WO2013056815A1 - Acrylic polyol resins compositions - Google Patents
Acrylic polyol resins compositions Download PDFInfo
- Publication number
- WO2013056815A1 WO2013056815A1 PCT/EP2012/004321 EP2012004321W WO2013056815A1 WO 2013056815 A1 WO2013056815 A1 WO 2013056815A1 EP 2012004321 W EP2012004321 W EP 2012004321W WO 2013056815 A1 WO2013056815 A1 WO 2013056815A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- glycidyl ester
- methyl
- dimethyl
- composition
- weight
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G65/00—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule
- C08G65/02—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule from cyclic ethers by opening of the heterocyclic ring
- C08G65/26—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule from cyclic ethers by opening of the heterocyclic ring from cyclic ethers and other compounds
- C08G65/2603—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule from cyclic ethers by opening of the heterocyclic ring from cyclic ethers and other compounds the other compounds containing oxygen
- C08G65/2615—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule from cyclic ethers by opening of the heterocyclic ring from cyclic ethers and other compounds the other compounds containing oxygen the other compounds containing carboxylic acid, ester or anhydride groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F212/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an aromatic carbocyclic ring
- C08F212/02—Monomers containing only one unsaturated aliphatic radical
- C08F212/04—Monomers containing only one unsaturated aliphatic radical containing one ring
- C08F212/06—Hydrocarbons
- C08F212/08—Styrene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/10—Esters
- C08F220/12—Esters of monohydric alcohols or phenols
- C08F220/14—Methyl esters, e.g. methyl (meth)acrylate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F283/00—Macromolecular compounds obtained by polymerising monomers on to polymers provided for in subclass C08G
- C08F283/002—Macromolecular compounds obtained by polymerising monomers on to polymers provided for in subclass C08G on to polymers modified by after-treatment
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F283/00—Macromolecular compounds obtained by polymerising monomers on to polymers provided for in subclass C08G
- C08F283/02—Macromolecular compounds obtained by polymerising monomers on to polymers provided for in subclass C08G on to polycarbonates or saturated polyesters
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F8/00—Chemical modification by after-treatment
- C08F8/14—Esterification
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/40—High-molecular-weight compounds
- C08G18/62—Polymers of compounds having carbon-to-carbon double bonds
- C08G18/6216—Polymers of alpha-beta ethylenically unsaturated carboxylic acids or of derivatives thereof
- C08G18/622—Polymers of esters of alpha-beta ethylenically unsaturated carboxylic acids
- C08G18/6237—Polymers of esters containing glycidyl groups of alpha-beta ethylenically unsaturated carboxylic acids; reaction products thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/40—High-molecular-weight compounds
- C08G18/62—Polymers of compounds having carbon-to-carbon double bonds
- C08G18/6216—Polymers of alpha-beta ethylenically unsaturated carboxylic acids or of derivatives thereof
- C08G18/625—Polymers of alpha-beta ethylenically unsaturated carboxylic acids; hydrolyzed polymers of esters of these acids
- C08G18/6254—Polymers of alpha-beta ethylenically unsaturated carboxylic acids and of esters of these acids containing hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G59/00—Polycondensates containing more than one epoxy group per molecule; Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups
- C08G59/14—Polycondensates modified by chemical after-treatment
- C08G59/1433—Polycondensates modified by chemical after-treatment with organic low-molecular-weight compounds
- C08G59/1438—Polycondensates modified by chemical after-treatment with organic low-molecular-weight compounds containing oxygen
- C08G59/1455—Monocarboxylic acids, anhydrides, halides, or low-molecular-weight esters thereof
- C08G59/1461—Unsaturated monoacids
- C08G59/1466—Acrylic or methacrylic acids
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D133/00—Coating compositions based on homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides, or nitriles thereof; Coating compositions based on derivatives of such polymers
- C09D133/04—Homopolymers or copolymers of esters
- C09D133/06—Homopolymers or copolymers of esters of esters containing only carbon, hydrogen and oxygen, the oxygen atom being present only as part of the carboxyl radical
- C09D133/062—Copolymers with monomers not covered by C09D133/06
- C09D133/066—Copolymers with monomers not covered by C09D133/06 containing -OH groups
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D151/00—Coating compositions based on graft polymers in which the grafted component is obtained by reactions only involving carbon-to-carbon unsaturated bonds; Coating compositions based on derivatives of such polymers
- C09D151/08—Coating compositions based on graft polymers in which the grafted component is obtained by reactions only involving carbon-to-carbon unsaturated bonds; Coating compositions based on derivatives of such polymers grafted on to macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D163/00—Coating compositions based on epoxy resins; Coating compositions based on derivatives of epoxy resins
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D171/00—Coating compositions based on polyethers obtained by reactions forming an ether link in the main chain; Coating compositions based on derivatives of such polymers
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D171/00—Coating compositions based on polyethers obtained by reactions forming an ether link in the main chain; Coating compositions based on derivatives of such polymers
- C09D171/02—Polyalkylene oxides
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D175/00—Coating compositions based on polyureas or polyurethanes; Coating compositions based on derivatives of such polymers
- C09D175/04—Polyurethanes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F222/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical and containing at least one other carboxyl radical in the molecule; Salts, anhydrides, esters, amides, imides, or nitriles thereof
- C08F222/10—Esters
- C08F222/12—Esters of phenols or saturated alcohols
- C08F222/14—Esters having no free carboxylic acid groups, e.g. dialkyl maleates or fumarates
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/31504—Composite [nonstructural laminate]
- Y10T428/31678—Of metal
- Y10T428/31692—Next to addition polymer from unsaturated monomers
- Y10T428/31699—Ester, halide or nitrile of addition polymer
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/31504—Composite [nonstructural laminate]
- Y10T428/31855—Of addition polymer from unsaturated monomers
- Y10T428/31935—Ester, halide or nitrile of addition polymer
Definitions
- the present invention relates to a composition of acrylic polyol resins based on a composition of hydroxyl functional acrylic resins (acrylic polyols) comprising a mixture of ⁇ , ⁇ -branched alkane carboxylic glycidyl esters derived from butene oligomers , which lead for example to improved hardness of the coatings derived thereof.
- the invention relates to acrylic polyol resins compositions comprising aliphatic tertiary saturated carboxylic acids or ⁇ , ⁇ -branched alkane carboxylic acids, which contain 9 or 13 carbon atoms and which provide glycidyl esters with a branching level of the alkyl groups depending on the olefin feedstock used and/or the oligomerisation process therof, and which is defined as below .
- the glycidyl ester derived from olefins containing 5 to 10 carbon atoms in the alkyl chain are used by the industry to introduce modified resins by reaction such a glycidyl ester with acrylic resins, such as given in US 6 136 991.
