WO2013068348A1 - Lna oligomers for improvement in hepatic function - Google Patents
Lna oligomers for improvement in hepatic function Download PDFInfo
- Publication number
- WO2013068348A1 WO2013068348A1 PCT/EP2012/071934 EP2012071934W WO2013068348A1 WO 2013068348 A1 WO2013068348 A1 WO 2013068348A1 EP 2012071934 W EP2012071934 W EP 2012071934W WO 2013068348 A1 WO2013068348 A1 WO 2013068348A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- liver function
- human subject
- miravirsen
- microrna
- lna
- Prior art date
Links
- 0 CC(C(*)OC1(C(*)(*)C(F)(F)F)C(*)(*)N)C1N Chemical compound CC(C(*)OC1(C(*)(*)C(F)(F)F)C(*)(*)N)C1N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/11—Antisense
- C12N2310/113—Antisense targeting other non-coding nucleic acids, e.g. antagomirs
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/32—Chemical structure of the sugar
- C12N2310/323—Chemical structure of the sugar modified ring structure
- C12N2310/3231—Chemical structure of the sugar modified ring structure having an additional ring, e.g. LNA, ENA
Definitions
- the present invention relates to the use of LNA antisense oligonucleotide inhibitors of microRNA-122 for use in improving blood liver function biomarkers in subjects which have or may have impaired liver function.
- the present invention also relates to use of miravirsen, a drug which is being developed for treatment of HCV, for use in improving liver function in non-HCV infected patients.
- WO2007/090071 many LNA's are toxic.
- WO2007/090071 uses 14mer LNA compounds targeting PTEN mRNA, in in vivo experiments in mice. The inventors of
- WO2007/090071 report that potent knock-down of PTEN using beta-D-oxy LNA (4 -CH2-0- 2' BNA) is associated with elevated biomarkers of hepatotoxicity, ALT and AST.
- WO 2007/027775 2'-MOE inhibitors of microRNA-122 are reported in WO 2007/027775, which further refers to methods for the treatment of cardiovascular or metabolic diseases characterized by elevated serum total cholesterol, elevated serum LDL-cholesterol, or elevated serum triglycerides, through the administration of an oligomeric compound which modulates the levels or activity of miR-122a.
- WO 2007/027775 discloses various metabolic diseases which the inventors allege may be treated using oligomeric compound which modulates the levels or activity of miR-122a, including diabetes, obesity, hyperlipidemia, hypercholesterolemia,
- WO 2007/027775 discloses various 23nt LNA/MOE mixmers, the in vivo examples of WO 2007/027775 are limited to fully modified 2'MOE compounds complementary to the entire miR-122 sequence. See also Esau et al. Cell. Metab. 2006 Feb;3(2):87-98.
- microRNA-122 (miR-122) is a liver specific microRNA. miR-122 is involved in cholesterol metabolism, and inhibition of miR-122 in vivo in mice, results in a reduction in serum cholesterol levels. How miR-122 regulated cholesterol metabolism is apparently, at present unknown.
- miR-122 is a host factor which is required for maintenance of hepatitis C (HCV) infection. Whilst many different hypotheses have been proposed as to how miR-122 interacts with the HCV virus, it has recently been proposed that miR-122 protects the 5' terminus of the HCV RNA from cytoplasmic sensors for viral RNA, and as such a key role of miR-122 in HCV infection may be in masking the vulnerable 5' terminal sequences of HCV from the innate cell immune response, a mechanism which is not related to HCV replication per se (Machlin et al., "Masking the 5' terminal nucleotides of the hepatitis C virus genome by an unconventional microRNA-target RNA complex". PNAS. 201 1 Feb 22;108(8):3193- 3198).
- Miravirsen (SPC3649) is the first therapeutic agent targeting a microRNA in clinical trials.
- Miravirsen is designed to specifically sequester miR-122 and has been shown to have activity in reducing viremia. Sequestering makes miR-122 unavailable, and may therefore unmask the 5'terminus of the HCV genome, allowing the cells' innate immunity access to the HCV virus.
- Miravirsen is a 15 nucleotide (15-mer) phosphorothioate oligonucleotide, consisting of beta-D-oxy-LNA (Locked Nucleic Acids) and DNA monomers respectively.
- the miravirsen molecule contains 8 LNA nucleotides and 7 DNA nucleotides arranged in the following sequence: 5'- m C c A 1 1 G T c a m C a m C t m C m C -3' (Capital letters denote beta-Doxy LNA modified nucleotides ( m C stands for LNA-5-Methyl-Cytidine) and lower case letters denote DNA nucleotides, all internucleoside linkages are phosphorothioate).
- the present invention relates to the use of LNA antisense oligonucleotide inhibitors targeting microRNA-122 for use in improving (such as e.g. lowering) blood liver function biomarkers in subjects which have or may have impaired liver function.
- the invention relates to the use of miravirsen, a drug being developed specifically for treatment of HCV, for use in the treatment of other hepatic diseases.
- the present invention is based upon the discovery that in human subjects with chronic HCV infection, administration of miravirsen at pharmacologically relevant doses produced a notable reduction in liver toxicity biomarkers associated with miravirsen treatment.
- liver transaminase enzymes whose presence in serum is widely used as a non-invasive test to assess liver function or vitality.
- ALT, AST and GGT level were most surprising as it was not always associated with a reduction in viremia, indicating that, in human subjects, there is an alternative HCV independent mechanism which results in improved liver function (as indicated by improved ALT, AST and GGT serum marker levels). This observation could not have been predicted from the pre-clinical non-human primate studies in which there was no general reduction in liver function biomarkers. Furthermore, no reduction of ALT or AST was identified in phase 1 clinical trials using healthy subjects.
- liver function biomarker scores was not correlated to reduction in HCV viremia, indicating that, in human subjects, miravirsen has a pronounced and independent effect on improving liver function in individuals who have general hepatitis
- HCV viremia disorders/symptoms, not necessarily related to HCV viremia. Indeed, reduction of blood serum biomarkers, ALT, AST and GGT was found in all dosage groups, and occurred at time points prior to and frequently independent of reduction in HCV titer.
- the invention provides a LNA antisense oligonucleotide inhibitor of microRNA-122 for use in a. improving (e.g. lowering) blood serum biomarkers of liver function in a human subject in need of improved liver function; and/or
- the invention provides a LNA antisense oligonucleotide inhibitor of microRNA-122 for use in the preparation of a medicament for
- liver function may be determined for example, by assessing blood serum biomarkers, such as miR-122, ALT, AST and/or GGT and/or for example via liver biopsy.
- blood serum biomarkers such as miR-122, ALT, AST and/or GGT and/or for example via liver biopsy.
- the blood serum biomarkers may, for example, be independently selected from the group consisting of ALT, AST and GGT, and as described herein, combinations thereof.
- microRNA-122 has been identified as a blood serum biomarker of necroinflammation (Bihrer et al., The American Journal of Gastroenterology, 201 1 , Su et al., "Serum MicroRNA-122 Level Correlates with Virologic Responses to Combination Therapy in Chronic Hepatitis C Patients" AASLD November 201 1 ).
- the invention provides LNA antisense oligonucleotide inhibitors targeting microRNA-122 for use in the treatment of necroinflammation.
- the invention relates to a LNA antisense oligonucleotide inhibitor of microRNA-122 for use in the preparation of a medicament for necroinflammation.
- the invention relates to a method of reducing the level of blood serum biomarkers of liver function in a human subject who is not infected with HCV and who is in need of improved liver function, said method comprising administering an effective dose of a LNA antisense oligonucleotide inhibitor of microRNA-122, such as miravirsen, to said human subject so as to reduce the level of blood serum biomarkers in the human subject.
- a LNA antisense oligonucleotide inhibitor of microRNA-122 such as miravirsen
- the invention relates to a method of preventing loss of liver function in a human subject who is not infected with HCV but is at risk of deteriorating liver function, said method comprising: administering an effective dose of a LNA antisense oligonucleotide inhibitor of microRNA- 122, such as miravirsen, to said human subject so as to preventing loss of liver function in the human subject.
- a LNA antisense oligonucleotide inhibitor of microRNA- 122 such as miravirsen
- the invention relates to a method of improving liver function in a human subject who is not infected with HCV, said method comprising administering an effective dose of a LNA antisense oligonucleotide inhibitor of microRNA-122, such as miravirsen, to said human subject so as to improve liver function in the human subject.
- a LNA antisense oligonucleotide inhibitor of microRNA-122 such as miravirsen
- the invention relates to a method of treating necroinflammation in a human subject who has or is suspected of having necroinflammation, said method comprising administering an effective dose of a LNA antisense oligonucleotide inhibitor of microRNA-122, such as miravirsen, to said human subject.
- a LNA antisense oligonucleotide inhibitor of microRNA-122 such as miravirsen
- the invention relates to a method of reducing the level of blood serum biomarkers of liver function in a human subject who is in need of improved liver-function, said method comprising
- a Determining the level of one or more blood serum biomarkers for liver function from the human subject to identify the subject is in need of improved liver function b. administering an effective dose of a LNA antisense oligonucleotide inhibitor of microRNA-122 , such as miravirsen, to said human subject so as to improve liver function in the human subject
- Figure 1 Dose-related increases in ALT in cynomologus monkeys illustrates a class effect of accumulation of oligonucleotides in the liver, were slightly more marked in females and almost reversible after the 12-week treatment-free period.
- FIG. 2 Treatment of HCV-infected chimpanzees with miravirsen was well tolerated.
- A Plasma trough levels of miravirsen.
- B alanine aminotransferase (ALT) levels in HCV- infected chimpanzees treated with miravirsen during the Lanford 2009 study.
- ALT alanine aminotransferase
- Figure 3 SPC3649-203 study ALT by treatment cohort (mean, U/L).
- Figure 4 SPC3649-203 study AST by treatment cohort (mean, U/L).
- FIG. 5 SPC3649-203 study GGT by treatment cohort (mean, U/L).
- FIG. 6 SPC3649-203 study LDH by treatment cohort (mean, U/L).
- Figure 7 Improvement in ALT serum level preceeds the reduction in HCV viremia in a human subject treated with 7mg/kg miravirsen.
- liver function Numerous serum biomarkers of liver function are known, by way of a non-limited example the following have been implicated as markers or likely markers of liver function: Lectin- reactive alpha fetoprotein (AFP-L3), Des-gamma-carboxy-prothrombin (DCP), ER6Q, Vimentin, actin alpha 1 skeletal muscle protein, hMFAP 4, tropomyosin, PTGES 2, amyloid P component, transgelin, calponin 1 , homo sapiens p20 protein, 17 kDa myosin light chain, H chain H Igg B12, prolyl 4-hydroxylase, beta subunit methylenetetrahydrofolate
- dehydrogenase 1 PR02619, aldehyde dehydrogenase 1 , fibrinogen alpha chain preproprotein, fructose-bisphosphate aldolase B, argininosuccinate synthetase, Eefla2, AT P 5 Al, alpha-2 actin, regucalcin, serum albumin, mitochondrial malate dehydrogenase, mitochondrial acetoacetyl-CoA thiolase, Hyaluronic Acid (HA), Hepascore, Prothrombin, Gamma Glutamyl Transpeptidase, Apolipoprotein A1 (PGA) index, Age platelet (AP) index, Bonacini index, Pohl score, Forns index, Aspartate aminotransferase/Platelets Ratio index (APRI), MP3 (MMP1 , PIINP) index, FIB4,and Fibrolndex.
- the blood serum biomarkers are typically measured in blood serum (or plasma) samples obtained from the subject (i.e. blood serum or blood plasma biomarkers).
- the blood biomarkers are markers of liver function. Typically elevated levels of the blood serum biomarkers are indicative of impaired liver function or vitality.
- Preferred blood serum biomarkers of liver function include for example, gamma
- GTT glutamyltransferase
- ALT alanine amino transferase
- AST aspartate amino transferase
- the blood biomarkers include AST; or GGT; or ALT; or ALT and AST; or ALT, AST and GGT; or AST & GGT; or ALT and GGT.
- microRNA-122(miR-122) may be used as the blood biomarker, either alone or in addition to the above sets of biomarkers.
- the blood biomarkers comprise of consist of miR-122 and ALT; or miR-122 and AST; or miR-122 and GGT; or miR-122 and ALT/AST ratio.
- alanine amino transferase also known as Alanine Transaminase (ALT) or serum glutamic pyruvic transaminase (sGPT)
- ALT Alanine Transaminase
- sGPT serum glutamic pyruvic transaminase
- ALT mediates conversion of major intermediate metabolites, catalyzing reversible transamination between alanine and oketoglutarate to form pyruvate and glutamate.
- ALT is widely distributed in many tissues but is found in greatest abundance in the liver.
- the major role of ALT in the liver is the conversion of alanine to glucose which is then exported to the body to be utilized in a multitude of processes.
- ALT Measurement of ALT activity is generally carried out by monitoring the rate of NADH oxidation in a coupled reaction system employing lactate dehydrogenase
- LDH Low Density Lipoprotein
- ALT activity is rate limiting, the rate decrease is directly proportional to the ALT activity in the sample.
- a protocol for measuring ALT may be the Advia Chemistry Systems ALT assay (03815151 Rev. B 2007-05) hereby incorportated by reference.
- the level of ALT in the blood (serum/plasma) indicative of impaired liver function is (e.g. when using the Advia Chemistry Systems assay) >69 IU/L, such as >70IU/L, such as >75IU/L, such as >80IU/L, such as >90IU/L, such as >100IU/L, such as >1 10IU/L, such as >120IU/L, such as >130IU/L, such as >150IU/L, such as >200IU/L.
- the level of ALT in the blood (serum/plasma) indicative of impaired liver function >1 .3x normal, such as >1.4x normal, such as >1.5x normal, such as > 2x normal, such as >3x normal, such as >4x normal, such as >5x normal.
- Normal is, as defined herein, refers to the upper range of normal.
- ASAT Aspartate aminotransferase
- AST Aspartate Transaminase
- SGOT serum glutamic oxaloacetic transaminase
- PDP pyridoxal phosphate
- AST catalyzes the reversible transfer of an a-amino group between aspartate and glutamate and, as such, is an important enzyme in amino acid metabolism.
- AST is found in the liver, heart, skeletal muscle, kidneys, brain, and red blood cells, and it is commonly measured clinically as a marker for liver health.
- AST activity assay is based on the quantification of oxaloacetate produced by AST.
- oxaloacetate and NADH are converted to malate and NAD by the enzyme malate dehydrogenase.
- the decrease in NADH absorbance at 340 nm is proportionate to AST activity.
- the level of AST in the blood (serum/plasma) indicative of impaired liver function is (e.g. when using the Advia Chemistry Systems assay) >50 IU/L, such as >55IU/L, such as >60IU/L, such as >65IU/L, such as >70IU/L, such as >75IU/L, such as >80IU/L, such as >85IU/L, such as >90IU/L, such as >95IU/L, such as >100IU/L.
- the level of AST indicative of impaired liver function is >1 .3x normal, such as >1.4x normal, such as >1.5x normal, such as > 2x normal, such as >3x normal, such as >4x normal, such as >5x normal.
- Normal is, as defined herein, refers to the upper range of normal.
- Gamma-glutamyl transpeptidase (also ⁇ -glutamyltransferase, GGT, GGTP, gamma-GT) is an enzyme that transfers gamma-glutamyl functional groups. It is found in many tissues, the most notable one being the liver. GGT catalyzes the transfer of the gamma-glutamyl moiety of glutathione to an acceptor that may be an amino acid, a peptide or water (forming glutamate). GGT plays a key role in the gamma-glutamyl cycle, a pathway for the synthesis and degradation of glutathione and drug and xenobiotic detoxification.
- the assay generally measures the cleavage of a specific GGT substrate (g- glutamyl-p-ntiroanilide) by the enzyme.
- the production of the p-nitroaniline (pNA) product, measured at 405 nm, is proportional to the level of GGT enzyme in the sample.
- the level of GGT in the blood (serum/plasma) indicative of impaired liver function is >1 .5x normal, such as > 2x normal, such as >3x normal, such as >4x normal, such as >5x normal.
- the indicative level of GGT for a female with impaired liver function is > 60IU/L, such as >70IU/L, such as >80IU/L, such as >90IU/L such as >100IU/L.
- the indicative level of GGT for a male impaired liver function is > 100IU/L, such as >1 10IU/L, such as >120IU/L, such as >130IU/L such as >140IU/L.
- kits and reagents are commercially available (e.g.
- microRNA-122 microRNA-122 is a microRNA which has been reported as liver specific (Lagos- Quintana et al (2002) Current Biol vol 12 pp 735-739) and is a distinct host cellular factor which is reported to be essential for HCV replication in hepatocytes (Jopling et al 2005).
- miR-122 exists in numerous forms, including the mature microRNA (SEQ ID NO 2) and precursors thereto, such as the sequence shown in SEQ ID NO 1 .
- the mature microRNA is known to exist in two forms, one 22nt microRNA sequence shown in SEQ ID NO 2, and a further form where there is an additional 3' U residue.
- the miR-122 seed sequence refers to nucleosides 2 through 8 from the
- the sequence of the human miR-122 sequence, hsa miR-122 is as follows:
- the subject has impaired, i.e. not normal (abnormal), liver function.
- normal refers to the central portion (95%) of the normal distribution (i.e. within the "normal range”).
- the upper limit cut off for normal (95%) level is referred to as the "upper limit of normal.” It should be recognized that the precise upper limit of normal can vary, e.g. depending upon the reference normal population, gender, age, and in some cases, the specific assay conditions used.
- normal liver function may be determined by the comparison to reference samples or values from a population of subjects with normal liver function.
- a subject whose blood serum biomarker levels are determined to be above the upper limit of normal may in some embodiments, or in general, be a subject who is in need to improved liver function.
- determination of normal liver function may be determined by selection of suitable pre-determined cut-of values for one or more of the biomarker levels, as described herein.
- the assays used to determine the specific cut off values are identified herein as the Advia Chemistry ALT, AST and GGT protocols. Non-limiting examples of such cut off values are provided herein.
- the normal range may be considered as being below the levels which are associated with healthy liver function, or cut off values provided herein. Levels above the cut off values may therefore be indicative of a subject who is in need of improved liver function.
- the subject prior to treatment, may have an ALT, AST, and/or GGT blood serum level which is above the upper limit of normal (elevated blood biomarkers).
- the level of blood biomarker(s) are assessed prior to treatment with the LNA antisense oligonucleotide inhibitor of microRNA-122, e.g. with miravirsen, such as within about 6 months, such as within about 5 months, such as within about 4 months, such as within about 3 months, such as within about 2 months, such as within about 1 month, such as within about 4 weeks, such as within about 3 weeks, such as within about 2 weeks, such as within about 1 week, such as within about 1 , 2, 3, 4, 5 or 6 days, prior to the first (or in some embodiments subsequent) administration with the microRNA-122 inhibitor.
- the assessment of the level of blood biomarkers involves the sequential steps of i) obtaining a blood sample from the human subject infected with HCV and, ii) determining the level of at least one biomarker in the blood sample.
- the subject is a human being who has been diagnosed with, or is suspected of having a impaired liver function or has been diagnosed with or is suspected of having a disease or disorder which results in or may result in impairment of hepatic function, or is otherwise in need of improved liver function or prevention of deterioration of liver function.
- the subject is typically not a normal healthy subject.
- the subject has or is suspected of having necroinflammation.
- such a subject may or may not have HCV infection, such as chronic HCV infection.
- the invention provides for a method of concurrent treatment of chronic HCV infection and
- the invention provides for a method of concurrent treatment of chronic HCV infection and improvement of liver function (or vitality), in a subject who is infected with HCV and has or is suspected of being in need of improved liver function, said method comprising the step of administering a (therapeutically) effective amount of the LNA antisense oligonucleotide inhibitor of microRNA-122 to the subject.
- the invention provides for a method of concurrent treatment of chronic HCV infection and improvement of blood serum biomarkers of liver function (e.g. ALT, AST and/or GGT), in a subject who is infected with HCV and is in need of improved blood serum biomarkers of liver function, said method comprising the step of administering a
- blood serum biomarkers of liver function e.g. ALT, AST and/or GGT
- the subject prior to treatment has a need for improved liver function.
- the subject, prior to treatment has impaired liver function.
- the human subject has been diagnosed with a disease or disorder selected from the group consisting of hepatitis B and hepatitis D.
- the human subject has been diagnosed with a disease or disorder selected from the group consisting of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis.
- the human subject has been diagnosed with a disease or disorder selected from the group consisting of cytomegalovirus infection, schistosomiasis infection and Leptospirosis infection.
- the subject is in need of prevention of loss of liver function.
- loss need not be full loss, but is typically used to refer to a partial loss in liver function, as determined, for example, by elevated blood biomarkers (as described herein), or by evaluation by liver biopsy.
- blood biomarkers or liver biopsy may be used in the assessment of, for example, the need for improved liver function or the treatment of impaired liver function.
- Biomarker assays/liver biopsies may also be used subsequent to or during treatment to monitor the improvement of liver function.
- Percutaneous liver biopsy is associated with potential complications, including bleeding (1 %-3%), pain (20%-30%), bile peritonitis ( ⁇ 1 %), pneumothorax( ⁇ 1 %), punctured viscera ( ⁇ 1 %), and death. It is preferred that the liver function of the subject is determined (e.g. prior to, during or after treatment, by assessment of blood biomarkers for liver function, such as those referred to herein, rather than the invasive liver biopsy).
- the subject has been diagnosed with or is suspected of having liver fibrosis or cirrhosis. In some embodiments, the subject has been diagnosed with necroinflammation or is suspected of having necroinflammation.
- liver biopsy an invasive and painful procedure which is widely used in the United States to provide information on the state of the liver and prognostic information for future disease progression (Comag and McHutchinson, AGA Vol 130, pp 231-264).
- Cirrhosis 4 6 Percutaneous liver biopsy is associated with potential complications, including bleeding (1 %-3%),pain (20%-30%), bile peritonitis ( ⁇ 1 %), pneumothorax( ⁇ 1 %), punctured viscera ( ⁇ 1 %), and death.
- Hepatitis is a medical condition defined by the inflammation of the liver and characterized by the presence of inflammatory cells in the tissue of the organ.
- the condition can be self- limiting (healing on its own) or can progress to fibrosis (scarring) and cirrhosis.
- Hepatitis may occur with limited or no symptoms, but often leads to jaundice, anorexia (poor appetite) and malaise.
- Hepatitis is acute when it lasts less than six months and chronic when it persists longer. Acute hepatitis typically lasts less than 2 months.
- Acute hepatitis may, for example be associated with liver damage caused by various poisons (e.g. carbon tetrachloride) or by certain drug overdoses. It is envisaged that miravirsen treatment may be unsuitable for treatment of acute hepatitis where the duration of the disease is less than 2 months. In some embodiments the hepatitis is sub-acute hepatitis, i.e. lasts between 2 - 6 months. It will be recognized that the type of hepatitis (acute, sub-acute or chronic) may be diagnosed either by the length of time since diagnosis and maintenance of the diseased state, or in some instances at the time of diagnosis (for example in the case of certain drug overdose or poisoning.
- hepatitis viruses cause most cases of hepatitis worldwide, but it can also be due to toxins (notably alcohol, certain medications, some industrial organic solvents and plants), other infections and autoimmune diseases.
- the hepatitis is not hepatitis C (HCV).
- the hepatitis is chronic hepatitis, which may be other than chronic HCV.
- Chronic hepatitis may, for example, be caused by viral infection: Hepatitis B, hepatitis C, hepatitis D may cause chronic or acute hepatitis, other causes of chronic hepatitis include autoimmune hepatitis, alcohol induced hepatitis, certain drugs may induce chronic hepatitis (e.g. methyldopa, nitrofurantoin, isoniazid and ketoconazole).
- the chronic hepatitis is steatohepatitis, such as non-alcoholic steatohepatitis.
- the hepatitis is infectious hepatitis, for example hepatitis B and D.
- HBV and HDV may occur in chronic or acute forms.
- Hepatitis B is an infectious illness caused by hepatitis B virus (HBV) which infects the liver and causes an inflammation called hepatitis. More than 2 billion people have been infected with the hepatitis B virus, and this includes 350 million chronic carriers of the virus.
- HBV hepatitis B virus
- Transmission of hepatitis B virus results from exposure to infectious blood or body fluids, such as semen and vaginal fluids, while viral DNA has been detected in the saliva, tears, and urine of chronic carriers with high titer DNA in serum. Perinatal infection is a major route of infection in endemic (mainly developing) countries.
- Acute hepatitis Approximately 70 percent of patients with acute hepatitis B have subclinical or anicteric hepatitis, while 30 percent develop icteric hepatitis. Fulminant hepatic failure is unusual, occurring in approximately 0.1 to 0.5 percent of patients.
- the method of acquiring HBV infection varies geographically. Perinatal transmission and occasionally horizontal transmission early in life are most common in high prevalence areas such as Southeast Asia and China, while sexual contact and percutaneous transmission (e.g., intravenous drug use) are most common in the United States, Canada, and Western Europe.
- the incubation period lasts one to four months.
- a serum sickness-like syndrome may develop during the prodromal period, followed by constitutional symptoms, anorexia, nausea, jaundice, and right upper quadrant discomfort.
