WO2012109296A1 - Antisense oligonucleotides - Google Patents
Antisense oligonucleotides Download PDFInfo
- Publication number
- WO2012109296A1 WO2012109296A1 PCT/US2012/024230 US2012024230W WO2012109296A1 WO 2012109296 A1 WO2012109296 A1 WO 2012109296A1 US 2012024230 W US2012024230 W US 2012024230W WO 2012109296 A1 WO2012109296 A1 WO 2012109296A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- seq
- exon
- antisense oligonucleotide
- oligonucleotide molecule
- sequence
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/7105—Natural ribonucleic acids, i.e. containing only riboses attached to adenine, guanine, cytosine or uracil and having 3'-5' phosphodiester links
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/04—Drugs for disorders of the muscular or neuromuscular system for myasthenia gravis
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/11—Antisense
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/31—Chemical structure of the backbone
- C12N2310/315—Phosphorothioates
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/32—Chemical structure of the sugar
- C12N2310/323—Chemical structure of the sugar modified ring structure
- C12N2310/3233—Morpholino-type ring
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/34—Spatial arrangement of the modifications
- C12N2310/346—Spatial arrangement of the modifications having a combination of backbone and sugar modifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2320/00—Applications; Uses
- C12N2320/30—Special therapeutic applications
- C12N2320/33—Alteration of splicing
Definitions
- DMD Duchenne muscular dystrophy
- DMD is caused by mutations in the dystrophin gene that preclude the synthesis of functional protein.
- the dystrophin gene is one of the largest genes in the human genome containing 79 exons spanning more than 2.3 million base pairs.
- AOs antisense oligonucleotides
- oligonucleotides have been shown to induce specific exon skipping and thereby restore the reading frame and expression of functional dystrophin. By skipping out-of-frame mutations of the dystrophin gene, the reading frame can be restored and a truncated, yet functional, Becker-like dystrophin protein is expressed.
- antisense oligonucleotides One critical factor determining the effect of targeted removal of specific exon to restore dystrophin reading frame is the identification of specific antisense oligonucleotides .
- antisense oligonucleotides normally have a sequence of only 20-30 nucleotides, there can be hundred or more potential candidate AOs for targeting each exon dystrophin exon. Practically, many antisense oligonucleotides do not produce efficient exon skipping. Therefore, to effectively remove an exon for the restoration of dystrophin reading frame for the treatment of DMD, antisense oligonucleotide targeting each human dystrophin exon needs to be screened and selected. However, there has been no roles to apply for the selection of most effective AO targeting individual exon.
- Embodiments of the present invention are directed generally to antisense compounds and compositions for the treatment of muscular dystrophy, and in particular, Duchenne muscular dystrophy (DMD).
- DMD Duchenne muscular dystrophy
- the invention is directed to antisense oligonucleotide molecules, pharmaceutical compositions and formulations comprising antisense oligonucleotide molecules, and methods of treating muscular dystrophy related diseases and disorders wherein the antisense oligonucleotide molecules comprises a base sequence selected from the group consisting of:
- GTCAATCCGACCTGAGCTTTGTTGTAGA SEQ ID NO: 14
- CTCCAACATCAAGGAAGATGGCA I I I CT (SEQ ID NO: 100);
- inventions are directed to polymers for the enhanced cell delivery of antisense oligonucleotide molecules.
- the present invention provides a class of polymers that are conjugates of poloxamer and polyethylemine (PEI). Poloxamer comprises a central hydrophobic chain of
- polyoxypropylene poly(propylene oxide)
- poly(ethylene oxide) poly(ethylene oxide)
- poly(ethylene oxide) poly(ethylene oxide)
- FIGS. 1-6 are images of western blots that show skipping of various exons using antisense oligonucleotide molecules in accordance with embodiments of the present invention.
- FIG. 7 shows structural chemistry of a polymer used in delivery of antisense oligonucleotide molecules in accordance with embodiments of the present invention.
- Embodiments of the present invention are directed to one or more antisense molecules that are capable of binding to specific targets to induce exon skipping. Certain embodiments of the present invention are also directed to methods of treating dystrophic related diseases including Duchenne muscular dystrophy (DMD).
- DMD Duchenne muscular dystrophy
- Antisense molecules in accordance with embodiments of the present invention include oligonucleotide sequences that are capable of inducing exon skipping in the human dystrophin gene of at least one of exon 43, exon 45, exon 50, exon 51 and exon
- antisense oligonucleotides in accordance with embodiments of the present invention have a high efficiency for inducing exon skipping in the targeted sequence.
- antisense oligonucleotide molecules can cause an exon skipping at an efficiency of at least 20%, more preferably, at least 40%, even more preferably, at least 50%, more preferably still, at least 60%, more preferably, at least
- the present invention is directed to antisense oligonucleotide molecule that is capable of inducing exon skipping of exon 43 of the dystrophin gene.
- Antisense oligonucleotide molecules for targeting of exon 43 may include one or more of the following sequences and analogs thereof:
- GTCAATCCGACCTGAGCTTTGTTGTAGA SEQ ID NO: 14
- the present invention is directed to antisense oligonucleotide molecule that is capable of inducing exon skipping of exon 45 of the dystrophin gene.
- Antisense oligonucleotide molecules for targeting of exon 45 in accordance with the present invention may include one or more of the following sequences and analogs thereof:
- the present invention is directed to antisense oligonucleotide molecule that is capable of inducing exon skipping of exon 50 of the dystrophin gene.
- Antisense oligonucleotide molecules in accordance with embodiments of the present invention for targeting of exon 50 may include one or more of the following sequences and analogs thereof:
- Antisense oligonucleotide molecules for targeting of exon 51 may include one or more of the following sequences and analogs thereof:
- Antisense oligonucleotide molecules for targeting of exon 53 may include one or more of the following sequences and analogs thereof:
- CAACTGTTGCCTCCGGTTCTGAAG SEQ ID NO: 1 16.
- the present invention also includes analogs of the aforementioned oligonucleotide sequences.
- the term oligonucleotide sequences include analogs of the aforementioned oligonucleotide sequences.
- analogs of the inventive oligonucleotide sequences may include sequences in which one or more thymine (T) bases have been substituted for uracil (U) base and vice versa.
- T thymine
- U uracil
- the presence of either base in a sequence generally still allows for the molecule to bind to the pre-mRNA of the dystrophin gene as it is a complementary sequence. Therefore, the presence of either base in the molecule will generally induce exon skipping.
- the above sequences of the molecule may contain all thymines, all uracils or a combination of the two.
- the selection of T or U in the sequences may be based, at least in part on the chemistry used to produce the molecule. For example, if the molecule is a phosphorodiamidate morpholino
- oligonucleotide PMO
- X may desirably be T as this base is used when producing PMOs.
- X may desirably be U as this base is used when producing 2'OMePSs.
- Additional analogs of the inventive oligonucleotide sequences may include sequences in which up to two of the bases have been deleted or substituted with other bases provided the molecule is capable of inducing exon skipping of the targeted exon.
- Further analogs in accordance with the present invention may include oligonucleotide sequences having an additional base added to one or both ends of the sequence, or in which one base at one or more of the opposite ends of the sequence have been deleted.
- analogs of CTGCTGTCTTCTTGCTATGAATAATGTC (SEQ ID NO. 7) may include the following:
- X and X' are independently are one of C, T, G, U, and A;
- CTGCTGTCTTCTTGCTATGAATAATGT SEQ ID NO 121 .
- Antisense oligonucleotide molecules in accordance with the present invention can be any type of molecule as long as it has the selected base sequence and can bind to a target site of the dystrophin pre-mRNA to cause exon skipping.
- the molecule can be an oligodeoxyribonucleotide, an oligoribonucleotide, a
- oligonucleotide is a PMO.
- the molecule is isolated so that it is free from other compounds or
- the antisense oligonucleotide molecules in accordance with the present invention include PMO and peptide nucleic acids (PNA).
- Morpholino oligomers are polymeric molecules having a backbone which supports bases capable of hydrogen bonding to typical polynucleotides, wherein the polymer lacks a pentose sugar backbone moiety, and more specifically a ribose backbone linked by phosphodiester bonds which is typical of nucleotides and nucleosides, but instead contains (a ring nitrogen with coupling through the ring nitrogen).
- Suitable morpholino oligomers include phosphorodiamidate morpholino oligomer
- Examples of morpholino oligomers that may be used in the practice of the present invention are described in greater detail in U.S. Patent. Nos. 5,698,685, 5,217,866, 5,142,047, 5,034,506,
- PNA Peptide nucleic acid polymers
- PNA has the backbone structurally homomorphous with the deoxyribose backbone and consists of N-(2-aminoethyl)glycine units where the nucleobases are attached.
- PNA has been investigated as antisense oligomers for the potential of targeting genes related to human diseases. The easy synthesis of PNA together with positively charged polymers has lead PNA and its conjugates being widely tested as drugs of experimental therapy to cancers, genetic disorders and other diseases.
- antisense oligonucleotide molecules and in particular, morpholino oligomers and PNAs, in accordance with the present invention can be conjugated with a wide variety of different positively charged polymers.
