methyl group.
- this invention provides a process for the preparation of a conjugated
- Aj to A n are conjugated monomers and said oligomer comprises at least 3 conjugated monomers;
- Aj to A n is independently an aryl
- Alk is independently the same or a different alkyl group
- n is an integer larger than 2;
- A is independently an aryl
- Alk is independently the same or a different alkyl group
- n is an integer; wherein said integer is at least 3.
- this invention provides a process for the preparation of a
- conjugated oligomer of formula Va wherein said process comprises the same steps as disclosed for the process of preparing a structure of formula V, wherein the Alk group is a methyl
- a compound of formula (1) or (3) is prepared by silylating a
- Alk is independently the same or a different alkyl group.
- (AlksSfbSiCl is (Me 3 Si) 3 SiCl.
- a compound of formula (1) or (3) is prepared by silylating a corresponding unsubstituted oligomer with (AlksSf SiCl and butyllithium (BuLi) followed by reaction with N-bromosuccinimide (NBS).
- N-bromosuccinimide N-bromosuccinimide
- terminated-bromo-oligomer (1) or (3) reacts with 2- tributyltin-oligomer of formula (2) or (4) respectively in the presence of tetrakis(triphenylphosphine)palladium [Pd(PPh 3 )/t] .
- this invention provides a process for the preparation of a conjugated oligomer wherein brominated oligomer is one of the reactants.
- the brominated oligomer (1), (3), (6) and (7) is prepared by bromination of the corresponding non-brominated oligomer.
- the bromination is in the presence of N-bromosuccinimide (NBS).
- NBS N-bromosuccinimide
- the bromination is according to Fumio et al., Bull. Chem. Soc. Jpn. 63, 2828 (1990), which is incorporated herein by reference.
- a of formula 1-7 is independently an aryl. In another embodiment, A of formula 1-7 is independently a phenyl. In another embodiment, A of formula 1-7 is independently a furan. In another embodiment, A of formula 1-7 is independently a selenophene. In another embodiment, A of formula 1-7 is independently a thiophene. In another embodiment, A of formula 1-7 is independently a naphthalene. In another embodiment, A of formula 1-7 is independently an anthracene. In another embodiment, A of formula 1-7 is independently an indole. In another embodiment, A of formula 1-7 is independently a perylene. In another embodiment, A of formula 1-7 is independently a fluorene.
- a of formula 1-7 is independently a pyridyl. In another embodiment, A of formula 1-7 is independently a phenyl, furan, selenophene, thiophene, naphthalene, anthracene, indole, perylene, fluorine, pyridyl or combination thereof. In another embodiment, A of formula 1-7 is the same. In another embodiment, A of formula 1-7 is different. In another embodiment, A of formula 1-7 is substituted or unsubstituted.
- the substituents include an alkyl, halide, hydroxyl, thiol, alkoxy, cyano, nitro, amino, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or combination thereof.
- L of formula 1-2 is independently a bond, linear or branched alkenyl, linear or branched alkynyl or combination thereof; wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom.
- L of formula 1-2 is a linear or branched C2-C2 0 alkenyl, linear or branched C2-C2 0 alkynyl or combination thereof.
- L of formula 1-2 is a linear or branched C2-C 10 alkenyl, linear or branched C2-C 10 alkynyl or combination thereof.
- L of formula 1-2 is independently a linear or branched C2-C6 alkenyl, linear or branched C2-C6 alkynyl or combination thereof.
- L of formula 1-2 is independently an alkenyl or alkynyl, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom.
- the heteroatom is S, P, N, O and/or Se.
- L of formula 1-2 is independently a bond.
- L of formula 1-2 is independently acetylene (-C ⁇ C-).
- L of formula 1-2 is the same.
- L of formula 1-2 is different. In another embodiment, L of formula 1-2 is substituted or unsubstituted. In another embodiments, the substituents include alkyl, halide, hydroxyl, thiol, alkoxy, cyano, nitro, amino, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or combination thereof.
- n of formula 2, 4 and 5 is an integer of at least 3. In one embodiment, n of formula 2, 4 and 5 is an integer of at least 4. In another embodiment, n of formula 2, 4 and 5 is an integer between 3 to 100. In another embodiment, n of formula 2, 4 and 5 is an integer between 3 to 50. In another embodiment, n of formula 2, 4 and 5 is an integer between 4 to 100. In another embodiment, n of formula 2, 4 and 5 is an integer between 4 to 50. In another embodiment, n of formula 2, 4 and 5 is an integer between 5 to 100. In another embodiment, n of formula 2, 4 and 5 is an integer between 5 to 50. In another embodiment, n of formula 2, 4 and 5 is an integer between 3 to 20.
- n of formula 2, 4 and 5 is an integer between 4 to 20. In another embodiment, n of formula 2, 4 and 5 is an integer between 5 to 20. In another embodiment, n of formula 2, 4 and 5 is an integer between 3 to 10. In another embodiment, n of formula 2, 4 and 5 is an integer between 4 to 10. In another embodiment, n of formula 2, 4 and 5 is an integer between 5 to 10.
- x of formula 1-4, 6 and 7 is an integer of at least 1. In one embodiment, x of formula 1-4, 6 and 7 is an integer of at least 2. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 1 to 100. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 2 to 100. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 1 to 50. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 2 to 50. In another embodiment, x of formula 1-4 is an integer between 1 to 90. . In another embodiment, x of formula 1-4 is an integer between 2 to 90.
- x of formula 1-4, 6 and 7 is an integer between 1 to 70. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 2 to 70. In another embodiment, x of formula 1-4 is an integer between 1 to 40. In another embodiment, x of formula 1-4 is an integer between 2 to 40. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 1 to 30. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 2 to 30. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 1 to 20. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 2 to 20. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 1 to 10. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 2 to 10.
- the oligomer of formula 1 and 3 is terminated with a Si[Si(Alk)3]3 group, wherein Alk is independently the same or a different alkyl group.
- the oligomer of formula 1 and/or 3 is terminated with a Si(SiMe 3 )3 group.
- the alkyl is independently a linear or a branched alkyl.
- the alkyl is independently a linear or a branched C -C alkyl.
- the alkyl is independently a linear or a branched C 1 -C4 alkyl.
- the alkyl is independently a linear or a branched C 1 -C3 alkyl.
- the alkyl group is methyl, ethyl, propyl, isopropyl, butyl, tert-butyl, pentyl or combination thereof.
- A is independently an aryl or a bond
- L is independently a bond, linear or branched C2-C2 0 alkenyl, linear or branched C2-C2 0 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom
- M is optionally Si[Si(Alk) 3 ] 3
- Alk is independently the same or a different alkyl group
- n is an integer of at least 3; are interchangeably herein, and both are encompassed as a conjugated oligomer of the present invention.
- Aj to A n and A are independently an aryl; and n is an integer of at least 3; Alk is independently the same or a different alkyl group; are interchangeably herein, and both are encompassed as a conjugated oligomer of the present invention.
- conjugated oligomers of this invention are prepared according to Examples 1 to 14.
- the conjugated oligomers of this invention comprise at least 3 conjugated monomeric units. In one embodiment, the conjugated oligomers of this invention comprise at least 3 conjugated aryl units. In one embodiment, the conjugated oligomers of this invention comprise at least 4 conjugated monomeric units. In another embodiment, the conjugated units are selected from aryl, alkenyl or alkynyl or combination thereof, wherein one or more carbons of the alkenyl or alkynyl is optionally replaced by a heteroatom. In another embodiment the monomeric units are substituted or unsubstituted.
- the monomeric units are substituted by an alkyl, halide, alkoxy, hydroxyl, thiol, cyano, nitro, amino, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or combination thereof.
- aryl refers to an aromatic group having at least one carbocyclic aromatic group or heterocyclic aromatic group. In one embodiment, the aryl group is a 3-12 membered ring. In another embodiment, the aryl group is a 3-8 membered ring. In another embodiment, the aryl group comprises 3-4 fused rings. In another embodiment, the aryl group comprises 3-4 fused rings.
- aryl groups include phenyl, furan, selenophene, thiophene, naphthalene, anthracene, perylene, fluorine, indole, pyridyl, bipyridyl.
- the aryl group is substituted or unsubstituted.
- the aryl is substituted by alkyl, halide, alkoxy, hydroxyl, thiol, cyano, nitro, amino, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or combination thereof.
- alkenyl refers to, in one embodiment, to an unsaturated hydrocarbon, including straight chain, branched chain and cyclic groups having one or more double bond.
- the alkenyl group may have one double bond, two double bonds, three double bonds etc. Examples of alkenyl groups are ethenyl, propenyl, butenyl, cyclohexenyl etc.
- the alkenyl is between 2 to 6 carbons.
- the alkenyl is between 2 to 8 carbons.
- the alkenyl is between 2 to 12 carbons.
- the alkenyl is between 2 to 20 carbons.
- the alkenyl group is substituted or unsubstituted.
- the alkenyl is substituted by alkyl, halide, alkoxy, hydroxyl, thiol, cyano, nitro, amino, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or combination thereof.
- alkynyl refers to, in one embodiment, to an unsaturated hydrocarbon, including straight chain, branched chain and cyclic groups having one or more triple bond.
- the alkynyl group may have one triple bond, two triple bonds, three triple bonds etc.
- Examples of alkynyl groups are ethynyl, propynyl, butynyl, etc.
- the alkynyl is between 2 to 6 carbons.
- the alkynyl is between 2 to 8 carbons.
- the alkynyl is between 2 to 12 carbons.
- the alkynyl is between 2 to 20 carbons.
- the alkynyl group is substituted or unsubstituted.
- the alkynyl is substituted by alkyl, halide, alkoxy, hydroxyl, thiol, cyano, nitro, amino, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or combination thereof.
- alkyl refers to linear and to branched alkyl. In one embodiment, the alkyl is interrupted by a heteroatom. In another embodiment, the term “alkyl” refers to a saturated linear aliphatic hydrocarbon chain. In another embodiment, the term “alkyl” refers to a saturated branched aliphatic hydrocarbon chain. In another embodiment, the alkyl group has 1-12 carbons. In another embodiment, the alkyl group has 2-8 carbons. In another embodiment, the alkyl group has 1-6 carbons. In another embodiment, the alkyl group has 1-4 carbons. In another embodiment, the alkyl group has 1-3 carbons.
- the branched alkyl is an alkyl substituted by alkyl side chains of 1 to 5 carbons.
- the alkyl group is methyl, ethyl, propyl, isopropyl, butyl, tert-butyl, pentyl or combination thereof.
- the alkyl group may be unsubstituted or substituted, wherein said substitutions include but are not limited to: halogen, alkyl of 1 to 6 carbons, alkoxy of 1 to 6 carbons, carboxy, cyano, nitro, hydroxyl, thiol, amine, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or any combination thereof.
- Conjugated monomers refers to same or different monomeric units which form together a backbone for conducting oligomer/polymer.
- conjugated oligomers of this invention provides conjugated oligomers (I-V and Ia-Va), with higher solubility compared to non silylated oligomers that improves the ease of processability, and enables drop casting/spin casting techniques to be used.
- the process of this invention provides conjugated oligomers with higher solubility compared to non silylated corresponding oligomer.
- this invention provides conjugated oligomers (I-V and Ia- Va) with higher solubility in organic solvents compared to non-silylated corresponding oligomer.
- the oligomers with higher solubility in organic solvent compared to non-silylated corresponding oligomer are selected from:
- the solubility of the oligomers of this invention depends on the length of the oligomer and the solvent.
- the solubility of 2Si-6F in hexane is between 0.1 to 0.4 g/mL.
- the solubility of 2Si-6F in dichloromethane is between 0.4 to 0.7 g/mL.
- the solubility of 2Si-6F in benzene is between 0.3 to 0.6 g/mL.
- the solubility of 2Si-9F in hexane is between 30 to 60 mg/mL.
- the solubility of 2Si-9F in dichloromethane is between 30 to 60 mg/mL.
- the solubility of 2Si-9F in benzene is between 20 to 50 mg/mL.
- the solubility of 2Si-4Ph in hexane is between 10 to 25 mg/mL. In another embodiment, the solubility of 2Si-4Ph in dichloromethane is between 10 to 20 mg/mL. In another embodiment, the solubility of 2Si-4Ph in benzene is between 10 to 20 mg/mL. In another embodiment, the solubility of 2Si-6Ph in hexane is between 10 to 25 mg/mL. In another embodiment, the solubility of 2Si-6Ph in dichloromethane is between 10 to 25 mg/mL. In another embodiment, the solubility of 2Si-6Ph in benzene is between 10 to 20 mg/mL.
- the conjugated oligomers of this invention provide fluorescence and better packing (i.e. shorter interplane distances) oligomers.
- the conjugated oligomers of this invention provide an efficient charge transport material (e.g., for OFETs) and as a luminescent material for organic light emission devices (OLEDs and OLETs).
- an efficient charge transport material e.g., for OFETs
- OLEDs and OLETs organic light emission devices
- the conjugated oligomers of this invention are fluorescent with high quantum yield. Short oligomers show a high fluorescent quantum yield in the deep blue spectral region that is very challenging for efficient electroluminescent devices.
- this invention provides a blue light emitting diode comprising a conjugated oligomer of this invention.
- the conjugated oligomers of this invention provide solid state fluorescence with high quantum yield.
- the solid state fluorescence of the conjugated oligomers of this invention is above 40%.
- the solid state fluorescence of the conjugated oligomers of this invention is above 50%.
- the solid state fluorescence of the conjugated oligomers of this invention is above 60%.
- the solid state fluorescence of the conjugated oligomers of this invention is above 70%.
- the solid state fluorescence of the conjugated oligomers of this invention is between 40% to 100%.
- the solid state fluorescence of the conjugated oligomers of this invention is between 40% to 90%.
- the solid state fluorescence of the conjugated oligomers of this invention is between
- the solid state fluorescence of 2Si-4Ph is between 65% to 75%.
- the solid state fluorescence of 2Si-5Ph is between 75% to 85%.
- the solid state fluorescence of 2Si-6Ph is between 60% to 70%.
- the solid state fluorescence of 2Si-7Ph is between 45% to 55%.
- the conjugated oligomers of this invention prepared by the processes of this invention can be used for the production of electrodes, catalysts, electrical storage systems, shielding materials, fluorescent markers, dyes, pigments, electrical switches, semiconductor components, electrochromic materials, electromagnetic interference materials, electro-optical devices such as light emitting diodes, field-effect transistors, solar cells, polarizing optical elements and batteries or for the antistatic treatment of plastics.
