BENZOXAZEPINES AS INHI ITORS OF PI3 /mTOR AND METHODS OF THEIR
USE AND MANUFACTURE
CROSS-REFERENCE/TO RELATED APPLICATIONS
[0001] This, application claims ihe benefit, of priority to U.S. Provisional Application No. 61/417.122, filed November 24, 2010. which is incorporated herein by reference.
SEQUENCE LISTING
1.0002] This application incorporates by reference in its entirety the Sequence Listing entitled." IO-025_Sequencc.txi" (16.2 KB) which was created November 2 2011 and Hied herewith on November 23.2011.
BACKOKQUND OF TI rii INVENTION
Field of the Invention
[0003] This invention relates to the field of protein kinases and inhibitors thereof. In particular, the invention relates to inhibitors of P1 K and/or the mammalian target of rapamycin (mTOR) signaling pathways, and methods of their use and preparation.
Background of the Invention.
|0004) The P13K pathway regulates cell growth, proliferation aiid survival, and is dysregulatcd with high frequency in human tumors. PI3K pathway activation in tumors occurs via multiple mechanisms includin prevalent mutation and amplification of the
PIK3CA gene (which encodes the pi 10 subunit of PI3Ka). or downrcgulation of the lipid phosphatase PTEN. Downstream of PI3K. mTOR controls cell growth and proliferation through its two distinct signaling complexes: mTORCI and niTORC2. Given the role of P13K signaling on critical cellular functions, an inhibitor that targets both P13K. and mTOR could provide therapeutic benefit to patient populations with 'tumors harboring activating mutations in PIK3CA or Ras. PTEN-delciion, or where minors arc upregulated in growth factor signaling.
|.()005] Recciit studies indicate that phosphalitlylinositol 3-kinase (P13K) signaling has significant effects on cancer cell growth, survival, motility, and metabolism. The P13K pathway is activated by several different mechanisms in cancers, including somatic mutation and amplification of genes encoding key components. In addition, PI3K signaling may serve integral functions for noncancerous cells in the tumor microenvironment. Consequently, there is continued. interest in developing inliibiiprs of PI3K isoforms as a means for treatin
various forms 'of -cancer, particularly the class II isoforms PI3K-alpha; Pl3K-bcia. anil PI3K- gamnia.
|000(ΐ| For example, phosphatidylinositol 3-kiiiasc (Ρ13Κα), a dual .specificity protein kinase, is composed of an 85 kDa regulatory suluiiiii and a 110 kDa catalytic subunil. The protein encoded by this gene represents the catalytic subunit. which uses ATP to
phosphorylaie Pidlns, Ptdlns4P and Ptdlns(4,5)P2. ΡΊΈ . a tumor suppressor which inhibits cell growth through multiple mechanisms, can depliosphorylatc PIP3. the major product of P1K3CA. PIP3. in turn, is required for translocation of protein kinase B (AKTI. PKB) to the cell membrane, where it is phosphorylnic.d and activated by upstream kinases. The effect of PTEN on cell death is mediated through the PI 3CA/AKT1 pathway,
[0007] ΡΙ3Κ has been implicated in the control of cytoskelctai reorganization, apoplosis. vesicular trafficking, proliferation and differentiation processes. Increased copy, number and expression of PIK3CA is associated with a number of malignancies such as ovarian cancer (Campbell el al.. Cancer Res 2004, 64.767S-76S 1 ; Levine ei al.. Clin Cancer Res 2005, 11.2875-2878; Wang el. a I.. Hum Mitlat 2005.25, 322; Lee el al.. Gynecol Oncol 2005, 97, 26-34). cervical cancer, breast cancer (Bachman. el al. Cancer Biol Titer 2004.3. 772-775; Levine. et al., supra; Li et al.. Breast Cancer Res Treat 2006, 96.91-95: Saal et al.. Cancer Res 2005, 65.2554-2559: Samuels and Veiculescu, Cell. Cycle 2004, 3, 1221-1224). colorectal cancer (Samuels, et al. Science 2004, 304, 554: Velho el al. E r J Cancer 2005, 4\. 1 49- 1 54). endometrial cancer (Oda el al. Cancer Res.2005, 65. 10669- 10673), gastric carcinomas (Byiin ei al., hit J Cancer 2003, 104, 318-327; Li et. al.. supra Velho el al., supra; Lee et al.. Oncogene 2005, 24. I477-14S0), hepatocellular carcinoma (Lee et al.. id.), small and non-small cell lung cancer (Tang el.nl.. Z,i</n> Cancer 2006, 51. 181-1 1: Massion et al., Am J Respir Crit Care Med 2004. I 70, 1088-1094). thyroid carcinoma (Wu et al.../ Clin Endocrinol Metab 2005, 90.4688-4693). acute myelogenous leukemia (AML) (Sujobert et al.. Blood 1997, 106. 1063-1066), chronic myelogenous leukemia (CML) (Hickcy and Colter ./ Biol Cliem 2006, 281.2441-2450). and glioblastomas (Harimann ei al. Acta Nciiropathol (Be ) 2005, 109.639-642; Samuels et al., supra).
OOOSj The mammalian, target. mTOR, is a protein kinase that integrates both
extracellular and intraccllular'signals of cellular growth, proli eration, and survival.
Extracellular mitogenic growth factor signaling from cell surface receptors and intracellular pathways that convey hypoxic stress, energy and nutrient status all converge at mTOR.
mTOR exists in two distinct complexes: mTOR complex 1 (niTORCI) and mTOR complex 2 (mTORC2). inTORCI is a key mediator of transcription and cell growth (via its substrates
p70S6 kinase and 4E-BPI) and promotes cell survival via the scrum and glucocoriicoid- aciivatcd kinase SGK, whereas. iuTORC2 promotes activation of the pro-survival kinase A T. Given its central role in cellular growth, proliferation and survival, it is perhaps not surprising that mTOR signaling is l'ret|uenlly dysrcgulated in cancer arid other diseases (Bjomsli and Houghton Rev Cancer 2004, 4(5), 335-48; Houghton and Hum* Microbiol Immunol 2004, 279.339-59; Inoki. Corradetii et al. Nat GateiHWS, 37(1). 19-24).
10009] mTOR is a member of the PIKK (PI3K-relaied Kinase) family of atypical kinases which includes ATM. ATR. and DNAPK. and its catalytic domain is homologous to that of PI3K. Dyregulalion of P13K signaling is a common function of tumor cells. In general.
mTOR inhibition may be considered as a strategy in many of the tumor types in which PI3 signaling is implicated such as those discussed below:
[0010] Inhibitors -of mTOR may be useful in treat ing a number of cancers, including the following: breast cancer (Nagala. Lan et al.. Cancer Cell 2004, 6(2 ). 117-27: Pandolfi /V Engl J Med 2004, 351(22).2337-8'; Nahta. Yu el al. Nat Clin Pract Oncol 2006, 3(5).269-280): antic cell lymphoma (MCL) (Dal Col. Zancai el al. Blood 2008.111(10).5142-51): renal cell carcinoma (Thomas. Tran el al. Nai Med 2006.12(1). 122-7: Atkins. Hidalgo ei al. J Clin Oncol 2004, 22(5).909-18; olzcr. Hudes ei al..1 Clin Oncol 2007, 25(25).3958-64); acute myelogenous leukemia (AML) (Sujoheit. Bardct et al. Blood 2005, 106(3). 1063-6; Billollcl. Grandagc et al. Oncogene 2006, 25(50).6648-6659: Tamburini. Elie ct al. Blood 2007,
110(3). 1025-8): chronic myelogenous leukemia (CML) (Skorski. Bellacosa ei al. EmboJ 1997, 16(20).61 1-61: Bai. Ouyang ei al. Blood 2000, 96(1 ), 4319-27: 1-!ickcy and Cotter Biol diem 2006, 2<S7(5).2441-50); diffuse large B cell lymphoma (DLBCL) (Uddin, Hussaiu el al. Blood 2006, 108( 13).4178-86): several subtypes of sarcoma (Hernando, Charytonowicz ct al. Nut Med 2007, 13(6).748-53: Wan and Helman Oncologist 2007, 12(S).1007-18); rhabdomyosarcoma (Cao, Yu et al. Cancer Res 2008, 68(19).8039-8048; Wan. Shcn et al. Neoplasia 2006, <S'(.5).394-401 ); ovarian cancer (Shayesteh, Lu et al. Nat Genet.1999, 21(1). 99- 1.02; (Lee, Choi et al. Gynecol Oncol 2005, 97( I ) 26-34): endometrial tumors (Obata. Morland ct al. Cancer Res WW, 58(10), 2095-7; Lu, Wu et al. Clin Cancer Res 2008, J 4(9). 2543-50): non small cell lung carcinoma (NSCLC) (Tang. He. ci al. Lung Cancer 2006, /(2). 181-91 : Marsit. Zheng ct al. Hum Pathol 2005, 36(7).76S-76); .small cell, sc|uamous, large cell and adenocarcinoma (Massion, Taflan el al. Am .1 Res ir Crit Care Med 2004, 170(10). 1088-94); lung tumors in general (Kokubo. Gemma et al. Br. I Cancer 2005, 92(9). 1711-9: Pao, Wang ei al. Pub Library of Science Med 2005, 2(1), el 7); colorectal tumors (Velho. Olivei a ct al. Eur J Cancer 2005, 41(11). 1649-54: Foukas. Claret et al. Nature, 2006,
441(7091), 366-370), particularly those that display mici salelliie instability (Gocl. Arnold cl al. Cancer Res 2004, 64(9), 3014-21; Nassif, Lobo cl al. Oncogene 2004, 23(2).617-28), KRAS-mutaied colorectal tumors (Bos Cancer Res 11)89.49(17), -1682-9; Pcaron Ann N Y Acad Sci 19)5, 768. 101-10): gastric carcinomas (B.yiin, Cho et al. hit .1 Cancer 2003, J 04(3). 318-27); hepatocellular tumors (Lee, Soung et al. Oncogene 2005, 24(8). 1477-S ); liver tumors (Hu. Huang el al. Cancer 2003, 97(8), 1929-40; Wan, Jiang et al. Cancer Res Clin Oncol 2003, 129(2), 100-6): primary- melanomas and associaied increased tumor thickness (Guldberg. tbor Stralen et al. Cancer Res 1997.57(17).3660-3: Tsao. Zhang cl al. Cancer Res 2000, 60(7). 1800-4: Whiicman. Zhou el al. hit J Canceri i, 99(1).63-7; Gocl. Lazar et al../ Invest Dermatol 126(1).2006, 154-60): pancreatic tumors. (Asano. Yao et al.
Oncogene 2004, 23(53).8571.-80.): prostate carcinoma (Cairns, Okaini el al. Cancer Res 1997, 57(22J.4997-5000; Gray. Stewart el . Br J Cancer 1998, 78(1.0). 1296-300:- Wang, Parsons et al. Clin Cancer Res 1998, 4(3).811 -5; Whang. Wu et al. Proc Natl Acad Sci U S A 1998, 95(9), 5246-50; ajumdcr and Sellers Oncogene 2005, 24(50) 7465-74: Wang. Garcia et al. Proc Natl Acad Sci U S A 2006, 103(5). 1480-5: (Lu, Ren et al. hit .1 Oncol 2006, 28(1). 245-51 : Mulholland, Dedhar el al. Oncogene 25(3).2006, 329-37: Xin. Teitell et al. Proc Natl Acad Sci USA /2006, 03(20). '7789-94: Mikhailova. Wang cl al. Adv Exp Med Biol 2008, 617.397-405: Wang. Mikhailova et al. Oncogene 2008, 27(56).7106- 117); thyroid carcinoma', particularly in ihc anaplastic subtype. (Garcia-Rpstan, Costa et al. Cancer Res 2005, 65(22). 10199-207); follicular thyroid carcinoma (Wu; Mambo el ol. J Clin Endocrinol Metab 2005, 90(8), .4688-93): anaplastic large cell lymphoma (ALCL): hamaratomas, angiomyelolipomas. TSC-associated and sporadic lymphangioleiom yomaiosis: Cowdcn's disease (multiple hamaraloma syndrome) (Bisslcr. McCormack et al. /V Engl J Med 2008, 358(2), 140-151); sclerosing hemangioma (Randa iVl. S. Amih Pathology International 2008, 58(1).38-44); Peulz-Jcghers syndrome (PJS): head and neck cancer (Gupia. McKenna et al. Clin Cancer es 2002, 8(3).885-892): neurofibromatosis (Fcrner Eur J Hum Genet 2006, 15(2), 131-138: Sabatini Nat Rev Cancer 2006, 6(9).729-734:.Johanncssen. Johnson et al, Current Biology 2008, 18(1), 56-62); macular degeneration: macular edema; myeloid leukemia; systemic lupus; and autoimmune lymphopiOlifcrativc syndrome ( ALPS).
SUMMARY OF THE INVENTION
[0011,| The following only summarizes certain aspects of the invention and is not intended to be limiting in nature. These aspects and other aspects and embodiments arc described more fully below. All references eilcd in this specification are hereby incorporated by reference in their entirely, In the event of a discrepancy between the express disclosure of
this specification' and the references i corporated by reference, the express disclosiirc.of ihis. specification shall control.
[ 00121 We recognized the important role of PI3K and ni TOR in biological processes and disease states and,, therefore,. realized.- that inhibitors of these protein
" kinases ; would be desirable, as evidenced in Serial Number PCT/US201,0/036032, filed May 25.201 , the entire contents of which is incorporated herein by reference. Accordingly, the invention provides compounds that inhibii. regulate, and/or modulate P13K and/or mTOR and are useful in the treatment of hyperproli erativc diseases, such as cancer, in mammals. This invention also provides methods of making the compound, methods of using such compounds in the treatment of hyperpro|ifcraiivc diseases in mammals, especially Intmans. and to pharmaceutical
|()(H3] A ftrsi;a.spect of theinveni provides a.Gompouiul o,i i¾imula I:
ff* R
2
or a single.stereoisomer or mixture
' .of stereoisomers thereof and additionally optionally as a phannaceiitieally- acceptable salt thereof, where.
R1 is phenyl optionally substituted with one. two. or three RF' groups; or
R1 is heteroaryl optionally substituted with one, two. or three R7; *
R2 is heteroaryl substituted with R\ R¾. R-,l?. R:,c. ;.nd R,J;
R'. R'''1. R3b, R C, and RM are independently hydrogen, cyano. nilro, alkyl. alkcnyl. alkynyl, halo, haloalkyl, hydroxyalkyl. alkoxyalkyl. cyanoalkyl. -SR'\ -S(0) R2". -C(0)H,
-C(0)ORJ. -C(0)NHR\ halocarbonyl. -NR"R"\ -OR, L:I. optionally substituted phenyl, optionally. substituted phenylalkyl, optionally substituted cycloalkyl, optionally substituted cycloalkylalkyj. optionally subsiituted lieterocyclp'alkyl. Optionally substituted hctcrocycloalkylalkyl, optionally substituted heteroaryl, opt ionally substituted hcteroarylalkyl, or alkyl substituted with One or two R1 ; or
two of R\ R '. RM . and RUI. when attached to the same carbon, form an optionally
substituted cycloalkyl, optionally substituted aryl. or an optionally substituted
hcicrocycoalkyl, or optionally substituted heteroaryl, and the other of R'\ R RA'. R'C. and R",J are independently hydrogen, cyano, nilro, alkyl, alkenyl, alkynyl. halo,. haloalkyl. hydroxyalkyl,;alkox.yalkyl, cyanoalkyl, -SR1", -S(0)2R20 -C(0)H, -C.(0)ORL
halocarbonyl, -C(0)NI-IR'. halocarbonyl, -N MR.'"A: -OR11'1, opiionally substiuiied phenyl; opiionally substiuiied phenylalkyi. optionally substiuiied cycioalkyi. optionally substituted cycioalkylalkyi. optionally substituted hcieroeycloalkyh opiionally substituted heiciocycloalkylalkyl. opiionally substituted hcteroaryl. opiionally substituted
hetcroarylalkyl. or alkyl substituted with one or two R16:
R'1 is alkyl, alkenyl. alkynyl. hydroxyalkyl. alkoxyalkyl. haloalkyl. aminoalkyl.
alkyianiinoalkyl. dialkylaminoalkyl. benzyl, or opiionally subsiiuiied
heiciocycloalkylalkyl:
R5:I and R5 are independently hydrogen, deuterium, or alkyl;
Rih is hydrogen, deuterium or halo;
R"1|v-is deuterium, (Ci.jja'lkyl, (Ci.jjalkoxy, luiIo(C|.; alkyl. or (C|.: haloaTkoxy:
R"D, R5C, RST, and R5? are hydrogen or deuterium:
each RF'. when R(' is present, is independently nitro: cyano; halo; alkyl: alkenyl; alkynyl: haloalkyl; -OR*3; -NRSRS ; -C(0)NRV:I: -S(0)2R': -NRXC(0)OR"; -NRSC(0)R":
-NR^CO^R*': ,NR¾(OjNR^R"; carboxy. -C(0)QR": halocarbonyl; alkylcarbonyl: alkyl substituted
or two -C(0)NR
SR
S:I: hcteroaryl optionally substituted with I.2, or 3 R'
:'; or opiionally substiuiied heierocyeloalkyl; or
two R(', together with the carbons to which ihey are'atlached, fprm an opiionally substituted 3, 4, 5, or 6-nicnibered cycioalkyi or licterocycloalkyl;
each R'. when R' is present, is independently oxo: nitro: cyano: alkyl: alkenyl; alkynyl: halo: haloalkyl; hydroxyalkyl; alkoxyalkyl; -OR" ; -SR13; -S(0)R'-': -S(0)2R' ',;'; -NR' RXN:
-C(0)NRsR'S;'; -NR'sC(0)OR ; - R'sC(0)R''; .NR8S(0)2RS : - RSC(0) RS:,R"; -C(0)0R"; halocarbonyl; alkylcarbonyl: -S(0) NR'sRy; alkylsulOnylalkyl; alkyl subsiiuiied with one or two -NRsRSa: alkyl substituted with one or two -NRsC(0')RSil; alkyl substituted with one or two -NR^CiOjOR*'; alkyl subsiiuiied with one or two -S(.0)2Rl¾; optionally subsiiuiied cycioalkyi; optionally subsiiuiied cycoalkylalkyi; opiionally substituted heierocycloalkyl; opiionally substituted heiciOcyeloalkylalkyl; opiionally .substituted phenyl: opiionally substiuiied phenylalkyi: opiionally substiuiied hcteroaryl: or opiionally subsiiuiied heieroarylalkyl;
each Rs, R11. R15, R17, and Rli! are independently hydrogen. Nl-I2, Nl-I(alkyl). N(alkyl)2. alkyl. alkenyl. alkynyl, hydroxyalkyl. alkoxyalkyl. or haloalkyl;
each RK;i. Rl l:\ and RI5:1 are independently hydrogen, alkyl. alkenyl. alkynyl. haloalkyk
hydroxyalkyl, cyaiioalkyl. aminoalkyl. alkyianiinoalkyl. dialkylaminoalkyl. alkoxyalkyl. carboxyalkyl. optionally subsiiuiied cycioalkyi. opiionally substiuiied cycioalkylalkyi,
optionally substituted heterocycloalkyi, optionally substituted hctcrocycloalkylalkyl. optionally substituted phenyl, optionally substituted plienylalkyl. optionally substituted heleroaryl. or optionally substituted heieioarylalkyl;
R"' is hydrogen; alkyl; alkenyl; alkyiiyl: hydroxyaikyl: alkoxyalkyl; aminoalkyl:
alkylamiiioalkyl: dialkylaminoalkyl; haloalkyi; hydioxyalkyl substituted with one. two, or three groups which are independently halo, amino, alkylaniino, or dialkylamino: alkyl substituted with one Or two amihocarbonyl; optionally substituted phenyl; optionally substituted plienylalkyl; optionally substituted cycloalkyl; optionally subsliluted cycloalkylalkyl; optionally substituted heteibaryl: optionally substituted heieioarylalkyl; optionally substituted heterocycloalkyi; or optionally substituted heterocycloalkylalkyl;
R1" is alkyl or optionall substituted plienylalkyl;
R1*' is alkyl. hydioxyalkyl. or haloalkyi: and
R1¾ is hydroxy, alkyl, haloalkyi. hyd oxyaikyl. or heterocycloalkyl optionally substituted with one or two groups which are independently halo, amino, alkylaniino. dialkylamino, hydroxy, alkyl, or hydroxyaikyl;
each R14, when R is present, is independently amino, alkylaniino. dialkylamino,; acylamino. halo, hydroxy, alkyl. haloalkyi. hydi xyalkyl. aniinoalkyl. alkyiaininoalkyl.
.dialkylaminoalkyl. alkoxycarbonyl. aniinocarbonyl. alkylaminocarbonyl.
dialkylaminocarbonyl. or optionally substituted phenyl;
each RLFL is independently halo, -NR"R";'. -NRI5S(0)RI5;I. -0C(0)R17. carboxy.
alkoxycarbonyl, -NHe(0)RL5;I. or -ORLS: and
R"° is alkyl. haloalkyi, hydroxyaikyl. amino, alkylaniino, dialkylamino, or heteiocycloalkyl: with the proviso that if One of 5a. R?c. R5'1. R3c. R3'. R5s. and R5harc deuterium, then R5" is H.
(C,.3)alkyl or halo(C|..;)alkyl.
[0014] In second aspect, the invention is directed to a pharmaceutical composition which comprises I ) a Compound of Formula I or a single stereoisomer or mixture of stereoisomers thereof, optionally as a pharmaceutically acceptable salt or solvate thereof and 2) a pharmaceutically acceptable carrier, cxcipicni. or diluent.
[0015'] In a third aspect of the invention is a method of inhibiting the in vivo activity of P13 and/or mTOR. the method comprisin administering to a patient an effective PI3K- inhibiting and/or inTOR-inhibiiing amount of a Compound of Formula la Compound of Formula I or a single stereoisomer or mixture of stereoisomers thereof, optionally as a pharmaceutically acceptable salt or solvate thereof or pharmaceutical composition thereof.
[0016] In a .fourth aspect, the Invention provides a method for treating a disease, d isorder, or syndrome which method comprises administering to a pat ient a therapeutical l y effect i e amount of a Compound of Formula I or a single stereoisomer or mixture of stereoisomers thereof, optionally as a pharmaceutically acceptable salt or solvate thereof, or a
pharmaceutical composition comprising a therapeutically effect ive amount of a Compound of Formula 1 or a single stereoisomer or mixture; of stereoisomers thereof, optionally as a pharmaceutical ly aeccplable.salt or sol vate" thereof, and a pharmaceutically acceptable carrier, excipicnt. or diluent.
1,0017') In a fifth aspect, the Invention provides a method for making a Compound of Formula 1(a) which method comprises
(a) reacting the fol lowing, or a salt thereof:
where 1 is as defined in the Summary of the Invention for a Compound of Formula I; with an intermediate of Formula R"X where X is halo, and R~ is as defined in the Summary of the Invent ion for a Compound of Formula I lo yield a Compound o ihe Invent ion of Formula 1(a)
1(a):
and opt ionally separating individual isomers: and optionally modifying any o ihe R 1 and R2 groups and opiionally forming a pharmaceuticall y acceptable salt thereof; or
(b) react ing the following, or a sail thereof: .Ί
where R is halo or -B(OR' h (where both R ' are hydrogen or the two R ' together form a boronic ester), and R" is as defined in the Summary of the Invention for a Compound of
Formula I: with an intermed iate of Fonnula R 1 Y where Y is halo when R is -li(OR ' )_ and Y is -B(OR*): when R is halo, and R2 is as defined in the Summary of the Invent ion for a
Compound of Formula I to yield a Compound of live Invention of Formula 1(a): and
opt ionall y separating individual isomers; and optional ly modifying any of the R 1 and R2 groups: and opt ionally forming a pharmaceutically acceptable salt, hydrate, solvate or combination thereof.
[ 1)0181 hi addit ional aspect of the invention is a method of inh ibiting the in vivo activit of rn'IOR, the method comprising administering to a pat ient an effect ive
PI3K/mTOR-inhihiling amount of a compound of formula f or of 'fable I or a single stereoisomer or mixture of isomers thereof, optional ly as a pharmaceutically acceptable salt or solvate thereof or pharmaceutical composition thereof. In this and other aspects and embodiments, as provided herein, the compound can be an inhibitor of ΡΙ3 Κα. PI3Kp\ Ρί3 Ι<γ, or other PI3 isoforms combinations thereof.
| ()()19] In an additional aspect of the Invention provides a meihbd for treat in a disease, disorder, or syndrome ..which method comprises administering to a patient therapeutical ly effective amount of a compound of formula I or a single stereoisomer or mixture of isomers thereof, optionally as a ' pharmaceutical l y acceptable salt or solvate thereof, or a
pharmaceut ical composition comprising a therapeutically effect ive amount of a compound of formula f or of Table I or a single stereoisomer or mixture of isomers thereof, optionally as a pharmaceut ically acceptable salt or sol vale thereof, and a pharmaceutically acceptable carrier, excipient, or d iluent.
[0020] In an addit ional aspect of the invention provides a method for treating a subject having a tumor the method comprising: (a ) administering a PI3 K-U select ive Inhibitor, a dual PI3 -u/mTO selective inhibitor, or a combination of a PI3 K- selective inhibitor and a inTOR select ive inhibitor to the subject if said tumor comprises a mutation in a PI3 K-a kinase domain: or (b) administering a combination of a PI3K-U selective inhibitor and a ΡΙ3 -β selecti ve inhibitor, a dual PI K.-u/mTOR . selective inhibitor, or a ΡΙ3 Κ-β selective inhibitor, to said subject if said tumor comprises a munition in a PI3 K-a hel ical domain, wherein the Ρ13 Κ-α selective inhibitor, the dual PI3 K-(i/mT0R selective inhibitor, or the combination of the P13 K-U selective inhibitor and a m'l'OR selective inhibitor is a compound of Formula I or of Table I .
| 0()2 l | In an additional aspect, the present invent ion provides a method for identi fying a selective inh ibitor of a ΡΓ3 isozyme, the method comprising: ( a) contacting a first cel l hearing a first mutation in a PI3K-«t with a candidate inhibitor: (b) contacting a second cel l bearing a wi ld type PI3 K-U, a PTEN null mutation, or a second mutation in said PI3 K-U with the candidate inhibitor: and (c) measuring AKT phosphorylation in said first and said second cells, wherein decreased AKT .phosphorylation in said first cell when compared to said
second cell identifies said candidate inhibitor as a selective PI3 K-a inhibitor, wherein the PI3 -U selective inhibitor, the dual PBK-rtVmTOR selective inhibitor, or l he combination of die PI3 K-U selective inhibitor and a mTOR selective inhibitor is a compound of Formula I or of Table 1.
|()()22 | In an additional aspect, the present invent ion provides for a method, for determining a treaimcnt regimen for a cancer patient having a tumor comprising a PI3 -U, the method comprising: determining the presence or absence of a mutation in amino acids I 0 7.and/or 545 of said P13 K-u; wherein if said PI3 K-U lias a mutat ion at position 1047, said method comprises. administering to the cancer patient a therapeut ically effective amount of a PI3 -H selective inliibitor compound, or a dual PI3K 7./mTOR . selective inh ibitor, or a combination of a PI3 - :sclective. iiiliibitoi ,and a inTOR select ive inhibitor; or wherein .if- said P13 K-a has a imitation at position 545. said method comprises administering to the cancer patient a therapeutically effective amount, of a combination of a ΡΙ3 Κ-ά selective inhibitor and a PI3 -|S select ive inhibitor, or a dual P13 -u/mTOR selective inhibitor, or a combination of a PI3 -a, sclcctive inhibitor and a mTOR select ive- inhibitor: . wherein the P13 K-U selective inhibitor, the dual PI3 K-u/mTOR elective inhibitor, or the combination of the PI3 -C. select ive inhibitor and a mTOR select ive inhibitor is a compound of Formula I or of Table I .
10023] In aii additional aspect, the cell used to diagnose, treat or screen against . includes a cancer or tumor cell obtained from a lumor.or cancer derived from: breast cancer, mantle cel l lymphoma, renal cell carcinoma, acute myelogenous leukemia, chronic myelogenous leukemia, NPM/AL -iransl rnied anaplastic large cell l ymphoma, diffuse large B cel l l ymphoma, rhabdomyosarcoma, ovarian cancer, endometrial cancer, cervical cancer, non- small cell lung carcinoma, small cel l lung carcinoma, adenocarcinoma, colon cancer, rectal cancer, gastric carcinoma, hepatocellular carcinoma, melanoma, pancreat ic cancer, prostate carcinoma, thyroid carcinoma, anaplastic large cell lymphoma, hemangioma, glioblastoma, or head and neck cancer, wherein the PI3. -cx selective inhibitor, the dual POK-u/mTOR selective inhibitor, or the combination of the PI3 K-U selective inliibitor and a mTOR selective inhibitor is a compound of Formula I or of Table I .
DETAI LED DESCRIFI'ION OF THE INVENTION
Abbreviations and Definitions
10024] The following abbreviations and terms have the indicated meanings throughout:
Abbreviation Meaning.
1 I
Abbreviation IV'Ieiiniiiy,
mol molc(s)
MS mass spectral analysis
N normal or normal ity
11 M nanomolar
NM P A'-inethyl-2-pyrrolidonc
NMR nuclear magnetic resonance spectroscopy
Quartet
rt Room temperature
s S inglet
t or tr Triplet
Tl-IF leirahydrofuran
10025) The symbol '-" means a single bond, means , a . double bond, ''= ' means; a triple bond, " -" means a single Or double bond. The symbol ">ΛΛΛ/ ': refers to a group on a double-bond as occupying either position on the terminus of a double bond to which the symbol is attached: that is. the geometry. E- or Z-. of the double bond is ambiguous. When a group is depicted removed from its parent Formula . the symbol will be used al the end of the bond which was theoretically cleaved in order to separate the group from its parent '.structural Formula.
[01)26] When chemical structures ..arc depicted or described. Unless explicitly stated otherwise, all carbons are assumed to have hydrogen - .substitution to conform to a valence of four. For example, in the structure on the left-hand side- f the schemat ic below there are nine hydrogens implied. The nine hydrogens are depicted in the right-hand structure. Somet imes a particular atom in a st ructure is described in textual Formula as hav ing a hydrogen or hydrogens as substitut ion (expressly defined hydrogen), for example. -CThCI-I?-. It is understood by one of ord inary skill in the art that the aforementioned descriptive techniques arc common in the chemical arts to provide brevity and simplicity to description of otherwise complex structures;
L<) 27.| If, a group
"IV is depict d as f loating'- on a riiigjsysteiii, as for example in ihe Formula :
then, unless otherwise defined, a substiluent "R" may reside on any atom of the ring system, assumin replacement of a depicted, implied, or expressly defined hydrogen from one of the ring atoms, so.long.as a stable structure, is' formed.
|()()281 If a group "R"
system, as for examp!e-in the Formul c:
then, unless otherwise defined, a substiluent "R" may reside on any atom of the fused ring system, assuming replacement of a depicted hydrogen (for example the -NM- in the Formida above), implicd hydrogen (for example as in the Formula above, where lite hydrogens arc rioushown but understood to be present), or expressly def ined hydrogen (for example where in the Formula above. "Z" equals =C1-1- ) from one of the rin atoms, so -long as a stable structure is formed. In. the cxaniplc depicted, the "R group may reside on either the 5- mcmbcred or the (remembered ring of the fused ring system.
[00291 When a group "R
" is depicted as existing on a ring system containing saturated carbons, as for example in the Formula :
where, in this example, "y" can be more than one. assuming each replaces a currently depicted, implied, or expressly defined hydrogen on the ring; then, unless Otherwise defined, where the resulting structure is stable, two "R's" may reside on the same carbon. In another example, two R's on the same carbon, including thai carbon, may form a ring, thus creating a spirocyclic ring structure with inc. de icted ring as for example in the Formula :
[00301 "Acyr means a -C(0)R radical where R is alkyl. alkenyl, cycloalkyl, cycloalkylalkyl. aryl. aralkyl. heteroaryl. hcicroaralkyl. hetcrocycloalkyl. or
1.3
hcterocycloalkylalkyl.. as■defined herein, e.g.. acetyl. ii il'hioi incihylcarbonyl, o
2-mcihoxyeihylcarboiiyl. and die l ike.
1003.1 1 "Acylamino" means a -NR R * radical where R is hydrogen, hydroxy, alkyl, or alkoxy and R ' is acyl. as defined herein.
100321 "Acylox y" means an -OR radical where R is acyl . as defined herein, e.g.
cyanomethylcarbpnyloxy. and the like.
[0033] " Administrat ion" and variants (hereof (e.g.. "administering" a compound) in reference to a Compound of the invent ion means int roducing the Compound or a prodrug of the Compound into the system of the animal in need of treatment: When a Compound of the 'invention oi' prodrug thereof is provided in combiiiai ion with one o -more oilier active;agejiis (e.g.. surgery, radiation, and chemotherapy, etc. ). "administration" aud its variants are each -understood to include concun eiu and sequential introduction of die Compound or prodrug thereof and other -agents.
[0034 | "Alkenyl" means a means a l inear monovalcni hydrocarbon radical of two to six carbon atoms or a . branched monovalent hydrocarbon radical of three to six carbon atoms which radical contains at least one double bond; e.g.. cthciiyl. pi penyl, l -bui-3-enyl, and l -penl-3-enyl. and the like.
[0035] "Alkoxy" means an -OR group where R is alkyl grou as defined herein.
Exaiiiples include mcthoxy, cthoxy, propoxy, isopropoxy, and the like.
[00361 "Alkoxyalkyl" means an alkyl group, as defined herein, substit uted with al least one, speci fical ly one. two. or three, alkoxy groups as defined herein. Representative examples include mcthoxymethyl and the like.
[0037 J "Alkoxycarbonyr* means a -C(0)R group where R is alkoxy, as defined herein.
[00381 "Alkyl" means a l inear saluraled, monovalent hydrocarbon radical of one to six carbon atoms or a branched salurated monovalent hydrocarbon- radical of three to six carbon atoms, e.g.. methyl, eihyl, propyl. 2-propyl, butyl (including al l isomeric forms), or pentyl (including all isomeric forms), and the like.
[00391 "Alkylam ino" means an -N HR group where R is alkyl. as defined herein.
|0040 | "Alkylamiiioalkyl" means an alkyl group subst ituted with one or two alkylamino groups, as defined herein.
[0041 1 "Alkylaminoalkyloxy" means an -OR group where R is alkylamiiioalkyl. as defined herein.
[00421 "Alkylcarbonyl" means a -C(0)R group where is alkyl, as defined herein.
[0043] ''Alkylsulfonyr means an -S(0):R group where R is alkyl, as defined herein.
[0044] "Alkylsiil fonylalkyl" means an al kyl group, as defined h rein, subst ituted with one or two -S(0)iR group where R is alkyl. as defined herein.
10045) "Alkynyl." means a linear monovalent hydrocarbon radical of two to six carbon atoms or a branched monovalent hydrocarbon radical of three to 6 carbon atoms which radical contains at least one triple bond. e.g.. eihynyl , propynyl. bui ynyl, peniyn-2-yl and the like.
|0046] ' Amino-* means -i\'H2.
Ι.Ό047] "Aminoalkyl" means an alkyl group subsumed with at least one., specifically one, two or three, amino groups.
|0048] "Aminoalkyloxy" means an -OR group where R is aminoalkyl . as defined herein.
[004 1 "Aniinocarbonyl" means a -C(0)N I-l2 group.
[(1050 J "Alkylaminocarbonyf ' means a C(0) l-I group where R is alkyl as defined herein.
[0051] "Aryl" means a monovalent six- to foiirleen-inembered. mono- or bi-carbocyclic ring, wherein the monocyclic ring is aromat ic and at least one of the rings in the bicyclic ring is aromat ic. Unless staled otherwise, the valency of the group may be located on any atom of any ring within the radical, valency rules permitting. Representat ive examples include phenyl, napluhyl. and indanyl, and the like.
[0052] "Arylalkyl" means an alkyl radical, as defined herein; substituted with one of two a yl groups, as defined herein, e.g., benzyl and phenethyl. and the l ike;
[0053] "Cyanoalkyl'* means an alkyl group, as defined herein, substituted with one or two cyano groups.
[0054] "Cycloalkyi" means a monocycl ic or fused bicycl ic, saturated or partially unsaturated (but not aromatic), monovalent hydrocarbon radical of threcto ten carbon ring atoms. Fused bicycl ic hydrocarbon radical includes spin) and bridged ring systems. U nless slated otherwise, the valency of the group may be located on any atom of any ring within the radical, valency rules permitting. One or two ring carbon atoms may be replaced by a -C(O)-, -C(S)-. or -C(=NM)- group. More specifically, the term cycloalkyi includes, but is not limited to, cyclopropyl. cyclobuiyl. cyclopentyl, cyclohexyl. cyclohexyl. or cycl hex-3-cnyl, and die like.
[0055] "Cycloalkylalkyl" means an alk yl group subst ituted with at least one.
spccificallyone or two. cycloalkyi group(s) as defined herein.
[ 0056 J "Dialkylumiho" means an -NR ' radical where R and ; are alkyl as defined herein, or an N-ox ide derivative, or a protected derivative thereof, e.g., dimethylaminb.
dieihylamino. /V./V-mclhylpropylamino or /V. V-melhylclhylamino, and the l ike.
[0057 ] "'Dialkylami oalkyr means an alkyl group subst ituted wit h one or two dialkylaniino groups, as defined herein.
[0058] "Dialkylaminoalkyloxy" means an -OR group where R is dialkylaminoalkyl , as defined herein. Representative examples include 2-(/V./V-diclhylamino)-elhyloxy, and the l ike.
[0059] '"Dialkylaminocarbonyl*' means a -C(0)NRR' group where R and R' are al kyl as defined herein.
|0060] '"Fused ring sysLcm" means a polycycl ie ring system lii.it contains bridged or fused rings: that is, where two rings have more than one shared atom in their ring structures. In this application, fused ring systems are not necessarily all aromatic ring systems. Typically, but not necessarily, fiiscd. ring systems share a vicinal set of atoms, for example naphthalene or 1.2,3,4-teti ahydtO-naphthalenCi Fused ring systems of the invent ion may themselves have spiro rings attached thereto via a single ring atom of the fused ring system. In some. examples. as appreciated by one of ordinary skill in the art. two adjacent groups oil an' aromatic system may be fused together lo fonin a ring structure. The fused ring structure may contain heteioatoms and may. be optional ly substituted with oiic or more groups:
[0061] "Halogen" or "halo" refers to fluorine, chlorine, bromine and iodine.
[0062J "Haloalkoxy" means an -OR : group where R' is haloalkyl as defined herein, e.g., iriflitoi mclhoxy or 2.2.2-trifluoi cthoxy, and the l ike.
[0063] "Haloalkyl" mean an alkyl group substituted with one or more halogens, specifical ly I , 2. 3. 4. 5. or 6 halo atoms, e.g.. trifluoromethyl, 2-chioroethyl, and
2,2-difluoi cthyl. and the like.
10064] "Halocarbonyl" means a -C(0)X group where X, is halo.
[0065] "l-icicr aryr means a monocyclic or fused bicycl ic or tricycl ic monovalent radical of 5 to 14 ring atoms containing one or more, specifically one, two, three, or four ring heieroatoms where each heteroatom is independently -0-, -S(0)„- ( n is 0. 1 . or 2). -N=, -NH-. or N-oxide, with the remaining ring atoms being carbon, wherein the ring comprising a monocycl ic radical is aromat ic and wherein at least one of the fused rings comprising (he bicyclic radical is aromatic. One or two ring carbon atoms of any nonaromat ic rings comprising a bicyclic radical may be replaced by a -C(O)-, -C(S)-. or -C(=NH )- group. Fused bicyclic radical includes bridged ring ystems. Unless stated otherwise, the. -valency-may be located on any atom of any ring of the hetcroaryl group, valency rules permitting; When the
point of valcncyis. loc l ell on ihc liilrogcn. R* is absent. More specifically, ihe lerm heteroafyl includes, but is not limited to, 1.2.4-triazolyl, 1,3.5-triazplyl. phihalimidyl, pyridinyl. pyrrol l. imida/.olyl. lliienvl. I'uianyl. indolyl.2.3-dihydro- 1 /V-indolyl (including, for example, 2.3-dihydro- 1 /7-indol-2-yl or 2.3-dihydro- 1 /7-indol-5-yl. anil the like), isoindolyl, indolinyl. isoiiidolinyl. benziniida/olyl. bcnzodioxol-4-yl, benzofuranyl, cinnolinyl, indolizinyl, naphthyridin-3-yl. phlhalazin-3-yl, phlhalazin-4-yl. pteridinyl.
purinyl, quinazolihyl, 5,6.7, S-ictrahydroi|uinazoIinyl, quinoxaliriyl. leirazoyl. pyrazol l, pyrazinyl. pyrifniclinyl. pyridazinyl. oxazolyl. isooxazolyl, oxadiazolyl, benzoxazolyl, quiiiolinyl.5,6,7,8-lctralrydroquinolinyl, isoquinolinyl, letralrydroispquinolinyl (including, -for example. ieiraliydroisoi|uinolin- -yl or teirahydroisoquinolih-fi-yl. and the like). pyrrolo|;3,2- lpyridinyl (including, for example, pyriolo|3.2-c]pyridin-2-yl or pyrrolo|3.2-c]pyridin-7-yl. and the like), benzopyranyl.2.3-diliydt benzoiiiranyl. benzo|i/|| l,3|dioxolyl.2.3- ,
dihydrobcivzo[fi|| 1 ,4 |dioxinyl, thiazolyl, isotbiazolyl. thiadiazolyl, bcnzolhiazolyl, beiizothienyl.6,7-dihydi -5//-cyclopciila|/»jpyridiiiyl.6.7-dihydro-5/7- ;cyclopcnta|clpyridinyl.6.7-dihydr -5/7-cyciopenta| </|pyriniidinyK 5;6,7.'8-ieirahydro-5,H- ethanoquinazplin-4-yl. and 6,7, 8,9Heiraliydrepyrimido| 4,5-b |indolizin-4-yl. and the N-oxide thereof and "a protected derivative thereof.
[OOfifij "i-Ieteroarylalkyl" means an alk l group, as defined herein, substituted with at least One. specifically one or two hcteroary! group(s). as defined herein.
(0()67| "Mctcrocycloalkyl" means a saturated or partially unsaturated (but not aromatic) monovalent monocyclic group of 3 to 8 ring atoms or a saturated or partially unsaturated (but not aromatic) monovalent fused or spirocyclic bicyclic group of 5 to 12 ring atoms in which one or more, specifically one. two. three, or four ring heteroatoms where each hcicroatom is independently Θ, S(0)„ (n is 0.1, of' 2). -NH-. or-N=. the remaining ring atoms being carbon. One or two ring carbon atoms may be replaced by a -CCO)-. -C(S)-, or -C(=NM)- group. Fused bicyclic radical includes bridged ring systems. Unless otherwise slated, the valency of the group may be located on any atom of any ring within the radical, valency rules permitting. When (he point of valency is located on a nitrogen atom, Ry is absent. More specifically the term helerocycloalkyl includes, but is not limited to. azelidinyl. pyrrolidinyl. 2-oxopyrrolidinyl, 2,5-dihvdro-l/7-pyrrolyl. pipcridinyl.4-pipcridonyl. morpholinyl.
pipera/.inyl.2-oxopipcrazinyl. tetrahydropyranyl.2-oxopipcridinyl. ihiomorpholinyl, thiamorpholinyl, perlvydroazepinyl. py azolidinyl. imidazolinyl. imidazolidinyl.
dihydropyridinyl. leirahydropyridinyl. oxazolinyl, oxazolidinyl, isoxazolidinyl. ihiazolinyl, thiazolidinyl. quinuclidinyl. isoihiazolidinyl. oclahydrocyclopcnia c]pyrrolyl.
octahydroindolyl, oc ihydroisoiiidolyl, deeahydroisoquinolyl, 2 dia .aspiro| 3.3 |hcplan-2-yl. lelrahydrpfuryl , and leirahydropyranyl. and the derivat ives thereof and N-ox iclc or a protected derivative■ thereof.
Γ0Θ68] "Hctcrocyclonlkylalkyl" means an alkyl radical, as defined' herein, substituted with one or two hcierocycloalkyl groups, as defined herein, e.g.. morphol inyhncih yl. /V-pyi rol idinylcthyl . and - V-a xl idinyl)propyl . and the like.
[0069] "Hydroxyalkyl" means an alkyl group, as defined herein, subst ituted with at least one. particularly. 1 , 2, 3, or 4. hydroxy groups.
[00701 "Phenylalkyl" means an alkyl group, as defined herein, substituted with one or two phenyl ' group's-
[0071 J "Optional" or "opt ional ly'' .means thai the subsequently described evenror circumstance may or may not occur, and that the dcscriplioh includes instances where said event or circumstance occurs and instances in which it does not. One of ord inary sk ill in the art would understand that with respect to any molecule described as containing one or more optional siibstituents, only sierical ly practical and/or syntheticall y feasible compounds are meant to be included. "Opt ionally substituted" refers to all .subsequent modifiers in a term, unless statedOtherwise. A list of exeniplary opt ional substitutions is presented below in the definition of "substituted."
[0072] 'Optionally substituted aryl" means an aryl group, as defined herein, optionally substituted with one, two, three, or four subsliiuenls where the siibstituenis are independent ly acyl. acylamino. acyloxy, alkyl, haloalkyl, hydroxyalkyl. alkox yalky!, aminoalkyl .
alkylaminoalkyl . lialkylaminoalkyl, alkenyl, alkoxy. alkenyloxy. halo, hydroxy,
alkoxyearbonyl. alkcnyloxycarbonyl , amino.' alkylamino, dialkylamino. nitro. aminocarhonyl. alky!amiiiocarbonyl, dialkylaminocarbonyl, carboxy, cyano, alkylthio, alkylsul finyl.
alkylsiil l nyl. aminosiilfonyl. alkylaminosulfonyl, dialkylaminosul fonyl, alky!sulfonylamino, or aminoalkoxy; or aryl is pentanuorophenyl. With in the optional siibstituents on ''aryl". the alkyl and -alkenyl, . cither alone or as part of another group. (including, for example, the alk yl in alkoxycarb nyl), are independently optional ly substituted with one, two. three, four, or five halo (e.g. alkoxyearbonyl includes trifhioromelhyloxycarbonyl).
[0073] "Opt ionally substituted arylalkyl" means an alkyl group, as defined herein, substituted with optional ly subst ituted aryl. as defined herein.
[0074 ] ' ptionally substituted cycloalkyl" means a cycloalkyl group, as defined herein, substituted with one, two, or three groups where the groups are independent ly acyl , acyloxy. acylamino. alkyl, haloalkyl , hydroxyalkyl. alkoxyalkyl. aminoalkyl , alkylaminoalkyl.
dialkylaminoalkyl. alkenyi, alkoxy. alkenyloxy. alkoxycarbonyl, alkcnyloxycai bonyl .
alkylihio. alkylsulfinyl, alkylsull'onyl. aniinosulfonyl, alkylaininosulfonyl.
dialkylaininosiilfonyl . al kylsulfonylainino. halo, hydroxy, amino, alkylamino, dialkyiamino, aminocarbonyl. alkylaminocarbonyl, dialkylaminocarbonyl , nitro, alkoxyalkyloxy.
aniinoalkoxy, alkylaminoalkdxy. d ialk ylaniinoalkoxy. carboxy. or cyano. Within the above optional stibsiiiuents o "cycloalkyl". the alkyl and alkcnyl. cither alone or as pai l of another siibstiliicnl on the cycloalkyl ring, are independent ly opt ional l y substituted with one. two, three, four, or five halo. e.g. haloalkyl. haloalkoxy. haloalkenyloxy, or haloalkylsul fonyl.
[0075] 'Optionally substituted cycloalk lalkyf" means an alkyl group substituted with. at least one. specifically one or .two. optionally substituted cycloalkyl groups, as dcfined herein.
[0076] "Optionally, substituted heteroaryl" means a heleroai yl group Optional ly substituted with ne, two, three, or four siibsi ilucnls where the substiluents are independently acyl, acylamino, acyloxy. alkyl. haloalkyl. hydroxyalkyl. alkoxyalkyl. aminoalkyl .
alkylaminoalkyl. dialkylaminoalkyl. alkcnyl, alkoxy. alkenyloxy. halo, hydroxy,
alkoxycarbonyl, alkcnyloxycai bonyl . amino, alky imino, dialkyiamino. nitro, aminocarbonyl. alkylaminoearboiiyl, dialkylaminocarbonyl, carboxy, cyano, alkylihio, alkylsulfinyl, alkylsulfony!. aniinosulfonyl, alkylaiui nosulfonyLdialkyla
aniinoalkoxy, aikylaiiiinoalkoxy. or dialkylaniinoalkoxy. Within the ptional substiluents on "hciciOaiyl", the alkyl and alkenyi, cither alone pi: as part of another group (including, for example, the alkyl in alkoxycarbonyl). are independent ly optional ly substituted with one. two, three, four, or five halo (e.g. alkox ycarbonyl includes iri fluoiOincthyloxycarbonyl).
[0077] "Optional ly substituted heicroar lalkyl" means an alkyl group, as defined herein, substituted with al least one, specifical ly one or two, opt ional ly substituted heteroaryl group(s), as defined herein.
[0078] "Optionally substituted heterocycloalkyl" means a hctcrocycloalkyl group, as defincd herein, optionally substituted with one, two. three, or .four, substiluents where the subsiilue is arc independentl y acyl. acylamino. acyloxy. alkyl. haloalkyl, hydroxyalkyl. alkoxyalkyl, aminoalkyl, alkylaminoalkyl. dialkylaminoalkyl , alkcnyl. alkoxy. alkenyloxy. halo, hydroxy, alkoxycarbonyl. alkcnyloxycai bonyl. amino, alkylamino. dialkyiamino. nitro. aminocarbonyl. alkylaminocarbonyl. dialkylaminocarbonyl. carboxy. cyano. alkylihio. alkylsulfinyl .
alkylsull'onyl , aniinosul fonyl. alkylaniinosulfonyl. dialkylaminosulfon l . alk ylsulfonylamino. aniinoalkoxy, or phenylalkyl. Within (lie optional substiuienls on "heterocycloalkyl", the alkyl and alkcnyl, cither alone or as part of another group (including, for example, the alkyl
in alkoxycarbonyl). arc independently optionally subst ituted with one, two. three, l our, or five halo (e.g. alkoxycarbonyl includes'lrinuroineiltyloxycarbonyl).
| 0079] 'Optionally subst ituted hcterocycloalkylalkyl" ' means an alkyl group, as derineil herein, substituted with at least one, specifically one or two, optionally .substituted
heiei cycloalkyl giOiip(s) as defined herein.
[ ()80 | "Optional ly substituted phenyl" means a phenyl group opt ionally subst ituted with one. two. or three subsi ituents where the sub.stiiueni.s are independently acyl, ac lamino. acyloxy, alkyl, haloalkyl, hydroxyalkyl. alkoxyalkyl . aminoalkvl . alkylaminoalkvl.
dialkylaminoalkyl. alkenyl. alkoxy. alkenyloxy, halo, hydroxy, alkoxycarbonyl.
aikenyloxycarbonyl, amino, alkylamino, dialkylamino. niiro. amiiiocat bonyl,
alkylaminocafbonyl, dialkylaminoearbonyl. carboxy. cyaiio, alkylthio, alkylsul fihyl, alkylsiilfonyl, ammosul fonyl, alkylaminosullOiiyl, dialkylaminosul fonyl, ajkylsujfonyJamino, or aminoalkoxy. "Optionally substituted phenyl" in addition includes peniafluorophenyl. Within the optional subsiituents on "phenyl", the alkyl and alkenyl, either alone or as part of another group ( including, lor example, the alkyl in alkoxycarbonyl ). are independently optionally substituted- with one. two. three, four, or five halo (e.g. alkoxycarbonyl includes iri fluoromethylpxycarbonyl ).
[0081] "Optionally substituted phcnylalkyl" means an alkyl group, as defined herein, subst ituted with one or two opt ionally substituted phenyl groups, as- defined herein.
1 082] "Oxo" means an oxygen which is attached via a double bond.
[0083] "Yield" for each of the reactions described herein is expressed as a percentage of the theoretical yield.
[0084] "Metabol ite" refers to the break-down or end product of a Compound or its salt produced by metabol ism or biotransformation in the animal or human body: for example, biotransformation" ίό it more polar molecule such as by ox idat ion, reduction, or hydrolysis, or to a conjugate (sec Goodman and Oilman. "The Pharmacological Basis of Therapeutics" 8.stip.ih Ed., Pergamon Press. Oi lman et al. (eds). 1990 for a discussion of
biotransformation). As used herein, the metabol ite of a Compound of the invention or its salt may be the biological ly active form of the Compound in the body. In one example, a prodrug may be used such that the biologically active form, a metabol ite, is released in vivo. In another example, a biological ly active metabolite is discovered screndipilously. that is, no proclaig design per se was undertaken. An assay for activity of a metabolite of a Compound of the present invention is known to one of skill in the art in light of the present disclosure.
100851 "Patient" for the purposes of t he present invent ion includes liuinai is and other animals, particularly mammals, and other organ isms. Thus the methods are appl icable to both human therapy and veterinary appl icat ions. In a specific embodiment the patient is a mammal, and in a more specific embodiment the pat ient is human.
| ()086j A "pharmaceutically acceptable salt" of a Compound . means a salt thai is pharmaceutical ly, cceptable and that possesses the desired pharmacological act ivity of the parent compound. It is understood that the pharmaceut ically acceptable salts arc non-tox ic. Additional information on suitable pharmaceut ical ly acceptable sal ts can be found i n Reniingwn 's Pharmaceutical Sciences. 17''' ed.. Mack Publ ishing Company. Easton. PA. 1985. which is incorporated herein by reference or S. M. Bei ge, et al., "Pharmaceutical Salts," J . Pharm.. Sct., 1977:66: 1 - 19 liot of which are incorporated herein by reference.
[0087] . Examples of pharmaceulicaliy acceptablt acidiaddit ion sails include those formed with inorganic acids such as hydrochloric aeidv'hydiObiOinic acid, sulfuric acid,™ti ic acid, phosphoric' acid, and the like; as well as organic acids such as acetic acid. irifiuoiOacelie acid, propionic acid, hexanoic acid', cyelopentancpropionie acid, gl ycol ie acid, pyruvic acid, lact ic- acid, oxalic acid, nialeic acid, malonic acid, succinic acid, l umaric acid, tartaric acid , citric acid, benzoic acid, cinnamie acid, 3-(4-hydroxyben/.oyl )hcn/.oic acid, niandel ie acid, methanesulfonie acid, ethanesul foiiic acid, 1 .2-eihahedisul fonic acid.
2-hydroxyelhanesulfonic acid, benzencsul fonic acid. 4-clvlproben7.enesulfpnic acid.
2-naphlhalenesul fonic acid. 4-tolucnesuliOnic acid, camphorsul fonic acid, glucohcptonic acid. 4-,4'-methylcnebis-(3-hydro'xy-2-ene- l -carboxylic acid). 3-phenylprop'ion.ic acid, irimeihylacetic acid, tertiary butylaceiic acid, lauryl sulfuric acid, gluconic acid, glutamic acid, hycli oxynaphthoic acid, sal icylic acid, stearic acid, muconic acid, p-toluenesulfonic acid, and salicyl ic acid and the l ike.
[0088 | Examples of a pharmaceutical l y acceptable base addit ion salts include those formed when an acidic proton present in the parent Compound is replaced by a metal ion, such as sodium, potassium, l ithium, ammonium, calcium, magnesium, iron. /inc. copper, manganese, aluminum salts and the like. Specific salts are the ammonium, potassium, sodium, calcium, and magnesium salts. Salts derived from pharmaceutical ly acceptable organic non-tox ic bases include, bul arc not l imited to, salts of primary, secondary, and icmiw amines, substituted amines including naturally occurring substituted amines, cyclic amines and basic ion exchange resins. Examples of organic bases include isopropylam ine. irimethylaminc, diethylamine, irieihylaminc, iripropylamine. eihanolainine,
2-dimethylaminoeihanol, 2-diethyiaminoethanol. dicyclohexylaniine. lysine, arginine.
hisiidinc. caffeine, procaine, hydrahainine. choline, belaine, elhyle cdiaminc, glucosamine. mcihylglUcaminc, -theobromine, purines, piperazine, pipcridine. N-eihylpiperidine.
tromcihamine. /V-uielhylglucamine. pol amine resins, and the l ike Exemplary organic bases, arc isopropylariiiuc. die!hylamine. clhanolamine. iriineihylainiric 'clicycl hcxyl;iinine, choline, and caffeine. "I¾tin(s)t" and "plalin-containing agenl(s)'' ,inclndc. for example, eisplaiin. carb.oplaiin, and oxal iplatin.
[0()89 |
'"Prod rug" refers to compounds thai are transformed ( typically rapidly) /; viva to yield the parent .Compound of ilic above Formula e. for example, by hydrol ysis in blood. Common examples include, but are not limited lo. ester and amide forms of a Conipouiid havin an active form bearin a carboxylic acid moiety. Examples -of pharmaceutical ly acceptable esters of the compounds of this invent ion include, bu are nol limited to. alkyl csiers ¾|qr . example with between aboul one and about six carbons) ilie- alkyl group is a .straight. or branched chain. Acceptable esters also inelude cyclPajkyl
1 esters and!arylal yT esters such as. but not limited to benzyl. Examples of harmaceut ically acceptable amides of the compounds of .this invention include, but are not limited to, primary amides, and secondary and icrliary alkyl amides ( for example with between aboui one and about six carbons ). Amides and esters of the compounds of the present invent ion may be prepared according to convent ional methods. A thorough discussion of prodrugs is provided ,in T. Higuehi and
sy..:$lcl la,
Dcliveiy¾ystemsV" Vdf,1 b the A;C;S.
Symposium Series, and in B ipreversiblc Gamers in Dru ■ 'Design', eel. Edward B. Roche, American X¾armacculical. Associat ion and Pergamoii Press, 1987. both of which are incorporated herein by reference for all purposes.
10090) 'Therapeutically effective amount" is an amount of a Compound of the invention, that when administered to a pat ient, ameliorates a symptom of the disease. The amount of a Compound of the invention -which constitutes a "lhcrapculically effective amount" wil l vary depending on the compound, die disease state and its severity, the age of the patient to be treated, and the like. The therapeutically effect ivc ainount can be deiermincd rout inel by-one of ordinary skill in ihe art having regard to their knowledge' and to ihis tlisclosuie.
[0091 J "Preventing" or "preveiil ioii" of a disease, disorder, or syndrome includes inhibiting the disease from occurrin in a human, i.e. causing the cl inical symptoms of ι he disease, disorder, or syndrome not to develop in an animal thai may be exposed to or predisposed to the disease, disorder, or syndrome but does nol yei experience or display symptoms of the disease, disorder, or syndrome.
10092| "Treating"' or "treatment" of a disease, disorder, or syndrome.. s used herein, includes ( i ) inhibiting the disease, disorder, or syndrome, i.e.. .arresting its development; and (ii ) relieving the disease, disorder, or syndrome, i.e. , causin regression of the disease, disorder, or syndrome. As is known in the an, adjustments for systemic versus local ized delivery, age, body weight, general health, sex. diet, time of adm in istrat ion, drug interaction and the severity of the condit ion may be necessary, and wil l be ascertainable with routine experimentation by one of ordinary skill in the art.
[009 J The compounds d isclosed herein also include al l pharmaceutically acceptable isoiopic variations, in wh ch' at- least one atom is replaced- by an atom having the same atomic number, but an atomic mass different from the atomic mass usual ly found in nature.
Examples of isotopes suitable for inclusion in the disclosed compounds include, without l imitation, isotopes of hydrogen, such as "H and Ή: isotopes of carbon, such as '"'C and '"''C: isotopes of nitrogen, such as | 3Ν; isotopes of oxygen, such as l 0 anil l!iO isotopes of phosphorus, such as '' P and Ή5; isotopes of sulfur, such as .sup '\S : isotopes of fluorine, such as I SF; and isotopes of chlorine, such as '"'CI. Use of isoiopic variations (e.g.. deuterium. ~H) may afford certain therapeutic advantages resulting from greater metabolic stability, for example, increased in vivo half-l ife or reduced dosage- requirements. Additionally', certain isotopic variations of the disclosed. compounds may incorporate a radioact i ve isotope- (e.g.. triiium, :iFI, Or C):, which may be useful, in drug and/or substrate t issue distribution studies.
1
Embodiments of the Invention
f 00941 The fol lowing paragraphs present a number of embod iments of compounds of the invention. In each instance ihe embodiment includes both the recited compounds, as well as a single stereoisomer or mixture of stereoisomers -thereof, as wel l as a pharmaceutical ly acceptable salt thereof.
[00951 Embodiments ( A.I ): In.oiie embodiment, the Compound of Formul 1 is that where Ryd is hydrogen or. alkyl and RIL'. R5'1. R5E, R5', and' R58 arc hydrogciu.and all other groups are independently as defined in the Summary of the Invent ion for a Compound of Formula \. In another embodiment, the Compound of Formula 1 is that where R5 ' is alkyl and R5 . R3J. R . R51. and R5S are hydrogen: and al l other groups are independent l y as defined in the Sum mary of the Invention for a Compound of Formula 1.
|0096 ] Embodiments ( Λ2 ): In another embodiment, die Compound of Formula I is that where R51' is (Cj .j)alkyl and R5A, R5\ RSD. R5L', RIR. R5-E. and R5H are hydrogen; and ail other groups are independently as defined in the Summary of the Invention for a Compound of
Formula I. In another cmboii iment. the Compound of Formula 1 is that where R L is halo(CY alkyl and R5A. R3 . R5d, RStf. RS(. R¾, ami R51' are hydrogen: and al l other groups arc independently as defined in the Summary of the Invention l or a Compound of Formula 1. In another embodiment, the Compound of Formula I is that, where R51' is methyl and R , R' . R5'1. R¾c, R3F, R5^ and R"1'1 are hydrogen; and al l other groups are independently as defined in the Summary of the Invention for a Compound of Formula 1 In another embodiment, the Compound of Formula I is that where R^1' is mediyl ; RSs. R ' . R5'1. R5", R-?r;,R¾, and R H are hydrogen: and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I.
| 0()97 | Embodiments (A3 ): In another embodiment, the Compound of Formula I is thai where R5 'is hydrogen or alkyl and RSA. R5'1. R5l;. R5f. and R3II are hydrogen; and all other groups arc independently as defined in the Summary of the Invent ion for a Compound of Formula I. In another embodiment, the Compound of Fo mula I is that where R5 is alkyl and R5 ', R5'1. R?F. and R3e arc hydrogen: and all other groups arc independently as defined in the Summary of the Invent i n for a Compound of Formula. I.
| ()098| Embodiments (A4 ): In another embodiment, the Compound of Formula I is that where R5H is hydrogen or halo and R5;\ RSc. R5D. R5C. R5\ Ss are hydrogen: and all other groups are independently as defined in the Summary ol' the Invention for a Compound of Fonnula I. In another embodiment, the Compound of Formula I is that where R3h is halo and R5:'. R5L . R?D, R . R51, R— are hydrogen: and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula i. In another embodiment , the Compound of Formula 1 is that where R51' is fluoro and R5 . R C. R5D. R5C. R5 R, R¾ are ■hydrogen;- arid al l other groups are independently as defined in the Summary of the Invent ion for a Compound of Formula I.
[009!)) Another embodiment o ihe Invention is directed to a Compound of Formula 1(a)
1(a)
where R 1 and R2 are independent ly as defined in the Summary of the Invention for a
Compound of Formula I.
IdO lOOJ In another embodiment of a compound of Formula la. R51' is methyl, ethyl, propyl. ' or trifluoromethyl.
IdOlOlj ln another embodiment of a compound of Formula la. R3'1 is methyl, or trifluoromciliyl.
100102] Embodiment ( Γ): In another embodiment, the Compound of Formula 1(a) is ihat where
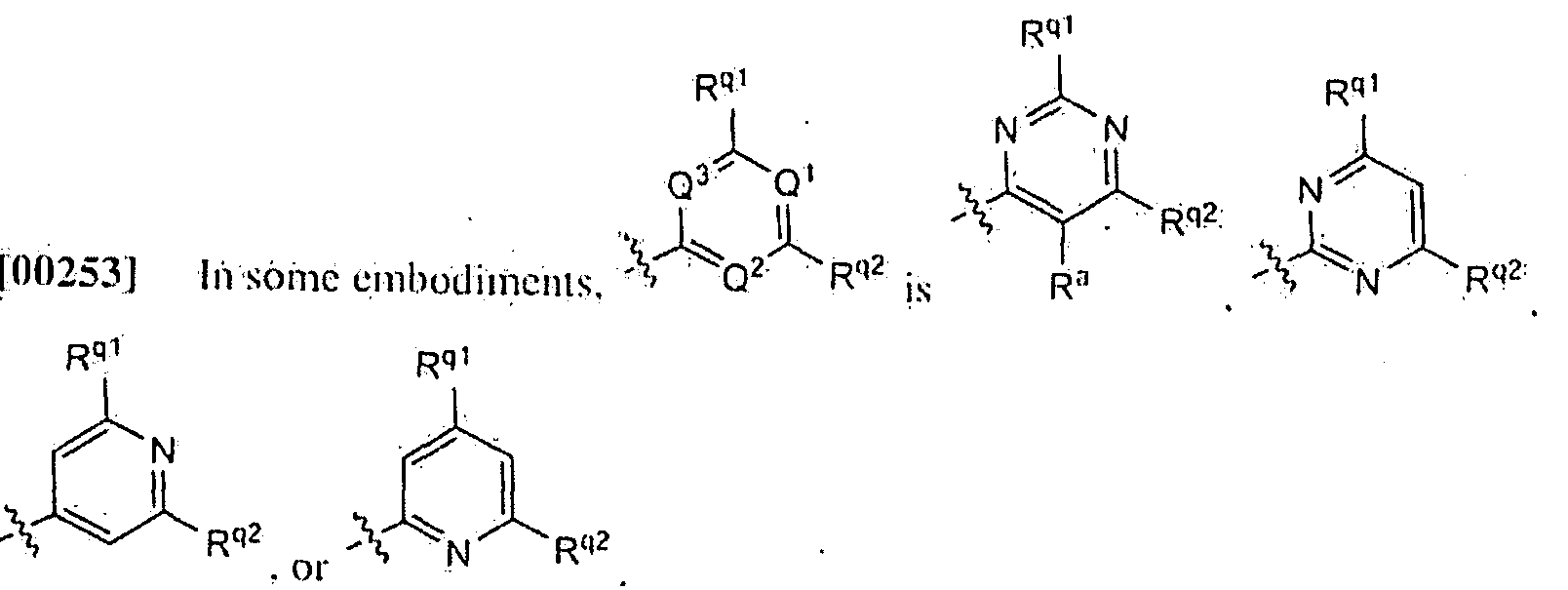
R1 is phenyl opiionall v .substituted with one. two, or three R* groups: or
R1 is hctcroaryl optionally subsiiiuied with one. two. or three R7:
R- is hctcroaryl substituted with R '. R¾ 3B. R . and R-,J;
R3. RJ1. Rjl', R'\ and R'"1 are independently hydrogen: cyano: alkyl; alkenyi: halo; haloalkyh hydipxyalkyl; alkoxyalkyl: cyanoalkyl: SR1 '; -S(0):R3 ; carboxy. alkoxycarbonyl:
lialoearbonyl; -NR".R":I: -ORII:i: phenyl optionally substituted with one or two groups winch arc independently alkyl or halo.; phcnylalkyl optionally substituted with one or-.two R19; cycloalkyl: cyeloalkvlalkyk heierocycloalkvl optionally substituted with one or two groups which are independently alkyl, alkoxycarbonyl. or bcn/.yloxycarbonyl:
hetciOcycloalkylalkyl optionally substituted with one or two groups which are
independently, alkyl, alkoxycarbonyl, or benzyloxycarbonyl; heteroaryl: hcicroarylalkyl; oralkyl substituted with one or two R1'1; or
two of R'\ R'M, R',h. R,c. and R'"\ wlien attached to the same carbon, form a cycloalkyl or a heiei cycoaikyl; and the other of R3, R; ..R31', R*', and R '1 are hydrogen;
eaCh-R"; when R6 is present, is independently niiro: cyanq; lialo; alkyl; halo; luiloalkyl:
-ORSn; -N ^ 8*;- -C(O¾NR*RSA; -Sl[0). -NRSC(0)R¾; ^NR'SS(0) RS!'; -NI-1CC0) HR"; carboxy. -C(0)OR''; or hctcroaryl optionally subsiiiuied with 1.2, or 3 R '''·.
each R7. when R' is present, is independently oxo: nitro: cyano: alkyl: alkenyi: halo:
haloalkyh hydroxyalkyl: alkoxyalkyl: -OR*1: -S 13: -S(0)KIJ: -S(0).Ri:i:i: -NR8RS":
-C(0)NR!IR!IA: -NRSC(OjOR': -N ^OJR'': -NRsS(0):R ': -NRSC(0)NRSI,R": -C(0)OR": lialoearbonyl: -S(0):>NRKR''; alkylsulfonylalkyl: alkyl substituted with one or two
-NR8R*'; alkyl substituted with one or two -NRSC(0)RXil: alkyl substituted with one or two -NR C(0')OR!': alkyl subsiiiuied with one or iwo -'SiO.' R1'^; cycloalkyl;
cycloalkyl alkyl; heterocycloalkyl opiionallv subsiituied with one or two groups which are independently alkyl or amino; phenyl; phcnylalkyl; helerocycloalkylalkyl; hctcroaryl; or hcicroarylalkyl:
RS. R11. R15. I*17, and RIS arc independently hydrogen, alkyl. alkenyi. alkynyl. hydroxyalkyl. alkoxyalkyl, or haloalkyl;
RS:|; Rlla; and RIS:| arc independently hydrogen: alkyl; alkenyi; alkynyl: haloalkyl;
hydroxyalkyl; cyanoalkyl: aminoalkyl: alkylaminoalkyl; dialkylaminoalkyl; alkoxyalkyl:
carboxyalkyk.cycloalkyl ; cycloalkylalkyl; heierocycloalkyl opi ional ly subst ituted with one or two groups which arc independentl y alkyl . alkox ycarbonyl. or benzylox y:
hcteiOcycloalkylalkyl opt ionally substituted with one or two groups which arc independently alkyl, alkoxycarbonyl. or benzyloxy. phenyl opi ionall y substituted with one or two groups which are independently halo, alkyl. or alkoxy: phenylalkyl ;
hetcroaryl; or hcleroar.ylalkyl;
Ry is hydrogen: alkyl ; alkenyl: alkynyl: hydroxyalkyl : alkoxyalkyl : aminoalkyl:
alkylaniiiioalkvl; dialkylaniiiioalkyi; haloalkyi ; hydiOxyalkyl subst ituted with one, two. or three groups which are indcpcnclently halo, amino, alkylamino, or dialkylamiiio: alkyl substituted with one or two aminocarbonyl; phenyl : phenylalkyl; cycloalkyl :
cycloalkylalkyl opiionally substituted with one or two groups which are independently amino or alkyl; heierocycloalkyl optional ly substituted with one or two groups which arc independently alkyl. alkoxycarbonyl, or benzyloxy: or heterocyeloalkylalkyl opiionally substituted with one or two groups which are independently alkyl. alkoxycarbonyl. or benzyloxy;
R12 is alkyl or phenylalkyl :
Ri is alkyl. hydroxyalkyl. or. haloalkyi; and
R 1 ' is hydroxy, alkyl, haloalkyi , hydroxyalkyl, or heierocycloalkyl optionally substituted with one or (wo groups which are independently halo, amino, alkylamino, dialkylamino. hydroxy, alkyl, or hydroxyalkyl :
each R H, wheii 1"' is present, is independently amino, alkylamino. dialkylamino. acy!amino. halo, hydroxy, alkyl. haloalkyi . hydroxyalkyl. aminoalkyl , alkylaminoalkyl.
dialkylaminoalkyl, alkoxycarbonyl, aminocarbonyl. alkylaminocarbonyl,
dialkylaminocarboiiyl. or phenyl ;
each R16 is independently halo. -NIl'V*. -NRI SS(0)R 151'. -OC(0)R 17. or -OR 1*;
each Riy is independently halo, alkyl. haloalkyi. amino, al kylamino. dialkylamino. or alkoxy: and
R"° is amino, alkylamino, dialkylamino. or heierocycloalkyl.
[00103J Embodimcni (B ): In another embodiment, the Compound of Formula 1(a) is that where R 1 is hcleroaryl optionally siibsiiliiied with one, two. or three R7 groups; where each R7 independently of each oilier (when R7 is present) and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ), (A2). (A3), (A4). and ( I ). In anoiher embodimeul . the Compound is according lo Formula 1(a) where R 1 is 3. -d ih dro-2 /-/- y l idoj 3 ,2-A> 11 1 .4 Joxazinyl.
pyrido|2.3-//|pyrazinyl, ii dazof 1 ,2-i/|pyriiiiidinyl, imidazo| 1 ,2-«|pyridinyl. iriazolof 1.5- r< Ipyridinyl, indolyl. 2,3-dihydiObenzofuranyl. benzo|/>|lhienyl, cjuinolinyl.. bcnziintdazolyl. indazolyl, I /7-pynolo| 2, 3-/> Ipyridinyl. pyridinyi. pyriniidinyl. pyridazinyl. ihicnyl. thiazOlyl. benzothiazolyl. imidazopy idinyl. pyrazolopyi idinyl, pyiTolopyridinyl, oiMlii zolopyridinyl' where R1 is oplionally subsliiiiied with one. two, or ihrec R7; where each R ' independently of eac!i oilier (when R7 is present) and all oilier groups arc independently as defined in ihe Summary of ihc Invention for a Compound of Formula I or as defined in any of
Embodiments (Al ), (A2). ( A3). ( A4). and ( I ).
100104 j Embodiments (H I ): In another embodiment, ihc Compound is according to Formula 1(a) where R1 is a )-membercd lieteroaiyl oplionally substituted with one. two. or
7 7 " 7
three R ; where: each" R independently of each other (when R -is -present) . 'and all oilier groups are indepcndenliy as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embocliments (A ! ). (Λ2). (A3). (Λ4 ). and ( I ). In another embodiment, the Compound is according to Formul 1(a) where R 1 is benzimidazolyl. iinidazo|4,5- /> Ipyridinyl. imidazo|4.5-c|pyridinyl. 3/7-imidazo[4.5-< pyridinyl, indazolyl. 1 H- pyrazolol ,4-/7 Ipyridinyl, indolyl, 1 A/-pyrmlo|2.3-/>|pyridinyl. I /7-pyrrolo| 3.2- > Ipyridinyl. benzo| d|ihiazolyl, lhiazolo| .5-/; Ipyridinyl. ihiazolo|4.5-i- Ipyridinyl. lhiazolo|5,4- |pyridiiiyl. or ihtazolo|5.4-/ |pyridinyl, and R1 is optionally .substituted with one, two, or three R'7; where each R' independently of each other (when R7 is present) and all other groups are
indepcndenliy as defined in the Summary of ihe Invention for a Compound of Formula 1 or as defined. in any of Embodiments (A l). (A 2). (A3). (A4). ancl (J ).
[00105] Embodimcnis (B 1 ): In another embodiment, the Compound is according to Formul 1(a) where R1 is 3/7-imidazo|4.5-/j|pyridinyl. l /7-imidazo|4 5-/>|pyridinyl. 3/7- imidazo|4,5-c|pyridinyl. or 1 /7-imidazo|4. i:|pyridinyl, where R1 is optionally substituted
7 7 ' 7 with one, two, or three R groups; where each R independently of each other (when R
' is · preseiii) and all other groups are indepcndenliy as defined in ihe Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ), (A2), (A3), (A4). and ( Γ). In anodier embodiment, the Compound is according lo Formula 1(a) where R
1 is 3/7-
3/7-imidazo|4,5-/>|pyriclin-6-yl, l /7-iniidazo|4,5-/;|pyridin-6-yl. 3/7-imidazo[4,5-r|pyridin-6-yl. l /7-imidazo|4,5-£. |pyridin-6- yl, 3/7-imidazo|4
",5-e|pyridin-5-yl, or l /7-iniidazo|4.5-f |pyriclin-5-yl. where R
1 is oplionally substituted with one. two. or ihrec R
7 groups; where each R
7 indepcndenliy of each other (when R
7 is present) and all other groups arc independently as defined in ihe Summary of the Invention for a Compound of Formula I or as defined in any of Embodimcnis ( A I ), (A2),
(A3), (A4), and (1). In anolher embodiment, the Compound is according 10 Formula 1(a) where R
1 is.3tf-imida/.o|4,5-/>|pyridin-5-yl. I /7-iinitla7.o|4.5-/jpyri(lin-5-yl.3/7-imidazo|4.5- /|pyiidi.n-6-yl, i/ -iniidazo|4.5-/j|pyridin-6-yl.3/7-imida7o|4,5-c|pyridin-6-yl, I //- iniida-ol^ - pyndin-^-yl.377-iiiiida2o|4.5- |pyridiii -yl, or l77-iniidazo|4.5- .||)yriclin- yl, where
1 is optionally substituted. with ,ojic or two
;R
7; each R
7. when R
7 is present, is
: independently halo, alkyl, cycloalkyl, haloalkyl, liydrexyallvyl.-alkoxyalkyl, alky I substituted with one or two -NR
'¾
8\ alkyl substituted with one or two -NR
SC(0)OR''. -NR
SR
S:i. or -NR
SC(0)OR
V: and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as. defined in any of Embodiments (Al ). (A2). (A3). (A4 )..and (1 ). In another embodiment, the Compound is according to Formula 1(a) where R
1 is 3/7-iniiclazo|4,5 |pyridiu >-yl.177- i midaxo[ 4 ,5-i | pyrid i n-5-y 1 , 3 //- im itlazo| 4,5- /ipyridih-6-yi. l/7-imida7. |4.5 |pyridin-6-yi.3/ - i m
" i d ΰκο [ 4: 5 - | py ri d ui - - y I , Ί /-
^ tniidiizo|4,5- - lpyridin- - 1.3// -ihiid zpl 4 ,5 - | pyrid in-'5-y 1 p :l/7-iinida7-p|4. lpyridin-5- yl, where R1 is ptionally stib>{titytedAyith¾nii'orl\yo'¾vj eaeh,:R7.vvhen R7 is present, is independently halo, alkyl. cycloalkyl, haloalkyl, hydroxyalkyl. alkoxyalkyl. alkyl substituted with one or two -NR'SRS;1. alkyl substituted with one or two -NRSC(O)OR\ -NRSRS\ or -NRSC(0)OR''; RS and RS:' are independently hydrogen or alkyl: R" is alkyl, benzyl. Or haloalkyl; and all other groups are independently as defined in tlic Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A. I ), (A2), (A3), (A4). and (1)..
[00106] Embodiments (B 2): In anolher ehibddimeiif. the Compound is ac'eprding.io
Formula i(b
I(b2)
where. R7. when R7 is present, is halo, alkyl. cycloalkyl. haloalkyl. hydroxyalkyl. alkoxyalkyl. alkyl substituted with one or two -NRsRSa. alkyl substituted with one or two -NRxC(0)ORy, -NRhRs\ or -NRlfC(0)OR'"'; and R" and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of
Embodiments (Al), (A2). (A3). (A4), and (I). In anolher embodiment, the Compound is according to Formula I( I ). or l(b2), where R7. when R' is present, is alkyl. cycloalkyl.
haloalkyl, hydroxyalkyl, alkyl substituted with one or two -NRsC(0)ORy. -NRSRS;>. or
-NRSC(0)OR''; R is hydrogen or alkyl; RS;' is hydrogen, alkyl. or haloalkyl; R-' is alkyl or benzyl; and R2 and all other groups are independently as defined in the Summary of die Invention for a Compound of Formula I or as defined in any of Embodiments (Al), (A2), (A3), (Λ4), and (I). In another embodiment, the Compound is according to Formula I(bl) or I(b2), where R7, when R' is present, is methyl, ethyl, n-propyl, isopropyl. eyclopropyl.
eyclobiilyl. monofluoromethvl. cliiluorpmciliyl, trifkioromeihyl, I -hyclroxyelhyl.
2-hydroxycihyl. amino, mcihylamino. eihylamino. incihoxycarbonylamino.
bcnzyloxyearbonylamino. aminoinethyl. meihylaminomethyl. or dimethylaininomethyl: and R" and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A l , (A2). (A3). (A4), and (.1).
[001071 Embodiments (R3-T: In another embodiment.' theCqmpound' f Formula I is according to Formula 1(a) where R1 is benzoi</|lhiazolyl. iliiazolo| .4-/p|pyridiiiyl', ihiazolo|5.4-t|pyridinyl, thiazolo|4.5-/ |pyridinyl, or thiazGlo|4.5-£-|pyridinyl. where R1 is optionally substituted with one, two. or three R' groups: where all other groups and each R . when R7 is present, arc.tiulcpendeitily as defined in the Summary of the Invention for a Compound of Formula I or.as defined in any of Embodiments (A I ), ( A2), (A3). ( A4), and ( l ) In another embodiment, the Compound of Formula I is according t Formula 1( a) where R1 is benzol i/|thiazol-5-yl. benzo|</|ihia/.ol-6-yl, thiazolo|5.4-/ |pyridin-5-yl. thiazolo(5,4- A>)pyridin.-6-yl. thia/.()lo|5.4-f;|pyr.idin-6-yl. thia/.ol)|4,5-/ |pyridui-5-yl. thiazolo|4,5- /|pyridin-6-yI. or thiazolo|4.5- |pyridin-fi-yl. where R1' is optionally substituted with one. two. or three R7 groups: where all other groups and each R7, when R7 is present, are independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in any of Embodiments (A I ). (Λ2), (A3), (A4). and ( 1 ). In another embodiment, the Compound of Formula 1 is according to Formula 1(a) where R1 is ihiazolo|5,4-/ |pyridin-6-yl or ihiazolo|4.5-/;|pyridin-6-yl optionally substituted with one R7 where R7 is alkyl. -NR'SRS:I. or -N'R!'C(0)OR'"'; and other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A 1), ,(A2), (A3), (A4). and ( 1 ). In another embodiment, the Compound of Formula I is according to Formula 1(a) where R1 is thiazo!o|5.4-/>|pyridin-6-yl or ihiazolo|4.5-/j]pyridin-6-yl optionally substituted with one R7 where R7 is -N ^ ^: and other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (Al ). (A2). (A3). (Λ4), and (1). in another embodiment, the Compound of Formula I is according to Formula 1(a) where R1 is tliiazoio|5.4-/>]pyridin-6-yl or
lliia¾:i>lo[4,5-/;|pyri'ilii>6-yl opiionally .subslimicd with one R7 where R 1 is all y), -NR^R^'. or -NRXC(0)OR,J; each RS. RS", and R'', independently of each other; are hydrogen or alkyl; and other groups are independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in any of Embodiments (A I ). (A2). (A3.). (Λ4). and ( I ).
|l)01081 Embodiments ( B4): In another embodiment, the Compound is according to Formula l(cl
where X 1 is N or CH; R7 (when present). R2, and all other groups arc independently as defined in the Summary of the Invention for a Compouiid of Formula lor as defined in any of Embodiments (A 1). (A2). (A3). (A4), and ( 1 ). In another embodiment, the Compound is accordin'g to Formula l(c l ) or l(c2) where X 1 is N or CH: R7. when R7 is present, is alkyl, -NRSRS:|. or -NR C(0)R": and R2 and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in any of
Embodiments (Al ), (A2). (A3).-(A4), and ( 1 ). In another embodiment, the Compound is according to; Formula l(c l ) or I(c2) where X' is N or CH: R ', wiieiv R ' is present, is alkyl. -NR- RS\ of -.NRSC(0)R'-; each RS and RSA are 'independently hydrogen or alkyl and R*' is alkyl; and R2 and all other groups are independently as dc fined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ). (A2 ), (A3), (A4). and ( I ). In another embodiment, the Compound of Formula I is according to Formula l(c l ) or I(c2) where X 1 is N or CH: R '. when R7 is present, is C| .j-alkyl. amino, or C| .3-alkylcarbonylamino: and R2 and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of
Embodiments (Al ). (A2). (A3), ( A4), and ( Γ). In another embodiment, the Compound is according to Formula l(c l j or .I(c2) where X1 is N or CH; R7, when R7 is present, is -NRSR'¾ where RS and RSA are indcpendcnlly hydrogen or alkyl; and R2 and all other groups arc . independently as defined in the Summary of the Invent ion for a Compound of Formula I or as defined in any of Embodiments (A l ). ( A2). (A3). (A4). and ( I ). In another embodiment, the Compound is according to Formula l(c l ) or I(c2) where X1 is N or CH; R '. when R ' is present, is -NR^R8'1 where R8 and R^' are independently hydrogen or C|..ralkyl; and R2 and all other groups are indcpendeiuly as defined in the Summary of the Invention for a
Compound of Formula I or as .defined in any of Embodimenis (A I). (A2). (A3). (A4). and (I).
[0()109| Embodimenis (B4a): lii inodiei einbodiineni. the Compound of Formula I is according to Formula I(cl ) or I(c2) where X
1 is N; R
7 (when present). R
2 and all other groups are independently as defined in the .Summary of the Invention for a Compound of Formula I or as 'defined in any of Embodiments (A I). (A2). (A3). (Λ4). and (I ). In another embodiment, the Compound: of Formula I is according lo Formula 1(c) where X is N; R , when R is present, is alkyl, -NR
SR
S:|. or -NR¾(0)R": and R
" and all oilier groups are independently as defined in the Summary of -
"the Invention for aCompound f
'Fornnila I or as defined in any of Embodiments (A 1). (A2). (A3). (A4), and (i). In another embodiment, the Compound of Formula I is according to Formula J(:c I ) or I(c2) where X
1 is ; R
7. when R
7 is present, is alkyl. -N
'R
XR
'S¾, or -NR
,sC(0)R'
:'; each R
s and R
Sa arc independently hydrogen or alkyl and R
' is alkyl; and R
" and all other groups are independently as defined in the Summary of die Invention for a Compound of Formula I or as defined in any of Embodimenis (A 1 ). (Λ2 ). (A3). (A4), and ( I ). In another embodiment, the Compound of .Formula I is according to Formula I(cl):;or I(c2) where X
1 is N; R'. when R' is present, is Ci.ralkyl, amino, or C|., alkyrcarbonyiaiiiino; and-R" and all
Summary of the Invention for a Compound of Formul l r as defined in any of
F.inbodiments (Al). (A2), (A3). (Ά4). and (I). In another embodi VeiU, the Compound of Formula I is according lo Formula l(cl) or I(c2) where X1 is N; R7 when R' is present, is -NR'^R^'i.each s and Rs? arc indepeiulcnlly liydrogeri or alkyl: and R" and all oilier groups arc independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodimenis (A I). (A2). (A3). (A4). and (1 )..ln another embodiment, the Compound of Formula I is according lo Formula l(cl ) or I(c2) where X1 is N: R7. when R' is present, is.-NR''1R!'n: each Rs and RS:1 are independenily hydrogen or Ci.;,-alkyl: and R" and all other groups are independenily as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A.I ), (A2), (A3), (A4), and 0).
1.00.110] Embodiments (B4b): lii another embotlimenl. lite Compound of Formula I is according to Formula I(c I ) or I(c2) where X1 is C : R7 (when present). R2. and all other groups are independenily as defined in the .Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A 1 ). ( A2). (A3). (A4). and ( I ). In another embodiment, ihe Compound of Formula I is according to Formula .[(c l) or I(c2) where X1 is C; R7, when R7 is present, is alkyl. -iNR'V", or - R C(0)R": and R2 and ll other groups are
independently as defined in the Summary of" the Invention for a Compound of Formula I or as defined in any of Embodiments (A 1). (A2). (A3). (Λ4). and (I). In another embodiment, the Compound of Formula I is according to Formula l'(cl) or l(c2) where X1 is C: R'. when R' is present, is alkyl, _-NRsR*\ or - RsC(0)Ry; each R8 and are independently hydrogen or alkyl and Ryis :alkyl: aiid R" and all other groups are independently as defined in the
Summary of the Invention for a.Compound of Formula f or a.s defined in any of
Embodiments (A I). (Λ2). ( A3 ), (Λ4), and (i). In another embodiment, the Compound of Formula I is according to Formula I(cl
') or i(c2) where X
1 is C; R
7. when R
7 is present, is C|. i-alkyl, amino, or
and R
2 and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in any of Embodiments. (A I ), ( A2), (A3). (A4). and ( I ).' In another embodiment, the Compound of Formula I is Rec ding; lb Formula I(cl) or l(c2) where X
1 is C; R
7, when R
7 is preseni
lis. -NR^R^.eaeh R
h and R
Su arc independently hydrogen br.alkyl;.and R
2,and all other group are independentl as defined in llie Siihiiiary of the: Ιιίνςηΐίοη I¾»ra
'iC3 nVppuiW
':of
;l?5nniila I or as defined in any of Embodiments (A I ). (A2). (A3). (A4). and ( I ). In another embodiment, the Compound of Formula I is according to Formula l(cl) or I(c2) where X
1 is C: R
7, when R
7 is present, is -NR
h'R
Sa; each R and R
j arc independently hydrogen or Ci-.valkyl; and R
* aiicl all other groups are independently as defined in. the Summary of the Invention for a Compound of Formula I or as defined in any■ of Embodiments (A I). (A2), (A3). (A4). and (I).
[001111 Embodiments:(B5): In another embodiment', the Compoiind of Formula I is according to. Formula 1(a) where. R1 is bcnzimidazolyl optionally. substituted with one, two. -or three R7 groups; where all other groups and each R' independently of each other (when R' is present) are independently as defined in the Summary of the Invention for a Compound of Formula I or a.s defined in any of Embodiments (A 1 ). (A2). (A3). (A4). and (I ). In another embodiment, the Compound of Formula I is according to Formula 1(a) where R1 is bcnzimidazolyl Optionally substituted with one or two R7 groups; and all other groups. and each R7 (wheii R7 is present.) arc independently as defined in die Summary of the Invention for a Compound of Formula 1 or as defined in any of Embodiments (A Γ). (A2), (A3), (A4). and (1). In another embodiment, the Compound of Formula I is according to Formula 1(a) where R1 is bcnzimidazolyl optionally substituted with one R7; and all other groups are independently a.s defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments -.(A I), (Λ2). (A3). (A4), and ( I ).
[00112 j; Embodiments (136): In another embodiment, the- Compound of Formula 1 is according to Formula l(d I ) or !(il2)
Kdl) I(d2) where R7 when R7 is present, is alkyl. haloalkyl. alkoxyalkyl, -SR'\ -NR8R*\ - RsC(0)RY - R¾(0)OR''..-N-R%(0)NR-aR"!, ! cyeloalkyl. lictcrocycloalkyl. or heieroaryl; and R* and all other group.s are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments ( A 1 ). (A2). (A3), (Λ4), and ( I ). In. another embodiment, the Compound is according to Formula Kdl ) or l(d2) where R'. when R' is present, is alkyl. alkoxyalkyl, -SRD; -NRV^- ¼^
hetcrocycloalkyl, or heieroaryl; R
H and
h3 are independently hydrogen or alkyl: R is alkyl. alkoxyalkyl. or optionally substituted heieroeycloalkylalk
'yl: 1¾' is alkyl; and R
2 and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in any of Embodiments ( A I ). (A2). ( A3). (A4), and (1). In another embodiment, the Compound is according to Formula l(dl) or I(d2) where R
7. when R
7 is present, is alkyl. alkoxyalkyl. -SR
1"'.•NR
sR
!,n. -NR
sC(0)R".
cyeloalkyl. hetcrocycloalkyl or heieroaryl; R and R*'
1 are independently hydrogen or alkyl; R
Y is alkyl: R
L> is alkyl; and R
2 and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula. I or as defined in any of Embodiments (A 1 ), (A2), (A3), ( A4). and ( I ). In another embodiment, the Compound is according to Fornuila l(d l ) or l(d2) where R
7. when R
7 is present, is Cvalkyl, alkoxyalkyl. -SR
13, -NR
SR
XA, -NR
sC(0)R
9, -ΝΙ¾¾0)01*
ι. cyeloalkyl. hetcrocycloalkyl. or heieroaryl: R
S aivd R
SA arc independently hydrogen or Ci -alkyl; R" is C|..i-;dkyl: is C|..i-alkyl: and R
2 and all other groups are independentl as defined in the Summary of the Invention for a Compound of Formula I or as defined in any ,of Embodiments (Al). (Λ2). (A3). (Λ4). anil ( ). In another embodiment, live Compound is accordin to Formula l(dl) or I(cl2) where R', when R' is present, is methyl, ethyl, n-p
'ropyl. isopropyl. mcthoxymelhyl. amino, mcibylamino. ethylamino.
isopropylamino. dimeihylamino.3-piperidinylpropylcarbonylammo, methoxycarbonylamino, 2-(methoxy)-c(hyloxycarbonylamino. cyclopropyl. cyclobuiyl. cyclopentyl. cyclohexyl. azetidinyl. piperidinyl. or pyridiuyl: and R and all other groups are independently as defined
in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodimenis. (AT), (A2), (A3). (Λ4), and (I).
100113] Embodiment (B7): Iii another embodiment, die Compound is- according to Formula l(d l) or I(d2) where R7 is present and is alkyl; and R 2 and all other groups arc independently as defined in the Summary of the Invention for a Compound of formiila I or as defined in any of Embodimenis (Al). (A2). (A3). (Λ4), and (I ). In another embodiment, the Compound is according to Formula l(dl) or I l2) where R7 is present and is C|..¾-alkyl; and R" and all other groups arc independently as defined in the Summary of the Inveniion for a Compound of Formula I or as defined in any of Embodiments ( A I ). ( A2). (A3). (Λ4), and (I). In another embodiment, the Compound is according to Formula I(dl) or I(cI2) where R7 is present, and is - R^R^; and ali pdicr groups are. independently as defined in the Summary of the Invention for a Compound of Formula lor as defined in any of Embodiments (Al). (A2). (A3), (A4), and (1). In another embodiment, the Compound is according to Formula I(dl ) or I(cl2) where R7 is present and is -NRsRSa; Rs and RS:' arc independently hydrogen or alkyl; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formul I or as defined in any of Embodimenis (A I ). ( A2). (A3). (A4), and (1). In another embodiment, the Compound is according to Formula I(dl) or I(d2) where R' is present and is -NR^R8*; ¾* and R 1 are independently hydrogen or Ci -alkyl; and all other groups are independently as defined iii the Summary of the. Invention for a Compound of Formul l or as defined in any of Embodiments (Al ), (A2). (A3). (Λ4), and ( 1 ). In: another embodiment, the Compound is according to Formula l(dl) or I(cl2) where R7 is present and is - R'sC(0)OR°; and all other groups are independently as defined in the Summary of the Invention for a Compound Of Formula 1 or as defined in any of Embodiments (A 1 ). (Ά2), (A3). (A4), and ( 1). In another embodimcni, the Compound is according to Formula l(dl) or I(d2) .where R7 is present and is - R^QC OR1"*: Rs and R° arc indepenclcnily hydrogen or alkyl; and all other-groups are independently as defined, in the Summary of the Invention for a Compound of Formul I or as defined in any of Embodiments (A 1 ). ( A2). ( A3). (Λ4). and (I). In another embodiment, the Compound is according to Formula l(dl ) or I(d2) where R' is present and is -NR'^QC OR0; R* and R" arc independently hydrogen or C|.;,-alkyl; and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (Al). (A2). (A3), ( A4 ). and ( I ). In another embodiment, the Compound is according to Formula I(dl ) or I(d2) where R' is present and is -SR1"1: and all other groups are independently as defined in the Summary of the Invention for
it Compound of Formula I or as defined in any of Embodiments (Λ 1 ). ( A2 ), ( A3). (Λ4). and ( D-
[001141 In another embodiment, the Compound is according to Formula I(d l ) or I(d2) where R7 is present and is haloalkyl: and all other groups are independently as defined in the Suniimiry of the Invention for a Compound of Formula I or as defined in any of
Embodiments (A 1 ). (A2), (A3). (A4). and ( I ). In another embodiment, the Compound is according to Formula I(d l ) or I(d2 ) where R7 is present and is cycloalUy!: and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any oi" Embodiments (A I ). (A2). (A3). (Λ4 ). and ( 1 ). In another embodiment, the Compound is according to Formula Kil l ) or I(d2) where 7 is present and is cyclopropyl". and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ). (A2): ( A3). (A4). and .( l ).
[00115] Embodiment ( BS): In another embodimenUtlie Compound is according to Formula 1(f).
1(f)
wherc ihe R7 at the 2-p si.iion is - RSRS;' or -NRSC(0)OR" and the other R " is halo: and R3 and all other groups are independently as defined in (he Summary of (lie Invention for a Compound of Formula I or as defined in any of Embodiments (A I ). (Λ2). (A3). (A4). and ( 1 ). In another embodiment, the Compound of Formula I is according to Formula 1(f) where the. R7' at the 2-posiiion is -N.RSR*' or -NRSC(0)OR" anil the other R7 is halo: R*. R8A, and R'} arc independently hydrogen or alkyl: and R: and all oilier groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A 1 ), (A2). (A3). (Λ4). and ( I ). In.anollicr embodiment, the Compound of Formula I is according to Formula 1( f) where the 7 at. the 2-posilion is -NI^R^' or
-NRXC(0)OR'j and the other R7 is halo: R\ RS:'. and R" are independcnl ly hydrogen or Chalky!; and R2 and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ), (A2 ). (A3). ( A4). and ( I ), In another embodiment, the Compound is according to Formula 1(f) where the R7 at the 2-posilion is meihoxycarbonylamino or amino and the other the R7 is
Πιιοΐ'ό: and R~ and all other groups are independentl as defined in the. Summary of die Invention for a Compound of Formula I or as defined in any of Embodiments (Al), (;A2). (A3). (Λ4). and ( I ).
|()0116) Embodiment (139): In anot er embodiment, the Compound is according to Formula 1(a) where R1 is a 5-incmbered hclcioaryl. where R1 is optionally substituted with one or two R7; each R7 (when present), and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of
Embodiments (Al). (A2), (A3). (A4), and (1).
[00117 J Embodiments ( B 10): In another. cmb'od intent, the Compound is according to Formula 1(a) where R1 is iliiay.al-2-yK ihiaz.ol-4-yl, or fhia.ol-5-yl. -where R' is optionally substituted with one or two R': each R7 (when present), and all other groups are
independently as defined in the Summary of the Invention for a Compound of Formul I o as defined in any of Embodiments (Al ). (Λ2). (A3). (A4), and (1). in another embodiment, the Compound is according to Formula 1(a) where R1 is ihiazol-2-yl, thiaz.ol-4-yl. or iltiaz.ol-.vyl, where R1 is optionally substituted with one R7; R7, all other groups are independently as .defined in the Summary of the- Inveniion for a Compound of Formula I or as defined in any of Embodiments (Al). (A2). (A3). (A4), and (I).