- the symptoms and jaundice generally disappear after one to three months, but some patients have prolonged fatigue even after normalization of serum aminotransferase concentrations.
- ALT and AST alanine and aspartate aminotransferase levels
- the serum bilirubin concentration may be normal in patients with anicteric hepatitis. In patients who recover, the normalization of serum aminotransferases usually occurs within one to four months. A persistent elevation of serum ALT for more than six months indicates a progression to chronic hepatitis.
- HBV HBV-specific cytotoxic T cells
- the rate of progression from acute to chronic hepatitis B is determined primarily by the age at infection. The rate is approximately 90 percent for a perinatally-acquired infection, 20 to 50 percent for infections between the age of one and five years, and less than 5 percent for an adult-acquired infection.
- a history of acute hepatitis is elicited in only a small percentage of patients with chronic HBV infection. In low or intermediate prevalence areas, approximately 30 to 50 percent of patients with chronic HBV infection have a past history of acute hepatitis; such a history is lacking in the remaining patients in these areas and in the majority of patients in high prevalence areas (predominantly perinatal infection). Many patients with chronic hepatitis B are asymptomatic (unless they progress to decompensated cirrhosis or have extrahepatic manifestations), while others have nonspecific symptoms such as fatigue. Some patients experience exacerbations of the infection which may be asymptomatic, mimic acute hepatitis, or manifest as hepatic failure. Physical examination may be normal, or there may be stigmata of chronic liver disease.
- Jaundice, splenomegaly, ascites, peripheral edema, and encephalopathy may be present in patients with decompensated cirrhosis. Laboratory tests may be normal, but most patients have a mild to moderate elevation in serum AST and ALT. During exacerbations, the serum ALT concentration may be as high as 50 times the upper limit of normal, and alfa-fetoprotein (AFP) concentrations as high as 1000 ng/mL may be seen.
- AFP alfa-fetoprotein
- a progression to cirrhosis is suspected when there is evidence of hypersplenism (decreased white blood cell and platelet counts) or impaired hepatic synthetic function
- Hepatitis D also referred to as hepatitis D virus (HDV) and classified as Hepatitis delta virus
- HDV hepatitis D virus
- HBV hepatitis B virus
- hepatitis D has the highest mortality rate of all the hepatitis infections of 20%.
- HDV hepatitis B core antigen
- Patients who are currently referred for HDV infection appear to represent cohorts infected years ago in whom the HDV-related disease rapidly developed to cirrhosis, but whose subsequent disease progression has been slow.
- Non-alcoholic fatty liver disease is one cause of fat accumulation (steatosis) in the liver not due to excessive alcohol use. It is related to insulin resistance and the metabolic syndrome and may respond to treatments originally developed for other insulin-resistant states.
- Nonalcoholic steatohepatitis is the term used to describe the distinct clinical entity in which patients lack a history of significant alcohol consumption but have liver biopsy findings indistinguishable from alcoholic steatohepatitis. The following criteria have been utilized for the diagnosis of NASH:
- NAFLD nonalcoholic fatty liver disease
- NAFLD liver disease
- NASH is frequently associated with obesity, type 2 diabetes mellitus, and hyperlipidemia. Most patients with NASH are asymptomatic although fatigue, malaise, and vague right upper abdominal discomfort bring some patients to medical attention. The most common presentation is elevation of liver aminotransferases detected on routine laboratory testing. Hepatomegaly is common. Serum AST and ALT are elevated in almost 90 percent of patients [12]. The AST/ALT ratio is usually less than 1 ; this is much lower than the ratio in alcoholic hepatitis, which is usually above 2. Alkaline phosphatase is less frequently elevated and hyperbilirubinemia is uncommon.
- DIAGNOSIS Various radiological methods can detect the presence of fat in the liver but no imaging modality is able to differentiate between the histological subtypes of relatively benign nonalcoholic hepatic steatosis or more aggressive NASH. Liver biopsy provides the definitive diagnosis. Patients with NAFLD have slightly lower overall survival than expected for the general population. Higher mortality is associated with increasing age, impaired fasting glucose, and cirrhosis. In most patients, there is little change in liver function tests throughout the course of the disease, although there may be histological progression to fibrosis and cirrhosis.
- Cytomegalovirus is a viral genus of the viral group known as Herpesviridae or
- herpesviruses The species that infects humans is commonly known as human CMV
- CMV human herpesvirus-5
- HMV human herpesvirus-5
- All herpesviruses share a characteristic ability to remain latent within the body over long periods. Although they may be found throughout the body, CMV infections are frequently associated with the salivary glands in humans and other mammals.
- CMV infections are frequently associated with the salivary glands in humans and other mammals.
- the spectrum of human illness caused by cytomegalovirus (CMV) is diverse and mostly dependent on the host-CMV infections in immunocompromised patients cause substantial morbidity and mortality, especially among transplant recipients and those infected with the human immunodeficiency virus (HIV). Infection in the immunocompetent host is generally asymptomatic or may present as a mononucleosis syndrome. However, occasionally primary CMV infection can lead to severe organ specific complications with significant morbidity and mortality.
- CMV establishes latent infection after the resolution of acute infection. Secondary, symptomatic disease may present later in the life of the host, reflecting one of two possibilities: reactivation of latent CMV or reinfection with a novel exogenous strain. Reactivation of CMV may occur at any time during the life of the human host, although the risk is higher in the setting of systemic immunosuppression, either iatrogenic or secondary to underlying medical conditions, such as the acquired
- AIDS immunodeficiency syndrome
- the bulk of CMV-related disease in immunocompetent hosts is related to primary infection.
- the proportion of humans with evidence of prior CMV infection varies throughout the world, with seroprevalence rates ranging between 40 to 100 percent of the adult population.
- the prevalence of CMV-specific antibody increases with age.
- a syndrome resembling infectious mononucleosis is the most common presentation of symptomatic CMV infection in immunocompetent adults.
- Two cardinal hematologic abnormalities help to define the syndrome of mononucleosis: an absolute lymphocytosis with greater than 50 percent mononuclear cells and the presence of more than 10 percent atypical lymphocytes on peripheral blood smear.
- Liver function abnormalities are frequently encountered in patients with symptomatic CMV infection.
- Subclinical transaminitis is the most common finding in immunocompetent patients; elevations of alkaline phosphatase and total bilirubin are less typical. Occasionally, patients will present with
- Schistosomiasis is a parasitic disease caused by several species of trematodes
- Schistosomiasis is a chronic illness that can damage internal organs and, in children, impair growth and cognitive development. Schistosomiasis is the second most socioeconomically devastating parasitic disease after malaria. This disease is most commonly found in Asia, Africa, and South America, especially in areas where the water contains numerous freshwater snails, which may carry the parasite. The disease affects many people in developing countries, particularly children who may acquire the disease by swimming or playing in infected water. Schistosomiasis can be associated with serious morbidity and mortality. Chronic complications are generally seen in those with a high parasite load, which usually occurs in individuals who live in endemic areas and have recurrent exposure.
- schistosomiasis can also cause complications in people with even brief exposures, such as travelers. It is estimated that more than 200 million people worldwide have schistosomiasis and that the infection is responsible for more than 200,000 deaths annually. On a global scale, 1 in every 30 individuals has schistosomiasis. Schistosomiasis is associated with significant morbidity including anemia, chronic pain, diarrhea, exercise intolerance, malnutrition, bladder cancer, portal hypertension and CNS complications.
- Inflammatory hepatic schistosomiasis is the main cause of hepatomegaly and severe splenomegaly in children and adolescents. The severity of disease is related to the intensity of the egg infestation.
- Leptospirosis is a disease caused by infection with bacteria of the genus Leptospira, and affects humans as well as other mammals, birds, amphibians, and reptiles. Though recognised among the world's most common diseases transmitted to people from animals, leptospirosis is nonetheless a relatively rare bacterial infection in humans. The infection is commonly transmitted to humans by allowing water that has been contaminated by animal urine to come in contact with unhealed breaks in the skin, the eyes, or with the mucous membranes. In the United States, most cases are reported from the southern and Pacific coastal states. Hawaii consistently reports the most cases of any state. Worldwide, endemic disease occurs in the tropics. The incidence of leptospirosis in some endemic countries appears to be increasing. Humans most often become infected after exposure to
- Leptospirosis is associated with a variable clinical course. The disease may manifest as a subclinical illness followed by seroconversion, a self-limited systemic infection, or a severe, potentially fatal illness accompanied by multiorgan failure. Leptospirosis presents with the abrupt onset of fever, rigors, myalgias and headache in 75 to 100 percent of patients, after an incubation period of two to 26 days (average 10 days).
- the LNA antisense oligonucleotide also referred to as an oligomer herein, may comprise at least 6, such as at least 7 consecutive nucleobases which are complementary to a part of a miR-122 sequence, such as the mature hsa-miR-122 sequence.
- the LNA antisense oligonucleotide may therefore comprise the complement of the miR-122 seed region.
- the LNA antisense oligonucleotide comprises at least one LNA monomer and typically at least 30%, such as at least 40%, such as at least 50% of the nucleoside monomers in the oligonucleotide are LNA oligonucleotides.
- Efficacy of a microRNA-122 inhibitor may be determined by measuring the effect on secondary or tertiary indices for microRNA-122 activity in vivo. For example, de-repression of known microRNA-122 mRNA targets
- inhibitors of microRNA-122 which may be effective in treatment of HCV in vivo typically are highly effective in de-repressing mRNA targets (e.g.
- the antimiR-122 oligonucleotide is designed as a mixmer or totalmer that is essentially incapable of recruiting RNAseH.
- Oligonucleotides that are essentially incapable of recruiting RNAseH are well known in the literature, in example see WO2007/1 12754, WO2007/1 12753, or WO2009/043353.
- Such compounds include LNA oligonucleotides which do not comprise a region of 5 or more consecutive DNA nucleosides, and is some instances do not comprise a region of 4 or more consecutive DNA nucleotides, and in some instances do not comprise a region of 3 or more consecutive DNA nucleotides.
- the LNA antisense oligonucleotide does not comprise a region of 5 or more non-LNA nucleosides, and is some instances do not comprise a region of 4 or more consecutive non-LNA nucleotides, and in some instances do not comprise a region of 3 or more consecutive non-LNA nucleotides. In some embodiments all the LNA nucleoside monomers present in the LNA antisense
- the oligonucleotide are beta-D-oxy LNA monomers.
- the LNA-antisense oligonucleotide inhibitor or microRNA-122 does not comprise 2' substituted nucleoside monomers, such as 2' MOE nucleoside monomers.
- the LNA- antisense oligonucleotide inhibitor or microRNA-122 is not one of compounds ISIS 387082, ISIS 387083, ISIS 387574, ISIS 387575, ISIS 387581 , ISIS 396604, ISIS 396605 and ISIS 387582, such as is not ISIS 387574, as disclosed in WO2007/027775 (specifically hereby incorporated by reference).
- the LNA-antisense oligonucleotide inhibitor or microRNA-122 does not have a nucleoside motif of 5'-(A-A-B)n(-A)nn-3' wherein A is 2'MOE and B is LNA, and n is 6 to 7 and nn is 0 to 2, such as (A-A-B)7(-A-A)1 .
- the antisense oligonucleotide has a length of 7 - 25 (contiguous) nucleotides, such as 8, 9, 10, 1 1 , 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 , 22, 23, or 24 (contiguous) nucleotides. In some embodiments, the antisense oligonucleotide has a length of 7 - 10 (contiguous) nucleotide, or in some instances 7 - 16 nucleotides.
- the antisense oligonucleotide at least 8 (contiguous) nucleotides in length, between 10-17 or 10 - 16 or 10-15 (contiguous) nucleotides, such as between 12 - 15 (contiguous) nucleotides.
- the oligomer is (essentially) incapable of recruiting RNaseH.
- EP 1 222 309 provides in vitro methods for determining RNaseH activity, which may be used to determine the ability to recruit RNaseH.
- a oligomer is deemed capable of recruiting RNase H if, when provided with the complementary RNA target, it has an initial rate, as measured in pmol/l/min, of at least 0.5%, such as at least 1 %, such as at least 5%, such as at least 10% or less than 20% of the equivalent DNA only oligonucleotide, with no 2' substitutions, with phosphorothioate linkage groups between all nucleotides in the oligonucleotide, using the methodology provided by Example 91 - 95 of EP 1 222 309.
- an oligomer is deemed essentially incapable of recruiting RNaseH if, when provided with the complementary RNA target, and RNaseH, the RNaseH initial rate, as measured in pmol/l/min, is less than 1 %, such as less than 5%, such as less than 10% or less than 20% of the initial rate determined using the equivalent DNA only oligonucleotide, with no 2' substitutions, with phosphorothioate linkage groups between all nucleotides in the oligonucleotide, using the methodology provided by Example 91 - 95 of EP 1 222 309.
- the complementary RNA target may be longer that the olgiomer to allow for easy detection of RNaseH activity.
- oligonucleotides which are mixmers or totalmers are usually essentially incapable of recruiting RNAseH and as such where we use the term essentially incapable or recruiting RNaseH herein, in some embodiments, such a term may be replaced with the term mixmer or totalmer, as defined herein.
- the oligomer may, in some embodiments, be either i) fully complementary to either the full microRNA-122 sequence, such as the mature miR-122 sequence, or ne complementary to a sub-sequence of contiguous nucleotides present in the miRNA-122 target, or ii) comprises no more than a single mismatch with the complement of a sub-sequence of contiguous nucleotides present in said RNA target.
- the oligonucleotide is an antisense oligonucleoitde - in that it is either fully complementary to the corresponding region of the target sequence, or comprises no more than a single mismatch with the corresponding region of the target sequence.
- the oligomer may therefore be an oligomer which targets (i.e. comprises or consists of a contiguous nucleotide sequence which is fully complementary to (a corresponding region of) microRNA-122 or comprises of no more than a single mismatch thereto.
- targets i.e. comprises or consists of a contiguous nucleotide sequence which is fully complementary to (a corresponding region of) microRNA-122 or comprises of no more than a single mismatch thereto.
- the oligonucleotides may be referred to as anti-microRNA-122 oligonucleotides.
- the LNA antisense oligonucleotide comprises only LNA and optionally also DNA nucleosides monomers.
- the LNA oligonucleotides comprise at least one phosphorothioatelinkage and may be fully phosphorothiolated - i.e. all
- internucleside linkages are phosphorothioate linkages.
- shorter LNA oligonucleotides for example compounds of 7, 8 or 9 nucleotides in length, it is preferred that at least 70% of the nucleosides present are LNA nucleosides, such as all nucleosides are LNA nucleosides.
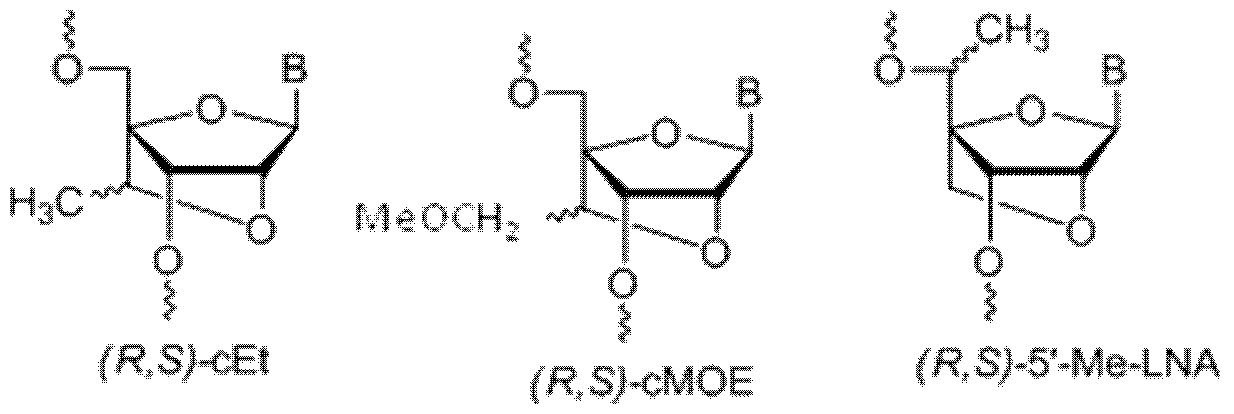
- Such short LNA oligonucleotides may comprise a sequence which is complementary to the miR-122 seed sequence. Examples of short LNA oligonucleotides which target miR-122 are provided in WO2009/043353. It some embodiments, the LNA monomers are beta-D-oxy LNA ( 4'-CH 2 -0-2' Bicyclic Nucleic Acid). Examples of modulators of microRNA-122 useful in the invention
- Specially preferred compounds for use in the present invention are those that target microRNA-122.
- the sequence of miR-122 can be found in the microRNA database
- the antisense oligomer is miravirsen (SPC3649) which has the
- a lowercase letter identifies a DNA unit
- an upper case letter identifies a LNA unit
- m C identifies a 5-methylcytosine LNA
- subscript s identifies a phosphorothioate internucleoside linkage
- LNA units are beta-D-oxy, as identified by a ° superscript after LNA residue.
- Miravirsen is the first microRNA-targeted drug to enter clinical trials. Miravirsen may be used as monotherapy or in combination with direct acting antiviral agents as an interferon-free treatment for chronic HCV infection in multiple genotypes.
- oligomer in the context of the present invention, refers to a molecule formed by covalent linkage of two or more nucleotides (i.e. an oligonucleotide).
- a single nucleotide (unit) may also be referred to as a monomer or unit.
- nucleoside a sequence of nucleotides or monomers
- sequence of bases such as A, T, G, C or U.
- the oligomer typically consists or comprises of a contiguous nucleotide sequence of from 7 - 25 units.
- the compound of the invention does not comprise RNA (units). It is preferred that the compound according to the invention is a linear molecule or is synthesised as a linear molecule.
- the oligomer is a single stranded molecule, and preferably does not comprise short regions of, for example, at least 3, 4 or 5 contiguous nucleotides, which are complementary to equivalent regions within the same oligomer (i.e. duplexes) - in this regards, the oligomer is not (essentially) double stranded. In some embodiments, the oligomer is essentially not double stranded, such as is not a siRNA. In various aspects, the oligomer is essentially not double stranded, such as is not a siRNA.
- the oligomer of the invention may consist entirely of the contiguous nucleotide region.
- the oligomer is not substantially self-complementary.
- nucleotide analogue and “corresponding nucleotide” are intended to indicate that the nucleotide in the nucleotide analogue and the naturally occurring nucleotide are identical.
- the "corresponding nucleotide analogue” contains a pentose unit (different from 2-deoxyribose) linked to an adenine.
- nucleoside analogue and “nucleotide analogue” are used interchangeably.
- nucleotide refers to a glycoside comprising a sugar moiety, a base moiety and a covalently linked group (linkage group), such as a phosphate or phosphorothioate internucleotide linkage group, and covers both naturally occurring nucleotides, such as DNA or RNA, and non-naturally occurring nucleotides comprising modified sugar and/or base moieties, which are also referred to as “nucleotide analogues" herein.
- a single nucleotide (unit) may also be referred to as a monomer or nucleic acid unit.
- nucleoside is commonly used to refer to a glycoside comprising a sugar moiety and a base moiety, and may therefore be used when referring to the nucleotide units, which are covalently linked by the internucleotide linkages between the nucleotides of the oligomer.
- nucleotide is often used to refer to a nucleic acid monomer or unit, and as such in the context of an oligonucleotide may refer to the base - such as the "nucleotide sequence”, typically refer to the nucleobase sequence (i.e. the presence of the sugar backbone and internucleoside linkages are implicit).
- nucleotide may refer to a "nucleoside” for example the term “nucleotide” may be used, even when specifiying the presence or nature of the linkages between the nucleosides.
- the 5' terminal nucleotide of an oligonucleotide does not comprise a 5' internucleotide linkage group, although may or may not comprise a 5' terminal group.
- Non-naturally occurring nucleotides include nucleotides which have modified sugar moieties, such as bicyclic nucleotides or 2' modified nucleotides, such as 2' substituted nucleotides.
- Nucleotide analogues are variants of natural nucleotides, such as DNA or RNA nucleotides, by virtue of modifications in the sugar and/or base moieties. Analogues could in principle be merely “silent” or “equivalent” to the natural nucleotides in the context of the oligonucleotide, i.e. have no functional effect on the way the oligonucleotide works to inhibit target gene expression. Such "equivalent” analogues may nevertheless be useful if, for example, they are easier or cheaper to manufacture, or are more stable to storage or manufacturing conditions, or represent a tag or label.
- the analogues will have a functional effect on the way in which the oligomer works to inhibit expression; for example by producing increased binding affinity to the target and/or increased resistance to intracellular nucleases and/or increased ease of transport into the cell.
- nucleoside analogues are described by e.g. Freier & Altmann; Nucl. Acid Res., 1997, 25, 4429-4443 and Uhlmann; Curr. Opinion in Drug Development, 2000, 3(2), 293-213, and in Scheme 1 :
- the oligomer may thus comprise or consist of a simple sequence of natural occurring nucleotides - preferably 2'-deoxynucleotides (referred to here generally as "DNA”), but also possibly ribonucleotides (referred to here generally as "RNA”), or a combination of such naturally occurring nucleotides and one or more non-naturally occurring nucleotides, i.e. nucleotide analogues.
- nucleotide analogues may suitably enhance the affinity of the oligomer for the target sequence.
- affinity-enhancing nucleotide analogues in the oligomer can allow the size of the specifically binding oligomer to be reduced, and may also reduce the upper limit to the size of the oligomer before non-specific or aberrant binding takes place.
- the oligomer comprises at least 1 nucleoside analogue. In some embodiments the oligomer comprises at least 2 nucleotide analogues. In some embodiments, the oligomer comprises from 3-8 nucleotide analogues, e.g. 6 or 7 nucleotide analogues. In the by far most preferred embodiments, at least one of said nucleotide analogues is a locked nucleic acid (LNA); for example at least 3 or at least 4, or at least 5, or at least 6, or at least 7, or 8, of the nucleotide analogues may be LNA. In some embodiments LNA may be LNA. In some LNA may be locked nucleic acid
- all the nucleotides analogues may be LNA.
- the oligomers of the invention which are defined by that sequence may comprise a corresponding nucleotide analogue in place of one or more of the nucleotides present in said sequence, such as LNA units or other nucleotide analogues, which raise the duplex stability/T m of the oligomer/target duplex (i.e. affinity enhancing nucleotide analogues).
- any mismatches between the nucleotide sequence of the oligomer and the target sequence are preferably found in regions outside the affinity enhancing nucleotide analogues, such as region B as referred to herein, and/or region D as referred to herein, and/or at the site of non modified such as DNA nucleotides in the oligonucleotide, and/or in regions which are 5' or 3' to the contiguous nucleotide sequence.
- modification of the nucleotide include modifying the sugar moiety to provide a 2'-substituent group or to produce a bridged (locked nucleic acid) structure which enhances binding affinity and may also provide increased nuclease resistance.
- a preferred nucleotide analogue is LNA, such as oxy-LNA (such as beta-D-oxy-LNA, and alpha-L-oxy-LNA), and/or amino-LNA (such as beta-D-amino-LNA and alpha-L-amino- LNA) and/or thio-LNA (such as beta-D-thio-LNA and alpha-L-thio-LNA) and/or ENA (such as beta-D-ENA and alpha-L-ENA). Most preferred is beta-D-oxy-LNA.
- oxy-LNA such as beta-D-oxy-LNA, and alpha-L-oxy-LNA
- amino-LNA such as beta-D-amino-LNA and alpha-L-amino- LNA
- thio-LNA such as beta-D-thio-LNA and alpha-L-thio-LNA
- ENA such as beta-D-ENA and alpha-L-ENA
- nucleotide analogues present within the oligomer of the invention are independently selected from, for example: 2'-0-alkyl-RNA units, 2'-amino-DNA units, 2'-fluoro-DNA units, LNA units, arabino nucleic acid (ANA) units, 2'-fluoro-ANA units, HNA units, INA (intercalating nucleic acid -Christensen, 2002. Nucl. Acids. Res. 2002 30: 4918-4925, hereby incorporated by reference) units and 2'MOE units.
- nucleotide analogues are 2'-0-methoxyethyl-RNA (2'MOE),
- 2'-fluoro-DNA monomers or LNA nucleotide analogues may comprise nucleotide analogues which are independently selected from these three types of analogue, or may comprise only one type of analogue selected from the three types.
- at least one of said nucleotide analogues is 2'-MOE- RNA, such as 2, 3, 4, 5, 6, 7, 8, 9 or 10 2'-MOE-RNA nucleotide units.
- At least one of said nucleotide analogues is 2'-fluoro DNA, such as 2, 3, 4, 5, 6, 7, 8, 9 or 10 2'-fluoro-DNA nucleotide units.
- the oligomer according to the invention comprises at least one Locked Nucleic Acid (LNA) unit, such as 1 , 2, 3, 4, 5, 6, 7, or 8 LNA units, such as from 3 - 7 or 4 to 8 LNA units, or 3, 4, 5, 6 or 7 LNA units.
- LNA Locked Nucleic Acid
- all the nucleotide analogues are LNA.
- the oligomer may comprise both beta-D-oxy- LNA, and one or more of the following LNA units: thio-LNA, amino-LNA, oxy-LNA, and/or ENA in either the beta-D or alpha-L configurations or combinations thereof.