- the glycidyl esters can be obtained according to PCT/EP2010/003334 or the US6433217. We have discovered that well chosen blend of isomers of the glycidyl ester of, for example, neononanoic acids give different and unexpected performance in combination with some particular polymers such as acrylic polyols.
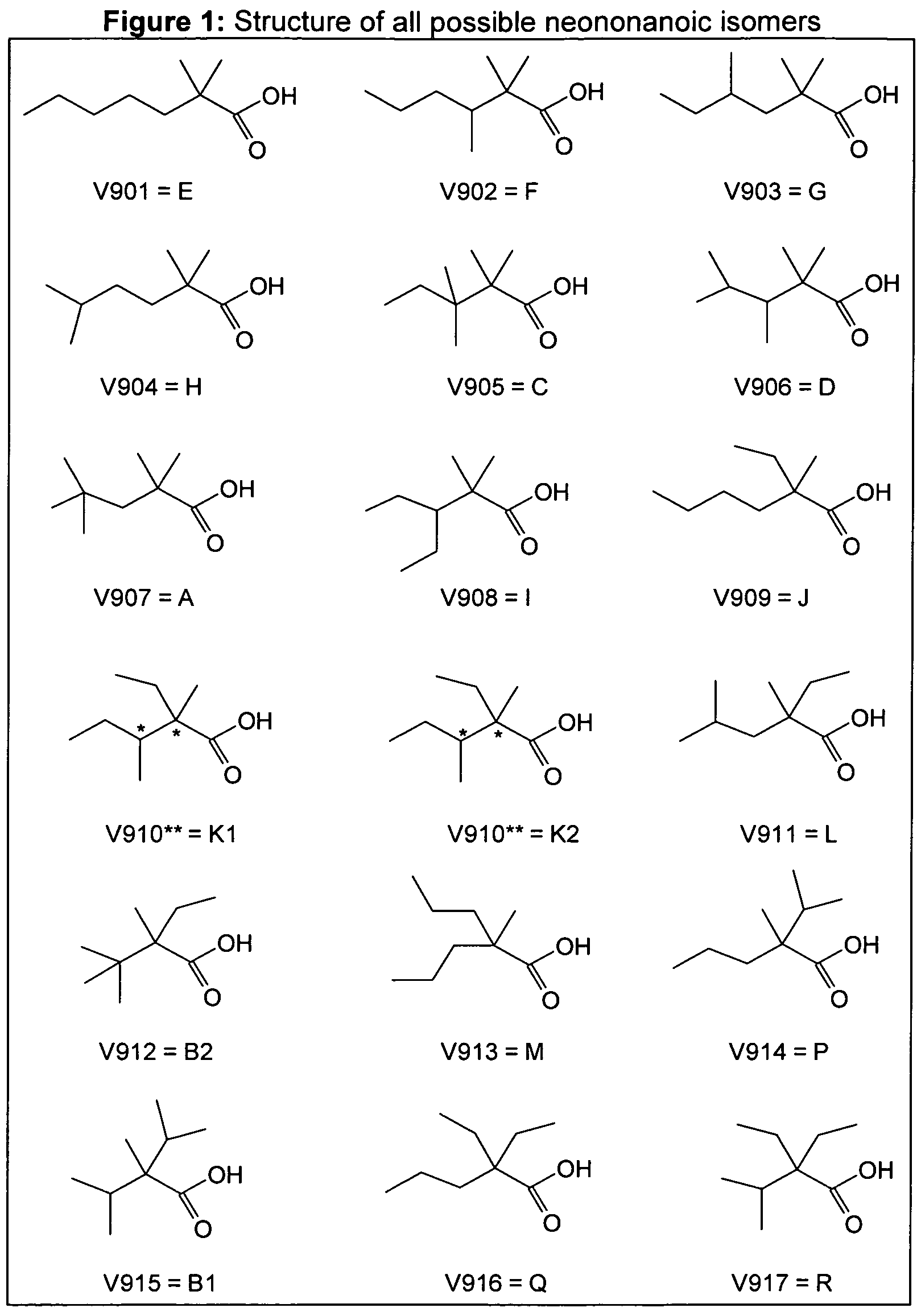
- the performance of the glycidyl ester compositions derived from the branched acid is depending on the branching level of the alkyl groups R 1 , R 2 and R 3 , for example the neononanoic acid has 3, 4 or 5 methyl groups.
- Highly branched isomers are defined as isomers of neo-acids having at least 5 methyl groups.
- Neo-acids for example neononanoic acids (V9) with a secondary or a tertiary carbon atoms in the ⁇ position are defined as blocking isomers.
- compositions of neononanoic acids glycidyl esters providing for example a high hardness of a coating is a mixture where the sum of the concentration of the blocked and of the highly branched isomers is at least 50%, preferably above 60% and most preferably above 75% on total composition .
- composition of the glycidyl ester mixture is comprising 2,2-dimethyl 3,3-dimethyl pentanoic acid glycidyl ester or 2-methyl 2-isopropyl 3-methyl butanoic acid glycidyl ester or 2-methyl 2-ethyl 3,3-dimethyl butanoic acid glycidyl ester .
- composition of the glycidyl ester mixture is comprising 2,2-dimethyl 3-methyl 4-methyl pentanoic acid glycidyl ester and 2,2-dimethyl 4,4-dimethyl pentanoic acid glycidyl
- composition of the glycidyl ester mixture in which the sum of the following content of glycidyl ester mixture, comprising 2,2-dimethyl 3,3-dimethyl pentanoic acid glycidyl ester and 2-methyl 2-isopropyl 3-methyl butanoic acid glycidyl ester and 2-methyl 2-ethyl 3,3-dimethyl butanoic acid glycidyl ester is above 10% weight, preferably above 15% weight and most preferably above 25% weight on total composition.
- composition of the glycidyl ester mixture in which the content of 2-methyl 2-ethyl hexanoic acid glycidyl ester is below 40% weight, preferably below 30% weight and most preferably below 20% weight on total composition.
- the above glycidyl esters compositions can be used for example, is as reactive diluent or as momomer in binder compositions for paints or adhesives.
- the glycidyl esters compositions can be used as reactive diluent for epoxy based formulations such as examplified in the technical brochure of Momentive (Product Bulletin: Cardura E10P The Unique Reactive Diluent MSC-521) .
- glycidyl ester uses of the glycidyl ester are the combinations with polyester polyols, or acrylic polyols, or polyether polyols.
- the combination with acrylic polyols such as the one used in the car industry coating leads to a fast drying coating system with attractive coating properties.