- this invention provides the use of the conjugated oligomers of this invention for imparting antistatic properties on plastic films.
- imparting antistatic properties on plastic films comprising a heat treatment of the coated films with mechanical deformation of the films, wherein said films comprise of oligophenylenes, oligofuran, polyfuran or copolymer of this invention. Simultaneous heat treatment and mechanical deformation of this type takes place in the production of plastic moldings from plastic films by thermoforming the films.
- this invention provides film coatings or layers of the conjugated oligomers of this invention in conjunction with a substrate.
- substrate include a metal foil, a graphite, gold, silicon, glass, a semiconductor, titanium.
- the conjugated oligomers of this invention are highly fluorescent, and can be used as fluorescent materials, markers, field effect transistors embedded in polymer matrices such as PMMA (polymethyl methacrylate).
- the conjugated oligomers of this invention are fluorescent.
- oligofuran Si-4F is fluorescent, with quantum yield of about 70%.
- oligofuran 2Si-4F is fluorescent, with quantum yield of about 68%.
- oligofuran 2Si-9F is fluorescent, with quantum yield of about 41%.
- the oligofuran of this invention are thermally stable.
- elongation of the chain length i.e. increase in number of monomeric units leads to decrease of the HUMO-LUMO gap and leads to increase in charge carrier mobility.
- the conjugated oligomers of this invention are deposited on a substrate by spin casting, drop casting, spraying, knife coating, brushing, subliming or printing.
- dopants are added.
- the oligomers of this invention comprise a dopant.
- the dopant is p-type.
- the p-type dopant is Br 3 " , I 3 " ,
- the dopant is n-type.
- the n-type dopant is Li + , Na + or K + .
- conductive oligomer films having holes can be formed via conventional p-dopants which include halogen atoms, e.g., 1 ⁇ 2, CI2, !3 ⁇ 4, IC1, ICI 3 , IBr and IF, Lewis acids, e.g., PF 5 , AsF 5 , SbF 5 , BF 3 , BC1 3 , SbCl 5 , BBr 3 and S0 3 , protonic acids, organic acids, or amino acids, e.g., HF, HC1, HNO 3 , H2SO4, HCIO4, FSO 3 H and CISO 3 H, transition metal compounds, e.g., FeCl 3 , Fe(OCl)3, Fe(C104)3, Fe(CH 3 C 6 H4S0 3 )3, T1CI4, ZrC , HfC , NbF 5 , NbCl 5 , TaCl 5 , MoF 5
- halogen atoms e.g., 1
- Conductive polymeric films employing electrons as carriers as in n-doped polymeric films utilize conventional n-dopants which include the alkali metals (e.g., Li, Na, K, Rb, and Cs), alkaline-earth metals e.g., Ca, Sr, and Ba.,
- alkali metals e.g., Li, Na, K, Rb, and Cs
- alkaline-earth metals e.g., Ca, Sr, and Ba.
- the oligomers of this invention may be doped with conventional p- and n- type dopants post polymerization of the respective monomers.
- the doping process typically involves treatment of the film material with an oxidizing or reducing agent in a redox reaction to form delocalized ionic centers in the material, with the corresponding counter ions derived from the applied dopants.
- Doping methods comprise for example exposure to a doping vapor in the atmospheric or at a reduced pressure, electrochemical doping in a solution containing a dopant, bringing the dopant in contact with the polymer to be thermally diffused, and ion-implantantion of the dopant into the semiconductor material.
- dopant refers, in one embodiment to a substance which is added to an oligomer of this invention in small quantities in order to cause the mixture of the oligomer and dopant to be electrically conductive.
- these oligomers are electrically conductive without a dopant, the magnitude of the conductivity can be increased by adding a dopant material.
- the conjugated oligomers of this invention are useful as layers and/or composites for thin film deposition which are useful in conjunction with the fabrication of thin film transistors and related devices as can be incorporated into an integrated circuit.
- this invention provides a use of the conjugated oligomers of this invention as a field-effect active layer in a semiconductor device which is a field-effect transistor.
- a field-effect transistor By determining current voltage characteristics at various gate voltages a field- effect is observed.
- a typical value of the field-effect charge-carrier mobility is approximately 10 ° - 10 "6 cm 2 /Vs) at a bulk conductivity. These values are typical of amorphous semiconducting polymers processed from solution.
- a good field-effect transistor combines a high mobility with a low bulk conductivity.
- this invention provides a semiconductor device having a semiconducting layer comprising the formation of a layer by drop casting, spin casting, spin spraying, sublimation, knife coating, brushing or printing (such as inkjet printing) using the conjugated oligomers of this invention.
- Inkjet printing is particularly suitable for high information content displays, in particular full color displays.
- Inkjet printing of OLEDs is described in, for example, EP
- this invention provides a use of the hypersilylated conjugated oligomers of this invention as a coating layer of an electrode.
- the coating thickness of the applied coating after drying is generally 0.1 to 100 ⁇ , depending on the conductivity desired and on the coating transparency desired.
- this invention provides a use conjugated oligomers of this invention as electrode material for rechargeable batteries.
- the conjugated oligomers of this invention are stable when used as electrode material for rechargeable batteries having a lower rate of self-discharge and can be re- and discharged (i.e. cyclised) frequently.
- this invention provides an electrochromic device comprising conjugated oligomers of this invention.
- electrolytic device refers to electrolytic cells that change their ability to transmit (or reflect) light in response to a small bias (typically 1-2 V) applied across the two electrodes.
- the electrochromic devices include displays, electronic ink, sensors, sun glasses, traffic signs or memory elements.
- this invention provides an organic light-emitting device, comprising: a first electrode; a second electrode; an emitting layer interposed between the first electrode and the second electrode; and at least one of a hole transporting layer and a hole injecting layer interposed between the emitting layer and the first electrode, said at least one of the hole transporting layer and the hole injecting layer obtained from a said conducting polymer.
- the layers are comprised of the conjugated oligomers of the invention.
- an electrical device for example, an optoelectronic device, comprising a conductive conjugated oligomer of this invention as a charge injecting layer in light emitting devices; as a component in electrochromic displays and as electrodes in field-effect transistors and as photovoltaic cells as the alternative for
- an electrical device for example, an optoelectronic device, comprising a conductive conjugated oligomer of this invention.
- the electrical device comprises an anode, a cathode, and an organic semi-conductive layer between the anode and cathode.
- the conductive polyfuran/ copolymer may be provided in a layer between the anode and cathode.
- the layer comprising the conductive polymer is preferably located between the anode and the organic semi- conductive layer.
- the layer comprising the conductive polymer is preferably located between the cathode and the organic semi-conductive layer or in the organic semi-conductive layer.
- the organic semi-conductive layer preferably is light-emissive.
- the anode preferably comprises indium- tin-oxide (ITO).
- the organic semi-conductive layer may comprise one or more of a hole transporter, an electron transporter and a light emissive material.
- One or more further organic semi- conductive layers may be provided between the anode and cathode.
- One or both of the anode and cathode independently may comprise the conductive polymer composition.
- one layer of the device is formed by solution processing then the skilled person will be aware of techniques to prevent intermixing of adjacent layers, for example by crosslinking of one layer before deposition of a subsequent layer or selection of materials for adjacent layers such that the material from which the first of these layers is formed is not soluble in the solvent used to deposit the second layer.
- one layer is preferably formed by deposition from solution followed by heat treatment in order to render it substantially insoluble in the solvent used for deposition for a subsequent layer. In this way, cross-linking may be avoided.
- the devices of this invention comprising the conjugated oligomers of this invention can be used in, e.g. imaging and electronic applications.
- the devices can be used as a field effect transistor, light emitting diode, light emitting transistors, photovoltaic cell, or as display backplanes.
- a Light Emitting Transistor is a form of transistor that emits light. Such a transistor has potential for digital displays and on-chip optical interconnects. LET is a new light-emission concept, providing planar light sources that can be easily integrated in substrates like silicon, glass, paper using standard microelectronic techniques. A transistor that emits light and is made from organic materials could lead to cheaper digital displays and fast-switching light sources on computer chips. A transistor-based light source would switch much faster than a diode, and because of its planar design it could be more easily integrated onto computer chips, providing faster data transmission across chips than copper wire. The key to higher efficiency is a three-layer structure, with thin films stacked on top of one another.
- this invention is directed to a field effect transistor (FET) device, comprising: (i) a gate electrode; (ii) a source electrode and a drain electrode, (iii) dielectric layer on top of the gate electrode; and a conjugated oligomer of this invention between said source and drain electrodes and in electrical contact therewith.
- the FET further comprises a substrate with the conjugated oligomer of this invention as a thin film thereon.
- the transistor is a junction field effect transistor.
- the gate electrode is in electrical contact with a p- type conjugated oligomer organic semiconductor.
- the conjugated oligomer is n-type semiconducting.
- the conjugated oligomer is p- type semiconducting.
- this invention is directed to a light effect transistor (LET) device, comprising: (i) a gate electrode; (ii) a source electrode and a drain electrode; (iii) dielectric layer on top of the gate electrode and a conjugated oligomer of this invention between said source and drain electrodes and in electrical contact therewith.
- the LET further comprising a substrate with the conjugated oligomer of this invention as a thin film thereon.
- the gate electrode is in electrical contact with a p-type conjugated oligomer organic semiconductor.
- the conjugated oligomer is n-type semiconducting.
- the conjugated oligomer is p-type semiconducting.
- this invention is directed to a complementary logic circuit, an active matrix display, an active matrix LED display containing organic transistor devices of this invention.
- Step 1 preparation of 4,4 , -fa ' 5 , (tabutylstannyl)-l, r-biphenyl Bu 3 Sn SnBu 3
- Step 2 preparation of fe 5 , -tris(trimethylsilyl)silane-te?ra-phenyl (4Ph-2Si)
- Si-2F J H NMR (CDC1 3 , 500 MHz). 0.21 (s, 27H), 6.41-6.52 (m, 4H), 7.36
- N- bromosuccinimide N- bromosuccinimide
- Step 2 bis-tris(trimethylsilyl)silane-te?ra— furan (2Si-4F)
- Step 2 preparation of bis-tris(trimethylsilyl)silane-»o»a-furan (2Si-9F)
- Quantum yield measurements were made using four excitation wavelengths, the quantum yields were averaged over 20 measurements, and the errors were estimated to be less than 5%.
- the solid state fluorescence quantum yields were measured with Hamamatsu, Quantaurus-QY CI 1347 spectrometer using an absolute PL quantum yield technique.
- Oligofurans and oligophenylenes substituted by tris(trialkylsilyl)silane are highly fluorescent in both solution and in solid state.
- Figure 1 provides the photophysical properties of oligomers of this invention, in solution:
Abstract
This invention is directed to conjugated oligomers with terminal tris(trialkylsilyl)silane group, process of preparation and uses thereof. The conjugated oligomers of this invention are well soluble in organic solvents.
Description
SOLUBLE CONJUGATED OLIGOMERS
FIELD OF THE INVENTION
[001] This invention is directed to conjugated oligomers with terminal tris(trialkyklsilyl)silyl group, process of preparation and uses thereof The conjugated oligomers of this invention are well soluble in organic solvents.
BACKGROUND OF THE INVENTION
[002] Organic molecules with long π-conjugation have received much attention as advanced materials and as the building blocks of nano-scale devices for use in solar cells, organic light emitting diodes (OLEDs), organic field effect transistors (OFETs), organic light emitting transistors (OLETs), batteries, electro-luminescent material and sensors.
[003] Unsubstituted conjugated oligomers are insoluble and difficult to process. Currently, most used organic semiconductors are based on small molecules which are insoluble in organic solvents and processed by thermal evaporation. Processing of insoluble materials is expensive and in many cases not possible.
[004] Introduction of solubilizing side chains forces consecutive aromatic units away and cause twisting of the backbone and loosing of semiconductor properties. Introduction of alkyl groups to the terminal positions of conjugated oligomers usually do not improve their solubility.
[005] The object of this invention is directed to the development of compounds which have good semiconducting properties and soluble in organic solvents.
SUMMARY OF THE INVENTION
[006] In one embodiment, this invention is directed to a conjugated oligomer comprising at least 3 conjugated monomers; wherein said oligomer is terminated with at least one tris(trialkyllsilyl)silyl group.
[007] In one embodiment, this invention is directed to a conjugated oligomer represented by the structure of formula I:
wherein
Ai to An and Li to Ln+i are conjugated monomers and said oligomer comprises at least 3 conjugated monomers;
Aj to An is independently an aryl or a bond;
Lj to Ln+1 is independently a bond, linear or branched C2-C20 alkenyl, linear or branched
C2-C20 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
n is an integer of at least 3;
M is optionally Si[Si(Aik)3]3; and
Alk is independently the same or a different alkyl group.
[008] In one embodiment, this invention is directed to a conjugated oligomer represented
(Π)
wherein Aj to An and Lj to Ln+1 is as described in formula I.
[009] In one embodiment, this invention provides a process for the preparation of a con ugated oligomer represented by the structure of formula II:
(ID
wherein
Aj to An and Lj to Ln+1 are conjugated monomers and said oligomer comprises at conjugated monomers;
Aj to An is independently an aryl or a bond;
Lj to Ln+1 is independently a bond, linear or branched C2-C20 alkenyl, linera or branched C2-C20 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
Alk is independently the same or a different alkyl group; and
n is an integer of at least 3; comprising reacting
(2) and Pd(PPli3)4 to obtain an oligomer of formula II;
wherein
A is independently an aryl or a bond;
L is independently a bond, linear or branched C2-C20 alkenyl, linear or branched C2-C20 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
at least one of A or L is not a bond;
x is an integer; wherein said integer is at least 1 ;
Alk is independently the same or a different alkyl group; and
n is an integer of at least 3.
[0010] In one embodiment, the conjugated oligomers of this invention are fluorescent.
[0011] In one embodiment, this invention provides a fluorescent marker comprising the conjugated oligomer of this invention. In one embodiment, this invention provides a field effect transistor device comprising the conjugated oligomer of this invention. In one embodiment, this invention provides a light emitting transistor device comprising the conjugated oligomer of this invention. In one embodiment, this invention provides a blue light emitting diodes comprising the conjugated oligomer of this invention. In one embodiment, this invention provides a solar cell comprising the conjugated oligomer of this invention.