- all LNA cytosine units are 5'methyl-Cytosine.
- the oligomer may comprise both LNA and DNA units.
- the combined total of LNA and DNA units is 10-25, such as 10 - 24, preferably 10-20, such as 10 - 18, even more preferably 12-16.
- the nucleotide sequence of the oligomer such as the contiguous nucleotide sequence consists of at least one LNA and the remaining nucleotide units are DNA units.
- the oligomer comprises only LNA nucleotide analogues and naturally occurring nucleotides (such as RNA or DNA, most preferably DNA nucleotides), optionally with modified internucleotide linkages such as phosphorothioate.
- nucleobase refers to the base moiety of a nucleotide and covers both naturally occuring a well as non-naturally occurring variants. Thus, “nucleobase” covers not only the known purine and pyrimidine heterocycles but also heterocyclic analogues and tautomeres thereof.
- nucleobases include, but are not limited to adenine, guanine, cytosine, thymidine, uracil, xanthine, hypoxanthine, 5-methylcytosine, isocytosine, pseudoisocytosine, 5-bromouracil, 5-propynyluracil, 6-aminopurine, 2-aminopurine, inosine, diaminopurine, and 2-chloro-6-aminopurine.
- At least one of the nucleobases present in the oligomer is a modified nucleobase selected from the group consisting of 5-methylcytosine, isocytosine, pseudoisocytosine, 5-bromouracil, 5-propynyluracil, 6-aminopurine, 2-aminopurine, inosine, diaminopurine, and 2-chloro-6-aminopurine.
- LNA refers to a bicyclic nucleoside analogue, known as “Locked Nucleic Acid”. It may refer to an LNA monomer, or, when used in the context of an "LNA
- LNA refers to an oligonucleotide containing one or more such bicyclic nucleotide analogues.
- LNA nucleotides are characterised by the presence of a linker group (such as a bridge) between C2' and C4' of the ribose sugar ring - for example as shown as the biradical R 4* - R 2* as described below.
- the LNA used in the oligonucleotide compounds of the invention preferably has the structure of the eneral formula I
- asymmetric groups may be found in either R or S orientation ;
- X is selected from -0-, -S-, -N(R N* )-, -C(R 6 R 6* )-, such as, in some
- B is selected from hydrogen, optionally substituted Ci -4 -alkoxy, optionally substituted Ci -4 -alkyl, optionally substituted Ci -4 -acyloxy, nucleobases including naturally occurring and nucleobase analogues, DNA intercalators, photochemically active groups, thermochemically active groups, chelating groups, reporter groups, and ligands; preferably, B is a nucleobase or nucleobase analogue;
- P designates an internucleotide linkage to an adjacent monomer, or a 5'-terminal group, such internucleotide linkage or 5'-terminal group optionally including the substituent R 5 or equally applicable the substituent R 5* ;
- P* designates an internucleotide linkage to an adjacent monomer, or a 3'-terminal group
- each of the substituents R 1* , R 2 , R 3 , R 5 , R 5* , R 6 and R 6* , which are present is independently selected from hydrogen, optionally substituted Ci-i 2 -alkyl, optionally substituted C 2- i2-alkenyl, optionally substituted C 2- i2-alkynyl, hydroxy, Ci-i 2 -alkoxy, C 2- i 2 - alkoxyalkyl, C 2- i2-alkenyloxy, carboxy, Ci-i 2 -alkoxycarbonyl, Ci-i 2 -alkylcarbonyl, formyl, aryl, aryloxy-carbonyl, aryloxy, arylcarbonyl, heteroaryl, heteroaryloxy-carbonyl, heteroaryloxy, heteroarylcarbonyl, amino, mono- and di(Ci -6 -alkyl)amino, carbamoyl, mono- and di(Ci -6 - alkyl)-amin
- R 4* and R 2* together designate a biradical consisting of a groups selected from the group consisting of C(R a R b )-C(R a R b )-, C(R a R b )-0-, C(R a R b )-NR a -, C(R a R b )-S-, and C(R a R b )-C(R a R b )-0-, wherein each R a and R b may optionally be
- R a and R b may be, optionally independently selected from the group consisting of hydrogen and C i- 6 alkyl, such as methyl, such as hydrogen.
- R 4* and R 2* together designate the biradical -0-CH(CH 2 OCH 3 )- (2'0-methoxyethyl bicyclic nucleic acid - Seth at al., 2010, J. Org. Chem) - in either the R- or S- configuration.
- R 4* and R 2* together designate the biradical -0-CH(CH 2 CH 3 )- (2'0-ethyl bicyclic nucleic acid - Seth at al., 2010, J. Org. Chem). - in either the R- or S- configuration.
- R 4* and R 2* together designate the biradical -0-CH(CH 3 )-. - in either the R- or S- configuration. In some embodiments, R 4* and R 2* together designate the biradical -0-CH 2 -0-CH 2 - - (Seth at al., 2010, J. Org. Chem).
- R 4* and R 2* together designate the biradical -0-NR-CH 3 - -
- the LNA units have a structure selected from the following group:
- R 1* , R 2 , R 3 , R 5 , R 5* are independently selected from the group consisting of hydrogen, halogen, Ci_ 6 alkyl, substituted Ci -6 alkyl, C 2-6 alkenyl, substituted C 2-6 alkenyl, C 2-6 alkynyl or substituted C 2-6 alkynyl, Ci -6 alkoxyl, substituted Ci -6 alkoxyl, acyl, substituted acyl, Ci -6 aminoalkyl or substituted Ci -6 aminoalkyl.
- asymmetric groups may be found in either R or S orientation.
- R 1* , R 2 , R 3 , R 5 , R 5* are hydrogen.
- R 1* , R 2 , R 3 are independently selected from the group consisting of hydrogen, halogen, Ci_ 6 alkyl, substituted Ci -6 alkyl, C 2-6 alkenyl, substituted C 2-6 alkenyl, C 2-6 alkynyl or substituted C 2-6 alkynyl, Ci -6 alkoxyl, substituted Ci -6 alkoxyl, acyl, substituted acyl, Ci -6 aminoalkyl or substituted Ci -6 aminoalkyl.
- asymmetric groups may be found in either R or S orientation.
- R 1* , R 2 , R 3 are hydrogen.
- R 5 or R 5* are hydrogen, where as the other group (R 5 or R 5*
- R 5 or R 5* is substituted Ci -6 alkyl.
- each J, and J 2 is, independently H or Ci -6 alkyl.
- either R 5 or R 5* is methyl, ethyl or methoxymethyl. In some embodiments either R 5 or R 5* is methyl.
- Such 5' modified bicyclic nucleotides are disclosed in WO 2007/134181 , which is hereby incorporated by reference in its entirety.
- B is a nucleobase, including nucleobase analogues and naturally occurring nucleobases, such as a purine or pyrimidine, or a substituted purine or substituted pyrimidine, such as a nucleobase referred to herein, such as a nucleobase selected from the group consisting of adenine, cytosine, thymine, adenine, uracil, and/or a modified or substituted nucleobase, such as 5-thiazolo-uracil, 2-thio-uracil, 5-propynyl-uracil, 2'thio-thymine, 5-methyl cytosine, 5-thiozolo-cytosine, 5-propynyl-cytosine, and 2,6- diaminopurine.
- nucleobase including nucleobase analogues and naturally occurring nucleobases, such as a purine or pyrimidine, or a substituted purine or substituted pyrimidine, such as
- R 4* and R 2* together designate the biradical C(R a R b )-N(R c )-0-, wherein R a and R b are independently selected from the group consisting of hydrogen, halogen, Ci_ 6 alkyl, substituted Ci -6 alkyl, C 2-6 alkenyl, substituted C 2-6 alkenyl, C 2-6 alkynyl or substituted C 2-6 alkynyl, Ci -6 alkoxyl, substituted Ci -6 alkoxyl, acyl, substituted acyl, Ci -6 aminoalkyl or substituted Ci -6 aminoalkyl, such as hydrogen, and; wherein R c is selected from the group consisting of hydrogen, halogen, Ci -6 alkyl, substituted Ci -6 alkyl, C 2-6 alkenyl, substituted C 2-6 alkenyl, C 2-6 alkynyl or substituted C 2-6 alkynyl, Ci -6 alkoxyl, substituted Ci -6 alkyl
- R 4* and R 2* together designate the biradical C(R a R b )-0-C(R c R d ) -0-, wherein R a , R b , R c , and R d are independently selected from the group consisting of hydrogen, halogen, Ci -6 alkyl, substituted Ci -6 alkyl, C 2-6 alkenyl, substituted C 2-6 alkenyl, C 2- 6 alkynyl or substituted C 2-6 alkynyl, Ci -6 alkoxyl, substituted Ci -6 alkoxyl, acyl, substituted acyl, C1-6 aminoalkyl or substituted Ci -6 aminoalkyl, such as hydrogen.
- R 4* and R 2* form the biradical -CH(Z)-0-, wherein Z is selected from the group consisting of Ci -6 alkyl, C 2-6 alkenyl, C 2- 6 alkynyl, substituted Ci -6 alkyl, substituted C 2 - 6 alkenyl, substituted C 2 - 6 alkynyl, acyl, substituted acyl, substituted amide, thiol or substituted thio; and wherein each of the substituted groups, is, independently, mono or poly substituted with optionally protected substituent groups independently selected from halogen, oxo, hydroxyl, OJ1 , NJiJz, SJi, N 3 , and CN, wherein each J 2 and J 3 is, independently, H or Ci -6 alkyl, and X is O, S or N ⁇ .
- Z is Ci -6 alkyl or substituted Ci -6 alkyl. In some embodiments Z is methyl. In some embodiments Z is substituted Ci -6 alkyl. In some embodiments said substituent group is Ci -6 alkoxy. In some embodiments Z is CH 3 OCH 2 -. For all chiral centers, asymmetric groups may be found in either R or S orientation. Such bicyclic nucleotides are disclosed in US 7,399,845 which is hereby incorporated by reference in its entirety.
- R 1* , R 2 , R 3 , R 5 , R 5* are hydrogen. In some some embodiments, R 1* , R 2 , R 3 * are hydrogen, and one or both of R 5 , R 5* may be other than hydrogen as referred to above and in WO 2007/134181 .
- R 4* and R 2* together designate a biradical which comprise a substituted amino group in the bridge such as consist or comprise of the biradical -CH 2 -N( R c )-, wherein R c is Ci _ i 2 alkyloxy.
- R 1* , R 2 , R 3 , R 5 , R 5* are independently selected from the group consisting of hydrogen, halogen, Ci_ 6 alkyl, substituted Ci -6 alkyl, C 2-6 alkenyl, substituted C 2-6 alkenyl, C 2-6 alkynyl or substituted C 2-6 alkynyl, Ci -6 alkoxyl, substituted Ci -6 alkoxyl, acyl, substituted acyl, Ci -6 aminoalkyl or substituted Ci -6 aminoalkyl.
- R 1* , R 2 , R 3 , R 5 , R 5* are hydrogen. In some embodiments, R 1* , R 2 , R 3 are hydrogen and one or both of R 5 , R 5* may be other than hydrogen as referred to above and in WO 2007/134181.
- R 4* and R 2* together designate a biradical (bivalent group) C(R a R b )-0-, wherein R a and R b are each independently halogen, C Ci 2 alkyl, substituted C Ci 2 alkyl, C2-C12 alkenyl, substituted C2-C12 alkenyl, C2-C12 alkynyl, substituted C2-C12 alkynyl, C1-C12 alkoxy, substituted C1-C12 alkoxy, OJ1 SJi, SOJ1 , S0 2 Ji, NJiJz, N 3 , CN,
- each J, and J 2 is, independently, H, C1 -C 6 alkyi, substituted C1 -C 6 alkyi, C 2 -C 6 alkenyl, substituted C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, substituted C 2 -C 6 alkynyl, C1 -C 6 aminoalkyl, substituted C1 -C 6 aminoalkyl or a protecting group.
- Such compounds are disclosed in WO2009006478A, hereby incorporated in its entirety by reference.
- R 4* and R 2* form the biradical - Q -, wherein Q is
- each J, and J 2 is, independently, H, Ci -6 alkyi, C 2-6 alkenyl, C 2-6 alkynyl, Ci -6 aminoalkyl or a protecting group; and, optionally wherein when Q is C(qi)(q 2 )(q 3 )(q 4 ) and one of q 3 or q 4 is CH 3 then at least one of the other of q 3 or q 4 or one of qi and q 2 is other than H.
- R 1* , R 2 , R 3 , R 5 , R 5* are hydrogen. For all chiral centers, asymmetric groups may be found in either R or S orientation.
- R 1* , R 2 , R 3 , R 5 , R 5* are independently selected from the group consisting of hydrogen, halogen, Ci -6 alkyi, substituted Ci -6 alkyi, C 2-6 alkenyl, substituted C 2-6 alkenyl, C 2-6 alkynyl or substituted C 2-6 alkynyl, Ci -6 alkoxyl, substituted Ci -6 alkoxyl, acyl, substituted acyl, Ci -6 aminoalkyl or substituted Ci -6 aminoalkyl.
- R 1* , R 2 , R 3 , R 5 , R 5* are hydrogen. In some embodiments, R 1* , R 2 , R 3 are hydrogen and one or both of R 5 , R 5* may be other than hydrogen as referred to above and in WO 2007/134181 or WO2009/067647 (alpha-L- bicyclic nucleic acids analogs).
- Y is selected from the group consisting of -0-, -CH 2 0-, -S-, -NH-, N(R e ) and/or - CH 2 -;
- Z and Z * are independently selected among an internucleotide linkage, R H , a terminal group or a protecting group;
- B constitutes a natural or non-natural nucleotide base moiety (nucleobase), and
- R H is selected from hydrogen and Ci -4 -alkyl;
- R a , R b R c , R d and R e are, optionally independently, selected from the group consisting of hydrogen, optionally substituted Ci-i 2 -alkyl, optionally substituted C 2- i2-alkenyl, optionally substituted C 2- i2-alkynyl, hydroxy, Ci-i 2 -alkoxy, C 2- i 2 -alkoxyalkyl, C 2- i 2 -alkenyloxy, carboxy, Ci-i
- R a , R b R c , R d and R e are, optionally independently, selected from the group consisting of hydrogen and Ci -6 alkyl, such as methyl.
- asymmetric groups may be found in either R or S orientation, for example, two exemplary
- stereochemical isomers include the beta-D and alpha-L isoforms, which may be illustrated as follows:
- thio-LNA comprises a locked nucleotide in which Y in the general formula above is selected from S or -CH 2 -S-.
- Thio-LNA can be in both beta-D and alpha-L- configuration.
- amino-LNA comprises a locked nucleotide in which Y in the general formula above is selected from -N(H)-, N(R)-, CH 2 -N(H)-, and -CH 2 -N(R)- where R is selected from hydrogen and Ci -4 -alkyl.
- Amino-LNA can be in both beta-D and alpha-L- configuration.
- Oxy-LNA comprises a locked nucleotide in which Y in the general formula above represents -0-. Oxy-LNA can be in both beta-D and alpha-L-configuration.
- ENA comprises a locked nucleotide in which Y in the general formula above is -CH 2 -0- (where the oxygen atom of -CH 2 -0- is attached to the 2'-position relative to the base B).
- R e is hydrogen or methyl.
- LNA is selected from beta-D-oxy-LNA, alpha-L-oxy-LNA, beta-D-amino-LNA and beta-D-thio-LNA, in particular beta-D-oxy-LNA.
- each monomer is linked to the 3' adjacent monomer via a linkage group.
- the 5' monomer at the end of an oligomer does not comprise a 5' linkage group, although it may or may not comprise a 5' terminal group.
- linkage group or "internucleotide linkage” are intended to mean a group capable of covalently coupling together two nucleotides. Specific and preferred examples include phosphate groups and phosphorothioate groups.
- nucleotides of the oligomer of the invention or contiguous nucleotides sequence thereof are coupled together via linkage groups.
- each nucleotide is linked to the 3' adjacent nucleotide via a linkage group.
- Suitable internucleotide linkages include those listed within WO2007/031091 , for example the internucleotide linkages listed on the first paragraph of page 34 of
- all remaining linkage groups are either phosphodiester or phosphorothioate, or a mixture thereof.
- all the internucleotide linkage groups are phosphorothioate.
- conjugate is intended to indicate a heterogenous molecule formed by the covalent attachment (“conjugation") of the oligomer as described herein to one or more non-nucleotide, or non-polynucleotide moieties.
- non-nucleotide or non- polynucleotide moieties include macromolecular agents such as proteins, fatty acid chains, sugar residues, glycoproteins, polymers, or combinations thereof.
- proteins may be antibodies for a target protein.
- Typical polymers may be polyethylene glycol.
- the oligomer of the invention may comprise both a polynucleotide region which typically consists of a contiguous sequence of nucleotides, and a further non-nucleotide region.
- the compound may comprise non-nucleotide
- oligomeric compound is linked to ligands/conjugates, which may be used, e.g. to increase the cellular uptake of oligomeric compounds.
- ligands/conjugates which may be used, e.g. to increase the cellular uptake of oligomeric compounds.
- WO2007/031091 provides suitable ligands and conjugates, which are hereby incorporated by reference.
- the oligomer of the invention may be used in pharmaceutical formulations and compositions.
- such compositions comprise a pharmaceutically acceptable diluent, carrier, salt or adjuvant.
- PCT/DK2006/000512 provides suitable and preferred
- Suitable dosages, formulations, administration routes, compositions, dosage forms, combinations with other therapeutic agents, pro-drug formulations are also provided in PCT/DK2006/000512 - which are also hereby incorporated by reference.
- Miravirsen sodium is a preferred pharmaceutical composition.
- [therapeutically] effective amount means an amount required to reduce symptoms (including but limited to curing) of the disease in an individual.
- Effectiveness of therapy may, for example be determined by improvement in indices of function (e.g., transaminases or liver biopsy/liver histology).
- the dose will be adjusted to the individual requirements in each particular case. That dosage can vary within wide limits depending upon numerous factors such as the severity of the disease to be treated, the age and general health condition of the patient, other medicaments with which the patient is being treated, the route and form of administration and the preferences and experience of the medical practitioner involved.
- treatment is initiated with smaller dosages which are less than the optimum dose of the compound. Thereafter, the dosage may be increased by small increments until the optimum effect for the individual patient is reached.
- One of ordinary skill in treating diseases described herein will be able, without undue experimentation and in reliance on personal knowledge, experience and the disclosures of this application, to ascertain a therapeutically effective amount of the compounds of the present invention for a given disease and patient.
- A (e.g. daily/weekly/monthly dose) may, for example be, between about 0.1 and about 500mg/kg body weight, such as between 0.1 and about 100mg/kg body weight such as between 0.1 and 1 mg/kg body weight per day, or between 1 .0 and about 10 mg/kg body weight per day.
- the dosage range may be about 7 mg to 0.7 g per day.
- each dose of the oligomer, such as miravirsen may, for example, be between about 0.1 mgs/kg or 1 mg/kg and about 10mg/kg of 20mg/kg, (i.e.
- Individual doses may therefore be, e.g. about 0.2mg/kg, such as about 0.3mg/kg, such as about 0.4mg/kg, such as about 0.5mg/kg, such as about 0.6mg/kg, such as about 0.7mg/kg, such as about 0.8mg/kg, such as about 0.9mg/kg, such as about 1 mg/kg, such as about 2mg/kg, such as about 3mg/kg, such as about 4mg/kg, such as about 5mg/kg, such as about 6mg/kg, such as about 7mg/kg, such as about 8mgs/kg, such as about 9mg/kg, such as about 10mg/kg.
- the dose of the oligomer is below 7mg/kg, such as below 5mg/kg or below 3mg/kg. In some embodiments the dose of the oligomer is above 0.5mg/kg, such as above 1 mg/kg.
- the time interval between each administration of the miR-122 inhibitor such as miravirsen during the treatment period may be for example, selected from the group consisting of 1 day, 2 days, 3 days, 4 days, 5 days, 6 days and weekly.
- the time interval between administration is at least every other day, such as at least every three days, such as at least every 4 days, such as at least every 5 days, such as at least every 6 days, such as weekly, such as at least every two weeks (biweekly) or at least every 3 or 4 weeks, or at least monthly.
- the oligomer e.g. miravirsen
- the formulation may include a sterile diluent, buffers, regulators of tonicity and antibacterials.
- the oligomer may, for example be administered i.v. or s.c. in a saline solution.
- the preferred carriers are physiological saline or phosphate buffered saline.
- Other methods of administration may be used, for example oral, nasal, rectal administration.
- miravirsen was administered as a single dose intravenously to a group of healthy volunteers. Ascending doses of miravirsen (0.2, 0.4, 0.8, 1.6, 3.2, 6.4, 9.0, and 12 mg/kg) were administered as single doses to different cohorts of subjects. Each cohort consisted of 6 subjects receiving miravirsen and 2 subjects receiving placebo. At the highest dose utilized in this study (12 mg/kg) 3 subjects of 6 administered miravirsen were noted to have increases in ALT (and AST) levels. The maximum increase (in a single individual) was 3 X the upper limit of normal (ULN).
- miravirsen was administered as multiple doses (weekly x 5 over 29 days) in ascending doses to a group of healthy volunteers. There were 6 cohorts of 5 subjects each (4 miravirsen and 1 placebo) in this study. Doses employed were 1 mg/kg iv, 1 mg/kg sc, 2 mg/kg sc, 3 mg/kg sc, 4 mg/kg sc, and 5 mg/kg sc.
- Two of 4 subjects receiving 2 mg/kg sc, 1 of 4 subjects receiving 3 mg/kg sc, 3 of 4 subjects receiving 4 mg/kg sc, and 3 of 4 subjects receiving 5 mg/kg sc of miravirsen had elevations on at least one occasion of ALT (or AST).
- the maximum increase was 1.95 x ULN (in a subject receiving 4 mg/kg sc).
- miravirsen was administered as multiple doses (weekly over 29 days) at 5 mg/kg sc to five healthy volunteers.
- Miravirsen administration occurred, in this study, after a single administration of peg-interferon 2a and ribavirin.
- Three of the 5 subjects were noted, on at least one occasion, to have an elevation of ALT.
- the maximum increase was 3.8 x ULN.
- Biomarker Assays The biomarker protocols used for measuring ALT was e.g. the Advia Chemistry Systems ALT assay (03903166 Rev. B 2007-05); the protocol for measuring ALT was e.g the Advia Chemistry Systems ALT assay (03815151 Rev. B 2007-05), the protocol for measuring GGT was the e.g. Advia Chemistry Systems ALT assay (04130756 Rev. B 2007-05).
- the objectives of the study were the following: to determine the safety and tolerability of a single dose of SPC3649 by observing any adverse events (AEs), clinical laboratory safety parameters, in particular complement system activity, coagulation, cytokines, liver and kidney function; to assess the pharmacokinetics (PK) of i.v. infusion of SPC3649 in healthy volunteers for the range of doses administered; and, to investigate if there were any effect on serum lipids as surrogate biomarkers of miR-122 inhibition after a single dose of SPC3649.
- AEs adverse events
- PK pharmacokinetics
- ALT and AST Treatment with SPC3649 resulted in a dose-related increase in ALT and AST. Values for both parameters began to increase around day 10 after infusion and reached their maximum around 40 to 70 days after infusion. In the dose groups of 9.0 mg/kg and below the values for both parameters remained within the normal range. In the 12.0 mg/kg group 3 subjects were noted to have increases in transaminases. In one subject the ALT increased to a maximum of three times ULN.
- ALT and AST Median values for ALT and AST were higher in the subjects receiving miravirsen compared to placebo. The increases were more pronounced in the higher dose groups (e.g., 54-64% increases with 6.4 and 9.0 mg/kg and 169% increase with 12.0 mg/kg).
- Group A only) or as a subcutaneous (s.c, Groups B through F) injection.
- Five subjects were allocated to each group; four received SPC3649 and one received placebo.
- the following doses of SPC3649 were administered: Group A: 1 mg/kg; Group B: 1 mg/kg; Group C: 2 mg/kg; Group D: 3 mg/kg; Group E: 4 mg/kg; and, Group F: 5 mg/kg.
- 30 subjects participated in the study.
- the objectives of the study were: to determine the safety and tolerability of multiple dosing of SPC3649; to assess the pharmacokinetics (PK) of multiple dosing of SPC3649 administered by i.v. and s.c. routes in healthy volunteers; to evaluate the bioavailability of s.c. administration of SPC3649; and, to investigate the effect of multiple dosing of SPC3649 on lipids as surrogate markers of miR-122 inhibition.
- PK pharmacokinetics
- Baseline 24.0 27 24.5 17.5 20.5 20 22.0 17.0
- Baseline 23.5 22.5 20.0 18.5 27.0 21.0 21.0 20.5
- PEG-IFNa and ribavirin were again administered on Day 37 followed by a 168-hour serial blood collection to assess the pharmacokinetic profile of PEG-IFNa and ribavirin following dosing with miravirsen.
- Subjects returned for a follow-up visit at Days 39, 41 , 44, 51 , 58, and 65 with a close-out visit on Day 77.
- the total study duration was planned to be approximately 14 weeks. In total, 5 subjects were enrolled and analyzed.
