Polymerisable compounds and the use thereof in liquid-crystal displays
The present invention relates to polymerisable compounds, to processes and intermediates for the preparation thereof, and to the use thereof for optical, electro-optical and electronic purposes, in particular in liquid- crystal (LC) media and LC displays, especially in LC displays of the PS ("polymer sustained") or PSA ("polymer sustained alignment") type. Background of the Invention
The liquid-crystal displays (LC displays) used at present are usually those of the TN ("twisted nematic") type. However, these have the disadvantage of a strong viewing-angle dependence of the contrast.
In addition, so-called VA ("vertically aligned") displays are known which have a broader viewing angle. The LC cell of a VA display contains a layer of an LC medium between two transparent electrodes, where the LC medium usually has a negative value of the dielectric (DC) anisotropy. In the switched-off state, the molecules of the LC layer are aligned perpendicular to the electrode surfaces (homeotropically) or have a tilted homeo- tropic alignment. On application of an electrical voltage to the two electrodes, a realignment of the LC molecules parallel to the electrode surfaces takes place.
Furthermore, OCB ("optically compensated bend") displays are known which are based on a birefringence effect and have an LC layer with a so- called "bend" alignment and usually positive (DC) anisotropy. On application of an electrical voltage, a realignment of the LC molecules perpendi- cular to the electrode surfaces takes place. In addition, OCB displays normally contain one or more birefringent optical retardation films in order to prevent undesired transparency to light of the bend cell in the dark state. OCB displays have a broader viewing angle and shorter response times compared with TN displays.
Also known are so-called IPS ("in-plane switching") displays, which contain an LC layer between two substrates, where the two electrodes are arranged on only one of the two substrates and preferably have
intermeshed, comb-shaped structures. On application of a voltage to the electrodes, an electric field which has a significant component parallel to the LC layer is thereby generated between them. This causes realignment of the LC molecules in the layer plane.
Furthermore, so-called FFS ("fringe-field switching") displays have been proposed (see, inter alia, S.H. Jung et al., Jpn. J. Appl. Phys., Volume 43, No. 3, 2004, 1028), which likewise contain two electrodes on the same substrate, but, in contrast to IPS displays, only one of these is in the form of an electrode which is structured in a comb-shaped manner, and the other electrode is unstructured. A strong, so-called "fringe field" is thereby gener- ated, i.e. a strong electric field close to the edge of the electrodes, and, throughout the cell, an electric field which has both a strong vertical component and also a strong horizontal component. Both IPS displays and also FFS displays have a low viewing-angle dependence of the contrast. In VA displays of the more recent type, uniform alignment of the LC molecules is restricted to a plurality of relatively small domains within the LC cell. Disclinations may exist between these domains, also known as tilt domains. VA displays having tilt domains have, compared with conventional VA displays, a greater viewing-angle independence of the contrast and the grey shades. In addition, displays of this type are simpler to produce since additional treatment of the electrode surface for uniform alignment of the molecules in the switched-on state, such as, for example, by rubbing, is no longer necessary. Instead, the preferential direction of the tilt or pretilt angle is controlled by a special design of the electrodes.
In so-called MVA ("multidomain vertical alignment") displays, this is usually achieved by the electrodes having protrusions which cause a local pretilt. As a consequence, the LC molecules are aligned parallel to the electrode surfaces in different directions in different, defined regions of the cell on application of a voltage. "Controlled" switching is thereby achieved, and the formation of interfering disclination lines is prevented. Although this
arrangement improves the viewing angle of the display, it results, however, in a reduction in its transparency to light. A further development of MVA uses protrusions on only one electrode side, while the opposite electrode has slits, which improves the transparency to light. The slitted electrodes generate an inhomogeneous electric field in the LC cell on application of a voltage, meaning that controlled switching is still achieved. For further improvement of the transparency to light, the separations between the slits and protrusions can be increased, but this in turn results in a lengthening of the response times. In so-called PVA ("patterned VA") displays, protrusions are rendered completely superfluous in that both electrodes are structured by means of slits on the opposite sides, which results in increased contrast and improved transparency to light, but is technologically difficult and makes the display more sensitive to mechanical influences ("tapping", etc.). For many applications, such as, for exam- pie, monitors and especially TV screens, however, a shortening of the response times and an improvement in the contrast and luminance (transmission) of the display are demanded.
A further development are the so-called PS or PSA ("polymer sustained" or "polymer sustained alignment") displays, for which the term "polymer stabilised" is also occasionally used. In these, a small amount (for example 0.3% by weight, typically < 1% by weight) of one or more polymerisable compound(s) is added to the LC medium and, after introduction into the LC cell, is polymerised or crosslinked in situ, usually by UV photopolymerisation, between the electrodes with or without an applied electrical voltage. The polymerisation is carried out at a
temperature where the LC medium exhibits a liquid crystal phase, usually at room temperature. The addition of polymerisable mesogenic or liquid- crystalline compounds, also known as reactive mesogens or "RMs", to the LC mixture has proven particularly suitable.
Unless indicated otherwise, the term "PSA" is used below as representative of PS displays and PSA displays. In the meantime, the PS(A) principle is being used in diverse classical LC displays. Thus, for example, PSA-VA, PSA-OCB, PSA-IPS, PSA-FFS and
PSA-TN displays are known. The polymerisation of the polymerisable compound(s) preferably takes place with an applied electrical voltage in the case of PSA-VA and PSA-OCB displays, and with or without, preferably without, an applied electrical voltage in the case of PSA-IPS displays. As can be demonstrated in test cells, the PS(A) method results in a pretilt in the cell. In the case of PSA-OCB displays, for example, it is possible for the bend structure to be stabilised so that an offset voltage is unnecessary or can be reduced. In the case of PSA-VA displays, the pretilt has a positive effect on response times. For PSA-VA displays, a standard MVA or PVA pixel and electrode layout can be used. In addition, however, it is also possible, for example, to manage with only one structured electrode side and no protrusions, which significantly simplifies production and at the same time results in very good contrast at the same time as very good transparency to light.
Furthermore, the so-called posi-VA displays ("positive VA") have proven to be a particularly suitable mode. Like in classical VA displays, the initial orientation of the LC molecules in posi-VA displays is homeotropic, i.e. substantially perpendicular to the substrates, in the initial state when no voltage is applied. However, in contrast to classical VA displays, in posi- VA displays LC media with positive dielectric anisotropy are used. Like in the usually used IPS displays, the two electrodes in posi-VA displays are arranged on only one of the two substrates, and preferably exhibit intermeshed and comb-shaped (interdigital) structures. By application of a voltage to the interdigital electrodes, which create an electrical field that is substantially parallel to the layer of the LC medium, the LC molecules are transferred into an orientation that is substantially parallel to the
substrates. In posi-VA displays, too, it a polymer stabilisation (PSA) has proven to be advantageous, i.e. the addition of RMs to the LC medium, which are polymerised in the cell, whereby a significant reduction of the switching times could be realised.
PSA-VA displays are described, for example, in JP 10-036847 A,
EP 1 170 626 A2, US 6,861 ,107, US 7,169,449, US 2004/0191428 A1 , US 2006/0066793 A1 and US 2006/0103804 A1. PSA-OCB displays are described, for example, in T.-J- Chen et al., Jpn. J. Appl. Phys. 45, 2006,
2702-2704 and S. H. Kim, L.-C- Chien, Jpn. J. Appl. Phys. 43, 2004, 7643-7647. PSA-IPS displays are described, for example, in
US 6,177,972 and Appl. Phys. Lett. 1999, 75(21), 3264. PSA-TN displays are described, for example, in Optics Express 2004, 12(7), 1221.
Like the conventional LC displays described above, PSA displays can be operated as active-matrix or passive-matrix displays. In the case of active- matrix displays, individual pixels are usually addressed by integrated, nonlinear active elements, such as, for example, transistors (for example thin- film transistors ("TFTs")), while in the case of passive-matrix displays, individual pixels are usually addressed by the multiplex method, as known from the prior art.
In particular for monitor and especially TV applications, optimisation of the response times, but also of the contrast and luminance (thus also transmission) of the LC display continues to be demanded. The PSA method can provide crucial advantages here. In particular in the case of PSA-VA, PSA-IPS, PSA-FFS and PSA-posi-VA displays, a shortening of the response times, which correlate with a measurable pretilt in test cells, can be achieved without significant adverse effects on other parameters.
In the prior art, use is made, for example, of polymerisable compounds of the following formula:
in which P denotes a polymerisable group, usually an acrylate or methacryl- ate group, as described, for example, in US 7,169,449.
However, the problem arises that not all combinations consisting of LC mixture (also referred to as "LC host mixture" below) + polymerisable component (typically RMs) are suitable for PSA displays since, for example, an inadequate tilt or none at all becomes established or since, for example, the so-called "voltage holding ratio" (VHR or HR) is inadequate for TFT display applications. In addition, it has been found that, on use in PSA displays, the
LC mixtures and RMs known from the prior art still have some disadvantages. Thus, not every known RM which is soluble in LC mixtures is suitable for use in PSA displays. In addition, it is often difficult to find a suitable selection criterion for the RM besides direct measurement of the pretilt in the PSA display. The choice of suitable RMs becomes even smaller if polymerisation by means of UV light without the addition of photoinitiators is desired, which may be advantageous for certain applications.
In addition, the selected combination of LC host mixture/RM should have the lowest possible rotational viscosity and the best possible electrical properties. In particular, it should have the highest possible VHR. In PSA displays, a high VHR after irradiation with UV light is particularly necessary since UV exposure is a requisite part of the display production process, but also occurs as normal exposure during operation of the finished display.
In particular, it would be desirable to have available novel materials for PSA displays which produce a particularly small pretilt angle. Preferred materials here are those which produce a lower pretilt angle during polymerisation for the same exposure time than the materials known to date, and/or through the use of which the (higher) pretilt angle that can be achieved with known materials can already be achieved after a shorter exposure time. The production time ("tact time") of the display could thus be shortened and the costs of the production process reduced. A further problem in the production of PSA displays is the presence or removal of residual amounts of unpolymerised RMs, in particular after the polymerisation step for production of the pretilt angle in the display. For example, unreacted RMs of this type may adversely affect the properties of the display by, for example, polymerising in an uncontrolled manner during operation after finishing of the display.
Thus, the PSA displays known from the prior art often exhibit the unde- sired effect of so-called "image sticking" or "image burn", i.e. the image produced in the LC display by temporary addressing of individual pixels still remains visible even after the electric field in these pixels has been switched off or after other pixels have been addressed.
This "image sticking" can occur on the one hand if LC host mixtures having a low VHR are used. The UV component of daylight or the backlighting can cause undesired decomposition reactions of the LC molecules therein and thus initiate the production of ionic or free-radical impurities. These may accumulate, in particular, at the electrodes or the alignment layers, where they may reduce the effective applied voltage. This effect can also be observed in conventional LC displays without a polymer component. In addition, an additional "image sticking" effect caused by the presence of unpolymerised RMs is often observed in PSA displays. Uncontrolled polymerisation of the residual RMs is initiated here by UV light from the environment or by the backlighting. In the switched display areas, this changes the tilt angle after a number of addressing cycles. As a result, a change in transmission in the switched areas may occur, while it remains unchanged in the unswitched areas.
It is therefore desirable for the polymerisation of the RMs to proceed as completely as possible during production of the PSA display and for the presence of unpolymerised RMs in the display to be excluded as far as possible or reduced to a minimum. To this end, materials are required which enable highly effective and complete polymerisation. In addition, controlled reaction of these residual amounts would be desirable. This would be simpler if the RM polymerised more rapidly and effectively than the materials known to date.
There is thus still a great demand for PSA displays, in particular of the VA and OCB type, and LC media and polymerisable compounds for use in such displays, which do not exhibit the disadvantages described above or only do so to a small extent and have improved properties. In particular, there is a great demand for PSA displays, and materials for use in PSA displays, which enable a high specific resistance at the same time as a large working-temperature range, short response times, even at low temperatures, and a low threshold voltage, a low pretilt angle, a multiplicity of grey shades, high contrast and a broad viewing angle, and have high values for the "voltage holding ratio" (VHR) after UV exposure.
The invention is based on the object of providing novel suitable materials, in particular RMs and LC media comprising same, for use in PSA displays, which do not have the disadvantages indicated above or do so to a reduced extent, polymerise as rapidly and completely as possible, enable a low pretilt angle to be established as quickly as possible, reduce or prevent the occurrence of "image sticking" in the display, and preferably at the same time enable very high specific resistance values, low threshold voltages and short response times.
A further object of the invention is the provision of novel RMs, in particular for optical, electro-optical and electronic applications, and of suitable processes and intermediates for the preparation thereof. In particular, the invention is based on the object of providing polymerisable compounds which produce a greater maximum pretilt after photopoly- merisation, which results in the desired pretilt being achieved more quickly and thus in significantly shortened times for production of the LC display. This object has been achieved in accordance with the invention by materials and processes as described in the present application. In particular, it has been found, surprisingly, that the use of multireactive polymerisable compounds according to the invention, which are based on coumarine or flavone derivatives and contain two or more, preferably three or more polymerisable groups, wherein at least two polymerizable groups are attached to the same ring, in PSA displays facilitates particularly low pretilt angles and fast establishment of the desired tilt angles. This has been demonstrated in connection with an LC medium by means of pretilt measurements. In particular, a pretilt has been achieved without the addition of photoinitiator. In addition, the compounds according to the invention exhibit significantly faster generation of the pretilt angle
compared with the materials known from the prior art, as has been demonstrated by exposure time-dependent measurements of the pretilt angle. In addition, the polymerisable compounds according to the invention exhibit a significantly higher polymerisation rate, causing smaller unreacted residual amounts to remain in the cell. The electro-optical
properties of the cell are thus improved, and in addition controlled reaction of these residual amounts becomes simpler.
Polymerisable coumarine derivatives have been described in the prior art for other purposes. WO 2004/003103 A1 discloses luminescent
polymerisable compounds which may also contain a coumarine group that is fused to a benzene ring like those of formula If 1 and If2
P-Sp alk l
wherein P is a polymerisable group and Sp is a spacer group, for use in organic light-emitting diodes. WO 2009/074520 A1 discloses coumarines the following formula
wherein R1 is acrylate or methacrylate, R2 is an organic straight-chain or branched alkyl or aryl substituent with up to 30 atoms selected from C, H, Si, O, N, P, S, F, CI, Br, R3-R5 are H or an organic straight-chain or branched alkyl or aryl substituent with up to 30 atoms selected from C, H, Si, O, N, P, S, F, CI, Br, X and Y are O, S, NH or NR, with R being an organic straight- chain or branched alkyl or aryl substituent with up to 30 atoms selected from C, H, Si, O, N, P, S, F, CI, Br, n is 0, 1 or 2 and m is 0 or 1 , for use as UV absorber in a copolymer for ophthalmological compositions.
However, these documents do neither disclose nor suggest the use of polymerisable coumarines in LC media of PS- or PSA-LCDs for creating a pretilt angle by in-situ polymerisation in an electric field.
WO 2010/049044 A1 discloses reactive mesogens based on coumarine or flavone for use in PSA displays, but does not explicitly disclose reactive mesogens as claimed in this invention which comprise three or more polymerizable groups or comprise two or more polymerizable groups attached to the same ring.
The compounds of the present invention are suitable for creating a high pretilt in PSA type displays. Compared to polymerizable compounds of prior art, they show a more rapid and complete polymerization reaction, and have higher solubility and enable higher VHR values in LC media.