BRIEF DESCRIPTION OF THE DRAWINGS
[0012] The subject matter regarded as the invention is particularly pointed out and distinctly claimed in the concluding portion of the specification. The invention, however, both as to organization and method of operation, together with objects, features, and advantages thereof, may best be understood by reference to the following detailed description when read with the accompanying drawings in which:
[0013] Fig. 1A and IB depict spectroscopic characterization of oligomers of this invention. Figure 1A depicts normalized absorption and Figure IB depicts fluorescence spectra of (Me3Si)3Si substituted oligofurans of this invention, measured in dioxane.
[0014] It will be appreciated that for simplicity and clarity of illustration, elements shown in the figures have not necessarily been drawn to scale. For example, the dimensions of some of the elements may be exaggerated relative to other elements for clarity. Further, where considered appropriate, reference numerals may be repeated among the figures to indicate corresponding or analogous elements. DETAILED DESCRIPTION OF THE PRESENT INVENTION
[0015] In the following detailed description, numerous specific details are set forth in order to provide a thorough understanding of the invention. However, it will be understood by those skilled in the art that the present invention may be practiced without these specific details. In other instances, well-known methods, procedures, and components have not been described in detail so as not to obscure the present invention.
[0016] In one embodiment, this invention is directed to conjugated oligomers, synthesis, characterization and uses thereof. The conjugated oligomers of this invention are soluble in organic solvents, have improved crystallinity, increased fluorescence, and lower HOMO- LUMO gap compared to non silylated oligomer.
[0017] In one embodiment, this invention provides a conjugated oligomer comprising at least 3 conjugated monomers; wherein said oligomer is terminated with at least one tris(trialkylsilyl)silyl group. In another embodiment, the oligomer of this invention is terminated with tris(trimethylsilyl)silyl. In another embodiment, the oligomer of this invention is terminated with one tris(trialkylsilyl)silyl group. In another embodiment, the oligomer of this invention is terminated with two tris(trialkylsilyl)silyl group.
[0018] In one embodiment, this invention provides a conjugated oligomer represented by the structure of formula I:
(I)
wherein
Aj to An and Lj to Ln+1 are conjugated monomers and said oligomer comprises at least 3 conjugated monomers;
Aj to An is independently an aryl or a bond;
Lj to Ln+1 is independently a bond, linear or branched C2-C20 alkenyl, linear or branched C2-C20 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
n is an integer of at least 3;
M is optionally Si[Si(Alk)3]3; and
Alk is independently the same or a different alkyl group.
[0019] In another embodiment, the oligomer of formula I comprises at least three aryl groups.
[0020] In one embodiment, this invention provides a conjugated oligomer represented by the structure of formula la:
da)
wherein
Aj to An and Lj to Ln+1 are as defined for the structure of formula I; and
M is optionally Si(SiMe3)3.
[0021] In another embodiment, the oligomer of formula la comprises at least three aryl groups.
[0022] In one embodiment M of formula I or formula la is nothing. In another embodiment, M of formula I is Si[Si(Alk)3]3. In another embodiment, M of formula I or formula la is Si(SiMe3)3
[0023] In one embodiment, this invention provides a conjugated oligomer represented by the structure of formula II:
(Π)
wherein
Aj to An and Lj to Ln+1 are conjugated monomers and said oligomer comprises at least 3 conjugated monomers;
Aj to An is independently an aryl or a bond;
Li to Ln+i is independently a bond, linear or branched C2-C20 alkenyl, linear or branched C2-C20 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
Alk is independently the same or a different alkyl group; and
n is an integer of at least 3.
[0024] In another embodiment, the oligomer of formula II comprises at least three aryl groups.
[0025] In one embodiment, this invention provides a conjugated oligomer represented by the structure of formula Ila:
(Ha)
wherein
Aj to An and Lj to Ln+1 are as defined for the structure of formula II.
[0026] In another embodiment, the oligomer of formula Ila comprises at least three aryl groups. [0027] In one embodiment, this invention provides a conjugated oligomer represented by the structure of formula III:
(HI)
wherein
Ai to An and Li to Ln+i are conjugated monomers and said oligomer comprises at least 3 conjugated monomers;
Aj to An is independently an aryl or a bond;
Lj to Ln+1 is independently a bond, linear or branched C2-C20 alkenyl, linear or branched C2-C20 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
Alk is independently the same or a different alkyl group; and
n is an integer of at least 3.
[0028] In another embodiment, the oligomer of formula III comprises at least three aryl groups.
[0029] In one embodiment, this invention provides a conjugated oligomer represented by the structure of formula Ilia:
(Ilia)
wherein
Ai to An and Li to Ln+i are as defined for the structure of formula III.
[0030] In another embodiment, the oligomer of formula Ilia comprises at least three aryl groups.
[0031] In one embodiment, this invention provides a conjugated oligomer represented by the structure of formula IV:
(IV) wherein
Ai to An is a conjugated monomer and said oligomer comprises at least 3 conjugated monomers;
Ai to An is independently an aryl;
Alk is independently the same or a different alkyl group; and
n is an integer of at least 3.
[0032] In another embodiment, the oligomer of formula IV comprises at least three aryl groups.
[0033] In one embodiment, this invention provides a conjugated oligomer represented by the structure of formula IVa:
(IVa)
wherein,
Ai to An are as defined for the structure of formula IV.
[0034] In another embodiment, the oligomer of formula IVa comprises at least three aryl groups.
[0035] In one embodiment, this invention provides a conjugated oligomer represented by the structure of formula V:
(V)
wherein
Ai to An is a conjugated monomer and said oligomer comprises at least 3 conjugated monomers; wherein Ai to An is independently an aryl;
Alk is independently the same or a different alkyl group; and
n is an integer of at least 3.
[0036] In another embodiment, the oligomer of formula V comprises at least three aryl groups. [0037] In one embodiment, this invention provides a conjugated oligomer represented by the structure of formula Va:
(Va)
wherein,
Aj to An are as defined for the structure of formula IV.
[0038] In another embodiment, the oligomer of formula Va comprises at least three aryl groups.
[0039] In one embodiment, this invention is directed to oligomers comprising at least three conjugated monomers. In another embodiment, three conjugated monomers are selected from an aryl, a linear or branched alkenyl, a linear or branched alkynyl, or combination thereof, wherein one or more carbons of the alkenyl or alkynyl are optionally replaced by a heteroatom. In another embodiment, the oligomer of this invention comprises at least three aryl groups.
[0040] In one embodiment, Ai to An of formula I-V and la-Va is independently an aryl. In another embodiment, Aj to An of formula I-V and la-Va is independently a phenyl. In another embodiment, Aj to An of formula I-V and la-Va is independently a furan. In another embodiment, Aj to An of formula I-V and la-Va is independently a selenophene. In another embodiment, Aj to An of formula I-V and la-Va is independently a thiophene. In another embodiment, Aj to An of formula I-V and la-Va is independently a naphthalene. In another embodiment, Ai to An of formula I-V and la-Va is independently an anthracene. In another embodiment, Aj to An of formula I-V and la-Va is independently a perylene. In another embodiment, Aj to An of formula I-V and la-Va is independently a fiuorene. In another embodiment, Aj to An of formula I-V and la-Va is independently an indole. In another embodiment, Aj to An of formula I-V and la-Va is independently a pyridyl. In another embodiment, Aj to An of formula I-V and la-Va is independently a phenyl, furan, selenophene, thiophene, naphthalene, anthracene, indole, pyridyl, perylene, fiuorene or combination thereof. In another embodiment, Aj to An of formula I-V and la-Va is the same. In another embodiment, Aj to An of formula I-V and la-Va is different. In another embodiment, Ai to An of formula I-V and la-Va is substituted or unsubstituted. In another
embodiments, the substituents include an alkyl, halide, alkoxy, hydroxyl, thiol, cyano, nitro, amino, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or combination thereof.
[0041] In one embodiment, Li to Ln+i of formula I-V and Ia-Va is independently a bond, linear or branched alkenyl, linear or branched alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom. In another Lj to Ln+1 of formula I-V and Ia-Va is a linear or branched C2-C20 alkenyl, linear or branched C2-C20 alkynyl or combination thereof. In another Lj to Ln+1 of formula I-V and Ia- Va is a linear or branched C2-C10 alkenyl, linear or branched C2-C10 alkynyl or combination thereof. In another embodiment, Lj to Ln+1 of formula I-V and Ia-Va is independently a linear or branched C2-C6 alkenyl, linear or branched C2-C6 alkynyl or combination thereof. In another embodiment, Lj to Ln+1 of formula I-V and Ia-Va is independently an alkenyl or alkynyl, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom. In another embodiment, the heteroatom is S, P, N, O and/or Se. In another embodiment, Li to Ln+i of formula I-V and Ia-Va is independently a bond. In another embodiment, Lj to Ln+1 of formula I-V and Ia-Va is independently an acetylene (-C≡C-). In another embodiment, Lj to Ln+1 of formula I-V is independently an ethylene (-C=C-). In another embodiment, Lj to Ln+1 of formula I-V and Ia-Va is the same. In another embodiment, Li to Ln+i of formula I-V is different. In another embodiment, Li to Ln+i of formula I-V and Ia-Va is substituted or unsubstituted. In another embodiments, the substituents include alkyl, halide, hydroxyl, thiol, alkoxy, cyano, nitro, amino, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or combination thereof.
[0042] In one embodiment, n of formula I-V and Ia-Va is an integer of at least 3. In one embodiment, n of formula I-V and Ia-Va is an integer of at least 4. In another embodiment, n of formula I-V and Ia-Va is an integer between 3 to 100. In another embodiment, n of formula I-V and Ia-Va is an integer between 3 to 50. In another embodiment, n of formula I-V and Ia-Va is an integer between 4 to 100. In another embodiment, n of formula I-V and Ia-Va is an integer between 4 to 50. In another embodiment, n of formula I-V and Ia-Va is an integer between 5 to 100. In another embodiment, n of formula I-V and Ia-Va is an integer between 5 to 50. In another embodiment, n of formula I-V and Ia-Va is an integer between 3 to 20. In another embodiment, n of formula I-V and Ia-Va is an integer between 4 to 20. In another embodiment, n of formula I-V and Ia-Va is an integer between 5 to 20. In another
embodiment, n of formula I-V and Ia-Va is an integer between 3 to 10. In another embodiment, n of formula I-V and Ia-Va is an integer between 4 to 10. In another embodiment, n of formula I-V and Ia-Va is an integer between 5 to 10.
[0043] In one embodiment the oligomer of formula I-V is terminated with a Si[Si(Alk)3]3 group, wherein Alk is independently the same or a different alkyl group. In another embodiment the oligomer of formula I-V is terminated with a Si(SiMe3)3 group. In another embodiment the alkyl is independently a linear or a branched alkyl. In another embodiment the alkyl is independently a linear or a branched C\-Ce alkyl. In another embodiment the alkyl is independently a linear or a branched C1-C4 alkyl. In another embodiment the alkyl is independently a linear or a branched C1-C3 alkyl. In another embodiment, the alkyl group is methyl, ethyl, propyl, isopropyl, butyl, tert-butyl, pentyl or combination thereof.
[0044] In one embodiment, this invention is directed to a hypersilylated conjugated oligomer represented
2Si-4Ph
[0045] In one embodiment, this invention is directed to a hypersilylated conjugated oligomer represe
2Si-5Ph
2Si-6Ph
[0048] In one embodiment, this invention is directed to a hypersilylated conjugated oligomer represented by the structure of 2Si-3F:
Me3Si SiMe3
Me3Si
[0049] In one embodiment, this invention is directed to a hypersilylated conjugated oligomer represented by the structure of 2Si-4F:
[0050] In one embodiment, this invention is directed to a hypersilylated conjugated oligomer represented by the structure of 2Si-6F:
2Si-6F
[0051] In one embodiment, this invention is directed to a hypersilylated conjugated oligomer represented by the structure of 2Si-9F:
In one embodiment, this invention is directed to a hypersilylated conjugated gomer represented by the structure of Si-3F:
Si-3F
[0053] In one embodiment, this invention is directed to a hypersilylated conjugated oligomer represented by the structure of Si-4F:
[0054] In one embodiment, this invention provides a process for the preparation of a
conjugated oligomer of formula II:
wherein
Ai to An and Li to Ln+i are conjugated monomers and said oligomer comprises at least 3 conjugated monomers;
Aj to An is independently an aryl or a bond;
Lj to Ln+1 is independently a bond, linear or branched C2-C20 alkenyl, linear or branched C2-C20 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
Alk is independently the same or a different alkyl group; and
n is an integer of at least 3;
comprising:
reacting
(2) and Pd(PPli3)4 to obtain an oligomer of formula
wherein
A is independently an aryl or a bond;
L is independently a bond, linear or branched C2-C20 alkenyl, linear or branched C2-C20 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
at least one of A or L is not a bond;
x is an integer; wherein said integer is at least 1 ;
Alk is independently the same or a different alkyl group; and
n is an integer of at least 3.
[0055] In one embodiment, this invention provides a process for the preparation of a conjugated oligomer of formula Ila, wherein said process comprises the same steps as disclosed for the process of preparing a structure of formula II, wherein the Alk group is a methyl group.
[0056] In one embodiment, this invention provides a process for the preparation of a conjugated oligomer of formula IV:
(IV) wherein
Aj to An are conjugated monomers and said oligomer comprises at least 3 conjugated monomers;
Aj to An is independently an aryl;
Alk is independently the same or a different alkyl group; and
n is an integer of at least 3;
comprising:
reacting
(3)
with
wherein
A is independently an aryl;
x is an integer; wherein said integer is at least 1 ;
n is an integer of at least 3; and
Alk is independently the same or a different alkyl group.
[0057] In one embodiment, this invention provides a process for the preparation of a conjugated oligomer of formula IVa, wherein said process comprises the same steps as disclosed for the process of preparing a structure of formula IV, wherein the Alk group is a methyl group.
[0058] In one embodiment, this invention provides a process for the preparation of a conjugated
herein
Aj to An are conjugated monomers and said oligomer comprises at least 3 conjugated monomers;
Aj to An is independently an aryl;
Alk is independently the same or a different alkyl group; and
n is an integer larger than 2;
comprising:
(5) with butyllithium (BuLi) and (AlksSf SiCl to yield a compound of formula V
wherein
A is independently an aryl;
Alk is independently the same or a different alkyl group; and
n is an integer; wherein said integer is at least 3.