- the objectives of the study were: To assess the effect of miravirsen on the pharmacokinetics of co-administered pegylated interferon-alpha and ribavirin, respectively; to evaluate the tolerability of co-administered miravirsen, pegylated interferon-alpha and ribavirin; and, to assess the role of miRNA-122 inhibition on the Vitamin K-dependent coagulation factors.
- AST liver function tests
- ALT liver function tests
- Doses of miravirasen administered were 3, 5, or 7 mg/kg, respectively. Following the dosing period, subjects were to return for follow-up visits for an overall study duration of 18 weeks.
- the objectives of the study were to determine the safety and tolerability of multiple doses of miravirsen in HCV genotype 1 infected subjects; to assess the pharmacokinetics of miravirsen administered subcutaneously to HCV infected subjects; and, to assess any effect on viral titer in subjects with HCV infection.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Biomedical Technology (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Molecular Biology (AREA)
- Biotechnology (AREA)
- General Engineering & Computer Science (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Wood Science & Technology (AREA)
- Microbiology (AREA)
- Plant Pathology (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- Physics & Mathematics (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
The present invention relates to the use of LNA antisense oligonucleotide inhibitors for use in improving blood liver function biomarkers in subjects which have or may have impaired liver function.
Description
LNA OLIGOMERS FOR IMPROVEMENT IN HEPATIC FUNCTION FIELD OF INVENTION
The present invention relates to the use of LNA antisense oligonucleotide inhibitors of microRNA-122 for use in improving blood liver function biomarkers in subjects which have or may have impaired liver function. The present invention also relates to use of miravirsen, a drug which is being developed for treatment of HCV, for use in improving liver function in non-HCV infected patients.
BACKGROUND
According to WO2007/090071 many LNA's are toxic. WO2007/090071 uses 14mer LNA compounds targeting PTEN mRNA, in in vivo experiments in mice. The inventors of
WO2007/090071 report that potent knock-down of PTEN using beta-D-oxy LNA (4 -CH2-0- 2' BNA) is associated with elevated biomarkers of hepatotoxicity, ALT and AST.
Swayze et al., reports that antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals (Nucleic Acids Research, 2007, Vol. 35, No. 2 687-700).
2'-MOE inhibitors of microRNA-122 are reported in WO 2007/027775, which further refers to methods for the treatment of cardiovascular or metabolic diseases characterized by elevated serum total cholesterol, elevated serum LDL-cholesterol, or elevated serum triglycerides, through the administration of an oligomeric compound which modulates the levels or activity of miR-122a. WO 2007/027775 discloses various metabolic diseases which the inventors allege may be treated using oligomeric compound which modulates the levels or activity of miR-122a, including diabetes, obesity, hyperlipidemia, hypercholesterolemia,
hypertriglyceridemia, hyperfattyacidemia, nonalcoholic fatty liver disease, non alcoholic steatohepatitis, or metabolic syndrome. Whilst 775 discloses various 23nt LNA/MOE mixmers, the in vivo examples of WO 2007/027775 are limited to fully modified 2'MOE compounds complementary to the entire miR-122 sequence. See also Esau et al. Cell. Metab. 2006 Feb;3(2):87-98.
microRNA-122 (miR-122) is a liver specific microRNA. miR-122 is involved in cholesterol metabolism, and inhibition of miR-122 in vivo in mice, results in a reduction in serum cholesterol levels. How miR-122 regulated cholesterol metabolism is apparently, at present unknown.
Furthermore, miR-122 is a host factor which is required for maintenance of hepatitis C (HCV) infection. Whilst many different hypotheses have been proposed as to how miR-122 interacts with the HCV virus, it has recently been proposed that miR-122 protects the 5' terminus of the HCV RNA from cytoplasmic sensors for viral RNA, and as such a key role of miR-122 in HCV infection may be in masking the vulnerable 5' terminal sequences of HCV from the innate cell immune response, a mechanism which is not related to HCV replication per se (Machlin et al., "Masking the 5' terminal nucleotides of the hepatitis C virus genome by an unconventional microRNA-target RNA complex". PNAS. 201 1 Feb 22;108(8):3193- 3198).
Miravirsen (SPC3649) is the first therapeutic agent targeting a microRNA in clinical trials. Miravirsen is designed to specifically sequester miR-122 and has been shown to have activity in reducing viremia. Sequestering makes miR-122 unavailable, and may therefore unmask the 5'terminus of the HCV genome, allowing the cells' innate immunity access to the HCV virus. Miravirsen is a 15 nucleotide (15-mer) phosphorothioate oligonucleotide, consisting of beta-D-oxy-LNA (Locked Nucleic Acids) and DNA monomers respectively. The miravirsen molecule contains 8 LNA nucleotides and 7 DNA nucleotides arranged in the following sequence: 5'- mC c A 1 1 G T c a mC a mC t mC mC -3' (Capital letters denote beta-Doxy LNA modified nucleotides (mC stands for LNA-5-Methyl-Cytidine) and lower case letters denote DNA nucleotides, all internucleoside linkages are phosphorothioate).
In pre-clinical animal experiments, there has been no evidence of serious miravirsen associated liver toxicities or histopathological changes in the study animals. There has not, prior to the present findings, been any evidence of improved hepatic function due to miravirsen treatment, other than that associated with reduction in HCV viral load. The preclinical results demonstrate potent and safe antagonism of miR-122 by miravirsen in livers of non -human primates. In African green monkeys, administration of 5 mg/kg LNA-antimiR® (miravirsen), resulted in no significant change in ALT and AST levels as compared to controls (Elmen et al, Nature 2008), whereas in a follow up study in cynomologus monkeys,
there was evidence of a mild and dose dependent elevation of ALT (Hildebrandt-Eriksen et al, OTS 2001 - see Figure 1 , and Hildebrandt-Eriksen et al, 201 1 in preparation).
In pre-clinical evaluation of miravirsen in HCV infected chimpanzee, in three out of four animals there was no change in ALT or AST level associated with miravirsen treatment. Although one high dose animal showed a pre-treatment spike in ALT level which may have been subsequently reduced due to the reduction in HCV viremia (reproduced in Figure 2).
SUMMARY OF INVENTION
The present invention relates to the use of LNA antisense oligonucleotide inhibitors targeting microRNA-122 for use in improving (such as e.g. lowering) blood liver function biomarkers in subjects which have or may have impaired liver function. The invention relates to the use of miravirsen, a drug being developed specifically for treatment of HCV, for use in the treatment of other hepatic diseases. The present invention is based upon the discovery that in human subjects with chronic HCV infection, administration of miravirsen at pharmacologically relevant doses produced a notable reduction in liver toxicity biomarkers associated with miravirsen treatment.
Specifically, there was a profound reduction in the serum levels of ALT, AST and GGT, liver transaminase enzymes whose presence in serum is widely used as a non-invasive test to assess liver function or vitality.
The reduction in ALT, AST and GGT level were most surprising as it was not always associated with a reduction in viremia, indicating that, in human subjects, there is an alternative HCV independent mechanism which results in improved liver function (as indicated by improved ALT, AST and GGT serum marker levels). This observation could not have been predicted from the pre-clinical non-human primate studies in which there was no general reduction in liver function biomarkers. Furthermore, no reduction of ALT or AST was identified in phase 1 clinical trials using healthy subjects. Therefore, we were most surprised to find that in the phase2a trial in HCV infected patients, the improvement in liver function biomarker scores was not correlated to reduction in HCV viremia, indicating that, in human subjects, miravirsen has a pronounced and independent
effect on improving liver function in individuals who have general hepatitis
disorders/symptoms, not necessarily related to HCV viremia. Indeed, reduction of blood serum biomarkers, ALT, AST and GGT was found in all dosage groups, and occurred at time points prior to and frequently independent of reduction in HCV titer.
The invention provides a LNA antisense oligonucleotide inhibitor of microRNA-122 for use in a. improving (e.g. lowering) blood serum biomarkers of liver function in a human subject in need of improved liver function; and/or
b. improving (e.g. lowering) blood serum biomarkers of liver function in a human subject in need of improved liver function; and/or
c. preventing loss of liver function in a human subject who is not infected with HCV but is at risk of deteriorating liver function; and/or
d. Improving liver function in a human subject who is not infected with HCV. The invention provides a LNA antisense oligonucleotide inhibitor of microRNA-122 for use in the preparation of a medicament for
a. improving (e.g. lowering) blood serum biomarkers of liver function in a human subject in need of improved liver function; and/or
b. improving (e.g. lowering) blood serum biomarkers of liver function in a human subject in need of improved liver function; and/or
c. preventing loss of liver function in a human subject who is not infected with HCV but is at risk of deteriorating liver function; and/or
d. improving liver function in a human subject who is not infected with HCV. Liver function may be determined for example, by assessing blood serum biomarkers, such as miR-122, ALT, AST and/or GGT and/or for example via liver biopsy.
The blood serum biomarkers may, for example, be independently selected from the group consisting of ALT, AST and GGT, and as described herein, combinations thereof. Recently microRNA-122 has been identified as a blood serum biomarker of necroinflammation (Bihrer et al., The American Journal of Gastroenterology, 201 1 , Su et al., "Serum MicroRNA-122 Level Correlates with Virologic Responses to Combination Therapy in Chronic Hepatitis C Patients" AASLD November 201 1 ).
The invention provides LNA antisense oligonucleotide inhibitors targeting microRNA-122 for use in the treatment of necroinflammation.
The invention relates to a LNA antisense oligonucleotide inhibitor of microRNA-122 for use in the preparation of a medicament for necroinflammation.
The invention relates to a method of reducing the level of blood serum biomarkers of liver function in a human subject who is not infected with HCV and who is in need of improved liver function, said method comprising administering an effective dose of a LNA antisense oligonucleotide inhibitor of microRNA-122, such as miravirsen, to said human subject so as to reduce the level of blood serum biomarkers in the human subject.
The invention relates to a method of preventing loss of liver function in a human subject who is not infected with HCV but is at risk of deteriorating liver function, said method comprising: administering an effective dose of a LNA antisense oligonucleotide inhibitor of microRNA- 122, such as miravirsen, to said human subject so as to preventing loss of liver function in the human subject.
The invention relates to a method of improving liver function in a human subject who is not infected with HCV, said method comprising administering an effective dose of a LNA antisense oligonucleotide inhibitor of microRNA-122, such as miravirsen, to said human subject so as to improve liver function in the human subject.
The invention relates to a method of treating necroinflammation in a human subject who has or is suspected of having necroinflammation, said method comprising administering an effective dose of a LNA antisense oligonucleotide inhibitor of microRNA-122, such as miravirsen, to said human subject.
The invention relates to a method of reducing the level of blood serum biomarkers of liver function in a human subject who is in need of improved liver-function, said method comprising
a. Determining the level of one or more blood serum biomarkers for liver function from the human subject to identify the subject is in need of improved liver function
b. administering an effective dose of a LNA antisense oligonucleotide inhibitor of microRNA-122 , such as miravirsen, to said human subject so as to improve liver function in the human subject
BRIEF DESCRIPTION OF FIGURES
Figure 1 : Dose-related increases in ALT in cynomologus monkeys illustrates a class effect of accumulation of oligonucleotides in the liver, were slightly more marked in females and almost reversible after the 12-week treatment-free period.
Figure 2: Treatment of HCV-infected chimpanzees with miravirsen was well tolerated. (A) Plasma trough levels of miravirsen. (B) alanine aminotransferase (ALT) levels in HCV- infected chimpanzees treated with miravirsen during the Lanford 2009 study.
Figure 3: SPC3649-203 study ALT by treatment cohort (mean, U/L).
Figure 4: SPC3649-203 study AST by treatment cohort (mean, U/L).
Figure 5: SPC3649-203 study GGT by treatment cohort (mean, U/L).
Figure 6: SPC3649-203 study LDH by treatment cohort (mean, U/L).
Figure 7: Improvement in ALT serum level preceeds the reduction in HCV viremia in a human subject treated with 7mg/kg miravirsen.
DETAILED DESCRIPTION OF INVENTION
Blood Serum Biomarkers
Numerous serum biomarkers of liver function are known, by way of a non-limited example the following have been implicated as markers or likely markers of liver function: Lectin- reactive alpha fetoprotein (AFP-L3), Des-gamma-carboxy-prothrombin (DCP), ER6Q, Vimentin, actin alpha 1 skeletal muscle protein, hMFAP 4, tropomyosin, PTGES 2, amyloid P component, transgelin, calponin 1 , homo sapiens p20 protein, 17 kDa myosin light chain, H chain H Igg B12, prolyl 4-hydroxylase, beta subunit methylenetetrahydrofolate
dehydrogenase 1 , PR02619, aldehyde dehydrogenase 1 , fibrinogen alpha chain preproprotein, fructose-bisphosphate aldolase B, argininosuccinate synthetase, Eefla2, AT P 5 Al, alpha-2 actin, regucalcin, serum albumin, mitochondrial malate dehydrogenase, mitochondrial acetoacetyl-CoA thiolase, Hyaluronic Acid (HA), Hepascore, Prothrombin, Gamma Glutamyl Transpeptidase, Apolipoprotein A1 (PGA) index, Age platelet (AP) index,
Bonacini index, Pohl score, Forns index, Aspartate aminotransferase/Platelets Ratio index (APRI), MP3 (MMP1 , PIINP) index, FIB4,and Fibrolndex.
The blood serum biomarkers are typically measured in blood serum (or plasma) samples obtained from the subject (i.e. blood serum or blood plasma biomarkers). The blood biomarkers are markers of liver function. Typically elevated levels of the blood serum biomarkers are indicative of impaired liver function or vitality.
Preferred blood serum biomarkers of liver function include for example, gamma
glutamyltransferase (GGT), alanine amino transferase (ALT), and aspartate amino transferase (AST).
In some embodiments, the blood biomarkers include AST; or GGT; or ALT; or ALT and AST; or ALT, AST and GGT; or AST & GGT; or ALT and GGT. In some embodiments, microRNA-122(miR-122) may be used as the blood biomarker, either alone or in addition to the above sets of biomarkers. In some embodiments, the blood biomarkers comprise of consist of miR-122 and ALT; or miR-122 and AST; or miR-122 and GGT; or miR-122 and ALT/AST ratio.
Alanine Amino Transferase (ALT)
Background: alanine amino transferase (ALAT), also known as Alanine Transaminase (ALT) or serum glutamic pyruvic transaminase (sGPT), is a homodimeric cytoplasmic pyridoxal phosphate-dependent enzyme involved in cellular nitrogen metabolism, amino acid metabolism, and liver gluconeogenesis. ALT mediates conversion of major intermediate metabolites, catalyzing reversible transamination between alanine and oketoglutarate to form pyruvate and glutamate. ALT is widely distributed in many tissues but is found in greatest abundance in the liver. The major role of ALT in the liver is the conversion of alanine to glucose which is then exported to the body to be utilized in a multitude of processes.
ALT Measurement: Measurement of ALT activity is generally carried out by monitoring the rate of NADH oxidation in a coupled reaction system employing lactate dehydrogenase
(LDH). The oxidation of NADH to NAD+ is accompanied by a decrease in absorbance at 340 nm. Under circumstances in which the ALT activity is rate limiting, the rate decrease is directly proportional to the ALT activity in the sample. A protocol for measuring ALT may be
the Advia Chemistry Systems ALT assay (03815151 Rev. B 2007-05) hereby incorportated by reference.
In some embodiments the level of ALT in the blood (serum/plasma) indicative of impaired liver function is (e.g. when using the Advia Chemistry Systems assay) >69 IU/L, such as >70IU/L, such as >75IU/L, such as >80IU/L, such as >90IU/L, such as >100IU/L, such as >1 10IU/L, such as >120IU/L, such as >130IU/L, such as >150IU/L, such as >200IU/L. In some embodiments the level of ALT in the blood (serum/plasma) indicative of impaired liver function >1 .3x normal, such as >1.4x normal, such as >1.5x normal, such as > 2x normal, such as >3x normal, such as >4x normal, such as >5x normal. Normal is, as defined herein, refers to the upper range of normal.
Aspartate Amino Transferase
Background: Aspartate aminotransferase (ASAT) , also known as Aspartate Transaminase (AST) or serum glutamic oxaloacetic transaminase (SGOT), is a pyridoxal phosphate (PLP)- dependent transaminase enzyme. AST catalyzes the reversible transfer of an a-amino group between aspartate and glutamate and, as such, is an important enzyme in amino acid metabolism. AST is found in the liver, heart, skeletal muscle, kidneys, brain, and red blood cells, and it is commonly measured clinically as a marker for liver health.
Measurement: In general, AST activity assay is based on the quantification of oxaloacetate produced by AST. In this assay, oxaloacetate and NADH are converted to malate and NAD by the enzyme malate dehydrogenase. The decrease in NADH absorbance at 340 nm is proportionate to AST activity.
Basic methodology: See for example, Bergmeyer H.U., Scheibe P. and Wahlefeld A.W. (1978). Optimization of methods for aspartate aminotransferase and alanine
aminotransferase. Clin. Chem. 24(1 ): 58-73 or Bowers Jr G.N. and McComb R.B. (1984). A unifying reference system for clinical enzymology: aspartate aminotransferase and the International Clinical Enzyme Scale. Clin. Chem. 30(7): 1 128-1 136. A protocol for measuring ALT may be the Advia Chemistry Systems ALT assay (03903166 Rev. B 2007- 05) hereby incorportated by reference.
In some embodiments the level of AST in the blood (serum/plasma) indicative of impaired liver function is (e.g. when using the Advia Chemistry Systems assay) >50 IU/L, such as >55IU/L, such as >60IU/L, such as >65IU/L, such as >70IU/L, such as >75IU/L, such as >80IU/L, such as >85IU/L, such as >90IU/L, such as >95IU/L, such as >100IU/L. In some embodiments the level of AST indicative of impaired liver function is >1 .3x normal, such as
>1.4x normal, such as >1.5x normal, such as > 2x normal, such as >3x normal, such as >4x normal, such as >5x normal. Normal is, as defined herein, refers to the upper range of normal.
Gamma-glutamyl transpeptidase
Background: Gamma-glutamyl transpeptidase (also γ-glutamyltransferase, GGT, GGTP, gamma-GT) is an enzyme that transfers gamma-glutamyl functional groups. It is found in many tissues, the most notable one being the liver. GGT catalyzes the transfer of the gamma-glutamyl moiety of glutathione to an acceptor that may be an amino acid, a peptide or water (forming glutamate). GGT plays a key role in the gamma-glutamyl cycle, a pathway for the synthesis and degradation of glutathione and drug and xenobiotic detoxification.
Measurement: The assay generally measures the cleavage of a specific GGT substrate (g- glutamyl-p-ntiroanilide) by the enzyme. The production of the p-nitroaniline (pNA) product, measured at 405 nm, is proportional to the level of GGT enzyme in the sample.
Basic methodology: Activity determination, kinetic analyses and isoenzyme identification of gamma glutamyltransferase in human neutrophils. Sener A, Yardimci T. J Biochem Mol Biol. 2005 May 31 ; 38(3):343-9. A protocol for measuring GGT may be the Advia Chemistry Systems GGT assay (04130756 Rev. B 2007-05) hereby incorporated by reference.
Blood test results for GGT suggest that the population 97.5th percentile (the so called "upper limit of normal") is around 45 IU/L for women and 75 IU/L for men. In some embodiments the level of GGT in the blood (serum/plasma) indicative of impaired liver function is >1 .5x normal, such as > 2x normal, such as >3x normal, such as >4x normal, such as >5x normal. In some embodiments, the indicative level of GGT for a female with impaired liver function is > 60IU/L, such as >70IU/L, such as >80IU/L, such as >90IU/L such as >100IU/L.
In some embodiments, the indicative level of GGT for a male impaired liver function is > 100IU/L, such as >1 10IU/L, such as >120IU/L, such as >130IU/L such as >140IU/L.
(suitable the specific values for GGT activity may be determined by using the Advia
Chemistry Systems assay).
ALT, AST and GGT assay methods, kits and reagents are commercially available (e.g.
Siemens ADVIA 1800 Chemistry System). Normal is, as defined herein, refers to the upper range of normal. microRNA-122
microRNA-122 (miR-122) is a microRNA which has been reported as liver specific (Lagos- Quintana et al (2002) Current Biol vol 12 pp 735-739) and is a distinct host cellular factor which is reported to be essential for HCV replication in hepatocytes (Jopling et al 2005). miR-122 exists in numerous forms, including the mature microRNA (SEQ ID NO 2) and precursors thereto, such as the sequence shown in SEQ ID NO 1 . The mature microRNA is known to exist in two forms, one 22nt microRNA sequence shown in SEQ ID NO 2, and a further form where there is an additional 3' U residue.
The miR-122 seed sequence refers to nucleosides 2 through 8 from the
5'-end of the mature miR-122 sequence, i.e. 5'-GGAGUGU-3'
According to miRBase, the sequence of the human miR-122 sequence, hsa miR-122, is as follows:
>hsa-mir-122 precursor sequence (miRBase) MI0000442:
CCUUAGCAGAGCUGUGGAGUGUGACAAUGGUGUUUGUGUCUAAACUAUCAAACGCC AUUAUCACACUAAAUAGCUACUGCUAGGC (SEQ ID NO 1 )
The mature hsa-miR-122 sequence (miRBase) MIMAT0000421 :
UGGAGUGUGACAAUGGUGUUUG(U) (SEQ ID NO 2)
Normal Liver function
Typically the subject has impaired, i.e. not normal (abnormal), liver function.
Typically, with reference to blood serum biomarkers, such as ALT, AST and GGT, normal refers to the central portion (95%) of the normal distribution (i.e. within the "normal range"). The upper limit cut off for normal (95%) level is referred to as the "upper limit of normal." It should be recognized that the precise upper limit of normal can vary, e.g. depending upon the reference normal population, gender, age, and in some cases, the specific assay conditions used.
Within the context of the present invention, it should therefore be recognised that "normal liver function" may be determined by the comparison to reference samples or values from a population of subjects with normal liver function. A subject whose blood serum biomarker levels are determined to be above the upper limit of normal may in some embodiments, or in general, be a subject who is in need to improved liver function.
Alternatively, determination of normal liver function may be determined by selection of suitable pre-determined cut-of values for one or more of the biomarker levels, as described
herein. The assays used to determine the specific cut off values are identified herein as the Advia Chemistry ALT, AST and GGT protocols. Non-limiting examples of such cut off values are provided herein. As such, the normal range may be considered as being below the levels which are associated with healthy liver function, or cut off values provided herein. Levels above the cut off values may therefore be indicative of a subject who is in need of improved liver function.
In the therapeutic methods of the invention, the subject, prior to treatment, may have an ALT, AST, and/or GGT blood serum level which is above the upper limit of normal (elevated blood biomarkers). .
Exemplary time-points for assessment of blood biomarkers
Suitably the level of blood biomarker(s) are assessed prior to treatment with the LNA antisense oligonucleotide inhibitor of microRNA-122, e.g. with miravirsen, such as within about 6 months, such as within about 5 months, such as within about 4 months, such as within about 3 months, such as within about 2 months, such as within about 1 month, such as within about 4 weeks, such as within about 3 weeks, such as within about 2 weeks, such as within about 1 week, such as within about 1 , 2, 3, 4, 5 or 6 days, prior to the first (or in some embodiments subsequent) administration with the microRNA-122 inhibitor. In some embodiments, the assessment of the level of blood biomarkers involves the sequential steps of i) obtaining a blood sample from the human subject infected with HCV and, ii) determining the level of at least one biomarker in the blood sample.
The Subject
The subject is a human being who has been diagnosed with, or is suspected of having a impaired liver function or has been diagnosed with or is suspected of having a disease or disorder which results in or may result in impairment of hepatic function, or is otherwise in need of improved liver function or prevention of deterioration of liver function. Suitably, the subject is typically not a normal healthy subject. In some embodiments the subject has or is suspected of having necroinflammation. In some embodiments such a subject may or may not have HCV infection, such as chronic HCV infection. In some embodiments the invention provides for a method of concurrent treatment of chronic HCV infection and
necroinflammation, in a subject who is infected with HCV and has or is suspected of having necroinflammation, said method comprising the step of administering a (therapeutically)
effective amount of the LNA antisense oligonucleotide inhibitor of microRNA-122 to the subject. In some embodiments the invention provides for a method of concurrent treatment of chronic HCV infection and improvement of liver function (or vitality), in a subject who is infected with HCV and has or is suspected of being in need of improved liver function, said method comprising the step of administering a (therapeutically) effective amount of the LNA antisense oligonucleotide inhibitor of microRNA-122 to the subject.
In some embodiments the invention provides for a method of concurrent treatment of chronic HCV infection and improvement of blood serum biomarkers of liver function (e.g. ALT, AST and/or GGT), in a subject who is infected with HCV and is in need of improved blood serum biomarkers of liver function, said method comprising the step of administering a
(therapeutically) effective amount of the LNA antisense oligonucleotide inhibitor of microRNA-122 to the subject. In some embodiments, the subject, prior to treatment has a need for improved liver function.
In some embodiments, the subject, prior to treatment has impaired liver function.
In some embodiments the human subject has been diagnosed with a disease or disorder selected from the group consisting of hepatitis B and hepatitis D.
In some embodiments the human subject has been diagnosed with a disease or disorder selected from the group consisting of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis.
In some embodiments the human subject has been diagnosed with a disease or disorder selected from the group consisting of cytomegalovirus infection, schistosomiasis infection and Leptospirosis infection.