- positively charged polymers include peptides, such as argine rich peptides (Examples of positively charged peptides that may be used in the practice of the invention include R9F2C; (RXR) 4 XB (where X can be any amino acid); R5F2R4C; (RFF)3; Tat proteins, such as TAT sequence CYGRKKRRQRRR; and (RFF)3R), cationic polymers, such as dendrimeric octaguanindine polymer, and other positively charged molecules as known in the art for conjugation to antisense oligonucleotide compounds.
- the antisense oligonucleotides are conjugated with positively charged polymer comprising a polymer having a molecular weight that is from about 1 ,000 to 20,000 Daltons, and preferably from about 5,000 to 10,000 Daltons.
- positively charged polymers is polyethylenimine (PEI) with multiple positively charged amine groups in its branched or unbranched chains. PEI has else been widely used as gene and oligomer delivery vesicle.
- Embodiments of the present invention are also directed to pharmaceutical formulations and compositions for the treating of DMD, and in particular, ameliorating the effects of DMD.
- Treatment or “treating” of an individual (e.g., a mammal, such as a human) or a cell may include any type of intervention used in an attempt to alter the natural course of the individual or cell.
- Treatment includes, but is not limited to, administration of a pharmaceutical composition, and may be performed either prophylactically or subsequent to the initiation of a pathologic event or contact with an etiologic agent.
- Treatment includes any desirable effect on the symptoms or pathology of a disease or condition associated with the dystrophin protein, as in certain forms of muscular dystrophy, and may include, for example, minimal changes or improvements in one or more measurable markers of the disease or condition being treated. Also included are “prophylactic” treatments, which can be directed to reducing the rate of progression of the disease or condition being treated, delaying the onset of that disease or condition, or reducing the severity of its onset. "Treatment” or “prophylaxis” does not necessarily indicate complete eradication, cure, or prevention of the disease or condition, or associated symptoms thereof.
- methods of treating muscular dystrophy such as DMD and BMD, by administering one or more antisense oligonucleotides of the present invention (e.g., SEQ ID NOS: 5-8, 10, 12, 14, 16, 24, 27, 28, 34, 35, 37, 40, 42, 44, 45, 46,, 79, 97, 100, 101 , and 1 16, and analogs and combinations thereof), optionally as part of a pharmaceutical formulation or dosage form, to a subject in need thereof.
- methods of inducing exon-skipping in a subject by administering one or more antisense oligomers, in which the exon is one of exons 42, 44, 45, 50, 51 , and 53, and combinations thereof from the human dystrophin gene.
- a "subject,” as used herein, includes any animal that exhibits a symptom, or is at risk for exhibiting a symptom, which can be treated with an antisense compound of the invention, such as a subject that has or is at risk for having DMD or BMD, or any of the symptoms associated with these conditions (e.g., muscle fiber loss).
- Suitable subjects include laboratory animals (such as mouse, rat, rabbit, or guinea pig), farm animals, and domestic animals or pets (such as a cat or dog) as long as such subjects contain the complementary sequences of the invention.
- Non-human primates and, preferably, human patients, are included.
- compositions comprising antisense oligonucleotide molecules in accordance with the present invention may be administered in any convenient physiologically acceptable vehicle.
- standard pharmaceutically accepted carriers include saline, phosphate buffered saline (PBS), water, aqueous ethanol, emulsions such as oil/water emulsions, triglyceride emulsions, wetting agents, tablets and capsules. It should be recognized that the choice of suitable physiologically acceptable carrier will vary dependent upon the chosen mode of administration.
- the pharmaceutical composition may comprise a plurality of molecules of the invention, each molecule directed to exon skipping in a different exon.
- the pharmaceutical composition may comprise a plurality of molecules of the invention, each molecule directed to exon skipping in the same exon.
- compositions of the present invention may be provided to target cells by any suitable means, including direct administration (e.g., in vitro by addition to culture medium, or in animals in vivo locally by injection or topical administration at a treatment site) or systemically (e.g., parenterally or orally, or intravenously,
- the compounds and compositions comprise part of a physiologically acceptable solution so that in addition to delivery of the desired agent to the target cells, the solution does not otherwise adversely affect the electrolyte and/or volume and/or metabolism of the cells or tissue or subject.
- compositions and compounds as utilized in this invention can be administered by intranasal, oral, inhalational, enteral, topical, intrauterine, vaginal, sublingual, rectal, intramuscular, intrapleural, intraventricular, intraperitoneal, ophthalmic, intravenous, or subcutaneous means.
- the pharmaceutical solution is provided via an intravenous injection.
- compositions in accordance with the present invention may be supplied in liquid or solid form.
- Compositions in accordance with the present invention may further include solvents, diluents, excipients, preservatives, emulsifiers, compounds for adjusting odor, taste, pH or the like.
- the pharmaceutical compositions of the invention may contain suitable excipients and auxiliaries which facilitate processing and delivery of the active compounds into preparations which can be used pharmaceutically.
- Suitable excipients include fillers such as sugars, for example, lactose, sucrose, mannitol or sorbitol, cellulose preparations, calcium phosphates, and binders such as starch, gelatin, methyl cellulose, hydroxypropyl methylcellulose, sodium carboxymethylcellulose, and/or polyvinyl pyrrolidone.
- disintegrating agents may be added, such as the above-mentioned starches as well as carboxymethyl starch, cross-linked polyvinyl pyrrolidone, agar, alginic acid, or a salt thereof.
- Auxiliaries include flow-regulating agents and lubricants, for example, silica, talc, stearic acid or salts thereof, and/or polyethylene glycol.
- formulations can be administered with or without additional carrier or diluent by the oral, systemic injections, percutaneous, transmucosal, or other typical route.
- compositions in accordance with the present invention may be any suitable pharmaceutical formulations in accordance with the present invention.
- caplet tablet, particle, granule, or powder forms.
- the present invention also provides a method of treating and/or ameliorating the effects one or more medical conditions by administering a therapeutically effective amount and/or a prophylactic amount of the aforementioned pharmaceutical formulations, to a sufferer in need thereof.
- a "therapeutically effective amount" of a compound, combination or pharmaceutical composition of the invention is an amount which is sufficient to achieve the desired pharmacological effect.
- the dosage required to provide an effective amount of the composition will vary, depending upon the age, health, physical condition, sex, weight and extent of disease, of the recipient. Additionally, the dosage may be determined by the frequency of treatment and the nature and scope of the desired effect.
- pharmaceutical formulations may range from about 0.05 to 1000 mg/ kg body weight, and in particular from about 5 to 500 mg/ kg body weight. In one embodiment, the dosage amount is from about 50 to 300 mg/ kg body weight once in 2 weeks, or once or twice a week, or any frequency required to achieve therapeutic effect.
- dosage administered will, of course, vary depending on the use and known factors such as the pharmacodynamic characteristics of the active ingredient; age, health, and weight of the recipient; nature and extent of symptoms, kind of concurrent treatment, frequency of treatment, and the effect desired.
- the recipient may be any type of mammal, but is preferably a human.
- dosage forms (compositions) of the inventive pharmaceutical composition may contain about 1 microgram to 20,000 micrograms of active ingredient per unit, and in particular, from about 10 to 1000 micrograms of active ingredient per unit.
- a unit dose of the pharmaceutical formulation will generally contain from 0. 5 to 500 micrograms per kg body weight and preferably will contain from 5 to 300 micrograms, in particular 10, 15, 20, 30, 40, 50, 100, 200, or 300 micrograms per kg body weight ⁇ g/kg body weight) of the antisense oligonucleotide molecule.
- Preferred intravenous dosage ranges from 10 ng to 2000 ⁇ g, preferably 3 to 300 ⁇ g, more preferably 10 to 100 ⁇ g of metal per kg of body weight.
- the unit dose may contain from 2 to 20 micrograms of the antisense oligonucleotide molecule and be administered in multiples, if desired, to give the preceding daily dose.
- the antisense oligonucleotide molecule in these pharmaceutical compositions, the antisense
- oligonucleotide molecule will ordinarily be present in an amount of about 0.5-95% by weight based on the total weight of the composition.
- the dosage can be raised or lowered based on individual patient response. It will be appreciated that the actual amounts of antisense oligonucleotide molecule used will vary according to the specific antisense oligonucleotide molecule being utilized, the particular compositions formulated, the mode of application, and the particular site of administration.
- the active ingredient can be combined with an oral, non-toxic, pharmaceutically acceptable, inert carrier, including but not limited to, lactose, starch, sucrose, glucose, methyl cellulose, magnesium stearate, dicalcium phosphate, calcium sulfate, mannitol, sorbitol and the like. Additionally, when desired or necessary, suitable binders, lubricants, disintegrating agents, and coloring agents can also be incorporated into the mixture.
- Suitable binders may include starch, gelatin, natural sugars such as glucose or beta-lactose, corn sweeteners, natural and synthetic gums such as acacia, tragacanth, or sodium alginate, carboxymethylcellulose, polyethylene glycol, waxes, and the like.