- the isomer distribution of neo-acid can be determined using gas chromatography, using a flame ionization detector (FID). 0.5 ml sample is diluted in analytical grade dichloromethane and n-octanol may be used as internal standard. The conditions presented below result in the approximate retention times given in table 1. In that case n-octanol has a retention time of approximately 8.21 minute .
- the GC method has the following settings:
- Carrier gas Helium
- Injection volume 1 ⁇ CP Wax 58 CB is a Gas chromatography column available from Agilent Technologies.
- the isomers of neononanoic acid as illustrative example have the structure (R 1 R 2 R 3 )-C-COOH where the three R groups are linear or branched alkyl groups having together a total of 7 carbon atoms.
- the isomers content is calculated from the relative peak area of the chromatogram obtained assuming that the response factors of all isomers are the same.
- Rl R2 R3 groups Blocking [Minutes]
- Table 1 Structure of all possible neononanoic isomers
- the isomer distribution of neo-acid can be determined using gas chromatography, using a flame ionization detector (FID).
- FID flame ionization detector
- 0.5 ml sample is diluted in analytical grade dichloromethane and n-octanol may be used as internal standard.
- the conditions presented below result in the approximate retention times given in Table 1. In that case n-octanol has a retention time of approximately 8.21 minute .
- the GC method has the following settings:
- Carrier gas Helium
- CP Wax 58 CB is a Gas chromatography column available from
- the isomers content is calculated from the relative peak area of the chromatogram obtained assuming that the response factors of all isomers are the same.
- GC-MS method can be used to identify the various isomers providing that the analysis is done by a skilled analytical expert .
- the molecular weights of the resins are measured with gel permeation chromatography (Perkin Elmer/ Water) in THF solution using polystyrene standards. Viscosity of the resins are measured with Brookfield viscometer (LVDV-I) at indicated temperature. Solids content are calculated with a function (Ww- Wd) / w x 100%.
- Ww is the weight of a wet sample
- d is the weight of the sample after dried in an oven at a temperature 110 °C for 1 hour.
- Tg glass transition temperature
- the carbon atom in alpha position of the carboxylic acid is always a tertiary carbon atom
- the carbon atom(s) in position can either be primary, secondary or tertiary.
- Neononanoic acids (V9) with a secondary or a tertiary carbon atoms in the ⁇ position are defined as blocking (blocked) isomers Figures 2 and 3) .
- Figure 3 Example of a Blocked V9 Structure
- the use of the glycidyl esters compositions, discussed here above, can be as momomer in binder compositions for paints and adhesives.
- These binders can be based on an acrylic polyol resin comprising the above composition glycidyl.
- the acrylic polyol resins of the invention are based on a composition of hydroxyl functional acrylic resins (acrylic polyols) comprising a mixture of ⁇ , ⁇ -branched alkane carboxylic glycidyl esters derived from butene oligomers characterized in that the sum of the concentration of the blocked and of the highly branched isomers is at least 50%, preferably above 60% and most preferably above 75% on total composition.
- hydroxyl functional acrylic resins (acrylic polyols) comprising a mixture of ⁇ , ⁇ -branched alkane carboxylic glycidyl esters derived from butene oligomers characterized in that the sum of the concentration of the blocked and of the highly branched isomers is at least 50%, preferably above 60% and most preferably above 75% on total composition.
- a prefer composition is that the glycidyl ester mixture is based on neononanoic (C9) acid mixture where the sum of the concentration of the blocked and of the highly branched isomers is at least 50%, preferably above 60% and most preferably above 75% on total composition.
- neononanoic (C9) glycidyl ester mixture is comprising 2,2-dimethyl 3,3-dimethyl pentanoic acid glycidyl ester or 2-methyl 2-isopropyl 3-methyl butanoic acid glycidyl ester or 2-methyl 2-ethyl 3,3-dimethyl butanoic acid glycidyl ester .
- composition of the glycidyl ester mixture is comprising 2,2-dimethyl 3-methyl 4-methyl pentanoic acid glycidyl ester or 2,2-dimethyl 4,4-dimethyl pentanoic acid glycidyl ester.
- composition of the glycidyl ester mixture is comprising the sum of the following content of glycidyl ester mixture, comprising 2,2-dimethyl 3,3-dimethyl pentanoic acid glycidyl ester and 2-methyl 2-isopropyl 3-methyl butanoic acid glycidyl ester and 2-methyl 2-ethyl 3,3-dimethyl butanoic acid glycidyl ester, is above 10% weight, preferably above 15% weight and most preferably above 25% weight on total composition .
- composition of the glycidyl ester mixture is comprising the sum of the following content of glycidyl ester mixture, comprising 2, 2-dimethyl 3, 3-dimethyl pentanoic acid glycidyl ester and 2-methyl 2-isopropyl 3-methyl butanoic acid glycidyl ester and 2-methyl 2-ethyl 3, 3-dimethyl butanoic acid glycidyl ester and 2, 2-dimethyl 3-methyl 4-methyl pentanoic acid glycidyl ester and 2, 2-dimethyl 4,4-dimethyl pentanoic acid glycidyl ester, is above 40% weight, preferably above 50% weight and most preferably above 60% weight on total composition.
- composition of the glycidyl ester mixture is comprising 2-methyl 2-ethyl hexanoic acid glycidyl ester is below 40% weight, preferably below 30% weight and most preferably below 20% weight on total composition.
- composition of the glycidyl ester mixture is comprising 2, 2-dimethyl 3, 3-dimethyl pentanoic acid glycidyl ester in 1 to 99 weight% or 2-methyl 2-isopropyl 3-methyl butanoic acid glycidyl ester in 1 to 99 weight% or 2- methyl 2-ethyl 3, 3-dimethyl butanoic acid glycidyl ester in 1 to 99 weight% on total composition and a prefer is in that the glycidyl ester mixture is comprising 2, 2-dimethyl 3, 3-dimethyl pentanoic acid glycidyl ester in 2 to 50 weight% or 2-methyl 2-isopropyl 3-methyl butanoic acid glycidyl ester in 5 to 50 weight% or 2-methyl 2-ethyl 3, 3-dimethyl butanoic acid glycidyl ester in 3 to 60 weight% on total composition, and a most prefer composition is that the glycidyl ester mixture is comprising 2,
- a further embodiment is that the composition of the glycidyl ester mixture is comprising 2, 2-dimethyl 3-methyl 4-methyl pentanoic acid glycidyl ester in 1 to 99 weight% or 2,2- dimethyl 4,4-dimethyl pentanoic acid glycidyl ester in 0.1 to 99 weight%
- a prefer composition is that the glycidyl ester mixture is comprising 2,2-dimethyl 3-methyl 4-methyl pentanoic acid glycidyl ester in 2 to 50 weight% or 2,2-dimethyl 4,4- dimethyl pentanoic acid glycidyl ester in 0.1 to 80 weight%
- a most prefer composition is that the glycidyl ester mixture is comprising 2,2-dimethyl 3-methyl 4-methyl pentanoic acid glycidyl ester in 4 to 25 weight% or 2,2-dimethyl 4,4- dimethyl pentanoic acid glycidyl ester in 0.2 to 45 weight%.
