WO2012107905A1 - Composition for use in the treatment of menopause problems/disorders and in the treatment of general aging of the organism. - Google Patents
Composition for use in the treatment of menopause problems/disorders and in the treatment of general aging of the organism. Download PDFInfo
- Publication number
- WO2012107905A1 WO2012107905A1 PCT/IB2012/050615 IB2012050615W WO2012107905A1 WO 2012107905 A1 WO2012107905 A1 WO 2012107905A1 IB 2012050615 W IB2012050615 W IB 2012050615W WO 2012107905 A1 WO2012107905 A1 WO 2012107905A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- composition
- accordance
- equol
- resveratrol
- menopause
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/48—Fabaceae or Leguminosae (Pea or Legume family); Caesalpiniaceae; Mimosaceae; Papilionaceae
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/105—Plant extracts, their artificial duplicates or their derivatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/045—Hydroxy compounds, e.g. alcohols; Salts thereof, e.g. alcoholates
- A61K31/05—Phenols
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/35—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom
- A61K31/352—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom condensed with carbocyclic rings, e.g. methantheline
- A61K31/353—3,4-Dihydrobenzopyrans, e.g. chroman, catechin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/87—Vitaceae or Ampelidaceae (Vine or Grape family), e.g. wine grapes, muscadine or peppervine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2009—Inorganic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2013—Organic compounds, e.g. phospholipids, fats
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/2027—Organic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyvinyl pyrrolidone, poly(meth)acrylates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/205—Polysaccharides, e.g. alginate, gums; Cyclodextrin
- A61K9/2054—Cellulose; Cellulose derivatives, e.g. hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/12—Drugs for genital or sexual disorders; Contraceptives for climacteric disorders
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P17/00—Preparation of heterocyclic carbon compounds with only O, N, S, Se or Te as ring hetero atoms
- C12P17/02—Oxygen as only ring hetero atoms
- C12P17/06—Oxygen as only ring hetero atoms containing a six-membered hetero ring, e.g. fluorescein
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2002/00—Food compositions, function of food ingredients or processes for food or foodstuffs
Definitions
- This invention concerns a composition for the treatment and prevention of disorders related to menopause and/or aging of the human organism.
- one aspect of this invention concerns a composition for the treatment of the problems/disorders associated with menopause, for example hot flushes, dismal mood/depression, insomnia, difficulty concentrating, general aging of the organism, aging of the skin, lowering of the metabolism, weight gain (excess weight).
- the invention composition may also be used as an adjuvant in the treatment of aging of the skin, in the lowering of metabolism, and in the treatment of weight gain.
- soy isoflavones
- red clover red clover
- flax seed The most widely known raw material to be used for menopause.
- soy a legislative limit on the daily quantities that products may contain does exist in Italy.
- the current legislation requires that 80 mg/day of soy isoflavones not be exceeded and the prevalent medical class believes that soy does not function efficaciously in the treatment of said disorders within this limit due to a problem of quantity, even if there is second portion of the medical class which believes that even these quantities may be effective.
- One of the purposes of the invention consists in preventing and/or in mitigating the effects on the human organism brought on by menopause.
- this invention provides a composition for the prevention and/or treatment of disorders and/or symptoms related to menopause including a biologically active and absorbable metabolite of daidzein and resveratrol in synergic association, and an edible carrier and/or an excipient, in which said biologically active and absorbable metabolite of daidzein is equol.
- the Applicant discovered the association of equol and resveratrol causes a synergic antioxidizing action at the level of certain cellular processes at the base of the disorders and/or of the symptoms associated with menopause and with cellular aging.
- resveratrol for example originating from an extract of Vitis vinifera, associated with equol, produces the synergic effect of activating the SIRT genes that code for sirtuin, enzymes capable of improving the cell efficiency counteracting the processes of oxidative stress that typically occur in menopause and/or in the aging processes of the human organism.
- the invention composition is a pharmaceutical composition.
- the invention composition is a nutraceutical composition. In certain embodiments, the invention composition is a dietetic product or a food supplement.
- the invention composition is a food product meant for animals.
- the resveratrol of the composition is obtained from Vitis vinifera.
- the daidzein metabolite is equol obtained through fermentation in the presence of non-pathogenic microorganisms, in particular bacteria, that belong to an equol-producing strain.
- the equol of the composition is obtained through a fermentative process comprising the transformation of daidzein into the inactive metabolite hydroxydaidzein, and the transformation of this metabolite into O- desmethylangolensin and equol.
- the environment in which the fermentative equol- production process takes place is the intestine, in particular the colon, typically an environment with reductive conditions.
- the invention composition comprises an extract of fermented soy with a total isoflavone content from 20 to 60%, in which the quantity of equol ranges from 5 to 20% and a Vitis vinifera extract with a resveratrol content from 5 to 15.2%.
- the resveratrol content of the extract of Vitis vinifera commonly known as “grapes,” it is understood to be the sum of monomers and oligomers, in which the resveratrol monomers amount to no less than 3 mg.
- this invention concerns the use of a composition containing a synergic association of equol and resveratrol in effective quantities and an edible carrier or excipient in the treatment or in the prevention of the menopause problems/disorders.
- the invention concerns the use of a composition, containing a synergic association of equol and resveratrol in effective quantities and an edible carrier or excipient, in the treatment and/or in the prevention of the aging of the organism.
- Figure 1 shows the results of the Mitotracker staining in the HUVEC endothelial cells of example 2;
- Figure 2 shows the results of the SIRT1 protein expression and SIRT1 enzymatic activity in the HUVEC cells of example 2;
- Figure 3 shows the results of the protein expression of porin and of the components of the respiratory chain in the HUVEC cells of example 2;
- Figure 4 shows the results relative to the genetic expression of the mitochondrial biogenesis factors PGC-1a, Nrf-1 , and Tfam in the HUVEC cells of example 2;
- Figure 5 shows the results relative to adipogenesis in the preadipocytes of example 2;
- Figure 6 refers to the results of the Mitotracker staining and the quantification of the mitochondrial DNA
- Figure 7 refers to the measurement of the mRNA expression of mitochondrial biogenesis factors via QRT-PCR
- Figure 8 refers to the quantification of the lipid content.
- the invention is directed to a composition for the prevention and/or the treatment of disorders related to menopause and/or with the aging of the organism containing a synergic association of equol and resveratrol in effective quantities and an edible carrier or excipient.
- resveratrol acts synergically with equol inducing a phytoestrogen ⁇ activity in the human or animal organism. This activity is particularly perceptible in female subjects in menopause.
- menopause is understood to be the time of the cessation of spontaneous menstruation that typically coincides with the end of the physiological reproductive life. This phenomenon, in particular in western women, may occur at an age between 48 and 53 years, inclusive.
- Menopause is typically very evident after an amenorrhea, a period of absence of menstruation of at least 6 months, in a female subject who has reached the average age of onset.
- menopause consists in the cessation of the activity of the ovary, the female gonad, located at the side of the uterus, which produces the female germ cells or oocytes and that secrete estrogens, the female hormones.
- menopause syndrome is understood to be the disorders and the symptoms that accompany menopause and that typically originate from the variation of female hormone levels, and estrogens in particular, which occur during or in the period prior to menopause.
- Equol ((3S)-3-(4-Hydroxyphenyl)-7-chromanol or 4',7-lsoflavandiol) present in the invention composition is an active metabolite obtained from daidzein (7-Hydroxy-3- (4-hydroxyphenyl) chromen-4-one or 4',7-Dihydroxyisoflavone), an isoflavonoid in aglyconic form naturally present in soy and in similar non-fermented products.
- the equol used in the invention composition is not present as such in the isoflavonoids, in the soy or soy shoots found in nature.
- the equol of the invention composition is obtained through metabolism or fermentation of daidzein by equol-producing microorganisms that are typically non-pathogenic bacteria, such as for example bifidus bacteria, lactobacilli, present in the intestinal ecosystem of certain individuals.
- equol is obtained through the fermentation of daidzein with a non-pathogenic, equol-producing microorganism.
- the equol-producing microorganisms are Pediococcus acidilactici bacteria, preferably of the genus Lactococcus lactis, particularly as described in the Italian patent application MI201 1A001829 filed on October 7, 2011 on behalf of the Applicant himself, the entire text of which is incorporated herein for reference.
- the equol of the invention is obtained from the fermentation of microorganisms belonging to the genus Enterococci thailandicus, as described in the Italian patent application ⁇ 2011 ⁇ 00 ⁇ 283 dated July 8, 201 1 on behalf of the Applicant, the entire text of which is incorporated herein for reference.
- the equol of the invention composition is contained in a fermented-soy extract, in particular by means of non-pathogenic, equol-producing microorganisms present in the bacteriological flora of the intestine.
- equol exists in two mirror-image isoforms, S-equol and R-equol.
- S-equol The form produced by certain equol-producing bacteria, present in particular in the intestinal microflora of certain individuals, is S-equol. This represents the most active form of equol in terms of biological activity.
- R-equol is the form obtained through chemical/fermentative synthesis starting from daidzein.
- the equol present in the invention composition is the S isomer.
- Equol has a bonding affinity in relation to alpha and beta estrogenic human receptors much greater than daidzein (from ten to eighty times) and induces the transcription much more strongly than any other isoflavonoid (N. Sathyamoorthy et a!., Eur. J. Cancer, 1977, 33, 2384-2389; G. Kuiper et al., Endocrinology, 1998, 139, 4252-4263; K. Morito et al., Biol. Phatm. Bull., 2001 , 24, 351-356; D. réelleac et al., J. Agr. Food Chem., 2003, 51 , 7632-7635; R.S. Muthyala et al., Bioorg. Med. Chem., 2004, 12, 1559-1567).
- Equol moreover, again with respect to the other isoflavonoids, has a significantly stronger antioxidant power (J.H. Mitchell et al., Arch. Biochem. Biophys., 1998, 360, 142-148).
- soy extracts with measured isoflavone content present on the market do not exhibit detectable levels of equol with standard methods.
- the fermentation of the soy via specific equol-producing strains of bacteria or strains conditioned to produce equol is capable of producing significant quantities of equol starting from daidzein.
- Daidzein is one of the major isoflavonoids. Daidzein is present in soy mainly in the biologically inactive forms of beta-glucoside, acetyl-glycoside, or malonyl- glycoside. It cannot be absorbed by the human intestine in these forms which, after ingestion, are hydrolyzed with aglycone only in small quantities at the level of the small intestine and, then, absorbed by the intestinal epithelium itself (A.J. Day et al., FEBS Lett., 2000, 468, 166-170).
- a large non-hydrolyzed fraction reaches the colon where the forms glycosylated, sulfated, and glucuronidated are deconjugated by the enzymes produced by the microorganisms (A.J. Day et al., 2000, ref. cit.; M. Richelle et al., J. Nutr., 2002, 132, 2587-2592; J. Chen et al., J. Pharmacol., Exp. Ther., 2003, 304, 1228-1235; K.D.R. Setchell et al., Am. J. Clin. Nutr., 2003, 77, 411-419).
- glycosidases of the bacteriological flora of the intestine cleave the beta-glycosidic bond, liberating the aglycone.
- the aglycones are then absorbed and subject to enterohepatic circulation or to further metabolism by the intestinal bacteria (H. Wiseman, Proc. Nutr. Soc, 1999, 58, 139-146).
- DHD dihydroxydaidzein
- Equol Equol
- O-DMA O- desmethylangolensin
- the soy-fermentation process results in an isoflavone content equal to 20-60%, mainly represented by genistein and daidzein.
- the invention composition contains equol in the form of a fermented-soy extract.
- the fermented-soy extract with a total isoflavone content in the interval from 20 to 60%, of which equol is 5 to 20% of the invention composition, corresponds to an interval from 1 mg to 100 mg of equol in the final composition.
- the soy extract of the invention contains from 1 to 30 mg of equol, normally about 9.6 mg of equol.
- the invention composition includes a quantity of fermented-soy extract from 50 to 1000 mg, preferably from 100 to 400 mg, more preferably about 200 mg of fermented-soy extract.
- the invention composition also contains resveratrol, preferably in the form of a Vitis vinifera extract.
- the Vitis vinifera extract has a resveratrol content from 5 to 15.2%.
- Resveratrol or trans-3,5,4'-trihydroxystilbene, is a polyphenolic compound present in various quantities in diverse species of plants, including grapes, peanuts, and Polygonum cuspidatum.
- the resveratrol preferably derives from an extract of grapes, typically from Vitis vinifera.
- the resveratrol content includes both monomers and oligomers thereof.
- the Vitis vinifera extract with a resveratrol content in the interval from 5 to 15.2% corresponds to an interval from 1 mg to 1000 mg of resveratrol in the final composition.
- the Vitis vinifera extract of the invention contains from 1.25 to 5 mg of resveratrol, for example approximately 3.4 mg of resveratrol monomer or 7.5 mg of resveratrol as the sum of monomers and oligomers.
- the invention composition includes a quantity of Vitis vinifera extract from 10 to 200 mg, preferably from 20 to 100 mg, for example, approximately 25 mg of Vitis vinifera extract.
- Certain embodiments of the invention composition contain a fermented-soy extract with a total isoflavone content in the interval from 20 to 60%, of which equol ranged from 5 to 20% and a Vitis vinifera extract with a resveratrol content in the interval from 5 to 15.2%.
- the invention composition may be administered orally and in the form of solid and liquid preparations.
- the preparation When the preparation is solid, the composition may be administered in the form of tablets, capsules, powders, or pastilles, preferably in tablet form.
- the preparation When the preparation is liquid, the invention composition may be administered in the form of a solution, suspension, drops, or syrup.
- the invention composition contains a fermented-soy extract with a total isoflavone content in the interval from 20 to 60%, of which equol is from 5 to 20% and a Vitis vinifera extract a resveratrol content in the interval from 5 to 15.2%.
- the invention composition includes one or more edible carriers and/or excipients.
- the term edible is understood to signify food- grade materials that are approved by the Regulatory authorities for use in pharmaceutical, food, nutritional, and/or dietetic applications.
- the invention composition also includes excipients chosen from the group consisting in bulking agents, anti-caking agents, stabilizing agents, thickening agents, humectants, tableting agents, and colorants commonly used within the scope of formulation techniques for pharmaceutical compositions or nutritional or dietetic preparations.
- composition in tablet form, it may be coated with glazing agents.
- the invention composition also includes plant extracts, for example of Pueraria lobata, and also, or alternatively, vitamins and/or minerals.
- the invention concerns a composition in accordance with any of the embodiments previously described for use as a medicinal, in particular for the use in the treatment of menopause symptoms/disorders, associated especially with hormonal changes consequential to the onset of menopause.
- the menopause disorders include hot flushes that typically manifest themselves as a sudden sensation of warmth on the dermis of the face, neck, and thorax, that may be accompanied by redness and sudation of the skin.