In some embodiments, the subject is in need of prevention of loss of liver function. In this respect loss need not be full loss, but is typically used to refer to a partial loss in liver function, as determined, for example, by elevated blood biomarkers (as described herein), or by evaluation by liver biopsy. In this respect blood biomarkers or liver biopsy may be used in the assessment of, for example, the need for improved liver function or the treatment of impaired liver function. Biomarker assays/liver biopsies may also be used subsequent to or during treatment to monitor the improvement of liver function.
Percutaneous liver biopsy is associated with potential complications, including bleeding (1 %-3%), pain (20%-30%), bile peritonitis (<1 %), pneumothorax(<1 %), punctured viscera
(<1 %), and death. It is preferred that the liver function of the subject is determined (e.g. prior to, during or after treatment, by assessment of blood biomarkers for liver function, such as those referred to herein, rather than the invasive liver biopsy).
In some embodiments, the subject has been diagnosed with or is suspected of having liver fibrosis or cirrhosis. In some embodiments, the subject has been diagnosed with necroinflammation or is suspected of having necroinflammation.
Liver Biopsy:
Traditionally, the level of necroinflammation in a subject is determined by liver biopsy, an invasive and painful procedure which is widely used in the United States to provide information on the state of the liver and prognostic information for future disease progression (Dienstag and McHutchinson, AGA Vol 130, pp 231-264).
Table 2. Histologic Scoring Systems for Fibrosis
Fibrosis ETAVIRiOE Is akiM
None 0 0
Portal fibrosis (some) i 1
Portal fibrosis (most) 1 2
Bridging fibrosis (occasional) 2 3
Bridging fibrosis (marked) 3 4
Incomplete cirrhosis 4 5
Cirrhosis 4 6 Percutaneous liver biopsy is associated with potential complications, including bleeding (1 %-3%),pain (20%-30%), bile peritonitis (<1 %), pneumothorax(<1 %), punctured viscera (<1 %), and death.
Disease or Disorders
In some embodiments the subject has or is suspected of having hepatitis. Hepatitis (plural hepatitides) is a medical condition defined by the inflammation of the liver and characterized by the presence of inflammatory cells in the tissue of the organ. The condition can be self- limiting (healing on its own) or can progress to fibrosis (scarring) and cirrhosis. Hepatitis may occur with limited or no symptoms, but often leads to jaundice, anorexia (poor appetite) and malaise. In the present invention, Hepatitis is acute when it lasts less than six months and chronic when it persists longer.
Acute hepatitis typically lasts less than 2 months. Acute hepatitis may, for example be associated with liver damage caused by various poisons (e.g. carbon tetrachloride) or by certain drug overdoses. It is envisaged that miravirsen treatment may be unsuitable for treatment of acute hepatitis where the duration of the disease is less than 2 months. In some embodiments the hepatitis is sub-acute hepatitis, i.e. lasts between 2 - 6 months. It will be recognized that the type of hepatitis (acute, sub-acute or chronic) may be diagnosed either by the length of time since diagnosis and maintenance of the diseased state, or in some instances at the time of diagnosis (for example in the case of certain drug overdose or poisoning.
A group of viruses known as the hepatitis viruses cause most cases of hepatitis worldwide, but it can also be due to toxins (notably alcohol, certain medications, some industrial organic solvents and plants), other infections and autoimmune diseases. In some embodiments, the hepatitis is not hepatitis C (HCV).
In some embodiments the hepatitis is chronic hepatitis, which may be other than chronic HCV. Chronic hepatitis may, for example, be caused by viral infection: Hepatitis B, hepatitis C, hepatitis D may cause chronic or acute hepatitis, other causes of chronic hepatitis include autoimmune hepatitis, alcohol induced hepatitis, certain drugs may induce chronic hepatitis (e.g. methyldopa, nitrofurantoin, isoniazid and ketoconazole). In some embodiments the chronic hepatitis is steatohepatitis, such as non-alcoholic steatohepatitis.
In some embodiments, the hepatitis is infectious hepatitis, for example hepatitis B and D. HBV and HDV may occur in chronic or acute forms.
Hepatitis B is an infectious illness caused by hepatitis B virus (HBV) which infects the liver and causes an inflammation called hepatitis. More than 2 billion people have been infected with the hepatitis B virus, and this includes 350 million chronic carriers of the virus. [WHO] Transmission of hepatitis B virus results from exposure to infectious blood or body fluids, such as semen and vaginal fluids, while viral DNA has been detected in the saliva, tears, and urine of chronic carriers with high titer DNA in serum. Perinatal infection is a major route of infection in endemic (mainly developing) countries. Other risk factors for developing HBV infection include working in a health care setting, transfusions, and dialysis, acupuncture, tattooing, extended overseas travel and residence in an institution.
Acute hepatitis: Approximately 70 percent of patients with acute hepatitis B have subclinical or anicteric hepatitis, while 30 percent develop icteric hepatitis. Fulminant hepatic failure is unusual, occurring in approximately 0.1 to 0.5 percent of patients.
The method of acquiring HBV infection varies geographically. Perinatal transmission and occasionally horizontal transmission early in life are most common in high prevalence areas such as Southeast Asia and China, while sexual contact and percutaneous transmission (e.g., intravenous drug use) are most common in the United States, Canada, and Western Europe.
The incubation period lasts one to four months. A serum sickness-like syndrome may develop during the prodromal period, followed by constitutional symptoms, anorexia, nausea, jaundice, and right upper quadrant discomfort. The symptoms and jaundice generally disappear after one to three months, but some patients have prolonged fatigue even after normalization of serum aminotransferase concentrations.
Laboratory testing during the acute phase reveals elevations in the concentration of alanine and aspartate aminotransferase levels (ALT and AST); values up to 1000 to 2000 IU/L are typically seen during the acute phase with ALT being higher than AST. The serum bilirubin concentration may be normal in patients with anicteric hepatitis. In patients who recover, the normalization of serum aminotransferases usually occurs within one to four months. A persistent elevation of serum ALT for more than six months indicates a progression to chronic hepatitis.
Traces of HBV are often detectable in the blood by PCR for many years after a clinical recovery from acute hepatitis, despite the presence of serum antibodies and HBV-specific cytotoxic T cells, which can be present at high levels. Thus, the complete eradication of HBV rarely occurs after recovery from acute HBV infection and latent infection can maintain the T cell response for decades following clinical recovery, thereby keeping the virus under control.
The rate of progression from acute to chronic hepatitis B is determined primarily by the age at infection. The rate is approximately 90 percent for a perinatally-acquired infection, 20 to
50 percent for infections between the age of one and five years, and less than 5 percent for an adult-acquired infection.
A history of acute hepatitis is elicited in only a small percentage of patients with chronic HBV infection. In low or intermediate prevalence areas, approximately 30 to 50 percent of patients with chronic HBV infection have a past history of acute hepatitis; such a history is lacking in the remaining patients in these areas and in the majority of patients in high prevalence areas (predominantly perinatal infection). Many patients with chronic hepatitis B are asymptomatic (unless they progress to decompensated cirrhosis or have extrahepatic manifestations), while others have nonspecific symptoms such as fatigue. Some patients experience exacerbations of the infection which may be asymptomatic, mimic acute hepatitis, or manifest as hepatic failure. Physical examination may be normal, or there may be stigmata of chronic liver disease.
Jaundice, splenomegaly, ascites, peripheral edema, and encephalopathy may be present in patients with decompensated cirrhosis. Laboratory tests may be normal, but most patients have a mild to moderate elevation in serum AST and ALT. During exacerbations, the serum ALT concentration may be as high as 50 times the upper limit of normal, and alfa-fetoprotein (AFP) concentrations as high as 1000 ng/mL may be seen.
A progression to cirrhosis is suspected when there is evidence of hypersplenism (decreased white blood cell and platelet counts) or impaired hepatic synthetic function
(hypoalbuminemia, prolonged prothrombin time, hyperbilirubinemia).
Hepatitis D, also referred to as hepatitis D virus (HDV) and classified as Hepatitis delta virus, is a disease caused by a small circular enveloped RNA virus. HDV is considered to be a subviral satellite because it can propagate only in the presence of the hepatitis B virus (HBV). Transmission of HDV can occur either via simultaneous infection with HBV
(coinfection) or superimposed on chronic hepatitis B or hepatitis B carrier state
(superinfection).
Both superinfection and coinfection with HDV results in more severe complications compared to infection with HBV alone. These complications include a greater likelihood of
experiencing liver failure in acute infections and a rapid progression to liver cirrhosis, with an increased chance of developing liver cancer in chronic infections. In combination with hepatitis B virus, hepatitis D has the highest mortality rate of all the hepatitis infections of 20%.
Due to the dependence of HDV on HBV, the presence of HBsAg is necessary for the diagnosis of HDV infection. The additional presence of IgM antibody to hepatitis B core antigen (IgM anti-HBc) is necessary for the diagnosis of acute HBV/HDV coinfection. The clinical sequelae of HDV infection encompass a spectrum of manifestations from fulminant liver failure to the asymptomatic carrier state. The severity of the clinical course is influenced by several factors. Persistent HDV replication is associated with annual rates of development of cirrhosis and hepatocellular carcinoma (HCC) of 4 and 2.8 percent, respectively.
In the Western world, where the predominant genotype is genotype I, acute hepatitis D has an increased risk of a fulminant course when compared to acute hepatitis B. Once chronic HDV infection is established, it usually exacerbates the preexisting liver disease due to HBV. Progression towards cirrhosis may be rapid. HDV-associated chronic liver disease may also run an indolent course and asymptomatic HDV carriers have been found.
Patients who are currently referred for HDV infection appear to represent cohorts infected years ago in whom the HDV-related disease rapidly developed to cirrhosis, but whose subsequent disease progression has been slow.
Non-alcoholic fatty liver disease: Non-alcoholic fatty liver disease (NAFLD) is one cause of fat accumulation (steatosis) in the liver not due to excessive alcohol use. It is related to insulin resistance and the metabolic syndrome and may respond to treatments originally developed for other insulin-resistant states.
Nonalcoholic steatohepatitis (NASH) is the term used to describe the distinct clinical entity in which patients lack a history of significant alcohol consumption but have liver biopsy findings indistinguishable from alcoholic steatohepatitis.
The following criteria have been utilized for the diagnosis of NASH:
• A liver biopsy showing steatosis
• Convincing evidence of negligible alcohol consumption (less than 20 g of ethanol per week).
• Absence of serologic evidence of infection with hepatitis B or hepatitis C.
The major risk factors for nonalcoholic fatty liver disease (NAFLD), central obesity, type 2 diabetes mellitus, dyslipidemia, and metabolic syndrome, are common in western societies. NAFLD is the most common liver disorder in Western industrialized countries, affecting 20 to 40 percent of the general population. NASH was confirmed by biopsy in 30 percent of ultrasound-positive patients.
The majority of cases occur between the ages of 40 and 60. However, NAFLD is probably the most common cause of liver disease in the preadolescent and adolescent age groups. NAFLD and NASH are more common in men.
NASH is frequently associated with obesity, type 2 diabetes mellitus, and hyperlipidemia. Most patients with NASH are asymptomatic although fatigue, malaise, and vague right upper abdominal discomfort bring some patients to medical attention. The most common presentation is elevation of liver aminotransferases detected on routine laboratory testing. Hepatomegaly is common. Serum AST and ALT are elevated in almost 90 percent of patients [12]. The AST/ALT ratio is usually less than 1 ; this is much lower than the ratio in alcoholic hepatitis, which is usually above 2. Alkaline phosphatase is less frequently elevated and hyperbilirubinemia is uncommon. DIAGNOSIS— Various radiological methods can detect the presence of fat in the liver but no imaging modality is able to differentiate between the histological subtypes of relatively benign nonalcoholic hepatic steatosis or more aggressive NASH. Liver biopsy provides the definitive diagnosis.
Patients with NAFLD have slightly lower overall survival than expected for the general population. Higher mortality is associated with increasing age, impaired fasting glucose, and cirrhosis. In most patients, there is little change in liver function tests throughout the course of the disease, although there may be histological progression to fibrosis and cirrhosis.
Certain infectious diseases with significant hepatic involvement Cytomegalovirus is a viral genus of the viral group known as Herpesviridae or
herpesviruses. The species that infects humans is commonly known as human CMV
(HCMV) or human herpesvirus-5 (HHV-5). All herpesviruses share a characteristic ability to remain latent within the body over long periods. Although they may be found throughout the body, CMV infections are frequently associated with the salivary glands in humans and other mammals. The spectrum of human illness caused by cytomegalovirus (CMV) is diverse and mostly dependent on the host-CMV infections in immunocompromised patients cause substantial morbidity and mortality, especially among transplant recipients and those infected with the human immunodeficiency virus (HIV). Infection in the immunocompetent host is generally asymptomatic or may present as a mononucleosis syndrome. However, occasionally primary CMV infection can lead to severe organ specific complications with significant morbidity and mortality. CMV establishes latent infection after the resolution of acute infection. Secondary, symptomatic disease may present later in the life of the host, reflecting one of two possibilities: reactivation of latent CMV or reinfection with a novel exogenous strain. Reactivation of CMV may occur at any time during the life of the human host, although the risk is higher in the setting of systemic immunosuppression, either iatrogenic or secondary to underlying medical conditions, such as the acquired
immunodeficiency syndrome (AIDS). The bulk of CMV-related disease in immunocompetent hosts is related to primary infection. The proportion of humans with evidence of prior CMV infection varies throughout the world, with seroprevalence rates ranging between 40 to 100 percent of the adult population. The prevalence of CMV-specific antibody increases with age. A syndrome resembling infectious mononucleosis is the most common presentation of symptomatic CMV infection in immunocompetent adults. Two cardinal hematologic abnormalities help to define the syndrome of mononucleosis: an absolute lymphocytosis with greater than 50 percent mononuclear cells and the presence of more than 10 percent
atypical lymphocytes on peripheral blood smear. Liver function abnormalities are frequently encountered in patients with symptomatic CMV infection. Subclinical transaminitis is the most common finding in immunocompetent patients; elevations of alkaline phosphatase and total bilirubin are less typical. Occasionally, patients will present with more significant laboratory abnormalities or signs of hepatic dysfunction.
Schistosomiasis is a parasitic disease caused by several species of trematodes
(platyhelminth infection, or "flukes"), a parasitic worm of the genus Schistosoma. Schistosomiasis is a chronic illness that can damage internal organs and, in children, impair growth and cognitive development. Schistosomiasis is the second most socioeconomically devastating parasitic disease after malaria. This disease is most commonly found in Asia, Africa, and South America, especially in areas where the water contains numerous freshwater snails, which may carry the parasite. The disease affects many people in developing countries, particularly children who may acquire the disease by swimming or playing in infected water. Schistosomiasis can be associated with serious morbidity and mortality. Chronic complications are generally seen in those with a high parasite load, which usually occurs in individuals who live in endemic areas and have recurrent exposure.
However, schistosomiasis can also cause complications in people with even brief exposures, such as travelers. It is estimated that more than 200 million people worldwide have schistosomiasis and that the infection is responsible for more than 200,000 deaths annually. On a global scale, 1 in every 30 individuals has schistosomiasis. Schistosomiasis is associated with significant morbidity including anemia, chronic pain, diarrhea, exercise intolerance, malnutrition, bladder cancer, portal hypertension and CNS complications.
Although most infections occur in residents of endemic areas, it has been clearly
documented that brief freshwater exposure is sufficient to establish infection; thus, travelers may also be infected. Most patients infected with schistosomes of all species are
asymptomatic. The clinical picture in individuals presenting in nonendemic countries tends to be different from manifestations seen in the developing world because of differences in immunity to the parasite and in intensity of infection. Acute symptoms tend to be more common in nonimmune individuals, such as travelers, due to a more intense immune response to exposure. By contrast, chronic complications require a higher burden of infection and, thus, are mainly seen in individuals from endemic areas.
Liver schistosomiasis tends to express itself in one of two distinct syndromes.
• Inflammatory hepatic schistosomiasis is the main cause of hepatomegaly and severe splenomegaly in children and adolescents. The severity of disease is related to the intensity of the egg infestation.
· Chronic hepatic schistosomiasis develops years later in young and middle-aged
adults with a long duration of intense infection with accompanying splenomegaly and portal hypertension. However, hepatocellular function remains normal. Leading causes of morbidity and mortality include the formation of ascites and esophageal bleeding from varices.
Leptospirosis is a disease caused by infection with bacteria of the genus Leptospira, and affects humans as well as other mammals, birds, amphibians, and reptiles. Though recognised among the world's most common diseases transmitted to people from animals, leptospirosis is nonetheless a relatively rare bacterial infection in humans. The infection is commonly transmitted to humans by allowing water that has been contaminated by animal urine to come in contact with unhealed breaks in the skin, the eyes, or with the mucous membranes. In the United States, most cases are reported from the southern and Pacific coastal states. Hawaii consistently reports the most cases of any state. Worldwide, endemic disease occurs in the tropics. The incidence of leptospirosis in some endemic countries appears to be increasing. Humans most often become infected after exposure to
environmental sources, such as animal urine, contaminated water or soil, or infected animal tissue. Portals of entry include cuts or abraded skin, mucous membranes or conjunctiva. While disease in humans is often sporadic, outbreaks may occur from common source exposures. Leptospirosis is associated with a variable clinical course. The disease may manifest as a subclinical illness followed by seroconversion, a self-limited systemic infection, or a severe, potentially fatal illness accompanied by multiorgan failure. Leptospirosis presents with the abrupt onset of fever, rigors, myalgias and headache in 75 to 100 percent of patients, after an incubation period of two to 26 days (average 10 days). From 25 to 35 percent of cases have an associated nonproductive cough and approximately 50 percent experience nausea, vomiting and diarrhea. Physical examination is often unrevealing but many patients have muscle tenderness, splenomegaly, lymphadenopathy, pharyngitis, hepatomegaly, muscle rigidity, abnormal respiratory auscultation, or skin rash. Jaundice may be observed in the most severe form of the disease and be associated with markedly elevated serum bilirubin concentrations. Routine laboratory tests are generally
nondiagnostic. White blood cell (WBC) counts may be elevated. Urinalysis frequently shows proteinuria, pyuria, granular casts and occasionally microscopic hematuria. Elevated creatine kinase is common. Approximately 40 percent of patients have minimal to moderate elevations of hepatic transaminases (usually <200 IU/L). Hyponatremia is common in severe leptospirosis.
LNA antisense oligonucleotide inhibitor of microRNA-122
The LNA antisense oligonucleotide, also referred to as an oligomer herein, may comprise at least 6, such as at least 7 consecutive nucleobases which are complementary to a part of a miR-122 sequence, such as the mature hsa-miR-122 sequence. The LNA antisense oligonucleotide may therefore comprise the complement of the miR-122 seed region. The LNA antisense oligonucleotide comprises at least one LNA monomer and typically at least 30%, such as at least 40%, such as at least 50% of the nucleoside monomers in the oligonucleotide are LNA oligonucleotides.
Efficacy of a microRNA-122 inhibitor, such as an LNA antisense oligonucleotide, may be determined by measuring the effect on secondary or tertiary indices for microRNA-122 activity in vivo. For example, de-repression of known microRNA-122 mRNA targets
(secondary indices) or blood serum cholesterol (tertiary indices) in vivo, for example in mice or in primates (Elmen et al. Nature 2008). However, the discovery that the mechanism of miR-122 interaction with the HCV virus is fundamentally distinct from the mechanism of miR- 122 mediated mRNA repression provides strong evidence that assays based on in vivo inhibition of mRNA targets of miR-122 are likely to be inadequate for prediction of clinically relevant inhibition of HCV replication. In this regard, inhibitors of microRNA-122 which may be effective in treatment of HCV in vivo typically are highly effective in de-repressing mRNA targets (e.g. at least 3 fold de-repression of AldoA or Ndrg3 in vivo in mouse), and/or highly effective in lowering blood serum cholesterol (e.g. by about at least 30 - 40% in mice - see Elmen et al, Nature 2008). In some embodiments, the antimiR-122 oligonucleotide is designed as a mixmer or totalmer that is essentially incapable of recruiting RNAseH.
Oligonucleotides that are essentially incapable of recruiting RNAseH are well known in the literature, in example see WO2007/1 12754, WO2007/1 12753, or WO2009/043353. Such compounds include LNA oligonucleotides which do not comprise a region of 5 or more consecutive DNA nucleosides, and is some instances do not comprise a region of 4 or more consecutive DNA nucleotides, and in some instances do not comprise a region of 3 or more
consecutive DNA nucleotides. In some embodiments, the LNA antisense oligonucleotide does not comprise a region of 5 or more non-LNA nucleosides, and is some instances do not comprise a region of 4 or more consecutive non-LNA nucleotides, and in some instances do not comprise a region of 3 or more consecutive non-LNA nucleotides. In some embodiments all the LNA nucleoside monomers present in the LNA antisense
oligonucleotide are beta-D-oxy LNA monomers. In some embodiments, the LNA-antisense oligonucleotide inhibitor or microRNA-122 does not comprise 2' substituted nucleoside monomers, such as 2' MOE nucleoside monomers. In some embodiments, the LNA- antisense oligonucleotide inhibitor or microRNA-122 is not one of compounds ISIS 387082, ISIS 387083, ISIS 387574, ISIS 387575, ISIS 387581 , ISIS 396604, ISIS 396605 and ISIS 387582, such as is not ISIS 387574, as disclosed in WO2007/027775 (specifically hereby incorporated by reference). In some embodiments, the LNA-antisense oligonucleotide inhibitor or microRNA-122 does not have a nucleoside motif of 5'-(A-A-B)n(-A)nn-3' wherein A is 2'MOE and B is LNA, and n is 6 to 7 and nn is 0 to 2, such as (A-A-B)7(-A-A)1 .
Length
In some embodiments the antisense oligonucleotide has a length of 7 - 25 (contiguous) nucleotides, such as 8, 9, 10, 1 1 , 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 , 22, 23, or 24 (contiguous) nucleotides. In some embodiments, the antisense oligonucleotide has a length of 7 - 10 (contiguous) nucleotide, or in some instances 7 - 16 nucleotides. In some embodiments, the antisense oligonucleotide at least 8 (contiguous) nucleotides in length, between 10-17 or 10 - 16 or 10-15 (contiguous) nucleotides, such as between 12 - 15 (contiguous) nucleotides.
Oligomers which are essentially incapable of recruiting RNAseH
In some embodiments, the oligomer is (essentially) incapable of recruiting RNaseH. EP 1 222 309 provides in vitro methods for determining RNaseH activity, which may be used to determine the ability to recruit RNaseH. A oligomer is deemed capable of recruiting RNase H if, when provided with the complementary RNA target, it has an initial rate, as measured in pmol/l/min, of at least 0.5%, such as at least 1 %, such as at least 5%, such as at least 10% or less than 20% of the equivalent DNA only oligonucleotide, with no 2' substitutions, with phosphorothioate linkage groups between all nucleotides in the oligonucleotide, using the methodology provided by Example 91 - 95 of EP 1 222 309.
In some embodiments, an oligomer is deemed essentially incapable of recruiting RNaseH if, when provided with the complementary RNA target, and RNaseH, the RNaseH initial rate,
as measured in pmol/l/min, is less than 1 %, such as less than 5%, such as less than 10% or less than 20% of the initial rate determined using the equivalent DNA only oligonucleotide, with no 2' substitutions, with phosphorothioate linkage groups between all nucleotides in the oligonucleotide, using the methodology provided by Example 91 - 95 of EP 1 222 309. Suitably, the complementary RNA target may be longer that the olgiomer to allow for easy detection of RNaseH activity.
It should be recognised that oligonucleotides which are mixmers or totalmers are usually essentially incapable of recruiting RNAseH and as such where we use the term essentially incapable or recruiting RNaseH herein, in some embodiments, such a term may be replaced with the term mixmer or totalmer, as defined herein.
The oligomer may, in some embodiments, be either i) fully complementary to either the full microRNA-122 sequence, such as the mature miR-122 sequence, or ne complementary to a sub-sequence of contiguous nucleotides present in the miRNA-122 target, or ii) comprises no more than a single mismatch with the complement of a sub-sequence of contiguous nucleotides present in said RNA target. As such the oligonucleotide is an antisense oligonucleoitde - in that it is either fully complementary to the corresponding region of the target sequence, or comprises no more than a single mismatch with the corresponding region of the target sequence.
The oligomer may therefore be an oligomer which targets (i.e. comprises or consists of a contiguous nucleotide sequence which is fully complementary to (a corresponding region of) microRNA-122 or comprises of no more than a single mismatch thereto. Such
oligonucleotides may be referred to as anti-microRNA-122 oligonucleotides. In some embodiments, the LNA antisense oligonucleotide comprises only LNA and optionally also DNA nucleosides monomers. Suitably the LNA oligonucleotides comprise at least one phosphorothioatelinkage and may be fully phosphorothiolated - i.e. all
internucleside linkages are phosphorothioate linkages. When shorter LNA oligonucleotides are used, for example compounds of 7, 8 or 9 nucleotides in length, it is preferred that at least 70% of the nucleosides present are LNA nucleosides, such as all nucleosides are LNA nucleosides. Such short LNA oligonucleotides may comprise a sequence which is complementary to the miR-122 seed sequence. Examples of short LNA oligonucleotides which target miR-122 are provided in WO2009/043353. It some embodiments, the LNA monomers are beta-D-oxy LNA ( 4'-CH2-0-2' Bicyclic Nucleic Acid).