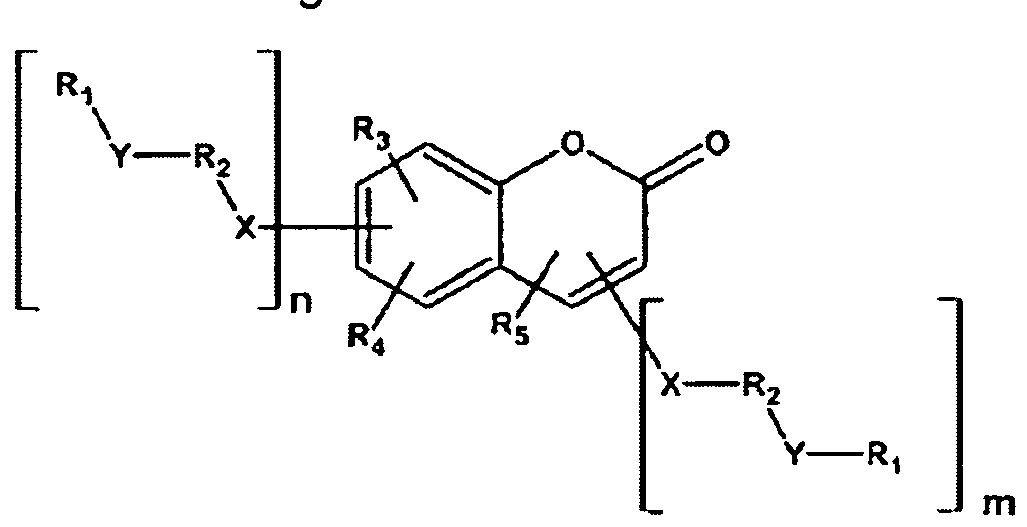
Summary of the Invention The invention relates to the use of compounds of the formula I
in which the individual radicals have the following meanings:
G denotes -CM=CR°-CO-, -CO-CRc=CM-, -CRc=CM-CO- or
-CO-CM=CRc-,
M denotes -X2-A4-(Z2-A3)n2-Rb,
A1"4 each, independently of one another, and on each occurrence identically or differently, denote an aromatic, heteroaromatic, alicyclic or heterocyclic group, preferably having 4 to 25 C atoms, which may also contain fused rings, and which is optionally mono- or polysubstituted by L, and A2 and A4 may also denote a single bond,
X1 and X2 each, independently of one another, and on each occurrence identically or differently, denote -O-, -S-, -CO-, -CO-O-, -O- CO-, -O-CO-O- or a single bond,
each, independently of one another, and on each occurrence identically or differently, denote -O-, -S-, -CO-, -CO-O-, -O- CO-, -O-CO-O-, -OCH2-, -CH2O-, -SCH2-, -CH2S-, -CF2O-, -OCF2-, -CF2S-, -SCF2-, -(CH2)„3-, -CF2CH2-, -CH2CF2-,
-(CF2)n3-, -CH=CH-, -CF=CF-, -CH=CF-, -CF=CH-, -C≡C-, -CH=CH-CO-O-, -O-CO-CH=CH-, -CH2-CH2-CO-O-, -O-CO- CH2-CH2-, -CR°R00- or a single bond, each, independently of one another, and on each occurrence identically or differently, denote P, P-Sp-, H, OH, F, CI, Br, I, -CN, -NO2, -NCO, -NCS, -OCN, -SCN, SF5 or straight-chain or branched alkyl having 1 to 25 C atoms, in which, in addition, one or more non-adjacent CH2 groups may each be replaced, independently of one another, by -C(R00)=C(R000)-, -C≡C-, -N(R00)-, -O-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O- in such a way that O and/or S atoms are not linked directly to one another, and in which, in addition, one or more H atoms may be replaced by F, CI, Br, I, CN or P-Sp-,
wherein at least two of the radicals Ra denote or contain a group P or P-Sp-, denotes on each occurrence, identically or differently, a poly- merisable group,
Sp denotes on each occurrence, identically or differently, a
spacer group or a single bond,
R and R each, independently of one another, denote H or alkyl having 1 to 12 C atoms,
L denotes on each occurrence, identically or differently, P-Sp-,
H, OH, CH2OH, halogen, SF5, NO2, a carbon group or hydrocarbon group, n1 and n2 each, independently of one another, denote 0, 1 , 2 or 3,
n3 denotes 1 , 2, 3 or 4, x denotes 2, 3 or 4, in liquid-crystal (LC) media and LC displays, preferably in LC media and displays of the PS (polymer stabilised) or PSA (polymer sustained alignment) type.
The invention furthermore relates to an LC medium comprising one or more polymerisable compounds according to the invention and one or more additional compounds, which may also be mesogenic, liquid- crystalline and/or polymerisable.
The invention furthermore relates to an LC medium comprising a polymer obtainable by polymerisation of one or more polymerisable compounds according to the invention and one or more additional compounds, which may also be mesogenic, liquid-crystalline and/or polymerisable.
The invention furthermore relates to an LC medium comprising
- a polymerisable component A) comprising one or more polymerisable compounds according to the invention, and
- a liquid-crystalline component B), also referred to below as "LC host mixture", comprising one or more, preferably two or more, low-molecular-weight (monomeric and unpolymerisable) compounds as described above and below.
The invention furthermore relates to a process for the preparation of an LC medium as described above and below in which one or more low- molecular-weight liquid-crystalline compounds, or an LC host mixture as described above and below, are mixed with one or more polymerisable compounds according to the invention and optionally with further liquid- crystalline compounds and/or additives.
The invention furthermore relates to the use of polymerisable compounds according to the invention and LC media according to the invention in PS and PSA displays, in particular the use in PS and PSA displays containing
an LC medium, for the production of a tilt angle in the LC medium by in- situ polymerisation of the compound(s) of the formula I in the PSA display, preferably with application of an electric or magnetic field. The invention furthermore relates to an LC display containing one or more polymerisable compounds according to the invention or an LC medium according to the invention, in particular a PS or PSA display, particularly preferably a PSA-VA, PSA-OCB, PSA-IPS, PS-FFS, PSA-posi-VA or PSA-TN display.
The invention furthermore relates to an LC display of the PS or PSA type containing an LC cell having two substrates and two electrodes, where at least one substrate is transparent to light and at least one substrate has one or two electrodes, and a layer, located between the substrates, of an LC medium comprising a polymerised component and a low-molecular- weight component, where the polymerised component is obtainable by polymerisation of one or more polymerisable compounds between the substrates of the LC cell in the LC medium, preferably with application of an electrical voltage to the electrodes, where at least one of the polymer- isable compounds is selected from polymerisable compounds according to the invention.
The invention furthermore relates to a process for the production of an LC display as described above and below in which an LC medium comprising one or more low-molecular-weight liquid-crystalline compounds or an LC host mixture as described above and below and one or more polymerisable compounds according to the invention is introduced into an LC cell having two substrates and two electrodes as described above and below, and the polymerisable compounds are polymerised, preferably with appli- cation of an electrical voltage to the electrodes.
The PS and PSA displays according to the invention have two electrodes, preferably in the form of transparent layers, which are applied to one or both of the substrates which form the LC cell. Either in each case one electrode is applied to each of the two substrates, as, for example, in PSA-VA, PSA-OCB or PSA-TN displays according to the invention, or
both electrodes are applied to only one of the two substrates, while the other substrate has no electrode, as, for example, in PSA-posi-VA, PSA- IPS or PSA-FFS displays according to the invention. The invention furthermore relates to novel compounds of the formula I in which G, A1, A2, Z1, X1, Ra, n1 and x have the meanings given above, and a) X1 and/or X2 is a single bond, and/or
b) one of n1 and n2 is different from 0 and/or
c) one of A2 and A4 is not a single bond.
The invention furthermore relates to processes for the preparation of compounds of formula I, and to novel intermediates used or obtained in these processes. Definitions of Terms
The terms "tilt" and "tilt angle" relate to a tilted alignment of the LC molecules of an LC medium relative to the surfaces of the cell in an LC display (here preferably a PS or PSA display). The tilt angle here denotes the average angle (< 90°) between the longitudinal molecular axes of the LC molecules (LC director) and the surface of the plane-parallel outer plates which form the LC cell. A low value for the tilt angle (i.e. a large deviation from the 90° angle) corresponds to a large tilt here. A suitable method for measurement of the tilt angle is given in the examples. Unless indicated otherwise, tilt angle values disclosed above and below relate to this measurement method.
The term "mesogenic group" is known to the person skilled in the art and is described in the literature, and denotes a group which, due to the ani- sotropy of its attracting and repelling interactions, essentially contributes to causing a liquid-crystal (LC) phase in low-molecular-weight or polymeric substances. Compounds containing mesogenic groups (mesogenic compounds) do not necessarily have to have an LC phase themselves. It is also possible for mesogenic compounds to exhibit LC phase behaviour only after mixing with other compounds and/or after polymerisation. Typical mesogenic groups are, for example, rigid rod- or disc-shaped units. An
overview of the terms and definitions used in connection with mesogenic or LC compounds is given in Pure Appl. Chem. 73(5), 888 (2001) and C. Tschierske, G. Pelzl, S. Diele, Angew. Chem. 2004, 116, 6340-6368. The term "spacer group", also referred to as "Sp" above and below, is known to the person skilled in the art and is described in the literature, see, for example, Pure Appl. Chem. 73(5), 888 (2001) and C. Tschierske, G. Pelzl, S. Diele, Angew. Chem. 2004, 116, 6340-6368. Unless indicated otherwise, the term "spacer group" or "spacer" above and below denotes a flexible group which connects the mesogenic group and the polymer- isable group(s) to one another in a polymerisable mesogenic compound.
The term "reactive mesogen" or "RM" denotes a compound containing one mesogenic group and one or more functional groups which are suit- able for polymerisation (also referred to as polymerisable group or group
P).
The terms "low-molecular-weight compound" and "unpolymerisable compound" denote compounds, usually monomeric, which contain no func- tional group which is suitable for polymerisation under the usual conditions known to the person skilled in the art, in particular under the conditions used for the polymerisation of RMs.
Detailed Description of the Invention
Above and below "organic group" denotes a carbon or hydrocarbon group.
"Carbon group" denotes a mono- or polyvalent organic group containing at least one carbon atom, where this either contains no further atoms (such as, for example, -C≡C-) or optionally contains one or more further atoms, such as, for example, N, O, S, P, Si, Se, As, Te or Ge (for example carbonyl, etc.). The term "hydrocarbon group" denotes a carbon group which additionally contains one or more H atoms and optionally one or more heteroatoms, such as, for example, N, O, S, P, Si, Se, As, Te or Ge.
"Halogen" denotes F, CI, Br or I.
"Conjugated radical" or "conjugated group" denotes a radical or group which contains principally sp2-hybridised (or possibly also sp-hybridised) carbon atoms, which may also be replaced by corresponding hetero- atoms. In the simplest case, this means the alternating presence of double and single bonds. "Principally" in this connection means that naturally (non-randomly) occurring defects which result in conjugation interruptions do not devalue the term "conjugated". Furthermore, the term "conjugated" is likewise used in this application text if, for example, arylamine units or certain heterocycles (i.e. conjugation via N, O, P or S atoms) are located in the radical or group.
A carbon or hydrocarbon group can be a saturated or unsaturated group. Unsaturated groups are, for example, aryl, alkenyl or alkynyl groups. A carbon or hydrocarbon radical having more than 3 C atoms can be straight-chain, branched and/or cyclic and may also contain spiro links or condensed rings.
The terms "alkyl", "aryl", "heteroaryl", etc., also encompass polyvalent groups, for example alkylene, arylene, heteroarylene, etc.
The term "aryl" denotes an aromatic carbon group or a group derived therefrom. The term "heteroaryl" denotes "aryl" as defined above, containing one or more heteroatoms.
Preferred carbon and hydrocarbon groups are optionally substituted alkyl, alkenyl, alkynyl, alkoxy, alkylcarbonyl, alkoxycarbonyl, alkylcarbonyloxy and alkoxycarbonyloxy having 1 to 40, preferably 1 to 25, particularly preferably 1 to 18, C atoms, optionally substituted aryl or aryloxy having 6 to 40, preferably 6 to 25, C atoms, or optionally substituted alkylaryl, aryl- alkyl, alkylaryloxy, arylalkyloxy, arylcarbonyl, aryloxycarbonyl, arylcarbon- yloxy and aryloxycarbonyloxy having 6 to 40, preferably 6 to 25, C atoms.
Further preferred carbon and hydrocarbon groups are Ci-C40 alkyl, C2-C40 alkenyl, C2-C40 alkynyl, C3-C40 allyl, C4-C40 alkyldienyl, C4-C40 polyenyl, C6-C 0 aryl, C6-C4o alkylaryl, C6-C40 arylalkyl, C6-C40 alkylaryloxy, C6-C4o
arylalkyloxy, C2-C4o heteroaryl, C4-C4o cycloalkyl, C4-C40 cycloalkenyl, etc. Particular preference is given to C C22 alkyl, C2-C22 alkenyl, C2-C22 alkynyl, C3-C22 allyl, C4-C22 alkyldienyl, C6-Ci2 aryl, C6-C2o arylalkyl and C2-C20 heteroaryl.
Further preferred carbon and hydrocarbon groups are straight-chain, branched or cyclic alkyl radicals having 1 to 40, preferably 1 to 25, C atoms, which are unsubstituted or mono- or polysubstituted by F, CI, Br, I or CN and in which one or more non-adjacent CH2 groups may each be replaced, independently of one another, by -C(RX)=C(RX)-, -C≡C-, -N(RX)-, -0-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O- in such a way that O and/or S atoms are not linked directly to one another.
Rx preferably denotes H, halogen, a straight-chain, branched or cyclic alkyl chain having 1 to 25 C atoms, in which, in addition, one or more non- adjacent C atoms may be replaced by -O-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O- and in which one or more H atoms may be replaced by fluorine, an optionally substituted aryl or aryloxy group having 6 to 40 C atoms, or an optionally substituted heteroaryl or heteroaryloxy group having 2 to 40 C atoms.
Preferred alkoxy groups are, for example, methoxy, ethoxy, 2-methoxy- ethoxy, n-propoxy, i-propoxy, n-butoxy, i-butoxy, s-butoxy, t-butoxy, 2- methylbutoxy, n-pentoxy, n-hexoxy, n-heptoxy, n-octoxy, n-nonoxy, n- decoxy, n-undecoxy, n-dodecoxy, etc.
Preferred alkyl groups are, for example, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, s-butyl, t-butyl, 2-methylbutyl, n-pentyl, s-pentyl, cyclo- pentyl, n-hexyl, cyclohexyl, 2-ethylhexyl, n-heptyl, cycloheptyl, n-octyl, cyclooctyl, n-nonyl, n-decyl, n-undecyl, n-dodecyl, dodecanyl, trifluoro- methyl, perfluoro-n-butyl, 2,2,2-trifluoroethyl, perfluorooctyl, perfluoro- hexyl, etc.
Preferred alkenyl groups are, for example, ethenyl, propenyl, butenyl, pentenyl, cyclopentenyl, hexenyl, cyclohexenyl, heptenyl, cycloheptenyl, octenyl, cyclooctenyl, etc.
Preferred alkynyl groups are, for example, ethynyl, propynyl, butynyl, pentynyl, hexynyl, octynyl, etc. Preferred alkoxy groups are, for example, methoxy, ethoxy, 2-methoxy- ethoxy, n-propoxy, i-propoxy, n-butoxy, i-butoxy, s-butoxy, t-butoxy, 2- methylbutoxy, n-pentoxy, n-hexoxy, n-heptoxy, n-octoxy, n-nonoxy, n- decoxy, n-undecoxy, n-dodecoxy, etc. Preferred amino groups are, for example, dimethylamino, methylamino, methylphenylamino, phenylamino, etc.
Aryl and heteroaryl groups can be monocyclic or polycyclic, i.e. they can contain one ring (such as, for example, phenyl) or two or more rings, which may also be fused (such as, for example, naphthyl) or covalently bonded (such as, for example, biphenyl), or contain a combination of fused and linked rings. Heteroaryl groups contain one or more hetero- atoms, preferably selected from O, N, S and Se. Particular preference is given to mono-, bi- or tricyclic aryl groups having 6 to 25 C atoms and mono-, bi- or tricyclic heteroaryl groups having 5 to 25 ring atoms, which optionally contain fused rings and are optionally substituted. Preference is furthermore given to 5-, 6- or 7-membered aryl and heteroaryl groups, in which, in addition, one or more CH groups may be replaced by N, S or O in such a way that O atoms and/or S atoms are not linked directly to one another.
Preferred aryl groups are, for example, phenyl, biphenyl, terphenyl,
[1 ,1,:3',1"]terphenyl-2,-yl, naphthyl, anthracene, binaphthyl, phenanthrene, pyrene, dihydropyrene, chrysene, perylene, tetracene, pentacene, benzo- pyrene, fluorene, indene, indenofluorene, spirobifluorene, etc.
Preferred heteroaryl groups are, for example, 5-membered rings, such as pyrrole, pyrazole, imidazole, 1 ,2,3-triazole, 1 ,2,4-triazole, tetrazole, furan, thiophene, selenophene, oxazole, isoxazole, 1 ,2-thiazole, ,3-thiazole, 1 ,2,3-oxadiazole, 1 ,2,4-oxadiazole, 1 ,2,5-oxadiazole, 1 ,3,4-oxadiazole,
1 ,2,3-thiadiazole, 1 ,2,4-thiadiazole, 1,2,5-thiadiazole, 1 ,3,4-thiadiazole, 6-membered rings, such as pyridine, pyridazine, pyrimidine, pyrazine, 1 ,3,5- triazine, 1 ,2,4-triazine, 1 ,2,3-triazine, 1 ,2,4,5-tetrazine, 1 ,2,3,4-tetrazine, 1 ,2,3,5-tetrazine, or condensed groups, such as indole, isoindole, indoli- zine, indazole, benzimidazole, benzotriazole, purine, naphthimidazole, phenanthrimidazole, pyridimidazole, pyrazinimidazole, quinoxalinimidazoie, benzoxazole, naphthoxazole, anthroxazole, phenanthroxazole, isoxazole, benzothiazole, benzofuran, isobenzofuran, dibenzofuran, quinoline, iso- quinoline, pteridine, benzo-5,6-quinoline, benzo-6,7-quinoline, benzo-7,8- quinoline, benzoisoquinoline, acridine, phenothiazine, phenoxazine, benzo- pyridazine, benzopyrimidine, quinoxaline, phenazine, naphthyridine, aza- carbazole, benzocarboline, phenanthridine, phenanthroline, thieno[2,3b]- thiophene, thieno[3,2b]thiophene, dithienothiophene, isobenzothiophene, dibenzothiophene, benzothiadiazothiophene, or combinations of these groups.