- the invention is also about the process to prepare the acrylic polyol resins compositions, which are obtained by the incorporation of the mixture of ⁇ , ⁇ -branched alkane carboxylic glycidyl esters, as characterized above, into a hydroxyl functional acrylic resins by the reaction of the epoxy group with the carboxylic acid group of ethylene carboxylic acid compounds from hydroxyl ethylene carboxylate ester monomers which are then reacted with one or more unsaturated monomers via a radical polymerization reaction, in one step or more.
- the ethylene carboxylic acid compounds are for example acrylic acid, methacrylic acid, and the like.
- the other unsaturated monomers are selected from the group consisting of octyl acrylate, octyl methacrylate, nonyl
- a further process to prepare the acrylic polyol resins of the invention are cooked it while having a polyester polyol or a polyether polyol or a mixture thereof as initial reactor charge .
- the acrylic polyol resins of the invention prepared according to the above processes will have a calculated hydroxyl value between 50 and 180 mgKOH/g on solid and or the number average molecular weight (Mn) is between 2500 and 50000 Dalton according polystyrene standard.
- the invention is also related to a binder composition useful for coating composition comprising at least any hydroxyl functional acrylic resins as prepared above.
- the said binder compositions are suitable for coating metal or plastic substrates.
- Neononanoic glycidyl ester of composition C (see Table 2)
- Neononanoic glycidyl ester of composition D (see Table 2)
- Neononanoic glycidyl ester of composition E (see Table 2)
- Table 2 Composition of the neononanoic glycidyl ester (according to the described gas chromatography method for glycidyl esters of neo-acid) GE5 : glycidyl ester of pivalic acid obtained by reaction of the acid with epichlorhydrin.
- Methylhexahydrophtalic anhydride available from Sigma Aldrich
- Hexahydrophtalic anhydride available from Sigma - Aldrich
- Methyl methacrylate available from Sigma - Aldrich
- Butyl acrylate available from Sigma - Aldrich
- Thinner A: is a mixture of Xylene 50wt%, Toluene 30wt%, ShellsolA 10wt%, 2-Ethoxyethylacetate 10wt%.
- Curing agents HDI : 1 , 6-hexamethylene diisocyanate trimer, Desmodur N3390 BA from Bayer Material Science or Tolonate
- HDT LV2 from Perstorp - Leveling agent 'BYK 10 wt % ' which is BYK-331 diluted at 10% in butyl acetate
- the following constituents were charged to a reaction vessel equipped with a stirrer, a condenser and a thermometer: 92.4 grams of GE9S, 24.0 grams of Butyl Acetate. That initial reactor charge has been heated up to 135°C. Then, the following mixture was added over a period of lh20 while keeping the temperature constant: 30.7 grams of acrylic acid, 1.2 grams of Di-t-Amyl Peroxide, 12.0 grams of n-Butyl Acetate. After further adding 1.2 grams of Di-t-Amyl Peroxide and 20.4 grams of n-Butyl Acetate, a post-cooking was pursued at 135°C for 1 h.
- the acrylic polyol had a molecular weight (Mw) of 11400 Daltons and a Tg of about -10°C.
- the acrylic polyol had a molecular weight (Mw) of 8600 Daltons and a Tg of about +26°C. Observations : Tg of acrylic polyols is impacted by the composition of the neononanoic glycidyl ester (see examples 01, 02) .
- a sample has been mixed in a test tube with Methanol (proportion 1:10) under magnetic agitation. Once solution was homogenous, drops of demi water were added in order to get homogenous precipitation (white coloration) . After ⁇ 2 hours of resting, the bottom of the test tube is recovered and dried in a vacuum oven at 120 °C for several days.
- the acrylic polyol had a molecular weight (Mw) of 19500 Daltons and a Tg of about + 27°C.
- a sample has been mixed in a test tube with Methanol (proportion 1:10) under magnetic agitation. Once solution was homogenous, drops of demi water were added in order to get homogenous precipitation (white coloration) . After ⁇ 2 hours of resting, the bottom of the test tube is recovered and dried in a vacuum oven at 120 °C for several days.
- the acrylic polyol had a molecular weight (Mw) of 15300 Daltons and a Tg of about + 57°C.
- the adducts of Glycidyl neononanoate GE9H (see Table 3) with acrylic acid (ACE-adduct) and with methacrylic acid (MACE- adduct) are acrylic monomers that can be used to formulate hydroxyl functional (meth) acrylic polymers.
- Table 3 Compositions of the adducts intakes in parts by weight
- Acrylic resins for high solids automotive refinish clearcoats A glass reactor equipped with stirrer was flushed with
- the initial reactor charge (see Table 4) heated to 160 °C.
- the monomer mixture including the initiator was then gradually added to the reactor via a pump over 4 hours at this temperature. Additional initiator was then fed into the reactor during another period of 1 hour at 160°C. Finally the polymer is cooled down to 135°C and diluted to a solids content of about 68% with xylene.
- GE9H based (28%) acrylic polymers for medium solids first- finish clear coats A reactor for acrylic polyols is flushed with nitrogen and the initial reactor charge (see Table 7) heated to 140°C. At this temperature the monomer mixture including the initiator is added over 4 hours to the reactor via a pump. Additional initiator is fed into the reactor during one hour, and then the mixture is kept at 140°C to complete the conversion in a post reaction. Finally the polymer is cooled down and diluted with butyl acetate to a solids content of about 60%.
- Clear lacquers are formulated (see Table 8) from the acrylic polymers by addition of Cymel 1158 (curing agent from CYTEC) , and solvent to dilute to spray viscosity.
- the acidity of the polymer is sufficient to catalyze the curing process, therefore no additional acid catalyst is added.
- the lacquer is stirred well to obtain a homogeneous composition.
- the coatings are applied with a barcoater on Q-panels to achieve a dry film thickness of about 40 m.
- the systems are flashed-off at room temperature for 15 minutes, then baked at 140°C for 30 minutes. Tests on the cured systems are carried out after 1 day at 23°C.
- a reactor equipped with an anchor stirrer, a thermometer, condenser and monomer/initiator feeding system 188.6g of GE9H and 90g of ethoxypropanol (EPR) were loaded and heated to about 150°C (see Table 9) .