[001181 Embodiments (B ID; In -another .-cmbodimcni. the Compound i according t Formula 1(a) where R1 is lhiazol-2-yl, lhiaz.ol-4-yl. or ihiazol-5-yl. where R1 is optionally
"7 7 7
substituted with one or two R : where each R (when present), where each R is
independently alkyl, -N *C(0)OR
*. -C
'(0)NR
8R
8a,or -. R
sR
Sa; each R
s and R
Xa.are iudcpeiulcnily hydrogen or alkyl and R is alkyl (in another embodiment each alkyl in R\ R'
Sa, and R
y are C|. -alkyl); and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments ( A 1 ). (A2). (λ\3), (A4). and (1), In another embodiment, the Compound is according to Formula 1(a) where R
1 is thiazol-2-yl. thiazol-4-yl. or ihiazol-5-yl. where R
1 is optionally substituted with one or two R
7: where each R
; (when present), where each R
7 is independently alkyl,
•-NR^CiQ R
9; -C(0)NR
sR
s,Vor - irV': each R
s and R
Sa are independently hydrogen or C|. .i-alkyl and is C|.;,-alkyl; and all othergroups arc indcpcndenlly as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ). (A2). ( A3), (A4), and (1). In another embodiment, the Compound is according to Formula 1(a) where R
1 is lhiaz.ol-2-yl. ihiazol-4-yl, or ihiazol-.vyl, where R
1 is optionally substituted with one or two R
7; each R
7. when R
; is present, is independently methyl, or amino; and all other groups are independently as defined in the Summary of the Invention for a Compound
of Formula I or us
'defined in any of Embodiments (A I ), (Λ2). (A3), (A4), and ( 1 ). In another embodiment, ilic Compound is according to Formula 1(a) where
1 is ihta.ol-2-yl. ihiazol-4- yl. or thiazol-5-yl. where R
1 is substituted with two R'; where one R
7, is alky! and the other R
7 - R^R^; and all oiher groups are independently as defined in tlie. Summai y of the Invention for a Compound of Formula for as defined in any ofEnH'odimenls (
;A | ), (A2).
[001191 Embodiments ("1312): In another embodiment, the Compound is according to Formula 1(a) where R1 is iliien-2-yl. lhicn-3-yI. ihien-4-yl. or tbien-5-yl. where R1 is optionally substituted with one or two R7 groups; where each R7 (when present), and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula I Or as defined in any of Embodiments (Al ). (A2 (A3). (A4); and ( 1 ). In. another eiribodiihent. the Cpmppuiul,is;aeeprding 1(a) where. R! ;isi l)ieii-2-yl.;lhien-3-yl.. lhicn-4-yl, or iliien-5-yl: and all other groups are independently as defined in the Summary of the Invent ion for a Compound of Formula 1 or as defined in any of Embodiments (ΑΓ), (Λ2). (A3). (Λ4). and(l).
[00120] Embodiments (.1313): In another embodiment, the Compound is according to Formula 1(a) where 5ί' is pyrazol-l-yl. pyra.ol-3-yl. pyra/.ol-4-yl. or pyrazol-5-yl. where R1 is optionally substituted with one or two R7 groups: where each R'' (when present), and all othe grpups are. independently as defined in the Summary of the Invention for a Compound of Formula I er as defined in any o Embodiments (A I ). (,A2); (A3), (A4 , and ( I ), In¾npihcr embodiment, the Conippund is aecoi ding to Formula Ifa) where R ' is pyra/pf- ί -yl, pyrazol-3- yl, pyrazol-4-yl, or pyrazol-5-yl: aiid all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of
Embodiments (Al ). (A2). (A3). (A4). and (1).
[00121 J Embodiment (1314): In another embodiment, the Compound is according to Formula 1(a) where R1 is a 6-membcretl heleroaryl. where R1 is optionally substituted with one or two R7 groups; where each R' (when present), and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A.I). (Λ2). (A3). (A4). and (1).
[00122] Embodiments (1315): In another embodiment; the Compound is according to Formula 1(a) where R1 is pyriinidin-2-yl. pyi imidin-4-yl. pyrimidin-5-yl. pyrimidin-6-yi. where R1 is optionally substituted with one or two R' groups; where each R' (when present), and all other groups are. independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ). (A2). (A3), (A4). and
(I). In another embodiment, the Compound is according to. Formula 1(a) where R1 is pyrimidin-2-yl, pyrimidin-4-yl, pyrimiclin-5-yl, pyiimidin-6-yl, where R1 is optionally siibstilulccl with one R7 where R7 is - RSRSA: R* and ' are independently hydrogen or alkyl: and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ), (Λ2). (A3). ( A4). and (I), hi another embodiment, the .Compound is accordin to Formula 1(a) where R1 is pyrimiclin-2-yl. pyrimidin-4-yl. pyrimidin-5-y'l, pyriinitlin-6-yl. where R1 is optionally substituted with one R7 where R7 is -NRXR A; ,RS and RK:' are independently hydrogen prC|..-,- alkyl; and all other groups arc independently as defined in the Summary of the Invention' for a Compound of Formula 1 or as defined in any of Embodiments (A 1 ), (A2), (A3), (A4), and (1). In another cmbodimcni. the Compound, is according lo Formula 1(a) where R1 is R1 is 2-amino-pyrimidin-5-yl; and all oilier groups arc independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in any of Embodiments (A I ). (Λ2). (A3), (A4). and (1).
[001231 Embodiments (B 16): In another embodiment, the Compound is according lo Formula 1(a) where R 1 is pyridin-2-yl. pyridin-3-yl. pyridin-4-yl. pyridin-5-yI. or yridin-6- yl, where R1 is optionally substituted with, one or two R7 groups.; .where, each R' (when present), and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula i or as defined in any of Embodiments (A I ). (A2). (A3-), (A4), and ( I). In another embodiment, the Compound is according to Formula 1(a) where R1 is pyridinyl where R1 is optionally subsiiiuted with one or two R' where each R' is
independently halo, cyano, alkylsull'onylalkyl. -OR '. -C(0) RSRS:'. S(0.) OH. -S(0)R' \ -S(;0;hRi:i:'. -S(0.)2M 8R9, -NR'SR'S . -NRsC(0)OR!', -NRsC(0)RY, -NRsS(0)2RI!A. or lictcrocycloalkyl optionally substituted with one amino; and all oilier groups are
independently as defined in lhe Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ). (A2), (A3), (A4). and ( 1 ).
[00124] Embodiments (B Ifia): In another cmbodimcni. the Compound is according to Formula 1(a) where R1 is pyridinyl where R1 is optionally substituted with one or two R7 where each R7 is independently halo, cyano. alkylsulfonylalkyl. -ORS:'. -C(0)NRXRS;I, S(0);OH. -S(0)R'-\ -S(O Rl3a. -S(0) RSR". -NRSRSA. -NRSC(.0)OR". - RSC(0)R".
-NRSS(0')2R j, lictcrocycloalkyl optionally substituted with one amino: where
each RS is independently hydrogen, haloalkyl, or alkyl;
each RSA is independently-hytli gen. alkyl, benzyl, or phenyl which phenyl is optionally
substituted with one or two groups which are independently halo or alkyl:
cacli R is independently hydrogen: alkyl hydroxyalkyl: alkoxyalkyl: aminoalkyl;
alkylaminoaikyl; dialkylaminoalkyl; haloalkyl: hydroxyalkyl substituted wii one. two, or ihrce halo, hetcrocycloalkvlalkyl optionally substituted with one alkyl: heierdcycloalkyl optionally subsiituied with one alkyl: cycloalkylalkyl optionally substituted with one amino: eyeloalkyl;
RIJ is alkyl or hydroxyalkyl-:
R1'1'1 is alkyl: hydroxyalkyl: heierocyeloalkyi optionally substituted with one or two groups which arc independently halo, amino, alkylamino. dialkylamino. hydroxy, alkyl, or hydroxyalkyl;
and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ). (A2), (A3), (A4), and (1).
[00125.] Embodiments (B 16b): In another embodiment, the. Compound of .Formula I is according to Formula 1(c)
1(e)
where each R7 and R2 are independently as defined in the Summary of the Invention for a Goinpound of Formula I or as defined in any of Embodiments (A l ), (A2). (A3), (A4), and (1). in another embodiment, the Compound ol' Formula 1 is according to Formula 1(e) where each R is independently as defined in embodiment ti 16a and R1 is as defined in the
Summary of the Invention lor a Compound of Formula I or as defined in any of
Embodiments (Al). (A2); (A3). (Λ4). and (I).
[001261 Emboclimenls.(B 16c): In another embodiment, the Compound of Formula I is according to Formula l(c I )
lie I)
where each R7 and R2 are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A l). (A2). (A3), (A4), and
( I ). In another embodiment, the Compound of Formula I is according t Formula 1(e) where each R7 is independently as defined in embodiment B I 6a and R3 is as defined in the
Summary of the Invention for a Compound of Formula I or as defined in any of
Embodiments (A.I), (A2), (A3). (A4). and ( I ). In another embodiment, the Compound of Formula I is according to Formula l(e l ) where the R ' in the 2 -position is hydrogen, halo, cyano. alkoxy. alkyl, or -NR
SR
N:I and the R
7 in the 3-position is -NR
s.S(0)
2R
x ': and R
2 and all other groups are as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I.). (A2), (A3). (Λ4). and (1). In another embodiment, the Compound of Formula I is according to Formula l(e I) where the R
7 in the 2-position is hydroxy or
R2 and all other groups are as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ). (Λ2 ). (A3). (Λ4). and (' I ). In another embodiment, the Compound of Formula I is according to Formula I(e l ) where the R ' in the 2-position is hydroxy or - RSR;S;' and the R7 in the 3-position is -S(0)R L\ -S(O R | !a.
-S(0)
2NR
-SR'
j; R
tcwis hydroxyalkyl: R
, 3a is alkyl or heieroeycloalkyl optionally substituted with one group which is amino, alkyl, hydroxyalkyl, or hydroxy; each R and ;R
S;1 arc independently hydrogen or alkyl; R''
amiiioalkyl. alk laminoalkyl. diajkylaminoalkyl. cycloalkyL heieroeycloalkyl,
heteiOcycloalkylalkyl. alkyl substituted with one aminoearbonyl, or hydroxyalkyl which is substituted with one amino or 3 halo; and R2 and al l other groups are as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of
Embodiments (A 1 ). (A2). (A3). (Λ4). and ( I ).
[001271 Embodiments (B I 7): In another embodiment, the Compound of Formula I is according to. Formula 1(a) wlterc R1 is pyridazin-3-yl. pyridazin-4-yl. pyrida7.in-5-yl, or pyridazin-6-yl, svhere R1 is optionally substituted with one or two R7 groups; where each R7 (when present); and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A l ). (A2), (A3), ( A4). and ( I ). In another embodiment, the Compound is according to Formula 1( a) where R 1 is pyridazin-3-yl. pyridazin-4-yl, pyridazin-5-yl. or pyridaziii-6-yl. where 1 is optionally subsliiiited with one or two R7 groups where each R7 is independently -NR'sRSa: Rx and .R^' arc independently hydrogen or alkyl; and R" and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A l), (A2), (A3), (A4). and ( I ). In another embodiment, the Compound is according to Fonnula 1(a) where R 1 is 3-amino-pyridazin-6-yl; and all other groups arc
independently as defined iii the Summary ol: the Invcniion for a Compound of Formula I or as defined in any of Embodiments (Al). (Λ2). (A3), (A4). and (I ).
[001281 Embodiments (B 18): In another embodiment, the Compound is according to Formula 1(a) where R1 is pyra/.in-2-yl. pyrazin-3-yl. pyra/.in -yl. or pyrazin-6-yL where R1 is optionally substituted with one or two R7 groups: where each R; (when present), and all oilier groups are independently as defined in the Summary of the Invention for a Compound of Formula I or asdefined in any of Embodiments (Al). (Λ2), (A3). (Λ4). and ( I). In another embodiment, the Compound is. according to Formula 1(a) where R1 is pyrazin-2-yl. pyra/Jn-3- yl. pyra/.in-5-yl. or pyrazin-6-yI, where R1 is optionally substituted with one R7 where R7 is -NR'R>'"1: Rs and RSj are independently hydrogen or alkyl : and R2 and all other groups are independently as defined in the Summary of the Invention for a Compound of Formul I or as defined in any of Embodiments (A 1 ), (A2). (A3). (Λ4). and (1). In another embodiment, the Compound is accordin to Formula 1(a) where R1 is 5-amiiio-pyrazin-2-yl: and R" and all other groups are independentl as defined in the Summary of the invention for a Compound of Formula lor as defined in any of Embodiments (Al ), (A2), (A3), (A4). and (Γ).
|0()I29| Embodiments ( B 19): In another embodiment, the Compound is according to Formula 1(a) where R1 is l/ -pyiTolo|2.3-/;|pyridinyl. optionally .substituted with one or two R' groups; whereeach R7. when R 7 is present, and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in any of Embodiments (A I ), (Λ2). (A3). (A4), and ( I). In another embodiment, the Compound is according to Formula 1(a) where R1 is I. /7- yrro 1 o| 2. -Λ | r i d i n- 5- I . optionally substituted with one or two R' groups; where each R7. when R' is present, and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ). (Λ2). (A3). (A4). and ( I ). In another embodiment, the Compound is according to Formula 1(a) where R1 is l./7-pyrrolo[2,3-/.?|pyridm-5-yl.
optionally substituted with one R7': where the R', when R7 is present, and all other groups arc independently as defined in the Summary. of the Invention for a Compound of Formula 1 or as defined in anyrof Embodiments (A I ). (A2). (A3). (A4). and ( 1). In another embodiment, the Compound is according to Formula 1(a) where R1 is l/V-pyrrolo|2.3-/>|pyridin -yl.
optionally substituted with one R': R7. when R' is present, is methyl or ethyl: and all other groups arc independently as defined in the Summary of the Invention for aCompound of Formula I or as defined in any of Embodiments (A I ). (A2). (A3). (Λ4). and ( 1 ).
[001301 Embodiments (B20): In another embodiment, the Compound is according to Formul 1(a) where R1 is iwlazolyl. optionally substituted with one or two R7 groups: where
R7, when R7 is present, mid all oilier groups are independently. ax defined in the S.tminiary of the Invent ion for a Compound of Formula I or as defined in any of Embodiments (A I ), (Λ2). (A3). (A4), and ( I ). In another embodiment, the Compound. is according to Formula 1(a) where R 1 is inda/ol-5-yl or inda ol-6-yl. where R 1 is opt ionally subst ituted with one or two R groups: where R . when R ' is present , and all other groups arc independent ly as defined in the Summary of the Inveniion for a Compound of Formula I or as defined in any of
Embodiments (A I ). (Λ2). (A3). (A4). and ( 1 ). In another embodiment, the Compound is according to Formula 1(a) where R 1 is indazol-5-yl or indazolr -y), where R 1 is optionally substituted with one R '; R7. when present, is alk-yl or amiho; aiid.R2 and al l oiliier groups arc independently as defined in the Summary of the Invent ion for a Compound of Formula 1 or as defined in any of Embodiments (Α Γ). (A2). (A3), (A4). and ( I ). In another embodiment', the Compound is according to Formula 1(a) where R 1 is indazol-5-yI, indazol -6-yl. or /V-mcthyl- indazol-5-yl; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A 1 ). (A2), (A3), (Λ4). and CI ).
[00131] Embodiment (132 1 ): In another embodiment, the Compound is according to Formula 1(a) where R 1 is benzimidazolyl substituted with two R7 groups where each R ' is alkyl: .and R2 and all other groups arc indepenclenlly as defined in the Summary of the Invent ion for a Compound of Formula 1 or as defined in any of Embodimcnts"(A 1 ), (A2); (A3). (A4), and ( I ). In another embodiment, the Compound is according to Formula 1(a) where R 1 is benzimidazolyl substituted with two R ' groups where each R7 is C-i.j-alkyl : and R2 and al l other groups arc independently as defined in the Summary of the Inveniion for a Compound of Formula I or as defined in any of Embodiments (A 1 ), ( Λ2), (A3). ( A4). and '( I ).
[001321 Embodiments ( B22): In another embodiment, the Compound is according to Formula 1(a) where R 1 is c|uinolin-2-yl. quinolin-3-yl, c|uinoTin-4-yl . c|uinolin-5-yl. c|uinolin- 6-yl. c|uinolin-7-yl, t|uinolin-8-yl, isoquinolin- l -yl. isot|iiinolin-3-yl. is(K|iiinol in-4-yl.
isoc|uinol in-5-yl. isoc]uinol in-6-yl. isoc|uinolin-7-yl. isoc|uinoIin-8-yl. quinazol in-2-yl.
qtiinazolin-3-yl. quinazolin-5-yl. qiiinazolin-6-yl. quinazolin-7-yl, or cjuina/olin-S-yl . where R 1 is opiionally substituted with one or two R7 groups: where each R ' . when R7 is present, and all other groups are independently as defined in the Summary of the Invention for a Compouiul of Formula I or as defined in any of Embodiments (A I.), (A2). (A3), (A4 ), and ( I ). In another embodiment, the Compound is according to Formula 1(a) where R 1 is quinol in-2-y], quinolin-3-yl, qiiinolin-4-yl, qiiinolin-5-yl , quino.l in-.6-yl . quinolin-7-yl.
quinolin-S-yl, quinazolin-2-yl. quinaz.olin-3-yl, quinazoiin-5-yl. quinazolin-6-yl, quinazolin- 7-yl, or quinazolin-8-yl; and all oilier groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (Λ1), (A2), (A3). (A4). and (I). In another embodiment, the Compound is according to Formula 1(a) where R1 is quinolin-3-yl or qiiiuazolin-6-yl: and R" and all other groups are independentl as defined in the Summary of the Invention for a Compound .of Formula I or astdefined in any of Embodiments (A I). (A2). (Λ3).(Λ4). and (I).
[00133] Embodiments (1324): In another embodiment, the Compound is accordin to Formula 1(a) where R1 is 2.3-dihydrobenzofuran-4-yl.2.3-dihydrobenzofuian-ci-yl.
2,3-dihyclrobenzofurnn-6-yl. or 2.3-dihydi bcnzofuran-7-yl. where R1 is optionally
.substituted' with one or two R' groups: where each R'. when R7 is present, and all other groups are independently as defined in the Summary of the Invention for a Compound o Formula I or as defined in an o Embodiments (A I ). (A2). (A3). (Λ4). and ( 1,·). Tn another embodiment, the Compound is --'according to Formula 1(a) where R1 is 2.3-dihydrobeiizontran- 4-yl. ^di drb cnzp.fiur n-S^
yl; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in any of Embodiments ( Al ), (A2), (A3), (A4). and (I). In another embodiment, the Compound is according to Formula 1(a) where R1 is 2.3- dihydrobcnzofiiran-5-yl". and R" and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as -defined in any of
Embodiments (A I). (Λ2). (A3), (Λ4), and ( I ).
1001341 Embodiments (B25): In another embodiment, the Compound is according- to; Formula 1(a) where R1 is iiidol-,l-yl, indol-2-yl. indol-3-yl, indol-4?.yl. indol-5-yl, indol-6-yl, or incl l-7-yl. where R1 is optionally substituted with one or two R7 groups: where each R'. when R' is |)iesenl. and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ). (A2). (A3), (A4), and (I). In another embodiment, the Compound is according to Formula 1(a) where R1 is indol-I-yl, indol-2-yl. inclol-3-yI. indol-4-yl. indol-5-yl, indoI-6-yl. or indol-7-yl where. R1 is optionally .substituted with one R' where R7 is alkyl: and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ). (A2), (A3). (A4). and ( I ). In another embodiment, the Compound is according to Formula 1(a) where R1 is indol-5-yl optionally substituted with
7 7
one R where R is alkyl; and all other groups are independently as defined in the Summary
o thc Invention for a Compouiul of Foriiuila I or as defined in any of Embodimenis (A I), (A2').(A3). (Ά4). and (1).
101)135] Embodimenis (B26): In another embodiment, ihe Compound is according IO
Formula 1(a) where R1 is | l.2.4|tria/.olo| l.5-a]pyridin-2-yl.11.2.4|triazolo| 1.5-a|pyridin-5-yl. |;l,2.4|liiazplo| l,5-a|pyridin-6-yl.11,2. 11 ri nx. l o [ l.,5-;i.| yri tt Ί n-7 - y ! , t >i- 11.2.4 |iria/.olo| L5- a|pyridin-8-yi, where R1 is optionally substituted with one or two R7 groups; where each R7. when R7 is present, arid all other groups are indeperideritly as defined in the Summary of thc Invention for a Compound of Formula I or as defined in any of Embodiments (A 1 ). (A2), (A3), (A4). and („l).; In another embodimeni. the Compound is according.to Formula 1(a) where R1 is 11 ,2.4|triazolp| 1 ,5-a|pyridin-2-yl. | IT2.4|tria/.olo( 1.5-a|pyridin-5-yl.
11 ,2,4|triazolo| 1.5-a]pyridin-6-yl.11.2,4 |iriaz.olo| I .. a|pyridin-7-vl. or 11 ,2. |triazolo| 1.5- a|pyridin-8-yl. where R1 is optionally substituted with one R' where R7 is -N ^ ^1: Rs and R arc independently hydrogen or alky!: and R" and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (Al), (A2). (A3), (Λ4). and ( I). In another embodiment, the Compound is according to Formula 1(a) where R1 is 11.2. |triazolo| l,5-a|pyridin-6-yl. or
I I.2.4|tria/.olo| 1 ,5-a|pyridin-7-yl. optionally substituted with one R
7 where.R'.is amino: and all
in the Summary of the Invention for a
Compound of Formula I. or as defined in any of Embodiments ( A I ), ( A2). ( A3). ( A4). and (I).
[Oil 136 J Embodiments (B27): In another embodiment, die Compound is according to Formula 1(g)
where Y is iN or CH; and R2 and R7 are independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in any of Embodiments (A I ). (A2), (A3). (Λ4). and (I ). In another embodiment the Compound of Formula 1(g) is that where R7. when present, is N'RsR8a or, -NRsC(0)Ry; and R" and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in any of Embodiments (ΑΓ). (A2), (A3), (Λ4), and ( I ). In another embodiment the Compound of Formula 1(g) is that where R'7. when present, is -NRW -M'R-8C(:0)-R,J': .RK and Rs" are
independently hydrogen oi alkyl; Ry is alkyl or haloalkyl; and R2 and nil Other groups are independently as defined in the .Summary of the Invention for a Compound of Formula 1 or as defined in any of Embodiments (A I ). (Λ2). (A3). (Λ4). and ( I ). In another embodiment the Compound of Formula Kg) is that where R7. wh n present, is -NR*RS:' or -NRsC(0)Ry; R's and R*1 are independently hydrogen or C|..¾-alkyl; R" is C|..;-alkyl or lialo-C|,-,-alkyl: and R: and all other groups arc independently as defined in the Summary of the Invention for a
Compound of Formula l or as defined in any of Embodiment's . (A.I), (Λ2). (A3), (Λ4), and ( I). In another embodiment the Compound of Formul 1(g) is that where R7. when present, is amino or mnuoromelhylcarbonylamino: and R2 and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ). (A2), (A3). (Λ4). and (I).
100137) Embodiments (B28): In another embodiment, the Compound of Formula I is according to Formula 1(a) where R1 is pyrido|2.3-b|pyrazinyl. optionally substituted with one or. two R' groups: where R' and all other groups are independently as defined in the. Summary of the Invention for a Compound ol" Formula I or as.defi cdjn any of Embodiments ( A 1). (A2), (A3), (A4).,and (1). In another embodiment; the Compound of Formula 1 is according to Formula 1(a) where R1 is unsubsiituted pyrido(2.3-b|pyrazinyl where all oilier groups are independently, as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ). (A2 ), (A3), (Λ4), and ( I ).
[00138J Embodiments (B29): In another embodiment. -the Compound of Formula 1 is according. to Formula 1(a) where R1 is 3,4-dihydro-2//-pyiido|3,2->|| l,4|oxazinyl optionally substituted with one or two R7 groups: where R7 and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I.). (A2), (A3). ( A4). and ( I ). In another embodiment, the Compound of Formula 1 is according to Formula 1(a) where R1 is unsubstitnted 3,4-dihydro-2/7-pyrido| 3.2- b\\ l.4|oxazinyl where all other groups are independently as defined in die Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ). ( A2), (A3), (A4), and (1).
1001391 Enibodimcnls (C): In another embodiment, the Compound of Formula I is according to Formula 1(a) where 1 is phenyl optionally substituted with one. two. or three Rf' groups: where each Re when R(1 is present, and all other groups are independently as defined in the Summary ol' the Invention lor a Compound of Formula I or as defined in any of Embodiments (A I ). (A2), (A3). (A4), and (1). In another embodiment, the Compound of Formula I is according to Formula 1(a) where R1 is phenyl optionally substituted with one or
two R"' roups: where each I '', when R L is present, and all other groups are independently as dellnccl in ihc Summary of the Invention for a .Compound of Formula I or as defined in any of Embodiments (A l . (A2). (A3). (A4). and ( 1 ).
[001401 Embodiments (C l ): In another embodiment, the Compound of Formula I is according to Formula 1(a) where R 1 is phenyl optionally substituted with one. two. or three R° groups; where each R*' is independently niuo. halo, alkoxy. -OR&. -S(0) >RS. -NR^R '.
-NRXS(0);RS:1. -NRSC(0)R". -C(0)N R8R :I. -ΝΙ*χαθ)ΝΙ<Κ:Ί¾· . carboxy. alkoxycarbonyl. or heteroaryl optionally subsiiiuied with one or two R L -!; aiul all other groups are independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in- any of Embodiments (A 1% (A2). (A3): (Α4'). and ( 1). In another embodiment.-lhe Compound of 'Formula'.! is according to Formula 1(a) where R 1 is phenyl optionally .subsiiiuied with one, two. or three R'' groups; where each R(' is. independently -S(C >RS, -C(0)NRXR'v'' or heteroaryl optionally substituted with one or two R1 '1; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A 1 ). (A2), (A3). (A4), and ( I ).
|() l4l] Embodiment (C2): In another embodiment, the Compound is according lo Formula 1(a) where R 1 is phenyl optionally substituted with one. two. or three R" groups: where each RFL is independently niuo, halo, alkoxy, -OR*'. -S(0)2 S, -NRV* -N'RSS(0) RS;1. -NRSC(Q)R9, -C(0)NRSRS:'; -NR¾0)NRS;,R9, carboxy. ;aikbxycarbonyl; or heteroaryl optionally subsiiiuied with one or two R 1'1: each RS is independently hydrogen or alkyl; each RX:1 is independently hydrogen, alkyl. Iialoalkyl, optionally subsiiiuied cycloalkyl. or optionally subsiiiuied heierocycloalkyl; R'' is alkyl; R 1 '1. when present, is hydroxyalkyl: and all other groups are independently as defined in ihe Summary of the Invention for a
Compound of Formula I or as defined in any of Embodiments (A l ), (A2), (A3). (A4), and ( 1 ). In another embodiment, liic Compound is according lo Formula 1(a) where R 1 is phenyl optionally substituted with one, two, or three R(' groups; where each R6 is independently niii . halo. alkoxy, -ORX\ -S(0):RS, - RV3, -NRsS(0)2R-S:'. - RSC(0)RL). -C(0) RSRSI'. -NR'SC(0)NRX ,R'', carboxy. alkoxycarbonyl. or heteroaryl optionally substituted with one or two R 1 *1; each RS is independently hydrogen or C|..i-alkyl; each RS:1 is independently hydrogen, alkyl. Iialoalkyl. optionally subsiiiuied cycloalkyl. or optionally substituted heierocycloalkyl; R is C|.;i-alkyl: R1 ". when present, is hydroxyalkyl: and all oilier groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in any of Embodiments (A I ). (Λ2), (A3). (Λ4), and ( 1 ).
[00142] Embodiment (C3): In another embodiment, the- Compound is according to Formula 1(a) where 1 is phenyl optionally .substituted with one or two R° groups where each R6 is independently nilro. clvloro. nteihoxy. nieihylsulfonyl. amino.
niethylannnocarbonylaniiiK mcthylamino. carboxy. melhylcarbpnylammo. aminpcar.bonvl. melhylaminocarbonyl. ctliylaniinoearlxMiyl, n-propylamiuOcarbonyl.
isopropylaminbcarbonyl.2HiK>nofIuoroeihylaminocarbonyl 2^
2,2,2-trilluoraelliylaiiiinQcarbonyi.1.1.1 -trifluoropiOp-2-ylaimnocarbony], cyclopiOpylaminoeaibonyl. pyrrol idinylaminocarbonyl. methoxycarbonyf iniidazolyl.
imidaxolyl siibsiiiutcd with hydroxymcihyl. or pyrazolyl: and R2 and all other groups are independently as.defincd in the Summary of 'the Invention lor a Compound of Formula I or as defined in any of Embodiments (A I >, ( A2), (A3). ( A4. and (I ).
[00143) lira Compound as described by any one of Formula I, 1(a), l(bl), I(b2), I(cl). I(c2), I(dl). I(d2).: l(cl), I(e2).1(f). and Kg), or by any ofthe above cmbodimenis ( 1). (A I). (A2). (A3). (A4). (B). (HI). (II2.I. (B 1 ). (B2). (B3.I. (84). (B4a). (B4b). ( B5). (B6). (B.S). (B9), (BIO).(Bl I). (B 12). (BI ). (B14). ( B 15). (Bib). (BI6a). (B16b). (B 16c). (BI7). (B18). (BI ), (B20), (B21), (B22),(B2i).:(B24). (B25). (B26). (mi:), (C). (C I ). (C2), and (C3). R2 can be described according to any of the following embodiments.
[00144] Embodiments (D): In another embodiment. R2 is a 6-membcred heierOaryl substitutcdwith R.3, R**, R3b. arid R3i: R'. R!l. R*. and R3e and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (1).
[001451 Emhodimcnls (PI ): In another embodiment, R2 is pyrimidinyi siibsiituied with R"\ Rja, and R-'1'; where R\ R'\ R-'1'. and all other.groups arc independently as defined in the Summary o the Invention for a Compound of Formula I or as defined in embodiment: (I).
[001461 Embodiments (D2): In another embodiment. R2 is according to Formula (a)
(a)
where. R'\ and R,H are independently hydrogen; alkyl: halo; hydroxyalkyl; eyanoalkyl;
-NR"R' -S(O)vR20;.o.pl'iOnally substituted cycloalkylalkyi; optionally substituted hclcroeycloaikyl; optionally substituted phenylalkyl: alkyl substituted with one or two RLF'; or
-ORLLN: and all other groups are independently as defined in the Summary ofthe Invention for a Compound of Formula I or as defined in embodiment ( I ). In another embodiment. R2 is
according to Formula (a) where R'\ R3:i. and R'"' are independently hydrogen: alkyl: halo: hydroxyalkyl; cyanoalkyl; - R"Ri|!': -S(O R20: cycloalkylalkyl: heierocycloalkyl oplionally substituted with one or two alkyl: phenylalkyl oplionally substituted with one or two R|l); alkyl substituted with one or two R1"; or -ORl ; and all other groups arc independently as defined in the Summary of die Invention for a Compound of Formula I or as defined in embodiment (I). In another embodiment, R2 is according to Formula (a) where R'\ Rs\ and RM,are independently hydrogen; alkyl halo; hydroxyalkyl: cyanoalkyl: - R"R":'; -S(Q)2I^'J cycloalkylalkyl; heierocycloalkyl oplionally substituted .with one or two alkyl; phenylalkyl Optionally substituted with one or Iwo R1'"'; alkyl substituted with one or two Rlf': or -ORll:': each Rlu is independently halo, alkyl. haloalkyl. alkoxy, amino, alkylamino. or dialkylamino: each R1" is independently halo. - R"R":' or -OC(0)R17: RIV is alkyl; each R" is
independently hydrogen, alkyl (in another embodiment each alkyl is Cj._i-alkyl), or
cycloalkyl; each R'"a is independently hydrogen; alkyl (in another embodiment each alkyl is C|. -alkyl): aminoalkyl; alkylaminoalkyl; dialkylaminoalkyl; phenyl: phenyl substituted with one alkoxy: -plieiiylalkyj.: licierocycloalkyl: hcterocycloalkyl substituted with one or two alkyl; helcrocycloalkylalkyl; licterocye!oalkylalkyl substituted vvith one or two alkyl; R2" is amino, alkylamino, dialkylamino, or heierocycloalkyl; and all other groups are independently as defined in Ihe Summary of the Invention for a Compound of Formula I or as defined in embodiment (1). In another embodiment, R2 is according lo Formula (a) where R'\ R' l. and 'R3h are independently hydrogen: alkyl (in another embodiment alkyl is Ci-j-alkyl):
phenylalkyl oplionally substiiuled with one or iwo groups which are independently halo, haloalkyk alkoxy. amino, alkylamino. Or dialkylamino: -NR"R'I:'; heierocycloalkyl:' cycloalkylalkyl; alkyl substituted wiih one or two R '; or hydroxyalkyl: where each R11 is independenily hydrogen or alkyl (in another embodiment each alkyl is C]..valkyl): each RU:i is independently alkyl (in another embodiment each alkyl is C|..;-alkyl), phenyl oplionally substituted with alkoxy. or is heierocycloalkyl optionally substiiuled wiih one or two alkyl: each RK' is independenily halo, amino, alkylamino. dialkylamino. or cyclopropyhimino: and all other groups are independenily as defined in the .Summary of the Invention for a
Compound of Formula I or as defined in embodiment ( 1 ).
100147) Embodiments (D3): In another embodiment. R2 is according lo Formula (a) where R3 is hydrogen, halo, alkyl. cycloalkylalkyl. or phenylalkyl oplionally substiiuled with one or iwo Riy; R~3 is hydrogen, alkyl. halo, oplionally substiiuled hcterocycloalkyl. or -INR'-R"11; and R3h is hydrogen, alkyl. hydroxyalkyl. cyanoalkyl. or alkyl substiiuled with one or two
4S
R '; aiid all other groups are independently as defined in the Summary of ihe Invent io fur a Compound of Formula I or as defined in embodiment (I).
[001481 Embodiments (P3a): In another embodimeni. R~ is according to Formula (a) where R( is phenylalkyl optionally substituted with one or two Ri : R',:' is alkyl: and R"'1' is hydrogen, alkyl, hydroxyalkyl, or alkyl substituted with one R""'; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodimeni (I). In another embodiment. R" is -according to · Formula (a) where R'1 is phenylalkyl optionally substituted with one or two R|IJ; each R1 is independently halo, alkyl. haloalkyl. alkoxy. amino, alkylamino. or dialkylamino: R':i is. alkyl (in another embodimeni alkyl is'C|.3-alkyl): and R',h is hydrogen, alkyl, hydroxyalkyl. or alkyl substituted with one >; R1'' is amino, alkylamino. dialkylamino. cyclopropylamino. or -OC(0)Clh; and all other groups arc independently as defined in the Summary of die Invention fora Compound of Formula 1 or as defined in embodiment ( 1 ).
[00149] Embod i men t s; (D 3 IV) : In anoiher enibodiiiien't. R'2 is according r vFqifhula (a) where R' is phenylalkyl opi )nallysubstil:iiied with oii^p t o 1'-; R-a and R3h afc' alkyl: aiid all other groups are independently as defined in the Summary of the Invention for
Compound of Formula 1 or as defined in embodiment ( I ). In anoiher embodiment. R" is according to Formula (a) where R1 is phenylalkyl optionally substituted with one or two R1'': each R1'' are independently halo, alkyl. haloalkyl. amino, alkylamino. dialkylamino. or alkoxy; R1'' and R'^'are alkyl (in another embodiment each alkyl is Ci.i-alkyl); and all other groups are independentl as defined in the Summary of the Invention for a ompound of Formula I or as defined in embodiment ( I). In anoiher embodiment. R2 is: according to Formula (a) where R"' is phenylalkyl ioptionally substituted with one r two halo: R,a and R31' arc alkyl (in anoiher embodiment each alkyl is C|..-alkyl ;-aiul all other groups are independently as defined in the Summary of the Invention for a Compound of Formula 1 or as denned" in embodimeni (1). In anoiher embodimeni. R: is according to Formula (a) where R' is phenylalkyl optionally substituted with one or two R1': each 1'1 arc independently halo, alkyl, haloalkyl; amino, alkylamino, dialkylamino, or alkoxy: R ,:' and R11' are methyl: and all other groups arc independently as defined in the Summary of the invention for a Compound of'Formula I or as defined in embodiment (1).
[00.150 J Embodiments ( D3c): In anoiher embodiment. R^ is accordin to Formida (a); where kJ and R,a are alkyl (in another embodiment each alkyl is Ci -alky!); R'b is hydrogen, alkyl. or alkyl substituted with one R1 : and all other groups are independently as defined in ihe Summary of the Invention for a Compound of Formula I or as defined in embodimeni (1 ).
In another. cnibodimcnl.. R'1 is according io Formula. (a) where R' and R" are alkyl (in another embodiment alkyl is C i -alkyl ): R'!h is hydrogen; and all other groups are i ndependently as defined in the Summary of the Invenlion for a Compound ol" Formula I or as defined in embodiment. ( I ). In another embodiment . R" is accordin to Formula (a) where R\ R ,n. and R '1' rc alkyl (iiT'anothcrenibod iment each alkyl is Chalky!); and al l other groups are independently as defined in the Summary of the In enlio for a Compound of Formula I or as defined in embodiment ( I ). In another embodiment, : isi according lo Formula (a) where R ' and R' are alkyl ( in another embodiment each alkyl is Ci.o-alkyl ); and R3 is alkyl substituted with one R 1"; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( 1 ). In another embodiment. R2 is according to Formula (a) where R"' and R ':i are alkyl ( in another embodiment each alkyl is C |.2-alkyl); and R"'1' is alkyl substituted with one R 1"; R "' is amino, alkylamino, dialkylamino, Or cycloalkylamino;.a d all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula I . or as defined in embodiment ( I ).
[00151 1 Embodiments (D3d): In another embodiment. R" is according to Formula (a) where R:' is alkyl : RJa and R3|v are hydrogen; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula l or as defined in cnibodiment ( l ). Iir another embodiment, R" is according to Formula (a) where R3 is Gi.->- alkyl : R"¾ and R"'l,'afe hydrogen; and al l other, groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( 1 ).
[.00-152']' Embodiments ("03c): In another embodiment. R" is according to Formula (a) where . 'R3 is phenylalkyl opiional ly substituted with one or two R 1": R",a is alkyl : and R31' is hydrogen: and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (T): M\ another embodiment. R~ is according- lo Formula (a) where R3 is. phenylalkyl opiional ly siibsiiiuted with one or two R19; each R | ,J is independently halo, alkyl, haloalkyl. amino, alkylamino. dialkylamino. or alkoxy: R :1 is alkyl; and R",b is hydrogen: and all other groups are independently as defined in the Summary of the Invent ion for a Compound of Formula I or as defi ned in embodi ment ( 1 ). 100153'] Embodiments (D3 f): In another embodiment, R" is according lo Formula (a) where R ' is phenylalkyl optional ly substituted with one or two R I J; R a is alkyl: and Rjl> is alkyl substituted with one Rui: and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I onas defined in embodiment ^). In another embodiment. R2 is according to Formula (a) where R3 is phenylalkyl optionall
substituted with oiic of- twoR19: each R1'' is independently halo, alkyl, haloalkyl. amino, alkyiamino. diaikylamino. or alkoxy: ¾ is alkyl (in another embodiment alkyl is C|.;-»ilkyl): and R' ' is alkyl substituted with one R1'': I*16 is amino, alkyiamino. diaikylamino. or cycloalkylamino: and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( I ).
[001541 Embodiments ( D3.g): In another .embodiment, R" is according to Formula (a) where R3 is, lkyl or phenylalkyl optionally substituted with one or two R^'-. '^' is alkyl; d' R'"' is hydrogen, alkyl, or alkyl substituted with Rlfi; and all. other groups are independently as- defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (I). In another -embodiment. R2 is according to Formula (a) where R is alkyl (in another embodiment alkyl is Ci. alkyl) or phenylalkyl optionally substituted with one or two Riy; R3'1 is alkyl (..in another embodiment alkyl is Ci.j-alkyl): and R'1'' is hydrogen, alkyl (in another embodiment' alkyl is C|.)-alkyl), or alkyl .substituted with R >; R"'.is amino, alkyiamino. diaikylamino. or-cyeloalkylamino: each R1'' is independently halo, alkyl.
haloalkyl. amino, alkyiamino, diaikylamino. or aikoxy: and all other groups are:
independently as defined iivihe Summary of the Invent ioir for a Compound of Formula lor a defined in embodiment (1).
[00155] Embodiments (D3h¾: In another embodiment. R" is according to Formula (a) where R'' is optionally substituted phcnyloxy: R'1 is alkyl: and R''1' is hydrogen: and all other groups arc independently as defined in the Summary of die Invention for a Compound of Formula I or as defined in embodiment ( I ). in another embodiment. R" is according to Formula (a) where R" is phenyloxy optionally substituted with one or two groups which groups are independently halo, alkyl. haloalkyl. amino, alkyiamino. diaikylamino. or alkoxy: R3a is alkyl (in another embodiment alkyl is C|.2-alkyl): and R '1' is hydrogen: and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (I). In another embodiment. R: is according to Formula (a) where R"' is phenyloxy: R,:i is alkyl (in another embodiment alkyl is C|.;-alkyl): and R31' is hydrogen; and all other groups are independently as defined in the Summary of the Invention lor a Compound of Formula I or as defined in embodiment (I).
[00.1561 Embodiments (D3t): In another embodiment. R2 is according to Formula (a) where R'1 is optionally substituted cycloalkylalkyl; R3a is alkyl: and Rjh is hydrogen or alkyl; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( I ). In another embodiment, R2 is according to Formula (a) where R3 is cycloalkylalkyl: R3:i is alkyl (in another embodiment
alkyl is C|.->-alkyl); and R'H is hydrogen or alkyl (tin another embodiment ''alkyl, is 0|.2-alkyl); and all Othe¾groiips arc independently as defined in. the Summary of the liv.venlibn l:or a- Compound of Foriiiiila or as ciefiived in cniliocliniciu ·.·.( Ί ).
100157] Enibodimens (D3i): In another
'embodiment, R
" is according to Formula (a) where R
"1 is alkyl
': R
'1:1 is phenylalkyl optionally substituted with one or two R
1''; and R""' is hydrogen; and all other groups arc independently as 'defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (1). hi another embodiment. R
2 is according to.Forimtia (a) where R ' isialkyl (iiv another
'em od iie
'nl alkyl is Gj i-alkyl);
' R
'5;' i phenylalkyl optionally substituted
1 with one
R
ig is iiutepe tulen t ly ha¼ alkyl,
oif alko y; and
3'
1 isVhydiOgeii; ahd'
jalfqthe groups . are ihd^R¾idciUi ¾S'¾efiii<^ in the Summary of the Invention fora Compound. of Formula.rl or as defined in embodiment ( l). In another embodiment. R
" is according to Formula (a) where R' is alkyl (in another embodiment alkyl is C|.
2-alkyl): R'
;I is phenylalkyl: and R
'h is hydrogen:. and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (1).
iP0158j Embodiments (D3k): In another embodiment, RJ is according to l-0nnida (;\ wheie; ? s alkyl; RJA is -NR^ R11"; and'-R^'-is hydiOgenOr.alkyl: and all other groups 'are independently as defined in the Summary of the jnycntioiV:l¾r'a Coinpoj.tiid of Formt a ;I nas defined in embodiment ( I ). In another embodiment. R~ is according to Formula (a) where R 1 is alkyl (in another embodiment alkyl is Ci -alkyl): R½ is -NR"RL LA; R5b is hydrogen or alkyl (in another embodiment alkyl is Ci^-alkyl): R1' is hydrogen or alkyl (in another embodiment alkyl is Ci-2-alkyl); R11'1 is alkyl, aminoalkyl, alkylainiuoalkyl. dialkylaminoalkyl. optionally substituted heterocycloalkyl. optionally substituted Jietereeycloalkylalky]. optionally substituted phenyl, or optionally substituted phenylalkyl; aiid , ll other group arc
independently s defined in ihe Summaiy of the. to i of as; defined in enibodinieiii (I): In ah the embod intent'. R" is acx- rdiiig ip Foiiniila fa) where :RIT is alkyl (in another embodiment alkyl is C|. alkyl); R¾ is -NR"R"A: R:,H is hydrogen or alkyl (in another embodiment alkyl is C|..2-alkyl); R11 is hydrogen or alkyl (in another embodiment alkyl is. C|.?-alkyl): RL L:I is alkyl. aminoalkyl. alkylaminoalkyl. dialkylaminoalkyl.
heleroeycloalkyl. hclcrocycloalkylalkyl (optionally substituted with one or two alkyl).
phenylalkyl, phenyl (optionally siibstituted.wilh onexir two groups which are independently halo, alkyl. '.haioaikyl, amino, alkylamino, dialkylamino.wr alkoxy); and'all other groups ate independently as defined in. the Summary of the Invention for a Compound of Formula I; r as defined in embodiment ( I ).
[001591 Embodiments (D4): In anolhcr embodiment. R2 is according lo Formula (a) where R¾ is alkyl (in anolhcr embodiment alkyl is C|. alkyl). or - R"RII:I: \V and R5B arc hydrogen: and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined ill embodiment ( I ).
[00160] Embodiments (D4a): In- another embodiment. R" is according to Formula (a) where R'VJ is alkyl (in another embodiment alkyl is"C|.-)-alkyl):,and R( and R'1' arc hydrogen and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in embodiment (I).
1001611 Embodiments (D4b): In another embodiment. R3 is according to Formula(a) where R'''1 is - R- 'R"'1; Ri and R',H are hydrogen: and all other groups are independently as defined iii the Summary of the Invention for a Compound of Formula I or as defined in embodiment (.1). In another embodiment, R2 is according to Formula (a) where R',A is -NR"RIIU; R3 and R"' are hydrogen: R11 is hydrogen or alkyl: RLL!' is optionally substituted phenyl: and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (1). In another embodiment. R2 is according to Formula (a) where R'1'' is -NR"R":1; R' and R 1' are hydrogen; I*" is hydrogen or alkyl (in 'another embodiment alkyl is Cij-alkyl): RL L:I is phenyl Optionally substituted with one or two groups which groups are independently halo, alkyl. haloalkyl. amino, alkylamiuo. clialkylamino, or alkoxy: aiul all other groups are independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in embodiment (1).
1001621 Embodiments (D.T): In another embodiment. R2 is according to Formula (a) where RJ and R',:I are hydrogen: R'1' is - R"RL LA; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (I). In anoihcr embodiment. R2 is according lo Formula (a) where R3 and R ' are hydrogen; R B is -NR"RII:I: R" is hydrogen or alkyl (in another embodiment alkyl is C|,2-alkyl); RL L:I is optionally substituted phenyl; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (I). In anolhcr embodiment, R2 is according to Formula (a) where R and R1:I arc hydrogen: R"'1' is -NRI 1R":': and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( 1 ). In another embodiment. R2 is according to Formula (a) where R and R' are hydrogen; R'1' is -NR"R":I: R" is hydrogcii or alkyl (in another embodiment alkyl is Ci -alkyl): RL,:I is hydrogen, alkyl (in another embodiment alkyl is Ci -alkyl), or optionally substituted phenyl; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as
defined in embodiment (1). In another embodiment. 2 is according t Formula (a) where R'( and R',n arc hydrogen; R31' is -NR 11 1'3; R11 is hydrogen* or alkyl (in .another embodiment alkyl is C|.2-alkyl); Rll:' is hydrogen, alkyl (in another embodiment alkyl is C| >-alkyl). or phenyl optionally substituted with one or two groups which groups are independently halo, alkyl. haloalkyl. amino, alkylaniino, dialkylaniino. or alkoxy; and all other groups arc independently as defined in the Summary of the Invention lor a Compound of Formula I or as defined in embodiment (1 ). hi another 'embodiment. R2 is according to Formula (a) svherc R'1 andR^ arc hydrogen; R'1' is -NR"Rlla; R11 is hydrogen or alkyl (in another 'embodiment, alkyl is C|-2-alkyl); R.1''* is hydrogen, alkyl (in -another embbdiiiieni'alkyLis C|.2- ikyl), r phenyl; aiid all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula I or as defined iii embodiment (1 ).
[00163] Embodiments (D6): In another embodiment. R" is according to Formula (a) where R3 is hydrogen; Rla is alkyl (in another embodiment alkyl is Ci -alkyl) or -NRllRll:': R'1' is hydrogen or alkyl (in another embodiment alkyl is Ci.i-alkyl); and all other groups are independently as defined in the Summary .of the Invention for a Compound- of Formula I or as defined in embodiment ( 1 ).
[.00.164J Embodiments. (D6a): In another enibodimcni, -R2 is according to Formula (a) where 3 is hydrogen; R'!:' is alkyl (in another embodiment alkyl is Ci -alkyl): R,1,! is" hydrogen; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in cmbodimeni ( 1 ).
[00165) Embodiments (D b): In another cmbodimeni. R2 is according to Formula (a) where R"1:' -NR"RIla:, ', and R*'1' are hydrogen; and all other groups arc independenily as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (I ). In another embodiment, R2 is according to Formula (a) where R3a is ' -NR"Rll ; R'1 aiid R b are hydrogen; R11 is hydrogen or alkyl (in another embodiment alkyl is C|.2-alkyl): Rlla is hydrogen, alkyl (in another embodiment alkyl is C-i.-j-alkyl); or optionally substituted phenyl; and all other groups are independently as defined in the Summary of the invention for a Compound of Formula 1 or as defined in embodiment ( I ). In another cmbodimeni, R2 is according lo Formula (a) where R-"1 is -NR"R":i; R"' and R",h arc hydrogen; R11 is hydrogen or alkyl (in another embodiment alkyl is C|.;-alkyl): R a is hydrogen, alkyl (in another embodiment, alkyl is C|.-;-alkyl),or phenyl optionally substiiuted with one or two groups which groups are independently halo; alkyl. haloalkyl, amino, alkylaniino, dialkylaniino, or alkoxy; and all other grbtips are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( 1 ). In
a 11 o 11 Vcr c in l o tl it ι i c Tit. " is according U) ¾rniuld (a) Svlierc R" is -NR" R ' '''': R and R?1' arc hydrogen: R 1 1 is hydrogen or alkyl (in another embodiment alkyl is C|.)-alkyl): R 1 1 ' is hydrogen, alkyl ( in another embodiment each alkyl is C|.2-:ilkyl). or phenyl optionally substituted with one alkoxy; and all other groups are independently as defined in the
Summary of the Invent ion for Compound of Formula I or as defined in embodiment ( 1 ). |()0166| Embodiments (D6c): In another embodiment. R" is according to Formula (a) where RJ. R'¾. and R""' are hydrogen; and all other groups- are independently as defined- in the Summary of the Invention for a Compound of Formula lor as defined in embodiment ( I ). |0()167 | Embodimcius- (D6d): hi another enibodimeni, R? is pyriniidin-2r.yl. pyriiri†diu-4^ yl, 5-(pheiiylmeihyl)^6Hrvcihyl-pyriniidin-4-.yl. 6-(phenylinclhyl)-5-nietliyl-pyriniidin-4-yl. 5- ( l -phen,ylelhyl)-6 netlvyl-pyriniidin- -yl? 2.6-diinelliyl-5-(phenylmeihyl)-pyriniidin-4-yl.
5- ( plienyliiielhyl)-6-ethyl-pyriniidin-4-yl. 2-meihyl-pyi imiilin-4-yl, 5-methyl-pyrimidin-4-yl.
6- methyl-pyrimidin-4-yl. 5,6-dimelhyl-pyrimidin-4-yl. 6-isopropyl-pyrimidin-4-yl. 5-methyl- 6-eihylrpynmidin-4.-.yl. 5-isopropyl-6-nieihyl-pynniidin-4-yl. 5 soamyl- nethyNpyrimiclin-
4- yI..5 -cthyf -6-is prop yl - jjyri in id i n-4-y J . 5-mcihyl-6-isp|)ropyi-|)yrimidin- -y
5- (p ciVyh fcthyI)-6-ciUoro py^
methyl-pyrimidin-4-yl. 5-(cyclopropyln -ihyl)-6-methyl-pyriinidin-4-yl. 2 uiniio-pyriniidin-
4- yl. 5-(2-chIo:ro^hcivylnieiliylj-6-methyl-pyrimidin-4-yl. 5-( 3-chloro-phenylmethyl)-6- niethyl-pyrimidin-4-yl, 5-(4-chloiO-phenylmcihyl)-fiHneilvyl-pyriniidiii-4-vl. 5-(2-fhioro- phcnylmclhyl)-6-meihyl-pyrimidin-4-yl. 5-(3-nuoro-phcnylinethyl)-6-methyl-pyriiiiidiii-4-yl.
5- (4-fkioiO-phei)ylinelliyl)-6-inethyl-pyi iiniciin-4-yl. 5-(3.4-diniioro-phcnylmethyl)-6- methyl-pyrimidin-4-yl. 5-(3.5 liniioiO-plienylmethyl )T6 nethyl-pyrimidin-4-yl. 5-(3-ehloro- 54 lu(iro-phenylmcthyi.)-6 neiliyl-pyriini(li!i-4-yl. 5-( 1 -(3-fluorophenyl )-cthyl)-6-meihyl- pyrimidin-4-yl. 2.6-dimet|iyl-5-(44lMW>^ 5'i(2-meih.yi- plienylnicthy!)-6-iiiciliyl-pyi"iniicliii-4-yl . '5-(3rineihyl j iciiylnte^Hyl)i6 'nelhyl^yrimiclin-4 yl, 5-(4-melhyr-phcnylnicihyl)-6-mctlvyl-pyrimidin-4-yl. (4-chloro-3-(dimethylaniino)- phcnylnieihyr)-6-mclhyl-pyrimidin-4- yl. 5-(2-niethoxy-pheiiylmcihyr)-d-mcthyl-pyrimicliii-4- yl , 5-(3-niethoxy-phenylmclhyl)-6-melhyl-pyrimiclin-4-yl . 5-(4-meth xy-phcnyl methyl )-(·>- mcihyl-pyrimidin-4-yl. 2-( phenylamino)-pyrimidin-4-yl. 6-(phenylamino)-pyrimidin-4-yl.
6- (4 iieilK)xy-phcnylaniiiio)-pyrimidin-4-yi. 5-mcihyl-6-(plienylamino pyrimidin-4-yl. 5-(2- triniioromethyl-phciiylniclhyl) 6-]iictlvyl-pyrimidin-4-yl. 5-(3-iriiliioiOhicihyl-phenylmelh l )- 6rmelhyl-pyriinidin-4-yl, 5-(4. ri
or 5- phenylineihyl-6-irinuoiOmcthyl-pyriniidin-4-yl: and all other groups arc independently as
defined. in the Summary of'lhe liivenlion for a Compound. of Formula I or as defined iii embodiment {\).
[0016S 1 Embodiments (D7): In another embodiment. -R" is pyridinyl substituted . with R3. R' j. R"1'. and R3c: where R3. R ':'. R*1'. and R"!C and al l other groups arc independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( I ).
[00169 ) ' Embodiments (D7a): In another embodiment. R2 is pyridinyl subst ituted with R3, R3\;R \ and R ½here R3. R-3a, R . and R3c are independently hydrogen, alkyl. or plienylalkyl opt ionally subst ituted with one or two R.'"': and al l other groups are
independently as defined in the Summary/of the 'I cniioni O^!a^GdtiipiBrundr f Formula I or as defined hi embodiment ( 1 ). In another embodiment. R2 is pyridinyl substituted with R\ R :l. R3b. and R"c: where R"\ R3:i. R '1'. and R are independently hydrogen, alkyl. plienylalkyl. or plienylalkyl substituted with one or two halo; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( I ).
[001701 Embodiments, (D7b): In another embodiment. R2 is; pyridinyl substituted with R ". R3:'. R31-, and R ; where R3 is alkyl (in another embodiment alkyl is Gi.ralkyi): R3, R¾.; R3I\ and R c are hydrogen; and all other groups are independently as defincd in the Summary of die Invent ion for a Compound of Formula I or as defined in embodiment ( 1 ).
[00171] Embodiments (D7c): In another embodiment, R2 is pyi idin-2-yl. pyridiii-3-yl. pyridin--l-yl 2-aniin0'-pyridiii-- -yl. 3-mcthyl-pyridin-2-yI, 2-methyl-3-( phcnylniethyl)- pyridin-4-yl. 3-(2-nuoro-phenylmethyl)-2-meihyl-pyridin-4-ylI 3-( 3-fhtoiO-pheiiyl mcihyl)-2- methyl-pyridiu-4-yl, or 3-(4-fluoi -phenylnielhyl)-2-nicihyl-pyri(lin-4-yl ; and al l other groups arc independently as defincd in the Summary of the Invention for a Compound of Formula I or as' defined in embodiment ( I ).
[00172] Embodiments (D7d): In ano o Formula ( b)
C )
where R'\ R3 '. and R3h are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( 1 ).
[001731 Embodiments (E): In another embodiment , R2 is a l O-mcmbercd hctcroaryl subst ituted with R3. R :i. R31', R3c. and R3'1: where R3, R :'. R3h. R3' . and R3'1 and all other
groups arc independently as defined in the Summary of the- Invention for a Compound of Formuja I or as defined in embodiment ( I ). In another embodiment., R" is a l()-meiubered lieieroaiyl anil the lO-mcmbcrcd lieteroaryl is quinazolin-2-yl. quiiKizolin-4-yl. quinazolin-5- yl, quinazolin-6-yl. qiiinazolin-7-yl. quinazolin-8-yl. pyrido| 3.2-r/|pyrimidtn-4-yl. p.yrido|4,3- <Y|pyrimiclin-4-yl. pyrido|3.4-f/|pyrimidin-4-yl. pyrido|2.3-r/|pyrimidin-4-yI.6.7-dihydro-5/7- cyclopcnta|//|pyrimidin-4-yl.5.6.7.S-lelrahydroquinazolin-4-yl. quinolin-2-yl. qiiinolin-3-yl. quinolin-4-yl. quiiiolin-5-yl. quinolin-6-yl. quinolin-7-yl. quinolin-8-yl. isoquinolin- l-yl. isoquinolin-3-yI. iso.quinolin-4-yl, isoquinolin-5-yl. isoquinolin-6-yl, isoquinolin-7-yl.
isoquinoIin-8-yl.
1/7- pyrrolof2.3-/
;|pyridin-4-yl. l/7-pynOlo[3.2-6|pyridin-4-yl. ihieiui|2.3-/>|pyridin-4-yl.
ihieno|32-f |pyndin-4- l.5.7HlihydioihieiH.)|3.4-</|pvriniidin-4-yl, 5.6.7.8- letrahydropyrido|3.4-i/|pyrimidin-4-yl< 5.6.7.S-tetrahycliopyri lo|4,3-i'/|pyrimidin-4-yl.
5.6.7.8-teii hydropyrido|2,3-i/lpyi iiiiidin-4-yl.5.6.7.S-tetrahycli pyrido|3,2-£/|pyrimidin-4- yl.6.7-diliydro-5/7-pyn lo|3,4-i/|pyrimidiii-4-yl.6.7-dihyd!O-5/7-pyiTolo|3.2-i/|pyrimidin-4- yl.6,7-dilvydi -5/7-pyriOlo|2.3-f/|pyriniidin-4-yl. or 5.6-dihydroquinazolinyl where R" is substituied with R3. R a. R31?, R3\ and R'w; where R*. R3'. R "\ R3C. and R3d and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in embodiment (1).
100174] Embodiincnls (El ): In another embodiment. R" is qiiinazolin-2-yl. quinazolin-4- yl. quinazolin-5-yl. quinazolin-6-yl. quinazolin-7-yl; or quinazolin-8-yl. where R" is substituted with R3, R N. R5b. R . and R3D: where R \ R¾. R* R . and R3d and all other groups are independently as defined in th Summary of the Invention for a Compound of Formula I or as defined in embodiment (I).
1001751 Embodiments (E2):. In another embodiment, R2 is quinazolin-4-yl substituted with R3, R3a. R h, R c. and R d; where R3. R3\ R '\ R¾. aiicl R U and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (1). In another embodimcni. R2 is qiiinazolin-4-yl substituted with R3, R3:'; R3h. R . and d: where R3, R3'1, R31'. R:\ and R arc independently hydrogen, halo, alkyl, haloalkyl. alkoxyearbonyl. optionally substituted plienyl. -S(0):R2". -NR"R":'. or -ORlla: and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( 1).
[001761 Embodiments (E2a): In another embodimcni. R2 is quinazolin-4-yl subsiituied with R\ R a. R3I\ R\ and R3d: Ri and R3d arc hydrogen and R \ R3a,.and R31' arc
independently cyano. alkyl. alkenyl, halo, haloalkyl. hydroxyalkyl, alkoxyalkyl. -SR12,
-S(0)2R2,\ -C(0)OR!. halocarbonyl, -NR"R":i. -O n optionally s stiiiucd phenyl optionally -substituted plienylalkyi, optionally substituted cycloalkyl. optionally. substituted cycloalkylalkyl. opiionally substituted hclcrocycloalkyl. opiionally substituted
hctcrocycloalkylalkyl, optionally substituted hclciOaryl. optionally substituted
heteroarylalkyl, or alkyl substituted with one or two Rir'; and all other .groups are
independently as defined in the -Summary of the Invention for a Compound of Formula I or as defined in embodiment (1). In another embodiment, R" is quinazolin-4-yl substituted with R"\ R: . R'1'. R'\ and R:'1': R k and R¾1 ar hydrogen and R\ R¾ and R1" are independently alkyl. halo, or -OR1'11; and all oilier groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (I ). In another embodiment, R: is quiiiazolin-4-yl substituted with R \ R':V R31'. R . and R3'1: R c and R d are hydrogen and R3, R"''1, and R,b are independently alkyl. halo, or -0RII;|; Rll:' is hydrogen, alkyl, or alkoxyalkyl: and all other groups arc -independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( I ).
[00177] Embodiments (E2b): In another embodiment, R2 is quinazoIin-4-yl substituted with R\ R3a. R31', R c. and R d; R31'. R . and RM are hydrogen, and R and R3n arc independently cyano. alkyl, alkenyl. halo, haloalkyl. hydro.xyalkyi, alkoxyalkyl. -SR12.
-S(0):R-". -C(0)ORL. haloearbon l. -NR"R";'. -OR":i. optionally substituted phenyl, optionally substituted plienylalkyi. opiionally substiliiied eyeloalkyl, opiionally substituted cycloalkylalkyl. optionally substituted helerocycloalkyl. optional ly substituted
hcter cycloalkylalkyl. optionally substituted heteroaryl, opiionally substituted
hcieroarylalkyl, or alkyl substituted with one or two RK': and all other groups are
independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (J ). In another embodiment, R" is quinazoltn-4-yl substituted with R3, R:,\ R31'. R3 , and R3<l; R""'. R , and R3'1 are hydrogen, and R3 and R ;| are independently alkyl. halo, -SfCJ R2", :0 ' or alkyl substituted with one R lsv and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in embodiment ( I ). In another embodiment, R2 is quinazolin-4-yl substituted with R'\ R a. R3", R . and R3d : R h, R c, and R d are hydrogen, and R3 and R3a arc independently alkyl. halo, -S(0):R2". -QRMa, or alkyl substituted wiih one R1''; Rll:i is hydrogen, alkyl.
aminoalkyl. alkylaminoalkyl. dialkylarninoalkyl. phenyl, cycloalkylalkyl. plienylalkyi, or heteroaryl: R1 is amino, alkylamino, dialkylamino, or cycloalkylamino; R " is alkyl: and all oilier groups arc independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (I). In another embodiment. R2 is quinazo|in-4-yl
substituted with R3, R \ R3", R¾, and R¾. R31'. RTc, and R:,,i:arc hydrogen, and R' .is -OR11:1 and R¾ is hydrogen, alkyl (in another emlxidinie ralkyl is Ci.ralkyl). or alkyl substituted ii!i one R"'; and all olhcr groups are independently as defined in die Summary of the Invention lor a Compound of Formula I or as defined in embodiment ( I ). In another embodiincntvR2 is quina/.olin-4-yl substituted with R3. R,:\ "\ R t', and R3'1; R31'. Rk, and R3>l arc hydrogen, and RJ is -OR 11:1 and R3:1 is hydrogen, alkyl. or alkyl substituted with one R|{>: Rltj is hydrogen or alkyl; R16 is amino, alkylaihino, dialkylamino. or cycloalkylamino: and all other groups are independently as defined in the Summary of the Invcnlion for a Compound of Formula 1 or as defined in emhodimenl (1).
(00178] Embodiments (E2c): In another cinbodimeut. " is quinazolin-4-yl substituted with R3, R a. R b. R3c. anil RM R \ R3h. and R3D are hydrogen and R3 is cyano. alkyl. alken.yl. halo, haloalkyl. hydroxyalkyl, alkoxyalkyl. -SR12. -S 0) R2". -C(0)OR'\
halocarbonyl, -NR"R"''. -O 11', optionally subsiiliiied phenyl, optionally substiluied phenylalkyl. optionally substituted cycloalkyl. optionally substituted cycloalkylalkyL optionally substituted heteroeycioalkyl, optionally substituted hetcrocycloalkyialkyl, optionally .'.substituted heicroaryl. optionally substiluied heteroarylalkyl, or alkyl substituted with one or two■ Rlft; and all other groups are independently as defined in the Summary of the Invcnlion for a Compound of Formula I or as defined in embodiment f 1). In another embodiment. R: is quinazolm-4-yl .substituted with R3. R3;i. R I\ R c. and R3": R :'. R3b. R3c. and R",d are hydrogen and R"1 is alkyl. halo, haloalkyl. alkylsulfonyl. optionally substituted phenyl, carboxy, alkoxycarbonyl, -NR"R":i, alkyl substiluied with one Rl(\ or -OR "a: and all olhcr groups are independently as defined in the Summary of the Invcnlion for a Compound of Formula I or as defined in embodiment (I). In another embodiment. R* is quinazolin-4-vI substituted with R3. R :'. R3". R3c,and R3,1; R :i. R . R3c. and R3'1 are hydrogen and R3 is alkyl. halo, haloalkyl. alkylsulfonyl. phenyl, carboxy. alkox'ycarbonyl. -NR."R"\ alkyl substituted with one R"'. or -ORL L:I: R11 is hydrogen or alkyl; Rlla is hydrogen, alkyl, alkoxyalkyl.
cyanoalkyl. or optionally substituted phenylalkyl: R1" is amino, alkylamino. dialkylamino. or cycloalkylamino: and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in embodiment (I). In another emhodimenl. R2 is quinazolin-4-yl subsiiliiied with R3, R ;\ R 1>. R3c, and R '1: R :\ R3I>. R2c. and R d are hydrogen and R*' is methyl, ethyl, n-propyl. isopropyl, n-buiyl. sec-butyl, isoamyl. bromo, chloro. flubro, iodo. trifluoiOmcthyl. mcihylsulfonyl. phenyl, mcthoxycarbonyl, cihoxycarbonyl, amino, incihylamino. ethylamino, n-propylamino, isopropylamino.
dimcthylamino. diethylamino. /V-methyl-A'-eihylamino. hydroxy, methoxy. cthyloxy, n-
piopoxy, isppropoxy, n-lnityloxy, sec-huiylaxy. i.soaiuyloxy, 2-amino-elhyloxy.
2- (iiiciliylamino)-ethyloxy.2-(dimeilvylamino)-eihyloxy.3÷au\ino-propyloxy.
3- (metliylamind)-pfop.yloxy 3-(cM^
cyanomethyloxy. and benzyloxy; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( I ). 10017 1 Embodiments (E2d): In another embodiment, R2 is quinazolin-4-yl, pyrido|3.2- i/|pyi'imidin-4-yl, pyrido|4,3-<-/|pyrimidin-4-yl. pyrido|3.4-f/|pyrimidin-4-yl. pyrido|2,3- i/|pyriii)idin-4-y|, 2-melbyl-quiiuizolin-4-yl.6-iHethyI-qtiinazolin-4-yl.7-melhyl-quinazolin-
4- yl, nctliyl-qui azolin-4-yl.2-etHyi-quinazolin-4-yl, 2-phenyl-{|iiinazo!in-4-yl.7- (quinoliiv-2-ylmelhyloxy)-8-n^^^^ 7^(2-diinethylainiitOiethyloxy)-S- methoxy-quinazolin-4-yl. -(3Hliiiietliylaiiiiiio-piopyk)xy)-8-incihoxy-(|uinazolin-4-yl.7- (cyelopropylniethyloxy)-X-tneihoxy- |iiinazoliii-4-\ , 6-(cya! )nvethyloxy)-quinazoliii-4-yl, 6- metlK)xy-qiiinazolin-4-yl.7-nielhoxy-quinazolin-4-yl.8-metlu) y-quinazolin-4-yl.6-cihoxy- quinazolin-4-yl, 6-(n-propoxy)-qtiinazolin-4-yl.6J-dimetho -quinazolin-4-yl.7,8- dimethoxy-qiiinazolin-4-yl.7-is(ximyloxy-8-niethoxy-quiiiazoliii-4-yl.5-bromo-qu inazol in-4- yl, fi-bromo-qiiinazolin-4-yl.74>romo-quinazolin-4-yl.8-bromo-i|uinazolin-4-yL 5-chloro- quinazoliti-4-yl, 6-ehloro-qiiinazolin-4-yl.7-elvlororquinazolin-4-yl, 8 ehloro- Liinazolm-4-yI,
5- nuoiO-q inazoltn-4-yl, 6-fhioiO-qutnazolin-4-yl.7-nuoro-qtiinazolin-4-yl, 8-fltior - quinazolin-4-yl, 5-iodo-quinazolin-4-yl.6-iodoHtiiiiazoliii-4-yl 7-iodoiquinazoh
S-iodo-quinazolin-4-yl, 6-bromo-7-chloiO-qiiin:izolin-4-yl.6-iodo-7-ehloiO-quinazolin-4-vl. 6,8-dieliloi -quinazolin-4-yl, 6.7-difluoro-quiiiazo!in-4-yi.6,8-dibi ino-quinazolin-4- l.2- melhyl-7-mcihoxy-quiiuizolin-4-yl, 2-eihyl-7-mcthoxy-quinazoliii-4-yl.2-methyl-6.7- dimctboxy-quinazolin-4-yl, 6-iodo-7-meilH)xy-quiriazolin-4-yl.6-chloro-7-methoxy- qiiinazolin-4-yl,
7-bromb-8vinetlio y-C|uin;»^)liiv4-yI, 7 ir0ino-6 ncthoxy-quiiiaz()lin-4-y!.6-elvloro-7.8- dimethoxy-quinazolin-4-yl, 6.7,8-trimetJioxy-qiiinazolin-4-yl, 6-(2riiieihoxy-ethyloxy)- quinazolin-4-yl, 6-(benzyoxy)-quinazolin-4-yl, 64iyclroxy-quinazolin-4-yh 7-(bciizyoxy)-8- methoxy-qiiinazolin-4-yl, 74iydroxy-8-melhoxy-quinazolin-4-yl, 7-(benzyoxy)-6-methoxy- qtiinazolin-4-yl.7-hydroxy-6-meihoxY-qiiinazolin-4-yl.6-iodo-8-meihyl-quinazolin-4-yl.6- mcthyl-84iiOmp-c|iiinazolin-4-yl, 2-ethoxyearbonyl-quinazolin-4-yl.2-mcihylaminn- quinazo!in-4-yl, 2-ethylamino-quinazolin-4-yl.2-(diethylamiiiO)-qiiinazolin-4-yl.2- (irinuoiOmctliylj-quinazolin-4-yl, 7-(U'ifhioiOmcihyl)-quinazolin-4-yi, 8-(trifiiioromcthyl)- quinazoIin-4-yl.6-melhylsulfonyl-quinazolin-4-yI.7-nieiliyIstil Γοηνΐ-qiii iiazol in-.4-yl ,
C|iiinazolin-4-yl. qiiinazolin-4-yl. or quinazolin-4-yl: and all other groups are independently as
defined in ihe Summary of the Invention lor a Compound of Formula' I or as '-defined in embodiment (1).
[00180] -Embodiments (E2e): In another embodiment. R" is pyridoi3 /|pyrihiidiii-4.-yl: and all other .groups are independently as defined in the Summary of th Invention for a Compound of Formula I or as defined in embodiment ( I).
[0 181] Embodiments (E3): hi another embodiment. R" is 5.6,7, 8-ieirahydroqumazolin-4- yl.6,7-dihydr(>-.i/7-eyelopciila|i/|pyriiiiidiii-4-yl.6.7,8.lMeirahydi -5/7- c dloh'cjp'ia |V/|'pyri in ί<ίί n^ - I . *5.6-.tt i h d itoqu inakol iii -4·- 1.7'.8'-dihydro-577-
embodiment, 2 is ;6,7,8-tctrahyd^^
cyclppe!Ua[^)pyriinidin-4-yl.6,7.H.y-tetrahydr0-5 5,6- dihycliOquinazolin-4-yl.7\8'-dihydro-577-spiro|cyclopiOpane- 1.6'-c|iiina/.oline|-4"-yl. or 6',8'- clihydro-577-spiro| cyclopropane- 1.7'-quinazoline |r4*-yl where R2 is substituted with R'\ R',:i. RJH, R and RM; where R3. R3a, R"\ R ', and R '1 are hydrogen; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (1).
[00182 | Enibodimehts(E3¾ Iii;ancHher;eniboclinicnt. R2 is 5v6,¾8rieirah
4-yl, 6,7 dihydro-5/7-cyel p'ent;i|(/|pynn dii
cyclohepta|</|pyrimiilin-4-yl.5,6-dihydroquiiiazolin-4-yl. or 7'.8'-dihydro-577- ^ .spiroicyclopi-op,anc-l,6'-quinazoline|-4'-yl. where R2 is substituted with R3. R \ R'"'. Rk. and R'd: where R3. R", , R ,l\ R'\ and R3'1 are independently hydrogen, alkyl. alkcnyl. halo, haloalkyl, hy.droxyalkyl, cyanoalkyl. -SRi . optionally substituted phenyl. -ORlla. alkyl substituted with cine I*"', optionally substituted hcterocycloalkyl, optionally substituted hcieroeyclpaJkylalkyl,;or optionally. substituted hcieroaryl: and all. other-groups are independently as.clei nied i = the Summary of the Iiiyentioi foi: a Compp}.uid'of Formula Fpras defined in cniWo.dimeni (1). In anothcr embodimeni. R2 is.5.6.7.8-ietraliydroquiiiaz0liri-4-yl. 6.7-dilrydro-5/7-cyclopeiHa[i7|pyriini(lin-4-yl.6.7,8, -tetrahydi -5/7-cyclohepia|i/|pyrimidin- 4-yl.5.6-dihydroquinazplin-4-yl. or 7'.8'-dihydiO-577-spiiO|cyclopro|)ane- 1.6'-quina/.olincl- 4-yl, where R2 is substituted with R;\ R3a, R b. R* and R I: where R3. R*\ R3". R3c. and R d are independently hydrogen, alkyl. alkenyl. halo, haloalkyl. hydroxyalkyl. cyanoalkyl, -SRU. phenyl, -OR."a, alkyl subsiiiuicd with one Rl' hcierocyeloalkyl (Optionally substituted with
alkoxycarbonyl. plienylalkyloxycarbonyl. or alky I). hetciOcyclpalkylalkyl (optionally substituted wilh one or two halo), or lieteiOar'yk R12 is alkyl or pheriylalkyl: 1" is NRL LRLLN. -NRL5S(0)Rl5V-ORLI< or -0G(:O)R''7-; R" is hydrogcuwalkyl: each R, l;' is independentl hydrogen. ilkyl. lialoalkyl, alkoxyalkyl. earboxyalkyl, cyel'oalkyl, or cycloalkylalkyl; and all oilier groups are independently as defined in the .Summary of the Invention for a Compound of Formula 1 or as defined in embodiment ( I ).
|()()183| Embodiments (E3b): In another embodiment. R" is 5.6,7.8-teimhydi quinazolin- 4-yl.6.7-dihydro-5/7-cyclopenta(<'/|pyrimidin--;l-yl.6J.8.9-lcirahydro-5/7- cydohcpta|y/JpyriWdih*4-yI,.5,^^ or. T'.S'-dihydro 'A/- spiro|cyclbpiOp.aiic- l ()'-t]iiiiiazoliiiej-4'-ylf .-where ? is siihsiitiited with R3 F R'':',1 "1|?. Ric,-.and R3'1; where R :I. R H. R'K. and R3'1 arc hydrogen, and R3 and a|l other groups are independently a definecl-in the Summary of the Invention for a Compound of Formula' l or as defined in embodiment (1). In aiiolher embodiment. R~ is 5.6.7.8-ie.irahydiO'qiiiiiazolin-.4-yl.6.7- dihydro-5W-cyclopenta|//|pyrimidin-4-yl, 6J,8:9-tetrabydi -5/-/-cyclohcpia|f/|pyriniidin-4-yl. 5;6-dihydrocjuinazolin-4-yl, or 7'.8'-dihydro-57/-spiro| cyclopropane- 1.6'-quinazoline|-4'-yl. where R- is stibstiluted with R3, A. RIH. R . and R3'1; where R3\ R3", R c,,.and R3'1 are hydrogen, and R3 is alkyl, alkenyl, hydroxyalkyl, alkoxyalkyl. lialoalkyl, , optionally substituted plienyl.alkyl substituted with oiie R"\ or -SR'-; and all other groups-are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (1). In another embodiment. R2 is 5.6.7.8-ietrohydrqquinazolin-4-yl, 6,7-dihydrQ-5/'/-cyclopenia|f/|pyritiiidin-4-yl.6.7.8.9-tetraliyd!O-5H-cycIohepta|i/|pyriiiiidin- 4-yl.5.6-dihydiO(|uinazolin-4-yl.7'.8'-dihydro-577-spiro| cyclopropane- 1.6'-quinazoline |-4'- yl. wliere R~ is substituted wilh R3. R\ R31'. R¾. and RJ": where RJA, R3", RJC. and R3'1 arc hydrogen, and R3 is alkyl, alkenyl, hydroxyalkyl, alkoxyalkyl. lialoalkyl. phenyl, alkyl substiiuted with one R1'1. or -SR12; R1" is alkyl or optionally substituted phenylalkyl: and all other groups are intlependenlly as defined in the Summary of the Invention for a Compound of Formiila I or as defined in embodiment (1).
[00184] Embodiments (E3c): In another embodiment. R
" is 5,6.7, 8-tctrahyiliOquinazolin- 4-yl.6,7-dihydi -5/7-cyclopcnla|i/|pynmidin-4-yI, 6,7.8,9-tetrahydro-5/7- cyelohepla|i/|pyrimidin-4- l, 5.6-dihydroquinazolin-4-yl. or 7',8'-dibydi -57/- spiiOleycl.opi panc-l.6-C|iiina'/.olinc
i|-4'-yl, where R
" is substituted with R
3, '. R
H. R
"'\ and R
3U; where R
31'. R
3C. R
M arc hydrogen, and R
3 and R
3A are independently alkyl. halo, optionally substituted phenyl, -SR
1". or alkyl substituted with one R
1'
1; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I
or as defined in embodiment ( 1 ). In another embodiment, " is 5.6 J.8-ieirahydroquiiiazoIin- 4-yl, j-diliydro-5/V yclopenla|(7||iyi iniidiii-4-yI. 6..7.8. -lcirahydro-5/7- ey.elolic|.na| f/|pyrimidin-4-yi. 5.6-dihydroc]itinazolin-4-yl
; or 7':8 diliydi -577- spiro|cyelo|}iOpane- l .6'-qiiiiia oline |-4'-yl, where R
" is substituted with R '. R
i'. R
'!h. R
3t". aiicl R
3'
1: where R '
1'. R
', . R
',J are hydrogen, and R
"' and R
,:i are independently alkyl. halo, phenyl, alkyl substituted with one R
1". or -SR
1 ': R
12 is alkyl or phenyl; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( I ). In -another embodiment. R
" is 5,6 J 8-ieirahydroquiiuizolin-4-yl. 6,7-dihydro-5/7-eyclopenla|i/|pyriiiiidiii-4-yl. 6J.8.y etrahydro-5/7-cycHohepta|i/|pyriniidin- 4-yl, eloptOpane- 1 .fr-qiiinazoline l- ^'-yl,
here R
3-; R
3c. R^ - are hydrogen. R ' is alkyl (in another embodiment alkyl is G| .2-alkyl), and R
3:1 is alkyl (in another embodiment alkyl js C
' l .^-alkyl). halo, phenyl, alkyl subsi iiuied with one R
lf'. or -SR
13: R
13 is alkyl or phenyl: and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as -defined in embodiment ( I ). In another embodiment. R
2 is 5;6,7.8-ietraliydroquinazolin-4-yl, 6,7-dihydro-5/7- cyelopenta|r7|pyrimidin-4-y^^
dihy lroquina/.olin-4-yl
;Or
where R
3 is substituted with R
3. R
:i,. R
h, R \ and R
3'
1; where R
3b,
c, R
3'
1 arc hydrogen, R
3 and R " are alkyl. (in another embodiment -each alkyl is
and all other grou s are independently as defined iii the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( I ). In another embodiment. R
" is 5.6.7,8-telrahydroquinazolin-4-yl. 6.7-dihycli o-5/7-eyelopciita|f/|pvriuiidin-4-yl. 6.7.S.9-icirahydro-5A/-cyclohepia| i/|pynmidin- 4-yl, 5.6-dihydroquinazolin-4-yl. or
.6'-quinazolinc|- 4'-yl
: where R- is substituted with R
3. R
3 ', R
3'\ R
3 . and R
3'
1; where R
3b. R* R
J arc hydrogen, R
3 and R
3:1 arc halo: and all other groups are independently as defined in the Summary of the Invention for Compound of Formula I or as defined in embodiment ( 1 ). In another embodiment. R
2 is 5.6.7.8-tetrahydroqiiinazolin-4-yl. 6,7-dihydi -5/7- cyelopcnta| i/|pyrimidin-4-yl. 6.7.8,9-letrahydro-5/7-eyclolicpta| i/|pyrimidiii-4-yl'. 5.6- dihydroquinazolin-4-yl, or 7\8'-dibydro-577-spiro| cyclopropane- 1 ,6
'-quina/.oline |-4
'-yl. where R
2 is substituted with R
3. R
:'. R
h. R
:\ and
3'
1: where R
:'". R
¾. R are hydrogen. R
3 is alkyl (in another embodiment alkyl is Cj -alkyl ). and R
3:i is hydrogen, alkyl . or alkyl substituted wiih R
1''; and all other groups ar independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in embodiment ( 1 ).
100185] Embodiments (E3d): In another embodiment, R~ is 5.6.7.8-icirahydiOquinazolin- 4-yl, 6J-dihydro-5.H-cyclopenta|i/|pyriniidiii--l-yl.6,7,8,9-letriihydiO-5/-/- cyclolicptal /.| yrimkliii-4-yl, 5j6-dil)ydiOC|iiiiiaxolin-'l-yl. or 7';8'-dihyclro-577- spiio|cyc opropane- l,6'-(|iiina7.oliiic|-4'-yl. Avliere R
2 is substituted with R
'\ R
' . R
'"'. R
' . and R
M: where R
3c.
'R
M are liydrogcn. and R
:\ R
A. and R
31' arc independently alkyl. alkenyl. halo, hydroxyalkyl. cyanoalkyl, alkyl substituted wiib R
1". heterocycloalkyl. or
helcibcycloalkylalkyl (optionally substituted with one or two halo); and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( I ), in another embodiment. R2 is 5.6.7,8-lctrahydroquinazolin-4-yl, 6.7-diliydro-5/-/-cycU)penla|i/|pyriniidin- -yl.6.7.8,9-lcirahydi -5/7-eyelohepta|i/|pyrimidin- 4-yl.5.6-dihydro(]uinazcilin-4-yl, or 7\8!-dihydro-577-spiro| cyclopropane- 1.6'-quinazolincl- 4-yl, where R2 is .substituted with R3. R¾. R31'. R:K\ and R3J; where RK'. R3'1 are hydrogen, and R'\ Rja. and R31' are independently alkyl. alkenyl. halo, hydroxyalkyl. cyanoalkyl. alkyl substituted with R"', heterocycloalkyl. or hcteroeycloalkylalkyl (optionally substituted with oncor two halo); RLF' is NR"R":1 where R11 is hydrogen or alkyl and Rll:i is alkyl, haloalkyf ajkoxyalkyl, cyelo ikyl, cycloalkylalkyl. or carboxyalkyl; or Rlf> is -Js!Rl5S(OJRlSa where R15 and R13a re independently hydrogen or alkyl: or R1" is -OC(0)R17 where R17 is alkyl: RLF' is -ORLS where Rlh is alkyl or alkoxyalkyl; and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in embodiment ( 1 ).
[00186] Embodiments (E3e): In another embodiment, R
2 is 5,6.7.S-tetrahydiOquinazoiin- 4-yl, 6;7-dibydror5/7-cyclopenia|i/Jpyrin)iclin-4-yl.6,7,8,9-teirahydro-5/7- cyclohep i[ /|pyrtinidinr^yl S,6-dihydro(jitiiva/Ailin-4-yl or.7'.8'-dilvydro-577- spiro|cyclopropaue-1.6'-quinazoliue|-4'-yl. where R
2 is substituted with R
'\ R
,a. R '
b. R
t. and R
3'
1; where R
"'
C. R
3'
1 are hydrogen, and R\ R'
A, and R
",L? are alkyl (in another embodiment each alkyl is Ci.j-alkyl): and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( Γ). In another embodiment, R
2 is 5.6.7.8-tcirahydroqiiinazolin-4-yl.6.7-dihydro-5/7- cyclopenla|i/|pyriinidin-4-yl.6.7.8,9-tcti"ahydro-5/7-cyclohcpta(c/|pyriniidin-4-yl.5.6- dihydiOquinazolin-4-yl. or 7\S'-dihydro-577-spiro| cyclopropane- 1.6'-quinazolincj-4'-yl, where R
2 is substituted with R
3, .R
-,a, R
3". R
¾, and R
3'
1; where R
3e, .R
3'
1 arc hydrogen. R
3 and R
3:> are alkyl (in another embodiment each alkyl is C|. -alk,yl). and R"'
1' is alkyl substituted with R
16; and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in embodiment
' (1 ). In another embodiment. R
2 is 5.6.7.8-teirahydi C|uinazolin-4-yl.6.7-dihydm-5/7-cyclopcnta|i/|pyriniidin-4-yl.6.7.8.9-
lciraliYclro-5/ -cyclohe| Ui|^/|pyriiviidiii-4-yl. 5,6-d ihydroquinazol in-4- l . or 7
'.8
'-clihydro-57-/- s|)i! oi cyc½propaiie-
and R
3'
1: where R '
1'. R
",D are hydrogen. R
'! and R ' arc a!kyl (in another embodiment each alk-yl is Ci--2-alkyl); and
J|1 is hetcrocyeloalkylalkyl; and all
'oilier grbiips arc indepeiidenily as defined in the Summary of die .Invent ion for a Compound of Formula I or as-defined in embodiment ( I ). In another embodiment. R
2 is f).6.7,8-leir hydi ot|uin:izolin- -yl, 6.7- clihydro-5/7-c
,yclopci'iia|f/|pyi iniidin-4-yl. 6,7,«
T -tctrahydro-5/:/-cyclohepla| i/|pyi iiiiklin-4-yL 5.6-dihydroc|uinazolin-4-yl. or 7'.S'-diliydro-577-spiro| cyclopropane- 1 .6'-quina/.oIine|-4'-yl. where R
2 is substituted with R\ R'
¾. R '
1'. R ' . and R
'">D; where R . R
M arc hydrogen, R ' and R
" arc alky I, ( in anoihencmbodihieni each alkyl is€.|.
2-alkyl),. aiul R*
i is hetei cycloalkyl:. and all.other groups are independentl y as defined in the Summary-of thc nA'Cniion fer a Compound of Formula I or as defined in embodiment ( I )·
100187] Embodiments ( Π3Ι ): In another embodiment, R2 is 6;7-dihydro-577- cyelopenta|i/|pyrimidin-4-yl, 6-inethyl-6J-dihydi -5//-cyclopenta /|pynmidiu-4-yl.
6,6-dimethyl-6J-dihydiO-5/7-cycI()penia| i/|pyriinidin-4-yl. 6-melhyl-2-(methylthio)-6.7- dihyclro-5/7-cyclopenta| /|pynniidin-4-yl, 2-(eihyllhio)-6J-dihyclro0/7- cyclopental i/)pyrimidin-4-yl. '2-(phenyliiicihylth'io)-6.7-clihydra-5/7-c clQpcnla| |pyrimid.in- 4-yl. 5-phenyl- 7-dihydro-5f/-cyclopentaL
cyclopenia|i/|pyrimidin-4-yl. - 6.7;H-tcirahy(lrdc|uiiiazolin^4-yl..6-uictliyl-3.6.7.S- tetrahydroqtnnaz;olin-4-yl, 6-ethyl-5X\7.S-tetrahydiOC|iiiiia7.olin-4-yl! 7-me,ihyl-5.6,7,S- tetrahydrQq'uinazoliiv4-yr, 7-ivieiliyl-7-plien.yl-5.6.7.8-teiraliydrociiiina olin-4-yl. '
6/v(rnnclhyl-5.6,7.8-tetrahydi'o(|iiina olin-4-yl. or 7,7-dimeihyl-5,6.7.H- ieiraliydroquinazolin-4-yl; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( I ).
|00188| Embodiments (E4); In another embodiment, R2 is according to Formula (c)
fc)
where m is 0 or I and R\ R',:i and al l other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( 1 ). In another embodiment. R2 is according to Formula (c) where m is 0 or I anil R'1 and R (:I.
together with die carbon to which they are attached, form an optionally .substituted cycloalkyl
or an optionally substituted heteroeycoalkyl: and all oilier groups arc independently as defined in the.Summar.y of the Invention for a Compound of 'Formula.1 or as-defined' in embodiment (1). In-aiiother embodiment. R2 is. ccordin .-to "Formula (c). where iii is.0. or I. and R' and R>,a are alkyl (in another embodiment each aikyl is C|.2-alkyl): and all other gr ups are independenlly as defined in the .Summary of the Invention for a Compound of formula I or as defined in embodiment ( I ). In another embodiment. R2 is according to Formula (e) where m is 0 or I anil J and R'¾ arc lialo; and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in embodiment (1).
[00189] Einbod i merits (E4a); 1 h another embodiment^ R2fe^
1. R"! and R are -as dgfined in any of the embodiments '(E4'd); and- all oilier groups are as defined in the Summary of the, Invention for a Compound of Formula I or as defined in embodiment ( 1).
100190] Embodiments (E4b): In another embodiment. R2 is6.(vdimeiliyl- 6.7.8- leiialiydroquinazolin-4-yl, 6.6-dichloro-5.6.7.8-ienahydrocjuina7.olin-4-yI.6,6-difluoro- 5.6,7, X-lelrahydroquinazolin-4-yl, 7.7-tlinielhyl-5,6,7,8-leirahydroquina/.olin-4-yl.7.7- dichloro-5,6.7;8-tetrahydro(|uiiiazbliii--l-yl.7\8'-dibydro-5¾-spirolcyclopiOpa[ie-l,6'- qiuna*oline|-4!-yl. pr¾ ;8' « ^9-5;^ where R2 is substituted with R'~b where- R31' is hydrogen-; alkyl, or haloalkyl; and all other groups arc • independently as defined in, the Summar of the Invention for a Compound of Formula I or as defined in embodiment (I ).
[00191] Embodiments (E4d): In another embodiment. R2 is according to Formula (d)
(d)
where m is 0 or I: R'\ R, . R'1', and all other groups are independently as defined in the Summary of the Invention for a Compound of Formul I or as defined in embodiment ( 1 ). In another embodiment. R2 is according to Formula (cl) where in is 0 or I ; R'' and RJJ arc alkyl (in another embodiment each alkyl is Ci-2-alkyl); and all other groups are independently as defined in. the Summary of the Invention for a Compound of Formula I of as defined in embodiment (I). In another embodiment, R2 is according to Formula (d) where m is O or 1;
R3 and R',A are halo: and all oilier groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( 1). In another embodiment, R" is according to Formula (d) where m is I : R' and ¾ are alkyl (in another embodiment each alkyl is Ci.^- lkyl); and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (1). In another embodiment, 2 is according. Co- Formula (d) where in is 1 ; R'' and R 1 arc halo;; and all other groups are independently as defined in the Summary of the Invention for a
Compound of Formula I or as defined in embodiment (I). In another embodiment. R2 is according to Formula (cl.) where m is 1: R'! and RJ:| arc alkyl (in' another embodiment each alkyl is C|.2-alkyl); R B is hydrogen, alkyl. alkenyl. hydroxyalkyl, cyanoalkyl.
heieorcyclQalkyl (optionally substituted with alkoxycarbonyl. benzyloxycarbonyl. or alkyl), hctcorcycloalkylalkyl (optionally substituted with one or two halo), or alkyl substituted with one RL('; and all other groups are independently as defined in the Summary of the Invention (or a Compound of Formula 1 or as defined in embodiment ( I ): I ii another embodiment. R2 is according to Formula (d) where m is I; R* and 3? arc alkyl (in another embodiment each alkyl is C|.2-alkyl); R"'1' is hydiOgcii, alkyl, alkenyl. 'hydroxyalkyl. cyanoalkyl.
hetcorcycioalkyl (optionally substituted with alkoxycarbonyl.. benzyloxycarbonyl, or alkyl), hctcorcycloalkylalkyl (optionall 'substituted with one or two halo), or alkyl substituted with one RK>; R1(I is - R1 'R11'1. -NR,5S.(0)5R,I:\ -OC(0)R17, or -OR18: and.all other groups are independently as defined in the Summary of the Invention for a Compound of Formul l or as defined in embodiment (I). In another embodiment, R3 is according to Formula (cl) where m is I ; ' and R3'1 arc alkyl (in another embodiment, each alkyl is Gi -alkyl): RM] is hydrogen, alkyl (in an therernbodiment alkyl is Ci.i-alkyt). cyanoalkyl, or alkyl substituted with one R : and all other groups are independently as defined in the, Summary, of the Invention for a Compound of Formula I or as defined in embodiment (1).
100192] In another embodiment, the Compound is according to Formula 1(a). R2 is according to embodiments (E4d) and R1 is according to embodiments (Z)-(Z5).
100193) Embodiments (E5a): In another embodiment. R
2 is according to Formula (e)
R3d
(e)
where >-''1 ? i η-'Ί
R
'\ R
"'. R", R"\ and R " arc positioned on any substitutable carbon of ring (c); and al other groups are independently as defined in the Summary of the Invention for a Compound
of Formula 1 or as defined in embodiment (I). In another embodiment. R
3 is according to Formula (e) where one of R
"\.R
" . R
',h. R
', . and R
"''
1 is hydrogen, alkyl (i another cmbodimciu each alkyl is Ci.->-alkyl). or alkyl substituted wit!i one R
,F' and the other Of R
5. '
V, R
''
1'. R
"'\ and R
"1'
1 and all oilier groups arc independently as defined in the Summary of the Inveiuion for a Compound of Formula Or as defined in embodiment ( 1). In another embodiment, R
2 is according to Formula (e) where one of R\ R
3A. R
,B. R
K. and R
3d is hydrogen, alkyl (in another embodiment alkyl is Ci -alkyl). or alkyl substituted with one R
1'' and the other of R
"\ R
"'
J. R
', . R' . and R
"'
U are independently hydrogen or alkyl (in another embodiment each alkyl is Ci.j-alkyl): and all other groups are as defined in the Summary of the Invention for a Compound of Formula or as defined in embodiment (\), In another embodiment, R
2 is according to Formula (e)
', whercone of R
"',.R
',:F. R
", .R
', ; andR-'- i hydrogen; alkyl (in another embodiment each alkyl is C|..>-alkyl); or alkyl
one R"' and the other of R'\ R ,;'. R',L>. R'K, and R'''1 arc alkyl, (in another embodiment each alkyl is G| .^-alkyl): and all oilier groups are as defined in the Summary of the Invention for a Compound of Formula or as defined in embodiment ( I ). In another embodiment. R2 is according to Formula (c) where one of R \ R ,J, R ''·'. R '\ and R'"1 is hydrogen, alkyl (in another embodiment alkyl is C|,i-alkyl), or alkyl substituted with one Rlf,.-a second of R\ R n RM R-\ and R"1'1 is hydrogen, and the other of R"\ R3a. R?['. R . and R'1'1 are alkyl (in •another embodiment each alkyl is C|¾alkyl^and"alI oth(¾vgroups are as defined injlVe Summary of the Invention for a.Compound of Formuh or as defined in embodiment ( I).
[001941 In another embodiment, the Compound is according to Formula 1(a). R" is according to embodiments (E5a) and R1 is according to embodiments (Z)-(Z5).
100195] Embodiments (E5b): In another embodiment. R2 is according to Formula (f)
CO
where Rib is hydrogen, alkyl (in another embodiment alkyl is C|..;-alkyl). cyanoalkyl. or alkyl substituted with one R1 ; and R is hydrogen, alkyl (in another embodiment alkyl is C|.j- alkyl). or alkenyl: and all other groups are as defined in the Summary of the Invention for a Compound of Formula or as defined in embodiment ( 1 ).
[00196] In another embodiment, the Compound is according to Formula 1(a), R2 is according to embodiments (E5b) and R1 is according to embodiments (Z)-(Z5).
|001!),7| Embodiments (E5c)> In anoth ent. R" is according to Formula; (g)
(g)
where R '1' is hydrogen, alkyl (in another embodiment alkyl is C|.;;-alkyl). cyanoalkyl. or alk l substituted with one R"-': and R~ is alkyl (in another embodiment alkyl.i.s Ci. 'aikyl);
hydroxyalkyl. alkoxyalkyl, or haloalkyl. and is located at the 6- or 7-position of the ring; and all other groups are as defined in the Summary of the Invention for a Compound of Formula or as defined in embodiment ( I ).
1001981 In another embodiment, the Compound is accordinglo Formula 1(a), R2 is according to embodiments (E5c) and R1 is according to-embodiments (Z)-(Z5).
|()0i'J9| Emhodinicnis (E d): In ano nt, R2 is according lo Formula (h)
(h)
where \ R"'-'. R". and R' L and all other groups are as defined in the Summary of the Invention for a Compound of Formula or as defined in embodiment (1). In another embodiment. R2 is according to Formula (Iv) where R '1' is hydrogen, alkyl. cyanoalkyl. or alkyl substituted with one R"': and all other groups are as defined in the Summary of the Invention for a Compound of Formula or as defined in embodiment (i ), In another embodiment.. R2 is according lo, Formula (h) where RJ|> is hydrogen, cyanoalkyl, alkyl (in another embodiment alkyl is C|.;s-alkyl). or alkyl substituted with one RICl; R'\ R"':i, and R arc independently hydrogen, alkyl (in another embodiment alkyl is C|.3-alkyl), alkenyl. halo, haloalkyl. hydroxyalkyl. -SR12. optionally substituted phenyl. -ORlla. alkyl subsiiiuted with one Rlfl, optionally substituted hcicrocycloalkyl, optionally substituted hcterocycloalkylalkyl, •or optionally substituted heieroaryl: and all other groups are as defined in the Summary of the Invention for a Compound of Formula or as defined in cinbodimenl (I ).
[002001 In another 'embodiment, the Compound is according to Formula 1(a), R2 is.
according lo embodiments (E5d) and R1 is according to embodiments (Z)-(Z5).