- these hot flushes may have an average duration of approximately 3 minutes and a frequency that generally decreases progressively over the course of years until it nearly disappears after approximately 10 years after the last menstruation.
- the invention composition is used in the treatment or prevention of genitourinary tissue alterations associated with a drop in circulating levels of estrogens.
- the invention composition is applied in the treatment or in the prevention of the following symptoms or disorders: dryness of the vagina, associated with burning or inconvenience during sexual intercourse, cystitis, burning sensation during urination, pelvic pains, frequent urination, and forms of urinary incontinence.
- the composition is applicable in cases where the dermis tends to thin out, and/or there is a tendency for relative dryness and there may be a slight increase of vellus hair in certain zones of the face.
- the composition is applicable in the cardiovascular disorders related to menopause in which the incidence of ischemic phenomena increase, and the lipid profile tends to worsen (increase of cholesterol and triglycerides, reduction of HDL).
- the composition can be applied in cases of menopause in which the bones tend to progressively decrease their density or in case of osteoporosis or in cases of increased risk of fracture, especially in relation to the vertebrae and femur.
- composition may also be applied in menopause associated with worsening of depressive moods.
- the invention composition is applied in the treatment or in the prevention of the symptoms or disorders in the premenopausal phase.
- premenopausal period is understood to be the period that precedes menopause. Typically, there is a progressive irregularity of the menstrual cycle in this period followed by the almost complete cessation of menstrual flow.
- the invention composition is applied in the treatment or in the prevention of the disorders of early menopause.
- early menopause is understood to be a menopause that manifests itself spontaneously in female subjects under the age of 40.
- the invention concerns the invention composition for the use in the treatment of general aging of the organism.
- the invention comprises a nutraceutical formulation containing the invention composition, preferably for use in the treatment of menopause problems/disorders and/or in the treatment of general aging of the organism, associated in particular with hormonal changes consequential to the occurrence of menopause.
- the invention composition is in fact capable of specifically activating mitochondrial biogenesis in different tissues.
- the invention composition is also capable of activating specific cellular-transcription factors that induce anti-inflammatory and antioxidant proteins.
- the invention composition is effective in promoting the viability and the functions of the cells, tissues, and of the organism, and is particularly effective in protecting the endothelial cells, skin cells, and nervous system cells against deterioration, against death due to degenerative processes and in aging in general.
- the invention composition is also active in terms of antiobesity effects, inducing the inhibition of adipogenesis and proapoptotic effects in human adipocytes.
- Table 1 Composition of a tablet of the invention.
- Fermented-soy extract 40% isoflavones 200.000 30.303
- Anti-caking agent 16.000 2.424
- Glazing agent 13.000 1. 970
- Anti-caking agent Glyceryl 12.000 1.818
- Stabilizing agent 10.000 1.515
- Anti-caking agent 10.000 1.515
- Anti-caking agent silicon 10.000 1.515
- Glazing agent 2.000 0.303
- Glazing agent stearic acid 2.000 0.303
- the material was compressed to a theoretical weight of 640 mg/tablet (17.5 x 8.8 mm shape without snap tab).
- the tablets were ten coated with a dispersion of the glazing agents indicated in table 1 .
- the effects were compared with the effects from equol alone and from resveratrol alone in the same quantities as the invention composition.
- Mitochondria are flexible and complex cellular entities that play crucial roles in pathological and normal cellular conditions. Mitochondrial biogenesis is an elaborate cellular process that relies on the strong bond of diverse regulatory controls, from gene transcription to the site-specific production of proteins. The pathways that govern mitochondrial biogenesis have recently emerged as potential therapeutic targets to improve endothelial dysfunction and vascular disorders observed in metabolic diseases (Ren. J., Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med. 2010 Oct; 88(10):993-1001 ).
- Mitochondrial mass was measured in HUVEC endothelial cells using the Mitotracker red staining technique as follows.
- the HUVEC cells were subdivided into three groups, which were treated with resveratrol, 10 pmol/l for 48 h, equol 10 pmol/l for 48 h, and with the composition containing resveratrol and equol, 10 pmol/l for 48 h, respectively.
- the mitochondrial mass in HUVEC was determined selectively loading mitochondria with Mitotracker fluorescent red dye (Invitrogen, Carlsbad, CA). Fluorescent calcein (green) and Hoechst 33258 (blue) dyes were used to stain the cytoplasm and the nuclei, respectively.
- Optical sections of HUVEC were captured at *60- magnification and the mitochondrial density-area ratio was calculated with respect to cytoplasmatic volume using the Zeiss Axiovision imaging software. Only cells with intact cytoplasmatic calcein stain were included in the analysis.
- the mitochondrial DNA content in the HUVEC cells was then measured as follows.
- the total DNA was isolated from the HUVEC cells (DirectPCR; Viagen Biotech).
- the number of mitochondrial DNA copies (mtDNA) was determined via QRT-PCR as described in Addabbo F, Ratliff B, Park HC, Kuo M, Ungvari Z, Csiszar A, Krasnikov B, Sodhi K, Zhang F, Nasjletti A, Goligorsky MS.
- the Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: proteomic approach. Am J Pathol 174: 34-43, 2009.
- cytochrome oxidase III and beta-actin as markers for the number of mtDNA and genomic DNA copies, respectively.
- the impact of the treatment with resveratrol, equol, and the invention composition containing both resveratrol and equol was evaluated.
- SIRT1 nuclear activity was measured in cells treated with resveratrol, equol, and the invention composition as described previously (Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008 Feb; 1 1 (1 ):139-50). Measurement of the protein expression of constituents of the electron transport chain.
- RNA was isolated with the isolation kit with Mini RNA (Zymo Research, Orange, CA) and was inverse-transcribed using Superscript III RT (Invitrogen) as described in Scapagnini G, D'Agata V, Calabrese V, Pascale A, Colombrita C, Alkon DL, Cavallaro S. (2002) Gene expression profiles of heme oxygenase isoforms in the rat brain Brain Res. 954(1 ): 31-39.
- the effectiveness of the PCR reaction was determined employing a series of standard sample dilutions.
- the housekeeping gene HPRT was used for internal normalization.
- the PCR reaction reproducibility was determined via melting point analysis and viewing the product on 2% agarose gel.
- Tests were performed in 96-well plates.
- the cells were seeded (5000 cells/well) and grew until maturing as described further above.
- the cells were seeded (2500 cells/well) and the assay was performed 4 days after seeding for the preadipocytes.
- Preadipocytes or mature adipocytes were incubated with 0.2% dimethyl sulfoxide (DMSO) or test compounds for 24 and 48 hours.
- DMSO dimethyl sulfoxide
- the cellular vitality assay was conducted in accordance with the instruction manual. Absorbance was measured at 490 nm in a plate reader (mQuant, Bio-Tek Instruments) to determine the formazan concentration, which is proportional to the number of live cells.
- the apoptosis detection kit ApoStrand ELISA was used to measure the apoptosis content.
- the cells were grown in 96-well plates, treated with the test compounds for 24 and 48 hours, and tested in accordance with the instruction manual.
- the assay selectively detects single-stranded DNA, which appears in apoptotic cells, but not in necrotic cells or cells with DNA cleavage in the absence of apoptosis. Assays were performed at least twice for 6 replicas for each treatment.
- the lipid content was quantified using the commercially available assay reagent AdipoRed.
- pre-confluent preadipocytes were grown in 96-well plates and incubators with 0.2% DMSO or test compounds during the adipogenic phase, and on the sixth day they were tested for lipid content in accordance with the instruction manual.
- the experiments were performed with at least 6 replicas per treatment and repeated 3 times.
- the mature adipocytes were treated with 0.2% DMSO or the test compounds for 5 h, and the free glycerol released was tested using an adipocyte-lipolysis assay kit (Zen-Bio) and in accordance with the instruction manual. The experiment was repeated twice with at least 4 replicas.
- the invention composition induces mitochondrial biogenesis in endothelial cells. This action is associated with the treatment of age-associated diseases and with correlated symptoms such as menopause (Tina Wenz Mitochondria and PGC-1a in aging and age-associated diseases, in the Journal of Aging Research Vo. 2011 Art. ID810619, pages 1-12).
- the Mitotracker staining showed that mitochondria were located in the perinuclear region in HUVEC.
- the density-area ratio with respect to cytoplasmatic volume in Mitotracker-labeled endothelial cells increased significantly after treatment with resveratrol (Fig. 1 ).
- the analysis of the Mitotracker intensity in HUVEC confirmed that treatment with resveratrol significantly increased the mitochondrial mass in treated cells compared with non-treated cells (Fig. 1 ). Equol alone was not effective in terms of activating the mitochondrial mass, however the invention composition (resveratrol + equol) was significantly more effective compared with resveratrol alone (Fig. 1 ).
- the invention composition also induces SIRT1 in endothelial cells.
- the invention composition proved capable of activating the protein expression of
- composition activated the constituents of the mitochondrial electron transport chain in endothelial cells.
- the invention composition activated the mitochondrial biogenesis factors.
- Mitochondrial biogenesis involves the integration of multiple transcriptional pathways, which control both nuclear and mitochondrial gene expression.
- the PPAR-gamma coactivator (PGC-1a), and the transcription factors Nrf-1 and Tfam are considered key regulators of mitochondrial biogenesis in multiples tissues.
- Measurements via QRT-PCR revealed that the expression of the mitochondrial biogenesis factors PGC-1a, Nrf-1 , and Tfam in HUVEC cells (Fig. 4) had increased significantly via treatment with the invention composition.
- Equol was unable to decrease cellular viability in preadipocytes at 24 h and at 48 h.
- Resveratrol slightly decreased cellular viability ( ⁇ 20% at 24 h and ⁇ 28% at 24 h).
- Table 2
- the invention composition induced mitochondrial biogenesis in human endothelial cells.
- the formation of new mitochondria was associated with the activation of SIRT1 and the induction of specific mitochondrial biogenesis factors.
- the invention composition had been shown to be capable of exerting an increased effect on apoptosis induction and on adipogenesis inhibition in adipocytes.
- the invention composition had likewise showed that the decrease in the number and dimension of the mature adipocytes entailed the loss of lipids and the loss of cells via apoptosis.
- Nrf-1 activates the transcription of many coded nuclear elements of the electron transport chain and also regulates Tfam, responsible for the transcription of mtDNA-coded genes.
- the regulatory function of Nrf-1 and of other mitochondrial biogenesis factors is modulated by PGC-1a. It was shown that resveratrol induced Nrf-1 , Tfam, and PGC-1a in human endothelial HUVEC cells (Fig. 4). It was also established that the altered expression of these factors modulated the mitochondrial biogenesis activity.
- the invention composition induced SIRT1 (Fig. 2) and SIRT1 similarly regulated multiple pathways involved in mitochondrial biogenesis in endothelial cells, including the fact that SIRT1 could also directly deacetylate PGC-1a, increasing its activity.
- the HUVA cells were subdivided into 6 groups, which were treated with 10 pmol/l equol for 48 h, 10 pmol/l daidzein for 48 h, 10 pmol/l of resveratrol for 48 h, fermented soy with a (18%) daidzein content and resveratrol for total of 10 ⁇ / ⁇ of said active ingredients for 48 h, and with the invention composition containing fermented-soy extract with an equol content of 4-5.6%, and Vitis vinifera extract with a resveratrol content of 5-15.2% for a total of ⁇ / ⁇ of said active ingredients for 48 h, and finally an untreated control group, respectively.
- the mitochondrial mass in HUVA was determined selectively loading mitochondria with Mitotracker fluorescent red dye (Invitrogen, Carlsbad, CA). Fluorescent calcein (green) and Hoechst 33258 (blue) dyes were used to stain the cytoplasm and the nuclei, respectively. Optical sections of HUVA were captured at x60 magnification and the mitochondrial density-area ratio was calculated with respect to cytoplasmatic volume using the for Zeiss Axiovision imaging software. Only cells with intact cytoplasmatic calcein stain were included in the analysis.
- the mitochondrial DNA content in the HUVA cells was then measured as follows. The total DNA was isolated from the HUVA cells (DirectPCR; Viagen Biotech). The number of mitochondrial DNA copies (mtDNA) was determined via QRT-PCR as described in Addabbo F, Ratliff B, Park HC, Kuo M, Ungvari Z, Csiszar A, Krasnikov B, Sodhi K, Zhang F, Nasjletti A, Goligorsky MS. The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: proteomic approach. Am J Pathol 174: 34-43, 2009.) using cytochrome oxidase III and beta-actin as markers for the number of mtDNA and genomic DNA copies, respectively.
- the QRT-PCR technique was used to determine the effect of equol, daidzein, resveratrol, fermented soy with a measured content of daidzein, and the invention composition (resveratrol + equol) (10 mol/l, for 24 hours) on the mRNA expression of mitochondrial biogenesis factors: nuclear respiratory factor (Nrf-1 ), mitochondrial transcription factor A (Tfam), and Peroxisome proliferator-activated receptor (PPAR)-a coactivator-1 (PGC-1 a) in HUVA using Light Cycler (Roche Molecular Biochemicals) as described in Scapagnini G, Butterfield DA, Colombrita C, Sultana R, Pascale A, Calabrese V. (2004) Ethyl ferulate, a lipophilic polyphenol, induces HO-1 and protects rat neurons against oxidative stress. Antioxid Redox Signal. 6(5):811-8.)
- RNA was isolated with the isolation kit with Mini RNA (Zymo Research, Orange, CA) and was inverse-transcribed using Superscript III RT (Invitrogen) as described in Scapagnini G, D'Agata V, Calabrese V, Pascale A, Colombrita C, Alkon DL, Cavallaro S. (2002) Gene expression profiles of heme oxygenase isoforms in the rat brain Brain Res. 954(1 ): 31-39
- the effectiveness of the PCR reaction was determined employing a series of standard sample dilutions.
- the housekeeping gene HPRT was used for internal normalization.
- the PCR reaction reproducibility was determined via melting point analysis and viewing the product on 2% agarose gel
- the lipid content was quantified using the commercially available assay reagent AdipoRed.
- pre-confluent preadipocytes were grown in 96-well plates and incubators with 0.2% DMSO or with equol, daidzein, resveratrol, fermented soy with a measured content of daidzein or the invention composition (resveratrol + equol) (10 mol/l, for 24 hours) during the adipogenic phase, and on the sixth day were assayed for lipid content in accordance with the instruction manual.
- the experiments were performed with at least 6 replicas per treatment and repeated 3 times. The results obtained are shown in Fig. 8.
- Table 4 demonstrates the absence of equol in I) a soy extract with a measured content of ginestein and daidzein, and in II) a supplement based on fermented soy with a measured content of aglyconic isoflavones.
- a third sample analyzed is III) a product of a fermentation with conditioned bacteria capable of producing S-equol, that represents a source that can be used as a food supplement of this molecule.