[0059] In one embodiment, this invention provides a process for the preparation of a
conjugated oligomer of formula Va, wherein said process comprises the same steps as disclosed for the process of preparing a structure of formula V, wherein the Alk group is a methyl
[0060] In one embodiment, a compound of formula (1) or (3) is prepared by silylating a
(6) (7) with (AiksSrbSiCl. In another embodiment, Alk is independently the same or a different alkyl group. In another embodiment, (AlksSfbSiCl is (Me3Si)3SiCl.
[0061] In one embodiment, a compound of formula (1) or (3) is prepared by silylating a corresponding unsubstituted oligomer with (AlksSf SiCl and butyllithium (BuLi) followed by reaction with N-bromosuccinimide (NBS).
[0062] In another embodiment terminated-bromo-oligomer (1) or (3) reacts with 2- tributyltin-oligomer of formula (2) or (4) respectively in the presence of tetrakis(triphenylphosphine)palladium [Pd(PPh3)/t] .
[0063] In one embodiment, this invention provides a process for the preparation of a conjugated oligomer wherein brominated oligomer is one of the reactants. In another embodiment, the brominated oligomer (1), (3), (6) and (7) is prepared by bromination of the corresponding non-brominated oligomer.
[0064] In another embodiment, the bromination is in the presence of N-bromosuccinimide (NBS). In another embodiment, the bromination is according to Fumio et al., Bull. Chem. Soc. Jpn. 63, 2828 (1990), which is incorporated herein by reference.
[0065] In one embodiment, A of formula 1-7 is independently an aryl. In another embodiment, A of formula 1-7 is independently a phenyl. In another embodiment, A of formula 1-7 is independently a furan. In another embodiment, A of formula 1-7 is independently a selenophene. In another embodiment, A of formula 1-7 is independently a thiophene. In another embodiment, A of formula 1-7 is independently a naphthalene. In another embodiment, A of formula 1-7 is independently an anthracene. In another embodiment, A of formula 1-7 is independently an indole. In another embodiment, A of formula 1-7 is independently a perylene. In another embodiment, A of formula 1-7 is independently a fluorene. In another embodiment, A of formula 1-7 is independently a pyridyl. In another embodiment, A of formula 1-7 is independently a phenyl, furan, selenophene, thiophene, naphthalene, anthracene, indole, perylene, fluorine, pyridyl or combination thereof. In another embodiment, A of formula 1-7 is the same. In another embodiment, A of formula 1-7 is different. In another embodiment, A of formula 1-7 is substituted or unsubstituted. In another embodiments, the substituents include an alkyl, halide, hydroxyl, thiol, alkoxy, cyano, nitro, amino, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or combination thereof.
[0066] In one embodiment, L of formula 1-2 is independently a bond, linear or branched alkenyl, linear or branched alkynyl or combination thereof; wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom. In another L of formula 1-2 is a linear or branched C2-C20 alkenyl, linear or branched C2-C20 alkynyl or combination thereof. In another embodiment, L of formula 1-2 is a linear or branched C2-C10 alkenyl, linear or branched C2-C10 alkynyl or combination thereof. In another embodiment, L of
formula 1-2 is independently a linear or branched C2-C6 alkenyl, linear or branched C2-C6 alkynyl or combination thereof. In another embodiment, L of formula 1-2 is independently an alkenyl or alkynyl, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom. In another embodiment, the heteroatom is S, P, N, O and/or Se. In another embodiment, L of formula 1-2 is independently a bond. In another embodiment, L of formula 1-2 is independently acetylene (-C≡C-). In another embodiment, L of formula 1- 2 is independently an ethylene (-C=C-). In another embodiment, L of formula 1-2 is the same. In another embodiment, L of formula 1-2 is different. In another embodiment, L of formula 1-2 is substituted or unsubstituted. In another embodiments, the substituents include alkyl, halide, hydroxyl, thiol, alkoxy, cyano, nitro, amino, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or combination thereof.
[0067] In one embodiment, n of formula 2, 4 and 5 is an integer of at least 3. In one embodiment, n of formula 2, 4 and 5 is an integer of at least 4. In another embodiment, n of formula 2, 4 and 5 is an integer between 3 to 100. In another embodiment, n of formula 2, 4 and 5 is an integer between 3 to 50. In another embodiment, n of formula 2, 4 and 5 is an integer between 4 to 100. In another embodiment, n of formula 2, 4 and 5 is an integer between 4 to 50. In another embodiment, n of formula 2, 4 and 5 is an integer between 5 to 100. In another embodiment, n of formula 2, 4 and 5 is an integer between 5 to 50. In another embodiment, n of formula 2, 4 and 5 is an integer between 3 to 20. In another embodiment, n of formula 2, 4 and 5 is an integer between 4 to 20. In another embodiment, n of formula 2, 4 and 5 is an integer between 5 to 20. In another embodiment, n of formula 2, 4 and 5 is an integer between 3 to 10. In another embodiment, n of formula 2, 4 and 5 is an integer between 4 to 10. In another embodiment, n of formula 2, 4 and 5 is an integer between 5 to 10.
[0068] In one embodiment, x of formula 1-4, 6 and 7 is an integer of at least 1. In one embodiment, x of formula 1-4, 6 and 7 is an integer of at least 2. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 1 to 100. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 2 to 100. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 1 to 50. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 2 to 50. In another embodiment, x of formula 1-4 is an integer between 1 to 90. . In another embodiment, x of formula 1-4 is an integer between 2 to 90. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 1 to 70. In another
embodiment, x of formula 1-4, 6 and 7 is an integer between 2 to 70. In another embodiment, x of formula 1-4 is an integer between 1 to 40. In another embodiment, x of formula 1-4 is an integer between 2 to 40. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 1 to 30. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 2 to 30. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 1 to 20. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 2 to 20. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 1 to 10. In another embodiment, x of formula 1-4, 6 and 7 is an integer between 2 to 10.
[0069] In one embodiment the oligomer of formula 1 and 3 is terminated with a Si[Si(Alk)3]3 group, wherein Alk is independently the same or a different alkyl group. In another embodiment the oligomer of formula 1 and/or 3 is terminated with a Si(SiMe3)3 group. In another embodiment the alkyl is independently a linear or a branched alkyl. In another embodiment the alkyl is independently a linear or a branched C -C alkyl. In another embodiment the alkyl is independently a linear or a branched C1-C4 alkyl. In another embodiment the alkyl is independently a linear or a branched C1-C3 alkyl. In another embodiment, the alkyl group is methyl, ethyl, propyl, isopropyl, butyl, tert-butyl, pentyl or combination thereof.
[0070] In one embodiment, the oligomer of formula I as described herein:
(I)
and the structure of formula Γ :
(Γ)
wherein A is independently an aryl or a bond; L is independently a bond, linear or branched C2-C20 alkenyl, linear or branched C2-C20 alkynyl or combination thereof, wherein
one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom; M is optionally Si[Si(Alk)3]3; Alk is independently the same or a different alkyl group; and n is an integer of at least 3; are interchangeably herein, and both are encompassed as a conjugated oligomer of the present invention.
[0071] In one embodiment, the oligomer of formula IV as described herein:
(IV)
and the structure of formula IV :
(IV)
wherein Aj to An and A are independently an aryl; and n is an integer of at least 3; Alk is independently the same or a different alkyl group; are interchangeably herein, and both are encompassed as a conjugated oligomer of the present invention.
[0072] In another embodiment, the conjugated oligomers of this invention are prepared according to Examples 1 to 14.
[0073] In one embodiment, the conjugated oligomers of this invention comprise at least 3 conjugated monomeric units. In one embodiment, the conjugated oligomers of this invention comprise at least 3 conjugated aryl units. In one embodiment, the conjugated oligomers of this invention comprise at least 4 conjugated monomeric units. In another embodiment, the conjugated units are selected from aryl, alkenyl or alkynyl or combination thereof, wherein one or more carbons of the alkenyl or alkynyl is optionally replaced by a heteroatom. In another embodiment the monomeric units are substituted or unsubstituted. In another embodiment, the monomeric units are substituted by an alkyl, halide, alkoxy, hydroxyl, thiol, cyano, nitro, amino, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or combination thereof.
[0074] The term "aryl" refers to an aromatic group having at least one carbocyclic aromatic group or heterocyclic aromatic group. In one embodiment, the aryl group is a 3-12 membered ring. In another embodiment, the aryl group is a 3-8 membered ring. In another embodiment, the aryl group comprises 3-4 fused rings. In another embodiment, the aryl group comprises 3-4 fused rings. Non limiting examples of aryl groups include phenyl, furan, selenophene, thiophene, naphthalene, anthracene, perylene, fluorine, indole, pyridyl, bipyridyl. In another embodiment, the aryl group is substituted or unsubstituted. In another embodiment, the aryl is substituted by alkyl, halide, alkoxy, hydroxyl, thiol, cyano, nitro, amino, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or combination thereof.
[0075] The term "alkenyl" refers to, in one embodiment, to an unsaturated hydrocarbon, including straight chain, branched chain and cyclic groups having one or more double bond. The alkenyl group may have one double bond, two double bonds, three double bonds etc. Examples of alkenyl groups are ethenyl, propenyl, butenyl, cyclohexenyl etc. In another embodiment the alkenyl is between 2 to 6 carbons. In another embodiment the alkenyl is between 2 to 8 carbons. In another embodiment the alkenyl is between 2 to 12 carbons. In another embodiment the alkenyl is between 2 to 20 carbons. In another embodiment, the alkenyl group is substituted or unsubstituted. In another embodiment, the alkenyl is substituted by alkyl, halide, alkoxy, hydroxyl, thiol, cyano, nitro, amino, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or combination thereof.
[0076] The term "alkynyl" refers to, in one embodiment, to an unsaturated hydrocarbon, including straight chain, branched chain and cyclic groups having one or more triple bond. The alkynyl group may have one triple bond, two triple bonds, three triple bonds etc. Examples of alkynyl groups are ethynyl, propynyl, butynyl, etc. In another embodiment the alkynyl is between 2 to 6 carbons. In another embodiment the alkynyl is between 2 to 8 carbons. In another embodiment the alkynyl is between 2 to 12 carbons. In another embodiment the alkynyl is between 2 to 20 carbons. In another embodiment, the alkynyl group is substituted or unsubstituted. In another embodiment, the alkynyl is substituted by alkyl, halide, alkoxy, hydroxyl, thiol, cyano, nitro, amino, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or combination thereof.
[0077] The term "alkyl" refers to linear and to branched alkyl. In one embodiment, the alkyl is interrupted by a heteroatom. In another embodiment, the term "alkyl" refers to a
saturated linear aliphatic hydrocarbon chain. In another embodiment, the term "alkyl" refers to a saturated branched aliphatic hydrocarbon chain. In another embodiment, the alkyl group has 1-12 carbons. In another embodiment, the alkyl group has 2-8 carbons. In another embodiment, the alkyl group has 1-6 carbons. In another embodiment, the alkyl group has 1-4 carbons. In another embodiment, the alkyl group has 1-3 carbons. In another embodiment, the branched alkyl is an alkyl substituted by alkyl side chains of 1 to 5 carbons. In another embodiment, the alkyl group is methyl, ethyl, propyl, isopropyl, butyl, tert-butyl, pentyl or combination thereof. The alkyl group may be unsubstituted or substituted, wherein said substitutions include but are not limited to: halogen, alkyl of 1 to 6 carbons, alkoxy of 1 to 6 carbons, carboxy, cyano, nitro, hydroxyl, thiol, amine, alkylamine, alkylhalide, alkylamide, hydroxyalkyl or any combination thereof.
[0078] "Conjugated monomers" refers to same or different monomeric units which form together a backbone for conducting oligomer/polymer.
[0079] Conjugated compounds, inherently possess low solubility. For many industrial applications processability from solution and therefore solubility is an essential requirement if an economically viable process is to be obtained. Therefore in one embodiment, the conjugated oligomers of this invention provides conjugated oligomers (I-V and Ia-Va), with higher solubility compared to non silylated oligomers that improves the ease of processability, and enables drop casting/spin casting techniques to be used. In another embodiment, the process of this invention provides conjugated oligomers with higher solubility compared to non silylated corresponding oligomer.
[0080] In another embodiment, this invention provides conjugated oligomers (I-V and Ia- Va) with higher solubility in organic solvents compared to non-silylated corresponding oligomer. In another embodiment, the oligomers with higher solubility in organic solvent compared to non-silylated corresponding oligomer are selected from:
Me3Si N— ' N— ' — ' — ' SiMe3
Me3Si r, f=\ f=\ f=\ f=\
Me3Si x— ' x— ' x— ' — ' x— ' x— ' x— ' SiMe3
2Si-7Ph
Si-3F
and
1] In one embodiment, the solubility of the oligomers of this invention depends on the length of the oligomer and the solvent. In another embodiment, the solubility of 2Si-6F in hexane is between 0.1 to 0.4 g/mL. In another embodiment, the solubility of 2Si-6F in dichloromethane is between 0.4 to 0.7 g/mL. In another embodiment, the solubility of 2Si-6F in benzene is between 0.3 to 0.6 g/mL. In another embodiment, the solubility of 2Si-9F in hexane is between 30 to 60 mg/mL. In another embodiment, the solubility of
2Si-9F in dichloromethane is between 30 to 60 mg/mL. In another embodiment, the solubility of 2Si-9F in benzene is between 20 to 50 mg/mL.
[0082] In another embodiment, the solubility of 2Si-4Ph in hexane is between 10 to 25 mg/mL. In another embodiment, the solubility of 2Si-4Ph in dichloromethane is between 10 to 20 mg/mL. In another embodiment, the solubility of 2Si-4Ph in benzene is between 10 to 20 mg/mL. In another embodiment, the solubility of 2Si-6Ph in hexane is between 10 to 25 mg/mL. In another embodiment, the solubility of 2Si-6Ph in dichloromethane is between 10 to 25 mg/mL. In another embodiment, the solubility of 2Si-6Ph in benzene is between 10 to 20 mg/mL.
[0083] In another embodiment, the conjugated oligomers of this invention provide fluorescence and better packing (i.e. shorter interplane distances) oligomers.
[0084] In one embodiment, the conjugated oligomers of this invention provide an efficient charge transport material (e.g., for OFETs) and as a luminescent material for organic light emission devices (OLEDs and OLETs).