[002011 Embodiments (Έ6): In another .embodiment, R~ is quiiiolin-2-yl, (|uinoliii-3-yl, quinolin-4-yl, quinoliii-5-yl. quiuolin-6-yl. quinojin-7-yl. quitio! in-S-yl . isoi|umolin-l-yl, isoquiiioIin-3-γΙ. isoquinoliii-4-yl. isoqtiiiiolin-5-yl. isoquiiiolin-6-yl. isoquinolin-7-vl. or isoquiiiolin-8-yl. where R~ is ubsiiuiicd with R"\ R,:'. R"'1'. and R : where R;\ R'5\ R11'. and R*'U and all other groups are independently as defined in (he Summary of the Invention lor a Compound of formula I or as defincd in embodiment ( I ). In another embodiment, R: is quinolin-4-yl or i's j L ι ί n ό 1 i n - 1 - I , where R2 is substituted with R \ R",:I. RM RM', and R3'1; where R''. R¾, ^'. '1'. and R"*'1 and all other groups are; independently as defined in (li Summary of the Invention for a Compound of Formul 1 or as defined in eiiibodin'ient ( Γ). |()0202| Embodiments (E6a): In another embodiment. R" is quinolin-4-yl. quinolin-3-yl. qitinolin-4-yl, quinolin-5-yI, quinolin-ft-yl. quinoliii-7-vl. qiiinolin-8-yl. isoquinolin- 1 -vl. isaquinolin-3-yl, isoquinolin-4-yl, isoquinoliu-5-yl. isoquiiioiin-6-yl, isoquinorin-7-yl. or isoquinoliu-.X-yl, where R3 is substituted with R\ \V R'1'. RJ . and RU; R' 1'. R¾. and RM are , hydrogen;. RL ahd R ,a ait independently hydrogen, cyaiio..alky I, halo, haloalkyl, -OR.1 phenyl, phcnyialkyl optionally substituted with one or two R1''. or alkyl substituted with one or two R-Vand all oilier groups are independently as defined in the Summary of the
Invention for a Compound of Formula l or as defined in; embodiment (1 ). In-anoiher einbodimeni. RJ is quinGliii-4-yl or isoquinolin- l-yl. where R 3 is substituted with R \ R',;I, R ,H. RK, and R;'D; R3H. R3\ and R l are hydrogen: R" anil R ' arc independently RJ and R ¾:i are independently hydrogen, eyano, alkyl fin another embodiment alkyl is C|. alkyl). halo, haloalkyl. -ORL . phenyl, phcnyialkyl optionally substituted with one or two RH'; or alkyl substituted with one or two R1^; ami all other groups are independently as defined iii the Summary of the Invention Cor a Compound of Formula I or-as defined in embodiment ( I ). |()0203| Embodiments (E6b): In another embodiment, R2 is 6.7-diniclhoxy-qiiinolin-4-yl.
7- cyano-quinolin-4-yl.5-fluoi -quinolin-4-yl, 6-fluoro-quinolin-4-yl, 7-fluoro-i|uinolin-4-yl,
8- ΠιιθΓο-ιμιίη ιη-4— y], 2-pheiiyl-quinolin-4-yl.2-mcihyl-quiiiolin-4-yl, 2-melhyl-7-inellioxy- quinolin-4-yl.2-lrifluoromelhyl-qiiinolin-4-yl. or isoquinolin- l-yl; and all oilier groups arc independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (1).
[00204] Embodiments (Έ7): In another embodiment. R" is 5H-pyrrolo|3.2-cl|pyrimidin-4- yl, thiciio|2.3-J|pyrimidin-4-yl.7W-pyrrolo|2.3-</|pyrimidin-4-yl. l/7-pyrrolo|2.3-/ |pyridin- 4-yl, l/7-pyirolo|3,2-(;|pyridin-4-yl, ihicn(j|2..V/>|pyridin-4-.yl, or iliicno|3.2-r;|pyridin-4-yl.. where R2 is .substituted with R R RM R\ and R;"'; R\ R':I, R'11', R C. and R-1'1 and all other groups are independently as defined in the Summary of the. Invention for a Compound o
Formula I or as defined in embodiment. (1 ). In another embodiment. is thicnol 2.3- J|pyrimidin-4-yl or 7J/-pviToio| 2.3-</|pyrinndin-4-yl. where R2 is substituted with R'\ R3 '. R3H. R3T'. and R¾L: R3. R :1. R H. R L\ and R'3'1 and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formul I or as defined in' embodiment (I )..· In another embodiment, R2 is ihlenoj 2.3-r/|pyri m id in.-4-yl or 7/7-pyrrolo| 2,3- r/|pyrimidin-4-yl, where R" is substituted' with R3. R¾. R31'. R . and R3'1; R3A. R3,\ R3 S and R3<L are hydrogen; R3 is hydrogen or alkyl (in another embodiment all yl is G|.;\-alkyl); and all other groups are independently as defined in the Summary of the Invention for a Compound ■of Formula I or as defined in embodiment ( I ). In another embodiment. R2 is thicnoj 2.3- i/|pyrimidin-4-yl, 5-mcihyl-lhieho| 2.3-r/]pyrimidin-4-yl, or 7/7-pyrrolo| 2,3-<:/|py-rimidin-4-yl': ■and all other groups are independently as defined in the Summary of the -Invention for a Compound of Formula" 1 or as -.defined in; embodiment ( 1 ),·
[002051 Emboditncnts .fES): In another embodiment. R3 is 5.!,7-di.hydi t'hienoj3';4- i/|pyrimidin-4-yl. 5,6.7.S-ietrahydropyriclo| 3.4-(7|pyiiniidin-4-yl. 5.6.7.8- telrahydiOpyrido|4.3-c/]pyrimidin-4-yi. 5.6,7.8-tetrahydropyrido| 2.3-r/|pyrimidin-4-yl.
5.6.7.8-telnibydropyndor3.2-i/]pyriniidin-4-yl. 6.7-(liliydr<i-5/7-pyrrolo| .4-i/|pyrimidin-4-yl. 6.7-dibydro-5/7-pyrrolo| 3,2-i/|pyrimidin-4-yl. or 6.7-dihydm-5/7-pyn lo|'2..3-i/]pyrimidin-4- yl. where R2 is substituted with R3. R3\. R3". R 'L. and R LI: where R3. R :'. R3B. R C. and R3D and all other groups are independently as defined in tlte Summary, of the Invention for a
Compound of Formula For as defined in embodiment ( 1 ).
J0()206| Embodiments (E8a): In another embodiment, R2 is 5,7-d i hydroi ienoj 3 ,4- i/|pyi iniidin-4-yl, 5,6,7,8-teltalvydropyndo| 3.4-f/|pyriinidin-4-yl. 5.6,7.8- ietrahydiOpyi ido|4,3-i/|pyrimidin-4-yl. or 6.7-dihydi -5/7-pyrrolo| 3.4-r/|pyrimiclin-4-yl, where R2 is substituted with R3. R :1. R31'. R-\ and R3D: R3. R :'. R3", R . and R3U and all other groups are independently as defined in the Summary of the Invent ion for a Compound of Formula I or as defined in embodiment ( I ).
[00207 ] Embodiments (ESb¾: In another embodiment. R2 is 5,7-dihydroihieno[ 3.4- i/jpyrimidin-4-yl, 5,6.7.8-ietrahydropyi ido|3.4-i/|pyrimidin-4-yl. 5,6.7.8- tctrahydi pyrido|4 3-i/|pyrimidin-4-\i. or 6.7-dibydi -5/7-pyrrolo| 3.4-i/|pyrimidin-4-yl. where R2 is substituted with R\ R ;I. R31'. R c. and R D: R3. R3A. R31'. R3C. and R3" and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( I ). In another embodiment. R2 is 5,7- dihydiOlhieno| 3,4-i/|pyrimiclin-4-yl, 5,6.7.8-icirahydropyrido| 3,4-f/|pyrimidin-4-yl. 5.6,7.8- lelrahydropyrido[.4.3-i/|pyntnidin-4-yK or 6,7-dihydiO-5/7-pyrrolo| 3.4-i/|pyrimidin-4-yl.
where R2 is substituted with R3, RJ\ R ,\ R . and R3d; R:,a. R3b, R-\ and R arc liydrogcn; R3 is hydrogen, alkyl-iin another embodiment alkyl is C|..-,-alkyl). Iialoalkyl. optionally
.substituted phenyl, optionally substituted plienylalkyl. optionally subsiiiuicd cycloalkyi. or optionally subsiiiuicd cycloalkylalkyl; and all oilier groups are independently as dcrincd in the Summary of the Invention for a Compound of Formula I or as denned in embodiment ( I'). 1002081 Embodiments (ESQ: In another embodiment. 2 is 5.7-dihydrothieno|3.4- </|pyrimidin-4-yl, 5,6.7, S-ieirahydiOpyrido| 3,4-r/|pyrimidin-4-yl , 7-cihyl-5.6.7, 8- ieirahydropyrido|3.4-f/|pyriniidin-4-yl.7-beir/.yl-5,fi.7.8-teinihyclropyriclof3. -i/|pyriinidin-4- yl.5.6.7.8-ieirahydropyridoj4,3--/|pyrimidin-4-yl.6-cycloprppyl-5,6.7.8- tetrahydropyrido|4.3-</|pyi imidin-4-yl.6.7-dihydi -5/7-pyri lo|3.4-f/)pyrimidin-4-yl.6-/- tolyl-6.7-dihyclro-5/ -pyriOlo|3.4-('/||)yrinii(lin-4-yl. or.6rcyclt^jtOpyl-6,7- l'ihydro-5ii/- pynOlo| 3,4-i/|pyrimidin-4-yl: and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula I or as dcrincd in embodiment (1).
[00209] Embodiments (B9): In another embodiment. R2 is 7/7-pyrrolo|2,3-i/|pyrimidin-4- yl substituted with R3, R3:1. R3", R3c. and R "1; R3".. R3h, R c, and R3d are hydrogen: R3 and all other groups arc independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (I ). In another embodiment. R2 is 7 /-pyrrolo|2.3- i/ipyrimidin-4-yl substituted with R\ R¾. R'"'. R3c. and R3'1; R3. R a, R h, R¾, and R d arc hydrogen; and another groups are independently as defined jn the Summary of the Invcniion for a Compound of Formula I Or as defined in embodiment ( I ).
[002M)] Embodiments (EIO): In another embodiment, R2 is lH-pyra olo|3.4 rfJpyriniidiii 4-yl subsiiiuicd with R3. R a, R h. R3e. and R3 ; R3:'. R31', R c. and R3d are hydrogen: R3 and all other groups are independently as defined in the Summary of the Invention for a Compound of .Formula I or as defined in embodiment (I). In another embodiment. R2 is 1/7- pyra/.olo|3.4-r/|pyrimidin-4-yl subsliluted with R \ R¾ R'h. R,c. and R3J: R3. R3:I. R b. Ric. and R', arc hydrogen: and all Other groups are independently as defined in the Summary of the Invcniion for a Compound of Formula 1 or as defined in embodiment. ( 1 ).
[00211.1 Embodiments (Ell): In another embodiment, R" is 6.7.S.9- lctrahydropyrimido|4.5-/ |indoli/.in-4-yl substituted with R \ R3i. R31', R c. and R3'1; where R3. R"'. R',h. R . and R3'1 and all other groups arc independently as defined in ihe Summary of the Invention for a Compound of Formula 1 or as defined in embodiment ( 1 ). In another embodiment. R2 is 6.7.S.y-teiraliydropyriniiclo|4.5-/»|indoli/.in-4-yl substituted with R'\ R' . R3b. R . and R3d; R¾. R31'. R3c. and R,d are hydrogen: R! is hydrogen or cyano: and all other groups arc independently as defined in the Summary of die Invention for a Compound of
Formula I or as defined in embodiment (I). In another embodiment. R" is 6.7.8.9- teirahydropyriniido|4.5-/>|indoli in- -yl or IO-cyano-6.7,8,9-ictrahydropyrimido|4.5-
/>|indolizin-4-yl: and all other groups are independently as defined in the Summary of the
Invention for a. Compound of 'Formula I or as defined in embodiment (1).
[00212] In anolhcr embodiment. the Compound is; according. to any¾f-enibodiinei ;(B) and (HI) and R2 is according to any one of embodiments (D)-(B2), 3)¾ k), D MD4b).
(D5), b6-D6d),.(Dl)-(D7d)T(E> E2) (E2ii^
(E6)-(E6b). (E7). (E8)-(E8c). and (E9)-(EI 1). In another embodiment, the Compound is according to any of embodiments (B) and (I I ! ) and R2 is according to any one of
embodiments (D2). (D3a)-(D3e). (D3g). (D3i). (E2). (E2b). (E3c). (E4a), (E4d). and (ESa)- (E5d).
[00213] ln :anolher e ibodi ienl. the Cohipotind is according to any of embodiments (B 1 )- (B2)and R;'is,accoiding to any one of embodiments (D)-(D2). (D3)-(D3k). (D4)-(D4b), (D'5), (D6-D6d) (D7)-(D7d), (E)-(E2). (E2a -(E2e)r (E3)-(E3f). (E4)-(E4d). (E a)-(E5d). (E6)-(E6b). (E7), (E8)-(E8c). and (E9)-(EI I). In another em odiment, the Compound is according to .any of embodiments (B 1 ) and R2 is according to any one of embodiments (D2). (D3a)-(D3c), (D-3'g), (D3i). (E2). (E2b), (E3c), (E4n), (E4d). and (E5a)-(E5d).
[00214] In anolher\embodimeni. the Compound is according to any of -embodiments (B3). (B4), (B4a). and (B4b) and R2 is according to any one of embodiments (D)-(D2). (D3)-(D3k}, (D4)-(D4b), (D5),.(D6-D6d), (D7)-(:D7d), (E)-(E2). (E2a)-(E2c). (E3>(E3lr). ;( E4)-(E4d); (E5a)-(E5d). (E6)-(E6b). (E7). (E8)-(E8c). and (E9)-(EI I). In another embodiment, the Compound is according to any of embodiments (B4a) and R2 is according to any' one o embodiments (D2), (D3a)-(D3c),.(D3g). (D3i). (E2). (E2b), (E3c). (E4a). (E4d). and (E5a)- (E5d).
[002.15J In another embodiment, the Coinpound is according to any of embodiments (B5). (B6). (B7). and (B8) and R2 is according to any one of embodiments (D)-(D2). (D3)-('D3k). (D4)-(D4b). (D'5), (D6-D6d), (D7)-fD7d). (E)-(E2), (E2a)-(E2c). (E3HE3Q. (E4)-(E4d), (E5a)-(.E5d), (E6)-(E6b), (E7), (ES)-(E8c). and fE9)-(El I), In another embodiment, the Compound is according to any of embodiments (B7) and R2 is according to any one of embodiments. CD2) (D3a)-(D3e), (D3g). (D3i). (E2), (E2b). (E3c), (E4a), (E4d), and (E5a)- (E5d).
[00216] I another embodiment, the Compound is according to any of embodiments (B9)- (B 13) and R2 is according to any one of embodiments (D)-(D2). (D3 )-(D3k). (D4)-(D4b). (D5). (D6-D6d). (D7)-(D7cl), (E)-(E2), (E2a)-(E2c). (E3)-(E3f). (E4)-(E4d). (E5a)-(E5d),
(E6')-(E6b), (E7). (E8)-(E8c). and (E9)-( I I ). In another embodiment, the Compound is according to any of embodiments (B9)-(B 13) and R2 is according to any one of embodiments' (Oil (D3a)-(D3c). (D3g) (D i). (E2). (E2b). (E3c). (E4a). (E4d). and (E5a)-(E5d);
|Ό02.17'| In another embodiment, the Compound is according to any of embodiments (B 16). (B16a)-(B16c). (1317). and (BIS) and R2 is according to any one of embodiments (D)- (D2). (D3)-(D3k), (D4)-(D4b). D5). (Dfi-Dfiil). (D7)-(D7d), (E)-(E2), (E2a)-(E2e). (E3)- (Ε3Γ), (E4>(:E4d), (E5a)-(E5cl). (E6)-(E6b). <E7). (E8)-(E8c). and (E9)-(E1 I). In another embodiment, the Compound is according to any of embodiments (B L6a)-(B 16c) and R: is accordin to any one of embodiments (D,)-(D2), (D3)-(D3k). ( D4)-(D4b), (D5). (D6-,D6cl). (D7)-(D7il). (E)-(E2). (E2a)-(E2e). (E3)-(E3f). (E4)-(E4d). ;(E5a)-(E5d), (E6)-(E6b), (E7), (E8)-(E8c). and (E9)-(E1 I ), in another 'embodiment-, the Compound, is according to any of embodiments (B 16a)-(B 16c) and R2 is according to any one of embodiments (D2). (D3a)- (D3c). (D3g). (D3i). (Έ2). (E2b). (E3c). (E4a). (E4d). and (.E5a)-(E5d).
[002 IS I In another embodiment, the Compound is according to any of embodiments (B19)-(B29) and R2 is according to any one of embodiments (D)-(D2), (D3)-(D3k), (D4)- (D4b), (D5). (D6-D6d)?:(D7j-(D7d). (£)-(E2), (E2a)-(E2c), (E3)-CE3f),i(E4)-(E4d). (E5a)- (E5d), (E6)-(E6b), (E7), (E8)-(E8c), and (E9)-(EI I ).. In another embodiment, the Compound is according to an of embodiments (B 19)-{B29) and R2 is accoidiug to any one of embodiments (D2), (D3a)-(D3c), (D3g), (D3i), (Έ2), (E2b), (E3c), (E4a). (E4d),and (E5a)- (E5d).
[00219] In another embodiment, the Compound is according to any of embodiments (C)- (C3) and R" is according to any one of enihodinieiils (D)-(D2). (D3)-(D3k). (D4 -(D4b). (D5). (D6-D6d). (D7)-(D7d). (E)-(E2). (E2a)-(E2e). (E3)-(E3f). (E4)-(E4d). (E5a)-(E5d). (E6)-(E6b), (E7), (E.S)-(E8c), and (E9)-( I I.). In another embodiment, the Compound is according to any of embodiments (C2) and R2 is according to any one of embodiments (D)- (D2), (D3)-(D3k), (D4)-(D4b), (D5). (D6-D6d), (D7)-(D7d), (E)-(E2), (E2a)-(.E2e), (E3)- iE3f), (E4)-(E4d), (E5a)-(E5d). (E6)-(E6b), (E7); (E8)-(E8c). and (E9)-(E1 I). In another embodiment, the Compound is according to any of embodiments (C2) and R2 is according to any one of embodiments (D2). (D3a)-(D3c). (D3g). (D3i). (E2), (E2b). (E3c). (E4a). (E4d). and (E5a)-(E5d).
[00220] Enibodimenis Z: In another embodiment, the Compound is thai where R1 is bcii7.imida/.ol-6-yl optionally substituted with one or two R7; and R' is as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (1). In another embodiment, the Compound is that where R1 is bcn iniidazol-6-yl optionally
substituted with one or iwo R7: each R7, when present, is alkyl. Iialoalkyl. - R RI>A.
-NRSC(0')OR'', or cycloalkyl; ami RS. R*\ and R" arc independently as defined in the Summary oP the Invention for a Compound of Formula I or as defined in embodiment ( 1 ). In another embodiment, the Compound is thai where R 1 is benz.imidazol-6-yl optionally substituted with one or two R'; each R '. when present, is independently alkyl (in another embodiment alkyl is C,.:,-alkyl ). haloalkyl. - V\ -N 8C(0)OR9, or cycloalkyl: RS is hydrogen: ^' is hydrogen. alkyl (in another embodiment alkyl is Ci.i-alkyl). or haloalkyl; R'' is hydrogen or alkyl (in another embodiment ;i I k 11."i s- i .-3- a ί k 1 ) .
100221] Embodiments Zl : In another embodiment, the Compound is that where R
1 is thiazolo| 5,-l-/i]pyridin-6-yl or ihiazolo|4.5-/ |pyridin-6-yl optionally substituted with one or two R
7: and R' is as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( 1 ). In another embodiment, the Compound is that where R
1 is thiazplo| 5,4-/ )pyridin-6-yl or thia/.olo|4,.v//|pyridin-6-yl optionally substituted with one or two R
7; each R
7. when present, is independently alkyl. haloalkyl. -NR
SR
S:1, -NR'
SC(0)OR'\ or cycloalkyl: and R
S. R
8:I. and R
<J are independently as defined in the Summary of the Invention* for a Compound of Formula I or as-defined in embodiment ( I ). In another embodiment, the Compound is thai where R
1 is thiazolo| 5.4-/;|pyridin-6-yl Or lhia/.olo|4,5-/;|pyridin-fi-yl optionally .substituted with one or two R
7: each R '. when present, is independently alkyl ( in another embodiment alkyl is C 1.3-a.lkyl). haloalkyl, -NRV".
or cycloalkyl: R
S is hydrogen: R*' is hydrogen, alkyl (in another embodiment alkyl is C |..i-alkyl), or haloalkyl; R
!L is hydrogen or alkyl (in another embodiment alkyl is C| .;.-alkyl).
100222] Embodiments Z2: In another embodiment, the. Compound is
R
;1 is ! /·/- imidazo|4.5-//|pyndin-5-yl, lJ/-iinidazo|4.5-/) |pyridin-6-yl. 37-/-imidazo|4.5-/j|pyridin-5-yl, or 3/- -imidazo|4,5-/>|pyridin-6-yl where R
1 is optionally substituted with R
7: and R
7 is as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( 1 ). In another embodiment, the Compound is that where R
1 is I /7-imidazo| 4.5- />|pyridin-5-yl. l /7-imidazo|4.5-/> |pyridin-6-yl, 3/7-imidazo| 4.5-/>|pyridin-5-yl. or 3/7- imidazo|4;5-/> |pyridin-6-yl where R
1 is optionally substituted with one or two R
7: each R
7. when present, is independently alkyl (in another embodiment alkyl is C i -alkyl), haloalkyl. -NR
8R
R:|, -NR
HC(0)OR
Y, or cycloalkyl; and R
X. R*\ and R
Y arc independently as defined in the Summary of the Invention for a Compound of Formula I or, as defined in embodiment ( I .). In another embodiment, the Compound is that, where R
1 is l /7-iinidazo|.4,5-b
'|pyridin-5-yl, l /7-imidazo|4,5-//|pyriclin-6-yl. 3/7-imidazo| 4.5-/>|pyridin-5-yl. or 3/7-imidazo|4.5-/> |pyridin- 6-yI where R
1 is optionally substituted with R
7; each R
'. when present, is independently alkyl
(in another embodiment alkyl i.s Ci.j-alkyl), haloalkyl. -N V\ - R
SC(0)OR
Y. or cycloa!kyl; R
S is.hydrogcn: R
'SN is hydrogen, alkyl f in another embodiment alkyl is Chalky!.), or haloalkyl; R ishyd rogen or alkyl (in another embodiment alkyl is C|.j-alkyl). 1002231 Embodiments Z3: In another embodiment, the Compound is thai where
1 is I II- imidazo|4,5-t-]pyridin-6-yl or 3/-/-imidazo|4,5-r;|pyridin-6-yl optionally substituted with one or two R
7; and R' is as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (I). In another embodiment, the Compound is that where R
1.is 1 //-imidazo|4.5-c|pyridin-6-yl or 3/-/-imid.azo[4.5-6|pyridin-6-yl optionally substituted- with one or two R
7: each R
7. when present, is independently alkyl (in another embodiment alkyl is C.j-alkyl), haloalkyl. -NR ", -NR
xC(Q)OR
Y. or cycloalkyl; and R
8, R
S:'. and R are independently as defined in the Summary of the Invention for- a Compound of Formula 1 or as defined in embodiment ( I). In another embodiment, the Compound is that where R
1 is l/7-imidazo|4,5-f |pyridin-6-yl or 3/-/-imidazo|4.5-t pyridiii-6-yl optionally substituted with one or two R
7;
'each R
7, when present, is independently alkyl (in another embodiment alkyl is C,.3-alkyl). haloalkyl. -.NRV
B. -NR
sC(0)OR". or cycloalkyl: R
S is hydrogen; R
SA is hydrogen, alkyl (in another embodiment alkyl is Ci.ralkyl), or haloalkyl; R
Y is hydrogen or alkyl (in another embodimeni alkyl is C|..i-alkyl).
I00224J Embodiments Z4: In another embodiment, tlie Compound is that where R1 is benzol </|.thiazol-5-yl or beira>|<7Jihiazoj-6-yl optionally substituted witli one or two R'; and R7 is as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (1). In another embodiment, the Compound is that where R1 is
ben/.o|^/|thiazol-5-yl or benzo| /|ihiazol-6-yl optionally substituted with one or two R7; each R', when present, is independently alkyl (in another embodiment alkyl is C|..ralkyl), haloalkyl, -NRSRSA. -NRsC(0)ORY. or cycloalkyl: and RS. RS:I. and R' arc independently as defined in the Summary of the Invention for a Compound; of Formula I or as defined in embodiment (1). In another embodiment, the Compound is that- where R1 is benzol djthiazol- 5-yl or benzol r/]thiazol-6-yl optionally substituted with one or two R7; each R7, when present, is independently alkyl (in anotherembodimeni alkyl is C|..valkyl). haloalkyl. -NRSRS:|, -NR,|C(0)ORY. or cycloalkyl: RS is hydrogen: RSJ is hydrogen, alkyl (in another embodiment alkyl is Ci.j-alkyl), or haloalkyl: RY is hydrogen or alkyl (in another embodiment alkyl is C|. .,-alkyl).
[01)225 J Embodiments Z5: In another embodiment, the Compound is that where R1 is pyridin-3-yl optionally substituted with One or two R7; and R7 is as defined in the Summary of the Invention for a Compound of Formula I or as defined in enihodinieni:(l ). in another
embodimenl. die Compound is dial where R1 is pyridin-3-yl optionally substituted with one or two R'; each R7. when present, is independently hydrogen, halo, cyano. hydroxy, alkoxy. alkyl. -NRV11. -NRSS(0)2R*\ -S(0)Ri:i. -S(0);Rl3s. or -SiOfcNRV: and all other groups are independently as defined in the Summary ol" the Invention for a Compound of Formula I or as defined in embodiment ( I), hi another embodiment, die Compound is that where R1 is pyriclin-3-yl optionally 'substituted with two R7; one R7 is hytlrogen,Jialo. cyano, alkoxy. alkyl (in another embodimenl alkyl is Ci -alkyl). or -NRSRft' and the other R7 is
-NRsS(0hR a; or one R7 is hydroxy or -NRSRS¾ and the oilier R7 is -S(G)R13, -S(O)2R,A; -S(0) NRhR''; and all oilier groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( I ). In another embodiment, the Compound is thai where R1 is pyridin-3-yl optionally substituted with two R7; one R; is hydrogen, halo, cyano. alkoxy. alkyl (in another embodiment, alkyl is C|..;- alkyl). or -NR^R*' andjhe other, R7 is - RSS(D)2R¾; .or one R7 is hydroxy or -NR¾S? and the other R7 is .SfO^R13. -S(0')2R,3\ -S(O)2NRsRy: R13 is hydroxyalkyl: R l¾ is alkyl or
Iieterocyeloalky] Optionally substituted with one .group which is amino, alkyl. hydroxyalkyl. or hydroxy: each R's and R*'1 are independently 'hydrogen or alkyl; R" is hydrogen, hahialkyl. alkoxyalkyl, hydroxyalkyl. aniinoalkyl. alkylaniinoalkyl. dialkylaminoalkyl. cycloalkvl. heicrocyeloalkyl. hcterocycloa!kylalkyl. alkyl substituted with one aminocarbbnyl, or hydroxyalkyl which is substituted with one amino or 3 halo: and all other groups are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (1).
100226] Embodiments (X): In another embodiment, the Compound is that where. Rf! is -S(0)2R's. -C(G)NRsRSa or heieroaryl optionally substituted with 1.2. or 3 R14; and-R*..R*\ and R11 are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodimenl ( I). In another embodiment, the Compound is thai where R'"is located in the para position of the phenyl ring lo which ii is attached; R6 is -C(0)NRKR8a or heieroaryl optionally substituted with 1.2. or 3 R1'1; and Rs. R*', and M arc independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( 1). In another embodiment, the Compound is that where R1' is located in die para position of the phenyl ring to which it is•attached: R" is -C ONR^R83 or heieroaryl optionally substituted with 1.2. or 3 R1'1: Rs is hydrogen': R' l is hydrogen, alkyl (in another embodiment alkyl is C1.3-alk.yl). haloalkyl. or optionally substituted heleroeycloalkyl: R1' is alkyl (in another embodimenl alkyl is C|..ralkyl) or alkoxyearbonyl. In another embodiment, the Compound is that where R" is located in the para position of the phenyl ring to which it is
attached: Rft is -C(0)NRsRSl'. imidazolyl. or pyrazolyl where die imidazolyl and pyrazolyl arc optionally substituted with 1 , 2. or 3 R |:1: ^ is hydrogen: RX:| is hydrogen, alkyl (in another embodiment alkyl is C |.3-alkyl). haloal kyl. or opt ionally substituted py rolidinyl : R 1'1 is alkyl ( in another embodiment alkyl is C| .;,-alkyl) or alkoxycat bonyl. In another embodiment, the Compound is thai where R ' is located in die mcla position of the phenyl ring to which it is attached; Rft is -S(O);>RS; and R is as defined in the Summary of the Invent ion for a
Compound of Formula 1 or as defined in embodiment ( I ). In another embodiment, the Compound is that where Rft is located in the meta position of the phenyl ring to which it is attached; Rfi is -SJpfcR8: Rs is alkyl.
[00227] Embodiments (J ): In another embodiment, the Compound is according to Formula 1( h)
where R 1. R \ R3'1. and R',b are independently as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in embodiment ( 1 ). In another embodiment, the Compound of Formula 1(h) is that where R3, R-*a, and R31' arc as described in any of embodiments. (D3a)-(D3c), (D3g). and ( 3 i); and al l oilier groups are as defined in die Summary of the Invention for a Compound of Formula 1 or as defined in embodiment ( 1 ).
[002281 In another embodiment of embodiments (J ), the Compound of Formula 1(h) is that where R 1 is according to any of embodiments (Z)-(Z5): and al l oilier groups are as defined in the Summary of the Invention for a Compound of Formula I or as denned in embod iment ( I ). Γ.00229.Ι In another embodiment of embodiments (K), the Compound of Formula I is according to Formula I(j):
where R
"\ R
:\ R
""!, and R
(' are independently as defined in the Summary of the Invention for a Compound of Formula I or as defined in emhodimeni (I ). In another embodiment, the Compound is of Formula l(j) where R
'\ R'
3. and R
''
1' arc as defined in embodiments (E2b); and all other groups arc as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment ( 1 ). In another embodiment, the Compound is of Formula I(j) where R
J is hydrogen, alkyl (in another embodiment alkyl is Ci -alkyl) halo. -OR
Ua,- or,.a
.lkyl. substituted with one R
x; M* is hydiOgcii: Rv' is hydrogen or alkoxy; and.R*' is as dcftiied in ihelSummar .dl' the Invention for a ConipouVid of Forinitl I or as defined in embodiment, (1).
|00230] hi another embodiment of.enibodimeius (K), the Compound of Formula I( ) is that where R* is;accordiiig to embodiments (X); and all othe -groups are as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (1 ). |()0231j hi another embodiment of embodiments (L). the Compound of Formula I is according to: Formula I(k):
where R'\ R ,;', R* and R(> are independently as .defined .'in. the Summary of the Invention for a Compound of Formula I or as defined in embodiment (I ): In another embodiment, the Compound'of Formula. I(lv) is thalwhere.R3, R ', and 3" are as descrb d in any;of embodiments (D3a)-{D3c). (D3g). and (D3i); and all other groups are as defined in the: Summary of the Invention for a Compound of Formula I or as defined in embodiment (I ).
1002321 In another embodiment of embodiments (L). ilie Compound of Formula l(k) is that where R6 is according lo enibodimeius (X); and all oilier groups are as defined in die Summary of the Invention for a Compound of Formula I or as defined in embodinieni (I ). 1002331 In another embodiment of embodiments (M ), lite Compound of Formula I is according to Formula I(m):
!(m)
where. R-V-R3*, R 1'- imd R6 are independently. s defined in the Summary of the Invention for a Compound of Fornuila 1 or as defined in embodiment ( I ). In another embodinveiu. the Compound is of Formula I(m) wheie R3 is -hydrogen, alkyl (in another embodiment alkyl is C,- -alky|). oralkyl substituted with one L(I. -OR1 R¾ is hydrogen or -OR1 and ^1'- is hydrogen oralkyl'. and R6 is as defined in die Summary of the Invention for a Compound of Formula 1 or as defined in cnibodimcni ( 1 ). In another embodiment, the Compound is of Formula J(ni) where R . R''1, and R H are as defined in cmbodimenis (E6a): and R6 is as defined in "the Summary of the Invention for a Compound of Fornuila I or as defined in embodiment (1).
1002341 In -another cmbodimeiu of eiiibocl iments ( ), the Compound of Formula I( in ) is that where R6 is; according to embodiments (X); and all other groups are as defined in the Summary of the Invention for a Compound of Formula l or as defined in embodiment ( I ).
[00235] In anolhcr cmbodime ). die Compound is of Formula l(n):
I(n)
where R1 is as defined in the Summary of the Invention for a Compound of Formula I .or as defined in embodiment (1); and one of R'\ R3:I, and R31' and all other groups are independently as defined in the Summary ol' ihe Invention for a Compound of Formula I or as defined in embodiment (1). In another embodiment of embodiments (N). the Compound is of Formula
SO
I(n) where R \ R-
;'. R-
,,\ and R
1 are independently as del inetl in the Summary of die Invention for a Compound of Formula I or as delineil in embodiment ( I ). In another embodiment, the Compound is ol
" Formula l(ii) where :R-',. R
~a, and R
21' is as defined in embodiments (£21).); and all other groups-are as defined in
of the Invention for a Compound of Formula I or as defined in embodiment ( I ). In. another embodiment, the Compound is of Formula l(n) where R ' is hydrogen, alkyl, (in another embodiment alkyl is Ci.j-alkyl). halo. -OR
1 1'
1, or alkyl substituted witli one R
1 : R'
1 is hydrogen; R
,:i is hydrogen or alkoxy; and R
1 is as defined in the Summary of the Invention
' for a Compound of Formula I or as defined in embodiment ( 1 ). In another embodiment, the Compound is of Formula l(u) where R
1 is as as,
define n the Summary of t e Invention or a Compound o ormula I ofeas e ine embodiment ( I). In another embodiment, the Compound is of Formula I(n) where Rl is as defined in the Summary of the Invcniion for a Compound of Formula I or as defined in embodiment ( I ); -and three of R'\ R'': and R'''\are hydrogen and the others are independently as defined in the Summary of the Invention for a.Conipound of Formula I or as defined in embodiment ( I ).
[00236] In another embodimcnt of embodiments (N),.ihc Compound of Formula l(n) is tliat where. R1 is aeeordih to any of enilxKlihienls (Z)^:Z3); and all btliengrpiips" areas defined in die Summary of the Invention for a Compound of Formula I or as d fined in embodiment ( I ).
[00237] Embodiments (P): In other embodiment, the Compound is of Formula Up):
Kp)
where R1 is as defined in the Summary ol" the Invention for a Compound of Formula 1: and one of R\ R"'1, and R'"' is hydrogen and the others are independently as defined in the Summary of the Invention for a . Compound of Formula I. In another embodiment, the Compound is of Formula I(p) where R 1 is as defined in the Summary of the Invention for a Compound of Formula 1; and one of R\ R ,:'. and R7'1' arc hydrogen and the others are independently as defined in the Summary of the Invention for a Compound of Formula I.. In
another embodmienf. tlic Compound is of Formula U p) where R 1 is as . defined in die
Summary of die Invention for a Compound of Formula I; and two of R'\ R1" and R"'1' aic hydrogen and iheiOlhers are independently as defined in lhc Summar of die invention for Compound of Formul 1. In anoiher embodiment, the Compound is of Formula I(p) where RJ is hydrogen, alkyl (in another cmbodimenl alkyl is Cj .j-alkyl ). or alk yl subst ituted wiib one R16. -OR l l :'-. R;,;i is hydrogen or -O M :'; and R;,h is hydrogen or alkyl ; and R" is as defined in the Summary of the Invention -for a Compound of Formula I or as defined in embodiment ( 1 ). In another embodiment, the Compound is of .Formula I(p) where R \ R'!\. and R 'h arc as defined . in embodiments . ( E6aj; and R° is as defined in ie Summary of the Invention- for , Compound of Formula or as defined in embodiment ( 1 ).
[002381 In anoiher embodiment o -embodi ments (P), the Compound of Formula l(p) is that, where R 1 is according to any of embodiments (Z)-(Z5): and all other groups arc as defined in the Summary of the Invention for a Compound of Formula I or as defined in cmbodimenl ( 1 ).
[00239] Embodiments Q: In anoiher embodi ment, ihe Compound is of Formula l( i|):
where R is as defined in the Summary of die Invention for a Compound of Formula I : and one of R"\. R 1, and Rib is hydrogen and the others are independently as defined in the Summary of the Invent ion for a Compound of Formula I. In anoiher cmbod imenl . the Compound is of Formula l(q) where 1 is as defined in the Summary of the .Invention for a Compound of Formula 1 ; and tw of R \ R3:'. ;uid l'\nrc hydrogen; and Ihe others arc independciii ly as defined in the Summary of the I nvention for a Compound of Formula I. lii another embodiment, the Compound is of Formula I(q) where R 1 is as 'defined in the
Summary of the Invent ion for a Compound of Formula 1: and llirce of R\ R ¾. and R-"' are hydrogen and ihe thers arc independently as defined in the Summary of ihe Invention for a Compound of Formula I.
[00240.1 In another embodiment of embodiments (Q). the Compound of Formula l( ) is that where R1 is according to any of embodiments (Z)-(Z5): and all other .groups are as defined in lite Summary of die Invention for a Compound of Formula I or as defined in embodiment (I.).
f 0024.1.1 Embodiment (F): hi another emb diment, the Compound is of Formula l(r):
l(r)
where R
1. R\ W
' and R '
1' are independently as defined in the Summary of the Invention for a Compound of Formula I. In another embodiment, the Compound of Formula l(r) is where R ' and R
S;1 are alkyl (in anothei embodiment alkyl is Ci-.j-alkyl and K ' is hydrogen, alkyl (in
other groups arc ?^f i.ried¾i'tlic Summary of "the Ijfveution-ior a biiippui l of Formula as defined in eiiib diinein ( I ), In anoiherembodimeni. the Compound of Formula I(r) is wlicre R' and R :i are halo and R ,h is hydrogen, alkyl (in another embodiment alkyl is C|.,u alkyl). haloalkyl. or alkyl substituted with one 1"; and all other groups arc as defined in the Summary of the Invention for a Compound of Formula I or as defined in embodiment (I ). In another, embodiment, the Comppjind of Formula l(r) is where R3 and R , together with the carbon to which the arc ittacbed form an optionally .subsiiirited oycloalkyl and R',b is hydfpgeh, alkyl (inanplher ehibodimcnl alkyl is G|r - |Iiyl¾ hal.oalkyLor alkyl substituted with Giie.. lfi; and all other groups are as defined in th Sununary -of the Invent ioiv for a Compound of Formula I or as defined in embodiment (1).
[002421 In another embodiment of embodiments (F). the Compound of Formula l(r) is that where R1 is according lo any of embodiments (Z)-(Z5): and all other groups are as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in embodiment ( I).
[00243] Embodiments CS): In another embodiment, the Compound is of Formula l(s):
Us)
where R
! is cyano, alkyl (in another embodiment, alkyl is Ci.
:!-;ilkyl), halo, haloalkyl. -SR
1". alkylsulfonyl. optionally subsiitiiled phenyl, optionally substituted phenylalkyl. optionally substituted cycloalkyl. optionally substituted eycloalkylalkyl. carboxy.
-NR"R"
''. or -OR"
:i; and R
1. R
:i. I*
31', R\ R". and R"
A are independently as defined in the Summary of the Invention for a Compound of Formula 1.
10024 1 In anoiher embodiment of embodiments S). the Compound of Formula l(s) is that where R1 is according to any of embodiments (Z)-(Z5); and all other groups are as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in embodiment ( 1 ).
[00245] Embodiments (T): In another embodiment the Compound is of Formula I(t):
1(0
where R1, R3, R"1'1, and R 1' arc independenily as defined in the Summary of the Invention for a Compound of Formula I.
|()024fi| In anoiher embodiment of embodiments (T). the Compound of Formula I(t) is that where R1 is according to any of embodiments (Z)-(Z5); and all other groups are as defined in the Summary of the Invention for a Compound of Formula 1 or as defined in embodiment ( I ).
[00247] Embodiment (U): In another embodiment, the Compound is according lo Formula 1(a) where R
1 is hcieroaryl optionally substituted with one or two R
7; each R
7. when present, is independently halo, alkyl, cycloalkyl, haloalkyl, hydroxyalkyl. alkoxyalkyl. -NR
sR
Sl. or -NR
hC(0)OR
9; ahd all other groups are independently as defined in, the Summary of the Invention for a Compound of Formula I. In anoiher embodiment, the Compound is according to Formula I(a)
R
1 is lieteroaryl optionally substituted with one or two R': each R
7. when present, is independently alkyl (in another embodiment alkyl is CYralkyl). cycloalkyl. haloalkyl. - R
sR
h:i. or -NR^OJOR
"': and all other groups arc independenily as defined in the Summary of the Invention for a Compound of Formula I. In another embodiment, the
Compound is according to Formula 1(a) where R
1 is hcieroaryl optionally .substituted with
7 7
one or two R : each R . when present, is independently alkyl (in anoihcr embodiment alky! is Cvalky). cycloalkyl. haloalkyl. -NR8RS\ or -NRSC(0)QR": Rs is hydrogen; RS:I is hydrogen, alkyl (in anoihcr embodiment alkyl is C|..valkyl). or haloalkyl: and I?" is hydrogen or alkyl (in another embodiment alkyl is C|.;(-alkyl): and all other groups arc independently as defined in the Summary oflhe Invention for a Compound of Formula I.
[002481 In another embodiment, the Compound is according to Formula 1(a) where R2 is 5.6J,8-ieirahydroc|Uiiiolin-4-yl or 5.6.7;8 cirah;ydroi.so^un liti-l-yl, where R2 is.subslitiited Willi R3. R :I. R31', R3C, aiid R3D; and.R1. R'A„ R33, R31'. R:k..and R L! areindependently a ide ineti in the Summary of the Invention for a Compound of Formula I. hi anoihcr embodiment, the Compound is according to Formula 1(a) where R: is 5.6,7,8-ieirahydroquinolin-4-yI or 5.6,7,8-ieirahydroiso(|uinolin-l-yl, where R2 is substituted wiih R3. R¾. R31'. R . and R3D: RM is hydrogen; and R1. R3. R"':|, R31', and RK are independently as defined in the Summary oflhe Invention for a. Compound of Formula 1. In anoihcr embodiment, the Compound is according to Formula 1(a)' where R is 5:6.7.8-letrahydroquiholin-4-yl or 5.6,7.8-ictrahydroisoqiiinoIin- I -yl. where R"2 is substittiied with R3, RB R31'. R C. and R3'1; R31'. R3C. and R D are hydrogen and R1. R3. and R are independently as defined in the Summary of ihe Invention for a.
Compound of Formula I. In another embodiment, the Compound" is according to Formula 1(a) where R2 is 5,6,7.8-ieirahydiOt|uinolin-4-yl or 5,6.7, S-tetrahydroisoquinolin- 1-yl, where R2 is substituted with R3. R . R""\ R\ and R TL: R . R I\ R3' . and R 'li are hydrogen: and R1. and R3 arc independently as defined in the Summary of the Invention for a Compound of Formula I. In another embodiment, the Compound is according to Formula 1(a) where R" is 5.6.7.8- tetiahydraquinolin-4-yl or 5.6.7, -icirahydroisoquinolin- l-yl, where R" is .substituted with R' . R \ R3". R C, and R D: R3, R3A. R '\ R3C. and R3'1 are hydrogen: and R1 is as defined in the Summary o he Invention for a Compound of Formula I
[00249] In another embodiment. R2 in the compound of formula I is optionally substituted
ihiazolyl. In some embodiments. R' is
[00250] In another embodiment. R2 is an optionally substituted dihydroihiazolo| 5.4- c.|pyridin-4(5H)-onc, or an optionally substituted dihydiOben/.o|d|thia/.ol-7(4H)-one.
100251] In another cni odimeni. R2 in the compound of formula I is optionally substituted
In another embodiment, R
" in the compound of formula I is
wherein
R"1 is H, (GrCf alkyl. (C,-Q,)alkyIeiic-OH. (C,-C(,)alkylcne-0(C,-C(l)alkyl. (C,- Cft)alkylene-N^
C jalkyi. N((Ci-Gtf)alkyl)2, (C-CoJaikylene-NHSOrfCi-Cf.ialkyh (C|-Gft)alkyJenCr
NH(C=OHC|-C¾)alkyh -(C=0)-NI÷f:, -[^^
NHSQHCi-C6)a)kyI..-CN.pr,^
R"2 is Hv¾CrG(l)alkyi. fC,-C,,)alkehyI. halo; halb(C|-C(,)alkyl. H2. NMfCrC^alkyl, N((CrCe)alkyl)2,
Q
1 is N, C-H. or C-(G|-C )alkyl:
C0
2H. a yl. halpCC|-C )alkyl, «¾C
7)cycloalkyl. (C,-C(i)alky!cite^C C7)eycloalkyl. CQH. GO
?H, GOifCrCdalkyl. CN. (C|-e
(0aIkylcne-CN. (C-C^alkylene-CsC-H. (G,-C
(,)aikyicnc- CsG-tCi-Ci,)silky.I,
or
R:i and R'1". jogeiherwiih the atoms 10 which they are attached, can be joined together to form an substituted 5.6,. or ? niembered saturated or unsaturated ring, optionally containing up 10 two lieteroatoms selected From N-H. -('C|-C,-,)alkyl. O. SO. SO?: and
3 i N or C-R , wherein R is H. halo-, or (Ci-C6)alkyl.
10025 J In some embodimenntts where
R
1'
1 is l-i. NI-l
2j (Ci-G )iilkyl. (Ci-Q,)alkyleiie-,OH,
(CrCV,)alkYlcne-NHS.0r(GrCf;);ilkyl (CrC(jalkylcik Nf-I2. NH(C,-
C)alkyl, W((C Go)alkyl)^ itr^ lkyl iie- HSDr C Giiialk l, (C,-Gr alfeyIene- NH(C=0)-(C
l-C(,)alkyl. -(C=0)- H
:. -fC=0)-NH(CrC(v)alkyl. -iG=0)-NH (.C,-G
fl)alkyr))
2. - NHS02-(G|-C(i)alkyl, -CN. or (Oi
wherein when any a
'lkylencis -CH
2-, then one of the hydrogens of the -Gi b- can optionally be replaced by (C|- C.v)haloalkyl:
R"2 is l-l, iC,-CV)alkyI. <C|-C(-/)alkenyl. halo. halo(C,-d„)alkyl. H?. NH<CrO«s)alkyl, N((C|-Cfi)alkyi)2; and
Ra and '1". together with the atoms to which they are attached, can be joined together to form an substituted 5, 6.or 7 inembered saturated r unsaturated ring, opii'ona.lly! containing up to, two hcteroatoms selected fiom N-l-I, N-(G|-G())alkyl. O. SO.502-
[00255] In some embodiments.
93
94
^wherein R'
|l:i and ft''"' are each independently I I. (C|-C,-,)alkyl. or halo(C
: |0025¾. In sonic eniboih'mciits where
. wherein R:i is clefiiictl a.s above; and
Rql is H. Ni l;;. (CrQ,)alky . (C Cjalkyleiie-Ol I. (C C(,)alkyIcne-OfC,-C(1)alkyl. (Gi-CfiJalkylcnerNHj. (C|-C(l)alkylcne-NHfC.|-C(,)alkyl, ( C j -C„.);i I k yl c I c 11 c- ( C , - Cf,)a I k y 1 )2 , (C - fi)alkylene-NHSOr.(Cj NIK Nl-IfC,-
Cf,-)aikyl, N(i¾j,¾Oalkyl)¼
NHSd.-(Ci-Ge)alkyl. -GN. or. (G i -€r,)alkylci.ic-(C3-C7)heierocyclo: and .
R"" is Hr (.C|-C(;)alkyl: (C|-C«)al.k'ciiyl. halo, halo(C|-C, alk.yl, N¾l2j NH(C,-Cf!)a)kyl, N((C|-C6)alkyl) .
1002611 In some embodiment w er
wherein :1 is defined as. above; and
R'" is H, Nl-I
2. fd-CV aikyl. (Ci-Q,)alkylene-OH. (C
rCr,)alkylen-0(C|-C
(jalkyl. (Ci-C
f,)alkylcnc-NI-l
2. (Ci-C
6)alkylciic-NH(C|-C
ft)alkyl, (C,-C
f))alkyleleiie-N(C,-C
(l)alkyl)
:.
Q,)alkyl. N((C,-C )alkyl)2. (CrCeJalkylene-NHSO.-fCi-Q.ialkyl. (C-Qjalkylenc-
(CrC
(i)alkyl))
2. - NHS0
2-(C,-Cr,)alkyl. -CN. or (Cr.¾)alkylenc-(C;rC )lreterGcyclo; and
R"- is H, CG|-Cf,)alkyl. (CrC(,)alkenyl, halo. halo(Ci-G )alkyl. ΝΙ-k NH(CrC(,)alkyl. N((C|-C„)alkyl)2. '
[00262] in some embodiments.
[00263.1 In some cmbodinieni /
wherein R:i is defined as above; and
R'1' is:H, NH , (C|-CV)alkyl, (CrC6)alkylene-OH. (CrC6)alkylene-0(C,-a)aIkyl, CCrC6)alkylen NH2. (C,-^
(CrC
6)aikylenc-NHS0
2-{CrCi
))alkyl.(C|-C
6)alkylcne-N -I(C=0)-(C
1-C )alkyl. NH
2. NH(C Cf,)alkyl. N((C|-C,,)alkyl)>. (CrC
(,)alkylene-NHS0
2-(C,-G
f,)alkyl. (C|-C,,)alkyle.ie-
-(C=0)-NH (C,-C„)alkyl))
2. - NHS02-(C,-C
6)alkyl. -CN. or (C,-Cf,)alkylcne-(C
3-C
7)heieiOcycIo: and
R<|2 is H, (C,-Cf,)alkyl. (C|.-C6)alkcnyl, halo, halo(C|-Cf,)alkyl, Nl-i2, NH(Ci-C(-,)alkyl. N((CrC())alkyl)2.
[00268] lii arioihcr cinboclimciii, ihc compound of Formula I is a■compound of formula 11(a) or ri( .
11(a) 11(b)
i.0026 j In sonic embodiments of Formula 11(a) or 11(b). R7 is hydrogen. (C|.C;,)alkyl. cyclopropyl, fluoromeihyl, difhioromethyl. trifluoromediyl. or NH?. In some embodimenis of Fornuila 11(a) or 11(b). R7 is methyl or ΝΙ-l·. In these and oilier embodimenis, R" can be any
of die dcfinilions provided herein. In some embodiments, R
* is
. More particularly. R
" is
I00270J In another . embodiment., the compound ol" Formula I is a compound of formula lllfa) or 11.1 b). wherein the variables can have any of the definitions provided herein.
II 1(a) 11 lib)
[00271J In some embodimenis. oi7 Formula II 1(a) or 111(b). R7 is hydrogen. (G|.G- )alkyl. cyclopropyl, fliioromethyl. dirinoromethyl. trifluoromeihyl. or H2. In some embodiments of Formula 11(a) o 11(b). R ' is methyl or Nl-k In these and other embodiments. R" can be any
1-03
[00272] Ιή another embodiment, the compound of Formula I is a compound of formula |V(a) or IV n.
lV(a) I V(b) 1002731 in;somc embodiments of Formula lV(a) and lV(b). one or both of ihe R7 groups arc optional ly present. In particular, when both R7 groups are present, one R is N H . chloro.
[002741 lii other embodiments, the compound of Formula IV(a) or I (b) is a compound of Formula I V(a l )
' or IV(
'b l )
? wherein the variables can have aiiy of the definitions provided herein.
IV(al) IV(lvl)
[00275] In some cmbodinicms of Formula I V(a I ).and IV(b|). R7 is -OW.-NH
100276] In other embodiments, the compound of Formula IV(a) or I V(b) is a compound of Formula lV(a2) or I V(b2). wherein die variables can have any of the definitions provided herein.
IV(a2) IV(b2)
7 is Nl-k chloro. hydroxy.
|'0()278| In oilier embodiments, llie compound of Formula I V(a) or I V(b) is a compound of Formula lV(b3). wherein R7 is joined together with the carbons to which they are aiiachcd lo form a 5 or 6-membcred helerocycloalkyl group and R" can have any of the definitions provided herein.
lV(b3)
1
I (10281 ) 11 some
•■embodiments of Formula IV(a2) nd IV(b2). R
7 is I
'luoro. chloro.
meihoxy, NHv; chloral hydroxy, -C.O
'iMc. or mclhoxy,
1002821 In another embodimeni, the compound of Formula I is a compound of formula V(a). V(b), V(e). or V(d).. wherein the variables can have any of the definitions provided herein.
V(c) V(d
[00283] In another embodiment, the compound of Formula 1 is a compound of formula - V'lfa) or VI(.b), wherein Ihc variables can have any of the definitions provided herein.
Vila) V l(b)
[00284] In another embodimeni. the compound of Formula I is. compound of formula' Vl(a) or VI(b). wherein the variables can have any of the .definitions provided herein.
VII
100285] In another embodiment, the compound of formula I.11(a), 11(b).111(a).111(b). IV(a). IV(li). Via),. V(b). V(c). V(d). is a compound .of formula Vlil:
Vlil
or a single stereoisomer or iiiixiure pf stereoisomers thereof and additionally optionally as a pharmaceutically acceptable salt thereof, where
R1 is aryl optionally .substituted with one. two, or three R(' groups: or heteroaryl optionally substiiuled with one, two, or three R';
R- is heteroaryl substituted with R\ R I;I. R;I|'. R . and R ·'''■;
R1, R \ RM RJC. and R""1 are independently hydrogen; cyaiio, alkyl. alkcnyl. halo; haloalkyl, hydr0xyalkyl alkpxyalkyl,eyanoalkyl. -SR12,•-S(P)2RA,--C(0.)QR:,:. -C(Q)NHR"' halocarboiiyl -NR.:11 R* LA. -OR "'\ optionally substituted phenyl, opt ional ly subst iutteci phenylalkyl. optionally substituted cycloalkyl. optionally substituted cycloalkylalkyl, optionally substiiuled lielcrocycloalkyl. optionally substiiuled heterocycloalkylalkyl, optionally substituted heteroaryl, optionally substiiuled hctcioarylalkyl. or alkyl substituted with one or two R16; or
two of R3, R¾, R;"1, R3l\ and R'"1. when attached lo the same carbon, .form an optionally
substituted cycloalkyl, optionally substituted aryl. or an optionally substituted
heieroeycoalkyl: or optionally .substituted heteroaryl. and ihe other of R"V, RJ", RJ|1. R3C. and R:1D arc independently hydrogen, cyano, alkyl, alkcnyl..halo., haloalkyl, hydroxyalkyl, alkoxyalkyl. cyanoaikyl. -SR1'. -S(0)2R ". -C(O)0R'. halocarbonyl. -NRNR"'\ -ORLLS. optionally substituted phenyl, optionally substiiuled phenylalkyl. optionally substiiuled cycloalkyl, optionally substiiuled cycloalkylalkyl. optionally substituted heterocycloalkyl, optionally substiiuled heterocycloalkylalkyl. optionally substiiuled heteroaryl. optionally substituted hcleroarylalkyl. or alkyl subsiiiutcd with one or iwo R1":
1.09
R'1 is alkyl, alkciiyl, alkynyl, hydroxyalkyl. alkoxyalkyl. lialoalkyi. aminoalkyl.
alkylaiiiinoalkyl. dialkylaminoalkyl. benzyl, or optionally subsiiiuiccl
heierocycloalkylalkyl:
R'S:| and R'^ue independently hydrogen or alkyl:
R l is hydrogen or halo;
RSI> is (C|.3)alkyl or halO(C|..?)alkyl:
R5'1, R5C..R5f,.and R5g are hydrogen;
each Rf\ when Rf' is present, is indepcndeiUly nitro: cyano; halo; alkyl: alkenyl: alkynyl; lialoalkyi: -ORSa: -NRsRS;i: -C(0)NRsRS;i; -S(O) RS; -NRSC(0)OR": - R¾(0)R':':
-NRsS(0)2RS:i: - R'sC(O)i\,RSnR!: carboxy. -C(0)ORl,: halocarbonyl: alky!carbonyl: alkyl substituted with one or two -C(0.)NRsRS:': hetcioaryl oplionally subsiiiuled with 1.2. or 3 R1': or optionally substituted heterocycloalkyi; or
two Rh. together with the carbons to which they are attached, form an optionally .substituted
3.4, .5, or 6-niembcred cycloalkyl or heierocycloalkyl;
each R7. when R.7 is present, is independently oxo: nitro; cyano; alkyl; alkenyl; alkynyl; halo: lialoalkyi: hydroxyalkyl: alkoxyalkyl: -ORS:i: -SR : -SfOjR1 : -Si JjR13": -N.RsRSa:
-C(0)NR
sR
S:'; - R
SC(0)OR''; -NR
SC(0)R";
-NR
SC(0)NR
SI,R": -C(0)0R": halocarbonyl; alkylearbonyl; -SfOhNR^R'': alkylsuliOnylalkyl: alkyl substiiuted with one or two -NR
SR
X''; alkyl substituted with one or two - R
8C(0)R
¾; alkyl substiiuted with one or two -r R
SC(O).0.R
Y; alkyl substituted with one or two -SfO.h
1 '; optionally substituted cycloalkyl: optionally .substituted cycloalkyl
'alkyl: optionally .substituted heterocycloalkyi; oplionally substituted heierocycloalkylalkyl: optionally substituted phenyl: optionally substituted phcnyialkyl; optionally substituted hetcioaryl: or oplionally substituted heteroarylalkyl;
each R8. R1', R15. R17. and R 18 are independently hydrogen. NI K; NH(alkyl). N(aikyl):. alkyl. alkenyl. alkynyl, hydroxyalkyl, alkoxyalkyl. or lialoalkyi:
each R'S:i. Rlla, and RI5;| are independently hydrogen, alkyl. alkenyl. alkynyl. lialoalkyi.
hydroxyalkyl, cyanoalkyl. aminoalkyl, alkylaiiiinoalkyl. dialkylaminoalkyl, alkoxyalkyl. carboxyalkyl. optionally substituted cycloalkyl. optionally substituted cycloalkylalkyl, optionally substituted heterocycloalkyi. oplionally .substituted heierocycloalkylalkyl.. optionally substiiuted' phenyl, optionally substituted phcnyialkyl, optionally substiiuted hctcroaryl. or oplionally substituted heteroarylalkyl;
R° is hydrogen: alkyl: alkenyl; alkynyl: hydroxyalkyl: alkoxyalkyl: aminoalkyl:
alkylaiiiinoalkyl: dialkylaniinoalkyl; lialoalkyi; hydroxyalkyl substituted with one, two, or
I 10
three groups which arc independently halo, amino, alkylamino. or dialkylamino'. alkyl substituted ii one or iwo aminocarboiiyl: optionally substituted phenyl: optionally substituted pheny.lalkyh.optioiially substituted cycloalkyl; optionally substituted cycloalk.ylajkyl; optionally substituted hcteroaryl; optionally subsiiuitccl heieroaryjalkyl: optionally substituted heierocycioalkyl; or optionally substituted hctcrocyeloalkylalkyl;
R12 is alkyl or optionally substituted phenylalkyl:
R13 is alkyl. hydroxyalkyl. or haloalkyl: and
I j is hydroxy, alkyl. haloalkyl. hydroxyalkyl, or heteroeycloalkyl optionally substituted with one or two. groups which are independently halo, amino, alkylamino, dialkylamino. hydroxy., alkyl, or hydroxyalkyl:
each RU, when R 1,1 is present, js independently amino, alkylaniiiio.:dialkyianiiiiO, acylaniino. halo, hydroxy, alkyh haloalkyl; hydfoxyalkylj aniiiioal.kylv.alky'raniinoalkyl.
dialkylan inpa|kyj, alkoxyearbonyl,
dialkyiaminocarbonyl. or optionally substituted phenyl;
each R16 is independently halo, -NR"RII:I. -NRI5S(0)RI5I\ -0C(0)R'7, or -OR1"; and
R2<l is alkyl. haloalkyl. hydroxyalkyl. amino, alkylamino. dialkylamino. or heteroeycloalkyl.
100286] Another embodiment: provides a pharmaceutical composit ion '-'which comprises 1) a compound, as-'avsinglc stereoisomer or .mixture oPster'epispmers lhcrco ,,aceprding:tp any one of Formula. I, (1(a); \(b\). I(b2)r I cl jv I(e2). .iCcl),.i(l¾. I(g).,I(h).I(j); l(k). I(m), 1(h); I(p), l(cj), I(r), l(s). and 1(0 or according to any one of the above
embodiments, optionally as a pharmaceutically acceptable salt thereof, and 2) a
pharmaceutically- acceptable carrier, excipient, and/or diluent thereof.
(002871 Another embodiment is a method of treating disease, disorder, or syndrome where the disease is associated with uncontrolled, abnormal, and/or unwanted cellular activities effected directly or indirectly by PI3 and/or mIO which- niethod 'comprises administering to a human in need thereof a therapeutically effective amount of αΌοηιρόιιικΓρΓ any of Formula I. (1(a). I(b I). I(b2). I(el). I(c2), l(dl). I(d2).1(e). I(c ).1(f), Kg), 1(h). I(j). !(k), Km), I(n). l(p).1((|). l(r-). l(s), and 1(1). a Compound of any oiie of the above embodiments, or a Compound from Table I. optionally as a pharniaccuiically acceptable salt or
pharmaceutical composition thereof. In another embodiment, the disease is cancer. In another embodiment, the disease is cancer and the Compound is of Formula 1(a) or a Compound from Table 1.
{002881 -Embodiment (G ): Another embodiment is irected to a method of treating a disease, disorder, or syndrome which method comprises administering to a patient a llierapciilically effect ive amount of a Compound of any of Formula L (1(a), I(b I ). I(b2), I(c 1 ). I(c2). l(d I ), I((I2). 1(e). 1(d ). 1( f). Kg), 1(h), l(j). I(k). I(m). I(n). I(p). I(q), l(r). I(s), and !(l). a Compound of any one of the above embodiments, or Compound from Table 1. optionally as a pharmaceutically acceptable salt thereof, or a pharmaceutical composition' comprising a therapeutically effective amount of a Compound of Po niu la I. ( 1(a). ((b l ), I(b2). I(c l ), I(c2). i(d l ), I(d2), 1(e), I(e l ). 1(f), Kg), 1(h). I(j), I(k). I( ni), l(u ), l( p), l(q), I(t). l(s), and I(i).. a Compound of any one of the above embodiments, or a Compound from Table 1 . and a pharmaceutically acceptable carrier, o.xcipicnt. or diluent . In another embodiment the disease is cancer.
[002891 I" another embodiment of any of the embod iments of Embodiment (G), the cancer is breast cancer, mantle cell lymphoma, renal cell carcinoma, acute myelogenous leukemia, chronic myelogenous leukemia, NPiVl/ALK-transfomied anaplastic large cell lymphoma, diffuse large B cell lymphoma, rhabdomyosarcoma, ovarian cancer, endometrial cancer, cervical cancer, non smal l ce l lung carcinoma, smal l cell lung carcinoma, adenocarcinoma, colon cancer, rectal cancer, gastric carcinoma, hepatocel lular carcinoma, melanoma, pancreatic cancer, prostate carcinoma, ' thyroid carcinoma, anaplastic large cell lymphoma, hemangioma, glioblastoma, or head and neck cancer.
100290] All Compounds in Table 1 were (esied in the assays described in Biological Examples I and 3.
100291 1 Embodiments- (
'V): In one .-embodiment the Compound of the Invention has an I3 -ii I li a- inhibitory activity of about 2.0 μιΥΙ or less and is inact ive for mTOR (when tested at a concentration of 3.0 μΜ or greater) or is select ive for PI -alpha over mTOR by about 5-fold or greater, about 7-fold or greater, or about l ()-fold or greater. In another embod iment the Compound of the Invention has an P I3 -alpha-inhibitory act ivity of about 1 .0 μΜ or less and is inactive for mTOR (when tested at a concentrat ion of 2.0 μ Μ or greater) or is select ive for PI -alpha over mTOR b about 5-fold or greater, about 7-fold or greater. or about 10- fold or
'greater. In another embodiment the Compound of the Invention has an P{3K-alpha- inhibitor.y activity of about 0.5 or less and is inactive for mTOR (when tested at a concentration of 2.0 μΜ or greater) or is selective for PI3 K-alpha over mTOR by about 5- foki or greater, about 7-fold or greater, or about 10-fold or greater. In another embodiment the Compound of the Invention has an P I3 -alpha-inhibilory activity of about 0.3 μΜ or less and is inactive for mTOR (when tested at a concentrat ion of 2.0 μΜ or greater) or is selective
for .PI3 -;ilpha over in
'I
'OR by aboiil. 5- folil or greater, about 7- fold or greater, or about 10- lolil or greater. .In another cnibodinicni the Compound f the Invention has an PI3 -alpha- inliibiioi y activity. -of about' 0.2-μ Μ or less and is sclecl ive- or
'P13 .--a
~l
.pba . over niTQR by about 5- fold or greater, about 7-fold or grea(ci\.pr about 10-fpkl or greater. In, another · enibodinieni the Compound of the Invent ion has,
;an PI3 K-alpha- inliibiiory .act ivity of about: 0. 1 u or less and is selective for PI3 -alpha over m
'l
'OR by about 5-fold or greater, about 7-fold or greater, r about 10-fold or greater. In another embodiment the Compouncl ol
' ihe Invent ion has an PI3 K-al pha-inhibitory act ivit y of about 0.05 uM or less and is selective for P13 K-alpha over niTOR by about 5-fold or greater, about 7-fold or greater, or about 1 -fold or greater. In
thc Compound of the Invention lias an P13
'K-alpha- inhibitory .activity of about 0.025 μΜ or less aiul is selective for Pl3 K-alph over mTOR by about 5-fold or greater, about 7-fold or greater, or about 10-fpjd or greater, Iiranothe
.r embodiment . the Compound of the Invention has an I 3 K-alplva-iiih ibitory: adl i v,iiy of-abou
'l 0.01 uM or less and is selective for PI3K-alpha over m
'l
'OR by about 5-fold or greater, about 7-fold or greater, or about. 10-fold or greater.
100292] Embodiments ( W): In one embodiment the Compound of the Invention has an PI3K-alpl)a:inhibiiory activity of about 2.0 μΜ or less and an mTOR-inliibitory act ivity of about 2.0 μ Μ or less ahd ihe select i viiy for one of the ^ target's Sve the other does not exceed. 3-fold. Iivanothei embodiment the Compound of - the Invention has an Pi3 k-aiplia-inhibiiorA'- activity of about 1 ) μΜ or less and an mTOR-inhibitory activity of about 1 .0 μ. or less and the selectivity for one of the targets over the other does not exceed 3-fold. |n another embodiment the Compound of the Invention has an PI3 K-alpha-inbibitor.y activit y of about 0.5 μΜ or less and an mTOR-inhibitory act i vity of about 0.5 μΜ or less and the selectivity for -one of the targets over the other does not exceed 3-fold. In another embodiment die Compound -of the Invention has an PI3 K-alpha-inhibiiory act ivit y of about 0.3 μΜ or less and an mTOR-inhibitory activity of about 0.3 μΜ or less and ihe select ivity for one of the targets over the other does not exceed 3-fold. In another embodiment ihe Compouncl of the Invent ion has an PI3 K-alpharinhibiiory activity of about 0. 15 μ or less and an 'mTOR-inhibitory activity of about 0. 15 μΜ or less and t he selectivity for -one of die targets over the other docs not exceed 2-fold. In another embodiment the Compouncl of the Invent ion has an P13 -alpha- inhibitory act ivity of about 0. 1 μ or less and an mTOR-inhibitory. activity of about 0. 1 μΜ or less. In anoihcr embodiment the Compound of the Invention has an PI3 K-alpha-inhibitory activity of about 0.05 μΜ or less .and an mTOR- inhibitory activity of about 0.05 μιΝΊ or less. In another embodiment the Compound of Ihe Invent ion has an PI3 -alpba-inhibilory act ivity
of about 0.02 μι\Ί or less and an mTOR-inhibito y activity of about. 0.02 μ or less. In another embodiment the Compound of ihe Invention has an PI3 -alpha-inhibiiory activity of about O.O l μΜ or less and an mTOR-inhibitory activit of about 0. 1 μΜ. of less.
100293] In another embodiment. Compounds of the invention are also useful as inh ibitors of PI3K.a and/or mTOR in vivtj for studying the /'// vivo role of PD Ka and/or mTOR in biological processes, including the diseases described herein. Accordingl y, the invent ion als comprises a method of inhibit ing PI3 Ku and/or mTOR in vivo comprising administering a compound or composition of the invention to a mammal.
100294] In another embodiment of any of the embodiments of Embodiment (W). the" cancer is breast cancer! mantle cel l lyinphoriia. renal cell carcinoma, acute myelogenous !c\ikcmiavehroniC;iiiy010geiieusilaikcini;i, NPM/ALK-tratisformeil , hapl stic. large cell lymphoma, diffuse large B cel l lymphoma, rhabdomyosarcoma, ovarian cancer, endometrial cancer, cervical cancer, non smal l cell lung carcinoma, small cell l ung carcinoma, adenocarcinoma, colon cancer, rectal cancer, gastric carcinoma, hepatocel lular carcinoma, melanoma, pancreatic cancer, prostate carcinoma, thyroid carcinoma, anaplast ic large cell lymphoma, hemangioma, gl ioblastoma, or head and neck cancer.
100295] Another embodiment is directed to a. incihpd for identi fying■ a- .selective . inhibitor of a PI3K isozyme, the method .comprising: (a). contacting - a first cell bearing a first mutalioiv in a PI3K- with a candidate inhibitor; (b) contact ing a second cell bearing a wild: type P13 K- a, a PTEN null mutation, or a second .mutation in said ΡΠ ^α with the candidate inhibitor; and (c) measuring A KT phosphorylation in said first and said second cel ls, wherein decreased A T phosphorylation in said first cell when compared to said second cell identifies said candidate inhibitor as a select ive PI3 K-U inhibitor.
100296] As noted above, the newly discovered association between selective genetic mutations and increased sensitivities of some cancers to speci fic inhibitors renders a particular genetic background more susceptible to one or more types of inhibitors than others. This association between genetic backgrounds and susceptibilities of certain cancers provides an attractive and convenient cel lular plat form for identificat ion of new selective inhibitors to PI3 K kinases (e.g. via screening assays to detect compounds or ent it ies that inhibit phosphorylat ion in a P1 K-udependcni manner). As will be appreciated by those of ord inary skill in llic art. any kind of compounds or agents can be tested using the invent ive screening methods. A candidate inhibitor compound may be a synthetic or natural compound: it may be a single molecule, a mixture of different molecules or a complex of at least two molecules. A candidate inhibitor can comprise functional groups necessary for structural interaction with
proteins, particularly hydrogen bonding and l ipoph i l ic binding, and typically include at least an amine, carb.pn.yl. hydroxyl. cl her. or carboxyl group, l or example at least two of the functional chemical groups. The candidate inhibitor often comprises cyclical carbon or heterocycloalkyl structures and/or aromat ic or hcicroaromat ic structures substituted with one or more of the above functional groups. Candidate inh ibitors arc also found amon biomoleculcs including .'peptides, saccharides. . fatty acids, steroids, purines, pyrimidines. derivat ives, structural analogs, or combinat ions thereof. In certain embodiments, the inventive methods arc used for testing one or more cand idate inhibitor compounds. In other embodiments, thciin entive methods are used for screening col lect ions or l ibraries of candidate inhibitor compounds. As used herein, the term "col lect ion" refers to any set of compounds, molecules or agents, while the term "l ibrary" refers to any set Of compounds, molecules or agents that are structural -analogs.
1002971 Libraries of candidate inhibitor compounds that can be su ecned using the:
methods-Of the present invention niay be cither prepared or purchased from a number of companies. Synthetic compound libraries are .commercially available, from, for example. Gomgenex ( PHncciOn. N.J .). Brandon Associaies (Merrimack. N.1-1.), M icrosource ( New Milfprd. Conn. ), and Aldricli ( Milwaukee. Wis.'). Libraries of candidate inhibitor compounds have also been developed by and are commercially avai lable from large chemical companies. Additionally, natural col lections, synthet ically produced libraries and compounds are readily modified through conventional chemical, physical, and biochemical means;
1002981 Cells to be used in the. practice of the screening methods described hercin½ay be primary cells, secondary cells, or immortalized.. cells (e.g.; established cell lines). The may he prepared by techniques well known in the an (for example, cel ls may be obtained by fine needle biopsy from a patient or a healthy donor) or purchased from immunological and microbiological commercial resources (for example, from the A merican Type Culture Collection ( ATCC). Manassas, Va. ). Alternat ively or addit ional ly, cel ls may be genetical ly engineered to contain, for example, a gene of interest. In a first set of cells, the cells possess a genetic- imitation in PI3 K-a kinase domain, for example. H I 047R. lira second set of cei ls to be used in the screening assays, the second set: of cel ls possess a genetic mutation in. a different kinase catalyt ic subunil. ( for example, a .mutation in a helical domain., for example, E545 . Or in a-differeni regulatory protein, for example Phosphatase and Tcnsin Homolog (ΡΊΈΝ). When a candidate inhibitor inhibits phosphorylat ion, ( for example AKT phosphorylat ion) to a higher degree in the cel l possessing the PI3 K-U kiiiase domain genetic mutation when compared to a cell possessing a genet ic mutation in a d ifferent kinase catalytic
subunil. (for example a mutation in a helical domain, for example. E545 K. or in a differeni regulatory- protein)., ihcn the candidaic inhibitor is a selective inhibitor for cancers or uimors thai harbor activation mutations in PI3 K-U. Conversely, PI3 -a -select i.vc compounds inhibit ΛΚ.Τ phosphorylat ion, PI3.K pathway act ivation, and cell proliferation with; greater potency in minor' cells harboring the PI3 .-U -H 1047R mutation compared to PTEN negative, PB -u wild-type, and PI3 K-a -E545K backgrounds. Both PTEN inactivalion and KRAS activation desensitize cells to the growth inhibitory effects of PI3 -« -select ive compounds. A wild- type P13 K-U is illustratively provided in S EQ ID NO; I and is encoded by a niRNA of SEQ ID NO: 2.
[ 00299] In some . embodiments, the first and second cells used in the screening assay have different genetic backgrounds. In one embodiment, the first cell group has a genetic mutation in a P13 K-K kinase domain. In aii illustrative embodiment, the genetic mutation in the first cell group includes a mutation in a niR NA (GenBank Accession No. NiVl 006218. version NM 00621 8.2 G l: 5479208 1 herein disclosed as S EQ I D NO: 2 which encodes a ful l length PI3 K-ct having a mutation in the kinase domain. In one embodimeni. an exemplary imitation is at a codon (3296. 3297 and 3298}. in the kinase domain of SEQ I D NO : 2. wherein die codon is mutated to provide an amino acid other than a histidine ai posit ion 1047 of PI3 K-u provided in SEQ ID NO: I . In one exemplary mutat ion, the histidine at 1047 is mutated to arginine (TI I 047R). This mutat ion has been previously reported to be a particularly oncogenic nuiiaiion in the PI3KMKT signaling pathway. The second cell group lacks the nuiiaiion of the first test cell group. In one embodiment, an exemplary nuiiaiion is at a codon ( 1790, 1791 and , 1792), in the hel ical domain of SEQ ID NO: 2. wherein the codon is mutaied to prov ide an amino acid other than a glutamic acid ai position 542 or 545 of P1 K- U provided in SEQ ID NO: I . In one exemplary mutation, the glutamic acid at 545 is mutated to lysine (for example, E542K or E545K). This mutation has also been previously reported to be a particularly oncogenic mutation in the PI3 K/A KT signaling pathway.
(00300 J In some embodiments, the second cell group can harbor a nuiiat ion in PTEN. 100301 ] In some embodiments, Ihe first cell group can include various cel l l ines, including Cancer cell l ines, for example breast cancer cell l ines (hat may be commercial ly available from ihe American Type Culture Collection ((ATCC) American Type Culture Col lection, Manassas. VA.) bearing the H 1047R lici genetic mutat ion of PI3K-<t. In some embodiments, the first cell can include HCT- I 16. T-47D. MDA-iVI B-453. S IGOV-3. BT-20 or LS H74T cel l lines. In some embodiments, the second cel l can include MCP-7. PC 3 MCI-H460, S K- B R-3. PC-3. MDA-MB-468. S K-BR-3. M DA-MB-2 I T. or A549. Each specific cel l l ine
can be maintained according to. instructions- rovided upon purchase and ate commonly available, through the ATCC.
100302] In some embodiments, the first cell group and second cell grpup,can also include noii-uimor cell l ines t t have been transformed with a mutant PI3 -a catalytic subuuii. for example. H I 047R hei or E545K PI3 K-U catalyt ic siibunit. Methods of int roducing nucleic acids and vectors into isolated cells and the culture and select ion of t ransformed host cel ls in vin o are known in. the art and include the use of calcium chloride-mediated transformation, transduction, conjugal ion. niparental mating. DEAE. dextran-niedialcd transfcciion.
infection, membrane fusion widi liposoiiies, high velocity bombardment with DNA-coated microprojectilcs. direct microinjection into single cells and eleciroporation {see. e.g., Sambr.oo.k-. et al., supra: Davis el ai.. Basic Methods in Molecular B iology. 2'"' cel., McGraw- Hil l Professional, 1995: and Neumann el al.. EM BO J .. 1 : 84.1 ( 1982)). There are several methods for eukaryotic cel l transformat ion, either transient ly or stably usi ng a variety of expression vectors. Methods for mut ating a cel l-l ine, for example N I H 3Ύ3 cel ls by ampl ifying a scciuence of- DNA encoding the mutated PI3 K-U catalytic subunil of interest, 'flic aiiipl ified PGR mutant PI3 K-U construct can be cloned into a viral expression vector, for example, pSX2neo, a Moloney murine leukemia virus (MLV) long terminal repeal-driven expression vector made by inserting a simian virus 40 early promoier-neomycin
phosphotransferase gene into pSX2. designed to express high lcvels f l'OA 1 MLV Env. TransformatioiT Of N IH 3T3 cells can be performed by transfcci ion with a different CaPO.i cop ecipitalion lechni(|ue. After reaching connuence the cells can be transferred into medium containing 5% PBS without dcxamethasone. Morphologically transformed cells can be separated and isolated from mixtures of transformed and noniransformcd Env-plasmid- iransfecicd cel ls b 'excising the. transformed foci from the cell layer with a small-bore pipette (a Pasteur pipette drawn out oyer a flame to give a fine lip) and aspiration of the foci by the use of a rubber btilb attached to a pipette.
[00303] In some embodiments, the methods described herein require that the eel Is- be. tested in the presence of a candidate inhibitor, wherein the cand idate inhibitor is added to separate exemplary assay wells, each well containing either the first or second cel ls. The amount of candidate inhibitor can vary, such that a range of inhibitory act i vities can be determined for the determination of an ICso for that candidate inhibitor. This can easil y be achieved by serially dilut ing the compound in an appropriate solvent , for example, DMSO and then in the culture medium in wh ich the first and second cel ls are being incubated in. In some embodiments, the concentration of the candidate inhibitor can range from about I pM
to about 1 nijVl concentration. In some embodiments, the cand idate inhibitors are added in amounts ranging from about 0.5 ntVI to about 10 μΝ'Ι. The incubat ion of caiulidate inhibitor with first and second cell . roups can-vary, typically ranging from about 30 minutes lb about 60 hours.;
| ()()304] In some embodiments, particularly with \PI3 -a mediated act ivity, the cel ls are stimulated with a growth factor. The selection of growth factor is mediated by the requirements of the cell l ine, for example, illustrat ive growth factors can include VEG F, IGF. insul in and licrcgulin.
|(Η)305] In some embodiments, lite inhibitory activity of the candidate -compounds can lie measured using a variety of cellular activities. When cancer cell l ines are being used, the tnh i i t ion oi* : I3 mediated activity, e.g./A T plibsphorylato arid T3'08) AKT activation, cellular prtilileratioit. a.n'cl apopiosis -resistance in the cell's can all be measured. In some embodiments, the amount of A KT phosphorylation in the first and second cell groups can b.e measured using a phophb-specific antibody ( for example ΑΚΊΊ (phospbo S473. Cat. No. abS 32. ΛΚΊΊ (phospb T308) Cat. No. ab66 l 34 ) which arc commercially available from AbCam. Cambridge, MA. Other methods for measuring the inh ibit ion of PI3K-C- activity in the first and second. cell groups are described in Donahue. A.G. et al., Meamrrngjihosphoryki xl Akt and oilier■ pliosplioiiio.siiiilc S-kiiHisc-re nlatcd
pli splinprolcins in primary, lymphocytes. Methods Enzymol: 2007(434): 13 1 - 154 which is incorporated herein by reference' in its entirety.
[00306] In another embodiment, the invention provides a method for determining a treatment regimen for a cancer patient having' a lumor comprising a PI3 K-«. the method comprising:
determining the presence or absence of a mutat ion in amino acids 1047 and/or 545 of ihc PI3 K-u;
wherein if the PI3.K-a has -a imitation at: position 10471 the method comprises administering to the cancer patient a therapeutically cffeciive.amounl of. a PI3 K-a selective inhibitor compound: or
wberein if the PI3 -U has a mutation al position 545, the method comprises administering to the cancer pat ient a therapeutical ly effective amount of a combination of a PI3 K-U sclcciive,inhibitor and a P13K-f) selective inhibitor, a dual PI3 K-c;7mTOR select i ve inhibitor, or a combination of a PI3 K-a selective inhibitor and a m'FOR select ive inbibilor.
(O0307] hv another embodi ment, the invention provides a method for determinin - treatment regimen far a cancer patient having a tumor comprising a PI3 K-U. the method comprising:
determining the presence or absence of a imitation in amino acids 1047 and/or 545 of wherein if the PI3 - has a mutation at posit ion 1 (347. the method comprises administering to the cancer patient a therapeut ical ly effective amount of a PI3 K-a select i ve inhibitor compound, a dual PB -ii/inTOR selective inhibitor, a combination of a PI3 -ct selective inhibitor and a mTOR select ive inhibitor. to the subject: or
wherein if the PI3 - . lias a mutat ion at position 545. llic method comprises administering lo.ihe cancer patient a therapeut ically effective aniount
'of a combination of a ΡΙ3 Κ-ά selective ihhibiior.and a PI3 K-|5 select ive inhibitor, a dual PI -<t/niTOR selective inhibitor, or a combination of a
selective inhibitor and a mTOR selective inhibitor. 1003081 The method of the invent ion can be used to identify cancer patient populations more likely to benefit from treatment with RD K -sclcciivc inhibitors as well as patient populations less likely to benefit.
100309] The invention can be used . to further define genet ic markers or gene expression signatures which identify PI3 Ka inhibito sensitive tumor subtypes by-extended iVi vitro cell line profil ing and in vivo pharmacodynamic and efficacy studies.
[00310] In some embodiments, a. method for determining a treatment regimen for a cancer patient having the exempl ified cancers herein can be readily performed oh the basis .of the differential activity of PI3 -U sclcciiye inhibitors in cancers having a PI3 K-U mutated background described herein. In patients in which a tumor cell has been anal yzed and assayed lo determine whether the tumor harbors a PI3 K.U mutation in the kinase domain, for example, a mutat ion resulting in 1-1 1047 . greater efficacy anil treatment improvement can be achieved b tai lorings treatment comprising a PI3 -U selective inhibitor. For patients, wh have a tumor which docs not harbor a mutation in PI3 u kinase domain, the t reatment may require adopting a different treatment regimen. Tor-example, by focusing on del ivery of a conibinaiion of Ρ13Κ-α select ive inhib tors and a P 13X41 select ive inhibitor, a dual PI3 K- tt mTOR selective inhibitor, or a combination of a P13 -a selective inhibitor and a mTOR selective inhibitor. As indicated above, t he PI3 -U selective inhibitors. mTOR selective inhibitors and dual P13 K-u/mTOR select ive inhibitors arc exempl i fied in Tables I . or 2, or 3. and in the detailed descript ion herein.
[0031.1.1 In■some embodiments, methods for determining a treatment regimen comprises determining the presence of a mutat ion in amino acids 1047 and/or 545 of the P! K-rt in the subject's tumor. This step can be achieved in a variety of ways, using nucleic acid
'approaches, protein .separat ion approaches or direct immunological' approaches using mutat ion . specific antibodies. In some embodiments, presence of a mutation in amino acids 1047 and/or 545 of the PI K.-U in the subject 's tumor can be determined using any suitable method for the sequence analysis of amino acids Examples of suitable techniques include, but are not limited to, western blot analysis, imniunoprecipiiation. radioimmunoassay (R IA) or enzyme-linked immunoabsorbent assay (ELI'S A ).
[003121 In the present invention, reference to position within the amino acid sequence of PO a is made referring to SEQ ID NO: 1 . Reference to positions within the nucleot ide sequence of the P13Ku is made referring to SEQ ID NO;2. Specific amino acids in the wild type protein sequence are described using single letter amino acid designation fol lowed by. the position in the protein sequence, for example .E545 indicates that. position 545 is.glutainic ■acid. To represent a substitution at a particular position, the substituted amino acid fol lows the posit ion, for example E545K indicates that the glutamic acid at posit ion 545 is replaced with a lysine.
[00313 j Determining the presence or absence of mutations in the sequence of the PI3 K-u pept ide sequence is general ly determined using in vitro methods wherein a tumor sample is used which has been removed from the body of a patient.
[00314] Determining the presence or absence of mutations in the amino; acid sequence of PI3Ka or a portion thereof, can be done using any suitable ; method. For example the nucleotide sequence of P13 a or a portion thereof maybe determined and the amino acid sequence deduced from the nucleotide sequence or a PI3K-U protein can be interrogated directl y.
[00315] The nucleotide sequence of the PI3 -a , or a portion thereof, may be determined using any method for the sequence analysis of nucleic acids. Methods for identification of sequence mutation in genes are well known in the art and the mutations iii die PI3 Ku can be identified by any suitable method. These methods include, but are not l imited to, dynamic allcle-specific hybridization: the use of molecular beacons: enzyme-based methods, using for example DNA ligasc, DNA polymerase or nucleases; PCR based methods, whole genome sequencing: partial genome sequencing: exome sequencing; nucleic acid probe hybridization: and restrict ion enzyme digestion analysis.
| 0031 fij Methods of Direct DNA sequencing are well known in the art, (se for example: Current Protocols in Molecular B iology, edited by Fred ;M. Ausubcl. Roger Brent. Robert. H., Kingston. David D. Moore. .1. G. Seidman. Johii A. S mith. Kevin Siruhl. and Molecular Cloning: A Laboratory Manual. Joe Sambrook. David W Russcl, 3llJ edit ion. Cold Spring Harbor Labonitory Press).) These sequencing protocols include for example, the use of radioactive I y! labeled nucleotides, and nucleot ides labeled with a fluorescent dye.
|00317 | For example. Barbi. S. et a I'. , used the fol lowing protocol to sequence the hel ical domain (exoii 9) and the kinase domain (exon 20) of PI3 K-ci.. Normal and tumor DNA was extracted from paraffin-embedded tissue, and ampl ified using fluorescent dyeilabeled primers. Primer sequences heed to be chosen to. uniquely select for a region Of ' .DNA. avoidin the possibil ity of iirislvybridizal ion to . a similar sequeiice.nearby. A commonly used method is B LAST search whereby al l the possible regions to which a primer may bind can be seen. Both the nucleotide sequence as well as the primer itsel f can be B LAS T searched. The free NCB I tool Primer-B LAST integrates primer design tool and B LAST search into one application, so docs commercial software product such as Beacon Designer, (Premier B i.osol'i •International.. Palo. Alto Cali fornia). Mononucleotide repeals should be avoided, as loop formation can occur and contribute to mishybridi/.ation. In addition, compiuer;programs are readil available, to aid in design of suitable: primers. 'In certain embodiments the nucleic acid probe is labeled for use in -Southern hybridization assay: The'.':iu»cl.eiCA»ci.d:jirbbc¾fjay be radioaeiivcly labeled, fluorcsccniiy labeled or is immunological l y delectab le, in particular is a digoxygcnin-labcled (Roche Diagnost ics GmbH . Mannheim).
(00318J In some embodiments, determining the presence of a hel ical domain mutation in exon 9 can include the use of forward primer and reverse primers:
GGG A A A A ATATG AC A A AG A A AG C (SEQ I D NO: 3) and
CTGAGATCA'GGCAAA ri'CAG'ri" (SEQ ID NO: 4) respeciivcly and a sequencing prinier can -include TAGCTAGAGACAATGAATTA AGGGA AA (S EQ ID NO: 5),
[00319) For determining a mutation in the kinase domain in exoii 20. an exemplary set of primers can include: forward and reverse primers CTCAATGATGCITG GCTCTG (S EQ ID NO: 6) and TGGAATCCAG AGTGAGCTITC (S EQ I D NO: 7) respeci i vcly and the sequencing primer can include ITGATGACAITGCATACATTCG (S EQ I D NO: S). The amplification products can then be sequenced. ( Barbi. S. et al. J . Experimental and Clinical Cancer Research 2010, 29:32) The sequences arc then compared and differences between the wild type P.I3K-U sequence and ihcsequcnce of the lumor PI3 K-U. are determined. The assay
could also be -performed by only amplifying the tumor DNA and comparing the ΡΙ3 -α sequence in the tumor with the sequence of SEQ ID NO: I .
100320] In some embodiments, the present invention provides polynucleotide sequences comprising polynucleotide sequences in whole or in part from SEQ ID NO: 2 that arc capable of hybridizing to the helical region, or the kinase domain of PI3 - under conditions of high stringency. In s nie embodiments, the polynucleotides can include sequences
complementary to nucleic acid sequences thai encode in whole or in part PI3K-n or Pl3K-i. having specific mutations as described herein. The terms "complementary" and
"complementarity" refer to polynucleotides (i.e.. a sequence of nucleotides) related by the base-pairing rules. For example, for the .sequence "A-G-'Γ," is complementary to the sequence "T-C-A." Complementarity may be "partial." in which only some of the nucleic acids' bases arc matched according to the base pairing rules. Or. there may be "complete" or "total" complementarity between the nucleic acids. The degree of complementarity between nucleic acid strands lias significant effects on the efficiency and strength of hybridization between nucleic acid slrands. This is. of particular importance in amplification read ions. as well as detection methods which depend upon binding between nucleic acids.
[00321] In some embodiments, the present invention provides polynucleotide sequences comprising polynucleotide sequences in whole or in part from SEQ ID NO: 2 that are capable of hybridizing to the helical region, or the kinase domain oP13 -a under conditions of high stringency. In some embodiments, the present method includes using isolated RNA from a subject's tumor in an assay lo determine whether there is a mutation at amino acid at position 1047, 542, or 545 of SEQ ID NO: 1 , the assay further comprises: (a) reverse transcribing said RNA sample into an equivalent cDNA: (b) amplifying a predetermined region of the cDNA using a pair of nucleic acid probes directed lo a predetermined region of the PI -<x gene: (c sequencing said amplified cDNA region to obtain a polynucleotide sequence of said amplified cDNA region; and (d) determining whether said amplified cDNA region contains a- -gene .mutation in a eoclon encoding the amino acid ai position 1047, 542, or 545 of SEQ ID NO: I .
|00322'| In some embodiments, the prcseni methods can employ amplifying a
predetermined region of the cDNA by amplifying the cDNA using a pair of nucleic acid primers, a first primer capable of hybridizing stringently to the cDNA upstream of a DNA codon encoding the amino acid at cither amino acid 1047 or 542 or 545 of SEQ ID O:.l , and second a nucleic acid primer operable to hybridize stringently to the cDNA downstream of a
ΟΝΛ codon encoding the amino acid at either amino acid 1047 or 542 or545 oTSEQ ID O: l
[U0323] In so ic embodiments, the polynucleot ides can include sequences complementary to nucleic acid sequences that encode in whole or in paiVPI3 -a or PI3 K.-U having speci fic mutations as described herein. The terms "complementary" and "complementarity" refer to polynucleotides (i.e., a sequence of nucleotides) related by the base-pairing rides. For example, for the sequence "A-G-T." is complementary, to the sequence "T-C-A."
Complementarity ma be, "part ial." in ..which only some of the nucleic acids' bases are matched according to the base pairing rules. Or. there may be "complete" or "total" complementarity. between the nucleic acids. The degree of complementarity bet ween nucleic acid strands has significant effects on the efficiency and strength of hybridization between nucleic acid strands. This is of particular importance in amplification react ions, as well as detection methods which depend upon binding between nucleic acids.
[00324] "High stringency conditions" when used in reference to nucleic acid hybridisation comprise conditions equivalent to binding or hybridization at 42C° in a .solution consisting of 5 x SSPE (43.8 g/l NaGl. 6.9 g/l aPbPO.-.l bO. and 1.85 g/l EDTA, pH adjusted to 7.4 with NaOH). 0.5% SDS. 5 x Dcnhardt's reagent and 100 μ¾ ι·ϊΐΊ. denatured salmon sperm DMA followed by washin in a solut ion comprising 0. 1 x SSPE. 1 .0% S DS at 42C° when a probe of about 500 nucleotides in length is employed.
[003251 The term "homology" when used in relation to nucleic acids refers to a degree of complementarity. There may be partial homology or complete homology ( i .e.. identity). "Sequence identity" refers to a measure of relateclness between two or more nucleic acids or proteins, and-is given as a percentage with reference to the total comparison length. The identity calculation takes into account those nucleotide or amino .acid residues' hai are ident ical arid in the same relative positions in their respect ive larger sequences. Calculat ions of identity may be performed by algorithms contained within computer programs such as "GAP" (Genet ics Computer Group, Madison. Wis.) and "A LIGN" ( DNASl ar. Madison, Wis.). A partially complementary sequence is one that at least part ially inhibits (or competes with) a completely complementary sequence from hybridizing to a target nucleic acid is referred to using the functional term "substantially homologous." The inh ibition of hybridization of the completely complementary sequence, to the target sequence may be examined using a hybridization assay (Southern or Northern blot, solution hybridization and the like) under conditions of low stringency. A substantially homologou.s sequence or probe will compeie for and inhibit the binding (i.e.. the hybridizat ion) of a sequence which is
completely homologous to a target under conditions of low- stringency. This is not to say that condition's of low.siriiigcucy. are suc that non-specific binding is permitted: low stringency conditions require that the binding of two sequences to one another be a speci fic ( i.e...
selective) interaction.. The -absenecOl: non-specific binding may be tested by the use of a second target which lacks even a partial degree of complementarity (e.g.. less than about 30% ident ity); in the absence of non-specific binding the probe will not hybridize to the second non-complementary target.
[003261 In preferred embodiments, hybridization-condit ions are based on the melting temperature (Tni) of the nucleic acid binding complex and confer a defined "stringency" The term "hybridization" refer to the pairing of complementary nucleic acids. Hybridization and. the strength of hybridizaiioir( i.e., the strength of the associat ioitbeiweenahe nuqliiic acids) us impacted by such factors as the degree of -complementary between the nucleic acids, stringency of the cond itions involved, ihe m of the formed hybrid, and the G:C ratio within the nucleic acids. A single molecule that contains pairing of complementary nucleic acids within its structure is said to be "sel f-hybridized."
[00327] The term "Tm" refers to the "melting temperature" of a nucleic acid. The melting temperature is the temperature at which a opuliit ioii of double-stranded nucleic acid molecules becomes half dissociated into single strands. The eciuat ipn fd caleulating
' the Tiri
in ihc art. As indicated by stanclard .references.. a simple: estimate of the Tiii value may be calculated by the equation: Tm =81 5*0.4 1 (% G+C). when a nucleic acid is in aqueous solution at 1 JV1 NaCI. The term "stringency" refers to the conditions of temperature, ionic strength, and the presence of other compounds such as organic solvents, under which nucleic acid hybridizations are conducted. With "high stringency" conditions, nucleic acid- base pairing will occur onl y between nucleic acid fragments that have a high frequency of complementary base "sequences.
100328] -In-addition, sequence mtitations.in the PI3 Ka can be determiiied iisihg any sequence-specific nuclcic.acid detection method allowing. delect ion of siuglc-nuclcotide- variation, in particular any such. method involving complementary base pairing. For example, to determine i f the P13 K comprises a E545 mutat ion, the sequence of P13 -a peptide or a p rl ion thereof comprising nucleot ides 1 790, 1 791 and 1 792 of SEQ I D NO:2 (codon corresponding with position 545 in the amino acid sequence), is used in a polymerase chain react ion (PGR) where the oligonucleot ide primers allow the ampl ification of PI3 a only if the nucleotide at position 1790 is G. If no.reaction product is formed then the amin acid at position 545 is mutated. In another example the .oligonucleotide- primers arc designed
lo allow the amplification of ihe to al low ampl ification i f the nucleotide at posit ion 3297 is A (codon comprising nucleotides 3296, 3297 and 3298 corresponds wit posit ion- 1047 of the ainino acid sequence')-. If no react ion product is formed using. those, primers then the amin acid at position 545 is mutated.. Methods for performing PGR are known in the art (.vet* Current Protocols in Molecular B iology, edited by Fred M. Ausubel. Roger B rent . Robert E. Kingston. David T}: Moore. J . G. Seiilman. John A. Smith, Kevin Sl ruhl . and ; Molecular Cloning:. A Laboratory Manual. J oe Sambrook . David VV Russel. 3"' edit ion. Cold Spring Harbor Laboratory Press).
101)3291: Dynamic allcU spe,cific liyl (lizatioi DAS I:I) genotyping takes advantage of the differences in the' meliing temperature in DNA that results from the instability of mismatched base pairs. This technique is well suited 'to automation. In the first step, a D A-segmeni is ampl ified and attached to a bead -through a PCI ' reactioti with a biotinylated ;priiiier.. In the second step, the ampl ified product is attached to a slreplaviclin column and washed with NaOH lo remove the un-biol inylaicd.sirahd. An sequence-specific oligonucleotide is then added. in the presence of a molecule that fluoresces when bound to double-stranded DNA. The intensity is then measured as. temperature is increased until the Tin can be '.determined. A single niicleotide.ehangc will restili in a lower than, expected Tin ( Howell. W.. J obs M ., Gyl lensien U., Brookes A. ( 1999) Dynamic allele-specifie hybridizat ion. A new method for scoring single nucleotide polymorph isms: Nat Bioic hnol. 1 7( l ):87-8). Because DAS H genolyping .is measuring a quantifiable change in Tin. it is capable of measuring al l types o mutations, not just SNPs. Other benefits of DASH include its abi lity to work with label, free probes and its simple design and performance conditions.