- the equol levels in the solutions to be tested were determined in triplicate via kits based on "Quantitative time-resolved fluoroimmunoassay" (Labmaster, Turku, Finland) technology.
- the first two samples I) soy extract and II) fermented soy standard exhibited values near the detectability limit of the method.
- Formulation of an invention composition in tablet form for the treatment of menopause syndrome (sample dosage 1 -3 tablets/day).
- Formulation of an invention composition for the treatment of the symptoms disorders related to aging (dosage 1 -3 tablets/day).
- Glazing agent microcrystalline cellulose 2.000 0.303
- Glazing agent stearic acid 2.000 0.303
- anti-caking agent crosslinked sodium carboxy methyl
- glazing agent hydroxypropyl methyl cellulose 13 mg
- anti-caking agent magnesium stearate - of vegetable origin
- anti-caking agent silicon dioxide
- thermoplastic material titanium dioxide
- glazing agent microcrystalline cellulose
- glazing agent stearic acid.
- glazing agent hydroxypropyl methyl cellulose 10.4 mg
- anti-caking agent silicon dioxide 40 mg
- anti-caking agent crosslinked sodium carboxy methylcellulose 6 mg
- glazing agent shellac 4 mg
- glazing agent microcrystalline cellulose 1.6 mg
- glazing agent stearic acid 1.6 mg.
- the subjects participating in the study are selected from a panel of healthy female subjects (between 50 and 55 years of age, inclusive) applying the inclusion and exclusion criteria reported below.
- Age between 50 and 55 years of age, inclusive
- Body mass index between 20 and 25, inclusive
- Anamnestic clinical picture characterized by psychological disorders (anxiety, emotional instability, depression) typical of the menopause syndrome, hot flushes, localized accumulations of fat, increase of mass
- the subjects are instructed how to use the product, and they are given the factsheet of the study and the product under discussion.
- Heart discomfort (unusual awareness of heart beat, heart None -Mild -Moderate -Severe -Very severe skipping, heart racing, tightness)
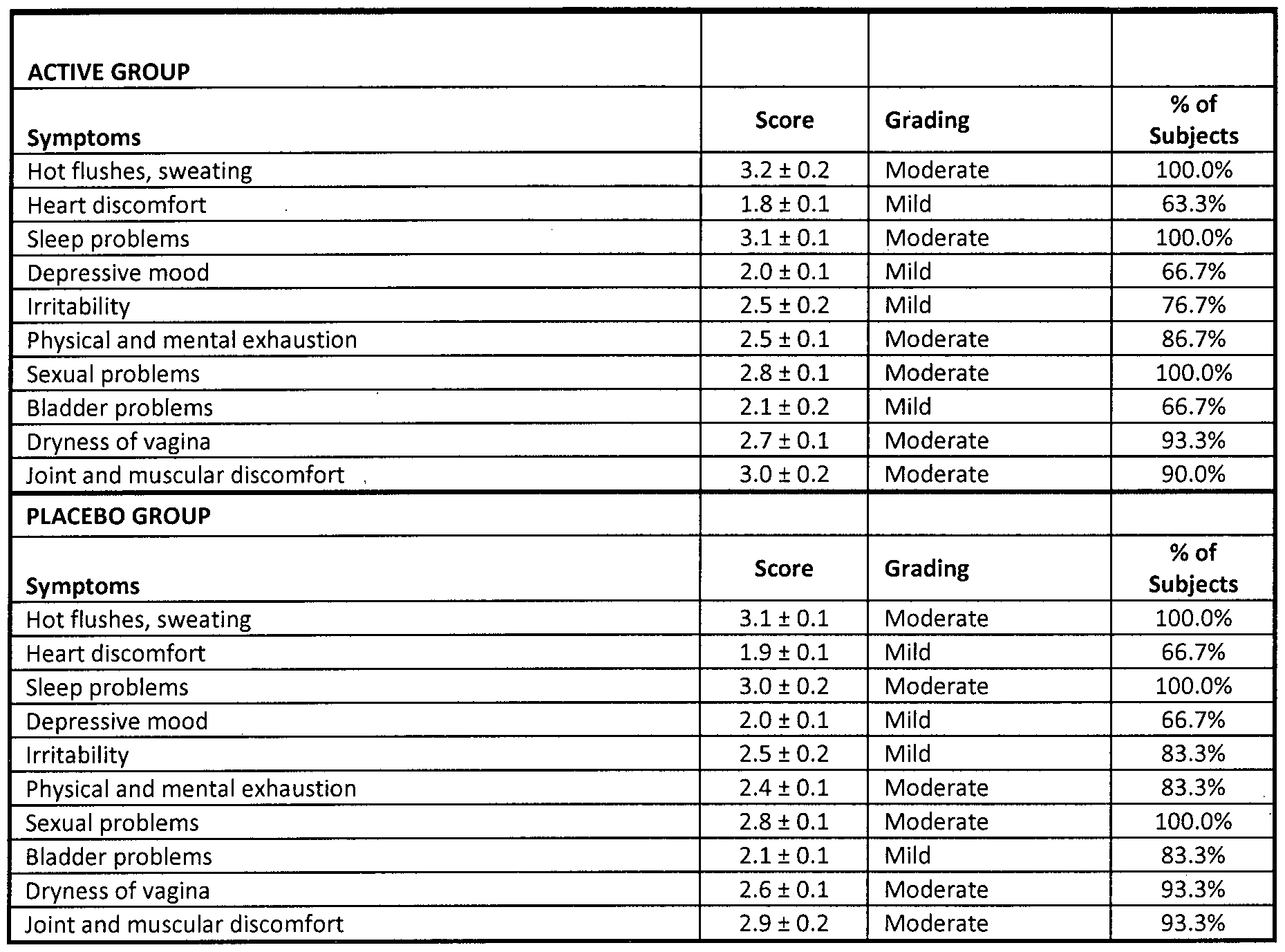
- Table 8 shows the evolution of menopausal symptomatology 1 to 3 months after taking the active product and the placebo product. The data show the percentage of subjects that exhibit an improvement * in the clinical symptomatology. Table 8a - Variation of the menopausal symptoms
- MENOPAUSE RATING SCALE, MRS FOR SYMPTOMS ASSOCIATED WITH MENOPAUSE (Lothar AJ Heinemann, Peter Potjthoff, and HermanPG Schneider. International versions of the Menopause Rating Scale (MRS). Health Qual. Life Outcomes. 2003;1 :28)
- Table 8b shows the intra- and intergroup statistical analysis.
- the intragroup statistical analysis is performed via the Wilcoxon test of the ranges indicated, whereas the intergroup analysis is performed via the Mann-Whitney U test.
- Table 8b Statistical analysis
- the two groups of subjects are homogeneous with reference to the initial symptoms anticipated by the Hamilton scale.
- the statistical analysis does not indicate significant differences (p>0.05) in the medians.
- Table 9 shows the evolution of the mood intensity 1 to 3 months after taking the active product and the placebo product.
- the data show the percentage of subjects that exhibit an improvement * in the clinical symptomatology.
- Table 9b shows the intra- and intergroup statistical analysis.
- the intragroup statistical analysis is performed via the Wilcoxon test of the ranges indicated, whereas the intergroup analysis is performed via the Mann-Whitney U test.
- the panel Upon enrollment, the panel is composed as described in table 10. Table 10 - Panel composition at TO
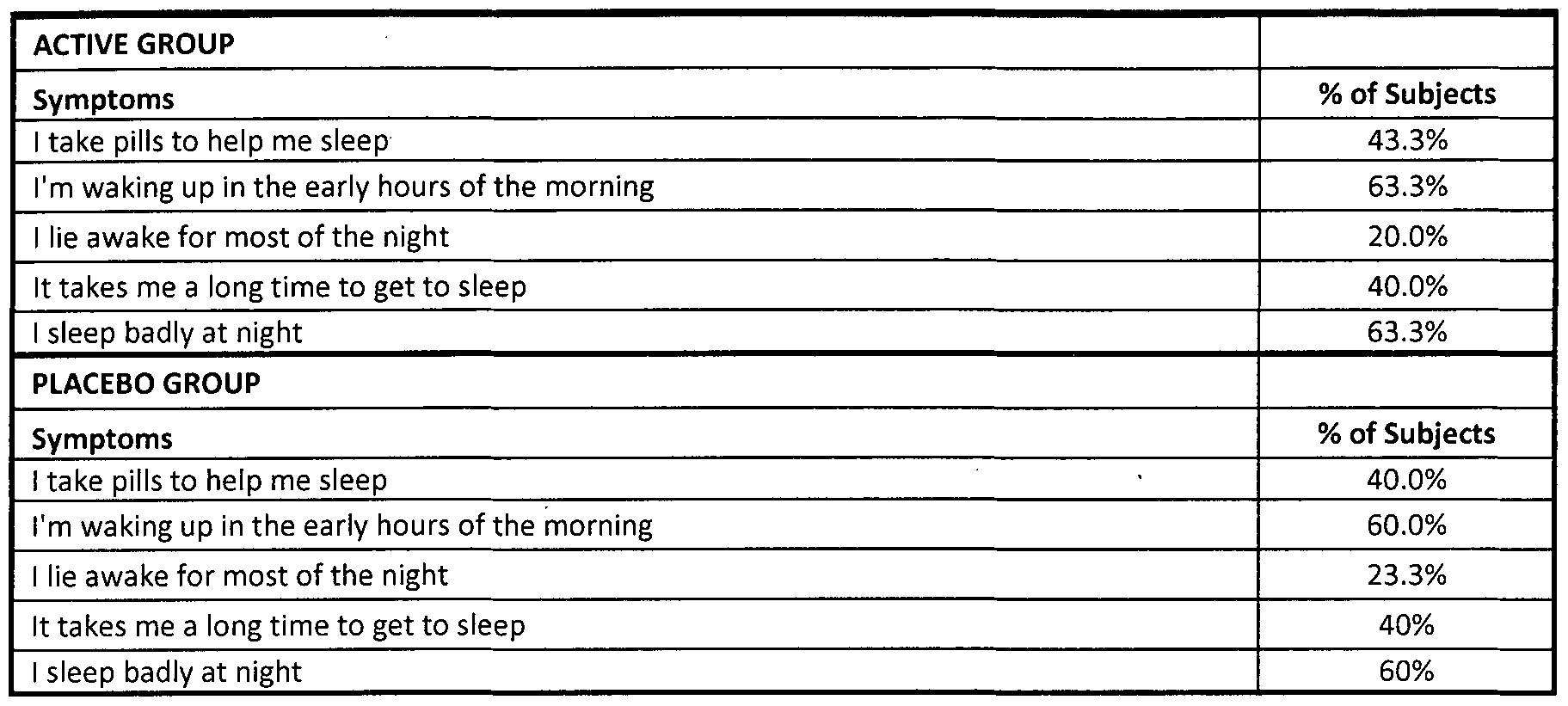
- Table 10 shows the evolution of the sleep disorders 1 to 3 months after taking the active product and the placebo product.
- the data show the percentage of subjects that exhibit an improvement * in the clinical symptomatology.
- Table 10b shows the intra- and intergroup statistical analysis.
- the intragroup statistical analysis is performed via the Wilcoxon test of the ranges indicated, whereas the intergroup analysis is performed via the Mann-Whitney U test.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Natural Medicines & Medicinal Plants (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Mycology (AREA)
- Biotechnology (AREA)
- Botany (AREA)
- Microbiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nutrition Science (AREA)
- General Chemical & Material Sciences (AREA)
- Alternative & Traditional Medicine (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Medical Informatics (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Polymers & Plastics (AREA)
- Food Science & Technology (AREA)
- Biochemistry (AREA)
- Reproductive Health (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biophysics (AREA)
- Molecular Biology (AREA)
- Endocrinology (AREA)
- Genetics & Genomics (AREA)
- General Engineering & Computer Science (AREA)
- Inorganic Chemistry (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Plant Substances (AREA)
Abstract
The invention concerns a composition for the prevention and/or the treatment of menopausal syndrome and/or of aging comprising an association of an equolbased active metabolite of daidzein with resveratrol, in an edible carrier or excipient. The invention composition may be in the form of a nutraceutical or pharmaceutical preparation.
Description
COMPOSITION FOR USE IN THE TREATMENT OF MENOPAUSE PROBLEMS/DISORDERS AND IN
THE TREATMENT OF GENERAL AGING OF THE ORGANISME
SCOPE OF THE INVENTION
This invention concerns a composition for the treatment and prevention of disorders related to menopause and/or aging of the human organism.
In particular, one aspect of this invention concerns a composition for the treatment of the problems/disorders associated with menopause, for example hot flushes, dismal mood/depression, insomnia, difficulty concentrating, general aging of the organism, aging of the skin, lowering of the metabolism, weight gain (excess weight).
Due to its capacity to counteract the general aging of the organism, the invention composition may also be used as an adjuvant in the treatment of aging of the skin, in the lowering of metabolism, and in the treatment of weight gain.
STATE OF THE ART
Currently the products present on the market for the treatment of menopause disorders/problems are predominantly composed of soy isoflavones, red clover, and flax seed. The most widely known raw material to be used for menopause is soy. For soy, however, a legislative limit on the daily quantities that products may contain does exist in Italy. Specifically, the current legislation requires that 80 mg/day of soy isoflavones not be exceeded and the prevalent medical class believes that soy does not function efficaciously in the treatment of said disorders within this limit due to a problem of quantity, even if there is second portion of the medical class which believes that even these quantities may be effective.
From the perspective of better understanding the conflicting data in the literature relative to soy, in recent years the scientific community identified a new molecule, equol, which derives from the metabolism, in the intestine, of daidzein, a soy isoflavone. It was thus learned that the population may be subdivided into equol- producers or non equol-producers depending on whether the right bacteria are present in the intestine to carry out the above-said transformation. Equol's importance would seem to reside in the fact that it is similar to estradiol (the female hormone estrogen), it therefore mimics estradiol's activity, but at the same
time it does not produce the side effects and contra-indications of estrogens.
Studies were thus conducted on fermented soy, which consequentially is naturally enriched with equol; these studies have demonstrated the effectiveness of the substance in reducing the symptoms associated with menopause at low doses (Takeshi et al, "Equol improves menopausal symptoms in Japanese women", The Journal of Nutrition; Supplement: equol, Soy and Menopause, 1386-1389).
The need to develop alternative and better products for treating menopause problems/disorders is still felt however.
SUMMARY OF THE INVENTION
One of the purposes of the invention consists in preventing and/or in mitigating the effects on the human organism brought on by menopause.
In accordance with a first aspect this invention provides a composition for the prevention and/or treatment of disorders and/or symptoms related to menopause including a biologically active and absorbable metabolite of daidzein and resveratrol in synergic association, and an edible carrier and/or an excipient, in which said biologically active and absorbable metabolite of daidzein is equol.