Examples of modulators of microRNA-122 useful in the invention
Specially preferred compounds for use in the present invention are those that target microRNA-122. The sequence of miR-122 can be found in the microRNA database
"mirbase" (http://microrna.sanger.ac.uk/sequences/). Inhibitors of microRNA-122 have been described in numerous patents and articles and are well known to the person skilled in the art. In a some embodiments, examples of such documents describing useful microRNA-122 modulators are WO2007/1 12754, WO2007/1 12753, or WO2009/043353 all of which are hereby incorporated by reference.
Miravirsen (SPC3649)
In a preferred embodiment the antisense oligomer is miravirsen (SPC3649) which has the
, . _., m_ o . o, , _ oT o m_ o m_ o. m_ om_o„,
formula: 5'- Cs csAg tstsGs Ts csas Cs as Cs ts Cs C -3'
wherein; a lowercase letter identifies a DNA unit, and an upper case letter identifies a LNA unit, mC identifies a 5-methylcytosine LNA, subscript s identifies a phosphorothioate internucleoside linkage, and wherein LNA units are beta-D-oxy, as identified by a ° superscript after LNA residue.
Miravirsen, is the first microRNA-targeted drug to enter clinical trials. Miravirsen may be used as monotherapy or in combination with direct acting antiviral agents as an interferon- free treatment for chronic HCV infection in multiple genotypes.
The term "oligomer" in the context of the present invention, refers to a molecule formed by covalent linkage of two or more nucleotides (i.e. an oligonucleotide). Herein, a single nucleotide (unit) may also be referred to as a monomer or unit. In some
embodiments, the terms "nucleoside", "nucleotide", "unit" and "monomer" are used interchangeably. It will be recognised that when referring to a sequence of nucleotides or monomers, what is referred to is the sequence of bases, such as A, T, G, C or U.
The oligomer typically consists or comprises of a contiguous nucleotide sequence of from 7 - 25 units.
In various embodiments, the compound of the invention does not comprise RNA (units). It is preferred that the compound according to the invention is a linear molecule or is synthesised as a linear molecule. The oligomer is a single stranded molecule, and preferably
does not comprise short regions of, for example, at least 3, 4 or 5 contiguous nucleotides, which are complementary to equivalent regions within the same oligomer (i.e. duplexes) - in this regards, the oligomer is not (essentially) double stranded. In some embodiments, the oligomer is essentially not double stranded, such as is not a siRNA. In various
embodiments, the oligomer of the invention may consist entirely of the contiguous nucleotide region. Thus, the oligomer is not substantially self-complementary.
The terms "corresponding nucleotide analogue" and "corresponding nucleotide" are intended to indicate that the nucleotide in the nucleotide analogue and the naturally occurring nucleotide are identical. For example, when the 2-deoxyribose unit of the nucleotide is linked to an adenine, the "corresponding nucleotide analogue" contains a pentose unit (different from 2-deoxyribose) linked to an adenine.
The terms "reverse complement", "reverse complementary" and "reverse
complementarity" as used herein are interchangeable with the terms "complement", "complementary" and "complementarity".
Nucleosides and Nucleoside analogues
In some embodiments, the terms "nucleoside analogue" and "nucleotide analogue" are used interchangeably.
The term "nucleotide" as used herein, refers to a glycoside comprising a sugar moiety, a base moiety and a covalently linked group (linkage group), such as a phosphate or phosphorothioate internucleotide linkage group, and covers both naturally occurring nucleotides, such as DNA or RNA, and non-naturally occurring nucleotides comprising modified sugar and/or base moieties, which are also referred to as "nucleotide analogues" herein. Herein, a single nucleotide (unit) may also be referred to as a monomer or nucleic acid unit.
In field of biochemistry, the term "nucleoside" is commonly used to refer to a glycoside comprising a sugar moiety and a base moiety, and may therefore be used when referring to the nucleotide units, which are covalently linked by the internucleotide linkages between the nucleotides of the oligomer. In the field of biotechnology, the term "nucleotide" is often used to refer to a nucleic acid monomer or unit, and as such in the context of an oligonucleotide may refer to the base - such as the "nucleotide sequence", typically refer to the nucleobase sequence (i.e. the presence of the sugar backbone and internucleoside linkages are implicit). Likewise, particularly in the case of oligonucleotides where one or more of the internucleoside linkage groups are modified, the term "nucleotide" may refer to a
"nucleoside" for example the term "nucleotide" may be used, even when specifiying the presence or nature of the linkages between the nucleosides.
As one of ordinary skill in the art would recognise, the 5' terminal nucleotide of an oligonucleotide does not comprise a 5' internucleotide linkage group, although may or may not comprise a 5' terminal group.
Non-naturally occurring nucleotides include nucleotides which have modified sugar moieties, such as bicyclic nucleotides or 2' modified nucleotides, such as 2' substituted nucleotides.
"Nucleotide analogues" are variants of natural nucleotides, such as DNA or RNA nucleotides, by virtue of modifications in the sugar and/or base moieties. Analogues could in principle be merely "silent" or "equivalent" to the natural nucleotides in the context of the oligonucleotide, i.e. have no functional effect on the way the oligonucleotide works to inhibit target gene expression. Such "equivalent" analogues may nevertheless be useful if, for example, they are easier or cheaper to manufacture, or are more stable to storage or manufacturing conditions, or represent a tag or label. Preferably, however, the analogues will have a functional effect on the way in which the oligomer works to inhibit expression; for example by producing increased binding affinity to the target and/or increased resistance to intracellular nucleases and/or increased ease of transport into the cell. Specific examples of nucleoside analogues are described by e.g. Freier & Altmann; Nucl. Acid Res., 1997, 25, 4429-4443 and Uhlmann; Curr. Opinion in Drug Development, 2000, 3(2), 293-213, and in Scheme 1 :
o
0=P-BH,"
Boranophosphates
Scheme 1
The oligomer may thus comprise or consist of a simple sequence of natural occurring nucleotides - preferably 2'-deoxynucleotides (referred to here generally as "DNA"), but also possibly ribonucleotides (referred to here generally as "RNA"), or a combination of such naturally occurring nucleotides and one or more non-naturally occurring nucleotides, i.e. nucleotide analogues. Such nucleotide analogues may suitably enhance the affinity of the oligomer for the target sequence.
Examples of suitable and preferred nucleotide analogues are provided by
WO2007/031091 or are referenced therein.
Incorporation of affinity-enhancing nucleotide analogues in the oligomer, such as LNA or 2'-substituted sugars, can allow the size of the specifically binding oligomer to be reduced, and may also reduce the upper limit to the size of the oligomer before non-specific or aberrant binding takes place.
In some embodiments, the oligomer comprises at least 1 nucleoside analogue. In some embodiments the oligomer comprises at least 2 nucleotide analogues. In some embodiments, the oligomer comprises from 3-8 nucleotide analogues, e.g. 6 or 7 nucleotide analogues. In the by far most preferred embodiments, at least one of said nucleotide
analogues is a locked nucleic acid (LNA); for example at least 3 or at least 4, or at least 5, or at least 6, or at least 7, or 8, of the nucleotide analogues may be LNA. In some
embodiments all the nucleotides analogues may be LNA.
It will be recognised that when referring to a preferred nucleotide sequence motif or nucleotide sequence, which consists of only nucleotides, the oligomers of the invention which are defined by that sequence may comprise a corresponding nucleotide analogue in place of one or more of the nucleotides present in said sequence, such as LNA units or other nucleotide analogues, which raise the duplex stability/Tm of the oligomer/target duplex (i.e. affinity enhancing nucleotide analogues).
In some embodiments, any mismatches between the nucleotide sequence of the oligomer and the target sequence are preferably found in regions outside the affinity enhancing nucleotide analogues, such as region B as referred to herein, and/or region D as referred to herein, and/or at the site of non modified such as DNA nucleotides in the oligonucleotide, and/or in regions which are 5' or 3' to the contiguous nucleotide sequence.
Examples of such modification of the nucleotide include modifying the sugar moiety to provide a 2'-substituent group or to produce a bridged (locked nucleic acid) structure which enhances binding affinity and may also provide increased nuclease resistance.
A preferred nucleotide analogue is LNA, such as oxy-LNA (such as beta-D-oxy-LNA, and alpha-L-oxy-LNA), and/or amino-LNA (such as beta-D-amino-LNA and alpha-L-amino- LNA) and/or thio-LNA (such as beta-D-thio-LNA and alpha-L-thio-LNA) and/or ENA (such as beta-D-ENA and alpha-L-ENA). Most preferred is beta-D-oxy-LNA.
In some embodiments the nucleotide analogues present within the oligomer of the invention (such as in regions A and C mentioned herein) are independently selected from, for example: 2'-0-alkyl-RNA units, 2'-amino-DNA units, 2'-fluoro-DNA units, LNA units, arabino nucleic acid (ANA) units, 2'-fluoro-ANA units, HNA units, INA (intercalating nucleic acid -Christensen, 2002. Nucl. Acids. Res. 2002 30: 4918-4925, hereby incorporated by reference) units and 2'MOE units. In some embodiments there is only one of the above types of nucleotide analogues present in the oligomer of the invention, or contiguous nucleotide sequence thereof.
In some embodiments the nucleotide analogues are 2'-0-methoxyethyl-RNA (2'MOE),
2'-fluoro-DNA monomers or LNA nucleotide analogues, and as such the oligonucleotide of the invention may comprise nucleotide analogues which are independently selected from these three types of analogue, or may comprise only one type of analogue selected from the three types. In some embodiments at least one of said nucleotide analogues is 2'-MOE-
RNA, such as 2, 3, 4, 5, 6, 7, 8, 9 or 10 2'-MOE-RNA nucleotide units. In some
embodiments at least one of said nucleotide analogues is 2'-fluoro DNA, such as 2, 3, 4, 5, 6, 7, 8, 9 or 10 2'-fluoro-DNA nucleotide units.
In some embodiments, the oligomer according to the invention comprises at least one Locked Nucleic Acid (LNA) unit, such as 1 , 2, 3, 4, 5, 6, 7, or 8 LNA units, such as from 3 - 7 or 4 to 8 LNA units, or 3, 4, 5, 6 or 7 LNA units. In some embodiments, all the nucleotide analogues are LNA. In some embodiments, the oligomer may comprise both beta-D-oxy- LNA, and one or more of the following LNA units: thio-LNA, amino-LNA, oxy-LNA, and/or ENA in either the beta-D or alpha-L configurations or combinations thereof. In some embodiments all LNA cytosine units are 5'methyl-Cytosine. In some embodiments of the invention, the oligomer may comprise both LNA and DNA units. Preferably the combined total of LNA and DNA units is 10-25, such as 10 - 24, preferably 10-20, such as 10 - 18, even more preferably 12-16. In some embodiments of the invention, the nucleotide sequence of the oligomer, such as the contiguous nucleotide sequence consists of at least one LNA and the remaining nucleotide units are DNA units. In some embodiments the oligomer comprises only LNA nucleotide analogues and naturally occurring nucleotides (such as RNA or DNA, most preferably DNA nucleotides), optionally with modified internucleotide linkages such as phosphorothioate.
The term "nucleobase" refers to the base moiety of a nucleotide and covers both naturally occuring a well as non-naturally occurring variants. Thus, "nucleobase" covers not only the known purine and pyrimidine heterocycles but also heterocyclic analogues and tautomeres thereof.
Examples of nucleobases include, but are not limited to adenine, guanine, cytosine, thymidine, uracil, xanthine, hypoxanthine, 5-methylcytosine, isocytosine, pseudoisocytosine, 5-bromouracil, 5-propynyluracil, 6-aminopurine, 2-aminopurine, inosine, diaminopurine, and 2-chloro-6-aminopurine.
In some embodiments, at least one of the nucleobases present in the oligomer is a modified nucleobase selected from the group consisting of 5-methylcytosine, isocytosine, pseudoisocytosine, 5-bromouracil, 5-propynyluracil, 6-aminopurine, 2-aminopurine, inosine, diaminopurine, and 2-chloro-6-aminopurine.
LNA
The term "LNA" refers to a bicyclic nucleoside analogue, known as "Locked Nucleic Acid". It may refer to an LNA monomer, or, when used in the context of an "LNA
oligonucleotide", LNA refers to an oligonucleotide containing one or more such bicyclic
nucleotide analogues. LNA nucleotides are characterised by the presence of a linker group (such as a bridge) between C2' and C4' of the ribose sugar ring - for example as shown as the biradical R4* - R2* as described below.
The LNA used in the oligonucleotide compounds of the invention preferably has the structure of the eneral formula I
wherein for all chiral centers, asymmetric groups may be found in either R or S orientation ;
wherein X is selected from -0-, -S-, -N(RN*)-, -C(R6R6*)-, such as, in some
embodiments -0-;
B is selected from hydrogen, optionally substituted Ci-4-alkoxy, optionally substituted Ci-4-alkyl, optionally substituted Ci-4-acyloxy, nucleobases including naturally occurring and nucleobase analogues, DNA intercalators, photochemically active groups, thermochemically active groups, chelating groups, reporter groups, and ligands; preferably, B is a nucleobase or nucleobase analogue;
P designates an internucleotide linkage to an adjacent monomer, or a 5'-terminal group, such internucleotide linkage or 5'-terminal group optionally including the substituent R5 or equally applicable the substituent R5*;
P* designates an internucleotide linkage to an adjacent monomer, or a 3'-terminal group;
R4* and R2* together designate a bivalent linker group consisting of 1 - 4 groups/atoms selected from -C(RaRb)-, -C(Ra)=C(Rb)-, -C(Ra)=N-, -0-, -Si(Ra)2-, -S-, -S02-, -N(Ra)-, and >C=Z, wherein Z is selected from -0-, -S-, and -N(Ra)-, and Ra and Rb each is independently selected from hydrogen, optionally substituted Ci-i2-alkyl, optionally substituted C2-i2-alkenyl, optionally substituted C2-i2-alkynyl, hydroxy, optionally substituted Ci-i2-alkoxy, C2-i2- alkoxyalkyl, C2-i2-alkenyloxy, carboxy, Ci-i2-alkoxycarbonyl, Ci-i2-alkylcarbonyl, formyl, aryl,
aryloxy-carbonyl, aryloxy, arylcarbonyl, heteroaryl, heteroaryloxy-carbonyl, heteroaryloxy, heteroarylcarbonyl, amino, mono- and di(Ci-6-alkyl)amino, carbamoyl, mono- and di(Ci-6- alkyl)-amino-carbonyl, amino-Ci-6-alkyl-aminocarbonyl, mono- and di(Ci-6-alkyl)amino-Ci-6- alkyl-aminocarbonyl, Ci-6-alkyl-carbonylamino, carbamido, Ci-6-alkanoyloxy, sulphono, Ci-6- alkylsulphonyloxy, nitro, azido, sulphanyl, Ci-6-alkylthio, halogen, DNA intercalators, photochemically active groups, thermochemically active groups, chelating groups, reporter groups, and ligands, where aryl and heteroaryl may be optionally substituted and where two geminal substituents Ra and Rb together may designate optionally substituted methylene (=CH2), wherein for all chiral centers, asymmetric groups may be found in either R or S orientation, and;
each of the substituents R1*, R2, R3, R5, R5*, R6 and R6*, which are present is independently selected from hydrogen, optionally substituted Ci-i2-alkyl, optionally substituted C2-i2-alkenyl, optionally substituted C2-i2-alkynyl, hydroxy, Ci-i2-alkoxy, C2-i2- alkoxyalkyl, C2-i2-alkenyloxy, carboxy, Ci-i2-alkoxycarbonyl, Ci-i2-alkylcarbonyl, formyl, aryl, aryloxy-carbonyl, aryloxy, arylcarbonyl, heteroaryl, heteroaryloxy-carbonyl, heteroaryloxy, heteroarylcarbonyl, amino, mono- and di(Ci-6-alkyl)amino, carbamoyl, mono- and di(Ci-6- alkyl)-amino-carbonyl, amino-Ci-6-alkyl-aminocarbonyl, mono- and di(Ci-6-alkyl)amino-Ci-6- alkyl-aminocarbonyl, Ci-6-alkyl-carbonylamino, carbamido, Ci-6-alkanoyloxy, sulphono, Ci-6- alkylsulphonyloxy, nitro, azido, sulphanyl, Ci-6-alkylthio, halogen, DNA intercalators, photochemically active groups, thermochemically active groups, chelating groups, reporter groups, and ligands, where aryl and heteroaryl may be optionally substituted, and where two geminal substituents together may designate oxo, thioxo, imino, or optionally substituted methylene; ; wherein RN is selected from hydrogen and Ci-4-alkyl, and where two adjacent (non-geminal) substituents may designate an additional bond resulting in a double bond; and RN*, when present and not involved in a biradical, is selected from hydrogen and Ci-4-alkyl; and basic salts and acid addition salts thereof. For all chiral centers, asymmetric groups may be found in either R or S orientation.
In some embodiments, R4* and R2* together designate a biradical consisting of a groups selected from the group consisting of C(RaRb)-C(RaRb)-, C(RaRb)-0-, C(RaRb)-NRa-, C(RaRb)-S-, and C(RaRb)-C(RaRb)-0-, wherein each Ra and Rb may optionally be
independently selected. In some embodiments, Ra and Rb may be, optionally independently selected from the group consisting of hydrogen and Ci-6alkyl, such as methyl, such as hydrogen.
In some embodiments, R4* and R2* together designate the biradical -0-CH(CH2OCH3)- (2'0-methoxyethyl bicyclic nucleic acid - Seth at al., 2010, J. Org. Chem) - in either the R- or S- configuration.
In some embodiments, R4* and R2* together designate the biradical -0-CH(CH2CH3)- (2'0-ethyl bicyclic nucleic acid - Seth at al., 2010, J. Org. Chem). - in either the R- or S- configuration.
In some embodiments, R4* and R2* together designate the biradical -0-CH(CH3)-. - in either the R- or S- configuration. In some embodiments, R4* and R2* together designate the biradical -0-CH2-0-CH2- - (Seth at al., 2010, J. Org. Chem).
In some embodiments, R4* and R2* together designate the biradical -0-NR-CH3- -
(Seth at al., 2010, J. Org. Chem) .
In some embodiments, the LNA units have a structure selected from the following group:
In some embodiments, R1*, R2, R3, R5, R5* are independently selected from the group consisting of hydrogen, halogen, Ci_6 alkyl, substituted Ci-6 alkyl, C2-6 alkenyl, substituted C2-6 alkenyl, C2-6 alkynyl or substituted C2-6 alkynyl, Ci-6 alkoxyl, substituted Ci-6 alkoxyl, acyl, substituted acyl, Ci-6 aminoalkyl or substituted Ci-6 aminoalkyl. For all chiral centers, asymmetric groups may be found in either R or S orientation.
In some embodiments, R1*, R2, R3, R5, R5* are hydrogen.
In some embodiments, R1*, R2, R3 are independently selected from the group consisting of hydrogen, halogen, Ci_6 alkyl, substituted Ci-6 alkyl, C2-6 alkenyl, substituted C2-6 alkenyl, C2-6 alkynyl or substituted C2-6 alkynyl, Ci-6 alkoxyl, substituted Ci-6 alkoxyl, acyl, substituted acyl, Ci-6 aminoalkyl or substituted Ci-6 aminoalkyl. For all chiral centers, asymmetric groups may be found in either R or S orientation.
In some embodiments, R1*, R2, R3 are hydrogen.
In some embodiments, R5 and R5* are each independently selected from the group consisting of H, -CH3, -CH2-CH3,- CH2-0-CH3, and -CH=CH2. Suitably in some
embodiments, either R5 or R5* are hydrogen, where as the other group (R5 or R5*
respectively) is selected from the group consisting of Ci-5 alkyl, C2-6 alkenyl, C2-6 alkynyl, substituted Ci-6 alkyl, substituted C2-6 alkenyl, substituted C2-6 alkynyl or substituted acyl (- C(=0)-); wherein each substituted group is mono or poly substituted with substituent groups independently selected from halogen, Ci-6 alkyl, substituted Ci-6 alkyl, C2-6 alkenyl, substituted C2-6 alkenyl, C2-6 alkynyl, substituted C2-6 alkynyl, OJi, SJi, NJiJ2, N3, COOJ1, CN, 0-C(=0)NJ1J2, N(H)C(=NH)NJ,J2 or N(H)C(=X)N(H)J2 wherein X is O or S; and each J, and J2 is, independently, H, Ci-6 alkyl, substituted Ci-6 alkyl, C2-6 alkenyl, substituted C2-6 alkenyl, C2-6 alkynyl, substituted C2-6 alkynyl, Ci-6 aminoalkyl, substituted Ci-6 aminoalkyl or a protecting group. In some embodiments either R5 or R5* is substituted Ci-6 alkyl. In some embodiments either R5 or R5* is substituted methylene wherein preferred substituent groups include one or more groups independently selected from F, NJ^, N3, CN, OJi, SJi, O- C(=0)NJ1J2, N(H)C(=NH)NJ, J2 or N(H)C(0)N(H)J2. In some embodiments each J, and J2 is, independently H or Ci-6 alkyl. In some embodiments either R5 or R5* is methyl, ethyl or methoxymethyl. In some embodiments either R5 or R5* is methyl. In a further embodiment either R5 or R5* is ethylenyl. In some embodiments either R5 or R5* is substituted acyl. In some embodiments either R5 or R5* is C(=0)NJ1J2. For all chiral centers, asymmetric groups may be found in either R or S orientation. Such 5' modified bicyclic nucleotides are disclosed in WO 2007/134181 , which is hereby incorporated by reference in its entirety.
In some embodiments B is a nucleobase, including nucleobase analogues and naturally occurring nucleobases, such as a purine or pyrimidine, or a substituted purine or substituted pyrimidine, such as a nucleobase referred to herein, such as a nucleobase selected from the group consisting of adenine, cytosine, thymine, adenine, uracil, and/or a modified or substituted nucleobase, such as 5-thiazolo-uracil, 2-thio-uracil, 5-propynyl-uracil, 2'thio-thymine, 5-methyl cytosine, 5-thiozolo-cytosine, 5-propynyl-cytosine, and 2,6- diaminopurine.
In some embodiments, R4* and R2* together designate a biradical selected from - C(RaRb)-0-, -C(RaRb)-C(RcRd)-0-, -C(RaRb)-C(RcRd)-C(ReRf)-0-, -C(RaRb)-0-C(RcRd)-, - C(RaRb)-0-C(RcRd)-0-, -C(RaRb)-C(RcRd)-, -C(RaRb)-C(RcRd)-C(ReRf)-, - C(Ra)=C(Rb)-C(RcRd)-, -C(RaRb)-N(Rc)-, -C(RaRb)-C(RcRd)- N(Re)-, -C(RaRb)-N(Rc)-0-, and - C(RaRb)-S-, -C(RaRb)-C(RcRd)-S-, wherein Ra, Rb, Rc, Rd, Re, and Rf each is independently selected from hydrogen, optionally substituted Ci-i2-alkyl, optionally substituted C2-i2-alkenyl,
optionally substituted C2-i2-alkynyl, hydroxy, Ci-12-alkoxy, C2-i2-alkoxyalkyl, C2-i2-alkenyloxy, carboxy, Ci-12-alkoxycarbonyl, Ci-12-alkylcarbonyl, formyl, aryl, aryloxy-carbonyl, aryloxy, arylcarbonyl, heteroaryl, heteroaryloxy-carbonyl, heteroaryloxy, heteroarylcarbonyl, amino, mono- and di(Ci-6-alkyl)amino, carbamoyl, mono- and di(Ci-6-alkyl)-amino-carbonyl, amino- Ci-6-alkyl-aminocarbonyl, mono- and di(Ci-6-alkyl)amino-Ci-6-alkyl-aminocarbonyl, Ci-6-alkyl- carbonylamino, carbamido, Ci-6-alkanoyloxy, sulphono, Ci-6-alkylsulphonyloxy, nitro, azido, sulphanyl, Ci-6-alkylthio, halogen, DNA intercalators, photochemically active groups, thermochemically active groups, chelating groups, reporter groups, and ligands, where aryl and heteroaryl may be optionally substituted and where two geminal substituents Ra and Rb together may designate optionally substituted methylene (=CH2). For all chiral centers, asymmetric groups may be found in either R or S orientation.
In a further embodiment R4* and R2* together designate a biradical (bivalent group) selected from -CH2-0-, -CH2-S-, -CH2-NH-, -CH2-N(CH3)-, -CH2-CH2-0-, -CH2-CH(CH3)-, - CH2-CH2-S-, -CH2-CH2-NH-, -CH2-CH2-CH2-, -CH2-CH2-CH2-O-, -CH2-CH2-CH(CH3)-, - CH=CH-CH2-, -CH2-O-CH2-O-, -CH2-N H-O-, -CH2-N(CH3)-0-, -CH2-0-CH2-, -CH(CH3)-0-, and -CH(CH2-0-CH3)-0-, and/or, -CH2-CH2-, and -CH=CH- For all chiral centers, asymmetric groups may be found in either R or S orientation.