[.00330]' Molecular beacons can also be used lo detect mutations in a DNA sequences Molecular beacons makes use of a specificall y engineered single-stranded ol igonucleot ide probe. he ol igonucleot ide is designed such that t here are complementar regions at each end and a probe sequence located in between. This design allows, the probe to lake on a hairpin, or stem-loop, structure in its natural, isolated stale. Attached to one end of t he probe is a fluorapliore and to the other end a fluorescence- uencher. Because of the stem-loop structure of the probe, the fluorophorc is in close proximit 'to the quencher, thus preventing the molecule from emitting any fluorescence. The molecule is also engineered such lhat onl y the probe sequence is complementary to Ihe lo ihe genomic DNA that will be used in the assay (Abravaya K.. Huff J ., Marshall R.. Merchant B.. Mullen C.. Schneider G .. and Robinson J . (2003) Molecular beacons as diagnost ic tools: technology and appl icat ions. Clin Clicni Lab Med., 41 :468-474).. If the probe sequence of ihe molecular beacon encounters ils largel
genomic D A during the assay, it will anneal and hybridize. Because of the length of the probe sequence, the hairpin segment of the probe will denatured in favor of forming a longer, more stable probe-target hybrid. This conformational change permits the fluorophore and quencher to be free of their l ight proximity due to the hairpin. association, allowing the molecule to fluoresce. If on the other hand, the probe sequence encounters a target sequence with as little as one noii-compleineiilary nucleotide, the molecular beacoiv wil l preferent ial l y stay in iis natural hairpin state and no fluorescence will be observed, as the fluorophore remains quenched. The unique design of these molecular beacons allows for a simple diagnostic assay to identify SNPs at a given location. I f a molecular beacon is designed to match a wild-lypp allele and another to match a mutant of the allele, the two can be used to identify the genotype of an individual. I f only the fust probe's fluorophore wavelength is delected during the assay then the individual is homozygous to the wild type. I only the second probe's wavelength1 is detected then the individual is honiozygous to. the mutant allele. Finally, if both wavelength are detected,; then both molecular beacons must be hybridizing to. their complements and thus the individual must contain bodi alleles and be heterozygous. ( 00331 J Enzyme-based nucleic acid methods are also suitable and contemplated for determining mutations in the PB K-u nucleot ide sequence. For example. Restriction fragment length polymorphism (R FLP) (discussed in greater detail below) can be used to detect single nucleotide differences. SNP-RFLP makes use of the many different restrict ion endonucleases and their high affinity to uhique and speci fic restriction site's.. By performing a digestion on a genomic sample and determining fragment; length's through a gel assay it is possible to ascertain whether or not the enzymes cut the expected restriction sites. A failure to' cut the genomic sample results in an ident i fiably larger than expected fragment implyi ng t hat there is a mutation at the point of the restrict ion site which is rendering it protected from nuclease activity.
100332 J The term "functional ly equivalent codon" is used herein to refer to codons that encode the same amino acid, such as the six codons for'arginine.
[00333] In one embodiment of the invention the method comprises at least one nucleic acid probe or ol igonucleotide for determining the sequence of the codon that encodes amino acid 1047. In another embodiment the method comprises at least one. nucleic acid probe or oligonucleotide for determining the sequence of the codon that encodes amino acid 545. The oligonucleotide is a PCR primer, preferably a set of PCR primers which allows ampl ification of a ΡΙ3 Κα nucleic acid sequence fragment only if the codon which encodes amino acid 1047 encodes a histidinc. In another method, the PCR primer or set of PCR primers allows
ilie ampl ification of nucleic acid sequence, fragment only i f the codon which encodes amino acid 545 encodes a glutamic acid. Determination of suitable PGR primers is routine in the art. (Current Protocols in Molecular B iology, edited by Fred M. Aiisubcl. Roger Brent, Robert E. Kingston. David D. Moore. J . G. Seidman. John A. Smith. Kevin Slruhl; Loosclea : 0-47 1 -650338-X: CD-ROM: 0-47 1 -306 1 -4). In addit ion, computer programs arc readily available to aid in design of suitable primers. In certain embodiments the nucleic acid probe is labeled for use in a Southern hybridization assay. The nucleic acid probe may be radioaciivcly labeled, fluoresceiilly labeled or is immunologically detectable, in particular is a digoxygenin-labcled (Roche Diagnostics GmbH. Mannheim).
[00334] U.S. Patent Publicat ion 20010016323 discloses methods for detecting point mutations using a fluorescently labeled oligonncleot idcnieric probe and fluorescence resonance energy transfer. A point mutat ion leading to a base mismatch between the probe and the target DNA strand causes the melt ing temperature of the complex to be lower than the melting temperature for the probe and the target if the probe and target were perfectly matched.
[00335] Other suitable methods for detecting single point mutations include those disclosed in. for example. U.S. Patent Publication 2002 1 665. which 'involves the use of oligonucleot ide probes in array formal . Such arrays can include one or more of SEQ I D NOs:3-8..U.S. Patent Publ icat ion 200201 77 157 discloses additional methods for delecting point mutations.
[00336] A polynucleotide carrying a point mutat ion leading to a mutat ion of PI3K kinase domain, for example, 1-1 1047 R that is the subject of this invent ion can be identified using one or more of a number of available techniques. 'However, detection i not limited to the techniques described herein and the methods and composition of the invention are not l imited to these methods, which are provided for exemplary purposes only. Polynucleot ide and oligonucleot ide probes arc also disclosed herein and are with in the scope of the invention, and these probes are suitable for one or more of the techniques described below. These include allcle-specilic ol igonucleotide hybridization ( ASO). wh ich, in one
embodiment , is a diagnostic mutation detect ion method wherein hybridization with a pair of oligonucleotides corresponding to alleles of a known initiation is usctl to detect the mulaiion. Another suitable method is denaturin high performance liquid chromatography (DHPLC). which is a liquid chromatography method designed to identi fy mutat ions and polymorphisms: based on detect ion of heteroduplcx formation between mismatched nucleotides. Under specified condit ions, heleroduplexes elntc from the column earl ier than homoduplcxcs
because bf reduced 'melting temperature. Analysis can then be performed on indi vidual samples.
[00337] An ampl ified region of the DNA containing the mutat ion or the wild-type sequence can be analyzed by DHPLC. Use of DH PLC is described in U.S. Pat. Nos.
5,795.976 and 6.453.244, both of which are incorporated herein by reference. A suitable method is that provided by Transgcnomic. Inc. (Omaha. Ncbr. ) using the T ransgenomic WAV E© System.
[00338] For ASO, a region of genomic DNA or cDNA containing the Pl.l -u mutat ion (H 1047R and/or E545K) is ampl ified by PGR and transferred onto duplicating membranes. This can be performed by dot/slot blotting, spotting by hand, ondigestion and . outhern, blotting. The membranes arc prehybridized. then hybridized with a radiolabeled or dcoxygenin (DIG) labeled ol igonucleot ide to cither the mutant or wild-type sequence's. For ihe.DIG lahel, detection is performed using chemiluminesccnl or colorimelric methods. The membranes arc then washed with increasin stringency until the ASO is washed from the non-specific sequence. Following autoradiographic exposure, the products are scored for the level of hybridization to each oligonucleot ide. Optimally, controls are included for the normal and mutant sequence o eaclv filter to confirm correct stringency, and a negative. PCR control is used to check for contaminat ion in the PGR.
[00339J The size of the ASO probe is not limited except by technical parameters of the art. Generally, too short a probe will not be unique to the locat ion, and too long a probe may cause loss of sensitivity. The oligonucleot ides are preferabl y 1 5-2 1 nucleot ides in length, with the mismatch twoards the center of the ol igonucleotide.
[00340] The region of sample DNA on which ASO hybridizat ion is performed to delect the mutation of this invention is preferably ampl i fied by PCR using a forward primer. Foi¬ exon 9 the forward primer and reverse primers were GGGAAAAATATGACAAAGAAAGC (SEQ ID NO: 3) and CTGAGATCAGCCAAATTCAGTT (SEQ I D NO: 4) respect ively and the sequencing.primcr was TAGCTAGAGACAATGAA'ITAAGGGAAA (SEQ ID NO: 5). for exon 20 the forward and reverse primers were CTCAATGATGCTTGGCTC TG (SEQ I D NO: 6) and TG G A ATCC AG A GTG A G C I TC (SEQ ID NO: 7 ) respectively. In this case, amplification by PCR or a comparable method is not necessary but can optional ly be performed.
[003411 Optionally, one or more than one of the amplified regions described above, (including the 306 nucleotide region generated using primers of SEQ I D NO:3-S. or shorter portions of either of these regions, can be analyzed by sequencing in order to detect the
mutation'. Sequencing can be performed .as- is routine in the art. The only l i mitation on choice of the region to be sequenced, in order to identify the presence of the mutat ion, is that the region selected for sequencing must include the nucleot ide t hat is the subject of the mutation. The size of the region selected for sequencing is not l imited except by technical parameters as is known in the art. and longer regions comprising part or al l of the DNA or RNA between selected ampl ified regions using the. rimers S17.Q ID NOs: 3 & 4 and 6 & 7 disclosed. herein can he sequenced.
[003421 Variations of the methods disclosed above are also suitable for delecting the mutation. For example, in a variation of ASO, the ASO's are given homopolymer tails with terminal deoxyfibonucleoiidyl transferase, spotted onto nylon membrane, and covalentl y bound by UV irradiation. The target DNA is amplified with bioiinylated primers and hybridized to the membrane containing the immobilized oligonucleotides, followed by detection. An example of this reverse dot blot technique- is the IiNNO-LIPA kit from umogcrielics ( Belgium).
1003431 With the identification and .-sequencing of the mutated gene and the ene product, i.e. SEQ ID NO: l having a mutation at H545 and I t I Q47R. probes and antibodies raised to the gene product can be used in a variety of hybridizat ion and immunological assays to screen for and detect the presence of either a normal or imitated gene or gene product.
[00344 J Expression of the mutated gene in heterologous cel l systems can be used to demonstrate structure function relationships. Ligat ing the DNA sequence into a plasmid expression vector to iransfcci cells is a useful method to test the influence of the mutation-cm various cellular biochemical parameters. Plasmid expression vectors. containin either the entire normal or mutant human or mouse sequence or port ions thereof, can be used in in vitr mutagenesis experiments which will ident i fy port ions of the protein crucial for regu latory function.
[00345] The DNA sequence can be manipulated in stud ies to understand the expression of the gene and its product, and to achieve production of large quantit ies of the protein for functional analysis, for ant ibody production, and for patient therapy. Changes in the sequence may or may not alter the expression pattern in terms of relat ive quantities, tissue-specificity and functional properties.
[0034(5] A number of methods arc available for analysis of variant (e.g.. mutant or polymorphic) nucleic acid sequences. Assays for detect ions polymorphisms or mutations fall into several categories, including, bin not l imited to direct sequencing assays, fragment polymorphism assays, hybridization assays, and. computer based data analysis. Protocols and
commercially available kits or services for performing multiple variations of these assays arc commercially available and known to ilio.se of skill in the-an. In some embodiments, assays arc performed in ^ combination or in combined pai ls (e.g., different reagents or technologies from several assays are combined to. yield one assay). The following illustrative assays may be used to screen and identify nucleic acid molecules containing the mutations of P IJ K-U mutation of interest.
Fragment Length Polymorphism Assays
[00347] lit some embodiments of the present invention, variant sequences arc detected using a fragment length; polymorphisirhassay. In a fragment length polymorphism assay; a unique DNA banding pattern based on cleaving the DNA at a scries of positions is generated using an enzyme (e.g., a restriction enzyme or a CI-EA V ASE 1 (Third Wave Technologies. Madison, Wis.] enzyme). DNA fragments/from a sampM'eVc0niaihihg .a 'SNP or a mutation will have a different banding. attern than wild type.
PGR Assays
[00348] In some embodiments of the present invention, variant sequences arc detected using a PCR-based assay. In some embodiments, the PCR assay comprises the use of oligonucleotide nucleic acid primers that hybridize only lo the variant or wild type allele o PI3Kd (e.g., to the region of mutation or multiple mutations). Both sets of primers are used to amplify a sample of DN A. If only the mutant primers result in a PGR product, then the subject' s tumor or cancer expresses a somatic imitation in an P13 K-ci mutation allele. PCR amplification conditions are tailored to the specific ol igonucleotide primers or
oligonucleotide probes used, the qual ity and type of DNA or RNA being .screened, and other well known variables that can be controlled using appropriate reagents and/or PCR cycling conditions known to those of ordinary skil l in the an.
RFLP Assays
[00349 ] In some embodiments of the present invent ion, variant sequences are detected using a.reslriction fragment length polymorphism assay (R FLP). The region of interest is first isolated using PCR. The PCR products arc then cleaved with restriction enzymes known lo give a unique length fragment for a given polymorphism. The restriction-enzyme digested PCR products are separated by agarose gel electrophoresis and visualized by eihidium bromide staining. The length of the fragments is compared lo molecular weight markers and
fragments generated from wild-type and mutant controls.
Direct Sequencing Assays
[0035 1 In some embodiments of die present invent inn. variant sequences arc detected using a direct sequencing technique. In these assays. DNA samples arc fi st isolated from a subject using any suitable method. In some embodiments, the region of interest is cloned into a suitable vcetor iid amplified by growtli in a .host cell (¾¾;·, a bacteria). In. other
embodiments; DNA in the region of interest is amplified using . PCR.
1003511 Followin amplification,- DNA in the region of interest ('e.g.. the region containin die SNP or imitation i of interest) is sequenced using any suitable method, including but not limited to manual sequencing using radioact i e marker nucleotides, or automated sequencing. The results of the sequencing are -displayed using any suitable method, flic sequence is examined and the presence or absence of a given SNP or mutation is determined.
CPU' Assays.
[00352] hi other embo i n^ using a'CLEAj SE fragment length polymorphism assay (GFLP; 'fliii l VVave Technologies. Madison'.- Wis.; Sec e.g.. U.S. Pat. Nos. 5,84X654: 5.843.669; 5.7 19.20.8; and 5,8-88.780; each of which is herein incorporated by reference). This assay is based on the observation that when single strands of DNA fold on themselves, they assume higher order structures that are highly individual to the precise sequence of the DNA molecule. These secondary 'structures involve partially duplexed regions of DNA such that single stranded regions are juxtaposed with double stranded DNA hairpins. The CLEAVASE I enzyme, is a structure-specific; thermostable nuclease thai recognizes and cleaves the junctions between these single-stranded- and double- stranded regions. The region of interest is first isolated, for example, using: PCR. Then, DNA strands are separated by healing. Next. . the reactions arc cooled to allow intra-strand secondary structure to form. The PCR products are then treated with the CLEAVASE I enzyme to generate a series of fragments that are unique to a given SNP or mulaiion. The CLEAVASE enzyme treated PCR products arc separated and delected (e.g., by agarose gel electrophoresis) and visualized (e.g., by elhidium bromide staining). The length of the -fragments is compared to molecular weight markers and fragments generated from wild-type and mutant controls.
Hybridization Assays
[00353] In so ic embodi ments of I lie present invention, variant sequences arc delected by hybridization analysis in a hybridization assay. In a hybridization assay, the presence or absence of a given imitation is determined based on tlic abil ity of the DNA from die sample to hybridize to a complementary DNA molecule (e.g., a oligonucleotide probe r 'probes'' as illustrated herein). A variety of hybridization assays using a variety of technologies for hybridization and detection are available. Relevant and useful hybridizat ion assays for practicing the methods of lhe prescnl invention are provided below.
Direct Detection of Hybridization
[003541 lii some embodiments, hybridization of a probe to the sequence of interest (e.g., a SNP or mutation) is detected direct ly by visual izing a bound probe (e.g., a Northern or Southern assay; See e.g.. Ausabel el al. (eds. ) ( 1 9 1 ) Current Protocols in Molecular Biology, John Wiley & Sons, NY). In a these assays, genomic DNA (Southern) or R NA (Northern) is isolated from a subject. The DNA or RNA is then cleaved with a series of restrict ion enzymes that cleave infrequently in the genome and not near any of the markers being assayed. The DNA or RNA is then separated (e.g.. on an agarose el ) and transferred to a membrane. A labeled (e.g., by incorporating a radionuclcolide) probe or probes specific for the SNP or imitation being detected is allowed to contact the membrane under a condition or low.
medium. Or high stringency conditions. The unbound probe is removed and the presence of binding is detected by visual izing the labeled probe.
Detection of Hybridizat ion Usinj> " DNA Chip" Assays
[003551 hi sonic embodi ments of the present invention, variant sequences are detected using a DNA ch ip hybridization assay. In this assay, a series of oligonucleotide probes are affixed to a sol id support . The oligonucleotide probes are designed to be unique to a given SNP or imitation.. The DNA sample of interest is. contacted with the DNA "chip" aiid hybridization is detected.
[00356] In some embodiments, an il lustrative and commercially available DNA chip assay can include a GENECH IP® (commercially available from Affymeirix. Santa Clara. CA. USA); See e.g., U.S. Pat. Nos. 6.045.996: 5,925.525; and 5.858,659; each of which is herein incorporated by reference) assay. The GENECH I P© technology uses miniaturized, h igh- density arrays of oligonucleot ide probes affixed to a "ch ip." Probe arrays are manufactured by Affymctrix's l ight-directed chemical synthesis process, wh ich combines sol id-phase
-chemical synthesis with photol ithographic fabricat ion techniques employed in the semiconductor industry. Using- a .series of photolithographic masks to define chip exposure sites, followed by specific chemical synthesis steps, the process constructs high-density arrays of ol igonucleotides, with each probe in a predefined position in the array. Mult iple probe arrays are synthesized simultaneously on a large glass wafer. The wafers are then diced, and ind ividual probe arrays are packaged in injection-molded plast ic cartridges, which protect Ihem from die environment and serve as chambers for hybrid ization.
100357] The nucleic acid to be analyzed is. isolated, ampli fied by PCR. and labeled with a
•fluorescent reporter-group. The labeled DNA is then ineubaied
thc array, using a fliiidics station. The array is then inserted into the scanner, where patterns of hybridization are detected. The hybridization data are collected as l ight emitted from the ' fluorescent reporter groups already incorporated into die target, which is bound to the probe array. Probes that perfectly match the target general ly produce stronger signals than those that have mismatches. -Since the sequence anil posit ion of each probe on the array are known, by complementarity, the identity of the target nucleic acid applied to the probe array can be determined;
Enzymatic Detection of Hybridization
[00358] In some embodiments of the present invention, hybridization can be detected by enzymatic cleavage of specific structures ( I N VADER assay, Th ird Wave Technologies: Sec e.g.. U.S. Pal . Nos. 5,846.7 17. 6.090.543 ; 6.001.567: 5.985,557 ; and 5,994.069: each of which is herein incorporated by reference). The INVADER assay detects specific DNA and RNA sequences by using structure-specific enzymes to cleave a complex formed by the hybridization of overlapping ol igonucleot ide probes. Elevated temperature and an excess of one of the probes enable multiple probes to be. cleaved for each target sequence present without temperature cycling. These cleaved probes then direct cleavage of a second labeled probe. The secondary probe oligonucleot ide can be 5'-end labeled with fluorescein that is quenched by an internal dye. Upon cleavage, the de-quenched fluorescein labeled product may be detected using a standard fluorescence plate reader. The INVA DER assay detects specific mutations in unaniplified genomic DNA. The isolated DNA sample is contacted with the first probe specific either for a mutat ion of the present invent ion' r wild type PI3K-a sequence and allowed to hybridize. Then a secondary probe, specific to the first probe, and containing the lliiorcscein label , is hybridized and the enzyme is added. B inding is detected by using a fluorescent plate reader and comparing ihe signal of the lest sample to known
positive and negative controls-.
[00359] In sonic embodiments, hybridization of a bound probe is detected using a TaqMan assay (PE B iosystems. Foster City. Calif'.: See e.g.. U.S. Pat. Nos. 5.962.233 and 5.538.848. each of which is herein incorporated by reference). The assay is performed during a PCR reaction. The TaqMan assay exploits the 5'-3' exonuclease act ivity of the AMPLI TAQ GOLD D A polymerase. A probe, specific for a given allele or mutation, is included in the PCR "reaction; The probe consists o an oligonucleotide vvitli a 5'-repdrteivdye (e.g.. a. fluorescein dye) and a 3'-qucneher dye. During PCR. if the probe is bound to its target, the 5'-3' nucleolyiic activity of ihe AMPLITAQ GOLD polymerase cleaves the probe between the reporter and the quencher dye. The separation of the reporter dye from the -quencher dye results in an increase of fluorescence. The signal accumulates with each cycle of PCR and can be monitored with a fluoronieter.
[003(50] In accordance with the present invention, diagnostic kits are also provided which will include the reagents necessary, for the a ove-describcd diagnostic sereens. For example, kits may be provided which include oligonucleot ide probes or PCR primer's are present for tlie dctcetion and/or amplification of mutant P13K-U. and comparable wi ld-type P'13-Κ-α - related nucleotide sequences. Again, such probes may be labeled for easier detection of specific hybridization. As appropriate to the various diagnostic embodiments described above, the oligonucleotide probes in such kits may be immobilized to substrates and appropriate controls , may be provided. Examples of such oligonucleotide probes include oligonucleotides comprising or consisting of at. feast one o SEQ ID NOs:3&4 and 6&7.
[00361] Determining the presence or absence :of imitations ii lhe: amino, acjd sci|ucnce of PI3K caii be determined using any method for the sequence analysis of amino acids. Non- limiting examples include: western blot analysis or ELISA assays, or direct protein sequencing of the PI3Ku in the subject's tumor, in some embodiments, particularly usefu l antibodies have selectivity for wild type P13 K-U versus the mutant P13 Ku . for example, an antibody useful in the assay would bind to wild type PI3 K-« . or a port ion wi ld type PI3 u. but not to a P13 u having a mutation at the amino acid of interest. Particularly useful antibodies could ..include antibodies which bind the wild type PI 3 « which has hist idine at position 1047 but does not bind a mutant P13 Ku which has an amino acid other than hisiidino. such as arginine, in other words the antibody specifically bind to an epitope comprising histidinc at position 1047 . Likewise, particularly useful arc antibodies which bind the wild type PI3Ka which has glutamic acid at position 545 but does not bind a mutant PI3 Kn which has an amino acid other than glutamic acid at posit ion 545. such as lysine at that position.
|0I)3C>2| Another embodiment of the invention provides a method comprising the use of at least one antibody which binds selectively to the wild type. PI3 u protein as compared with binding to a mutated form of P13 u . Alternately the antibody binds selectively lo a mulaicd form of PI3 a as compared with binding lo the wild type PI3 u protein and can differentiate between wild-type PI3 Ka and Pl3 Ku-M I 047R or lieiwcen wild-type PI3 a and PI3 K«-E545 . Methods for isolat ing suitable amounts of target protein from a complex mixture in relatively small amounts ( less than 1 mg) arc commonly known by those skilled in the an. In one illustrative embodiment, a tumor cell or plural ity of Junior cells from a subject's tumor or cancer are .lysed using commonly available lysing reagents in the presence of protease inhibitors. The lysate is cleared and the supernatant is either electraphorcsed and -subjected to a Western Blot using mutation specific antibodies, or alternatively, the mutated PI3 (.-H 1047R or PI3 Ka-E545K arc selectively imirmnoprecipitaied and further dissociated from the capture antibody and subjected to Western Blotting or protein sequenced directly. 1003631 "Antibody" includes, any immunoglobul in molecule that recognizes and specifically binds to a target, such as a protein, polypeptide, peptide-, carbohydrate, polynucleotide. lipid, etc., through at least one antigen recognit ion site within the variable region of the immunoglobulin molecule. As used herein, the term is used in the broadest sense and encompasses intact polyclonal antibodies, intact monoclonal antibodies, ant ibod fragment's (such as Fab, Fab', F(ab'h. and Fv fragments), single chain Fv (scFv) inuiants. multispccific antibodies such as bispecific antibodies generated from at least two intact ant ibodies, fusion proteins comprising an antibody port ion, and any other modified immunoglobul in molecule comprising an ant igen recognition site so long as the ant ibodies exhibit lite desired biological acti vity, An antiho ly can be of any the five major classes of immunoglobulins: IgA. IgD. lgE. IgG. and IgM, or subclasses ( isoiypcs.) thereof (e.g. IgG I , IgG2. lgG3, IgG4. IgA I and lgA2). based on the ident ity of their heavy-chain constant 'domains referred to as alpha, delta, ep.silon, gamma, and mu, respectively. The di fferent classes of immunoglobul ins have different and well known subunit structures and three- dimensional configurations. Ant ibodies can be naked or conjugated lo other molecules such as loxins, radioisotopes and the l ike.
[00364J "Antibody fragment" can refer to a port ion of an intact antibody. Examples of antibody fragments include, but are not limited to. l inear antibodies: single-chain antibody molecules: . Fe orFc' pept ides, Fab and Fab. fragments, and multispccific antibodies formed from antibody fragments.,
[003651 "Chimeric ant ibodies" refers to an I i botl i cs wlierei n the amino acid sequence-Gi llie immunoglobulin molecule is derived from two or niorc species. Typicall y, the variable region of both light and heavy chains corresponds to the variable region of antibodies derived from one species of mammals (e.g. mou.se. rai, rabbit, etc) with the desired specificity, affinity, and capability while the. coiistani regions are homologous lo 'lhc sequences in antibodies derived front anothe (usually human) to avoid el iciting an imnuihe' response in that species.
[00366] "Humanized" forriis of non-hiiman (e.g., rabbit) antibodies include chimeric antibodies that contain minimal sequence, or no sequence, derived from non-hitman imnumoglob ilin; Fo the most pari, humanized. ntibodies are/human immunoglobul ins (recipient antibody) in which residues from a hypervariable region of the recipient arc replaced by residue's from a hypervariable region of a ηοή-humaii species (donor antibody) such. as mouse, rat. rabbit or nonhuman primate having the desired specificity, affinity, and capacity. In some instances. Fv framework region (FR) residues of the human
immunoglobulin arc replaced b corresponding non-human residues. Furthermore, hiinianix.ed ntibodies can comprise residues that are not found in the recipient ant ibody or in the donor antibody. Most often, the humanized antibody can comprise substantially . all of at least oric. ahd typically two, variable domains, in which all or substantially alL of the hypervariable loops eprrcspond to those of a nonhuman immunoglobulin and all or substant ially all of the FR residues are those of a human immunoglobul in sequence. The humanized antibody can also comprise at least a portion of an immunoglobulin constant region ( Fc), typically that of a human immunoglobulin. Methods used to generate humanized antibodies are well known in the field of immunology and molecular biology.
[003.671 "Hybrid antibodies" can include immunoglobul in molecules in which pairs of heavy and l ight chains from antibodies with different antigenic determinant region's arc: assembled together so thai two different epitopes or two different antigens .ean .be recognized and bound by the resulting letramcr:
[00368 ) The term "epitope" or "antigenic determinant" are used interchangeably herein and refer to that portion of an ant igen capable of being recognized and speci fical ly bound by a part icular ant ibody. When the ant igen is a polypeptide, epitopes can be formed both from contiguous amino acids and noncontiguous amino acids juxtaposed by tertiary folding of a protein. Epitopes formed from contiguous amino acids arc typical ly retained upon protein denaturing, whereas epitopes, formed by tertiary folding are typically lost upon protein denaturing. An epitope typical ly includes at least 3 . and more usually, at least 5 or'S- l O amino acids in a unique spatial conformation.
[0()36'1)J "Specifically binds" lo or shows "specific binding" Iwoards an epitope means thai the antibody reacts or associates more frequently, and/or more rapidly, and/or greater duration, and/o with greater affinity with lite epitope than with alternative substances.
Prepa ra t ion of A n 1 i bod ies
Polyclonal Antibodies
[003701 Polyclonal antibodies are preferably raised in animals by multiple subcutaneous (sc) or intraperitoneal (ip) injections of the relevant antigen and an adjuvant. Alternatively, antigen may be injected directly into the animal's lymph node (see ilpatfick ei al .,
Hybridonia, 16:381 -389. 1.9973.. Ail 'improved antibody response may be obtained by.
conjugating the relevant antigen to a protein ial- is immunogenic in the species to be immunized, e.g.. keyhole limpet hemocyanin. serum albumin, bovine ihyroglobiilin. or soybean trypsin inhibitor using a bil'uiiciional or dcrivalizing agent , for example, maleimidobenzoyl sulfosuccinimide esier (conjugation through cysteine residues), - h di xysuccinimidc (through lysine residues), glularaldebyde. succinic anhydride or other agcnls known in the art.
[00371 J Animals are immunized against the antigen, immunogenic conjugates -or derivatives by combining, e.g., 100 μ¾ of the protein -or .conjugate: (for mice) with 3 volumes, of Freuud's complete adjuvant and injecting the solution intradermal! at multiple sites. One month later, the animals are boosted with 1 /5 to 1 / 1-0 the original amount of peptide or conjugate in Freuud's complete adjuvant by subcutaneous injection al multiple sites. At 7- 14 days posi-bopslcr injection, the animals are bled and the serum is assayed for ant ibody liter. Animals are boosted until the liter plateaus. Preferably, the animal is boosted with the conjugate of the same ant igen, but conjugated through a - different cross-l inking reagent. Conjugates also: can be made in recombinant cell culture as protein fusions. Also, aggregating agents such as alum are suitably used to enhance the immune response.
Monoclonal Antibodies
[00372] Monoclonal ant ibodies can be made using the hybridonia method first described by Kohlcr ct al.. Nature, 256:495 ( 1 75). or by recombinant DNA methods. In the hybridonia method, a mouse or other appropriate host animal, such as rats, hamster or macaque monkey, is immunized to elicit lymphocytes that produce or are capable of producing antibodies that will specifically bind to the protein used for immunization. Alternatively, lymphocytes may be immunized in vitro. Lymphocytes; then are fused with myeloma cells using a suitable
fusing agent, such as polyethylene glycol, to orm a hy ridoma cel l (Coding, Monoclonal Antibodies: Principles and Pract ice, pp. 59- 103 (Academ ic Press, 1986)). The hyhridoma cells thus prepared arc seeded and grown in a suitable culture medium that preferably contains one or more substances that inhibit the growth or survival of the unfused, parental myeloma cells. For example, if the parental myeloma cells lack the cn/.ymc hypoxanthine guanine phosphoribosyl transferase ( l-IG PRT or HPRT), the culture medium for the hybridomas typically wil l include hypoxanthine, amiuopterin, and thymidine (HAT medium), which substances prevent the growth of HGPRT-dcficieni cells.
[00373] Preferred myeloma cells are those that fuse efficiently, support stable high-level production of antibody by the selected antibody-producing cells and arc sensitive to medium. Human myeloma and mouse-human heteroniyeloma cell l ines also have been described for the production of human monoclonal antibodies (Ko bor. J . Immunol., 1 3: 3001 ( I S4); Brodeur ct al., Monoclonal Antibody Production Techniques and Applications, pp. 51 -63 (Marcel Dekker, Inc., New York, 1987)): Exemplary -murine myeloma l ines include those derived from MOP-2 1 and M. C.- l I mouse tumors available from the Salk Institute Cell Distribution Center, San Diego. Cal if. USA. and S P-2 or X63-Ag8-653 cells available from the American Type Culture Col leeiion. Rockvil lc, Md. USA. Culture medium in which hybridoma cells arc growing is1 assayed for production of monoclonal antibodies directed against the antigen. Preferably, the binding specificity o ''monoclonal antibodies produced by hybridoma cel ls is determined by immunoprccipital ion or by an in vitro binding assjiy, uch as radioimmunoassay (RIA) or enzymc-.l inked immunoabsorbenr assay (ELISA). The binding affinity of the monoclonal ant ibody can be determined, for. example, by B IAcore or Scaichard analysis (Munson el al.. Anal. B iochem.. 107:220 ( 1 80)).
[00374 ] After hybridoma cel ls arc ident ified that produce ant ibod ies of the desired specificity, affinity, and/or act ivi ty, the clones can be subcloned by l imit ing dilut ion procedures and grown by standard methods (Goding. Monoclonal Antibodies: Principles and Practice, pp. 59- 103 (Academic Press, 1986)). Suitable culture media for this purpose include, for example. D-MEMO or RPM I 1640 medium. In addition, the hybridoma cells can be grown in vivo as ascites tumors in an animal . The monoclonal antibodies secreted by the subclones arc suitably separated from the culture medium, ascites fluid, or serum by conventional immunoglobulin purification procedures such as protein A-Sepharose.
hydroxylapal ilc chromatography, gel electrophoresis, dialysis, or affinity chromatography.
Recombinant Production of Antibodies
|0I) 75] The amino- acid sequence of an immunoglobulin of interest can be determined. by direct protein .sequenc g, and suitable ci odiiig nucleotide sequences can be designed according to a universal codon table.
[00376] Alternat ivel y, DNA encoding the monoclonal ant ibodies can be isolated and sequenced from the hybridoma cel ls using conventional procedures (e.g.. by using oligonucleot ide probes that arc capable of binding specifical l y to genes encod ing the heavy and light chains of the monoclonal ant ibodies). Sequence determination wi l l general ly require isolation of at least a port ion of the gene or cDNA of interest . Usually this requires cloning the DNA or mRNA encoding the monoclonal antibodies. Cloning is carried ou using standard ' techniques (scc, .e.g., Sambro6k el al. ( 1989) Molecular Cloning: A Laboratory Guide, Vols 1 -3. Cold Sprin Harbor Press, which is incorporated herein by reference), for example, a cDNA l ibrary can be constructed by reverse transcription of polyA+ mRNA. preferably membrane-associated mRNA, and the l ibrary screened using probes specific for human immunoglobulin polypeptide gene sequences. In a preferred embod iment, the polymerase chain react ion (PCR) is. used to ampl ify cDNAs (or portions of full-length cDNAs) encoding an immunoglobulin gene segment. of interest (e.g., a light chain variable segment). The amplified sequences can be cloned readily into any suitable vector, e.g..
expression vectors, minigeuc veciors. or phage display vectors. It will be appreciated thai the particular method of cloning used is not critical, so long as it is possible to determine the sequence of some portion of the immunoglobul in polypeptide of interest.
| ()0377] One source for RNA used for cloning and sequencing is a hybridoma produced by obtaining a B cell from the transgenic mouse and fusing the 13 cel l to an immortal cell . An advantage of using hybridomas is that thcy can. be easily screened, and a hybridoma that produces a human monoclonal ant ibody of interest selected. Alternativel y. R NA can be isolated from IB cells (or whole spleen) of the immunized animal. When sources oilier than hybridomas. arc used, ii may be desirable to screen for sequences encoding immunoglobul ins or immunqglobulin polypeptides with specific binding characteristics. One method for such screening is the use of phage display technology. Phage display is. described in e.g., Dower et al.. WO 91 / 1727 1 , McCaffcrty el al.. WO 92/01047. and Caton and oprowski, Proc. Nai l. Acad. Sci. USA. 87:6450-6454 .( 1990). each of which is incorporated herein by reference. In one embodiment using phage display technology, cDNA from an immunized t ransgenic ■ mouse (e.g., total spleen cDNA) is isolated, PCR is used to ampl ify cDNA sequences that encode a port ion of an immunoglobulin polypeptide, e.g., CDR regions, and the ampl i fied
sequences arc inserted into a p!uige vector. cDNAs encoding pept ides of interest , e.g..
variable region peptides with desired binding characteristics, arc identified by standard icchnic|iics such as panning. The sequence of the ampl ified or cloned nucleic acid is then determined. Typically the sequence encoding an entire variable region of the immunoglobulin polypeptide is determined, however,. sometimes only-a port ion of a variable region need be sequenced, for example, t he CDR-cncoding portion. Typical ly t he sequenced portion will be at least 30 bases in length, and more often bases coding for at least about one-th ird or at least about one-half of the length of the variable region will be sequenced. Sequencing can be carried out on clones isolated from a cDNA library or, when PCR is used, after subcloning the amplified sequence or by direct PCR sequencing of the ampl ified segment . Sequencing is carried out using standard techniques (see. e.g., Sainbrook ct al. (.1089) MolccuhirC!oning: A Laboratory Guide, Vols 1 -3,-Gold Spring Harbor Press, and Sanger. F. ct al. ( 1977) Proc. Natl. Acad. Sci. USA 74: 5463-5467. which is. incorporated herein by reference). By comparing live sequence of the cloned nucleic acid with publ ished sequences of human immunoglobulin genes and cDNAs, an artisan can determine readily, depending on the region sequenced, (i) the germl ine segment usage of the hybridoma immunoglobu l in pol ypeptide (including the isotypc of the heavy chain) and (ii) the sequence of the heavy and l ight chain variable regions, including sequences resulting from N-region addition and die process of somatic mutat ion. One source of immunoglobulin gene sequence information is the National Center for Biotechnology Information, National Library of Medicine, National Institutes of Health, Bethcsda, Md.
100378] Once isolated, the DNA may be operably linked to ex pression cont rol sequences or placed into expression vectors, which are then transfected into host cel ls such as E. coli cells, simian COS cells. Chinese hamster ovary (C HO) cells, or myeloma cel ls that do not otherwise produce immunoglobulin protein, to direct the synthesis of monoclonal antibodies in the recombinant host cel ls.
1003791 Expression control sequences denote DNA sequences necessary for the expression of an operably linked coding sequence in a particular host organism., The control sequences that are suitable for prokaryotes, for example, include promoter. Optional ly an operator sequence, and a ribosomc-binding site. Eukaryotic cells are known to utilize promoters, polyadcnylalion signals, and enhancers.
100380] Nucleic acid is operably l inked when it is placed i nto a functional relationship with another nucleic acid sequence. For example, DNA for a presequencc or secretory leader is operably l inked to DNA for a polypeptide i f it is expressed as a preprotein that participates
in the secreiiou of the polypeptide; a prompter or enhancer is operably linked lo a coding sequence i f it. affects the transcription of the sequence: or a ribosome-binding site' is operably linked to a coding sequence if it is positioned so as to facilitate translation. General ly, operably l inked means that the DNA sequences being l inked are cont iguous, and, in the case of a secretory leader, contiguous and in reading phase. However, enhancers do not have to be contiguous. Linking can be accomplished by l igat ion at convenient restrict ion sites. If such sites, do not exist, synthet ic oligonucleotide adaptors or l inkers can be used in accordance with conventional practice.
[00381] Cell, cell l ine, and cel l culture are often used interchangeabl y and al l such designations include progeny. Transformants and transformed cells include the pri mary subject cel l and cultures deri ved therefrom without regard for the number of transfers. It also is understood that al l progeny may not be precisely ident ical in DNA content, due to deliberate or inadvertent mutations. Mutant progeny that have the. same function or biological activity as screened for in the originally transformed cell arc included..
ΙΪ00382] Isolated nucleic acids also are provided that encode specific antibodies, optionally operably linked to control sequences recognized by a host cel l, vectors and host' cel ls comprising the nucleic acids, and recombinant techniques for the production of the antibodies, which may comprise culiiiring the host cell so that the nucleic acid is expressed and. optional ly, recovering the antibody from the host cel l culture or culture medium.
[00383] A variety of vectors are known in the art. Vector components can include one or niore of the following: a signal sequence (thai , for example, can direct, ecret ion of the ant ibody), an origin of repl ication, one or more. selective marker genes (that, for example; can confer antibiotic or other drug resistance, complement auxotrophic deficiencies, or supply critical nutrients not available in the media), an enhancer element, a promoter, and a transcription' termination sequence, all of which are well known in the art.
[00384] Suitable host cells include prokaryote, yeast, or h igher cukaryote cells. Suitable prokaryoies. include eubacteria, such as Gram-negative or G ram-positive organisms, for example, Enterohacicriaccae. such as Escherichia, e.g., E. col i, Entcrobuctcr. Erwinia.
Klebsiella, Proteus, Salmonel la, e.g.. Salmonella typhimuHum, Serrat ia, e.g., Scrrat ia marccseans. and Shigella, as wel l as Bacill i such as B . subtilis and B . l icheniformis,
Pseudomonas, and Slreptomyces. In addition to prokaryoies. eukaryotic microbes such as filamentous fungi or yeast are suitable cloning or expression hosts for antibody-encoding vectors. Saccharomyces cercvisiae, or common baker's yeast, is the most commonl y used among lower eukaryotic host microorganisms. However, a number of other genera, species,
and strains are commonly available, such as Pichia. e.g. P. pasioris, Schizosaceharomyces pombe: Kluy.yeromyces, Y-arrowia: Candida: Tricliodcrma reesia; Neurospora crassa:
Schvvanniomyc'es such as Schwann ioniyccs occidentalis; and filamentous fungi such as. e.g.. Neurospora, Pcnicil liuiii, Tolypocladium, and Aspergillus hosts-such as A:. iiidulans and A. nigcr.
100385] Suitable host cells for the expression of glycosylated anlibodies'are derived from multicellular organisms. Examples of invertebrate cells include plant and insect cells.
Numerous baculoviral strains and variants and corresponding permissive insect host cells from -hosts such as Spodoptera frugiperda (caterpillar). Aedes aegypti (mosquito). Aedes albopiclus (mosquito), Drosophila mclanogasler (f uilfiy), and Bombyx mori have been identified. A variety of viral strains for iransfeclion of such cells are publicly available, e.g., the L-I variant of Autograplia californic ; P-V and'lhc Bm- strain ol^Bonibyx niori PV. 100386] However, interest has been greatest: iii vertebrate cells, and propagation o vertebrate cells in culture (tissue culture) has become routine. Examples of useful mammalian host cell-lines are'Chinese hamster ovary cells, including CHOK I cells (ATCC CCL61) and Chinese hamster ovary ccllsADHFR (DXB- 11, DG-44; Urlaub et al. Proc. Natl. Acad. Sci. USA 77:.4216;( 1980)) monkey kidney CV 1 line transformed by SV40 (CQS-7. ATCC CRL 1651): human embryonic kidney line (293 or 293 cells subcloned for growth in suspension culture, [Graham ct a)., J. Gen Virol.36: 59 (1977)J: baby hamster kidney cells (BHK, A TCC CGL 10); mouse Sertoli cells (TM4. Mather, Biol. Reprod.23: 243-251 (198.0)); monkey kidney cells (CV1 ATCC CCL 70): African green, monkey kiilney cells (VERO-76; ATCC CRL- 1587): human cervical carcinoma cells (HELA. ATCC CCL 2); canine kidney cells (MDCK. ATCC CCL 34); buffalo rat liver cells (BRL 3 A, ATCC CRL 1442); human lung cells (W138: ATCC CCL 75); human hepatoma cells (Hep G2. HB 8065): mouse mammary minor (MMT 060562, ATCC CCL5I): TRI cells (Mather el al.. Annals N.Y. Acad. Sci.383: 44-68 (1982)); -MRC 5 cells and FS4 cells.
[00387] The host cells can be cultured in. a variety of media. Commercially available media such as Ham's FK) (Sigma). Minimal Essential Medium ((MEM). (Sigma). RPMI- 1640 (Sigma), and Dulbccco's Modified Eagle's Medium ((DMEM). Sigma) are suitablc for culluring.lhe host cells. In addition, any of the" media described, in Hamlet al., Meth. 'Eii/-..58: 44 (1979),. B arnes et al., Anal. Biocliem.102: 255 (1-980). U.S. Pat. Nos.4.767,704;
4.657.S66; 4,927762; 4.560,655; or 5.122.469; WO90I 3430; WO 87/00195; or U.S. Pal. Re. No.30.985 can be used as culture media for the host cells. Any of these media can be supplemented as necessary with hormones and/or other growth factors (such as insulin.
transferrin, or epidermal growth factor), salts (such as sodium chloride, calcium, magnesium, and phosphate), buffers (such as■ HE PES), nucleotides (such as adenosine and thymidine), antibiotics (sueh as Gentamycin.TM. drug), trace elements (defined as inorganic compounds usually present at final concentrations in the micromolar range), and glucose or an equivalent energy source. Any other necessary supplements also can be included at appropriate concentrations that would be known to t hose skil led in the an. The culture condit ions, such as temperature, pl-l , and the like, arc those previously used with the host cell selected l or expression, and will be apparent to the art isan.
100388] The antibody composition can be purified using, for example, hydroxylapatile chromatography, cation pr anion exchange chromatography, or preferabl y affinity chromatography, using jhc antigen of interest or protein A or protein G as an affinity ligand. Protein A can be used lb purify antibodies that arc based on human .gamma. 1.. .gamma.2. or .gamma.4:heavy chains (Lindmark cl al .. .1. Immunol. eth. 62: 1 - 1 3 ( 1.983)). Protein G is recommended for all mouse isotypes and for human .gamma. (Guss el al.. 2U EM BO J . 5: 15671575 ( 1986)). The matrix to which the affinity ligand is attached is most often agarose, but other matrices are available. Mechanical ly stable matrices such as controlled pore glass or poly(siyrciicdi vinyl )benzene allo w; for faster How rates and shorter .processing t imes than can be achieved with agarose. Where the antibody, comprises a GH3 domain, the Bakerbond ΛΒΧ.ΤΜ. resin (J . T. Baker. Phillipsburg, 25 NJ .) is useful for puri ficat ion. Other techniques for protein purification such as eihanol precipitation. Reverse Phase Hl'LC,
chiOmaiol ciising, SDS-PAGE, and ammonium sul fate precipitation are also possible depending on the specific binding agent or antibody to be recovered.
1.00389] The term "epitope'' or "antigenic determinant" are used interchangeably herein and refer to that portion of an ant igen capable of being recognised and specificall y bound by a particular antibody. When the antigen is a polypeptide, epitopes can be formed both from contiguous amino acids and noncontiguous amino acids juxtaposed by tertiary folding of a protein. Epitopes formed from contiguous amino acids arc typical ly retained upon protein denaturing, whereas epitopes formed by tert iary folding arc typical ly lost upon protein denaturing. An epitope typically includes at least 3-5. and more usually, ai least 5 or 8- 10 amino acids in a unique spatial conformat ion.
(00390] "Specifically binds" to or shows "specific binding" iwoards an epitope means that the antibody reacts or associates more frequently, and/or more rapidly, and/or greater duration, and/or with greater affinity with the epitope than with alternative substances.
[003911 In ome embodiments, once the subject's tumor has been analyzed to determine whether the tumor harbors a wild type P13 K-a versus a mutant PI3 K-K, Tor example. PI3 K-a E545K or PI3 K-U H 1047R . using any one or more οΓ the assays and methods described above, a treatment, regimen can be prepared for- the subject. If (he subject's minor harbors a P13K-U having a mutation at position 1047, (for example. H I047R), the treatment regimen comprises administering to the subject a therapeut ically effective amount of a PI3 -U selective inhibitor compound, or a dual PB -u/inTOR selective inhibitor, or a combination of a PI3 K-H selective inhibitor or a mTOR selective inhibitor. If the subject's tumor harbors a Ρ.13 -Γ. having a mutat ion al posit ion 545. (for example. E545K). the treatment regimen comprises administering to the subject a therapeut ically effective amount of a combination of a P13 K-a selective inhibitor and a Ρ13 -β select ive inhibitor, a dual PI3 -a/mTOR selective inhibitor, or a combination of a PI3 - i select ive- inhibitor and a; niTOR select iye inh ibitor. Γ00392] In another embodiment, the present invention provides kits comprising materials useful for carrying. out the methods of the invent ion. The diagnost ic/screening procedures described herein may be performed by diagnostic laboratories, experimental laboratories, or practitioners. The invention provides kits which can be used in these different sett ings.
[00393] Basic materials and reagents required for identifying a Ρ1 Κ-α mutation in a subject's tumor or cancer according to riicthods of lhe present invention may be assembled together in a kit. In certain embodiments, . the kit comprises at least one PI3 K-a amino acid sequence determining reagent that specifically detects a mutation in a nucleic acid or protein obtained from a subject 's tumor disclosed herein, and instructions for using the k it accordin to one or more methods of the invention. Each kit necessarily comprises reagents which render the procedure speci fic. Thus, for delecting inRNA harboring the PI3 K-U H I 047R or E545 mutation, the reagent wil l comprise a nucleic acid probe complementary to niR NA, such as. for example, a cDNA or an oligonucleotide. The nucleic acid probe may or may not be immobil ized on a substrate surface (e.g., a niicroarray). For detecting a polypeptide product encoded by at least one PDK-a.mutat ion gene, the reagent wil l comprise an anlibody that. specifically . binds to . the mutated PI3K-rx .or a wild-type PI3K-(i.
[00394] Depending on the procedure, the kit may further comprise one or more of:
extract ion bu ffer and/or reagents, amplificat ion bu ffer and/or reagents, hybridization buffer and/or reagents, immunodetect ion buffer and/or reagents, labeling buffer and/or reagents, and detection means. Protocols for using these buffers and reagents for performing difl'ereni steps of the procedure niay also be included in the kit.
1003951 Reagents may be suppl ied in a solid (e.g.. l yophilizcd) or l iquid form. Kits of the present invention' may -optionally comprise one or more receptacles for mix ing samples and/or reagents (e.g.. vial, ampoule, lesi l ube. LISA plate, culture plate, flask or bottle) .for each individual buffer .and/or reagent. Each component will generall y be suitable as aliquoied in its respective-container or provided in a concentrated form. Other containers suitable for conduct ihg" certain steps for the disclosed n hodsunay-also be provided'. The individual containers of the kit are preferably maintained in close confinement for commercial sale. 100396] In certain - embodiments-, the kits of the present; invent ion furthepcOmprisc control samples. For example, a kit may include samples of total: mR NA derived from tissue, of various physiological states, such as. for example, wild-type PI3 K-U. I3 K-H H I 047R m'R A or ΡΙ3 -α E545K niRNA to be used as controls. In other embodiments, the i nvent ive kits comprise at least one prostate disease e.xpression .prol'ile map as described herein for use as comparison template. Preferably, the expression profile map is digital informat ion stored in computer-readable medium;
[00397] Instructions for using the kit according to one or more methods of the invention may comprise instructions for processing the prostate tissue sample and/or perforin in the test, instructions for interpret ing the results as well as a notice in the form prescribed by a governmental agency (e^g.. FDA ) regulat ing the manufacture, use or sale of pharmaceuticals or biological products.
Representative Compounds
100398] Representative compounds of Formul I arc depicted in the following tables: The examples are merely il lustrati ve and do not limit the scope of the invent ion in anyway.. Compounds of the invention arc named according to systematic appl icai ion' of the nomenciaiure rules agreed upon by the International Union of Pure and Applied Chemistry ( iUPAC). International. Union of B iochemist ry and Molecular Biology ( lUB M B). and the Chemical Abstracts Service (CAS). Specifical l , the names in the tables below were generated using ACD/Labs naming software 8.00 release, product version 8.08 or later. 100399] In one embodiment, .compounds of the invent ion are l isted below.
100400] In one embodiment, compounds of the invention arc l isted in Table I
Table I
(00401 J In other cmbodiinents. the coinpoiinds.or ihe invention include the. compounds depicted below:
ı73
ı74
ı75
ı76
181
I S2
ı83
100402] Useful Intermediates:
tctrahydfo- 1 ,4-bcnzoxazcpine; 4.- { 4-| 6.7-/> .v(mcthyioxy)quinol^^
l
t4-he!izoxa/x-piii-7-yl ) -2-iuLi aniIine: 4-
lctrahydro- 1 ^-bcnzoxazepin^-yl }
'benzene- 1 .2-diaminc: Λ'-| 5-(4- j 5-| (4 - fluoiOphenyDmelhyl |-(vmcthylpynmidine-4-yl J -2.3.4.5-leirah ydro- i .4
1 .3-Uiiazol-2 yl |acetaniide; 7-bi mq-4- (:5-| (4-l1uoropl)enyl)mcibyl | - β- 1 viel li y I p r i ιτι i tl i n -4 -
2,3.4.5-tcirahydro- l ,4-bcnzoxazepinc: 7-bronio-4-| 6-(niediyloxy)c|uinazol in-4-yl |-2.3,4.5- tctnihydro-.l ,4-bcnzoxazcpinc.
General Administration
[004031 In one aspect, the .invention provides pharmaceutical eohiposiliohs coiiiprising an inhibitor of PI3 and/or niTOR according to the invention and a phai inaccia ically-accepiable carrier. excipi.enl . or diluent. In certain other specific embodiments, administrat ion is by the oral route. Administration of the compounds of the invention, or their pharmaceuticall y acceptable sails, in pure form or in an appropriate pharmaceutical composition, can be carried out via any of the accepted modes of administration or agents for serving similar uti l it ies. Thus, administration can be. for example, orally, nasally, parenieral ly ( intravenous, intramuscular, or subcutaneous), topically, transdcrmally, ihiravaginally. intravesical jy, intracislemally, or rectal ly. in the form of solid, semi-sol id, lyophilized powder, or l iquid dosage forms, such as for example, tablets, suppositories, pills, soft clastic and hard gelatin capsules, powders, solutions. 'suspensions, or aerosols, or the l ike, specifically in unit dosage forms suitable for simple administration of precise dosages.
1,00404] The compositions will include a convent ional pharmaceut ical carrier or excipienl and a Compound of the invention as the/an active agent, and. in addition, may include carriers and adjuvants, etc.
100405] Adjuvants include preserving, wett ing, '.suspending, sweetening, flavoring, perfuming, emulsifying, and, dispensing agents. Prevention opthe action of microorganisms can be ensured by various ant ibacterial and antifungal agents, for example, parabeiis.
chlorobutanol. phenol, so bic acid, and the like. Ii may also be desirable lo include isotonic agents, for example sugars, sodium chloride, and the l ike. Prolonged absorpt ion of the injectable pharmaceutical form can be brought about by die use of agents delaying absorption, for example, aluminum monostearatc and gelat in.
100406] If desired, a pharmaceutical composition of the invention may also contain minor amounts of auxiliary, substances such as. welting or emulsifying agents. pl-I buffering agents,
anl iox.icl.inis, and the like, sueli as. for example, cilrie acid, sorbilan monolauralc, triclliaiiolami e oleale, luilylalted hydroxytolucne. etc.
1.004071 The choice of lOrmulal ion depends on various factors such as the mode of drug administration (e.g.. for oral administration, formulations in the form of tablets, pills or capsules) and the bioavailabil ity of (lie dru substance, Recently, pharmaceut ical formulations have been developed especially for drugs that show poor bioavailabil ity based upon the principle that bioavai labil ity can be increased by increasin the surface-area i.e., decreasing particle size. For example, U.S. Pat . No. 4.107.288 describes a pharmaceutical formulation having panicles in the size range from 10 to 1 .000 nm in which the active material is supported on a crossl inked matrix of macromolccules. U .S. Pat. No. 5. 145,684 describes the production of a pharmaceutical formulation in which the drug substance is pulverized to nanopariicles (average particle size of 400 nm) in the presence of a surface modifier and then dispersed in a l iquid: medium to give a pharmaceutical formulat ion that exhibits remarkably high bioavailability.
[00408] Compositions suitable for parenteral injection may comprise physiological ly acceptable sterile aqueous or nonaqueous solutions, dispersions, suspensions or emulsions', and sterile powders for reconst ilut ion into steri le injectable solut ions or dispersions.
Examples of suitable aqueous and nonaqueous carriers, diluents, sol vents or veh icles include water, ethanol, polyols (propyleneglycol. polyclhylcneglycol . glycerol, and the l ike), suitable mixtures thereof, vegetable oils (such as olive oil) and injectable organic esters such as ethyl oleale. Proper fluidity can be . maintained, for exampje, by the use of a coating such as lecithin, by the maintenance of the required panicle size in the case of dispersions and by the use of surfactants.
| 0()40l/| One spccific route of administration is oral, using a convenient dai ly dosage regimen that can be adjusted according to the degree of severity of the disease-state to be treated.
100410] Solid dosage forms for oral administration include capsules, tablets, pil ls, powders, and granules. In such sol id dosage forms, the acl ivc Compound is admixed with al least one inert customary excipient (or carrier) such as sodium citrate or dicalciuni phosphate or (a) fillers or extenders, as for example, starches, lactose, sucrose, glucose, mannitol, and silicic acid, (b) binders, as for example, cellulose derivatives, starch, alignates. gelatin, polyvinylpyrrolidone, sucrose, and gum acacia, (c) humeciants. as for example, glycerol, (d) disintegrating agents, as for example, agar-agar, calcium carbonate, potato or tapioca starch, alginic acid, croscarnicllose sodium, complex silicates, and sodium carbonate, (e) solution
ictiirtlci s. as for example paraffin, (T) absorption accelerators, as for example. quaternary ammonium -compounds, (g) welling agenls. as for example, cclyl alcohol, and gl ycerol monosiearale. magnesium stearaie and the like (h) adsorbenis. as for example, kaolin and bentonitc, and (i) . lubricants, as for example, laic, calcium stearaie, magnesium siearaie. sol id polyeihylcne glycols, sodium lauryl sulfate, or mixtures thereof. In the case of capsules, tablets, and pills, the dosage forms may also comprise buffering agcnis.
[00 11 1 Sol id dosage forms as described..above' can be prepared. ith eoalirigs-.and shells, uch as ciueric coat ings and others well known in the; art. They may conlain pacifying agcnis, and can also be of such composit ion thai they release the active Compound or compounds in a certain part Of the intest inal tract in a delayed manner. Examples of embedded compositions that can be used are polymeric substances and waxes. The active compounds can also be in microencapsulated form, if appropriate, with one or more of the above-mentioned excipicnis.
[00412) Liquid dosage forms for oral administration include pharmaceutically acceptable emulsions, solutions, suspensions; syrups, and elixirs. Such dosage forms arc prepared, for example, by dissolving, dispersing, etc.. a compound(s);of the invention, or a
pharmaceutically acceptable salt thereof, and optional pharmaceutical adjuvants in a carrier, such as. for example, water, sal ine, aqueous dextrose, glycerol, eihanol and the like;
solubi l izing agenls and emulsifiers. as for example, ethyl alcohol, isopropyl alcohol, eth yl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoale. propylenegl yeol.
1.3-biityleneglycol. d imethylformamide; oi ls, in part icular, cottonseed oi l . groundnut oi l . corn germ oil . olive oil, castor oil and sesaine oil. glycerol, letrahydrofurfuryl alcohol, polycthyleneglyeols and fatty acid esters of sorbiian; or mixtures o these substances, and the like, to thereby form a solution or suspension.
[004.13 ] Suspensions, in addit ion to the active-compounds, may contain suspending agents, as for example, ethoxylaied isostearyl alcohols, polyoxycihylcne sorbitol and sorbitan esters, microcrystal l inc cel lulose, al uminum mciahydrox idc. beiuoniie. agar-agar and tragacanth. or mixtures of these substances, and the l ike.
[004141 Composit ions. for rectal administrations are. for example, suppositories that can be prepared by mixing the compounds of the present invention with for example suitable non- irritating excipicnis or carriers such as cocoa butter, polycihylencglycol .or -.suppository- wax. which are solid al ordinary temperatures but liquid al body icmperalurc and therefore, melt while in a suitable body cavity and release the active component therein.
[00415) Dosage forms for topical administration of a Compound of this invention include ointments, powders, sprays, and inhalants. The active component is admixed under sterile
1.86
conditions with a physiological ly acceptable carrier and any preservatives, buffers, or propellants as may be rec|tiired. Ophthalmic formulat ions, eye oint ments, powders, and solutions arc also contemplated as being with in the scope of this invent ion.
100416] Compressed gases may be used to disperse a Compound of this invention in aerosol form. Inert gases suitable for this purpose are nitrogen, carbon diox ide, etc.
100 17] Generally, depending on the intended mode of adniinisi raiion, the
pharmaccuiicaHy acceptable compositions will contain about 1 % lo about 99% by weight of a eompound(s) of the -invention, or a pharmaceutically acceptable sail ihcreol", and 9.9% lo 1 % by weight of a suitable pharmaceutical excipieni. In one example, the composition will be- between about 5% and about 75% by weight of a conipound(s') of the invention, or a pharmaccuiicaHy acceptable salt thereof, with the resi being 'suitable pharmaceut ical excipicnls.
[00418] Actual methods of preparing such dosage forms are known, or wil l lie apparent, to those. skilled in this art: for example, see Remington's Pharmaceutical Sciences, 1 SUV Ed., (Mack Publishing Company. Easion. Pa.. 1990). The composition lo be administered wil l , in any event, contain,^ therapeuticall y effective amount of a Compound of the invention, or a pharmaceut ically acceptable salt thereof, for treatment of a disease-state in accordance with the teachings of this invention.
[00419] The.eompounds of the invention, or their pharmaceutically acceptable salts or solvates, are administered in a therapeutically effective .'.amount which will vary depending upon a variety of factors including the act ivity of the specific Compound employed, ihc metabol ic stability and length of act ion of the compound, the age. body weight, general health, sex , diet, mode and l ime of adniinisirai ion. rale of excret ion, drug combinai ion, the severity of the particular disease-states, and the host undergoing therapy. The compounds of the present invention can he administered to a patient at dosage levels in the range of about 0. 1 to about 1 ,000 nig per day. For a normal human adult having a body weight of -about. 70 kilograms, a dosage in the range of about 0.01 to about 100 m per kilogram of body weight per day is an example. The specific dosage used, however, can vary. For example, the dosage can depend on a number of factors including ihe requ irements of ι he patient, the severit y of (he condition being treated, and the pharmacological activity of (he Compound being used. The determination of optimum dosages for a particular patient is wel l known lo one of ordinary skill in the art.
[004201 I formulated as a fixed dose, such combinat ion products employ the compounds of this invention within ihe dosage range described above and ihe other pharmaceutically
active ageni(s) within its approved dosage range. Compounds of the instant invent ion, niay alternatively he used sequentially with known pharmaceutical l y acceptable ageni( s) when a combination formulation is inappropriate.
General Synthesis
[0()421j Compounds of this invent ion can be made by the synthetic procedures described below. The starting materials and reagents used in preparing these compounds are either available from coiiimcreiaksuppi ifcrs. such as Aklrich Chemical Co. ( i lwaukee. Wis.), or Baehem (Torrance, Calif.). Or arc prepared by methods known to those .skilled in the art following procedures set forth in references such as Fieser and Fieser's Reagents for Organic Synthesis. Volumes 1 - 1 7 (John Wi ley and Sons, 1 91 ): odd' s Chemistry of Carbon Compounds; Volumes 1 -5 and Supplementals ( Elsevier Science Publ ishers, 1 ,89): Organic Reactions. Volumes 1 -40 (John Wiley and Sons. 1991 ). March's Advanced Organic Chemistry. (John Wiley and Sons. 411' Edition) and Laroek' s . Comprehensive Organic Transformations (VCW Publishers inc., 1989). These schemes arc merel il lustrative of some methods by which the compounds, of this invent ion can be synthesized, and various modifications to these schemes can be. made and will be suggested to one skilled in the art having referred to this disclosure. The starting materials and the intermediates of the reaction may be isolated. and purified i f desired using conventional techniques, including but not limited to filtration, distillation, crystal l ization, chromatography and the l ike. Such materials may be characterized using conventional means, including physical constants and spectral data.
[00422] Unless specified to the contrary, the reactions described herein lake, place at .atmospheric pressure and over a temperature range from about -78 "C to about 150 "C, more, speci fically from about 0 "C. to about 125 "C and more specifically at. about room (or ambient ) temperature, e.g., about 20 "C. Unless olherwisc stated (as in the case of an hydrogenation). all reactions arc performed under an atmosphere of nitrogen.
[00423.1 Prodrugs can be prepared by techniques known to one skilled in the art. These, techniques generally modify appropriate functional groups in a given compound. These modi fied functional groups regenerate original functional groups by routine manipulation or in vivo. Amides and esters of the compounds of the present invention may be prepared according lo convent ional methods. A thorough discussion of prodrugs is provided . in T. Higuchi and V. Stella, "Pro-drugs as Novel Del ivery Systems.*' Vol 14 of the A.C.S.
Symposium Series, and in B iorcvcrsiblc Carriers in Drug Design, ed. Edward B. Roche.
American Pharmaceutical Associat ion and Pergamon Press. 1 87. both of which are incorporated herein by reference for al l purposes.
[004241 The compounds of the 'invention, or their pharmaceutically acceptable salts, may have asymmcirie carbon atoms or qtiaiernked niirogen atoms in their st ructure. Compounds of the Invention may ex ist as single stereoisomers, raccmates. and as mixtures of enani iomers and diastcreoniers. The compounds may also ex ist as geometric isomers. Al l such single stereoisomers; raccinates .and mixtures thereof, and geometric isomers arc intended to be within the scope of this invent ion.
[004251 Some of the .compounds of the invent ion coniai a active kclone -QO)CF-, and may exist in pin t or in whole as the -C(OH2)CF,¾ form. Regardless of whether the Compound is drawn as the -G(0)CF;¾ or -C'fOi-U -F form, both arc included within the scope of the Invent ion. Although an individual Compound may be drawn as the -C(0)CF;v form, one of ordinary skill in the art would understand that the Compound may ex ist in part or in whole as Llic -QOI-hjCFj form and that the rat io of the two forms may vary depending on the
Compound and the condit ions in which it exists.
[004261 Some of the compounds of the m^cnU n^na- esisUa^ lautomers; For example, where a ketone or aldehyde is present, the molecule may ex ist in the cnol form: where an amide is.preseril. the molecule liVay exist as ihe imidic acid; andVhcrc an enamitie is present, the molecule may exist as an inline. Al l such lautomers .ai e within the scope of the invention. Further, for example; in this application R
1 can be 5-oxo- 1 /7- 1 .2.4-t riaz.ol-3-yl . depicted structurally
5-oxo- 1 H- 1..2.4-iriazolr3-yl and the structure 100
hydroxy-pyrimiclin-5-yl and the structure 101 include, and are equivalent to, N-ox ide of 2-
amino-pyrimidin-5-yl aiid its .structure 2 1:
structure or which terminology is used, each laiiiomcr is included within the scope of the Invention.
[1)0427] The present invention also includes N-ox ide derivatives and protected derivat ives of compounds -of the Invention. For example, .when compounds of the Invention contain an ox idizable nitrogen atom, the nitrogen atom.can be converted to.an N-oxide by methods, well known in the art, When compounds of the Invention contain groups siich. as hydroxy, carboxy, thiol .
'or: any group containing a nitrogen atOin(s)i lhe e-grdiips. ait bS
iproteeted
a suitable "protecting group
" or "protect ive group
". A comprehensive l ist of suitable protective groups can be found in T.W. Greene. Protective Croups in Organic Synthesis. John Wiley & Sons, Inc. 1991 , the disclosure of which is incorporated herein by reference in its entirely. The protected derivatives of compounds of the Invention can be prepared by methods well known in the art.
100428] Methods for the preparation and/or separat ion and isolation of. single
stereoisomers 'from racemic mixtures or non-racemic mixtures of stereoisomers arc well known in the art. For example, opt ical ly active (R )- and (S)- isoniers may be prepared using chiral synthons or chiral reagents, or resolved using conventional techniques. Enantiomers ( R- and S-isomers) may be resolved by methods known to one of ordinary sk i l l in the art. for exaniple by: formation of cliastereoisomcricsalis or complexes which may be separated , for example, by crystall ization; via formation of diastereoisomeric derivatives which may be separated, for example, by crystallization, selective, reaction of one enanliomcr w th an eiKiiitibnicr-specific reagenl, for example enzymatic oxidatioiKor reduction, followed by separation of the modified and unmodified enantiomers; or gas-liquid or l iquid
chromatography in a chiral environnienl, for example on a chiral support, such as silica with a bound chiral ligand or in the presence of a chiral solvent. It will be appreciated that where a desired enanliomcr. is convened, into another chemical entity by one of the separation procedures described above, a-further step may be required to l iberate the desired
enantiomeric form. Alternat ively, specific enanl iomcr may be synthesized by asymmetric synihesis using optically active reagents, substrates, catalysts or solvents or by converting on enantioincr to the other by asymmetric transformation. For a mixture of enantiomers, enriched in a particular enanliomcr. the major component cnantibmer may be further enriched ( with .concomitant loss in yield) by recryslallizaiion.
1.004291 hi addition, the compounds of the present invention can exist in unsolvatcd as wel l as solvated forms with pharmaceutical ly acceptable solvents such as water, cthanol , and the
like. In general, the solvaied forms are considered equivalent to the nsolvalcd forms for the purposes of the present invent ion.
j 1)0430 J The chemistry l or the preparation of the compounds of th is invent ion is known to those skilled in the art . In fact, there may be more than one process to prepare the compounds of the. invention. The following examples i llustrate but do not l imit the invention. Al l references cited herein . arc incorporated by reference -in their entirely.
|'0043l] " An ihlcnnedi'aie of foniriila 4 where PC is a hitTOgen-protcciing group, R5:i and
5lv
are independently hydrogen or alkyl. R is hydrogen or halo. R31> is (C i alkyl, and R > ,5uJ
R5 . R5 ', and R'1£ are hydrogen can he prepared accord ing to Scheme 1 .
Scheme 1
100432] In part icular, an intermediate of formula 4a can be prepared according to Scheme l a.
Scheme la
[00433] An intermediate of formula 2a where R5 | is hydrogen or methyl is commercially available. The intermediate of formula l a is treated with an intermediate of formula 2a in the presence of a reducing agent such as sodium borohydi idc. in a solvent( s) such as tcirahydrofuran and/or methanol and al lowed to react at a temperature of about 40 °C for approximately 4 hours. The solvent is then removed and the reaction is taken up in a .solvciu(s) such as ethyl acetate and/or saturated sodium bicarbonate. To this suspension' . nitrogen-protecting group precursor, such as di-/e 74iulyl dicarbonatc, is added and the mixture is allowed to stir at room. lemperature overnight to yield an intermediate of formula 3a where PG is a nitrogen-protect ing group.
I 00434J Intermediate 3a is then treated wit h a catal yst , such as triphcnylphosph ine. in the presence of a dehydrating agent such as diisopropyl azodicarboxylate. in a sol vent such as DCM. The -react ion is allowed to proceed at room temperature for approximately 12 hours
and the resulting product is opiionally puii ricd by column chromatography to yield an intermediate ol' fomuiia 4a. Alternat ivel , the intermediate of formula 4a can be prepared by treating the intermediate of formula 3a with Burgess' reagent.
[00435] An intermediate of formula 5 where each R is hydrogen or bot h R \s when taken together form a cycl ic boronic ester. PG is a nit rogen-protecting group. R^' and R are independently hydrogen or alkyl . R5" is hydrogen or halo. R5h is (C , .;,)alkyl . R5c. R5'. and Rsp are hydrogen, and R 1 is as defined in the Summary of the Invention for a Compound of Formula 1 can be prepared according to Scheme 2.
Scheme 2
where, the intermediate of formul 4 is prepared as described in Scheme I .
100436] In particular, an inlermed inle f formula 5a where 5:i is hydrogen or alkyl. R51' is hydrogen or halo, R31' is (Cj. jalkyf and R 1 is as defined in the Summary of the Invention for a Compound of Formula I. can be prepared accord ing to Scheme 2a.
5a
The intermediate of formula 4a, prepared as described in Scheme l a. is treated with a boronic
acid of formula R ' l3(OH or
which are commercial ly available or can be prepared using procedures known to one of ordinary skil l in the art . The reaction is carried out in the presence of a catalyst such as PdfdppfhCl:. a base such as potassium carbonate, and in a solvent such as D E at about 80 °C for about 2 hours. The product can then be purified by chromatography to yield an intermediate of formula 5a.
[004371 Alternatively; an intermediate of formula 5. as defined above, can be prepared as described in Scheme 4.
1 4
[00438] In particular, an intermediate of formula 5b where-PG is a niirogcn-protcciing group and R 1 and R51' arc as defined in the Summary of the Invention for a Compound of Formula 1 can be prepared according to Scheme 4a.
An iiHenncdi;a.le:0f formula I 3, where PG is a nitrogen-protecting group, is. prepared as described in Scheme l . .13 is treated with IriisopiOpylborate in a solvent such as THF at a temperature of about -60 °C, followed by dropwisc addit ion of a base such as /i-luiiyli iihium in leirahydrofuran. The reaction was al lowed to proceed for about 30 minutes, was treated with an acid such as hydroch loric acid, and al lowed to warm to room temperature to yield an intermediate of formula 14a. Intermediate 1 a is then treated with an intermediate of formula R ' X (where X is a halide. and wh ich is commerci lly available or-.can be prepared using procedures known to one of ordinary skill in the art), in the presence of a base such as potassium carbonate, in the presence of a catalyst such as
tclrakis(i riphcnylph sphine)pal ladium(O). and in a solveni(s) such as 1 ,2-dimelhox yethane and/or water. The reaction is al lowed lo proceed under nitrogen and sl irred at reflux for about 3 hours to yield an intermediate of formula 5b.
[00439] In particular, a Compound of the Invent ion where Y is =CH- or =N-. R
5 '. R
'11. R
>d, R
JC, R
if ? R
5l\. and R -
1' are hydrogen: R
1 is benzimida/.ol-6-yl substituted at the 2-posiiion with One R
7: R
7 is alkyl; R" and R
51' and all other groups arc independently as defined in the Summary of the Invention fo a Compound of Formula I. can be prepared accordin
'g«t
'c> Scheme 6a.
The nitro.of the intermediate of formula 17a. prepared as described above in Scheme 4. is reduced in (he presence of H- and palladium on carbon in a solvcnt(s) such as nethanol and/or acetic acid to yield an intermediate of formula I S;t. The inter edia'ie -of formula 18a i then treated with an intermediate of formula R7C(0)OH. ;iivllie presence of a.coiipling agent such as HATU, in the presence of a base such as DIEA. in a sol vciu(s) such as DMF and/or acetic acid. The product can be purified by column chromatography to yield a Compound -of Formula I(x).
[00440] A Compound of the Invention of Formula I where R3:i and R are independently hydrogen or alkyl. R l is hydrogen or halo, is (C|.; alkyl. R5 , R5f, and R5t: are hydrogen, and R 1 and R" are .-independently as defined in the Summary .of the' Invention for a Compound of Formula 1 can be prepared as described in Scheme 5.
Sc
w here X is halo or hydroxy.
[00441] In particular, n Compound of Formula l(w) where RSa is hydrogen or alkyl. R5h is hydrogen or halo, R51' is (Ci.3-)a1kyl, and 1 and R2 are independently as defined in the Summary of the Invention for a Compound of Formula I can be prepared as described in Scheme 5a.
Scheme
The protecting group on the iiucrmediaic of formula 5a is removed. When the protecting group is Boc. it can be removed with HCl lo yield an intermediate of formula 6a. The intermediate of formula R2X (where X is a leaving group such as halo) is commercially
available or can be prepared using procedures described herein or procedures known 10 one or" ordinary skill in the art. The intermediate o 'formula 6a is then treated with R2X. in the presence of a base such as Hiinig's base or'NMP. in a solvent such as.DMF. at a temperature of about 50 °C. The product can be purified by column chromatography to yield an intermediate. of Formula l(w).
1004421 In particular, a Compound of Formula 1(a) where R . R~. and R' are
independently as defined in the Summary of the Invention for a Compound of Formula I can
The protecting group on intermediate of formula 5b, prepared as described in Scheme 4a. is removed. When the protecting group is Boc, it can be removed with HCI to yield an intermediaie-of formula 6b. Intermediate 6b is tlien treated with- n inicrmediaicsof formul R"X where X is a leavin group such as hale tisiiig stiiikla'rd alkylating conditions to yield a Compound of Formula 1(a).
[00443] A Compound of -Formula l(aa) whore one of Y| and Y> is =CH- and the other is = -, R1 is benzimidazol-6-γΙ substituted at the 2-posiiion with one R ': R51'. R ' and R" are independently as defined itt the Summary of the Invention for a Compound of Formula I can be prepared according to Scheme 6a using conditions known to one of ordinary skill in the
100444) An intermediate of formula 17 is prepared by I ) treating an intermediate of formula 14a. prepared as described in Scheme 4a. with an intermediate of formula
X where X is halo using standard Suzuki' coupling- conditions: 'followed by 2) ' treating the with and intermediate of formula R"X using. standard alkylating conditions. 17 is
then Irydrogcnaicd in tlie presence: of palladium on carbon in-aispivcnl sueh as- acetic acid .to yield the intermediate of formula 1 8. 1:8 is then treated with an acid olM'ornuila R 'C(0)OH to yield the Compound of Formula l(aa).
100445] Alternat ively, a Compound ol* Formula I(aa') can be prepared according to Scheme 6b.
Sclieme
The intermediate of formula I S is treated with an .intermediate of formula 23 in the presence of glacial, acetic acid, optional ly in the presence of irielhyl orthofoi nuiic. and heated to yield an a Compound of Formula l(aa).
[0044ti | A Compound of Formula I( v) where R^ and R51' arc as defined in the Summary of the Invention for a Compound of Formula I can be prepared according t Scheme 7a, Scheme 7a
The Compound of Formula I(u) where R is alkyl. prepared using procedures according ιο Scheme 5b, is treated with a base such as LiOH, in a solvcnt(s) such as TI-I F and/or water to yield the hydrofyzed Compound o Formula l(y),
[004471 A Compound of Formul !( .) where R
2.
R
s. and R
X:1 are independently as defined in the Summary of ihe Invention for a Compound of Formula I can be prepared according to Scheme 7b.
Schem
The Compound of Formula l(v l ) where X is halo or hydrox y can be prepared according to Scheme 7a or prepared by making the acid chloride from. a Compound of Formula l(v). The
Compound of Formula I(v l ) is then treated with an amine of formula HR^R ' optionally in the presence of a base such as DIEA in a solvcnl such as THF to yield a Coiiipoiind of Formula l(z).
100 81 A Compound of Formula I where R 1. R2. R3:'. RM'. RSc. R5U, R5", R5'. R5i\ and Rs" arc as defined above can he prepared according in the following scheme (where R is - 13(01-1 )? and Y is halo, or R is halo and Y is -13(01-1)2) using Suzuki coupling procedures known to one of ordinary skill in the art.
Scheme 8
[00449 J In particular, a Compound of Formula 1(a ) where R 1. R5b. and R" are
independently as defined in ihe Summary of the Invent ion for a Compound of Formula I can be prepared as described in Scheme 8a.
Scheme ttsi
An intermediate of formula 19 (where each R is hydrogen or the two R ' s together forma boronic ester), which can be prepared by fol lowing step I of Scheme 4a and subsequent depi'Qlcction, is treated wiih an intermediate of formula R3X in a solvent such as dioxahe/l- O and in the presence of a base such as DI PEA. The resulting mixture is heated to about 90 °C to yield an intermediate of formula 20. 20 is treated with an intermediate of formula R' X where X is halo and R1 is as defined in the Summary of the Invention for a Compound of Formula I in a solvent such as DM F/watcr. in the presence of a base such as D I F.A. in the presence of a catalyst such as ( 1 .1 '-b is( diphenylpli(isph ino)l'ern)cene |dichloiOpal ladiuni( I I ). The reaction is heated to about 95 "C. 20 is then opt ionally purified to yield a Compound of Formula 1(a).
I00450J Alternatively, a Compound of Formula 1(a) where R 1 . R51', and R" arc
independently as defined in the Summary of the Invention for Compound of Formula 1 can be prepared as described in Scheme Sb.
Scheme 8b
An intcrmediaie of formula 21 where Y is halo, which can be prepared by following Scheme l a followed by deprplcclion, is treated with an intermediate of formula R~X where X is halo, a base such as D I EA in a solvenl such as 1 -biilanol and heated to yield an intermediate of formula 22. 22 is then treated with an intermediate of I rnuila R ' B.(0R )_ (where each R is hydrogen or the two R together form a boronic ester), in the presence of a base such as potassium carbonate and in the presence of a catalyst such as dicbloro| l . l -/? .v(diphcuyl- phosphino.|l'errocenepalladium ( I I ) dichloromcihane adduci in a solvenl such as
dimcthoxyclhanc/water. The reaction was heated and yielded a Compound of Formula 1(a).
S v t H c t i c E x m p I cs
[00451] STEP I : A solution of methyl 2-amino-5-bromo-4- mcthoxybenx.oaie (75 nig. 0.29 mmol) and ammonium formate (38 mg, 0.8 mmol ) in formamide ( I inL) was heated at 165 "C for 18li. The mixture was allowed to cool to room temperature then dilulcd with an excess of waler. The solid formed was collected by filtration aiul washed with water then cihyl acctaic and dried to give 6-broirK>-7-mcthox yquinazolin- (3/-A)-onc (53 mg, 72% yield) as a pale yellow sol id. MS (EI) for
255, 257 ( IT
1).
[ 1)0452] STEP 2: 6-biOmo-7-melhoxyc|uinazol in-4(3//)-onc (53 mg, 0.2 1 mmol) was taken into thionyl chloride ( 1 .5 mL) followed by addition of catalyt ic DM F. The mixiure was lieated lo 80 "C for 2 h then conceniraied. The residue was .part itioned with ethyl acctaic and saturated aqueous sodium bicarbonate. The organic phase was washed with brine then dried over anhydrous sodium sulfate, filtered and conceniraied to give 6-bromo-4-chloro-7- mcthoxyquinazoline (36 mg, 62 % yield) as a brown sol id. MS ( EI) for Cul-li.BrCI NaO: 275 (Μ Ι-Γ).
[00453] Using analogous synthetic techniques and substituting with alternative starl ing reagents in step 1 the following reagents were prepared.
['004541 4-cliloro^7-(inclliyl.stillOnyl)quin;i/.nliiic..Synlhesizcd according lo the method of reagent preparation I using 7-(methylsiil(onyl)quinazolin-4(3/-/)-one in step 2. Ή NMR ('400 MM/., d(,-DMSO): 8.36 (d. I I I), 8.34 (s, 11-1).8.1.8 (d. I H), S02 (dd. III).3.36 (s.31-1).
[004551 4.7-dkhloro-6-iodoqiiinazolinc. Synthesized according to the meihod of reagent preparation I using methyl 2-amino-4.-chkm>- iodobenzoalc in ste I. MS (ET) for
CsH;.CI I .: 325 (Mi l").
[004561 4-chloiO-6-iodo-8-meihylquinazoline. Synthesized according to the meihod of reagent preparation 1 usin 2-amiiio- iodo-3-ineihylbenzoic acid in step 1. MS (El) for C..,l-I(,Gil v: 305 (Ml-f ).
[00457 J 4-ehloro-6- plicnyhnetlH)xyjH|iiiiKizol n frepared according to the melhotl of reagent preparation I Using 2-ainitio-5-beiizylo .bcnzoic.acitl nieth l csfeii(J. Qig. Chein. 2001, 66(8), 2784-2788) in step I. MS (EI) for CI5H,|C1N20: 271 ( l ).
[00458] 4.6-dicliloro-7-methoxy-quinazoline. Prepared according to the method of reagent preparation 1 using 5-chloro-4-methoxyaiuhranilic acid (US 80-126838) in ste I. MS (EI)
[00459] 4-chloro-7.8-dinietlu)xy-{iuinazoline, Prepared accordin to the meihod of reagent preparation I using 2-amino-3,4-dimethoxybcivzoic acid methyl ester (US 4287341) in step 1. MS (El) for G, I¾GlN2€).: 225 IV).
[00460] 7-(bcir/.yloxy)-4-chlor()-8-ntelhoxyt|uinazolinc. Prepared according to live method of reagent preparation 1 using 2-anvino-3-nieih¾xy-4-(pheny;lmelhoxy)benzoic acid ( J . Med. Chem.1992.35(14), 2703-10) in step 1. MS (El) for C,eH.|jCIN:0_: 301 MM*).
[00461] 4,6-dichloiO-7.8;dimelhoxyquina.olinc. Prepared according to the method of reagent preparation 1 using 2-amino-5-chloro-3.4-dimelhoxybcnzoic acid (US 42873 1 ) in step 1. MS (El) for C,r,HsCbN202: 260 ΜΙ-Γ).
[00462] 6-biOmo-4.7-dichloro()uinazolinc. Synthesized according to .the meihod o reagent preparation 1 by using 2Hunino-543romp.-4-chlor0bcnzoie acid in step 1. MS (EI) for
Gxl-l3BiChN2: 277. (MH").
[00463] 4-chloro-6-iodo-7-nieihoxyquina/oline. Synthesized accordin to the method of reagent preparation I by Λ'-iodosuccinimide iodinalion of methyl 2-amino-4- inethoxybe zoate to give methyl 5-iodo-2-amino-4-methoxybenzoaie then proceeding with step 1. Ί-Ι NMR (400 MHz. CDGh): 8.97. (s.11-1).8.75.7.31 (s.1H), 4.08 (s.3H). GC-MS forC9l-l()CIIN20: 319 (Μ ').
[00464] 7-lKpnio-4-clvior'o-8-iTicihoxyc|uinazoline and 7-biOino-4-chloi .-6- meihoxyquinazbline. Synthesized according to the method of reagent preparation 1 by
nitrat ion anil hydrogenalion of methyl 4-liromo-3-iiicthoxybenzoate to give a separable mixture of methyl 4-biOmo-3-meilioxy-2-aininobetv oaic and methyl 4-bromo-5-mei hoxy-2- aminobehzontc then proceeding with step I individually. 7-bromo-4-ch loro-8- methoxyquinazol ine: Ή NMR (400 M Hz. CDCI3): 9.09. (s. I I I). 7.92 (d. I l-l). 7.87 (d. 1 1-1 ). 4.21 (s. 3H). CC-MS for CyHcBrCI iO: 272 (M+). 7-bronio-4-chloro-6-mcthoxyquinazoline: Ή NMR (400 MH/.. CD.CI3): 8.95, (s. I IT), 8.40 (d, 1 1 1). 7-45 (d, I H). ,.i:8-(s, - 3 r)s CC-MS for e.< Hf,BrCI 20: -272 (M+).
[00465] 8-bronK>-4 -ehIoro-6-nielhyl-quinazolinc. Synthesizcdkiecording to the method of reagent preparation I using 2-amino-3- i-on >-5-mcihy.lbenzGic acid in step f. GC-MS (El) for C9H6BrCiN,: 257 ( *).
[00466] 4-ehlor0-6-(itiethylstilf0iiyljC|uit)a- )liiie. Synthesized according 10 the method of reagent preparation I using 6-(.niethylsul fotiyl)quinazoliii-4(3/7 -one in step 2.
6-(methylsulfoiiyl)(|iiinazGlin-4(3/7)-one was obtained by the one step oxidation of
6-(nielhylthio)cjuinazolin-4(3/7)-one (./. Mai Client. 1983, 26 ). 420-5). MS ( El) for C9H7ClN;O 242 ( M4 ).
Reagent Preparation; 2
4-chloro-5-nicthyl'r6-iphen linet-hy-l)pyri'nii(]inc' 1004671 Prepared frpnv4,6-dichlor()-5-iiieihylpyriniidinc and benzyl zinc bromide (0.5 M solution in tetrahydrofiiran) according to the procedure ...described in WO 2007/146824 as a colorless oil. Ή NMR (400 MHz. CDCI.;): 8.78 (s. I I I ). 7.33-7. 1 8 (in. 5H). 4. 19 (s. 2 H). 2.36 (s. 3H): MS (El) for CnH , ,CIN2: 2 19 ( Μ Ι-Γ).
Reagent Preparation 3: 4-chlon)-6,6-(liniethyl-5,6,7,8-tet nihydroquiiuizoline
[00468] STEP I : To a cooled (0 °C) solution of 4.4-climcthylcyclohexanone (2 1 g. 0. 1 7 mol) and dimethyl carbonate (45 g. 0.50 mol) in THF (400 niL) was added NaH ( 60% wt/wt in mineral oil. 17 g. 0.43 mol) pori ionwi e over 30 minutes. The result ing slurry was al lowed to stir at ambient temperature lor 30 minutes followed by two hours at reflux . The reaction mixture was cooled (0 °C) and McOH (30 ml_) was added dropwise over 20 minutes. The resulting slurry was partitioned between i0% aqueous citric acid and ethyl acetate. The organic layer was washed with brine, dried over .magnesium sul fate. and concentrated in vacuo. Purification by vacuum distillation provided methyl ^-hydroxy- .S-dimediy'lcyclohcx
l-enccarboxyIatc(22.5 g.75% yield). Ή N (400. MHz, C Ch) δ 12.15 (s, III), 3.75 (s. 3H), 2.29 (l, 2H), 2,03 (s, 2H), I, .44 (i, 21-1), 0.96 (s, 61-I); MS (El) for Cj0H:i6(¾: 184 (M .
[004691 STEP 2: A solution of ineiliyl 24iydioxy-5,5-dii)iclhyleyclohex-l-enecarl)oxylatc (10.0 g, 54 mmol) and 'ammonium acetate (.10 g, 1 0 mmol)an ethanol (50 mL) was heated to reflux for 2 hours. The reactio was concentrated to onelhird original volume, and then -diluted wiihycUvyLacetatc (.1 OOjnL).. The organicsolulion was washed with vvaten (TOO, mL) and brine (50 mL) and then dried ovcr'anhydroiis sodium sulfate: After nitration arid- conccntration, the residue was purified by silica gel column chromatography ".(.ethyl aceiale/hexanes, 1:8) to afford methyl 2-aniino-5.5-diinethylcycl0hex-l-ciiecarboxylale (7.42 g, 75% yield) as a yellow solid. MS (El) ΓΟΓ€ΙΟΗ,7Ν02: 184 (MH+).
100470] STEP 3: 2-aniino-5,5-dimetlvyleyclohe.x.- 1 -enecarboxylate (7.42:¾g.4 mmol) was dissolved in M/V-dimethylloiinamide dimcth lnccial (50 mL;) and heated t 11 C for 8 hours. The resulliiig solutidiv was cooled to room lempcrauire and concentrated to provide methyl 2^(dimeihylamino)nie^
98% yield) as* an oil. Ί-Ι NM (400 MHz. CDCl3): 3,65 (s.314).3-4 is; 1T1), 2.95 (s,.6B), 2.35 (in.2H), 2,15 (br s.2H), 1.41 (t.2H).0.95^(s, 6.Η)· MS (El) for Ci3H« 3Q2 239 (Μ1-Γ).
[00471] STEP 4: A solution of methyl "2-(;(diinethylainino)mclhyleneatuiiio)-5,5- dimethylcyclohex- 1 -enecarboxylate (9.5 g, 40 mol) in 7.0M ammonia in methan l^ mL) was stirred at 25 °C for 90 minutes then concentrated to aivoil. The residue was purified by silica gel column chromatography (ethyl aceiatc/hexanes.1:8) to givc 6;6-d.iinethyl-5,6,7v8- tctrahydroc]uinazolin-4(3H)-one (6.41 g, 90% yield) as a white solid.. Ή NMR (400 MHz. dfi- DMS0): 7.96 (s.111).2.52 (1.2H).2.14 (s, 2H).1.48 (l.2H).0.93 (s, 6H); MS (El) for CioHuN.O: 179 (Ml-f ).
[004721 . STEP 5: To 6,6HlimethyI-5.6.7,8Hctrahydrot|uina/.oIiii-4(3//)-one (6.41 g, 36 mmol) in chloroform (10 mL) added phosphorus oxychloride (10 mL) and refluxed for 2 hours. The mixture was concentrated to an oil, thcii diluted with ethyl acetate (80 mL) and washed with saturated sodium carbonate (50 mL) and brine (25 mL). Thcsolulion was dried over anhydrous sodium sulfate, filtered and concentrated, then the residue purified by silica gel column chromatography (ethyl aceiatc/hexanes.1:8) to give 4-chloiO-6,6-climethyl- 5,6,7,8-tetrahydroquinazoline (5.3 g.75% yield) as a yellow solid. 'I-! NMR (400 MHz. CDC ): 8.72 (s. ΠΓ).2.52 (t.2H),2.14 (s, 2H).1.48 (t 2H).0.93 (s.6H); MS (El). for . CoH ClN .197(Μ1-Γ).
[00473] Using analogous .synthetic techniques and substituting with alternat ive starling reagents in ste 1 or 2 the following reagents were prepared. Alternative starting materials were available commercially unless otherwise indicated;
|0 474| 4-chloro-6-methyl-6,7-(lihydro-5/^ y lopenta| /|pyiimjc!ine, Prepared according to the method of reagent preparation 3; usin 4-niclhyl-2-oxo-eycippcntanecarboxylic acid methyl ester (J. Chcm. Soc. Perkin Trans I 1987.7, .1485-8) in step 2. Ί-Ι N R (400 MHz. CDCIj): 8.78 (s, I B), 3.20 (in.2H), 2.70(ni.31-1), 1.22 (d.3H). GC/MS (El) for CsHyCIN>: 168 (M+),
[00475] 4-chl0ro-6-cycl6propyl-5,6,7,8-ietra^^
according to the method of reagent -preparation 3 using i-cyclopropyl-4-oxo v
p'iperidinecarboxylic acid methyl ester (Hcicrocyelcs.1999, 50(2), 867-874) in step 2. Ή NM (400 MHz, CDGli): 8.7S (s. IH).3.79 (s.2H).2.98 (m. H).1,88 (in. I H), 0.60 (m. 21-1).0.54 (m.2H). MS (EI) for C0H12CI 3: 210 (ΜΙ ).
[004761 4-cldoro-6-cyclopropyi-6.7Hlihydro-5/7-pyiTolo[3.4-i|pyrinTidine. Prepared according to the method of cagcnt rcp'aratibn'3-iisiiig.J-cycldpro.pyl-40 -3.- pyn lidinecaihoxylic acid methyl ester in step 2. MS (El) for .CyHjiiCI j:.1% (ΜΙ- ).
I O477I 4-chloi -6-p-tolyl-6,7-dihydro-5/7-pyriOlo|3,4- |pyHniidine^P
to the method reagent preparation 3 usin l-(4-mcihylphcnyl)-4-6xb-3- pyi roli(liivecarboxilic acid ethyl ester in step 2. Ή NMR (400 MHz, CDCI3): 8.92 (s, I H).7.14.(11.2H), 6.62 (d, 2H)..4.70.(hi.4H).2.30 (s, 3H). MS (EI) for CijHi^CI ^: 246 (MH+).
[004781 4-chloio-7-melhyl-7;-phcnyl-5.6,7,8-tetrahydro(|iiinazolinc. Prepared according to the method of reagent preparation 3 using 4-mcthyl-2-oxo-4 phcnyl cyclohcxanecarboxylic acid methyl ester (J. Org. Chem. 1991, 56(21).6199-205) in stcpLMS (EI) for C|5H|5C1N2: 259 (M Ι-Γ).
[00479] 4-chloro-5-phenyl-6,7-dihydiO-5//-cyclopciita| /|pyriniidinc:> Synthesized according to the method of reagent preparation 3 using ethyl 2-oxo-5- phenylcyclopeiuanecarbqxylate in step 2. MS (El) for CoH 1 iCIN?: 231 (ΜΙ- ).
[00480J 4-cliIoro-7.7-diniethyl75;6 J,8-teirahydrociuinazoline: Synthesized according to the method of reagent preparation 3 using ethyl 4,4-diinethyl-2-qxocyclohexanccarboxylatc in step 2. Ή NMR (400 MHz, CDCI.v): 8.91 (s, IH), 2.9 (s.2H).2.88 (Ir, 211).1.73 (tr.211). 1.07 (s, 611); MS (El), for C,0H 13CIN2: 197 (ΜΙ-Γ).
[004811 4'-cldoro-78'-dihydro-57 -.spiro|cyclopropane-'l ,6'-quinazoline|. Prepared according to the method of reagent preparation 3 using spiro[2.5|ocian-6-onc in step 1. Ή
NiVIR (400 MHz, CDC ) 68.73 (s. I l-l).3.00 (t, 2H).2.63 (s, 211).1,69 (I.211), 0.52 (s; 4H); MS (EI) for CVoHnClNj: 194 (M*).
|()0482| 4-chloiO-6,6-difliu)i -5,6.7.S-tciiahy-lroc|iiiiiazoline. Synthesized according to the method of reagent preparation using 4.4-difhioi cyclolvexanane in step I . MS (ΈΙ) for GSH7C1F2 .: 204 ( ).
[004831 (R) -chloro-7-met.hy^ according to the method of reagent preparation 3 using ( ?)-3-methylcycIolicxanonc in step I. MS (El) for
[004841 4-chl0ro-2,6Hlinietliyl-5.6.7.S clialiydr0cjuihazoliiic. Synthesized according to the method of reagent preparation 3: using 4-methylcycIohexanone in step 1 and 1.1-dimclhoxy- /V.Aklimeihylethanamine in step . MS (El) 'For CinH|jCINi:: 1 6 (M+).
[00485] 4 chlqro-6-cthyl-2 nethyl-5.6;7.8-tetraliydi quinazpline. Synthesized. according to the method of reagent preparation 3 using 4-eihyleyclohexanone in step 1 arid
l.l-diiiictlioxy-A'.Ndinietliylcthariainine in step 3, MS (EI) for CriHij Nj; 210 (M+).
[004.86] 4-chloro-7-(trilluoroiii.eil)yl)-5,6.7,8 eirahydroquiiia'z()line. Synthesized according to the method of reagent preparation 3 using methyl 2-hyar.oxy-4-0rlITuormcihyl)cyc}61iex- 1 - cneearboxylate in step 2. MS (EI) for C:yHsClF.-,N2: 236 (M+).
[004871 (ti¾ns -chlord;6,7rdimethyl-5,6,7.8-letrahyd
according to the method of reagent preparation 3 using (trans) 3,4-ditiiethylcyeiohexanone in step 1. MS (ED for C,0Hi.-iCINi: 1.96 (M*).
[00488] 4-chloro-6-(irilliioiO]ncihyl)-5,6,7,8-leirahythOC|iiinazormc. Synthesized according to lire method of reagent preparation 3 using 4-(lrifluormcthyl )cyclohcxanone in step 1. MS (El) for CyHgCIFjNa: 236 (M ").
1 048 1 (S)-4 iiloro-7-iiieilrYl-5,6.7>8-tetrahydiOC|iiihazolinc. Synthesized accordirig.to the method of reagent preparation using (S)-3-methyleyclohexanonc (US2006Q293364) in step 1. MS (El) fprCyHuGIN:: 182 (M+).
[00490] 4-cliloro-5-(trinuor0inethyl)-5;6,7.8-tetrahy.di C|uinazolinc. Synthesized according to the method of reagent preparation 3 using methyl 2-hydroxy-6-(irifluorniethyl)cyclohex-l- enccarboxylate in step 2. MS (El) for CyHxCIF.^: 236 (M+).
1004911 4-chloiO-7-vinyl-5.6,7.8-tclrahydiO(|tiinazoline. Synthesized according to the method of reagent preparation 3 using 3-vinylcyclohexanone (J. Med. Chem.1.987.30, 1177- 1186) in step 1. MS (El) for CoHiiClN,: 194 (M
+).
(00492] 4-clilorq-8,8 ]iim the method of reagent preparation 3 using
in step I,. MS (EI);tor do.HiaCl ,: 196 (M ").
[00493] 4- h!oiOr6,6,7-tiiniethyI-5T6-(:liliydiO(|iiiiia/.()linc. Syntliesi/xd.'aecoiding to the method of reagcnl preparation 3 usin 3,4;4 rini'ethylcyeloiiexT2-enoiic ( Am. Cliem. Soc. 1994.1.16, 2902-2913) in step I. MS (EI) for C||H|3eiN2: 208 (M+).
[00494] (S)-4-ehloro-8-vinyl-6,7.8.9-tetrahydro0^
^^ Synthesized according to the method o reagent prepaf ii0ir3 usin
^ (S -3-viny[cyelblicptaiione (p epared using procedurc-for
;(S ^v
;inylcyGloh¾x-anone in O.rg. Lett.2003, 5, 97-99,
: but starting, wit
[00495] 4-qhlor0-66-dimcd^
method of reagent preparation 3 using 4.4-clinietlVy]cyc0hex-2-enone iii-step I, MS (ES) for CioHifClNj: !95(:ΜΙ-Γ).
[00496] 4clvloro-6,6,8-ir iinciliyl-5.6.-clihytli'otjuin'azol inc. Synthesized according to the method. of reagent preparation 3 usin 2.4,4-triinethylcyclphex-2-enone in step I. MS (El) for C,iH|;iCI :: 209 ( MM').
[00497] 4-chloro-6,6,7;8-tetrameih^ according to the method, of reagent preparation 3 using 2,3,4,4 etrmmthyjcy^ Chem.
1981, 46.1515- 1521) in step L MS (EI) fottC,2l l,5eiN2: 223 (Μ1Π).
[00498] (S)-4-eIiloro-7-ethyl-5.6;7,8-tetnthydrtH^iiiiaz0line; Syntliesized aecording to the method of reagent preparation 3
1997, 8, 1253-1257) in ste 1. MS (EI) for CioHuCIN2:.197 (MH+).