[0085] In one embodiment, the conjugated oligomers of this invention are fluorescent with high quantum yield. Short oligomers show a high fluorescent quantum yield in the deep blue spectral region that is very challenging for efficient electroluminescent devices. In another embodiment, this invention provides a blue light emitting diode comprising a conjugated oligomer of this invention.
[0086] In one embodiment, the conjugated oligomers of this invention provide solid state fluorescence with high quantum yield. In another embodiment, the solid state fluorescence of the conjugated oligomers of this invention is above 40%. In another embodiment, the solid state fluorescence of the conjugated oligomers of this invention is above 50%. In another embodiment, the solid state fluorescence of the conjugated oligomers of this invention is above 60%. In another embodiment, the solid state fluorescence of the conjugated oligomers of this invention is above 70%. In another embodiment, the solid state fluorescence of the conjugated oligomers of this invention is between 40% to 100%. In another embodiment, the solid state fluorescence of the conjugated oligomers of this invention is between 40% to 90%. In another embodiment, the solid state fluorescence of the conjugated oligomers of this invention is between
50% to 80%. In another embodiment, the solid state fluorescence of 2Si-4Ph is between 65% to 75%. In another embodiment, the solid state fluorescence of 2Si-5Ph is between
75% to 85%. In another embodiment, the solid state fluorescence of 2Si-6Ph is between 60% to 70%. In another embodiment, the solid state fluorescence of 2Si-7Ph is between 45% to 55%. Methods of use.
[0087] In some embodiments of this invention the conjugated oligomers of this invention prepared by the processes of this invention can be used for the production of electrodes, catalysts, electrical storage systems, shielding materials, fluorescent markers, dyes, pigments, electrical switches, semiconductor components, electrochromic materials, electromagnetic interference materials, electro-optical devices such as light emitting diodes, field-effect transistors, solar cells, polarizing optical elements and batteries or for the antistatic treatment of plastics.
[0088] In one embodiment, this invention provides the use of the conjugated oligomers of this invention for imparting antistatic properties on plastic films. In another embodiment, imparting antistatic properties on plastic films comprising a heat treatment of the coated films with mechanical deformation of the films, wherein said films comprise of oligophenylenes, oligofuran, polyfuran or copolymer of this invention. Simultaneous heat treatment and mechanical deformation of this type takes place in the production of plastic moldings from plastic films by thermoforming the films.
[0089] In one embodiment, this invention provides film coatings or layers of the conjugated oligomers of this invention in conjunction with a substrate. In another embodiment, non limiting examples of substrate include a metal foil, a graphite, gold, silicon, glass, a semiconductor, titanium.
[0090] In another embodiment, the conjugated oligomers of this invention are highly fluorescent, and can be used as fluorescent materials, markers, field effect transistors embedded in polymer matrices such as PMMA (polymethyl methacrylate).
[0091] In one embodiment, the conjugated oligomers of this invention are fluorescent. In another embodiment, oligofuran Si-4F is fluorescent, with quantum yield of about 70%. In another embodiment, oligofuran 2Si-4F is fluorescent, with quantum yield of about 68%. In another embodiment, oligofuran 2Si-9F is fluorescent, with quantum yield of about 41%. In another embodiment, the oligofuran of this invention are thermally stable.
[0092] In another embodiment, elongation of the chain length (i.e. increase in number of monomeric units) leads to decrease of the HUMO-LUMO gap and leads to increase in charge carrier mobility.
[0093] In another embodiment, the conjugated oligomers of this invention are deposited on a substrate by spin casting, drop casting, spraying, knife coating, brushing, subliming or printing.
[0094] In one embodiment, to increase or improve the electrical conductivity of the conjugated oligomers of this invention, dopants are added.
[0095] In one embodiment, the oligomers of this invention comprise a dopant. In another embodiment, the dopant is p-type. In another embodiment, the p-type dopant is Br3 ", I3 ",
AsF6~, CIO4", BF4" or FeCLt · In another embodiment, the dopant is n-type. In another embodiment, the n-type dopant is Li+, Na+ or K+.
[0096] In one embodiment, conductive oligomer films having holes (p-doped) can be formed via conventional p-dopants which include halogen atoms, e.g., ½, CI2, !¾, IC1, ICI3, IBr and IF, Lewis acids, e.g., PF5, AsF5, SbF5, BF3, BC13, SbCl5, BBr3 and S03, protonic acids, organic acids, or amino acids, e.g., HF, HC1, HNO3, H2SO4, HCIO4, FSO3H and CISO3H, transition metal compounds, e.g., FeCl3, Fe(OCl)3, Fe(C104)3, Fe(CH3C6H4S03)3, T1CI4, ZrC , HfC , NbF5, NbCl5, TaCl5, MoF5, MoCl5, WF5, WC16, UF6 and LnX3 wherein Ln is a lanthanoid and X is an anion, e.g., CI", Br", Γ, ¾", HSO4", S04 2", N03 ", CIO4", BF4", B12F12 2", PF6 ", AsF6 ", SbF6 ", FeCl4 ", Fe(CN)6 3", and anions of various sulfonic acids, such as aryl-SCV. Also, O2, as well as O3, may be used. Conductive polymeric films employing electrons as carriers as in n-doped polymeric films utilize conventional n-dopants which include the alkali metals (e.g., Li, Na, K, Rb, and Cs), alkaline-earth metals e.g., Ca, Sr, and Ba.,
[0097] In one embodiment, the oligomers of this invention may be doped with conventional p- and n- type dopants post polymerization of the respective monomers. The doping process typically involves treatment of the film material with an oxidizing or reducing agent in a redox reaction to form delocalized ionic centers in the material, with the corresponding counter ions derived from the applied dopants. Doping methods comprise for example exposure to a doping vapor in the atmospheric or at a reduced pressure, electrochemical doping in a solution containing a dopant, bringing the dopant in contact with the polymer to be thermally diffused, and ion-implantantion of the dopant into the semiconductor material.
[0098] The term "dopant" refers, in one embodiment to a substance which is added to an oligomer of this invention in small quantities in order to cause the mixture of the oligomer and dopant to be electrically conductive. However, though these oligomers are electrically conductive without a dopant, the magnitude of the conductivity can be increased by adding a dopant material.
[0099] In one embodiment, the conjugated oligomers of this invention are useful as layers and/or composites for thin film deposition which are useful in conjunction with the fabrication of thin film transistors and related devices as can be incorporated into an integrated circuit.
[00100] In one embodiment, this invention provides a use of the conjugated oligomers of this invention as a field-effect active layer in a semiconductor device which is a field-effect transistor. By determining current voltage characteristics at various gate voltages a field- effect is observed. A typical value of the field-effect charge-carrier mobility is approximately 10 ° - 10"6 cm2/Vs) at a bulk conductivity. These values are typical of amorphous semiconducting polymers processed from solution. A good field-effect transistor combines a high mobility with a low bulk conductivity.
[00101] In one embodiment, this invention provides a semiconductor device having a semiconducting layer comprising the formation of a layer by drop casting, spin casting, spin spraying, sublimation, knife coating, brushing or printing (such as inkjet printing) using the conjugated oligomers of this invention.
[00102] Spin-coating is particularly suitable for devices wherein patterning of the electroluminescent material is unnecessary - for example for lighting applications or simple monochrome segmented displays.
[00103] Inkjet printing is particularly suitable for high information content displays, in particular full color displays. Inkjet printing of OLEDs is described in, for example, EP
0880303, which is incorporated herein by reference.
[00104] In one embodiment, this invention provides a use of the hypersilylated conjugated oligomers of this invention as a coating layer of an electrode. The coating thickness of the applied coating after drying is generally 0.1 to 100 μπι, depending on the conductivity desired and on the coating transparency desired.
[00105] In one embodiment, this invention provides a use conjugated oligomers of this invention as electrode material for rechargeable batteries. In another embodiment the conjugated oligomers of this invention are stable when used as electrode material for
rechargeable batteries having a lower rate of self-discharge and can be re- and discharged (i.e. cyclised) frequently.
[00106] In one embodiment, this invention provides an electrochromic device comprising conjugated oligomers of this invention.
[00107] In one embodiment, the term "electrochromic device" refers to electrolytic cells that change their ability to transmit (or reflect) light in response to a small bias (typically 1-2 V) applied across the two electrodes.
[00108] In another embodiment, the electrochromic devices include displays, electronic ink, sensors, sun glasses, traffic signs or memory elements.
[00109] In one embodiment, this invention provides an organic light-emitting device, comprising: a first electrode; a second electrode; an emitting layer interposed between the first electrode and the second electrode; and at least one of a hole transporting layer and a hole injecting layer interposed between the emitting layer and the first electrode, said at least one of the hole transporting layer and the hole injecting layer obtained from a said conducting polymer. In another embodiment the layers are comprised of the conjugated oligomers of the invention.
[00110] In one embodiment, there is provided an electrical device, for example, an optoelectronic device, comprising a conductive conjugated oligomer of this invention as a charge injecting layer in light emitting devices; as a component in electrochromic displays and as electrodes in field-effect transistors and as photovoltaic cells as the alternative for
ITO.
[00111] In one embodiment, there is provided an electrical device, for example, an optoelectronic device, comprising a conductive conjugated oligomer of this invention. In another embodiment, the electrical device comprises an anode, a cathode, and an organic semi-conductive layer between the anode and cathode. The conductive polyfuran/ copolymer may be provided in a layer between the anode and cathode. When the conductive conjugated oligomer is used as a hole injection material, the layer comprising the conductive polymer is preferably located between the anode and the organic semi- conductive layer. When the conductive polymer is used as an electron transport material, the layer comprising the conductive polymer is preferably located between the cathode and the organic semi-conductive layer or in the organic semi-conductive layer. The organic
semi-conductive layer preferably is light-emissive. The anode preferably comprises indium- tin-oxide (ITO).
[00112] The organic semi-conductive layer may comprise one or more of a hole transporter, an electron transporter and a light emissive material. One or more further organic semi- conductive layers may be provided between the anode and cathode. One or both of the anode and cathode independently may comprise the conductive polymer composition.
[00113] In one embodiment, if multiple layers of the device are formed by solution processing then the skilled person will be aware of techniques to prevent intermixing of adjacent layers, for example by crosslinking of one layer before deposition of a subsequent layer or selection of materials for adjacent layers such that the material from which the first of these layers is formed is not soluble in the solvent used to deposit the second layer. Alternatively, one layer is preferably formed by deposition from solution followed by heat treatment in order to render it substantially insoluble in the solvent used for deposition for a subsequent layer. In this way, cross-linking may be avoided.
[00114] In another embodiment, the devices of this invention comprising the conjugated oligomers of this invention can be used in, e.g. imaging and electronic applications. In another embodiment, the devices can be used as a field effect transistor, light emitting diode, light emitting transistors, photovoltaic cell, or as display backplanes.
[00115] A Light Emitting Transistor (LET) is a form of transistor that emits light. Such a transistor has potential for digital displays and on-chip optical interconnects. LET is a new light-emission concept, providing planar light sources that can be easily integrated in substrates like silicon, glass, paper using standard microelectronic techniques. A transistor that emits light and is made from organic materials could lead to cheaper digital displays and fast-switching light sources on computer chips. A transistor-based light source would switch much faster than a diode, and because of its planar design it could be more easily integrated onto computer chips, providing faster data transmission across chips than copper wire. The key to higher efficiency is a three-layer structure, with thin films stacked on top of one another. Current flows horizontally through the top and bottom layers one carrying electrons and the other holes while carriers that wander into the central layer recombine and emit photons. Because they're segregated into their own layer of material, the recombined carriers, known as singlets, don't run into other carriers, and their energy states change to the point where they won't emit photons. Such quenching is one of the major limitations of OLED efficiency.
[00116] In one embodiment, this invention is directed to a field effect transistor (FET) device, comprising: (i) a gate electrode; (ii) a source electrode and a drain electrode, (iii) dielectric layer on top of the gate electrode; and a conjugated oligomer of this invention between said source and drain electrodes and in electrical contact therewith. In another embodiment, the FET further comprises a substrate with the conjugated oligomer of this invention as a thin film thereon. In another embodiment, the transistor is a junction field effect transistor. In another embodiment, the gate electrode is in electrical contact with a p- type conjugated oligomer organic semiconductor. In another embodiment, the conjugated oligomer is n-type semiconducting. In another embodiment, the conjugated oligomer is p- type semiconducting.
[00117] In one embodiment, this invention is directed to a light effect transistor (LET) device, comprising: (i) a gate electrode; (ii) a source electrode and a drain electrode; (iii) dielectric layer on top of the gate electrode and a conjugated oligomer of this invention between said source and drain electrodes and in electrical contact therewith. In another embodiment, the LET further comprising a substrate with the conjugated oligomer of this invention as a thin film thereon. In another embodiment, the gate electrode is in electrical contact with a p-type conjugated oligomer organic semiconductor. In another embodiment, the conjugated oligomer is n-type semiconducting. In another embodiment, the conjugated oligomer is p-type semiconducting.
[00118] In one embodiment, this invention is directed to a complementary logic circuit, an active matrix display, an active matrix LED display containing organic transistor devices of this invention.
[00119] Without further elaboration, it is believed that one skilled in the art can, using the preceding description, utilize the present invention to its fullest extent. The following examples and preferred specific embodiments are, therefore, to be construed as merely illustrative, and not limitative of the remainder of the disclosure in any way whatsoever.
EXAMPLES EXAMPLE 1
Synthesis of tris(trimethylsilyl)silane-benzene
[00120] A solution of n-BuLi (9 mL, 1.6 M in hexanes, 1.4 mmol, 0.88 equivalents) was added dropwise to a solution of 1 ,4-dibromobenzene (3.76 g, 1.6 mmol) in dry tetrahydrofuran (THF, 30 mL) at -30°C under nitrogen. The reaction mixture was allowed to reach 0°C and stirred for 2 h. To the resulting yellow suspension at 0°C, (Me3Si)3SiCl (3.96 g 1.4 mmol) was added in three portions and the reaction mixture was stirred for 2 hours at 0°C. The mixture was quenched with water, extracted with ether, dried (MgSO/t), and evaporated. Flash chromatography using hexane as the eluent gave Br-Ph-Si: 1.8 g, 31.9% yield) and 2Si-Ph (0.03 g, 0.4 % yield).