In some embodiments, R4* and R2* together designate the biradical C(RaRb)-N(Rc)-0-, wherein Ra and Rb are independently selected from the group consisting of hydrogen, halogen, Ci_6 alkyl, substituted Ci-6 alkyl, C2-6 alkenyl, substituted C2-6 alkenyl, C2-6 alkynyl or substituted C2-6 alkynyl, Ci-6 alkoxyl, substituted Ci-6 alkoxyl, acyl, substituted acyl, Ci-6 aminoalkyl or substituted Ci-6 aminoalkyl, such as hydrogen, and; wherein Rc is selected from the group consisting of hydrogen, halogen, Ci-6 alkyl, substituted Ci-6 alkyl, C2-6 alkenyl, substituted C2-6 alkenyl, C2-6 alkynyl or substituted C2-6 alkynyl, Ci-6 alkoxyl, substituted Ci-6 alkoxyl, acyl, substituted acyl, Ci-6 aminoalkyl or substituted Ci-6 aminoalkyl, such as hydrogen.
In some embodiments, R4* and R2* together designate the biradical C(RaRb)-0-C(RcRd) -0-, wherein Ra, Rb, Rc, and Rd are independently selected from the group consisting of hydrogen, halogen, Ci-6 alkyl, substituted Ci-6 alkyl, C2-6 alkenyl, substituted C2-6 alkenyl, C2-6 alkynyl or substituted C2-6 alkynyl, Ci-6 alkoxyl, substituted Ci-6 alkoxyl, acyl, substituted acyl, C1-6 aminoalkyl or substituted Ci-6 aminoalkyl, such as hydrogen.
In some embodiments, R4* and R2* form the biradical -CH(Z)-0-, wherein Z is selected from the group consisting of Ci-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, substituted Ci-6 alkyl,
substituted C2-6 alkenyl, substituted C2-6 alkynyl, acyl, substituted acyl, substituted amide, thiol or substituted thio; and wherein each of the substituted groups, is, independently, mono or poly substituted with optionally protected substituent groups independently selected from halogen, oxo, hydroxyl, OJ1 , NJiJz, SJi, N3,
and CN, wherein each J2 and J3 is, independently, H or Ci-6 alkyl, and X is O, S or N^ . In some embodiments Z is Ci-6 alkyl or substituted Ci-6 alkyl. In some embodiments Z is methyl. In some embodiments Z is substituted Ci-6 alkyl. In some embodiments said substituent group is Ci-6 alkoxy. In some embodiments Z is CH3OCH2-. For all chiral centers, asymmetric groups may be found in either R or S orientation. Such bicyclic nucleotides are disclosed in US 7,399,845 which is hereby incorporated by reference in its entirety. In some embodiments, R1*, R2, R3, R5, R5* are hydrogen. In some some embodiments, R1*, R2, R3 * are hydrogen, and one or both of R5, R5* may be other than hydrogen as referred to above and in WO 2007/134181 .
In some embodiments, R4* and R2* together designate a biradical which comprise a substituted amino group in the bridge such as consist or comprise of the biradical -CH2-N( Rc)-, wherein Rc is Ci _ i2 alkyloxy. In some embodiments R4* and R2* together designate a biradical -Cq3q4-NOR -, wherein q3 and q4 are independently selected from the group consisting of hydrogen, halogen, Ci_6 alkyl, substituted Ci-6 alkyl, C2-6 alkenyl, substituted C2-6 alkenyl, C2-6 alkynyl or substituted C2-6 alkynyl, Ci-6 alkoxyl, substituted Ci-6 alkoxyl, acyl, substituted acyl, Ci-6 aminoalkyl or substituted Ci-6 aminoalkyl; wherein each substituted group is, independently, mono or poly substituted with substituent groups independently selected from halogen, OJi, SJi, NJiJ2, COOJ1 , CN, 0-C(=0)NJ1J2, N(H)C(=NH)N J-,J2 or N(H)C(=X=N(H)J2 wherein X is O or S; and each of Ji and J2 is, independently, H, d-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, Ci-6 aminoalkyl or a protecting group. For all chiral centers, asymmetric groups may be found in either R or S orientation. Such bicyclic nucleotides are disclosed in WO2008/150729 which is hereby incorporated by reference in its entirity. In some embodiments, R1*, R2, R3, R5, R5* are independently selected from the group consisting of hydrogen, halogen, Ci_6 alkyl, substituted Ci-6 alkyl, C2-6 alkenyl, substituted C2-6 alkenyl, C2-6 alkynyl or substituted C2-6 alkynyl, Ci-6 alkoxyl, substituted Ci-6 alkoxyl, acyl, substituted acyl, Ci-6 aminoalkyl or substituted Ci-6 aminoalkyl. In some embodiments, R1*, R2, R3, R5, R5* are hydrogen. In some embodiments, R1*, R2, R3 are hydrogen and one or both of R5, R5* may be other than hydrogen as referred to above and in WO 2007/134181. In some embodiments R4* and R2* together designate a biradical (bivalent group) C(RaRb)-0-, wherein Ra and Rb are each independently halogen, C Ci2 alkyl, substituted C Ci2 alkyl,
C2-C12 alkenyl, substituted C2-C12 alkenyl, C2-C12 alkynyl, substituted C2-C12 alkynyl, C1-C12 alkoxy, substituted C1-C12 alkoxy, OJ1 SJi, SOJ1 , S02Ji, NJiJz, N3, CN,
N(H)C(=S)NJ1J2; or Ra and Rb together are =C(q3)(q4); q3 and q4 are each, independently, H, halogen, Ci-Ci2alkyl or substituted C Ci2 alkyi; each substituted group is, independently, mono or poly substituted with substituent groups independently selected from halogen, d- C6 alkyi, substituted Ci-C6 alkyi, C2- C6 alkenyl, substituted C2-C6 alkenyl, C2-C6 alkynyl, substituted C2-C6 alkynyl, OJ1 , SJi, NJiJ2, N3, CN,
O- C(=0)NJ1J2, N(H)C(=0)NJ1J2 or N(H)C(=S)NJ1J2. and; each J, and J2 is, independently, H, C1 -C6 alkyi, substituted C1 -C6 alkyi, C2-C6 alkenyl, substituted C2-C6 alkenyl, C2-C6 alkynyl, substituted C2-C6 alkynyl, C1 -C6 aminoalkyl, substituted C1 -C6 aminoalkyl or a protecting group. Such compounds are disclosed in WO2009006478A, hereby incorporated in its entirety by reference.
In some embodiments, R4* and R2* form the biradical - Q -, wherein Q is
C(qi)(q2)C(q3)(q4), C(qi)=C(q3), C[=C(qi)(q2)]-C(q3)(q4) or C(qi)(q2)-C[=C(q3)(q4)]; qi, q2, q3, q4 are each independently. H, halogen, CM2 alkyi, substituted CM2 alkyi, C2-i2 alkenyl, substituted Ci-i2 alkoxy, OJ1 , SJi, SOJ1 , SO2J1 , NJiJz, N3, CN,
C(=0) Ji,
each J, and J2 is, independently, H, Ci-6 alkyi, C2-6 alkenyl, C2-6 alkynyl, Ci-6 aminoalkyl or a protecting group; and, optionally wherein when Q is C(qi)(q2)(q3)(q4) and one of q3 or q4 is CH3 then at least one of the other of q3 or q4 or one of qi and q2 is other than H. In some embodiments, R1*, R2, R3, R5, R5* are hydrogen. For all chiral centers, asymmetric groups may be found in either R or S orientation. Such bicyclic nucleotides are disclosed in WO2008/154401 which is hereby incorporated by reference in its entirity. In some embodiments, R1*, R2, R3, R5, R5* are independently selected from the group consisting of hydrogen, halogen, Ci-6 alkyi, substituted Ci-6 alkyi, C2-6 alkenyl, substituted C2-6 alkenyl, C2-6 alkynyl or substituted C2-6 alkynyl, Ci-6 alkoxyl, substituted Ci-6 alkoxyl, acyl, substituted acyl, Ci-6 aminoalkyl or substituted Ci-6 aminoalkyl. In some embodiments, R1*, R2, R3, R5, R5* are hydrogen. In some embodiments, R1*, R2, R3 are hydrogen and one or both of R5, R5* may be other than hydrogen as referred to above and in WO 2007/134181 or WO2009/067647 (alpha-L- bicyclic nucleic acids analogs).
In some embodiments the LNA used in the oligonucleotide compounds of the invention preferably has the structure of the general formula II:
wherein Y is selected from the group consisting of -0-, -CH20-, -S-, -NH-, N(Re) and/or - CH2-; Z and Z* are independently selected among an internucleotide linkage, RH, a terminal group or a protecting group; B constitutes a natural or non-natural nucleotide base moiety (nucleobase), and RH is selected from hydrogen and Ci-4-alkyl; Ra, Rb Rc, Rd and Re are, optionally independently, selected from the group consisting of hydrogen, optionally substituted Ci-i2-alkyl, optionally substituted C2-i2-alkenyl, optionally substituted C2-i2-alkynyl, hydroxy, Ci-i2-alkoxy, C2-i2-alkoxyalkyl, C2-i2-alkenyloxy, carboxy, Ci-i2-alkoxycarbonyl, Ci_i2- alkylcarbonyl, formyl, aryl, aryloxy-carbonyl, aryloxy, arylcarbonyl, heteroaryl, heteroaryloxy- carbonyl, heteroaryloxy, heteroarylcarbonyl, amino, mono- and di(Ci-6-alkyl)amino, carbamoyl, mono- and di(Ci-6-alkyl)-amino-carbonyl, amino-Ci-6-alkyl-aminocarbonyl, mono- and di(Ci-6-alkyl)amino-Ci-6-alkyl-aminocarbonyl, Ci-6-alkyl-carbonylamino, carbamido, Ci-6- alkanoyloxy, sulphono, Ci-6-alkylsulphonyloxy, nitro, azido, sulphanyl, Ci-6-alkylthio, halogen, DNA intercalators, photochemically active groups, thermochemically active groups, chelating groups, reporter groups, and ligands, where aryl and heteroaryl may be optionally substituted and where two geminal substituents Ra and Rb together may designate optionally substituted methylene (=CH2); and RH is selected from hydrogen and Ci-4-alkyl. In some embodiments Ra, Rb Rc, Rd and Re are, optionally independently, selected from the group consisting of hydrogen and Ci-6 alkyl, such as methyl. For all chiral centers, asymmetric groups may be found in either R or S orientation, for example, two exemplary
stereochemical isomers include the beta-D and alpha-L isoforms, which may be illustrated as follows:
β-D-amino-LNA
The term "thio-LNA" comprises a locked nucleotide in which Y in the general formula above is selected from S or -CH2-S-. Thio-LNA can be in both beta-D and alpha-L- configuration.
The term "amino-LNA" comprises a locked nucleotide in which Y in the general formula above is selected from -N(H)-, N(R)-, CH2-N(H)-, and -CH2-N(R)- where R is selected from hydrogen and Ci-4-alkyl. Amino-LNA can be in both beta-D and alpha-L- configuration.
The term "oxy-LNA" comprises a locked nucleotide in which Y in the general formula above represents -0-. Oxy-LNA can be in both beta-D and alpha-L-configuration.
The term "ENA" comprises a locked nucleotide in which Y in the general formula above is -CH2-0- (where the oxygen atom of -CH2-0- is attached to the 2'-position relative to the base B). Re is hydrogen or methyl.
In some exemplary embodiments LNA is selected from beta-D-oxy-LNA, alpha-L-oxy-LNA, beta-D-amino-LNA and beta-D-thio-LNA, in particular beta-D-oxy-LNA.
Internucleotide Linkages
The monomers of the oligomers described herein are coupled together via linkage groups. Suitably, each monomer is linked to the 3' adjacent monomer via a linkage group.
The person having ordinary skill in the art would understand that, in the context of the present invention, the 5' monomer at the end of an oligomer does not comprise a 5' linkage group, although it may or may not comprise a 5' terminal group.
The terms "linkage group" or "internucleotide linkage" are intended to mean a group capable of covalently coupling together two nucleotides. Specific and preferred examples include phosphate groups and phosphorothioate groups.
The nucleotides of the oligomer of the invention or contiguous nucleotides sequence thereof are coupled together via linkage groups. Suitably each nucleotide is linked to the 3' adjacent nucleotide via a linkage group.
Suitable internucleotide linkages include those listed within WO2007/031091 , for example the internucleotide linkages listed on the first paragraph of page 34 of
WO2007/031091 (hereby incorporated by reference).
It is, in some embodiments, preferred to modify the internucleotide linkage from its normal phosphodiester to one that is more resistant to nuclease attack, such as
phosphorothioate or boranophosphate .
In some embodiments, such as the embodiments referred to above, where suitable and not specifically indicated, all remaining linkage groups are either phosphodiester or phosphorothioate, or a mixture thereof.
In some embodiments all the internucleotide linkage groups are phosphorothioate.
Conjugates
In the context the term "conjugate" is intended to indicate a heterogenous molecule formed by the covalent attachment ("conjugation") of the oligomer as described herein to one or more non-nucleotide, or non-polynucleotide moieties. Examples of non-nucleotide or non- polynucleotide moieties include macromolecular agents such as proteins, fatty acid chains, sugar residues, glycoproteins, polymers, or combinations thereof. Typically proteins may be antibodies for a target protein. Typical polymers may be polyethylene glycol.
Therefore, in various embodiments, the oligomer of the invention may comprise both a polynucleotide region which typically consists of a contiguous sequence of nucleotides, and a further non-nucleotide region. When referring to the oligomer of the invention consisting of a contiguous nucleotide sequence, the compound may comprise non-nucleotide
components, such as a conjugate component.
In various embodiments of the invention the oligomeric compound is linked to ligands/conjugates, which may be used, e.g. to increase the cellular uptake of oligomeric compounds. WO2007/031091 provides suitable ligands and conjugates, which are hereby incorporated by reference.
Compositions
The oligomer of the invention may be used in pharmaceutical formulations and compositions. Suitably, such compositions comprise a pharmaceutically acceptable diluent, carrier, salt or adjuvant. PCT/DK2006/000512 provides suitable and preferred
pharmaceutically acceptable diluent, carrier and adjuvants - which are hereby incorporated by reference. Suitable dosages, formulations, administration routes, compositions, dosage forms, combinations with other therapeutic agents, pro-drug formulations are also provided in PCT/DK2006/000512 - which are also hereby incorporated by reference. Miravirsen sodium is a preferred pharmaceutical composition. Dosing
The term "[therapeutically] effective amount" as used herein means an amount required to reduce symptoms (including but limited to curing) of the disease in an individual.
Effectiveness of therapy may, for example be determined by improvement in indices of function (e.g., transaminases or liver biopsy/liver histology). The dose will be adjusted to the individual requirements in each particular case. That dosage can vary within wide limits depending upon numerous factors such as the severity of the disease to be treated, the age and general health condition of the patient, other medicaments with which the patient is being treated, the route and form of administration and the preferences and experience of the medical practitioner involved. Generally, in some embodiments, treatment is initiated with smaller dosages which are less than the optimum dose of the compound. Thereafter, the dosage may be increased by small increments until the optimum effect for the individual patient is reached. One of ordinary skill in treating diseases described herein will be able, without undue experimentation and in reliance on personal knowledge, experience and the disclosures of this application, to ascertain a therapeutically effective amount of the compounds of the present invention for a given disease and patient.
A (e.g. daily/weekly/monthly dose) may, for example be, between about 0.1 and about 500mg/kg body weight, such as between 0.1 and about 100mg/kg body weight such as between 0.1 and 1 mg/kg body weight per day, or between 1 .0 and about 10 mg/kg body
weight per day. Thus, for administration to a 70 kg person, in some embodiments, the dosage range may be about 7 mg to 0.7 g per day. In some embodiments each dose of the oligomer, such as miravirsen may, for example, be between about 0.1 mgs/kg or 1 mg/kg and about 10mg/kg of 20mg/kg, (i.e. a range of between e.g.0.1 and 20mg/kg, such as between 1 mg/kg and 12mg/kg). Individual doses may therefore be, e.g. about 0.2mg/kg, such as about 0.3mg/kg, such as about 0.4mg/kg, such as about 0.5mg/kg, such as about 0.6mg/kg, such as about 0.7mg/kg, such as about 0.8mg/kg, such as about 0.9mg/kg, such as about 1 mg/kg, such as about 2mg/kg, such as about 3mg/kg, such as about 4mg/kg, such as about 5mg/kg, such as about 6mg/kg, such as about 7mg/kg, such as about 8mgs/kg, such as about 9mg/kg, such as about 10mg/kg. In some embodiments the dose of the oligomer is below 7mg/kg, such as below 5mg/kg or below 3mg/kg. In some embodiments the dose of the oligomer is above 0.5mg/kg, such as above 1 mg/kg.
In some embodiments, the time interval between each administration of the miR-122 inhibitor such as miravirsen during the treatment period may be for example, selected from the group consisting of 1 day, 2 days, 3 days, 4 days, 5 days, 6 days and weekly. In some embodiments the time interval between administration is at least every other day, such as at least every three days, such as at least every 4 days, such as at least every 5 days, such as at least every 6 days, such as weekly, such as at least every two weeks (biweekly) or at least every 3 or 4 weeks, or at least monthly.
The oligomer, e.g. miravirsen, may, for example, be administered parentally. For parenteral, subcutaneous, intradermal, transdermal administration the formulation may include a sterile diluent, buffers, regulators of tonicity and antibacterials. The oligomer may, for example be administered i.v. or s.c. in a saline solution. For intravenous or subcutaneous administration the preferred carriers are physiological saline or phosphate buffered saline. Other methods of administration may be used, for example oral, nasal, rectal administration.
EXAMPLES
Summary of Examples 1 - 3:
In clinical trials SPC3649-201 , SPC3649-202 and SPC3649-204, increasing doses (single dose administration) and increasing frequency of administration (weekly administration X 5 at weekly intervals), in subjects who were healthy volunteers was associated, in a few subjects, with increased levels of ALT (ALAT) and AST (ASAT).
In study SPC3649-201 , miravirsen was administered as a single dose intravenously to a group of healthy volunteers. Ascending doses of miravirsen (0.2, 0.4, 0.8, 1.6, 3.2, 6.4, 9.0, and 12 mg/kg) were administered as single doses to different cohorts of subjects. Each cohort consisted of 6 subjects receiving miravirsen and 2 subjects receiving placebo. At the highest dose utilized in this study (12 mg/kg) 3 subjects of 6 administered miravirsen were noted to have increases in ALT (and AST) levels. The maximum increase (in a single individual) was 3 X the upper limit of normal (ULN).
In study SPC3649-202, miravirsen was administered as multiple doses (weekly x 5 over 29 days) in ascending doses to a group of healthy volunteers. There were 6 cohorts of 5 subjects each (4 miravirsen and 1 placebo) in this study. Doses employed were 1 mg/kg iv, 1 mg/kg sc, 2 mg/kg sc, 3 mg/kg sc, 4 mg/kg sc, and 5 mg/kg sc. Two of 4 subjects receiving 2 mg/kg sc, 1 of 4 subjects receiving 3 mg/kg sc, 3 of 4 subjects receiving 4 mg/kg sc, and 3 of 4 subjects receiving 5 mg/kg sc of miravirsen had elevations on at least one occasion of ALT (or AST). In this study, the maximum increase was 1.95 x ULN (in a subject receiving 4 mg/kg sc).
In study SPC3649-204, miravirsen was administered as multiple doses (weekly over 29 days) at 5 mg/kg sc to five healthy volunteers. Miravirsen administration occurred, in this study, after a single administration of peg-interferon 2a and ribavirin. Three of the 5 subjects were noted, on at least one occasion, to have an elevation of ALT. The maximum increase was 3.8 x ULN.
The purposes of studies SPC3649-201 and SPC3649-202 were to evaluate the
pharmacokinetics of miravirsen with single and multiple dosing, respectively. The purpose of study SPC3649-204 was to evaluate the impact, if any, of miravirsen on the
pharmacokinetic profiles of peg-interferon 2a and ribavirin.
None of the subjects noted above were symptomatic with the increases in ALT. None of the increases was accompanied by increases in direct or total bilirubin.
Biomarker Assays:
The biomarker protocols used for measuring ALT was e.g. the Advia Chemistry Systems ALT assay (03903166 Rev. B 2007-05); the protocol for measuring ALT was e.g the Advia Chemistry Systems ALT assay (03815151 Rev. B 2007-05), the protocol for measuring GGT was the e.g. Advia Chemistry Systems ALT assay (04130756 Rev. B 2007-05).
Example 1 : SPC 3649-201
This was a single-center, randomized, double-blind, placebo-controlled, single dose, dose escalation trial. Dose levels were 0.2, 0.4, 0.8, 1 .6, 3.2, 6.4, 9.0, and 12.0 mg/kg. Eight subjects were recruited to each dose group. Six subjects had active SPC3649 and two had placebo. SPC3649 and placebo was administered as an intravenous infusion over 2 hours. Following the infusion each subject was followed for 3 months. Each subject had 12 visits to the clinic - a screening visit to assess eligibility, a treatment visit including an overnight stay at the clinic, and 10 follow-up visits. In total, 64 subjects were enrolled and analyzed.
The objectives of the study were the following: to determine the safety and tolerability of a single dose of SPC3649 by observing any adverse events (AEs), clinical laboratory safety parameters, in particular complement system activity, coagulation, cytokines, liver and kidney function; to assess the pharmacokinetics (PK) of i.v. infusion of SPC3649 in healthy volunteers for the range of doses administered; and, to investigate if there were any effect on serum lipids as surrogate biomarkers of miR-122 inhibition after a single dose of SPC3649.
Study Flow Chart (SPC3649-201 )
* Clinical Chemistry includes AST, ALT, GGT
An increase in circulating transaminases was noted in 3 subjects in the highest dose group (12.0 mg/kg). The maximum ALT elevation was no greater than 3 times ULN. This increase was temporally associated with cholesterol lowering. There was no increase in bilirubin at
any time, and the increase in liver enzymes had a delayed onset similar to that observed for the decrease in lipids.
ALT and AST: Treatment with SPC3649 resulted in a dose-related increase in ALT and AST. Values for both parameters began to increase around day 10 after infusion and reached their maximum around 40 to 70 days after infusion. In the dose groups of 9.0 mg/kg and below the values for both parameters remained within the normal range. In the 12.0 mg/kg group 3 subjects were noted to have increases in transaminases. In one subject the ALT increased to a maximum of three times ULN.
Median values for ALT and AST were higher in the subjects receiving miravirsen compared to placebo. The increases were more pronounced in the higher dose groups (e.g., 54-64% increases with 6.4 and 9.0 mg/kg and 169% increase with 12.0 mg/kg).
Example 2: SPC 3649-202
This was a placebo-controlled, double-blind, randomized, multiple dose-escalating safety study in healthy volunteers. Each subject received a dose of SPC3649 or placebo on study Days 1 , 8, 15, 22, and 29 (total of 5 doses) as either a 2-hour intravenous infusion (i.v.,
Group A only) or as a subcutaneous (s.c, Groups B through F) injection. Five subjects were allocated to each group; four received SPC3649 and one received placebo. The following doses of SPC3649 were administered: Group A: 1 mg/kg; Group B: 1 mg/kg; Group C: 2 mg/kg; Group D: 3 mg/kg; Group E: 4 mg/kg; and, Group F: 5 mg/kg. In total, 30 subjects participated in the study. The objectives of the study were: to determine the safety and tolerability of multiple dosing of SPC3649; to assess the pharmacokinetics (PK) of multiple dosing of SPC3649 administered by i.v. and s.c. routes in healthy volunteers; to evaluate the bioavailability of s.c. administration of SPC3649; and, to investigate the effect of multiple dosing of SPC3649 on lipids as surrogate markers of miR-122 inhibition.
Study Flow Chart (SPC3649-202)
In
Screen Dosing & Follow-up
Clinic
1 2 / 3 4 5 6 7 8 9 10 1 1 12 13 14 15 16 17
Visit #
-21 -1 1 0 2 5 8 15 22 29 31 33 43 57 85 1 13 141 169
Day #
Dosing X X X X X
Clinical
Chemistry X X X X X X X X X X X X X X X X
*
* Clinical Chemistry includes AST, ALT, GGT
Compared to placebo, subjects receiving miravirsen were noted to have slight elevations in ALT and AST from Baseline to the End-of-Study visit. Similar changes were noted in GGT but were not associated with symptoms or changes in total bilirubin.