The aryl and heteroaryl groups mentioned above and below may also be substituted by alkyl, alkoxy, thioalkyl, fluorine, fluoroalkyl or further aryl or heteroaryl groups.
The (non-aromatic) alicyclic and heterocyclic groups encompass both saturated rings, i.e. those containing exclusively single bonds, and also partially unsaturated rings, i.e. those which may also contain multiple bonds. Heterocyclic rings contain one or more heteroatoms, preferably selected from Si, O, N, S and Se.
The (non-aromatic) alicyclic and heterocyclic groups can be monocyclic, i.e. contain only one ring (such as, for example, cyclohexane), or poly- cyclic, i.e. contain a plurality of rings (such as, for example, decahydro- naphthalene or bicyclooctane). Particular preference is given to saturated groups. Preference is furthermore given to mono-, bi- or tricyclic groups having 5 to 25 ring atoms, which optionally contain fused rings and are optionally substituted. Preference is furthermore given to 5-, 6-, 7- or 8- membered carbocyclic groups, in which, in addition, one or more C atoms may be replaced by Si and/or one or more CH groups may be replaced by
N and/or one or more non-adjacent CH2 groups may be replaced by -O- and/or -S-.
Preferred alicyclic and heterocyclic groups are, for example, 5-membered groups, such as cyclopentane, tetrahydrofuran, tetrahydrothiofuran, pyrrolidine, 6-membered groups, such as cyclohexane, silinane, cyclohexene, tetrahydropyran, tetrahydrothiopyran, 1 ,3-dioxane, 1 ,3-dithiane, piperidine, 7-membered groups, such as cycloheptane, and fused groups, such as tetrahydronaphthalene, decahydronaphthalene, indane, bicyclo[1.1.1]- pentane-1 ,3-diyl, bicyclo[2.2.2]octane-1,4-diyl, spiro[3.3]heptane-2,6-diyl, octahydro-4,7-methanoindane-2,5-diyl.
Preferred substituents are, for example, solubility-promoting groups, such as alkyl or alkoxy, electron-withdrawing groups, such as fluorine, nitro or nitrile, or substituents for increasing the glass transition temperature (Tg) in the polymer, in particular bulky groups, such as, for example, t-butyl or optionally substituted aryl groups.
Preferred substituents, also referred to as "L" above and below, are, for example, F, CI, Br, I, -CN, -N02, -NCO, -NCS, -OCN, -SCN, -C(=0)N(Rx)2, -C(=0)Y1, -C(=O)Rx, -N(RX)2, in which R has the meaning indicated above, and Y1 denotes halogen, optionally substituted silyl or aryl having 6 to 40, preferably 6 to 20, C atoms, and straight-chain or branched alkyl, alkoxy, alkylcarbonyl, alkoxycarbonyl, alkylcarbonyloxy or alkoxycarbonyl- oxy having 1 to 25 C atoms, in which one or more H atoms may optionally be replaced by F or CI.
"Substituted silyl or aryl" preferably means substituted by halogen, -CN, R°, -OR0, -CO-R0, -CO-O-R0, -O-CO-R0 or -O-CO-O-R0, in which R° has the meaning indicated above.
Particularly preferred substituents L are, for example, F, CI, CN, NO
2, CH
3, C
2H
5, OCH
3, OC
2H
5, COCH3, COC
2H
5, COOCH3, COOC
2H
5, CF
3, OCF
3, OCHF
2, OC
2F
5, furthermore phenyl.
in which L has one of the meanings indicated above.
The polymerisable group P is a group which is suitable for a polymerisation reaction, such as, for example, free-radical or ionic chain polymerisation, polyaddition or polycondensation, or for a polymer-analogous reaction, for example addition or condensation onto a main polymer chain. Particular preference is given to groups for chain polymerisation, in particular those containing a C=C double bond or -C≡C- triple bond, and groups which are suitable for polymerisation with ring opening, such as, for example, oxetane or epoxide groups.
Preferred roups P are selected from the group consisting of CH2=CW1-
CW =CH-CO-NH-, CH2=CW1-CO-NH-, CH3-CH=CH-O-, (CH2=CH)2CH- OCO-, (CH2=CH-CH2)2CH-OCO-, (CH2=CH)2CH-0-, (CH2=CH-CH2)2N-, (CH2=CH-CH2)2N-CO-, HO-CW2W3-, HS-CW2W3-, HW2N-, HO-CW2W3- NH-, CH2=CW1-CO-NH-, CH2=CH-(COO)k1-Phe-(0)k2-, CH2=CH-(CO)ki- Phe-iO)^-, Phe-CH=CH-, HOOC-, OCN- and wWWSi-, in which W1 denotes H, F, CI, CN, CF3, phenyl or alkyl having 1 to 5 C atoms, in particular H, F, CI or CH3, W2 and W3 each, independently of one another, denote H or alkyl having 1 to 5 C atoms, in particular H, methyl, ethyl or n-propyl, W4, W5 and W6 each, independently of one another, denote CI, oxaalkyl or oxacarbonylalkyl having 1 to 5 C atoms, W7 and W8 each, independently of one another, denote H, CI or alkyl having 1 to 5 C atoms, Phe denotes 1 ,4-phenylene, which is optionally substituted by one or more radicals L as defined above which are other than P-Sp-, ki, k2 and k3 each, independently of one another, denote 0 or 1 , k3 preferably denotes 1 , and k4 denotes an integer from 1 to 10.
Particularly preferred roups P are selected from the group consisting of
, CH
2=CW
2-0-, CH
2=CW
2-, CW
1=CH-
CO-(0)k3-, CW1=CH-CO-NH-, CH2=CW1-CO-NH-, (CH2=CH)2CH-OCO-, (CH2=CH-CH2)2CH-OCO-, (CH2=CH)2CH-0-, (CH2=CH-CH2)2N-f (CH2=CH- CH2)2N-CO-, CH2=CW1-CO-NH-, CH2=CH-(COO)k1-Phe-(0)k2-, CH2=CH- (0Ο)κι-Ρίιβ-(Ο)κ2-, Phe-CH=CH- and V^wWSi-, in which W1 denotes H, F, CI, CN, CF3, phenyl or alkyl having 1 to 5 C atoms, in particular H, F, CI or CH3, W2 and W3 each, independently of one another, denote H or alkyl having 1 to 5 C atoms, in particular H, methyl, ethyl or n-propyl, W4, W5 and W6 each, independently of one another, denote CI, oxaalkyl or oxa- carbonylalkyl having 1 to 5 C atoms, W7 and W8 each, independently of one another, denote H, CI or alkyl having 1 to 5 C atoms, Phe denotes 1 ,4- phenylene, k-i, k2 and k3 each, independently of one another, denote 0 or 1 , k3 preferably denotes 1 , and l¾ denotes an integer from 1 to 10.
Very particularly preferred groups P are selected from the group consisting of CH2=CW -CO-0-, in particular CH2=CH-CO-0-, CH2=C(CH3)-CO-0- and CH2=CF-CO-0-, furthermore CH2=CH-0-, (CH2=CH)2CH-0-CO-
Further very particularly preferred groups P are selected from the group consisting of vinyl, vinyloxy, acrylate, methacrylate, fluoroacrylate, chloro- acrylate, oxetane and epoxide groups, and particularly preferably denote an acrylate or methacrylate group.
Preferred spacer groups Sp other than a single bond are selected from the formula Sp"-X", so that the radical P-Sp- conforms to the formula P-Sp"-X"-, where
Sp" denotes alkylene having 1 to 20, preferably 1 to 12, C atoms, which is optionally mono- or polysubstituted by F, CI, Br, I or CN and in which, in addition, one or more non-adjacent CH2 groups may each be replaced, independently of one another, by -0-, -S-, -NH-, -N(R°)->
-S R^R000)-, -CO-, -CO-O-, -O-CO-, -O-CO-O-, -S-CO-, -CO-S-, -N(R00)-CO-O-, -O-CO-N(R00)-, -N(R00)-CO-N(R00)-, -CH=CH- or -C≡C- in such a way that O and/or S atoms are not linked directly to one another,
X" denotes -O-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O-, -CO-N(R00)-,
-N(R00)-CO-, -N(R00)-CO-N(R00)-, -OCH2-, -CH2O-, -SCH2-, -CH2S-, -CF2O-, -OCF2-, -CF2S-, -SCF2-, -CF2CH2-, -CH2CF2-, -CF2CF2-, .CH=N-, -N=CH-, -N=N-, -CH=CR0-, -CY2=CY3-, -C≡C-, -CH=CH-CO- O-, -O-CO-CH=CH- or a single bond,
R00 and R000 each, independently of one another, denote H or alkyl having 1 to 12 C atoms, and Y2 and Y3 each, independently of one another, denote H, F, CI or CN.
X' is preferably -O-, -S-, -CO-, -COO-, -OCO-, -O-COO-, -CO-NR0-, -NR°- CO-, -NR°-CO-NR°- or a single bond. Typical spacer groups Sp" are, for example, -(CH2)pi-,
-(CH2CH2O)qi-CH2CH2-, -CH2CH2-S-CH2CH2-, -CH2CH2-NH-CH2CH2- or -(SiR00R000-O)pi-, in which p1 is an integer from 1 to 12, q1 is an integer from 1 to 3, and R00 and R000 have the meanings indicated above.
Particularly preferred groups -Sp"-X"- are -(CH2)p1-, -(CH2)p -O-, -(CH2)p1-O- CO-, -(CH2)pi-O-CO-O-, in which p1 and q1 have the meanings indicated above.
Particularly preferred groups Sp" are, for example, in each case straight- chain ethylene, propylene, butylene, pentylene, hexylene, heptylene, octylene, nonylene, decylene, undecylene, dodecylene, octadecylene,
ethyleneoxyethylene, methyleneoxybutylene, ethylenethioethylene, ethyl- ene-N-methyliminoethylene, 1-methylalkylene, ethenylene, propenylene and butenylene.
In a further preferred embodiment of the invention, Ra and/or Rb in formula I denote a radical containing two or more polymerisable groups (multifunctional polymerisable radicals). Suitable radicals of this type and polymerisable compounds containing them and the preparation thereof are described, for example, in US 7,060,200 B1 or US 2006/0172090 A1. Particular preference is given to multifunctional polymerisable radicals selected from the following formulae:
-X-alkyl-CHP1-CH2-CH2P2 l*a -X-alkyl-C(CH2P1)(CH2P2)-CH2P3 l*b
-X-alkyl-CHP1CHP2-CH2P3 l*c
-X-alkyl-C(CH2P1)(CH2P2)-CaaH2aa+i l*d
-X-alkyl-CHP1-CH2P2 l*e
-X-alkyl-CHP1P2 l*f -X-alkyl-CP1P2-CaaH2aa+i l*g
-X-alkyl-C(CH2P1)(CH2P2)-CH2OCH2-C(CH2P3)(CH2P4)CH2P5 l*h
-X-alkyl-CH((CH2)aaP1)((CH2)bbP2) l*i
-X-alkyl-CHP1CHP2-CaaH2aa+i l*k
-X'-alkyl-C(CH3)(CH2P1)(CH2P2) l*m in which
alkyl denotes a single bond or straight-chain or branched alkylene having 1 to 12 C atoms, in which one or more non-adjacent CH2 groups may each be replaced, independently of one another, by -C(R00)=C(R000)-, -C≡C-, -N(R00)-, -0-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O- in such a way that O and/or S atoms are not linked directly to one another and in which, in addition, one or more H atoms may be replaced by F, CI or CN, where R00 and R000 have the meanings indicated above, aa and bb each, independently of one another, denote 0, 1 , 2, 3, 4, 5 or 6, has one of the meanings indicated for X', and
.1-5 each, independently of one another, have one of the meanings indicated for P.
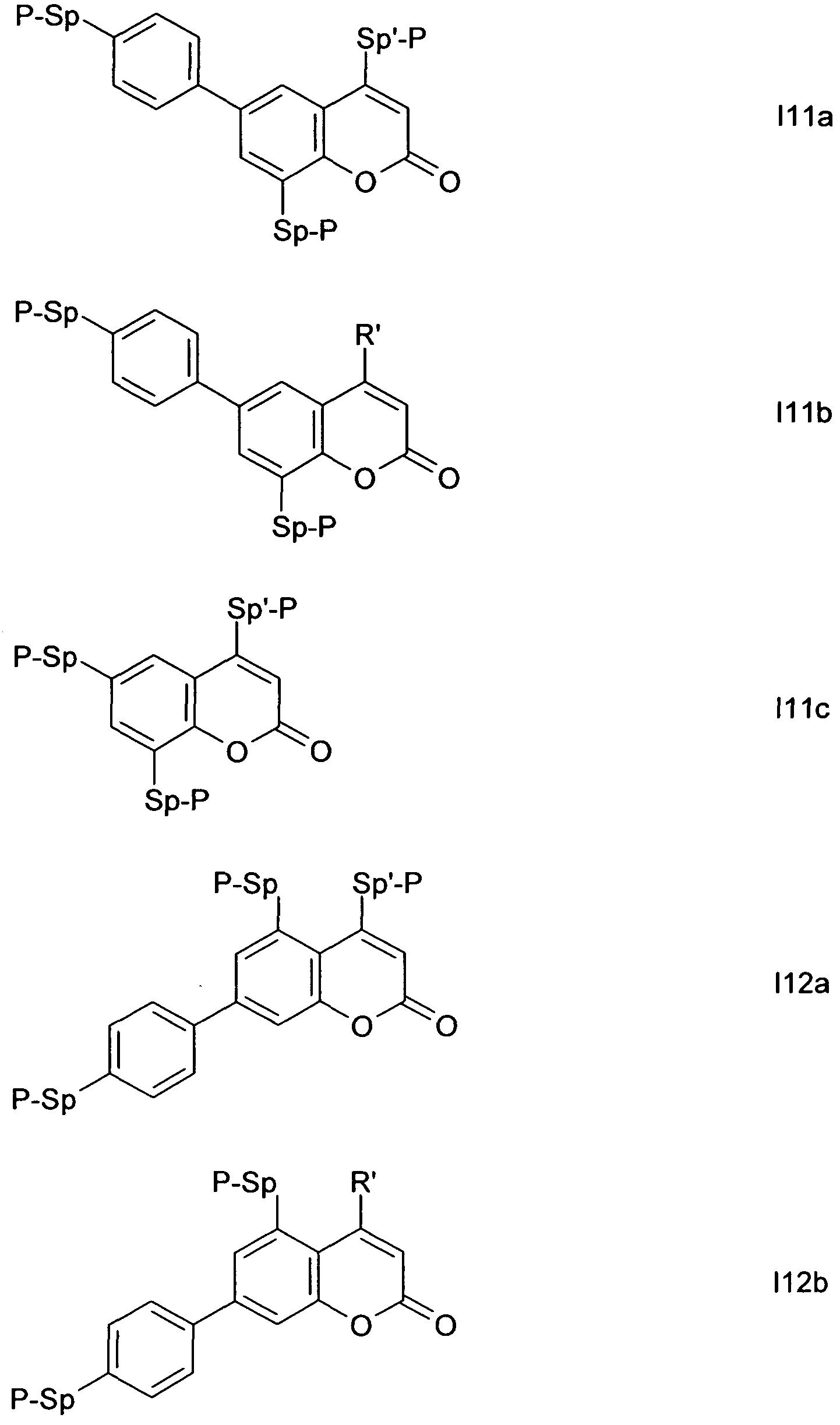
Formula I covers the following compounds
wherein R
a, R°, A
1, A
2, Z , X
1, M, n1 and x are as defined in formula I. Compounds of formula lb and lc are especially preferred.
Particularly preferred compounds of the formula I and sub-formulae thereof indicated above and below are those which contain more than two, preferably three, four, five or six groups Ra or Rb which denote P or P-Sp-.