- a mixture of 52g of hydroxyethylmethacrylate (HEMA) , 160g of styrene, 68g of acrylic acid (AA) , lOg of dicumylperoxide (DCP), 37.7g of GE9H and 40g of ethoxypropanol (EPR) were added over 2 hours 30 minutes to the reactor while keeping its content at 150°C.
- HEMA hydroxyethylmethacrylate
- DCP dicumylperoxide
- EPR ethoxypropanol
- the reactor content was held for 30 minutes at this temperature. After the 30 minutes hold period, 108g of HEMA, 30g of AA, 142g of isobutyl methacrylate (IBMA), 5g of DCP and 45 grams of EPR were added over 2 hours and 30 minutes at about 150 °C followed by a rinsing step for the feed system with 5 g of EPR. After the rinsing step, the content of the reactor was held for 2 hours at 150°C. The reactor content was cooled down to 100°C and 100 parts of EPR were distilled off at atmospheric pressure.
- the polyacrylate polyol has a solids content of the solution of 90% by weight.
- Example 10 Example 11
- Example 12 Example 13
- a clear coat is formulated with one of the polyester based acrylic polyol (from examples 10, 11 or 12, the curing agent (HDI, Desmodur N3390), the thinner, the levelling agent (BYK- 331) and the catalyst (dibutyltin dilaurate, DBTDL) according to the amounts indicated in Table 11.
- the curing agent HDI, Desmodur N3390
- the thinner the levelling agent
- BYK- 331 levelling agent
- the catalyst dibutyltin dilaurate, DBTDL
- the clearcoat formulations (from Table 11) are applied with a barcoater on degreased Q-panel.
- the panels are dried at room temperature, optionally with a preliminary stoving at 60°C for 30 min.
- Clear coats have been characterized among others by measuring the dust free time and Koenig hardness development
- Maleic anhydride was reacted with the selected alcohol (3,3,5 trimethyl cyclohexanol ) in an equimolar ratio at 110°C to form a maleate monoester in presence of around 5 wt % butyl acetate. The reaction was continued until conversion of the anhydride had reached at least 90 % (Conversion of the anhydride is monitored by acid-base titration.). Methanol was added to open the remaining anhydride in a 1.2/1 molar ratio of methanol/anhydride and the reaction was continued for 30 minutes .
- the reactor was flushed with nitrogen and the initial reactor charge was heated to the polymerization temperature of 150 °C.
- the first charge of Di ter-amylperoxide was then added in one shot.
- the monomer-initiator mixture was dosed continuously to the reactor in 330 minutes at the same temperature.
- the monomer addition feed rate was halved during the last hour of monomer addition.
- the third charge of Di ter-amylperoxide was then fed together with a small amount of the butyl acetate to the reactor in 15 minutes.
- the reactor was kept at this temperature for 60 more minutes.
- the polymer was cooled down. Resin characteristics are in Table 14.
- the mixture was heated to a temperature of about 110 °C for about 1 hour and then steadily increased to 150°C in 3 hours and then cooled down.
- the polyester-ether had an epoxy group content of 4 mmol/kg, a solids content of about 99% a viscosity of 254000 cP an acid value of 1.3 mg KOH/g and a theoretical OH content of 285 mg KOH/g.
- polyester-ether was then formulated in high solids and very high solids 2K polyurethane topcoats either as sole binder or as reactive diluent for an acrylic polyol.
- polyol dispersion is formulated in 2K waterborne polyurethane topcoats .
- the aqueous dispersion is made in the presence of additional other one polyol (s) , or the aqueous dispersion is combined with additional other polyol dispersion (s) , then formulated waterborne polyurethane topcoats .
- the obtained polyol has an Acid Value of about 30mgKOH, a TgFox of 33°C and a solids content of about 90%.
- the obtained polyol was then cooled down to 80°C, and a quantity of N, -di-methyl ethanolamine was added into the vessel to neutralize 80% of the acid groups.
- the vessel was stirred for another 15 minutes before starting the preparation of the aqueous dispersion.
- the aqueous dispersion is obtained by adding demi water preheated at 70°C gradually into the vessel over a period of 2 hours under adequate agitation.
- the dispersion obtained has a solid content of about 40%, a pH of about 7.5 and a particle size of 146nm.
- the following constituents were charged into a reaction vessel under nitrogen flush equipped with a stirrer, a condenser and a thermometer: 300 grams of CarduraTM E10, 100 grams of butyl glycol. That initial reactor charge has been heated up to 140°C. Then, the following mixture was added over a period of 4.5hrs while keeping the temperature constant: 137 grams of acrylic acid, 140 grams of Hydroxyethyl methacrylate, 200 grams of Styrene, 90 grams of Butyl Acrylate, 133 grams of Methyl methacrylate, 25 grams of Di-t-Amyl Peroxide. After further adding 5 grams of Di-t-Amyl Peroxide, a post-cooking was pursued at the same temperature for 2 hrs . The obtained polyol has an Acid Value of about 30mgKOH, a TgFox of 33°C and a solids content of about 90%.
- the obtained polyol was then cooled down to 80 °C, and a quantity of N, N-di-methyl ethanolamine was added into the vessel to neutralize 80% of the acid groups.
- the vessel was stirred for another 15 minutes before starting the preparation of the aqueous dispersion.
- the aqueous dispersion is obtained by adding demi water preheated at 70°C gradually into the vessel over a period of 2 hours under adequate agitation.
- the dispersion obtained has a solid content of about 40%, a pH of about 7.5 and a particle size of 147nm.
- the obtained polyol has an Acid Value of about 30mgKOH, a TgFox of 49°C and a solids content of about 90%.
- the obtained polyol was then cooled down to 80°C, and a quantity of N, N-di-methyl ethanolamine was added into the vessel to neutralized 80% of the acid groups.
- the vessel was stirred for another 15 minutes before starting the preparation of the aqueous dispersion.
- the aqueous dispersion is obtained by adding demi water preheated at 70°C gradually into the vessel over a period of 2 hours under adequate agitation.
- the dispersion obtained has a solid content of about 40%, a pH of about 7.5 and a particle size of 161nm.
- the following constituents were charged into a reaction vessel under nitrogen flush equipped with a stirrer, a condenser and a thermometer: 300 grams of GE9H, 100 grams of butyl glycol. That initial reactor charge has been heated up to 140°C. Then, the following mixture was added over a period of 4.5hrs while keeping the temperature constant: 143 grams of acrylic acid, 135 grams of Hydroxyethyl methacrylate, 200 grams of Styrene, 240 grams of Methyl methacrylate, 25 grams of Di-t-Amyl Peroxide. After further adding 5 grams of Di-t-Amyl Peroxide, a post-cooking was pursued at the same temperature for 2 hrs. The obtained polyol has an Acid Value of about 30mgKOH, a TgFox of about 64 °C and a solids content of about 90%.