Br-Ph-Si
[00121] JH NMR (C6D6, 300 MHz): δ 7.29-7.22 (4H), 0.22 (s, 27H). JH NMR
(CD2C12, 500 MHz): δ 7.35 (dd, 2H), 7.40 (dd, 2H), 0.23 (s, 27H). 13C NMR (CD2C12, 500 MHz): δ 1.1, 122.0, 130.8, 134.9, 138.1. FD HRMS for C15H3iBrSi4 [M+] calcd 404.0666, found 404.0652.
Ph-2Si :
Ph-2Si
[00122] m.p. 270°C. JH NMR (CD2C12, 500 MHz): δ 7.34 (s, 4H), 0.22 (s, 54H). 13C
NMR (CD2C12, 125.8 MHz): δ 1.2, 134.4, 136.2. FD HRMS for C24H58Si8 [M+] calcd 570.2693, found 570.2684. max abs 267 nm (CH2C12)
EXAMPLE 2
Synthesis of tris(trimethylsilyl)silane-biphenyl
[00123] A solution of n-BuLi (6.9 mL, 2.5 M in hexanes, 1.7 mmol,) was added dropwise to a solution of 4,4'-dibromobiphenyl (5g, 165 mmol) in dry THF (100 mL) at
-78 C under nitrogen. The reaction mixture was stirred at -78 C for 45 min. To the resulting yellow suspension was added (Me3Si)3SiCl (4.81 g, 1.7 mmol) at -78°C as three portions and the reaction mixture was stirred for 2 h at room temperature (RT). The mixture was quenched with water, extracted with ether, washed with brine, dried (MgSO/t), and evaporated. Flash chromatography using hexane as the eluent gave Br- 2Ph-Si (0.82 g, 11.2% yield) and 2Ph-2Si (0.01 g, 0.1 % yield).
Br-2Ph-Si [00124] JH NMR (C6D6, 300 MHz): δ 7.63 (d /=8.1 Hz, 2H), 7.28 (d, /=8.7 Hz, 2H),
7.24 (d, /=8.1 Hz, 2H), 7.07 (d, /=8.4 Hz, 2H), 0.31 (s, 27H). JH NMR (CD2C12, 500 MHz): δ 7.58 -7.48 (m, 8H), 0.25 (s, 27H). 13C NMR (CD2C12, 500 MHz): δ 1.2, 121.6, 126.3, 128.8, 132.1, 135.6, 137.5, 138.9, 140.2. FD HRMS for C21H35BrSi4 [M+] calcd 478.0999, found 478.1010.
2Ph-2Si:
2Ph-2Si
[00125] m.p. 338°C. JH NMR (C6D6, 300 MHz): δ 7.67 (d, /=8.4 Hz, 2H), 7.49 (d,
/=8.1 Hz, 2H), 0.31 (s, 54 H). JH NMR (CD2C12, 500 MHz): δ 7.52 (8H), 0.25 (s, 54H). 13C NMR (CD2C12, 125.8 MHz): δ 1.2, 126.3, 134.8, 137.5, 140.0. FD HRMS for C3oH62Si8 [M+] calcd 646.3006, found 646.3011. max abs 294 nm (CH2C12).
EXAMPLE 3
Synthesis of Ais-tris(trimethylsilyl)silane-tetra-phenyl (4Ph-2Si) [00126] Step 1 : preparation of 4,4,-fa'5,(tabutylstannyl)-l, r-biphenyl
Bu3Sn SnBu3
4,4' -bis(tributylstannyl)- 1,1 ' -biphenyl
[00127] 4,4'-Dibromo-/?-biphenyl (3g, 9.6 mmol) was dissolved in 100 mL of dry THF and cooled to -78 C. To this solution was added a 2.5 M solution of «-buthyllithium in hexane (8.5 mL, 21.3 mmol), and resulting solution was stirred for 45 min at -78°C. Bu3SnCl (5.7 mL, 21 mmol) was added, and the mixture was stirred 15 min at -78°C, and then 2 h at room temperature. The reaction quenched with EtOAc (20 mL) and the solvent was removed in vacuum. The residue was dissolved in 20 mL of ether and filtered through a neutral alumina. The filtrate was concentrated. The product was purified by basic silica gel chromatography using hexane as an eluent to give title product in 91.7 % yield (6.45g).
[00128] H NMR (CD2C12, 300 MHz), δ 0.88 (t, 18H), 1.07 (t, 12H), 1.33 (12H), 1.50-
1.58 (m, 4H), 7.531 (s, 4H), 7.533 (s, 4H). [00129] Step 2: preparation of fe 5,-tris(trimethylsilyl)silane-te?ra-phenyl (4Ph-2Si)
2Si-4Ph
[00130] Pd(PPli3)4 (61 mg, 0.53 mmol) was added to a solution of bromophenyl-
(trimethylsilyl)trisilane (Br-Ph-Si) (0.43 g, 1.1 mmol) (see Example 2) and 4,4'- bis(tributylstannyl)- 1,1 ' -biphenyl (0.39 g, 1.13 mmol) in dry toluene (35 mL), and the reaction mixture was refluxed under nitrogen overnight. The mixture was then cooled and evaporated. Purification of the residue by flash chromatography on silica gel (elution with hexane:ethyl acetate= 10:0.25) afforded 2Si-4Ph (0.16 g, 9.1% yield), m.p. is higher than 390 C.
[00131] JH NMR (CD2C12, 500 MHz), 7.77-7.72 (8H), 7.60-7.57 (8H), 0.27 (s, 54H).
13C NMR (CD2CI2, 125.8 MHz): δ 1.2, 126.4, 127.55, 127.58, 135.2, 137.5, 139.62, 139.64, 140.2. 317 nm (CH2C12); 317 nm (dioxane). max,flu 371, 389 nm
(dioxane). QY 0.77 (in dioxane). FD HRMS for C42H70S18 [M+] calcd 798.3632, found 798.3621.
EXAMPLE 4
4,4"-bis(tributylstannyl)-l,l':4',l"-terphenyl
[00133] 4,4'-Dibromo-/?-terphenyl (1.5g, 3.9 mmol) was partially dissolved in 200 mL of dry THF and cooled to -78 C. To this solution was added a 2.5 M solution of n- buthyllithium in hexane (4.6 mL, 11.6 mmol), and resulting solution was stirred for 45 min at -78°C. Bu3SnCl (3.1 mL, 11.6 mmol) was added, and the mixture was stirred 15 min at -78°C, and then 2 h at room temperature. The reaction quenched with EtOAc (20 mL) and the solvent was removed in vacuum. The residue was dissolved in 20 mL of ether and filtered through a neutral alumina. The filtrate was concentrated. The product was purified by flash chromatography using basic silica gel and hexane as an eluent and was used without further purification (~lg of crude mixture was obtained, purity -80% pure). [00134]
2Si-5Ph
[00135] 2Si-5Ph. Pd(PPh3)4 (76 mg, 0.66 mmol) was added to a solution of bromophenyl-(trimethylsilyl)trisilane (Br-Ph-Si) (See Example 2) (0.53 g, 1.3 mmol) and 4,4□□-bis(tributylstannyl)-l,Γ :4',l "-te henyl (0.53 g, 0.66 mmol) in dry toluene (35 mL), and the reaction mixture was refluxed under nitrogen overnight. The mixture was then cooled and evaporated. Purification of the residue by flash chromatography on silica gel (elution with hexane:ethyl acetate=10:0.25)) afforded 2Si-5Ph (0.053 g, 9.1% yield), m.p. is higher than 390°C.
[00136] JH NMR (CD2C12, 500 MHz): δ 7.81-7.58 (m, 20H), 0.27 (s, 54H). 13C NMR (CD2C12, 125.8 MHz): δ 1.2, 126.4, 127.57, 127.61, 127.64, 135.2, 137.5, 139.61,
139.65, 139.85, 140.3. FD HRMS for C48H74S18 [M+] calcd 874.3945, found 874.3932. max abs 321 nm (CH2CI2); 322 nm (dioxane). max flu 381, 399 nm (dioxane). QY 0.78 (in dioxane). EXAMPLE 5
[00137] Pd(PPh3)4 (55 mg, 0.44 mmol) was added to a solution of 2-(4'-bromo-[l,l '- biphenyl]-(trimethylsilyl)trisilane (Br-2Ph-Si) (See Example 2) (0.4 g, 8.7 mmol) and
4,4' -bis(tributylstannyl)- 1,1 '-biphenyl (See Example 3) (0.32 g, 0.44 mmol) in dry toluene (35 mL), and the reaction mixture was refluxed under nitrogen overnight. The mixture was then cooled and evaporated. Purification of the residue by flash chromatography on silica gel (elution with hexane:ethyl acetate=10:0.5) afforded 2Si- 6Ph (0.13 g, 31.7% yield), m.p. is higher than 390°C. JH NMR (CD2C12, 500 MHz): δ
7.79 (s, 4H), 7.78-7.73 (m, 16H), 7.59 (d, /=3.5 Hz, 4H), 0.28 (s, 54H). 13C NMR (CD2CI2, 125.8 MHz): δ 1.2, 126.4, 127.53, 127.56, 127.61 (two carbons), 135.2, 137.5, 139.53, 139.56, 139.74, 139.84, 140.3. FD HRMS for
[M+] calcd 950.4258, found 950.4264. max,abs 324.5 nm (CH2C12); 326 nm (dioxane). max>flu 387, 404 nm (dioxane). QY 0.80 (in dioxane).
EXAMPLE 6
[00138] Pd(PPh3)4 (66 mg, 0.57 mmol) was added to a solution of 2-(4'-bromo-[l,l '- biphenyl]- (trimethylsilyl)trisilane (Br-2Ph-Si) (See Example 2) (0.4 g, 8.7 mmol) and
4,4"-bis(tributylstannyl)-l,Γ :4',l "-teφhenyl (0.32 g, 0.44 mmol) (See Example4) in dry toluene (35 mL), and the reaction mixture was refluxed under nitrogen overnight. The mixture was then cooled and evaporated. Purification of the residue by flash
chromatography on silica gel (elution with hexane:ethyl acetate=10:0.5) afforded 2Si- 7Ph (0.06 g, 10.2% yield), m.p. higher than 390°C.
[00139] JH NMR (CD2C12, 500 MHz): δ 7.80-7.73 (b, 20H), 7.61-7.57 (m, 8H), 0.27
(s, 54H). 13C NMR (CD2C12, 125.8 MHz): δ 1.3, 126.4, 127.6, 127.63, 127.69 (broad signal, three carbons), 135.2, 137.6, 139.61, 139.64, 139.81, 139.88, 139.94, 140.4. FD
HRMS for C6oH82Si8 [M+] calcd 1026.4571, found 1026.4520. max,abs 329 nm (CH2C12); 328 nm (dioxane). max,flu 389, 409 nm (dioxane). QY 0.79 (in dioxane).
EXAMPLE 7
Synthesis of tris(trimethylsilyl)silane-Z»i's~furan (Si-2F)
{ ~ Si-SiMe3
O O 1
SiMe3
Si-2F
[00140] A solution of n-BuLi (5.4 mL, 2.5 M in hexanes, 13.4 mmol, 1.2 equivalents) was added drop wise to a solution of bifuran (1.5 g, 11 mmol) in dry THF (75 mL) at -
78°C under nitrogen. The reaction mixture was allowed to reach room temperature and was stirred for 1 h. The resulting red-brown solution was cooled to -78UC, (Me3Si)3SiCl (3.79g, 13.4 mmol) was added in four portions, and the reaction mixture was stirred at room temperature overnight. The mixture was quenched with water, extracted with hexane, dried (MgSO/t), and evaporated. Flash chromatography using hexane as eluent gave 2Si-2F (1.2g, 17.4% yield) and Si-2F (1.4g, 34 % yield, oil).
[00141] Si-2F: JH NMR (CDC13, 500 MHz). 0.21 (s, 27H), 6.41-6.52 (m, 4H), 7.36
(s, 1H). 13C NMR (CDCI3, DEPT, 126 MHz). 0.8, 104.5, 105.5, 111.3, 122.8, 141.4, 147.5, 150.6, 155.1. FD HRMS for C17H3202Si4 [M+] calcd 380.1479, found 380.1483.
305 nm (dioxane).
EXAMPLE 8
[00142] A solution of n-BuLi (3.2 mL, 1.6 M in hexanes, 4.9 mmol, 2.2 equivalents) was added dropwise to a solution of bifuran (0.3 g, 2.2 mmol) in dry tetrahydrofuran (THF, 15 mL) at -78 C under nitrogen. The reaction mixture was allowed to reach room temperature and stirred for 1 h. The resulting white suspension was cooled to -78 C,
(Me3Si)3SiCl (1.39 g 4.9 mmol) was added in one portion and the reaction mixture stirred at room temperature overnight. The mixture was quenched with water, extracted with hexane, dried (MgSC ), and evaporated. Flash chromatography using hexane as eluent gave 2Si-2F (0.3 g, 21.7% yield) and Si-2F (0.48 g, 57% yield).
[00143] 2Si-2F: m.p. (DSC) 126 °C. m.p. (cap) 193 °C (softening from 122°C).1H
NMR (CDC13, 500 MHz). 0.20 (s, 54H), 6.45 (d, 2H, /=3.5Hz), 6.50 (d, 2H, /=3.5 Hz). 13C NMR (CDCI3, DEPT, 126 MHz). 0.8, 105.1, 122.9, 151.4, 154.5. FD HRMS for C26H5802Si8 [M+] calcd 625.2591, found 626.2579. max,abs 323 nm (dioxane). QY 0.59 (in dioxane).
EXAMPLE 9
Synthesis of bromo-tris(trimethylsilyl)silane-Z»i's~furan (Br-2F-Si)
SiMe
-Si— SiMe3
SiMe3
Br-2F-Si
[00144] Into a solution of Si-2F (1.4 g, 3.7 mmol) (See Example 7) in benzene (45 mL) was added N-bromosuccinimide (NBS, 0.79 g, 4.4 mmol) and the mixture was stirred for 2 h in the dark at room temperature. The mixture was then extracted with hexane, washed with a saturated solution of hydrogen bicarbonate and brine, dried (MgSO/t), and evaporated. Flash chromatography using hexane as eluent gave Br-2F-Si ( 0.83 g, 49% yield).
[00145] Br-2F-Si :JH NMR (CDCI3, 500 MHz). 0.20 (s, 27 H), 6.35 (d, /=3.3 Hz,
2H), 6.5 (d, /=3.6 Hz, 2H). FD HRMS for C17H3i02Si4Br [M+] calcd 458.0585, found 458.0598.