Particularly preferred compounds of the formula I and sub-formulae thereof indicated above and below are those in which
A1, A2, A3, A4 each, independently of one another, when being different from a single bond, denote 1 ,4-phenylene, naphthalene-1 ,4- diyl or naphthalene-2,6-diyl, where one or more CH groups in these groups are optionally replaced by N, cyclohexane-1 ,4- diyl, in which, in addition, one or more non-adjacent CH2 groups are optionally replaced by O and/or S, 1 ,4- cyclohexenylene, bicyclo[1.1.1]pentane-1 ,3-diyl, bicyclo- [2.2.2]octane-1 ,4-diyl, spiro[3.3]heptane-2,6-diyl, piperidine- 1 ,4-diyl, decahydronaphthalene-2,6-diyl, 1 ,2,3,4-tetrahydro- naphthalene-2,6-diyl, indane-2,5-diyl or octahydro-4,7- methanoindane-2,5-diyl, where all these groups are un- substituted or mono- or polysubstituted by L,
L denotes P, P-Sp-, OH, CH2OH, F, CI, Br, I, -CN, -NO2, -NCO,
-NCS, -OCN, -SCN, -C(=O)N(Rx)2, -C(=O)Y1, -C(=O)Rx, -N(RX)2, optionally substituted silyl, optionally substituted aryl having 6 to 20 C atoms, straight-chain or branched alkyl or alkoxy having 1 to 25 C atoms, or straight-chain or branched alkenyl, alkynyl, alkylcarbonyl, alkoxycarbonyl, alkylcarbonyl- oxy or alkoxycarbonyloxy having 2 to 25 C atoms, in which,
in addition, one or more H atoms in all these groups may be replaced by F, CI, P or P-Sp-,
Y1 denotes halogen, and
Rx denotes P, P-Sp-, H, halogen, straight-chain, branched or cyclic alkyl having 1 to 25 C atoms, in which, in addition, one or more non-adjacent CH2 groups may be replaced by -0-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O- in such a way that O and/or S atoms are not linked directly to one another, and in which, in addition, one or more H atoms may be replaced by F, CI or P-Sp-.
Further preferred compounds of the formula I and sub-formulae thereof indicated above and below are those in which
x is 2 or 3,
x is 2,
Ra and Rb denote identical or different radicals P-Sp-,
Ra and Rb denote identical or different radicals P-Sp- in which one or more radicals Sp denote a single bond,
x is 2 or 3 and all radicals Ra denote identical groups P-Sp-,
Rb denotes an unpolymerisable group, preferably selected from straight-chain or branched alkyl having 1 to 25 C atoms, in which, in addition, one or more non-adjacent CH2 groups may each be replaced, independently of one another, by -C(R00)=C(R000)-, -C≡C-, -N(R00)-, -O-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O- in such a way that O and/or S atoms are not linked directly to one another, and in which, in addition, one or more H atoms may be replaced by F, CI,
Br, I or CN,
Rb denotes P-Sp-,
Rb denotes P-Sp- wherein Sp denotes a single bond,
- Rc denotes H, Ci-8-alkyl or Ci-8-alkoxy, preferably H,
one or more of the radicals Sp denote a single bond,
one or more of the radicals Sp denotes -(CH2)pi-, -(CH2)pi-0-,
-(CH2)pi-OCO- or -(CH2)Pi-OCOO-, in which p1 denotes an integer from 1 to 12 and r1 denotes an integer from 1 to 8,
L does not denote or contain a polymerisable group,
A1 and A3 are selected from the group consisting of 1 ,4-phenylene and naphthalene-2,6-diyl, where, in addition, one or two CH groups in these rings are optionally replaced by N, and where these rings are optionally mono- or polysubstituted by L, as described above and below,
A2 is a single bond,
A4 is a single bond,
A2 and A4, when being different from a single bond, are selected from the group consisting of 1 ,4-phenylene and naphthalene-2,6-diyl, where, in addition, one or two CH groups in these rings are optionally replaced by N, and where these rings are optionally mono- or polysubstituted by L, as described above and below,
n1 = n2 = 0, and one of A2 and A4 is a single bond and the other is not a single bond, and is preferably 1,4-phenylene or naphthalene- 2,6-diyl, where, in addition, one or two CH groups in these rings are optionally replaced by N, and where these rings are optionally mono- or polysubstituted by L, as described above and below
Z1 and Z2 are selected from the group consisting of -0-, -CO-0-, -OCO-, -OCH2-, -CH20-, -CF20-, -OCF2-, -CH2CH2-, -CH=CH-, -CF=CF-, -CH=CF-, -CF=CH-, -C≡C-, single bond,
n1 is 0 or 1 , preferably 0 and A2 is a single bond,
n2 is 0 and A4 is a single bond,
n2 is 0 and A4 is not a single bond,
n2 is not 0 and is preferably 1 or 2, very preferably 1 , and A4 is a single bond,
-X2-A4-(Z2-A3)n2- does not denote -CO-C6H - (where C6H4 stands for 1 ,4-phenylene),
X2-A4-(Z2-A3)n2-Rb does not denote -CO-C6H5,
L is an unpolymerisable group, preferably selected from F, CI, -CN and straight-chain or branched alkyl having 1 to 25, particularly preferably 1 to 10, C atoms, in which, in addition, one or more non-adjacent CH2 groups may each be replaced, independently of one another, by -C(R00)=C(R000)-, -C≡C-, -N(R00)-. -0-, -S-, -CO-, -CO-O-,
-O-CO-, -O-CO-O- in such a way that O and/or S atoms are not linked directly to one another, and in which, in addition, one or more H atoms may be replaced by F, CI, Br, I or CN. Particularly preferred compounds of the formula I are selected from the group consisting of the following sub-formulae:
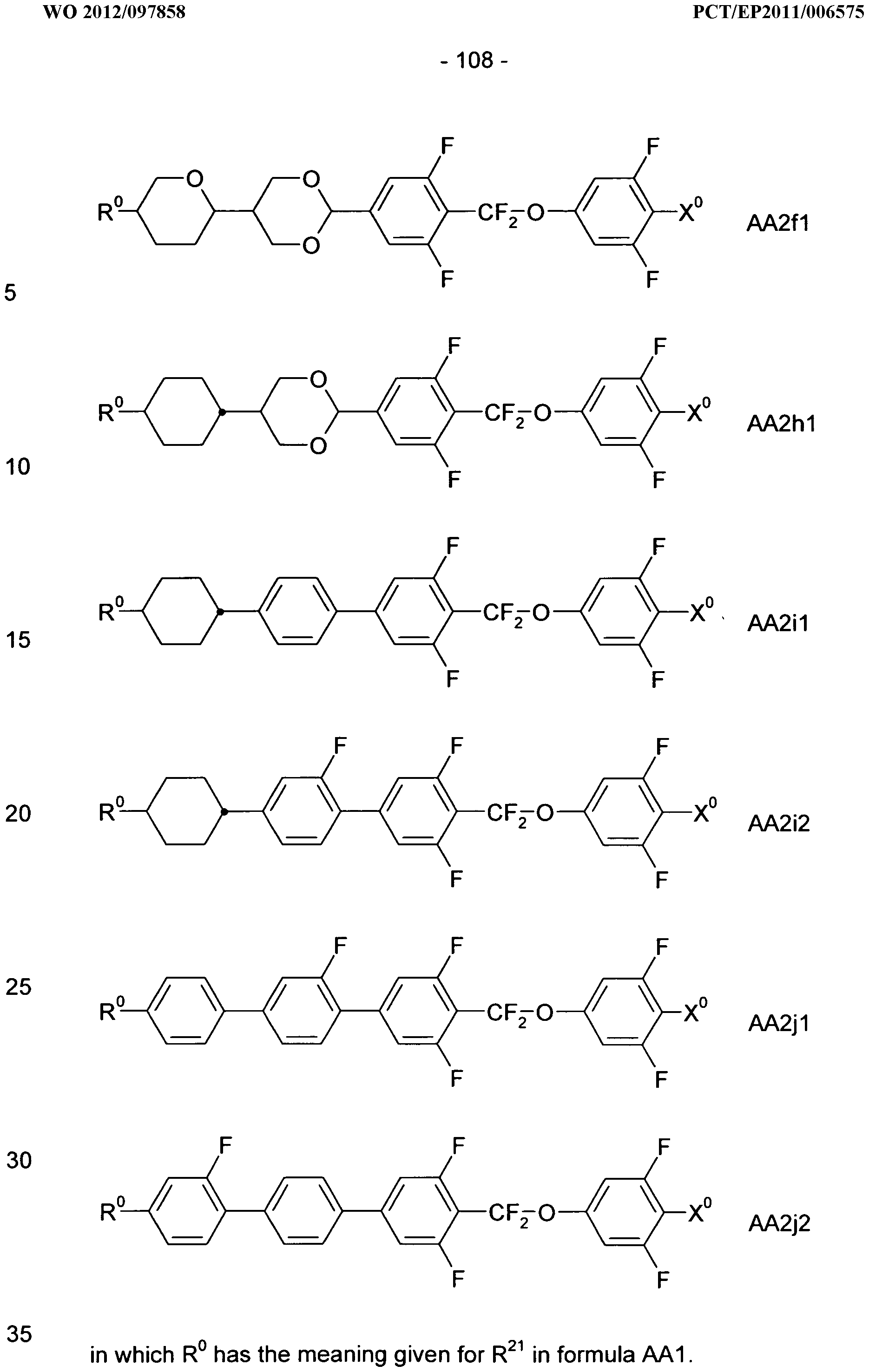
in which R
a, R
b, A
1"4, Z
1 ,2, X
1'
2, n1 and n2 each, independently of one another, have one of the meanings indicated in formula I or as indicated above and below.
Especially preferred are compounds of formulae 11 -114, in which Ra is P- Sp-, and P, Sp, Rb, A1"4, Z1,2, X1,2, n1 and n2 each, independently of one another, have one of the meanings indicated in formula I or as indicated above and below.
In case n1 or n2, respectively, is different from 0, the group -(A1-Z1)ni-A2- X1- and X2-A -(Z2-A2)n2-, respectively, in the compounds of the formulae I and 11-114 preferably denotes 1 ,4-phenylene or naphthalene-2,6-diyl, where, in addition, one or two CH groups in these rings are optionally replaced by N, and where, in addition, these rings are optionally mono- or polysubstituted by L, as described above and below.
Very preferred compounds of formulae 11 to 114 are those wherein n1 is 0 and A2 is a single bond.
Further preferred compounds of formulae 11 to 114 are those wherein n2 is 1 and A4 is a single bond.
Further preferred compounds of formulae 11 to 114 are those wherein Rb denotes P-Sp-.
Further preferred compounds of formulae 11 to 114 are those wherein Rb denotes an unpolymerisable group, preferably selected from straight-chain or branched alkyl having 1 to 25 C atoms, in which, in addition, one or more non-adjacent CH2 groups may each be replaced, independently of one another, by -C(R00)=C(R000)-, -C≡C-, -N(R00)-, -O-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O- in such a way that O and/or S atoms are not linked directly to one another, and in which, in addition, one or more H atoms may be replaced by F, CI, Br, I or CN.
Very preferred compounds of formulae 11 to 114 are those wherein Ra is P-Sp- and Sp is different from a single bond.
Further preferred compounds of formulae 11 to 114 are those wherein Ra is P-Sp- and one or both groups Sp denote a single bond.
Further preferred compounds of formulae 11 to 114 are those wherein R
b is P-Sp- and Sp denotes a single bond. Further preferred compounds of formulae 11 to 114 are those wherein R
b is P-Sp- and Sp is different from a single bond.
Very particularly preferred compounds of the formulae 11 to 114 are selected from the group consisting of the following sub-formulae:
35
in which P and Sp have one of the meanings indicated in formula I or above and below, Sp' has one of the meanings given for Sp, and is identical to or different from Sp, and R' has one of the meanings indicated for R
b in formula I or above and below which is different from H, P- and P-Sp-, and wherein the phenylene and naphthalene groups are optionally substituted with one, two, three of four F atoms.
P in the compounds of the formulae I, 11 to 114 and 11a to 114c preferably denote an acrylate, fluoroacrylate or methacrylate group.
Sp in the compounds of the formulae I, 11 to 114 and 11a to 114c preferably denote -(CH2)pi-, -0-(CH2)Pi-, -(CH2)p1-O-, -OCO-(CH2)p1-, -(CH2)p1-OCO-, -OCOO-(CH2)pi- or -(CH2)p1-OCOO-, in which p1 denotes an integer from 1 to 12, preferably from 1 to 6, and r1 denotes an integer from 1 to 8, preferably 1 , 2 or 3, where these groups are linked to P in such a way that O atoms are not linked directly to one another.
Preference is given to compounds of the formulae I, 11 to 114 and 11a to 114c wherein P denotes an acrylate, fluoroacrylate or methacrylate group, and Sp denotes -(CH2)p1-, -0-(CH2)p1-, -(CH2)pi-0-, -OCO-(CH2)p1-,
-(CH2)p1-OCO-, -OCOO-(CH2)p1- or -(CH2)Pi-OCOO-, in which p1 denotes an integer from 1 to 12, preferably from 1 to 6, and r1 denotes an integer from 1 to 8, preferably 1 , 2 or 3, where these groups are linked to P in such a way that O atoms are not linked directly to one another.
Preference is furthermore given to compounds of the sub-formulae I1a-I14c in which one or more of the radicals Sp denote a single bond and one or more of the radicals Sp are not a single bond.
Preference is furthermore given to compounds of the sub-formulae I1a-I14c in which one or more of the radicals Sp denote a single bond and one or more of the radicals Sp are not a single bond, and P denotes an acrylate, fluoroacrylate or methacrylate group.
The invention furthermore relates to novel compounds of the formula I and sub-formulae 11 to 114 in which the individual radicals have the meaning indicated in formula I or as given above and below, and wherein
a) X1 and/or X2 is a single bond, and/or
b) one of n1 and n2 is different from 0 and/or
c) one of A2 and A4 is not a single bond.
Particular preference is given to novel compounds of the formula I and sub-formulae 11 to 114 as defined above, in which n2 is not 0 and preferably denotes 1 or 2. Particular preference is furthermore given to novel compounds selected from the sub-formulae 11a to 114c as defined above.
The invention furthermore relates to novel intermediates for the preparation of compounds of the formula I, selected from the following formula:
in which G, A
1'
2, Z
1, X
1, x and n1 have the meaning indicated in formula I or above and below, and the radicals R
a and R
b each, independently of one another, denote -Sp-O-Sg, where Sp has the meaning indicated in formula I or above and below, and Sg denotes an H atom or a protecting group, and wherein preferably
a) X1 and/or X2 is a single bond, and/or
b) one of n1 and n2 is different from 0 and/or
c) one of A2 and A4 is not a single bond.
Suitable protecting groups Sg are known to the person skilled in the art. Preferred protecting groups are alkyl, acyl and alkyl- or arylsilyl groups, 2-tetrahydropyranyl or methoxymethyl.
Particularly preferred intermediates are selected from the sub-formulae 11 to 114 as indicated above, where Ra and Rb are as defined in formula I*.
Very particularly preferred intermediates are selected from the group consisting of the following sub-formulae:
I13
*a
Sp-O-Sg
in which Sp and Sg have the meanings indicated above, and Sp' has one of the meanings given for Sp, and is identical to or different from Sp. Sg particularly preferably denotes H. Particular preference is given to compounds of the formulae shown above in which one of the radicals Sp and Sp' denotes -(CH
2)
pi- or a single bond and the other denotes a single bond, where p1 is as defined above.
The compounds and intermediates of the formulae I and I* and sub-formulae thereof can be prepared analogously to processes known to the person skilled in the art and described in standard works of organic chemistry, such as, for example, in Houben-Weyl, Methoden der organi- schen Chemie [Methods of Organic Chemistry], Thieme-Verlag, Stuttgart. For example, compounds of the formula I are synthesised by esterification or etherification of the intermediates of the formula I using corresponding acids, acid derivatives, or halogenated compounds containing a group P, such as, for example, (meth)acryloyl chloride or methacrylic anhydride in the presence of a base, or (meth)acrylic acid in the presence of a dehydrating reagent, such as, for example, DCC (dicyclohexylcarbodiimide).
Particularly suitable and preferred processes for the preparation of compounds and intermediates of the formulae I and I* are depicted by way of example in the following schemes and preferably comprise one or more of the steps described below.
General access to coumarines is provided, for example, by condensation of salicylic aldehydes with phenylacetic acid derivatives in the sense of a Perkin reaction [cf. e.g. M. J. Matos et al., Bioorg. Med. Chem. Lett. 20 (2010) 5157-5160 and literature cited therein], or from salicylic aldehydes and benzonitriles (N. P. Buu-Ηοϊ, N. Hoan, M. R. Khenissi, J. Chem. Soc. 1951 , 2307) in accordance with scheme 1 for M = H (Formula 1). For substituents M other than H the aldehyde group is simply replaced by the corresponding ketone.