- the obtained polyol was then cooled down to 80 °C, and a quantity of N, -di-methyl ethanolamine was added into the vessel to neutralized 80% of the acid groups.
- the vessel was stirred for another 15 minutes before starting the preparation of the aqueous dispersion.
- the aqueous dispersion is obtained by adding demi water preheated at 70°C gradually into the vessel over a period of 2 hours under adequate agitation.
- the dispersion obtained has a solid content of about 40%, a pH of about 7.5 and a particle size of 150nm.
- the obtained polyol has an Acid Value of about 30mgKOH, a TgFox of about 49°C and a solids content of about 90%.
- the obtained polyol was then cooled down to 80°C, and a quantity of N, -di-methyl ethanolamine was added into the vessel to neutralized 80% of the acid groups.
- the vessel was stirred for another 15 minutes before starting the preparation of the aqueous dispersion.
- the aqueous dispersion is obtained by adding demi water preheated at 70°C gradually into the vessel over a period of 2 hours under adequate agitation.
- Example 20 comparative The following constituents were charged into a reaction vessel under nitrogen flush equipped with a stirrer, a condenser and a thermometer: 100 grams of Mono PentaErythritol and 376 grams of MethylHexaHydroPhthalic Anhydride. That initial reactor charge has been heated up to 150 °C under agitation until the resulting mixture became homogenous and transparent. Then, 624 grams of CarduraTM E10 is added into the vessel.
- the following mixture is then added over a period of 4 hours while keeping the temperature constant: 110 grams of acrylic acid, 200 grams of Hydroxyethyl methacrylate, 400 grams of Styrene, 190 grams of Methyl methacrylate, 60 grams of Di-t-Amyl Peroxide. After further adding 10 grams of Di-t-Amyl Peroxide, a post-cooking was pursued at the same temperature for 2 hrs .
- the obtained polyol has an Acid Value of about 30mgKOH, a solids content of about 100%.
- the obtained polyol was then cooled down to 80 °C, and a quantity of N, N-di-methyl ethanolamine was added into the vessel to neutralized 80% of the acid groups.
- the vessel was stirred for another 15 minutes before starting the preparation of the aqueous dispersion.
- the aqueous dispersion is obtained by adding demi water preheated at 70°C gradually into the vessel over a period of 2 hours under adequate agitation.
- the dispersion obtained has a solid content of about 40%.
- Example 21 The following constituents were charged into a reaction vessel under nitrogen flush equipped with a stirrer, a condenser and a thermometer: 103 grams of Mono PentaErythritol and 389 grams of MethylHexaHydroPhthalic Anhydride. That initial reactor charge has been heated up to 150 °C under agitation until the resulting mixture became homogenous and transparent. Then, 608 grams of GE9H is added into the vessel.
- the following mixture is then added over a period of 4 hours while keeping the temperature constant: 112 grams of acrylic acid, 200 grams of Hydroxyethyl methacrylate, 400 grams of Styrene, 190 grams of Methyl methacrylate, 60 grams of Di-t-Amyl Peroxide. After further adding 10 grams of Di-t-Amyl Peroxide, a post-cooking was pursued at the same temperature for 2 hrs .
- the obtained polyol has an Acid Value of about 30mgKOH, a solids content of about 100%.
- the obtained polyol was then cooled down to 80 °C, and a quantity of N, N-di-methyl ethanolamine was added into the vessel to neutralized 80% of the acid groups.
- the vessel was stirred for another 15 minutes before starting the preparation of the aqueous dispersion.
- the aqueous dispersion is obtained by adding demi water preheated at 70°C gradually into the vessel over a period of 2 hours under adequate agitation.
- the dispersion obtained has a solid content of about 40%.
- the following mixture is then added over a period of 4 hours while keeping the temperature constant: 112 grams of acrylic acid, 200 grams of Hydroxyethyl methacrylate, 400 grams of Styrene, 190 grams of Methyl methacrylate, 60 grams of Di-t-Amyl Peroxide. After further adding 10 grams of Di-t-Amyl Peroxide, a post-cooking was pursued at the same temperature for 2 hrs.
- the obtained polyol has an Acid Value of about 30mgKOH, a solids content of about 100%.
- the obtained polyol was then cooled down to 80 °C, and a quantity of N, N-di-methyl ethanolamine was added into the vessel to neutralized 80% of the acid groups.
- the vessel was stirred for another 15 minutes before starting the preparation of the aqueous dispersion.
- the aqueous dispersion is obtained by adding demi water preheated at 70°C gradually into the vessel over a period of 2 hours under adequate agitation.
- the dispersion obtained has a solid content of about 40%.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Materials Engineering (AREA)
- Wood Science & Technology (AREA)
- General Chemical & Material Sciences (AREA)
- Emergency Medicine (AREA)
- Paints Or Removers (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Polymerisation Methods In General (AREA)
- Graft Or Block Polymers (AREA)
Abstract
Description
Claims
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020147012000A KR101630102B1 (en) | 2011-10-19 | 2012-10-16 | Acrylic polyol resins compositions |
| JP2014536133A JP6307433B2 (en) | 2011-10-19 | 2012-10-16 | Acrylic polyol resin composition |
| US14/352,258 US9803051B2 (en) | 2011-10-19 | 2012-10-16 | Acrylic polyol resins compositions |
| BR112014009484A BR112014009484A8 (en) | 2011-10-19 | 2012-10-16 | COMPOSITION OF ACRYLIC RESINS WITH HYDROXYL FUNCTION, PROCESS FOR PREPARING A COMPOSITION, BINDER COMPOSITION, METAL OR PLASTIC SUBSTRATE, ACRYLIC POLYOL, ACRYLIC POLYOL COPOLYMER RESIN, AND, COATING COMPOSITION |
| EP12783108.9A EP2768881B1 (en) | 2011-10-19 | 2012-10-16 | Acrylic polyol resins compositions |
| CN201280051233.3A CN103890037B (en) | 2011-10-19 | 2012-10-16 | acrylic polyol resin composition |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP11075233 | 2011-10-19 | ||
| EP11075233.4 | 2011-10-19 | ||
| EP12002491.4 | 2012-04-05 | ||