- Lubricants used in these dosage forms may include sodium oleate, sodium stearate, magnesium stearate, sodium benzoate, sodium acetate, sodium chloride, and the like.
- Disintegrators include, without limitation, starch, methyl cellulose, agar, bentonite, xanthan gum, and the like.
- antisense oligonucleotide molecules formulations of the present invention may also be coupled with soluble polymers as targetable drug carriers.
- soluble polymers can include, for example, polyvinylpyrrolidone, pyran copolymer, polyhydroxypropylmethacrylamide-phenol, polyhydroxyethylaspartamidephenol, or polyethyleneoxide-polylysine substituted with palmitoyl residues, or Poloxamer and Polyethylemine (PEI), or a conjugated polymer of Poloxamer and PEL
- the Poloxamer can be any such as L44, L64, P85, P123 and F127.
- FIG. 7 shows a polymer that can be used for cell delivery in accordance with certain embodiments of the present invention.
- antisense oligonucleotide molecule formulations in accordance with the present invention may be coupled to a class of biodegradable polymers useful in achieving controlled release of a drug, for example, polylactic acid, polyglycolic acid, copolymers of polylactic and polyglycolic acid, polyepsilon caprolactone, polyhydroxy butyric acid, polyorthoesters, polyacetals, polydihydropyrans,
- polycyanoacylates a conjugated polymer of Poloxamer and PEI and crosslinked or amphipathic block copolymers of hydrogels.
- compositions described herein may be administered as part of a sustained- release formulation (i.e., a formulation such as a capsule or resin or sponge that effects a slow release of modulating agent following administration).
- sustained-release formulations may generally be prepared using well known technology and administered by, for example, oral, rectal or subcutaneous implantation, or by implantation at the desired target site.
- Sustained-release formulations may contain a modulating agent dispersed in a carrier matrix and/or contained within a reservoir surrounded by a rate controlling membrane.
- Carriers for use within such formulations are bio-compatible, and may also be
- biodegradable preferably the formulation provides a relatively constant level of modulating agent release.
- the length of the treatment generally may be proportional to the length or intensity or prior duration of the disease or pathophysiological process, and may further depend on the animal species, drug effectiveness and degree of effect required or recommended.
- the doses may be single doses or multiple doses over a period of one day, one week, one year to entire life span.
- the pharmaceutical compositions and compounds of the present invention are administered topically, especially when the target of treatment includes areas or organs readily accessible by topical application, including diseases of the eye, the skin, or the lower intestinal tract.
- Topical application may also be readily used to administer the combinations, compounds and compositions of the invention to tissue below the skin, such as muscle. Suitable topical formulations may be prepared for each of these areas or organs.
- Topical application for the lower intestinal tract may be effected in a rectal suppository formulation or in a suitable enema formulation. Topically-transdermal patches may also be used.
- the pharmaceutical compositions may be formulated in a suitable ointment containing the active component suspended or dissolved in one or more carriers.
- Carriers for topical administration of the compounds of this invention include, but are not limited to, mineral oil, liquid petrolatum, white petrolatum, propylene glycol, polyoxyethylene, polyoxypropylene compound, emulsifying wax and water.
- the pharmaceutical compositions may be formulated in a suitable lotion or cream containing the active components suspended or dissolved in one or more pharmaceutically acceptable carriers. Suitable carriers include, but are not limited to, mineral oil, sorbitan monostearate, polysorbate 60, cetyl esters wax, cetearyl alcohol, 2-octyldodecanol, benzyl alcohol and water.
- the pharmaceutical compositions may be formulated as micronized suspensions in isotonic, pH adjusted sterile saline, or, preferably, as solutions in isotonic, pH adjusted sterile saline, either with our without a preservative such as benzylalkonium chloride.
- the pharmaceutical compositions may be formulated in an ointment such as petrolatum.
- Formulations suitable for topical administration to the eye also include eye drops wherein the active ingredients were dissolved or suspended in a suitable carrier, especially an aqueous solvent for the active ingredients.
- the active ingredients were preferably present in such formulations in a concentration of 0.5 to 20%, advantageously 0.5 to 10% and particularly about 1.5% w/w.
- the skin sites include anatomic regions for transdermal ⁇ administering the drug, such as the forearm, abdomen, chest, back, buttock, thigh and retroauricular area.
- the compound is administered to the skin by placing on the skin either a topical formulation comprising the compound or a transdermal drug delivery device that administers the compound.
- the delivery vehicle is designed, shaped, sized, and adapted for easy placement and comfortable retention on the skin, or the formulation is applied directly on the skin in a prescribed amount and schedule.
- Formulations suitable for topical administration include liquid or semi-liquid preparations suitable for penetration through the skin (e.g., liniments, lotions, ointments, creams, gels or pastes) and drops suitable for administration to the eye, ear, or nose.
- a suitable topical dose of active ingredient of a compound of the invention is expected to be similar to the dose used by any other local and systemic route
- the antisense oligonucleotides When formulated in an ointment, the antisense oligonucleotides may be employed with either paraffinic or a water-miscible ointment base. Alternatively, the antisense oligonucleotides may be formulated in a cream with an emulsified cream base. If desired, the aqueous phase of the cream base may include, for example at Least 30% w/w of a polyhydric alcohol such as propylene glycol, butane-1 ,3-diol, mannitol, sorbitol, glycerol, polyethylene glycol and mixtures thereof.
- a polyhydric alcohol such as propylene glycol, butane-1 ,3-diol, mannitol, sorbitol, glycerol, polyethylene glycol and mixtures thereof.
- the topical formulation may desirably include a compound which enhances absorption or penetration of the active ingredient through the skin or other affected areas.
- dermal penetration enhancers include methocarbamol, longer-chain alcohols, dimethylsulfoxide and related analogs.
- transdermal drug delivery devices can be employed with the pharmaceutical formulations of this invention.
- a simple adhesive patch comprising a backing material and an acrylate adhesive can be prepared.
- the drug and any penetration enhancer can be formulated into the adhesive casting solution.
- the adhesive casting solution can be cast directly onto the backing material or can be applied to the skin to form an adherent coating.
- Transdermal administration may be accomplished using a patch either of the reservoir and porous membrane type or of a solid matrix variety.
- the active agent is delivered continuously from the reservoir or microcapsules through a membrane into the active agent permeable adhesive, which is in contact with the skin or mucosa of the recipient. If the active agent is absorbed through the skin, a controlled and
- the encapsulating agent may also function as the membrane.
- the compound of the invention will be delivered using a liquid reservoir system drug delivery device.
- a liquid reservoir system drug delivery device typically comprise a backing material, a membrane, an acrylate based adhesive, and a release liner.
- the membrane is sealed to the backing to form a reservoir.
- the drug or compound and any vehicles, enhancers, stabilizers, gelling agents, and the like are then incorporated into the reservoir.
- Matrix patches comprising a backing, a drug/penetration enhancer matrix, a membrane, and an adhesive can also be employed to deliver a compound of the invention transdermally.
- the matrix material typically will comprise a polyurethane foam.
- the drug, any enhancers, vehicles, stabilizers, and the like are combined with the foam precursors.
- the foam is allowed to cure to produce a tacky, elastomeric matrix which can be directly affixed to the backing material.
- preparations for topical application to the skin comprising a compound of the invention, typically in concentrations in the range from about 0.001 % to 10%, together with a non-toxic, pharmaceutically acceptable topical carrier.
- topical preparations can be prepared by combining an active ingredient according to this invention with conventional pharmaceutical diluents and carriers commonly used in topical dry, liquid, and cream formulations.
- Ointment and creams may, for example, be formulated with an aqueous or oily base with the addition of suitable thickening and/or gelling agents.
- bases may include water and/or an oil, such as liquid paraffin or a vegetable oil, such as peanut oil or castor oil.
- Thickening agents that may be used according to the nature of the base include soft paraffin, aluminum stearate, cetostearyl alcohol, propylene glycol, polyethylene glycols, woolfat, hydrogenated lanolin, beeswax, and the like.
- Lotions may be formulated with an aqueous or oily base and will, in general, also include one or more of the following: stabilizing agents, emulsifying agents, dispersing agents, suspending agents, thickening agents, coloring agents, flavoring agents, coloring agents, perfumes, and the like.
- Powders may be formed with the aid of any suitable powder base, e.g., talc, lactose, starch, and the like.
- Drops may be formulated with an aqueous base or non-aqueous base also comprising one or more dispersing agents, suspending agents, solubilizing agents, flavoring agents, coloring agents, and the like.
- the oily phase of the emulsions of this invention may be constituted from known ingredients in a known manner. While the phase may comprise merely an emulsifier, it may comprise a mixture of at least one emulsifier with a fat or an oil or with both a fat and an oil. Preferably, a hydrophilic emulsifier is included together with a lipophilic emulsifier which acts as a stabilizer. It is also preferred to include both an oil and a fat.