[00499] Step 1 : A solution of methyl 4-mcilvyl-2-oxocycIopentanecarboxylale;.(0.42 g, 2.69 mmol).2rmcthyl-2-thiopseudourea sulfate (J lO g, 7- nimol) and potassium hydroxide (0.50 g, 8,9 mmol) in water (12 L) was stirred at 25 °C for 30 minutes, and lhcn heated to reflux. for 4 hours. The reaction was cooled to 0 "C by adding ice and a precipitate was formed. The solid product was removed by filtration and the filler cake dried to give 6- incthyl-2-(methylthio)-6,7-clihydro-3W-eyclopenia| |pyritni'din^(5 l)-one (0.19 g.43% yield) as a white solid. Ή NMR (400 MHz, d6-DMSG): 2.87 (m, 211).2.53 (s. H), 2-37 (m, 211).2.28 (s.311), 1.49 (m, 1 I I), 1,02 (d, 311).
[00500] Step 2: A solut ion ol;i6-iuethyi-2-(nietliyllln
cyciopenta| rf|pyriiiiidin- (5H)i-onc (0: 19 g..0, 7 inmol) in phosphorous oxychloride (5.0 niL) was healed κν9 I
'or I hour. After cooling the rcaei¼HAVSis
dissolved in.cihyl. acetate (50 niL) and washed with cold water (25 ml.). 0. I M ai|iieous sodium hydroxide (25 inL) and brine (20 mL). The organic phase was dried over anhydrous sodiuni sulfaic. filtered and concentrated., The residue was clVromalpgraphed on silica gel (diethyl eihcr/hexanes. 1 : 10) and the product .containingTraetions concentrated; The residue thu obtained Was purified 'further by preparative reverse plfasc MPLC (0: 1 % aqucpus;
amnionium accjatc-acetonit^
cyeloperita[<-/|pyrimi{line (25 g. 12% yield) as oil. ' H NMR (400 MHz. d6-DMSO): 3. 1.2 (ra, 2H), 2.6 1 (m, 2H), 2:56 (s. 3H), l .25 m, lj-1), l ;l8;(d,3 H) S (EI) for¾H i ,C]N?S: 215 (Mff
[00501] Using analogous synthetic techniques and
reagents the following reagents were prepared.
[00.502]' 4-ehloro-2-(niethyllhio)r6.7-dilVycliO-5H-ey
according to the method of reageiit preparation 4 by replacement of step 1 with :|,2,3¾;5ί6,·7-- liexahydro-2-thioxo-4/7 -yclopeniapyriinidin-4-qnc S-alkylation w ith iodoniethane and proceeding to step 2. Ή NMR (400 M Hz. CDCly): 3.00 (tr. 2H). 2.92 (tr, 2H). 2.56 (s, 3H). 2. 14 (m. 2H).
[00503] 2-(benzyllhio)÷4-:cM^ Synthesized aeeordihg t the method of ieftg'cflt preparation 4 by replacci fcril. of step 1 with 1 ,2,3.,5 6.7- l^xahydr0-2-thi'oxo-4W-cyelopeiHapyriniid Svalkylation with benzyl bromide and proceeding to step 2. Ή NMR (400 Hz. CDCI; ): 7:43 (d! .2H). 7.27 (lr..2 H). 7.22-7; 18 (m, 1 H). 4.38 (s, 2H). 2.95 (tr. 2H). 2.S6 (t r, 2H), 2.08 (m. 2H).
[00504] 4-chloro-2-(eihyliln^o)-6J-dihydiO-5W-cyclopeiita[<7|pyrin Synthesized according to the method of reagent preparation 4 by replacement of step I with l,2,3..5j6;7- licxahydro -.lhiox(>4H-c cloperita|xyrinii(tin-4-one S alkylalion with iodoethanc and proceeding to step 2. Ή NMR .(400 M Hz. CDC ) 3.08 (q, 2H). 2.93 (tr, 21 1), 2.86 (tr, 2H). 2M (m. 2H ), 1 .32 (ir; 31 1 ).
Rengent Preparation 5
[00505] STEP I : A solution of ethyl 4-mcthyl-3-oxopcntanoate (3.0 g, 1 .0 minol) and potassium carbonate (7.86 g, 56.9 mmol) in THF (40 mL) was stirred at room temperature l or 3 It under N
2 (g). The mixture was cooled to 0 "C and methyl iodide (3.23 g, 22.8 nimol) as added dropwise over 5 min. The reaction mixture was allowed to warm to room temperature and stirred for 16 h. Subsequent filtration and concentration provided ethyl 2.4-dimethyl-3- :pxopentanoaie-(2.8 g, 89% yield) as a clear, yel low oil that was used without further purification. MS (El) for (¼-¼(¾: 172 (MB*).
[00506] STEP 2: To anhydrous elhanol ( 1 10 mL) was added sodium metal ( 1. 16 g. 50.4 mmol) and the mixture was stirred until dissolution was complete. To this solution was added thiourea ( 1 .79 g. 23.5 mmol) and ethyl 2,4-dimethyl-3-oxopenianoale (2.89 g. I 6.S mmol). The reaction mixture was stirred at 85 "G for 20 h then cooled and concentrated. The residue was diluted with water, the pH adjusted, to 4 with 1 N.hydroeltloric acid then extracted with ethyl aeetaie (3 80 mL-). The combined organic -layers.. were washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated to provide 6
iisopropyl-5-mcthyl-2- thioxo-2,3-dihydropyrimidin-4( l H)-one (2;40 g. 78% yield) as a tan solid that was used without further purification.
1 85 (MH
+).
[00507] STEP 3: To a solution of 30% hydrogen perox ide ( 12 mL) and water (23 mL) was slowly added 6-isopiOpyl-5-met yl-2-^.ioxo-2,3^djhydrop.yi niidin^(.l.H)-one 1 ;.0 g. 5.4 mmol). The reaction mixture was. st irred at 70 "C for 3 -h. After cowlin to room temperature, saturated sodium carbonate was slowly added until the pH reached 10. To this mixture was slowly added a I M . solution of sodium ihiosulfate until residual peroxide was quenched, whereupon the aqueous solution was concentrated to dryness. The residue was suspended in chloroform ( 100 mL), filtered to, remove inorganic salts and the filtrate concentrated to provide 6-isopi pyl-5-methylpyrimidin-4-ol (0.25 g, 30% yield) as a white solid that was used without further purification. MS (El) for CsH ii .O: 15 (Mli+).
[00508 ] STEP 4: To 6-isopropyl-5-mcthylpyriniidin-4-ol (0.25 g, 1 .6 mmol) was added neat phosphorous o ychloricle (5 mL) and the mixture stirred at 70 °C for 3 h. After cool ing to room temperature the solution was concentrated, diluted with water then neutral ized by portion-wise- addition of saturated sodium carbonate solution. The aqueous mixture was extracted with ethyl acetate and the organic solution -washed with brine then dried over anhydrous sodium sulfate. Filtration and concentration provided 4-chloiO-6-isopropyi-5- methylpyrimidine (30 mg, 1 1 % yield) as a brown oil that was used without further purification. MS (EI) for CsH nCl :: 170 (MH+).
[00509] Using analogous synthetic . techniques and substitutin with alternative starting reagents in step I the following reagents were prepared.
[005101 4-chlor075-(cyclopropylnieihyl)-6Tnieihylpyriniidine. Synthesized/according to the method of reagcntprcparation 5 using methyl 3-oxobutanpate and
(biOmoinelhyl)cyclopropane in ste I . MS (El) for Cyl½Cl!¾. 182 (MH).
[00511 ] 4-clnoro-5-(4-chloiObenzyl)-6^melhylpyrjinidi to the methPd of reagent preparat ion 5 using methyl 3 -oxobulanoate and l-(biOin0melhyl)-4- chlorobenzenc in step 1 . MS (El) for C I^H HJCI NO.: 254 (M I f ).
[00512] 4-chk)ro-5-(3.5-difluorobenzyl)-6-methylpyriinidiiie'. Synthesized according to; the method of reagent preparation 5 using, methyl 3-oxobutanoate and 1 -(bromomelhyI)-3,5- difluGi benzene, in step 1 . MS (EI ) for GfiH^GlFi i; 255 (MH+).
[00513] 4rchloiO-6 niethyl-5'-(.3-(lrinii0rpm Synthesized acepi'djiig to,;ihe method of. eagent, preparation^ tisingrnVetlvyJ, 3-ox'pbl aii0aie and! l-(chloronicdiyl)-3Htrifto
(MH+).
[00514] 4-chloro-5-( l -(3-fkioiOphcnyl)ethyl)-6-metliylpynmidine. Synthesized according to the method of reagent preparation 5 using methyl 3-oxobuianoate and l -(3- fhiorophenyl)cthyl mcthanesul fonaie in step I . MS ;(El) for CuI-I |2ClFN2: 251 (MH*).
[00515] 4-chIoi -5-(4-chlor.o-3-nuoiObeuzyI)-6-mcthylpyrimidinc. Synthesized according to the method of reagent preparation 5 using- !nethy,l-3-Px0butariPaie and 4s(brom0methyl)- 1 -
272 (MH*)..
[00516] -cl pro:
:5-^ the method of reagcnli preparation 5 using methyl, 3-oxpbuianpale and l -(biOmomethyl)-4- I
'hiorobcnzenc in step 1 . MS (EI) for
237 (MH
+).
[00517] 4-chloro-5-(2-nu0iObenzy!)-6-niethylpyrimidine. Prepared according to the method of reagent preparation 5 by using methyl 3-oxobutanoate and l -(bromomethyl)-2- fluorobcnzehc in step 1. Ή NMR (400 MHz, CDCI3): 8.79 ( I H), 7.28 to 7. l 2 (m, I H), 7. 14 to 6.97 (m, 2H), 6.82 (dd, I H). 4. 19 (s, 2H). 2AJ (s,.3H), GC-MS for C| 2H 10GlFN2: 236 (M+).
[00518J 4-chl0ip-5-ethyl-6-isppropylpyrimidine. Prepared; according to reagent preparation 5 by using ethyl isobulyrylacctate and iodoethanc in step I . MS (El) for
[00519] 5-benzyl-4-chloiO-6-metliylpyriinidine. Prepared. according to reagent preparation 5 by using ethyl 2-benzylacctoacetate in step 2. MS (El) for C.|2H | |C1N2: 219 (MH+).
[00520] 4 hloro-6-eihyl-5-methyl-pyriinidine. Prepared according to reagent preparation 5 by using methyl 3-oxopcnianoate in step I . Ή NMR' (4Q0 MHz, CDCI?): 8.74. (s. I H). 2.85 ((|. 21 1), 2.39 (s, 3H), 1.30 (t, 3H)i MS (El) for C7H9CI.N2: 158 (MH+).
|0()521J 4-chloro-5,6s7.X-tctralvydr i|uiiui x)linc..Syiiilicsizeti according to. the method of reagent preparation 5 using ethyl 2-oxoeyclohexaiiecarbpxylalc in .step 2. Ή N'MR (400 M Hz. CDC ); 8.7 (s, l H), 2.90 (m. 2M), 2.78 (in, 211); l,SS (m. 4H). MS ( El) for CsH.;CIN2: 169 ( i l').
[005221 4-chloiO-5,6-diethyl-pyriinidine. Prepared ' .-according to reagent picparal ion 5 by using iethyl 3r0xo.pentanoatc .aiul iodoethane in step I .
[00523] 4 -hlorp-6rmetliyl-5-( l -mcthylethyl)-pyrimidinc; Prepared according to reagent preparation 5 by using methyl ..B-oxObiitaribate^ Ί-1 ISJMR (400
MHz. DMSO-dfi):: 8,70 (s, Η% 3.49 (h.. l H), 2.60 (s, 3H¾ 1 34 (cl, 6H); MS (El) for
CsH i iCINN: 171 (MH+).
{00524] 4-chloro-5-isobiuyl-6-niethyIpyrinmlinc. Prepared according to reagent preparation 5 by using methyl.3-ox b'ul-a'iioafe ;and l -i0do-2-metlryIpf0panc iii step 1. MS (El) for'CiWi-j'ClNo: 184 (M+).
[00525 J 5-benzyl-4-chloro-6-elhylpyriniidine. Prepared according.to reagent preparation 5 by using methyl 3-ox pentanoaic and benzyl bromide in step 1. Ί-Ι N' R (400. MHz, CDCl.i): S.83;(s; I B), 7.27 (m, 3H), 7.08 (m, 2H), 4.22 (s, 2H . 2¾ (¾ 2H), 1.20 <α. 3R); MS (El) ior CuHuC-lN;: 234 (MH+):
[0052(5] 4vchI'Qro-5-(3rnuordbcnzyii-6-mctl.iy'lR.yr aceordiirg to reagent preparation 5 by using melhyl 3-oxobuliinoale'and 3-flubrobeiYzylbrbmide in' step I . MS (El) for C i 2l-l |i)dFN2: 237 (Ml-f).
[00527.1 4-chloro-5-(3-chlojObcnzyl )-6-mcihylpyrimidine. Prepared according to reagent preparation 5 by using methyl 3-oxobulanoatc and 3-chlorobenzylbromidc in step 1. MS (El) for' Ci.2H ioCliN2.: 253 (MH+).
[005281 4-chloiO-6-nicthyl-5-plienoxy-|)yriinidinc. Prepared according lo rcagent preparation 5 by- using ethyl 3r;bxb-2pheiu)xybutanoale.iri.step 2. MS (EI) lor CM HyCIN20: 221 (MH*). ,
[0052 1 4-chloro-6-methyl-5-( 1 -phenylelhyOpyrimidihc. Prepared according to reagent preparation 5 by using methyl 3-oxobulanoatc and ( l -biOmoeihyl)benzenc in step 1. MS (El) for CnHi.¾ClN2: 233 (MH+).
[00530] 4-chloro-5-(2-chlorobcnzyl)-6-methylpyrimidine. Prepared according to reagent preparation .5 by using melhyl 3-oxobulanoatc. and 2-ehlorobenzyl bromide in step I .
100531) 4 lilorp-fvniclliyl-5-(4 netliy!benzy))pyi iinidinc. Prepared according to .reagent preparation 5 by using mctliyl 3-oxoluitanoate and 4-methylbeuzyl bromide in step 1. Ή
MR (400 MHz. CDCI:(): 8.76 (s. I I I).7.10 (d.2H).6:99 (d; 2H), 4.l5 (s.2M).2.50 (s.3H). 2.32 (s.31-0; MS (El) for C|3H|3CIN3: 233.-(MH*).
[00532J 4-diloro-5-(4^niellioxybenzyl)6-nicil)ylpynrViidi
preparation' 5 by using-mcihyl 3-oxobutanoate and 4-n thoxyben-zyl bromide in step- 1.
1 H NMRt(400 MHz: ClDGlj): 8;76J(S, ¾|¾ 7:02(d, 2Hj;.6:S3 (dv^) 44¾:(s, 2H);3v7:8 ®iW),
Pfcjiafctl
:acepYKn
:g:t
; reagent preparation 5 by using mctliyl 3.-oxobutanoaie and
'^methox.ybenzyl bromide in-step I. Ή
NMR (400 MHz. DMSO-d,,): 8.81 (s. I H).7.22 (m.1H).6.8! (m.111).6.70 (s.1H).6.63 (d,
I !-l), 4.17 (s, 21-1), 3.71 (s, 3H), 2;47 (s.3H);MS (El), for C1?HnClN20: 249 (MH+).
101)5341 4-ehloro-6 neibyl-5-.(3 iieiliylbenzyl)pyriinidine. Prepared according to reagent preparation 5 by Using mctliyl
&rpjnijd/e in step J ..Ή
NMRpO/ Hz.C Clj): 8.77 is. III).7.18 (m. l ^^O Cd.1H).6.88 (m.2H),4.I6 (s,
2H¾ 2f50 (s^H),3.31 (s, 311); :MS (El) for CuH^CIN;,: 23 ¾MH*
100535] 5-benzyl-4-chlorc)pyriniidirie. Prepared accottling wr eagent preparation 5 b using ethyl 2-behzyl-3-hyliOxyacrylaic (J-- Ai . Cli in. Soc. W4, 96, 2121 -2129) in step 2.
MS (El) for C, , Hg.iCIN,: 205 (Μ1-Γ).
[00536) 4-c1iloro-5-(3-clilorc)-5-l1uordbenzyl)-6-ntcthylpyriniidine. Prepared according to reagent preparation 5 by using methyl 3-oxobutanoate and 3-chloro-5-fluoTObenzyl bromide in step I. MS (ΕΓ) for C, H.;C1-4: 2: 271 (M!-f).
I00537J. 4-chloro-5-(2-niethdxybenzyl^ according to reagent prcparaiipnvS jby using ; mctliyl
instep 1. Ή
NMR (400 MHz; mctlianol-d,,) 8.71 (s, III).7.23 (m, I H).6.9.8 (cl.1H).6.83 (m. III).6.71 (d.1 H), 4.16 (s, 2H).3.85 Cs, 3H); 2.45 (s, 3H>.
[00538] 4-cldoro-6 iiclhyl-5^(2-iiietIiylbenzyl)pyriinicline. Prepared according to reagent, preparation 5 by using methyl 3-oxobulanoate and 2Vnieth'yll>cn¾yl bromide in step 1. Ή NMR (400 MHz, methanol !.,): 8.77 (s.1H).7.23 (d. IH), 7.12 (m. ΓΙ-Ι).7.03 (m, 1H), 6.45 (d. IH).4.16 (s, 2H), 2.43 (s.31-1), 2.42 (s.3H).
10()539|: 4-diloro-5-(3,4-din.uoiObenzyl)-6-mcihylpyriniidinc. Prepared according to reagent, reparation.5. b using.; methyl 3-oxobutanoate and 3,4-:diim0robenzyl bromide: in step I. MS (E TorCpJ-L!CIF-Ni: 255 (ΜΙ-Γ).
[005 01 4-chloro-6-nicihyl-5-(4-(irifliioFOiiicthyljbGn/. l)|Jyriini<lin'c. Prepared according lo reagent preparation 5 by using methyl 3rOxobutanoalc and .l -(cliloiOiiiethyl)-4-(irifluqro-niethyl)benzene in step I . MS (El) for Ci¾H|.oClF3N2 20 (MH+).
1005 1 1 5-:ben yl- -e luro-6-(lt if uGroinel y yiiriiidliic. Prepared according Lp reagent preparation 5 by using ciliyl 4,4,4 rilluoiOaceioacelatc and benzyl broniidertn step I . MS (EI ) lor C,.HSC1F.,N2: 272 (M+).
[00542] 4-ehloro-6;6Hlimethyl-6'J-.dih^^ Synthesized according to the method of reagent preparation 5 using ethyl 4,4-dimctliyU2-ox0- cyclopcntanecarboxylate in step 2. MS (E ) for G.M nCI j: 183 (iVl H+).
'Reagent Preparation 6
(i 1iK)n)-5-niethyI V-plieiiylpyrinudin-4-iimine
[0()543| .STEP 1 : To a mixture of 4;6-dtchlord-5
:hictliylp,ymnnclitie (2.27 gyl¾,9 mmol)
hydrochloric acid ( 1.5 niL) and heated to reflux for 2;5 It. The nuxiure was then concentrated and the residue triturated with ethyl aeetate:isopropanol:4: 1. The solid was collected by filtration and washed with additional ethyl acetaterisopropanoi 4: 1 then" dried lo give 6- diloro-5-niethyl-N-phenylpyri midin-4-aminc (2.0 g. 67% yield). Ή NMR (400 MHz, dfl- DMSO): 8.85 (s, 11 1). 8.26 (s, I I I), 7:60 (d. 2H). 7.35 (ir. 2H ), 7. 1 1 (tr, 1 H), 2.3.1 (s, 31 1). MS (El) for C| ,Hioei 3: 220 (M l ).
100544] STEP I : To a suspension of potassium /e/7-bui'oxidc ( 10.6 g, 95.0 mmol) in ictrahyclrofuran ( 1 0 niL) were added methyl accloacetatc ( 10.0 g, 86.0 mmol) and lcrl- l)Li inol (0.83 mL, 8.6 mmol) al room temperature. The resulting solution was slirred for 1 h, and then 4-,nuoiObcnzylbi mitle ( 1 1.2 niL, 90 mmol) was added. The reaction mixture was slirred at room tefnperattire for 18 h. aivcl iheii partitioned betweeii water and ethyl acetate. The aqueous layer was extracted with ethyl acetate (3 x), the combined organic extracts were washed with brine, dried over sod'aihi sulfate, filtered and concentrated. Column
ehromatography.of the rcsiduc on silica (5-20% ethyl acetate . in hcxanes) gave methyl 2-(4- fhiorobenzyl)-3-oxobutanoaie ( 14.5 g, 75% yield) as a colorless oil which was used in. the next step without further purificat ion.
[00545] STEP 2: To a suspension of acctamidinc "hydrochloride (0 4 g, 5.7 1 mmol) in methanol (8 mL) was added a 30% solution of sodium methoxide iiv nicihanoi ( 1 .1 mL. 5.7 iriinol), and the result ing solution as st irred at room temperature for 45 liiin. Then, a solution of methyl 2-(4-riuorobcnzyl )-3H>xobuianoate (0.80 g, 3.57 nimpl) in methanol (3 mL) was added dropwise. and.lhe resulting mixture was stirred at room temperature for 22 h. Water ( 100 inLj was added, and the mixture was cxtracied. with Chlorolbriii (4 x 50 mL). The combined organic cxii acis were dried over sodium sulfate;, filtered and concentrated to provide: 5-(4-11TO (0.74'g, '8 % yield) as; . colorless, solid. Ή NM (400 H/, methanol-dj): 7:2 1 (m. 21T), 6v 6.-Cni, -2K).:3,84 (s, 2H). 2.35 (s. 3H). 2.25 (s. 3 H): MS (El), for C nH i.,FN20: 233 (M H+).
[00546] STEP 3: A solution of 5-(44'luorobetizyl)-2.6-dimcthylpyritiiidiii-4- r(730 nig, 3. 14 mmol ) in phosphorus o ychloridc (.1 mL) was stirred at 60 °C for 90 mill. The reaction mixture was.coiieeni rated and. ethyl acetate (50 mL) was added to the residucThe organic solution vvas washed with saturated sodiimi bicafbptiate:(5PviiiL), water (50 mL), and 'brine (50 mL), dried over sodium sulfate, filtered and' concentrated. Column chromatography of the residue on silic '(5-40% ethyl acetate in hcxane ) afforded 4-cW
diiiiethylpyrimidinc (527 mg, 6.7% yield) as a colorless solid.. 1 H; NM R (400 MHz, CDC ): 7.21 (m. 2H). 6 im. 2H). 4. 1 (s. 2H); 2;67 (s. 3I ),2.45 (s 3H); MS (E ) or
C ,.rH |2ClFN2: 250 (M+).
[00547J Using analogous synthetic techniques and substituting with alternative starting reagents in step I the following reagents ere prepared.
[00548) 4-ChloiO-7-mcthyl-5,6.7,8-tetrahydio(|tiiiiazoIine. Prepared according to the method of reagent preparation 8 by using ethyl 4Hiiethyl-2^oxpcyclohexaiiecarboxylate and fprmamidine formate in step 2..GC-MS for Gyl-l nGIN : 182 (M+).
[005.49] 4-Ghl0ro 6-cthyl-5 i7^8 clrahy.clroqiiiiia/.oline.. Prepared according to the method of reagent preparat ion by usiiig mcillyl.5-eihyl-2-oxoeyelolre and formamidine 'formate in step 2. GC-MS for.CVoM 13CIN2: 196 (M*).
[00550] 4-Cliloro-5-eihyl-2,6-dimeihylpyrimidinc. Synthesized according to the method of reagent preparat ion S by using ethyliodide in step I . MS (EI) for CS1-1 | |CIN2: 17 1 (MH+).
[00551] 4-Cliloi -5-(cyclopropylinethyl)-2.6-dimeihylpyriniidiiic. Synthesized according to the method of reagent preparation 8 by using cyclopropylmcthylbromidc in step 1 . MS (EI) fo CioH CIN2: 197 (MH+).
[00552.1 4-Chloro-2.6,ivirimeihyl-5.6,7,8-ictraliydroquinazolinc. Synthesized-. According to the method of reagent preparation 8 by using methyl 5,5-dimclhyl-2-oxocyclohexane- earboxylutc in. step 2. MS (El) for Cu H|3eiN3: 21 1 (MH+).
100553] 4-Chlorp-6.5-(liinel.liyl-2-(pyi-id.in-2-yl)-5
Synthesized according t the method of reagent preparation, 8 by using 2-hycli;o.\y-5^- dinietliylcyclohcx- l -enccarboxylate and picoliuimidamide 'hydrochloride in step 2; -MS (ES) for Ci5H|f,CIN:,: 274 (MH+).
[005541 2-(4-cliloro-6,6-climethyl-5 iJ,8-ieti aliydroquinazolii^2-yl)piOpan-2-ol.
Synthesized according to the method of reagent preparation 8 using 2-liydroxy-5,5- dimethylcyclohcx- l -eneearboxylate and 2,-hydroxy-2H)ieihyipropanimi'dainide hydrochloride in step 2. MS (OS) for C,.d liyClN;: 255 ( MHf).
[00555] -meUiyletlvyl)pyrimidinc. Synthesized according to the
method of reagent preparatioh :8 by u irig l-iodqpjqp^ MS* (EI) :Γ G¾HBC1NJ:
185 (MH+). ■
[00556] (7 )-4-cldoi -7-eihyl-2-niellvyl-5Xi.7.8 eira]iydroquinazoline. Synthesized according to the method o reagent preparation 8 by using methyl (4.S
')-4-ethyl-2- oxocyclohcxaneearboxylaie (reagent preparation 3) in step 2. MS (EI) for
21 1
(Ml-f).
Synthesized according.to the method of reagent preparation 8 by using l - pyrrolidinepropanimidamide in s.tcp 2. MS (El) for Gi6 ¾Cl i: 294 (MH+).
[00558] STEP 1 : To a solution of phcnylmclhyl 2-melhyl-4-oxo-3.4-dihydropyridine- l (2H)-carboxylalc (./. Biaor^. Mccl. Chein, 2007, 1 106- 1 1:16) (2.4 g,
.9.7.8 mmol) in THE (35 mL) added dropwise a 1 M solution of liihiunrbis(lriniethyisilyl)amide in THE ( 1 1 inL) at -78 °C. The solution was warmed up to 0 °C. stirred at this temperature for j h, then cooled again to -78
' °C. 3-Eluoi benzadehyde ( 1.3 mL, 12.7 mmol) was added in one' portion. The reaction was stirred for 4 h while allowing it to slowly warm up to 0 °C. Then, saturated ammonium chloride (20 mL) was added, and the layers were separated. The aqueous layer was extracted with ethyl acetate (2 x 20 mL) and the combined organic layers were washed with aturated sodium chloride (50 mL), dried over sodium sulfate, filtered and concentrated.
Column chromatography on silica (gradient 20 to 100% clhyl acelalc in hcxancs) afforded phcnylmclhyl 3-| (3-fhiorop.henyl)(hydroxy)inethyl |-2 ne.lhyl-4-oxp-3,4- I (2H)-
'carboxylatc. (2.4 g, 66% yield) as mixture of;diastereomers. MS (EI)' for C21 20FNO4:
[005591' STEP 2: Mesyl chloride (0.31 mL. 3.97 mmol); was added in one portion to a solution of phcnylme.thyl.3-|(3-^
dil)ydropyridine- l (2H)-carboxylate (0.73..'g. 1.98 liimol) in anhydnnis pyridine (5 mL) at 0 °C. The reaction, mixture was warmed u ao o m tcmp.crauire and stirrcd or I h. Water (5 inL) and eihyl acciate (5 111 L)- ere added, the layers were separated, and the aqueous layer was extracted with ethyl acetate (3 x 5 mL). The combined organic layers were- washed with saturated sodium chloride ( 15 hiU)-'dried' ovcr'sodium-'s/ul falc„'fi <5fcd and concentrated to afford phcnylmclhyl 3- ( (3-I1uorophcnyl)| meihylsu^^
dihydropyridine- l(2H)-carboxylale. MS (EI) for G2-H-2FNOf)S: 48.1 ( lT ).
[00560], STEP 3: Phenylmelhyl 3-|(3-nuoropheny|)[mcihylsulforiy])oxy|niethyl }-2- inelhyl-4-oxo-3.4-dihydropyridinc- 1 (2H)-caiboxylaic from step 2 was dissolved in THE (30 mL) arid potassium tei t-butoxidc (.1.1 1 2. 9- mmol) was ;addcd in one portion. After 15 min the reaction mixture was .
•quenched
', with saturated 'ainnionium cliloride (20 tnL); The layers were separated and the aqueous layer was extracted with 5: 1 chloroform/isopropanol (3 x 20 mL). The combined organic layers were dried over sodium sulfate, filtered arid concentrated. Column chromatography in silica-( 10 methanol iiY lichloromeihanc) afforded 3-| (3-
('ltiorophciiyl)hiclhyl)-2 iiethylpyridin-4(l H)-oiic (0.230 g, 53% for two steps) Ή NMR (400 MHz, CDCI3): 7.30 (d, I H). 7.18-7.13 (m, I H). 6.97 (d. 1 1-1), :6.87-6.79 (ni. 21 1). 6,35 (d. I H). 3.91 (sv 2H) 2.22 (i;, 3H). MS (El)
(MH
+).
(005611 STEP 4: A solution οΓ 34 (3 ;luoroplienyl)!iielhyl)-2- I H)-one
(0.0.7 g, 0.32.mmol) in phosphorous oxydiloride (3 mL). was healed 10 55 C for 16 h. Then Ihc solution was cooled lo room temperature and concentrated. The remaining residue was dissolved in ethyl acetate ( 10 mL). washed with 5% sodium bicarbonate (2 x 5 mL). and saturated sodium chloride (5 mL), dried over sodium sulfate, filtered and concentrated to afford 4-chk)ro-3-| (3-lluoropheiiyl)nieihyl |-2-inclhylpyridinc. Ί-Ι NM (400 MHz. CDCI3): 8.33 (d. 1 IT), 7.30-7.23 (m. 2H), 6.92-6.85 (m,.2H). 6.76 (d. I I I ). 4.22.(s, 2H). 2.5 (s, 3H). MS (El) for C|?H, |CIF: 236.0 (MH+).
[00562] Using analogous synthetic techniques and substituting with-allcrnative starling reagents in step 1 the following reagents were prepared.
Ϊ00563] 3-benzyl-4-chloro-2-niclliylpyri(liiic. Synthesized according to the method of reagent preparation 9 using bcnzaldchyde in step I . "HI NMR (400'MHZ. £i½| &3l0.(d. 11-1), 7.29-7:'19 (m, 4H), 7.0.8 (d.2H;), 4.22 (s, 2H).2.5Ϊ (s,-3W);;! Si(E! .?f0r G^H^CIN^IS ( Ι-Γ').
100564] 4-chlotO-3-(4.-niioiOben yl)-2-meihylpyridine. S nl!iesizcd-according to the method of eage ^ Hz, CDCb): 8:32 (d, 1*1); 7.29 (d.1 H).7.05-6.95 Am, 4H),4.l9(s, 2H) 2:54 (s, 311): MS (EI) for GuHnCIFN: 236 (MH+).
Reagent Preparation 10
[00565] STEP 1 : To a solution of elhyl 3-brompbutanoate (6i0 mL, 42 mm l) in Λ'.V- diniethyiformamide (20 mL) at 0 *C was added pipcridine 8:0!ίτϊΕ.ν80 mniOl¾;arid the mixture' was warmed to rob'fti.ieinpciaiurc tlicii stirred 16 li. The^eaction nix(urc was diluted with;et!hyl acetate (200'mL);aiKl washed with a solution of brine-and . .0 aqueous sodium hydroxide (4: 1 v/v). The organic phas was then ϋ'π'6¾ργέ^
filtered, and concentrated lo give elhyl 4-p'ip.er'idin-l - i¼'ai'an ^t£ -(&&.&:<8J:$-< yield)' as brown oil. MS (El.) ror C||H2lN02: 200 (Ml Γ)
1 0566] Step 2: To a solution of potassium hydroxide ( 11 g.0.20 iivol) in water (40 mL) was added a solution of ethyl 4-pipei itlin-l-ylbutanpate (6.8 g, 34 mmol) in cthanpl (30 mL) and the mixture was stirred at .3 "C for 2 hours. The reaction was: quenched by drppwise add it ioii of:¾-7¾¾ aqueous hydrochloric aeid/( ί·5 i mL) and the i i ire was concentrated then dried under vacuum. The residue was suspended in
addition of catalytic N.NKlimelhylfurmamide (0.2 mL) then dropwise addition of oxalyl chloride (15 mL, 170 mm l) and the mixture was stiiTCdai! 25 "C for 1-8. hours. The reaction mixture was concentrated to afford crude 4-piperidin- 1 -yl utaiioyl '-chloride hydrochloride. To a suspension of the 4-piperin-l-ylbutanpy| chloride hydrochloride (ca.40 mmol) and 2- melhyI-2-thiopseudoiirea sulfate (5.6 g.20! mmol) in aeetonitrile (100 mL) was added tricthylaminc (20 mL.0.27 mol) in portions while cooling in an! ice bath. The reaction was then allowed to warm lo 25 "C over 1 h. The rcaciion mixture was filtered, through Celtic with an aeetonitrile wash (100 mL). The filtrate was concentrated to afford methyl iV:N'-bis-(4- piperidin- 1 -ylbuuinoyl)imidothiocarbainatc (10.6 g, 79% yield) as a brown oil that was used without; further purification. MS (El) lor e2o¾ 402S: 397 ,(MM+).
1 05671 Using analogous synthetic techniques: and substituting with alternative starting reagents /;i'.v-|2-( methoxy)elhoxy || (mcihylthio)melhylideiie]biscarbamale was prepared
according to the method of reagent preparation 10 ii.sing2-mcthoxycthyl chloroformaie in step 2. MS (El) for C,oH,s 206S:.295 (ΜΙ-Γ).
Reagent Preparation 11
|00568| STEP 1: To a solution of 6-broni( 2Hnclltyl-l/7-.iiriidazo|4
?5-/|pyridine (3.40 g, 16:0 niniol) and diisopropyl
'cthylamine (6.5 inL, 65 nim i) in /V./V-diriictliylformamide (20 inL) cooled in an ice bath was added diopvvi.se isobutyl ehlorolOrniate (2.51 ,mL, 19.2 mmol) and the mixture was warmed to room temperalLirc. After I hour the reaction was diluted with ethyl acetate (80 mL) and washed with water (60 mL), |:0¾·aqu(røμsci ρ·aci ·t4U.·mL)· ncl brine (20 inL). The organic phase was dried . over anhydrous sodiunvsiilfate. filtered and concentrated to a slurry. The residue was triturated diethyl ether (100 mil) and the solid isolated by fillratipn to
carboxylaic (¾3 g, 46% yield). MS (El) lbr C, I I uBrN¾C¾: 313 (MH*).
[00569J Using analogous synthetic techniques and subsntutih¾i\viUvMWmaiivC'Siart-in'g reagents in step 1 isobiily! 2-(4-bromophenyf^ \v;as prepared according to the method of reagent preparation 11 using 2-(4:bromophenyl)- IH-imidazolc and isobutyl chloroformate in step I . MS (El) for e|ltHi5Br 2( >: 324 (ΜΙ- ).
100570 J Isobutyl 6-bromo-lW-ben/.o|d|imida/.olc-l -carboxylatc. Prepared according to the method of reagent preparation I I using S-broinb- 1 //-benzol d |inlidazolc in ste L MS (EI) IbrCi.Hi.iBrN.Oa: 297/299 (ΜΙ- ).
Reagent repiinit ion 12
5-Bronib -etliyl-l/ -benzimida/.0le
100571 J 5-bronib-l -ethyl- l/7-beiiziiiiidazolc was prcpared:ifr3 steps from 1 ,4.-dibrbmo-2- hitrobenzenc according to the method described" in (Bioorg, and Med. Chem. Leu.2003, 13. 2485-2488). MS (EI) for CgHyBrNi: 226 (MH+).
Reagent Preparation 13
yV-(5-bronvotIiia7.()lor5,4 ;]pyridi^i-2-yl)benzaniide I00572J STEP 1: To a .solution of ammonium thiocyanatc (0.4 g, 5.0 mmol) in acetone ( mL) was slowly added benzoyl chloride (0.6 mL, 5.0 mmol); and the suspension as heated to reflux for te minutes. A..solution of 6-bromo-2-cldoro-3^yndinamine (1.0 g, 4.8 mmol) in acetone (10 mL) was then added and the reaction mixture was refluxed for one hour. After cooling to room temperature the mixture was poured into water and partitioned with ethyl acetate (250 mL). The layers were separated and the aqueous layer was further extracted with ethyl acetate (2x, 100 mL). The combined organic layers were washed with brine (2x.100 mL), dried over sodium sulfate, filtered and concentrated until a suspension formed. The
white solid was collected by nitration lo.give 7\^(6 ino 2-cldor pyri(Jin-3- .ylcarbainolhioyObcnzaniide ( L<5 g, 89%). Ή NMR (400 Mz. do-DMSO): 12:62 (br s, I H), 12.00 (br s. I M). 8.37 (d, I H). 8.00 (2cl, 2l l), 7.79 (d; I I I), 7.69 :(l, I IT) , 7.5 (t, 2H)..MS (El) for C|.,HyBrCIN.,OS: 370 (MH+).
[005731 STEP 2: A solution of /V-(6-brpnio-2-chIoropyrid»i-3-ylcarbampthioyl)benzamidc ( 1.5 g, 4.0 mmol) and sodium ethoxide.(0.54 g, S.OMiimol) in l-meihyl-2-pyrrolidinone ( 10 mL) was heated to 120 "C for 8 hours. After cooling the reaction .mixture to room
give ; ( br0mothia7Olol5,4-bJpyrid'in -y bciv/.am'idie (1 .02 g,:76%) 1 H .NMR- (400 MM/.. d6-DMSO): 13.2 (br . I H), 8. 16-S. f() (m. 3IT). 7,72 (d, I H). 7.70 Ct. I I I). 7.59 (t. 2H). MS (EI) for C.iHsB K OS: 336 (MH+).
Reagent Preparation 14
[110574] STEP 1 : To a solution of 2-amino-5-bromopyridine (5.0 g, 29 mmol) in dioxane (60 mL) was added elhoxycarbonylisothioeyanate (3.4 mL. 29 mmol) in a dropwise -manner and ' the■mixture was allowed to. stir for I 8h at.room temperature. The -mixture.' was then concentrated and the residue triturated with 10% ethyl acetate i hexanes. The solid was collected by filtration and dried to afford ethyl { |(5-biOmopyridin- 2yl)ainino|earboii0thioyl [carbamate (6.2 g, 69%) as a colorless solid. MS (EI) for
C)I-1 loBr jGiS: 305 (M 1-1*).
[00575] STEP 2: { [(5-Broniopyridiii-2yl)aininolearbonPthioy converted to 6-broiiio-| 1 ,2,4 |triazolo| l ,5-«|pyridin÷2-aminc according to methods in the literature, sec I ) Monatx efr fuer Chemic. 1983, 114(6-7). 789-98 and 2) Synthesis. 2003, / /. 1649-1652. Thus, a mixture of hydroxylaminc hydrochloride (375 mg, 5.4 mmol) and DIPEA (560 uL, 3.2 mmol) in 1 : 1 methanol:ethanol (8 mL) was stirred for 10 minutes at room temperature followed by addition of | |
'(5-brb
'mopyridiii-.2yl)annn6jcai¾onpth|oyl [carbamate (500 mg, 1.62 mmol) and the resulting suspension was stirred for 2 h at room temperature then brought to 60 °C for an additional 2 h. The resulting solution was then cooled to room temperature and concentrated! The residue was then partitioned with ethyl acetate and saturated aqueous sodium bicarbonate. The organic solution was washed with brine, dried over anhydrous sodium sulfate then filtered and concentrated to give 6-bi mo-[ l ,2,4.|iria/.olo|
' I ,5-«|pyridin-2- amine (340 mg, 98 % yield) as a colorless crystalline solid. MS (EI) for C,;H
5BrN.i: 214 (MH
+).
[00576.1 S TEP 3: A solution of 6-bronio-f 1 ,2, |lriazolo| 1 5-n|pyi:idm-2-amine (340 trig, 1 .6 mmol), di-/e/Y-lniiyl dicarbonate (370 mg. 1 .6 mmol) aiid catalytic DM AP was stirred at 35 "C in THF (5 inL) for 1 8h. An additional -equivalent of di- t'/7-butyl dicarbonate was then added and stirring was cont inued for 48 h. The solution was then part itioned with ethyl acetate and water. The organic phase was. washed with brine, dried over anhydrous sodium sulfate then filtered and concentrated. The. esidue was taken into diehloiOmclhane and insoluble starting.maicrial was removed by filtration. The filirate was concentrated and purified by silica gel -chrohuitqgraphy to afford bis-( I , l -dimelhylethyl) (6-
white solid. Ή NMR (400 M Hz, d„-DMSO): 9:45 (s. 1 1 1 ). 7.91 (d. I l l), 7 86 (cl, 1 H). 1: 1 (s. i SH).
|(K)577] Using analogous synthetic techniques and subsliluting with alternative starting reagents bis ( 1. 1 -dimethylelhyl) (5-lm>mo-4 nelhyl- l ,3-thia^
prepared, according to the method of reagent preparation 14 using .5 bi mo-4-rnethylthiazol- 2-amine in step 3 and conducting the protection step at reflux temperature. Ή NMR (400 MHz, CDCI.v): 2:30 (s. 3 H). L53 fs. 1 8H).
Reagent. Preparation 15
6 )romOrl-trit) -l/;/-nn lazb[4,5-ftlpyridine and 6-lmmio-34rit} -3H-imidazol4,5-
/jjpyridine
[00578] STEP 1 : A suspension of 2.3rdiainino-5-brbmopyrid inc (3.0 g, 16.00 mmol ) in formic acid (30 mL) was heated lo reflux for 3 hours.. A ter cool ing the reaction mixture to room
'.temperature it was concentrated and the residue, was taken; into 50% elhyl acetate in toluene ( 1Ό0 mL) then concentrated and , the process, repeated Once more to remove excess formic acid. The resulting solid was triturated with ethyl acetate and the solid residue collected by filtration lo give 6-bromo- l -imidazo|4, 5-/> |pyridine (3,7 g, 95%). GCMS (EI)
[0057 1 STEP 2: To a solul ion of 6-bromo^ 1 H-imidazo|4.5-//|pyridinc (2.7 g, 1 1 :0 mmol) in dimethylformamicle (30 mL) at 0 "C was added 60% sodium hydride in mineral oil (0.53 g, 13.2 inniol ) and the reaction mixture was stirred for 30 minutes, fol lowed by the addition of a solution of triphenylmeihyl chloride (3,2 g, 1 1.55 mmol) in dimcthyllbrmamide (5 mL). The reaction mixture was stirred at room lempcraiure for 24 hours then quenched by the careful addition of water then part itioned with ethyl acetate (250 mL). The organic phase was washed with 10% aqueous citric acid (2x. 100 mL). brine ( 100: mL), saturated sodium bicarbonate ( 100 mL), brine ( 100 mL) then dried over anhydi tis. sodium sulfate, filtered and
coiiccniraiecl. Silica gel. chromatography (hcxane elhyl acetate 9: 1 lo 4:1) provided 6-bromo- 3-trit l-3/7-imidazo|4.5-/|p rrdine (1.8 g, 37%). Ή NMR (400 MHz. CDCI3): S.IS (d. lH), S.14 (d.1 H), 8.02 (s, I H), 7.36-7.28 (m, 10H), 7.18-7.14 (m.5H) and 6-bromo- 1 -irilyl- 1 H- i mid axo[ ,5-/j? Ipyrid i no (2.9 g, 60%) 1 H NMR (400 MHz, .CDCI3 : 8.50 (d. III).8.14 (s, IH). 7.38-7.34 (in. l l-l), 7.16-7.12 (m, 511), 6.84 (d, 111).
Reagent Preparation 16
.A'-(7-I*iOino-[l,2,4]tr:ia/.olqlJ
1.00580) S TEP 1 : To a solution ol:7-I3romo-l 1 ,2,4)ΐι'κιζοΓο| 1 ,5-ri|pyridin-2-.ylaihine (prepared using the procedu e ii'r WO2006038116) (0,15().g„ 0.704 mmol),
diisopiopylethylaminc (0.363 g, 2.81 mmol), catalytic DMAP (0.09 g, 0.07 mmol) iii anhydrous ΊΊ-IF (4 niL) was added acetic anhydride (0.21 g, 2.1:1 mmol). The reaction mixture was stirred at 50 "C for 22 h under N2 (g). After cooling to room temperature the mixture was: concentrated, diluted . i i ^ salurated sodium bicarbonate (40 niL). brine (40 m L). and dried over- anhydrous sodium sulfate. 'Filtration and concentration followed bycoluriin chromatography of the residue on silic (95:5
yl)acetairiide (0.170 g, 95% yield) as a brown oil. MS (EI)! for Csl-l7BrR,0; 256 (MH+). Reagent Preparation 17: l-(4-chIoro-¾,6-.dimethyl-5;6J,8-tetrahydrot|inna7.olin-2-yl)-
N,V-d i m eth l in et an am i 11 e
1005811 Step I : To a solution of methyl :5,5-diiiielhyl-2-oxocyclqhexanccarboxy.lale (.6.0 g, 33 mmol). and 2-chloiOaccliniidairiide hydrochloride (4.6 g, 36 inmol) in methanol (30 mL.) was added sodium niclhoxidc (4.4 M in MeOM, 9.0' mL, 40 mmol),.. The. reaction■•.•mixture was stirred at ambient temperature for three hours and ihcii concentrated. The resulting residue was partitioned between ethyl acetate and aqueous sodoiin bicarbonate. The organic layer was washed with brine, dried over magnesium sulfate and concentrated.. Purification by silica gel chromatography provided 2-(chloromethyl)-6,6-dimethyl-5.6,7,8-tetrahycli ciuinazolin-4- ol (4.2 g, 57% yield) as a white solid. MS (ES) for CnHisCl jO: 227 (MIT).
(00582) Step 2: To a solution of 2-(chloi meihyl)-6,6-dimcthyl-5.6.7.8- icirahydroquinazoIin-4-ol (2.5 g¾ 11 mmol) inTHF (l() mL) was added dimethyl amine (2M in THP, 16.5 mL, 33 mmol). The reaction mixture was healed (60 °C) for two hours and then partitioned b.cl.ween ethyl acetate and sodium bicarbonate. The organic layer was washed
brine, dried' over magnesium sulfate, filtered aiid'Coi 'cninileclio.p.rovidc 2- ((dimcihylamino')mcihyl)^ which was used in step 3 without -further purification. MS (ES) for Gtffl'ai }Θ.? 23'6· (MH*
|()()5 3| Step 3: To a solittipn of the final residue from step 2 in CHCfs (.10 ml_) was added I'OCI.i (10 inL); 'The-rcactiidn mixture washeaied (90 °C) for two hours and concentrated. This residue was: partitioned between dichloromethanc and aqueous sodium hicarhpnate and the resulting organic layer was washed with brine, dried over magnesium sulfate, filtered and concentrated in vacuo. Purification by silica, gel chroniatography'(5-IO concentrated aqueous ammonia in methanol), in chloroform provided i-tf-chior eje-dmjcih l^fe,?.,^ ictrahydroquina7.olin-2-yl)-A'^ yiel l). 'H NMR (400'
Mi l/. CD3OD 64.S2:(s.211).3-02 (s.6M).2,9.8 (t.2W); 2.61 (s.2H), 1.71 (l.2H).1:06 (s. 61-1); MS (ES) for CaHao l j: 254. (ΜΙ:Γ)
[00584] Using;analOgpus synthetic techniques and substiluting viih-altcniaiivc startin reagents the following compounds of the invention were prepared. Alternative starting materials were obtained commercially unless otherwise indicated.
[005851 (S)-4-chltiro-2-((3-riiKm>pyrrylidin^
ictrahydrpquinazoline. Synthesized according to the method of reagent preparation 17 using (S)-3-fkiorPpyn lidinc in step 2. MS (ES) Tor CisH'aiGlFNa': 298 (MlT).
100586] (R)-4-cMoro-2-(( 'fliioropyrrolidm
teirahydroquinazoline. Synlhesizcd.according to the method of reagent preparation 17 using (R)-3.-fluoropyrrolidine in step 2. MS (ES) for CisHiiClFN3: 298 (ΜΙ-Γ).
1.00587] 4-chloro-2-((3,3-difluoiOpyrrolidin-l-yI)nKthyl)-6,6-dimclhyl-5.6.^
lelrahydroquinazolinc. Synlhcsizcil according to the method of reagent preparation 17 usin 3.3--di fluoropyrrol idine in step 2. MS (ES) for C|5H2oCIF2 3: 316 (Mi l4).
|()0588| N-((4-chloro-6.6-clinielhyl-5XT,7.;8-tctrahydrpquinazolin-2-yl)niclhyl^ mclhylclhanamine. Synthesizcd according .to.lhe method of reagent preparation 17 using N- mcthylethanamine in step 2. MS (ES) for GuI- CINs: 268 (MA*).
100589] 4-chloro-6,6-climcihyl-2-(piperM^
Synthesized according to the method of reagent preparation 17 using piperidinc in step 2. MS (ES)..fi>r CftHi C! j: 294. (Μΐ ).
[00590] N-((4 hloro-6.6 liniethyl-5,6.7.8^ctrahydi quinazolin-2-yl)incthyl)-N- melhylpropan-2-am
.inc. Synthesized according to the method of reagent preparation 17 usin N-meihylpropan-2-amine in step 2. MS (ES) for C|
5l½eiN
3: 282 (ΜΙ- ):
mel ylcycopiOpanamne. ynt esized according lo.lhe.method of icageni preparation 17 sing NTii iUylcyclopiOpanahvine in step 2: MS (ES) lor I)5I-l22CI 3: 280 (MHf).
|00592| Benzyl (4 hloi;o-6,6^dimethyl-5.6J,8^^
yl)mcthyi(isopropyl)carbamate. Synthesized according to the method of reagent preparation 17 using propane-2-aminc in step 2 followed by Cbz protection. MS (ES) for C22M:,sGI 302: 402 (Ml-|+).
[00593.1 4-chlpiO-6.6-c1iniclhyl-2-(py! oliLliiv- 1 -yinictlryl)-5,6;7.S-tciraliydroqiiinax.0iiiie. Synthesized according to the method of reagenl preparation 17 usi g pyrrolidine in step 2. MS (ES) for.C , 5I½C I j: 280 (MH+).
1005941 (S),;l-(4-chIoiO-7-cth^
dimethylm^ according- to ,lhc method ofreagcnl preparation 17 using
(S)- methyl 4--ellvyi-2-hytliOxycyciohexrl-cneearboxylate in step I . MS (ES) for CijHioCl j: 254(Μ1-Γ).
[00595] (4-ehloro-5-[(4 1uorophenyl)methyl]-6^^ acetate. Synthesized according to the method of reagcnl preparation 17 using.2--(ch'l.oromeihyl)-5-|(4- fluoro.phcnyl)meih.yI |-6-methylpyrimidih-4-dl and sodium. acetate in acetic acid in step 2. MS (ES) ΙΌι·ει5ΗΜαΕ 0.: 30.9 ( H+).
[00596] 4.≤ciiloiO-2-(inetIvoxymetiiyl)-6;6-
Syulhcsizcd. ccording lo
'lhe method of reagent preparation 1 using sodium methoxidc in step 2. MS (ES)
241 (MH
+).
[00597] Benzyl (4 -hloro-6,6-climeilvyl.-5,6 ,8-letrahydroquinazoliir-2-yl)in
carbamate. Prepared according to the method of rcageiU preparation 17 by using elhylamiiic in step 2 followed by Cbz protection. MS (El) for C2|H2(-1CIN,0 : 388 (MH+).
[00598] Benzyl (4 hloro-6,6-dimelhyl-5.6,7,8-tcirahydi C|uinazolin-2-yl)mcthyl(2- fluoroeiIiyl)carbamate. Prepared according to the method ofreagcnl preparation 17 by using fliiorocihylaminc in step 2 followed by Cbz protection. MS (EI) for ^iH sClF jO.: 406
( :H+)v
[005991 (4 hloro^6,6-di[nelhyl-5',6,7.8-ictrahydroquinazolin-2- yl)metlvyl|eyclopropanamine. Prepared according to the method of reagent preparation 17 by using cyclopropylamine in step 2. MS (El) for C14H20CIN3: 266 (MH+).
[00600] Benzyl (4-chlorc)-6,6-dimetliyl-5,6.7.8-lcirahydi quinazolin-2- yl)mcihyl(cyclpbulyl)carbamate. Prepared according to the method of reagent preparation 17
by using cyclobtltylaniinc in step 2 followed by Cbz- protcctitinJ S; (Ef). for G iHasGI' jOj: 414 ( H+).
|l) 6() ] l-(4-Chloro-5-(cyelo|m)pylmcthyl 6 w^ .
dimcthylmcihanamine. Prepared according to llie .method of reagent preparation 17 by using methyl 2-(.cyciapr&"p ifn^ihyl 3'Oxdbutart0atc (reagent preparation 8) in ste 1. MS: (El) for Ci2HmClr¾:240;(Ml ),
;cjIi thSi^ preparation 17¾ using dicihyiaminc in step 2. MS (El) lor CijH2.iCIN3: 282 (Mi l*).
[00603] 4-((4-Chkm>-n,(vdinicihyl-5,6j;8-tctrahyd
Prepared accordin to the method of reagent preparation 17 by using morpholine in ste 2. MS (El) for Ci5ll
22CIN.
(0: 2 (M(P).
-using eiliylis.oprppylaminc in step 2. MS (El) for .C,,,I½,CiN,: 2¾'0νΐί-ί
+):
metliylprppan-2Taminc. Preparedaccording,t the nietlrod of.reagent preparation 17 by using lert-bulylaminc in step 2. MS (El) for dsl-ktClN. 282 (ΜΙ- ).
[006061 N-((4-chloro-6,6-dinicthyl-5.6,7,8-(e.lrahydroii.uina/^)lin-2-y0
methylpiOpan-1 -amine. Prepared according to the method of reagent preparation 17 by using iso-buiylaiiiinc in step 2. MS (El) lor C H.MCI -,: 2S2 ί H+).
[00607] Benzyl W-chloro^oydurieth l-S^^
clifliiorocthyl)carbaniate. Prepared according to the nicilibd of reagent preparation 17 by using 2,2 liflllol' clhylainine.in.step 2 followed by.G.bz,proiection..MS (EI) for
C2|H2;1CIF2N;(0 : 424 (Mi l*).
|0()6()8| N-((4-chIoro-6)6-diincthyl-5.6J.8-ieirahyclrociuii)azoliii-2-^
irifluoroethanaminc. Prepared according to the method of reagent preparation 17 by usin 2.2.2-trinuproethylamine in ste .2. MS ( E I) for C u M |7G1 FJ'N.-I : 308 (MH+).
[00609] Ν-(( - Ιί1οι·ο-6.6-^ιηϊοιΙιν1-5,678^ιεΐΓ«ί.ΐ γιΐΓθΐ π^
cyclopropylethanamine. Prepared according to the method of reagent preparation 17 by using
in step 2. MS(EI) f6rlC,<
yl¾CIN
3>.294 (MH
');
(00610] (4-Chlorp-6/>-dime
Prepared according, lo.tic method of rcngeiitpreparation 17 by using poiassium acetate iii step 2. MS (EI) l rGi H,7CI 202: 269 (MH+)..
[006 I I ] Benzyl (4-chloro-6,6-dimcihyl-5.6.7,8-lcviahydiG()iiiiia'/,«lin-2- yl)mclhyl(eye!opentyl)earbaniale. Prepared accordin lo the method of reagenl preparation 17 by usiiig cyclopcntyla'mine in step 2 followed by'Cb/ proiceti0ii.. iVlS (EI) for
C2.! Hj,iCI N302: 42S (iVI H+).
[00612] Elhyl 2-((4 liloi -6.6-dinieihyI-5.6J,S,tctrahydro(|iiinax.olin-2- yl)mcihylamino)propanoaic. Prepared accordingao the method of reagent, preparation 17 by using alanine ethyl ester in step 2. MS (El) for Cifil-lyCl
326 (Μ1-Γ).
[006131 l -( -eiiloro-5.6-climelhylpyrimidin-2-:y -A';A/-cliiriclliy Prepared according lo the method of reagent' preparation 17 by usin methyl 2-niethyl-3-oxObutnnoatc in step 1 i step 2. MS (El) for GIM MCI N^: 200 (M H+).
100614 ] l - 1 clrIoro 5r.(4-nu0rbbenzyl -6-m ·
dinicthylmethaiiaminc. Synthesized according to the method of reagenl preparation 17 using methyl 2-(4-fliiaiObenzyl)-3.rOxobitlaiioatc in step 1 . Ή NMR (400MHz, CDC¾): 7.08-7.05
(in, 2H ). 7.00-6.96 (m, 2H), 4: 14 (s, 2H),.3.68 (s. 2H), 2.5.1 (s, 31 1). 2.38 (s, 6H).
[00615] l -(4-chloro-5-isopropyl-6-iiiethyIpyriniidin-2-yl)- /V-dinie
Synthesized according to the method of reagent preparation 1 7 using methyl 2-acclyl-3- nicthylbuianoatc in step 1. MS (EI) for C
n HisNjC!:
'228. 230·( Η
f. CI isotope pattern).
(S)-butan-2-aniiuc in step 2 followed Cbz-proteciion prior to step 3. MS (ES) for
[00617 ] (R)-bcnzyl sec-bulyl((4-chIoro^6;6-diniethyl-5;6^
yl )me(hyl)carbamate Synthesized according to the method of reagent preparat ion 17 iising (R)-bulan-2-amiuc in step 2 followed Cbz-protcclion prior lo slcp 3. MS (ES) for
C^l fioCINiiCh: 416 ( Ι- ).
[00618] l -(4rchloror6-ethyl-5-nicthylpyriiiiidiii-2-.yl)-AW-d
Synthesized according to the method of reagenl preparation J using methyl 2-mcthyl-3- oxopentanoate iii step 1 . MS (ES) for :GioH|(,G)N3: 214 (MH+).
[00619] l -(4-chloro-5-isopropylpyrimidin-2-yl)-yV.N-dimcihyinicihaiianii e Synthesized according to the method of reagent preparatio 17 using methyl 2Tniclhyl-3-oxopcnianoaic (Elaridi et al. Tetrahedron: Asymmetry 2005, 16(7), 1309- 1 19) in step 1.
[006201 N-((4-chloro-6.6-dimeihyl-5.6,7.8-lctrahydro(|iiinazoIin 2-yl )mcthyl)-N-incihyl-2- nitrobenzcncsulfonamide Synthesized according lo the method of reagent preparation 17 using melhylamine in step 2 followed by protection a.vihe 2-niirobenzcncsuilbnamidc prior to
step 3. Ή NMR (400 MHz. CDCI.v) δ 8.I8-K.1 (m: IH)..7.71-7.62 (m.2H), 7.61-7.57 (in, III), 4.69 (s, 2H), 3.08 (cl.3H).2.73 (t.21-1).2.47 (s.2H), 1.60 (l, 2H), 1.01 (s.6H); MS (ES) forCi«H2|CIN404S: 425 (MM*).-.
1006211 N-((4-chloiO-6.6-cliinci!iyl-56.7.S-iciraliy(li t|uinax.olin-2- yl)metltyl)nieihane.siilfoiiami(le Synthesized according to the method of reagent, preparation 17 using ammonia iivstcp 2 followed by niesylation rior to . step 3. Ή N R (400 MHz. CDGI.i) 84.49 (d.211).3.01 (s.3ll).2.90 (l.211).2.54 (s, 2H), 1.67 (t.2H)V1:05 (s, 6H): MS (ES) for C|2HiSCIN.t02$: 304 (Ml-f),
[00622] l-(4-chloiO-5-ethyl-.6-me^
Synthesized according to the method of reagent preparation 17 using ethyl 2-ethyl-3- oxobutanoate in step I. Ή NMR (400 MHz. CDCI3) § 3.64 (s, 211).2:78 (q.2H).2.58 (s. 3H),2.36 (s, 6H).1.19 (t.3H); MS (ES) for C,oI½CIN;,: 214 ( Ι- ).
[006231 4-chloiO-6.6-diniellVyl-2-({|2-(jnell)>½xy)cth"ylloxy}inelh\4)-5.6.7.8- teirahydroquinazgl inc. S.ynlliesized according to the method of reagent preparation 17 using sodium hydride and 2-rinelhoxyelhanol in'NN-dinietlvyl forma in step 2. MS (ES) for C|.H2|CIN20 : 285 (Μ1- ).
[0062 | N-l(4-chk)ro-6;6-diinetlvyl-5 i;7,8-ieirahyclroquinaz:^
(meihyloxY)cthanaiuiuc. Synthesized according to the method of reagent preparation 17 using 2-methoxycthanamiiie in step 2. MS (ES) for C|,Hi2Cli ,0: 284 (MH+).
[006251 N-((4-chloiO-5-(4-nuoiObenzylh6-nicihylpyrimidin-2- yl)incihyl)cyelopro|umamine. Prepared according to ihe method. f reagent preparation 17 by Using methyl 2T.( -fluatoib'eiv/.5'l j and cycloprop laniiiic in step 2.
MS (El) fqrC|0H,7CIFN;,: 306 (MH+).
dinielhylmethanamine. Prepared according to the -met od of reagent preparation 17 using inethyl 5.5-dimethyl-2-oxocyclohex-3 enecarhoxylale(Can. J. Ch
'cm., 1981.59, 601-608) in step 1. MS (ES) for
284 (Ml-f).
Reagent Preparation 18: Phcnylmcthyl (2/i)-2-(4-ehlor0-6,6-dimetljyl-5,6,7,8- teti'ahydn)(|uina/oliii-2-yI)pyrrolidiiie-l-carl)oxylate.
100627) STEP I : To sodium mclhoxidc (30wt% in methanol.8 mg, 0.05. mmol) was added a solution of?(R)^benzyl-2-cyaiiopyrr0lidine- I-earboxylaie (189 mg, 0.82 nimoj) in methanol (1 niL) at room temperature and the reaction mixture was stirred for one -houf: Aniinoniun chloride (44 mg.0.82 mmol) was introduced
'and the stirring was continued for an additional two hours, followed by the addition of me.lhyl 5,5-dimelhyl-2-oxoeyclohexancearboxylate
( 100 mg, 0.5 mniol) and sodium melhoxidc (30wl in methanol. 29.1 nig, 1.63 mmol). The stirring was continued for two more hours. The reacliOiv.mixturc was ..quenched with water ( 10 inL), neutral ized with I N hydrohloric acid and extracted with ethyl . cetate (;3 10 inL). The . combined extract was washed with water (20 inL) and briiic, dried over sodium sulfate, filtered, concentrated and purified by gradient flash chromatography (25% t.q 95 cthyl acetate in hexane) to give
lelraliydrpquinazolin-2-.yl)^ mg. 90%);. MS (EI) for
Ci2l- 7Noi03: 3. 1 ( i l ').
| (K)628| STEP 2: A mixture phenyiiiiediyf (2/v')-2-(4-hydroxy-6;6-dimeth
leirahydroc|uiiiazolin-2-yl)pynOlidine- l -carboxylaie ( 1 0 mg. 0.39 mniol). and phosphorous oxyeh.lor.ide (1 niL) in chloroform: (3 mL) was. stirred at 80"e forgone hour: After cooling- to room temperature the reaction mixture was coneehtratcd and llie residue was partitioned between saturated sodium bicarbonate (20 niL): nd ethyl acetate (20 mL). The mixture was stirred for 15 minutes and p'H was-maintainecl above 7 by the addit ion of solid sodium bicarbonate. The organic layer was separated and washed with water ( 10 niL) and brine, dried over sodium sulfate, filtered and concentrated to give phenyl methyl (2 ?)-2-(4-chloro-6:6- diniethyI-5,6 8 etrahydroquii izoUn-2-yi)pyi olidine- 1 -carboxylate ( 1.17 mg. 74%). MS' (El) for C22H26CI 3O.: 400 ( 1- ).
100629] Using analogous synthetic techniques and substitut in with alternative starting materials in step f the fol lowing reagents of the invention were prepared. Alternative starling niaicrials were Obtained commercially unless otherwise indicated.
[0.0630] PhenyJmeihyl (2iS')-2-(4-cliloip-6,6-diinethyl-5,6.7.8 etrahydroq^
y pyrrdlidine- 1 -carboxylate. Prepared according to the method f reagent preparation I S by using (S)-benzyl 2-cyanqpynOlidinc- l -carboxylate in step 1.( 1 1 8 mg, 75%). MS (EI) for C22H26C1N302: 400 (Μ Ι-Γ).
[00631 ] Phcnylmethyl 2-(4-chloiO-6,6-dimcihyl-5,6.7,8-lctrahydiOquina/.olin-2- y pyrrolidine- 1 -carboxylate. Prepared according to the method of reagent preparation 1 8 by usiii (R,S)-bcnzyl 2-cyanopyrrolidine- r-carboxylaie in step 1 ( 1 1 8 mg, .75%). MS (El) for
Reagent Preparation 19: Plicnylinctliyl { |6-bromo-3-({ t2- (trinictliylsilyI)t'tliylJ()xy}nie(liyr)-3//-imida/.()(4,5-ilpyridi»
yl jmcthyljmcthylcurbaniatc.
[00632] STEP 1 ; To a mixture of 2-[(benzyIoxycarbonyl)(meihyl)anrinojacciic acid (0.42 g, I .S8 inmol), 0-(7-azabcnzotriazol- l.-yl)-/VjV,/V',/V'-ietramcthyluronitim
licxariuoropiibsplKUC '(Q.75¾. 1.'97. mmol) in /V.yV-d i nict h I Iwma ide ( 3.0 in L),
V.A'-diisopropylctliyjaniine (0.72 niL. 4. 12 mmol) was added and the reaction mixture was: stirred for 30 minute's al room temperature, followed by the addition of 5-bronio-2y3- diaminopyridinc (0.35 g, 1 .86 mmol). then stirred for 16 hours. It was diluted with ethyl acetate (50 mL). washed with aqueous lithium chloride (2 x.20 niL) aitd brine, dried over sodium sul ate, filtered and .cpncenintie(l,:.Gradicni.i'lash chromatography (35% to 85% ethyl acetate in hcxaiie)■ provided pliehyliTiethyl :{2-.| (2-a
pxocthyt}mcthylcarba.maie (:0; O}g 9 ¾ .,M ^ ΦΪ ΙΪ7¾Γ¾<¾: 394 ( l-f );
[110633] STEP 2: A'soliitioivo pheuylmeUiyl {2- (2-a iii 75¾ m
2-oxoethyl ) mcihylearbaniate (Q.30.g, 0.76 mmol) in acetic acid (7.5 niL) was healed in a microwave apparatus (250 VV) for 30 iniri. at 120 °C. After coolihgiil to room tempcrauire the reaction mixture was concentrated and the pH was ad justed to S by die addition of. saturated aqueous sodium bicarbonate. The precipitating solid was collected by, filtration, washed with
[00634] STEP 3: To a solutioji of plieiiylniethyl
yf)methyl |methylcarbamaie (0:22 g. 0 59, nVtivol) itt Y. iy-dinietliyl formain ide (3:0 in L was added 60% sodium hydride in mineral oil (56
"mg 1.48 mmol) and the reaction
'..mixture was stirred for 30 minutes at i()oni temperature, jOlIowcd by the addition of 2- (irimethylsilyl)eihoxynielhyl chloride (C i 1 mL, 0.62 minol); The reaction mixture was stirred at room temperature for 16 hours then it was quenehed by tlicicareful addition o saturated
water (20 niL). The organic layer was separated and washed With .10% aqueous citric ncid (2 20 mL) and brine (20 mL), dried over sodium sulfate, filtered .
'and concentrated.
'Gradient flash chromatography ( 15% to 35%: ethyl acetate in hcxane) gave phcnylmethyl { |6 bromo-3-( { 12- (iriniethylsilyI)elhyl ]oxy } oieinyl)-3H-im^
(0:28 g, 93%). MS (EI) for C I- BrR^Si: 506 (.Vllrf ).
[00635] Using analogous .synthetic techniques' and substituting with -alternative starting materials and reagents in step I or step 2. nd step.3 the following reagents of the invention were prepared. Alternative stalling materials, were obtained coninicrciaily unless otherwise indicated.
1.00636] Phcnylmethyl 1 ( I /i)- l -1 -bromo-3-(| [ 2-(tnmcthyls'ily)')elhyl lox } methyl )-3/·/- iniida2o[4,5-/>|j>yfidm^
Synthesized according to the method of reagent preparation 19,
'by using.5-biOmo-2,3-
diaininopyridine and N-(beivzyloxycarbonyl)-D-alanihe in step 1 and 2- (trimeihylsilyl)ethoxymethyl chloride in step 3. MS (121) for
636. (MH
+).
[00637] Phenylmethyl { < l.> 1 -|6-hronio-3-(J |2-(iriinei ylsilyl)eihyi |oxy iicihyl)-3/V- iimdazo|4.5-/>|pyridin-2-yl|ethyl |(.||2-(trinictliylsilyl)cthy |pxy}methyl)carbainaic.
Synthesized according lo the method of reagent, preparatioii by using 5-bi ino-2
;3- diaminopyridinc
'and N-Cbcnzy!oxycarUonyl)-L-alaiiine. in step I and 2- (ti'imcthylsilyl)eihoxynielhyl chloride insiep3. MS (El)
636
i.(MH
+) 1006381 7-Brbmo-2-methyl-3-V{|2-(methy!oxy
clpyrldine and ?-brom -2TUielhyi-l ÷(†|'2^
t lpyridinc. Synthesized aecordirtgio the nicthodof reagent, preparation 19 by using 5- broinopyridincr3,4-clianiine.and tricthyl orthoacetate in step 2 and mellioxyethoxymeihyl chloride in step 3. Ή NMR (400 Ml-Iz. CDCI v): 8.83' (s, 211). -8.44 (s, 211), 5.88 (s, 2H).5:66 (s.2H),3,36 (s, 3.H).3.37 (s, 3 H), 2.98 (s.41-1% 2:91 (s, 414), 2.73 (s.3H).2.75 (s.3H): MS (El) forCnHmBi jO:: 301 (MH+).
[006391 l-(6-BiOnio-3/-/-iniidazo|4.5-/;|pyridin-2-.yl)eihanc)l. Synthesized according to the method -of reagent 'preparation 1,9 by using D.L-laelie acid in step 1. MS (El) fo CsHsBrNjO: 241 (M/7-).
Synthesized, according, to the method of reagent preparaiipn 1 using 4-bromobcnzene- 1 ,2τ diamine and difluoroacelic acid in step I and BOC protection
in step 3- MS (El) for 6-biOmp-2-(difliK)rpmetliyl)-l/74enzo|tl)imidazolc (step 2)
CSHJBIF N.: 247.249 (ΜΙ- , Br isotope pattern).
[006411 1 1-Dinicthylcthyl 6-bromo-2,4-dimcthyU 1 /7-benzimidazolc- 1 -cat boxylate.
Synthesized according to the method of reagent. reparation 19 using 5-bromo-3- methy!benzene- l ,2-diaminc and acetylaiidn using acetyl chloride in lcirahydiOl'uran .in stcp 1 the BOC protection with di-lerl-butyl dicarbonate in step 3. MS (EI) for CijHnB^Oz: 267. 269 (M-Boc. Br isotope pattern).
[00642] 1.1 -Dimcthylethyl 5-bromo-6-lluofo-2-mcthyl- 1 /7-benzimidazole- ί -carboxylnte. Synthesized according to the method of reagent preparation 19 using 4-bromo-5- fluoi benzcne-l,2-dianiine and tricthyl onhoacelalc in step 2 and BOC protection with di- tert-butyl dicarbonate in step 3. MS (EI) for C|3H|,BrFN:02: 271.273 (M-Boc. Br isotope pattern).
[00643] 2TMethylpropyl 5 )rbmo-4-niioro-2 ncihyl-17/-bcnzimidazole:l^carboxylaie. Synthesized according to the method f reagent. preparation 19 using 54-bromo-3-
fluorobenzcne- l ,2-diaminc and aectylalion witlracclic aiiliyclridc iii icirahydiOPurane in step 1 then treatment with isobuiyl chiproformalc in slcp:"3. MS (El) for C i-luB NiC : 328..330 (M IT1. Br isotope pattern).
1006441 6-Bronio-2-clliyl-3-({ | 2'-(trinielltylsilyl)ctbyrjoxy ) incthyO
/>|pyriclinc. Synlbcsi/.ed according to the method of reagent preparation 19 by using.5-bromo- 2,3-diaminopyridine and irimethyl orthopropionaie in step 2 and 2- (tnmcthylsilyOcthoxyiiietltyl chloride in step 3. MS (El), l or Gu H-BrNiOSi: 357 (MH+).
[00645J 2-fVlethy I propyl ^-brpino-2-eyclqprqpyl-37^-imidaz0[ ;5÷/ p>M,idj c-3-''
carboxylatc. S ynthesized according to the method. of reagent preparation 19 by using 5- broni0-2 -dianuhopyritline aiul acylation wiih cycloprppylc^i'bpnyl, chloride in step I and treatment with isobuiyl chlbiOforniaie in step 3. MS (Ei) -forGiaHiftBr jOi-: 339 (MH+).
[00646] 2-Mcthylpropyl 5-bromo-2-(fIuoiOmethyI )- 1 / -benzimidazole- 1 -carboxylatc. Synthesized according lo the method of reagent preparation 19 using 4-bromobenzene- 1 ,2- diaminc and Fluoroacctic acid in step I then treatment with isobutyj chlorolormatc in step 3. . MS (El) l:or C 13H uBrFN202: 330. (M H4>.
[006471 STEP 1 : To a solution of 4-methoxyaiuhranil ic acid (5.0 g, 30.0 mmol) in a mixture iOT 10% methanol in tctrahydrofurau ( 100 itiL) was added dropwisc
(trimethylsilyl)diazomethane (2.0 M solution in diethyl-ciher. 18.0 mL. 36.0 mmol) at 0 "C. The reaction mixture was stirred for 1 , hours at room temperature then quenched by the addition of glacial acet ic acid .(0. 1 mL). The reaction mix lure was concent rated and the residue was partitioned between .saturated sodium bicarbonate (50 mL) and ethyl acetate (250 mL). The organic layer was separated and washed with water (50 mL). saturated sodium bicarbonate (50 mL) and brine (50 mL). dried over sodium sulfate, filtered and concentrated to give methyl 2-amino-4-mcihoxybenzoate as an oil (5.4 g,. quantitative). MS (EI) for e Hu N(¾:J 82 (M H+).
[00648] STEP 2: To a mixture of methyl 2-amino-4-methoxybenzoatc (5.4 g, 30.0 mmol) and chloroacelonilrile (2.8 mL, 45.0 mmol) was added ..anhydrous hydrogen chloride (4M solution in 1 ,4-dioxane. 20.0 mL, 80 mmol) and the reaction mixture was stirred at 50 °C for 30 minutes. After cooling it to room temperature the resulting slurry was diluted with diethyl ether ( 100 mL) and the st irring was continued for an additional 30. minutes. The off-white
precipitate was collected by filtration, washed with diethyl ether and, dried in vaciio to provide 2-(chloromeihyl)-7-(nicihyloxy)c|iiinaz l in )l hydrochloride (7:5 g, 96%). MS (EI) for C|ol¾CIN202: ; 225 (ΜΊ-Γ).
[00649] STEP 3: To a solution of dimcihylaniine (2M solution in tcirahydrofuran. 40.0 mL, 80.0 mmol) was added 2-(chloroniclhyl)-7-(incthyloxy)c|uina/oliii-4-ol hydrochloride (7.5 g. 29 mmol) and the rcaclion mixture was stirred for 90 minutes al 50 "C. Al ter cooling it to room temperature the reaction mixture was concentrated and (lie residue was partit ioned between water ( 100 mL) and ethyl acetate (250 niL). The organic layer was separated and washed with water (J 00 niL), saturated sodium , bicarbonate ( l 00 niL).and bi ine,( l 00 niL), dried over sodium sulfate, filtered and concentrated to give 2-| (dinietlvyiannn(¾
(meihyloxy)quinazo]in-4-ol (6.6 , 97%). MS (El) for C.|.2M½N302:- 234 ( H+)
[00650] STE 4: A solution of 2-t(dinieihylaniino)ineihyj J-7-^
(6.6 g, 28.0 mmbl) in a mixture of chloroform ( 1 5.0 mL).and phosphorous oxychloride ( 15.0 mL) was heated to reflux for 90 minutes. After cooling it to room temperature the reaction mixture was concentrated and the residue was partitioned bciwccii saturated sodium bicarbonate ( 100 mL) and ethyl acetate (400 mL) and the mixture was
' stirred for 30 minutes. The , organic layer was separated' and washcd wilh saturated sodiuni bicarbonate (2 100-mL) and brine (20Q. mL);
J:lllqred and concentrated., Purification by silica gel column chromatography using 15% methanol containing 0.5% triethylammc in ethyl acetate provided l -| 4-ehl()ro-7-(methyloxy)quinazpl in-2-ylj-/V. '-di
(7.Ό g, quantitat ive). MS ( El) for C^H uCii^O: 252 (MH+).
[00651 J Using analogous synthetic techniques and substituting with alternative starting materials in step 2 the following reagents of the invention were prepared. Alternat ive starling materials were obtained commercially unless otherwise indicated..
[00652 J l -(4-chloro-6-lluoroi|innazolin-2^ Prepared according to the method of reagent preparation 20 by using, methyl 2-aminq-5-fluoi obcnzoate in step 2. MS (EI) for C, , H | ,CIFN3: 240 (ΜΗ· ).
Reagent Preparation 21 : 5-Bromo- l-niethyI-lH-pyrrolo[2,3-b]pyridine
[00653J STEP I : To a mixture of 5-bromo- l / -pyi i lo| 2,3'-b|pyridine (207 mg. 1 :05 mmol), sodium hydride (29 mg, 1.2 1 mmol) in letrahydiOfuran (5 mL) was added
iodomelhanc ( 164 mg, 1 . 1 mot) then stiri ed, for 2h at room temperature. The reaction mixture was carefully quenched with water then extracted with ethyl acetate (3x). The combined organic layers were dried over magnesium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by silica gel chromatography in give 5-
broino-l-nielhyl-l /7-pyi iolo|2 ^b|pyritlinc. MS (CI) for CsH7Br 2: 209.211 (ΜΙ-Γ, Br pattern')..
|00654] Usitig analogous synllietic techniques and substi utiiig
alternative starting reagents in step 1 the following reagent was prepared.
b|pyridine. Synthesized according to the method of reagent preparation 21 using iodoethane. MS (EI) for CM.,BrN2: 223.225 (ΜΙ-Γ. Br pattern).
Reagent Preparation 22: (4-(4-iir<Jit>ophenyl)-l//ihiidazol-2yl)nK,t!iariol
[00655] STEP 1: To a solution of ethyl ihiooxamaie (10.0 g, 75 mmol). in
di i'loromcthanc (400 mL) was slowly added- Irimethyloxbnium tcirafhioroborale (13.1 g, 8 minol) 'at 0 "C. After 1 min the ice hath was removed, and the reaction mixture was stirred overnight. The solvent was removed to afford ethyl 2-imino-2-(methylthio)acctate (i2;0g, 66.6%) as tetrafluoroboiatc salt which was used without further purification.
[00656] STEP 2: A mixture of 2-amino-4-broinoacetoplVenone hydroeltloiide (4.0 g.16.0 mmol), sodium acetate (6. Ig, 90.0 nimol), acetic acid (4,6 mL, 80.0 mriiol) and ethyl 2- imino-2-(meihylthio)acefate .(7.7g, 32.0:>mmol) iiV'dioxanc C40:niL) was "stirred at-95 "C overnight. The reaction mixture was carefully iieiitfalized vyilli saturated NaHC03 solution and extracted with ethyl acetate. The organic sqhition-was dried.over sodium sulfate and concentrated. Purification by silica gel column chromatography (ethyl aee uedicxancs 1:1) afforded ethyl 4-r4-l>rpinophenyl)i rH-imidazolci2-eaitoxylarevi3..53ig; 75.0%). MS (EI) for C,2HiiBrN2Q2: 296·(.Μ.Β),.
[00657] STEP 3: To a solution of ethyl ÷e-1jrQinQphcivyl)-iH-ini azplc--2rcarb'ox lalc (1.30'g, 4.40 mmol) iivTlTF (30 mL) was slowly added Red-AI( 65 vvt% in toluene, 2.0 mL, 6.16 minol) at - 25 "C: The rcaelion. niixtiire was stirred for'4h at. the same temperature then slowly warmed to 0 "C over Ih and quenched with 20 % sodium tartrate solution (30 mL). The reaction was extracted with ethyl acetate (70 mL) and the organic layer was left for 3h at room temperature. A solid separated and was collected by filtration, washed with ethyl acetate and dried to afford (4-(4-bi mophenyl)-l/7-imidazpl-2yl)methanol (778 ing.71.0·%). MS (E I) for C I ()Hy B rNiO : 254.1 (MH+).
R' = NH2, NHGH3, CI
[00658] STEP 1 : To a solution of (R)^pyrrolidih-3-ol (32 mg, 0.3 mmol) and potassium carbonate (102 mg, 0.74 mmol) in dioxaiic (2 mL) and water (400 uL) was added 2-aniino-5-
biOmopyridine-3-sulfonyl ch loride f 100 nig, 0.37 mmol. prepared according to the methods in WO2008 144463). Tlie reaction mixture was stirred for 2 h at i t. Saturated sodium bicarbonate was then added, and ihe aqueous solution was extracted twice with ethyl acetate. The combined organic extracts were dricd.ovcr magnesium ' sulfate, filtered, and concentrated in vacuo to provide ( )- l -(2-aniino-5-bronK)pyridin-3-ylsulfonyI )pyrroIidin-3-ol (87.3 mg. 0.27 mmol. 73% yield) as a white sol id. Ή NMR (400 MHz. D .SOD6-d6) δ 8.3 1 .(d, .1 I I ), 7.92 (d. 1 H). 6.85 (br s, 2H), 5.02 (br s. 1 H). 4.23 (dt, I H). 3.38-3.25 (m. 31 1). 3.14-3.06 (m. I H ). 1 .92- 1.81 (m. I H). 1 .77- 1 .67 (in, I I I); MS (EI) for Ο)Η ,:Βι Ν.,0;(δ: 322, 324 (Br isotopes. MH÷).
|()()6591 Usirig analogous synthetic techniques and substituting; with alternative..starting reagcnts^ i^sf^ ; 1, the following reagejiLs were prepared. /Alternative stallin materials- were obtained commercially unless otherwise indicated.
[00660] Prepared
according to the methods described in reagent . 'preparation 25 using 2-inelhbxyelhanamihe in step I .
[00661] 2-aniino-5-bronio-N-(2.2.2-trinuoroetlvyl)pyridine-3-stili¾n Prepared according to the riielhods described in reagent preparation 25 using 2,2.2-t ifluoroethanamine in. ste 1 .
[00662] 2ramino-5-bromo-N-(2-hydroxyethyl)-N-niethylp>Ttdine-3-siilfonamide. Prepared according to the methods described in reagent preparation 25 using 2-(metliylamino)ethanol i ste 1.
[00663] 2-amino-5-broino-N-(2-hydroxypropyl)pyridine-3-stillOnamidc. Prepared according to the methods described in reagent preparation 25 using, l -aminopropan-2-ol in step 1. MS (EI) for C
sH
3 10. 3 1 2 (Br isotopes. 1- ).
[00664] 2-amino-N-(azctidin-3-yl )-5-biOniopyridihe -sitlfonaniide Prepared according to the methods described in reagent preparation 25 using icrt-butyl 3-aminoazctidinc- l - carboxylale in step 1 .
[00665] . 2-amino-5-l Onia-N-(2,3-dihy.drox Prepared according to the methods described in reagent preparation 25 using 3-aniinopropanc- l ,2-diol in step I . MS (El) for CxH l2Bi N:tO.,S: 326. 328 (Br isotopes. MH+);
100666] l -(2-amino-5-biOmopyridin-3-ylsulfonyl)piperidin-3-ol. Prepared according to the methods described in reagent preparation 25 using pipcridinO-ol in step I . MS (El) for CoH uBrNsOjS: 336. 338 (Br Isotopes. Μ1-Γ).
(00667] 2-uminb-N-(3-ainmo-2.2Tdim^^
Prepared according 10 die methods described in reagent preparation 25 using 2.2- dimethylpropane-I.3-diamine in step I. MS (El) for CiJIivBrNiiGi : 337.339 (Br isotopes; MH ).
[ 066S] 2-ami!io-5-broiiio. N-(3-li.ydroxy-2,2-dinie.ll)ylpropy
Prepared according to (lie methods described in reagent preparation 25 using 3-3ιηίηο-2.2 dimeihy.lpropaii-l.-όΐ in step I. MS (El) for Gi H|ftBrN50.,S: 338: 40 (Br isptopes. MH+).
[00669] 2-amino-5-bronw-N-(l-iiydrox
Prepared according iodic methods described in reagent preparation 25 usin 2-amino-2- mcthylprOpaml-ol in step I . MS (El) for CoHuBrN3OjS: 324.326 (Br isotopes, MH+). (00670] icrl-butyl 4-((2-amino-5-lmmiopyridm^
carboxylate. Prepared according.to the methods. described in reagent preparation.25. using lei hbutyl 4-(aniinonvelhyl)piper'idinc- 1 -carboxylate in step I . MS (El)fof CifiHjsBr uOaS:
393, 395 (Br isotopes. MI f-t-butyl).
[00671] 2-amino-5-l>romo-N-((l-mclhyIpiperidin-4-y^
Prepared according to the methods described in 'reagent, preparation 25'iising (1- mcihy,lpiperidin-4-yl)meihanamine in step 1. MS (EI) for Ci Hiy r jOjS: 363.365 (Br isotopes. ΜΙ- ).
100672] leri-biityl I -:((2ianiino-5-biOniopyn,dine^3- sullbnainido)mctliyl cyclopropyrcarbaiiiale. Prepared; ccording to the methods described in reagent .preparation 25 using tcrt-biilyl 1 -(aminpnietlvyl)GycI propylearl)aniate;:in ste I . MS. (EI) for CW½Br :t(¾S: 365.3 7< (Brisotbpes; ΜΐΓ-1-bulyl).
[00673] ten-butyl lraiis-4-(2-amino-5-bromopyridinc-3-siilfpnM^
Prepared according to the methods described in reagent preparation 25 using, tert-butyl trans- 4.-amin0cycl0hcxylcarbamale in step I .
100674] benzyl l-(2-aniino-5-br( iiiopyridihe-3-sulfonaniido)propan-2-yIcarbamate.
Prepared according to the methods described in reagent preparation 25 using benzyl I- aminopropan^-ylcarbamate iii step I .
[00675] 2-amino-5-bramo N-ethylpyridine-3-sulfonamide. Prepared according to the methods described in reagent preparation 25 using clhylamine in step I. Ή NMR (400 MHz. CDCb) 58.28 (d. IH).8:07 (d. lH).5.63 (brs.2H).4.61 (t.11:1).3.06-2.97 (m, 2H), 1.14 (t. 3H); MS (EI) for C7l½BrN3O2S::280.282 (Br isotopes.. MH").
[00676] 2-amino-5-bronio-N-iso|iiOpylpyridine-3-su]fonamide. Prepared according to the methods. described in reagent preparation 25 using isppiOpylamine in step 1. Ή NMR (400
MHz. CDC ) δ 8.28 (d.111).8.09 (d, IH), 5.59 (hr s.211).4.52 (d. I H).3.50-3.39 (m. I H). 1.1 I (d, 6H): MS (EI) lor CxH|2BrN;,0:S: 294.296 (Br isotopes. Μ1-Γ).
[00677] 2-aniino-5-broino-N-(2-(dinielhylainiiK))ctl)yl)pyridiiie-3-sul Prepared according to the methods clcscrihcd in reagent preparation 25 using /V,/V-dimctliyleihanc-l,2- diamine in step I. 'H.NMR (400 MHz. CDC ) δ 8.27 (d, IH).8.08 (d, IH), 5.66 (br s, 2H). 2.99,-2.93 (m, 2H).2.36-2-30 (in.2H).2,12 (s.611); MS (El) for Cc,HlsBrN.i02S: 323.325 (Br isotopes. MH+).
[00678] 2-ainino-5-^mnm-N-(2-lvydrox'yciliyl)pyridin'Cr3-siiil¾nain'ide; Prepared according lo the methods described iii reagent preparation 25 using 2-aminocthanoI in step 1. Ή NMR (400 MHz, CQCIrs) δ 8.29 (cl.1 II), 8.08 td, I H), 5.65 (br s.3H), 5.23 (bi s, IH), 376-3.67 (in. 3H).3.16-3.07 (in, 311); MS (El) for C7H,oBf NjOjS: 296, 298 (Br isotopes, ΜΙ- ).
[00679] l-(2-amiiK 5-l.nt)n )pyndin-3-yl.sulfonyl)-3-(hydroxyineihyl)azetidin-3-oi.
Prepared according to the .methods described in reagent preparation 25 using
3-(hydi xymeihyl)azetidin-3-ol (prepared ^according to procedures described in
WO2007044515) in step I. ' i NMR (400 MHz. CDjOD) δ 8:28 (d. IH).8.00 (d. IH).3.90- 3.S4 (m.2H)t 3.70-3.64 (in, 2H), 3.32-3.29 (m, 2H); MS ( El) for CyH 12B rNiOaS : 338.340 (Br isotopes. MH*).
[00680] 2-(2-amino-5-broiiiopyridinc-3-.sulfonainido)accianude. Prepared according to the methods described in reagent preparation' 25 using ^^ 2-ainihoacctainid :lvydi Ghlondc in .ste I . Ί-Ι NMR (400 MHz. DMSO-d6) δ 8.26 (d,. IH), 8.1,8 (br s. IH), 7:90 (d, I'M).7:34 (br s. I H). 7.12,(br s, 1 H), 6:84 (br s.2H).3.45 (s.2H); MS ;(E1) .for C HsBrNaQ jS-: 309; 311 (Br isotopes. MH+).
[00681] lerl-butyl 3-(2-ainiii0T5-bromdpyridiner3-.sulI'onainido)-2- hydroxypiqpylcarbamaic. Prepared according to the methods described in reagent preparation 25 using lerl-butyl 3-ainin -2-hydroxypiOpylcarbamale in step I. Ή NMR (400 MHz.
DMSO-d6) 58.26 (cl. IH).7.88 (d, IH).6,82 (bi s, 2H).6.74 (l, I H).5.02 (d, IH), 3.50-3.42 (m. I H).2.88 (l.2H), 2.82 (dd, 1 H).2.57 (dd.1 H)„ 1.37 (s, 9H); MS (EI) for C13H2, Br jOjS: 369.371 (Br isotopes. MH+-l-Bu).
[00682] 5-bromo-3-(3-(dinicthylannno)azeiidii^l-ylsulfonyl)pyridin-2-amine. Prepared according to the methods described in reagent preparation 25 using A'./V÷dimeihy!azeiidin-3- aniinc hydrochloride in step I. Ή NMR (400 MHz. DMSO-d6) δ 8.39 (d, l H).7.92 (d, IH). 6.90 (br s, 2H), 3.88-3.76 (m.2H).3.63-3.54 (m, 2H).3.07-2.97 (m. IH), 1.96 (s.6H): MS (EI) for Ci«HisBrN.|0
2S: 335.337 (Br isotopes, MH
+).
1,00683]
Prepared according lo the 'methods described in reagent preparation 25 using 5-bromo-2- '( ine 111 y I ; i n l i i i t) ) y ii cl i n c - 3 - s 1111 Όί ι y I chloride (prepared from 5.-bromo-N-metliylpyridin-2- aminc using analogous conditions to those described in WO200SI44463) nd 2->iminoethanol in step l . Ή N R (400 MHz. GDC ) 88.28 (<1. lH).S.00(d.1 H), 7.10-7.03. (m, IH),6.4S- 6,3.9 Cm..1H), 3.93 ( I I I), 3.60 (cj.211).3.04-2.96 (m, 5H); MS (El) for CsHi.Br -iOisS: 310. 12 (Br isotopes.; M l-O.
niUObenzenesulfdiiamide. Prepared aceordingto.the methods described iii reagent preparation 25· using' N-(azctidin-3-yl)-^ in step "I. Ί-Ι NMR (400
MHz. CDCli) δ 8.32 (d. lH),.8.06-8,03 (m.111).8.00 (d. I H).7.77-7.72 (m.2H).7.70-7.65 (in.1H).5.78 (br , 2H).4.90-4.80 (m. l H). .i:9-4'0S rn,,2H).4,01 (dd.2H), 291 (s, 3H): •MS (EI) for Cr5Hi6BrN5O6S:..06.508 (Br isotopes. H+).
100685] tcrt-buiyl 4.-(2-amino-5-bromopyridin-3-yls^
Prepared according to the methods described in reagent preparation 25 using tcrt-buiyl piperazine-l-carboxylatc in step I . Ή NMR (400 MHz, DMSO-cl6) δ 834 (d.1 H).7.86 (d. 1Ή), 6.90 (br s, 2H).3.40^35 (m.41-1).3.09¾02 (ni^H).1.37 (s.9H): MS (EI) for
C|.|H;iBrNj*OjS: 367.365 (Br isotopes. Μΐ -t-Bu).
100686] 3-(3-ainiiio-3-niethylazctidiii-T-ylsuirpn
according to the nvelhods described in reageni preparal ion 25 usiiig 3-nicthyl_azelidin-3-aniiiie hydrochloride (prepared by procedures described in WO2007007057 followed by
benzylidenc dcpraieciion) in ste I . Ή NMR (400 MHz. DMSCRI6) δ 837 (d.1H).7.88 (d. 1 H); 6.86 (br s.2H).3.5S-3.47 (ni.4H)..2.06 (br s, 2H).1.22 (s.31-1); MS (El) for
Ca-l Br iC S: 321.323 (Br isotopes. Ι ).
100687] tcrt-buiyl 2-(2-aniino-5-bronii)pyri(linc-3-suIfonamido)-2-inethylpi pylcarbamatc. Prepared according to the methods described in reagent preparation 25. using tei-t-butyl 2- amiiio-2-niethylpropylcarbainalc in step 1. Ή NMR (400 MHz, CDCI.v) δ S.26 (d. lH), 8.08 (d.1 H), 5.89 (br s.1 H).5.60 (br s.211).5.04 (t.11-1). '3.112 (d, 21-Ϊ)..1.46 (s.91-1), 1 ,19 (s.6H); MS (EI) for Ci l jBrNjOjS: 367.369 (Br isotopes. MH+-i-Bu).
[00688] ten-butyl 5-((2-amino-5-biOmopyridinc-3- sulfonamido)niclhyr)hexahydrocyclopLMUa|c|pyri lc-2(.lH)-carboxylaie. Prepared accordin to the methods described in reagent preparation 25 using ten-butyl
5-(aminoniethyl)hexahytliOcyclopenta|c|pyrr(ilc-2( I H^carboxylale (prepared from substrates described in WO2004006846) iii step 1. Ή NMR (400 MHz. CDC).,) δ 8.28 (d, l-H), 8.06. (d.
IH).5.65 (br s.2H).5.03 (t. IH).3.41 (br s, 2H), 3.17 (br s, 21-1), 2.9.3 (t, 2H).2.63-2.54 (m, 211), 2.1.4-1.^)8 (m, 3H), 1.4 (s, 9H).1.09-0.98 (:m, 2I-I); MS (El).I r. G|XH27Br 404S: 41 , 421 (Br isotopes. MI-^-l-Bu).
[00689] lert-buiyl l-(2-ainino-5-bronmpyridiiic-3^siillOn;iniido)buliiii-2-ylcai )aniatc. Prepared according to die meihods described in reagent preparation 25 using tcrt-butyl 1- aminobuian-2-ylcarbamate in step I. Ή NMR (400 Hz, DMSO-d6) δ 8.28 (d.111).7.89; (d, 11-0.6.78 (br .211).6.57 (d, I I-l).3.33-3.26 (m, I H), 2.77-2.65 (m.2H).1.53-1.39 (m. IH). 1.37 (s.911).1,28-1.1.5 (m. I H), 0.76 (i.3H): MS (El) for
367, 369 (Br isotopes, MH
+-t-Bu),
[00690] tcrt-butyl 4-(2-aniino-5-broitiopyridine-3-sidronarnido)-2-inellVylbuian-2- ylcarbamate. Prepared according to the method
's described iii reagent preparation 2 using tcrt-butyl 4-amino-2-mcthyIbuian-2-ylcarbamale in step I . Ή NMR (400 MHz. CDC ) δ 8.27 (d, li-O.8.06 (d. H-l).5.-6
'4 (brs.2H), 5.07 (br s..IH).4.41 (br s.111).2.98 (q.2H).1.93- 1.85 (m.2H), 1.41 (s, 9H).1.22 (s.6H): MS (EI) for
381, 383 (Br isotopes. ΜΙ-Γ-t-Bu).
[00691] 2-aiiiino-N-(2-aniiiio-2-nietbylpiOpy -5-broinppyiid Prepared according to the methods described in reagent preparation 25 using 2- ieihyIpropane- 1,2- diaminc iirstcp 1. :,H NMR (400 MHz. CDC¾) S 8.27 (d, 111).8.07 (d. I II), 5,69 (br s.2H). 2.73 (s, 2H).1.12 (s, 6H); MS (El) for CoHiiBrKjCbS: 323.325 (Br isotopes. MH+).
[00692] lert-butyl l-(2-ariiino-5-bron )pyridin-3^yl.sulfony0azetidin-3-ylc;u"b
Prepared according to the methods described in reagent preparation 25 using .-ten-butyl azetidin-3-ylcarbamate in step I . Ή NMR (400 MHz. CDCI3) δ 8.31 (d, IH), 8.00 (d, IH), 5.76 (br s, 2H), 4.80 (br s, I H), 4.50-4.3.6 (m.1 H), 4- 11 (i.2H), 3.75 (l, 21-1).1.42 (s, 9H).: MS (EI) for CnH|yBrNa0 S: 407.409 (Br isotope's, .'MHO.
[00693] lert-butyl l-(2-amino-5-broinopyridin-3-ylsullOnyr)piperidin-4-ylcarbai]iate sulfonamide. Prepared according to the methods described in reagent preparation 25 using lert-butyl piperidin-4-yIcarbainate in step 1.
[00694] 2-amino-5-bromo-N-(2-hydroxy-2-m^
Prepared according to the methods described in reagent preparation 25 using l-amino-2- mcthylprbpan-2-ol instep 1.
[00695] 2-Aniino-5-biOmp-/V./V-diinethylpyridine-3-SLdfonamide. Prepared according to the method ol' reagent preparation 25 by using dimethylamine in step.1. MS (E ) for
C7Hl„BrN,02S: 280 (MH+).
|0069 | 5-Bron i-3-(inorplK)liiiosulfonyl)pyridin-2-:iiiiine. Prepared according lo the method of reagent preparation 25 by using morpholine in ste I . MS (EI) for C.)Hi2Br :,0-,S: 322 ( f-Γ);
[00697J
-ylsiilfonyl)pyridiii-2-ainiiic. Prepared according to the method of reagenl preparation 25 by using N-methylpiperazine in step 1. MS (EI) for C|(iHi5Bn .,0,S: 335 (:ΜΙ·Γ).
|()()698] 3-(Azctidiii- 1 -ylsuIlbiiyl)-5-bi:0iir0pyfidin-2-aniine. Prepared according to the method of reagent preparation 25 by using N-melhylpipcrazine in step I. MS (El) for CsI-li Br jO.S: 292 (MM*).