The Applicant discovered the association of equol and resveratrol causes a synergic antioxidizing action at the level of certain cellular processes at the base of the disorders and/or of the symptoms associated with menopause and with cellular aging.
In particular, the inventors of this invention discovered that resveratrol, for example originating from an extract of Vitis vinifera, associated with equol, produces the synergic effect of activating the SIRT genes that code for sirtuin, enzymes capable of improving the cell efficiency counteracting the processes of oxidative stress that typically occur in menopause and/or in the aging processes of the human organism.
In certain embodiments, the invention composition is a pharmaceutical composition.
In some embodiments, the invention composition is a nutraceutical composition. In certain embodiments, the invention composition is a dietetic product or a food supplement.
In some embodiments, the invention composition is a food product meant for
animals.
In one embodiment, the resveratrol of the composition is obtained from Vitis vinifera.
In certain embodiments, the daidzein metabolite is equol obtained through fermentation in the presence of non-pathogenic microorganisms, in particular bacteria, that belong to an equol-producing strain.
In certain embodiments, the equol of the composition is obtained through a fermentative process comprising the transformation of daidzein into the inactive metabolite hydroxydaidzein, and the transformation of this metabolite into O- desmethylangolensin and equol.
In some embodiments, the environment in which the fermentative equol- production process takes place is the intestine, in particular the colon, typically an environment with reductive conditions.
In some embodiments, the invention composition comprises an extract of fermented soy with a total isoflavone content from 20 to 60%, in which the quantity of equol ranges from 5 to 20% and a Vitis vinifera extract with a resveratrol content from 5 to 15.2%.
In this invention, when indicating the resveratrol content of the extract of Vitis vinifera, commonly known as "grapes," it is understood to be the sum of monomers and oligomers, in which the resveratrol monomers amount to no less than 3 mg.
In accordance with a second aspect, this invention concerns the use of a composition containing a synergic association of equol and resveratrol in effective quantities and an edible carrier or excipient in the treatment or in the prevention of the menopause problems/disorders.
Under a third aspect, the invention concerns the use of a composition, containing a synergic association of equol and resveratrol in effective quantities and an edible carrier or excipient, in the treatment and/or in the prevention of the aging of the organism.
BRIEF DESCRIPTION OF THE FIGURES
This invention shall be described in detail further on with reference to the figures, in which:
Figure 1 shows the results of the Mitotracker staining in the HUVEC endothelial cells of example 2;
Figure 2 shows the results of the SIRT1 protein expression and SIRT1 enzymatic activity in the HUVEC cells of example 2;
Figure 3 shows the results of the protein expression of porin and of the components of the respiratory chain in the HUVEC cells of example 2;
Figure 4 shows the results relative to the genetic expression of the mitochondrial biogenesis factors PGC-1a, Nrf-1 , and Tfam in the HUVEC cells of example 2; Figure 5 shows the results relative to adipogenesis in the preadipocytes of example 2;
Figure 6 refers to the results of the Mitotracker staining and the quantification of the mitochondrial DNA;
Figure 7 refers to the measurement of the mRNA expression of mitochondrial biogenesis factors via QRT-PCR;
Figure 8 refers to the quantification of the lipid content.
DETAILED DESCRIPTION OF THE INVENTION
In accordance with a first aspect, the invention is directed to a composition for the prevention and/or the treatment of disorders related to menopause and/or with the aging of the organism containing a synergic association of equol and resveratrol in effective quantities and an edible carrier or excipient.
The inventors of this invention have observed that resveratrol acts synergically with equol inducing a phytoestrogen^ activity in the human or animal organism. This activity is particularly perceptible in female subjects in menopause.
The term "menopause" is understood to be the time of the cessation of spontaneous menstruation that typically coincides with the end of the physiological reproductive life. This phenomenon, in particular in western women, may occur at an age between 48 and 53 years, inclusive.
Menopause is typically very evident after an amenorrhea, a period of absence of menstruation of at least 6 months, in a female subject who has reached the average age of onset.
From a biological perspective, menopause consists in the cessation of the activity of the ovary, the female gonad, located at the side of the uterus, which produces
the female germ cells or oocytes and that secrete estrogens, the female hormones.
With menopause, estrogen levels typically reach very low values while there is a progressive increase of FSH.
Within the scope of this invention, the term menopause syndrome is understood to be the disorders and the symptoms that accompany menopause and that typically originate from the variation of female hormone levels, and estrogens in particular, which occur during or in the period prior to menopause.
Equol ((3S)-3-(4-Hydroxyphenyl)-7-chromanol or 4',7-lsoflavandiol) present in the invention composition is an active metabolite obtained from daidzein (7-Hydroxy-3- (4-hydroxyphenyl) chromen-4-one or 4',7-Dihydroxyisoflavone), an isoflavonoid in aglyconic form naturally present in soy and in similar non-fermented products. Typically, the equol used in the invention composition is not present as such in the isoflavonoids, in the soy or soy shoots found in nature.
In certain embodiments, the equol of the invention composition is obtained through metabolism or fermentation of daidzein by equol-producing microorganisms that are typically non-pathogenic bacteria, such as for example bifidus bacteria, lactobacilli, present in the intestinal ecosystem of certain individuals. In some embodiments of the invention composition, equol is obtained through the fermentation of daidzein with a non-pathogenic, equol-producing microorganism. In certain embodiments, the equol-producing microorganisms are Pediococcus acidilactici bacteria, preferably of the genus Lactococcus lactis, particularly as described in the Italian patent application MI201 1A001829 filed on October 7, 2011 on behalf of the Applicant himself, the entire text of which is incorporated herein for reference.
In certain embodiments, the equol of the invention is obtained from the fermentation of microorganisms belonging to the genus Enterococci thailandicus, as described in the Italian patent application ΜΙ2011Α00Ί283 dated July 8, 201 1 on behalf of the Applicant, the entire text of which is incorporated herein for reference.
In certain embodiments, the equol of the invention composition is contained in a fermented-soy extract, in particular by means of non-pathogenic, equol-producing
microorganisms present in the bacteriological flora of the intestine.
Typically, equol exists in two mirror-image isoforms, S-equol and R-equol.
The form produced by certain equol-producing bacteria, present in particular in the intestinal microflora of certain individuals, is S-equol. This represents the most active form of equol in terms of biological activity.
Typically, R-equol is the form obtained through chemical/fermentative synthesis starting from daidzein.
In certain embodiments, the equol present in the invention composition is the S isomer.
Equol has a bonding affinity in relation to alpha and beta estrogenic human receptors much greater than daidzein (from ten to eighty times) and induces the transcription much more strongly than any other isoflavonoid (N. Sathyamoorthy et a!., Eur. J. Cancer, 1977, 33, 2384-2389; G. Kuiper et al., Endocrinology, 1998, 139, 4252-4263; K. Morito et al., Biol. Phatm. Bull., 2001 , 24, 351-356; D. Kostenac et al., J. Agr. Food Chem., 2003, 51 , 7632-7635; R.S. Muthyala et al., Bioorg. Med. Chem., 2004, 12, 1559-1567).
Equol, moreover, again with respect to the other isoflavonoids, has a significantly stronger antioxidant power (J.H. Mitchell et al., Arch. Biochem. Biophys., 1998, 360, 142-148).
The soy extracts with measured isoflavone content present on the market do not exhibit detectable levels of equol with standard methods.
Preparations based on fermented soy are available on the market, both as foods (natto, tempeh) and as food supplements, in which the bacteriological fermentation action is capable of increasing aglyconic isoflavone levels. In this preparations also, no significant quantities of equol are observed.
Typically, the fermentation of the soy via specific equol-producing strains of bacteria or strains conditioned to produce equol is capable of producing significant quantities of equol starting from daidzein.
Daidzein is one of the major isoflavonoids. Daidzein is present in soy mainly in the biologically inactive forms of beta-glucoside, acetyl-glycoside, or malonyl- glycoside. It cannot be absorbed by the human intestine in these forms which, after ingestion, are hydrolyzed with aglycone only in small quantities at the level of
the small intestine and, then, absorbed by the intestinal epithelium itself (A.J. Day et al., FEBS Lett., 2000, 468, 166-170). A large non-hydrolyzed fraction reaches the colon where the forms glycosylated, sulfated, and glucuronidated are deconjugated by the enzymes produced by the microorganisms (A.J. Day et al., 2000, ref. cit.; M. Richelle et al., J. Nutr., 2002, 132, 2587-2592; J. Chen et al., J. Pharmacol., Exp. Ther., 2003, 304, 1228-1235; K.D.R. Setchell et al., Am. J. Clin. Nutr., 2003, 77, 411-419).
In particular, the glycosidases of the bacteriological flora of the intestine cleave the beta-glycosidic bond, liberating the aglycone. The aglycones are then absorbed and subject to enterohepatic circulation or to further metabolism by the intestinal bacteria (H. Wiseman, Proc. Nutr. Soc, 1999, 58, 139-146).
Upon returning to the intestine, they are then transformed in the colon by the microorganisms present:
- genistein is converted into dihydroxygenistein
- daidzein is converted into dihydroxydaidzein (DHD), Equol and O- desmethylangolensin (O-DMA)
The capacity to carry out these transformations varies depending on single individuals. While approximately 80-90% of the population is able to produce O- desmethylangolensin, only 30-50% is able to synthesize equol from daidzein.
In certain embodiments, the soy-fermentation process results in an isoflavone content equal to 20-60%, mainly represented by genistein and daidzein. In certain embodiments, the invention composition contains equol in the form of a fermented-soy extract.
In certain embodiments, the fermented-soy extract, with a total isoflavone content in the interval from 20 to 60%, of which equol is 5 to 20% of the invention composition, corresponds to an interval from 1 mg to 100 mg of equol in the final composition.
In some embodiments, the soy extract of the invention contains from 1 to 30 mg of equol, normally about 9.6 mg of equol.
In certain embodiments, the invention composition includes a quantity of fermented-soy extract from 50 to 1000 mg, preferably from 100 to 400 mg, more preferably about 200 mg of fermented-soy extract.
The invention composition also contains resveratrol, preferably in the form of a Vitis vinifera extract. In certain embodiments, the Vitis vinifera extract has a resveratrol content from 5 to 15.2%.
Resveratrol, or trans-3,5,4'-trihydroxystilbene, is a polyphenolic compound present in various quantities in diverse species of plants, including grapes, peanuts, and Polygonum cuspidatum.
In this invention, the resveratrol preferably derives from an extract of grapes, typically from Vitis vinifera. The resveratrol content includes both monomers and oligomers thereof.
In some embodiments of the invention composition, the Vitis vinifera extract with a resveratrol content in the interval from 5 to 15.2% corresponds to an interval from 1 mg to 1000 mg of resveratrol in the final composition.
In certain embodiments, the Vitis vinifera extract of the invention contains from 1.25 to 5 mg of resveratrol, for example approximately 3.4 mg of resveratrol monomer or 7.5 mg of resveratrol as the sum of monomers and oligomers.
In some embodiments, the invention composition includes a quantity of Vitis vinifera extract from 10 to 200 mg, preferably from 20 to 100 mg, for example, approximately 25 mg of Vitis vinifera extract.
Certain embodiments of the invention composition contain a fermented-soy extract with a total isoflavone content in the interval from 20 to 60%, of which equol ranged from 5 to 20% and a Vitis vinifera extract with a resveratrol content in the interval from 5 to 15.2%.
The invention composition may be administered orally and in the form of solid and liquid preparations. When the preparation is solid, the composition may be administered in the form of tablets, capsules, powders, or pastilles, preferably in tablet form. When the preparation is liquid, the invention composition may be administered in the form of a solution, suspension, drops, or syrup.
In some embodiments, the invention composition contains a fermented-soy extract with a total isoflavone content in the interval from 20 to 60%, of which equol is from 5 to 20% and a Vitis vinifera extract a resveratrol content in the interval from 5 to 15.2%.
In certain embodiments, the invention composition includes one or more edible
carriers and/or excipients.
For the purpose of this invention, the term edible is understood to signify food- grade materials that are approved by the Regulatory Authorities for use in pharmaceutical, food, nutritional, and/or dietetic applications.
In certain embodiments, the invention composition also includes excipients chosen from the group consisting in bulking agents, anti-caking agents, stabilizing agents, thickening agents, humectants, tableting agents, and colorants commonly used within the scope of formulation techniques for pharmaceutical compositions or nutritional or dietetic preparations.
In the embodiments in which the invention composition is in tablet form, it may be coated with glazing agents.
In certain embodiments, the invention composition also includes plant extracts, for example of Pueraria lobata, and also, or alternatively, vitamins and/or minerals. Under another aspect, the invention concerns a composition in accordance with any of the embodiments previously described for use as a medicinal, in particular for the use in the treatment of menopause symptoms/disorders, associated especially with hormonal changes consequential to the onset of menopause.
In certain embodiments, the menopause disorders include hot flushes that typically manifest themselves as a sudden sensation of warmth on the dermis of the face, neck, and thorax, that may be accompanied by redness and sudation of the skin. Typically, these hot flushes may have an average duration of approximately 3 minutes and a frequency that generally decreases progressively over the course of years until it nearly disappears after approximately 10 years after the last menstruation.
In certain embodiments, the invention composition is used in the treatment or prevention of genitourinary tissue alterations associated with a drop in circulating levels of estrogens.
In certain embodiments, the invention composition is applied in the treatment or in the prevention of the following symptoms or disorders: dryness of the vagina, associated with burning or inconvenience during sexual intercourse, cystitis, burning sensation during urination, pelvic pains, frequent urination, and forms of urinary incontinence.
For example, the composition is applicable in cases where the dermis tends to thin out, and/or there is a tendency for relative dryness and there may be a slight increase of vellus hair in certain zones of the face.
In certain embodiments, the composition is applicable in the cardiovascular disorders related to menopause in which the incidence of ischemic phenomena increase, and the lipid profile tends to worsen (increase of cholesterol and triglycerides, reduction of HDL).
In certain embodiments, the composition can be applied in cases of menopause in which the bones tend to progressively decrease their density or in case of osteoporosis or in cases of increased risk of fracture, especially in relation to the vertebrae and femur.
The composition may also be applied in menopause associated with worsening of depressive moods.
In certain embodiments, the invention composition is applied in the treatment or in the prevention of the symptoms or disorders in the premenopausal phase.
The term premenopausal period is understood to be the period that precedes menopause. Typically, there is a progressive irregularity of the menstrual cycle in this period followed by the almost complete cessation of menstrual flow.
In certain embodiments, the invention composition is applied in the treatment or in the prevention of the disorders of early menopause.
The term early menopause is understood to be a menopause that manifests itself spontaneously in female subjects under the age of 40.
Again, under an additional aspect, the invention concerns the invention composition for the use in the treatment of general aging of the organism.
Under another aspect, the invention comprises a nutraceutical formulation containing the invention composition, preferably for use in the treatment of menopause problems/disorders and/or in the treatment of general aging of the organism, associated in particular with hormonal changes consequential to the occurrence of menopause.