| EP12002491 | 2012-04-05 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013056815A1 true WO2013056815A1 (en) | 2013-04-25 |
Family
ID=47143811
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2012/004321 WO2013056815A1 (en) | 2011-10-19 | 2012-10-16 | Acrylic polyol resins compositions |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US9803051B2 (en) |
| EP (1) | EP2768881B1 (en) |
| JP (1) | JP6307433B2 (en) |
| KR (1) | KR101630102B1 (en) |
| CN (1) | CN103890037B (en) |
| BR (1) | BR112014009484A8 (en) |
| WO (1) | WO2013056815A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2896635A1 (en) * | 2014-01-17 | 2015-07-22 | Fujifilm Corporation | Flexible tube, endoscopic medical apparatus, and resin composition for top coat layer |
| EP3808823A1 (en) * | 2019-10-14 | 2021-04-21 | Hexion Research Belgium SA | Glycidyl esters of alpha, alpha branched acids from renewable sources and formulation thereof |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9751982B2 (en) | 2011-10-19 | 2017-09-05 | Hexion Inc | Polyether polyol resins compositions |
| EP2768882B1 (en) * | 2011-10-19 | 2015-08-26 | Hexion Research Belgium SA | Acrylic polyol resins compositions |
| US10767073B2 (en) * | 2016-10-18 | 2020-09-08 | Ppg Industries Ohio, Inc. | Curable film-forming compositions containing hydroxyl functional, branched acrylic polymers and multilayer composite coatings |
| KR102206436B1 (en) * | 2019-04-18 | 2021-01-22 | 주식회사 케이씨씨 | Coating Composition |
| JP2022552919A (en) * | 2019-10-14 | 2022-12-20 | ヘキシオン・インコーポレイテッド | Glycidyl esters of alpha, alpha branched acids and blends thereof from renewable sources |
| CN112920308A (en) * | 2021-03-31 | 2021-06-08 | 重庆三峡油漆股份有限公司 | AOE intermediate, hydroxy acrylic resin containing AOE intermediate and preparation method thereof |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2831877A (en) | 1952-03-24 | 1958-04-22 | Studiengesellschaft Kohel Mit | Production of carboxylic acids from olefins |
| US2876241A (en) | 1954-05-15 | 1959-03-03 | Studiengesellschaft Kohle Mit | Process for the production of carboxylic acids |
| US2967873A (en) | 1957-04-24 | 1961-01-10 | Studiengesellschaft Kohle Mbh | Process for the production of aliphatic and cycloaliphatic monocarboxylic acid alkyl esters |
| US3053869A (en) | 1959-12-31 | 1962-09-11 | Standard Oil Co | Carboxylic acids |
| US3061621A (en) | 1959-01-26 | 1962-10-30 | Studiengesellschaft Kohle Mbh | Process for producing carboxylic acids from olefins, carbon monoxide and water |
| US6136991A (en) | 1996-05-21 | 2000-10-24 | Exxon Chemical Patents Inc. | Glycidyl ester adducts having increased glass transition temperatures |
| EP1227113A1 (en) * | 1999-11-30 | 2002-07-31 | DAICEL CHEMICAL INDUSTRIES, Ltd. | Lowly lactone-modified reactive monomer composition, acrylic polyol resins produced with the same, curable resin compositions and coating compositions |
| US6433217B1 (en) | 1998-09-23 | 2002-08-13 | Gerrit Gerardus Rosenbrand | Process for the preparation of glycidylesters of branched carboxylic acids |
| WO2005040241A1 (en) | 2003-10-15 | 2005-05-06 | Hexion Specialty Chemicals Research Belgium S.A. | Fast drying coating composition comprising an unsaturated hydroxydiester |
Family Cites Families (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3479416A (en) | 1967-05-19 | 1969-11-18 | Petro Tex Chem Corp | Production of isobutylene from isobutane |
| US3849364A (en) | 1973-01-10 | 1974-11-19 | Hercules Inc | Finely divided crystalline polymers of 1,4-dihalo-2,3-epoxybutane |
| DE2362115C3 (en) | 1973-12-14 | 1978-05-24 | Chemische Werke Huels Ag, 4370 Marl | Process for the separation and recovery of isobutene from C4 hydrocarbon mixtures |
| US4086151A (en) | 1974-02-20 | 1978-04-25 | The Dow Chemical Company | Curable mixtures and cured resins made from linear polymers of glycidol |
| JPS6032803A (en) | 1983-07-30 | 1985-02-20 | Cosmo Co Ltd | Alkyletherified hydroxystyrene polymer containing ester group and its manufacture |
| JPS60262821A (en) | 1984-06-08 | 1985-12-26 | Osaka Soda Co Ltd | Glycidyl ester copolymer |
| US5051492A (en) | 1990-06-19 | 1991-09-24 | Shell Oil Company | Polyether resins and process for preparing the same |
| IL116255A (en) | 1995-01-05 | 2004-02-19 | Du Pont | High-solids coating composition |
| US5681906A (en) | 1995-04-19 | 1997-10-28 | Exxon Chemical Patents Inc. | Thermoset coating compositions having improved hardness |
| WO1998023661A1 (en) | 1996-11-25 | 1998-06-04 | Shell Internationale Research Maatschappij B.V. | Acid functional and epoxy functional polyester resins |
| JP2000063484A (en) * | 1998-04-07 | 2000-02-29 | Yuka Shell Epoxy Kk | Low-viscosity composition of epoxy functional polyester resin |
| GB9828445D0 (en) | 1998-12-24 | 1999-02-17 | Ici Plc | Coating composition |
| DE19906518A1 (en) | 1999-02-17 | 2000-08-31 | Oxeno Olefinchemie Gmbh | Dibutene fractionation process |
| DE19908320A1 (en) | 1999-02-26 | 2000-08-31 | Oxeno Olefinchemie Gmbh | Process for the production of vinyl esters from butene oligomers |
| DK1171536T3 (en) | 1999-03-17 | 2003-03-03 | Du Pont | Acid etch and scratch resistant clear coating composition with high solids content |
| WO2001025225A2 (en) | 1999-09-30 | 2001-04-12 | Resolution Research Nederland B.V. | Adducts of glycidylesters of alpha, alpha-branched carboxylic acids and acrylic acids and poly(ortho ester) as intermediate for their preparation |
| AU2001254628A1 (en) * | 2000-02-02 | 2001-08-14 | Resolution Research Nederland B.V. | Manufacturing process for the preparation of alpha-branched carboxylic acids having a decreased content of beta-branched isomers |
| EP1283226A1 (en) * | 2001-07-31 | 2003-02-12 | Resolution Research Nederland B.V. | Hydroxyl-functional copolymer and coating compositions formed therewith |
| EP1281700A1 (en) * | 2001-07-31 | 2003-02-05 | Resolution Research Nederland B.V. | Manufacturing process for the preparation of alpha, alpha-branched alkane carboxylic acids providing esters with an improved softness |
| JP3780254B2 (en) * | 2002-12-25 | 2006-05-31 | 東洋インキ製造株式会社 | Polyester resin for toner, toner for developing electrostatic image, and image forming method |