(006991 2-Aiiiino-5-bronio- -nic!hylpyridinc-3-sulfonan ide. Prepared according to the method of reagent preparation 25 by using-methylamine in step I . MS (El) for
266 (MH+).
[00700] :l-(2-Aminor5?.bro'mopyridiiv-3-^
method of reagent preparation 25'hy using azetidinol. in step 1...MS (EI) for OxHioBr 3.0;¾S: 308 (ΜΙ- ).
[00701]; 5 Br0ni( 3-(pyrrolidin-l-ylsiilf0ifyl)py according to the meihod of reagent preparation 25 by using pyrrolidine in step I . MS (EI) for
306 (ΜΙ-Γ)..
|007()2| (2-Anvino-5-bron )pyridin-3-.yisulfonyi)pyrrolidin-3rol. Prepared according to the method of reagent preparatioii 25 by, using 3-pyrrolidinol in step 1. MS (EI). for
0.)Η|2Βι·ΝιΘ 5: 322 (Μ1-Ι+).
• [00703] 2-Aniino-5-bronio- -cyclobulylpyridine-3-sulf()nan ide. Prepared according to the method of reagent preparation 25 by using cyclobulylamine in step I . MS (El) for
[00704] 2-Aiiiino-5-broniopyridinc-3-si)llOnamidc. Prepared according to the method of reagent preparation 25 by using ammoniumhydroxide in step I. MS (EI) for
252 (ΜΙ- ).
[007.05] 2-Aniino-5-bronK N-clhyl-N.-ntell\ylpyridiiie-3-sul(Onaniidc. Prepared according to the meihod of reagent preparation 25 by using N-metliylethylainine in step 1. MS (EI) for C,sH,2BrN:i02S:294(MH+).
[00706] 5-Bronio -(33HlinLior( xetidinrl-ylsulfonyI)pyridinr2-aniine. Prepared according to the method of reagent prepara.ti.oa25 by using 3,3-difluoiOazelidine in step I. MS (El) for CsHsB F.N'^O^S: 328 (,MH+).
[00707] 2-Ainino-5-brpmo-N-( 1 -hy^;uxypippan- --y|)pyiidiiic -suirQnaniidc. Prepared according to the method of reagent preparation 25 b using 2^'amino.propan-l-ol in. Step I. MS (El): Tor Csl½BrN;iO;iS: 3.10 (MH*).
[007081
Prepared according to the method of reagent preparation 25 by using 2-fluoraethyIaminc in step J. MS (EI) for C
7 Ηή B r F , Oi$ : 298 ( H
+) .
[007091 terl-Buiyl l.-(2-amino-5-bromo yridiii-3-yls^^
Prepared according, to the method of reagent preparation'.25 by using tcii-bulyl pyrrolidin-3- . ylearhamaie in step I. MS (EI) ΙΌΓΟ|.|Η2|ΒΓ 4Ρ:|5: 365 (MH+-U3u).
[00710) I-(2-Amino: -bromopyriclin-3-ylsid( iiyl)piperidiiV -ol. Prepared according to the method of reagent preparation 25 by using 4-hydrcixypipcndine hi step 1. MS (EI) for
C1,1lI|.;Br .(0.iS:336(MH+)
[00711 J tert-Buiyl I-(2-amino -bromopyridin-3-ylsulfoiiy!)pipcridiiv-3-ylcarbamaie. Prepared according to the method of reagent preparation 25 by usin teit-buiyl pipcridin-3- ylearbamaie in step I. MS (EI) for G15H23B N.1O.1S: 379 (Μΐ -tBu).
[00712] lert-Butyi 2-(.2>amino.T5;-brqmopyridiiic-¾ Prepared according; to the method of reagent preparation-25!by LIS i ti : tei i- b iii I 2 uninocthy]carbamaie in step 1. MS (EI) for C HiuB jQaS: 339 (Mll+-lBu).
100713J 2TAniiiio-5-bromo-N^(3-hydroxypropyl)pyridiiic-3-sulfoiKUTiide
according, lo'the- method -of reagent preparat ion 25by using 3-hydroxypiOpylaniine in step I .
MS (EI) for CsH,2 i ;,0.iS: 310 (MH+).
[00714] terl-Bulyl 3-(2-aniiho-5-bi !nopyridinc-3-sulfoiiaiiiido)propylcarbamate. Prepared according to the method of reagent preparation 25 by using tcrt-btityl
2- aiiiinopropylcarbamalc in step.1. MS (EI) for Ci:d-l;2,Br 0.iS: 35 (MH+-tBu).
[00715] 2-Ainino-5-broniO N-(3.3.3-lrinuoro-2-Iiydroxypro|iyl)|iyridinc-3-sulfoiiamid Prepared according to the method of reagent preparation 25 by using 3-amino- 1.1,1 - trifluoropropan-2-ol in step 1. MS (ΈΙ) for CsH BrF3 -,0.vS: 364 (MH*).
[00716] tert-Butyl 5-(2-anuno-;5-bromopyridin-3-yl.sulfony^
c|pyrrolc-2(l H)-carboxyIatc. Prepared according to the method of reagent preparation 25 by using ten-butyl hexahydropyrroIo[-3.4-c|pyriOlc-2( I H)-carb0xylate in step 1. MS (EI) for Cif.H33BrN4O.1S: 391 (MI-I+-lBu)
100717] tert-Butyl l-(2-amin0-5-bromopyridin-3-ylsuif nyl)-3-nicthylpynOlidin-3- ylcarbainale. Prepared according to the method of reagent preparation 25 by using tert-butyl
3- mcthylpynOlidin-3-ylcarbamate in ste 1. MS {EI) for dsI^Br tS: 379 (MH+-lBii).
[007 J ] (lS,4S)-tert-BuiyI 5-(2-ainiiu)-5-broinopyriclin-3-ylsuironyl)-2.5- (Iia/.abicyclpf2.2,l |liept.niic-2-c;ii¾oxy!atc. Prepared ^ according ip the method -of reagent preparation 25 by using ( I S.4S)-lcn-butyl 2.5-dtazabteyclo[2."2.1 |hcptane-2-carboxylatc in step l.MS(El) forC|SH2,BrN.,0.tS:377 (ΜΐΓ-ιΒιι).
[00719J (R)-terl-Buiyl 2-((2-amiiH)-5-l Oniopyiidiner3-siHfonamido)incthy))|ryriOlidine- 1- earboxylalc. Prepared according ,10 'the- method of rcagen prcparation 25 by using. (R)-teri- biityl 2-(amino'mclhyl)pyiTOlidine- 1 -carboxylaic in step I. MS (EI) for C15H BrN.1O.1S: 335 (ΜΙ-Γ-Boc).
[00720 j (S)-tcrt-Buiyl 2-((2 uriiiio-5-br(.imopyridine^
carboxylate., Prepared according to the method of reagent preparation 25; by using (S)-tert- butyl 2-(aminomcihyl)pyrrolidihe- 1 -carboxylate in step 1.
335 ( H*-Boe).,
1007211 ( lR,4R)-ten- Butyl 5-(2raniiiib-5-bi:omopyr;idin-3-ylsulIbnyl)-2.5- diazabicyclo|2.2.l |hepianc-2-carboxylatc. Prepared according to the method of reagent preparation 25 by using (1 R,4R)-tert-bulyl 2V5-tlia/.abicycloj2.2.1 |hcptancr2-carboxylate in step I . MS (EI) for C|5H2|Br .,0,tS: 377 (ΜΙ Γ-Boc).
Prepared according to the method of reagent preparation-25 b using tert-buiyl 4- amiiYopiperidiiic-l-carboxylate in ste I . MS (EI) for CisHiiB Q S: 379 (MM+-Boc).
[00723] 5-Bromb-3-((TS.4S)^^^^^
ylsidfoiiyl)pyriclin-2-aminc. Prepared according to. the method of reagent preparation 25 by using ( IS.4S)-2-methyl-2,5-dia/.abicycIo|2.2,l ]heptarie in step 1, MS (EI) for
[00724] (S)-lerl-Buiyl l-(2-aniiiK 54irniioiiyridin-3-.ylsulfo yl)'pynOlidiii-3-y
Prepared according to the method ofrcagent preparation 25 by using (S)-tcrt-butyl pyrrolidin- 3-ylcarbamate in step I,. MS (El) for CuM2iBrN.1O.iS: 421 (MH+).
[00725] (Rr)-tefl-Biityl l
Prepared according to the method of reagent preparation 25 by using (R)-teri-butyl
pyrrolidiib3-yicarbamate in step I . MS (EI) for ΟΜΗ2|ΒΓΝ. Ι5; 421.(MH*).
[00726] tert-Butyl 8-(2 uiiino-54K(miopyridin-3^vIsuironyl)-8-azabicyclo| 3.2.1 Jocian-3- ylcarbamate. Prepared according^) the method of reagent preparation 25 by using icrt-butyl 8-azabicyclo|3.2.11octan-3-ylcarbaiiialc (WO 2009055077) in step 1. MS (El) for
Ο^ΗΜΒΓ ,Ο.^^] (ΜΙ-Γ).
100727] 2.2.2-Trichlorocthyl 3-(2-:niiiiu)-5-broinopyriclinc-3'-siilfon;intido)-S- azabicyc|o| 3.2.1 |pctanc-8-carboxylaie. Prepared according to the method of reagent preparation 25 by using 2.2',2-trichlomcthyl 3-aniinp-S-azabicyclo|3.2.1 Jociane-8- carbpxylale (WO 2009055.077) in step 1. MS (El) fbr.Cy'HisB^ ^S: 535'-( l-I+).,
[00728) (R)-teri-Biilyl 3-((2;aniino-54ironK^yndine-3-sull naiiii
carboxylalc... Prepared according to the•method of reageni repaniliPn 25 by using (S)-leri- buiyl 3-(aminometbyl)pyri lidiiie-l-carb xylale in step 1. MS (El) for CisI Nj r aO S: 435 (ΜΙ-Γ),
1 07291 (S)-terl-Buiyl 3.-((2-aniino-5.-broriiopyridi c-3-sulfonamido)mcihyl)pyiToli earboxylate. Prepared according to the method of reagent. preparation 25 by using (R)-lert- butyl .3-(aminonieUiyl)pyriOl idiiic- 1 -earboxylate in ste I . MS (El) fo C 15 Wi.Br iOiiS: 435 (Mil*).
[00730] (R)rlert-Buiyl-3>(2-'amntt 5^^^
earboxylate., Prepared according to the method of reagent preparation 25. by iising;(R)-„tert- biityl 3-aminopyrrolidine- 1 -earboxylate in step.1. MS (EI) lor G l iBrtt tS: 421 (MH*). 100731] (S)-tert-Buiyl 3-(2-ainino-5--l)romppyridine-3-siilfonamido)pyrr<)li'dinc-l- carboxylate. Prepared according to the method of reagent preparation 25 by using (S)-tcrt- butyl 3-an.iiiiOpyn;olidine-l-carbpxyhue^ (MH+). '[0.0732J tcrt-Butyl 3'Η(2-αηιϊιίο-5·-1>ω^
earboxylate. Prepared ac'cording.toHlie method of reagent preparation 2 by using tert-butyl 3,-(anmioniethyi)pipcridine- 1 -earboxylate in step 1. MS (EI) for <Dir,H25.BrN;,©4S: 449 (MH+). 100733] tert-Butyl 2-((2-aniino-5-broniopyi'idinc-3-siilfonamido)nicihyl)piperidinc-l-i earboxylate. Prepared according to the method of reagent preparation 25 by using tcrl-buiyl 2-(aminomcihyl)pipciidine-l. -carboxylalc in tep 1. MS (EI) for Cif -lTj r tO S: 44 (MH+).
[00734] (R)-lert-Bulyl 3-((2-aininp-5-broni(^yridine-3-suIl nainido)inethyl)piperidihe-l- carboxylaie. Prepared according to the method of reagent preparation 25 by using (S)-tert- btityl,3:-(aininomeibyl)pipcridine-l -earboxylate in step: 1..MS (EI) for C .H sBr aOjS: 449 (ΜΙ-Γ).
[007351 (S)-lerirButyl 3-(
'(2-aniiiU)-5-l^aniopyridine-3-suli nairiid())inetbyl)piperidiive-l^ carboxylalc. Prepared according to the method of reagent preparation 25 by using (R)rlcrt- buiyl 3-(aminometbyl)pipcridine- 1 -carboxylalc in step 1. MS (EI) for Ci/ - jBrN-iOjS: 449 (MH
+).
Prepared according to die method of reagent prcparati;on:.25:by -usy ng. (<R)-(1 -m.cthylpipertdin- 3-yl)melhananiinc in step .1. MS (EI) for C,2H |.,BrN,,02S: 363 (MH+).
100737] 2-ainiiKr-54iroino-N-[(3/ )- l Hiicllvylpyrroliiliii-3-yl |pyridin
Synthesized according t the method of reagent preparation 25 by tising (R)- 1 - meihylpyiTqlidin-3-aminc. hydrochloride (symhesizccl according to- lire method of Journal of Medicinal Ghcimsiry (2002). 45(3). 72 1 -73 ) i step I . MS.{EI) ibr.ei Hi5i3r ;,02S: 334v 336 (Ml- . Br isotope pattern).
(00738] 2-ninitK)-5-lir in -/V-{ | (3.S')- 1 -niclhylpyi >l idin-3-yl jmcthyl } pyridine-3- sulforianiide. Synthesized according to the method of reageiit preparation 2'5 byu.sing (R)-( l - niethylpyriOlidiiV-3-yl)ineihanamine liydrobroiiude (synthesized according to the method* of WO 2006028904 for the synthesis of benzyl | |(R)- l -(lert4 itoxycarbonyi)pyn lidin-3- yljmethyl (carbamate, WO 2006002047 for (he synthesis of (S)-benzyl pyrrolidi -3- ylniethylcarbainate and Journal of Medicinal Cliemistry (2002), 45(3); 721 -739:for the synthesis of (R)-benzyl ( 1 -niethylpyrrc)iidin-3-yl)itiethyicarbainate; using (R)-3- (amin0methyl)- l--(lcr^lniiyloxycarbonyl)'pyrrolidinc as starting material) in step 1. MS (EI) o Cu HnBi aOiS: 348. 350 (*M H+. Br isotope pattern);
(00739] ten-Butyl 6-(2-anuno-5-brotn )pyridih-3-yIsiilfonyl)-:2.6-cl^
carboxylatc. Prepared according to the method of reagent preparation 25 by using terl-buiyl 2.6-diazaspiro|3.3 |hcptane-2-earboxylate iii step I . MS (EI) for CisHji BrN^S: 377 (MH+- tB.it).
1 0740] (S)-teri-Butyl l -(5-bronH)-2 iloropyridii 3-ylsulfonyl)pynOlidii>3-ylcarbamatc. Prepared:acc0R)iiig,ip the methods idescribcd in reagent, preparation 25 using !5-bromo-2- chloropyridine-3-sulfo yl chloride and (S -tcri-butyl pyrrol iUin-3 ylcarbamatc in step 1. 1 H NMR (400 MHz. CDCI3) δ 8.61 .'(d 1 H). 8.52 (d, I H), 4,67 (s, 1 H). 4.25 (s. I H). 3.57 (m, 41-1), 3.34 (m, I H). 2.22 (m, I I-l), 1.92 (m, I H). 1.45 (s, 9H); MS (ES) for C,.1H ,flB.rClN304S: 440. 442 (Br isotopes. M l-f).
[00741] tcrl-Buiyl 3-((2-ainino-5-bromopyridiiie-3-siil( namido)methyl)azciidihc- 1 - carboxylatc. Prepared according to the methods described in reagent preparation 25 using tcrt-buiyl 3-(aminomethyl)azctidine- 1 -carboxylatc in step 1. MS (ES.) for C|.iHi| BrN.,04S: .421 ,,423 (Br isotopes. l-f).
Reagent Preparation 26: N-(5-brom -2-iiielhylpyridin'-3>yl)ifiethanesuironamide' L00742] STEP 1 : A solution of 5-broino-2-ii hylpyridin-3-amine ( 187 ing. 1.0 mmol) and diisopropylcthylamine (523 uL, 3.0 imol) in diehloromclhane (5 i L) was cooled to O °C,
and ihcri meihanesulfonyl chloride ( 1 55 uL, 2:0' mmo!) .was added slowly. The reaction mixture was stirred. al 0 °C lor 8 min and was then warmed to rt. After stirring- for I h, the volatile materials were removed iii vacuo. The residue was then dissolved in methanol (2.5 mL) and aqueous sodium hydrox ide (2 M, 1.5 tub, 3 mmol) was added. The reaction mixture was stirred lor 1 40 min at i t. VVater was then added to the mixture which was -subsequently extracted twice with dichloiOmclhanc. The combined organic extracts were extracted with aqueous citric acid ( 10%). The organic phase was discarded, and the aqueous phase was basified to pH ~ 7.5 with aqueous sodium hydrox ide ( 1 M). Tlic aqiieous mixture was extracted three times with dicliloromethane. The. combined organic extracts \verc dried over magnesium sulfatc, filtered, and concentrated in vacuo. The residue was purified by flash chromatography (50% hexanes : 50% ethyl acetate) to provide N-(5-broiiH 2-meihylpyridin- 3-yl)methanesulfonamide ( 1 1 1 nig. 0.42 mmol, 42% yield) as a white solid. Ή NMR (400 M Hz. DMSO-d6) δ 9.58 (s. I H). 8.44 (d, I I I). 7:87 (d. IH), 3. 10 (s, 3H). 2.47 (s. 3 H); MS (El) for C7H9B1 N2O2S: 265, 267 (Br isotopes, ΜΙ-Γ.).
(007431 Using analogous synthetic techniques and substituting. wit alternat ive starting reagents in step 1 the following reagents were prepared. Alternative .starting materials were obtained commercially unless otherwise indicated.
1007441 N-(5^Bronio-2 chloroplienyl)iiielhancsulfonamidc. Prepared according to the methods described in reagent preparation 26 using 5-brdmor2-ehlor a ilinc in ste Ί . Ή
NMR (400 MHz. CDCIj) δ 7.83 (d. I H), 7.32-7.23 (m, 2H). 6.80 (br s. I H), 3.06. (s, 3 H); MS (EI) for C7H7BrCIN02S: 282, 284, 286 (Br + CI isotopes, MH+).
[00745] N-(5-Bi mo-2-mcthoxypyridiii-3-yl)mcthanesulfonamidc. Prepared according to the methods described in reagent preparation 26 using 5-bromo-2-iiiethoxypyridin-3-amine in step I . Ή NMR (400 MHz. CDCl;,) S 7.97 (d. I I I), 7.90 (d. I I I), 6.73 (br s. I H). 4.00 (s, 3H). 3.05 (s. '3 IT);. MS (EI) For C7H.jBrN2.O3S: 281 . 2S3 (Br isotopes, MH+).
[00746] N-(5-Br0nio-2-cyanopyri(lin-3-yl)nicthanesull namide. Prepared according to the methods described in reagent preparation 26 using 3.-amino-5-bromopicolinonitriic in step 1. Ή NMR (40 MHz. CDCI3) 0 8.55 (d, ΓΗ). 8.29 (d, I H). 7.00 (br s. I I I), 3.2 1 (s. 3H); MS (El) for C7H(iBrN302S: 276, 278 (Br isotopes. Μ Ι-Γ).
[00747] N-(5-Bi mopyridin-3-yl)melhancsulfonamidc. Prepared according to the methods described in reagent preparation 26 using 5-bromopyrklin-3-aminc in step 1 . MS (EI) for Cr,H7BrN202S: 251 . 253 (Br isotopes. MH+).
[00748] N-(5-Bronio-2 iloropyridin -yl)-2-chloro-6-nicthylbciizciicsullonamide.
Prepared according to the methods described in reagent preparation 26 using 5-bromo-2-
c iGiOpyridin-3-aminc uiul 2-.chloro-6-meihyll)Cir/.enc- l.-siilfoviyi chloride in sicp' 1 . MS (El) r r eiil-l BrC jO.S: 393. 395. 397 ( r + Cl isotopes. Μ1-Γ).
100749] N-(5-Broino-2-ilii6r0p.yridin-3-y[)iuelluiiiestilf iiaiiii(le. Prepared accord ing lo the methods described in reagent preparation 26 using 5-brQmo-2-l1uoropyritlin-3-ainine in step I . MS (EI) for CeHeBrFNaOjS.: 269. 27 1 (Br isotopes, Mi l4).
[00750] N-(5-Bronuv2 il0ropyi idin-3-yl)acctami(le. Prepared according to the methods, described iii .rcagcnl preparation 26 using 5-bromo-2-chloiOpyridiir-3-amiiic and acetyl chloride in step 1 .
[00751] Methyl 5 ironio-2-c4ilor(jpyridiiiT3-ylcarl)aniate. Prepared according to the methods described in reagent preparat ion 26 using 5-brpnio-2-cldoropyridin-3-aminc and methyl chlorol'ormate in step 1 .
Reagent Preparation 27: 5-Ι)π>ηιρ-2-ΰΙιΙ(ΐΓ()-3-(ηιι·11ΐ) «ίι1Γοη)'ΙιυςΙΙΐ}'1)ρ} ί ίιιε 1007521 STEP 1 : A mixture of 5-l)roiiuv-2-chloro;3 chloroiivethyl)pyridine ( 124 mg. 0.52 mmol) and sodium meihaiiesulfmalP (52 mg, 0:52 mmol ) in dioxane (J :4 niL) and water ( 1 .4 niL) was heated to 1 10 °C in a microwave reactor for 15 min. After cool ing to rt, water was added to the reaction mixture which was subseciticntly extracted twice with ethyl acetate. The combined , organic extracts were dried oyer magnesium sulfate,, filtered, and; concentrated in vacuo to provide 5-brdino-2-chloiO-3-(iiicthyisuiron mmol. 94% yield) as a yellow solid. Ή NMR (400 Mi l/. DMSCHI6) δ 8.63 (d, I H), 8.21 (d, I H), 4.70 (s, 2H), 3. 10 (s, 31-I); :MS (EI) for C7H7BtGltM02S: 284, 286, 288 (Br * CI isotopes, MH+).
[Ό0753] Using analogous synthet ic techniques and substitutin with alternative starting reagents in step 1 the following reagent was prepared. Alternat ive starting materials were obtained commercially unless otherwise indicated.
[00754] 5-Br5mo-3-(mcthylsul fonylmethyl)py.ridin-2-amine. Prepared according to the methods described in reagent preparation 27 using 5.4 romo-3r|bromomethyl)pyricliiv2-ainine hydrochloride in step I . Ή N MR (400 MHz. DMSO-d6) S 8.03 (d. I H). 7.59 (d. 1 H). 6.35 (br s, 2H). 4.44 (s, 2H), 2.95 (s, 3 H); MS (El) for C7H.jBr 2O2S: 265, 267 (Br isotopes. ' ΜΙ-Γ).
Reagent Preparation 28: N-(5-bromo-2-chloropyridin-3-yl)-N- met hylmelhancsull namide
100755] STEP I : A solut ion of N-(54.iromo-2-clvlorppyridiiv-3-yl)mclhancsulfonainide (96 nig. 0.34 mmol, reagent preparation 24 in DMF ( 1 niL) .was treated with potassium carbonate (93 nig, 0:68 niniol) and iodbmeilianc (33 uL, 0.51 'mmol) at rt for 18 h. Water was
then added, and the result ing aqueous mixture was extracted twice with ethyl acetate. The combined organic extracts were washed with aqueous l ithium chloride ( 10%) followed by Water, dried over magnesium sulfate, filtered, and concentrated in vacuo to provide N-(5- bromo-2-chloropyridin-3-yl)- -nieihylinethancsulfonainidc (9 .2 mg, 0.304 mmol, 90% yield) as a light yellow solid, Ή NMR (400 MHz, CDCI. 6 ' 8,46 (d, I I-l), 8.00 (d, I I I), 3.32 (s. 3I-I). 3.07 (s. 3H): MS (El) for CTl lsBfClNiO.S: 299, 301 , 303 (Br + CI isotopes. Μ Ι-Γ).
Reagent Preparation 29: 5-l)roni()-2-chloro-3-((linuoroniotIioxy)pyridine |0()756 | To a solution of 54)roirvo-2-chloropyridih-3-ol ( 150 nig, 0.72 mmol) in DMF (5 niL) was added poiassium carbonate (298 mg, 2.2 mmol). The mixture was heated to 70 °C and bromodifluoi nieihane was bubbled through for 3 min. After cooling to rt. water was added, and the resulting aqueous mixture was extracted twice with ethyl acetate. The organic extracts were washed with aqueous lithium chloride ( 1.0%): followed by water, dried over magnesium; .sulfate, filtered, and concentrated in vacuo io^r0V.i'dc..5-Btomi3^2Hililoro-3- (di fluoromclhoxy)pyridine ( 159 mg, 0.61 mmol. 85 yield) as a brown oil. ' H' NMR (400 MHz. CDCb) 6 8.36 (d. 1 H). 7.76 (d. I H), 6.61 (t. IH); MS (EI) for Cf(H3BrClF2 O: 258 ( +).
Reagent Preparation 30: N-(5-bromor2-elhoxypyridin-3-Yl)mctltanesuli namide [007571 STEP 1 : A solution of 5-bromo-2-chloiO-3-nitropyridine ( 1 0 mg, 0.42 mmol) and L,8-diazabicyclo| 5.4.0 |undec-7-ciie.(3 15 uL, 2. 1 1 mmol) in cthanol (1 niL) was healed to 50 °C for 50 min and then cooled to rt. Water was added and (he resulting aqueous mixture was extracted twice with ethyl acetate. The combined organic extracts were washed with 1 N HC1, dried over magnesium sulfate, filtered, and concentrated in vacuo. The residue, was purified by flash chromatography ( gradient, 100% hexancs to 90% hcxanes : 10% ethyl acetate) to provide 5-bromo-2-ethoxy-3-nitropyridine (52.2 mg, 0.2 1 1 mmol, 50% yield) as a yellow oil. Ή NMR (400 MHz. CDC-b) δ 8.42 (d. I H), 8.36 (d. I H). 4.55 (q. 2H). 1 .45 (t. 3 H); MS ( El) for C7H7 B 1 N2O : 246. 248 (M).
[00758J STEP 2: To a solution of 5-bromo-2-elhoxy-3-nitropyridinc (75.2 mg, 0.304 mmol) in ethyl acetate (3 mL) was added lin( II ) chloride (289 mg, 1 .52 mmol). and the mixture was heated 10 reflux for 2 h. After cooling lo rt, 50% aqueous sodium hydroxide was added dropwise until a sticky brown solid completely "formed. Sodium sulfate was then added, and the mixture was stirred for several minutes. The solids were then removed by filtration. The filtrate was dried over sodium sulfate, filtered, and concentrated in vacuo to provide 5-bi mo-2-clhoxypyriclin-3-amine (53 mg. 0.25 mmol. 80% yield) as a dark blue
film, Ή NMR (400 MHz. CDCIj) δ 7.56 (d. I H). 6.97 ( l. I I ). 4.37 (q, 2H ). 3.85 (br s. 21 1). 1 .40 (del. 3 H): MS (EI) for C7HyBrN20: 2 17. 2 19 ( Br isotopes, M H+).
|()0759| STEP 3 : A solut ion of 5-liiOiiio-2-cilioxypyridiii-3-ainine (53 mg, :0.25 mmol) and cl i i s 0 p i (? p y I c 11 iy I am i n c (9.6 tiL, '0.55.mmol : in dicblorpmelhaiie ( I mL) was copied to 0 °C and nielhaiiesulfonyl chl0i de¾(39 :u L. 0ΐ mmol) wasjadded. The mixture was' allowed to' warm to' it over 1 5Πι, and then wate was added. The resuli iiig tnixture was extracted wit dichloiOmeihane. The organic extract was dried over magnesium sulfate, filtered, and concentrated in vacuo. The residue was dissolved in, methanol (500 uL) and dioxanc (500 uL). and i en sodium hydro ide (2 M. 1 0 uL. 0.3.8 mmol) was added. The mixture wits heated to 60 °C and 3 drops of aqueous sodium hydroxide (50%) were added. After stirring a further 30 min, the niixture was cooled to rl. Dilution with water was. followed. b
acidification with aqueous citric acid .( 10%) and then two extractions with ethyl acetate. The ■combined organic ex t racts; w^en washed wiiliwaicr.' dried over.inagrtesiiihr sulfate, filtered, and coiieeiitfalecl in vacuo. Tiie residue was piiri iedj by Iash chrpmatpgraphy grad 100% hcxancs to 70% hexanes : 3.0% ethyl acetate) to provide N-(5-bromo-2-eihoxypyridin-3- y inetluincsulfonamide (32.1 nig. 0.1 1 mmol, 43% yield) as a colorless film. Ή NMR (400 MHz. CDCI3) δ 7.95 (d, I H). 7.89 (d, I B), .75 (br . I I I). 4.42 (q. 2H). 3.05 (s, 3 H). 1 .41 (t. 3H): MS (El) for CsH | , Bi N20>S: 295. 297 ( Br isotopes, M ff ).
100760] Using analogous synthetic techniques and substitut ing with alternative starting reagents in step I ihefollowing reagent was prepared. Alternative starting materials were obta'i ucd cpiri e rc tal 1 y U nless ot her wi se inc| ieaiccl .
100761 ] N-l^-CBenzylox^-S-broinopyridm
■to the methods described in reagent preparation 30 Using benzyl alcohol in step I . Ή NMR (400 Hz, CDCb) δ 8.00 (d. I H). 7. 1 (d. I l l), 7.44-7.34 (in, 5H), 6.7 1 (br s. I H). 5.40 (s, 2H). 2.99 (s, 31 1): MS (El) for C, iH i3BrN203S : 357. 359 ( Br isotopes, Μ Ι-Γ).
Reagent Preparation 31 : N-(2-amino-5-br<)mopyriclin-3-yl)metli:ine.sulfonamidu (00762] STEP 1 : To a solution of 5-bromo-3-nili pyi idin-2-aniihe (21 8 nig, 1 mmol) in THF (5 mL) was added DM AP ( 1 83 mg, 1 .5 mmol) and di-7<.'r/-butyl ,dicarbonate (655 mg. 3 mmol). After . stirring 40 min at i t. the volatile materials were removed in vacuo, and the resulting residue was purified by flash chromatography (gradient, 100% hexanes to 70% hcxancs. : 30% ethyl acetate). The isolated material indicated the addition of two Boc groups by Ή NMR. This.material was dissolved in cihyl.acetate (8 mL) and was treated with excess Λ',/V-dimcthylcthylcnediamine. After stirring for 17 h at rt. the reaction niixture was diluted with ethyl acetate. The resulting solution was washed with aqueous citric acid ( 10%)
followed by -water, dried over liiagncsiiiiivsulfate, jTiUcrcd
vacuo to provide lcit-lnilyl 5-bronid-3^ni -opyridih-2-ylc ibaiiiate 0.85 ηίηίο
'Ι. 85%. yield).- as an orange . solid. Ί-Ι NMR (400 MHz, CD.Cl
3) δ
'9.48 (br s. 1 l),.S 74 (d. I I I), 8.63.(d, I I I). 1 .56 (s. 9H); MS (EI) for-C iftH 12B.rN.1O4: 3 16, 318 (Br isotopes. M-H).
[00763J STEP 2: Iron powder (293 nig, 5.2 mmol) was added to a solution of tert-buiyl 5- bromo-3-nili pyridin-2-ylcarbamalc ( 167 nig, 0.52 mmol) in acetic acid (2.5 mL). The mixture was stirred at 60 CC for I h 20 mill before cooling to rl. The mixture was then diluted wiih elhyl aeeiaie, and solids were removed by filtration through celite. The filtrate was washed with water followed by saturated aqueous sodium bicarbonate. The organic phase was dried - oyer -ma'gncsiiun: sulfate, filtered, and concentrated in vacuo' to provide ten-butyl 3- •amino^b'^ 64¾r yie!d)vas a. ra solid.. Ή
NMR (400 MHz. CDCIj) 5 7:83 (d, I I I ). 7.20 (d I I I). 6.95 (bi s. l H), 4.42 (br s. 21 1). 1.51 (s, 9H): MS (EI) for Ci«HhiBrN;iO2: 2.32. 234 ( Br isotopes. MH+-/-.buiyl).
[00764] STEP 3: A solution of tcrt-butyl 3-aniiilo-54.iromopyridih-2-ylcaibaniaie (96.3 mg, 0.33 mmol) and diisopropylelhylamiiie (128 uL. 074 inmol) iii diehioroniethane (2 mL) was cooled to 0 °e, and to ii was. added methanesul fonyl. chloride (52 uL. 0.67 mmol). The mixture was allowed to warm 10 rt over 2 h. The mixture was then diluted with
organic, phase.was then dried over- magnesium sulfate, filtered, and concentrated in vacuo. The residue, was purified by flash - chromatograph (gradient, 10Q.¾ he ncs to 70% hexancs : 30% ethyl acetate) to provide ten-butyl 5-bromo-3-(N-
(methylsullbnyl)inelhylsulfonaniido)pyridin-2-ylcarbainalc (77 mg, 0. 17 mmol. 52% yield) as a colorless film. Ή NM R (400 M Hz. CDCb) δ 8.64 (d, I H), 7.79 (d, I I I). 7. 10 (s. 1 H), 3.44 (s, 61 1), 1 .52 (s. 9H ); MS (El) for Ci2H i.s.BrN30(,S2: 388. 390 (Br isotopes. MH+-/-butyl).
[00765] STEP 4: A solution of tcrt-butyl 5-bromo-3-(N-,
(niet ylsulI¾nyI)niet yls'utfonarii idt))pyindin-2rylcarbaniatc (68: mg, 0.15 mmol) and NM- dihiethylelhyrcncdiamine .( l 69 uL. 1 .5 inmol) in dioxaiic ( I mL) was stirred' at r for 70 in in. After diluting wiih ethyl acetate, the mixture: was washed witlv aqueous. citr c acid (.10 ) followed by water. The organic phase was then dried over magnesium sulfate, filtered, and concentrated in vacuo. The residue was then diluted with dichloromeihane which was then washed with I N HCI. After partitioning, th organic phase, was dried over magnesium sulfate, filtered, and concentrated in vacuo to provide tcrt-butyl 5-bromo-3- (iiiethy]sulfoiianiklo)pyndin-2-ylcarbaniatc (57 mg, 0. 1 5 mmol. quantitative yield) as a white
sol id. Ή NM (400 H¾. CDCb) δ 8.24 (d. I H). 8.07 (d, I H), 2.98 (s. 3H). 1.54 (s, 9H): MS (EI) for C,oH ,f,BrN;,0,.S: 3 10. 3 12 ( Br isotopes. M H+-/-buty|).
100766] STEP 5: A solul ioiiiof tcrt-butyl 5-broinor3 -(ineihylsul lXMiaiiiido)pyridin-2- ylcarbamaic (57 mg, 0.1 iiL, 1 .5 iiimol) was. healed to .60
vaciio to royidcjvi-^^ hydrochloridc'salt in qiumtiiaiivc yield. Ή NM R (400 Mi l/.. DMSO-d6) δ 9; iG (br s, I I I). 7.95 (d, I I-l). 7.54 (d. I IT). 6.42 (br s, I FT). 3.02 (s. 3I-I): MS ( El)
'for C
6I
:l
sBrN
30;S: 266. 268 (Br isotopes. MW).
Reagent Preparation 32: 5-brom()- l -(letrahy(lr()-2//-pyran-2- l)- lH-pyra ulo[3,4-
//|pyridiiic.
100767] To a solution of 5-bromo- 1 //-pyra/.oIo| 3.4-/.>|pyridiiic ( 1 -A g. 7.2 mmol) and dihydropyran (3.3 niL. 36. mmol) in tctrahydrofur.au (20 niL) wa : added. (±).- camphorsul fpiiie acid (250 mg) and the reaction niixiurc-wa's stirred al '65 "© for 16 hours. After cooling to fooili temperature it was diluiecl with . cihyj; acetate (25.0 mL), washed with •sattu-ated aqueous sodium .bie.arb'Oiiaic '■($ x 10 mL) and brine ( 100. in L), dried over sodium sulfate, filtered and concentrated. Gradient cbliiinri chromatography ( 1 % to 30% ethyl acetate in hcxane) provided 5-bromo- l -(lcirahy(jiO-2/7-pyraii-2-yl)- I //^pyrazolo| 3.4- •ftjpyiidine ( 1,8 g. 90%). MS ( El) for Cn H 12BrN30: 283 (MH+).
Reagent Preparation 33: 2-Ainiiio 5-broiiH)- / :(liinethyInicotinamide
[00768] To a suspension of 2-amino-5-bromonicotinic acid (0.35 g. 1-.61 mmol ) in tetraliydrofuraii (5 niL) was added diincthylaniiiie (0.8 niL of a 2M solution in
tcirahydrof ran, 1 .60 mmol). diethylphosphoryl cyanide (0.29 g; 1 .77 mmol). and iiieihylamine- (0.34 g, 3.38 mmol),at 0 C. The mix-lure was stirred at 0 °C for 30 min and then at room temperature for 4 h: Cohceiilratioii and purification by column eliromalography on sil ica (5- 10% methanol in dichloiOiiictliaiie) gave the title Compound as a white solid. MS ( El) for CsHinBi-N.xO: 244 ( Ι- ).
Reagent Preparation 34: 5-Hromo-3-(ctliylsiilfonyl)pyridiii-2-amine [00769] STEP 1 : 2-Aniino-5-l)iOmop.yi idiiic-3-sulfonyl chloride (94 mg, 0.35 iiimol) was taken inloTHF (2 mL) fol lowed by addition of anhydrous hydrazine (40 iiL. 1.4 mmol) and the mixture was stirred for 1 minutes at room temperature. The mixture was concentrated and dried to give
a whiic .solid, which was then taken into ethanol (2 ml.) followed by addilion of sodium aceiale (320 mg. 3.9 mmol) and ethyl iodide ( 140 uL. 1 .75 mmol). The mixture was rcfluxcd for 12h then cooled to room temperature and concentrated. The residue was partitioned with ethyl acetate
'and water and
I he organic phase waslied with brine then dried over sodium sulfate, filtered and concentrated to give 5-bronu)-3-(ciliylsullOnyl)pyridin-2-aniinc (67 mg, 72%) as a yellow oil. MS (El) for C
7l-l
yN
2S0
2Br: 265, 267 (MI Γ).
[00770] Using -analogous synthetic techniques and substitutin with alternative starling reagents in step 1 the following rcagcnts wcre prepared.
1007711 5-Brpnio-3-(meihylsiilfonyl)pyridin-2-amihc. Synthesized according to the method "of reagent preparation 34 using iodomcthanc. GCMS (EI) for 'CftHyNi'SO.Br: 250, 252 (M÷).
[00772] 3-(2-aniino-5-biOiiiopyndin-3-ylsulfonyl)propane- 1 ,2-diol. Synthesized according to the method of reagent preparation 34 using 3-biOmopiOpane- l ,2-diol followed by silica gel chromatography using ethyl ether then ethyl acetate as e icnt. MS (EI) for QHijN.SOi r: 3 1 1 , 3 13 ( Ι ).
.100773] 3r(2-amin0-5-bron1opyr to the method of reagent preparation 34 using 3-biQniopropan- 1 -ol. followed by silica gel chromatography using ethyl ether as elueiit. MS (El) for Cvl^VSG Br: 295; 297 (MH
+). (00774] (S)-3-(2^amino-5-biOiiiopyridin-3-ylsiill nyl)-2-inclhylpiOpan- l -ol. Synthesized according to the method of reagent preparation 34 using (S)-3-br0m0-2-uicihylpropaii- i -pl followed by silica gel chromatography using 4: 1 ethyl ethenhcxanes as elue t. MS (EI) for
|()0775] (R)-3-(2-amino-5-broiiippyridin-3-ylsulfonyl)-2-m^ Synthesized according to the method of reagent preparation 34 using (R)-3-broino-2-nicthylpropan- 1 -ol followed by silica gel chromatography using 4: 1 ethyl ethenhcxanes as eluent. MS (El) for C7I IyN2S02Br: 309. 1 1 (Μΐ ).
Reagent Preparation 35: 6-lH-omo-2-metIiyl-I-({[2-(triiiK*thylsilyl)cthyl |oxy}metliyl)-l//- imida/.o[4,5-/; |pyndine.
[<)()776] To a solution of 6-biOino-2-nielhyl- 1 /7-imidazo|4,5-/ |pyridine (3.0 g, 14. 1 nimol) in a mixture of Λ'./V-dimcthylformamide and teirahydrofuran (30 mL, 2: 1 ) at 0 °C was added 60% sodiuni hydride in mineral oil (0.68 g, I 7;0 nimol) and the reaction mixture was st irred for 30 minutes, followed by the addition of 2-(irimeliiylsilyl)clhoxymcthyl chloride (2.7 mL. I 4; nimol). The reaction mixture was stirred for 16 hotirs at room temperature then it was quenched by the careful addition of water and diluted with ethyl acetate (250 mL); washed with brine (3x 150 mL), dried over sodium sulfate, filtered and concentrated. Gradient column chromatography ( 10% to 30% ethyl acetate in hcxane) provided 6-bromo-2-mcthyl- l -({ | 2-(trimeihylsilyI)elhyr|oxy ) meihyl)- l //-iniidazo|4.5-/? |pyridiiie (4.4 g, 92%). Ή NMR
(400 MHz, <ZOCU): 8:4-1 (s. 1 H). 8. 12 (s. 1 H)..5.67 (s. 2H), 3.62 (m. 2I-I). 2.76 (s, 3H), ().% (i . 2H). 0.00 (s. '9H). MS ( EI) for C nH3,l3rN3OSi: 342, 344 ( l-l \ Br loiope pattern).
Reagent Preparation 36: 6- n»nio-N-t,(liyl-3-(methoxymetliyl)-3//-imidazo|4,5- bJpyricIin-2-;imine and 6-bn)m()-N-ethyl V -l)is(methoxymelhyI)-3//-iniidazoI4.5- b lpyridin-2-ainine.
[00777] Step I : To a cooled (() °C) solution o!' 5-bromopyridinc-2.3-diaivtine (5.0 g. 27 nimbi) in NMP (20 mL) was added isothiocyanatoclliane (2.3' mL. 26 mniol). The resulting solution was heated (65 °C) for'-lbur hours and thcn cp()Ieci to aiiibient icnipcraltire before 1 ,3-diisopropylciirbodiiiiiidc (4.2 mL, 27 nimol) was added. The reaction mixture was stirred for fS hours..diluted with water and tile -resulting suspension was .collected by-filtration. Trituration with ethyl acetate provided 6 irbtiK)-N-'eiliyl-3 / niidazo| 4,5-b |pyridiii-2:-amiiie (4.8 g, 75% yield) as a . brown sol id. Ή N MR . (400 MHz, d6-DMSO) ό I J .4 1 (bs, I H). 7.91 (s. I H), 7.53 (s, 1 H ). 7. 17 (s, I H), 3,33 (q, 2H)S 1 . 17 ({, 3H) MS (ES) for/CgHoBrMi: 241 (MH+).
[00778] Step 2: To a cooled (0 °C ) solution of 6¾rbmb^ -6tliyI 3H-mridazbj4,5- b]pyridiii-2-¾mine (0;36 g; 1.3 nmbl) in DM}? w s added NaH (¾0¾ dispersion ^ mineral oil, ,0.060 g, 1 5 mniol) portion wise over 15 minutes. The; reaction mixture was stirred for 1 5 minutes and!tlieii chlbro(nieihbxy)inethane (0. 12 mL. _1..5 iimpl):-\yas added dropwise over 15 minutes. The resulting slurry svas allowed to warm to ambient temperature and was stirred for two hours and was partitioned between ethyl acetate and saturated aqueous- sodium bicarbonate. The organic layer was washed with brine, dried over magnesium sulfate, filtered and concentrated in vacuo. Purification by silica gel chromatography provided both 6-bromo- N-ethyl-N,3-bis( iiicihoxymethyl) A/-iniidazo|4,5-b |pyridin-2-a (0.0 1 g; 18%) and 6- bromo-N-etliyl -(melhoxymcthyl)-3/^ g, 35% yield).
Bi piOtectcdLproduet:
Mbnoprotecied.piOduct: Ή NMR (400 MHz. CDC¾) δ 8.03 (d. I H), 7.73 (d, I H), 5.42 (s. 21-1), 4.98 (s, I H), 3.59 (q, 21 1), 3.36.(s, 3 H), 1 .34 (l. 3H); MS (ES) for C|
(lH ,
3BrN.iO: 285 (MI-P).
Rcajjeul Preparation 37: 7-Bromu-2/7-pyrid()|2,3-e|[ l,2,4 ]tliiadia/.in-3(4H)-one 1,1- dioxide
100779] STEP I : 2-Amino-5-bromopyridinc-3-suIfonyl chloride (reagent preparation 25) (95.5 mg, 0.35 mmol) was treated with 0.5M ammonia in dioxane solution (7 mL) and the mixture was stirred for I h at room temperature. Concentrated aqueous ^ammonia (2 mL) was then added to the mixlure then stirred an additional 12h. The mixture was then concentrated
and the residue suspended in water (5 niL). The solid was: collected by filtration and dried to give 2-ainino"-5-:bromopyricIine-3-.sulf()naniide (55:7 livg, 89%).
(00780] STEP 2: 2-Ainino-5 5rqniopyridine-3-sulfonaniide as Obtained above (0.22 nirnol) was taken/into THP(2 inL) followed by addition ol'diisbprbpylethylaininc ( I l 5'uL. 0.66 mmol): Phosgene (20W% in toluene, l 20uL, 0.22 mmol) was added carefully, nd the mixture was allowed to stir for I h al room temperature. The mixture was partitioned with cihyl acetate and 0.5M aqueous hydrochloric acid. The organic phase was then extracted once with .saturated aqueous sodium bicarbonate. he organic layer was discarded and the aqueous phase carefully acidified to plT I -2 with concentrated aqueous hydrochloric acid. The aqueous mixture was then , exlraelcd once with ethyl acetate, dried over sodium sulfate, filtered and c nceito to give:7-broinp-2W-pyfidp|2^c||^
l , I -dioxide (17, g; 28%) as a sol id. -MS (EI) for CAN^SB : 277. 279 (M ).
Reagent '. Preparation 38: 2-aniiiio-5-broniopyri{Iiiie-3-.siilloiuc acid |0078J.J STEP 1 : 2-Ainino-5-broiii( pyridine-3-sulfonyl chloride ( 1 0 nig, 0.37 mmol) was taken into 1 : 1 aqueous dioxanc (3 inL) and the mixture was basificd to pH 14 by drop wise addition of 50% aqueous sodium hydroxide .solution. The mixture was wa med to 75 "C for 0.5h then cooled to room temperature and concentrated. The residue was taken into water (2 niL) and carefully acidified to pi I I -2 by concentrated aqueous hydrochloric acid addition and cooled to 0 "C. After 111 at 0 ,}G the.crysialline sblid^
and dried to give
a.solid. Ή NMR (DMSO-df,):
8.24 (d, 1 H), 8.06 (d, 1 M ). MS (EI) for G5H5N2SO3B1 : 253, 255 (MH+. Br pattern):
Reagent Preparation 39: N-(5-bi (nno-2- dimetIiyJainin( pyridin-3- yOmcthanesulf'onamide
[00782] STEP 1 : 5-Bromo-2-chloro-3-niiippyridine (J. Heterocyclic Chcm. 2003.. 40, 261 ) ( 128 mg, 0.54 mmol) was taken into TH E (0.25 niL) followed by addition of 40W% aqueous dimethylaminc (0.25 inL) and. the resulting solution was stirred for l h at . room temperature. The mixture was then partitioned with ethyl ether and 1 M aqucous'hydrochloric acid. The xirganic solution was then washed with additional I M aqueous hydrochloric acid C3x) then dried over magnesium sulfatc, filtered and .concentrated to g'ivc 5-bro'ino W;N-di'meth:yl-3- nitiOpyridiri-2ramine. MS (El) for C7I-I.sNi02Br: 246, 248'(ΜΙ- , Br pattern).
100.783] STEP 2: 5-BiOmo-jV, V-diincthyl-3-nilropyridin-2-amine as obtained in step I (0.54 mmol) was taken into ethyl acetate ( 10 niL) followed by addition of t in (II) chloride (522 mg, 2.8 mmol) and the mixture was heated to reflux for 15 minutes then cooled to room temperature. 50W% aqueous sodium hydroxide was added drop wise to the mixture until a
precipitate. formed then solid sodium sulfate was added. The ihixuirc was filtered and the filter cake washed with ethyl acetate. The Organic, filtrate was concentrated to give 5-bronio- N2. 2-dinicthylpyridinc-2.3-dianiinc (53· nig, 45%).was aii amorphous residue. MS (El) for C7H |0N.iBr: 216. 218 (ΜΙ- . Br pattern).
11)0784) STEP 3: 5-Broin(>-N2.N2-dinietliyipyndine-2
<3-dianiine (S3 nig, 0.25 minol) was taken into TI-IF (2 niL) followed by addition of diisoprppylethylamine (213 liL, I .25 mmol) and ethanesulfonyl chloride (95 iil. 1.25 mmol). The mixture was allowed to stir.for 4Sh at room temperature then partitioned with ethyl-acetate and water. The organic phase was washed with brine then dried over sodium sulfate, filtered: anci concentrated. Tlve.residuc was taken into methanol (3 mL) followed by addition of potassium hydiOxide (.IOS nig, lQ eq) in
; a minimum of water. The mixture was stirred for 15 minutes at room temperature
' then partitioned with ethyl acetate and 10% aqueous citric acid. The organic solution was dried over magnesium sulfate, filtered and concentrated. The residue was purified by silica gel
(27.9 nig, 39%); MS (El) for CKU jNiSbiBr: 294. 296 ( Η+,. Br pattern).
[00785] Using analogous synthetic techniques and substituting wilh alternative startin . reagents in sic ll the Jol losin reagents were prepared!,
[00786J N-(,2÷(Benzylamino)-5^^^ Synthesized according lo die method of reagent preparation 39 using bcnzylamine in step I ... MS (EI) for C^ lLi jSOiB : 356. 358 ( i f. Br patlern).
[007871 N 5-Bromo-2-(phenylamino)pyridin -yl)m
according to the method of reagent preparation 39 using neat aniline at 75 "G in step I . MS (El) for .C H'iiNjSQjBr: 342. 344 (MH*, Br pattern).
[00788] N-(5-Bromo-2-(incihylannno)pyridin-3-yl)niethanesulfo^ -Synthesized according to the method of reagent preparation 39 using methyamine in step I . MS (El) for C7H|0 .uSO2Br: 280. 282 ( Ι- , Br pattern).
Reagent Preparation 40: 1,1-dinicthylethyl {(35)- l-[ (5-bronio-2-hydroxypyndin-3- yl).sulfonyl|pyrrolidin-3-yl}c:ii baniiitc and 1 ,1-dimethylefhyl |(3.S)-l-(.{5-bromo-2-|(3i')- 3-({[(;l,l-dimethyletbyr)oxy]ca^^
yl }.siill'()iiyf)pyrrolidiii-3-yl |carbamate.
[00789.1 STEP I : To a solution of 3-amino-54>romo-2-chloropyridine (0.23 g, j .1 mmol) in accionitrile (3.0 mL) at - 15 "C was added a solution of sodium nii;ile (0.091 g, 1.3 mmol) in water ( 1.20 mL), followed by ihe addition of concentrate hydrochloric acid (1.8 mL, 21.3 mmol) and the reaction mixture was stirred for 5 minutes. A 30 vvt% solution of sulfur
dioxide in acetic acid 3.0 mL, 1.3 ininol) was prepared and introduced into the reaction mixture, followed by the addition of" solution of eppper(il) chloride 0.09 ί g, 0.68 mmPl) in water ( 1.2 mL). he stirring: was commuted for an additional 3 hours at -5 "C. The pH'of the mixture was adjusted to 8 by the addition of a solution of potassium hydrogenphosphatc and 2M ac|iieous sodium hydroxide and partitioned wiih ethyl acetate (50 mL). The organic layer was separated and washed with water (10 mL) and brine ( 10 mL), dried over sodium sulfate, filtered and concentrated to give:54:>romo-2-diloropyridinc-3-sullbnyl chloride (0.20 g. 63%).
[00790] STE .2:; A. mixture, of 5-bi iiio-2-,chloropyridinc-3-sulfonyl chloride (0:19 g, 0.65 ιηιήόΊ). (3S)-( -3-(terlTbiU0xycarbqnylamino) 8 g, 0.98 mmol) andNN- diisopropylethylaininc (0.34 mL. 1.95 inniol) in dichloromcthane ( 1 :5 in L) was stirred, for '16 hours at room temperature. The reaction mixture was partitioned between didiloromethane (50 mL) and brine ( 10 mL.). The organic layer was separated, dried over sodium sulfate, filtered and concentrated. The resulting crude product was- dissolved in a mixture of 1 ,4- dioxane ( 1.5 mL) and 2 aqueous sodium hydroxide ( 1.5 mL) and stirred at 100 °C for 2 hours. After cooling to room temperature the reaction mixture was concentrated and the residue was partitioned between brine (20 mL) and ethyl acetate (50 mL). The organic- layer was separated and washed with brine (20 mL). dried over sodium sulfate, filtered and concentrated. Gradient flash chromatpgraphy (25%:tp ,50% ethyl . cetate in licxane) followed by 10% methanol in dichl rometiianc provided 1, 1 -dimelliylelhyl
3-( { [( l . l -diniethylcthyl)pxy|carbonyl |amino)pyrroIidin- l-yl|p,yri
yl }sulfonyl)pyrrolidin-3-yl |carbamate (80 mg. 21 %), MS (EI) for
(MI-I+); and 1 .1 -di'mct'liylcthyl { (3.V)- 1 -| (5-bromo-2-hydroxypyridin-3-yl).sulfonyl Jpyrrolidin- 3-yl Carbamate (35 mg, 13·%); MS (El) fpr C1J I.0BrN.3O5S: 423 (ΜΙ-Γ).
Reagent P
[00791] STEP 1 : To a solution of 2-aniino-545iOmopyridinc-3-sulfonyl chloride (reagent preparation 25, ste I ) (0.40 g, '( 47 mmol) in .a mixture of 1.4 dioxanc (8.0 mL) and water (1.0 mL) was added triphenylphPspliinc (1.64 ; 6.25 nimol) aiid the reaction mixture was stirred for 50 minutes at room temperature. Potassium carbonate (0.35 g, 2.50 mmol) was introduced, fol lowed by 4-biOnio-2-methyl-2-butan0l (Tetrahedron Letters 2000. 41(38), 7337-7340) (0.3 1 g, 1.86 mmol) and the reaction mixture was stirred at 80 °C for 1.6 hours. After cooling to room temperature the reaction mixture was concentrated and the residue was partitioned between brine (50 mL) and ethyl acetate ( 100 mL). The organic layer was
separaictl and washed with brine (50 mL). dried over sodium sul fate, filtered and
concentrated. Gradient flash chromatography (25 to 50% ethyl acetate in hcxanc) provided 4-| (2-ainino-5-broiuopyridin-3-yl)thio |-2-niethylbulaii-2-ol (0. 1 8.g. 42%); MS (EI) for C ioH,5BrN2OS: 292 ( M M+).
[00792] STEP 2 A: To a solution of 4-( (2-amino-5 )rouHipyridm
2-dl (90 mg, 0.3 1. mmol) in a mixture of mcthanoL(750 μϋ), acetone (750 μί) and water (450 L) was added potassium peroxymonosulfatc (285 mg. 0.46 mmol) and the. eaction mixture was stirred for 15 minutes at room temperature. Tlic reaction
between water (20 mL) and ethyl acetate (50 mL). The organic layer was separated and washed with water (20 mL) and brine (20 mL), dried over-sodium sulfate, filtered and concentrated. Purification" y flash chromatography (35% to 80% ethyl acetate in -hcxanc) gave 4-j (2-aniin -5-bromdp.yriclin-3-yl)sullb 48%); MS (EI) for C I oH is B r 20.,S: 323 (M H+).
[00793] 5 ΕΡ;2Βί Τό η ;«>1ύΐ^
2-ol (S3 mg. 0.28 mmol) in a mixtuic of methanol (750 μ
'-L), acetone (750 uL) and water (450 μΕ) was added potassium pcroxymonosulfate ( 13 1 mg. 0.2-1 mmol) and the reaction mixture
' was stirred for 90 minutes at 0 "C. The reaction mixture -was partitioned between water (20 mL) and ethyl acetate (50 mL). The organic layer was separated and washed with water (2 mL) and brine (20 mL), dried over sodium sulfate, filtered and conceniraied. .Purification by flash chromatography (35% to 80% ethyl acetate in
broniOpyriclin-3-yl )sidiiiiyfj-2Tmethylbuiaii-2^ol (5 mg, 60%); MS (EI) for CioH isBrNiCbS: 308 (ΜΙ-Γ).
[00794] Using analogous synthetic techniques and substituting with alternative start ing materials in step 1 the following reagents of the invention were prepared. Alternat ive starling .materials were obtained commercially unless otherwise indicated.
[00795] (2.S')-3-| (2-amino-5-bromopyridin-3-yl)sulfonyl |-2-meihylpropan- 1 -ol. Prepared according to the method of reagent preparation 41 by using (S)-(+)-3-br0mo-2 mcthy| l - propanol in step I . MS (EI ) for C. W |.tBrN >0 S: 310 (Μ ΐ: ).
[00796] (2.$ 3 (2-amino-5-broimjpyridiiv-3-y^ Prepared according to the method of reagent preparation 41 by using (S,)-(+)-3-biOmo-2-methyl- I - propanol in step 1 . MS (El) for G4-I uBrN.O;>S: 294 (MH+).
Reagent Preparation 42: (4-chloro-5,6,7,8-letrahydroquin /oIin-7-yl)mt'thaii()l.
[00797] Ozone was bubbled through a cooled (-78 °C) solution of 4-cliloro.-7-vinyl- S.iSJ.S-tcirahydr.ociuinazoline ireageiu preparation 3, 0.35 g, 1 .8 mmol) in methanol (5 mL)
and dichloromcthane (30 riiL) until a blue color persisted. The solution was then sparged with N Tor 10 minutes and sodium borohydride (().14 g. 3'.6 mmol) was added portionwise. After 30 minutes the reaction mixture was partitioned between dichloi inclhane and saturated aqueous sodium bicarbonate. The organic layer was washed with brine, dried over magnesium sulfate, filtered and then concentrated in vacuo to provide (4-chlorc>0;6,7,8- tetrahydroquinazolin-7-yl)mcihangl (0.32 g, 90% yield) as a waxy solid. MS (ES) for GjH| ,CIN 0: 199 (MI-! K).
Reagent example 43: l-(4-chloro-5,<),7,8-tctrahydroqiiina olin-7-j )elhaiiol 100798] Step 1 : Ozone wa bubbled through a cooled (-78 0C),sqlution of 4-ehloro-7- vinyl-5,6,7,8-tcirahydiO |uina7.olinc (reagent preparation 3. 0.3S g. 2.0 mmol) in
dichioroniethane (45 mL) until a blue color persisted. The solution was then sparged with N2 for 10 minutes and triphenylphosphine (0.52 g. 2.0 mmol) was added portionwise. After one. hour, the reaction ..mixture was partitioned between dichloromcthane and .saturated aqueous sodium;biearbonatc. The organic layer was washed with brine, dried oyer magnesium sulfate, filtered and then concentrated in vacuo. Purification by silica gel chromatography provided 4- chloro-5,6,7,8-icirahydroquinazoline-7-carbaklchyde (0.33 g. 85% yield) as a viscous oil. MS (ES) for CjHgCIN.O: 1 7 (ΜΙ-Γ).
[00799] Step 2: To a cooled (0 °C) solution of 4-chloiO-5i6;7,8-tctrahydroquina/.oline-7- carbaldchyde (0.10 g, 0.51 mmol) in THF (5 mL) was added a solution of MeMgBr (3.0 M iii ethyl ether, 0.40 mL, Γ.2 mmol). The resulting mixture was stirred at ambient temperature for 30 minutes and then partitioned between ethyl acetate and salurated sodium bicarbonate. The organic layer -was washed with brine, dried over magnesium sulfate,, filtered and concentrated in vacuo. Purification by silica gel chromatograph provided' l-(4-ehloro-5,6.7.8- tetrahydroquinazolin-7-yI)ethanol (0.09 g, 83% yield) as a waxy solid. MS (ES) for
C,oH,3CIN20: 2 l3 (MH+).
Reagent example 44: 4-chloro-7-(methoxymetliyl)-5,6,7,8-tetrahydroquinazoline.
[00800,1 To a slurry of (4-chloi -5.,6,7,8-icirahycliOquinazolin-7-yl)methanol (reagent preparation 42, 0.80 g. 0.40 mmol), potassium carbonate (0.1 1 g. 0,81 mmol) and THF (15 mL) was added iodomcthane (0 09 mL. 0.60 mmol). The reaction mixture was stirred for 18 hours and then partitioned between ethyl acetate and water. The organic layer was washed with brine, dried over magnesium sulfate, filtered and concentrated in vacuo. Purification by silica gel chromatography provided 4-ehloro-7-(mcihoxyme-hyl)-5,6,7.8- tclrahydroquinazoHnc (0.03 g. 35% yield) as a waxy solid. MS (ES) lor C||)H|3C1 20: 213 (MH+).
Reagent Preparation 45: 2-(a i(lonietli l) -chloro-6,6-dinicthyl-5,6.7,8- tel rahyd roi| uin azol i n
[00801 1 STEP 1 : To a solution of 2-(eliloroiiiclliyl )-6;6-.dinicihyl:5.6,7;8- len-ahydroquiiia/.olin-4(31T)-one ( 150 nig, 0.66 mniol. reagent preparation 17) in DMF (3 inL) was added sodium azidc (2 15 mg. 3.3 minor). The resultin mixture was slirred al.ri for.35.- min. Water was added and the result ing mixture was extracted twice. with ethyl acetate. The combined organic extracts were washed with aqueous l ithium chloride ( 10.%). dried over magiiesiuin.suli'aie, filtered, andicoiiecnlrated in vacuo to provide 2-(a/.idomethyl)-6,6- diniethyl-5,6,7,8 ett ahydi qiiinaz()lin-4(3H )-one (.1,51 mg. 0.65 riunol, 98% yield) as a 'waxy yellow solid, 'H NMR (400 Mi l/., CDCh) 6 1 l .7() (b,r s. I H), 4:41 (s. 2Ή). 2.66 (1. 2H). 2.33 (S. 2HI), 1,58 (1, 3H), 1,00 £s, 61 MS :(EI) for C IJ H ^ JO: 23&ξΜΜ
1(I0802| STEP 2: A solution of 2-(a/idoniethyl)-6.6-dimethyI-5.6.7.8- ieu ahydroquinazolin-4(3 H one { 1 5 l ' mg. 0:65 minol) iivchloroforin ( 1 .2 niL) was treated wild phosphorus oxycliloridc (600 tiL) al 60 0C for 1 1120 i iii. After cooling to n, the volatile materials were removed in vacuo, and the resulting residue was dissolved in ethyl acetate. The organic solution was washed with saturated aqueous sodium bicarbonate, and the aqueous phase, was back extracted with ethyl acetate. The -combined organic extracts were .dried over magnesiurii sulfate, filtered, and concentrated in vacuo to provide 2-(azidomethyl)- 4-ehlorq-6i6-dim mg, 0.54 mmol, 83% yield) as, an orange oil. ',Η ΝΜΙΪ (400 MHz, CDCh) δ 4:47 (s. 21 1). 2:94 (U;2W), 2:55 (k; 2H), 1 .68 (t, 2H), 1.05 (s. 6H): MS (El) for C H i iCl j: 252 (ΜΙ- ).
Reagent Preparation 46: I-C4-chIoro-fi,6-dimetlyyl-5,6J,8-te^
VjV-diniethyletliaiiamine.
100803] STEP 1 : To a solulion of dimcthylamine (2M solulion in telrahydrofiiran. 4.0 niL. 8.0 nimbi) was added 2-( l-chloroelhyl )-6,6Hlimcthyl-5.6,7,8-tetrahydiOCiuina/.olin-4-ol (.synthesized according to, the method of reagent preparation 1 8 using 2-ehl0i bpropionitrilc in step I) (50 mg, 0.21 nimol) and ilie reaction mixture was stirred in a sealed tube for 16 hours at 80 "C. After cooling tp room temperature the reaction mixture was concentrated and life residue was partitioned between brine (50 niL) and ethyl acetate (50 niL). The organic layer was separated and washed with brine (20 niL). dried over sodium sulfate. filtered and conecnlratcd to give 2-| 1 -(diniethylamino)eihyl |-6.6-dimethyl-5.6;7;S-teirahydi quin.azoIin- 4-ol (50 mg. 96%). MS (El) for Ο,.,Η^Ν,Ο: 250 (ΜΙ-Γ).
100804] STEP 2: A solution of 2-| 1 -(diincthylaniiiHi)elhyl]-6;6 iimethyl-5,6,7,8- ietrahydroquinazolin-4-όΙ (50 mg. 0.20 mmol) in a■mixture of chloroform ( 1 .5 mL) and
•phosphorous oxyehloridc (0.5 mL) was healed to reflux for. .0.minutes. After cooling to room temperature the react ion mixture was concentrated and the residue was, artitioned -between saturated aqueous sodium bicarbonate (20 mL) and ethyl acetate (20 mL). The mixture was stirred for 15 minutes and pH was maintained above 7- by the addition of solid sodium bicarbonate. The organic l yer was separated and washed with water ( 10 mL) and brine, dried over .sodium sulfate, filtered and concentrated to give, 1 -(4-chloro-6,6-dinieihyl-5,6,7i8 tcirahyclroc|uiha ;tmnr2-yl)-N7y-diiiiethyletliaiiainiiic (46 nig. 85%)'. MS- (El) for C1 1H I .V 268 (MH+).
100805) Using analogous .synthetic techniques and substituting .with' ltehiative starting materials in step I the fol lowing reagenl was prepared. Alternative starting materials were obtained commercially unless otherwise indicated.
[00806] 4-cbloro-6/vdime!hyl-2-( r^yrrol idin
Prepared according to the mclJiod of reagent preparation 46 by using pyrrolidine in step 1 . MS (EI)' forCifiI½CINs: 294 (Μ1- ).
Reagent- Preparation 47: methyl 6-bromo'ii^imida'z0^4^5:-c] yridi
[008071 A soltiti n of 2-bromo-5-nit.ro .yridin-4-amihe;( 1.5' mmol) in acetic acid (20 mL) was added in portions into a 75 °C suspchsion of iron powder (1.5;g^27 minol ) in acetic acid (20 mL). The reaction mixture was .st irred at 75 °C for 2 h, coolcd io r.oonvtcniperalurc, and filtered through ccl iie. To. the filtrate was added 1 -bis(incih0xycarbo"nyl)-2'-niethyl-2- thtopseudourca ( 1 .4 g. 6. mmol), and the mixture was stirred at 65 °C for 60 h. The reaction mixture was cooled 10 room temperature and' concentrated. The solid residue was triturated with dichloromethane and dried to give the title Compound (1.8 g, 'quantitative yield) as an orange solid. MS (El) for Cxl-l7Br ,0 : 27 1/273 (MH+).
Reagent Preparation 48: tert-butyl 3 bi.sCtert-but0xycarbonyl)amino)-5-bpomo-]/7- indazok'-ircarboxylatc.
[008081 To a cooled (0 °C) solution of 5-bromo- fW-indazol-3-aminc (0:30 g. 1.4 mmol). DIPEA (2.5 mL, 14 mmol) and dUlcrl-bulyl dicarbonalc ( 1.5 g, 7.0- mmol) in THE ( 15 mL) was. added DMAP (0.09 g. 0.70 mm l). The reaction mixture was then stirred at ambient temperature for three hours. The resulting solution was diluted with ethyl acetate (75 mL) and washed with saturated aqueous ammonium chloride (2 x 50 mL). The organic layer was washed with brine, dried over magnesium sulfate, filtered and concentrated in vacuo.
Puri f icat ion by sil ica gel chromatography provided tert-butyl 3-(bis(tcrt- buioxycarbonyl)amino)r5-bromo- 1 W-inda .olc- 1 -par 6χγ,ΓαΐΙέ·,(0 4 :β* 61 %) as a waxy solid.
Ή NMR (400 MHz. CD.CIj) δ 8.04 (l. 1 H), 7Ϊ68 (cld, I H). 7.66-7.5S (in, I I I), 1.53 (s, 18H), 1.43 (s, 91 1); MS (El) for C:2l-hoBrN;,Of>: 12 (Μΐ ).
Reagent Preparation 49: 6-clilon)- -p enyIijyrimidinc-4-;in>inc
[008091 STEP 1 : 6-Clilorop riraidin-4-.ol (500mg. 3.85 mmol). aniline (420 μΐ , 4.62 mmol): and N. N-diisopropyleihylamine ( I mL) in dicihylene glycol diinelliyl ether (5 mL) :wa's heated to 120 "C . and .stirred :l'or 8h. The mixture. was.-c p.lcd -is-room temperature then diluted with actoue:diethyl ether -.solution ( 1 : 1. l SiiVil) to given precipitate. The solid collected by nitration and washed with acctoiie then dried . iojafford 6- (pheylaniino)pyriinidii 4-ol ( 255 nig. 35.5 '¾). MS (EI) for GipHg jO: 188.2 (MH+);
[00810] STEP 2: 6-(PhcnyIamino)pyfimidin-4-of(253 mg, 1.3,5 mmol) was dissolved in neat phosphorous oxychloridc (5 mL) and -stirred for 3h at 95 "C then cooled to room temperature and concentrated. The residue was poured into an ice water slurry and extracted with diehl.or.omelhane. The extract was washed 'saturated aqiieous sodium hiearbonate 'solution: dried over sodium sulfate, filtered and the solvent evaporated to afford 6-chloro-N- phenyIpyriiiii(liiic-4-ainitie (220 mgHvhieh was used. ithout further purification.
[008111 Using analogous synthetic techniques' and substituting- with alternative starting reagents in step I the following reagents were prepared.
[00812] fi-Chloro-NT-(4-nicllK)xyphenyl)pyriniidiu- -aniine. Synthesized according to the metliod of reagent preparation 4 using 4-meihoxyaniline in step I .
[00813] 6-ChIoro-N-(3-mcthoxyphcnyl)pyrimidin-4-amine. Synthesized according to the method of reagent preparation 4 using 3:mclhoxyaniline in step I .
according to the method of reagent 'preparation 49 using 6-ehloro-5÷methylpyrimiclin-4- l and 4-melhoxyanilinc in step 1.
[00815] 6-Chl0i T5-mclhyl-N-plienyIpyrimidin-4-amine. Synthesized according to the method of reagent -preparation.49 using 6-chioro-'5-meth.ylpynmidin-4-ol and. aniline in step 1.
Reagent Preparation 50: 5-(4,4,5,5 ctramctliyl-'l,'3,2-dioxabo'r0lanr2-yl)-l-mcthyl-lH- indaznlc
[00816] STEP 1 : A suspension of 5-hromo- 1 //-indazplc (200 mg, 1 .02 mmol), cesium carbonate (661 nig, 2.00 mmol). and iodomcthane ( 156 mg, 1.10 mmol) in
dimclhylformamide (3 mL) was stirrcd at room temperature for 15 h. The mixture was partitioned between 5% lithium chloride and ethyl acetate, the aqueous layer was extracted with ethyl acetate (2 x). the combined organic extracts were washed with 1 N sodium
hydroxide, -anti brine, dried over anhydrous sodium sul fate, l iltered and concentrated. Column chromatography on silica (hexanes/eihyl acetate 4: 1 ) gave 5-bromo- l -methyl- Γ/7-indazble ( 150 mg, 70% yield) as an orange sol id. MS (El) for Gxl lvBi N,: 2 12 (MH+).
[00817] STEP 2: A suspension of 5-hromo- 1 -methyl- r//-indazole ( 1 50. mg, 0.7 1 nimol). bis'(pinacolatb)diboron (200 mg,: 0.7S.mmbI), potassium acctate. (206 liig, 2. 10 nimol), and dichlorol 1 , 1 -/>/x(dip'henylphqspln^ ( I I) dichloromcihane adduet (36 mg.
0:04 nimbi) in dimciiiyf sul fox ide (4 niL) \vas degassed withriiitrqgciv, aiicl then stirred; at 80 °C for 18 hi The reaction . mixture was cooled' to, room temperature and partitioned between water and ethyl acetate. The mixture was filtered through ec lite and then the layers were separated. The aqiicous layer was extracted with ethyl acetate (2 x), the combined organic extracts were washed with brine, dried over anhydrous sodiuin sulfate, filtered and concentrated. Column chromatography on silica (hexancs/clhyl acetate 7:3) provided 5-
mg, 86% yield) as a yellow oil. MS (EI) for C| M iyB
20
2: 259 (ΜΙ Γ).
Reagent Preparation 51 : 1 , 1 -diinelhyletliyl 7-broino-9-meihyI-2,3-<lihydro- l ,4- -4(5H)-carboxylate
a mixture oTT;l¾F (SO.niL) andj-mefl^ ' mechanical .stirring apparatus aiid the mixture was gently warmed' until Iv ii inogencous. solution was.obtaincd. On cooling to room temperature, elhanolamine (23 niL. 0.38 mol). was added over 5 minutes. The resulting solution was st irred for l h at room temperature then cooled to 0 "C. Sodium boi ohydride (4.26 g, 1 12 mmol ) was added in port ions followed by THF (65 mL) and the mixture was stirred for l h. Di-tcrl-buiyl dicarbonaie (82 g. 0.375 mol) was then added as. a conceniraied solution in THF over 30 minutes. The resulting mixture was then allowed to warm to room temperature. and and stirred an :additidhal 2h. The mixture was concentrated.. I'd a! thick residue and partitioned with ethyl acciate and water. The organic phase- was, washed twice with l¼:at|ueousihydrochloric acid. once with water then brine,, dried .over anhydrous sodium sulfate then-filtered and concentrated. The residue was taken into a minimum of warm hcxanes and allowed to stand. The crystalline solid obtained was
collected by niiralioii, washed wiili hexancs and dried to '.afford 1 , 1,-dimclhylcihyl | (5-brpmo- 2-hydroxy-3-methylphenyl)|(2-hy<lrt)xyclliyl)carbaiiuitc (.40 g): The mother l iquor was concentrated and further purified by gradient silica. gel chromatography using 3: 1 hcxanes:cthyl acetate to 100% ethyl acetate and the combined product fractions combined and concentrated. The residue was crystallized from a minimum of warm hcxanes and combined with the previous crop lo give 1 .1 -dimethylethyl | f5¾omo-2-hydroxy-3- meihylphenyl) |(2-hydroxycthyl Carbamate (61 .87g. 57% yield) as a colorless crystalline solid.
STEP 2: 1 , 1 dimethylethyl |(5-bi()mo-2-hydiOxy-3-nielhylphenyl)|(2- ;lvydr0xyethyJ)eaH>aniate't 27.8 mniol) was taken into dichloroniethane (50 nil) and the resulting solution cooled t 0"°C. Diisopro ylelhylamiiie (5.8 m'L; 33.4 πίηιοΊ)· was added to the solution followed by tosyl chloride (5: g, 27.8 mmol) and the mixture vyas allowed to warm to room temperature then stirred for I 2h. The resulting slurry was concentrated and partitioned with ethyl ether and l iVI aqueous hydrochloric acid. The organic solution was dried over sodium sulfate, filtered and concentrated to a colorless amouphous residue. The residue obtained was taken into Tl-IF (50 niL) and cooled. to 0 "C. Sodium
bis(trimethyl.silyl)aniide (5.3 g, 28.9 mmol) was added and stirring was continued for 1 h at which point additional sodium b is( t r i 11 ί ethyl si i y 1) a ini cJc (5.3 g) was added and the mixture was al lowed to warm to room temperature and stirred for 12 h. The rcsulting slufry was partitioned witlr ethyl ether and 1 at|ucous hydrochloric acid ahd the organic solution was dried over sodium sulfate, filtered and concentrated to a colorless amouphous residue. The residue was purified by silica gel chromatography to afford 1 . 1 -dimethylethyl 7-bromo-9- methyl-2,3-dihydro- l ,4-benzoxa/.cpine-4(5H)-carboxylatc (6.9 g. 73 % yield) as a colorless oil that slowly crystall ized. ' l INM R (400 MHz, CDCI3): 7.22 (s, 1 .5H). 7. 19 (s, 0.5H), 4.41 (br.s, 0.6Ή). 4.34 (br. s, L4H), 3:99 (m, 21-1). 3.79 (m, 21-1), 2.20 (s, 3H). 1 .4<) (s. 9H);
' HNM R (400 MHz, DMSO-dfi): 7.3 1 (br. s. 1 H), 7.22 (br. s. 1 H), 4.38 (br. s. 0.6H). 4.32 (s, 1.4H), 4.03 (in, I H),.3.96 (m, I H), 3.68 (in, 2H). 2, 16 (s. 31-1), 1.32 (s, 9H ); MS (EI) for
Proceeding according to the method of reagent preparation 1 and replacement of 5- bromo-2-hydroxy-3-methylbcn7.aldehvdc in step 1 with alternative reagents, the fol lowing were prepared:
I , I -diinelhylethyl 7-bi ino-9-fluoro-2'.3-dihydro- 1 .4-ben/.oxazepine-4(5/7)- carboxylaie. Ί-INM R (400 MHz, CDCI.V): 7. 19 (m, I .5H), 7. 10 (s, 0.5H), 4.46 (br. s, 0.6H), 4,3.9 (br.s, 1.41-1), 4.09 (m, 2H), 3.8 1 ( m. 2H). I .40. (s, 9H ): Ί-INMR (400 M Hz, DMSO-d6):
7.50 (d. 1 H), 7.27 (s, I H), 4.45 (in. 21-1). 4. 1 1 (in, 21-1), 3.92 (br.s, 2H), 1 .29 (S. 91-1); MS (El) for . C M-H f7B.rFN03: 290 (M+÷BOC).
1 , 1 -dimclliyleihyl 7- r0nio-9-fehl0r0-2,3TdihycliOT l ,4 )cnzbxa^
carboxylate. Ή NMR (400 MHz, CDClj) δ 7.43 (d, 1 M). 7.26 (d, I H). 4.40 (s. 21 1). 4.10 (m. 2H). 3.82 (nu 21-1), 1.42 (s. 9H): MS (ES) for CMl-l ,7Bi CIN< ,: 362 ( H+).
1 , 1 -dimelhylclhyl 7-bromo-9-cdiyl -2,3-diliydro- 1 .4-bcnzoxazepinc-4(5//)- carboxylatc. MS (ES) for Cu,H2:>BrNOy. 356, 358 ( i l ").
1 , l -dimclhylelhyl 7-bromo-9-nicthyIoxy-2,3-dihydiO- 1 ,4.-benzoxazcpinc-4(5/7)- carboxylate. ' i lNMR (400 MHz. CDC ): 7.06-6.94 (m. 2H), 4.44 (bs. 2H), 4.04 (del. 2H). 3.84 (s. 3H), 3.82 - 3.78 (ms.2.1-1), 1.42 (s, 9H), -CliIoro-5-isopropyl-6-inelhyIpj,rimidiu-2-amine
[00818] STEP I : To a solution of ethyl 2-i.sopiOpy!aceloacctaie (22.0 g, 0. 1 8 mol) . and guanidine hydrochloride ( 18.0 g. 0. 19 mol) in methanol ( 100 mL) was added sodium methoxide (0.3S mol, 86.4 niL, 25 % methanol solution), at 0 ''G via dropping funnel over 30 niiii. Th reaction mixture was allowed to room temperature, hen heated to 50 "C for 18 hrs. The mixtur was concentrated, diluted with ethyl acetate (20 mL) .arid adjusted lo pH 6-7 with 6N aqueous hydrochloric acid'. The resulting solid was filtered and washed with water. The filtrates were concentrated and repealed filtration afforded a second crop of solid. The combined solids were dried under vacuum to give 2-aniino-5- isopropyl-6-nielhylpyrimidin- 4( l H)-one as a pale yellow sol id ( 16.8 g, 56 %); Ή NM R (400 MHz. DMSO-dfl): δ 10.5 (s. 1 H). 6. 17 (s, 21 1). 2.85 (in, 1 1-1), 2.03 (s, 31 1), 1 .15 (d, 6H): MS (El) for Cx!-InN30: 168.2
[00819] STEP 2: To a solution of 2-aminp-5-isopropyl-6-mcthylpyrimidin-4( I M)-one (4.93 g, 29.5 mmol) in phosphorus oxychloride (50 mL) was rcfluxed for 1 8 hrs. The reaction mixture was concentrated and the residue partitioned with a mixture of ethyl acetate and water ( 10 mL each). The biphasic mixture was quenched with solid sodium bicarbonate addition until the aqueous phase pl-l was 6-7. The aqueous layer was extracted with ethyl acetate (3 x 100 mL) and the combined organic solutions dried over magnesium sulfate, filtered and concentrated to afford 4-chloro-5-isopi pyl-6-incUiylpyrimidin-2-amine as a pale
brown solid (4.92 g, 90 ): Ή NMR (400 MHz. DMSO-dr,): δ .6 1 (s, 2H).3.26 (in.1 H). 3.25(s.-3H).1.21 (d.6H): MS (El) lor CsHuCI y: 186.1 (Ml-f).
100820] Proceeding according ιο llic incihod of reagent preparation 2 and replacing ethyl 2-isopropylaccloaceiaie in step I wit alternative reagents, the following were prepared:
4-Chloro-5.6^dimcthylpyrimidin-2-annne. Ή NMR (400 MHz. DMSO^df,): 56.69 (brs,2M), 2.27 (s.3H) .2.l0(.s, .3.ΙΨ): MS (ΕΪ) for€
6Η
8Ν;
(ά: I58.2.(MH
'4.-Chloio-6-cthy^ 1 H N R' (400 MHz, CDCl.s): 6
4:98 (br s.2H).3.42 - 3.26 (m. I H).2.72 (q, 2H).1.34 (cl.611).1.27 fi, 3H).
4-Chlbn^5 nhyl-6 nelhylpyriinidin-2-aininc. Ή NM (400 MHz. DMSO-d6): δ 6.73 (brs.'2H).2.60-2.47 (ni, 2H).2.30 (s.3H), 1.04 (v, 3H): MS (El) for C7H,nN3CI: 172.1 (MH+).
4-Chloi -5-
'isopropylpyrimidin-2-amine. MS (Ei) for C HIUCI J: 172.1 (Mi l*).
1 H N R (400 MHz; pMSO-d
6): 6.82
(s; 2H), 254-2.48 (m, 2H, overlapped).2.32 (s, 3H¾ 1.56;- 1.37 (in, 2H¾ 0.93 (du 3H); MS (El forCxH-ijGINj: 186.1 (ΜΐΓ).
4-Chloi -5-(cyck>propylincthyl^ MHz. DMSC W: 4.03 (br s.2H), 2.55 (d.21-1).2.35 (s.3H).0.99 - 0-.88 ( i.1 H).0.49 - 0.34 (m., 2H).0.22 (m.21-1); MS (El) for CyHi.CIN-,: 198.1 (MH ).
4-Cliloro-6.6-diniethyl-5.6-.7.S-lciraliydr0qiiinazoliii-2-rainiiie. MS (El) for
GIOH|L,C}N3: 212 (MM*).
.5-Allyl-4-chloro-6'inetliylpyriinidiii-2-amine. MS (El) for CsHioClN3: 184 (MH*).
4,¾-DicliloiO-5-cihylpyrinii(lin-2-aiiiinc. Ή NMR (.400 MHz, QMSO-do): 11.11 (s. l;H.),,7.33(s,'lH)y.6.71 (s, 2H.)V.2;62 (q.21-1).2.33 (q.«,2H)5- 1.07 (t.3I:r),O.96'(t.3H):
Reagent Preparation 53: l-(4-Chl(>ro-5-isopropyl-6-inethylpyrimidin-2-yl)-yV;N-
100821] STEP I: A pressure vessel was charged with methyl acctpaceiatc (40;0 g.34.4 miiiol), potassium carbonate (48.0 g.34.7 nimol), and TI-IF (200 iiiL). The heterogeneous
mixture was stirred at n for 45 niiii heroic adding 2-iodopropanc (36.6 niL. 3.6.6 mmol). sealed and heated to SO "Q for 7-2 h widi mixing. The reaction was then coolcd-to rt. Water was added in portions until all solid was dissolved to afford a homogeneous biphasic mixture. The mixture was partitioned, and the organic layer was/dried over sodium sulfate, filtered and concentrated under vacuum to afford a yellow oil. Distillation under vacuum afforded methyl 2-acciyl-3-melhylbutanoaic as a clear colorless oil. (20.0 g. 8:2 mix of methyl 2-aceiyl-3- methylbulanoaie: methyl aceloacclaic. Ή NMR (400 MH'/.,-CDCI¾): 5. 3.73 (s 3 H)\ 3.20 (d. I H), 2.50 - 2;3.5 (m. J'M), 2.22 (s. 3H). 0.95 (dd, 6H), MS (EI) for CsHy.O.,: Γ59.2 ( H+).
[00822] STEP 2: A round' bottom flask "was charged with metliy!.2-aeelyr-3- nielhylbutanoate-as obiairicd -ill step I ( 14:3 g. 72.4 mmol). nielhanol:(30 niL), and 2- chloroacetamidine hydrochlori(le «( 12.S g,'99;5 mmol). The mixture was cooled to 0"C followed by addition of 25 wt% sodium meihoxideM methanol (4.8.8 niL, 181- mmol. 2.5 eq.). The reaction was warmed to i t. allowed to stir overnight, then filtered. The filter cake was rinsed with ethyl acetate and the organic solutions were combined, concentrated to a slurry, and the residue was purified by gradient silica gel chromatography (60:40 hcxanes : ethyl acetate to 1 : 1 hexanes : ethyl acetate) to afford pure 2-(chloromcihyl):-5-isopropyl-6- mcthylpyrimidin-4-ol as a yello solid (3.17,g. 22 % yield). 1 H NMR (400 MHz. CDC1. ): 54.43 (s. 2H). 3. 18 - 3.00 (m, I H). 2.34 (s. 31 1). 1 .33 (d, Η);¾$ (Ei) for --e9Hi ½0*Cl:-. 201 .1 CM H .
[00823] STEP 3:. A .round-bottom flask was charged with .2- ehroroniethyl)-5-isopropyl-6- nietliylpyrimidin-4-ol (500 nig, 2.5 mmol ), THE (7 niL), and 2.0M dimethylamine in TH F (2.5 niL. 5.0 mmol). The reaction was heated to 60"C overnight, cooled to rt and
concentrated under vacuum to afford crude.2-[ (dinielhylaiiiino)nieihyl |-5-i.sopi pyl-6- incihylpyrimidin-4-ol as a brown oil. Neat phosphorous oxychloridc (3 mL) was added and heated to 60"C for 2 h. The react ion was cooled lo rt, and concentrated under, vacuum. Ice cold .watcr was: added to the residue and then basified widi 6N aqueous sodiuni hydroxide to pl-l 7. The aqueous mixture was extracted four times with ethyl acetate. The organic layers ere combined, dried over sodium sulfate, filtered and concentrated. The residue was purified by silica gel plug filtration', eluiing with 95:5 ethyl acetate : methanol, then 90: 10 ethyl acetate : methanol to afford pure l -(4-cliloro-5-isopropyl-6-methylpyrimidin-2-yl)-N/V- dimcthylmcllianamine as a brown oi l (459 nig, 80 % yield). Ή NMR (400 M Hz. DMSO- tl„): δ 4.52 (,s. 2H). 3.61 - 3.46 (in. 1 11 ). 2.89 (s, 6H). 2.65 (s. 3M). 1 .35 (d. 61 1): MS (El) for C, |H|KN CI: 228.2 (MH*).
[00824] Proceeding according ιο the method of Reagent Preparation 3 and isolating the second cluiing compound in step 2 to afford 2-(methoxymcthyl)-5-isopiOpyl-6- irielhylpyrimidin-4-τΌΐ. then proc'ecding.wiih step.3, 4-ehk)ro-5-isopiO|iyl-2-(nicthoxymethyl)- 6-methylpyrimidine vyas. prepared.
[00825] Proceeding according: to the method of Reagent Preparation 3 and replacing methyl 2-acetyl-3-methylbiilanoalc in step 2 with alternative reagents, the following- were prepared:
l-(4.6-dichloro-5-isopropylpyriinidin-2-yl)- /V-di!nctlryltnethanamine MS (EI) for CoH.sN h: 248.1 ( H+).
l-(4-Ch|oro-5-isopropylpyrin)idiiv2-yl)-A'./V-diincthylinelhanannnc. Ή NMR (400 MHz, CDC ) δ·8.59 (s, 1 H), 3.87 (s.2H), 3.38-3.19 (m, I H), 2.52 (s, 6H).1.40-1.23 (m. 6H); MS (El) for 0|!1Η1(,αΝ;!:·2Ι4.216 (MH+, CI isotopes).
1 -(4-Chloro-5-ethylpyriniidin-2-yl)-/V;A/-diineihylinclhananiine.1 H NMR (400-MHz. GDCh) 68,52 (s.1H).3.70 (s.2H).2.74 (q, 2H); 2.37 (s, 6H).1.28 (t.3H); MS (EI) for CvH|.,CiN3: 200, 202 (MH+, GI isotopes);
l-(4-CWQra-5,6-dieihylpyrimidin-2-yO^^ Ή NMR (400
MHz, CDCb) δ 3;65 (s.2H), 2.84 (q.211).2.77 (q, 2H).2.36 (s.61-1), 1.29 (1, 3H), 1.20 (I, 3H): MS (EI) for CnHigCl .,: 228.230 (Mil*. CI isotopes).
l-(4 Ihlorq-6-etliyl-5-jsoprx>pylpyrimldm^ 'I"! NMR
(400 MHz, DMSQ-dfi): 3.68 (s.2H).3.48 (dl, 1 H).2.88 (q, 2Ή).2.33 (s.6H), 1.35 (d, 6H). 1.20(q,3H): MS (EI) for Ci^iC'l ,: 242.1 (ΜΙ-Γ).
.l (4TGhIoi T5- ,2i2-t inii0r0ethyl)p MS (El)for CqH I |Cl.F¾N-.¾:.254 (MH+).
l-(4,6-Dichloro-5-ethylpyriinidin-2-yl)-A'./V-dim Ή NMR (400
MHz, DMSO-df,): 3.58 (s, 2H), 2.82 (q, 2H), 2.23 (s.6H).1.16 (I, 3H); MS (El) for
l-(4-:Chloro-5-cthyl-6-melhylpyriniidin-2-yl)-/V.A'-diinelhylmclhanamine. MS (El) for C, HI(lClN¾: 214.I(MH+)
r(4-Chlbro-6-!.spp*ropyl-5-]iiethylpyrinudin^2-yi^
MS (EI) for C| j HIKCINJ:-.228.2/(ΜΙ-Γ)
[00826], Proceeding accordin to the method of Reagent Preparation 3 and replacing dimethylamine in step .3 with alternative reagents, the following were prepared:
4-Chloro-2-((3.3-difliiorapyrroIidin-l-yl)m
MS (EI) for C^Hii^CIF:: 272.2 (Μ1 ).
4-Ch!biO-5-isopropyl-6^nicihyl-2 Ή NMR (400 Hz, CDCI. δ 3.72 (s.21-1), 3.58-3.44 (in, 1H), 2.78-2.34 (in.1111).2.28 (s.3H), 1.37 (d.6H); MS (El) for C|.|HBCIN.,: 283.285 (Μ1- , CI isotopes).
4-|(4-ClMoro-5-isopropyl-6-nunhylpyr'mi ^ (EI) for
C.-.Hj CI .O: 270.0 (ΜΙ:Γ).
4-Chlorc 5-i'sqp.ropyl-6-m MS (El) for
■C HioClNi: 254 (ΜΙ-Γ).
N-|(4-Chloro-5-isopmpyl-6-meiliylpyrimidin^
Ή NMR (400 MHz. DMSO-dfl): 3.76 (s.2H).3.30 - 3.24 (m..1H).2.57 (s.31:1).1.32 (d.6H). 1.06 (s.9H); MS (El) for Crd-I^CIN;,: 256.( H+).
[00827j Proceeding according 10 the method of Reagent Preparation 3 and replacin methyl 2-acctyl-3-melhyl ulanoate in .step 2 and dimelhylaminc in step 3 with alternative reagents, the following were prepared;
4-Chl;oro-5-isopropyI-2-(|y olidi Ή NMR (400 MH .
CDCI.0 i) 8.56 (s, III).3^88 (S..2H).3.40-3.;i5f( i 1H), 2.82-2.54 (m,,4H), 1.99-1.79 (m.411). 1,31 (d, 61-1); MS (EI) for C|2H|«CINj: 240, 242 (MH+, CI isotopes).
[00828] Proceeding according to the method of Reagent Preparation 3 and replacing methyl 2-acclyl-3-methylbutanoate and 2-chloroacetamidine hydrochloride in step 2 with alternative rcugcnls.-ihc following were; prepared:
4-Ghloro-26?6Hriincthyl-5 7.8-LeirahydtOiiiinaz0 MS (EI) for C1II15CI 2: 211
(ΜΙ-Γ),
101)829] STEP 1 : Λ solution of 5-bromo-2-chloropyridin-3-amine ( 1.0 g, 4.8 mmol) and diisopropylcthylaminc ( .85 niL, 10.6 mmol) in diehloromelhane (25111L) was cooled lo 0 °C, and then methancsulfonyl chloride (750 iiL.9.6 nimol) was added slowly. The reaction mixture was stirred at 0 °G for 15 min and was then warmed to rl. After stirring for 2 h, water was added, and the biphaslc mixture vvas partitioned. The organic phase was dried over magnesium sulfate, filtered, and concentrated in vacuo. The residue was then dissolved
in dioxanc ( 10 nil-) ηικΐ water ( 10 mL). Potassium carbonate (2.76 g. 20 mi ol) was added, and the reaction mixture was slirrcd for, 15 h at i t. Water was then added to the mixture which was subsequently acidified with aqueous citric acid ( 10%). The aqueous mixture was extracted twice with ethyl acetate. The combined organic extracts were dried over magnesium sul fate, filtered, and concentrated in vacuo. The residue was purified by flash chromatography (gradient. 100% hcxanes to 50% hcxanes in ethyl acetate) to provide N-(5- luomo-2 1iloropyridin -yl)mcihancsulfonaniide.(520 irig, 1 .82 mnipl„3S% yield) as a light pink solid, Ή R. (400 MH/. CDCIj) δ 8.27 (d, I H). 8. 14 (d. I H). 6.83 (br s. I l l), 3. 1 1 (s. 3H): MS (El) for E(iH6BreiN202S: 285; 287. 28? (Br. CI isotope-pattern. M H*).
[ 00830"] Proceedin according to t ie.nieihpd; of Reagent Preparation 4 and replacing Methanesulfonyl chloride with irinuoroinclhancsiill'onic ahliydride. the following was prepared:
N-(5-Bbrorito-2-chloi pyricliii-3-yl)- 1 . 1 , 1 -irifluoroinethanestillOiiamidc. MS (EI) for C6H3Bi CI Fa ,02S: 33.8.9 (M H*).
100831 ] STEP 1 : To a 50 niL pressure vessel were added 4-chloro-5-iodo-6- methylpyrimidin-2-amine (2.0 g. 7.43 mmol), 4.4.5,5-tetramethyl-2-vinyl- 1 ,3,2- dioxaborolanc (" 1 .37 g, 8. 17 mmol). diehloro| 1 , 1 -bi.s(diphenyI)phosphino]fcrrocchepalladiuin (I I) dichloromcthane adduct (2S5 mg. 0.37 mmol. 5 mpl.%) and 2M sodium carbonate solution (7 mL) and 1 ,2-dimelhpxycthanc (20 mL). The reaction mixture was purged with nitrogen for 5. minutes and heated to 95 °C for 12 hours. The reaction was then cooled to room temperature and filtered through a pad of sil ica gel using-elhyl acetate and the eluent concentrated. The residue was purified by gradient silica gel chromatography (hcxanes : ethyl acetate 80:20 to 70:30) to afford 628 mg of 4-chloro-6-methyI-5-vinylpyrimidin-2-amine (53% yield). Ή NM R (400 M H/. CDG1j):6.60 (dd. I H). 5.58 (dd, I H). 5.47 (dd, I H), 5.04 (s. 21 1), 2.44 (s. 31 1); MS (El) for CJHXCINJ: 170.0 (MH+).
| ()()832] Proceeding according lo the method of reagent preparation 5 and replacing 4- clilOiO-5-iodo-6-mcthyIpyrimidin-2-aminc with alternative reagents, the following were prepared:
.4 v(licliloro-5-vinyipyriniic!in-2-amine. Ή Nfyl (400 H/. DMSO-df)): S 7.59 (br in. 2H). 6.53 (del. I H). 5.66 (ddr 2H).
4-C1ilor0-5-viiiy]pyriinidi -2-aininer 'l-I N.MR (400 MHz, DMSQ-dY): 8.57 (s. I B). 7.25 (s, 3H). 6.66 (del. I H). 5.77 (d. 1 H). 5.23 (d. I I I): MS ( El) forQI-lfiClNi: 156.1 (ΜΗ ').
[00833J Proceeding according to the method of reagent preparation 5 and replacing 4.4.5.5-lelramelhyl-2-vinyl- 1.3.2-dioxaborolanc with alternative reagents, the following were prepared:
4-Chloro-5 3-niioropheiiyl)-6 incthylpyriinidin-2-ain 1 I I NMR (400 MHz, DM.SO-dft): δ 7.54 - 7.43 (m. ( IT), 7.28 - 7,01 (iii. 5H), 2.02 (s. 311): MS (El). or
'Cii-HylSljFCi::..23:e..-l (MH+).
Reagent Preparation 56: (S)-4-Chloro-7-mcthy^
[00834] STE I : To a cooled (0 °C) solution of .(S)-3-tncjhylcycloliexanone
(US200602y3364) (2.0 g, 18 tnmof) and dimethyl carbonate (2.0 mL..22 mmol) in dielhyleiher (40 m'L) was addco" sodium hydride (60% wt/wt in mineral oil, 1.0 g. 25 "-mmol). porl ionwise over 30 minutes. The resulting slurry was allowed to stir at ambient temperature for 30 minutes followed by two-hours at reflux. The reaction /mixture was cooled (0 °C) and methanol (30. mL) was added dropwise over - 2.0 minutes. The resulting slurry was partitioned between 10% aqueous citric acid and ethyl acetate. The organic layer was washed with brine, dried over magnesium sulfate and concentrated in vacuo, Purificalion b silica gel column chromatography provided (4S)-methyl 4-inethylr2-0xocyclohexanecarboxylatc (3,0 g, 100% yield). MS (ES) for CyI-Ι ,, : 17 1 (M+).