Values of ALT and AST by Treatment Group (SPC 3649-202) - Baseline & End of Study (IU/L, mean and median)
Treatment group
1 mg/kg 1 mg/kg 2 mg/kg 3 mg/kg 4 mg/kg 5 mg/kg Miravirsen Placebo (inf) (s.c.) (s.c.) (s.c.) (s.c.) (s.c.) Total Total
ALT (U/L) N = 4 N = 4 N = 4 N = 4 N = 4 N = 4 N = 24 N = 6
Mean
values
Baseline 23.3 25.5 22.5 21.0 19.3 20.8 22.0 23.7
End of
23.5 34.8 37.5 41.5 51.0 42.5 38.5 25.5 Study
Median
values
Baseline 24.0 27 24.5 17.5 20.5 20 22.0 17.0
End of
25.0 29.5 38.0 43.5 65.5 44.5 38.0 29.0 Study
AST (U/L) N = 4 N = 4 N = 4 N = 4 N = 4 N = 4 hi = 24 N = 6
Mean
values
Baseline 23.0 20.3 21.8 19.3 25.8 23.0 22.2 20.5
End of
21.5 23.0 31.0 36.3 38.0 37.8 31.3 21.8 Study
Median
values
Baseline 23.5 22.5 20.0 18.5 27.0 21.0 21.0 20.5
End of
22.0 20.5 29.0 36.0 44.5 34.5 31.0 22.5 Study
Nine of 24 subjects (38%) receiving miravirsen sodium were noted to have increases of ALT > ULN, the maximal increase in any subject was 1.84 x ULN. No subject experienced symptoms associated with these increases and no subject was noted to have an increase in serum bilirubin
Example 3: SPC 3649-204
This was a Phase 1 , open-label drug interaction study to assess the safety, tolerability, and pharmacokinetics of miravirsen, pegylated interferon-alpha (PEG-IFNa) and ribavirin when co-administered in healthy subjects. Five subjects were administered PEG-IFNa and ribavirin on Day 1 and serial blood draws were taken over a 168-hour period to assess the
single-dose pharmacokinetic profile. Subjects then received 5 weekly SC doses of miravirsen (5 mg/kg) on Day 8, Day 15, Day 22, Day 29, and Day 36. PEG-IFNa and ribavirin were again administered on Day 37 followed by a 168-hour serial blood collection to assess the pharmacokinetic profile of PEG-IFNa and ribavirin following dosing with miravirsen. Subjects returned for a follow-up visit at Days 39, 41 , 44, 51 , 58, and 65 with a close-out visit on Day 77. The total study duration was planned to be approximately 14 weeks. In total, 5 subjects were enrolled and analyzed. The objectives of the study were: To assess the effect of miravirsen on the pharmacokinetics of co-administered pegylated interferon-alpha and ribavirin, respectively; to evaluate the tolerability of co-administered miravirsen, pegylated interferon-alpha and ribavirin; and, to assess the role of miRNA-122 inhibition on the Vitamin K-dependent coagulation factors.
Study Flow Chart (SPC3649-204)
* Clinical Chemistry includes AST, ALT, GGT
There was an increase in the liver function tests (AST, ALT and, to a lesser extent, GGT). Three of the 5 subjects showed increases in AST (median 23 U/L at Screening to median 35 U/L at Day 77) and ALT (median 21 U/L at Screening to median 44 U/L at Day 77). No subject had associated symptoms or increases in total bilirubin.
Values of ALT and AST by Visit (SPC 3649-204) - (IU/L, mean and median)
This was a randomized, double-blind, placebo-controlled, sequential cohort, ascending multiple-dose study of miravirsen in subjects with chronic hepatitis C (genotype 1 ) infection. Three sequential cohorts of 12 subjects each were randomized to miravirsen or placebo (9 active: 3 placebo). Miravirsen or placebo was administered as subcutaneous (SC) injections on Days 1 , 8, 15, 22, and 29 of the study. Doses of miravirasen administered were 3, 5, or 7 mg/kg, respectively. Following the dosing period, subjects were to return for follow-up visits for an overall study duration of 18 weeks. All subjects were eligible for pegylated-interferon and ribavirin dosing (standard of care (SOC) at the time of study initiation) after completion of the dosing period. For Cohort 1 , SOC could have been administered any time after Week 7 and for Cohorts 2 and 3 any time after Week 10. At the time of this report, 7 subjects in Cohort 1 , 3 subjects in Cohort 2 and 2 subjects in Cohort 3 went on to SOC after dosing with study drug was completed.
In total, 36 subjects were dosed with either miravirsen or placebo.
The objectives of the study were to determine the safety and tolerability of multiple doses of miravirsen in HCV genotype 1 infected subjects; to assess the pharmacokinetics of miravirsen administered subcutaneously to HCV infected subjects; and, to assess any effect on viral titer in subjects with HCV infection.
Study Flow Chart (SPC3649-203)
* Clinical Chemistry includes AST, ALT, GGT
SPC3649-203 Summary: Study SPC3649-203 was conducted to evaluate use of miravirsen in patients with hepatitis C virus (HCV) infections. Three cohorts of 12 subjects each were enrolled sequentially. In each cohort, 9 subjects received miravirsen and 3 received placebo. Miravirsen was administered weekly x 5 over 29 days as either 3 mg/kg sc (Cohort 1 ), 5 mg/kg sc (Cohort 2), or 7 mg/kg sc (Cohort 3).
All subjects receiving miravirsen whose ALT value was increased at baseline showed a decrease in ALT levels into the normal range during and following miravirsen treatment (4/4 in Cohort 1 ; 7/7 in Cohort 2; 5/5 in Cohort 3). Only 1 of 8 subjects receiving placebo whose ALT value was increased at baseline showed a decrease (1 subject's ALT value was within normal limits at baseline). None of the individuals receiving miravirsen whose ALT value was within normal limits at baseline showed an increase in ALT during or following miravirsen treatment.
Thus, in individuals with HCV infection and abnormal transaminase levels (suggestive of hepatic dysfunction), administration of miravirsen weekly (x 5) over 29 days was associated with uniform improvement in the transaminase levels (See figures 3 - 5). This effect was found to be independent of the degree of response of HCV RNA levels to administration of miravirsen, indicating that miravirsen improved hepatic function independent of HCV viral load (see figure 7). This independence of viral load reduction that provides direct teaching that miravirsen may be used in general for the treatment of hepatic disorders which are characterized by elevated ALT, AST and/or GGT blood serum levels.
Claims
A LNA antisense oligonucleotide inhibitor of microRNA-122 for use in
a. improving blood serum biomarkers of liver function in a human subject in need of improved liver function; and/or
b. preventing loss of liver function in a human subject who is not infected with
HCV but is at risk of deteriorating liver function; and/or
c. Improving liver function in a human subject who is not infected with HCV; and/or
d. the treatment of necroinflammation in a subject who has, or is suspected of having, necroinflammation.
A LNA antisense oligonucleotide inhibitor of microRNA-122 for use in the preparation of a medicament for
a. improving blood serum biomarkers of liver function in a human subject in need of improved liver function; and/or
b. preventing loss of liver function in a human subject who is not infected with
HCV but is at risk of deteriorating liver function; and/or
c. improving liver function in a human subject who is not infected with HCV; and/or
d. the treatment of necroinflammation in a subject who has, or is suspected of having, necroinflammation.
The LNA antisense oligonucleotide inhibitor of microRNA-122 according to claim 1 or 2, wherein the human subject is identified for treatment by the presence of elevated one or more blood serum biomarkers selected from the group consisting of miR-122, ALT, AST and/or GGT, or by liver biopsy.
The LNA antisense oligonucleotide inhibitor of microRNA-122 according to any one of claims 1 - 3, wherein said human subject is not infected with hepatitis C.
The LNA antisense oligonucleotide inhibitor of microRNA-122 according to any one of claims 1 - 4, wherein the human subject has been diagnosed with a disease or disorder selected from the group consisting of hepatitis B and hepatitis D.
The LNA antisense oligonucleotide inhibitor of microRNA-122 according to any one of claims 1 - 5 wherein the human subject has been diagnosed with a disease or disorder selected from the group consisting of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis.
7. The LNA antisense oligonucleotide inhibitor of microRNA-122 according to any one of claims 1 - 6 wherein the human subject has been diagnosed with a disease or disorder selected from the group consisting of cytomegalovirus infection,
schistosomiasis infection and Leptospirosis infection.
8. The LNA antisense oligonucleotide inhibitor of microRNA-122 according to any one of claims 1 - 7, wherein the LNA antisense oligonucleotide inhibitor of microRNA-122 is miravirsen (SPC3649).
9. A method of reducing the level of blood biomarkers of liver function in a human
subject who is not infected with HCV and who is in need of improved liver-function, said method comprising administering an effective dose of a LNA antisense oligonucleotide inhibitor of microRNA-122, such as miravirsen, to said human subject so as to reduce the level of blood biomarkers in the human subject.
10. A method of preventing loss of liver function in a human subject who is not infected with HCV but is at risk of deteriorating liver function, said method comprising administering an effective dose of a LNA antisense oligonucleotide inhibitor of microRNA-122, such as miravirsen, to said human subject so as to preventing loss of liver function in the human subject.
1 1 . A method of improving liver function in a human subject who is not infected with
HCV, said method comprising administering an effective dose of a LNA antisense oligonucleotide inhibitor of microRNA-122, such as miravirsen, to said human subject so as to improve liver function in the human subject.
12. A method of reducing the level of blood biomarkers of liver function in a human
subject who is in need of improved liver-function, said method comprising
a. Determining the level of one or more blood serum biomarkers for liver function from the human subject to identify the subject is in need of improved liver function
b. administering an effective dose of a LNA antisense oligonucleotide inhibitor of microRNA-122 , such as miravirsen, to said human subject so as to improve liver function in the human subject
13. The method according to claim 12, wherein the subject is as defined in any one of the proceeding claims.
14. A method for treating a subject infected with HCV, wherein the subject has blood serum biomarkers which are associated with non-responsiveness to miravirsen, said method comprising a pre-treatment period wherein the subject is administered one or
more first doses of miravirsen, followed by a treatment period wherein the subject is administered one or more second dose or a series of second doses of miravirsen, wherein the dosage level of miravirsen during the pre-treatment period is lower than the dosage level of miravirsen during the treatment period.
15. The method according to claim 14, wherein the dosage level of miravirsen during the pre-treatment period is less than about 5mg/kg, such as less than about 4mg/kg, such as less than about 3mg/kg, such as less than about 2mg/kg, such as less than about 1 mg/kg, such as less than about 0.5mg/kg.
16. The method according to claim 14 or 15, wherein the dosage level of miravirsen during the treatment period is greater than or equal to about 3mg/kg, such as greater than or equal to about 4mg/kg, such as greater than or equal to about 5mg/kg, such as greater than or equal to about 6mg/kg, such as greater than or equal to about 7mg/kg.
17. The method according to any one of claims 14 - 16, wherein the time interval
between each first does and/or between each second dose is at least about 1 week, such as at least about 2 weeks, such as at least about 3 weeks, such as at least about monthly or at least about bimonthly.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161556309P | 2011-11-07 | 2011-11-07 | |
| US61/556,309 | 2011-11-07 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013068348A1 true WO2013068348A1 (en) | 2013-05-16 |
Family
ID=47143103
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2012/071934 WO2013068348A1 (en) | 2011-11-07 | 2012-11-06 | Lna oligomers for improvement in hepatic function |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2013068348A1 (en) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9157083B2 (en) | 2013-05-01 | 2015-10-13 | Regulus Therapeutics Inc. | MicroRNA compounds and methods for modulating miR-122 |
| US9506030B2 (en) | 2013-05-01 | 2016-11-29 | Regulus Therapeutics Inc. | Compounds and methods for enhanced cellular uptake |
| WO2017046181A1 (en) * | 2015-09-14 | 2017-03-23 | Genfit | Methods for diagnosing and evaluating non-alcoholic steatohepatitis |
| RU2639388C1 (en) * | 2016-10-11 | 2017-12-21 | Федеральное государственное бюджетное учреждение "Государственный научный центр "Институт иммунологии" Федерального медико-биологического агентства России | Composition for viral hepatitis c therapy |
| US11155816B2 (en) | 2012-11-15 | 2021-10-26 | Roche Innovation Center Copenhagen A/S | Oligonucleotide conjugates |
| WO2022226313A1 (en) * | 2021-04-23 | 2022-10-27 | University Of Cincinnati | Therapeutics for hyponatremia and polycystic kidney disease |
| US12005120B2 (en) | 2018-05-08 | 2024-06-11 | Regulus Therapeutics Inc. | Galnac conjugated modified oligonucleotides as miR-122 inhibitor having HCV antiviral activity with reduced hyperbilirubinemia side-effect |
Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1222309A1 (en) | 1999-09-30 | 2002-07-17 | Isis Pharmaceuticals, Inc. | Human rnase h and oligonucleotide compositions thereof |
| WO2007027775A2 (en) | 2005-08-29 | 2007-03-08 | Isis Pharmaceuticals, Inc. | Methods for use in modulating mir-122a |
| WO2007031091A2 (en) | 2005-09-15 | 2007-03-22 | Santaris Pharma A/S | Rna antagonist compounds for the modulation of p21 ras expression |
| WO2007090071A2 (en) | 2006-01-27 | 2007-08-09 | Isis Pharmaceuticals, Inc. | 6-modified bicyclic nucleic acid analogs |
| WO2007112753A2 (en) | 2006-04-03 | 2007-10-11 | Santaris Pharma A/S | Pharmaceutical composition comprising anti-mirna antisense oligonucleotides |
| WO2007134181A2 (en) | 2006-05-11 | 2007-11-22 | Isis Pharmaceuticals, Inc. | 5'-modified bicyclic nucleic acid analogs |
| WO2008150729A2 (en) | 2007-05-30 | 2008-12-11 | Isis Pharmaceuticals, Inc. | N-substituted-aminomethylene bridged bicyclic nucleic acid analogs |
| WO2008154401A2 (en) | 2007-06-08 | 2008-12-18 | Isis Pharmaceuticals, Inc. | Carbocyclic bicyclic nucleic acid analogs |
| WO2009006478A2 (en) | 2007-07-05 | 2009-01-08 | Isis Pharmaceuticals, Inc. | 6-disubstituted bicyclic nucleic acid analogs |
| WO2009043353A2 (en) | 2007-10-04 | 2009-04-09 | Santaris Pharma A/S | Micromirs |
| WO2009067647A1 (en) | 2007-11-21 | 2009-05-28 | Isis Pharmaceuticals, Inc. | Carbocyclic alpha-l-bicyclic nucleic acid analogs |
| WO2010122538A1 (en) * | 2009-04-24 | 2010-10-28 | Santaris Pharma A/S | Pharmaceutical compositions for treatment of hcv patients that are non-responders to interferon |
-
2012
- 2012-11-06 WO PCT/EP2012/071934 patent/WO2013068348A1/en active Application Filing
Patent Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1222309A1 (en) | 1999-09-30 | 2002-07-17 | Isis Pharmaceuticals, Inc. | Human rnase h and oligonucleotide compositions thereof |
| WO2007027775A2 (en) | 2005-08-29 | 2007-03-08 | Isis Pharmaceuticals, Inc. | Methods for use in modulating mir-122a |
| WO2007031091A2 (en) | 2005-09-15 | 2007-03-22 | Santaris Pharma A/S | Rna antagonist compounds for the modulation of p21 ras expression |
| WO2007090071A2 (en) | 2006-01-27 | 2007-08-09 | Isis Pharmaceuticals, Inc. | 6-modified bicyclic nucleic acid analogs |
| WO2007112753A2 (en) | 2006-04-03 | 2007-10-11 | Santaris Pharma A/S | Pharmaceutical composition comprising anti-mirna antisense oligonucleotides |
| WO2007112754A2 (en) | 2006-04-03 | 2007-10-11 | Santaris Pharma A/S | Pharmaceutical compositions comprising anti-mirna antisense oligonucleotides |
| WO2007134181A2 (en) | 2006-05-11 | 2007-11-22 | Isis Pharmaceuticals, Inc. | 5'-modified bicyclic nucleic acid analogs |
| WO2008150729A2 (en) | 2007-05-30 | 2008-12-11 | Isis Pharmaceuticals, Inc. | N-substituted-aminomethylene bridged bicyclic nucleic acid analogs |
| WO2008154401A2 (en) | 2007-06-08 | 2008-12-18 | Isis Pharmaceuticals, Inc. | Carbocyclic bicyclic nucleic acid analogs |
| WO2009006478A2 (en) | 2007-07-05 | 2009-01-08 | Isis Pharmaceuticals, Inc. | 6-disubstituted bicyclic nucleic acid analogs |
| WO2009043353A2 (en) | 2007-10-04 | 2009-04-09 | Santaris Pharma A/S | Micromirs |
| WO2009067647A1 (en) | 2007-11-21 | 2009-05-28 | Isis Pharmaceuticals, Inc. | Carbocyclic alpha-l-bicyclic nucleic acid analogs |
| WO2010122538A1 (en) * | 2009-04-24 | 2010-10-28 | Santaris Pharma A/S | Pharmaceutical compositions for treatment of hcv patients that are non-responders to interferon |
Non-Patent Citations (23)
| Title |
|---|
| BERGMEYER H.U.; SCHEIBE P.; WAHLEFELD A.W.: "Optimization of methods for aspartate aminotransferase and alanine aminotransferase", CLIN. CHEM., vol. 24, no. 1, 1978, pages 58 - 73 |
| BIHRER ET AL., THE AMERICAN JOURNAL OF GASTROENTEROLOGY, 2011 |
| BOWERS JR G.N.; MCCOMB R.B.: "A unifying reference system for clinical enzymology: aspartate aminotransferase and the International Clinical Enzyme Scale", CLIN. CHEM., vol. 30, no. 7, 1984, pages 1128 - 1136 |
| CHRISTENSEN, NUCL. ACIDS. RES., vol. 30, 2002, pages 4918 - 4925 |
| CHUN-GUANG FAN ET AL.: "miR-122 inhibits viral replication and cell proliferation in hepatitis B virus-related hepatocellular carcinoma and targets NDRG3", ONCOLOGY REPORTS, 1 November 2011 (2011-11-01), pages 1281 - 1286, XP055047966, ISSN: 1021-335X, DOI: 10.3892/or.2011.1375 * |
| DIENSTAG; MCHUTCHINSON, AGA, vol. 130, pages 231 - 264 |
| EIMEN ET AL., NATURE, 2008 |
| ELMEN ET AL., NATURE, 2008 |
| ELMÉN J ET AL: "Antagonism of microRNA-122 in mice by sistemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver", NUCLEIC ACIDS RESEARCH, OXFORD UNIVERSITY PRESS, SURREY, GB, vol. 36, no. 4, 23 December 2007 (2007-12-23), pages 1153 - 1162, XP002533568, ISSN: 0305-1048, DOI: 10.1093/NAR/GKM1113 * |
| ESAU ET AL., CELL. METAB., vol. 3, no. 2, February 2006 (2006-02-01), pages 87 - 98 |
| F. JI ET AL: "Circulating microRNAs in hepatitis B virus-infected patients", JOURNAL OF VIRAL HEPATITIS, vol. 18, no. 7, 1 July 2011 (2011-07-01), pages e242 - e251, XP055048004, ISSN: 1352-0504, DOI: 10.1111/j.1365-2893.2011.01443.x * |
| FREIER; ALTMANN, NUCL. ACID RES., vol. 25, 1997, pages 4429 - 4443 |
| HILDEBRANDT-ERIKSEN ET AL., OTS, 2001 |
| LAGOS-QUINTANA ET AL., CURRENT BIOL, vol. 12, 2002, pages 735 - 739 |
| MACHLIN ET AL.: "Masking the 5' terminal nucleotides of the hepatitis C virus genome by an unconventional microRNA-target RNA complex", PNAS, vol. 108, no. 8, 22 February 2011 (2011-02-22), pages 3193 - 3198 |
| NUCLEIC ACIDS RESEARCH, vol. 35, no. 2, 2007, pages 687 - 700 |
| QIU L. ET AL.: "miR-122-induced down-regulation of HO-1 negatively affects miR-122-mediated suppression of HBV", BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS, ACADEMIC PRESS INC. ORLANDO, FL, US, vol. 398, no. 4, 6 August 2010 (2010-08-06), pages 771 - 777, XP027192773, ISSN: 0006-291X, [retrieved on 20100713], DOI: 10.1016/J.BBRC.2010.07.021 * |
| SAIFENG WANG ET AL.: "Loss of microRNA 122 expression in patients with hepatitis B enhances hepatitis B virus replication through cyclin G1-modulated P53 activity", HEPATOLOGY, vol. 55, no. 3, 1 March 2012 (2012-03-01), pages 730 - 741, XP055047997, ISSN: 0270-9139, DOI: 10.1002/hep.24809 * |
| SENER A; YARDIMCI T., J BIOCHEM MOL BIOL., vol. 38, no. 3, 31 May 2005 (2005-05-31), pages 343 - 9 |
| SETH, J. ORG. CHEM, 2010 |
| SU ET AL.: "Serum MicroRNA-122 Level Correlates with Virologic Responses to Combination Therapy in Chronic Hepatitis C Patients", AASLD, November 2011 (2011-11-01) |
| UHLMANN, CURR. OPINION IN DRUG DEVELOPMENT, vol. 3, no. 2, 2000, pages 293 - 213 |
| YANG FU ET AL: "Modulation of the Unfolded Protein Response Is the Core of MicroRNA-122-Involved Sensitivity to Chemotherapy in Hepatocellular Carcinoma", NEOPLASIA (NEW YORK), vol. 13, no. 7, July 2011 (2011-07-01), pages 590, XP008158864, ISSN: 1522-8002 * |
Cited By (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11155816B2 (en) | 2012-11-15 | 2021-10-26 | Roche Innovation Center Copenhagen A/S | Oligonucleotide conjugates |
| US10240151B2 (en) | 2013-05-01 | 2019-03-26 | Regulus Therapeutics Inc. | Compounds and methods for enhanced cellular uptake |
| US9309513B2 (en) | 2013-05-01 | 2016-04-12 | Regulus Therapeutics Inc. | MicroRNA compounds and methods for modulating miR-122 |
| US9506030B2 (en) | 2013-05-01 | 2016-11-29 | Regulus Therapeutics Inc. | Compounds and methods for enhanced cellular uptake |
| US9574194B2 (en) | 2013-05-01 | 2017-02-21 | Regulus Therapeutics Inc. | MicroRNA compounds and methods for modulating miR-122 |
| US9157083B2 (en) | 2013-05-01 | 2015-10-13 | Regulus Therapeutics Inc. | MicroRNA compounds and methods for modulating miR-122 |
| US10941400B2 (en) | 2013-05-01 | 2021-03-09 | Regulus Therapeutics Inc. | Compounds and methods for enhanced cellular uptake |
| US10150967B2 (en) | 2013-05-01 | 2018-12-11 | Regulus Therapeutics Inc. | MicroRNA compounds and methods for modulating miR-122 |
| WO2017046181A1 (en) * | 2015-09-14 | 2017-03-23 | Genfit | Methods for diagnosing and evaluating non-alcoholic steatohepatitis |
| EP3712279A1 (en) * | 2015-09-14 | 2020-09-23 | Genfit | Methods for diagnosing and evaluating non-alcoholic steatohepatitis |
| CN108138232A (en) * | 2015-09-14 | 2018-06-08 | 基恩菲特公司 | For diagnosing and assessing the method for nonalcoholic fatty liver disease |
| US11371098B2 (en) | 2015-09-14 | 2022-06-28 | Genfit | Methods for diagnosing and evaluating non-alcoholic steatohepatitis |
| IL257680B (en) * | 2015-09-14 | 2022-11-01 | Genfit | Methods for diagnosing and evaluating non-alcoholic steatohepatitis |
| AU2016323380B2 (en) * | 2015-09-14 | 2023-01-05 | Genfit | Methods for diagnosing and evaluating non-alcoholic steatohepatitis |
| IL257680B2 (en) * | 2015-09-14 | 2023-03-01 | Genfit | Methods for diagnosing and evaluating non-alcoholic steatohepatitis |
| RU2639388C1 (en) * | 2016-10-11 | 2017-12-21 | Федеральное государственное бюджетное учреждение "Государственный научный центр "Институт иммунологии" Федерального медико-биологического агентства России | Composition for viral hepatitis c therapy |
| US12005120B2 (en) | 2018-05-08 | 2024-06-11 | Regulus Therapeutics Inc. | Galnac conjugated modified oligonucleotides as miR-122 inhibitor having HCV antiviral activity with reduced hyperbilirubinemia side-effect |
| WO2022226313A1 (en) * | 2021-04-23 | 2022-10-27 | University Of Cincinnati | Therapeutics for hyponatremia and polycystic kidney disease |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20210108205A1 (en) | MicroRNA Compounds and Methods for Modulating MIR-122 | |
| KR101953074B1 (en) | Oligomers and oligomer conjugates | |
| WO2013068348A1 (en) | Lna oligomers for improvement in hepatic function | |
| ES2856266T3 (en) | Modulation of Hepatitis B Virus (HBV) Expression | |
| JP5773535B2 (en) | Pharmaceutical composition for the treatment of HCV patients unresponsive to interferon | |
| JP2012524068A (en) | Modulation of inflammatory response by factor XI | |
| US20120295961A1 (en) | Modulation of hepatitis b virus (hbv) expression | |
| US12005120B2 (en) | Galnac conjugated modified oligonucleotides as miR-122 inhibitor having HCV antiviral activity with reduced hyperbilirubinemia side-effect | |
| CN114828852A (en) | HBV-targeted antiviral and/or immunomodulatory agent pharmaceutical combinations for the treatment of HBV | |
| WO2012007477A1 (en) | Anti hcv oligomers | |
| TWI854980B (en) | Microrna compounds and methods for modulating mir-122 | |
| CA3234478A1 (en) | Pharmaceutical combinations for treatment of hbv | |
| NZ732101B2 (en) | Microrna compounds and methods for modulating mir-122 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12781321 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 12781321 Country of ref document: EP Kind code of ref document: A1 |