EXAMPLE 10
Synthesis of bis-tris(trimethylsilyl)silane-ter--furan (2Si-3F)
Me3Six n Λ Π Λ Π Λ SiMe3
Me3Si-Si— >— <(, ^— < . ^ -Si— SiMe3
Me3Si' O O O "siMe3
2Si"3F
[00146] A solution of n-BuLi (2.1 mL, 1.6 M in hexanes, 3.3 mmol, 2.2 equivalents) was added dropwise to a solution of terfuran (0.3 g, 1.5 mmol) in dry THF (15 mL) at - 78 C under nitrogen. The reaction mixture was allowed to reach room temperature and stirred for 1 h. The resulting white suspension was cooled to -78°C, (Me3Si)3SiCl (0.93 g, 3.3 mmol) was added as a single portion and the reaction mixture was stirred for 3 hours at room temperature. The mixture was quenched with water, extracted with hexane, dried (MgSC ), and evaporated. Flash chromatography using hexane as the eluent gave 2Si-3F (0.38 g, 36.5% yield) m.p. (DSC) 126°C, m.p. (cap) 122-124 °C, and mono product Si-3F (0.11 g, 16% yield).
[00147] 2Si-3F JH NMR (CDC13, 500 MHz). 0.22 (s, 54H), 6.52 (s, 2H), 6.53 (4H).
13C NMR (CDC13, DEPT, 126 MHz). 0.8, 105.6, 106.4, 122.9, 146.2, 150.4, 155.4. FD HRMS for C3oH6o03Si8 [M+] calcd 692.2697, found 692.2668. max,abs 361 nm (dioxane). QY 0.70 (in dioxane).
EXAMPLE 11
Si-3F
[00148] A solution of n-BuLi (3 mL, 2.5 M in hexanes, 7.4 mmol, 1.2 equivalents) was added drop wise to a solution of terfuran (1.24 g, 6.2 mmol) in dry tetrahydrofuran (THF, 50 mL) at -78 C under nitrogen. The reaction mixture was allowed to reach room temperature and stirred for 1 h. The resulting white suspension was cooled to -78 C, (Me3Si)3SiCl (2.1g, 7.4 mmol) was added as a single portion and the reaction mixture was stirred for room temperature overnight. The mixture was quenched with water,
extracted with hexane, dried (MgSO/t), and evaporated. Flash chromatography using hexane as eluent gave Si-3F (1.18 g, 42.6 % yield) and 2Si-3F (0.18 g, 4.2% yield).
[00149] Si-3F: JH NMR (CDC13, 300 MHz). 0.23 (s, 27H), 6.44-6.46 (m, 1H), 6.53- 6.59 (m, 5H), 7.40 (1H). 13C NMR (CDC13, DEPT, 126 MHz). 0.8, 105.1, 105.9, 106.3, 107.0, 111.5, 122.9, 141.8, 146.4, 146.50, 146.53, 150.2, 155.6. FD HRMS for C21H34O3S14 [M+] calcd 446.1585, found 446.1573. max>abs 347 nm (dioxane).
EXAMPLE 12
Br-3F-Si
[00150] Into a solution of Si-3F (1.18 g, 2.2 mmol) in benzene (25 mL) was added N- bromosuccinimide (NBS, 0.48 g, 2.7 mmol), and the mixture was stirred for 2 h in the dark at room temperature. The mixture was then extracted with hexane, washed with a saturated solution of hydrogen bicarbonate and brine, dried (MgSO/t), and evaporated.
Flash chromatography using hexane as eluent gave Br-3F-Si (0.73 g, 63% yield).
[00151] Br-3F-Si: JH NMR (CDC13, 500 MHz). 0.22 (s, 27 H), 6.35 (d, /=3.5 Hz, 1H), 6.50-6.54 (m, 3H), 6.56 (d, J=3 Hz, 1H), 6.59 (d, /=3.5 Hz, 1H). FD HRMS for C2iH3303Si4Br [M+] calcd 526.0670, found 526.0667.
EXAMPLE 13
Synthesis of bis-tris(trimethylsilyl)silane-te£ra--furan (2Si-4F)
[00153] Pd(PPh3)4 (60mg, 0.05 mmol, 5% mol) was added to 2,5'-(dibromo)bisfuran
(289 mg, 1 mmol) and 2-tributyltinfuran (790 mg, 2.2 mmol) in dry toluene (20 mL), and the mixture was refluxed under N2 for 5 h. The mixture was then cooled,
evaporated and extracted with dichloromethane. The organic extract was dried (MgSO/ , evaporated, and the product was separated over a basified silica (NEt3) column, using hexane as eluent (R/ = 0.2) to yield white, crystalline product. Characterization of 4F was described elsewhere (Kauffmann et al., Chemische Berichte 114, (11), 3667-73 (1981)).
[00154] Step 2: bis-tris(trimethylsilyl)silane-te?ra— furan (2Si-4F)
Me3Six n- Λ Λ— Λ Λ-Λ Λ-Λ SiMe3
Me3Si-Si— y— { >— < — ^-Si— SiMe3
Me3Si' O O O O ¾iMe3
2Si-4F
[00155] A solution of n-BuLi (2.1 mL, 1.6 M in hexanes, 3.3 mmol, 2.2 equivalents) was added dropwise to a solution of a-quaterfuran (0.4 g, 1.5 mmol) in dry THF (15 mL) at -78 C under nitrogen. The reaction mixture was allowed to reach room temperature and stirred for 1 h. The resulting white suspension was cooled to -78 C, (Me3Si)3SiCl (0.93 g, 3.3 mmol) was added as one portion and the reaction mixture was stirred at room temperature overnight. The mixture was quenched with water, extracted with hexane, dried (MgSC ), and evaporated. Flash chromatography using hexane as eluent gave 2Si-4F (0.17 g, 15% yield) and Si-4F (0.02 g, 2.6 % yield).
[00156] 2Si-4F. m.p. (DSC) 161° C, m.p. (cap) 155-157 °C. JH NMR (CDC13, 500
MHz). 0.23 (s, 54H), 6.55 (d, /=3.6 Hz, 4H), 6.58 (d, /=3.3Hz, 2H), 6.62 (d, /=3.6 Hz, 2H). 13C NMR (CDCI3, 100.7 MHz). 0.8, 105.9, 106.5, 107.0, 122.9, 145.2, 146.6,
150.2, 155.6. FD HRMS for C34H6204Si8 [M+] calcd 758.2802, found 758.2796. max,abs 386 nm (dioxane). max tiu 386, 419, 446 (dioxane). QY 0.71 (in dioxane).
0 0 0 0 SiMe3
Si-4F
[00157] Si-4F. JH NMR (CDCI3, 500 MHz). 0.21(s, 27H), 6.47 (dd, /=1.8 Hz, /=3.3
Hz, 1H), 6.54-6.66 (m, 7H), 7.42 (d, J=\ .2 Hz, 1H). 13C NMR (CDCI3, DEPT, 126 MHz). 0.9, 105.5, 106.1 , 106.5, 107.0, 107.1 , 107.3, 111.6, 123.0, 142.0, 145.1 , 145.7, 145.8, 146.3, 146.7, 150.2, 155.8. FD HRMS for C25H3604Si4 [M+] calcd 512.1691 ,
found 512.1701. maXia s 376 nm (dioxane), maXitiu 376, 408, 434 nm (dioxane). QY 0.72 (in dioxane).
EXAMPLE 13
Synthesis of bis-tris(trimethylsilyl)silane-ftexa--furan (2Si-6F)
[00159]
in hexanes, 15.7 mmol) was added dropwise to a solution of bifuran (1.0 g, 7.5 mmol) in dry tetrahydrofuran (THF, 40 mL) at -78°C under N2. The reaction mixture was allowed to reach to room temperature and stirred for 1 h. The resulting white suspension was cooled to 0°C, Bu3SnCl (4.26 mL, 15.7 mmol) was added dropwise and the reaction mixture was allowed to reach room temperature and stirred overnight. The mixture was quenched with water, extracted with hexane, dried (MgSO/t), and evaporated. Flash chromatography on a basified (NEt3) silica, using hexane as eluent gave 5,5- bis(tributylstannyl)-2,2'-bifuran (2.44 g, 47% yield) as a colorless oil and 2- (Tributylstannyl)-2,2' -bifuran (0.96 g, 30% yield) as a colorless oil.
[00160] 5,5-bis(tributylstannyl)-2,2'-bifuran H NMR (300 MHz, CDC13) δ 6.55
(dd, / = 9.6, 3.1 Hz, 4H), 1.69 - 1.45 (m, 12H), 1.41 - 1.23 (m, 12H), 1.10 - 0.99 (m,
12H), 0.88 (t, / = 7.3 Hz, 18H) ppm.
[00161] 2-(Tributylstannyl)-2,2'-bifuran JH NMR (300 MHz, CDC13): δ 7.34 (dd, /
= 0.7, 1.8 Hz, 1H), 6.57 (dd, / = 3.3, 6.0 Hz, 2H), 6.50 (d, / = 3.3 Hz, 1H), 6.40-6.42 (m, 1H), 1.48 - 1.63 (m, 6H), 1.26 - 1.39 (m, 6H), 1.03 - 1.13 (m, 6H), 0.85 - 0.91 (t, / =
7.3 Hz, 9H), ppm. 13C NMR (75 MHz, CDC13): δ 10.2, 13.7, 27.2, 28.9, 104.6, 105.0,
111.3, 123.0, 141.4, 147.5, 151.0, 160.9 ppm.
[00162] Step 2: Preparation of 2Si-6F
[00163] Pd(PPri3)4 (130 mg, 0.11 mmol) was added to a solution of 5-bromo-2-
(trimethylsilyl)trisilan-bifuran (Br-2F-Si) (See Example 9) (0.52 g, 1.13 mmol) and 5,5-bis(tributylstannyl)-2,2-bifuran (0.37 g, 1.13 mmol) in dry toluene (30 mL), and the reaction mixture was refluxed under nitrogen overnight. The mixture was then cooled and evaporated. Purification of the residue by flash chromatography on basified (Et3N) silica gel (elution with hexane) afforded the title product (2Si-6F: 0.16 g, 36% yield), m.p. (DSC) 124°C (broad peak), m.p. (cap) 155-161° C (softening from 122°C), and 60 mg 2Si-4F. JH NMR (C6D6, 500 MHz). 0.29 (s, 54H), 6.49 (d, /=3.5 Hz, 2H), 6.53 (d, /=3Hz, 2H), 6.56 (d, J=2.5 Hz, 4H), 6.59 (d, J=3 Hz, 2H), 6.65 (d, /=3.5 Hz, 2H). 13C NMR (C6D6, DEPT, 126 MHz,) 0.6, 106.4, 106.9, 107.5, 107.68, 107.73, 123.3, 145.3, 145.69, 145.71, 145.9, 146.9, 155.5. FD HRMS for C42H6606Si8 [M+] calcd 890.3014, found 890.3002. max abs 416 nm (dioxane). QY 0.66 (in dioxane).
EXAMPLE 14
Synthesis of bis-tris(trimethylsilyl)silane-«o«a~furan (2Si-9F)
[00164]
B
[00165] 2-(tributylstannyl)terfuran (A) and 5,5"-Bis(tributylstannyl) 2,2':5',2"- terfuran (B) A 2.5M solution of «-BuLi in hexanes (3.2 mL, 8 mmol, 1.6 equivalents) was added dropwise to a solution of terfuran (1 g, 5 mmol) in dry THF (50 mL) at -78°C under N2. The mixture was allowed to come to room temperature and stirred for 30 min. To the white suspension was added dropwise trimethyltin chloride (1.5 mL, 5.5 mmol) at 0°C and the reaction was allowed to reach room temperature and stirred for 2 h. The mixture was extracted with hexane, dried (MgSC ) and evaporated.
Separation using basified silica (Nets) and hexane gave B (1.05 g, 27% yield), A (890 mg, 48% yield) and starting material (110 mg, 11% yield).
[00166] 2-(tributylstannyl)terfuran (A)JH NMR (300 MHz, CDC13): δ 0.83 - 0.90 (t, / = 7.3 Hz, 9H), 1.03 - 1.08 (m, 6H), 1.22 - 1.38 (dq, / = 14.3, 7.2 Hz, 6H), 1.49 - 1.59 (m, 6H), 6.41 - 6.43 (dd, / = 3.4, 1.8 Hz, 1H), 6.52 - 6.60 (m, 5H), 7.37 (d, / = 1.2 Hz, 1H) ppm. 13C NMR (300 MHz, CDCI3): δ 10.3, 13.7, 27.2, 28.9, 105.1, 105.4, 106.5, 107.0, 111.4, 123.1, 141.8, 145.4, 146.5, 146.6, 150.6, 161.4 ppm. HRMS (FD): m/z calcd for C24H3403Sn: 490.1535; found 490.1534.
[00167] 5,5"-Bis(tributylstannyl) 2,2':5',2"-terfuran (B) JH NMR (300 MHz, CDCI3): δ 0.82 - 0.90 (t, / = 7.2 Hz, 18H), 1.01 - 1.11 (m, 12H), 1.23 - 1.37 (dq, / = 7.2, 14.2 Hz, 12H), 1.48 - 1.60 (m, 12H), 6.53 (s, 2H), 6.54 - 6.58 (q, / = 3.2 Hz, 4H) ppm. 13C NMR (300 MHz, CDCI3): δ 10.3, 13.7, 27.2, 28.9, 105.1, 106.5, 123.1, 146.3, 150.8, 161.1 ppm. HRMS (FD): m/z calcd for C36H6o03Sn2: 778.2595; found 778.2599.
[00169] Pd(PPh3)4 (80 mg, 0.07 mmol) was added to a solution of 5-bromo-2-
(trimethylsilyl)trisilan-terfuran (Br-3F-Si) (0.73 g, 1.4 mmol) (see Example 12) and 5,5- bis(tributylstannyl)-2,2-terfuran (0.54 g, 7 mmol) in dry toluene (50 mL), and the reaction mixture was refluxed under nitrogen overnight. The mixture was then cooled and evaporated. Purification of the residue by flash chromatography on basified (Et3N) silica gel (elution with hexane) afforded the title product 2Si-9F (0.02 g, 2.6% yield) and 20 mg of 2Si-6F.