The invention composition is in fact capable of specifically activating mitochondrial biogenesis in different tissues. The invention composition is also capable of activating specific cellular-transcription factors that induce anti-inflammatory and
antioxidant proteins. By means of these actions, the invention composition is effective in promoting the viability and the functions of the cells, tissues, and of the organism, and is particularly effective in protecting the endothelial cells, skin cells, and nervous system cells against deterioration, against death due to degenerative processes and in aging in general. The invention composition is also active in terms of antiobesity effects, inducing the inhibition of adipogenesis and proapoptotic effects in human adipocytes.
All of these results of effectiveness will be evident from the experimental part that follows which presents experimental results in terms of an increase in mitochondrial biogenesis, an increase in lipolysis, an induction of apoptosis in mature adipocytes, and the activation of SIRT 1 in endothelial cells.
These experimental results may be attributed to benefits such as:
- general anti-aging activity on the organism
- cutaneous anti-aging action
- inhibition of the lowering of metabolism with the resulting inhibition of weight gain
- modulation of estrogen receptors with the reduction of typical menopause symptoms.
Below are examples of invention embodiments and the evaluation of the invention's effects, provided for the sake of example and not restrictively for the invention itself.
EXAMPLES
Example 1 :
The ingredients indicated in the table below were used in the quantities shown to prepare the invention composition.
Table 1 : Composition of a tablet of the invention.
Description Active Ingredient mg/un. g/100 g
of the raw
material
Bulking agent: 207.000 31.364
microcrystalline cellulose
Fermented-soy extract 40% isoflavones 200.000 30.303
(equol content: 4-5.6%) and total
derivatives
Bulking agent: dicalcium 150.000 22.727
phosphate
Vitis vinifera extract 30% monomers 25.000 3.788
Resveratrol content: and oligomers
5-15.2%
Anti-caking agent: 16.000 2.424
crosslinked sodium carboxy
methyl cellulose
Glazing agent: 13.000 1. 970
hydroxypropyl methyl
cellulose
Anti-caking agent: Glyceryl 12.000 1.818
behenate
Stabilizing agent: 10.000 1.515
polyvinylpyrrolidone
Anti-caking agent: 10.000 1.515
magnesium stearate of
vegetable origin
Anti-caking agent: silicon 10.000 1.515
dioxide
Colorant: titanium dioxide 3.000 0.455
Glazing agent: 2.000 0.303
microcrystalline cellulose
Glazing agent: stearic acid 2.000 0.303
Total 660.00 100.000
The ingredients above were mixed and sifted on a 1 .0 mm mesh. All components with the exception of magnesium stearate were been transferred in the mixer and mixed for 10 minutes. Magnesium stearate was then transferred in the mixer and mixed for 5 minutes.
The material was compressed to a theoretical weight of 640 mg/tablet (17.5 x 8.8 mm shape without snap tab). The tablets were ten coated with a dispersion of the glazing agents indicated in table 1 .
660 mg tablets having the following characteristic were obtained:
Tablet 36
weight 660 mg Validity months
Appearance oval
Color white
EXAMPLE 2
Evaluating the effects of the invention composition.
The effects of the invention composition were evaluated using the composition of example 1.
The effects were compared with the effects from equol alone and from resveratrol alone in the same quantities as the invention composition.
Mitochondria are flexible and complex cellular entities that play crucial roles in pathological and normal cellular conditions. Mitochondrial biogenesis is an elaborate cellular process that relies on the strong bond of diverse regulatory controls, from gene transcription to the site-specific production of proteins. The pathways that govern mitochondrial biogenesis have recently emerged as potential therapeutic targets to improve endothelial dysfunction and vascular disorders observed in metabolic diseases (Ren. J., Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med. 2010 Oct; 88(10):993-1001 ).
Mitochondrial mass was measured in HUVEC endothelial cells using the Mitotracker red staining technique as follows.
The HUVEC cells were subdivided into three groups, which were treated with resveratrol, 10 pmol/l for 48 h, equol 10 pmol/l for 48 h, and with the composition containing resveratrol and equol, 10 pmol/l for 48 h, respectively. The mitochondrial mass in HUVEC was determined selectively loading mitochondria with Mitotracker fluorescent red dye (Invitrogen, Carlsbad, CA). Fluorescent calcein (green) and Hoechst 33258 (blue) dyes were used to stain the cytoplasm and the nuclei, respectively. Optical sections of HUVEC were captured at *60- magnification and the mitochondrial density-area ratio was calculated with respect to cytoplasmatic volume using the Zeiss Axiovision imaging software. Only cells with intact cytoplasmatic calcein stain were included in the analysis.
The mitochondrial DNA content in the HUVEC cells was then measured as follows.
The total DNA was isolated from the HUVEC cells (DirectPCR; Viagen Biotech). The number of mitochondrial DNA copies (mtDNA) was determined via QRT-PCR as described in Addabbo F, Ratliff B, Park HC, Kuo M, Ungvari Z, Csiszar A, Krasnikov B, Sodhi K, Zhang F, Nasjletti A, Goligorsky MS. The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: proteomic approach. Am J Pathol 174: 34-43, 2009.
Using cytochrome oxidase III and beta-actin as markers for the number of mtDNA and genomic DNA copies, respectively. The impact of the treatment with resveratrol, equol, and the invention composition containing both resveratrol and equol was evaluated.
SIRT1 activity assay
SIRT1 nuclear activity was measured in cells treated with resveratrol, equol, and the invention composition as described previously (Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008 Feb; 1 1 (1 ):139-50). Measurement of the protein expression of constituents of the electron transport chain.
To explain the effect of resveratrol, equol, and of the invention composition (resveratrol + equol) on the protein expression of constituents of the electron transport chain Western blotting was performed as described in Nicoletti VG, Caruso A, Tendi EA, Privitera A, Console A, Calabrese V, Spadaro F, Ravagna A, Copani A, Stella AM. Effect of nitric oxide synthase induction on the expression of mitochondrial respiratory chain enzyme subunits in mixed cortical and astroglial cell cultures. Biochimie. 1998 Oct; 80(10):871-81. Direct primary antibodies against complex II, complex V (Molecular Probes/I nvitrogen) and cytochrome oxidase were used (COX-IV, no. 4844; Cell Signaling). The levels of porin, the most abundant protein of the outer mitochondrial membrane, were also measured. Because of its abundance, porin is often used as a marker for the cell mitochondrial mass. Anti-P-actin (no. 6276; Abeam) was used for normalization purposes.
Measuring the mRNA expression of mitochondrial biogenesis factors via QRT- PCR.
The QRT-PCR technique was used to determine the effect of resveratrol, equol, and of the invention composition (resveratrol + equol) (10 mol/l, for 24 hours) on the mRNA expression of mitochondrial biogenesis factors: nuclear respiratory factor (Nrf-1 ), mitochondrial transcription factor A (Tfam), and Peroxisome proliferator-activated receptor (PPAR)-a coactivator-1 (PGC-1 a) in HUVEC using Light Cycler (Roche Molecular Biochemicals) as described in Scapagnini G, Butterfield DA, Colombrita C, Sultana R, Pascale A, Calabrese V. (2004) Ethyl ferulate, a lipophilic polyphenol, induces HO-1 and protects rat neurons against oxidative stress. Antioxid Redox Signal. 6(5):811-8.)
The total RNA was isolated with the isolation kit with Mini RNA (Zymo Research, Orange, CA) and was inverse-transcribed using Superscript III RT (Invitrogen) as described in Scapagnini G, D'Agata V, Calabrese V, Pascale A, Colombrita C, Alkon DL, Cavallaro S. (2002) Gene expression profiles of heme oxygenase isoforms in the rat brain Brain Res. 954(1 ): 31-39.
The effectiveness of the PCR reaction was determined employing a series of standard sample dilutions. The housekeeping gene HPRT was used for internal normalization. The PCR reaction reproducibility was determined via melting point analysis and viewing the product on 2% agarose gel.
Adipocyte viability and apoptosis assays.
Tests were performed in 96-well plates. For the mature adipocytes, the cells were seeded (5000 cells/well) and grew until maturing as described further above. The cells were seeded (2500 cells/well) and the assay was performed 4 days after seeding for the preadipocytes. Preadipocytes or mature adipocytes were incubated with 0.2% dimethyl sulfoxide (DMSO) or test compounds for 24 and 48 hours. The cellular vitality assay was conducted in accordance with the instruction manual. Absorbance was measured at 490 nm in a plate reader (mQuant, Bio-Tek Instruments) to determine the formazan concentration, which is proportional to the number of live cells. The apoptosis detection kit ApoStrand ELISA was used to measure the apoptosis content. The cells were grown in 96-well plates, treated with the test compounds for 24 and 48 hours, and tested in accordance with the instruction manual. The assay selectively detects single-stranded DNA, which appears in apoptotic cells, but not in necrotic cells or cells with DNA cleavage in
the absence of apoptosis. Assays were performed at least twice for 6 replicas for each treatment.
Quantification of the lipid content.
The lipid content was quantified using the commercially available assay reagent AdipoRed. In short, post-confluent preadipocytes were grown in 96-well plates and incubators with 0.2% DMSO or test compounds during the adipogenic phase, and on the sixth day they were tested for lipid content in accordance with the instruction manual. The experiments were performed with at least 6 replicas per treatment and repeated 3 times.
Lipolvsis assay.
To determine the lipolysis content induced by the test compounds, the mature adipocytes were treated with 0.2% DMSO or the test compounds for 5 h, and the free glycerol released was tested using an adipocyte-lipolysis assay kit (Zen-Bio) and in accordance with the instruction manual. The experiment was repeated twice with at least 4 replicas.
Results
The invention composition induces mitochondrial biogenesis in endothelial cells. This action is associated with the treatment of age-associated diseases and with correlated symptoms such as menopause (Tina Wenz Mitochondria and PGC-1a in aging and age-associated diseases, in the Journal of Aging Research Vo. 2011 Art. ID810619, pages 1-12).
The Mitotracker staining showed that mitochondria were located in the perinuclear region in HUVEC. The density-area ratio with respect to cytoplasmatic volume in Mitotracker-labeled endothelial cells increased significantly after treatment with resveratrol (Fig. 1 ). The analysis of the Mitotracker intensity in HUVEC confirmed that treatment with resveratrol significantly increased the mitochondrial mass in treated cells compared with non-treated cells (Fig. 1 ). Equol alone was not effective in terms of activating the mitochondrial mass, however the invention composition (resveratrol + equol) was significantly more effective compared with resveratrol alone (Fig. 1 ).
The invention composition also induces SIRT1 in endothelial cells.
The invention composition proved capable of activating the protein expression of
SIRT1 and of increasing the SIRT1 enzymatic activity in HUVEC endothelial cells
(Fig 2). Equol was not effective in terms of activating SIRT1 , however the invention composition was significantly more effective in comparison with resveratrol alone (Fig. 2).
The composition activated the constituents of the mitochondrial electron transport chain in endothelial cells.
Through Western blotting it was shown that in the cells treated with the invention composition, porin following the instruction manual had increased significantly when compared with untreated HUVEC (Fig. 3A). The porin levels (which control the diffusion of small metabolites across the outer membrane) were correlated with the cellular mitochondrial volume. The expression of complex II, complex IV, and complex V in HUVEC cells (Fig. 3 B, C, D) also increased through treatment with the invention composition.
The invention composition activated the mitochondrial biogenesis factors.
Mitochondrial biogenesis involves the integration of multiple transcriptional pathways, which control both nuclear and mitochondrial gene expression. The PPAR-gamma coactivator (PGC-1a), and the transcription factors Nrf-1 and Tfam are considered key regulators of mitochondrial biogenesis in multiples tissues. Measurements via QRT-PCR revealed that the expression of the mitochondrial biogenesis factors PGC-1a, Nrf-1 , and Tfam in HUVEC cells (Fig. 4) had increased significantly via treatment with the invention composition.
Cellular viability of preadipocvtes and mature adipocytes.
Equol was unable to decrease cellular viability in preadipocytes at 24 h and at 48 h. Resveratrol slightly decreased cellular viability (~ 20% at 24 h and ~ 28% at 24 h). The invention composition further decreased cellular viability by 42% at 24 h and by 57% (P = 0.001 ), after a 48 hours of treatment in accordance with Table 2 below.
Table 2
Similarly, the mature adipocytes were treated with the invention composition (resveratrol and equol), and resveratrol and equol as individual compounds for 24 and 48 hours. Equol was unable to decrease viability. Resveratrol alone slightly decreased cellular viability, while the invention composition appreciably decreased cellular viability by 46% (P = 0.001 ) after 24 hours by 58% (P = 0.001 ) after 48 hours (Table 2).
Induction of apoptosis in mature adipocytes
Neither resveratrol nor equol increased apoptosis as individual treatments, whereas the association of the compounds increased apoptosis by 32% at 24 h and by 56% at 48 h (Table 3).
Table 3
Inhibition of lipid accumulation
In maturing preadipocytes, preliminary experiments with resveratrol and equol showed that the combined effect on adipogenesis was very powerful. The effect on adipogenesis was much more powerful than the effect on viability, indicating a true decrease in lipid accumulation (fig 5).
Effects on lipolvsis
Neither resveratrol nor equol induced lipolysis as individual treatments whereas the combination increased it by 40% (P = 0.01 ).
Therefore, the invention composition induced mitochondrial biogenesis in human endothelial cells. The formation of new mitochondria was associated with the activation of SIRT1 and the induction of specific mitochondrial biogenesis factors. Moreover, the invention composition had been shown to be capable of exerting an increased effect on apoptosis induction and on adipogenesis inhibition in adipocytes. The invention composition had likewise showed that the decrease in the number and dimension of the mature adipocytes entailed the loss of lipids and the loss of cells via apoptosis.
The results reported above support the inventive nature of the composition that increased the mitochondrial content in endothelial cells (Fig. 1 ). The increased mitochondrial biogenesis in the cells treated with the invention composition was also indicated by the increased cellular mtDNA content (Fig. 1 ) and by the increased expression of proteins of the respiratory-chain components (Fig. 3). Multiple mechanisms could explain that the invention composition induced mitochondrial biogenesis and its contribution to cellular health. To determine if the increased number of mitochondria in endothelial cells treated with the invention composition was a consequence of the induction of mitochondrial biogenesis factors, QRT-PCR was used to examine the expression of Nrf-1 , Tfam, and PGC- 1a. Nrf-1 activates the transcription of many coded nuclear elements of the electron transport chain and also regulates Tfam, responsible for the transcription of mtDNA-coded genes. The regulatory function of Nrf-1 and of other mitochondrial biogenesis factors is modulated by PGC-1a. It was shown that resveratrol induced Nrf-1 , Tfam, and PGC-1a in human endothelial HUVEC cells (Fig. 4). It was also established that the altered expression of these factors modulated the mitochondrial biogenesis activity. Furthermore, the invention composition induced SIRT1 (Fig. 2) and SIRT1 similarly regulated multiple pathways involved in mitochondrial biogenesis in endothelial cells, including the fact that SIRT1 could also directly deacetylate PGC-1a, increasing its activity.