Scheme 1
Y = COOH, COCI, CN
An alternative approach especially for non-aromatic acetic acid derivatives goes via a Wittig reaction as published by R. S. Mali, S. G. Tilve, S. N. Yeola, A. R. Manekar, Heterocycles (1987), 26(1), 121-7 (scheme 2).
Scheme 2
Y = COOH, COCI, CN
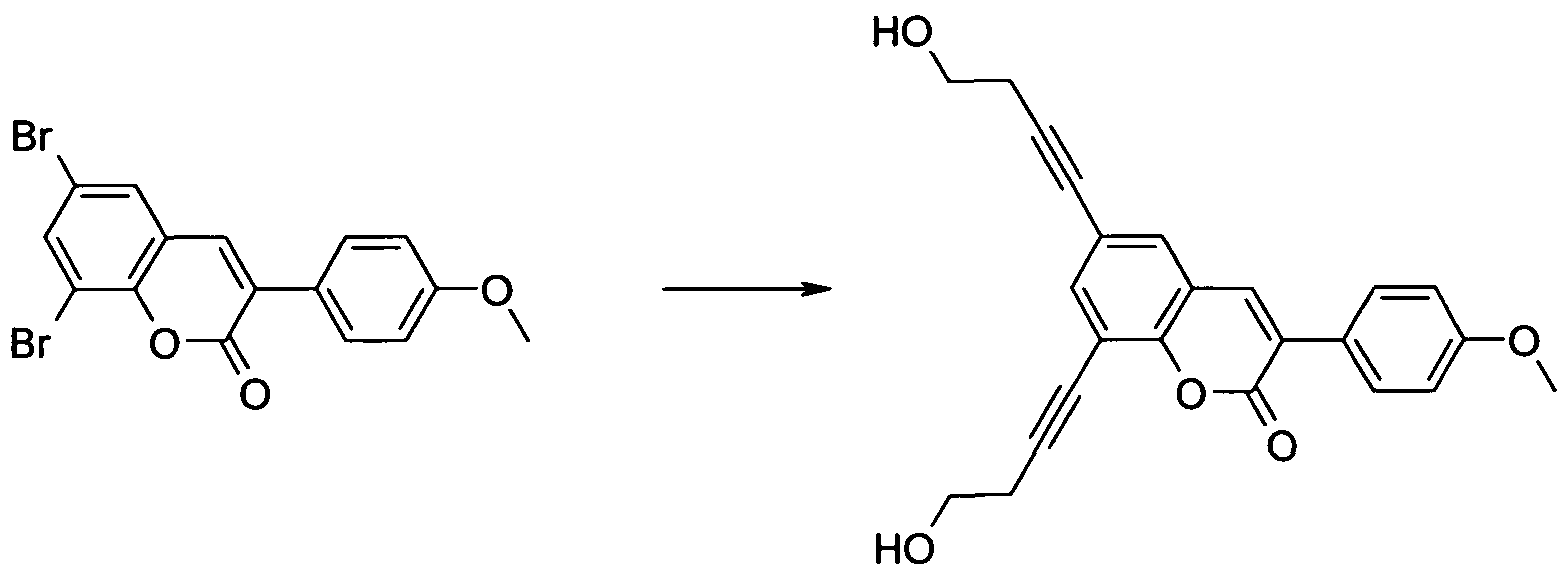
Spacer groups can be introduced e.g. by Sonogashira coupling of hydroxy alkynes and subsequent hydrogenation either before or after the Perkin reaction (scheme 3 and 4).
Scheme 3
Scheme 4
Introduction of a hydroxypropyl group by Stille coupling and subsequent hydroboration is desribed in B.S. Moon et al., Bioorg. Med. Chem. 2009, 17(9), 3479-3488. For a Heck reaction with allyl alcohol cf. R.A. Lerner et al., J. Am. Chem. Soc. 1996, 118, 11720-11724. Another general approach is given by in situ hydroboration of terminally unsaturated alcohols and Suzuki coupling as descibed e.g. in A. R. de Lera,
Tetrahedron 2001 , 57, 3125-3130. These reactions are exemplified by reactions to give the propyl homologue starting from a dibromosalicylic aldehyde (scheme 5) but are generally applicable to other isomers or congeners of salicylic aldehydes.
Scheme 5
9-BBN
In the above scheme it can be advantageous to protect the salicylic aldehyde, esp. the aldehyde function, e.g. as an acetal. A method of protecting both the aldehyde and the hydroxyl group together is desaibed in A. Hadfield, Synth. Comm. 1994, 24(7), 1025-1028 (Scheme 6).
Scheme 6
Isoflavones are accessible by the method of R. J. Bass, Chem. Comm. 1976, 78, by treatment of benzyl aryl ketones with boron trifluoride ether- ate in the presence of mesyl chloride in dimethylformamide. The ketones themselves can be prepared in a simple manner by carbonylating Suzuki reaction by the method of A. Suzuki et al., J. Org. Chem. 1998, 63, 4762, as shown by way of example in scheme 7:
Alternatively, isoflavones can also be obtained by the method of K.M. Dawood, Tetrahedron 2007, 63, 9642, from chromanones via the corresponding bromoisoflavones by Suzuki reaction, as shown by way of example in scheme 8:
Scheme 8
The flavones according to the invention are prepared, for example, by Baker-Venkataraman rearrangement (W. Baker, Nature 1952, 169, 706) or they can be prepared by the method of E.U. Mughal et al., Bioorg. Med. Chem. 2006, 14, 4704, from o-acylphenols by aldol condensation with aromatic aldehydes and subsequent oxidative cyclisation, as shown by way of example in Scheme 9:
Scheme 9
For the production of PSA displays, the polymerisable compounds are polymerised or crosslinked (if one compound contains two or more polymerisable groups) by in-situ polymerisation in the LC medium between the substrates of the LC display with application of a voltage. The polymerisation can be carried out in one step. It is also possible firstly to carry out the polymerisation with application of a voltage in a first step in order to produce a pretilt angle, and subsequently, in a second polymerisation step without an applied voltage, to polymerise or crosslink the compounds which have not reacted in the first step ("end curing").
Suitable and preferred polymerisation methods are, for example, thermal or photopolymerisation, preferably photopolymerisation, in particular UV photopolymerisation. One or more initiators can optionally also be added here. Suitable conditions for the polymerisation and suitable types and
amounts of initiators are known to the person skilled in the art and are described in the literature. Suitable for free-radical polymerisation are, for example, the commercially available photoinitiators Irgacure651®, Irga- cure184®, Irgacure907®, Irgacure369® or Darocure1173® (Ciba AG). If an initiator is employed, its proportion is preferably 0.001 to 5% by weight, particularly preferably 0.001 to 1% by weight.
The polymerisable compounds according to the invention are also suitable for polymerisation without an initiator, which is accompanied by consider- able advantages, such, for example, lower material costs and in particular less contamination of the LC medium by possible residual amounts of the initiator or degradation products thereof. The polymerisation can thus also be carried out without the addition of an initiator. In a preferred embodiment, the LC medium thus comprises no polymerisation initiator.
The polymerisable component A) or the LC medium may also comprise one or more stabilisers in order to prevent undesired spontaneous polymerisation of the RMs, for example during storage or transport. Suitable types and amounts of stabilisers are known to the person skilled in the art and are described in the literature. Particularly suitable are, for example, the commercially available stabilisers from the Irganox® series (Ciba AG), such as, for example, Irganox® 1076. If stabilisers are employed, their proportion, based on the total amount of RMs or the polymerisable component A), is preferably 10-500,000 ppm, particularly preferably 50- 50,000 ppm.
The LC media according to the invention for use in PSA displays preferably comprise from >0 to < 5% by weight, particularly preferably from >0 to < 1% by weight, very particularly preferably from 0.01 to 0.5% by weight, of polymerisable compounds, in particular polymerisable compounds of the formulae indicated above.
Particular preference is given to LC media comprising one, two or three polymerisable compounds according to the invention.
Preference is furthermore given to LC media in which the polymerisable component (component A) comprises exclusively polymerisable compounds according to the invention. Preference is furthermore given to LC media in which component B) is an LC compound or an LC mixture which has a nematic liquid-crystal phase.
Preference is furthermore given to achiral polymerisable compounds according to the invention and LC media in which the compounds of com- ponent A) and/or B) are selected exclusively from the group consisting of achiral compounds.
Preference is furthermore given to LC media in which the polymerisable component or component A) comprises one or more polymerisable com- pounds according to the invention containing one polymerisable group (monoreactive) and one or more polymerisable compounds according to the invention containing two or more, preferably two, polymerisable groups (di- or multireactive). Preference is furthermore given to PSA displays and LC media in which the polymerisable component or component A) comprises exclusively polymerisable compounds according to the invention containing two polymerisable groups (direactive). The proportion of the polymerisable component or component A) in the LC media according to the invention is preferably from > 0 to < 5%, particularly preferably from > 0 to < 1%, very particularly preferably from 0.01 to 0.5%. The proportion of the liquid-crystalline component or component B) in the LC media according to the invention is preferably from 95 to < 100%, particularly preferably from 99 to <100%.
The polymerisable compounds according to the invention can be polym- erised individually, but it is also possible to polymerise mixtures which comprise two or more polymerisable compounds according to the inven-
tion, or mixtures comprising one or more polymerisable compounds according to the invention and one or more further polymerisable compounds (comonomers), which are preferably mesogenic or liquid- crystalline. In the case of polymerisation of such mixtures, copolymers form. The invention furthermore relates to the polymerisable mixtures mentioned above and below. The polymerisable compounds and co- monomers are mesogenic or non-mesogenic, preferably mesogenic or liquid-crystalline. Suitable and preferred mesogenic comonomers, particularly for use in PSA displays, are selected, for example, from the following formulae:
in which the individual radicals have the following meanings:
P1 and P2 each, independently of one another, denote a polymerisable group, preferably having one of the meanings indicated above and below for P, particularly preferably an acrylate, methacrylate, fluoroacrylate, oxetane, vinyl, vinyloxy or epoxide group,
Sp1 and Sp2 each, independently of one another, denote a single bond or a spacer group, preferably having one of the meanings indicated above and below for Sp, and particularly preferably denote -(CH2)pi-, -(CH2)Pi-0-, -(CH2)pi-CO-0- or -(CH2)p1-O- CO-0-, in which p1 is an integer from 1 to 12, and where the linking to the adjacent ring in the last-mentioned groups takes place via the O atom,
where, in addition, one or more of the radicals P1-Sp1- and P2-Sp2- may denote Raa, with the proviso that at least one of the radicals P1-Sp1- and P2-Sp2- present does not denote Raa,
Raa denotes H, F, CI, CN or straight-chain or branched alkyl hav- ing 1 to 25 C atoms, in which, in addition, one or more non- adjacent CH2 groups may each be replaced, independently of one another, by C(R°)=C(R00)-> -C≡C-, -N(R0)-, -0-, -S-, -CO-, -CO-O-, -O-CO-, -O-CO-O- in such a way that O and/or S atoms are not linked directly to one another, and in which, in addition, one or more H atoms may be replaced by
F, CI, CN or P1-Sp1-, particularly preferably straight-chain or branched, optionally mono- or polyfluorinated alkyl, alkoxy, alkenyl, alkynyl, alkylcarbonyl, alkoxycarbonyl, alkylcarbonyl- oxy or alkoxycarbonyloxy having 1 to 12 C atoms (where the alkenyl and alkynyl radicals have at least two C atoms and the branched radicals have at least three C atoms),
R°, R00 each, independently of one another and identically or differently on each occurrence, denote H or alkyl having 1 to 12 C 3t0mS'
Ry and Rz each, independently of one another, denote H, F, CH3 or CF3,
Z1 denotes -O-, -CO-, -C(RyRz)- or -CF2CF2-,
Z2 and Z3 each, independently of one another, denote -CO-O-, -O-CO-,
-CH2O-, -OCH2-, -CF2O-, -OCF2- or -(CH2)n-, where n is 2, 3 or 4,
L on each occurrence, identically or differently, denotes F, CI,
CN or straight-chain or branched, optionally mono- or poly- fluorinated alkyl, alkoxy, alkenyl, alkynyl, alkylcarbonyl, alkoxycarbonyl, alkylcarbonyloxy or alkoxycarbonyloxy having 1 to 12 C atoms, preferably F,
L' and L" each, independently of one another, denote H, F or CI, r denotes 0, 1 , 2, 3 or 4,
s denotes 0, 1 , 2 or 3,
t denotes 0, 1 or 2,
x denotes 0 or 1.
In the compounds of formulae M1 to M29
wherein L on each occurrence, identically or differently, has one of the meanings given above or below, and is preferably F, CI, CN, NO
2, CH
3, C2H5, C(CH
3)
3, CH(CH
3)
2> CH
2CH(CH
3)C
2H5, OCH
3, OC
2H
5> COCH
3,
COC2H5, COOCH3, COOC2H5, CF3, OCF3, OCHF2, OC2F5 or P-Sp-, very preferably F, CI, CN, CH3, C2H5, OCH3, COCH3, OCF3 or P-Sp-, more preferably F, CI, CH3, OCH3l COCH3 oder OCF3 , especially F or CH3.
Besides the polymerisable compounds described above, the LC media for use in the LC displays according to the invention comprise an LC mixture ("host mixture") comprising one or more, preferably two or more, low-molecular-weight (i.e. monomeric or unpolymerised) compounds. The latter are stable or unreactive to a polymerisation reaction under the conditions used for polymerisation of the polymerisable compounds. In principle, any LC mixture which is suitable for use in conventional VA and OCB displays is suitable as host mixture. Suitable LC mixtures are known to the person skilled in the art and are described in the literature, for example mixtures in VA displays in EP 1 378 557 A1 and mixtures for OCB displays in EP 1 306 418 A1 and DE 102 24 046 A1.
The polymerisable compounds of formula I are especially suitable for use in an LC host mixture that comprises one or more compounds having a terminal alkenyl group, where they show improved properties, like solubility, reactivity or capability of generating a tilt angle, compared to reactive mesogens known from prior art.
In a first preferred embodiment the LC medium contains an LC host mixture based on compounds with negative dielectric anisotropy. Such LC media are especially suitable for use in PSA-VA displays. Particularly preferred embodiments of such an LC medium are those of sections a)-x) below: a) LC medium which comprises one or more compounds of the formu- lae CY and/or PY:
wherein a denotes 1 or 2,
denotes 0 or 1 ,
R1 and R2 each, independently of one another, denote alkyl having 1 to 12 C atoms, where, in addition, one or two non-adjacent CH2 groups may be replaced by -0-, -CH=CH-, -CO-, -OCO- or -COO- in such a way that O atoms are not linked directly to one another, preferably alkyl or alkoxy having 1 to 6 C atoms,
Zx and Zy each, independently of one another, denote -CH2CH2-,
-CH=CH-, -CF2O-, -OCF2-, -CH2O-, -OCH2-, -CO-O-, -O-CO-, -C2F4-, -CF=CF-, -CH=CH-CH2O- or a single bond, preferably a single bond, each, independently of one another, denote F, CI, OCF3 CF3, CH3, CH2F, CHF2.
Preferably, both L1 and L2 denote F or one of L1 and L2 denotes F and the other denotes CI, or both L3 and L4 denote F or one of L3 and L4 denotes F and the other denotes CI.