(008351 STEP 2:. Λ solution of (4S)-methyl.4-melhyl-2-oxocyclohcxanccarboxylaic (3.0 g. 18 mmol) and ammonium acetate (3.4 g, 45 nimol) in clhauol (50.mL)' was. healed to, reflux for 2 hours. The reaction was concentrated to one lhird original v lunic, and then diluted with cth.yl -acctaic (J O .niL). The organic solution was washed with water ( 100 mL) and brine (50 mL) and then dried over anhydrous sodium sulfate After filtration and
concentration, the residue was dissolved in MN-dimethyilOrmamidc d.imcthylacelal (50 mL) and heated to 1 10 °C for 18 hours. The resulting solution was cooled lo room temperature and concentrated to provide (S,Z)-mcthyl 2-((dimethylamirio)methyleneamino)-4-
mcihylcyclohcx- I -enecarboxylatc (3.0 g. 88% yield) as an oil. MS (El) for C12H20 2O2: 224
|0()83ή| STEP 3: A solution of StZ)-melhyi 2-((d mellvylanmH>)irieihyleiicainino)-4- inctliylcyclohcx- l -eneearl) xylaie (3.5 :g, 16 mmol) in 7.0M ammonia in methanol (35.- n.iL) was stirred at 25 °C for 90 minutes then concentrated. The resulting oil was 'dissolved, in. chloroform (5 mL) and phosphorus oxycliloridc (5 'niL) and rcfhi.xed for .2 hours. The mixture was concentrated to an oil. diluted with ethyl acetate (50 mL) and washed with saturated sodium carbonate (50 mL) and brine (25 mL). The solution was dried over anhydrous sodium sulfate, filtered and concentrated. The residue was purified by silica gel column chromatography (ethyl acetate: hexancs, 1 :8) to give (S)-4-ehl0ro-7-methyl-:5;6.7;8- tetrahydroquinazoline (O^ g,^ yield) as a yellow oil MS (ES): for CjiWi jGINj: 183 (MH+).
[00837;) Using analogous synthetic techniques and substiuit ing (S)-3-inelhylcycIohex.an0nc with 4,4-^methylcycloliex-2-en .ne in step 1 , 4-chloro-6,6rdiiviethyl-5. -dihydrociuina7.oline was prepared. MS (ES) for C |„H , |CI N.: 1 5 (MH+).
Reage iclirie
100838] STEP
, 2:01 mmol, example 5, step 1 ) was added in portions. into a mixture of 12% aqueous hydrogen peroxide (6 mL) and teirahydrofuran (5 mL) at 70 °C. and the resuliing solution was stilted at this temperature for 30 min. After cooling to room temperature the pH -was adjuslcd to 9 with saturated aqueous sodium carbonate, and. the resulting mixture was stirred at room
temperature for 30 mih. A 10% aqueous solution ofspdium tniosulfaic was added until the reaction with iodine-starch paper was negative. The mixture was extracted with ediyl acciatc (3 x 50 111 L), and die combined organic layers were washed with brine (50 mL),- dried over sodium sulfate, filtered and concent rated 10 provide 5-isopiOpyl-6-methyipyrimidiri-4-ol (0.32 g, quantitative yield)- as a colorless .solid. MS (EI) for CxI½N20: 153. ( H*).
100839] STEP 2: A solut ion of 5-isopropyI-6-melhyipyriinidin-4-ol (0.32 g, 2.01 mmol) in phosphorus oxycliloridc (5 mL) u;as stirred at 60 °C for 2 h. The reaction mixture was concentrated, ethyl acetate ( 10 mL) and saturated sodium bicarbonate ( 10 mL) was added to die residue, and the mixture was stirred at room temperature for I h. More ethyl acetate (50
mL) was added, the layers were separated, and the organic layer was washed with saturated sodium bicarbonate (5 mL), and brine (5 mL). dried over odiiim sulfate, filtered and concentrated. Column' chromatography of the residue :οϊι silica (0-30% ethyl acetate in hcxanes) afforded 4-ChIoi -5-isopi pyl-6-niethylpyrinii liiie (.46 mg. 13% yield) as a colorless oil. MS (El)lor CsH , ,GI N2: 171 (M*).
Reagent l'rc]]araliui.i"58: M 7-Ikomo-9.Hncthyl-2^3-di!iydrp^
[00840] STEP 1 : Dess-Martin periodinanc (0.3 1 g, 0.74 mmo!) was added to a cooled solut;ibh\0T [4-(7.-bromo-9-m^
.metliylpypi.nii l iiyi2= n'mpt ano'l (0.20 g, :0-49" mnio;]) and chlqiOfOrm ( IjO inDv The reaction mixture was allowed to warrii lo nibienLteinperalure over I hour and was partitioned between saturated aqueous sodium bicarbonate and diehloromethanc. The organic phase was then washed with brine, dried over magnesium .sulfate, filtered and "concentrated in vacuo. Purification by. column chromatography provided 4-(745ron;jo-9-inethyl-2,3-ciihydiO- 1 ,4- benz0xazepin-4(5/7)-yl)-5-isopi ^iyl-6-[iielIvyi|xyrimiclinc-2-carbald^ (0. 15 g, 75% yield)' as a waxy solid. MS (ES). C|<>H22BrN;¾02: 404, 406 ( ΜΙ- ).
[00841] STEP 2: To a cooled (0 "Q solution of 4-(74)romo-9-metliyl-2,3-xlihydiO-1.4- benxoxazepi 4(5/-/)-.yl)-5-isopro g, 0.20
•mmoi);and cesium carbonate (0. 19g. 0.57 mniol) in TI-IF (5 in L) was added
trinuoroincthyltriihethylsilane (0.08 mL, 0.54 mmdl). The resulting mixture was stirred at ambient temperature for 24 hours and methanol ( I mL) was added. The resulting slurry was concentrated in vacuo and partit ioned between diehloromethanc and water. The organic layer was washed with brine, dried over MgSO,t, filtered and concentrated in vacuo. Purification by silica gel column chromatography provided l -[4-(7-bromo-9-methyl-2,3-dihydro- l ,4- benzoxa/,epin-4(5//)-yl)-5-isopropyl-6-m^ (25 nig,
.28% yield) as a clear waxy sol id. MS
476 ( H -):
Reagent Preparation 59: l-[4-(7 5romo-9 iicthyl-2 -dih^
yl)-5-i.sopropyl-6-methylpyrimidii»-2-yl]ethan()l (008421 STEP I : To a cooled (0 "Q solution of 4-(74ii mo-9-methyl-2.3-dihydro- 1,4- beir/.oxa/.cpiiv4(.W)-yl)-5-isopropyl-6-i^^ (0,09, g.0.20 iiundl) in THE (5 mL) was added meihylmagensium bromide (200 pL.3.0 M solution in hexancs). The reaction mixture was allowed to stir at room temperature Tor I hour and saturated aqueous ammonium chloride (5 mL) was added. The resulting slurry-was partitioned bctweeirdichlorgmethane and water and, the organic layer was washed with brine, dried over. magnesium sulfate.. filtered and. concentrated in vacuo. Purification of the residue by silica gel
■■column diromatography provided l-|4-(7-broino-9-nietliyl-2.3-dihy(lro-1.4- bcn-oxazcpin
^4(5/7)-yl)-5risopropyl-6-mw (3 mg, 38% yield).
Example 1: 6-{4-|2-AmiiH)-6-niethyl-5-(l-methylethyl)pyrimidin-4-yl]-9 i
[00843.1 STEP I : To ad 00 mL pressure vessel were acldcdtcrl-butyl 7-bromo-9^ mcthy 2.3-id'ihy(lro-'l ^-bcny-oxazcpinc iSHJ-earbo!x late (9.2 g, 0.026 mol).
bispinacolaio(dibon)u) (8.2 g.0.032 mol) aiid. otassium acetate (7.6 g, 0.078 mol) in dioxanc (50 mL). Dichloroj 1.1 -bis(diplienyl)phosphino|fcnOcenepalladiunv (II) dichloromelhane adduci (530 mg, 0.65 mmol, 2.-5 mol %) was added and nitrogen was- bubbled through the reaction mixture for 5 minutes. The reaction 'mixture was'heaiecl lo 95 °C for 12. hours, cooled to room temperature and filtered through a pad of Cclite®.'The Celitc® pad was washed with ethyl acetate. (2 x 100 mL) and the combined , organic layers were dried over sodium silfalc. filtered,. nd concentrated-. The residue w.as.punficd by gradient silica gel flash
chromatography (hcxanc's:cthyl acetate '80:20 ΐο70:3Ρ) Itvafford lcil-bui l 9-inethy 7- ( A,5,5-tetramcthyl-l.,3.2'-d'ioxa^
carboxylatc (10. 1 g, quantitative yield). '.H.NMR (400 MHz, CDCIj): δ 7.66 -7.4.3 (m.2.H). 4.45 (d, 21-1).4.03 (s.2H).3.87 - 3.75 (m.2H), 2.23 (s.3H), 1. 1 (s, 911).1.36 - 1.26 (s. I2H); MS (EI) for C;il½BN05: 288.2.290.2 (MH+-Boc).
[008441 STEP 2: A glass pressure vessel was charged
' with icrt-bulyl 9-mcthyl-7.-(4,4,5,5- ieiramcthyl-l,3.2Hlioxaborolan-2-yl
:)-2 Hlihydrobeii.o| l,4|oxa/
"pine-4(5H)-carboxylatc (12.0 g.30.8 inniol), -(6-bromothiazolo|5,4-b|pyridiii-2-yl)acctainidc
(8.35 g.30.8 mmol). DME (75mL)..and 2M Na:CO.i(aq) ( 1.2 mL. 1.6 mmol). The reaction mixture was purged with nitrogen followed by addition of diehloi'o| 1.1 -his- (diphcnyl)ph()sphiiid|ferroccnepalIadium (II) dicliloromelhane addiict (1.27 g.5 mol%) then licated to 80 °C for 2.3 b. The vessel was, then cooled to rt, and the reaction mixture was filtered through Gcliic<¾and the filler ealic rinsed with ethyl acetate., Tlic edinbihcd Grganic filtrate was cpiiccntralcd to a brown residue whicli .was lukcci-iiitb eihanol (30 mL) and ethyl acetate (10 mL and allowed to stir at rt for I h until a fine precipitate formed. The precipitate was collected by filtration, rinsed with eihanol and dried under 'vacuum to afford' pure product as a light tan solid (5:9g). The filtrate was concentrated and purified by silica gel flash chromatograph (1:1 hcxanes:ethyl. acetate) to afford additional ten-butyl 7-(2- acctamidolliiazolo|5,4-b)pyridin-^
carboxylaic (0.74 ) to give a combined yield of (6.67 g.48% yield), 1 H N R (:400 MHz,
DMSO-dr.) :■ :δ L2.56 (s, IH), 8.73 (s.1 H),,s: 5 (m I I I), 7:54 (m, 2W¾.4.47 : (m.2H).4:20- 3.97 (in, 2H); 3:73 (s, 2H), 2.26 (s.3H),2.24 (s, 3! I), 1.34 (s, 9M): MS (El) for
C2.1l-i„N.10.,S: 555.I.(MH+).
[008451 STEP 3: A 500 mL round bottom liask; was charged with lcr buiyl 7-(2- acctainidoiliiaz0l0|5.4-b|pyridtn-6-yl)-9-nictl^
carboxylatc (6.67 g, 14.7 mmol), eihanol (8 mL). and concentrated aqueous hydrochloric acid (37 mL). The resulting slurry was stirred at ft for 20 min then healed to reflux overnight with stirring. The reaction mixture was then cooled to rt. and concentrated to 1/3 the volume. Acclonitrilc (20 mL) was added to produce a fine precipitate that was collected by filtration, rinsed with acctonitrile and dried under vacuum to give pure 6-(9-mcihyl-2.3.4,5-lcirahydro- l,4-be!izox;i/.cpin-7-yl)[ l,3]lhiazolo[5,4 b|pyridin-2-ainiiie hydrochloride salt (5.65 g, 14.7 mmol, quantitative yield).. 'l-LNMR.(400 MHz,.DMSO-d„): ft 9.67 (br s, 2H), 8.47 (m, 2H), 7. 1 (d, 1H).7.70 (dd.2H), 4,36 (br s, 2H), 4.23 (br s.211), 3.49 (br s.211).2.29 (s.3H); MS (El) lor Cir,W|f, .)OS: 313.1 (MH
+).
00846Ί STEP 4: A mixture of 6-(9-melliy!-2.3.4,5-tetraliytlro- 1.4-benzoxazepin-7- yl)[,l , jthiazolol 5A-b jpyridin-2-aminc dihydmchloridc (0.26 g.0.67 mmol), 4-ΟΗΓΟΙΌ-5- isbpropyl-6-mcllrylpyrimidiii-2-aminc (0.12. .0:67 m
'inol) ami
(0.6 niL.3.35 mniol) in N./V-dimethylacetamide,(3.0 mL) was heated at reflux for 30 minutes. Afler.cGoliivg.to room temperature the reaction mixture was diluted with water (7 niL) and the precipitate thus formed was collected by filtration, washed with hexanes and dried 'in vacuo. Gradient silica gel chromatography (diehloromethan : methanol 95:5 to 85:1 ) provided 6-^-[2-ainino-6 iieihyl-5-(l-niethylethyl)pyrimiclin-4-yl|-9
ieirahydro-1.44>enzoxazepin-7-yl) 11.3 |lhiazolo| 5,4-b jpyridin-2-aniine (0.16 g.52%). Ή
NMR (400 MHz, CD.?OD) S.66 (d.1 H), 8:01 (d. III).7.52 (d, 1 H).7.47 (d. HI).4.89 (s. 2H).4.45 m.,2H).4.04 (m.2H).3,08 (m.1 H).2.4 (s, ¾¾, 2.30 (s; 3H), 1 'Xd.6H): MS (1£S) for C. H27 OS: 462 (iVI If).
[0084 1 Proceeding according to the method of Example 1 and replacing
isopropyl-6-melhylpyriniidin-2-aniin alternative reagents, the; following compounds of the invention were prepared:
6f 4 2-Amino-5.6-dimcthylpyrm^
bcnzoxazcpm-7-yll| 1.31lhiazolo|5_.4-blpyridin-2-amine. Ή N R (400 MHz. DMSO-d6): ί 8.37 (d.1 H), 7.90 - 7.77 (m, 3Ή), 7.50 (dd, 2H), 6.51 (br s, 211), 4.63 (s,2H).4.32 (m.2H), 3.83 (m, 211).2.25 (s.3H), 2.1.8 (s.3H), 2.05 (s, 3H); MS (El) for C22H23N7QS: 434.2 (MH*).
6-f4-(2-Amindr5-elhyl-6-ihcthylpyn iidm^
benzoxazepiii-7-yl|| l,3;|t!iiazolol5;4-b|pyridiiV-2-amine. Ή NMR (400 MHz, DMSO-dfi): δ 12.50 (s, H-l).8.41 (s, ΙΗ)· 7.93 (s.2H).7.86 (s, ΙΉ), 7:62 ¾s, IH).7.49 (s,,2H), 4:92 (s.2H), 4.39 (s.2H).4.08 (s.2H).3.40 (buried q.2H), 2.29 (s.3H).2.24 (s.3H).1.08 (t. 311); MS (EI) for C2.d-l25 7OS: 448.2 (MH4).
6-|4-{2-Ammo-5-cihenyi-6-mediylpyrinudm^^
benzoxazepin-7-yll| 1 lthiazolo[5,4-b|pyridin-2-amine. Ή NMR (400 MHz. DMSO-d«): 833 (d, 11-1).7.86 (s, 2H).7.78.(d. I'M), 7-44 (s.1Η),.7.36;(χ, I If).6.56 (dd, 1H), 6.12 (s, 2H), 5.37 (dd.1H); 5.23 (dd.1H)..4:66 (s.2H).4.23 (s.2H).3.83 (s, 2H).2.24 (s.3H), 2.19 (s, 3H); 1.90 (s.3H. OAc) MS (El) lor C23H23 7OS: 446.1 (ΜΙ ).
6-(4 '2-Aniino-5-(l nethyIethyl)pyrimidih,4-yl]-9rmeih
benzoxazepin-7-yUI l.3|thiazolo|5.4-b|pyridin-2-amine. Ή NMR (400 MHz. DMSO-d,;): 8.35 (d. IH).7.94 (s.1 If), 7.87 (s.2H).7.79 (d. III).7.49 (s. III).7.40 (d.1H).6.02 (d.2H).
4.44 (s.2H), 4.27 (s, 2H).3.69 (s, 2H), 2.97 (di.1 li).2.20 (s, 311).1.17 (d, 6H); MS (El) for C2.1H25N7OS: 448.2 (MH+).
-Amin0-2-[7-(2-iimi
jio| 1.3 |tliin/.olo| 5,4- |pyridin-6-yl -9-nieihylr2,.1-dihydro- 1.4-
I l-l).8.28 (d, III).8.01 (s.2H), 7,84 (d, 1H).7,64 (s, IH), 7.47 (s, 211), 7.42 - 7.23.(m. I H). 4.87.(in, 2H), 4; 14 (i,.4 l¼ 2.23¾ 3H>: ,MS (EI) for G2i I liX ii©S 31.1 (MH ).
4-Amino-2-|7^
bcnz<)xazcpin-4(5H)-yf|| ^ DMSO-do): δ 8.38
(d, 311).7.84 (di.4H).7.61 (s, 2M), 7.08-6.84 (111. tH).4.82'(s,2H), .4,13 (d.4H), 2.23 (s. 3H): MS (El) forC2,H2o s02S: 449.1 (MH+).
6-|7-.(2-Amino| l,3.|ihiazolo|5.4-b'|pyridin-6-yl_)-9-methyI-2,3-di ylrorl,4- i)eiizoxazcpin-4(5H)-yl |pyridine-3-carbonitrilc. 'vH NM (400 MHz. DMSO-do): 8.47 (d. 111).8.37 (d. I H), 7.93 - 7.80 (in.4H); 7.75 (s, 1H).7.48 (s, IH), 7.13 (d.1H).4:87 (s.211), 4:23 (s.211), 4.1 (s.2H), 2.2 (s.3H); MS (EI) for C22Hi. OS: 415.1 (MH■).
benzoxazcpin-7-yl || l ,3|HiiazQlQ^ DMSO-d(l): 8.36 (d, Hi), 7:85 (s, 211).7.80 (d. J l-l).7.54 (s, 2H).7.40 (d, IH).6.32 (s, 2H).4.76 (s.211). 4.05 (cl.41-1), 2.21 (.s.3H).1.88 (s.31-1. OAc).1.79 (s, 3*1); .MS (EI) for C^Hn^OS: 420.1 (MH*).
6-|4-(2-Amino-6-meihyl-5-piOpylpyrim^ .4- .beirzo'xazcpii1-7-yl|| I ,3|lliiaz l0|;5,44)lpyridih-2-airi Ή NMR (400 MHz, DMSO-do): 8.36 (d.1 H), 7:85 (s; 2H).7.81 (d, I H), 7.47 (d, 2H), 5.92 (s.2H).4.43 (s.2H).427 (s.2H). 3.63 (s.211).2.46 -2.36 (m..2H).2,26.1s- 3H).2.18 (s, 3;H).1.49-J.30 (m.2H).0.73 (l.311): MS; (El) fo C2.1H27N7OS: 4 1.9 (MH*).
6-(4-[2-Aniii >-5-(cyclppropylnietliyl)-'6-mclhyl'pyriinidi
tciraliydro-l,4-bcnzc)xazcpin-7-yl )| 1.3|ihiazolo[5:4-b|pyridin-2ramiiic. Ή NMR (400 Hz. DMSO-dft): 8.42 (d, I H).7.93 (s, 2H).7.87 (d, I H).7.56 (d..2H), 6.04 (s.214), 4.47 (s, 211). 4.31 (s.2H), 3.71 (s, 2H), 2.53 (d, 2H).2.33 (s.3H)'.2.30 (s, 3Ή).1.89 (d, 2H. OAc), 0.8S (s. I H)s 0.47 - 0.27 (in.2H), 0.00 (q.2H); MS (El) for C25H27N7OS: 473.9 (MH*).
Cv(4-{2-l(Diiiiciliylaniino)mcihyf|-6-cdiyl-5-(l-incihylcihyl)pyn
inctlryl-2i3,4.5-tetiahydro-li4-,bcnzoxazcpiii-7-yl)lI,3jtliiazo Ή NMR (400 MHz, DMSO-do): 8.34 (d, 1 H), 7.87 (s, 2H).7:78 (d, HI).7.49 (s, IH), 7.41 (s. ,11-1).4.39 (s, 2H): 4.29 (s, 2H).'3.67 (s, 3H).2.80 - 2.67 (in.2H).2.53 (d. I H).2.26 (s.311). 2.16 (s.6H).1.31 (d.6H).1.20 (t~ 3H): MS (El) for C2KH35N7OS: 518.3 (MH*).
6 4-(2-Αηϊίηο-5τθΐΙΐ(_ιιγΙ|)γπ^
b.enzp'xazcpi -7--yW
8.34 (eld.11-1), 7,88 (d, 1 H).7.86 (,s.211).7.7?; (cK I H'h 7.46 (cl.1 H), 7.37 (d, 1 H).6:56 (dd. Ill), 6.26 (s.2H).5:43 (del.11:1).5.08 (dd.1 H).4.69 (s.2H), 4.24 (d, 2H), 3.92 (d.211), 2.24 (s.3H).1. 0 fd.2H..0AC); MS (El) for Ci;R2iN7OS: 4 1.9 (MH+).
6-(9-Mediyl-4-|6-mclliyl-5-( l nchylctliyl)-2-(morplK)lin-4-yJmclliyl)pyri'midin-4 yl]-2,3.4,5-leiiahydiO- 1 ,4-bcn'/.oxazcpin-7-yl || 1.3 |ihiazol |5,4-b|pyridin-2-aniine. Ή NMR (400 MHz., DMSO-d()): 8.34 (d. III).7.88 (s.2H), 7.77 (t. IH), 7.48 (d, III).7,39 (d, 111). 4:45 (S-, 21-1), 4.32 (S..2H); 3£&{«, 2H), 3.51 -3.41 tm,4H), S.27 (dd, 2H), 2:51 :(s=, IH).2.46 (s;4H),.2.3Rs..3H).2.22 (d.3H).1. 1 (s.21-1. OAc), 1.31 (d.6H); MS (EI) for
C.(JI½N703S: 546.2 (ΜΙ- ).
••6( -.{,2 A'niino^
tetraliycliO-t.4-bcir/.oxazepiii-7-yl)| l.3]Uiiazolo|
'5.4-b|p.yridin-2-anii e-v Ή NMR (400 MHz. DMSO-cU: δ S.38 (d.1 H).7.93 - 7.79 (m, 3H).7.50 (s.2H).6.03 (s.2H), 4.39 (s.2H).4.24 (nv, 2H).3.64 (hi.2H).3.45 (i.2H).3.20.(.s, 31-1), 2.75 (i, 2H).2.27 (s.3H),
:2,21 (s
;3H), 2.07 (s, 21-I-OAc peak); MS. (EI) for
(MH
+).
6-|4;-(4-Aminop)a niidm^ ,4-ben/.0xazepih-7- ylll l iiliiazololS^-blpyridin^-aiiiiiic. Ή NMR (400 MHz. DMSO-dfl): δ 8.2S (d. IH). 7:i9. (s, 21-1), 7.73 (d. lH),7i63:(d. IH), 745 (s, IH), 7.35 (d, IH), 6.39 (s.2H).5.61 (d..IH), 4.72 (s.2H), 4.01 (br d, 4M), 2.16(s.3H); 1.75 (si 2H,QAc peak); MS CE1) for'QoH'ioNjOS: 406.1 (Ml Γ).
347 2-Amino|l,3|ihia/.olo|5.44)Jpyridin-6-yl)-9 hclby
beiv:0xazcpin-4C5H)-yl'|pyrazinc-2-carbonitrile. Ή NMR (400 MHz, DMSO-ci6): 58.41 (d, IH); S.34 (cl. HI), S.06 (d. I H).7.87 (s.2H>, 7.79 ( l. IH), 7.57 (s, IH).7.48 (s, IH , 5.08 (s, 2H), 4.38 (m, 211), 4.23 (m.21-1).2.23 (s.3H); MS (El) for C2i H17N7QS: 416.1 (MH+).
6-|4^4-Ammo-5-I"li!oropyrjniidm^
benzoxa/.cpiii-7-yl|| 1.3|lhiazolo|5.4-b;|pyridiii-2-amiiie. Ή NMR (400 MHz, DMSO-d6): δ 8.36 (d. I H).7.86 (s.2H), 7. 1 (d.1 H).7.76 (d.1 l-l).7.52 (s. I H).7.42 (cl. I H).6.91 (br s. 2I-I), 4.75 (s.21-1).4.06 (brs.4H), 2.25 (s.3H); MS (EI) for C_0HisN7OSF: 424.1 ( H*)..
2-|7-(2-Amiiio|l |tliiazolo|5.4-b|pyiidin-6-yl)-9-mctbyl-2,3-(lihydiO-l,4- bcnzoxazcpin-4(5H)-yl|pyndiiic-3-carbon rile. Ή NMR (4.00 MHz, DMSO-dfi): δ 8J9- 8.29 (ni.2H), 8.03 (dd, IH).7.87 (s.2H).7.78 (d. lH).7.55 (d. IH).7.45 (d. IH), 6.82 (dd, IH), 5.06 (s, 2H), 4.32 (m, 2H), 4.17 (m.2H).2.23 (s.3'H): MS (El) for Cr_HinNflOS: 415.1 (MH*).
2-ΐ;7-(2-Αηπηϋ|Ί,3]ι] ηζο1οΓ5,^
benzoxa'Cpin-4(5H).-^ ΜΙ-Γζ. DMSO-d<y): δ 8,33 (d,
III), 8.08 (dd. III), 8,02 (s, lH), 7.85 (s; 2W), 7-79 (d.1Ή), 7.05 - 7.5 fi , 21-1), 7.41 (d, 2H). 6.71 (dd, I H), 4.76 (s, 211).4,29 (m.2H), 3;84 (in.21-1).2.21 (s.311); MS (EI) for
CjiHmNeOiS: 433.1 (MH+).
6-|4-(2-A!niiio-6-chloro-5-clhcnylpyiiii)idi
b;ciiz0xazcpin*7^yi;|J;l,,3'|ihi 'Ή R (400 MHz., DMSO-dfl): δ
8.33 (d.1H).7.91 -7.77 (in,.3H).7.41 (d, 211).6.6Ί ( r s.211).6.55 (dd, I I I), 5.40 (dd,2H). 4.71 (s, 2H).4.29-4.11 (m, 2H)..3-75 (in.21:1).2.22 (s.311): MS (EI) for C32H2uN7OSGl: 466.1 (MH+).
6^ { 4- 1 -A'in ino-5-(3-nu0rop!iciiy l)-6-mct li^
letrahydro- 1 ,4-bciizoxazcpim7-yi }[ 1 ,31ihiazok)| 5.4.-bfpyridin-2-aniiiic. Ή NMR (400 MHz. DMSO-dfi): δ 8.31 (d, I H), 7,87 (s.2H).7.76 (s.1 H).7.49 - 7.33 (m.211).7.09 (d.2H).7.02 (t, l H).6.95(s. lH),6.l8(s,2H), 4.34 (s, 2H).4.06(s.2H), 2,20 (s.3H). i,86(s,3H); MS (EI) forC27H24N7O.SE: 514.1 (MH+).
6-Aniino-24, 7-(2-aniino( i .3 |lliiazolo|5.4-b]pyrW
benzoxazcpiii-4(5H)-yI lpyridme-3-carbonHrilc, 1 I I NMR (400;;MHz.;DMSO-d6); δ 8:34 :(d. 1H).7l86(s:,2I¾ 7,79 (l, 11-1).7.59 (d.1H). ^8- 7.38 (ni, 2H), 6.69 (s.2H).5.81 (d, H I). 4.87 s,:
(ΜΙ-Γ).
tctrahydiO-l l-beii oxazcpiii-^^yl}! 1.3|diiazolo|,5,4-b|pyridin-2-amiiic: Ή NMR (400 MHz. DMSO-df,): δ 8,34 (s. I H), 7 S7 (s.2H).7.78 (s. .1*1)-, 7.51 (s.1 M) 7.38 (s.1 I I).6.02 (br s. 2H), 4.23 (brs, 4H).3.53 (br s.2H).3.22 (m, ΙΗ)· 2.58 ,(q, 2H), 2.26 (s.3H).1.91 (s.2H- OAc peak), 1.31 (d, 6H).1.16 (t.3 H); MS (EI) for C25H2 N7OS: 476.2 (MR*).
6444,2-Ainiiio-6 ]|pi ^5-(l nclliylcdiyl)pynm.idin-4-yl|-9-mcdiyl-2,3,4,5- tctraliydro- 1 ,4-b.enz0xazepin-7-yl ) | .1.3 |thiazdlo| 5,44)|pyndih-2-amirie. 1 H NMR' (400'M Hz, DMSP-dfi): δ 8.45 (d, H I), 7.55 - 7.48 (m, 2H).7.33 (d, 1Ή), 6.54 (s.2H).4.37 (s, 2H), 4.26 (111.2H).3.65 (m.21-1).3.1 - 3.02 (111.1 H).2.27 (s.3H), 1.30 (d.61-1); MS (EI) for
Cv-IjaNvOSCI: 482.1 (M H+):
6-(4-{ 6-Ciiloro-2-Htlmieihy!nni io;)nicihyl 5-(l-nict y )-9- methyl-2s3.4,5 cirahydro-l.,4-Benzoxa7.epin-7-.yl)(1v3Ji Ή NMR (400 MHz. DMSO-d,,): δ 8.33 (s, IH).7.85 s„lH), 7.77 fs, I H), 7.44 (d, 2H), 4.56 (s.
2H).4.31 (brs, 2H), 3.77 (br s.2H); 3.25 (buried s, 211).3:23 -.3.04 (in.; 1H).2.21 (s.3H). 2.07 (s, 611).1.35 (d.6H): MS.(EI) for C;«I IM 70S I: 524.2 (MH+).
6-(4-{2 (3,3-Difku>ropynOliclh^^
4-yl )-9-mediyl-2;3,4.5-tctraliydi -1.4 icn7.oxazcpiii-7-yl)| 1,3 J.llvia/.plo[ 5,44) |pyriclin-2- aniine. Ή NMR (400,Ml l7.. DMSO-cln): δ 8,32 (s, I H).7.S4 (s.,2H).7.75 (s l.H), 7.4 (s. 11-1), 7.37 (s, 1,1:1), 4.42 (s.2I-I),4.30 (s,.2l-f)/3.66 (s, 2H).3.52 ;(s 211), 3.12 (m. I H).2.85 (l, 2H).2.6 (l.21:1).2.47 (buried s.3Ή).245 (s.3H).2,21 (m, 211).1.88 (s-. lH-OAc peak); 1.2 ■(<!, 6H): MS (El) for CyiH-jx iPSFi: -566Λ (MH*):
;4-|7-(2-Aniinc)|.l .3 lihiazoU 5.44).)pyridiii-6-yl)-9-ineihyl 2,3-(lihydr .1.4- beivzoxazepin-4(5H)-yl |-6.6-dimethyl-5'.6.7,8-tctral!ydroc|iiina/.olin-2-aiiiine.1 Η NMR (400 MH/.. DMSO-dfi): 8.36 (d.1 Η).7-87 (s.2H).7.81 (d.1 H); 7.48 (s, 2H), 5.84 (s.2H).4.46 (s. 2H), 4.27 (s.2H), 3.73 (s, 2H), 2.50 (l, 21-1), 2.34 (s, 2H),'2.26 (s, 3H) J.54 (t, 2M), 0.86 (s, 611); MS (El) for C:6H2yN7OS: 48S (MH*).
6-{4.-|2-Aniinp-5-(lrinirojpincdiyl)p 1.4- bcn/.oxazepin-7-yl)| ?3:llliiazolo|5,44 -Ή NMR (400 MHz, DMSOrd6):
8.38 (d, IH).8.19 (s. I H).7.94.- 7.70 (m.3H),7.57 (s, l H).7.45 (s, IH).6.93 (s, 2B), 4.84 (s.211).4.27 (s.2H).3.90 (s.2H), 2.22 (s.3 H): MS (EI) for C.iHisWOS: 474 (MH*).
■6-{4-|4-amino-5-(iriniuyromclhy1) 1,4- benzoxazepin-7-yl}| lT3|lhiazoloI5.4-b|pyridin-2-ainiiie; Ή NMR (400 MHz. DMSO-dft): 8. 1 (s, 1 H), 8.07 (s, 11-1).7.85 (s.41-1).7:69 (s.1 H).7.46 (s, 2H); 4,81 (s, 2H).4.11 (d, 4H).. 2.23 (s.3H); MS (El) for C2iHik¾ 7OS: 474 (Mi l4).
yl|| l.3|tliiazolo|5;4-b|pyridii)-2-amiiic. rH NMR (400 MHz. DMSO-dfl): 8.39 (s.1H).8.18 (s.2H), 7.85.(m, 3H).7.59 (s. I Ή), 7. 1 (s.1 H), 6.91 (s, 1 H).4.41 (s, 2H), 4.30 - 4.22 (m. 2H); 3.65-3.51 (in, 2H).2.26 (s.3H), 2.24 (s.3H): MS (EI) for C22H.1N5QS: 404 (MH*).
64,4-(2-Amino-6-nicthyl-5-|mip-2-en-l-ylpyr^
teliahydro-l,4- enzoxazepin-7-y|| l,3|lHiazolo|5,4-b'|pyridin-2-aininc. Ή NMR (400 MHz. DMSO-d6): 8.33 (d, III).7.87 (s, 2H).7.77 (d. III).7.47 (s, IH).7.34 (.¾ lH).6.00 (m, 311). 5.20 (d, IH), 4.98 (d, II I).4.43 (s.2H).4.25 (s.21-1), 3.68.(s, 21-1).3.1 (s, 2H), 2.25 (s.3H), 2.10 (s, 3H); MS (EI) for C:.,H:5N7OS: 460 (MH*).
6-[4-(2-Amino-6 hloro-5-cihyIpyrimidih^^
benzoxazepin-7-yl|| l,3|thiazplo|;5.44i|pyridin-2-ainiiie. Ή NMR (400 MHz, DMSO-d6): 8.36 (d, 1 7.8 (s, 211).7.81 ("d, 1 H), 7.48 (s, 2H), 6.46 (s, 211), 4.57 (s.2H), 4,31 (s, 2H). 3.77 (s.2H), 2.53 (m, 2H), 2.25 (s.3H).1.14.(1, 31-1); MS (EI) for C22H22CIN7OS: 468 (MH*)
6-(4-{6-Chloro-2-[(dimc.thylamiho)^
DMSO-d,,): 8,37 (d. I H), 7;87 (s, 2H).7.81 (d.1H).7:54 (d. I l); 7.46 (d, I I I).4.74 (s.2Ή).
4.42 - 4.26 (m.2H), 3.98 - 3.82 (in.211).3l34 (s, 2H).2.68 (tj.2H).2.24 (d.31-1).2.11 (s. 6H).1.21 (t. H): MS (EI) lor C25H2SCIN7OS: 510(ΜΙ-Γ).
6-{ 4-| 2- j |( 1 , 1 -Dimethyle[hyl)amino|mct yl }-6-met yl-5-(.l -mei y'lcihyl )pyrimidin- 4-yl ]-9-met yl-2,3.4,5-tcirahydro^ I. ,4 -ben zox aze'pi n-7-y 1 | [1 ,3 |lhiazolo|5,4-b|pyridin-2- amine. Ή NMR (400 Hz, DMS'0-dft): 8.35 (d.1H),.7.S6 (s, 21-1), 7.79 (d, IH).7.48 (,s.1H).
7.43 (s. I H).4.46 (s.2H), 4.3 (s, 2H).3.70 (s.2H), 3.56 (s, 2H).3.27 (r , 1 H), 2.46 (s.3H), 2.25 (,s, 3H).1. 1 (d.6H), 0.94 (s„9H ; MS
53 (ΜΙ-Γ).
i,4-benzoxazepin-7-yl|| ^ Ή NMR (400 MHz. methanol- d.,): 8.34 (d, 11-1).7.82 (d, IH), 7.42.(d. Ill), 7.40 (d. I H), 466 ( ,,2H).4.30 (m,2H), 3,97 (m. 2H).2,74 (I.211).2.46 (s, 2'M, 2.40 (s, 3H), 2, 1 (s, 3M).1.66 (I, 2H).0. 1 (s.6H); MS (EI) for C:7HwN>OS: 487 (ΜΙ-Γ).
6 4-(6,6-Dimcthyl-5,6,-dil^ ,4- bcnzoxazepin-7-y!|| l.3|dnazolo|5,4 i|pyridin-2-amine. 'I:! NMR (400 MHz/methanbi- d.,): 8.54 (s, 1 H), 8.37 (d, 1H),7.83 (d, I H), 7.52 (d, IH).7,44 (d. IH), 6,59 (d, HI).6;34 (d, IH).5.12 (s.2H)..4.47.(m.2H)V4.30 (in, 2H).2^92 (s, 2H).2.2 (s.3H).1.0S(s.611): MS (EI) for.C¾I- ,N(iOS: 471 (MH+).
6-{9-Mcihyi-4-l'6-methyl-:5-(l-mctity'leihyl^
yl|-2,3.4.5-icirahyclro-l'.4-bcnzoxazepin-7-y|.}[ l,3jdiiazold|;5.44r|pyridin-2-aminc. Ή NMR (400 MHz. methanol -d.,): 8.35 (d. I l-l), 7.82 (d. I H), 7.41 (m, 214).4.61 (s, 2H), 4.38 (m, 2H). 4.03 (s.21-1).3.85 (m.2H).3.3S (in, 11 Γ), 301 (m, 4H).2.56 (s.3H).2.31 (s.3H).1.91 (s. 3H).1.88 (m.41-1), 1.39 (d.6H); MS (EI) for C29H35N7OS: 530 (MH+).
6-(4-{24.(Diineihylamihp)inethyl|^5-(2.2,2-irifluorocthyl)pyrn^
2,3;4,5.-tcli'ahydrp-l,4 )enzoxaz^ Ή NM
(400 MHz, niethanol-d ): 8.44 (d, 1Η),·8.27 (s IH).7.60 (d, I H), 7.49 (d, I H), 7.38 (d, IH). 4.91 (s, 2H), 4.44 (in.2H).4.04 (m, 2H).3.83 (s, 2H), 3:
'73.((|.2H).2.47 (s.6H).2.27 (s, 3H), 1.94 (s.311); MS (EI)
531 (ΜΙ-Γ).
6-(4-{2-|(Dicthylamino)melhyl|-6-meihyI-5-(l-melhylcihyl)pyTimidin-4-yl |-9- meUiyl-2.3,4.5-tctrahyclro-l A-bcnzoxazepin^^ Ή NMR (400 MHz. methanol'df): 8.33 (cl, IH), 7.8.1 (d, IH).7.39 (m, 2M).4,58 (s, 2H), 4.36
(m.2H).3.93 (s, 2H).3.84 (m.21-1).3.39 (m.1H): 2.90 (q.4H), 2,56 (8,311).2.31 (s.31-1). 1.90 (s.31-1)..1.39 (cl.6H), 1.1 (t.611); M (EI) for C20H37 7 S: 532 (MH+).
6-{9-rVlelhyl-4-(6-iiiet yl-5-( 1 -nictliylct yl)pyrimiclin-4-y_l |-2,3.4,5-tctrahydro-i .4- benzoxazepin-7-yl}| l.3'|iliiazolo|5T4-b|pyridin-2-aiiiinc. 'll NMR (400 MHz, inclhanol- ,d.,): 8.66 (ti I H), 8.45 (s, H I), 8:01 (cl, 1 H).7.56 (d, I hi), 7.46 (d, 11-1), 5.10 (s..2H), 4.52 (in, 2H).4.1 (in, 2Ϊ-Ι).3.21 (m. I I I).2.62 (s.3H).2.27 (s.3H), 1.43 (cl, 6I-I); MS (EJ) for
C24H26NftOS: 447 (ΜΙ-Γ).
6-(4-| 2-|(DimctIiylamini>)mcthyl j-5-( I -niclliylei yljpyninitlin-4ryl }τ9-ηιοΐΙιν1- 2,3,4,5-iciraliydro- 1.4-benzoxazep'm-7-yl)| 1.3 |i iazolO| 5.4-b|pyridin-2-aminc. Ή NMR (400 Ml-lz. DMSO-dft) δ 8.35 (d, 11-1), 8.34 (s, 1 H), 7.87 (brs, 2H).7.79 (d, 1 H), JA6 (s.2H), 4.61 (s, 211), 4.35-4.28 (in, 2H).3.87-3.81 (in, 2H), 3.38 (s.2H).3.14-3.04 (111. I H), 2.24 (s. 3H), 2.12 (s, 6H), 1.23 (d.611); MS (El) for C2,H3,N7OS: 490. (MH+),
6-(;4.-(2-.|(DiinCthyra;iriinc)meiliyr|-5-ethylpyr
.3|thiazolo[5,4-b |pyridin-2-aminc. Ή NM (400 Hz. DMSO-d„) 68.36 (d. I II).8.12 (s. III).7.86 (s.2H).7.80 (d, lH).7.56(d.1 H).7.44 (d. II I). 4.72 (s.2H), 4.33-4.24 (m, 2H), 3.99-3.92 (in, 2
'H), 3.36 (s.2.H.), 2.67 (q.2H).2.23 (s, 31-1), 2.12 (s.61-1).1.14 (1.311); MS (III) lor C25H29N7OS: 476 (MH
+).
ictrahydro- 1 ,4-bcir/.oxazepin-7-yl)| 1.3]lhiazoloi ;4-b|pyridin-2-aininc. Ή NMR (400 MHz. DMSO-cl(,) δ 8.35 (d, IH).7.86 (s.211).7.7 :(d, I H).7.49 (s, 11-1).7.46 (s. I II ), 4.57 (s, 2H), 4.34-4,27 (m.2H).3.83-3.76 (m, 2H).3.36 (s.2H), 2.70,2.58' (m.4H).2.24 (s.3H), 2.15 (s, 6H), 1.1 - 1.09 (m, 61-1); MS (EI) fo 027ΐ¾Ν705: 504 (MH+).
6-{9-Mclhyl-4-|5-(.l-incdiylediyl)-2'-(pyrrolidii)-l- tetrahydiO-l.4-beriz0xazepin-7-yl|| l,3|llnazolo|5.4-b|pyridiii-2-ainiiie. Ή NMR (400 MHz. D SO-dft) δ 8.34 (d. I H).8.33 (s. I H).7.86 (s.2H).7.78 (d. H I), 7.48-7.43 (in.2H), 4.62 (s, 2H), 4.36-4.29 (m.2H), 3.86-3.80 (m, 21-1), 3.52 (s.211).3.15-3.05 (in, I H).2.44-2.38 (in. 4H)T 2.24 (s.3H).1.61-1.53 (in.41-1).1.23 (d.6H); MS (EI) for Q-sH^ OS: 516 (MH+).
6-(9-Metbyl-4- ( 6-meihyl-5-( 1 -mctliylclhyl)-2-|(4-inclhylpipcrazin- 1 - yl)mclhyl]pyrimidin-4-yl-}-2,3A5-teir^
blpyridin-2-aminc. Ή NMR (400 MHz. DMSO-dfl) δ 8.33 (d, IH).7.88 (s, 2H).7.77 (d, IH), 7.47 (d, IH).7.38 (cl, 1 H), 4.43 (s, 2H), 4.38-4.24 (in, 2H), 3.76-3.57 (m.21-1).3.36 (s, 21-0,3.31-3.23 (in, IH).2.46 (s, 3H), 2.41-2.14 (in.1 IH), 2.10 (s.3H).1.31 (d.6H); MS (El) forCwI-bsNsOS: 559 (ΜΙ-Γ).
6-(4-{2r|(Dinicthylamin0 inelliyl]-5-ciliyl-)-'n
ietrahycIro-1 -bcnzoxazcpin-7-yl)|i,3|di^^ Ή NMR (400 MHz.
D SO-d6) δ 8.33 (m. I H).7.85 (s.2H), 7.77(m, 1 H).7.44 (t.2H).4.54 (d, 2H).4.27 (d.211), 3.76 (d, 2H), 2.59 (q, 21-1), 2.33 (s, 31-1), 2.22 (d.3H), 2.11 (s, 6H), 1,88 (s, 2H).1.11. (m, 3H): MS (El) for C2r,H3iN7OS: 490.2 (Μ1-Γ).
6-(4r 2-| (P'niiet ylainin;o)nicllvyl )-6-is
2,3,4,5-tet'^hydro- 14*enzoxazepin-7-yl) l ,3:|ilviazold|5,44jlpyfidiiv2-amirie; Ή NMR (400 MHz. DMSO-d6) δ 8.33(1. Ill), ,7.86 (s, 2H), 7.78 (t. H I).7.51 (s. I l l), 7.44 (d. II-I), 4.57 (s. •2H). 30 (ss 2H)i 3.81 (s, 2H),-362 (s; 2H) 3.1S (ni; 1 H);¾32¾s 6PI); 2.21 (dsGFl), 1.14 (l, 6H): MS (EI) for C27H3:,N7OS: 490.2 ( H*>,
6-(4-{2-| (Diniclliyluinin )incUiyl|-6-nicthyI-5-( i -rnclhylcihyl)pyriniicIin-4-.yl } -9- methyI-2,3.4.5-lctraliydro-l -bcnzoxazcpin-7-yl)| l.3|Uiiazolo|5.4-b|pyridin-2-amiiic.
'HNMR.(400 MHZ. DMSO-d*}: 8,35 (d, I B), 7.88 (br..s^H)v 7.78 (cl, IH), 7.48 (d. IK), 7.4.0 (cl.1 H), 4.40 (s, 2H), 4.28 (in, 211).3.67 (in.2H).3.35 (s.211).3.27 (m, 111).2.45 (s, 3ft), 2.25: (s, 3H), 2,l4,:(s, 6E), 1.30 (cl, 6H); MS (El) forCijHgj .?©? 5Q4( | ),
6-(9 Ieilvyl-4-{6 ncdiyl-5 lHnediyl^
2.3,4.5-lctrahydro-
1 HNiVlR (400 MHz, DMS -dfi): 8.35 (d, 1 H), 7:87 (s, 2H), 7.79r(d., ΪΗ), 749 (d, 11¾, 74 (d, 1 % 441 (s, 2H).4.30 (s.2H), 4.27 (m.2H).3.68 (in, 2H).3.32 (in, IH).3.26 (s.3H).2.47 (s.3H).2.26 (s,3H), 1.29 (d,6H); MS (EI) for C.
¾H.)
6pS 4 1 (MH*).
6-( 9-Mcihyl-4-| (7S)-7 iieiliyl-5;6,7.8Hcirahydroqiiinazdlin-4-yl ]-2,3,4,5-teirahydro- 1 ,4-benzoxazepin-7-yl } J 1 ,3|diiazolo| 5,4,b|pyridin-2-amine, 1 M NMR (400. MHz..CD3O.D.) δ 8.58 (d. IH), 8.52 (s. IH).7.96 (d, ill).7.57 (d, 1 H).7.46 (d.111).5.20 (d. IH), 5.13 (d; 1 H). 4.52 (in.2H).4.29 (m, 2H), 2.98 (in, 1 H), 2.93 (m. IH), 2.74 (.111. IH), 2.38 (m. HI).2.29 (s, 311).2.00 fm, 2H), 1.27 (ni, IH), 1.12.(d, 3H); MS (ES) C25H2fiN(,dS::45 (ΜΗ').
2-Amino-6 7-(2-ainiiib i.3|l!iiazold^
l)cnz xazepin-4(5H)-yl;|pyridincr3i5. licarbonitrie. 1 H NMR (400 MHz. df)-DMSO) δ 8.38 (d, I H).8.12 (cl. I l-l).7.S5 (s.2H).7.84 (d, IH), 7:68 (d, 1 H), 7.48 (d.1 H); 748 (bs.2H), 5.00 (s, 2H), 4.27 (m.4H), 2.24;(s, 311); MS (ES) C2.-,H1SNKQS: 455 (Ml- ).
2-Amin -6-| 7-(2-amino| 1 ,3 |ihiazolo|544T)pyridin-6-yI)-9-melliyl-2,3-diliydro- 1 - bcivzoxazcpi]>4(5H)-yl|pyridinc-3.5-(licarbonitrilc. Ή NMR (400 MHz. d6-DMSO) δ 9.34 (m. IH).8.43 (d. IH), 8.16 (bs, 211), 7.87 (d. IH).7.66 (d, ΓΗ), 7.45 (d. IH), 7.42 (bs, 2H), 5()0 (s.2H).4.28 (m,4H), 2:37 (s, 3H), 2.23 (s.3H); MS (ESVCyHaiNsOS: 469 (MH+).
[00848] Proceeding according to the method of example 1 and replacing ft'/7-biuyl 7- l)romo÷9Hiiethyl-2.3Hlihydro- l^ /m-biityl 7-bipnio-
9-elhyl-2,3-dihydiO- 1 ,^ cn½ xaz(i iiic-4C5H)rtarboxylalc, the following compounds of (lie invention were prepared:
6-{4-[2-Anii o-6-nicihyl-5-f r-iuclhylcilVyl)pyiiiiii
let rail yjdiO-1/ ' i NM (400-MHz.
8,35 cK I I Hy 7.S7 (s, 2H). ΊΜ{ά.11 ί>.7 , 9 (d, IH).7,39 {jd.1H),6.06 («, 2Η·). 4.23 (m.2W).4.23 (s.2H).3.54 (m, 211).3.22 (in, 111).2.68 (q.2Ή), 230
'(s.3H).1.26 (d. 6H), 1.20 (i, 311); MS (ES) for C
25H .>N
7OS: 476 ( il
4).
100849] Proceeding according to the method of example 1 and rcplaeenieiU of starting reagents in, step 2 with /e/ -biiiyl
carboxylalc and A'-|6-(4;4,5v5^tctraniethyl-l,3.2-di6x bo¾
/ |pyridin-2-y||aceiamide, the
6-{4-|2-amiiio-6=me^
ictrahydro-l ,4-benzoxazepin-7-yl;}[';l.^^^
Ή NMR (400 MHz, Ct¾QD) ό 8.63 (d. IH),7:99 (d. IW 7,70 (d, I H).7.66 (s. IH), 4.94 (s. 21-1).4.51 (m, 2H).4.06 (m.211), 3.06 (in; IH).2.45 ( .3H).1.37 (d.6Η)· MS (ES) for C3iHMCIN7OS: 482 (ΜΙ Γ).
6-{4-|2-lKU -dihjei ylcdiyJj meift
metlrylelliyl)pyrimidin-4-yl|-9Hncihyl-2,3.4.5-ictraliy^^
yl}|;i. :]thiazo]p|5.4-bjpyridiiu2^nnine. Ή NMR (ΛΟΟ'ΜΉζ, dh-Diy[S©) δ ¾.34 (d, IH), 7:86 (s, 2H),7.77 (d. lM),7,47 (s, I W)r 7,39 (s. IH);4.40(s, 2H)v 4 0 (s.2H , 3:68 (s, 2H), 3.39 (m, 3H), 2.46 (s.3H): 2:25 (s.3H), 2. l3 (s 3H).1.31 (d, 6H)„0;98 (s,¾'M) MS (El) for C,nl½N70S:.546 (MH+).
6V(4-[2-( |(2,2-cliniioroethyl)ai iho|nicth^
Ή NMR (400 MHz. methaiiol-d.,): 8.70 (d. III).8.07 (d, II I).7.62 (cl, I I I ).7.55 (d, IH), 6.29 (in, IH), 5:10 (,s, 21-1).4.60 (m.21-1).4:53 (s.211).4.20 (in.211).3:65 (in.2H).3; 16 (m. IH).2.65 (s, 3H), 2.31 (s, 311), 1.41 (cl.6H). MS (EI) for 27H31 E2N7OS:, 54.0 (MH+).
6- { -melliyl-4-| 6-melliyl-5-( 1 -melliyleihyl)-2-( | (2,2,2- irifluoroelhyl)aniinolmeihyll pyriinidin-4-yl |-2.3.4.5-ietrahyclro- 1 ,4-benzoxazepin-7- yl)| l,3.|thiazolo|5,4-blpyridin-2-aminc. Ή NMR (400 MHz. dr,-DMSO): 8.35 (d. IB), 7.86 (s.2H), 7.79 (cl. IH), 7:49 (s, IH)",- 7.44 (s; IH).4.46 (s, 2H).4.31 (s.2H), 3.70 (s, 4H).3.17
(d.31-1), 2.70 (d, 21-1), 2.47 (s, 3H), 2.25 (s, 3H), 1.89 (s, 1 I I), 1.34 (s, I H), 1.31 (d.6H). MS (El) for C:7H3oF3N7OS: 558.2 (ΜΙ-Γ').
6-{4-|.2,6-diinclhyI-5-(l-melhyte^
1 ,4-bcM/.0xazcpin-7-yI } 11 ,3|Lhia/. lo|5.4-h|pyiidin-2-aniinc. Ί-Ι NMR (400 MHz, d(l- DMSO): 8.35 (d. IH), 7.88 (s, 2H), 7.79(d. IH), 751 (d, I H).7.42 (d, III).4.35 (s.2H), 4.31-4.1 (in, 2H), 3;69-3.59 (in, 2H).3.32- 3.22 (ιϊι, I H); 2M (s, 3H).2.35 (s, 3Ή), 2.27 (s. 3Ή).1.29 (d, 6H). MS' (EI) for C25I½N60S: 461 (,ΜΗ').
{-4-|7-(2-amiiio| 1 ,3 |diiazolo| 5,4¾pyridi -6-y^
bcnzoxazcpin-4(5l-l)-yl|-6-nicihyl-5-(l-methylethyl)py MS (ES) for C2(,H2i OS: 486 (MH+) 1 H NMR'(4f)0 MHz. d6-DMSO) δ 8.36 (s.1 H),7.83 (s.2H). 7.80 (s, IH).7.51 (s, IH).7.42 (s, III).4.46 (s, 211).4.32 (in, IH).4.09 (s, 2H), 3.7 (in, 2H), :3· 23; (in,, I l-l), 2.48 (s, 3H.)>2.27.;(s.'.3H), 1.31 (d, 6H>.
:Ντ<5-| *|2*άη¾(^
l,4-lenzoxazepiii-7-ylHj,3-tliiaz0l-2-yl)acciamilc. Ί·"Μ' NMR W. MHz, d6 DMSG)): 7.6 (s, I I-I).7.38 (s. I H).7.23 (s, IH),.5.97 (s, 2H), 4.19 (br ir, 2H), 4.16 (s, 2H), 3.49 (br ir, 2H). 3.15 (m. IH), 2.27 (s.311).2.21 (s, 311).2.12 (s.311).1.22 (cl, 6H). MS (EI) for
6- ( 9-methyl-4-|2-ineihyl-5-( I -mcihylciivyl)pyriinidin-4-yl ]-2.3.4.5-lcirahydiO- 1.4- benzoxazepin-7-yl } 1.1 ,3 llhiazol |¾4- j yridin-2,amine.
1 l-l NMR (400 MHz, d^DMS
'G):, 8.35 (d, I H), 8.28 (s. I I I), 7.S9 (s, 21-1), 7.79 (d, I H), 7. 1-7.47 (in.1 H), 7.47-7.43 (m. I H). 4.56 (s, 2H 4.37
^4:24 (jm.2H)v 3vS7-3:¾ (in, 2H), 3
:l3-298¾m, IH).2.38 (s 3H).2.25 (s, 3H), 1.21 (t, 6H),
447 (MH*)!
6- ( 4-[6-cblorc)-5-( 1 -incdiylclhyl)pyrimidiii-4-yI |-9-.metivyl-2,3,4,5-leliabydiO-l ,4- benzoxazcpiri-7-yl}| 1.3|tb'iazoIo|5.4-b|pyridin-2-aminc. Ί-Ι NMR (400 MHz, d-fi DMSO): 8.33 (m.21-1), 7.86 (s, 2H).7.78 (d. I H); 7.50 (s, I H).7145 (s, I H).4.56 (s.2H).4.34 (br s. 2H), 3.7S (br s, 2H), 3.19 (in, 1 H), 2.25 (s.3H).1.37 (d, 6H). MS (El) for C^I^CI r.OS: 467.1 (MH+). 6-|4-(5-ellicivyl-6-nictl)yIpynmidin-4-yl)-9-inet]vyl-2,3,4,5-lelrahydiO-1.4- bciizo ii/-epin-7-y! ) 11. 1 th iazqloj 5. -b jpyrid in-2-aniinc. Ή NMR (400 MHz, d6-DMSO): 8.32l(dd: 21-1), 7.86 (s.2H), 7,78 (l. IH).7.44 (d, IH).7.38 (d, IH).6.75 (dd, IH), 5.58 (dd, I H), 5.37 (dd, IH).4.82 (s.2H), 4.29 (s, 21-1).3.90 (s; 211).2.33 (s.31-1), 2.22 (s, 311). MS (EI) for C23H22Nf)OS: 431.1 (MH+).
6- { 9-inctbyl-4-| 5-( 1 -mcthyleihyl)p>Tiinidin-4-yl |-2.3,4.5-tetrahydro 1 ,4- benzoxazcpin-7-yl }| 1 ,31tliiazolo|5,4-b|pyridin-2-aminc. Ή NMR (400 MHz. d
6-DMSO): 8.48 (cl. IH), 8.41 (d. Il-I).8.35 (t. IH).7.87 (s.2H), 7.79(1. lH).7.49(d, IH), 7.42 (cl, IH),
MS (El) for
6-{4-[2-amino-G-methyj-5-( l-ii^
benzoxazcpin-7-yl}| l,3|lliiazoU|5.44 pyiidin-2-:uiiine-cl.,. Ή NM (400 MHz. DMSO-dG): δ 8.32 (l.1 H).7.85 (s.2H).7.77 (t. I H).7.55 (d, 211).7.05. (i.1 H).5.98 (s.2H).4.26 (s
; 2H), 3.15 (m. IH).2.27 (s, 3H).1.87 (s.3.1-1).1.22 (d. fil-lj. MS (El) for
452.2 (MH
+).
6-(4-|2-aniino-6-nictliyl-5-( l-me i lel^
δ 8;25:(s.11-1).7.84 (s, 2H)
!.?
T79 (¾ Jl-l), 7.05 (cl. IH).6.1 (bs. IH).4.01 (s.. IH), 3.l2(m. 1H),.2.28 (s, 3H).1.21 (·.¾ 6Ή), MS
454.2 (MH
+).
6 {9-meihyl-4-l6-nicl!iyl-5-( 1 -nicthyleihenyl)pyriniiclin-4-y! |-2.3.4.5-tctrahydro- 1 ,4- benzoxaz pin-7-yl } 11.3 |ibiazolo| .5.4-lV|p.yridin-2-aminc. 1 H NMR (400 MHz. d6-DMSO): 8.32 (d.2H).7.86 (s.2H).7.77 (l.1 H).7.44 (s; 1 II).7.39 (d.1 H).5.48 (s, I H).5.13 (s, 1 H). 4.84 (s, 211).4.23 (d, 2H).4.05 (s.211).2.28 (d.3Ή).2.22 (s.3H), 1,92 (s.3H).1.88 (s.2H). MS (EI) for CaF iNeOS: 445.2 (ΜΙ- ).
!-Η-7-(¾¾ι ϊιιρ|¾ '|ϊίη^ζο(^^
benzoxazcpinr45H)-yl|-6-aKUhy|xyniiiid 'i-I
8:40;(s. lH).8.35.(d, IH).7.87¾, 2H), 7.80 (cl. I H), 7.45;(d. H).721 (d. I H), 4.80 (s.2H). 4.38 -4.25 (m.2H), 3,90 - 3.69 (in.2H), 2.43 (s.3H), 2:25 (s.3H).2:20 (s, 3H). MS (EI) for C23H22 (,0> : 447.1 ( H+).
6- ( 4-| 2- ( I (2-.fluoroeihyl)aniiiie |mcihyl } -6-mcihylr5-( 1 -mclhylethyl)pyrirnidiu-4-yl|- 9-meiliyl-2,3,4,5-icirahydrOr 1 ,4-benzoxazepiii7-yl 111.3;|thiazoib| 5.4-b |pyridin-2-ainine. 1 H NMR (400 MHz, CD.,OD) δ 8.71 (d. HI); 8,11 (d, IH),7.69 (s. IH); 7.35 (s. IH), 5.16 (s. 2H, 4.75. (m, 21-1); 4.62 , 2 H), 452;(s; 2H), :;23;(l, 211), 3.50 (dl, 2H).3.16 (m, I H), 2.66 (s.3H), 2:30 (s, 3H).1.43 (d, 61-1). MS'(ES) for C27H32p 7OS:,522i; H:).
6-(9-mcdiyl-4-|6-mcthyl-5-|2-(niethy^^
4-yl |-2.3.4.5-tctrahydrc>- 1.4-bciizoxazcpin-7-yI)| 1 ,3|lliiaz0lo|5.4-b|pyridin-2-aminc. Ή NMR (400 MHz. df,-DMSO): 8.37 (d. IH), 7.87 (s.2H), 7.83 (d} IH).7.49 (d.2H).4.56 (s, 2H), 4.35-4.24 (m, 2H), 3.83-3.72 (in.2H), 3.54 (I.2H).3.50 (s, 2H).3.21 (s.3H), 2.91 (I, 2H).2.48-2.41 (m.4H).2.38 (s.3H).2.25 (s,3H), 1.64-1.55 (m, 4H). MS (EI) for C29H35N7O2S: 554 (MH+).
6-{9-mcthyl-4-[6-mellvylr'5r(l-!nediylclhyl)-2-Ctrinuo^
2.3,4,5^etrahydro-1.4-benzoxazepin-7-yl}| l,3ilhi Ή NMR
(400 MHz. c -DMSO) 58.35 (cl. I H).7.88 (s.2Η).7.79 (d, IH), 7.48 (d, 2Η).4.58 (s, 211). 4.32 (s, 2H),.3.8.I (s, 2H).3.24 (dd, IH).2,55 (s, 3H).2.24 (d.3H).1.34 (d, 611). MS (El) for C25H25F3 (,OS: 5I5(MH+).
.-benzbxazcp'm-7-yl)| 1 )tlviazolo|5.4 i|pyridiii-2-amine. 'I:! NMR (40.0 MI-lz, melhanol- d..): 8.38. (s, lH), 8.36 (d, HI), 7.84 (d. Ill), 7=45 (d. IH).7.41 (c!, IH), 4.66 (s, 2H).4.62 (br s.2H), 4.35 (in, 211), 3.89 (m.2H), 3.58.(1, 2H).3.24 (;s.3H).3.04 (t, 2H), 2.47 (s, 311), 2. 1 (s, 3H). MS (El) forC2,|l- f,N6Q2S: 463 (ΜΙ-Γ).
6-{4-[.2-iiniino.-6-inelhyl-=5r( l-inclhylellicnyl)pynmidiii-4-.yl|-9-iiicthy|i2.3.4T5- lelrahydra- 1 ,4-benzoxazepin-7-yI )| 1.3jllnazolo[5,4-b]pyi¾ljiv-2-aniine. Ή NMR (400 MHz. df,-DMSO): 8.34 (cl. IH).7.85 (s.2H).7:78 (t. ΊΗ).7.53 - 7.36 (m.2H), 5.98 (s, 2H).5.36 (s. IH), 4.99 (s, IH), 4.72 (s.2H), 4.15 (s, 2H), 3.97 (s, 2H), 2.23 (s.311).2.09 (s.3H).1.89 (s. I H), 1.87 (S.3H). MS (EI) for G2.i H 25 N7OS : 60.2 (MH+) .
2-{4H7-(2-amiiio| 1 ,3:|thiazolo|.5,4-blpy^
bcnzoxazcpiiv4(5H)-yll-6-el^ Ή N R (400 MHz, methanoi-d.,): 8.33 (d. IH).8.09 (s.1 If).7.80 (cl. IH), 7.43 (d.1 If).7.36 (d.1 H).4.80 (s, 2H).4.62 (s.3H).4.22 (m, H).3.92 (in.214), 2.26 (s.3H).1:80 (s.6H). MS (El) far C2.?H23C1 602S: 483 (MH+).
6-'(4- { 2,6-diincthyI-5-| 2-.(mcdvyloxy)e^
tcUaliydro-1.4;-bcnzoxazcpin-7-yl)l^^ Ή N (400 MHz, d6-DMSO): 8.38 (d, I B).7-.S7 (s,,2H)! 7.84 (cl. B); 7.55,7.47 (m, 2H), 4.51 (s, 2M), 4.30- 4.22 (in, 2H), 3.80-3.73 tm, H) 3,51 (t.-2H); 3.20 (s.3H), 2:89 (t.2H), 2136 (s.6H).2.26 (s. 31-1). MS (EI) for (¾ Hjx fcOjS : 47 (Mil*)
6-{4-l2-a.clic!in-3-yl-6-m¾ iyU5-( l-incllvylcthyl)pyriinidin-4-yl|-9-methyl;-2.3;4,5- leLiahydro- 1 ,4-benzoxazepin-7-yl } 11.3 |thiazolo| 5,4-b|pyndin-2-ainitie. 1 I I NMR (400 MHz, dc-DMSO): 8.36 (d.2H).7.88 (s, 2H).7:80 (d. I l-I), 7.50 (s. IH), 7.45 (s, IH), 4.46 (s.2H), 4.32 (m, 2H), 3.80 (s, 21-1), 3.72 (m, 211).3.60 (m.2H), 3.26 (m. I H), 2.46 (s.311).2.25 (s. 3H).1.31 (d.6H). MS (EI) for C27H.HN7OS: 502 (MH+).
6-{4-|2-(amiiiomeihyl)-6-mediyk5^
teirahydro!- l..4-b6nzbx;(zcpin-7-yi.) |
' 1 ,3 |ihiazolo| 5,4-lil ymiIiri-2-!iini e. Ή NMR (400 MHz. d
6-DMSO):.8,35 (d, I I I), 7:87 (br s, 2H),
'7,80'(d, I H), 7.50 (br.d. I H).7:45 (br d, I ), 4.45 (s, 2H), 4.29 (br t.211), 3:72 (br-s, 2H).3:60 (s, 2H .3.28 (111.1 I I ).2.47 (s.3H).2.26 (s, 3H). 1.86 (s, 6H-OAc peak), .1.31 (d, 6H)..MS (EI) for C23H29N7OS: 476.2 (Μ1- ).
L4-bcnzoxaze|nn-7-yl)| l,3)lM 'l-I.N R' (400 MHz. d6-
D SO): 8.37 (d. Ill), 8. I I (s. IH), 7.87 (s.211).7.82 (d. IH), 7.53(c). IH), 7.48 (d, IH).4.66 (s, 2W).4.35-4.23 (in, 21-1).3.96-3.85 (in.211).3.54 (t.2H), 3.21 fs, 3H"), 2.87 (t, 2H).2.37 (s. 31-1).2.24 (s.3H). MS (El) for C^Hz^Nr.C S: 463 ( H+).
6-(9-incilryl-4-{6-methyl-2-|(methylamm
yl}-2,3,4,5-iciral^^ Ή NMR
(400 Hz. d()-DMSO) 88.3.6 (<1, I H).7,88 (s.21-1), 7.80 (d. I I I).7.58 - 7:43 (in, 2H).4.48 (s, 2H), 4.31 (d, 2H), 3.72 (s.2H), 3 ·56 (s, 2H). 3.26 (in. I H).2,45 (s.311), 2:24 (s.3H).2.18 (s. 3M).1.31 (d;6H). MS (EI) for.C^-l.n vOS: 490(MH").
-|4-(2,6Γ^ϊη)ο ιγ1-5^ ι-ρ -2->'η-Ι -yJ yriiiii'dtii4- lJ-9-iii6llv l.^
ben oxa/cpiii-7-ylll l,3|tbiazolo|5.4-b|pyridin-2-aniinc. Ή NMR (400 MHz. dc-DMSO): 8.36 (d, I H).7.88 (s.2H), 7.80 (d.1 H).7.67 (d. I H)..7.53 (d, I
'M).4.62 (s, 2H).4.35 -4.29 (in.2H), 3.88-3.82 (in, 2H).3.39-3.35 (in.2H), 3.28-3.24(in. I H).2.41 (s, 3H).2.37 (s.3H). 2.27 (s.3H). MS (EI) for
457 (MH
f).
6-|4-(5-bui-2-yn7:l-yl-2;6-diineliiylpyriinidin-4-yl)-9-nicihy 1,4- benzoxazepin-7-yl || l . |ihiazolo| 5.4-b|pyridin-2-aminc, Ή NMR (400 MHz. DMSO-d„) i S;34 (t, U-l), 7188 (S H), 7.77 (ly IH).7,64 (l., IH), 7.50 (d;
2B), 3.81
*(s,2H), 3.28 (s, 2H\2.38¾, 3H) .34i(s,.3H), 2,24 (s.3i l), l.85(s, 3H). MS (EI) for C:
f,H2
(iN
7OS: 47l.2(MH
+).
6-(4- ( 2,6-dimethyl-5- I -(inciliyloxy)cihyl |pyriinidiii-4-yl }-9-mclliyl-2,3,4,5- teirahydro- 1 ,4-bcnzoxazepin-7-y.l)I l .3|thiazolb|5,4-b|pyridin-2-aminc. Ή NMR (400 Hz, dft-D SO): 8.36 (d. IH); 7.88 (s; 2H).7.8 (d. IH).7.54 -7,41 (in, 21-1), 4.66- 4.50 (m.2H). 4.39 (eld, 2H).4.24 - 4.07 (ni. I H).3,88 - 3.77 (in.1 H), 3.70 (del. I H), 2.94 (s.3H), 2.45 (s. 31-1).2.35 (d.3H), 2.30 - 2.18 (in, 3H), 1.58 (d, 3H). MS (El) for C sI- xNeOiS; 477.2 (Μ1- ).
6-(4- [2.6-dimetbyl-5-|.(inctliyl0xy')incthyl lp.yriniiciiii- ryl }- ^met yl-2,3,4,5- iclraliydro- 1 ,4-beir/.oxazepin-7-yl)| I .3 ]thia. l [5.4-b|pyricIiii-2-aminc.. ! H NMR (400 MHz, df,-DMSO): 8.36 (d.1 H), 7.87 (s, 2H).7.79 (d, 1 H), 7.47 (s, 211).4.75 (s.2H), 4.37 - 4.26 (in.2H), 4.20 (s.2H).3.94 (d, 2H).3.34 (s.31-1), 2.32 (d, 611).2.24 (s, 3H). MS (EI) for C2.,H2f, (,02S: 463.2 (MI-f).
6-(4-|2-(difliioromctlryl)-6-incdiyl-5-(l-mciliylctbyl)pyrimidin-4-yH
2,3,4,5-letrabydro- 1 ,4-benzoxazcpiii-7-yl H I , |diiazolo| 5.4-b|pyridhi-2-aminc. Ή NMR (400 MHz. dd-DMSO) δ 8.36 (d, IH).7.86 (s, 2H), 7.80 (d. lH).7.50 (cl. IH), 7.45 (d.1 H),
6.64. (t. I H), 4.49 (s.2H), 4.29 (l, 2H), 3:75 (l.2H); 3:25 (in, 1 H).2.54 (s.3H), 2:26 ;(s.3H),
1.33 (d.61-1). MS (ES) for QsH^N OS: 497 ( i l*).
6-|4-(2aniino-5-ethyny 6=ineiliyl^^^
beiizoxazcpiii-7-yI|| l3;ji iazpIo|.5,44)'|pyrdin-2:-anuiie. ι Ι·ΙΝΜΚ·(400'ΜΜζ. dfi-DMSO) δ
8.34 (d, IH). »(*, 2 H), 7.78 d, IH).7 8 1H);7;44(*.11:1); 6:4? (s, 2H), 5,07 (s, 2H). 4,67(s, 1 H).4.32 (s, }ΛΜΧ$,:Ζ®),- -23 ($,:6H). MS (m$far iffi2$&S:' 4A(Mtf).
6^{9-iiteth l- Hj6-m&½l-5-tl-m&l^
iclrahydra- 1 ,4-bcnzoxazepi(i-7-yl } [ 1 ,3|l iazolo[5.4-b|pyridin-2-aniinc. 1 H NMR (400 Hz. d,,-DMSO): 8.37 (d. IH), 7.87 (s, 2H).7.81 (d. ΓΗ).7.47 (s.2H).4.54 (s, 2H), 4.32 (m.2H). 3.91 (in.111), 3.73 (m.2H.), 3.23 (m, 1.H).2.84 (in.111).2.63 (m.111).2.45 (s, 3H).2.22 (s, 311); 1.98(ni. IH).1.52 (in.3H), 1.30 (dd.6H). MS (El) lor C2xH.«?N7OS: 16 (MH+).
6-(4-{;2-|:(2S 4,4^dl¾
'l -NMR (400MHz. dj-McOH): 8Ί36¾Ι, M l {<\. IH).7. 1 (s. IH).7.40 (s. IH).4.54 s? 2H).4.36 (in.2H).4:21 (ir. IH).3.84 (tr.2H).3.35 (m. I H).3.l0(q. IH).2.96 (q, 1 H). 2.58-2.47 (in, lH),. 52-(s, 3H), 2.27 (s, 3H); 2.23-2.11 (m, Hi), 1.35 (dd.6H), MS (El) for C28H3|.F2N70S:.553 (MH*).
6-{9 netliyI-4*|6-(metliylaniino)-5-rtitrop,yi
f H NMR (400 MHz, d DMSO):
9.98 (s, lH)v 7:36 (s, IH), 7.2
" hi, 2H).7;;I0 (d,.2;!H,:6.¾2 IH), 6.S0M 1¾, 4
:40 (s^ 2H); 4.22 (in.211).3.53 (m, 2H).2.80 (s, 311).1.99 (s.311). MS (EI) for CjiH-o sChS: 465
6-{ 9-nicUi> -4-l6-metlvyi-5-(l-mctlvylclhyl)^2(.l-niella
yl |-2.3,4,5-ictrahydro- 1 ,4-benzoxazepin-7-yl } 11.3 jihia2olo|'5.4-b jpyridin-2-annne: 1 H NMR (400 Hz, d(i-DMSO); 8.35 (cl, IH), 7.87 (s.2H).7.79 (d, IH).7,48 (d, IH), 7,42 (d, IH). 4.42 (s.2H), 4.28 (in.2M), 3.70 (in, 211), 3,26 (in. I H)', 3;20 (in.2H), 2.98 (m IH).2.45 (s, 311).2.25 (s, 3H), 2:.l,2...(s, 3H), 1.96 (m.2H), 1.77 (in, 1 H), 1.66 (in, IH), 1.30 (dd, 6H). MS (EI) for G29H35N7OS: 531 (ΜΗ¾ MS (El) for C»HMN7OS: 53,1 (MH+);
6-{4-|2-cyclopropyI-6-niethyl-5-(l-.inclliylclbyl)pyriiiiidin-4^
tctraliydro-l,4-benzoxazepi -:7-yl}ri,31diiazolo|5,4-b Ή NMR (40GMHz,
CD3O.D) β 8.58 (s, IH).7.97 (d, IH), 7.50 (s, IH), 7.41 (s, IH), 5.03 (s, 2H), 4.54 (m, 2H). 4.05 (t.211).3.20 (m. H I), 2.58 (s, 3H), 2.22 (s,3H).1.94 (in. IH), 1.42 (1, 6H), .1:01 (d.2H), 0.82 (d, 211)., MS (ES) for C37¾oN6OS:.487 (Μ1- ).
(5-(4-{2-|(2S.4R)-4-f ioropyir lilin-2-yl |-6-meUiy!- -(l-iiieihyVethyl)pyriniidin- - yl}-9-mclhyl-2,3.4.5-leira ydiO^
Ή NMR (400 MHz, CDjQ'D) S.8.36 (d, I II), 7.S3 (d, I H¾ 7,41 (d, 2H), 5.17 (dt, 111), 4.66 (s, 2H), 4.42 (cld, 1 H), 4.37 (l, 2H).3.85 (I, 2H).3.34 (in, I H).3.18..(in, 2-H), 2.54 (s.311). 2.44 (m, 2H), 2.27 (s, 3 i t > , 1.39 (d, 611). MS (ES) l:orG.sH32FN7OS: 534 (MH+).
6- {'9-nicth yl - ^| 6-1 netlv 1 -5 -( I iicthylclhyl)-2-(niclliylpxy)pyriiniclin-4-yl 1-2.3.4.5- teliahydiO- 1 ,4 iciizoxazcpm-7-yl ) 11 ,3|diiazol()|5.4- |pyridin-2-ainiiic, Ή NMR (400 MHz. d6-DMS0): 8.34 (t, I H), 7.87 (s.2H).7.78 (I. I H).7.49 (d.1 H), 7.42 (d. I H).4.47 (s.21-1). 4.31 (s, 2H), 3.68 (s.5H. overlapped).3.25 -3.11 (in. I Ή), 2.40.(s, 3H).2.25 (s, 3H).1.27. (l. 6H). MS (EI) for C25HsNf,p S: 477.2 (MH+).
6-(4-{2,6-dimethyl-5-[ h-inethyl-2÷(iivcllvyloxy)(;lhyl ]pyrimidiii-4-yi }-9rinediyl- 2;,3,4.5-te ahydro- l -benzoxazcpui-7-yl) Ή NMR
(400 MHz.do-DMSO): 8,37 (d. I H), 7.89 (s; 2H): 7:81 (d, I II ), 7.52 (d.1H).7.46 (d. I II), 4:41 (d' TH).4:34 -4.10 (m, 3H).3.71-3v63;(m,,2H).:3.6r (dj 2 ,;3.53-3:4Q¾m, I H).3.20 (s, 3H), 2.41 (s, 3H), 2.39 (s, 3H).2,28 (s..3;H)f L23 (d.3H). MS:(El).for G26l-bnNf,02S: 491 (ΜΙ-Γ).
6-{9-nvelhyl-4-i6-inediyl-5-(l-!ncthylelhyl)-2-( |2-(methyl0xy)cl yl|o.\y}pyrimidin- 4-yll-2.3.4.5-lcirabydro- l.4-benzoxa/epin-7-yl || l, jilviazold|5.4-b|pyridin-2-amiiic. Ή NMR (400 MHz, d
f,-DMSO): 8.35 (d. IH).7.87 (s.211).7.79 (d, 1 H).7.48 (s.11-1).7.45 (d. H), 449 (s, 2H), 4.31 (s, 2H)v ;l8 -4.02 (n 2H , 3:69 (s: 2H¾3.49 - 3.40 (ill, 2H).3.22 - 3.13 (m, 411, overlapped).2.39 (s. M), 24 $W), 1 -27 (t, 6H). MS (El)
521.2 (MH+).
6-(9-meihyl-4-(6-meihylr5-( l-mcthylctliyl)-2- 2r(met yIoxy)etlvyr|pyrimidin-4-yl )- 2,3,4.5-tctrahydro-1.4-beirzo.\azepiii-7-yl)| 1.3]lhiazolo|5.4-b|pyridiii-2-aininc. Ή NMR (400 Hz, dc-DMSO): 8.37 (d. IH).7.92 (s.2H)
; 7.79 (d. IH).7.52 (d. Ill), 744 (d, IH). 4.42 (s
s 2H), 4.28 (m.2H), 3.6 (m.211).3.63 (m.2H).3.24 (in. IH).3.12 (s.3H), 2.79 (m 2H), 2.43 (s.3H).2.26 (s.3H).1.28 (d.6H). MS (EI)
(ΜΙ- ).
6-{4-[2-{ {(2-fluoroct yt)(mcthyl)amiiio|nictliyl } -6-mct yl-5-( 1 - metlvyleihyOpyrimic^^
yl } 11 ,3 ithiazolpi 5.4-b ipyridi -2-aniitic. Ή-NMR (400MHz, d.i-MeOH): 8.33 (d, IH).7.79 (d. IH).7.3 ¾ IH), 7.36 (s. IH), 4.57 (ir. IH).4.54.(s: 2H), 4.45 (ir, IH), 4.34 (Ir.2H), 3.82 (ir.2H).3.67 (d.2H).3.38 (m. IH), 2.86 (in. IH), 2.80 (in. I H).2.53 (s.3H).2.36 (d.3H). 2.30 (s, 3H), 1.38 (d, 6Ή). MS (El) for CsHwFN7OS: 537 (MH+).
6T|4-{2-|(dimelhylamin
.(inctlvylGxy)-2,3,4,5tetialiyclio- ; τϋοηζο
!H N R (400 MHz, DMSO-d6)'8 S.37 (dd, 1H).7.86 (s..2H).7.83 (d, 1H .7.16 (dt.2H). 4.39 (s.2H), 4.20 (d.2Hj.3.84 (s, 3H).3.63 (d.2H).3.37 (d, 2H), 3.25 (in. I H).2.44 (s, 3ΊΙ). 2.1 (s,.6H).1,30 (I, 6H). MS (El) For C^HnM^S: 520.2 (MM).
2.3.4,5 eirahydro-l,4 raizoxazepiii-7-yl)| l,3|to Ή NMR
(400 Mi l/.. φ-,-DMSO): 8.35 (d. I H), 7.87 (s, 211), 7.79 (d.1 H), 7.49 (d. I H).7.44 (d.1 H). 4.46 (s.:2H); 4.33-4.23'Cm, 2H), 3.76-3:64 (m,2H), 3.56 (s.2H).3:2 - : 18 (m,; lH , 2.46 (s. 3H); 2:43 ((|.21 J),;2.25 (s, 3H)..I;S9. (s, 3H), 1.31 (d,6H).0.90.(1.31-1). MS (El) for C27H.v 7OS: 504-(MI-f:)..
4-yl |-9-mcthyl-2 ,3.4,5-tctfaliydi - 1 ,4-bcnz0xazepm-7-yl 111 ,31thiazolo|5.4-Tb|pyridin-2- aminc. 1 H-NMR (400MHz d.i-McOH): 8.32 (d. lH),7.79 (dt I I I).7.40 (s. I.M).7.35.(s, 1 H), 4.54 (tr. 1 H).4.53 is, 2H), 4.42 (tr, 1 H).4.34 (tr, 211).3. 1 (tr, 2H).3.77 (s, 211), 3.38 (in. Ill), 2.98 Or.1 H), 2.91 (tr, IH), 2.72 (q, 2H), 2.53 (S..3H), 2,29 s; 3H), 1,38 (<| 6H) 1.04(tr.3H). MS (EI) for < H^N70S: 55:1 (ΜΪ- ).
N 2-cliloro-5-(9-mcthy^
(niethyioxy)ciliyi|pyrimiife^^
yi|metiiaiiesulfonamide. Ή NMR (400 MHz. d,,-DMSO): 9.89 (s, 1 H).8.51 (d. IH), 8.02 (d, 1 H), 7.54 (d, 1 H), 7.44 (d, IH).4:45 (s.2 H).4.30 (m, 2H).3:69 (in, 21-1), 3.60 (m, 2H), 3.33 (s.3H).3.24 (in. I I I).3.12 (s.3H).2.79 (in.2H), 2.44 (s.3H), 2.24 (s, 3H), 1.27 (d, 6H). MS (El) io Q>7l-k,CIN50.,S: 560 (Μ1- ).
EXAMPLE 2: { 7-(2-Amino|i^
1.00850,1 STEP I : A mixture of N-(6-broniort ,3;|tliiazolo|5^^ (4.6 g, 17.0 miiiol). bis(pinacolalo)'diboroii (8.6 g, 39.0 mniol).
bis(diphenylphospbiiio)fcnOeene]dichloiOpalladiuiii(II) (1.2 g.1.75 inmol), and potassium acetate (0.25 g, 0.75 inmol) in 1 ,4τϋΐθχαηβ (50 mL) was degassed with liitiogcn and heated al 1 0 °C in a microwave apparatus for 1 hour. The reaction mixture was cooled to room temperature and diluted with diethyl edier (100 niL). The solid was collected by. filtration. The crude filler cake wa suspended in water, filtered and washed with hexanc lo give Λ'-|6- (4,4.5.5-tetramcthyl- 1 ,3,2-dioxaborolan-2-.yl)| 1,3 |lhiazolo| 5.4-/>|pyridm-2-yl Jacciamide as
liglil brown solid (2.8 g.52%). Ί-INiV! (400 MHz. DMSO-df,}: 12.60 (s. I l l), 8.60 (s. IH). 17 (s.1H).2.19 (s.3H).1.29 (s. I2H); MS (El) (or Ci.iHisBiN:iQ;,S: 320.(MH+).
1 0851) STEP 2: A n xU e, 2-(clil0roniclbyl)-6 UcUiyl-5-(l -metliylcihyl)pyiiiiiidin-4- ol'(1.20g, 6.00 niniol). csium acclatc (11.46 g.60 mmpl) in glacial aceiic acid (.2.0 mL) was healed at 1309G in. a microwave apparatus for 1 hour. After cooling to room temperature the reaction mixture was partitioned between water and. ethyl acetate. The. rganic layer was separated washed with saturated aqiieoiis sodium hydrogen carbonate, brine, dried over anhydrous magnesium sulfate, filtered, and concentrated to give |4-hydroxy-6-mcihyl-5-( 1 - mcihylethyl')pyrimidin-2-yl|methyl acetate which was used without further purification in the next step. (1.3 g, quant.). MS (EI) for C,|H'|„N;0:,: 225 (ΜΙ-Γ).
[00852] STEP 3.: A solution bfl4-hydroxy-6-inclhyl-5-(1-methyleiliyl)pyrimi'di'n-2- •yljmethyj acetate,(l;3 g.6:00 mmol), prepared in step 2, invplipHphonis^.ychloriicle;C8:0 mL, 5.S4 mmol) was heated to reflux fo I hour. : After "cooling to ropih temperature, the reaction mixture was concentrated and the resultin residue \vas . partitioned between saturated aqueous sodium hydrogen carbonate.and elhyl acetate. The organic layer wasseparated. washed with 'brine. -dried over anhydrous riiaghesiurn sulfate, filtered and concentrated. The residue was purified by gradient silica gel chromatography (hexaiie : ethyl acetate 9: 1 to 1:1) to provide |4-chloro-6-mcthyl-5-( 1 -methyIethyl)pyrimidin-2-yl Imethyl acetate. MS (EI) for C||H|5C1N,02: 243 (ΜΙ-Γ).
100853] STEP 4: A solution of 1.1 -dimeihylcih.yl 7-bi mo-9-niethyl-2,3-dihydi - 1.4- benzoxazepine-4(5//)-carboxylate (9.0 g.26.3 mmol) ;in amixture o methanol (30 mL) and 4N hydrogen chloride in 1 ,4 libxanc (:10 mL) was refluxcdTpr 30 nnnutcs:;Aftcr cooling to room teinpcfature,.. tlic reaclio'n. jifi iuiic'- was concentrated to a reduced volume: and. the precipitate that-formed was collected Ivy filtration, washed several riiiics witlvethyl acetate and hexanes then dried in vacuo to give 7-biOmo-9-melhyl-2,3,4,5-teirahydi -l ,4- benzo azepinc hydrochloride (5.7 g.78%) as a while solid. '.HNMR (400 MHz. DMSO-d(i): 9.57 (br.s.1 H).7.52 (d. I H).7.47 (d. I H), 4.26 (s, 2H), 4.18 (m, 2H).3.43 (m, 2H).2.1 (s, 3H); MS (El) .fp CioHi:BrNO: 243 (ΜΙ-Γ).
100854] STEP 5: A mixture 7^l)t m0-9:-mcth^l 2,3.4.5-tctFa¾ydi:o- 1 ,4-bcnzoxazcpinc hydrochloride (0,82 g, 2.90 mmol). |4-clvIoro-6.-niethyl-5-( Hncthylethyl)pyrimidin-2- yljmethyi -acetate (0:70 g.2.90 mmol) prepared in step 3 and./V.N-diisppiOpylcthylamine (1.9 mL, 10:9 mm l) in A'.yV-dimcthylacciamidc (3.0 mL) was heated at 130 °C for 3 hours. After cooling to room temperature, the reaction mixture was partitioned between water and dichloromcihanc. The organic layer was separated washed with brine, dried over anhydrous
sodium sulfatc,:fiHcrcU and concentrated. Gradient silica gel chromatography oi; the residue (liexane : ethyl acetate 9: 1 to pro\ ded[4-(7.¾omo-9.-mctliy ^^
bcii/.oxazcphv4{5H)-yi)T6-iu'e^ (0.65 g.
50%). MS (El) for C2,H¾BrN.,Oj: 44S (MH+).
[00855] S TEP 6: Λ mixture of |4-(7-br.omo-9-mcthyl-2.3-dihydro-l ,44icnzoxazepin- 4(5/7)-yl)-6-iticlhyi-5-( 1 -niclhyletliyl)pyi-imidin-2-yl |niethyl acetate (68 mg, 0.15 niiiiol) prepared in step 5, N-.[.6-(4,4,5.54cm^^
/;|pyridiiv2-yllacetam.idc'(48;iiig, 0.15 inmol) preparedin ste 1.
bi.s(dipheiiy,lphospliirio)ferr0ceiicJ and cesium carbonate (0.25.g, 0.75 mmdl) in a vmixtiir O i, ^dioxane. \6:ni )i:atidAvaier ('0.4 niL) was healed at 130 °G usinga. microwave apparatus for 2' hours. The reaction mixture was cooled to room temperature, and parlilioned bclwecn ciliyl acel.ate and saturated .aqueous- sodium hydrogen carbonate. The organic layer was separated washed with brine, dried over anhydrous magnesium sulfate, filtered and conceiitratcd./Silica gel ehrqmalography of the reside (chloroform : methanol 95:5). provided {.4 7-(--aniinp| 1.3|tliiazolo|5.4-//[p.yridin-6^ . yI)-9-mcihyl-2.3.-dihydro-l ,4 }enzoxazcpiiT-4(5W)-yi|÷6-metI^
2-yl [methanol (24 mg.34%). 'PI NMR (400 MHz. CD;iOD) 88.56 (d, III).7.95 (d, 1H).7.56 (d.1H), 7.45 (d. I l l), 5.05 (s.2H), 4.56 (s.2H), 4.48 (in, 2H), 4.15 (m, 211).3,21 (m. I H). 2.6.3 (s, 3H).2.27 (s.3H), 1,43 (d.6H); MS (ES) for C25H2s t-.0:S: 477 (Mlf).
[ 00856] Proceeding accprdingto the method of Example 2 and replacing |4-(7^bromo-9- incthyl-2.3-dihydro-1.44icnzoxazepin-4(5/i)-yl)-6-methyl-5-( l-melhylethyl)pyrimidiii-2- yl|mediyl acetate in step 6 with l-|4-(7-bromo-9-mcthyl-2,3-dihydro-l,4-bcnzoxazepin- 4(5/ )-yl)-5-isopiOpyl-6-mclhylpyrimidin-2-yl )ethanol, the following compound of die invention was prepared:
l-{4-|7-(2-Aniino| l.31thiazolo|54-b]pyridiiit6-yl)-9-mciliyl-2.3-di
bcir/bxazepin-4(5H)-yl|-6-mclhyl0-( l-ihcthyletliyl)pyriinidin-2-yl |ethanql. 'HNM (400 MHz. DMSO-df,): 8.36 (d, 1 H).7,88 (s.2H), 7.80 (d. I H), 7.49 (d. I H).7.45 (d, I H).-4.7() (br.s. IH), 4.48 (m. IH), 4.47 (s.211).4.30 (in.211).3.72 (in, 2H).3.26 (m.1 H).2.49 (s.3H). 2.24 (s.3Ή), 1.32 (dd, 3H), 1.26 (d, 611). MS (El) for C26H.ii) f,02S 491 (Ml-I+).
100857] Proceeding according lo tlie method of Example 2 and replacing [4-(7-brqmo-9- methyl-2,3-dihydtO- 1.4-bcnzoxazcpin-4(5/)-yl)-6-inethyl-5-( I -melhylethyl)pyrimidin-2- yl|meihyl acetate in step 6 with l-|4-(7-biOnio-9-mclhyl-2.3-diliydiO-l,4-bcnzoxazcpiiiT 4(5H)-yl)-5-isopropyl-6-nicihylpynmidin-2-yr]-2,2,2-irif]uoi Cllianol. the following compound of the invention was prepared:
|-{4 7-(2-Aminp| l,3|lhiazolo|5,4-b|pyridin^^
benzoxazepin-4(5H)-yr|-6-methyl-5-(l ne
'HNM (400 MHz. D SO-dfi): 8.35 (cl, I H), 7.88 (s, 2H), 7.80 (d, l.H), 7!49 (d, 1 H), 7.42 (d, IH), 6.57 (br. s, 1 H), 4.88 (in, IH), 4.51 (s.2H).4.32 (m.2H), 3.74 (in.2H), 3.26 (m, IH), 2.52 (s, 3H), 2.25 (s,3H ,.1,47 (I, 6H); MS (El) lor C2(1H:7F1Nf)02S. 43 (MH+).
l^{4H7-(2-amiao j,31lhiazolo|5A-b|pyridin
benzoxazepiii-4(5H)-yl|-6-mcihyl-5-( l-methyleihyl)pyrimi
.din-2-yl Jethanol. Ή NMR (400 MHz, d
f,-DMSO): 8.36 (d, I H), 7.87 (s.2H), 7.80 (d, IH), 7.49 (d, 11-1), 7.45 (d, 1H),4.72 (brS.1 H), 4.50 (in, I H), 4.47 (s, 2H), 4,30 (m.2H), 3.76 ( i, 2H), 3.24 (m, 1 H), 2.46 (s, 3H). 2.25 (s, 3H), 1.32 (dd, 3H), 1 :24 (d, 6H). MS (EI) for-
491 (MH
+).
6-{4-[2-amino-6-incihyl-5-(l-incdiylelhyl)pyrijnid
tetrahydro-l,4-benz(>xazcpin-7-yl)| I,3|lhiazolo|5,4-b]pyridin-2'-aintne. Ή NMR (400 MHz, DMSQ-dft) δ 8.33 (s.1H): 7.79(s..3H), 7.20 (s, IH).7.07 (s, IH).6.24 (bs, 2H), 4.30 (s, 2H), 4.18 (s, 2H), 3.80 (s, 3H), 354 (s.2H).3.09 (m, I H), 2.24 (s, 3H), 1.18 (d, 6H). MS (EI) for G2 H27 7O2S: 478.2 (Ml-f).
EXAMPLE 3
6-yl)-2 -diliydro^l,4-benzoxazepin-4(5H)-yl]py iniidin-2-yi}
[00858] STEP 1 : A mixture of 7Hiromp-9-methyl-2 ,3:;4,5^etraliy.dra- l ,4-benzoxazepine hydrochloride (1.0 g, 3.6.1 minoi),
/V.N-dimethylineUianamine (0:82 g, 3. 1 irimbl), and M/V-diisoprppylethylamine (3.1 mL. 18.1 mmol) in W.N-dimcthylacctamide (5.0 mL) was. heated at 130 °C for 3 hours. After cooling to room temperature, the reaction mixture was partitioned between water and ethyl acetate. The organic layer was separated washed with brine (2 x 100 mL), dried over anhydrous sodium sulfate, filtered and concentrated. Gradient silica gel chromatography of the residue (chloroform : methanol 95:5. to 9:1) provided .l-| -(7-bfomO-9-mel yl-2.3- dihydro- 1 ,4-benzoxazepin-4(5//)-yl)-6-meth^
dimethylmethanamine (0.64 g
: 41%). MS (El) for
433 (MH
+).
[00859] STEP 2: A mixture of ]-|4-(7-bromo-9-methyl-2,3-dihydro-l ,4-benzpxazepin- 4(5H)-yl)-6-mcthyI-5-(l-methylelhyl)pyriniidin-2-yl|-N.A'-diniethylmeihanaminc (90 mg, 0.21 mmol), (l-{r(l,l-dimethylethyl)oxy|ea^^
acid (60 mg, 0.22 mmol), bis(diphenylphpsphino)ferrocenc)dichlpropalladium (II) (32 mg, 0.042 mmol) and cesium carbonate. (0.36 g, 1.10 mmol) in a mixture of 1 ,4-dioxane (2.7 mL),
and water (0.3 niL) was healed at 130 °C using a microwave apparatus for I hour. The reaction mixture was cooled to room temperature and partitioned between water and ethyl acetate. The organic layer was separated washed with- brine.-. dried over anhydrous magnesium sulfate, filtered, and coriccnlraied. The resulting residue was dissolved in methanol (5.0 niL) and concentrated hydrochloric acid (0.5 imL) was added io the solution. The reaction mixture was heated to reflux, for 5 minutes. After cooling to room temperature, the mixture was concentrated and the residue purified by preparative reverse phase I-IPLC (.0.1% aqueous ammonium acetate buffered aqueous acctonitrile mbile phase) to give MA'-dimcthyl-l-^- niclhyl-5 l-meihylediyl)-6-l'9-meihyl-7-:(2^^
benzoxazepin-4(5H)-yl|pyriinidin-2-yl lincthanamiiie (42 mg.-42%). Ί-I MR (400 MHz. DMSO-d6): 7,62 (s, IH), 7.49 (d, I H).7.41 (d. IH).7.35 (dd. I ll), 7.34 (s.1H).7,30 (br.s. 1 H), 4.37 (S.211), 4.26 (s, 2H), 3.66 (s,.2H).3.38 (s.2H).3.33 (m, III).2.47 (s.311); 2.26 (s, 3H).2.16 (s, 6H).1.86 (s, 3Ή), 1,32 (d.61-1): MS (El) For C2.,R,f>Nf,0: 485 (MH+).
[00860] Proceeding according to the method of Example 3 and replacing 1 -|4-chloro-6- methyl-5-( 1 -mellrylcthyl)pyrimidin-2-ylJ-N.W-diinclliylinelhanaininc in ste 1 with 4-CIIIOIO 5-isopiOpyl-6-mclhyIpyrimidiii-2-aminc and replacing ( I - { | ( 1.1 - diinelhylethyl'jox jcarbonyl Ι-2-niclhyl· l7-/-bcnzimtclazol-6-yl)bpiOnic acid in step 2 with alternative reagents, the following compounds of the invention Were prepared:
Methyl 4-{4-|2-Amino-6Hiiethy^
tctrahydro-1.4-benzoxazepin-7-yl|-2(inethyloxy)benzoatc. Ή NMR (400 MHz. d<-,-DMSO) δ 7.73 (d, IH).7.55 (d, I'M).7.43 (d, IH), 7.3,1 (d, IH), 7.26 (dd, IH), 6.05 (s, 2H), 4.25 (s, 4H), 3.91 (s,.3H), 3.79 (s, 3H), 3.53 (s, 311).3.19 (dd, I H).2.29 (cl, 6H), 1.24 (d, 6H). MS (EI) for C^H^N^: 477.11 (MM*).
4-|7-(3-Am"mophcnyl)-9-mcthyl-2.3-dihyclro-l ,4-benzoxazepin-4(5H)-yl)-6-meihyl- 5-(i-methyleihyl)pyrimidin-2-amine, Ή NMR (400 MHz, d6-DMSO) δ 7.30 (s, IH), 7.20 (d. IH), 7.06 (t, IH).6.77 (d. I H).6.70 (d, IH), 6.51 (dd, IH).6.01 (s.2H), 4.20 (d.4H).3.53 (s. 2H).3.24.-3.16 (m.I H), 2.30 (s.3H).2.25 (s, 3H).1.25 (d.6H). MS (El) .for CMH .N50: 404.13 (ΜΙ-Γ).
3-{4-|2-Amino-6-meihyl-5-(lHiieihylcthyl)pyrimidin-4^yl|-9-meihyl-2.3.4,5- tctrahydro-1.4-bcnzoxazepin-7-yllphenoI. Ή NMR (400 MHz, dr.-DMSQ) 67,35 (s. IH). 7.26 - 7.18 (m.2H).7.00 (d. IH).6.95 (d, 11-1), 6.72 (dd, IH).6.02 (s.2H).4.21 (d.4H).3.54 (s, 2H).3.23 (dd, IH), 2.30 (s.3H), 2.26 (s, 3H).1.26 (d, 6H). MS (EI) for C2.1H2s .1O2: 405.08 (MH
+).
benzoxa7 pin-4(5H)-yl)pyrimidih-2^aniinc. Ή NM.R (400 M;Hz, clfi-DMS.O) δ;9.14 (s, I H), 9.08 (s.2Η), 7.60 (d, 1 H), 7.48 (d. I I f).6.02 (s, 2H), 4.28 (s, 4H), 3.53 (s, 2H).3.2-1 - 3; 11 (in, 111), 2.28 (d, 6M), 1.23 (d, 6H). MS (EI) f r ^I-hft ^O: 391.10 (MH+).