EXAMPLE 3
The HUVA cells were subdivided into 6 groups, which were treated with 10 pmol/l equol for 48 h, 10 pmol/l daidzein for 48 h, 10 pmol/l of resveratrol for 48 h,
fermented soy with a (18%) daidzein content and resveratrol for total of 10 μιτιοΙ/Ι of said active ingredients for 48 h, and with the invention composition containing fermented-soy extract with an equol content of 4-5.6%, and Vitis vinifera extract with a resveratrol content of 5-15.2% for a total of μιτιοΙ/Ι of said active ingredients for 48 h, and finally an untreated control group, respectively.
A) The mitochondrial mass in HUVA was determined selectively loading mitochondria with Mitotracker fluorescent red dye (Invitrogen, Carlsbad, CA). Fluorescent calcein (green) and Hoechst 33258 (blue) dyes were used to stain the cytoplasm and the nuclei, respectively. Optical sections of HUVA were captured at x60 magnification and the mitochondrial density-area ratio was calculated with respect to cytoplasmatic volume using the for Zeiss Axiovision imaging software. Only cells with intact cytoplasmatic calcein stain were included in the analysis.
B) The mitochondrial DNA content in the HUVA cells was then measured as follows. The total DNA was isolated from the HUVA cells (DirectPCR; Viagen Biotech). The number of mitochondrial DNA copies (mtDNA) was determined via QRT-PCR as described in Addabbo F, Ratliff B, Park HC, Kuo M, Ungvari Z, Csiszar A, Krasnikov B, Sodhi K, Zhang F, Nasjletti A, Goligorsky MS. The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: proteomic approach. Am J Pathol 174: 34-43, 2009.) using cytochrome oxidase III and beta-actin as markers for the number of mtDNA and genomic DNA copies, respectively. The impact of the treatment with equol, daidzein, resveratrol, fermented soy with a measured content of daidzein and resveratrol, and the invention composition containing both resveratrol and equol was evaluated.
The results obtained are shown in figure 6.
EXAMPLE 4
Measuring the mRNA expression of mitochondrial biogenesis factors via QRT- PCR.
The QRT-PCR technique was used to determine the effect of equol, daidzein, resveratrol, fermented soy with a measured content of daidzein, and the invention composition (resveratrol + equol) (10 mol/l, for 24 hours) on the mRNA expression of mitochondrial biogenesis factors: nuclear respiratory factor (Nrf-1 ), mitochondrial transcription factor A (Tfam), and Peroxisome proliferator-activated receptor (PPAR)-a coactivator-1 (PGC-1 a) in HUVA using Light Cycler (Roche
Molecular Biochemicals) as described in Scapagnini G, Butterfield DA, Colombrita C, Sultana R, Pascale A, Calabrese V. (2004) Ethyl ferulate, a lipophilic polyphenol, induces HO-1 and protects rat neurons against oxidative stress. Antioxid Redox Signal. 6(5):811-8.)
The total RNA was isolated with the isolation kit with Mini RNA (Zymo Research, Orange, CA) and was inverse-transcribed using Superscript III RT (Invitrogen) as described in Scapagnini G, D'Agata V, Calabrese V, Pascale A, Colombrita C, Alkon DL, Cavallaro S. (2002) Gene expression profiles of heme oxygenase isoforms in the rat brain Brain Res. 954(1 ): 31-39
The effectiveness of the PCR reaction was determined employing a series of standard sample dilutions. The housekeeping gene HPRT was used for internal normalization. The PCR reaction reproducibility was determined via melting point analysis and viewing the product on 2% agarose gel
These results are summarized and illustrated in Figure 7
EXAMPLE 5
Quantification of the lipid content
The lipid content was quantified using the commercially available assay reagent AdipoRed. In short, post-confluent preadipocytes were grown in 96-well plates and incubators with 0.2% DMSO or with equol, daidzein, resveratrol, fermented soy with a measured content of daidzein or the invention composition (resveratrol + equol) (10 mol/l, for 24 hours) during the adipogenic phase, and on the sixth day were assayed for lipid content in accordance with the instruction manual. The experiments were performed with at least 6 replicas per treatment and repeated 3 times. The results obtained are shown in Fig. 8.
EXAMPLE 6
Table 4 demonstrates the absence of equol in I) a soy extract with a measured content of ginestein and daidzein, and in II) a supplement based on fermented soy with a measured content of aglyconic isoflavones.
A third sample analyzed is III) a product of a fermentation with conditioned bacteria capable of producing S-equol, that represents a source that can be used as a food supplement of this molecule.
The equol levels in the solutions to be tested were determined in triplicate via kits based on "Quantitative time-resolved fluoroimmunoassay" (Labmaster, Turku, Finland) technology.
In accordance with the recommended procedures, read via Victor 3 Multilabel Readers (PerkinElmer, Milan, Italy) and interpolated by means of Sigma Plot (Systat Software).
RESULTS
The first two samples I) soy extract and II) fermented soy standard, exhibited values near the detectability limit of the method.
In sample III) with a soy base fermented with conditioned strains, it was possible to detect significant quantities of equol (approximately 8 mg/100 mg of fermented extract). Table 4
EXAMPLE 7
Formulation of an invention composition in tablet form for the treatment of menopause syndrome (sample dosage 1 -3 tablets/day).
EXAMPLE 8
Formulation of an invention composition for the treatment of the symptoms disorders related to aging (dosage 1 -3 tablets/day).
Glazing agent: microcrystalline cellulose 2.000 0.303
Glazing agent: stearic acid 2.000 0.303
Total Tot. 885.00 100.000
EXAMPLE 9
A randomized clinical study was conducted and the effectiveness of a food supplement on disorders and menopause events was controlled with placebos.
1.2. Nature and scope of the study. The study is intended to evaluate the effectiveness of a food supplement capable of preventing or treating the menopause syndrome.
For this purpose, a randomized clinical study, controlled with placebos, was conducted on 60 female subjects (30 subjects per line of treatment) of age between 50 and 55 years in menopause for less than one year.
In particular, it was studied whether or not the product was able to improve the symptoms accompanying the cessation of gonadal activity, such as: hot flushes, insomnia, and psychological disorders (anxiety, emotional instability, depression).
1 .3. Product studied: Menopause supplement:
bulking agent: microcrystalline cellulose 207 mg,
equol originating from fermented soy (40% isoflavones and total derivatives) 200 mg,
bulking agent: dicalcium phosphate 150 mg,
Resveratrol from Vitis vinifera 25 mg,
anti-caking agent: crosslinked sodium carboxy methyl
cellulose 16 mg,
glazing agent: hydroxypropyl methyl cellulose 13 mg,
anti-caking agent: Glyceryl behenate 12 mg,
stabilizing agent: polyvinylpyrrolidone,
anti-caking agent: magnesium stearate - of vegetable origin,
anti-caking agent: silicon dioxide,
colorant: titanium dioxide,
glazing agent: microcrystalline cellulose,
glazing agent: stearic acid.
Menopause supplement (placebo):
bulking agent: dicalcium phosphate 341 mg,
bulking agent: microcrystalline cellulose 273 mg,
glazing agent: hydroxypropyl methyl cellulose 10.4 mg,
anti-caking agent: magnesium stearate - of vegetable origin 40 mg,
anti-caking agent: silicon dioxide 40 mg,
anti-caking agent: crosslinked sodium carboxy methylcellulose 6 mg,
glazing agent: shellac 4 mg,
colorant: E171 2.4 mg,
glazing agent: microcrystalline cellulose 1.6 mg,
glazing agent: stearic acid 1.6 mg.
1.4. Ethical requirements
The study was approved by the "Independent Ethics Committee for Non Pharmacological Clinical Investigations" (on March 30, 2011 , ref. 2011/02) and conducted in observance of the following ethical requirements.
1.4.1. All subjects participating in the study are healthy volunteers at least 18 years of age.
1.4.2. All the subjects participating in the study are selected under the supervision of a dermatologist with the application of inclusion/exclusion criteria (see § 1.5.1.1..-2.).
1.4.3. All the subjects participating in the study are volunteers informed of the purpose and nature of the study.
1.4.4. All the subjects participating in the study were informed of any risk entailed in the performance of the study.
1.4.5. All the subjects participating in the study gave their informed consent signing a document prior to the start of the study.
1.4.6. Before the volunteers are exposed to the product under study, all the health and safety information relevant to the product and its individual components are evaluated.
1.4.7. All the study procedures are conducted in conformity with the ethical principles for research in the medical field (Ethical principles for medical research that involves human subjects, adopted by the 18th General Assembly of the WMA at Helsinki, Finland, in June 1964) and subsequent amendments.
1.4.8 All the precautions necessary to prevent the occurrence of adverse cutaneous reactions were taken.
1.4.9. Should there be unexpected/adverse reactions, the experimenter physician judges the severity thereof
(reporting it in detail) and accordingly implements appropriate care.
5. Subjects participating in the study
1.5.1. Selecting the subjects
The subjects participating in the study are selected from a panel of healthy female subjects (between 50 and 55 years of age, inclusive) applying the inclusion and exclusion criteria reported below.
1.5.1.1. Inclusion criteria
Healthy female subjects
Age: between 50 and 55 years of age, inclusive
Race: Caucasian
Body mass index between 20 and 25, inclusive
Slightly overweight (10-20% with respect to the ideal weight)
Onset of menopause (date of the last menstrual cycle) no longer than 1 year with respect to date of enrollment in the study
Anamnestic clinical picture characterized by psychological disorders (anxiety, emotional instability, depression) typical of the menopause syndrome, hot flushes, localized accumulations of fat, increase of mass
Subjects who have not participated in similar studies
Absence of pathologies
Commitment to not change the normal daily routine
Commitment to not use products with activity that could superimpose that of the product under study for the entire period of the study
Commitment to not change eating habits
Subjects knowledgeable of the study who have signed an informed consent document
1.5.1.2. Exclusion criteria
Subjects that do not satisfy the inclusion criteria
Subjects in both local and systemic pharmacological therapy
Subjects whose medical history do not exhibit past episodes of endometrial hyperplasia
Hormone replacement therapy
Metabolic syndrome
Depressive syndrome not correlated with menopause
Subjects with food intolerances
1.6. MATERIALS AND METHODS
1.6.1. Conducting the study
1.6.1.1 PHASE I - Selecting the subjects
In this phase, 60 female subjects are recruited from a panel of healthy subjects in menopause for less than one year. The experimenter evaluates: the general state of health of the subject and her eligibility to participate in the study (compliance with the inclusion criteria/exclusion).
1.6.1.2 Phase II - Start and end of the study
After enrollment, the subjects are subdivided into the following experimental groups:
Group A: subjects (n =30) who take the active ingredient belong to this group.
Group B: subjects (n =30) who take the placebo belong to this group.
For each volunteer, the experimenter evaluates the basal conditions (TO).
Afterwards, the subjects are instructed how to use the product, and they are given the factsheet of the study and the product under discussion.
Subsequent controls at 1 (T1 ) and 3 (T3) months of treatment are then scheduled.
1.6.2. Ratings
1.6.2.1. Product effectiveness in improving psychological disorders (anxiety, emotional instability, depression) and the hot flushes that accompany the menopause syndrome.
The following parameters are monitored before the study and at a distance of 1 and 3 months of treatment:
-Via the Hamilton Depression Rating scale (Hamilton M.A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62) the improvement of the mood intensity is rated (reduction of the symptoms correlated with the depression). The questionnaire given to the volunteers is shown in Table 5.
- The change in sleep disorders is rated via the "Nottingham Health profile" rating scale. The questionnaire given to the volunteers is shown in Table 6.
-The change in the symptoms associated with menopause is evaluated via the
"Menopause Rating Scale, MRS" (Lothar AJ Heinemann, Peter Potthoff, and
HermanPG Schneider. International versions of the Menopause Rating Scale
(MRS). Health Qual Life Outcomes. 2003;1 :28). The questionnaire given to the volunteers is shown in Table 7.
Table 5 Hamilton M.M. rating scale for depression
Intensity
Symptoms
Hot flushes, sweating (episodes of sweating) None -Mild -Moderate -Severe -Very severe
Heart discomfort (unusual awareness of heart beat, heart None -Mild -Moderate -Severe -Very severe skipping, heart racing, tightness)
Sleep problems (difficulty in falling asleep, difficulty in None -Mild -Moderate -Severe -Very severe sleeping through, waking up early)
Depressive mood (feeling down, sad, on the verge of tears, None -Mild -Moderate -Severe -Very severe lack of drive, mood swings)
Irritability (feeling nervous, inner tension, feeling None -Mild -Moderate -Severe -Very severe aggressive)
Physical and mental exhaustion (general decrease in None -Mild -Moderate -Severe -Very severe performance, impaired memory, decrease in concentration,
forgetfulness)
Sexual problems (change in sexual desire, in sexual activity None -Mild -Moderate -Severe -Very severe and satisfaction)
Bladder problems (difficulty in urinating, increased need to None -Mild -Moderate -Severe -Very severe urinate, bladder incontinence) Dryness of vagina (sensation
of dryness or burning in the vagina, difficulty with sexual
intercourse)
Dryness of vagina (sensation of dryness or burning in the None -Mild -Moderate -Severe -Very severe vagina, difficulty with sexual intercourse)
Joint and muscular discomfort (pain in the joints, None -Mild -Moderate -Severe -Very severe rheumatoid complaints)
Table 6 Hamilton M.M. rating scale for depression
1.6.2.2. Results
The results of the questionnaires are expressed in percentage values and shown in the respective tables.
"MENOPAUSE RATING SCALE, MRS" FOR SYMPTOMS ASSOCIATED WITH MENOPAUSE
(Lothar AJ Heinemann, Peter Potthoff, and HermanPG Schneider. International versions of the Menopause Rating Scale (MRS). Health Qual. Life Outcomes. 2003;1 :28)
The severity of the symptoms associated with menopause was assessed by means of the "Menopause Rating Scale, MRS." Upon enrollment, the panel is composed as described in table 8.
Table 8 Panel composition at TO
The two groups of subjects (Active and Placebo) are homogeneous with reference to the initial symptoms anticipated by MRS. Statistical analysis (Mann-Whitney U test) does not reveal significant differences (p>0.05) in the medians.
"MENOPAUSE RATING SCALE, MRS" FOR SYMPTOMS ASSOCIATED WITH MENOPAUSE
(Lothar AJ Heinemann, Peter Potthoff, and HermanPG Schneider. International versions of the Menopause Rating Scale (MRS). Health Qual. Life Outcomes. 2003; 1 :28)
Table 8 shows the evolution of menopausal symptomatology 1 to 3 months after taking the active product and the placebo product. The data show the percentage of subjects that exhibit an improvement* in the clinical symptomatology.