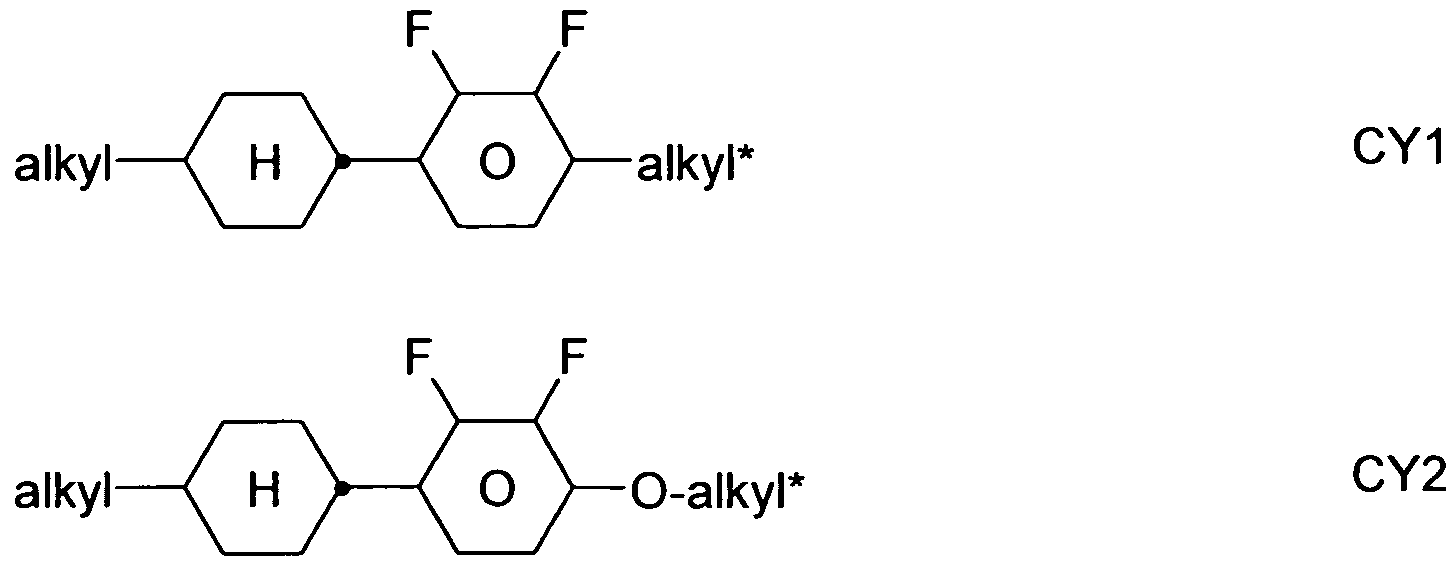
The compounds of the formula CY are preferably selected from the group consisting of the following sub-formulae:
in which a denotes 1 or 2, alkyl and alkyl
* each, independently of one another, denote a straight-chain alkyl radical having 1-6 C atoms, and alkenyl denotes a straight-chain alkenyl radical having 2-6 C atoms, and (O) denotes an oxygen atom or a single bond. Alkenyl preferably denotes CH
2=CH-, CH2=CHCH
2CH
2-, CH
3-CH=CH-,
CH
3-CH
2-CH=CH-, CH3-(CH
2)2-CH=CH-, CH
3-(CH
2)
3-CH=CH- or CH
3-CH=CH-(CH
2)
2-.
The compounds of the formula PY are preferably selected from the group consisting of the following sub-formulae:
F F alkyl— ( H >— C2H4— O O )— (O)alkyl* PY18
in which alkyl and alkyl* each, independently of one another, denote a straight-chain alkyl radical having 1-6 C atoms, and alkenyl denotes a straight-chain alkenyl radical having 2-6 C atoms, and (O) denotes an oxygen atom or a single bond. Alkenyl preferably denotes CH
2=CH-, CH
2=CHCH
2CH
2-, CH
3-CH=CH-, CH
3-CH
2- CH=CH-, CH
3-(CH
2)2-CH=CH-, CH
3-(CH
2)
3-CH=CH- or CH
3-CH=CH- (CH
2)
2-.
LC medium which additionally comprises one or more compounds of the following formula:
R° D y~ R ZK
in which the individual radicals have the following meanings:
denotes— ( H >— or — O
R3 and R4 each, independently of one another, denote alkyl having 1 to 12 C atoms, in which, in addition, one or two non-adjacent CH2 groups may be replaced by -0-, -CH=CH-, -CO-, -O-CO- or -CO-O- in such a way that O atoms are not linked directly to one another, denotes -CH2CH2-, -CH=CH-, -CF2O-, -OCF2-, -CH2O- -OCH2-, -CO-O-, -O-CO-, -C2F4-, -CF=CF-, -CH=CH- CH2O- or a single bond, preferably a single bond.
The compounds of the formula ZK are preferably selected from the group consisting of the following sub-formulae:
alkyl— ( H — Η - O-alkyl* ZK2
alkenyl— lH >— ( H V- alkyl ZK3
in which alkyl and alkyl* each, independently of one another, denote a straight-chain alkyl radical having 1-6 C atoms, and alkenyl denotes a straight-chain alkenyl radical having 2-6 C atoms. Alkenyl preferably denotes CH
2=CH-, CH
2=CHCH
2CH
2-, CH
3-CH=CH-, CH
3- CH
2-CH=CH-, CH
3-(CH
2)
2-CH=CH-, CH
3-(CH
2)
3-CH=CH- or CH
3- CH=CH-(CH
2)
2-.
LC medium which additionally comprises one or more compounds of the following formula:
in which the individual radicals on each occurrence, identically or
differently, have the following meanings: each, independently of one another, have one of the meanings indicated above for R1, or — ( o
The compounds of the formula DK are preferably selected from the group consisting of the following sub-formulae:
alkyl— < H → O )—
( O )— alkyl* DK4
in which alkyi and alkyi
* each, independently of one another, denote a straight-chain alkyi radical having 1-6 C atoms, and alkenyl and alkenyl
* each, independently of one another, denote a straight-chain alkenyl radical having 2-6 C atoms. Alkenyl and alkenyl
* preferably denote CH
2=CH-, CH2=CHCH
2CH
2-, CH
3-CH=CH-, CH
3-CH
2-
CH=CH-, CH3-(CH
2)2-CH=CH-, CH
3-(CH
2)
3-CH=CH- or CH
3-CH=CH- (CH
2)
2-.
LC medium which additionally comprises one or more compounds of the following formula:
in which the individual radicals have the following meanings:
R1 and R2 each, independently of one another, denote alkyl having 1 to 12 C atoms, where, in addition, one or two non- adjacent CH2 groups may be replaced by -0-, -CH=CH-, -CO-, -OCO- or -COO- in such a way that O atoms are not linked directly to one another,
Zx and Zy each, independently of one another, denote -CH2CH2-,
-CH=CH-, -CF2O-, -OCF2-, -CH2O-, -OCH2-, -CO-O-, -O-CO-, -C2F4-, -CF=CF-, -CH=CH-CH2O- or a single bond, preferably a single bond,
L and L2 each, independently of one another, denote F, CI, OCF3,
CF3, CH3, CH2F, CHF2.
Preferably, both radicals L1 and L2 denote F or one of the radicals L1 and L2 denotes F and the other denotes CI.
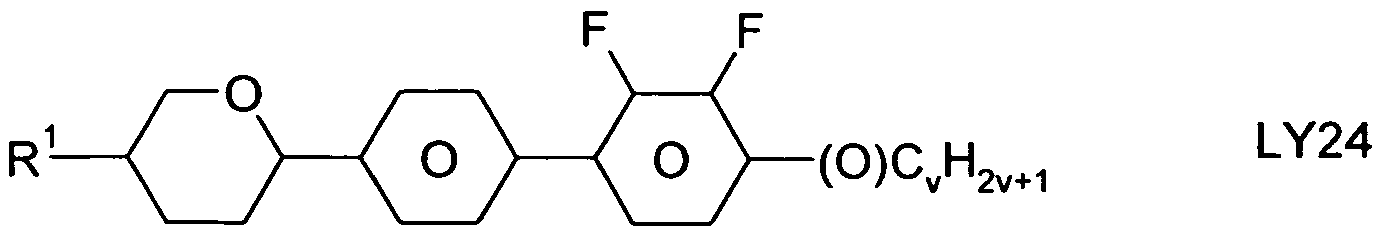
The compounds of the formula LY are preferably selected from the group consisting of the following sub-formulae:
in which R
1 has the meaning indicated above, alkyl denotes a straight-chain alkyl radical having 1-6 C atoms, (O) denotes an oxygen atom or a single bond, and v denotes an integer from 1 to 6. R
1 preferably denotes straight-chain alkyl having 1 to 6 C atoms or straight-chain alkenyl having 2 to 6 C atoms, in particular CH
3, C2H5, n-C
3H
7> n-C
4H
9, n-CsHn, CH
2=CH-, CH2=CHCH
2CH
2-, CH
3-CH=CH-, CH3-CH2-CI- CH-, CH
3-(CH
2)
2-CH=CH-, CH
3-(CH
2)
3-CH=CH- or CH
3-CH=CH-(CH
2)
2-. LC medium which additionally comprises one or more compounds selected from the group consisting of the following formulae:
in which alkyl denotes Ci-e-alkyl, L
x denotes H or F, and X denotes F, CI, OCF
3, OCHF
2 or OCH=CF
2. Particular preference is given to compounds of the formula G1 in which X denotes F.
LC medium which additionally comprises one or more compounds selected from the group consisting of the following formulae:
F F
R H >-OCF2- o )— (0)d-alkyl Y2
F F
CF20→: o >— (0)d-alkyl Y3
F F
OCF -C O >- (0)d-alkyl Y4
F F
R— < H O V-OCF2- O - (0)d-alkyl Y6
Y7
in which R
5 has one of the meanings indicated above for R
1, alkyl denotes Ci-e-alkyl, d denotes 0 or 1 , and z and m each, independently of one another, denote an integer from 1 to 6. R
5 in these compounds is particularly preferably Ci-6-alkyl or -alkoxy or C
2-6-alkenyl, d is preferably 1. The LC medium according to the invention preferably comprises one or more compounds of the above-mentioned formulae in amounts of > 5% by weight.
LC medium which additionally comprises one or more biphenyl compounds selected from the group consisting of the following formulae:
in which alkyl and alkyl* each, independently of one another, denote a straight-chain alkyl radical having 1-6 C atoms, and alkenyl and alkenyl* each, independently of one another, denote a straight-chain alkenyl radical having 2-6 C atoms. Alkenyl and alkenyl* preferably denote CH2=CH-, CH2=CHCH2CH2-, CH3-CH=CH-, CH3-CH2- CH=CH-, CH3-(CH2)2-CH=CH-, CH3-(CH2)3-CH=CH- or CH3-CH=CH- (CH2)2-.
The proportion of the biphenyls of the formulae B1 to B3 in the LC mixture is preferably at least 3% by weight, in particular > 5% by weight.
The compounds of the formula B2 are particularly preferred.
The compounds of the formulae B1 to B3 are preferably selected from the group consisting of the following sub-formulae:
in which alkyl
* denotes an alkyl radical having 1-6 C atoms. The medium according to the invention particularly preferably comprises one or more compounds of the formulae B1a and/or B2c. LC medium which additionally comprises one or more terphenyl compounds of the following formula:
jn which R
5 and R
6 each, independently of one another, have one of the meanings indicated above for R
1, and
each, independently of one another, denote
in which L
5 denotes F or CI, preferably F, and L
6 denotes F, CI, OCF
3, CF
3, CH
3, CH
2F or CHF
2, preferably F.
The compounds of the formula T are preferably selected from the group consisting of the following sub-formulae:
in which R denotes a straight-chain alkyl or alkoxy radical having 1-7 C atoms, R* denotes a straight-chain alkenyl radical having 2-7 C atoms, (O) denotes an oxygen atom or a single bond, and m denotes an integer from 1 to 6. R* preferably denotes CH2=CH-,
CH2=CHCH2CH2-, CH3-CH=CH-, CH3-CH2-CH=CH-, CH3-(CH2)2- CH=CH-, CH3-(CH2)3-CH=CH- or CH3-CH=CH-(CH2)2-.
R preferably denotes methyl, ethyl, propyl, butyl, pentyl, hexyl, meth- oxy, ethoxy, propoxy, butoxy or pentoxy.
The LC medium according to the invention preferably comprises the terphenyls of the formula T and the preferred sub-formulae thereof in an amount of 0.5-30% by weight, in particular 1-20% by weight.
Particular preference is given to compounds of the formulae T1 , T2, T3 and T21. In these compounds, R preferably denotes alkyl, furthermore alkoxy, each having 1-5 C atoms.
The terphenyls are preferably employed in mixtures according to the invention if the Δη value of the mixture is to be≥ 0.1. Preferred mixtures comprise 2-20% by weight of one or more terphenyl compounds of the formula T, preferably selected from the group of compounds T1 to T22.
LC medium which additionally comprises one or more compounds selected from the group consisting of the following formulae:
in which R1 and R2 have the meanings indicated above and preferably each, independently of one another, denote straight-chain alkyl having 1 to 6 C atoms or straight-chain alkenyl having 2 to 6 C atoms.
Preferred media comprise one or more compounds selected from the formulae 01 , 03 and 04.
LC medium which additionally comprises one or more compounds of the following formula:
R9 denotes H, CH3, C2H5 or n-C3H7, (F) denotes an optional fluorine substituent, and q denotes 1 , 2 or 3, and R7 has one of the meanings
indicated for R1, preferably in amounts of > 3% by weight, in particular > 5% by weight and very particularly preferably 5-30% by weight.
Particularly preferred compounds of the formula Fl are selected from the group consisting of the following sub-formulae:
in which R
7 preferably denotes straight-chain alkyl, and R
9 denotes CH
3, C
2H
5 or n-C
3H
7. Particular preference is given to the compounds of the formulae FI1 , FI2 and FI3.
LC medium which additionally comprises one or more compounds selected from the group consisting of the following formulae:
in which R
8 has the meaning indicated for R
1, and alkyl denotes a straight-chain alkyl radical having 1-6 C atoms.
LC medium which additionally comprises one or more compounds which contain a tetrahydronaphthyl or naphthyl unit, such as, for example, the compounds selected from the group consisting of the following formulae:
in which R
10 and R
1 each, independently of one another, have one of the meanings indicated for R
1, preferably denote straight-chain alkyl or alkoxy having 1 to 6 C atoms or straight-chain alkenyl having 2 to 6 C atoms, and Z
1 and Z
2 each, independently of one another, denote -C
2H
4-, -CH=CH-, -(CH
2)
4-, -(CH
2)
3O-, -O(CH
2)
3-, -CH=CH- CH
2CH
2-, -CH
2CH
2CH=CH-, -CH
20-, -OCH
2-, -CO-O-, -0-CO-, -C
2F
4-, -CF=CF-, -CF=CH-, -CH=CF-, -CH
2- or a single bond.
LC medium which additionally comprises one or more difluoro- dibenzochromans and/or chromans of the following formulae:
in which
R11 and R12 each, independently of one another, have the meanings indicated above,
ring M is trans-1 ,4-cyclohexylene or 1 ,4-phenylene,
Zm -C2H4-, -CH2O-, -OCH2-, -CO-O- or -O-CO-,
c is 0 or 1 ,
preferably in amounts of 3 to 20% by weight, in particular in amounts of 3 to 15% by weight.
Particularly preferred compounds of the formulae BC, CR and RC are selected from the group consisting of the following sub-formulae:
in which alkyi and alkyi
* each, independently of one another, denote a straight-chain alkyi radical having 1-6 C atoms, (O) denotes an oxygen atom or a single bond, c is 1 or 2, and alkenyl and alkenyl
* each, independently of one another, denote a straight-chain alkenyl radical having 2-6 C atoms. Alkenyl and alkenyl
* preferably denote CH
2=CH-, CH
2=CHCH
2CH
2-, CH
3-CH=CH-, CH
3-CH
2-CH=CH-, CH
3- (CH
2)
2-CH=CH-, CH
3-(CH
2)
3-CH=CH- or CH
3-CH=CH-(CH
2)
2-.
Very particular preference is given to mixtures comprising one, two or three compounds of the formula BC-2.
LC medium which additionally comprises one or more fluorinated phenanthrenes and/or dibenzofurans of the following formulae:
PH
in which R
11 and R
2 each, independently of one another, have the meanings indicated above, b denotes 0 or 1 , L denotes F, and r denotes 1, 2 or 3.
Particularly preferred compounds of the formulae PH and BF are selected from the group consisting of the following sub-formulae:
in which R and R" each, independently of one another, denote a straight-chain alkyl or alkoxy radical having 1-7 C atoms.
LC medium which, apart from the polymerisable compounds according to the invention, in particular of the formula I or sub-formulae
thereof and the comonomers, comprises no compounds which contain a terminal vinyloxy group (-0-CH=CH2). r) LC medium which comprises 1 to 5, preferably 1 , 2 or 3, polymeri- sable compounds, preferably selected from polymerisable compounds according to the invention, in particular of the formula I or sub-formulae thereof . s) LC medium in which the proportion of polymerisable compounds, in
particular of the formula I or sub-formulae thereof, in the mixture as a whole is 0.05 to 5%, preferably 0.1 to 1%. t) LC medium which comprises 1 to 8, preferably 1 to 5, compounds of the formulae CY1 , CY2, PY1 and/or PY2. The proportion of these compounds in the mixture as a whole is preferably 5 to 60%, particularly preferably 10 to 35%. The content of these individual compounds is preferably in each case 2 to 20%. u) LC medium which comprises 1 to 8, preferably 1 to 5, compounds of the formulae CY9, CY10, PY9 and/or PY10. The proportion of these compounds in the mixture as a whole is preferably 5 to 60%, particularly preferably 10 to 35%. The content of these individual compounds is preferably in each case 2 to 20%. v) LC medium which comprises 1 to 10, preferably 1 to 8, compounds of the formula ZK, in particular compounds of the formulae ZK1 , ZK2 and/or ZK6. The proportion of these compounds in the mixture as a whole is preferably 3 to 25%, particularly preferably 5 to 45%. The content of these individual compounds is preferably in each case 2 to 20%. w) LC medium in which the proportion of compounds of the formulae
CY, PY and ZK in the mixture as a whole is greater than 70%, preferably greater than 80%. x) LC medium in which the LC host mixture contains one or more
compounds containing an alkenyl group, preferably selected from the
group consisting of formula CY, PY and LY, wherein one or both of R1 and R2 denote straight-chain alkenyl having 2-6 C atoms, formula ZK and DK, wherein one or both of, R3 and R4 denote straight-chain alkenyl having 2-6 C atoms, and formula B2 and B3, very preferably selected from formulae CY15, CY16, CY34, CY32, PY15, PY16,
ZK3, ZK4, DK3, DK6, B2 and B3, most preferably selected from formulae ZK3, ZK4, B2 and B3. The concentration of these compounds in the LC host mixture is preferably from 2 to 70%, very preferably from 3 to 55%. y) PSA-VA display in which the pretilt angle is preferably < 85°, particularly preferably < 80°.