- Emulsifiers and emulsion stabilizers suitable for use in the formulation of the present invention include TWEENTM 60, SPANTM 80, cetostearyl alcohol, myristyl alcohol, glyceryl monostearate, sodium lauryl sulfate, glyceryl distearate alone or with a wax, or other materials well known in the art.
- the choice of suitable oils or fats for the formulation is based on achieving the desired cosmetic properties, since the solubility of the active compound in most oils likely to be used in pharmaceutical emulsion formulations is very low.
- the cream should preferably be a non-greasy, non-staining and washable product with suitable consistency to avoid leakage from tubes or other containers.
- Straight or branched chain, mono- or dibasic alkyl esters such as di-isoadipate, isocetyl stearate, propylene glycol diester of coconut fatty acids, isopropyl myristate, decyl oleate, isopropyl palmitate, butyl stearate, 2-ethylhexyl palmitate or a blend of branched chain esters may be used. These may be used alone or in combination depending on the properties required. Alternatively, high melting point lipids such as white soft paraffin and/or liquid paraffin or other mineral oils can be used.
- the topical pharmaceutical compositions according to this invention may also include one or more preservatives or bacteriostatic agents, e.g., methyl hydroxybenzoate, propyl hydroxybenzoate, chlorocreosol, benzalkonium chlorides, and the like.
- the topical pharmaceutical compositions also can contain other active ingredients such as antimicrobial agents, particularly antibiotics, anesthetics, analgesics, and antipruritic agents, as well as anti-fungal agents.
- formulation/application may also be included.
- compositions of the present invention can also be delivered through mucosal membranes.
- Transmucosal (i.e., sublingual, buccal, and vaginal) drug delivery provides for an efficient entry of active substances to systemic circulation and reduces immediate metabolism by the liver and intestinal wall flora.
- Transmucosal drug dosage forms e.g., tablet, suppository, ointment, pessary, membrane, and powder
- the method is carried out by administering to the subject an antisense oligonucleotide, and in particular a morpholino oligomer that is selected to target a specific gene region aiming to restore specific gene expression or switch the isoforms of the gene.
- the method comprises administering to a subject, in a suitable pharmaceutical carrier, an amount of the antisense oligonucleotide molecule effective to interfere with the integrety, transcription or splicing, and thus suppress or restore normal expression of the protein, or switch the expression of different isoforms.
- the method results in expression of a dominant negative variant of the protein.
- the subject is a human subject.
- various systemic routes of delivery including oral and parenteral routes, e.g., intravenous, subcutaneous, intraperitoneal, and intramuscular, as well as inhalation, transdermal and topical delivery, can be used.
- oral and parenteral routes e.g., intravenous, subcutaneous, intraperitoneal, and intramuscular, as well as inhalation, transdermal and topical delivery.
- one or more doses of antisense oligonucleotide molecules are administered, generally at regular intervals, preferably once a day to once a month.
- Preferred doses for oral administration are from about 1 mg per kg of bodyweight antisense oligonucleotide molecule to about 600 mg antisense oligonucleotide molecule per kg body weight, and more preferably, from about 30 mg per kg of bodyweight antisense oligonucleotide molecule to about 300 mg antisense oligonucleotide molecule per kg body weight.
- the preferred doses are similar to the doses described above. Dosages will vary in accordance with such factors as the age, health, sex, size and weight of the patient, the route of administration, and the efficacy of the antisense oligonucleotide molecule with respect to the particular disease state.
- An effective in vivo treatment regimen using the antisense oligonucleotides of the invention will vary according to the frequency and route of administration, as well as the condition of the subject under treatment. Optimum dosages for a given route can be determined by routine experimentation according to methods known in the art. Such in vivo therapy is generally monitored by tests appropriate to the particular type of ailment being treated, and a corresponding adjustment in the dose or treatment regimen can be made in order to achieve an optimal therapeutic outcome.
- the invention provides a kit for the amelioration of DMD in a patient, the kit comprising a molecule of the invention and instructions for its use.
- the kit may contain a plurality of molecules for use in causing exon skipping in the same exon or a plurality of exons.
- mice containing the entire human dystrophin gene which expresses near normal levels of dystrophin.
- the three models were used to screen antisense oligomers targeting the specific human dystrophin exons.
- the DMD myoblasts and normal human myoblasts were grown in 24 well plates and 2.5 ⁇ PMO or 2'-0-methyl oligonucleotide (2'OMePS) was used for initial screening in duplicate for each antisense oligonucleotide. 24 hours after AO treatment, the cells were then cultured in differentiation medium for further 2 days.
- 2'OMePS 2'-0-methyl oligonucleotide
- PMO antisense oligonucleotides were prepared in 40 ⁇ saline and administrated by intramuscular injections in duplicates.
- the antisense oligonucleotides were delivered together with polymers comprising conjugates of poloxamer and polyethylemine (PEI), or PMOs conjugated with dendrimeric
- Poloxamer comprises a central hydrophobic chain of polyoxypropylene (polypropylene oxide)) and two flanking hydrophilic chains of polyoxyethylene (poly(ethylene oxide). See FIG. 7.
- Treated muscles were harvested 7 days after single injection and the muscles were snap-frozen in liquid nitrogen-cooled isopentane and stored at -80°C. Sections were cut from the muscles and collected into microcentrafuge tubes and then RNAs were extracted for RT-PCR.
- RNA from oligomer-treated cells or muscle tissues was extracted with TriZol reagent (Invitrogene, Carlsbad, CA) according to
- RNA was stored at -80°C for later use.
- RT-PCR was performed with RT-Fidelitaq MasterMix (USB, Cleveland, OH) to amplify the sequence of interest. 100 ng of template RNA was used for each 25 ⁇ RT-PCR reaction.
- the PCR conditions were 43°C for 15 minutes, 94°C for 2 minutes, then cycled 30 times at 94°C for 30 seconds, 65°C for 30 seconds, and 68°C for 1 minute.
- the conditions for nested PCR were 94°C for 2 minutes, then cycled 30 times at 94°C for 30 seconds, 55°C for 30 seconds, 72° C for 1 minute, and 72°C for 5 minutes.
- RT-PCR primers 1 1 :
- Nested PCR primers 1 1 :
- Reverse primer Ex52R2, 5' - TTTTGGGCAGCGGTAATGAG - 3'.
- Reverse primer E49R71 1 1/7137, 5'- C AC ATCCG GTTGTTTAG CTTG AACTG C -3' Nested PCR primers 3: Forward primer: E43F6265/6290, 5'-AACAAAATGTACAAGGACCGACAAGG-3' Reverse primer: E48R7034/7017, 5'-CTAATAGGAGATAACCACAGCAGCAGA-3'.
- hDysEx41 F1 GGGAAATTGAGAGCAAATTTGC;
- hDysEx45R1 GAGGATTGCTGAATTATTTCTTCC.
- hDysEx41/42F2 C AACTTTG C AC AAATTC AC AC ;
- hDysEx45R2 CAATGTTCTGACAACAGTTTGCCG.
- the PCR products were examined by electrophoresis on a 2% agarose gel.
- the intensity of the PCR products demonstrated by the Ethidium bromide staining was measured with NIH image J 1.42 software.
- the PCR product representing the mRNA with the targeted exon skipped will be compared to the PCR product representing the mRNA without skipping of the targeted exon.
- the total signal intensity from the 2 bands will be considered 100%. Therefore, exon skipping efficiency will be considered 50% if the signal intensity of the transcript with targeted exon skipped is equal to that of normal mRNA without exon skipping.
- exon skipping efficiency will be considered 90% if the signal intensity of the transcript with targeted exon skipped is 90% of total signal intensity representing both transcripts with and without the targeted exon skipped.
- preferred AO sequences for targeting dystrophin exon 43 include HE43(+156-10) , HE43(+26+53), HE43(+40+67),
- preferred sequences for targeting dystrophin exon 45 include: HE45(-3+22), HE45(-2+20), HE45(- 8+21 ), HE45(-1 +27), HE45(-9+21), HE45(-3+27), HE45(-7+23), HE45(-7+21), HE45(-5+23), HE45(-3+25); SEQ ID NOs 27, 27, 34, 35, 37, 40, 42, 44, 45,46.