Table 8a - Variation of the menopausal symptoms
* the percentage of subjects that exhibit an improvement is calculated relative to number of subjects who demonstrate a degree of symptomatology greater than "absent" at the start (subjects reporting the symptoms to be absent are excluded from the calculation).
"MENOPAUSE RATING SCALE, MRS" FOR SYMPTOMS ASSOCIATED WITH MENOPAUSE (Lothar AJ Heinemann, Peter Potjthoff, and HermanPG Schneider. International versions of the Menopause Rating Scale (MRS). Health Qual. Life Outcomes. 2003;1 :28)
Table 8b shows the intra- and intergroup statistical analysis. The intragroup statistical analysis is performed via the Wilcoxon test of the ranges indicated, whereas the intergroup analysis is performed via the Mann-Whitney U test.
Table 8b Statistical analysis
HAMILTON DEPRESSION RATING SCALE
(Hamilton M.A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62)
The mood intensity was evaluated by means of the "Hamilton Depression Rating Scale" Upon enrollment, the panel is composed as described in table 2.
Table 9 Panel composition at TO
The two groups of subjects (Active and Placebo) are homogeneous with reference to the initial symptoms anticipated by the Hamilton scale. The statistical analysis (Mann-Whitney U test) does not indicate significant differences (p>0.05) in the medians.
HAMILTON DEPRESSION RATING SCALE
(Hamilton M.A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62)
Table 9 shows the evolution of the mood intensity 1 to 3 months after taking the active product and the placebo product. The data show the percentage of subjects that exhibit an improvement* in the clinical symptomatology.
Table 9a Variation of the mod
ACTIVE GROUP
Symptoms Tl T3
Work and activities 76.5% 94.1%
Agitation 23.8% 52.4%
Anxiety (psycological) 31.6% 68.4%
Anxiety somatic 31.6% 63.2%
PLACEBO GROUP
Symptoms Tl T3
Work and activities 33.3% 38.9%
Agitation 19.0% 28.6%
Anxiety (psycological) 15.0% 20.0%
Anxiety somatic 19.0% 23.8%
* the percentage of subjects that exhibit an improvement is calculated relative to number of subjects who demonstrate a degree of symptomatology greater than "absent" at the start (subjects reporting the symptoms to be absent are excluded from the calculation).
HAMILTON DEPRESSION RATING SCALE
(Hamilton M.A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62)
Table 9b shows the intra- and intergroup statistical analysis. The intragroup statistical analysis is performed via the Wilcoxon test of the ranges indicated, whereas the intergroup analysis is performed via the Mann-Whitney U test.
Table 9b Statistical analysis
"NOTTINGHAM HEALTH PROFILE" SLEEP DISORDER RATING SCALE
The severity of the sleep disorders associated with menopause was evaluated by means of the "Nottingham Health Profile."
Upon enrollment, the panel is composed as described in table 10.
Table 10 - Panel composition at TO
The two groups of subjects (Active and Placebo) are homogeneous with reference to the initial symptoms anticipated by the MRS. The statistical analysis (Mann- Whitney U test) does not indicate significant differences (p>0.05) in the medians. "NOTTINGHAM HEALTH PROFILE" SLEEP DISORDER RATING SCALE
Table 10 shows the evolution of the sleep disorders 1 to 3 months after taking the active product and the placebo product. The data show the percentage of subjects that exhibit an improvement* in the clinical symptomatology.
Table 10a - Variation of the sleep disorders
* the percentage of subjects that exhibit an improvement is calculated relative to number of subjects who demonstrate a degree of symptomatology at the start (subjects reporting the symptoms to be absent are excluded from the calculation).
"NOTTINGHAM HEALTH PROFILE" SLEEP DISORDER RATING SCALE
Table 10b shows the intra- and intergroup statistical analysis. The intragroup statistical analysis is performed via the Wilcoxon test of the ranges indicated, whereas the intergroup analysis is performed via the Mann-Whitney U test.
Table 10b - Statistical analysis
n.s. not significant - *, p<0.05 - **, p<0.01 - ***, p<0.001
CONCLUSIONS
The ingestion (1 tablet/day) of the product (food supplement) previously identified results in an improvement of the clinical symptomatology of menopause. The product's effect can already be seen after the first month of use. This effect is superior to that recorded for the placebo product, as shown by the statistical analysis reported in the table below:
Table 11
DEMOGRAPHY
The table below shows the panel characteristics
Table 12
Menopausal food supplement Menopausal food supplement Batch FH226 (Equopausa Tablets) Batch FH314 (Equopausa placebo)
Sample size 30 subjects 30 subjects
Sex Female Female
BMI 2CKBMK25 20<BMI<25
Overweight >10 e I and <20 >10 e 1 and <20
Menopause onset < 1 year from enrolment date < 1 year from enrolment date
Table 13 - AVERAGE DATA
The data are reported as average +/- SEM
MRS - MENOPAUSE RATING 0 MONTHS 1MONTHS 3 MONTHS SCALE ACTIVE PLACEBO ACTIVE PLACEBO ACTIVE PLACEBO
1- Hot flushes, sweating 3,2 ± 0,2 3,1 ± 0,1 2,8 ± 0,2 3,1 ± 0,1 2,3 ± 0,2 3,0 ± 0,1 (episodes of sweating)
2- Heart discomfort
(unusual awareness of heart 1,8 ± 0,1 1,9 ± 0,1 1,2 ± 0,1 1,9 ± 0,1 1,1 ± 0,1 1,7 ± 0,1 beat, heart skipping, heart
racing, tightness)
3- Sleep problems
Sleep problems (difficulty in 3,1 ± 0,1 3,0 ± 0,2 2,8 ± 0,2 2,8 ± 0,1 2,6 ± 0,1 2,9 ± 0,2 falling asleep, difficulty in
sleeping through, waking up
early)
4- Depressive mood
(feeling down, sad, on the verge 2,0 ± 0,1 2,0 ± 0,1 2,0 ± 0,1 2,0 ± 0,2 1,4 ± 0,1 1,7 ± 0,2 of tears, lack of drive, mood
swings)
5- Irritability
Irritability (feeling nervous, 2,5 ± 0,2 2,5 ± 0,2 2,1 ± 0,2 2,2 ± 0,2 1,9 ± 0,2 2,3 ± 0,2 inner tension, feeling
aggressive)
6- Physical and mental
exhaustion 2,5 ± 0,1 2,4 ± 0,1 2,2 ± 0,2 2,8 ± 0,1 1,8 ± 0,1 2,3 ± 0,2 (general decrease in
performance, impaired
memory, decrease in
concentration, forgetfulness)
7- Sexual problems
Sexual problems (change in 2,8 ± 0,1 2,8 ± 0,1 2,7 ± 0,2 2,6 ± 0,1 2,0 ± 0,1 3,0 ± 0,1 sexual
desire, in sexual activity and
satisfaction)
8- Bladder problems
Bladder problems (difficulty in 2,1 ± 0,2 2,1 ± 0,1 1,8 ± 0,2 2,0 ± 0,1 1,4 ± 0,1 1,9 ± 0,1 urinating, increased need to
urinate, bladder incontinence)
9- Dryness of vagina
Dryness of vagina (sensation of 2,7 ± 0,1 2,6 ± 0,1 2,5 ± 0,1 2,6 ± 0,1 1,9 ± 0,1 2,6 ± 0,1 dryness or burning in the
vagina, difficulty with sexual
intercourse)
10- Joint and muscular
discomfort 3,0 ± 0,2 2,9 ± 0,2 2,3 ± 0,1 2,7 ± 0,2 2,2 ± 0,2 2,7 ± 0,2
(pain in the joints, rheumatoid
complaints)
Table 14 - Average data
The data are reported as % average of
NHP - NOTTINGHAM 0 MONTHS 1 MONTHS 3 MONTHS HEALTH PROFILE ACTIVE PLACEBO ACTIVE PLACEBO ACTIVE PLACEBO
Subarea "sleep"
1 1 take pills to help me 43,3% 40,0% 40,0% 40,0% 23,3% 36,7% sleep
2 I'm waking up in the 63,3% 60,0% 50,0% 60,0% 30,0% 53,3% early hours of the
morning
3 1 lie away for most of 20,0% 23,3% 16,7% 23,3% 3,3% 20,0% the night
4 It takes me a long time 40,0% 40,0% 36,7% 36,7% 16,7% 33,3% to get to sleep
5 1 sleep badly at night 63,3% 60,0% 40,0% 56,7% 23,3% 46,7%
Table 15 - Average data
The data are reported as average +/- SEM
HAMILTON DEPRESSION 0 MONTHS 1 MONTHS 3 MONTHS SCALE ACTIVE PLACEBO ACTIVE PLACEBO ACTIVE PLACEBO
1 Work and activities 2,1 ± 0,2 2,0 ± 0,1 0,8 ± 0,1 1,6 ± 0,1 0,5 ± 0,1 1,5 ± 0,1
2 Agitation 1,4 ± 0,1 1,5 ± 0,1 1,2 ± 0,2 1,4 ± 0,1 0,7 ± 0,2 1,5 ± 0,2
3 Anxiety (psycological) 1,6 ± 0,2 1,4 ± 0,1 1,4 ± 0,2 1,3 ± 0,1 0,9 ± 0,2 1,3 ± 0,1
4 Anxiety somatic 1,8 ± 0,2 1,7 ± 0,1 1,3 ± 0,1 1,6 ± 0,1 0,7 ± 0,1 1,3 ± 0,1
Claims
1. A composition for the prevention and/or the treatment of menopausal syndrome and/or aging comprising an association of a biologically active metabolite of daidzein absorbable from the intestine and resveratrol in effective quantities, at least one edible carrier, and/or excipient, wherein the biologically active metabolite of daidzein absorbable from the intestine is equol.
2. Composition in accordance with claim 1 , wherein equol is obtained by the fermentation of daidzein by non-pathogenic, equol-producing microorganisms.
3. Composition in accordance with claim 1 or 2, containing equol in an amount ranging from 1 to 100 mg.
4. Composition in accordance with claim 1 or 2 wherein equol is contained in a fermented-soy extract.
5. Composition in accordance with claim 4 wherein the fermented-soy extract has a titre from 20 to 60% in total isoflavones.
6. Composition in accordance with claim 1 , wherein the resveratrol is contained in a Vitis vinifera extract.
Composition in accordance with claim 6 wherein said Vitis vinifera extract has a resveratrol titre from 5% to 15.2%.
7. Composition in accordance with claim 6, wherein the Vitis vinifera extract comprises 1 .25-5 mg of resveratrol.
8. Composition in accordance with claim 1 , wherein the composition comprises a Vitis vinifera extract in a quantity of 10-200 mg.
9. Composition in accordance with any one of the claims from 1 to 8 for medicinal use.
10. Composition in accordance with any one of the claims from 1 to 8 for use in the treatment or in the prevention of menopausal syndrome.
1 1. Composition in accordance with claim 10, wherein said menopausal syndrome includes one or more affections selected from depression, insomnia, anxiety, cardiovascular disorders, osteoporosis, urinary ailments, pelvic pains, imbalances of blood lipid fractions.
12. Composition in accordance with any one of the claims 1-8 in the form of a nutraceutical preparation or food supplement.
13. Use of a composition in accordance with claim 12 for reducing the menstrual syndrome disorders selected from hot flushes, dryness of the skin, redness of the skin, perspiration, excess weight, and combinations thereof.
14. The use of a composition in accordance with claim 12 for reducing the disorders and/or symptoms related to the aging of the organism.