In a second preferred embodiment the LC medium contains an LC host mixture based on compounds with positive dielectric anisotropy. Such LC media are especially suitable for use in PSA-OCB-, PSA-TN-, PSA-Posi- VA-, PSA-IPS- Oder PSA-FFS-displays.
Particularly preferred is an LC medium of this second preferred
embodiment, which contains one or more compounds selected from the group consisting of compounds of formula AA and BB
and optionally contains, in addition to the compounds of formula AA and/or BB, one or more compounds of formula CC
in which the individual radicals have the following meanings: each, independently
of one another, and on each occurrence, identically or differently
on each occurrence, identically or differently
R21, R31, R41, R42 each, independently of one another, alkyl, alkoxy, oxaalkyl or fluoroalkyl having 1 to 9 C atoms or alkenyl having 2 to 9 C atoms,
X° F, CI, halogenated alkyl or alkoxy having 1 to 6 C atoms or halogenated alkenyl or alkenyloxy having 2 to 6 C atoms,
Z31 -CH2CH2-, -CF2CF2-, -COO-, irans-CH=CH-, trans-
CF=CF-, -CH20- or a single bond, preferably -CH2CH2-, -COO-, trans-CH=CH- or a single bond, particularly preferably -COO-, frans-CH=CH- or a single bond,
Z41, Z42 -CH2CH2-, -COO-, frans-CH=CH-, frans-CF=CF-, -CH20-,
-CF2O-, -C≡C- or a single bond, preferably a single bond,
L21 L 22 L31 L 32 |-| or g 1 , 2 or 3, h 0, 1 , 2 or 3.
X° is preferably F, CI, CF3) CHF2, OCF3, OCHF2, OCFHCF3, OCFHCHF2, OCFHCHF2, OCF2CH3, OCF2CHF2, OCF2CHF2) OCF2CF2CHF2,
OCF2CF2CHF2, OCFHCF2CF3, OCFHCF2CHF2, OCF2CF2CF3,
OCF2CF2CCIF2, OCCIFCF2CF3 or CH=CF2, very preferably F or OCF3
The compounds of formula AA are preferably selected from the group consisting of the following formulae:
AA1
in which A21, R21, X°, L21 and L22 have the meanings given in formula AA, L2' and L24 each, independently of one another, are H or F, and X° is preferably F. Particularly preferred are compounds of formulae AA1 and AA2.
Particularly preferred compounds of formula AA1 are selected from the group consisting of the following subformulae
AA1a
in which R° has one of the meanings given for R21 in formula AA1 , X°, L21 and L22 have the meaning given in formula AA1 , L23, L24, L25 and L26 are each, independently of one another, H or F, and X° is preferably F.
Very particularly preferred compounds of formula AA1 are selected from the group consisting of the following subformulae:
In which R° has the meaning given for R21 in formula AA1.
Very preferred compounds of formula AA2 are selected from the group consisting of the following subformulae
in which R° has the meaning given for R
21 in formula AA1 , X°, L
21 and L
22 have the meaning given in formula AA, L
23, L
24, L
25 and L
26 each, independently of one another, are H or F, and X° is preferably F.
Very particularly preferred compounds of formula AA2 are selected from the group consisting of the following subformulae
Particularly preferred compounds of formula AA3 are selected from the group consisting of the following subformulae
in which R° has the meaning given for R
21 in formula AA1 , X°, L
21 and L have the meaning given in formula AA3, and X° is preferably F.
Particularly preferred compounds of formula AA4 are selected from the group consisting of the following subformulae
in which R° has the meaning given for R
21 in formula AA .
The compounds of formula BB are preferably selected from the group consisting of the following formulae:
in which A
31, A
32, R
31, X°, L
31 and L
32 have the meanings given in formula BB, and X° is preferably F. Particularly preferred are compounds of formulae BB1 and BB2.
Particularly preferred compounds of formula BB1 are selected from the group consisting of the following subformulae
in which R
3 has the meaning given for R
31 in formula BB1 , X°, L
31 and L
32 have the meaning given in formula BB1 , and X° is preferably F. Very particularly preferred compounds of formula BB1a are selected from the group consisting of the following subformulae
in which R
3 has the meaning given for R
3 in formula BB1.
Very particularly preferred compounds of formula BB1b are selected from the group consisting of the following subformulae
" Vco-o- 0-CF
3 BB1 b1
in which R
3 has the meaning given for R
31 in formula BB1.
Particularly preferred compounds of formula BB2 are selected from the group consisting of the following subformulae
in which R° has one of the meanings given for R
21 in formula BB2, X°, L
31 and L
32 have the meaning given in formula BB2, L
33, L
34, L
35 and L
36 are each, independently of one another, H or F, and X° is preferably F.
Very particularly preferred compounds of formula BB2 are selected from the group consisting of the following subformulae:
in which R
3 has the meaning given for R
31 in formula BB2.
Very particularly preferred compounds of formula BB2b are selected from the group consisting of the following subformulae
in which R
3 has the meaning given for R in formula BB2.
Very particularly preferred compounds of formula BB2c are selected from the group consisting of the following subformulae
« / 0-CF
3
in which R has the meaning given for R in formula BB2.
Very particularly preferred compounds of formula BB2d and BB2e are selected from the group consisting of the following subformulae
in which R
3 has the meaning given for R
31 in formula BB2.
Very particularly preferred compounds of formula BB2f are selected from the group consisting of the following subformulae
in which R has the meaning given for R in formula BB2.
Very particularly preferred compounds of formula BB2g are selected from the group consisting of the following subformulae
in which R
3 has the meaning given for R
31 in formula BB2.
Very particularly preferred compounds of formula BB2h are selected from the group consisting of the following subformulae
BB2h1
in which R
3 has the meaning given for R
31 in formula BB2.
Very particularly preferred compounds of formula BB2i are selected from the group consisting of the following subformulae
in which R
3 has the meaning given for R in formula BB2.
Very particularly preferred compounds of formula BB2k are selected from the group consisting of the following subformulae
in which R
3 has the meaning given for R
31 in formula BB2.
Alternatively to, or in addition to, the compounds of formula BB1 and/or BB2 the LC media may also comprise one or more compounds of formula BB3 as defined above.
Particularly preferred compounds of formula BB3 are selected from the group consisting of the following subformulae
in which R
3 has the meaning given for R
31 in formula BB3.
Preferably the LC media according to this second preferred embodiment comprise, in addition to the compounds of formula AA and/or BB, one or more dielectrically neutral compounds having a dielectric anisotropy in the range from -1.5 to +3, preferably selected from the group of compounds of formula CC as defined above. Particularly preferred compounds of formula CC are selected from the group consisting of the following subformulae
35
ln which R
41 and R
42 have the meanings given in formula CC, and preferably denote each, independently of one another, alkyl, alkoxy, fluorinated alkyl or fluorinated alkoxy with 1 to 7 C atoms, or alkenyl, alkenyloxy, alkoxyalkyi or fluorinated alkenyl with 2 to 7 C atoms, and L
4 is H or F.
Preferably the LC media according to this second preferred embodiment comprise, in addition or alternatively to the dielectrically neutral compounds of formula CC, one or more dielectrically neutral compounds having a dielectric anisotropy in the range from -1.5 to +3, selected from the group of compounds of formula DD.
In which A
41, A
42, Z
41, Z
42, R
41, R
42 and h have the meanings given in formula CC.
Particularly preferred compounds of formula DD are selected from the group consisting of the following subformulae
in which R
41 and R
42 have the meanings given in formula DD and R
41 preferably denotes alkyl bedeutet, and in formula DD1 R preferably denotes alkenyl, particularly preferably vorzugsweise -(CH
2)2-CH=CH-CH3, aanndd iinn ffoorrmmuullaa DDDD22 I R preferably denotes alkyl, -(CH
2)2-CH=CH
2 or (CH
2)
2-CH=CH-CH
3.
The compounds of formula AA and BB are preferably used in the LC medium according to the invention in a concentration from 2% to 60%, more preferably from 3% to 35%, and very particularly preferably from 4% to 30% in the mixture as a whole.
The compounds of formula CC and DD are preferably used in the LC medium according to the invention in a concentration from 2% to 70%, more preferably from 5% to 65%, even more preferably from 10% to 60%, and very particularly preferably from10%, preferably 15%, to 55% in the mixture as a whole.
The combination of compounds of the preferred embodiments a)-y) mentioned above with the polymerised compounds described above causes low threshold voltages, low rotational viscosities and very good low-temperature stabilities in the LC media according to the invention at the same
time as constantly high clearing points and high HR values, and allows the rapid establishment of a particularly low pretilt angle in PSA displays. In particular, the LC media exhibit significantly shortened response times, in particular also the grey-shade response times, in PSA displays compared with the media from the prior art.
The liquid-crystal mixture preferably has a nematic phase range of at least 80 K, particularly preferably at least 100 K, and a rotational viscosity of not greater than 250 mPa-s, preferably not greater than 200 mPa s, at 20°C.
In the VA-type displays according to the invention, the molecules in the layer of the LC medium in the switched-off state are aligned perpendicular to the electrode surfaces (homeotropically) or have a a tilted homeotropic alignment. On application of an electrical voltage to the electrodes, a re- alignment of the LC molecules takes place with the longitudinal molecular axes parallel to the electrode surfaces.
LC media according to the invention based on compounds with negative dielectric anisotropy according to the first preferred embodiment, in particular for use in displays of the PSA-VA type, have a negative dielectric anisotropy Δε, preferably from -0.5 to -10, in particular from -2.5 to -7.5, at 20°C and 1 kHz.
The birefringence Δη in LC media according to the invention for use in displays of the PSA-VA type is preferably below 0.16, particularly preferably from 0.06 to 0.14, very particularly preferably from 0.07 to 0,12.
In the OCB-type displays according to the invention, the molecules in the layer of the LC medium have a "bend" alignment. On application of an electrical voltage, a realignment of the LC molecules takes place with the longitudinal molecular axes perpendicular to the electrode surfaces.
LC media according to the invention for use in displays of the PSA-OCB type are preferably those based on compounds with positive dielectric anisotropy according to the second preferred embodiment, and preferably have a positive dielectric anisotropy Δε from +4 to +17 at 20°C and 1 kHz.
The birefringence Δη in LC media according to the invention for use in displays of the PSA-OCB type is preferably from 0.14 to 0.22, particularly preferably from 0.16 to 0.22.
LC media according to the invention, based on compounds with positive dielectric anisotropy according to the second preferred embodiment, for use in displays of the PSA-TN-, PSA-posi-VA-, PSA-IPS- Oder PSA-FFS- type, preferably have a positive dielectric anisotropy Δε from +2 to +30, particularly preferably from +3 to +20, at 20°C and 1 kHz.
The birefringence Δη in LC media according to the invention for use in displays of the PSA-TN-, PSA-posi-VA-, PSA-IPS- Oder PSA-FFS-type is preferably from 0.07 to 0.15, particularly preferably from 0.08 to 0.13.
The LC media according to the invention may also comprise further additives which are known to the person skilled in the art and are described in the literature, such as, for example, polymerisation initiators, inhibitors, stabilisers, surface-active substances or chiral dopants. These may be polymerisable or non-polymerisable. Polymerisable additives are accordingly ascribed to the polymerisable component or component A). Non- polymerisable additives are accordingly ascribed to the non-polymerisable component or component B). In a preferred embodiment the LC media contain one or more chiral dopants, preferably in a concentration from 0.01 to 1 %, very preferably from 0.05 to 0.5%. The chiral dopants are preferably selected from the group consisting of compounds from Table B below, very preferably from the group consisting of R- or S-1011 , R- or S-2011 , R- or S-3011 , R- or S- 4011 , and R- or S-501 1.
In another preferred embodiment the LC media contain a racemate of one or more chiral dopants, which are preferably selected from the chiral dopants mentioned in the previous paragraph.
Furthermore, it is possible to add to the LC media, for example, 0 to 15% by weight of pleochroic dyes, furthermore nanoparticles, conductive salts, preferably ethyldimethyldodecylammonium 4-hexoxybenzoate, tetrabutyl- ammonium tetraphenylborate or complex salts of crown ethers (cf., for example, Haller et al., Mol. Cryst. Liq. Cryst. 24, 249-258 (1973)), for improving the conductivity, or substances for modifying the dielectric anisotropy, the viscosity and/or the alignment of the nematic phases. Substances of this type are described, for example, in DE-A 22 09 127, 22 40 864, 23 21 632, 23 38 281 , 24 50 088, 26 37 430 and 28 53 728.
The individual components of the preferred embodiments a)-z) of the LC media according to the invention are either known or methods for the preparation thereof can readily be derived from the prior art by the person skilled in the relevant art, since they are based on standard methods described in the literature. Corresponding compounds of the formula CY are described, for example, in EP-A-0 364 538. Corresponding compounds of the formula ZK are described, for example, in DE-A-26 36 684 and DE-A-33 21 373. The LC media which can be used in accordance with the invention are prepared in a manner conventional per se, for example by mixing one or more of the above-mentioned compounds with one or more polymerisable compounds as defined above, and optionally with further liquid-crystalline compounds and/or additives. In general, the desired amount of the com- ponents used in lesser amount is dissolved in the components making up the principal constituent, advantageously at elevated temperature. It is also possible to mix solutions of the components in an organic solvent, for example in acetone, chloroform or methanol, and to remove the solvent again, for example by distillation, after thorough mixing. The invention furthermore relates to the process for the preparation of the LC media according to the invention.
It goes without saying to the person skilled in the art that the LC media according to the invention may also comprise compounds in which, for example, H, N, O, CI, F have been replaced by the corresponding isotopes.
The structure of the LC displays according to the invention corresponds to the usual geometry for PSA displays, as described in the prior art cited at the outset. Geometries without protrusions are preferred, in particular those in which, in addition, the electrode on the colour filter side is unstructured and only the electrode on the TFT side has slots. Particularly suitable and preferred electrode structures for PSA-VA displays are described, for example, in US 2006/0066793 A1. The following examples explain the present invention without restricting it. However, they show the person skilled in the art preferred mixture concepts with compounds preferably to be employed and the respective concentrations thereof and combinations thereof with one another. In addition, the examples illustrate which properties and property combinations are accessible.
The following abbreviations are used:
(n, m, z: in each case, independently of one another, 1 , 2, 3, 4, 5 or 6)
CVC-n-m CVY-V-m
CCY-V-Om CCY-n-OmV
CCOY-n-Om CCOY-n-m
F F F F CA. ( H cf
2o-
→ ( o >-(0)-c
mH
; Cn
H2rv1 <
H OCF
2→( (0)-C
mH
2m+1
YPY-n-m YPY-n-mV
BCH-nm BCH-nmF
CnH2mf~ H O ,HO)CmH2n 1 H o )— o CmH2nn1
CPYP-n-(O)m CPGP-n-m
CCYY-n-m CPYG-n-(0)m
CBC-nm CBC-nmF
CETNap-n-Om CK-n-F
C
nH
2n+1(0)- (0)C
mH
2m+1
DFDBC-n(0)-(O)m C-DFDBF-n-(O)m
In a preferred embodiment of the present invention, the LC media according to the invention comprise one or more compounds selected from the group consisting of compounds from Table A.
Table B
Table B shows possible chiral dopants which can be added to the LC media according to the invention.
CM 44 CM 45
CM 47
R/S-4011 R/S-5011
R/S-1011
The LC media preferably comprise 0 to 10% by weight, in particular 0.01 to 5% by weight, particularly preferably 0.1 to 3% by weight, of dopants. The LC media preferably comprise one or more dopants selected from the group consisting of compounds from Table B.
Table C
Table C shows possible stabilisers which can be added to the LC media according to the invention.
(n here denotes an integer from 1 to 12, preferably 1 , 2, 3, 4, 5, 6, 7 or 8, terminal methyl groups are not shown).
- 135-
The LC media preferably comprise 0 to 10% by weight, in particular 1 ppm to 5% by weight, particularly preferably 1 ppm to 1% by weight, of stabilisers. The LC media preferably comprise one or more stabilisers selected from the group consisting of compounds from Table C.