4-Methyl-5-(l - ctliyleihyl)-6 9-incthyl-7-( 1 H-pyraz0l75-yl)-2,3-dihydiO- 1 ,4, bcnzbxa/.cpin-4(5H)-yj pyriinidjn-2-aniiiic. Ή NM.R (400 MH/., dc-DMSO.) δ.8.29 (s, IH), 7.67 (s. IH), 7.54 (s.1Ϊ-Ι), 7,49 I H), 6.59 (d, III), 6:.0I (s< 2H), 4.20 (s, 4H); 3.52 (s, 2H). 3.25^-3.13(ϊη, IH).2.29 (s, 311), 2.24 (s.31-1), 1.24 (d, 6H),MS (EI) for ¾,Η¾Ν60: 379.08 (Μΐ-Γ).
4-|7-(J,3-Benzodi0XOl-5-yl)-9-methyN
melhyi -(l-methyleihyl)pyrimidin-2-amine. Ή N MR- (400 MHz. dr.-DMSO) δ 7.36 (d, IH), 7.24 (d, 1 H), 7.16 (d, 1 H).7.07 (Ud. I H).6.98 (d, 1 H), 6.05 (s, 2H), 6.02 (s, 1 H), 4.19 (s, 4H, 3.52 (s.2H), 3.25 - 3.15 (m, 211), 2.29 (s.3H),2.25(s.3H), 1.24 (d.6H). MS (EI) for C25H
28N.,Q
3:-433.09 (MH
4;).
8.60 (dd.
2H), 7.63 (d, 2H), 7.48 (s, 1 H), 7.60(s.1 H), 6.02 (s, 2H), 4.26 (s,4H), 3.45 (ni, 2H), 3.17 (m, III).2.29. (s.6H), 1.23 (d, 6H); MS (El): or ¾H.7N50. found 390.09 (MH*).
4-MctlryI-5-(l-meihy!ethyl)-649-metliyl-7-pyiidin-3-yl-2,3-d
benzoxazepin-4(5H)-yr)pyriniidih-2-amirie. '-H-NMR (400 MHz, D6-DMSO): δ 8.84 (d, IH), 8.53 (dd, IH), 8.01 (m, IH), 7.48 (m, IH), 7:42 (in, IH).7.39 (d, I H).6.02 (s, 2H), 4.25 (s, 4H).3.60 (s, 2H), 3.20 (q, IH).2.29 (m.6H).1.24 (cl, 6H); MS (EI) for C23H27N5O, found 390.09 (MH*).
3- { 4> \2- A in iiio-6- meili 1 τ5
Γ- 1 - nietlv iethyii)pyiini id i n-4- 11-9- met HyI-2,3 ,4,5- leliairydrb-l,4-bcnzoxazepin-7-yl}bcnz.ainiilc. Ί-1-NMR (400 MHz, D
6-DMSpj: δ 8,09 (d. 2H), 7.78 (dd, 2H), 7.52 (1, 2H), 7.46 - 7.25 (nr.2)1), 6.02 (s, 2H), 425 (s, 4H).3.53 (s, 2H), 3.21 (q, IH).2.29 (s, 6H).1.24 (d, 6H); MS (EI) for
found 432.09 (MH*).
4- {7 3.4-Bis(mediyloxy)pUeiiyl|-9-nvei yl-2,3-diliydro-l,4-bc zoxazcpiiv4(5H 6-inelliyl-5-(l-incthylelhyl)pyrimidiii-2-amine. Ί-1-NMR (400MMz, D„-DMSO): δ 7.41 (d, IH), 7.29 (d, IH), 7:13 (in, 2H), 7.02 (d, IH), ,6.02.(s*.2H), 4.19 (s, 4H), 3.83 (s, 3H).3.78 (s. 3H), 3:52 (s, 2H), 3.24 (q, I H), 230 (s, 3H).2.26 (s, 3H), 1.26 (d, 6H); MS (EI) for
G26H12N j0.v, Tpuiid 449,09 (MH*).
4^1edTyl-5-(lHnelhylethyl)-6-{9-ineiKyl-7,- 1.4-benzoxazcpiii-4(5H)-yl }pyrimidin-2-amine. Ή- MR (400 MHz, D6-DMSO): δ 8.43 (d.
IH).8.25 (d. I I I).7.55 (m.2H), 7.41 (d, II I), 6.03 (s,.2H), 4.24 (s, 411), 3.90 (s.311), 3.53 .Cm, 2H), 3.20 (q, H I), 2.29 (s, 3H), 2.28 (s, 311), 1.24 (d.611); MS (EI) Ibr C2.|H2«;N502, ;|Oii0d'.420.l2(MI-I+).
lrai/.oxazepii 4(5I I)-yl|pynmidiii-2-yininc. Ή-NMR (400 MHz, D()-PMSO): δ 8.34 (s, 1 I I). 7.93 (s, 2H), 7.35 (s, 1 H), 7.25 -(s; 1 I I).6.02 (s, 2H), 4.17 (s, 4H), 3.49 (s.2R).3.19 (q, I H). 2.29 (s, 3H), 2.21 (s.3H), 1.23 (d.6H); MS (EI) Ibr ς2|Η2ή ήΟ. Pound 379.14 (Mlf).
4-|7-(2-Aniinopyriinidin-5-yl)-9-methyl-2.3-clihydrc l,44ien/.o a c
methy]-5-(l-ineUiylcihyl)pyrimidiii-2-aniinc.
1 H-NMR (400 MHz, D
6-DMSO):.88.50 (s. 2H), 7.38 (s. IH), 7.25 (s, 1 I-l).6.72 (s, 2H), 5.99 (s, 2H), 4.20 (s, 4H).3. 1 (s.2H); 3.1 (q. l-H), 2.29 (s, 3H), 2.24 (s-, 3Μ),· l;.23.(d,..6H); MS (EI)
4-Mcthyl-5 l-nicdiylctlvyl)-6-:i9 ircdiyl-7-|2-(iiic
dih;ydf0Hj\4-beiizoxazepin^C5H Ή-NMR (400 MHz, D6-DMSO): δ
8.86 (s.2H).7.50 (s, IH), 7.37 (s, 111).6.02 (s, 211), 4.25 (s, 411).3:95 (s.3H).3.52 (s.211). 3.17 (q, IH), 2.28 (s.3H), 2,26(s, 3H), I ; 23 (d.6H): MS
found 420:51 (MH
+).
4-|7-(2-Fluoropyridin-4-yf)-9-metbyI-2,3-dihydro-^^
mcthyl-5-( l-nicthylctliyl)pyrimidiii-2-ami.nc. 1 H-NMR (400 MHz, Dr,-DMSO): δ 8.27 (l. IH), 7.66 (m, 2H), 7.56 (d, I H), 7.46 (s.1M).6.01 (s, 2H), 4.29 (s.4H), 3.53 (s, 211).3.14 (q, 1H¾2,28 (s.6H), 1.89 (s, I H).1.22 (d, ¾H): MS (EI) for Cr^E sC). found 408. 1 (MM4.
mcthyl-5-( l-mcihyle.tbyl)pyriniidin-2-mninc, 1 H-NMR (400 MHz. Df,-DMSO): δ 7.24 (m. 21-1), 7.06 (m, 31-1); 6:01 (s, 2H).4.17(m.2H).4.11 (s.2H), 3.51 (s.2H).3.19 (q, 111); 2.30 (s. 3H).2.20 (s, 3H), 1.25 (d, 6H); MS (El) for C2|H2ftN(,OS. found 411.50 (Ml Γ).
4-|7-(6-Aminopyridin-3-yl)-9-methyI-2,3-dihydro-l .4-beiizoxazcpin-4(5H)-yl|-6- nicihyl-5-(l-melhylcdiyl)pyrimtdin-2-aminc. Ή NMR (400 MHz, mcthanpl-di): 8.09 (d, IH), 7.71 (dd, IH), 7.25 (m.2H).6.66,(d. IH).4.47 (s.2H), 4:29 (m, 2H).3,78.(m.2H).3.24 (m; IH), 2.39 (s, 3H), 2.28 (s.3H),.1.95 (s.3H).1.32 (d.6H); MS (EI) for C^H.s^O: 405 (MH+).
4-Mciliyl-5-(l -nicUiyleihyl)-6-{9-inethyl-7 6-(riiclhyloxy)pyridin-3-y
1 H).7.94 (dd, 1 H).7.42 (s.1 H), 7.30 (s..IH); 6.90 (d, I H).6.02 (s.2H).4.22 (s.4H).3.88 (s. 3H), 3.53 (s, 2H).3.19 (dd, IH), 2.28 (d, 6H), 1.24.(d, 6H). MS (EI) for
420.12 (MH
+).
[00861] Proceeding according lo the nietliod of Example 3 aiuhrcplaeiiig N|4-chloiG-6-
iiv sic
' .1 with 4-cliloro- 5-is6pi pyl-6-nietliylpyrimidin-2-aniiiic, the following compound of the invention was prepared:
4- clhyl-5-(l--niethylelhyl)-6 9-"ielli^^
dihydro- 1 ,4
rben/Oxa7.epiii-4(5H)-'yl pyrinj
:i(lin-2-an»iic. NMR (400 MHz, dc-DMSO) δ 7,66 (s.1 H).7.50 (s, 1 H).7.43 (d, I 7.35 (d.111).7.31 (s. I H).6.02 (s, 2H).4.21 (s, 4!-|), 3.54(m.2H).3.26 (m. I H).2.50 (s.31-1).2,30 (s, 3H)
:, 2.28 (s..3H).1,27 (d, 6H): MS (ES) for
4-[7^1',3 dimetliy|ii:i;{rpyra7.ol-4-y^ ,4-bc»¾ox'ajcpin-4'(5Hj- yl |-6^iielliyl-5-(I ineihylelhyl)pyjiiii^ Ή NMR (400 MHz, d^DMSO): 7.76 (s,
1 H).7.14 (s. I H).7.03 (s.1 H).6.00 (s, 2H).4.19 (brtr.21-1).4.13 (s.21-1).3.76 (s.3H).3.51 (br tr.2H).3.23 (m.1 H).2.29 (s.311).2.24 (s.3H),2.22 (s.211).1.25 (d.611). MS (El) for C23H»N¾0: 40S (Μ1- ).
4-|7H1.5-diniethyklH^yrazol-4-yl)-9-m
yl|-6 iiediyl-5^1-iiielhylcdiyl)pyriiiiidiii-2-aminc: Ή N R.( 0a H d6-¾M¾;G); 7:47 (s. I H).7.13 (s, IH).7.00 (s.1 H).6.00 is.2H).4.20 (br tr.211).4.15¾κ.2H).3:76 (s. H).3.52 (br tr.2H).3.24 (ηϊ. IH); 2.33 (s.311), 2-29 3Ή%2.2 (s.3H). i:25;(d.6H). MS (El) for .CMHVIN,,0: 408 (MM*).
4rl1-( ] -ethyl - } Η ρ^3^οΊ^-^'Ι).-9Ηΐκ'ιΙν^|.2;3^ίΙι>¾Ιίό* lv4>bcnzo^azcpin-4(5H)-.yU-6- inethyl-5-(lHiieihylethyi)pyiin)idin-2-aniine. Ή NMR (400 MHz, d(l-DMSO):: 8.06 (s, IH), 7.74 (s.1 H).7.32 (s, IH).7.21 (s. U-l).6:01 (s. Iff).4.17 (br s, 4.H), 4.13 (q.2H).3.49 (br tr. 2H).3.19 (m. IH), 2.29 (s.3H).2.21 (s.3H), 1.38 (ir.3H).1.24 (d, 6H). MS (EI) for C33I- t)Nf,0: 408 (MH+).
4-mctliyl-5-(l iiethyletlvyl)-6H9-iiicihyl-7-|2-(iiieiliylamh ,3-lliiazo!-4-yl|-2.3- dihydro-1.4-beiizoxazcpin-4(5l-f)-yl}pyriini(lin-2-aniinc. Ή NMR (400 MHz. dc-DMSO): 7.78 s, J H),.7.69 (s, 1 H).7.63 (s. I H).6.00 (s, 2H).4.22 (br s, 411), 4.03 (s, 3H).3.52 (br tr, 2H), 3.18 (in, I II), 2.29.,(s, 31-1).2.25 (s.311).1.24 (d.6H). MS (EI) for C22H2(jNi,OS: 426 (MH+).
4-iiielhyl-5-(]-melhylethyI)-6-|9-niclhyl-7-(l-mclhyl-l H-pyrazol-4-.yl)-2.3-dihydro- l.4-bcnzoxazepiii-4(5H)-ylJpyriniidiii-2-amine. Ή NMR (400 MHz. d,,-DMSO): 8.0,1 (s, 1 H), 7.74 (s, 1,1-1), 7.31 (s.111).7.20 (s, il l).6.07 (br s.21-1), 4.20(br s.41-1), 3.85 (s, 3H). 3.52 (brs,3H), 3.17 (m. IH), 2.30 (s, 31-1), 2:21 (s.31-1), 1.24 (d, 6H). MS (El) for
C22H2s 60: 394 (MH+).
4-iiiciliyl-5-(l-meiliyleihyl)-6-|9-inclhyl-^^
l.,4-benzoxa7.cpin-4(5H)--yl.|p:yriinidiii-2-aniiiic. 'W MR (400 MHz, d6-DMSO): 7:90 (s: 111), 7;42 (s. IH), 7.25 (s, I H).6.00 (s, IH).4.22 (br ti;,;2H)„4,18 (s, 2H)S 3,52 (s, 2H), 3.16 (in. IH), 2.66 is.3H).2.29 (s.3H).2.23 (s.3H).1.24(d.611). MS (EI) for C22II27N5OS: 411 (MH+).
benzoxazepin-4(5H)-yl|-6-mctliy^ Ή NMR (400 MHz. d„-DMSO) δ 8.35 (d. IH), 8.08 (i. IH).7.87 (s. IH), 7.79 (d. IH), 7.51 (s. 1 H).7.40 (s. I H).4. 1 (s, 2H).4.30 (m.21-1).4.19 (d, 2H), 3.70 (m.2H)..3.26 (m, I H), 2.47 (s, 3H).2.27 (s, 31-1), 1,85 (s.3H).1.31 (d,6H). MS (ES) for C27H31N7O2S: 518 (MH÷).
tcinilvydi -I ,4-bcnzoxazcpiii-7-yl |I I .3jthiazolo|5.4-b|pyridin-27ariiiue. 'l-l MR (400 MHz, CD OD) 8.61 (d, IH).7-98 (d, 1 H), 7.56 (s.. IH.),, 7.46 (s, IH).5;40 (d, 2H), 5:08 (s, 2H). 4.50 (m,2H).4.17 (in, 2H).3.22(m. I H), 2.64 (s, 3H), 2.27 (s.3H).1.44 (d; 6H). MS (ES) foi C35H27 6OS: 479 (MH+).
6-(4-{ 2-((diinclhyli)niino)mclliyl l-6-methy!-5- I-methylethyl)pyriniidin-4-yl }-9- (iiicthyloxy)-2.3.4.5-tclrahydiO-I,4-bciiz .\azcpin-7-yl]|l,3]thiazolo|5
Ή NMR (400MHz, D SO-d,,) δ 8.37 (dd, H I).7.86 (s.2H), 7.83 (d, IH), 7.16 (dl.2H). 4.39 (s,.2H).4.20 (d.2H).3.84 (s, 311).3.63 (d.2H).3.37 (d.2H), 3.25 (in.111).2.44 (s.3H), 2.15 (x, 61-1).1.30(1, 6I-I). MSV(E!).lor
520.2 (MH
+).
4-mcdvyl-54l --methyleihy
yl)-2,3-dihydra-l,4-b.enzoxazcpin-4^^ Ή NMR (400 MHz, d0-
DMSO):8:48(br s. ill).8.00 (br s. H I).7.50 (d, IH), 7.36 (cl. IH), 6.02 (s-i 2H).4.30-4. |7 (111.4H).3.58-3.48 (111; 2H), 3.33 (s, 3H): 3.29-3. \-9 (m, HI).2.53 (s.3H), 2.29 (s.3H), 2.29 (s.3H), 1.26 (l.6H). MS (El) for C25H2.JN7O: 444 (MH†).
4-|7-(l I-l-in)idazo|4.5-b|pyriclin-6-yI)-9-incibyI-2.3-dihydro-l,4-bcnzoxazcpi yl|-6-inelliyl-5-( l-inclhyletliyl)pyiiinidin-2-amiiie. Ή NMR (400 MHz, metbari l-d,|): 8.61 (l, IH), 8.41 (cl. III).8.16 (l, IH), 7.44 (cl, IH), 7.37 (d.. I H), 4.37 (s, 2H), 4.28 (in.2H), 3.68 (m.211).3.38 (111, H I), 2.3S :(s, 3H), 2.33 (s.311), 1.33 (d.61-1). MS (EI) for G24H27N7O: 430.1 (ΜΙ- ). N-(2-clil0ro-5-14-|2-(j(2.2-dinuorocthyl)aminolmctbyl}-6-meibyI-5-(l^ melhylelbyl)pyrimidin-4-yl |-9-melhyl-2,3,4.5-tctrahydro- 1 ,4-benzoxazcpin-7-yl}pyridin-3- yOincdiancsulfoiKimidc. Ή NMR (400'MHz, CD3OD) δ 8.48 (ά\ IH).8.17 (d, .IH), 7.50 (s. M-D..7.5Q (s.1 H).6.29 (1.1 I I).5.04 (s, 2H), 4.57 (1.2H), 4.50 (s, 2H).4.19 (t.2H), 3.64 (del.
2H).3.19 (in, IH), 3.13 (s, 3H), 2.66 (s, 3H).2.30 (s.3H), 1.43 (d, 6H). MS (ES) for
C27.H33CIF2NeQ.1S·: 596 (MI-I+).
beir//)xazcpin-4(5H)-yl|-6-niethyl-5-(l-in^ Ή NMR (400 MHz. Cl¾OD) δ 9.44 (s. IH), 8.99 (d. IH).8.59 (cl, I H).7.6 (s, IH).7,60 (s. IH).6.26 (!, 1H).5.09 (sj:2H); ^S Ci.2H;), 4.48 (s.2H), 4.1,8 (u 2II>, 3.61 (del.21-1).3,1:8 (in, XH),2.65 (s.3H), 2.33 (s, 31-1), 1.44 (d, 6H). MS (ES) lor C27H31E2 7O: 508 ( Ι- ).
imidaz0[4,5-b|pyndin-6-yl)-2.3"-di^^^
yl)nicthyl)ethaiiamiiie. Ή R. (400 Hz. CD3OD) 08.8S (d.1 H).8.44 (d..IH).7.65 (d. 1 H).7,58 (d.1 H).6.26 (t. I H).5.08 (s.2H).4.59 (l, 21-1).4.49 (s.2H).4.18(1, 2H); 3.62 (id. 21-1), 3.18 (m, IH), 2.91 (s, 311).2.65 (s..3H).2.32 (s. H), 1.41 (d.6H). MS.(ES) for C2XH33F2 7O: 522 (MH+).
2,2 difluoro÷ -({4 |7-(;tt^m^
mctliylcihanamine. Ή NMR (400 MHz. clfc-D SO): 8.61 (bi s. I H).8.47 (s, ill).8.17 (br s, 1 H).7.51 (d. I H), 7.42 (d. I H).6:03 (It. H I), 4.42 (s.2H).4.36-4.2 (m.211).3.71.-3.66 (m. 2H).3.66 (s, 2H), 3.38-3.23 (m.3H).2.92 (id.2Ί Ι).2,4S'(s, 3H), 2.33{s,.3H).2.27 (s..3H). 1.32 (d, 6H). MS (El) for CjgWjjFs'NiO':- 522 (MH+).
N-ethyl-2.2-difluoro-N-( { -17-( I H-imidaz0|4.5-b|pyridin-6-yl)^
dihydro- 1 ,4-benzoxazcpin-4(5M)-yl|-6-mcihyl-5-( 1 -niethylethyl)pyrimidin-2- yl I methyl )elhanaiuinc. Ή NMR (400 MHz. CD.,OD) 69.48 (s. Ill), 9.01 (d. IH).8.60 (d. IH), 7.71 (d, IH).7.56(t. IH).6,08(i.1 H);.5J7
;(s.2H).;4.60 (t.2H), 4.24 (s.2H).4.180. 2H), 3v31 Cell.2H).3.19 (m. lH).3:06 (q.2H).2166 (OH).2.29 (s, 3H).1.44 (d, 6Ή).1.11 (m, 311). MS (ES) for
536 (MM*).
6-{4-[2-( 1 -aniinoetliyl)-6-nicihyl-5-( 1 -tiicihylcthyDpyi-imiclin-4-yl |-9-mclhyl-2.3.4.5- tetrahydro-1.4-bcnzoxazepin-7-yl}| 1.3|lluazolo|5,4-b|pyriclin-2-amine. Ή NMR- (400 MHz. de-D SC) δ 8.36 (d.1 H).7.88; (s.2H).7.80 (d. I H).7.47 (s.1 l-l).7:46 (s, 1 H).4.48 (s.2H). 4.31 (t, 2H).3.75 (111, IH).3.72 (t.2H).3.26 (m, IH), 2.47 (s, 311).2.25 (s.3H).1.32 (d.3H). 1.29 (d.3H).1.18 (d.3H). MS (ES) for CMH3lN7OS:490 (MH+).
EXAMPLE 4: 4-[7-{6-Chloro.5-|;(!nelliylsi^
carboxamide
[00862] STEP I : A. mixture of i , 1 -dinietliyleiliyl,9-nicliiyl-7-(4,4.5;5-letiaincthyl- 1 ,3.2- dioxaborolan-2-yl)-2.3-dihydr.o- l ,4 ^^ ( 1 .0 g, 2,5 mmol), -
(5-l Gmo-2rCliloropy idin-3-yl:)inclhaiicsidibn mg, 2.5 mmol), cesium carbonate
diclijorpmeiliane complex (204 mg. 0.25 mniol) in diox ine.(2.4 inLJiand water (600 uL) was stirred at l i b °G for 2 h and was then cooled to n. The mixture was then diluted with water and extracted three times with ethyl acetate. The organic extracts were coinb'iiied. dricd over sodiiim-sulfate. filtered, and concentrated in vacuo. The residue
-gradient silica gel .chromatography ( i 00%-hcxanes ;lo.,5()¾ ciliyl acei to provide
1 , 1 -dimeihylethyl 7 ^( 6-chlofo-5 (m^
dihydro- 1 ,4^bcnzoxazcpiner4(5H =c rboxylatc ( 12 m , 1.52 mmol, 6 ) % yield) as a light brewiv film; 'if NMR (4f)0 MHz, CDCI3) δ 8.39 (d, 1 H). 8, 1 -8.06 (m, 1 H), 7.37-7.18 (m, 2Ή), 6,86 (br s, I H), 4.56-4.43 (m, 2H). 4,09-4.05 (m,
3.09 (s, 3H), 2.33 (d. 3 H),, 1 .44¾1 j H I); MS (Ef) for C
2, I½Cir¾Q5S: 468, 70 (MH*. CI isotopes).
[0086 1 STEP 2: To a solut ion of 1 .1 -dimelhylethyl 7- { 6-chloro-5- I (tiiethyl.siilfonyl)amino lpyridinr3-yl,} -9-nicUhyl-2.3-dihydro- l ,4-bcn/.oxazepiiic- (5H) earboxylale (712· mg, 1 .5 πιηιό1) ίιί nictlianql (5 niL) vvas added hytlrogen .chloride in dioxane (4 M, 3175 niL. fS minol); and the resulting solution w<xs heated to 60 °G -for 3.0· ηϊϋίν After cooling to- r the volatile materials were removed in vacuo to provide N | 2-cliloro-5-(9-
hydrochloride salt in quantitative yield. Ή NMR (400 MHz, DMSO-df)) δ 9.90 (s, .I H). 9.39 (br s, 2H), 8.57 (d, 1 H). 8.Q5 (d, I H), 7;70 (d, 2Η),.4.5 · :ϋ ί (ιη, 4ΐ-Ι). 3 55-3.47 (in, 2H), 3.17 (s, 31-1), 2.30 (s, 31:1); MS (EI) for G , r.l l 1 sCl 30:iS:, 368, 370:(Μ1- ;.Ο isotopes).
100864] STE 3: A mixture of:N-j 2^chloro-5-(9-nielhyl-2,3,4,5-ietrahydro- 1;4- benzoxazepin-7-yl)pyridiii-3-yi |nietlranesiil I¾iiai)iide ( 1 mg, 0.94 mmol), |4-chloro-6- mcthyl-5-( I -meihylethyl)pyrimidin-2-yl Jmclhyl acetate (21 1 ni * 0.94 mmol), and diisoprapylethyiamirie (491 uL. 2.8 iiimol) in NMP (940 uL) was.hcatcd to 120 °C for 16 h before cooling to rt. The mixture was then diluted with water and extracted three times vyith ethyl acetate. The organic extracts were combined, dried over sodium sul fate, filtered, and concentrated in vacuo. The residue was purified by gradient sil ica gel chromatography (100%. iiexanes to 25% liexanes in ethyl acetate) to provide {4-I'7-|6-chloro-5-
|(mellTylsiilfonyl)amiiK)|
mcthyi-5-{ 1 -mcihyIcthyl)pyriniittin-2-y! } methyl acetate (298 mg.0.519 ήιίηοί.55% yield) as a brown oil. Ή NMR (400 MHz. CDCIj) δ 8.40 (d, 1 H).8.14 (d, 111), 7.33 (d, 1 H).7.23 (d, 111), 5.09 (s.2H).4.37 (s.2H).4.32-4.26 (m.21-1), 3.80-3.73 (m.2H).3:43-3.33 (in, 1 H).3.10 (s.3H).2.56 (s.311).2.33. (s.3H).2.18 (s.311), 1.36 (d, 61-1); (EI) for C27l½CIN505S: 574, 576 (MH+, CI isotopes).
1 0865 J STEP 4: To a solution of ( 4-| 7- { -ehl0ro-5-|(nipthylsulfonyl)aniino|pyridin-3- yl )-9-ineihyl-2,3.-dihydiO-1.4-;hcn7.oxazepm
2-yl}
' methyl acetate (298 mg.0
'.
'51 mmol) in methanol (2 mL) was. added aqueous potassium hydroxide (.1 M, 1.56 mL, 1.56 mmol). The solution was stirred at rt for 50 min nd -was then diluted with ethyl. acetate. Brine and saturated aqueous ammonium chloride were :ihcn> added and the;phascs were partitioned. The aqueous layer .was -extracted with ethyl acetate. The combined organic extracts -were dried over sodium sulfate, filtered, arid
' concentrated in vacuo to provide N-(2-chloro-5- {
'4-|2-(hydroxyiiietlvyl)-6-nieth.yl-5-( I -methylethyl)pyrimidin-4-yl |-
mg.0.417 mmol, 80% yield) as a yellow-orange, film: Ί;ί N (400 Mlrl/,.CDCIj) 88. 1 (d, 11-1), 8.L5 (d. IH), 7.32.(d.1H), 7-29 cl. IH). ^6 {s.2H), 4.47 (s, 2H;i, 4:32-4.25 (in.2H), 3.84-3.77 (m.211).3.43-3.29 (m. I H), 3.11 (s.311), 2.56 (s.311), 2.32 (d, 3W).1.38 (d.6H). 1.29-1.20 (m.7H);.(El) for CasI-boClNjOiiS: 532.534 ( H+. CI isotopes).
[00866] STEP 5: A solution of N-(2-chloro-5- { 4-| 2-(hydroxymelhyl)-6-methyl-5-( I - meliiylcthyl)pyrimidin74-yl|-9-ineilvyl-2
^ }pyridih-3- yl)methane.suifonamide (210 mg, 0.3 himol) in djchloromethanc (2 mL) was treated with 1,1.1 -lri(acetyloxy)- 1 , 1 -dihyclro- l 2-benziodoxol-3r( l H)-onc (251 nig, 0.59 mmol ) for 30 miii at it. The mixture was diluted. with dichloromcthanc and washed with aqueous spdium bisulfatc. The aqueous phase was extracted with dichloromcthane. The combined organic extracts were dried over sodium sul ate, filtered, and concentrated in vacuo. The residue was dissolved in /er/-butanol (1.5 mL) and acctoniliilc (500 uL). To this solution were added 2- meihyl-2-butcne ( approximately I mL) and a solution of sodium chlorite (176 mg.1.95 mmol) and potassium dihydrogen phosphate (212 mg, 1.56 mmpl) in water (1.5 mL). The mixture was stirred for 1 h at i t and then water and ethyl acetate were added. An insoluble white precipitate was removed by filtration and was discarded. The filtrate was. partitioned, and the aqueous phase was extracted with ethyl acelatc. The combined organic extracts were dried over sodium sulfate, filtered, and concentrated in vacuo. The residue was purified by gradient sjlica gc| chromatography (100% dichloromethanc to 20% methanol- in
dichloromelhanc) to provide 4-|7- { 6-cldoro-5-|(nieilvy]siill¾V)yl)amiii |pyridiii-3-yl ) -9- meihyl-2,3
'-dihydro-
I -niethyleihyl )p.yrimiditie-2- carboxylic acid ( 136 mg, 0.25 mmol. 64% yield) as a yellow solid. Ή NMR.(400 MHz, DMSO-d
ft) δ 8.13 (hi' s, I H). 7.S7 (s. 1 H). 7.46-7.35 (m. 21 1). 4.44 (s, 21 1). 4.34-4.23 (m. 21 1). 3.80-3.70 (in, 2H), 3.33-3.23 (in, 1 1-1). 2.92 (s. 31 1); 2.53 (s, 3H), 2.26 (s, 3H). 1.34 (d. 6H): ( E I) for. C
'25 i-IigCI NjOsS ; 546, 548 (MH
+ . CI isotopes),
1.008671 STEP 6: To a solution of 4-| 7- { 6-chloro,^
9-meihyl-2,3-dihydro- 1 ;4-l nzoxazepin-4(5H)-yl |-6-methy.K5-( 1 -nic!hylethyl)pyrimidinc-2- carboxylic adid (73 nig, 0.13 riimol) in DMF (400,uL) vas added <¾C ^ bchzoif^ yU NA/ V'-tcirainciliylui niuni hexafluorppk^
ammonia (28-30%. 35 uL. 0.67 mmol ). After stirring 40 min. additional 0-(7- azabcnzoiriazol- 1 -yl)/V,N/V',/V'-teiramelhykiiOnitim hexafluoiOphosphate ( 103 mg. 0.27 mmoI) and ..aqueous ammonia (28-30%. 35 uL, 0.67 mmol) were added. The reaction mixture was stirred a further .1.5 h at it and was then diluted with -aqueous lithium chloride ( 10%). The aqueous solution was extracted twice with ethyl acetate. The coritoine organic extracts were dried over sodium sulfate, filtered, and concentrated in vacuo, The, residue was purified by preparative reverse phase MPLC to provide .4-|7.-{6-chloro-5r.
|(methylsulipnyl)ainino|pyridi;n l\4-bcnzox-azepin-4(5H^'Ij-^ nicilvyl-5-(l-incihylctlvyi)pyriinicliiic-2-carboxam 0X)46 mmol. 35% yield) as a pale yellow solid. Ή R (400 MHz. DMSO-d
fl) δ 9.82 (s, 1 H), 8.55 (s, 1 H), 8.03 (d, 1 H). 7.75 (d, 1 1-1). 7.54 (s. 2-H). 7.50 (d. I H), 4.55 (s. 2Ή). 4.36-4.2S (m. 2H). 3.83-3.75 (m. 2H), 3.31 -3.21 (m. I H). 3.17 (s, 3H). 2:53 (s, 3H), 2:25 (s, 3Ή), 1.33 (
'd; 6H): MS (EI)Tor
547 (MH
+. CI isotopes).
00868J Proceeding according to the method of Example 4 and replacing.ammonia in step 6 with N,/V-dimediylcthylenediamine the following compound of the inventioii .was 'prepared:
.4-|7-.{ 6-Cldoro-5-|(melhylsulfoiiyl)amiiK)]pyridin-3-yl ) -9-m
bcnzoxazepin-4(5H)-yl |-N-|2-:(dinicthylamino)cihyU
2-ciirboxamide. Ή NMR (400 MHz. DMSO-d0) δ 8:39 (t. 1 H). 8.29 (s. 1 H), 7.94 (d, I H). 7.51 -7.42 (m, 2H), 4.55 (s, 2H). 4.36-4.29 (m, 2H), 3.85-3.74 .(in. 211). 3.37-3.22 (m. 311). 2.99 (s. 311). 2.61 -2.53 (in. 514), 2.34 (s, 6H). 2.25 (s, 3H). 1.34 (d; 61-1); MS (EI) for C:9¾.sCIN70.jS: 616. 618 (MH*S CI isotopes).
[00869] Proceeding according to the method of Example 4 and rcplaemg,|4-chlpro-6- mcthyl-5^( 1 -inethylcihyl)pyriniidin-2-yl|mclhyl acetate in step 3 with alternative reagents and omission of steps 4-6. the following compounds of the invention were prepared:
N-(;5-J -l2-Aniiiu^6-niotliyl-5-i l-nictliylc iyl)pyrniiidin-4-yli-9-mctlryl-2.3. ?5- tciraliydro- l,,4- cn.xoxazcpin-7-yl }.-2-c loropyriclin-3.-yl)nicllKincsLiironaiiiidc. ?'H NMR (400 MHz, DMSO-dfi) 89 4t rs. Ill), 8.49 (s. III). Si00:(d, IH), 7.52 (d. ΙΉ), 7.39 (d, IH), 6.08 (br s.2H).4.32-4.19 (m.41-1), 36.|-3.49 (m, 2Ή),·3\2'3·-3·.07 (m; 4H).¾.30'(s, 3H).2.28 (s, 31-1).1.25 (d.611) MS (E ) lor C¾¾eiN60,S:5l7~51 (; I-I* CI isotopes).
N-(5-(4r|2-Amino-5^(lniluoronielhyl)pyrimi(liiv-4-ylJ
I,4-bcnzoxa/.cpii 7-yU-2-chloropyridm Ή NMR (400 MHz.
DMSO-dr,): 9.81 (s. IH).8.19 (s.2H). S.00 (s. IH).7.57 (s. IH), 7.43 (s, IH), 7.11 -6.62 (m. 2W), 4.83 (s, 2H):.4.29 ,(s.211).3.89 (s.2H), 3.13 (s.3H), 2.22 (s, 3M); MS.(El) fof
£v H2ofiF3 ri03Sr529 (Μ1-Γ).
N-(5-{4-|:4-Amiiio-5-(triniioroiTiell)yl
I.4-benzqxazcpin-7-yl:h2-eM 1 H NMR (400 MHz.
D SO^do):^. 1.(s, 1¾ 8.44 (s. lH).;8:()8 ;(s^l H), 7.96 (s, IH); 7ΐ67 ψ, IH). 0 (s, I H), 7. IS - 6.71 (m.2H).4.S0 (s.2H).4.12 (d. H).3:07 (s.3.11).2.23 (s.311): MS (El) for
2-17-{6-Ghloro^5-j(nicl yKsuironyl)aiiiino|pyritliii-3-y! } -9-mel yl-2,3-dihydio- 1.4- benzoxa2epin-4(5H)÷yl|-N^netliy -(l-m Ή NMR
(400 MHz. DMSO-d6) 68.35 (s.111).7.95 (1. Ill), ΊΑΊ :(dd, 4Η),:4·73 (s, 2H).4.19 (d.2H). 4.01 (s, 2H).3.62 (m.1Ή).3.05 (s.3H).2.62 (d.3H), 2.24 (s.3H).1.08 (d.6H): MS (EI) for
2-[7-{'6-Cbloroi54i(nictbyisulfonyl)aniiiiojpyri
bciv/.oxazepiiv4(5H)-yij-N-cthyl-4-(]-nictliylcthyl)r
(400: MHz. DMSO-d6) δ 9.84 (s. I H).8.48 (d, I H), 7.97 (cK 1 I I) , 7.49 (t, 3H).4.68 (d.2H). 4.20 (s, 21-1).3.95 (d.2H).3.59 (in, 1 H).3.12 (m.5H), 2.25 (s.3H).1.09 (d, 6H).1.01 (t.3H); MS (EI) foi Qd-IjoCI sO-LS,: 564.1 (MH4).
N-{5-(4-(4-Amino,-5-eyanopyrimidi^^^
bcnzoxazcpin- 7-yl|-2-chloropyi-idiii-3-yi [iTieihaiicsuIfonamidc. Ή NM (400 MHz. DMSO- de): 9;82( . IH).8.51 (d, I I I).8.25 (d. lH)„8.00 (d. I H).7.63 (s. IH).7.37 id, 3H).4.87 (d. 2I-D..4.1.3 (d.4H).3.15 (cl, 3 I I >, 2.23 (s.3H); MS (El) l' r C:) FyCI vCbS: 486.1 ,(MI-f).
[00870] Proceeding according to the method of example 4 and replacin N-(54iromo-2- chloropyridin-3-yl)meihancsulfonaniidc in step 1 wilh alternative reagents and |4-chloro-6- methyl-5-( l-mclhylcihyl)pyrimidin-2-yl|incthyl acetate in step 3 with alternative reagents and omission of steps 4-6. the following compounds o ihc invention were prepared:
2-Amino-5-{4-|2-annrio-6Miielh^
2,3,4.5-tcirahydm-l,4-bcnzoxazc|)iiir7-yl}'pyri(linc-3 sulfG Ή NMR (400 MHz,
DMSO-d
6) δ 8.44 fcl. I H).8.07 (d. I H).7.48 (br s.2H), 7.36 (d. I I I ).7.24 (d. I 14), 6.61 (br s. 211).6.01 (s, 211), 4.26-4.15 (m.411).3.57-3.47 (in.2H), 3.25-3.14 (in, | H).2.30 (s, 3Ή), 2.26 (s.3H).1.25 (cl.6I-I): MS (El) for
484 (MH
+).
Ν^(5 {4-|2-Αηιίηρ-6 ΐηδΐΙιγ 5÷(;ί.-ιη^
tciraliydro-l^bcnzoxazepiri-J^yl jiyridin-3-yl)inciluniesiilf0na^nide; 'H NMR (400 MHz. DMSO-d6) 68.57 (s. IH), 8,37 (s. IH).7,77 (s, I H).7,47. (s,, 111).7.34.(s, lH).5.96.(d, IH), 4.24 (a.4H), 3.52 (d
; 211), 3.10 (s.3H).2.29 (d, 6H).1.26 (d.6H): MS (EI) lor
483.2 (Ml-O.
N-(5-{4-|2-Aininb-6-metliylT5-(l-^
tcirahydro- 1 ,4-bciizoxazepin-7-yl,) -2-hydroxypyi diiv-3-yl)nieiliancsultonanii ' H NMR (400 MHz, DMSO-d6) δ 12.2 (s, 1 I I).8.79 (d, I II), 7.64 (t. HI), 7.43 (d. IH), 7.30 (d. IH). 7.17 (d. IH), 6.20 (bs.2H), 4.24 (d, 414).3.57 (s, 2H), 316 (ni, 1 H), 3. 1 (s, 3H), 2.31 (s, H).2.
ieualvydrQ , - ^^ Ή NMR (400 MHz, 'DMSO,d6)-69,33 (d, 1Ή), 8.27 (t.114), 7.82 (t. ΓΗ), 7.40 (in, 311), 4,67 (s,. 2I-D.4.36 (d, 2H).3.96 (s.3H), 3.86 (s, 2H).3:07 (s.314), 3.00 (in, I H).2.37 (s.314).2.24 (s. 3H).1.27 (d.6H): MS (EI) for C^I- . f.O S: 13.2 (MH+):
4-{ 7 4-( rH-Fmidazol-2-yljphcnyIJ-9-niel yi-2.3÷diliydiO- 14- enzo azcpin-4(5H)- yl )-6-mcthyl-5-( 1 -iiiclliylei]iyi)pyriinidiii-2-aininc,
1 I I NMR (400 MHz, DMS.Q-d6) δ 7.97 (d, 2M)/7:67 (d.2H).7.49 (s. IH).7.36 (s. IH), 7.13 (bs.2H).6÷00:(s„214).4,22 (s.4H).3.51 (S.2H).3.20 (in. I H), 2.26 (I.614).1.23 (d.61-1): MS (EI) for C
27H;,(.N
f,0: 455.2 (ΜΙ- ).
teiraIiydiO-l-,445cnzoxazepiii-7-yl } -2-clilor0 "yridinV3-yI)- 1,1. | -irinubrQiiieUKincsuironumidc. Ή NMR (400 MHz. DMSO-d6) δ 8.01 (l, IH).7.81 (i, 1 H).7.33 (d.2H).7.14 (bs.114).4.63 (s, 214), 4.35 (s.2H).3.80 (d, 2H).2.99 (clt. I H).2.37 (d, 314).2.22 (s.3H), 1.26 (d.6H); MS (El) forC2.iH2f,CIF;,N60.-iS: 571.1 (Ml-I+).
4-(6-Iodoc|uiiiazoliii-4-yl)-9-inethylr7-(2-nieiliyl-lHrbenziniidazol-6-yl)
ieiral ydro-l,4,bcnzoxazepine. Ή NMR (400 MHz, nieUiaiipl-d:,): 8.57 (s, I H), 8,44:(d.114), 8.04 (dd, IH).7.8;l (s, IH), 7.57-7:45 (m,.5H).5;00 (s, 2H);-4,5.1 ¾IW,.2H|, 4.23 (in, 214), 2.58 (s, 314).2.34 (s.314).1.96 (s.3H); MS (El) for C^r.l-MNsO: 547 (MH+).
4-| 7-(2^nnino| 1 ,3 jiliiazolol 5^
bcir/.oxazepin-4(5H)-ylj-N-ei!iyl-6-nicihyl-5-( ^^^l-nicihyleihyI)pyrimidinc-2-earboxamidc. Ή NMR (400 MHz. d6-DMSO): 8.40 (d. I I-I).8.25 (I.1 H).7.87 (s.2H), 7;84 (d, 1 H).7.57 (d. I H).7.50 (d, I H), 4.59' (s.21-1), 4.35-4.28 (m.21-1), 3.82-3.76 (m.2H).3.31 -3.23 (m.2H). 3.17-3.08 (in, I H), 2.53 (s.31:1), 2.24 (s, 311). I.33,(d, 611).0.93 (t, 3H). MS (EI) for
C27H31N702S: 518 (MH+).
N-(2- hl i -5-(4-|2-{|(2-niK)roclhyl)ainiiK)|niclliyl}-6-melhyl-5-(l- mciliylcthyl)pyi'imidin-4-yl |-9-mclhyI-2. 5-tetrahydro- 1 ,4-benzoxazepin-7-yi }pyridin-3- yl)melhane.suli namidc. Ή NMR (400 MHz. CDjOD) δ 8.39 (d, 1 H), 8.13 (cU IH), 7.41 (s. 1 I I), 7.41 (s. ITI), 4.60 (s.2H), 4.50 (dt.2H).4.35 (i , 21-1).3.85 (in, 3 I I).3.84 (s, 3H), 3.35 (in, 11-1), 3.08 (s, 3H).2,94 (hi.2H).2,54 (s, 3H¾ 2.29 (Sv3H), 1.39 (d, 6Ή). MS (ES) for C27 H.«C 1 FNf,OiS : 577 ( H+) .
N-(2-chloro-5.-{4-|2-{|(2.2-dinuorocdi>4)amino|inel y
mclhylcthyl)pyrimidm-4-yl |-9-mcthyl-2,3,4,5-teirahydro- 1 ,4-bcnzoxazepin-7-yl}pyriclin-3- yl)mclluuiesul onamide. Ή NMR (400 MHz. CDjOD) δ 8.48 (d. IH).8.17 (d. I H).7.50 (s. I H).7.50 (s. I H").6.2 (t, 1 H), 5.04 (s.21-1).4.57 (Ί.211), 4.50 (s.2H), 4.19 (1, 211), 3.64 (dd. 211), 3.19 (in, IH), 3.13 (s,.3H).2.66 (s, 311), 2.30.(s.3H), 1.43 (d, 6H). MS (ES) for
C27HMClF2 flO.-)S: 596 (MH+).
EXAMPLE'S: 6-(9-Mcthyl-4-r;6-methyI-5-(l-nrclhylcthyl).2-(mcthylsuIf
4-yI|-2,3,4,5-tetraIiydr<Kl,4-ben/^
101)871] STEP 1 : Sodium metal (640 mg.27.8 mmol ) was added to ethanoi (40 inL) and was stirred at rt until it completely reacted to form a sodium ethoxi.de .solution. Ethyl 2- acciyl-3-methylbuianoatc (2.0.8 mL. I 1.6 mmol) and thiourea (1.06 g, 14 mmol) were then added. The resulting reaction mixture was heated 1080 °C and stirred for 6 h before being cooled to rt. The volatile materials were removed iii vacuo: The residue Was dissolved in water which was subsequently acidified by addition of acetic aeid. The while precipitate that formed was isolated by filtration and then dried in vacuo to provide 6-meihyl-5-(l- methylethyl)-2-thioxo-2,3-dihydropyrimidin-4(lH)-one (1.19 g, 6.46 mmol.56% yield.) as a white crystalline solid. Ή NMR (400 MHz, DMSO-dfl) δ 12.29-11.88 (in.2H).2.89-2.77 (m. IH).2;13 (s, 3H), 1.16 (d.6H); (EI) for CKl-l|.N2OS: 185 (ΜΙ-Γ).
1008721 STEP 2: To a solution of 6-mcthyl-5-( I -mcthylethyl)-2-thioxo-2,3- dihydropyrimidin-4(lH)-one (1.19 g.6.46 mmol) in D F (7 mL) was added iodamelhanc (811 uL, .13' mmol), and the resulting mixture was stirred at rt for 90 min. The mixture was
then diluted with 1.0%;ac|iieoiis liihiuit chloi idc and extracted twice with ethyl acetate. The organic extracts were combined, washed oiicc with 10% aqueous lithium chloride, dried over sodium sulfate, filtered, and conceniraicsd in vacuo to provide 6-meih"yl-5-(l Miicthylcthyl)-2- (mcthylthio)pyrimidin-4(3H)-one (1.11 g, 5.6 mmol, 86% yield) as. a pale yellow solid. Ή NMR (400 MHz, CDCh) δ 11.73 (s.1 I I), 3.09-2.96 (m, 11:1).2,5.6 (s, 311).2.32 (s.3H).1.31 (d, 6H); (EI) for Cyl l H2QS 1 9 (ΜΙ-Γ).
(1.11 g„5;6 mmol) was adcled clilOrofoim (8 mL) and phosplVoais.oxychloride (8 mL), and the resulling inixliirc was healed to 70. °C for .45 min. After cooling to rt, the mixture was concentrated in vacuo. The residue was dilulcd.with dieliloromcthanc arid then washed with saturated aqueous sodium bicarbonate. The aqueous wash was extracted with
dichloromcihane. The organic extracts were combined, dried over sodium sulfate, filtered, and concentrated in vacuo to provide 4-chloiO-6-meihyl-5- l-methyleihyl).-2- (methyllh'io)pyri idine (ί '.20-:g,.'5.55: imnoiv¾9% yield) as a ycl low oil. 'T MR (400 MHz. CDC ) S 3.51-3.39 (m, l H), 2.5=7-2.51 (m, 6H).1,36 (d: 6H); (El) for CyHi:,CIN2OS: 217. 2I9(MH+.CI isotopes):
100874J STEP 4: .A. jxtufe;-o.f^(9^iicihy ;4-bcnzoxa'zepin-7- yl)| l.3|lhiazoIo|5.4-b|pyridin-2-amiiie hydrochloride (400 mg; 1.04 m iol).4-chloro-6- mcihyl-5-( 1 -inethylethyl)-2-;(nVeihylthi(>)pyriniidinc (226 mg, 1.04 mmol), -and
diisopropyicthylamine (724 uL.4,16 mmol) in NMP (2 mL) was heated to 120 °G for 18 h then cooled to it. The mixture was theu diluted with water and extracted several times, with 10% methanol in ethyl acetate; The organic extracts were combined, dried over sodium sulfate, filtered, and concentrated in vacuo. The residue was purified b gradient silica gel chromatography (100% dichlorometharie to 10% methanol in dichloromeihane) to provide 70%. pure 6-.{ 9-methyl-4-|;6-riielhyl-5-( t.-mcth"y]clliyl)-2-('tncthylthip)pyriiniclin-4-yr|-2.3.4-.5- lelrahydro- 1 ,4-benzoxazepin-7-yl }| 1.3|thiazoIo|5,4-b|pyridin-2-amine (235 nig, 0.47 mmol, 46% yield) as a viscous brovvii syrup. Ή NMR (.400 MHz, CDC1. δ 8:42 (d, 1 H).7.83 (d, I H), 7.34 (d, 1H), 7.26. (1, 1 H).5,66 (b s. I IT).4.41 (s.2H), 4.35-4.27 (m.2H), 3.85-3.74 (m, 21-1).3.37-3.26 (m, I H), 2.50 (s,3H).2.44 (s, 3H), 2.34 (s, 311).1.36 (tl, 611); (EI) for
C2sH2s (i05S2: 493 (ΜΙ-Γ).
|(H)875] STEP 5: To a solutioh of 6-{9-mclliyl-4-|6-incihyl-5-( l -mcthyleihyl)-2-
(iiicthyltIiio)pyrimidin-4-ylj-2,3;,4,5-teir;ihydro-l,4-bcivzoxazcpin-7-ylH
b|pyridin-2-amine(235 mg, 0476 mmol) in dichloronielhane (5 mL) was added 3- chloroperbenzoic acid (207 mg, 1.2 mmol). The mixture was stirred for 1 h at n and then
was diluted willi dichloromethane. The organic mixture was washed. ith: aqueous saturated sodium bicarbonate. The aqueous wash was extracted with dichlqromclhane. The combined organic extracts were dried over sodium sulfate, filtered, and concentrated in -vacuo-. A portion of the residue was purified by gradient silica gel chromatography ( 100% hcxanes to 100% ethyl acetate) t provide 6- ( 9-mcihyl-4-|6-mcthyl-5-( ί -niethyleihyl)-2-
b |pyridin-2-atninc as a yellow solid. Ή NM R (400 MHz, D SO-d 5 8.38 (d, I H), 7.86 (s, 2H), 7.83 (d. I H), 7,5 1 (s, 1 H), 7.48 (s, I H), 4.68 (s, 2H), 4:4'N4.3'4 (m, 21-1), 3.87-3.79 (m. 2H), 3.29-3, Ι 8. (ηκ I H). 3:07 -:(ss,3.H).-.2 56 (s, 3Ή), 2:23 (s. 3H>, 1 .35 (d; 6H); MS (El) for eisHaeNoPi'Sit 525 ( ΜΙ- ).
100876] Proceeding accordingjto the method of example 5; the following compound -of Ulc invention .was prepared:
6- { 9-methyl-4-|6-mcthyl-5-( I -nVclhylelhyl)-2T(inethylsulfinyl)pyrimidin-4-yl |- 2,3 ,4,5-lclrahydro- l .4-benzoxazcpin-7-yl j| l ,3 |thiazolo[ 5.4-lr|pyridin-2^amme. Ή NMR (400 MHz, mclhano.l-d.,): 8.37 (cl. 1 H). 7.84 (d. I H). 7.45 (d. I H ). 7.39 (d. I H). 4.70 (m, 2H). 4.37 (m. 2H). 3.91 (m. 2 H), 2.66 (s. 3 H). 2.60 (s. 3H), 2.28 (s. 31 1). 1 . 1. (m. 6H). MS (EI) for C3.5-H2sNftO.2S2·: 509 (MH+).
EXAMPLE; 6': ^
dimethylet hane-l,2-diamine
[008771 STEP I : A solution of 6-{ 9-methyl-4-[ 6-methyl-5-( hnicihylelhyl)-2- (nieUiylsuli nyl)pyrimidin-4-yl |-2.3.4.5-tctrahydro- 1 i4-benzoxazcpin-7-yl } [ 13 )thiazoIo| 5,4- b'lpyridin-2-amine ( 100 mg, 0. 19,mmol) in AW-dimcthyleihyicricdiam'ine ( 1 riiL) was heated to 100 °C for 2 h. After cooling. to r(. the. volatile materials were removed in vacuo. The reisduc was purified by preparative reverse phase HPLC to provide Ν'-{4-| -(2- .
aniinoj 1 ,3]tliiazold| 5,4-b|pyridiiv-6-yl)-9-i.neth
meihyl-5-( 1 -mcthyle!hyi)pyri.mid'm-2-yl } - V,A'-dimeihylethanc- 1 .2-diamiric (35.0 mg. 0.066 mmol. 35% yield) as a white powder. Ή NMR (400 MHz. DMSO-dr.) δ S.34 (d. 1 H), 7.87 (s, 2H), 7.78 (d, I l-l), 7.48 (s, I H), 7.39 (s. 1 H), 6.24-6. 1 (m, I H). 4,33 (br s. 2H), 4.29-4.23 (m. 2H), 3.64-3.55 ( m. 2H). 3.24-3. 1 I (m. 3H). 2.29 (s, 3H). 2.27-2.22 (m, 5H), 2.09 (s. 61-1 ), 1.26 (d, 6H); MS (EI) for QsH f,N.sOS: 533 ( Ι- ).
[00878] Proceeding according io the method of Example 6 and replacing /V,N- dimcihylethylcnediamine with mclhyiamine the · following compound of' the invention was prepared:
6-{ 9-n lhyl-4-r6-methyl-2-;(nielhylanm
tctrahydro- 1 ,4-bcnzoxazcpin-7-yl ) 1 1 , |thia7.olo|5,4^b|pyriclin-2-am.ine. 1 I I NM (400 MHz, DMSO-do) δ 8.34 (d, I I I), 7.87 (s. 211). 7.77 (d. 1 H), 7.49 (d; I I I). 7.3 (d. 1 H), 6.40 (cj, I H), 4.32 (s. 21 1). 4.30-4.1 (m. 2H), 3.68-3.50 (m, 211), 3.26^3.09 (in, I l-l), 2.66 (d, 31 1). 2,30 (s, 31 1). 2.26 (s, 3H), 1.26 (d, 6H): MS (El) for Cji HijN^OS :.476 ( Ι- ):
1 - l)pyri
.iiiicIin-4- yl 1-2.3.4,5-teirahy.diO- 1 ,4-benzqxazepin-7-yl ] | 1.3]thiazoloI5.4-b|pyridin-2-amine.
1 H NMR (400 MHz. tncihanol-d.,): 8.34 (d. 1 l-l), 7;S 1 (d. 1 H). 7.38 (in, 2H). 462 fs, 211), 4.50 (s, 211). 4.30 (m. 211), 3.74 (m, 211). 3.62 (m. 4H). 2.40 (m, 411), 2.38 (s. 31 1). 2.3 1 (s, 3H), 2.29 (s. 3H), 1 .34 (d, 6H). MS (El) for CA'NxOS: 545 (MH*).
l - { 4-| 7-(2-a!ninp| I;3.|!i)ia/.olo| 5,4-b|pyridn
ben/.oxazcpin-4(5,W:)-yl|-6-nietli^ ' H NMR:
(400 MHz. CD;,OD) δ.8,35 (d, 1 H). 7.82 ( l. 1 H), 7.44 (s. I I I). 7.38 (s, I H ). 4. 1 (s, 2H). 4.83 (m. 1 I I), 4.50 (m. 3H), 4.24 (nr. 21 1). 4.01 (m. 2H), 3.80 (m. 211). 3J 2 (m. 1 H). 2.47 (s. 3H). 2.26 (s. 311). 1.39 (d, 6H). MS (ES) for
(Μΐ ).
EXAMPLE 7: 4-t7-(5-nmiiio-l,3,4 iiiadiaz^
bei>zox zepii]-4(5//)->^]-6-mc
[00879] STEP 1 : A .mix ture of 1:, I -dinicthylcthyU7Hiromo-9-nielhyl-2,3-dihydro- 1 ,4- I. iizoxazepiiie-4(5H)-carboxylate, (4.90 g, 14.92 mniol) and zinc cyanide ( 1.76 g, 14.92 mmol) in .N. -dimethyfo namide (30 mL), was degassed with nitrogen then.
terakis(triphcnylphosphinc)palladiiim(0) (0.86 g, 0.75 mmol) was added to the mixture and it was heated at 85 °C for 2.5 hours. After cooling to room temperature the reaction mixture was partitioned between water ( 100 niL) and ethyl acetate (300 mL). The organic layer was separated washed with water (2x 150 mL) and brine, dried over anhydrous magnesium sulfate then filtered and concentrated. Gradient silica.gcl column chroma graphy (hexanc:ethyl acetate 99: 1 to 9:1 ) provided l , l ^dimelhylethyl-7-cyaiio-9-mcthyl-2>3-dih.ydro- l ,4- bcirzoxazepine-4(5 -/)-carboxylatc (4.02 g, 93%). MS (EI) for Ο,Λ1½Ν20;,: 232 (M-l13u*).
[008801 STEP 2: A mixture of l, l-dinie.lhylcthylr7-cyatib-9-nicih:yl-2.3-dihydro- 1 ,4- bcnzoxazcpinc-4(5/f)-carboxylatc.(2.3 g, 7.98 mmol) and ihtoscmicarbazidc (0.76 g, 8.38 mmol) in irifluoroacetic acid (20 mL) was heated to reflux-, for 6 hours. A ter cooling to room
icmperaturcihe reaction mixture was concentrated then taken into 1,4-dibxane aiid concentrated (3x 50 mL) to give crude 5-Cy-niethyl-2; T3, ,5 tetralvydiO.-l,4-benzoxazepin-7- yl)- i ,3,4-tlviatlia7.ol-2-amine. MS (ΈΙ) for Gr2ll|:, .i0S: 263 (ΜΙ- ). To a.solution of 5-(9-. nicihyl-2.3.4,5-letraliydi - 1.4-benzoxazcpin-7-yl)- 1.•3.4-lhiadia/.ol-'2-aininc as obtained above in a mixture of water (70 mL) and lctrahyroluran ( lO.mL) was added 2M aqueous sodium hydroxide (20 mL, 40 mmol) and the reaction mixture was cooled to 0 °C, followed by the addition pf'di-/e /i-biilyldicarbonate ( 1 2 a.8.7 mmol) then stirred at room
.temperature for I.8 liours. The reaction
'mixture; was^in titiohecl between water (200 mL) and •clhyl acetate (^^--mLX/Thcor^anicla er-wa!!i separatcd-washed' with vvatef.(250 mL) and brine then dried over anhydrous magnesium sulfate. I
'iUered and concentrated. Gradient, column chromatography (hexane:eiliyl acetate 9:1 to 3:2) provided I.1 -dimetIiyletliiyl-7-(5- amina- 13.4-ihiadiazol-2-yl)-9-meth.y!-2.3-dihydro- 1 ;4-bLMizpxazcpinc 4(
'5./¾
icarbox
'ylat
'e (1.22 g, 41%). ' I-! NMR (400 l-Iz. D SO-df,): 7.42 brs, (2IT), 7.3 (s, 21-Γ .42 (brs.2H). 4.02 (ni 2Η·) 3.72 (brs.2H).2.22(s.3H).1.35 (s.9H): MS (El) for
363 (MH
+).
100881] STEP 3: A solui ion of 1.1 -dimcihyleihyl-7-(5-amino- 1.3.4-thiacIi:r.ol-2-yl)-9- mL4hyl-2 -dihydi;o-l^ mmol) in a mixture of methanol (25 mL) ami 4N liydrochloric acid in 1.4-dioxanc (5 mL) was rcflii cdfoi: 30 minutes. After cooling to room temperature :die reaction mixture was .concentrated and the precipitate was collected by filtration, washed-with ethyl acetate and hcxanes then dried in vacuo to give 5-(9-meihyl-2,3,4!5-ictrahycliO- 1 ,4 :>enzoxazepin-7-yI)- l.,3,4Hhiadiazol-2- aminedihydrochloridc sail (1.0 g,94%) as a white solid. 'H N R (400 MHzvDMSO-df,): 9.71 (s, 2H).7.78 (s, 1 H), 7.68 is, I H).4.38 (brs.2H).4.24 (m.2H), 3.45 (brs.2H).2.24 (s. 3H): MS (El) for C|2HMN4OS: 263 (ΜΙ-Γ).
(0.0882] STEP 4: A mixture of
'5-(9 i)cthyl-2,3.4.5-tcirahy(liO- l,4-benzoxazcpin-7-yl)- 1.3,4:lhiadiazol-2-aniine dihydrochloridc salt (0.22 g, 0.70 mmoi)..4-.chloro-6-meihyl-5-(l- incthylethyl)pyriniidin-2-aniiiie
' (0.12 g, 0.65 mmol) aiid N.N-diisopropylethylaminc (0.60 mL.3.5 mmol) in I-mcthyl-2-pyrroiidinone (2 mL) was heated t 110
qC for 18 hours. After cooling to room temperature the reaction mixture was diluted with methanol (6 mL) and water (4 mL) and the pH was adjusted lo 5 by the addition of glacial acetic acid then purified by preparative reverse phase HPLC (0.1 '¾ aqueous -ammonium acetate and acetonitrile mobile phase). Product fractions were concentrated and the residue was partitioned between 2M aqueous sodium hydroxide (100 mL) and. ethyl -acetate (250 mL). The organic layer was separated washed with 2M aqueous sodium hydroxide (100 mL) and brine, dried over
anhydrous magnesium sulfate then filtered and concentrated. The residue was dissolved in ethanol (20 mL) and concentrated aqueous hydrochloric acid (1.0 niL) was added and the solvent was partially concentrated. The solid precipitate was collected by filtration washed with ethyl, acetate and hcxanes then dried in vacuo to give 4-|7-
'(5-amino-
2-aminc: hydrochloride (78 mg; 27%). Ή N R (400 MHz, methanol-d.,):.7.67 (d. IH), 7.54 (d, 11-1), 4.84 (s, 2H), 4.50 (m.2Η), 4.02 (m.2H).3.0l.(m. I H).2:44 (s.3Η).2;25 (s.3H). 1.34 (d..6H). MS (El) for C2oI½NvOS: 412 (Mi l*),
00883 j Proceeding according to the method of example 7 and replacement of 4-chloro-6- melhyl-5-(l-methyleil]yl)pyriniidin-2-aminc with alternative reagents the following compounds of die/invention were prepared:
5-(4-|2-{|(2.2-dinuoroethyl)(inclhyl)anm^
1 H- NMR (400 M Hz, d
fl-DMSO) : 7.50 (clyi H).7:47 (d.1HJ,73?;(s,; H), 6.03 (tt. I H), 4;.?9 (s, 2 H), 4.35-4.28 (m.2H).3,72-3:66 (m.21-1), 3.64 (s.2H), 3.27-3.15 (m. 11¾.2.91 (id, 2H), 2.47 (s, 3Hi), 2.32 (s.3H), 222;(s.31:1), f.3 (d.6H)> MS (El) for C2-iH
3|F
2N
70S: 504 (Μ1-Γ).
5- (4-| 2-{| (2,2-dinuoroeihyl)amino |niethyl }--6-nicth*yl-5-^C 1 -nielli lcthyl)pyrimidin-4- yl |-9-methyl-2,3,4,5-ietrahydro- 1 ,4-benzoxazepin-7-yl |- 1 ,3,4-diiadiazol-2-aminc.1 II NMR (400 MHz. CD3OD) δ 7.78 (s, IH), 7.61 (s, IH), 6.34 (l, I H).5.01 (s.2H).4.60 (d.2H).4.44 (s, 2H).4.14 (t, 21-1).3,62 (m.2H), 3.14 (m. IH), 2.64 (s,3H), 2.27 (s, 3Ή), 1.41 (d, 6H). MS (ES) fx)rC?3H¾F.N7QS::490 (ΜΙ-Γ).
5- ( ^13-^ |:(2,2-<liftiioroetHyl)(etliyl)aniiii6]m - meUiyleihyl)pynmidin-4-yr|-9-mclhyl-2.¾4,5-ieiralv
thiadiazol-2-amine. Ή NMR (400 MHz. CD3OD) δ 7.79 (s, IH).7.58 (s. I H).6.07 (l. Ill), 5.12 (s, 2I-D.4.6I (Ί, 2H).4.15 (t, 2H).4.I3 (s, 2H), 3.19 (m, 2H), 3.15 (m.1H).2.98 (q.2H), 2.65 (s.3H), 2.23 (s.311), 1.43 (d.611).1.11 (t, 3H). MS (ES) for CisI-l iFi OS: 518 (ΜΙ-Γ).
N-elhyl-2,2-cliniK>ro-N-((4-|7-(H-I-imidazo
yl) methyl )elhanamine. 1 II NMR (400 MHz. CD3OD) δ 9.48 (s, IH); 9.01 (d. III).8.60 (cl. IH).7. 1 (d,.lH),7.56 (1, IH), 6.08 (l, I I I).5.17 (s, 2H), 4.60 (t, 2Η),·4.24 (s, 2H).4.1 (t. 211).3.31 (dt.2H), 3.19 (m. IH), 3.06 (q.2H).2.66 (s.3H).2.29 (s, 3H).1,44 (d, 6H).1.11 (m,3H). MS.(ES) for Ο^.,Η^Ε,^Ο: 536 (MH+).
5- {4-| 2,6-climethyl-5-( I -inciliyleihyl)pyriniidin-4-yl |-9-niclhyl-2.3.4,5-lcirahydro- 1 ,4-benzoxazcpiii-7-yl I - 1 ,3.4-diiadiazol-2-amiiie. 1 I I NMR (400 MHz. mcthano.l-di : 7.53 (in, 2H).4.47 (s.2H).4.32 (in, 2H), 3.81 (m.21-1).2.50 (x.3H), 2.40 (s.31-1).2.27 (s, 3H), 1.36 (d, 611). MS (El) lor C;iH-.6N(lOS: 411 ( i l').
5-{.9-inethyl-4'-|6-iiiei yl-5-( t -nicthylcih>^)pyiuiiidiii-4-.yV]-2,3.4.5-tctt-ahydro- 1.4- benm\azcpin-7-yl|-l.3,4-ihiadiazol-2-aniinc. Ή N (4(H) MHz, melluinol-cl.,): 8.35 (s. IH).7.52 (m.2H).4.47 (s.2H).4.3:5 ("ni.21-1).3.81 (m.2H).2.54 (s, 3H).2.28 (s.3H).1.37 (d, 6H). MS (EI) lor C. HyNftOS: 397 (ΜΗ').
5-|4-(2,5-dimediylpyrimidin-4-yl)-9-n^
yl|-l,3.4-diiadiazol-2-aminc. 1 H NMR (4.00 MHz. mclhanol-d.,): 7.99 (s. IH), 7.76 (d, IH), 7.50 (d, 11-1).5.14 (s.2H).4.42 (m.4H), 2.57 (s, 111), 2.42 (s, 3H).2.25 (s.3H). MS (EI) lor CiSH.2oNi,OS: 369 (ΜΙ-Γ).
-netli leCliyl)pyriiiiidin-4-.yl j 2.3.4,5
'-icirahytlr0- 1.4- berizoxazcpuv7-yl}-1.3.4-ihiadi zol-2-ainin^ Ή NMR (400 MHz, melhanol-d.:): 8.16 (s, IH).7.55 (d, IH), 7.50 (d, I H).4.64 fs, 2H).4,34 (m, 2H).3.94 (in, 211).2. 1 (m. IH).2.41 (s.3Ή).2.26 (s.3H).1.26 (d.61-1). MS (El) for
(ΜΙ-Γ).
5-|4-(5,6-dinicdiylpyriiindin-4-yl)-9-m
yl|-l,3.4-ihiadiazol-2-aminc. Ή NMR (400 MHz. medianol-di): 8.29,(s, IH), 7.52 (d, IH). 7.46 (d, IH), 4.65 (s.2H), 4.36 (m, 2H).3,92 (in.2H), 2.39 (s, 3H), 2.26 (s.3H).2.24 (s, 3H MS (EI) for CisHjo f.OS: 369 (MH+). ·
5-{9-nicthyl-445-(I-mcthylcil^
benzoxazepin-7-yl]-1.3.4-lhiadiazoIr2-aininc. Ή NMR (400 MHz, meUianol-d.|): 8.42 (s. Ill), 8.30 (s, IH), 7.51 (m.21-1), 4.67 (s, 2H).4.39 (in.2H), 3.94 (m, 2H), 3.14 (m, I H), 2.26 (s, 3H), 1,29 (d.6H). MS (EI) for
383 (Μΐ ).
4-[7-(5-amiiio-l.3.4-ihiadiazol-2-yr)-9-mediyl^
yl|-5-mcihylpyrimidin-2-amine. Ή NMR (400 MHz. mctlianol-d.,): 7.66 (d. IH).7.56 (s. 111).7.42 (d. III).4.83 (s.2H).4.32 (in.2H), 4.13 (in.211).2.26 (s, 3H), 2.21 (s, 3H).1.95 (s, 3H).
MS (El) for G|7H|yN7OS: 370 (MH+).
4-|7-(5-annii l,3,4-Un'adiiizol-2-^^
yl|-5.6-dimcLhylpyriinidin-2-amine. Ή NMR (400 MHz. mcihanoi-di): 7.68 (d, 11-1), 7.55 (d. IH), 4.98 (s.2H).4.48 (t.2Ή), 4.15 (I.2H).2.33 (s.3H), 2.26 (s, 3H).2:25 (s, 3H). MS (EI) for C,sH2iN7OS: 384 (ΜΙ-Γ).
4-[7-.(5-amino- 1 ,3, -iliiiidi:izol-2--.yl)-9-inc.tl'iyl-2,3-dihydro- 1 ,4rb.ciizpxazepin-4.(51-l)- yl |-5-.( l--me"Uiy1eihyl>pyrimitlin-2-aiiiiiie. Ή NMR (400 MHz. mcihanol-dj.): 7.80 (s. 1 1-1); 7.57 (d, I H), 7.46 (d. 1 H). 4.66 (s, 211), 4.36 (m, 2H), 3.93 (in, 21-1); 3:0 (m, 1 H), 2.26 (s, 3H), 1,95 (s, 31-1), 1.22 (d, 6H). MS (El) for C19H.23N7.OS: 398 (ΜΙ-Γ).
4-|7-(5-amino- 1 ,3.4-lhiadia/.ol-2-yl)-9-mcthyr-2,3-(JihydiO- 1 ,4-benzoxazcpin-4(5H)- yl |-5-cihcnyl-6-nicihylpyrimidin-2-aminc. ' HTNMR (400MHz. d.i-McOH): 7.6S (s. I H). 7.57 (s. I H), 6.55 (dd. I H). 5.65 (d, I I I). 5.33 (d, I H), 5.05 (s. 2H). 4.41 (lr. 2H). 4.19 Or. 21 1), 2:32 is, 3H), 2.25 (s. 3H). MS (El) for ^Η3|Ν705: 396 (ΜΙ ).
Biological Example 1
mTOR/GblJR aplor (mTORC 1 :) E LIS A. Assay
[008841 The measurement of mTORC I enzyme activity was performed in an ELISA assay format following the phosphorylation of 4E-BP 1 protein. All experiments were performed in the 384- cll format. Generally, 0.5 p L DMSO containing varying concentrations of the test Compound was mixed with 15 p L enzyme solution. Kinase reactions were initiated with the addition of 15 pL of siibstrates-containing solution. The issay conditions were -as follows; 0.2 nM mTORC 1. 10 p ATP and 50 iiM NHis-tagged 4E-BP 1 in 20 inM Hepes. pH 7.2, I mM D'lT. 50 m aCf 10 mM Mud;, 0.02 mg/niL BSA, 0.01 % CHAPS. 50 mM
P-glycerophospIiate. Following an incubation of 120 minutes" at anibicnt icmperature, 20 p L of the reaction volume was transferred to a Ni-Chelale-eoaled 384-wcll plate. The binding step of thc 4E-BPl protein proceeded for 60 minutes, 'fol lowed by washing 4 times each with 50 p L of Tris-buffered saline solution (TBS). Anti-phospho-4E-BP l rabbit-IgG (20 ^i L. 1 :5000) in 5% BSA-TBST (0.2% Tween-20 in TBS) was added and further incubated for 60 minutes. Incubation with a secondary HRP-tagged anti-IgG was similarly performed after washing off the primary antibody (4 washes of 50 p L). Following the final wash step with TBST, 20 pL of SuperSignal ELISA Fciiilo (Pierce Biotechnology) was added and the luminescence ''.measured using an EnVision plate reader.
1.00885] As numbered in Table I , Compounds 2-3, 5-6. 8-12. 14. 16. 17, 19-23, 28, 30-32, 34, 40-44, 46, 49-59. 61 -67. 80, 84. 85-94. 98-99. 104- 108. 1 13.. 1 15- 1 16, 119- 130, 132- 139. 141 - 147. 149- 156, 158- 166. 168- 169. 172- 181 have an ICS„ in this assay of iess than or equal to 100 nM.
100886] As numbered in Table 1. Compounds 7. 1 , 1 . 18. 24-27, 33, 35-39. 45. 47-48, 60, 68-79, 81 -83. 100, 103, 109-112, 1 14. 1 17- 1 18, 131 , 140, 148, 157, 167, 170- m have an IC50 i» this assay of greater than 100 nM but less than or equal to 500 nM.
Biological Example 2
Imnume-Complcx mTORC2 Kinase (mTORC2 IP-Kiiiase) Assay
[00887J I-leLa (ATCC) cells are grown in suspension culture and lysed in ice-cold lysis buffer containing 40 niM H EPES pH 7.5, 1 20 mM NaCI. I mM EDTA. 10 mM sodium pyrophosphate, I O nM'|J-glyccrophosphatc, 10 mM NaF, 10 mM NaNi, one tablet of protease inhibitor Cdiii leiCT i i. EDTA-frec, Roche), 0.3%
cholamidoprqpyldinicihylannnoniopi opaiicsiilloiiate (CHAPS). I. niM AEBSF. 0.5 mM benzamidine. HCI, 20 Hg inL heparin; and 1 :5 niM a3VG).i. The ni ORG? complex is immunoprecipitatcd with anti-R ICTOR antibody for 2 h. The immune complexes arc immobil ized on Protein A sepharose (GE Healthcare. 17-5280- 1 ), washed sequentially 3 limes with wash buffer (40 mM HEPES pH 7.5. 120 mM NaCI. 10 mM
β-glyccrophosphaie. 0.3% CHAPS, I mM AEBSF. 20 Mg/mL heparin. 1 .5 mM Na.^VO,). and Complete-Mini, EDTA-frec) and resuspended in kinase buffer (40 niM HEPES, pH 7.5. 120 mM NaCI, 0.3% p lARS, 20 pg L heparin, 4 luM. gCU, 4 m nCU. 10% Glycerol, and 10 mM DTT); The immune complexes (equivalent to l xl 0? cells) are. re-incubaicd at 37 °C with a test Compound or 0:6% DMSOT0i: min, ahd hen
for 8 min in a final volume of 33 Ε (including 5 μΙ_ bed volume) containing kinase buffer. 50 μΜ ATP, and 0.75 jig ful l lcaglli clephasph ryiated AKT I . Kinase reactions arc tcnninated by addition of 1 1 μΕ 4x SDS sample buffer containing 20% β-mercaptoethanol and resolved in a 10% Tris Glycine gels. The gels arc transferred onio PVDF membrane at 50' V for 20 h at 4 °C. The membranes are blocked in 5% non-fat milk iii TBST for 1 h and incubated overnight at 4 °C wiili l / l 000 dilutidn ;of rabbit anti;-pAKT'(S473) (Cell Signal ing Technology. 4060) in, 3% BS.AyT.RST. The membranes arc washed 3 times in TBST and incubated for 1- h with a 1 /10000 dilution of secondary goat anti-rabbit HRP antibody (Cell Signaling Technology. ,2125) iiv 5% non-fat niilk/TBST. The signal is detected using
Amcrsham ECL-plus. The scanned data arc analyzed using ImagcQuanl software. .IC50 for the test Compound is determined relative to DMSO treated sample using XLfil4 software.
Biological Example 3
P13K B iochemical Assays
[00S88J P13Ku activity was measured as the percent of ATP consumed following the kinase reaciioivusiiig liici fer;Lse-lucifcnn-cpupIcd chcmilmni'iiesccnc.e. Reactions were conducted in 384-wcII white, medium binding microliter plates (Greiricr). Kinase reactions were initiated by combining test compounds. ATP. substrate (PIP2), and kinase in a 20 p L
volume ina buffer solution. The .'standard PDKalpha assay buffer was-eo.mposed 50 niM Tris. pH 7.5, 1 niM EGTA, lO niM MgCI2.. I ni PTT and 0.03% CHAPS. Thc:standard assay concentrations for cnzynie, ATP, andsubstrate were 3 nM, ΙμΜ, and 10 μΜ. respectively. The rcaclion mixture was incubated at ambient temperature for approximately 2 h. Following the kinase reaction, a 10 yiL aliquot of luciferase-lticiferin mi (Promega inase-Glo) was added and the chemiiumincscenec signal measured using a Victor2 or EnVision (Perkin Elmer). Total ATP consumption was limited. to 40-60% and IC50 values of control compounds .-correlate well with literature references. Substituting ΡΙ3ί<α with ΡΒ β, Ι3Κγ, or ΡΙ3 δ. the inhibitory activity of the compounds for the other isoforms of PI3K were measured. For the ΡΙ3Κβ and Ρ13Κδ assays, enzyme concentrations were .10 nM. and 4 nM. respectively. For the. ΡΪ3 Κγ assay, enzyme concentration was 40nM, the incubation time was I h, and the concentration of M'g'Clj in the assay buffer was 5 niM.
(008891 As numbered in Table I . Compounds 2.3.5- 12.14- 1 , 18, 20, 21.23.26, 2S, 30- 32, 35, 40.41, 43-59, 61-67.70, 72-76, 78-94 98-105.107-109.113.115-11 . I 19-130.132- 147.149-173.175-182 have an IC5o in the PI3K-aipha assay of lcss than or equal to 100 nM.
[008901 As numbered in Table I . Compounds 13.22, 24-25.27.34.36-39.42.60.71 , 77. 106, 111-112.114.117-118, 131.148.174, have an ICsoirijhe PI3K¾ipha; assay of greater than.100 nM but lcss.ihan.-. re„qu.al-;io 0'n .
[00891 As numbered in Table 1 , Compounds- 17, 19; 33, 68,69.109.110 have an IC5o in the P13 -alpha assayof greater I haiv 500 nM but less than or equal to '2500 nM.
[00892] Embodiments 1: In one embodiment the invention comprises a compound of the invention having a PI3 -alpha-inhibiiory activity of about 0.5 μΜ or less and is inactive for mTOR (when tested at a concentration of 2.0 μΜ or greater) or is selective for PI3 -alpha over niTOR by about 5-fold or greater, about 7-fold or greater, or about 10-fold or greater. In another embodiment, the invention comprises a compound of the invention having a PI3K- alpl i-mhibiiory activity of about 0.35 μΜ or less and is inactive for mTOR (whcnicslcd at a concentration of 2.Ό μΜ or.grcater) or is selective for PBK-alpha ove nvlOR by about 5- fold or greater, about 7-fold or greater, oraboiit 0-fdld orgreatcr. In another embodiment, the invention comprises a compound of the invention having a Pl3K-alpha-inhibiloiy activity of about 0.25 μΜ or less and is inactive for mTOR (when tested at a concentration of 2.0 μΜ or greater) or is selective for PI3K-alpha over mTOR by about 5-fold or greater, about 7-fold or greater, or about 10-fold or greatcr. In another embodiment the compounds of the invention have an PI3 -alpha-inhibitory acliviiy of about 0.1 μΜ or less and.is inactive for mTOR (when tested at a concentration o 2.0 μΜ or greater) or is selective lor PI3K-alpha
over inTOR by about 5-folti or greater, about 7-fold or greater, or about 10- fold or greater. In another embodiment the invention comprises a compound o the invention having an PI3 K- alpha-inhibilory activity of aboulO.()5 μΜ or less and is selective for PI3 K-alpha over mTOR by about 5-fold or greater, about 7- fold or greater, or about 10-fold or greaicr.
100893] Embodimcnts.2:. In otic embodiment the invention comprises a compound of the invention haying. a PO K-alplui-inhibiiory activity of about 2.0 μΜ or Jess and. an mTOR- mhibilOry aetivity of about 2.0 μΜ .οΐ" lcss .aiid the selectivity, for one of the targets over the other does not exceed 3-fpld. In . another embodiment the invention cotnpriscs a epmppund of the invention having a PLl Kralpha-inliibil ry activity of about Ι .Ο or less.'ahd an mTOR- inhibilpry activity of about 1.0 μΜ or less and the selectivity for one of the targets over the other does not exceed 3-fold. In another embodiment the invention comprises a compound of the invention liaving a P[3 -alpha-inhibilory activity of about 0.5. μΜ .οι; less and an mTOR- ■ inhibitory aeliviiy of about 0.5 μ or less aiul live selectivity for oiic of the targets over the dllier does riot exceed old. In .iin'plhcr: embodiment the invention comprises a compound of the invcnlipn haying a P13 K-alpha-inhibitory activity of about 0.3 μΜ or less and an hiTOR- inhibiiory activity.of ab iil 0.3 uiVI or less and the selectivity for one of "the targets over the other does not exceed 3-fold. In another embodinveni the invention comprises a compound of the invention having a P13 -alpha-inhibiipry activity of about 0.2 μΜ or less and an mTOR- inhibitory activity of about 0.2 Μ or less and the selectivity for one of the largetsiover the other does not exceed 2-fold. 1 n another embodiment the invent ion comprises a compound of the invention having a P13K-a!pha-inhibitory activity of about 0. Γ5 μΜ or less and an mTQR- inhibitory activity of about.0.15 μΜ or less and the selectivity for oiic of the targets over, the other does not exceed 2-fold. In another embodiment the invention comprises a compound of the invention having a P13.K-alpha-.inhibi.tory activity of about 0, 1 μΜ or less and ail mTOR- inliibitory activity of about 0; 1 μΜ or less. In another embpcl imcnl thc invention comprises a compound of.lhe invention having. a PI3K-alpha-inhibitor .activity of about 0.05 μΜ or less and an mTOR-iiihibitory activity of about 0.05 μΜ or less. Iivanother embodiment the invention comprises a compound of the invent ion have a P ! 3 - a 1 ph a - i ri h i b i to ry activity of about 0.02 u or less and an mTOR-inhibitory activity of about 0.02 μΜ or less. In another embodiment the invention comprises a compound of the invention have a PI3K-alpha- inhibitory activity of about 0.01 μι\·1 or less and ail mTOR-inhibilory activiiy of about 0.01. μΜ or less.
Biological Example 5
pS6 (S240/244) ELISA Assay.
[00894] MCF-7 ccl Is (ATCC) ceils were seeded at 24000 eel Is per well in 9 -we 11 plates (Corning, 3904) in DiVIEM (Cel lgro) containing 10% FBS (Cellgro), 1 % NEAA (Ccl lgro) and 1 % penicillin-streptomycin (Cellgro). Cells were incubated at 37°C. 5% C02 Tor 48 h. and the growth medium was replaced with serum-frcc DiVIIZiVl or in medium containing 0.4% BSA. Serial dilutions of the lest Compound in 0.3% DMSO (vehicle) were added to the cells and incubated or 3h. To fix i e cells, medium was removed and ΙΟΟμίΛνεΙΙ of 4% formaldehyde (S igma Aldi ich, F8775) in TBS (20 niM Tris, 5()0 miM NaCI) was added to each well at RT for 30 min. Cel ls were washed 4 times wiilr.200jiLTB'S containing 0. 1 % Triton X-.1.00;(Sigma, catalog # T9284). Plates were blocked with Ι ΟΟμΙ. Odyssey blocking buffer (Li-Cor Biosciences. 927,40000) for l h at RT. Anti-pS6 (S240/244) . antibody (Cell Signaling Technology, 22 15) and anti-iota I -S6 antibody (R&D systems, MAB5436) were diluted 1 :400 in Odyssey blocking buffer, and 50μΙ- of the antibody solution containing both antibodies wa added io one plate lo detect pS6 and total S6. After incubation overnight at 4 C, plates were washed 4 limes with 200^L BS containing 0.1 % Tween20 ( Bio-Rad. catalog # 170-6351 ) (TB-ST). Goal anti-rabbit and Goat anti-mouse. secondary antibody (Li- Cor B iosciences, catalog it 926-32221 and 926-32210) conjugated to IRDye were diluted 1 :400 in Odyssey blocking buffer containing 0. 1 % Twecn20. 50uL.of antibody solution containing both antibodies was added to each well and incubated for l h at RT.- Plates were washed 3 limes with 20ϋμΙ_ ΤΒ5Τ and 2 times with 200'j.iL TBS. Fluorescence was read on an Odyssey plate reader. IC50 values were determined bascd.on the ratio of pS6 to total S6 signal for Compound treated wells, normalized to the DMSO-ireat¾l control wells.
{00895] In one embodiment, the Compounds of the Invention tested in this assay in MGF- 7 cells had an inhibilory activity of 1 .5 μ or less. In another embodiment, the Compounds of the Invention tested in this assay in MCF-7 cells had an inhibilory activity of 1 .0 μ or less. In another embodiment, the Compounds of the Invention tested in this assay in MCF-7 cells had an inhibilory activity of 0.5 μΜ or less. In one embodiment, the Compounds, of the Invention tested in this assay in MCF-7 cells had an inhibilory activity of 0.3 μΜ or less. In one embodimcnl. the Compounds of the Invention - tested in this assay in MCF-7 cells had an inhibilory activity of 0. 1 μΜ or less. In one embodiment, tlic Compounds of the Invention tested in this assay in MCF-7 cells had an inhibilory activity of 0.03 μΜ or less.
[00896] In one embodiment, the Compound of the Invention tesied in this assay in PC-3 cells had an inhibitory activity of about 1.7 μΜ or less. In another embodiment, the
Compound of ihc Invention tested in this assay in PC-3 cells had an inhibitory activity of about 0.55 u or less. In another embodiment, the Compound of the Invention tested in ibis assay in PC-3 cells had an inhibitory activity of about 0:55 μ or less. In another embodiment, the Compound of the Invention tested in this assay in PC-3 cells had an inhibitory activity of about 0.3 μΜ or less. In another embodiment, the Compound of die Invention tested in this assay in PC-3 cells had 'an inhibitory activity of about 0. 1 μΜ or less. In another embodiment, ihc Compound of the Invention tested in this assa in PC-3 cells bad an inhibitory "activity of about O.OS .u or less.
Biological Kxample 6
AKT (T308) ELISA Assay
[00897] MCF-7 cells ( ATCC) cells were seeded at 24000 cells per well in -well plates (Corning, 3904) in DM EM (Cel lgro) containing 10% FBS (Ccllgro), 1 % ΝΕΛΑ (Ccllgro) and 1 % pcnicillin-stfcpioniycin (Cellgro). Cells were incubated at 37°C, 5% C02 for 48 h, and the growth medium was replaced with serum-free DMEM or in medium containing 0.4% BSA. Serial .dilutions of the tcsi Compound in 0.3% DMSO ( vehicle) were added to the cells and incubated for 3 It. At the end of the incubation period, cells were stimulated for 10 minutes by the addit ion of L-IGF (S igma, I- 127 1 ) at a final concentration of l OOng/ml. Afterwards, media was discarded from cell plates and I ΙΟμΙ/well of cold lysis buffer (sec table below) were added. Cell plates were incubated on ice and then put on shaker in 4°C cold room for l b. Two capture plates (Thermo Scientific. Reacti-bind plate. 1 5042) were prepared for each cell plate by pre-coating with capture Akl antibody from the two sandwich ELISA antibody pairs used (Cell Signaling Technology 7 142 and 7144). The Akl capture antibodies were diluted 1 : 100 in PBS and Ι Ο Ι of diluted capture antibody was added per well. Capture plates were incubated at 4C overnight. Prior to use, capture. lates were washed 3 limes in TBS containing 0. 1 % Tween20 (Bio-Rad, 170-63 1 ) (TBST) and blocked in blocking buffer (Thermo Scientific. Starling' Block T20. 37543) for 1 - 2 h at room temperature. After 111 of cel l lysis, 85j.il of cell lysalc/wcll was transferred to the capture plate for detection of pAkt(T308 ). 1 μΙ of cell lysate was transferred from same well to the second capture plate for detection of total Aktl . After incubat ion -overnight at 4°C. plates were washed 3 limes with 20(tyL TBST. Primary antibodies, diluted 1 : 100 in blocking buffer, were added to the corresponding capture plates for pAkt(T308) (Cell S ignaling Technology, 7 144) and total Akt l (Cell Signaling Technology. 7 142) detection and incubated at room temperature for J h. Plates were washed 3 times with 200pL of TBST. Goal a ti-mouse secondary antibody (Cel l Signaling Technology, 7076) conjugated to I-IRP was diluted
1 : 1000 in blocking-buffer and Ι ΟμΙ were added to' each well and incubated "for 30 minutes at room temperature. Plates were then washed 3 times with 200μΙ_ of TBST. Ι Ομί O
SiipcrSignal ELISA Fcmto stable peroxidase solution (Thermo Scientific. 37075) was added to each well. After I minute incubat ion, chemilumincscenee was read on a Wallac V ictor2 1420 multilabel counter. IC50 values were determined based on the rat io of pAkt(T308) to total Akt l signal for Compound treated wells, normalized to the DMSO-trealed conLrol wells.
[00898] In one embodiment, the Compounds of the Invention tested in this assay in PC-3 cells had an. inhibitory activity of about 2.0 μΜ or less In another embodiment, the
Compounds of the Invention tested in this assay in PC-3 cells had an inhibitory activity, of about 1 .0 μΜ or less. In another embodiment, the Compounds of the Invention tested in this assay in PC-3 cells had an inhibitory activity of about 0.3 μΜ or less; In another embodiment, the Compounds of the Invention tested in this assay in PC-3 cel ls had an inhibitory activity of about 0.2 μΜ or less.
|()0899) In one embodiment, the Compounds of the Invention tested in this assay in MCF- 7 cells had an inhibitory activity of about 3.0 μΜ or less. In another embotiiment, the Compound of the Invention tested in this assay in MCF-7 cells had an inhibitory activity of about 3.0 μΜ or less: In another embodiment, the Compounds of the Invention tested in this assay in MCF-7 cells had an inhibitory activity of about 1 .5 μΜ or less. In another embodiment, the Compounds of the Invention tested in ibis assay in MCF-7 cells had an inhibitory activity of about 0.75 μΜ or less. In another embodiment, the Compounds of the Invention tested in this assay in MCF-7 cells had an inhibitory activity of about 0.5 μΜ or less. In another embodiment, the Compounds of the Invention tested in this assay in MCF-7 cells had an inhibitory activity of about 0.25 μΜ or less. In another embodiment, the
Compounds of the Invention tested in this assay in MCF-7 cells had an inhibitory activity of about 0.1 μΜ or less.
Biological Example 7- 13
Pharmacodynamic xenograft tumor models
1 0.9001 Female and male athymic nude mice ( NCr) 5-8 weeks of age and weighing approximately 20-25 g are .■used in the Following -models. Prior to inilialion.oF a study, the animals are allowed lo aecl imaie'For a minimum οΓ 8 h. During these '.studies, -animals are provided Food andHvalcr ad libitum and housed ii rooniicoiiditioped at 70-75cF and 50% relative luiinidity..A 12 h Itght aikl 12 li dark cycle is maintained with automatic timers. All animals are examined daily For compound-induced or tumor-related deaths.
MCF-7 Breast adenocarcinoma model
[009011 MCF7 human mammary adenocarcinoma cells are cultured in vitro in DMEM (Ccllgro) supplemented with 10% Fetal Bovine Serum (Cell ro). Pcnicillin-Slrepidriiycin and non-essential amino acids at 37 °C in a , humidified 5% CQi atmosphere. On day 0. cells are harvested by irypsiiiixat ion. aiul 5 x 10'' cells iii 100 p L oF a solution made oF 50 cold Hanks balanced salt solution with 50% growth Factor reduced matrigcl (Beeton Dickinson) implanted subcutaneously into the hindl'larik of Female nude mice. - --A irahspbiider is implanted into' each mouse For idcnliFication and data tracking, and aiiiinals arc moi it rcd daily For clinical symptoms and survival.
[00902] Tumors are established in Female athymic nude mice and staged when the average tumor weight reached 1 0-200 nig... A Compound of the Invention is orally administered s a solution/Fine suspension in water (with 1 : 1 molar rat io of I N HCL) once-daily (qd) or twice-daily (bid) at 10, 25. 50 and 100 mg/kg For I4 days. During ihc dosing period of 14- 1 days, tumor weights are 'determined twice-weekly aiid body weights arc recorded daily.
Colo-205 colon model
[00903] Colo-205: human colorectal•carcinoma c^
(Mediatcch) supplemented willi. 10% Fetal Bovine .Scruni (Hyclone), Pciiicil lin-Streptomycin and non-essential amino acids at 37 °C in a - luintidificd. 5% COv tmosphere. On day 0, cells arc harvested by irypsinization. and 3x 10'' cells ( passage 10- 1 . >95% viability) in 0.1 ml. ice-cold Hank's balanced salt solut ion are implanted intradermally in the hind-Flank oF 5-8 week old Female athymic nude mice. A transponder is implanted in each mouse For identification, and animals are monitored daily For clinical symptoms and survival .
[00904] Tumors are established in Female' athymic nude mice and staged when the average tumor weight reached 100-200 nig. A Compound oF thc Invention is orally administered as a solution/Fine suspension in water (with I : I. molar ratio oF I N 1-lCL) oncc^daily (qd) or
twice-daily (bid) at 10. 25. 50 and 100 mg/kg ("or 14 days. During the dosing period of 14 days, tumor weights are determined twice-weekly and body weights arc recorded daily.
I'C-3 prostate adenocarcinoma model
[00905J PC-3 human prostate adenocarcinoma cells are cultured in vitro in DMEM (Mediated)) .supplemented with 20% Fetal Bovine Serum (Hyelone). Penicillin-Streptomycin and non-essential amino acids at 37 °C in a humidified 5% C02 atmosphere. On day 0. cells are harvested by trypsinizai ion and 3x 10ft cells (passage 10- 14. >95% viability) in 0. 1 mL of ice-cold flank's balanced salt solution are implanted subcuiancously into the hindflank of 5-8 week old male nude mice. A transponder is implanted in each mouse for identification, and animals arc monitored daily for clinical symptoms and survival;
['0090<>] Tumors -arc established in male athyniic ntide inice and staged when the average tunior we iglit, reached 1.00¾().0 nig. A Compound of. the Invention is orally administered as a solution fine suspension in water (with 1 : 1 molar ratio of 1 N HC1) once-daily (qd) or twice-daily (bid). at 10, 25, 50, or 100-ing/kg for 19 days. During the closing period of 14- 19 days, tumor weights are determined twice-weekly and body weights arc recorded daily.
U-87 NIG human glioblastoma model
[009071 U-87 MG human glioblastoma cclls are cultured in vitro in DMEM (Mediatech) supplemented with 10% Fetal Bovine Serum .(Hyelone), Pciiicillin-Strepiomyein and nonessential amino acids at 37 °C in uiniidified 5% GO? atmosphere. On day (.). cells arc harvested by irypsinization and 2x 106 cells (passage 5, 96% viability) in 0. 1 mL of ice-cold Hank's balanced salt solution are implanted intradermally into the hindflank of 5-8 week old female nude mice. A transponder is implanted in each mouse for identification, and animals arc monitored daily for cl inical symptoms and survival. Body sveights are recorded daily.
A549 human lung carcinoma model
[00908] A549 human lung carcinoma cells. are cultured in vitro in DMEM (Mediatech) supplemented with 10% Fetal Bovine Serum (Hyelone), Penicillin-Streptomycin and nonessent ial .-amino acids at 37 °C in -a humidified 5% CO atmosphere. On day 0, cells arc harvested by trypsinizaiion and 1 Ox 10f> cells (passage 12. 9.9% viability) in 0.1 mL of ice-cold Hank's balanced salt solution, are implanted intradermally into the hindflank of 5-8 week old female nude mice. A transponder is implanted in each mouse for identification, and animals are monitored daily for clinical symptoms and survival. Body weights are recorded daily.
Λ2058 human melanoma model
[00909] A2058 human melanoma cells are cultured in vitro in DMEM (Mediatech) supplemented with 10% Fetal Bovine Scrum . (Hyelone), Penicil lin-Streptomycin and non-
essential amino acids ai 37 "C in , a humidified, 5% C02 atmosphere. On day 0, cells are harvested by trypsinization and 3x 10° cells (passage 3. 95% viabil ity) in 0. 1 niL ice-cold Hank's balaiice'd salt solution : are implanted iniradcrmally in the hind-flank of 5-8 week old female aihymie nude mice. Λ transponder is implanted in each mouse for identi fication, and animals are monitored daily for clinical symptoms and survival. Body weights, are recorded daily.
WM-266-4' human melanoma model
[009101 WM.- 2.66-4 human melanoma cells arc cultured in vitro in DM EM ( ediatcch) supplemented with 10% Fetal Bovine Sciiim ( Hyclonc). Penicillin-Streptomycin and nonessential amino acids at 37 °C in a humidified, 5% C02 atmosphere. On-day 0, cells are hai'.vcsicd by irypsihizaliou and 3x 10r' cells (passage 5, .9% viabil ity), in 0. 1 niL ice-cold Hank 's balanced salt soUilipn are implanted intradcrmally in ihe hind-flank 5.-8 week old female athymic nude mice. A transponder is implanted inieaeh mouse for identification, and ariiniiils; arc monitored daily for- clinical symptoms and survival.. Body weights are recorded daily.
[0091 1,| Tumor Weight (TW.) in. the above models is determined by measuring
perpendicular diameters with a caliper, using the followin formula:
tumor weight (mg) = [tumor volume = length (mm) x width2 (mm2) |/2
These data were recorded and plotted on a tumor weight vs. days post-iniplantatioiVl iiie graph · and presented graphical ly s an indication of tumor growth rates. Percent inhibition of tumor growth (TGI) is determined with the following formula:
where X¾ = average TW of al l tumors on group day
Xr = TVV of treated group on Day f
Yi = TW of vehicle control group on Day f
If tumors regress below their starting sizes, then the percent tumor regression is determined with the following formula:
Tumor size, is calculated individually for each tumor to obtain a mean ± SEM value for each experimental group. Statistical significance is determined using, the 2-iailcd Student's t-test (significance defined as P<().()5).
100912] The foregoing invention has been described in some detail by way of illustration and example,. or purposes of clarity and understaiiding..The invent ion has been described with reference to: various specific embodiments and icehniqucs. However- it should be understood that many variations and modifications may be made while remaining within the spirit and scope of the invention. It will be obvious to one of skill in the art lhat changes and modifications may be practiced within the scope of the .-appended claims. Therefore, it is to be understood that the above dcscriplion is intended to be illustrative and not restrictive. The scope of the in ention should, therefore, be determined not with reference to the above description, btii shou!d insteacl be determined with reference lo .tlic following appended claims, along with the- ull scope of equivalent's to which such claims ar.e.ciuitled. Ail patents, patent applications -and' publications eifed'-'in this application" are licrcby 'incorporal.eil by reference in their entirety for all purposes to ihc same extenl as if each individual patent, patent application or publ icaiion were so individually denoted.