15. The use in accordance with claim 14, wherein reducing the disorders and/or symptoms related to the aging of the organism comprises the aging of the skin, the lowering of metabolism, the increase in weight, and memory or cognitive deficits.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP12708937.3A EP2672964B1 (en) | 2011-02-10 | 2012-02-10 | Composition for use in the treatment of menopause problems/disorders |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ITMI2011A000198 | 2011-02-10 | ||
| ITMI2011A000198A IT1404171B1 (en) | 2011-02-10 | 2011-02-10 | COMPOSITION FOR USE IN THE TREATMENT OF PROBLEMS / DISORDERS OF THE MENOPAUSE AND IN THE TREATMENT OF THE GENERAL AGING OF THE ORGANISM |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012107905A1 true WO2012107905A1 (en) | 2012-08-16 |
Family
ID=43976103
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IB2012/050615 Ceased WO2012107905A1 (en) | 2011-02-10 | 2012-02-10 | Composition for use in the treatment of menopause problems/disorders and in the treatment of general aging of the organism. |
Country Status (3)
| Country | Link |
|---|---|
| EP (1) | EP2672964B1 (en) |
| IT (1) | IT1404171B1 (en) |
| WO (1) | WO2012107905A1 (en) |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103070849A (en) * | 2013-01-29 | 2013-05-01 | 江苏省中国科学院植物研究所 | Application of oxyresveratrol in preparing ovary aging resistant medicine |
| US8937170B2 (en) | 2009-04-30 | 2015-01-20 | Ab Enzymes Oy | Nucleic acids encoding fungal protease |
| KR101692849B1 (en) * | 2015-07-30 | 2017-01-06 | 이화여자대학교 산학협력단 | Pharmaceutical composition for preventing or treating menopausal diseases comprising Resveratrol or pharmaceutically acceptable salts thereof |
| ITUA20162575A1 (en) * | 2016-04-13 | 2017-10-13 | S&R Farm S P A | Pharmaceutical or nutraceutical composition for use in the treatment of polycystic ovary syndrome or diseases or disorders related to it |
| WO2018053595A1 (en) * | 2016-09-23 | 2018-03-29 | The University Of Newcastle | Methods for improving cerebrovascular function and cognition in peri- and post-menopausal women |
| CN107922979A (en) * | 2015-07-21 | 2018-04-17 | Tak循环株式会社 | The screening technique of the material of the evaluation method of physical condition, the reminding method of information and improvement or prevention physical condition |
| JPWO2022050308A1 (en) * | 2020-09-04 | 2022-03-10 | ||
| US12171728B2 (en) | 2018-04-16 | 2024-12-24 | S&R Farmaceutici S.P.A. | Mixture of resveratrol supported on metal hydroxide and non-supported pure resveratrol for treating female fertility |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IT201800020932A1 (en) | 2018-12-21 | 2020-06-21 | Kolinpharma S P A | Multicomponent composition based on L-tryptophan, resveratrol and crocus sativus extract for the treatment of menstrual cycle disorders |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001070212A2 (en) * | 2000-03-23 | 2001-09-27 | Interhealth Nutraceuticals Incorporated | Method and composition for preventing or reducing the symptoms of menopause |
| EP1656942A2 (en) * | 1997-08-08 | 2006-05-17 | Otsuka Pharmaceutical Co., Ltd. | Isoflavone-containing composition |
| US20080254055A1 (en) * | 2007-04-11 | 2008-10-16 | John Erich Oblong | Compositions for Regulation of Hair Growth |
| EP2014282A2 (en) * | 2007-06-22 | 2009-01-14 | E-Therapeutics plc | Treatment of depression |
| WO2009114525A1 (en) * | 2008-03-10 | 2009-09-17 | University Of Louisville Research Foundation, Inc. | Methods and compositions for controlled delivery of phytochemical agents |
-
2011
- 2011-02-10 IT ITMI2011A000198A patent/IT1404171B1/en active
-
2012
- 2012-02-10 WO PCT/IB2012/050615 patent/WO2012107905A1/en not_active Ceased
- 2012-02-10 EP EP12708937.3A patent/EP2672964B1/en not_active Not-in-force
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1656942A2 (en) * | 1997-08-08 | 2006-05-17 | Otsuka Pharmaceutical Co., Ltd. | Isoflavone-containing composition |
| WO2001070212A2 (en) * | 2000-03-23 | 2001-09-27 | Interhealth Nutraceuticals Incorporated | Method and composition for preventing or reducing the symptoms of menopause |
| US20080254055A1 (en) * | 2007-04-11 | 2008-10-16 | John Erich Oblong | Compositions for Regulation of Hair Growth |
| EP2014282A2 (en) * | 2007-06-22 | 2009-01-14 | E-Therapeutics plc | Treatment of depression |
| WO2009114525A1 (en) * | 2008-03-10 | 2009-09-17 | University Of Louisville Research Foundation, Inc. | Methods and compositions for controlled delivery of phytochemical agents |
Non-Patent Citations (32)
| Title |
|---|
| "Estropiu' Bustine", 1 September 2010 (2010-09-01), XP002679815, Retrieved from the Internet <URL:http://www.saninforma.it/Sezione.jsp?idSezione=13458&sez=prodotti&cat=dietetici-e-integratori&titolo=estropiu-bustine> [retrieved on 20120712] * |
| A.J. DAY ET AL., FEBS LETT., vol. 468, 2000, pages 166 - 170 |
| ADDABBO F; RATLIFF B; PARK HC; KUO M; UNGVARI Z; CSISZAR A; KRASNIKOV B; SODHI K; ZHANG F; NASJLETTI A: "The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: proteomic approach", AM J PATHOL, vol. 174, 2009, pages 34 - 43 |
| D. KOSTENAC ET AL., J. AGR. FOOD CHEM., vol. 51, 2003, pages 7632 - 7635 |
| ETHICAL PRINCIPLES FOR MEDICAL RESEARCH THAT INVOLVES HUMAN SUBJECTS, ADOPTED BY THE 18TH GENERAL ASSEMBLY OF THE WMA AT HELSINKI, FINLAND, June 1964 (1964-06-01) |
| FAURE EVELYNE DRAPIER ET AL: "Effects of a standardized soy extract on hot flushes: a multicenter, double-blind, randomized, placebo-controlled study.", MENOPAUSE (NEW YORK, N.Y.) 2002 SEP-OCT LNKD- PUBMED:12218721, vol. 9, no. 5, September 2002 (2002-09-01), pages 329 - 334, XP002659776, ISSN: 1072-3714 * |
| FERRARA N; RINALDI B; CORBI G; CONTI V; STIUSO P; BOCCUTI S; RENGO G; ROSSI F; FILIPPELLI A: "Exercise training promotes SIRT1 activity in aged rats", REJUVENATION RES., vol. 11, no. 1, February 2008 (2008-02-01), pages 139 - 50, XP055130752, DOI: doi:10.1089/rej.2007.0576 |
| G. KUIPER ET AL., ENDOCRINOLOGY, vol. 139, 1998, pages 4252 - 4263 |
| H. WISEMAN, PROC. NUTR. SOC., vol. 58, 1999, pages 139 - 146 |
| HAMILTON M.A: "rating scale for depression", J NEUROL NEUROSURG PSYCHIATRY, vol. 23, 1960, pages 56 - 62 |
| HEINEMANN LOTHAR AJ ET AL: "International versions of the Menopause Rating Scale (MRS)", HEALTH AND QUALITY OF LIFE OUTCOMES, BIOMED CENTRAL, LONDON, GB, vol. 1, no. 1, 30 July 2003 (2003-07-30), pages 28, XP021008835, ISSN: 1477-7525, DOI: 10.1186/1477-7525-1-28 * |
| INDEPENDENT ETHICS COMMITTEE FOR NON PHARMACOLOGICAL CLINICAL INVESTIGATIONS, 30 March 2011 (2011-03-30) |
| J. CHEN ET AL., J. PHARMACOL., EXP. THER., vol. 304, 2003, pages 1228 - 1235 |
| J.H. MITCHELL ET AL., ARCH. BIOCHEM. BIOPHYS., vol. 360, 1998, pages 142 - 148 |
| K. MORITO ET AL., BIOL. PHATM. BULL., vol. 24, 2001, pages 351 - 356 |
| K.D.R. SETCHELL ET AL., AM. J. CLIN. NUTR., vol. 77, 2003, pages 411 - 419 |
| LANGE SKOVGAARD G R ET AL: "Effect of a novel dietary supplement on skin aging in post-menopausal women", EUROPEAN JOURNAL OF CLINICAL NUTRITION, XX, XX, vol. 60, 1 January 2006 (2006-01-01), pages 1201 - 1206, XP008080469 * |
| LOTHAR AJ HEINEMANN; PETER POTTHOFF; HERMANPG SCHNEIDER: "International versions of the Menopause Rating Scale (MRS", HEALTH QUAL. LIFE OUTCOMES, vol. 1, 2003, pages 28, XP021008835, DOI: doi:10.1186/1477-7525-1-28 |
| LOTHAR AJ HEINEMANN; PETER POTTHOFF; HERMANPG SCHNEIDER: "Menopause Rating Scale, MRS", INTERNATIONAL VERSIONS OF THE MENOPAUSE RATING SCALE (MRS). HEALTH QUAL LIFE OUTCOMES, vol. 1, 2003, pages 28 |
| M. RICHELLE ET AL., J. NUTR., vol. 132, 2002, pages 2587 - 2592 |
| N. SATHYAMOORTHY ET AL., EUR. J. CANCER, vol. 33, 1977, pages 2384 - 2389 |
| NARUMOL PHOSRITHONG ET AL: "Molecular docking study on anticancer activity of plant-derived natural products", MEDICINAL CHEMISTRY RESEARCH, vol. 19, no. 8, 28 July 2009 (2009-07-28), pages 817 - 835, XP055007493, ISSN: 1054-2523, DOI: 10.1007/s00044-009-9233-5 * |
| NICOLETTI VG; CARUSO A; TENDI EA; PRIVITERA A; CONSOLE A; CALABRESE V; SPADARO F; RAVAGNA A; COPANI A; STELLA AM: "Effect of nitric oxide synthase induction on the expression of mitochondrial respiratory chain enzyme subunits in mixed cortical and astroglial cell cultures", BIOCHIMIE., vol. 80, no. 10, October 1998 (1998-10-01), pages 871 - 81 |
| PEARSON K J ET AL: "Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span", CELL METABOLISM 20080806 US LNKD- DOI:10.1016/J.CMET.2008.06.011, vol. 8, no. 2, 6 August 2008 (2008-08-06), pages 157 - 168, XP002659775, ISSN: 1550-4131 * |
| R.S. MUTHYALA ET AL., BIOORG. MED. CHEM., vol. 12, 2004, pages 1559 - 1567 |
| REN. J.; PULAKAT L; WHALEY-CONNELL A; SOWERS JR: "Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease", J MOL MED., vol. 88, no. 10, October 2010 (2010-10-01), pages 993 - 1001, XP019823114 |
| SCAPAGNINI G; BUTTERFIELD DA; COLOMBRITA C; SULTANA R; PASCALE A; CALABRESE V: "Ethyl ferulate, a lipophilic polyphenol, induces HO-1 and protects rat neurons against oxidative stress", ANTIOXID REDOX SIGNAL., vol. 6, no. 5, 2004, pages 811 - 8, XP008132367, DOI: doi:10.1089/ars.2004.6.811 |
| SCAPAGNINI G; D'AGATA V; CALABRESE V; PASCALE A; COLOMBRITA C; ALKON DL; CAVALLARO S: "Gene expression profiles of heme oxygenase isoforms in the rat brain", BRAIN RES., vol. 954, no. 1, 2002, pages 31 - 39 |
| SIMPLY NATURAL: "Phyto Soya Night & Day @ Simply Natural", 28 August 2008 (2008-08-28), XP002659774, Retrieved from the Internet <URL:http://web.archive.org/web/20080828131358/http://www.simplynatural.org.uk/acatalog/Phyto_Soya_Night_Day.html> [retrieved on 20110922] * |
| T. ASO: "Equol Improves Menopausal Symptoms in Japanese Women", THE JOURNAL OF NUTRITION, vol. 140, no. 7, 19 May 2010 (2010-05-19), pages 1386S - 1389S, XP055007310, ISSN: 0022-3166, DOI: 10.3945/jn.109.118307 * |
| TAKESHI ET AL.: "Equol improves menopausal symptoms in Japanese women", THE JOURNAL OF NUTRITION; SUPPLEMENT: EQUOL, SOY AND MENOPAUSE, pages 1386 - 1389 |
| TINA WENZ: "Mitochondria and PGC-1a in aging and age-associated diseases", JOURNAL OF AGING RESEARCH, vol. 2011, pages 1 - 12 |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8937170B2 (en) | 2009-04-30 | 2015-01-20 | Ab Enzymes Oy | Nucleic acids encoding fungal protease |
| CN103070849A (en) * | 2013-01-29 | 2013-05-01 | 江苏省中国科学院植物研究所 | Application of oxyresveratrol in preparing ovary aging resistant medicine |
| CN107922979A (en) * | 2015-07-21 | 2018-04-17 | Tak循环株式会社 | The screening technique of the material of the evaluation method of physical condition, the reminding method of information and improvement or prevention physical condition |
| CN107922979B (en) * | 2015-07-21 | 2022-04-05 | Tak循环株式会社 | Method for evaluating physical condition, method for presenting information, and method for screening substance for improving or preventing physical condition |
| KR101692849B1 (en) * | 2015-07-30 | 2017-01-06 | 이화여자대학교 산학협력단 | Pharmaceutical composition for preventing or treating menopausal diseases comprising Resveratrol or pharmaceutically acceptable salts thereof |
| ITUA20162575A1 (en) * | 2016-04-13 | 2017-10-13 | S&R Farm S P A | Pharmaceutical or nutraceutical composition for use in the treatment of polycystic ovary syndrome or diseases or disorders related to it |
| WO2017179012A1 (en) * | 2016-04-13 | 2017-10-19 | S&R Farmaceutici S.P.A. | Nutraceutical or pharmaceutical composition for use in the treatment of polycystic ovary syndrome or of diseases or disorders related thereto |
| US11246834B2 (en) | 2016-04-13 | 2022-02-15 | S&R Farmaceutici S.P.A. | Nutraceutical or pharmaceutical composition for use in the treatment of polycystic ovary syndrome or of diseases or disorders related thereto |
| WO2018053595A1 (en) * | 2016-09-23 | 2018-03-29 | The University Of Newcastle | Methods for improving cerebrovascular function and cognition in peri- and post-menopausal women |
| US12171728B2 (en) | 2018-04-16 | 2024-12-24 | S&R Farmaceutici S.P.A. | Mixture of resveratrol supported on metal hydroxide and non-supported pure resveratrol for treating female fertility |
| JPWO2022050308A1 (en) * | 2020-09-04 | 2022-03-10 |
Also Published As
| Publication number | Publication date |
|---|---|
| IT1404171B1 (en) | 2013-11-15 |
| ITMI20110198A1 (en) | 2012-08-11 |
| EP2672964A1 (en) | 2013-12-18 |
| EP2672964B1 (en) | 2016-06-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2672964B1 (en) | Composition for use in the treatment of menopause problems/disorders | |
| Trinchese et al. | Human, donkey and cow milk differently affects energy efficiency and inflammatory state by modulating mitochondrial function and gut microbiota | |
| Lambert et al. | Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: a randomized controlled trial | |
| Justice et al. | Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women | |
| Cassidy | Potential risks and benefits of phytoestrogen-rich diets | |
| Jackson et al. | Emerging evidence of the health benefits of S-equol, an estrogen receptor β agonist | |
| Simbalista et al. | Consumption of a flaxseed-rich food is not more effective than a placebo in alleviating the climacteric symptoms of postmenopausal women | |
| Sutanto et al. | The impact of 5-hydroxytryptophan supplementation on sleep quality and gut microbiota composition in older adults: A randomized controlled trial | |
| EP3850954A1 (en) | Anti-aging agent and anti-aging method | |
| KR101867768B1 (en) | Composition for preventing or treating of climacterium comprising Lactobacillus acidophilus | |
| CN101977596A (en) | New uses for cannabinoids | |
| Fayed | Health benefits of some physiologically active ingredients and their suitability as yoghurt fortifiers | |
| CA2902248A1 (en) | Activated soy pod fiber | |
| KR101915904B1 (en) | Composition for preventing or treating of climacterium comprising Lactobacillus intestinalis | |
| CN104981241A (en) | Product comprising red clover extract and methods for producing the same | |
| Noone et al. | The impact of 60 days of‐6° head down tilt bed rest on mitochondrial content, respiration and regulators of mitochondrial dynamics | |
| Blonde-Cynober et al. | Use of ornithine [alpha]-ketoglutarate in clinical nutrition of elderly patients | |
| Hemmeter et al. | Effect of flumazenil-augmentation on microsleep and mood in depressed patients during partial sleep deprivation | |
| Tampanna et al. | The role of kratom (Mitragyna speciosa Korth.) extract in medical foods for obese patients: Effects on gut microbiota in a colon model | |
| Lee et al. | Supplementation of Korean fermented soy paste doenjang reduces visceral fat in overweight subjects with mutant uncoupling protein-1 allele | |
| WO2021250661A1 (en) | Shan-zha for the treatment of depression and anxiety disorders | |
| Fouad et al. | Bee Products for Relieving Menopausal Symptoms | |
| Tanwar et al. | Ayurvedic approach to manage Polycystic Ovarian Syndrome with Yoga Basti-A Case Study | |
| Nishimura et al. | A randomized, double-blind, placebo-controlled study to examine the effects of high-isoflavone soybeans “Yukipirika” in climacteric women | |
| Mukai et al. | Effect of Continuous Intake of Onion Powder on Oxidative Stress Biomarkers and Skeletal Muscle Maintenance in Elderly Bedridden Individuals: A Pilot Study |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12708937 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012708937 Country of ref document: EP |