Table D
Table D shows illustrative compounds which can be used in the LC media in accordance with the present invention, preferably as reactive meso- genic compounds.
35
ln a preferred embodiment of the present invention, the mesogenic media comprise one or more compounds selected from the group of the compounds from Table D. In addition, the following abbreviations and symbols are used:
Vo threshold voltage, capacitive [V] at 20°C,
ne extraordinary refractive index at 20°C and 589 nm, n0 ordinary refractive index at 20°C and 589 nm,
Δη optical anisotropy at 20°C and 589 nm,
si dielectric permittivity perpendicular to the director at 20°C and 1 kHz,
8 | | dielectric permittivity parallel to the director at 20°C and
1 kHz,
Δε dielectric anisotropy at 20°C and 1 kHz,
cl.p., T(N,I) clearing point [°C],
yi rotational viscosity at 20°C [mPa-s],
Ki elastic constant, "splay" deformation at 20°C [pN],
K2 elastic constant, "twist" deformation at 20°C [pN],
K3 elastic constant, "bend" deformation at 20°C [pN].
Unless explicitly noted otherwise, all concentrations in the present application are quoted in per cent by weight and relate to the corresponding mixture as a whole, comprising all solid or liquid-crystalline components, without solvents.
Unless explicitly noted otherwise, all temperature values indicated in the present application, such as, for example, for the melting point T(C,N), the transition from the smectic (S) to the nematic (N) phase T(S,N) and the clearing point T(N,I), are quoted in degrees Celsius (°C). M.p. denotes melting point, cl.p. = clearing point. Furthermore, C = crystalline state, N = nematic phase, S = smectic phase and I = isotropic phase. The data between these symbols represent the transition temperatures.
All physical properties are and have been determined in accordance with "Merck Liquid Crystals, Physical Properties of Liquid Crystals", Status Nov. 1997, Merck KGaA, Germany, and apply for a temperature of 20°C,
and Δη is determined at 589 nm and Δε at 1 kHz, unless explicitly indicated otherwise in each case.
The term "threshold voltage" for the present invention relates to the capa- citive threshold (V0), also known as the Freedericks threshold, unless explicitly indicated otherwise. In the examples, the optical threshold may also, as generally usual, be quoted for 10% relative contrast (V10).
Unless stated otherwise, the process of polymerizing the polymerisable compounds in the PSA displays as described above and below is carried out at a temperature where the LC medium exhibits a liquid crystal phase, preferably a nematic phase, and most preferably is carried out at room temperature. The display used for measurement of the capacitive threshold voltage consists of two plane-parallel glass outer plates at a separation of 20 pm, each of which has on the inside an electrode layer and an unrubbed poly- imide alignment layer on top, which effect a homeotropic edge alignment of the liquid-crystal molecules.
The display or test cell used for measurement of the tilt angles consists of two plane-parallel glass outer plates at a separation of 4 pm, each of which has on the inside an electrode layer and a polyimide alignment layer on top, where the two polyimide layers are rubbed antiparallel to one another and effect a homeotropic edge alignment of the liquid-crystal molecules.
The polymerisable compounds are polymerised in the display or test cell by irradiation with UVA light of defined intensity for a prespecified time, with a voltage simultaneously being applied to the display (usually 10 V to 30 V alternating current, 1 kHz). In the examples, unless indicated otherwise, a metal halide lamp and an intensity of 100 mW/cm2 is used for polymerisation. The intensity is measured using a standard UVA meter (Hoenle UV-meter high end with UVA sensor).
The tilt angle is determined by crystal rotation experiment (Autronic- Melchers TBA-105). A low value (i.e. a large deviation from the 90° angle) corresponds to a large tilt here. The VHR value is measured as follows: 0.3% of a polymerisable mono- meric compound is added to the LC host mixture, and the resultant mixture is introduced into VA-VHR test cells (not rubbed, VA-polyimide alignment layer, LC-layer thickness d « 6 pm). The HR value is
determined after 5 min at 100°C before and after UV exposure at 1 V, 60 Hz, 64 ps pulse (measuring instrument: Autronic-Melchers VHRM-105).
Example 1
2-Methylacrylic acid-4 8-f4-(2-methyl-acryloyloxy -butvn-3-r4-(2-methyl- acryloyloxy)-phenyl1-2-oxo-2H-chromen-6-yl}-butyl ester (1)
1.1 6,8-Dibromo-3-(4-methoxy-phenyl)-chromen-2-one
3,5-Dibromo-2-hydroxybenzaldehyd (30.0 g, 107 mmol) [CAS-Nr. 90-59-5] and 4-methoxyphenylacetic acid (35.7 g, 166 mmol) are heated in 51 ml
acetic anhydride and 60 ml triethylamine overnight at reflux. The reaction mixture is cooled down to 80°C and poured onto 1000 ml 2 M sodium hydroxide under stirring. After 5 min the precipitated product is filtered by vacuum, washed with water and digested two times with 100 ml ethanol. 6,8-Dibromo-3-(4-methoxy-phenyl)-chromen-2-one is obtained as a yellow solid.
1H-NMR (300 MHz, CDCI3)
δ = 3.86 (s, 3 H, OCH3), 6,98 (AB-mc, therein: J = 8.9 Hz, 2 H, Ph-H), 7.66 (AB-mc, therein: J = 8.9 Hz, 2 H, Ph-H), 7.60 (d, J = 2.2, Hz, 1 H, Ar-H), 7.62 (s, 1 H, Ar-H), 7.84 (d, J = 2.2 Hz, 1 H, Ar-H).
1.2 6,8-Bis-(4-hydroxy-but-1-vnyl)-3-(4-methoxy-phenyl)-chromen-2-one
6,8-Dibromo-3-(4-methoxy-phenyl)-chromen-2-one (30.8 g, 75.1 mmol), copper(l)iodide (1.50 g, 7.88 mmol) and bis(acetonitril)palladium(ll) chloride (5.00 g, 7.12 mmol) are dissolved in 300 ml DMF and 50 ml diisopropylamin at 70 °C, and a solution of butyne-1-ol (20.0 g, 285 mmol) in 50 ml DMF is added dropwise within 30 min. The reaction mixture is stirred overnight at 80°C, then poured onto 300 ml water and acidified with 2 N hydrochloric acid. The precipitated product is filtered by vacuum, then filtered through silica gel with THF/toluene (4:1) and addition of a small amount of ethanol, and the crude product is
recrystallized from toluene. 6,8-Bis-(4-hydroxy-but-1-ynyl)-3-(4-methoxy- phenyl)-chromen-2-one is obtained as light brown solid, which is pure enough to be used as starting material for the next step.
1.3 6.8-Bis-(4-hvdroxy-bu I)-3-(4-methoxy-phenyl)-chromen-2-one
6,8-Bis-(4-hydroxy-but-1-ynyl)-3-(4-methoxy-phenyl)-chromen-2-one is hydrogenated in THF on Pd/activated charcoal until the reaction is completed. The catalyst is filtered off, the solvent removed in vacuo and the crude product is filtered through silica gel with THF/toluene (3:2) and recrystallized from toluene. 6,8-Bis-(4-hydroxy-butyl)-3-(4-methoxy- phenyl)-chromen-2-one is obtained as a colourless solid.
1H-NMR (300 MHz, CDCI3)
δ = 1.47 (s, br., 1 H, OH), 1.55-1.86 ppm (m, 8 H, CH2), 2.68 (t, J = 7.4 Hz, 2 H, Ar-CH^), 2.89 (t, J = 7.3 Hz, 2 H, Ar-CH^), 3.69 (mc, 4 H, -CH2-O-) 3.85 (s, 3 H, OCH3), 6.97 (AB-mc, therein: J = 8.9Hz, 2 H, Ph-H), 7.17 (dd, J = 2.0 Hz, J = 9.9 Hz, 2 H, Ar-H), 7.67 (AB-mc, therein: J = 8.9 Hz, 2 H, Ph-H), 7.70 (s, br., 1 H, Ar-H).
1.4 6,8-Bis-(4-hvdroxy-butyl)-3-(4-hvdroxy-phenyl)-chromen-2-one
6,8-Bis-(4-hydroxy-butyl)-3-(4-methoxy-phenyl)-chromen-2-one (14.3 g, 36.0 mmol) is dissolved in 270 ml dichloromethane and a solution of boron tribromide (12 ml, 126 mmol) in 30 ml dichloromethane is added under ice-cooling. The cooling is removed and the reaction mixture is stirred overnight at room temp. The solution is poured onto ice/water, acidified with 2 N hydrochloric acid, and extracted three times with ethyl acetate. The combined organic layers are washed with water and dried over sodium sulfate. The solvent is removed in vacuo and the residue is filtered through silica gel with toluene/THF (7:3). 6,8-Bis-(4-hydroxy-butyl)-3-(4- hydroxy-phenyl)-chromen-2-one is obtained as a viscous oil.
1.5 2-Methyl-acrylic acid-4 8-r4-(2-methyl-acryloyloxyVbutvn-3-r4-(2- methyl-acryloyloxy)-phenvn-2-oxo-2H-chromen-6-yl>-butyl ester
6,8-Bis-(4-hydroxy-butyl)-3-(4-hydroxy-phenyl)-chromen-2-one (10.1 g, 25.1 mmol) is suspended in 230 ml dichloromethane, methacrylic acid (8.5 g, 99.0 mmol) and DMAP (0.5 g) are added, and then a solution of 1-(3- Dimethylaminopropyl)-3-ethylcarbodiimid (15.0 g, 96.6 mmol) in 20 ml dichloromethane is added under ice-cooling. After 1 h the cooling is removed and the reaction mixture is stirred obvernight at room temp. The solvent is removed in vacuo and the residue is purified by column chromatography on silica with dichloromethane/ethyl acetate (95:5) as eluent. 2-Methyl-acrylic acid-4-{8-[4-(2-methyl-acryloyloxy)-butyl]-3-[4-(2- methyl-acryloyloxy)-phenyl]-2-oxo-2H-chromen-6-yl}-butyl ester is obtained as a colourless oil.
1H-NMR (300 MHz, CDCI3)
δ = 1.70-1.85 ppm (m, 8 H, CH2), 1.94 (mc> 6 H, 2 Me), 2.08 (mc, 3 H, Me), 2.70 (mC) 2 H, Ar-CH^), 2.92 (mc, 2 H, Ar-CH^), 4.20 (mc, -CH^O-), 5.55 (mCl 2 H, 2 CHH=C(Me)COO-), 5.78 (mc, 1 H, CHH=C(Me)COO-), 6.10 (mc, mc> 2 H, 2 CHH=C(Me)COO-), 6.37 (mC) 1 H, CHH=C(Me)COO- ), 7.15-7.24 (m, 3 H, Ar-H), 7.71-7.80(m, 4 H, Ar-H).
Example 2
2-Methyl-acrylic acid-3-{3-f4-(2-methyl-acryloyloxy)-phenyll-6-f3-(2-methyl- acryloyloxy)-propyn-2-oxo-2H-chromen-8-yl>-propyl ester (2)
(2)
In analogy to example 1 , 2-methyl-acrylic acid-3-{3-[4-(2-methyl- acryloyloxy)-phenyl]-6-[3-(2-methyl-acryloyloxy)-propyl]-2-oxo-2H- chromen-8-yl}-propyl ester (2) is obtained from 3-(4-Hydroxy-phenyl)-6,8- bis-(3-hydroxy-propyl)-chromen-2-one as colourless crystals with a melting point of 49 °C.
Example 3
2-Methylacrylic acid-2-(6-r2-(2-methyl-aci loyloxy)-ethyl1-3-i4-(2-methyl- acryloyloxy)-phenvn-2-oxo-2H-chromen-8-yl}-ethyl ester (3)
(3)
In analogy to example 1 , 2-methyl-acrylic acid-2-{6-[2-(2-methyl- acryloyloxy)-ethyl]-3-[4-(2-methyl-acryloyloxy)-phenyl]-2-oxo-2H-chromen- 8-yl}-ethyl ester is obtained from 3-(4-hydroxy-phenyl)-6,8-bis-(2-hydroxy- ethyl)-chromen-2-one as colourless crystals.
Example 4
2-Methylacrylic acid-4-{6.7-bis-[3-(2-methyl-acryloyloxy)-butyl1-2-oxo-2H- chromen-3-yl)-phenyl ester (4)
(4)
In analogy to the synthesis sequence as described in example 1 , 2- methyl-acrylic acid 4-{6,7-bis-[3-(2-methyl-acryloyloxy)-butyl]-2-oxo-2H- chromen-3-yl}-phenyl ester is obtained from 5-bromo-4-chlor-2-hydroxy- benzaldehyde [CAS-No. 876492-31-8] as a colourless oil.
Example 5
2-Methyl-acrylic acid-4-{5.7-bis-[3-(2-methyl-acryloyloxy)-butvn-2-oxo-2H- chromen-3-yl}-phenyl ester (5)
(5)
In analogy to the synthesis sequence as described in Example 1 , 2- methyl-acrylic acid-4-{5,7-bis-[3-(2-methyl-acryloyloxy)-butyl]-2-oxo-2H- chromen-3-yl}-phenyl ester is obtained from 2,4-dibromo-6-hydroxy- benzaldehyde [CAS-No. 73289-92-6] as a colourless oil.
Example 6
2-Methyl-acrylic acid-4-{7,8-bis-[3-(2-methyl-acryloyloxy)-butyl1-2-oxo-2H- chromen-3-yl)-phenyl ester (6)
(6)
In analogy to the synthesis sequence as described in Example 1 , 2- methyl-acrylic acid-4-{7,8-bis-[3-(2-methyl-acryloyloxy)-butyl]-2-oxo-2H- chromen-3-yl}-phenyl ester is obtained from 3,4-dibromo-2-hydroxy- benzaldehyde [CAS-No. 51042-20-7] as a colourless oil.
The following compounds are obtained in analogy to the synth described in Example 1 :
(11) (12)
- 157-
(21) (22)
(23)
Mixture Example A
The nematic LC mixture N1 is formulated as follows.
CY-3-O2 18.00% cl.p. + 74.5
CPY-2-02 10.00% Δη 0.1021
CPY-3-02 10.00% Δε - 3.1
CCY-3-02 9.00% ειι 3.5
CCY-4-02 4.00% 1.16
CC-3-V 40.00% Υι 86
PYP-2-3 9.00% Vo 2.29
For each measured sample, the polymerisable monomeric compound (1) or (2) from Example 1 or 2, respectively (hereinafter referred to as monomer M1 or M2, see below), is added to the LC mixture N1 at a concentration of 0.3% by weight. Each resultant polymerisable mixture is inserted into a VA e/o test cell (rubbed antiparallel, VA-polyimide alignment layer, LC-layer thickness d « 4 pm). The cells are irradiated with UV light having an intensity of 100 mW/cm2 for the time indicated with
application of a voltage of 24 V (alternating current), causing
polymerisation of the monomeric compound. The tilt angle is determined before and after UV irradiation by a crystal rotation experiment (Autronic- Melchers TBA-105).
In order to determine the polymerisation rate, the residual content of unpolymerised RM (in % by weight) in the test cells is measured by HPLC after various exposure times. For this purpose each mixture is polymerised in the test cell under the stated conditions. The mixture is then rinsed out of the test cell using MEK (methyl ethyl ketone) and measured. in addition, the VHR values of the polymerisable LC mixtures comprising N1 and monomer M1 or M2, respectively, are before and after UV exposure are measured as described above.
For comparative purposes, the experiments as described above are also carried out with monomer C1 of prior art.
The tilt-angle results for monomers M1 , M2 and C1 in N1 are shown in Table 1. The residual concentrations of monomer M1 , M2 and C1 in N1 after different exposure times are shown in Table 2. The VHR values of mixtures containing N1 together with M1 , M2 or C1 are shown in Table 3
Table 1 (t = exposure time)
Table 2 (t = exposure time)
C1 M1 M2
t / s RM concentration / %
0 0.300 0.300 0.300
20 - 0.164 0.138
40 - 0.0375 0.027
60 - 0.035 0.015
90 - 0.061 0.008
120 0.1105 0.009 0.0055
180 - - 0
360 0.067 - -
Table 3 (t = exposure time)
As can be seen from Table 1 , a small tilt angle after polymerisation is achieved more quickly in PSA displays containing monomers 1 and M2 according to the invention, compared to PSA displays containing the monomer C1 from prior art.
As can be seen from Table 2, significantly more rapid and complete polymerisation is achieved in PSA displays containing the monomers M1 and M2 according to the invention, compared to PSA displays containing the monomer C1 from prior art.
As can be seen from Table 3, the VHR values of monomers M1 and M2 according to the invention in LC mixture N1 after UV exposure are significantly higher than the VHR values of monomer C1 in N1.