| US8197905B2 (en) | 2005-10-05 | 2012-06-12 | E I Du Pont De Nemours And Company | Method of applying high solids coating composition to multilayer coating |
| DE102007057145A1 (en) | 2007-11-28 | 2009-06-04 | Evonik Goldschmidt Gmbh | Process for the preparation of polyether alcohols with DMC catalysts using compounds bearing SiH groups as additives |
| EP2261220A1 (en) | 2009-06-11 | 2010-12-15 | Hexion Specialty Chemicals Research Belgium S.A. | Process for preparing glycidyl esters of branched monocarboxylic acids |
| KR20130101093A (en) * | 2010-10-19 | 2013-09-12 | 모멘티브 스페셜티 케미칼즈 인코포레이티드 | Glycidyl esters of alpha, alpha branched neononanoic acids, synthesis and uses |
| EP2476672A1 (en) * | 2010-12-22 | 2012-07-18 | Momentive Specialty Chemicals Research Belgium S.A. | Glycidyl esters of alpha , alpha branched acids compositions |
| EP2474537A1 (en) | 2010-12-22 | 2012-07-11 | Momentive Specialty Chemicals Research Belgium S.A. | glycidyl esters of alpha, alpha branched acids compositions |
| BR112014009504A2 (en) * | 2011-10-19 | 2017-05-09 | Momentive Specialty Chem Inc | polyether polyol resin composition, process for preparing a composition, binder composition, metal or plastic substrate, and ether polyester resin |
| EP2768882B1 (en) | 2011-10-19 | 2015-08-26 | Hexion Research Belgium SA | Acrylic polyol resins compositions |
| US20140287252A1 (en) | 2011-10-19 | 2014-09-25 | Momentive Specialty Chemicals Inc. | Polyester polyol resins compositions |
| US9751982B2 (en) | 2011-10-19 | 2017-09-05 | Hexion Inc | Polyether polyol resins compositions |
| WO2013056816A1 (en) | 2011-10-19 | 2013-04-25 | Momentive Specialty Chemicals Research Belgium Sa | Polyester polyol resins compositions |
| JP6032803B2 (en) | 2012-11-19 | 2016-11-30 | 株式会社タカキタ | Compost spreader |
-
2012
- 2012-10-16 BR BR112014009484A patent/BR112014009484A8/en not_active Application Discontinuation
- 2012-10-16 KR KR1020147012000A patent/KR101630102B1/en active IP Right Grant
- 2012-10-16 WO PCT/EP2012/004321 patent/WO2013056815A1/en active Application Filing
- 2012-10-16 US US14/352,258 patent/US9803051B2/en active Active
- 2012-10-16 JP JP2014536133A patent/JP6307433B2/en active Active
- 2012-10-16 CN CN201280051233.3A patent/CN103890037B/en active Active
- 2012-10-16 EP EP12783108.9A patent/EP2768881B1/en active Active
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2831877A (en) | 1952-03-24 | 1958-04-22 | Studiengesellschaft Kohel Mit | Production of carboxylic acids from olefins |
| US2876241A (en) | 1954-05-15 | 1959-03-03 | Studiengesellschaft Kohle Mit | Process for the production of carboxylic acids |
| US2967873A (en) | 1957-04-24 | 1961-01-10 | Studiengesellschaft Kohle Mbh | Process for the production of aliphatic and cycloaliphatic monocarboxylic acid alkyl esters |
| US3061621A (en) | 1959-01-26 | 1962-10-30 | Studiengesellschaft Kohle Mbh | Process for producing carboxylic acids from olefins, carbon monoxide and water |
| US3053869A (en) | 1959-12-31 | 1962-09-11 | Standard Oil Co | Carboxylic acids |
| US6136991A (en) | 1996-05-21 | 2000-10-24 | Exxon Chemical Patents Inc. | Glycidyl ester adducts having increased glass transition temperatures |
| US6433217B1 (en) | 1998-09-23 | 2002-08-13 | Gerrit Gerardus Rosenbrand | Process for the preparation of glycidylesters of branched carboxylic acids |
| EP1227113A1 (en) * | 1999-11-30 | 2002-07-31 | DAICEL CHEMICAL INDUSTRIES, Ltd. | Lowly lactone-modified reactive monomer composition, acrylic polyol resins produced with the same, curable resin compositions and coating compositions |
| WO2005040241A1 (en) | 2003-10-15 | 2005-05-06 | Hexion Specialty Chemicals Research Belgium S.A. | Fast drying coating composition comprising an unsaturated hydroxydiester |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2896635A1 (en) * | 2014-01-17 | 2015-07-22 | Fujifilm Corporation | Flexible tube, endoscopic medical apparatus, and resin composition for top coat layer |
| EP3808823A1 (en) * | 2019-10-14 | 2021-04-21 | Hexion Research Belgium SA | Glycidyl esters of alpha, alpha branched acids from renewable sources and formulation thereof |
| WO2021073764A1 (en) * | 2019-10-14 | 2021-04-22 | Hexion Research Belgium Sa | Glycidyl esters of alpha, alpha branched acids from renewable sources and formulations thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| US9803051B2 (en) | 2017-10-31 |
| CN103890037A (en) | 2014-06-25 |
| JP2015501347A (en) | 2015-01-15 |
| BR112014009484A8 (en) | 2018-02-06 |
| JP6307433B2 (en) | 2018-04-04 |
| KR20140078720A (en) | 2014-06-25 |
| BR112014009484A2 (en) | 2017-05-09 |
| EP2768881B1 (en) | 2016-03-23 |
| CN103890037B (en) | 2018-01-26 |
| US20140248503A1 (en) | 2014-09-04 |
| KR101630102B1 (en) | 2016-06-13 |
| EP2768881A1 (en) | 2014-08-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2768881B1 (en) | Acrylic polyol resins compositions | |
| EP2661430B1 (en) | Glycidyl esters of alpha, alpha branched acids compositions | |
| EP2655351B1 (en) | Glycidyl esters of alpha, alpha branched acids compositions | |
| EP2630113B1 (en) | Glycidyl esters of alpha, alpha branched neononanoic acids, synthesis and uses | |
| EP2768882B1 (en) | Acrylic polyol resins compositions | |
| EP2768915A1 (en) | Polyester polyol resins compositions | |
| EP2768901A1 (en) | Polyester polyol resins compositions | |
| EP2780418A1 (en) | Polyether polyol resin compositions | |
| US20190119510A1 (en) | Glycidyl esters of alpha, alpha branched acids compositions |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12783108 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14352258 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: 2014536133 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012783108 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 20147012000 Country of ref document: KR Kind code of ref document: A |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112014009484 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112014009484 Country of ref document: BR Kind code of ref document: A2 Effective date: 20140417 |