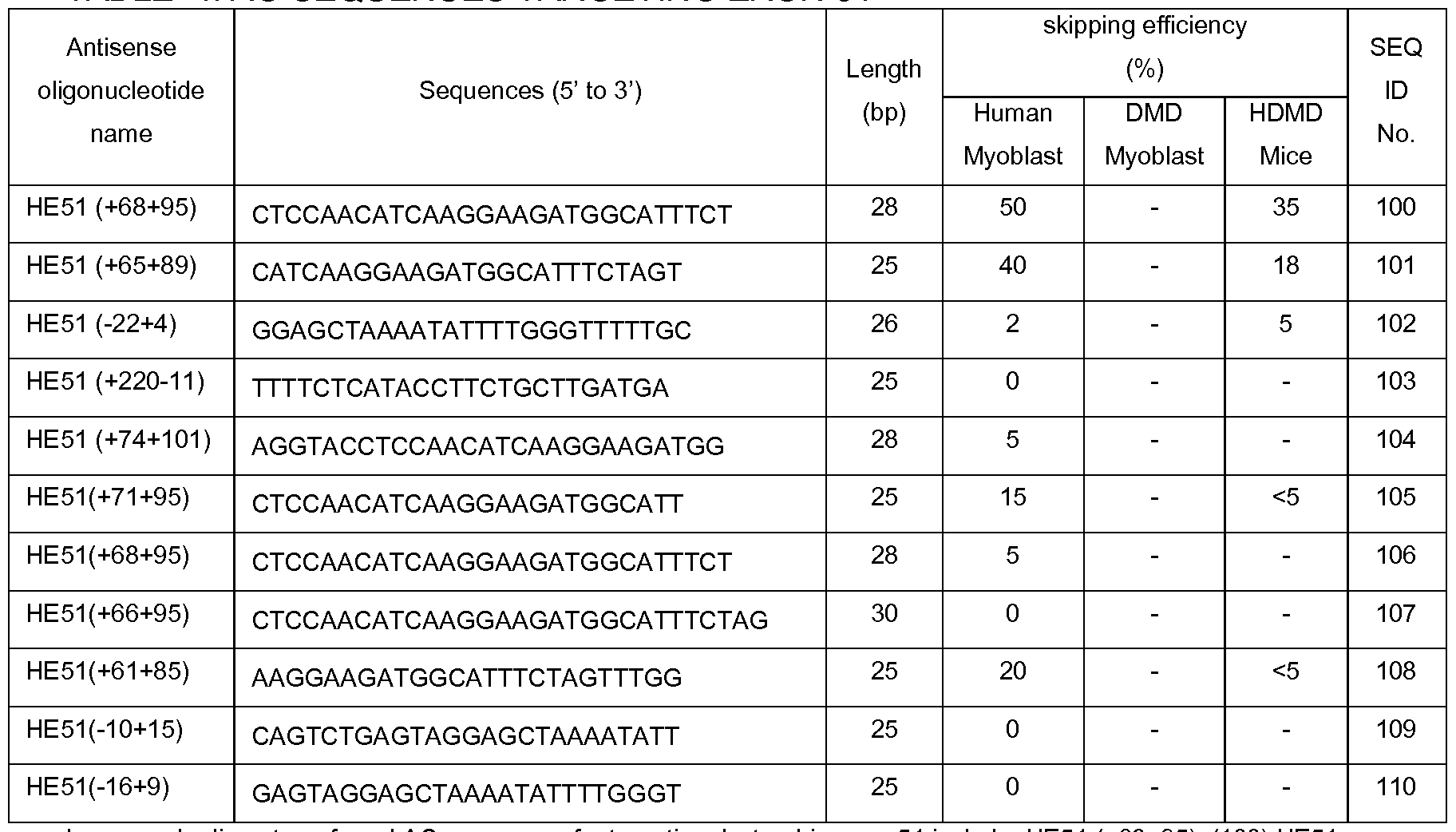
- preferred AO sequences for targeting dystrophin exon 50 HE50(-19+8) (79), HE50(+103-18) (SEQ ID NO 97). TABLE 4: AO SEQUENCES TARGETING EXON 51
- preferred AO sequences for targeting dystrophin exon 51 include: HE51 (+68+95), (100) HE51 (+65+89) (SEQ ID NO 101). TABLE 5: AO SEQUENCES TARGETING EXON 53
- preferred AO sequences for targeting dystrophin exon 53 include: HE53 (+42+65) (SEQ ID NO 116).
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- Epidemiology (AREA)
- Biotechnology (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- General Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Physics & Mathematics (AREA)
- Plant Pathology (AREA)
- Microbiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Physical Education & Sports Medicine (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Neurology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicinal Preparation (AREA)
Abstract
Description
Claims
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| BR112013020273A BR112013020273A2 (en) | 2011-02-08 | 2012-02-08 | antisense oligonucleotides |
| EP12705552.3A EP2672977A1 (en) | 2011-02-08 | 2012-02-08 | Antisense oligonucleotides |
| CA2826836A CA2826836A1 (en) | 2011-02-08 | 2012-02-08 | Antisense oligonucleotides |
| MX2013009191A MX2013009191A (en) | 2011-02-08 | 2012-02-08 | Antisense oligonucleotides. |
| CN201280015038.5A CN103501793A (en) | 2011-02-08 | 2012-02-08 | Antisense oligonucleotides |
| KR20137023595A KR20140052963A (en) | 2011-02-08 | 2012-02-08 | Antisense oligonucleotides |
| JP2013553506A JP2014507143A (en) | 2011-02-08 | 2012-02-08 | Antisense oligonucleotide |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161440603P | 2011-02-08 | 2011-02-08 | |
| US61/440,603 | 2011-02-08 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012109296A1 true WO2012109296A1 (en) | 2012-08-16 |
Family
ID=45755532
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2012/024230 WO2012109296A1 (en) | 2011-02-08 | 2012-02-08 | Antisense oligonucleotides |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US9078911B2 (en) |
| EP (1) | EP2672977A1 (en) |
| JP (1) | JP2014507143A (en) |
| KR (1) | KR20140052963A (en) |
| CN (1) | CN103501793A (en) |
| BR (1) | BR112013020273A2 (en) |
| CA (1) | CA2826836A1 (en) |
| MX (1) | MX2013009191A (en) |
| WO (1) | WO2012109296A1 (en) |
Cited By (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013040417A1 (en) * | 2011-09-16 | 2013-03-21 | The Charlotte-Mecklenburg Hospital Authority D/B/A Carolinas Medical Center | Amphiphilic cationic polymers for the delivery of therapeutic agents |
| US8450474B2 (en) | 2004-06-28 | 2013-05-28 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US8637483B2 (en) | 2009-11-12 | 2014-01-28 | The University Of Western Australia | Antisense molecules and methods for treating pathologies |
| US8865883B2 (en) | 2008-10-24 | 2014-10-21 | Sarepta Therapeutics, Inc. | Multiple exon skipping compositions for DMD |
| US9217148B2 (en) | 2013-03-14 | 2015-12-22 | Sarepta Therapeutics, Inc. | Exon skipping compositions for treating muscular dystrophy |
| WO2015194520A1 (en) * | 2014-06-17 | 2015-12-23 | 日本新薬株式会社 | Antisense nucleic acid |
| US9506058B2 (en) | 2013-03-15 | 2016-11-29 | Sarepta Therapeutics, Inc. | Compositions for treating muscular dystrophy |
| WO2018005805A1 (en) | 2016-06-30 | 2018-01-04 | Sarepta Therapeutics, Inc. | Exon skipping oligomers for muscular dystrophy |
| WO2018118662A1 (en) | 2016-12-19 | 2018-06-28 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| WO2018118599A1 (en) | 2016-12-19 | 2018-06-28 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| WO2018118627A1 (en) | 2016-12-19 | 2018-06-28 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| JP2019030330A (en) * | 2012-07-03 | 2019-02-28 | バイオマリン テクノロジーズ ベー.フェー. | Oligonucleotide for the treatment of muscular dystrophy patients |
| WO2019059973A1 (en) | 2017-09-22 | 2019-03-28 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| WO2019067981A1 (en) | 2017-09-28 | 2019-04-04 | Sarepta Therapeutics, Inc. | Combination therapies for treating muscular dystrophy |
| WO2019067979A1 (en) | 2017-09-28 | 2019-04-04 | Sarepta Therapeutics, Inc. | Combination therapies for treating muscular dystrophy |
| US10385092B2 (en) | 2010-09-01 | 2019-08-20 | Nippon Shinyaku Co., Ltd. | Antisense nucleic acids |
| EP2870246B1 (en) * | 2012-07-03 | 2019-09-11 | BioMarin Technologies B.V. | Oligonucleotide for the treatment of muscular dystrophy patients |
| US10450568B2 (en) | 2015-10-09 | 2019-10-22 | Wave Life Sciences Ltd. | Oligonucleotide compositions and methods thereof |
| WO2020123574A1 (en) * | 2018-12-13 | 2020-06-18 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| US10758629B2 (en) | 2018-05-29 | 2020-09-01 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| EP3655535A4 (en) * | 2017-07-21 | 2021-04-14 | The Governors of the University of Alberta | Antisense oligonucleotides that bind to exon 51 of human dystrophin pre-mrna |
| USRE48960E1 (en) | 2004-06-28 | 2022-03-08 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| EP4215614A1 (en) | 2022-01-24 | 2023-07-26 | Dynacure | Combination therapy for dystrophin-related diseases |
| US12064483B2 (en) | 2017-01-06 | 2024-08-20 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and methods of inducing exon skipping |
Families Citing this family (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006086667A2 (en) | 2005-02-09 | 2006-08-17 | Avi Bio Pharma, Inc. | Antisense composition and method for treating muscle atrophy |
| CN101896186A (en) | 2007-10-26 | 2010-11-24 | 莱顿教学医院 | The mode and the method for antagonism disorder of muscle |
| EP2119783A1 (en) | 2008-05-14 | 2009-11-18 | Prosensa Technologies B.V. | Method for efficient exon (44) skipping in Duchenne Muscular Dystrophy and associated means |
| MX2013012593A (en) | 2011-04-29 | 2014-08-21 | Selecta Biosciences Inc | Tolerogenic synthetic nanocarriers to reduce antibody responses. |
| US20130085139A1 (en) | 2011-10-04 | 2013-04-04 | Royal Holloway And Bedford New College | Oligomers |
| EP4043039A1 (en) | 2012-01-27 | 2022-08-17 | BioMarin Technologies B.V. | Rna modulating oligonucleotides with improved characteristics for the treatment of duchenne and becker muscular dystrophy |
| KR20220025907A (en) | 2013-05-03 | 2022-03-03 | 셀렉타 바이오사이언시즈, 인크. | Tolerogenic synthetic nanocarriers to reduce or prevent anaphylaxis in response to a non-allergenic antigen |
| TR201901939T4 (en) * | 2014-03-12 | 2019-03-21 | Nat Center Neurology & Psychiatry | Antisense nucleic acid. |
| CN106714845A (en) * | 2014-08-11 | 2017-05-24 | 得克萨斯州大学系统董事会 | Prevention of muscular dystrophy by crispr/cas9-mediated gene editing |
| MX2017002931A (en) * | 2014-09-07 | 2017-05-30 | Selecta Biosciences Inc | Methods and compositions for attenuating anti-viral transfer vector immune responses. |
| GB201504124D0 (en) * | 2015-03-11 | 2015-04-22 | Proqr Therapeutics B V | Oligonucleotides |
| MA41795A (en) | 2015-03-18 | 2018-01-23 | Sarepta Therapeutics Inc | EXCLUSION OF AN EXON INDUCED BY ANTISENSE COMPOUNDS IN MYOSTATIN |
| WO2017047707A1 (en) * | 2015-09-15 | 2017-03-23 | 日本新薬株式会社 | Antisense nucleic acid |
| US10543286B2 (en) | 2015-10-07 | 2020-01-28 | The Research Foundation For The State University Of New York | Methods for increasing platelet count by inhibiting biliverdin IXβ reductase |
| CN108699555A (en) | 2015-10-09 | 2018-10-23 | 萨勒普塔医疗公司 | Composition for treating Duchenne's dystrophy and associated disease and method |