[00170] 2Si-9F JH NMR (C6D6, 500 MHz). 0.31 (s, 54H), 6.49-6.58 (m, 14H), 6.60 (d, /=3.5 Hz, 2H), 6.66 (d, /=3.5 Hz, 2H). 13C NMR (C6D6, DEPT, 126 MHz,). 0.6, 106.4, 106.9, 107.5, 107.75 (2 carbons), 107.77, 107.79, 107.81, 123.3, 145.3, 145.63, 145.69, 145.74, 145.81, 146.1, 147.0, 150.6, 155.6. FD HRMS for C54H72O9S18 [M+] calcd 1088.3331, found 1088.3315. max,abs 435 nm (dioxane), 434, 478,512
(dioxane). QY 0.41 (in dioxane).
EXAMPLE 15
Photophysical properties of the oligofurans and oligophenylenes of this invention.
[00171] UV-vis absorption measurements were made on a Cary-50 spectrometer (Varian). Steady state fluorescence measurements were performed on a Cary Eclipse fluorimeter (Varian) with the excitation/emission geometry at right angles. Fluorescence quantum yields (Of) were determined using a standard procedure (Lakowicz, J. R., Principles of Fluorescence spectroscopy. 2nd ed., Kluwer Academic/Plenum: New York, 1999), and coumarine 30 in MeCN ( abS = 403 nm, ^m = 480 nm, Of = 0.67) was used as a reference (Jones, G., Jackson, W. R., Choi, C, Bergmark, W. R. J. Phys. Chem., 1985, 89, 294-300). Quantum yield measurements were made using four excitation wavelengths, the quantum yields were averaged over 20 measurements, and the errors were estimated to be less than 5%. The solid state fluorescence quantum yields were measured with Hamamatsu, Quantaurus-QY CI 1347 spectrometer using an absolute PL quantum yield technique.
[00172] Oligofurans and oligophenylenes substituted by tris(trialkylsilyl)silane are highly fluorescent in both solution and in solid state.
[00173] Figure 1 provides the photophysical properties of oligomers of this invention, in solution:
2Si-9F
[00174] While certain features of the invention have been illustrated and described herein, many modifications, substitutions, changes, and equivalents will now occur to those of ordinary skill in the art. It is, therefore, to be understood that the appended claims are intended to cover all such modifications and changes as fall within the true spirit of the invention.
Claims
WHAT IS CLAIMED IS:
1. A conjugated oligomer comprising at least 3 conjugated monomers; wherein said oligomer is terminated with at least one tris(trialkylsilyl)silyl group.
2. The oligomer of claim 1, wherein said tris(trialkylsilyl)silyl group is tris(trimethyllsilyl)silyl.
3. The conjugated oligomer of claim 1, wherein said conjugated oligomer is represented by the structure of formula I:
(I)
wherein
Ai to An and Li to Ln+i are conjugated monomers and said oligomer comprises at least 3 conjugated monomers;
Aj to An is independently an aryl or a bond;
Lj to Ln+1 is independently a bond, linear or branched C2-C20 alkenyl, linerar or branched C2-C20 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
n is an integer of at least 3; and
M is optionally Si[Si(Alk)3]3; and
Alk is independently the same or a different alkyl group.
4. The conjugated oligomer of claim3, wherein said oligomer is represented by
(Π)
wherein Aj to An , Lj to Ln+ 1 and Alk are as described in formula I.
5. The conjugated oligomer of claim 3, wherein said oligomer is represented by the structure of formula III:
(III)
wherein Aj to An and Lj to Ln+1 is as described in formula I.
The conjugated oligomer of claim 3, wherein said oligomer is represented by the structure of formula IV:
(IV)
wherein
Aj to An is a conjugated monomer and said oligomer comprises at least 3 conjugated monomers; wherein Aj to An is independently an aryl; Alk is independently the same or a different alkyl group; and
n is an integer of at least 3.
The conjugated oligomer of claim5, wherein said oligomer is represented by the structure of formula V:
(V)
wherein
Aj to An is a conjugated monomer and said oligomer comprises at least 3 conjugated monomers; wherein Aj to An is independently an aryl; Alk is independently the same or a different alkyl group; and
n is an integer of at least 3.
8. The conjugated oligomer of claim 3, wherein said aryl is selected from phenyl, thiophene, furan, selenophene, perylene, fluorine, pyridyl and combination thereof. 9. The conjugated oligomer of claim 6, wherein said oligomer is selected from:
SiMe3
Me3Si =\ =\ /=\ 7""'C3
Me3S SiMe3
2Si-4Ph
2Si-6Ph
Me3Si SiMe3
Me3Si-Si— V Si-SiMe3
Me3Si' ff f/ // % // X // // SiMe3
2Si-3F
Me3Si SiMe3
Me3Si-Si- -Si— SiMe3
Me3Si' Ό' Ό' T SiMe3
2Si-4F
Me3Siv * SiMe3
Me3Si-Si— N Si— SiMe3
Me3Si' SiMe3
2Si-6F ; and
Me3Si^ V SiMe.
Me3Si— Si x II X 11 X II X II X II X II X II X II X -Si— SiMe:
Me3Si' SiMe,
2Si-9F
The conjugated oligomer of claim 7, wherein said oligomer is selected from:
O 0 Ό SiMe3
Si-3F
11. The conjugated oligomer of any one of claims 1 to 10, wherein said oligomer is fluorescent.
12. A fluorescent marker comprising said oligomer of any one of claims 1 to 10.
13. A field effect transistor device comprising said oligomer of any one of claims 1 to 10.
14. A light emitting transistor device comprising said oligomer of any one of claims 1 to 10. 15. A organic solar cell comprising said oligomer of any one of claims 1 to 10.
16. A blue light emitting diode comprising said oligomer of any one of claims 1 to 10. 17. A process for the preparation of hypersilylated conjugated oligomer represented by the structure of formula II:
(Π)
wherein
Aj to An and Lj to Ln+1 are conjugated monomers and said oligomer comprises at least 3 conjugated monomers;
Aj to An is independently an aryl or a bond;
Lj to Ln+1 is independently a bond, linear or branched C2-C20 alkenyl, linear or branched C2-C20 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
n is an integer of at least 3; and
Alk is independently the same or a different alkyl group;
comprising reacting
(1) with
(2)
and Pd(PPri3)4 to obtain an oligomer of formula II;
wherein
A is independently an aryl or a bond;
L is independently a bond, linear or branched C2-C20 alkenyl, linear or branched C2-C20 alkynyl or combination thereof, wherein one or more carbons of said alkenyl or alkynyl are optionally replaced by a heteroatom;
Alk is independently the same or a different alkyl group;
at least one of A or L is not a bond;
x is an integer; wherein said integer is at least 1 ; and
n is an integer of at least 3.
18. The process of claim 17, wherein said alkyl is a methyl.
19. The process of claim 17, wherein said aryl is selected from phenyl, thiophene, furan, selenophene, perylene, fluorine, pyridyl and combination thereof.
20. The process of claim 17, wherein said structure of formula (1) is prepared by silylating a corresponding dibromo derivative with (Alk^Si^SiCl. 21. The process of claim 17, wherein said structure of formula (1) is prepared by silylating a corresponding unsubstituted oligomer with (Alk3Si)3SiCl and butyllithium (BuLi) followed by reaction with N-bromosuccinimide (NBS).
Br-Si-2Ph
and Pd(PPri3)4 to obtain an oligomer of 2Si-7Ph.
comprising reacting
,SiMe3
-Si— SiMe3
SiMe3
Br-Si-3F
with
and Pd(PPh3)4 to obtain an oligomer of 2Si-9F.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261714779P | 2012-10-17 | 2012-10-17 | |
| US61/714,779 | 2012-10-17 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014061019A1 true WO2014061019A1 (en) | 2014-04-24 |
Family
ID=49546597
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IL2013/050835 WO2014061019A1 (en) | 2012-10-17 | 2013-10-16 | Soluble conjugated oligomers |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2014061019A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023080042A1 (en) * | 2021-11-02 | 2023-05-11 | 国立大学法人群馬大学 | Method for producing oligofuran compound containing reactive silyl group, and oligofuran compound containing reactive silyl group |
| WO2023100914A1 (en) * | 2021-12-02 | 2023-06-08 | 国立大学法人群馬大学 | Method for manufacturing oligofuran skeleton-containing polycarbosilane and oligofuran skeleton-containing polycarbosilane |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH07325329A (en) * | 1994-06-01 | 1995-12-12 | Mitsubishi Electric Corp | Organic superlattice material, its production and element using that material |
| EP0880303A1 (en) | 1996-11-25 | 1998-11-25 | Seiko Epson Corporation | Method of producing organic el elements, organic el elements and organic el display device |
| WO2011024171A1 (en) * | 2009-08-27 | 2011-03-03 | Yeda Research And Development Co. Ltd | Oligo- and polyfurans, preparation and uses thereof |
| JP2011122077A (en) * | 2009-12-11 | 2011-06-23 | Sony Corp | Liquid crystal material, liquid crystal display element, and liquid crystal optical space modulation element |
| JP2012049352A (en) * | 2010-08-27 | 2012-03-08 | Konica Minolta Holdings Inc | Organic photoelectric conversion element, and solar cell and optical sensor array utilizing the same |
-
2013
- 2013-10-16 WO PCT/IL2013/050835 patent/WO2014061019A1/en active Application Filing
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH07325329A (en) * | 1994-06-01 | 1995-12-12 | Mitsubishi Electric Corp | Organic superlattice material, its production and element using that material |
| EP0880303A1 (en) | 1996-11-25 | 1998-11-25 | Seiko Epson Corporation | Method of producing organic el elements, organic el elements and organic el display device |
| WO2011024171A1 (en) * | 2009-08-27 | 2011-03-03 | Yeda Research And Development Co. Ltd | Oligo- and polyfurans, preparation and uses thereof |
| JP2011122077A (en) * | 2009-12-11 | 2011-06-23 | Sony Corp | Liquid crystal material, liquid crystal display element, and liquid crystal optical space modulation element |
| JP2012049352A (en) * | 2010-08-27 | 2012-03-08 | Konica Minolta Holdings Inc | Organic photoelectric conversion element, and solar cell and optical sensor array utilizing the same |
Non-Patent Citations (5)
| Title |
|---|
| JONES, G.; JACKSON, W. R.; CHOI, C.; BERGMARK, W. R., J. PHYS. CHEM., vol. 89, 1985, pages 294 - 300 |
| KAUFFMANN ET AL., CHEMISCHEBERICHTE, vol. 114, no. 11, 1981, pages 3667 - 73 |
| LAKOWICZ, J. R: "Principles of Fluorescence spectroscopy", 1999, KLUWER ACADEMIC/PLENUM |
| SOICHIRO KYUSHIN ET AL: "Hyperchromic Effect of Silyl Groups on the UV-Visible Spectrum of 5,10,15,20-Tetraphenylporphyrin", CHEMISTRY LETTERS, vol. 38, no. 4, 1 January 2009 (2009-01-01), pages 324 - 325, XP055094341, ISSN: 0366-7022, DOI: 10.1246/cl.2009.324 * |
| ZHANG W ET AL: "Free radical reactions for heterocycle synthesis. Part 7: 2-Bromobenzoic acids as building blocks in the construction of spirobenzolactones and spirobenzolactams", TETRAHEDRON, ELSEVIER SCIENCE PUBLISHERS, AMSTERDAM, NL, vol. 59, no. 24, 9 June 2003 (2003-06-09), pages 4237 - 4247, XP004429336, ISSN: 0040-4020, DOI: 10.1016/S0040-4020(03)00655-0 * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023080042A1 (en) * | 2021-11-02 | 2023-05-11 | 国立大学法人群馬大学 | Method for producing oligofuran compound containing reactive silyl group, and oligofuran compound containing reactive silyl group |
| WO2023100914A1 (en) * | 2021-12-02 | 2023-06-08 | 国立大学法人群馬大学 | Method for manufacturing oligofuran skeleton-containing polycarbosilane and oligofuran skeleton-containing polycarbosilane |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| TWI638823B (en) | Organic light emitting device | |
| Zou et al. | Unexpected Propeller‐Like Hexakis (fluoren‐2‐yl) benzene Cores for Six‐Arm Star‐Shaped Oligofluorenes: Highly Efficient Deep‐Blue Fluorescent Emitters and Good Hole‐Transporting Materials | |
| CN106117522B (en) | Hole mobile material | |
| KR101556095B1 (en) | Novel heterocyclic compound and use thereof | |
| Prachumrak et al. | Novel bis [5-(fluoren-2-yl) thiophen-2-yl] benzothiadiazole end-capped with carbazole dendrons as highly efficient solution-processed nondoped red emitters for organic light-emitting diodes | |
| KR101817808B1 (en) | Electroactive materials | |
| TW201829416A (en) | Organic semiconducting compounds | |
| US7906724B2 (en) | N-type conjugated materials based on 2-vinyl-4,5-dicyanoimidazoles and their use in organic photovoltaics | |
| EP2814817B1 (en) | Electronic devices using organic small molecule semiconducting compounds | |
| TWI588150B (en) | Heterocyclic compound and use thereof | |
| KR20160065885A (en) | Photoactive organic material for optoelectronic components | |
| JP2013523931A (en) | Fused ring dithiophene copolymer | |
| CN112236882B (en) | Organic semiconductor compound | |
| KR20170078637A (en) | Electroactive materials | |
| Li et al. | Molecular engineering by σ-Bond spacer enables solution-processable host materials for TADF emitter towards high-performance OLEDs | |
| JP2013503152A (en) | Oligofurans, polyfurans, their production and use | |
| Ban et al. | Spirobifluorene/sulfone hybrid: highly efficient solution-processable material for UV–violet electrofluorescence, blue and green phosphorescent OLEDs | |
| JP2017531047A (en) | Hole transport compound and composition | |
| KR20180030220A (en) | Hole transport material | |
| CN111247651A (en) | Organic light emitting device | |
| EP3537490A1 (en) | Organic electronics material, organic layer, organic electronics element, organic electroluminescence element, display element, illumination device, and display device | |
| Ou et al. | Dumbbell effects of solution-processed pyrene-based organic semiconductors on electronic structure, morphology and electroluminescence | |
| KR101387065B1 (en) | Conjugated polymer having electron donor and acceptor alternately, organic photoelectric device and organic solar cell comprising the same | |
| CN112352328B (en) | Organic semiconductor compound | |
| WO2014061019A1 (en) | Soluble conjugated oligomers |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13786536 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 13786536 Country of ref document: EP Kind code of ref document: A1 |