| MA45328A (en) | 2016-04-01 | 2019-02-06 | Avidity Biosciences Llc | NUCLEIC ACID-POLYPEPTIDE COMPOSITIONS AND USES THEREOF |
| FI3464306T3 (en) * | 2016-05-24 | 2024-05-16 | Sarepta Therapeutics Inc | Processes for preparing phosphorodiamidate morpholino oligomers |
| SG10202101830WA (en) * | 2016-05-24 | 2021-04-29 | Sarepta Therapeutics Inc | Processes for preparing oligomers |
| KR20190124295A (en) | 2017-03-11 | 2019-11-04 | 셀렉타 바이오사이언시즈, 인크. | Methods and compositions related to combination treatment with synthetic nanocarriers comprising anti-inflammatory agents and immunosuppressants |
| AU2018378812A1 (en) | 2017-12-06 | 2020-07-09 | Avidity Biosciences, Inc. | Compositions and methods of treating muscle atrophy and myotonic dystrophy |
| US20220251551A1 (en) * | 2018-06-13 | 2022-08-11 | Sarepta Therapeutics, Inc. | Exon skipping oligomers for muscular dystrophy |
| US11168141B2 (en) | 2018-08-02 | 2021-11-09 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| EP3830259A4 (en) | 2018-08-02 | 2022-05-04 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating facioscapulohumeral muscular dystrophy |
| EP3829595A4 (en) | 2018-08-02 | 2022-08-24 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| CA3172111A1 (en) | 2020-03-19 | 2021-09-23 | Barbora MALECOVA | Compositions and methods of treating facioscapulohumeral muscular dystrophy |
| US11771776B2 (en) | 2021-07-09 | 2023-10-03 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating dystrophinopathies |
| US11638761B2 (en) | 2021-07-09 | 2023-05-02 | Dyne Therapeutics, Inc. | Muscle targeting complexes and uses thereof for treating Facioscapulohumeral muscular dystrophy |
| US12071621B2 (en) | 2022-04-05 | 2024-08-27 | Avidity Biosciences, Inc. | Anti-transferrin receptor antibody-PMO conjugates for inducing DMD exon 44 skipping |
Citations (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5034506A (en) | 1985-03-15 | 1991-07-23 | Anti-Gene Development Group | Uncharged morpholino-based polymers having achiral intersubunit linkages |
| US5142047A (en) | 1985-03-15 | 1992-08-25 | Anti-Gene Development Group | Uncharged polynucleotide-binding polymers |
| US5166315A (en) | 1989-12-20 | 1992-11-24 | Anti-Gene Development Group | Sequence-specific binding polymers for duplex nucleic acids |
| US5185444A (en) | 1985-03-15 | 1993-02-09 | Anti-Gene Deveopment Group | Uncharged morpolino-based polymers having phosphorous containing chiral intersubunit linkages |
| US5217866A (en) | 1985-03-15 | 1993-06-08 | Anti-Gene Development Group | Polynucleotide assay reagent and method |
| US5506337A (en) | 1985-03-15 | 1996-04-09 | Antivirals Inc. | Morpholino-subunit combinatorial library and method |
| US5521063A (en) | 1985-03-15 | 1996-05-28 | Antivirals Inc. | Polynucleotide reagent containing chiral subunits and methods of use |
| US6124271A (en) | 1997-01-24 | 2000-09-26 | Avi Biopharma, Inc. | Method and conjugate for treating H. pylori infection |
| EP1160318A2 (en) * | 2000-04-26 | 2001-12-05 | Jcr Pharmaceuticals Co., Ltd. | Medicament for treatment of Duchenne muscular dystrophy |
| US6784291B2 (en) | 2000-05-04 | 2004-08-31 | Avi Biopharma, Inc. | Splice-region antisense composition and method |
| WO2006000057A1 (en) * | 2004-06-28 | 2006-01-05 | SMITHKLINE BEECHAM CORPORATION, doing business as GLAXOSMITHKLINE | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US7049431B2 (en) | 2000-01-04 | 2006-05-23 | Avi Biopharma, Inc. | Antisense antibacterial cell division composition and method |
| US7582615B2 (en) | 2006-03-07 | 2009-09-01 | Avi Biopharma, Inc. | Antisense antiviral compound and method for treating arenavirus infection |
| US7625873B2 (en) | 2004-07-02 | 2009-12-01 | Avi Biopharma, Inc. | Antisense antibacterial method and compound |
| WO2010050802A2 (en) * | 2008-10-27 | 2010-05-06 | Academisch Ziekenhuis Leiden | Methods and means for efficient skipping of at least one of the following exons of the human duchenne muscular dystrophy gene: 43, 46, 50- 53. |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1857548A1 (en) * | 2006-05-19 | 2007-11-21 | Academisch Ziekenhuis Leiden | Means and method for inducing exon-skipping |
| CN101896186A (en) * | 2007-10-26 | 2010-11-24 | 莱顿教学医院 | The mode and the method for antagonism disorder of muscle |
| SI3133160T1 (en) * | 2008-10-24 | 2019-05-31 | Sarepta Therapeutics, Inc. | Exon skipping compositions for dmd |
| ES2593836T3 (en) * | 2009-04-24 | 2016-12-13 | Biomarin Technologies B.V. | Oligonucleotide comprising an inosine to treat DMD |
| ES2693459T3 (en) | 2009-11-12 | 2018-12-11 | The University Of Western Australia | Antisense molecules and methods for the treatment of pathologies |
-
2012
- 2012-02-08 WO PCT/US2012/024230 patent/WO2012109296A1/en active Application Filing
- 2012-02-08 CA CA2826836A patent/CA2826836A1/en not_active Abandoned
- 2012-02-08 EP EP12705552.3A patent/EP2672977A1/en not_active Withdrawn
- 2012-02-08 KR KR20137023595A patent/KR20140052963A/en not_active Application Discontinuation
- 2012-02-08 BR BR112013020273A patent/BR112013020273A2/en not_active IP Right Cessation
- 2012-02-08 US US13/369,050 patent/US9078911B2/en active Active
- 2012-02-08 MX MX2013009191A patent/MX2013009191A/en not_active Application Discontinuation
- 2012-02-08 JP JP2013553506A patent/JP2014507143A/en not_active Ceased
- 2012-02-08 CN CN201280015038.5A patent/CN103501793A/en active Pending
Patent Citations (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5034506A (en) | 1985-03-15 | 1991-07-23 | Anti-Gene Development Group | Uncharged morpholino-based polymers having achiral intersubunit linkages |
| US5142047A (en) | 1985-03-15 | 1992-08-25 | Anti-Gene Development Group | Uncharged polynucleotide-binding polymers |
| US5185444A (en) | 1985-03-15 | 1993-02-09 | Anti-Gene Deveopment Group | Uncharged morpolino-based polymers having phosphorous containing chiral intersubunit linkages |
| US5217866A (en) | 1985-03-15 | 1993-06-08 | Anti-Gene Development Group | Polynucleotide assay reagent and method |
| US5506337A (en) | 1985-03-15 | 1996-04-09 | Antivirals Inc. | Morpholino-subunit combinatorial library and method |
| US5521063A (en) | 1985-03-15 | 1996-05-28 | Antivirals Inc. | Polynucleotide reagent containing chiral subunits and methods of use |
| US5698685A (en) | 1985-03-15 | 1997-12-16 | Antivirals Inc. | Morpholino-subunit combinatorial library and method |
| US5166315A (en) | 1989-12-20 | 1992-11-24 | Anti-Gene Development Group | Sequence-specific binding polymers for duplex nucleic acids |
| US6124271A (en) | 1997-01-24 | 2000-09-26 | Avi Biopharma, Inc. | Method and conjugate for treating H. pylori infection |
| US7049431B2 (en) | 2000-01-04 | 2006-05-23 | Avi Biopharma, Inc. | Antisense antibacterial cell division composition and method |
| EP1160318A2 (en) * | 2000-04-26 | 2001-12-05 | Jcr Pharmaceuticals Co., Ltd. | Medicament for treatment of Duchenne muscular dystrophy |
| US6784291B2 (en) | 2000-05-04 | 2004-08-31 | Avi Biopharma, Inc. | Splice-region antisense composition and method |
| WO2006000057A1 (en) * | 2004-06-28 | 2006-01-05 | SMITHKLINE BEECHAM CORPORATION, doing business as GLAXOSMITHKLINE | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US7625873B2 (en) | 2004-07-02 | 2009-12-01 | Avi Biopharma, Inc. | Antisense antibacterial method and compound |
| US7582615B2 (en) | 2006-03-07 | 2009-09-01 | Avi Biopharma, Inc. | Antisense antiviral compound and method for treating arenavirus infection |
| WO2010050802A2 (en) * | 2008-10-27 | 2010-05-06 | Academisch Ziekenhuis Leiden | Methods and means for efficient skipping of at least one of the following exons of the human duchenne muscular dystrophy gene: 43, 46, 50- 53. |
Non-Patent Citations (6)
| Title |
|---|
| AARTSMA-RUS A ET AL: "Functional analysis of 114 exon-internal AONs for targeted DMD exon skipping: indication for steric hindrance of SR protein binding sites", OLIGONUCLEOTIDES, MARY ANN LIEBERT, NEW YORK, NY, US, vol. 15, no. 4, 1 December 2005 (2005-12-01), pages 284 - 297, XP002575217, ISSN: 1545-4576, DOI: 10.1089/OLI.2005.15.284 * |
| AARTSMA-RUS ANNEMIEKE ET AL: "Antisense-induced multiexon skipping for Duchenne muscular dystrophy makes more sense", AMERICAN JOURNAL OF HUMAN GENETICS, AMERICAN SOCIETY OF HUMAN GENETICS, CHICAGO, IL, US, vol. 74, no. 1, 1 January 2004 (2004-01-01), pages 83 - 92, XP002616474, ISSN: 0002-9297, DOI: 10.1086/381039 * |
| ANNEMIEKE AARTSMA-RUS ET AL: "Exonic Sequences Provide Better Targets for Antisense Oligonucleotides Than Splice Site Sequences in the Modulation of Duchenne Muscular Dystrophy Splicing", OLIGONUCLEOTIDES, vol. 20, no. 2, 1 April 2010 (2010-04-01), pages 69 - 77, XP055024186, ISSN: 1545-4576, DOI: 10.1089/oli.2009.0215 * |
| ANNEMIEKE AARTSMA-RUS: "Antisense-mediated modulation of splicing: Therapeutic implications for Duchenne muscular dystrophy", RNA BIOLOGY, vol. 7, no. 4, 1 July 2010 (2010-07-01), pages 453 - 461, XP055024159, ISSN: 1547-6286, DOI: 10.4161/rna.7.4.12264 * |
| See also references of EP2672977A1 * |
| STEVE D WILTON ET AL: "Antisense Oligonucleotide-induced Exon Skipping Across the Human Dystrophin Gene Transcript", MOLECULAR THERAPY, NATURE PUBLISHING GROUP, GB, vol. 15, no. 7, 1 July 2007 (2007-07-01), pages 1288 - 1296, XP002625829, ISSN: 1525-0024, [retrieved on 20070206], DOI: 10.1038/SJ.MT.6300095 * |
Cited By (89)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9024007B2 (en) | 2004-06-28 | 2015-05-05 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US10995337B2 (en) | 2004-06-28 | 2021-05-04 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US9605262B2 (en) | 2004-06-28 | 2017-03-28 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US8455634B2 (en) | 2004-06-28 | 2013-06-04 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US8455635B2 (en) | 2004-06-28 | 2013-06-04 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US8476423B2 (en) | 2004-06-28 | 2013-07-02 | The University of Western Austrailia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US8486907B2 (en) | 2004-06-28 | 2013-07-16 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US8524880B2 (en) | 2004-06-28 | 2013-09-03 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| USRE47691E1 (en) | 2004-06-28 | 2019-11-05 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| USRE47751E1 (en) | 2004-06-28 | 2019-12-03 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US10421966B2 (en) | 2004-06-28 | 2019-09-24 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US9018368B2 (en) | 2004-06-28 | 2015-04-28 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US8455636B2 (en) | 2004-06-28 | 2013-06-04 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US8450474B2 (en) | 2004-06-28 | 2013-05-28 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US10781451B2 (en) | 2004-06-28 | 2020-09-22 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| USRE47769E1 (en) | 2004-06-28 | 2019-12-17 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| USRE48960E1 (en) | 2004-06-28 | 2022-03-08 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US10266827B2 (en) | 2004-06-28 | 2019-04-23 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US10227590B2 (en) | 2004-06-28 | 2019-03-12 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US9249416B2 (en) | 2004-06-28 | 2016-02-02 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US9422555B2 (en) | 2004-06-28 | 2016-08-23 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US9175286B2 (en) | 2004-06-28 | 2015-11-03 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US9441229B2 (en) | 2004-06-28 | 2016-09-13 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US9994851B2 (en) | 2004-06-28 | 2018-06-12 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US9447415B2 (en) | 2004-06-28 | 2016-09-20 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US10968450B2 (en) | 2004-06-28 | 2021-04-06 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US9035040B2 (en) | 2004-06-28 | 2015-05-19 | The University Of Western Australia | Antisense oligonucleotides for inducing exon skipping and methods of use thereof |
| US9453225B2 (en) | 2008-10-24 | 2016-09-27 | Sarepta Therapeutics, Inc. | Multiple exon skipping compositions for DMD |
| US8865883B2 (en) | 2008-10-24 | 2014-10-21 | Sarepta Therapeutics, Inc. | Multiple exon skipping compositions for DMD |
| US9447417B2 (en) | 2008-10-24 | 2016-09-20 | Sarepta Therapeutics, Inc. | Multiple exon skipping compositions for DMD |
| US9447416B2 (en) | 2008-10-24 | 2016-09-20 | Sarepta Therapeutics, Inc. | Multiple exon skipping compositions for DMD |
| US8871918B2 (en) | 2008-10-24 | 2014-10-28 | Sarepta Therapeutics, Inc. | Multiple exon skipping compositions for DMD |
| US9434948B2 (en) | 2008-10-24 | 2016-09-06 | Sarepta Therapeutics, Inc. | Multiple exon skipping compositions for DMD |
| US9234198B1 (en) | 2008-10-24 | 2016-01-12 | Sarepta Therapeutics, Inc. | Multiple exon skipping compositions for DMD |
| US9228187B2 (en) | 2009-11-12 | 2016-01-05 | The University Of Western Australia | Antisense molecules and methods for treating pathologies |
| US8637483B2 (en) | 2009-11-12 | 2014-01-28 | The University Of Western Australia | Antisense molecules and methods for treating pathologies |
| US9758783B2 (en) | 2009-11-12 | 2017-09-12 | The University Of Western Australia | Antisense molecules and methods for treating pathologies |
| US11447776B2 (en) | 2009-11-12 | 2022-09-20 | The University Of Western Australia | Antisense molecules and methods for treating pathologies |
| US10287586B2 (en) | 2009-11-12 | 2019-05-14 | The University Of Western Australia | Antisense molecules and methods for treating pathologies |
| US10781450B2 (en) | 2009-11-12 | 2020-09-22 | Sarepta Therapeutics, Inc. | Antisense molecules and methods for treating pathologies |
| US10385092B2 (en) | 2010-09-01 | 2019-08-20 | Nippon Shinyaku Co., Ltd. | Antisense nucleic acids |
| US10662217B2 (en) | 2010-09-01 | 2020-05-26 | Nippon Shinyaku Co., Ltd. | Antisense nucleic acids |
| US10683322B2 (en) | 2010-09-01 | 2020-06-16 | Nippon Shinyaku Co., Ltd. | Antisense nucleic acids |
| US10647741B2 (en) | 2010-09-01 | 2020-05-12 | Nippon Shinyaku Co., Ltd. | Antisense nucleic acids |
| US10870676B2 (en) | 2010-09-01 | 2020-12-22 | Nippon Shinyaku Co., Ltd. | Antisense nucleic acids |
| US10407461B2 (en) | 2010-09-01 | 2019-09-10 | Nippon Shinyaku Co., Ltd. | Antisense nucleic acids |
| US10487106B2 (en) | 2010-09-01 | 2019-11-26 | Nippon Shinyaku Co., Ltd. | Antisense nucleic acids |
| US11028122B1 (en) | 2010-09-01 | 2021-06-08 | Nippon Shinyaku Co., Ltd. | Antisense nucleic acids |
| WO2013040417A1 (en) * | 2011-09-16 | 2013-03-21 | The Charlotte-Mecklenburg Hospital Authority D/B/A Carolinas Medical Center | Amphiphilic cationic polymers for the delivery of therapeutic agents |
| JP2019030330A (en) * | 2012-07-03 | 2019-02-28 | バイオマリン テクノロジーズ ベー.フェー. | Oligonucleotide for the treatment of muscular dystrophy patients |
| JP2021105049A (en) * | 2012-07-03 | 2021-07-26 | バイオマリン テクノロジーズ ベー.フェー. | Oligonucleotide for treatment of muscular dystrophy patients |
| EP2870246B1 (en) * | 2012-07-03 | 2019-09-11 | BioMarin Technologies B.V. | Oligonucleotide for the treatment of muscular dystrophy patients |
| US10907154B2 (en) | 2013-03-14 | 2021-02-02 | Sarepta Therapeutics, Inc. | Exon skipping compositions for treating muscular dystrophy |
| US11932851B2 (en) | 2013-03-14 | 2024-03-19 | Sarepta Therapeutics, Inc. | Exon skipping compositions for treating muscular dystrophy |
| US9217148B2 (en) | 2013-03-14 | 2015-12-22 | Sarepta Therapeutics, Inc. | Exon skipping compositions for treating muscular dystrophy |
| US10364431B2 (en) | 2013-03-15 | 2019-07-30 | Sarepta Therapeutics, Inc. | Compositions for treating muscular dystrophy |
| US10337003B2 (en) | 2013-03-15 | 2019-07-02 | Sarepta Therapeutics, Inc. | Compositions for treating muscular dystrophy |
| US9506058B2 (en) | 2013-03-15 | 2016-11-29 | Sarepta Therapeutics, Inc. | Compositions for treating muscular dystrophy |
| US12060556B2 (en) | 2014-06-17 | 2024-08-13 | Nippon Shinyaku Co., Ltd. | Antisense nucleic acids |
| WO2015194520A1 (en) * | 2014-06-17 | 2015-12-23 | 日本新薬株式会社 | Antisense nucleic acid |
| US11193125B2 (en) | 2014-06-17 | 2021-12-07 | Nippon Shinyaku Co., Ltd. | Antisense nucleic acids |
| JPWO2015194520A1 (en) * | 2014-06-17 | 2017-04-20 | 日本新薬株式会社 | Antisense nucleic acid |
| US9840706B2 (en) | 2014-06-17 | 2017-12-12 | Nippon Shinyaku Co., Ltd. | Antisense nucleic acids |
| US10450568B2 (en) | 2015-10-09 | 2019-10-22 | Wave Life Sciences Ltd. | Oligonucleotide compositions and methods thereof |
| WO2018005805A1 (en) | 2016-06-30 | 2018-01-04 | Sarepta Therapeutics, Inc. | Exon skipping oligomers for muscular dystrophy |
| WO2018118627A1 (en) | 2016-12-19 | 2018-06-28 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| EP4122497A1 (en) | 2016-12-19 | 2023-01-25 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| EP4406601A2 (en) | 2016-12-19 | 2024-07-31 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| US10888578B2 (en) | 2016-12-19 | 2021-01-12 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| US11000600B2 (en) | 2016-12-19 | 2021-05-11 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| WO2018118599A1 (en) | 2016-12-19 | 2018-06-28 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| US11642364B2 (en) | 2016-12-19 | 2023-05-09 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| WO2018118662A1 (en) | 2016-12-19 | 2018-06-28 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| EP4115908A1 (en) | 2016-12-19 | 2023-01-11 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| US11395855B2 (en) | 2016-12-19 | 2022-07-26 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| US11382981B2 (en) | 2016-12-19 | 2022-07-12 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| US12064483B2 (en) | 2017-01-06 | 2024-08-20 | Avidity Biosciences, Inc. | Nucleic acid-polypeptide compositions and methods of inducing exon skipping |
| US11142767B2 (en) | 2017-07-21 | 2021-10-12 | The Governors Of The University Of Alberta | Antisense oligonucleotides that bind to exon 51 of human dystrophin pre-mRNA |
| EP3655535A4 (en) * | 2017-07-21 | 2021-04-14 | The Governors of the University of Alberta | Antisense oligonucleotides that bind to exon 51 of human dystrophin pre-mrna |
| US11891603B2 (en) | 2017-07-21 | 2024-02-06 | The Governors Of The University Of Alberta | Antisense oligonucleotides that bind to exon 51 of human dystrophin pre-mRNA |
| WO2019059973A1 (en) | 2017-09-22 | 2019-03-28 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| WO2019067981A1 (en) | 2017-09-28 | 2019-04-04 | Sarepta Therapeutics, Inc. | Combination therapies for treating muscular dystrophy |
| WO2019067979A1 (en) | 2017-09-28 | 2019-04-04 | Sarepta Therapeutics, Inc. | Combination therapies for treating muscular dystrophy |
| US10765760B2 (en) | 2018-05-29 | 2020-09-08 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| US11491238B2 (en) | 2018-05-29 | 2022-11-08 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| US11338041B2 (en) | 2018-05-29 | 2022-05-24 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| US10758629B2 (en) | 2018-05-29 | 2020-09-01 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| WO2020123574A1 (en) * | 2018-12-13 | 2020-06-18 | Sarepta Therapeutics, Inc. | Exon skipping oligomer conjugates for muscular dystrophy |
| EP4215614A1 (en) | 2022-01-24 | 2023-07-26 | Dynacure | Combination therapy for dystrophin-related diseases |
Also Published As
| Publication number | Publication date |
|---|---|
| MX2013009191A (en) | 2013-11-04 |
| EP2672977A1 (en) | 2013-12-18 |
| CA2826836A1 (en) | 2012-08-16 |
| US20120202752A1 (en) | 2012-08-09 |
| US9078911B2 (en) | 2015-07-14 |
| KR20140052963A (en) | 2014-05-07 |
| BR112013020273A2 (en) | 2016-10-18 |
| CN103501793A (en) | 2014-01-08 |
| JP2014507143A (en) | 2014-03-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9078911B2 (en) | Antisense oligonucleotides | |
| JP7459691B2 (en) | RNA regulatory oligonucleotides with improved characteristics for the treatment of neuromuscular disorders | |
| US11395855B2 (en) | Exon skipping oligomer conjugates for muscular dystrophy | |
| US10947541B2 (en) | Treatment of atopic dermatitis and asthma using RNA complexes that target IL4Rα, TRPA1, or F2RL1 | |
| TW201828996A (en) | Exon skipping oligomer conjugates for muscular dystrophy | |
| TW201840339A (en) | Exon skipping oligomer conjugates for muscular dystrophy | |
| JP2011510678A (en) | Methods and means for treating DNA repeat instability related genetic disorders | |
| JP5674923B2 (en) | Pharmaceutical compositions containing antisense oligonucleotides and methods of using the same | |
| US20170009230A1 (en) | Compositions for inhibiting dux4 gene expression and uses thereof | |
| JP2021526017A (en) | Exon skipping oligomeric conjugate for muscular dystrophy | |
| CN114901823A (en) | Antisense nucleic acids inducing skipping of exon 50 | |
| US11312959B2 (en) | Antisense oligonucleotides and their use for treating Pendred syndrome | |
| US20240110181A1 (en) | Substances and methods for treating dystrophic epidermolysis bullosa | |
| WO2024043833A1 (en) | Splice-switching oligonucleotides targeting il-4ra | |
| US20230416734A1 (en) | Umlilo antisense transcription inhibitors | |
| CN115484986A (en) | Composition for preventing or treating macular degeneration containing cell-permeable nucleic acid complex as active ingredient | |
| JP2021526807A (en) | Exon skipping oligomers and oligomeric conjugates for muscular dystrophy |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12705552 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2826836 Country of ref document: CA Ref document number: 2013553506 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2013/009191 Country of ref document: MX |
|
| ENP | Entry into the national phase |
Ref document number: 20137023595 Country of ref document: KR Kind code of ref document: A |
|
| REEP | Request for entry into the european phase |
Ref document number: 2012705552 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012705552 Country of ref document: EP |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112013020273 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112013020273 Country of ref document: BR Kind code of ref document: A2 Effective date: 20130808 |